EP0144748A2 - Imidazolinones, process for their preparation and their use in plant ptotection - Google Patents
Imidazolinones, process for their preparation and their use in plant ptotection Download PDFInfo
- Publication number
- EP0144748A2 EP0144748A2 EP84113259A EP84113259A EP0144748A2 EP 0144748 A2 EP0144748 A2 EP 0144748A2 EP 84113259 A EP84113259 A EP 84113259A EP 84113259 A EP84113259 A EP 84113259A EP 0144748 A2 EP0144748 A2 EP 0144748A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- alkyl
- halogen
- formula
- phenyl
- substituted
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- CAAMSDWKXXPUJR-UHFFFAOYSA-N 3,5-dihydro-4H-imidazol-4-one Chemical class O=C1CNC=N1 CAAMSDWKXXPUJR-UHFFFAOYSA-N 0.000 title claims abstract description 8
- 238000000034 method Methods 0.000 title claims description 10
- 238000002360 preparation method Methods 0.000 title claims description 7
- -1 cyanomethyl Chemical group 0.000 claims abstract description 32
- 229910052736 halogen Inorganic materials 0.000 claims abstract description 22
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims abstract description 22
- 150000002367 halogens Chemical class 0.000 claims abstract description 17
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 11
- 125000004453 alkoxycarbonyl group Chemical group 0.000 claims abstract description 10
- 125000003342 alkenyl group Chemical group 0.000 claims abstract description 6
- 125000004849 alkoxymethyl group Chemical group 0.000 claims abstract description 5
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims abstract description 5
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 claims abstract description 4
- 239000003630 growth substance Substances 0.000 claims abstract description 4
- 239000004009 herbicide Substances 0.000 claims abstract description 4
- 150000001875 compounds Chemical class 0.000 claims description 30
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 25
- 229910052739 hydrogen Inorganic materials 0.000 claims description 15
- 239000001257 hydrogen Substances 0.000 claims description 15
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 14
- 239000002253 acid Substances 0.000 claims description 13
- 230000012010 growth Effects 0.000 claims description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 12
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 claims description 11
- 125000004400 (C1-C12) alkyl group Chemical group 0.000 claims description 8
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 8
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims description 8
- 150000001408 amides Chemical class 0.000 claims description 8
- 230000002363 herbicidal effect Effects 0.000 claims description 8
- 125000003944 tolyl group Chemical group 0.000 claims description 8
- 125000006529 (C3-C6) alkyl group Chemical group 0.000 claims description 7
- 239000000460 chlorine Substances 0.000 claims description 7
- 150000003839 salts Chemical class 0.000 claims description 7
- 229910052801 chlorine Inorganic materials 0.000 claims description 6
- 125000000068 chlorophenyl group Chemical group 0.000 claims description 6
- 239000011737 fluorine Substances 0.000 claims description 6
- 229910052731 fluorine Inorganic materials 0.000 claims description 6
- 239000003795 chemical substances by application Substances 0.000 claims description 5
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 claims description 4
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 4
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 claims description 4
- 125000004399 C1-C4 alkenyl group Chemical group 0.000 claims description 4
- 125000003282 alkyl amino group Chemical group 0.000 claims description 4
- 150000002431 hydrogen Chemical class 0.000 claims description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 4
- 125000004454 (C1-C6) alkoxycarbonyl group Chemical group 0.000 claims description 3
- 125000006650 (C2-C4) alkynyl group Chemical group 0.000 claims description 3
- 230000010933 acylation Effects 0.000 claims description 3
- 238000005917 acylation reaction Methods 0.000 claims description 3
- 230000029936 alkylation Effects 0.000 claims description 3
- 238000005804 alkylation reaction Methods 0.000 claims description 3
- 230000015572 biosynthetic process Effects 0.000 claims description 3
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 claims description 3
- 229910052794 bromium Inorganic materials 0.000 claims description 3
- 229910052799 carbon Inorganic materials 0.000 claims description 3
- 150000001721 carbon Chemical group 0.000 claims description 3
- 230000008030 elimination Effects 0.000 claims description 3
- 238000003379 elimination reaction Methods 0.000 claims description 3
- 125000005843 halogen group Chemical group 0.000 claims description 3
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 3
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 3
- 230000003287 optical effect Effects 0.000 claims description 3
- 230000003647 oxidation Effects 0.000 claims description 3
- 238000007254 oxidation reaction Methods 0.000 claims description 3
- 238000006277 sulfonation reaction Methods 0.000 claims description 3
- 230000001276 controlling effect Effects 0.000 claims 2
- 230000001105 regulatory effect Effects 0.000 claims 2
- 125000001188 haloalkyl group Chemical group 0.000 abstract description 4
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical compound O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 abstract 1
- 125000003545 alkoxy group Chemical group 0.000 abstract 1
- 150000001733 carboxylic acid esters Chemical class 0.000 abstract 1
- 125000000753 cycloalkyl group Chemical group 0.000 abstract 1
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 abstract 1
- 241000196324 Embryophyta Species 0.000 description 21
- 239000004480 active ingredient Substances 0.000 description 19
- 239000000203 mixture Substances 0.000 description 13
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 9
- 230000000694 effects Effects 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 235000014113 dietary fatty acids Nutrition 0.000 description 7
- 239000000194 fatty acid Substances 0.000 description 7
- 229930195729 fatty acid Natural products 0.000 description 7
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 6
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 6
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 239000011734 sodium Substances 0.000 description 6
- 229910052708 sodium Inorganic materials 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 244000038559 crop plants Species 0.000 description 5
- 239000004495 emulsifiable concentrate Substances 0.000 description 5
- 239000003995 emulsifying agent Substances 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- 239000002904 solvent Substances 0.000 description 5
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 4
- 229920000151 polyglycol Polymers 0.000 description 4
- 239000010695 polyglycol Substances 0.000 description 4
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 239000012141 concentrate Substances 0.000 description 3
- 239000002270 dispersing agent Substances 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 239000003337 fertilizer Substances 0.000 description 3
- 238000000227 grinding Methods 0.000 description 3
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 238000007363 ring formation reaction Methods 0.000 description 3
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 3
- 235000017557 sodium bicarbonate Nutrition 0.000 description 3
- 239000000080 wetting agent Substances 0.000 description 3
- 239000008096 xylene Substances 0.000 description 3
- NOKZRORHBQSMJX-UHFFFAOYSA-N 4-methyl-4-propan-2-yl-2-[2-(trifluoromethyl)phenyl]-1h-imidazol-5-one Chemical compound N1C(=O)C(C(C)C)(C)N=C1C1=CC=CC=C1C(F)(F)F NOKZRORHBQSMJX-UHFFFAOYSA-N 0.000 description 2
- 239000005995 Aluminium silicate Substances 0.000 description 2
- 0 CC(C)(/C=C1)/C(/*)=C(\*)/C(/*)=N/C1(C)C(N*(*)*(*)CC=CC)=O Chemical compound CC(C)(/C=C1)/C(/*)=C(\*)/C(/*)=N/C1(C)C(N*(*)*(*)CC=CC)=O 0.000 description 2
- 240000005979 Hordeum vulgare Species 0.000 description 2
- 235000007340 Hordeum vulgare Nutrition 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical class NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- 241001300479 Macroptilium Species 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- 150000001204 N-oxides Chemical class 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- 235000007238 Secale cereale Nutrition 0.000 description 2
- 244000082988 Secale cereale Species 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 244000098338 Triticum aestivum Species 0.000 description 2
- 235000012211 aluminium silicate Nutrition 0.000 description 2
- 238000009835 boiling Methods 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 235000013339 cereals Nutrition 0.000 description 2
- 150000001805 chlorine compounds Chemical class 0.000 description 2
- 150000002170 ethers Chemical class 0.000 description 2
- 241001233957 eudicotyledons Species 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 150000002191 fatty alcohols Chemical class 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 230000009036 growth inhibition Effects 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 230000017066 negative regulation of growth Effects 0.000 description 2
- UHZYTMXLRWXGPK-UHFFFAOYSA-N phosphorus pentachloride Chemical compound ClP(Cl)(Cl)(Cl)Cl UHZYTMXLRWXGPK-UHFFFAOYSA-N 0.000 description 2
- 229910052938 sodium sulfate Inorganic materials 0.000 description 2
- 235000011152 sodium sulphate Nutrition 0.000 description 2
- 239000002689 soil Substances 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 230000009105 vegetative growth Effects 0.000 description 2
- 239000004563 wettable powder Substances 0.000 description 2
- YCPQUHCGFDFLSI-LURJTMIESA-N (2s)-2-amino-2,3-dimethylbutanamide Chemical compound CC(C)[C@](C)(N)C(N)=O YCPQUHCGFDFLSI-LURJTMIESA-N 0.000 description 1
- XUJLWPFSUCHPQL-UHFFFAOYSA-N 11-methyldodecan-1-ol Chemical compound CC(C)CCCCCCCCCCO XUJLWPFSUCHPQL-UHFFFAOYSA-N 0.000 description 1
- MXIUWSYTQJLIKE-UHFFFAOYSA-N 2-(trifluoromethyl)benzoyl chloride Chemical compound FC(F)(F)C1=CC=CC=C1C(Cl)=O MXIUWSYTQJLIKE-UHFFFAOYSA-N 0.000 description 1
- FOGYNLXERPKEGN-UHFFFAOYSA-N 3-(2-hydroxy-3-methoxyphenyl)-2-[2-methoxy-4-(3-sulfopropyl)phenoxy]propane-1-sulfonic acid Chemical compound COC1=CC=CC(CC(CS(O)(=O)=O)OC=2C(=CC(CCCS(O)(=O)=O)=CC=2)OC)=C1O FOGYNLXERPKEGN-UHFFFAOYSA-N 0.000 description 1
- YYROPELSRYBVMQ-UHFFFAOYSA-N 4-toluenesulfonyl chloride Chemical compound CC1=CC=C(S(Cl)(=O)=O)C=C1 YYROPELSRYBVMQ-UHFFFAOYSA-N 0.000 description 1
- 241000219310 Beta vulgaris subsp. vulgaris Species 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 241000219146 Gossypium Species 0.000 description 1
- 101000913968 Ipomoea purpurea Chalcone synthase C Proteins 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- 241000209510 Liliopsida Species 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 101000907988 Petunia hybrida Chalcone-flavanone isomerase C Proteins 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 235000021536 Sugar beet Nutrition 0.000 description 1
- 235000021307 Triticum Nutrition 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000016383 Zea mays subsp huehuetenangensis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- GYJDNTLCTPWPIK-UHFFFAOYSA-N [N].N1C=NCC1 Chemical compound [N].N1C=NCC1 GYJDNTLCTPWPIK-UHFFFAOYSA-N 0.000 description 1
- 206010000269 abscess Diseases 0.000 description 1
- 230000000895 acaricidal effect Effects 0.000 description 1
- 239000000642 acaricide Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 125000006193 alkinyl group Chemical group 0.000 description 1
- 125000002877 alkyl aryl group Chemical group 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 238000010533 azeotropic distillation Methods 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 239000007859 condensation product Substances 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- VAYGXNSJCAHWJZ-UHFFFAOYSA-N dimethyl sulfate Chemical compound COS(=O)(=O)OC VAYGXNSJCAHWJZ-UHFFFAOYSA-N 0.000 description 1
- 229940071161 dodecylbenzenesulfonate Drugs 0.000 description 1
- 238000010410 dusting Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- SRCZQMGIVIYBBJ-UHFFFAOYSA-N ethoxyethane;ethyl acetate Chemical compound CCOCC.CCOC(C)=O SRCZQMGIVIYBBJ-UHFFFAOYSA-N 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000000417 fungicide Substances 0.000 description 1
- 230000035784 germination Effects 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 208000037824 growth disorder Diseases 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 150000003840 hydrochlorides Chemical class 0.000 description 1
- 239000012433 hydrogen halide Substances 0.000 description 1
- 229910000039 hydrogen halide Inorganic materials 0.000 description 1
- 239000002917 insecticide Substances 0.000 description 1
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 1
- 239000012948 isocyanate Substances 0.000 description 1
- 150000002513 isocyanates Chemical class 0.000 description 1
- 229910052622 kaolinite Inorganic materials 0.000 description 1
- 235000009973 maize Nutrition 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- SNQQPOLDUKLAAF-UHFFFAOYSA-N nonylphenol Chemical class CCCCCCCCCC1=CC=CC=C1O SNQQPOLDUKLAAF-UHFFFAOYSA-N 0.000 description 1
- 229920002113 octoxynol Polymers 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 230000000885 phytotoxic effect Effects 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920001522 polyglycol ester Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 239000004576 sand Substances 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 238000009331 sowing Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 230000036435 stunted growth Effects 0.000 description 1
- ZBZJXHCVGLJWFG-UHFFFAOYSA-N trichloromethyl(.) Chemical compound Cl[C](Cl)Cl ZBZJXHCVGLJWFG-UHFFFAOYSA-N 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D233/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings
- C07D233/54—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members
- C07D233/66—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings having two double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D233/70—One oxygen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
Definitions
- the cyclization of the amides (II) can e.g. with phosphorus pentachloride, advantageously in the presence of a solvent which is inert under the reaction conditions.
