VVID-747PC RAS-PI3K INHIBITORS AND USES THEREOF CROSS-REFERENCE TO RELATED APPLICATIONS [001] This application claims the benefit of U.S. provisional application serial no. 63/595,998 filed November 03, 2023, the content of which is incorporated by reference in its entirety. FIELD OF THE DISCLOSURE [002] The disclosure relates to compounds and methods for modulating RAS-PI3K. BACKGROUND OF THE DISCLOSURE [003] There is a need for improved cancer therapeutics and methods of treatment, particularly for modulation of RAS-PI3K. SUMMARY [004] Disclosed herein in some aspects are modulators of RAS-PI3K. Some such aspects relate to a RAS- PI3K agonist. Some aspects relate to a RAS-PI3K inhibitor. The RAS-PI3K modulators may include a compound described herein. The RAS-PI3K modulators may be useful in a method described herein. [005] In another aspect, provided herein is a method of modulating RAS-PI3K, the method comprising administering to a subject in need thereof a therapeutically effective amount of a compound disclosed herein, or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a tautomer thereof, or a pharmaceutically acceptable salt of a stereoisomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of a tautomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of an atropisomer thereof. In some embodiments, the method comprises inhibition of RAS-PI3K. In some embodiments, the method comprises activation of RAS-PI3K. [006] The present disclosure provides a compound of Formula (I), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof: wherein:
R
1 is C
1-C
6 alkyl or C
3-C
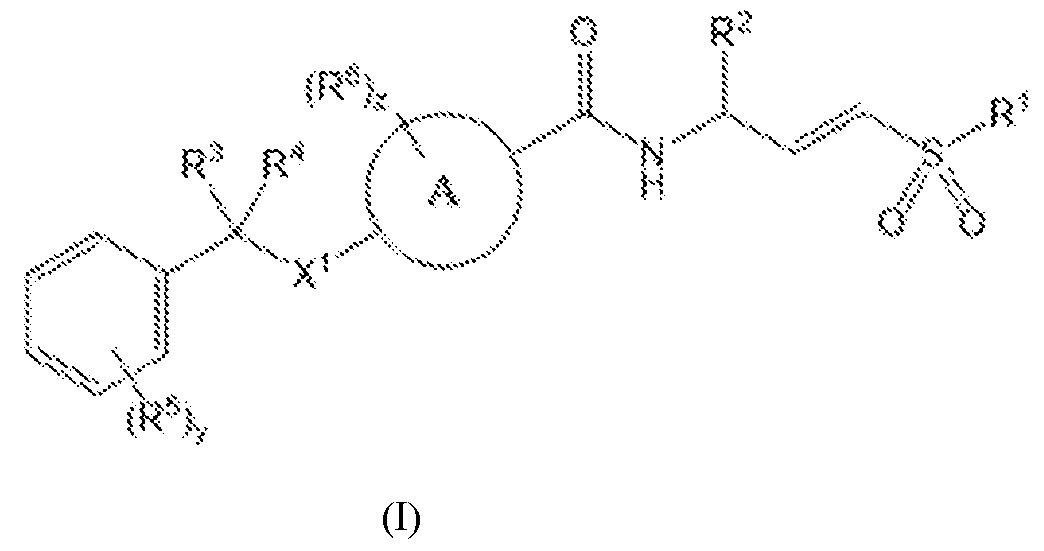
6 cycloalkyl; R
2 is hydrogen or C
1-C
6 alkyl; 1 QB\184200.00081\93036187.2
VVID-747PC ring A is phenyl, a 6-membered monocyclic heteroaryl having 1 to 2 nitrogen atoms, or a 9- membered bicyclic heteroaryl ring having 1 to 4 nitrogen atoms; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, C
3-C
6 cycloalkyl, or R
9 together with R
6 form a 5 to 6-membered heteroaryl or heterocyclic ring having 1 to 2 nitrogen atoms and 0 to 1 oxygen atom; wherein said 5 to 6- membered heteroaryl or heterocyclic ring is fused with ring A; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are substituted with 0-2 substituents independently selected from halogen, hydroxy, cyano, C
1-C
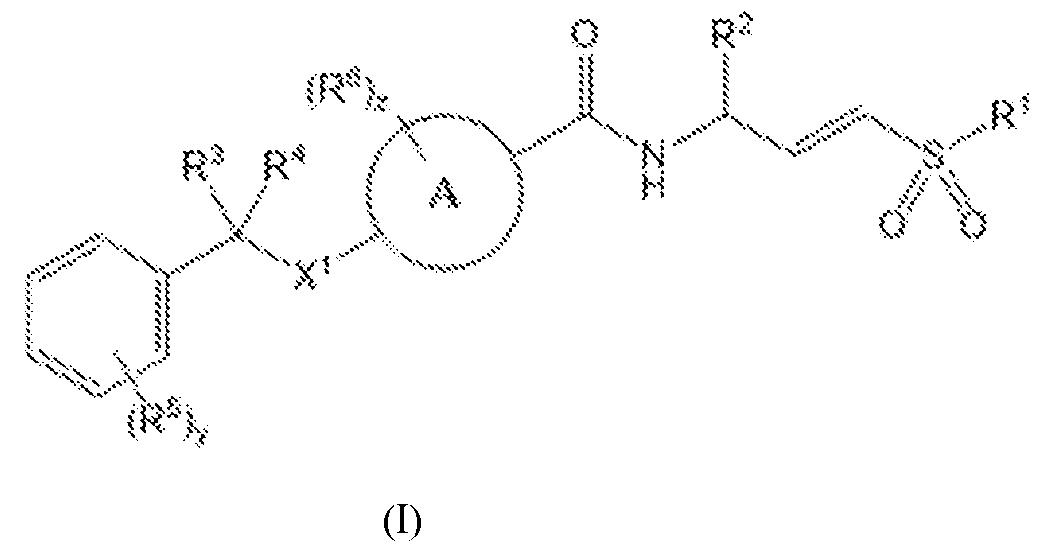
3 alkyl, C
1- C
3 alkoxy, or C
1-C
3 haloalkyl; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, -C(O)NR
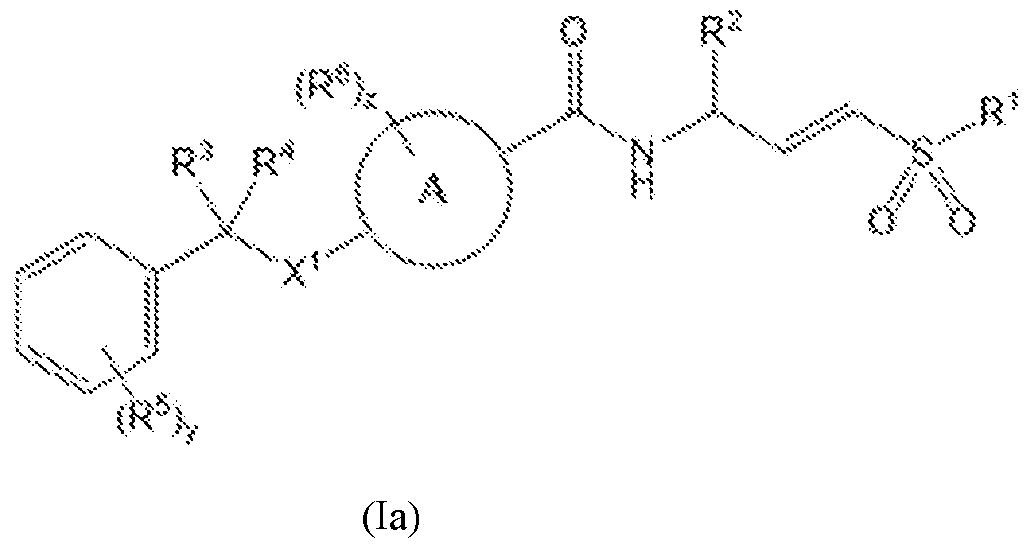
7R
8, -O-, -CH=, -CH
2-, -N=, a 5 to 6-membered heteroaryl ring, -C
1-C
3alkylene- OCH
3, or C
3-C
6 cycloalkyl, wherein said C
3-C
6 cycloalkyl is substituted with 0-2 substituents independently selected from halogen and C
1-C
3 haloalkyl; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [007] The present disclosure also provides a compound of Formula (Ia), or a pharmaceutically acceptable salt or solvate thereof: wherein:
R
1 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl; R
2 is hydrogen or C
1-C
6 alkyl; ring A is phenyl or a monocyclic or bicyclic heteroaryl ring having 1 to 3 nitrogen atoms; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; 2 QB\184200.00081\93036187.2
VVID-747PC each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [008] In some embodiments, R
1 and R
2 are methyl. [009] In some embodiments, ring A is phenyl. In some embodiments, ring A is a monocyclic heteroaryl having 1 or 2 nitrogen atoms. In some embodiments, A is pyrimidinyl or pyrazinyl. In some embodiments, ring A is a bicyclic heteroaryl having 1 or 2 nitrogen atoms. [0010] In some embodiments, R
6 is halogen, C
1-C
6 alkyl or C
1-C
6 haloalkyl. [0011] In some embodiments, z is 0, 1 or 2. [0012] In some embodiments, X
1 is NH or O. In some embodiments, X
1 is NH. In some embodiments, X
1 is O. [0013] In some embodiments, R
3 is hydrogen and R
4 is C
1-C
6 alkyl, C
1-C
6 haloalkyl or C
3-C
6 cycloalkyl. [0014] In some embodiments, R
4 is -CH
3, -CF
3 or cyclopropyl. [0015] In some embodiments, each R
5 is halogen. [0016] In some embodiments, each R
5 is independently chloro or fluoro. [0017] In some embodiments, y is 1 or 2. [0018] The present disclosure further provides a compound of Formula (IIa), or a pharmaceutically acceptable salt or solvate thereof: wherein:
R
1 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl; R
2 is hydrogen or C
1-C
6 alkyl; 3 QB\184200.00081\93036187.2
VVID-747PC X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C3-C6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C1-C6 alkyl. [0019] The present disclosure further provides a compound of Formula (IIIa), or a pharmaceutically acceptable salt or solvate thereof: wherein: 1
R is C
1-C
6 or C
3-C
6 R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; 4 QB\184200.00081\93036187.2
VVID-747PC z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0020] The present disclosure further provides a compound of Formula (IVa), or a pharmaceutically acceptable salt or solvate thereof: wherein: R
1 is C
1-C6 R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0021] The present disclosure further provides a compound of Formula (Va), or a pharmaceutically acceptable salt or solvate thereof: QB\184200.00081\93036187.2
VVID-747PC (Va) wherein: R
1 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl; R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0022] The present disclosure further provides a compound of Formula (VIa), or a pharmaceutically acceptable salt or solvate thereof: wherein:
R
1 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl; R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C3-C6 cycloalkyl, or C3-C6 heterocyclyl, wherein said C3-C6 cycloalkyl and C3-C6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; 6 QB\184200.00081\93036187.2
VVID-747PC each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0023] The present disclosure further provides a compound of Formula (VIIa), or a pharmaceutically acceptable salt or solvate thereof: wherein: 1
R is C
1-C
6 R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 hydroxyalkyl, or C1-C6 alkoxy; y is 0, 1, 2 or 3; each of X
2, X
3, X
4 and X
5 is independently nitrogen, NR
9, carbon, or -CR
9-, wherein not more than 3 of X
2, X
3, X
4 and X
5 are nitrogen or NR
9; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0024] The present disclosure further provides for a pharmaceutical composition comprising a compound of the present disclosure, such as without limitation a compound of any of Formula (I) through Formula 7 QB\184200.00081\93036187.2
VVID-747PC (VII) or of Formula (Ia) through Formula (VIIa), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof, and a pharmaceutically acceptable excipient or carrier. [0025] The present disclosure further provides for a method of inhibiting RAS-PI3K in a subject in need of such inhibition, comprising administering to the subject a therapeutically effective amount of a compound of the present disclosure, such as without limitation a compound of any of Formula (I) through Formula (VII) or of Formula (Ia) through Formula (VIIa), or a pharmaceutically salt, solvate, stereoisomer, or tautomer thereof. [0026] The present disclosure further provides for a method of treating a disease or condition comprising administering to a subject in need thereof a therapeutically effective amount of a compound of the present disclosure, such as without limitation a compound of any of Formula (I) through Formula (VII) or of Formula (Ia) through Formula (VIIa) or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof. [0027] In some embodiments, the disease is cancer. In some embodiments, the cancer is selected from the group of bladder cancer, uterine cancer, head and neck cancer, esophageal cancer, ovarian cancer, liver cancer, cervical cancer, lung cancer, colorectal cancer, cholangiocarcinoma, gastric cancer, kidney cancer, and pancreatic cancer. [0028] In some embodiments, the disease or condition is an immunological disease or condition. In some embodiments, the immunological condition is wound healing deficiency. [0029] The present disclosure provides a method of inhibiting RAS-PI3K in a subject in need of such inhibition, comprising administering to the subject a therapeutically effective amount of a compound of the present disclosure, such as without limitation a compound of Formula (I) through Formula (VII) or of Formula (Ia) through (VIIa), or a pharmaceutically salt, solvate, stereoisomer, or tautomer thereof. [0030] In some aspects, the present disclosure provides for the use of the compounds of the present disclosure, such as without limitation the compounds of Formula (I) through Formula (VII) or Formula (Ia) through Formula (VIIa) in the treatment of cancer or the promotion of wound healing. [0031] In some aspects, the present disclosure provides for the use of the compounds of the present disclosure, such as without limitation the compounds of Formula (I) through Formula (VII) or of Formula (Ia) through Formula (VIIa) in the formulation of a medicament useful for the treatment of cancer or the promotion of wound healing. [0032] Additional aspects and advantages of the present disclosure will become readily apparent to those skilled in this art from the following detailed description, wherein only illustrative embodiments of the present disclosure are shown and described. As will be realized, the present disclosure is capable of other and different embodiments, and its several details are capable of modifications in various obvious respects, all without departing from the disclosure. Accordingly, the drawings and description are to be regarded as illustrative in nature, and not as restrictive. INCORPORATION BY REFERENCE 8 QB\184200.00081\93036187.2
VVID-747PC [0033] All publications, patents, and patent applications mentioned in this specification are herein incorporated by reference to the same extent as if each individual publication, patent, or patent application was specifically and individually indicated to be incorporated by reference. To the extent publications and patents or patent applications incorporated by reference contradict the disclosure contained in the specification, the specification is intended to supersede and/or take precedence over any such contradictory material. DETAILED DESCRIPTION [0034] RAS proteins are small GTPases known for their involvement in oncogenesis. RAS operates in a complex signaling network with multiple activators and effectors, which allows them to regulate cellular functions such as cell proliferation, differentiation, apoptosis, and senescence. Phosphatidylinositol 3- kinase (PI3K) is one of the main effector pathways of RAS, regulating cell growth, cell cycle entry, cell survival, cytoskeleton reorganization, and metabolism. It is the involvement of this pathway in human tumors that has attracted most attention. PI3K has proven to be necessary for RAS-induced transformation in vitro. Mice with mutations in the PI3K catalytic subunit p110α that block its ability to interact with RAS are highly resistant to endogenous oncogenic KRAS-induced lung tumorigenesis and HRAS-induced skin carcinogenesis. These animals also have a delayed development of the lymphatic vasculature. [0035] Disclosed herein are compounds and methods for modulating RAS-PI3K activity. Some embodiments relate to a compound or method of inhibiting RAS-PI3K activity. Some embodiments relate to a compound or method of activating RAS-PI3K activity. The modulating of RAS-PI3K activity may be in vitro or in vivo. The compound for modulating RAS-PI3K may be formulated for administration to a subject. The RAS-PI3K modulation may be performed in a subject. [0036] The compounds disclosed herein may be useful for treatment of diseases where RAS-PI3K activity may be a concern. In some embodiments, the compound is used for wound healing. In some embodiments, the compound is used for treatment of cancer. Compounds of the Disclosure [0037] In one aspect, the present disclosure provides for a compound of Formula (I), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof: wherein:
9 QB\184200.00081\93036187.2
VVID-747PC R
1 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl; R
2 is hydrogen or C
1-C
6 alkyl; ring A is phenyl, a 6-membered monocyclic heteroaryl having 1 to 2 nitrogen atoms, or a 9- membered bicyclic heteroaryl ring having 1 to 4 nitrogen atoms; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, C
3-C
6 cycloalkyl, or R
9 together with R
6 form a 5 to 6-membered heteroaryl or heterocyclic ring having 1 to 2 nitrogen atoms and 0 to 1 oxygen atom; wherein said 5 to 6- membered heteroaryl or heterocyclic ring is fused with ring A; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are substituted with 0-2 substituents independently selected from halogen, hydroxy, cyano, C
1-C
3 alkyl, C
1- C
3 alkoxy, or C
1-C
3 haloalkyl; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, -C(O)NR
7R
8, -O-, -CH=, -CH
2-, -N=, a 5 to 6-membered heteroaryl ring, -C
1-C
3alkylene- OCH
3, or C
3-C
6 cycloalkyl, wherein said C
3-C
6 cycloalkyl is substituted with 0-2 substituents independently selected from halogen and C
1-C
3 haloalkyl; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0038] In some embodiments, R
1 and R
2 are methyl. [0039] In some embodiments, ring A is phenyl. [0040] In some embodiments, ring A is a 6-membered monocyclic heteroaryl having 1 or 2 nitrogen atoms. [0041] In some embodiments, ring A is pyrimidinyl or pyrazinyl. [0042] In some embodiments, ring A is pyridinyl. [0043] In some embodiments, ring A is a 9-membered bicyclic heteroaryl having 1 or 2 nitrogen atoms. [0044] In some embodiments, ring A is a 9-membered bicyclic heteroaryl having 3 or 4 nitrogen atoms. [0045] In some embodiments, R
6 is halogen, C
1-C
6 alkyl or C
1-C
6 haloalkyl. [0046] In some embodiments, R
6 is -O-, -CH=, -CH
2-, or -N=. [0047] In some embodiments, z is 0 or 1. [0048] In some embodiments, X
1 is NH or O. [0049] In some embodiments, X
1 is NH. [0050] In some embodiments, X
1 is O. 10 QB\184200.00081\93036187.2
VVID-747PC [0051] In some embodiments, X
1 is NR
9, and R
9 together with R
6 form a 5 to 6-membered heteroaryl or heterocyclic ring having 1 to 2 nitrogen atoms and 0 to 1 oxygen atom; wherein said 5 to 6-membered heteroaryl or heterocyclic ring is fused with ring A. [0052] In some embodiments, R
3 is hydrogen and R
4 is C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl. [0053] In some embodiments, R
4 is -CH3, -CH(CH3)2, -CF3, cyclopropyl, or oxetanyl. [0054] In some embodiments, each R
5 is halogen. [0055] In some embodiments, each R
5 is independently chloro or fluoro. [0056] In some embodiments, y is 1 or 2. [0057] In some embodiments, the compound is a compound of Formula (II), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof: wherein: 1
R is C
1-C
6 or R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, C
3-C
6 cycloalkyl, or R
9 together with R
6 form a 5 to 6-membered heteroaryl or heterocyclic ring having 1 to 2 nitrogen atoms and 0 to 1 oxygen atom; wherein said 5 to 6- membered heteroaryl or heterocyclic ring is fused with ring A; each of R
3 and R
4 is independently hydrogen, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are substituted with 0-2 substituents independently selected from halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, -C(O)NR
7R
8, -O-, -CH=, -CH
2-, -N=, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and 11 QB\184200.00081\93036187.2
VVID-747PC R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0058] In some embodiments, the compound is a compound of Formula (III), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof: wherein: R
1 is C
1-C
6
R
2 is hydrogen or C1-C6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, C
3-C
6 cycloalkyl, or R
9 together with R
6 form a 5 to 6-membered heteroaryl or heterocyclic ring having 1 to 2 nitrogen atoms and 0 to 1 oxygen atom; wherein said 5 to 6- membered heteroaryl or heterocyclic ring is fused with ring A; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are substituted with 0-2 substituents independently selected from halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 hydroxyalkyl, C1-C6 alkoxy, cyano, -C(O)NR
7R
8, -O-, -CH=, -CH
2-, -N=, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0059] In some embodiments, the compound is a compound of Formula (IV), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof: 12 QB\184200.00081\93036187.2
VVID-747PC wherein: R
1 is C
1-C
6 alkyl
R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are substituted with 0-2 substituents independently selected from halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, -C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0060] In some embodiments, the compound is a compound of Formula (V), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof: wherein:
R
1 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl; 13 QB\184200.00081\93036187.2
VVID-747PC R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are substituted with 0-2 substituents independently selected from halogen, hydroxy, cyano, C1-C3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, -C(O)NR
7R
8, or a 5- to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0061] In some embodiments, the compound is a compound of Formula (VI), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof: wherein:
R
1 is C1-C6 alkyl or C3-C6 cycloalkyl; R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are substituted with 0-2 substituents independently selected from halogen, hydroxy, cyano, C
1-C
3 alkyl, C
1- C3 alkoxy, or C1-C3 haloalkyl; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; 14 QB\184200.00081\93036187.2
VVID-747PC y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, -C(O)NR
7R
8, a 5 to 6-membered heteroaryl ring, -C
1-C
3alkylene-OCH
3, or C
3-C
6 cycloalkyl, wherein said C
3-C
6 cycloalkyl is substituted with 0-2 substituents independently selected from halogen and C
1-C
3 haloalkyl; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0062] In some embodiments, the compound is a compound of Formula (VII), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof: wherein: 1
R is C
1-C
6 R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are substituted with 0-2 substituents independently selected from halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each of X
2 and X
6 is independently nitrogen or carbon, X
3 is -CR
9=, each of X
4 and X
5 is independently nitrogen, NR
9, or-CR
9=, X
7 is nitrogen or -CR
9=; wherein not more than 4 of X
2, X
4, X
5, X
6, and X
7 are nitrogen or NR
9; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, -C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; 15 QB\184200.00081\93036187.2
VVID-747PC z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0063] In some embodiments, the compound is selected from the group consisting of: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide; 2,5-Difluoro-4-(((S)-1-(2-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 2,5-Difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4-(((S)-1-(o- tolyl)ethyl)amino)benzamide; 4-(((S)-1-(2-Chloro-6-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide; 5-Chloro-4-(((S)-1-(2-chlorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide; 4-(((S)-1-(2-Chloro-6-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide; 4-(((*S)-1-(2-Chloro-5-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide; 4-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide; 4-(((*R)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide; 4-(((*S)-1-(2-Chloro-4-methylphenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide; 4-(((*S)-1-(2-Chloro-5-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide; 4-(((*R)-1-(2-Chloro-5-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide; (R,E)-4-((2-(2-Chlorophenyl)propan-2-yl)amino)-2,5-difluoro-N-(4-(methylsulfonyl)but-3-en-2- yl)benzamide; (R,E)-4-((1-(2-Chlorophenyl)cyclopropyl)amino)-2,5-difluoro-N-(4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((*S)-1-(2-Chlorophenyl)-2-methylpropyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide; 4-(((*R)-1-(2-Chlorophenyl)-2-methylpropyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide; 4-((1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide; 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 16 QB\184200.00081\93036187.2
VVID-747PC 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((R)-1-(2-Chlorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-((1-(2-Chlorophenyl)-2,2,2-trifluoroethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2,3-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((R)-1-(2-Chlorophenyl)ethyl)amino)-2,3-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-3-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)picolinamide; 4-((1-(2-Chlorophenyl)propyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((*R)-1-(2-Chlorophenyl)propyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((*S)-1-(2-Chlorophenyl)propyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-((1-(2-Chloro-4-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((*S)-1-(2-Chloro-4-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide; 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3- (trifluoromethyl)picolinamide; N-((R,E)-4-(Methylsulfonyl)but-3-en-2-yl)-5-(((S)-1-(2- (trifluoromethyl)phenyl)ethyl)amino)pyrimidine-2-carboxamide; 4-((1-(2-Chlorophenyl)-2-hydroxyethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((R)-1-(2-Chlorophenyl)-2-hydroxyethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide; 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine- 2-carboxamide; 5-((1-(2,3-Difluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide; 5-(((*S)-1-(2,3-Difluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 17 QB\184200.00081\93036187.2
VVID-747PC 5-(((*R)-1-(2,3-Difluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 4-((1-(2-Chloro-4-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide; 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2-methoxy-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2,6-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N1-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)isophthalamide; 2-Fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4-(((S)-1-(o-tolyl)ethyl)amino)benzamide; 2-Fluoro-4-(((S)-1-(2-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-((1-(2-Chlorophenyl)-2,2,2-trifluoroethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide; 4-(((*R)-1-(2-Chlorophenyl)-2,2,2-trifluoroethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide; 4-(((*S)-1-(2-Chlorophenyl)-2,2,2-trifluoroethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide; 4-((1-(2-Chlorophenyl)propyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((*S)-1-(2-Chlorophenyl)propyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((*R)-1-(2-Chlorophenyl)propyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-(((S)-1-(2-Chloro-4-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide; 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3- (trifluoromethyl)benzamide; 5-((1-(2-Chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 4-(((*S)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide; 4-(((*R)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide; 18 QB\184200.00081\93036187.2
VVID-747PC 4-((1-(2-Chlorophenyl)ethyl)amino)-1-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H- indazole-7-carboxamide; 8-((1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)imidazo[1,2- a]pyridine-5-carboxamide; 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3-(oxazol-2- yl)benzamide; 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2- carboxamide; 5-(((S)-1-(2-Chloro-4-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-((*S)-1-(2-Chloro-3,6-difluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 7-((1-(2-Chlorophenyl)ethyl)amino)-1-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H- indazole-4-carboxamide; 7-((1-(2-Chlorophenyl)ethyl)amino)-2-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-2H- indazole-4-carboxamide; 5-(((*R)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide; 5-(((*S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-Chloro-6-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide; 5-(((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrazine-2-carboxamide; 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-(((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide; 6-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)nicotinamide; 6-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)nicotinamide; 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-3,5-difluoro-N-((R, E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 19 QB\184200.00081\93036187.2
VVID-747PC 5-((S)-1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide; 5-((S)-1-(2-Chloro-4-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((1-(2-Chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-(((S)-1-(2-Chloro-4-methoxyphenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-((2-Chlorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*R)-(2-Chlorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-(2-Chlorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-3-cyano-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 5-(2-Chloro-3-fluorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide; 5-((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide; 5-(((*S)-1-(2-Chlorophenyl)ethyl)(cyclopropyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((2-Chlorophenyl)(tetrahydrofuran-3-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide 5-((*S)-(2-Chlorophenyl)((*R)-tetrahydrofuran-3-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide; 5-((*S)-(2-Chlorophenyl)((*S)-tetrahydrofuran-3-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide; 5-((*R)-(2-Chlorophenyl)((R)-tetrahydrofuran-2-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide; 5-((*R)-(2-Chlorophenyl)((S)-tetrahydrofuran-2-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide; 4-(1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide; 20 QB\184200.00081\93036187.2
VVID-747PC 4-((*S)-1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide; 4-(1-(2-Chlorophenyl)ethoxy)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide; 4-((S)-1-(2-Chlorophenyl)ethoxy)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-((S)-1-(2-Chloro-4-fluorophenyl)ethoxy)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 4-((S)-1-(2-Chlorophenyl)ethoxy)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 5-(((S)-1-(2-Chlorophenyl)ethyl)(methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 4-(((S)-1-(2-Chlorophenyl)ethyl)(methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 5-(((S)-1-(2-Chlorophenyl)ethyl)(ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 4-(((*R)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide; 4-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide; 6-((1-(2-Chlorophenyl)ethyl)amino)-4-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)nicotinamide; 6-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)nicotinamide; 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-3,6-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)picolinamide; 5-((*S)-1-(2-Chloro-3-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-Chloro-3,4-difluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(1-(2,3-Dichlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide; 5-((S)-1-(2-Chlorophenyl)ethoxy)-3-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)picolinamide; 5-((*R)-1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide; 21 QB\184200.00081\93036187.2
VVID-747PC 5-((*R)-1-(2-Chloro-3-fluorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-Chloro-3-fluorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 4-((S)-1-(2-Chlorophenyl)ethoxy)-3-(1H-imidazol-2-yl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide; 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)picolinamide; 8-(((S)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)imidazo[1,2- a]pyridine-5-carboxamide; 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-N2-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine- 2,4-dicarboxamide; 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-4-cyano-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((S)-1-(2-chlorophenyl)ethoxy)-4-methoxy-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((*S)-(2-chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-(((*R)-(2-chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-(((*S)-1-(3-fluoro-2-methylphenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-(((*R)-1-(3-fluoro-2-methylphenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-(((*S)-(2-chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-(((*R)-(2-chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-(((*S)-(2-chloro-3-fluorophenyl)((*R)-2,2-difluorocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide; 5-(((*R)-(2-chloro-3-fluorophenyl)((*S)-2,2-difluorocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide; 5-(((*S)-(2-chloro-3-fluorophenyl)((*S)-2,2-difluorocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide; 5-(((*R)-(2-chloro-3-fluorophenyl)((*R)-2,2-difluorocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide; 22 QB\184200.00081\93036187.2
VVID-747PC 5-(((*S)-1-(2-chloro-3-fluorophenyl)-2-methylpropyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrazine-2-carboxamide; 5-(((*R)-1-(2-chloro-3-fluorophenyl)-2-methylpropyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrazine-2-carboxamide; 5-(((*S)-1-(2-chloro-3-fluorophenyl)propyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-(((*R)-1-(2-chloro-3-fluorophenyl)propyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 4-(((S)-1-(2-chlorophenyl)ethyl)amino)-3-(1H-imidazol-2-yl)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide; 5-(((*S)-1-(2-chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide; 5-(((*R)-1-(2-chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide; 8-(((S)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)imidazo[1,2- c]pyrimidine-5-carboxamide; 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-4-(difluoromethyl)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide; 5-(((R)-1-(2-chloro-6-fluorophenyl)ethyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide; 5-(((S)-1-(2-chloro-6-fluorophenyl)ethyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide; 5-(((*R)-1-(2-chlorophenyl)-2,2-difluoroethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((*R)-(2-chloro-3-fluorophenyl)(1-cyanocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide; 5-(((*S)-(2-chloro-3-fluorophenyl)(1-cyanocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide; 5-((*S)-(2-chloro-3-fluorophenyl)(1-fluorocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*R)-(2-chloro-3-fluorophenyl)(1-fluorocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-(((*R)-(2-chloro-6-fluorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-(((*S)-(2-chloro-6-fluorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 23 QB\184200.00081\93036187.2
