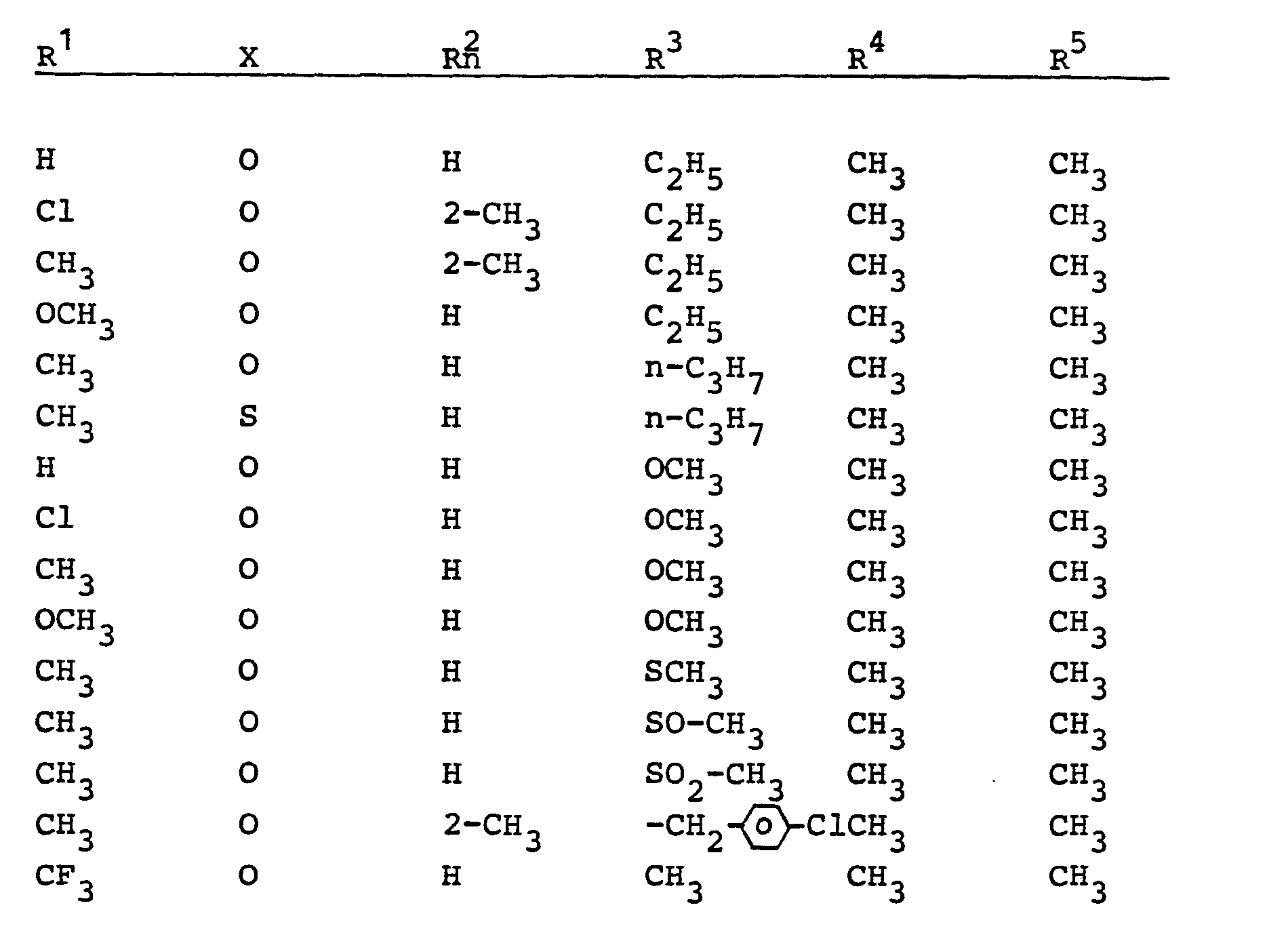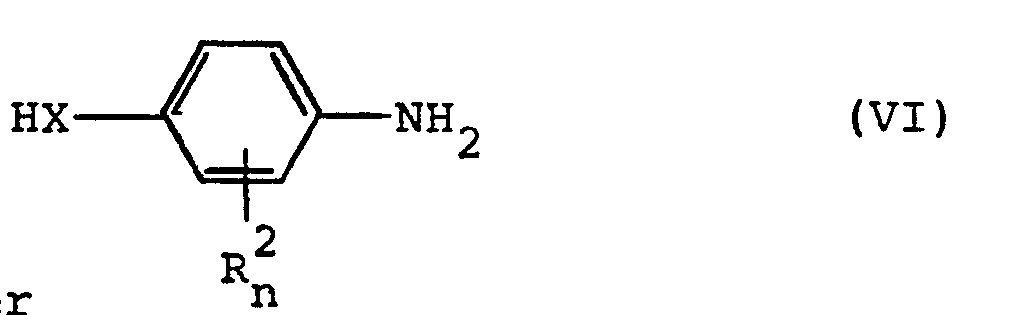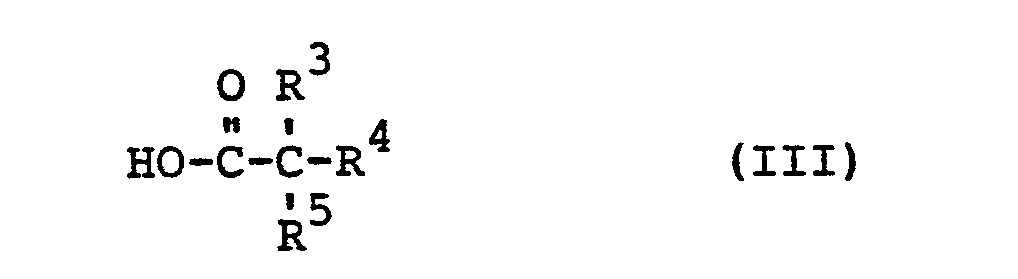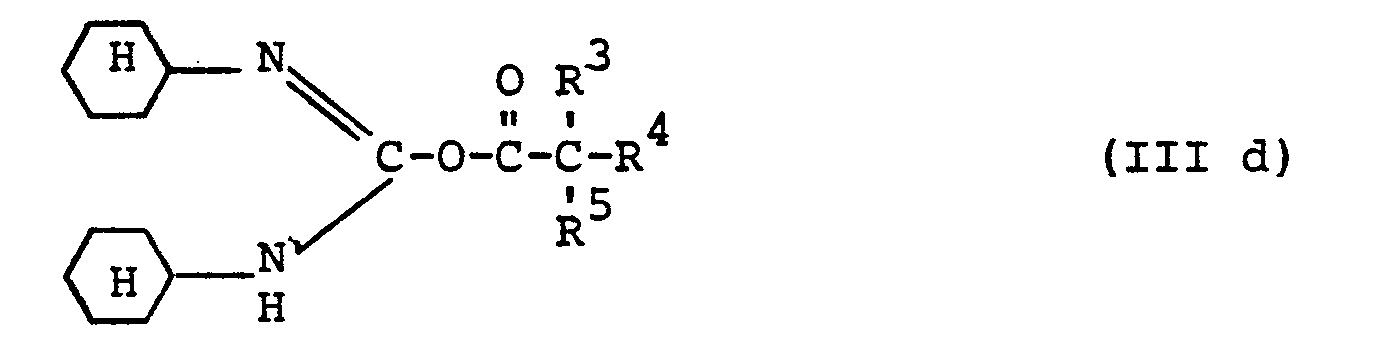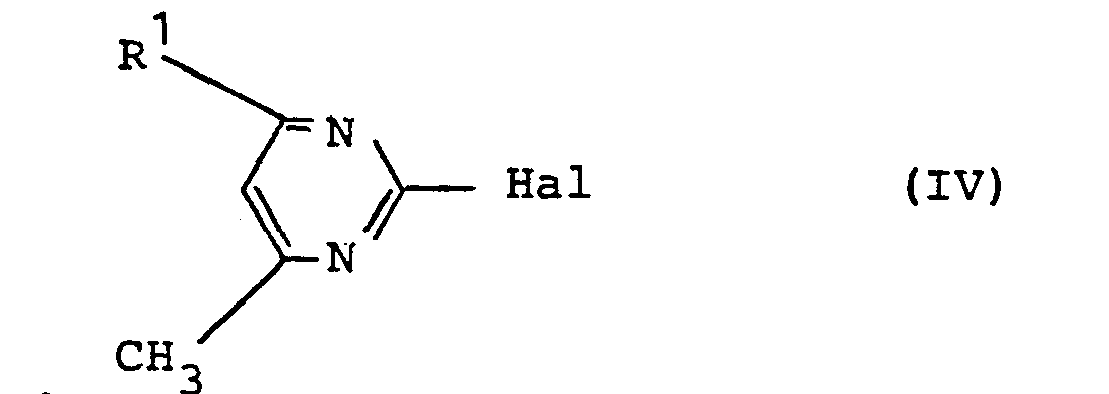EP0168608A2 - Substituted carboxylic-acid anilides - Google Patents
Substituted carboxylic-acid anilides Download PDFInfo
- Publication number
- EP0168608A2 EP0168608A2 EP85106804A EP85106804A EP0168608A2 EP 0168608 A2 EP0168608 A2 EP 0168608A2 EP 85106804 A EP85106804 A EP 85106804A EP 85106804 A EP85106804 A EP 85106804A EP 0168608 A2 EP0168608 A2 EP 0168608A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- carbon atoms
- optionally substituted
- formula
- alkyl
- chlorine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- -1 carboxylic-acid anilides Chemical class 0.000 title claims abstract description 31
- 229940051881 anilide analgesics and antipyretics Drugs 0.000 title claims abstract description 12
- 238000000034 method Methods 0.000 claims abstract description 85
- 125000004432 carbon atom Chemical group C* 0.000 claims abstract description 56
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 29
- 229910052736 halogen Inorganic materials 0.000 claims abstract description 27
- 150000002367 halogens Chemical group 0.000 claims abstract description 27
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims abstract description 19
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims abstract description 17
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims abstract description 16
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 14
- 239000001257 hydrogen Substances 0.000 claims abstract description 14
- 150000002431 hydrogen Chemical group 0.000 claims abstract description 13
- 125000000547 substituted alkyl group Chemical group 0.000 claims abstract description 12
- 238000002360 preparation method Methods 0.000 claims abstract description 11
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims abstract description 9
- 125000003545 alkoxy group Chemical group 0.000 claims abstract description 9
- 239000004009 herbicide Substances 0.000 claims abstract description 9
- 229910052760 oxygen Inorganic materials 0.000 claims abstract description 9
- 239000001301 oxygen Substances 0.000 claims abstract description 9
- 125000003107 substituted aryl group Chemical group 0.000 claims abstract description 8
- 125000004414 alkyl thio group Chemical group 0.000 claims abstract description 7
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims abstract description 7
- 229910052799 carbon Inorganic materials 0.000 claims abstract description 7
- 150000001721 carbon Chemical group 0.000 claims abstract description 7
- 229920006395 saturated elastomer Polymers 0.000 claims abstract description 7
- 229910052717 sulfur Chemical group 0.000 claims abstract description 7
- 239000011593 sulfur Chemical group 0.000 claims abstract description 7
- 125000002837 carbocyclic group Chemical group 0.000 claims abstract description 6
- 125000003710 aryl alkyl group Chemical group 0.000 claims abstract description 5
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 39
- 239000002253 acid Substances 0.000 claims description 36
- 239000000460 chlorine Substances 0.000 claims description 36
- 229910052801 chlorine Inorganic materials 0.000 claims description 36
- 239000003085 diluting agent Substances 0.000 claims description 34
- 150000001875 compounds Chemical class 0.000 claims description 33
- 229910052731 fluorine Inorganic materials 0.000 claims description 31
- 239000011737 fluorine Substances 0.000 claims description 31
- 241000196324 Embryophyta Species 0.000 claims description 26
- 239000000203 mixture Substances 0.000 claims description 25
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 claims description 23
- 229910052794 bromium Inorganic materials 0.000 claims description 23
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 claims description 21
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims description 21
- 239000011230 binding agent Substances 0.000 claims description 20
- 125000001153 fluoro group Chemical group F* 0.000 claims description 19
- 150000003230 pyrimidines Chemical class 0.000 claims description 11
- 229940083082 pyrimidine derivative acting on arteriolar smooth muscle Drugs 0.000 claims description 9
- 150000004820 halides Chemical class 0.000 claims description 8
- 150000008065 acid anhydrides Chemical class 0.000 claims description 7
- 150000001244 carboxylic acid anhydrides Chemical class 0.000 claims description 7
- 230000002363 herbicidal effect Effects 0.000 claims description 7
- 239000013543 active substance Substances 0.000 claims description 4
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 4
- 239000004606 Fillers/Extenders Substances 0.000 claims description 3
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims description 2
- 125000001309 chloro group Chemical group Cl* 0.000 claims description 2
- 150000003931 anilides Chemical class 0.000 claims 1
- 125000002490 anilino group Chemical class [H]N(*)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 claims 1
- 239000000126 substance Substances 0.000 abstract description 29
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 24
- 238000006243 chemical reaction Methods 0.000 description 20
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 16
- 238000002844 melting Methods 0.000 description 16
- 230000008018 melting Effects 0.000 description 16
- 239000007858 starting material Substances 0.000 description 16
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 16
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 15
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 14
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 14
- 239000004480 active ingredient Substances 0.000 description 13
- 150000003254 radicals Chemical class 0.000 description 13
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 12
- 238000001953 recrystallisation Methods 0.000 description 12
- 239000002904 solvent Substances 0.000 description 12
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 11
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 10
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 9
- 0 CC1=CC(*=C)=NC(*C(CC2)=C(*3)C(*)C3C2NC(C(*)(*)*)=O)*1 Chemical compound CC1=CC(*=C)=NC(*C(CC2)=C(*3)C(*)C3C2NC(C(*)(*)*)=O)*1 0.000 description 9
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 9
- 239000002689 soil Substances 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- PLIKAWJENQZMHA-UHFFFAOYSA-N 4-aminophenol Chemical compound NC1=CC=C(O)C=C1 PLIKAWJENQZMHA-UHFFFAOYSA-N 0.000 description 8
- 239000003995 emulsifying agent Substances 0.000 description 8
- 239000008187 granular material Substances 0.000 description 8
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 7
- 238000009472 formulation Methods 0.000 description 7
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 7
- RZVPFDOTMFYQHR-UHFFFAOYSA-N 2-chloro-4,6-dimethylpyrimidine Chemical compound CC1=CC(C)=NC(Cl)=N1 RZVPFDOTMFYQHR-UHFFFAOYSA-N 0.000 description 6
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 239000003960 organic solvent Substances 0.000 description 6
- 150000002989 phenols Chemical class 0.000 description 6
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 6
- 238000012545 processing Methods 0.000 description 6
- 150000003839 salts Chemical class 0.000 description 6
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 6
- 241000209117 Panicum Species 0.000 description 5
- 235000006443 Panicum miliaceum subsp. miliaceum Nutrition 0.000 description 5
- 235000009037 Panicum miliaceum subsp. ruderale Nutrition 0.000 description 5
- 150000001448 anilines Chemical class 0.000 description 5
- 239000013078 crystal Substances 0.000 description 5
- 150000002170 ethers Chemical class 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 5
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 4
- 235000005781 Avena Nutrition 0.000 description 4
- 244000075850 Avena orientalis Species 0.000 description 4
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 4
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- 235000021506 Ipomoea Nutrition 0.000 description 4
- 241000207783 Ipomoea Species 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 4
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 4
- 241000209072 Sorghum Species 0.000 description 4
- 235000011684 Sorghum saccharatum Nutrition 0.000 description 4
- 239000000370 acceptor Substances 0.000 description 4
- 229910052783 alkali metal Inorganic materials 0.000 description 4
- 150000001340 alkali metals Chemical class 0.000 description 4
- 229910000287 alkaline earth metal oxide Inorganic materials 0.000 description 4
- 239000012141 concentrate Substances 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 239000011541 reaction mixture Substances 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 238000003756 stirring Methods 0.000 description 4
- 230000009885 systemic effect Effects 0.000 description 4
- 239000008096 xylene Substances 0.000 description 4
- JVSFQJZRHXAUGT-UHFFFAOYSA-N 2,2-dimethylpropanoyl chloride Chemical compound CC(C)(C)C(Cl)=O JVSFQJZRHXAUGT-UHFFFAOYSA-N 0.000 description 3
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- ASZGGIFGRFMKAF-UHFFFAOYSA-N 4,6-dimethyl-2-phenoxypyrimidine Chemical compound CC1=CC(C)=NC(OC=2C=CC=CC=2)=N1 ASZGGIFGRFMKAF-UHFFFAOYSA-N 0.000 description 3
- TWQPYTHNDVDCLI-UHFFFAOYSA-N 4-(4,6-dimethylpyrimidin-2-yl)oxyaniline Chemical compound CC1=CC(C)=NC(OC=2C=CC(N)=CC=2)=N1 TWQPYTHNDVDCLI-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 241000192043 Echinochloa Species 0.000 description 3
- 241000748465 Galinsoga Species 0.000 description 3
- 241001101998 Galium Species 0.000 description 3
- 241000209510 Liliopsida Species 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- 241000209094 Oryza Species 0.000 description 3
- 241000209048 Poa Species 0.000 description 3
- XBDQKXXYIPTUBI-UHFFFAOYSA-N Propionic acid Substances CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 3
- 241000220261 Sinapis Species 0.000 description 3
- 235000002634 Solanum Nutrition 0.000 description 3
- 241000207763 Solanum Species 0.000 description 3
- 240000006694 Stellaria media Species 0.000 description 3
- 241000209140 Triticum Species 0.000 description 3
- 235000021307 Triticum Nutrition 0.000 description 3
- 240000008042 Zea mays Species 0.000 description 3
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 125000002877 alkyl aryl group Chemical group 0.000 description 3
- BRPQOXSCLDDYGP-UHFFFAOYSA-N calcium oxide Chemical compound [O-2].[Ca+2] BRPQOXSCLDDYGP-UHFFFAOYSA-N 0.000 description 3
- 239000000292 calcium oxide Substances 0.000 description 3
- ODINCKMPIJJUCX-UHFFFAOYSA-N calcium oxide Inorganic materials [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 241001233957 eudicotyledons Species 0.000 description 3
- 239000000417 fungicide Substances 0.000 description 3
- 229930195733 hydrocarbon Natural products 0.000 description 3
- 150000002430 hydrocarbons Chemical class 0.000 description 3
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 3
- 239000002798 polar solvent Substances 0.000 description 3
- 229920000151 polyglycol Polymers 0.000 description 3
- 239000010695 polyglycol Substances 0.000 description 3
- 229910000027 potassium carbonate Inorganic materials 0.000 description 3
- 239000011435 rock Substances 0.000 description 3
- 229910000029 sodium carbonate Inorganic materials 0.000 description 3
- HXJUTPCZVOIRIF-UHFFFAOYSA-N sulfolane Chemical compound O=S1(=O)CCCC1 HXJUTPCZVOIRIF-UHFFFAOYSA-N 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- CXWXQJXEFPUFDZ-UHFFFAOYSA-N tetralin Chemical compound C1=CC=C2CCCCC2=C1 CXWXQJXEFPUFDZ-UHFFFAOYSA-N 0.000 description 3
- RFFLAFLAYFXFSW-UHFFFAOYSA-N 1,2-dichlorobenzene Chemical compound ClC1=CC=CC=C1Cl RFFLAFLAYFXFSW-UHFFFAOYSA-N 0.000 description 2
- BQHWATVEWGHHHF-UHFFFAOYSA-N 2,2-dimethyl-3-phenylpropanoic acid Chemical compound OC(=O)C(C)(C)CC1=CC=CC=C1 BQHWATVEWGHHHF-UHFFFAOYSA-N 0.000 description 2
- BTLKROSJMNFSQZ-UHFFFAOYSA-N 2,4-dichloro-6-methylpyrimidine Chemical compound CC1=CC(Cl)=NC(Cl)=N1 BTLKROSJMNFSQZ-UHFFFAOYSA-N 0.000 description 2
- GFKKWENTMDBEPJ-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)-2-methylpropanoic acid Chemical compound CC1=CC(Cl)=CC=C1OC(C)(C)C(O)=O GFKKWENTMDBEPJ-UHFFFAOYSA-N 0.000 description 2
- XXSPGBOGLXKMDU-UHFFFAOYSA-N 2-bromo-2-methylpropanoic acid Chemical compound CC(C)(Br)C(O)=O XXSPGBOGLXKMDU-UHFFFAOYSA-N 0.000 description 2
- UZIJEBOLOXOVFY-UHFFFAOYSA-N 2-bromo-4-methylpyrimidine Chemical compound CC1=CC=NC(Br)=N1 UZIJEBOLOXOVFY-UHFFFAOYSA-N 0.000 description 2
- MYCXCBCDXVFXNE-UHFFFAOYSA-N 2-chloro-2-methylpropanoic acid Chemical compound CC(C)(Cl)C(O)=O MYCXCBCDXVFXNE-UHFFFAOYSA-N 0.000 description 2
- PYVIXLOQDVXPSD-UHFFFAOYSA-N 2-chloro-4-(4-chlorophenyl)-6-methylpyrimidine Chemical compound ClC1=NC(C)=CC(C=2C=CC(Cl)=CC=2)=N1 PYVIXLOQDVXPSD-UHFFFAOYSA-N 0.000 description 2
- HBGCZKKCKKDPOI-UHFFFAOYSA-N 2-chloro-4-methoxy-6-methylpyrimidine Chemical compound COC1=CC(C)=NC(Cl)=N1 HBGCZKKCKKDPOI-UHFFFAOYSA-N 0.000 description 2
- URADHXDRUDGYPQ-UHFFFAOYSA-N 2-chloro-4-methyl-6-(4-methylphenyl)pyrimidine Chemical compound C1=CC(C)=CC=C1C1=CC(C)=NC(Cl)=N1 URADHXDRUDGYPQ-UHFFFAOYSA-N 0.000 description 2
- MHJYYMZHEQUQLJ-UHFFFAOYSA-N 2-chloro-4-methyl-6-(trifluoromethyl)pyrimidine Chemical compound CC1=CC(C(F)(F)F)=NC(Cl)=N1 MHJYYMZHEQUQLJ-UHFFFAOYSA-N 0.000 description 2
- ZRLKMSQHBYUETA-UHFFFAOYSA-N 2-chloro-4-methyl-6-[3-(trifluoromethyl)phenyl]pyrimidine Chemical compound ClC1=NC(C)=CC(C=2C=C(C=CC=2)C(F)(F)F)=N1 ZRLKMSQHBYUETA-UHFFFAOYSA-N 0.000 description 2
- GLWCUQHONLZTOE-UHFFFAOYSA-N 2-chloro-4-methyl-6-methylsulfanylpyrimidine Chemical compound CSC1=CC(C)=NC(Cl)=N1 GLWCUQHONLZTOE-UHFFFAOYSA-N 0.000 description 2
- XLNXAKAMPPMGDS-UHFFFAOYSA-N 2-chloro-4-methyl-6-phenylpyrimidine Chemical compound ClC1=NC(C)=CC(C=2C=CC=CC=2)=N1 XLNXAKAMPPMGDS-UHFFFAOYSA-N 0.000 description 2
- URSQXNVFZREMFE-UHFFFAOYSA-N 2-chloro-4-methyl-6-propan-2-ylpyrimidine Chemical compound CC(C)C1=CC(C)=NC(Cl)=N1 URSQXNVFZREMFE-UHFFFAOYSA-N 0.000 description 2
- COCZTGBQCHPXSZ-UHFFFAOYSA-N 2-chloro-4-methyl-6-propylpyrimidine Chemical compound CCCC1=CC(C)=NC(Cl)=N1 COCZTGBQCHPXSZ-UHFFFAOYSA-N 0.000 description 2
- BHAKRVSCGILCEW-UHFFFAOYSA-N 2-chloro-4-methylpyrimidine Chemical compound CC1=CC=NC(Cl)=N1 BHAKRVSCGILCEW-UHFFFAOYSA-N 0.000 description 2
- LHJPKLWGGMAUAN-UHFFFAOYSA-N 2-ethyl-2-methyl-butanoic acid Chemical compound CCC(C)(CC)C(O)=O LHJPKLWGGMAUAN-UHFFFAOYSA-N 0.000 description 2
- JQWBTFGGAKGQPA-UHFFFAOYSA-N 2-fluoro-4,6-dimethylpyrimidine Chemical compound CC1=CC(C)=NC(F)=N1 JQWBTFGGAKGQPA-UHFFFAOYSA-N 0.000 description 2
- BKBZFJRHYSCZQA-UHFFFAOYSA-N 2-methoxy-2-methylpropanoic acid Chemical compound COC(C)(C)C(O)=O BKBZFJRHYSCZQA-UHFFFAOYSA-N 0.000 description 2
- CDFNSDKSYUXWQO-UHFFFAOYSA-N 2-methyl-2-methylsulfanylpropanoic acid Chemical compound CSC(C)(C)C(O)=O CDFNSDKSYUXWQO-UHFFFAOYSA-N 0.000 description 2
- LVWJRYVSTKPXMQ-UHFFFAOYSA-N 2-methyl-2-methylsulfonylpropanoic acid Chemical compound OC(=O)C(C)(C)S(C)(=O)=O LVWJRYVSTKPXMQ-UHFFFAOYSA-N 0.000 description 2
- ILPUOPPYSQEBNJ-UHFFFAOYSA-N 2-methyl-2-phenoxypropanoic acid Chemical compound OC(=O)C(C)(C)OC1=CC=CC=C1 ILPUOPPYSQEBNJ-UHFFFAOYSA-N 0.000 description 2
- WLAMNBDJUVNPJU-UHFFFAOYSA-N 2-methylbutyric acid Chemical compound CCC(C)C(O)=O WLAMNBDJUVNPJU-UHFFFAOYSA-N 0.000 description 2
- NYDAEBWSGUNHIF-UHFFFAOYSA-N 3-(4-methoxyphenyl)-2,2-dimethylpropanoic acid Chemical compound COC1=CC=C(CC(C)(C)C(O)=O)C=C1 NYDAEBWSGUNHIF-UHFFFAOYSA-N 0.000 description 2
- SYBYTAAJFKOIEJ-UHFFFAOYSA-N 3-Methylbutan-2-one Chemical compound CC(C)C(C)=O SYBYTAAJFKOIEJ-UHFFFAOYSA-N 0.000 description 2
- IFJDPIPLLBRMGH-UHFFFAOYSA-N 4,6-dimethyl-2-(4-nitrophenoxy)pyrimidine Chemical compound CC1=CC(C)=NC(OC=2C=CC(=CC=2)[N+]([O-])=O)=N1 IFJDPIPLLBRMGH-UHFFFAOYSA-N 0.000 description 2
- RVLKXVCJBJCTCE-UHFFFAOYSA-N 4-amino-2,5-dichlorophenol Chemical compound NC1=CC(Cl)=C(O)C=C1Cl RVLKXVCJBJCTCE-UHFFFAOYSA-N 0.000 description 2
- QPVZPORQYZKIRM-UHFFFAOYSA-N 4-amino-2-chloro-5-methylphenol Chemical compound CC1=CC(O)=C(Cl)C=C1N QPVZPORQYZKIRM-UHFFFAOYSA-N 0.000 description 2
- ZYAMARVMEHHNHM-UHFFFAOYSA-N 4-amino-2-chlorobenzenethiol Chemical compound NC1=CC=C(S)C(Cl)=C1 ZYAMARVMEHHNHM-UHFFFAOYSA-N 0.000 description 2
- ZYZQSCWSPFLAFM-UHFFFAOYSA-N 4-amino-2-chlorophenol Chemical compound NC1=CC=C(O)C(Cl)=C1 ZYZQSCWSPFLAFM-UHFFFAOYSA-N 0.000 description 2
- RESJHCCPSZNMBZ-UHFFFAOYSA-N 4-amino-2-methylbenzenethiol Chemical compound CC1=CC(N)=CC=C1S RESJHCCPSZNMBZ-UHFFFAOYSA-N 0.000 description 2
- HDGMAACKJSBLMW-UHFFFAOYSA-N 4-amino-2-methylphenol Chemical compound CC1=CC(N)=CC=C1O HDGMAACKJSBLMW-UHFFFAOYSA-N 0.000 description 2
- NXFCPFFXRRLFHV-UHFFFAOYSA-N 4-amino-3-chlorobenzenethiol Chemical compound NC1=CC=C(S)C=C1Cl NXFCPFFXRRLFHV-UHFFFAOYSA-N 0.000 description 2
- PNLPXABQLXSICH-UHFFFAOYSA-N 4-amino-3-chlorophenol Chemical compound NC1=CC=C(O)C=C1Cl PNLPXABQLXSICH-UHFFFAOYSA-N 0.000 description 2
- CRRYCJCMAGAZIH-UHFFFAOYSA-N 4-amino-3-methylbenzenethiol Chemical compound CC1=CC(S)=CC=C1N CRRYCJCMAGAZIH-UHFFFAOYSA-N 0.000 description 2
