CN1064538A - Palladium partial combustion catalyst and using method thereof - Google Patents
Palladium partial combustion catalyst and using method thereof Download PDFInfo
- Publication number
- CN1064538A CN1064538A CN91111207A CN91111207A CN1064538A CN 1064538 A CN1064538 A CN 1064538A CN 91111207 A CN91111207 A CN 91111207A CN 91111207 A CN91111207 A CN 91111207A CN 1064538 A CN1064538 A CN 1064538A
- Authority
- CN
- China
- Prior art keywords
- catalyst
- palladium
- temperature
- carrier
- combustion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Landscapes
- Catalysts (AREA)
Abstract
The present invention relates to a kind of palladium-containing catalyst and a kind of partial combustion method that is placed on the carrier, fuel utilizes this catalyst and partial combustion in the method.Palladium catalyst can comprise that also palladium mixes mutually with the metal that is selected from VIII or IB family; It can be segmentation, makes to have greater activity in the catalyst forward position; Also can place on the carrier of zirconia formation.Select such catalyst and carrier for use, solved the problem that many and palladium interrelate as the long-time stability of partial combustion catalyst.It is stable that this catalyst structure is in operation, and it has lower operating temperature, low beginning oxidizing temperature, and be difficult for occurrence temperature " out of control ".
Description
The present invention is that the part of following patent continues: Dalla Betta, the U.S.Ser.S.No.07/617 of Tsurumi and Shoji, 974, " a kind of segmentation contain palladium partial combustion catalyst and using method (PA-0029) thereof "; Dalla Betta, Shoji, the U.S.Ser.No.07/617 of Tsurumi and Ezawa, 975, " a kind of partial combustion method and a kind of catalyst (PA-0006) that is used for this method "; Dalla Betta, Tsurumi, the U.S.Ser.No.07/617 of Shoji and Garten, 979, " a kind of catalyst and using method (PA-0026) thereof " in the burning of zirconium carrier top; And Dalla Betta, Ezawa, Tsurumi, the U.S.Ser of Shoji and Ribeiro, No.07/617,981, " a kind of partial combustion catalyst and using method (PA-0025) thereof that contains the palladium hybrid metal ", each patent is all merged into integral body in submission on November 26 nineteen ninety and by announcement.
The present invention relates to a kind of catalyst and a kind of partially combusted method that contains palladium on carrier, fuel is by the sort of catalyst and partially combusted in the method.This palladium catalyst also can comprise and is mixed with the palladium that is selected from VIII family or IB family metal, can be segmentation so that the forward position of its catalyst has higher active or place and contain on the zirconic carrier.The selection of above-mentioned catalyst and carrier has all solved many long term stability problem as the partial combustion palladium catalyst.It is stable that this catalyst structure is in operation, and has low relatively operating temperature, low beginning oxidizing temperature, and it is out of control to be difficult for occurrence temperature.The combustion gas that typical catalytic process is produced is in the temperature that is lower than self-ignition point, and can use under this temperature, perhaps is transported to other burning zone to be further used for gas turbine, kiln, boiler etc.
In the U.S. and all over the world, along with the appearance of modern antipollution regulation, studying all contaminations is being reduced to minimum effective new method.Though fuel-be wooden, coal, oil or natural gas, their burning all is the reason of current many pollution problems.Some pollutant (as if SO
2, it is owing to the pollutant that exists in the fuels sources produces) both can remove by handling fuel, also can remove by the waste gas of handling last generation.Other pollutant, for example carbon monoxide (it is owing to imperfect combustion produces) can be removed by the oxidation after the burning or by improving combustion method.The pollutant that other is main, NO
x(a kind of equilibrium mixture, great majority are NO, but also comprise very a spot of NO
2) can remove by the control combustion process or by post processing, to reduce NO as far as possible
xGeneration.NO
xIn case produce,, remove it and be not easy because its relative stability and it are low concentrations in most of waste gas.A kind of solution of finding in automobile is to utilize carbon monoxide with NO
xBe reduced to nitrogen, carbon monoxide then is oxidized to carbon dioxide.Even so, in some combustion process, the concentration of (for example gas turbine) carbon monoxide is to be not enough to and NO
xReaction is also removed its, and must see: different with amounts of sulphur contaminants, amounts of sulphur contaminants can be removed from fuel, obviously is unpractiaca and remove nitrogen from the air of input combustion process.Different with the carbon monoxide situation, owing to have higher temperature in the combustion process, improve the NO that combustion reaction may increase new generation
xContent.
Yet, in combustion process, reduce NO
xChallenge still exist, some diverse ways have now been proposed.Selected minimizing NO
xMethod must not contradict basically with the target that produces burning gases, produce burning gases and just reclaim the calorific value of combustion gas with turbine, boiler or kiln.
Many people recognize: be used for the turbine feed gas, NO in the control combustion process
xA kind of effective method that produces is that local and bulk temperature is restricted to and is lower than 1800 ℃ with the combustion zone.For example see people's such as Furuya U.S. Patent No. 4,731,989 first row, people's such as the capable and Hindin of 52-59 U.S. Patent No. 4,088,135 the 12nd row.
Many control method of temperature are arranged,,, or use various fuel mixtures barren or enrichment to carry out sectional combustion with one or more catalyst control oxidations for example with excessive air dilution.Being used in combination of these methods also is known.
A kind of extensive trial method is to use multistage catalytic combustion, greatly partly has been the multistage catalyst that uses metal oxide on ceramic catalyst carrier in the disclosed method.These disclosed typical cases are as follows:
First section second section the 3rd section of public document
Day disclosure 60-205129 Pt family/Al
2O
3La/SiO
2Al
2O
3
SiO
2
Day disclosure 60-147243 La and Pd and ferrite/Al
2O
3
Pt/Al
2O
3
Day disclosure 60-66022 Pd and Pt/ZrO
2Ni/ZrO
2
Day disclosure 60-60424 Pd/ CaO and Al
2O
3With
The expensive gold of NiO and W/
Belong to
Day disclosure 60-51545 Pd/* Pt/* LaCO
3/ *
Day disclosure 60-51543 Pd/* Pt/*
Day disclosure 60-51544 Pd/* Pt/*? Base Metal oxygen
Change thing/*
Day disclosure 60-54736 Pd/* Pt or Pt-Rh or
The oxidation of Ni Base Metal
Thing
Or LaCO
3/ *
Day disclosure 60-202235 MoO
4/-CoO
3And ZrO
2
And noble metal
Day disclosure 60-200021 Pd and Al
2O
3/ Pd and AO
2O
3/ * Pt/**
+*??*
Day disclosure 60-147243 noble metal/heat-resisting ferrite/heat-resisting year
The carrier body
Day disclosure 60-60424 La or Nd/ Pd or Pt/Nio
2Or
Al
2O
3Al
2O
3Or
0.5%SiO
2CaO0.5%SiO
Day disclosure 60-14938 Pd/? Pt/?
Be day disclosure 60-14939 Pd and Pt/ anti-?
The fire material
Day disclosure 61-252409 Pd and Pt/** Pd and Ni/*** Pd and Pt/**
*??????????????????????????*
Day disclosure 62-080419 Pd and Pt Pd, Pt and NiO Pt or Pt and Pd
Day disclosure 62-080420 Pd and Pt and Pt Pt or Pd
NiO
Day disclosure 63-080848 Pd and Pd Pd and Pt and NiO Pt or Pt and Pd
Day disclosure 63-080849 Pd, Pt, NiO/? Pd and Pt(or Pt or Pd and
NiO)/??????????Pt/
* aluminium oxide on mullite or cordierite or zirconia
* Co is at ground floor; Zr, Sr, one or more of Ba are at the second layer, and La and Nd are at least a at the 3rd layer
* * is with the monolithic carrier of lanthanide series or alkaline earth oxide stabilisation
Annotate: catalyst indicates with " a " "/b " in the table, and wherein a is a reactive metal and " b " is carrier.
Yet in these methods, control medium temperature or inter-stage temperature are difficult.Because the target of these methods all is to produce heat as much as possible, as to be convenient to the form that can effectively utilize in some back process, combustion phases is adiabatic basically.Therefore, combustion rate, air speed or operating process any point point change the significant variation that all will cause intersegmental temperature.Very high temperature causes producing thermal strain on its follow-up catalyst element.
This table clearly illustrates that: the platinum group metal that comprises palladium is useful in catalyticing combustion process, yet, traditional catalyticing combustion process normally mixes fuel and air, makes this mixture it that completing combustion basically take place by the catalyst on the catalytic bed then.This causes extremely high temperature, generally is 1100 ℃ to 1500 ℃.For this reason, those catalyst and carriers that can stand this high temperature and energy retentive activity are pointed in more catalyst development work.Some research depends on control method, and in these methods, air in the middle of introducing between different catalytic stages or fuel stream, its flow velocity are according to the overall gas temperature and in check.People such as above-mentioned Furuya have described a kind of method that overcomes the high problem of catalyst temperature, and it is to dilute fuel/air mixture by air is input to catalyst, and the mixture that is produced to cause has 900 ℃ to 1000 ℃ adiabatic combustion temperature.By catalyst, reaction partially or completely makes that catalyst temperature is the highest to be no more than 1000 ℃, and gas temperature is no more than 1000 ℃ with this mixture.After evenly burning has obtained temperature required (1200 ℃ to 1500 ℃), add the fuel that appends at catalyst and this mixture.Yet the shortcoming of this method is to add fuel two stages, and need mix this fuel that appends with hot gas and mustn't go to traditional High temperature diffusion flame and corresponding N O
xProduct.
Our this inventive method at beginning one end of combustion chamber in certain proportion with air and fuel mix, make that this final ignition temperature (after further burning or several combustion step) is to reclaim hot device for some subsequent processes or from combustion gas, be gas turbine, needed.Typical mixture can be methane and AIR MIXTURES, its mixing ratio, and volume of fuel/volume of air is 0.043.This mixture (after being preheating to 350 ℃) can produce about 1300 ℃ ignition temperature.This mixture is by branch on the catalyst and utilize catalyst to make its generating unit divided combustion, and the catalytic temperature maximum is limited in a remarkable adiabatic combustion temperature less than this gas.The effect of restriction is considered to because following reaction:
During carrying out, reaction have oxygen partial pressure to exist.The temperature that transforms takes place in the palladium that the temperature of having found to be limited obtains in heat Jie gravimetric analysis (TGA) exactly/palladium oxide.Say that roughly pure palladium is about 780 ℃ to 800 ℃ in the airborne this conversion temperature of 1atm, and is about 930 ℃ to 950 ℃ in 10 atm air.