VVID-747PC 5-(((*R)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)-4-(trifluoromethyl)pyrimidine-2-carboxamide; 5-(((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)-4-(trifluoromethyl)pyrimidine-2-carboxamide; 8-(((S)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)- [1,2,4]triazolo[4,3-a]pyridine-5-carboxamide; 8-(((*R)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)imidazo[1,2-c]pyrimidine-5-carboxamide; 8-(((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)imidazo[1,2-c]pyrimidine-5-carboxamide; 8-(((S)-1-(2-chlorophenyl)ethyl)amino)-2-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)imidazo[1,2-c]pyrimidine-5-carboxamide; 4-(((S)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazolo[1,5- a]pyrazine-7-carboxamide; 7-(((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-1-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-1H-pyrazolo[4,3-c]pyridine-4-carboxamide; 7-(((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-2-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-2H-pyrazolo[4,3-c]pyridine-4-carboxamide; 8-(((*R)-(2-chloro-3-fluorophenyl)(oxetan-3-yl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)imidazo[1,2-c]pyrimidine-5-carboxamide; 8-(((*S)-(2-chloro-3-fluorophenyl)(oxetan-3-yl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)imidazo[1,2-c]pyrimidine-5-carboxamide; 8-(((*R)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)-[1,2,4]triazolo[4,3-c]pyrimidine-5-carboxamide; 8-(((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)-[1,2,4]triazolo[4,3-c]pyrimidine-5-carboxamide; 5-(((*R)-(2-chlorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-(((*S)-(2-chlorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-(((*S)-1-(2-chloro-3-fluorophenyl)ethyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide; 5-(((*R)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-(((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 24 QB\184200.00081\93036187.2
VVID-747PC 5-(((*S)-(2-chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-4-(trifluoromethyl)pyrimidine-2-carboxamide; 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(cyclopropylsulfonyl)but-3-en-2-yl)-4- methylpyrimidine-2-carboxamide; 5-(((*S)-1-(2-chloro-3-fluorophenyl)ethyl)amino)-4-(difluoromethyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-(((*S)-1-(3-fluoro-2-methylphenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((*R)-(2-chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(cyclopropylsulfonyl)but-3- en-2-yl)pyrazine-2-carboxamide; 5-(((*S)-(2-chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(cyclopropylsulfonyl)but-3- en-2-yl)pyrazine-2-carboxamide; 5-(((*S)-(2-chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide; 5-(((*R)-(2-chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide; 5-((2-chloro-3-fluorophenyl)(oxetan-3-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((S)-1-(2-chlorophenyl)ethoxy)-4-(hydroxymethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chloro-3-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4-(1- (trifluoromethyl)cyclopropyl)pyrimidine-2-carboxamide; 5-((*S)-(2-chloro-3-fluorophenyl)(3-methyloxetan-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*R)-(2-chloro-3-fluorophenyl)(3-methyloxetan-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*R)-1-(2-chloro-3-fluorophenyl)ethoxy)-4-((*S)-2,2-difluorocyclopropyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chloro-3-fluorophenyl)ethoxy)-4-((*R)-2,2-difluorocyclopropyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chloro-3-fluorophenyl)ethoxy)-4-((*S)-2,2-difluorocyclopropyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((S)-1-(2-chlorophenyl)ethoxy)-4-(methoxymethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*R)-(2-chlorophenyl)(1-cyanocyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 25 QB\184200.00081\93036187.2
VVID-747PC 5-((*S)-(2-chlorophenyl)(1-cyanocyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*R)-(2-chlorophenyl)((*R)-2,2-difluorocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*S)-(2-chlorophenyl)((*S)-2,2-difluorocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*R)-1-(2-chlorophenyl)-2-methylpropoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chlorophenyl)-2-methylpropoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*R)-(2-chlorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide; 5-((*S)-(2-chlorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide; 5-((*R)-1-(2-chloro-3-fluorophenyl)-2-methylpropoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chloro-3-fluorophenyl)-2-methylpropoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*R)-(2-chloro-3-fluorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*S)-(2-chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-((*R)-(2-chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; 5-(((S)-1-(2-chlorophenyl)ethyl)(methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-((S)-1-(2-chlorophenyl)ethoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*R)-(2-chlorophenyl)(cyclobutyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-(2-chlorophenyl)(cyclobutyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chlorophenyl)-2-methylpropoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 26 QB\184200.00081\93036187.2
VVID-747PC 5-((*R)-1-(2-chlorophenyl)-2-methylpropoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chlorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide; 5-((*R)-1-(2-chlorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide; 5-((*R)-1-(2-chloro-3-fluorophenyl)-2-methylpropoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chloro-3-fluorophenyl)-2-methylpropoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-4-cyclopropyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 1-((*R)-1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-indazole-5- carboxamide; 1-((*S)-1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-indazole-5- carboxamide; 3-((*R)-1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5- c]pyridine-6-carboxamide; 3-((*S)-1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5- c]pyridine-6-carboxamide; 1-((*R)-1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-pyrazolo[3,4- c]pyridine-5-carboxamide; 1-((*S)-1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-pyrazolo[3,4- c]pyridine-5-carboxamide; 1-(1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)indoline-5-carboxamide; 4-((*R)-1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3,4-dihydro-2H- benzo[b][1,4]oxazine-7-carboxamide; 4-((*S)-1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3,4-dihydro-2H- benzo[b][1,4]oxazine-7-carboxamide; 1-((*R)-(2-chlorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H- pyrazolo[3,4-c]pyridine-5-carboxamide; 1-((*S)-(2-chlorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H- pyrazolo[3,4-c]pyridine-5-carboxamide; 1-((*R)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-1H-pyrazolo[3,4-c]pyridine-5-carboxamide; 1-((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-1H-pyrazolo[3,4-c]pyridine-5-carboxamide; 27 QB\184200.00081\93036187.2
VVID-747PC 3-((*R)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide; 3-((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide; 3-((*R)-(2-chloro-3-fluorophenyl)(oxetan-3-yl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide; 3-((*S)-(2-chloro-3-fluorophenyl)(oxetan-3-yl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide; 3-((*S)-(2-chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide; 3-(((*R)-(2-chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide; 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-4-methoxy-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((2-chlorophenyl)(cyclopropyl)methyl)amino)-4-methoxy-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide; 5-(((2-chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-6- oxo-1,6-dihydropyrimidine-2-carboxamide; and 5-((*S)-(2-Chloro-3-fluorophenyl)(1-(2,2,2-trifluoroethyl)azetidin-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide; or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof. [0064] The present disclosure also provides a compound of Formula (Ia), or a pharmaceutically acceptable salt or solvate thereof: wherein:
R
1 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl; R
2 is hydrogen or C1-C6 alkyl; ring A is phenyl or a monocyclic or bicyclic heteroaryl ring having 1 to 3 nitrogen atoms; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; 28 QB\184200.00081\93036187.2
VVID-747PC each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0065] In some embodiments, R
1 and R
2 are methyl. [0066] In some embodiments, ring A is phenyl. In some embodiments, ring A is a monocyclic heteroaryl having 1 or 2 nitrogen atoms. In some embodiments, A is pyrimidinyl or pyrazinyl. In some embodiments, ring A is a bicyclic heteroaryl having 1 or 2 nitrogen atoms. [0067] In some embodiments, R
6 is halogen, C
1-C
6 alkyl or C
1-C
6 haloalkyl. [0068] In some embodiments, z is 0, 1 or 2. [0069] In some embodiments, X
1 is NH or O. In some embodiments, X
1 is NH. In some embodiments, X
1 is O. [0070] In some embodiments, R
3 is hydrogen and R
4 is C
1-C
6 alkyl, C
1-C
6 haloalkyl or C
3-C
6 cycloalkyl. [0071] In some embodiments, R
4 is -CH
3, -CF
3 or cyclopropyl. [0072] In some embodiments, each R
5 is halogen. [0073] In some embodiments, each R
5 is independently chloro or fluoro. [0074] In some embodiments, y is 1 or 2. [0075] The present disclosure further provides a compound of Formula (IIa), or a pharmaceutically acceptable salt or solvate thereof: wherein:
R
1 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl; R
2 is hydrogen or C
1-C
6 alkyl; 29 QB\184200.00081\93036187.2
VVID-747PC X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C3-C6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C1-C6 alkyl. [0076] The present disclosure further provides a compound of Formula (IIIa), or a pharmaceutically acceptable salt or solvate thereof: wherein: 1
R is C
1-C
6 or C
3-C
6 R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; 30 QB\184200.00081\93036187.2
VVID-747PC z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0077] The present disclosure further provides a compound of Formula (IVa), or a pharmaceutically acceptable salt or solvate thereof: wherein: R
1 is C
1-C6 R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0078] The present disclosure further provides a compound of Formula (Va), or a pharmaceutically acceptable salt or solvate thereof: QB\184200.00081\93036187.2
VVID-747PC (Va) wherein: R
1 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl; R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0079] The present disclosure further provides a compound of Formula (Va), or a pharmaceutically acceptable salt or solvate thereof, wherein: R
1 is C
1-C
4 alkyl; R
2 is C
1-C
4 alkyl; X
1 is NH; each of R
3 and R
4 is independently hydrogen, C
1-C
4 alkyl, or C
3-C
6 cycloalkyl, each R
5 is independently halogen or C
1-C
4 alkoxy; y is 1 or 2; and z is 0. [0080] According to further embodiments, the compounds of the disclosure are selected from the group consisting of: 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2- carboxamide; 5-(((S)-1-(2-chloro-4-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-((1-(2-chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-(((S)-1-(2-chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 32 QB\184200.00081\93036187.2
VVID-747PC 5-(((S)-1-(2-chloro-4-methoxyphenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide; 5-(((*R)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrazine-2-carboxamide; 5-(((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrazine-2-carboxamide. [0081] The present disclosure further provides a compound of Formula (VIa), or a pharmaceutically acceptable salt or solvate thereof: wherein: 1
R is C
1-C
6 R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0082] The present disclosure further provides a compound of Formula (VI), or a pharmaceutically acceptable salt or solvate thereof, wherein: R
1 is C
1-C
6 alkyl; R
2 is C
1-C
6 alkyl; 33 QB\184200.00081\93036187.2
VVID-747PC X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
4 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
4 alkyl, or C
3-C
6 cycloalkyl; each R
5 is independently halogen, or C
1-C
4 haloalkyl; y is 0, 1, 2, or 3; each R
6 is independently C
1-C
4 alkyl or C
1-C
4 haloalkyl; and z is 0 or 1. [0083] According to further embodiments, the compounds of the disclosure are selected from the group consisting of: 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide; 5-((S)-1-(2-chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide; N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-5-(((S)-1-(2- (trifluoromethyl)phenyl)ethyl)amino)pyrimidine-2-carboxamide; 5-((S)-1-(2-chloro-4-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((1-(2,3-difluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide; 5-((S)-1-(2-chloro-3-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((S)-1-(2,3-difluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((R)-1-(2,3-difluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((1-(2-chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((*S)-1-(2-chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chloro-3,6-difluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chloro-3,4-difluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((*R)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide; 5-(((*S)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide; 34 QB\184200.00081\93036187.2
VVID-747PC 5-((*R)-1-(2-chloro-3-fluorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chloro-3-fluorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*R)-1-(2-chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide; 5-(((S)-1-(2-chlorophenyl)ethyl)(ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*R)-(2-chlorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-(2-chlorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((2-chlorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-((*S)-1-(2-chloro-6-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(1-(2,3-dichlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide; 5-((*R)-(2-chloro-3-fluorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide; 5-((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide; 5-((2-chloro-3-fluorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((*S)-1-(2-chlorophenyl)ethyl)(cyclopropyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide; 5-(((*R)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide; 5-(((*S)-(2-chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide; and 5-(((S)-1-(2-chlorophenyl)ethyl)(methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. 35 QB\184200.00081\93036187.2
VVID-747PC [0084] The present disclosure further provides a compound of Formula (VIIa), or a pharmaceutically acceptable salt or solvate thereof: wherein: R
1 is C
1-C
6
R
2 is hydrogen or C
1-C
6 alkyl; X
1 is NR
9 or O; R
9 is hydrogen, C
1-C
6 alkyl, or C
3-C
6 cycloalkyl; each of R
3 and R
4 is independently hydrogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, wherein said C
3-C
6 cycloalkyl and C
3-C
6 heterocyclyl are optionally substituted with halogen, hydroxy, cyano, C
1-C
3 alkyl, or C
1-C
3 alkoxy; or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 cycloalkyl; each R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
1-C
6 alkoxy; y is 0, 1, 2 or 3; each of X
2, X
3, X
4 and X
5 is independently nitrogen, NR
9, carbon, or -CR
9-, wherein not more than 3 of X
2, X
3, X
4 and X
5 are nitrogen or NR
9; each R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
1-C
6 alkoxy, cyano, C(O)NR
7R
8, or a 5 to 6-membered heteroaryl ring; z is 0, 1 or 2; and R
7 and R
8 are each independently hydrogen or C
1-C
6 alkyl. [0085] The present disclosure provides for a pharmaceutical composition comprising a compound of the present disclosure, including without limitation a compound of Formula (I) through Formula (VII), or of Formula (Ia) through Formula (VIIa), or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof, and a pharmaceutically acceptable excipient or carrier. [0086] The present disclosure provides a method of inhibiting RAS-PI3K in a subject in need of such inhibition, comprising administering to the subject a therapeutically effective amount of a compound of the present disclosure, or a pharmaceutically salt, solvate, stereoisomer, or tautomer thereof. 36 QB\184200.00081\93036187.2
VVID-747PC [0087] The present disclosure provides a method of treating a disease or condition comprising administering to a subject in need thereof a therapeutically effective amount of a compound of the present disclosure, or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof. [0088] In some embodiments, the disease is cancer. [0089] In some embodiments, the cancer is selected from the group of bladder cancer, uterine cancer, head and neck cancer, esophageal cancer, ovarian cancer, liver cancer, cervical cancer, lung cancer, colorectal cancer, cholangiocarcinoma, gastric cancer, kidney cancer, and pancreatic cancer. [0090] In some embodiments, the disease or condition is an immunological disease or condition. [0091] In some embodiments, the immunological condition is wound healing deficiency. [0092] The present disclosure provides for use of a compound of the present disclosure, or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof for the treatment of cancer. [0093] In some embodiments, the use of the compound is to treat bladder cancer, uterine cancer, head and neck cancer, esophageal cancer, ovarian cancer, liver cancer, cervical cancer, lung cancer, colorectal cancer, cholangiocarcinoma, gastric cancer, kidney cancer, or pancreatic cancer. [0094] The present disclosure provides for use of a compound of the present disclosure, or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof for the treatment of an immunological disease or condition. [0095] In some embodiments, the immunological condition addressed by the use is wound healing deficiency. [0096] In some aspects, the present disclosure provides for a method treating cancer comprising administering a sufficient amount of a compound of Formula (I) or Formula (Ia) to a subject in need thereof. In some aspects, the subject is a human subject. In some aspects, the cancer is selected from the group consisting of bladder cancer, uterine cancer, head and neck cancer, esophageal cancer, ovarian cancer, liver cancer, cervical cancer, lung cancer, colorectal cancer, cholangiocarcinoma, gastric cancer, kidney cancer, and pancreatic cancer. [0097] In some aspects, the present disclosure provides for methods of promoting wound healing, comprising administering to a subject in need thereof a compound of Formula (I) or of Formula (Ia) in an amount sufficient to promote wound healing. [0098] In some aspects, the present disclosure provides for the use of the compounds of Formula (I) through Formula (VII) and Formula (Ia) through Formula (VIIa) in the treatment of cancer or the promotion of wound healing. [0099] In some aspects, the present disclosure provides for the use of the compounds of Formula (I) through Formula (VII) and Formula (Ia) through Formula (VIIa) in the formulation of a medicament useful for the treatment of cancer or the promotion of wound healing. [00100] In some aspects, the present disclosure provides for a method for treating cancer comprising administering a sufficient amount of a compound of Formula (I) or Formula (Ia) to a subject in need thereof. In some aspects, the subject is a human subject. In some aspects, the cancer is selected from the group 37 QB\184200.00081\93036187.2
VVID-747PC consisting of bladder cancer, uterine cancer, head and neck cancer, esophageal cancer, ovarian cancer, liver cancer, cervical cancer, lung cancer, colorectal cancer, cholangiocarcinoma, gastric cancer, kidney cancer, and pancreatic cancer. [00101] In some aspects, the present disclosure provides for methods of promoting wound healing, comprising administering to a subject in need thereof a compound of the present disclosure such as without limitation a compound of Formula (I) through Formula (VII) or Formula (Ia) through Formula (VIIa) in an amount sufficient to promote wound healing. [00102] Any combination of the groups described above for the various variables is contemplated herein. Throughout the specification, groups and substituents thereof are chosen by one skilled in the field to provide stable moieties and compounds. Further Forms of Compounds [00103] In some aspects, a compound disclosed herein possesses one or more stereocenters and each stereocenter exists independently in either the R or S configuration. The compounds presented herein include all diastereomeric, enantiomeric, and epimeric forms as well as the appropriate mixtures thereof. Furthermore, the compounds may exist as atropisomers. The compounds and methods provided herein include all cis, trans, syn, anti, entgegen (E), and zusammen (Z) isomers as well as the appropriate mixtures thereof. In certain embodiments, compounds described herein are prepared as their individual stereoisomers by reacting a racemic mixture of the compound with an optically active resolving agent to form a pair of diastereoisomeric compounds/salts, separating the diastereomers and recovering the optically pure enantiomers. In some embodiments, resolution of enantiomers is carried out using covalent diastereomeric derivatives of the compounds described herein. In another embodiment, diastereomers are separated by separation/resolution techniques based upon differences in solubility. In other embodiments, separation of stereoisomers is performed by chromatography or by the forming diastereomeric salts and separation by recrystallization, or chromatography, or any combination thereof. Jacques J., et al., “Enantiomers, Racemates and Resolutions”, John Wiley and Sons, Inc., 1981. In one aspect, stereoisomers are obtained by stereoselective synthesis. [00104] In some embodiments, compounds described herein are prepared as prodrugs. A “prodrug” refers to an agent that is converted into the parent drug in vivo. Prodrugs are often useful because, in some situations, they may be easier to administer than the parent drug. They may, for instance, be bioavailable by oral administration whereas the parent is not. The prodrug may also have improved solubility in pharmaceutical compositions over the parent drug. In some embodiments, the design of a prodrug increases the effective water solubility. An example, without limitation, of a prodrug is a compound described herein, which is administered as an ester (the “prodrug”) to facilitate transmittal across a cell membrane where water solubility is detrimental to mobility, but which then is metabolically hydrolyzed to the carboxylic acid, the active entity, once inside the cell where water solubility is beneficial. A further example of a prodrug might be a short peptide (polyaminoacid) bonded to an acid group where the peptide is metabolized 38 QB\184200.00081\93036187.2
VVID-747PC to reveal the active moiety. In certain embodiments, upon in vivo administration, a prodrug is chemically converted to the biologically, pharmaceutically or therapeutically active form of the compound. In certain embodiments, a prodrug is enzymatically metabolized by one or more steps or processes to the biologically, pharmaceutically or therapeutically active form of the compound. [00105] In one aspect, prodrugs are designed to alter the metabolic stability or the transport characteristics of a drug, to mask side effects or toxicity, to improve the flavor of a drug or to alter other characteristics or properties of a drug. By virtue of knowledge of pharmacokinetic, pharmacodynamic processes and drug metabolism in vivo, once a pharmaceutically active compound is known, the design of prodrugs of the compound is possible. (See, for example, Nogrady (1985) Medicinal Chemistry A Biochemical Approach, Oxford University Press, New York, pages 388-392; Silverman (1992), The Organic Chemistry of Drug Design and Drug Action, Academic Press, Inc., San Diego, pp. 352-401, Rooseboom et al., Pharmacological Reviews, 56:53–102, 2004; Aesop Cho, “Recent Advances in Oral Prodrug Discovery”, Annual Reports in Medicinal Chemistry, Vol.41, 395-407, 2006; T. Higuchi and V. Stella, Pro-drugs as Novel Delivery Systems, Vol.14 of the A.C.S. Symposium Series). [00106] In some embodiments, some of the herein-described compounds may be a prodrug for another derivative or active compound. [00107] In some embodiments, sites on the aromatic ring portion of compounds described herein are susceptible to various metabolic reactions. Therefore incorporation of appropriate substituents on the aromatic ring structures will reduce, minimize or eliminate this metabolic pathway. In specific embodiments, the appropriate substituent to decrease or eliminate the susceptibility of the aromatic ring to metabolic reactions is, by way of example only, a halogen, or an alkyl group. [00108] In another embodiment, the compounds described herein are labeled isotopically (e.g., with a radioisotope) or by another other means, including, but not limited to, the use of chromophores or fluorescent moieties, bioluminescent labels, or chemiluminescent labels. [00109] The compounds disclosed herein, in some embodiments, are used in different enriched isotopic forms, e.g., enriched in the content of
2H,
3H,
11C,
13C and/or
14C. In one particular embodiment, the compound is deuterated in at least one position. Such deuterated forms can be made by the procedure described in U.S. Patent Nos.5,846,514, and 6,334,997. As described in U.S. Patent Nos.5,846,514, and 6,334,997, deuteration can improve the metabolic stability and or efficacy, thus increasing the duration of action of drugs. [00110] Unless otherwise stated, compounds described herein are intended to include compounds which differ only in the presence of one or more isotopically enriched atoms. For example, compounds having the present structures except for the replacement of a hydrogen by a deuterium or tritium, or the replacement of a carbon by
13C- or
14C-enriched carbon are within the scope of the present disclosure. [00111] The compounds of the present disclosure optionally contain unnatural proportions of atomic isotopes at one or more atoms that constitute such compounds. For example, the compounds may be labeled with isotopes, such as for example, deuterium (
2H), tritium (
3H), iodine-125 (
125I) or carbon14 (
14C). 39 QB\184200.00081\93036187.2
VVID-747PC Isotopic substitution with
2H,
11C,
13C,
14C,
15C,
12N,
13N,
15N,
16N,
16O,
17O,
14F,
15F,
16F,
17F,
18F,
33S,
34S,
35S,
36S,
35Cl,
37Cl,
79Br,
81Br, and
125I are all contemplated. All isotopic variations of the compounds of the present invention, whether radioactive or not, are encompassed within the scope of the present invention. [00112] In some embodiments of a compound disclosed herein, one or more of R
1, R
2, R
3, R
4, R
5, R
5, R
6, R
7, R
8 groups comprise deuterium at a percentage higher than the natural abundance of deuterium. [00113] In some embodiments of a compound disclosed herein, one or more hydrogens are replaced with one or more deuteriums in one or more of the following groups R
1, R
2, R
3, R
4, R
5, R
5, R
6, R
7, R
8, R
9. [00114] In some embodiments of a compound disclosed herein, the abundance of deuterium in each of R
1, R
2, R
3, R
4, R
5, R
5, R
6, R
7, R
8, R
9 is independently at least 1%, at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, or 100% of a total number of hydrogen and deuterium. [00115] In certain embodiments, the compounds disclosed herein have some or all of the
1H atoms replaced with
2H atoms. The methods of synthesis for deuterium-containing compounds are known in the art and include, by way of non-limiting example only, the following synthetic methods. [00116] Deuterium substituted compounds are synthesized using various methods such as described in: Dean, Dennis C.; Editor. Recent Advances in the Synthesis and Applications of Radiolabeled Compounds for Drug Discovery and Development. [In: Curr., Pharm. Des., 2000; 6(10)] 2000, 110 pp; George W.; Varma, Rajender S. The Synthesis of Radiolabeled Compounds via Organometallic Intermediates, Tetrahedron, 1989, 45(21), 6601-21; and Evans, E. Anthony. Synthesis of radiolabeled compounds, J. Radioanal. Chem., 1981, 64(1-2), 9-32. [00117] Deuterated starting materials are readily available and are subjected to the synthetic methods described herein to provide for the synthesis of deuterium-containing compounds. Large numbers of deuterium-containing reagents and building blocks are available commercially from chemical vendors, such as Aldrich Chemical Co. [00118] In additional or further embodiments, the compounds described herein are metabolized upon administration to an organism in need to produce a metabolite that is then used to produce a desired effect, including a desired therapeutic effect. [00119] “Pharmaceutically acceptable” as used herein, refers a material, such as a carrier or diluent, which does not abrogate the biological activity or properties of the compound, and is relatively nontoxic, i.e., the material may be administered to an individual without causing undesirable biological effects or interacting in a deleterious manner with any of the components of the composition in which it is contained. [00120] The term “pharmaceutically acceptable salt” refers to a formulation of a compound that does not cause significant irritation to an organism to which it is administered and does not abrogate the biological activity and properties of the compound. In some embodiments, pharmaceutically acceptable salts are obtained by reacting a compound disclosed herein with acids. Pharmaceutically acceptable salts are also obtained by reacting a compound disclosed herein with a base to form a salt. 40 QB\184200.00081\93036187.2
VVID-747PC [00121] Compounds described herein may be formed as, and/or used as, pharmaceutically acceptable salts. The type of pharmaceutical acceptable salts, include, but are not limited to: (1) acid addition salts, formed by reacting the free base form of the compound with a pharmaceutically acceptable: inorganic acid, such as, for example, hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid, metaphosphoric acid, and the like; or with an organic acid, such as, for example, acetic acid, propionic acid, hexanoic acid, cyclopentanepropionic acid, glycolic acid, pyruvic acid, lactic acid, malonic acid, succinic acid, malic acid, maleic acid, fumaric acid, trifluoroacetic acid, tartaric acid, citric acid, benzoic acid, 3-(4- hydroxybenzoyl)benzoic acid, cinnamic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, 1,2-ethanedisulfonic acid, 2-hydroxyethanesulfonic acid, benzenesulfonic acid, toluenesulfonic acid, 2- naphthalenesulfonic acid, 4-methylbicyclo-[2.2.2]oct-2-ene-1-carboxylic acid, glucoheptonic acid, 4,4’- methylenebis-(3-hydroxy-2-ene-1-carboxylic acid), 3-phenylpropionic acid, trimethylacetic acid, tertiary butylacetic acid, lauryl sulfuric acid, gluconic acid, glutamic acid, hydroxynaphthoic acid, salicylic acid, stearic acid, muconic acid, butyric acid, phenylacetic acid, phenylbutyric acid, valproic acid, and the like; (2) salts formed when an acidic proton present in the parent compound is replaced by a metal ion, e.g., an alkali metal ion (e.g., lithium, sodium, potassium), an alkaline earth ion (e.g., magnesium, or calcium), or an aluminum ion. In some cases, compounds described herein may coordinate with an organic base, such as, but not limited to, ethanolamine, diethanolamine, triethanolamine, tromethamine, N-methylglucamine, dicyclohexylamine, tris(hydroxymethyl)methylamine. In other cases, compounds described herein may form salts with amino acids such as, but not limited to, arginine, lysine, and the like. Acceptable inorganic bases used to form salts with compounds that include an acidic proton, include, but are not limited to, aluminum hydroxide, calcium hydroxide, potassium hydroxide, sodium carbonate, sodium hydroxide, and the like. [00122] It should be understood that a reference to a pharmaceutically acceptable salt includes the solvent addition forms, particularly solvates. Solvates contain either stoichiometric or non-stoichiometric amounts of a solvent, and may be formed during the process of crystallization with pharmaceutically acceptable solvents such as water, ethanol, and the like. Hydrates are formed when the solvent is water, or alcoholates are formed when the solvent is alcohol. Solvates of compounds described herein can be conveniently prepared or formed during the processes described herein. In addition, the compounds provided herein can exist in unsolvated as well as solvated forms. In general, the solvated forms are considered equivalent to the unsolvated forms for the purposes of the compounds and methods provided herein. Pharmaceutical Compositions [00123] In another aspect, provided herein is a compound of the present disclosure, such as without limitation a compound of Formula (I) through Formula (VII) or a compound of Formula (Ia) through Formula (VIIa), or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a tautomer thereof, or a pharmaceutically acceptable salt of a stereoisomer thereof, or an atropisomer thereof, or a 41 QB\184200.00081\93036187.2