- WCDSVWRUXWCYFN-UHFFFAOYSA-N 4-aminobenzenethiol Chemical compound NC1=CC=C(S)C=C1 WCDSVWRUXWCYFN-UHFFFAOYSA-N 0.000 description 2
- KGEXISHTCZHGFT-UHFFFAOYSA-N 4-azaniumyl-2,6-dichlorophenolate Chemical compound NC1=CC(Cl)=C(O)C(Cl)=C1 KGEXISHTCZHGFT-UHFFFAOYSA-N 0.000 description 2
- QGNGOGOOPUYKMC-UHFFFAOYSA-N 4-hydroxy-6-methylaniline Chemical compound CC1=CC(O)=CC=C1N QGNGOGOOPUYKMC-UHFFFAOYSA-N 0.000 description 2
- 241000219144 Abutilon Species 0.000 description 2
- 241000209136 Agropyron Species 0.000 description 2
- 241000743339 Agrostis Species 0.000 description 2
- 241000234282 Allium Species 0.000 description 2
- 241000743985 Alopecurus Species 0.000 description 2
- 241000219318 Amaranthus Species 0.000 description 2
- 244000099147 Ananas comosus Species 0.000 description 2
- 241000404028 Anthemis Species 0.000 description 2
- 241001666377 Apera Species 0.000 description 2
- 235000003911 Arachis Nutrition 0.000 description 2
- 244000105624 Arachis hypogaea Species 0.000 description 2
- 235000005340 Asparagus officinalis Nutrition 0.000 description 2
- 241000611157 Brachiaria Species 0.000 description 2
- 241000339490 Brachyachne Species 0.000 description 2
- 235000011331 Brassica Nutrition 0.000 description 2
- 241000219198 Brassica Species 0.000 description 2
- 240000007124 Brassica oleracea Species 0.000 description 2
- 241000209200 Bromus Species 0.000 description 2
- 241000132570 Centaurea Species 0.000 description 2
- 241000219312 Chenopodium Species 0.000 description 2
- 235000000509 Chenopodium ambrosioides Nutrition 0.000 description 2
- 244000098897 Chenopodium botrys Species 0.000 description 2
- 235000005490 Chenopodium botrys Nutrition 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 244000192528 Chrysanthemum parthenium Species 0.000 description 2
- 241000207892 Convolvulus Species 0.000 description 2
- 244000024469 Cucumis prophetarum Species 0.000 description 2
- 235000010071 Cucumis prophetarum Nutrition 0.000 description 2
- 241000219122 Cucurbita Species 0.000 description 2
- 241000234653 Cyperus Species 0.000 description 2
- 241000320605 Dactyloctenium Species 0.000 description 2
- 241000208296 Datura Species 0.000 description 2
- 241000208175 Daucus Species 0.000 description 2
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 2
- 235000017896 Digitaria Nutrition 0.000 description 2
- 241001303487 Digitaria <clam> Species 0.000 description 2
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 2
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 2
- 241000209215 Eleusine Species 0.000 description 2
- 235000007351 Eleusine Nutrition 0.000 description 2
- 244000294661 Emex spinosa Species 0.000 description 2
- 235000006369 Emex spinosa Nutrition 0.000 description 2
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 2
- 241000234642 Festuca Species 0.000 description 2
- 241001290564 Fimbristylis Species 0.000 description 2
- 241000816457 Galeopsis Species 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 235000009438 Gossypium Nutrition 0.000 description 2
- 241000219146 Gossypium Species 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- 241000209219 Hordeum Species 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- 241000208822 Lactuca Species 0.000 description 2
- 241000520028 Lamium Species 0.000 description 2
- 241000801118 Lepidium Species 0.000 description 2
- 241000064140 Lindernia Species 0.000 description 2
- 241000208204 Linum Species 0.000 description 2
- 241000209082 Lolium Species 0.000 description 2
- 235000002262 Lycopersicon Nutrition 0.000 description 2
- 241000227653 Lycopersicon Species 0.000 description 2
- 235000017945 Matricaria Nutrition 0.000 description 2
- 235000007232 Matricaria chamomilla Nutrition 0.000 description 2
- 235000003990 Monochoria hastata Nutrition 0.000 description 2
- 240000000178 Monochoria vaginalis Species 0.000 description 2
- 241000721621 Myzus persicae Species 0.000 description 2
- JLTDJTHDQAWBAV-UHFFFAOYSA-N N,N-dimethylaniline Chemical compound CN(C)C1=CC=CC=C1 JLTDJTHDQAWBAV-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- 241000208125 Nicotiana Species 0.000 description 2
- 235000011096 Papaver Nutrition 0.000 description 2
- 240000001090 Papaver somniferum Species 0.000 description 2
- 241000219833 Phaseolus Species 0.000 description 2
- 241000746981 Phleum Species 0.000 description 2
- 241000219843 Pisum Species 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- 241000205407 Polygonum Species 0.000 description 2
- 241000219295 Portulaca Species 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- 239000007868 Raney catalyst Substances 0.000 description 2
- NPXOKRUENSOPAO-UHFFFAOYSA-N Raney nickel Chemical compound [Al].[Ni] NPXOKRUENSOPAO-UHFFFAOYSA-N 0.000 description 2
- 229910000564 Raney nickel Inorganic materials 0.000 description 2
- 241000341978 Rotala Species 0.000 description 2
- 241000209051 Saccharum Species 0.000 description 2
- 240000009132 Sagittaria sagittifolia Species 0.000 description 2
- 241000209056 Secale Species 0.000 description 2
- 241000780602 Senecio Species 0.000 description 2
- 244000275012 Sesbania cannabina Species 0.000 description 2
- 235000005775 Setaria Nutrition 0.000 description 2
- 241000232088 Setaria <nematode> Species 0.000 description 2
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 2
- 244000273618 Sphenoclea zeylanica Species 0.000 description 2
- 235000017967 Sphenoclea zeylanica Nutrition 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 241000219422 Urtica Species 0.000 description 2
- 240000005592 Veronica officinalis Species 0.000 description 2
- 241000219873 Vicia Species 0.000 description 2
- 241000405217 Viola <butterfly> Species 0.000 description 2
- 241001506766 Xanthium Species 0.000 description 2
- 241000209149 Zea Species 0.000 description 2
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 125000002252 acyl group Chemical group 0.000 description 2
- 244000193174 agave Species 0.000 description 2
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 150000008051 alkyl sulfates Chemical class 0.000 description 2
- 150000008052 alkyl sulfonates Chemical class 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- CODNYICXDISAEA-UHFFFAOYSA-N bromine monochloride Chemical compound BrCl CODNYICXDISAEA-UHFFFAOYSA-N 0.000 description 2
- 229910000019 calcium carbonate Inorganic materials 0.000 description 2
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 2
- 150000001735 carboxylic acids Chemical class 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 2
- 235000005822 corn Nutrition 0.000 description 2
- NZNMSOFKMUBTKW-UHFFFAOYSA-N cyclohexanecarboxylic acid Chemical compound OC(=O)C1CCCCC1 NZNMSOFKMUBTKW-UHFFFAOYSA-N 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 2
- USIUVYZYUHIAEV-UHFFFAOYSA-N diphenyl ether Chemical compound C=1C=CC=CC=1OC1=CC=CC=C1 USIUVYZYUHIAEV-UHFFFAOYSA-N 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 239000000975 dye Substances 0.000 description 2
- 235000013399 edible fruits Nutrition 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 239000000706 filtrate Substances 0.000 description 2
- 235000013312 flour Nutrition 0.000 description 2
- 239000003502 gasoline Substances 0.000 description 2
- 150000004678 hydrides Chemical class 0.000 description 2
- 150000004679 hydroxides Chemical class 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 150000002576 ketones Chemical class 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000000395 magnesium oxide Substances 0.000 description 2
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 150000002736 metal compounds Chemical class 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- FSWKNXQMWWGBEJ-UHFFFAOYSA-N n-(4-hydroxyphenyl)-2,2-dimethylpropanamide Chemical compound CC(C)(C)C(=O)NC1=CC=C(O)C=C1 FSWKNXQMWWGBEJ-UHFFFAOYSA-N 0.000 description 2
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 150000002825 nitriles Chemical class 0.000 description 2
- LQNUZADURLCDLV-UHFFFAOYSA-N nitrobenzene Chemical compound [O-][N+](=O)C1=CC=CC=C1 LQNUZADURLCDLV-UHFFFAOYSA-N 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 239000006072 paste Substances 0.000 description 2
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 2
- ODZPKZBBUMBTMG-UHFFFAOYSA-N sodium amide Chemical compound [NH2-].[Na+] ODZPKZBBUMBTMG-UHFFFAOYSA-N 0.000 description 2
- QDRKDTQENPPHOJ-UHFFFAOYSA-N sodium ethoxide Chemical compound [Na+].CC[O-] QDRKDTQENPPHOJ-UHFFFAOYSA-N 0.000 description 2
- 239000012312 sodium hydride Substances 0.000 description 2
- 229910000104 sodium hydride Inorganic materials 0.000 description 2
- 239000012265 solid product Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 description 2
- 229960000278 theophylline Drugs 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- 239000008158 vegetable oil Substances 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- DURPTKYDGMDSBL-UHFFFAOYSA-N 1-butoxybutane Chemical compound CCCCOCCCC DURPTKYDGMDSBL-UHFFFAOYSA-N 0.000 description 1
- ZYVYEJXMYBUCMN-UHFFFAOYSA-N 1-methoxy-2-methylpropane Chemical group COCC(C)C ZYVYEJXMYBUCMN-UHFFFAOYSA-N 0.000 description 1
- VOVPSLMZFGVAIS-UHFFFAOYSA-N 1-methyl-4-propan-2-ylcyclohexane-1-carboxylic acid Chemical compound CC(C)C1CCC(C)(C(O)=O)CC1 VOVPSLMZFGVAIS-UHFFFAOYSA-N 0.000 description 1
- REHQLKUNRPCYEW-UHFFFAOYSA-N 1-methylcyclohexane-1-carboxylic acid Chemical compound OC(=O)C1(C)CCCCC1 REHQLKUNRPCYEW-UHFFFAOYSA-N 0.000 description 1
- MNIBBVOEXUQHFF-UHFFFAOYSA-N 1-methylcyclopentanecarboxylic acid Chemical compound OC(=O)C1(C)CCCC1 MNIBBVOEXUQHFF-UHFFFAOYSA-N 0.000 description 1
- DIZKLZKLNKQFGB-UHFFFAOYSA-N 1-methylcyclopropane-1-carboxylic acid Chemical compound OC(=O)C1(C)CC1 DIZKLZKLNKQFGB-UHFFFAOYSA-N 0.000 description 1
- 150000008319 1H-pyrimidin-2-ones Chemical class 0.000 description 1
- LTBPRPATSZONGJ-UHFFFAOYSA-N 2-(1-methylcyclohexyl)acetic acid Chemical compound OC(=O)CC1(C)CCCCC1 LTBPRPATSZONGJ-UHFFFAOYSA-N 0.000 description 1
- ZHQAIBYWBFMHHQ-UHFFFAOYSA-N 2-(1-methylcyclopentyl)acetic acid Chemical compound OC(=O)CC1(C)CCCC1 ZHQAIBYWBFMHHQ-UHFFFAOYSA-N 0.000 description 1
- AOPATQAZIRXNOX-UHFFFAOYSA-N 2-(1-methylcyclopropyl)acetic acid Chemical compound OC(=O)CC1(C)CC1 AOPATQAZIRXNOX-UHFFFAOYSA-N 0.000 description 1
- OVSKIKFHRZPJSS-DOMIDYPGSA-N 2-(2,4-dichlorophenoxy)acetic acid Chemical compound OC(=O)[14CH2]OC1=CC=C(Cl)C=C1Cl OVSKIKFHRZPJSS-DOMIDYPGSA-N 0.000 description 1
- MZHCENGPTKEIGP-UHFFFAOYSA-N 2-(2,4-dichlorophenoxy)propanoic acid Chemical compound OC(=O)C(C)OC1=CC=C(Cl)C=C1Cl MZHCENGPTKEIGP-UHFFFAOYSA-N 0.000 description 1
- WNTGYJSOUMFZEP-UHFFFAOYSA-N 2-(4-chloro-2-methylphenoxy)propanoic acid Chemical compound OC(=O)C(C)OC1=CC=C(Cl)C=C1C WNTGYJSOUMFZEP-UHFFFAOYSA-N 0.000 description 1
- 150000005006 2-aminopyrimidines Chemical class 0.000 description 1
- KVVDRQDTODKIJD-UHFFFAOYSA-N 2-cyclopropylacetic acid Chemical compound OC(=O)CC1CC1 KVVDRQDTODKIJD-UHFFFAOYSA-N 0.000 description 1
- XJOFPJZWSSQZMN-UHFFFAOYSA-N 2-methyl-2-[3-(trifluoromethyl)phenyl]propanoic acid Chemical compound OC(=O)C(C)(C)C1=CC=CC(C(F)(F)F)=C1 XJOFPJZWSSQZMN-UHFFFAOYSA-N 0.000 description 1
- YYEROYLAYAVZNW-UHFFFAOYSA-N 2-methyl-2-phenylpropanoic acid Chemical compound OC(=O)C(C)(C)C1=CC=CC=C1 YYEROYLAYAVZNW-UHFFFAOYSA-N 0.000 description 1
- UPMXNNIRAGDFEH-UHFFFAOYSA-N 3,5-dibromo-4-hydroxybenzonitrile Chemical compound OC1=C(Br)C=C(C#N)C=C1Br UPMXNNIRAGDFEH-UHFFFAOYSA-N 0.000 description 1
- YBJGQSNSAWZZHL-UHFFFAOYSA-N 3-chloro-2,2-dimethylpropanoic acid Chemical compound ClCC(C)(C)C(O)=O YBJGQSNSAWZZHL-UHFFFAOYSA-N 0.000 description 1
- XWSSUYOEOWLFEI-UHFFFAOYSA-N 3-phenylpyridazine Chemical compound C1=CC=CC=C1C1=CC=CN=N1 XWSSUYOEOWLFEI-UHFFFAOYSA-N 0.000 description 1
- FPLNYQRUEFPJPX-UHFFFAOYSA-N 4-(4,6-dimethylpyrimidin-2-yl)sulfanylaniline Chemical compound CC1=CC(C)=NC(SC=2C=CC(N)=CC=2)=N1 FPLNYQRUEFPJPX-UHFFFAOYSA-N 0.000 description 1
- CSDQQAQKBAQLLE-UHFFFAOYSA-N 4-(4-chlorophenyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine Chemical compound C1=CC(Cl)=CC=C1C1C(C=CS2)=C2CCN1 CSDQQAQKBAQLLE-UHFFFAOYSA-N 0.000 description 1
- ADZSGNDOZREKJK-UHFFFAOYSA-N 4-amino-6-tert-butyl-3-ethylsulfanyl-1,2,4-triazin-5-one Chemical compound CCSC1=NN=C(C(C)(C)C)C(=O)N1N ADZSGNDOZREKJK-UHFFFAOYSA-N 0.000 description 1
- BTJIUGUIPKRLHP-UHFFFAOYSA-N 4-nitrophenol Chemical compound OC1=CC=C([N+]([O-])=O)C=C1 BTJIUGUIPKRLHP-UHFFFAOYSA-N 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- RGCKGOZRHPZPFP-UHFFFAOYSA-N Alizarin Natural products C1=CC=C2C(=O)C3=C(O)C(O)=CC=C3C(=O)C2=C1 RGCKGOZRHPZPFP-UHFFFAOYSA-N 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N Alumina Chemical compound [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 235000007119 Ananas comosus Nutrition 0.000 description 1
- 241000235349 Ascomycota Species 0.000 description 1
- 241000221198 Basidiomycota Species 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 241001465180 Botrytis Species 0.000 description 1
- 235000011303 Brassica alboglabra Nutrition 0.000 description 1
- 235000011302 Brassica oleracea Nutrition 0.000 description 1
- 235000003899 Brassica oleracea var acephala Nutrition 0.000 description 1
- 235000011301 Brassica oleracea var capitata Nutrition 0.000 description 1
- 235000001169 Brassica oleracea var oleracea Nutrition 0.000 description 1
- BMTAFVWTTFSTOG-UHFFFAOYSA-N Butylate Chemical compound CCSC(=O)N(CC(C)C)CC(C)C BMTAFVWTTFSTOG-UHFFFAOYSA-N 0.000 description 1
- XIPFMBOWZXULIA-UHFFFAOYSA-N CC(C)(C)C(N)=O Chemical compound CC(C)(C)C(N)=O XIPFMBOWZXULIA-UHFFFAOYSA-N 0.000 description 1
- HICJECYUZNPQGG-KIYNQFGBSA-N CC(CCN1C)[C@@H](C)C=C1Sc(cc1)ccc1N Chemical compound CC(CCN1C)[C@@H](C)C=C1Sc(cc1)ccc1N HICJECYUZNPQGG-KIYNQFGBSA-N 0.000 description 1
- KBFYNCAJEGTMFI-UHFFFAOYSA-N CC1(CCC(CC1)C(C)C)CC(=O)O Chemical compound CC1(CCC(CC1)C(C)C)CC(=O)O KBFYNCAJEGTMFI-UHFFFAOYSA-N 0.000 description 1
- QJXNCDKICRYINC-UHFFFAOYSA-N CC1NC(OCc(cc2)ccc2[N+]([O-])=O)=NC(C)=C1 Chemical compound CC1NC(OCc(cc2)ccc2[N+]([O-])=O)=NC(C)=C1 QJXNCDKICRYINC-UHFFFAOYSA-N 0.000 description 1
- FEIHCAFTYDPAHR-UHFFFAOYSA-N CNc(cc1)ccc1S Chemical compound CNc(cc1)ccc1S FEIHCAFTYDPAHR-UHFFFAOYSA-N 0.000 description 1
- 229910021532 Calcite Inorganic materials 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 241000320316 Carduus Species 0.000 description 1
- HCATUIMBJIDQDT-UHFFFAOYSA-N Cc1nc(C)nc(C)c1 Chemical compound Cc1nc(C)nc(C)c1 HCATUIMBJIDQDT-UHFFFAOYSA-N 0.000 description 1
- 241000760356 Chytridiomycetes Species 0.000 description 1
- 241000132536 Cirsium Species 0.000 description 1
- 241000207199 Citrus Species 0.000 description 1
- 244000060011 Cocos nucifera Species 0.000 description 1
- 235000013162 Cocos nucifera Nutrition 0.000 description 1
- 240000007154 Coffea arabica Species 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- NDUPDOJHUQKPAG-UHFFFAOYSA-N Dalapon Chemical compound CC(Cl)(Cl)C(O)=O NDUPDOJHUQKPAG-UHFFFAOYSA-N 0.000 description 1
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical class S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 description 1
- 235000001950 Elaeis guineensis Nutrition 0.000 description 1
- 244000127993 Elaeis melanococca Species 0.000 description 1
- 241000202829 Eleocharis Species 0.000 description 1
- CWYNVVGOOAEACU-UHFFFAOYSA-N Fe2+ Chemical class [Fe+2] CWYNVVGOOAEACU-UHFFFAOYSA-N 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 241001327265 Ischaemum Species 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- 241001330975 Magnaporthe oryzae Species 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- RRVIAQKBTUQODI-UHFFFAOYSA-N Methabenzthiazuron Chemical compound C1=CC=C2SC(N(C)C(=O)NC)=NC2=C1 RRVIAQKBTUQODI-UHFFFAOYSA-N 0.000 description 1
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 1
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- 240000005561 Musa balbisiana Species 0.000 description 1
- 235000018290 Musa x paradisiaca Nutrition 0.000 description 1
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 1
- PAFZNILMFXTMIY-UHFFFAOYSA-N NC1CCCCC1 Chemical compound NC1CCCCC1 PAFZNILMFXTMIY-UHFFFAOYSA-N 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- IOVCWXUNBOPUCH-UHFFFAOYSA-N Nitrous acid Chemical compound ON=O IOVCWXUNBOPUCH-UHFFFAOYSA-N 0.000 description 1
- 241000233654 Oomycetes Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 241001268782 Paspalum dilatatum Species 0.000 description 1
- 241001503460 Plasmodiophorida Species 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 241000490453 Rorippa Species 0.000 description 1
- 241000202758 Scirpus Species 0.000 description 1
- 239000004113 Sepiolite Substances 0.000 description 1
- 241000488874 Sonchus Species 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 244000269722 Thea sinensis Species 0.000 description 1
- 244000299461 Theobroma cacao Species 0.000 description 1
- 235000009470 Theobroma cacao Nutrition 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 1
- WHKUVVPPKQRRBV-UHFFFAOYSA-N Trasan Chemical compound CC1=CC(Cl)=CC=C1OCC(O)=O WHKUVVPPKQRRBV-UHFFFAOYSA-N 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- 241000221577 Uromyces appendiculatus Species 0.000 description 1
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical class ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 1
- 241000607479 Yersinia pestis Species 0.000 description 1
