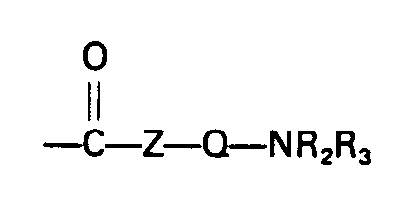EP0140274B2 - Lubricating oil additives - Google Patents
Lubricating oil additives Download PDFInfo
- Publication number
- EP0140274B2 EP0140274B2 EP84112445A EP84112445A EP0140274B2 EP 0140274 B2 EP0140274 B2 EP 0140274B2 EP 84112445 A EP84112445 A EP 84112445A EP 84112445 A EP84112445 A EP 84112445A EP 0140274 B2 EP0140274 B2 EP 0140274B2
- Authority
- EP
- European Patent Office
- Prior art keywords
- mol
- esters
- proportion
- component
- acrylic acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M145/00—Lubricating compositions characterised by the additive being a macromolecular compound containing oxygen
- C10M145/02—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C10M145/10—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to a carboxyl radical, e.g. acrylate
- C10M145/12—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to a carboxyl radical, e.g. acrylate monocarboxylic
- C10M145/14—Acrylate; Methacrylate
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M143/00—Lubricating compositions characterised by the additive being a macromolecular hydrocarbon or such hydrocarbon modified by oxidation
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M149/00—Lubricating compositions characterised by the additive being a macromolecular compound containing nitrogen
- C10M149/02—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M157/00—Lubricating compositions characterised by the additive being a mixture of two or more macromolecular compounds covered by more than one of the main groups C10M143/00 - C10M155/00, each of these compounds being essential
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/02—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/06—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing conjugated dienes
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2209/00—Organic macromolecular compounds containing oxygen as ingredients in lubricant compositions
- C10M2209/02—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C10M2209/08—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to a carboxyl radical, e.g. acrylate type
- C10M2209/084—Acrylate; Methacrylate
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2217/00—Organic macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2217/02—Macromolecular compounds obtained from nitrogen containing monomers by reactions only involving carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2217/00—Organic macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2217/02—Macromolecular compounds obtained from nitrogen containing monomers by reactions only involving carbon-to-carbon unsaturated bonds
- C10M2217/022—Macromolecular compounds obtained from nitrogen containing monomers by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to an amino group
- C10M2217/023—Macromolecular compounds obtained from nitrogen containing monomers by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to an amino group the amino group containing an ester bond
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2217/00—Organic macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2217/02—Macromolecular compounds obtained from nitrogen containing monomers by reactions only involving carbon-to-carbon unsaturated bonds
- C10M2217/024—Macromolecular compounds obtained from nitrogen containing monomers by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to an amido or imido group
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2217/00—Organic macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2217/02—Macromolecular compounds obtained from nitrogen containing monomers by reactions only involving carbon-to-carbon unsaturated bonds
- C10M2217/028—Macromolecular compounds obtained from nitrogen containing monomers by reactions only involving carbon-to-carbon unsaturated bonds containing monomers having an unsaturated radical bound to a nitrogen-containing hetero ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2217/00—Organic macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2217/06—Macromolecular compounds obtained by functionalisation op polymers with a nitrogen containing compound
Definitions
- the invention relates to multifunctional lubricating oil additives based on polyalkyl acrylates and polyalkyl methacrylates as well as systems formed from olefin copolymers (OCP) or hydrogenated styrene-diene copolymers (HSD) and PAMA.
- OCP olefin copolymers
- HSD hydrogenated styrene-diene copolymers
- Lubricating oils generally contain n-paraffin hydrocarbons, which on the one hand have a positive effect on the setting of a good viscosity / temperature behavior, but on the other hand fail in crystalline form on cooling and thereby impair the flow of the oils or completely prevent them ("sticking").
- An improvement in the low-temperature flow properties can be achieved by dewaxing. Since the costs increase considerably if one wants to lower the "pour point" beyond certain values, one generally only performs a partial dewaxing of the oils up to a pour point in the range of -15 ° C. and makes use of the further reduction Pour point (down to about -40 ° C) so-called pour point depressants, which effectively reduce the pour point even in concentrations between 0.05 and 1%. The following idea is decisive:
- Paraffin-like compounds are built into the growing paraffin crystal surfaces and thus prevent the crystals from growing further and the formation of extensive crystal groups.
- pour point improvers For the mode of operation of such pour point improvers, it applies that they have certain structural elements, namely sufficiently long alkyl groups to be incorporated into the growing paraffin crystals from the nucleation and side chains or side groups at greater intervals to disrupt the crystal growth. (See Ullmanns, Encyklopedia of technical chemistry, 4th edition, volume 20, Verlag Chemie, 1981, p. 548).
- Technically applicable pour point depressants on the other hand, must be required to have good thermal, oxidative and chemical stability, shear strength and the like. have.
- the currently preferred pour point depressants are polymethacrylates, which lower the pour point of lubricating oils sufficiently in a concentration of 0.1-0.5% (cf. US Pat. No. 2,091,627, US Pat. No. 2 100 993, US Pat. PS 2 114 233).
- the carbon number of the alkyl radicals is between 12 and 18, the branching wheel between 10 and 30 mol%.
- Polymethacrylates in the M range between approx. 5,000 and 500,000 are available, which allow the flow behavior of light, low-molecular to heavy, high-molecular lubricating oils to be improved.
- FR-A 2 407 259 has set itself the task of the pour point of a so-called non-classic oil, namely a hydrocracked base oil with a particularly high V.I. (XHVI- ⁇ I with V.I. of at least 140 according to ASTM D-2270).
- non-classic oil namely a hydrocracked base oil with a particularly high V.I.
- Such oils are not special cases directly comparable to "classic" oils. thereby proving that polyalkyl methacrylates with an average carbon number of 12.4-13.7 in the range of the C9-C18 alkyl esters and at least 6 alkyl chains with a different C number in the range of the alkyl esters with 9-18 carbon atoms, which are especially for HVI oils were developed according to which FR-A did not produce any useful results with these non-classical oils.
- multifunctional additives for mineral oils are also said to improve the viscosity / temperature behavior at high and low temperatures. This requires, compared to pure pour point improvers, larger additional amounts in the range between 1-30% by weight.
- multifunctional viscosity index improvers can also have dispersing / detergent properties (cf. Ullmanns Encyklopadie der Technische Chemie, 4th edition, volume 20, loc.cit., Pp. 457-671) .
- These multifunctional VI improvers are mostly based on polymethacrylic acid esters (PAMA) and combinations (mixed polymers) of PAMA with olefin copolymers (OCP) or hydrogenated styrene-diene copolymers (HSD) and less on the basis of OCP or HSD alone.
- PAMA polymethacrylic acid esters
- OCP olefin copolymers
- HSD hydrogenated styrene-diene copolymers
- the object on which the present invention is based relates to improving the viscosity / temperature behavior of mineral oils containing n-paraffin in the broadest sense, especially at low temperatures, caused by the tendency of the n-paraffins to crystallize.
- This task in one of its particularly acute forms is explained in more detail using the example of lubricating oils containing n-paraffin:
- base oils quality poorer mineral oils
- Lubricating oils in the sense of the present invention are understood to mean paraffin-based and naphthenic-based vacuum distillate oils.
- the additives according to the invention also contain VI-improving polyolefins / olefin copolymers (OCP), preferably of the type of the combination of polyalkyl (meth) acrylates (PAMA) and OCP (mixed polymers) as described in DE-PS 29 05 954 or US Pat. No. 4,290,925 is described.
- OCP VI-improving polyolefins / olefin copolymers
- the proportion of the olefin copolymers or the polymers according to the cited DE-PS 29 05 954 or US Pat. No. 4,290,925 in the additives can be 10-70% by weight.
- the proportion of the polymers (P) in the additives according to the invention is 10-80% by weight, the total content of polymers is 20-80% by weight.
- the proportion of component a) in the polymer P 1 is preferably 50-100 mol%, especially 100 mol%.
- the proportion of component b ') in the polymer P 2 is preferably 20-40 mol%.
- the polymer P 2 is composed only of components a ') and b').
- components b) and b ') are preferably acrylic or methacrylic acid esters of straight-chain, unbranched C 16 -C 24 alcohols, especially C 18 -C 22 alcohols.
- the tallow fatty alcohols and Alfole® (products from Condea) may be mentioned.
- the molecular weights Mw of the polymers P 1 are generally in the range from 50,000 to 500,000, and those of the polymers P 2 in the range from 50,000 to 500,000.
- the polymers P 1 and P 2 can be prepared by the customary free-radical polymerization processes.
- Component e) of the polymers P 1 is by definition understood to mean free-radically polymerizable monomers with functional groups in the molecule, in particular those whose positive effect on oil additives in the sense of dispersing or detergent activity is known.
- R 1 is hydrogen or methyl and Bs is an (inert) heterocyclic 5- or 6-membered ring or a radical means, where Z is oxygen or a radical -NR 4 and Q are an optionally alkylated hydrocarbon bridge with a total of 2 to 10 carbon atoms and R 2 and R 3 are each an alkyl radical with 1 to 6 carbon atoms or together including nitrogen and optionally others Heteroatoms can form a heterocyclic 5- or 6-membered ring and in which R 4 represents hydrogen or an alkyl radical having 1 to 6 carbon atoms.
- Examples include C- and N-vinylpyridine-, vinylpyrrolidone, vinylcarbazole, vinylimidazole and their alkyl derivatives, in particular the N-vinyl compounds, also the dialkylaminoalkyl esters of (meth) acrylic acid, especially dimethylaminoethyl acrylate and methacrylate, dimethylaminopropylacrylate, methacrylate and the corresponding amide (methacrylate) - or -methacrylamides) such as dimethylaminopropyl (meth) acrylamide.
- the above definitions (formula I) also apply to e ') in the polymer P 2 .
- the solvents (L) used in the additives according to the invention are the paraffin- or naphthene-based mineral oils known in the art for lubricating oil additives or the known ester oils or poly-a-olefins. (See Ullmanns Encyklopadie der techn. Chemie, Volume 20, loc.cit., Pp. 483-529).
- the preparation of the polymers follows the polymerization processes of the prior art.