VVID-747PC pharmaceutically acceptable salt of a tautomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of an atropisomer thereof, for use in the manufacture of a medicament. [00124] In one aspect, the compounds described herein (e.g., compound of Formula (I), or pharmaceutically acceptable salts thereof, or a stereoisomer thereof, or a tautomer thereof, or a pharmaceutically acceptable salt of a stereoisomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of a tautomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of an atropisomer thereof) are formulated into pharmaceutical compositions. Pharmaceutical compositions are formulated in a conventional manner using one or more pharmaceutically acceptable inactive ingredients that facilitate processing of the active compounds into preparations that can be used pharmaceutically. Proper formulation is dependent upon the route of administration chosen. A summary of pharmaceutical compositions described herein can be found, for example, in Remington: The Science and Practice of Pharmacy, Nineteenth Ed (Easton, Pa.: Mack Publishing Company, 1995); Hoover, John E., Remington’s Pharmaceutical Sciences, Mack Publishing Co., Easton, Pennsylvania 1975; Liberman, H.A. and Lachman, L., Eds., Pharmaceutical Dosage Forms, Marcel Decker, New York, N.Y., 1980; and Pharmaceutical Dosage Forms and Drug Delivery Systems, Seventh Ed. (Lippincott Williams & Wilkins1999), herein incorporated by reference for such disclosure. [00125] A pharmaceutical composition, as used herein, refers to a mixture of a compound disclosed herein with other chemical components (i.e., pharmaceutically acceptable inactive ingredients), such as carriers, excipients, binders, filling agents, suspending agents, flavoring agents, sweetening agents, disintegrating agents, dispersing agents, surfactants, lubricants, colorants, diluents, solubilizers, moistening agents, plasticizers, stabilizers, penetration enhancers, wetting agents, anti-foaming agents, antioxidants, preservatives, or one or more combination thereof. The pharmaceutical composition facilitates administration of the compound to an organism. [00126] Pharmaceutical formulations described herein are administrable to a subject in a variety of ways by multiple administration routes, including but not limited to, oral, parenteral (e.g., intravenous, subcutaneous, intramuscular, intramedullary injections, intrathecal, direct intraventricular, intraperitoneal, intralymphatic, intranasal injections), intranasal, buccal, topical or transdermal administration routes. The pharmaceutical formulations described herein include, but are not limited to, aqueous liquid dispersions, self-emulsifying dispersions, solid solutions, liposomal dispersions, aerosols, solid dosage forms, powders, immediate release formulations, controlled release formulations, fast melt formulations, tablets, capsules, pills, delayed release formulations, extended release formulations, pulsatile release formulations, multiparticulate formulations, and mixed immediate and controlled release formulations. [00127] In some embodiments, the compounds disclosed herein are administered orally. [00128] In some embodiments, the compounds disclosed herein are administered topically. In such embodiments, the compound disclosed herein is formulated into a variety of topically administrable compositions, such as solutions, suspensions, lotions, gels, pastes, shampoos, scrubs, rubs, smears, 42 QB\184200.00081\93036187.2
VVID-747PC medicated sticks, medicated bandages, balms, creams or ointments. In one aspect, the compounds disclosed herein are administered topically to the skin. [00129] In another aspect, the compounds disclosed herein are administered by inhalation. [00130] In another aspect, the compounds disclosed herein are formulated for intranasal administration. Such formulations include nasal sprays, nasal mists, and the like. [00131] In another aspect, the compounds disclosed herein are formulated as eye drops. [00132] In any of the aforementioned aspects are further embodiments in which the effective amount of the compound disclosed herein is: (a) systemically administered to the mammal; and/or (b) administered orally to the mammal; and/or (c) intravenously administered to the mammal; and/or (d) administered by inhalation to the mammal; and/or (e) administered by nasal administration to the mammal; or and/or (f) administered by injection to the mammal; and/or (g) administered topically to the mammal; and/or (h) administered by ophthalmic administration; and/or (i) administered rectally to the mammal; and/or (j) administered non- systemically or locally to the mammal. [00133] In any of the aforementioned aspects are further embodiments comprising single administrations of the effective amount of the compound disclosed herein, including further embodiments in which (i) the compound is administered once; (ii) the compound is administered to the mammal multiple times over the span of one day; (iii) the compound is administered continually; or (iv) the compound is administered continuously. [00134] In any of the aforementioned aspects are further embodiments comprising multiple administrations of the effective amount of the compound disclosed herein, including further embodiments in which (i) the compound is administered continuously or intermittently: as in a single dose; (ii) the time between multiple administrations is every 6 hours; (iii) the compound is administered to the mammal every 8 hours; (iv) the compound is administered to the mammal every 12 hours; (v) the compound is administered to the mammal every 24 hours. In further or alternative embodiments, the method comprises a drug holiday, wherein the administration of the compound disclosed herein is temporarily suspended or the dose of the compound being administered is temporarily reduced; at the end of the drug holiday, dosing of the compound is resumed. In one embodiment, the length of the drug holiday varies from 2 days to 1 year. [00135] In certain embodiments, the compound disclosed herein is administered in a local rather than systemic manner. [00136] In some embodiments, the compound disclosed herein is administered topically. In some embodiments, the compound disclosed herein is administered systemically. [00137] In some embodiments, the pharmaceutical formulation is in the form of a tablet. In other embodiments, pharmaceutical formulations of the compounds disclosed herein are in the form of a capsule. [00138] In one aspect, liquid formulation dosage forms for oral administration are in the form of aqueous suspensions or solutions selected from the group including, but not limited to, aqueous oral dispersions, emulsions, solutions, elixirs, gels, and syrups. 43 QB\184200.00081\93036187.2
VVID-747PC [00139] For administration by inhalation, a compound disclosed herein is formulated for use as an aerosol, a mist or a powder. [00140] For buccal or sublingual administration, the compositions may take the form of tablets, lozenges, or gels formulated in a conventional manner. [00141] In some embodiments, compounds disclosed herein are prepared as transdermal dosage forms. [00142] In one aspect, a compound disclosed herein is formulated into a pharmaceutical composition suitable for intramuscular, subcutaneous, or intravenous injection. [00143] In some embodiments, the compound disclosed herein is be administered topically and can be formulated into a variety of topically administrable compositions, such as solutions, suspensions, lotions, gels, pastes, medicated sticks, balms, creams or ointments. [00144] In some embodiments, the compounds disclosed herein are formulated in rectal compositions such as enemas, rectal gels, rectal foams, rectal aerosols, suppositories, jelly suppositories, or retention enemas. Methods of Treatment [00145] In another aspect, provided herein is a compound of the present disclosure, such as without limitation a compound of Formula (I) through Formula (VII) or a compound of Formula (Ia) through Formula (VIIa), or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a tautomer thereof, or a pharmaceutically acceptable salt of a stereoisomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of a tautomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of an atropisomer thereof, for use in a method of modulating RAS-PI3K. In some embodiments, the method comprises inhibiting RAS-PI3K. In some embodiments, the method comprises activating RAS-PI3K. [00146] In another aspect, provided herein is a compound of Formula (I), or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a tautomer thereof, or a pharmaceutically acceptable salt of a stereoisomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of a tautomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of an atropisomer thereof, for use in a method of treating a disease or condition. In some embodiments, provided herein is a method of treating a disease or condition, the method comprising administering to a subject in need thereof, a therapeutically effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a tautomer thereof, or a pharmaceutically acceptable salt of a stereoisomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of a tautomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of an atropisomer thereof. In some embodiments, the disease or condition is mediated by RAS-PI3K. [00147] In another aspect, provided herein is a compound of the present disclosure, such as without limitation a compound of Formula (I) through Formula (VII), or a compound of Formula (Ia) through Formula (VIIa), or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a tautomer thereof, or a pharmaceutically acceptable salt of a stereoisomer thereof, or an atropisomer thereof, or a 44 QB\184200.00081\93036187.2
VVID-747PC pharmaceutically acceptable salt of a tautomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of an atropisomer thereof, for use in treating cancer in a patient in need thereof. [00148] In some embodiments, the disease is a cancer. In some embodiments, the cancer is selected from the group consisting of bladder cancer, uterine cancer, head and neck cancer, esophageal cancer, ovarian cancer, liver cancer, lung cancer, colorectal cancer, cervical cancer, cholangiocarcinoma, gastric cancer, kidney cancer, and pancreatic cancer. [00149] In another aspect, provided herein is a compound of the present disclosure, such as without limitation a compound of Formula (I) through Formula (VII), or a compound of Formula (Ia) through Formula (VIIa), or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a tautomer thereof, or a pharmaceutically acceptable salt of a stereoisomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of a tautomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of an atropisomer thereof, for use in a method of treating an immunological condition. In some embodiments, the immunological condition is a wound healing. [00150] In another aspect, provided herein is a compound of the present disclosure, such as without limitation a compound of Formula (I) through Formula (VII), or a compound of Formula (Ia) through Formula (VIIa), or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a tautomer thereof, or a pharmaceutically acceptable salt of a stereoisomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of a tautomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of an atropisomer thereof, for use in a method of treating a disease or condition. [00151] In some embodiments, administration of a compound of the present disclosure, such as without limitation a compound of Formula (I) through Formula (VII), or a compound of Formula (Ia) through Formula (VIIa), or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a tautomer thereof, or a pharmaceutically acceptable salt of a stereoisomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of a tautomer thereof, or an atropisomer thereof, or a pharmaceutically acceptable salt of an atropisomer thereof, promotes wound healing. Dosing and Treatment Regimens [00152] In one aspect, the compounds disclosed herein are used in the preparation of medicaments for the treatment of diseases or conditions described herein. In addition, a method for treating any of the diseases or conditions described herein in a subject in need of such treatment, involves administration of pharmaceutical compositions that include at least one compound disclosed herein or a pharmaceutically acceptable salt, active metabolite, prodrug, solvate, stereoisomer, or tautomer thereof, in therapeutically effective amounts to said subject. [00153] In some embodiments, the compositions containing the compound disclosed herein are administered for prophylactic and/or therapeutic treatments. In certain therapeutic applications, the compositions are administered to a patient already suffering from a disease or condition, in an amount sufficient to cure or at least partially arrest at least one of the symptoms of the disease or condition. 45 QB\184200.00081\93036187.2
VVID-747PC Amounts effective for this use depend on the severity and course of the disease or condition, previous therapy, the patient's health status, weight, and response to the drugs, and the judgment of the treating physician. Therapeutically effective amounts are optionally determined by methods including, but not limited to, a dose escalation clinical trial. [00154] In prophylactic applications, compositions containing the compounds disclosed herein are administered to a patient susceptible to or otherwise at risk of a particular disease, disorder or condition. [00155] In some embodiments, the dose of drug being administered may be temporarily reduced or temporarily suspended for a certain length of time (i.e., a “drug holiday”). [00156] Doses employed for adult human treatment are typically in the range of 0.01mg - 5000 mg per day or from about 1 mg to about 1000 mg per day. In one embodiment, the desired dose is conveniently presented in a single dose or in divided doses. Combination Treatments [00157] In certain instances, it is appropriate to administer at least one compound disclosed herein, or a pharmaceutically acceptable salt, solvate, stereoisomer, or tautomer thereof, in combination with another therapeutic agent. [00158] In one specific embodiment, a compound disclosed herein is co-administered with a second therapeutic agent, wherein the compound disclosed herein and the second therapeutic agent modulate different aspects of the disease, disorder or condition being treated, thereby providing a greater overall benefit than administration of either therapeutic agent alone. [00159] For combination therapies described herein, dosages of the co-administered compounds vary depending on the type of co-drug(s) employed, on the specific drug(s) employed, on the disease or condition being treated and so forth. In additional embodiments, when co-administered with one or more other therapeutic agents, the compound provided herein is administered either simultaneously with the one or more other therapeutic agents, or sequentially. [00160] If administration is simultaneous, the multiple therapeutic agents are, by way of example only, provided in a single, unified form, or in multiple forms. Definitions [00161] As used in this specification and the appended claims, the singular forms “a,” “an,” and “the” include plural referents unless the content clearly dictates otherwise. It should also be noted that the term “or” is generally employed in its sense including “and/or” unless the content clearly dictates otherwise. Further, headings provided herein are for convenience only and do not interpret the scope or meaning of the claimed invention. [00162] The terms below, as used herein, have the following meanings, unless indicated otherwise: [00163] “Oxo” refers to the =O substituent. 46 QB\184200.00081\93036187.2
VVID-747PC [00164] “Alkyl” refers to a straight or branched hydrocarbon chain radical, having from one to twenty carbon atoms, and which is attached to the rest of the molecule by a single bond. An alkyl comprising up to 10 carbon atoms is referred to as a C
1-C
10 alkyl, likewise, for example, an alkyl comprising up to 6 carbon atoms is a C
1-C
6 alkyl. Alkyls (and other moieties defined herein) comprising other numbers of carbon atoms are represented similarly. Alkyl groups include, but are not limited to, C
1-C
10 alkyl, C
1-C
9 alkyl, C
1-C
8 alkyl, C
1-C
7 alkyl, C
1-C
6 alkyl, C
1-C
5 alkyl, C
1-C
4 alkyl, C
1-C
3 alkyl, C
1-C
2 alkyl, C
2-C
8 alkyl, C
3-C
8 alkyl and C
4-C
8 alkyl. Representative alkyl groups include, but are not limited to, methyl, ethyl, npropyl, 1methylethyl (ipropyl), nbutyl, i-butyl, s-butyl, npentyl, 1,1dimethylethyl (tbutyl), 3methylhexyl, 2methylhexyl, 1-ethyl-propyl, and the like. In some embodiments, the alkyl is methyl or ethyl. Unless stated otherwise specifically in the specification, an alkyl group may be optionally substituted as described below. [00165] “Alkylene” refers to a straight or branched divalent hydrocarbon chain linking the rest of the molecule to a radical group. In some embodiments, the alkylene is -CH
2-, -CH
2CH
2-, or -CH
2CH
2CH
2-. In some embodiments, the alkylene is -CH
2-. In some embodiments, the alkylene is -CH
2CH
2-. In some embodiments, the alkylene is -CH
2CH
2CH
2-. [00166] “Alkoxy” refers to a radical of the formula OR where R is an alkyl radical as defined. Unless stated otherwise specifically in the specification, an alkoxy group may be optionally substituted as described below. Representative alkoxy groups include, but are not limited to, methoxy, ethoxy, propoxy, butoxy, pentoxy. In some embodiments, the alkoxy is methoxy. In some embodiments, the alkoxy is ethoxy. [00167] “Heteroalkyl” refers to an alkyl radical as described above where one or more carbon atoms of the alkyl is replaced with a O, N (i.e., NH, N-alkyl) or S atom. “Heteroalkylene” refers to a straight or branched divalent heteroalkyl chain linking the rest of the molecule to a radical group. Unless stated otherwise specifically in the specification, the heteroalkyl or heteroalkylene group may be optionally substituted as described below. Representative heteroalkyl groups include, but are not limited to -OCH
2OMe, - OCH
2CH
2OMe, or -OCH
2CH
2OCH
2CH
2NH
2. Representative heteroalkylene groups include, but are not limited to -OCH2CH2O-, -OCH2CH2OCH2CH2O-, or -OCH2CH2OCH2CH2OCH2CH2O-. [00168] “Alkylamino” refers to a radical of the formula -NHR or -NRR where each R is, independently, an alkyl radical as defined above. Unless stated otherwise specifically in the specification, an alkylamino group may be optionally substituted as described below. [00169] The term “aromatic” refers to a planar ring having a delocalized p-electron system containing 4n+2 p electrons, where n is an integer. Aromatics can be optionally substituted. The term “aromatic” includes both aryl groups (e.g., phenyl, naphthalenyl) and heteroaryl groups (e.g., pyridinyl, quinolinyl). [00170] “Aryl” refers to an aromatic ring wherein each of the atoms forming the ring is a carbon atom. Aryl groups can be optionally substituted. Examples of aryl groups include, but are not limited to phenyl, and naphthyl. In some embodiments, the aryl is phenyl. Depending on the structure, an aryl group can be a monoradical or a diradical (i.e., an arylene group). Unless stated otherwise specifically in the 47 QB\184200.00081\93036187.2
VVID-747PC specification, the term “aryl” or the prefix “ar” (such as in “aralkyl”) is meant to include aryl radicals that are optionally substituted. [00171] “Carboxy” refers to -CO
2H. In some embodiments, carboxy moieties may be replaced with a “carboxylic acid bioisostere”, which refers to a functional group or moiety that exhibits similar physical and/or chemical properties as a carboxylic acid moiety. A carboxylic acid bioisostere has similar biological properties to that of a carboxylic acid group. A compound with a carboxylic acid moiety can have the carboxylic acid moiety exchanged with a carboxylic acid bioisostere and have similar physical and/or biological properties when compared to the carboxylic acid-containing compound. For example, in one embodiment, a carboxylic acid bioisostere would ionize at physiological pH to roughly the same extent as a carboxylic acid group. Examples of bioisosteres of a carboxylic acid include, but are not limited to: , atoms
a may or partially unsaturated. Cycloalkyls may be fused with an aromatic ring (in which case the cycloalkyl is bonded through a non-aromatic ring carbon atom). Cycloalkyl groups include groups having from 3 to 10 ring atoms. Representative cycloalkyls include, but are not limited to, cycloalkyls having from three to ten carbon atoms, from three to eight carbon atoms, from three to six carbon atoms, or from three to five carbon atoms. In some embodiments, a cycloalkyl is a C
3-C
6cycloalkyl. In some embodiments, the cycloalkyl is monocyclic, bicyclic or polycyclic. In some embodiments, cycloalkyl groups are selected from among cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cycloheptyl, cyclooctyl, spiro[2.2]pentyl, bicyclo[1.1.1]pentyl, bicyclo[3.3.0]octane, bicyclo[4.3.0]nonane, bicyclo[2.1.1]hexane, bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane, bicyclo[3.2.2]nonane, bicyclo[3.3.2]decane, norbornyl, decalinyl and adamantyl. In some embodiments, the cycloalkyl is monocyclic. Monocyclic cyclcoalkyl radicals include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl. In some embodiments, the monocyclic cyclcoalkyl is cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl. In some embodiments, the cycloalkyl is bicyclic. Bicyclic cycloalkyl groups include fused bicyclic cycloalkyl groups, spiro bicyclic cycloalkyl groups, and bridged bicyclic cycloalkyl groups. In some embodiments, cycloalkyl groups are selected from among spiro[2.2]pentyl, bicyclo[1.1.1]pentyl, bicyclo[3.3.0]octane, bicyclo[4.3.0]nonane, bicyclo[2.1.1]hexane, bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane, bicyclo[3.2.2]nonane, bicyclo[3.3.2]decane, norbornyl, 3,4-dihydronaphthalen- 1(2H)-one and decalinyl. In some embodiments, the cycloalkyl is polycyclic. Polycyclic radicals include, 48 QB\184200.00081\93036187.2
VVID-747PC for example, adamantyl, and. In some embodiments, the polycyclic cycloalkyl is adamantyl. Unless otherwise stated specifically in the specification, a cycloalkyl group may be optionally substituted. [00173] “Fused” refers to any ring structure described herein which is fused to an existing ring structure. When the fused ring is a heterocyclyl ring or a heteroaryl ring, any carbon atom on the existing ring structure which becomes part of the fused heterocyclyl ring or the fused heteroaryl ring may be replaced with a nitrogen atom. [00174] “Halo” or “halogen” refers to bromo, chloro, fluoro or iodo. [00175] “Haloalkyl” refers to an alkyl radical, as defined above, that is substituted by one or more halo radicals, as defined above, e.g., trifluoromethyl, difluoromethyl, fluoromethyl, trichloromethyl, 2,2,2trifluoroethyl, 1,2difluoroethyl, 3bromo2fluoropropyl, 1,2dibromoethyl, and the like. Unless stated otherwise specifically in the specification, a haloalkyl group may be optionally substituted. [00176] “Haloalkoxy” refers to an alkoxy radical, as defined above, that is substituted by one or more halo radicals, as defined above, e.g., trifluoromethoxy, difluoromethoxy, fluoromethoxy, trichloromethoxy, 2,2,2trifluoroethoxy, 1,2difluoroethoxy, 3bromo2fluoropropoxy, 1,2dibromoethoxy, and the like. Unless stated otherwise specifically in the specification, a haloalkoxy group may be optionally substituted. [00177] “Hydroxyalkyl” refers to an alkyl radical, as defined above, that is substituted by one or more hydroxyl radicals. Unless stated otherwise specifically in the specification, a hydroxyalkyl group may be optionally substituted. [00178] “Heterocycloalkyl” or “heterocyclyl” or “heterocyclic ring” refers to a stable 3 to 14 membered nonaromatic ring radical comprising 2 to 10 carbon atoms and from one to 4 heteroatoms selected from the group consisting of nitrogen, oxygen, and sulfur. Unless stated otherwise specifically in the specification, the heterocycloalkyl radical may be a monocyclic, bicyclic ring (which may include a fused bicyclic heterocycloalkyl (when fused with an aryl or a heteroaryl ring, the heterocycloalkyl is bonded through a non-aromatic ring atom), bridged heterocycloalkyl or spiro heterocycloalkyl), or polycyclic. In some embodiments, the heterocycloalkyl is monocyclic or bicyclic. In some embodiments, the heterocycloalkyl is monocyclic. In some embodiments, the heterocycloalkyl is bicyclic. The nitrogen, carbon or sulfur atoms in the heterocyclyl radical may be optionally oxidized. The nitrogen atom may be optionally quaternized. The heterocycloalkyl radical is partially or fully saturated. Examples of such heterocycloalkyl radicals include, but are not limited to, dioxolanyl, thienyl[1,3]dithianyl, decahydroisoquinolyl, imidazolinyl, imidazolidinyl, isothiazolidinyl, isoxazolidinyl, morpholinyl, octahydroindolyl, octahydroisoindolyl, 2oxopiperazinyl, 2oxopiperidinyl, 2oxopyrrolidinyl, oxazolidinyl, piperidinyl, piperazinyl, 4piperidonyl, pyrrolidinyl, pyrazolidinyl, quinuclidinyl, thiazolidinyl, tetrahydrofuryl, trithianyl, tetrahydropyranyl, thiomorpholinyl, thiamorpholinyl, 1oxothiomorpholinyl, 1,1dioxothiomorpholinyl. The term heterocycloalkyl also includes all ring forms of carbohydrates, including but not limited to monosaccharides, disaccharides and oligosaccharides. Unless otherwise noted, heterocycloalkyls have from 2 to 10 carbons in the ring. In some embodiments, heterocycloalkyls have from 2 to 8 carbons in the ring. In some embodiments, heterocycloalkyls have from 2 to 8 carbons in the 49 QB\184200.00081\93036187.2
VVID-747PC ring and 1 or 2 N atoms. In some embodiments, heterocycloalkyls have from 2 to 10 carbons, 0-2 N atoms, 0-2 O atoms, and 0-1 S atoms in the ring. In some embodiments, heterocycloalkyls have from 2 to 10 carbons, 1-2 N atoms, 0-1 O atoms, and 0-1 S atoms in the ring. It is understood that when referring to the number of carbon atoms in a heterocycloalkyl, the number of carbon atoms in the heterocycloalkyl is not the same as the total number of atoms (including the heteroatoms) that make up the heterocycloalkyl (i.e., skeletal atoms of the heterocycloalkyl ring). Unless stated otherwise specifically in the specification, a heterocycloalkyl group may be optionally substituted. [00179] “Heteroaryl” refers to an aryl group that includes one or more ring heteroatoms selected from nitrogen, oxygen and sulfur. The heteroaryl is monocyclic or bicyclic. Illustrative examples of monocyclic heteroaryls include pyridinyl, imidazolyl, pyrimidinyl, pyrazolyl, triazolyl, pyrazinyl, tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl, pyrrolyl, pyridazinyl, triazinyl, oxadiazolyl, thiadiazolyl, furazanyl, indolizine, indole, benzofuran, benzothiophene, indazole, benzimidazole, purine, quinolizine, quinoline, isoquinoline, cinnoline, phthalazine, quinazoline, quinoxaline, 1,8-naphthyridine, and pteridine. Illustrative examples of monocyclic heteroaryls include pyridinyl, imidazolyl, pyrimidinyl, pyrazolyl, triazolyl, pyrazinyl, tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl, pyrrolyl, pyridazinyl, triazinyl, oxadiazolyl, thiadiazolyl, and furazanyl. Illustrative examples of bicyclic heteroaryls include indolizine, indole, benzofuran, benzothiophene, indazole, benzimidazole, purine, quinolizine, quinoline, isoquinoline, cinnoline, phthalazine, quinazoline, quinoxaline, 1,8-naphthyridine, and pteridine. In some embodiments, heteroaryl is pyridinyl, pyrazinyl, pyrimidinyl, thiazolyl, thienyl, thiadiazolyl or furyl. In some embodiments, a heteroaryl contains 0-4 N atoms in the ring. In some embodiments, a heteroaryl contains 1-4 N atoms in the ring. In some embodiments, a heteroaryl contains 0-4 N atoms, 0-1 O atoms, and 0-1 S atoms in the ring. In some embodiments, a heteroaryl contains 1-4 N atoms, 0-1 O atoms, and 0-1 S atoms in the ring. In some embodiments, heteroaryl is a C
1-C
9heteroaryl. In some embodiments, monocyclic heteroaryl is a C
1-C
5heteroaryl. In some embodiments, monocyclic heteroaryl is a 5-membered or 6-membered heteroaryl. In some embodiments, a bicyclic heteroaryl is a C
6- C9heteroaryl. [00180] The term “optionally substituted” or “substituted” means that the referenced group may be substituted with one or more additional group(s) individually and independently selected from alkyl, haloalkyl, hydroxyalkyl, cycloalkyl, aryl, heteroaryl, heterocycloalkyl, -OH, alkoxy, aryloxy, alkylthio, arylthio, alkylsulfoxide, arylsulfoxide, alkylsulfone, arylsulfone, -CN, alkyne, C
1-C
6alkylalkyne, halogen, acyl, acyloxy, -CO
2H, -CO
2alkyl, nitro, and amino, including mono and disubstituted amino groups (e.g., -NH
2, -NHR, -NR
2), and the protected derivatives thereof. In some embodiments, optional substituents are independently selected from alkyl, alkoxy, haloalkyl, cycloalkyl, halogen, -CN, -NH
2, -NH(CH
3), - N(CH
3)
2, -OH, -CO
2H, and -CO
2 alkyl. In some embodiments, optional substituents are independently selected from fluoro, chloro, bromo, iodo, -CH
3, -CH
2CH
3, -CF
3, -OCH
3, and -OCF
3. In some embodiments, substituted groups are substituted with one or two of the preceding groups. In some embodiments, an optional substituent on an aliphatic carbon atom (acyclic or cyclic) includes oxo (=O). 50 QB\184200.00081\93036187.2
VVID-747PC [00181] A “tautomer” refers to a proton shift from one atom of a molecule to another atom of the same molecule. The compounds presented herein may exist as tautomers. Tautomers are compounds that are interconvertible by migration of a hydrogen atom, accompanied by a switch of a single bond and adjacent double bond. In bonding arrangements where tautomerization is possible, a chemical equilibrium of the tautomers will exist. All tautomeric forms of the compounds disclosed herein are contemplated. The exact ratio of the tautomers depends on several factors, including temperature, solvent, and pH. Some examples of tautomeric interconversions include: .