We find, can become unstable at partial combustion palladium catalyst in service.Through after a while, reaction has stopped and also having improved for the required preheat temperature level of energy stable operation.To this problem, we have found the way of many solutions.For example, we have observed the present invention by adopting following one or more measures of the present invention, have stable temp autocontrolled characteristics.
A. use palladium (and optional other VIII family noble metal, as platinum, osmium, rhodium, ruthenium; Platinum preferably; Or optional IB family metal, as copper, gold, silver; Preferably silver-colored) as active catalytic metal.
B. apply one deck diffusion impervious layer at catalyst surface, be diffused into catalyst surface, thereby limited the speed of catalytic reaction, and then make the palladium can limited maximum temperature with fuel limitation.
C. use contain zirconic carrier (preferably being placed on the metal substrate) thus the bearing catalyst layer provides a kind of catalyst structure that tolerates very much thermal shock, or
D. on carrier, place catalyst metals so that contain the catalyst material of greater activity in the forward position part of the catalyst structure of contact flow air-flow.
The front for example people such as Furuya at U.S patent No.4,731989 have described the mutual conversion of palladium oxide and palladium about 800 ℃, yet, this patent is described this conversion as shortcoming, because the active oxidation palladium is converted into active less palladium, thereby has stoped the combustion reaction on catalyst to be carried out fully.The process that the inventive method utilizes this palladium oxide/palladium to transform mutually makes it stable in a different manner and promotes its generation, here in order to the limiting catalyst temperature, thereby can use (very) high activity and stable catalyst.
Significantly be lower than this adiabatic combustion temperature by catalyst temperature is maintained, then some problems that interrelate with the thermal shock of the evaporation of the thermal sintering of catalyst, palladium and carrier are just significantly reduced or are eliminated.
Mentioned in passing metallic catalyst carrier has been used for platinum metal catalysts, for example see people's such as Hindin U.S. patent No.4.088,435 " platinums group metal " are in the 4th to 63 row and later a few row thereof, and " carrier can be metal or ceramic ... " is listed as the 45th row the 6th.On the contrary, at people's such as Hindin U.S. patent No.4,287,856 the 1st be listed as the 65th row and wait the place to mention that usefulness platinum metal alloy monolith catalyst is as combustion catalyst.Other similarly open U.S. patent NoS.3966391 as people such as previous Hindin; 3,956,188; 4,008,037 and 4,021,185 all can find.At people's such as Madgavkar U.S. patent No.4, having mentioned in 366,688 will be at the combustion catalyst of the platinum on steel (" the Fecralloy ") carrier as low-BTU gas.
Disclosed other metal and the metallic carrier that is mainly used in the catalytic converter of automobile comprises:
The public document patentee
The U.S. 3,920,583 Pugh
The U.S. 3,988, people such as 082 Cairns
The U.S. 4,279, people such as 782 Chapman
The U.S. 4,318,828, people such as Chapman
The U.S. 4,331, people such as 631 Chapman
The U.S. 4,414, people such as 023 Aggen
The U.S. 4,521,532 Cho
The U.S. 4,601, people such as 999 Retalliok
The U.S. 4,673,663 Mangnier
The U.S. 4,742,038 Matsumoto
The U.S. 4,752, people such as 599 Nakamura
The U.S. 4,784, people such as 984 Yamanaka
Britain 1,528, people such as 455 Cairns
As gang, these patents are discussed the ferrate catalyst carrier usually, on this carrier aluminium oxide as crystallite, coat, whisker or the like and exist.Many patent disclosures the platinum group metal be to be suitable for being placed on those carriers as catalyst.Nobody proposes to contain the catalyst of palladium, does not also propose them and has the ability of limiting catalyst temperature stably.
Yet, on practice significance, the use of metallic carrier has been limited to the situation that adiabatic combustion temperature is lower than 1100 ℃ or 1000 ℃, in this case, the liner temperature that the completing combustion of fuel/air mixture causes will can not destroyed metal, and this block has limited the temperature that final gas can reach or needed operational phase fuel or add air and make Combustion chamber design further complicated.Even be 1300 ℃ to 1600 ℃ fuel/air mixture for adiabatic combustion temperature, the use of the inventive method has limited the metal gasket temperature and be lower than 850 ℃ when 1atm, and be lower than 950 ℃ when 16atm.
The inventive method also provides the advantage of using ceramic substrate owing to stably limit the liner temperature, because restriction liner temperature has reduced thermal stress, and when when starting and closing the combustion chamber, has reduced the caused fault of thermal shock.It is 1300 ℃ to 1600 ℃ that this protection is equivalent to adiabatic combustion temperature to the fuel/air mixture ratio, and it is important to seem especially.
Generally speaking, though document has proposed the various non-relative section of the inventive method and catalyst structure, these files all do not propose the advantage of disclosed palladium-containing catalyst (except stably having limited the liner temperature).
The present invention relates to a kind of combustion catalyst that contains palladium.This combustion catalyst also can comprise IB family or VIII family noble metal, and can be placed at and comprise on the zirconium carrier.In addition, can be with this combustion catalyst segmentation, can there be a higher part of activity in the forward position of this catalyst structure in other words, the present invention includes a kind of partial combustion method, wherein fuel is to utilize this kind catalyst and partially combusted, and the selecting for use of above-mentioned catalyst and carrier solved in the present technique field palladium as the long term stability problem of partial combustion catalyst.It is stable that this catalyst structure is in operation, and has low relatively operating temperature, has low " lighting " temperature, but it is that to be difficult for occurrence temperature out of control.The combustion gas that catalytic process produced can be in below the autoignition temperature, and it can use under this temperature, perhaps is transported to other combustion phases to be further used for gas turbine, kiln, boiler etc.
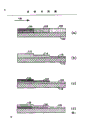
Fig. 1 and 2 is the section particular illustration of some catalyst in the scope of the invention.
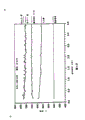
Fig. 3 is a palladium and platinum catalyst separately operating temperature comparison diagram under different fuel/air mixture ratios.
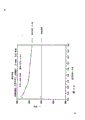
Fig. 4 A is that palladium oxide/palladium is at the airborne TGA figure of 1atm.
Fig. 4 B is the TGA figure of palladium oxide/palladium in the 1atm pure oxygen
Fig. 5 is for the various procedure exit temperature of a certain specific uncoated catalyst and the graph of a relation of catalyst warm-up temperature.
Fig. 6 is for the various procedure exit temperature of a certain specific catalyst with diffusion impervious layer and the graph of a relation of catalyst warm-up temperature.
Fig. 7 A and 7B are the LOT figure and steady-state operation hygrogram that is coated with zirconic cordierite monolithic.
Fig. 8 A and 8B are the LOT figure and steady-state operation hygrogram that is coated with zirconic metal monolithic.
Fig. 9 A and 9B are when use is coated with zirconic ceramic monolith, the hygrogram of expression working condition of the present invention.
Figure 10 A and 10B are the hygrograms of making comparisons with Fig. 9 A and 9B.
Figure 11 A and 11B are when use is coated with zirconic metallic carrier, the hygrogram of expression working condition of the present invention.
Figure 12 A and 12B are the hygrograms of making comparisons with Figure 11 A and 11B.
Figure 13 A-13D and Figure 14 A-14D show that the platinum of varying number is to the influence of its working condition in the palladium combustion catalyst that with the cordierite is carrier.
Figure 15 A-15B and Figure 16 A-16B show that platinum is to the influence of its working condition in the palladium combustion catalyst that with the metal is carrier.
Figure 17 and Figure 18 are the hygrograms that shows graded catalyst of the present invention and comparative catalyst's service behaviour.
The present invention is a kind of combustion catalyst that contains palladium, and it also can contain 1B or VIII family noble metal, and can be placed on the carrier that contains zirconium. And this combustion catalyst can be segmentation, namely can have in the forward position of catalyst structure active higher part. The present invention includes a kind of partial combustion method, this catalyst of fuel applications wherein and partial combustion. Select such catalyst and carrier to solve in the art about the long term stability problem of palladium as the partial combustion catalyst. This catalyst structure is stable at work, and operating temperature is lower, has low " lighting-extinguish " temperature, but is difficult for occurrence temperature " out of control ". The burning gases that produced by catalytic process can be in below the autoignition temperature, and it can use under this temperature, or is transported to other combustion phases, to be further used for gas turbine, kiln, boiler etc.
This catalyst contains palladium, also can contain any one or more VIII family noble metal (platinum, ruthenium, rhodium, osmium, iridium are take platinum as better) or IB family metal (take silver as better), and its quantity equates with the molal quantity of palladium at most. Be 325 ℃ and following in temperature, palladium is quite active as oxidation catalyst, so it can be used for the partial combustion process as a kind of " lighting-extinguish " catalyst. As previously discussed, palladium it is believed that it is owing to there is palladium oxide as the catalytic activity of combustion oxidation catalyst. Metal Palladium as catalyst except under the quite high temperature (as at 750 ° to more than 800 ℃) be not very active. Metal Palladium exists under the condition of excess of oxygen, just is easy to be oxidized to palladium oxide by following balanced reaction under 325 ℃ temperature low:
1/2O
2+Pd←→PdO
When but temperature raise, balance was moved to the left, and namely palladium oxide decomposes. Such transformation makes reaction temperature become self limit. In 1 atmospheric air, burning makes temperature easily reach 750 ℃ to 800 ℃, and at this moment palladium oxide becomes the species that have negligible amounts, thereby makes reaction slack-off.
Palladium oxide is converted into the temperature of palladium, partly depends on the dividing potential drop of oxygen. Conversion temperature can easily be measured by thermogravimetry (" TGA "-namely measures the method for palladium oxide example weight loss when temperature increases). The PdO-Pd inversion point is established the underlayer temperature of self limit under this condition of work. Palladium catalyst as combustion catalyst generally will be restricted to this TGA restriction conversion temperature to underlayer temperature.
Yet we have found that, use palladium (those substrates of the aluminium oxide that particularly contains) can obtain unpredictable partial oxidation catalyst of a kind of life-span at some substrate. Although the outlet temperature that produces reduces can be very remarkable, deactivated reason is still unclear, and this effect both can be at the metallic carrier of coating alumina, also can be in sight in the situation of pure alumina. The VIII family noble metal of certain tittle or IB family metal (for example platinum or silver) joined go to make catalyst combination deposits yields long-time stability in the palladium catalyst, and substantially do not affect low " lighting-extinguish " temperature that meets the requirements that palladium catalyst has, we have found that also a kind of catalyst carrier that comprises zirconia (reasonable be with zirconium form) also can make the steady-state operation stable performance of palladium base section oxidation catalyst.
