EP0140000A2 - Entmetallisierung und Dekarbonisierung umfassende kombinierte Verfahren zur Verbesserung von Rohöl - Google Patents
Entmetallisierung und Dekarbonisierung umfassende kombinierte Verfahren zur Verbesserung von Rohöl Download PDFInfo
- Publication number
- EP0140000A2 EP0140000A2 EP84110165A EP84110165A EP0140000A2 EP 0140000 A2 EP0140000 A2 EP 0140000A2 EP 84110165 A EP84110165 A EP 84110165A EP 84110165 A EP84110165 A EP 84110165A EP 0140000 A2 EP0140000 A2 EP 0140000A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- acid
- oil
- precipitate
- catalyst
- liquid phase
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G55/00—Treatment of hydrocarbon oils, in the absence of hydrogen, by at least one refining process and at least one cracking process
- C10G55/02—Treatment of hydrocarbon oils, in the absence of hydrogen, by at least one refining process and at least one cracking process plural serial stages only
- C10G55/06—Treatment of hydrocarbon oils, in the absence of hydrogen, by at least one refining process and at least one cracking process plural serial stages only including at least one catalytic cracking step
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G17/00—Refining of hydrocarbon oils in the absence of hydrogen, with acids, acid-forming compounds or acid-containing liquids, e.g. acid sludge
- C10G17/02—Refining of hydrocarbon oils in the absence of hydrogen, with acids, acid-forming compounds or acid-containing liquids, e.g. acid sludge with acids or acid-containing liquids, e.g. acid sludge
Definitions
- the invention is concerned with upgrading crude oil by zeolite catalytic cracking and particularly the residual portion of atmospheric distillation or that portion of crude oil boiling above about a middle distillate fraction after treatment by the combination of vacuum distillation, demetallizing and decarbonizing a vacuum resid portion with concentrated acid thereby concentrating metal contaminants in an asphalt product thereof separated from a liquid product more suitable for catalytic upgrading with vacuum gas oil.
- gasoline and other liquid hydrocarbon fuels boil in the range of about 38°C (100°F) to about 343°C (650°F).
- the crude oil from which these fuels are made contains a diverse mixture of hydrocarbons and other compounds which vary widely in molecular weight and therefore boil over a wide range.
- crude oils are known in which 30 to 60% or more of the total volume of oil is composed of compounds boiling at temperatures above 343°C (650°F).
- crudes in which about 10% to about 30% or more of the total volume consists of compounds so heavy in molecular weight that they boil above 552°C (1025°F) or at least will not boil below 552°C (1025°F) at atmospheric pressure.
- Crude oil in the natural state contains a variety of materials which tend to have quite troublesome effects on fluid catalytic cracking processes.
- these troublesome materials are coke precursors (such as asphaltenes, polynuclear aromatics, etc.), heavy metals (such as nickel, vanadium, iron, copper, etc.), lighter metals (such as sodium, potassium, etc.), sulfur, nitrogen and others.
- coke precursors such as asphaltenes, polynuclear aromatics, etc.
- heavy metals such as nickel, vanadium, iron, copper, etc.
- lighter metals such as sodium, potassium, etc.
- sulfur nitrogen and others.
- Certain of these, such as the lighter metals can be removed substantially by desalting operations, which are part of the normal procedure for pretreating crude oil for fluid catalytic cracking.
- the heavy metals accumulate on the catalyst to the point that they unfavorably alter the composition of the catalyst and/or its catalytic effect upon the feedstock.
- vanadium tends to form fluxes with certain components of commonly used FCC catalysts, lowering the melting point of portions of the catalyst particles sufficiently so that they begin to sinter and become ineffective cracking catalysts.
- Accumulations of vanadium and other heavy metals, especially nickel, are considered "poison" to the catalyst. They tend in varying degrees to promote excessive dehydrogenation and aromatic condensation, resulting in excessive production of carbon and gases with consequent impairment of liquid fuel yield.
- Such heavy oil fractions also comprise relatively large quantities of coke precursors, referred to in the prior art as metallo-organic compounds or as carbo-metallic containing oils.
