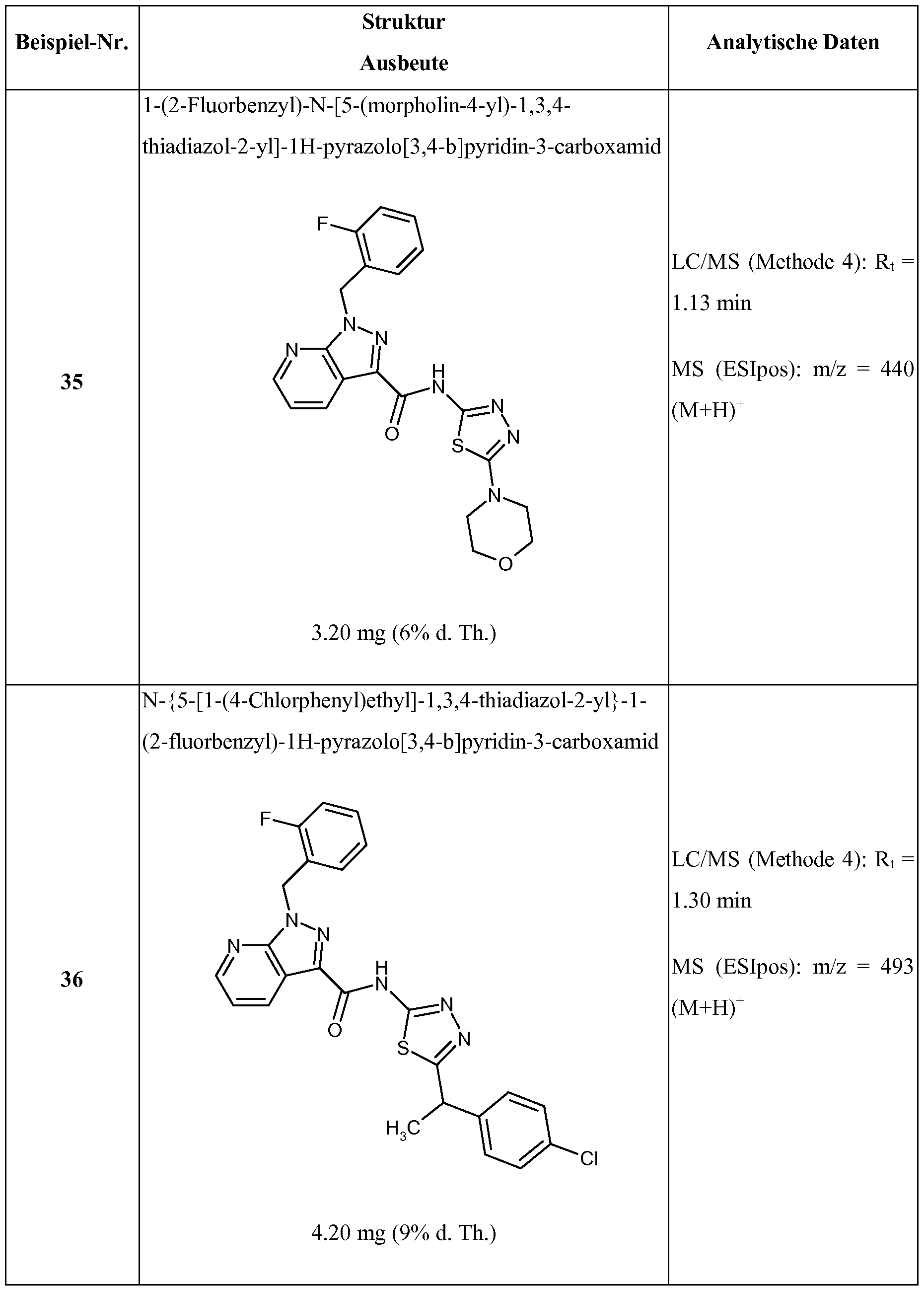
WO2017121693A1 - Substituted thiazole and thiadiazole amides, and use thereof - Google Patents
Substituted thiazole and thiadiazole amides, and use thereof Download PDFInfo
- Publication number
- WO2017121693A1 WO2017121693A1 PCT/EP2017/050311 EP2017050311W WO2017121693A1 WO 2017121693 A1 WO2017121693 A1 WO 2017121693A1 EP 2017050311 W EP2017050311 W EP 2017050311W WO 2017121693 A1 WO2017121693 A1 WO 2017121693A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- substituted
- phenyl
- hydrogen
- group
- Prior art date
Links
- QOMRCMAKWINLAN-UHFFFAOYSA-N OC(c1n[n](Cc(cccc2)c2F)c2ncccc12)=O Chemical compound OC(c1n[n](Cc(cccc2)c2F)c2ncccc12)=O QOMRCMAKWINLAN-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
Definitions
- the present application relates to novel substituted thiazole and thiadiazolamides, processes for their preparation, their use alone or in combinations for the treatment and / or prophylaxis of diseases and their use for the preparation of medicaments for the treatment and / or prophylaxis of diseases, in particular Treatment and / or prophylaxis of cardiovascular diseases.
- cyclic guanosine monophosphate cGMP
- NO nitric oxide
- the guanylate cyclases catalyze the biosynthesis of cGMP from guanosine triphosphate (GTP).
- GTP guanosine triphosphate
- the previously known members of this family can be divided into two groups according to both structural features and the nature of the ligands: the particulate guanylate cyclases stimulable by natriuretic peptides and the soluble guanylate cyclases stimulable by NO.
- the soluble guanylate cyclases consist of two subunits and most likely contain one heme per heterodimer that is part of the regulatory center. This is central to the activation mechanism. NO can bind to the iron atom of the heme and thus significantly increase the activity of the enzyme. On the other hand, heme-free preparations can not be stimulated by NO. Also, carbon monoxide (CO) is able to bind to the central iron atom of the heme, with stimulation by CO being significantly less than by NO.
- CO carbon monoxide
- guanylate cyclase plays a crucial role in various physiological processes, in particular in the relaxation and proliferation of smooth muscle cells, platelet aggregation and adhesion, neuronal signaling and diseases based on a disturbance of the above operations.
- the NO / cGMP system may be suppressed, which may, for example, lead to hypertension, platelet activation, increased cell proliferation, endothelial dysfunction, arteriosclerosis, angina pectoris, heart failure, myocardial infarction, thrombosis, stroke and sexual dysfunction.
- a NO-independent treatment option for such diseases which is aimed at influencing the cGMP pathway in organisms, is a promising approach on account of the expected high efficiency and low side effects.
- WO 00/06568 and WO 00/06569 disclose fused pyrazole derivatives and WO 03/095451 discloses carbamate-substituted 3-pyrimidinyl-pyrazolopyridines. 3-Pyrimidinyl-pyrazolopyridines with phenylamide substituents are described in E.M. Becker et al, BMC Pharmacology, 2001, 1 (13).
- WO 2004/009590 describes pyrazolopyridines with substituted 4-aminopyrimidines for the treatment of CNS diseases.
- WO 2010/065275 and WO 2011/149921 disclose substituted pyrrolo and dihydropyridopyrimidines as sGC activators.
- the sGC stimulators described in WO 2012/004259 are fused aminopyrimidines and in WO 2012/004258, WO 2012/143510 and WO 2012/152629 fused pyrimidines and triazines.
- WO 2012/28647 discloses pyrazolopyridines with various azaheterocycles for the treatment of cardiovascular diseases.
- WO 2008/052898 describes indazoles with thiazolamide substituents for the treatment of diabetes and related diseases.
- WO 2006/015263 discloses N-benzyl-indazoles having various amide substituents for the treatment of cancer and benign prostatic hyperplasia.
- the object of the present invention was to provide novel substances which act as stimulators of soluble guanylate cyclase.
- the present invention relates to compounds of the general formula (I)
- V is CR 3 or N
- R is hydrogen, trifluoromethyl, (C 1 -C 6 ) -alkyl, (C 3 -C 7 ) -cycloalkyl, (C 1 -C 4 ) -alkoxycarbonyl, phenyl, pyridyl, -NR 5 R 6 , -S (O) n R 7 , -S0 2 NR 8 R 9 or -OR 10 , wherein (Ci-C6) alkyl having 1 to 3 substituents independently selected from the group fluorine, trifluoromethyl, hydroxy, trifluoromethoxy, (Ci-C i) - Alkoxy, hydroxycarbonyl, (C 1 -C 4) -alkoxycarbonyl, (C 1 -C 4) -alkoxycarbonylamino, mono (C 1 -C 4) -alkylamino, di- (C 1 -C 4) -alkylamino, phenyl, phenoxy, 4 to
- R 5 is hydrogen or (Ci-C 6 ) alkyl, wherein (Ci-C6) alkyl in turn with 1 or 2 substituents independently selected from the group fluorine, trifluoromethyl, hydroxy, trifluoromethoxy and (Ci-C4) alkoxy may be substituted
- R 6 is hydrogen, (Ci-C 6 ) alkyl, (C 3 -C 7 ) -cycloalkyl, phenyl or 4- to 7-membered heterocyclyl, wherein phenyl in turn having 1 or 2 substituents independently selected from the group halogen , (C 1 -C 4) -alkyl, (C 1 -C 4) -alkoxy and (C 1 -C 4) -alkylcarbonylamino, or
- R 5 and R 6 together with the nitrogen atom to which they are attached form a 4- to 7-membered heterocycle in which 4- to 7-membered heterocycle may be substituted by (C 1 -C 4) -alkyl, in which C4) -alkyl may be substituted by hydroxy,
- R 7 is (C 1 -C 6 ) -alkyl, (C 3 -C 7 ) -cycloalkyl or phenyl
- R 8 is hydrogen or (C 1 -C 6 ) -alkyl
- R 9 is hydrogen, (C 1 -C 6 ) -Alkyl or (C 3 -C 7 ) -cycloalkyl
- R 10 is (Ci-C 6 ) -alkyl, (C 3 -C 7 ) -cycloalkyl or phenyl, is CR 4 or N, wherein R 4 is hydrogen, (C 1 -C 4 ) -alkyl, (C 3 -C 7 ) -cycloalkyl or 3 to 7-membered heterocyclyl, wherein (C 1 -C 4 ) -alkyl having 1 to 3 substituents selected independently of one another the group fluorine, trifluoromethyl, hydroxy, trifluoromethoxy, (Ci-C4)
- Di- (C 1 -C 4) -alkylamino in which mono- (C 1 -C 4) -alkylamino and di- (C 1 -C 4) -alkylamino in turn have 1 or 2 substituents independently selected from the group of fluorine, trifluoromethyl and phenyl in which 4- to 7-membered heterocyclyl may in turn be substituted by 1 or 2 substituents independently of one another selected from the group of fluoro, (C 1 -C 4) -alkyl, hydroxyl, oxo and (C 1 -C 4) -alkoxy, in which (C 1 -C 4) -alkyl in turn contains 1 or 2 substituents independently of one another selected from the group of fluorine, trifluoromethyl, hydroxy,
- Trifluoromethoxy and (Ci-C4) alkoxy may be substituted, or
- R 3 and R 4 together with V and W to which they are attached form a 4- to 7-membered heterocyclyl ring wherein the 4- to 7-membered heterocyclyl ring is substituted with (C 1 -C 4) -alkyl can
- R 1 is hydrogen or fluorine
- R 2 is (C 1 -C 6 ) -alkyl or benzyl, where (C 1 -C 6 ) -alkyl is substituted by a substituent trifluoromethyl, where (C 1 -C 6) -alkyl having 1 to 3 Substituents fluorine may be substituted, and wherein benzyl is substituted by 1 to 3 substituents fluorine, with the proviso that when V is N, W is CR, with the proviso that when W is N, V is CR 3 , and their N-oxides, salts, solvates, salts of N-oxides and solvates of N-oxides and salts.
- Compounds according to the invention are the compounds of the formula (I) and their salts, solvates and solvates of the salts comprising the compounds of the formulas below and their salts, solvates and solvates of the salts and of the formula (I) encompassed by formula (I), hereinafter referred to as exemplary compounds and their salts, solvates and solvates of the salts, as far as the compounds of formula (I), the following compounds are not already salts, solvates and solvates of the salts.
- Salts used in the context of the present invention are physiologically acceptable salts of the compounds according to the invention. Also included are salts which are themselves unsuitable for pharmaceutical applications but can be used, for example, for the isolation or purification of the compounds of the invention.
- Physiologically acceptable salts of the compounds of the invention include acid addition salts of mineral acids, carboxylic acids and sulfonic acids, e.g. Salts of hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid, methanesulfonic acid, ethanesulfonic acid, toluenesulfonic acid, benzenesulfonic acid, naphthalenedisulfonic acid, formic acid, acetic acid, trifluoroacetic acid, propionic acid, lactic acid, tartaric acid, malic acid, citric acid, fumaric acid, maleic acid and benzoic acid.
- salts of hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid, methanesulfonic acid, ethanesulfonic acid, toluenesulfonic acid, benzenesulfonic acid, naphthalenedisulfonic acid formic acid, acetic acid, trifluoro
- Physiologically acceptable salts of the compounds according to the invention also include salts of customary bases, such as, by way of example and by way of preference, alkali metal salts (for example sodium and potassium salts), alkaline earth salts (for example calcium and magnesium salts) and ammonium salts derived from ammonia or organic amines having 1 to 16 carbon atoms, for example and preferably ethylamine, diethylamine, triethylamine, ethyldiisopropylamine, monoethanolamine, diethanolamine, triethanolamine, dicyclohexylamine, dimethylaminoethanol, procaine, dibenzylamine, N-methylmorpholine, arginine, lysine, ethylenediamine and N-methylpiperidine.
- customary bases such as, by way of example and by way of preference, alkali metal salts (for example sodium and potassium salts), alkaline earth salts (for example calcium and magnesium salts) and ammonium salts
- Solvates in the context of the invention are those forms of the compounds according to the invention which form a complex in the solid or liquid state by coordination with solvent molecules. Hydrates are a special form of Solvates where coordination with water occurs. As solvates, hydrates are preferred in the context of the present invention.
- the compounds of the invention may exist in different stereoisomeric forms depending on their structure, i. in the form of configurational isomers or optionally also as conformational isomers (enantiomers and / or diastereomers, including those in atropisomers).
- the present invention therefore includes the enantiomers and diastereomers and their respective mixtures. From such mixtures of enantiomers and / or diastereomers, the stereoisomerically uniform components can be isolated in a known manner; Preferably, chromatographic methods are used for this, in particular HPLC chromatography on achiral or chiral phase.
- the present invention encompasses all tautomeric forms.
- the present invention also encompasses all suitable isotopic variants of the compounds according to the invention.
- An isotopic variant of a compound according to the invention is understood to mean a compound in which at least one atom within the compound according to the invention is exchanged for another atom of the same atomic number but with a different atomic mass than the atomic mass that usually or predominantly occurs in nature.
- isotopes which can be incorporated into a compound of the invention are those of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, fluorine, chlorine, bromine and iodine, such as 2 H (deuterium), 3 H (tritium), 13 C, 14 C, 15 N, 17 0, 18 0, 32 P, 33 P, 33 S, 34 S, 35 S, 36 S, 18 F, 36 Cl, 82 Br, 123 I, 124 I, 129 I and 131 I.
- isotopic variants of a compound of the invention such as, in particular, those in which one or more radioactive isotopes are incorporated, may be useful, for example, for the study of the mechanism of action or distribution of the drug in the body; Due to the comparatively easy production and detectability, compounds labeled with 3 H or 14 C isotopes in particular are suitable for this purpose.
- the incorporation of isotopes such as deuterium may result in certain therapeutic benefits as a result of greater metabolic stability of the compound, such as prolonging the body's half-life or reducing the required effective dose; Such modifications of the compounds of the invention may therefore optionally also constitute a preferred embodiment of the present invention.
- iso- Topical variants of the compounds according to the invention can be prepared by the processes known to the person skilled in the art, for example by the methods described below and the instructions given in the exemplary embodiments, by using appropriate isotopic modifications of the respective reagents and / or starting compounds.
- the present invention also includes prodrugs of the compounds of the invention.
- prodrugs refers to compounds which themselves may be biologically active or inactive, but are converted during their residence time in the body to compounds of the invention (for example metabolically or hydrolytically).
- alkyl is a linear or branched alkyl radical having 1 to 4 carbon atoms.
- alkyl radical having 1 to 4 carbon atoms.
- Cycloalkyl in the context of the invention is a monocyclic, saturated carbocycle having 3 to 7 ring carbon atoms. Examples which may be mentioned by way of example include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl and cycloheptyl.
- Alkoxy in the context of the invention is a linear or branched alkoxy radical having 1 to 4 carbon atoms. Examples which may be mentioned by way of example include: methoxy, ethoxy, n-propoxy, isopropoxy, 1-methylpropoxy, n-butoxy, isobutoxy and tert. Butoxy.
- Alkoxycarbonyl in the context of the invention are a linear or branched alkoxy radical having 1 to 4 carbon atoms and an oxygen-bonded carbonyl group. Examples which may be mentioned by way of example include: methoxycarbonyl, ethoxycarbonyl, n-propoxycarbonyl, isopropoxycarbonyl and tert. Butoxycarbonyl.
- Heterocyclyl in the context of the invention is a saturated heterocycle having a total of 4 to 7 ring atoms, which contains one or two ring heteroatoms from the series N, O and / or S and is linked via a ring carbon atom or optionally a ring nitrogen atom.
- Examples which may be mentioned are: azetidinyl, oxetanyl, pyrrolidinyl, pyrazolidinyl, tetrahydrofuranyl, piperidinyl, Piperazinyl, tetrahydropyranyl, morpholinyl and thiomorpholinyl.
- Preferred are azetidinyl, pyrrolidinyl, piperidinyl, piperazinyl and morpholinyl.
- Heteroaryl is in the context of the invention for a monocyclic aromatic heterocycle (heteroaromatic) with a total of 5 or 6 ring atoms containing up to three identical or different ring heteroatoms from the series N, O and / or S and via a ring carbon atom or optionally linked via a ring nitrogen atom.
- heterocycle monocyclic aromatic heterocycle (heteroaromatic) with a total of 5 or 6 ring atoms containing up to three identical or different ring heteroatoms from the series N, O and / or S and via a ring carbon atom or optionally linked via a ring nitrogen atom.
- Examples which may be mentioned are: furyl, pyrrolyl, thienyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, isoxazolyl, isothiazolyl, triazolyl, oxadiazolyl, thiadiazolyl, pyridyl, pyrimidinyl, pyridazinyl, pyrazinyl and triazinyl.
- Halogen is in the context of the invention for fluorine, chlorine, bromine and iodine.
- An oxo group in the context of the invention is an oxygen atom which is bonded via a double bond to a carbon atom.
- radicals are substituted in the compounds according to the invention, the radicals can, unless otherwise specified, be monosubstituted or polysubstituted. In the context of the present invention, the meaning is independent of each other for all radicals which occur repeatedly. Substitution with one, two or three identical or different substituents is preferred.
- V is CR 3 or N
- R 3 is hydrogen, trifluoromethyl, (Ci-C 6) -alkyl, (C 3 -C 6) cycloalkyl, (Ci-C4) - alkoxycarbonyl, phenyl, pyridyl, -NR 5 R 6, -S (0) n is R 7 or -SO 2 NR 8 R 9 , wherein (Ci-C6) alkyl having 1 or 2 substituents independently selected from the group fluorine, trifluoromethyl, trifluoromethoxy, (Ci-C i) alkoxy, methylamino, ethylamino , Dimethylamino, diethylamino, phenoxy, azetidinyl, pyrrolodinyl, piperidinyl, morpholinyl, piperazinyl and pyrazolyl may be substituted, wherein pyrazolyl in turn may be substituted with 1 or 2 substituents independently selected from the group of fluoro,
- R 5 is hydrogen or (C 1 -C 4 ) -alkyl, in which (C 1 -C 4 ) -alkyl in turn may be substituted by 1 or 2 substituents independently of one another selected from the group consisting of fluorine, trifluoromethyl, hydroxyl, trifluoromethoxy, methoxy and ethoxy,
- R 6 is (C 1 -C 4 ) -alkyl, (C 3 -C 6 ) -cycloalkyl, phenyl, azetidinyl, pyrrolidinyl, tetrahydrofuranyl, dioxotetrahydrothiolanyl, piperidinyl, tetrahydropyranyl, in which phenyl is in turn independently selected with 1 or 2 substituents from the group of fluorine, chlorine, methyl, ethyl, methoxy, ethoxy and methylcarbonylamino, or R 5 and R 6 together with the nitrogen atom to which they are bonded form an azetidinyl, pyrrolidinyl, piperidinyl, piperazinyl , Morpholinyl or dioxothiomorpholinyl ring, wherein the piperazinyl ring on the nitrogen may be substituted with ethyl wherein ethyl is substitute
- R 7 is (C 1 -C 4 ) -alkyl or (C 3 -C 6 ) -cycloalkyl, is hydrogen, methyl or ethyl, R 9 is hydrogen, methyl or ethyl; W is CR 4 or N, wherein
- R 4 is hydrogen, (C 1 -C 4 ) -alkyl, cyclopropyl, cyclobutyl or cyclopentyl, in which (C 1 -C 4) -alkyl with trifluoromethyl, hydroxy, hydroxycarbonyl,
- Methoxycarbonyl, ethoxycarbonyl, methylamino, ethylamino, dimethylamino or diethylamino may be substituted, wherein methylamino, ethylamino, dimethylamino and diethylamino may be substituted by phenyl, R 1 is hydrogen,
- R 2 is 2-fluorobenzyl, provided that when V is N, W is CR 4 , with the proviso that when W is N, V is CR 3 , and their salts, solvates and solvates salts.
- Particularly preferred in the context of the present invention are compounds of the formula (I) in which
- U is S, V is CR 3 or N, where R 3 is hydrogen, trifluoromethyl, (C 1 -C 6) -alkyl, cyclopropyl, cyclohexyl, methoxycarbonyl, phenyl, pyridyl, -NR 5 R 6 , -S (0) n is R 7 or -SO 2 NR 8 R 9 , in which (C 1 -C 6) -alkyl may be substituted by trifluoromethyl, (C 1 -C 4) -alkoxy, methylamino, ethylamino, dimethylamino, diethylamino, phenoxy, pyrrolodinyl or pyrazolyl, wherein pyrazolyl in turn may be substituted with 1 or 2 substituents independently selected from the group consisting of methyl and ethyl, wherein phenyl and pyridyl may be substituted with 1 or 2 substituents independently selected from the group flu
- R 5 is hydrogen or (C 1 -C 4 ) -alkyl, in which (C 1 -C 4 ) -alkyl may in turn be substituted by hydroxyl,
- R 6 is (C 1 -C 4) -alkyl, cyclohexyl, phenyl or dioxotetrahydrothiolanyl, in which phenyl in turn may be substituted by methoxy, ethoxy or methylcarbonylamino, or
- R 5 and R 6 together with the nitrogen atom to which they are attached form a pyrrolidinyl, piperazinyl or morpholinyl ring in which the piperazinyl ring on the nitrogen is substituted by ethyl, in which ethyl is substituted by hydroxy,
- R 7 is (C 1 -C 4 ) -alkyl or cyclohexyl
- R 8 is hydrogen, methyl or ethyl
- R 9 is hydrogen, methyl or ethyl, CR 4 or N, wherein
- R 4 is hydrogen, (C 1 -C 4 ) -alkyl or cyclopropyl, in which (C 1 -C 4) -alkyl or methylamino may be substituted, wherein methylamino may be substituted with phenyl,
- R is hydrogen
- R 2 is 2-fluorobenzyl, provided that when V is N, W is CR, with the proviso that when W is N, V is CR 3 , and their salts, solvates and solvates of the salts ,
- R 1 is hydrogen, and their salts, solvates and solvates of the salts.
- R 2 is 2-fluorobenzyl, and their salts, solvates and solvates of the salts. Particularly preferred in the context of the present invention are also compounds of the formula (I) in which
- U is S, and their salts, solvates and solvates of the salts.
- V is N, and their salts, solvates and solvates of the salts.
- R 3 is hydrogen, trifluoromethyl, (C 1 -C 6) -alkyl, cyclopropyl, cyclohexyl, methoxycarbonyl, phenyl, pyridyl, -NR 5 R 6 , -S (O) n R 7 or -SO 2 NR 8 R 9 in which (C 1 -C 6) -alkyl with trifluoromethyl, (C 1 -C 4) -alkoxy, methylamino, ethylamino,
- pyrazolyl Dimethylamino, diethylamino, phenoxy, pyrrolodinyl or pyrazolyl, in which pyrazolyl may in turn be substituted with 1 or 2 substituents independently selected from the group consisting of methyl and ethyl, wherein phenyl and pyridyl having 1 or 2 substituents independently selected from the group fluorine , Chloro, methyl, methoxy and ethoxy, and wherein n is a number 1 or 2,
- R 5 is hydrogen or (C 1 -C 4 ) -alkyl, in which (C 1 -C 4 ) -alkyl may in turn be substituted by hydroxyl,
- R 6 is (C 1 -C 4) -alkyl, cyclohexyl, phenyl or dioxotetrahydrothiolanyl, in which phenyl in turn may be substituted by methoxy, ethoxy or methylcarbonylamino, or
- R 5 and R 6 together with the nitrogen atom to which they are attached form a pyrrolidinyl, piperazinyl or morpholinyl ring in which the piperazinyl ring on the nitrogen is substituted by ethyl, in which ethyl is substituted by hydroxy,
- R 7 is (C 1 -C 4 ) -alkyl or cyclohexyl
- R 8 is hydrogen, methyl or ethyl
- R 9 is hydrogen, methyl or ethyl
- W is N, and their salts, solvates and solvates of the salts.
- R 4 is hydrogen, (C 1 -C 4 ) -alkyl or cyclopropyl, in which (C 1 -C 4) -alkyl or methylamino may be substituted, in which methylamino may be substituted by phenyl, and their salts, solvates and solvates of the salts.
- the invention further provides a process for the preparation of the compounds of the formula (I) according to the invention which comprises reacting a compound of the formula (II) in which R 1 and R 2 each have the meanings given above, in an inert solvent in the presence of a suitable base and a suitable coupling reagent with a compound of the formula (III)
- Inert solvents are alcohols such as methanol, ethanol, n-propanol, isopropanol, n-butanol, tert-butanol or 1, 2-ethanediol, ethers such as diethyl ether, dioxane, tetrahydrofuran, glycol dimethyl ether or diethylene glycol dimethyl ether, hydrogen halides such as dichloromethane, trichloromethane, Tetrachloromethane, trichlorethylene, chlorobenzene or 1, 2-dichlorobenzene, hydrocarbons such as benzene, toluene, xylene, hexane, cyclohexane or petroleum fractions, or other solvents such as acetone, methyl ethyl ketone, ethyl acetate, acet
- N- (3-dimethylaminoisopropyl) -N'-ethylcarbodiimide hydrochloride EDC
- N, N-diisopropylethylamine or O- (benzotriazol-1-yl) -N, N, N ', N'-tetramethyluronium tetrafluoroborate (TBTU) in combination with 4-methylmorpholine.
- the reaction (II) + (III) -> (I) is generally carried out in a temperature range from 0 ° C to + 140 ° C, preferably carried out in a temperature range from + 20 ° C to + 60 ° C.
- the reaction may be carried out at normal, elevated or reduced pressure (e.g., from 0.5 to 5 bar). Generally, one works at normal pressure.
- the compounds of the formula (II) are known from the literature (cf., for example, WO 2007/124854), and can be prepared in analogy to processes known from the literature.
- Other compounds of the invention may optionally also be prepared by conversions of functional groups of individual substituents, in particular those listed under R 3 and R 4 , starting from the compounds of the formula (I) obtained by the above methods.
- transformations are carried out as described in the present experimental part, according to customary methods known to the person skilled in the art, and include, for example, reactions such as nucleophilic and electrophilic substitutions, oxidations, reductions, hydrogenations, transition metal-catalyzed coupling reactions, eliminations, alkylation, amination, esterification, ester cleavage, Etherification, ether cleavage, formation of carbonamides, and introduction and removal of temporary protecting groups.
- reactions such as nucleophilic and electrophilic substitutions, oxidations, reductions, hydrogenations, transition metal-catalyzed coupling reactions, eliminations, alkylation, amination, esterification, ester cleavage, Etherification, ether cleavage, formation of carbonamides, and introduction and removal of temporary protecting groups.
- the compounds of the invention act as stimulators of soluble guanylate cyclase and possess valuable pharmacological, and are therefore suitable for the treatment and / or prophylaxis of diseases in humans and animals.
- the compounds of the invention cause vasorelaxation and inhibition of platelet aggregation and lead to a reduction in blood pressure and to an increase in coronary blood flow. These effects are mediated by direct stimulation of soluble guanylate cyclase and an intracellular cGMP increase.
- the compounds according to the invention enhance the action of substances which increase cGMP levels, such as, for example, EDRF (endothelium-derived relaxing factor), NO donors, protoporphyrin IX, arachidonic acid or phenylhydrazine derivatives.
- the compounds according to the invention can therefore be used in medicaments for the treatment and / or prophylaxis of cardiovascular diseases such as hypertension, acute and chronic heart failure, heart failure, coronary heart disease, stable and unstable angina pectoris, peripheral and cardiac vascular diseases, arrhythmias, arrhythmias of the atria and the chambers and Transient disorders such as atrio-ventricular blockades grade I-III (AB block I-III), supraventricular tachyarrhythmia, atrial fibrillation, atrial flutter, ventricular fibrillation, ventricular tachyarrhythmia, torsades de pointes tachycardia, atrial and ventricular extrasystoles, AV junctional Extrasystoles, sick sinus syndrome, syncope, AV nodal reentrant tachycardia, Wolff-Parkinson-White syndrome, acute coronary syndrome (ACS), autoimmune heart disease (pericarditis, endocarditis, valvolitis, aortiti
- cardiac insufficiency also encompasses more specific or related forms of disease such as acute decompensated heart failure, right heart failure, left heart failure, global insufficiency, ischemic cardiomyopathy, dilated cardiomyopathy, hypertrophic cardiomyopathy, idiopathic cardiomyopathy, congenital heart defects, valvular heart failure, cardiac valvulopathy, mitral valve stenosis, mitral valve insufficiency, Aortic valve stenosis, aortic valve insufficiency, tricuspid stenosis, tricuspid regurgitation, pulmonary valve stenosis, pulmonary valvular insufficiency, combined heart valve defects, myocarditis, chronic myocarditis, acute myocarditis, viral myocarditis, diabetic heart failure, alcoholic cardiomyopathy, cardiac storage disorders, diastolic heart failure and systolic heart failure.
- ischemic cardiomyopathy dilated cardiomyopathy
- the compounds according to the invention may also be used for the treatment and / or prophylaxis of arteriosclerosis, lipid metabolism disorders, hypolipoproteinemias, dyslipidemias, hypertriglyceridemias, hyperlipidemias, hypercholesterolemias, abetelipoproteinaemia, sitosterolemia, xanthomatosis, Tangier's disease, obesity (obesity) and combined hyperlipidemias and the metabolic syndrome.
- the compounds of the invention may be used for the treatment and / or prophylaxis of primary and secondary Raynaud's phenomenon, microcirculatory disorders, claudication, peripheral and autonomic neuropathies, diabetic microangiopathies, diabetic retinopathy, diabetic ulcers on the extremities, gangrenous, CREST syndrome, Erythematosis, onychomycosis, rheumatic diseases and to promote wound healing.
- the compounds according to the invention are suitable for the treatment of urological diseases such as benign prostatic syndrome (BPS), benign prostatic hyperplasia (BPH), benign prostate enlargement (BPE), bladder emptying disorder (BOO), lower urinary tract syndromes (LUTS, including Feiine's urological syndrome ( FUS)), diseases of the urogenital system including neurogenic overactive bladder (OAB) and (IC), incontinence (UI) such as mixed, urge, stress, or overflow incontinence (MUI, UUI, SUI, OUI), Pelvic pain, benign and malignant diseases of the organs of the male and female urogenital system.
- BPS benign prostatic syndrome
- BPH benign prostatic hyperplasia
- BPE benign prostate enlargement
- BOO bladder emptying disorder
- LUTS lower urinary tract syndromes
- FUS lower urinary tract syndromes
- UI incontinence
- MUI mixed, urge, stress, or overflow incontinence
- UUI UUI
- SUI S
- kidney diseases in particular of acute and chronic renal insufficiency, as well as of acute and chronic renal failure.