The quantity of the palladium metal that adds should be able to be enough to provide catalytic activity. The concrete quantity that adds depends on many conditions, used fuel for example, economy, activity, life-span, existence of pollutant etc. (palladium) metal maximum quantity in theory causing that not unsuitable metal small crystal growth and the activity of following reduce in the situation, approximately is to be enough to topped most of substrate. Following obviously is the factor of confronting with each other: the maximum higher surperficial topped thickness of catalyst activity sexual needs, and higher surperficial topped thickness can promote the growth between the adjacent small crystals. And, also must consider the form of catalyst carrier. If carrier is to use in the environment of a high space velocity, the catalyst loading amount should be high, even so that in short situation of the time of staying, also can keep enough conversion ratios, total as it target, wish to use economically the catalyst metals of minimum number, just can finish needed task. At last, the existence of pollutant requires to use the catalyst of higher useful load to offset owing to catalyst activity reduces factor in the burning.
The content of palladium metal generally should be from about 0.01% to about 25%(% by weight in this catalyst composites), this quantity also is subjected to the impact of charging composition.
Palladium can by the dispersion with palladium complex, compound or this metal, be incorporated on the carrier with various method. This compound or complex can water-soluble or hydrocarbon. Palladium metal can be precipitated out from solution. Liquid-carrier usually only needs by volatilization or decomposes and remove from catalyst carrier, and with the form of disperseing palladium is stayed on the carrier. Being applicable to prepare the complex of palladium of catalyst system therefor of the present invention and the example of compound is the chloride of palladium: diamino two nitrite anions close the chloride of palladium, palladium nitrate, four ammino palladiums; Sodium chloride palladium, 2 ethyl hexanoic acid palladium and various other palladium salt or complex. Although the prepared general activity of catalyst of chloride is very high,, under this catalyst was situation for the burner of gas turbine, chloride was not a best selection just. Because chloride (even with very little amount) can significantly cause the corrosion of turbo blade and cylindrical shell. Thereby the parent of nitrogenous palladium is the most desirable.
As previously mentioned, catalyst can comprise a kind of catalyst of interpolation, for example a kind of IB family's metal (such as resembling silver) or a kind of VIII family's noble metal (such as resembling platinum), and its quantity can reach the mole that is slightly more than platinum in carbon monoxide-olefin polymeric. The molar ratio of palladium and interpolation metal is effective between 0.95-0.25. Although the metal that adds can add as a kind of complex, compound or metal dispersion by being incorporated in the liquid-carrier that contains palladium, if the metal that adds is to add in step subsequently, then the stability of resulting catalyst just more can expect. The platinum complex and the compound that are suitable for preparing the selected catalyst of the satisfactory hope of the present invention are the chloride of platinum: the chloride of two nitrite anions, two ammino platinum, platinum nitrate, four ammino platinum, sodium chloride platinum and various other platinum salt or complex. Similar salt and the complex of other VIII family noble metal or IB family metal are known.
Also as previously mentioned, we have seen use a kind of catalyst structure (its forward position has the tail portion greater activity than it), then can produce a kind of structure with following advantages: lower " a little-extinguish " temperature, this catalyst structure do not have " focus " than the rear section, and be the stable catalyst of a kind of behavior in service generally.
The structure of segmentation can make with many different modes, as shown in Figure 1, substrate metal or pottery (102) can be with three kinds of different catalyst (104,106 and 108) apply, every kind of catalyst has different activity: 104 catalyst have high activity, 106 catalyst activities are medium, and 108 catalyst have lowest activity. 104,106 and 108 catalyst can obtain by the useful load that changes the active catalyst material. For example, 104 catalyst contain 20% palladium, and 106 catalyst contain 10% palladium, and 108 catalyst contain 5% palladium. Another method is that the palladium dispersion can have the 104 different with 108 variation of minimum dispersion of a best result prose style free from parallelism because of contained.
Another method is the catalyst that obtains segmentation with shallow layer, and this shallow layer not only comprises a kind of material of oxidation but also comprise a kind of catalyst material with constant activity, and changes thickness along catalyst. In figure IB, a kind of catalyst shallow layer with constant activity is applied to substrate (110), (112) apply thick-layer (having greater activity) in the porch, and are thinner in middle (114), and (116) are very thin in the exit.
The third method is to use the catalyst shallow layer of different activities, (such as Fig. 1 a) but make these shallow layers become overlayer (such as Fig. 1 C), it is to be applied to above the whole substrate (118) that low activity catalyst has coating (120), use subsequently the part (122) of the shallow layer coated substrate of medium activity, at last only at entrance coated high activity shallow layer (124). In addition, a kind of structure is illustrated in Fig. 1 d, and wherein high activity shallow layer (126) at first is applied to the porch, uses subsequently medium activity (128) and low activity (130) shallow layer.
Can make the shallow layer of high, medium and low activity by many methods. Active component (as if palladium) can change from the high concentration to the low concentration. In addition, the dispersion of active palladium can be by heat-treating to obtain to change to catalyst with different preparation methods or in different temperatures. Another kind method is the surface that changes the active component deposition. For example, for a kind of palladium/Al2O
3Or palladium/ZrO2Catalyst adopts the long-pending Al of different surfaces2O
3Or ZrO2Carrier will produce the catalyst of different activities, and the carrier with high surface area will have higher activity.
The catalyst structure of these segmentations can prepare by several method. For example, the structure shown in Fig. 1 d can make at the peak of pottery nest monolithic, by this monolithic is partly immersed in the coating solution, and excessive coating solution is blown out passage. Then, repeat this process, this monolithic is immersed in coated holds glue more deeply. This identical universal method can be used for preparing the structure of Fig. 1 c. This same method can be used for the metal monolithic, and this metal monolithic is by the rolling metal forming or for spiral-tube or folded metal paper tinsel become needed shape, as makes as described in the ceramic monoliths the sort of with its dipping.
Another kind method is the catalyst layer with segmentation, be applied to suitably to become on the undulatory metal forming, thereby then rolling becomes helical structure to form last catalyst elements. Coating solution is sprayed or be brushed to metal foil surface, perhaps apply with other common known methods, for example use chemical vapour deposition (CVD), sputter etc. In order to obtain desirable segmental structure catalyst, paillon foil can partly be covered with limiting catalyst and only arrive desirable zone. In this way, such as Fig. 1 a, the seen structure of 1b and 1d can make by spraying on paillon foil or brushing, and one side or both sides that coating solution can only be applied to tinsel all apply.
Should be noted that: in these combined methods, catalyst can be used as a kind of compound mixture and the high surface area carrier (Al of active catalyst (as if palladium)2O
3,ZrO
2And SiO2Deng) apply. These will be by being impregnated into palladium on high surface oxide powder surface, and then calcining transforms into colloid with it and make. In the second approach, the high surface coating solution can at first be applied on monolithic or the tinsel, and the location. Then, catalyst (such as palladium) can be applied in by same dipping or sprinkling process. Structure shown in Fig. 1 d can be by using single coating solution oxide sol and single catalyst (palladium) solution, and repeat the method and make.
Fig. 2 illustrates the catalyst of another kind of series, and catalyst coat wherein makes with the above-mentioned either method of discussing, and in Fig. 2 a, the catalyst (202) of thicker (therefore having more activity) is arranged on the upstream of thinner catalyst layer (202). Similarly be in Fig. 2 b, used one and lacked but thick catalyst layer (208). Different from Fig. 2 a, a kind of like this configuration also can adopt catalyst or the color preheating with higher level of namely adopting greater activity. Fig. 2 c represents the catalyst that a kind of segmentation changes, and Fig. 2 d represents constant variation.
Preferably carrier for this catalyst is metal. Spiral shell cellular-shaped, corrugated plate execute volume (it can and flat shim between stack), column (" one straw " shape) or other have that vertical passage can access high space velocity and the structure of Pressure Drop minimum all is suitable for purposes of the present invention. They are malleable, can easily install and be connected on the surrounding structure, and less to the resistance of air-flow because its wall than ceramic monolith the wall that can easily make thinner.
Another practical advantage of metallic carrier is its ability that stands thermal shock. It is in service that such thermal shock betides the combustion turbine, when turbine starts or stops, particularly when the necessary quick closedown of turbine. In this rear situation, fuel is cut off, and perhaps because the physical load (for example generating set) of turbine is removed, turbine by " unhook " (tripped). The fuel of supplying with turbine will cut off to prevent from overrunning immediately. Temperature in the combustion chamber (process of the present invention just occurs therein) is very fast to drop to compressed-air actuated temperature from ignition temperature. This decline may be crossed over more than 1000 ℃ within less than 1 second time. Catalyst always deposits on (or otherwise placing) wall in the metallic carrier passage in any case, and its quantity as mentioned above. Have the carrier material of several types can make satisfactorily this purposes: aluminium, contain aluminium or with steel and some stainless steel or any high-temperature metal alloys that aluminium was processed, comprise that catalyst can be deposited on cobalt on the metal surface or the alloy of nickel.
Material is the steel that contains aluminium preferably, such as United States Patent (USP), people such as 4,414,023(Aqgen), United States Patent (USP) 4,331, the people such as 631(Chopman), with people such as 3,969,082(Calrn) etc. document introduce. These steel, and (the River Lite 20-5 SR) that sold by Kawasaki iron company, (the Aluchrom 1RE) of Vereinigte Deutehse Metallwerke AG, and Allegheny Ludlum Steel (Alfa-1V) all contains enough dissolved aluminums, so that when oxidized, these aluminium are at Surface Creation alumina whisker or the crystal of steel, thereby a coarse and chemically active surface is provided, so that coating is better adhered thereto.
After alumina whisker generates, can process these steel with a kind of zirconium-containing compound, perhaps process with suspension or the colloidal sol of zirconia or hydrous zirconium oxide(HZO) better. Palladium compound and any other catalyst precursor generally all are applied on the zirconia coat, although also can will mix palladium in the zirconium coating preparation. Zirconium coating can apply (mixed oxide comprises additive and many other components such as silicon, titanium or barium, selenium, lanthanum, chromium) with one or more zirconia sols or mixed oxide colloidal sol. Apply after the suspension, can be with its oven dry and calcining, to generate the adhesion oxide skin(coating) of high surface in the metal surface.
Coat can apply the same method that lacquer is applied to a certain surface by people. For example spray, directly spread, it is medium that carrier is immersed in coating material. Another kind of catalyst layer being added to method on the carrier structure, is at first catalyst metals to be joined in the indifferent oxide powder. By heat treatment or chemical treatment catalyst metals is fixed on the oxide. Then palladium/indifferent oxide mixture is milled to generate colloid solution. With sprinkling, impregnating method colloidal sol is put on the liner again.
