CN102992982A - Synthesis method of p-hydroxybenzaldehyde - Google Patents
Synthesis method of p-hydroxybenzaldehyde Download PDFInfo
- Publication number
- CN102992982A CN102992982A CN2012105343723A CN201210534372A CN102992982A CN 102992982 A CN102992982 A CN 102992982A CN 2012105343723 A CN2012105343723 A CN 2012105343723A CN 201210534372 A CN201210534372 A CN 201210534372A CN 102992982 A CN102992982 A CN 102992982A
- Authority
- CN
- China
- Prior art keywords
- hydroxybenzaldehyde
- phenol
- synthetic method
- reaction
- reagent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- RGHHSNMVTDWUBI-UHFFFAOYSA-N 4-hydroxybenzaldehyde Chemical compound OC1=CC=C(C=O)C=C1 RGHHSNMVTDWUBI-UHFFFAOYSA-N 0.000 title claims abstract description 103
- 238000001308 synthesis method Methods 0.000 title abstract 4
- 238000006243 chemical reaction Methods 0.000 claims abstract description 37
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 claims abstract description 34
- 239000002994 raw material Substances 0.000 claims abstract description 19
- 238000000034 method Methods 0.000 claims abstract description 17
- QQVDYSUDFZZPSU-UHFFFAOYSA-M chloromethylidene(dimethyl)azanium;chloride Chemical compound [Cl-].C[N+](C)=CCl QQVDYSUDFZZPSU-UHFFFAOYSA-M 0.000 claims abstract description 15
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 claims description 96
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 23
- 238000010189 synthetic method Methods 0.000 claims description 19
- 239000003153 chemical reaction reagent Substances 0.000 claims description 10
- 238000001035 drying Methods 0.000 claims description 10
- 239000007787 solid Substances 0.000 claims description 10
- 238000003756 stirring Methods 0.000 claims description 10
- 239000000243 solution Substances 0.000 claims description 7
- 238000009413 insulation Methods 0.000 claims description 6
- 238000005406 washing Methods 0.000 claims description 6
- 239000000376 reactant Substances 0.000 claims description 5
- 239000002904 solvent Substances 0.000 claims description 4
- 230000035484 reaction time Effects 0.000 claims description 3
- 239000004615 ingredient Substances 0.000 claims description 2
- 239000011259 mixed solution Substances 0.000 claims description 2
- RLOWWWKZYUNIDI-UHFFFAOYSA-N phosphinic chloride Chemical compound ClP=O RLOWWWKZYUNIDI-UHFFFAOYSA-N 0.000 claims description 2
- 239000000047 product Substances 0.000 abstract description 8
- 238000000746 purification Methods 0.000 abstract description 6
- 239000006227 byproduct Substances 0.000 abstract description 4
- 238000000926 separation method Methods 0.000 abstract description 4
- 230000015572 biosynthetic process Effects 0.000 abstract description 3
- 238000009776 industrial production Methods 0.000 abstract description 3
- 238000003786 synthesis reaction Methods 0.000 abstract description 3
- 238000009835 boiling Methods 0.000 description 17
- 238000005303 weighing Methods 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 3
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- LSQZJLSUYDQPKJ-NJBDSQKTSA-N amoxicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 LSQZJLSUYDQPKJ-NJBDSQKTSA-N 0.000 description 2
