EP0593952A1 - Product for releasing treatment agents into the wash liquid of an automatic washing or dishwashing machine - Google Patents
Product for releasing treatment agents into the wash liquid of an automatic washing or dishwashing machine Download PDFInfo
- Publication number
- EP0593952A1 EP0593952A1 EP93115693A EP93115693A EP0593952A1 EP 0593952 A1 EP0593952 A1 EP 0593952A1 EP 93115693 A EP93115693 A EP 93115693A EP 93115693 A EP93115693 A EP 93115693A EP 0593952 A1 EP0593952 A1 EP 0593952A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- weight
- treatment agent
- chamber
- product according
- water
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Classifications
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06F—LAUNDERING, DRYING, IRONING, PRESSING OR FOLDING TEXTILE ARTICLES
- D06F39/00—Details of washing machines not specific to a single type of machines covered by groups D06F9/00 - D06F27/00
- D06F39/02—Devices for adding soap or other washing agents
- D06F39/024—Devices for adding soap or other washing agents mounted on the agitator or the rotating drum; Free body dispensers
-
- A—HUMAN NECESSITIES
- A47—FURNITURE; DOMESTIC ARTICLES OR APPLIANCES; COFFEE MILLS; SPICE MILLS; SUCTION CLEANERS IN GENERAL
- A47L—DOMESTIC WASHING OR CLEANING; SUCTION CLEANERS IN GENERAL
- A47L15/00—Washing or rinsing machines for crockery or tableware
- A47L15/42—Details
- A47L15/44—Devices for adding cleaning agents; Devices for dispensing cleaning agents, rinsing aids or deodorants
- A47L15/4445—Detachable devices
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D81/00—Containers, packaging elements, or packages, for contents presenting particular transport or storage problems, or adapted to be used for non-packaging purposes after removal of contents
- B65D81/32—Containers, packaging elements, or packages, for contents presenting particular transport or storage problems, or adapted to be used for non-packaging purposes after removal of contents for packaging two or more different materials which must be maintained separate prior to use in admixture
- B65D81/3261—Flexible containers having several compartments
- B65D81/3272—Flexible containers having several compartments formed by arranging one flexible container within another
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D17/00—Detergent materials or soaps characterised by their shape or physical properties
- C11D17/04—Detergent materials or soaps characterised by their shape or physical properties combined with or containing other objects
- C11D17/041—Compositions releasably affixed on a substrate or incorporated into a dispensing means
- C11D17/042—Water soluble or water disintegrable containers or substrates containing cleaning compositions or additives for cleaning compositions
- C11D17/044—Solid compositions
Definitions
- the present invention relates to a product for the release of treatment agents into the washing liquid of an automatic washing machine or dishwasher in the form of a bag, which comprises at least one chamber containing a treatment agent.
- Conventional cleaning agents for dishwashers are widely known in the prior art. They consist essentially of the components alkali tripolyphosphate, alkali metasilicate and alkali carbonate.
- organic additives such as non-foaming surfactants, polycarboxylates (homopolymers of acrylic acid or copolymers with maleic acid), chlorine releasers (eg sodium dichloroisocyanurate) are used.
- Such cleaners are not only used for cleaning the dishes, but also in larger quantities for basic cleaning and degreasing of the dishwasher itself.
- the machine cleaner used for cleaning and degreasing the machine is added to the dishwasher in larger quantities that cannot be added to the metering chamber, the machine cleaner must be added to the interior of the dishwasher in powder form. If used improperly, when the powder was poured on the sieve inside the machine, the powder in the circulation pump would clump and cause high repair costs. This was also due to the powdery consistency of dust in the material, which leads to irritation of the respiratory tract when inhaled.
- the portioned cleaners offer several advantages over the loose powder, such as constant dosing, no or less dust formation, no or less skin contact, no or less clumping and no spilling of powder. There are two different solutions.
- sachets Another option for providing portioned cleaners is through the use of sachets.
- a sachet is described, for example, in DE-A-4011508, which discloses a textile detergent for a washing machine, the sachet consisting of insoluble, water-permeable fabric.
- the grain size distribution of the granular content is chosen so that the mesh size of the fabric is smaller than the grain size. The product can only be released after penetration by water.
- FR-A-2616796 sachets with liquid detergents are described.
- both embodiments of the detergent tablets have the disadvantage that the tablets have a delayed solubility, so that they only become fully effective slowly, whereas powdered detergents have a high dissolution rate.
- the tablet In the case of tablets which, due to their size, can be placed in the metering chamber of the machine, the tablet normally falls freely onto the inside of the dishwasher when the metering chamber is opened and dissolves there only slowly, since the water does not use much mechanical energy there.
- Tablets which due to their size can no longer be placed in the dosing chamber, are placed directly in the Cutlery basket or a separate holder. This means that part of the detergent is already dissolved in the pre-rinse cycle. This means that the tablet must be so hard that only a little substance is dissolved in the pre-rinse cycle and the main amount is available in the main rinse cycle. However, this increases the risk of caustic solution being carried over to rinse-aid, since it is difficult to precisely maintain the tablet's dissolving speed.
- the object of the present invention was to provide a portioned cleaner for the dishwasher, which is easy and safe to use and does not have the disadvantages mentioned above.
- the object of the invention is achieved by providing a product for releasing treatment agents into the washing liquid of an automatic washing machine or dishwasher, comprising a bag with at least one chamber containing a treatment agent, the chamber walls consisting of water-soluble film material with such water solubility that the treatment agent is released within the period up to the start of rinse aid according to the type of treatment agent.
- the bag according to the invention depending on its size, which can contain from 10 to 500 g of treatment agent depending on the intended use, can be placed in the dosing chamber or in the crockery basket.
- the bag can be taken up like a powder or granulate from the dosing chamber of the dishwasher. For larger bags, this is placed in the basket.
- the water solubility can be chosen arbitrarily so that the treatment agent is released at the beginning of the washing process in the pre-rinse cycle or during the hot rinse cycle.
- Water-soluble films based on synthetic or natural polymers such as starch, polyvinyl alcohol or modified polyvinyl alcohol, which can also be biodegradable, can be used as the bag material. The use of polyvinyl alcohol or acetylated polyvinyl alcohol is preferred.
- polyvinyl alcohol does not result in excessive foaming, which usually occurs in dishwashers when the alkali is circulated and which strongly inhibits or even prevents the detergent solution from being continuously fed onto the dishes, since the circulating pump only sucks in air or foam.
- polyvinyl alcohol as the film material is also preferred for the reason that the polyvinyl alcohol of the film is completely biodegraded in the sewage treatment plant and is completely non-toxic. This makes the environmentally friendly formulation of the portioned while using the above-mentioned detergent composition Reinerers greatly upgraded ecologically. At the same time, waste is significantly reduced compared to the individual packaging of tablets, for example, and the dangers in the household when using the cleaner are also minimized.
- a composition consisting of is used as the preferred treatment agent for cleaning dishes
- water-soluble alkali silicate with a ratio of silicon dioxide to alkali oxide of greater than 1: 1, 0 - 50% by weight alkali carbonate, 2 to 20% by weight of polymeric sequesters or dispersants, preferably of the polycarboxylate type, in particular homopolymers of acrylic acid or copolymers with other organic acids or ethers which contain vinyl groups, 0 - 40% by weight alkali salts of organic acids with sequestering action on calcium ions such as polycarboxylic acids, in particular citric acid or acids obtained fermentatively from sugar, in particular gluconic acid.
- a boron-free, oxygen-based bleaching agent from the group of peroxo compounds or peroxohydrates or mixtures thereof, which release hydrogen peroxide in the water
- a bleach activator from the group of compounds which liberate reactive peracids, in particular peracetic acid, under the action of hydrogen peroxide
- enzymes or enzyme mixtures from the group of hydrolases, in particular proteases, amylases and lipases 0.5 - 5% by weight of a low-foaming, non-ionic or anionic surfactant, the sum of the individual components being 100% by weight.
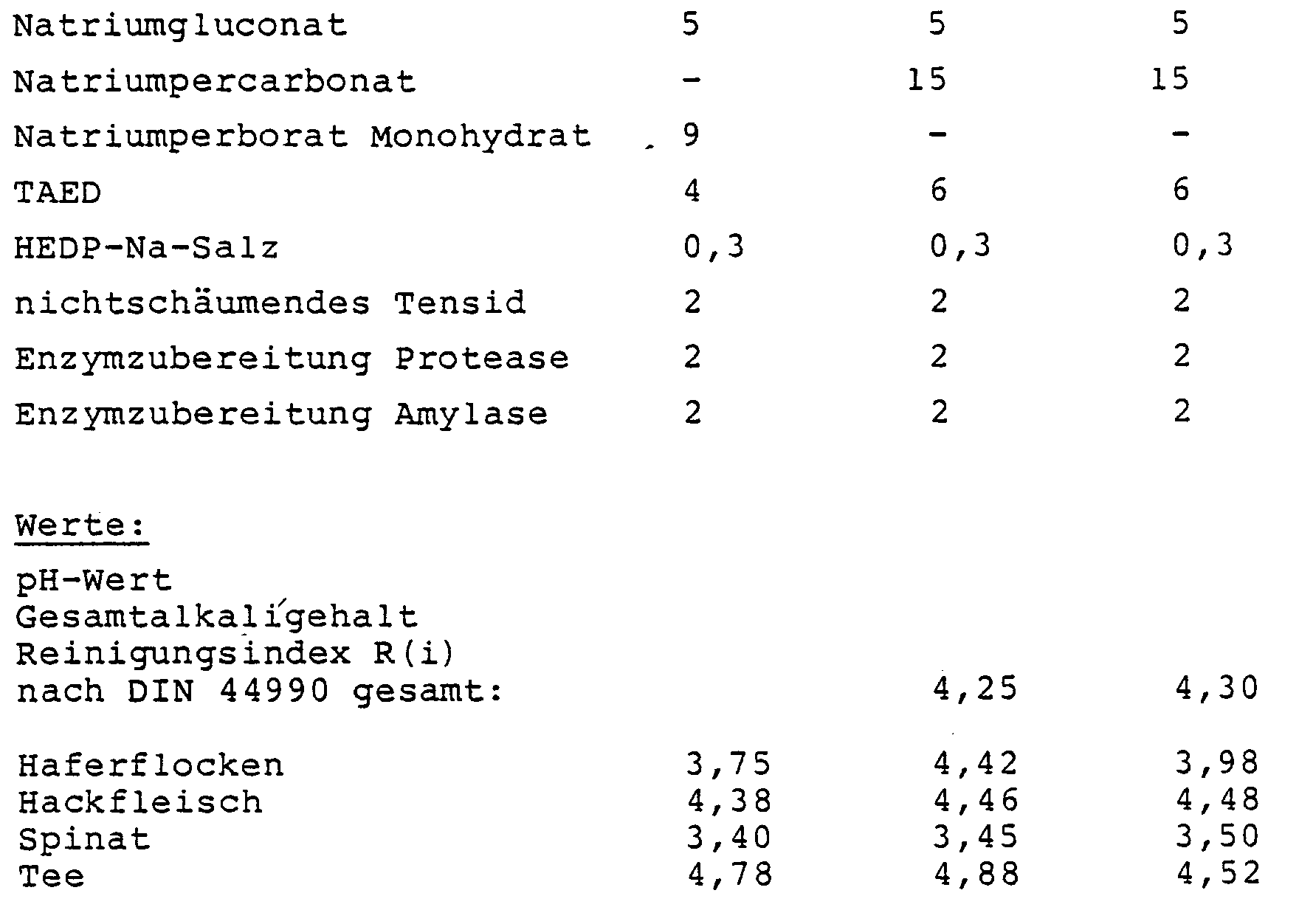
- amorphous sodium disilicate with SiO2: Na2O from 1.9: 1 to 2.1: 1, 10 - 40% by weight, in particular 25-35% by weight, sodium carbonate, 3 - 10 wt.%, In particular 4-8 wt.%, Polyacrylate maleate (7: 3) as sodium salt, 3 - 10% by weight, in particular 4-7% by weight, sodium gluconate, 5 - 25% by weight, in particular 5-15% by weight, sodium percarbonate, 0 - 15 wt.%, In particular 3-7 wt.%, TAED (tetraacetylethylene diamine), 0-2% by weight, in particular 0.2-0.5% by weight, of HEDP (hydroxyethane-1,1-diphosphonic acid) as the sodium salt, 0 - 5% by weight mixture of stabilized enzymes, in particular proteases, amylases and lipases, 0 - 5% by weight of low-foaming, i
- the silicates and carbonates serve as mild alkalis to improve dirt removal by swelling, and the silicates also as corrosion protection.