- 235000016383 Zea mays subsp huehuetenangensis Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 230000000895 acaricidal effect Effects 0.000 description 1
- 239000000642 acaricide Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- HFVAFDPGUJEFBQ-UHFFFAOYSA-M alizarin red S Chemical compound [Na+].O=C1C2=CC=CC=C2C(=O)C2=C1C=C(S([O-])(=O)=O)C(O)=C2O HFVAFDPGUJEFBQ-UHFFFAOYSA-M 0.000 description 1
- 229910000272 alkali metal oxide Inorganic materials 0.000 description 1
- 150000008055 alkyl aryl sulfonates Chemical class 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- ZRYCZAWRXHAAPZ-UHFFFAOYSA-N alpha,alpha-dimethyl valeric acid Chemical compound CCCC(C)(C)C(O)=O ZRYCZAWRXHAAPZ-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 125000005228 aryl sulfonate group Chemical group 0.000 description 1
- MXWJVTOOROXGIU-UHFFFAOYSA-N atrazine Chemical compound CCNC1=NC(Cl)=NC(NC(C)C)=N1 MXWJVTOOROXGIU-UHFFFAOYSA-N 0.000 description 1
- 229960000892 attapulgite Drugs 0.000 description 1
- 235000021028 berry Nutrition 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 150000001733 carboxylic acid esters Chemical class 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 238000009903 catalytic hydrogenation reaction Methods 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 150000008422 chlorobenzenes Chemical class 0.000 description 1
- JXCGFZXSOMJFOA-UHFFFAOYSA-N chlorotoluron Chemical compound CN(C)C(=O)NC1=CC=C(C)C(Cl)=C1 JXCGFZXSOMJFOA-UHFFFAOYSA-N 0.000 description 1
- 235000020971 citrus fruits Nutrition 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 235000016213 coffee Nutrition 0.000 description 1
- 235000013353 coffee beverage Nutrition 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- VZFUCHSFHOYXIS-UHFFFAOYSA-N cycloheptane carboxylic acid Natural products OC(=O)C1CCCCCC1 VZFUCHSFHOYXIS-UHFFFAOYSA-N 0.000 description 1
- LJOODBDWMQKMFB-UHFFFAOYSA-N cyclohexylacetic acid Chemical compound OC(=O)CC1CCCCC1 LJOODBDWMQKMFB-UHFFFAOYSA-N 0.000 description 1
- VSLSUBGNFPEGKU-UHFFFAOYSA-N cyclopentanecarboxylic acid 2,2-dichloro-1-methylcyclopropane-1-carboxylic acid Chemical compound OC(=O)C1CCCC1.OC(=O)C1(C)CC1(Cl)Cl VSLSUBGNFPEGKU-UHFFFAOYSA-N 0.000 description 1
- YMGUBTXCNDTFJI-UHFFFAOYSA-N cyclopropanecarboxylic acid Chemical compound OC(=O)C1CC1 YMGUBTXCNDTFJI-UHFFFAOYSA-N 0.000 description 1
- 239000002837 defoliant Substances 0.000 description 1
- 239000002274 desiccant Substances 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 239000010459 dolomite Substances 0.000 description 1
- 229910000514 dolomite Inorganic materials 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- VUWPFWPXBJVSQO-UHFFFAOYSA-N ethyl hydrogen carbonate;hydrochloride Chemical compound Cl.CCOC(O)=O VUWPFWPXBJVSQO-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 150000008282 halocarbons Chemical class 0.000 description 1
- 239000012433 hydrogen halide Substances 0.000 description 1
- 229910000039 hydrogen halide Inorganic materials 0.000 description 1
- 229910000037 hydrogen sulfide Inorganic materials 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 239000001023 inorganic pigment Substances 0.000 description 1
- 230000000749 insecticidal effect Effects 0.000 description 1
- 239000002917 insecticide Substances 0.000 description 1
- NRXQIUSYPAHGNM-UHFFFAOYSA-N ioxynil Chemical compound OC1=C(I)C=C(C#N)C=C1I NRXQIUSYPAHGNM-UHFFFAOYSA-N 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- LSACYLWPPQLVSM-UHFFFAOYSA-N isobutyric acid anhydride Chemical compound CC(C)C(=O)OC(=O)C(C)C LSACYLWPPQLVSM-UHFFFAOYSA-N 0.000 description 1
- PUIYMUZLKQOUOZ-UHFFFAOYSA-N isoproturon Chemical compound CC(C)C1=CC=C(NC(=O)N(C)C)C=C1 PUIYMUZLKQOUOZ-UHFFFAOYSA-N 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229920005610 lignin Polymers 0.000 description 1
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 1
- 235000009973 maize Nutrition 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical class [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 239000004579 marble Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- VHCNQEUWZYOAEV-UHFFFAOYSA-N metamitron Chemical compound O=C1N(N)C(C)=NN=C1C1=CC=CC=C1 VHCNQEUWZYOAEV-UHFFFAOYSA-N 0.000 description 1
- FOXFZRUHNHCZPX-UHFFFAOYSA-N metribuzin Chemical compound CSC1=NN=C(C(C)(C)C)C(=O)N1N FOXFZRUHNHCZPX-UHFFFAOYSA-N 0.000 description 1
- 230000003641 microbiacidal effect Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- AOTVFPGINKQSKR-UHFFFAOYSA-N n-(2-chloro-4-hydroxyphenyl)-2,2-dimethylpropanamide Chemical compound CC(C)(C)C(=O)NC1=CC=C(O)C=C1Cl AOTVFPGINKQSKR-UHFFFAOYSA-N 0.000 description 1
- KEFHQWSSFNXBHQ-UHFFFAOYSA-N n-[2-chloro-4-(4,6-dimethylpyrimidin-2-yl)oxyphenyl]-2,2-dimethylpropanamide Chemical compound CC1=CC(C)=NC(OC=2C=C(Cl)C(NC(=O)C(C)(C)C)=CC=2)=N1 KEFHQWSSFNXBHQ-UHFFFAOYSA-N 0.000 description 1
- FNVHBFLQGGNMQZ-UHFFFAOYSA-N n-[4-(4,6-dimethylpyrimidin-2-yl)oxyphenyl]-2,2-dimethylpropanamide Chemical compound CC1=CC(C)=NC(OC=2C=CC(NC(=O)C(C)(C)C)=CC=2)=N1 FNVHBFLQGGNMQZ-UHFFFAOYSA-N 0.000 description 1
- IOTJIJDDRVFCOV-UHFFFAOYSA-N n-[4-(4,6-dimethylpyrimidin-2-yl)sulfanylphenyl]-2-methoxy-2-methylpropanamide Chemical compound C1=CC(NC(=O)C(C)(C)OC)=CC=C1SC1=NC(C)=CC(C)=N1 IOTJIJDDRVFCOV-UHFFFAOYSA-N 0.000 description 1
- 229940042880 natural phospholipid Drugs 0.000 description 1
- 239000005645 nematicide Substances 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229910052755 nonmetal Chemical class 0.000 description 1
- 235000014571 nuts Nutrition 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 239000012074 organic phase Substances 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- 229910052625 palygorskite Inorganic materials 0.000 description 1
- 239000000575 pesticide Substances 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- UHZYTMXLRWXGPK-UHFFFAOYSA-N phosphorus pentachloride Chemical compound ClP(Cl)(Cl)(Cl)Cl UHZYTMXLRWXGPK-UHFFFAOYSA-N 0.000 description 1
- 239000001007 phthalocyanine dye Substances 0.000 description 1
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 1
- 239000004476 plant protection product Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 1
- LFULEKSKNZEWOE-UHFFFAOYSA-N propanil Chemical compound CCC(=O)NC1=CC=C(Cl)C(Cl)=C1 LFULEKSKNZEWOE-UHFFFAOYSA-N 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- FVSKHRXBFJPNKK-UHFFFAOYSA-N propionitrile Chemical compound CCC#N FVSKHRXBFJPNKK-UHFFFAOYSA-N 0.000 description 1
- 239000003531 protein hydrolysate Substances 0.000 description 1
- 239000008262 pumice Substances 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 239000013558 reference substance Substances 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 229910052624 sepiolite Inorganic materials 0.000 description 1
- 235000019355 sepiolite Nutrition 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 238000009331 sowing Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000013616 tea Nutrition 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- UAXOELSVPTZZQG-UHFFFAOYSA-N tiglic acid Natural products CC(C)=C(C)C(O)=O UAXOELSVPTZZQG-UHFFFAOYSA-N 0.000 description 1
- 239000011135 tin Substances 0.000 description 1
- 229910052718 tin Inorganic materials 0.000 description 1
- IUTCEZPPWBHGIX-UHFFFAOYSA-N tin(2+) Chemical class [Sn+2] IUTCEZPPWBHGIX-UHFFFAOYSA-N 0.000 description 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 1
- 125000003944 tolyl group Chemical group 0.000 description 1
- YNJBWRMUSHSURL-UHFFFAOYSA-N trichloroacetic acid Chemical compound OC(=O)C(Cl)(Cl)Cl YNJBWRMUSHSURL-UHFFFAOYSA-N 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 235000014101 wine Nutrition 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/46—Two or more oxygen, sulphur or nitrogen atoms
- C07D239/58—Two sulfur atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/54—1,3-Diazines; Hydrogenated 1,3-diazines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/32—One oxygen, sulfur or nitrogen atom
- C07D239/34—One oxygen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/32—One oxygen, sulfur or nitrogen atom
- C07D239/38—One sulfur atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/46—Two or more oxygen, sulphur or nitrogen atoms
- C07D239/52—Two oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/46—Two or more oxygen, sulphur or nitrogen atoms
- C07D239/56—One oxygen atom and one sulfur atom
Definitions
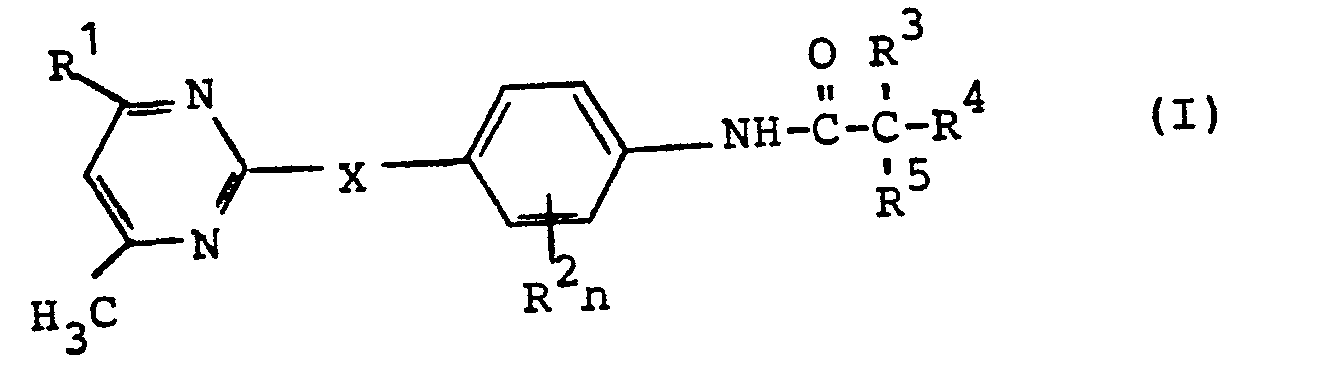
- the present invention relates to new substituted carboxylic acid anilides, several processes for their preparation and their use as herbicides.
- pyrimidinyl-2-ethers and thioethers are suitable as herbicides (cf. JP-OS 9 474/1967, US Pat. Nos. 3, 126, 271 and US Pat. Nos. 3, 250, 775).
- the 2-phenoxy-4,6-dimethyl-pyrimidine and the 2- (4-chloro-benzylmer- capto) -4,6-dimethyl-pyrimidine can be used to control weeds.
- the herbicidal potency of these substances is not always sufficient.
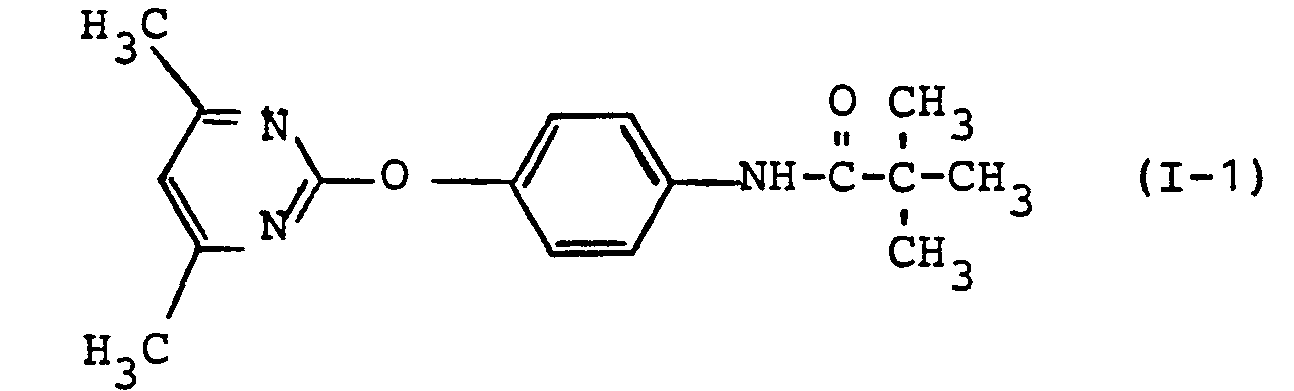
- the substituted carboxylic acid anilides of the formula (I) according to the invention have significantly better herbicidal properties than the constitutionally most similar previously known substances.
- the carboxylic acid anilides of the formula (I) according to the invention can be used much better for weed control than the 2-phenoxy-4,6-dimethyl-pyrimidine, which is a structurally similar previously known active ingredient of the same type of action.
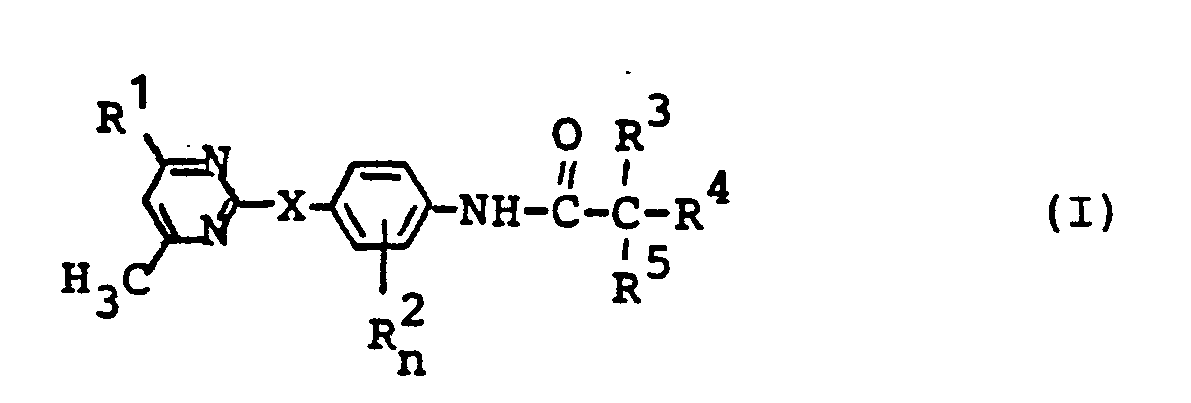
- the substituted carboxylic acid anilides according to the invention are generally defined by the formula (I).
- X stands for oxygen or sulfur.
- the radical R 1 preferably represents hydrogen, fluorine, chlorine, Bromine, straight-chain or branched alkyl having 1 to 4 carbon atoms, trifluoromethyl, phenyl optionally substituted by chlorine, trifluoromethyl and / or methyl, alkoxy having 1 to 4 carbon atoms or alkyl mercapto having 1 to 4 carbon atoms.
- R preferably represents chlorine, bromine or methyl.
- the index n preferably stands for 0 or 1.
- R 3 preferably stands for hydrogen, fluorine, chlorine, bromine, alkyl with 1 to 4 carbon atoms optionally substituted by fluorine, chlorine and / or bromine, optionally with fluorine, chlorine, bromine, trifluoromethyl, Methoxy and / or methyl substituted phenyl, optionally substituted by fluorine, chlorine, bromine, trifluoromethyl, methoxy and / or methyl or for the radicals -OR 6 or -SO -R 6 , where R is preferably for optionally by fluorine, chlorine and / or bromine-substituted alkyl having 1 to 4 carbon atoms or phenyl optionally substituted by fluorine, chlorine, bromine and / or alkyl having 1 to 4 carbon atoms and the index m being 0, 1 or 2.
- R 4 and R 5 independently of one another are preferably fluorine, chlorine, bromine or alkyl having 1 to 4 carbon atoms which is optionally substituted by fluorine, chlorine and / or bromine.
- R 4 and R 5 together with the adjacent carbon atom, preferably represent a saturated or unsaturated carbocyclic ring with 3 to 7 ring carbon atoms which is optionally substituted by fluorine, chlorine and / or alkyl having 1 to 4 carbon atoms.
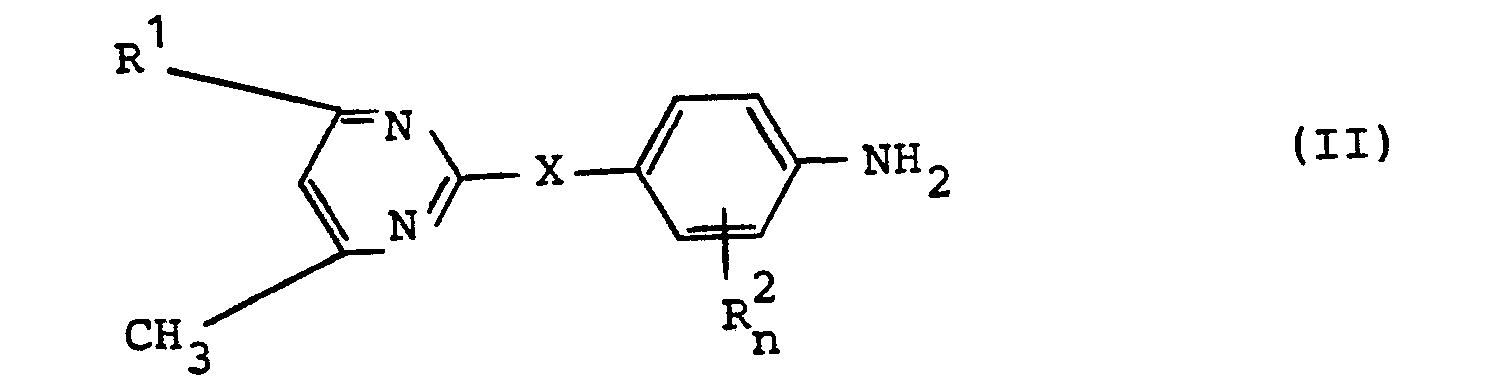
- Formula (II) provides a general definition of the aniline derivatives required as starting materials in process (a) according to the invention.
- R ', R 2 , X and n preferably have those meanings which have already been mentioned preferably in connection with the description of the substances of the formula (I) according to the invention for these radicals or this index.
- Formula (IV) defines the pyrimidine derivatives required as starting materials in process (c) above.
- R 1 preferably has those meanings which have preferably been mentioned for this radical in connection with the description of the substances of the formula (I) according to the invention.
- Hal preferably represents fluorine, chlorine or bromine.
- pyrimidine derivatives of the formula (IV) are known or can be prepared in a simple manner by methods known in principle.
- pyrimidine derivatives of the formula (IV) are obtained by reacting 2-hydroxy-pyrimidine derivatives (dihydro-pyrimidone-2 derivatives) with inorganic acid halides, such as phosphorus oxychloride or phosphorus pentachloride, or else by reacting corresponding 2-amino-pyrimidine derivatives with nitrous acid in the presence of hydrohalic acids.
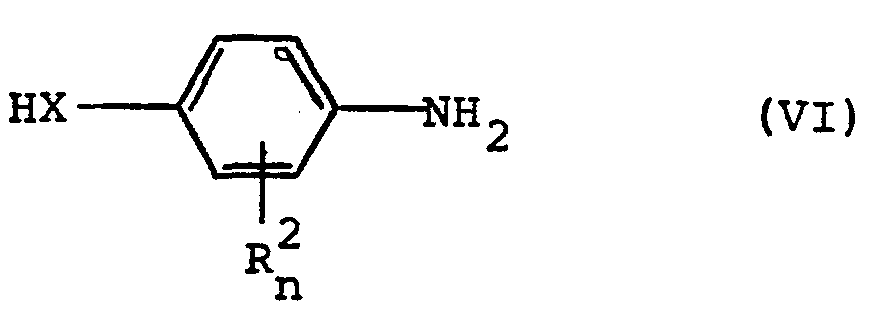
- Formula (VI) defines the 4-amino (thio) phenols which are further required as starting materials in process (c).
- R 2 , X and n preferably have those meanings which have already been mentioned preferably in connection with the description of the substances of the formula (I) according to the invention for these radicals or for this index.
- the 4-amino (thio) phenols of the formula (VI) are known or can be prepared in a simple manner by methods known in principle.
- Alkali and alkaline earth oxides, hydroxides and carbonates such as sodium hydroxide, potassium hydroxide, calcium oxide, sodium carbonate and potassium carbonate are preferably usable, furthermore alkali alcoholates, amides and hydrides such as sodium methylate, sodium ethylate, potassium tert. butylate, sodium amide and sodium hydride.
- All customary inert organic solvents can be used as diluents when carrying out process (c).
- Hydrocarbons such as gasoline, toluene and xylene, ethers such as dioxane, glycol dimethyl ether and diglycol dimethyl ether, furthermore nitriles such as acetonitrile, and also strongly polar solvents such as dimethyl sulfoxide, sulfolane and dimethylformamide are preferred.
- reaction temperatures can be varied within a substantial range when carrying out process (c). In general, temperatures between 0 ° C and 200 ° C, preferably between 50 ° C and 150 ° C.
- reaction according to process (c) is generally carried out under normal pressure.
- Formula (VII) defines the 2- (4-nitro (thio) phenoxy) pyrimidine derivatives required as starting materials in process (d).
- R 1 , R 2 , X and n preferably have those meanings which are already in connection with the description the substances of the formula (I) according to the invention were preferably mentioned for these radicals or for this index.
- Suitable as reducing agents in process (d) are all those substances which are usually used for the reduction of aromatic nitro compounds.
- Elemental metals such as iron, zinc and tin
- metal compounds in lower valence levels such as iron (II) and tin (II) salts
- non-metal compounds in low valence levels such as, for example, salts of hydrogen sulfide, alkali metal sulfites and alkalidithionites.
- the reduction can also be carried out by catalytic hydrogenation with hydrogen in the presence of a catalyst, such as Raney nickel.
- Suitable diluents in process (d) are all customary organic solvents suitable for such reductions.
- the reaction temperatures can be varied within a substantial range. They correspond to the temperatures used in analog reactions.
- the acid halides of the formula (III a) are known or can be prepared in a simple manner by methods known in principle.
- Suitable acid binders for the reaction according to process (a, variant ⁇ ) are all customary acid acceptors.
- Tertiary amines such as triethylamine, pyridine and N, N-dimethylaniline, furthermore alkaline earth metal oxides, such as magnesium and calcium oxide, and also alkali metal and alkaline earth metal carbonates, such as sodium carbonate, potassium carbonate and calcium carbonate, are preferably usable.
- alkaline earth metal oxides such as magnesium and calcium oxide
- alkali metal and alkaline earth metal carbonates such as sodium carbonate, potassium carbonate and calcium carbonate
- Diluents which can be used in process (a, maniadl) according to the invention are all solvents which are inert to acid halides.
- Hydrocarbons such as gasoline, benzene, toluene, xylene and tetralin are preferably usable, furthermore halogenated hydrocarbons such as methylene chloride, chloroform, carbon tetrachloride, chlorobenzene and o-dichlorobenzene, also ketones such as acetone and methyl isopropyl ketone, furthermore ethers such as diethyl ether, tetrahydrofuran and Dioxane, moreover carboxylic acid esters, such as ethyl acetate, and also strongly polar solvents, such as dimethyl sulfoxide and sulfolane. If the hydrolysis stability of the acid halide permits, the reaction can also be carried out in the presence of water.
- reaction temperatures can be varied within a substantial range when carrying out the process (a, avadj). If one works without solvents and acid binders, the procedure is generally such that the components are initially at temperatures between -20 ° C. and +20 ° C can react and then heated to temperatures between 70 and 200 ° C. If one works in the presence of a diluent and an acid binder, the are Reaction temperatures in general between -20 ° C and + 100 ° C, preferably between 0 ° C and 50 ° C.
- the process (a, variant ⁇ ) according to the invention is generally carried out under normal pressure.
- the starting materials of the formulas (II) and (III a) are generally used in approximately equivalent amounts. However, it is also possible to use one or the other component in a larger excess.
- the processing is then carried out using customary methods.