- renal insufficiency includes both acute and chronic manifestations of renal insufficiency, as well as underlying or related renal diseases such as renal hypoperfusion, intradialytic hypotension, obstructive uropathy, glomerulopathies, glomerulonephritis, acute glomerulonephritis, glomerulosclerosis, tubulo-interstitial disorders, nephropathic disorders such as primary and congenital kidney disease, nephritis, immunological renal diseases such as renal transplant rejection, immune complex-induced kidney disease, nephropathy induced by toxic substances, contrast agent-induced nephropathy, diabetic and nondiabetic nephropathy, pyelonephritis, renal cysts, nephrosclerosis, hypertens
- the present invention also encompasses the use of the compounds of the invention for the treatment and / or prophylaxis of sequelae of renal insufficiency, such as pulmonary edema, cardiac insufficiency, uremia, anemia, electrolyte imbalances (e.g., hyperkalemia, hyponatremia) and disorders in bone and carbohydrate metabolism.
- sequelae of renal insufficiency such as pulmonary edema, cardiac insufficiency, uremia, anemia, electrolyte imbalances (e.g., hyperkalemia, hyponatremia) and disorders in bone and carbohydrate metabolism.
- the compounds according to the invention are also suitable for the treatment and / or prophylaxis of asthmatic diseases, pulmonary arterial hypertension (PAH) and others Forms of pulmonary hypertension (PH), chronic obstructive pulmonary disease (COPD), acute respiratory tract syndrome (ARDS), acute lung injury (ALI), alpha-1-antitrypsin deficiency (AATD), pulmonary fibrosis, pulmonary emphysema (eg cigarette smoke-induced pulmonary emphysema) and cystic fibrosis (CF).
- PAH pulmonary arterial hypertension
- COPD chronic obstructive pulmonary disease
- ARDS acute respiratory tract syndrome
- ALI acute lung injury
- AATD alpha-1-antitrypsin deficiency
- pulmonary fibrosis pulmonary emphysema
- CF cystic fibrosis
- the compounds described in the present invention are also agents for controlling diseases in the central nervous system, which are characterized by disorders of the NO /
- they are suitable for improving the perception, concentration performance, learning performance or memory performance after cognitive disorders such as occur in situations / diseases / syndromes such as mild cognitive impairment, age-associated learning and memory disorders, age-associated memory loss, vascular dementia, cranial brain -Trauma, stroke, post-stroke dementia, post-traumatic traumatic brain injury, generalized concentration disorder, difficulty concentrating in children with learning and memory problems, Alzheimer's disease, dementia with Lewy bodies , Dementia with degeneration of the frontal lobes including Pick's syndrome, Parkinson's disease, progressive nuclear palsy, dementia with corticobasal degeneration, amyolateral sclerosis (ALS), Huntington's disease, demyelinization, multiple sclerosis, thalamic degeneration, Creutzfeld-Jacob dementia, HIV dementia, schizophrenia with dementia or Korsakoff's psychosis. They are also suitable for the treatment and / or prophylaxis of diseases of the central nervous system such as states of anxiety, tension and depression, central nervous conditional sexual dysfunction
- the compounds according to the invention are also suitable for regulating cerebral blood flow and are effective agents for combating migraine. They are also suitable for the prophylaxis and control of the consequences of cerebral infarct events (Apoplexia cerebri) such as stroke, cerebral ischaemias and craniocerebral trauma , Likewise, the compounds of the invention can be used to combat pain and tinnitus.
- the compounds of the invention have anti-inflammatory action and can therefore be used as anti-inflammatory agents for the treatment and / or prophylaxis of sepsis (SIRS), multiple organ failure (MODS, MOF), inflammatory diseases of the kidney, chronic inflammatory bowel disease (IBD, Crohn's Disease, UC), pancreatitis , Peritonitis, rheumatoid diseases, inflammatory skin diseases as well as inflammatory eye diseases.
- SIRS sepsis
- MODS multiple organ failure
- IBD chronic inflammatory bowel disease
- UC chronic inflammatory bowel disease
- pancreatitis inflammatory skin diseases as well as inflammatory eye diseases.
- the compounds of the invention can also be used for the treatment and / or prophylaxis of autoimmune diseases.
- the compounds according to the invention are suitable for the treatment and / or prophylaxis of fibrotic disorders of the internal organs such as, for example, the lung, the heart, the kidney, the bone marrow and in particular the liver, as well as dermatological fibroses and fibrotic disorders of the eye.
- fibrotic disorders includes in particular the following terms: liver fibrosis, cirrhosis, pulmonary fibrosis, endomyocardial fibrosis, nephropathy, glomerulonephritis, interstitial renal fibrosis, fibrotic damage as a result of diabetes, bone marrow fibrosis and similar fibrotic disorders, scleroderma, morphea, keloids, hypertrophic scarring (also after surgical procedures), nevi, diabetic retinopathy and proliferative vitroretinopathy.
- the compounds of the invention are useful for controlling postoperative scarring, e.g. as a result of glaucoma surgery.
- the compounds according to the invention can likewise be used cosmetically for aging and keratinizing skin.
- the compounds according to the invention are suitable for the treatment and / or prophylaxis of diabetes, hepatitis, neoplasm, osteoporosis, glaucoma and gastroparesis.
- Another object of the present invention is the use of the compounds of the invention for the treatment and / or prophylaxis of diseases, in particular the aforementioned diseases.
- Another object of the present invention is the use of the compounds of the invention for the treatment and / or prophylaxis of heart failure, angina pectoris, hypertension, pulmonary hypertension, ischaemia, vascular disease, renal insufficiency, thromboembolic disorders, fibrotic diseases and arteriosclerosis.
- the present invention furthermore relates to the compounds according to the invention for use in a method for the treatment and / or prophylaxis of cardiac insufficiency, angina pectoris, hypertension, pulmonary hypertension, ischaemias, vascular disorders, renal insufficiency, thromboembolic disorders, fibrotic disorders and atherosclerosis.
- Another object of the present invention is the use of the compounds of the invention for the manufacture of a medicament for the treatment and / or prophylaxis of diseases, in particular the aforementioned diseases.
- Another object of the present invention is the use of the compounds of the invention for the manufacture of a medicament for the treatment and / or prophylaxis of heart failure, angina pectoris, hypertension, pulmonary hypertension, ischemia, vascular diseases, renal insufficiency, thromboembolic disorders, fibrotic diseases and arteriosclerosis.
- Another object of the present invention is a method for the treatment and / or prophylaxis of diseases, in particular the aforementioned diseases, using an effective amount of at least one of the compounds of the invention.
- the present invention further provides a method for the treatment and / or prophylaxis of cardiac insufficiency, angina pectoris, hypertension, pulmonary hypertension, ischaemias, vascular diseases, renal insufficiency, thromboembolic disorders, fibrotic diseases and atherosclerosis, using an effective amount of at least one of the compounds according to the invention ,
- Another object of the present invention is a method for the treatment and / or prophylaxis of diseases, in particular the aforementioned diseases, using an effective amount of at least one of the compounds of the invention.
- the compounds of the invention may be used alone or as needed in combination with other agents.
- Another object of the present invention are pharmaceutical compositions containing at least one of the compounds of the invention and one or more other active ingredients, in particular for the treatment and / or prophylaxis of the aforementioned diseases.
- suitable combination active ingredients are: organic nitrates and NO donors, such as, for example, sodium nitroprusside, nitroglycerin, isosorbide mononitrate, isosorbide dinitrate, molsidomine or SIN-1, and inhaled NO;
- cGMP cyclic guanosine monophosphate
- PDE phosphodiesterases
- sildenafil, vardenafil and tadalafil inhibitors of phosphodiesterases 1, 2 and / or 5 inhibitors such as sildenafil, vardenafil and tadalafil
- antithrombotic agents by way of example and preferably from the group of platelet aggregation inhibitors, anticoagulants or profibrinolytic substances
- antihypertensive agents by way of example and preferably from the group of calcium antagonists, angiotensin all-antagonists, ACE inhibitors, endothelin antagonists, renin Inhibitors, alpha-receptor blockers, beta-receptor blockers, mineralocorticoid receptor antagonists and diuretics
- lipid metabolism-altering agents by way of example and with preference from the group of thyroid receptor agonists, cholesterol synthesis inhibitors such as, by way of example and preferably, HMG-
- Antithrombotic agents are preferably understood as meaning compounds from the group of platelet aggregation inhibitors, anticoagulants or profibrinolytic substances.
- the compounds according to the invention are administered in combination with a platelet aggregation inhibitor, such as, by way of example and by way of preference, aspirin, clopidogrel, ticlopidine or dipyridamole.
- a platelet aggregation inhibitor such as, by way of example and by way of preference, aspirin, clopidogrel, ticlopidine or dipyridamole.
- the compounds according to the invention are administered in combination with a thrombin inhibitor, such as, by way of example and by way of preference, ximelagatran, dabigatran, melagatran, bivalirudin or Clexane.
- the compounds according to the invention are administered in combination with a GPIIb / IIIa antagonist, such as, by way of example and by way of preference, tirofiban or abciximab.
- a GPIIb / IIIa antagonist such as, by way of example and by way of preference, tirofiban or abciximab.
- the compounds according to the invention are used in combination with a factor Xa inhibitor, such as by way of example and preferably rivaroxaban (BAY 59-7939), DU-176b, apixaban, otamixaban, fidexaban, razaxaban, fondaparinux, idraparinux, PMD No. 3112, YM-150, KFA-1982, EMD-503982, MCM-17, MLN-1021, DX 9065a, DPC 906, JTV 803, SSR-126512 or SSR-128428.
- a factor Xa inhibitor such as by way of example and preferably rivaroxaban (BAY 59-7939), DU-176b, apixaban, otamixaban, fidexaban, razaxaban, fondaparinux, idraparinux, PMD No. 3112, YM-150, KFA-1982, EMD-503982, MCM
- the compounds according to the invention are administered in combination with heparin or a low molecular weight (LMW) heparin derivative.
- LMW low molecular weight
- the compounds according to the invention are administered in combination with a vitamin K antagonist, such as by way of example and preferably coumarin.
- a vitamin K antagonist such as by way of example and preferably coumarin.
- the antihypertensive agents are preferably compounds from the group of calcium antagonists, angiotensin AII antagonists, ACE inhibitors, endothelin antagonists, renin inhibitors, alpha-receptor blocker, beta-receptor blocker, mineralocorticoid receptor - understood antagonists and diuretics.
- the compounds according to the invention are administered in combination with a calcium antagonist, such as, by way of example and by way of preference, nifedipine, amlodipine, verapamil or diltiazem.
- the compounds according to the invention are administered in combination with an alpha-1-receptor blocker, such as by way of example and preferably prazosin.
- the compounds according to the invention are used in combination with a beta-receptor blocker, such as by way of example and preferably propranolol, atenolol, timolol, pindolol, alprenolol, oxprenolol, penbutolol, bupranolol, metipranolol, nadolol, mepindolol, carazalol, sotalol, Metoprolol, betaxolol, celiprolol, bisoprolol, carteolol, esmolol, labetalol, carvedilol, adaprolol, landiolol, nebivolol, epanolol or bucindolol.
- a beta-receptor blocker such as by way of example and preferably propranolol, atenolol, timolol
- the compounds according to the invention are administered in combination with an angiotensin AII antagonist, such as by way of example and preferably losartan, candesartan, valsartan, telmisartan or embursatan.
- an angiotensin AII antagonist such as by way of example and preferably losartan, candesartan, valsartan, telmisartan or embursatan.
- the compounds according to the invention are administered in combination with an ACE inhibitor such as, by way of example and by way of preference, enalapril, captopril, lisinopril, ramipril, delapril, fosinopril, quinopril, perindopril or trandopril.
- an ACE inhibitor such as, by way of example and by way of preference, enalapril, captopril, lisinopril, ramipril, delapril, fosinopril, quinopril, perindopril or trandopril.
- the compounds according to the invention are administered in combination with an endothelin antagonist such as, by way of example and by way of preference, bosentan, darusentan, ambrisentan or sitaxsentan.
- an endothelin antagonist such as, by way of example and by way of preference, bosentan, darusentan, ambrisentan or sitaxsentan.
- the compounds of the invention are administered in combination with a renin inhibitor, such as by way of example and preferably aliskiren, SPP-600 or SPP-800.
- the compounds according to the invention are administered in combination with a mineralocorticoid receptor antagonist, such as by way of example and preferably spironolactone or eplerenone.
- a diuretic such as by way of example and preferably furosemide.
- lipid metabolizing agents are preferably compounds from the group of CETP inhibitors, thyroid receptor agonists, cholesterol synthesis inhibitors such as HMG-CoA reductase or squalene synthesis inhibitors, the ACAT inhibitors, MTP inhibitors, PPAR-alpha, PPAR gamma and / or PPAR delta agonists, cholesterol Abso ⁇ tionhemmer, polymeric Benklareadsorber, bile acid Reabso ⁇ tionshemmer, lipase inhibitors and the lipoprotein (a) understood antagonists.
- CETP inhibitors such as HMG-CoA reductase or squalene synthesis inhibitors
- ACAT inhibitors such as HMG-CoA reductase or squalene synthesis inhibitors
- MTP inhibitors MTP inhibitors
- PPAR-alpha PPAR-alpha
- PPAR gamma and / or PPAR delta agonists cholesterol Abso ⁇ tionhemmer
- polymeric Benklareadsorber bil
- the compounds according to the invention are administered in combination with a CETP inhibitor, such as, for example and preferably, dalcetrapib, BAY 60-5521, anacetrapib or CETP vaccine (CETi-1).
- a CETP inhibitor such as, for example and preferably, dalcetrapib, BAY 60-5521, anacetrapib or CETP vaccine (CETi-1).
- the compounds of the invention are administered in combination with a thyroid receptor agonist such as, by way of example and by way of preference, D-thyroxine, 3,5,3'-triiodothyronine (T3), CGS 23425 or axitirome (CGS 26214).
- a thyroid receptor agonist such as, by way of example and by way of preference, D-thyroxine, 3,5,3'-triiodothyronine (T3), CGS 23425 or axitirome (CGS 26214).
- T3 3,5,3'-triiodothyronine
- CGS 23425 CGS 23425
- axitirome CGS 26214
- the compounds according to the invention are administered in combination with an HMG-CoA reductase inhibitor from the class of statins, such as by way of example and preferably lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin, rosuvastat
- the compounds according to the invention are administered in combination with a squalene synthesis inhibitor, such as by way of example and preferably BMS-188494 or TAK-475.
- a squalene synthesis inhibitor such as by way of example and preferably BMS-188494 or TAK-475.
- the compounds according to the invention are administered in combination with an ACAT inhibitor, such as by way of example and preferably avasimibe, melinamide, pactimibe, eflucimibe or SMP-797.
- an MTP inhibitor such as, for example and preferably, implitapide, BMS-201038, R-103757 or JTT-130.
- the compounds of the invention are administered in combination with a PPAR gamma agonist such as, by way of example and by way of preference, pioglitazone or rosiglitazone.
- a PPAR delta agonist such as by way of example and preferably GW 501516 or BAY 68-5042.
- the compounds according to the invention are administered in combination with a cholesterol absorption inhibitor, such as by way of example and preferably ezetimibe, tiqueside or pamaqueside.
- a cholesterol absorption inhibitor such as by way of example and preferably ezetimibe, tiqueside or pamaqueside.
- the compounds according to the invention are administered in combination with a lipase inhibitor, such as, for example and preferably, orlistat.
- a lipase inhibitor such as, for example and preferably, orlistat.
- the compounds of the invention are administered in combination with a polymeric bile acid adsorbent such as, by way of example and by way of preference, cholestyramine, colestipol, colesolvam, cholesta gel or colestimide.
- a polymeric bile acid adsorbent such as, by way of example and by way of preference, cholestyramine, colestipol, colesolvam, cholesta gel or colestimide.
- ASBT IBAT
- AZD-7806 S-8921
- AK-105 AK-105
- BARI-1741 AK-105
- SC-435 SC-635.
- the compounds of the invention are administered in combination with a lipoprotein (a) antagonist such as, by way of example and by way of preference, gemcabene calcium (CI-1027) or nicotinic acid.
- a lipoprotein (a) antagonist such as, by way of example and by way of preference, gemcabene calcium (CI-1027) or nicotinic acid.
- compositions containing at least one compound of the invention usually together with one or more inert, non-toxic, pharmaceutically suitable excipients, and their use for the purposes mentioned above.
- the compounds according to the invention can act systemically and / or locally.
- they may be applied in a suitable manner, e.g. oral, parenteral, pulmonary, nasal, sublingual, lingual, buccal, rectal, dermal, transdermal, conjunctival, otic or as an implant or stent.
- the compounds according to the invention can be administered in suitable administration forms.
- the compounds according to the invention quickly and / or modified donating application forms, the Compounds according to the invention in crystalline and / or amorphised and / or dissolved form, such as tablets (uncoated or coated tablets, for example, with enteric or delayed-dissolving or insoluble coatings, which control the release of the compound of the invention) in the oral cavity quickly disintegrating tablets or films / wafers, films / lyophilisates, capsules (for example hard or soft gelatin capsules), dragées, granules, pellets, powders, emulsions, suspensions, aerosols or solutions.
- Parenteral administration can be accomplished by bypassing a resorption step (e.g., intravenous, intraarterial, intracardiac, intraspinal, or intralumbar) or by resorting to absorption (e.g., intramuscular, subcutaneous, intracutaneous, percutaneous, or intraperitoneal).
- a resorption step e.g., intravenous, intraarterial, intracardiac, intraspinal, or intralumbar
- absorption e.g., intramuscular, subcutaneous, intracutaneous, percutaneous, or intraperitoneal.
- parenteral administration are suitable as application forms u.a. Injection and infusion preparations in the form of solutions, suspensions, emulsions, lyophilisates or sterile powders.
- Inhalation medicaments including powder inhalers, nebulizers
- nasal drops solutions or sprays
- lingual, sublingual or buccal tablets films / wafers or capsules
- suppositories ear or eye preparations
- vaginal capsules aqueous suspensions (lotions, shake mixtures)
- lipophilic suspensions ointments
- creams transdermal therapeutic systems (eg plasters)
- milk pastes, foams, powdered powders, implants or stents.
- compositions according to the invention can be converted into the stated administration forms. This can be done in a conventional manner by mixing with inert, non-toxic, pharmaceutically suitable excipients.
- adjuvants include, among others.
- Excipients for example microcrystalline cellulose, lactose, mannitol
- solvents for example liquid polyethylene glycols
- emulsifiers and dispersants or wetting agents for example sodium dodecyl sulfate, polyoxysorbitol oleate
- binders for example polyvinylpyrrolidone
- synthetic and natural polymers for example albumin
- stabilizers For example, antioxidants such as ascorbic acid
- dyes eg, inorganic pigments such as iron oxides
- flavor and / or odoriferous for example microcrystalline cellulose, lactose, mannitol
- solvents for example liquid polyethylene glycols
- emulsifiers and dispersants or wetting agents for example sodium dodecyl sulfate, polyoxysorbitol oleate
- binders for example polyvinylpyrrolidone
- synthetic and natural polymers for example albumin
- stabilizers for example, antioxidants such as
- the dosage is about 0.01 to 100 mg / kg, preferably about 0.01 to 20 mg / kg and most preferably 0.1 to 10 mg / kg of body weight.
- Instrament Waters ACQUITY SQD UPLC System; Column: Waters Acquity UPLC HSS T3 1,8 ⁇ 50 x 1mm; Eluent A: 1 l of water + 0.25 ml of 99% formic acid, eluent B: 1 l of acetonitrile + 0.25 ml of 99% formic acid; Gradient: 0.0 min 90% A -> 1.2 min 5% A -> 2.0 min 5% A; Oven: 50 ° C; Flow: 0.40 ml / min; UV detection: 210 - 400 nm.
- Instrument MS Waters
- Instrument HPLC Waters (column Phenomenex Luna 5 ⁇ C18 (2) 100A, AXIA Tech 50 x 21.2 mm
- eluent A water + 0.05% formic acid
- eluent B acetonitrile (ULC) + 0.05% formic acid
- gradient 0.0 min 95% A - 0.15 min 95% A - 8.0 min 5% A - 9.0 min 5% A
- flow 40 ml / min
- UV detection DAD, 210 - 400 nm).
- Instrument MS Waters
- Instrument HPLC Waters (column Phenomenex Luna 5 ⁇ C18 (2) 100A, AXIA Tech 50 x 21.2 mm
- eluent A water + 0.05% formic acid
- eluent B acetonitrile (ULC) + 0.05% formic acid
- gradient 0.0 min 95% A - 0.15 min 95% A - 8.0 min 5% A - 9.0 min 5% A
- flow 40 ml / min
- UV detection DAD, 210 - 400 nm).
- Example 1 Exemplary embodiments: Example 1
- Example 1A 500 mg (1.843 mmol) of Example 1A were initially charged in 5 ml of dichloromethane and 5 ml of dimethylformamide and then with 312 mg (2.212 mmol) of 2-amino-5-cyclopropyl-l, 3,4-thiadiazole, 530 mg (2.765 mmol) - (3-dimethylaminopropyl) -3-ethylcarbodiimide hydrochloride and 0.963 ml (5.530 mmol) of ⁇ , ⁇ -diisopropylethylamine. Thereafter, it was stirred for 1.5 hours at room temperature. It was then diluted with water and dichloromethane and the phases separated.
- the aqueous phase was extracted twice with dichloromethane and the combined organic phases were concentrated. Further purification was carried out by preparative HPLC (acetonitrile: water (+0.05% formic acid) gradient). 146 mg of the target compound were obtained (19% of theory).
- the multi-well plate was covered and shaken at room temperature for 18 h. It was then filtered off and the filtrate purified directly via preparative LC-MS method 2 or 3.
- the product-containing fractions were concentrated by means of a centrifugal dryer in vacuo. The residue of the individual fractions was dissolved in 0.6 ml of DMSO and combined. Subsequently, the solvent was completely evaporated in a centrifugal dryer. There were obtained 4.2 mg (10% of theory) of the title compound.
- the multi-well plate was covered and shaken at room temperature for 18 h. It was then filtered off and the filtrate was purified directly via preparative LC-MS according to Method 2 or 3.
- the product-containing fractions were concentrated by means of a centrifugal dryer in vacuo. The residue of the individual fractions was dissolved in 0.6 ml of DMSO and combined. Subsequently, the solvent was completely evaporated in a centrifugal dryer. 5.80 mg (12% of theory) of the title compound were obtained.
- the force of contraction is detected with Statham UC2 cells, amplified and digitized via A / D converter (DAS-1802 HC, Keithley Instruments Munich) and registered in parallel on a chart recorder.
- a / D converter DAS-1802 HC, Keithley Instruments Munich
- phenylephrine is added cumulatively to the bath in increasing concentration.
- the substance to be examined is added in each subsequent course in increasing dosages and the height of the contraction is compared with the height of the contraction achieved in the last predistortion. This is used to calculate the concentration required to reduce the level of the control value by 50% (IC50 value).
- the standard application volume is 5 ⁇ , the DMSO content in the bath solution corresponds to 0.1%.
- the cellular activity of the compounds of the invention is measured on a recombinant guanylate cyclase reporter cell line as described in F. Wunder et al., Anal. Biochem.339, 104-112 (2005).
- a commercially available telemetry system from DATA SCIENCES INTERNATIONAL DSI, USA is used for the blood pressure measurement on awake rats described below.
- the system consists of 3 main components:
- Implantable transmitters Physiotel® telemetry transmitters
- Physiotel® receivers connected to a data acquisition computer through a multiplexer (DSI Data Exchange Matrix).
- the telemetry system allows a continuous recording of blood pressure heart rate and body movement on awake animals in their habitual habitat.
- the experimental animals are kept individually in macroion cages type 3 after transmitter implantation. You have free access to standard food and water.
- the day - night rhythm in the experimental laboratory is changed by room lighting at 6:00 in the morning and at 19:00 in the evening.
- the TAH PA - C40 telemetry transmitters are surgically implanted into the experimental animals under aseptic conditions at least 14 days before the first trial.
- the animals so instrumented are repeatedly used after healing of the wound and ingrowth of the implant.
- the fasting animals are anesthetized with pentobabital (Nembutal, Sanofi: 50 mg / kg i.p.) and shaved and disinfected on the ventral side.
- pentobabital Nembutal, Sanofi: 50 mg / kg i.p.
- tissue adhesive VetBonD TM, 3M.
- the transmitter housing is fixed intraperitoneally to the abdominal wall musculature and the wound is closed in layers.
- an antibiotic is administered for infection prevention (Tardomyocel COMP Bayer 1ml / kg s.c.)
- a solvent-treated group of animals is used as a control.
- the existing telemetry measuring device is configured for 24 animals. Each trial is registered under a trial number (VYear month day).
- the instrumented rats living in the plant each have their own receiving antenna (1010 receivers, DSI).
- the implanted transmitters can be activated externally via a built-in magnetic switch. They will be put on the air during the trial run.
- the emitted signals can be recorded online by a data acquisition system (Dataquest TM A.R.T. for Windows, DSI) and processed accordingly. The storage of the data takes place in each case in a folder opened for this purpose which carries the test number.
- SBP Systolic blood pressure
- DBP Diastolic blood pressure
- MAP Heart rate
- HR Activity
- the measured value acquisition is repeated computer-controlled in 5-minute intervals.
- the absolute value of the source data is corrected in the diagram with the currently measured barometric pressure (Ambient Pressure Reference Monitor, APR-1) and stored in individual data. Further technical details can be found in the extensive documentation of the manufacturer (DSI).
- test substances will take place at 9 o'clock on the day of the experiment. Following the application, the parameters described above are measured for 24 hours.
- the collected individual data are sorted with the analysis software (DATAQUEST TM A.RT. TM ANALYSIS).
- the blank value is assumed here 2 hours before application, so that the selected data record covers the period from 7:00 am on the day of the experiment to 9:00 am on the following day.
- the data is smoothed over a presettable time by averaging (15 minutes average) and transferred as a text file to a disk.
- the so presorted and compressed measured values are transferred to Excel templates and displayed in tabular form.
- the filing of the collected data takes place per experiment day in a separate folder that bears the test number. Results and test reports are sorted in folders and sorted by paper. literature
- the compounds according to the invention can be converted into pharmaceutical preparations as follows:
- composition
- the mixture of compound of the invention, lactose and starch is granulated with a 5% solution (m / m) of the PVP in water.
- the granules are mixed after drying with the magnesium stearate for 5 minutes.
- This mixture is compressed with a conventional tablet press (for the tablet format see above).
- a pressing force of 15 kN is used as a guideline for the compression.
- the rhodigel is suspended in ethanol, the compound according to the invention is added to the suspension. While stirring, the addition of water. Until the completion of the swelling of Rhodigels is stirred for about 6 h.
- the compound of the invention is suspended in the mixture of polyethylene glycol and polysorbate with stirring. The stirring is continued until complete dissolution of the compound according to the invention. i.v. -Solution:
- the compound of the invention is dissolved at a concentration below saturation solubility in a physiologically acceptable solvent (e.g., isotonic saline, 5% glucose solution, and / or 30% PEG 400 solution).
- a physiologically acceptable solvent e.g., isotonic saline, 5% glucose solution, and / or 30% PEG 400 solution.
- the solution is sterile filtered and filled into sterile and pyrogen-free injection containers.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Cardiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Heart & Thoracic Surgery (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The invention relates to novel substituted thiazole and thiadiazole amides, to a method for producing same, and to the use of same alone or in combinations for treating and/or preventing diseases, as well as to their use for producing medication for treating and/or preventing diseases, particularly for treating and/or preventing cardiovascular diseases.
Description
Substituierte Thiazol- und Thiadiazolamide und ihre Verwendung Substituted thiazole and thiadiazolamides and their use
Die vorliegende Anmeldung betrifft neue substituierte Thiazol- und Thiadiazolamide, Verfahren zu ihrer Herstellung, ihre Verwendung allein oder in Kombinationen zur Behandlung und/oder Prophylaxe von Krankheiten sowie ihre Verwendung zur Herstellung von Arzneimitteln zur Behand- lung und/oder Prophylaxe von Krankheiten, insbesondere zur Behandlung und/oder Prophylaxe von Herz-Kreislauf-Erkrankungen. The present application relates to novel substituted thiazole and thiadiazolamides, processes for their preparation, their use alone or in combinations for the treatment and / or prophylaxis of diseases and their use for the preparation of medicaments for the treatment and / or prophylaxis of diseases, in particular Treatment and / or prophylaxis of cardiovascular diseases.
Eines der wichtigsten zellulären Übertragungssysteme in Säugerzellen ist das cyclische Guanosin- monophosphat (cGMP). Zusammen mit Stickstoffmonoxid (NO), das aus dem Endothel freigesetzt wird und hormonelle und mechanische Signale überträgt, bildet es das NO/cGMP-System. Die Guanylatcyclasen katalysieren die Biosynthese von cGMP aus Guanosintriphosphat (GTP). Die bisher bekannten Vertreter dieser Familie lassen sich sowohl nach strukturellen Merkmalen als auch nach der Art der Liganden in zwei Gruppen aufteilen: Die partikulären, durch natriuretische Peptide stimulierbaren Guanylatcyclasen und die löslichen, durch NO stimulierbaren Guanylatcyclasen. Die löslichen Guanylatcyclasen bestehen aus zwei Untereinheiten und enthalten höchstwahrscheinlich ein Häm pro Heterodimer, das ein Teil des regulatorischen Zentrums ist. Dieses hat eine zentrale Bedeutung für den Aktivierungsmechanismus. NO kann an das Eisenatom des Häms binden und so die Aktivität des Enzyms deutlich erhöhen. Hämfreie Präparationen lassen sich hingegen nicht durch NO stimulieren. Auch Kohlenmonoxid (CO) ist in der Lage, an das Eisen-Zentralatom des Häms zu binden, wobei die Stimulierung durch CO deutlich geringer ist als die durch NO. Durch die Bildung von cGMP und der daraus resultierenden Regulation von Phosphodiesterasen, Ionenkanälen und Proteinkinasen spielt die Guanylatcyclase eine entscheidende Rolle bei unterschiedlichen physiologischen Prozessen, insbesondere bei der Relaxation und Proliferation glatter Muskelzellen, der Plättchenaggregation und -adhäsion, der neuronalen Signalübertragung sowie bei Erkrankungen, welche auf einer Störung der vorstehend genannten Vorgänge beruhen. Unter patho- physiologischen Bedingungen kann das NO/cGMP-System supprimiert sein, was zum Beispiel zu Bluthochdruck, einer Plättchenaktivierung, einer vermehrten Zellproliferation, endothelialer Dysfunktion, Arteriosklerose, Angina pectoris, Herzinsuffizienz, Myokardinfarkt, Thrombosen, Schlaganfall und sexueller Dysfunktion führen kann. One of the most important cellular transmission systems in mammalian cells is cyclic guanosine monophosphate (cGMP). Together with nitric oxide (NO), which is released from the endothelium and transmits hormonal and mechanical signals, it forms the NO / cGMP system. The guanylate cyclases catalyze the biosynthesis of cGMP from guanosine triphosphate (GTP). The previously known members of this family can be divided into two groups according to both structural features and the nature of the ligands: the particulate guanylate cyclases stimulable by natriuretic peptides and the soluble guanylate cyclases stimulable by NO. The soluble guanylate cyclases consist of two subunits and most likely contain one heme per heterodimer that is part of the regulatory center. This is central to the activation mechanism. NO can bind to the iron atom of the heme and thus significantly increase the activity of the enzyme. On the other hand, heme-free preparations can not be stimulated by NO. Also, carbon monoxide (CO) is able to bind to the central iron atom of the heme, with stimulation by CO being significantly less than by NO. Through the formation of cGMP and the resulting regulation of phosphodiesterases, ion channels and protein kinases, guanylate cyclase plays a crucial role in various physiological processes, in particular in the relaxation and proliferation of smooth muscle cells, platelet aggregation and adhesion, neuronal signaling and diseases based on a disturbance of the above operations. Under pathophysiological conditions, the NO / cGMP system may be suppressed, which may, for example, lead to hypertension, platelet activation, increased cell proliferation, endothelial dysfunction, arteriosclerosis, angina pectoris, heart failure, myocardial infarction, thrombosis, stroke and sexual dysfunction.
Eine auf die Beeinflussung des cGMP-Signalweges in Organismen abzielende NO-unabhängige Behandlungsmöglichkeit für derartige Erkrankungen ist aufgrund der zu erwartenden hohen Effizienz und geringen Nebenwirkungen ein vielversprechender Ansatz. A NO-independent treatment option for such diseases, which is aimed at influencing the cGMP pathway in organisms, is a promising approach on account of the expected high efficiency and low side effects.
