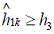CN115646396B - Safety parameter detection method, control method and device in reaction kettle reaction process - Google Patents
Safety parameter detection method, control method and device in reaction kettle reaction process Download PDFInfo
- Publication number
- CN115646396B CN115646396B CN202211688292.3A CN202211688292A CN115646396B CN 115646396 B CN115646396 B CN 115646396B CN 202211688292 A CN202211688292 A CN 202211688292A CN 115646396 B CN115646396 B CN 115646396B
- Authority
- CN
- China
- Prior art keywords
- temperature
- gas
- reaction kettle
- pressure
- liquid level
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Abstract
The invention relates to the technical field of reaction kettle monitoring, in particular to a safety parameter detection method, a safety parameter control method and a safety parameter control device in a reaction process of a reaction kettle. The invention eliminates the noise influence caused by sensor error and environmental interference by combining the liquid level height of reactants in the reaction kettle and the gas temperature to the gas pressure through the extended Kalman filtering based on the measured value of the gas pressure, and performs data fusion on the corrected and optimized pressure value so as to ensure the accuracy of gas pressure detection in the reaction process. Wherein, liquid level height, gas temperature also rectify the optimization, have guaranteed the detection accuracy of safety parameter.
Description
Technical Field
The invention relates to the technical field of reaction kettle monitoring, in particular to a safety parameter detection method in a reaction process of a reaction kettle, a safety parameter detection device using the safety parameter detection method, a control method of the reaction process of the reaction kettle using the safety parameter detection method, and a control device of the reaction process of the reaction kettle using the control method.
Background
The safety parameter detection/monitoring of the reaction kettle in chemical production is always a key problem of the reaction kettle. The safety parameters of the reaction kettle comprise the temperature in the kettle, the liquid level height (material quantity) in the kettle and the pressure (air pressure) in the kettle, especially the safety problem that the monitoring of the pressure directly influences the reaction kettle, and the kettle is exploded due to the overlarge pressure to cause the occurrence of production accidents. Therefore, the pressure value must be accurately measured, the sealing performance in the whole reaction process is ensured, and potential safety hazards caused by overlarge pressure in the kettle cannot be caused.
However, in the prior art, an electronic liquid level sensor, a temperature sensor and a pressure sensor are generally adopted to directly acquire the liquid level, the temperature and the pressure in the kettle. This approach does not reduce the noise during the reaction, and the effect of sensor noise on the measurement results. And the electronic level sensor needs to be in contact with materials, so that the electronic level sensor can be in failure due to corrosion or short circuit.
Therefore, the inventor considers that an ultrasonic liquid level sensor is used for detecting the liquid level, and the prediction, correction and optimization are carried out on the pressure in the kettle on the basis of the gas volume calculated by the gas temperature in the kettle and the liquid level height in the kettle. In general, the temperature of the gas inside the materials in the kettle is the same as that of the gas above the materials by default, but the two have certain errors in practice. Moreover, the detection result of the ultrasonic liquid level sensor is influenced by temperature, and the gas state is also related to the temperature. If the data of the temperature sensor and the ultrasonic liquid level sensor are directly referred to, the accuracy of the pressure measurement result in the kettle can be influenced.
Disclosure of Invention
Therefore, a method, a control method and a device for detecting safety parameters in a reaction process of a reaction kettle are needed to solve the problem that the pressure in the reaction kettle is not accurately measured in the reaction kettle reflection process of the existing reaction kettle.
The invention is realized by adopting the following technical scheme:
in a first aspect of the present disclosure, a method for detecting safety parameters in a reaction kettle reaction process is provided, which is used for detecting material temperature, gas temperature, liquid level height and gas pressure in the reaction kettle reaction process.
The method for detecting the safety parameters in the reaction process of the reaction kettle comprises the following steps:
the method comprises the following steps: obtainingCurrent time of dayt k The material temperature of different heights in the reactants in the reaction kettleT pk To is aligned withT pk Processing to obtain average material temperature(ii) a Obtaining the gas temperature of the gas at different heights above the reactant in the reaction kettle at the current momentT qk To is aligned withT qk The average value of the gas temperature is obtained after correction and optimization;
Wherein the content of the first and second substances,is the current timet k The material temperature of the reaction kettle in the reaction process;is the current timet k The gas temperature in the reaction process of the reaction kettle;
step two: obtaining the current timet k The liquid level height of the reactants in the reaction kettleh k1 Based onFor is toh k1 Correcting and optimizing to obtain corrected liquid level height;
Wherein, the first and the second end of the pipe are connected with each other,is the current timet k The liquid level height of the reaction kettle in the reaction process;h k1 indirectly by ultrasonic measurement;
step three:obtaining the current timet k Gas pressure at two different positions above reactants in the reaction kettleα k1 、α k2 Based on、To pairα k1 、α k2 Respectively corrected to obtain corrected gas pressure、And will be、Fusing to obtain optimized gas pressure;
Wherein the content of the first and second substances,is the current timet k And the gas pressure in the reaction kettle during the reaction process.
The method for detecting safety parameters in the reaction process of the reaction kettle realizes the method or process according to the embodiment of the disclosure.
In a second aspect of the present disclosure, a device for detecting safety parameters in a reaction kettle reaction process is provided, and the method for detecting safety parameters in a reaction kettle reaction process disclosed in the first aspect is used.
Safety parameter detection device includes material temperature data detection module, gas temperature data detection module, liquid level height data detection module, gas pressure data detection module among the reation kettle reaction process.
The material temperature data detection module is used for acquiring the material temperatures at different heights in reactants in the reaction kettle in real timeT pk Are combined with each otherT pk Processing to obtain average temperature value of materials(ii) a The gas temperature data detection module is used for acquiring the gas temperatures of different heights of gas above reactants in the reaction kettle in real timeT qk Are combined with each otherT qk After optimization, the average value is taken to obtain an optimized value(ii) a Liquid level height data detection module is used for gathering measuring distance in reation kettle in real timeL k To, forL k Optimizing to obtain optimized valueAnd is based onObtaining the corrected liquid level height(ii) a The gas pressure data detection module is used for acquiring gas pressure data above reactants in the reaction kettle in real timeα k1 、α k2 To, forα k1 、α k2 Optimizing to obtain a data fusion value。
The security parameter detection apparatus implements methods or processes in accordance with embodiments of the present disclosure.
In a third aspect of the present disclosure, there is provided a method of controlling a reaction tank reaction process, comprising the steps of:
the material temperature in the reaction kettle reaction process is obtained by adopting the method for detecting the material temperature in the reaction kettle reaction process disclosed in the first aspectGas temperatureHeight of liquid levelAnd gas pressure.
if it isIf not in the preset temperature range, judgingA relationship with an upper limit temperature and a lower limit temperature of the temperature range;
wherein, ifIf the temperature is higher than the upper limit temperature of the temperature range, an alarm is sent out, the temperature of the heat exchange medium flowing through the jacket is reduced, and the reaction kettle is cooled;
if it isThe temperature of the heat exchange medium flowing through the jacket is increased when the temperature is lower than the lower limit temperature of the temperature range, and the temperature of the reaction kettle is increased;
judgment ofSafety value of liquid levelh 1 Liquid level early warning valueh 2 Liquid level alarm valueh 3 The size of (d); wherein, the first and the second end of the pipe are connected with each other,h 3 >h 2 >h 1 ;
if it isReducing the feeding to the reaction kettle and increasing the discharging of the reaction kettle untilStopping discharging the reaction kettle;
if it isSending an alarm, stopping feeding to the reaction kettle, stopping stirring by the reaction kettle, and discharging the reaction kettle at the maximum flow until the reaction kettle is dischargedStopping discharging the reaction kettle, and restarting stirring the reaction kettle;
if it isOut of the preset pressure range, judgingThe relationship between the pressure and the upper limit pressure and the lower limit pressure of the pressure range;
wherein, ifWhen the pressure is higher than the upper limit pressure of the pressure range, an alarm is given out to release the pressure of the reaction kettle;
if it isAnd if the pressure is lower than the lower limit pressure of the pressure range, giving an alarm to pressurize the reaction kettle.