of agents to a are to treatment in which the agents are administered by the same or different route of administration or at the same or different time. [00183] The terms “effective amount” or “therapeutically effective amount,” as used herein, refer to a sufficient amount of an agent or a compound being administered which will relieve to some extent one or more of the symptoms of the disease or condition being treated. The result can be reduction and/or alleviation of the signs, symptoms, or causes of a disease, or any other desired alteration of a biological system. For example, an “effective amount” for therapeutic uses is the amount of the composition comprising a compound as disclosed herein required to provide a clinically significant decrease in disease symptoms. An appropriate “effective” amount in any individual case may be determined using techniques, such as a dose escalation study. An “effective amount” is an amount sufficient for a compound to accomplish a stated purpose relative to the absence of the compound (e.g., achieve the effect for which it is administered, treat a disease, reduce enzyme activity, increase enzyme activity, reduce a signaling pathway, or reduce one or more symptoms of a disease or condition). An example of an “effective amount” is an amount sufficient to contribute to the treatment, prevention, or reduction of a symptom or symptoms of a disease, which could also be referred to as a “therapeutically effective amount.” A “reduction” of a symptom or symptoms (and grammatical equivalents of this phrase) means decreasing of the severity or frequency of the symptom(s), or elimination of the symptom(s). A “prophylactically effective amount” of a drug is an amount of a drug that, when administered to a subject, will have the intended prophylactic effect, e.g., preventing or delaying the onset (or reoccurrence) of an injury, disease, pathology or condition, or reducing the likelihood of the onset (or reoccurrence) of an injury, disease, pathology, or condition, or 51 QB\184200.00081\93036187.2
VVID-747PC their symptoms. The full prophylactic effect does not necessarily occur by administration of one dose, and may occur only after administration of a series of doses. Thus, a prophylactically effective amount may be administered in one or more administrations. An “activity decreasing amount,” as used herein, refers to an amount of antagonist required to decrease the activity of an enzyme relative to the absence of the antagonist. A “function disrupting amount,” as used herein, refers to the amount of antagonist required to disrupt the function of an enzyme or protein relative to the absence of the antagonist. The exact amounts will depend on the purpose of the treatment, and will be ascertainable by one skilled in the art using known techniques (see, e.g., Lieberman, Pharmaceutical Dosage Forms (vols.1-3, 1992); Lloyd, The Art, Science and Technology of Pharmaceutical Compounding (1999); Pickar, Dosage Calculations (1999); and Remington: The Science and Practice of Pharmacy, 20th Edition, 2003, Gennaro, Ed., Lippincott, Williams & Wilkins). [00184] The term “pharmaceutical combination” as used herein, means a product that results from the mixing or combining of more than one active ingredient and includes both fixed and non-fixed combinations of the active ingredients. The term “fixed combination” means that the active ingredients, e.g., a compound of Formula (I) and a co-agent, are both administered to a patient simultaneously in the form of a single entity or dosage. The term “non-fixed combination” means that the active ingredients, e.g., a compound of Formula (I) and a co-agent, are administered to a patient as separate entities either simultaneously, concurrently or sequentially with no specific intervening time limits, wherein such administration provides effective levels of the two compounds in the body of the patient. The latter also applies to cocktail therapy, e.g., the administration of three or more active ingredients. [00185] The term “subject” or “patient” encompasses mammals. Examples of mammals include, but are not limited to, humans. In one embodiment, the mammal is a human. [00186] The terms “treat,” “treating” or “treatment,” as used herein, include alleviating, abating or ameliorating at least one symptom of a disease or condition, preventing additional symptoms, inhibiting the disease or condition, e.g., arresting the development of the disease or condition, relieving the disease or condition, causing regression of the disease or condition, relieving a condition caused by the disease or condition, or stopping the symptoms of the disease or condition either prophylactically and/or therapeutically. [00187] All stereocenters labeled with (S) or (R) are known, absolute stereochemistry. In cases where the stereochemistry is pure but unknown, the stereocenter is drawn and defined as (*S) or (*R). Stereochemistry that is drawn but not labeled is in the correct cis or trans configuration but is racemic and designated as “rac”. Stereocenters that are not defined are racemic. Table 1. Compounds of the Disclosure. 52 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name N-
l)- - - 5-
QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name 5- - 5- 5- - d
e - d
e ,5-
54 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name ,5- N-
N- - N- N-
55 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name e E
)- N- N- -N- -
56 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name 2- r
o- e - i
de ide -
4- i
de -
4- i
de
57 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name -
N- - 4- 5- o)- -
58 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name o)- - -
o- e r
o- e - -
4- i
de
59 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name - - )- - )- - )- -5- i
de de
60 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name - )- )- - - 4- i
de 4- de
61 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name 4- de - - 4- ide 4- ide N-
62 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name i
de 4- i
de -
4- d
e - - 4- i
de - -
63 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name N- - ide ide )- 2- N- - - -
QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name - - 4-
N- E)-
65 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name - E)- - - -
4- N- e N- e
QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name 4- i
de - i
de 4- )-
67 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name -
4- i
de l
)- e - e -5- - - -
4- i
de
68 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name -N- -N- - - ide ide de de
69 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name * * de de 3- -3- - - ol- - -
70 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name
5-((*S)-(2-chloro-3-fluorophenyl)(1- 145 fluorocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide 5-((*R)-(2-chloro-3-fluorophenyl)(1- 146 fluorocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide 71 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name 5-(((*R)-(2-chloro-6- N- - N- - 4- 4- 4- ne- 4- ne- - - -7-
72 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name 7-(((*S)-(2-chloro-3- N- N- n- n- 4- 4- -4- -4- -
73 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name N- - N- - -N- ne- - -2- - - -N- 2- -N- 2-
QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name - n- - n- - - N- - 4- de - - - - ,2- 3-
75 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name ,2- 3- 2- 3- N- - y)- 2- y)- 2- ide ide yl- 2- yl- 2-
76 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name )- -4- E)- y)- -
77 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name - - 4- i
de E)- E)- )- E)- i
de ide
78 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name )- 2- )- 2- -N- -
79 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name e E)- E)- l)-
80 QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name l)- l)- l)- l)- l)-
QB\184200.00081\93036187.2
VVID-747PC Structure Ex No. IUPAC Name - - ide
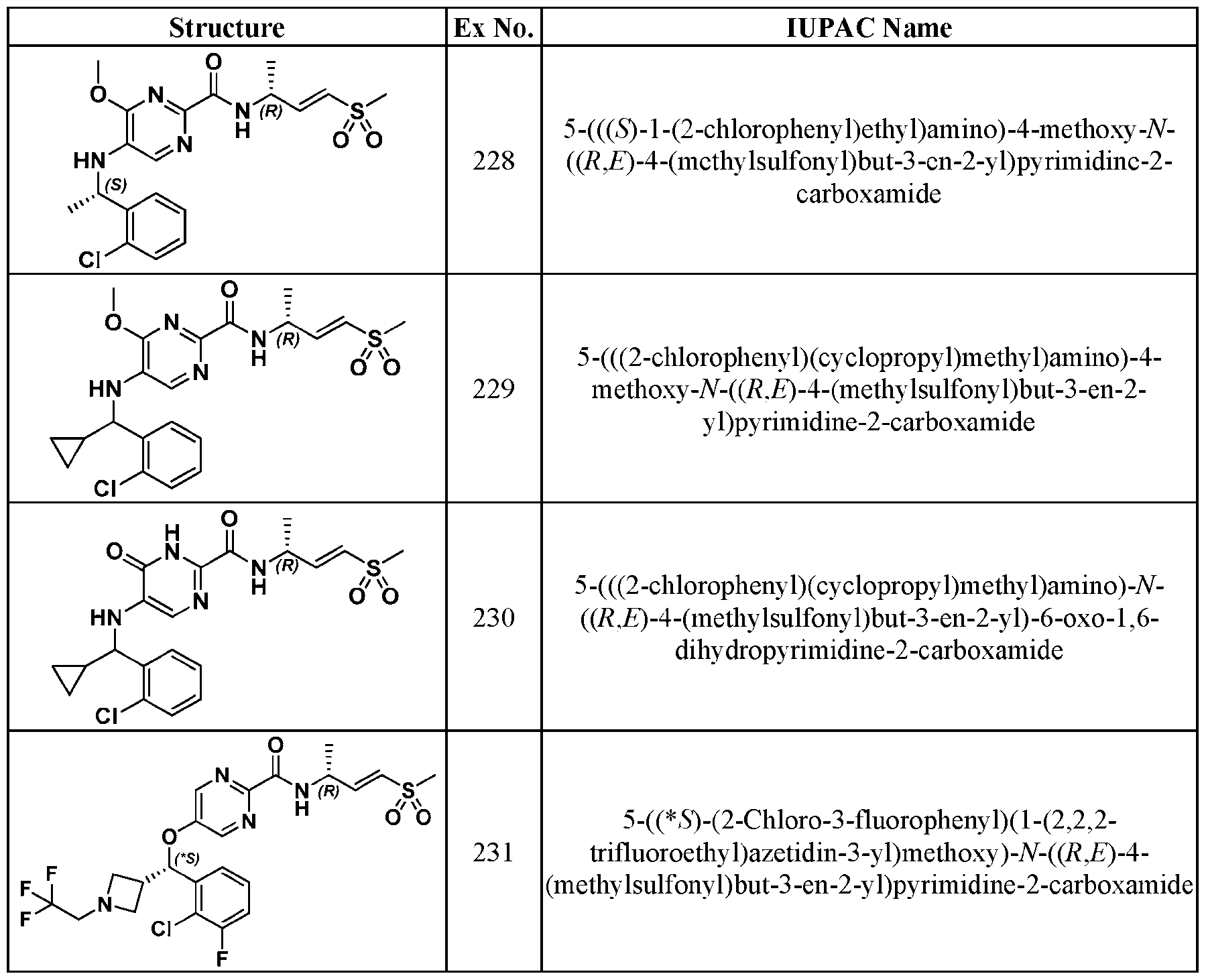
[00188] The following examples are offered to illustrate, but not to limit the claimed invention. The following examples further illustrate the invention but, of course, should not be construed as in any way limiting its scope. [00189] The following synthetic schemes are provided for purposes of illustration, not limitation. The following examples illustrate the various methods of making compounds described herein. It is understood that one skilled in the art may be able to make these compounds by similar methods or by combining other methods known to one skilled in the art. It is also understood that one skilled in the art would be able to make, in a similar manner as described below by using the appropriate starting materials and modifying the synthetic route as needed. In general, starting materials and reagents can be obtained from commercial vendors or synthesized according to sources known to those skilled in the art or prepared as described herein. [00190] In further embodiments, the compounds described herein, and other related compounds having different substituents are synthesized using techniques and materials described herein as well as those that are recognized in the field, such as described, for example, in Fieser and Fieser’s Reagents for Organic Synthesis, Volumes 1-17 (John Wiley and Sons, 1991); Rodd’s Chemistry of Carbon Compounds, Volumes 1-5 and Supplementals (Elsevier Science Publishers, 1989); Organic Reactions, Volumes 1-40 82 QB\184200.00081\93036187.2
VVID-747PC (John Wiley and Sons, 1991), Larock’s Comprehensive Organic Transformations (VCH Publishers Inc., 1989), March, Advanced Organic Chemistry 4th Ed., (Wiley 1992); Carey and Sundberg, Advanced Organic Chemistry 4th Ed., Vols. A and B (Plenum 2000, 2001), and Green and Wuts, Protective Groups in Organic Synthesis 3rd Ed., (Wiley 1999) (all of which are incorporated by reference for such disclosure). General methods for the preparation of compounds as disclosed herein may be derived from reactions and the reactions may be modified by the use of appropriate reagents and conditions, for the introduction of the various moieties found in the formulae as provided herein. As a guide the following synthetic methods may be utilized. Scheme 1
[00191]
in three steps from a compound of Formula (2) and a compound of Formula (3). In a first step, a compound of Formula (2), wherein ring A is phenyl or a monocyclic or bicyclic heteroaryl ring, R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 alkoxyl, cyano or a 5 to 6-membered heteroaryl ring, z is 0, 1 or 2, and Y is Br, is reacted under conventional Buchwald-Hartwig coupling conditions in the presence of a commercially available or synthetically accessible compound of Formula (3), wherein R
3 is C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, or C
3-C
6 cycloalkyl and R
4 is hydrogen or methyl or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 spirocycle, R
5 is independently halogen, C
1-C
6 alkyl, or C
1-C
6 haloalkyl, y is 1 or 2, and X is NH
2. The reaction takes place in the presence of a ligand/catalyst system such as SPhos Pd G4, RuPhos Pd G4, XantPhos/Pd
2(dba)
3, and the like; a base such as Cs
2CO
3, and the like; in a suitable solvent such as toluene; and the like; at temperatures of 80-110 °C for a period of 16-24 hours to provide a compound of Formula (4), where X
1 is NH. [00192] Alternatively, a compound of Formula (4) can be synthesized from a compound of Formula (2) and a compound of Formula (3) under nucleophilic aromatic substitution conditions. In this case, a compound of Formula (2), wherein ring A is phenyl or a monocyclic or bicyclic heteroaryl ring, R
6 is 83 QB\184200.00081\93036187.2
VVID-747PC independently halogen, C
1-C
6 alkyl, C
3-C
6 cycloalkyl, C
1-C
6 haloalkyl, C
1-C
6 alkoxyl, cyano, C
1-C
6 hydroxyalkyl, C
1-C
3 alkylene-OCH
3 or a 5 to 6-membered heteroaryl ring, z is 0, 1 or 2, and Y is F or Cl, is reacted under conventional S
NAr coupling conditions in the presence of a commercially available or synthetically accessible compound of Formula (3), wherein R
3 is C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, R
4 is hydrogen, R
5 is independently halogen, C
1-C
6 alkyl or C
1-C
6 alkoxyl, y is 1, 2 or 3, and X is NH
2 or OH. The reaction takes place using a suitable base such as K
2CO
3, Cs
2CO
3, KOtBu, DIPEA, TEA, pyridine, NaH, and the like; in a solvent such as DMSO, DMF, NMP, 1,4- dioxane, THF, and the like; at temperatures of 25-120 °C for a period of 3-20 hours to provide a compound of Formula (4), where X
1 is NH or O. In some cases, a compound of Formula (5) may be obtained directly from these reaction conditions. [00193] Alternatively, a compound of Formula (4) can be synthesized from a compound of Formula (2) and a compound of Formula (3) under nucleophilic substitution conditions. In this case, a compound of Formula (2), wherein ring A is a monocyclic heteroaryl ring, z is 0, and Y is NHcPr, is reacted under conventional S
N2 coupling conditions in the presence of a commercially available or synthetically accessible compound of Formula (3), wherein R
3 is methyl, R
4 is hydrogen, R
5 is halogen, y is 1, and X is Cl. The reaction takes place using a suitable base such as K
2CO
3, Cs
2CO
3, and the like; in a solvent such as DMSO, DMF, and the like; at a temperature of 85-100 °C for a period of 16 hours to provide a compound of Formula (4), where X
1 is NcPr. In another embodiment, R
6 together with Y, which is NH, forms a 5 to 6-membered heteroaryl or heterocyclic ring fused with ring A and is reacted under the conditions described to form a compound of Formula (4), where X
1 is N and forms a bicyclic ring with A. [00194] Alternatively, a compound of Formula (4) can be synthesized from a compound of Formula (2) and a compound of Formula (3) under Mitsunobu reaction conditions. In this case, a compound of Formula (2), wherein ring A is phenyl, R
6 is halogen, z is 0, 1 or 2, and Y is OH, is reacted under conventional Mitsunobu conditions in the presence of a commercially available or synthetically accessible compound of Formula (3), wherein R
3 is C
1-C
6 alkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl, R
4 is hydrogen, R
5 is halogen, y is 1 or 2, and X is OH. Under conditions known to one skilled in the art, the reaction takes place in the presence of DIAD, and the like; with PPh
3 in a suitable solvent such as THF, and the like; at rt for a period of 16-24 hours to provide a compound of Formula (4), where X
1 is O. In another embodiment, R
6 together with Y, which is NH, forms a 5 to 6-membered heteroaryl or heterocyclic ring fused with ring A and is reacted under the conditions described to form a compound of Formula (4), where X
1 is N and forms a bicyclic ring with A. [00195] Alternatively, a compound of Formula (4) can be synthesized from a compound of Formula (2) and a compound of Formula (3) under reductive amination conditions. In this case, a compound of Formula (2), wherein ring A is phenyl, R
6 is halogen, z is 1, and Y is NH
2, is reacted under conventional reductive amination conditions in the presence of a commercially available or synthetically accessible compound of Formula (3), wherein R
3 is methyl, R
4 does not exist, R
5 is halogen, y is 2, and X is O and forms a ketone with the carbon to which it is attached. Under conditions known to one skilled in the art, the reaction takes 84 QB\184200.00081\93036187.2
VVID-747PC place in the presence of TsOH and molecular sieves in a solvent such as dimethoxyethane, EtOH, and the like; at a temperature of 120 °C for 4 hours before addition of AcOH and sodium cyanoborohydride at rt reacting for a period of 2-12 hours to provide a compound of Formula (4), where X
1 is NH. [00196] In a second step, a compound of Formula (5), wherein ring A is phenyl or a monocyclic or bicyclic heteroaryl ring, R
6 is independently halogen, C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 alkoxyl, cyano, or a 5 to 6-membered heteroaryl ring, z is 0, 1 or 2, X
1 is NR
9 or O, R
9 is hydrogen, C
1-C
6 alkyl, C
3-C
6 cycloalkyl or together with R
6 forms a 5 to 6-membered heteroaryl or heterocyclic ring fused with ring A, R
3 is C
1-C
6 alkyl, C
1-C
6 haloalkyl, C
1-C
6 hydroxyalkyl, C
3-C
6 cycloalkyl, or C
3-C
6 heterocyclyl and R
4 is hydrogen or methyl or R
3 and R
4 together with the carbon atom to which they are attached form a C
3-C
6 spirocyclyl, R
5 is independently halogen, C
1-C
6 alkyl, C
1-C
6 alkoxyl, or C
1-C
6 haloalkyl, and y is 1, 2 or 3, is prepared from a compound of Formula (4). Using hydrolysis conditions known to one skilled in the art, a compound of Formula (4) is reacted with an appropriate hydroxide such as aqueous LiOH, and the like; in a suitable solvent such as MeOH, ACN, and the like; at temperatures of 0-80 °C for a period of 1-16 hours to provide a compound of Formula (5). [00197] In a third step, a compound of Formula (I), fully defined in Claim 1, is prepared from a compound of Formula (5) under amide coupling conditions known to one skilled in the art. Employing conventional amide bond forming techniques, a carboxylic acid such as a compound of Formula (5) is reacted with a coupling agent such as HATU, T
3P
®, EDC/DMAP, and the like; in the presence of an amine such as a compound of Formula (6), wherein R
2 is hydrogen or C
1-C
6 alkyl and R
1 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl, and a base such as DIPEA, TEA, and the like; in a solvent such as DMF, DCM, and the like; at rt for a period of 30 minutes to 16 hours to provide a compound of Formula (I). 85 QB\184200.00081\93036187.2
VVID-747PC Scheme 2
is formed from a compound of Formula (7) under S
N2 conditions using iodomethane, iodoethane, and the like; a suitable base such as NaH, and the like; in a solvent such as DMF, THF, and the like; at temperatures of 0-25 °C for a period of 30 min to 16 hours. The reaction provides a compound of Formula (8), where R
a is methyl or hydrogen. Similarly, a compound of Formula (9), wherein Y is F or Br, and X
4, X
5, and X
7 are independently N, NH, or CH, is reacted under the same conditions to provide a compound of Formula (10), where one of X
4 or X
5 is NR
9. Scheme 3 [00199]
a compound of Formula (11). A compound of Formula (11), wherein ring A is a monocyclic heteroaryl ring, R
6 is halogen, z is 1 or 2, X
1 is NH, R
3 is C
1-C
6 alkyl, R
4 is hydrogen, R
5 is halogen, and y is 1, is treated with NBS in a suitable solvent such as ACN, and the like; at a temperature of 80 °C for a period of 12 h to provide a compound of Formula (12), where Y is Br. In a second step, a compound of Formula (12), wherein Y is Br or I, is reacted under an atmosphere of carbon monoxide in the presence of a suitable base such as TEA, and the like; a ligand/catalyst system such as Pd(dppf)Cl
2, and the like; in a solvent such as DMF, MeOH, and the like; at temperatures of 70-80 °C for a period of 12-16 h to provide a compound of Formula (4). Similarly, a compound of Formula (2) is formed under the same reaction conditions from a compound of Formula (13), wherein ring A is a monocyclic heteroaryl ring, R
6 is C
1-C
6 alkyl, C
1-C
6 haloalkyl, or substituted C
3-C
6 cycloalkyl, z is 1, Y
1 is Cl or Br, and Y is Cl or F. 86 QB\184200.00081\93036187.2
VVID-747PC Scheme 4
be formed from a compound of Formula (14) or a compound of Formula (23) in three steps. In a first step, a compound of Formula (14), wherein R
5 is halogen and y is 1 or 2, is treated with 2-methylpropane-2- sulfinamide and Ti(OiPr)
4 in an appropriate solvent such as THF, and the like; at rt for a period of 16 h to provide a compound of Formula (15). The same chemistry is used to transform a compound of Formula (23), wherein R
3 is C
1-C
6 alkyl, C
3-C
6 cycloalkyl, or a substituted C
3-C
4 heterocycle, to a compound of Formula (24). In a second step, a compound of Formula (15) is reacted under conventional Grignard coupling conditions using iPrMgCl, cPrMgBr, EtMgBr, and the like; in a solvent such as THF, and the like; at a temperature of -78 °C for 1-4 hours to form a compound of Formula (16), where R
3 is C
1-C
6 alkyl, C
3-C
6 cycloalkyl, or a substituted C
3-C
4 heterocycle. A compound of Formula (24) is reduced using NaBH
4 in a solvent such as THF, MeOH, and the like; at rt for 3 h to provide a compound of Formula (16). Finally, a compound of Formula (16) is treated with an acid such as HCl, and the like; in a suitable solvent such as MeOH, and the like; at rt for a period of 2 h to provide a compound of Formula (3), where R
4 is hydrogen and X is NH
2. Scheme 5
or C
3-C
4 heterocyclyl, R
4 is hydrogen, R
5 is halogen, y is 1 or 2, and X is OH, can be synthesized from a compound of Formula (14) or from a compound of Formula (19) using Grignard coupling conditions with a compound of Formula (17) or a compound of Formula (18) as previously described in Scheme 4. 87 QB\184200.00081\93036187.2
VVID-747PC Alternatively, 2-bromochlorobenzene is treated with n-butyllithium in THF at -65 °C followed by treatment of a compound of Formula (19) for 1 h to provide a compound of Formula (3). A compound of Formula (3), wherein X is OH and R
4 is hydrogen, can be oxidized using DMP in DCM at rt for 16 h to provide a compound of Formula (23). Scheme 6
, or a a of Formula (20) is reacted under amide coupling conditions previously described in Scheme 1 with N,O- dimethylhydroxylamine to form a compound of Formula (21). Next, an aryl bromide such as a compound of Formula (22), wherein R
5 is halogen and y is 1 or 2, is treated with n-BuLi in a solvent such as THF, and the like; at a temperature of -78 °C for 30 minutes. This solution is then treated with a compound of Formula (21) at -78 °C, stirring for 3 hours to provide a compound of Formula (23). Alternatively, a compound of Formula (21) can be reacted under Grignard conditions as described in Scheme 4 with a compound of Formula (17) to provide a compound of Formula (23). Finally, reduction of a compound of Formula (23) with NaBH
4, NaBH
4CN, and the like; in a solvent such as MeOH, and the like; at 0-25 °C for a period of 1-2 hours provides a compound of Formula (3), where R
3 is C
3-C
6 cycloalkyl or C
3-C
6 heterocyclyl, R
4 is hydrogen, R
5 is halogen, y is 1 or 2, and X is OH. Scheme 7
(a compound of Formula (4)) can be synthesized from methyl 4-(1-(2-chlorophenyl)ethoxy)-3- formylbenzoate using glyoxal and ammonium acetate in a suitable solvent such as EtOH, and the like; at rt for a period of 16 hours. The same conditions are used to convert methyl 4-fluoro-3-formylbenzoate to methyl 4-fluoro-3-(1H-imidazol-2-yl)benzoate, a compound of Formula (2). Scheme 8 88 QB\184200.00081\93036187.2
VVID-747PC two steps
described in Scheme 1, 2-bromo-5-(methoxycarbonyl)benzoic acid is coupled with 2,2-dimethoxyethan- 1-amine to provide methyl 4-bromo-3-((2,2-dimethoxyethyl)carbamoyl)benzoate. Cyclization using P
2O
5 in methanesulfonic acid at 140 °C for a period of 2 hours provides methyl 4-bromo-3-(oxazol-2- yl)benzoate, a compound of Formula (2). Scheme 9 [00205] According to carboxylate (a compound of
Formula (2)) can be 5- 2- and cyclopropylamine using S
NAr conditions previously described in Scheme 1. Scheme 10 [00206] According to
ethan-1-amine (a compound of Formula (3)) can be synthesized from 2-amino-2-(2-chlorophenyl)ethan-1-ol using tert- butyldimethylsilyl chloride and DMAP in the presence of a base such as TEA, and the like; in a solvent such as DCM, and the like; at rt for a period of 10 hours. Scheme 11
or cyclopropyl, can be formed from tert-butyl (1-oxopropan-2-yl)carbamate in two steps. In a first step, the appropriate phosphonate such as diethyl ((methylsulfonyl)methyl)phosphonate or diethoxyphosphorylmethylsulfonylcyclopropane is treated with a base such as NaH, and the like; in a solvent such as THF, and the like; at 0 °C for 30 min before addition of tert-butyl (1-oxopropan-2- yl)carbamate. Reaction at 0 °C for a period of 1 hour provides a compound of Formula (25). In a second 89 QB\184200.00081\93036187.2
VVID-747PC step, a compound of Formula (25) is treated with TsOH in a solvent such as ACN, and the like; at 60 °C for a period of 12 h to provide a compound of Formula (6), where R
2 is methyl and R
1 is methyl or cyclopropyl. Scheme 12
[00208] According to a , is C
3-C
4 heterocyclyl, R
5 is halogen, and y is 1 or 2, is treated with ammonium acetate, sodium cyanoborohydride, and ammonia in EtOH at 110 °C for 16 h to provide a compound of Formula (3), where R
4 is hydrogen and X is NH
2. Scheme 13
as 1- carboxylic acid, 2,2-difluoroacetic acid, cyclopropanecarboxylic acid, and the like; with AgNO
3 and ammonium persulfate in ACN/water at 85-100 °C for 16 h to provide a compound of Formula (13), where ring A is pyrimidine, R
6 is C
1-C
6 haloalkyl or C
3-C
6 cycloalkyl, z is 1, Y
1 is Cl, and Y is F. Under the same conditions, 5-fluoropyrimidine-2-carbonitrile can also be converted to a compound of Formula (13), where Y
1 is cyano. Scheme 14
5- fluoropyrimidine in three steps. First, 2-chloro-5-fluoropyrimidine is reacted with BPO and TFA in MeOH at 65 °C for 16 h to form (2-chloro-5-fluoro-pyrimidin-4-yl)methanol. Second, (2-chloro-5-fluoro- pyrimidin-4-yl)methanol is converted to the methyl carboxylate using conditions previously described in Scheme 3 to provide methyl 5-fluoro-4-(hydroxymethyl)pyrimidine-2-carboxylate. Finally, methyl 5- fluoro-4-(hydroxymethyl)pyrimidine-2-carboxylate is treated with either Ag
2O and iodomethane or acetic anhydride and TEA in DCM at 20 °C for 16 h to provide a compound of Formula (26), where R
a is methyl or acetyl respectively. Scheme 15 90 QB\184200.00081\93036187.2
VVID-747PC O O N N O O [00211] According to 2-carboxylate is converted to methyl 8-fluoroimidazo
of Formula (2)) using chloroacetaldehyde in MeOH at 100 °C for 12 h. Scheme 16 Cl Cl Cl N N F N [00212] (a compound of Formula (13))
2,6-dichloro-3- fluoropyridine is reacted with hydrazine hydrate in EtOH at 80 °C for 16 h to provide (6-chloro-3-fluoro- 2-pyridyl)hydrazine. Second, (6-chloro-3-fluoro-2-pyridyl)hydrazine is reacted with trimethoxymethane and TFA at 100 °C for 16 h to give 5-chloro-8-fluoro-[1,2,4]triazolo[4,3-a]pyridine. Scheme 17 of
can steps 2,4- 5- 2,4- 5- fluoropyrimidine is reacted under S
NAr conditions with 2-aminopropan-1-ol and DIPEA in ACN at 75 °C for 24 h to give 2-((2-chloro-5-fluoropyrimidin-4-yl)amino)propan-1-ol. 2-((2-Chloro-5-fluoropyrimidin- 4-yl)amino)propan-1-ol is treated with TEA and Ms
2O in DCM at 45 °C for 12 h to give 5-chloro-8-fluoro- 2-methyl-2,3-dihydroimidazo[1,2-c]pyrimidine. 5-Chloro-8-fluoro-2-methyl-2,3-dihydroimidazo[1,2- c]pyrimidine is oxidized using activated MnO
2 in 1,4-dioxane at 90 °C for 12 h to give 5-chloro-8-fluoro- 2-methylimidazo[1,2-c]pyrimidine. Scheme 18 H H N O N O N Br [00214]
of Formula (13)) can be prepared in two steps from pyrazolo[1,5-a]pyrazin-4(5H)-one. Pyrazolo[1,5-a]pyrazin-4(5H)- one is treated with NCS and acetic acid in DMF at 80 °C for 30 min to provide 7-chloropyrazolo[1,5- a]pyrazin-4(5H)-one. 7-Chloropyrazolo[1,5-a]pyrazin-4(5H)-one is reacted with phosphorus oxybromide in toluene at 110 °C for 4 h to give 4-bromo-7-chloropyrazolo[1,5-a]pyrazine. 91 QB\184200.00081\93036187.2
VVID-747PC Scheme 19 O N Cl N Cl N O N N [00215] (a compound
2,4- Dichloro-5-fluoropyrimidine is reacted under S
NAr conditions with tert-butyl hydrazinecarboxylate and DIPEA in THF at 20 °C for 2 h to provide tert-butyl 2-(2-chloro-5-fluoropyrimidin-4-yl)hydrazine-1- carboxylate. tert-Butyl 2-(2-chloro-5-fluoropyrimidin-4-yl)hydrazine-1-carboxylate is reacted under conditions previously described in Scheme 3 to transform the chloride into methyl carboxylate. Methyl 4- (2-(tert-butoxycarbonyl)hydrazinyl)-5-fluoropyrimidine-2-carboxylate is Boc deprotected using standard conditions known to those skilled in the art. Treatment with TFA in DCM at rt for 2 hours provides methyl 5-fluoro-4-hydrazinylpyrimidine-2-carboxylate. Methyl 5-fluoro-4-hydrazinylpyrimidine-2-carboxylate is treated with trimethoxymethane and TFA at 100 °C for 16 h to give methyl 8-fluoro-[1,2,4]triazolo[4,3- c]pyrimidine-5-carboxylate. Scheme 20 [00216] According to
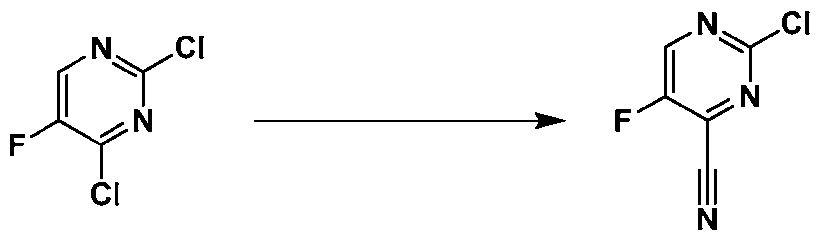
(a compound of Formula (13)) is prepared from 2,4-dichloro-5-fluoropyrimidine using 1,4-diazabicyclo[2.2.2]octane and sodium cyanide in DMF/water at rt for 5 h. Scheme 21 6 N O R N (R
6)
z OH [00217] According
C
1-C
6 alkoxyl or C
3-C
6 cycloalkyl and R
3 is C
1-C
6 alkyl or C
3-C
6 cycloalkyl, is treated with 10% aq. NaOH at 100 °C for 16 h to provide a compound of Formula (5), where ring A is pyrimidine, R
4 is hydrogen, and X
1 is NH. In the 92 QB\184200.00081\93036187.2
VVID-747PC instance of a compound of Formula (27) wherein R
6 is -OCH
3, treatment under these conditions can provide a compound of Formula (5) where R
6 is oxo. Scheme 22 3-
methyl 5-((1-(tert-butoxycarbonyl)azetidin-3-yl)(2-chloro-3-fluorophenyl)methoxy)pyrimidine-2- carboxylate. First, Boc deprotection as previously described in Scheme 19 is employed. The TFA salt is removed with an aq. NaHCO
3 wash to provide methyl 5-(azetidin-3-yl-(2-chloro-3- fluorophenyl)methoxy)pyrimidine-2-carboxylate. Treatment with TEA and 2,2,2-trifluoroethyl trifluoromethanesulfonate in toluene at 80 ℃ for 3 h provides methyl 5-((2-chloro-3-fluorophenyl)(1- (2,2,2-trifluoroethyl)azetidin-3-yl)methoxy)pyrimidine-2-carboxylate (a compound of Formula (4)). Abbreviation Term 1H NMR Proton nuclear magnetic resonance
QB\184200.00081\93036187.2
VVID-747PC Abbreviation Term m/z Mass-to-charge ratio NBS N-Bromosuccinimide )
Intermediate 1: (R,E)-4-Methylsulfonylbut-3-en-2-amine.
Butyl (R,E)-(4-(methylsulfonyl)but-3-en-2-yl)carbamate. To a solution of diethyl ((methylsulfonyl)methyl)phosphonate (2.7 g, 11.5 mmol, 1.0 eq) in THF (60 mL, 0.19 M) was added NaH (462 mg, 11.5 mmol, 1.0 eq, 60% in mineral oil) at 0°C. The mixture was stirred at 0°C for 30 min before addition of (R)-tert-butyl (1-oxopropan-2-yl)carbamate (2.0 g, 11.5 mmol, 1.0 eq) in THF (10 mL). The reaction was stirred at 0°C for 1 h before being quenched with water and extracted with EtOAc. The combined organic layers were dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by prep-HPLC to give tert-butyl (R,E)-(4-(methylsulfonyl)but-3-en-2- yl)carbamate (950 mg, 33% yield) as a white solid. [00220] Step B: (R,E)-4-Methylsulfonylbut-3-en-2-amine. To a solution of tert-butyl (R,E)-(4- (methylsulfonyl)but-3-en-2-yl)carbamate (200 mg, 0.800 mmol, 1.0 eq) in ACN (3.0 mL, 0.27 M) was added 4-methylbenzenesulfonic acid (138 mg, 0.800 mmol, 1.0 eq). The mixture was stirred at 60°C for 12 h. After cooling to rt, the reaction was concentrated to give (R,E)-4-(methylsulfonyl)but-3-en-2-amine, 94 QB\184200.00081\93036187.2
VVID-747PC 4-methylbenzenesulfonic acid (200 mg, 78% yield) as a white solid.
1H NMR (400 MHz, Methanol-d
4) δ 7.71 (d, J = 8.1 Hz, 2H), 7.24 (d, J = 8.0 Hz, 2H), 6.92 - 7.01 (m, 1H), 6.81 - 6.89 (m, 1H), 4.18 (q, J = 6.5 Hz, 1H), 3.02 (s, 3H), 2.37 (s, 3H), 1.47 (d, J = 6.8 Hz, 3H). Intermediate 2: (*S)-1-(2-Chlorophenyl)-2-methylpropan-1-amine. A: (R,E)-N-(2-Chlorobenzylidene)-2-methylpropane-2-sulfinamide. To a solution of 2-
(1.5 g, 10.7 mmol, 1.0 eq) in THF (15 mL, 0.71 M) was added (R)-2-methylpropane- 2-sulfinamide (1.4 g, 11.7 mmol, 1.1 eq) and Ti(OiPr)
4 (5.0 mL). The reaction was stirred at 20°C for 16 h before being diluted with H
2O and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, and filtered. The filtrate was concentrated under vacuum to give (R,E)-N- (2-chlorobenzylidene)-2-methylpropane-2-sulfinamide (2.6 g, quant. yield) as a yellow oil, which was used in the next step without further purification. [00222] Step B: (R)-N-((*S)-1-(2-Chlorophenyl)-2-methylpropyl)-2-methylpropane-2-sulfinamide. To a solution of (R,E)-N-(2-chlorobenzylidene)-2-methylpropane-2-sulfinamide (2.6 g, 10.7 mmol, 1.0 eq) in THF (75 mL, 0.14 M) at -78°C was added iPrMgCl (2M, 21.3 mL, 42.7 mmol, 4.0 eq) dropwise over a period of 0.5 h. The reaction was stirred at -78°C for 1 h. After warming to rt, the reaction was diluted with H
2O and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The crude material was purified via RP-HPLC (40-70% ACN in 10 mM aq. NH
4HCO
3 (second eluting product)) to obtain (R)-N-((*S)-1-(2- chlorophenyl)-2-methylpropyl)-2-methylpropane-2-sulfinamide (800 mg, 26% yield) as a white solid. MS (ESI): mass calcd. for C
14H
22ClNOS, 287.1; m/z found, 288.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.34 - 7.38 (m, 1H), 7.30 - 7.34 (m, 1H), 7.22 - 7.27 (m, 1H), 7.17 - 7.22 (m, 1H), 4.64 - 4.75 (m, 1H), 3.50 - 3.57 (m, 1H), 2.06 - 2.19 (m, 1H), 1.18 (s, 9H), 1.03 (d, J = 6.8 Hz, 3H), 0.92 (d, J = 6.9 Hz, 3H). [00223] Step C: (*S)-1-(2-Chlorophenyl)-2-methylpropan-1-amine. To a solution of (R)-N-((*S)-1-(2- chlorophenyl)-2-methylpropyl)-2-methylpropane-2-sulfinamide (800 mg, 2.78 mmol, 1.0 eq) in MeOH (8.0 mL, 0.35 M) was added concentrated HCl (4.0 mL, 50.4 mmol, 18 eq). The reaction was stirred at 20°C for 2 h before being concentrated under vacuum to provide (*S)-1-(2-chlorophenyl)-2-methylpropan- 1-amine (600 mg, 98% yield) as a yellow oil. This was used without further purification. MS (ESI): mass calcd. for C10H14ClN, 183.1; m/z found, 184.0 [M+H]
+.
1H NMR (400 MHz, CDCl3) δ 8.80 (br s, 2H), 7.63 - 7.75 (m, 1H), 7.36 - 7.46 (m, 1H), 7.27 (br s, 2H), 4.51 - 4.68 (m, 1H), 2.36 (br s, 1H), 1.13 - 1.18 (m, 3H), 0.86 - 0.96 (m, 3H). Intermediate 3: (*R)-1-(2-Chlorophenyl)-2-methylpropan-1-amine. 95 QB\184200.00081\93036187.2
VVID-747PC ((*R)-1-(2-Chlorophenyl)-2-methylpropyl)-2-methylpropane-2-sulfinamide was isolated
of Intermediate 2, Step B (first eluting product) and elaborated in an analogous manner to provide the title compound. MS (ESI): mass calcd. for C
10H
14ClN, 183.1; m/z found, 184.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.62 - 8.95 (m, 2H), 7.61 - 7.76 (m, 1H), 7.37 - 7.43 (m, 1H), 7.27 - 7.30 (m, 1H), 7.24 - 7.27 (m, 1H), 4.58 (br s, 1H), 2.35 (br d, J = 6.4 Hz, 1H), 1.13 - 1.17 (m, 3H), 0.90 (br d, J = 6.5 Hz, 3H). Intermediate 4: (*S)-(2-Chlorophenyl)(cyclopropyl)methanamine. compound was prepared in a manner analogous to Intermediate 2 using cPrMgBr instead
Step B.
1H NMR (400 MHz, CDCl
3) δ 8.87 (br s, 3H), 7.93 (dd, J = 1.6, 7.7 Hz, 1H), 7.50 - 7.55 (m, 1H), 7.39 - 7.49 (m, 2H), 3.96 - 4.09 (m, 1H), 1.41 (ddd, J = 4.4, 8.1, 12.6 Hz, 1H), 0.57 - 0.73 (m, 2H), 0.46 - 0.55 (m, 1H), 0.32 - 0.42 (m, 1H). Intermediate 5: (*R)-(2-Chlorophenyl)(cyclopropyl)methanamine.
compound was prepared in a manner analogous to Intermediate 2 using (S)-2- methylpropane-2-sulfinamide instead of (R)-2-methylpropane-2-sulfinamide in Step A and cPrMgBr instead of iPrMgCl in Step B.