Zur therapeutischen Stimulation der löslichen Guanylatcyclase wurden bisher ausschließlich Verbindungen wie organische Nitrate verwendet, deren Wirkung auf NO beruht. Dieses wird durch Bio-
konversion gebildet und aktiviert die lösliche Guanylatcyclase durch Angriff am Eisen-Zentralatom des Häms. Neben den Nebenwirkungen gehört die Toleranzentwicklung zu den entscheidenden Nachteilen dieser Behandlungsweise. For therapeutic stimulation of soluble guanylate cyclase, only compounds such as organic nitrates have been used, whose action is based on NO. This is converts and activates the soluble guanylate cyclase by attack on the iron central atom of the heme. In addition to the side effects, tolerance development is one of the decisive disadvantages of this treatment.
In den letzten Jahren wurden einige Substanzen beschrieben, die die lösliche Guanylatcyclase direkt, d.h. ohne vorherige Freisetzung von NO stimulieren, wie beispielsweise 3-(5'-Hydroxymethyl-2'- furyl)-l-benzylindazol [YC-1 ; Wu et al., Blood 84 (1994), 4226; Mülsch et al., Brit. J. Pharmacol. 120 (1997), 681], Fettsäuren [Goldberg et al., J. Biol. Chem. 252 (1977), 1279], Diphenyl- iodonium-hexafluorophosphat [Pettibone et al., Eur. J. Pharmacol. 116 (1985), 307], Iso- liquiritigenin [Yu et al., Brit. J. Pharmacol. 114 (1995), 1587] sowie verschiedene substituierte Pyrazol-Derivate (WO 98/16223). Zudem werden in WO 00/06568 und WO 00/06569 annellierte Pyrazol-Derivate und in WO 03/095451 Carbamat-substitutierte 3-Pyrimidinyl-Pyrazolopyridine offenbart. 3-Pyrimidinyl-Pyrazolopyridine mit Phenylamid-Substituenten werden in E. M. Becker et al, BMC Pharmacology, 2001, 1 (13), beschrieben. WO 2004/009590 beschreibt Pyrazolopyridine mit substituierten 4-Aminopyrimidinen zur Behandlung von ZNS -Erkrankungen. WO 2010/065275 und WO 2011/149921 offenbaren substituierte Pyrrolo- und Dihydropyridopyrimidine als sGC Aktivatoren. Als sGC Stimulatoren werden in WO 2012/004259 annellierte Aminopyrimidine und in WO 2012/004258, WO 2012/143510 und WO 2012/152629 annellierte Pyrimidine und Triazine beschrieben. WO 2012/28647 offenbart Pyrazolopyridine mit verschiedenen Azaheterocyclen zur Behandlung kardiovaskulärer Erkrankungen. WO 2008/052898 beschreibt Indazole mit Thiazolamid-Substituenten zur Behandlung von Diabetes und verwandten Erkrankungen. WO 2006/015263 offenbart N-Benzyl-Indazole mit verschiedenartigen Amid-Substituenten zur Behandlung von Krebs und benigner Prostata- Hyperplasie. In recent years, some substances have been described which directly release the soluble guanylate cyclase, i. without stimulating NO, such as 3- (5'-hydroxymethyl-2'-furyl) -l-benzylindazole [YC-1; Wu et al., Blood 84 (1994), 4226; Mülsch et al., Brit. J. Pharmacol. 120 (1997), 681], fatty acids [Goldberg et al., J. Biol. Chem. 252 (1977), 1279], diphenyliodonium hexafluorophosphate [Pettibone et al., Eur. J. Pharmacol. 116 (1985), 307], iso-liquiritigenin [Yu et al., Brit. J. Pharmacol. 114 (1995), 1587] and various substituted pyrazole derivatives (WO 98/16223). In addition, WO 00/06568 and WO 00/06569 disclose fused pyrazole derivatives and WO 03/095451 discloses carbamate-substituted 3-pyrimidinyl-pyrazolopyridines. 3-Pyrimidinyl-pyrazolopyridines with phenylamide substituents are described in E.M. Becker et al, BMC Pharmacology, 2001, 1 (13). WO 2004/009590 describes pyrazolopyridines with substituted 4-aminopyrimidines for the treatment of CNS diseases. WO 2010/065275 and WO 2011/149921 disclose substituted pyrrolo and dihydropyridopyrimidines as sGC activators. The sGC stimulators described in WO 2012/004259 are fused aminopyrimidines and in WO 2012/004258, WO 2012/143510 and WO 2012/152629 fused pyrimidines and triazines. WO 2012/28647 discloses pyrazolopyridines with various azaheterocycles for the treatment of cardiovascular diseases. WO 2008/052898 describes indazoles with thiazolamide substituents for the treatment of diabetes and related diseases. WO 2006/015263 discloses N-benzyl-indazoles having various amide substituents for the treatment of cancer and benign prostatic hyperplasia.
Aufgabe der vorliegenden Erfindung war die Bereitstellung neuer Substanzen, die als Stimulatoren der löslichen Guanylatcyclase wirken. The object of the present invention was to provide novel substances which act as stimulators of soluble guanylate cyclase.
Gegenstand der vorliegenden Erfindung sind Verbindungen der allgemeinen Formel (I)
The present invention relates to compounds of the general formula (I)
U für O oder S steht, U stands for O or S,
V für CR3 oder N steht, wobei V is CR 3 or N, where
R für Wasserstoff, Trifluormethyl, (Ci-C6)-Alkyl, (C3-C7)-Cycloalkyl, (Ci-C4)- Alkoxycarbonyl, Phenyl, Pyridyl, -NR5R6, -S(0)nR7, -S02NR8R9 oder -OR10 steht, worin (Ci-C6)-Alkyl mit 1 bis 3 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Hydroxy, Trifluormethoxy, (Ci-C i)-Alkoxy, Hydroxycarbonyl, (Ci-C i)-Alkoxycarbonyl, (Ci-C4)-Alkoxycarbonylamino, Mono- (Ci-C4)-Alkylamino, Di-(Ci-C4)-Alkylamino, Phenyl, Phenoxy, 4- bis 7-gliedriges Heterocyclyl und 5- oder 6-gliedriges Heteroaryl substituiert sein kann, worin Phenyl mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Halogen, (Ci-C4)-Alkyl und (Ci-C4)-Alkoxy substituiert sein kann, worin 4- bis 7-gliedriges Heterocyclyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, (Ci-C4)-Alkyl, Hydroxy, Oxo und (Ci-C4)-Alkoxy substituiert sein kann, und worin Phenoxy und 5- oder 6-gliedriges Heteroaryl ihrerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Halogen, (Ci-C4)-Alkyl und (Ci-C4)-Alkoxy substituiert sein können,
worin Phenyl und Pyridyl mit 1 bis 3 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Halogen, (Ci-C i)-Alkyl und (Ci-C4)-Alkoxy substituiert sein können, und worin n für eine Zahl 0, 1 oder 2 steht, R is hydrogen, trifluoromethyl, (C 1 -C 6 ) -alkyl, (C 3 -C 7 ) -cycloalkyl, (C 1 -C 4 ) -alkoxycarbonyl, phenyl, pyridyl, -NR 5 R 6 , -S (O) n R 7 , -S0 2 NR 8 R 9 or -OR 10 , wherein (Ci-C6) alkyl having 1 to 3 substituents independently selected from the group fluorine, trifluoromethyl, hydroxy, trifluoromethoxy, (Ci-C i) - Alkoxy, hydroxycarbonyl, (C 1 -C 4) -alkoxycarbonyl, (C 1 -C 4) -alkoxycarbonylamino, mono (C 1 -C 4) -alkylamino, di- (C 1 -C 4) -alkylamino, phenyl, phenoxy, 4 to 7 be substituted heterocyclyl and 5- or 6-membered heteroaryl may be substituted, wherein phenyl may be substituted with 1 or 2 substituents independently selected from the group consisting of halogen, (Ci-C4) alkyl and (Ci-C4) alkoxy, wherein 4 - to 7-membered heterocyclyl in turn may be substituted with 1 or 2 substituents independently selected from the group fluorine, (Ci-C4) alkyl, hydroxy, oxo and (Ci-C4) alkoxy, and wherein phenoxy and 5- or 6-membered heteroaryl of hers ts having 1 or 2 substituents independently of one another can be substituted from the group halogen, (C 1 -C 4) -alkyl and (C 1 -C 4) -alkoxy, in which phenyl and pyridyl having 1 to 3 substituents independently of one another can be substituted from the group halogen, (Ci-C i) -alkyl and (Ci-C4) -alkoxy, and wherein n is a number 0, 1 or 2,
R5 für Wasserstoff oder (Ci-C6)-Alkyl steht, worin (Ci-C6)-Alkyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Hydroxy, Trifluormethoxy und (Ci-C4)-Alkoxy substituiert sein kann, R 5 is hydrogen or (Ci-C 6 ) alkyl, wherein (Ci-C6) alkyl in turn with 1 or 2 substituents independently selected from the group fluorine, trifluoromethyl, hydroxy, trifluoromethoxy and (Ci-C4) alkoxy may be substituted
R6 für Wasserstoff, (Ci-C6)-Alkyl, (C3-C7)-Cycloalkyl, Phenyl oder 4- bis 7- gliedriges Heterocyclyl steht, worin Phenyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Halogen, (Ci-C4)-Alkyl, (Ci-C4)-Alkoxy und (Ci-C4)-Alkylcarbonylamino substituiert sein kann, oder R 6 is hydrogen, (Ci-C 6 ) alkyl, (C 3 -C 7 ) -cycloalkyl, phenyl or 4- to 7-membered heterocyclyl, wherein phenyl in turn having 1 or 2 substituents independently selected from the group halogen , (C 1 -C 4) -alkyl, (C 1 -C 4) -alkoxy and (C 1 -C 4) -alkylcarbonylamino, or
R5 und R6 bilden zusammen mit dem Stickstoffatom, an das sie gebunden sind, einen 4- bis 7-gliedrigen Heterocyclus, worin 4- bis 7-gliedriger Heterocyclus mit (Ci-C4)-Alkyl substituiert sein kann, worin (Ci-C4)-Alkyl mit Hydroxy substituiert sein kann, R 5 and R 6 together with the nitrogen atom to which they are attached form a 4- to 7-membered heterocycle in which 4- to 7-membered heterocycle may be substituted by (C 1 -C 4) -alkyl, in which C4) -alkyl may be substituted by hydroxy,
R7 für (Ci-C6)-Alkyl, (C3-C7)-Cycloalkyl oder Phenyl steht, R8 für Wasserstoff oder (Ci-C6)-Alkyl steht, R9 für Wasserstoff, (Ci-C6)-Alkyl oder (C3-C7)-Cycloalkyl steht, R10 für (Ci-C6)-Alkyl, (C3-C7)-Cycloalkyl oder Phenyl steht, für CR4 oder N steht, wobei
R4 für Wasserstoff, (Ci-C4)-Alkyl, (C3-C7)-Cycloalkyl oder 3- bis 7-gliedriges Heterocyclyl steht, worin (Ci-C4)-Alkyl mit 1 bis 3 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Hydroxy, Trifluormethoxy, (Ci-C4)-Alkoxy, Hydroxycarbonyl, (Ci-C4)-Alkoxycarbonyl, Amino, Mono-(Ci-C4)-Alkylamino undR 7 is (C 1 -C 6 ) -alkyl, (C 3 -C 7 ) -cycloalkyl or phenyl, R 8 is hydrogen or (C 1 -C 6 ) -alkyl, R 9 is hydrogen, (C 1 -C 6 ) -Alkyl or (C 3 -C 7 ) -cycloalkyl, R 10 is (Ci-C 6 ) -alkyl, (C 3 -C 7 ) -cycloalkyl or phenyl, is CR 4 or N, wherein R 4 is hydrogen, (C 1 -C 4 ) -alkyl, (C 3 -C 7 ) -cycloalkyl or 3 to 7-membered heterocyclyl, wherein (C 1 -C 4 ) -alkyl having 1 to 3 substituents selected independently of one another the group fluorine, trifluoromethyl, hydroxy, trifluoromethoxy, (Ci-C4) alkoxy, hydroxycarbonyl, (Ci-C4) alkoxycarbonyl, amino, mono (Ci-C4) alkylamino and
Di-(Ci-C4)-Alkylamino substituiert sein kann, worin Mono-(Ci-C4)-Alkylamino und Di-(Ci-C4)-Alkylamino ihrerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl und Phenyl substituiert sein können, und worin 4- bis 7-gliedriges Heterocyclyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, (Ci-C4)-Alkyl, Hydroxy, Oxo und (Ci-C4)-Alkoxy substituiert sein kann, worin (Ci-C4)-Alkyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Hydroxy,Di- (C 1 -C 4) -alkylamino, in which mono- (C 1 -C 4) -alkylamino and di- (C 1 -C 4) -alkylamino in turn have 1 or 2 substituents independently selected from the group of fluorine, trifluoromethyl and phenyl in which 4- to 7-membered heterocyclyl may in turn be substituted by 1 or 2 substituents independently of one another selected from the group of fluoro, (C 1 -C 4) -alkyl, hydroxyl, oxo and (C 1 -C 4) -alkoxy, in which (C 1 -C 4) -alkyl in turn contains 1 or 2 substituents independently of one another selected from the group of fluorine, trifluoromethyl, hydroxy,
Trifluormethoxy und (Ci-C4)-Alkoxy substituiert sein kann, oder Trifluoromethoxy and (Ci-C4) alkoxy may be substituted, or
R3 und R4 bilden zusammen mit V und W, an die sie gebunden sind, einen 4- bis 7-gliedrigen Heterocyclyl-Ring, worin der 4- bis 7-gliedrige Heterocyclyl-Ring mit (Ci-C4)-Alkyl substituiert sein kann, R 3 and R 4 together with V and W to which they are attached form a 4- to 7-membered heterocyclyl ring wherein the 4- to 7-membered heterocyclyl ring is substituted with (C 1 -C 4) -alkyl can
R1 für Wasserstoff oder Fluor steht, R2 für (Ci-C6)-Alkyl oder Benzyl steht, wobei (Ci-C6)-Alkyl mit einem Substituenten Trifluormethyl substituiert ist, wobei (Ci-C6)-Alkyl mit 1 bis 3 Substituenten Fluor substituiert sein kann, und wobei Benzyl mit 1 bis 3 Substituenten Fluor substituiert ist, mit der Maßgabe, dass wenn V für N steht, W für CR steht,
mit der Maßgabe, dass wenn W für N steht, V für CR3 steht, sowie ihre N-Oxide, Salze, Solvate, Salze der N-Oxide und Solvate der N-Oxide und Salze. R 1 is hydrogen or fluorine, R 2 is (C 1 -C 6 ) -alkyl or benzyl, where (C 1 -C 6 ) -alkyl is substituted by a substituent trifluoromethyl, where (C 1 -C 6) -alkyl having 1 to 3 Substituents fluorine may be substituted, and wherein benzyl is substituted by 1 to 3 substituents fluorine, with the proviso that when V is N, W is CR, with the proviso that when W is N, V is CR 3 , and their N-oxides, salts, solvates, salts of N-oxides and solvates of N-oxides and salts.
Erfindungsgemäße Verbindungen sind die Verbindungen der Formel (I) und deren Salze, Solvate und Solvate der Salze, die von Formel (I) umfassten Verbindungen der nachfolgend genannten Formeln und deren Salze, Solvate und Solvate der Salze sowie die von Formel (I) umfassten, nachfolgend als Ausfuhrungsbeispiele genannten Verbindungen und deren Salze, Solvate und Solvate der Salze, soweit es sich bei den von Formel (I) umfassten, nachfolgend genannten Verbindungen nicht bereits um Salze, Solvate und Solvate der Salze handelt. Compounds according to the invention are the compounds of the formula (I) and their salts, solvates and solvates of the salts comprising the compounds of the formulas below and their salts, solvates and solvates of the salts and of the formula (I) encompassed by formula (I), hereinafter referred to as exemplary compounds and their salts, solvates and solvates of the salts, as far as the compounds of formula (I), the following compounds are not already salts, solvates and solvates of the salts.
Als Salze sind im Rahmen der vorliegenden Erfindung physiologisch unbedenkliche Salze der erfindungsgemäßen Verbindungen bevorzugt. Umfasst sind auch Salze, die für pharmazeutische Anwendungen selbst nicht geeignet sind, jedoch beispielsweise für die Isolierung oder Reinigung der erfindungsgemäßen Verbindungen verwendet werden können. Salts used in the context of the present invention are physiologically acceptable salts of the compounds according to the invention. Also included are salts which are themselves unsuitable for pharmaceutical applications but can be used, for example, for the isolation or purification of the compounds of the invention.
Physiologisch unbedenkliche Salze der erfindungsgemäßen Verbindungen umfassen Säureadditionssalze von Mineralsäuren, Carbonsäuren und Sulfonsäuren, z.B. Salze der Chlorwasserstoffsäure, Bromwasserstoffsäure, Schwefelsäure, Phosphorsäure, Methan- sulfonsäure, Ethansulfonsäure, Toluolsulfonsäure, Benzolsulfonsäure, Naphthalin- disulfonsäure, Ameisensäure, Essigsäure, Trifluoressigsäure, Propionsäure, Milchsäure, Weinsäure, Äpfelsäure, Zitronensäure, Fumarsäure, Maleinsäure und Benzoesäure. Physiologically acceptable salts of the compounds of the invention include acid addition salts of mineral acids, carboxylic acids and sulfonic acids, e.g. Salts of hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid, methanesulfonic acid, ethanesulfonic acid, toluenesulfonic acid, benzenesulfonic acid, naphthalenedisulfonic acid, formic acid, acetic acid, trifluoroacetic acid, propionic acid, lactic acid, tartaric acid, malic acid, citric acid, fumaric acid, maleic acid and benzoic acid.
Physiologisch unbedenkliche Salze der erfindungsgemäßen Verbindungen umfassen auch Salze üblicher Basen, wie beispielhaft und vorzugsweise Alkalimetallsalze (z.B. Natrium- und Kaliumsalze), Erdalkalisalze (z.B. Calcium- und Magnesiumsalze) und Ammoniumsalze, abgeleitet von Ammoniak oder organischen Aminen mit 1 bis 16 C- Atomen, wie beispielhaft und vorzugsweise Ethylamin, Diethylamin, Triethylamin, Ethyl- diisopropylamin, Monoethanolamin, Diethanolamin, Triethanolamin, Dicyclohexylamin, Dimethylaminoethanol, Prokain, Dibenzylamin, N-Methylmorpholin, Arginin, Lysin, Ethylendiamin und N-Methylpiperidin. Physiologically acceptable salts of the compounds according to the invention also include salts of customary bases, such as, by way of example and by way of preference, alkali metal salts (for example sodium and potassium salts), alkaline earth salts (for example calcium and magnesium salts) and ammonium salts derived from ammonia or organic amines having 1 to 16 carbon atoms, for example and preferably ethylamine, diethylamine, triethylamine, ethyldiisopropylamine, monoethanolamine, diethanolamine, triethanolamine, dicyclohexylamine, dimethylaminoethanol, procaine, dibenzylamine, N-methylmorpholine, arginine, lysine, ethylenediamine and N-methylpiperidine.
Als Solvate werden im Rahmen der Erfindung solche Formen der erfindungsgemäßen Verbindungen bezeichnet, welche in festem oder flüssigem Zustand durch Koordination mit Lösungsmittelmolekülen einen Komplex bilden. Hydrate sind eine spezielle Form der
Solvate, bei denen die Koordination mit Wasser erfolgt. Als Solvate sind im Rahmen der vorliegenden Erfindung Hydrate bevorzugt. Solvates in the context of the invention are those forms of the compounds according to the invention which form a complex in the solid or liquid state by coordination with solvent molecules. Hydrates are a special form of Solvates where coordination with water occurs. As solvates, hydrates are preferred in the context of the present invention.
Die erfindungsgemäßen Verbindungen können in Abhängigkeit von ihrer Struktur in unterschiedlichen stereoisomeren Formen existieren, d.h. in Gestalt von Konfigurationsisomeren oder gegebenenfalls auch als Konformationsisomere (Enantiomere und/oder Diastereomere, einschließlich solcher bei Atropisomeren). Die vorliegende Erfindung umfasst deshalb die Enantiomere und Diastereomere und ihre jeweiligen Mischungen. Aus solchen Mischungen von Enantiomeren und/ oder Diastereomeren lassen sich die stereoisomer einheitlichen Bestandteile in bekannter Weise isolieren; vorzugsweise werden hierfür chromatographische Verfahren verwendet, insbesondere die HPLC-Chromatographie an achiraler bzw. chiraler Phase. The compounds of the invention may exist in different stereoisomeric forms depending on their structure, i. in the form of configurational isomers or optionally also as conformational isomers (enantiomers and / or diastereomers, including those in atropisomers). The present invention therefore includes the enantiomers and diastereomers and their respective mixtures. From such mixtures of enantiomers and / or diastereomers, the stereoisomerically uniform components can be isolated in a known manner; Preferably, chromatographic methods are used for this, in particular HPLC chromatography on achiral or chiral phase.
Sofern die erfindungsgemäßen Verbindungen in tautomeren Formen vorkommen können, umfasst die vorliegende Erfindung sämtliche tautomere Formen. If the compounds according to the invention can occur in tautomeric forms, the present invention encompasses all tautomeric forms.
Die vorliegende Erfindung umfasst auch alle geeigneten isotopischen Varianten der erfin- dungsgemäßen Verbindungen. Unter einer isotopischen Variante einer erfindungsgemäßen Verbindung wird hierbei eine Verbindung verstanden, in welcher mindestens ein Atom innerhalb der erfindungsgemäßen Verbindung gegen ein anderes Atom der gleichen Ordnungszahl, jedoch mit einer anderen Atommasse als der gewöhnlich oder überwiegend in der Natur vorkommenden Atommasse ausgetauscht ist. Beispiele für Isotope, die in eine erfindungsgemäße Verbindung inkorporiert werden können, sind solche von Wasserstoff, Kohlenstoff, Stickstoff, Sauerstoff, Phosphor, Schwefel, Fluor, Chlor, Brom und Iod, wie 2H (Deuterium), 3H (Tritium), 13C, 14C, 15N, 170, 180, 32P, 33P, 33S, 34S, 35S, 36S, 18F, 36C1, 82Br, 123I, 124I, 129I und 131I. Bestimmte isotopische Varianten einer erfindungsgemäßen Verbindung, wie insbesondere solche, bei denen ein oder mehrere radioaktive Isotope inkor- poriert sind, können von Nutzen sein beispielsweise für die Untersuchung des Wirkmechanismus oder der Wirkstoff- Verteilung im Körper; aufgrund der vergleichsweise leichten Herstell- und Detektierbarkeit sind hierfür insbesondere mit 3H- oder 14C-Isotopen markierte Verbindungen geeignet. Darüber hinaus kann der Einbau von Isotopen, wie beispielsweise von Deuterium, zu bestimmten therapeutischen Vorteilen als Folge einer größeren metabolischen Stabilität der Verbindung führen, wie beispielsweise eine Verlängerung der Halbwertszeit im Körper oder eine Reduktion der erforderlichen Wirkdosis; solche Modifikationen der erfindungsgemäßen Verbindungen können daher gegebenenfalls auch eine bevorzugte Ausführungsform der vorliegenden Erfindung darstellen. Iso-
topische Varianten der erfmdungsgemäßen Verbindungen können nach den dem Fachmann bekannten Verfahren hergestellt werden, so beispielsweise nach den weiter unten beschriebenen Methoden und den bei den Ausführungsbeispielen wiedergegebenen Vorschriften, indem entsprechende isotopische Modifikationen der jeweiligen Reagentien und/oder Ausgangsverbindungen eingesetzt werden. The present invention also encompasses all suitable isotopic variants of the compounds according to the invention. An isotopic variant of a compound according to the invention is understood to mean a compound in which at least one atom within the compound according to the invention is exchanged for another atom of the same atomic number but with a different atomic mass than the atomic mass that usually or predominantly occurs in nature. Examples of isotopes which can be incorporated into a compound of the invention are those of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, fluorine, chlorine, bromine and iodine, such as 2 H (deuterium), 3 H (tritium), 13 C, 14 C, 15 N, 17 0, 18 0, 32 P, 33 P, 33 S, 34 S, 35 S, 36 S, 18 F, 36 Cl, 82 Br, 123 I, 124 I, 129 I and 131 I. Certain isotopic variants of a compound of the invention, such as, in particular, those in which one or more radioactive isotopes are incorporated, may be useful, for example, for the study of the mechanism of action or distribution of the drug in the body; Due to the comparatively easy production and detectability, compounds labeled with 3 H or 14 C isotopes in particular are suitable for this purpose. Moreover, the incorporation of isotopes such as deuterium may result in certain therapeutic benefits as a result of greater metabolic stability of the compound, such as prolonging the body's half-life or reducing the required effective dose; Such modifications of the compounds of the invention may therefore optionally also constitute a preferred embodiment of the present invention. iso- Topical variants of the compounds according to the invention can be prepared by the processes known to the person skilled in the art, for example by the methods described below and the instructions given in the exemplary embodiments, by using appropriate isotopic modifications of the respective reagents and / or starting compounds.
Außerdem umfasst die vorliegende Erfindung auch Prodrugs der erfindungsgemäßen Verbindungen. Der Begriff "Prodrugs" bezeichnet hierbei Verbindungen, welche selbst biologisch aktiv oder inaktiv sein können, jedoch während ihrer Verweilzeit im Körper zu erfindungsgemäßen Verbindungen umgesetzt werden (beispielsweise metabolisch oder hydrolytisch). In addition, the present invention also includes prodrugs of the compounds of the invention. The term "prodrugs" refers to compounds which themselves may be biologically active or inactive, but are converted during their residence time in the body to compounds of the invention (for example metabolically or hydrolytically).
Im Rahmen der vorliegenden Erfindung haben die Substituenten, soweit nicht anders spezifiziert, die folgende Bedeutung: Unless otherwise specified, in the context of the present invention, the substituents have the following meaning:
Alkyl steht im Rahmen der Erfindung für einen linearen oder verzweigten Alkylrest mit 1 bis 4 Kohlenstoffatomen. Beispielhaft und vorzugsweise seien genannt: Methyl, Ethyl, n-Propyl, Isopropyl, n-Butyl, iso-Butyl, 1 -Methylpropyl, tert-Butyl. In the context of the invention, alkyl is a linear or branched alkyl radical having 1 to 4 carbon atoms. By way of example and preferably mention may be made of: methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, 1-methylpropyl, tert-butyl.
Cycloalkyl steht im Rahmen der Erfindung für einen monocyclischen, gesättigten Carbocyclus mit 3 bis 7 Ring-Kohlenstoffatomen. Beispielhaft und vorzugsweise seien genannt: Cyclopropyl, Cyclobutyl, Cyclopentyl, Cyclohexyl und Cycloheptyl. Alkoxy steht im Rahmen der Erfindung für einen linearen oder verzweigten Alkoxyrest mit 1 bis 4 Kohlenstoffatomen. Beispielhaft und vorzugsweise seien genannt: Methoxy, Ethoxy, n-Propoxy, Isopropoxy, 1 -Methylpropoxy, n-Butoxy, iso-Butoxy und tert. -Butoxy. Cycloalkyl in the context of the invention is a monocyclic, saturated carbocycle having 3 to 7 ring carbon atoms. Examples which may be mentioned by way of example include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl and cycloheptyl. Alkoxy in the context of the invention is a linear or branched alkoxy radical having 1 to 4 carbon atoms. Examples which may be mentioned by way of example include: methoxy, ethoxy, n-propoxy, isopropoxy, 1-methylpropoxy, n-butoxy, isobutoxy and tert. Butoxy.
Alkoxycarbonyl stehen im Rahmen der Erfindung für einen linearen oder verzweigten Alkoxyrest mit 1 bis 4 Kohlenstoffatomen und einer am Sauerstoff angebundenen Carbonylgruppe. Beispielhaft und vorzugsweise seien genannt: Methoxycarbonyl, Ethoxycarbonyl, n-Propoxycarbonyl, Isopropoxycarbonyl und tert. -Butoxycarbonyl. Alkoxycarbonyl in the context of the invention are a linear or branched alkoxy radical having 1 to 4 carbon atoms and an oxygen-bonded carbonyl group. Examples which may be mentioned by way of example include: methoxycarbonyl, ethoxycarbonyl, n-propoxycarbonyl, isopropoxycarbonyl and tert. Butoxycarbonyl.
Heterocyclyl steht im Rahmen der Erfindung für einen gesättigten Heterocyclus mit insgesamt 4 bis 7 Ringatomen, der ein oder zwei Ring-Heteroatome aus der Reihe N, O und/oder S enthält und über ein Ring-Kohlenstoffatom oder gegebenenfalls ein Ring-Stickstoffatom verknüpft ist. Beispielhaft seien genannt: Azetidinyl, Oxetanyl, Pyrrolidinyl, Pyrazolidinyl, Tetrahydrofüranyl, Piperidinyl,
Piperazinyl, Tetrahydropyranyl, Morpholinyl und Thiomorpholinyl. Bevorzugt sind Azetidinyl, Pyrrolidinyl, Piperidinyl, Piperazinyl und Morpholinyl. Heterocyclyl in the context of the invention is a saturated heterocycle having a total of 4 to 7 ring atoms, which contains one or two ring heteroatoms from the series N, O and / or S and is linked via a ring carbon atom or optionally a ring nitrogen atom. Examples which may be mentioned are: azetidinyl, oxetanyl, pyrrolidinyl, pyrazolidinyl, tetrahydrofuranyl, piperidinyl, Piperazinyl, tetrahydropyranyl, morpholinyl and thiomorpholinyl. Preferred are azetidinyl, pyrrolidinyl, piperidinyl, piperazinyl and morpholinyl.
Heteroaryl steht im Rahmen der Erfindung für einen monocyclischen aromatischen Heterocyclus (Heteroaromaten) mit insgesamt 5 oder 6 Ringatomen, der bis zu drei gleiche oder verschiedene Ring-Heteroatome aus der Reihe N, O und/oder S enthält und über ein Ring-Kohlenstoffatom oder gegebenenfalls über ein Ring- Stickstoffatom verknüpft ist. Beispielhaft und vorzugsweise seien genannt: Furyl, Pyrrolyl, Thienyl, Pyrazolyl, Imidazolyl, Thiazolyl, Oxazolyl, Isoxazolyl, Iso- thiazolyl, Triazolyl, Oxadiazolyl, Thiadiazolyl, Pyridyl, Pyrimidinyl, Pyridazinyl, Pyrazinyl und Triazinyl. Heteroaryl is in the context of the invention for a monocyclic aromatic heterocycle (heteroaromatic) with a total of 5 or 6 ring atoms containing up to three identical or different ring heteroatoms from the series N, O and / or S and via a ring carbon atom or optionally linked via a ring nitrogen atom. Examples which may be mentioned are: furyl, pyrrolyl, thienyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, isoxazolyl, isothiazolyl, triazolyl, oxadiazolyl, thiadiazolyl, pyridyl, pyrimidinyl, pyridazinyl, pyrazinyl and triazinyl.
Halogen steht im Rahmen der Erfindung für Fluor, Chlor, Brom und Iod. Halogen is in the context of the invention for fluorine, chlorine, bromine and iodine.
Eine Oxo-Gruppe steht im Rahmen der Erfindung für ein Sauerstoffatom, das über eine Doppelbindung an ein Kohlenstoffatom gebunden ist. An oxo group in the context of the invention is an oxygen atom which is bonded via a double bond to a carbon atom.
Wenn Reste in den erfindungsgemäßen Verbindungen substituiert sind, können die Reste, soweit nicht anders spezifiziert, ein- oder mehrfach substituiert sein. Im Rahmen der vorliegenden Erfindung gilt, dass für alle Reste, die mehrfach auftreten, deren Bedeutung unabhängig voneinander ist. Eine Substitution mit ein, zwei oder drei gleichen oder verschiedenen Substituenten ist bevorzugt. If radicals are substituted in the compounds according to the invention, the radicals can, unless otherwise specified, be monosubstituted or polysubstituted. In the context of the present invention, the meaning is independent of each other for all radicals which occur repeatedly. Substitution with one, two or three identical or different substituents is preferred.