The control method implements a method or process according to an embodiment of the present disclosure.
In a fourth aspect of the present disclosure, a control device for a reaction process of a reaction kettle is provided, which includes a data detection module, a mechanism execution module, and a controller.
The data detection module comprises a temperature detection module, a liquid level detection module and a pressure detection module; the temperature detection module comprises a material temperature detection submodule and a gas temperature detection submodule; the material temperature detection submodule is used for acquiring the material temperature in the reaction process of the reaction kettle; the gas temperature detection submodule is used for acquiring the gas temperature of the reaction kettle in the reaction process; the liquid level detection module is used for acquiring the liquid level height of the reaction process in the reaction kettle; the pressure detection module is used for acquiring the gas pressure in the reaction process in the reaction kettle.
The mechanism execution module comprises a temperature execution module, a liquid level execution module and a pressure execution module; the temperature execution module is used for adjusting the temperature of reactants; the liquid level execution module is used for controlling the liquid level height of the reactant; the pressure execution module is used for adjusting the gas pressure.
The controller is used for receiving the data acquired by the data detection module, wirelessly transmitting the data to the remote monitoring terminal, receiving an instruction sent by the remote monitoring terminal and controlling the corresponding execution module to work; wherein, the remote monitoring end issues an instruction to the controller according to the control method of the reaction process of the reaction kettle in the first aspect.
The control device implements methods or processes according to embodiments of the disclosure.
It should be understood that what is described in this summary section is not intended to limit key features or essential features of the embodiments of the disclosure, nor is it intended to limit the scope of the disclosure. Other features of the present disclosure will be readily apparent from the following description.
Compared with the prior art, the invention has the following beneficial effects:
the invention is based on the measured value of gas pressure, and eliminates the noise influence caused by sensor error and environmental interference by combining the liquid level height of reactants in a reaction kettle and the gas temperature on the gas pressure through extended Kalman filtering, thereby ensuring the accuracy of gas pressure detection in the reaction process. Wherein, liquid level height, gas temperature also rectify and optimize, have guaranteed the detection accuracy of safety parameter.
2, when the invention is used for measuring the gas pressure, two pressure data at different positions are selected, corrected and optimized and then fused, so that the error of the gas pressure measurement can be further reduced.
3, the invention realizes more accurate detection of the temperature of the materials in the kettle, the temperature of the gas in the kettle and the liquid level height of the materials by utilizing the process of detecting the pressure; the invention transmits the detected safety parameter data from the controller to the remote monitoring end through the wireless network, and the remote monitoring end sends an instruction to the controller, thereby realizing the automation of remote monitoring.
Drawings
FIG. 1 is a flow chart of a method for detecting safety parameters in a reaction process of a reaction kettle according to the present invention;
FIG. 2 is a structural diagram of a reaction vessel to which the method for detecting safety parameters of FIG. 1 is specifically applied;
FIG. 3 is a structural view of a control device used in the reaction process of the reaction vessel in FIG. 2;
FIG. 4 is a flow chart of the liquid level height detection and control based on the liquid level height of the reaction kettle of FIG. 2;
FIG. 5 is a flow chart of the gas pressure detection and control based on the gas pressure in the reaction vessel of FIG. 2;
in the drawings, the components represented by the respective reference numerals are listed below:
1. a kettle body; 2. a discharge valve; 3. a water inlet; 4. a jacket; 5. an effluent temperature detector; 6. a water outlet; 7. a temperature sensor; 8. a first pressure sensor; 9. an exhaust valve; 10. a stirring blade; 11. a gas densitometer; 12. a servo motor; 13. a second pressure sensor; 14. an intake valve; 15. a feed valve; 16. an ultrasonic liquid level sensor.
The present invention is described in further detail with reference to the drawings and the detailed description.
Detailed Description
The technical solutions in the embodiments of the present invention will be clearly and completely described below with reference to the drawings in the embodiments of the present invention, and it is obvious that the described embodiments are only a part of the embodiments of the present invention, and not all of the embodiments. All other embodiments, which can be derived by a person skilled in the art from the embodiments given herein without making any creative effort, shall fall within the protection scope of the present invention.
It will be understood that when an element is referred to as being "mounted on" another element, it can be directly on the other element or intervening elements may also be present. When a component is referred to as being "disposed on" another component, it can be directly on the other component or intervening components may also be present. When an element is referred to as being "secured to" another element, it can be directly secured to the other element or intervening elements may also be present.
Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. The terminology used in the description of the invention herein is for the purpose of describing particular embodiments only and is not intended to be limiting of the invention. As used herein, the term "or/and" includes any and all combinations of one or more of the associated listed items.
Example 1
Please refer to fig. 1, which is a flow chart of a method for detecting safety parameters in a reaction process of a reaction kettle according to the present invention. This example 1 discloses a method for detecting safety parameters in a reaction process of a reaction kettle, which is used for detecting a liquid level height and a gas pressure in the reaction process of the reaction kettle. It should be noted that the liquid level height is compensated with respect to the use temperature. The gas pressure is compensated by using liquid level height and temperature. Referring to fig. 2, a structure diagram of a reaction kettle to which the method for detecting safety parameters of embodiment 1 is specifically applied is shown.
As shown in fig. 2, the reaction kettle comprises a kettle body 1 and a jacket 4 for accommodating a heat exchange medium. The jacket 4 is arranged at the periphery of the kettle body 1 and is used for enabling the heat exchange medium to exchange heat for the kettle body 1. The heat exchange medium can be heat conduction oil or water. The jacket 4 may be connected to a temperature adjusting means such as a thermostatic bath through which the temperature of the jacket 4 is adjusted.
The bottom of the jacket 4 is provided with a water inlet 3, the top is provided with a water outlet 6, and both positions are provided with control valves; wherein, a water outlet 6 of the jacket 4 is provided with a water outlet temperature detector 5 for detecting the outlet temperature of the heat exchange medium.
The top of the kettle body 1 is provided with a feeding valve 15 for controlling the feeding of the reaction kettle. The bottom of the kettle body 1 is provided with a discharge valve 2 for controlling the discharge of the reaction kettle and promoting the reaction process of the materials.
The reactor body 1 is internally provided with a stirring paddle 10, the stirring paddle 10 is connected with an external servo motor 12, and is driven by the servo motor 12 to stir the reactant in the reactor body 1.
The inner top of the kettle body 1 is provided with a gas densimeter 11 and two pressure sensors, wherein the gas densimeter is used for detecting the density of gas above the reactantρ p . It should be noted that the two gas pressure sensors are arranged at two different positions on the top of the kettle and used for detecting the gas pressure at the two different positionsα k1 、α 2 。
The top of the kettle body 1 is also provided with an air inlet valve 14 and an air outlet valve 9 which are used for adjusting the pressure of the reaction kettle. Specifically, when both valves are kept closed, the air tightness in the kettle body 1 is ensured. The exhaust valve 9 is opened to perform exhaust and pressure relief, and the intake valve is opened to perform intake pressurization.
An ultrasonic liquid level sensor 16 is arranged in the kettle body 1 and is used for detecting the distance between the kettle body and the liquid level of the reactantL k . It should be noted that, in order to facilitate the calculation related to the subsequent gas, it is suggested to install the ultrasonic liquid level sensor 16 at the junction of the kettle top and the kettle wall.
The kettle body 1 is internally provided withnA temperatureThe sensor 7 is used for acquiring the temperatures of different positions of the kettle body 1; wherein the reactant is above the liquid levelmA temperature sensor 7 for collecting the gas temperature at different heights above the reactant in the reaction kettleT qk . Below the liquid level of the reactant isn-m A temperature sensor 7 for collecting the material temperature at different heights in the reactant in the reaction kettleT pk 。
Referring to fig. 2, since the real-time reaction of the reactant and the agitation of the agitating blades 10 cause the disturbance of the gas at the liquid level of the reactant, the temperature sensors 7 are installed on the inner wall of the kettle body 1, and are arranged at equal intervals from top to bottomnAnd (4) respectively. Of course, the temperature sensors 7 can be covered at the top and the bottom of the reaction kettle, so as to more accurately reflect the real value of the temperature of each layer of the reaction kettle.