1H NMR (400 MHz, DMSO-d
6) δ 8.86 - 8.64 (m, 2H), 7.98 - 7.80 (m, 1H), 7.57 - 7.34 (m, 3H), 4.10 - 3.96 (m, 1H), 1.44 - 1.31 (m, 1H), 0.73 - 0.33 (m, 4H). Intermediate 6: (*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methanamine.
compound was prepared in a manner analogous to Intermediate 2 using 2-chloro-3- fluorobenzaldehyde instead of 2-chlorobenzaldehyde in Step A and cPrMgBr instead of iPrMgCl in Step 96 QB\184200.00081\93036187.2
VVID-747PC B.
1H NMR (400 MHz, Methanol-d
4) δ 7.48 - 7.56 (m, 2H), 7.30 - 7.40 (m, 1H), 4.15 (d, J = 9.8 Hz, 1H), 1.42 - 1.54 (m, 1H), 0.82 - 0.92 (m, 1H), 0.60 - 0.72 (m, 2H), 0.44 - 0.53 (m, 1H). Intermediate 7: (*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methanamine. compound was prepared in a manner analogous to Intermediate 2 using 2-chloro-3-
instead of 2-chlorobenzaldehyde and (S)-2-methylpropane-2-sulfinamide instead of (R)-2-methylpropane-2-sulfinamide in Step A and cPrMgBr instead of iPrMgCl in Step B.
1H NMR (400 MHz, Methanol-d
4) δ 7.50 - 7.58 (m, 2H), 7.29 - 7.42 (m, 1H), 4.15 (d, J = 9.9 Hz, 1H), 1.41 - 1.56 (m, 1H), 0.80 - 0.92 (m, 1H), 0.56 - 0.72 (m, 2H), 0.42 - 0.55 (m, 1H). Intermediate 8: (R)-2-((tert-Butyldimethylsilyl)oxy)-1-(2-chlorophenyl)ethan-1-amine.
of (R)-2-amino-2-(2-chlorophenyl)ethan-1-ol (170 mg, 0.991 mmol, 1.0 eq) in DCM (5.0 mL, 0.19 M) was added TEA (200 mg, 1.98 mmol, 2.0 eq), tert-butyldimethylsilyl chloride (149 mg, 0.991 mmol, 1.0 eq), and DMAP (24 mg, 0.198 mmol, 0.2 eq). The mixture was stirred at 25°C for 10 h before being quenched with water and extracted with DCM. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by RP-HPLC (65% - 95% ACN in 10 mM aq. NH
4HCO
3) to give (R)-2-((tert- butyldimethylsilyl)oxy)-1-(2-chlorophenyl)ethan-1-amine (150 mg, 53% yield) as a yellow solid.
1H NMR (400 MHz, CDCl
3) δ 7.68 - 7.76 (m, 1H), 7.35 - 7.42 (m, 1H), 7.28 - 7.29 (m, 1H), 7.26 (br s, 1H), 4.79 - 4.92 (m, 1H), 4.04 (dd, J = 10.5, 3.8 Hz, 1H), 3.78 - 3.89 (m, 1H), 0.85 (s, 9H), 0.01 (s, 3H), -0.04 (s, 3H). Intermediate 9: 1-(2-Chloro-3-fluorophenyl)propan-1-ol.
of 2-chloro-3-fluorobenzaldehyde (1.0 g, 6.31 mmol, 1.0 eq) in THF (40 mL, 0.16 M) was added dropwise ethylmagnesium bromide (6.3 mL, 18.9 mmol, 3.0 eq) at 0°C. The mixture was stirred at 0°C for 3 h before being quenched with sat. aq. NH
4Cl and extracted with EtOAc. The combined 97 QB\184200.00081\93036187.2
VVID-747PC organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (0-20% EtOAc in PE) to give 1-(2-chloro- 3-fluorophenyl)propan-1-ol (650 mg, 55% yield) as a colorless oil.
1H NMR (400 MHz, CDCl
3) δ 7.28 (d, J = 7.9 Hz, 1H), 7.23 - 7.14 (m, 1H), 7.03 - 6.94 (m, 1H), 5.01 (dd, J = 4.8, 7.6 Hz, 1H), 1.95 - 1.84 (m, 1H), 1.83 - 1.59 (m, 2H), 0.92 (t, J = 7.4 Hz, 3H). Intermediate 10: (2-Chloro-3-fluorophenyl)(cyclopropyl)methanol. compound was prepared in a manner analogous to Intermediate 9 using
instead of 2-chloro-3-fluorobenzaldehyde and (2-chloro-3- fluorophenyl)magnesium bromide instead of ethylmagnesium bromide.
1H NMR (400 MHz, CDCl
3) δ 7.43 (d, J = 7.9 Hz, 1H), 7.27 - 7.32 (m, 1H), 7.09 (td, J = 8.5, 1.5 Hz, 1H), 4.67 (d, J = 7.6 Hz, 1H), 1.26 - 1.31 (m, 1H), 0.59 - 0.69 (m, 1H), 0.43 - 0.58 (m, 3H). Intermediate 11: (2-Chlorophenyl)(tetrahydrofuran-3-yl)methanol.
Methoxy-N-methyltetrahydrofuran-3-carboxamide. To the solution of tetrahydrofuran- 3-carboxylic acid (10 g, 86.1 mmol, 1.0 eq) in DCM (100 mL, 0.86 M) was added N,O- dimethylhydroxylamine hydrochloride (12.6 g, 129 mmol, 1.5 eq), DIPEA (13 g, 129 mmol, 1.5 eq), EDC hydrochloride (30 g, 129 mmol, 1.5 eq), and 4-(dimethylamino)pyridine (105 mg, 0.861 mmol, 0.01 eq) at 0℃. The solution was stirred at 25℃ for 12 h before being poured into water and extracted with DCM. The combined organic layers were washed with brine, dried over Na
2SO
4, and filtered. The filtrate was concentrated in vacuo to give N-methoxy-N-methyltetrahydrofuran-3-carboxamide (11 g, 80% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 4.05 (t, J = 8.4 Hz, 1H), 3.75 - 3.94 (m, 3H), 3.71 (s, 3H), 3.36 - 3.47 (m, 1H), 3.20 (s, 3H), 2.21 - 2.27 (m, 1H), 2.01 - 2.14 (m, 1H). [00233] Step B: (2-Chlorophenyl)(tetrahydrofuran-3-yl)methanone. A solution of 1-bromo-2- chlorobenzene (11.3 g, 59.3 mmol, 1.5 eq) in THF (90 mL, 0.33 M) was degassed, purged with N
2, and cooled to -78℃. To the solution was added n-BuLi (12.6 mL, 31.5 mmol, 0.8 eq) and the mixture was stirred at -78℃ for 0.5 h. A solution of N-methoxy-N-methyltetrahydrofuran-3-carboxamide (6.3 g, 39.6 mmol, 1.0 eq) in THF (30 mL, 0.33 M) was added and the reaction was stirred at -78℃ for 3 h. The reaction was quenched with sat. aq. NH
4Cl and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting 98 QB\184200.00081\93036187.2
VVID-747PC residue was purified by FCC on silica (0-25% EtOAc in PE) to give (2-chlorophenyl)(tetrahydrofuran-3- yl)methanone (2.0 g, 24% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 7.32 - 7.47 (m, 4H), 3.92 - 4.05 (m, 4H), 3.87 (td, J = 7.9, 6.4 Hz, 1H), 2.22 - 2.33 (m, 1H), 2.12 - 2.22 (m, 1H). [00234] Step C: (2-Chlorophenyl)(tetrahydrofuran-3-yl)methanol. A solution of (2- chlorophenyl)(tetrahydrofuran-3-yl)methanone (2.0 g, 9.49 mmol, 1.0 eq) in methanol (20 mL, 0.47 M) was degassed, purged with N
2, and cooled to 0℃. Sodium borohydride (720 mg, 19.0 mmol, 2.0 eq) was added and the reaction was stirred at 20℃ for 1 h. The reaction was quenched with water at 0℃ and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, and filtered. The filtrate was concentrated under reduced pressure to give (2-chlorophenyl)(tetrahydrofuran-3- yl)methanol (1.6 g, 79% yield) as a colorless oil, which was used in the next step without further purification.
1H NMR (400 MHz, CDCl
3) δ 7.56 (d, J = 7.7 Hz, 1H), 7.28 - 7.39 (m, 2H), 7.20 - 7.26 (m, 1H), 5.07 - 5.17 (m, 1H), 3.65 - 4.01 (m, 4H), 2.52 - 2.97 (m, 1H), 1.68 - 2.03 (m, 2H). Intermediate 12: (*R)-(2-Chlorophenyl)((R)-tetrahydrofuran-2-yl)methanol.
compound was prepared in a manner analogous to Intermediate 11 using (R)- tetrahydrofuran-2-carboxylic acid instead of tetrahydrofuran-3-carboxylic acid in Step A. Separation of (2-chlorophenyl)((R)-tetrahydrofuran-2-yl)methanol (Step C) via FCC on silica (0-20% EtOAc in PE (second eluting product)) provided the title compound.
1H NMR (400 MHz, CDCl
3) δ 7.57 (dd, J = 7.7, 1.6 Hz, 1H), 7.29 - 7.39 (m, 2H), 7.18 - 7.26 (m, 1H), 5.05 (d, J = 6.2 Hz, 1H), 4.07 (q, J = 6.5 Hz, 1H), 3.93 - 4.02 (m, 1H), 3.78 - 3.90 (m, 1H), 1.69 - 2.09 (m, 5H). Intermediate 13: (*R)-(2-Chlorophenyl)((S)-tetrahydrofuran-2-yl)methanol.
compound was prepared in a manner analogous to Intermediate 11 using (S)- tetrahydrofuran-2-carboxylic acid instead of tetrahydrofuran-3-carboxylic acid in Step A. Separation of (2-chlorophenyl)((S)-tetrahydrofuran-2-yl)methanol (Step C) via FCC on silica (0-20% EtOAc in PE (first eluting product)) provided the title compound.
1H NMR (400 MHz, CDCl
3) δ 7.63 (dd, J = 7.7, 1.4 Hz, 1H), 7.28 (s, 2H), 7.19 - 7.26 (m, 1H), 5.38 (d, J = 3.5 Hz, 1H), 4.22 - 4.35 (m, 1H), 3.93 - 3.98 (m, 1H), 3.83 - 3.87 (m, 1H), 1.84 - 1.96 (m, 5H). Intermediate 14: Methyl 7-fluoro-1-methyl-1H-indazole-4-carboxylate. 99 QB\184200.00081\93036187.2
VVID-747PC dried flask under N
2, sodium hydride (124 mg, 3.09 mmol, 3.0 eq) was taken up in
M) and cooled to 0°C. To this was added methyl 7-fluoro-1H-indazole-4-carboxylate (200 mg, 1.03 mmol, 1.0 eq) and the reaction was allowed to stir for 5 min. Finally, iodomethane (0.19 mL, 3.09 mmol, 3.0 eq) was added and the reaction was stirred at 0°C for 10 min then rt for 2 h. The reaction was quenched with water and extracted with EtOAc. The combined organic extracts were dried over Na
2SO
4, filtered, and concentrated under reduced pressure. Purification by FCC on silica (0-100% EtOAc in heptanes (first eluting product)) provided methyl 7-fluoro-1-methyl-1H-indazole-4-carboxylate (127 mg, 59% yield). MS (ESI): mass calcd. for C
10H
9FN
2O
2, 208.1; m/z found, 209.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.46 (d, J = 2.4 Hz, 1H), 7.85 (dd, J = 8.1, 4.2 Hz, 1H), 7.05 (dd, J = 11.3, 8.1 Hz, 1H), 4.28 (d, J = 1.1 Hz, 3H), 3.99 (s, 3H). Intermediate 15: Methyl 7-fluoro-2-methyl-2H-indazole-4-carboxylate.
was isolated from the reaction described in Intermediate 14 (52 mg, 24% yield (second eluting product)). MS (ESI): mass calcd. for C
10H
9FN
2O
2, 208.1; m/z found, 209.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.45 (d, J = 2.7 Hz, 1H), 7.86 (dd, J = 7.9, 4.3 Hz, 1H), 6.97 (dd, J = 10.6, 7.9 Hz, 1H), 4.28 (s, 3H), 3.96 (s, 3H). Intermediate 16: Methyl 4-bromo-1-methyl-1H-indazole-7-carboxylate.
was prepared in a manner analogous to Intermediate 14 using methyl 4-bromo- 1H-indazole-7-carboxylate instead of methyl 7-fluoro-1H-indazole-4-carboxylate. MS (ESI): mass calcd. for C
10H
9BrN
2O
2, 268.0; m/z found, 269.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.08 (s, 1H), 7.78 (d, J = 7.8 Hz, 1H), 7.31 (d, J = 7.9 Hz, 1H), 4.27 (s, 3H), 3.98 (s, 3H). Intermediate 17: Methyl 5-chloro-4-(trifluoromethyl)pyrimidine-2-carboxylate. 100 QB\184200.00081\93036187.2
VVID-747PC of 2,5-dichloro-4-(trifluoromethyl)pyrimidine (4.0 g, 18.4 mmol, 1.0 eq) in
80 mL, 0.23 M) was added TEA (5.6 g, 55.3 mmol, 3.0 eq) and Pd(dppf)Cl
2 (667 mg, 0.920 mmol, 0.05 eq). The mixture was degassed and purged with CO (50 psi) then stirred at 50°C for 5 h. After cooling to rt, the reaction mixture was concentrated under reduced pressure, poured into H
2O, and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (PE:EtOAc = 85:15) to give methyl 5-chloro-4-(trifluoromethyl)pyrimidine-2-carboxylate (1.5 g, 34% yield) as a yellow solid.
1H NMR (400 MHz, CDCl
3) δ 9.10 (s, 1H), 4.09 (s, 3H). Intermediate 18: Methyl 5-fluoro-4-methylpyrimidine-2-carboxylate.
compound was prepared in a manner analogous to Intermediate 17 using 2-chloro-5- fluoro-4-methyl-pyrimidine instead of 2,5-dichloro-4-(trifluoromethyl)pyrimidine.
1H NMR (400 MHz, CDCl
3) δ 8.61 (d, J = 0.8 Hz, 1H), 4.06 (s, 3H), 2.67 (d, J = 2.6 Hz, 3H). Intermediate 19: Methyl 4-bromo-3-(oxazol-2-yl)benzoate.
4-bromo-3-((2,2-dimethoxyethyl)carbamoyl)benzoate. To a solution of 2-bromo- 5-(methoxycarbonyl)benzoic acid (1.0 g, 3.86 mmol, 1.0 eq) in DCM (15 mL, 0.26 M) was added HATU (2.2 g, 5.79 mmol, 1.5 eq), DIPEA (1.5 g, 11.6 mmol, 3.0 eq) and 2,2-dimethoxyethan-1-amine (487 mg, 4.63 mmol, 1.2 eq). The mixture was stirred at 25°C for 16 h before being concentrated under reduced pressure. The mixture was diluted with water and extracted with DCM. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (0-50% EtOAc in PE) to give methyl 4-bromo-3-((2,2- dimethoxyethyl)carbamoyl)benzoate (1.0 g, 75% yield) as a white solid.
1H NMR (400 MHz, CDCl
3) δ 8.07 - 8.19 (m, 1H), 7.84 - 7.95 (m, 1H), 7.63 - 7.72 (m, 1H), 6.24 (br s, 1H), 4.53 (t, J = 5.3 Hz, 1H), 3.92 (d, J = 2.1 Hz, 3H), 3.62 (td, J = 5.5, 2.8 Hz, 2H), 3.44 (d, J = 2.1 Hz, 6H). [00243] Step B: Methyl 4-bromo-3-(oxazol-2-yl)benzoate. To a solution of methyl 4-bromo-3-((2,2- dimethoxyethyl)carbamoyl)benzoate (500 mg, 1.44 mmol, 1.0 eq) in methanesulfonic acid (4.9 g, 50.6 101 QB\184200.00081\93036187.2
VVID-747PC mmol, 35 eq) was added P
2O
5 (820 mg, 5.78 mmol, 4.0 eq). The mixture was stirred at 140°C for 2 h. After cooling to rt, the reaction mixture was poured into sat. aq. NaHCO
3 and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by prep-TLC (PE:EtOAc = 5:1, Rf = 0.5) to give methyl 4-bromo-3-(oxazol-2-yl)benzoate (180 mg, 44% yield) as a white solid.
1H NMR (400 MHz, CDCl
3) δ 8.60 (d, J = 2.1 Hz, 1H), 7.94 (dd, J = 8.4, 2.1 Hz, 1H), 7.79 - 7.88 (m, 2H), 7.37 (s, 1H), 3.95 (s, 3H). Intermediate 20: Methyl 5-(cyclopropylamino)pyrimidine-2-carboxylate. cyclopropylamine (183 mg, 3.20 mmol, 1.0 eq) in DMSO (6.0 mL, 0.53 M) was
2-carboxylate (500 mg, 3.20 mmol, 1.0 eq) and pyridine (760 mg, 9.61 mmol, 3.0 eq). The reaction was degassed and purged with N
2 then stirred at 100°C for 16 h. After cooling to rt, the reaction mixture was poured into water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (PE:EtOAc = 0:1) to give methyl 5- (cyclopropylamino)pyrimidine-2-carboxylate (400 mg, 65% yield) as a yellow solid. MS (ESI): mass calcd. for C
9H
11N
3O
2, 193.1; m/z found, 194.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.37 (s, 2H), 4.01 (s, 3H), 2.56 (tt, J = 6.7, 3.5 Hz, 1H), 0.84 - 0.96 (m, 2H), 0.56 - 0.68 (m, 2H). Intermediate 21: (R,E)-4-(Cyclopropylsulfonyl)but-3-en-2-amine.
sodium cyclopropanesulfinate (922 mg, 7.19 mmol, 2.0 eq) in DMSO (6.0 mL, 0.6M) was stirred for 1 hour at rt. Diethyl iodomethylphosphonate (0.60 mL, 3.60 mmol, 1.0 eq) was added and the reaction was heated to 80 °C for 16 hours. Another aliquot of sodium cyclopropanesulfinate (922 mg, 7.19 mmol, 2.0 eq) was added and the reaction was heated to 100 °C for 1 hour. After cooling to rt, the reaction was diluted with EtOAc, washed with 1M HCl and brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC (0-10% MeOH in DCM) to afford diethoxyphosphorylmethylsulfonylcyclopropane (432 mg, 47% yield) as a yellow oil. [00246] The title compound was prepared in a manner analogous to Intermediate 1 using diethoxyphosphorylmethylsulfonylcyclopropane instead of diethyl ((methylsulfonyl)methyl)phosphonate in Step A.
1H NMR (400 MHz, DMSO-d
6) δ 8.18 (br s, 2H), 6.98 (d, J = 15.5 Hz, 1H), 6.72 (dd, J = 15.4, 5.6 Hz, 1H), 4.12 (br d, J = 5.0 Hz, 1H), 2.59 - 2.73 (m, 1H), 1.33 (d, J = 6.8 Hz, 3H), 0.93 - 1.10 (m, 4H). 102 QB\184200.00081\93036187.2
VVID-747PC Intermediate 22: 1-(2-Chloro-3-fluorophenyl)-2-methylpropan-1-amine. compound was prepared in a manner analogous to Intermediate 2 using 2-chloro-3-
instead of 2-chlorobenzaldehyde and 2-methylpropane-2-sulfinamide instead of (R)- 2-methylpropane-2-sulfinamide in Step A. Intermediate 23: 1-(2-Chloro-3-fluorophenyl)propan-1-amine. compound was prepared in a manner analogous to Intermediate 2 using 2-chloro-3-
instead of 2-chlorobenzaldehyde and 2-methylpropane-2-sulfinamide instead of (R)- 2-methylpropane-2-sulfinamide in Step A and using EtMgBr instead of iPrMgCl in Step B. Intermediate 24: (2-Chloro-3-fluorophenyl)(cyclopropyl)methanamine.
compound was prepared in a manner analogous to Intermediate 2 using 2-chloro-3- fluorobenzaldehyde instead of 2-chlorobenzaldehyde and 2-methylpropane-2-sulfinamide instead of (R)- 2-methylpropane-2-sulfinamide in Step A and using cPrMgBr instead of iPrMgCl in Step B. Intermediate 25: (2-Chloro-6-fluorophenyl)(cyclopropyl)methanamine.
compound was prepared in a manner analogous to Intermediate 2 using 2-chloro-6- fluorobenzaldehyde instead of 2-chlorobenzaldehyde and 2-methylpropane-2-sulfinamide instead of (R)- 2-methylpropane-2-sulfinamide in Step A and using cPrMgBr instead of iPrMgCl in Step B.
1H NMR (400 103 QB\184200.00081\93036187.2
VVID-747PC MHz, CDCl
3) δ 7.19 - 7.12 (m, 2H), 7.05 - 6.94 (m, 1H), 3.66 (d, J = 9.3 Hz, 1H), 1.47 (ddd, J = 12.1, 5.7, 2.6 Hz, 1H), 0.72 - 0.60 (m, 1H), 0.47 - 0.38 (m, 2H), 0.37 - 0.28 (m, 1H). Intermediate 26: (2-Chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methanamine. NH
2 O compound was prepared in a manner analogous to Intermediate 2 using 3-fluorooxetane-
instead of 2-chlorobenzaldehyde and 2-methylpropane-2-sulfinamide instead of (R)-2- methylpropane-2-sulfinamide in Step A and using (2-chloro-3-fluorophenyl)magnesium bromide instead of iPrMgCl in Step B. MS (ESI): mass calcd. for C
10H
10ClF
2NO, 233.1; m/z found, 234.1 [M+H]
+. Intermediate 27: (2-Chlorophenyl)(3-methyloxetan-3-yl)methanol. compound was prepared in a manner analogous to Intermediate 9 using 3-methyloxetane-
3- instead of 2-chloro-3-fluorobenzaldehyde and (2-chlorophenyl)magnesium bromide instead of ethylmagnesium bromide.
1H NMR (400 MHz, CDCl
3) δ 7.44 - 7.41 (m, 1H), 7.31 - 7.28 (m, 1H), 7.27 - 7.23 (m, 1H), 7.18 (dd, J = 7.8, 1.6 Hz, 1H), 5.23 (d, J = 2.4 Hz, 1H), 4.88 (dd, J = 11.8, 5.8 Hz, 2H), 4.18 (dd, J = 12.6, 5.8 Hz, 2H), 1.30 (s, 3H). Intermediate 28: (2-Chlorophenyl)(cyclobutyl)methanol.
compound was prepared in a manner analogous to Intermediate 9 using cyclobutanecarbaldehyde instead of 2-chloro-3-fluorobenzaldehyde and (2-chlorophenyl)magnesium bromide instead of ethylmagnesium bromide.
1H NMR (400 MHz, CDCl
3) δ 7.47 (dd, J = 7.7, 1.7 Hz, 1H), 7.34 (dd, J = 7.8, 1.3 Hz, 1H), 7.29 - 7.25 (m, 1H), 7.23 - 7.16 (m, 1H), 5.11 (d, J = 6.9 Hz, 1H), 2.86 - 2.69 (m, 1H), 2.03 - 1.84 (m, 6H). Intermediate 29: 1-(2-Chloro-3-fluorophenyl)-2-methylpropan-1-ol. 104 QB\184200.00081\93036187.2
VVID-747PC OH compound was prepared in a manner analogous to Intermediate 9 using isobutyraldehyde
3-fluorobenzaldehyde and (2-chloro-3-fluorophenyl)magnesium bromide instead of ethylmagnesium bromide.
1H NMR (400 MHz, CDCl
3) δ 7.27 (s, 2H), 7.11 - 7.01 (m, 1H), 4.93 (d, J = 5.8 Hz, 1H), 2.14 - 1.97 (m, 1H), 0.96 (t, J = 6.4 Hz, 6H). Intermediate 30: (2-Chloro-3-fluorophenyl)(oxetan-3-yl)methanol. compound was prepared in a manner analogous to Intermediate 9 using oxetane-3-
carbaldehyde instead of 2-chloro-3-fluorobenzaldehyde and (2-chloro-3-fluorophenyl)magnesium bromide instead of ethylmagnesium bromide.
1H NMR (400 MHz, CDCl
3) δ 7.37 – 7.18 (m, 2H), 7.17 – 7.01 (m, 1H), 5.38 (dd, J = 6.3, 4.0 Hz, 1H), 4.77 (t, J = 6.2 Hz, 1H), 4.73 – 4.63 (m, 2H), 4.60 (t, J = 6.3 Hz, 1H), 3.42 (tq, J = 8.1, 6.3 Hz, 1H), 3.09 (d, J = 4.1 Hz, 1H). Intermediate 31: tert-Butyl 3-((2-chloro-3-fluorophenyl)(hydroxy)methyl)azetidine-1-carboxylate. OH
was prepared in a manner analogous to Intermediate 9 using tert-butyl 3- formylazetidine-1-carboxylate instead of 2-chloro-3-fluorobenzaldehyde and (2-chloro-3- fluorophenyl)magnesium bromide instead of ethylmagnesium bromide. MS (ESI): mass calcd. for C
15H
19ClFNO
3, 315.1; m/z found, 260.1 [M+2H-tBu]
+.
1H NMR (400 MHz, CDCl
3) δ 7.27 (m, 2H), 7.04 (t, J = 8.1 Hz, 1H), 5.28 - 5.15 (m, 1H), 4.09 - 3.74 (m, 4H), 2.99 - 2.87 (m, 1H), 2.53 (br s, 1H), 1.38 (s, 9H). Intermediate 32: (2-Chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methanol. 105 QB\184200.00081\93036187.2
VVID-747PC compound was prepared in a manner analogous to Intermediate 9 using 3-fluorooxetane-
instead of 2-chloro-3-fluorobenzaldehyde and (2-chloro-3-fluorophenyl)magnesium bromide instead of ethylmagnesium bromide.
1H NMR (400 MHz, CDCl
3) δ 7.43 (d, J = 8.4 Hz, 1H), 7.33 (dt, J = 8.1, 5.3 Hz, 1H), 7.22 - 7.15 (m, 1H), 5.69 - 5.56 (m, 1H), 4.88 - 4.65 (m, 4H), 2.47 - 2.34 (m, 1H). Intermediate 33: 1-((2-Chlorophenyl)(hydroxy)methyl)cyclopropane-1-carbonitrile. (500 mg, 2.61 mmol, 1.0 eq) was taken up in THF (5.0 mL, 0.37 M),
and cooled to -65 °C. n-Butyllithium (1.3 mL, 3.25 mmol, 1.2 eq, 2.5 M) was added dropwise followed by a solution of 1-formylcyclopropanecarbonitrile (300 mg, 3.15 mmol, 1.2 eq) in THF (2.0 mL, 0.37 M). The reaction was stirred at -65 °C for 1 h before being diluted with sat. aq. NH
4Cl and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4 and filtered. The filtrate was concentrated under reduced pressure to give 1-((2- chlorophenyl)(hydroxy)methyl)cyclopropane-1-carbonitrile (100 mg, 18% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 7.79 (dd, J = 8.0, 1.5 Hz, 1H), 7.42 - 7.35 (m, 2H), 7.34 - 7.28 (m, 1H), 5.07 (s, 1H), 1.42 - 1.20 (m, 4H). Intermediate 34: (2-Chlorophenyl)(2,2-difluorocyclopropyl)methanol.
A: 2,2-Difluoro-N-methoxy-N-methylcyclopropane-1-carboxamide. 2,2- Difluorocyclopropanecarboxylic acid (10 g, 81.9 mmol, 1.0 eq) was taken up in DCM (50 mL, 1.6 M). T
4P (88 g, 123 mmol, 1.5 eq), DIPEA (43 mL, 246 mmol, 3.0 eq) and N,O-dimethylhydroxylamine hydrochloride (8.0 g, 81.9 mmol, 1.0 eq) were added and the reaction was allowed to stir at 25 °C for 2 h. The mixture was diluted with water and extracted with DCM. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 10:0 - 9:1) to provide 2,2-difluoro-N-methoxy-N-methylcyclopropane-1- carboxamide (3.5 g, 50% yield) as a colorless oil.
1H NMR (400 MHz, CDCl
3) δ 3.75 (s, 3H), 3.23 (s, 3H), 3.00 - 2.86 (m, 1H), 2.19 - 2.07 (m, 1H), 1.67 (dddd, J = 11.9, 10.9, 7.5, 4.8 Hz, 1H). 106 QB\184200.00081\93036187.2
VVID-747PC [00260] Step B: (2-Chlorophenyl)(2,2-difluorocyclopropyl)methanone. 2,2-Difluoro-N-methoxy-N- methylcyclopropane-1-carboxamide (5.0 g, 30.2 mmol, 1.0 eq) was taken up in THF (30 mL, 1.0 M), placed under N
2, and cooled to 0 °C. (2-Chlorophenyl)magnesium bromide (45 mL, 45.4 mmol, 1.5 eq, 1M) was added dropwise and the reaction was stirred at 0 °C for 30 min, then allowed to warm to rt and stirred 12 h. The mixture was quenched with sat. aq. NH
4Cl and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 1:0 - 10:1) to give (2-chlorophenyl)(2,2- difluorocyclopropyl)methanone (1.0 g, 15% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 7.62 - 7.56 (m, 1H), 7.47 (br d, J = 3.1 Hz, 2H), 7.39 (td, J = 5.1, 2.7 Hz, 1H), 3.41 - 3.26 (m, 1H), 2.53 - 2.39 (m, 1H), 1.90 - 1.77 (m, 1H). [00261] Step C: (2-Chlorophenyl)(2,2-difluorocyclopropyl)methanol. (2-Chlorophenyl)-(2,2- difluorocyclopropyl)methanone (550 mg, 2.54 mmol, 1.0 eq) was taken up in MeOH (10 mL, 0.25 M) and placed under N
2. Sodium borohydride (144 mg, 3.81 mmol, 1.5 eq) was added and the reaction was allowed to stir at 25 °C for 3 h. The mixture was concentrated under reduced pressure to provide (2- chlorophenyl)(2,2-difluorocyclopropyl)methanol (550 mg, quant. yield) as a yellow oil. The product was used in the next step without purification. Intermediate 35: (2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methanol.
compound was prepared in a manner analogous to Intermediate 34 using 1- fluorocyclopropane-1-carboxylic acid instead of 2,2-difluorocyclopropanecarboxylic acid in Step A and (2-chloro-3-fluorophenyl)magnesium bromide instead of (2-chlorophenyl)magnesium bromide in Step B. Intermediate 36: (2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methanamine.