Bevorzugt im Rahmen der vorliegenden Erfindung sind Verbindungen der Formel (I), in welcher Preferred in the context of the present invention are compounds of the formula (I) in which
U für O oder S steht, U stands for O or S,
V für CR3 oder N steht, wobei V is CR 3 or N, where
R3 für Wasserstoff, Trifluormethyl, (Ci-C6)-Alkyl, (C3-C6)-Cycloalkyl, (Ci-C4)- Alkoxycarbonyl, Phenyl, Pyridyl, -NR5R6, -S(0)nR7 oder -S02NR8R9 steht, worin (Ci-C6)-Alkyl mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Trifluormethoxy, (Ci-C i)-Alkoxy, Methylamino, Ethylamino, Dimethylamino, Diethylamino, Phenoxy, Azetidinyl, Pyrrolodinyl, Piperidinyl, Morpholinyl, Piperazinyl und Pyrazolyl substituiert sein kann,
worin Pyrazolyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Methyl und Ethyl substituiert sein kann, worin Phenyl und Pyridyl mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Chlor, Methyl, Ethyl, Methoxy und Ethoxy substituiert sein können, und worin n für eine Zahl 1 oder 2 steht, R 3 is hydrogen, trifluoromethyl, (Ci-C 6) -alkyl, (C 3 -C 6) cycloalkyl, (Ci-C4) - alkoxycarbonyl, phenyl, pyridyl, -NR 5 R 6, -S (0) n is R 7 or -SO 2 NR 8 R 9 , wherein (Ci-C6) alkyl having 1 or 2 substituents independently selected from the group fluorine, trifluoromethyl, trifluoromethoxy, (Ci-C i) alkoxy, methylamino, ethylamino , Dimethylamino, diethylamino, phenoxy, azetidinyl, pyrrolodinyl, piperidinyl, morpholinyl, piperazinyl and pyrazolyl may be substituted, wherein pyrazolyl in turn may be substituted with 1 or 2 substituents independently selected from the group of fluoro, methyl and ethyl, wherein phenyl and pyridyl substituted with 1 or 2 substituents independently selected from the group fluorine, chlorine, methyl, ethyl, methoxy and ethoxy and where n is a number 1 or 2,
R5 für Wasserstoff oder (Ci-C4)-Alkyl steht, worin (Ci-C4)-Alkyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Hydroxy, Trifluormethoxy, Methoxy und Ethoxy substituiert sein kann, R 5 is hydrogen or (C 1 -C 4 ) -alkyl, in which (C 1 -C 4 ) -alkyl in turn may be substituted by 1 or 2 substituents independently of one another selected from the group consisting of fluorine, trifluoromethyl, hydroxyl, trifluoromethoxy, methoxy and ethoxy,
R6 für (Ci-C4)-Alkyl, (C3-C6)-Cycloalkyl, Phenyl, Azetidinyl, Pyrrolidinyl, Tetrahydrofuranyl, Dioxotetrahydrothiolanyl, Piperidinyl, Tetrahydro- pyranyl steht, worin Phenyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Chlor, Methyl, Ethyl, Methoxy, Ethoxy und Methylcarbonylamino substituiert sein kann, oder R5 und R6 bilden zusammen mit dem Stickstoffatom, an das sie gebunden sind, einen Azetidinyl-, Pyrrolidinyl-, Piperidinyl-, Piperazinyl-, Morpholinyl- oder Dioxothiomorpholinyl-Ring, worin der Piperazinyl-Ring am Stickstoff mit Ethyl substituiert sein kann, worin Ethyl mit Hydroxy substituiert ist, R 6 is (C 1 -C 4 ) -alkyl, (C 3 -C 6 ) -cycloalkyl, phenyl, azetidinyl, pyrrolidinyl, tetrahydrofuranyl, dioxotetrahydrothiolanyl, piperidinyl, tetrahydropyranyl, in which phenyl is in turn independently selected with 1 or 2 substituents from the group of fluorine, chlorine, methyl, ethyl, methoxy, ethoxy and methylcarbonylamino, or R 5 and R 6 together with the nitrogen atom to which they are bonded form an azetidinyl, pyrrolidinyl, piperidinyl, piperazinyl , Morpholinyl or dioxothiomorpholinyl ring, wherein the piperazinyl ring on the nitrogen may be substituted with ethyl wherein ethyl is substituted with hydroxy,
R7 für (Ci-C4)-Alkyl oder (C3-C6)-Cycloalkyl steht, für Wasserstoff, Methyl oder Ethyl steht,
R9 für Wasserstoff, Methyl oder Ethyl steht, W für CR4 oder N steht, wobei R 7 is (C 1 -C 4 ) -alkyl or (C 3 -C 6 ) -cycloalkyl, is hydrogen, methyl or ethyl, R 9 is hydrogen, methyl or ethyl; W is CR 4 or N, wherein
R4 für Wasserstoff, (Ci-C4)-Alkyl, Cycloproyl, Cyclobutyl oder Cyclopentyl steht, worin (Ci-C4)-Alkyl mit Trifluormethyl, Hydroxy, Hydroxycarbonyl,R 4 is hydrogen, (C 1 -C 4 ) -alkyl, cyclopropyl, cyclobutyl or cyclopentyl, in which (C 1 -C 4) -alkyl with trifluoromethyl, hydroxy, hydroxycarbonyl,
Methoxycarbonyl, Ethoxycarbonyl, Methylamino, Ethylamino, Dimethylamino oder Diethylamino substituiert sein kann, worin Methylamino, Ethylamino, Dimethylamino und Diethylamino mit Phenyl substituiert sein können, R1 für Wasserstoff steht, Methoxycarbonyl, ethoxycarbonyl, methylamino, ethylamino, dimethylamino or diethylamino may be substituted, wherein methylamino, ethylamino, dimethylamino and diethylamino may be substituted by phenyl, R 1 is hydrogen,
R2 für 2-Fluorbenzyl steht, mit der Maßgabe, dass wenn V für N steht, W für CR4 steht, mit der Maßgabe, dass wenn W für N steht, V für CR3 steht, sowie ihre Salze, Solvate und Solvate der Salze. Besonders bevorzugt im Rahmen der vorliegenden Erfindung sind Verbindungen der Formel (I), in welcher R 2 is 2-fluorobenzyl, provided that when V is N, W is CR 4 , with the proviso that when W is N, V is CR 3 , and their salts, solvates and solvates salts. Particularly preferred in the context of the present invention are compounds of the formula (I) in which
U für S steht, V für CR3 oder N steht, wobei R3 für Wasserstoff, Trifluormethyl, (Ci-C6)-Alkyl, Cyclopropyl, Cyclohexyl, Methoxycarbonyl, Phenyl, Pyridyl, -NR5R6, -S(0)nR7 oder -S02NR8R9 steht, worin (Ci-C6)-Alkyl mit Trifluormethyl, (Ci-C4)-Alkoxy, Methylamino, Ethylamino, Dimethylamino, Diethylamino, Phenoxy, Pyrrolodinyl oder Pyrazolyl substituiert sein kann,
worin Pyrazolyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Methyl und Ethyl substituiert sein kann, worin Phenyl und Pyridyl mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Chlor, Methyl, Methoxy und Ethoxy substituiert sein können, und worin n für eine Zahl 1 oder 2 steht, U is S, V is CR 3 or N, where R 3 is hydrogen, trifluoromethyl, (C 1 -C 6) -alkyl, cyclopropyl, cyclohexyl, methoxycarbonyl, phenyl, pyridyl, -NR 5 R 6 , -S (0) n is R 7 or -SO 2 NR 8 R 9 , in which (C 1 -C 6) -alkyl may be substituted by trifluoromethyl, (C 1 -C 4) -alkoxy, methylamino, ethylamino, dimethylamino, diethylamino, phenoxy, pyrrolodinyl or pyrazolyl, wherein pyrazolyl in turn may be substituted with 1 or 2 substituents independently selected from the group consisting of methyl and ethyl, wherein phenyl and pyridyl may be substituted with 1 or 2 substituents independently selected from the group fluorine, chlorine, methyl, methoxy and ethoxy, and where n is a number 1 or 2,
R5 für Wasserstoff oder (Ci-C4)-Alkyl steht, worin (Ci-C4)-Alkyl seinerseits mit Hydroxy substituiert sein kann, R 5 is hydrogen or (C 1 -C 4 ) -alkyl, in which (C 1 -C 4 ) -alkyl may in turn be substituted by hydroxyl,
R6 für (Ci-C4)-Alkyl, Cyclohexyl, Phenyl oder Dioxotetrahydrothiolanyl steht, worin Phenyl seinerseits mit Methoxy, Ethoxy oder Methylcarbonylamino substituiert sein kann, oder R 6 is (C 1 -C 4) -alkyl, cyclohexyl, phenyl or dioxotetrahydrothiolanyl, in which phenyl in turn may be substituted by methoxy, ethoxy or methylcarbonylamino, or
R5 und R6 bilden zusammen mit dem Stickstoffatom, an das sie gebunden sind, einen Pyrrolidinyl-, Piperazinyl- oder Morpholinyl-Ring, worin der Piperazinyl-Ring am Stickstoff mit Ethyl substituiert ist, worin Ethyl mit Hydroxy substituiert ist, R 5 and R 6 together with the nitrogen atom to which they are attached form a pyrrolidinyl, piperazinyl or morpholinyl ring in which the piperazinyl ring on the nitrogen is substituted by ethyl, in which ethyl is substituted by hydroxy,
R7 für (Ci-C4)-Alkyl oder Cyclohexyl steht, R 7 is (C 1 -C 4 ) -alkyl or cyclohexyl,
R8 für Wasserstoff, Methyl oder Ethyl steht, R 8 is hydrogen, methyl or ethyl,
R9 für Wasserstoff, Methyl oder Ethyl steht, für CR4 oder N steht, wobei R 9 is hydrogen, methyl or ethyl, CR 4 or N, wherein
R4 für Wasserstoff, (Ci-C4)-Alkyl oder Cycloproyl steht, worin (Ci-C4)-Alkyl oder Methylamino substituiert sein kann,
worin Methylamino mit Phenyl substituiert sein kann, R 4 is hydrogen, (C 1 -C 4 ) -alkyl or cyclopropyl, in which (C 1 -C 4) -alkyl or methylamino may be substituted, wherein methylamino may be substituted with phenyl,
R für Wasserstoff steht, R is hydrogen,
R 2 für 2-Fluorbenzyl steht, mit der Maßgabe, dass wenn V für N steht, W für CR steht, mit der Maßgabe, dass wenn W für N steht, V für CR3 steht, sowie ihre Salze, Solvate und Solvate der Salze. R 2 is 2-fluorobenzyl, provided that when V is N, W is CR, with the proviso that when W is N, V is CR 3 , and their salts, solvates and solvates of the salts ,
Besonders bevorzugt im Rahmen der vorliegenden Erfindung sind auch Verbindungen der Formel (I), in welcher Particularly preferred in the context of the present invention are also compounds of the formula (I) in which
R1 für Wasserstoff steht, sowie ihre Salze, Solvate und Solvate der Salze. R 1 is hydrogen, and their salts, solvates and solvates of the salts.
Besonders bevorzugt im Rahmen der vorliegenden Erfindung sind auch Verbindungen der Formel (I), in welcher Particularly preferred in the context of the present invention are also compounds of the formula (I) in which
R2 für 2-Fluorbenzyl steht, sowie ihre Salze, Solvate und Solvate der Salze. Besonders bevorzugt im Rahmen der vorliegenden Erfindung sind auch Verbindungen der Formel (I), in welcher R 2 is 2-fluorobenzyl, and their salts, solvates and solvates of the salts. Particularly preferred in the context of the present invention are also compounds of the formula (I) in which
U für S steht, sowie ihre Salze, Solvate und Solvate der Salze. U is S, and their salts, solvates and solvates of the salts.
Besonders bevorzugt im Rahmen der vorliegenden Erfindung sind auch Verbindungen der Formel (I), in welcher Particularly preferred in the context of the present invention are also compounds of the formula (I) in which
V für N steht, sowie ihre Salze, Solvate und Solvate der Salze. V is N, and their salts, solvates and solvates of the salts.
Besonders bevorzugt im Rahmen der vorliegenden Erfindung sind auch Verbindungen der Formel (I), in welcher
für CR3 steht, wobei Particularly preferred in the context of the present invention are also compounds of the formula (I) in which stands for CR 3 , where
R3 für Wasserstoff, Trifluormethyl, (Ci-C6)-Alkyl, Cyclopropyl, Cyclohexyl, Methoxy- carbonyl, Phenyl, Pyridyl, -NR5R6, -S(0)nR7 oder -S02NR8R9 steht, worin (Ci-C6)-Alkyl mit Trifluormethyl, (Ci-C i)-Alkoxy, Methylamino, Ethylamino,R 3 is hydrogen, trifluoromethyl, (C 1 -C 6) -alkyl, cyclopropyl, cyclohexyl, methoxycarbonyl, phenyl, pyridyl, -NR 5 R 6 , -S (O) n R 7 or -SO 2 NR 8 R 9 in which (C 1 -C 6) -alkyl with trifluoromethyl, (C 1 -C 4) -alkoxy, methylamino, ethylamino,
Dimethylamino, Diethylamino, Phenoxy, Pyrrolodinyl oder Pyrazolyl substituiert sein kann, worin Pyrazolyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Methyl und Ethyl substituiert sein kann, worin Phenyl und Pyridyl mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Chlor, Methyl, Methoxy und Ethoxy substituiert sein können, und worin n für eine Zahl 1 oder 2 steht, Dimethylamino, diethylamino, phenoxy, pyrrolodinyl or pyrazolyl, in which pyrazolyl may in turn be substituted with 1 or 2 substituents independently selected from the group consisting of methyl and ethyl, wherein phenyl and pyridyl having 1 or 2 substituents independently selected from the group fluorine , Chloro, methyl, methoxy and ethoxy, and wherein n is a number 1 or 2,
R5 für Wasserstoff oder (Ci-C4)-Alkyl steht, worin (Ci-C4)-Alkyl seinerseits mit Hydroxy substituiert sein kann, R 5 is hydrogen or (C 1 -C 4 ) -alkyl, in which (C 1 -C 4 ) -alkyl may in turn be substituted by hydroxyl,
R6 für (Ci-C4)-Alkyl, Cyclohexyl, Phenyl oder Dioxotetrahydrothiolanyl steht, worin Phenyl seinerseits mit Methoxy, Ethoxy oder Methylcarbonylamino substituiert sein kann, oder R 6 is (C 1 -C 4) -alkyl, cyclohexyl, phenyl or dioxotetrahydrothiolanyl, in which phenyl in turn may be substituted by methoxy, ethoxy or methylcarbonylamino, or
R5 und R6 bilden zusammen mit dem Stickstoffatom, an das sie gebunden sind, einen Pyrrolidinyl-, Piperazinyl- oder Morpholinyl-Ring, worin der Piperazinyl-Ring am Stickstoff mit Ethyl substituiert ist, worin Ethyl mit Hydroxy substituiert ist, R 5 and R 6 together with the nitrogen atom to which they are attached form a pyrrolidinyl, piperazinyl or morpholinyl ring in which the piperazinyl ring on the nitrogen is substituted by ethyl, in which ethyl is substituted by hydroxy,
R7 für (Ci-C4)-Alkyl oder Cyclohexyl steht,
R8 für Wasserstoff, Methyl oder Ethyl steht, R9 für Wasserstoff, Methyl oder Ethyl steht, sowie ihre Salze, Solvate und Solvate der Salze. R 7 is (C 1 -C 4 ) -alkyl or cyclohexyl, R 8 is hydrogen, methyl or ethyl, R 9 is hydrogen, methyl or ethyl, and their salts, solvates and solvates of the salts.
Besonders bevorzugt im Rahmen der vorliegenden Erfindung sind auch Verbindungen der Formel (I), in welcher Particularly preferred in the context of the present invention are also compounds of the formula (I) in which
W für N steht, sowie ihre Salze, Solvate und Solvate der Salze. W is N, and their salts, solvates and solvates of the salts.
Besonders bevorzugt im Rahmen der vorliegenden Erfindung sind auch Verbindungen der Formel (I), in welcher W für CR4 steht, wobei Also particularly preferred in the context of the present invention are compounds of the formula (I) in which W is CR 4 , where
R4 für Wasserstoff, (Ci-C4)-Alkyl oder Cycloproyl steht, worin (Ci-C4)-Alkyl oder Methylamino substituiert sein kann, worin Methylamino mit Phenyl substituiert sein kann, sowie ihre Salze, Solvate und Solvate der Salze. R 4 is hydrogen, (C 1 -C 4 ) -alkyl or cyclopropyl, in which (C 1 -C 4) -alkyl or methylamino may be substituted, in which methylamino may be substituted by phenyl, and their salts, solvates and solvates of the salts.
Die in den jeweiligen Kombinationen bzw. bevorzugten Kombinationen von Resten im Einzelnen angegebenen Reste-Definitionen werden unabhängig von den jeweiligen angegebenen Kombinationen der Reste beliebig auch durch Reste-Definitionen anderer Kombinationen ersetzt. The residue definitions given in detail in the respective combinations or preferred combinations of residues are also replaced by residue definitions of other combinations, regardless of the particular combinations of the residues indicated.
Besonders bevorzugt sind Kombinationen von zwei oder mehreren der oben genannten Vorzugs- bereiche. Particularly preferred are combinations of two or more of the preferred ranges mentioned above.
Weiterer Gegenstand der Erfindung ist ein Verfahren zur Herstellung der erfindungsgemäßen Verbindungen der Formel (I) dadurch gekennzeichnet, dass man eine Verbindung der Formel (II)
in welcher R1 und R2 jeweils die oben angegebenen Bedeutungen haben, in einem inerten Lösungsmittel in Gegenwart einer geeigneten Base und eines geeigneten Kupplungsreagenzes mit einer Verbindung der Formel (III) The invention further provides a process for the preparation of the compounds of the formula (I) according to the invention which comprises reacting a compound of the formula (II) in which R 1 and R 2 each have the meanings given above, in an inert solvent in the presence of a suitable base and a suitable coupling reagent with a compound of the formula (III)
in welcher R die oben angegebenen Bedeutungen hat, umsetzt, und gegebenenfalls die resultierenden Verbindungen der Formel (I) mit den entsprechenden (i) Lösungsmitteln und/oder (ii) Säuren oder Basen in ihre Solvate, Salze und/oder Solvate der Salze überführt. in which R has the meanings given above, and, if appropriate, converting the resulting compounds of the formula (I) with the appropriate (i) solvents and / or (ii) acids or bases into their solvates, salts and / or solvates of the salts.
Die Umsetzung (II) + (III) — »■ (I) erfolgt in einem Lösungsmittel, welches unter den Reaktionsbedingungen inert ist. Inerte Lösungsmittel sind Alkohole wie Methanol, Ethanol, n-Propanol, Isopropanol, n-Butanol, tert.-Butanol oder 1 ,2-Ethandiol, Ether wie Diethylether, Dioxan, Tetrahydrofuran, Glykoldimethylether oder Diethylenglykoldimethylether, Halogenkohlen- Wasserstoffe wie Dichlormethan, Trichlormethan, Tetrachlormethan, Trichlorethylen, Chlorbenzol oder 1 ,2-Dichlorbenzol, Kohlenwasserstoffe wie Benzol, Toluol, Xylol, Hexan, Cyclohexan oder Erdölfraktionen, oder andere Lösungsmittel wie Aceton, Methylethylketon, Essigsäureethylester, Acetonitril, NN-Dimethylformamid, Dimethylsulfoxid, NN'-Dimethylpropylenharnstoff (DMPU), N-Methylpyrrolidon (NMP), Sulfolan oder Pyridin. Ebenso ist es möglich, Gemische der genannten Lösungsmittel einzusetzen. Bevorzugt ist Dichlormethan, DMF oder DMSO.
Beim Verfahrensschritt (II) + (III)— »■ (I) eignen sich als Kupplungsreagenzien beispielsweise Carbo- diimide wie NN'-Diethyl-, NN'-Dipropyl-, NN'-Diisopropyl-, NN'-Dicyclohexylcarbodiimid (DCC) oder N-(3-Dimethylaminoisopropyl)-N'-ethylcarbodiirnid-Hydrochlorid (EDC), Phosgen- Derivate wie NN'-Carbonyldiimidazol (CDI), 1 ,2-Oxazoliumverbindungen wie 2-Ethyl-5-phenyl- 1 ,2-oxazolium-3 -sulfat oder 2-fert.-Butyl-5-methylisoxazolium-perchlorat, Acylaminoverbindungen wie 2-Ethoxy-l-ethoxycarbonyl-l,2-dihydrochinolin, oder Isobutylchlorformiat, Propanphosphon- säureanhydrid, Cyanophosphonsäurediethylester, Bis-(2-oxo-3 -oxazolidinyl)-phosphorylchlorid, Benzotriazol- 1 -yloxy-tris(dimethylarnino)phosphonium-hexafluorophosphat, Benzotriazol- 1 -yloxy- tris(pyrrolidino)phosphonium-hexafluorophosphat (PyBOP), 0-(Benzotriazol- 1 -yl)-N,N,N',N'-tetra- methyluronium-tetrafluoroborat (TBTU), 2-(lH-Benzotriazol-l-yl)-l,l,3,3-tetramethyluronium- hexafluorophosphat (HBTU), 2-(2-Oxo-l-(2H)-pyridyl)-l,l,3,3-tetramethyluronium-tetrafluoro- borat (TPTU), 0-(7-Azabenzotriazol- 1 -yl)-N,N,N',N'-tetramethyluronium-hexafluorophosphat (HATU) oder 0-(lH-6-Chlorbenzotriazol-l -yl)-l , 1 ,3,3-tetramethyluronium-tetrafluoroborat (TCTU), gegebenenfalls in Kombination mit weiteren Hilfsstoffen wie 1 -Hydroxybenzotriazol (HOBt) oder N-Hydroxysuccinimid (HOSu). Bevorzugt wird N-(3-Dimethylaminoisopropyl)-N'- ethylcarbodiimid-Hydrochlorid (EDC) in Kombination mit NN-Diisopropylethylamin oder O- (Benzotriazol-l-yl)-N,N,N',N'-tetramethyluronium-tetrafluoroborat (TBTU) in Kombination mit 4- Methylmorpholin eingesetzt. The reaction (II) + (III) -> (I) takes place in a solvent which is inert under the reaction conditions. Inert solvents are alcohols such as methanol, ethanol, n-propanol, isopropanol, n-butanol, tert-butanol or 1, 2-ethanediol, ethers such as diethyl ether, dioxane, tetrahydrofuran, glycol dimethyl ether or diethylene glycol dimethyl ether, hydrogen halides such as dichloromethane, trichloromethane, Tetrachloromethane, trichlorethylene, chlorobenzene or 1, 2-dichlorobenzene, hydrocarbons such as benzene, toluene, xylene, hexane, cyclohexane or petroleum fractions, or other solvents such as acetone, methyl ethyl ketone, ethyl acetate, acetonitrile, N, N-dimethylformamide, dimethyl sulfoxide, N, N'-dimethylpropyleneurea (DMPU ), N-methylpyrrolidone (NMP), sulfolane or pyridine. It is likewise possible to use mixtures of the solvents mentioned. Preference is given to dichloromethane, DMF or DMSO. In process step (II) + (III) -> ■ (I) are suitable as coupling reagents, for example carbodiimides such as NN'-diethyl, NN'-dipropyl, NN'-diisopropyl, NN'-dicyclohexylcarbodiimide (DCC) or N- (3-dimethylaminoisopropyl) -N'-ethylcarbodiirnide hydrochloride (EDC), phosgene derivatives such as NN'-carbonyldiimidazole (CDI), 1,2-oxazolium compounds such as 2-ethyl-5-phenyl-1,2-oxazolium 3 -sulfate or 2-tert-butyl-5-methylisoxazolium perchlorate, acylamino compounds such as 2-ethoxy-l-ethoxycarbonyl-l, 2-dihydroquinoline, or isobutyl chloroformate, propanephosphonic anhydride, diethyl cyanophosphonate, bis (2-oxo-3 -oxazolidinyl) -phosphoryl chloride, benzotriazole-1-ynyloxy-tris (dimethylamino) phosphonium hexafluorophosphate, benzotriazole-1-ylxytris (pyrrolidino) phosphonium hexafluorophosphate (PyBOP), 0- (benzotriazol-1-yl) -N, N , N ', N'-tetramethyluronium tetrafluoroborate (TBTU), 2- (1H-benzotriazol-1-yl) -1,3,3-tetramethyluronium hexafluorophosphate (HBTU), 2- (2-oxo) l- (2H) -pyrido dyl) -l, l, 3,3-tetramethyluronium tetrafluoroborate (TPTU), 0- (7-azabenzotriazol-1-yl) -N, N, N ', N'-tetramethyluronium hexafluorophosphate (HATU) or 0 - (lH-6-chlorobenzotriazol-1-yl) -1,1,3,3-tetramethyluronium tetrafluoroborate (TCTU), optionally in combination with other excipients such as 1-hydroxybenzotriazole (HOBt) or N-hydroxysuccinimide (HOSu). Preference is given to N- (3-dimethylaminoisopropyl) -N'-ethylcarbodiimide hydrochloride (EDC) in combination with N, N-diisopropylethylamine or O- (benzotriazol-1-yl) -N, N, N ', N'-tetramethyluronium tetrafluoroborate ( TBTU) in combination with 4-methylmorpholine.
Die Reaktion (II) + (III) —> (I) wird im Allgemeinen in einem Temperaturbereich von 0°C bis +140°C durchgeführt, bevorzugt in einem Temperaturbereich von +20°C bis +60°C durchgeführt. Die Umsetzung kann bei normalem, erhöhtem oder bei erniedrigtem Druck erfolgen (z.B. von 0.5 bis 5 bar). Im Allgemeinen arbeitet man bei Normaldruck. The reaction (II) + (III) -> (I) is generally carried out in a temperature range from 0 ° C to + 140 ° C, preferably carried out in a temperature range from + 20 ° C to + 60 ° C. The reaction may be carried out at normal, elevated or reduced pressure (e.g., from 0.5 to 5 bar). Generally, one works at normal pressure.
Das beschriebene Herstellverfahren kann durch das folgende Syntheseschema (Schema 1) beispielhaft verdeutlicht werden: Schema 1 The described preparation process can be exemplified by the following synthesis scheme (Scheme 1): Scheme 1
[a): EDC, Diisopropylethylamin, DMF/CH2C12 (1 : 1), RT].
Die Verbindungen der Formel (III) sind kommerziell erhältlich, literaturbekannt oder können in Analogie zu literaturbekannten Verfahren hergestellt werden. [a): EDC, diisopropylethylamine, DMF / CH 2 Cl 2 (1: 1), RT]. The compounds of the formula (III) are commercially available, known from the literature or can be prepared in analogy to processes known from the literature.
Die Verbindungen der Formel (II) sind literaturbekannt (vgl. z.B. WO 2007/124854), und können in Analogie zu literaturbekannten Verfahren hergestellt werden. Weitere erfindungsgemäße Verbindungen können gegebenenfalls auch hergestellt werden durch Umwandlungen von funktionellen Gruppen einzelner Substituenten, insbesondere den unter R3 und R4 aufgeführten, ausgehend von den nach obigen Verfahren erhaltenen Verbindungen der Formel (I). Diese Umwandlungen werden wie im vorliegenden experimentellen Teil beschrieben, nach üblichen, dem Fachmann bekannten Methoden durchgeführt, und umfassen beispielsweise Reaktionen wie nukleophile und elektrophile Substitutionen, Oxidationen, Reduktionen, Hydrierungen, Übergangsmetall-katalysierte Kupplungsreaktionen, Eliminierungen, Alkylierung, Aminierung, Veresterung, Esterspaltung, Veretherung, Etherspaltung, Bildung von Carbonamiden, sowie Einführung und Entfernung temporärer Schutzgruppen. The compounds of the formula (II) are known from the literature (cf., for example, WO 2007/124854), and can be prepared in analogy to processes known from the literature. Other compounds of the invention may optionally also be prepared by conversions of functional groups of individual substituents, in particular those listed under R 3 and R 4 , starting from the compounds of the formula (I) obtained by the above methods. These transformations are carried out as described in the present experimental part, according to customary methods known to the person skilled in the art, and include, for example, reactions such as nucleophilic and electrophilic substitutions, oxidations, reductions, hydrogenations, transition metal-catalyzed coupling reactions, eliminations, alkylation, amination, esterification, ester cleavage, Etherification, ether cleavage, formation of carbonamides, and introduction and removal of temporary protecting groups.
Die erfindungsgemäßen Verbindungen wirken als Stimulatoren der löslichen Guanylatcyclase und besitzen wertvolle pharmakologische, und eignen sich daher zur Behandlung und/ oder Prophylaxe von Erkrankungen bei Menschen und Tieren. The compounds of the invention act as stimulators of soluble guanylate cyclase and possess valuable pharmacological, and are therefore suitable for the treatment and / or prophylaxis of diseases in humans and animals.
Die erfindungsgemäßen Verbindungen bewirken eine Gefäßrelaxation und eine Hemmung der Thrombozytenaggregation und führen zu einer Blutdrucksenkung sowie zu einer Steigerung des koronaren Blutflusses. Diese Wirkungen sind über eine direkte Stimulation der löslichen Guanylat- cyclase und einen intrazellulären cGMP -Anstieg vermittelt. Außerdem verstärken die erfindungsgemäßen Verbindungen die Wirkung von Substanzen, die den cGMP-Spiegel steigern, wie beispielsweise EDRF (endothelium-derived relaxing factor), NO-Donatoren, Protoporphyrin IX, Arachidon- säure oder Phenylhydrazin-Derivate. The compounds of the invention cause vasorelaxation and inhibition of platelet aggregation and lead to a reduction in blood pressure and to an increase in coronary blood flow. These effects are mediated by direct stimulation of soluble guanylate cyclase and an intracellular cGMP increase. In addition, the compounds according to the invention enhance the action of substances which increase cGMP levels, such as, for example, EDRF (endothelium-derived relaxing factor), NO donors, protoporphyrin IX, arachidonic acid or phenylhydrazine derivatives.