Constructed of aluminium also is applicable to the present invention, and can process or apply with substantially the same method. The ductility of aluminium alloy is larger, may be out of shape even melt in the operating temperature range of this method. Therefore they are not bery desirable carriers, if but the satisfiable words of temperature profile also can use.
In a single day the catalyst metals of coat, palladium and other any interpolation puts on the metallic carrier and after calcining, and can add one deck or which floor refractory oxide cover layer, as diffusion impervious layer, to prevent temperature discussed above " out of control ". This barrier layer can be silica, zirconia, titanium oxide, the perhaps oxide of other oxidation to fuel with low catalytic activity, or to top coat in similar mixed oxide or the oxide of additive is arranged. Aluminium oxide be it seems and is not suitable as the barrier layer very much, but also can be accepted under some environment. The thickness range on barrier layer can from apply bed thickness 1% until significantly greater than coat thickness; Be preferably 10% to 100% of coat thickness. Better thickness depends on the condition of work of catalyst, comprises the type of fuel, gas flow rate, and preheat temperature, and the catalytic activity of coat etc. Also finding only needs apply diffusion barrier coating in the downstream part of catalyst, and (for example 30% of total length to 70%) just can provide enough protections to catalyst under certain condition. Identical with coat, the barrier layer also can apply with paint technology.
Coat, catalyst and diffusion impervious layer can be applied on all surfaces of catalyst carrier as said here, also can only be applied on the surface relative with a uncoated surface. For example in the helical ripple structure above-mentioned, can only cover its one side with coat, catalyst and diffusion impervious layer. Then the ripple struction that will process is rolled into an integral body. Also can be coated with shim with the corrugated plate analog material in catalysis material one side, and roll into the helical form monolithic with corrugated plate. No matter when, put the monolithic surface that catalyst is arranged on it, will in combustion process, produce heat. This heat can pass to the gas that flows through, and is transmitted on the adjacent on-catalytic surface (and being colder surface) by catalyst structure. Heat will pass to the unburned gas that passes through along this surface therefrom. This is just so that the temperature of the catalyst surface in the catalyst structure is controlled by whole heat exchange, need not to seek help from the measures such as the dilution of air or external heat switching fabric. Such control ability may wish to obtain very much, for example when entering the very high and gas flow rate of preheat temperature that implication makes when unstable.
The size of catalyst structure and structure should make the gas by the catalyst structure axial passage, its average linear speed in whole catalyst structure greater than about 0.2 meter per second less than 40 meter per seconds. This lower limit is greater than the flame front speed of methane, and higher limit is a practical value to present commercially available bearer type. For the fuel beyond the methane, these two average speeds can be different with it.
Method
This method can be used various fuel, and can work in wider condition of work scope.
Although normal gaseous hydrocarbon (such as methane, ethane and propane) is suitable as the fuels sources of this method very much, most of carbon-containing fuels of vaporizing under the process temperature that can be discussed below also all are suitable for. For example, fuel can be liquid or gas under room temperature and normal pressure. Example comprises above-mentioned low-molecular-weight aliphatic hydrocarbon, and butane, pentane, hexane, heptane, octane; Gasoline; Aromatic hydrocarbon such as benzene, toluene, ethylbenzene and dimethylbenzene; Naphtha; Diesel oil and kerosene; Jet fuel; Other middle distillate; Heavier fuel (preferably the process hydrotreatment is to remove nitrogen compound and sulphur compound); The alcohols oxygenated fuel comprises methyl alcohol, ethanol, isopropyl alcohol, butanols etc.; And diethyl ether, ethylbenzene ether, the ethers such as MTBE. Other low-BTU gas such as town gas or synthetic gas also can be used as fuel.
In typical case, the quantity of fuel mix in the combustion air is to produce the mixture of its adiabatic combustion temperature greater than temperature that the inventive method reaches.Adiabatic combustion temperature is advisable to be higher than 800 ℃, is preferably in more than 1000 ℃.Non-gaseous fuel should be vaporized to small part before the contact catalyst district.Combustion air can be normal pressure or lower (0.25atm), and also can be compressed to pressure is 35atm or higher.Land-based gas turbine engine (gas that it can finally use this method to produce) is generally worked in gauge pressure is 8 to 35atm scope.Therefore, this method can be worked in 0.25 to 35atm scope.Preferably between 0 to 17atm.
The fuel/air mixture that is transported to catalyst should be through well-mixed, and the gas access temperature can change with fuel used difference.This temperature can be by heat exchange with the gas preheating or gas is carried out adiabatic compression reach.
What this method was used certain catalytic quantity contains the palladium material, and it is placed on (preferably metallic carrier) on the catalyst carrier, and carrier has the zirconium of containing coat, and low to the resistance of gas stream.The gas of partial combustion leaves the whole outlet temperature when containing catalyst area, and the wall temperature of catalyst, all will be markedly inferior to the thermal insulation or the autoignition temperature of this gas.In general, outlet temperature and wall temperature are not higher than about 800 ℃, preferably are not higher than 550 ° to 650 ℃.In addition, catalyst temperature should not surpass 1000 ℃, preferably is no more than 950 ℃.These temperature will depend on various factors, and they comprise pressure, the partial pressure of oxygen of system, calorific value of fuel etc.But catalyst will make the fuel meat oxidation, and its final temperature will be limited in be lower than a certain numerical value of its adiabatic combustion temperature.
Embodiment
These embodiment represent the preparation of scope of the invention inner catalyst and application in the method for the invention thereof.Comparative catalyst and control methods also have been described.
Measured two parameters of catalyst: the stable state duration of work stability and " lighting-extinguish temperature " (LOT).
By inserting catalyst in the combustion reactor and introducing fuel and air flows record LOT on catalyst.Although these tests are carried out under an atmospheric pressure, and other under elevated pressures, carry out.The temperature of gas/air mixture increases with constant speed.Monitor to such an extent that leave the temperature of some points in the gas temperature of catalyst and the catalyst.If catalyst is active, when this temperature raises, on some point, catalyst will begin oxygenated fuel, and can see that catalyst outlet place gas temperature and middle temperature raise.For ease of comparing, LOT gets preheat temperature and limits the average temperature value of temperature value certainly.
After suitable (and being constant) is selected to the preheat temperature of fuel and air mixture,,, draw the stability of catalyst by measuring in catalyst and the gas stream with the temperature of sampling point as the measurement of LOT.
This embodiment is divided into several portions, and has shown that relatively more similar platinum base combustion catalyst limits the temperature of the performance of palladium base combustion catalyst of the present invention.
A part (relatively using the preparation of platinum catalyst)
By a kind of platinum catalyst of following preparation: dense (70.3%) nitric acid of the low alkali gama-aluminas of 250 grams, 422 milliliters distilled water and 42 milliliters is placed the ball mill of the polymer linner of a pottle capacity, the abrasive media of the gama-alumina of half capacity of dress in this ball milling.
With said mixture ball milling 8 hours, obtain content and be about 35%(weight) Al
2O
3Alumina sol.
With one 100 hole inches
2(opsi) cordierite material (piece, sheet) (2 inches diameter, 2 inches in length) immerses this alumina sol, and with air the colloidal sol of surplus is blown out this building stones duct.Then with these building stones 100 ℃ down dry, and in Muffle furnace under 850 ℃ with its roasting 10 hours.Final building stones contain about 20%(weight) the aluminum oxide film coating.
The H that this building stones immersion one that scribbles aluminum oxide coating layer is contained about 0.14 gram platinum/gram solution
2PtCl
6In the solution.Superfluous solution is blown out with air, dry these building stones and 500 ℃ of roastings.Repetition is with more than the platinum dipping secondary.This final catalyst of 850 ℃ of roastings 10 hours.Final catalyst contains 4.5%(weight) platinum.
B part (preparation of palladium catalyst)
Prepare a palladium catalyst, preparation has the cordierite material of (washcoated) aluminum oxide coating layer, and carries out roasting as above-mentioned, with PdCl
2Be dissolved in the hydrochloric acid of two equivalents and be diluted to 0.042 gram palladium/milliliter and make a palladium solution.Cated building stones are immersed this solution, and blow out redundant solution with air, dry catalyst, then under 850 ℃ with catalyst calcination 10 hours.Final catalyst contains the 0.5%(weight of having an appointment) palladium.
The C part
In this part, will place a combustion test reaction unit from two kinds of catalyst of A part and B part gained.This reactor has 2 inches internal diameter, and with before catalyst contacts, can carefully control CH
4The preheat temperature of/air mixture.This reaction unit also is equipped with thermocouple to measure the variation of various gas and catalyst wall temperature.
Platinum catalyst
Will be prepared in the A part, relatively place this reaction unit with platinum catalyst.The air of per minute 500 standard liter (SLPM) flows flow through an electric heater, gas at rest blender and this catalyst of flowing through.The natural gas that contains about 93% methane is introduced into the air stream that tightly is in this gas mixer upstream.Be suspended from that the thermocouple that porcelain bushing is arranged in this gas stream was measured before this catalyst and after gas temperature.The underlayer temperature of this catalyst places near the thermocouple of a passage of the ceramic monoliths catalyst of this catalyst outlet by one and measures.
With air heat to 550 ℃, methane flow is increased to 1.1SLPM, corresponding fuel/air mixture ratio is 0.0022.Monitor this underlayer temperature.Substep is increased to 0.002 with this fuel/air mixture ratio, and each fuel/air mixture ratio is noted its underlayer temperature.These data are shown in Fig. 3.At the fuel/air mixture ratio is 0.010 o'clock, and catalyst has enough activity, and underlayer temperature is increased to 740 ℃.760 ℃ of this value adiabatic combustion temperatures that approach to calculate, this mixture.Because the increase of fuel/air mixture ratio, underlayer temperature then equate with this adiabatic combustion temperature is approaching.This explanation platinum catalyst has all burnt at all fuel of catalyst surface.
Palladium catalyst
With the above-mentioned palladium catalyst of the same manner test.Equally, when improve fuel/air mixture than the time, underlayer temperature rises and immediately following the adiabatic combustion temperature that calculates.(its fuel/air mixture is than being 0.013-0.020) its underlayer temperature remains on 800 ℃ but, as shown in Figure 3.
As shown in Figure 3, this embodiment has illustrated that a combustion catalyst that contains palladium has limited the temperature of this catalyst combination to about 780 ℃.And on the other hand, the temperature of platinum catalyst then follows closely significantly in the adiabatic combustion temperature that calculates.
This embodiment has shown the measurement of the temperature when palladium oxide changes palladium metal into, thereby has also shown the temperature limit of the catalyst substrate when methane burns in excess air.