- the pH of the cleaner can be adjusted between about 9.5 and 11.5 and buffered.
- Both the polycarboxylates or other polymers mentioned and the organic acids or their salts serve primarily to bind the water hardness, which can still be present in the dishwasher despite the ion exchanger.
- the polymeric material of the bag itself like the other polymers, also contributes to the dispersion of solids, e.g. Spinach particles, at.
- the bleaching system consists of the active oxygen donor from the group of the per compounds, the activator, such as TAED and the stabilizer, preferably from the group of the phosphates.
- the activator such as TAED
- the stabilizer preferably from the group of the phosphates.
- nitrogen bases can also be used as complexing agents for iron and heavy metals as in the case of glycine derivatives are even biodegradable.
- These stabilizer compounds delay the decomposition of the active oxygen compounds catalyzed by the metal ions by complexing the metal ions.
- the oxygen carrier In the case of polyvinyl alcohol as film material, the oxygen carrier must be boron-free, since the borates react with the polyvinyl alcohol to form poorly soluble complexes, which has a negative effect on the solubility of the bag.
- the low-foaming surfactants contribute to cleaning performance through their defoaming effect and the solubilization of fats.
- the enzymes have a very specific effect on food residues. Starch residues are targeted by the amylase and protein residues by the protease. Since they show strong effects even in extremely small quantities and are biodegradable, they contribute to the low environmental impact of the portioned cleaner. The same applies to lipase, which can be used for better degreasing.
- the bag according to the invention has a larger capacity of approx. 250 g
- the product according to the invention can suitably be used for cleaning and degreasing the machine itself.
- the compositions mentioned above can be used as cleaning agents.
- the bag according to the invention can also be used for cleaning washing machines.
- the descaling treatment agents primarily free the heating elements of the washing machine, whereas the alkaline cleaners remove the so-called fat lice.
- the latter are deposits of constituents of the detergents, grease and hardness formers of the water, which often result from underdosing of the detergents.
- the product comprises a second chamber containing a second treatment agent, which can be adjacent to, separated from or contained in the first chamber via a common seam.
- the inner bag can also be completely separated from or attached to the outer bag.
- An integrated construction, created by folding, is also possible.
- the second chamber can be present in a separate, additional third chamber, separate from the first chamber.
- the water solubilities of the chamber walls of the two chambers are expediently so different that the dissolution of the respective chamber wall and the release of the treatment agent contained therein take place at different times in the course of the cleaning process before the rinse process begins.
- the water solubility of the chamber walls can be influenced, for example, by the chamber walls of the two chambers being made of water-soluble film material with different thicknesses and / or of different polymers or mixtures thereof. In this way it can be achieved, for example, that the content of the first chamber is carried out with cold water Pre-rinse and the contents of the second chamber are only released in the course of the actual cleaning step carried out with hot water.
- the film material used can have a thickness of 1 ⁇ m to 2 mm.
- a decalcifying agent can then be used as the ingredient in the first chamber, for example, which is selected from the group consisting of citric acid, urea phosphate and sulfamic acid or other solid inorganic or organic acids or acid salts.
- any form of direct contact with the cleaner is avoided in the formulation with the composition given above as a result of the alkali reduction using the following procedure. No dust is released and the respiratory tract is not irritated.
- a product according to the invention can accordingly be provided with two chambers, the decalcifier being packed in the chamber made of cold water-soluble film and the alkaline basic cleaner in the chamber made of hot water-soluble film.
- the decalcifying agent works in the pre-rinse cycle, whereas the alkaline cleaner only works when the main rinse cycle heats up.
- the decalcifying agent works in the pre-rinse cycle
- the alkaline cleaner only works when the main rinse cycle heats up.
- a corresponding result can also be achieved when using two separate bags, each of which comprises chambers made of different film materials with different ingredients.
- the water-soluble adhesives which can usually be used in this field can be used as the adhesive for the film material forming the chamber.
- Sodium carboxymethyl cellulose is mentioned as an example of a suitable water-sensitive adhesive.
- a product according to the invention is provided, which is characterized in that the bag comprising at least one chamber is connected to one or more bags comprising at least one chamber via film areas which have tear-off areas between adjacent bags, which may or may not be perforated, and which allow the individual bags to be separated from one another. The consumer can thus easily portion the amount of treatment agent.
- the recipe from Example II is packed with 250 g in sachets made of cold water-soluble polyvinyl alcohol with a wall thickness of 40 m ⁇ .
- the alkalinity of the cleaner is adjusted so that the film material is not affected.
- a machine with heavy deposits of food residues in the sieve of the circulation pump is treated as follows: At the beginning of the main wash cycle without items to be washed, the machine is opened and the original sealed bag is placed in the upper basket. After closing, the water is immediately circulated again and the contents of the bag practically at first contact with water released and evenly distributed. After the program has ended, the sieve is free of leftovers.
- a parallel test with the same powder, which is improperly poured directly onto the sieve leads to the fact that the cleaner hardens in the suction hose of the circulation pump and a repair is necessary.
- Example 1 The same bag material as in Example 1) is filled with 250 g of citric acid. With the same procedure as in 1), limescale deposits are removed in the machine, especially on the heating elements.
- a combination pack When connecting the dishwasher to cold water, a combination pack can be used, which is made up of different foils.
- the bag from 2) serves as a descaler.
- the cleaner as in 1) is in a bag made of a polyvinyl acetate, which is only partially converted to polyvinyl alcohol. This can affect the water solubility.
- the degree of conversion is chosen so that the film only dissolves completely from a temperature of 45 ° to 50 ° C.
- the wall thickness is approx. 100 m ⁇ so that the bag does not burst open prematurely and the product is released.
- a dishwasher with leftovers in the sieve and limescale deposits on the heating elements is treated as follows: The two separate bags (a descaler and a cleaner) are placed unopened next to each other in the upper basket. Then a 65 ° C program with pre-rinse chosen. This is the normal program for the Siemens dishwasher type Lady Plus 260 used. In the pre-rinse cycle with cold water (inlet approx. 17 ° C), the bag with the citric acid dissolves and the lime on the heating elements is removed. The bag with the alkaline cleaner is only slightly dissolved. The acidic solution is pumped off and fresh water for the main wash cycle runs in. After the circulation and heating have started, the second pouch with the cleaner opens when it reaches approx. 40 ° to 50 ° C. Now the alkaline cleaner can attack the leftovers in the sieve. Result: The machine is descaled and cleaned of food residues in one operation. This saves water and energy compared to separate use.
- recipe I can advantageously be used, since the more stable perborate is present as oxygen bleach. However, since part of the protease is used up by the bag material, the removal of the oatmeal is somewhat worse. If mixture I uses polyvinyl alcohol as the bag material, you will find slimy residues in the machine and on the dishes.
- the cleaning performance of formulation I was determined using pouches made of starch, the others using pouches made of polyvinyl alcohol which was soluble in cold water.
- the dosage was 25 g.
- the reference cleaner according to the DIN 44990 standard achieved a cleaning index of 3.98.
Landscapes
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Mechanical Engineering (AREA)
- Detergent Compositions (AREA)
Abstract
Description
Die vorliegende Erfindung betrifft ein Produkt für die Freisetzung von Behandlungsmitteln in die Waschflüssigkeit einer automatischen Wasch- oder Geschirrspülmaschine in Form eines Beutels, der wenigstens eine ein Behandlungsmittel enthaltende Kammer umfaßt.The present invention relates to a product for the release of treatment agents into the washing liquid of an automatic washing machine or dishwasher in the form of a bag, which comprises at least one chamber containing a treatment agent.
Konventionelle Reinigungsmittel für Geschirrspülmaschinen sind im Stand der Technik vielfach bekannt. Sie bestehen im wesentlichen aus den Komponenten Alkalitripolyphosphat, Alkalimetasilikat und Alkalicarbonat. Daneben sind organische Additive wie nicht schäumende Tenside, Polycarboxylate (Homopolymere der Acrylsaäure oder Copolymere mit Maleinsäure), Chlorabspalter (z.B. Natriumdichlorisocyanurat) im Einsatz.Conventional cleaning agents for dishwashers are widely known in the prior art. They consist essentially of the components alkali tripolyphosphate, alkali metasilicate and alkali carbonate. In addition, organic additives such as non-foaming surfactants, polycarboxylates (homopolymers of acrylic acid or copolymers with maleic acid), chlorine releasers (eg sodium dichloroisocyanurate) are used.
Neuere Entwicklungen zielen auf den Ersatz von Phosphat, um die Eutrophierung der Oberflächengewässer zu reduzieren.Recent developments aim to replace phosphate in order to reduce the eutrophication of surface waters.
Neben dieser ökologischen Seite ist auch die Toxikologie der verwendeten Reinigungsmittel von Bedeutung. Gesetzliche Maßnahmen führen dazu, daß hochalkalische Produkte ab 1991 in der BRD nur in kindergesicherten Verpackungen auf den Markt gebracht werden dürfen. Dies führte zur Entwicklung von Geschirreinigern mit verminderter Alkalität, um die Gefährdung des Verbrauchers, vor allem von Kindern, zu verringern, ohne andererseits das Müllproblem durch die aufwendigere Verpackung zu vergößern. Gleichzeitig verzichten die neuen Reiniger auf Chlorverbindungen.In addition to this ecological aspect, the toxicology of the cleaning agents used is also important. Legal measures mean that from 1991 onwards, highly alkaline products in the FRG may only be brought onto the market in child-resistant packaging. This led to the development of dishwashers with reduced alkalinity in order to reduce the risk to consumers, especially children, without, on the other hand, increasing the waste problem due to the more complex packaging. At the same time, the new cleaners do without chlorine compounds.
Derartige Reiniger werden nicht nur zur Reinigung des Geschirrs, sondern ebenso in größeren Mengen zur Grundreinigung und Entfettung der Spülmaschine selbst eingesetzt. Aufgrund der Tatsache jedoch, daß der zur Reinigung und Entfettung der Maschine eingesetzte Maschinenreiniger in größeren Mengen in die Geschirrspülmaschine gegeben wird, die nicht in die Dosierkammer gegeben werden können, muß der Maschinenreiniger in Pulverform in den Innenraum der Geschirrspülmaschine gegeben werden. Bei falscher Anwendung, wenn das Pulver gehäuft auf das in der Maschine befindliche Sieb geschüttet wurde, waren Verklumpungen des Pulvers in der Umwälzpumpe die Folge, und hohe Reparaturkosten wurden verursacht. Damit verbunden waren ebenfalls durch die pulverförmige Konsistenz Verstaubungen des Materials, die beim Einatmen zu einer Reizung der Atemwege führen.Such cleaners are not only used for cleaning the dishes, but also in larger quantities for basic cleaning and degreasing of the dishwasher itself. However, due to the fact that the machine cleaner used for cleaning and degreasing the machine is added to the dishwasher in larger quantities that cannot be added to the metering chamber, the machine cleaner must be added to the interior of the dishwasher in powder form. If used improperly, when the powder was poured on the sieve inside the machine, the powder in the circulation pump would clump and cause high repair costs. This was also due to the powdery consistency of dust in the material, which leads to irritation of the respiratory tract when inhaled.