Since the temperature sensors 7 are installed at equal intervals, the height of the liquid level is equal to the number of the temperature sensors 7 above the liquid level of the reactantmThe number of temperature sensors 7 below the liquid level of the reactant(s) ((n-m) Is related and can be obtained according to the liquid level heightm、(n-m). Wherein, initially obtainedm、(n-m) Can be directly obtained from the measurement value of the ultrasonic level sensor 16.
Referring to fig. 1, the method for detecting safety parameters in the reaction process of the reaction kettle comprises the following steps:
the method comprises the following steps: obtaining the current timet k Material temperature at different height in reaction kettleT pk To, forT pk Processing to obtain average material temperature(ii) a Obtaining the current timet k Gas temperature at different heights of gas above reactants in reaction kettleT qk To, forT qk The average value of the gas temperature is obtained after correction and optimization。
For theFirst, pass throughn-m) The temperature sensors 7 acquire the material temperature at different heights in the reactantT ipk ,T ipk Indicating the current time of dayt k From top to bottomiThe temperature values collected by the individual temperature sensors 7. Then, the current time is comparedt k All ofT ipk Obtaining the average valueI.e. by。
To pairT qk The method for predicting and correcting by adopting the extended Kalman filtering comprises the following steps:
s101, constructing a gas temperature prediction equation above the reactant based on a heat balance equation.
First, for thermal reactions inside a reaction vessel, which are generally exothermic, there is a heat balance equation:Q 1 =Q 2 -Q 3 -Q 4 ;
Q 1 heat is accumulated for the materials in the kettle body 1,Q 2 the heat is released for the material reaction in the kettle body 1,Q 3 the heat transferred into the jacket 4 for the kettle body 1,Q 4 for transferring heat to the gas above the reactants.
Assuming that both the volume and density of the reactor jacket 4 per unit time remain constant and other heat losses during the heat exchange are neglected, the above heat balance equation is converted into:
the simplification is as follows:
and then obtaining a prediction equation of the gas temperature above the reactant as follows:
wherein the content of the first and second substances,as the current timet k The gas temperature prior predicted value of (a);V p is the volume of the reactant, ΔHThe heat of the molar reaction is used as the heat of the molar reaction,rfor the speed of chemical reaction, byr=KD p Is calculated to obtainKIn order to reflect the frequency constant, the frequency of the reaction,D p is the concentration of the material in the reaction kettle;tcharacterizing time;Uis the heat transfer coefficient between the reaction kettle and the jacket 4,A R the contact area of the jacket 4 and the reaction kettle,is the temperature of the materials in the reaction kettle,,T c is the outlet temperature of the heat exchange medium,Mwhich is the mass of the reactants, is,C p is the specific heat capacity of the reactants,C q is the specific heat capacity of the gas above the reactants;m q mass of gas above the reactant;dT q is the amount of change in the temperature of the gas per unit time,is as followsAt a momentt k-1 A posterior estimate of the gas temperature of (a),w q k-(1) is the last momentt k-1 The process noise of (1).
For gas above the reaction vessel, massm q Is represented by the formula:m q =ρ q V q and (4) calculating.
ρ q Is the density of the gas, measured on-line by a gas densitometer 11.V q Is the current volume of gas. Referring to fig. 2, in this embodiment 1, the main body of the kettle 1 is a cylindrical structure, and a kettle top with a spherical crown structure is disposed above the main body of the kettle 1.
V q From the sum of the volumes of a cylinder plus a spherical cap, as in fig. 2, it can be determined that:V q = V 1 +V 2 wherein, in the step (A),V 1 is the volume of the spherical cap,V 2 is the volume of a cylinder.
According to the volume calculation formula of the spherical cap, the volume of the gas contained in the curved surface part of the spherical cap is as follows:
wherein the content of the first and second substances,R 1 the radius of a ball of the spherical crown is intercepted;H 1 is the height of the spherical cap.
V 2 =L·S;
Wherein the content of the first and second substances,Sis the cross-sectional area of the cylinder,Lthe distance from the top of the cylinder to the liquid surface of the reactant.
Of course, if the kettle body 1 has other shapes, the gas volume above the reaction kettle needs to be expressed by adopting a corresponding shape calculation formula.
S102, establishing an observation equation of the temperature of the gas above the reactantZ qk :
Z qk =T qk +v qk ;
Wherein the content of the first and second substances,T qk is the current timet k A desired gas temperature value of;v qk as the current timet k Measurement of gas temperature noise.
S103, carrying out prior estimation on the gas temperature of each position:
the influence of noise, process noise, is not considered when carrying out the prior estimation calculationwIs 0 and is therefore estimated a prioriNot embodied in the calculation.
S104, calculating the gas temperature covariance:
wherein the content of the first and second substances,as the current timet k A predicted value of gas temperature covariance;P q k(-1) is the last momentt k-1 A posterior estimate of the gas temperature covariance;A q k(-1) is the last momentt k-1 The posterior estimated value is linearized to obtain a Jacobian matrix;
w qk Q q w qk T the kalman filter process noise is extended for the gas temperature,w qk is the current timet k The process noise of the temperature of the gas,Q q is process noisew qk The covariance matrix of (2).
S105, calculating a gas temperature gain coefficient:
wherein the content of the first and second substances,K qk as the current timet k The gain factor of (a) is determined,H q for the equation of observationZ qk A coefficient matrix of (a);v qk R q v qk T the measurement noise of the kalman filter is extended for the gas temperature,v qk is the noise of the gas temperature measurement of the current step,R q for measuring noisev qk The covariance matrix of (2).
S106, carrying out posterior estimation on the gas temperature of each position:
wherein, the first and the second end of the pipe are connected with each other,as the current timet k A posterior estimate of the gas temperature of;h q (T qk ) The gas temperature desired value is a non-linear expression.
S107, updating the gas temperature covariance:
wherein the content of the first and second substances,P qk is as followsTime of dayt k A posteriori estimate of the gas temperature covariance,Iis an identity matrix.
S108, estimating the posterior temperature of all the gasesTaking the mean valueAs the current timet k Average value of gas temperature。
Based on the above description, the liquid level of the reactant is abovemA temperature sensor 7 at the current timet k Correspond tomAn。
Step two: obtaining the current timet k Liquid level height of reactant in reaction kettleh k1 Based onTo pairh k1 Corrected liquid level height is obtained by correction optimization;
Wherein the content of the first and second substances,as the current timet k The liquid level height of the reaction process in the reaction kettle.
h k1 Indirectly by means of ultrasonic measurements: referring to FIG. 2, the ultrasonic level sensor 16 is used for the ultrasonic measurement in this embodiment 1. An ultrasonic liquid level sensor 16 is arranged on the inner wall of the reaction kettle and is positioned above the liquid level of the reactant for measuringDistance from the liquid surface of the reactantL k 。
Therefore, the temperature of the molten metal is controlled,h k1 =h-L k (ii) a Wherein the content of the first and second substances,hto the mounting height of the ultrasonic level sensor 16,L k as the current timet k The measured distance of (2). In this example 1, the ultrasonic level sensor 16 is installed at the junction of the kettle top and the kettle wall, so that the distance from the top end of the cylinder to the liquid level of the reactantLI.e. detected by the ultrasonic level sensor 16L k And the calculation is convenient.
Thus, with the ultrasonic level sensor 16, the material level height can be measured without contacting the material and the volume of gas above the reactant can be calculated. But due to detection by the ultrasonic level sensor 16L k Is subject to temperature effects and is therefore subject to corrective optimization.
To pairh k1 The method for performing correction optimization comprises the following steps:
to pairL k Adopting extended Kalman filtering to carry out prediction correction to obtain corrected measurement distance;
I.e. if the exact level is to be obtainedThe accurate measurement distance needs to be obtained first。
Specifically, forL k The method for predicting and correcting by adopting the extended Kalman filtering comprises the following steps:
s201, establishing ultrasonic sound velocityV s And the current timet k Average value of gas temperatureAnd then establishing a prediction equation of the measured distance.