21.9 milligrams palladium oxide powdered samples are stated from a thermogravimetry (TGA) device, and in the vestibule of this sample, charge into the dry air of 40 ml/min flows.With 10 ℃ of/minute temperature that increase this sample, the continuous monitoring sample weight obtains the TGA curve shown in Fig. 4 A.Divide the liberation palladiums at 795 ℃ of these palladium oxides, and the oxygen that produces when emitting weightlessness.The weightlessness that records sample is 2.74 milligrams, is equivalent to 12.5% of initial palladium oxide samples weighed.As shown in the formula theoretical weightlessness be 13.1%.
PdO…→→Pd+O
2
Repeating the TGA test with 5 ℃/minute the rates of heat addition, also to have drawn the knee pointy temperature that palladium oxide is transformed into palladium be 795 ℃.
When using palladium, in air, occur in approximately as the same temperature of underlayer temperature (approximating 780 ℃) of the restriction that records by the transformation that TGA surveyed under the atmospheric pressure from palladium oxide to palladium as the catalyst of the foregoing description 1.
New sample with palladium oxide repeats the TGA test, but this sample vestibule cleans with pure oxygen.Shown in Fig. 4 B, measured is 880 ℃ by palladium oxide to the transition temperature of palladium.As under higher partial pressure of oxygen, then should will take place in higher temperature by the transition point of palladium oxide to palladium.
This embodiment shows that the TGA that palladium oxide/palladium is done is the function of this specific partial pressure of oxygen.
Embodiment 3
Present embodiment divides two parts.A partly is depicted as and uses palladium, but the preparation of the steel monolithic of unprotect diffusion impervious layer on Catalytic Layer; The use that B partly is depicted as this monolithic reaches when using at low temperatures the tendency that it is out of control.
The A part
With 75.5 inches long of a slices, 2 inches wide Kawasaki River Lite20-5SR ripple (wrinkle) steel sample and one 73 inches long, 2 inches wide Kawasaki River Lite20-5SR band steel sheet sample, be exposed in the air at 950 ℃ and heat-treat.The heat treatment result is owing to contain aluminium in the steel, the growth that produces alumina whisker on the steel surface.
With 5%(weight) false-boehmite (psseudo-boehmite) colloid water slurry spray in the two sides of band steel sheet and steel corrugated sheet, as primer coating, obtain having 1%(weight) coating of left and right sides weight metal.At 90 ℃ of these metals of drying.
Spray-20%(weight) the colloidal suspension of gama-alumina make the shallow layer of high surface, carry out drying at 90 ℃, and in air, 850 ℃ of calcinings 5 hours down.Final shallow layer has 20% final catalyst weight.
With Pd(NH
3)
2(NO
2)
2Being dissolved in nitric acid makes one and contains palladium solution.With spray-on process this is contained palladium solution and is coated on this thin metal foil sheet, one be loaded with about 2%(weight) the final catalyst of palladium metal.At 90 ℃ of these steel discs of drying, and in air, calcined 4 hours down for 850 ℃.
This ripple (wrinkle) steel disc and band steel sheet are laminated together, and be rolled into about 2 inch diameters, geometric area has the spiral monolithic (Spiralmonolith) of about 300 passages per square inch.The perforated area of this monolithic is about 2.36 inches
2(or perforate of about 77%).
The B part
This part has shown in the normal entry air temperature ranges in 325 ℃~400 ℃, the operation of the catalyst monolithic that A partly prepares.
Catalyst structure is placed above-mentioned reaction unit system.2 thermocouples place the downstream of this monolithic to measure the wall temperature of monolithic.Equally, also monitor the overall gas temperature in exit.
With the air stream of 1500SLPM and the CH of 70SLPM
4Stream is introduced this monolithic, this mist is preheated to 300 ℃ initial temperature.And with about 20 ℃/minute speed preheat temperature that raises lentamente.
Reached before 350 ℃~355 ℃ in this gas preheat temperature, not seeing has any noticeable response." light-extinguish " point (lif-off) promptly at catalyst, the overall gas temperature in exit increases to about 550 ℃.The temperature of one of this monolithic wall thermocouple increases to 1000 ℃ very soon, then, as shown in Figure 5, begins to swing fast between about 700 ℃-1100 ℃.
This test program finishes subsequently.Cooled catalyst.
Use same test program on this monolithic, to carry out second test.This catalyst " is lighted-extinguish " between 325 ℃ and 335 ℃.It is obvious that the swing of its wall temperature becomes again.
Therefore, although it is generally acknowledged that in this area this palladium component can limit the temperature increase of this catalyst, only uses palladium always can not limit its wall temperature fully.
This embodiment has shown the preparation of using palladium and having diffusion impervious layer steel monolithic.
The A part
In the air that exposes, under 950 ℃, the Kawasaki River Lite20-5SR steel corrugated sheet of one section 70.0 inches of heat treatments and the flat steel disc of Kawasaki River Lite20-5SR of one section 70.0 inches section in stove heat to make in 16 hours and generate alumina whisker on the surface.
Use the program of embodiment 3, these two metals are handled false boehmite, gama-alumina coating and the palladium that sprays bottom respectively.It is described to press embodiment 3 then, correctly finishes drying and calcining step.
By the gama-alumina colloidal sol of spraying one 30%, 90 ℃ of dryings, and under 85 ℃, calcined 5 hours, this catalyst surface is applied diffusion impervious layer.This coating is about 5% of catalyst gross weight.
These two through being rolled into volume together then, makes the spiral monolithic (spiral monolith) of about 2 inch diameters.
The free perforated area of this monolithic is 2.36 inches
2(or perforate of about 78%).
The B part
This part is depicted as, and, uses as same temperature increase rate used in embodiment 3 application operating of prepared catalyst monolithic in the A part in same temperature range.
The monolithic that rolling is crossed inserts reactor assembly.The air velocity rate is 1500SLPM, the CH that acts as a fuel
4Be 80.5SLPM.The lighting-extinguish of this catalyst about 385 ℃.The overall gas temperature at catalyst outlet place reaches to 600 ℃ rapidly and is stabilized, and its wall temperature does not produce swing as once taking place among the embodiment 4.
Cool off this catalyst structure body then, repeat the test process more than four times.Lighting-extinguish at every turn all in 335 ℃ of-345 ℃ of scopes of this catalyst, and wall temperature is not swung in normal, 325 °~410 ℃ pre-heat rating.
Embodiment 5
This embodiment is depicted as under the preheat temperature of a single fuel/air mixture lasting raising when, the limit temperature effect of catalyst of the present invention.Although partially combusted gas temperature is located in the gas outlet of improving the air intake temperature and being produced, this catalyst structure wall temperature still is maintained at about 800 ℃.
Prepared a kind of highly enriched palladium catalyst, with a 100cpsi, the cordierite sheet of 50mm diameter and 50mm length is by being coated with aluminum oxide coating layer as mentioned above.At 850 ℃ this shallow layer was calcined 10 hours.With PdCl
2Be dissolved in the hydrochloric acid of two equivalents and make PdCl
4Solution.Final solution concentration is 0.081 palladium/milliliter.This shallow layer monolithic is dipped in this palladium solution, uses the air scavenging redundant solution.Feed H
2S gas in this single chip architecture until with whole PdCl
4Be converted to PdS.In air, calcine this monolithic then under 500 ℃.Repeat this palladium dipping process.At last, calcined 10 hours down at 850 ℃.
This catalyst is placed an above-mentioned test reaction device.From air intake 10mm, 25mm and 48mm place, in a single hole road, insert thermocouple respectively.This duct seals with vitrified bonding, and like this, thermocouple can record the substrate ceramic temperature.
The natural gas flow that makes the air of 1000SLPM and 40SLPM is through this catalyst.Heat the admixture of gas to 300 ℃ of this charging, and slowly increase temperature, the monitoring catalyst activity as shown in Figure 6.On 360 ℃, this catalyst is lighted-is extinguished, and its temperature rises on the gas temperature.At about 390 ℃, the underlayer temperature at (10mm is to outlet 48mm place) is constant in about 800 ℃ apart from the gas outlet.When the air intake gas temperature further improves, this underlayer temperature still is limited in about 800 ℃.
At 400 ℃, with this fuel/air mixture ratio, calculate and adiabatic combustion temperature be about 1240 ℃.This high activated catalyst does not cause underlayer temperature to increase to 1240 ℃ the fact, is because the very strong inhibition temperature performance of palladium.
Embodiment 6
This embodiment has shown LOT, and has a steady-state operation of palladium catalyst that is coated with the cordierite carrier of zirconium.
Make palladium/zirconium/cordierite catalyst by at first preparing a zirconium colloidal sol.Contain ZrO one
2The ball mill of polymer linner of abrasive media in, having of 125gm is 95m
2The ZrO that/gm is more long-pending than table
2Sample and 211 ml waters and 15 milliliters of red fuming nitric acid (RFNA)s mix.This mixture was ground 8 hours.
The honeycomb-like cordierite monolithic that will have 100opsi is dipped in this colloidal sol, dry and as above-mentioned the calcining.Repeat this process, contain the 78%(weight of having an appointment until this monolithic) ZrO
2Coating.
With Pd(NO
2)
2(NH
3)
2Be dissolved in HNO
3The aqueous solution and be diluted with water to concentration be 0.083 the gram palladium/milliliter make palladium solution.This monolithic is immersed this palladium solution, and redundant solution is with air scavenging, and drying is calcined under 850 ℃ in air again.Repeat this process, contain 2.2% palladium until this carbon monoxide-olefin polymeric.
This carbon monoxide-olefin polymeric is inserted in the adiabatic combustion reactor.Beginning through this catalyst, improves this mist temperature (" preheating ") with constant rate of speed with the natural gas flow of the air of flow velocity 1500SLPM and flow velocity 60SLPM, and at 350 ℃, this catalyst becomes and has activity.Shown in Fig. 7 A, when preheating was 370 ℃, the catalyst gas outlet began constant in about 800 ℃.Further improving preheat temperature does not cause the catalyst air outlet temperature to increase.Used palladium has limited the catalyst air outlet temperature on this aspect.
This catalyst has carried out in addition the test of steady-state operation at the fuel of the air of 1000SLPM and 40SLPM.Catalyst is employed under constant 400 a ℃ preheating.Shown in Fig. 7 B, this catalyst is highly stable, and keeps about 770 ℃ of catalyst outlet place temperature.Do not see the reduction that any activity is arranged.
This embodiment is similar to embodiment 6, but it has shown the useful effect of the partial combustion catalyst of the zirconium that uses us on metallic carrier.
Make and have ZrO
2The partial combustion catalyst of the metal foil-based monolithic of coating, and with following program do steady-state stability can test.