- the general procedure is to remove precipitated salts and to concentrate the remaining reaction mixture by stripping off the diluent. If one works in the presence of water or of water-miscible solvents, the procedure can also be such that the reaction mixture is diluted with water, the resulting mixture is suctioned off or extracted with a water-immiscible organic solvent, the organic phase is washed, concentrated and the the remaining residue may be subjected to customary cleaning procedures.
- the symmetrical carboxylic anhydrides of the formula (III b) are known or can be prepared in a simple manner by methods known in principle.
- the diluents which can be used in carrying out the process (a, variant ⁇ ) are preferably those diluents which are also preferred in the process (a, variant ⁇ ). Otherwise, an excess carboxylic acid anhydride of the formula (III b) can also act as a diluent.
- reaction temperatures can also be varied within a substantial range in the process according to the invention (a, variant ⁇ ). In general, temperatures between -20 ° C and + 150 ° C, preferably between 0 and 100 ° C.
- the process (a, variant ⁇ ) according to the invention is generally carried out under normal pressure.
- the starting materials of the formulas (II) and (III b) are generally used in approximately equivalent amounts. However, it is also possible to use the carboxylic anhydride in a larger excess. The processing takes place according to usual methods.
- the procedure is to remove diluent and excess carboxylic acid anhydride and the carboxylic acid formed by distillation or by washing with an organic solvent or with water.
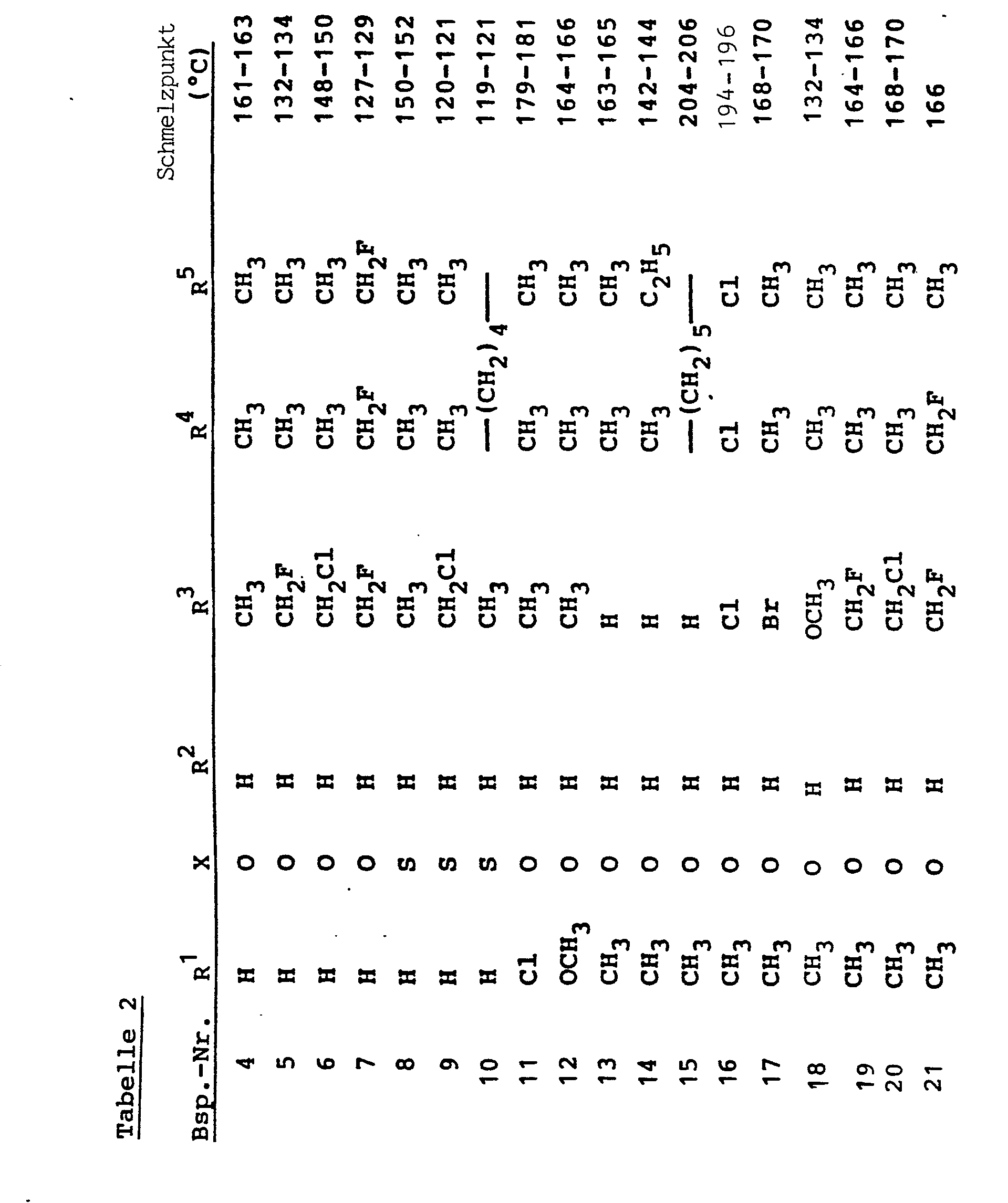
- R 3 , R 4 and R 5 preferably have those meanings which have already been mentioned for these radicals in connection with the description of the substances of the formula (I) according to the invention.
- R preferably represents alkyl having 1 or 2 carbon atoms or phenyl.
- the diluents which can be used in carrying out the process (a, variant) according to the invention are preferably those diluents which are also preferred in the process (a, variant ⁇ ).
- excess acid anhydride of the formula (III c) can also act as a diluent.
- reaction temperatures can also be varied within a substantial range in the process (a, variant f) according to the invention. In general one works with temperature fittings between -20 ° C and + 150 ° C, preferably between 0 and 100 ° C.
- the process (a, variant f) according to the invention is generally carried out under normal pressure.
- Diluents which can be used when carrying out process (a, variant ⁇ ) according to the invention are preferably those diluents which are also preferred in process (a, variant ⁇ ).
- reaction temperatures can also be varied within a substantial range in the process (a, variant) according to the invention. In general, temperatures between -20 ° C and + 150 ° C, preferably between 0 and 100 ° C.
- the process (a, variant ⁇ ) according to the invention is generally carried out under normal pressure.
- Formula (V) clearly defines the acylaniline derivatives required as starting materials in process (b) according to the invention.
- R 2 , R 3 , R 4 , R 5 , X and n preferably have those meanings which have already been mentioned in connection with the description of the substances of the formula (I), preferably for these radicals or for this index.
- All customary acid acceptors can be used as acid binders when carrying out process (b) according to the invention.
- Alkali metal and alkaline earth metal oxides, hydroxides and carbonates such as sodium hydroxide, potassium hydroxide, magnesium oxide, calcium oxide, sodium carbonate, potassium carbonate and calcium carbonate, furthermore alkali metal and alkaline earth metal amides and hydrides, such as sodium amide, sodium hydride and calcium hydride, and also also are preferably usable Alkali metal alcoholates, such as sodium methylate, sodium ethylate and potassium tert-butoxide.
- Hydrocarbons such as toluene and xylene are preferably usable, furthermore ethers such as dibutyl ether, tetrahydrofuran, dioxane, glycol dimethyl ether and diglycol dimethyl ether, also nitriles such as acetonitrile and propionitrile and also polar ones Solvents such as nitrobenzene, dimethyl sulfoxide, sulfolane, dimethylformamide and N-methyl-pyrrolidone.
- ethers such as dibutyl ether, tetrahydrofuran, dioxane, glycol dimethyl ether and diglycol dimethyl ether, also nitriles such as acetonitrile and propionitrile and also polar ones
- Solvents such as nitrobenzene, dimethyl sulfoxide, sulfolane, dimethylformamide and N-methyl-pyrrolidone.
- reaction temperatures can be varied within a substantial range when carrying out process (b) according to the invention. In general, temperatures between 0 and 200 ° C, preferably between 50 and 150 ° C.
- Process (b) according to the invention is generally carried out under normal pressure.
- reaction components of the formulas (IV) and (V) are generally employed in approximately equimolar amounts. However, it is also possible to use one or the other component in a larger excess. In addition, an equimolar amount of acid-binding agent is generally also used. However, it may also be advantageous to add the acid binder in an excess of up to one molar. In particular, the general procedure is to add the acid-binding agent to a mixture of the reaction components in a suitable diluent.
- a salt is first produced from the acylaniline derivative of the formula (V) and the acid binder and this is then reacted with a pyrimidine derivative of the formula (IV).
- a salt is first produced from the acylaniline derivative of the formula (V) and the acid binder and this is then reacted with a pyrimidine derivative of the formula (IV).
- the acylaniline derivative of the formula (V) it is also possible to use the acylaniline derivative of the formula (V) to produce a salt separately from an acid binder, then to isolate it and then to react it with a pyrimidine derivative of the formula (IV) in the presence of a suitable diluent without further addition of an acid binder.
- the processing is carried out according to the usual methods.
- the active compounds according to the invention can be used as defoliants, desiccants, haulm killers and in particular as weed killers. Weeds in the broadest sense are understood to mean all plants that grow up in places where they are undesirable. Whether the substances according to the invention act as total or selective herbicides depends essentially on the amount used.
- the compounds are suitable for total weed control, e.g. on industrial and track systems and on paths and squares with and without tree cover.
- the compounds for weed control in permanent crops e.g. Forestry, ornamental trees, fruit, wine, citrus, nut, banana, coffee, tea, rubber, oil palm, cocoa, berry fruit and hop plants and for selective weed control in annual crops.
- the active compounds according to the invention are particularly suitable for the selective control of mono- and dicotyledon weeds in monocotyledon crops, such as, for example, maize and cereals.
- the active compounds according to the invention also have a strong microbicidal action and can be used practically to combat unwanted microorganisms.
- the active ingredients are suitable for use as pesticides.
- Fungicidal agents in crop protection are used to control Plasmodiophoromycetes, Oomycetes, Chytridiomycetes, Zygomycetes, Ascomycetes, Basidiomycetes, Deuteromycetes.
- the active compounds according to the invention can be used with particular advantage for combating Pyricularia oryzae in rice, against botrytis and for combating bean rust.
- the substances according to the invention are also distinguished by an insecticidal activity. They have a good root systemic effectiveness.
- the active ingredients can be converted into the customary formulations, such as solutions, emulsions, wettable powders, suspensions, powders, dusts, pastes, soluble powders, granules, suspension emulsion concentrates, active ingredient-impregnated natural and synthetic substances and very fine encapsulations in polymeric substances.
- formulations are made in a known manner, e.g. by mixing the active ingredients with extenders, that is to say liquid solvents and / or solid carriers, optionally using surface-active agents, that is to say emulsifiers and / or dispersants and / or foam-generating agents.
- extenders that is to say liquid solvents and / or solid carriers
- surface-active agents that is to say emulsifiers and / or dispersants and / or foam-generating agents.
- organic solvents can also be used as auxiliary solvents.
- auxiliary solvents e.g. organic solvents
- aromatics such as xylene, toluene, or alkylnaphthalenes
- chlorinated aromatics and chlorinated aliphatic hydrocarbons such as chlorobenzenes, chlorethylenes or methylene chloride
- aliphatic hydrocarbons such as cyclohexane or paraffins, e.g.
- Petroleum fractions mineral and vegetable oils, alcohols such as butanol or glycol and their ethers and esters, ketones such as acetone, methyl ethyl ketone, methyl isobutyl ketone or cyclohexanone, strongly polar solvents such as dimethylformamide and dimethyl sulfoxide, and water.
- alcohols such as butanol or glycol and their ethers and esters
- ketones such as acetone, methyl ethyl ketone, methyl isobutyl ketone or cyclohexanone
- strongly polar solvents such as dimethylformamide and dimethyl sulfoxide, and water.
- Adhesives such as carboxymethyl cellulose, natural and synthetic powdery, granular or latex-shaped polymers, such as gum arabic, polyvinyl alcohol, polyvinyl acetate, and natural phospholipids, such as cephalins and lecithins and synthetic phospholipids, can be used in the formulations.
- Other additives can be mineral and vegetable oils.
- Dyes such as inorganic pigments, e.g. Iron oxide, titanium oxide, ferrocyan blue and organic dyes such as alizarin, azo and metal phthalocyanine dyes and trace nutrients such as salts of iron, manganese, boron, copper, cobalt, molybdenum and zinc can be used.
- the formulations generally contain between 0.1 and 95 percent by weight of active compound, preferably between 0.5 and 90%.
- the active compounds according to the invention can also be used for weed control in a mixture with known herbicides, finished formulations or tank mixes being possible.
- a mixture with other known active compounds such as fungicides, insecticides, acaricides, nematicides, bird repellants, plant nutrients and agents which improve soil structure, is also possible.
- the active compounds can be used as such, in the form of their formulations or in the use forms prepared therefrom by further dilution, such as ready-to-use solutions, suspensions, emulsions, powders, pastes and granules.
- the application happens in the usual way, e.g. B. by pouring, spraying, spraying, scattering.
- the active compounds according to the invention can be applied both before and after emergence of the plants. They can also be worked into the soil before sowing.
- the amount of active ingredient used can vary over a wide range when the substances according to the invention are used as herbicides. It essentially depends on the type of effect desired. In general, the application rates are between 0.01 and 10 kg of active ingredient per hectare of soil, preferably between 0.05 and 5 kg per ha.
- the application rate can be varied within a substantial range depending on the type of application.
- the active substance concentrations in the treatment of parts of plants in the use forms are generally between 1 and 0.0001% by weight, preferably between 0.5 and 0.001% by weight.
- active ingredient concentrations of 0.00001 to 0.1% by weight, preferably 0.0001 to 0.02% are required at the site of action.
- active compound 1 part by weight of active compound is mixed with the stated amount of solvent, the stated amount of emulsifier is added and the concentrate is diluted with water to the desired concentration.
- Test plants which have a height of 5-15 cm are sprayed with the active substance preparation in such a way that the desired amounts of active substance are applied per unit area.
- the concentration of the spray liquor is chosen so that the desired amounts of active compound are applied in 2000 l of water / ha.
- the degree of damage to the plants is rated in% damage compared to the development of the untreated control.
- the compound according to Example 45 in the selective control of galinsoga, galium, sinapis, stellaria, echinochloa, panicum and poa in wheat and corn shows a substantially better herbicidal activity than the comparison substance (A).
- active compound 1 part by weight of active compound is mixed with the stated amount of solvent, the stated amount of emulsifier is added and the concentrate is diluted with water to the desired concentration.
- the active ingredient preparation is intimately mixed with soil.
- the treated soil is filled into pots and planted with cabbage (Brassica oleracea).
- the active ingredient can be taken up from the soil by the plant roots and transported to the leaves.
- the root systemic effect of the active ingredient is derived from the number of killings. It is 100% when all test animals have been killed and 0% when as many test insects are still alive as in the untreated control.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Neue substituierte Carbonsaureanilide der Formel <IMAGE> in welcher X für Sauerstoff oder Schwefel steht, R¹ für Wasserstoff, Halogen, Alkyl mit 1 bis 6 Kohlenstoffatomen, Trifluormethyl, gegebenenfalls substituiertes Phenyl, Alkoxy mit 1 bis 6 Kohlenstoffatomen oder Alkylmercapto mit 1 bis 6 Kohlenstoffatomen steht, R² für Halogen oder Methyl steht, n für 0, 1 oder 2 steht, R³ für Wasserstoff, Halogen, gegebenenfalls substituiertes Alkyl mit 1 bis 6 Kohlenstoffatomen, gegebenenfalls substituiertes Aryl, gegebenenfalls substituiertes Aralkyl oder für die Reste -OR<6> oder -SOm -R<6> steht, wobei R<6> für gegebenenfalls substituiertes Alkyl mit 1 bis 6 Kohlenstoffatomen oder für gegebenenfalls substituiertes Aryl steht und m für 0, 1 oder 2 steht, und R<4> und R<5> unabhängig voneinander für Halogen oder gegebenenfalls substituiertes Alkyl mit 1 bis 6 Kohlenstoffatomen stehen oder R<4> und R<5> gemeinsam mit dem angrenzenden Kohlenstoffatom für einen gegebenenfalls substituierten, gesättigten oder ungesättigten carbocyclischen Ring mit 3 bis 8 Ring-Kohlenstoffatomen stehen, mehrere Verfahren zur Herstellung der neuen Stoffe und deren Verwendung als Herbizide.New substituted carboxylic acid anilides of the formula <IMAGE> in which X represents oxygen or sulfur, R¹ represents hydrogen, halogen, alkyl having 1 to 6 carbon atoms, trifluoromethyl, optionally substituted phenyl, alkoxy having 1 to 6 carbon atoms or alkyl mercapto having 1 to 6 carbon atoms , R² is halogen or methyl, n is 0, 1 or 2, R³ is hydrogen, halogen, optionally substituted alkyl having 1 to 6 carbon atoms, optionally substituted aryl, optionally substituted aralkyl or for the radicals -OR <6> or - SOm -R <6>, where R <6> stands for optionally substituted alkyl having 1 to 6 carbon atoms or for optionally substituted aryl and m stands for 0, 1 or 2, and R <4> and R <5> independently of one another stand for halogen or optionally substituted alkyl having 1 to 6 carbon atoms or R 4 and R 5 together with the adjacent carbon atom for an optionally substituted seeded saturated or unsaturated carbocyclic ring with 3 to 8 ring carbon atoms, several processes for the preparation of the new substances and their use as herbicides.
Description
Die vorliegende Erfindung betrifft neue substituierte Carbonsäureanilide, mehrere Verfahren zu ihrer Herstellung und ihre Verwendung als Herbizide.The present invention relates to new substituted carboxylic acid anilides, several processes for their preparation and their use as herbicides.
Es ist bereits bekannt, daß bestimmte Carbonsäureanilide herbizide Eigenschaften besitzen (vgl. R. Wegler "Chemie der Pflanzenschutz- und Schädlingsbekämpfungsmittel" Bd. 2, Seiten 311-314, Springer-Verlag, Berlin 1970). So kann zum Beispiel das Propionsäure-3,4-dichloranilid zur Unkrautbekämpfung eingesetzt werden. Die Wirkung dieser Verbindung ist gut, jedoch werden bei geringen Aufwandmengen einige Unkräuter nicht immer voll erfaßt. Außerdem läßt auch die Selektivität in manchen Fällen zu wünschen übrig.It is already known that certain carboxanilides have herbicidal properties (cf. R. Wegler "Chemistry of Plant Protection Products and Pest Control" Vol. 2, pages 311-314, Springer-Verlag, Berlin 1970). For example, propionic acid 3,4-dichloroanilide can be used to control weeds. The effect of this compound is good, but some weeds are not always fully detected when small amounts are applied. In addition, the selectivity also leaves something to be desired in some cases.
Ferner ist bekannt, daß zahlreiche Pyrimidinyl-2-ether und -thioether als Herbizide geeignet sind (vgl. JP-OS 9 474/ 1967, US-PS 3, 126, 271 und US-PS 3, 250, 775). Zum Beispiel können das 2-Phenoxy-4,6-dimethyl-pyrimidin und das 2- (4-Chlor-benzylmer- capto)-4,6-dimethyl-pyrimidin zur Bekämpfung von Unkräutern verwendet werden. Die herbizide Potenz dieser Stoffe ist aber nicht immer ausreichend.It is also known that numerous pyrimidinyl-2-ethers and thioethers are suitable as herbicides (cf. JP-OS 9 474/1967, US Pat. Nos. 3, 126, 271 and US Pat. Nos. 3, 250, 775). For example, the 2-phenoxy-4,6-dimethyl-pyrimidine and the 2- (4-chloro-benzylmer- capto) -4,6-dimethyl-pyrimidine can be used to control weeds. However, the herbicidal potency of these substances is not always sufficient.
Weiterhin ist bekannt, daß niedere Acylderivate von 4-Pyridyloxy-(bzw. thio)-anilinen herbizide Eigenschaften aufweisen (vgl. DE-OS 2, 501, 648, JP-OS 55-122 763 und JP-OS 56-123 970). Darüberhinaus sind auch herbizid wirksame Acylderivate von 4-Pyrimidyloxy-anilinen bekannt, die in der 5-Stellung des Pyrimidylrestes durch Halogen oder Trifluormethyl substituiert sind, dagegen in den Positionen 4 und 6 keinen Substituenten enthalten (vgl. JP-OS 56-029 576). Auch die Wirksamkeit dieser Stoffe ist jedoch für praktische Zwecke nicht immer befriedigend.It is also known that lower acyl derivatives of 4-pyridyloxy (or thio) anilines have herbicidal properties (cf. DE-OS 2, 501, 648, JP-OS 55-122 763 and JP-OS 56-123 970) . In addition, herbicidally active acyl derivatives of 4-pyrimidyloxy-anilines are known which are substituted by halogen or trifluoromethyl in the 5-position of the pyrimidyl radical, but do not contain any substituents in positions 4 and 6 (cf. JP-OS 56-029 576) . However, the effectiveness of these substances is not always satisfactory for practical purposes.
Es wurden nun neue substituierte Carbonsäureanilide der Formel
in welcher
- X für Sauerstoff oder Schwefel steht,
- R1 für Wasserstoff, Halogen, Alkyl mit 1 bis 6 Kohlenstoffatomen, Trifluormethyl, gegebenenfalls substituiertes Phenyl, Alkoxy mit 1 bis 6 Kohlenstoffatomen oder Alkylmercapto mit 1 bis 6 Kohlenstoffatomen steht,
- R 2 für Halogen oder Methyl steht,
- n für 0,1 oder 2 steht,
- R3 für Wasserstoff, Halogen, gegebenenfalls substituiertes Alkyl mit 1 bis 6 Kohlenstoffatomen, gegebenenfalls substituiertes Aryl, gegebenenfalls substituiertes Aralkyl oder für die Reste -OR6 oder -SOm-R6 steht, wobei
- R6 für gegebenenfalls substituiertes Alkyl mit 1 bis 6 Kohlenstoffatomen oder für gegebenenfalls substituiertes Aryl steht und
- m für 0,1 oder 2 steht,
und - R 4 und R 5 unabhängig voneinander für Halogen oder gegebenenfalls substituiertes Alkyl mit 1 bis 6 Kohlenstoffatomen stehen oder
- R 4 und R 5 gemeinsam mit dem angrenzenden Kohlenstoffatom für einen gegebenenfalls substituierten, gesättigten oder ungesättigten carbocyclischen Ring mit 3 bis 8 Ring-Kohlenstoffatomen stehen,
gefunden.There have now been new substituted carboxanilides of the formula
in which
- X represents oxygen or sulfur,
- R 1 represents hydrogen, halogen, alkyl having 1 to 6 carbon atoms, trifluoromethyl, optionally substituted phenyl, alkoxy having 1 to 6 carbon atoms or alkyl mercapto having 1 to 6 carbon atoms,
- R 2 represents halogen or methyl,
- n represents 0.1 or 2,
- R 3 represents hydrogen, halogen, optionally substituted alkyl having 1 to 6 carbon atoms, optionally substituted aryl, optionally substituted aralkyl or the radicals -OR 6 or -SO m -R 6 , where
- R 6 represents optionally substituted alkyl having 1 to 6 carbon atoms or optionally substituted aryl and
- m represents 0.1 or 2,
and - R 4 and R 5 independently of one another represent halogen or optionally substituted alkyl having 1 to 6 carbon atoms or
- R 4 and R 5 together with the adjacent carbon atom represent an optionally substituted, saturated or unsaturated carbocyclic ring having 3 to 8 ring carbon atoms,
found.
Weiterhin wurde gefunden, daß man substituierte Carbonsäureanilide der Formel (I) erhält, wenn man
- a) Anilin-Derivate der Formel
in welcher- R1, R2, X und n die oben angegebene Bedeutung haben,
entweder - α) mit Säurehalogeniden der Formel
in welcher - R 3, R 4 und R 5 die oben angegebene Bedeutung haben
und - Y für Halogen steht,
gegebenenfalls in Gegenwart eines Verdünnungsmittels und gegebenenfalls in Gegenwart eines Säurebindemittels umsetzt,
oder - R1, R2, X und n die oben angegebene Bedeutung haben,
- ß) mit symmetrischen Carbonsäureanhydriden der Formel
in welcher- R 3,R 4 und R5 die oben angegebene Bedeutung haben,
- gegebenenfalls in Gegenwart eines Verdünnungsmittels umsetzt,
oder - mit asymmetrischen Säureanhydriden der Formel
in welcher - R 3,R 4 und R 5 die oben angegebene Bedeutung haben und
- R für Alkyl mit 1 bis 4 Kohlenstoffatomen oder Phenyl steht,
gegebenenfalls in Gegenwart eines Verdünnungsmittels umsetzt,
oder - ) mit Verbindungen der Formel
in welcher- R 3, R 4 und R 5 die oben angegebene Bedeutung haben,
- gegebenenfalls in Gegenwart eines Verdünnungsmittels umsetzt,
oder - b) Pyrimidin-Derivate der Formel
in welcher- R1 die oben angegebene Bedeutung hat und
- Hal für Halogen steht,
- mit Acylanilin-Derivaten der Formel
in welcher - R2, R3, R4, R , X und n die oben angegebene Bedeutung haben,
- in Gegenwart eines Verdünnungsmittels umsetzt.