- 229960003022 amoxicillin Drugs 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 238000005265 energy consumption Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- LSQZJLSUYDQPKJ-UHFFFAOYSA-N p-Hydroxyampicillin Natural products O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)C(N)C1=CC=C(O)C=C1 LSQZJLSUYDQPKJ-UHFFFAOYSA-N 0.000 description 2
- IWDCLRJOBJJRNH-UHFFFAOYSA-N p-cresol Chemical compound CC1=CC=C(O)C=C1 IWDCLRJOBJJRNH-UHFFFAOYSA-N 0.000 description 2
- 239000002304 perfume Substances 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- SATCULPHIDQDRE-UHFFFAOYSA-N piperonal Chemical compound O=CC1=CC=C2OCOC2=C1 SATCULPHIDQDRE-UHFFFAOYSA-N 0.000 description 2
- 238000004080 punching Methods 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- WVLBCYQITXONBZ-UHFFFAOYSA-N trimethyl phosphate Chemical compound COP(=O)(OC)OC WVLBCYQITXONBZ-UHFFFAOYSA-N 0.000 description 2
- DYHOLQACRGJEHX-CYBMUJFWSA-N (-)-Farrerol Natural products C1([C@@H]2OC3=C(C)C(O)=C(C(=C3C(=O)C2)O)C)=CC=C(O)C=C1 DYHOLQACRGJEHX-CYBMUJFWSA-N 0.000 description 1
- UPMXNNIRAGDFEH-UHFFFAOYSA-N 3,5-dibromo-4-hydroxybenzonitrile Chemical compound OC1=C(Br)C=C(C#N)C=C1Br UPMXNNIRAGDFEH-UHFFFAOYSA-N 0.000 description 1
- YRSSHOVRSMQULE-UHFFFAOYSA-N 3,5-dichloro-4-hydroxybenzonitrile Chemical compound OC1=C(Cl)C=C(C#N)C=C1Cl YRSSHOVRSMQULE-UHFFFAOYSA-N 0.000 description 1
- ZPTVNYMJQHSSEA-UHFFFAOYSA-N 4-nitrotoluene Chemical compound CC1=CC=C([N+]([O-])=O)C=C1 ZPTVNYMJQHSSEA-UHFFFAOYSA-N 0.000 description 1
- 239000005489 Bromoxynil Substances 0.000 description 1
- 235000014493 Crataegus Nutrition 0.000 description 1
- 241001092040 Crataegus Species 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- DZTRDRPCROOSOG-UHFFFAOYSA-N Matteucinol Natural products C1=CC(OC)=CC=C1C1OC2=C(C)C(O)=C(C)C(O)=C2C(=O)C1 DZTRDRPCROOSOG-UHFFFAOYSA-N 0.000 description 1
- NPWGWQRXHVJJRD-UHFFFAOYSA-N N-hydroxyglycine Chemical compound ONCC(O)=O NPWGWQRXHVJJRD-UHFFFAOYSA-N 0.000 description 1
- 240000007651 Rubus glaucus Species 0.000 description 1
- 235000011034 Rubus glaucus Nutrition 0.000 description 1
- 235000009122 Rubus idaeus Nutrition 0.000 description 1
- 230000003190 augmentative effect Effects 0.000 description 1
- UCKZMPLVLCKKMO-LHLIQPBNSA-N cephamycin Chemical compound S1CC(C)=C(C(O)=O)N2C(=O)[C@@H](C)[C@]21OC UCKZMPLVLCKKMO-LHLIQPBNSA-N 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000004807 desolvation Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- DYHOLQACRGJEHX-ZDUSSCGKSA-N farrerol Chemical compound C1([C@H]2OC3=C(C)C(O)=C(C(=C3C(=O)C2)O)C)=CC=C(O)C=C1 DYHOLQACRGJEHX-ZDUSSCGKSA-N 0.000 description 1
- 239000012847 fine chemical Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 230000002363 herbicidal effect Effects 0.000 description 1
- 239000004009 herbicide Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000004973 liquid crystal related substance Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- ZRSNZINYAWTAHE-UHFFFAOYSA-N p-methoxybenzaldehyde Chemical compound COC1=CC=C(C=O)C=C1 ZRSNZINYAWTAHE-UHFFFAOYSA-N 0.000 description 1