Firstly, the speed of sound of ultrasonic wave is measured by Newton's interpolation methodV s And upper corrected optimized gas temperatureEstablishing a fitting relation:
assume a temperature sampling interval of ΔTSample interval [ 2 ]T 0 , T n ],T 0 In order to sample the temperature at the start point,T n in order to sample the end point temperature,n=1,2,3, \8230;. The sampling point number can be set automatically according to the sampling precision requirement.
Acquiring the sound velocity value of the ultrasonic sensor corresponding to the gas temperature value at each sampling point, and constructing an equation:
due to the fact thatIn whicht s Establishing a prediction equation of the measurement distance for the total propagation time of the ultrasonic wave back and forth:
wherein, the first and the second end of the pipe are connected with each other,as the current timet k A priori predicted value of the distance is measured,is the last momentt k-1 A posterior estimate of the distance is measured,is the last momentt k-1 The gas is corrected to an optimized temperature value,V s k-(1) is the last momentt k-1 The speed of sound of the ultrasonic waves,t s k-(1) is the last momentt k-1 The total time for the ultrasonic sound velocity to travel back and forth,w s k-(1) for the last timet k-1 The system process noise.
S202, establishing an observation equation of the measured distanceZ sk :
Z sk =L k +v sk ;
Wherein, the first and the second end of the pipe are connected with each other,L k as the current timet k The expected value of the distance is measured and,v sk as the current timet k Measurement noise of the measurement distance.
S203, carrying out prior estimation on the measurement distance:
the influence of noise, process noise, is not considered when carrying out the prior estimation calculationwIs 0 and is therefore estimated a prioriNot reflected in the calculation.
S204, calculating the covariance of the measurement distance:
wherein the content of the first and second substances,measuring a predicted value of the distance covariance for the current time;P s k-(1) is the last momentt k-1 Measuring a posterior estimate of the distance covariance;A s k-(1) is the last momentt k-1 Carrying out linearization to obtain a Jacobian matrix;
w sk Q s w sk T in order to measure the distance extended kalman filter process noise,w sk is the current time of dayt k The process noise of the measured distance is,Q s is process noisew sk The covariance matrix of (c).
S205, calculating a measurement distance gain coefficient:
wherein the content of the first and second substances,K sk is the current timet k The gain factor of (a) is determined,H s for the equation of observationZ sk A coefficient matrix of (a);v sk R s v sk T in order to measure the measurement noise of the extended kalman filter,v sk is the current step measurement distance measurement noise,R s for measuring noisev sk The covariance matrix of (2).
S206, calculating a posterior estimated value of the measured distance:
wherein the content of the first and second substances,as the current timet k The posterior estimate of the measured distance, i.e. the corrected measured distance,h s (L k ) A non-linear expression for measuring distance expectation.
S207, updating the covariance of the measurement distance:
wherein the content of the first and second substances,P sk as the current timet k A posteriori estimate of the distance covariance is measured,Iis an identity matrix.
Thus, based on the corrected measured distanceThe current time can be obtainedt k Liquid level height of reaction process in reaction kettle,。
Step three: obtaining the current timet k Gas pressure at two different positions above reactants in reaction kettleα k1 、α k2 Based on、To pairα k1 、α k2 Respectively corrected to obtain corrected gas pressure、And will be、Fusing to obtain optimized gas pressure;
Wherein the content of the first and second substances,as the current timet k The gas pressure in the reaction kettle during the reaction process;
referring to fig. 2, in the present embodiment 1, two pressure sensors (including a first pressure sensor 8 and a second pressure sensor 13) are used for measuring the gas pressureα k1 、α k2 And (6) detecting. Due to the existence of sensor errors and the influence of environmental interference, the extended Kalman filter pair is utilized according to the gas volume (namely liquid level height) and the temperatureα k1 、α k2 Correction is carried out to obtain a corrected value、And fusing to obtain optimized gas pressure。
To pairα k1 、α k2 Correcting to obtain corrected value、The method is the same as the method in the following steps:
s301, establishing a prediction equation of the gas pressure above the reactant.
Assume that in a unit time intervalt k -1, t k ]And van der waals gas equation is adopted for the gas state equation under the non-ideal state,t k andt k-1 the pressure value of the gas at the moment is respectivelyα k Andα k-1 。
from the above equation, a prediction equation for the gas pressure above the reactants is established as:
wherein the content of the first and second substances,is a priori predicted value of the gas pressure at the current moment,the last time gas pressure posterior estimation value is obtained;R g is a constant of the gas and is,v q k(-1) is the last momentt k-1 The specific volume of the gas is such that,v qk as the current timet k The specific volume of gas;w g k(-1) is the last momentt k-1 The process noise of (1);a、bis a correction number.
Specific volumeUnit ofm 3 /kg。m q Is gas mass per unitkg;V q Is the volume of gas, unitm 3 ;R g Is a gas constant, unitJ/(kg·K)。
aFor the correction number, it is related to the nature of the gas itself and has the unit ofm 6 ·Pa)/kg 2 According to the following steps:thus obtaining the product.bThe corrected number is related to the nature of the gas itself and has the unit ofm 3 /kgAccording to the following:thus obtaining the product.T c Is the critical temperature of the gas inK;P c Is the critical pressure of the gas inpa。
S302, establishing a gas pressure observation equationZ gk :
Z gk =α k +v gk ;
Wherein the content of the first and second substances,α k as the current timet k The desired value of the pressure of the gas,v gk the measurement of the gas pressure at the present moment is noisy.
S303, carrying out prior estimation on the gas pressure:
the influence of noise, process noise, is not taken into account when carrying out the prior estimation calculationwIs 0 and is therefore estimated a prioriNot reflected in the calculation.
S304, calculating the covariance of the gas pressure:
wherein the content of the first and second substances,is the current timet k A predicted value of gas pressure covariance;P g k(-1) is the last momentt k-1 A posterior estimate of the gas pressure covariance;A g k(-1) is the last momentt k-1 Carrying out linearization to obtain a Jacobian matrix;
w gk Q g w gk T the kalman filter process noise is extended for the gas pressure,w gk is the current timet k The process noise of the gas pressure is such that,Q g is process noisew gk The covariance matrix of (2).
S305, calculating a gas pressure gain coefficient:
wherein the content of the first and second substances,K gk as the current timet k The gain factor of (a) is determined,H g for the equation of observationZ gk A coefficient matrix of (a);v gk R g v gk T the measurement noise of the gas pressure extended kalman filter,v gk is the noise of the gas pressure measurement of the current step,R g for measuring noisev gk The covariance matrix of (c).
S206, calculating a posterior estimated value of the gas pressure:
wherein the content of the first and second substances,as the current timet k The posterior estimate of the gas pressure, i.e. the corrected gas pressure,h g (α k ) Is a non-linear expression of the gas pressure desired value.
S207, updating the gas pressure covariance:
wherein the content of the first and second substances,P sk as the current timet k A posteriori estimate of the covariance of the gas pressure,Iis an identity matrix.
To show more specifically, the method is as followsInstead of using,Is replaced by,α k Is replaced byα yk ,Instead of using,Is replaced by,y=1,2。
andthe correction values of the first pressure sensor 8 and the second pressure sensor 13 are respectively.α k1 Andα k2 the measurement expectation values of the first pressure sensor 8 and the second pressure sensor 13 are respectively. Determining weighting factors of measured values of two sensors by calculating the proportion of the deviation between the corrected optimized value and the expected value of the two pressure sensors at the same moment to the total deviationλ k,1 Andλ k,2 . It is emphasized that the weighting factorsλ k,1 、λ k,2 According toAndthe design and the self-adaptive association exist with the design and can be based on the real-timeAndand the change can reflect the internal relation of the pressure so as to reduce the error of the fusion pressure.
Based on the detection method, the sensors of gas temperature detection, ultrasonic liquid level detection and gas pressure detection are respectively reduced by using extended Kalman filteringSelf-error of the device and noise influence caused by environmental interference. Can obtain more accurate material temperature for detecting the reaction process of the reaction kettleGas temperatureHeight of liquid levelGas pressureTherefore, the monitoring of safety parameters in the reaction process is more accurate, and the data source accuracy for controlling the reaction kettle in the follow-up process is convenient.