(E)-N-((2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methylene)-2-methylpropane-2- sulfinamide. (2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methanone (Intermediate 35 Step B, 1.0 g, 4.62 mmol, 1.0 eq) was taken up in THF (2.0 mL, 2.3 M) and placed under N
2. 2-Methylpropane-2- sulfinamide (1.1 g, 9.23 mmol, 2.0 eq) and Ti(OEt)
4 (3.2 g, 13.9 mmol, 3.0 eq) were added and the reaction was stirred at 85 °C for 16 h. After cooling to rt, the crude product was used directly in the next step without work up or purification. 107 QB\184200.00081\93036187.2
VVID-747PC [00264] Step B: N-((2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)-2-methylpropane-2- sulfinamide. (Z)-N-((2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methylene)-2-methylpropane-2- sulfinamide (1.5 g, 4.62 mmol, 1.0 eq) was taken up in THF (10 mL, 0.46 M) and placed under N
2. Sodium borohydride (349 mg, 9.23 mmol, 2.0 eq) was added portionwise and the reaction was allowed to stir at 25 °C for 3 h. The mixture was quenched with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, and filtered. The filtrate was concentrated under reduced pressure to give N-((2-chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)-2-methylpropane-2- sulfinamide (1.4 g, 94% yield) as a brown oil, which was used in the next step directly. MS (ESI): mass calcd. for C
14H
18ClF
2NOS, 321.1; m/z found, 322.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.49 (d, J = 7.8 Hz, 1H), 7.34 - 7.27 (m, 1H), 7.16 - 7.10 (m, 1H), 5.42 (d, J = 16.8 Hz, 1H), 1.24 (s, 9H), 1.07 – 0.80 (m, 3H), 0.73 - 0.60 (m, 1H). [00265] Step C: (2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methanamine. N-((2-Chloro-3- fluorophenyl)(1-fluorocyclopropyl)methyl)-2-methylpropane-2-sulfinamide (1.4 g, 4.35 mmol, 1.0 eq) was taken up in MeOH (2.0 mL, 2.2 M). To this was added conc. HCl (1.5 mL) and the reaction was stirred at 25 °C for 2 h. The solvent was removed under reduced pressure and the residue was diluted with sat. aq. NaHCO
3 and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, and filtered. The filtrate was concentrated under reduced pressure to give (2-chloro-3- fluorophenyl)(1-fluorocyclopropyl)methanamine (900 mg, 95% yield) as a brown oil, which was used in the next step directly. Intermediate 37: (2-Chloro-3-fluorophenyl)(2,2-difluorocyclopropyl)methanamine.
compound was prepared in a manner analogous to Intermediate 36 using (2-chloro-3- fluorophenyl)(2,2-difluorocyclopropyl)methanone instead of (2-chloro-3-fluorophenyl)(1- fluorocyclopropyl)methanone in Step A. MS (ESI): mass calcd. for C
10H
9ClF
3N, 235.0; m/z found, 236.1 [M+H]
+. Intermediate 38: 1-(2-Chlorophenyl)-2,2-difluoroethan-1-amine.
compound was prepared in a manner analogous to Intermediate 36 using 1-(2- chlorophenyl)-2,2-difluoroethanone instead of (2-chloro-3-fluorophenyl)(1-fluorocyclopropyl)methanone 108 QB\184200.00081\93036187.2
VVID-747PC in Step A.
1H NMR (400 MHz, CDCl3) δ 7.53 (dd, J = 7.6, 1.2 Hz, 1H), 7.41 (dd, J = 7.7, 1.5 Hz, 1H), 7.37 - 7.28 (m, 2H), 6.14 - 5.77 (m, 1H), 4.76 (ddd, J = 16.8, 8.4, 3.2 Hz, 1H). Intermediate 39: 1-(Amino(2-chloro-3-fluorophenyl)methyl)cyclopropane-1-carbonitrile. 1-((2-Chloro-3-fluorophenyl)(hydroxy)methyl)cyclopropane-1-carbonitrile. 1-
(1.0 g, 10.5 mmol, 1.0 eq) was taken up in THF (20 mL, 0.53 M), placed under N
2, and cooled to 0 °C. (2-Chloro-3-fluorophenyl)magnesium bromide (16 mL, 16.0 mmol, 1.5 eq) was added dropwise and the reaction was allowed to warm to rt and stir for 16 h. The mixture was diluted with sat. aq. NH
4Cl and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 1:4) to give 1-((2-chloro-3-fluorophenyl)(hydroxy)methyl)cyclopropane-1-carbonitrile (2.5 g, 95% yield) as a colorless oil.
1H NMR (400 MHz, CDCl
3) δ 7.61 (d, J = 8.0 Hz, 1H), 7.37 (td, J = 8.1, 5.4 Hz, 1H), 7.18 (td, J = 8.5, 1.3 Hz, 1H), 5.03 (s, 1H), 1.44 - 1.26 (m, 4H). [00269] Step B: 1-(2-Chloro-3-fluorobenzoyl)cyclopropane-1-carbonitrile. 1-((2-Chloro-3- fluorophenyl)(hydroxy)methyl)cyclopropane-1-carbonitrile (2.0 g, 8.86 mmol, 1.0 eq) was taken up in DCM (20 mL, 0.44 M). Dess-Martin periodinane (3.8 g, 8.86 mmol, 1.0 eq) was added and the reaction was stirred at 25 °C for 16 h. The mixture was diluted with sat. aq. Na
2S
2O
3 and filtered. The filtrate was poured into sat. aq. NaHCO
3 and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na2SO4 and filtered. The filtrate was concentrated under reduced pressure to give 1-(2- chloro-3-fluorobenzoyl)cyclopropane-1-carbonitrile (1.7 g, 86% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 7.43 - 7.29 (m, 2H), 7.25 (d, J = 7.5 Hz, 1H), 2.05 - 1.99 (m, 2H), 1.91 - 1.86 (m, 2H). [00270] The title compound was prepared in a manner analogous to Intermediate 36 using 1-(2-chloro-3- fluorobenzoyl)cyclopropane-1-carbonitrile instead of (2-chloro-3-fluorophenyl)(1- fluorocyclopropyl)methanone in Step A. Intermediate 40: (2-Chloro-3-fluorophenyl)(oxetan-3-yl)methanamine.
(2-Chloro-3-fluorophenyl)(oxetan-3-yl)methanone. (2-Chloro-3-fluorophenyl)(oxetan-3- yl)methanol (Intermediate 30, 600 mg, 2.77 mmol, 1.0 eq) was taken up in DCM (6.0 mL, 0.46 M) and cooled to 0 °C. DMP (2.3 g, 5.54 mmol, 2.0 eq) was added and the reaction was allowed to warm to rt over 109 QB\184200.00081\93036187.2
VVID-747PC 2 h. The mixture was quenched with sat. aq. Na
2S
2O
3 and sat. aq. NaHCO
3 then extracted with DCM. The organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated in vacuo. The residue was purified by FCC (PE:EtOAc = 3:1 - 2:1) to give (2-chloro-3-fluorophenyl)(oxetan-3-yl)methanone (500 mg, 84% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 7.39 - 7.29 (m, 3H), 5.00 - 4.84 (m, 4H), 4.69 - 4.55 (m, 1H). [00272] Step B: (2-Chloro-3-fluorophenyl)(oxetan-3-yl)methanamine. (2-Chloro-3-fluorophenyl)(oxetan- 3-yl)methanone (250 mg, 1.16 mmol, 1.0 eq) was taken up in EtOH (2.0 mL, 0.58 M) and placed under N
2. Ammonium acetate (1.8 g, 23.3 mmol, 20 eq), sodium cyanoborohydride (183 mg, 2.91 mmol, 2.5 eq) and NH
3 ^H
2O (25% H
2O, 1.0 mL) were added and the reaction was stirred at 110 °C for 16 h. After cooling to rt, the mixture was quenched with water and extracted with EtOAc. The organic layers were washed with brine, dried over Na
2SO
4, filtered and concentrated under reduced pressure. The residue was purified by RP-HPLC (15-45% ACN in 10 mM aq. NH
4HCO
3) to afford (2-chloro-3-fluorophenyl)(oxetan-3- yl)methanamine (180 mg, 72% yield) as a white solid.
1H NMR (400 MHz, CDCl
3) δ 7.19 - 7.12 (m, 1H), 7.06 - 6.94 (m, 2H), 4.80 (t, J = 6.9 Hz, 1H), 4.70 - 4.61 (m, 2H), 4.58 (t, J = 7.1 Hz, 1H), 4.29 (t, J = 6.3 Hz, 1H), 3.41 - 3.24 (m, 1H). Intermediate 41: Methyl 4-fluoro-3-(1H-imidazol-2-yl)benzoate. 3-formylbenzoate (1.0 g, 5.49 mmol, 1.0 eq) was taken up in EtOH (20 mL, 0.27
M). Glyoxal (1.6 g, 27.5 mmol, 5.0 eq) and ammonium acetate (4.2 g, 54.9 mmol, 10 eq) were added and the reaction was allowed to stir at 25 °C for 16 h. The mixture was concentrated under reduced pressure and the crude product was diluted with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 1:0 - 3:1) to give methyl 4-fluoro-3-(1H-imidazol-2-yl)benzoate (500 mg, 41% yield) as a yellow solid. Intermediate 42: Methyl 4-(2,2-difluorocyclopropyl)-5-fluoropyrimidine-2-carboxylate.
(3.0 g, 22.6 mmol, 1.0 eq) was taken up in ACN/water (v/v 1:1, 90 mL, 0.22 M). AgNO
3 (7.7 g, 45.3 mmol, 2.0 eq) and 2,2-difluorocyclopropane-1-carboxylic acid (8.3 g, 67.9 mmol, 3.0 eq) were added and the mixture was heated to 85 °C before a solution of ammonium persulfate (10.3 g, 45.3 mmol, 2.0 eq) in water (12 mL, 0.22 M) was added dropwise. The reaction was 110 QB\184200.00081\93036187.2
VVID-747PC stirred at 85 °C for 16 h. After cooling to rt, EtOAc and brine were added. The mixture was filtered and the filtrate was separated. The organic layers were washed with water and brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 100:1 - 9:1) to give 2-chloro-4-(2,2-difluorocyclopropyl)-5-fluoropyrimidine (1.4 g, 30% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 8.42 (d, J = 0.8 Hz, 1H), 3.17 - 3.00 (m, 1H), 2.65 - 2.52 (m, 1H), 2.09 - 1.93 (m, 1H). [00275] The title compound was prepared in a manner analogous to Intermediate 17 using 2-chloro-4-(2,2- difluorocyclopropyl)-5-fluoropyrimidine instead of 2,5-dichloro-4-(trifluoromethyl)pyrimidine.
1H NMR (400 MHz, CDCl
3) δ 8.67 (s, 1H), 4.07 - 4.02 (m, 3H), 3.23 - 3.10 (m, 1H), 2.80 - 2.66 (m, 1H), 2.09 - 1.96 (m, 1H). Intermediate 43: Methyl 5-fluoro-4-(1-(trifluoromethyl)cyclopropyl)pyrimidine-2-carboxylate. was prepared in a manner analogous to Intermediate 42 using 1-
1-carboxylic acid instead of 2,2-difluorocyclopropane-1-carboxylic acid in Step A.
1H NMR (400 MHz, CDCl
3) δ 8.75 (s, 1H), 4.06 (s, 3H), 1.65 - 1.60 (m, 2H), 1.43 (br s, 2H). Intermediate 44: Methyl 4-(difluoromethyl)-5-fluoropyrimidine-2-carboxylate.
was prepared in a manner analogous to Intermediate 42 using 2,2- difluoroacetic acid instead of 2,2-difluorocyclopropane-1-carboxylic acid in Step A.
1H NMR (400 MHz, CDCl
3) δ 9.23 – 8.74 (m, 1H), 7.00 – 6.63 (m, 1H), 4.10 (s, 3H). Intermediate 45: Methyl 5-fluoro-4-(methoxymethyl)pyrimidine-2-carboxylate.
5-fluoropyrimidin-4-yl)methanol. 2-Chloro-5-fluoropyrimidine (5.0 g, 37.7 mmol, 1.0 eq) was taken up in MeOH (200 mL, 0.2 M). TFA (8.6 g, 75.5 mmol, 2.0 eq) and BPO (18 g, 75.5 mmol, 2.0 eq) were added and the reaction was stirred at 65 °C for 16 h. After cooling to rt, the mixture was slowly quenched with sat. aq. NaHCO
3 and extracted with DCM. The combined organic layers were 111 QB\184200.00081\93036187.2
VVID-747PC washed with brine, dried over Na
2SO
4, filtered, and concentrated in vacuo. The residue was purified by FCC (PE:EtOAc = 10:1 to 5:1) to give (2-chloro-5-fluoro-pyrimidin-4-yl)methanol (2.0 g, 33% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 8.44 (s, 1H), 4.87 (d, J = 1.1 Hz, 2H), 3.36 - 3.15 (m, 1H). [00279] Step B: Methyl 5-fluoro-4-(hydroxymethyl)pyrimidine-2-carboxylate. (2-Chloro-5-fluoro- pyrimidin-4-yl)methanol (2.0 g, 12.3 mmol, 1.0 eq) was taken up in MeOH/DMF (v/v 1:1, 30 mL, 0.4M). Pd(dppf)Cl
2 (445 mg, 0.615 mmol, 0.05 eq) and TEA (3.7 g, 36.9 mmol, 3.0 eq) were added. The reaction was placed under CO (50 psi) and stirred at 80 °C for 16 h. After cooling to rt, the mixture was concentrated in vacuo to give methyl 5-fluoro-4-(hydroxymethyl)pyrimidine-2-carboxylate (1.7 g, 74% yield) as a brown oil. [00280] Step C: Methyl 5-fluoro-4-(methoxymethyl)pyrimidine-2-carboxylate. Methyl 5-fluoro-4- (hydroxymethyl)pyrimidine-2-carboxylate (1.0 g, 5.37 mmol, 1.0 eq) was taken up in DCM (10 mL, 0.54 M) and cooled to 0 °C. Ag
2O (6.2 g, 26.9 mmol, 5.0 eq) and iodomethane (3.8 g, 26.9 mmol, 5.0 eq) were added and the reaction was allowed to stir at 20 °C for 16 h. The mixture was filtered then diluted with water and extracted with DCM. The organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated in vacuo. The residue was purified by FCC (PE:EtOAc = 3:1 to 1:1) to afford methyl 5- fluoro-4-(methoxymethyl)pyrimidine-2-carboxylate (550 mg, 51% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 8.73 (d, J = 1.1 Hz, 1H), 4.74 (d, J = 1.6 Hz, 2H), 4.06 (s, 3H), 3.52 (s, 3H). Intermediate 46: Methyl 4-(acetoxymethyl)-5-fluoropyrimidine-2-carboxylate.
was prepared in a manner analogous to Intermediate 45 using acetic anhydride and TEA instead of iodomethane and Ag
2O in Step C.
1H NMR (400 MHz, CDCl
3) δ 8.73 (d, J = 1.3 Hz, 1H), 5.36 (d, J = 1.5 Hz, 2H), 4.07 (s, 3H), 2.19 (s, 3H). Intermediate 47: Methyl 8-fluoroimidazo[1,2-c]pyrimidine-5-carboxylate.
amino-5-fluoropyrimidine-2-carboxylate was prepared in a manner analogous to Intermediate 17 using 2-chloro-5-fluoropyrimidin-4-amine instead of 2,5-dichloro-4- (trifluoromethyl)pyrimidine.
1H NMR (400 MHz, CDCl
3) δ 8.25 (d, J = 2.3 Hz, 1H), 6.15 (br d, J = 3.9 Hz, 2H), 4.01 (s, 3H).
[00283] Step B: Methyl 4-amino-5-fluoropyrimidine-2-carboxylate (700 mg, 4.09 mmol, 1.0 eq) was taken up in MeOH (14 mL, 0.29 M). Chloroacetaldehyde (2.4 g, 12.2 mmol, 3.0 eq) was added and the reaction 112 QB\184200.00081\93036187.2
VVID-747PC was stirred at 100 °C for 12 h. After cooling to rt, the mixture was diluted with sat. aq. NaHCO
3 and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC (PE:EtOAc = 1:1 - 2:3) to give methyl 8-fluoroimidazo[1,2-c]pyrimidine-5-carboxylate (180 mg, 22% yield) as a white solid.
1H NMR (400 MHz, Methanol-d
4) δ 8.88 (dd, J = 3.4, 1.4 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.86 (d, J = 1.3 Hz, 1H), 4.08 (s, 3H). Intermediate 48: Methyl 8-fluoro-[1,2,4]triazolo[4,3-a]pyridine-5-carboxylate. Chloro-3-fluoro-2-pyridyl)hydrazine.2,6-Dichloro-3-fluoropyridine (5.0 g, 30.1 mmol,
up in EtOH (50 mL, 0.6 M). Hydrazine hydrate (6.0 g, 120 mmol, 4.0 eq) was added and the reaction was stirred at 80 °C for 16 h. After cooling to rt, the mixture was concentrated in vacuo then diluted with water and stirred at rt for 30 min before being filtered. The precipitate was collected to provide (6-chloro-3-fluoro-2-pyridyl)hydrazine (2.9 g, 60% yield) as a yellow solid.
1H NMR (400 MHz, DMSO- d
6) δ 8.21 (s, 1H), 7.37 (dd, J = 10.9, 8.0 Hz, 1H), 6.56 (dd, J = 8.0, 2.5 Hz, 1H), 4.17 (br s, 2H). [00285] Step B: 5-Chloro-8-fluoro-[1,2,4]triazolo[4,3-a]pyridine. (6-Chloro-3-fluoro-2-pyridyl)hydrazine (2.0 g, 12.4 mmol, 1.0 eq) was taken up in trimethoxymethane (19 g, 183 mmol, 15 eq). TFA (56 mg, 0.495 mmol, 0.04 eq) was added and the reaction was stirred at 100 °C for 16 h. After cooling to rt, the mixture was concentrated in vacuo. The resulting residue was purified by FCC (PE:EtOAc = 5:1 - 0:1) to give 5- chloro-8-fluoro-[1,2,4]triazolo[4,3-a]pyridine (1.2 g, 57% yield) as a yellow solid.
1H NMR (400 MHz, CDCl
3) δ 9.01 (d, J = 2.8 Hz, 1H), 7.01 (dd, J = 9.2, 7.9 Hz, 1H), 6.87 (dd, J = 7.9, 3.5 Hz, 1H). [00286] The title compound was prepared in a manner analogous to Intermediate 17 using 5-chloro-8- fluoro-[1,2,4]triazolo[4,3-a]pyridine instead of 2,5-dichloro-4-(trifluoromethyl)pyrimidine. MS (ESI): mass calcd. for C
8H
6FN
3O
2, 195.0; m/z found, 196.1 [M+H]
+. Intermediate 49: Methyl 8-fluoro-2-methylimidazo[1,2-c]pyrimidine-5-carboxylate.
A: 2-((2-Chloro-5-fluoropyrimidin-4-yl)amino)propan-1-ol. 2,4-Dichloro-5- fluoropyrimidine (5.0 g, 29.9 mmol, 1.0 eq) was taken up in ACN (50 mL, 0.6 M).2-Aminopropan-1-ol (2.2 g, 29.9 mmol, 1.0 eq) and DIPEA (7.7 g, 59.9 mmol, 2.0 eq) were added and the reaction was stirred at 75 °C for 24 h. After cooling to rt, the mixture was concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 1:0 - 65:35) to give 2-((2-chloro-5-fluoropyrimidin-4-yl)amino)propan- 113 QB\184200.00081\93036187.2
VVID-747PC 1-ol (3.8 g, 61% yield) as a white solid. MS (ESI): mass calcd. for C
7H
9ClFN
3O, 205.0; m/z found, 206.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.81 (d, J = 2.8 Hz, 1H), 5.37 (br s, 1H), 4.37 - 4.24 (m, 1H), 3.75 (dd, J = 11.0, 3.8 Hz, 1H), 3.61 (dd, J = 11.0, 5.0 Hz, 1H), 1.25 (d, J = 6.8 Hz, 3H). [00288] Step B: 5-Chloro-8-fluoro-2-methyl-2,3-dihydroimidazo[1,2-c]pyrimidine. 2-((2-Chloro-5- fluoropyrimidin-4-yl)amino)propan-1-ol (2.6 g, 12.6 mmol, 1.0 eq) was taken up in DCM (30 mL, 0.42 M). TEA (2.6 g, 25.3 mmol, 2.0 eq) and Ms
2O (2.6 g, 15.2 mmol, 1.2 eq) were added and the reaction was stirred at 45 °C for 12 h. Upon cooling, the mixture was diluted with sat. aq. NaHCO
3 and extracted with DCM. The organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC (PE:EtOAc = 1:0 - 1:1) to give 5-chloro-8- fluoro-2-methyl-2,3-dihydroimidazo[1,2-c]pyrimidine (1.3 g, 55% yield) as a light yellow oil.
1H NMR (400 MHz, CDCl
3) δ 7.18 (d, J = 3.0 Hz, 1H), 4.44 (q, J = 10.3, 7.0 Hz, 1H), 4.33 - 4.23 (m, 1H), 3.72 (dd, J = 11.2, 8.2 Hz, 1H), 1.40 (d, J = 6.6 Hz, 3H). [00289] Step C: 5-Chloro-8-fluoro-2-methylimidazo[1,2-c]pyrimidine. 5-Chloro-8-fluoro-2-methyl-2,3- dihydroimidazo[1,2-c]pyrimidine (1.3 g, 6.93 mmol, 1.0 eq) was taken up in 1,4-dioxane (25 mL, 0.27 M). Activated MnO
2 (6.0 g, 69.3 mmol, 10 eq) was added and the mixture was stirred at 90 °C for 12 h. After cooling to rt, the solution was filtered and concentrated under reduced pressure. The resulting residue was purified by FCC (PE:EtOAc = 10:0 - 7:3) to give 5-chloro-8-fluoro-2-methylimidazo[1,2-c]pyrimidine (260 mg, 20% yield) as a colorless oil.
1H NMR (400 MHz, CDCl
3) δ 7.74 (d, J = 2.1 Hz, 1H), 7.53 (dd, J = 3.0, 0.8 Hz, 1H), 2.54 (d, J = 0.9 Hz, 3H). [00290] The title compound was prepared in a manner analogous to Intermediate 17 using 5-chloro-8- fluoro-2-methylimidazo[1,2-c]pyrimidine instead of 2,5-dichloro-4-(trifluoromethyl)pyrimidine.
1H NMR (400 MHz, CDCl
3) δ 8.61 (d, J = 2.8 Hz, 1H), 7.96 (d, J = 1.6 Hz, 1H), 4.12 (s, 3H), 2.59 (s, 3H). Intermediate 50: Methyl 7-chloropyrazolo[1,5-a]pyrazine-4-carboxylate.
[1,5-a]pyrazin-4(5H)-one.1H-Pyrazole-5-carboxylic acid (20 g, 178 mmol, 1.0 eq) was taken up in 1,4-dioxane (200 mL, 0.9 M) and placed under N
2. CDI (32 g, 196 mmol, 1.1 eq) was added and the mixture was stirred at 50 °C for 30 min before 2,2-dimethoxyethan-1-amine (21.4 mL, 196 mmol, 1.1 eq) was added, continuing to stir for 30 min at 50 °C. HCl (74 mL, 892 mmol, 5.0 eq) was added and the reaction was heated to 100 °C for 16 h. After cooling to rt, the mixture was concentrated under reduced pressure and the residue was diluted with water and stirred for 30 min. The mixture was filtered and the precipitate was collected to give pyrazolo[1,5-a]pyrazin-4(5H)-one (7.8 g, 32% yield) as a brown solid. MS (ESI): mass calcd. for C
6H
5N
3O, 135.0; m/z found, 136.0 [M+H]
+. [00292] Step B: 7-Chloropyrazolo[1,5-a]pyrazin-4(5H)-one. Pyrazolo[1,5-a]pyrazin-4(5H)-one (1.0 g, 7.40 mmol, 1.0 eq) was taken up in DMF (10 mL, 0.74 M) and placed under N
2. Acetic acid (1.3 g, 22.2 114 QB\184200.00081\93036187.2
VVID-747PC mmol, 3.0 eq) and NCS (988 mg, 7.40 mmol, 1.0 eq) were added and the mixture was stirred at 80 °C for 30 min. After cooling to rt, the mixture was diluted with water and extracted with EtOAc. The organic phase was washed with water, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. i- PrOH was added and the mixture was stirred for 15 min before being filtered and the precipitate collected to give 7-chloropyrazolo[1,5-a]pyrazin-4(5H)-one (500 mg, 40% yield) as a brown solid. MS (ESI): mass calcd. for C
6H
4ClN
3O, 169.0; m/z found, 170.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 11.56 (br s, 1H), 8.01 (d, J = 2.1 Hz, 1H), 7.24 (s, 1H), 7.15 (d, J = 2.1 Hz, 1H). [00293] Step C: 4-Bromo-7-chloropyrazolo[1,5-a]pyrazine. 7-Chloropyrazolo[1,5-a]pyrazin-4(5H)-one (100 mg, 0.590 mmol, 1.0 eq) was taken up in toluene (1.0 mL, 0.6 M) and placed under N
2. Phosphorus oxybromide (845 mg, 2.95 mmol, 5.0 eq) was added and the reaction was stirred at 110 °C for 4 h. After cooling to rt, the mixture was concentrated under reduced pressure before water was added and stirring continued for 30 min. The mixture was filtered and the precipitate was collected to give 4-bromo-7- chloropyrazolo[1,5-a]pyrazine (30 mg, 22% yield) as a brown solid. MS (ESI): mass calcd. for C
6H
3BrClN
3, 230.9; m/z found, 232.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.38 (d, J = 2.4 Hz, 1H), 8.04 (s, 1H), 7.16 (d, J = 2.4 Hz, 1H). [00294] The title compound was prepared in a manner analogous to Intermediate 17 using 4-bromo-7- chloro-pyrazolo[1,5-a]pyrazine instead of 2,5-dichloro-4-(trifluoromethyl)pyrimidine. MS (ESI): mass calcd. for C
8H
6ClN
3O
2, 211.0; m/z found, 212.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.30 (d, J = 2.3 Hz, 1H), 8.14 (s, 1H), 7.55 (d, J = 2.4 Hz, 1H), 4.11 (s, 3H). Intermediate 51: Methyl 7-fluoro-1-methyl-1H-pyrazolo[4,3-c]pyridine-4-carboxylate.
Chloro-5-fluoronicotinaldehyde. 4-Chloro-3-fluoropyridine (50 g, 380 mmol, 1.0 eq) was taken up in THF (600 mL, 0.6 M), placed under N
2, and cooled to -78 °C. LDA (285 mL, 570 mmol, 1.5 eq, 2M in THF) was added dropwise and the solution was allowed to stir for 2 h at -78 °C. DMF (44 mL, 570 mmol, 1.5 eq) in THF (40 mL, 0.6 M) was added and the reaction was allowed to warm to rt over 2 h. The mixture was cooled to 0 °C, quenched with sat. aq. NH
4Cl and water, and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 3:1) to give 4-chloro-5- fluoronicotinaldehyde (26 g, 39% yield) as a brown solid.
1H NMR (400 MHz, CDCl
3) δ 10.47 (s, 1H), 8.89 (s, 1H), 8.70 (s, 1H). [00296] Step B: 7-Fluoro-1H-pyrazolo[4,3-c]pyridine. 4-Chloro-5-fluoronicotinaldehyde (10 g, 62.7 mmol, 1.0 eq) was taken up in DMSO (100 mL, 0.6 M). Hydrazine monohydrate (9.0 g, 144 mmol, 2.3 eq) was added dropwise at 15 °C then the reaction was heated to 120 °C and stirred for 16 h. After cooling to rt, the mixture was diluted with water and extracted with EtOAc. The combined organic layers were washed 115 QB\184200.00081\93036187.2
VVID-747PC with brine, dried over Na
2SO
4, filtered and concentrated under reduced pressure to give 7-fluoro-1H- pyrazolo[4,3-c]pyridine (6.0 g, 70% yield) as a brown solid.
1H NMR (400 MHz, CDCl
3) δ 14.16 (br s, 1H), 8.97 (d, J = 2.5 Hz, 1H), 8.44 (br s, 1H), 8.33 (br d, J = 2.6 Hz, 1H). [00297] Step C: 7-Fluoro-1H-pyrazolo[4,3-c]pyridine 5-oxide. 7-Fluoro-1H-pyrazolo[4,3-c]pyridine (5.8 g, 42.3 mmol, 1.0 eq) was taken up in THF (250 mL, 0.17 M).3-Chloroperbenzoic acid (17 g, 84.6 mmol, 2.0 eq, 85%) was added portionwise at 0 °C. The reaction was stirred at rt for 16 h before being filtered. The filtrate was diluted with Na
2S
2O
3. The filter cake was triturated with EtOAc at 20 °C and the solid was dried to give 7-fluoro-1H-pyrazolo[4,3-c]pyridine 5-oxide (4.0 g, 62% yield) as a brown solid.
1H NMR (400 MHz, CDCl
3) δ 14.49 - 14.28 (m, 1H), 8.74 (s, 1H), 8.42 (br s, 1H), 8.24 (br s, 1H). [00298] Step D: 4-Chloro-7-fluoro-1H-pyrazolo[4,3-c]pyridine. (2-Chloro-3- fluorophenyl)(cyclopropyl)methanamine 5-oxide (3.5 g, 22.9 mmol, 1.0 eq) was taken up in POCl
3 (35 mL) and the reaction was stirred at 110 °C for 30 min. After cooling to rt, the mixture was concentrated under reduced pressure. The residue was quenched with water and extracted with EtOAc. The combined organic layers were washed with sat. aq. NaHCO
3 and brine, dried over Na
2SO
4, filtered and concentrated under reduced pressure. The resulting residue was purified by FCC (PE:EtOAc = 1:0 to 2:1) to give 4- chloro-7-fluoro-1H-pyrazolo[4,3-c]pyridine (2.0 g, 51% yield) as a pale yellow solid.
1H NMR (400 MHz, CDCl
3) δ 8.27 (d, J = 3.0 Hz, 1H), 8.11 (d, J = 2.4 Hz, 1H). [00299] Step E: 4-Chloro-7-fluoro-1-methyl-1H-pyrazolo[4,3-c]pyridine. 4-Chloro-7-fluoro-1H- pyrazolo[4,3-c]pyridine (1.0 g, 5.83 mmol, 1.0 eq) was taken up in THF (20 mL, 0.3 M) and placed under N
2 at 0 °C. Sodium hydride (280 mg, 6.99 mmol, 1.2 eq, 60% in mineral oil) was added and the mixture was stirred for 30 min. Iodomethane (1.6 g, 11.7 mmol, 2.0 eq) was added and the reaction was stirred for 1 h at 20 °C. The solution was quenched with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 4:1 to 2:1) to give 4-chloro-7-fluoro-1-methyl-1H-pyrazolo[4,3- c]pyridine (450 mg, 42% yield, first eluting product) as a white solid.
1H NMR (400 MHz, CDCl
3) δ 8.11 (d, J = 2.1 Hz, 1H), 8.01 (d, J = 3.1 Hz, 1H), 4.26 (s, 3H). [00300] The title compound was prepared in a manner analogous to Intermediate 17 using 4-chloro-7- fluoro-1-methyl-pyrazolo[4,3-c]pyridine instead of 2,5-dichloro-4-(trifluoromethyl)pyrimidine. MS (ESI): mass calcd. for C
9H
8FN
3O
2, 209.1; m/z found, 210.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.64 (d, J = 1.9 Hz, 1H), 8.37 (d, J = 2.8 Hz, 1H), 4.32 (s, 3H), 4.10 (s, 3H). Intermediate 52: Methyl 7-fluoro-2-methyl-2H-pyrazolo[4,3-c]pyridine-4-carboxylate.
VVID-747PC [00301] 4-Chloro-7-fluoro-2-methyl-2H-pyrazolo[4,3-c]pyridine was isolated from the separation of 4- chloro-7-fluoro-1-methyl-1H-pyrazolo[4,3-c]pyridine (Intermediate 51, Step E, second eluting product) and elaborated to the title compound in a manner analogous to Intermediate 17.
1H NMR (400 MHz, CDCl
3) δ 8.15 (d, J = 2.3 Hz, 1H), 7.91 (d, J = 3.1 Hz, 1H), 4.31 (s, 3H). Intermediate 53: Methyl 8-fluoro-[1,2,4]triazolo[4,3-c]pyrimidine-5-carboxylate. Butyl 2-(2-chloro-5-fluoropyrimidin-4-yl)hydrazine-1-carboxylate. 2,4-Dichloro-5-
(1.0 g, 5.99 mmol, 1.0 eq) was taken up in THF (15 mL, 0.4 M). tert-Butyl hydrazinecarboxylate (871 mg, 6.59 mmol, 1.1 eq) and DIPEA (1.2 mL, 7.19 mmol, 1.2 eq) were added and the reaction was allowed to stir at 20 °C for 2 h. The mixture was diluted with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 1:0 - 3:1) to provide tert-butyl 2-(2-chloro-5-fluoropyrimidin-4-yl)hydrazine-1-carboxylate (1.3 g, 86% yield) as a white solid.