- a solvent which is inert under the reaction conditions. Examples of the latter are: toluene, xylene, chloroform or phosphorus oxychloride.
- the reaction temperature is not critical and can be varied between -10 ° C and + 150 ° C. Reaction temperatures between 0 and 100 ° C. are particularly advantageous.
- the hydrochlorides of the imidazolinones (I) are primarily obtained. According to usual methods, e.g. by reaction with sodium carbonate or sodium hydrogen carbonate, the free bases can be produced therefrom.
- the cyclization can also take place in the presence of strong organic or inorganic acids, such as sulfuric acid or p-toluenesulfonic acid, with simultaneous removal of the water formed at temperatures from 0 ° C. to 150 ° C.
- the reaction can be carried out particularly advantageously in such a way that the water formed is separated off by azeotropic distillation with a solvent such as toluene, xylene or chloroform. After neutralization, the products can be isolated using customary methods.
- the compounds according to the invention are tautomeric, so that they can be in one of the two forms (I a) / (I b) or as a mixture of (I a) and (I b). These isomers also occur in the derivatives with Z ⁇ H.
- Both the acid addition salts and the N-oxides of the compounds of the formula (I) are readily accessible in a generally known manner, the latter, for example, by reaction with peroxides or H 2 O 2 .
- the amides of the formula (II) can easily be obtained from the aminoamides (III) and the correspondingly substituted carboxylic acid derivatives (IV).
- the present compounds according to the invention have excellent herbicidal activity against a broad spectrum of economically important mono- and dicotyledonous harmful plants. Perennial root weeds that are difficult to control are also well captured by the active ingredients. It does not matter whether the substances are applied in pre-sowing, pre-emergence or post-emergence spraying. If the compounds according to the invention are applied to the earth's surface before germination, the emergence of the seedlings is not completely prevented. The weeds grow to the cotyledon stage, but then stop growing and eventually die completely after 3 weeks.
- the active ingredients When the active ingredients are applied to the green parts of the plant in the post-emergence process, there is also a drastic growth stop very quickly after the treatment and the weed plants remain in the growth stage at the time of application or die completely after a certain time, so that one for the crop plants U harmful nkrautkonkurrenz very early and can be permanently eliminated through the use of new compositions of the invention.
- the compounds according to the invention have excellent herbicidal activity against monocotyledonous and dicotyledon weeds, crop plants of economically important crops such as, for. B. wheat, barley, rye, rice, maize, sugar beet, cotton and soy only insignificantly or even not harmed.
- the substances according to the invention thus have excellent selectivity in crop plants and, for these reasons, are very suitable for controlling unwanted vegetation in agricultural crops.
- the present invention therefore also relates to herbicidal and growth-regulating compositions which comprise the active compound of the formula (I) in addition to customary formulation auxiliaries.
- the agents according to the invention can be used as wettable powders, emulsifiable concentrates, sprayable solutions, dusts, mordants, dispersions, granules or microgranules in the usual preparations.
- Wettable powders are preparations which are uniformly dispersible in water and which, in addition to the active ingredient, in addition to, if appropriate, a diluent or inert substance, are also wetting agents, for.
- ligninsulfonic acid sodium 2,2'-di-naphthylmethane-6,6'-disulfonic acid sodium, dibutylnaphthalenesulfonic acid sodium or oleoylmethyl tauric acid sodium.
- the production takes place in the usual way, for. B. by grinding and mixing the components.
- Emulsifiable concentrates can e.g. B. by dissolving the active ingredient in an inert organic solvent, e.g. Example butanol, cyclohexanone, dimethylformamide, xylene or else higher-boiling aromatics or K ohlenwasserstof- fen with addition of one or more emulsifiers her.- are provided.
- an inert organic solvent e.g. Example butanol, cyclohexanone, dimethylformamide, xylene or else higher-boiling aromatics or K ohlenwasserstof- fen with addition of one or more emulsifiers her.- are provided.
- the solvent content can also be omitted entirely or in part.
- alkyl-arylsulfonic acid calcium salts such as Ca-dodecylbenzenesulfonate or nonionic emulsifiers
- alkyl-arylsulfonic acid calcium salts such as Ca-dodecylbenzenesulfonate or nonionic emulsifiers
- fatty acid polyglycol esters alkyl aryl polyglycol ethers, fatty alcohol polyglycol ethers, propylene oxide-ethylene oxide condensation products, fatty alcohol propylene oxide or ethylene oxide polyorbityl ester polyorbityl acid fatty acid products, ethylene oxide polyorbityl ester fatty acid products, ethylene oxide polyorbityl ester fatty acid products, ethylene oxide polyorbityl ester fatty acid products, ethylene oxide polyorbityl ester fatty acid products, ethylene oxide polyorbityl ester fatty acid products, ethylene oxide polyorbityl ester fatty acid products, ethylene oxide
- Dusts can be obtained by grinding the active ingredient with finely divided, solid substances, e.g. B. talc, natural clays such as kaolin, bentonite, pyrophillite or diatomaceous earth.
- finely divided, solid substances e.g. B. talc, natural clays such as kaolin, bentonite, pyrophillite or diatomaceous earth.
- Granules can either be produced by spraying the active ingredient onto adsorbable, granulated inert material or by applying active ingredient concentrates by means of binders, e.g. As polyvinyl alcohol, sodium polyacrylic acid or mineral oils on the surface of carriers such as sand, kaolinite or granulated inert material. Suitable active ingredients can also be granulated in the manner customary for the production of fertilizer granules, if desired in a mixture with fertilizers.
- binders e.g. As polyvinyl alcohol, sodium polyacrylic acid or mineral oils on the surface of carriers such as sand, kaolinite or granulated inert material.
- Suitable active ingredients can also be granulated in the manner customary for the production of fertilizer granules, if desired in a mixture with fertilizers.
- the active ingredient concentration in wettable powders is e.g. B. about 10 to 90 wt .-%, the rest of 100 wt .-% consists of conventional formulation components. In the case of emulsifiable concentrates, the active substance concentration can be approximately 10 to 80% by weight. Dust-like formulations usually contain 5 to 20 wt .-% of active ingredient, sprayable solutions about 2 to 20 wt .-%. In the case of granules, the active ingredient content depends in part on whether the active compound is in liquid or solid form and which granulation aids, fillers, etc. are used.
- the active ingredient formulations mentioned optionally contain the customary adhesives, wetting agents, dispersants, emulsifiers, penetrants, solvents, fillers or carriers.
- the concentrates present in the commercial form are optionally diluted in a conventional manner, for. B. with wettable powders, emulsifiable concentrates, dispersions and sometimes also with microgranules using water. Dusty and granular Preparations and sprayable solutions are usually no longer diluted with other inert substances before use.
- the application rates of active ingredient of the formula (I) vary, depending on the indication, between 0.01 and 10 kg of active ingredient / ha.
- a wettable powder which is readily dispersible in water is obtained by mixing 25 parts by weight of active compound, 64 parts by weight of kaolin-containing quartz as an inert substance, 10 parts by weight of lignosulfonic acid potassium and 1 part by weight of oleoylmethyl tauric acid sodium as wetting and dispersing agent and grinding in a pin mill.
- a dispersion concentrate which is readily dispersible in water is obtained by mixing 20 parts by weight of active compound with 6 parts by weight of alkylphenol polyglycol ether (for example (R) Triton X 207 from Rohm & Haas Co.), 3 parts by weight.
- alkylphenol polyglycol ether for example (R) Triton X 207 from Rohm & Haas Co.
- An emulsifiable concentrate is obtained from 15 parts by weight of active ingredient, 75 parts by weight of cyclohexanone as a solvent and 10 parts by weight of ethylated nonylphenol (10 AeO) as an emulsifier.
- Seeds or rhizome pieces of mono- and dicotyledon weeds were placed in clay soil in plastic pots (0 9 cm) and covered with soil.
