EP0643123A2 - Verfahren zur Behandlung von halogenierten Kohlenwasserstoffen - Google Patents
Verfahren zur Behandlung von halogenierten Kohlenwasserstoffen Download PDFInfo
- Publication number
- EP0643123A2 EP0643123A2 EP94308591A EP94308591A EP0643123A2 EP 0643123 A2 EP0643123 A2 EP 0643123A2 EP 94308591 A EP94308591 A EP 94308591A EP 94308591 A EP94308591 A EP 94308591A EP 0643123 A2 EP0643123 A2 EP 0643123A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- stream
- hydrogen
- halogenated organic
- compounds
- hydrogen halide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G45/00—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G45/00—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds
- C10G45/02—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to eliminate hetero atoms without changing the skeleton of the hydrocarbon involved and without cracking into lower boiling hydrocarbons; Hydrofinishing
Definitions
- the field of art to which this invention pertains is the production of a hydrogenated hydrocarbonaceous product and an anhydrous hydrogen halide stream from a halogenated organic stream.
- the preferred hydrogen halide stream is anhydrous which minimizes the cost of building the processing plant based upon metallurgical considerations.
- the invention provides an improved process to convert organic waste streams containing halide compounds to hydrogenated organic compounds and to recover the resulting hydrogen halide as an anhydrous product stream.
- the invention provides a process for treating a halogenated organic stream to produce a hydrogenated hydrocarbonaceous stream having a reduced level of halogen and an anhydrous stream comprising a hydrogen halide which process comprises the steps of: (a) contacting the halogenated organic stream, a hydrogen-rich gaseous recycle stream and a recycle stream comprising unreacted halogenated organic compounds with a hydrogenation catalyst in a hydrogenation reaction zone at hydrogenation conditions to increase the hydrogen content of the halogenated organic stream and to thereby produce hydrogen halide; (b) condensing at least a portion of the resulting effluent from the hydrogenation reaction zone to produce the hydrogen-rich gaseous recycle stream and a liquid stream comprising hydrogenated hydrocarbonaceous compounds and hydrogen halide; (c) separating the liquid stream comprising hydrogenated hydrocarbonaceous compounds and hydrogen halide to produce an anhydrous stream comprising hydrogen halide and a stream comprising hydrogenated hydrocarbonaceous compounds and unreacted halogenated organic compounds; and (
- Embodiments of the present invention encompass further details such as preferred feedstocks, hydrogenation catalyst and operating conditions, all of which are hereinafter disclosed in the following discussion of each of these facets of the invention.
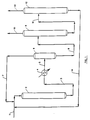
- the drawing is a simplified process flow diagram of a preferred embodiment of the present invention.
- the present invention provides an improved integrated process for the conversion of a halogenated hydrocarbonaceous stream to produce a hydrogenated hydrocarbonaceous stream having a reduced level of halogen and an anhydrous stream comprising a hydrogen halide.
- halogenated organic compounds both unsaturated and saturated, are candidates for feed streams in accordance with the process of the present invention.
- organic streams comprising halogenated organic compounds which are suitable for treatment by the process of the present invention are dielectric fluids, hydraulic fluids, heat transfer fluids, used lubricating oil, used cutting oils, used solvents, halogenated hydrocarbonaceous by-products, oils contaminated with polychlorinated biphenyls (PCB), halogenated wastes, petrochemical by-products and other halogenated hydrocarbonaceous industrial wastes.
- PCB polychlorinated biphenyls
- halogenated wastes petrochemical by-products and other halogenated hydrocarbonaceous industrial wastes.
- the halogenated organic compounds may also contain hydrogen and are therefore then referred to as hydrocarbonaceous compounds.
- the halogenated organic feedstock preferably contains less than 500 ppm by weight of water and, in certain contexts, or water precursors.
- water precursors are oxygenated compounds which, when subjected to hydrogenation conditions in the presence of certain of the contemplated catalysts, are converted into hydrogenated compounds and water.