- a mixture of mineral oil and a monomer mixture of a), b), c), d) and e) is placed in a reaction vessel which is suitably equipped with a stirrer, thermometer, reflux condenser and metering line.
- the mixture is heated to about 90-100 ° C. under a CO 2 atmosphere and with stirring. After this temperature has been reached and initiator (preferably per compounds such as peresters, peroxides or azo compounds) has been added, a mixture of the monomers a), b), c), d) and e) and further initiator are metered in; approx. 2 hours after the end of the feed, further initiator is fed.
- the total amount of initiator is generally 1-3% by weight, based on the total amount of monomers.
- the total polymerization time is generally 8-9 hours.
- a viscous solution with a polymer content of generally 40-70% by weight is obtained.
- One component is placed in a suitable container and heated to approx. 80-120 ° C with stirring.
- the admixing components are also heated to approx. 80-120 ° C and metered in as quickly as possible to the component while stirring.
- the additive according to the invention possibly together with other additives, such as DI package and OCP-VI improver, is dissolved in the base oil at 50-60 ° C. with stirring.
- the additives according to the invention can be added to the lubricating oils in a manner known per se.
- Oil formulations containing the additives according to the invention in addition to the required viscosity data at 100 ° C., show very favorable values for pour point and stable pour point as well as excellent viscosity data at -15 ° C. to 40 ° C.
- Feed 1 is metered in uniformly within 210 minutes. 120 minutes after the end of the inlet, inlet 2 is started:
Description
Die Erfindung betrifft multifunktionelle Schmieröladditive auf Basis von Polyalkylacrylaten und Polyalkylmethacrylaten sowie aus Olefincopolymerisaten (OCP) bzw. hydrierten Styrol-Dien-Copolymerisaten (HSD) und PAMA gebildeten Systemen. Zur Verbesserung des "Pour point", des Viskositäts-/Temperaturverhaltens bei hohen und tiefen Temperaturen und gegebenenfalls der Dispergiert-/Detergent-Eigenschaften.The invention relates to multifunctional lubricating oil additives based on polyalkyl acrylates and polyalkyl methacrylates as well as systems formed from olefin copolymers (OCP) or hydrogenated styrene-diene copolymers (HSD) and PAMA. To improve the "pour point", the viscosity / temperature behavior at high and low temperatures and, if necessary, the dispersing / detergent properties.
Schmieröle enthalten in der Regel n-Paraffinkohlenwasserstoffe, die sich zwar einerseits auf die Einstellung eines guten Viskositäts-/Temperaturverhaltens günstig auswirken, andererseits aber beim Abkühlen in kristalliner Form ausfallen und dadurch das Fließen der Öle beeinträchtigen odervöllig verhindern ("Stocken"). Eine Verbesserung der Tieftemperatur- Fließeigenschaften kann durch Entparaffinierung erreicht werden. Da die Kosten erheblich ansteigen, wenn man den "Pour point" über bestimmte Werte hinweg herabsetzen will, führt man im allgemeinen nur eine partielle Entparaffinierung der Öle bis zu einem Pour point im Bereich von -15°C durch und bedient sich zur weiteren Herabsetzung des Pour point (bis etwa -40°C) sogenannter Pour point-Erniedriger, die den Pour point bereits in Konzentrationen zwischen 0,05 und 1% wirksam herabsetzen. Dabei ist in etwa folgende Vorstellung maßgeblich:Lubricating oils generally contain n-paraffin hydrocarbons, which on the one hand have a positive effect on the setting of a good viscosity / temperature behavior, but on the other hand fail in crystalline form on cooling and thereby impair the flow of the oils or completely prevent them ("sticking"). An improvement in the low-temperature flow properties can be achieved by dewaxing. Since the costs increase considerably if one wants to lower the "pour point" beyond certain values, one generally only performs a partial dewaxing of the oils up to a pour point in the range of -15 ° C. and makes use of the further reduction Pour point (down to about -40 ° C) so-called pour point depressants, which effectively reduce the pour point even in concentrations between 0.05 and 1%. The following idea is decisive:
Paraffinähnliche Verbindungen werden in die wachsenden Paraffinkristallflächen eingebaut und verhindern so das Weiterwachsen der Kristalle und die Bildung ausgedehnter Kristallverbände.Paraffin-like compounds are built into the growing paraffin crystal surfaces and thus prevent the crystals from growing further and the formation of extensive crystal groups.
Für die Wirkungsweise derartiger Pour-Point-Verbesserer gilt, daß sie bestimmte Strukturelemente aufweisen, nämlich hinreichend lange Alkylgruppen, um von der Keimbildung ab in die wachsenden Paraffinkristalle eingebaut zu werden und in größeren Abständen Seitenketten bzw. Seitengruppen um das Kristallwachstum zu stören. (Vgl. Ullmanns, Encyklopädie der technischen Chemie, 4. Auflage, Band 20, Verlag Chemie, 1981, S. 548). Von technisch anwendbaren Pour-point-Erniedrigern muß andererseits verlangt werden, daß sie gute thermische, oxidative und chemische Stabilität, Scherfestigkeit u.ä. besitzen.For the mode of operation of such pour point improvers, it applies that they have certain structural elements, namely sufficiently long alkyl groups to be incorporated into the growing paraffin crystals from the nucleation and side chains or side groups at greater intervals to disrupt the crystal growth. (See Ullmanns, Encyklopedia of technical chemistry, 4th edition, volume 20, Verlag Chemie, 1981, p. 548). Technically applicable pour point depressants, on the other hand, must be required to have good thermal, oxidative and chemical stability, shear strength and the like. have.
Die zur Zeit bevorzugten Pour-point-Erniedriger sind Polymethacrylate, die bereits in Konzentration von 0,1-0,5% den Fließpunkt von Schmierölen hinreichend erniedrigen (vgl. US-PS 2 091 627, US-PS 2 100 993, US-PS 2 114 233). Die Kohlenstoffzahl der Alkylreste liegt dabei zwischen 12 und 18, der Verzweigungsrad zwischen 10 und 30 Mol-%. Zur Verfügung stehen Polymethacrylate im Bereich M zwischen ca. 5 000 und 500 000, die eine Verbesserung des Fließverhaltens von leichten, niedermolekularen bis zu schweren, hochmolekularen Schmierölen gestatten.The currently preferred pour point depressants are polymethacrylates, which lower the pour point of lubricating oils sufficiently in a concentration of 0.1-0.5% (cf. US Pat. No. 2,091,627, US Pat. No. 2 100 993, US Pat. PS 2 114 233). The carbon number of the alkyl radicals is between 12 and 18, the branching wheel between 10 and 30 mol%. Polymethacrylates in the M range between approx. 5,000 and 500,000 are available, which allow the flow behavior of light, low-molecular to heavy, high-molecular lubricating oils to be improved.
Die FR-A 2 407 259 hat sich zur Aufgabe gesetzt, den Pour Point eines sogenannten nicht-klassischen Öls, nämlich eines hydrogekrackten Grundöls mit speziell hohem V.I. (XHVI-ÖI mit V.I. von mindestens 140 nach ASTM D-2270) zu verbessern. Derartige Öle stellen nicht unmittelbar mit "klassischen" Ölen vergleichbare Sonderfälle dar. Diese Tatsache wird u.a. dadurch belegt, daß Polyalkylmethacrylate mit einer Durchschnitts-Kohlenstoffzahl von 12,4-13.7 im Bereich der C9-C18-Alkylester und mindestens 6 Alkylketten mit einer abweichenden C-Zahl im Bereich der Alkylester mit 9-18 Kohlenstoffatomen, die speziell für HVI-Öle entwickelt worden waren, laut der FR-A bei diesen nichtklassichen Öle keine brauchbaren Ergebnisse erbrachten.FR-A 2 407 259 has set itself the task of the pour point of a so-called non-classic oil, namely a hydrocracked base oil with a particularly high V.I. (XHVI-ÖI with V.I. of at least 140 according to ASTM D-2270). Such oils are not special cases directly comparable to "classic" oils. thereby proving that polyalkyl methacrylates with an average carbon number of 12.4-13.7 in the range of the C9-C18 alkyl esters and at least 6 alkyl chains with a different C number in the range of the alkyl esters with 9-18 carbon atoms, which are especially for HVI oils were developed according to which FR-A did not produce any useful results with these non-classical oils.
Multifunktionelle Additive für Mineralöle sollen neben einer Herabsetzung des Stockpunktes auch das Viskositäts-/Temperaturverhalten und zwar bei hohen und tiefen Temperaturen verbessern. Dazu zind, verglichen mit reinen Stockpunktverbesserern, größere Zusatzmengen im Bereich zwischen 1-30 Gew.-% notwendig. Außerdem können solche multifunktionelle Viskositäts-Index-Verbesserer (VI-Verbesserer) noch Dispergier-/Detergenz-Eigenschaften besitzen (vgl. Ullmanns Encyklopädie der technischen Chemie, 4. Aufl., Band 20, loc.cit., S. 457-671). Diese multifunktionellen VI-Verbesserer sind meist auf Basis von Polymethacrylsäureestern (PAMA) und Kombinationen (Mixed Polymers) aus PAMA mit Olefincopolymeren (OCP) oder hydrierten Styrol-Dien-Copolymeren (HSD) und weniger auf Basis von OCP oder HSD alleine.In addition to reducing the pour point, multifunctional additives for mineral oils are also said to improve the viscosity / temperature behavior at high and low temperatures. This requires, compared to pure pour point improvers, larger additional amounts in the range between 1-30% by weight. In addition, such multifunctional viscosity index improvers (VI improvers) can also have dispersing / detergent properties (cf. Ullmanns Encyklopadie der Technische Chemie, 4th edition, volume 20, loc.cit., Pp. 457-671) . These multifunctional VI improvers are mostly based on polymethacrylic acid esters (PAMA) and combinations (mixed polymers) of PAMA with olefin copolymers (OCP) or hydrogenated styrene-diene copolymers (HSD) and less on the basis of OCP or HSD alone.