At first, the different peroxide of the zirconium of water hydrolysis 66gm is with the ZrO of 100gm
2Powder and the 100gm water that adds in addition mix the mixture that generates, to produce a ZrO
2Colloidal solution.This zirconia powder has 100m
2The specific area of/gm.With cylindrical ZrO
2Medium was in a polymer linner these slurries of grinding in ball grinder 8 hours.The colloidal sol of other dilute with water gained is to 15%ZrO again
2Concentration.
One Fe/Cr/Al thin plate ripple is processed into herringbone pattern, and 900 ℃ of following oxidations in air, to form alumina whisker on the surface.Use an air atomizer to spray this colloidal sol on this thin slice, dry, in 850 ℃ of air, calcine.The gained thin slice contains 2mgZrO on its surface
2/ cm
2
The palladium 2 ethyl hexanoic acid is dissolved in toluene contains 0.1gm palladium/ml with formation solution.This solution is sprayed on the metal forming of bag coated.This paper tinsel drying, calcining contain about 0.5mg palladium/cm
2The surface.
This undulatory paillon foil is rolled into one has the spiral structure that wherein passes through axial passage, about 2 inches of last diameter of movement.
With the roughly same quadrat method of being done to test with above-mentioned catalyst, this catalyst has been done the test of steady-state operation.Porch 1,2.5 and 4.8cm place at the distance catalyst structure insert thermocouple respectively.The temperature of the gas at 15cm place stream after the temperature at other thermocouple measurement catalyst outlet place and the catalyst.
With the air stream of a 1000SLPM and this catalyst of natural gas stream tying-in of 40SLPM.With the temperature (" preheating ") of constant rate of speed raising mist, at 400 ℃, catalyst becomes and has activity.Shown in Fig. 8 A, be 440 ℃ in preheating, the catalyst outlet place, temperature is maintained at about 770 ℃.Further improve preheat temperature, do not cause catalyst outlet place temperature to rise.Palladium has wherein limited this catalyst outlet place temperature in this point.
With the fuel of the air of 1000SLPM and 40SLPM this catalyst is done the test of steady-state operation again.Shown in Fig. 8 A, catalyst is in 500 ℃ of operations down of a constant preheat temperature, and this catalyst is highly stable, and keeps about 760 ℃-770 ℃ of catalyst outlet temperature.Do not see its activated decline.
Embodiment 8
The A part
This part A has shown the reduction with hydrazine, scribbles the method that generates palladium catalyst on the zirconic cordierite carrier one.
ZrO with 125 grams
2Powder (tool 95m
2The surface area of/gm), the red fuming nitric acid (RFNA) of 211 milliliters water and 15 milliliters puts into the ball mill of a polymer linner, this ball mill is full of ZrO
2Medium ground this mixture 8 hours.
With Pd(NH
3)
2(NO
2)
2Be dissolved in nitric acid, formation one contains the solution of 0.083 gram palladium/milliliter.With this palladium solution of 42 milliliters, add the ZrO of 50 grams to
2Regulate pH to 9.0 in the colloidal sol, add the hydrazine of 1.0 grams.Stirring this solution reduces fully until palladium.This colloidal sol contains 20%(weight) palladium.
With a 50mm diameter, 50mm are long, the cordierite honeycomb monolithic of 100 square holes is dipped in palladium/ZrO per square inch
2In the colloidal sol, unnecessary colloidal sol blows out cellular duct with air, drying, and, repeat this process 850 ℃ of calcinings down, contain palladium/ZrO of 12% until this monolithic
2Final catalyst contains 2.3%(weight) palladium and shallow layer has 26m
2/ gm surface area.
It is the adiabatic section of combustion reactor 2 inches, the same that this catalyst is placed an internal diameter.With the air of flow velocity per minute 1000 standard liters (SLPM) flow through heater, gas at rest blender, the catalyst of flowing through then.Natural gas is introduced near in the air of the gas mixer upstream stream, measures gas temperature before or after this catalyst by hanging on thermocouple in the gas stream.The thermocouple measurement of catalyst substrate temperature.This thermocouple places apart from the porch to be the duct of 25mm and 48mm and to seal with vitrified bonding.
The natural gas of 40SLPM is introduced in the air stream, air themperature is increased to 400 ℃.The catalyst substrate temperature is stable after rising to about 750 ℃.The exit gas temperature remains on 560 ℃.Test period at 3.5 hours, these temperature are highly stable.
The data of LOT data and steady-state operation are shown in Fig. 9 A and 9B respectively.
The B part
This comparative example has shown the production method of the catalyst of alumina support.
As above-mentioned A part, contain the total impurities amount to one and make ball milling less than the very highly purified aluminium oxide of 50ppm.Add Pd(NH
3)
2(NO
2)
2Solution with hydrazine reduction (equally as A part as described in), contains 20%(weight to form one) palladium/Al of palladium
2O
3Colloidal sol.Cordierite monolithic coating is contained 11%(weight to generate one) palladium/Al
2O
3With 2.2%(weight) the final catalyst of palladium.Its final shallow layer has 50m
2The surface area of/gm.
Described as the A part, test this catalyst.Test LOT(Figure 10 A of this catalyst simultaneously) and stable operating performance (Figure 10 B).Stable state application performance when preheating is 400 ℃ (Figure 10 B) shows, when only dropping to 420 ℃ at its underlayer temperature of this catalyst center within 3 hours, and 560 ℃ of its exit gas temperatures show the rapid inactivation of this catalyst when dropping to 485 ℃.
Load and preparation process that the catalyst tool of testing in the present embodiment is similar.The catalyst of the alumina support of test has higher surface area in the B part, and can expect to have more activity.But, palladium/Al
2O
3The rapid inactivation of catalyst.This explanation, Al
2O
3Not really suitable carrier as the palladium in catalyst for catalytic combustion; Be ZrO better
2
Embodiment 9
The A part
This embodiment has shown the formation method of the cellular corrugated metal paper tinsel substrate that contains zirconia coating.
One ZrO
2Colloidal sol is prepared as follows.The different peroxide of zirconium with about 66 grams of 75cc water hydrolysis.Then with itself and tool 100m
2100 gram ZrO of/gm surface area
2Powder and other 56 ml waters mix.Use ZrO
2Cylindrical pellet grinding media these slurries of ball milling 8 hours in the ball mill of a polymer linner.Again in addition this colloidal sol of thin up to 15%(weight) ZrO
2Concentration.
One Fe/Cr/Al tinsel is crumpled into herringbone pattern, and 900 ℃ of following oxidations in air then are to generate alumina whisker at sheet surface.With ZrO
2Colloidal sol is sprayed on the one side of this corrugated foil.Paillon foil dry and that this coating of calcining is crossed under 850 ℃.Final paillon foil contains 2.0mg ZrO on its surface
2/ cm
2
The 2-ethene caproic acid of palladium is dissolved in toluene, to 0.1 the gram palladium/ml concentration, with this solution spraying at ZrO
2Containing on the paillon foil of coating, drying and this paillon foil of calcining in 850 ℃ air.Final paillon foil contains about 0.5 milligram of palladium/cm on its surface
2
This undulatory paillon foil is rolled into volume, makes ripple not produce interlock,, two inch diameters one final, two inches long to form, and axially connect a duct radially in its structure.
This catalyst is placed embodiment 8 described combustion reactors, and make LOT and stable state application testing.Air flow rate is 1000SLPM, and the methane flowing velocity is 40SLPM, and the stable state preheat temperature is 450 ℃.This Application of Catalyst operation is shown in Figure 11 A and 11B.
The B part
Preparing this catalyst, but use Al with present embodiment A part similar methods
2O
3Make carrier.
Prepare an Al
2O
3Colloidal sol.The red fuming nitric acid (RFNA) of the gama-aluminas of 250 grams, 422 milliliters water and 21 milliliters is placed the ball mill of a polymer linner.Add cylindric zirconia media, with this mixture ball milling 8 hours.
This alumina sol of dilute with water is sprayed into it on corrugated metal foil to containing 15% solid approximately, calcines 10 hours down at 850 ℃.Final Al
2O
3Useful load is 2.2mg/cm
2
With the thylhexoic acid solution spraying of a palladium to scribbling ZrO
2Tinsel on, dry also this paillon foil of calcining in 850 ℃ of air.Final paillon foil contains about 0.5mg/cm on its surface
2
This catalyst is placed above-mentioned reactor and test.Air flow rate is 1000SLPM, and natural gas flow speed is 40SLPM, and the stable state preheat temperature is 450 ℃.This Application of Catalyst performance is shown in Figure 12 A and 12B.Palladium/ZrO wherein
2Stability shown that this salic catalyst has significantly and improved.
Embodiment 10
This embodiment shown the palladium on one group of cordierite carrier of the present invention, contain the formation and the use of a series of catalyst of the palladiums of different amounts.Two groups of parameters to this catalyst: " light and extinguish temperature " (LOT) and the stability of stable state in using done test.
The monolithic honeycomb shape structure that has 100 square holes per square inch, is made up of cordierite ceramic is by with Al
2O
3Colloidal sol (Catabal B) carries out coating, this monolithic of calcining in 800 ℃ of air.The monolithic that generates contains 23.7% Al
2O
3Repeat monolithic is immersed a Pd(NO
2)
2(NH
3)
2In the solution, drying, calcining is 10 hours in 850 ℃ air.This catalyst contains 1.0% the palladium that accounts for this aluminium oxide book coating.
Excise the cylindrical piece (long 25mm) of 4 sections 20mm diameters from the single chip architecture of above preparation.In these 4 sections 3 sections soak method with dripping, with different Pd(NO
2)
2(NH
3)
2Consumption handle.Every section drying and in 850 ℃ air, calcining 1~10 hour all.The catalyst of gained contains 0.5%, 0.30% and 1.2% the platinum that accounts for this aluminum oxide film coating.
This catalyst is placed a combustion reactor respectively, 3 thermocouples be placed in along catalyst, apart from its porch be respectively 1,25 and the single duct at 4.8cm place in.This duct is full of vitrified bonding, so that thermocouple can record underlayer temperature (wall temperature) rather than gas temperature.
Make air flow to an electric heater,, and flow through catalyst with methane blended with the flow velocity of 1500 standard liters/minute (SLPM).