- anhydrous stream comprising hydrogen halide connotes a stream having less than 50 ppm by weight of water.
- Preferred feedstocks comprise fractionation column bottoms in the production of allyl chloride, fractionation column bottoms in the production of ethylene dichloride, fractionation column bottoms in the production of trichloroethylene or perchloroethylene, used dielectric fluids containing polychlorinated biphenyls (PCB) or halogenated benzene, used solvents, fractionation bottoms from the purification column in epichlorohydrin production, carbon tetrachloride, 1,1,1-trichloroethane, halogenated alcohols, halogenated ethers, chlorofluorocarbons or admixtures thereof.
- PCB polychlorinated biphenyls
- halogenated organic compounds which are contemplated as feedstocks in the present invention may contain chlorine, bromine, fluorine or iodine.
- Preferred halogen compounds contain chlorine or fluorine.
- a feedstock containing halogenated organic compounds is introduced in admixture with a hydrogen-rich, gaseous recycle stream and a recycle stream comprising unreacted halogenated hydrocarbonaceous compounds into a catalytic hydrogenation zone containing hydrogenation catalyst and maintained at hydrogenation conditions.
- This catalytic hydrogenation zone may contain a fixed, ebulliated or fluidized catalyst bed. This reaction zone is preferably maintained at conditions which are chosen to dehalogenate the halogenated organic compounds which are introduce thereto.
- the catalytic hydrogenation zone is preferably maintained under an imposed pressure from atmospheric to 2000 psig (14 MPa, gauge) and more preferably under a pressure from 100 psig (0.7 MPa, gauge) to 1800 psig (12.5 MPa, gauge).
- a maximum catalyst bed temperature in the range of 122°F. (50°C.) to 850°F. (455°C) selected to perform the desired dehalogenation conversion to reduce or eliminate the concentration of halogenated organic compounds contained in the combined feed stream and to consequently increase the hydrogen content of the stream.
- the desired hydrogenation conversion includes, for example, dehalogenation and hydrocracking.
- Further preferred operating conditions include liquid hourly space velocities in the range from 0.05 h ⁇ 1 to 20 h ⁇ 1 and hydrogen circulation rates from 200 standard cubic feet per barrel (SCFB) (35 normal m3/m3) to 100,000 SCFB (18,000 normal m3/m3), preferably from 200 SCFB (35 normal m3/m3) to 50,000 SCFB (9,000 normal m3/m3).
- SCFB standard cubic feet per barrel
- hydrotreating or “hydrogenation” is meant to include reactions whereby the organic reactants achieve an increased hydrogen content, regardless of whether this is achieved by olefin saturation, diolefin saturation, desulfurization, denitrification or dehalogenation, for example.
- the hydrocarbonaceous effluent containing at least one hydrogen halide compound from the hydrogenation zone is cooled and introduced into a vapor-liquid separator to produce a hydrogen-rich, gaseous recycle stream and a liquid stream comprising hydrogenated hydrocarbonaceous compounds and hydrogen halide.
- the resulting liquid stream comprising hydrogenated hydrocarbonaceous compounds and hydrogen halide is separated to produce an anhydrous stream comprising hydrogen halide and a liquid stream comprising hydrogenated hydrocarbonaceous compounds and unreacted organic compounds.
- This resulting liquid stream is then separated to produce a recycle stream comprising unreacted halogenated organic compounds which is introduced into the hydrogenation reaction zone and a hydrogenated hydrocarbonaceous stream having a reduced level of halogen.
- the hydrogen halide compound is recovered as an anhydrous product stream. This permits the subsequent recovery and use of a desirable and valuable hydrogen halide compound.
- the preferred catalytic composite disposed within the hereinabove described hydrogenation zone can be characterized as containing a metallic component having hydrogenation activity, which component is combined with a suitable refractory carrier material of either synthetic or natural origin, especially a refractory inorganic oxide.
