EP0482253A1 - Environmentally friendly fuel compositions and additives therefor - Google Patents
Environmentally friendly fuel compositions and additives therefor Download PDFInfo
- Publication number
- EP0482253A1 EP0482253A1 EP90311609A EP90311609A EP0482253A1 EP 0482253 A1 EP0482253 A1 EP 0482253A1 EP 90311609 A EP90311609 A EP 90311609A EP 90311609 A EP90311609 A EP 90311609A EP 0482253 A1 EP0482253 A1 EP 0482253A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fuel
- hydrocarbyl group
- carbon atoms
- soluble
- ppm
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 0 *C(CCCC1*)C1C1CCCCCCCC1 Chemical compound *C(CCCC1*)C1C1CCCCCCCC1 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/19—Esters ester radical containing compounds; ester ethers; carbonic acid esters
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/143—Organic compounds mixtures of organic macromolecular compounds with organic non-macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/221—Organic compounds containing nitrogen compounds of uncertain formula; reaction products where mixtures of compounds are obtained
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/222—Organic compounds containing nitrogen containing at least one carbon-to-nitrogen single bond
- C10L1/2222—(cyclo)aliphatic amines; polyamines (no macromolecular substituent 30C); quaternair ammonium compounds; carbamates
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/222—Organic compounds containing nitrogen containing at least one carbon-to-nitrogen single bond
- C10L1/2222—(cyclo)aliphatic amines; polyamines (no macromolecular substituent 30C); quaternair ammonium compounds; carbamates
- C10L1/2225—(cyclo)aliphatic amines; polyamines (no macromolecular substituent 30C); quaternair ammonium compounds; carbamates hydroxy containing
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/222—Organic compounds containing nitrogen containing at least one carbon-to-nitrogen single bond
- C10L1/224—Amides; Imides carboxylic acid amides, imides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/232—Organic compounds containing nitrogen containing nitrogen in a heterocyclic ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/238—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds
- C10L1/2383—Polyamines or polyimines, or derivatives thereof (poly)amines and imines; derivatives thereof (substituted by a macromolecular group containing 30C)
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/02—Use of additives to fuels or fires for particular purposes for reducing smoke development
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/182—Organic compounds containing oxygen containing hydroxy groups; Salts thereof
- C10L1/183—Organic compounds containing oxygen containing hydroxy groups; Salts thereof at least one hydroxy group bound to an aromatic carbon atom
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/182—Organic compounds containing oxygen containing hydroxy groups; Salts thereof
- C10L1/183—Organic compounds containing oxygen containing hydroxy groups; Salts thereof at least one hydroxy group bound to an aromatic carbon atom
- C10L1/1832—Organic compounds containing oxygen containing hydroxy groups; Salts thereof at least one hydroxy group bound to an aromatic carbon atom mono-hydroxy
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/182—Organic compounds containing oxygen containing hydroxy groups; Salts thereof
- C10L1/183—Organic compounds containing oxygen containing hydroxy groups; Salts thereof at least one hydroxy group bound to an aromatic carbon atom
- C10L1/1835—Organic compounds containing oxygen containing hydroxy groups; Salts thereof at least one hydroxy group bound to an aromatic carbon atom having at least two hydroxy substituted non condensed benzene rings
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/192—Macromolecular compounds
- C10L1/198—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds homo- or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon to carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid
- C10L1/1981—Condensation polymers of aldehydes or ketones
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/192—Macromolecular compounds
- C10L1/198—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds homo- or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon to carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid
- C10L1/1983—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds homo- or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon to carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid polyesters
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/236—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derivatives thereof
- C10L1/2364—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derivatives thereof homo- or copolymers derived from unsaturated compounds containing amide and/or imide groups
Definitions
- This invention relates to middle distillate fuel compositions such as diesel fuel, home heating oil, kerosene, jet fuel, aviation fuel, gas turbine engine fuels, light cycle oils, etc. having improved combustion characteristics.
- This invention involves the surprising discovery that it is possible to provide a middle distillate fuel composition which results on combustion in almost immediate substantial reductions of noxious emissions such as, unburned hydrocarbons, particulates, and/or carbon monoxide. Moreover, it has been found that such fuel compositions can provide substantial improvements in fuel economy in the operation, for example, of diesel engines.
- the fuel compositions preferably contain one or more sterically hindered phenolic compounds, most preferably a 2,6-dihydrocarbyl-a-dihydrocarbylamino-p- cresol such as 2,6-di-tert-butyl-a-dimethylamino-p-cresol which is available from Ethyl Corporation as ETHYL@ antioxidant 703.
- a 2,6-dihydrocarbyl-a-dihydrocarbylamino-p- cresol such as 2,6-di-tert-butyl-a-dimethylamino-p-cresol which is available from Ethyl Corporation as ETHYL@ antioxidant 703.
- this invention provides a fuel composition which comprises liquid middle distillate hydrocarbonaceous fuel containing at least one fuel-soluble ashless dispersant in an amount of at least 50 ppm sufficient to cause a prompt reduction in emissions released upon combustion of said fuel composition.
- the fuel contains 75 to 1,000 ppm and most preferably 100-250 ppm of the ashless dispersant.
- Another embodiment of this invention is a method of achieving an almost immediate reduction in the amount of noxious emissions formed on combustion of a hydrocarbonaceous fuel in the middle distillate boiling range which comprises blending in such fuel an ashless dispersant in an amount of at least 50 ppm sufficient to achieve such reduction in emissions.
- Ashless Dispersants are described in numerous patent specifications, mainly as additives for use in lubricant compositions, but their use in hydrocarbon fuels has also been described. Ashless dispersants leave little or no metal-containing residue on combustion. They generally contain only carbon, hydrogen, oxygen and in most cases nitrogen, but sometimes contain in addition other non-metallic elements such as phosphorus, sulphur or boron.
- the preferred ashless dispersant is an alkenyl succinimide of an amine having at least one primary amino group capable of forming an imide group.
- alkenyl succinimides may be formed by conventional methods such as by heating an alkenyl succinic anhydride, acid, acid-ester, acid halide, or lower alkyl ester with an amine containing at least one primary amino group.
- the alkenyl succinic anhydride may be made readily by heating a mixture of olefin and maleic anhydride to about 180°-220°C.
- the olefin is preferably a polymer or copolymer of a lower monoolefin such as ethylene, propylene, isobutene and the like.
- the more preferred source of alkenyl group is from polyisobutene having a molecular weight up to 10,000 or higher.
- the alkenyl group is a polyisobutenyl group having a molecular weight of about 500-5,000, and preferably about 700-2,000, especially 800-1,200.
- the isobutene used in making the polyisobutene is usually (but not necessarily) a mixture of isobutene and other C 4 isomers such as 1-butene.
- the acylating agent formed from maleic anhydride and "polyisobutene” made from such mixtures of isobutene and other C 4 isomers such as 1-butene can be termed a "polybutenyl succinic anhydride” and a succinimide made therewith can be termed a “polybutenyl succinimide”.
- polyisobutenyl and “polybutenyl” are used interchangably to denote the alkenyl moiety whether made from a highly pure isobutene or a more impure mixture of isobutene and other C 4 isomers such as 1-butene.
- Amines which may be employed in forming the ashless dispersant include any that have at least one primary amino group which can react to form an imide group.
- a few representative examples are: methylamine, 2-ethylhexylamine, n-dodecylamine, stearylamine, N,N-dimethylpropanediamine, N-(3-aminopropyl)morpholine, N-dodecylpropanediamine, N-aminopropyl-piperazine, ethanolamine, N-ethanolethylenediamine and the like.
- the preferred amines are the alkylene polyamines such as propylene diamine, dipropylene triamine, di-(1,2-butylene)triamine, and tetra-(1,2-propylene)pentamine.
- the most preferred amines are the ethylene polyamines which can be depicted by the formula wherein n is an integer from one to about ten. These include: ethylene diamine, diethylene triamine, triethylene tetramine, tetraethylene pentamine, pentaethylene hexamine, and the like, including mixtures thereof in which case n is the average value of the mixture. These ethylene polyamines have a primary amine group at each end so can form mono-alkenylsuccinimides and bis-alkenylsuccinimides.
- ethylene polyamine mixtures usually contain minor amounts of branched species and cyclic species such as N-aminoethyl piperazine, N,N'-bis(aminoethyl)piperazine, N,N'-bis- (piperazinyl)ethane, and like compounds.
- the preferred commercial mixtures have approximate overall compositions falling in the range corresponding to diethylene triamine to pentaethylene hexamine, mixtures generally corresponding in overall makeup to tetraethylene pentamine being most preferred.
- especially preferred ashless dispersants for use in the present invention are the products of reaction of a polyethylene polyamine, e.g. triethylene tetramine or tetraethylene pentamine, with a hydrocarbon-substituted carboxylic acid or anhydride made by reaction of a polyolefin, preferably polyisobutene, having a number average molecular weight of 500 to 5,000, preferably 700 to 2,000 and especially 800 to 1,200, with an unsaturated polycarboxylic acid or anhydride, e.g., maleic anhydride, maleic acid, fumaric acid, or the like, including mixtures of two or more such substances.
- a polyethylene polyamine e.g. triethylene tetramine or tetraethylene pentamine
- a hydrocarbon-substituted carboxylic acid or anhydride made by reaction of a polyolefin, preferably polyisobutene, having a number average molecular weight of 500 to 5,000,
- uccinimide is meant to encompass the completed reaction product from reaction between components (i) and (ii) and is intended to encompass compounds wherein the product may have amide, amidine, and/or salt linkages in addition to the imide linkage of the type that results from the reaction of a primary amino group and an anhydride moiety.
- alkenyl succinic acid esters and diesters of alcohols containing 1-20 carbon atoms and 1-6 hydroxyl groups Representative examples are described in U.S. Pat. Nos. 3,331,776; 3,381,022; and 3,522,179.
- the alkenyl succinic portion of these esters corresponds to the alkenyl succinic portion of the succinimides described above including the same preferred and most preferred subgenus, e.g., polyisobutenyl succinic acids wherein the polyisobutenyl group has a number average molecular weight of 500 to 5,000, preferably 700-2,000, especially 800 to 1,200.
- Alcohols useful in preparing the esters include methanol, ethanol, isobutanol, octadecanol, eicosanol, ethylene glycol, diethylene glycol, tetraethylene glycol, diethylene glycol monoethylether, propylene glycol, tripropylene glycol, glycerol, sorbitol, 1,1,1-trimethylol ethane, 1,1,1-trimethylol propane, 1,1,1-trimethylol butane, pentaerythritol, dipentaerythritol, and the like.
- the succinic esters are readily made by merely heating a mixture of alkenyl succinic acid, anhydrides or lower alkyl (e.g., C 1 -C 4 ) ester with the alcohol while distilling out water or lower alkanol. In the case of acid-esters less alcohol is used. In fact, acid-esters made from alkenyl succinic anhydrides do not evolve water. In another method the alkenyl succinic acid or anhydrides can be merely reacted with an appropriate alkylene oxide such as ethylene oxide, propylene oxide, and the like, including mixtures thereof.
- an appropriate alkylene oxide such as ethylene oxide, propylene oxide, and the like, including mixtures thereof.
- the ashless dispersant is an alkenyl succinic ester-amide mixture.
- alkenyl succinic ester-amide mixture may be made by heating the above-described alkenyl succinic acids, anhydrides or lower alkyl esters with an alcohol and an amine either sequentially or in a mixture.
- the alcohols and amines described above are also useful in this embodiment.
- amino alcohols can be used alone or with the alcohol and/or amine to form the ester-amide mixtures.
- the amino alcohol can contain 1-20 carbon atoms, 1-6 hydroxy groups and 1-4 amine nitrogen atoms. Examples are ethanolamine, diethanolamine, N-ethanol-diethylene triamine, and trimethylol aminomethane.
- ester-amide mixtures are described in U.S. Pat. Nos. 3,184,474; 3,576,743; 3,632,511; 3,804,763; 3,836,471; 3,862,981; 3,936,480; 3,948,800; 3,950,341; 3,957,854; 3,957,855; 3,991,098; 4,071,548; and 4,173,540.
- Such ashless dispersants containing alkenyl succinic residues may, and as is well known, be post- reacted with boron compounds, phosphorus derivatives and/or carboxylic acid acylating agents, e.g. maleic anhydride.
- ashless dispersants includes the Mannich condensates of hydrocarbyl- substituted phenols, formaldehyde or formaldehyde precursors (e.g. paraformaldehyde) and an amine having at least one primary amine group and containing 1-10 amine groups and 1-20 carbon atoms.
- Mannich condensates useful in this invention are described in U.S. Pat. Nos.
- Mannich condensates are those made by condensing a polyisobutenyl phenol wherein the polyisobutenyl group has an average molecular weight of about 800-3,000 with formaldehyde or a formaldehyde precursor and an ethylene polyamine having the formula: wherein n is an integer from one to ten or mixtures thereof especially those in which n has an average value of 3-5.
- a further type of ashless dispersants which can be used comprises interpolymers of oil-solubilising monomers such as decyl methacrylate, vinyl decyl ether and high molecular weight olefins with monomers containing polar substituents, e.g., aminoalkyl acrylates or acrylamides and poly(oxyethylene)-substituted acrylates.
- polar substituents e.g., aminoalkyl acrylates or acrylamides and poly(oxyethylene)-substituted acrylates.
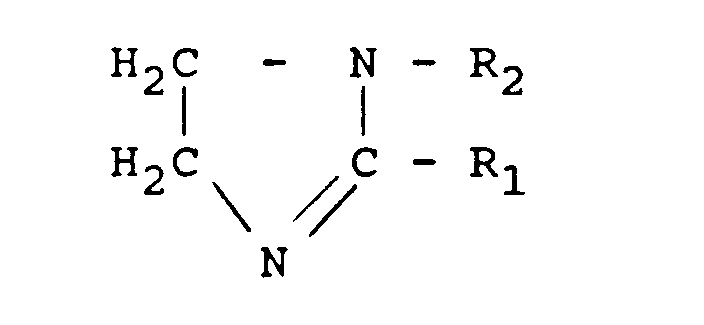
- Another class of ashless dispersants which can advantageously be used in the fuel compositions of this invention are the imidazoline dispersants which can be represented by the formula: wherein R 1 represents a hydrocarbon group having 1 to 30 carbon atoms, e.g. an alkyl or alkenyl group having 7 to 22 carbon atoms, and R 2 represents a hydrogen atoms or a hydrocarbon radical of 1 to 22 carbon atoms, or an aminoalkyl, acylaminoalkyl or hydroxyalkyl radical having 2 to 50 carbon atoms.