Die der vorliegenden Erfindung zugrundeliegende Aufgabe bezieht sich auf die Verbesserung des Viskositäts-/Temperatur-Verhaltensvon n-Paraffinhaltigen Mineralölen im weitesten Sinne, besonders bei tiefen Temperaturen, hervorgerufen durch die Kristallisationsneigung der n-Paraffine. Diese Aufgabe in einer ihrer besonders akuten Formen sie am Beispiel der n-Paraffin-haltigen Schmieröle näher erläutert: Die Erschöpfung bestehender Öllagerstätten hat bekanntlich dazu geführt, daßwenigerergiebige bzw. geringerwertige Ölvorkommen ausgebeutet werden. Man trifft daher in zunehmenden Maße auf ein Angebot an qualitativ schlechteren Mineralölen (Grundölen). Kritisch kann sich z.B. der Umstand auswirken, daß diese Öle immer weniger entparaffiniert sind und sich technologisch schwerer handhaben lassen; (sogenannte "kritische Grundöle"). Es bestand daher ein Bedürfnis nach pour-point- bzw. fließverbessernden Additiven für Mineralöle, welche die Verwertung auch der technologisch schwer handhabbaren Mineralöle erleichtern. Den oben aufgezeigten Problemen überlagern sich noch spezielle Anwendungsprobleme. So treten bei Motoren-Mehrbereichsölen, die OCP als VI-Verbesserer enthalten, vermehrte Schwierigkeiten hinsichtlich des Pour-point auf, da die OCP's offensichtlich einen negativen Effekt auf den Pour-point ausüben. Weiter beobachtet man Schwierigkeiten bei Verwendung von OCP-haltigen Schmierölen bei Dieselmotoren, dann nämlich, wenn Diesel-Kraftstoff in OCP-haltige Motorenöle gelangt. Trotz der eintretenden Verdünnung erfolgt in der Regel ein Ansteigen des Pour-point unter dem Einfluß des Dieselkraftstoffs. Die zur Verfügung stehenden Mittel konnten den auf die Technik zukommenden, neuen Anforderungen nur unvollkommen gerecht werden.The object on which the present invention is based relates to improving the viscosity / temperature behavior of mineral oils containing n-paraffin in the broadest sense, especially at low temperatures, caused by the tendency of the n-paraffins to crystallize. This task in one of its particularly acute forms is explained in more detail using the example of lubricating oils containing n-paraffin: As is well known, the exhaustion of existing oil deposits has led to the exploitation of less-efficient or lower-quality oil deposits. There is therefore an increasing amount of quality poorer mineral oils (base oils). For example, the fact that these oils are less and less dewaxed and are more difficult to handle technologically can have a critical impact; (so-called "critical base oils"). There was therefore a need for pour-point or flow-improving additives for mineral oils, which also facilitate the recycling of technologically difficult to handle mineral oils. The problems shown above are overlaid by special application problems. For motor multigrade oils that contain OCP as a VI improver, there are increased difficulties with regard to the pour point, since the OCP's obviously have a negative effect on the pour point. Difficulties are also observed when using OCP-containing lubricating oils in diesel engines, namely when diesel fuel gets into OCP-containing engine oils. Despite the thinning that occurs, the pour point usually rises under the influence of the diesel fuel. The funds available could only incompletely meet the new technical requirements.
Es wurde nun gefunden, daß sich Additive dem Paraffingehalt der Schmieröle anpassen lassen und damit die Lösung der gestellten Aufgabe ermöglichen, die außer den üblichen Lösungsmitteln Mischungen aus den Polymeren (P), die
- I. 10-99 Gew.-% eines oder mehrerer Polymeren P1, die
- a) aus Estern der Methacryl-, der Acrylsäure oder beiden mit geradkettigen, unverzweigten Alkoholen mit mindestens 6 und höchstens 15 Kohlenstoffatomen und
- b) aus Estern der Methacryl-, der Acrylsäure oder beiden mit geradkettigen unverzweigten Alkoholen mit 16 bis 30 Kohlenstoffatomen und
- c) aus Estern der Methacryl-, der Acrylsäure oder beiden mit verzweigten Alkoholen mit 8 bis 40 Kohlenstoffatomen,
- d) aus Estern der Methacryl-, der Acrylsäure oder beiden mitAlkoholem mit 1 bis 5 Kohlenstoffatomen,
- e) aus radikalisch copolymerisierbaren Monomeren, die funktionelle Gruppen im Molekül aufweisen, aufgebaut sind,
wobei der Anteil des Bestandteils a) 10-100 Mol-%, der Anteil des Bestandteils b) 0-5 Mol-%, vorzugsweise 0,5-5 Mol-%, speziell 1-5 Mol-%, der Anteil des Bestandteils c) 0-90 Mol-%, vorzugsweise 0,5-90 Mol-%, besonders bevorzugt 0,5-60 Mol-%, der Anteil des Bestandteils d) 0-50 Mol-%, vorzugsweise 5-30 Mol-% und der Anteil des Bestandteils e) 0-20 Mol-%, vorzugsweise 2-15 Mol-%, jeweils bezogen auf das Polymere P1 ausmacht, und
- II. 90-1 Gew.-% eines oder mehrerer Polymerer P2, die
- a') aus Estern der Methacryl-, der Acrylsäure oder beiden mit geradkettigen unverzweigten Alkoholen mit mindestens 6 und höchstens 15 Kohlenstoffatomen und
- b') aus Estern der Methacryl-, der Acrylsäure oder beiden mit geradkettigen, unverzweigten Alkoholen mit 16 bis 30 Kohlenstoffatomen und
- c') aus Estern der Methacryl-, der Acrylsäure oder beiden mit verzweigten Alkoholen mit 8 bis 40 Kohlenstoffatomen und
- d') aus Estern der Methacryl-, der Acrylsäure oder beiden mit Alkoholen mit 1 bis 5 Kohlenstoffatomen, e') aus radikalisch copolymerisierbaren Monomeren, die funktionelle Gruppen im Molekül aufweisen, aufgebaut sind,
wobei der Anteil des Bestandteils a') 30-90 Mol.-%, der Anteil des Bestandteils b') 10-70 Mol.-%, der Antei des Bestandteils c') 0-90 Mol.-%, vozugsweise 10-90 Mol.-%, besonders bevorzugt 10-30 Mol.- %, und der Anteil des Bestandteils d') 0-50 Mol.-%, vorzugsweise 5-30 Mol.-%, und der Anteil des Bestandteils e') 0-20 Mol-%, vorzugsweise 2-15 Mol.-%, jeweils bezogen auf das Polymerisat P2 ausmacht, und einer zweiten Polymerkomponente, ausgewählt aus der Gruppe der VI-verbessernden Olefincopolymerisate mit der Maßgabe, daß die Olefincopolymerisate aus Ethylen, Propylen, Butylen oder Isobutylen aufgebaut sind, auf welche Monomeren aus der Gruppe der Bestandteile a), b), c) gepfropft wurden, oder in Form einer konzentrierten Polymeremulsion, die als kontinuierliche Phase Poly(meth)acrylate, ein Trägermedium, das gegenüber den Poly(meth)acrylestern als gutes Lösungsmitel gegenüber den Olefincopolymerisaten aufgrund des Gehalts an Poly(meth)acrylsäureester als weniger gutes Lösungsmittel wirkt und als Stabilisator für die Phasenverteilung ein Pfropf- oder ein Blockpolymerisat aus Olefincopolymerisaten und (Meth)acrylsäure-estern enthält, neben dem Lösungsmittel L ausgewählt aus der Gruppe bestehend aus paraffin- oder naphthenbasischen Mineralölen, Esterölen oder Poly-a-Olefinen enthalten. Mit der Maßgabe, daß an Anteil der Polymeren (P) und der Olefincopolymerisate 20-80 Gew.-%, bezogen auf die Additive beträgt.
- I. 10-99 wt .-% of one or more polymers P 1 , the
- a) from esters of methacrylic acid, acrylic acid or both with straight-chain, unbranched alcohols having at least 6 and at most 15 carbon atoms and
- b) from esters of methacrylic acid, acrylic acid or both with straight-chain unbranched alcohols having 16 to 30 carbon atoms and
- c) from esters of methacrylic acid, acrylic acid or both with branched alcohols having 8 to 40 carbon atoms,
- d) from esters of methacrylic acid, acrylic acid or both with alcohols having 1 to 5 carbon atoms,
- e) are built up from free-radically copolymerizable monomers which have functional groups in the molecule,
wherein the proportion of component a) 10-100 mol%, the proportion of component b) 0-5 mol%, preferably 0.5-5 mol%, especially 1-5 mol%, the proportion of component c ) 0-90 mol%, preferably 0.5-90 mol%, particularly preferably 0.5-60 mol%, the proportion of component d) 0-50 mol%, preferably 5-30 mol% and the proportion of component e) is 0-20 mol%, preferably 2-15 mol%, in each case based on the polymer P 1 , and
- II. 90-1 wt .-% of one or more polymers P 2 , the
- a ') from esters of methacrylic acid, acrylic acid or both with straight-chain unbranched alcohols having at least 6 and at most 15 carbon atoms and
- b ') from esters of methacrylic acid, acrylic acid or both with straight-chain, unbranched alcohols having 16 to 30 carbon atoms and
- c ') from esters of methacrylic acid, acrylic acid or both with branched alcohols having 8 to 40 carbon atoms and
- d ') from esters of methacrylic acid, acrylic acid or both with alcohols having 1 to 5 carbon atoms, e') from free-radically copolymerizable monomers which have functional groups in the molecule,
wherein the proportion of component a ') 30-90 mol%, the proportion of component b') 10-70 mol%, the proportion of component c ') 0-90 mol%, preferably 10-90 Mol%, particularly preferably 10-30 mol%, and the proportion of component d ') 0-50 mol%, preferably 5-30 mol%, and the proportion of component e') 0- 20 mol%, preferably 2-15 mol%, each based on the polymer P 2 , and a second polymer component selected from the group of VI-improving olefin copolymers, with the proviso that the olefin copolymers from ethylene, propylene, butylene or isobutylene, onto which monomers from the group of constituents a), b), c) have been grafted, or in the form of a concentrated polymer emulsion which, as a continuous phase, comprises poly (meth) acrylates, a carrier medium which is opposite the poly (meth ) acrylic esters as a good solvent compared to the olefin copolymers because of the content of poly (meth) acrylic acid ester as a less good solution acts medium and contains as a stabilizer for the phase distribution a graft or a block polymer from olefin copolymers and (meth) acrylic esters, in addition to the solvent L selected from the group consisting of paraffin or naphthenic mineral oils, ester oils or poly-a-olefins. With the proviso that the proportion of the polymers (P) and the olefin copolymers is 20-80% by weight, based on the additives.
(Die Summe aus a-e bzw. a'-e' beträgt jeweils 100 Mol.-%).(The sum of ae and a'-e 'is 100 mol%).
Unter Schmierölen im Sinne der vorliegenden Erfindung seien paraffinbasische und naphthenbasische Vakuumdestillatöle verstanden.Lubricating oils in the sense of the present invention are understood to mean paraffin-based and naphthenic-based vacuum distillate oils.
Hervorzuheben ist, daß die erfindungsgemäßen Additive neben den Lösungsmitteln noch VI-verbessernde Polyolefine/Olefincopolymerisate (OCP) enthalten, vorzugsweise vom Typ der Kombination von Polyalkyl-(meth)acrylaten (PAMA) und OCP (Mixed Polymere) wie er in der DE-PS 29 05 954 bzw. der US-PS 4 290 925 beschrieben ist.It should be emphasized that, in addition to the solvents, the additives according to the invention also contain VI-improving polyolefins / olefin copolymers (OCP), preferably of the type of the combination of polyalkyl (meth) acrylates (PAMA) and OCP (mixed polymers) as described in DE-PS 29 05 954 or US Pat. No. 4,290,925 is described.
Der Anteil der Olefincopolymerisate bzw. der Polymeren gemäß der zitierten DE-PS 29 05 954 bzw. der US-PS 4 290 925 an den Additiven kann 10-70 Gew.-% betragen.The proportion of the olefin copolymers or the polymers according to the cited DE-PS 29 05 954 or US Pat. No. 4,290,925 in the additives can be 10-70% by weight.
Der Anteil der Polymeren (P) an den erfindungsgemäßen Additiven liegt bei 10-80 Gew.-%, der Gehalt an Polymeren insgesamt bei 20-80 Gew.-%.The proportion of the polymers (P) in the additives according to the invention is 10-80% by weight, the total content of polymers is 20-80% by weight.
Der Anteil der Komponente a) im Polymerisat P1 liegt vorzugsweise bei 50-100 Mol-%, speziell bei 100 Mol-%. Der Anteil der Komponente b') im Polymerisat P2 liegt vorzugsweise bei 20-40 Mol-%.The proportion of component a) in the polymer P 1 is preferably 50-100 mol%, especially 100 mol%. The proportion of component b ') in the polymer P 2 is preferably 20-40 mol%.
Bevorzugt ist auch die Ausführungsform, bei der das Polymerisat P2 nur aus den Komponenten a') und b') aufgebaut ist.Also preferred is the embodiment in which the polymer P 2 is composed only of components a ') and b').
Für die Komponenten a) und a') gilt gleichermaßen: Bevorzugt sind Acryl-bzw. Methacrylsäureester mit geradkettigen unverzweigten C1O-C14-Alkoholen, z.B. hergestellt nach dem Ziegler-Verfahren durch Hydrolyse von Aluminiumalkoxiden. Genannt seien z.B. die Produkte Lorole® der Fa. Henkel KG. Düsseldorf und Alfole®, Produkte der Firma Condea, Hamburg).The following applies equally to components a) and a '): Acrylic or. Methacrylic acid esters with straight-chain unbranched C 10 -C 14 alcohols, for example prepared by the Ziegler process by hydrolysis of aluminum alkoxides. Examples include the Lorole® products from Henkel KG. Düsseldorf and Alfole®, products from Condea, Hamburg).
Für die Komponenten b) und b') gilt, daß sie vorzugsweise Acryl- bzw. Methacrylsäureester von geradkettigen, unverzweigten C16-C24-Alkoholen, besonders von C18-C22-Alkoholen darstellen. Genannt seien die Talgfettalkohole und Alfole® (Produkte der Fa. Condea).It applies to components b) and b ') that they are preferably acrylic or methacrylic acid esters of straight-chain, unbranched C 16 -C 24 alcohols, especially C 18 -C 22 alcohols. The tallow fatty alcohols and Alfole® (products from Condea) may be mentioned.
Für die Komponenten c) und c') gilt, daß sie vorzugsweise aus Estern derAcryl- bzw. der Methacrylsäure mit verzweigten C8-C20-Alkoholen des Iso-Alkanol-Typs, insbesondere aus Isodecyl-, Isotridecyl- und Isooctodecylalkoholen besteht.For components c) and c ') it applies that it preferably consists of esters of acrylic or methacrylic acid with branched C 8 -C 20 alcohols of the isoalkanol type, in particular of isodecyl, isotridecyl and isooctodecyl alcohols.
Die Molgewichte Mw der Polymerisate P1 liegen im allgemeinen im Bereich 50 000 bis 500 000, die der Polymerisate P2 im Bereich 50 000 bis 500 000.The molecular weights Mw of the polymers P 1 are generally in the range from 50,000 to 500,000, and those of the polymers P 2 in the range from 50,000 to 500,000.
Die Herstellung der Polymerisate P1 bzw. P2 kann nach den üblichen radikalischen Polymerisationsverfahren vorgenommen werden.The polymers P 1 and P 2 can be prepared by the customary free-radical polymerization processes.
Unter der Komponente e) der Polymeren P1 seien definitionsgemäß radikalisch polymerisierbare Monomere mit funtionellen Gruppen im Molekül verstanden, insbesondere solchen, deren positive Wirkung bei Öladditiven im Sinne von Dispergier- bzw. Detegenzaktivität bekannt ist. Genannt seien z.B. Verbindungen der allgemeinen Formel
Genannt seien z.B. C- und N-Vinylpyridin-, Vinylpyrrolidon, Vinylcarbazol, Vinylimidazol sowie deren Alkylderivate, insbesondere die N-Vinylverbindungen ferner die Dialkylaminoalkylester der (Meth)acrylsäure, speziell Dimethylaminoäthylacrylat und -methacrylat, Dimethylaminopropylacrylat, -methacrylat sowie die entsprechenden Amide (Dialkylaminoalkylacryl- bzw. -methacrylamide) wie z.B. das Dimethylaminopropyl(meth)acrylamid. Die vorstehenden Definitionen (Formel I) gelten auch für e') im Polymeren P2.Examples include C- and N-vinylpyridine-, vinylpyrrolidone, vinylcarbazole, vinylimidazole and their alkyl derivatives, in particular the N-vinyl compounds, also the dialkylaminoalkyl esters of (meth) acrylic acid, especially dimethylaminoethyl acrylate and methacrylate, dimethylaminopropylacrylate, methacrylate and the corresponding amide (methacrylate) - or -methacrylamides) such as dimethylaminopropyl (meth) acrylamide. The above definitions (formula I) also apply to e ') in the polymer P 2 .
Als Lösungsmittel (L) finden in den erfindungsgemäßen Additive die einschlägig für Schmierölzusätze bekannten verwendung paraffin- oder naphthenbasische Mineralöle oder die bekannten Esteröle oder Poly-a-Olefine. (Vgl. Ullmanns Encyklopädie der techn. Chemie, Band 20, loc.cit., S. 483-529).The solvents (L) used in the additives according to the invention are the paraffin- or naphthene-based mineral oils known in the art for lubricating oil additives or the known ester oils or poly-a-olefins. (See Ullmanns Encyklopadie der techn. Chemie, Volume 20, loc.cit., Pp. 483-529).
Die Herstellung der Polymerisate schließt an die Polymerisationsverfahren des Standes der Technik an.The preparation of the polymers follows the polymerization processes of the prior art.
In einem Reaktionsgefäß, das zweckmäßig mit Rührer, Thermometer, Rückflußkühler und Dosierleitung ausgestattet ist, wird eine Mischung aus Mineralöl und einer Monomerenmischung aus a), b), c), d) und e) vorgelegt.A mixture of mineral oil and a monomer mixture of a), b), c), d) and e) is placed in a reaction vessel which is suitably equipped with a stirrer, thermometer, reflux condenser and metering line.
Unter CO2-Atmosphäre und Rühren wird auf ca. 90-100°C erhitzt. Nach Erreichen dieser Temperatur und Zugabe von Initiator (vorzugsweise Perverbindungen wie Perester, Peroxiden oderAzoverbindungen) wird eine Mischung aus den Monomeren a), b), c), d) und e) sowie weiterer Initiator zudosiert; ca. 2 Stunden nach Ende des Zulaufs wird weiterer Initiator nachgefüttert. Die Gesamtinitiatormenge liegt in der Regel bei 1-3 Gew.-%, bezogen auf die Gesamtmenge der Monomeren. Die Gesamtpolymerisationszeit beträgt im allgemeinen 8-9 Stunden. Man erhält eine viskose Lösung mit einem Polymerisatgehalt von im allgemeinen 40-70 Gew.-%. (Die Herstellung der zweiten Polymerkomponente vom Typ der Kombination von Polyalkyl-(meth)acrylaten (PAMA) und OCP wird nach der DE-PS 29 05 954 bzw. der US-PS 4 290 925 vorgenommen.)The mixture is heated to about 90-100 ° C. under a CO 2 atmosphere and with stirring. After this temperature has been reached and initiator (preferably per compounds such as peresters, peroxides or azo compounds) has been added, a mixture of the monomers a), b), c), d) and e) and further initiator are metered in; approx. 2 hours after the end of the feed, further initiator is fed. The total amount of initiator is generally 1-3% by weight, based on the total amount of monomers. The total polymerization time is generally 8-9 hours. A viscous solution with a polymer content of generally 40-70% by weight is obtained. (The production of the second polymer component of the type of combination of polyalkyl (meth) acrylates (PAMA) and OCP is carried out according to DE-PS 29 05 954 and US-PS 4,290,925.)
Dabei kann wie folgt vorgegangen werden:You can do this as follows:
Eine Komponente wird in einem geeigneten Behälter vorgelegt und unter Rühren auf ca. 80-120°C erhitzt. Die Zumischkomponenten werden ebenfalls auf ca. 80-120°C erhitzt und zur vorgelegten Komponente unter Rühren möglichst rasch zudosiert.One component is placed in a suitable container and heated to approx. 80-120 ° C with stirring. The admixing components are also heated to approx. 80-120 ° C and metered in as quickly as possible to the component while stirring.
