EP0091047B1 - Fuel for a carburettor engine - Google Patents
Fuel for a carburettor engine Download PDFInfo
- Publication number
- EP0091047B1 EP0091047B1 EP83103030A EP83103030A EP0091047B1 EP 0091047 B1 EP0091047 B1 EP 0091047B1 EP 83103030 A EP83103030 A EP 83103030A EP 83103030 A EP83103030 A EP 83103030A EP 0091047 B1 EP0091047 B1 EP 0091047B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- phenol
- oil
- gasoline
- extraction
- boiling
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/04—Liquid carbonaceous fuels essentially based on blends of hydrocarbons
- C10L1/06—Liquid carbonaceous fuels essentially based on blends of hydrocarbons for spark ignition
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G19/00—Refining hydrocarbon oils in the absence of hydrogen, by alkaline treatment
- C10G19/02—Refining hydrocarbon oils in the absence of hydrogen, by alkaline treatment with aqueous alkaline solutions
Definitions
- the invention relates to a method for producing a low-benzene, environmentally friendly gasoline from light coal oil.
- the conversion of coal to gasoline (gasoline) is preferably done in two stages. In the first stage, the bottom phase, the coal is converted into an intermediate product (medium oil and crude gasoline) in the presence of finely divided catalysts using hydrogen under suitable pressure and temperature, which is then converted into gasoline fuel in subsequent stages using further catalysts.
- Carburetor fuels obtained in this way are highly aromatic because of the molecular structure of the coal from condensed aromatics.
- the benzene content in particular is between 10 and 20% by weight. This high benzene content is undesirable because of the toxic properties of benzene.
- the benzene content in carburetor fuels is or is being striven for.
- the benzene content in gasoline in the Federal Republic of Germany should be limited to 5 percent by weight.
- the invention has for its object to produce a low-benzol gasifier fuel from light coal oil, which is independent of the availability of low-benzol admixture components.
- a core fraction of the boiling point 145-185 ° C. is distilled off from the crude light coal oil.
- the fraction contains the majority of the phenol contained in the coal oil.
- the phenol can be separated from this core fraction and extracted by extraction with aqueous sodium hydroxide solution or sodium phenolate solution, preferably in a three-stage mixer-settler extractor.
- the remaining phenol-free raffinate of the core fraction is mixed with the portion of light coal oil, boiling up to 145 ° C, and by refining z.
- B pressure 60 bar, temperature 410 ° C load 1.5 kg oil / kg cat. H) prepared for reformer application specification. Reforming (e.g. pressure 15 bar, temperature 480 ° C, load 1.5 kg oil / kg cat. H) results in a low-gasoline gasoline fuel with a high octane number.
- the benzene content of the reformate is reduced to approximately 5% by weight and the process stages of refining and reformer are relieved in terms of quantity.
- the phenol is not converted into worthless water and benzene by the hydrogenation reactions or, depending on the degree of hydrogenation, into cyclohexane, valuable hydrogen is saved in this stage.
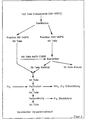
- FIG. 1 shows the procedure of the method according to the invention.
- Examples 1 to 3 give experimental data on how the benzene content is reduced in the process according to the invention, the quantity of product flow in the refining and in the reformer is relieved and how the hydrogen consumption is reduced for the hydrogenating removal of the phenols while simultaneously hydrogenating the benzene to cyclohexane.
- a core fraction in the boiling range 145-185 ° C. which contains the majority of the phenol present in the light coal oil, are distilled off from 100 parts of crude light coal oil by refractionation.
- This core fraction is de-phenolized by 3-stage extraction with 69 parts of 12% aqueous NaOH in a mixer-settler extractor and the fraction freed from phenol is then mixed again with the light coal oil (boiling point - 145 ° C).
- the phenol-free light coal oil is processed by refining and reforming on gasoline. The procedure described lowers the benzene content of the gasoline fuel to 2.6% by weight. If the light coal oil is not de-phenolic before processing in the refiner and reformer, the benzene content in the gasoline is 17.1% by weight.
- 100 parts of light coal oil which contain 16% by weight of phenol, are broken down into two fractions - boiling from the beginning of boiling to 145 ° C, 55 parts or 45 parts from 145 to 185 ° C.
- the phenol is enriched to 35% by weight.