- a suitable refractory carrier material of either synthetic or natural origin, especially a refractory inorganic oxide.
- Preferred carrier materials are alumina, silica, carbon and mixtures thereof.
- Suitable metallic components having hydrogenation activity are those selected from the group comprising the metals of Groups VIB and VIII of the Periodic Table, as set forth in the Periodic Table of Elements, E. H. Sargent and Company, 1964.
- the catalytic composite may comprise one or more metallic components from the group of molybdenum, tungsten, chromium, iron, cobalt, nickel, platinum, palladium, iridium, osmium, rhodium, ruthenium, and mixtures thereof.
- concentration of the catalytically active metallic component, or components is primarily dependent upon a particular metal as well as the physical and/or chemical characteristics of the particular hydrocarbon feedstock.
- the metallic components of group VIB are generally present in an amount within the range of from 1 to 20 weight percent, the iron-group metals in an amount within the range of from 0.2 to 10 weight percent, whereas the noble metals of Group VIII are preferably present in an amount within the range of from 0.1 to 5 weight percent, all of which are calculated as if these components existed within the catalytic composite in the elemental state.
- hydrogenation catalytic composites may comprise one or more of the following components: cesium, francium, lithium, potassium, rubidium, sodium, copper, gold, solver, cadmium, mercury and zinc.
- Preferred hydrogenation catalysts comprise alumina and palladium.
- the resulting hydrogenated hydrocarbonaceous effluent from the hydrogenation reaction zone is preferably separated to produce a hydrogen-rich gas phase and a liquid hydrocarbonaceous stream in a separation zone which is maintained at essentially the same pressure as the hydrogenation reaction zone and at a temperature in the range from -70°F. (-57°C.) to 40°F. (4.5°C.), and as a consequence, the liquid hydrocarbonaceous stream contains dissolved hydrogen, dissolved hydrogen halide and low molecular weight normally gaseous hydrocarbons if present.
- the hydrogenated liquid phase comprising the hydrogen chloride is separated to produce an anhydrous hydrogen halide stream by separating, for example, by stripping, flashing or fractionating.
- a resulting hydrocarbonaceous stream is separated to produce a hydrocarbonaceous stream, primarily comprising hydrogenated hydrocarbonaceous compounds and a stream primarily comprising halogenated organic compounds which may then be recycled to the hydrogenation conversion zone if desired.
- Such as separation may be conducted in any convenient manner such as, for example, stripping, flashing or fractionating.
- a halogenated organic feed stream containing halogenated organic compounds is introduced into the process via conduit 1 and is contacted with a hydrogen-rich gaseous recycle stream which is provided via conduit 7 and is hereinafter described.
- the halogenated organic feed stream containing halogenated organic compounds, the hydrogen-rich gaseous recycle stream and an unconverted halogenated organic recycle stream provided via conduit 14 and hereinafter described are introduced into hydrogenation reaction zone 2.
- the resulting hydrogenated organic stream is removed from the hydrogenation reaction zone 2 via conduit 3, is cooled in heat exchanger 4 and introduced into vapor liquid separator 6 via conduit 5.
- a hydrogen-rich gaseous stream is removed from vapor-liquid separator 6 via conduit 7 and recycled as described hereinabove.
- make-up hydrogen may be introduced into the system at any convenient and suitable point but is not shown on the drawing.
- a liquid hydrogenated hydrocarbonaceous stream containing hydrogen and a hydrogen halide in solution is removed from vapor liquid separator 6 and is introduced into fractionation zone 9 via conduit 8.
- a product stream containing hydrogen halide is removed from fractionation zone 9 via conduit 10 and recovered.
- a liquid distillable hydrogenated hydrocarbonaceous stream is removed from fractionation zone 9 via conduit 11 and is introduced into fractionation zone 12.
- a product stream containing hydrocarbonaceous compounds having a reduced concentration of halogen is removed from fractionation zone 12 via conduit 13 and recovered.