- R 1 represents a hydrocarbon group having 1 to 30 carbon atoms, e.g. an alkyl or alkenyl group having 7 to 22 carbon atoms
- R 2 represents a hydrogen atoms or a hydrocarbon radical of 1 to 22 carbon atoms, or an aminoalkyl, acylaminoalkyl or hydroxyalkyl radical having 2 to 50 carbon atoms.
- Such long-chain alkyl (or long-chain alkenyl) imidazoline compounds may be made by reaction of a corresponding long-chain fatty acid (of formula Ri-COOH), for example oleic acid, with an appropriate polyamine.
- the imidazoline formed is then ordinarily called, for example, oleylimidazoline where the radical R 1 represents the oleyl residue of oleic acid.
- Other suitable alkyl substituents in the 2-position of these imidazolines include undecyl, heptadecyl, lauryl and erucyl.
- Suitable N-substituents of the imidazolines i.e.
- radicals R 2 include hydrocarbyl groups, hydroxyalkyl groups, aminoalkyl groups, and acylaminoalkyl groups. Examples of the foregoing groups include methyl, butyl, decyl, cyclohexyl, phenyl, benzyl, tolyl, hydroxyethyl, aminoethyl, oleylaminoethyl and stearylaminoethyl.
- polyamines examples include N-oleyl-1,3-propanediamine, N-stearyl-1,3-propanediamine, N-oleyl-1,3-butanediamine, N-oleyl-2-methyl-1,3-propanediamine, N-oleyl-1,3-pentanediamine, N-oleyl-2-ethyl-1,3-propanediamine, N-stearyl-1,3-butanediamine, N-stearyl-2-methyl-1,3-propanediamine, N-stearyl-1,3-pentanediamine, N-stearyl-2-ethyl-1,3-propanediamine, N-oleyl-dipropylenetriamine and N-stearyl- dipropylenetriamine.
- Such linear N-alkylpolyamines are condensed with, e.g., a succinic, maleic, phthalic or hexahydrophthalic acid anhydride which may be substituted by one or more radicals of up to 5 carbon atoms each.
- Another class of ashless dispersant which can be incorporated in the compositions of the present invention are the products of reaction of an ethoxylated amine made by reaction of ammonia with ethylene oxide with a carboxylic acid of 8 to 30 carbon atoms.
- the ethoxylated amine may be, for example, mono-, di- or tri-ethanolamine or a polyethoxylated derivative thereof, and the carboxylic acid may be, for example, a straight or branched chain fatty acid of 10 to 22 carbon atoms, a naphthenic acid, a resinic acid or an alkyl aryl carboxylic acid.
- Still another type of ashless dispersants which can be used in the practise of this invention are the a-olefin-maleimide copolymers such as are described in U.S. Pat. No. 3,909,215.
- Such copolymers are alternating copolymers of N-substituted maleimides and aliphatic a-olefins of from 8 to 30 carbon atoms.
- the copolymers may have an average of 4 to 20 maleimide groups per molecule.
- the substituents on the nitrogen of the maleimide may be the same or different and are organic radicals composed essentially of carbon, hydrogen and nitrogen having a total of 3 to 60 carbon atoms.
- a commercially available material which is highly suitable for use in this invention is Chevron OFA 425B, and this material is believed to be or comprise an a-olefin maleimide copolymer of the type described in U.S. Pat. No. 3,909,215.
- ashless dispersants which contain little, if any, halogen atoms such as chlorine atoms.
- the additive composition contains no detectable amount of halogen.
- Typical halogen (chlorine)-free ashless dispersants suitable for use in the compositions of this invention include, in addition to various types described hereinabove, those described in the following recently- published applications: WO 9003359 and EP 365288.
- Hindered Phenolic Antioxidants As noted above it is desirable to include in the fuel compositions at least one fuel-soluble hindered phenolic antioxidant.
- One preferred type of compound is comprised of compounds of the formula wherein R 1 is a hydrocarbyl group containing 1 to 12 carbon atoms, R 2 is a secondary or tertiary hydrocarbyl group containing from 3 to 12 carbon atoms, R 3 is a hydrocarbyl group or a group of the formula and R 4 is a hydrocarbyl group.
- R 3 and R 4 when hydrocarbyl groups, can contain 50 or more carbon atoms but usually contain 18 or less carbon atoms. Methods applicable for the synthesis of such compounds are described for example in U.S. Patent No. 2,962,531.
- phenolic antioxidant for use in the practise of this invention is comprised of methylene bridged phenolic compounds including 2,2'-methylenebis(4,6-dihydrocarbylphenols), 4,4'-methylenebis(2,6-dihydrocarbylphenols), and various mixtures of methylene bridged alkylphenols such as are described in U.S. Patent No. 3,211,652.
- Typical 2,2'-methylenebis(4,6-dihydrocarbylphenols) include 2,2'-methylenebis(4-methyl-6-tertbutylphenol), 2,2'-methylenebis(4-ethyl-6-tert-butyl-phenol), 2,2'-methylenebis(4,6-di-tert-butylphenol), and the like.
- Exemplary 4,4'-methylenebis(2,6-dihydrocarbyl-phenols) include 4,4'-methylenebis(2,6-di-tert-butylphenol), 4,4'-methylenebis(2-methyl-6-tert-butylphenol), 4,4'-methylenebis(2,6-diisopropyl-phenol), 4,4'-methylenebis(2-tert-amyl-o-cresol), and similar compounds. Synthesis of such compounds are described for example in U.S. Patent No. 2,807,653.
- Commercially available methylene-bridged alkyl phenols include ETHYL@ antioxidant 728 and ETHYL@ antioxidant 702, both available from Ethyl Corporation.
- sterically hindered phenolic antioxidants which can be used include 2,6-di-tert-butylphenol, 4-methyl-2,6-di-tert-butylphenol, 2,4,6-tri-tert-butylphenol, 2-tert-butylphenol, 2,6-diisopropylphenol, 2-methyl-6-tert-butylphenol, 2,4-dimethyl-6-tert-butylphenol, 4-ethyl-2,6-di-tert-butylphenol, 2-methyl-6-styrylphenol, 2,6-di-styryl-4-nonylphenol, and their analogs and homologs. Mixtures of two or more such phenolic compounds are also suitable such as for example, ETHYL@ antioxidant 733 available from Ethyl Corporation.
- one or more additional components are included in the fuel compositions. These include demulsifying agents, metal deactivators, antifoam agents, corrosion inhibitors, lubricity additives, friction modifiers, and/or solvents.
- the demulsifying agent improves the water tolerance level of the fuel compositions by minimizing or preventing excessive emulsion formation.
- Exemplary demulsifiers which may be employed in the practise of this invention include poly-(alkylphenol) formaldehyde condensates and the polyalkylenoxy modified reaction products thereof. These compounds are prepared by reacting an alkylphenol with formaldehyde and thereafter reacting the reaction product of the above with a C 2 to C 6 alkylene oxide such as ethylene oxide and propylene oxide.
- the demulsifiers have a generalized structural formula wherein U is an alkylene of 2 to 6 carbons; y is an integer averaging between 4 and 10; x is an integer averaging between 4 and 10; and R 5 is an alkyl having from 4 to 15 carbon atoms.
- Preferred demulsifiers described by the above formula are polyethyleneoxy modified methylene bridged poly(alkylphenol) polymers having a polyethyleneoxy chain of 8 to 20 carbons and preferably from 10 to 16 carbons and at least about 75 number percent of the polyethyleneoxy chains being within the range specified.
- the methylene bridged poly(alkylphenol) portion of the polymer has from 4 to 10 and preferably from 5 to 8 repeating methylene bridged alkylphenol units with 4 to 15 and preferably 6 to 12 carbons in the alkyl group.
- the alkyl groups are a mixture of alkyls having between 4 and 12 carbon atoms.
- Illustrative alkylphenols include p-isobutylphenol, p-diisobutylphenol, p-hexylphenol, p-heptylphenol, p-octylphenol, p-tripropylenephenol, and p-dipropylenephenol, etc.
- demulsifier component is an ammonia-neutralised sulphonated alkylphenol.
- R 1 is a hydrocarbyl group having from 4 to 15 carbon atoms, preferably from 6 to 12.
- These compounds are prepared by sulphonating an alkylated phenol and thereafter neutralising the sulphonated product with ammonia.
- demulsifier is an oxyalkylated glycol.
- These compounds are prepared by reacting a polyhydroxy alcohol such as ethylene glycol, trimethylene glycol, etc., with ethylene or propylene oxide.
- ethylene glycol trimethylene glycol, etc.
- propylene oxide ethylene or propylene oxide.
- Many of the compounds are commercially available from BASF-Wyandotte Chemical Company under the PLURONIC trademark. They are polyethers terminated by hydroxy groups and produced by the block copolymerisation of ethylene oxide and propylene oxide.
- the ethylene oxide blocks act as the hydrophiles and the propylene oxide blocks as the hydrophobes. They are available in a wide range of molecular weights and with varying ratios of ethylene oxide to propylene oxide.
- a further type of commercially available demulsifiers comprises a mixture of alkylaryl sulphonates, polyoxyalkylene glycols and oxyalkylated alkylphenolic resins.
- Such products are supplied by Petrolite Corporation under the TOLAD trademark.
- One such propriety product, identified as TOLAD 286K, is understood to be a mixture of these components dissolved in a solvent composed of alkyl benzenes.
- a related product, TOLAD 286, is also suitable. In this case the product apparently contains the same kind of active ingredients dissolved in a solvent composed of heavy aromatic naphtha and isopropanol.
- other known demulsifiers can be used.
- Metal Deactivators Generally speaking, metal deactivators fall into two broad categories. One category comprises the passivators which are considered to react with the metal surface and thereby passivate the surface. The other category comprises the chelators, i.e., substances which have the capability of reacting or complexing with dissolved metal and/or metal ions.
- the passivator type is the thiadiazoles such as HITEC@ 314 additive (Ethyl Petroleum Additives, Ltd.; Ethyl Petroleum Additives, Inc.).
- Examples of the chelator type of metal deactivators include 8-hydroxyquinoline, ethylene diamine tetraacetic acid, ;8-diketones such as acetylacetone, ⁇ 3-ketoesters such as octyl acetoacetate, and the like.
- the preferred metal deactivators which are generally regarded as chelators, are Schiff bases, such as N,N'-disalicylidene-1,2-ethanediamine, N,N'-disalicylidene-1,2-propanediamine, N,N'-disalicylidene-1,2-cyclohexane-diamine, and N,N"-disalicylidene-N'-methyl-dipropylene-triamine.
- Schiff bases such as N,N'-disalicylidene-1,2-ethanediamine, N,N'-disalicylidene-1,2-propanediamine, N,N'-disalicylidene-1,2-cycl
- Suitable antifoam agents include silicones and organic polymers such as acrylate polymers. Various antifoam agents are described in Foam Control Agents by H. T. Kerner (Noyes Data Corporation, 1976, pages 125-176). The use of silicone oils such as are available as articles of commerce is generally preferred.
- Corrosion Inhibitors It is also preferred pursuant to this invention to employ in the fuel compositions a suitable quantity of a corrosion or rust inhibitor.
- a corrosion or rust inhibitor This may be a single compound or a mixture of compounds having the property of inhibiting corrosion of metallic surfaces. In some cases compounds are known which act not only as corrosion inhibitors but as metal deactivators in passivating the surface of the metal and thereby inhibiting corrosion.
- thiazoles for use in accordance with preferred embodiments of this invention are the thiazoles, triazoles and thiadiazoles.
- examples of such compounds include benzotriazole, tolyltriazole, octyltriazole, decyltriazole, dodecyltriazole, 2-mercapto benzothiazole, 2,5-dimercapto-1,3,4-thiadiazole, 2-mercapto-5-hydrocarbylthio-1,3,4-thiadiazoles, 2-mercapto-5-hydrocarbyldithio-1,3,4-thiadiazoles, 2,5-bis(hydrocarbylthio)-1,3,4-thiadiazoles, and 2,5-(bis)hydrocarbyldithio)-1,3,4-thiadiazoles.
- a number of these materials are available as articles of commerce.
- dimer and trimer acids such as are produced from tall oil fatty acids, oleic acid, linoleic acid, etc.
- alkenyl succinic acid and alkenyl succinic anhydride corrosion inhibitors such as tetrapropenylsuccinic acid, tetrapropenylsuccinic anhydride, dodecenylsuccinic acid, dodecenylsuccinic anhydride, hexadecenylsuccinic acid, and similar compounds.
- alkenyl succinic acids and anhydrides such as half esters of alkenyl succinic acids having 8 to 24 carbon atoms in the alkenyl group with alcohols such as diols and polyglycols and imides and amides of alkenyl succinic acids and anhydrides having 8 to 24 carbon atoms in the alkenyl group, for example the reaction product of dodecenyl succinic acid or anhydride with a polyethylene polyamine, further reacted with a fatty acid such as oleic acid.
- aminosuccinic acids or derivatives thereof represented by the formula: wherein each of R 1 , R 2 , R 5 , R 6 and R 7 is, independently, a hydrogen atom or a hydrocarbyl group containing 1 to 30 carbon atoms, and wherein each of R 3 and R 4 is, independently, a hydrogen atom, a hydrocarbyl group containing 1 to 30 carbon atoms, or an acyl group containing from 1 to 30 carbon atoms.
- the groups R 1 , R 2 , R 3 , R 4 , R 5 , R 6 and R 7 when in the form of hydrocarbyl groups, can be, for example, alkyl, cycloalkyl or aromatic containing groups.
- R 1 and R 5 are the same or different straight-chain or branched-chain hydrocarbon radicals containing 1-20 carbon atoms. Most preferably, R 1 and R 5 are saturated hydrocarbon radicals containing 3-6 carbon atoms.
- R 2 , either R 3 or R 4 , R 6 and R 7 , when in the form of hydrocarbyl groups, are preferably the same or different straight-chain or branched-chain saturated hydrocarbon radicals.
- a dialkyl ester of an aminosuccinic acid is used in which R 1 and R 5 are the same or different alkyl groups containing 3-6 carbon atoms, R 2 is a hydrogen atom, and either R 3 or R 4 is an alkyl group containing 15-20 carbon atoms or an acyl group which is derived from a saturated or unsaturated carboxylic acid containing 2-10 carbon atoms.
- aminosuccinic acid derivative is a dialkylester of an aminosuccinic acid of the above formula wherein R 1 and R 5 are isobutyl, R 2 is a hydrogen atom, R 3 is octadecyl and/or octadecenyl and R 4 is 3-carboxy-1-oxo-2-propenyl.
- R 6 and R 7 are most preferably hydrogen atoms.