Diese Nachteile können dadurch beseitigt werden, daß die lose geschütteten Pulver in Form von portionierten Wasch- oder Geschirreinigungsmitteln angeboten werden. Die portionierten Reiniger bieten gegenüber dem lose geschütteten Pulver einige Vorteile wie z.B. gleichbleibende Dosierung, keine oder weniger Staubbildung, kein oder geringerer Hautkontakt, kein oder geringeres Verklumpen sowie kein Verschütten von Pulver. Dabei werden zwei verschiedene Lösungswege beschritten.These disadvantages can be eliminated in that the loosely poured powder in the form of portioned washing or Dishwashing detergents are offered. The portioned cleaners offer several advantages over the loose powder, such as constant dosing, no or less dust formation, no or less skin contact, no or less clumping and no spilling of powder. There are two different solutions.
Die pulverförmigen Bestandteile werden mit oder ohne spezielle Tablettierhilfsmittel zu Tabletten (Tabs) verpreßt. Es entstehen teilweise sehr harte Preßlinge, die deshalb auch als kindersicher bezeichnet werden, weil sie weniger leicht aufgenommen werden können als Pulver. Es bleibt jedoch insbesondere bei erhöhter Alkalität die Gefahr des Verätzens der Zunge bestehen. Derartige Reinigungsmittel in Tablettenform für Spül- oder Waschmaschinen sind beispielsweise in der DE-A-2926253, DE-A-4010524 und DE-A-4010533 beschrieben.The powdery constituents are pressed into tablets (tabs) with or without special tabletting aids. Sometimes very hard compacts are formed, which are also called child-safe because they are less easily absorbed than powder. However, there is still a risk of caustic burns, especially if the alkalinity is increased. Such cleaning agents in tablet form for dishwashers or washing machines are described, for example, in DE-A-2926253, DE-A-4010524 and DE-A-4010533.
Eine weitere Möglichkeit zur Bereitstellung von portionierten Reinigern ist durch die Verwendung von Portionsbeuteln gegeben. Ein derartiger Portionsbeutel ist beispielsweise in der DE-A-4011508 beschrieben, die ein Textilwaschmittel für eine Waschmaschine offenbart, wobei der Portionsbeutel aus unlöslichem wasserdurchlässigem Gewebe besteht. Dabei ist die Korngrößenverteilung des granulatförmigen Inhalts so gewählt, daß die Maschenweite des Gewebes kleiner als die Korngröße ist. Das Produkt kann somit erst nach dem Auflösen durch eindringendes Wasser freigesetzt werden. In der FR-A-2616796 werden Portionsbeutel mit flüssigen Waschmitteln beschrieben.Another option for providing portioned cleaners is through the use of sachets. Such a sachet is described, for example, in DE-A-4011508, which discloses a textile detergent for a washing machine, the sachet consisting of insoluble, water-permeable fabric. The grain size distribution of the granular content is chosen so that the mesh size of the fabric is smaller than the grain size. The product can only be released after penetration by water. In FR-A-2616796 sachets with liquid detergents are described.
Während die portionierten Waschmittel für die Waschmaschine meist direkt in die Trommel auf die Wäsche gegeben werden und dann ein Programm ohne Vorwäsche durchgeführt wird, liegen bei der Spülmaschine andere Vorausetzungen vor. Beutel aus unlöslichem Gewebe, wie sie für Waschpulver eingesetzt werden, sind für die Spülmaschine ungeeignet, da die Gefahr besteht, daß sich der Beutel in der Maschine, im Sieb oder auf dem Geschirr festsetzen und so einen einwandfreien Ablauf des Prozesses verhindern würde.While the portioned detergents for the washing machine are usually put directly into the drum on the laundry and then a program without prewash is carried out, the dishwasher has other requirements. Bags made of insoluble fabric, such as those used for washing powder, are unsuitable for the dishwasher, since there is a risk that the bag will get stuck in the machine, in the sieve or on the dishes and thus prevent the process from running smoothly.
Wenn auch die oben genannten Reinigertabletten für die Spülmaschine die bereits erwähnten Vorteile von portionierten Reinigern aufweisen, so zeigen sie doch erhebliche Nachteile, die bei den gebräuchlichen Ausführungen noch unterschiedlich sind. Bei den Reinigertabletten ist zwischen den Tabs solcher Größe zu unterscheiden, die in die Dosierkammer der Maschine gegeben werden können, und solchen Reinigertabletten, die aufgrund ihrer Größe nur in den Besteckkorb oder in eigens dafür vorgesehene Halter eingebracht werden.Even if the above-mentioned detergent tablets for the dishwasher have the advantages of portioned detergents already mentioned, they nevertheless show considerable disadvantages which are still different in the customary designs. In the case of detergent tablets, a distinction must be made between tabs of such size that can be placed in the metering chamber of the machine, and detergent tablets that, due to their size, can only be placed in the cutlery basket or in a holder provided for this purpose.
Beide Ausführungsformen der Reinigertabletten haben jedoch den Nachteil, daß die Tabletten eine verzögerte Löslichkeit besitzen, so daß sie erst langsam voll wirksam werden, wohingegen pulverförmige Reiniger eine hohe Lösegeschwindigkeit aufweisen. Bei Tabletten, die aufgrund der Größe in die Dosierkammer der Maschine gegeben werden können, fällt die Tablette normalerweise bei Öffnung der Dosierkammer ungehindert auf den Innenboden der Spülmaschine und löst sich dort nur langsam auf, da das Wasser dort nur wenig mechanische Energie aufbringt.However, both embodiments of the detergent tablets have the disadvantage that the tablets have a delayed solubility, so that they only become fully effective slowly, whereas powdered detergents have a high dissolution rate. In the case of tablets which, due to their size, can be placed in the metering chamber of the machine, the tablet normally falls freely onto the inside of the dishwasher when the metering chamber is opened and dissolves there only slowly, since the water does not use much mechanical energy there.
Tabletten, die aufgrund ihrer Größe nicht mehr in die Dosierkammer gegeben werden können, werden direkt in den Besteckkorb oder einen separaten Halter eingelegt. Dadurch wird ein Teil des Reinigers bereits im Vorspülgang aufgelöst. Das bedeutet, daß die Tablette so hart sein muß, daß nur wenig Substanz im Vorspülgang aufgelöst wird und die Hauptmenge im Hauptspülgang zur Verfügung steht. Dadurch erhöht sich jedoch die Gefahr einer Laugenverschleppung bis zur Klarspülung, da die genaue Einhaltung der Lösegeschwindigkeit der Tablette schwierig ist.Tablets, which due to their size can no longer be placed in the dosing chamber, are placed directly in the Cutlery basket or a separate holder. This means that part of the detergent is already dissolved in the pre-rinse cycle. This means that the tablet must be so hard that only a little substance is dissolved in the pre-rinse cycle and the main amount is available in the main rinse cycle. However, this increases the risk of caustic solution being carried over to rinse-aid, since it is difficult to precisely maintain the tablet's dissolving speed.
Die Aufgabe der vorliegenden Erfindung bestand nun darin, einen portionierten Reiniger für die Geschirrspülmaschine bereitzustellen, der einfach und sicher handhabbar ist und die oben erwähnten Nachteile nicht mit sich bringt.The object of the present invention was to provide a portioned cleaner for the dishwasher, which is easy and safe to use and does not have the disadvantages mentioned above.
Die Aufgabe er Erfindung wird gelöst durch Bereitstellung eines Produktes zur Freisetzung von Behandlungsmitteln in die Waschflüssigkeit einer automatischen Wasch- oder Geschirrspülmaschine, umfassend einen Beutel mit wenigstens einer ein Behandlungsmittel enthaltenden Kammer, wobei die Kammerwände aus wasserlöslichem Folienmaterial mit einer solchen Wasserlöslichkeit bestehen, daß das Behandlungsmittel innerhalb des Zeitraums bis zum Beginn des Klarspülvorgangs entsprechend der Art des Behandlungsmittels freigesetzt wird. Dabei kann der erfindungsgemäße Beutel entsprechend seiner Größe, der je nach Verwendungszweck von 10 bis 500 g Behandlungsmittel enthalten kann, in die Dosierkammer oder in den Geschirrkorb gegeben werden.The object of the invention is achieved by providing a product for releasing treatment agents into the washing liquid of an automatic washing machine or dishwasher, comprising a bag with at least one chamber containing a treatment agent, the chamber walls consisting of water-soluble film material with such water solubility that the treatment agent is released within the period up to the start of rinse aid according to the type of treatment agent. The bag according to the invention, depending on its size, which can contain from 10 to 500 g of treatment agent depending on the intended use, can be placed in the dosing chamber or in the crockery basket.
Bei entsprechender Größe mit einem Inhalt bis ca. 25 g kann der Beutel wie ein Pulver oder Granulat von der Dosierkammer der Spülmaschine aufgenommen werden. Bei größeren Beuteln wird dieser in den Geschirrkorb gegeben. Durch Wahl des geeigneten Beutelmaterials kann die Wasserlöslichkeit beliebig gewählt werden, so daß das Behandlungsmittel zu Beginn des Waschprozesses im Vorspülgang oder während des Heißspülgangs freigesetzt wird. Als Beutelmaterial können hierbei wasserlösliche Folien auf Basis synthetischer oder natürlicher Polymere, wie Stärke, Polyvinylalkohol oder modifiziertem Polyvinylalkohol, die auch biologisch abbabar sein können, verwendet werden. Bevorzugt ist hierbei die Verwendung von Polyvinylalkohol oder acetyliertem Polyvinylalkohol.With the appropriate size and a content of up to approx. 25 g, the bag can be taken up like a powder or granulate from the dosing chamber of the dishwasher. For larger bags, this is placed in the basket. By choosing the appropriate bag material, the water solubility can be chosen arbitrarily so that the treatment agent is released at the beginning of the washing process in the pre-rinse cycle or during the hot rinse cycle. Water-soluble films based on synthetic or natural polymers, such as starch, polyvinyl alcohol or modified polyvinyl alcohol, which can also be biodegradable, can be used as the bag material. The use of polyvinyl alcohol or acetylated polyvinyl alcohol is preferred.
Überraschenderweise bringt hier die Verwendung von Polyvinylalkohol keine starke Schaumbildung mit sich, die üblicherweise in Spülmaschinen bei Umwälzung der Lauge auftritt und die eine ständige Zuführung der Reinigerlösung auf das Geschirr stark hemmt oder sogar unterbindet, da die Umwälzpumpe nur Luft oder Schaum ansaugt.Surprisingly, the use of polyvinyl alcohol does not result in excessive foaming, which usually occurs in dishwashers when the alkali is circulated and which strongly inhibits or even prevents the detergent solution from being continuously fed onto the dishes, since the circulating pump only sucks in air or foam.
Bei dem erfindungsgemäßen Beutel mit Kammerwänden aus Polyvinylalkohol und einem Reiniger als Behandlungsmittel treten nach Auflösung des Beutels in der Spülmaschine keinerlei Schaumprobleme auf und der Druck der Laugenpumpe sinkt nicht ab, so daß die Mechanik der Spülmaschine nicht behindert wird. Dabei wird sogar die Schmutztragefähigkeit der Reinigerlösung in der Spülmaschine durch den Polyvinylalkohol unterstützt. Dieser synergistische Effekt wirkt sich vor allem bei der Entfernung von dispergierbarem Schmutz positiv aus.In the bag according to the invention with chamber walls made of polyvinyl alcohol and a cleaner as a treatment agent, no foam problems occur after the bag has been dissolved in the dishwasher and the pressure of the drain pump does not drop, so that the mechanics of the dishwasher are not hindered. Even the dirt-carrying capacity of the cleaning solution in the dishwasher is supported by the polyvinyl alcohol. This synergistic effect has a positive effect especially when removing dispersible dirt.
Die Verwendung von Polyvinylalkohol als Folienmaterial ist auch aus dem Grund bevorzugt, da der Polyvinylalkohol der Folie in der Kläranlage vollständig biologisch abgebaut wird und vollkommen ungiftig ist. Dadurch wird bei gleichzeitiger Verwendung der oben erwähnten Reinigerzusammensetzung die umweltschonende Formulierung des portionierten Reinigers ökologisch stark aufgewertet. Gleichzeitig wird gegenüber einer Einzelverpackung von z.B. Tabletten der Abfall wesentlich reduziert und die Gefahren im Haushalt beim Umgang mit dem Reiniger ebenfalls minimiert.The use of polyvinyl alcohol as the film material is also preferred for the reason that the polyvinyl alcohol of the film is completely biodegraded in the sewage treatment plant and is completely non-toxic. This makes the environmentally friendly formulation of the portioned while using the above-mentioned detergent composition Reinerers greatly upgraded ecologically. At the same time, waste is significantly reduced compared to the individual packaging of tablets, for example, and the dangers in the household when using the cleaner are also minimized.