Die erfindungsgemäßen Verbindungen können daher in Arzneimitteln zur Behandlung und/oder Prophylaxe von kardiovaskulären Erkrankungen wie beispielsweise Bluthochdruck, akute und chronische Herzinsuffizienz, Herzversagen, koronare Herzerkrankung, stabile und instabile Angina pectoris, periphere und kardiale Gefäßerkrankungen, Arrhythmien, Rhythmusstörungen der Vorhöfe und der Kammern sowie Überleitungsstörungen wie beispielsweise atrio-ventrikuläre Blockaden Grad I-III (AB-Block I-III), supraventrikuläre Tachyarrhythmie, Vorhofflimmern, Vorhoffflattern, Kammerflimmern, Kammerflattern, ventrikuläre Tachyarrhytmie, Torsade de pointes-Tachykardie, Extrasystolen des Vorhoffs und des Ventrikels, AV-junktionale Extrasystolen, Sick-Sinus Syndrom, Synkopen, AV-Knoten-Reentrytachykardie, Wolff-Parkinson- White- Syndrom, von akutem Koronarsyndrom (ACS), autoimmune Herzerkrankungen (Perikarditis, Endokarditis, Valvolitis, Aortitis,
Kardiomyopathien), kardiogenem Schock, Aneurysmen, Boxerkardiomyopathie (premature ventricular contraction (PVC)), zur Behandlung und/oder Prophylaxe von thromboembolischen Erkrankungen und Ischämien wie myokardiale Ischämie, Myokardinfarkt, Hirnschlag, Schock, Herzhypertrophie, transistorischen und ischämischen Attacken, Präeklampsie, entzündliche kardiovaskuläre Erkrankungen, Spasmen der Koronararterien und peripherer Arterien, Ödembildung wie beispielsweise pulmonales Ödem, Hirnödem, renales Ödem oder Herzinsuffizienz-bedingtes Ödem, peripheren Durchblutungsstörungen, Reperfusionsschäden, arterielle und venöse Thrombosen, Mikroalbuminurie, Herzmuskelschwäche, endotheliale Dysfunktion, zur Verhinderung von Restenosen wie nach Thrombolysetherapien, percutan-transluminalen Angioplastien (PTA), transluminalen Koronarangioplastien (PTCA), Herztransplantationen und Bypass-Operationen, sowie mikro- und makrovaskuläre Schädigungen (Vasculitis), erhöhte Spiegel von Fibrinogen und von LDL geringer Dichte sowie erhöhte Konzentrationen von Plasminogenaktivator-Inhibitor 1 (PAI-1), sowie zur Behandlung und/oder Prophylaxe von erektiler Dysfunktion und weiblicher sexueller Dysfunktion eingesetzt werden. Im Sinne der vorliegenden Erfindung umfasst der Begriff Herzinsuffizienz auch spezifischere oder verwandte Krankheitsformen wie akut dekompensierte Herzinsuffizienz, Rechtsherzinsuffizienz, Linksherzinsuffizienz, Globalinsuffizienz, ischämische Kardiomyopathie, dilatative Kardiomyopathie, hypertrophe Kardiomyopathie, idiopathische Kardiomyopathie, angeborene Herzfehler, Herzklappenfehler, Herzinsuffizienz bei Herzklappenfehlern, Mitralklappenstenose, Mitralklappeninsuffizienz, Aortenklappenstenose, Aortenklappeninsuffizienz, Trikuspidalstenose, Trikuspidalinsuffizienz, Pulmonalklappenstenose, Pulmonalklappeninsuffizienz, kombinierte Herzklappenfehler, Herzmuskelentzündung (Myokarditis), chronische Myokarditis, akute Myokarditis, virale Myokarditis, diabetische Herzinsuffizienz, alkoholtoxische Kardiomyopathie, kardiale Speichererkrankungen, diastolische Herzinsuffizienz sowie systolische Herzinsuffizienz. Darüber hinaus können die erfindungsgemäßen Verbindungen auch zur Behandlung und/oder Prophylaxe von Arteriosklerose, Lipidstoffwechselstörungen, Hypolipoproteinämien, Dyslipidämien, Hypertriglyceridämien, Hyperlipidämien, Hypercholesterolämien, Abetelipoproteinämie, Sitosterolämie, Xanthomatose, Tangier Krankheit, Fettsucht (Adipositas), Fettleibigkeit (Obesitas) und von kombinierten Hyperlipidämien sowie des Metabolischen Syndroms eingesetzt werden. Außerdem können die erfindungsgemäßen Verbindungen zur Behandlung und/oder Prophylaxe von primärem und sekundärem Raynaud-Phänomen, von Mikrozirkulationsstörungen, Claudicatio, peripheren und autonomen Neuropathien, diabetischen Mikroangiopathien, diabetischer Retinopathie, diabetischen Geschwüren an den Extremitäten, Gangren, CREST-Syndrom,
Erythematose, Onychomykose, rheumatischen Erkrankungen sowie zur Förderung der Wundheilung verwendet werden. The compounds according to the invention can therefore be used in medicaments for the treatment and / or prophylaxis of cardiovascular diseases such as hypertension, acute and chronic heart failure, heart failure, coronary heart disease, stable and unstable angina pectoris, peripheral and cardiac vascular diseases, arrhythmias, arrhythmias of the atria and the chambers and Transient disorders such as atrio-ventricular blockades grade I-III (AB block I-III), supraventricular tachyarrhythmia, atrial fibrillation, atrial flutter, ventricular fibrillation, ventricular tachyarrhythmia, torsades de pointes tachycardia, atrial and ventricular extrasystoles, AV junctional Extrasystoles, sick sinus syndrome, syncope, AV nodal reentrant tachycardia, Wolff-Parkinson-White syndrome, acute coronary syndrome (ACS), autoimmune heart disease (pericarditis, endocarditis, valvolitis, aortitis, Cardiomyopathies), cardiogenic shock, aneurysms, premature ventricular contraction (PVC), for the treatment and / or prophylaxis of thromboembolic disorders and ischaemias such as myocardial ischemia, myocardial infarction, stroke, shock, cardiac hypertrophy, transitory and ischemic attacks, preeclampsia, inflammatory cardiovascular Diseases, spasms of the coronary arteries and peripheral arteries, edema formation such as pulmonary edema, cerebral edema, renal edema or heart failure-related edema, peripheral circulatory disorders, reperfusion injury, arterial and venous thrombosis, microalbuminuria, myocardial insufficiency, endothelial dysfunction, for the prevention of restenosis such as after thrombolytic therapy, percutaneous transluminal angioplasties (PTA), transluminal coronary angioplasties (PTCA), heart transplants and bypass operations, as well as microvascular and macrovascular lesions (vasculitis), increased levels of fibrinogen and low-density LDL and elevated levels of plasminogen activator inhibitor 1 (PAI-1), and for the treatment and / or prophylaxis of erectile dysfunction and female sexual dysfunction. For the purposes of the present invention, the term cardiac insufficiency also encompasses more specific or related forms of disease such as acute decompensated heart failure, right heart failure, left heart failure, global insufficiency, ischemic cardiomyopathy, dilated cardiomyopathy, hypertrophic cardiomyopathy, idiopathic cardiomyopathy, congenital heart defects, valvular heart failure, cardiac valvulopathy, mitral valve stenosis, mitral valve insufficiency, Aortic valve stenosis, aortic valve insufficiency, tricuspid stenosis, tricuspid regurgitation, pulmonary valve stenosis, pulmonary valvular insufficiency, combined heart valve defects, myocarditis, chronic myocarditis, acute myocarditis, viral myocarditis, diabetic heart failure, alcoholic cardiomyopathy, cardiac storage disorders, diastolic heart failure and systolic heart failure. In addition, the compounds according to the invention may also be used for the treatment and / or prophylaxis of arteriosclerosis, lipid metabolism disorders, hypolipoproteinemias, dyslipidemias, hypertriglyceridemias, hyperlipidemias, hypercholesterolemias, abetelipoproteinaemia, sitosterolemia, xanthomatosis, Tangier's disease, obesity (obesity) and combined hyperlipidemias and the metabolic syndrome. In addition, the compounds of the invention may be used for the treatment and / or prophylaxis of primary and secondary Raynaud's phenomenon, microcirculatory disorders, claudication, peripheral and autonomic neuropathies, diabetic microangiopathies, diabetic retinopathy, diabetic ulcers on the extremities, gangrenous, CREST syndrome, Erythematosis, onychomycosis, rheumatic diseases and to promote wound healing.
Weiterhin eignen sich die erfindungsgemäßen Verbindungen zur Behandlung urologischer Erkrankungen wie beispielsweise benignes Prostata-Syndrom (BPS), benigne Prostata-Hyperplasie (BPH), benigne Prostata Vergrösserung (BPE), Blasenentleerungsstörung (BOO), untere Harnwegssyndrome (LUTS, einschließlich Feiines Urologisches Syndrom (FUS)), Erkrankungen des Urogenital- Systems einschliesslich neurogene überaktive Blase (OAB) und (IC), Inkontinenz (UI) wie beispielsweise Misch-, Drang-, Stress-, oder Überlauf-Inkontinenz (MUI, UUI, SUI, OUI), Beckenschmerzen, benigne und maligne Erkrankungen der Organe des männlichen und weiblichen Urogenital-Systems. Furthermore, the compounds according to the invention are suitable for the treatment of urological diseases such as benign prostatic syndrome (BPS), benign prostatic hyperplasia (BPH), benign prostate enlargement (BPE), bladder emptying disorder (BOO), lower urinary tract syndromes (LUTS, including Feiine's urological syndrome ( FUS)), diseases of the urogenital system including neurogenic overactive bladder (OAB) and (IC), incontinence (UI) such as mixed, urge, stress, or overflow incontinence (MUI, UUI, SUI, OUI), Pelvic pain, benign and malignant diseases of the organs of the male and female urogenital system.
Weiterhin eignen sich die erfindungsgemäßen Verbindungen zur Behandlung und/oder Prophylaxe von Nierenerkrankungen, insbesondere von aktuer und chronischer Niereninsuffizienz, sowie von akutem und chronischem Nierenversagen. Im Sinne der vorliegenden Erfindung umfasst der Begriff Niereninsuffizienz sowohl akute als auch chronische Erscheinungsformen der Niereninsuffizienz, wie auch zugrundeliegende oder verwandte Nierenerkrankungen wie renale Hypoperfusion, intradialytische Hypotonie, obstruktive Uropathie, Glomerulopathien, Glomerulonephritis, akute Glomerulonephritis, Glomerulosklerose, tubulointerstitielle Erkrankungen, nephropathische Erkrankungen wie primäre und angeborene Nierenerkrankung, Nierenentzündung, immunlogische Nierenerkrankungen wie Nierentransplantatabstoßung, Immunkomplex-induzierte Nierener- krankungen, durch toxische Substanzen induzierte Nephropathie, Kontrastmittel-induzierte Nephropathie, diabetische und nicht-diabetische Nephropathie, Pyelonephritis, Nierenzysten, Nephrosklerose, hypertensive Nephrosklerose und nephrotisches Syndrom, welche diagnostisch beispielsweise durch abnorm verminderte Kreatinin- und/oder Wasser- Ausscheidung, abnorm erhöhte Blutkonzentrationen von Harnstoff, Stickstoff, Kalium und/oder Kreatinin, veränderte Aktivität von Nierenenzymen wie z.B. Glutamylsynthetase, veränderte Urinosmolarität oder Urinmenge, erhöhte Mikroalbuminurie, Makroalbuminurie, Läsionen an Glomerula und Arteriolen, tubuläre Dilatation, Hyperphosphatämie und/oder die Notwendigkeit zur Dialyse charakterisiert werden können. Die vorliegende Erfindung umfasst auch die Verwendung der erfindungsgemäßen Verbindungen zur Behandlung und/oder Prophylaxe von Folgeerscheinungen einer Niereninsuffizienz, wie beispielsweise Lungenödem, Herzinsuffizienz, Urämie, Anämie, Elektrolytstörungen (z.B. Hyperkalämie, Hyponaträmie) und Störungen im Knochen- und Kohlenhydrat-Metabolismus. Furthermore, the compounds according to the invention are suitable for the treatment and / or prophylaxis of kidney diseases, in particular of acute and chronic renal insufficiency, as well as of acute and chronic renal failure. For the purposes of the present invention, the term renal insufficiency includes both acute and chronic manifestations of renal insufficiency, as well as underlying or related renal diseases such as renal hypoperfusion, intradialytic hypotension, obstructive uropathy, glomerulopathies, glomerulonephritis, acute glomerulonephritis, glomerulosclerosis, tubulo-interstitial disorders, nephropathic disorders such as primary and congenital kidney disease, nephritis, immunological renal diseases such as renal transplant rejection, immune complex-induced kidney disease, nephropathy induced by toxic substances, contrast agent-induced nephropathy, diabetic and nondiabetic nephropathy, pyelonephritis, renal cysts, nephrosclerosis, hypertensive nephrosclerosis and nephrotic syndrome which are diagnostic for example, abnormally decreased creatinine and / or water excretion, abnormally elevated blood levels of urine toff, nitrogen, potassium and / or creatinine, altered activity of renal enzymes, e.g. Glutamyl synthetase, altered urinary or urinary output, increased microalbuminuria, macroalbuminuria, glomerular and arteriolar lesions, tubular dilation, hyperphosphatemia, and / or the need for dialysis. The present invention also encompasses the use of the compounds of the invention for the treatment and / or prophylaxis of sequelae of renal insufficiency, such as pulmonary edema, cardiac insufficiency, uremia, anemia, electrolyte imbalances (e.g., hyperkalemia, hyponatremia) and disorders in bone and carbohydrate metabolism.
Weiterhin eignen sich die erfindungsgemäßen Verbindungen auch zur Behandlung und/oder Prophylaxe von asthmatischen Erkrankungen, pulmonaler arterieller Hypertonie (PAH) und anderen
Formen der pulmonalen Hypertonie (PH), der chronisch-obstruktive Lungenerkrankung (COPD), des akuten Atemwegssyndrom (ARDS), der akuten Lungenschädigung (ALI), der alpha- 1 -Antitrypsin- Defizienz (AATD), der Lungenfibrose, des Lungenemphysem (z.B. durch Zigarettenrauch induziertes Lungenemphysem) und der zystischen Fibrose (CF). Die in der vorliegenden Erfindung beschriebenen Verbindungen stellen auch Wirkstoffe zur Bekämpfung von Krankheiten im Zentralnervensystem dar, die durch Störungen des NO/cGMP- Systems gekennzeichnet sind. Insbesondere sind sie geeignet zur Verbesserung der Wahrnehmung, Konzentrationsleistung, Lernleistung oder Gedächtnisleistung nach kognitiven Störungen, wie sie insbesondere bei Situationen/Krankheiten/Syndromen auftreten wie "Mild cognitive impairment", altersassoziierten Lern- und Gedächtnisstörungen, altersassoziierten Gedächtnisverlusten, vaskulärer Demenz, Schädel-Hirn-Trauma, Schlaganfall, Demenz, die nach Schlaganfällen auftritt ("post stroke dementia"), post-traumatischem Schädel-Hirn-Trauma, allgemeinen Konzentrationsstörungen, Konzentrationsstörungen bei Kindern mit Lern- und Gedächtnisproblemen, Alzheimer'scher Krankheit, Demenz mit Lewy-Körperchen, Demenz mit Degeneration der Frontallappen einschliesslich des Pick's-Syndroms, Parkinson'scher Krankheit, progressiver nuclear palsy, Demenz mit corticobasaler Degeneration, Amyolateralsklerose (ALS), Huntington'scher Krankheit, Demyelinisation, Multipler Sklerose, Thalamischer Degeneration, Creutzfeld- Jacob-Demenz, HIV- Demenz, Schizophrenie mit Demenz oder Korsakoff-Psychose. Sie eignen sich auch zur Behandlung und/oder Prophylaxe von Erkrankungen des Zentralnervensystems wie Angst-, Spannungs- und Depressionszuständen, zentral-nervös bedingten Sexualdysfunktionen und Schlafstörungen sowie zur Regulierung krankhafter Störungen der Nahrungs-, Genuss- und Suchtmittelaufnahme. Furthermore, the compounds according to the invention are also suitable for the treatment and / or prophylaxis of asthmatic diseases, pulmonary arterial hypertension (PAH) and others Forms of pulmonary hypertension (PH), chronic obstructive pulmonary disease (COPD), acute respiratory tract syndrome (ARDS), acute lung injury (ALI), alpha-1-antitrypsin deficiency (AATD), pulmonary fibrosis, pulmonary emphysema (eg cigarette smoke-induced pulmonary emphysema) and cystic fibrosis (CF). The compounds described in the present invention are also agents for controlling diseases in the central nervous system, which are characterized by disorders of the NO / cGMP system. In particular, they are suitable for improving the perception, concentration performance, learning performance or memory performance after cognitive disorders such as occur in situations / diseases / syndromes such as mild cognitive impairment, age-associated learning and memory disorders, age-associated memory loss, vascular dementia, cranial brain -Trauma, stroke, post-stroke dementia, post-traumatic traumatic brain injury, generalized concentration disorder, difficulty concentrating in children with learning and memory problems, Alzheimer's disease, dementia with Lewy bodies , Dementia with degeneration of the frontal lobes including Pick's syndrome, Parkinson's disease, progressive nuclear palsy, dementia with corticobasal degeneration, amyolateral sclerosis (ALS), Huntington's disease, demyelinization, multiple sclerosis, thalamic degeneration, Creutzfeld-Jacob dementia, HIV dementia, schizophrenia with dementia or Korsakoff's psychosis. They are also suitable for the treatment and / or prophylaxis of diseases of the central nervous system such as states of anxiety, tension and depression, central nervous conditional sexual dysfunctions and sleep disorders as well as for the regulation of pathological disorders of food, consumption and addiction.
Weiterhin eignen sich die erfindungsgemäßen Verbindungen auch zur Regulation der cerebralen Durchblutung und stellen wirkungsvolle Mittel zur Bekämpfung von Migräne dar. Auch eignen sie sich zur Prophylaxe und Bekämpfung der Folgen cerebraler Infarktgeschehen (Apoplexia cerebri) wie Schlaganfall, cerebraler Ischämien und des Schädel-Hirn-Traumas. Ebenso können die erfindungsgemäßen Verbindungen zur Bekämpfung von Schmerzzuständen und Tinnitus eingesetzt werden. Furthermore, the compounds according to the invention are also suitable for regulating cerebral blood flow and are effective agents for combating migraine. They are also suitable for the prophylaxis and control of the consequences of cerebral infarct events (Apoplexia cerebri) such as stroke, cerebral ischaemias and craniocerebral trauma , Likewise, the compounds of the invention can be used to combat pain and tinnitus.
Zudem besitzen die erfindungsgemäßen Verbindungen antiinflammatorische Wirkung und können daher als entzündungshemmende Mittel zur Behandlung und/oder Prophylaxe von Sepsis (SIRS), multiplem Organversagen (MODS, MOF), entzündlichen Erkrankungen der Niere, chronischen Darmentzündungen (IBD, Crohn's Disease, UC), Pankreatitis, Peritonitis, rheumatoiden Erkrankungen, entzündlichen Hauterkrankungen sowie entzündlichen Augenerkrankungen eingesetzt werden.
Des weiteren können die erfindungsgemäßen Verbindungen ebenfalls zur Behandlung und/ oder Prophylaxe von Autoimmunerkrankungen eingesetzt werden. In addition, the compounds of the invention have anti-inflammatory action and can therefore be used as anti-inflammatory agents for the treatment and / or prophylaxis of sepsis (SIRS), multiple organ failure (MODS, MOF), inflammatory diseases of the kidney, chronic inflammatory bowel disease (IBD, Crohn's Disease, UC), pancreatitis , Peritonitis, rheumatoid diseases, inflammatory skin diseases as well as inflammatory eye diseases. Furthermore, the compounds of the invention can also be used for the treatment and / or prophylaxis of autoimmune diseases.
Weiterhin sind die erfindungsgemäßen Verbindungen zur Behandlung und/ oder Prophylaxe fibrotischer Erkrankungen der inneren Organe, wie beispielsweise der Lunge, des Herzens, der Niere, des Knochenmarks und insbesondere der Leber, sowie dermatologischer Fibrosen und fibrotischer Erkrankungen des Auges, geeignet. Im Sinne der vorliegenden Erfindungen umfasst der Begriff fibrotischer Erkrankungen insbesondere die folgenden Begriffe Leberfibrose, Leberzirrhose, Lungenfibrose, Endomyocardfibrose, Nephropathie, Glomerulonephritis, interstitielle Nierenfibrose, fibrotische Schäden in Folge von Diabetes, Knochenmarksfibrose und ähnliche fibrotische Erkrankungen, Sklerodermie, Morphaea, Keloide, hypertrophe Narbenbildung (auch nach chirurgischen Eingriffen), Naevi, diabetische Retinopathie und proliferative Vitroretinopathie. Furthermore, the compounds according to the invention are suitable for the treatment and / or prophylaxis of fibrotic disorders of the internal organs such as, for example, the lung, the heart, the kidney, the bone marrow and in particular the liver, as well as dermatological fibroses and fibrotic disorders of the eye. For the purposes of the present invention, the term fibrotic disorders includes in particular the following terms: liver fibrosis, cirrhosis, pulmonary fibrosis, endomyocardial fibrosis, nephropathy, glomerulonephritis, interstitial renal fibrosis, fibrotic damage as a result of diabetes, bone marrow fibrosis and similar fibrotic disorders, scleroderma, morphea, keloids, hypertrophic scarring (also after surgical procedures), nevi, diabetic retinopathy and proliferative vitroretinopathy.
Weiterhin eignen sich die erfindungsgemäßen Verbindungen zur Bekämpfung postoperativer Narbenbildung, z.B. in Folge von Glaukom-Operationen. Furthermore, the compounds of the invention are useful for controlling postoperative scarring, e.g. as a result of glaucoma surgery.
Die erfindungsgemäßen Verbindungen können ebenfalls kosmetisch bei alternder und verhornender Haut eingesetzt werden. The compounds according to the invention can likewise be used cosmetically for aging and keratinizing skin.
Außerdem sind die erfindungsgemäßen Verbindungen zur Behandlung und/ oder Prophylaxe von Diabetes, Hepatitis, Neoplasma, Osteoporose, Glaukom und Gastroparese geeignet. In addition, the compounds according to the invention are suitable for the treatment and / or prophylaxis of diabetes, hepatitis, neoplasm, osteoporosis, glaucoma and gastroparesis.
Weiterer Gegenstand der vorliegenden Erfindung ist die Verwendung der erfindungsgemäßen Verbindungen zur Behandlung und/oder Prophylaxe von Erkrankungen, insbesondere der zuvor genannten Erkrankungen. Another object of the present invention is the use of the compounds of the invention for the treatment and / or prophylaxis of diseases, in particular the aforementioned diseases.
Weiterer Gegenstand der vorliegenden Erfindung ist die Verwendung der erfindungsgemäßen Verbindungen zur Behandlung und/oder Prophylaxe von Herzinsuffizienz, Angina pectoris, Hypertonie, pulmonaler Hypertonie, Ischämien, Gefäßerkrankungen, Niereninsuffizienz, thromboembolischen Erkrankungen, fibrotischen Erkrankungen und Arteriosklerose. Weiterer Gegenstand der vorliegenden Erfindung sind die erfindungsgemäßen Verbindungen zur Verwendung in einem Verfahren zur Behandlung und/ oder Prophylaxe von Herzinsuffizienz, Angina pectoris, Hypertonie, pulmonaler Hypertonie, Ischämien, Gefäßerkrankungen, Niereninsuffizienz, thromboembolischen Erkrankungen, fibrotischen Erkrankungen und Arteriosklerose. Another object of the present invention is the use of the compounds of the invention for the treatment and / or prophylaxis of heart failure, angina pectoris, hypertension, pulmonary hypertension, ischaemia, vascular disease, renal insufficiency, thromboembolic disorders, fibrotic diseases and arteriosclerosis. The present invention furthermore relates to the compounds according to the invention for use in a method for the treatment and / or prophylaxis of cardiac insufficiency, angina pectoris, hypertension, pulmonary hypertension, ischaemias, vascular disorders, renal insufficiency, thromboembolic disorders, fibrotic disorders and atherosclerosis.
Weiterer Gegenstand der vorliegenden Erfindung ist die Verwendung der erfindungsgemäßen Verbindungen zur Herstellung eines Arzneimittels zur Behandlung und/oder Prophylaxe von Erkrankungen, insbesondere der zuvor genannten Erkrankungen.
Weiterer Gegenstand der vorliegenden Erfindung ist die Verwendung der erfindungsgemäßen Verbindungen zur Herstellung eines Arzneimittels zur Behandlung und/oder Prophylaxe von Herzinsuffizienz, Angina pectoris, Hypertonie, pulmonaler Hypertonie, Ischämien, Gefäßerkrankungen, Niereninsuffizienz, thromboembolischen Erkrankungen, fibrotischen Erkrankungen und Arteriosklerose. Another object of the present invention is the use of the compounds of the invention for the manufacture of a medicament for the treatment and / or prophylaxis of diseases, in particular the aforementioned diseases. Another object of the present invention is the use of the compounds of the invention for the manufacture of a medicament for the treatment and / or prophylaxis of heart failure, angina pectoris, hypertension, pulmonary hypertension, ischemia, vascular diseases, renal insufficiency, thromboembolic disorders, fibrotic diseases and arteriosclerosis.
Weiterer Gegenstand der vorliegenden Erfindung ist ein Verfahren zur Behandlung und/oder Prophylaxe von Erkrankungen, insbesondere der zuvor genannten Erkrankungen, unter Verwendung einer wirksamen Menge von mindestens einer der erfindungsgemäßen Verbindungen. Another object of the present invention is a method for the treatment and / or prophylaxis of diseases, in particular the aforementioned diseases, using an effective amount of at least one of the compounds of the invention.
Weiterer Gegenstand der vorliegenden Erfindung ist ein Verfahren zur Behandlung und/oder Prophylaxe von Herzinsuffizienz, Angina pectoris, Hypertonie, pulmonaler Hypertonie, Ischämien, Gefäßerkrankungen, Niereninsuffizienz, thromboembolischen Erkrankungen, fibrotischen Erkrankungen und Arteriosklerose, unter Verwendung einer wirksamen Menge von mindestens einer der erfindungsgemäßen Verbindungen. The present invention further provides a method for the treatment and / or prophylaxis of cardiac insufficiency, angina pectoris, hypertension, pulmonary hypertension, ischaemias, vascular diseases, renal insufficiency, thromboembolic disorders, fibrotic diseases and atherosclerosis, using an effective amount of at least one of the compounds according to the invention ,
Weiterer Gegenstand der vorliegenden Erfindung ist ein Verfahren zur Behandlung und/oder Prophylaxe von Erkrankungen, insbesondere der zuvor genannten Erkrankungen, unter Verwendung einer wirksamen Menge von mindestens einer der erfindungsgemäßen Verbindungen. Another object of the present invention is a method for the treatment and / or prophylaxis of diseases, in particular the aforementioned diseases, using an effective amount of at least one of the compounds of the invention.
Die erfindungsgemäßen Verbindungen können allein oder bei Bedarf in Kombination mit anderen Wirkstoffen eingesetzt werden. Weiterer Gegenstand der vorliegenden Erfindung sind Arzneimittel, enthaltend mindestens eine der erfindungsgemäßen Verbindungen und einen oder mehrere weitere Wirkstoffe, insbesondere zur Behandlung und/oder Prophylaxe der zuvor genannten Erkrankungen. Als geeignete Kombinationswirkstoffe seien beispielhaft und vorzugsweise genannt: organische Nitrate und NO-Donatoren, wie beispielsweise Natriumnitroprussid, Nitroglycerin, Isosorbidmononitrat, Isosorbiddinitrat, Molsidomin oder SIN-1, sowie inhalatives NO; The compounds of the invention may be used alone or as needed in combination with other agents. Another object of the present invention are pharmaceutical compositions containing at least one of the compounds of the invention and one or more other active ingredients, in particular for the treatment and / or prophylaxis of the aforementioned diseases. Examples of suitable combination active ingredients are: organic nitrates and NO donors, such as, for example, sodium nitroprusside, nitroglycerin, isosorbide mononitrate, isosorbide dinitrate, molsidomine or SIN-1, and inhaled NO;
Verbindungen, die den Abbau von cyclischem Guanosinmonophosphat (cGMP) inhibieren, wie beispielsweise Inhibitoren der Phosphodiesterasen (PDE) 1, 2 und/oder 5, insbesondere PDE 5- Inhibitoren wie Sildenafil, Vardenafil und Tadalafil; antithrombotisch wirkende Mittel, beispielhaft und vorzugsweise aus der Gruppe der Thrombozytenaggregationshemmer, der Antikoagulantien oder der profibrinolytischen Substanzen; den Blutdruck senkende Wirkstoffe, beispielhaft und vorzugsweise aus der Gruppe der Calcium- Antagonisten, Angiotensin All- Antagonisten, ACE-Hemmer, Endothelin- Antagonisten, Renin-
Inhibitoren, alpha-Rezeptoren-Blocker, beta-Rezeptoren-Blocker, Mineralocorticoid-Rezeptor- Antagonisten sowie der Diuretika; und/oder den Fettstoffwechsel verändernde Wirkstoffe, beispielhaft und vorzugsweise aus der Gruppe der Thyroidrezeptor-Agonisten, Cholesterinsynthese-Inhibitoren wie beispielhaft und vorzugsweise HMG-CoA-Reduktase- oder Squalensynthese-Inhibitoren, der ACAT-Inhibitoren, CETP- Inhibitoren, MTP-Inhibitoren, PPAR-alpha-, PPAR-gamma- und/oder PPAR-delta-Agonisten, Cholesterin-Absoφtionshemmer, Lipase-Inhibitoren, polymeren Gallensäureadsorber, Gallensäure- Reabsorptionshemmer und Lipoprotein(a)-Antagonisten. Compounds which inhibit the degradation of cyclic guanosine monophosphate (cGMP), such as inhibitors of phosphodiesterases (PDE) 1, 2 and / or 5, in particular PDE 5 inhibitors such as sildenafil, vardenafil and tadalafil; antithrombotic agents, by way of example and preferably from the group of platelet aggregation inhibitors, anticoagulants or profibrinolytic substances; antihypertensive agents, by way of example and preferably from the group of calcium antagonists, angiotensin all-antagonists, ACE inhibitors, endothelin antagonists, renin Inhibitors, alpha-receptor blockers, beta-receptor blockers, mineralocorticoid receptor antagonists and diuretics; and / or lipid metabolism-altering agents, by way of example and with preference from the group of thyroid receptor agonists, cholesterol synthesis inhibitors such as, by way of example and preferably, HMG-CoA reductase or squalene synthesis inhibitors, ACAT inhibitors, CETP inhibitors, MTP inhibitors, PPAR alpha, PPAR gamma and / or PPAR delta agonists, cholesterol absorption inhibitors, lipase inhibitors, polymeric bile acid adsorbers, bile acid reabsorption inhibitors, and lipoprotein (a) antagonists.
Unter antithrombotisch wirkenden Mittel werden vorzugsweise Verbindungen aus der Gruppe der Thrombozytenaggregationshemmer, der Antikoagulantien oder der profibrinolytischen Substanzen verstanden. Antithrombotic agents are preferably understood as meaning compounds from the group of platelet aggregation inhibitors, anticoagulants or profibrinolytic substances.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Thrombozytenaggregationshemmer, wie beispielhaft und vorzugsweise Aspirin, Clopidogrel, Ticlopidin oder Dipyridamol, verabreicht. Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Thrombin-Inhibitor, wie beispielhaft und vorzugsweise Ximelagatran, Dabigatran, Melagatran, Bivalirudin oder Clexane, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a platelet aggregation inhibitor, such as, by way of example and by way of preference, aspirin, clopidogrel, ticlopidine or dipyridamole. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a thrombin inhibitor, such as, by way of example and by way of preference, ximelagatran, dabigatran, melagatran, bivalirudin or Clexane.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem GPIIb/IIIa-Antagonisten, wie beispielhaft und vorzugsweise Tirofiban oder Abciximab, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a GPIIb / IIIa antagonist, such as, by way of example and by way of preference, tirofiban or abciximab.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Faktor Xa-Inhibitor, wie beispielhaft und vorzugsweise Rivaroxaban (BAY 59-7939), DU-176b, Apixaban, Otamixaban, Fidexaban, Razaxaban, Fondaparinux, Idrapa- rinux, PMD-3112, YM-150, KFA-1982, EMD-503982, MCM-17, MLN-1021, DX 9065a, DPC 906, JTV 803, SSR-126512 oder SSR-128428, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are used in combination with a factor Xa inhibitor, such as by way of example and preferably rivaroxaban (BAY 59-7939), DU-176b, apixaban, otamixaban, fidexaban, razaxaban, fondaparinux, idraparinux, PMD No. 3112, YM-150, KFA-1982, EMD-503982, MCM-17, MLN-1021, DX 9065a, DPC 906, JTV 803, SSR-126512 or SSR-128428.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit Heparin oder einem low molecular weight (LMW)-Heparin-Derivat verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with heparin or a low molecular weight (LMW) heparin derivative.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Vitamin K-Antagonisten, wie beispielhaft und vorzugsweise Coumarin, verabreicht.