- compositions of this invention may also contain lubricity agents such as sulfurized fats, sulfurized isobutylene, dialkyl polysulfides, and sulphur-bridged phenols such as nonylphenol polysulfide.
- lubricity agents such as sulfurized fats, sulfurized isobutylene, dialkyl polysulfides, and sulphur-bridged phenols such as nonylphenol polysulfide.
- fatty acids and their derivatives such as dimer or trimer acids such as are produced from tall oil fatty acids, oleic acid, linoleic acid, etc.
- Friction Modifiers Another type of additives which may be included in the compositions of this invention are friction modifiers such as aliphatic amines or ethoxylated aliphatic amines, aliphatic fatty acid amides, aliphatic carboxylic acids, aliphatic carboxylic esters, aliphatic carboxylic ester-amides, aliphatic phosphonates, aliphatic phosphates, aliphatic thiophosphonates, aliphatic thiophosphates, etc., wherein the aliphatic group usually contains above about eight carbon atoms so as to render the compound suitably soluble in hydrocarbons.
- aliphatic substituted succinimides formed by reacting one or more aliphatic succinic acids or anhydrides with ammonia such as are described in EP 20,037.
- Solvents Various types of solvents or carriers are available for use in formulating the compositions of this invention. These include hydrocarbons of suitable viscosities and boiling ranges such as high boiling aromatic naphtha, process oil, kerosene, and the like including oligomers and hydrogenated oligomers of alkenes such as hydrogenated decene-1 dimer or trimer. Also useful are alcohols and esters especially higher alcohols such as liquid alkanols having at least eight carbon atoms. An especialy useful solvent is isodecanol.
- Still other components may be utilised in their customary amounts in the compositions of this invention.
- aromatic amine antioxidants or stabilizers such as N,N'-di-sec-butyl-p-phenylenediamine, phenyl-a-naphthylamine, and 4-isopropylaminodiphenylamine
- cetane improvers such as organic nitrate esters or fuel soluble peroxides, hydroperoxides or peroxyesters
- metal-containing combustion improvers such as ferrocene and ferrocene derivatives, cyclopentadienyl manganese carbonyl compounds such as cyclopentadienyl manganese tricarbonyl and methylcyclopentadienyl manganese tricarbonyl, and metallic salts such as manganese oleate, iron naphthenate, copper naphthenate, cobalt naphthenate, nickel oleate, and manganese naphthenate;
- a critical feature of this invention is to employ a sufficiently high concentration of the fuel-soluble ashless dispersant to achieve prompt reduction in noxious emissions on combustion of the fuel.
- this invention achieves virtually immediate, dramatic reductions in noxious emissions because of the high concentration of ashless dispersant employed in the fuel.
- the minimum amount of ashless dispersant needed to achieve such a substantially instantaneous reduction in emissions will vary to some extent depending upon the character and makeup of the hydrocarbonaceous middle distillate fuel in which the dispersant is employed. However, generally speaking, the minimum amount will fall within the range of 50 to 60 ppm. Ordinarily the fuels will contain no more than about 5,000 ppm of the dispersant although even higher concentrations may be used in situtations where such higher concentrations can be justified or are needed.
- the other additive components of the compositions of this invention are employed in minor amounts sufficient to improve the performance characteristics and properties of the base fuel.
- the amounts will thus vary in accordance with such factors as the type of base fuel, the type of service for which the fuel composition is intended, and the performance characteristics desired in the finished fuel.
- concentrations (parts per million by weight) of the components (active ingredients) in the fuels are typical:
- the individual components employed can be separately blended into the fuel or can be blended therein in various subcombinations, if desired. Ordinarily, the particular sequence of such blending steps is not critical. Moreover, such components can be blended in the form of separate solutions in a diluent. It is preferable, however, to blend the components used in the form of an additive concentrate of this invention, as this simplifies the blending operations, reduces the likelihood of blending errors, and takes advantage of the compatibility and solubility characteristics afforded by the overall concentrate.
- the additive concentrates of this invention will contain the components used in amounts proportioned to yield finished fuels consistent with the concentrations tabulated above.
- the additive concentrate will contain one or more diluents such as light mineral oils and/or higher alcohols to facilitate handling and blending of the concentrate.
- concentrates containing up to 90% by weight of one or more diluents or solvents can be used.
- Hydrocarbonaceous Fuels In principle, the advantages of this invention may be achieved in any distilled or distillable liquid hydrocarbonaceous fuel derived from petroleum, coal, shale and/or tar sands. In most instances, at least under present circumstances, the base fuels will be derived primarily, if not exclusively, from petroleum.
- the invention is thus applicable to such fuels as kerosene, jet fuel, aviation fuel, diesel fuel, home heating oil, light cycle oil, heavy cycle oil, light gas oil, heavy gas oil, and in general, any liquid hydrocarbonaceous product suitable for combustion either in an engine (e.g., diesel fuel, gas turbine fuels, etc.) or in a burner apparatus (e.g., gas oils, home heating oils, etc.).
- fuels as kerosene, jet fuel, aviation fuel, diesel fuel, home heating oil, light cycle oil, heavy cycle oil, light gas oil, heavy gas oil, and in general, any liquid hydrocarbonaceous product suitable for combustion either in an engine (e.g., diesel fuel, gas turbine fuels, etc.) or in a burner apparatus (e.g., gas oils, home heating oils, etc.).
- suitable fuels may include liquid fuels derived from biomass, such as vegetable oils (e.g., rapeseed oil, jojoba oil, cottonseed oil, etc.); or refuse-derived liquid fuels such as fuels derived from municipal and/or industrial wastes; or waste oils and/or liquid waste biomass and its derivatives; or mixtures of any of the foregoing substances.
- vegetable oils e.g., rapeseed oil, jojoba oil, cottonseed oil, etc.
- refuse-derived liquid fuels such as fuels derived from municipal and/or industrial wastes; or waste oils and/or liquid waste biomass and its derivatives; or mixtures of any of the foregoing substances.
- An additive concentrate of this invention was formed by blending together the following components in the proportions specified:
- the additive concentrate was blended into commercially available diesel fuels at a concentration of 400 ppm.
- the fuels contained approximately 112.5 ppm of succinimide ashless dispersant (active ingredient) and 15 ppm of 2,6-di-tert-butyl-a-dimethylamino-p-cresol.
- These blended fuels were used in the operation of two different diesel engines.
- One was a Volvo TD121 F 12 litre turbo-charged intercooled truck engine.
- the other engine was a 4-cylinder Mercedes-Benz 200D passenger car engine with a displacement of 1.997 litres and a rated power of 53 kw.
- the base fuel used in the Volvo truck engine tests had a cetane number of 48.9 (per ASTM D 976-80), a density at 15°C of 0.853 (per ASTM D 1298), an API gravity of 34.2, a total sulphur content of 0.125 weight percent, a content of aromatics of 39% by volume (per ASTM D 1019-68) (Mod)), an initial boiling point of 176 °C and a final boiling point of 362 ° C (per ASTM D 86).
- the Volvo truck engine test was run utilising the ECE R49 13-mode cycle test firstly to give triplicate results on the clear (unadditised) diesel fuel (the commercially available diesel fuel described above) and then immediately in triplicate on the above diesel fuel of this invention. Thereafter, a further triplicate series of tests were run on the additised fuel, but for this triplicate series, the measurements were made only after 60 hours of running and the cycle used only for this 60 hours of running employed three typical modes rather than the full 13 modes employed in the previous two series. A summary of the results so obtained is set forth in Table 1.
- the Mercedes-Benz 200D passenger car tests were road tests using a vehicle which had already been driven in normal service for 135,500 km with service intervals of 10,000 km and without injector replacement.
- the fuel was switched to using the fuel of this invention and the emissions and fuel consumption change were measured both immediately and after an additional 1,000 km of driving.
- Four different standard driving cycle systems were evaluated; namely Federal Test Procedure 75 ("FTP 75"), Federal Test Procedure 72 (“FTP 72"), Highway Fuel Economy Test (“HW FET”), and ECE Regulation 15-04 (“ECE 15"). The results of these tests are summarized in Table 2.
- Tests were also conducted using a diesel fuel not of this invention containing a very similar additive formulation as described above, but at a lower concentration of ashless dispersant.
- the fuel was evaluated in a Peugeot 309 GLD diesel passenger car equipped with a 1,905 cc 4-cylinder, indirect injection diesel engine.
- the additive formulation (deemed suitable for the practise of this invention if utilised at a suitably high concentration) was as follows:
- This additive concentrate was blended into the fuel at a concentration of 200 ppm. Accordingly, the fuel contained approximately 26.2 ppm of the active ashless dispersant and 5.0 ppm of the 2,6-di-tert-butyl-a-dimethylamino-p-cresol.
- the test operation involved 967 km dynamometer distance accumulation using a mixed urban/highway driving cycle. This was followed by measurements of emissions according to the ECE 15-04 cycle. The results, expressed in terms of grams of emissions per test, of duplicate tests performed in this manner are as follows:
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Combustion & Propulsion (AREA)
- Liquid Carbonaceous Fuels (AREA)
- Lubricants (AREA)
Abstract
Compositions and methods for achieving almost immediate reductions in emissions produced during the operations of engines or burners on middle distillate hydrocarbonaceous fuels. This is effected by including in the fuel a sufficiently high concentration of at least one fuel-soluble ashless dispersant. The amount used is equivalent to at least 50 ppm, preferably 75 to 1,000 ppm, and most preferably 100 to 250 ppm of ashless dispersant (active ingredient). It is preferred to include in the fuel at least one fuel-soluble hindered phenolic antioxidant, such as 2,6-di-tert-butyl- alpha -dimethylamino-p-cresol.
Description
- This invention relates to middle distillate fuel compositions such as diesel fuel, home heating oil, kerosene, jet fuel, aviation fuel, gas turbine engine fuels, light cycle oils, etc. having improved combustion characteristics.
- It has long been known to use small quantities of various additives to improve the properties and/or performance characteristics of middle distillate fuels. For example, small quantities of dispersants have been used in order to improve engine or burner cleanliness and thereby obtain in time a reduction in the amount of noxious emissions formed on combustion of the fuel. Likewise, antioxidants, corrosion inhibitors, antifoam agents, demulsifiers and similar types of additives have been employed in such fuels.
- This invention involves the surprising discovery that it is possible to provide a middle distillate fuel composition which results on combustion in almost immediate substantial reductions of noxious emissions such as, unburned hydrocarbons, particulates, and/or carbon monoxide. Moreover, it has been found that such fuel compositions can provide substantial improvements in fuel economy in the operation, for example, of diesel engines.
- In the practise of this invention use is made of a higher concentration of fuel-soluble ashless dispersant than was customarily employed in fuels heretofore. In addition, the fuel compositions preferably contain one or more sterically hindered phenolic compounds, most preferably a 2,6-dihydrocarbyl-a-dihydrocarbylamino-p- cresol such as 2,6-di-tert-butyl-a-dimethylamino-p-cresol which is available from Ethyl Corporation as ETHYL@ antioxidant 703.
- Accordingly, in one of its embodiments this invention provides a fuel composition which comprises liquid middle distillate hydrocarbonaceous fuel containing at least one fuel-soluble ashless dispersant in an amount of at least 50 ppm sufficient to cause a prompt reduction in emissions released upon combustion of said fuel composition. Preferably the fuel contains 75 to 1,000 ppm and most preferably 100-250 ppm of the ashless dispersant.
- Another embodiment of this invention is a method of achieving an almost immediate reduction in the amount of noxious emissions formed on combustion of a hydrocarbonaceous fuel in the middle distillate boiling range which comprises blending in such fuel an ashless dispersant in an amount of at least 50 ppm sufficient to achieve such reduction in emissions.
- Other embodiments of this invention will be apparent from the ensuing description and appended claims.
- Ashless Dispersants. Ashless dispersants are described in numerous patent specifications, mainly as additives for use in lubricant compositions, but their use in hydrocarbon fuels has also been described. Ashless dispersants leave little or no metal-containing residue on combustion. They generally contain only carbon, hydrogen, oxygen and in most cases nitrogen, but sometimes contain in addition other non-metallic elements such as phosphorus, sulphur or boron.
- The preferred ashless dispersant is an alkenyl succinimide of an amine having at least one primary amino group capable of forming an imide group. Representative examples are given in U.S. Pat. Nos. 3,172,892; 3,202,678; 3,216,936; 3,219,666; 3,254,025; 3,272,746; and 4,234,435. The alkenyl succinimides may be formed by conventional methods such as by heating an alkenyl succinic anhydride, acid, acid-ester, acid halide, or lower alkyl ester with an amine containing at least one primary amino group. The alkenyl succinic anhydride may be made readily by heating a mixture of olefin and maleic anhydride to about 180°-220°C. The olefin is preferably a polymer or copolymer of a lower monoolefin such as ethylene, propylene, isobutene and the like. The more preferred source of alkenyl group is from polyisobutene having a molecular weight up to 10,000 or higher. In a still more preferred embodiment the alkenyl group is a polyisobutenyl group having a molecular weight of about 500-5,000, and preferably about 700-2,000, especially 800-1,200. The isobutene used in making the polyisobutene is usually (but not necessarily) a mixture of isobutene and other C4 isomers such as 1-butene. Thus, strictly speaking, the acylating agent formed from maleic anhydride and "polyisobutene" made from such mixtures of isobutene and other C4 isomers such as 1-butene, can be termed a "polybutenyl succinic anhydride" and a succinimide made therewith can be termed a "polybutenyl succinimide". However, it is common to refer to such substances as "polyisobutenyl succinic anhydride" and "polyisobutenyl succinimide", respectively. As used herein "polyisobutenyl" and "polybutenyl" are used interchangably to denote the alkenyl moiety whether made from a highly pure isobutene or a more impure mixture of isobutene and other C4 isomers such as 1-butene.
- Amines which may be employed in forming the ashless dispersant include any that have at least one primary amino group which can react to form an imide group. A few representative examples are: methylamine, 2-ethylhexylamine, n-dodecylamine, stearylamine, N,N-dimethylpropanediamine, N-(3-aminopropyl)morpholine, N-dodecylpropanediamine, N-aminopropyl-piperazine, ethanolamine, N-ethanolethylenediamine and the like.
- The preferred amines are the alkylene polyamines such as propylene diamine, dipropylene triamine, di-(1,2-butylene)triamine, and tetra-(1,2-propylene)pentamine.