Das erfindungsgemäße Additive wird, eventuell zusammen mit weiteren Zusatzstoffen, wie DI-Paket und OCP-VI-Verbesserer, bei 50-60°C unter Rühren im Grundöl gelöst.The additive according to the invention, possibly together with other additives, such as DI package and OCP-VI improver, is dissolved in the base oil at 50-60 ° C. with stirring.
Die erfindungsgemäßen Additive können den Schmierölen in an sich bekannter Weise zugesetzt werden.The additives according to the invention can be added to the lubricating oils in a manner known per se.
Für Motoren-Schmieröle und ATF-Öle empfiehlt sich ein Zusatz von 1-10 Gew.-%, vorzugsweise 2-6 Gew.- %, bei Hydraulik- und Getriebeölen ist ein Zusatz von 5-30 Gew.-%, vorzugsweise 10-20 Gew.-%, zu empfehlen.For engine lubricating oils and ATF oils, an addition of 1-10% by weight, preferably 2-6% by weight is recommended; for hydraulic and gear oils, an addition of 5-30% by weight, preferably 10- 20% by weight, recommended.
Vorteilhafte Wirkungen der Erfindung liegen in der flexiblen Anpassung an jedes spezielle Grundöl, besonders an kritische Grundöle und bei Mitverwendung von OCP. Ölformulierungen, die die erfindungsgemäßen Additive enthalten, zeigen neben den erforderlichen Viskositätsdaten bei 100°C sehr günstige Werte für Pour-point und Stable Pour-point sowie ausgezeichnete Viskositätsdaten bei -15°C bis 40°C.Advantageous effects of the invention lie in the flexible adaptation to any special base oil, especially critical base oils and when using OCP. Oil formulations containing the additives according to the invention, in addition to the required viscosity data at 100 ° C., show very favorable values for pour point and stable pour point as well as excellent viscosity data at -15 ° C. to 40 ° C.
Die Charakterisierung kann durch folgende Meßgrößen erfolgen:
In einem 1 1-Vierhalskolben mit Rührer, Thermometer, Rückflußkühlerund Dosierleitung wird folgende Mischung vorgelegt:
- 252 g Mineralöl (η100°C=5.3 mm2/s)
- 26,6 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 1,4 g Methylmethacrylat
- 1,6 g tert.-Butylperoctoat
- 252 g mineral oil (η 100 ° C = 5.3 mm 2 / s)
- 26.6 g methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 1.4 g methyl methacrylate
- 1.6 g of tert-butyl peroctoate
Nach Lösen der Komponenten wird bei 90°C nachstehendes Gemisch üner einen Zeitraum von 210 Min. gleichmäßig zudosiert.
- 304 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 16 g Methylmethacrylat
- 2,56 g tert.-Butylperoctoat
- 304 g of methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 16 g methyl methacrylate
- 2.56 g of tert-butyl peroctoate
Zwei Stunden nach Zulaufende wird mit 0,7 g tert.-Butylperoctoat nachgefüttert. Gesamtpolymerisationszeit 8 Stunden. Es wird eine klare, viskose Lösung erhalten.
- Polymerisatgehalt=58 Gew.-%
- Viskosität (100°C, 58 Gew.-%ig)=500 mm2/s
- Viskosität (100°C, 5,8 Gew.-%ig in Mineralöl mit η100°C=5,3 mm2/s)=11,0 mm2/s SS!1> (5,8 Gew.-% ig in Mineralöl mit η100°C=5,3 mm2/s)=7,5
- Polymer content = 58% by weight
- Viscosity (100 ° C, 58% by weight) = 500 mm 2 / s
- Viscosity (100 ° C, 5.8% by weight in mineral oil with η 100 ° C = 5.3 mm 2 / s) = 11.0 mm 2 / s SS! 1 > (5.8% by weight in mineral oil with η 100 ° C = 5.3 mm 2 / s) = 7.5
Vorlage:
- 252 g Mineralöl (η100°C=5,3 mm2/s)
- 6,2 g Methacrylsäureester eines n-C16-C18-Alkoholgemisches
- 20,4 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 1,4 g Methacrylsäuremethylester
- 1,6 g tert.-Butylperoctoat
- 252 g mineral oil ( η100 ° C = 5.3 mm 2 / s)
- 6.2 g of methacrylic acid ester of an nC 16 -C 18 alcohol mixture
- 20.4 g of methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 1.4 g of methyl methacrylate
- 1.6 g of tert-butyl peroctoate
Zulauf:
- 71 g Methacrylsäureester eines n-C16-C18-Alkoholgemisches
- 233 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 16 g Methylmethacrylat
- 2,56 g tert.-Butylperoctoat
- 71 g of methacrylic acid ester of an nC 16 -C 18 alcohol mixture
- 233 g of methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 16 g methyl methacrylate
- 2.56 g of tert-butyl peroctoate
Vorlage:
- 252 g Mineralöl (η100°C=5,3 mm2/s)
- 6,75 g Methacrylsäureester eines n-C,8-C22-Alkoholgemisches
- 19,85 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 1,4 g Methylmethacrylat
- 1,6 g tert.-Butylperoctoat
- 252 g mineral oil ( η100 ° C = 5.3 mm 2 / s)
- 6.75 g of methacrylic acid ester of an nC, 8 -C 22 alcohol mixture
- 19.85 g of methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 1.4 g methyl methacrylate
- 1.6 g of tert-butyl peroctoate
Zulauf:
- 77,2 g Methacrylsäureester eines n-C12-C22-Alkoholgemisches
- 226,8 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 16 g Methacrylsäuremethylester
- 2,56 g tert.-Butylperoctoat
- 77.2 g methacrylic acid ester of an nC 12 -C 22 alcohol mixture
- 226.8 g of methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 16 g of methyl methacrylate
- 2.56 g of tert-butyl peroctoate
Vorlage:
- 252 g Mineralöl (η100°C=5,3 mm2/s)
- 26,6 g Methacrylsäureester eines iso-C10-Alkohols
- 1,4 g Methylmethacrylat
- 1,6 g tert.-Butylperoctoat
- 252 g mineral oil (η 100 ° C = 5.3 mm 2 / s)
- 26.6 g of methacrylic acid ester of an iso-C 10 alcohol
- 1.4 g methyl methacrylate
- 1.6 g of tert-butyl peroctoate
Zulauf:
- 304 g Methacrylsäureester eines iso-C10-Alkohols
- 16 g Methylmethacrylat
- 2,56 g tert.-Butylperoctoat
- 304 g of methacrylic acid ester of an iso-C 10 alcohol
- 16 g methyl methacrylate
- 2.56 g of tert-butyl peroctoate
Es wird eine klare, viskose Lösung erhalten.
- Polymerisatgehalt=58 Gew.-%
- Polymer content = 58% by weight
1) SSI=Scherstabilitätsindex=Verlust an Verdickungswirkung in % bei Scherstabilitätsprüfung nach DIN 51 382
- Viskosität (100°C, 58 Gew.-%ig)=1000 mm2/s
- Viskosität (100°C, 5,8 Gew.-%ig in Mineralöl mit η100°C=5,3 mm2/s=11,0 mm2/s
- SSI1) (58 Gew.-%ig in Mineralöl mit η100°C=5,3 mm2/S)=7,5
- Viscosity (100 ° C, 58% by weight) = 1000 mm 2 / s
- Viscosity (100 ° C, 5.8% by weight in mineral oil with η 100 ° C = 5.3 mm 2 / s = 11.0 mm 2 / s
- SSI 1) (58% by weight in mineral oil with η 100 ° C = 5.3 mm 2 / S) = 7.5
Vorlage:
- 252 g Mineralöl (η100°C=5,3 mm2/s)
- 11,76 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 14,84 g Methacrylsäureester eines iso-C18-Alkohols
- 1,4 g Methylmethacrylat
- 1,6 g tert.-Butylperoctoat
- 252 g mineral oil ( η100 ° C = 5.3 mm 2 / s)
- 11.76 g of methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 14.84 g of methacrylic acid ester of an iso-C 18 alcohol
- 1.4 g methyl methacrylate
- 1.6 g of tert-butyl peroctoate
Zulauf:
- 134,4 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 169,6 g Methacrylsäureester eines iso-C18-Alkohols
- 16,0 g Methylmethacrylat
- 2,56 tert.-Butylperoctoat
- 134.4 g methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 169.6 g of methacrylic acid ester of an iso-C 18 alcohol
- 16.0 g methyl methacrylate
- 2.56 tert-butyl peroctoate
Vorlage:
- 252 g Mineralöl (η100°C=5,3 mm2/s)
- 26,6 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 1,4 g Methmethacrylat
- 1,6 g tert.-Butylperoctoat
- 252 g mineral oil ( η100 ° C = 5.3 mm 2 / s)
- 26.6 g methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 1.4 g meth methacrylate
- 1.6 g of tert-butyl peroctoate
Zulauf 1:
- 152,9 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 8,1 g Methylmethacrylat
- 1,29 g tert.-Butylperoctoat
- 152.9 g of methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 8.1 g methyl methacrylate
- 1.29 g of tert-butyl peroctoate
Zulauf 1 wird innerhalb 210 Min. gleichmäßig zudosiert. 120 Min. nach Zulaufende wird mit Zulauf 2 begonnen:Feed 1 is metered in uniformly within 210 minutes. 120 minutes after the end of the inlet, inlet 2 is started:
Zulauf 2:
- 151,1 g Methacrylsäureester eines iso-C10-Alkohols
- 7,9 g Methylmethacrylat
- 1,27 g tert.-Butylperoctoat
- 151.1 g of methacrylic acid ester of an iso-C 10 alcohol
- 7.9 g methyl methacrylate
- 1.27 g of tert-butyl peroctoate
Zwei Stunden nach Ende vom Zulauf 2 wird mit 0,7 g tert.-Butylperoctoat nachgefüttert. Gesamtpolymerisationszeit 12 Stunden.Two hours after the end of feed 2, 0.7 g of tert-butyl peroctoate is added. Total polymerization time is 12 hours.