This embodiment 1 still provides safety parameter detection device among reation kettle reaction process, detects module, liquid level height data detection module, gas pressure data detection module including material temperature data.
The material temperature data detection module is used for acquiring the material temperatures at different heights in reactants in the reaction kettle in real timeT pk And is combined withT pk To be processed to obtain the average value of the material temperature. The gas temperature data detection module is used for acquiring the gas temperatures of different heights of gas above reactants in the reaction kettle in real timeT qk And are combined toT qk After optimization, the average value is taken to obtain an optimized value. Liquid level height data detection module is used for gathering measuring distance in reation kettle in real timeL k To, forL k Optimizing to obtain optimized valueAnd is based onObtaining a corrected liquid level height. The gas pressure data detection module is used for acquiring gas pressure data above reactants in the reaction kettle in real timeα k1 、α k2 To is aligned withα k1 、α k2 Optimizing to obtain a data fusion value。
According to the method and the device for detecting the safety parameters in the reaction process of the reaction kettle, the temperature sensor 7 is used for collecting the material temperature and the gas temperature, and the noise influence caused by the sensor error and the environmental interference is eliminated from the gas temperature through the extended Kalman filtering; collecting the distance from the ultrasonic sensor to the liquid level, eliminating noise influence caused by sensor error and environmental interference by combining the temperature on the measured distance through extended Kalman filtering, and indirectly calculating to obtain the liquid level height of reactants in the reaction kettle; the gas pressure at two different positions is acquired by utilizing the pressure sensor, and the gas pressure is subjected to extended Kalman filtering and data fusion by combining the liquid level height and temperature of reactants in the reaction kettle, so that the noise influence caused by sensor errors and environmental interference is eliminated, and the accuracy of safety parameter detection in the reaction process is ensured.
Example 2
This embodiment 2 provides a control method and a control system for a reaction process of a reaction vessel. Referring to fig. 3, the control system employs a remote monitoring design.
As shown in fig. 3, the control system for the reaction process of the reaction vessel comprises a data detection module, a controller, an execution module, and a remote monitoring end.
The data detection module comprises a temperature detection module, a liquid level detection module and a pressure detection module.Wherein, the temperature detection module includes that material temperature detects submodule piece and gas temperature and detects the submodule piece. The data detection module is the safety parameter detection device in the reaction kettle reaction process in the embodiment 1. The material temperature detection submodule is a material temperature data detection module which passes through (A) in embodiment 1n-m) The temperature sensor 7 detects and processes the material temperature data to obtain the material temperature of the reaction kettle in the reaction process. The gas temperature detection submodule is a gas temperature data detection module which passes through the module in the embodiment 1mThe temperature sensor 7 realizes the gas temperature detection and processes to obtain the gas temperature in the reaction process of the reaction kettle. The liquid level detection module is a liquid level height data detection module which detects the liquid level height through the ultrasonic liquid level sensor 16 in the embodiment 1 and processes the liquid level height to obtain the liquid level height of the reaction process in the reaction kettle. The pressure detection module is a gas pressure data detection module, and realizes gas pressure detection through the pressure sensor in embodiment 1 and processes the gas pressure to obtain the gas pressure of the reaction process in the reaction kettle。
The mechanism execution module comprises a temperature execution module, a liquid level execution module and a pressure execution module.
Wherein the temperature performing module is used to adjust the temperature of the reactant, for example, to increase or decrease the temperature of the jacket 4. The temperature execution module comprises a jacket 4 and a temperature adjusting device connected with the jacket 4. Specifically, the temperature of the reactant can be increased by increasing the temperature of the heat exchange medium flowing through the jacket 4 by the temperature adjusting device, and the temperature of the reactant can be decreased by decreasing the temperature of the heat exchange medium flowing through the jacket 4 by the temperature adjusting device.
The liquid level execution module is used for controlling the liquid level height of reactants, such as controlling the feeding flow of the kettle body 1 and the outward discharging flow of the kettle body 1. The liquid level execution module comprises a feed valve 15 and a discharge valve 2. Specifically, the feeding valve 15 is adjusted to reduce the feeding flow to the kettle body 1 or stop feeding to the kettle body 1, and the discharging valve 2 is adjusted to increase the outward discharging flow of the kettle body 1 and reduce the liquid level height of the reactant. The reverse operation is performed to increase the reactant level.
The pressure execution module is used for adjusting the gas pressure. Such as exhaust pressure relief or intake pressurization. The pressure actuator module comprises an inlet valve 14 and an outlet valve 9. Specifically, when both valves are kept closed, the air tightness in the kettle body 1 is ensured. When the exhaust valve 9 is opened, the air is exhausted and decompressed, and when the air inlet valve is opened, the air is introduced and pressurized.
The controller is used for receiving the data acquired by the data detection module, transmitting the data to the remote monitoring terminal through wireless transmission, receiving the instruction issued by the remote monitoring terminal and controlling the corresponding execution module to work. Wherein, the remote monitoring end issues an instruction to the controller according to a set control method of the reaction process of the reaction kettle.
Namely, the input of the controller is a data detection module for acquiring related safety parameter data, and the output is a mechanism execution module for performing online execution of the remote monitoring end instruction.
The control method of the reaction process of the reaction kettle comprises the following steps:
the method for detecting the safety parameters in the reaction kettle reaction process in example 1 was used to obtain the material temperature in the reaction kettle reaction processGas temperatureHeight of liquid levelPressure of gas。
If it isAnd in a preset temperature range, indicating that the temperature is normal, and keeping detection.
If it isOut of the preset temperature range, judgingA relationship with an upper limit temperature and a lower limit temperature of the temperature range;
wherein, ifWhen the temperature is higher than the upper limit temperature of the temperature range, an alarm is sent out, the temperature of the heat exchange medium flowing through the jacket 4 is reduced, and the reaction kettle is cooled;
if it isThe temperature of the heat exchange medium flowing through the jacket 4 is increased when the temperature is lower than the lower limit temperature of the temperature range, and the temperature of the reaction kettle is increased;
specifically, the alarm is sent out by prompting at a remote monitoring end. And adjusting the temperature of the reaction kettle, namely, controlling the temperature execution module to operate.
judge the height of the liquid level in the reaction process of the reaction kettleSafety value of liquid levelh 1 Liquid level early warning valueh 2 Liquid level alarm valueh 3 The size of (d); wherein, the first and the second end of the pipe are connected with each other,h 3 >h 2 >h 1 。
If it isReducing feeding to the reaction kettle and increasing discharging of the reaction kettle untilStopping discharging the reaction kettle;
if it isSending an alarm, stopping feeding to the reaction kettle, stopping stirring by the reaction kettle, and discharging the reaction kettle at the maximum flow untilStopping discharging the reaction kettle, and restarting stirring the reaction kettle;
specifically, the alarm is sent out by prompting at a remote monitoring end. The feeding and discharging adjustment of the reaction kettle is to control the liquid level execution module to work. It should be noted that the stirring blade 10 of the reaction kettle and the external servo motor 12 connected thereto are essential components of the reaction kettle, and may also form a stirring execution module, and the stirring execution module is also controlled to operate in the above process.
Referring to fig. 4, a flow chart of the liquid level detection and control based on the liquid level in the reaction kettle in fig. 2 is shown.
If it isIf not in the preset pressure range, judgingThe relationship between the pressure and the upper limit pressure and the lower limit pressure of the pressure range;
wherein, ifThe upper limit pressure which is greater than the pressure range gives an alarm, and the pressure of the reaction kettle is released through the exhaust valve until the pressure is up toAnd dropping to a preset pressure range.
If it isWhen the pressure is less than the lower limit pressure of the pressure range, an alarm is sent out, and the reaction kettle is pressurized through the air inlet valve until the pressure is less than the lower limit pressure of the pressure rangeRising to a preset pressure range.
Wherein, the alarm is sent out by prompting at a remote monitoring end. The pressure of the reaction kettle is adjusted to control the pressure execution module to work.