Unter den Blutdruck senkenden Mitteln werden vorzugsweise Verbindungen aus der Gruppe der Calcium-Antagonisten, Angiotensin AII-Antagonisten, ACE-Hemmer, Endothelin-Antagonisten, Renin-Inhibitoren, alpha-Rezeptoren-Blocker, beta-Rezeptoren-Blocker, Mineralocorticoid-Rezep- tor- Antagonisten sowie der Diuretika verstanden. Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Calcium-Antagonisten, wie beispielhaft und vorzugsweise Nifedipin, Amlodipin, Verapamil oder Diltiazem, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a vitamin K antagonist, such as by way of example and preferably coumarin. Among the antihypertensive agents are preferably compounds from the group of calcium antagonists, angiotensin AII antagonists, ACE inhibitors, endothelin antagonists, renin inhibitors, alpha-receptor blocker, beta-receptor blocker, mineralocorticoid receptor - understood antagonists and diuretics. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a calcium antagonist, such as, by way of example and by way of preference, nifedipine, amlodipine, verapamil or diltiazem.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem alpha- 1 -Rezeptoren-Blocker, wie beispielhaft und vorzugsweise Prazosin, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with an alpha-1-receptor blocker, such as by way of example and preferably prazosin.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem beta-Rezeptoren-Blocker, wie beispielhaft und vorzugsweise Propranolol, Atenolol, Timolol, Pindolol, Alprenolol, Oxprenolol, Penbutolol, Bupranolol, Metipranolol, Nadolol, Mepindolol, Carazalol, Sotalol, Metoprolol, Betaxolol, Celiprolol, Bisoprolol, Carteolol, Esmolol, Labetalol, Carvedilol, Adaprolol, Landiolol, Nebivolol, Epanolol oder Bucindolol, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are used in combination with a beta-receptor blocker, such as by way of example and preferably propranolol, atenolol, timolol, pindolol, alprenolol, oxprenolol, penbutolol, bupranolol, metipranolol, nadolol, mepindolol, carazalol, sotalol, Metoprolol, betaxolol, celiprolol, bisoprolol, carteolol, esmolol, labetalol, carvedilol, adaprolol, landiolol, nebivolol, epanolol or bucindolol.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Angiotensin AII-Antagonisten, wie beispielhaft und vorzugsweise Losartan, Candesartan, Valsartan, Telmisartan oder Embursatan, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with an angiotensin AII antagonist, such as by way of example and preferably losartan, candesartan, valsartan, telmisartan or embursatan.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem ACE-Hemmer, wie beispielhaft und vorzugsweise Enalapril, Captopril, Lisinopril, Ramipril, Delapril, Fosinopril, Quinopril, Perindopril oder Trandopril, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with an ACE inhibitor such as, by way of example and by way of preference, enalapril, captopril, lisinopril, ramipril, delapril, fosinopril, quinopril, perindopril or trandopril.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Endothelin-Antagonisten, wie beispielhaft und vorzugsweise Bosentan, Darusentan, Ambrisentan oder Sitaxsentan, verabreicht. Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Renin-Inhibitor, wie beispielhaft und vorzugsweise Aliskiren, SPP-600 oder SPP-800, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with an endothelin antagonist such as, by way of example and by way of preference, bosentan, darusentan, ambrisentan or sitaxsentan. In a preferred embodiment of the invention, the compounds of the invention are administered in combination with a renin inhibitor, such as by way of example and preferably aliskiren, SPP-600 or SPP-800.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Mineralocorticoid-Rezeptor-Antagonisten, wie beispielhaft und vorzugs- weise Spironolacton oder Eplerenon, verabreicht.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Diuretikum, wie beispielhaft und vorzugsweise Furosemid, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a mineralocorticoid receptor antagonist, such as by way of example and preferably spironolactone or eplerenone. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a diuretic, such as by way of example and preferably furosemide.
Unter den Fettstoffwechsel verändernden Mitteln werden vorzugsweise Verbindungen aus der Gruppe der CETP-Inhibitoren, Thyroidrezeptor-Agonisten, Cholesterinsynthese-Inhibitoren wie HMG-CoA-Reduktase- oder Squalensynthese-Inhibitoren, der ACAT-Inhibitoren, MTP-Inhibitoren, PPAR-alpha-, PPAR-gamma- und/oder PPAR-delta-Agonisten, Cholesterin- Absoφtionshemmer, polymeren Gallensäureadsorber, Gallensäure-Reabsoφtionshemmer, Lipase-Inhibitoren sowie der Lipoprotein(a)-Antagonisten verstanden. Among the lipid metabolizing agents are preferably compounds from the group of CETP inhibitors, thyroid receptor agonists, cholesterol synthesis inhibitors such as HMG-CoA reductase or squalene synthesis inhibitors, the ACAT inhibitors, MTP inhibitors, PPAR-alpha, PPAR gamma and / or PPAR delta agonists, cholesterol Absoφtionhemmer, polymeric Bensäureadsorber, bile acid Reabsoφtionshemmer, lipase inhibitors and the lipoprotein (a) understood antagonists.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem CETP-Inhibitor, wie beispielhaft und vorzugsweise Dalcetrapib, BAY 60- 5521, Anacetrapib oder CETP -Vaccine (CETi-1), verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a CETP inhibitor, such as, for example and preferably, dalcetrapib, BAY 60-5521, anacetrapib or CETP vaccine (CETi-1).
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Thyroidrezeptor-Agonisten, wie beispielhaft und vorzugsweise D- Thyroxin, 3,5,3'-Triiodothyronin (T3), CGS 23425 oder Axitirome (CGS 26214), verabreicht. Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem HMG-CoA-Reduktase-Inhibitor aus der Klasse der Statine, wie beispielhaft und vorzugsweise Lovastatin, Simvastatin, Pravastatin, Fluvastatin, Atorvastatin, Rosuvastatin oder Pitavastatin, verabreicht. In a preferred embodiment of the invention, the compounds of the invention are administered in combination with a thyroid receptor agonist such as, by way of example and by way of preference, D-thyroxine, 3,5,3'-triiodothyronine (T3), CGS 23425 or axitirome (CGS 26214). In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with an HMG-CoA reductase inhibitor from the class of statins, such as by way of example and preferably lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin or pitavastatin.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Squalensynthese-Inhibitor, wie beispielhaft und vorzugsweise BMS- 188494 oder TAK-475, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a squalene synthesis inhibitor, such as by way of example and preferably BMS-188494 or TAK-475.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem ACAT-Inhibitor, wie beispielhaft und vorzugsweise Avasimibe, Melinamide, Pactimibe, Eflucimibe oder SMP-797, verabreicht. Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem MTP-Inhibitor, wie beispielhaft und vorzugsweise Implitapide, BMS- 201038, R-103757 oder JTT-130, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with an ACAT inhibitor, such as by way of example and preferably avasimibe, melinamide, pactimibe, eflucimibe or SMP-797. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with an MTP inhibitor such as, for example and preferably, implitapide, BMS-201038, R-103757 or JTT-130.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem PPAR-gamma- Agonisten, wie beispielhaft und vorzugsweise Pioglitazone oder Rosiglitazone, verabreicht.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem PPAR-delta-Agonisten, wie beispielhaft und vorzugsweise GW 501516 oder BAY 68-5042, verabreicht. In a preferred embodiment of the invention, the compounds of the invention are administered in combination with a PPAR gamma agonist such as, by way of example and by way of preference, pioglitazone or rosiglitazone. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a PPAR delta agonist, such as by way of example and preferably GW 501516 or BAY 68-5042.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Cholesterin-Absorptionshemmer, wie beispielhaft und vorzugsweise Ezetimibe, Tiqueside oder Pamaqueside, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a cholesterol absorption inhibitor, such as by way of example and preferably ezetimibe, tiqueside or pamaqueside.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Lipase-Inhibitor, wie beispielhaft und vorzugsweise Orlistat, verabreicht. In a preferred embodiment of the invention, the compounds according to the invention are administered in combination with a lipase inhibitor, such as, for example and preferably, orlistat.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem polymeren Gallensäureadsorber, wie beispielhaft und vorzugsweise Cholestyramin, Colestipol, Colesolvam, CholestaGel oder Colestimid, verabreicht. In a preferred embodiment of the invention, the compounds of the invention are administered in combination with a polymeric bile acid adsorbent such as, by way of example and by way of preference, cholestyramine, colestipol, colesolvam, cholesta gel or colestimide.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Gallensäure-Reabsoφtionshemmer, wie beispielhaft und vorzugsweise ASBT (= IBAT)-Inhibitoren wie z.B. AZD-7806, S-8921, AK-105, BARI-1741, SC-435 oder SC- 635, verabreicht. In a preferred embodiment of the invention, the compounds of the invention are used in combination with a bile acid reabsorption inhibitor, such as by way of example and preferably ASBT (= IBAT) inhibitors such as e.g. AZD-7806, S-8921, AK-105, BARI-1741, SC-435 or SC-635.
Bei einer bevorzugten Ausführungsform der Erfindung werden die erfindungsgemäßen Verbindungen in Kombination mit einem Lipoprotein(a)-Antagonisten, wie beispielhaft und vorzugsweise Gemcabene calcium (CI-1027) oder Nicotinsäure, verabreicht. In a preferred embodiment of the invention, the compounds of the invention are administered in combination with a lipoprotein (a) antagonist such as, by way of example and by way of preference, gemcabene calcium (CI-1027) or nicotinic acid.
Weiterer Gegenstand der vorliegenden Erfindung sind Arzneimittel, die mindestens eine erfindungs- gemäße Verbindung, üblicherweise zusammen mit einem oder mehreren inerten, nichttoxischen, pharmazeutisch geeigneten Hilfsstoffen enthalten, sowie deren Verwendung zu den zuvor genannten Zwecken. Another object of the present invention are pharmaceutical compositions containing at least one compound of the invention, usually together with one or more inert, non-toxic, pharmaceutically suitable excipients, and their use for the purposes mentioned above.
Die erfindungsgemäßen Verbindungen können systemisch und/oder lokal wirken. Zu diesem Zweck können sie auf geeignete Weise appliziert werden, wie z.B. oral, parenteral, pulmonal, nasal, sublingual, lingual, buccal, rectal, dermal, transdermal, conjunctival, otisch oder als Implantat bzw. Stent. The compounds according to the invention can act systemically and / or locally. For this purpose, they may be applied in a suitable manner, e.g. oral, parenteral, pulmonary, nasal, sublingual, lingual, buccal, rectal, dermal, transdermal, conjunctival, otic or as an implant or stent.
Für diese Applikationswege können die erfindungsgemäßen Verbindungen in geeigneten Applikationsformen verabreicht werden. For these administration routes, the compounds according to the invention can be administered in suitable administration forms.
Für die orale Applikation eignen sich nach dem Stand der Technik funktionierende, die erfindungs- gemäßen Verbindungen schnell und/oder modifiziert abgebende Applikationsformen, die die
erfindungsgemäßen Verbindungen in kristalliner und/oder amorphisierter und/oder gelöster Form enthalten, wie z.B. Tabletten (nicht-überzogene oder überzogene Tabletten, beispielsweise mit magensaftresistenten oder sich verzögert auflösenden oder unlöslichen Überzügen, die die Freisetzung der erfindungsgemäßen Verbindung kontrollieren), in der Mundhöhle schnell zerfallende Tabletten oder Filme/Oblaten, Filme/Lyophylisate, Kapseln (beispielsweise Hart- oder Weichgelatinekapseln), Dragees, Granulate, Pellets, Pulver, Emulsionen, Suspensionen, Aerosole oder Lösungen. For the oral administration are according to the prior art functioning, the compounds according to the invention quickly and / or modified donating application forms, the Compounds according to the invention in crystalline and / or amorphised and / or dissolved form, such as tablets (uncoated or coated tablets, for example, with enteric or delayed-dissolving or insoluble coatings, which control the release of the compound of the invention) in the oral cavity quickly disintegrating tablets or films / wafers, films / lyophilisates, capsules (for example hard or soft gelatin capsules), dragées, granules, pellets, powders, emulsions, suspensions, aerosols or solutions.
Die parenterale Applikation kann unter Umgehung eines Resorptionsschrittes geschehen (z.B. intravenös, intraarteriell, intrakardial, intraspinal oder intralumbal) oder unter Einschaltung einer Resorption (z.B. intramuskulär, subcutan, intracutan, percutan oder intraperitoneal). Für die parenterale Applikation eignen sich als Applikationsformen u.a. Injektions- und Infusionszubereitungen in Form von Lösungen, Suspensionen, Emulsionen, Lyophilisaten oder sterilen Pulvern. Parenteral administration can be accomplished by bypassing a resorption step (e.g., intravenous, intraarterial, intracardiac, intraspinal, or intralumbar) or by resorting to absorption (e.g., intramuscular, subcutaneous, intracutaneous, percutaneous, or intraperitoneal). For parenteral administration are suitable as application forms u.a. Injection and infusion preparations in the form of solutions, suspensions, emulsions, lyophilisates or sterile powders.
Für die sonstigen Applikationswege eignen sich z.B. Inhalationsarzneiformen (u.a. Pulverinhalatoren, Nebulizer), Nasentropfen, -lösungen oder -sprays, lingual, sublingual oder buccal zu applizierende Tabletten, Filme/Oblaten oder Kapseln, Suppositorien, Ohren- oder Augen- präparationen, Vaginalkapseln, wäßrige Suspensionen (Lotionen, Schüttelmixturen), lipophile Suspensionen, Salben, Cremes, transdermale therapeutische Systeme (z.B. Pflaster), Milch, Pasten, Schäume, Streupuder, Implantate oder Stents. For the other routes of administration are suitable, for example Inhalation medicaments (including powder inhalers, nebulizers), nasal drops, solutions or sprays, lingual, sublingual or buccal tablets, films / wafers or capsules, suppositories, ear or eye preparations, vaginal capsules, aqueous suspensions (lotions, shake mixtures), lipophilic suspensions, ointments, creams, transdermal therapeutic systems (eg plasters), milk, pastes, foams, powdered powders, implants or stents.
Bevorzugt sind die orale oder parenterale Applikation, insbesondere die orale Applikation. Die erfindungsgemäßen Verbindungen können in die angeführten Applikationsformen überführt werden. Dies kann in an sich bekannter Weise durch Mischen mit inerten, nichttoxischen, pharmazeutisch geeigneten Hilfsstoffen geschehen. Zu diesen Hilfsstoffen zählen u.a. Trägerstoffe (beispielsweise mikrokristalline Cellulose, Lactose, Mannitol), Lösungsmittel (z.B. flüssige Poly- ethylenglycole), Emulgatoren und Dispergier- oder Netzmittel (beispielsweise Natriumdodecylsulfat, Polyoxysorbitanoleat), Bindemittel (beispielsweise Polyvinylpyrrolidon), synthetische und natürliche Polymere (beispielsweise Albumin), Stabilisatoren (z.B. Antioxidantien wie beispielsweise Ascorbinsäure), Farbstoffe (z.B. anorganische Pigmente wie beispielsweise Eisenoxide) und Geschmacks- und/oder Geruchskorrigentien. Preference is given to oral or parenteral administration, in particular oral administration. The compounds according to the invention can be converted into the stated administration forms. This can be done in a conventional manner by mixing with inert, non-toxic, pharmaceutically suitable excipients. These adjuvants include, among others. Excipients (for example microcrystalline cellulose, lactose, mannitol), solvents (for example liquid polyethylene glycols), emulsifiers and dispersants or wetting agents (for example sodium dodecyl sulfate, polyoxysorbitol oleate), binders (for example polyvinylpyrrolidone), synthetic and natural polymers (for example albumin), stabilizers ( For example, antioxidants such as ascorbic acid), dyes (eg, inorganic pigments such as iron oxides) and flavor and / or odoriferous.
Im Allgemeinen hat es sich als vorteilhaft erwiesen, bei parenteraler Applikation Mengen von etwa 0.001 bis 1 mg/kg, vorzugsweise etwa 0.01 bis 0.5 mg/kg Körpergewicht zur Erzielung wirksamer Ergebnisse zu verabreichen. Bei oraler Applikation beträgt die Dosierung etwa 0.01 bis 100 mg/kg, vorzugsweise etwa 0.01 bis 20 mg/kg und ganz besonders bevorzugt 0.1 bis 10 mg/kg Körpergewicht.
Trotzdem kann es gegebenenfalls erforderlich sein, von den genannten Mengen abzuweichen, und zwar in Abhängigkeit von Körpergewicht, Applikationsweg, individuellem Verhalten gegenüber dem Wirkstoff, Art der Zubereitung und Zeitpunkt bzw. Intervall, zu welchem die Applikation erfolgt. So kann es in einigen Fällen ausreichend sein, mit weniger als der vorgenannten Mindestmenge auszu- kommen, während in anderen Fällen die genannte obere Grenze überschritten werden muss. Im Falle der Applikation größerer Mengen kann es empfehlenswert sein, diese in mehreren Einzelgaben über den Tag zu verteilen. In general, it has proven to be advantageous, when administered parenterally, to administer amounts of about 0.001 to 1 mg / kg, preferably about 0.01 to 0.5 mg / kg of body weight, in order to achieve effective results. When administered orally, the dosage is about 0.01 to 100 mg / kg, preferably about 0.01 to 20 mg / kg and most preferably 0.1 to 10 mg / kg of body weight. Nevertheless, it may be necessary to deviate from the stated amounts, depending on body weight, route of administration, individual behavior towards the active ingredient, type of preparation and time or interval at which the application is carried out. Thus, in some cases it may be sufficient to make do with less than the aforementioned minimum quantity, while in other cases the above upper limit must be exceeded. In the case of the application of larger quantities, it may be advisable to distribute these in several single doses throughout the day.
Die nachfolgenden Ausführungsbeispiele erläutern die Erfindung. Die Erfindung ist nicht auf die Beispiele beschränkt. Die Prozentangaben in den folgenden Tests und Beispielen sind, sofern nicht anders angegeben, Gewichtsprozente; Teile sind Gewichtsteile. Lösungsmittelverhältnisse, Verdünnungsverhältnisse und Konzentrationsangaben von flüssig/flüssig-Lösungen beziehen sich jeweils auf das Volumen.
The following embodiments illustrate the invention. The invention is not limited to the examples. The percentages in the following tests and examples are by weight unless otherwise indicated; Parts are parts by weight. Solvent ratios, dilution ratios and concentration data of liquid / liquid solutions are based on volume.
A. Beispiele A. Examples
Abkürzungen und Akronyme: aq. wässrige Lösung Abbreviations and acronyms: aq. Aqueous solution
ber. Berechnet Calculated
DCI direkte chemische Ionisation (bei MS) DCI direct chemical ionization (in MS)
DMF Dimethylformamid DMF dimethylformamide
DMSO Dimethylsulfoxid DMSO dimethyl sulfoxide
d. Th. der Theorie (bei Ausbeute) d. Th. Of theory (at yield)
eq. Äquivalent(e) eq. Equivalent (s)
ESI Elektrospray-Ionisation (bei MS) ESI electrospray ionization (in MS)
Et Ethyl Et ethyl
gef. Gefunden gef. Found
h Stunde(n) h hour (s)
HPLC Hochdruck-, Hochleistungsflüssigchromatographie HPLC high pressure, high performance liquid chromatography
HRMS hochaufgelöste Massenspektrometrie HRMS high-resolution mass spectrometry
konz. konzentriert conc. concentrated
LC/MS Flüssigchromatographie-gekoppelte Massenspektrometrie LC / MS liquid chromatography-coupled mass spectrometry
LiHMDS Lithiumhexamethyldisilazid LiHMDS lithium hexamethyldisilazide
Me Methyl Me methyl
min Minute(n) min minute (s)
MS Massenspektrometrie MS mass spectrometry
NMR Kernresonanzspektrometrie NMR nuclear magnetic resonance spectrometry
Pd2dba3 Tris-(dibenzylidenaceton)-dipalladium Pd2dba3 tris (dibenzylideneacetone) dipalladium
Ph Phenyl Ph phenyl
RT Raumtemperatur RT room temperature
Rt Retentionszeit (bei HPLC) R t retention time (by HPLC)
THF Tetrahydrofuran THF tetrahydrofuran
UV Ultraviolett-Spektrometrie UV ultraviolet spectrometry
v/v Volumen zu Volumen- Verhältnis (einer Lösung)v / v volume to volume ratio (of a solution)
XPHOS Dicyclohexyl-(2',4',6'-triisopropylbiphenyl-2-yl)-phosphin
LC/MS-Methoden: XPHOS dicyclohexyl- (2 ', 4', 6'-triisopropylbiphenyl-2-yl) -phosphine LC / MS methods:
Methode 1 (LC-MS): Method 1 (LC-MS):
Instrament: Waters ACQUITY SQD UPLC System; Säule: Waters Acquity UPLC HSS T3 1,8μ 50 x 1mm; Eluent A: 1 1 Wasser + 0.25 ml 99%ige Ameisensäure, Eluent B: 1 1 Acetonitril + 0.25 ml 99%ige Ameisensäure; Gradient: 0.0 min 90% A -> 1.2 min 5% A -> 2.0 min 5% A; Ofen: 50°C; Fluss: 0.40 ml/min; UV-Detektion: 210 - 400 nm. Instrament: Waters ACQUITY SQD UPLC System; Column: Waters Acquity UPLC HSS T3 1,8μ 50 x 1mm; Eluent A: 1 l of water + 0.25 ml of 99% formic acid, eluent B: 1 l of acetonitrile + 0.25 ml of 99% formic acid; Gradient: 0.0 min 90% A -> 1.2 min 5% A -> 2.0 min 5% A; Oven: 50 ° C; Flow: 0.40 ml / min; UV detection: 210 - 400 nm.
Methode 2 (LC-MS): Method 2 (LC-MS):
Instrument MS: Waters, Instrument HPLC: Waters (Säule Phenomenex Luna 5μ C18(2) 100A, AXIA Tech. 50 x 21.2 mm, Eluent A: Wasser + 0.05% Ameisensäure, Eluent B: Acetonitril (ULC) + 0.05% Ameisensäure, Gradient: 0.0 min 95%A - 0.15 min 95%A - 8.0 min 5%A - 9.0 min 5%A; Fluss: 40 ml/min; UV-Detektion: DAD; 210 - 400 nm). Instrument MS: Waters, Instrument HPLC: Waters (column Phenomenex Luna 5μ C18 (2) 100A, AXIA Tech 50 x 21.2 mm, eluent A: water + 0.05% formic acid, eluent B: acetonitrile (ULC) + 0.05% formic acid, gradient : 0.0 min 95% A - 0.15 min 95% A - 8.0 min 5% A - 9.0 min 5% A, flow: 40 ml / min, UV detection: DAD, 210 - 400 nm).
Methode 3 (LC-MS): Method 3 (LC-MS):
Instrument MS: Waters, Instrument HPLC: Waters (Säule Phenomenex Luna 5μ C18(2) 100A, AXIA Tech. 50 x 21.2 mm, Eluent A: Wasser + 0.05% Ameisensäure, Eluent B: Acetonitril (ULC) + 0.05% Ameisensäure, Gradient: 0.0 min 95%A - 0.15 min 95%A - 8.0 min 5%A - 9.0 min 5%A; Fluss: 40 ml/min; UV-Detektion: DAD; 210 - 400 nm). Instrument MS: Waters, Instrument HPLC: Waters (column Phenomenex Luna 5μ C18 (2) 100A, AXIA Tech 50 x 21.2 mm, eluent A: water + 0.05% formic acid, eluent B: acetonitrile (ULC) + 0.05% formic acid, gradient : 0.0 min 95% A - 0.15 min 95% A - 8.0 min 5% A - 9.0 min 5% A, flow: 40 ml / min, UV detection: DAD, 210 - 400 nm).
Methode 4 (LC-MS): LC-MS-Analytik-Methode SCDK SQD SB AQ: Method 4 (LC-MS): LC-MS Analytical Method SCDK SQD SB AQ:
Instrument MS: Waters SQD; Instrument HPLC: Waters UPLC; Säule: Zorbax SB-Aq (Agilent), 50 mm x 2.1 mm, 1.8 μιη; Eluent A: Wasser + 0.025%> Ameisensäure, Eluent B: Acetonitril (ULC) + 0.025% Ameisensäure; Gradient: 0.0 min 98%A - 0.9 min 25%A - 1.0 min 5%A - 1.4 min 5%A - 1.41 min 98%A - 1.5 min 98%A; Ofen: 40°C; Fluss: 0.600 ml/min; UV-Detektion: DAD; 210 nm.
Instrument MS: Waters SQD; Instrument HPLC: Waters UPLC; Column: Zorbax SB-Aq (Agilent), 50 mm x 2.1 mm, 1.8 μιη; Eluent A: water + 0.025%> formic acid, eluent B: acetonitrile (ULC) + 0.025% formic acid; Gradient: 0.0 min 98% A - 0.9 min 25% A - 1.0 min 5% A - 1.4 min 5% A - 1.41 min 98% A - 1.5 min 98% A; Oven: 40 ° C; Flow: 0.600 ml / min; UV detection: DAD; 210 nm.
Ausgangsverbindungen und Intermediate: Starting compounds and intermediates:
Beispiel 1A Example 1A
1 -(2-Fluorbenzyl)- 1 H-pyrazolo [3 ,4-b]pyridin-3 -carbonsäure 1 - (2-fluorobenzyl) -1 H -pyrazolo [3,4-b] pyridine-3-carboxylic acid
Die Synthese dieser Verbindung ist beschrieben in WO 2007/124854 Beispiel 29A Stufe a).
The synthesis of this compound is described in WO 2007/124854 Example 29A step a).
Ausführungsbeispiele : Beispiel 1 Exemplary embodiments: Example 1
N-(5-Cyclopropyl- 1 ,3 ,4-thiadiazol-2-yl)- 1 -(2-fluorbenzyl)- 1 H-pyrazolo [3 ,4-b]pyridin-3 -carboxamid N- (5-Cyclopropyl-1,3,3,4-thiadiazol-2-yl) -1- (2-fluorobenzyl) -1 H -pyrazolo [3,4-b] pyridine-3-carboxamide
500 mg (1.843 mmol) Beispiel 1A wurden in 5 ml Dichlormethan und 5 ml Dimethylformamid vorgelegt und anschliessend mit 312 mg (2.212 mmol) 2-Amino-5-cyclopropyl-l,3,4-thiadiazol, 530 mg (2.765 mmol) l-(3-Dimethylaminopropyl)-3-ethylcarbodiimid-Hydrochlorid und 0.963 ml (5.530 mmol) Ν,Ν-Diisopropylethylamin versetzt. Danach wurde 1.5 Stunden bei Raumtemperatur gerührt. Es wurde dann mit Wasser und Dichlormethan verdünnt und die Phasen separiert. Die wässrige Phase wurde zweimal mit Dichlormethan extrahiert und die vereinigten organischen Phasen wurden eingeengt. Weitere Reinigung erfolgte mittels präparativer HPLC (Acetonitril: Wasser (+0.05 % Ameisensäure) - Gradient). Es wurden 146 mg der Zielverbindung erhalten (19 % d. Th.). 500 mg (1.843 mmol) of Example 1A were initially charged in 5 ml of dichloromethane and 5 ml of dimethylformamide and then with 312 mg (2.212 mmol) of 2-amino-5-cyclopropyl-l, 3,4-thiadiazole, 530 mg (2.765 mmol) - (3-dimethylaminopropyl) -3-ethylcarbodiimide hydrochloride and 0.963 ml (5.530 mmol) of Ν, Ν-diisopropylethylamine. Thereafter, it was stirred for 1.5 hours at room temperature. It was then diluted with water and dichloromethane and the phases separated. The aqueous phase was extracted twice with dichloromethane and the combined organic phases were concentrated. Further purification was carried out by preparative HPLC (acetonitrile: water (+0.05% formic acid) gradient). 146 mg of the target compound were obtained (19% of theory).
LC-MS (Methode 1): Rt = 1.08 min; MS (ESIpos): m/z = 395 (M+H)+. LC-MS (Method 1): R t = 1.08 min; MS (ESIpos): m / z = 395 (M + H) + .
'H-NMR (400 MHz, DMSO-de): δ [ppm] = 1.00-1.04 (m, 2H), 1.13-1.21 (m, 2H), 2.40-2.47 (m, 1H), 5.88 (s, 2H), 7.16 (t, 1H), 7.24 (t, 1H), 7.34-7.40 (m, 2H), 7.49 (dd, 1H), 8.59 (dd, 1H), 8.72 (dd, 1H), 12.91 (s br, 1H). 'H-NMR (400 MHz, DMSO-de): δ [ppm] = 1.00-1.04 (m, 2H), 1.13-1.21 (m, 2H), 2.40-2.47 (m, 1H), 5.88 (s, 2H ), 7.16 (t, 1H), 7.24 (t, 1H), 7.34-7.40 (m, 2H), 7.49 (dd, 1H), 8.59 (dd, 1H), 8.72 (dd, 1H), 12.91 (s br , 1H).
Beispiel 2 Example 2
N- [5-(2-Ethoxyethyl)- 1 ,3 ,4-thiadiazol-2-yl] - 1 -(2-fluorbenzyl)- 1 H-pyrazolo [3 ,4-b]pyridine-3 - carboxamid
N- [5- (2-Ethoxyethyl) -1,3,4-thiadiazol-2-yl] -1- (2-fluorobenzyl) -1 H -pyrazolo [3,4-b] pyridine-3-carboxamide
H3C H 3 C
17 mg (0.1 mmol) 5-(2-Ethoxyethyl)-l,3,4-thiadiazol-2-amin wurden in einem well einer 96er Multititerplatte vorgelegt und mit 0.2 ml DMSO versetzt. Zu dieser Lösung wurden 27.1 mg (0.1 mmol) l-(2-Fluorbenzyl)-lH-pyrazolo [3 ,4-b]pyridin-3 -carbonsäure und O-(Benzotriazol-l-yl)- N,N,N',N'-tetramethyluronium-tetrafluoroborat (TBTU), jeweils gelöst in 0.2 ml DMSO, sowie 20 mg (0.2 mmol) 4-Methylmorpholin gegeben. Die Multititerplatte wurde abgedeckt und bei Raumtemperatur 18 h geschüttelt. Dann wurde ab filtriert und das Filtrat direkt via präparativer LC- MS nach Methode 2 oder 3 gereinigt. Die produkthaltigen Fraktionen wurden mittels Zentrifugaltrockner im Vakuum eingeengt. Der Rückstand der einzelnen Fraktionen wurde in je 0.6 ml DMSO gelöst und vereint. Anschließend wurde im Zentrifugaltrockner das Lösungsmittel vollständig abgedampft. Es wurden 4.2 mg (10% d. Th.) der Titelverbindung erhalten. 17 mg (0.1 mmol) of 5- (2-ethoxyethyl) -1,3,4-thiadiazol-2-amine were placed in a well of a 96-well multititer plate and treated with 0.2 ml of DMSO. To this solution was added 27.1 mg (0.1 mmol) of 1- (2-fluorobenzyl) -1H-pyrazolo [3,4-b] pyridine-3-carboxylic acid and O- (benzotriazol-1-yl) -N, N, N '. , N'-tetramethyluronium tetrafluoroborate (TBTU), each dissolved in 0.2 ml of DMSO, and 20 mg (0.2 mmol) of 4-methylmorpholine. The multi-well plate was covered and shaken at room temperature for 18 h. It was then filtered off and the filtrate purified directly via preparative LC-MS method 2 or 3. The product-containing fractions were concentrated by means of a centrifugal dryer in vacuo. The residue of the individual fractions was dissolved in 0.6 ml of DMSO and combined. Subsequently, the solvent was completely evaporated in a centrifugal dryer. There were obtained 4.2 mg (10% of theory) of the title compound.
LC-MS (Methode 4): Rt = 1.19 min; MS (ESIpos): m/z = 427 (M+H)+, LC-MS (Method 4): R t = 1.19 min; MS (ESIpos): m / z = 427 (M + H) + ,
In Analogie zu Beispiel 2 wurden die in Tabelle 1 aufgeführten Beispiele durch Umsetzung von hergestellt.
In analogy to Example 2, the examples listed in Table 1 were prepared by reaction of.
Tabelle 1 Table 1
Bei diesen Ansätzen wurde in der Reaktion DMF statt DMSO als Lösungsmittel und HATU (N- In these reactions, DMF was used in the reaction instead of DMSO as the solvent and HATU (N-
[(Dimethylamino)(3H- [ 1 ,2,3 Jtriazolo [4,5-b]pyridin-3 -yloxy)methyliden] -N-methylmethanaminium hexafluorophosphat) anstelle von TBTU als Kupplungsreagenz verwendet. [(Dimethylamino) (3H- [1,2,3-jtriazolo [4,5-b] pyridine-3-yloxy) methylidene] -N-methylmethanaminium hexafluorophosphate) was used instead of TBTU as coupling reagent.
