EP0058751B1 - Use of n-oxyalkylated derivatives of aniline as polymer dissolving components in floor cleaning compositions - Google Patents
Use of n-oxyalkylated derivatives of aniline as polymer dissolving components in floor cleaning compositions Download PDFInfo
- Publication number
- EP0058751B1 EP0058751B1 EP81108390A EP81108390A EP0058751B1 EP 0058751 B1 EP0058751 B1 EP 0058751B1 EP 81108390 A EP81108390 A EP 81108390A EP 81108390 A EP81108390 A EP 81108390A EP 0058751 B1 EP0058751 B1 EP 0058751B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- aniline
- floor cleaning
- cleaning compositions
- dissolving components
- polymer dissolving
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 238000004140 cleaning Methods 0.000 title claims description 11
- 239000000203 mixture Substances 0.000 title claims description 4
- 229920000642 polymer Polymers 0.000 title claims description 4
- 125000002490 anilino group Chemical class [H]N(*)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 title 1
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 claims description 15
- 150000001448 anilines Chemical class 0.000 claims description 7
- 239000001257 hydrogen Substances 0.000 claims description 6
- 229910052739 hydrogen Inorganic materials 0.000 claims description 6
- 125000000217 alkyl group Chemical group 0.000 claims description 4
- 125000004432 carbon atom Chemical group C* 0.000 claims description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 2
- 125000004435 hydrogen atom Chemical class [H]* 0.000 claims 3
- 239000002904 solvent Substances 0.000 claims 1
- 239000000047 product Substances 0.000 description 7
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 6
- AFBPFSWMIHJQDM-UHFFFAOYSA-N N-methyl-N-phenylamine Natural products CNC1=CC=CC=C1 AFBPFSWMIHJQDM-UHFFFAOYSA-N 0.000 description 6
- 239000012459 cleaning agent Substances 0.000 description 6
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 4
- RNVCVTLRINQCPJ-UHFFFAOYSA-N o-toluidine Chemical compound CC1=CC=CC=C1N RNVCVTLRINQCPJ-UHFFFAOYSA-N 0.000 description 4
- RZXMPPFPUUCRFN-UHFFFAOYSA-N p-toluidine Chemical compound CC1=CC=C(N)C=C1 RZXMPPFPUUCRFN-UHFFFAOYSA-N 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 239000007795 chemical reaction product Substances 0.000 description 3
- CBCKQZAAMUWICA-UHFFFAOYSA-N 1,4-phenylenediamine Chemical compound NC1=CC=C(N)C=C1 CBCKQZAAMUWICA-UHFFFAOYSA-N 0.000 description 2
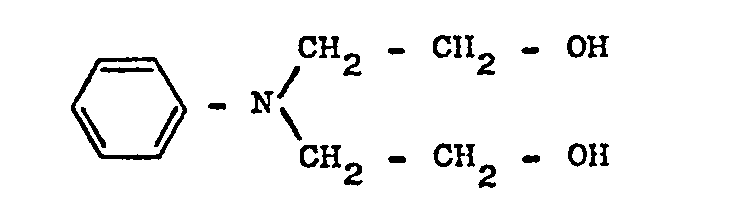
- OJPDDQSCZGTACX-UHFFFAOYSA-N 2-[n-(2-hydroxyethyl)anilino]ethanol Chemical compound OCCN(CCO)C1=CC=CC=C1 OJPDDQSCZGTACX-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000004040 coloring Methods 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- OAYXUHPQHDHDDZ-UHFFFAOYSA-N 2-(2-butoxyethoxy)ethanol Chemical compound CCCCOCCOCCO OAYXUHPQHDHDDZ-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 239000003945 anionic surfactant Substances 0.000 description 1
- 150000004982 aromatic amines Chemical class 0.000 description 1
- 150000007514 bases Chemical class 0.000 description 1
- 238000005282 brightening Methods 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- YRIUSKIDOIARQF-UHFFFAOYSA-N dodecyl benzenesulfonate Chemical compound CCCCCCCCCCCCOS(=O)(=O)C1=CC=CC=C1 YRIUSKIDOIARQF-UHFFFAOYSA-N 0.000 description 1
- 239000003651 drinking water Substances 0.000 description 1
- 235000020188 drinking water Nutrition 0.000 description 1
- 238000007046 ethoxylation reaction Methods 0.000 description 1
- 229940071106 ethylenediaminetetraacetate Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- -1 fatty acid triethanolamine salt Chemical class 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 239000012299 nitrogen atmosphere Substances 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229920000151 polyglycol Polymers 0.000 description 1
- 239000010695 polyglycol Substances 0.000 description 1
- BDAWXSQJJCIFIK-UHFFFAOYSA-N potassium methoxide Chemical compound [K+].[O-]C BDAWXSQJJCIFIK-UHFFFAOYSA-N 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- NESLWCLHZZISNB-UHFFFAOYSA-M sodium phenolate Chemical compound [Na+].[O-]C1=CC=CC=C1 NESLWCLHZZISNB-UHFFFAOYSA-M 0.000 description 1
- RPACBEVZENYWOL-XFULWGLBSA-M sodium;(2r)-2-[6-(4-chlorophenoxy)hexyl]oxirane-2-carboxylate Chemical compound [Na+].C=1C=C(Cl)C=CC=1OCCCCCC[C@]1(C(=O)[O-])CO1 RPACBEVZENYWOL-XFULWGLBSA-M 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000004575 stone Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000004018 waxing Methods 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/26—Organic compounds containing nitrogen
- C11D3/30—Amines; Substituted amines ; Quaternized amines
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/43—Solvents
Definitions
- N-phenyldiethanolamine is mainly used to dissolve the polymers in floor cleaning agents (self-wax wax removal) (prepared by adding 2 moles of ethylene oxide to aniline without adding any contact) (WE Draper, LP Johnson, Soap & Chemical Specialties January 1971, Page 38 to 44, 74 and 75).