1H NMR (400 MHz, CDCl
3) δ 8.06 (d, J = 3.3 Hz, 1H), 1.55 - 1.43 (m, 9H). [00303] Step B: Methyl 4-(2-(tert-butoxycarbonyl)hydrazineyl)-5-fluoropyrimidine-2-carboxylate. tert- Butyl 2-(2-chloro-5-fluoropyrimidin-4-yl)hydrazine-1-carboxylate (1.3 g, 4.95 mmol, 1.0 eq) was taken up in MeOH/DMF (v/v 1:1, 30 mL, 0.16 M). TEA (1.4 mL, 9.90 mmol, 2.0 eq) and Pd(dppf)Cl2 (358 mg, 0.495 mmol, 0.1 eq) were added. The reaction was placed under CO (50 psi) and stirred at 80 °C for 12 h. After cooling to rt, the mixture was diluted with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 3:1 - 1:2) to give methyl 4-(2-(tert-butoxycarbonyl)hydrazinyl)- 5-fluoropyrimidine-2-carboxylate (1.2 g, 85% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 8.31 (d, J = 2.6 Hz, 1H), 7.17 (br s, 1H), 6.78 (br s, 1H), 3.98 (s, 3H), 1.49 (br s, 9H). [00304] Step C: Methyl 5-fluoro-4-hydrazineylpyrimidine-2-carboxylate. Methyl 4-(2-(tert- butoxycarbonyl)hydrazinyl)-5-fluoropyrimidine-2-carboxylate (1.2 g, 4.19 mmol, 1.0 eq) was taken up in DCM/TFA (v/v 3:1, 16 mL, 0.26 M) and the reaction was allowed to stir at 20 °C for 2 h. The mixture was concentrated under reduced pressure to provide methyl 5-fluoro-4-hydrazinylpyrimidine-2-carboxylate (1.2 g, quant. yield) as a brown oil, which was used directly without purification. [00305] Step D: Methyl 8-fluoro-[1,2,4]triazolo[4,3-c]pyrimidine-5-carboxylate. Methyl 5-fluoro-4- hydrazinylpyrimidine-2-carboxylate (1.2 g, 6.45 mmol, 1.0 eq) was taken up in trimethoxymethane (19 mL, 177 mmol, 27 eq). TFA (147 mg, 1.29 mmol, 0.2 eq) was added and the reaction was stirred at 100 °C for 16 h. After cooling to rt, the mixture was concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 3:1 - 1:1) to give methyl 8-fluoro-[1,2,4]triazolo[4,3-c]pyrimidine-5- 117 QB\184200.00081\93036187.2
VVID-747PC carboxylate (500 mg, 40% yield) as a yellow solid.
1H NMR (400 MHz, CDCl
3) δ 9.93 (d, J = 2.9 Hz, 1H), 8.00 (d, J = 1.4 Hz, 1H), 4.00 (s, 3H). Intermediate 54: Ethyl 5-fluoro-4-methoxypyrimidine-2-carboxylate. 2-pyrimidinecarboxylic acid (166 mg, 0.964 mmol, 1.0 eq) was taken up
M). To this was added, oxalyl chloride (245 µL, 2.89 mmol, 3.0 eq) and 1 drop of DMF. The reaction was allowed to stir at rt for 2 hours, then concentrated under a stream of air. EtOH (1 mL) was added and the mixture was allowed to stir for 15 min at rt before being concentrated under reduced pressure. The product was taken onto the next step without purification. MS (ESI): mass calcd. for C
8H
9FN
2O
3, 200.1; m/z found, 201.0 [M+H]
+. Intermediate 55: Ethyl (S)-5-((1-(2-chlorophenyl)ethyl)amino)-4-cyanopyrimidine-2-carboxylate. O N N 5-fluoropyrimidine-4-carbonitrile.2,4-Dichloro-5-fluoropyrimidine (5.0 g, 29.9
mmol, 1.0 eq) and 1,4-diazabicyclo[2.2.2]octane (336 mg, 2.99 mmol, 0.1 eq) were taken up in DMF/water (v/v 5:1, 60 mL, 0.5 M). Sodium cyanide (1.9 g, 39.2 mmol, 1.3 eq) was added at 0 °C and the reaction was warmed to rt and stirred for 5 h. The mixture was diluted with water and extracted with EtOAc. The combined organic layers were washed with brine and water, dried over Na
2SO
4, filtered, and concentrated in vacuo. The residue was purified by FCC (PE:EtOAc = 1:0 - 8:1) to give 2-chloro-5-fluoropyrimidine-4- carbonitrile (2.2 g, 47% yield) as a colorless oil.
1H NMR (400 MHz, CDCl
3) δ 8.81 (s, 1H). [00308] Step B: (S)-2-Chloro-5-((1-(2-chlorophenyl)ethyl)amino)pyrimidine-4-carbonitrile. 2-Chloro-5- fluoropyrimidine-4-carbonitrile (1.0 g, 6.35 mmol, 1.0 eq) was taken up in DMSO (10 mL, 0.6 M). (S)-1- (2-Chlorophenyl)ethan-1-amine (1.0 g, 6.67 mmol, 1.05 eq) and TEA (2.6 mL, 19.0 mmol, 3.0 eq) were added and the reaction was heated at 100 °C for 2 h. After cooling to rt, the mixture was diluted with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (5-15% EtOAc in PE) to give (S)-2-chloro-5-((1-(2-chlorophenyl)ethyl)amino)pyrimidine-4-carbonitrile (1.5 g, 81% yield) as a yellow solid. MS (ESI): mass calcd. for C
13H
10Cl
2N
4, 292.0; m/z found, 293.0 [M+H]
+.
1H NMR (400 118 QB\184200.00081\93036187.2
VVID-747PC MHz, CDCl
3) δ 7.93 (s, 1H), 7.46 - 7.40 (m, 1H), 7.34 - 7.27 (m, 2H), 7.26 - 7.19 (m, 1H), 5.12 - 4.99 (m, 2H), 1.67 (d, J = 6.4 Hz, 3H). [00309] The title compound was prepared in a manner analogous to Intermediate 17 using (S)-2-chloro-5- ((1-(2-chlorophenyl)ethyl)amino)pyrimidine-4-carbonitrile instead of 2,5-dichloro-4- (trifluoromethyl)pyrimidine and EtOH instead of MeOH.
1H NMR (400 MHz, CDCl
3) δ 8.16 (s, 1H), 7.45 - 7.39 (m, 1H), 7.34 - 7.27 (m, 2H), 7.26 - 7.22 (m, 1H), 5.45 (br d, J = 5.6 Hz, 1H), 5.19 (q, J = 6.5 Hz, 1H), 4.47 (q, J = 7.1 Hz, 2H), 1.70 (d, J = 6.6 Hz, 3H), 1.41 (t, J = 7.1 Hz, 3H). 119 QB\184200.00081\93036187.2
VVID-747PC Example 1: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide. (1-(2-chlorophenyl)ethyl)amino)benzoate. Methyl 4-bromobenzoate (120
up in toluene (2.8 mL, 0.2 M) under N
2. To this was added (S)-1-(2- chlorophenyl)ethan-1-amine hydrochloride (113 mg, 0.590 mmol, 1.05 eq), Cs
2CO
3 (545 mg, 1.67 mmol, 3.0 eq), and SPhos Pd G4 (44 mg, 0.059 mmol, 0.1 eq). The reaction was stirred at 100°C for 24 hours. After cooling to room temperature, the reaction was quenched with water and extracted with EtOAc. The combined organic layers were dried over Na
2SO
4, filtered, and concentrated under reduced pressure. Purification by FCC on silica (0-25% EtOAc in heptanes) provided methyl (S)-4-((1-(2- chlorophenyl)ethyl)amino)benzoate (120 mg, 74% yield). MS (ESI): mass calcd. for C
16H
16ClNO
2, 289.1; m/z found, 290.0 [M+H]
+. [00311] Step B: (S)-4-((1-(2-Chlorophenyl)ethyl)amino)benzoic acid. Methyl (S)-4-((1-(2- chlorophenyl)ethyl)amino)benzoate (20 mg, 0.068 mmol, 1.0 eq) was taken up in methanol (0.4 mL, 0.16 M). To this was added lithium hydroxide (2N in water, 0.27 mL, 0.550 mmol, 8.0 eq) and the reaction was stirred at 60°C for 3 hours. After cooling to rt, the reaction was adjusted to pH ∼2 with 1N HCl. The precipitated product was collected by filtration to provide (S)-4-((1-(2-chlorophenyl)ethyl)amino)benzoic acid (14 mg, 76% yield) as a white solid. MS (ESI): mass calcd. for C15H14ClNO2, 275.1; m/z found, 276.0 [M+H]
+. [00312] Step C: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide. (S)-4-((1-(2-Chlorophenyl)ethyl)amino)benzoic acid (14 mg, 0.050 mmol, 1.0 eq) and HATU (24 mg, 0.060 mmol, 1.2 eq) were taken up in DMF (0.26 mL, 0.2 M). To this was added N,N-diisopropylethylamine (27 µL, 0.160 mmol, 3.0 eq) and the reaction was allowed to stir for 5 minutes. (R,E)-4-(Methylsulfonyl)but-3-en-2-amine, 4-methylbenzenesulfonic acid (Intermediate 1, 17 mg, 0.050 mmol, 1.0 eq) was added and the reaction was stirred for 30 min at rt. The crude reaction was filtered through a PTFE filter with MeOH and purified by RP-HPLC (5-95% ACN in 0.1% aq. HCOOH) to provide 4-(((S)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide (17 mg, 79% yield). MS (ESI): mass calcd. for C
20H
23ClN
2O
3S, 406.1; m/z found, 407.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.59 – 7.49 (m, 2H), 7.42 – 7.32 (m, 2H), 7.22 – 7.12 (m, 2H), 6.90 (dd, J = 15.1, 4.6 Hz, 1H), 6.52 – 6.37 (m, 3H), 5.93 (d, J = 7.7 Hz, 1H), 5.01 – 4.89 (m, 2H), 4.56 (s, 1H), 2.90 (s, 3H), 1.54 (d, J = 6.7 Hz, 3H), 1.37 (d, J = 7.2 Hz, 3H). 120 QB\184200.00081\93036187.2
VVID-747PC Example 2: 2,5-Difluoro-4-(((S)-1-(2-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide. was prepared in a manner analogous to Example 1 using (S)-1-(2-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4- bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
21F
3N
2O
3S, 426.1; m/z found, 427.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.64 (dd, J = 12.2, 7.0 Hz, 1H), 7.31 – 7.21 (m, 2H), 7.13 – 7.04 (m, 2H), 6.90 (dd, J = 15.1, 4.6 Hz, 1H), 6.54 (dd, J = 14.6, 7.6 Hz, 1H), 6.47 (dd, J = 15.1, 1.7 Hz, 1H), 6.11 (dd, J = 14.0, 6.7 Hz, 1H), 5.02 – 4.89 (m, 1H), 4.87 - 4.78 (m, 2H), 2.93 (s, 3H), 1.61 (d, J = 6.0 Hz, 3H), 1.36 (d, J = 7.1 Hz, 3H). Example 3: 2,5-Difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4-(((S)-1-(o- tolyl)ethyl)amino)benzamide.
prepared in a manner analogous to Example 1 using (S)-1-(o-tolyl)ethan- 1-amine hydrochloride instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4-bromo-2,5- difluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
21H
24F
2N
2O
3S, 422.1; m/z found, 423.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.63 (dd, J = 12.3, 7.0 Hz, 1H), 7.31 – 7.27 (m, 1H), 7.22 – 7.13 (m, 3H), 6.90 (dd, J = 15.2, 4.6 Hz, 1H), 6.53 (dd, J = 14.6, 7.7 Hz, 1H), 6.46 (dd, J = 15.1, 1.7 Hz, 1H), 5.93 (dd, J = 14.2, 6.7 Hz, 1H), 5.01 – 4.90 (m, 1H), 4.81 (s, 1H), 4.68 (p, J = 6.3 Hz, 1H), 2.92 (s, 3H), 2.44 (s, 3H), 1.55 (d, J = 6.6 Hz, 3H), 1.36 (d, J = 7.1 Hz, 3H). Example 4: 4-(((S)-1-(2-Chloro-6-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide. 121 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 1 using (S)-1-(2-chloro-6-
of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-bromo-2- fluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 7.90 (dd, J = 8.1, 3.3 Hz, 1H), 7.27 - 7.47 (m, 3H), 7.03 - 7.23 (m, 2H), 6.62 - 6.82 (m, 2H), 6.39 (dd, J = 8.6, 1.9 Hz, 1H), 6.22 (dd, J = 14.3, 1.5 Hz, 1H), 5.00 (q, J = 6.8 Hz, 1H), 4.68 - 4.81 (m, 1H), 3.00 (s, 3H), 1.58 (d, J = 7.0 Hz, 3H), 1.25 (d, J = 7.0 Hz, 3H). Example 5: 5-Chloro-4-(((S)-1-(2-chlorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide.
prepared in a manner analogous to Example 1 using methyl 4-bromo-5- chloro-2-fluorobenzoate instead of methyl 4-bromobenzoate and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
20H
21Cl
2FN
2O
3S, 458.1; m/z found, 459.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.15 (dd, J = 7.8, 1.7 Hz, 1H), 7.58 (d, J = 7.5 Hz, 1H), 7.47 (ddd, J = 7.5, 3.5, 1.6 Hz, 2H), 7.21 - 7.37 (m, 2H), 6.63 - 6.83 (m, 2H), 6.53 (br d, J = 6.9 Hz, 1H), 6.02 (d, J = 13.5 Hz, 1H), 4.91 (t, J = 6.9 Hz, 1H), 4.64 - 4.79 (m, 1H), 3.00 (s, 3H), 1.56 (d, J = 6.8 Hz, 3H), 1.24 (d, J = 7.1 Hz, 3H). Example 6: 4-(((S)-1-(2-Chloro-6-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide.
VVID-747PC [00317] The title compound was prepared in a manner analogous to Example 1 using (S)-1-(2-chloro-6- fluorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-bromo-2,5- difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
20H
20ClF
3N
2O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.07 (dd, J = 8.0, 2.8 Hz, 1H), 7.28 - 7.38 (m, 3H), 7.18 (ddd, J = 11.1, 6.2, 3.4 Hz, 1H), 6.73 - 6.80 (m, 1H), 6.61 - 6.72 (m, 2H), 6.17 (dd, J = 13.1, 7.0 Hz, 1H), 4.95 - 5.17 (m, 1H), 4.64 - 4.81 (m, 1H), 3.00 (s, 3H), 1.63 (d, J = 6.9 Hz, 3H), 1.24 (d, J = 7.1 Hz, 3H). Example 7: 4-(((*S)-1-(2-Chloro-5-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide. prepared in a manner analogous to Example 1 using (*S)-1-(2-chloro-5-
fluorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-bromo-2- fluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 7.94 (br dd, J = 7.9, 2.6 Hz, 1H), 7.52 (dd, J = 8.6, 5.0 Hz, 1H), 7.40 (t, J = 8.7 Hz, 1H) ,7.11 - 7.25 (m, 3H), 6.71 -6.79 (m, 1H), 6.61 - 6.70 (m, 1H), 6.30 (br d, J = 8.4 Hz, 1H), 6.12 (br d, J = 13.9 Hz, 1H), 4.72 - 4.83 (m, 2H), 2.99 (s, 3H), 1.44 (d, J = 6.6 Hz, 3H), 1.24 (d, J = 7.0 Hz, 3H). 123 QB\184200.00081\93036187.2
VVID-747PC Example 8: 4-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. prepared in a manner analogous to Example 1 using 1-(2-chloro-3-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4- bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. Separation of 4-(1-(2-chloro-3-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide (Step C) via SFC (Stationary phase: OZ (3x25 cm); Mobile phase: 30% MeOH/CO
2; Rt = 5.50 min) provided the title compound. MS (ESI): mass calcd. for C
20H
20ClF
3N
2O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 7.99 - 8.14 (m, 1H), 7.26 - 7.45 (m, 4H), 7.07 (br d, J = 7.1 Hz, 1H), 6.59 - 6.84 (m, 2H), 5.93 - 6.17 (m, 1H), 4.90 (q, J = 6.7 Hz, 1H), 4.65 - 4.79 (m, 1H), 2.90 - 3.05 (m, 3H), 1.51 (d, J = 6.6 Hz, 3H), 1.24 (d, J = 7.0 Hz, 3H). Example 9: 4-(((*R)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide.
prepared in a manner analogous to Example 1 using 1-(2-chloro-3- fluorophenyl)ethan-1-amine hydrochloride instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4- bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. Separation of 4-(1-(2-chloro-3-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide (Step C) via SFC (Stationary phase: OZ (3x25 cm); Mobile phase: 30% MeOH/CO
2; Rt = 6.84 min) provided the title compound. MS (ESI): mass calcd. for C
20H
20ClF
3N
2O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.03 (dd, J = 7.9, 3.2 Hz, 1H), 7.26 - 7.43 (m, 4H), 7.01 - 7.14 (m, 1H), 6.64 - 6.83 (m, 2H), 6.01 (dd, J = 12.9, 6.9 Hz, 1H), 4.84 - 4.96 (m, 1H), 4.68 - 4.80 (m, 1H), 2.94 - 3.03 (m, 3H), 1.51 (d, J = 6.6 Hz, 3H), 1.25 (d, J = 7.0 Hz, 3H). 124 QB\184200.00081\93036187.2
VVID-747PC Example 10: 4-(((*S)-1-(2-Chloro-4-methylphenyl)ethyl)amino)-2-fluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. prepared in a manner analogous to Example 1 using (*S)-1-(2-chloro-4-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-bromo-2- fluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
21H
24ClFN
2O
3S, 438.1; m/z found, 439.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 7.89 (dd, J = 7.9, 3.3 Hz, 1H), 7.38 (t, J = 8.7 Hz, 1H), 7.23 - 7.31 (m, 2H), 7.05 - 7.21 (m, 2H), 6.72 - 6.84 (m, 1H), 6.62 - 6.70 (m, 1H), 6.30 (dd, J = 8.6, 1.5 Hz, 1H), 6.08 (br d, J = 14.1 Hz, 1H), 4.67 - 4.82 (m, 2H), 2.99 (s, 3H), 2.25 (s, 3H), 1.42 (d, J = 6.6 Hz, 3H), 1.24 (d, J = 7.0 Hz, 3H). Example 11: 4-(((*S)-1-(2-Chloro-5-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide.
prepared in a manner analogous to Example 1 using (*S)-1-(2-chloro-5- fluorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-bromo-2,5- difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
20H
20ClF
3N
2O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.09 (dd, J = 7.8, 2.6 Hz, 1H), 7.53 (dd, J = 8.8, 5.2 Hz, 1H), 7.29 - 7.41 (m, 2H), 7.16 (td, J = 8.4, 3.1 Hz, 1H), 7.01 (br d, J = 7.6 Hz, 1H), 6.65 - 6.82 (m, 2H), 6.04 (dd, J = 12.9, 7.0 Hz, 1H), 4.85 (br t, J = 6.9 Hz, 1H), 4.74 (br d, J = 4.8 Hz, 1H), 3.00 (s, 3H), 1.50 (d, J = 6.8 Hz, 3H), 1.25 (d, J = 7.0 Hz, 3H). Example 12: 4-(((*R)-1-(2-Chloro-5-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. 125 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 1 using (*R)-1-(2-chloro-5-
of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-bromo-2,5- difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
20H
20ClF
3N
2O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.04 (br dd, J = 7.8, 2.8 Hz, 1H), 7.53 (dd, J = 8.8, 5.1 Hz, 1H), 7.28 - 7.42 (m, 2H), 7.15 (td, J = 8.4, 3.1 Hz, 1H), 7.01 (br d, J = 7.4 Hz, 1H), 6.62 - 6.82 (m, 2H), 6.02 (dd, J = 12.8, 6.9 Hz, 1H), 4.85 (br t, J = 6.8 Hz, 1H), 4.71 - 4.78 (m, 1H), 2.98 (s, 3H), 1.50 (d, J = 6.6 Hz, 3H), 1.25 (d, J = 7.0 Hz, 3H). Example 13: (R,E)-4-((2-(2-Chlorophenyl)propan-2-yl)amino)-2,5-difluoro-N-(4-(methylsulfonyl)but-3- en-2-yl)benzamide.
was prepared in a manner analogous to Example 1 using 2-(2- chlorophenyl)propan-2-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-bromo-2,5- difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
21H
23ClF
2N
2O
3S, 456.1; m/z found, 457.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 7.98 (dd, J = 8.0, 3.0 Hz, 1H), 7.69 (dd, J = 7.9, 1.3 Hz, 1H), 7.38 - 7.45 (m, 1H), 7.26 - 7.38 (m, 3H), 6.70 - 6.78 (m, 1H), 6.62 - 6.70 (m, 1H), 6.29 (br s, 1H), 5.68 (dd, J = 13.7, 7.3 Hz, 1H), 4.64 - 4.77 (m, 1H), 2.98 (s, 3H), 1.79 (s, 6H), 1.23 (d, J = 7.0 Hz, 3H). 126 QB\184200.00081\93036187.2
VVID-747PC Example 14: (R,E)-4-((1-(2-Chlorophenyl)cyclopropyl)amino)-2,5-difluoro-N-(4-(methylsulfonyl)but-3- en-2-yl)benzamide. was prepared in a manner analogous to Example 1 using 1-(2-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-bromo-2,5- difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
21H
21ClF
2N
2O
3S, 454.1; m/z found, 455.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.06 (dd, J = 7.8, 2.8 Hz, 1H), 7.79 (dd, J = 7.3, 1.8 Hz, 1H), 7.42 (dd, J = 7.5, 1.6 Hz, 1H), 7.21 - 7.36 (m, 3H), 6.61 - 6.91 (m, 4H), 4.68 - 4.79 (m, 1H), 2.95 - 3.03 (m, 3H), 1.16 - 1.33 (m, 7H). Example 15: 4-(((*S)-1-(2-Chlorophenyl)-2-methylpropyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide.
prepared in a manner analogous to Example 1 using (*S)-1-(2- chlorophenyl)-2-methylpropan-1-amine (Intermediate 2) instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
22H
25ClF
2N
2O
3S, 470.1; m/z found, 471.2 [M+H]
+. 1H NMR (400 MHz, DMSO-d
6) δ 8.02 (br dd, J = 2.7, 7.8 Hz, 1H), 7.60 (d, J = 7.6 Hz, 1H), 7.43 (d, J = 7.9 Hz, 1H), 7.21 - 7.36 (m, 3H), 6.91 (br d, J = 8.4 Hz, 1H), 6.64 - 6.80 (m, 2H), 6.18 - 6.31 (m, 1H), 4.67 - 4.80 (m, 1H), 4.37 - 4.49 (m, 1H), 3.00 (s, 3H), 2.14 - 2.29 (m, 1H), 1.20 - 1.28 (m, 3H), 1.13 (d, J = 6.4 Hz, 3H), 0.69 - 0.78 (m, 3H). 127 QB\184200.00081\93036187.2
VVID-747PC Example 16: 4-(((*R)-1-(2-Chlorophenyl)-2-methylpropyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. prepared in a manner analogous to Example 1 using (*R)-1-(2-
- amine (Intermediate 3) instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
22H
25ClF
2N
2O
3S, 470.1; m/z found, 471.2 [M+H]
+. 1H NMR (400 MHz, DMSO-d
6) δ 7.98 (dd, J = 3.5, 8.0 Hz, 1H), 7.60 (dd, J = 1.6, 7.8 Hz, 1H), 7.43 (dd, J = 1.2, 7.9 Hz, 1H), 7.19 - 7.37 (m, 3H), 6.92 (br d, J = 8.5 Hz, 1H), 6.61 - 6.79 (m, 2H), 6.24 (dd, J = 7.0, 13.4 Hz, 1H), 4.66 - 4.80 (m, 1H), 4.42 (t, J = 8.9 Hz, 1H), 2.98 (s, 3H), 2.13 - 2.28 (m, 1H), 1.25 (d, J = 7.0 Hz, 3H), 1.13 (d, J = 6.5 Hz, 3H), 0.73 (d, J = 6.9 Hz, 3H). Example 17: 4-((1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide.
was prepared in a manner analogous to Example 1 using 1-(2- chlorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine in Step A. MS (ESI): mass calcd. for C
20H
23ClN
2O
3S, 406.1; m/z found, 407.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.59 – 7.49 (m, 2H), 7.42 – 7.32 (m, 2H), 7.23 – 7.13 (m, 2H), 6.90 (ddd, J = 15.1, 4.5, 1.9 Hz, 1H), 6.50 – 6.38 (m, 3H), 5.87 (d, J = 7.8 Hz, 1H), 5.06 – 4.86 (m, 2H), 4.53 (s, 1H), 2.91 (d, J = 1.8 Hz, 3H), 1.55 (t, J = 5.9 Hz, 3H), 1.38 (dd, J = 7.1, 2.7 Hz, 3H). 128 QB\184200.00081\93036187.2
VVID-747PC Example 18: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide. prepared in a manner analogous to Example 1 using methyl 4-bromo-2-
4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
22ClFN
2O
3S, 424.1; m/z found, 425.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.81 (t, J = 8.9 Hz, 1H), 7.42 – 7.37 (m, 1H), 7.35 – 7.31 (m, 1H), 7.24 – 7.17 (m, 2H), 6.92 (dd, J = 15.1, 4.5 Hz, 1H), 6.55 – 6.42 (m, 2H), 6.34 (dd, J = 8.7, 2.3 Hz, 1H), 6.06 (dd, J = 15.2, 2.3 Hz, 1H), 5.02 – 4.89 (m, 2H), 4.65 (d, J = 5.8 Hz, 1H), 2.92 (s, 3H), 1.55 (d, J = 6.7 Hz, 3H), 1.38 (d, J = 7.1 Hz, 3H). Example 19: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide.
prepared in a manner analogous to Example 1 using methyl 4-bromo-2,5- difluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.66 (dd, J = 12.2, 7.0 Hz, 1H), 7.45 – 7.37 (m, 1H), 7.37 – 7.29 (m, 1H), 7.25 – 7.18 (m, 2H), 6.91 (dd, J = 15.1, 4.6 Hz, 1H), 6.59 – 6.41 (m, 2H), 5.98 (dd, J = 14.0, 6.7 Hz, 1H), 5.04 – 4.89 (m, 2H), 4.86 (d, J = 4.8 Hz, 1H), 2.93 (s, 3H), 1.59 (d, J = 6.6 Hz, 3H), 1.36 (d, J = 7.1 Hz, 3H). 129 QB\184200.00081\93036187.2
VVID-747PC Example 20: 4-(((R)-1-(2-Chlorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide. was prepared in a manner analogous to Example 1 using (R)-1-(2-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4- bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.65 (dd, J = 12.2, 7.0 Hz, 1H), 7.46 – 7.36 (m, 1H), 7.37 – 7.28 (m, 1H), 7.25 – 7.17 (m, 2H), 6.88 (dd, J = 15.1, 4.6 Hz, 1H), 6.52 (dd, J = 14.8, 7.7 Hz, 1H), 6.42 (dd, J = 15.1, 1.7 Hz, 1H), 5.97 (dd, J = 14.0, 6.8 Hz, 1H), 5.04 – 4.78 (m, 3H), 2.90 (s, 3H), 1.59 (d, J = 6.6 Hz, 3H), 1.39 (d, J = 7.1 Hz, 3H). Example 21: 4-((1-(2-Chlorophenyl)-2,2,2-trifluoroethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide.
in a manner analogous to Example 1 using (1-(2-chlorophenyl)- 2,2,2-trifluoroethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine in Step A. MS (ESI): mass calcd. for C
20H
20ClF
3N
2O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.71 – 7.56 (m, 2H), 7.56 – 7.41 (m, 2H), 7.32 (tt, J = 7.3, 5.4 Hz, 2H), 6.90 (dd, J = 15.1, 4.6 Hz, 1H), 6.72 – 6.56 (m, 2H), 6.45 (dt, J = 15.1, 2.3 Hz, 1H), 6.05 (d, J = 7.8 Hz, 1H), 5.68 (p, J = 7.2 Hz, 1H), 5.04 – 4.89 (m, 1H), 4.83 (d, J = 8.1 Hz, 1H), 2.90 (s, 3H), 1.39 (dd, J = 7.1, 1.9 Hz, 3H). 130 QB\184200.00081\93036187.2
VVID-747PC Example 22: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2,3-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide. prepared in a manner analogous to Example 1 using methyl 4-bromo-2,3-
methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.54 (td, J = 8.6, 2.1 Hz, 1H), 7.42 – 7.35 (m, 1H), 7.35 – 7.29 (m, 1H), 7.24 – 7.15 (m, 2H), 6.90 (dd, J = 15.1, 4.6 Hz, 1H), 6.51 – 6.33 (m, 2H), 6.16 (ddd, J = 9.0, 7.3, 1.5 Hz, 1H), 5.08 – 4.88 (m, 2H), 4.78 (dd, J = 6.2, 3.0 Hz, 1H), 2.91 (s, 3H), 1.60 (d, J = 6.7 Hz, 3H), 1.41 (d, J = 7.1 Hz, 3H). Example 23: 4-(((R)-1-(2-Chlorophenyl)ethyl)amino)-2,3-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide.
was prepared in a manner analogous to Example 1 using (R)-1-(2- chlorophenyl)ethan-1-amine hydrochloride instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4- bromo-2,3-difluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.53 (td, J = 8.6, 2.1 Hz, 1H), 7.42 – 7.35 (m, 1H), 7.35 – 7.29 (m, 1H), 7.23 – 7.15 (m, 2H), 6.92 (dd, J = 15.2, 4.7 Hz, 1H), 6.53 – 6.34 (m, 2H), 6.16 (ddd, J = 9.0, 7.4, 1.5 Hz, 1H), 5.09 – 4.89 (m, 2H), 4.79 (dd, J = 6.1, 3.0 Hz, 1H), 2.93 (s, 3H), 1.59 (d, J = 6.7 Hz, 3H), 1.40 (d, J = 7.1 Hz, 3H). 131 QB\184200.00081\93036187.2
VVID-747PC Example 24: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-3-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)picolinamide. prepared in a manner analogous to Example 1 using methyl 5-bromo-3-
4-bromobenzoate and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
19H
21ClFN
3O
3S, 425.1; m/z found, 426.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.75 – 7.63 (m, 2H), 7.42 – 7.29 (m, 2H), 7.24 – 7.14 (m, 2H), 6.92 (dd, J = 15.1, 4.5 Hz, 1H), 6.48 (dd, J = 15.1, 1.8 Hz, 1H), 6.34 (dd, J = 13.2, 2.3 Hz, 1H), 5.32 – 5.16 (m, 1H), 5.03 – 4.82 (m, 2H), 2.90 (s, 3H), 1.56 (s, 3H), 1.39 (d, J = 7.1 Hz, 3H). Example 25: 4-((1-(2-Chlorophenyl)propyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide.