- a) Aniline derivatives of the formula
in which- R 1 , R 2 , X and n have the meaning given above,
either - α) with acid halides of the formula
in which - R 3 , R 4 and R 5 have the meaning given above
and - Y represents halogen,
if appropriate in the presence of a diluent and if appropriate in the presence of an acid binder,
or - R 1 , R 2 , X and n have the meaning given above,
- ß) with symmetrical carboxylic anhydrides of the formula
in which- R 3 , R 4 and R 5 have the meaning given above,
- if appropriate in the presence of a diluent,
or - with asymmetric acid anhydrides of the formula
in which - R 3 , R 4 and R 5 have the meaning given above and
- R represents alkyl having 1 to 4 carbon atoms or phenyl,
if appropriate in the presence of a diluent,
or - ) with compounds of the formula
in which- R 3 , R 4 and R 5 have the meaning given above,
- if appropriate in the presence of a diluent,
or - b) pyrimidine derivatives of the formula
in which- R 1 has the meaning given above and
- Hal stands for halogen,
- with acylaniline derivatives of the formula
in which - R 2 , R 3 , R 4 , R, X and n have the meaning given above,
- in the presence of a diluent.
Schließlich wurde gefunden, daß sich die neuen substituierten Carbonsäureanilide der Formel (I) durch hervorragende herbizide Wirksamkeit auszeichnen.Finally, it was found that the new substituted carboxanilides of the formula (I) are notable for excellent herbicidal activity.
Überraschenderweise besitzen die erfindungsgemäßen substituierten Carbonsäureanilide der Formel (I) wesentlich bessere herbizide Eigenschaften als die konstitutionell ähnlichsten vorbekannten Stoffe. So lassen sich die erfindungsgemäßen Carbonsäureanilide der Formel (I) wesentlich besser zur Unkrautbekämpfung verwenden als das 2-Phenoxy-4,6-dimethyl-pyrimidin, welches ein strukturell ähnlicher vorbekannter Wirkstoff gleicher Wirkungsart ist.Surprisingly, the substituted carboxylic acid anilides of the formula (I) according to the invention have significantly better herbicidal properties than the constitutionally most similar previously known substances. Thus, the carboxylic acid anilides of the formula (I) according to the invention can be used much better for weed control than the 2-phenoxy-4,6-dimethyl-pyrimidine, which is a structurally similar previously known active ingredient of the same type of action.
Die erfindungsgemäßen substituierten Carbonsäureanilide sind durch die Formel (I) allgemein definiert. In dieser Formel steht X für Sauerstoff oder Schwefel. Der Rest R1 steht vorzugsweise für Wasserstoff, Fluor, Chlor, Brom, geradkettiges oder verzweigtes Alkyl mit 1 bis 4 Kohlenstoffatomen, Trifluormethyl, gegebenenfalls durch Chlor, Trifluormethyl und/oder Methyl substituiertes Phenyl, Alkoxy mit 1 bis 4 Kohlenstoffatomen oder Alkylmercapto mit 1 bis 4 Kohlenstoffatomen. R steht vorzugsweise für Chlor, Brom oder Methyl. Der Index n steht vorzugsweise für 0 oder 1. R3 steht vorzugsweise für Wasserstoff, Fluor, Chlor, Brom, gegebenenfalls durch Fluor, Chlor und/oder Brom substituiertes Alkyl mit 1 bis 4 Kohlenstoffatomen, gegebenenfalls durch Fluor, Chlor, Brom, Trifluormethyl, Methoxy und/ oder Methyl substituiertes Phenyl, gegebenenfalls durch Fluor, Chlor, Brom, Trifluormethyl, Methoxy und/oder Methyl substituiertes Benzyl oder für die Reste -OR6 oder -SO -R6, worin R vorzugsweise für gegebenenfalls durch Fluor, Chlor und/oder Brom substituiertes Alkyl mit 1 bis 4 Kohlenstoffatomen oder für gegebenenfalls durch Fluor, Chlor, Brom und/oder Alkyl mit 1 bis 4 Kohlenstoffatomen substituiertes Phenyl steht und der Index m für 0, 1 oder 2 steht. R4 und R5 stehen unabhängig voneinander vorzugsweise für Fluor, Chlor, Brom oder gegebenenfalls durch Fluor, Chlor und/oder Brom substituiertes Alkyl mit 1 bis 4 Kohlenstoffatomen. Außerdem stehen R4 und R5 auch gemeinsam mit dem angrenzenden Kohlenstoffatom vorzugsweise für einen gegebenenfalls durch Fluor, Chlor und/oder Alkyl mit 1 bis 4 Kohlenstoffatomen substituierten gesättigten oder ungesättigten carbocyclischen Ring mit 3 bis 7 Ring -Kohlenstoffatomen.The substituted carboxylic acid anilides according to the invention are generally defined by the formula (I). In this formula, X stands for oxygen or sulfur. The radical R 1 preferably represents hydrogen, fluorine, chlorine, Bromine, straight-chain or branched alkyl having 1 to 4 carbon atoms, trifluoromethyl, phenyl optionally substituted by chlorine, trifluoromethyl and / or methyl, alkoxy having 1 to 4 carbon atoms or alkyl mercapto having 1 to 4 carbon atoms. R preferably represents chlorine, bromine or methyl. The index n preferably stands for 0 or 1. R 3 preferably stands for hydrogen, fluorine, chlorine, bromine, alkyl with 1 to 4 carbon atoms optionally substituted by fluorine, chlorine and / or bromine, optionally with fluorine, chlorine, bromine, trifluoromethyl, Methoxy and / or methyl substituted phenyl, optionally substituted by fluorine, chlorine, bromine, trifluoromethyl, methoxy and / or methyl or for the radicals -OR 6 or -SO -R 6 , where R is preferably for optionally by fluorine, chlorine and / or bromine-substituted alkyl having 1 to 4 carbon atoms or phenyl optionally substituted by fluorine, chlorine, bromine and / or alkyl having 1 to 4 carbon atoms and the index m being 0, 1 or 2. R 4 and R 5 independently of one another are preferably fluorine, chlorine, bromine or alkyl having 1 to 4 carbon atoms which is optionally substituted by fluorine, chlorine and / or bromine. In addition, R 4 and R 5 , together with the adjacent carbon atom, preferably represent a saturated or unsaturated carbocyclic ring with 3 to 7 ring carbon atoms which is optionally substituted by fluorine, chlorine and / or alkyl having 1 to 4 carbon atoms.
Eine besonders bevorzugte Gruppe erfindungsgemäßer Stoffe sind diejenigen substituierten Carbonsäureanilide der Formel (I), in denen
- X für Sauerstoff oder Schwefel steht,
- R1 für Wasserstoff, Alkyl mit 1 bis 4 Kohlenstoffatomen, Alkoxy mit 1 bis 4 Kohlenstoffatomen oder Trifluormethyl steht,
- R 2 für Chlor, Brom oder Methyl steht,
- n für 0 oder 1 steht,
- R 3 für Fluor, Chlor, Brom, gegebenenfalls durch Fluor und/oder Chlor substituiertes Alkyl mit 1 bis 4 kohlenstoffatomen, gegebenenfalls durch Fluor, Chlor und/oder Methyl substituiertes Phenyl, gegebenenfalls durch Fluor, Chlor und/oder Methyl substituiertes Benzyl oder für die Reste -OR6 oder -SOm R6 steht, worin
- R6 für gegebenenfalls durch Fluor und/oder Chlor substituiertes Alkyl mit 1 bis 4 Kohlenstoffatomen oder für gegebenenfalls durch Fluor, Chlor und/ oder Alkyl mit 1 bis 4 Kohlenstoffatomen substituiertes Phenyl steht und
- m für 0, 1 oder 2 steht,
und - R 4 und R5 unabhängig voneinander für Fluor, Chlor oder gegebenenfalls durch Fluor und/oder Chlor substituiertes Alkyl mit 1 bis 4 Kohlenstoffatomen stehen
oder - R 4 und R5 gemeinsam mit dem angrenzenden Kohlenstoffatom für einen gegebenenfalls durch Fluor, Chlor, Methyl, Ethyl, n-Propyl, iso-Propyl, n-Butyl, isoButyl, sek.-Butyl und/oder tert.-Butyl substituierten gesättigten oder ungesättigten carbocyclischen Ring mit 3 bis 7 Ring-Kohlenstoffatomen stehen.
- X represents oxygen or sulfur,
- R 1 represents hydrogen, alkyl having 1 to 4 carbon atoms, alkoxy having 1 to 4 carbon atoms or trifluoromethyl,
- R 2 represents chlorine, bromine or methyl,
- n represents 0 or 1,
- R 3 for fluorine, chlorine, bromine, optionally substituted by fluorine and / or chlorine alkyl having 1 to 4 carbon atoms, optionally substituted by fluorine, chlorine and / or methyl phenyl, optionally substituted by fluorine, chlorine and / or methyl, or for the Radicals -OR 6 or -SO m R 6 , wherein
- R 6 represents optionally substituted by fluorine and / or chlorine alkyl having 1 to 4 carbon atoms or represents optionally substituted by fluorine, chlorine and / or alkyl having 1 to 4 carbon atoms and
- m represents 0, 1 or 2,
and - R 4 and R 5 independently of one another represent fluorine, chlorine or alkyl having 1 to 4 carbon atoms which is optionally substituted by fluorine and / or chlorine
or - R 4 and R 5 together with the adjacent carbon atom for a saturated or optionally substituted by fluorine, chlorine, methyl, ethyl, n-propyl, iso-propyl, n-butyl, iso-butyl, sec-butyl and / or tert-butyl unsaturated carbocyclic ring having 3 to 7 ring carbon atoms.
Als Beispiele für substituierte Carbonsäureanilide der Formel (I) seien die in der folgenden Tabelle 1 aufgeführten Stoffe genannt.The substances listed in Table 1 below may be mentioned as examples of substituted carboxanilides of the formula (I).
Verwendet man 4-(4,6-Dimethyl-pyrimidyl-2-oxy)-anilin und Pivalsäurechlorid als Ausgangsstoffe, so kann der Verlauf des erfindungsgemäßen Verfahrens (a, Variante α) durch das folgende Formelschema wiedergegeben werden:If 4- (4,6-dimethyl-pyrimidyl-2-oxy) aniline and pivaloyl chloride are used as starting materials, the course of the process according to the invention (a, variant α) can be represented by the following formula:
Verwendet man 4-(4,6-Dimethyl-pyrimidyl-2-mercapto)-anilin undα-Methoxy-isobuttersäure-kohlensäure-ethylester-anhydrid als Ausgangsstoffe, so kann der Verlauf des erfindungsgemäßen Verfahrens (a, Variante ) durch das folgende Formelschema wiedergegeben werden:If 4- (4,6-dimethyl-pyrimidyl-2-mercapto) aniline and α-methoxy-isobutyric acid-carbonic acid-ethyl ester-anhydride are used as starting materials, the course of the process (a, variant) according to the invention can be represented by the following formula will:
Die Anilin-Derivate der Formel (II) sind teilweise bekannt. Sie lassen sich herstellen, indem man
- c) Pyrimidin-Derivate der Formel
in welcher- R1 und Hal die oben angegebene Bedeutung haben,
- mit 4-Amino-(thio)-phenolen der Formel
in welcher - R 2, X und n die oben angegebene Bedeutung haben,
- in Gegenwart eines Säurebindemittels sowie gegebenenfalls in Gegenwart eines Verdünnungsmittel umsetzt,
oder - d) 2-(4-Nitro-(thio)-Phenoxy)-pyrimidin-Derivate der Formel
in welcher- R1, R2, X und n die oben angegebene Bedeutung haben,
- c) pyrimidine derivatives of the formula
in which- R 1 and Hal have the meaning given above,
- with 4-amino (thio) phenols of the formula
in which - R 2 , X and n have the meaning given above,
- in the presence of an acid binder and optionally in the presence of a diluent,
or - d) 2- (4-nitro- (thio) phenoxy) pyrimidine derivatives of the formula
in which- R 1 , R 2 , X and n have the meaning given above,
Die bei dem obigen Verfahren (c) als Ausgangsstoffe benötigten Pyrimidin-Derivate sind durch die Formel (IV) definiert. In dieser Formel hat R1 vorzugsweise diejenigen Bedeutungen, die bereits im Zusammenhang mit der Beschreibung der erfindungsgemäßen Stoffe der Formel (I) vorzugsweise für diesen Rest genannt wurden. Hal steht vorzugsweise für Fluor, Chlor oder Brom.Formula (IV) defines the pyrimidine derivatives required as starting materials in process (c) above. In this formula, R 1 preferably has those meanings which have preferably been mentioned for this radical in connection with the description of the substances of the formula (I) according to the invention. Hal preferably represents fluorine, chlorine or bromine.
Als Beispiele für Pyrimidin-Derivate der Formel (IV) seien genannt:
- 2-Chlor-4-methyl-pyrimidin
- 2-Brom-4-methyl-pyrimidin
- 2,4-Dichlor-6-methyl-pyrimidin
- 2-Chlor-4-methoxy-6-methylpyrimidin
- 2-Chlor-4-methylmercapto-6-methyl-pyrimidin
- 2-Chlor-4,6-dimethyl-pyrimidin
- 2-Fluor-4,6-dimethyl-pyrimidin
- 2-Chlor-4-methyl-6-propyl-pyrimidin
- 2-Chlor-4-methyl-6-isopropyl-pyrimidin
- 2-Chlor-4-methyl-6-trifluormethyl-pyrimidin
- 2-Chlor-4-methyl-6-phenyl-pyrimidin
- 2-Chlor-4-methyl-6-(4-chlor-phenyl)-pyrimidin
- 2-Chlor-4-methyl-6-(4-methyl-phenyl)-pyrimidin
- 2-Chlor-4-methyl-6-(3-trifluormethyl-phenyl)-pyrimidin
- 2-chloro-4-methyl-pyrimidine
- 2-bromo-4-methyl-pyrimidine
- 2,4-dichloro-6-methyl-pyrimidine
- 2-chloro-4-methoxy-6-methylpyrimidine
- 2-chloro-4-methylmercapto-6-methyl-pyrimidine
- 2-chloro-4,6-dimethyl-pyrimidine
- 2-fluoro-4,6-dimethyl-pyrimidine
- 2-chloro-4-methyl-6-propyl-pyrimidine
- 2-chloro-4-methyl-6-isopropyl-pyrimidine
- 2-chloro-4-methyl-6-trifluoromethyl-pyrimidine
- 2-chloro-4-methyl-6-phenyl-pyrimidine
- 2-chloro-4-methyl-6- (4-chlorophenyl) pyrimidine
- 2-chloro-4-methyl-6- (4-methylphenyl) pyrimidine
- 2-chloro-4-methyl-6- (3-trifluoromethylphenyl) pyrimidine
Die Pyrimidin-Derivate der Formel (IV) sind bekannt oder lassen sich nach im Prinzip bekannten Methoden in einfacher Weise herstellen. So erhält man Pyrimidin-Derivate der Formel (IV) zum Beispiel dadurch, daß man 2-Hydroxy-pyrimidin-Derivate (Dihydro-pyri- midon-2-Derivate) mit anorganischen Säurehalogeniden, wie zum Beispiel Phosphoroxychlorid oder Phosphorpentachlorid, umsetzt, oder auch dadurch, daß man entsprechende 2-Amino-pyrimidin-Derivate mit Salpetriger Säure in Gegenwart von Halogenwasserstoffsäuren umsetzt.The pyrimidine derivatives of the formula (IV) are known or can be prepared in a simple manner by methods known in principle. For example, pyrimidine derivatives of the formula (IV) are obtained by reacting 2-hydroxy-pyrimidine derivatives (dihydro-pyrimidone-2 derivatives) with inorganic acid halides, such as phosphorus oxychloride or phosphorus pentachloride, or else by reacting corresponding 2-amino-pyrimidine derivatives with nitrous acid in the presence of hydrohalic acids.
Die bei dem Verfahren (c) weiterhin als Ausgangsstoffe benötigten 4-Amino-(thio)-phenole sind durch die Formel (VI) definiert. In dieser Formel haben R2, X und n vorzugsweise diejenigen Bedeutungen, die bereits im Zusammenhang mit der Beschreibung der erfindungsgemäßen Stoffe der Formel (I) vorzugsweise für diese Reste bzw. für diesen Index genannt wurden.Formula (VI) defines the 4-amino (thio) phenols which are further required as starting materials in process (c). In this formula, R 2 , X and n preferably have those meanings which have already been mentioned preferably in connection with the description of the substances of the formula (I) according to the invention for these radicals or for this index.
Als Beispiele für 4-Amino-(thio)-phenole der Formel (VI) seien genannt:
- 4-Amino-phenol
- 2-Chlor-4-amino-phenol
- 3-Chlor-4-amino-phenol
- 2,5-Dichlor-4-amino-phenol
- 2,6-Dichlor-4-amino-phenol
- 2-Methyl-4-amino-phenol
- 3-Methyl-4-amino-phenol
- 2-Chlor-5-methyl-4-amino-phenol
- 4-Amino-thiophenol
- 2-Chlor-4-amino-thiophenol
- 3-Chlor-4-amino-thiophenol
- 2-Methyl-4-amino-thiophenol
- 3-Methyl-4-amino-thiophenol
- 4-aminophenol
- 2-chloro-4-aminophenol
- 3-chloro-4-aminophenol
- 2,5-dichloro-4-aminophenol
- 2,6-dichloro-4-aminophenol
- 2-methyl-4-aminophenol
- 3-methyl-4-aminophenol
- 2-chloro-5-methyl-4-aminophenol
- 4-amino-thiophenol
- 2-chloro-4-aminothiophenol
- 3-chloro-4-aminothiophenol
- 2-methyl-4-aminothiophenol
- 3-methyl-4-aminothiophenol
Die 4-Amino-(thio)-phenole der Formel (VI) sind bekannt oder lassen sich nach im Prinzip bekannten Methoden in einfacher Weise herstellen.The 4-amino (thio) phenols of the formula (VI) are known or can be prepared in a simple manner by methods known in principle.
Als Säurebindemittel können bei der Durchführung des Verfahrens (c) alle üblicherweise für derartige Umsetzungen verwendbaren Säureakzeptoren verwendet werden. Vorzugsweise verwendbar sind Alkali- und Erdalkalioxide, -hydroxide und -carbonate, wie Natriumhydroxid, Kaliumhydroxid, Calciumoxid, Natriumcarbonat und Kaliumcarbonat, ferner Alkali-alkoholate, -amide und -hydride, wie zum Beispiel Natrium-methylat, Natriumethylat, Kalium-tert.-butylat, Natrium-amid und Natrium-hydrid.All acid acceptors that can usually be used for such reactions can be used as acid binders when carrying out process (c). Alkali and alkaline earth oxides, hydroxides and carbonates such as sodium hydroxide, potassium hydroxide, calcium oxide, sodium carbonate and potassium carbonate are preferably usable, furthermore alkali alcoholates, amides and hydrides such as sodium methylate, sodium ethylate, potassium tert. butylate, sodium amide and sodium hydride.
Als Verdünnungsmittel können bei der Durchführung des Verfahrens (c) alle üblichen inerten organischen Solventien verwendet werden. Vorzugsweise in Frage kommen Kohlenwasserstoffe, wie Benzin, Toluol und Xylol, ferner Ether, wie Dioxan, Glykoldimethylether und Diglykoldimethylether, außerdem Nitrile, wie Acetonitril, und auch stark polare Solventien, wie Dimethylsulfoxid, Sulfolan und Dimethylformamid.All customary inert organic solvents can be used as diluents when carrying out process (c). Hydrocarbons such as gasoline, toluene and xylene, ethers such as dioxane, glycol dimethyl ether and diglycol dimethyl ether, furthermore nitriles such as acetonitrile, and also strongly polar solvents such as dimethyl sulfoxide, sulfolane and dimethylformamide are preferred.
Die Reaktionstemperaturen können bei der Durchführung des Verfahrens (c) innerhalb eines größeren Bereiches variiert werden. Im allgemeinen arbeitet man bei Temperaturen zwischen 0°C und 200°C, vorzugsweise zwischen 50°C und 150°C.The reaction temperatures can be varied within a substantial range when carrying out process (c). In general, temperatures between 0 ° C and 200 ° C, preferably between 50 ° C and 150 ° C.
Die Umsetzung nach dem Verfahren (c) wird im allgemeinen unter Normaldruck vorgenommen.The reaction according to process (c) is generally carried out under normal pressure.
Bei der Durchführung des Verfahrens (c) setzt man die Ausgangsstoffe der Formeln (IV) und(VI) im allgemeinen in angenähert äquimolaren Mengen um. Es ist jedoch auch möglich, die eine oder andere Komponente in einem größeren Überschuß zu verwenden. Die Aufarbeitung erfolgt nach üblichen Methoden.When carrying out process (c), the starting materials of the formulas (IV) and (VI) are generally reacted in approximately equimolar amounts. However, it is also possible to use one or the other component in a larger excess. The processing takes place according to usual methods.
Die bei dem Verfahren (d) als Ausgangsstoffe benötigten 2-(4-Nitro-(thio)-phenoxy)-pyrimidin-Derivate sind durch die Formel (VII) definiert. In dieser Formel haben R1, R2, X und n vorzugsweise diejenigen Bedeutungen, die bereits im Zusammenhang mit der Beschreibung der erfindungsgemäßen Stoffe der Formel (I) vorzugsweise für diese Reste bzw. für diesen Index genannt wurden.Formula (VII) defines the 2- (4-nitro (thio) phenoxy) pyrimidine derivatives required as starting materials in process (d). In this formula, R 1 , R 2 , X and n preferably have those meanings which are already in connection with the description the substances of the formula (I) according to the invention were preferably mentioned for these radicals or for this index.
Die Verbindungen der Formel (VII) sind bekannt oder lassen sich nach im Prinzip bekannten Methoden in einfacher Weise herstellen. So erhält man Verbindungen der Formel (VII) zum Beispiel dadurch, daß man Pyrimidin-Derivate der Formel
in welcher
- R und Hal die oben angegebene Bedeutung haben,
- mit 4-Nitro-(thio)-phenolen der Formel
in welcher - R 2, X und n die oben angegebene Bedeutung haben,
in Gegenwart eines Säurebindemittels und gegebenenfalls in Gegenwart eines Verdünnungsmittels bei Temperaturen zwischen 0°C und 200°C, vorzugsweise zwischen 50°C und 150°C, umsetzt. Als Säurebindemittel und Verdünnungsmittel kommen hierbei vorzugsweise diejenigen Stoffe in Betracht, die bereits im Zusammenhang mit dem Verfahren (c) als vorzugsweise verwendbare Säureakzeptoren und Solventien genannt wurden.The compounds of the formula (VII) are known or can be prepared in a simple manner by methods known in principle. Compounds of formula (VII) are thus obtained, for example, by using pyrimidine derivatives of the formula
in which
- R and Hal have the meaning given above,
- with 4-nitro (thio) phenols of the formula
in which - R 2 , X and n have the meaning given above,
in the presence of an acid binder and optionally in the presence of a diluent at temperatures between 0 ° C. and 200 ° C., preferably between 50 ° C. and 150 ° C. As an acid binder and diluent means here are preferably those substances which have already been mentioned in connection with process (c) as preferably usable acid acceptors and solvents.
Als Reduktionsmittel kommen bei dem Verfahren (d) alle diejenigen Stoffe in Frage, die üblicherweise zur Reduktion aromatischer Nitroverbindungen eingesetzt werden. Vorzugsweise verwendbar sind elementare Metalle, wie Eisen, Zink und Zinn, ferner Metallverbindungen in niederen Wertigkeitsstufen, wie Eisen (II)- und Zinn (II)-Salze, und außerdem Nichtmetall-Verbindungen in niederen Wertigkeitsstufen, wie zum Beispiel Salze des Schwefelwasserstoffes, Alkalisulfite und Alkalidithionite. Im übrigen kann die Reduktion auch durch katalytische Hydrierung mit Wasserstoff in Gegenwart eines Katalysators, wie zum Beispiel Raney-Nickel, erfolgen.Suitable as reducing agents in process (d) are all those substances which are usually used for the reduction of aromatic nitro compounds. Elemental metals, such as iron, zinc and tin, are preferably usable, furthermore metal compounds in lower valence levels, such as iron (II) and tin (II) salts, and also non-metal compounds in low valence levels, such as, for example, salts of hydrogen sulfide, alkali metal sulfites and alkalidithionites. Otherwise, the reduction can also be carried out by catalytic hydrogenation with hydrogen in the presence of a catalyst, such as Raney nickel.
Als Verdünnungsmittel kommen bei dem Verfahren (d) alle üblichen für derartige Reduktionen geeigneten organischen Solventien in Betracht. Die Reaktionstemperaturen können innerhalb eines größeren Bereiches variiert werden. Sie entsprechen den Temperaturen, die bei analogen Reaktionen angewandt werden.Suitable diluents in process (d) are all customary organic solvents suitable for such reductions. The reaction temperatures can be varied within a substantial range. They correspond to the temperatures used in analog reactions.