- Such heavy residual oil feeds represent a particular challenge for upgrading to liquid fuel products by the petroleum refiner.
- the coke-forming tendency or coke precursor content of an oil fraction can be ascertained by determining the weight percent of carbon remaining after a sample of that oil has been pyrolyzed.
- the industry accepts this value as a measure of the extent to which a given oil tends to form non-catalytic coke when employed as feedstock in a catalytic cracker.
- Two established tests are recognized, the Conradson Carbon and Ramsbottom Carbon tests, the former being described in ASTM D189-76 and the latter being described in ASTM Test No. D524-76.
- Conradson carbon values on the order of about 0.05 to about 1.0 are regarded as indicative of acceptable feed.
- the present invention is particularly concerned with the use of petroleum hydrocarbon feedstocks and residual portions thereof which provide relatively high Conradson carbon values and thus exhibit substantially greater potential for coke formation than lower boiling gas oil feeds.
- the heavy metal content of feedstock for FCC processing is controlled at a relatively low level, e.g. about 0.25 ppm Nickel Equivalents or less.
- the present invention is concerned with the processing of feedstocks containing metals substantially in excess of this value and which therefore have a significantly greater potential for accumulating on and poisoning catalyst.
- the metal content of the catalyst is maintained at a level which may for example be in the range of about 200 to about 600 ppm Nickel Equivalents.
- the process of the present invention is concerned with the use of equilibrium catalyst having a substantial metals content up to 5000 or 6000 ppm of Ni + V or higher and which therefore has a tendency to promote dehydrogenation, aromatic condensation, gas production and/or coke formation. Therefore, high metals accumulation on catalyst is normally regarded as quite undesirable in FCC processing.
- Erdmond 3,190,829 removes heavy metals from petroleum oils by treating with methyl or ethyl sulfonic acids.
- Adams 3,245,902 removes nickel and vanadium from petroleum fractions by treatment with hydrofluoric acid at a temperature in the range of 121°C-191°C (250-375°F) for 10 to 60 minutes.
- Schulze 4046687 is concerned with treating aqueous solution of arsenic, antimony and bismuth with a water insoluble or low solubility salt of phosphoric acid or an ester thereof.
- Blytas 4048061 uses acidified active carbon to remove lead, copper, nickel, vanadium and iron.
- Kluksdahl 4192736 relies upon alumina promoted with phosphorous oxide to remove nickel and vanadium from petroleum resid.
- Gould 4197192 uses an organic peroxyacid to oxidize a petroleum feed to remove vanadium and nickel.
- VGO vacuum gas oil
- feedstock known as “vacuum gas oil” (VGO) is generally prepared from crude oil by distilling off the fractions boiling below about 343°C (650°F) at atmospheric pressure and then separating the 343°C (650°F) plus fraction by vacuum distillation from the heavier resid fraction as vacuum gas oil boiling between about 343°C (650°F) up to about 482°C (900°F) or 552°C (1025°F).
- the vacuum gas oil plus atmospheric gas oils is used as the oil feedstock in conventional FCC processing.
- the heavier resid fraction of vacuum distillation is normally employed for a variety of other purposes, such as for instance the production of asphalt, #6 fuel oil, or used as marine Bunker C fuel oil.
- the present invention is concerned with recovering a partially demetallized portion of these heavier oil fractions containing substantial quantities of both coke precursors and heavy metals contaminants as well as other troublesome components.
- the recovery partially demetallized resid portion of vacuum distillation is then used with a lower boiling gas oil fraction thereby increasing the volume of charge oil whereby an increased overall yield of gasoline and other hydrocarbon liquid fuels may be realized from a given quantity of crude oil.
- the oil feeds capable of being catalytically cracked following the acid treatment of this invention are those which include at least about 70 percent of which boil above 343°C (650°F) and contain a carbon residue on pyrolysis and up to about 4 parts per million of nickel equivalents of heavy metals.
- Examples of these oil feeds are fractions of crude oils such as topped crudes, residual or reduced crudes, residua, and extracts from solvent de-asphalting.