- the feed oil for the refiner / reformer still contains 0.8% by weight of phenol. Due to the extraction of the phenol, only 84 parts of the original 100 parts of light coal oil have to be processed in the refiner / reformer, ie this system is relieved in terms of quantity.
- the hydrogen consumption in refining is reduced by 130 I / kg feed oil compared to processing of the crude, non-de-phenolic light coal oil, since the phenol has been removed and thus the hydrogen requirement for the hydrogenating phenol removal is eliminated.
- a 3-stage mixer-settling extraction apparatus is used to remove phenols from light coal oil using aqueous sodium hydroxide solution.
- stage I fresh sodium hydroxide solution is introduced into the mixer zone by means of a metering pump and pre-extracted light coal oil via a forced flow in stage 11.
- stage II the simply pre-extracted light coal oil from stage 111 is extracted with the stage 1 extract, which is partially loaded with phenols.
- stage III the raw coal light oil is conveyed by means of a metering pump and pre-extracted with the extract (phenol-containing sodium hydroxide solution) from the first two stages.
- the lighter, organic phase is led to stage 11.
- the heavy phase is removed from the process as a phenol-containing extract to recover the phenols.
- the light coal oil to be refined is introduced into the reaction system by means of a metering pump 1.
- Compressed make-up hydrogen is metered in before the preheater 2, in which the components are heated to a temperature slightly below the reaction temperature.
- the implementation takes place in a fixed bed reactor, which is electrically heated by a heating jacket with several control loops.
- On the catalytic converter all organic sulfur, nitrogen and oxygen compounds contained in the oil are converted into hydrogen sulfide, ammonia, water and hydrocarbons, and gaseous decomposition products are also formed.
- the product stream leaving the reactor is separated into gas and liquid phases in high-pressure separator 5.
- the gas phase is expanded via a gas expansion valve 8. It mainly consists of excess hydrogen, hydrogen sulfide and small amounts of C i -C 4 gases.
- the liquid phase flows through an expansion valve 6 into a water-oil separating container 7.
- the raffinate separates from the water of reaction due to the density differences, and also small amounts of dissolved gases are released as stripping gas.
- the system according to FIG. 4 includes control loops with mass measurement WIR, volume measurement FIR, pressure measurement PI, temperature measurement TIRC, maintenance LIRC and pressure control PRC. Since, when the refined light coal oil is reformed, the reformer contact is quickly deactivated by oxygen and excessive water vapor partial pressure, the feed oil according to FIG. 5 is pumped through a deoxidizer 11 and dryer 12 by means of a pump 10. Subsequently, compressed hydrogen is added before a preheater 13, which heats the mixture to the reaction temperature. The following fixed bed reactor 14 must be heated from the outside to maintain the reaction. In the reforming, aromatics are formed from paraffinic and naphthenic hydrocarbons with the evolution of hydrogen.
- the oil After expansion, the oil is pumped into a stabilizing column 18 by means of expansion valve 17, in which low-boiling hydrocarbons are removed by distillation.
- the associated pump is designated 19.
- the distillation sump is the aromatic-rich reformate.
- the gaseous phase from the high-pressure separator is freed of organic constituents under system pressure in a gas scrubber and gas dryer 20 and is returned as a cycle gas by means of a cycle gas compressor 21.
- the hydrogen formed during the reforming leaves the process via a valve 22 as excess gas.
- control devices belonging to the system according to FIG. 5 have the same designations as the control devices according to FIG. 4.