Als bevorzugte Behandlungsmittel zur Reinigung von Geschirr wird dabei eine Zusammensetzung verwendet, die besteht ausA composition consisting of is used as the preferred treatment agent for cleaning dishes
10 - 70 Gew.% wasserlöslichem Alkalisilikat mit einem Verhältnis von Siliciumdioxid zu Alkalioxid von größer als 1:1,
0 - 50 Gew.% Alkalicarbonat,
2 - 20 Gew.% polymere Sequester bzw. Dispergatoren, vorzugsweise vom Typ der Polycarboxylate, insbesondere Homopolymere der Acrylsäure oder Copolymere mit anderen organischen Säuren oder Äthern, die Vinylgruppen enthalten,
0 - 40 Gew.% Alkalisalze von organischen Säuren mit sequestrierender Wirkung auf Calciumionen wie Polycarbonsäuren, insbesondere Zitronensäure oder aus Zucker fermentativ gewonnene Säuren, insbesondere Glukonsäure,,
2 - 15 Gew.% eines borfreien Bleichmittels auf Sauerstoffbasis aus der Gruppe der Peroxoverbindungen oder Peroxohydrate oder Mischungen daraus, die im Wasser Wasserstoffperoxid freisetzen,
0 - 15 Gew.% eines Bleichaktivators aus der Gruppe von Verbindungen, die unter Einwirkung von Wasserstoffperoxid reaktive Persäuren, insbesondere Peressigsäure freisetzen,
0 - 5 Gew.% eines Alkalisalzes einer Phosphonsäure zur Stabilisierung des Bleichmittels bei der Lagerung,
0 - 5 Gew.% Enzyme oder Enzymgemische aus der Gruppe der Hydrolasen, insbesondere Proteasen, Amylasen und Lipasen,
0,5 - 5 Gew.% eines schwachschäumenden, nicht-ionischen oder anionischen Tensides,
wobei die Summe der einzelnen Komponenten 100 Gew.% beträgt.10-70% by weight of water-soluble alkali silicate with a ratio of silicon dioxide to alkali oxide of greater than 1: 1,
0 - 50% by weight alkali carbonate,
2 to 20% by weight of polymeric sequesters or dispersants, preferably of the polycarboxylate type, in particular homopolymers of acrylic acid or copolymers with other organic acids or ethers which contain vinyl groups,
0 - 40% by weight alkali salts of organic acids with sequestering action on calcium ions such as polycarboxylic acids, in particular citric acid or acids obtained fermentatively from sugar, in particular gluconic acid.
2-15% by weight of a boron-free, oxygen-based bleaching agent from the group of peroxo compounds or peroxohydrates or mixtures thereof, which release hydrogen peroxide in the water,
0 - 15% by weight of a bleach activator from the group of compounds which liberate reactive peracids, in particular peracetic acid, under the action of hydrogen peroxide,
0 - 5% by weight of an alkali salt of a phosphonic acid for stabilizing the bleaching agent during storage,
0 - 5% by weight of enzymes or enzyme mixtures from the group of hydrolases, in particular proteases, amylases and lipases,
0.5 - 5% by weight of a low-foaming, non-ionic or anionic surfactant,
the sum of the individual components being 100% by weight.
Besonders bevorzugt ist die Verwendung einer Zusammensetzung, die besteht ausIt is particularly preferred to use a composition consisting of
25 - 60 Gew.%, insbesondere 40-50 Gew.%, amorphes Natriumdisilikat mit SiO₂:Na₂O von 1,9:1 bis 2,1:1,
10 - 40 Gew.%, insbesondere 25-35 Gew.%, Natriumcarbonat,
3 - 10 Gew.%, insbesondere 4-8 Gew.%, Polyacrylatmaleinat (7:3) als Natriumsalz,
3 - 10 Gew.%, insbesondere 4-7 Gew.%, Natriumglukonat,
5 - 25 Gew.%, insbesondere 5-15 Gew.%, Natriumpercarbonat,
0 - 15 Gew.%, insbesondere 3-7 Gew.%, TAED (Tetraacetylethylendiamin),
0 - 2 Gew.%, insbesondere 0,2-0,5 Gew.%, HEDP (Hydroxyethan-1,1-diphosphonsäure) als Natriumsalz,
0 - 5 Gew.% Mischung aus stabilisierten Enzymen, insbesondere Proteasen, Amylasen und Lipasen,
0 - 5 Gew.% schwachschäumende, ionische oder anionische Tenside,
wobei die Summe der einzelnen Komponenten 100 Gew.% beträgt.25-60% by weight, in particular 40-50% by weight, amorphous sodium disilicate with SiO₂: Na₂O from 1.9: 1 to 2.1: 1,
10 - 40% by weight, in particular 25-35% by weight, sodium carbonate,
3 - 10 wt.%, In particular 4-8 wt.%, Polyacrylate maleate (7: 3) as sodium salt,
3 - 10% by weight, in particular 4-7% by weight, sodium gluconate,
5 - 25% by weight, in particular 5-15% by weight, sodium percarbonate,
0 - 15 wt.%, In particular 3-7 wt.%, TAED (tetraacetylethylene diamine),
0-2% by weight, in particular 0.2-0.5% by weight, of HEDP (hydroxyethane-1,1-diphosphonic acid) as the sodium salt,
0 - 5% by weight mixture of stabilized enzymes, in particular proteases, amylases and lipases,
0 - 5% by weight of low-foaming, ionic or anionic surfactants,
the sum of the individual components being 100% by weight.
Dabei dienen die Silikate und Carbonate als milde Alkalien zur Verbesserung der Schmutzentfernung durch Aufquellen, und die Silikate zusätzlich als Korrosionsschutz. Durch gezielte Wahl der Carbonate z.B. aus der Gruppe Natriumcarbonat, Natriumsesquicarbonat und Natriumhydrogencarbonat bzw. Mischungen daraus läßt sich der pH-Wert des Reinigers zwischen ca. 9,5 und 11,5 einstellen und puffern.The silicates and carbonates serve as mild alkalis to improve dirt removal by swelling, and the silicates also as corrosion protection. Through the targeted choice of carbonates e.g. from the group of sodium carbonate, sodium sesquicarbonate and sodium bicarbonate or mixtures thereof, the pH of the cleaner can be adjusted between about 9.5 and 11.5 and buffered.
Sowohl die genannten Polycarboxylate bzw. andere Polymere als auch die organischen Säuren bzw. deren Salze dienen vor allem zur Bindung der Wasserhärte, die trotz Ionenaustauscher immer noch in der Spülmaschine vorhanden sein kann. Auch das polymere Material des Beutels selbst trägt wie die anderen Polymeren zusätzlich zur Dispergierung von Feststoffen, wie z.B. Spinat-Partikeln, bei.Both the polycarboxylates or other polymers mentioned and the organic acids or their salts serve primarily to bind the water hardness, which can still be present in the dishwasher despite the ion exchanger. The polymeric material of the bag itself, like the other polymers, also contributes to the dispersion of solids, e.g. Spinach particles, at.
Das Bleichsystem besteht aus dem Aktivsauerstoff-Spender aus der Gruppe der Perverbindungen, dem Aktivator, wie z.B. TAED und dem Stabilisator, vorzugsweise aus der Gruppe der Phosphate. Allerdings kommen auch Stickstoffbasen als Komplexbildner für Eisen und Schwermetalle in Frage, die wie im Falle von Glycinabkömmlingen sogar biologisch abbaubar sind. Diese Stabilisatoren-Verbindungen verzögern den durch die Metallionen katalysierten Zerfall der Aktivsauerstoffverbindungen durch Komplexierung der Metallionen. Im Falle von Polyvinylalkohol als Folienmaterial muß der Sauerstoffträger unbedingt borfrei sein, da die Borate mit dem Polyvinylalkohol zu schwerlöslichen Komplexen reagieren, was die Löslichkeit des Beutels negativ beeinflußt.The bleaching system consists of the active oxygen donor from the group of the per compounds, the activator, such as TAED and the stabilizer, preferably from the group of the phosphates. However, nitrogen bases can also be used as complexing agents for iron and heavy metals as in the case of glycine derivatives are even biodegradable. These stabilizer compounds delay the decomposition of the active oxygen compounds catalyzed by the metal ions by complexing the metal ions. In the case of polyvinyl alcohol as film material, the oxygen carrier must be boron-free, since the borates react with the polyvinyl alcohol to form poorly soluble complexes, which has a negative effect on the solubility of the bag.
Die schaumarmen Tenside tragen durch ihre entschäumende Wirkung und die Solubilisierung von Fetten zur Reinigungsleistung bei. Die Enzyme wirken sehr spezifisch auf Speisereste. So werden durch die Amylase Stärkereste und durch die Protease Eiweißreste gezielt angegriffen. Da sie bereits in äußerst geringen Mengen starke Wirkung zeigen und biologisch abbaubar sind, tragen sie zur geringen Umweltbelastung des portionierten Reinigers bei. Gleiches gilt auch für die Lipase, die zur besseren Entfettung eingesetzt werden kann.The low-foaming surfactants contribute to cleaning performance through their defoaming effect and the solubilization of fats. The enzymes have a very specific effect on food residues. Starch residues are targeted by the amylase and protein residues by the protease. Since they show strong effects even in extremely small quantities and are biodegradable, they contribute to the low environmental impact of the portioned cleaner. The same applies to lipase, which can be used for better degreasing.
Um auch borhaltige Perverbindungen, wie z.B. Perborat, einsetzen zu können, wäre auch der Einsatz von Stärke oder modifizierter Stärke als Beutelmaterial möglich.To also contain boron-containing per-compounds, e.g. To be able to use perborate, it would also be possible to use starch or modified starch as the bag material.
Wenn der erfindungsgemäße Beutel ein größeres Fassungsvermögen von ca. 250 g aufweist, kann das erfindungsgemäße Produkt geeigneterweise zur Reinigung und Entfettung der Maschine selbst verwendet werden. Dabei können als Reinigungsmittel die zuvor genannten Zusammensetzungen verwendet werden.If the bag according to the invention has a larger capacity of approx. 250 g, the product according to the invention can suitably be used for cleaning and degreasing the machine itself. The compositions mentioned above can be used as cleaning agents.
Selbstverständlich kann der erfindungsgemäße Beutel auch zur Reinigung von Waschmaschinen verwendet werden. Hier befreien die entkalkenden Behandlungsmittel vor allem die Heizelemente der Waschmaschine, wohingegen die alkalischen Reiniger die sogenannten Fettläuse entfernen. Letztere sind Ablagerungen aus Bestandteilen der Waschmittel, Fett und Härtebildnern des Wassers, die oftmals durch Unterdosierung der Waschmittel entstehen.Of course, the bag according to the invention can also be used for cleaning washing machines. Here The descaling treatment agents primarily free the heating elements of the washing machine, whereas the alkaline cleaners remove the so-called fat lice. The latter are deposits of constituents of the detergents, grease and hardness formers of the water, which often result from underdosing of the detergents.
Bei einer weiteren Ausführungsform des erfindungsgemäßen Produktes umfaßt das Produkt eine zweite, ein zweites Behandlungsmittel enthaltende Kammer, die der ersten Kammer über einen gemeinsamen Saum benachbart, von dieser getrennt oder in dieser enthalten sein kann. Der innere Beutel kann auch vollständig vom äußeren Beutel getrennt oder daran befestigt sein. Eine integrierte Konstruktion, erzeugt durch Falten, ist ebenso möglich. Entsprechend einer weiteren Möglichkeit kann die zweite Kammer in einer getrennten, zusätzlichen dritten Kammer, getrennt von der ersten Kammer vorliegen.In a further embodiment of the product according to the invention, the product comprises a second chamber containing a second treatment agent, which can be adjacent to, separated from or contained in the first chamber via a common seam. The inner bag can also be completely separated from or attached to the outer bag. An integrated construction, created by folding, is also possible. According to a further possibility, the second chamber can be present in a separate, additional third chamber, separate from the first chamber.