Referring to fig. 5, a flow chart of gas pressure detection and control based on gas pressure in the reaction vessel in fig. 2 is shown. It should be noted that the exhaust valve 9 and the intake valve 14 used in the present embodiment are both electrically controlled, and are controlled by the controller to open and close. If the opening faults of the exhaust valve 9 and the air inlet valve 14 occur in the remote control, the field worker is informed to manually openOpening the valve untilAnd returning to the preset pressure range and then closing the valve again.
More specifically, the feeding rate is controlled by the feeding valve 15, and the liquid level sensor continuously monitors the liquid level at the current moment when the material height reachesh 1 And in time, the valve is closed, so that the overflow risk caused by overfilling of materials is prevented, or the production progress is influenced too little. At this moment, the remote monitoring end transmits a signal to the controller, the controller controls the servo motor 12 to start rotating to drive the stirring paddle blade 10 to start rotating, the reaction materials are continuously stirred to start exothermic reaction, and the online detection and optimization of the material temperature and the gas temperature are carried out in real time.
And the liquid level height is detected and optimized in real time, the controller makes a judgment according to the obtained liquid level height and uploads information to the remote monitoring end, and the remote monitoring end controls the operation of the liquid level execution module. If the liquid level is not high enoughh 2 The liquid level execution module does not operate, and the liquid level sensor continuously monitors the liquid level height. If the liquid level reaches the heighth 2 The opening degree of the feeding valve 15 at the next time is reduced, the feeding amount is reduced, the opening degree of the discharging valve 2 is increased, the discharging amount is increased, and redundant materials are discharged. When the liquid level is lowered toh 1 Thereafter, the inlet/outlet valve 2 was closed again to carry out the reaction. If the liquid level height reaches, threatens the safety production of the reaction kettle, the remote monitoring end receives alarm information and controls the operation of the liquid level executing mechanism, the servo motor 12 receives a signal to stop running, the stirring paddle blade 10 stops rotating, the discharging valve 2 is completely opened to discharge redundant materials until the liquid level height is reduced to the levelh 1 And then, the discharge valve 2 is closed again, the servo motor 12 is restarted to drive the stirring paddle 10 to rotate, and the reaction continues.
And detecting and optimizing the gas pressure in real time, and judging by the controller according to the pressure safety interval input on line. When the real-time gas pressure is in a normal range, the system continues to react; when the real-time gas pressure exceeds the upper limit of the pressure safety interval, the system immediately gives an alarm and uploads alarm information to the remote monitoring end and automatically opens the exhaust valve 9, the remote monitoring end monitors the opening state of the exhaust valve 9, if the exhaust valve 9 fails to open, field workers are informed to manually open the exhaust valve 9, and the exhaust valve 9 is closed again until the real-time gas pressure is reduced to the preset pressure range. When the real-time gas pressure exceeds the lower limit of the pressure safety interval, the system immediately gives an alarm and uploads alarm information to the remote monitoring end and automatically opens the air inlet valve 14, the remote monitoring end monitors the opening state of the air inlet valve 14, if the air inlet valve 14 fails to open, field workers are informed to manually open the air inlet valve 14 until the real-time gas pressure rises to a preset pressure range, and then the air inlet valve 14 is closed again.
The technical features of the embodiments described above may be arbitrarily combined, and for the sake of brevity, all possible combinations of the technical features in the embodiments described above are not described, but should be considered as being within the scope of the present specification as long as there is no contradiction between the combinations of the technical features.
The above-mentioned embodiments only express several embodiments of the present invention, and the description thereof is more specific and detailed, but not construed as limiting the scope of the invention. It should be noted that, for a person skilled in the art, several variations and modifications can be made without departing from the inventive concept, which falls within the scope of the present invention. Therefore, the protection scope of the present patent should be subject to the appended claims.
Claims (10)
1. The safety parameter detection method in the reaction process of the reaction kettle is used for detecting the material temperature, the gas temperature, the liquid level height and the gas pressure in the reaction process of the reaction kettle, and is characterized by comprising the following steps:
the method comprises the following steps: obtaining the current timet k The material temperature at different heights in the reactants in the reaction kettleT pk To, forT pk Processing to obtain average material temperature(ii) a Obtaining the current timet k The gas temperature of the gas at different heights above the reactant in the reaction kettleT qk To is aligned withT qk Correcting and optimizing to obtain the average value of the gas temperature;
Wherein the content of the first and second substances,is the current timet k The material temperature of the reaction kettle in the reaction process;as the current timet k The gas temperature in the reaction process of the reaction kettle;
step two: obtaining the current timet k The liquid level height of reactants in the reaction kettleh k1 Based onTo pairh k1 Corrected liquid level height is obtained by correction optimization;
Wherein, the first and the second end of the pipe are connected with each other,as the current timet k The liquid level height of the reaction kettle in the reaction process;h k1 indirectly by ultrasonic measurement;
step three: obtaining the current timet k Gas pressure at two different positions above reactants in the reaction kettleα k1 、α k2 Based on、To pairα k1 、α k2 Respectively corrected to obtain corrected gas pressure、And will be、Fusing to obtain optimized gas pressure;
Wherein the content of the first and second substances,is the current timet k The gas pressure in the reaction kettle during the reaction process;
in the third step, the pressure of gasα yk The prediction correction is carried out by adopting the extended Kalman filtering,y=1,2;
for is toα yk The method for predicting and correcting by adopting the extended Kalman filtering comprises the following steps:
s301, establishing a prediction equation of the gas pressure above the reactant as follows:
wherein, the first and the second end of the pipe are connected with each other,is the current timet k Is predicted a priori by the gas pressure of (a),is the last momentt k-1 A posterior estimate of the gas pressure of (a);R g is a constant of the gas and is,v q k(-1) is the last momentt k-1 The specific volume of the gas (es),v qk as the current timet k The specific volume of gas;w g k(-1) is the last momentt k-1 The process noise of (1);a、bis a correction number;
s302, establishing a gas pressure observation equationZ gk :
Z gk =α yk +v gk ;
Wherein the content of the first and second substances,v gk is the current timet k Measurement noise of gas pressure;
s303, carrying out prior estimation on the gas pressure:
s304, calculating the gas pressure covariance:
wherein, the first and the second end of the pipe are connected with each other,as the current timet k Gas pressure covarianceThe predicted value of (2);P g k(-1) is the last momentt k-1 A posterior estimate of gas pressure covariance;A g k(-1) is the last momentt k-1 Linearizing the posterior estimated value to obtain a Jacobian matrix;
w gk Q g w gk T the kalman filter process noise is expanded for the gas pressure,w gk is the current timet k The process noise of the gas pressure of (c),Q g is process noisew gk The covariance matrix of (a);
s305, calculating a gas pressure gain coefficient:
wherein the content of the first and second substances,K gk as the current timet k The gain factor (c) of (a) is,H g for the equation of observationZ gk A coefficient matrix of (a);v gk R g v gk T the measurement noise of the gas pressure extended kalman filter,v gk is the noise of the gas pressure measurement of the current step,R g for measuring noisev gk The covariance matrix of (a);
s306, calculating a posterior estimation value of the gas pressure:
wherein the content of the first and second substances,is the current timet k A posterior estimate of the gas pressure,h g (α yk ) I.e. a non-linear expression of the gas pressure desired value;
s307, updating the gas pressure covariance:
wherein the content of the first and second substances,P gk as the current timet k A posteriori estimate of the covariance of the gas pressure,Iis an identity matrix.
2. The method for detecting the safety parameter in the reaction process of the reaction kettle according to claim 1, wherein the reaction kettle comprises a kettle body; two pressure sensors are arranged at two different positions in the kettle top of the kettle body and are used for detecting gas pressure at the two different positionsα k1 、α k2 ;
3. The method for detecting the safety parameters in the reaction process of the reaction kettle according to claim 1, wherein the reaction kettle further comprises a jacket for accommodating a heat exchange medium, and the jacket is arranged at the periphery of the kettle body and used for enabling the heat exchange medium to exchange heat with the kettle body;
the kettle body is internally provided withnThe temperature sensors are used for acquiring the temperatures of different positions of the kettle body; wherein the reactant is above the liquid levelmA temperature sensor for collecting gas temperatures at different heights of gas above the reactant in the reaction kettleT qk (ii) a Below the liquid level of the reactants aren-mA temperature sensor for collecting the material temperature at different heights in the reactant in the reaction kettleT pk 。
4. The method for detecting the safety parameter in the reaction process of the reaction kettle according to claim 3, characterized in that: in the step one, theT pk The treatment method comprises the following steps:
wherein, the first and the second end of the pipe are connected with each other,T ipk indicating the current time of dayt k From top to bottomiThe temperature value collected by each temperature sensor.