- the mulsionskonzentrate as wettable powders or as E formulated compounds of the invention were applied as aqueous suspensions or emulsions on the surface.
- the amount of water applied per pot corresponded to the equivalent of 600 1 / ha.
- the test pots were placed in the greenhouse and the test plants were cultivated under good growth conditions (temperature: 23 ⁇ 1 ° C; relative humidity 60-80%). After approximately 3 weeks, the damage to the plants was assessed visually. Untreated controls served as a comparison.
Abstract
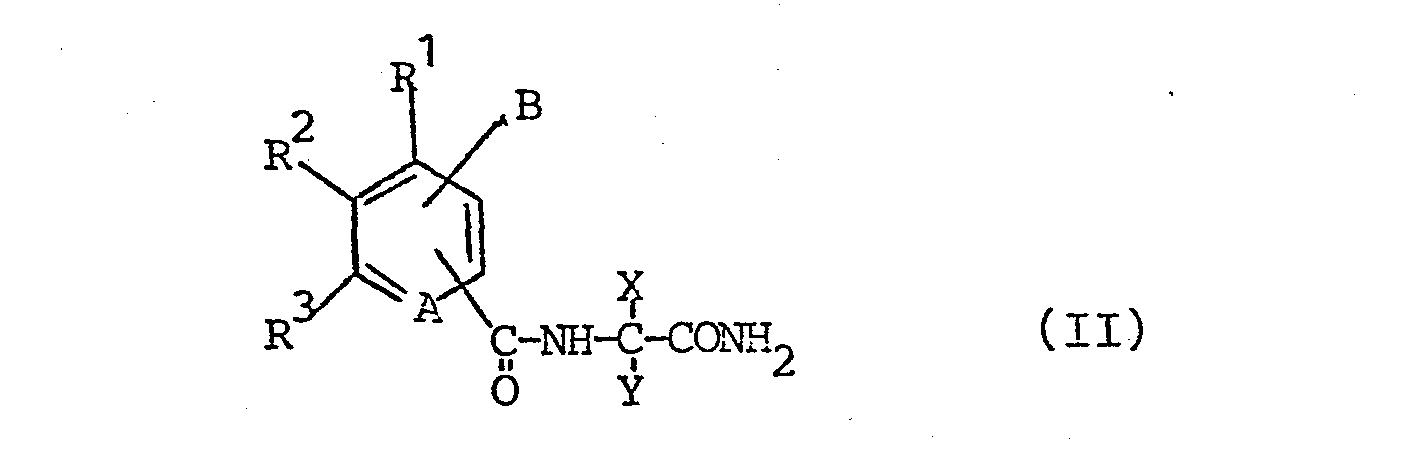
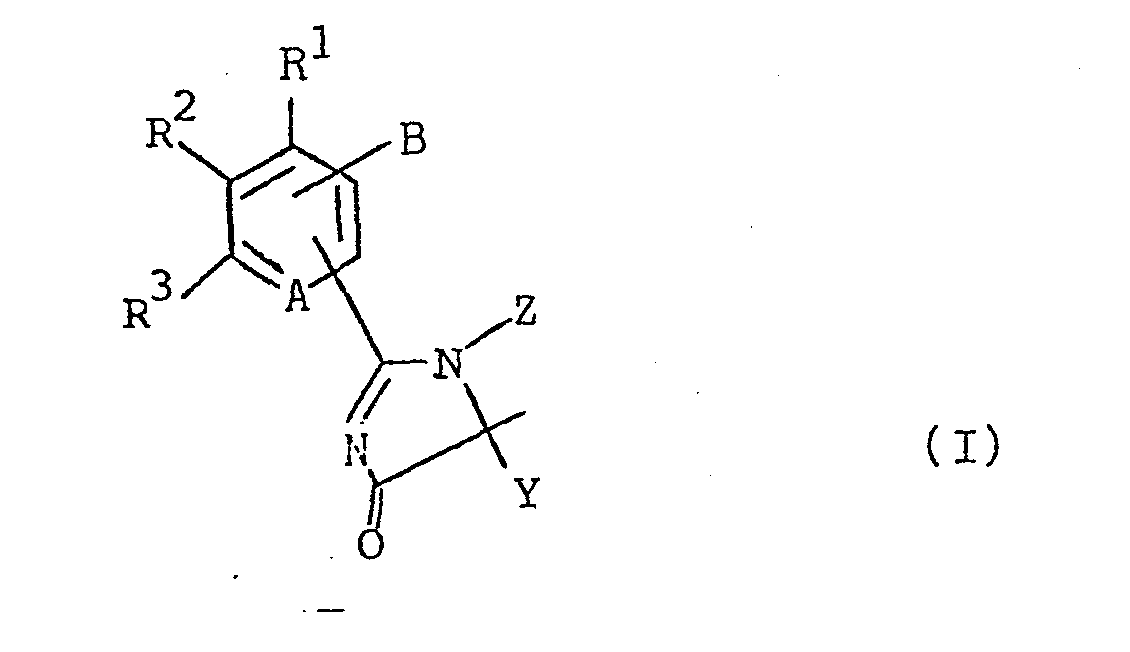
Imidazolinone der Formel <IMAGE> worin A N oder C-R<4>; B Halogenalkyl, Alkoxymethyl, Cyanmethyl oder Thiocyanatomethyl; X Alkyl; Y Alkyl, Cycloalkyl, Alkenyl, Phenyl oder Benzyl; oder X und Y zusammen mit C eine Spiro-cycloalkylgruppe; Z H, (subst.) Alkyl, Alkenyl, Propargyl, eine Alkyl-, Carbonester- oder Sulfoestergruppe; R¹-R<4> H, Halogen, Alkyl, Alkoxy, Alkoxycarbonyl, Halogenalkyl, NO2, CN, (subst.) Phenoxy, (subst.) Phenyl oder je zwei benachbarte zusammen den Rest -CH=CH-CH-CH- bedeuten, sind wirksame Herbizide und Wachstumsregulatoren.Imidazolinones of the formula <IMAGE> wherein A N or C-R <4>; B haloalkyl, alkoxymethyl, cyanomethyl or thiocyanatomethyl; X alkyl; Y is alkyl, cycloalkyl, alkenyl, phenyl or benzyl; or X and Y together with C represent a spiro-cycloalkyl group; Z H, (subst.) Alkyl, alkenyl, propargyl, an alkyl, carboxylic ester or sulfoester group; R¹-R <4> H, halogen, alkyl, alkoxy, alkoxycarbonyl, haloalkyl, NO2, CN, (subst.) Phenoxy, (subst.) Phenyl or two adjacent groups together mean the radical -CH = CH-CH-CH- , are effective herbicides and growth regulators.
Description
Die'vorliegende Erfindung betrifft neue, herbizid wirksame Imidazolinone der Formel (I)
- A N oder C-R4;
- B Halogen-(C1-C2)alkyl wobei unter Halogen Fluor, Chlor und Brom, vorzugsweise Fluor und Chlor zu verstehen sind, (C1-C4) Alkoxymethyl, Cyanmethyl und Thiocyanatomethyl;
- X (C1-C4)Alkyl;
- Y (C1- C6)Alkyl, Cyclo(C3-C6)alkyl, (C2-C4) Alkenyl , (C2-C4)Alkinyl, Phenyl oder Benzyl;
- X und Y zusammen mit dem Kohlenstoffatom, an das sie gebunden sind, eine gegebenenfalls durch -CH3 substituierte Spirocyclo(C3-C6)alkylgruppe;
- Z Wasserstoff, (C1-C4)Alkyl, welches durch (C1-C4)Alkoxycarbonyl substituiert sein kann, (C3-C4)Alkenyl, Propargyl, -CO-R5 oder -SO2-R6 ;
- R1, R2, R3 und R4 unabhängig voneinander Wasserstoff, Halogen, (C1-C4)Alkyl, (C1-C6)Alkoxy, (C1-C6) Alkoxycarbonyl, Halogen-(C1-C2)alkyl, Nitro, Cyano, Phenoxy und Phenyl, das gegebenenfalls mit (C1-C4)Alkyl, (C1-C4)Alkoxy oder Halogen substituiert sein kann, wobei jeweils zwei o-ständige Reste R1, R2, R 3 oder R4 auch gemeinsam die Gruppierung -CH=CH-CH=CH bilden können;
- R5 (C1-C12)Alkyl, das gegebenenfalls mit bis zu zwei (C1-C4)Alkoxygruppen oder mit bis zu drei Halogen substituiert ist; Phenyl, das mit bis zu zwei Halogenatomen, einer Methyl-, einer Nitro- oder einer Methoxygruppe substituiert sein kann; Cyclo(C3-C7)alkyl, (C1-C4)Alkoxy, (C1-C4)Alkoxycarbonyl, Benzyloxy, Phenoxy oder -NR7R8, insbesondere (C1-C12) Alkyl ;
- R6 (C1-C4)Alkyl, CF3, CCl3, Phenyl, Chlorphenyl oder Methylphenyl;
- R 7 Wasserstoff oder (C1-C4)Alkyl; und
- R8 (C1-C4)Alkyl, Phenyl, Chlorphenyl, Methylphenyl, Amino, Mono-oder Di(C1-C4)alkylamino, bedeuten, sowie deren optischen Isomere (falls X ‡.Y), ihre Säureadditionssalze und N-Oxide (für den Fall, daß A für N steht).