With above-mentioned LOT and all 4 sections catalyst of steady state activity program test.Resulting data show with chart:
Catalyst is formed LOT figure steady state picture
1%Pd??13A??14A
1%Pd,0.15%Pt??13B??14B
1%Pd,0.30%Pt??13C??14C
1%Pd,1.20%Pt??13D??14D
Comparison diagram 13A and Figure 13 B, 13C and 13D as seen, the LOT of each occasion is maintained at about 350-360 ℃, no matter and the recruitment of platinum how.Be used for limitting temperature shockingly to be lower than certainly to be used to and not having platinum catalyst of catalyst made from platonic, and none temperature reaches adiabatic combustion temperature.The adiabatic combustion temperature of this admixture of gas is about 1180 ℃ in theory.The temperature of leaving the gas of this catalyst all is constant concerning the catalyst of each platiniferous, demonstrates overall stability, although can see some deactivation phenomenom (Figure 13 B and 13C) in the lower catalyst early region of platinum containing amount.
Embodiment 11
This embodiment has shown the useful effect that platinum is added on the catalyst of the palladium on the metallic carrier.
Two kinds of catalyst have been prepared.First kind of catalyst is for only doing " control " of catalyst with palladium.Second kind of catalyst is for using the catalyst of platinum and palladium simultaneously.
Only be loaded with the Pd metal carrier catalyst
One contains the one side of aluminum steel salt sheet (CAMET), forms the little coating of an aluminium oxide after the oxidation, after spraying on the one side is with two layers of alumina sol (Catapal-B) drying, 850 ℃ of calcinings.This paillon foil is sprayed with Pd(NH
3)
2(NO
2)
2Solution, drying were calcined 10 hours down at 850 ℃.Then this paillon foil is rolled into a monolithic with about per square inch 150 holes (opsi).This coating contains the Pd/cm based on about 1.3mg of aluminum oxide film coating
2
The catalyst of carrying Pd/Pt
One contains the one side of the steel foil sheet (CAMET) of aluminium, forms little coating of an aluminium oxide after the oxidation, spraying two-layer alumina sol layer (Catapal-B) on one side, drying.850 ℃ of calcinings down.This paillon foil is sprayed with Pd(NH
3)
2(NO
2)
2Solution, drying sprays with Pd(NH again
3)
2(NO
2)
2Dry behind the solution.Dried paillon foil was calcined 10 hours down at 850 ℃.This paillon foil volume of gained is for having the monolithic in about per square inch 150 holes (opsi).Be coated in coating on the aluminum oxide film coating and contain the Pd/cm of 1.3mg approximately
2Pt/cm with about 0.13mg
2
Use above-mentioned steady-state method of test to test each catalyst.Air flow rate is 1000SLPM, and the fuel/air mixture ratio is 0.019 or 0.040.Preheating level in each example is 500 ℃.Record gas temperature and the preheat temperature of leaving catalyst respectively.
Comparison diagram 15A and 15B, this two occasion is all used 0.019 fuel-air ratio, and the back catalytic temperature of palladium catalyst reduces to 590 ℃ level, and when three hours EOT, this temperature is still descending.Palladium/the platinum catalyst that is shown in Figure 15 B of the present invention by comparison, rises to described level, and when test finished afterwards in 1.7 hours, roughly constant.
The operation of Figure 16 A and Figure 16 B shows, is 0.040 o'clock at the fuel/air mixture ratio, and the stable palladium catalyst of platinum shows that ground is more stable.In Figure 16 B, this catalyst still moves under about 650 a ℃ maintenance level.Otherwise the stability of the unstabilized catalyst in Figure 16 A causes the exit temperature to reduce to 600 ℃ in about 3 hours.
Present embodiment has illustrated the different of independent use palladium and the catalyst that uses palladium and platinum simultaneously.Metallic carrier in the present embodiment is to be coated with aluminium oxide.
This embodiment has shown the palladium prepared at concentrations palladium/ZrO with classification
2The method of cordierite catalyst and with the comparison of stepless catalyst.
The A part
Has 45m with one
2125 gram ZrO of/gm surface area
2Be dipped in 45 milliliters contain 0.0834 the gram palladium/milliliter solution in.This solution is with Pd(NH
3)
2(NO
2)
2Being dissolved in nitric acid makes.Dry palladium/ZrO
2Mixture, and 500 ℃ of calcinings down.
125 palladium/the ZrO that restrain pack in the ball milling of a polymer linner
2Mixture, 230 ml waters, 2.0 milliliter 70% HNO
3(nitric acid) and ZrO
2Medium.
The colloidal sol sample of 50 milliliters of generations is mixed with other 36 milliliters 0.0834 gram palladium/ml soln, use NH
4OH regulates pH to 9.0, under agitation adds 0.64 gram hydrazine, and after a few hours, palladium is reduced.Colloidal sol (after fully calcined) generates one at ZrO
2On the shallow layer that contains 13.6% palladium.
With 100 hole/inches
2The cordierite monolithic of (2 inch diameters, 2 inches long) immerses above-mentioned palladium/ZrO
2In the colloidal sol, in advance to blow down, also calcine under 850 ℃ by dry this monolithic with air stream for unnecessary colloidal sol.The single coating that generates contains palladium/ZrO of 7.69% on monolithic
2With 1.03% palladium.
Again this catalyst is immersed palladium/ZrO
2Colloidal sol, but its depth of immersion 12mm, unnecessary colloidal sol blows down from the duct, dry and this monolithic of calcining.Palladium concentration is on this monolithic: being 4.2% palladium on the entrance of 12mm, is 1.0% palladium on the exit portion of 39mm.Average palladium concentration is 1.8% palladium.
The B part
This embodiment has shown the stepless palladium/ZrO that is used for comparison
2-cordierite catalyst.
Described as the A part, flood another cordierite single chip architecture, and do similar blowing, calcine final catalyst down at 850 ℃.This final catalyst has more uniform comparatively speaking 1.9% palladium concentration from its porch to exit.
This embodiment has compared the relative activity of two catalyst of embodiment 12.This two catalyst is through test, and in test, methane and air are preheated.This preheat temperature originates in 325 ℃, keeps constant speed to increase, and measures and write down the outlet temperature that is used for partial combustion gas and the variation of internal temperature.
This catalyst is placed an adiabatic reactor respectively, introduce air, introduce methane with 36SLPM with the speed of 800 standard liters/minute (SLPM).
The A part
As visible among Figure 17, at 350 ℃ pine in advance of embodiment 12, A part, (lit off) lighted-extinguished to the catalyst of classification, and become and have activity.The wall temperature of monolithic rises to 780 ℃ and Outlet Gas Temperature is stabilized in about 550 ℃.When continuous rising preheat temperature, its temperature of this catalyst outlet is obviously evenly got up, and peony is made in change.448 ℃ preheat temperature, catalyst part temperature is increased to 800 catalyst, bright white point occurs.This white portion begins to enlarge, and fuel is emitted to prevent that catalyst from burning.
The catalyst of this classification have one between 350 ℃ and 445 ℃ working window or 98 ℃ working width.
The B part
Test in a similar manner by gained in the B part of embodiment 12 and relatively use catalyst.As shown in Figure 18, this catalyst is lighted at about 350 ℃ and is extinguished, and has showed the focus (>800 ℃) on about 390 ℃ preheat temperature.This catalyst has one 350 °-390 ℃ working window, or about 40 a ℃ working width is only arranged.
Although two kinds of catalyst have identical palladium load, the catalyst of classification has big many working windows.
The present invention is disclosed by description and the use of embodiment.These embodiment only are embodiment, are not to be used for any limitation of the invention.And those of ordinary skill in the art can draw appropriate section of the present invention described herein, but does not exceed the written contained scope of appended claims.We think that also these appropriate sections belong to the present invention.
Claims (56)
1, a kind of partial combustion method is characterized in that it may further comprise the steps:
A. with the oxygen-containing gas of some and flammable fuel mix, to produce a kind of flammable admixture of gas; And
B. this combustible gas mixture is fed the combustion zone that comprises catalyst structure, catalyst structure contains palladium catalyst in the part of catalyst carrier at least, the combustible gas mixture of allowing is passed through and partially combusted passage and this carrier has, the waste gas of crossing with the generating unit divided combustion, the bulk temperature of waste gas is lower than the adiabatic combustion temperature of combustible gas mixture, and catalyst temperature is not higher than the TGA conversion temperature of palladium catalyst.
2, the method for claim 1 is characterized in that catalyst carrier wherein comprises a kind of material that is selected from metal and has the metal of oxide cover layer.
3, method as claimed in claim 2 is characterized in that catalyst carrier wherein comprises iron containing alloy.
4, method as claimed in claim 3 is characterized in that catalyst carrier wherein comprises the iron containing alloy that is coated with zirconia, titanium oxide, silica, aluminium oxide, non-catalytic oxidation thing, refractory metal oxides or their mixture.
5, the method for claim 1 is characterized in that wherein palladium catalyst also comprises the catalyst metals of one or more interpolations that are selected from silver, gold, platinum, ruthenium, rhodium, iridium or osmium.
6, method as claimed in claim 5 is characterized in that interpolation catalyst wherein is silver or platinum.
7, the method for claim 1 is characterized in that palladium catalyst wherein also additionally comprises silver.
8, method as claimed in claim 4 is characterized in that palladium catalyst is wherein covered by the oxide skin(coating) of a diffusion layer or another catalytically inactive at least in part.
9, method as claimed in claim 8 is characterized in that diffusion impervious layer wherein comprises zirconia.
10, method as claimed in claim 8 is characterized in that diffusion layer wherein is a kind of shallow layer (washcoat).
11, the method for claim 1 is characterized in that fuel gas wherein comprises methane, and combustible gas mixture feeding temperature is at least about 325 ℃ partial combustion district.
12, the method for claim 1, the gauge pressure that it is characterized in that partial combustion district wherein is between 0.25 to 35atm.
13, method as claimed in claim 2 is characterized in that wherein catalyst carrier has cellular, tubulose, rolls the vertical passage of forms such as corrugated.
14, method as claimed in claim 13 is characterized in that only some comprises palladium for the surface of vertical passage wherein.
15, the method for claim 1 it is characterized in that the channel surface that wherein contains the vertical passage surface of palladium and do not contain palladium is adjacent, and these surfaces is in whole heat exchange relationship each other.
16, the method for claim 1 is characterized in that passage wherein has a forward position district and a forward position district and a back tail region that contains than the low activity palladium-containing catalyst that contains than the low activity palladium-containing catalyst that contains the greater activity palladium-containing catalyst.
17, method as claimed in claim 16 is characterized in that the concentration of forward position district palladium wherein is higher than the concentration of palladium in the tail region, back.
18, method as claimed in claim 16 is characterized in that wherein the forward position district contains the thickness of palladium floor greater than the thickness in the follow-up district.
19, method as claimed in claim 16 is characterized in that also additionally comprising a diffusion impervious layer on the palladium-containing catalyst in the wherein back tail region.
20, the method for claim 1 is characterized in that wherein catalyst structure comprises and also comprises a kind of zirconium compounds beyond the palladium that places on the catalyst carrier.