Beispiel 14 Example 14
N- [5-(Dimethylsulfamoyl)-4-methyl- 1 ,3 -thiazol-2-yl] - 1 -(2-fluorbenzyl)- 1 H-pyrazolo [3 ,4-b]pyridin- 3 -carboxamid
N- [5- (Dimethylsulfamoyl) -4-methyl-1,3-thiazol-2-yl] -1- (2-fluorobenzyl) -1 H -pyrazolo [3,4-b] pyridine-3-carboxamide
22 mg (0.1 mmol) 2-Amino-N,N,4-trimethyl-l,3-thiazol-5-sulfonamid wurden in einem well einer 96er Multititerplatte vorgelegt. Es wurden 27.1 mg (0.1 mmol) N-[(Dimethylamino)(3H- [ 1 ,2,3 Jtriazolo [4,5-b]pyridin-3 -yloxy)methyliden] -N-methylmethanaminium-Hexafluorophosphat HATU) und l-(2-Fluorbenzyl)-lH-pyrazolo [3 ,4-b]pyridin-3 -carbonsäure, jeweils gelöst in 0.3 ml DMF, sowie 32 mg (0.4 mmol) Pyridin zugegeben. Die Multititerplatte wurde abgedeckt und bei Raumtemperatur 18 h geschüttelt. Dann wurde abfiltriert und das Filtrat direkt via präparativer LC- MS nach Methode 2 oder 3 gereinigt. Die produkthaltigen Fraktionen wurden mittels Zentrifugaltrockner im Vakuum eingeengt. Der Rückstand der einzelnen Fraktionen wurde in je 0.6 ml DMSO gelöst und vereint. Anschließend wurde im Zentrifugaltrockner das Lösungsmittel vollständig abgedampft. Es wurden 5.80 mg (12% d. Th.) der Titelverbindung erhalten. 22 mg (0.1 mmol) of 2-amino-N, N, 4-trimethyl-l, 3-thiazole-5-sulfonamide were placed in a well of a 96-well multititer plate. 27.1 mg (0.1 mmol) of N - [(dimethylamino) (3H- [1,2,3-jtriazolo [4,5-b] pyridine-3-oxy) methylidene] -N-methylmethanaminium hexafluorophosphate HATU) and (2-fluorobenzyl) -LH-pyrazolo [3,4-b] pyridine-3-carboxylic acid, each dissolved in 0.3 ml of DMF, and 32 mg (0.4 mmol) of pyridine was added. The multi-well plate was covered and shaken at room temperature for 18 h. It was then filtered off and the filtrate was purified directly via preparative LC-MS according to Method 2 or 3. The product-containing fractions were concentrated by means of a centrifugal dryer in vacuo. The residue of the individual fractions was dissolved in 0.6 ml of DMSO and combined. Subsequently, the solvent was completely evaporated in a centrifugal dryer. 5.80 mg (12% of theory) of the title compound were obtained.
LC/MS (Methode 4): Rt = 1.24 min LC / MS (Method 4): R t = 1.24 min
MS (ESIpos): m/z = 475 MS (ESIpos): m / z = 475
In Analogie zu Beispiel 14 wurden die in Tabelle 2 aufgeführten Verbindungen hergestellt.
In analogy to Example 14, the compounds listed in Table 2 were prepared.
Tabelle 2: Table 2:
N-(5-Ethyl-l,3,4-oxadiazol-2-yl)-l-(2-fluorbenzyl)-lH-pyrazolo[3,4-b]pyridin-3-carboxamid
N- (5-ethyl-l, 3,4-oxadiazol-2-yl) -l- (2-fluorobenzyl) -lH-pyrazolo [3,4-b] pyridine-3-carboxamide
150 mg (0.553 mmol) der Verbindung aus Beispiel 1A wurden in 5 ml Dimethylformamid vorgelegt und anschließend mit 284 mg (0.664 mmol) N-[({[(lZ)-l-Cyan-2-ethoxy-2- oxoethyliden]amino}oxy)(mo^holin-4-yl)methylen]-N-methylmethanaminiumhexafluorophosphat (COMU) sowie 187 mg (1.327 mmol) 2,2,6,6-Tetramethylpiperidin versetzt. Danach wurde 5 Min. bei Raumtemperatur gerührt und 75 mg (0.664 mmol) 5-Ethyl-l,3,4-oxadiazol-2-yl-amin (beschrieben in WO 00/63166, Beispiel 48b) addiert. Es wurde für 3 d bei Raumtemperatur gerührt, mit 10 ml IN wässriger Salzsäure versetzt und mit Essigsäureethylester extrahiert. Die vereinigten organischen Phasen wurden über Natriumsulfat getrocknet und am Rotationsverdampfer eingeengt. Der Rückstand wurde mittels präparativer HPLC (Eluent: Wasser/Acetonitril/Wasser + 1% Trifluoressigsäure, Gradient 55:40:5 — »■ 0:95:5) gereinigt. Es wurden 20 mg (10% d. Th.) der Titelverbindung erhalten. 150 mg (0.553 mmol) of the compound from Example 1A were initially charged in 5 ml of dimethylformamide and then with 284 mg (0.664 mmol) of N - [({[(LZ) -l-cyano-2-ethoxy-2-oxoethylidene] amino} oxy) (molybin-4-yl) methylene] -N-methylmethanaminium hexafluorophosphate (COMU) and 187 mg (1.327 mmol) of 2,2,6,6-tetramethylpiperidine. Thereafter, it was stirred for 5 min. At room temperature and 75 mg (0.664 mmol) of 5-ethyl-l, 3,4-oxadiazol-2-yl-amine (described in WO 00/63166, Example 48b) added. The mixture was stirred for 3 d at room temperature, treated with 10 ml of 1N aqueous hydrochloric acid and extracted with ethyl acetate. The combined organic phases were dried over sodium sulfate and concentrated on a rotary evaporator. The residue was purified by preparative HPLC (eluent: water / acetonitrile / water + 1% trifluoroacetic acid, gradient 55: 40: 5 → 0.95 : 5). 20 mg (10% of theory) of the title compound were obtained.
LC-MS (Methode 1): Rt = 0.93 min; MS (ESIpos): m/z = 367 (M+H)
LC-MS (Method 1): R t = 0.93 min; MS (ESIpos): m / z = 367 (M + H)
B. Bewertung der pharmakologischen Wirksamkeit B. Evaluation of Pharmacological Activity
Die pharmakologische Wirkung der erfindungsgemäßen Verbindungen kann in folgenden Assays gezeigt werden: The pharmacological activity of the compounds according to the invention can be demonstrated in the following assays:
B-l . Gef ßrelaxierende Wirkung in vitro Kaninchen werden durch Nackenschlag betäubt und entblutet. Die Aorta wird entnommen, von anhaftendem Gewebe befreit, in 1.5 mm breite Ringe geteilt und einzeln unter einer Vorspannung in 5 ml-Organbäder mit 37°C warmer, Carbogen-begaster Krebs-Henseleit-Lösung folgender Zusammensetzung gebracht (jeweils mM): Natriumchlorid: 119; Kaliumchlorid: 4.8; Calciumchlorid- Dihydrat: 1 ; Magnesiumsulfat-Heptahydrat: 1.4; Kaliumdihydrogenphosphat: 1.2; Natriumhydrogencarbonat: 25; Glucose: 10. Die Kontraktionskraft wird mit Statham UC2-Zellen erfasst, verstärkt und über A/D-Wandler (DAS- 1802 HC, Keithley Instruments München) digitalisiert sowie parallel auf Linienschreiber registriert. Zur Erzeugung einer Kontraktion wird Phenylephrin dem Bad kumulativ in ansteigender Konzentration zugesetzt. Nach mehreren Kontrollzyklen wird die zu untersuchende Substanz in jedem weiteren Durchgang in jeweils steigender Dosierung zugesetzt und die Höhe der Kontraktion mit der Höhe der im letzten Vordurchgang erreichten Kontraktion verglichen. Daraus wird die Konzentration errechnet, die erforderlich ist, um die Höhe des Kontrollwertes um 50% zu reduzieren (ICso-Wert). Das Standardapplikationsvolumen beträgt 5 μΐ, der DMSO-Anteil in der Badlösung entspricht 0.1%. B-l. Vascular Relaxation In Vitro Rabbits are stunned and bled by a stroke of the neck. The aorta is harvested, detached from adherent tissue, divided into 1.5 mm wide rings and placed individually under bias in 5 ml organ baths with 37 ° C warm, carbogen-fumed Krebs-Henseleit solution of the following composition (in each case mM): Sodium chloride: 119; Potassium chloride: 4.8; Calcium chloride dihydrate: 1; Magnesium sulfate heptahydrate: 1.4; Potassium dihydrogen phosphate: 1.2; Sodium hydrogencarbonate: 25; Glucose: 10. The force of contraction is detected with Statham UC2 cells, amplified and digitized via A / D converter (DAS-1802 HC, Keithley Instruments Munich) and registered in parallel on a chart recorder. To create a contraction, phenylephrine is added cumulatively to the bath in increasing concentration. After several control cycles, the substance to be examined is added in each subsequent course in increasing dosages and the height of the contraction is compared with the height of the contraction achieved in the last predistortion. This is used to calculate the concentration required to reduce the level of the control value by 50% (IC50 value). The standard application volume is 5 μΐ, the DMSO content in the bath solution corresponds to 0.1%.
Repräsentative IC50- Werte für die erfindungsgemäßen Verbindungen sind in der nachstehenden Tabelle (Tabelle 1) wiedergegeben: Representative IC50 values for the compounds according to the invention are given in the table below (Table 1):
Tabelle 1 : Table 1 :
Beispiel Nr. IC50 [nM] Example No. IC50 [nM]
1 767 1 767
2 363 2 363
4 1000 4 1000
7 602 7 602
9 805
Beispiel Nr. IC50 [nM] 9,805 Example No. IC 50 [nM]
10 345 10,345
11 454 11 454
13 1990 13 1990
B-2. Wirkung an rekombinanter Guanylatcyclase-Reporterzelllinie B-2. Effect on recombinant guanylate cyclase reporter cell line
Die zelluläre Wirkung der erfindungsgemäßen Verbindungen wird an einer rekombinanten Guanylat- cyclase-Reporterzelllinie, wie in F. Wunder et al., Anal. Biochem.339, 104-112 (2005) beschrieben, bestimmt. The cellular activity of the compounds of the invention is measured on a recombinant guanylate cyclase reporter cell line as described in F. Wunder et al., Anal. Biochem.339, 104-112 (2005).
Repräsentative Werte (ECso-Werte) für die erfindungsgemäßen Verbindungen sind in der nachstehenden Tabelle (Tabelle 2) wiedergegeben: Representative values (EC 50 values) for the compounds according to the invention are given in the table below (Table 2):
Tabelle 2 Table 2
Beispiel Nr. MECso [μΜ] Beispiel Nr. MECso [μΜ] Example No. MECso [μΜ] Example No. MECso [μΜ]
1 0.3 16 3.0 1 0.3 16 3.0
2 0.3 17 3.0 2 0.3 17 3.0
3 1.0 18 1.0 3 1.0 18 1.0
4 1.0 19 1.0 4 1.0 19 1.0
5 1.0 20 1.0 5 1.0 20 1.0
6 1.0 21 1.0 6 1.0 21 1.0
7 0.3 22 1.0 7 0.3 22 1.0
8 0.3 23 1.0 8 0.3 23 1.0
9 1.0 24 1.0 9 1.0 24 1.0
10 0.3 25 1.0 10 0.3 25 1.0
11 0.3 26 1.0 11 0.3 26 1.0
12 0.3 27 1.0 12 0.3 27 1.0
13 1.0 28 1.0 13 1.0 28 1.0
14 3.0 29 1.0 14 3.0 29 1.0
15 3.0 30 1.0
Beispiel Nr. MECso [μΜ] Beispiel Nr. MECso [μΜ] 15 3.0 30 1.0 Example No. MECso [μΜ] Example No. MECso [μΜ]
31 1 .0 43 1 .0 31 1 .0 43 1 .0
32 1 .0 44 1 .0 32 1 .0 44 1 .0
33 1 .0 45 1 .0 33 1 .0 45 1 .0
34 1 .0 46 1 .0 34 1 .0 46 1 .0
35 1 .0 47 1 .0 35 1 .0 47 1 .0
36 1 .0 48 1 .0 36 1 .0 48 1 .0
37 1 .0 49 1 .0 37 1 .0 49 1 .0
38 1 .0 50 1 .0 38 1 .0 50 1 .0
39 1 .0 51 1 .0 39 1 .0 51 1 .0
40 1 .0 52 1 .0 40 1 .0 52 1 .0
41 1 .0 53 3.0 41 1 .0 53 3.0
42 1 .0 42 1 .0
B-3. Radiotelemetrische Blutdruckmessung an wachen, spontan hypertensiven Ratten B-3. Radiotelemetric blood pressure measurement on awake, spontaneously hypertensive rats
Für die im Folgenden beschriebene Blutdruckmessung an wachen Ratten wird ein im Handel erhältliches Telemetriesystem der Firma DATA SCIENCES INTERNATIONAL DSI, USA eingesetzt. A commercially available telemetry system from DATA SCIENCES INTERNATIONAL DSI, USA is used for the blood pressure measurement on awake rats described below.
Das System besteht aus 3 Hauptkomponenten: The system consists of 3 main components:
Implantierbare Sender (Physiotel® Telemetrietransmitter) Implantable transmitters (Physiotel® telemetry transmitters)
Empfänger (Physiotel® Receiver), die über einen Multiplexer (DSI Data Exchange Matrix ) mit einem Datenakquisitionscomputer verbunden sind. Receivers (Physiotel® receivers) connected to a data acquisition computer through a multiplexer (DSI Data Exchange Matrix).
Die Telemetrieanlage ermöglicht eine kontinuierliche Erfassung von Blutdruck Herzfrequenz und Körperbewegung an wachen Tieren in ihrem gewohnten Lebensraum. The telemetry system allows a continuous recording of blood pressure heart rate and body movement on awake animals in their habitual habitat.
Tiermaterial Die Untersuchungen werden an ausgewachsenen weiblichen spontan hypertensiven Ratten (SHR Okamoto) mit einem Körpergewicht von >200 g durchgeführt. SHR/NCrl von Okamoto Kyoto
School of Medicine, 1963 wurden aus männlichen Wistar Kyoto Ratten mit stark erhöhtem Blutdruck und weiblichen mit leicht erhöhtem Blutdruck gekreuzt und in der Fl 3 an die U.S. National Institutes of Health abgegeben. Animal material Investigations are carried out on adult female spontaneously hypertensive rats (SHR Okamoto) weighing> 200 g. SHR / NCrl from Okamoto Kyoto School of Medicine, 1963 were crossed male Wistar Kyoto rats with high blood pressure and female with slightly elevated blood pressure and delivered in the Fl 3 to the US National Institutes of Health.
Die Versuchstiere werden nach Senderimplantation einzeln in Makroion - Käfigen Typ 3 gehalten. Sie haben freien Zugang zu Standardfutter und Wasser. The experimental animals are kept individually in macroion cages type 3 after transmitter implantation. You have free access to standard food and water.
Der Tag - Nacht - Rhythmus im Versuchslabor wird per Raumbeleuchtung um 6:00 Uhr morgens und um 19:00 Uhr abends gewechselt. The day - night rhythm in the experimental laboratory is changed by room lighting at 6:00 in the morning and at 19:00 in the evening.
Senderimplantation transmitter implantation
Die eingesetzten Telemetriesender TAH PA - C40 werden den Versuchstieren mindestens 14 Tage vor dem ersten Versuchseinsatz unter aseptischen Bedingungen chirurgisch implantiert. Die so instrumentierten Tiere sind nach Abheilen der Wunde und Einwachsen des Implantats wiederholt einsetzbar. The TAH PA - C40 telemetry transmitters are surgically implanted into the experimental animals under aseptic conditions at least 14 days before the first trial. The animals so instrumented are repeatedly used after healing of the wound and ingrowth of the implant.
Zur Implantation werden die nüchternen Tiere mit Pentobabital (Nembutal, Sanofi: 50mg/kg i.p. ) narkotisiert und an der Bauchseite weiträumig rasiert und desinfiziert. Nach Eröffnung des Bauchraumes entlang der Linea alba wird der flüssigkeitsgefüllte Meßkatheter des Systems oberhalb der Bifurcation nach cranial in die Aorta descendens eingesetzt und mit Gewebekleber (VetBonD TM, 3M) befestigt. Das Sendergehäuse wird intraperitoneal an der Bauchwandmuskulatur fixiert und die Wunde wird schichtweise verschlossen. For implantation, the fasting animals are anesthetized with pentobabital (Nembutal, Sanofi: 50 mg / kg i.p.) and shaved and disinfected on the ventral side. After opening the abdominal cavity along the alba line, the system's fluid-filled measuring catheter above the bifurcation is inserted cranially into the descending aorta and secured with tissue adhesive (VetBonD ™, 3M). The transmitter housing is fixed intraperitoneally to the abdominal wall musculature and the wound is closed in layers.
Postoperativ wird zur Infektionsprophylaxe ein Antibiotikum verabreicht (Tardomyocel COMP Bayer 1ml/kg s.c.) Postoperatively, an antibiotic is administered for infection prevention (Tardomyocel COMP Bayer 1ml / kg s.c.)
Substanzen und Lösungen Substances and solutions
Wenn nicht anders beschrieben werden die zu untersuchenden Substanzen jeweils einer Gruppe von Tieren (n = 6 ) per Schlundsonde oral verabreicht. Entsprechend einem Applikationsvolumen von 5 ml/kg Körpergewicht werden die Testsubstanzen in geeigneten Lösungsmittelgemischen gelöst oder in 0.5 %-iger Tylose suspendiert. Unless otherwise described, the substances to be tested are each administered orally to a group of animals (n = 6) by gavage. According to an application volume of 5 ml / kg body weight, the test substances are dissolved in suitable solvent mixtures or suspended in 0.5% Tylose.
Eine Lösungsmittel- behandelte Gruppe von Tieren wird als Kontrolle eingesetzt. A solvent-treated group of animals is used as a control.
Versuchsablauf experimental procedure
Die vorhandene Telemetrie - Meßeinrichtung ist für 24 Tiere konfiguriert. Jeder Versuch wird unter einer Versuchsnummer registiert (VJahr Monat Tag).
Den in der Anlage lebenden instrumentierten Ratten ist jeweils eine eigene Empfangsantenne zugeordnet (1010 Receiver, DSI ). The existing telemetry measuring device is configured for 24 animals. Each trial is registered under a trial number (VYear month day). The instrumented rats living in the plant each have their own receiving antenna (1010 receivers, DSI).
Die implantierten Sender sind über einen eingebauten Magnetschalter von außen aktivierbar. Sie werden bei Versuchsvorlauf auf Sendung geschaltet. Die ausgestrahlten Signale können durch ein Datenakquisitionssystem (Dataquest TM A.R.T. for WINDOWS, DSI ) online erfasst und entsprechend aufgearbeitet werden. Die Ablage der Daten erfolgt jeweils in einem hierfür eröffneten Ordner der die Versuchsnummer trägt. The implanted transmitters can be activated externally via a built-in magnetic switch. They will be put on the air during the trial run. The emitted signals can be recorded online by a data acquisition system (Dataquest ™ A.R.T. for Windows, DSI) and processed accordingly. The storage of the data takes place in each case in a folder opened for this purpose which carries the test number.
Im Standardablauf werden über je 10 Sekunden Dauer gemessen: Systolischer Blutdruck (SBP) Diastolischer Blutdruck (DBP) Arterieller Mitteldruck (MAP) Herzfrequenz (HR) Aktivität (ACT). The standard procedure measures duration of 10 seconds each: Systolic blood pressure (SBP) Diastolic blood pressure (DBP) Arterial mean pressure (MAP) Heart rate (HR) Activity (ACT).
Die Messwerterfassung wird rechnergesteuert in 5 Minuten Abständen wiederholt. Die als Absolutwert erhobenen Quelldaten werden im Diagramm mit dem aktuell gemessenen Barometerdruck (Ambient Pressure Reference Monitor; APR-1) korrigiert und in Einzeldaten abgelegt. Weitere technische Details sind der umfangreichen Dokumentation der Herstellerfirma (DSI) zu entnehmen. The measured value acquisition is repeated computer-controlled in 5-minute intervals. The absolute value of the source data is corrected in the diagram with the currently measured barometric pressure (Ambient Pressure Reference Monitor, APR-1) and stored in individual data. Further technical details can be found in the extensive documentation of the manufacturer (DSI).
Wenn nicht anders beschrieben erfolgt die Verabreichung der Prüfsubstanzen am Versuchstag um 9.00 Uhr. Im Anschluss an die Applikation werden die oben beschriebenen Parameter 24 Stunden gemessen. Unless otherwise stated, the administration of the test substances will take place at 9 o'clock on the day of the experiment. Following the application, the parameters described above are measured for 24 hours.
Auswertung evaluation
Nach Versuchsende werden die erhobenen Einzeldaten mit der Analysis-Software (DATAQUEST TM A. R.T. TM ANALYSIS) sortiert. Als Leerwert werden hier 2 Stunden vor Applikation angenommen, so dass der selektierte Datensatz den Zeitraum von 7:00 Uhr am Versuchstag bis 9:00 Uhr am Folgetag umfasst. After the end of the test, the collected individual data are sorted with the analysis software (DATAQUEST TM A.RT. TM ANALYSIS). The blank value is assumed here 2 hours before application, so that the selected data record covers the period from 7:00 am on the day of the experiment to 9:00 am on the following day.
Die Daten werden über eine voreinstellbare Zeit durch Mittelwertbestimmung geglättet (15 Minuten Average) und als Textdatei auf einen Datenträger übertragen. Die so vorsortierten und
komprimierten Messwerte werden in Excel- Vorlagen übertragen und tabellarisch dargestellt. Die Ablage der erhobenen Daten erfolgt pro Versuchstag in einem eigenen Ordner, der die Versuchsnummer trägt. Ergebnisse und Versuchsprotokolle werden in Papierform nach Nummern sortiert in Ordnern abgelegt. Literatur The data is smoothed over a presettable time by averaging (15 minutes average) and transferred as a text file to a disk. The so presorted and compressed measured values are transferred to Excel templates and displayed in tabular form. The filing of the collected data takes place per experiment day in a separate folder that bears the test number. Results and test reports are sorted in folders and sorted by paper. literature
Klaus Witte, Kai Hu, Johanna Swiatek, Claudia Müssig, Georg Ertl and Björn Lemmer: Experimental heart failure in rats: effects on cardio vascular circadian rhythms and on myocardial ß- adrenergic signaling. Cardiovasc Res 47 (2): 203-405, 2000; Kozo Okamoto: Spontaneous hypertension in rats. Int Rev Exp Pathol 7: 227- 270, 1969; Maarten van den Buuse: Circadian Rhythms of Blood Pressure, Heart Rate, and Locomotor Activity in Spontaneously Hypertensive Rats as Measured With Radio-Telemetry. Physiology & Behavior 55(4): 783-787, 1994
Klaus Witte, Kai Hu, Johanna Swiatek, Claudia Müssig, Georg Ertl and Björn Lemmer: Experimental heart failure in rats: effects on cardiac vascular circadian rhythms and on myocardial beta-adrenergic signaling. Cardiovasc Res 47 (2): 203-405, 2000; Kozo Okamoto: Spontaneous hypertension in rats. Int Rev. Exp Pathol 7: 227-270, 1969; Maarten van den Buuse: Circadian Rhythms of Blood Pressure, Heart Rate, and Locomotor Activity in Spontaneously Hypertensive Rats as Measured with Radio Telemetry. Physiology & Behavior 55 (4): 783-787, 1994
C. Ausführungsbeispiele für pharmazeutische Zusammensetzungen C. Embodiments of Pharmaceutical Compositions
Die erfindungsgemäßen Verbindungen können folgendermaßen in pharmazeutische Zubereitungen überführt werden: The compounds according to the invention can be converted into pharmaceutical preparations as follows:
Tablette: Zusammensetzung: Tablet: composition:
100 mg der erfindungsgemäßen Verbindung, 50 mg Lactose (Monohydrat), 50 mg Maisstärke (nativ), 10 mg Polyvinylpyrrolidon (PVP 25) (Fa. BASF, Ludwigshafen, Deutschland) und 2 mg Magnesiumstearat. 100 mg of the compound according to the invention, 50 mg of lactose (monohydrate), 50 mg of corn starch (native), 10 mg of polyvinylpyrrolidone (PVP 25) (BASF, Ludwigshafen, Germany) and 2 mg of magnesium stearate.
Tablettengewicht 212 mg. Durchmesser 8 mm, Wölbungsradius 12 mm. Herstellung: Tablet weight 212 mg. Diameter 8 mm, radius of curvature 12 mm. production:
Die Mischung aus erfindungsgemäßer Verbindung, Lactose und Stärke wird mit einer 5%-igen Lösung (m/m) des PVPs in Wasser granuliert. Das Granulat wird nach dem Trocknen mit dem Magnesiumstearat 5 Minuten gemischt. Diese Mischung wird mit einer üblichen Tablettenpresse verpresst (Format der Tablette siehe oben). Als Richtwert für die Verpressung wird eine Presskraft von 15 kN verwendet. The mixture of compound of the invention, lactose and starch is granulated with a 5% solution (m / m) of the PVP in water. The granules are mixed after drying with the magnesium stearate for 5 minutes. This mixture is compressed with a conventional tablet press (for the tablet format see above). As a guideline for the compression, a pressing force of 15 kN is used.
Oral applizierbare Suspension: Orally administrable suspension:
Zusammensetzung: Composition:
1000 mg der erfindungsgemäßen Verbindung, 1000 mg Ethanol (96%), 400 mg Rhodigel® (Xanthan gum der Firma FMC, Pennsylvania, USA) und 99 g Wasser. Einer Einzeldosis von 100 mg der erfindungsgemäßen Verbindung entsprechen 10 ml orale Suspension. 1000 mg of the compound of the invention, 1000 mg of ethanol (96%), 400 mg of Rhodigel ® (xanthan gum of the firm FMC, Pennsylvania, USA) and 99 g of water. A single dose of 100 mg of the compound of the invention corresponds to 10 ml of oral suspension.
Herstellung: production:
Das Rhodigel wird in Ethanol suspendiert, die erfindungsgemäße Verbindung wird der Suspension zugefügt. Unter Rühren erfolgt die Zugabe des Wassers. Bis zum Abschluß der Quellung des Rhodigels wird ca. 6 h gerührt.
Oral applizierbare Lösung: The rhodigel is suspended in ethanol, the compound according to the invention is added to the suspension. While stirring, the addition of water. Until the completion of the swelling of Rhodigels is stirred for about 6 h. Orally administrable solution:
Zusammensetzung: Composition:
500 mg der erfindungsgemäßen Verbindung, 2.5 g Polysorbat und 97 g Polyethylenglycol 400. Einer Einzeldosis von 100 mg der erfindungsgemäßen Verbindung entsprechen 20 g orale Lösung. 500 mg of the compound according to the invention, 2.5 g of polysorbate and 97 g of polyethylene glycol 400. A single dose of 100 mg of the compound according to the invention correspond to 20 g of oral solution.
Herstellung: production:
Die erfindungsgemäße Verbindung wird in der Mischung aus Polyethylenglycol und Polysorbat unter Rühren suspendiert. Der Rührvorgang wird bis zur vollständigen Auflösung der erfindungsgemäßen Verbindung fortgesetzt. i.V. -Lösung: The compound of the invention is suspended in the mixture of polyethylene glycol and polysorbate with stirring. The stirring is continued until complete dissolution of the compound according to the invention. i.v. -Solution:
Die erfindungsgemäße Verbindung wird in einer Konzentration unterhalb der Sättigungslöslichkeit in einem physiologisch verträglichen Lösungsmittel (z.B. isotonische Kochsalzlösung, Glucoselösung 5% und/oder PEG 400-Lösung 30%) gelöst. Die Lösung wird steril filtriert und in sterile und pyrogenfreie Injektionsbehältnisse abgefüllt.
The compound of the invention is dissolved at a concentration below saturation solubility in a physiologically acceptable solvent (e.g., isotonic saline, 5% glucose solution, and / or 30% PEG 400 solution). The solution is sterile filtered and filled into sterile and pyrogen-free injection containers.
Claims
Patentansprüche claims
1. Verbindung der Formel (I) 1. Compound of formula (I)
U für O oder S steht, U stands for O or S,
V für CR3 oder N steht, wobei für Wasserstoff, Trifluormethyl, (Ci-C6)-Alkyl, (C3-C7)-Cycloalkyl, (Ci- C4)-Alkoxycarbonyl, Phenyl, Pyridyl, -NR5R6, -S(0)nR7, -S02NR8R9 oder - OR10 steht, worin (Ci-C6)-Alkyl mit 1 bis 3 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Hydroxy, Trifluormethoxy, (Ci-C i)-Alkoxy, Hydroxycarbonyl, (C1-C4)- Alkoxycarbonyl, (Ci-C4)-Alkoxycarbonylamino, Mono-(Ci-C4)-Alkylamino, Di-(Ci-C4)-Alkylamino, Phenyl, Phenoxy, 4- bis 7-gliedriges Heterocyclyl und 5- oder 6-gliedriges Heteroaryl substituiert sein kann, worin Phenyl mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Halogen, (Ci-C4)-Alkyl und (C1-C4)- Alkoxy substituiert sein kann, worin 4- bis 7-gliedriges Heterocyclyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, (Ci-C4)-Alkyl, Hydroxy, Oxo und (Ci-C4)-Alkoxy substituiert sein kann,
und worin Phenoxy und 5- oder 6-gliedriges Heteroaryl ihrerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Halogen, (Ci-C i)-Alkyl und (Ci-C4)-Alkoxy substituiert sein können, worin Phenyl und Pyridyl mit 1 bis 3 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Halogen, (Ci-C4)-Alkyl und (Ci-C4)-Alkoxy substituiert sein können, und worin n für eine Zahl 0, 1 oder 2 steht, V represents CR 3 or N, wherein for hydrogen, trifluoromethyl, (C 1 -C 6 ) -alkyl, (C 3 -C 7 ) -cycloalkyl, (C 1 -C 4 ) -alkoxycarbonyl, phenyl, pyridyl, -NR 5 R 6 , -S (O) n R 7 , -SO 2 NR 8 R 9 or - OR 10 , wherein (Ci-C6) alkyl having 1 to 3 substituents independently selected from the group fluorine, trifluoromethyl, hydroxy, trifluoromethoxy , (C 1 -C 4) -alkoxy, hydroxycarbonyl, (C 1 -C 4) -alkoxycarbonyl, (C 1 -C 4) -alkoxycarbonylamino, mono (C 1 -C 4) -alkylamino, di- (C 1 -C 4) -alkylamino, phenyl, Phenoxy, 4- to 7-membered heterocyclyl and 5- or 6-membered heteroaryl may be substituted, wherein phenyl having 1 or 2 substituents independently selected from the group halogen, (Ci-C4) alkyl and (C1-C4) - Alkoxy can be substituted, wherein 4- to 7-membered heterocyclyl in turn with 1 or 2 substituents independently selected from the group fluorine, (Ci-C 4 ) alkyl, hydroxy, oxo and (Ci-C 4 ) alkoxy substituted can and wherein phenoxy and 5- or 6-membered heteroaryl may in turn be substituted with 1 or 2 substituents independently selected from the group consisting of halogen, (Ci-C i) -alkyl and (Ci-C4) -alkoxy, wherein phenyl and pyridyl with 1 to 3 substituents independently of one another can be substituted from the group halogen, (C 1 -C 4) -alkyl and (C 1 -C 4) -alkoxy, and in which n is a number 0, 1 or 2,
R5 für Wasserstoff oder (Ci-C6)-Alkyl steht, worin (Ci-C6)-Alkyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Hydroxy, Trifluormethoxy und (Ci-C4)-Alkoxy substituiert sein kann, R 5 is hydrogen or (Ci-C 6 ) alkyl, wherein (Ci-C6) alkyl in turn with 1 or 2 substituents independently selected from the group fluorine, trifluoromethyl, hydroxy, trifluoromethoxy and (Ci-C4) alkoxy may be substituted
R6 für Wasserstoff, (Ci-C6)-Alkyl, (C3-C7)-Cycloalkyl, Phenyl oder 4- bis 7-gliedriges Heterocyclyl steht, worin Phenyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Halogen, (Ci-C4)-Alkyl, (Ci-C4)-Alkoxy und (Ci-C4)-Alkylcarbonylamino substituiert sein kann, oder R 6 is hydrogen, (Ci-C 6 ) alkyl, (C 3 -C 7 ) -cycloalkyl, phenyl or 4- to 7-membered heterocyclyl, wherein phenyl in turn having 1 or 2 substituents independently selected from the group halogen , (C 1 -C 4) -alkyl, (C 1 -C 4) -alkoxy and (C 1 -C 4) -alkylcarbonylamino, or
R5 und R6 bilden zusammen mit dem Stickstoffatom, an das sie gebunden sind, einen 4- bis 7-gliedrigen Heterocyclus, worin 4- bis 7-gliedriger Heterocyclus mit (Ci-C4)-Alkyl substituiert sein kann, worin (Ci-C4)-Alkyl mit Hydroxy substituiert sein kann,
R7 für (Ci-C6)-Alkyl, (C3-C7)-Cycloalkyl oder Phenyl steht, R8 für Wasserstoff oder (Ci-C6)-Alkyl steht, R9 für Wasserstoff, (Ci-C6)-Alkyl oder (C3-C7)-Cycloalkyl steht, R10 für (Ci-C6)-Alkyl, (C3-C7)-Cycloalkyl oder Phenyl steht, für CR4 oder N steht, wobei R 5 and R 6 together with the nitrogen atom to which they are attached form a 4- to 7-membered heterocycle in which 4- to 7-membered heterocycle may be substituted by (C 1 -C 4) -alkyl, in which C4) -alkyl may be substituted by hydroxy, R 7 is (C 1 -C 6 ) -alkyl, (C 3 -C 7 ) -cycloalkyl or phenyl, R 8 is hydrogen or (C 1 -C 6 ) -alkyl, R 9 is hydrogen, (C 1 -C 6 ) -Alkyl or (C 3 -C 7 ) -cycloalkyl, R 10 is (Ci-C 6 ) -alkyl, (C 3 -C 7 ) -cycloalkyl or phenyl, is CR 4 or N, wherein
R4 für Wasserstoff, (Ci-C4)-Alkyl, (C3-C7)-Cycloalkyl oder 3- bis 7-gliedriges Heterocyclyl steht, worin (Ci-C4)-Alkyl mit 1 bis 3 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Hydroxy, Trifluormethoxy, (Ci-C i)-Alkoxy, Hydroxycarbonyl, (C1-C4)- Alkoxycarbonyl, Amino, Mono-(Ci-C4)-Alkylamino und Di-(Ci-C4)- Alkylamino substituiert sein kann, worin Mono-(Ci-C4)-Alkylamino und Di-(Ci-C4)-Alkylamino ihrerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl und Phenyl substituiert sein können, und worin 4- bis 7-gliedriges Heterocyclyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, (Ci-C4)-Alkyl, Hydroxy, Oxo und (Ci-C4)-Alkoxy substituiert sein kann, worin (Ci-C4)-Alkyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Hydroxy, Trifluormethoxy und (Ci-C4)-Alkoxy substituiert sein kann, oder R 4 is hydrogen, (C 1 -C 4 ) -alkyl, (C 3 -C 7 ) -cycloalkyl or 3 to 7-membered heterocyclyl, wherein (C 1 -C 4 ) -alkyl having 1 to 3 substituents selected independently of one another the group fluorine, trifluoromethyl, hydroxy, trifluoromethoxy, (Ci-C i) alkoxy, hydroxycarbonyl, (C 1 -C 4) - alkoxycarbonyl, amino, mono- (C 1 -C 4) -alkylamino and di- (C 1 -C 4) -alkylamino in which mono- (C 1 -C 4) -alkylamino and di- (C 1 -C 4) -alkylamino may themselves be substituted by 1 or 2 substituents independently of one another selected from the group consisting of fluorine, trifluoromethyl and phenyl, and in which 4 to 7-membered heterocyclyl may in turn be substituted by 1 or 2 substituents independently of one another selected from the group fluorine, (C 1 -C 4) -alkyl, hydroxy, oxo and (C 1 -C 4) -alkoxy, in which (C 1 -C 4) -alkyl in turn with 1 or 2 substituents independently selected from the group fluorine, trifluoromethyl, hydroxy, trifluoromethoxy and (Ci-C4) alkoxy substituted can be, or
R3 und R4 bilden zusammen mit V und W, an die sie gebunden sind, einen 4- bis 7- gliedrigen Heterocyclyl-Ring,
worin der 4- bis 7-gliedrige Heterocyclyl-Ring mit (Ci-C i)-Alkyl substituiert sein kann, R 3 and R 4 together with V and W to which they are attached form a 4- to 7-membered heterocyclyl ring, wherein the 4- to 7-membered heterocyclyl ring may be substituted by (Ci-C i) -alkyl,
R1 für Wasserstoff oder Fluor steht, R2 für (Ci-C6)-Alkyl oder Benzyl steht, wobei (Ci-C6)-Alkyl mit einem Substituenten Trifluormethyl substituiert ist, wobei (Ci-C6)-Alkyl mit 1 bis 3 Substituenten Fluor substituiert sein kann, und wobei Benzyl mit 1 bis 3 Substituenten Fluor substituiert ist, mit der Maßgabe, dass wenn V für N steht, W für CR4 steht, mit der Maßgabe, dass wenn W für N steht, V für CR3 steht, sowie ihre N-Oxide, Salze, Solvate, Salze der N-Oxide und Solvate der N-Oxide und Salze. R 1 is hydrogen or fluorine, R 2 is (C 1 -C 6 ) -alkyl or benzyl, where (C 1 -C 6 ) -alkyl is substituted by a substituent trifluoromethyl, where (C 1 -C 6) -alkyl having 1 to 3 Substituents fluorine may be substituted, and wherein benzyl is substituted with 1 to 3 substituents fluorine, provided that when V is N, W is CR 4 , with the proviso that when W is N, V is CR 3 and their N-oxides, salts, solvates, salts of N-oxides and solvates of N-oxides and salts.