- floor cleaning agents self-wax wax removal
- This blue color is unpleasant because it makes the original color bluish. Due to the cleaning, this blue coloring is not uniform, but leads to more or less intense coloring. Splashes of drops that are not removed result in deep blue color dots. These blue discolorations can only be removed very poorly or not at all.
- n assumes a value from 1 to 5, in particular from 1 to 3.
- N-oxalkylated derivatives of aniline can thus be used according to the invention as a polymer-dissolving component in floor cleaning agents: reaction products of o-toluidine, p-toluidine, p-phenylenediamine, N-methylaniline with the corresponding number of moles of ethylene oxide and / or propylene oxide, preferably reaction products of Aniline with the corresponding mole number of ethylene oxide and / or propylene oxide.
- the N-oxalkylated aniline derivatives in question can be prepared by adding the corresponding number of moles of ethylene oxide and / or propylene oxide to aniline and substituted aromatic amines in the presence of alkaline catalysts.
- alkaline catalysts Na methylate, Na ethylate, Na n-propylate, sodium hydroxide, potassium hydroxide, potassium methylate and sodium phenolate.
- aniline derivatives can be used as starting materials for the addition of ethylene oxide and / or propylene oxide: o-toluidine, p-toluidine, p-phenylenediamine, N-methylaniline.
- the most important effect of the products to be used according to the invention is that they do not show the disturbing blue color of the agent of the prior art. Furthermore, some of them have a significantly improved dissolving effect on the polymers used in self-waxing floors.
- PVC sheets that had been pretreated with commercially available floor care products were used for cleaning.
- the care products were colored with a dark color (R 28032 ex. Conc. BASF) for better evaluation of the cleaning tests.
- the Gardner scrubber was used for cleaning.
- the cleaning effect was determined with the filter color measuring device RFC 3 from Zeiss.
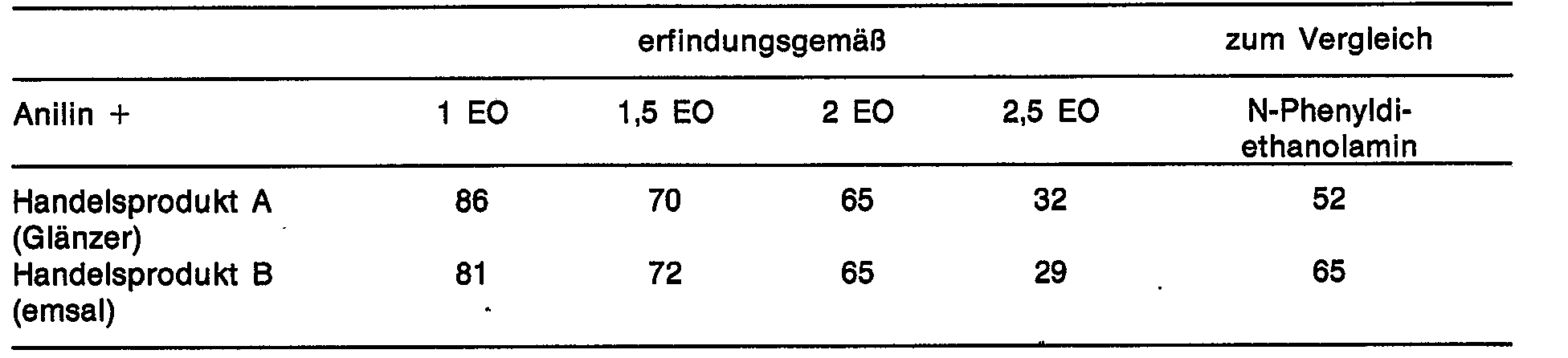
- Figure 1 shows the cleaning effect of various N-oxyalkylated aniline derivatives compared to N-phenyldiethanolamine.