Description
Die Erfindung betrifft ein Verfahren zur Herstellung eines benzolarmen, umweltfreundlichen Vergaserkraftstoffes aus Kohleleichtöl. Die Umwandlung von Kohle zu Vergaserkraftstoffen (Benzin) erfolgt vorzugsweise in zwei Stufen. In der ersten Stufe, der Sumpfphase wird die Kohle in Gegenwart fein verteilter Katalysatoren mittels Wasserstoff unter geeignetem Druck und Temperatur in ein Zwischenprodukt (Mittelöl und Rohbenzin) übergeführt, das dann in nachgeschalteten Stufen mittels weiterer Katalysatoren in Vergaserkraftstoff umgewandelt wird. Solchermaßen gewonnene Vergaserkraftstoffe sind wegen des molekularen Aufbaus der Kohle aus kondensierten Aromaten hocharomatisch. Insbesondere der Benzolgehalt liegt je nach den Verfahrensbedingungen im Reformer zwischen 10 und 20 Gew.- %. Dieser hohe Benzolgehalt ist wegen der toxischen Eigenschaften von Benzol unerwünscht. In einigen Ländern besteht eine Limitierung des Benzolgehaltes in Vergaserkraftstoffen bzw. wird das angestrebt. Z. B. soll der Benzolgehalt im Benzin in der Bundesrepublik Deutschland auf 5 Gewichtsprozente beschränkt werden.The invention relates to a method for producing a low-benzene, environmentally friendly gasoline from light coal oil. The conversion of coal to gasoline (gasoline) is preferably done in two stages. In the first stage, the bottom phase, the coal is converted into an intermediate product (medium oil and crude gasoline) in the presence of finely divided catalysts using hydrogen under suitable pressure and temperature, which is then converted into gasoline fuel in subsequent stages using further catalysts. Carburetor fuels obtained in this way are highly aromatic because of the molecular structure of the coal from condensed aromatics. Depending on the process conditions in the reformer, the benzene content in particular is between 10 and 20% by weight. This high benzene content is undesirable because of the toxic properties of benzene. In some countries, the benzene content in carburetor fuels is or is being striven for. For example, the benzene content in gasoline in the Federal Republic of Germany should be limited to 5 percent by weight.
Maßnahmen zur Senkung des Benzolgehaltes laufen darauf hinaus, daß durch Verschneiden mit benzolarmen Vergaserkraftstoffen die Konzentration von Benzol abgesenkt wird. Das setzt voraus, daß benzolarmer Vergaserkraftstoff, meist mineralölstämmigen Ursprungs, zur Verfügung steht, was bei einer autark arbeitenden Kohleverflüssigungsanlage nicht der Fall sein wird.Measures to lower the benzene content boil down to the fact that the concentration of benzene is reduced by blending with low-gasoline gasoline. This presupposes that low-gasoline gasoline, mostly of mineral oil origin, is available, which will not be the case with a self-sufficient coal liquefaction plant.
Der Erfindung liegt die Aufgabe zugrunde, einen benzolarmen Vergaserkraftstoff aus Kohleleichtöl herzustellen, der unabhängig von der Verfügbarkeit benzolarmer Zumischkomponenten ist.The invention has for its object to produce a low-benzol gasifier fuel from light coal oil, which is independent of the availability of low-benzol admixture components.
Nach der Erfindung wird das dadurch erreicht, daß aus dem rohen Kohleleichtöl eine Kernfraktion der Siedelage 145-185 °C, vorzugsweise über eine Füllkörperkolonne, abdestilliert wird. Die Fraktion enthält die Hauptmenge des im Kohleöl enthaltenen Phenols. Durch Extraktion mit wässriger Natronlauge oder Natriumphenolatlauge, vorzugsweise in einem dreistufigen Mixer-Settler-Extraktor, läßt sich das Phenol aus dieser Kernfraktion abtrennen und gewinnen. Das verbleibende phenolfreie Raffinat der Kernfraktion wird dem Anteil des Kohleleichtöls, Siedebeginn bis 145 °C, zugemischt und durch Raffination z. B. Druck 60 bar, Temperatur 410 °C Belastung 1,5 kg Öl/kg Kat. h) auf Reformer einsatzspezifikation aufbereitet. Durch Reforming (z. B. Druck 15 bar, Temperatur 480 °C, Belastung 1,5 kg Öl/kg Kat. h) entsteht ein benzoiarmer Vergaserkraftstoff hoher Oktanzahl.According to the invention this is achieved in that a core fraction of the boiling point 145-185 ° C., preferably via a packed column, is distilled off from the crude light coal oil. The fraction contains the majority of the phenol contained in the coal oil. The phenol can be separated from this core fraction and extracted by extraction with aqueous sodium hydroxide solution or sodium phenolate solution, preferably in a three-stage mixer-settler extractor. The remaining phenol-free raffinate of the core fraction is mixed with the portion of light coal oil, boiling up to 145 ° C, and by refining z. B. pressure 60 bar, temperature 410 ° C load 1.5 kg oil / kg cat. H) prepared for reformer application specification. Reforming (
Vorteilhafterweise wird bei dem erfindungsgemäßen Verfahren zugleich ein wertvoller Chemierohstoff (Phenol) gewonnen, der im großen Maßstab in der Kunststoffindustrie Verwendung findet.In the process according to the invention, a valuable chemical raw material (phenol) is advantageously obtained at the same time, which is used on a large scale in the plastics industry.
Durch die beschriebene Arbeitsweise wird der Benzolgehalt des Reformates auf ca. 5 Gew.-% abgesenkt und werden die Verfahrensstufen der Raffination und Reformer mengenmäßig entlastet. Da außerdem das Phenol nicht durch die Hydrierreaktionen in wertloses Wasser und Benzol bzw. je nach Hydrierschärfe in Cyclohexan überführt wird, wird in dieser Stufe wertvoller Wasserstoff eingespart.As a result of the procedure described, the benzene content of the reformate is reduced to approximately 5% by weight and the process stages of refining and reformer are relieved in terms of quantity. In addition, since the phenol is not converted into worthless water and benzene by the hydrogenation reactions or, depending on the degree of hydrogenation, into cyclohexane, valuable hydrogen is saved in this stage.
Im Blockschema nach Figur 1 ist die Verfahrensführung des erfindungsgemäßen Verfahrens dargestellt.The block diagram according to FIG. 1 shows the procedure of the method according to the invention.
Beispiel 1 bis 3 geben experimentelle Daten wieder, wie beim erfindungsgemäßen Verfahren der Benzolgehalt abgesenkt, der Produktstrom in der Raffination und im Reformer mengenmäßig entlastet wird und wie für die hydrierende Entfernung der Phenole bei gleichzeitiger Hydrierung des Benzols zu Cyclohexan der Wasserstoffverbrauch reduziert wird.Examples 1 to 3 give experimental data on how the benzene content is reduced in the process according to the invention, the quantity of product flow in the refining and in the reformer is relieved and how the hydrogen consumption is reduced for the hydrogenating removal of the phenols while simultaneously hydrogenating the benzene to cyclohexane.
Gemäß dem in Figur 2 dargestellten Mengenfließschema werden aus 100 Teilen rohem Kohleleichtöl durch Refraktionierung 45 Teile einer Kernfraktion im Siedebereich 145-185 °C abdestilliert, welche die Hauptmenge des im Kohleleichtöl vorliegenden Phenols enthält. Diese Kernfraktion wird durch 3stufige Extraktion mit 69 Teilen 12 %iger wässriger NaOH in einem Mixer-Settler-Extraktor entphenolt und die von Phenol befreite Fraktion anschließend dem Kohleleichtöl (Siedebeginn - 145 °C) wieder zugemischt. Das phenolfreie Kohleleichtöl wird durch Raffination und Reformierung auf Vergaserkraftstoff verarbeitet. Durch die beschriebene Arbeitsweise wird der Benzolgehalt des Vergaserkraftstoffes auf 2,6 Gew.-% abgesenkt. Wird das Kohleleichtöl vor der Verarbeitung im Refiner und Reformer nicht entphenolt, so liegt der Benzolgehalt im Vergaserkraftstoff bei 17,1 Gew.-%.According to the flow diagram shown in FIG. 2, 45 parts of a core fraction in the boiling range 145-185 ° C., which contains the majority of the phenol present in the light coal oil, are distilled off from 100 parts of crude light coal oil by refractionation. This core fraction is de-phenolized by 3-stage extraction with 69 parts of 12% aqueous NaOH in a mixer-settler extractor and the fraction freed from phenol is then mixed again with the light coal oil (boiling point - 145 ° C). The phenol-free light coal oil is processed by refining and reforming on gasoline. The procedure described lowers the benzene content of the gasoline fuel to 2.6% by weight. If the light coal oil is not de-phenolic before processing in the refiner and reformer, the benzene content in the gasoline is 17.1% by weight.
100 Teile Kohleleichtöl, welche 16 Gew.-% Phenol enthalten, werden in zwei Fraktionen - siedend von Siedebeginn bis 145 °C 55 Teile bzw. 45 Teile von 145 bis 185 °C - zerlegt. In der Kernfraktion 145 bis 185 °C ist das Phenol auf 35 Gew.-% angereichert. Das Einsatzöl für den Refiner/Reformer enthält nach der Vereinigung des Raffinats aus der Extraktion mit der Fraktion Siedebeginn- 145 °C noch 0,8 Gew.-% Phenol. Bedingt durch die Extraktion des Phenols müssen im Refiner/Reformer nur noch 84 Teile der ursprünglichen 100 Teile Kohleleichtöl verarbeitet werden, d. h. diese Anlage wird mengenmäßig entlastet. Der Wasserstoffverbrauch in der Raffination vermindert sich um 130 I/kg Einsatzöl gegenüber der Verarbeitung des rohen, nicht entphenolten Kohleleichtöls, da das Phenol entfernt wurde und damit der Wasserstoffbedarf für die hydrierende Phenolentfernung entfällt.100 parts of light coal oil, which contain 16% by weight of phenol, are broken down into two fractions - boiling from the beginning of boiling to 145 ° C, 55 parts or 45 parts from 145 to 185 ° C. In the core fraction 145 to 185 ° C, the phenol is enriched to 35% by weight. After the raffinate from the extraction has been combined with the fraction at the beginning of boiling at 145 ° C., the feed oil for the refiner / reformer still contains 0.8% by weight of phenol. Due to the extraction of the phenol, only 84 parts of the original 100 parts of light coal oil have to be processed in the refiner / reformer, ie this system is relieved in terms of quantity. The hydrogen consumption in refining is reduced by 130 I / kg feed oil compared to processing of the crude, non-de-phenolic light coal oil, since the phenol has been removed and thus the hydrogen requirement for the hydrogenating phenol removal is eliminated.
Aus 100 Teilen rohem Kohleleichtöl mit 16 Teilen Phenol werden durch Destillation 45 Teile einer phenolreichen Fraktion 145-185 °C siedend gewonnen. Der Phenolgehalt in dieser Fraktion ist durch die Destillation auf 35 Gew.-% angereichert. Durch Extraktion mit 12 %iger Natronlauge wird Phenol aus dieser Kernfraktion extrahiert und durch Ansäuern mit stöchiometrischen Mengen verdünnter Schwefelsäure werden die vorher extrahierten Phenole, das sind 15 Teile Phenol freigesetzt. Bezogen auf Kohleleichtöl sind dies 94 Gew.-% des im rohen Kohleleichtöl vorhandenen Phenols.From 100 parts of crude light coal oil with 16 parts of phenol, 45 parts of a phenol-rich fraction at 145-185 ° C. are boiled by distillation. The phenol content in this fraction is enriched to 35% by weight by distillation. Phenol is extracted from this core fraction by extraction with 12% sodium hydroxide solution and the previously extracted phenols, that is 15 parts of phenol, are released by acidification with stoichiometric amounts of dilute sulfuric acid. Based on light coal oil, this is 94% by weight of the phenol present in the raw light coal oil.
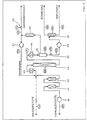
Zur Entfernung von Phenolen aus Kohleleichtöl mittels wässriger Natronlauge wird gemäß Figur 3 eine 3-stufige-Mixer-Settier-Extraktionsapparatur benutzt.According to FIG. 3, a 3-stage mixer-settling extraction apparatus is used to remove phenols from light coal oil using aqueous sodium hydroxide solution.
In die Stufe I wird frische Natronlauge mittels einer Dosierpumpe und vorextrahiertes Kohleleichtöl über einen Zwangsablauf der Stufe 11 in die Mixer-Zone eingebracht.In stage I, fresh sodium hydroxide solution is introduced into the mixer zone by means of a metering pump and pre-extracted light coal oil via a forced flow in
Durch intensives Rühren wird der Stoffaustausch, zwischen den beiden, ineinander fast unlöslichen Phasen bis zur Phasengleichgewichtseinstellung herbeigeführt, d. h. die im Kohleöl enthaltenen Phenole lösen sich in der wässrigen Natronlauge. Im Mittelteil des Extraktors trennt sich das inhomogene Gemisch aufgrund unterschiedlicher Dichten in das leichtere, organische Raffinat (entphenoltes Kohleleichtöl) und den schweren Extrakt (phenolhaltige Natronlauge). Die beiden Phasen werden getrennt abgezogen. Die obere Phase ist das angestrebte, phenolfreie Raffinat, die untere Phase fließt zur Stufe 11.By intensive stirring, the mass transfer between the two, almost insoluble, phases into each other is brought about until the phase equilibrium is reached, i.e. H. The phenols contained in the coal oil dissolve in the aqueous sodium hydroxide solution. In the middle section of the extractor, the inhomogeneous mixture separates into the lighter, organic raffinate (de-phenolic carbon oil) and the heavy extract (phenol-containing sodium hydroxide solution) due to different densities. The two phases are subtracted separately. The upper phase is the target, phenol-free raffinate, the lower phase flows to
In Stufe II wird nach der bereits beschriebenen Arbeitsweise das einfach vorextrahierte Kohleleichtöl aus Stufe 111 mit dem teilweise mit Phenolen beladenen Extrakt der Stufe 1 extrahiert.In stage II, the simply pre-extracted light coal oil from stage 111 is extracted with the stage 1 extract, which is partially loaded with phenols.
In die Stufe III wird mittels Dosierpumpe das rohe Kohleleichtöl befördert und mit dem Extrakt (phenolhaltige Natronlauge) der ersten beiden Stufen vorextrahiert. Die leichtere, organische Phase wird zur Stufe 11 geführt. Die schwere Phase wird als phenolhaltiger Extrakt aus dem Prozess ausgeschleust, zur Rückgewinnung der Phenole.In stage III, the raw coal light oil is conveyed by means of a metering pump and pre-extracted with the extract (phenol-containing sodium hydroxide solution) from the first two stages. The lighter, organic phase is led to
Nach Figur 4 wird das zu raffinierende Kohleleichtöl mittels Dosierpumpe 1 in das Reaktionssystem eingebracht. Vor dem Vorheizer 2, in dem die Komponenten auf eine Temperatur wenig unterhalb der Reaktionstemperatur erhitzt werden, wird komprimierter make up Wasserstoff zudosiert. Die Umsetzung erfolgt in einem Festbettreaktor, der durch einen Heizmantel mit mehreren Regelkreisen elektrisch beheizt wird. Am Katalysator werden sämtliche im Öl enthaltenen organischen Schwefel-, Stickstoff- und Sauerstoffverbindungen in Schwefelwasserstoff, Ammoniak, Wasser und Kohlenwasserstoffe umgewandelt, außerdem bilden sich gasförmige Abbauprodukte. Der den Reaktor verlassende Produktstrom wird nach Kühlung 4, im Hochdruckabscheider 5 in Gas- und Flüssigphase getrennt. Die Gasphase wird über ein Gasentspannunngsventil 8 entspannt. Sie besteht vorwiegend aus überschüssigem Wasserstoff, Schwefelwasserstoff und geringen Anteilen Ci-C4-Gasen.According to FIG. 4, the light coal oil to be refined is introduced into the reaction system by means of a metering pump 1. Compressed make-up hydrogen is metered in before the
Die Flüssigphase fließt durch ein Entspannungsventil 6 in einen Wasser-ÖI-Trennbehälter 7. Hier trennt sich aufgrund der Dichteunterschiede das Raffinat vom Reaktionswasser, außerdem werden geringe Mengen gelöste Gase als Abstreifergas frei.The liquid phase flows through an expansion valve 6 into a water-oil separating
Zu der Anlage nach Figur 4 gehören Regelkreise mit Massenmessung WIR, Volumenmessung FIR, Druckmessung Pl, Temperaturmessung TIRC, Standhaltung LIRC und Drucksteuerung PRC. Da beim Reformieren des raffinierten Kohleleichtöls der Reformerkontakt durch Sauerstoff und zu hohen Wasser dampfpartialdruck schnell desaktiviert, wird das Einsatzöl nach Figur 5 mittels einer Pumpe 10 durch einen Entoxidierer 11 und Trockner 12 gepumpt. Anschließend erfolgt die Zumischung von komprimiertem Wasserstoff vor einem Vorheizer 13, der das Gemisch auf die Reaktionstemperatur erhitzt. Der folgende Festbettreaktor 14 muß zur Erhaltung der Reaktion von außen beheizt werden. Bei der Reformierung werden aus paraffinischen und naphthenischen Kohlenwasserstoffen unter Wasserstoffentwicklung Aromaten gebildet.The system according to FIG. 4 includes control loops with mass measurement WIR, volume measurement FIR, pressure measurement PI, temperature measurement TIRC, maintenance LIRC and pressure control PRC. Since, when the refined light coal oil is reformed, the reformer contact is quickly deactivated by oxygen and excessive water vapor partial pressure, the feed oil according to FIG. 5 is pumped through a
Nach Kühlung des Reaktionsgemisches in einem Kühler 15 trennt sich im Hochdruckabscheider 16 das Öl von der Gasphase.After cooling the reaction mixture in a
Das Öl wird nach Entspannung mittels Entspannungsventil 17 in eine Stabilkolonne 18 gepumpt, in welcher leichtsiedende Kohlenwasserstoffe destillativ entfernt werden. Die zugehörige Pumpe ist mit 19 bezeichnet. Der Destillationssumpf ist das aromatenreiche Reformat. Die gasförmige Phase aus dem Hochdruckabscheider wird unter Systemdruck in einer Gaswäsche und Gastrockner 20 von organischen Bestandteilen befreit und mittels Kreisgaskompressor 21 als Kreislaufgas zurückgefahren. Der bei der Reformierung gebildete Wasserstoff verläßt über ein Ventil 22 als Überschußgas den Prozeß.After expansion, the oil is pumped into a stabilizing
Die zur Anlage nach Figur 5 gehörenden Regeleinrichtungen tragen die gleichen Bezeichnungen wie die Regeleinrichtungen nach Figur 4.The control devices belonging to the system according to FIG. 5 have the same designations as the control devices according to FIG. 4.