2. Verbindung der Formel (I) nach Anspruch 1, in welcher 2. A compound of formula (I) according to claim 1, in which
U für O oder S steht, U stands for O or S,
V für CR3 oder Ν steht, wobei V is CR 3 or Ν, where
R3 für Wasserstoff, Trifluormethyl, (Ci-C6)-Alkyl, (C3-C6)-Cycloalkyl, (Ci- C4)-Alkoxycarbonyl, Phenyl, Pyridyl, -NR5R6, -S(0)nR7 oder -S02NR8R9 steht, worin (Ci-C6)-Alkyl mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Trifluormethoxy, (Ci-R 3 is hydrogen, trifluoromethyl, (Ci-C 6) -alkyl, (C 3 -C 6) cycloalkyl, (Ci C 4) -alkoxycarbonyl, phenyl, pyridyl, -NR 5 R 6, -S (0) n is R 7 or -SO 2 NR 8 R 9 , in which (C 1 -C 6) -alkyl having 1 or 2 substituents independently of one another selected from the group consisting of fluorine, trifluoromethyl, trifluoromethoxy,
C i)-Alkoxy, Methylamino, Ethylamino, Dimethylamino, Diethylamino, Phenoxy, Azetidinyl, Pyrrolodinyl, Piperidinyl, Morpholinyl, Piperazinyl und Pyrazolyl substituiert sein kann, worin Pyrazolyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Methyl und Ethyl substituiert sein kann,
worin Phenyl und Pyridyl mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Chlor, Methyl, Ethyl, Methoxy und Ethoxy substituiert sein können, und worin n für eine Zahl 1 oder 2 steht, C i) alkoxy, methylamino, ethylamino, dimethylamino, diethylamino, phenoxy, azetidinyl, pyrrolodinyl, piperidinyl, morpholinyl, piperazinyl and pyrazolyl may be substituted, wherein pyrazolyl in turn having 1 or 2 substituents independently selected from the group fluorine, methyl and ethyl may be substituted wherein phenyl and pyridyl may be substituted with 1 or 2 substituents independently selected from the group of fluorine, chlorine, methyl, ethyl, methoxy and ethoxy, and wherein n is a number 1 or 2,
R5 für Wasserstoff oder (Ci-C4)-Alkyl steht, worin (Ci-C4)-Alkyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Trifluormethyl, Hydroxy, Trifluormethoxy, Methoxy und Ethoxy substituiert sein kann, R 5 is hydrogen or (C 1 -C 4 ) -alkyl, in which (C 1 -C 4 ) -alkyl in turn may be substituted by 1 or 2 substituents independently of one another selected from the group consisting of fluorine, trifluoromethyl, hydroxyl, trifluoromethoxy, methoxy and ethoxy,
R6 für (Ci-C4)-Alkyl, (C3-C6)-Cycloalkyl, Phenyl, Azetidinyl, Pyrrolidinyl, Tetrahydrofuranyl, Dioxotetrahydrothiolanyl, Piperidinyl, Tetrahydropyranyl steht, worin Phenyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Chlor, Methyl, Ethyl, Methoxy, Ethoxy und Methylcarbonylamino substituiert sein kann, oder R 6 is (C 1 -C 4 ) -alkyl, (C 3 -C 6 ) -cycloalkyl, phenyl, azetidinyl, pyrrolidinyl, tetrahydrofuranyl, dioxotetrahydrothiolanyl, piperidinyl, tetrahydropyranyl, in which phenyl is in turn independently selected from the group consisting of 1 or 2 substituents Group fluorine, chlorine, methyl, ethyl, methoxy, ethoxy and methylcarbonylamino may be substituted, or
R5 und R6 bilden zusammen mit dem Stickstoffatom, an das sie gebunden sind, einen Azetidinyl-, Pyrrolidinyl-, Piperidinyl-, Piperazinyl-, Morpholinyl- oder Dioxothiomoφholinyl- Ring, worin der Piperazinyl-Ring am Stickstoff mit Ethyl substituiert sein kann, worin Ethyl mit Hydroxy substituiert ist, R 5 and R 6 together with the nitrogen atom to which they are attached form an azetidinyl, pyrrolidinyl, piperidinyl, piperazinyl, morpholinyl or dioxothiomoφholinyl ring in which the piperazinyl ring on the nitrogen may be substituted with ethyl, wherein ethyl is substituted with hydroxy,
R7 für (Ci-C4)-Alkyl oder (C3-C6)-Cycloalkyl steht, R 7 is (C 1 -C 4 ) -alkyl or (C 3 -C 6 ) -cycloalkyl,
R8 für Wasserstoff, Methyl oder Ethyl steht, R 8 is hydrogen, methyl or ethyl,
R9 für Wasserstoff, Methyl oder Ethyl steht,
W für CR4 oder N steht, wobei R 9 is hydrogen, methyl or ethyl, W is CR 4 or N, where
R4 für Wasserstoff, (Ci-C4)-Alkyl, Cycloproyl, Cyclobutyl oder Cyclopentyl steht, worin (Ci-C4)-Alkyl mit Trifluormethyl, Hydroxy, Hydroxycarbonyl, Methoxycarbonyl, Ethoxycarbonyl, Methylamino, Ethylamino, Dimethylamino oder Diethylamino substituiert sein kann, worin Methylamino, Ethylamino, Dimethylamino und Diethylamino mit Phenyl substituiert sein können, R 4 is hydrogen, (C 1 -C 4 ) -alkyl, cyclopropyl, cyclobutyl or cyclopentyl, in which (C 1 -C 4) -alkyl may be substituted by trifluoromethyl, hydroxy, hydroxycarbonyl, methoxycarbonyl, ethoxycarbonyl, methylamino, ethylamino, dimethylamino or diethylamino, wherein methylamino, ethylamino, dimethylamino and diethylamino may be substituted by phenyl,
R1 für Wasserstoff steht, R 1 is hydrogen,
R2 für 2-Fluorbenzyl steht, mit der Maßgabe, dass wenn V für N steht, W für CR4 steht, mit der Maßgabe, dass wenn W für N steht, V für CR3 steht, sowie ihre Salze, Solvate und Solvate der Salze. Verbindung der Formel (I) nach Anspruch 1 oder 2, in welcher U für S steht, V für CR3 oder N steht, wobei R 2 is 2-fluorobenzyl, provided that when V is N, W is CR 4 , with the proviso that when W is N, V is CR 3 , and their salts, solvates and solvates salts. A compound of the formula (I) according to claim 1 or 2, in which U is S, V is CR 3 or N, where
R3 für Wasserstoff, Trifluormethyl, (Ci-C6)-Alkyl, Cyclopropyl, Cyclohexyl, Methoxycarbonyl, Phenyl, Pyridyl, -NR5R6, -S(0)nR7 oder -S02NR8R9 steht, worin (Ci-C6)-Alkyl mit Trifluormethyl, (Ci-C4)-Alkoxy, Methylamino, Ethylamino, Dimethylamino, Diethylamino, Phenoxy, Pyrrolodinyl oder Pyrazolyl substituiert sein kann,
worin Pyrazolyl seinerseits mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Methyl und Ethyl substituiert sein kann, worin Phenyl und Pyridyl mit 1 oder 2 Substituenten unabhängig voneinander ausgewählt aus der Gruppe Fluor, Chlor, Methyl, Methoxy undR 3 is hydrogen, trifluoromethyl, (C 1 -C 6) alkyl, cyclopropyl, cyclohexyl, methoxycarbonyl, phenyl, pyridyl, -NR 5 R 6 , -S (O) n R 7 or -SO 2 NR 8 R 9 , in which (C 1 -C 6) -alkyl may be substituted by trifluoromethyl, (C 1 -C 4) -alkoxy, methylamino, ethylamino, dimethylamino, diethylamino, phenoxy, pyrrolodinyl or pyrazolyl, wherein pyrazolyl in turn may be substituted with 1 or 2 substituents independently selected from the group of methyl and ethyl, wherein phenyl and pyridyl having 1 or 2 substituents independently selected from the group fluorine, chlorine, methyl, methoxy and
Ethoxy substituiert sein können, und worin n für eine Zahl 1 oder 2 steht, Ethoxy can be substituted, and wherein n is a number 1 or 2,
R5 für Wasserstoff oder (Ci-C4)-Alkyl steht, worin (Ci-C4)-Alkyl seinerseits mit Hydroxy substituiert sein kann, R 5 is hydrogen or (C 1 -C 4 ) -alkyl, in which (C 1 -C 4 ) -alkyl may in turn be substituted by hydroxyl,
R6 für (Ci-C4)-Alkyl, Cyclohexyl, Phenyl oder Dioxotetrahydrothiolanyl steht, worin Phenyl seinerseits mit Methoxy, Ethoxy oder Methylcarbonylamino substituiert sein kann, oder R 6 is (C 1 -C 4 ) -alkyl, cyclohexyl, phenyl or dioxotetrahydrothiolanyl, in which phenyl in turn may be substituted by methoxy, ethoxy or methylcarbonylamino, or
R5 und R6 bilden zusammen mit dem Stickstoffatom, an das sie gebunden sind, einen Pyrrolidinyl-, Piperazinyl- oder Morpholinyl-Ring, worin der Piperazinyl-Ring am Stickstoff mit Ethyl substituiert ist, worin Ethyl mit Hydroxy substituiert ist, R 5 and R 6 together with the nitrogen atom to which they are attached form a pyrrolidinyl, piperazinyl or morpholinyl ring in which the piperazinyl ring on the nitrogen is substituted by ethyl, in which ethyl is substituted by hydroxy,
R7 für (Ci-C4)-Alkyl oder Cyclohexyl steht, R 7 is (C 1 -C 4 ) -alkyl or cyclohexyl,
R8 für Wasserstoff, Methyl oder Ethyl steht, R 8 is hydrogen, methyl or ethyl,
R9 für Wasserstoff, Methyl oder Ethyl steht, W für CR4 oder N steht, wobei
R4 für Wasserstoff, (Ci-C4)-Alkyl oder Cycloproyl steht, worin (Ci-C i)-Alkyl oder Methylamino substituiert sein kann, worin Methylamino mit Phenyl substituiert sein kann, R1 für Wasserstoff steht, R 9 is hydrogen, methyl or ethyl; W is CR 4 or N, wherein R 4 is hydrogen, (C 1 -C 4 ) -alkyl or cyclopropyl, in which (C 1 -C 4) -alkyl or methylamino may be substituted, in which methylamino may be substituted by phenyl, R 1 is hydrogen,
R 2 für 2-Fluorbenzyl steht, mit der Maßgabe, dass wenn V für N steht, W für CR steht, mit der Maßgabe, dass wenn W für N steht, V für CR steht, sowie ihre Salze, Solvate und Solvate der Salze. R 2 is 2-fluorobenzyl, provided that when V is N, W is CR, with the proviso that when W is N, V is CR, and their salts, solvates and solvates of the salts.
4. Verfahren zur Herstellung von Verbindungen der Formel (I), wie in den Ansprüchen 1 bis 3 definiert, dadurch gekennzeichnet, dass man eine Verbindung der Formel (II) 4. A process for the preparation of compounds of the formula (I) as defined in claims 1 to 3, which comprises reacting a compound of the formula (II)
in welcher R1 und R2 jeweils die in den Ansprüchen 1 bis 3 angegebenen Bedeutungen haben, in einem inerten Lösungsmittel in Gegenwart einer geeigneten Base und eines geeigneten Kupplungsreagenzes mit einer Verbindung der Formel (III) in which R 1 and R 2 each have the meanings given in claims 1 to 3, in an inert solvent in the presence of a suitable base and a suitable coupling reagent with a compound of the formula (III)
H
in welcher R die in den Ansprüchen 1 bis 4 angegebenen Bedeutungen hat, umsetzt, und
gegebenenfalls die resultierenden Verbindungen der Formel (I) mit den entsprechenden (i) Lösungsmitteln und/oder (ii) Säuren oder Basen in ihre Solvate, Salze und/oder Solvate der Salze überführt. H in which R has the meanings given in claims 1 to 4, and, if appropriate, the resulting compounds of the formula (I) with the corresponding (i) solvents and / or (ii) acids or bases are converted into their solvates, salts and / or solvates of the salts.
5. Verbindung der Formel (I), wie in einem der Ansprüche 1 bis 3 definiert, zur Behandlung und/oder Prophylaxe von Krankheiten. 5. A compound of formula (I) as defined in any one of claims 1 to 3 for the treatment and / or prophylaxis of diseases.
6. Verwendung einer Verbindung der Formel (I), wie in einem der Ansprüche 1 bis 3 definiert, zur Herstellung eines Arzneimittels zur Behandlung und/oder Prophylaxe von Herzinsuffizienz, Angina pectoris, Hypertonie, pulmonaler Hypertonie, Ischämien, Gefäß- erkrankungen, Niereninsuffizienz, thromboembolischen Erkrankungen, fibrotischen6. Use of a compound of the formula (I) as defined in any one of claims 1 to 3 for the manufacture of a medicament for the treatment and / or prophylaxis of cardiac insufficiency, angina pectoris, hypertension, pulmonary hypertension, ischaemias, vascular diseases, renal insufficiency, thromboembolic disorders, fibrotic
Erkrankungen und Arteriosklerose. Diseases and arteriosclerosis.
7. Arzneimittel enthaltend eine Verbindung der Formel (I), wie in einem der Ansprüche 1 bis 3 definiert, in Kombination mit einem inerten, nicht-toxischen, pharmazeutisch geeigneten Hilfsstoff. 8. Arzneimittel enthaltend eine Verbindung der Formel (I), wie in einem der Ansprüche 1 bis 3 definiert, in Kombination mit einem weiteren Wirkstoff ausgewählt aus der Gruppe bestehend aus organischen Nitraten, NO-Donatoren, cGMP-PDE-Inhibitoren, antithrombotisch wirkenden Mitteln, den Blutdruck senkenden Mitteln sowie den Fettstoffwechsel verändernden Mitteln. 9. Arzneimittel nach Anspruch 7 oder 8 zur Behandlung und/oder Prophylaxe von Herzinsuffizienz, Angina pectoris, Hypertonie, pulmonaler Hypertonie, Ischämien, Gefäßerkrankungen, Niereninsuffizienz, thromboembolischen Erkrankungen, fibrotischen Erkrankungen und Arteriosklerose. 7. A pharmaceutical composition comprising a compound of the formula (I) as defined in any one of claims 1 to 3, in combination with an inert, non-toxic, pharmaceutically suitable excipient. 8. A medicament containing a compound of formula (I) as defined in any one of claims 1 to 3, in combination with another active ingredient selected from the group consisting of organic nitrates, NO donors, cGMP-PDE inhibitors, antithrombotic agents , antihypertensives and lipid metabolising agents. 9. Medicament according to claim 7 or 8 for the treatment and / or prophylaxis of heart failure, angina pectoris, hypertension, pulmonary hypertension, ischaemias, vascular diseases, renal insufficiency, thromboembolic diseases, fibrotic diseases and arteriosclerosis.
10. Verfahren zur Behandlung und/oder Prophylaxe von Herzinsuffizienz, Angina pectoris, Hypertonie, pulmonaler Hypertonie, Ischämien, Gefäßerkrankungen, Niereninsuffizienz, thromboembolischen Erkrankungen, fibrotischen Erkrankungen und Arteriosklerose bei Menschen und Tieren unter Verwendung einer wirksamen Menge mindestens einer Verbindung der Formel (I), wie in den Ansprüchen 1 bis 3 definiert, oder eines Arzneimittels, wie in einem der Ansprüche 7 bis 9 definiert.
10. A method for the treatment and / or prophylaxis of heart failure, angina pectoris, hypertension, pulmonary hypertension, ischaemias, vascular diseases, renal insufficiency, thromboembolic disorders, fibrotic diseases and arteriosclerosis in humans and animals using an effective amount of at least one compound of formula (I) as defined in claims 1 to 3, or a pharmaceutical composition as defined in any one of claims 7 to 9.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP16151585 | 2016-01-15 | ||
| EP16151585.3 | 2016-01-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017121693A1 true WO2017121693A1 (en) | 2017-07-20 |
Family
ID=55168210
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2017/050311 WO2017121693A1 (en) | 2016-01-15 | 2017-01-09 | Substituted thiazole and thiadiazole amides, and use thereof |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2017121693A1 (en) |
Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998016223A1 (en) | 1996-10-14 | 1998-04-23 | Bayer Aktiengesellschaft | Use of condensated (hetaryl-substituted) 1-benzal-3-pyrazol derivates for treating special diseases of the cardiovascular and the central nervous systems |
| WO2000006568A1 (en) | 1998-07-29 | 2000-02-10 | Bayer Aktiengesellschaft | Substituted pyrazole derivatives |
| WO2000006569A1 (en) | 1998-07-29 | 2000-02-10 | Bayer Aktiengesellschaft | Substituted pyrazole derivatives condensed with six-membered heterocyclic rings |
| WO2000063166A1 (en) | 1999-04-20 | 2000-10-26 | F. Hoffmann-La Roche Ag | Carbamic acid derivatives and their use as metabotropic glutamate receptor ligands |
| WO2003095451A1 (en) | 2002-05-08 | 2003-11-20 | Bayer Healthcare Ag | Carbamate-substituted pyrazolopyridines |
| WO2004009590A1 (en) | 2002-07-18 | 2004-01-29 | Bayer Healthcare Ag | 4-amino-substituted pyrimidine derivatives |
| WO2006015263A2 (en) | 2004-07-29 | 2006-02-09 | Threshold Pharmaceuticals, Inc. | Lonidamine analogs |
| WO2007124854A1 (en) | 2006-04-27 | 2007-11-08 | Bayer Healthcare Ag | Heterocyclic substituted, anellated pyrazole derivative and its uses |
| WO2008052898A1 (en) | 2006-11-01 | 2008-05-08 | F. Hoffmann-La Roche Ag | Indazole derivatives useful as l-cpt1 inhibitors |
| WO2010065275A1 (en) | 2008-11-25 | 2010-06-10 | Merck Sharp & Dohme Corp. | Soluble guanylate cyclase activators |
| WO2011149921A1 (en) | 2010-05-27 | 2011-12-01 | Merck Sharp & Dohme Corp. | Soluble guanylate cyclase activators |
| WO2012004259A1 (en) | 2010-07-09 | 2012-01-12 | Bayer Pharma Aktiengesellschaft | Ring-fused 4 -aminopyrimidines and use thereof as stimulators of soluable guanylate cyclases |
| WO2012004258A1 (en) | 2010-07-09 | 2012-01-12 | Bayer Pharma Aktiengesellschaft | Ring-fused pyrimidines and triazines and use thereof for the treatment and/or prophylaxis of cardiovascular diseases |
| WO2012028647A1 (en) | 2010-09-03 | 2012-03-08 | Bayer Pharma Aktiengesellschaft | Bicyclic aza heterocycles, and use thereof |
| WO2012143510A1 (en) | 2011-04-21 | 2012-10-26 | Bayer Intellectual Property Gmbh | Fluoroalkyl-substituted pyrazolopyridines and use thereof |
| WO2012152629A1 (en) | 2011-05-06 | 2012-11-15 | Bayer Intellectual Property Gmbh | Substituted imidazopyridines and imidazopyridazines and the use thereof |
| WO2013131923A1 (en) * | 2012-03-06 | 2013-09-12 | Bayer Intellectual Property Gmbh | Substituted azabicycles and use thereof |
-
2017
- 2017-01-09 WO PCT/EP2017/050311 patent/WO2017121693A1/en active Application Filing
Patent Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998016223A1 (en) | 1996-10-14 | 1998-04-23 | Bayer Aktiengesellschaft | Use of condensated (hetaryl-substituted) 1-benzal-3-pyrazol derivates for treating special diseases of the cardiovascular and the central nervous systems |
| WO2000006568A1 (en) | 1998-07-29 | 2000-02-10 | Bayer Aktiengesellschaft | Substituted pyrazole derivatives |
| WO2000006569A1 (en) | 1998-07-29 | 2000-02-10 | Bayer Aktiengesellschaft | Substituted pyrazole derivatives condensed with six-membered heterocyclic rings |
| WO2000063166A1 (en) | 1999-04-20 | 2000-10-26 | F. Hoffmann-La Roche Ag | Carbamic acid derivatives and their use as metabotropic glutamate receptor ligands |
| WO2003095451A1 (en) | 2002-05-08 | 2003-11-20 | Bayer Healthcare Ag | Carbamate-substituted pyrazolopyridines |
| WO2004009590A1 (en) | 2002-07-18 | 2004-01-29 | Bayer Healthcare Ag | 4-amino-substituted pyrimidine derivatives |
| WO2006015263A2 (en) | 2004-07-29 | 2006-02-09 | Threshold Pharmaceuticals, Inc. | Lonidamine analogs |
| WO2007124854A1 (en) | 2006-04-27 | 2007-11-08 | Bayer Healthcare Ag | Heterocyclic substituted, anellated pyrazole derivative and its uses |
| WO2008052898A1 (en) | 2006-11-01 | 2008-05-08 | F. Hoffmann-La Roche Ag | Indazole derivatives useful as l-cpt1 inhibitors |
| WO2010065275A1 (en) | 2008-11-25 | 2010-06-10 | Merck Sharp & Dohme Corp. | Soluble guanylate cyclase activators |
| WO2011149921A1 (en) | 2010-05-27 | 2011-12-01 | Merck Sharp & Dohme Corp. | Soluble guanylate cyclase activators |
| WO2012004259A1 (en) | 2010-07-09 | 2012-01-12 | Bayer Pharma Aktiengesellschaft | Ring-fused 4 -aminopyrimidines and use thereof as stimulators of soluable guanylate cyclases |
| WO2012004258A1 (en) | 2010-07-09 | 2012-01-12 | Bayer Pharma Aktiengesellschaft | Ring-fused pyrimidines and triazines and use thereof for the treatment and/or prophylaxis of cardiovascular diseases |
| WO2012028647A1 (en) | 2010-09-03 | 2012-03-08 | Bayer Pharma Aktiengesellschaft | Bicyclic aza heterocycles, and use thereof |
| WO2012143510A1 (en) | 2011-04-21 | 2012-10-26 | Bayer Intellectual Property Gmbh | Fluoroalkyl-substituted pyrazolopyridines and use thereof |
| WO2012152629A1 (en) | 2011-05-06 | 2012-11-15 | Bayer Intellectual Property Gmbh | Substituted imidazopyridines and imidazopyridazines and the use thereof |
| WO2013131923A1 (en) * | 2012-03-06 | 2013-09-12 | Bayer Intellectual Property Gmbh | Substituted azabicycles and use thereof |
Non-Patent Citations (10)
| Title |
|---|
| CARDIOVASC RES, vol. 47, no. 2, 2000, pages 203 - 405 |
| E. M. BECKER ET AL., BMC PHARMACOLOGY, vol. 1, no. 13, 2001 |
| F. WUNDER ET AL., ANAL. BIOCHEM., vol. 339, 2005, pages 104 - 112 |
| GOLDBERG ET AL., J. BIOL. CHEM., vol. 252, 1977, pages 1279 |
| KOZO OKAMOTO: "Spontaneous hypertension in rats", INT REV EXP PATHOL, vol. 7, 1969, pages 227 - 270 |
| MAARTEN VAN DEN BUUSE: "Circadian Rhythms of Blood Pressure, Heart Rate, and Locomotor Activity in Spontaneously Hypertensive Rats as Measured With Radio-Telemetry", PHYSIOLOGY & BEHAVIOR, vol. 55, no. 4, 1994, pages 783 - 787 |
| MÜLSCH ET AL., BRIT. J. PHARMACOL., vol. 120, 1997, pages 681 |
| PETTIBONE ET AL., EUR. J. PHARMACOL., vol. 116, 1985, pages 307 |
| WU ET AL., BLOOD, vol. 84, 1994, pages 4226 |
| YU ET AL., BRIT. J. PHARMACOL., vol. 114, 1995, pages 1587 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2635577B1 (en) | Substituted 6-fluoro-1h-pyrazolo[4,3-b]pyridines and use thereof | |
| EP2576547B1 (en) | Substituted 5-fluoro-1h-pyrazolopyridines and use thereof | |
| EP2635576B1 (en) | Benzyl-substituted carbamates and use thereof | |
| EP2802580B1 (en) | Substituted triazine derivatives and their use as stimulators of soluble guanylate cyclase | |
| EP2914595A1 (en) | Carboxy-substituted imidazo[1,2-a]pyridinecarboxamides and their use as soluble guanylate cyclase stimulants | |
| EP3119777A1 (en) | Cyano-substituted imidazo[1,2-a]pyridinecarboxamides and their use | |
| EP3119778A1 (en) | Substituted imidazo[1,2-a]pyridinecarboxamides and their use | |
| EP3137464A1 (en) | Imidazo[1,2-a]pyridines as stimulators of soluble guanylate cyclase for treating cardiovascular diseases | |
| EP3227286B1 (en) | Substituted pyrazolo[1,5-a]pyridines and imidazo[1,2-a]pyrazines and their use | |
| WO2012010578A1 (en) | Substituted methyl-pyrimidin-5-yl carbamates and use thereof | |
| WO2012010577A1 (en) | Substituted oxazolidinones and oxazinanones and use thereof | |
| WO2015165933A2 (en) | 6-substituted imidazo[1,2-a]pyrazinecarboxamides and use thereof | |
| WO2017121700A1 (en) | 1,3-disubstituted 1h-pyrazolo[3,4-b]pyridine derivatives and use thereof as stimulators of soluble guanylate cyclase | |
| WO2012010576A1 (en) | Carbamate-substituted diaminopyrimidines and use thereof | |
| WO2015165970A1 (en) | 6-chlorine-substituted imidazo[1,2-a]pyridine carboxamides and the use thereof as soluble guanylate cyclase stimulators | |
| EP3227287B1 (en) | Heteroaryl-substituted imidazo[1,2-a]pyridines and their use | |
| EP3137463A1 (en) | Enantiomers of the n-(2-amino-5-fluoro-2-methylpentyl)-8-[(2,6-difluorobenzyl)oxy]-2-methylimidazo[1,2-a]pyridine-3-carboxamide, as well as of the di- and trifluoro derivatives for the treatment of cardiovascular diseases | |
| EP3253758B1 (en) | N-substituted 8-[(2,6-difluorobenzyl)oxy]-2,6-dimethylimidazo[1,2-a]pyrazine-3-carboxamide-derivatives as stimulators of the soluble guanylatcyclase (sgc) for the treatment of cardiovascular diseases | |
| WO2017121693A1 (en) | Substituted thiazole and thiadiazole amides, and use thereof | |
| WO2017121692A1 (en) | Substituted sulfamides and use of same | |
| DE102011075399A1 (en) | New substituted imidazopyrid(az)ine compounds are soluble guanylate cyclase stimulators, useful to treat e.g. heart disease, angina pectoris, hypertension, pulmonary hypertension, ischemia, vascular disease or renal insufficiency | |
| DE102011007890A1 (en) | New fluoroalkyl-substituted pyrazolopyridine compounds are soluble guanylate cyclase stimulators, useful to treat e.g. heart disease, angina pectoris, hypertension, pulmonary hypertension, ischemia, vascular disease or renal insufficiency | |
| WO2018184976A1 (en) | Substituted imidazo[1,2-a]pyridinecarboxamides and use of same | |
| DE102011082041A1 (en) | New substituted fused pyrimidine compounds are guanylate cyclase stimulators useful to treat and/or prevent e.g. heart failure, hypertension, ischemia, vascular disease, renal failure, thromboembolic disorders and fibrotic diseases | |
| DE102011078715A1 (en) | New substituted pyrazolo-pyridine derivatives useful for treating and/or prophylaxis of e.g. congestive heart failure, angina pectoris, hypertension, pulmonary hypertension, ischemia, vascular diseases, renal failure, and fibrotic diseases |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17700004 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17700004 Country of ref document: EP Kind code of ref document: A1 |