- a liquid stream containing unconverted halogenated organic compounds is removed from fractionation zone 12 via conduit 14 and is recycled to hydrogenation reaction zone 2 via conduit 14 as described hereinabove.
- a halogenated organic feedstock having the characteristics presented in Table 1 is charged at a rate of 47.8 mol/h to a hydrogenation reaction zone containing a palladium on alumina catalyst which is conducted at hydrogenation conditions which includes a temperature of 176°F. (80°C.), a pressure of 750 psig (5.2 MPa, gauge) and a hydrogen circulation rate of 20,000 SCFB (3,600 normal m3/m3).
- HABLE 1 HALOGENATED ORGANIC FEEDSTOCK PROPERTIES Composition Weight Percent Chlorinated Propane 91.1 Unsaturated Propane 4.7 Other 4.2
- a recycle stream containing unconverted feedstock and hereinafter described is also charged to the hydrogenation reaction zone in an amount of 30 mol/h.
- the resulting effluent from the hydrogenation reaction zone is cooled to a temperature of -15°C. and introduced into a vapor-liquid separator.
- a hydrogen-rich gaseous stream is removed from the vapor-liquid separator and which stream contains 2000 mol/h of hydrogen, 850 mol/h of hydrogen chloride and 70 mol/h of chlorinated hydrocarbons.
- a liquid stream is removed from the vapor-liquid separator in an amount of 140 mol/h including 50 mol/h of propane, 60 mol/h of hydrogen chloride and 25 mol/h of chlorinated hydrocarbons.
- This resulting liquid stream is fractionated to produce a hydrogen chloride product stream containing 60 mol/h of hydrogen chloride, a propane product stream containing 48 mol/h propane and a liquid recycle stream containing 30 mol/h of chlorinated hydrocarbons.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP94308591A EP0643123A3 (de) | 1994-11-21 | 1994-11-21 | Verfahren zur Behandlung von halogenierten Kohlenwasserstoffen. |
| CN94118716.0A CN1123268A (zh) | 1994-11-21 | 1994-11-21 | 卤化烃的处理方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP94308591A EP0643123A3 (de) | 1994-11-21 | 1994-11-21 | Verfahren zur Behandlung von halogenierten Kohlenwasserstoffen. |
| CN94118716.0A CN1123268A (zh) | 1994-11-21 | 1994-11-21 | 卤化烃的处理方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0643123A2 true EP0643123A2 (de) | 1995-03-15 |
| EP0643123A3 EP0643123A3 (de) | 1995-08-23 |
Family
ID=37097981
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP94308591A Withdrawn EP0643123A3 (de) | 1994-11-21 | 1994-11-21 | Verfahren zur Behandlung von halogenierten Kohlenwasserstoffen. |
Country Status (2)
| Country | Link |
|---|---|
| EP (1) | EP0643123A3 (de) |
| CN (1) | CN1123268A (de) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2780300A1 (fr) * | 1998-06-25 | 1999-12-31 | Rhodia Chimie Sa | Procede de traitement de gaz ou de liquides issus de reformage catalytique |
| WO2025214947A1 (en) | 2024-04-08 | 2025-10-16 | Topsoe A/S | Fluorine removal from renewable feedstocks |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3623430A1 (de) * | 1986-07-11 | 1988-01-28 | Veba Oel Entwicklungs Gmbh | Verfahren zur hydrierenden behandlung von mit chlorbiphenylen u. dgl. kontaminierten mineraloelen |
| US5316663A (en) * | 1992-01-13 | 1994-05-31 | Uop | Process for the treatment of halogenated hydrocarbons |
-
1994
- 1994-11-21 CN CN94118716.0A patent/CN1123268A/zh active Pending
- 1994-11-21 EP EP94308591A patent/EP0643123A3/de not_active Withdrawn
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2780300A1 (fr) * | 1998-06-25 | 1999-12-31 | Rhodia Chimie Sa | Procede de traitement de gaz ou de liquides issus de reformage catalytique |