Zweckmäßigerweise sind die Wasserlöslichkeiten der Kammerwände der beiden Kammern derart unterschiedlich, daß die Auflösung der jeweiligen Kammerwand und die Freisetzung des darin enthaltenen Behandlungsmittels zu unterschiedlichen Zeitpunkten im Verlauf des Reinigungsprozesses vor Beginn des Klarspülvorgangs erfolgt.The water solubilities of the chamber walls of the two chambers are expediently so different that the dissolution of the respective chamber wall and the release of the treatment agent contained therein take place at different times in the course of the cleaning process before the rinse process begins.
Die Wasserlöslichkeit der Kammerwände kann dadurch beispielsweise beeinflußt werden, daß die Kammerwände der beiden Kammern aus wasserlöslichem Folienmaterial mit unterschiedlichen Dicken und/oder aus unterschiedlichen Polymeren oder Mischungen davon bestehen. Dadurch kann beispielsweise erreicht werden, daß der Inhaltsstoff der ersten Kammer im Verlauf des mit kaltem Wasser durchgeführten Vorspülgangs und der Inhaltsstoff der zweiten Kammer erst im Verlauf des mit heißem Wasser durchgeführten eigentlichen Reinigungsschrittes freigesetzt werden. Das verwendete Folienmaterial kann dabei eine Dicke von 1 µm bis 2 mm besitzen.The water solubility of the chamber walls can be influenced, for example, by the chamber walls of the two chambers being made of water-soluble film material with different thicknesses and / or of different polymers or mixtures thereof. In this way it can be achieved, for example, that the content of the first chamber is carried out with cold water Pre-rinse and the contents of the second chamber are only released in the course of the actual cleaning step carried out with hot water. The film material used can have a thickness of 1 µm to 2 mm.
Als Inhaltsstoff der ersten Kammer kann dann beispielsweise ein Entkalker eingesetzt werden, der ausgewählt wird aus der Gruppe, die besteht aus Zitronensäure, Harnstoffphosphat- und Sulfaminsäure oder anderen festen anorganischen oder organischen Säuren bzw. sauren Salzen.A decalcifying agent can then be used as the ingredient in the first chamber, for example, which is selected from the group consisting of citric acid, urea phosphate and sulfamic acid or other solid inorganic or organic acids or acid salts.
Zusätzlich wird bei der Formulierung mit der oben angegebenen Zusammensetzung in Folge der Alkalireduktion bei folgender Vorgehensweise jeglicher direkte Kontakt mit dem Reiniger vermieden. Dabei wird kein Staub freigesetzt und die Atemwege werden nicht gereizt.In addition, any form of direct contact with the cleaner is avoided in the formulation with the composition given above as a result of the alkali reduction using the following procedure. No dust is released and the respiratory tract is not irritated.
Durch geeignete Wahl verschiedener wasserlöslicher Folien auf Basis modifizierten Polyvinyl-Alkohols kann demzufolge ein erfindungsgemäßes Produkt mit zwei Kammern bereitgestellt werden, wobei der Entkalker in der Kammer aus kaltwasserlöslicher Folie und der alkalische Grundreiniger in der Kammer aus heißwasserlöslicher Folie abgepackt ist. Bei einem Programmablauf der Spülmaschine wirkt dann im Vorspülgang der Entkalker, wogegen der alkalische Reiniger erst beim Aufheizen im Hauptspülgang seine Wirkung entfaltet. Bei dieser Arbeitsweise ist zur Reinigung und Entfettung der Geschirrspülmaschine nur ein Arbeitsgang notwendig. Ein entsprechendes Ergebnis läßt sich ebenso bei Verwendung von zwei getrennten Beuteln erzielen, die jeweils Kammern aus unterschiedlichen Folienmaterialien mit unterschiedlichen Inhaltsstoffen umfassen.By suitable selection of various water-soluble films based on modified polyvinyl alcohol, a product according to the invention can accordingly be provided with two chambers, the decalcifier being packed in the chamber made of cold water-soluble film and the alkaline basic cleaner in the chamber made of hot water-soluble film. When the dishwasher runs, the decalcifying agent works in the pre-rinse cycle, whereas the alkaline cleaner only works when the main rinse cycle heats up. With this method of operation, only one operation is necessary to clean and degrease the dishwasher. A corresponding result can also be achieved when using two separate bags, each of which comprises chambers made of different film materials with different ingredients.
Als Kleber für das die Kammer bildende Folienmaterial können die üblicherweise auf diesem Gebiet verwendbaren wasserlöslichen Kleber verwendet werden. Als Beipiel eines geeigneten wasserempfindlichen Klebers ist Natriumcarboxymethylcellulose genannt.The water-soluble adhesives which can usually be used in this field can be used as the adhesive for the film material forming the chamber. Sodium carboxymethyl cellulose is mentioned as an example of a suitable water-sensitive adhesive.
Als eine weitere Ausführungsform wird ein erfindungsgemäßes Produkt bereitgestellt, das dadurch gekennzeichnet ist, daß der wenigstens eine Kammer umfassende Beutel mit einem oder mehreren, wenigstens eine Kammer umfassenden Beuteln über Folienbereiche verbunden ist, die zwischen benachbarten Beuteln Abrißbereiche aufweisen, die gegebenenfalls perforiert sein können und die eine Trennung der einzelnen Beutel voneinander erlauben. So ist dem Verbraucher eine einfache Portionierung der Menge des Behandlungsmittels möglich.As a further embodiment, a product according to the invention is provided, which is characterized in that the bag comprising at least one chamber is connected to one or more bags comprising at least one chamber via film areas which have tear-off areas between adjacent bags, which may or may not be perforated, and which allow the individual bags to be separated from one another. The consumer can thus easily portion the amount of treatment agent.
Die vorliegende Erfindung wird anhand der nachfolgenden Beispiele näher erläutert.The present invention is illustrated by the following examples.
Die Rezeptur aus Beispiel II wird mit 250 g in Portionsbeutel aus kaltwasserlöslichem Polyvinylalkohol mit einer Wandstärke von 40 mµ abgepackt. Die Alkalität des Reinigers ist so eingestellt, daß das Folienmaterial nicht beeinträchtigt wird. Eine Maschine mit starken Ablagerungen von Essensresten im Sieb der Umwälzpumpe wird wie folgt behandelt:
Zu Beginn des Hauptspülgangs ohne Spülgut wird die Maschine geöffnet und der originalverschlossene Beutel in den oberen Geschirrkorb gelegt. Nach dem Schließen wird das Wasser sofort wieder umgewälzt und der Beutelinhalt praktisch beim ersten Kontakt mit dem Wasser freigesetzt und gleichmäßig verteilt. Nach Beenden des Programms ist das Sieb wieder frei von Essensresten. Ein Parallelversuch mit dem gleichen Pulver, das unsachgemäß angehäuft direkt auf das Sieb geschüttet wird, führt dazu, daß der Reiniger sich in dem Ansaugschlauch der Umwälzpumpe verhärtet und eine Reparatur nötig wird.The recipe from Example II is packed with 250 g in sachets made of cold water-soluble polyvinyl alcohol with a wall thickness of 40 mµ. The alkalinity of the cleaner is adjusted so that the film material is not affected. A machine with heavy deposits of food residues in the sieve of the circulation pump is treated as follows:
At the beginning of the main wash cycle without items to be washed, the machine is opened and the original sealed bag is placed in the upper basket. After closing, the water is immediately circulated again and the contents of the bag practically at first contact with water released and evenly distributed. After the program has ended, the sieve is free of leftovers. A parallel test with the same powder, which is improperly poured directly onto the sieve, leads to the fact that the cleaner hardens in the suction hose of the circulation pump and a repair is necessary.
Das gleiche Beutelmaterial wie in Beispiel 1) wird mit 250 g Citronensäure befüllt. Bei gleicher Vorgehensweise wie unter 1) werden Kalkablagerungen in der Maschine, vor allem auf den Heizstäben entfernt.The same bag material as in Example 1) is filled with 250 g of citric acid. With the same procedure as in 1), limescale deposits are removed in the machine, especially on the heating elements.
Beim Anschluß der Spülmaschine an Kaltwasser kann eine Kombipackung zum Einsatz gelangen, die aus verschiedenen Folien aufgebaut ist. Als Entkalker dient der Beutel aus 2). Der Reiniger wie unter 1) befindet sich in einem Beutel aus einem Polyvinylacetat, das nur teilweise zu Polyvinylalkohol umgesetzt ist. Dadurch kann die Wasserlöslichkeit beeinflußt werden. Der Grad der Umsetzung wird so gewählt, daß die Folie sich erst ab einer Temperatur von 45° bis 50°C vollständig auflöst. Die Wandstärke beträgt ca. 100 mµ, damit der Beutel nicht vorzeitig aufplatzt und das Produkt freisetzt.When connecting the dishwasher to cold water, a combination pack can be used, which is made up of different foils. The bag from 2) serves as a descaler. The cleaner as in 1) is in a bag made of a polyvinyl acetate, which is only partially converted to polyvinyl alcohol. This can affect the water solubility. The degree of conversion is chosen so that the film only dissolves completely from a temperature of 45 ° to 50 ° C. The wall thickness is approx. 100 mµ so that the bag does not burst open prematurely and the product is released.
Eine Spülmaschine mit Essensresten im Sieb und Kalkablagerungen auf den Heizstäben wird wie folgt behandelt:
In den oberen Geschirrkorb werden die beiden getrennten Beutel (ein Entkalker und ein Reiniger) ungeöffnet nebeneinander gelegt. Danach wird ein 65°C-Programm mit Vorspülgang gewählt. Bei der eingesetzten Siemens Spülmaschine Typ Lady Plus 260 ist dies das Normalprogramm. Im Vorspülgang mit Kaltwasser (Zulauf ca. 17°C) löst sich der Beutel mit der Citronensäure auf und der Kalk auf den Heizstäben wird entfernt. Der Beutel mit dem alkalischen Reiniger wird dabei nur wenig angelöst. Die saure Lösung wird abgepumpt und frisches Wasser für den Hauptspülgang läuft ein. Nach Beginn des Umwälzens und Aufheizens öffnet sich der zweite Beutel mit dem Reiniger bei Erreichen von ca. 40° bis 50°C. Nun kann der alkalische Reiniger die Essensreste im Sieb angreifen. Ergebnis: In einem Arbeitsgang ist die Maschine entkalkt und von Essensresten gereinigt. Dies spart gegenüber getrennte Anwendung Wasser und Energie.A dishwasher with leftovers in the sieve and limescale deposits on the heating elements is treated as follows:
The two separate bags (a descaler and a cleaner) are placed unopened next to each other in the upper basket. Then a 65 ° C program with pre-rinse chosen. This is the normal program for the Siemens dishwasher type Lady Plus 260 used. In the pre-rinse cycle with cold water (inlet approx. 17 ° C), the bag with the citric acid dissolves and the lime on the heating elements is removed. The bag with the alkaline cleaner is only slightly dissolved. The acidic solution is pumped off and fresh water for the main wash cycle runs in. After the circulation and heating have started, the second pouch with the cleaner opens when it reaches approx. 40 ° to 50 ° C. Now the alkaline cleaner can attack the leftovers in the sieve. Result: The machine is descaled and cleaned of food residues in one operation. This saves water and energy compared to separate use.
Beim Einsatz von Stärke als Beutelmaterial läßt sich vorteilhaft die Rezeptur I einsetzen, da das stabilere Perborat als Sauerstoffbleiche enthalten ist. Da ein Teil der Protease allerdings durch das Beutelmaterial aufgebraucht wird, ist die Entfernung der Haferflocken etwas schlechter. Wird bei Mischung I Polyvinylalkohol als Beutelmaterial verwendet, findet man schleimige Rückstände in der Maschine und auf dem Geschirr.When starch is used as the bag material, recipe I can advantageously be used, since the more stable perborate is present as oxygen bleach. However, since part of the protease is used up by the bag material, the removal of the oatmeal is somewhat worse. If mixture I uses polyvinyl alcohol as the bag material, you will find slimy residues in the machine and on the dishes.