- 238000002161 passivation Methods 0.000 description 1
- 239000000575 pesticide Substances 0.000 description 1
- 229940081310 piperonal Drugs 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 238000011403 purification operation Methods 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 235000013599 spices Nutrition 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- KCDXJAYRVLXPFO-UHFFFAOYSA-N syringaldehyde Chemical compound COC1=CC(C=O)=CC(OC)=C1O KCDXJAYRVLXPFO-UHFFFAOYSA-N 0.000 description 1
- YIBXWXOYFGZLRU-UHFFFAOYSA-N syringic aldehyde Natural products CC12CCC(C3(CCC(=O)C(C)(C)C3CC=3)C)C=3C1(C)CCC2C1COC(C)(C)C(O)C(O)C1 YIBXWXOYFGZLRU-UHFFFAOYSA-N 0.000 description 1
- 150000004684 trihydrates Chemical class 0.000 description 1
- IEDVJHCEMCRBQM-UHFFFAOYSA-N trimethoprim Chemical compound COC1=C(OC)C(OC)=CC(CC=2C(=NC(N)=NC=2)N)=C1 IEDVJHCEMCRBQM-UHFFFAOYSA-N 0.000 description 1
- 229960001082 trimethoprim Drugs 0.000 description 1
- MWOOGOJBHIARFG-UHFFFAOYSA-N vanillin Chemical compound COC1=CC(C=O)=CC=C1O MWOOGOJBHIARFG-UHFFFAOYSA-N 0.000 description 1
- FGQOOHJZONJGDT-UHFFFAOYSA-N vanillin Natural products COC1=CC(O)=CC(C=O)=C1 FGQOOHJZONJGDT-UHFFFAOYSA-N 0.000 description 1
- 235000012141 vanillin Nutrition 0.000 description 1
Landscapes
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
Claims (9)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201210534372.3A CN102992982B (en) | 2012-12-12 | 2012-12-12 | Synthesis method of p-hydroxybenzaldehyde |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201210534372.3A CN102992982B (en) | 2012-12-12 | 2012-12-12 | Synthesis method of p-hydroxybenzaldehyde |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN102992982A true CN102992982A (en) | 2013-03-27 |
| CN102992982B CN102992982B (en) | 2015-01-14 |
Family
ID=47922167
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201210534372.3A Active CN102992982B (en) | 2012-12-12 | 2012-12-12 | Synthesis method of p-hydroxybenzaldehyde |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN102992982B (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103787945A (en) * | 2014-01-20 | 2014-05-14 | 天津市敬业精细化工有限公司 | Preparation method of aromatic aldehyde |
| CN104230748A (en) * | 2014-11-04 | 2014-12-24 | 洛阳市三诺化工有限公司 | Synthetic process of copper extraction agent 5-nonyl salicylaldoxime |
| CN104230748B (en) * | 2014-11-04 | 2017-01-04 | 洛阳市三诺化工有限公司 | A kind of synthesis technique of copper extractant 5-nonyl salicyl aldooxime |
| CN106916051A (en) * | 2017-02-21 | 2017-07-04 | 新乡学院 | A kind of preparation method of the hydroxy-benzyl alcohol of 3,5 di-t-butyl 4 |
| CN112205405A (en) * | 2020-11-11 | 2021-01-12 | 浙江新安化工集团股份有限公司 | Weeding composition containing vanillin and glufosinate-ammonium and herbicide |
| CN112616837A (en) * | 2020-10-28 | 2021-04-09 | 贵州省中国科学院天然产物化学重点实验室(贵州医科大学天然产物化学重点实验室) | Use of o-hydroxy-p-methoxybenzaldehyde derivatives as herbicides |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101456853A (en) * | 2009-01-05 | 2009-06-17 | 江苏省农业科学院 | Bionic novel series compounds 7-alkoxyl-8-(3,3'-disubstituted propyl) benzo pyran-2-ones synthesis and its application as pesticides |