- the floor cleaning agents in which the agent to be used according to the invention is used, consist i. a. from an anionic and / or nonionic surfactant and optionally an inorganic basic compound.
Description
Für die Lösung der Polymeren in Fußbodenreinigungsmitteln (Fußbodenselbstglanzwachsentfernung) wird hauptsächlich N-Phenyldiethanolamin
Es wurde nun gefunden, daß diese störenden Blaufärbungen nicht auftreten bei Verwendung von N-oxalkylierten Derivaten des Anilins der allgemeinen Formel
Bevorzugt eingesetzt werden Verbindungen der allgemeinen Formel
Es lassen sich erfindungsgemäß somit beispielsweise folgende N-oxalkylierte Derivate des Anilins als polymerlösende Komponente in Fußbodenreinigungsmitteln einsetzen : Umsetzungsprodukte von o-Toluidin, p-Toluidin, p-Phenylendiamin, N-Methylanilin mit der entsprechenden Molzahl Ethylenoxid und/oder Propylenoxid, vorzugsweise Umsetzungsprodukte von Anilin mit der entsprechenden Molzahl Ethylenoxid und/oder Propylenoxid.The following N-oxalkylated derivatives of aniline can thus be used according to the invention as a polymer-dissolving component in floor cleaning agents: reaction products of o-toluidine, p-toluidine, p-phenylenediamine, N-methylaniline with the corresponding number of moles of ethylene oxide and / or propylene oxide, preferably reaction products of Aniline with the corresponding mole number of ethylene oxide and / or propylene oxide.
Die betreffenden N-oxalkylierten Anilinderivate lassen sich herstellen durch Anlagerung der entsprechenden Molzahl Ethylenoxid und/oder Propylenoxid an Anilin sowie substituierte aromatische Amine in Gegenwart von alkalischen Katalysatoren. Als alkalische Katalysatoren lassen sich verwenden : Na-methylat, Na-ethylat, Na-n-propylat, Natriumhydroxid, Kaliumhydroxid, Kaliummethylat und Natriumphenolat.The N-oxalkylated aniline derivatives in question can be prepared by adding the corresponding number of moles of ethylene oxide and / or propylene oxide to aniline and substituted aromatic amines in the presence of alkaline catalysts. The following can be used as alkaline catalysts: Na methylate, Na ethylate, Na n-propylate, sodium hydroxide, potassium hydroxide, potassium methylate and sodium phenolate.
Unter Stickstoffatmosphäre wurden an 465 g (5 Mole) Anilin im Autoklaven bei ca. 140 °C und 3 bar in Gegenwart von 2,3 g Na-Methylat 440 g (10 Mole) Ethylenoxid angelagert. Nach beendeter Reaktion wurde eine halbe Stunde mit Stickstoff gespült und das angefallene Reaktionsprodukt ohne weitere Aufarbeitung eingesetzt.Under a nitrogen atmosphere, 440 g (10 moles) of ethylene oxide were added to 465 g (5 moles) of aniline in an autoclave at approx. 140 ° C. and 3 bar in the presence of 2.3 g of Na methylate. After the reaction had ended, the mixture was flushed with nitrogen for half an hour and the reaction product obtained was used without further workup.
Als Ausgangsstoffe für die Anlagerung von Ethylenoxid und/oder Propylenoxid können folgende Anilin-Derivate herangezogen werden : o-Toluidin, p-Toluidin, p-Phenylendiamin, N-Methylanilin.The following aniline derivatives can be used as starting materials for the addition of ethylene oxide and / or propylene oxide: o-toluidine, p-toluidine, p-phenylenediamine, N-methylaniline.
Der wichtigste Effekt der erfindungsgemäß zu verwendenden Produkte besteht darin, daß sie die störende Blaufärbung des Mittels des Standes der Technik nicht zeigen. Ferner haben sie zum Teil eine deutlich verbesserte lösende Wirkung auf die in Fußbodenselbstglanzwachsen eingezetzten Polymeren.The most important effect of the products to be used according to the invention is that they do not show the disturbing blue color of the agent of the prior art. Furthermore, some of them have a significantly improved dissolving effect on the polymers used in self-waxing floors.