Die Leistung der Reiniger ist aus der nachfolgenden Tabelle zu entnehmen:
Zu den Versuchen ist anzumerken, daß die Reinigungsleistung der Formulierung I mit Beutel aus Stärke ermittelt wurde, die anderen mit Beuteln aus kaltwasserlöslichem Polyvinylalkohol. Die Dosierung betrug 25 g. Bei gleicher Dosierung wurde mit dem Referenz-Reiniger nach der Vorschrift DIN 44990 ein Reinigungsindex von 3,98 erreicht.Regarding the experiments, it should be noted that the cleaning performance of formulation I was determined using pouches made of starch, the others using pouches made of polyvinyl alcohol which was soluble in cold water. The dosage was 25 g. With the same dosage, the reference cleaner according to the DIN 44990 standard achieved a cleaning index of 3.98.
Claims (16)
10 - 70 Gew.% wasserlöslichem Alkalisilikat mit einem Verhältnis von Siliciumdioxid zu Alkalioxid von größer als 1:1,
0-50 Gew.% Alkalicarbonat,
2-20 Gew.% polymere Sequester bzw. Dispergatoren, vorzugsweise vom Typ der Polycarboxylate insbesondere Homopolymere der Acrylsäure oder Copolymere mit anderen organischen Säuren oder Äthern, die Vinylgruppen enthalten,
0 - 40 Gew.% Alkalisalze von organischen Säuren mit sequestrierender Wirkung auf Calciumionen wie Polycarbonsäuren, insbesondere Zitronensäure oder aus Zucker fermentativ gewonnene Säuren, insbesondere Glukonsäure,
2-15 Gew.% eines borfreien Bleichmittels auf Sauerstoffbasis aus der Gruppe der Peroxoverbindungen oder Peroxohydrate oder Mischungen daraus, die in Wasser Wasserstoffperoxid freisetzen,
0-15 Gew.% eines Bleichaktivators aus der Gruppe von Verbindungen, die unter Einwirkung von Wasserstoffperoxid reaktive Persäuren, insbesondere Peressigsäure freisetzen,
0-5 Gew.% eines Alkalisalzes einer Phosphonsäure zur Stabilisierung des Bleichmittels bei der Lagerung,
0-5 Gew.% Enzyme oder Enzymgemische aus der Gruppe der Hydrolasen, insbesondere Proteasen, Amylasen und Lipasen
0,5 - 5 Gew.% eines schwachschäumenden, nicht-ionischen oder anionischen Tensides,
wobei die Summe der einzelnen Komponenten 100 Gew.% beträgt.Product according to one of claims 1 to 6, characterized in that a granular phosphate-free treatment agent for automatic dishwashing with a composition consisting of is used as the treatment agent
10-70% by weight of water-soluble alkali silicate with a ratio of silicon dioxide to alkali oxide of greater than 1: 1,
0-50% by weight alkali carbonate,
2-20% by weight of polymeric sequesters or dispersants, preferably of the polycarboxylate type, in particular homopolymers of acrylic acid or copolymers with other organic acids or ethers which contain vinyl groups,
0 - 40% by weight alkali salts of organic acids with sequestering action on calcium ions such as polycarboxylic acids, in particular citric acid or acids obtained from sugar by fermentation, in particular gluconic acid,
2-15% by weight of a boron-free oxygen-based bleaching agent from the group of the peroxo compounds or peroxohydrates or mixtures thereof which release hydrogen peroxide in water,
0-15% by weight of a bleach activator from the group of compounds which liberate reactive peracids, in particular peracetic acid, under the action of hydrogen peroxide,
0-5% by weight of an alkali salt of a phosphonic acid for stabilizing the bleaching agent during storage,
0-5% by weight of enzymes or enzyme mixtures from the group of hydrolases, in particular proteases, amylases and lipases
0.5 - 5% by weight of a low-foaming, non-ionic or anionic surfactant,
the sum of the individual components being 100% by weight.
25-60 Gew.%, insbesondere 40-50 Gew.%, amorphes Natriumdisilikat mit SiO₂:Na₂O von 1,9:1 bis 2,1:1,
10-40 Gew.%, insbesondere 25-35 Gew.%, Natriumcarbonat
3-10 Gew.%, insbesondere 4-8 Gew.%, Polyacrylatmaleinat (7:3) als Natriumsalz
3-10 Gew.%, insbesondere 4-7 Gew.%, Natriumglukonat,
5-25 Gew.%, insbesondere 5-15 Gew.%, Natriumpercarbonat,
0-15 Gew.%, insbesondere 3-7 Gew.%, TAED (Tetraacetylethylendiamin),
0-2 Gew.%, insbesondere 0,2-0,5 Gew.%, HEDP (Hydroxyethan-1,1-diphosphonsäure) als Natriumsalz
0-5 Gew.% Mischung aus stabilisierten Enzymen, insbesondere Proteasen, Amylasen und Lipasen
0-5 Gew.%, schwachschäumende, ionische oder anionische Tenside,
wobei die Summe der einzelnen Komponenten 100 Gew.% beträgt.Product according to claim 7, characterized in that the treatment agent used is a granular phosphate-free treatment agent for automatic dishwashing with a composition consisting of
25-60% by weight, in particular 40-50% by weight, amorphous sodium disilicate with SiO₂: Na₂O from 1.9: 1 to 2.1: 1,
10-40% by weight, especially 25-35% by weight, sodium carbonate
3-10% by weight, in particular 4-8% by weight, polyacrylate maleate (7: 3) as the sodium salt
3-10% by weight, in particular 4-7% by weight, sodium gluconate,
5-25% by weight, in particular 5-15% by weight, sodium percarbonate,
0-15% by weight, in particular 3-7% by weight, TAED (tetraacetylethylene diamine),
0-2% by weight, in particular 0.2-0.5% by weight, of HEDP (hydroxyethane-1,1-diphosphonic acid) as the sodium salt
0-5% by weight mixture of stabilized enzymes, especially proteases, amylases and lipases
0-5% by weight, low-foaming, ionic or anionic surfactants,
the sum of the individual components being 100% by weight.
10 - 70 Gew.% wasserlöslichem Alkalisilikat mit einem Verhältnis von Siliciumdioxid zu Alkalioxid von grösser als 1:1,
0-50 Gew.% Alkalicarbonat,
2-20 Gew.% polymere Sequester bzw. Dispergatoren, vorzugsweise vom Typ der Polycarboxylate insbesondere Homopolymere der Acrylsäure oder Copolymere mit anderen organischen Säuren oder Ätzern, die Vinylgruppen enthalten,
0 - 40 Gew.% Alkalisalze von organischen Säuren mit sequestrierender wirkung auf Calciumionen wie Polycarbonsäuren, insbesondere Zitronensäure oder aus Zucker fermentativ gewonnene Säuren, insbesondere Glukonsäure,
2-15 Gew.% eines borfreien Bleichmittels auf Sauerstoffbasis aus der Gruppe der Peroxoverbindungen oder Peroxohydrate oder Mischungen daraus, die in Wasser Wasserstoffperoxid freisetzen,
0-15 Gew.% eines Bleichaktivators aus der Gruppe von Verbindungen, die unter Einwirkung von Wasserstoffperoxid reaktive Persäuren, insbesondere Peressigsäure freisetzen,
0-15 Gew.% eines Alkalisalzes einer Phosphonsäure zur Stabilisierung des Bleichmittels bei der Lagerung,
0-15 Gew.% Enzyme oder Enzymgemische aus der Gruppe der Hydrolasen, insbesondere Proteasen, Amylasen und Lipasen,
0-5 Gew.% eines schwachschäumenden, nicht-ionischen oder anionischen Tensides,
wobei die Summe der einzelnen Komponenten 100 Gew.% beträgt.Product according to one of claims 2 to 5, characterized in that the bag comprises 2 chambers, the first chamber as treatment agent a powdered acid decalcifier, selected from the group consisting of Contains citric acid, sulfamic acid, urea phosphate or other solid organic or inorganic acids or acid salts, and the second chamber contains as treatment agent a granular, phosphate-free dishwashing agent with a composition consisting of
10 - 70% by weight of water-soluble alkali silicate with a ratio of silicon dioxide to alkali oxide of greater than 1: 1,
0-50% by weight alkali carbonate,
2-20% by weight of polymeric sequesters or dispersants, preferably of the polycarboxylate type, in particular homopolymers of acrylic acid or copolymers with other organic acids or etchers, which contain vinyl groups,
0 - 40% by weight alkali salts of organic acids with sequestering action on calcium ions such as polycarboxylic acids, in particular citric acid or acids obtained fermentatively from sugar, in particular gluconic acid,
2-15% by weight of a boron-free oxygen-based bleaching agent from the group of the peroxo compounds or peroxohydrates or mixtures thereof which release hydrogen peroxide in water,
0-15% by weight of a bleach activator from the group of compounds which liberate reactive peracids, in particular peracetic acid, under the action of hydrogen peroxide,
0-15% by weight of an alkali metal salt of a phosphonic acid for stabilizing the bleaching agent during storage,
0-15% by weight of enzymes or enzyme mixtures from the group of hydrolases, in particular proteases, amylases and lipases,
0-5% by weight of a low-foaming, non-ionic or anionic surfactant,
the sum of the individual components being 100% by weight.
25-60 Gew.%, insbesondere 40-50 Gew.%, amorphes Natriumdisilikat mit SiO₂:Na₂O von 1,9:1 bis 2,1:1,
10-40 Gew.%, insbesondere 25-35 Gew.%, Natriumcarbonat,
3-10 Gew.%, insbesondere 4-8 Gew.%, Polyacrylat-Maleinat (7:3) als Natriumsalz,
3-10 Gew.%, insbesondere 4-7 Gew.%, Natriumglukonat,
5-25 Gew.%, insbesondere 5-15 Gew.%, Natriumpercarbonat,
0-15 Gew.%, insbesondere 3-7 Gew.%, TAED (Tetraacetylethylendiamin),
0-2 Gew.%, insbesondere 0,2-0,5 Gew.%, HEDP (Hydroxyethan-1,1-diphosphonsäure) als Natriumsalz,
0-5 Gew.% Mischung aus stabilisierten Enzymen, insbesondere Proteasen, Amylasen und Lipasen,
0-5 Gew.%, schwachschäumende, ionische oder anionische Tenside,
wobei die Summe der einzelnen Komponenten 100 Gew.% beträgt.Product according to claim 11, characterized in that a composition consisting of is used as the granular, phosphate-free treatment agent
25-60% by weight, in particular 40-50% by weight, amorphous sodium disilicate with SiO₂: Na₂O from 1.9: 1 to 2.1: 1,
10-40% by weight, in particular 25-35% by weight, sodium carbonate,
3-10% by weight, in particular 4-8% by weight, polyacrylate maleinate (7: 3) as sodium salt,
3-10% by weight, in particular 4-7% by weight, sodium gluconate,
5-25% by weight, in particular 5-15% by weight, sodium percarbonate,
0-15% by weight, in particular 3-7% by weight, TAED (tetraacetylethylene diamine),
0-2% by weight, in particular 0.2-0.5% by weight, of HEDP (hydroxyethane-1,1-diphosphonic acid) as the sodium salt,
0-5% by weight mixture of stabilized enzymes, especially proteases, amylases and lipases,
0-5% by weight, low-foaming, ionic or anionic surfactants,
the sum of the individual components being 100% by weight.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE9214065U DE9214065U1 (en) | 1992-10-17 | 1992-10-17 | Product for the release of treatment agents into the washing liquid of an automatic washing or dishwasher machine |
| DE9214065U | 1992-10-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0593952A1 true EP0593952A1 (en) | 1994-04-27 |
Family
ID=6884955
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP93115693A Ceased EP0593952A1 (en) | 1992-10-17 | 1993-09-29 | Product for releasing treatment agents into the wash liquid of an automatic washing or dishwashing machine |
Country Status (2)
| Country | Link |
|---|---|
| EP (1) | EP0593952A1 (en) |
| DE (1) | DE9214065U1 (en) |
Cited By (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999041442A1 (en) * | 1998-02-14 | 1999-08-19 | Henkel-Ecolab Gmbh & Co. Ohg | Method for dosing detergent and device for carrying out said method |
| DE19845602A1 (en) * | 1998-10-05 | 2000-04-06 | Henkel Kgaa | Detergent tab pack for dishwashers |
| EP1026230A1 (en) * | 1999-02-05 | 2000-08-09 | Unilever Plc | A machine dishwashing kit |
| US6281183B1 (en) | 1999-03-17 | 2001-08-28 | Unilever Home & Personal Care, Division Of Conopco, Inc. | Process for producing a water soluble package |
| GB2361688A (en) * | 2000-04-28 | 2001-10-31 | Procter & Gamble | Multi-compartment water soluble pouch for detergents |
| WO2001083669A1 (en) * | 2000-04-28 | 2001-11-08 | The Procter & Gamble Company | Pouched compositions |
| WO2002006431A2 (en) * | 2000-07-14 | 2002-01-24 | Henkel Kommanditgesellschaft Auf Aktien | Hollow body with a compartment, containing a portion of a washing, cleaning or rinsing agent |
| GB2365018A (en) * | 2000-07-24 | 2002-02-13 | Procter & Gamble | Water soluble pouches |
| US6363693B1 (en) | 1999-03-17 | 2002-04-02 | Unilever Home & Personal Care, Usa | Process for producing a water soluble package |
| WO2002026928A1 (en) * | 2000-09-28 | 2002-04-04 | The Dow Chemical Company | Polymer composite structures useful for controlled release systems |
| EP1197546A1 (en) * | 2000-05-12 | 2002-04-17 | Unilever Plc | Unit dose cleaning product |
| US6378274B1 (en) | 1999-03-17 | 2002-04-30 | Unilever Home & Personal Care Usa Division Of Conopco, Inc. | Process for producing a water soluble package |
| GB2370554A (en) * | 1999-11-17 | 2002-07-03 | Reckitt Benckiser | Rigid water-soluble containers |
| WO2002053696A1 (en) * | 2000-12-28 | 2002-07-11 | Unilever Plc | Laundry product |
| EP1223113A1 (en) * | 2001-01-10 | 2002-07-17 | Buck-Chemie GmbH . | Lavatory cleansing product packaged in a water soluble package |
| US6451750B2 (en) | 2000-04-14 | 2002-09-17 | Unilever Home & Personal Care Usa Division Of Conopco, Inc. | Water soluble package and liquid contents thereof |
| US6479448B2 (en) | 2000-05-15 | 2002-11-12 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Liquid detergent composition |
| EP1256623A1 (en) * | 2001-05-08 | 2002-11-13 | The Procter & Gamble Company | Kit of water-soluble or water dispersible pouches |
| DE10081213C1 (en) * | 1999-05-10 | 2002-11-21 | Bbt Bergedorfer Biotech Gmbh | Cleaner, especially for cleaning drain pipe for greasy waste water by adding nitrogen compounds to bacteria that degrade and/or emulsify grease contains urea, derivative and/or salt and/or magnesium sulfate |
| JP2003504491A (en) * | 1999-07-09 | 2003-02-04 | ヘンケル・コマンディットゲゼルシャフト・アウフ・アクチエン | Packaging of laundry detergent / dishwasher detergent |
| WO2002042408A3 (en) * | 2000-11-27 | 2003-04-17 | Procter & Gamble | Detergent products, methods and manufacture |
| EP1364610A1 (en) * | 2002-05-24 | 2003-11-26 | The Procter & Gamble Company | Detergent system |
| WO2004005156A1 (en) * | 2002-07-03 | 2004-01-15 | Crown Packaging Technology, Inc. | Water-soluble container |
| US6831051B2 (en) | 2000-04-28 | 2004-12-14 | The Procter & Gamble Company | Pouched compositions |
| US6946501B2 (en) | 2001-01-31 | 2005-09-20 | The Procter & Gamble Company | Rapidly dissolvable polymer films and articles made therefrom |
| WO2006045450A1 (en) * | 2004-10-22 | 2006-05-04 | Henkel Kommanditgesellschaft Auf Aktien | Detergent or cleaning agent dosing unit |
| US7083047B2 (en) | 2002-10-03 | 2006-08-01 | Unilever Home & Personal Care Usa Division Of Conopco, Inc. | Polymeric film for water soluble package |
| WO2008000567A1 (en) * | 2006-06-30 | 2008-01-03 | Unilever Plc | Laundry articles |
| WO2008104023A1 (en) * | 2007-02-27 | 2008-09-04 | Amcor Limited | Packaging films |
| US7439215B2 (en) | 2000-11-27 | 2008-10-21 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| EP2011856A1 (en) * | 2007-07-02 | 2009-01-07 | The Procter and Gamble Company | Method of treating laundry |
| DE102007036769A1 (en) * | 2007-08-03 | 2009-02-05 | Erlen Gmbh | Assembly to clean bedpans/urine bottles has a cleaning chamber and a steam chamber, with a dosing unit to feed water softener and disinfectant into the water tank |
| US7830263B2 (en) | 2006-04-28 | 2010-11-09 | Obrist Closures Switzerland Gmbh | Closure with RFID device |
| USD630093S1 (en) | 2010-06-11 | 2011-01-04 | Obrist Closures Switzerland Gmbh | Closure |
| US8283300B2 (en) | 2000-11-27 | 2012-10-09 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US8357647B2 (en) | 2000-11-27 | 2013-01-22 | The Procter & Gamble Company | Dishwashing method |
| US8413830B2 (en) | 2008-04-04 | 2013-04-09 | Obrist Closures Switzerland Gmbh | Closure |
| US8453856B2 (en) | 2007-07-13 | 2013-06-04 | Obrist Closures Switzerland Gmbh | Tamper-evident closure |
| US8490804B2 (en) | 2007-10-31 | 2013-07-23 | Obrist Closures Switzerland Gmbh | Closure with movable tamper-evident member |
| US8522991B2 (en) | 2003-10-31 | 2013-09-03 | Obrist Closures Switzerland Gmbh | Tamper evident closure |
| WO2014031308A3 (en) * | 2012-08-21 | 2014-08-28 | Premark Feg L.L.C. | Warewash machine with descaling/deliming system and method |
| US8940676B2 (en) | 2000-11-27 | 2015-01-27 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| EP1404801B2 (en) † | 2001-07-11 | 2015-08-05 | Reckitt Benckiser N.V. | Dishwashing composition |
| US9102448B2 (en) | 2007-07-13 | 2015-08-11 | Obrist Closures Switzerland Gmbh | Tamper-evident closure |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE69427696T2 (en) * | 1994-09-12 | 2002-05-08 | Procter & Gamble | Portion-packed detergent |
| DE19831703A1 (en) * | 1998-07-15 | 2000-01-20 | Henkel Kgaa | Portions of detergent or washing composition packaged in water-soluble film containers with most of the composition above a specified particle size to prevent container sealing and storage problems |
| DE102012014268B4 (en) * | 2012-07-19 | 2014-02-20 | Brauns-Heitmann Gmbh & Co. Kg | Device for cleaning and / or descaling for use in dishwashers or washing machines |
| DE102018208649A1 (en) * | 2018-05-30 | 2019-12-05 | Henkel Ag & Co. Kgaa | Multi-component automatic dishwashing detergent |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0132792A1 (en) * | 1983-07-20 | 1985-02-13 | DISPO-Kommerz AG | Water soluble powdery cleaning agent for hard surfaces |
| EP0236136A2 (en) * | 1986-03-07 | 1987-09-09 | Unilever Plc | Product for dispensing treatment agents in a washing or dishwashing machine |
| EP0337568A2 (en) * | 1988-04-11 | 1989-10-18 | Colgate-Palmolive Company | Detersive article |
| EP0414462A2 (en) * | 1989-08-23 | 1991-02-27 | Unilever Plc | Laundry treatment product |
-
1992
- 1992-10-17 DE DE9214065U patent/DE9214065U1/en not_active Expired - Lifetime
-
1993
- 1993-09-29 EP EP93115693A patent/EP0593952A1/en not_active Ceased
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0132792A1 (en) * | 1983-07-20 | 1985-02-13 | DISPO-Kommerz AG | Water soluble powdery cleaning agent for hard surfaces |
| EP0236136A2 (en) * | 1986-03-07 | 1987-09-09 | Unilever Plc | Product for dispensing treatment agents in a washing or dishwashing machine |
| EP0337568A2 (en) * | 1988-04-11 | 1989-10-18 | Colgate-Palmolive Company | Detersive article |
| EP0414462A2 (en) * | 1989-08-23 | 1991-02-27 | Unilever Plc | Laundry treatment product |
Cited By (81)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999041442A1 (en) * | 1998-02-14 | 1999-08-19 | Henkel-Ecolab Gmbh & Co. Ohg | Method for dosing detergent and device for carrying out said method |
| DE19845602A1 (en) * | 1998-10-05 | 2000-04-06 | Henkel Kgaa | Detergent tab pack for dishwashers |
| US6610643B1 (en) | 1998-10-05 | 2003-08-26 | Henkel Kommanditgesellschaft Auf Aktien | Detergent tablet packaging for dishwashers |
| EP1026230A1 (en) * | 1999-02-05 | 2000-08-09 | Unilever Plc | A machine dishwashing kit |
| WO2000046341A1 (en) * | 1999-02-05 | 2000-08-10 | Unilever Plc | A machine dishwashing kit |
| US6378274B1 (en) | 1999-03-17 | 2002-04-30 | Unilever Home & Personal Care Usa Division Of Conopco, Inc. | Process for producing a water soluble package |
| US6363693B1 (en) | 1999-03-17 | 2002-04-02 | Unilever Home & Personal Care, Usa | Process for producing a water soluble package |
| US6281183B1 (en) | 1999-03-17 | 2001-08-28 | Unilever Home & Personal Care, Division Of Conopco, Inc. | Process for producing a water soluble package |
| DE10081213C1 (en) * | 1999-05-10 | 2002-11-21 | Bbt Bergedorfer Biotech Gmbh | Cleaner, especially for cleaning drain pipe for greasy waste water by adding nitrogen compounds to bacteria that degrade and/or emulsify grease contains urea, derivative and/or salt and/or magnesium sulfate |
| JP2003504491A (en) * | 1999-07-09 | 2003-02-04 | ヘンケル・コマンディットゲゼルシャフト・アウフ・アクチエン | Packaging of laundry detergent / dishwasher detergent |
| EP1647494A3 (en) * | 1999-11-17 | 2015-09-23 | Reckitt Benckiser (UK) Limited | Injection moulded water-soluble container |
| EP2266889A3 (en) * | 1999-11-17 | 2014-12-10 | Reckitt Benckiser (UK) Limited | Injection-moulded water-soluble container |
| GB2370554A (en) * | 1999-11-17 | 2002-07-03 | Reckitt Benckiser | Rigid water-soluble containers |