| WO2025214947A1 (en) | 2024-04-08 | 2025-10-16 | Topsoe A/S | Fluorine removal from renewable feedstocks |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0643123A3 (de) | 1995-08-23 |
| CN1123268A (zh) | 1996-05-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0432323B1 (de) | Gleichzeitige Hydrodehalogenierung von zwei halogenierte organische Verbindungen enthaltenden Strömen | |
| US4899001A (en) | Process for the simultaneous hydroconversion of a first feedstock comprising unsaturated, halogenated organic compounds and a second feedstock comprising saturated, halogenated organic compounds | |
| US4929781A (en) | Process for the simultaneous hydroconversion of a first feedstock comprising unsaturated, halogenated organic compounds and a second feedstock comprising saturated, halogenated organic compounds | |
| US5013424A (en) | Process for the simultaneous hydrogenation of a first feedstock comprising hydrocarbonaceous compounds and having a non-distillable component and a second feedstock comprising halogenated organic compounds | |
| US5401894A (en) | Process for the treatment of halogenated organic feedstocks | |
| US5354931A (en) | Process for hydrotreating an organic feedstock containing oxygen compounds and a halogen component | |
| EP0682100B1 (de) | Unterdrückung von kohlehaltigen Niederschlägen in Hydrobehandlungsverfahren von instabile olefinische Verbindungen enthaltende organischen Einsätzen | |
| EP0541871B1 (de) | Hydrierende Konversion eines Abfalleinsatzstoffes aus hochreaktiven organischen Verbindungen | |
| EP0390985A1 (de) | Behandlung eines temperaturempfindlichen Kohlenwasserstoffeinsatzes | |
| EP0360406B1 (de) | Behandlung eines temperaturempfindlichen Kohlenwasserstoffstromes, der eine nicht destillierbare Komponente enthält | |
| US5723706A (en) | Process for the treatment of halogenated organic feedstocks | |
| US4840721A (en) | Process for treating a temperature-sensitive hydrocarbonaceous stream containing a non-distillable component to produce a hydrogenated distillable hydrocarbonaceous product | |
| US5316663A (en) | Process for the treatment of halogenated hydrocarbons | |
| EP0306164B1 (de) | Verfahren zum Hydrieren von temperaturempfindlichen Abfall-Kohlenwasserstoffmaterialien | |
| EP0643123A2 (de) | Verfahren zur Behandlung von halogenierten Kohlenwasserstoffen | |
| US5773549A (en) | Process for hydrotreating an organic feedstock containing a halogenated component and contaminated with distillable oxygen and nitrogen compounds having boiling points lower than the halogenated compounds | |
| US5004533A (en) | Process for treating an organic stream containing a non-distillable component to produce an organic vapor and a solid | |
| US5780695A (en) | Process for the selective saturation of olefin-containing halogenated organic streams | |
| US5552037A (en) | Process for the treatment of two halogenated hydrocarbon streams | |
| US5637782A (en) | Process for the selective saturation of olefin-containing halogenated organic streams | |
| US5176816A (en) | Process to produce a hydrogenated distillable hydrocarbonaceous product | |
| EP0642810A2 (de) | Verfahren zur Wasserstoffbehandlung von organischen Einsätzen, die olefinische Verbindungen und einen Halogenteil enthalten | |
| KR940009044B1 (ko) | 고반응성 유기 화합물로 이루어진 공급 원료의 수소화 전환방법 | |
| JP2569273B2 (ja) | 触媒への炭化物析出を抑制するハイドロトリーティング方法 | |
| JPH08141366A (ja) | ハロゲン化炭化水素の水素化処理法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL PT SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL PT SE |
|
| 17P | Request for examination filed |
Effective date: 19951016 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN WITHDRAWN |
|
| 18W | Application withdrawn |
Withdrawal date: 19960611 |