- AN or CR 4 ;
- B halogen (C 1 -C 2 ) alkyl, where halogen means fluorine, chlorine and bromine, preferably fluorine and chlorine, (C 1 -C 4 ) alkoxymethyl, cyanomethyl and thiocyanatomethyl;
- X (C 1 -C 4 ) alkyl;
- Y (C 1 -C 6 ) alkyl, cyclo (C3-C6) alkyl, (C 2 -C 4 ) alkenyl, (C 2 -C 4 ) alkynyl, phenyl or benzyl;
- X and Y together with the carbon atom to which they are attached represent an optionally -CH 3 substituted spirocyclo (C3-C6) alkyl group;
- Z is hydrogen, (C 1 -C 4 ) alkyl, which can be substituted by (C 1 -C 4 ) alkoxycarbonyl, (C 3 -C 4 ) alkenyl, propargyl, -CO-R 5 or -SO 2 -R 6 ;
- R 1 , R 2 , R 3 and R 4 independently of one another are hydrogen, Halogen, (C 1 -C 4 ) alkyl, (C 1 -C 6 ) alkoxy, (C 1 -C 6 ) alkoxycarbonyl, halogen (C 1 -C 2 ) alkyl, nitro, cyano, phenoxy and phenyl, which are optionally can be substituted with (C 1 -C 4 ) alkyl, (C 1 -C 4 ) alkoxy or halogen, where in each case two o-radicals R 1 , R 2 , R 3 or R 4 together also form the group --CH = CH -C can form H = CH;
- R 5 (C 1 -C 12 ) alkyl which is optionally substituted with up to two (C 1 -C 4 ) alkoxy groups or with up to three halogen; Phenyl which can be substituted with up to two halogen atoms, one methyl, one nitro or one methoxy group; C y c l o (C 3 -C 7 ) alkyl, (C 1 -C 4 ) alkoxy, (C 1 -C 4 ) alkoxycarbonyl, benzyloxy, phenoxy or -NR 7 R 8 , in particular (C 1 -C 12 ) Alkyl;
- R 6 (C 1 -C 4 ) alkyl, CF3 , CCl 3 , phenyl, chlorophenyl or methylphenyl;
- R 7 is hydrogen or (C 1 -C 4 ) alkyl; and
- R 8 is (C 1 -C 4 ) alkyl, phenyl, chlorophenyl, methylphenyl, amino, mono- or di (C 1 -C 4 ) alkylamino, and their optical isomers (if X ‡ .Y), their acid addition salts and N -Oxides (if A is N).
Man erhält die erfindungsgemäßen Verbindungen, indem man die Amide der Formel (II)
Die Cyclisierung der Amide (II) kann z.B. mit Phosphorpentachlorid, vorteilhaft in Gegenwart eines unter den Reaktionsbedingungen inerten Lösemittels erfolgen. Als Beispiele für letztere seien genannt: Toluol, Xylol, Chloroform oder Phosphoroxychlorid. Die Reaktionstemperatur ist unkritisch und kann zwischen -10°C und +150°C variiert werden. Besonders vorteilhaft sind Reaktionstemperaturen zwischen 0 und 100°C. Man erhält dabei primär die Hydrochloride der Imidazolinone (I). Nach üblichen Methoden, z.B. durch.Umsetzung mit Natriumcarbonat oder Natriumhydrogencarbonat, lassen sich daraus die freien Basen herstellen.The cyclization of the amides (II) can e.g. with phosphorus pentachloride, advantageously in the presence of a solvent which is inert under the reaction conditions. Examples of the latter are: toluene, xylene, chloroform or phosphorus oxychloride. The reaction temperature is not critical and can be varied between -10 ° C and + 150 ° C. Reaction temperatures between 0 and 100 ° C. are particularly advantageous. The hydrochlorides of the imidazolinones (I) are primarily obtained. According to usual methods, e.g. by reaction with sodium carbonate or sodium hydrogen carbonate, the free bases can be produced therefrom.
Die Cyclisierung kann ebenfalls in Gegenwart starker organischer oder anorganischer Säuren, wie Schwefelsäure oder p-Toluolsulfonsäure, unter gleichzeitiger Abtrennung des gebildeten Wassers bei Temperaturen von 0°C bis 150°C erfolgen. Besonders vorteilhaft läßt sich die Reaktion so führen, daß das gebildete Wasser durch Azeotropdestillation mit einem Lösemittel, wie Toluol, Xylol oder Chloroform, abgetrennt wird. Nach Neutralisation können die Produkte nach üblichen Methoden isoliert werden.The cyclization can also take place in the presence of strong organic or inorganic acids, such as sulfuric acid or p-toluenesulfonic acid, with simultaneous removal of the water formed at temperatures from 0 ° C. to 150 ° C. The reaction can be carried out particularly advantageously in such a way that the water formed is separated off by azeotropic distillation with a solvent such as toluene, xylene or chloroform. After neutralization, the products can be isolated using customary methods.
Die Imidazolinone der Formel (I) (Z = Wasserstoff) werden dabei im allgemeinen in guten Ausbeuten erhalten. Dies war für die halogenalkylsubstituierten Vertreter (B = Halogenalkyl) überraschend, da vielmehr angenommen werden mußte, daß sich die Imidazolinone (I), auf Grund der sterisch sehr günstigen Anordnung der Halogenalkylgruppe, zum basischen Imidazolinstickstoff unter Halogenwasserstoff abspaltung zersetzen würden. Die Cyclisierungsprodukte der Formel (I) mit Z = Wasserstoff können in einfacher Weise nach an sich bekannten Verfahren mit Alkylierungsmitteln, (Methyljodid, Dimethylsulfat) oder Acylierungsmitt-eln (wie Säurechloriden) in Gegenwart von Basen, oder mit Isocyanaten umgesetzt werden.The imidazolinones of the formula (I) (Z = hydrogen) are generally obtained in good yields. This was surprising for the haloalkyl-substituted representatives (B = haloalkyl), since it had to be assumed that the imidazolinones (I), owing to the sterically very favorable arrangement of the haloalkyl group, became the basic imidazoline nitrogen under hydrogen halide would decompose. The cyclization products of the formula (I) with Z = hydrogen can be reacted in a simple manner by processes known per se with alkylating agents (methyl iodide, dimethyl sulfate) or acylating agents (such as acid chlorides) in the presence of bases, or with isocyanates.
Die erfindungsgemäßen Verbindungen sind, wenn Z für Wasserstoff steht, tautomer, so daß sie in einer der beiden Formen (I a)/(I b) oder als Gemisch von (I a) und (I b) vorliegen können. Diese Isomeren treten auch bei den Derivaten mit Z ‡ H auf.
Die Definitionen der Formel (I) umfassen stets beide isomeren Strukturen der Formel (I a) und (I b).The definitions of the formula (I) always include both isomeric structures of the formulas (I a) and (I b).
Sowohl die Säureadditionssalze, als auch die N-Oxide der Verbindungen der Formel (I) sind auf allgemein bekanntem Weg gut zugänglich, letztere z.B. durch Umsetzung mit Peroxiden oder H2O2.Both the acid addition salts and the N-oxides of the compounds of the formula (I) are readily accessible in a generally known manner, the latter, for example, by reaction with peroxides or H 2 O 2 .
Die Amide der Formel (II) lassen sich leicht aus den Aminoamiden (III) und den entsprechend substituierten Carbonsäurederivaten (IV) erhalten. Als Derivate geeignet sind z.B. Säurechloride oder Kohlensäurealkylester (R9 = Cl, -O-COO-Alkyl).
Die vorliegenden erfindungsgemäßen Verbindungen weisen eine ausgezeichnete herbizide Wirksamkeit gegen ein breites Spektrum wirtschaftlich wichtiger mono- und dikotyler Schadpflanzen auf. Auch schwer bekämpfbare perennierende Wurzelunkräuter werden durch die Wirkstoffe gut erfaßt. Dabei ist es gleichgültig, ob die Substanzen in Vorsaat-, Vorauflauf- oder Nachauflaufspritzung ausge-bracht werden. Werden die erfindungsgemäßen Verbindungen vor dem Keimen auf die Erdoberfläche appliziert, so wird das Auflaufen der Keimlinge nicht vollständig verhindert. Die Unkräuter wachsen bis zum Keimblattstadium heran, stellen jedoch dann ihr Wachstum ein und sterben schließlich nach 3 Wochen vollkommen ab.The present compounds according to the invention have excellent herbicidal activity against a broad spectrum of economically important mono- and dicotyledonous harmful plants. Perennial root weeds that are difficult to control are also well captured by the active ingredients. It does not matter whether the substances are applied in pre-sowing, pre-emergence or post-emergence spraying. If the compounds according to the invention are applied to the earth's surface before germination, the emergence of the seedlings is not completely prevented. The weeds grow to the cotyledon stage, but then stop growing and eventually die completely after 3 weeks.
Bei Applikation der Wirkstoffe auf die grünen Pflanzenteile im Nachauflaufverfahren tritt ebenfalls sehr rasch nach der Behandlung ein drastischer Wachstumsstop ein und die Unkrautpflanzen bleiben in dem zum Applikationszeitpunkt vorhandenen Wachstumsstadium stehen oder sterben nach einer gewissen Zeit ganz ab, so daß auf diese Weise eine für die Kulturpflanzen schädliche Unkrautkonkurrenz sehr früh und nachhaltig durch den Einsatz der neuen erfindungsgemäßen Mittel beseitigt werden kann. Obgleich die erfindungsgemäßen Verbindungen eine ausgezeichnete herbizide Aktivität gegenüber mono- und dikotylen Unkräutern aufweisen, werden Kulturpflanzen wirtschaftlich bedeutender Kulturen wie z. B. Weizen, Gerste, Roggen, Reis, Mais, Zuckerrübe, Baumwolle und Soja nur unwesentlich oder gar nicht geschädigt. Die erfindungsgemäßen Substanzen besitzen somit ausgezeichnete Selektivität bei Kulturpflanzen und eignen sich aus diesen Gründen sehr gut zur Bekämpfung von unerwünschtem Pflanzenwuchs in landwirtschaftlichen Nutzungspflanzungen..When the active ingredients are applied to the green parts of the plant in the post-emergence process, there is also a drastic growth stop very quickly after the treatment and the weed plants remain in the growth stage at the time of application or die completely after a certain time, so that one for the crop plants U harmful nkrautkonkurrenz very early and can be permanently eliminated through the use of new compositions of the invention. Although the compounds according to the invention have excellent herbicidal activity against monocotyledonous and dicotyledon weeds, crop plants of economically important crops such as, for. B. wheat, barley, rye, rice, maize, sugar beet, cotton and soy only insignificantly or even not harmed. The substances according to the invention thus have excellent selectivity in crop plants and, for these reasons, are very suitable for controlling unwanted vegetation in agricultural crops.
Darüberhinaus weisen sie wachstumsregulatorische Eigenschaften bei Kulturpflanzen auf. Sie greifen regulierend in den pflanzene-igenen Stoffwechsel ein und können damit zur Ernteerleichterung wie z.B. durch Auslösen von Desikkation, Abzession und Wuchsstauchung eingesetzt werden. Desweiteren eignen sich auch zur generellen Steuerung und Hemmung von unerwünschtem vegetativen Wachstum, ohne dabei die Pflanzen abzutöten. Eine Hemmung des vegetativen Wachstums spielt bei vielen mono-und dikolylen Kulturen eine große Rolle, da das Lagern hierdurch verringert oder völlig verhindert werden kann. Gegenstand der vorliegenden Erfindung sind daher auch herbizide und wachstumsregulatorische Mittel, welche den Wirkstoff der Formel (I) neben üblichen Formulierungshilfsmitteln enthalten.In addition, they have growth-regulating properties in crop plants. They intervene to regulate the plant's own metabolism and can thus be used to facilitate harvesting, for example by triggering desiccation, abscess and stunted growth. Furthermore, they are also suitable for general control and inhibition of undesired vegetative growth without killing the plants. An inhibition of vegetative growth plays a major role in many mono- and dicolylene cultures, since storage can thereby be reduced or completely prevented. The present invention therefore also relates to herbicidal and growth-regulating compositions which comprise the active compound of the formula (I) in addition to customary formulation auxiliaries.
Die erfindungsgemäßen Mittel können als Spritzpulver, emulgierbare Konzentrate, versprühbare Lösungen, Stäubemittel, Beizmittel, Dispersionen, Granulate oder Mikrogranulate in den üblichen Zubereitungen angewendet werden. Spritzpulver sind in Wasser gleichmäßig dispergierbare Präparate, die neben dem Wirkstoff außer gegebenenfalls einem Verdünnungs- oder Inertstoff noch Netzmittel, z. B. polyoxethylierte Alkylphenole, polyoxethylierte Fettalkohole, Alkyl- oder Alkylphenylsulfonate und Dispergiermittel, z. B. ligninsulfonsaures Natrium, 2,2'-di- naphthylmethan-6,6'-disulfonsaures Natrium, dibutylnaphthalinsulfonsaures Natrium oder auch oleoylmethyltaurinsaures Natrium enthalten. Die Herstellung erfolgt in üblicher Weise, z. B. durch Mahlen und Vermischen der Komponenten.The agents according to the invention can be used as wettable powders, emulsifiable concentrates, sprayable solutions, dusts, mordants, dispersions, granules or microgranules in the usual preparations. Wettable powders are preparations which are uniformly dispersible in water and which, in addition to the active ingredient, in addition to, if appropriate, a diluent or inert substance, are also wetting agents, for. B. polyoxethylated alkylphenols, polyoxethylated fatty alcohols, alkyl or alkylphenyl sulfonates and dispersants, e.g. B. ligninsulfonic acid sodium, 2,2'-di-naphthylmethane-6,6'-disulfonic acid sodium, dibutylnaphthalenesulfonic acid sodium or oleoylmethyl tauric acid sodium. The production takes place in the usual way, for. B. by grinding and mixing the components.
Emulgierbare Konzentrate können z. B. durch Auflösen des Wirkstoffes in einem inerten organischen Lösungsmittel, z. B. Butanol, Cyclohexanon, Dimethylformamid, Xylol oder auch höhersiedenden Aromaten oder Kohlenwasserstof- fen unter Zusatz von einem oder mehreren Emulgatoren her.- gestellt werden. Bei flüssigen Wirkstoffen kann der Lösungsmittelanteil auch ganz oder teilweise entfallen. Als Emulgatoren können beispielsweise verwendet werden: Alkyl-arylsulfonsaure Calciumsalze wie Ca-dodecylbenzolsulfonat oder nichtionische Emulgatoren wie Fettsäurepolyglykolester, Alkyl-arylpolyglykolether, Fettalkoholpolyglykolether, Propylenoxid-Ethylenoxid-Kondensationsprodukte, Fettalkohol-Propylenoxid-Ethylenoxid-Kondensationsprodukte, Alkylpolyglykolether, Sorbitanfettsäureester, Polyoxethylensorbitanfettsäureester oder Polyoxethylensorbitester.Emulsifiable concentrates can e.g. B. by dissolving the active ingredient in an inert organic solvent, e.g. Example butanol, cyclohexanone, dimethylformamide, xylene or else higher-boiling aromatics or K ohlenwasserstof- fen with addition of one or more emulsifiers her.- are provided. In the case of liquid active ingredients, the solvent content can also be omitted entirely or in part. Examples of emulsifiers that can be used are: alkyl-arylsulfonic acid calcium salts such as Ca-dodecylbenzenesulfonate or nonionic emulsifiers such as fatty acid polyglycol esters, alkyl aryl polyglycol ethers, fatty alcohol polyglycol ethers, propylene oxide-ethylene oxide condensation products, fatty alcohol propylene oxide or ethylene oxide polyorbityl ester polyorbityl acid fatty acid products, ethylene oxide polyorbityl ester fatty acid products, ethylene oxide polyorbityl ester fatty acid products, ethylene oxide polyorbityl ester fatty acid products, ethylene oxide polyorbityl ester fatty acid products, ethylene oxide polyorbityl ester fatty acid products,
Stäubemittel kann man durch Vermahlen des Wirkstoffes mit fein verteilten, festen Stoffen, z. B. Talkum, natürlichen Tonen wie Kaolin, Bentonit, Pyrophillit oder Diatomeenerde erhalten.Dusts can be obtained by grinding the active ingredient with finely divided, solid substances, e.g. B. talc, natural clays such as kaolin, bentonite, pyrophillite or diatomaceous earth.
Granulate können entweder durch Verdüsen des Wirkstoffes auf adsorptionsfähiges, granuliertes Inertmaterial hergestellt werden oder durch Aufbringen von Wirkstoffkonzentraten mittels Bindemitteln, z. B. Polyvinylalkohol, polyacrylsaurem Natrium oder auch Mineralölen auf die Oberfläche von Trägerstoffen wie Sand, Kaolinite oder von granuliertem Inertmaterial.Auch können geeignete Wirkstoffe in der für die Herstellung von Düngemittelgranulaten üblichen Weise, gewünschtenfalls in Mischung mit Düngemitteln, granuliert werden.Granules can either be produced by spraying the active ingredient onto adsorbable, granulated inert material or by applying active ingredient concentrates by means of binders, e.g. As polyvinyl alcohol, sodium polyacrylic acid or mineral oils on the surface of carriers such as sand, kaolinite or granulated inert material. Suitable active ingredients can also be granulated in the manner customary for the production of fertilizer granules, if desired in a mixture with fertilizers.
In Spritzpulvern beträgt die Wirkstoffkonzentration z. B. etwa 10 bis 90 Gew.-%, der Rest zu 100 Gew.-% besteht aus üblichen Formulierungsbestandteilen. Bei emulgierbaren Konzentraten kann die Wirkstoffkonzentration etwa 10 bis 80 Gew.-% betragen. Staubförmige Formulierungen enthalten meistens 5 bis 20 Gew.-% an Wirkstoff, versprühbare Lösungen etwa 2 bis 20 Gew.-%. Bei Granulaten hängt der Wirkstoffgehalt zum Teil davon ab, ob die wirksame Verbindung flüssig oder fest vorliegt und welche Granulierhilfsmittel, Füllstoffe usw. verwendet werden.The active ingredient concentration in wettable powders is e.g. B. about 10 to 90 wt .-%, the rest of 100 wt .-% consists of conventional formulation components. In the case of emulsifiable concentrates, the active substance concentration can be approximately 10 to 80% by weight. Dust-like formulations usually contain 5 to 20 wt .-% of active ingredient, sprayable solutions about 2 to 20 wt .-%. In the case of granules, the active ingredient content depends in part on whether the active compound is in liquid or solid form and which granulation aids, fillers, etc. are used.
Daneben enthalten die genannten Wirkstofformulierungen gegebenenfalls die jeweils üblichen Haft-, Netz-, Dispergier-, Emulgier-, Penetrations-, Lösungsmittel, Füll- oder Trägerstoffe.In addition, the active ingredient formulations mentioned optionally contain the customary adhesives, wetting agents, dispersants, emulsifiers, penetrants, solvents, fillers or carriers.
Zur Anwendung werden die in handelsüblicher Form vorliegenden Konzentrate gegebenenfalls in üblicher Weise verdünnt, z. B. bei Spritzpulvern, emulgierbaren Konzentraten, Dispersionen und teilweise auch bei Mikrogranulaten mittels Wasser. Staubförmige und granulierte Zubereitungen sowie versprühbare Lösungen werden vor der Anwendung üblicherweise nicht mehr mit weiteren inerten Stoffen verdünnt.For use, the concentrates present in the commercial form are optionally diluted in a conventional manner, for. B. with wettable powders, emulsifiable concentrates, dispersions and sometimes also with microgranules using water. Dusty and granular Preparations and sprayable solutions are usually no longer diluted with other inert substances before use.
Die Aufandmengen an Wirkstoff der Formel (I)variieren je nach Indikation zwischen 0,01 und 10 kg Wirkstoff/ha.The application rates of active ingredient of the formula (I) vary, depending on the indication, between 0.01 and 10 kg of active ingredient / ha.
Auch Mischungen oder Mischformulierungen mit anderen Wirkstoffen, wie z. B. Insektiziden, Akariziden, Herbiziden, Düngemitteln, Wachstumsregulatoren oder Fungiziden sind gegebenenfalls möglich.Mixtures or mixed formulations with other active ingredients, such as. B. insecticides, acaricides, herbicides, fertilizers, growth regulators or fungicides are optionally possible.
Nachstehend seien einige Formulierungsbeispiele aufgeführt:
- Ein Stäubemittel wird erhalten, indem man 10 Gewichtsteile Wirkstoff und 90 Gewichtsteile Talkum oder Inertstoff mischt und in einer Schlagmühle zerkleinert.
- A dusting agent is obtained by mixing 10 parts by weight of active ingredient and 90 parts by weight of talc or inert material and comminuting them in a hammer mill.
Ein in Wasser leicht dispergierbares, benetzbares Pulver wird erhalten, indem man 25 Gewichtsteile Wirkstoff, 64 Gewichtsteile kaolinhaltigen Quarz als Inertstoff, 10 Gewichtsteile ligninsulfonsaures Kalium und 1 Gewichtsteil oleoylmethyltaurinsaures Natrium als Netz- und Dispergiermittel mischt und in einer Stiftmühle mahlt.A wettable powder which is readily dispersible in water is obtained by mixing 25 parts by weight of active compound, 64 parts by weight of kaolin-containing quartz as an inert substance, 10 parts by weight of lignosulfonic acid potassium and 1 part by weight of oleoylmethyl tauric acid sodium as wetting and dispersing agent and grinding in a pin mill.
Ein in Wasser leicht dispergierbares Dispersionskonzentrat wird erhalten, indem man 20 Gewichtsteile Wirkstoff mit 6 Gewichtsteilen Alkylphenolpolyglykolether (z.B. (R)Triton X 207 der Rohm & Haas Co.), 3 Gew.-Tl. Isotridecanol polyglykolether (8 AeO) und 71 Gewichtsteilen paraffinischem Mineralöl (Siedebereich z. B. ca. 255 bis über 377 °C) mischt und in einer Reibkugelmühle auf eine Feinheit von unter 5 Mikron vermahlt.A dispersion concentrate which is readily dispersible in water is obtained by mixing 20 parts by weight of active compound with 6 parts by weight of alkylphenol polyglycol ether (for example (R) Triton X 207 from Rohm & Haas Co.), 3 parts by weight. Isotridecanol polyglycol ether (8 AeO) and 71 parts by weight of paraffinic mineral oil (boiling range e.g. approx. 255 to over 377 ° C) are mixed and ground in a friction ball mill to a fineness of less than 5 microns.
Ein emulgierbares Konzentrat wird erhalten aus 15 Gewichtsteilen Wirkstoff, 75 Gewichtsteilen Cyclohexanon als Lösungsmittel und 10 Gewichtsteilen oxethyliertes Nonylphenol (10 AeO) als Emulgator.An emulsifiable concentrate is obtained from 15 parts by weight of active ingredient, 75 parts by weight of cyclohexanone as a solvent and 10 parts by weight of ethylated nonylphenol (10 AeO) as an emulsifier.
Die folgenden Beispiele dienen der weiteren Erläuterung der Erfindung:
- A. Herstellungsbeispiele
- A. Manufacturing examples
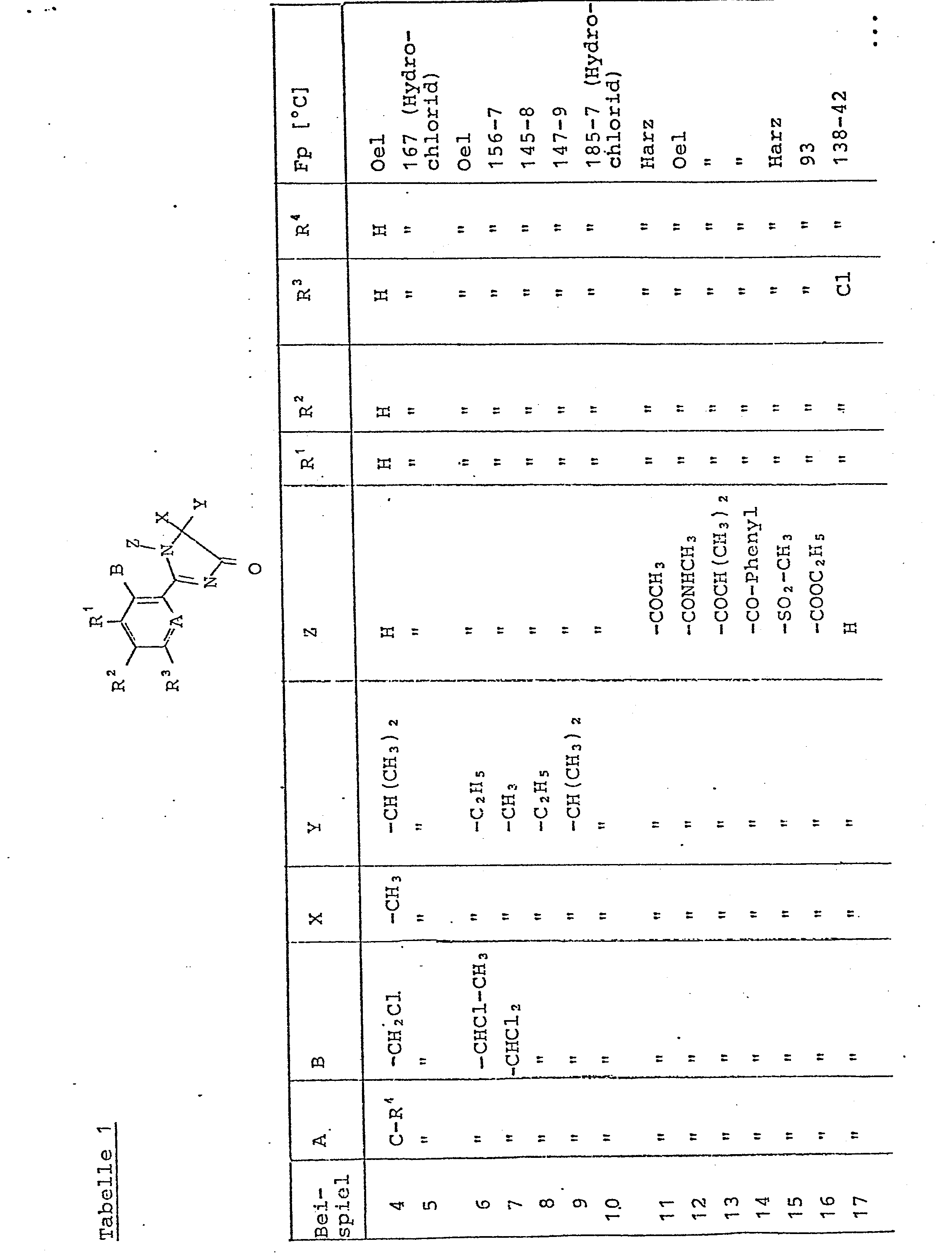
Zu einer Lösung aus 10,3g(0,079 mol) 2-Methylvalinamid und 8,7 g (0,086 mol) Triethylamin in 50 ml abs. Methylenchlorid tropft man bei 0 - 5°C 15 g (0,072 mol) 2-Trifluormethylbenzoylchlorid zu. Nach 1 h Reaktionszeit bei Raumtemperatur wird auf 500 ml 0,5 n Natriumhydrogencarbonatlösung gegossen, nachgerührt und abgesaugt. Nach dem Trocknen erhält man 18,3 g (85 % der Theorie) 2-Trifluormethylbenzoesäure-N-2-(2-carbamoyl-3-methyl)-butylamid als farbloser Feststoff vom Fp. 124 bis 126°C.To a solution of 10.3 g (0.079 mol) of 2-methylvalinamide and 8.7 g (0.086 mol) of triethylamine in 50 ml abs. Methylene chloride is added dropwise at 0 - 5 ° C 15 g (0.072 mol) of 2-trifluoromethylbenzoyl chloride. After a reaction time of 1 h at room temperature, the mixture is poured onto 500 ml of 0.5N sodium hydrogen carbonate solution, stirred and suctioned off. After drying, 18.3 g (85% of theory) of 2-trifluoromethylbenzoic acid-N-2- (2-carbamoyl-3-methyl) -butylamide are obtained as a colorless solid, mp. 124 to 126 ° C.
18,3 g (0,060 mol) 2-Trifluormethylbenzoesäure-N-2-(2- carbamoyl-3-methyl)-butylamid werden in 100 ml Phosphoroxychlorid gelöst und bei Raumtemperatur portionsweise mit 14,0 g (0,067 mol) Phosphorpentachlorid versetzt. Nach 5 h bei Raumtemperatur wird im Vakuum eingedampft, mit Eis hydrolysiert, mit Natriumhydrogencarbonat neutralisiert und die Lösung 2mal mit je 100 ml Essigester extrahiert. Nach dem Trocknen über Natriumsulfat und Eindampfen erhält man 15,5 g (91 % der Theorie) 5-Isopropyl-5-methyl-4-oxo-2-(2-trifluormethylphenyl)-2-imidazolin als farblosen Feststoff vom Fp. 109 bis 110°C.18.3 g (0.060 mol) of 2-trifluoromethylbenzoic acid-N-2- ( 2 -carbamoyl-3-methyl) butylamide are dissolved in 100 ml of phosphorus oxychloride, and 14.0 g (0.067 mol) of phosphorus pentachloride are added in portions at room temperature. After 5 h at room temperature, the mixture is evaporated in vacuo, hydrolyzed with ice, neutralized with sodium hydrogen carbonate and the solution is extracted twice with 100 ml of ethyl acetate. After drying over sodium sulfate and Evaporation gives 15.5 g (91% of theory) of 5-isopropyl-5-methyl-4-oxo-2- (2-trifluoromethylphenyl) -2-imidazoline as a colorless solid, mp 109 to 110 ° C.
Zu 4,5 g (0,015 mol) 2-(2-Dichlormethylphenyl)-5-5-diethyl-4-oxo-2-imidazolin in 30 ml abs. Pyridin gibt man bei 0 - 5°C 3,2 g (0,017 mol) 4-Toluolsulfonsäurechlorid zu, läßt 1 d bei Raumtemperatur stehen, gießt auf 500 ml Wasser, extrahiert 2mal mit je 100 ml Toluol, wäscht die org. Phase lmal mit 2 n Natronlauge und 1mal mit Wasser. Nach dem Trocknen (Natriumsulfat) und Ein-Eindampfen wird der Rückstand chromatographisch (Kieselgel, LM Petrolether-Essigester 7 : 3) gereinigt. Man erhält 4,4 g (66 % der Theorie) 2-(2-Dichlormethylphenyl) -5-diethyl-4-oxo-1-(4-toluolsulfonyl)-2-imidazolin als farbloser Feststoff vom Fp. 139 - 140°C.
Die Schädigung der Unkrautpflanzen bzw. die Kulturpflanzenverträglichkeit wurde in einem Schlüssel von 0 - 5 bonitiert. Dabei bedeutet
- 0 = ohne Wirkung (Schaden)
- 1 = 0 - 20 % Wirkung
- 2 = 20 - 40 % Wirkung
- 3 = 40 - 60 % Wirkung
- 4 = 60 - 80 % Wirkung
- 5 = 80 - 100 % Wirkung
- 0 = without effect (damage)
- 1 = 0 - 20% effect
- 2 = 20 - 40% effect
- 3 = 40 - 60% effect
- 4 = 60 - 80% effect
- 5 = 80 - 100% effect
Samen bzw. Rhizomstücke mono- und dikotyler Unkräuter wurden in Lehmerde in Plastiktöpfen (0 9 cm) ausgelegt und mit Erde abgedeckt. Die als benetzbare Pulver bzw. als Emulsionskonzentrate formulierten erfindungsgemäßen Verbindungen wurden in Form wäßriger Suspensionen bzw. Emulsionen auf die Erdoberfläche appliziert. Die Wasseraufwandmenge pro Topf entsprach dabei umgerechnet 600 1/ha. Nach der Behandlung wurden die Versuchstöpfe im Gewächshaus aufgestellt und die Versuchspflanzen unter guten Wachstumsbedingungen (Temperatur: 23 ± 1 °C; rel. Luftfeuchte 60 - 80 %) kultiviert. Nach ca. 3 Wochen wurde die Pflanzenschädigung visuell bonitiert. Als Vergleich dienten dabei unbeharidelte Kontrollen.Seeds or rhizome pieces of mono- and dicotyledon weeds were placed in clay soil in plastic pots (0 9 cm) and covered with soil. The mulsionskonzentrate as wettable powders or as E formulated compounds of the invention were applied as aqueous suspensions or emulsions on the surface. The amount of water applied per pot corresponded to the equivalent of 600 1 / ha. After the treatment, the test pots were placed in the greenhouse and the test plants were cultivated under good growth conditions (temperature: 23 ± 1 ° C; relative humidity 60-80%). After approximately 3 weeks, the damage to the plants was assessed visually. Untreated controls served as a comparison.
Wie aus den Werten der Tabelle 2 hervorgeht, weisen die erfindungsgemäßen Verbindungen im Vorauflaufverfahren eine zum Teil ausgezeichnete herbizide Wirksamkeit gegen wirtschaftlich bedeutende mono-und dikotyle Schadpflanzen auf.As can be seen from the values in Table 2, show the compounds according to the invention have a partially excellent herbicidal activity against economically important mono- and dicotyledonous harmful plants in the pre-emergence process.
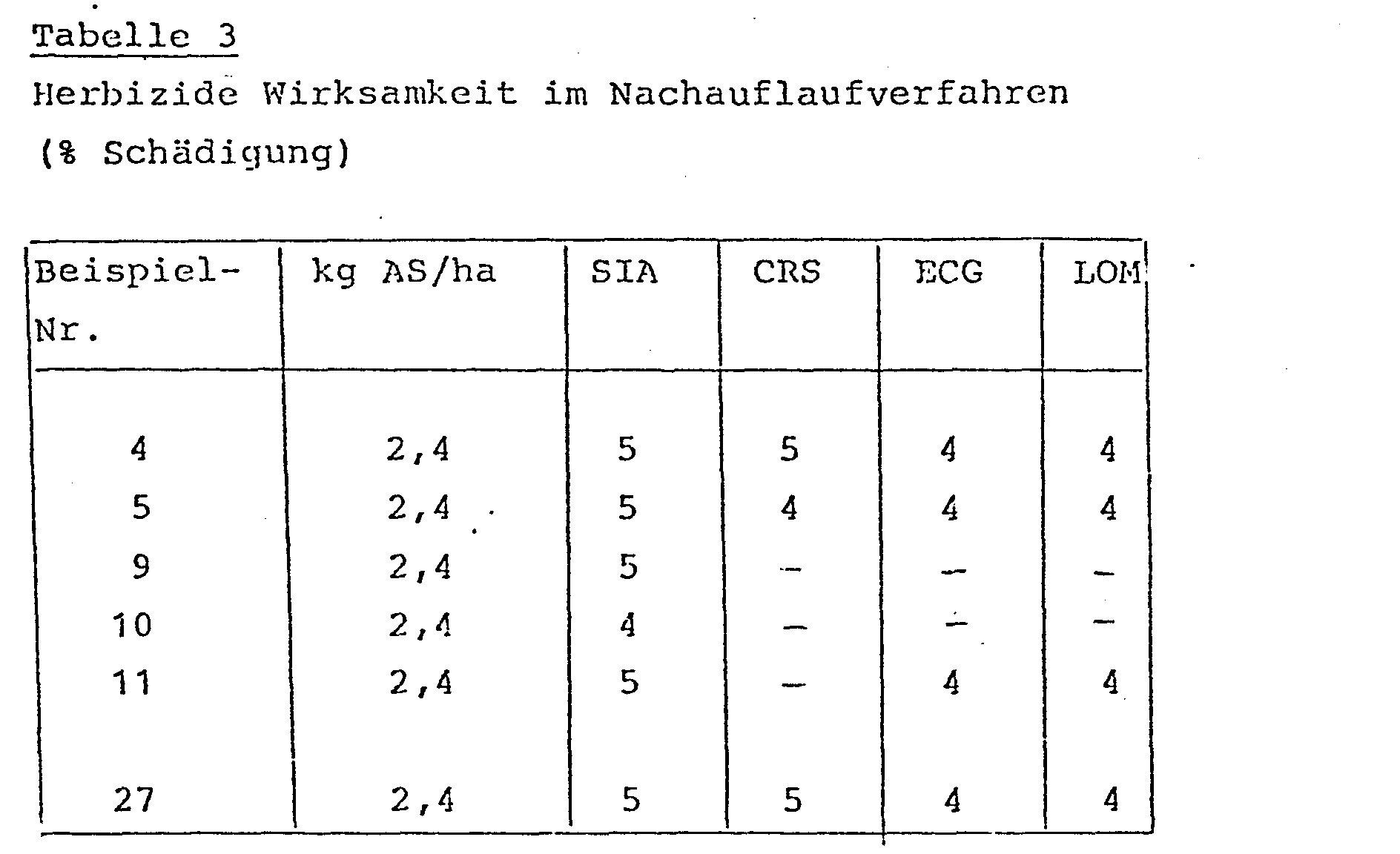
In ähnlicher Weise wurden verschiedene Unkräuter in Töpfen im Gewächshaus bis zum 3-6-Blattstadium herangezogen und dann im Nachauflauf verfahren mit den erfindungsgemäßen Verbindungen (formuliert als Spritzpulver) behandelt. 4 Wochen später wurden die Versuchspflanzen im Vergleich zu unbehandelten Kontrollpflanzen visuell bonitiert, indem die Schädigung geschätzt wurde.Similarly, various weeds were grown in pots in the greenhouse to the 3-6 leaf stage and then post-emergence with the compounds according to the invention (formulated as wettable powder). 4 weeks later, the test plants were visually rated in comparison to untreated control plants by estimating the damage.
Die Ergebnisse des Versuchs (Tabelle 3) belegen die guten herbiziden Eigenschaften der Verbindungen.
In Schalenversuchen im Gewächshaus wurden junge Getreidepflanzen (Weizen, Gerste und Roggen) im 3-Blattstadium mit den zu prüfenden Verbindungen in den in Tabelle 4 angegebenen Wirkstoffkonzentrationen (kg/ha) tropfnaß gespritzt. Nachdem die unbehandelten Kontrollpflanzen eine Wuchshöhe von etwa 55 cm erreicht hatten, wurde bei allen Pflanzen der Zuwachs gemessen und die Wuchshemmung in % des Zuwachses der Kontrollpflanzen berechnet. Es wurde außerdem die phytotoxische Wirkung der Verbindungen beobachtet. Die Ergebnisse sind in Tabelle 4 zusammengefaßt. Bei der Angabe der Wuchshemmung bedeuten 100 % den Stillstand des Wachstums und 0 % ein Wachstum entsprechend dem der unbehandelten Kontrollpflanzen.
10 - 15 cm Buschbohnen wurden mit den Wirkstoffzubereitungen tropfnaß bespritzt. Nach 2 Wochen wird der Zuwachs gemessen und die Wuchshemmung in % des Zuwachses der Kontrollpflanzen berechnet. Die Ergeb- nisse sind in Tabelle 5 zusammengefaßt.
Claims (9)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3340595 | 1983-11-10 | ||
| DE19833340595 DE3340595A1 (en) | 1983-11-10 | 1983-11-10 | IMIDAZOLINONES, METHOD FOR THEIR PRODUCTION AND THEIR USE IN PLANT PROTECTION |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0144748A2 true EP0144748A2 (en) | 1985-06-19 |
| EP0144748A3 EP0144748A3 (en) | 1985-07-03 |
Family
ID=6213909
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP84113259A Withdrawn EP0144748A3 (en) | 1983-11-10 | 1984-11-03 | Imidazolinones, process for their preparation and their use in plant ptotection |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US4614535A (en) |
| EP (1) | EP0144748A3 (en) |
| JP (1) | JPS60123475A (en) |
| KR (1) | KR850003722A (en) |
| CN (1) | CN85101424A (en) |
| AU (1) | AU3528884A (en) |
| BR (1) | BR8405750A (en) |
| DD (1) | DD231481A5 (en) |
| DE (1) | DE3340595A1 (en) |
| ES (1) | ES8600248A1 (en) |
| HU (1) | HUT36341A (en) |
| IL (1) | IL73470A0 (en) |
| PH (1) | PH20098A (en) |
| ZA (1) | ZA848760B (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2174395A (en) * | 1986-05-09 | 1986-11-05 | American Cyanimid Co | Herbicidal 2-(2-imidazolin-2-yl)pyridine derivatives |
| EP0270760A1 (en) * | 1986-10-14 | 1988-06-15 | Bayer Ag | 2-(Oxo-5-imidazolin-2-yl)-pyridine derivatives |
| WO1991014679A1 (en) * | 1990-03-20 | 1991-10-03 | Sanofi | Heterocyclic n-substituted derivatives, their preparation and thepharmaceutical compositions containing them |
| EP0519831A1 (en) * | 1991-06-21 | 1992-12-23 | Sanofi | N-Substituted heterocyclic derivatives, their preparation, and pharmaceutical compositions containing them |
| AP405A (en) * | 1991-12-20 | 1995-09-03 | Bayer Sas | Fungicidal 2-imidazolin-5-one and 2-imidazoline-5-thione derivatives. |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4798619A (en) * | 1980-06-02 | 1989-01-17 | American Cyanamid Co. | 2-(2-imidazolin-2-yl)-pyridines and quinolines and use of said compounds as herbicidal agents |
| GR861747B (en) * | 1985-07-25 | 1986-09-23 | Nissan Chemical Ind Ltd | Pyridine derivatives process for their production and herbicidal compositions |
| CN86106435A (en) * | 1985-09-24 | 1987-05-27 | 舍林股份公司 | Imidazolinyl derivative, its preparation method and the preparation that contains this compounds with herbicide effect |
| EP0261705A1 (en) * | 1986-08-25 | 1988-03-30 | Shell Internationale Researchmaatschappij B.V. | Herbicidal imidazolinyl benzoic acids and derivatives |
| EP0303863A3 (en) * | 1987-08-17 | 1991-10-23 | American Cyanamid Company | Benzenesulfonyl carboxamide compounds, intermediate compounds and methods of preparation thereof and use of said compounds and intermediate compounds as herbicidal agents |
| US5034532A (en) * | 1988-01-27 | 1991-07-23 | American Cyanamid Company | Method for the preparation of quinolyl and pyridyl substituted imidazolinones |
| US5062881A (en) * | 1989-12-20 | 1991-11-05 | American Cyanamid Company | 2-(1-substituted-2-imidazolin-2-yl)benzoic and nicotinic acids and a method for their preparation |
| US5270317A (en) * | 1990-03-20 | 1993-12-14 | Elf Sanofi | N-substituted heterocyclic derivatives, their preparation and the pharmaceutical compositions in which they are present |
| US5076832A (en) * | 1990-07-20 | 1991-12-31 | Ici Americas Inc. | Certain 3-(substituted phenyl)-5-(substituted phenyl)-1-ethylimidazolidine-4-ones as herbicides |
| US5108485A (en) * | 1990-08-31 | 1992-04-28 | American Cyanamid Company | Herbicidal 2-(2-imidazolin-2-yl)-benzazoles |
| FR2706456B1 (en) * | 1993-06-18 | 1996-06-28 | Rhone Poulenc Agrochimie | Optically active derivatives of 2-imidazoline-5-ones and 2-imidazoline-5-thiones fungicides. |
| US6002016A (en) * | 1991-12-20 | 1999-12-14 | Rhone-Poulenc Agrochimie | Fungicidal 2-imidazolin-5-ones and 2-imidazoline-5-thiones |
| US6008370A (en) * | 1992-11-25 | 1999-12-28 | Rhone-Poulenc Agrochimie | Fungicidal-2-alkoxy/haloalkoxy-1-(mono- or disubstituted)amino-4,4-disubstituted-2-imidazolin-5-ones |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2400018A1 (en) * | 1977-08-08 | 1979-03-09 | American Cyanamid Co | NEW IMIDAZOLINYLBENZOIC ACIDS, THEIR ESTERS AND THEIR SALTS, THEIR PREPARATION PROCESS AND THEIR APPLICATION AS INSECTICIDES |
| GB2011876A (en) * | 1978-01-09 | 1979-07-18 | American Cyanamid Co | Plant growth regulating methods |
| US4188487A (en) * | 1977-08-08 | 1980-02-12 | American Cyanamid Company | Imidazolinyl benzoic acids, esters and salts and their use as herbicidal agents |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4125727A (en) * | 1976-10-18 | 1978-11-14 | American Cyanamid Company | Method of preparing imidazoisoindolediones |