- the unusually large amount of coke which deposits on the catalyst in carbo-metallic oil processing presents critical problems, the primary problem arising from the fact that the reactions in the regenerator which convert coke to water, carbon monoxide and carbon dioxide are highly exothermic.
- Using a carbo-metallic feed with its unusually high content of coke precursors as compared to gas oil FCC feeds, can substantially increase the amount of coke to be burned in the regenerator and thus the regeneration temperatures ran become excessive if there is thorough burning of deposited coke. Excessive regeneration temperatures can permanently deactivate the catalyst and/or damage the regenerating equipment.
- the heat of combustion of coke depends upon the concentration of hydrogen in the coke and the ratio of C0 2 to CO in the products of combustion. Carbon produces 13,910 BTU per pound when burned to C0 2 and only 3,962 BTU per pound when burned to CO. Hydrogen produces 61,485 BTU per pound when burned to H 2 0.
- the heats of combustion of coke for three representative levels of hydrogen and four different ratios of CO 2 /CO are given in the following table:
- one object of this invention is to provide a method for converting carbo-metallic containing residual oils to liquid fuels.
- Another object is to provide a carbo-metallic containing residual oil conversion process which effectively reduces the temperatures encountered in the provided catalyst regeneration operation.
- the metal contaminants in the crude oil fraction to be upgraded are removed as a function of two variables.
- the variables are (1) oxidation of a chelated metals to give a water soluble metal and (2) precipitation of asphaltenes and/or aromatic materials with which the metals are chelated.
- the amount of heavy hydrocarbon material removed from the feedstock is in the range of 0.1% to 20% by weight depending on the feed stock being processed.
- the metals removal accomplished by the method of this invention includes from 15% up to 82% nickel; from 5% up to 95% vanadium and about 50% ⁇ 10% iron.
- a heavy oil fraction from 0.5 to 2.0% (weight/weight) of a concentrated acid such as 85% phosphoric acid, concentrated sulfuric and nitric acid.
- a concentrated acid such as 85% phosphoric acid, concentrated sulfuric and nitric acid.
- the mixture thus formed of the heavy oil fraction and phosphoric acid is heated to a temperature of at least 66°C (150°F) and preferably from about 104°C to 177°C (220°F to 350°F) for a period of time sufficient for reaction to occur.
- an organo metal complex is formed with contaminants and asphaltenes which precipitate out.
- the thus treated heavy oil fraction is then separated from the precipitate at the temperature at which it is formed.
- This procedure produces a heavy oil fraction with a lower metals content of lower Ramsbottom carbon value, the density of the heavy oil fraction is thus improved and the crackability of the thus treated resid heavy oil portion to form liquid transportation fuels is greatly enhanced.
- the substantial metals removal accomplished by the method and process of this invention substantially improves the cracking catalyst on stream life by reducing the rate of metals deposition thereon.
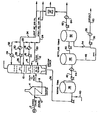
- the drawing is a schematic flow diagram of a preferred system for practicing the invention.
- a topped crude oil fraction obtained from the bottom of an atmospheric distillation tower is charged to the combination process of this invention.
- the topped crude is separated from an atmospheric distillation tower (not shown) with an initial boiling point within the range of 316°C to 371°C (600 to 700°F) and more usually about 343°C (650°F).
- the thus obtained topped crude is charged by conduit 2 to furnace 4.
- Stream is charged by conduit 6 for admixture with the heavy residual portion of the crude oil in conduit 2 to reduce coking thereof within the furnace tubes during heating thereof to a temperature within the range of about 388°C (730°F) up to about 454°C (850°F) before discharge by conduit 8 into a lower bottom portion of a vacuum distillation tower 10.
- a pressure is maintained within a bottom portion of tower 10 within the range of 25 to 50 mm of Hg.
- Steam is added to the tower 10 by conduit 12 to assist with obtaining separation of light and heavy vacuum gas oils from the residual oil to topped crude fraction charged to tower 10.