Die Durchführung der Reduktion nach dem Verfahren (d) und die Aufarbeitung des anfallenden Reaktionsgemisches erfolgen nach üblichen Methoden.The reduction is carried out according to process (d) and the reaction mixture obtained is worked up using customary methods.
Die bei dem erfindungsgemäßen Verfahren (a, Variante α) als Reaktionskomponenten benötigten Säurehalogenide sind durch die Formel (III a) eindeutig definiert. In dieser Formel haben R3, R4 und R5 vorzugsweise diejenigen Bedeutungen, die bereits im Zusammenhang mit der Beschreibung der erfindungsgemäßen Stoffe der Formel (I) vorzugsweise für diese Reste genannt wurden. Y steht vorzugsweise für Fluor, Chlor und Brom.The process (a, variant α) Acid halides required as reaction components are clearly defined by the formula (III a). In this formula, R 3 , R 4 and R 5 preferably have those meanings which have already been mentioned for these radicals in connection with the description of the substances of the formula (I) according to the invention. Y preferably represents fluorine, chlorine and bromine.
Als Beispiele für Säurehalogenide der Formel (III a) seien die Chloride der folgenden Säuren genannt:
- Trichloressigsäure
- Trifluoressigsäure
- α,α-Dichlorpropionsäure
- Isobuttersäure
- α -Chlor-isobuttersäure
- α-Brom-isobuttersäure
- α-Methoxy-isobuttersäure
- α-Phenoxy-isobuttersäure
- d-(4-Chlor-phenoxy)-isobuttersäure
- α-(2-Methyl-4-chlor-phenoxy)-isobuttersäure
- α-Methylmercapto-isobuttersäure
- α-Methylsulfonyl-isobuttersäure
- α-Methyl-buttersäure
- Pivalinsäure
- ß-Fluor-pivalinsäure
- ß-Chlor-pivalinsäure
- ß,ß'-Difluor-pivalinsäure
- ß,ß'-Dichlor-pivalinsäure
- β,β'β"-Trifluor-pivalinsäure
- ß,ß'ß"-Trichlor-pivalinsäure
- α,α-Dimethyl-valeriansäure
- α-Methyl-α-ethyl-buttersäure
- α,α-Dimethyl-phenylessigsäure
- α,α-Dimethyl-(4-chlor-phenyl)-essigsäure
- α,α-Dimethyl-(3,4-dichlor-phenyl)-essigsäure
- α,α-Dimethyl-(3-trifluormethyl-phenyl)-essigsäure
- α-Benzyl-isobuttersäure
- o(--(4-Chlor-benzyl)-isobuttersäure
- α-(4-Methoxy-benzyl)-isobuttersäure
- Cyclopropan-carbonsäure
- 1-Methyl-cyclopropan-carbonsäure
- 2,2-Dichlor-1-methyl-cyclopropan-carbonsäure Cyclopentan-carbonsäure
- 1-Methyl-cyclopentan-carbonsäure
- Cyclohexan-carbonsäure
- 1-Methyl-cyclohexan-carbonsäure
- 1-Methyl-4-isopropyl-cyclohexan-carbonsäure
- Trichloroacetic acid
- Trifluoroacetic acid
- α, α-dichloropropionic acid
- Isobutyric acid
- α-chloro-isobutyric acid
- α-bromo-isobutyric acid
- α-methoxy-isobutyric acid
- α-phenoxy-isobutyric acid
- d- (4-chlorophenoxy) isobutyric acid
- α- (2-Methyl-4-chlorophenoxy) isobutyric acid
- α-methylmercapto isobutyric acid
- α-methylsulfonyl isobutyric acid
- α-methyl butyric acid
- Pivalic acid
- ß-fluoro-pivalic acid
- ß-chloro-pivalic acid
- ß, ß'-difluoropivalic acid
- ß, ß'-dichloropivalic acid
- β, β'β "-trifluoropivalic acid
- ß, ß'ß "-Trichlor-pivalic acid
- α, α-dimethylvaleric acid
- α-methyl-α-ethyl-butyric acid
- α, α-dimethyl-phenylacetic acid
- α, α-Dimethyl- (4-chlorophenyl) acetic acid
- α, α-dimethyl- (3,4-dichlorophenyl) acetic acid
- α, α-Dimethyl- (3-trifluoromethyl-phenyl) acetic acid
- α-benzyl isobutyric acid
- o (- (4-chlorobenzyl) isobutyric acid
- α- (4-methoxy-benzyl) isobutyric acid
- Cyclopropane carboxylic acid
- 1-methyl-cyclopropane-carboxylic acid
- 2,2-dichloro-1-methyl-cyclopropane-carboxylic acid cyclopentane-carboxylic acid
- 1-methyl-cyclopentane carboxylic acid
- Cyclohexane carboxylic acid
- 1-methyl-cyclohexane-carboxylic acid
- 1-methyl-4-isopropyl-cyclohexane-carboxylic acid
Die Säurehalogenide der Formel (III a) sind bekannt oder lassen sich nach im Prinzip bekannten Methoden in einfacher Weise herstellen.The acid halides of the formula (III a) are known or can be prepared in a simple manner by methods known in principle.
Als Säurebindemittel kommen bei der Umsetzung nach dem erfindungsgemäßen Verfahren (a, Variante α) alle üblichen Säureakzeptoren in Betracht. Vorzugsweise verwendbar sind tertiäre Amine, wie Triethylamin, Pyridin und N,N-Dimethyl-anilin, ferner Erdalkalimetalloxide, wie Magnesium- und Calciumoxid, außerdem Alkali- und Erdalkali-metall-carbonate, wie Natriumcarbonat, Kaliumcarbonat und Calciumcarbonat. Es ist auch möglich, die jeweiligen Anilin-Derivate der Formel (II) gleichzeitig als Säurebindemittel zu verwenden. Dazu muß die betreffende Anilin-Verbindung dann zumindest in solcher Menge eingesetzt werden, daß der freiwerdende Halogenwasserstoff gebunden werden kann.Suitable acid binders for the reaction according to process (a, variant α) are all customary acid acceptors. Tertiary amines, such as triethylamine, pyridine and N, N-dimethylaniline, furthermore alkaline earth metal oxides, such as magnesium and calcium oxide, and also alkali metal and alkaline earth metal carbonates, such as sodium carbonate, potassium carbonate and calcium carbonate, are preferably usable. It is also possible, to use the respective aniline derivatives of the formula (II) at the same time as acid binders. For this purpose, the aniline compound in question must then be used at least in such an amount that the hydrogen halide released can be bound.
Als Verdünnungsmittel können bei dem erfindungsgemäßen Verfahren (a, Variantedl) alle gegenüber Säurehalogeniden inerten Solventien eingesetzt werden. Vorzugsweise verwendbar sind Kohlenwasserstoffe, wie Benzin, Benzol, Toluol, Xylol und Tetralin, ferner Halogenkohlenwasserstoffe, wie Methylenchlorid, Chloroform, Tetrachlorkohlenstoff, Chlorbenzol und o-Dichlorbenzol, außerdem Ketone, wie Aceton und Methyl-isopropylketon, weiterhin Ether, wie Diethylether, Tetrahydrofuran und Dioxan, darüberhinaus Carbonsäureester, wie Ethylacetat, und auch stark polare Solventien, wie Dimethylsulfoxid und Sulfolan. Wenn die Hydrolysestabilität des Säurehalogenids es zuläßt, kann die Umsetzung auch in Gegenwart von Wasser durchgeführt werden.Diluents which can be used in process (a, Variantedl) according to the invention are all solvents which are inert to acid halides. Hydrocarbons such as gasoline, benzene, toluene, xylene and tetralin are preferably usable, furthermore halogenated hydrocarbons such as methylene chloride, chloroform, carbon tetrachloride, chlorobenzene and o-dichlorobenzene, also ketones such as acetone and methyl isopropyl ketone, furthermore ethers such as diethyl ether, tetrahydrofuran and Dioxane, moreover carboxylic acid esters, such as ethyl acetate, and also strongly polar solvents, such as dimethyl sulfoxide and sulfolane. If the hydrolysis stability of the acid halide permits, the reaction can also be carried out in the presence of water.
Die Reaktionstemperaturen können bei der Durchführung des erfindungsgemäßen Verfahrens (a, Variantedj innerhalb eines größeren Bereiches variiert werden. Arbeitet man ohne Lösungsmittel und Säurebindemittel, so geht man im allgemeinen so vor, daß man die Komponenten zunächst bei Temperaturen zwischen -20°C und +20°C reagieren läßt und danach auf Temperaturen zwischen 70 und 200°C erhitzt. Arbeitet man in Gegenwart eines Verdünnungsmittels und eines Säurebindemittels, so liegen die Reaktionstemperaturen im allgemeinen zwischen -20°C und +100°C, vorzugsweise zwischen 0°C und 50°C.The reaction temperatures can be varied within a substantial range when carrying out the process (a, Variantedj). If one works without solvents and acid binders, the procedure is generally such that the components are initially at temperatures between -20 ° C. and +20 ° C can react and then heated to temperatures between 70 and 200 ° C. If one works in the presence of a diluent and an acid binder, the are Reaction temperatures in general between -20 ° C and + 100 ° C, preferably between 0 ° C and 50 ° C.
Das erfindungsgemäße Verfahren (a, Variante α) wird im allgemeinen unter Normaldruck durchgeführt.The process (a, variant α) according to the invention is generally carried out under normal pressure.
Bei der Durchführung des erfindungsgemäßen Verfahrens (a, Variante J) werden die Ausgangsstoffe der Formeln (II) und (III a) im allgemeinen in angenähert äquivalenten Mengen verwendet. Es ist jedoch auch möglich, die eine oder andere Komponente in einem größeren Überschuß einzusetzen. Die Aufarbeitung erfolgt danach üblichen Methoden. Im allgemeinen geht man so vor, daß man ausgefallene Salze entfernt und das verbleibende Reaktionsgemisch durch Abziehen des Verdünnungsmittels einengt. Arbeitet man in Gegenwart von Wasser oder von mit Wasser mischbaren Solventien, so kann man auch so verfahren, daß man das Reaktionsgemisch mit Wasser verdünnt, das entstehende Gemisch abgesaugt oder mit einem Wasser wenig mischbaren organischen Lösungsmittel extrahiert, die organische Phase wäscht, einengt und den verbleibenden Rückstand gegebenenfalls üblichen Reinigungsverfahren unterwirft.When carrying out process (a, variant J) according to the invention, the starting materials of the formulas (II) and (III a) are generally used in approximately equivalent amounts. However, it is also possible to use one or the other component in a larger excess. The processing is then carried out using customary methods. The general procedure is to remove precipitated salts and to concentrate the remaining reaction mixture by stripping off the diluent. If one works in the presence of water or of water-miscible solvents, the procedure can also be such that the reaction mixture is diluted with water, the resulting mixture is suctioned off or extracted with a water-immiscible organic solvent, the organic phase is washed, concentrated and the the remaining residue may be subjected to customary cleaning procedures.
Die bei dem erfindungsgemäßen Verfahren (a, Variante ß) als Reaktionskomponenten benötigten symmetrischen Carbonsäure-anhydride sind durch die Formel (III b) eindeutig definiert. In dieser Formel haben R 3, R 4 und R 5 vorzugsweise diejenigen Bedeutungen, die bereits im Zusammenhang mit der Beschreibung der erfindungsgemäßen Stoffe der Formel (I) vorzugsweise für diese Reste genannt wurden.The symmetrical carboxylic acid anhydrides required as reaction components in the process (a, variant β) according to the invention are clearly defined by the formula (III b). In this formula, R 3 , R 4 and R 5 preferably have those meanings which are already in connection with the description of the invention Substances of formula (I) were preferably mentioned for these radicals.
Die symmetrischen Carbonsäureanhydride der Formel (III b) sind bekannt oder lassen sich nach im Prinzip bekannten Methoden in einfacher Weise herstellen.The symmetrical carboxylic anhydrides of the formula (III b) are known or can be prepared in a simple manner by methods known in principle.
Als Verdünnungsmittel können bei der Durchführung des erfindungsgemäßen Verfahrens (a, Variante ß) vorzugsweise diejenigen Verdünnungsmittel verwendet werden, die auch bei dem Verfahren (a, Variante δ) vorzugsweise in Betracht kommen. Im übrigen kann auch ein im Überschuß eingesetztes Carbonsäureanhydrid der Formel (III b) gleichzeitig als Verdünnungsmittel fungieren.The diluents which can be used in carrying out the process (a, variant β) are preferably those diluents which are also preferred in the process (a, variant δ). Otherwise, an excess carboxylic acid anhydride of the formula (III b) can also act as a diluent.
Die Reaktionstemperaturen können auch bei dem erfindungsgemäßen Verfahren (a, Variante ß) innerhalb eines größeren Bereiches variiert werden. Im allgemeinen arbeitet man bei Temperaturen zwischen -20°C und +150°C, vorzugsweise zwischen 0 und 100°C.The reaction temperatures can also be varied within a substantial range in the process according to the invention (a, variant β). In general, temperatures between -20 ° C and + 150 ° C, preferably between 0 and 100 ° C.
Das erfindungsgemäße Verfahren (a, Variante ß) wird im allgemeinen unter Normaldruck durchgeführt.The process (a, variant β) according to the invention is generally carried out under normal pressure.
Bei der Durchführung des erfindungsgemäßen Verfahrens (a, Variante ß) werden die Ausgangsstoffe der Formeln (II) und (III b) im allgemeinen in angenähert äquivalenten Mengen verwendet. Es ist jedoch auch möglich, das Carbonsäureanhydrid in einem größeren Überschuß einzusetzen. Die Aufarbeitung erfolgt nach üblichen Methoden.When carrying out the process (a, variant β) according to the invention, the starting materials of the formulas (II) and (III b) are generally used in approximately equivalent amounts. However, it is also possible to use the carboxylic anhydride in a larger excess. The processing takes place according to usual methods.
Im allgemeinen geht man so vor, daß man Verdünnungsmittel und im überschuß vorhandenes Carbonsäureanhydrid sowie die entstehende Carbonsäure durch Destillation oder durch Waschen mit einem organischen Lösungsmittel oder mit Wasser entfernt.In general, the procedure is to remove diluent and excess carboxylic acid anhydride and the carboxylic acid formed by distillation or by washing with an organic solvent or with water.
Die bei dem erfindungsgemäßen Verfahren (a, Varianter) als Reaktionskomponenten benötigten asymmetrischen Säureanhydride sind durch die Formel (III c) eindeutig definiert. In dieser Formel haben R3, R 4 und R 5 vorzugsweise diejenigen Bedeutungen, die bereits im Zusammenhang mit der Beschreibung der erfindungsgemäßen Stoffe der Formel (I) vorzugsweise für diese Reste genannt wurden. R steht vorzugsweise für Alkyl mit 1 oder 2 Kohlenstoffatomen oder für Phenyl.The asymmetric acid anhydrides required as reaction components in the process (a, variant) according to the invention are clearly defined by the formula (III c). In this formula, R 3 , R 4 and R 5 preferably have those meanings which have already been mentioned for these radicals in connection with the description of the substances of the formula (I) according to the invention. R preferably represents alkyl having 1 or 2 carbon atoms or phenyl.
Die asymmetrischen Säureanhydride der Formel (III c) sind bekannt oder lassen sich nach im Prinzip bekannten Methoden in einfacher Weise herstellen. So erhält man Verbindungen der Formel (III c) dadurch, daß man Carbonsäuren der Formel
in welcher
- R 3, R 4 und R 5 die oben angegebene Bedeutung haben,
- mit Kohlensäure-ester-chloriden der Formel
in welcher - R die oben angegebene Bedeutung hat,
in Gegenwart eines Verdünnungsmittels, wie zum Beispiel Methylenchlorid, und in Gegenwart eines Säurebindemittels, wie zum Beispiel Triethylamin, bei Temperaturen zwischen -20°C und +100°C, vorzugsweise zwischen 0 und 50°C, umsetzt. Die asymmetrischen Säureanhydride der Formel (III c) werden im allgemeinen nicht in reiner Form isoliert, sondern in der anfallenden Form, gegebenenfalls nach vorheriger Entfernung von Verdünnungsmittel und/ oder Salzen, weiter verwendet.The asymmetric acid anhydrides of the formula (III c) are known or can be prepared in a simple manner by methods known in principle. Thus, compounds of formula (III c) are obtained by carboxylic acids of the formula
in which
- R 3 , R 4 and R 5 have the meaning given above,
- with carbonic acid ester chlorides of the formula
in which - R has the meaning given above,
in the presence of a diluent, such as methylene chloride, and in the presence of an acid binder, such as triethylamine, at temperatures between -20 ° C and + 100 ° C, preferably between 0 and 50 ° C. The asymmetric acid anhydrides of the formula (III c) are generally not isolated in pure form, but are used further in the form obtained, if appropriate after removing diluent and / or salts beforehand.
Als Verdünnungsmittel können bei der Durchführung des erfindungsgemäßen Verfahrens (a, Variante ) vorzugsweise diejenigen Verdünnungsmittel verwendet werden, die auch bei dem Verfahren (a, Variante α) vorzugsweise in Betracht kommen. Im übrigen kann auch im Überschuß eingesetztes Säureanhydrid der Formel (III c) gleichzeitig als Verdünnungsmittel fungieren.The diluents which can be used in carrying out the process (a, variant) according to the invention are preferably those diluents which are also preferred in the process (a, variant α). In addition, excess acid anhydride of the formula (III c) can also act as a diluent.
Die Reaktionstemperaturen können auch bei dem erfindungsgemäßen Verfahren (a, Variantef) innerhalb eines größeren Bereiches variiert werden. Im allgemeinen arbeitet man bei Tempearaturen zwischen -20°C und +150°C, vorzugsweise zwischen 0 und 100°C.The reaction temperatures can also be varied within a substantial range in the process (a, variant f) according to the invention. In general one works with temperature fittings between -20 ° C and + 150 ° C, preferably between 0 and 100 ° C.
Das erfindungsgemäße Verfahren (a, Variantef) wird im allgemeinen unter Normaldruck durchgeführt.The process (a, variant f) according to the invention is generally carried out under normal pressure.
Bei der Durchführung des erfindungsgemäßen Verfahrens (a, Variante δ) werden die Ausgangsstoffe der Formeln (II) und (III c) im allgemeinen in angenähert äquivalenten Mengen verwendet. Es ist jedoch auch möglich, das Säureanhydrid in einem größeren Überschuß einzusetzen. Die Aufarbeitung erfolgt nach üblichen Methoden.When carrying out process (a, variant δ) according to the invention, the starting materials of the formulas (II) and (III c) are generally used in approximately equivalent amounts. However, it is also possible to use the acid anhydride in a larger excess. The processing takes place according to usual methods.
Die bei dem erfindungsgemäßen Verfahren (a, Variante ) als Reaktionskomponenten benötigten Verbindungen sind durch die Formel (III d) eindeutig definiert. In dieser Formel haben R3, R4 und R5 vorzugsweise diejenigen Bedeutungen, die bereits im Zusammenhang mit der Beschreibung der erfindungsgemäßen Stoffe der Formel (I) vorzugsweise für diese Reste genannt wurden.The compounds required as reaction components in the process (a, variant) according to the invention are clearly defined by the formula (III d). In this formula, R 3 , R 4 and R 5 preferably have those meanings which have already been mentioned for these radicals in connection with the description of the substances of the formula (I) according to the invention.
Die Verbindungen der Formel (III d) sind bekannt oder lassen sich nach im Prinzip bekannten Verfahren in einfacher Weise herstellen. So erhält man Verbindungen der Formel (III d) dadurch, daß man Carbonsäuren der Formel
in welcher
- R 3, R 4 und R die oben angegebene Bedeutung haben,
mit Dicyclohexylcarbodiimid der Formel
umsetzt.The compounds of the formula (III d) are known or can be prepared in a simple manner by processes which are known in principle. Thus, compounds of formula (III d) are obtained by carboxylic acids of the formula
in which
- R 3 , R 4 and R have the meaning given above,
with dicyclohexylcarbodiimide of the formula
implements.
Als Verdünnungsmittel können bei der Durchführung des erfindungsgemäßen Verfahrens (a, Variante δ) vorzugsweise diejenigen Verdünnungsmittel verwendet werden, die auch bei dem Verfahren (a, Variante α) vorzugsweise in Betracht kommen.Diluents which can be used when carrying out process (a, variant δ) according to the invention are preferably those diluents which are also preferred in process (a, variant α).
Die Reaktionstemperaturen können auch bei dem erfindungsgemäßen Verfahren (a, Variante ) innerhalb eines größeren Bereiches variiert werden. Im allgemeinen arbeitet man bei Temperaturen zwischen -20°C und +150°C, vorzugsweise zwischen 0 und 100°C.The reaction temperatures can also be varied within a substantial range in the process (a, variant) according to the invention. In general, temperatures between -20 ° C and + 150 ° C, preferably between 0 and 100 ° C.
Das erfindungsgemäße Verfahren (a, Variante δ) wird im allgemeinen unter Normaldruck durchgeführt.The process (a, variant δ) according to the invention is generally carried out under normal pressure.
Bei der Durchführung des erfindungsgemäßen Verfahrens (a, Variante δ) werden die Ausgangsstoffe der Formeln (II) und (III d) im allgemeinen in angenähert äquivalenten Mengen verwendet. Die Aufarbeitung erfolgt nach üblichen Methoden.When carrying out process (a, variant δ) according to the invention, the starting materials of the formulas (II) and (III d) are generally used in approximately equivalent amounts. The processing takes place according to usual methods.
Die bei dem erfindungsgemäßen Verfahren (b) als Ausgangsstoffe benötigten Pyrimidin-Derivate wurden bereits im Zusammenhang mit der Beschreibung des Verfahrens (c) abgehandelt.The pyrimidine derivatives required as starting materials in process (b) according to the invention have already been dealt with in connection with the description of process (c).
Die bei dem erfindungsgemäßen Verfahren (b) weiterhin als Ausgangsstoffe benötigten Acylanilin-Derivate sind durch die Formel (V) eindeutig definiert. In dieser Formel haben R 2, R 3, R 4, R 5, X und n vorzugsweise diejenigen Bedeutungen, die bereits im Zusammenhang mit der Beschreibung der Stoffe der Formel (I) vorzugsweise für diese Reste bzw. für diesen Index genannt wurden.Formula (V) clearly defines the acylaniline derivatives required as starting materials in process (b) according to the invention. In this formula, R 2 , R 3 , R 4 , R 5 , X and n preferably have those meanings which have already been mentioned in connection with the description of the substances of the formula (I), preferably for these radicals or for this index.
Die Verbindungen der Formel (V) sind bekannt oder lassen sich nach im Prinzip bekannten Methoden in einfacher Weise herstellen. So erhält man Acylanilin-Derivate der Formel (V) zum Beispiel dadurch, daß man 4-Amino-(thio)-phenole der Formel
in welcher
- R2, X und n die oben angegebene Bedeutung haben,
mit Säurehalogeniden der Formel
in welcher - R 3, R 4, R 5 und Y die oben angegebene Bedeutung haben,
gegebenenfalls in Gegenwart eines Verdünnungsmittels und gegebenenfalls in Gegenwart eines Säurebindemittels umsetzt. Die Reaktionsbedingungen entsprechen dabei denjenigen, die auch bei der Durchführung des Verfahrens (a, VariantecL) angewandt werden.The compounds of the formula (V) are known or can be prepared in a simple manner by methods known in principle. For example, acylaniline derivatives of the formula (V) are obtained by adding 4-amino (thio) phenols of the formula
in which
- R 2 , X and n have the meaning given above,
with acid halides of the formula
in which - R 3 , R 4 , R 5 and Y have the meaning given above,
if appropriate in the presence of a diluent and if appropriate in the presence of an acid binder. The reaction conditions correspond to those which are also used when carrying out process (a, variant cL).
Als Säurebindemittel können bei der Durchführung des erfindungsgemäßen Verfahrens (b) alle üblichen Säureakzeptoren eingesetzt werden. Vorzugsweise verwendbar sind Alkalimetall- und Erdalkalimetall-oxide, -hydroxide und -carbonate, wie Natriumhydroxid, Kaliumhydroxid, Magnesiumoxid, Calciumoxid, Natriumcarbonat, Kaliumcarbonat und Calciumcarbonat, ferner Alkalimetall-und Erdalkalimetallamide und -hydride, wie Natriumamid, Natriumhydrid und Calciumhydrid, und außerdem auch Alkalimetall-alkoholate, wie Natrium-methylat, Natriumethylat und Kalium-tert.-butylat.All customary acid acceptors can be used as acid binders when carrying out process (b) according to the invention. Alkali metal and alkaline earth metal oxides, hydroxides and carbonates, such as sodium hydroxide, potassium hydroxide, magnesium oxide, calcium oxide, sodium carbonate, potassium carbonate and calcium carbonate, furthermore alkali metal and alkaline earth metal amides and hydrides, such as sodium amide, sodium hydride and calcium hydride, and also also are preferably usable Alkali metal alcoholates, such as sodium methylate, sodium ethylate and potassium tert-butoxide.