21, method as claimed in claim 20 is characterized in that zirconium compounds wherein comprises zirconia.
22, a kind of partial combustion method is characterized in that it may further comprise the steps:
A. with the air and the methane blended of some, be higher than 900 ℃ combustible gas mixture haply to produce adiabatic combustion temperature; And
B. combustible gas mixture is fed the combustion zone, this district comprises the catalyst carrier that spiral helicine corrugated iron alloy sheet is made, this carrier has the combustible gas mixture of allowing and passes through also partially combusted vertical passage, and on the portion of channel of this carrier, has the catalyst that wherein contains palladium catalyst at least, the waste gas of crossing with the generating unit divided combustion, the bulk temperature of waste gas is lower than the adiabatic combustion temperature of combustible gas mixture, and catalyst temperature is lower than the TGA temperature of palladium catalyst.
23, method as claimed in claim 22 is characterized in that palladium-containing catalyst is wherein covered by a kind of diffusion impervious layer of on-catalytic activating oxide that comprises at least in part.
24, method as claimed in claim 22 is characterized in that diffusion impervious layer wherein comprises zirconia.
25, method as claimed in claim 22 is characterized in that wherein palladium catalyst also comprises the catalyst metals of one or more interpolations that are selected from silver, gold, platinum, ruthenium, rhodium, iridium or osmium.
26, method as claimed in claim 25 is characterized in that the catalyst that wherein adds is a silver.
27, method as claimed in claim 22 is characterized in that combustible gas mixture was preheating to temperature in the past in the feeding combustion zone and is about more than 325 ℃.
28, method as claimed in claim 22 is characterized in that only some comprises palladium for the surface of vertical passage wherein.
29, method as claimed in claim 24 is characterized in that only some comprises palladium for wherein vertical passage.
30, method as claimed in claim 28 it is characterized in that the channel surface that wherein contains the vertical passage surface of palladium and do not contain palladium is adjacent, and these surfaces is in whole heat exchange relationship each other.
31, method as claimed in claim 22 is characterized in that passage wherein has a forward position district and a back tail region that contains than the low activity palladium-containing catalyst that contains the greater activity palladium-containing catalyst.
32, method as claimed in claim 31 is characterized in that the concentration of forward position district palladium wherein is higher than the concentration of palladium in the tail region, back.
33, method as claimed in claim 31 is characterized in that wherein the forward position district contains the thickness of palladium floor greater than the thickness that contains the palladium floor in the follow-up district.
34, a kind of catalyst structure is characterized in that it comprises:
A. metallic catalyst carrier, it has and is suitable for the passage that fuel gas passes through, and have in these passages can be for the surface of placing catalysis material;
B. catalysis material that contains palladium, it is placed on the portion of channel of metallic carrier at least; And
C. diffusion layer, it comprises the on-catalytic activating oxide that is positioned on the palladium catalysis material.
35, structure as claimed in claim 34 is characterized in that metallic catalyst carrier wherein comprises iron containing alloy.
36, structure as claimed in claim 35 is characterized in that wherein passage has a part to have to be selected from the cover layer of following one group of material at least: aluminium oxide, zirconia, silica, titanium oxide, fire-resistant metal oxide, non-catalytic oxidation thing or their mixture.
37, structure as claimed in claim 36 is characterized in that cover layer wherein comprises zirconia.
38, structure as claimed in claim 37 is characterized in that diffusion impervious layer wherein comprises zirconia.
39, structure as claimed in claim 34 is characterized in that wherein catalysis material also comprises the catalyst metals of one or more interpolations that are selected from silver, gold, platinum, ruthenium, rhodium, iridium or osmium.
40, structure as claimed in claim 39 is characterized in that the catalyst that wherein adds is a silver.
41, structure as claimed in claim 35 is characterized in that metallic catalyst carrier wherein comprises steel corrugated sheet, and its facing surfaces is rolled into a monoblock.
42, structure as claimed in claim 41 is characterized in that metallic catalyst carrier wherein also comprises the steel shim adjacent with steel corrugated sheet, and they are rolled into a monoblock entirely.
43, structure as claimed in claim 41 is characterized in that wherein catalysis material is that each that be placed on steel corrugated sheet replaces on the surface.
44, structure as claimed in claim 34 is characterized in that passage wherein has a forward position district and a back tail region that contains than the low activity palladium-containing catalyst that contains the greater activity palladium-containing catalyst.
45, structure as claimed in claim 44 is characterized in that wherein the concentration of forward position district palladium is higher than the concentration of tail region palladium afterwards.
46, structure as claimed in claim 44 is characterized in that wherein the forward position district contains the thickness of the thickness of palladium floor greater than follow-up district palladium floor.
47, a kind of catalyst structure is characterized in that it comprises:
A. catalyst carrier, it has and is suitable for the passage that fuel gas passes through, and have in these passages can be for the surface of placing catalysis material;
B. catalysis material, it comprises and is positioned at the palladium that contains on the zirconia material, and is placed at least on the portion of channel of carrier.
48, structure as claimed in claim 47 is characterized in that catalyst carrier wherein comprises iron containing alloy.
49, structure as claimed in claim 47 is characterized in that at least comprising diffusion impervious layer in the part of passage that the barrier layer is made of fire-resistant metal oxide, non-catalytic oxidation thing or their mixture.
50, structure as claimed in claim 49 is characterized in that diffusion impervious layer wherein comprises zirconia.
51, structure as claimed in claim 48 is characterized in that catalyst carrier wherein comprises steel corrugated sheet, and its facing surfaces is rolled into a monoblock.
52, structure as claimed in claim 47 is characterized in that wherein catalysis material also comprises and can reach 50% mole the interpolation catalyst that is selected from VIII family noble metal and 1B family metal.
53, structure as claimed in claim 47 is characterized in that this structure is substantially free of chlorine.
54, structure as claimed in claim 47 is characterized in that passage wherein has a forward position district and a back tail region that contains than the low activity palladium-containing catalyst that contains the greater activity palladium-containing catalyst.
55, structure as claimed in claim 54 is characterized in that wherein the concentration of forward position district palladium is higher than the concentration of tail region palladium afterwards.
56, structure as claimed in claim 54 is characterized in that wherein the forward position district contains the thickness of the thickness of palladium floor greater than follow-up district palladium floor.
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US61798190A | 1990-11-26 | 1990-11-26 | |
| US617,981 | 1990-11-26 | ||
| US07/617,979 US5259754A (en) | 1990-11-26 | 1990-11-26 | Partial combustion catalyst of palladium on a zirconia support and a process for using it |
| US07/617,975 US5326253A (en) | 1990-11-26 | 1990-11-26 | Partial combustion process and a catalyst structure for use in the process |
| US617,979 | 1990-11-26 | ||
| US07/617,973 US5258349A (en) | 1990-11-26 | 1990-11-26 | Graded palladium-containing partial combustion catalyst |
| US617,975 | 1990-11-26 | ||
| US617,973 | 1990-11-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1064538A true CN1064538A (en) | 1992-09-16 |
Family
ID=51356922
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN91111207A Pending CN1064538A (en) | 1990-11-26 | 1991-11-26 | Palladium partial combustion catalyst and using method thereof |
Country Status (2)
| Country | Link |
|---|---|
| CN (1) | CN1064538A (en) |
| TW (1) | TW209270B (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101138703B (en) * | 2006-07-13 | 2012-12-26 | 株式会社Ict | Method for purifying internal combustion engine exhaust |
| CN112570044A (en) * | 2020-11-18 | 2021-03-30 | 苏州西热节能环保技术有限公司 | Pilot plant and method for catalyst regeneration drying and roasting process |
-
1991
- 1991-11-26 CN CN91111207A patent/CN1064538A/en active Pending
-
1992
- 1992-05-23 TW TW081104048A patent/TW209270B/zh active
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101138703B (en) * | 2006-07-13 | 2012-12-26 | 株式会社Ict | Method for purifying internal combustion engine exhaust |
| TWI423848B (en) * | 2006-07-13 | 2014-01-21 | Umicore Shokubai Japan Co Ltd | Method for purification of exhaust gas from internal-combustion engine |
| US9429055B2 (en) | 2006-07-13 | 2016-08-30 | Umicore Shokubai Usa Inc. | Method for purification of exhaust gas from internal-combustion engine |
| CN112570044A (en) * | 2020-11-18 | 2021-03-30 | 苏州西热节能环保技术有限公司 | Pilot plant and method for catalyst regeneration drying and roasting process |
Also Published As
| Publication number | Publication date |
|---|---|
| TW209270B (en) | 1993-07-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1195669C (en) | Process for abatement of nitrogen oxides in exhaust from gas turbine power generation | |
| US5183401A (en) | Two stage process for combusting fuel mixtures | |
| US5232357A (en) | Multistage process for combusting fuel mixtures using oxide catalysts in the hot stage | |
| CN1229313C (en) | Autothermal process for production of olefins | |
| KR100261782B1 (en) | Palladium partial combustion catalysts and a process for using them | |
| CN1835795A (en) | Microchannel with internal fin support for catalyst or sorption medium | |
| CN1310828C (en) | Method of producing synthetic gas | |
| CN1296463C (en) | Method and composition for improving fuel combustion | |
| CN1195172C (en) | Method for burning fuel with oxidant and burner device | |
| CN1502546A (en) | Catalyst for manufacturing hydrogen or synthesis gas and manufacturing method of hydrogen or synthesis gas | |
| CN1674985A (en) | Catalyst for clarifying exhaust gas | |
| CN1064420A (en) | A kind of catalyst structure with complete heat exchange | |
| CN1415411A (en) | Purification catalyst for waste gas | |
| CN1218423A (en) | Exhaust gas catalyst comprising multilayered upstream zones | |
| TW198743B (en) | ||
| CN1457320A (en) | Hydrogen purification device and fuel cell power generation system | |
| CN1089232A (en) | Catalytic process for producing synthesis gas | |
| CN1055302A (en) | The catalyst of purifying exhaust air and production method thereof | |
| CN1266028C (en) | Hydrogen purification apparatus | |
| CN1064538A (en) | Palladium partial combustion catalyst and using method thereof | |
| CN1239903A (en) | Catalytic metal plate | |
| CN1189386C (en) | Hydrogen producer | |
| CN1867652A (en) | Method for producing liquefied petroleum gas containing propane or butane as main component | |
| CN1147288A (en) | Improved process and catalyst structure employing integral heat exchange with optional downstream flameholder | |
| US20040131984A1 (en) | Low NOx burner |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C01 | Deemed withdrawal of patent application (patent law 1993) | ||
| WD01 | Invention patent application deemed withdrawn after publication |