- a heavy gas oil fraction is withdrawn by conduit 14 from a tray within tower 10, passed to pump 16 for passage to heat exchange 18. Thereafter a portion of the heavy vacuum gas oil is recycled to the tower by conduit 20, another portion thereof is recycled by conduit 22 to the tower 10 and a portion is withdrawn by conduit 24 for use as discussed below.
- a light gas oil fraction is withdrawn from an upper liquid accumulation tray in tower 10 by conduit 26 for passage to pump 28 and heat exchanger 30.
- a portion of the withdrawn light cycle oil or light vacuum gas oil is recycled to an upper portion of tower 10 as reflux by conduit 32.
- Another portion is withdrawn by conduit 34 for use as discussed below.
- a vacuum resid is withdrawn from the bottom of tower 10 by conduit 36 and passed to a mixing tank 38.
- Phosphoric acid of about 85% concentration is charged by conduit 40 to mixing tank 38 maintained after 104°C (220°F) after admixture with vacuum resid in conduit 36.
- a weight ratio of resid to phosphoric acid in a range of 0.5 to 1 is maintained in mixing tank 38.
- the mixture formed in tank 38 is then passed by conduit 42 to heat exchanger 44 and thence to a first settling tank 46.
- a first precipitate comprising an organometallic phosphorous complex formed in settling tank 46 maintained at a temperature of at least 104°C (220°F) up to about 177°C (350°F) is collected in the bottom of the tank.
- Means are provided in the bottom of tank 46 for scraping or otherwise providing for removal of the asphaltic precipitate as by conduit 48 for passage to asphalt processing.
- a portion of the material charged to tank 46 and not completely reacted to form the metal complex precipitate is passed from tank 46 by conduit 50 to a second settling tank 52.
- Tank 52 is maintained at a temperature of at least 104°C (220°F) wherein an additional metal complex asphaltic precipitate is formed and which settles to the bottom of the tank.
- Means are provided in the bottom of the tank for collecting and removing the formed precipitate as by conduit 54.
- the precipitates removed by conduits 48 and 54 are joined together and passed by conduit 56 to a heat exchanger 58 providing heat sufficient to maintain the precipitate phase fluid for passage by conduit 60 to asphaltic material processing.
- the vacuum resid thus treated to remove metal contaminants and some asphaltenes along with some Conradson carbon or Ramsbottom carbon producing components is withdrawn from tank 52 by conduit 62 comprising heat exchanger 64 for passage to a fluid catalyst cracking unit (not shown) along with vacuum gas oils recovered by conduits 24 and 34 as above discussed.
- a reduced crude or vacuum resid treated as herein described provides a partially demetallized and decarbonized feed or heavy oil fraction more suitable for catalytic cracking beneficiaation.
- 0.895% phosphoric acid was added to the resid fraction, removed 5.06% of material from the feed. Of this removed material, 58.33% is hexane insolubles and 0.18% THF insolubles.
- Processing by catalytic cracking such acid treated material admixed with atmospheric and vacuum gas oils boiling above about 288°C (550°F) provides a feed suitable to obtain an increase of 18.34 vol % of gasoline and a 19 percent reduction in coke make.
- the metals removal capability and feed conversion attributed to the method and process of the invention is represented by the following data.
- an equilibrium catalyst (GRZ-1 and described in the Table) is used in a cracking operation in which highly contaminated, carbon contaminated residual feedstock, (a heavy vacuum gas oil prepared in apparatus as described in the Figure but deleting all elements numbered higher than 40) is fed to a conventional fluid catalytic cracking apparatus in which the catalyst circulates between a riser cracking zone and a regeneration zone in which carbon is removed by oxidation.
- the phosphoric acid is mixed with the vacuum tower bottoms as shown in the Figure and the mixture then moves (without the need for mixing tank 38 which may optionally be provided) directly into the FCC or RCC unit shown at the right-hand side of the drawing.
- the phosphoric acid will generally be of the commercial 85% concentration but may be of lower or higher concentration and may be premixed with a small amount of emulsifying agent and oil to aid in dispersion.
- the phosphoric acid: vacuum bottoms weight ratio is 0.01 in the tests shown in Table 1. This ratio will preferably be in the range of about 0.001 to about 0.05, more preferably from about 0.003 to about 0.03 and most preferably from about 0.005 to about 0.015 weight ratio.