Als Verdünnungsmittel können bei dem erfindungsgemäßen Verfahren (b) alle üblichen inerten organischen Solventien eingesetzt werden. Vorzugsweise verwendbar sind Kohlenwasserstoffe, wie Toluol und Xylol, ferner Ether, wie Dibutylether, Tetrahydrofuran, Dioxan, Glykoldimethylether und Diglykoldimethylether, außerdem Nitrile, wie Acetonitril und Propionitril, und weiterhin polare Lösungsmittel, wie Nitrobenzol, Dimethylsulfoxid, Sulfolan, Dimethylformamid und N-Methyl-pyrrolidon.All customary inert organic solvents can be used as diluents in process (b) according to the invention. Hydrocarbons such as toluene and xylene are preferably usable, furthermore ethers such as dibutyl ether, tetrahydrofuran, dioxane, glycol dimethyl ether and diglycol dimethyl ether, also nitriles such as acetonitrile and propionitrile and also polar ones Solvents such as nitrobenzene, dimethyl sulfoxide, sulfolane, dimethylformamide and N-methyl-pyrrolidone.
Die Reaktionstemperaturen können bei der Durchführung des erfindungsgemäßen Verfahrens (b) innerhalb eines größeren Bereiches variiert werden. Im allgemeinen arbeitet man bei Temperaturen zwischen 0 und 200°C, vorzugsweise zwischen 50 und 150°C.The reaction temperatures can be varied within a substantial range when carrying out process (b) according to the invention. In general, temperatures between 0 and 200 ° C, preferably between 50 and 150 ° C.
Das erfindungsgemäße Verfahren (b) wird im allgemeinen unter Normaldruck durchgeführt.Process (b) according to the invention is generally carried out under normal pressure.
Bei der Durchführung des erfindungsgemäßen Verfahrens (b) setzt man die Reaktionskomponenten der Formeln (IV) und (V) im allgemeinen in etwa äquimolaren Mengen ein. Es ist jedoch auch möglich, die eine oder andere Komponente in einem größeren Uberschuß zu verwenden. Außerdem setzt man im allgemeinen auch eine äquimolare Menge an säurebindendem Mittel ein. Es kann jedoch auch von Vorteil sein, das Säurebindemittel in einem bis zu einmolaren Uberschuß hinzuzufügen. Im einzelnen geht man im allgemeinen so vor, daß man das säurebindende Mittel zu einer Mischung der Reaktionskomponenten in einem geeigneten Verdünnungsmittel gibt. Man kann aber auch in der Weise verfahren, daß man zunachst aus dem Acylanilin-Derivat der Formel (V) und dem Säurebindemittel ein Salz erzeugt und dieses dann mit einem PyrimidinDerivat der Formel (IV) umsetzt. Weiterhin ist es auch möglich, aus dem Acylanilin-Derivat der Formel (V) mit einem Säurebindemittel zunächst separat ein Salz herzustellen, dieses dann zu isolieren und anschließend in Gegenwart eines geeigneten Verdünnungsmittels ohne weitere Zugabe eines Säurebindemittels mit einem Pyrimidin-Derivat der Formel (IV) umzusetzen. -Die Aufarbeitung erfolgt jeweils nach üblichen Methoden.When carrying out process (b) according to the invention, the reaction components of the formulas (IV) and (V) are generally employed in approximately equimolar amounts. However, it is also possible to use one or the other component in a larger excess. In addition, an equimolar amount of acid-binding agent is generally also used. However, it may also be advantageous to add the acid binder in an excess of up to one molar. In particular, the general procedure is to add the acid-binding agent to a mixture of the reaction components in a suitable diluent. However, one can also proceed in such a way that a salt is first produced from the acylaniline derivative of the formula (V) and the acid binder and this is then reacted with a pyrimidine derivative of the formula (IV). Furthermore, it is also possible to use the acylaniline derivative of the formula (V) to produce a salt separately from an acid binder, then to isolate it and then to react it with a pyrimidine derivative of the formula (IV) in the presence of a suitable diluent without further addition of an acid binder. - The processing is carried out according to the usual methods.
Die erfindungsgemäßen Wirkstoffe können als Defoliants, Desiccants, Krautabtötungsmittel und insbesondere als Unkrautvernichtungsmittel verwendet werden. Unter Unkraut im weitesten Sinne sind alle Pflanzen zu verstehen, die an Orten aufwachsen, wo sie unerwünscht sind. Ob die erfindungsgemäßen Stoffe als totale oder selektive Herbizide wirken, hängt im wesentlichen von der angewendeten Menge ab.The active compounds according to the invention can be used as defoliants, desiccants, haulm killers and in particular as weed killers. Weeds in the broadest sense are understood to mean all plants that grow up in places where they are undesirable. Whether the substances according to the invention act as total or selective herbicides depends essentially on the amount used.
Die erfindungsgemäßen Wirkstoffe können z.B. bei den folgenden Pflanzen verwendet werden:
- Dikotyle Unkräuter der Gattungen: Sinapis, Lepidium, Galium, Stellaria, Matricaria, Anthemis, Galinsoga, Chenopodium, Urtica, Senecio, Amaranthus, Portulaca, Xanthium, Convolvulus, Ipomoea, Polygonum, Sesbania, Ambrosia, Cirsium, Carduus, Sonchus, Solanum, Rorippa, Rotala, Lindernia, Lamium, Veronica, Abutilon, Emex, Datura, Viola, Galeopsis, Papaver, Centaurea.
- Dikotyle Kulturen der Gattungen: Gossypium, Glycine, Beta, Daucus, Phaseolus, Pisum, Solanum, Linum, Ipomoea, Vicia, Nicotiana, Lycopersicon, Arachis, Brassica, Lactuca, Cucumis, Cucurbita.
- Monokotyle Unkräuter der Gattungen: Echinochloa, Setaria, Panicum, Digitaria, Phleum, Poa, Festuca, Eleusine, Brachiaria, Lolium, Bromus, Avena, Cyperus, Sorghum, Agropyron, Cynodon, Monochoria, Fimbristylis, Sagittaria, Eleocharis, Scirpus, Paspalum, Ischaemum, Sphenoclea, Dactyloctenium, Agrostis, Alopecurus, Apera.
- Monokotyle Kulturen der Gattungen: Oryza, Zea, Triticum, Hordeum, Avena, Secale, Sorghum, Panicum, Saccharum, Ananas, Asparagus, Allium.
- Dicotyledon weeds of the genera: Sinapis, Lepidium, Galium, Stellaria, Matricaria, Anthemis, Galinsoga, Chenopodium, Urtica, Senecio, Amaranthus, Portulaca, Xanthium, Convolvulus, Ipomoea, Polygonum, Sesbania, Ambrosia, Cirsorus, Carduusanum, Sonuanum , Rotala, Lindernia, Lamium, Veronica, Abutilon, Emex, Datura, Viola, Galeopsis, Papaver, Centaurea.
- Dicotyledon cultures of the genera: Gossypium, Glycine, Beta, Daucus, Phaseolus, Pisum, Solanum, Linum, Ipomoea, Vicia, Nicotiana, Lycopersicon, Arachis, Brassica, Lactuca, Cucumis, Cucurbita.
- Monocotyledonous weeds of the genera: Echinochloa, Setaria, Panicum, Digitaria, Phleum, Poa, Festuca, Eleusine, Brachiaria, Lolium, Bromus, Avena, Cyperus, Sorghum, Agropyron, Cynodon, Monochoria, Fimbristylis, Sagittaria, Iochasemirumum, Scalumum, Scalumumum , Sphenoclea, Dactyloctenium, Agrostis, Alopecurus, Apera.
- Monocot cultures of the genera: Oryza, Zea, Triticum, Hordeum, Avena, Secale, Sorghum, Panicum, Saccharum, Pineapple, Asparagus, Allium.
Die Verwendung der erfindungsgemäßen Wirkstoffe ist jedoch keineswegs auf diese Gattungen beschränkt, sondern erstreckt sich in gleicher Weise auch auf andere Pflanzen.However, the use of the active compounds according to the invention is by no means restricted to these genera, but extends in the same way to other plants.
Die Verbindungen eignen sich in Abhängigkeit von der Konzentration zur Totalunkrautbekämpfung z.B. auf Industrie-und Gleisanlagen und auf Wegen und Plätzen mit und ohne Baumbewuchs. Ebenso können die Verbindungen zur Unkrautbekämpfung in Dauerkulturen, z.B. Forst-, Ziergehölz-, Obst-, Wein-, Citrus-, Nuß-, Bananen-, Kaffee-, Tee-, Gummi-, ölpalm-, Kakao-, Beerenfrucht- und Hopfenanlagen und zur selektiven Unkrautbekämpfung in einjährigen Kulturen eingesetzt werden.Depending on the concentration, the compounds are suitable for total weed control, e.g. on industrial and track systems and on paths and squares with and without tree cover. Likewise, the compounds for weed control in permanent crops, e.g. Forestry, ornamental trees, fruit, wine, citrus, nut, banana, coffee, tea, rubber, oil palm, cocoa, berry fruit and hop plants and for selective weed control in annual crops.
Die erfindungsgemäßen Wirkstoffe eignen sich besonders gut zur selektiven Bekämpfung mono- und dikotyler Unkräuter in monokotylen Kulturen, wie zum Beispiel Mais und Getreide.The active compounds according to the invention are particularly suitable for the selective control of mono- and dicotyledon weeds in monocotyledon crops, such as, for example, maize and cereals.
Die erfindungsgemäßen Wirkstoffe weisen auch eine starke mikrobizide Wirkung auf und können zur Bekämpfung von unerwünschten Mikroorganismen praktisch eingesetzt werden. Die Wirkstoffe sind für den Gebrauch als Pflanzenschutzmittel geeignet.The active compounds according to the invention also have a strong microbicidal action and can be used practically to combat unwanted microorganisms. The active ingredients are suitable for use as pesticides.
Fungizide Mittel im Pflanzenschutz werden eingesetzt zur Bekämpfung von Plasmodiophoromycetes, Oomycetes, Chytridiomycetes, Zygomycetes, Ascomycetes, Basidiomycetes, Deuteromycetes.Fungicidal agents in crop protection are used to control Plasmodiophoromycetes, Oomycetes, Chytridiomycetes, Zygomycetes, Ascomycetes, Basidiomycetes, Deuteromycetes.
Die gute Pflanzenverträglichkeit der Wirkstoffe in den zur Bekämpfung von Pflanzenkrankheiten notwendigen Konzentrationen erlaubt eine Behandlung von oberirdischen Pflanzenteilen, von Pflanz- und Saatgut, und des Bodens.The fact that the active compounds are well tolerated by plants in the concentrations required to combat plant diseases permits treatment of above-ground parts of plants, of propagation stock and seeds, and of the soil.
Die erfindungsgemäßen Wirkstoffe lassen sich mit besonderem Vorteil zur Bekämpfung von Pyricularia oryzae bei Reis, gegen Botrytis und zur Bekämpfung von Bohnenrost verwenden.The active compounds according to the invention can be used with particular advantage for combating Pyricularia oryzae in rice, against botrytis and for combating bean rust.
Im übrigen zeichnen sich die erfindungsgemäßen Stoffe auch durch eine insektizide Wirksamkeit aus. Sie besitzen dabei eine gute wurzelsystemische Wirksamkeit. Die Wirkstoffe können in die üblichen Formulierungen überführt werden, wie Lösungen, Emulsionen, Spritzpulver, Suspensionen, Pulver, Stäubemittel, Pasten, lösliche Pulver, Granulate, Suspensions-Emulsions-Konzentrate, wirkstoffimprägnierte Natur- und synthetische Stoffe sowie Feinstverkapselungen in polymeren Stoffen.Otherwise, the substances according to the invention are also distinguished by an insecticidal activity. They have a good root systemic effectiveness. The active ingredients can be converted into the customary formulations, such as solutions, emulsions, wettable powders, suspensions, powders, dusts, pastes, soluble powders, granules, suspension emulsion concentrates, active ingredient-impregnated natural and synthetic substances and very fine encapsulations in polymeric substances.
Diese Formulierungen werden in bekannter Weise hergestellt, z.B. durch Vermischen der Wirkstoffe mit Streckmitteln, also flüssigen Lösungsmitteln und/oder festen Trägerstoffen, gegebenenfalls unter Verwendung von oberflächenaktiven Mitteln, also Emulgiermitteln und/oder Dispergiermitteln und/oder schaumerzeugenden Mitteln.These formulations are made in a known manner, e.g. by mixing the active ingredients with extenders, that is to say liquid solvents and / or solid carriers, optionally using surface-active agents, that is to say emulsifiers and / or dispersants and / or foam-generating agents.
Im Falle der Benutzung von Wasser als Streckmittel können z.B. auch organische Lösungsmittel als Hilfslösungsmittel verwendet werden. Als flüssige Lösungsmittel kommen im wesentlichen in Frage: Aromaten, wie Xylol, Toluol, oder Alkylnaphthaline, chlorierte Aromaten und chlorierte aliphatische Kohlenwasserstoffe, wie Chlorbenzole, Chlorethylene oder Methylenchlorid, aliphatische Kohlenwasserstoffe, wie Cyclohexan oder Paraffine, z.B. Erdölfraktionen, mineralische und pflanzliche öle, Alkohole, wie Butanol oder Glycol sowie deren Ether und Ester, Ketone wie Aceton, Methylethylketon, Methylisobutylketon oder Cyclohexanon, stark polare Lösungsmittel, wie Dimethylformamid und Dimethylsulfoxid, sowie Wasser.If water is used as an extender, e.g. organic solvents can also be used as auxiliary solvents. The following are essentially suitable as liquid solvents: aromatics, such as xylene, toluene, or alkylnaphthalenes, chlorinated aromatics and chlorinated aliphatic hydrocarbons, such as chlorobenzenes, chlorethylenes or methylene chloride, aliphatic hydrocarbons, such as cyclohexane or paraffins, e.g. Petroleum fractions, mineral and vegetable oils, alcohols such as butanol or glycol and their ethers and esters, ketones such as acetone, methyl ethyl ketone, methyl isobutyl ketone or cyclohexanone, strongly polar solvents such as dimethylformamide and dimethyl sulfoxide, and water.
Als feste Trägerstoffe kommen in Frage:
- z. B. Ammoniumsalze und natürliche Gesteinsmehle, wie Kaoline, Tonerden, Talkum, Kreide, Quarz, Attapulgit, Montmorillonit oder Diatomeenerde und synthetische Gesteinsmehle, wie hochdisperse Kieselsäure, Aluminiumoxid und Silikate, als feste Trägerstoffe für Granulate kommen in Frage: z.B. gebrochene und fraktionierte natürliche Gesteine wie Calcit, Marmor, Bims, Sepiolith, Dolomit sowie synthetische Granulate aus anorganischen und organischen Mehlen sowie Granulate aus organischem Material wie Sägemehl, Kokosnußschalen, Maiskolben und Tabakstengeln; als Emulgier- und/oder schaumerzeugende Mittel kommen in Frage: z.B. nichtionogene und anionische Emulgatoren, wie Polyoxyethylen-Fettsäure-Ester, Polyoxyethylen-FettalkoholEther, z.B. Alkylaryl-polyglykolether, Alkylsulfonate, Alkylsulfate, Arylsulfonate sowie Eiweißhydrolysate; als Dispergiermittel kommen in Frage: z.B. Lignin-Sulfitablaugen und Methylcellulose.
- e.g. B. ammonium salts and natural rock powders such as kaolins, clays, talc, chalk, quartz, attapulgite, Montmorillonite or diatomaceous earth and synthetic rock flours, such as highly disperse silica, aluminum oxide and silicates, are suitable as solid carriers for granules: e.g. broken and fractionated natural rocks such as calcite, marble, pumice, sepiolite, dolomite as well as synthetic granules from inorganic and organic flours as well as granules from organic material such as sawdust, coconut shells, corn cobs and tobacco stalks; Suitable emulsifying and / or foam-generating agents are: for example nonionic and anionic emulsifiers, such as polyoxyethylene fatty acid esters, polyoxyethylene fatty alcohol ethers, for example alkylaryl polyglycol ethers, alkyl sulfonates, alkyl sulfates, aryl sulfonates and protein hydrolyzates; The following may be used as dispersants: for example lignin sulfite waste liquor and methyl cellulose.
Es können in den Formulierungen Haftmittel wie Carboxymethylcellulose, natürliche und synthetische pulvrige, körnige oder latexförmige Polymere verwendet werden, wie Gummiarabicum, Polyvinylalkohol, Polyvinylacetat, sowie natürliche Phospholipide, wie Kephaline und Lecithine und synthetische Phospholipide. Weitere Additive können mineralische und vegetabile öle sein.Adhesives such as carboxymethyl cellulose, natural and synthetic powdery, granular or latex-shaped polymers, such as gum arabic, polyvinyl alcohol, polyvinyl acetate, and natural phospholipids, such as cephalins and lecithins and synthetic phospholipids, can be used in the formulations. Other additives can be mineral and vegetable oils.
Es können Farbstoffe wie anorganische Pigmente, z.B. Eisenoxid, Titanoxid, Ferrocyanblau und organische Farbstoffe, wie Alizarin-, Azo- und Metallphthalocyaninfarbstoffe und Spurennährstoffe wie Salze von Eisen, Mangan, Bor, Kupfer, Kobalt, Molybdän und Zink verwendet werden.Dyes such as inorganic pigments, e.g. Iron oxide, titanium oxide, ferrocyan blue and organic dyes such as alizarin, azo and metal phthalocyanine dyes and trace nutrients such as salts of iron, manganese, boron, copper, cobalt, molybdenum and zinc can be used.
Die Formulierungen enthalten im allgemeinen zwischen 0,1 und 95 Gewichtsprozent Wirkstoff, vorzugsweise zwischen 0,5 und 90 %.The formulations generally contain between 0.1 and 95 percent by weight of active compound, preferably between 0.5 and 90%.
Die erfindungsgemäßen Wirkstoffe können als solche oder in ihren Formulierungen auch in Mischung mit bekannten Herbiziden zur Unkrautbekämpfung Verwendung finden, wobei Fertigformulierungen oder Tankmischungen möglich sind.The active compounds according to the invention, as such or in their formulations, can also be used for weed control in a mixture with known herbicides, finished formulations or tank mixes being possible.
Für die Mischungen kommen bekannte Herbizide wie z. B. N-(2-Benzthiazolyl)-N,N'-dimethyl-harnstoff, 3-(3-Chlor-4-methylphenyl)-1,1-dimethylharnstoff, 3-(4-Isopropylphenyl)-1,1-dimethylharnstoff, 4-Amino-6-(1,1-dimethylethyl)-3-methylthio-1,2,4-triazin-5(4H)-on, 4-Amino-6-(1,1-dimethyl-ethyl)-3-ethylthio-1,2,4-triazin-5(4H)-on, 1-Amino-6-ethylthio-3-(2,2-dimethylpropyl)-1,3,5-triazin-2,4-(1H, 3H)-dion, 4-Amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-on, 2-Chlor-4-ethylamino-6-isopropyl-amino-1,3,5-triazin, das R-Enantiomere des 2-L4-(3,5-Dichlor-pyridin-2-oxy)-phenoxY/-propionsäure-(trimethylsilyl)-methylesters, das R-Enantiomere des 2-[4-(3,5-Dichlorpyridyl-2-oxy)-phenoxy]-propionsäure-(2-benzyloxy)-ethylesters, 2,4-Dichlorphenoxyessigsäure, 2-(2,4-Dichlorphenoxy)-propionsäure, 4-Chlor-2-methyl-phenoxy-essigsäure, 2-(2-Methyl-4-chlor-phenoxy)-propionsäure, 3,5-Dijod-4-hydroxy-benzonitril, 3,5-Dibrom-4-hydroxy-benzonitril sowie Diphenylether und Phenylpyridazine, wie zum Beispiel Pyridate. Einige Mischungen zeigen überraschenderweise auch synergistische Wirkung.Known herbicides such as z. B. N- (2-Benzothiazolyl) -N, N'-dimethylurea, 3- (3-chloro-4-methylphenyl) -1,1-dimethylurea, 3- (4-isopropylphenyl) -1,1-dimethylurea , 4-amino-6- (1,1-dimethylethyl) -3-methylthio-1,2,4-triazin-5 (4H) -one, 4-amino-6- (1,1-dimethyl-ethyl) - 3-ethylthio-1,2,4-triazin-5 (4H) -one, 1-amino-6-ethylthio-3- (2,2-dimethylpropyl) -1,3,5-triazine-2,4- ( 1H, 3H) -dione, 4-amino-3-methyl-6-phenyl-1,2,4-triazin-5 (4H) -one, 2-chloro-4-ethylamino-6-isopropylamino-1, 3,5-triazine, the R-enantiomer of 2-L4- (3,5-dichloropyridine-2-oxy) -phenox Y / -propionic acid (trimethylsilyl) -methyl ester, the R-enantiomer of 2- [4th - (3,5-dichloropyridyl-2-oxy) phenoxy] propionic acid (2-benzyloxy) ethyl ester, 2,4-dichlorophenoxyacetic acid, 2- (2,4-dichlorophenoxy) propionic acid, 4-chloro-2- methyl-phenoxy-acetic acid, 2- (2-methyl-4-chlorophenoxy) propionic acid, 3,5-diiodo-4-hydroxy-benzonitrile, 3,5-dibromo-4-hydroxy-benzonitrile as well as diphenyl ether and phenylpyridazine, such as pyridates. Surprisingly, some mixtures also show a synergistic effect.
Auch eine Mischung mit anderen bekannten Wirkstoffen, wie Fungiziden, Insektiziden, Akariziden, Nematiziden, Schutzstoffen gegen Vogelfraß, Pflanzennährstoffen und Bodenstrukturverbesserungsmitteln ist möglich.A mixture with other known active compounds, such as fungicides, insecticides, acaricides, nematicides, bird repellants, plant nutrients and agents which improve soil structure, is also possible.
Die Wirkstoffe können als solche, in Form ihrer Formulierungen oder den daraus durch weiteres Verdünnen bereiteten Anwendungsformen, wie gebrauchsfertige Lösungen, Suspensionen, Emulsionen, Pulver, Pasten und Granulate angewandt werden. Die Anwendung geschieht in üblicher Weise, z. B. durch Gießen, Spritzen, Sprühen, Streuen.The active compounds can be used as such, in the form of their formulations or in the use forms prepared therefrom by further dilution, such as ready-to-use solutions, suspensions, emulsions, powders, pastes and granules. The application happens in the usual way, e.g. B. by pouring, spraying, spraying, scattering.
Die erfindungsgemäßen Wirkstoffe können bei einer Verwendung als Herbizide sowohl vor als auch nach dem Auflaufen der Pflanzen appliziert werden. Sie können auch vor der Saat in den Boden eingearbeitet werden.When used as herbicides, the active compounds according to the invention can be applied both before and after emergence of the plants. They can also be worked into the soil before sowing.
Die angewandte Wirkstoffmenge kann beim Einsatz der erfindungsgemäßen Stoffe als Herbizide in einem größeren Bereich schwanken. Sie hängt im wesentlichen von der Art des gewünschten Effektes ab. Im allgemeinen liegen die Aufwandmengen zwischen 0,01 und 10 kg Wirkstoff pro Hektar Bodenfläche, vorzugsweise zwischen 0,05 und 5 kg pro ha.The amount of active ingredient used can vary over a wide range when the substances according to the invention are used as herbicides. It essentially depends on the type of effect desired. In general, the application rates are between 0.01 and 10 kg of active ingredient per hectare of soil, preferably between 0.05 and 5 kg per ha.
Auch beim Einsatz der erfindungsgemäßen Stoffe als Fungizide kann die Aufwandmenge je nach Art der Applikation in einem größeren Bereich variiert werden. So liegen die Wirkstoffkonzentrationen bei der Behandlung von Pflanzenteilen in den Anwendungsformen im allgemeinen zwischen 1 und 0,0001 Gew.-%, vorzugsweise zwischen 0,5 und 0,001 Gew.-%. Bei der Saatgutbehandlung werden im allgemeinen Wirkstoffmengen von 0,001 bis 50 g je kg Saatgut, vorzugsweise 0,01 bis 10 g, benötigt. Bei Behandlung des Bodens sind Wirkstoffkonzentrationen von 0.00001 bis 0,1 Gew.-%, vorzugsweise von 0,0001 bis 0,02 %, am Wirkungsort erforderlich.When using the substances according to the invention as fungicides, the application rate can be varied within a substantial range depending on the type of application. The active substance concentrations in the treatment of parts of plants in the use forms are generally between 1 and 0.0001% by weight, preferably between 0.5 and 0.001% by weight. When treating seeds, in general Active ingredient amounts of 0.001 to 50 g per kg of seed, preferably 0.01 to 10 g, are required. When treating the soil, active ingredient concentrations of 0.00001 to 0.1% by weight, preferably 0.0001 to 0.02%, are required at the site of action.