Einige der erfindungsgemäßen Produkte wurden in nachfolgender Rezeptur eines Fußbodenreinigungsmittels eingearbeitet und für Reinigungsversuche verwendet :
- 2,5 % n-Dodecylbenzolsulfonat-Na-Salz
- 3,0 % Fettsäuretriethanolaminsalz
- 2,0 % Fettsäureamidpolyglykolether
- 2,0 % Butyldiglykol
- 3,Q % Triethanolamin
- 3,0 % Ethylendiamintetraacetat
- 5,0% N-oxethyliertes Anilin
Rest zu 100% TrinkwasserSome of the products according to the invention were incorporated into the following formulation of a floor cleaning agent and used for cleaning tests:
- 2.5% n-dodecylbenzenesulfonate Na salt
- 3.0% fatty acid triethanolamine salt
- 2.0% fatty acid amide polyglycol ether
- 2.0% butyl diglycol
- 3, Q% triethanolamine
- 3.0% ethylenediaminetetraacetate
- 5.0% N-oxyethylated aniline
Rest 100% drinking water
Zur Reinigung wurden PVC-Platten, die mit handelsüblichen Fußbodenpflegemitteln (Glänzer, emsal, eingetragene Warenzeichen) vorbehandelt waren, verwendet. Die Pflegemittel wurden zur besseren Auswertung der Reinigungsversuche mit einem dunklen Farbton (R 28032 ex. conc. Fa. BASF) eingefärbt.PVC sheets that had been pretreated with commercially available floor care products (gloss, emsal, registered trademarks) were used for cleaning. The care products were colored with a dark color (R 28032 ex. Conc. BASF) for better evaluation of the cleaning tests.
Zur Reinigung wurde das Scheuergerät nach Gardner benutzt. Die Reinigungswirkung wurde mit dem Filterfarbmeßgerät RFC 3 der Fa. Zeiss festgestellt.The Gardner scrubber was used for cleaning. The cleaning effect was determined with the filter color measuring device RFC 3 from Zeiss.
Ergebnisse:
- Reinigungswirkung in Prozent Aufhellung
- Cleaning effect in percent brightening
In der Abbildung 1 wird die Reinigungswirkung verschiedener N-oxalkylierter Anilin-Derivate im Vergleich zum N-Phenyldiethanolamin dargestellt.Figure 1 shows the cleaning effect of various N-oxyalkylated aniline derivatives compared to N-phenyldiethanolamine.
Dabei zeigt sich, daß die erfindungsgemäß einzusetzenden Produkte mit 1 bis 2 Mol EO deutlich bessere Reinigungsergebnisse ergeben als der Stand der Technik. Höher oxethylierte Produkte sind weniger gut.It shows that the products to be used according to the invention with 1 to 2 mol EO give significantly better cleaning results than the prior art. Products with higher levels of ethoxylation are less good.
Es muß jedoch darauf hingewiesen werden, daß unter praxisnahen Gebrauchsbedingungen auch nach mehreren Wochen keine unerwünschte Blaufärbung festgestellt wurde.However, it must be pointed out that, under practical conditions of use, no undesirable blue color was found even after several weeks.
Die Fußbodenreinigungsmittel, in welchen das erfindungsgemäß zu verwendende Mittel eingesetzt wird, bestehen i. a. aus einem anionischen und/oder nichtionischen Tensid und gegebenenfalls einer anorganischen basischen Verbindung.The floor cleaning agents, in which the agent to be used according to the invention is used, consist i. a. from an anionic and / or nonionic surfactant and optionally an inorganic basic compound.