- phosphoric acid is not the only acid which may be used. Instead, other acids, e.g., sulfuric acid (either oleum or lower concentration of H2SO4), or other strong mineral acid may be utilized though nitric and hydrohalic acids will be less preferred because of their contaminating nitrogen and halogen anions which can cause damage to the catalyst and/or apparatus. Acidic acid may also be employed in place of phosphoric acid as the principal objective is to provide regeneration of acid sites on the zeolite in the catalyst material.
- acids e.g., sulfuric acid (either oleum or lower concentration of H2SO4), or other strong mineral acid may be utilized though nitric and hydrohalic acids will be less preferred because of their contaminating nitrogen and halogen anions which can cause damage to the catalyst and/or apparatus.
- Acidic acid may also be employed in place of phosphoric acid as the principal objective is to provide regeneration of acid sites on the zeolite in the catalyst material.
- the zeolite is embedded in an inorganic matrix which can be readily penetrated by the acids and contact between the acid and the zeolite though not wishing to be bound by any theory, apparently the combination presence of the zeolite, the inorganic matrix, e.g., alumina or silica and the acid acts to provide composite molecules in the inorganic matrix which molecules provide acid sites. These acid sites excellerate the cracking of the heavy molecules contained in the vacuum tower bottoms or other residuums being processed. This cracking provides products of smaller molecular weight which can then enter through the inorganic matrix and contact the zeolite portion of the catalyst contained within the matrix. Upon contact between the vapor and the zeolite, further cracking occurs and the final products are largely in the valuable transportation fuel range of boiling points, e.g. light cycle oil, gasoline, and C3-C4 hydrocarbons.
- boiling points e.g. light cycle oil, gasoline, and C3-C4 hydrocarbons.
- Table 2 on the other hand identifies a feed stock processed by the method of the invention and identified as 735 tank (2/7/81).
- the feed is a mixed composition of crude oil as identified.
- Table 3 presented below identifies the yields obtained when processing by catalytic cracking the feedstock of Table 2 with and without acid treatment.
- the data presented in Table 3 above shows quite clearly the improved results obtained when practicing the method and concepts of the inventions. That is, the acid treated feed used in test PDU168 shows a lower C 3 - gas yield, an increased yield of C 5 -221°C (430°F) gasoline product and higher selectivity, a higher yield of 332°C (630 0 F) - cycle oil and a lower coke and slurry oil (>332°C) (>630°F) yield.
- the data of Table 3 are substantially self explanitory with respect to the improvements obtained when cracking acid treated feed to effect partical demetallizing and decarbonizing thereof over that obtained with a similar feed which has not been acid treated as herein provided.
- Table 4 below is presented to show the effect of different acid percent treatment on different metal content feeds with respect to metals content before and after acid treatment.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US53262283A | 1983-09-15 | 1983-09-15 | |
| US532622 | 1983-09-15 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0140000A2 true EP0140000A2 (de) | 1985-05-08 |
| EP0140000A3 EP0140000A3 (de) | 1987-02-04 |
Family
ID=24122513
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP84110165A Withdrawn EP0140000A3 (de) | 1983-09-15 | 1984-08-25 | Entmetallisierung und Dekarbonisierung umfassende kombinierte Verfahren zur Verbesserung von Rohöl |
Country Status (2)
| Country | Link |
|---|---|
| EP (1) | EP0140000A3 (de) |