Claims (3)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19823213220 DE3213220A1 (en) | 1982-04-08 | 1982-04-08 | CARBURETOR FUEL |
| DE3213220 | 1982-04-08 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0091047A2 EP0091047A2 (en) | 1983-10-12 |
| EP0091047A3 EP0091047A3 (en) | 1985-01-09 |
| EP0091047B1 true EP0091047B1 (en) | 1987-03-04 |
Family
ID=6160604
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP83103030A Expired EP0091047B1 (en) | 1982-04-08 | 1983-03-26 | Fuel for a carburettor engine |
Country Status (8)
| Country | Link |
|---|---|
| EP (1) | EP0091047B1 (en) |
| AU (1) | AU556607B2 (en) |
| CA (1) | CA1206908A (en) |
| DE (2) | DE3213220A1 (en) |
| IN (1) | IN158910B (en) |
| NZ (1) | NZ203807A (en) |
| SU (1) | SU1172452A3 (en) |
| ZA (1) | ZA832467B (en) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3410455A1 (en) * | 1984-03-22 | 1985-10-03 | Ruhrkohle Ag, 4300 Essen | METHOD FOR PRODUCING A GASIFYING FUEL FROM CARBON OIL |
| CN101429449B (en) * | 2007-11-09 | 2012-10-10 | 丁冉峰 | System and method for catalyzing hydrocarbon for recombinant production of high-quality gasoline |
| CN101429446B (en) * | 2007-11-09 | 2012-10-10 | 丁冉峰 | System and method for catalyzing hydrocarbon for recombinant production of high-quality gasoline |
| CN101429442B (en) * | 2007-11-09 | 2013-02-06 | 丁冉峰 | System and method for catalyzing hydrocarbon for recombinant production of high-quality gasoline |
| CN101429447B (en) * | 2007-11-09 | 2012-11-14 | 丁冉峰 | System and method for catalyzing hydrocarbon for recombinant production of high-quality gasoline |
| CN101429444B (en) * | 2007-11-09 | 2012-11-14 | 丁冉峰 | System and method for catalyzing hydrocarbon for recombinant production of high-quality gasoline |
| CN101429443B (en) * | 2007-11-09 | 2012-08-22 | 丁冉峰 | System and method for catalyzing hydrocarbon for recombinant production of high-quality gasoline |
| CN101429448B (en) * | 2007-11-09 | 2012-11-14 | 丁冉峰 | System and method for catalyzing hydrocarbon for recombinant production of high-quality gasoline |
| CN101475835B (en) * | 2009-01-22 | 2012-09-05 | 北京金伟晖工程技术有限公司 | System and method for preparing high quality petrol by hydrogenation after component oil refining hydrocarbon recombination |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB107454A (en) * | 1916-07-31 | 1917-07-05 | George Edward Heyl | Improvements in Liquid Fuels. |
| US4319981A (en) * | 1980-11-12 | 1982-03-16 | The United States Of America As Represented By The United States Department Of Energy | Process for preparing a liquid fuel composition |
-
1982
- 1982-04-08 DE DE19823213220 patent/DE3213220A1/en not_active Withdrawn
-
1983
- 1983-03-26 EP EP83103030A patent/EP0091047B1/en not_active Expired
- 1983-03-26 DE DE8383103030T patent/DE3370022D1/en not_active Expired
- 1983-03-31 AU AU13094/83A patent/AU556607B2/en not_active Ceased
- 1983-04-06 NZ NZ203807A patent/NZ203807A/en unknown
- 1983-04-07 IN IN234/DEL/83A patent/IN158910B/en unknown
- 1983-04-07 CA CA000425386A patent/CA1206908A/en not_active Expired
- 1983-04-07 SU SU833582281A patent/SU1172452A3/en active
- 1983-04-08 ZA ZA832467A patent/ZA832467B/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| NZ203807A (en) | 1986-02-21 |
| EP0091047A2 (en) | 1983-10-12 |
| ZA832467B (en) | 1983-12-28 |
| AU556607B2 (en) | 1986-11-13 |
| AU1309483A (en) | 1983-10-13 |
| SU1172452A3 (en) | 1985-08-07 |
| EP0091047A3 (en) | 1985-01-09 |
| CA1206908A (en) | 1986-07-02 |
| DE3213220A1 (en) | 1983-10-13 |
| DE3370022D1 (en) | 1987-04-09 |
| IN158910B (en) | 1987-02-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0091047B1 (en) | Fuel for a carburettor engine | |
| US3105095A (en) | Process of liquefaction of lignin | |
| US8197678B2 (en) | Refining coal-derived liquid from coal gasification, coking and other coal processing operations | |
| US2662843A (en) | Shale oil refining | |
| DE60208420T2 (en) | SULFUR REMOVAL PROCESS | |
| US3974073A (en) | Coal liquefaction | |
| US4761222A (en) | Method for separating normally liquid organic compounds | |
| US2847362A (en) | Two-stage treating process | |
| US2645655A (en) | Recovery and purification of oil soluble oxygenated compounds | |
| US1844362A (en) | Process of producing an antiknock compound | |
| DE2612449A1 (en) | PROCESS FOR THE REMOVAL OF SULFUR COMPOUNDS FROM HYDROCARBON FEED PRODUCTS | |
| US4382855A (en) | Process for removal of hydroxy- and/or mercapto-substituted hydrocarbons from coal liquids | |
| DE1270017B (en) | Process for the production of highly purified benzene | |
| US2754248A (en) | Refining hydrocarbon oils with sulfur dioxide | |
| CN1077870C (en) | Saturated hydrocarbon recovering and utilizing technology | |
| EP0209665B1 (en) | Coal hydrogenation process by liquid phase and fixed-bed catalyst hydrogenation | |
| US2719106A (en) | Champagnat | |
| US5964987A (en) | Neutral oil removal from natural cresylic acid mixtures | |
| DE3022158C2 (en) | Process for hydrogenating coal liquefaction | |
| DE1272921B (en) | Process for the catalytic high-pressure hydrogenation of a starting material containing essentially polynuclear aromatic hydrocarbons in two stages | |
| US3017346A (en) | Solvent extraction process using dimethyl hydrogen phosphite | |
| US2953523A (en) | Chemical process | |
| US2754252A (en) | Refining hydrocarbon oils with bf3 and perfluoroalkanoic acid | |
| EP0155456B1 (en) | Process for the preparation of a fuel for a carburetter engine from a light coal oil | |
| US2257547A (en) | Solvent treating mineral oils |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): BE DE FR GB IT NL |
|
| 17P | Request for examination filed |
Effective date: 19830928 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: VEBA OEL AG Owner name: RUHRKOHLE AKTIENGESELLSCHAFT |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): BE DE FR GB IT NL |
|
| ITF | It: translation for a ep patent filed |
Owner name: FIAMMENGHI - DOMENIGHETTI |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE DE FR GB IT NL |
|
| ET | Fr: translation filed | ||
| REF | Corresponds to: |
Ref document number: 3370022 Country of ref document: DE Date of ref document: 19870409 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19930911 Year of fee payment: 11 |
|
| ITTA | It: last paid annual fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19941201 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19950317 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19960331 Year of fee payment: 14 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19960507 Year of fee payment: 14 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Effective date: 19961129 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19970213 Year of fee payment: 15 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19970331 |
|
| BERE | Be: lapsed |
Owner name: VEBA OEL A.G. Effective date: 19970331 Owner name: RUHRKOHLE A.G. Effective date: 19970331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19971001 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 19971001 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980326 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19980326 |