5. The method for detecting the safety parameter in the reaction process of the reaction kettle according to claim 4, characterized in that: in step one, toT qk Performing prediction correction by adopting extended Kalman filtering;
to pairT qk The method for predicting and correcting by adopting the extended Kalman filtering comprises the following steps:
s101, constructing a gas temperature prediction equation above the reactant based on a heat balance equation as follows:
wherein the content of the first and second substances,as the current timet k Is predicted a priori by the gas temperature of (c),V p is the volume of the reactant, ΔHIs the heat of the molar reaction,rin order to speed up the chemical reaction, the reaction solution,tthe time is characterized by the fact that,Uis the heat transfer coefficient between the reaction kettle and the jacket,A R is the contact area of the jacket and the reaction kettle,is the temperature of the materials in the reaction kettle,,T c is the outlet temperature of the heat exchange medium,Mwhich is the mass of the reactants, is,C p is the specific heat capacity of the reactants,C q is the specific heat capacity of the gas above the reactants;m q mass of gas above the reactant;dT q is the amount of change in the temperature of the gas per unit time,is the last momentt k-1 A posterior estimate of the gas temperature of (a),w q k-(1) is the last momentt k-1 The process noise of (1);
s102, establishing an observation equation of the temperature of the gas above the reactantZ qk :
Z qk =T qk +v qk ;
Wherein the content of the first and second substances,v qk as the current timet k Measurement noise of gas temperature;
s103, carrying out prior estimation on the gas temperature of each position:
s104, calculating the gas temperature covariance:
wherein the content of the first and second substances,as the current timet k A predicted value of gas temperature covariance;P q k(-1) is the last momentt k-1 A posterior estimate of the gas temperature covariance;A q k(-1) is the last momentt k-1 The posterior estimated value is linearized to obtain a Jacobian matrix;
w qk Q q w qk T the kalman filter process noise is extended for the gas temperature,w qk is the current timet k The temperature of the gas (es) process noise,Q q is process noisew qk The covariance matrix of (a);
s105, calculating a gas temperature gain coefficient:
wherein the content of the first and second substances,K qk as the current timet k The gain factor of (a) is determined,H q for the equation of observationZ qk A coefficient matrix of (a);v qk R q v qk T the measurement noise of the kalman filter is extended for the gas temperature,v qk is the noise of the gas temperature measurement of the current step,R q for measuring noisev qk The covariance matrix of (a);
s106, carrying out posterior estimation on the gas temperature of each position:
wherein, the first and the second end of the pipe are connected with each other,is the current timet k A posterior estimate of the gas temperature of (a);h q (T qk ) A gas temperature desired value nonlinear expression;
s107, updating the gas temperature covariance:
wherein the content of the first and second substances,P qk is the current timet k A posteriori estimate of the covariance of the gas temperature,Iis a unit matrix;
6. The method for detecting the safety parameters in the reaction process of the reaction kettle according to claim 5, wherein the ultrasonic wave measurement adopts an ultrasonic wave liquid level sensor; the ultrasonic liquid level sensor is arranged on the inner wall of the reaction kettle, is positioned above the liquid level of the reactant and is used for measuring the distance to the liquid level of the reactantL k ;
The liquid level height of reactants in the reaction kettleh k1 =h-L k ;
Wherein the content of the first and second substances,his the installation height of the ultrasonic liquid level sensor,L k as the current timet k The measured distance of (2).
7. The method for detecting safety parameters in the reaction process of a reaction kettle according to claim 6, wherein in the second step, the step ofh k1 The method for performing correction optimization comprises the following steps:
for is toL k Adopting extended Kalman filtering to carry out prediction correction to obtain corrected measurement distance;
Wherein, it is toL k The method for predicting and correcting by adopting the extended Kalman filtering comprises the following steps:
s201, establishing ultrasonic sound velocityV s And the current timet k Average value of gas temperatureAnd further establishing a prediction equation of the measured distance:
wherein, the first and the second end of the pipe are connected with each other,as the current timet k A priori predicted values of the distances are measured,is the last momentt k-1 A posterior estimate of the distance is measured,is the last momentt k-1 The gas is corrected to an optimized temperature value,V s k-(1) is at the last momentCarving toolt k-1 The speed of sound of the ultrasonic waves,t s k-(1) is the last momentt k-1 The total time for the ultrasonic sound velocity to travel back and forth,w s k-(1) for the last timet k-1 The system process noise of (a);
s202, establishing an observation equation of the measured distanceZ sk :
Z sk =L k +v sk ;
Wherein, the first and the second end of the pipe are connected with each other,v sk is the current timet k Measurement noise of the measurement distance;
s203, carrying out prior estimation on the measurement distance:
s204, calculating the covariance of the measurement distance:
wherein, the first and the second end of the pipe are connected with each other,as the current timet k Measuring a predicted value of the distance covariance;P s k-(1) is the last momentt k-1 Measuring a posterior estimate of the distance covariance;A s k-(1) is the last momentt k-1 Linearizing the posterior estimated value to obtain a Jacobian matrix;
w sk Q s w sk T in order to measure the distance extended kalman filter process noise,w sk is the current timet k The process noise of the measured distance is,Q s is process noisew sk The covariance matrix of (a);
s205, calculating a measurement distance gain coefficient:
wherein the content of the first and second substances,K sk is the current timet k The gain factor of (a) is determined,H s for observing the equationZ sk A coefficient matrix of (a);v sk R s v sk T in order to measure the measurement noise of the extended kalman filter,v sk is the current step measurement distance measurement noise,R s for measuring noisev sk The covariance matrix of (a);
s206, calculating a posterior estimated value of the measured distance:
wherein the content of the first and second substances,as the current timet k The posterior estimated value of the measured distance is the corrected measured distance;h s (L k ) A nonlinear expression for ultrasonic measurement expected values;
s207, updating the covariance of the measurement distance:
wherein the content of the first and second substances,P sk as the current timet k A posterior estimate of the range covariance is measured,Iis an identity matrix.