- The most preferred amines are the ethylene polyamines which can be depicted by the formula
wherein n is an integer from one to about ten. These include: ethylene diamine, diethylene triamine, triethylene tetramine, tetraethylene pentamine, pentaethylene hexamine, and the like, including mixtures thereof in which case n is the average value of the mixture. These ethylene polyamines have a primary amine group at each end so can form mono-alkenylsuccinimides and bis-alkenylsuccinimides. Commercially available ethylene polyamine mixtures usually contain minor amounts of branched species and cyclic species such as N-aminoethyl piperazine, N,N'-bis(aminoethyl)piperazine, N,N'-bis- (piperazinyl)ethane, and like compounds. The preferred commercial mixtures have approximate overall compositions falling in the range corresponding to diethylene triamine to pentaethylene hexamine, mixtures generally corresponding in overall makeup to tetraethylene pentamine being most preferred. - Thus especially preferred ashless dispersants for use in the present invention are the products of reaction of a polyethylene polyamine, e.g. triethylene tetramine or tetraethylene pentamine, with a hydrocarbon-substituted carboxylic acid or anhydride made by reaction of a polyolefin, preferably polyisobutene, having a number average molecular weight of 500 to 5,000, preferably 700 to 2,000 and especially 800 to 1,200, with an unsaturated polycarboxylic acid or anhydride, e.g., maleic anhydride, maleic acid, fumaric acid, or the like, including mixtures of two or more such substances.
- As used herein the term "succinimide" is meant to encompass the completed reaction product from reaction between components (i) and (ii) and is intended to encompass compounds wherein the product may have amide, amidine, and/or salt linkages in addition to the imide linkage of the type that results from the reaction of a primary amino group and an anhydride moiety.
- Another class of useful ashless dispersants includes alkenyl succinic acid esters and diesters of alcohols containing 1-20 carbon atoms and 1-6 hydroxyl groups. Representative examples are described in U.S. Pat. Nos. 3,331,776; 3,381,022; and 3,522,179. The alkenyl succinic portion of these esters corresponds to the alkenyl succinic portion of the succinimides described above including the same preferred and most preferred subgenus, e.g., polyisobutenyl succinic acids wherein the polyisobutenyl group has a number average molecular weight of 500 to 5,000, preferably 700-2,000, especially 800 to 1,200.
- Alcohols useful in preparing the esters include methanol, ethanol, isobutanol, octadecanol, eicosanol, ethylene glycol, diethylene glycol, tetraethylene glycol, diethylene glycol monoethylether, propylene glycol, tripropylene glycol, glycerol, sorbitol, 1,1,1-trimethylol ethane, 1,1,1-trimethylol propane, 1,1,1-trimethylol butane, pentaerythritol, dipentaerythritol, and the like.
- The succinic esters are readily made by merely heating a mixture of alkenyl succinic acid, anhydrides or lower alkyl (e.g., C1-C4) ester with the alcohol while distilling out water or lower alkanol. In the case of acid-esters less alcohol is used. In fact, acid-esters made from alkenyl succinic anhydrides do not evolve water. In another method the alkenyl succinic acid or anhydrides can be merely reacted with an appropriate alkylene oxide such as ethylene oxide, propylene oxide, and the like, including mixtures thereof.
- In another embodiment the ashless dispersant is an alkenyl succinic ester-amide mixture. These may be made by heating the above-described alkenyl succinic acids, anhydrides or lower alkyl esters with an alcohol and an amine either sequentially or in a mixture. The alcohols and amines described above are also useful in this embodiment. Alternatively, amino alcohols can be used alone or with the alcohol and/or amine to form the ester-amide mixtures. The amino alcohol can contain 1-20 carbon atoms, 1-6 hydroxy groups and 1-4 amine nitrogen atoms. Examples are ethanolamine, diethanolamine, N-ethanol-diethylene triamine, and trimethylol aminomethane.
- Representative examples of suitable ester-amide mixtures are described in U.S. Pat. Nos. 3,184,474; 3,576,743; 3,632,511; 3,804,763; 3,836,471; 3,862,981; 3,936,480; 3,948,800; 3,950,341; 3,957,854; 3,957,855; 3,991,098; 4,071,548; and 4,173,540.
- Such ashless dispersants containing alkenyl succinic residues may, and as is well known, be post- reacted with boron compounds, phosphorus derivatives and/or carboxylic acid acylating agents, e.g. maleic anhydride.
- Another useful class of ashless dispersants includes the Mannich condensates of hydrocarbyl- substituted phenols, formaldehyde or formaldehyde precursors (e.g. paraformaldehyde) and an amine having at least one primary amine group and containing 1-10 amine groups and 1-20 carbon atoms. Mannich condensates useful in this invention are described in U.S. Pat. Nos. 3,442,808; 3,448,047; 3,539,633; 3,591,598; 3,600,372; 3,634,515; 3,697,574; 3,703,536; 3,704,308; 3,725,480; 3,726,882; 3,736,357; 3,751,365; 3,756,953; 3,793,202; 3,798,165; 3,798,247; 3,803,039; and 3,413,347.
- More preferred Mannich condensates are those made by condensing a polyisobutenyl phenol wherein the polyisobutenyl group has an average molecular weight of about 800-3,000 with formaldehyde or a formaldehyde precursor and an ethylene polyamine having the formula:
wherein n is an integer from one to ten or mixtures thereof especially those in which n has an average value of 3-5. - Typical post-treated ashless dispersants such as succinimides and Mannich condensates are described in U.S. Pat. Nos. 3,036,003; 3,087,936; 3,200,107; 3,216,936; 3,254,025; 3,256,185; 3,278,550; 3,280,234; 3,281,428; 3,282,955; 3,312,619; 3,366,569; 3,367,943; 3,373,111; 3,403,102; 3,442,808; 3,455,831; 3,455,832; 3,493,520; 3,502,677; 3,513,093; 3,533,945; 3,539,633; 3,573,010; 3,579,450; 3,591,598; 3,600,372; 3,639,242; 3,649,229; 3,649,659; 3,658,846; 3,697,574; 3,702,575; 3,703,536; 3,704,308; 3,708,422; and 4,857,214.
- A further type of ashless dispersants which can be used comprises interpolymers of oil-solubilising monomers such as decyl methacrylate, vinyl decyl ether and high molecular weight olefins with monomers containing polar substituents, e.g., aminoalkyl acrylates or acrylamides and poly(oxyethylene)-substituted acrylates. These may be characterised as "polymeric dispersants" and examples thereof are disclosed in the following U.S. Pat. Nos.: 3,329,658; 3,449,250; 3,519,565; 565; 3,666,730; 3,687,849; and 3,702,300.
- Another class of ashless dispersants which can advantageously be used in the fuel compositions of this invention are the imidazoline dispersants which can be represented by the formula:
wherein R1 represents a hydrocarbon group having 1 to 30 carbon atoms, e.g. an alkyl or alkenyl group having 7 to 22 carbon atoms, and R2 represents a hydrogen atoms or a hydrocarbon radical of 1 to 22 carbon atoms, or an aminoalkyl, acylaminoalkyl or hydroxyalkyl radical having 2 to 50 carbon atoms. Such long-chain alkyl (or long-chain alkenyl) imidazoline compounds may be made by reaction of a corresponding long-chain fatty acid (of formula Ri-COOH), for example oleic acid, with an appropriate polyamine. The imidazoline formed is then ordinarily called, for example, oleylimidazoline where the radical R1 represents the oleyl residue of oleic acid. Other suitable alkyl substituents in the 2-position of these imidazolines include undecyl, heptadecyl, lauryl and erucyl. Suitable N-substituents of the imidazolines (i.e. radicals R2) include hydrocarbyl groups, hydroxyalkyl groups, aminoalkyl groups, and acylaminoalkyl groups. Examples of the foregoing groups include methyl, butyl, decyl, cyclohexyl, phenyl, benzyl, tolyl, hydroxyethyl, aminoethyl, oleylaminoethyl and stearylaminoethyl. - Other suitable ashless dispersants which may be incorporated in the fuel compositions of this invention include the products of condensation of a cyclic anhydride with a straight-chain N-alkylpolyamine of the formula:
where n is an integer at least equal to 1, usually 3 to 5, R is a saturated or unsaturated linear hydrocarbon radical of 10 to 22 carbon atoms and R' is a divalent alkylene or alkylidene radical of 1 to 6 carbon atoms. Examples of such polyamines include N-oleyl-1,3-propanediamine, N-stearyl-1,3-propanediamine, N-oleyl-1,3-butanediamine, N-oleyl-2-methyl-1,3-propanediamine, N-oleyl-1,3-pentanediamine, N-oleyl-2-ethyl-1,3-propanediamine, N-stearyl-1,3-butanediamine, N-stearyl-2-methyl-1,3-propanediamine, N-stearyl-1,3-pentanediamine, N-stearyl-2-ethyl-1,3-propanediamine, N-oleyl-dipropylenetriamine and N-stearyl- dipropylenetriamine. Such linear N-alkylpolyamines are condensed with, e.g., a succinic, maleic, phthalic or hexahydrophthalic acid anhydride which may be substituted by one or more radicals of up to 5 carbon atoms each. - Another class of ashless dispersant which can be incorporated in the compositions of the present invention are the products of reaction of an ethoxylated amine made by reaction of ammonia with ethylene oxide with a carboxylic acid of 8 to 30 carbon atoms. The ethoxylated amine may be, for example, mono-, di- or tri-ethanolamine or a polyethoxylated derivative thereof, and the carboxylic acid may be, for example, a straight or branched chain fatty acid of 10 to 22 carbon atoms, a naphthenic acid, a resinic acid or an alkyl aryl carboxylic acid.
- Still another type of ashless dispersants which can be used in the practise of this invention are the a-olefin-maleimide copolymers such as are described in U.S. Pat. No. 3,909,215. Such copolymers are alternating copolymers of N-substituted maleimides and aliphatic a-olefins of from 8 to 30 carbon atoms. The copolymers may have an average of 4 to 20 maleimide groups per molecule. The substituents on the nitrogen of the maleimide may be the same or different and are organic radicals composed essentially of carbon, hydrogen and nitrogen having a total of 3 to 60 carbon atoms. A commercially available material which is highly suitable for use in this invention is Chevron OFA 425B, and this material is believed to be or comprise an a-olefin maleimide copolymer of the type described in U.S. Pat. No. 3,909,215.
- All the aforesaid types of ashless dispersants are described in the literature and many are available commercially. Mixtures of various types of ashless dispersants can, of course, be used.
- Because of environmental concerns it is desirable to employ ashless dispersants which contain little, if any, halogen atoms such as chlorine atoms. Thus, in order to satisfy such concerns, it is desirable (although not necessary from a performance standpoint) to select ashless dispersants (as well as the other components used in the compositions of this invention) such that the total halogen content of the overall fuel composition does not exceed 10 ppm. Indeed, the lower the better. Most desirably, the additive composition contains no detectable amount of halogen.
- Typical halogen (chlorine)-free ashless dispersants suitable for use in the compositions of this invention include, in addition to various types described hereinabove, those described in the following recently- published applications: WO 9003359 and EP 365288.
- Hindered Phenolic Antioxidants. As noted above it is desirable to include in the fuel compositions at least one fuel-soluble hindered phenolic antioxidant. One preferred type of compound is comprised of compounds of the formula
wherein R1 is a hydrocarbyl group containing 1 to 12 carbon atoms, R2 is a secondary or tertiary hydrocarbyl group containing from 3 to 12 carbon atoms, R3 is a hydrocarbyl group or a group of the formula - Another preferred type of phenolic antioxidant for use in the practise of this invention is comprised of methylene bridged phenolic compounds including 2,2'-methylenebis(4,6-dihydrocarbylphenols), 4,4'-methylenebis(2,6-dihydrocarbylphenols), and various mixtures of methylene bridged alkylphenols such as are described in U.S. Patent No. 3,211,652. Typical 2,2'-methylenebis(4,6-dihydrocarbylphenols) include 2,2'-methylenebis(4-methyl-6-tertbutylphenol), 2,2'-methylenebis(4-ethyl-6-tert-butyl-phenol), 2,2'-methylenebis(4,6-di-tert-butylphenol), and the like. Exemplary 4,4'-methylenebis(2,6-dihydrocarbyl-phenols) include 4,4'-methylenebis(2,6-di-tert-butylphenol), 4,4'-methylenebis(2-methyl-6-tert-butylphenol), 4,4'-methylenebis(2,6-diisopropyl-phenol), 4,4'-methylenebis(2-tert-amyl-o-cresol), and similar compounds. Synthesis of such compounds are described for example in U.S. Patent No. 2,807,653. Commercially available methylene-bridged alkyl phenols include ETHYL@ antioxidant 728 and ETHYL@ antioxidant 702, both available from Ethyl Corporation.
- Other sterically hindered phenolic antioxidants which can be used include 2,6-di-tert-butylphenol, 4-methyl-2,6-di-tert-butylphenol, 2,4,6-tri-tert-butylphenol, 2-tert-butylphenol, 2,6-diisopropylphenol, 2-methyl-6-tert-butylphenol, 2,4-dimethyl-6-tert-butylphenol, 4-ethyl-2,6-di-tert-butylphenol, 2-methyl-6-styrylphenol, 2,6-di-styryl-4-nonylphenol, and their analogs and homologs. Mixtures of two or more such phenolic compounds are also suitable such as for example, ETHYL@ antioxidant 733 available from Ethyl Corporation.
- In preferred embodiments of this invention one or more additional components are included in the fuel compositions. These include demulsifying agents, metal deactivators, antifoam agents, corrosion inhibitors, lubricity additives, friction modifiers, and/or solvents.
- Demulsifying Agents. The demulsifying agent improves the water tolerance level of the fuel compositions by minimizing or preventing excessive emulsion formation.
- Exemplary demulsifiers which may be employed in the practise of this invention include poly-(alkylphenol) formaldehyde condensates and the polyalkylenoxy modified reaction products thereof. These compounds are prepared by reacting an alkylphenol with formaldehyde and thereafter reacting the reaction product of the above with a C2 to C6 alkylene oxide such as ethylene oxide and propylene oxide. The demulsifiers have a generalized structural formula
wherein U is an alkylene of 2 to 6 carbons; y is an integer averaging between 4 and 10; x is an integer averaging between 4 and 10; and R5 is an alkyl having from 4 to 15 carbon atoms. - Preferred demulsifiers described by the above formula are polyethyleneoxy modified methylene bridged poly(alkylphenol) polymers having a polyethyleneoxy chain of 8 to 20 carbons and preferably from 10 to 16 carbons and at least about 75 number percent of the polyethyleneoxy chains being within the range specified. The methylene bridged poly(alkylphenol) portion of the polymer has from 4 to 10 and preferably from 5 to 8 repeating methylene bridged alkylphenol units with 4 to 15 and preferably 6 to 12 carbons in the alkyl group. In preferred embodiments, the alkyl groups are a mixture of alkyls having between 4 and 12 carbon atoms.
- Illustrative alkylphenols include p-isobutylphenol, p-diisobutylphenol, p-hexylphenol, p-heptylphenol, p-octylphenol, p-tripropylenephenol, and p-dipropylenephenol, etc.
-
- These compounds are prepared by sulphonating an alkylated phenol and thereafter neutralising the sulphonated product with ammonia.
- Another type of demulsifier is an oxyalkylated glycol. These compounds are prepared by reacting a polyhydroxy alcohol such as ethylene glycol, trimethylene glycol, etc., with ethylene or propylene oxide. Many of the compounds are commercially available from BASF-Wyandotte Chemical Company under the PLURONIC trademark. They are polyethers terminated by hydroxy groups and produced by the block copolymerisation of ethylene oxide and propylene oxide. The ethylene oxide blocks act as the hydrophiles and the propylene oxide blocks as the hydrophobes. They are available in a wide range of molecular weights and with varying ratios of ethylene oxide to propylene oxide.
- A further type of commercially available demulsifiers comprises a mixture of alkylaryl sulphonates, polyoxyalkylene glycols and oxyalkylated alkylphenolic resins. Such products are supplied by Petrolite Corporation under the TOLAD trademark. One such propriety product, identified as TOLAD 286K, is understood to be a mixture of these components dissolved in a solvent composed of alkyl benzenes. A related product, TOLAD 286, is also suitable. In this case the product apparently contains the same kind of active ingredients dissolved in a solvent composed of heavy aromatic naphtha and isopropanol. However, other known demulsifiers can be used.
- Metal Deactivators. Generally speaking, metal deactivators fall into two broad categories. One category comprises the passivators which are considered to react with the metal surface and thereby passivate the surface. The other category comprises the chelators, i.e., substances which have the capability of reacting or complexing with dissolved metal and/or metal ions. An example of the passivator type is the thiadiazoles such as HITEC@ 314 additive (Ethyl Petroleum Additives, Ltd.; Ethyl Petroleum Additives, Inc.). Examples of the chelator type of metal deactivators include 8-hydroxyquinoline, ethylene diamine tetraacetic acid, ;8-diketones such as acetylacetone, {3-ketoesters such as octyl acetoacetate, and the like. The preferred metal deactivators which are generally regarded as chelators, are Schiff bases, such as N,N'-disalicylidene-1,2-ethanediamine, N,N'-disalicylidene-1,2-propanediamine, N,N'-disalicylidene-1,2-cyclohexane-diamine, and N,N"-disalicylidene-N'-methyl-dipropylene-triamine. Thus a wide variety of known metal deactivators are available for use in the compositions of this invention. Mixtures of metal deactivators can be used.
- Antifoam Agents. Suitable antifoam agents include silicones and organic polymers such as acrylate polymers. Various antifoam agents are described in Foam Control Agents by H. T. Kerner (Noyes Data Corporation, 1976, pages 125-176). The use of silicone oils such as are available as articles of commerce is generally preferred.
- Corrosion Inhibitors. It is also preferred pursuant to this invention to employ in the fuel compositions a suitable quantity of a corrosion or rust inhibitor. This may be a single compound or a mixture of compounds having the property of inhibiting corrosion of metallic surfaces. In some cases compounds are known which act not only as corrosion inhibitors but as metal deactivators in passivating the surface of the metal and thereby inhibiting corrosion.
- Among suitable corrosion inhibitors for use in accordance with preferred embodiments of this invention are the thiazoles, triazoles and thiadiazoles. Examples of such compounds include benzotriazole, tolyltriazole, octyltriazole, decyltriazole, dodecyltriazole, 2-mercapto benzothiazole, 2,5-dimercapto-1,3,4-thiadiazole, 2-mercapto-5-hydrocarbylthio-1,3,4-thiadiazoles, 2-mercapto-5-hydrocarbyldithio-1,3,4-thiadiazoles, 2,5-bis(hydrocarbylthio)-1,3,4-thiadiazoles, and 2,5-(bis)hydrocarbyldithio)-1,3,4-thiadiazoles. A number of these materials are available as articles of commerce.
- Other types of useful corrosion inhibitors include dimer and trimer acids such as are produced from tall oil fatty acids, oleic acid, linoleic acid, etc.; alkenyl succinic acid and alkenyl succinic anhydride corrosion inhibitors such as tetrapropenylsuccinic acid, tetrapropenylsuccinic anhydride, dodecenylsuccinic acid, dodecenylsuccinic anhydride, hexadecenylsuccinic acid, and similar compounds. Also useful are derivatives of alkenyl succinic acids and anhydrides such as half esters of alkenyl succinic acids having 8 to 24 carbon atoms in the alkenyl group with alcohols such as diols and polyglycols and imides and amides of alkenyl succinic acids and anhydrides having 8 to 24 carbon atoms in the alkenyl group, for example the reaction product of dodecenyl succinic acid or anhydride with a polyethylene polyamine, further reacted with a fatty acid such as oleic acid. Also useful are aminosuccinic acids or derivatives thereof represented by the formula:
wherein each of R1, R2, R5, R6 and R7 is, independently, a hydrogen atom or a hydrocarbyl group containing 1 to 30 carbon atoms, and wherein each of R3 and R4 is, independently, a hydrogen atom, a hydrocarbyl group containing 1 to 30 carbon atoms, or an acyl group containing from 1 to 30 carbon atoms. The groups R1, R2, R3, R4, R5, R6 and R7, when in the form of hydrocarbyl groups, can be, for example, alkyl, cycloalkyl or aromatic containing groups. Preferably R1 and R5 are the same or different straight-chain or branched-chain hydrocarbon radicals containing 1-20 carbon atoms. Most preferably, R1 and R5 are saturated hydrocarbon radicals containing 3-6 carbon atoms. R2, either R3 or R4, R6 and R7, when in the form of hydrocarbyl groups, are preferably the same or different straight-chain or branched-chain saturated hydrocarbon radicals. Preferably a dialkyl ester of an aminosuccinic acid is used in which R1 and R5 are the same or different alkyl groups containing 3-6 carbon atoms, R2 is a hydrogen atom, and either R3 or R4 is an alkyl group containing 15-20 carbon atoms or an acyl group which is derived from a saturated or unsaturated carboxylic acid containing 2-10 carbon atoms. - Most preferred of the aminosuccinic acid derivative is a dialkylester of an aminosuccinic acid of the above formula wherein R1 and R5 are isobutyl, R2 is a hydrogen atom, R3 is octadecyl and/or octadecenyl and R4 is 3-carboxy-1-oxo-2-propenyl. In such ester R6 and R7 are most preferably hydrogen atoms.
- Lubricity Agents. The compositions of this invention may also contain lubricity agents such as sulfurized fats, sulfurized isobutylene, dialkyl polysulfides, and sulphur-bridged phenols such as nonylphenol polysulfide. Also useful as lubricity agents are fatty acids and their derivatives such as dimer or trimer acids such as are produced from tall oil fatty acids, oleic acid, linoleic acid, etc.
- Friction Modifiers. Another type of additives which may be included in the compositions of this invention are friction modifiers such as aliphatic amines or ethoxylated aliphatic amines, aliphatic fatty acid amides, aliphatic carboxylic acids, aliphatic carboxylic esters, aliphatic carboxylic ester-amides, aliphatic phosphonates, aliphatic phosphates, aliphatic thiophosphonates, aliphatic thiophosphates, etc., wherein the aliphatic group usually contains above about eight carbon atoms so as to render the compound suitably soluble in hydrocarbons. Also suitable are aliphatic substituted succinimides formed by reacting one or more aliphatic succinic acids or anhydrides with ammonia such as are described in EP 20,037.
- Solvents. Various types of solvents or carriers are available for use in formulating the compositions of this invention. These include hydrocarbons of suitable viscosities and boiling ranges such as high boiling aromatic naphtha, process oil, kerosene, and the like including oligomers and hydrogenated oligomers of alkenes such as hydrogenated decene-1 dimer or trimer. Also useful are alcohols and esters especially higher alcohols such as liquid alkanols having at least eight carbon atoms. An especialy useful solvent is isodecanol.
- Still other components may be utilised in their customary amounts in the compositions of this invention. These include aromatic amine antioxidants or stabilizers such as N,N'-di-sec-butyl-p-phenylenediamine, phenyl-a-naphthylamine, and 4-isopropylaminodiphenylamine; cetane improvers such as organic nitrate esters or fuel soluble peroxides, hydroperoxides or peroxyesters; metal-containing combustion improvers such as ferrocene and ferrocene derivatives, cyclopentadienyl manganese carbonyl compounds such as cyclopentadienyl manganese tricarbonyl and methylcyclopentadienyl manganese tricarbonyl, and metallic salts such as manganese oleate, iron naphthenate, copper naphthenate, cobalt naphthenate, nickel oleate, and manganese naphthenate; dyes; and the like.
- Concentrations and Proportions. As noted above, a critical feature of this invention is to employ a sufficiently high concentration of the fuel-soluble ashless dispersant to achieve prompt reduction in noxious emissions on combustion of the fuel. In other words, rather than relying upon a gradual clean up of engine or burner parts with concomitant gradual reduction in emissions, this invention achieves virtually immediate, dramatic reductions in noxious emissions because of the high concentration of ashless dispersant employed in the fuel. The minimum amount of ashless dispersant needed to achieve such a substantially instantaneous reduction in emissions will vary to some extent depending upon the character and makeup of the hydrocarbonaceous middle distillate fuel in which the dispersant is employed. However, generally speaking, the minimum amount will fall within the range of 50 to 60 ppm. Ordinarily the fuels will contain no more than about 5,000 ppm of the dispersant although even higher concentrations may be used in situtations where such higher concentrations can be justified or are needed.
- In general, the other additive components of the compositions of this invention are employed in minor amounts sufficient to improve the performance characteristics and properties of the base fuel. The amounts will thus vary in accordance with such factors as the type of base fuel, the type of service for which the fuel composition is intended, and the performance characteristics desired in the finished fuel. However, generally speaking, the following concentrations (parts per million by weight) of the components (active ingredients) in the fuels are typical:
- It will be appreciated that the individual components employed can be separately blended into the fuel or can be blended therein in various subcombinations, if desired. Ordinarily, the particular sequence of such blending steps is not critical. Moreover, such components can be blended in the form of separate solutions in a diluent. It is preferable, however, to blend the components used in the form of an additive concentrate of this invention, as this simplifies the blending operations, reduces the likelihood of blending errors, and takes advantage of the compatibility and solubility characteristics afforded by the overall concentrate.
- The additive concentrates of this invention will contain the components used in amounts proportioned to yield finished fuels consistent with the concentrations tabulated above. In most cases, the additive concentrate will contain one or more diluents such as light mineral oils and/or higher alcohols to facilitate handling and blending of the concentrate. Thus concentrates containing up to 90% by weight of one or more diluents or solvents can be used.
- Hydrocarbonaceous Fuels. In principle, the advantages of this invention may be achieved in any distilled or distillable liquid hydrocarbonaceous fuel derived from petroleum, coal, shale and/or tar sands. In most instances, at least under present circumstances, the base fuels will be derived primarily, if not exclusively, from petroleum.
- The invention is thus applicable to such fuels as kerosene, jet fuel, aviation fuel, diesel fuel, home heating oil, light cycle oil, heavy cycle oil, light gas oil, heavy gas oil, and in general, any liquid hydrocarbonaceous product suitable for combustion either in an engine (e.g., diesel fuel, gas turbine fuels, etc.) or in a burner apparatus (e.g., gas oils, home heating oils, etc.). Other suitable fuels may include liquid fuels derived from biomass, such as vegetable oils (e.g., rapeseed oil, jojoba oil, cottonseed oil, etc.); or refuse-derived liquid fuels such as fuels derived from municipal and/or industrial wastes; or waste oils and/or liquid waste biomass and its derivatives; or mixtures of any of the foregoing substances.
- In many cases, specifications exist for various hydrocarbonaceous fuels or grades thereof, and in any event the nature and character of such fuels are wellknown and reported in the literature. Generally speaking, so-called middle distillate fuels boil within the range of about 140 ° C to about 400 C.
- The practise and advantages of this invention will be still further apparent from the following illustrative example wherein all percentages are by weight.
-
- The additive concentrate was blended into commercially available diesel fuels at a concentration of 400 ppm. Thus, the fuels contained approximately 112.5 ppm of succinimide ashless dispersant (active ingredient) and 15 ppm of 2,6-di-tert-butyl-a-dimethylamino-p-cresol. These blended fuels were used in the operation of two different diesel engines. One was a Volvo TD121 F 12 litre turbo-charged intercooled truck engine. The other engine was a 4-cylinder Mercedes-Benz 200D passenger car engine with a displacement of 1.997 litres and a rated power of 53 kw. The base fuel used in the Volvo truck engine tests had a cetane number of 48.9 (per ASTM D 976-80), a density at 15°C of 0.853 (per ASTM D 1298), an API gravity of 34.2, a total sulphur content of 0.125 weight percent, a content of aromatics of 39% by volume (per ASTM D 1019-68) (Mod)), an initial boiling point of 176 °C and a final boiling point of 362 ° C (per ASTM D 86).
- The Volvo truck engine test was run utilising the ECE R49 13-mode cycle test firstly to give triplicate results on the clear (unadditised) diesel fuel (the commercially available diesel fuel described above) and then immediately in triplicate on the above diesel fuel of this invention. Thereafter, a further triplicate series of tests were run on the additised fuel, but for this triplicate series, the measurements were made only after 60 hours of running and the cycle used only for this 60 hours of running employed three typical modes rather than the full 13 modes employed in the previous two series. A summary of the results so obtained is set forth in Table 1.
- It can be seen from Table 1 that the fuel of this invention gave an almost immediate dramatic reduction in emissions of hydrocarbons, CO, and particulates. In addition, the fuel of this invention gave an almost immediate improvement in fuel economy. Moreover, these improvements continued and in some cases became even greater after 60 hours of test.
- The Mercedes-Benz 200D passenger car tests were road tests using a vehicle which had already been driven in normal service for 135,500 km with service intervals of 10,000 km and without injector replacement. The fuel was switched to using the fuel of this invention and the emissions and fuel consumption change were measured both immediately and after an additional 1,000 km of driving. Four different standard driving cycle systems were evaluated; namely Federal Test Procedure 75 ("FTP 75"), Federal Test Procedure 72 ("FTP 72"), Highway Fuel Economy Test ("HW FET"), and ECE Regulation 15-04 ("ECE 15"). The results of these tests are summarized in Table 2.
- Tests were also conducted using a diesel fuel not of this invention containing a very similar additive formulation as described above, but at a lower concentration of ashless dispersant. In this test the fuel was evaluated in a Peugeot 309 GLD diesel passenger car equipped with a 1,905 cc 4-cylinder, indirect injection diesel engine. The additive formulation (deemed suitable for the practise of this invention if utilised at a suitably high concentration) was as follows:
- This additive concentrate was blended into the fuel at a concentration of 200 ppm. Accordingly, the fuel contained approximately 26.2 ppm of the active ashless dispersant and 5.0 ppm of the 2,6-di-tert-butyl-a-dimethylamino-p-cresol.
- The test operation involved 967 km dynamometer distance accumulation using a mixed urban/highway driving cycle. This was followed by measurements of emissions according to the ECE 15-04 cycle. The results, expressed in terms of grams of emissions per test, of duplicate tests performed in this manner are as follows:
-
- It can be seen that in each instance the treated fuel not of this invention resulted in an increase in noxious emissions.
Claims (12)
1. A fuel composition which comprises a liquid middle distillate hydrocarbonaceous fuel containing at least one fuel-soluble ashless dispersant in an amount of at least 50 ppm sufficient to cause a prompt reduction in emissions released upon combustion of said fuel composition.
2. A fuel composition as claimed in claim 1 wherein the amount of ashless dispersant in said fuel composition is in the range of 75 to 1,000 ppm.
3. A fuel composition as claimed in claim 1 wherein the amount of ashless dispersant in said fuel composition is in the range of 100 to 250 ppm.
4. A fuel composition as claimed in any of claims 1-3 additionally containing at least one fuel-soluble hindered phenolic antioxidant.
5. A fuel composition as claimed in any of claims 1-3 additionally containing at least one fuel-soluble hindered phenolic compound of the formula
wherein R1 is a hydrocarbyl group containing 1 to 12 carbon atoms, R2 is a secondary or tertiary hydrocarbyl group containing from 3 to 12 carbon atoms, R3 is a hydrocarbyl group or a group of the formula
and R4 is a hydrocarbyl group.
wherein R1 is a hydrocarbyl group containing 1 to 12 carbon atoms, R2 is a secondary or tertiary hydrocarbyl group containing from 3 to 12 carbon atoms, R3 is a hydrocarbyl group or a group of the formula
and R4 is a hydrocarbyl group.
6. A fuel composition as claimed in any of claims 1-3 additionally containing 2,6-di-tert-butyl-a-dimethylamino-p-cresol.
7. A fuel composition as claimed in any of claims 1-6 additionally containing at least one or more of the following components:
a) at least one fuel-soluble metal deactivator of the chelation type;
b) at least one fuel-soluble demulsifying agent;
c) at least one fuel-soluble antifoam agent;
d) at least one lubricity additive;
e) as a solvent or carrier fluid, at least one liquid alcohol and/or at least one liquid aromatic hydrocarbon.
8. A fuel composition as claimed in any of claims 1-7 wherein the ashless dispersant is a succinimide formed by reacting at least one polybutenyl succinic acylating agent with a mixture of cyclic and acyclic polyethylene polyamines having an approximate overall composition falling in the range of diethylene triamine to pentaethylene hexamine.
9. A method of achieving an almost immediate reduction in the amount of noxious emissions formed on combustion of a hydrocarbonaceous fuel in the middle distillate boiling range which comprises blending in said fuel at least one ashless dispersant in an amount of at least 50 ppm sufficient to achieve said reduction in emissions.
10. A method according to claim 11 wherein there is also blended into said fuel at least one fuel-soluble hindered phenolic compound of the formula
wherein R1 is a hydrocarbyl group containing 1 to 12 carbon atoms, R2 is a secondary or tertiary hydrocarbyl group containing from 3 to 12 carbon atoms, R3 is a hydrocarbyl group or a group of the formula
and R4 is a hydrocarbyl group.
wherein R1 is a hydrocarbyl group containing 1 to 12 carbon atoms, R2 is a secondary or tertiary hydrocarbyl group containing from 3 to 12 carbon atoms, R3 is a hydrocarbyl group or a group of the formula
and R4 is a hydrocarbyl group.
11. A method of minimizing fuel consumption of a compression ignition engine operated on a liquid hydrocarbonaceous compression ignition fuel which method comprises supplying to said engine a liquid hydrocarbonaceous compression ignition fuel containing at least one ashless dispersant in an amount of at least one 50 ppm sufficient to minimize such fuel consumption.
12. A method according to Claim 11 wherein the fuel supplied to said engine also contains at least one fuel-soluble hindered phenolic compound of the formula
wherein R1 is a hydrocarbyl group containing 1 to 12 carbon atoms, R2 is a secondary or tertiary hydrocarbyl group containing from 3 to 12 carbon atoms, R3 is a hydrocarbyl group or a group of the formula
and R4 is a hydrocarbyl group.
wherein R1 is a hydrocarbyl group containing 1 to 12 carbon atoms, R2 is a secondary or tertiary hydrocarbyl group containing from 3 to 12 carbon atoms, R3 is a hydrocarbyl group or a group of the formula
and R4 is a hydrocarbyl group.
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP90311609A EP0482253A1 (en) | 1990-10-23 | 1990-10-23 | Environmentally friendly fuel compositions and additives therefor |
| CA002053825A CA2053825A1 (en) | 1990-10-23 | 1991-10-21 | Environmentally-friendly fuel compositions and additives therefor |
| FI914980A FI914980A7 (en) | 1990-10-23 | 1991-10-22 | Environmentally friendly fuel blends and their additives |
| AU86056/91A AU8605691A (en) | 1990-10-23 | 1991-10-22 | Environmentally-friendly fuel compositions and additives therefor |
| JP3301174A JPH04272995A (en) | 1990-10-23 | 1991-10-22 | Fuel composition having mild effect on environment, and additive for it |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP90311609A EP0482253A1 (en) | 1990-10-23 | 1990-10-23 | Environmentally friendly fuel compositions and additives therefor |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0482253A1 true EP0482253A1 (en) | 1992-04-29 |
Family
ID=8205585
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP90311609A Withdrawn EP0482253A1 (en) | 1990-10-23 | 1990-10-23 | Environmentally friendly fuel compositions and additives therefor |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP0482253A1 (en) |
| JP (1) | JPH04272995A (en) |
| AU (1) | AU8605691A (en) |
| CA (1) | CA2053825A1 (en) |
| FI (1) | FI914980A7 (en) |
Cited By (84)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994009092A1 (en) * | 1992-10-09 | 1994-04-28 | RWE-DEA Aktiengesellschaft für Mineraloel und Chemie | Fuel additives for spark ignition engines and fuels containing them |
| WO1995033021A1 (en) * | 1994-05-31 | 1995-12-07 | Exxon Chemical Patents Inc. | Oil additives and compositions |
| WO1996021709A1 (en) * | 1995-01-10 | 1996-07-18 | Exxon Chemical Patents Inc. | Fuel compositions |
| WO1996025473A1 (en) * | 1995-02-17 | 1996-08-22 | Bp Chemicals (Additives) Limited | Diesel fuels |
| EP0780460A1 (en) * | 1995-12-22 | 1997-06-25 | Exxon Research And Engineering Company | Gasoline additive concentrate |
| EP0807676A3 (en) * | 1996-05-17 | 1998-01-07 | Ethyl Petroleum Additives Limited | Fuel additives and compositions |
| EP0829527A1 (en) * | 1996-09-12 | 1998-03-18 | Exxon Research And Engineering Company | Additive concentrate for fuel compositions |
| WO1998016597A1 (en) * | 1996-10-11 | 1998-04-23 | Infineum Usa L.P. | Fuel composition containing lubricity additive |
| WO1998042808A1 (en) * | 1997-03-21 | 1998-10-01 | Infineum Holdings Bv | Fuel oil compositions |
| EP0890631A3 (en) * | 1995-02-02 | 1999-04-14 | Exxon Chemical Patents Inc. | Additives and fuel oil compositions |
| WO1999036489A1 (en) * | 1998-01-13 | 1999-07-22 | Baker Hughes Incorporated | Composition and method to improve lubricity in fuels |
| US5968211A (en) * | 1995-12-22 | 1999-10-19 | Exxon Research And Engineering Co. | Gasoline additive concentrate |
| US6010545A (en) * | 1994-12-13 | 2000-01-04 | Exxon Chemical Patents, Inc. | Fuel oil compositions |
| EP1081210A1 (en) * | 1999-08-31 | 2001-03-07 | Ethyl Corporation | Fuels with enhanced lubricity |
| US6200359B1 (en) | 1998-12-23 | 2001-03-13 | Shell Oil Company | Fuel oil composition |
| WO2003044134A3 (en) * | 2001-11-21 | 2003-08-07 | Shell Int Research | Diesel fuel compositions |
| WO2003091365A1 (en) * | 2002-04-23 | 2003-11-06 | The Lubrizol Corporation | Method of operating internal combustion engine by introducing antioxidant into combustion chamber |
| WO2004013260A1 (en) * | 2002-08-06 | 2004-02-12 | The Associated Octel Company Limited | Jet fuel composition comprising a phenol derivative |
| US6733550B1 (en) | 1997-03-21 | 2004-05-11 | Shell Oil Company | Fuel oil composition |
| US6866690B2 (en) | 2002-04-24 | 2005-03-15 | Ethyl Corporation | Friction modifier additives for fuel compositions and methods of use thereof |
| ES2245872A1 (en) * | 2004-03-22 | 2006-01-16 | Repsol Ypf, S.A. | Composition of gas oil or fuel disposal stabilized means. (Machine-translation by Google Translate, not legally binding) |
| ES2261063A1 (en) * | 2005-02-28 | 2006-11-01 | Repsol Ypf, S.A. | Formulation of stabilized automobile gas-oil presents sulphur content of less than 350 milligrams per kilogram of fuel |
| US7189269B2 (en) | 2002-10-18 | 2007-03-13 | Shell Oil Company | Fuel composition comprising a base fuel, a fischer tropsch derived gas oil, and an oxygenate |
| US7229481B2 (en) | 2002-11-13 | 2007-06-12 | Shell Oil Company | Diesel fuel compositions |
| WO2007102948A3 (en) * | 2006-02-03 | 2007-12-21 | Eastman Chem Co | Antioxidant compositions useful in biodiesel and other fatty acid and acid ester compositions |
| EP1728847A3 (en) * | 2005-04-04 | 2008-04-30 | Evonik Degussa GmbH | Process for increasing the oxidative stability of biodiesel. |
| US7402185B2 (en) | 2002-04-24 | 2008-07-22 | Afton Chemical Intangibles, Llc | Additives for fuel compositions to reduce formation of combustion chamber deposits |
| US7435272B2 (en) | 2002-04-24 | 2008-10-14 | Afton Chemical Intangibles | Friction modifier alkoxyamine salts of carboxylic acids as additives for fuel compositions and methods of use thereof |
| EP2055761A2 (en) | 2007-10-31 | 2009-05-06 | Chevron Oronite Company LLC | Lubricating oil compositions comprising a biodiesel fuel and a detergent |
| EP2055762A2 (en) | 2007-10-26 | 2009-05-06 | Chevron Oronite Company LLC | Lubricating oil compositions comprising a biodiesel fuel and an antioxidant |
| EP2065367A1 (en) | 2007-11-29 | 2009-06-03 | Chevron Oronite Company LLC | Sulfurized metal alkyl phenate compositions having a low alkyl phenol content |
| EP2077315A1 (en) | 2007-12-20 | 2009-07-08 | Chevron Oronite Company LLC | Lubricating oil compositions containing a tetraalkyl-napthalene-1,8 diamine antioxidant |
| EP2078744A1 (en) | 2008-01-10 | 2009-07-15 | Shell Internationale Researchmaatschappij B.V. | Fuel compositions |
| EP2078743A1 (en) | 2008-01-10 | 2009-07-15 | Shell Internationale Researchmaatschappij B.V. | Fuel composition |
| US7638661B2 (en) | 2003-12-01 | 2009-12-29 | Shell Oil Company | Power increase and increase in acceleration performance of diesel fuel compositions |
| EP2147966A1 (en) * | 2008-07-25 | 2010-01-27 | Total Raffinage Marketing | Additive for liquid fuel, liquid fuel containing same and its use for energy generation and/or heating and/or cooking devices |
| US7737311B2 (en) | 2003-09-03 | 2010-06-15 | Shell Oil Company | Fuel compositions |
| EP0956328B2 (en) † | 1996-10-11 | 2010-07-07 | Infineum USA L.P. | Fuel compositions with lubricity additives |
| WO2010076304A1 (en) | 2008-12-29 | 2010-07-08 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| WO2010076303A1 (en) | 2008-12-29 | 2010-07-08 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| US7846224B2 (en) | 2002-04-24 | 2010-12-07 | Afton Chemical Intangibles, Llc | Methods to improve the low temperature compatibility of amide friction modifiers in fuels and amide friction modifiers |
| WO2011076948A1 (en) | 2009-12-24 | 2011-06-30 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| WO2011080250A1 (en) | 2009-12-29 | 2011-07-07 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| EP2371931A1 (en) | 2010-03-23 | 2011-10-05 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| US8152869B2 (en) | 2007-12-20 | 2012-04-10 | Shell Oil Company | Fuel compositions |
| US8152868B2 (en) | 2007-12-20 | 2012-04-10 | Shell Oil Company | Fuel compositions |
| WO2012098258A1 (en) | 2011-01-21 | 2012-07-26 | Shell Internationale Research Maatschappij B.V. | Test kit and method for detection of additives in fuel compositions |
| WO2012162403A1 (en) | 2011-05-23 | 2012-11-29 | Virent, Inc. | Production of chemicals and fuels from biomass |
| WO2012163935A2 (en) | 2011-05-30 | 2012-12-06 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| WO2013034617A1 (en) | 2011-09-06 | 2013-03-14 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| WO2013086138A1 (en) | 2011-12-07 | 2013-06-13 | Igp Energy, Inc. | Fuels and fuel additives comprising butanol and pentanol |
| WO2013092533A1 (en) * | 2011-12-21 | 2013-06-27 | Total Raffinage Marketing | Additive compositions that improve the lacquering resistance of superior quality diesel or biodiesel fuels |
| US8475647B2 (en) | 2005-08-22 | 2013-07-02 | Shell Oil Company | Diesel fuel and a method of operating a diesel engine |
| US8541635B2 (en) | 2006-03-10 | 2013-09-24 | Shell Oil Company | Diesel fuel compositions |
| US8663344B2 (en) | 2007-08-24 | 2014-03-04 | Albemarle Corporation | Antioxidant blends suitable for use in biodiesels |
| GB2507675A (en) * | 2012-12-13 | 2014-05-07 | Fuel Additive Science Technologies Ltd | Fuel additive composition |
| DE102013112821A1 (en) | 2012-11-30 | 2014-06-05 | Shell Internationale Research Maatschappij B.V. | Fuel Compositions |
| WO2014091231A1 (en) * | 2012-12-13 | 2014-06-19 | Fuel Additive Science Technologies Limited | Fuel additive composition |
| WO2014096234A1 (en) | 2012-12-21 | 2014-06-26 | Shell Internationale Research Maatschappij B.V. | Liquid diesel fuel compositions containing organic sunscreen compounds |
| US8987537B1 (en) | 2014-05-22 | 2015-03-24 | Shell Oil Company | Fuel compositions |
| US9057035B1 (en) | 2014-02-17 | 2015-06-16 | Shell Oil Company | Fuel compositions |
| WO2015091458A1 (en) | 2013-12-16 | 2015-06-25 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| EP2889361A1 (en) | 2013-12-31 | 2015-07-01 | Shell Internationale Research Maatschappij B.V. | Diesel fuel formulation and use thereof |
| EP2907867A1 (en) | 2014-02-17 | 2015-08-19 | Shell International Research Maatschappij B.V. | Fuel compositions |
| EP2949732A1 (en) | 2014-05-28 | 2015-12-02 | Shell Internationale Research Maatschappij B.V. | Use of an oxanilide compound in a diesel fuel composition for the purpose of modifying the ignition delay and/or the burn period |
| EP2732012B1 (en) | 2011-07-12 | 2015-12-09 | Total Marketing Services | Compositions of additives improving stability and engine performance of diesel fuels. |
| WO2016188850A1 (en) | 2015-05-22 | 2016-12-01 | Shell Internationale Research Maatschappij B.V. | Fuel composition |
| WO2016188858A1 (en) | 2015-05-22 | 2016-12-01 | Shell Internationale Research Maatschappij B.V. | Fuel composition |
| WO2017081199A1 (en) | 2015-11-11 | 2017-05-18 | Shell Internationale Research Maatschappij B.V. | Process for preparing a diesel fuel composition |
| US9663735B2 (en) | 2013-10-24 | 2017-05-30 | Shell Oil Company | Liquid fuel compositions |
| EP3184612A1 (en) | 2015-12-21 | 2017-06-28 | Shell Internationale Research Maatschappij B.V. | Process for preparing a diesel fuel composition |
| WO2017134251A1 (en) | 2016-02-05 | 2017-08-10 | Shell Internationale Research Maatschappij B.V. | Fuel composition |
| US9752092B2 (en) | 2015-10-30 | 2017-09-05 | Chevron Oronite Company Llc | Lubricating oil compositions containing amidine antioxidants |
| WO2017202735A1 (en) | 2016-05-23 | 2017-11-30 | Shell Internationale Research Maatschappij B.V. | Use of a wax anti-settling additive in automotive fuel compositions |
| WO2018077976A1 (en) | 2016-10-27 | 2018-05-03 | Shell Internationale Research Maatschappij B.V. | Process for preparing an automotive gasoil |
| WO2018206729A1 (en) | 2017-05-11 | 2018-11-15 | Shell Internationale Research Maatschappij B.V. | Process for preparing an automotive gas oil fraction |
| WO2020070246A1 (en) | 2018-10-05 | 2020-04-09 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| WO2020109184A1 (en) | 2018-11-26 | 2020-06-04 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| WO2020120416A1 (en) | 2018-12-11 | 2020-06-18 | Shell Internationale Research Maatschappij B.V. | Use and method to reduce deposits in compression ignition internal combustion engines |
| WO2021018895A1 (en) | 2019-07-30 | 2021-02-04 | Shell Internationale Research Maatschappij B.V. | Fuel compositions with enhanced stability and methods of making same |
| US11390821B2 (en) * | 2019-01-31 | 2022-07-19 | Afton Chemical Corporation | Fuel additive mixture providing rapid injector clean-up in high pressure gasoline engines |
| US20220333027A1 (en) * | 2019-09-10 | 2022-10-20 | Chevron Oronite Company Llc | Reducing friction in combustion engines through fuel additives |
| WO2022228990A1 (en) | 2021-04-26 | 2022-11-03 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| WO2022228989A1 (en) | 2021-04-26 | 2022-11-03 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102004005108A1 (en) * | 2004-02-02 | 2005-10-27 | Basf Ag | Process for the preparation of polyisobutenylphenols |
| EP1612256B1 (en) * | 2004-06-30 | 2012-06-13 | Infineum International Limited | Fuel additives comprising a colloidal metal compound. |
| DE102004038113A1 (en) * | 2004-08-05 | 2006-03-16 | Basf Ag | Nitrogen-containing heterocyclic compounds as Reibverschleißvermindernder addition to fuels |
| EP1705234A1 (en) * | 2005-03-24 | 2006-09-27 | Basf Aktiengesellschaft | Use of detergent additives to inhibit or reduce the formation of injection system deposits in direct injection diesel engines |
| DE102009033161A1 (en) * | 2009-07-13 | 2011-01-27 | Schülke & Mayr GmbH | Additive for the bactericidal and anticorrosive finishing of fuels |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1914338A1 (en) * | 1968-04-05 | 1970-04-23 | Exxon Research Engineering Co | Fuel additive and method of making the same |
| US4022589A (en) * | 1974-10-17 | 1977-05-10 | Phillips Petroleum Company | Fuel additive package containing polybutene amine and lubricating oil |
| EP0078249A1 (en) * | 1981-10-12 | 1983-05-04 | Lang & Co., chemisch-technische Produkte Kommanditgesellschaft | Additive with a combustion promoting and soot inhibiting activity for furnace oils, diesel fuels and other liquid combustion and fuel substances, as well as the aforesaid liquid combustion and fuel substances |
| EP0203692A1 (en) * | 1985-04-26 | 1986-12-03 | Exxon Chemical Patents Inc. | Fuel oil compositions |
| EP0207560A1 (en) * | 1985-06-24 | 1987-01-07 | Shell Internationale Researchmaatschappij B.V. | Gasoline composition |
| EP0235868A1 (en) * | 1986-03-06 | 1987-09-09 | Shell Internationale Researchmaatschappij B.V. | Fuel composition |
| EP0251419A1 (en) * | 1983-12-30 | 1988-01-07 | Ethyl Corporation | Fuel composition and additive concentrates, and their use in inhibiting engine coking |
| EP0355895A2 (en) * | 1988-08-05 | 1990-02-28 | Shell Internationale Researchmaatschappij B.V. | Process for the preparation of succinic anhydride derivatives |
| EP0385633A1 (en) * | 1989-03-02 | 1990-09-05 | Ethyl Petroleum Additives, Inc. | Middle distillate fuel having improved storage stability |
-
1990
- 1990-10-23 EP EP90311609A patent/EP0482253A1/en not_active Withdrawn
-
1991
- 1991-10-21 CA CA002053825A patent/CA2053825A1/en not_active Abandoned
- 1991-10-22 JP JP3301174A patent/JPH04272995A/en active Pending
- 1991-10-22 FI FI914980A patent/FI914980A7/en not_active Application Discontinuation
- 1991-10-22 AU AU86056/91A patent/AU8605691A/en not_active Abandoned
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1914338A1 (en) * | 1968-04-05 | 1970-04-23 | Exxon Research Engineering Co | Fuel additive and method of making the same |
| US4022589A (en) * | 1974-10-17 | 1977-05-10 | Phillips Petroleum Company | Fuel additive package containing polybutene amine and lubricating oil |
| EP0078249A1 (en) * | 1981-10-12 | 1983-05-04 | Lang & Co., chemisch-technische Produkte Kommanditgesellschaft | Additive with a combustion promoting and soot inhibiting activity for furnace oils, diesel fuels and other liquid combustion and fuel substances, as well as the aforesaid liquid combustion and fuel substances |
| EP0251419A1 (en) * | 1983-12-30 | 1988-01-07 | Ethyl Corporation | Fuel composition and additive concentrates, and their use in inhibiting engine coking |
| EP0203692A1 (en) * | 1985-04-26 | 1986-12-03 | Exxon Chemical Patents Inc. | Fuel oil compositions |
| EP0207560A1 (en) * | 1985-06-24 | 1987-01-07 | Shell Internationale Researchmaatschappij B.V. | Gasoline composition |
| EP0235868A1 (en) * | 1986-03-06 | 1987-09-09 | Shell Internationale Researchmaatschappij B.V. | Fuel composition |
| EP0355895A2 (en) * | 1988-08-05 | 1990-02-28 | Shell Internationale Researchmaatschappij B.V. | Process for the preparation of succinic anhydride derivatives |
| EP0385633A1 (en) * | 1989-03-02 | 1990-09-05 | Ethyl Petroleum Additives, Inc. | Middle distillate fuel having improved storage stability |
Cited By (123)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994009092A1 (en) * | 1992-10-09 | 1994-04-28 | RWE-DEA Aktiengesellschaft für Mineraloel und Chemie | Fuel additives for spark ignition engines and fuels containing them |
| WO1995033021A1 (en) * | 1994-05-31 | 1995-12-07 | Exxon Chemical Patents Inc. | Oil additives and compositions |
| US5733346A (en) * | 1994-05-31 | 1998-03-31 | Exxon Chemical Patents Inc. | Oil additives and compositions |
| EP1050573A3 (en) * | 1994-12-13 | 2001-01-03 | Infineum USA L.P. | Fuel oil compositions |
| US6010545A (en) * | 1994-12-13 | 2000-01-04 | Exxon Chemical Patents, Inc. | Fuel oil compositions |
| WO1996021709A1 (en) * | 1995-01-10 | 1996-07-18 | Exxon Chemical Patents Inc. | Fuel compositions |
| US6280488B1 (en) | 1995-02-02 | 2001-08-28 | Exxon Chemical Patents Inc | Additives and fuel oil compositions |
| EP0890631A3 (en) * | 1995-02-02 | 1999-04-14 | Exxon Chemical Patents Inc. | Additives and fuel oil compositions |
| EP0885947A3 (en) * | 1995-02-02 | 1999-04-14 | Exxon Chemical Patents Inc. | Additives and fuel oil compositions |
| WO1996025473A1 (en) * | 1995-02-17 | 1996-08-22 | Bp Chemicals (Additives) Limited | Diesel fuels |
| EP0780460A1 (en) * | 1995-12-22 | 1997-06-25 | Exxon Research And Engineering Company | Gasoline additive concentrate |
| US5968211A (en) * | 1995-12-22 | 1999-10-19 | Exxon Research And Engineering Co. | Gasoline additive concentrate |
| EP0807676A3 (en) * | 1996-05-17 | 1998-01-07 | Ethyl Petroleum Additives Limited | Fuel additives and compositions |
| EP0829527A1 (en) * | 1996-09-12 | 1998-03-18 | Exxon Research And Engineering Company | Additive concentrate for fuel compositions |
| US6277158B1 (en) | 1996-09-12 | 2001-08-21 | Exxon Research And Engineering Company | Additive concentrate for fuel compositions |
| WO1998011175A1 (en) * | 1996-09-12 | 1998-03-19 | Exxon Research And Engineering Company | Additive concentrate for fuel compositions |
| US6248142B1 (en) | 1996-10-11 | 2001-06-19 | Exxon Chemical Patents Inc | Fuel composition containing lubricity additive |
| WO1998016597A1 (en) * | 1996-10-11 | 1998-04-23 | Infineum Usa L.P. | Fuel composition containing lubricity additive |
| EP0956328B2 (en) † | 1996-10-11 | 2010-07-07 | Infineum USA L.P. | Fuel compositions with lubricity additives |
| WO1998042808A1 (en) * | 1997-03-21 | 1998-10-01 | Infineum Holdings Bv | Fuel oil compositions |
| US6733550B1 (en) | 1997-03-21 | 2004-05-11 | Shell Oil Company | Fuel oil composition |
| WO1999036489A1 (en) * | 1998-01-13 | 1999-07-22 | Baker Hughes Incorporated | Composition and method to improve lubricity in fuels |
| US6129772A (en) * | 1998-01-13 | 2000-10-10 | Baker Hughes Incorporated | Composition and method to improve lubricity in fuels |
| US6200359B1 (en) | 1998-12-23 | 2001-03-13 | Shell Oil Company | Fuel oil composition |
| EP1081210A1 (en) * | 1999-08-31 | 2001-03-07 | Ethyl Corporation | Fuels with enhanced lubricity |
| WO2003044134A3 (en) * | 2001-11-21 | 2003-08-07 | Shell Int Research | Diesel fuel compositions |
| WO2003091365A1 (en) * | 2002-04-23 | 2003-11-06 | The Lubrizol Corporation | Method of operating internal combustion engine by introducing antioxidant into combustion chamber |
| US7435272B2 (en) | 2002-04-24 | 2008-10-14 | Afton Chemical Intangibles | Friction modifier alkoxyamine salts of carboxylic acids as additives for fuel compositions and methods of use thereof |
| US7846224B2 (en) | 2002-04-24 | 2010-12-07 | Afton Chemical Intangibles, Llc | Methods to improve the low temperature compatibility of amide friction modifiers in fuels and amide friction modifiers |
| US6866690B2 (en) | 2002-04-24 | 2005-03-15 | Ethyl Corporation | Friction modifier additives for fuel compositions and methods of use thereof |
| US7402185B2 (en) | 2002-04-24 | 2008-07-22 | Afton Chemical Intangibles, Llc | Additives for fuel compositions to reduce formation of combustion chamber deposits |
| WO2004013260A1 (en) * | 2002-08-06 | 2004-02-12 | The Associated Octel Company Limited | Jet fuel composition comprising a phenol derivative |
| US7189269B2 (en) | 2002-10-18 | 2007-03-13 | Shell Oil Company | Fuel composition comprising a base fuel, a fischer tropsch derived gas oil, and an oxygenate |
| US7229481B2 (en) | 2002-11-13 | 2007-06-12 | Shell Oil Company | Diesel fuel compositions |
| US7737311B2 (en) | 2003-09-03 | 2010-06-15 | Shell Oil Company | Fuel compositions |
| US7638661B2 (en) | 2003-12-01 | 2009-12-29 | Shell Oil Company | Power increase and increase in acceleration performance of diesel fuel compositions |
| ES2245872A1 (en) * | 2004-03-22 | 2006-01-16 | Repsol Ypf, S.A. | Composition of gas oil or fuel disposal stabilized means. (Machine-translation by Google Translate, not legally binding) |
| ES2261063A1 (en) * | 2005-02-28 | 2006-11-01 | Repsol Ypf, S.A. | Formulation of stabilized automobile gas-oil presents sulphur content of less than 350 milligrams per kilogram of fuel |
| EP1728847A3 (en) * | 2005-04-04 | 2008-04-30 | Evonik Degussa GmbH | Process for increasing the oxidative stability of biodiesel. |
| US8475647B2 (en) | 2005-08-22 | 2013-07-02 | Shell Oil Company | Diesel fuel and a method of operating a diesel engine |
| US9422508B2 (en) | 2006-02-03 | 2016-08-23 | Eastman Chemical Company | Antioxidant compositions useful in biodiesel and other fatty acid and acid ester compositions |
| US8075804B2 (en) * | 2006-02-03 | 2011-12-13 | Eastman Chemical Company | Antioxidant compositions useful in biodiesel and other fatty acid and acid ester compositions |
| WO2007102948A3 (en) * | 2006-02-03 | 2007-12-21 | Eastman Chem Co | Antioxidant compositions useful in biodiesel and other fatty acid and acid ester compositions |
| US8541635B2 (en) | 2006-03-10 | 2013-09-24 | Shell Oil Company | Diesel fuel compositions |
| US8663344B2 (en) | 2007-08-24 | 2014-03-04 | Albemarle Corporation | Antioxidant blends suitable for use in biodiesels |
| EP2055762A2 (en) | 2007-10-26 | 2009-05-06 | Chevron Oronite Company LLC | Lubricating oil compositions comprising a biodiesel fuel and an antioxidant |
| EP2055761A2 (en) | 2007-10-31 | 2009-05-06 | Chevron Oronite Company LLC | Lubricating oil compositions comprising a biodiesel fuel and a detergent |
| EP2065367A1 (en) | 2007-11-29 | 2009-06-03 | Chevron Oronite Company LLC | Sulfurized metal alkyl phenate compositions having a low alkyl phenol content |
| EP2077315A1 (en) | 2007-12-20 | 2009-07-08 | Chevron Oronite Company LLC | Lubricating oil compositions containing a tetraalkyl-napthalene-1,8 diamine antioxidant |
| US8152869B2 (en) | 2007-12-20 | 2012-04-10 | Shell Oil Company | Fuel compositions |
| US8152868B2 (en) | 2007-12-20 | 2012-04-10 | Shell Oil Company | Fuel compositions |
| EP2078744A1 (en) | 2008-01-10 | 2009-07-15 | Shell Internationale Researchmaatschappij B.V. | Fuel compositions |
| EP2078743A1 (en) | 2008-01-10 | 2009-07-15 | Shell Internationale Researchmaatschappij B.V. | Fuel composition |
| US8273137B2 (en) | 2008-01-10 | 2012-09-25 | Shell Oil Company | Fuel composition |
| FR2934276A1 (en) * | 2008-07-25 | 2010-01-29 | Total France | LIQUID FUEL ADDITIVE, LIQUID FUEL CONTAINING THE SAME, AND USE THEREOF FOR ENERGY AND / OR HEATING AND / OR COOKING APPARATUS |
| EP2147966A1 (en) * | 2008-07-25 | 2010-01-27 | Total Raffinage Marketing | Additive for liquid fuel, liquid fuel containing same and its use for energy generation and/or heating and/or cooking devices |
| WO2010076303A1 (en) | 2008-12-29 | 2010-07-08 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| WO2010076304A1 (en) | 2008-12-29 | 2010-07-08 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| US8771385B2 (en) | 2008-12-29 | 2014-07-08 | Shell Oil Company | Fuel compositions |
| US9017429B2 (en) | 2008-12-29 | 2015-04-28 | Shell Oil Company | Fuel compositions |
| WO2011076948A1 (en) | 2009-12-24 | 2011-06-30 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| WO2011080250A1 (en) | 2009-12-29 | 2011-07-07 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| EP2371931A1 (en) | 2010-03-23 | 2011-10-05 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| US8876923B2 (en) | 2010-03-23 | 2014-11-04 | Shell Oil Company | Fuel compositions |
| WO2012098258A1 (en) | 2011-01-21 | 2012-07-26 | Shell Internationale Research Maatschappij B.V. | Test kit and method for detection of additives in fuel compositions |
| WO2012162403A1 (en) | 2011-05-23 | 2012-11-29 | Virent, Inc. | Production of chemicals and fuels from biomass |
| WO2012163935A2 (en) | 2011-05-30 | 2012-12-06 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| EP2732012B1 (en) | 2011-07-12 | 2015-12-09 | Total Marketing Services | Compositions of additives improving stability and engine performance of diesel fuels. |
| WO2013034617A1 (en) | 2011-09-06 | 2013-03-14 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| WO2013086138A1 (en) | 2011-12-07 | 2013-06-13 | Igp Energy, Inc. | Fuels and fuel additives comprising butanol and pentanol |
| EA030972B1 (en) * | 2011-12-21 | 2018-10-31 | Тотал Маркетинг Сервисез | Additive compositions for improving the lacquering resistance of higher grade fuels of the diesel and biodiesel types |
| CN103998581A (en) * | 2011-12-21 | 2014-08-20 | 道达尔销售服务公司 | Additive compositions that improve the lacquering resistance of superior quality diesel or biodiesel fuels |
| WO2013092533A1 (en) * | 2011-12-21 | 2013-06-27 | Total Raffinage Marketing | Additive compositions that improve the lacquering resistance of superior quality diesel or biodiesel fuels |
| CN103998581B (en) * | 2011-12-21 | 2017-02-22 | 道达尔销售服务公司 | Additive compositions that improve the lacquering resistance of superior quality diesel or biodiesel fuels |
| US20150033617A1 (en) * | 2011-12-21 | 2015-02-05 | Total Marketing Services | Additive compositions for improving the lacquering resistance of higher grade fuels of the diesel or biodiesel type |
| DE102013112821A1 (en) | 2012-11-30 | 2014-06-05 | Shell Internationale Research Maatschappij B.V. | Fuel Compositions |
| GB2507675A (en) * | 2012-12-13 | 2014-05-07 | Fuel Additive Science Technologies Ltd | Fuel additive composition |
| GB2507675B (en) * | 2012-12-13 | 2014-11-12 | Fuel Additive Science Technologies Ltd | Fuel additive composition |
| WO2014091231A1 (en) * | 2012-12-13 | 2014-06-19 | Fuel Additive Science Technologies Limited | Fuel additive composition |
| WO2014096234A1 (en) | 2012-12-21 | 2014-06-26 | Shell Internationale Research Maatschappij B.V. | Liquid diesel fuel compositions containing organic sunscreen compounds |
| US9222047B2 (en) | 2012-12-21 | 2015-12-29 | Shell Oil Company | Liquid fuel compositions |
| US9663735B2 (en) | 2013-10-24 | 2017-05-30 | Shell Oil Company | Liquid fuel compositions |
| US9587195B2 (en) | 2013-12-16 | 2017-03-07 | Shell Oil Company | Liquid composition |
| WO2015091458A1 (en) | 2013-12-16 | 2015-06-25 | Shell Internationale Research Maatschappij B.V. | Liquid fuel compositions |
| EP2889361A1 (en) | 2013-12-31 | 2015-07-01 | Shell Internationale Research Maatschappij B.V. | Diesel fuel formulation and use thereof |
| US9057035B1 (en) | 2014-02-17 | 2015-06-16 | Shell Oil Company | Fuel compositions |
| US10577551B2 (en) | 2014-02-17 | 2020-03-03 | Shell Oil Company | Fuel compositions |
| EP2907867A1 (en) | 2014-02-17 | 2015-08-19 | Shell International Research Maatschappij B.V. | Fuel compositions |
| US9487718B2 (en) | 2014-02-17 | 2016-11-08 | Shell Oil Company | Fuel compositions |
| EP3581637A1 (en) | 2014-02-17 | 2019-12-18 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| US9499758B2 (en) | 2014-05-22 | 2016-11-22 | Shell Oil Company | Fuel compositions |
| EP2990465A1 (en) | 2014-05-22 | 2016-03-02 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| US8987537B1 (en) | 2014-05-22 | 2015-03-24 | Shell Oil Company | Fuel compositions |
| EP2947135A1 (en) | 2014-05-22 | 2015-11-25 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| US10457881B2 (en) | 2014-05-22 | 2019-10-29 | Shell Oil Company | Fuel compositions |
| WO2015179017A2 (en) | 2014-05-22 | 2015-11-26 | Shell Oil Company | Fuel compositions |
| EP2949732A1 (en) | 2014-05-28 | 2015-12-02 | Shell Internationale Research Maatschappij B.V. | Use of an oxanilide compound in a diesel fuel composition for the purpose of modifying the ignition delay and/or the burn period |
| WO2016188850A1 (en) | 2015-05-22 | 2016-12-01 | Shell Internationale Research Maatschappij B.V. | Fuel composition |
| US11001775B2 (en) | 2015-05-22 | 2021-05-11 | Shell Oil Company | Fuel composition |
| US11104857B2 (en) | 2015-05-22 | 2021-08-31 | Shell Oil Company | Fuel composition |
| WO2016188858A1 (en) | 2015-05-22 | 2016-12-01 | Shell Internationale Research Maatschappij B.V. | Fuel composition |
| US9752092B2 (en) | 2015-10-30 | 2017-09-05 | Chevron Oronite Company Llc | Lubricating oil compositions containing amidine antioxidants |
| WO2017081199A1 (en) | 2015-11-11 | 2017-05-18 | Shell Internationale Research Maatschappij B.V. | Process for preparing a diesel fuel composition |
| US11084997B2 (en) | 2015-11-11 | 2021-08-10 | Shell Oil Company | Process for preparing a diesel fuel composition |
| EP3184612A1 (en) | 2015-12-21 | 2017-06-28 | Shell Internationale Research Maatschappij B.V. | Process for preparing a diesel fuel composition |
| WO2017134251A1 (en) | 2016-02-05 | 2017-08-10 | Shell Internationale Research Maatschappij B.V. | Fuel composition |
| US11254885B2 (en) | 2016-02-05 | 2022-02-22 | Shell Oil Company | Fuel composition |
| US11359155B2 (en) | 2016-05-23 | 2022-06-14 | Shell Usa, Inc. | Use of a wax anti-settling additive in automotive fuel compositions |
| WO2017202735A1 (en) | 2016-05-23 | 2017-11-30 | Shell Internationale Research Maatschappij B.V. | Use of a wax anti-settling additive in automotive fuel compositions |
| WO2018077976A1 (en) | 2016-10-27 | 2018-05-03 | Shell Internationale Research Maatschappij B.V. | Process for preparing an automotive gasoil |
| WO2018206729A1 (en) | 2017-05-11 | 2018-11-15 | Shell Internationale Research Maatschappij B.V. | Process for preparing an automotive gas oil fraction |
| WO2020070246A1 (en) | 2018-10-05 | 2020-04-09 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| WO2020109184A1 (en) | 2018-11-26 | 2020-06-04 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| US11499106B2 (en) | 2018-11-26 | 2022-11-15 | Shell Usa, Inc. | Fuel compositions |
| WO2020120416A1 (en) | 2018-12-11 | 2020-06-18 | Shell Internationale Research Maatschappij B.V. | Use and method to reduce deposits in compression ignition internal combustion engines |
| US11867117B2 (en) | 2018-12-11 | 2024-01-09 | Shell Usa, Inc. | Use and method to reduce deposits in compression ignition internal combustion engines |
| US11390821B2 (en) * | 2019-01-31 | 2022-07-19 | Afton Chemical Corporation | Fuel additive mixture providing rapid injector clean-up in high pressure gasoline engines |
| WO2021018895A1 (en) | 2019-07-30 | 2021-02-04 | Shell Internationale Research Maatschappij B.V. | Fuel compositions with enhanced stability and methods of making same |
| US12241033B2 (en) | 2019-07-30 | 2025-03-04 | Shell Usa, Inc. | Fuel compositions with enhanced stability and methods of making same |
| US20220333027A1 (en) * | 2019-09-10 | 2022-10-20 | Chevron Oronite Company Llc | Reducing friction in combustion engines through fuel additives |
| WO2022228990A1 (en) | 2021-04-26 | 2022-11-03 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| WO2022228989A1 (en) | 2021-04-26 | 2022-11-03 | Shell Internationale Research Maatschappij B.V. | Fuel compositions |
| US12338405B2 (en) | 2021-04-26 | 2025-06-24 | Shell Usa Inc. | Fuel compositions |
Also Published As
| Publication number | Publication date |
|---|---|
| FI914980A0 (en) | 1991-10-22 |
| AU8605691A (en) | 1992-04-30 |
| CA2053825A1 (en) | 1992-04-24 |
| JPH04272995A (en) | 1992-09-29 |
| FI914980L (en) | 1992-04-24 |
| FI914980A7 (en) | 1992-04-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0482253A1 (en) | Environmentally friendly fuel compositions and additives therefor | |
| EP0476196B1 (en) | Hydrocarbonaceous fuel compositions and additives therefor | |
| US5944858A (en) | Hydrocarbonaceous fuel compositions and additives therefor | |
| EP0435631B1 (en) | Diesel fuel compositions | |
| EP0476197B1 (en) | Hydrocarbonaceous fuel compositions and additives therefor | |
| US5551957A (en) | Compostions for control of induction system deposits | |
| EP0890632B1 (en) | Use of additives in diesel fuel oil compositions | |
| CA2095683C (en) | Composition for control of induction system deposits | |
| US8425627B2 (en) | Fuel additive concentrate composition and fuel composition and method thereof | |
| JP4786123B2 (en) | Gasoline additive concentrated compositions and fuel compositions and methods thereof | |
| US5482521A (en) | Friction modifiers and antiwear additives for fuels and lubricants | |
| AU2002250378A1 (en) | Gasoline additive concentrate composition and fuel composition and method thereof | |
| JPH1053777A (en) | Fuel additive and composition | |
| EP1713889A1 (en) | Fuel composition containing a solvent substantially free of sulphur and process thereof | |
| US8709108B2 (en) | Fuel compositions | |
| GB2261441A (en) | Fuel compositions | |
| GB2279965A (en) | Additive compositions for control of deposits, exhaust emissions and/or fuel consumption in internal combustion engines | |
| KR20080009753A (en) | Use of fatty acid alkoxylate as a method to remove adhesion of the engine suction valve | |
| GB2259522A (en) | Compositions for control of induction system deposits | |
| EP2169034A2 (en) | Fuel compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE DE ES FR GB IT NL |
|
| 17P | Request for examination filed |
Effective date: 19920416 |
|
| 17Q | First examination report despatched |
Effective date: 19930317 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 19940825 |