Es wird eine leicht trübe, viskose Lösung erhalten.
- Polymerisatgehalt=58 Gew.-%ig
- Viskosität (100°C 58 Gew.-%ig)=800 mm2/s
- Viskosität (100°C, 5,8 Gew.-% in Mineralöl mit η100°C=5,3 mm2/s)=11,0 mm2/s
- SSI1) (5,8 % ig in Mineralöl mit η100°C =5,3 mm2/s)=7,5
- Polymer content = 58% by weight
- Viscosity (100 ° C 58% by weight) = 800 mm 2 / s
- Viscosity (100 ° C, 5.8% by weight in mineral oil with η 100 ° C = 5.3 mm 2 / s) = 11.0 mm 2 / s
- SSI 1) (5.8% in mineral oil with η 100 ° C = 5.3 mm 2 / s) = 7.5
In einem 1 I-Vierhalskolben mit Rührer, Thermometer, Rückflußkühler und Dosierleitung werden vorgelegt.
- 17,4 g eines Copolymerisats, bestehend aus 70 Gew.-% Äthylen und 30 Gew.-% Propylen mit Mw=80 000
- 252 g Mineralöl (η100°C=5,3 mm2/s)
- 17.4 g of a copolymer consisting of 70% by weight of ethylene and 30% by weight of propylene with Mw = 80,000
- 252 g mineral oil (η 100 ° C = 5.3 mm 2 / s)
Nach Lösen des Copolymerisats innerhalb 10 Stunden bei 90°C wird nachstehendes Gemisch zugesetzt:
- 1) SSI=Scherstabilitätsindex=Verlust an Verdickungswirkung in % bei Scherstabilitätsprüfung nach DIN 51 382
- 1) SSI=Scherstabilitätsindex=Verlust an Verdickungswirkung in % bei Scherstabilitätsprüfung nach DIN 51 382
- 28,4 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 1,5 g Methylmethacrylat
- 1,0 g tert.-Butylperoctoat
- 1) SSI = shear stability index = loss of thickening effect in% with shear stability test according to DIN 51 382
- 1) SSI = shear stability index = loss of thickening effect in% with shear stability test according to DIN 51 382
- 28.4 g methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 1.5 g methyl methacrylate
- 1.0 g of tert-butyl peroctoate
Nach Lösen der vorgelegten Komponenten wird bei 90°C nachstehendes Gemisch über einen Zeitraum von 210 Min. gleichmäßig zudosiert.
- 285,7 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 15,0 g Methylmethacrylat
- 1,5 g tert.-Butylperoctoat
- 285.7 g of methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 15.0 g methyl methacrylate
- 1.5 g of tert-butyl peroctoate
2 Stunden nach Zulaufende wird mit 0,66 g tert.-Butylperoctoat nachgefüttert. Nach einer Gesamtpolymerisationszeit von 8 Stunden werden dem Polymerisat
- 7,8 Mineralöl (η100°C=5.3 mm2/s)
- 10,76 g N-Vinylpyrrolidon-2
- 0,9 g tert.-Butylperbenzoat
- Nach 1 bzw. 2 Stunden wird mit je
- 0,4 g tert.-Butylperbenzoat nachgefüttert.
- Der Versuch wird noch weitere 5 Stunden bei 130°C gehalten. Es wird eine trübe viskose Lösung erhalten.
- Polymerisatgehalt: 58 Gew.-%
- Viskosität (100°C, 58 Gew.-%ig)=2000 mm2/s
- Viskosität (100°C, 5,8 Gew.-%ig in Mineralöl mit η100°C=5,3 mm2/s)=17,0 mm2/s
- SSI1) 5,8 Gew.-% ig in Mineralöl mit η100°C=5,3 mm2/s)=32
- 7.8 mineral oil (η100 ° C = 5.3 mm 2 / s)
- 10.76 g of N-vinyl pyrrolidone-2
- 0.9 g of tert-butyl perbenzoate
- After 1 or 2 hours with
- 0.4 g of tert-butyl perbenzoate was fed.
- The experiment is held at 130 ° C. for a further 5 hours. A cloudy, viscous solution is obtained.
- Polymer content: 58% by weight
- Viscosity (100 ° C, 58% by weight) = 2000 mm 2 / s
- Viscosity (100 ° C, 5.8% by weight in mineral oil with η 100 ° C = 5.3 mm 2 / s) = 17.0 mm 2 / s
- SSI 1) 5.8% by weight in mineral oil with η100 ° C = 5.3 mm 2 / s) = 32
In einem 1 1-Vierhalskoben mit Rührer, Thermometer, Rückflußkühler und Dosierleitung werden vorgelegt:
- 258 g Mineralöl (η100°C=5,3 mm2/s)
- 25,8 g Methacrylsäureesters eines C12-C18-Alkoholgemisches mit 20% iso-Anteilen
- 2,9 g Methylmethacrylat
- 1,2 g tert.-Butylperoctoat
- 258 g mineral oil (η 100 ° C = 5.3 mm 2 / s)
- 25.8 g of methacrylic acid ester of a C 12 -C 18 alcohol mixture with 20% iso shares
- 2.9 g methyl methacrylate
- 1.2 g of tert-butyl peroctoate
Nach Lösen der Komponenten wird bei 90°C nachstehendes Gemisch über einen Zeitraum von 210 Min. gleichmäßig zudosiert:
- 282 g Methacrylsäureester eines C12-C18-Alkoholgemisches mit 20% iso-Anteilen
- 31,3 g Methylmethacrylat
- 1,9 g tert.-Butylperoctoat
- 282 g of methacrylic acid ester of a C 12 -C 18 alcohol mixture with 20% iso shares
- 31.3 g methyl methacrylate
- 1.9 g of tert-butyl peroctoate
Zwei Stunden nach Zulaufende wird mit 0,7 g tert.-Butylperoctoat nachgefüttert une weitere 5 Stunden bei 90°C gerührt. Danach erfolgt die Zugabe von:
- 7,97 g Mineralöl (η100°C=5,3 mm2/s)
- 10,57 g N-Vinylpyrrolidon-2
- 7.97 g mineral oil ( η100 ° C = 5.3 mm 2 / s)
- 10.57 g of N-vinyl pyrrolidone-2
Jetzt erfolgt der Zusatz von:
- 0,9 g tert.-Butylperbenzoat
- 0.9 g of tert-butyl perbenzoate
Nach weiteren 1 bzw. 2 Stunden wird mit jeweils 0,4 g tert.-Butylperbenzoat nachgefüttert und danach noch 5 Stunden bei 130°C gerührt.After a further 1 or 2 hours, 0.4 g of tert-butyl perbenzoate are added and the mixture is then stirred at 130 ° C. for 5 hours.
Es wird eine klare, viskose Lösung erhalten.
- Polymerisatgehalt=57 Gew.-%
- Viskosität (100°C, 57 Gew.-%ig)=1300 mm2/s
- Viskosität (100°C, 5,7 Gew.-%ig in Mineralöl mit η100°C=5,3 mm2/s)=14,4 mm2/s
- SSI1) (5,7 Gew.-%ig in Mineralöl mit η100°C=5,3 mm2/s)=24
- Polymer content = 57% by weight
- Viscosity (100 ° C, 57% by weight) = 1300 mm 2 / s
- Viscosity (100 ° C, 5.7% by weight in mineral oil with η 100 ° C = 5.3 mm 2 / s) = 14.4 mm 2 / s
- SSI 1) (5.7% by weight in mineral oil with η100 ° C = 5.3 mm 2 / s) = 24
Vorlage:
- 252 g Mineralöl (η100°C=5,3 mm2/s)
- 1) SSI=Scherstabilitätsindex=Verlust an Verdickungswirkung in % bei Scherstabilitätsprüfung nach DIN 51 382
- 1) SSI=Scherstabilitätsindex=Verlust an Verdickungswirkung in % bei Scherstabilitätsprüfung nach DIN 51 382
- 28 g Methacrylsäureester eines C12-C18-Alkoholgemisches mit 13% iso-Anteilen
- 1,6 g tert.-Butylperoctoat
- 252 g mineral oil (η 100 ° C = 5.3 mm 2 / s)
- 1) SSI = shear stability index = loss of thickening effect in% with shear stability test according to DIN 51 382
- 1) SSI = shear stability index = loss of thickening effect in% with shear stability test according to DIN 51 382
- 28 g methacrylic acid ester of a C 12 -C 18 alcohol mixture with 13% iso shares
- 1.6 g of tert-butyl peroctoate
Zulauf:
- 320 g Methacrylsäureester eines C12-C18-Alkoholgemisches mit 13% iso-Anteilen
- 2,56 g tert.-Butylperoctoat
- 320 g of methacrylic acid ester of a C 12 -C 18 alcohol mixture with 13% iso shares
- 2.56 g of tert-butyl peroctoate
Vorlage:
- 252 g Mineralöl (η100°C=5,3 mm2/s)
- 11,7 g Methacrylsäureester eines n-C16-C18-Alkoholgemisches
- 14,9 g Methacrylsäureester eines iso-C10-Alkohols
- 1,4 g Methylmethacrylat
- 1,6 g tert.-Butylperoctoat
- 252 g mineral oil (η 100 ° C = 5.3 mm 2 / s)
- 11.7 g of methacrylic acid ester of an nC 16 -C 18 alcohol mixture
- 14.9 g of methacrylic acid ester of an iso-C 10 alcohol
- 1.4 g methyl methacrylate
- 1.6 g of tert-butyl peroctoate
Zulauf:
- 133,4 g Methacrylsäureester eines n-C16-C18-Alkoholgemisches
- 170,6 g Methacrylsäureester eines iso-C10-Alkohols
- 16,0 g Methmethacrylat
- 2,56 g tert.-Butylperoctoat
- 133.4 g of methacrylic acid ester of an nC 16 -C 18 alcohol mixture
- 170.6 g of methacrylic acid ester of an iso-C 10 alcohol
- 16.0 g meth methacrylate
- 2.56 g of tert-butyl peroctoate
In einem 1 I-Vierhalskolben mit Rührer, Thermometer, Rückflußkühler und Dosierleitung werden vorgelegt:
- 17,4 g eines Copolymerisats, bestehend aus 70 Gew.-% Äthylen und 30 Gew.-% propylen mit Mw=80 000
- 252 g Mineralöl (η100°C=5,3 mm2/s)
- 17.4 g of a copolymer consisting of 70% by weight of ethylene and 30% by weight of propylene with Mw = 80,000
- 252 g mineral oil (η 100 ° C = 5.3 mm 2 / s)
Nach Lösen des Copolymerisats innerhalb 10 Stunden bei 90°C wird nachstehendes Gemisch zugesetzt:
- 11,4 g Methacrylsäureester eines n-C16-C18-Alkoholgemisches
- 6,7 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 10,7 g Methacrylsäureester eines iso-C13-Alkohols
- 1,5 Methylmethacrylat
- 1,7 g tert.-Butylperoctoat
- 11.4 g of methacrylic acid ester of an nC 16 -C 18 alcohol mixture
- 6.7 g of methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 10.7 g of methacrylic acid ester of an iso-C 13 alcohol
- 1.5 methyl methacrylate
- 1.7 g of tert-butyl peroctoate
Nach Lösen der vorgelegten Komponenten wird bei 90°C nachstehendes Gemisch über einen Zeitraum von 210 Min. gleichmäßig zudosiert:
- 113,4 g Methacrylsäureester eines n-C16-C18-Alkoholgemisches
- 66,2 g Methacrylsäureester eines n-C12-C14-Alkoholgemisches
- 106,1 g Methacrylsäureester eines iso-C13-Alkohols
- 15,0 g Methylmethacrylat
- 2,7 g tert.-Butyloctoat
- 113.4 g of methacrylic acid ester of an nC 16 -C 18 alcohol mixture
- 66.2 g methacrylic acid ester of an nC 12 -C 14 alcohol mixture
- 106.1 g of methacrylic acid ester of an iso-C 13 alcohol
- 15.0 g methyl methacrylate
- 2.7 g of tert-butyl octoate
2 Stunden nach Zulaufende wird mit 0,66 g tert.-Butylperoctoat nachgefüttert.2 hours after the end of the feed, 0.66 g of tert-butyl peroctoate is added.
Nach einer Gesamtpolymerisationszeit von 8 Stunden werden dem Polymerisat
- 7,8 g Mineralöl (η100°C=5,3 mm2/s)
- 10,76 g N-Vinylpyrrolidon-2
- 0,9 g tert.-Butylperbenzoat
- 7.8 g mineral oil (η 100 ° C = 5.3 mm 2 / s)
- 10.76 g of N-vinyl pyrrolidone-2
- 0.9 g of tert-butyl perbenzoate
Nach 1 bzw. 2 Stunden wird mit je
- 0,4 g tert.-Butylperbenzoat nachgefüttert.
- 0.4 g of tert-butyl perbenzoate was fed.
Der Versuch wird noch weitere 5 Stunden bei 130°C gehalten. Es wird eine trübe, viskose Lösung erhalten.
- Polymerisatgehalt: 58 Gew.-%
- Viskosität (100°C, 58 Gew.-%ig)=1000 mm2/s
- Viskosität (100°C, 5,8 Gew.-%ig in Mineralöl mit η100°C=5,3 mm2/s)=14,3 mm2/s
- SSI1) (5,8 Gew.-% ig in Mineralöl mit η100°C=5,3 mm2/s)=22
- Polymer content: 58% by weight
- Viscosity (100 ° C, 58% by weight) = 1000 mm 2 / s
- Viscosity (100 ° C, 5.8% by weight in mineral oil with η 100 ° C = 5.3 mm 2 / s) = 14.3 mm 2 / s
- SSI 1) (5.8% by weight in mineral oil with η 100 ° C = 5.3 mm 2 / s) = 22
1) SSI=Scherstabilitätsindex=Verlust an Verdickungswirkung in % bei Scherstabilitätsprüfung nach DIN 51 382 1 ) SSI = shear stability index = loss of thickening effect in% with shear stability test according to DIN 51 382
Die Beispiele zeigen, daß mit den erfindungsgemäßen Mischungen (Beispiele 1-2) günstigere Pour-point-Werte in beiden Grundlölen erhalten wurden als nach dem Stand der Technik (s. Vergleichsbeispiele 1-16).The examples show that with the mixtures according to the invention (Examples 1-2), more favorable pour point values were obtained in both base oils than in the prior art (see Comparative Examples 1-16).
Claims (1)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19833339103 DE3339103A1 (en) | 1983-10-28 | 1983-10-28 | ADDITIVES FOR LUBRICANTS |
| DE3339103 | 1983-10-28 |
Publications (4)
| Publication Number | Publication Date |
|---|---|
| EP0140274A2 EP0140274A2 (en) | 1985-05-08 |
| EP0140274A3 EP0140274A3 (en) | 1987-05-13 |
| EP0140274B1 EP0140274B1 (en) | 1990-12-05 |
| EP0140274B2 true EP0140274B2 (en) | 1994-06-22 |
Family
ID=6212932
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP84112445A Expired - Lifetime EP0140274B2 (en) | 1983-10-28 | 1984-10-16 | Lubricating oil additives |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US4968444A (en) |
| EP (1) | EP0140274B2 (en) |
| JP (1) | JPH0631382B2 (en) |
| DE (2) | DE3339103A1 (en) |
Families Citing this family (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3544061A1 (en) * | 1985-12-13 | 1987-06-19 | Roehm Gmbh | HIGHLY STABLE MULTI-RANGE LUBRICANTS WITH IMPROVED VISCOSITY INDEX |
| DE3607444A1 (en) * | 1986-03-07 | 1987-09-10 | Roehm Gmbh | ADDITIVES FOR MINERAL OILS WITH IMPROVEMENT EFFECT |
| DE3613992C2 (en) * | 1986-04-25 | 2000-05-04 | Roehm Gmbh | Additives for paraffinic lubricating oils |
| WO1989001507A1 (en) * | 1987-08-19 | 1989-02-23 | Pennzoil Products Company | Methacrylate pour point depressants and compositions |
| US4956111A (en) * | 1987-08-19 | 1990-09-11 | Pennzoil Products Company | Methacrylate pour point depressants and compositions |
| US4844829A (en) * | 1987-08-19 | 1989-07-04 | Pennzoil Products Company | Methacrylate pour point depressants and compositions |
| US5349019A (en) * | 1988-12-24 | 1994-09-20 | Hoechst | New copolymers, mixtures thereof with poly(meth)acrylate esters and the use thereof for improving the cold fluidity of crude oils |
| DE3905681A1 (en) * | 1989-02-24 | 1990-08-30 | Basf Ag | CONCENTRATED MIXTURES OF GAPPOPOLYMERISATS FROM ESTERS OF UNSATURATED ACIDS AND ETHYLENE-VINYLESTER COPOLYMERISATS |
| DE3930142A1 (en) * | 1989-09-09 | 1991-03-21 | Roehm Gmbh | DISPERGING VISCOSITY INDEX IMPROVERS |
| US5149452A (en) * | 1990-12-19 | 1992-09-22 | Exxon Research And Engineering Company | Wax isomerate having a reduced pour point |
| FR2679444B1 (en) * | 1991-07-25 | 1995-04-07 | Oreal | USE AS OIL THICKENING AGENTS, IN AN OILY COSMETIC COMPOSITION, OF A COMBINATION OF TWO COPOLYMERS. |
| US5229021A (en) * | 1991-12-09 | 1993-07-20 | Exxon Research & Engineering Company | Wax isomerate having a reduced pour point |
| US5534175A (en) * | 1992-12-28 | 1996-07-09 | The Lubrizol Corporation | Copolymers of unsaturated fatty esters, their use as viscosity improver and lubricating oil containing said copolymers |
| HUT69298A (en) | 1993-07-23 | 1995-09-28 | Rohm & Haas | Method of making a copolymer useful as viscosity index improving additive for hydraulic fluids |
| US5416162A (en) * | 1993-09-20 | 1995-05-16 | Rohm And Haas Company | Compatibilizer for a viscosity index improving polymer blend |
| JP2748104B2 (en) * | 1994-03-08 | 1998-05-06 | 三洋化成工業株式会社 | Viscosity index improver and lubricating oil |
| US6228819B1 (en) | 1994-04-14 | 2001-05-08 | Rohm And Haas Company | Process for making a viscosity index improving copolymer |
| IT1270673B (en) * | 1994-10-19 | 1997-05-07 | Euron Spa | MULTIFUNCTIONAL ADDITIVE FOR LUBRICANTS COMPATIBLE WITH FLUOROELASTOMERS |
| US5520832A (en) * | 1994-10-28 | 1996-05-28 | Exxon Research And Engineering Company | Tractor hydraulic fluid with wide temperature range (Law180) |
| US5821313A (en) | 1995-06-19 | 1998-10-13 | The Lubrizol Corporation | Dispersant-viscosity improvers for lubricating oil compositions |
| US5969068A (en) * | 1995-06-19 | 1999-10-19 | The Lubrizol Corporation | Dispersant-viscosity improvers for lubricating oil compositions |
| US6140431A (en) * | 1997-02-27 | 2000-10-31 | Rohm And Haas Company | Process for preparing continuously variable-composition copolymers |
| US5807815A (en) * | 1997-07-03 | 1998-09-15 | Exxon Research And Engineering Company | Automatic transmission fluid having low Brookfield viscosity and high shear stability |
| KR100517190B1 (en) | 1997-08-22 | 2005-09-28 | 로막스 아디티페스 게엠베하 | Method for maintaining low-temperature fluidity of lubricating oil composition, a concentrate for use in lubricating oil composition and a lubricating oil composition |
| US6124249A (en) | 1998-12-22 | 2000-09-26 | The Lubrizol Corporation | Viscosity improvers for lubricating oil compositions |
| US6255261B1 (en) * | 1999-09-22 | 2001-07-03 | Ethyl Corporation | (Meth) acrylate copolymer pour point depressants |
| US6323164B1 (en) | 2000-11-01 | 2001-11-27 | Ethyl Corporation | Dispersant (meth) acrylate copolymers having excellent low temperature properties |
| DE10335360B4 (en) * | 2002-08-02 | 2010-09-09 | Sanyo Chemical Industries, Ltd. | Use of an oil-soluble copolymer as a viscosity index improver |
| US7378379B2 (en) * | 2003-06-10 | 2008-05-27 | The Lubrizol Corporation | Functionalized polymer composition for grease |
| US20060252660A1 (en) * | 2005-05-09 | 2006-11-09 | Akhilesh Duggal | Hydrolytically stable viscosity index improves |
| JP5488893B2 (en) * | 2007-06-08 | 2014-05-14 | 東邦化学工業株式会社 | Pour point depressant for lubricant |
| WO2011084997A1 (en) * | 2010-01-05 | 2011-07-14 | Novomer Inc. | Hydrocarbon additives |
| US9481849B2 (en) | 2010-04-26 | 2016-11-01 | Evonik Oil Additives Gmbh | Polymer useful as viscosity index improver |
| KR20140066968A (en) | 2010-10-29 | 2014-06-03 | 에보니크 오일 아디티페스 게엠베하 | A diesel motor having improved properties |
| WO2012076285A1 (en) | 2010-12-10 | 2012-06-14 | Evonik Rohmax Additives Gmbh | A lubricant composition |
| WO2013062924A2 (en) * | 2011-10-27 | 2013-05-02 | The Lubrizol Corporation | Lubricating composition containing an esterified polymer |
| US20130340325A1 (en) * | 2012-06-22 | 2013-12-26 | Baker Hughes Incorporated | Charged Block Co-polymers as Pour Point Depressants |
| BR112016016713A2 (en) * | 2014-01-21 | 2018-05-08 | Evonik Oil Additives Gmbh | pour point depressants to improve low temperature viscosity of aged lubricating oil. |
| CN105585657B (en) * | 2014-10-24 | 2018-03-20 | 中国石油化工股份有限公司 | A kind of pour depressant for lubricating oil and preparation method thereof |
| CN105524209B (en) * | 2014-10-24 | 2017-09-29 | 中国石油化工股份有限公司 | Acrylate based copolymer and its application and pour depressant for lubricating oil and preparation method thereof |
| JP6438069B2 (en) * | 2016-04-26 | 2018-12-12 | 三洋化成工業株式会社 | Lubricating oil composition |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2114233A (en) * | 1933-05-22 | 1938-04-12 | Rohm & Haas | Polymeric materials |
| US2091627A (en) * | 1934-06-08 | 1937-08-31 | Rohm & Haas | Composition of matter and process |
| US2100993A (en) * | 1934-12-14 | 1937-11-30 | Rohm & Haas | Process for preparing esters and products |
| US2655479A (en) * | 1949-01-03 | 1953-10-13 | Standard Oil Dev Co | Polyester pour depressants |
| US3251775A (en) * | 1962-05-24 | 1966-05-17 | Rohm & Haas | Lubricating oil compositions |
| US3386998A (en) * | 1964-05-19 | 1968-06-04 | Rohm & Haas | Nu-alkenoyloxy-2-morpholinones and their corresponding hydrolysis products |
| US3513096A (en) * | 1968-12-03 | 1970-05-19 | Exxon Research Engineering Co | Oil concentrate containing a compatible mixture of polyisobutylene and ethylene-alpha olefin copolymer |
| US3772196A (en) * | 1971-12-03 | 1973-11-13 | Shell Oil Co | Lubricating compositions |
| PH10685A (en) * | 1972-12-29 | 1977-08-10 | Texaco Development Corp | Oil compositions having improved viscosity index and pour paint properties |
| US4146492A (en) * | 1976-04-02 | 1979-03-27 | Texaco Inc. | Lubricant compositions which exhibit low degree of haze and methods of preparing same |
| US4071407A (en) * | 1976-11-16 | 1978-01-31 | The Board Of Trustees Of The University Of Alabama | Novel maltase enzyme produced by a new yeast strain |
| DE2657570C3 (en) * | 1976-12-18 | 1980-11-20 | Bayer Ag, 5090 Leverkusen | Electrochemical cell for the detection of hydrogen sulfide in a gas mixture |
| GB1559952A (en) * | 1977-10-26 | 1980-01-30 | Shell Int Research | Lubricating oil compositions |
| DE2835192C2 (en) * | 1978-08-11 | 1986-12-11 | Röhm GmbH, 6100 Darmstadt | Lubricating oil additives |
| DE2905954C2 (en) * | 1979-02-16 | 1982-10-28 | Röhm GmbH, 6100 Darmstadt | Concentrated polymer emulsions as viscosity index improvers for mineral oils |
| DE3544061A1 (en) * | 1985-12-13 | 1987-06-19 | Roehm Gmbh | HIGHLY STABLE MULTI-RANGE LUBRICANTS WITH IMPROVED VISCOSITY INDEX |
-
1983
- 1983-10-28 DE DE19833339103 patent/DE3339103A1/en not_active Ceased
-
1984
- 1984-10-16 DE DE8484112445T patent/DE3483714D1/en not_active Expired - Lifetime
- 1984-10-16 EP EP84112445A patent/EP0140274B2/en not_active Expired - Lifetime
- 1984-10-24 JP JP59222331A patent/JPH0631382B2/en not_active Expired - Fee Related
-
1988
- 1988-12-27 US US07/291,387 patent/US4968444A/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| DE3339103A1 (en) | 1985-05-09 |
| US4968444A (en) | 1990-11-06 |
| EP0140274A3 (en) | 1987-05-13 |
| JPH0631382B2 (en) | 1994-04-27 |
| EP0140274A2 (en) | 1985-05-08 |
| JPS60110790A (en) | 1985-06-17 |
| DE3483714D1 (en) | 1991-01-17 |
| EP0140274B1 (en) | 1990-12-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0140274B2 (en) | Lubricating oil additives | |
| EP0418610B1 (en) | Viscosity index improver, with a dispersant activity | |
| DE3607444A1 (en) | ADDITIVES FOR MINERAL OILS WITH IMPROVEMENT EFFECT | |
| EP0225598B1 (en) | High shear-stable multifunctional lubricating oil with an improved viscosity index | |
| DE3613247C2 (en) | Concentrated emulsions of ethylene-vinyl acetate copolymers, processes for their preparation and their use as pour point improvers | |
| DE2905954C2 (en) | Concentrated polymer emulsions as viscosity index improvers for mineral oils | |
| DE3613992C2 (en) | Additives for paraffinic lubricating oils | |
| DE3207291C2 (en) | ||
| DE2658952A1 (en) | ALKYL ACRYLATE OR IN PARTICULAR ALKYL METHACRYLATE POLYMERIZATE MIXTURE AND A LUBRICATING OIL FORMULATION CONTAINING THE SAME | |
| EP0436872B1 (en) | Mineral oil based transmission fluid | |
| DE1520634B2 (en) | Process for the preparation of mixed polymers from acrylic acid esters and poly mensates of N vinyl 2 pyrrohdons | |
| EP0008327A1 (en) | Lubricating oil additives and their preparation | |
| EP0744457A2 (en) | Lubricant additive | |
| DE3905681A1 (en) | CONCENTRATED MIXTURES OF GAPPOPOLYMERISATS FROM ESTERS OF UNSATURATED ACIDS AND ETHYLENE-VINYLESTER COPOLYMERISATS | |
| EP0406684B1 (en) | Diesel fuel additive | |
| DE3725059A1 (en) | POLYMER FLOW IMPROVERS FOR MEDIUM DISTILLATES | |
| EP0890589B1 (en) | Solutions or dispersions based on copolymers of olefins and unsaturated carboxylic esters and their use as additives for mineral oils | |
| EP0090168A1 (en) | Concentrated dispersions of olefinic copolymers | |
| EP0721475B1 (en) | Ethylene-based copolymers and their use as viscosity improvers in petroleum distillates | |
| DE3917815A1 (en) | POLYMER MODIFIED BITUMEN | |
| EP0421256A1 (en) | Process for dewaxing waxy mineral-oil products | |
| DE4333680A1 (en) | Copolymers based on ethylene, and the use thereof as flow improvers in petroleum middle distillates | |
| EP0925274B1 (en) | Flow improver for crude-oil middle distillates | |
| DE4341528A1 (en) | Ethylene] copolymers for reducing flow temp. of petroleum middle distillate | |
| DE1520634C (en) | Process for the production of mixed polymers from acrylic acid esters and poly mensates of N vinyl 2 pyrrolidone |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): BE DE FR GB IT NL |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): BE DE FR GB IT NL |
|
| 17P | Request for examination filed |
Effective date: 19870807 |
|
| 17Q | First examination report despatched |
Effective date: 19880322 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE DE FR GB IT NL |
|
| ITF | It: translation for a ep patent filed |
Owner name: JACOBACCI & PERANI S.P.A. |
|
| ET | Fr: translation filed | ||
| REF | Corresponds to: |
Ref document number: 3483714 Country of ref document: DE Date of ref document: 19910117 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) | ||
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: BASF AKTIENGESELLSCHAFT, LUDWIGSHAFEN Effective date: 19910829 |
|
| NLR1 | Nl: opposition has been filed with the epo |
Opponent name: BASF AG. |
|
| ITTA | It: last paid annual fee | ||
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| 27A | Patent maintained in amended form |
Effective date: 19940622 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): BE DE FR GB IT NL |
|
| GBTA | Gb: translation of amended ep patent filed (gb section 77(6)(b)/1977) |
Effective date: 19940608 |
|
| NLR2 | Nl: decision of opposition | ||
| ET3 | Fr: translation filed ** decision concerning opposition | ||
| ITF | It: translation for a ep patent filed |
Owner name: JACOBACCI CASETTA & PERANI S.P.A. |
|
| NLR3 | Nl: receipt of modified translations in the netherlands language after an opposition procedure | ||
| NLT1 | Nl: modifications of names registered in virtue of documents presented to the patent office pursuant to art. 16 a, paragraph 1 |
Owner name: ROEHM GMBH & CO. KG |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: CJ |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20030929 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20030930 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20031009 Year of fee payment: 20 Ref country code: BE Payment date: 20031009 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20031010 Year of fee payment: 20 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20041015 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20041016 |
|
| BE20 | Be: patent expired |
Owner name: *ROHM G.M.B.H. Effective date: 20041016 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 |
|
| NLV7 | Nl: ceased due to reaching the maximum lifetime of a patent |
Effective date: 20041016 |