| CN102617313A (en) * | 2012-03-15 | 2012-08-01 | 华东理工大学 | Sharing synthesis method for vanillin and isovanillin |
-
2012
- 2012-12-12 CN CN201210534372.3A patent/CN102992982B/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101456853A (en) * | 2009-01-05 | 2009-06-17 | 江苏省农业科学院 | Bionic novel series compounds 7-alkoxyl-8-(3,3'-disubstituted propyl) benzo pyran-2-ones synthesis and its application as pesticides |
| CN102617313A (en) * | 2012-03-15 | 2012-08-01 | 华东理工大学 | Sharing synthesis method for vanillin and isovanillin |
Non-Patent Citations (3)
| Title |
|---|
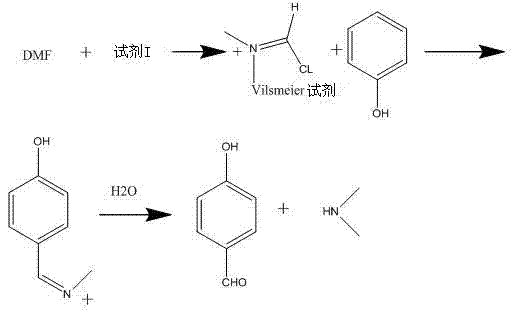
| GURNOS JONES ET AL.: "The vilsmeier reaction of fully conjugated carbocycles and heterocycles", 《ORGANIC REACTIONS》, vol. 49, 31 December 1997 (1997-12-31) * |
| 厍梦尧等: "对羟基苯甲醛的合成研究新进展", 《化学试剂》, vol. 33, no. 12, 31 December 2011 (2011-12-31), pages 1087 - 1094 * |
| 李东凤等: "《有机化学》", 31 August 2007, article "醛、酮、醌", pages: 326 * |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103787945A (en) * | 2014-01-20 | 2014-05-14 | 天津市敬业精细化工有限公司 | Preparation method of aromatic aldehyde |
| CN104230748A (en) * | 2014-11-04 | 2014-12-24 | 洛阳市三诺化工有限公司 | Synthetic process of copper extraction agent 5-nonyl salicylaldoxime |
| CN104230748B (en) * | 2014-11-04 | 2017-01-04 | 洛阳市三诺化工有限公司 | A kind of synthesis technique of copper extractant 5-nonyl salicyl aldooxime |
| CN106916051A (en) * | 2017-02-21 | 2017-07-04 | 新乡学院 | A kind of preparation method of the hydroxy-benzyl alcohol of 3,5 di-t-butyl 4 |
| CN112616837A (en) * | 2020-10-28 | 2021-04-09 | 贵州省中国科学院天然产物化学重点实验室(贵州医科大学天然产物化学重点实验室) | Use of o-hydroxy-p-methoxybenzaldehyde derivatives as herbicides |
| CN112616837B (en) * | 2020-10-28 | 2024-03-01 | 贵州省中国科学院天然产物化学重点实验室(贵州医科大学天然产物化学重点实验室) | Use of o-hydroxy-p-methoxybenzaldehyde derivatives as herbicides |
| CN112205405A (en) * | 2020-11-11 | 2021-01-12 | 浙江新安化工集团股份有限公司 | Weeding composition containing vanillin and glufosinate-ammonium and herbicide |
Also Published As
| Publication number | Publication date |
|---|---|
| CN102992982B (en) | 2015-01-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102174040B (en) | Preparation method of electronic grade triglycidyl isocyanurate | |
| CN102992982B (en) | Synthesis method of p-hydroxybenzaldehyde | |
| CN104387291B (en) | Preparation method of 1,3,6-hexanetricarbonitrile | |
| CN104788632B (en) | A kind of preparation method of high-purity polyether-ether-ketone | |
| CN106117128A (en) | A kind of micro passage reaction prepares the method for pyridone chlorine addition product continuously | |
| CN102285649A (en) | Method for preparing monopotassium phosphate | |
| CN107459472A (en) | Method for refining dimethyl sulfoxide solvent in carbon fiber precursor production process | |
| CN105503513B (en) | The method of silicon dioxide carried catalysis of phosphotungstic acid synthesis 4,4 '-dichloromethyl biphenyl | |
| CN102976929A (en) | Method for synthesizing (4-chloro-2-phenoxy phenyl)-acetic acid | |
| CN104250218B (en) | A kind of tert-butyl acrylamide sulfonate production method | |