8. The device for detecting the safety parameters in the reaction process of the reaction kettle is characterized by using the method for detecting the safety parameters in the reaction process of the reaction kettle according to any one of claims 1 to 7,
safety parameter detection device includes in the reation kettle reaction process:
the material temperature data detection module is used for acquiring the material temperatures at different heights in reactants in the reaction kettle in real timeT pk And are combined toT pk Processing to obtain average material temperature;
The gas temperature data detection module is used for acquiring the gas temperatures of different heights of the gas above the reactants in the reaction kettle in real timeT qk Are combined with each otherT qk After optimization, the average value is taken to obtain an optimized value;
Liquid level height data detection module for collecting measurement distance in reaction kettle in real timeL k To, forL k Optimizing to obtain optimized valueAnd is based onObtaining the corrected liquid level height;
9. The control method of the reaction process of the reaction kettle is characterized by comprising the following steps:
obtaining the material temperature of the reaction kettle by using the method for detecting the safety parameter in the reaction kettle reaction process as claimed in any one of claims 1 to 7Gas temperatureHeight of liquid levelPressure of gas;
if it isIf not in the preset temperature range, judgingThe relation between the temperature and the upper limit temperature and the lower limit temperature of the temperature range;
wherein, ifWhen the temperature is higher than the upper limit temperature of the temperature range, an alarm is sent out, the temperature of the heat exchange medium flowing through the jacket is reduced, and the reaction kettle is cooled;
if it isThe temperature of the heat exchange medium flowing through the jacket is increased when the temperature is lower than the lower limit temperature of the temperature range, and the temperature of the reaction kettle is increased;
judgment ofSafety value of liquid levelh 1 Liquid level early warning valueh 2 Liquid level alarm valueh 3 The size of (d); wherein, the first and the second end of the pipe are connected with each other,h 3 >h 2 >h 1 ;
if it isReducing feeding to the reaction kettle and increasing discharging of the reaction kettle untilStopping discharging the reaction kettle;
if it isSending an alarm, stopping feeding to the reaction kettle, stopping stirring by the reaction kettle, and discharging the reaction kettle at the maximum flow until the reaction kettle is dischargedStopping discharging the reaction kettle, and restarting stirring the reaction kettle;
if it isIf not in the preset pressure range, judgingThe relationship with the upper limit pressure and the lower limit pressure of the pressure range;
wherein, ifWhen the pressure is higher than the upper limit pressure of the pressure range, an alarm is given out to release the pressure of the reaction kettle;
10. Control device of reation kettle reaction process, its characterized in that includes:
the data detection module comprises a temperature detection module, a liquid level detection module and a pressure detection module; the temperature detection module comprises a material temperature detection submodule and a gas temperature detection submodule; the material temperature detection submodule is used for acquiring the material temperature in the reaction process of the reaction kettle; the gas temperature detection submodule is used for acquiring the gas temperature in the reaction process of the reaction kettle; the liquid level detection module is used for acquiring the liquid level height of the reaction process in the reaction kettle; the pressure detection module is used for acquiring gas pressure in the reaction process in the reaction kettle;
the mechanism execution module comprises a temperature execution module, a liquid level execution module and a pressure execution module; the temperature execution module is used for adjusting the temperature of the reactant; the liquid level execution module is used for controlling the liquid level height of the reactant; the pressure execution module is used for adjusting the gas pressure; the controller is used for receiving the data acquired by the data detection module, wirelessly transmitting the data to the remote monitoring terminal, receiving the instruction sent by the remote monitoring terminal and controlling the corresponding execution module to work; wherein the remote monitoring end issues an instruction to the controller according to the control method of the reaction process of the reaction kettle as claimed in claim 9.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211688292.3A CN115646396B (en) | 2022-12-28 | 2022-12-28 | Safety parameter detection method, control method and device in reaction kettle reaction process |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211688292.3A CN115646396B (en) | 2022-12-28 | 2022-12-28 | Safety parameter detection method, control method and device in reaction kettle reaction process |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN115646396A CN115646396A (en) | 2023-01-31 |
| CN115646396B true CN115646396B (en) | 2023-03-10 |
Family
ID=85022455
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202211688292.3A Active CN115646396B (en) | 2022-12-28 | 2022-12-28 | Safety parameter detection method, control method and device in reaction kettle reaction process |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN115646396B (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117008481B (en) * | 2023-10-08 | 2023-12-22 | 安徽省特种设备检测院 | Pressure parameter optimization-based reaction kettle process control method and device |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102548650A (en) * | 2009-09-17 | 2012-07-04 | 巴斯夫欧洲公司 | Two-degree-of-freedom control having an explicit switching for controlling chemical engineering processes |
| CN104582829A (en) * | 2012-08-29 | 2015-04-29 | 博里利斯股份公司 | Reactor assembly and method for polymerization of olefins |
| CN107649079A (en) * | 2017-09-26 | 2018-02-02 | 中安信科技有限公司 | A kind of carbon fiber produces polymerization reactor control device and method |
| CN111580570A (en) * | 2020-05-28 | 2020-08-25 | 爱瑟福信息科技(上海)有限公司 | Container liquid level monitoring method and system |
| CN112068617A (en) * | 2019-06-11 | 2020-12-11 | 中国石油化工股份有限公司 | Safety control system and control method for oxidation unit of anthraquinone method hydrogen peroxide device |
| EP3967738A1 (en) * | 2020-09-15 | 2022-03-16 | Socar Turkey Enerji A.S. | Estimation method of nitrogen content in the hydrocracker reactor feedstock for temperature optimization |
| CN115007084A (en) * | 2022-08-04 | 2022-09-06 | 安徽建筑大学 | Reaction kettle reaction process temperature detection method and device, control method and reaction kettle |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9310137B2 (en) * | 2013-04-29 | 2016-04-12 | Chevron Phillips Chemical Company, Lp | Unified cooling in multiple polyolefin polymerization reactors |
| EP3696619A1 (en) * | 2019-02-15 | 2020-08-19 | Basf Se | Determining operating conditions in chemical production plants |
| CN210752600U (en) * | 2019-09-06 | 2020-06-16 | 成都硅特自动化设备有限公司 | Reaction kettle capable of regulating and controlling reaction temperature |
-
2022
- 2022-12-28 CN CN202211688292.3A patent/CN115646396B/en active Active
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102548650A (en) * | 2009-09-17 | 2012-07-04 | 巴斯夫欧洲公司 | Two-degree-of-freedom control having an explicit switching for controlling chemical engineering processes |
| CN104582829A (en) * | 2012-08-29 | 2015-04-29 | 博里利斯股份公司 | Reactor assembly and method for polymerization of olefins |
| CN107649079A (en) * | 2017-09-26 | 2018-02-02 | 中安信科技有限公司 | A kind of carbon fiber produces polymerization reactor control device and method |
| CN112068617A (en) * | 2019-06-11 | 2020-12-11 | 中国石油化工股份有限公司 | Safety control system and control method for oxidation unit of anthraquinone method hydrogen peroxide device |
| CN111580570A (en) * | 2020-05-28 | 2020-08-25 | 爱瑟福信息科技(上海)有限公司 | Container liquid level monitoring method and system |
| EP3967738A1 (en) * | 2020-09-15 | 2022-03-16 | Socar Turkey Enerji A.S. | Estimation method of nitrogen content in the hydrocracker reactor feedstock for temperature optimization |
| CN115007084A (en) * | 2022-08-04 | 2022-09-06 | 安徽建筑大学 | Reaction kettle reaction process temperature detection method and device, control method and reaction kettle |
Also Published As
| Publication number | Publication date |
|---|---|
| CN115646396A (en) | 2023-01-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN115646396B (en) | Safety parameter detection method, control method and device in reaction kettle reaction process | |
| CN102692248B (en) | The deteriorated sensor realized in transmitter detects | |
| CN115007084B (en) | Reaction kettle reaction process temperature detection method and device, control method and reaction kettle | |
| US20140096836A1 (en) | Method and automated system for control of oil well production and modular skid for use in said method | |
| EP3783346A1 (en) | Scale thickness estimation system, scale thickness estimation method, and scale thickness estimation program | |
| JP4374814B2 (en) | Treatment method for perfluoride treatment | |
| CN115591493A (en) | Reaction kettle temperature control method | |
| CN108469390B (en) | Detachable loop type single-phase flow erosion test device | |
| US20150260423A1 (en) | Thermal Source Instrument Controlling Device and Air-Conditioning System | |
| JP2020011230A (en) | Liquid mixing device with electronic control of high dynamic regulation and operating method thereof | |
| CN211159717U (en) | Vanadyl sulfate solution preparation device and production system | |
| US6109096A (en) | Methods and apparatus for monitoring water process equipment | |
| KR100862859B1 (en) | Gas holder using seal oil level compensation | |
| CN210018927U (en) | Saucepan with automatic temperature control detection function | |
| CN208627240U (en) | A kind of high-temperature high-pressure reaction kettle | |
| US20180321066A1 (en) | Real-time vessel monitoring system | |
| CN215864243U (en) | Novel circulating cooling water system | |
| CN102070290A (en) | Methods for making a glass material and apparatus | |
| CN115747877A (en) | Temperature control method for electrolytic cell and alkaline water electrolysis hydrogen production system | |
| CN202631490U (en) | On-line industrial ion chromatography analysis and measurement device | |
| JP4788963B2 (en) | Chemical reaction calorimeter and measuring method | |
| JP5299042B2 (en) | Silicon cutting powder oxidation inhibiting method and silicon cutting powder oxidation inhibiting apparatus | |
| CN111912740A (en) | Density measuring device and method and wet desulphurization tower comprising same | |
| CN208109780U (en) | A kind of solid particle high-temperature material pH detection device | |
| RU2809036C1 (en) | System for precision control of production parameters of titanium sponge restoration process and control method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |