EP0103487A2 - Electrical insulation - Google Patents
Electrical insulation Download PDFInfo
- Publication number
- EP0103487A2 EP0103487A2 EP83305380A EP83305380A EP0103487A2 EP 0103487 A2 EP0103487 A2 EP 0103487A2 EP 83305380 A EP83305380 A EP 83305380A EP 83305380 A EP83305380 A EP 83305380A EP 0103487 A2 EP0103487 A2 EP 0103487A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- polymer
- radical
- carbon atoms
- aromatic
- article according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 238000010292 electrical insulation Methods 0.000 title abstract description 5
- 229920000642 polymer Polymers 0.000 claims abstract description 53
- 125000003118 aryl group Chemical group 0.000 claims abstract description 21
- 229920001577 copolymer Polymers 0.000 claims abstract description 15
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 claims abstract description 10
- 239000005977 Ethylene Substances 0.000 claims abstract description 10
- 230000009477 glass transition Effects 0.000 claims abstract description 5
- 125000004432 carbon atom Chemical group C* 0.000 claims description 16
- 239000004020 conductor Substances 0.000 claims description 13
- 229910052731 fluorine Inorganic materials 0.000 claims description 12
- 239000011737 fluorine Substances 0.000 claims description 12
- 229920000098 polyolefin Polymers 0.000 claims description 12
- 238000002844 melting Methods 0.000 claims description 7
- 230000008018 melting Effects 0.000 claims description 6
- 229920000620 organic polymer Polymers 0.000 claims description 4
- 125000002877 alkyl aryl group Chemical group 0.000 claims description 3
- NBVXSUQYWXRMNV-UHFFFAOYSA-N fluoromethane Chemical group FC NBVXSUQYWXRMNV-UHFFFAOYSA-N 0.000 claims description 3
- 125000005843 halogen group Chemical group 0.000 claims description 3
- 239000000155 melt Substances 0.000 claims description 3
- UUFQTNFCRMXOAE-UHFFFAOYSA-N 1-methylmethylene Chemical compound C[CH] UUFQTNFCRMXOAE-UHFFFAOYSA-N 0.000 claims description 2
- HIXDQWDOVZUNNA-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-hydroxy-7-methoxychromen-4-one Chemical compound C=1C(OC)=CC(O)=C(C(C=2)=O)C=1OC=2C1=CC=C(OC)C(OC)=C1 HIXDQWDOVZUNNA-UHFFFAOYSA-N 0.000 claims description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 claims description 2
- 150000001491 aromatic compounds Chemical class 0.000 claims description 2
- 125000000751 azo group Chemical group [*]N=N[*] 0.000 claims description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 claims description 2
- 125000006575 electron-withdrawing group Chemical group 0.000 claims description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 2
- AUONHKJOIZSQGR-UHFFFAOYSA-N oxophosphane Chemical compound P=O AUONHKJOIZSQGR-UHFFFAOYSA-N 0.000 claims description 2
- 229920000412 polyarylene Polymers 0.000 claims description 2
- 229920001169 thermoplastic Polymers 0.000 claims description 2
- 239000004416 thermosoftening plastic Substances 0.000 claims description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 claims description 2
- 229920002554 vinyl polymer Chemical group 0.000 claims description 2
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 claims 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims 1
- ATTZFSUZZUNHBP-UHFFFAOYSA-N Piperonyl sulfoxide Chemical group CCCCCCCCS(=O)C(C)CC1=CC=C2OCOC2=C1 ATTZFSUZZUNHBP-UHFFFAOYSA-N 0.000 claims 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims 1
- 125000001174 sulfone group Chemical group 0.000 claims 1
- 238000009413 insulation Methods 0.000 abstract description 9
- 239000004696 Poly ether ether ketone Substances 0.000 abstract description 5
- 229920002530 polyetherether ketone Polymers 0.000 abstract description 5
- 229920006393 polyether sulfone Polymers 0.000 abstract description 4
- 239000000779 smoke Substances 0.000 abstract description 4
- BQCIDUSAKPWEOX-UHFFFAOYSA-N 1,1-Difluoroethene Chemical compound FC(F)=C BQCIDUSAKPWEOX-UHFFFAOYSA-N 0.000 abstract description 3
- UUAGAQFQZIEFAH-UHFFFAOYSA-N chlorotrifluoroethylene Chemical group FC(F)=C(F)Cl UUAGAQFQZIEFAH-UHFFFAOYSA-N 0.000 abstract description 3
- 229920001643 poly(ether ketone) Polymers 0.000 abstract description 3
- 239000004695 Polyether sulfone Substances 0.000 abstract description 2
- 229920006037 cross link polymer Polymers 0.000 abstract 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 11
- 239000000203 mixture Substances 0.000 description 9
- 229920002313 fluoropolymer Polymers 0.000 description 8
- BFKJFAAPBSQJPD-UHFFFAOYSA-N tetrafluoroethene Chemical group FC(F)=C(F)F BFKJFAAPBSQJPD-UHFFFAOYSA-N 0.000 description 7
- 239000000178 monomer Substances 0.000 description 5
- 150000001336 alkenes Chemical class 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 230000005855 radiation Effects 0.000 description 3
- 238000002834 transmittance Methods 0.000 description 3
- 239000002033 PVDF binder Substances 0.000 description 2
- 239000004697 Polyetherimide Substances 0.000 description 2
- 229920006355 Tefzel Polymers 0.000 description 2
- 229920004738 ULTEM® Polymers 0.000 description 2
- JUPQTSLXMOCDHR-UHFFFAOYSA-N benzene-1,4-diol;bis(4-fluorophenyl)methanone Chemical compound OC1=CC=C(O)C=C1.C1=CC(F)=CC=C1C(=O)C1=CC=C(F)C=C1 JUPQTSLXMOCDHR-UHFFFAOYSA-N 0.000 description 2
- 238000005336 cracking Methods 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- QHSJIZLJUFMIFP-UHFFFAOYSA-N ethene;1,1,2,2-tetrafluoroethene Chemical compound C=C.FC(F)=C(F)F QHSJIZLJUFMIFP-UHFFFAOYSA-N 0.000 description 2
- 238000001125 extrusion Methods 0.000 description 2
- HCDGVLDPFQMKDK-UHFFFAOYSA-N hexafluoropropylene Chemical group FC(F)=C(F)C(F)(F)F HCDGVLDPFQMKDK-UHFFFAOYSA-N 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 229920002492 poly(sulfone) Polymers 0.000 description 2
- 229920001601 polyetherimide Polymers 0.000 description 2
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 2
- 150000003457 sulfones Chemical class 0.000 description 2
- QMIWYOZFFSLIAK-UHFFFAOYSA-N 3,3,3-trifluoro-2-(trifluoromethyl)prop-1-ene Chemical group FC(F)(F)C(=C)C(F)(F)F QMIWYOZFFSLIAK-UHFFFAOYSA-N 0.000 description 1
- 229920001780 ECTFE Polymers 0.000 description 1
- JIGUQPWFLRLWPJ-UHFFFAOYSA-N Ethyl acrylate Chemical compound CCOC(=O)C=C JIGUQPWFLRLWPJ-UHFFFAOYSA-N 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 239000006057 Non-nutritive feed additive Substances 0.000 description 1
- 229920004888 Victrex® 200P Polymers 0.000 description 1
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 1
- 239000000370 acceptor Substances 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- BAPJBEWLBFYGME-UHFFFAOYSA-N acrylic acid methyl ester Natural products COC(=O)C=C BAPJBEWLBFYGME-UHFFFAOYSA-N 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 125000005250 alkyl acrylate group Chemical group 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 150000001733 carboxylic acid esters Chemical class 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 150000003949 imides Chemical class 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000000034 method Methods 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- PNJWIWWMYCMZRO-UHFFFAOYSA-N pent‐4‐en‐2‐one Natural products CC(=O)CC=C PNJWIWWMYCMZRO-UHFFFAOYSA-N 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 229920006162 poly(etherimide sulfone) Polymers 0.000 description 1
- 229920003208 poly(ethylene sulfide) Polymers 0.000 description 1
- 229920001470 polyketone Polymers 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 150000003462 sulfoxides Chemical group 0.000 description 1
- 239000003017 thermal stabilizer Substances 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 229920001567 vinyl ester resin Polymers 0.000 description 1
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B3/00—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties
- H01B3/18—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances
- H01B3/30—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B3/00—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties
- H01B3/18—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances
- H01B3/30—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes
- H01B3/44—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins
- H01B3/441—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins from alkenes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B7/00—Insulated conductors or cables characterised by their form
- H01B7/17—Protection against damage caused by external factors, e.g. sheaths or armouring
- H01B7/29—Protection against damage caused by extremes of temperature or by flame
- H01B7/295—Protection against damage caused by extremes of temperature or by flame using material resistant to flame
Definitions
- operably is used herein to include a single electrically insulated elongate conductor often referred to in the art as "wire"), an article comprising a plurality of separate elongate conductors each of which is separately insulated, and an article comprising a plurality of elongate conductors which are physically joined together but electrical.ly insulated from each other by insulating material, e.g. ribbon cable.
- Fluorocarbon polymers especially ethylene/tetrafluoroethylene (ETFE) copolymers such as Tefzel, are used extensively for electrical insulation, in particular for aircraft wire. Particularly when cross-linked, such polymers can exhibit an excellent combination of physical and electrical properties under normal service conditions.
- EFE ethylene/tetrafluoroethylene
- Tefzel ethylene/tetrafluoroethylene copolymers
- U.S. Patents Nos. 3,580,829, 3,738,923, 3,763,222, 3,840,619, 3,894,118, 3,911,192, 3,947,525, 3,970,770, 3,985,716, 3,995,091, 4,031,167, 4,155,823, 4,121,001, and 4,176,027 Other polymers which have been used for electrical insulation include other olefin polymers (both homopolymers and copolymers) and various high-melting aromatic polymers.
- the olefin polymer forming the inner layer preferably has a tensile (Young's) modulus of at least 138 MPa (20,000 p.s.i.) especially at least 207 MPa (30,000 p.s.i.) and particularly at least 276 MPa (40,000 p.s.i.) in order to minimize wrinkling of the outer layer when the article, e.g. in the form of a wire, is bent.
- a tensile (Young's) modulus of at least 138 MPa (20,000 p.s.i.) especially at least 207 MPa (30,000 p.s.i.) and particularly at least 276 MPa (40,000 p.s.i.) in order to minimize wrinkling of the outer layer when the article, e.g. in the form of a wire, is bent.
- the insulation of the article to the invention provides a valuable combination of physical and electrical properties.
- the outer layer provides excellent resistance to. physical abuse.
- the inner layer is more flexible than the outer layer and thus provides insulation which is more flexible, for a particular dielectric strength, than insulation which is composed only of the aromatic polymer.
- the-aromatic polymers often have poor resistance to stress-cracking which can seriously reduce their dielectric strength, the olefin polymers do not suffer from this disadvantage, and the inner jacket will' therefore provide continuous insulation even in environments which cause stress-cracking of the outer jacket.
- olefin polymer as used herein is defined as being a polymer of one or more unsubstituted and/or substituted olefins. Where the polymer includes substituted olefins as monomers or comonomers they are preferably polar monomers and especially fluorine-containing monomers, e.g. tetrafluorethylene, or a carboxylic ester, in particular an alkyl acrylate, e.g. methyl or ethyl acrylate, or a vinyl ester, e.g. vinyl acetate.
- the olefin is preferably a fluorcarbon polymer as explained below.
- the inner layer is composed of a cross-linked fluorocarbon layer.
- electrical wire which, when tested for smoke evolution by ASTM E 662-79 (flaming mode), has a D m value of less than 50, preferably less than 35, where D m is the maximum specific optical density.
- fluorocarbon polymer is used herein to denote a polymer or mixture of polymers which contains more than 10%, preferably more than 25%, by weight of fluorine.
- the fluorocarbon polymer may be a single fluorine-containing polymer, a mixture of two or more fluorine-containing polymers, or a mixture of one of more fluorine-containing polymers with one or mere polymers which do not contain fluorine.
- the fluorocarbon polymer comprises at least 50%, particularly at least 75% especially at least 85%, by weight of one or more thermoplastic crystalline polymers each containing at least 25% by weight of fluorine, a single such crystalline polymer being preferred.
- Such a fluorocarbon polymer may contain, for example, a fluorine-containing elastomer and/or a polyolefin, preferably a crystalline polyolefin, in addition to the crystalline fluorine-containing polymer or polymers.
- the fluorine-containing polymers are generally homo- or copolymers of one or more fluorine-containing olefinically unsaturated monomers, or copolymers of one or more such monomers with one or more. olefins.
- the fluorocarbon polymer usually has a melting point of at least 150°C, and will often have a melting point of at least 250°C, e.g.
- the melting point being defined for crystalline polymers as the temperature above which no crystallinity exists in the polymer (or when a mixture of crystalline polymers is used, in the major crystalline component in the mixture).
- the polymeric composition, prior to cross-linking has a viscosity of less than 10 poise at a temperature not more than 60°C above its melting point.
- a preferred fluorocarbon polymer is a copolymer of ethylene and tetrafluoroethylene and optionally one or more other comonomers'(known as ETFE polymers), especially a copolymer comprising 35 to 60 mole percent of ethylene, 35 to 60 mole percent of tetrafluoro-ethylene and up to 10 mole percent of one or more other comonomers.
- ETFE polymers comonomers
- polymers which can be used include copolymers of ethylene and chlorotrifluoroethylene; polyvinylidene fluoride; copolymers of vinylidene fluoride with one or both of hexafluoropropylene and tetrafluoroethylene, or with hexafluoroisobutylene; and copolymers of tetrafluoro- ethylene and hexafluoropropylene.
- Either or both of the inner and outer insulating layers can optionally contain suitable additives such as pigments, antioxidants, thermal stabilisers, acid acceptors and processing aids.
- Such polymers include polyketones, polyether ketones, polyether ether ketones and polyether sulfones, polyether ketone/ sulfone copolymers and polyether imides. Blends of different polymers can be used.
- Preferred aromatic polymers are crystalline polymers with a melting point of at least 250°C, particularly at least 300°C.
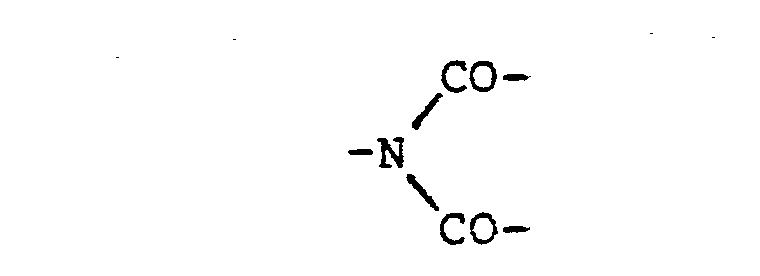
- the polymer comprises, and preferably consists essentially of, units of the formula the units being the same or different, Ar being a divalent aromati-c radical and Q being -O-, -S-, -SO 2 -, -CO-, -NH-CO- or -COO-, or Ar being a polyvalent radical and Q being the Q radical preferably being directly bonded to aromatic carbon atoms in the Ar radical.
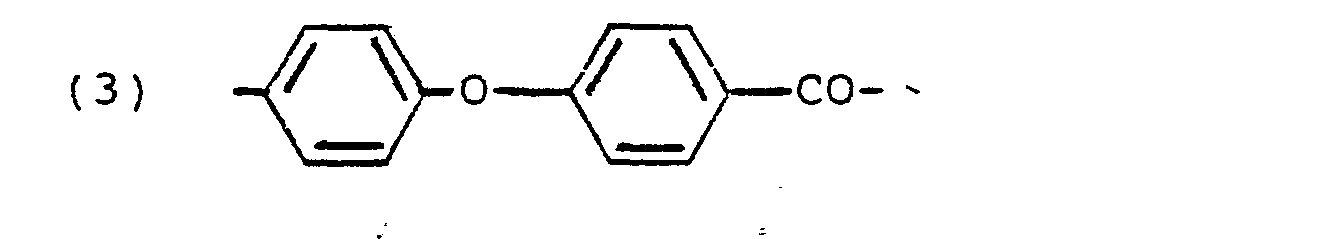
- the aromatic polymer is a crystalline polyarylene ether comprising recurring units of the formula where E is the residue of a dihydric phenol and E' is the residue of an aromatic compound having an electron withdrawing group in at least one of the positions ortho and para to the valence bonds, the E and E' radicals being linked to the -0- radicals through aromatic carbon atoms.
- E is a radical of the formula wherein R is a divalent radical; x is 0 or 1; Y is a radical selected from halogen atoms, alkyl radicals.
- R' is a radical selected from halogen atoms, alkyl radicals containing 1 to 4 carbon atoms and alkoxy radicals containing 1 to 4 carbon atoms; z is 0, 1, 2, 3 or 4, and E' is a radical of the formula wherein R' is a sulfone, carbonyl, vinyl, sulfoxide, azo, saturated fluorocarbon, organic phosphine oxide or ethylidene radical.
- polysulfones are those in which y and z are 0, x is 1, R' is a sulfone radical and R is a radical of the formula wherein each of R'' and R''' is independently selected from the group consisting of hydrogen; alkyl radicals containing 1 to 4 carbon atoms; halogen-substituted alkyl radical containing 1 to 4 carbon atoms; aryl, alkaryl and aralkyl radicals containing 6 to 10 carbon atoms; and halogen-substituted aryl alkaryl and aralkyl radicals containing 6 to 10 carbon atoms.
- the polymer is a polyether imide or polysulfone imide which comprises recurring units of the formula where Q is -O- or -SO 2 , Z is a trivalent aromatic radical, R is a divalent aromatic radical and R' is a divalent organic radical.
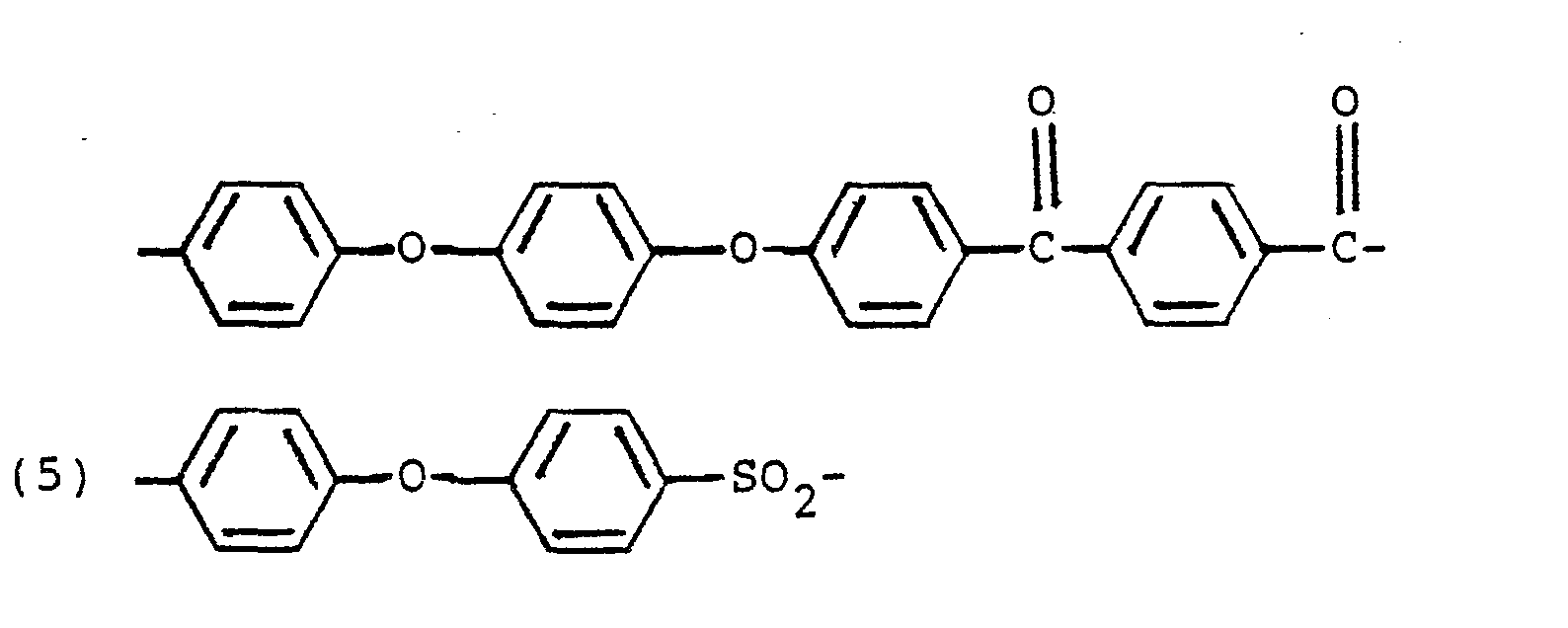
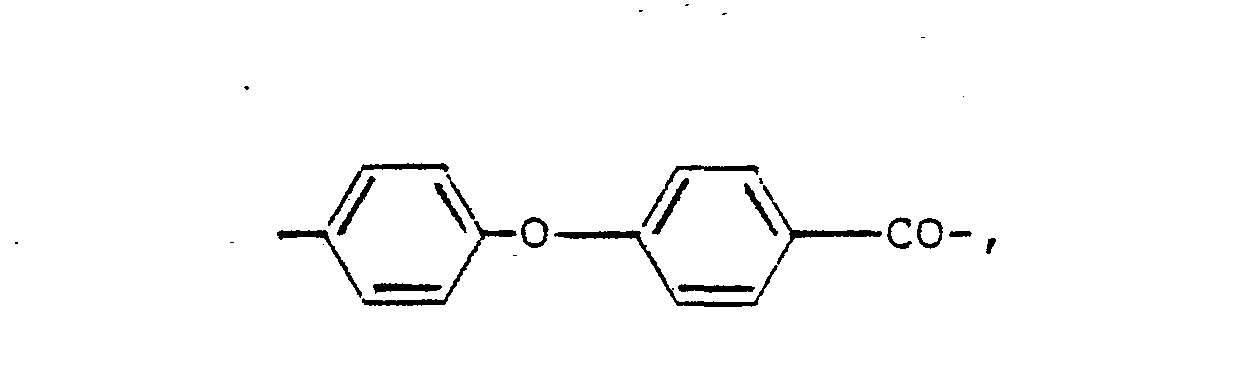
- Preferred aromatic polymers consist essentially of repeating units having one of the following formulae wherein each of x, m and n is 0 or 1, with n beinq 0 when x. is 1, p is an integer from 1 to 4, with m being 1 and x being 0 when p is greater than 1, e.g.,
- the insulated articles of the present invention can be produced by conventional techniques; the inner layer usually contacts the conductor, and the inner and outer layers generally constitute the total insulation of the article; however, other insulating layers can be present.
- the olefin polymer is preferably cross-linked by radiation, and cross-linking can be effected before or after the aromatic polymer (which is generally not cross-linked by radiation) is applied.
- the inner layer will usually be of annular cross-section of thickness for example 76.2 to 381 micrometres (3 to 15 mils), preferably 101.6 to 177.8 micrometres (4 to 7 mils).
- the cable can comprise a plurality of conductors, each of which has an inner insulating layer around it, with the conductors being joined together and further insulated by the outer insulating layer.
- the invention is illustrated by the following Examples, Examples 1, 2, 3 and 8 of which are comparative.
- a 20 AWG stranded (19/32) conductor was extrusion-coated with an inner insulating layer having the composition and thickess shown in the Table. Except in Examples 1 and 2, the inner insulating layer was then extrusion-coated with an outer insulating layer having the composition and thickness shown in the Table.
- the coated conductor was irradiated to a dosage of about 10 Megarads to cross-link the inner coating; in these Examples, the inner coating also contained, when it was irradiated, a suitable amount of a radiation cross-linking agent.
- the outer coating was substantially unaffected by this irradiation.
- the coated conductor was annealed at 180°C for 1 hour.
- Tefzel 280 is a copolymer of ethylene and tetrafluoro- ethylene available from du Pont.
- Halar 300 is a copolymer of ethylene and chlorotrifluoroethylene available from Allied Chemical.
- Kynar 450 is polyvinylidene fluoride available from Pennwalt.
- PEEK is a polyether ether ketone available from ICI.
- Ultem is a polyetherimide available from General Electric.
- Victrex 200P a polyethersulphone available from ICI.
- PEEK, Ultem and PES are substantially linear aromatic polymers.
Landscapes
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Organic Insulating Materials (AREA)
- Insulated Conductors (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Polymers With Sulfur, Phosphorus Or Metals In The Main Chain (AREA)
Abstract
Description
- Electrical insulation must meet a variety of electrical and physcial requirements under normal service conditions. In addition, for many purposes the insulation must meet test requirements which are intended to ensure that if the insulation is exposed to very high temperatures, e.g. in a fire, it will not evolve excessive amounts of toxic products or smoke. These requirements are particularly severe for electrical cable which is to be used in aircraft and similar equipment. The term "cable" is used herein to include a single electrically insulated elongate conductor often referred to in the art as "wire"), an article comprising a plurality of separate elongate conductors each of which is separately insulated, and an article comprising a plurality of elongate conductors which are physically joined together but electrical.ly insulated from each other by insulating material, e.g. ribbon cable.
- Fluorocarbon polymers, especially ethylene/tetrafluoroethylene (ETFE) copolymers such as Tefzel, are used extensively for electrical insulation, in particular for aircraft wire. Particularly when cross-linked, such polymers can exhibit an excellent combination of physical and electrical properties under normal service conditions. In this connection, reference may be made to U.S. Patents Nos. 3,580,829, 3,738,923, 3,763,222, 3,840,619, 3,894,118, 3,911,192, 3,947,525, 3,970,770, 3,985,716, 3,995,091, 4,031,167, 4,155,823, 4,121,001, and 4,176,027. Other polymers which have been used for electrical insulation include other olefin polymers (both homopolymers and copolymers) and various high-melting aromatic polymers.
- We have discovered that insulation which has improved properties and which can be efficiently manufactured comprises an inner layer of a cross-linked melt-extruded olefin polymer covered by a layer of a melt extruded aromatic polymer having a glass transition temperature of at least 100°C. Accordingly, the present invention provides an insulated electrical article, especially an insulated electrical wire or cable comprising:
- (a) a conductor;
- (b) a melt-shaped, preferably melt-extruded, inner insulating layer which preferably contacts the con ductor and compris-es a first organic polymer component which is a cross-linked olefin polymer, particularly an ETFE copolymer, and
- (c) a melt-shaped, preferably melt-extruded, outer insulating layer which contacts the inner insulating layer and which comprises a second organic polymer component which is a substantially linear aromatic polymer having a glass transition temperature of at least 100°C, preferably at least 130°C.
- The olefin polymer forming the inner layer preferably has a tensile (Young's) modulus of at least 138 MPa (20,000 p.s.i.) especially at least 207 MPa (30,000 p.s.i.) and particularly at least 276 MPa (40,000 p.s.i.) in order to minimize wrinkling of the outer layer when the article, e.g. in the form of a wire, is bent.
- The insulation of the article to the invention provides a valuable combination of physical and electrical properties. The outer layer provides excellent resistance to. physical abuse. The inner layer is more flexible than the outer layer and thus provides insulation which is more flexible, for a particular dielectric strength, than insulation which is composed only of the aromatic polymer. Furthermore, the-aromatic polymers often have poor resistance to stress-cracking which can seriously reduce their dielectric strength, the olefin polymers do not suffer from this disadvantage, and the inner jacket will' therefore provide continuous insulation even in environments which cause stress-cracking of the outer jacket.
- The term "olefin polymer" as used herein is defined as being a polymer of one or more unsubstituted and/or substituted olefins. Where the polymer includes substituted olefins as monomers or comonomers they are preferably polar monomers and especially fluorine-containing monomers, e.g. tetrafluorethylene, or a carboxylic ester, in particular an alkyl acrylate, e.g. methyl or ethyl acrylate, or a vinyl ester, e.g. vinyl acetate. The olefin is preferably a fluorcarbon polymer as explained below.
- Particularly useful properties are obtained when the inner layer is composed of a cross-linked fluorocarbon layer. We have discovered that the combination of an inner layer of a cross-linked fluorocarbon polymer and an outer layer of an aromatic polymer results in a completely unexpected reduction in the smoke evolved under standard test condictions. Thus it is possible, through use of the present invention, to manufacture electrical wire which, when tested for smoke evolution by ASTM E 662-79 (flaming mode), has a D m value of less than 50, preferably less than 35, where Dm is the maximum specific optical density.
- The term "fluorocarbon polymer" is used herein to denote a polymer or mixture of polymers which contains more than 10%, preferably more than 25%, by weight of fluorine. Thus the fluorocarbon polymer may be a single fluorine-containing polymer, a mixture of two or more fluorine-containing polymers, or a mixture of one of more fluorine-containing polymers with one or mere polymers which do not contain fluorine. In one preferred class, the fluorocarbon polymer comprises at least 50%, particularly at least 75% especially at least 85%, by weight of one or more thermoplastic crystalline polymers each containing at least 25% by weight of fluorine, a single such crystalline polymer being preferred. Such a fluorocarbon polymer may contain, for example, a fluorine-containing elastomer and/or a polyolefin, preferably a crystalline polyolefin, in addition to the crystalline fluorine-containing polymer or polymers. The fluorine-containing polymers are generally homo- or copolymers of one or more fluorine-containing olefinically unsaturated monomers, or copolymers of one or more such monomers with one or more. olefins. The fluorocarbon polymer usually has a melting point of at least 150°C, and will often have a melting point of at least 250°C, e.g. up to 350°C, the melting point being defined for crystalline polymers as the temperature above which no crystallinity exists in the polymer (or when a mixture of crystalline polymers is used, in the major crystalline component in the mixture). Preferably the polymeric composition, prior to cross-linking, has a viscosity of less than 10 poise at a temperature not more than 60°C above its melting point. A preferred fluorocarbon polymer is a copolymer of ethylene and tetrafluoroethylene and optionally one or more other comonomers'(known as ETFE polymers), especially a copolymer comprising 35 to 60 mole percent of ethylene, 35 to 60 mole percent of tetrafluoro-ethylene and up to 10 mole percent of one or more other comonomers. Other specific polymers which can be used include copolymers of ethylene and chlorotrifluoroethylene; polyvinylidene fluoride; copolymers of vinylidene fluoride with one or both of hexafluoropropylene and tetrafluoroethylene, or with hexafluoroisobutylene; and copolymers of tetrafluoro- ethylene and hexafluoropropylene.
- Either or both of the inner and outer insulating layers can optionally contain suitable additives such as pigments, antioxidants, thermal stabilisers, acid acceptors and processing aids.
- The aromatic polymers which are used in this invention are will known to those skilled in the art, and reference may be made for example to U.S. Patents Nos. 3,025,605, 3,354,129, 3,441,538, 3,442,538, 3,446,654, 3,658,938, 3,838,097, 3,847,867, 3,953,400, 3,956,240, 4,107,147, 4,108,837, 4,111,908, 4,175,175, 4,293,670, 4,320,224, and 3,446,654 and British Patents Nos. 971,227, 1,369,210 and 1,599,106. Such polymers include polyketones, polyether ketones, polyether ether ketones and polyether sulfones, polyether ketone/ sulfone copolymers and polyether imides. Blends of different polymers can be used. Preferred aromatic polymers are crystalline polymers with a melting point of at least 250°C, particularly at least 300°C. In one class of such polymers the polymer comprises, and preferably consists essentially of, units of the formula
- In another class of aromatic polymers the aromatic polymer is a crystalline polyarylene ether comprising recurring units of the formula
-
-
- The insulated articles of the present invention can be produced by conventional techniques; the inner layer usually contacts the conductor, and the inner and outer layers generally constitute the total insulation of the article; however, other insulating layers can be present. The olefin polymer is preferably cross-linked by radiation, and cross-linking can be effected before or after the aromatic polymer (which is generally not cross-linked by radiation) is applied. For electrical cable, the inner layer will usually be of annular cross-section of thickness for example 76.2 to 381 micrometres (3 to 15 mils), preferably 101.6 to 177.8 micrometres (4 to 7 mils). Alternatively, the cable can comprise a plurality of conductors, each of which has an inner insulating layer around it, with the conductors being joined together and further insulated by the outer insulating layer.
- The invention is illustrated by the following Examples, Examples 1, 2, 3 and 8 of which are comparative.
- In each of the Examples, a 20 AWG stranded (19/32) conductor was extrusion-coated with an inner insulating layer having the composition and thickess shown in the Table. Except in Examples 1 and 2, the inner insulating layer was then extrusion-coated with an outer insulating layer having the composition and thickness shown in the Table. In some of the Examples, as designated in the Table, the coated conductor was irradiated to a dosage of about 10 Megarads to cross-link the inner coating; in these Examples, the inner coating also contained, when it was irradiated, a suitable amount of a radiation cross-linking agent. The outer coating was substantially unaffected by this irradiation. The coated conductor was annealed at
180°C for 1 hour. Samples of the resulting cable were tested in accordance with the procedure of ASTM E662-79 (flaming mode), and the Table shows the values obtained for the minimum transmittance, the transmittance after 10 minutes, the time taken to reach the point of minimum transmittance, and the maximum optical density . (D m). - The various polymers used in the Examples are further indentified below
Tefzel 280 is a copolymer of ethylene and tetrafluoro- ethylene available from du Pont. - Halar 300 is a copolymer of ethylene and chlorotrifluoroethylene available from Allied Chemical.
- Kynar 450 is polyvinylidene fluoride available from Pennwalt.
- PEEK is a polyether ether ketone available from ICI.
- Ultem is a polyetherimide available from General Electric.
- Victrex 200P a polyethersulphone available from ICI.
-
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT83305380T ATE21462T1 (en) | 1982-09-15 | 1983-09-14 | ELECTRICAL INSULATION. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US41835582A | 1982-09-15 | 1982-09-15 | |
| US418355 | 1982-09-15 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0103487A2 true EP0103487A2 (en) | 1984-03-21 |
| EP0103487A3 EP0103487A3 (en) | 1984-08-01 |
| EP0103487B1 EP0103487B1 (en) | 1986-08-13 |
Family
ID=23657778
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP83305380A Expired EP0103487B1 (en) | 1982-09-15 | 1983-09-14 | Electrical insulation |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP0103487B1 (en) |
| JP (1) | JPS5973807A (en) |
| AT (1) | ATE21462T1 (en) |
| CA (1) | CA1214528A (en) |
| DE (1) | DE3365309D1 (en) |
| GB (1) | GB2127210B (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0151904A1 (en) * | 1984-02-08 | 1985-08-21 | HUBER & SUHNER AG KABEL-, KAUTSCHUK-, KUNSTSTOFF-WERKE | Insulated electric cord |
| WO1989000757A1 (en) * | 1987-07-10 | 1989-01-26 | Raychem Limited | Electrical wire |
| WO1989000758A1 (en) * | 1987-07-10 | 1989-01-26 | Raychem Limited | Electrical wire |
| EP0301543A3 (en) * | 1987-07-29 | 1990-05-09 | Sumitomo Electric Industries, Limited | Resinous composition and its use |
| WO1999004402A1 (en) * | 1997-07-14 | 1999-01-28 | Draka Uk Limited | Co-axial cable |
| US6296935B1 (en) * | 1996-08-22 | 2001-10-02 | The Furukawa Electric Co., Ltd. | Multilayer insulated wire and transformer using the same |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3073545B2 (en) * | 1990-05-23 | 2000-08-07 | 株式会社フジクラ | Insulated wire and cable using this |
| JPH04108810U (en) * | 1991-03-07 | 1992-09-21 | 古河電気工業株式会社 | insulated wire |
| JP3233655B2 (en) * | 1991-05-24 | 2001-11-26 | 株式会社フジクラ | Flame retardant electrical cable |
| JP5258022B2 (en) * | 2008-02-18 | 2013-08-07 | 古河マグネットワイヤ株式会社 | Insulated wire for coil |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3294604A (en) * | 1960-12-20 | 1966-12-27 | Anaconda Wire & Cable Co | Method of making electric cable having compressed insulation |
| US3217084A (en) * | 1960-12-20 | 1965-11-09 | Anaconda Wire & Cable Co | Electric cable having compressed insulation |
| US4184001A (en) * | 1978-04-19 | 1980-01-15 | Haveg Industries, Inc. | Multi layer insulation system for conductors comprising a fluorinated copolymer layer which is radiation cross-linked |
| EP0040034A1 (en) * | 1980-05-08 | 1981-11-18 | BICC Limited | Insulated wires and electric cables |
| JPS57130304A (en) * | 1981-02-02 | 1982-08-12 | Chiyanpurein Cable Corp | Insulating system for wire or cable |
-
1983
- 1983-09-14 DE DE8383305380T patent/DE3365309D1/en not_active Expired
- 1983-09-14 JP JP58170544A patent/JPS5973807A/en active Granted
- 1983-09-14 GB GB08324662A patent/GB2127210B/en not_active Expired
- 1983-09-14 CA CA000436688A patent/CA1214528A/en not_active Expired
- 1983-09-14 AT AT83305380T patent/ATE21462T1/en not_active IP Right Cessation
- 1983-09-14 EP EP83305380A patent/EP0103487B1/en not_active Expired
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0151904A1 (en) * | 1984-02-08 | 1985-08-21 | HUBER & SUHNER AG KABEL-, KAUTSCHUK-, KUNSTSTOFF-WERKE | Insulated electric cord |
| WO1989000757A1 (en) * | 1987-07-10 | 1989-01-26 | Raychem Limited | Electrical wire |
| WO1989000758A1 (en) * | 1987-07-10 | 1989-01-26 | Raychem Limited | Electrical wire |
| EP0301543A3 (en) * | 1987-07-29 | 1990-05-09 | Sumitomo Electric Industries, Limited | Resinous composition and its use |
| US6296935B1 (en) * | 1996-08-22 | 2001-10-02 | The Furukawa Electric Co., Ltd. | Multilayer insulated wire and transformer using the same |
| WO1999004402A1 (en) * | 1997-07-14 | 1999-01-28 | Draka Uk Limited | Co-axial cable |
Also Published As
| Publication number | Publication date |
|---|---|
| CA1214528A (en) | 1986-11-25 |
| DE3365309D1 (en) | 1986-09-18 |
| EP0103487B1 (en) | 1986-08-13 |
| GB8324662D0 (en) | 1983-10-19 |
| GB2127210B (en) | 1986-01-22 |
| ATE21462T1 (en) | 1986-08-15 |
| EP0103487A3 (en) | 1984-08-01 |
| JPS5973807A (en) | 1984-04-26 |
| JPH0517642B2 (en) | 1993-03-09 |
| GB2127210A (en) | 1984-04-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4521485A (en) | Electrical insulation | |
| US4678709A (en) | Electrical insulation | |
| US6359230B1 (en) | Automotive-wire insulation | |
| US3269862A (en) | Crosslinked polyvinylidene fluoride over a crosslinked polyolefin | |
| CA1221146A (en) | Flame retarded cladding | |
| US5296558A (en) | Polymeric composition | |
| EP0103487B1 (en) | Electrical insulation | |
| WO1998005046A1 (en) | Insulated electrical conductors | |
| US3607387A (en) | Flame resistant polyimide-coated conductor having a linear polyimide layer covered by an aromatic polyamide | |
| DE3888537T2 (en) | ELECTRIC WIRE. | |
| EP0606319A1 (en) | Electrical wire | |
| US4705823A (en) | Extrudable blend | |
| US5437930A (en) | Cable for high operating temperatures | |
| EP0366700B1 (en) | Electrical wire | |
| US4592955A (en) | Insulating covering for strand material | |
| EP0040034A1 (en) | Insulated wires and electric cables | |
| EP0222507B1 (en) | Shaped articles of crosslinked polymers | |
| AU606731B2 (en) | Electrical wire with polyamide/fluoropolymer insulation | |
| JP3233655B2 (en) | Flame retardant electrical cable | |
| EP0010586B2 (en) | Flame retardant insulating material for an electrical cable and electrical cable made with such a material | |
| US4656091A (en) | Insulating material for telephone cords and telephone cords incorporating same | |
| JPH04341709A (en) | Insulated wire | |
| JPH11504365A (en) | Polyester composition | |
| JP3654536B2 (en) | coaxial cable | |
| CA1195031A (en) | Flame retardant wire with high insulation resistance |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19830927 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR IT LI NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR IT LI NL SE |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR IT LI NL SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Effective date: 19860813 |
|
| REF | Corresponds to: |
Ref document number: 21462 Country of ref document: AT Date of ref document: 19860815 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3365309 Country of ref document: DE Date of ref document: 19860918 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19900921 Year of fee payment: 8 |
|
| ITTA | It: last paid annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19900930 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19901005 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19910915 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19910930 |
|
| BERE | Be: lapsed |
Owner name: RAYCHEM CORP. Effective date: 19910930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19920401 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19920909 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19920918 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19920923 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Effective date: 19930930 Ref country code: CH Effective date: 19930930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19940531 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19940601 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| EUG | Se: european patent has lapsed |
Ref document number: 83305380.4 Effective date: 19920408 |