| GB2370554B (en) * | 1999-11-17 | 2002-11-20 | Reckitt Benckiser | Rigid water-soluble containers |
| US6451750B2 (en) | 2000-04-14 | 2002-09-17 | Unilever Home & Personal Care Usa Division Of Conopco, Inc. | Water soluble package and liquid contents thereof |
| US6831051B2 (en) | 2000-04-28 | 2004-12-14 | The Procter & Gamble Company | Pouched compositions |
| GB2361688A (en) * | 2000-04-28 | 2001-10-31 | Procter & Gamble | Multi-compartment water soluble pouch for detergents |
| WO2001083669A1 (en) * | 2000-04-28 | 2001-11-08 | The Procter & Gamble Company | Pouched compositions |
| US7169740B2 (en) | 2000-04-28 | 2007-01-30 | The Procter & Gamble Company | Pouched compositions |
| EP1197546A1 (en) * | 2000-05-12 | 2002-04-17 | Unilever Plc | Unit dose cleaning product |
| US6479448B2 (en) | 2000-05-15 | 2002-11-12 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Liquid detergent composition |
| US7601679B2 (en) | 2000-07-14 | 2009-10-13 | Henkel Ag & Co. Kgaa | Process for producing a hollow body with a compartment, containing a portion of a washing, cleaning or rinsing agent |
| US7417019B2 (en) | 2000-07-14 | 2008-08-26 | Henkel Kommanditgesellschaft Auf Aktien | Hollow body with a compartment, containing a portion of a washing, cleaning or rinsing agent |
| WO2002006431A3 (en) * | 2000-07-14 | 2002-05-30 | Henkel Kgaa | Hollow body with a compartment, containing a portion of a washing, cleaning or rinsing agent |
| WO2002006431A2 (en) * | 2000-07-14 | 2002-01-24 | Henkel Kommanditgesellschaft Auf Aktien | Hollow body with a compartment, containing a portion of a washing, cleaning or rinsing agent |
| EP1586631A3 (en) * | 2000-07-14 | 2005-10-26 | Henkel Kommanditgesellschaft auf Aktien | Hollow bodies with compartments comprising a portion of washing, cleaning or rinsing agent |
| EP1586631A2 (en) * | 2000-07-14 | 2005-10-19 | Henkel Kommanditgesellschaft auf Aktien | Hollow bodies with compartments comprising a portion of washing, cleaning or rinsing agent |
| EP1303585B2 (en) † | 2000-07-24 | 2012-04-18 | The Procter & Gamble Company | Articles containing enclosed compositions |
| GB2365018A (en) * | 2000-07-24 | 2002-02-13 | Procter & Gamble | Water soluble pouches |
| WO2002026928A1 (en) * | 2000-09-28 | 2002-04-04 | The Dow Chemical Company | Polymer composite structures useful for controlled release systems |
| US9434916B2 (en) | 2000-11-27 | 2016-09-06 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US8435935B2 (en) | 2000-11-27 | 2013-05-07 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US7439215B2 (en) | 2000-11-27 | 2008-10-21 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US8156713B2 (en) | 2000-11-27 | 2012-04-17 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US9382506B2 (en) | 2000-11-27 | 2016-07-05 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US8940676B2 (en) | 2000-11-27 | 2015-01-27 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US10889786B2 (en) | 2000-11-27 | 2021-01-12 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US8658585B2 (en) | 2000-11-27 | 2014-02-25 | Tanguy Marie Louise Alexandre Catlin | Detergent products, methods and manufacture |
| US8518866B2 (en) | 2000-11-27 | 2013-08-27 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| EP1484389A1 (en) * | 2000-11-27 | 2004-12-08 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US10081786B2 (en) | 2000-11-27 | 2018-09-25 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US8357647B2 (en) | 2000-11-27 | 2013-01-22 | The Procter & Gamble Company | Dishwashing method |
| US8283300B2 (en) | 2000-11-27 | 2012-10-09 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| US8250837B2 (en) | 2000-11-27 | 2012-08-28 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| EP1790713A3 (en) * | 2000-11-27 | 2007-09-05 | The Procter & Gamble Company | Detergent products, methods and manufacture |
| WO2002042408A3 (en) * | 2000-11-27 | 2003-04-17 | Procter & Gamble | Detergent products, methods and manufacture |
| WO2002053696A1 (en) * | 2000-12-28 | 2002-07-11 | Unilever Plc | Laundry product |
| EP1223113A1 (en) * | 2001-01-10 | 2002-07-17 | Buck-Chemie GmbH . | Lavatory cleansing product packaged in a water soluble package |
| US7547737B2 (en) | 2001-01-31 | 2009-06-16 | The Procter & Gamble Company | Rapidly dissolvable polymer films and articles made therefrom |
| US6946501B2 (en) | 2001-01-31 | 2005-09-20 | The Procter & Gamble Company | Rapidly dissolvable polymer films and articles made therefrom |
| EP1256623A1 (en) * | 2001-05-08 | 2002-11-13 | The Procter & Gamble Company | Kit of water-soluble or water dispersible pouches |
| EP1724206A3 (en) * | 2001-05-08 | 2007-03-07 | The Procter and Gamble Company | Kit of water-soluble or water dispersible pouches |
| WO2002090486A1 (en) * | 2001-05-08 | 2002-11-14 | The Procter & Gamble Company | Kit of water-soluble or water dispersible pouches |
| EP1721838A2 (en) * | 2001-05-08 | 2006-11-15 | The Procter and Gamble Company | Kit of water-soluble or water dispersible pouches |
| EP1724206A2 (en) * | 2001-05-08 | 2006-11-22 | The Procter and Gamble Company | Kit of water-soluble or water dispersible pouches |
| US7166566B2 (en) | 2001-05-08 | 2007-01-23 | The Procter & Gamble Company | Kit of water-soluble or water dispersible pouches |
| EP1721838A3 (en) * | 2001-05-08 | 2007-03-07 | The Procter and Gamble Company | Kit of water-soluble or water dispersible pouches |
| EP1404801B2 (en) † | 2001-07-11 | 2015-08-05 | Reckitt Benckiser N.V. | Dishwashing composition |
| AU2003239617B2 (en) * | 2002-05-24 | 2007-06-28 | The Procter & Gamble Company | Detergent system |
| WO2003099977A3 (en) * | 2002-05-24 | 2004-07-08 | Procter & Gamble | Detergent system |
| EP1364610A1 (en) * | 2002-05-24 | 2003-11-26 | The Procter & Gamble Company | Detergent system |
| WO2003099977A2 (en) * | 2002-05-24 | 2003-12-04 | The Procter & Gamble Company | Detergent system |
| WO2004005156A1 (en) * | 2002-07-03 | 2004-01-15 | Crown Packaging Technology, Inc. | Water-soluble container |
| US7083047B2 (en) | 2002-10-03 | 2006-08-01 | Unilever Home & Personal Care Usa Division Of Conopco, Inc. | Polymeric film for water soluble package |
| US9242768B2 (en) | 2003-10-31 | 2016-01-26 | Obrist Closures Switzerland Gmbh | Tamper evident closure |
| US8522991B2 (en) | 2003-10-31 | 2013-09-03 | Obrist Closures Switzerland Gmbh | Tamper evident closure |
| WO2006045450A1 (en) * | 2004-10-22 | 2006-05-04 | Henkel Kommanditgesellschaft Auf Aktien | Detergent or cleaning agent dosing unit |
| US7830263B2 (en) | 2006-04-28 | 2010-11-09 | Obrist Closures Switzerland Gmbh | Closure with RFID device |
| US7977298B2 (en) | 2006-06-30 | 2011-07-12 | The Sun Products Corporation | Laundry articles |
| WO2008000567A1 (en) * | 2006-06-30 | 2008-01-03 | Unilever Plc | Laundry articles |
| WO2008104023A1 (en) * | 2007-02-27 | 2008-09-04 | Amcor Limited | Packaging films |
| EP2011856A1 (en) * | 2007-07-02 | 2009-01-07 | The Procter and Gamble Company | Method of treating laundry |
| US9102448B2 (en) | 2007-07-13 | 2015-08-11 | Obrist Closures Switzerland Gmbh | Tamper-evident closure |
| US8453856B2 (en) | 2007-07-13 | 2013-06-04 | Obrist Closures Switzerland Gmbh | Tamper-evident closure |
| DE102007036769A1 (en) * | 2007-08-03 | 2009-02-05 | Erlen Gmbh | Assembly to clean bedpans/urine bottles has a cleaning chamber and a steam chamber, with a dosing unit to feed water softener and disinfectant into the water tank |
| US8490804B2 (en) | 2007-10-31 | 2013-07-23 | Obrist Closures Switzerland Gmbh | Closure with movable tamper-evident member |
| US8413830B2 (en) | 2008-04-04 | 2013-04-09 | Obrist Closures Switzerland Gmbh | Closure |
| USD630093S1 (en) | 2010-06-11 | 2011-01-04 | Obrist Closures Switzerland Gmbh | Closure |
| CN104661576A (en) * | 2012-08-21 | 2015-05-27 | 浦瑞玛柯Feg有限责任公司 | Warewash machine with descaling/deliming system and method |
| WO2014031308A3 (en) * | 2012-08-21 | 2014-08-28 | Premark Feg L.L.C. | Warewash machine with descaling/deliming system and method |
| CN104661576B (en) * | 2012-08-21 | 2017-03-22 | 浦瑞玛柯Feg有限责任公司 | Warewash machine with descaling/deliming system and method |
Also Published As
| Publication number | Publication date |
|---|---|
| DE9214065U1 (en) | 1993-06-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0593952A1 (en) | Product for releasing treatment agents into the wash liquid of an automatic washing or dishwashing machine | |
| EP0692020B1 (en) | Stable, bifunctional, phosphate-, metasilicate- and polymer-free low alkaline detergent tablets for dishwashing machines, and process for producing the same | |
| DE69726605T2 (en) | WASHING SYSTEM CONTAINING NON-IONIC SURFACTANT WITH ANOTHER CLEANING AND COATING EFFECT AND A WASHING PROCESS | |
| DE69229366T2 (en) | Phosphate-free dishwasher detergent | |
| DE69108927T2 (en) | METHOD FOR PRODUCING OXYGEN BLEACHING SYSTEMS CONTAINING PHOSPHATE-FREE DISHWASHER COMPOSITIONS. | |
| DE4112075C2 (en) | ||
| EP0743978B1 (en) | Bleaching tablet with builder substances | |
| EP0264701B1 (en) | Cleaning agent tablets for the automatic dishwashing | |
| DE69210669T2 (en) | Item containing detergent | |
| DE69408530T2 (en) | SOLID DETERGENT BRIQUETTES | |
| DE69724336T2 (en) | Water softening tablets | |
| DE60308935T2 (en) | Detergent tablet with two reactive coatings separated by a barrier layer | |
| EP1113070A2 (en) | Automatic dishwashing compositions comprising MGDA having low alkalinity | |
| DE3853612T3 (en) | Process for dispensing a granulated functional substance from a water-soluble container | |
| US4127496A (en) | Non-phosphate automatic dishwasher detergent | |
| EP0523095B1 (en) | Stable, bifunctional, phosphate-free detergent tablets for use in dishwashing machines | |
| EP0512371B1 (en) | Granular phosphate-free agents for automatic dishwashing | |
| DE2036673A1 (en) | Detergent preparation containing amorphous sodium silicate and methods for washing fabrics | |
| WO1996037596A1 (en) | Diswasher detergent with agents providing protection against the corrosion of silver | |
| EP0569365A1 (en) | Phosphate-free cleaning agent. | |
| DE102018222239A1 (en) | Cleaning and care products for automatic dishwashers | |
| EP0846155B1 (en) | Use of lipases in low-alkaline mechanical dishwashing agents | |
| US5232620A (en) | Sodium tripolyphosphate composition and method of producing it | |
| WO1996022353A1 (en) | Cleaning product containing an anti-corrosion agent for silver | |
| DE19547730A1 (en) | Weakly alkaline dish washing |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FR GB IT LI LU NL PT SE |
|
| 17P | Request for examination filed |
Effective date: 19940914 |
|
| 17Q | First examination report despatched |
Effective date: 19970225 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: CHEMOLUX S.A.R.L. |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: HENKEL KOMMANDITGESELLSCHAFT AUF AKTIEN |
|
| APAB | Appeal dossier modified |
Free format text: ORIGINAL CODE: EPIDOS NOAPE |
|
| APAB | Appeal dossier modified |
Free format text: ORIGINAL CODE: EPIDOS NOAPE |
|
| APAD | Appeal reference recorded |
Free format text: ORIGINAL CODE: EPIDOS REFNE |
|
| APBZ | Receipt of observations in appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNOBA4E |
|
| APBT | Appeal procedure closed |
Free format text: ORIGINAL CODE: EPIDOSNNOA9E |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN REFUSED |
|
| 18R | Application refused |
Effective date: 20040525 |
|
| APAF | Appeal reference modified |
Free format text: ORIGINAL CODE: EPIDOSCREFNE |