| JP (1) | JPS6096688A (de) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103102936A (zh) * | 2011-11-10 | 2013-05-15 | 中国石油化工股份有限公司 | 一种高含酸原油的加工方法 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2682496A (en) * | 1951-12-07 | 1954-06-29 | Standard Oil Dev Co | Deashing residual oils with an acid of phosphorus |
| US3245902A (en) * | 1962-02-28 | 1966-04-12 | Exxon Research Engineering Co | Demetallization of high boiling petroleum fractions |
| US3190829A (en) * | 1962-11-29 | 1965-06-22 | Gulf Research Development Co | Process for removing metals from a mineral oil with an alkyl sulfonic acid |
| US3622505A (en) * | 1969-12-24 | 1971-11-23 | Union Oil Co | Demetallization of residual oils with polyphosphoric acids |
| US4197192A (en) * | 1978-10-23 | 1980-04-08 | Exxon Research & Engineering Co. | Vanadium and nickel removal from petroleum utilizing organic peroxyacid |
| US4390416A (en) * | 1981-12-07 | 1983-06-28 | W. R. Grace & Co. | Catalytic cracking of hydrocarbons |
-
1984
- 1984-08-25 EP EP84110165A patent/EP0140000A3/de not_active Withdrawn
- 1984-09-14 JP JP19382884A patent/JPS6096688A/ja active Pending
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103102936A (zh) * | 2011-11-10 | 2013-05-15 | 中国石油化工股份有限公司 | 一种高含酸原油的加工方法 |
| CN103102936B (zh) * | 2011-11-10 | 2015-04-01 | 中国石油化工股份有限公司 | 一种高含酸原油的加工方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| JPS6096688A (ja) | 1985-05-30 |
| EP0140000A3 (de) | 1987-02-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4336160A (en) | Method and apparatus for cracking residual oils | |
| US4434044A (en) | Method for recovering sulfur oxides from CO-rich flue gas | |
| EP1332199B8 (de) | Durch schnelle thermische behandlung von schweren kohlenwassterstoffeinsätzen hergestelltes produkt | |
| US4435272A (en) | Process for upgrading crude oil and residual fractions thereof by vaporizing the charge in a falling curtain of contact particles | |
| US4443325A (en) | Conversion of residua to premium products via thermal treatment and coking | |
| JPS5953591A (ja) | 供給油の転化方法および装置 | |
| EP0134924B1 (de) | Zugabe von Wasser zur Regenerationsluft | |
| US4551231A (en) | Ammonia contacting to passivate metals deposited on a cracking catalyst during reduced crude processing | |
| JPS6337155B2 (de) | ||
| US4437981A (en) | Immobilization and neutralization of contaminants in crude oil | |
| US4256567A (en) | Treatment of petroleum stocks containing metals | |
| EP0433026B1 (de) | Verfahren zur Entfernung von metallischen Verunreinigungen aus Kohlenwasserstoffölen | |
| US4148717A (en) | Demetallization of petroleum feedstocks with zinc chloride and titanium tetrachloride catalysts | |
| US4390416A (en) | Catalytic cracking of hydrocarbons | |
| EP0072873A1 (de) | Raffinationsprozess zur Ausbeutesteigerung von Destillaten aus schweren Erdöleinsätzen | |
| US3165462A (en) | Pretreatment and cracking of heavy mineral oils | |
| EP0097829B1 (de) | Umwandlung von carbometallischen Ölen mittels Wasserstoff in einem Steigrohr unter Verwendung eines Katalysators mit hohem Metallgehalt | |
| NL8105848A (nl) | Werkwijze voor de kwaliteitsverbetering van koolwaterstofhoudende olien met een water bevattende vloeistof. | |
| US5919352A (en) | Integrated residua upgrading and fluid catalytic cracking | |
| EP0140000A2 (de) | Entmetallisierung und Dekarbonisierung umfassende kombinierte Verfahren zur Verbesserung von Rohöl | |
| EP0123014A1 (de) | Verfahren zur Crackung hochsiedender Kohlenwasserstoffe unter kontinuierlicher Zugabe aciditätserhöhender Zusätze | |
| JP2002542375A (ja) | 高沸点物質の反応再循環による残油の改良された脱歴方法 | |
| JPH11509259A (ja) | 統合された残油品質向上及び流動接触分解 | |
| US3061539A (en) | Hydrogen fluoride treatment of coking and cracking feed stock | |
| CA1250243A (en) | Selective vaporization process |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE DE FR GB IT NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE DE FR GB IT NL SE |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 19870805 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: GOOLSBY, TERRY L. Inventor name: HOBBS, ESTEL M. |