| CN105315232A (en) | Method for preparing acryloyl morpholine | |
| CN105837791B (en) | Preparation method of hydantoin epoxy resin | |
| CN104910032A (en) | Preparation method of anilino-acetate | |
| CN103951590B (en) | The preparation method of N, O-dimethyl-N '-nitro isourea | |
| CN107253916B (en) | A kind of environmentally protective new method for preparing amion acetic acid | |
| Souza et al. | Structural characterization of a new dioxamic acid derivative by experimental (FT-IR, NMR, and X-ray) analyses and theoretical (HF and DFT) investigations | |
| CN105330605B (en) | A kind of industrialized preparing process of the fluoropyrimidine of 2 first sulfydryl 4,6 | |
| CN108409768A (en) | A kind of preparation method of boron trifluoride benzylamine complex compound | |
| CN103524291A (en) | Continuous synthetic method of chloralkane | |
| CN115960133A (en) | Comprehensive recovery and utilization method of alkyl dichlorophosphine production by-products | |
| CN104117389A (en) | Preparation method capable of improving yield of catalyst raw powder for methanol to olefin | |
| CN104945234B (en) | The preparation method of the methoxy benzophenone of 2,2 ' dihydroxy 4 | |
| CN103319355B (en) | Separation and purification method of amino glycerol | |
| CN205653373U (en) | System for synthetic 2, 4 - dinitroanisole of serialization | |
| CN104045525A (en) | Production technology for synthesizing m-bromoanisole |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| ASS | Succession or assignment of patent right |
Owner name: CHAMBROAD AGROCHEMICAL TECHNOLOGY CO., LTD. Free format text: FORMER OWNER: YELLOW RIVER DELTA JINGBO CHEMICAL RESEARCH INSTITUTE CO., LTD. Effective date: 20141201 |
|
| C41 | Transfer of patent application or patent right or utility model | ||
| TA01 | Transfer of patent application right |
Effective date of registration: 20141201 Address after: 256500 Shandong city of Binzhou province Hu Zhen Boxing County Chen Jingbo Industrial Park Beijing Bo agricultural Polytron Technologies Inc Applicant after: JINGBO AGROCHEMICALS TECHNOLOGY Co.,Ltd. Address before: 256500 Boxing Economic Development Zone, Binzhou, Shandong Applicant before: CHAMBROAD CHEMICAL INDUSTRY RESEARCH INSTITUTE Co.,Ltd. |
|
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CP03 | Change of name, title or address | ||
| CP03 | Change of name, title or address |
Address after: 256500 Boxing County Economic Development Zone, Binzhou, Shandong Patentee after: JINGBO AGROCHEMICALS TECHNOLOGY Co.,Ltd. Address before: 256500 Jingbo Agrochemical Technology Co., Ltd. of Jingbo Industrial Park, Chenhu Town, Boxing County, Binzhou City, Shandong Province Patentee before: JINGBO AGROCHEMICALS TECHNOLOGY Co.,Ltd. |
|
| CP01 | Change in the name or title of a patent holder | ||
| CP01 | Change in the name or title of a patent holder |
Address after: 256500 Boxing Economic Development Zone, Shandong, Binzhou Patentee after: Shandong Jingbo Agrochemical Technology Co.,Ltd. Address before: 256500 Boxing Economic Development Zone, Shandong, Binzhou Patentee before: JINGBO AGROCHEMICALS TECHNOLOGY Co.,Ltd. |