Claims (3)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3106491 | 1981-02-21 | ||
| DE3106491A DE3106491C2 (en) | 1981-02-21 | 1981-02-21 | Use of N-oxalkylated derivatives of aniline as polymer-dissolving components in floor cleaning agents |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0058751A1 EP0058751A1 (en) | 1982-09-01 |
| EP0058751B1 true EP0058751B1 (en) | 1984-04-11 |
Family
ID=6125420
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP81108390A Expired EP0058751B1 (en) | 1981-02-21 | 1981-10-16 | Use of n-oxyalkylated derivatives of aniline as polymer dissolving components in floor cleaning compositions |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US4425266A (en) |
| EP (1) | EP0058751B1 (en) |
| DE (2) | DE3106491C2 (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4891160A (en) * | 1982-12-23 | 1990-01-02 | The Proctor & Gamble Company | Detergent compositions containing ethoxylated amines having clay soil removal/anti-redeposition properties |
| US4857114A (en) * | 1987-04-13 | 1989-08-15 | Amway Corporation | Floor polish remover |
| DE19718065A1 (en) * | 1997-04-29 | 1998-11-05 | Henkel Kgaa | Detergent for post-curing polyurethane hotmelts |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA714018A (en) | 1961-05-26 | 1965-07-20 | Colgate-Palmolive Company | Hard surface cleaning compositions |
| US3954648A (en) | 1969-12-22 | 1976-05-04 | Pennwalt Corporation | Coatings removal composition containing an alkali metal hydroxide, an oxygenated organic solvent, and an amine |
| US3766076A (en) | 1970-11-05 | 1973-10-16 | Oxy Metal Finishing Corp | Stripping composition and process |
| US3723148A (en) | 1971-03-15 | 1973-03-27 | Oxy Metal Finishing Corp | Process for recovering coating materials |
| GB1438948A (en) | 1972-08-11 | 1976-06-09 | Unilever Ltd | Solvent type cleaners |
| US4077896A (en) | 1975-01-15 | 1978-03-07 | Minnesota Mining And Manufacturing Company | Wax-stripping cleaning composition |
-
1981
- 1981-02-21 DE DE3106491A patent/DE3106491C2/en not_active Expired
- 1981-10-16 DE DE8181108390T patent/DE3163102D1/en not_active Expired
- 1981-10-16 EP EP81108390A patent/EP0058751B1/en not_active Expired
-
1982
- 1982-02-22 US US06/350,931 patent/US4425266A/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| DE3163102D1 (en) | 1984-05-17 |
| EP0058751A1 (en) | 1982-09-01 |
| US4425266A (en) | 1984-01-10 |
| DE3106491C2 (en) | 1982-12-09 |
| DE3106491A1 (en) | 1982-09-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE3417912C1 (en) | Siloxanes containing betaine groups, their production and use in cosmetic preparations | |
| EP2778170B1 (en) | Phosphoric acid esters, their preparation and use | |
| DE2203141C3 (en) | Colored particles and their use in all-purpose cleaning agents | |
| EP2363422A1 (en) | Silicon-organic graft copolymers containing nitrogen | |
| DE102004053384A1 (en) | Liquid, viscous agent based on an organofunctional silane system for the production of weather-resistant protective coatings to prevent contamination of surfaces | |
| DE1291749B (en) | Process for the preparation of amine oxides | |
| DE10147650A1 (en) | Hydrophilic emulsifiers based on polyisobutylene | |
| DE2155004C3 (en) | Carpet and upholstery cleaning agents | |
| DE1771548A1 (en) | Metalworking and anti-corrosion agents | |
| WO2002083626A2 (en) | Alkyl and/or alkenyl glycerol carbamates | |
| EP0058751B1 (en) | Use of n-oxyalkylated derivatives of aniline as polymer dissolving components in floor cleaning compositions | |
| DE1020144B (en) | Detergents and cleaning agents | |
| DE602004006552T2 (en) | Gemini glycidyl ether adducts of polyhydroxyalkylalkylenediamines | |
| EP1354905A2 (en) | Method for producing ether-carboxylic acids having low pour point | |
| DE1172791B (en) | Detergent made from synthetic detergent substances | |
| DE3541813C2 (en) | Thickener for preparations containing surfactants based on polyether derivatives | |
| DE2711000A1 (en) | PROCESS FOR THE PRODUCTION OF Aqueous Coating Agents or PAINT | |
| DE102004010505A1 (en) | Process for the solvent-free production of low residual salt ether carboxylic acids | |
| WO2003040073A1 (en) | Alkyl(en)ylglycerinether carboxylic acids | |
| DE102014216380A1 (en) | Emulsions of aminosiloxanes and silicates | |
| DE1053139B (en) | Textile oil based on mineral oil | |
| DE69915747T2 (en) | Process for the treatment of fruits and vegetables after harvest, with the purification of phytosanitary products contaminated with primary aromatic amines | |
| WO1996009763A1 (en) | Use of surfactants to boost the anti-microbial properties of a carboxylic acid amide | |
| EP0670299A1 (en) | Dedusting agent | |
| DE1959652A1 (en) | Acid detergents |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB IT NL |
|
| 17P | Request for examination filed |
Effective date: 19820805 |
|
| ITF | It: translation for a ep patent filed |
Owner name: SOCIETA' ITALIANA BREVETTI S.P.A. |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB IT NL |
|
| REF | Corresponds to: |
Ref document number: 3163102 Country of ref document: DE Date of ref document: 19840517 |
|
| ET | Fr: translation filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19841001 Year of fee payment: 4 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19841031 Year of fee payment: 4 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19841106 Year of fee payment: 4 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19860501 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19860630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19860701 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19881121 |