Die Herstellung und die Verwendung der erfindungsgemäßen Wirkstoffe geht aus den nachfolgenden Beispielen hervor.The preparation and use of the active compounds according to the invention can be seen from the examples below.
21,5 g (0,1 Mol) 2-(4-Amino-phenoxy)-4,6-dimethyl-pyrimidin wurden in 150 ml Tetrahydrofuran gelöst. Man gab 10,1 g (0,1Mol) Triethylamin hinzu und tropfte dann bei einer Temperatur von 10 bis 15°C unter Rühren 12 g (0,1 Mol) Pivalsäurechlorid ein. Das Gemisch wurde 2 Stunden bei Raumtemperatur nachgerührt. Danach wurde aufgearbeitet, indem man das abgeschiedene Festprodukt absaugte und das Filtrat unter vermindertem Druck eindampfte. Es verblieben 27,5 g (92 % der Theorie) an 2-(4-Pivaloylamino-phenoxy)-4,6-dimethyl-pyrimidin in Form eines Festproduktes.21.5 g (0.1 mol) of 2- (4-aminophenoxy) -4,6-dimethyl-pyrimidine were dissolved in 150 ml of tetrahydrofuran. 10.1 g (0.1 mol) of triethylamine were added and 12 g (0.1 mol) of pivaloyl chloride were then added dropwise at a temperature of 10 to 15 ° C. with stirring. The mixture was stirred at room temperature for 2 hours. The mixture was then worked up by sucking off the solid product which had separated off and evaporating the filtrate under reduced pressure. There remained 27.5 g (92% of theory) of 2- (4-pivaloylamino-phenoxy) -4,6-dimethyl-pyrimidine in the form of a solid product.
Schmelzpunkt: 190-191°C (nach Umkristallisation aus Essigester)Melting point: 190-191 ° C (after recrystallization from ethyl acetate)
11,8 g (0,1 Mol)o6-Methoxy-isobuttersäure wurden in 150 ml Tetrahydrofuran gelöst und mit 10,1 g (0,1 Mol) Triethylamin versetzt. Hierzu tropfte man bei 10 - 15°C unter Rühren 10,85 g (0,1 Mol) Kohlensäureethylesterchlorid. Die Mischung wurde 2 Stunden bei Raumtemperatur nachgerührt und anschließend portionsweise mit 23,1 g (0,1 Mol)2-(4-Amino-phenylmercapto)-4,6-dimethyl-pyrimidin versetzt. Man rührte 1 Stunde bei Raumtemperatur nach und kochte noch 2 Stunden unter Rückfluß. Das Reaktionsgemisch wurde abgekühlt und dann in 1 Liter schwach mit Salzsäure angesäuertes Wasser eingerührt. Die abgeschiedenen Kristalle wurden abgesaugt, mit Wasser gewaschen und an der Luft getrocknet. Man erhielt 27,4 g (83 % der Theorie) 2-(4-(α-Methoxy-isobutyrylamino)-phenylmercapto)-4,6-dimethyl-pyrimidin.11.8 g (0.1 mol) of o6-methoxy-isobutyric acid were dissolved in 150 ml of tetrahydrofuran, and 10.1 g (0.1 mol) of triethylamine were added. For this, 10.85 g (0.1 mol) of ethyl carbonate chloride were added dropwise at 10-15 ° C. with stirring. The mixture was stirred for 2 hours at room temperature and then 23.1 g (0.1 mol) of 2- (4-aminophenylmercapto) -4,6-dimethyl-pyrimidine were added in portions. The mixture was stirred at room temperature for 1 hour and refluxed for a further 2 hours. The reaction mixture was cooled and then stirred into 1 liter of water slightly acidified with hydrochloric acid. The deposited crystals were suction filtered, washed with water and air dried. 27.4 g (83% of theory) of 2- (4- (α-methoxy-isobutyrylamino) phenylmercapto) -4,6-dimethyl-pyrimidine were obtained.
Schmelzpunkt: 123 - 125°C (nach Umkristallisation aus Waschbenzin)Melting point: 123 - 125 ° C (after recrystallization from white spirit)
22,8 g (0,1 Mol) 3-Chlor-4-pivaloylamino-phenol wurden in 150 ml Dimethylsulfoxid gelöst. Hierzu gab man bei Raumtemperatur unter Rühren portionsweise 5,6 g (0,1 Mol) pulverisiertes Kaliumhydroxid. Man rührte 30 Minuten bei Raumtemperatur nach und fügte dann portionsweise 14,25 g (0,1 Mol) 2-Chlor-4,6-dimethyl-pyrimidin hinzu. Die Mischung wurde innerhalb einer Stunde auf 120°C erhitzt und noch 5 Stunden bei dieser Temperatur nachgerührt. Anschließend wurde das Gemisch abgekühlt und in 1 Liter Wasser eingegossen. Die Kristalle wurden abgesaugt und an der Luft getrocknet. Man erhielt 25,5 g (76,5 % der Theorie) 2-(3-Chlor-4-pivaloylamino-phenoxy)-4,6-dimethylpyrimidin.22.8 g (0.1 mol) of 3-chloro-4-pivaloylamino-phenol were dissolved in 150 ml of dimethyl sulfoxide. 5.6 g (0.1 mol) were added in portions at room temperature with stirring powdered potassium hydroxide. The mixture was stirred at room temperature for 30 minutes and then 14.25 g (0.1 mol) of 2-chloro-4,6-dimethyl-pyrimidine were added in portions. The mixture was heated to 120 ° C. within one hour and stirred at this temperature for a further 5 hours. The mixture was then cooled and poured into 1 liter of water. The crystals were suctioned off and air dried. 25.5 g (76.5% of theory) of 2- (3-chloro-4-pivaloylamino-phenoxy) -4,6-dimethylpyrimidine were obtained.
Nach den in den vorausgehenden Beispielen und in der Beschreibung angegebenen Methoden wurden auch die in der folgenden Tabelle formelmäßig aufgeführten Stoffe der Formel (I) hergestellt.According to the methods given in the preceding examples and in the description, the substances of the formula (I) listed in the following table were also prepared.
24,5 g (0,1 Mol) 2-(4-Nitro-phenoxy)-4,6-dimethylpyri- midin wurden in 150 ml Dioxan gelöst und mit Wasserstoff in Gegenwart von Raney-Nickel unter einem Druck von 50 bar bei einer Temperatur zwischen 20 und 50°C erschöpfend hydriert. Danach wurde der Katalysator abgesaugt und das Filtrat unter vermindertem Druck eingedampft. Man erhielt 20,4 g (95 % der Theorie) an 2-(4-Amino- phenoxy)-4,6-dimethylpyrimidin in Form einer Festsubstanz.24.5 g (0.1 mol) of 2- (4-nitro-phenoxy) -4,6-dimethylpyrimidine were dissolved in 150 ml of dioxane and hydrogenated in the presence of Raney nickel under a pressure of 50 bar at a Temperature between 20 and 50 ° C exhaustively hydrogenated. The catalyst was then suction filtered and the filtrate was evaporated under reduced pressure. 20.4 g (95% of theory) of 2- (4-aminophenoxy) -4,6-dimethylpyrimidine were obtained in the form of a solid substance.
Schmelzpunkt: 170 - 171°C (nach Umkristallisation aus Essigester)Melting point: 170 - 171 ° C (after recrystallization from ethyl acetate)
Nach der im Beispiel 75 angegebenen Methode wurden auch die in den folgenden Beispielen aufgeführten Stoffe der Formel (II) hergestellt.The substances of the formula (II) listed in the following examples were also prepared by the method given in Example 75 .
Schmelzpunkt: 170 - 172°C (nach Umkristallisation aus Essigester)Melting point: 170 - 172 ° C (after recrystallization from ethyl acetate)
109 g (1 Mol) 4-Amino-phenol wurden in 500 ml Dimethylsulfoxid gelöst. Nach Zugabe von 56 g (1 Mol) pulverisiertem Kaliumhydroxid verrührte man das Gemisch 30 Minuten bei Raumtemperatur und trug dann portionsweise 143 g (1 Mol) 2-Chlor-4,6-dimethyl-pyrimidin ein.109 g (1 mol) of 4-aminophenol were dissolved in 500 ml of dimethyl sulfoxide. After the addition of 56 g (1 mol) of powdered potassium hydroxide, the mixture was stirred for 30 minutes at room temperature and then 143 g (1 mol) of 2-chloro-4,6-dimethyl-pyrimidine were added in portions.
Die Mischung wurde 5 Stunden auf 100°C erwärmt, abgekühlt und dann in 3 Liter Wasser eingerührt. Das Kristallisat wurde abgesaugt, mehrfach mit Wasser gewaschen und getrocknet. Man erhielt 119 g (55,3 % der Theorie) 2-(4-Amino-phenoxy)-4,6-dimethyl-pyrimidin.The mixture was heated at 100 ° C for 5 hours, cooled and then stirred into 3 liters of water. The crystals were filtered off, washed several times with water and dried. 119 g (55.3% of theory) of 2- (4-amino-phenoxy) -4,6-dimethyl-pyrimidine were obtained.
Schmelzpunkt: 170 - 171°C (nach Umkristallisation aus Ethanol)Melting point: 170 - 171 ° C (after recrystallization from ethanol)
Nach der im Beispiel 78 angegebenen Methode wurden auch die in den folgenden Beispielen aufgeführten Stoffe der Formel (II) hergestellt.The substances of the formula (II) listed in the following examples were also prepared by the method given in Example 78.
Schmelzpunkt: 108 - 110°C.Melting point: 108-110 ° C.
Schmelzpunkt: 168 - 170°C.Melting point: 168-170 ° C.
Nach der im Beispiel 83 angegebenen Methode wurden auch die in den folgenden Beispielen aufgeführten Stoffe der Formel (V) hergestellt.The substances of the formula (V) listed in the following examples were also prepared by the method given in Example 83.
Schmelzpunkt: 150 - 152°C (nach Umkristallisation aus Toluol)Melting point: 150 - 152 ° C (after recrystallization from toluene)
In dem nachfolgenden Verwendungsbeispiel wurde die folgende Verbindung als Vergleichssubstanz eingesetzt:
2-Phenoxy-4,6-dimethyl-pyrimidin (bekannt aus US-PS 3, 126, 271)2-phenoxy-4,6-dimethyl-pyrimidine (known from US Pat. No. 3,126,271)
Post-emergence-Test
- Lösungsmittel: 5 Gewichtsteile Aceton
- Emulgator: 1 Gewichtsteil Alkylarylpolyglykolether
- Solvent: 5 parts by weight of acetone
- Emulsifier: 1 part by weight of alkylaryl polyglycol ether
Zur Herstellung einer zweckmäßigen Wirkstoffzubereitung vermischt man 1 Gewichtsteil Wirkstoff mit der angegebenen Menge Lösungsmittel, gibt die angegebene Menge Emulgator zu und verdünnt das Konzentrat mit Wasser auf die gewünschte Konzentration.To produce a suitable preparation of active compound, 1 part by weight of active compound is mixed with the stated amount of solvent, the stated amount of emulsifier is added and the concentrate is diluted with water to the desired concentration.
Mit der Wirkstoffzubereitung spritzt man Testpflanzen, welche eine Höhe von 5 - 15 cm haben so, daß die jeweils gewünschten Wirkstoffmengen pro Flächeneinheit ausgebracht werden. Die Konzentration der Spritzbrühe wird so gewählt, daß in 2000 1 Wasser/ha die jeweils gewünschten Wirkstoffmengen ausgebracht werden. Nach drei Wochen wird der Schädigungsgrad der Pflanzen bonitiert in % Schädigung im Vergleich zur Entwicklung der unbehandelten Kontrolle.Test plants which have a height of 5-15 cm are sprayed with the active substance preparation in such a way that the desired amounts of active substance are applied per unit area. The concentration of the spray liquor is chosen so that the desired amounts of active compound are applied in 2000 l of water / ha. After three weeks, the degree of damage to the plants is rated in% damage compared to the development of the untreated control.
Es bedeuten:
- 0 % = keine Wirkung (wie unbehandelte Kontrolle)
- 100 % = totale Vernichtung
- 0% = no effect (like untreated control)
- 100% = total annihilation
In diesem Test zeigt die Verbindung gemäß Beispiel45 bei der selektiven Bekämpfung von Galinsoga, Galium, Sinapis, Stellaria, Echinochloa, Panicum und Poa in Weizen und Mais eine wesentlich bessere herbizide Wirksamkeit als die Vergleichssubstanz (A).In this test, the compound according to Example 45 in the selective control of galinsoga, galium, sinapis, stellaria, echinochloa, panicum and poa in wheat and corn shows a substantially better herbicidal activity than the comparison substance (A).
Grenzkonzentrations-Test / Wurzelsystemische Wirkung
- Testinsekt: Myzus persicae
- Lösungsmittel: 3 Gewichtsteile Aceton
- Emulgator: 1 Gewichtsteil Alkylarylpolyglykolether
- Test insect: Myzus persicae
- Solvent: 3 parts by weight of acetone
- Emulsifier: 1 part by weight of alkylaryl polyglycol ether
Zur Herstellung einer zweckmäßigen Wirkstoffzubereitung vermischt man 1 Gewichtsteil Wirkstoff mit der angegebenen Menge Lösungsmittel, gibt die angegebene Menge Emulgator zu und verdünnt das Konzentrat mit Wasser auf die gewünschte Konzentration.To produce a suitable preparation of active compound, 1 part by weight of active compound is mixed with the stated amount of solvent, the stated amount of emulsifier is added and the concentrate is diluted with water to the desired concentration.
Die Wirkstoffzubereitung wird innig mit Boden vermischt. Dabei spielt die Konzentration des Wirkstoffs in der Zubereitung praktisch keine Rolle, entscheidend ist allein die Wirkstoffzubereitung pro Volumeneinheit Boden, welche in ppm (= mg/1) angegeben wird. Man füllt den behandelten Boden in Töpfe und bepflanzt diese mit Kohl (Brassica oleracea). Der Wirkstoff kann so von den Pflanzenwurzeln aus dem Boden aufgenommen und in die Blätter transportiert werden.The active ingredient preparation is intimately mixed with soil. The concentration of the active ingredient in the preparation is practically irrelevant, the only decisive factor is the active ingredient preparation per unit volume of soil, which is given in ppm (= mg / 1). The treated soil is filled into pots and planted with cabbage (Brassica oleracea). The active ingredient can be taken up from the soil by the plant roots and transported to the leaves.
Für den Nachweis des wurzelsystemischen Effektes werden nach 7 Tagen ausschließlich die Blätter mit den obengenannten Testtieren besetzt. Nach weiteren 2 Tagen erfolgt die Auswertung durch Zählen oder Schätzen der toten Tiere. Aus den Abtötungszahlen wird die wurzelsystemische Wirkung des Wirkstoffs abgeleitet. Sie ist 100 %, wenn alle Testtiere abgetötet sind und 0 %, wenn noch genau so viele Testinsekten leben wie bei der unbehandelten Kontrolle.To prove the root systemic effect, only the leaves are populated with the test animals mentioned above after 7 days. After another 2 days, the evaluation is carried out by counting or estimating the dead animals. The root systemic effect of the active ingredient is derived from the number of killings. It is 100% when all test animals have been killed and 0% when as many test insects are still alive as in the untreated control.
In diesem Test zeichnen sich die Verbindungen gemäß Beispielen (14), (20) und (38) durch eine sehr gute Wirksamkeit aus.In this test, the compounds according to Examples (14), (20) and (38) are notable for very good activity.
Claims (7)
in welcher
und
in which
and
und
and
in welcher
und
dadurch gekennzeichnet, daß man
in welcher
entweder
in welcher
gegebenenfalls in Gegenwart eines Verdünnungsmittels und gegebenenfalls in Gegenwart eines Säurebindemittels umsetzt,
oder
in welcher
gegebenenfalls in Gegenwart eines Verdünnungsmittels umsetzt,
in welcher
gegebenenfalls in Gegenwart eines Verdünnungsmittels umsetzt.
oder
in wlcher
gegebenenfalls in Gegenwart eines Verdünnungsmittels umsetzt,
in welcher
in welcher
in Gegenwart eines Säurebindemittels und gegebenenfalls in Gegenwart eines Verdünnungsmittels umsetzt.3) Process for the preparation of substituted carboxylic acid anilides of the formula
in which
and
characterized in that one
in which
either
in which
if appropriate in the presence of a diluent and if appropriate in the presence of an acid binder,
or
in which
if appropriate in the presence of a diluent,
in which
if appropriate in the presence of a diluent.
or
in more
if appropriate in the presence of a diluent,
in which
in which
in the presence of an acid binder and optionally in the presence of a diluent.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19843422077 DE3422077A1 (en) | 1984-06-14 | 1984-06-14 | SUBSTITUTED CARBONIC ACID ANILIDE |
| DE3422077 | 1984-06-14 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0168608A2 true EP0168608A2 (en) | 1986-01-22 |
| EP0168608A3 EP0168608A3 (en) | 1986-03-19 |
Family
ID=6238341
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP85106804A Withdrawn EP0168608A3 (en) | 1984-06-14 | 1985-06-03 | Substituted carboxylic-acid anilides |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US4627871A (en) |
| EP (1) | EP0168608A3 (en) |
| JP (1) | JPS6117572A (en) |
| DE (1) | DE3422077A1 (en) |
| DK (1) | DK268485A (en) |
| HU (1) | HU195194B (en) |
| IL (1) | IL75483A (en) |
| ZA (1) | ZA854450B (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0219835A2 (en) * | 1985-10-18 | 1987-04-29 | Stauffer Chemical Company | Acylaminoaryloxy and arylthio pyrimidines |
| EP0226885A1 (en) * | 1985-12-13 | 1987-07-01 | Bayer Ag | Pyrimidylmercapto-acylanilides |
| EP0229283A1 (en) * | 1985-12-13 | 1987-07-22 | Bayer Ag | Anilides of pyrimidyl-thio-carboxylic acids |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4888047A (en) * | 1985-10-18 | 1989-12-19 | Ici Americas Inc. | Acylaminoaryloxy and arylthio pyrimidines as herbicides |
| US4847258A (en) * | 1986-08-26 | 1989-07-11 | Ciba-Geigy Corporation | Substituted benzoylphenylureas compounds useful as pesticides |
| JPH01109312U (en) * | 1988-01-18 | 1989-07-24 | ||
| AU2001241128A1 (en) * | 2000-03-14 | 2001-09-24 | Fujisawa Pharmaceutical Co. Ltd. | Novel amide compounds |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2501648A1 (en) * | 1974-01-22 | 1975-07-24 | Dow Chemical Co | SUBSTITUTED PYRIDINYLOXY (THIO) PHENYL ALKANAMIDES AND UREA |
| EP0015124B1 (en) * | 1979-02-16 | 1983-06-01 | Ici Australia Limited | Parasiticidal heterocyclic ether derivatives, processes for the manufacture thereof and compositions thereof |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3126271A (en) * | 1964-03-24 | Method of controlling weeds | ||
| US3250775A (en) * | 1963-02-06 | 1966-05-10 | Stauffer Chemical Co | Process for preparing benzylmercapto pyrimidines |
| NZ188244A (en) * | 1977-09-13 | 1981-04-24 | Ici Australia Ltd | 2-substituted pyrimidines compositions growth regulating processes |
-
1984
- 1984-06-14 DE DE19843422077 patent/DE3422077A1/en not_active Withdrawn
-
1985
- 1985-05-24 US US06/737,977 patent/US4627871A/en not_active Expired - Fee Related
- 1985-06-03 EP EP85106804A patent/EP0168608A3/en not_active Withdrawn
- 1985-06-11 IL IL75483A patent/IL75483A/en unknown
- 1985-06-13 DK DK268485A patent/DK268485A/en not_active Application Discontinuation
- 1985-06-13 ZA ZA854450A patent/ZA854450B/en unknown
- 1985-06-14 HU HU852366A patent/HU195194B/en unknown
- 1985-06-14 JP JP60128398A patent/JPS6117572A/en active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2501648A1 (en) * | 1974-01-22 | 1975-07-24 | Dow Chemical Co | SUBSTITUTED PYRIDINYLOXY (THIO) PHENYL ALKANAMIDES AND UREA |
| EP0015124B1 (en) * | 1979-02-16 | 1983-06-01 | Ici Australia Limited | Parasiticidal heterocyclic ether derivatives, processes for the manufacture thereof and compositions thereof |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0219835A2 (en) * | 1985-10-18 | 1987-04-29 | Stauffer Chemical Company | Acylaminoaryloxy and arylthio pyrimidines |
| EP0219835A3 (en) * | 1985-10-18 | 1988-02-10 | Stauffer Chemical Company | Acylaminoaryloxy and arylthio pyrimidines |
| EP0226885A1 (en) * | 1985-12-13 | 1987-07-01 | Bayer Ag | Pyrimidylmercapto-acylanilides |
| EP0229283A1 (en) * | 1985-12-13 | 1987-07-22 | Bayer Ag | Anilides of pyrimidyl-thio-carboxylic acids |
| US4765824A (en) * | 1985-12-13 | 1988-08-23 | Bayer Aktiengesellschaft | Pyrimidyl-thio-carboxanilides |
| US4797146A (en) * | 1985-12-13 | 1989-01-10 | Bayer Aktiengesellschaft | Herbicidal 2-[4-substituted carboxyamino-phenyl mercapto)]-subsituted pyrimidines |
Also Published As
| Publication number | Publication date |
|---|---|
| IL75483A0 (en) | 1985-10-31 |
| HU195194B (en) | 1988-04-28 |
| IL75483A (en) | 1988-11-30 |
| US4627871A (en) | 1986-12-09 |
| ZA854450B (en) | 1986-02-26 |
| HUT38321A (en) | 1986-05-28 |
| DK268485D0 (en) | 1985-06-13 |
| DK268485A (en) | 1985-12-15 |
| DE3422077A1 (en) | 1985-12-19 |
| EP0168608A3 (en) | 1986-03-19 |
| JPS6117572A (en) | 1986-01-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0137358A2 (en) | 1,3-Diaryl-5-methylene-perhydropyrimidin-2-one | |
| EP0096354B1 (en) | Substituted pyridyl-phenyl ethers | |
| EP0256396A1 (en) | Triazolo-pyrimidine-2-sulfon amides | |
| EP0168608A2 (en) | Substituted carboxylic-acid anilides | |
| EP0068260B1 (en) | Substituted phenoxypropionates, process for their preparation and their use as herbicides | |
| EP0037526A1 (en) | Substituted acetanilides, process for their preparation and their utilization as herbicides | |
| EP0173319A1 (en) | Substituted arylsulfonyl guanidines | |
| DE3335477A1 (en) | 2-ARYL-2-HALOGENALKYL-OXIRANE | |
| EP0037527A1 (en) | Substituted acetanilides, process for their preparation and their utilization as herbicides | |
| DE3219789A1 (en) | PHENOXYPROPIONIC ACID DERIVATIVES, METHOD FOR THE PRODUCTION THEREOF AND THEIR USE AS HERBICIDES | |
| EP0226885A1 (en) | Pyrimidylmercapto-acylanilides | |
| DE3425123A1 (en) | Optically active phenoxypropionic acid derivatives | |
| EP0127808A1 (en) | Optically active derivatives of phenoxy propionic acid | |
| EP0243868A2 (en) | Pyridylthio-acylanilides | |
| EP0060423B1 (en) | 2-pyridyloxyacetanilides, process for their preparation and their use as herbicides | |
| EP0307753A2 (en) | Sulfonyl isothiourea and process and intermediates for their preparation | |
| DE3724467A1 (en) | 6-Chlorobenzoxazolyloxyacetanilides | |
| DE3502266A1 (en) | OPTICALLY ACTIVE PHENOXYPROPIONIC ACID DERIVATIVES | |
| DE3431933A1 (en) | HETEROARYLTHIOHURANE | |
| EP0037525A1 (en) | Substituted acetanilides, process for their preparation and their utilization as herbicides | |
| EP0126361A1 (en) | Optically active propionic-acid derivatives | |
| DE3035021A1 (en) | 3-Di:methylamino-4-methyl-6-substd. phenyl-1,2,4-triazinone derivs. - with plant growth regulant, herbicidal and fungicidal activity | |
| EP0089538A1 (en) | Optically active derivatives of phenoxy-benzoic acid, process for their preparation and their use as herbicides | |
| EP0220635A1 (en) | Phenoxybenzoic-acid derivatives | |
| DE3510794A1 (en) | 6-t-Butyl-3-methylthio-4-sulphimido-1,2,4-triazin-5-ones |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19850603 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI NL |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE CH DE FR GB IT LI NL |
|
| 17Q | First examination report despatched |
Effective date: 19860822 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN WITHDRAWN |
|
| 18W | Application withdrawn |
Withdrawal date: 19890227 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: EUE, LUDWIG, DR. Inventor name: SCHMIDT, ROBERT, R. DR. Inventor name: SANTEL, HANS-JOACHIM Inventor name: SASSE, KLAUS, DR. |