-
1983
- 1983-11-10 DE DE19833340595 patent/DE3340595A1/en not_active Withdrawn
-
1984
- 1984-11-02 HU HU844076A patent/HUT36341A/en unknown
- 1984-11-03 EP EP84113259A patent/EP0144748A3/en not_active Withdrawn
- 1984-11-08 US US06/669,620 patent/US4614535A/en not_active Expired - Fee Related
- 1984-11-08 ES ES537502A patent/ES8600248A1/en not_active Expired
- 1984-11-08 DD DD84269265A patent/DD231481A5/en unknown
- 1984-11-08 PH PH31423A patent/PH20098A/en unknown
- 1984-11-09 KR KR1019840007012A patent/KR850003722A/en not_active Application Discontinuation
- 1984-11-09 BR BR8405750A patent/BR8405750A/en unknown
- 1984-11-09 ZA ZA848760A patent/ZA848760B/en unknown
- 1984-11-09 AU AU35288/84A patent/AU3528884A/en not_active Abandoned
- 1984-11-09 JP JP59235343A patent/JPS60123475A/en active Pending
- 1984-11-09 IL IL73470A patent/IL73470A0/en unknown
-
1985
- 1985-04-01 CN CN198585101424A patent/CN85101424A/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2400018A1 (en) * | 1977-08-08 | 1979-03-09 | American Cyanamid Co | NEW IMIDAZOLINYLBENZOIC ACIDS, THEIR ESTERS AND THEIR SALTS, THEIR PREPARATION PROCESS AND THEIR APPLICATION AS INSECTICIDES |
| US4188487A (en) * | 1977-08-08 | 1980-02-12 | American Cyanamid Company | Imidazolinyl benzoic acids, esters and salts and their use as herbicidal agents |
| GB2011876A (en) * | 1978-01-09 | 1979-07-18 | American Cyanamid Co | Plant growth regulating methods |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2174395A (en) * | 1986-05-09 | 1986-11-05 | American Cyanimid Co | Herbicidal 2-(2-imidazolin-2-yl)pyridine derivatives |
| EP0270760A1 (en) * | 1986-10-14 | 1988-06-15 | Bayer Ag | 2-(Oxo-5-imidazolin-2-yl)-pyridine derivatives |
| WO1991014679A1 (en) * | 1990-03-20 | 1991-10-03 | Sanofi | Heterocyclic n-substituted derivatives, their preparation and thepharmaceutical compositions containing them |
| EP0454511A1 (en) * | 1990-03-20 | 1991-10-30 | Sanofi | N-substituted heterocycle derivatives, their preparation, compositions containing them |
| EP0519831A1 (en) * | 1991-06-21 | 1992-12-23 | Sanofi | N-Substituted heterocyclic derivatives, their preparation, and pharmaceutical compositions containing them |
| FR2677984A1 (en) * | 1991-06-21 | 1992-12-24 | Sanofi Elf | N-SUBSTITUTED IMIDAZOLINE DERIVATIVES, THEIR PREPARATION, PHARMACEUTICAL COMPOSITIONS CONTAINING THE SAME |
| AP405A (en) * | 1991-12-20 | 1995-09-03 | Bayer Sas | Fungicidal 2-imidazolin-5-one and 2-imidazoline-5-thione derivatives. |
Also Published As
| Publication number | Publication date |
|---|---|
| KR850003722A (en) | 1985-06-26 |
| EP0144748A3 (en) | 1985-07-03 |
| ES537502A0 (en) | 1985-10-16 |
| HUT36341A (en) | 1985-09-30 |
| BR8405750A (en) | 1985-09-17 |
| DD231481A5 (en) | 1986-01-02 |
| JPS60123475A (en) | 1985-07-02 |
| US4614535A (en) | 1986-09-30 |
| DE3340595A1 (en) | 1985-05-23 |
| CN85101424A (en) | 1987-01-17 |
| AU3528884A (en) | 1985-05-16 |
| ZA848760B (en) | 1985-06-26 |
| PH20098A (en) | 1986-09-24 |
| ES8600248A1 (en) | 1985-10-16 |
| IL73470A0 (en) | 1985-02-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0126254B1 (en) | N-(2-nitrophenyl)-4-aminopyrimidine derivatives as microbicides | |
| EP0131258A2 (en) | N-alkoxy- and N-alkylsulfonylaminosulfonyl ureas, and pyrimido- or triazino-thiatriazine oxides as intermediates | |
| EP0144748A2 (en) | Imidazolinones, process for their preparation and their use in plant ptotection | |
| EP0137868B1 (en) | 1-substituted imidazole-5-carboxylic-acid derivatives, their preparation and their use as biocides | |
| DD264844A5 (en) | HERBICIDE MEDIUM | |
| EP0248968A1 (en) | (R)-2-[4-(5-chloro-3-fluoropyridin-2-yloxy)-phenoxy]-propionic acid propynyl ester endowed with a herbicidal activity | |
| EP0264865A2 (en) | Herbicides containing alpha-iminoanilide, these compounds and process for their preparation | |
| EP0075840B1 (en) | Heterocyclic phenyl ethers, process for their preparation and herbicides containing them | |
| DD261084A5 (en) | 1,2-DISUBSTITUTED PIPERIDINES, PROCESS FOR THEIR PREPARATION AND THEIR USE IN PLANT PROTECTION | |
| EP0216243A2 (en) | N-substituted 3,4,5,6-tetrahydrophthalimide, process for their preparation and their use as herbicides | |
| DD273436A5 (en) | METHOD FOR PRODUCING SUBSTITUTED TETRAHYDROPHTHOLIMIDES AND THEIR USE OF THE HERBIDE | |
| EP0151744B1 (en) | 1-acylimidazolinones, process for their preparation and their agricultural application | |
| EP0071572B1 (en) | Derivatives of 2-nitro-4- or-5-pyridyloxy-phenylphosphonic acid, process for their preparation, their use as herbicides and/or regulators of plant growth and/or microbicides, as well as the intermediates used for their preparation, the process for their preparation and their use as herbicides | |
| EP0391849B1 (en) | Pyridyl-methylene amino-1,2,4-triazinones | |
| DE3818848A1 (en) | PYRIDAZINE DERIVATIVES, METHOD FOR THE PRODUCTION THEREOF, MEANS CONTAINING IT AND THEIR USE IN PLANT PROTECTION | |
| EP0227045B1 (en) | Heterocyclic phenyl ethers, process for their preparation and herbicidal compositions containing them | |
| EP0144749A2 (en) | Substituted 2-aryl-imidazolinones, process for their preparation and their use in plant protection | |
| DE3408403A1 (en) | Substituted 2-arylimidazolinones, their preparation, and their use as agents for treating plants | |
| DE3403730A1 (en) | ISOINDOLES, METHOD FOR THEIR PRODUCTION AND THEIR USE IN AGRICULTURE | |
| EP0009555A1 (en) | Herbicidal agents based on thiolcarbamate, containing a haloacylamide as antagonist, and their use in a process for selectively controlling the growth of unwanted plants | |
| EP0133162A2 (en) | Phosphine oxide derivatives and their use as herbicidal and plant-growth regulating agents | |
| EP0144001A1 (en) | Substituted phenoxypropionyl isothioureas, method for their preparation and their use in plant protection | |
| DE3624859A1 (en) | NEW CHINOLINOXY COMPOUNDS, METHOD FOR THEIR PRODUCTION AND THEIR USE AS ANTIDOTS | |
| EP0221439A1 (en) | Herbicidal compositions which contain nitrogen-containing heterocycles with arylcarbamoyl radicals, and heterocyclic derivatives with arylcarbamoyl radicals | |
| DD219656A5 (en) | HERBICIDES AND / OR FUNGICIDES COMPOSITION |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI NL SE |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI NL SE |
|
| RTI1 | Title (correction) | ||
| 17P | Request for examination filed |
Effective date: 19851115 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN WITHDRAWN |
|
| 18W | Application withdrawn |
Withdrawal date: 19860829 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: MILDENBERGER, HILMAR, DR. Inventor name: BIERINGER, HERMANN, DR. Inventor name: BUERSTELL, HELMUT, DR. Inventor name: SCHMIERER, ROLAND, DR. Inventor name: LIEBL, RAINER, DR. Inventor name: BAUER, KLAUS, DR. Inventor name: HANDTE, REINHARD, DR. |