prepared in a manner analogous to Example 1 using 1-(2- chlorophenyl)propan-1-amine hydrochloride instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4-bromo-2-fluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
21H
24ClFN
2O
3S, 438.1; m/z found, 439.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.85 – 7.73 (m, 1H), 7.43 – 7.34 (m, 1H), 7.33 – 7.25 (m, 1H), 7.26 – 7.14 (m, 2H), 6.91 (ddd, J = 15.2, 4.5, 2.8 Hz, 1H), 6.60 – 6.32 (m, 3H), 6.11 (ddd, J = 15.4, 5.4, 2.3 Hz, 1H), 5.04 – 4.92 (m, 1H), 4.89 – 4.64 (m, 2H), 2.90 (d, J = 3.1 Hz, 3H), 1.98 – 1.71 (m, 2H), 1.37 (dd, J = 7.1, 3.8 Hz, 3H), 1.11 – 0.94 (m, 3H). 132 QB\184200.00081\93036187.2
VVID-747PC Example 26: 4-(((*R)-1-(2-Chlorophenyl)propyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide. via separation of 4-((1-(2-chlorophenyl)propyl)amino)-2-fluoro-
- en-2-yl)benzamide (Example 25) by SFC (Stationary phase: AD-H (3x25 cm); Mobile phase: 40% MeOH/CO
2; Rt = 3.34 min). MS (ESI): mass calcd. for C
21H
24ClFN
2O
3S, 438.1; m/z found, 439.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.78 (t, J = 9.0 Hz, 1H), 7.42 – 7.33 (m, 1H), 7.33 – 7.24 (m, 1H), 7.24 – 7.11 (m, 2H), 6.92 (dd, J = 15.1, 4.5 Hz, 1H), 6.62 – 6.42 (m, 2H), 6.37 (dd, J = 8.8, 2.3 Hz, 1H), 6.11 (dd, J = 15.3, 2.3 Hz, 1H), 5.02 – 4.91 (m, 1H), 4.86 (d, J = 6.4 Hz, 1H), 4.76 (dt, J = 7.8, 5.8 Hz, 1H), 2.91 (s, 3H), 1.97 – 1.74 (m, 2H), 1.37 (d, J = 7.1 Hz, 3H), 1.03 (t, J = 7.4 Hz, 3H). Example 27: 4-(((*S)-1-(2-Chlorophenyl)propyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide.
via separation of 4-((1-(2-chlorophenyl)propyl)amino)-2-fluoro- N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide (Example 25) by SFC (Stationary phase: AD-H (3x25 cm); Mobile phase: 40% MeOH/CO
2; Rt = 4.37 min). MS (ESI): mass calcd. for C
21H
24ClF
2N
2O
3S, 438.1; m/z found, 439.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.78 (t, J = 8.9 Hz, 1H), 7.42 – 7.34 (m, 1H), 7.34 – 7.23 (m, 1H), 7.26 – 7.11 (m, 2H), 6.91 (dd, J = 15.1, 4.5 Hz, 1H), 6.63 – 6.30 (m, 3H), 6.10 (dd, J = 15.3, 2.2 Hz, 1H), 5.04 – 4.92 (m, 1H), 4.87 (d, J = 6.4 Hz, 1H), 4.75 (dt, J = 7.7, 5.8 Hz, 1H), 2.90 (s, 3H), 1.98 – 1.70 (m, 2H), 1.38 (d, J = 7.1 Hz, 3H), 1.02 (t, J = 7.4 Hz, 3H). 133 QB\184200.00081\93036187.2
VVID-747PC Example 28: 4-((1-(2-Chloro-4-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide. O N
(R) S prepared in a manner analogous to Example 1 using 1-(2-chloro-4-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4- bromo-2-fluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.78 (t, J = 8.9 Hz, 1H), 7.31 (ddd, J = 8.8, 6.0, 0.9 Hz, 1H), 7.13 (ddd, J = 8.4, 2.6, 1.7 Hz, 1H), 6.97 – 6.81 (m, 2H), 6.62 – 6.40 (m, 2H), 6.32 (dt, J = 8.8, 2.6 Hz, 1H), 6.03 (ddd, J = 15.2, 3.3, 2.3 Hz, 1H), 5.02 – 4.92 (m, 1H), 4.91 – 4.75 (m, 2H), 2.90 (d, J = 2.8 Hz, 3H), 1.51 (d, J = 6.2 Hz, 3H), 1.37 (dd, J = 7.1, 3.3 Hz, 3H). Example 29: 4-(((*S)-1-(2-Chloro-4-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide.
prepared via separation of 4-((1-(2-chloro-4-fluorophenyl)ethyl)amino)- 2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide (Example 28) by SFC (Stationary phase: AD-H (3x25 cm); Mobile phase: 40% MeOH/CO
2; Rt = 3.09 min (first eluting product)). MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.80 (t, J = 8.9 Hz, 1H), 7.31 (dd, J = 8.7, 6.0 Hz, 1H), 7.14 (dd, J = 8.4, 2.6 Hz, 1H), 6.99 – 6.80 (m, 2H), 6.61 – 6.41 (m, 2H), 6.32 (dd, J = 8.8, 2.3 Hz, 1H), 6.04 (dd, J = 15.2, 2.2 Hz, 1H), 5.05 – 4.92 (m, 1H), 4.88 (p, J = 6.4 Hz, 1H), 4.74 (d, J = 5.6 Hz, 1H), 2.91 (s, 3H), 1.52 (d, J = 6.6 Hz, 3H), 1.37 (d, J = 7.1 Hz, 3H). 134 QB\184200.00081\93036187.2
VVID-747PC Example 30: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3- (trifluoromethyl)picolinamide. prepared in a manner analogous to Example 1 using methyl 5-bromo-3-
of methyl 4-bromobenzoate and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
20H
21ClF
3N
3O
3S, 475.1; m/z found, 476.0 [M+H]
+.
1H NMR (499 MHz, CDCl
3) δ 7.85 (d, J = 2.7 Hz, 1H), 7.72 (d, J = 8.4 Hz, 1H), 7.44 – 7.39 (m, 1H), 7.36 – 7.31 (m, 1H), 7.25 – 7.20 (m, 2H), 7.15 (d, J = 2.7 Hz, 1H), 6.90 (dd, J = 15.1, 4.5 Hz, 1H), 6.48 (dd, J = 15.1, 1.8 Hz, 1H), 5.04 (q, J = 6.7 Hz, 1H), 4.97 – 4.83 (m, 2H), 2.91 (s, 3H), 1.61 (d, J = 6.7 Hz, 3H), 1.39 (d, J = 7.1 Hz, 3H). Example 31: N-((R,E)-4-(Methylsulfonyl)but-3-en-2-yl)-5-(((S)-1-(2- (trifluoromethyl)phenyl)ethyl)amino)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 1 using (S)-1-(2- (trifluoromethyl)phenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5- bromopyrimidine-2-carboxylate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
19H
21F
3N
4O
3S, 442.1; m/z found, 443.2 [M+H]
+.
1H NMR (499 MHz, CDCl
3) δ 8.11 (s, 2H), 7.75 – 7.68 (m, 2H), 7.61 (d, J = 7.9 Hz, 1H), 7.52 (dd, J = 9.4, 6.1 Hz, 1H), 7.39 (t, J = 7.6 Hz, 1H), 6.95 – 6.85 (m, 1H), 6.49 (dd, J = 15.1, 1.7 Hz, 1H), 5.25 – 4.85 (m, 3H), 2.92 (s, 3H), 1.63 (d, J = 6.5 Hz, 3H), 1.41 (d, J = 7.1 Hz, 3H). 135 QB\184200.00081\93036187.2
VVID-747PC Example 32: 4-((1-(2-Chlorophenyl)-2-hydroxyethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide. in a manner analogous to Example 1 using 2-amino-2-(2-
(S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4-bromo-2- fluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
22ClFN
2O
4S, 440.1; m/z found, 441.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.81 (t, J = 8.9 Hz, 1H), 7.49 – 7.41 (m, 1H), 7.37 (dd, J = 7.1, 2.4 Hz, 1H), 7.25 (ddt, J = 7.7, 5.0, 1.9 Hz, 2H), 6.93 (ddd, J = 15.2, 4.6, 2.3 Hz, 1H), 6.61 – 6.43 (m, 2H), 6.42 – 6.35 (m, 1H), 6.11 (ddd, J = 15.2, 6.5, 2.3 Hz, 1H), 5.04 – 4.94 (m, 2H), 4.08 (dd, J = 11.3, 3.6 Hz, 1H), 3.85 (ddd, J = 11.4, 5.8, 1.3 Hz, 1H), 3.51 (s, 2H), 2.94 (d, J = 2.8 Hz, 3H), 1.41 (dd, J = 7.1, 3.6 Hz, 3H). Example 33: 4-(((R)-1-(2-Chlorophenyl)-2-hydroxyethyl)amino)-2-fluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide.
in a manner analogous to Example 1 using (R)-2-((tert- butyldimethylsilyl)oxy)-1-(2-chlorophenyl)ethan-1-amine (Intermediate 8) instead of (S)-1-(2- chlorophenyl)ethan-1-amine, methyl 4-bromo-2-fluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
20H
22ClFN
2O
4S, 440.1; m/z found, 441.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 7.89 (dd, J = 7.9, 3.4 Hz, 1H), 7.41 - 7.52 (m, 1H), 7.34 - 7.41 (m, 2H), 7.25 - 7.31 (m, 2H), 7.13 (br d, J = 6.8 Hz, 1H), 6.73 - 6.80 (m, 1H), 6.63 - 6.70 (m, 1H), 6.34 (dd, J = 8.6, 1.8 Hz, 1H), 6.17 (br d, J = 13.0 Hz, 1H), 5.10 - 5.29 (m, 1H), 4.69 - 4.85 (m, 2H), 3.63 - 3.73 (m, 1H), 3.51 - 3.60 (m, 1H), 2.99 (s, 3H), 1.24 (d, J = 7.0 Hz, 3H). Example 34: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. 136 QB\184200.00081\93036187.2
VVID-747PC was prepared in a manner analogous to Example 1 using methyl 5-
instead of methyl 4-bromobenzoate and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
18H
21ClN
4O
3S, 408.1; m/z found, 409.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.01 (s, 2H), 7.79 (d, J = 8.3 Hz, 1H), 7.41 – 7.30 (m, 2H), 7.22 – 7.10 (m, 2H), 6.90 (dd, J = 15.1, 4.5 Hz, 1H), 6.48 (dd, J = 15.1, 1.7 Hz, 1H), 5.32 (s, 1H), 5.07 – 4.85 (m, 2H), 2.90 (s, 3H), 1.59 (s, 3H), 1.39 (d, J = 7.1 Hz, 3H). Example 35: 5-((1-(2,3-Difluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
was prepared in a manner analogous to Example 1 using 1-(2,3- difluorophenyl)ethan-1-amine hydrochloride instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5- bromopyrimidine-2-carboxylate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
18H
20F
2N
4O
3S, 410.1; m/z found, 411.0 [M+H]
+.
1H NMR (499 MHz, CDCl
3) δ 8.15 (s, 2H), 7.76 (d, J = 8.3 Hz, 1H), 7.16 – 6.98 (m, 3H), 6.91 (ddd, J = 15.1, 4.5, 2.8 Hz, 1H), 6.49 (ddd, J = 15.1, 5.5, 1.7 Hz, 1H), 5.01 – 4.87 (m, 2H), 2.92 (d, J = 2.3 Hz, 3H), 1.67 (d, J = 6.7 Hz, 3H), 1.43 (dd, J = 7.1, 2.8 Hz, 3H). 137 QB\184200.00081\93036187.2
VVID-747PC Example 36: 5-(((*S)-1-(2,3-Difluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. prepared via separation of 5-((1-(2,3-difluorophenyl)ethyl)amino)-N-
- en-2-yl)pyrimidine-2-carboxamide (Example 35) by SFC (Stationary phase: C9-5 (2x25 cm); Mobile phase: 40% MeOH/CO
2; Rt = 2.54 min). MS (ESI): mass calcd. for C
18H
20F
2N
4O
3S, 410.1; m/z found, 411.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.13 (d, J = 3.3 Hz, 2H), 7.79 (s, 1H), 7.20 – 7.01 (m, 3H), 6.93 (dd, J = 15.1, 4.4 Hz, 1H), 6.50 (dd, J = 15.1, 1.6 Hz, 1H), 5.19 – 4.73 (m, 3H), 2.93 (s, 3H), 1.70 (s, 3H), 1.45 (d, J = 7.0 Hz, 3H). Example 37: 5-(((*R)-1-(2,3-Difluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
prepared via separation of 5-((1-(2,3-difluorophenyl)ethyl)amino)-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 35) by SFC (Stationary phase: C9-5 (2x25 cm); Mobile phase: 40% MeOH/CO
2; Rt = 4.76 min). MS (ESI): mass calcd. for C
18H
20F
2N
4O
3S, 410.1; m/z found, 411.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.09 (d, J = 5.9 Hz, 2H), 7.76 (s, 1H), 7.16 – 6.98 (m, 3H), 6.91 (dd, J = 15.1, 4.4 Hz, 1H), 6.53 – 6.41 (m, 1H), 5.05 – 4.85 (m, 2H), 4.76 (s, 1H), 2.91 (s, 3H), 1.67 (s, 3H), 1.42 (d, J = 7.1 Hz, 3H). 138 QB\184200.00081\93036187.2
VVID-747PC Example 38: 4-((1-(2-Chloro-4-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide. O F N
(R) S prepared in a manner analogous to Example 1 using 1-(2-chloro-4-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4- bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
20ClF
3N
2O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.70 – 7.60 (m, 1H), 7.33 (dd, J = 8.7, 6.0 Hz, 1H), 7.17 (dt, J = 8.3, 2.3 Hz, 1H), 7.02 – 6.83 (m, 2H), 6.61 – 6.39 (m, 2H), 5.94 (ddd, J = 13.9, 6.8, 1.1 Hz, 1H), 5.05 – 4.78 (m, 3H), 2.92 (d, J = 10.4 Hz, 3H), 1.57 (s, 3H), 1.39 (dd, J = 9.0, 7.1 Hz, 3H). Example 39: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2-methoxy-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide.
prepared in a manner analogous to Example 1 using methyl 4-bromo-2- methoxybenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
21H
25ClN
2O
4S, 436.1; m/z found, 437.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.90 (d, J = 8.6 Hz, 1H), 7.69 (d, J = 7.7 Hz, 1H), 7.41 – 7.34 (m, 2H), 7.24 – 7.14 (m, 2H), 6.93 (dd, J = 15.1, 4.3 Hz, 1H), 6.51 – 6.40 (m, 1H), 6.23 – 6.15 (m, 1H), 5.93 (d, J = 2.2 Hz, 1H), 5.07 – 4.93 (m, 2H), 3.77 (s, 3H), 2.91 (s, 3H), 1.53 (s, 3H), 1.35 (d, J = 7.2 Hz, 3H). 139 QB\184200.00081\93036187.2
VVID-747PC Example 40: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2,6-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide. prepared in a manner analogous to Example 1 using methyl 4-bromo-2,6-
methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.41 – 7.34 (m, 1H), 7.33 – 7.27 (m, 1H), 7.20 (dt, J = 5.6, 3.5 Hz, 2H), 6.88 (dd, J = 15.1, 4.4 Hz, 1H), 6.50 (dd, J = 15.1, 1.8 Hz, 1H), 6.17 (dt, J = 8.0, 2.5 Hz, 1H), 5.97 (d, J = 11.6 Hz, 2H), 5.00 – 4.80 (m, 3H), 2.89 (s, 3H), 1.51 (s, 3H), 1.36 (d, J = 7.2 Hz, 3H). Example 41: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N
1-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)isophthalamide.
in a manner analogous to Example 1 using methyl 4-bromo-3- cyanobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
21H
24ClN
3O
4S, 449.1; m/z found, 450.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.90 (d, J = 6.1 Hz, 1H), 8.08 (d, J = 2.2 Hz, 1H), 7.51 (dd, J = 8.8, 2.1 Hz, 1H), 7.41 – 7.28 (m, 2H), 7.16 (dd, J = 5.9, 3.4 Hz, 2H), 6.86 (dd, J = 15.1, 4.7 Hz, 1H), 6.52 – 6.37 (m, 2H), 6.29 (d, J = 9.0 Hz, 1H), 5.00 (p, J = 6.6 Hz, 1H), 4.95 – 4.83 (m, 1H), 2.90 (s, 3H), 1.56 (d, J = 6.6 Hz, 3H), 1.34 (d, J = 7.1 Hz, 3H). 140 QB\184200.00081\93036187.2
VVID-747PC Example 42: 2-Fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4-(((S)-1-(o- tolyl)ethyl)amino)benzamide. prepared in a manner analogous to Example 1 using (S)-1-(o-tolyl)ethan-
of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4-bromo-2- fluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
21H
25FN
2O
3S, 404.2; m/z found, 405.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.79 (t, J = 9.0 Hz, 1H), 7.34 – 7.27 (m, 1H), 7.22 – 7.10 (m, 3H), 6.96 – 6.87 (m, 1H), 6.57 – 6.40 (m, 2H), 6.33 (dd, J = 8.7, 2.3 Hz, 1H), 6.04 (dd, J = 15.4, 2.2 Hz, 1H), 5.03 – 4.93 (m, 1H), 4.76 – 4.53 (m, 2H), 2.91 (s, 3H), 2.43 (s, 3H), 1.51 (d, J = 6.5 Hz, 3H), 1.37 (d, J = 7.1 Hz, 3H). Example 43: 2-Fluoro-4-(((S)-1-(2-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide.
was prepared in a manner analogous to Example 1 using (S)-1-(2- fluorophenyl)ethan-1-amine hydrochloride instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4- bromo-2-fluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
22F
2N
2O
3S, 408.1; m/z found, 409.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.81 (t, J = 8.9 Hz, 1H), 7.34 – 7.19 (m, 2H), 7.15 – 7.02 (m, 2H), 6.92 (dd, J = 15.1, 4.5 Hz, 1H), 6.60 – 6.34 (m, 3H), 6.14 (dd, J = 15.3, 2.3 Hz, 1H), 5.07 – 4.89 (m, 1H), 4.83 (p, J = 6.7 Hz, 1H), 4.69 – 4.50 (m, 1H), 2.92 (s, 3H), 1.59 (s, 3H), 1.38 (d, J = 7.1 Hz, 3H). 141 QB\184200.00081\93036187.2
VVID-747PC Example 44: 4-((1-(2-Chlorophenyl)-2,2,2-trifluoroethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. in a manner analogous to Example 1 using 1-(2-chlorophenyl)-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4- bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
18ClF
5N
2O
3S, 496.1; m/z found, 497.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.68 (dd, J = 11.9, 6.9 Hz, 1H), 7.57 – 7.43 (m, 2H), 7.40 – 7.29 (m, 2H), 6.89 (ddd, J = 15.1, 7.5, 4.7 Hz, 1H), 6.60 (dd, J = 13.9, 7.7 Hz, 1H), 6.53 – 6.29 (m, 2H), 5.68 – 5.50 (m, 1H), 5.19 – 5.07 (m, 1H), 5.01 – 4.88 (m, 1H), 2.91 (d, J = 10.3 Hz, 3H), 1.44 – 1.29 (m, 3H). Example 45: 4-(((*R)-1-(2-Chlorophenyl)-2,2,2-trifluoroethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide.
was prepared via separation of 4-((1-(2-chlorophenyl)-2,2,2- trifluoroethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide (Example 44) by SFC (Stationary phase: A6-5 (2x25 cm); Mobile phase: 35% MeOH/CO
2; Rt = 2.41 min). MS (ESI): mass calcd. for C
20H
18ClF
5N
2O
3S, 496.1; m/z found, 497.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.69 (dd, J = 11.9, 6.9 Hz, 1H), 7.57 – 7.42 (m, 2H), 7.36 (ddd, J = 6.2, 3.2, 2.0 Hz, 2H), 6.89 (dd, J = 15.2, 4.6 Hz, 1H), 6.59 (dd, J = 14.1, 7.7 Hz, 1H), 6.49 – 6.28 (m, 2H), 5.61 (p, J = 7.0 Hz, 1H), 5.19 – 5.04 (m, 1H), 5.03 – 4.89 (m, 1H), 2.90 (s, 3H), 1.40 (d, J = 7.1 Hz, 3H). 142 QB\184200.00081\93036187.2
VVID-747PC Example 46: 4-(((*S)-1-(2-Chlorophenyl)-2,2,2-trifluoroethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. was prepared via separation of 4-((1-(2-chlorophenyl)-2,2,2-
- N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide (Example 44) by SFC (Stationary phase: A6-5 (2x25 cm); Mobile phase: 35% MeOH/CO
2; Rt = 2.91 min). MS (ESI): mass calcd. for C
20H
18ClF
5N
2O
3S, 496.1; m/z found, 497.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.71 (dd, J = 11.9, 6.9 Hz, 1H), 7.58 – 7.43 (m, 2H), 7.42 – 7.30 (m, 2H), 6.92 (dd, J = 15.1, 4.7 Hz, 1H), 6.60 (dd, J = 14.1, 7.7 Hz, 1H), 6.49 (dd, J = 15.2, 1.7 Hz, 1H), 6.38 (dd, J = 13.4, 6.6 Hz, 1H), 5.73 – 5.51 (m, 1H), 5.20 – 5.05 (m, 1H), 5.03 – 4.90 (m, 1H), 2.94 (s, 3H), 1.40 (d, J = 7.1 Hz, 3H). Example 47: 4-((1-(2-Chlorophenyl)propyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide.
prepared in a manner analogous to Example 1 using 1-(2- chlorophenyl)propan-1-amine hydrochloride instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4-bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
21H
23ClF
2N
2O
3S, 456.1; m/z found, 457.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.64 (dd, J = 12.3, 7.0 Hz, 1H), 7.46 – 7.35 (m, 1H), 7.32 – 7.18 (m, 3H), 6.90 (ddd, J = 15.2, 9.4, 4.6 Hz, 1H), 6.63 – 6.36 (m, 2H), 6.04 (ddd, J = 14.1, 6.8, 2.7 Hz, 1H), 5.04 – 4.83 (m, 2H), 4.81 – 4.68 (m, 1H), 2.91 (d, J = 12.1 Hz, 3H), 2.03 – 1.79 (m, 2H), 1.38 (dd, J = 10.4, 7.1 Hz, 3H), 1.05 (t, J = 7.4 Hz, 3H). 143 QB\184200.00081\93036187.2
VVID-747PC Example 48: 4-(((*S)-1-(2-Chlorophenyl)propyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide. prepared via separation of 4-((1-(2-chlorophenyl)propyl)amino)-2,5-
- but-3-en-2-yl)benzamide (Example 47) by SFC (Stationary phase: A6-5 (2x25 cm); Mobile phase: 35% MeOH/CO
2; Rt = 3.51 min). MS (ESI): mass calcd. for C
21H
23ClF
2N
2O
3S, 456.1; m/z found, 457.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.64 (dd, J = 12.2, 7.0 Hz, 1H), 7.48 – 7.35 (m, 1H), 7.34 – 7.14 (m, 3H), 6.89 (dd, J = 15.1, 4.6 Hz, 1H), 6.55 (dd, J = 14.7, 7.7 Hz, 1H), 6.42 (dd, J = 15.2, 1.7 Hz, 1H), 6.04 (dd, J = 14.1, 6.7 Hz, 1H), 5.06 – 4.82 (m, 2H), 4.83 – 4.67 (m, 1H), 2.90 (s, 3H), 2.05 – 1.79 (m, 2H), 1.39 (d, J = 7.1 Hz, 3H), 1.05 (t, J = 7.4 Hz, 3H). Example 49: 4-(((*R)-1-(2-Chlorophenyl)propyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide.
prepared via separation 4-((1-(2-chlorophenyl)propyl)amino)-2,5- difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide (Example 47) by SFC (Stationary phase: A6-5 (2x25 cm); Mobile phase: 35% MeOH/CO
2; Rt = 4.40 min). MS (ESI): mass calcd. for C
21H
23ClF
2N
2O
3S, 456.1; m/z found, 457.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.64 (dd, J = 12.2, 7.0 Hz, 1H), 7.46 – 7.35 (m, 1H), 7.34 – 7.26 (m, 1H), 7.26 – 7.17 (m, 2H), 6.91 (dd, J = 15.1, 4.6 Hz, 1H), 6.63 – 6.41 (m, 2H), 6.05 (dd, J = 14.0, 6.7 Hz, 1H), 5.04 – 4.84 (m, 2H), 4.82 – 4.67 (m, 1H), 2.93 (s, 3H), 2.04 – 1.77 (m, 2H), 1.36 (d, J = 7.1 Hz, 3H), 1.05 (t, J = 7.4 Hz, 3H). 144 QB\184200.00081\93036187.2
VVID-747PC Example 50: 4-(((S)-1-(2-Chloro-4-fluorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. prepared in a manner analogous to Example 1 using (S)-1-(2-chloro-4-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4- bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
20H
20ClF
3N
2O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.67 (dd, J = 12.2, 7.0 Hz, 1H), 7.32 (dd, J = 8.7, 5.9 Hz, 1H), 7.17 (dd, J = 8.3, 2.6 Hz, 1H), 7.02 – 6.84 (m, 2H), 6.60 – 6.39 (m, 2H), 5.94 (dd, J = 13.9, 6.7 Hz, 1H), 5.02 – 4.75 (m, 3H), 2.94 (s, 3H), 1.59 (s, 3H), 1.37 (d, J = 7.1 Hz, 3H). Example 51: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3- (trifluoromethyl)benzamide.
prepared in a manner analogous to Example 1 using methyl 4-bromo-3- (trifluoromethyl)benzoate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
21H
22ClF
3N
2O
3S, 474.1; m/z found, 475.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.94 (d, J = 2.2 Hz, 1H), 7.63 (dd, J = 8.8, 2.2 Hz, 1H), 7.45 – 7.35 (m, 1H), 7.30 (dt, J = 7.7, 3.4 Hz, 1H), 7.24 – 7.13 (m, 2H), 6.89 (dd, J = 15.2, 4.6 Hz, 1H), 6.45 (dd, J = 15.1, 1.7 Hz, 1H), 6.36 (d, J = 8.8 Hz, 1H), 6.15 (d, J = 7.8 Hz, 1H), 5.16 – 5.00 (m, 2H), 5.01 – 4.89 (m, 1H), 2.91 (s, 3H), 1.59 (d, J = 6.5 Hz, 3H), 1.39 (d, J = 7.1 Hz, 3H). 145 QB\184200.00081\93036187.2
VVID-747PC Example 52: 5-((1-(2-Chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 1 using 1-(2-chloro-3-
of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-bromopyrimidine- 2-carboxylate instead of methyl 4-bromobenzoate, and Pd
2(dba)
3/XantPhos instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
18H
20ClFN
4O
3S, 426.1; m/z found, 427.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d6) δ 8.60 - 8.72 (m, 1H), 7.99 (s, 2H), 7.52 (d, J = 6.8 Hz, 1H), 7.23 - 7.44 (m, 3H), 6.61 - 6.84 (m, 2H), 4.90 - 5.01 (m, 1H) 4.63 - 4.82 (m, 1H), 2.97 (d, J = 3.9 Hz, 3H), 1.50 (d, J = 6.6 Hz, 3H), 1.27 (dd, J =7.0, 2.6 Hz, 3H). Example 53: 5-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
prepared via separation 5-((1-(2-chloro-3-fluorophenyl)ethyl)amino)-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 52) by SFC (Stationary phase: IH (3x25 cm); Mobile phase: 45% iPrOH/CO
2; Rt = 7.98 min (second eluting product)). MS (ESI): mass calcd. for C
18H
20ClFN
4O
3S, 426.1; m/z found, 427.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.56 - 8.75 (m, 1H), 7.92 - 8.01 (m, 2H), 7.47 - 7.57 (m, 1H), 7.20 - 7.39 (m, 3H), 6.61 - 6.85 (m, 2H), 4.91 - 5.05 (m, 1H), 4.69 - 4.82 (m, 1H), 2.90 - 3.02 (m, 3H), 1.51 (d, J = 6.8 Hz, 3H), 1.28 (d, J = 6.9 Hz, 3H). 146 QB\184200.00081\93036187.2
VVID-747PC Example 54: 4-(((*S)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. prepared in a manner analogous to Example 1 using (*S)-(2-
(Intermediate 4) instead of (S)-1-(2-chlorophenyl)ethan-1- amine, methyl 4-bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
22H
23ClF
2N
2O
3S, 468.1; m/z found, 469.2 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.04 (dd, J = 2.9, 7.9 Hz, 1H), 7.67 (dd, J = 1.6, 7.7 Hz, 1H), 7.45 (dd, J = 1.1, 7.9 Hz, 1H), 7.24 - 7.40 (m, 3H), 7.10 (br d, J = 7.5 Hz, 1H), 6.65 - 6.82 (m, 2H), 6.11 (dd, J = 7.0, 13.1 Hz, 1H), 4.69 - 4.79 (m, 1H), 4.21 (t, J = 8.3 Hz, 1H), 3.00 (s, 3H), 1.37 - 1.52 (m, 1H), 1.24 (d, J = 7.1 Hz, 3H), 0.59 - 0.70 (m, 1H), 0.40 - 0.52 (m, 3H). Example 55: 4-(((*R)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-2,5-difluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide.
prepared in a manner analogous to Example 1 using (*R)-(2- chlorophenyl)(cyclopropyl)methanamine (Intermediate 5) instead of (S)-1-(2-chlorophenyl)ethan-1- amine, methyl 4-bromo-2,5-difluorobenzoate instead of methyl 4-bromobenzoate, and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
22H
23ClF
2N
2O
3S, 468.1; m/z found, 469.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 7.99 (dd, J = 2.8, 8.0 Hz, 1H), 7.62 - 7.70 (m, 1H), 7.44 (d, J = 7.6 Hz, 1H), 7.22 - 7.37 (m, 3H), 7.12 (br d, J = 7.6 Hz, 1H), 6.63 - 6.77 (m, 2H), 6.10 (dd, J = 6.8, 13.2 Hz, 1H), 4.64 - 4.79 (m, 1H), 4.19 (t, J = 8.4 Hz, 1H), 2.98 (s, 3H), 1.35 - 1.48 (m, 1H), 1.25 (d, J = 7.2 Hz, 3H), 0.62 (br s, 1H), 0.39 - 0.51 (m, 3H). Example 56: 4-((1-(2-Chlorophenyl)ethyl)amino)-1-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)- 1H-indazole-7-carboxamide. 147 QB\184200.00081\93036187.2
VVID-747PC was prepared in a manner analogous to Example 1 using 1-(2-
of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4-bromo-1- methyl-1H-indazole-7-carboxylate (Intermediate 16) instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
22H
25ClN
4O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.03 (s, 1H), 7.41 – 7.33 (m, 2H), 7.22 (dd, J = 7.9, 1.1 Hz, 1H), 7.19 – 7.11 (m, 2H), 6.91 (ddd, J = 15.1, 4.8, 4.1 Hz, 1H), 6.51 (ddd, J = 15.2, 13.5, 1.7 Hz, 1H), 6.01 (dd, J = 7.8, 5.1 Hz, 1H), 5.77 (dd, J = 8.1, 1.1 Hz, 1H), 5.12 (q, J = 6.8 Hz, 1H), 4.99 – 4.87 (m, 2H), 4.09 (d, J = 1.0 Hz, 3H), 2.90 (d, J = 8.7 Hz, 3H), 1.63 (d, J = 6.6 Hz, 3H), 1.40 (t, J = 6.9 Hz, 3H). Example 57: 8-((1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)imidazo[1,2-a]pyridine-5-carboxamide. O N N
was prepared in a manner analogous to Example 1 using 1-(2- chlorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 8- bromoimidazo[1,2-a]pyridine-5-carboxylate instead of methyl 4-bromobenzoate in Step A. MS (ESI): mass calcd. for C
21H
23ClN
4O
3S, 446.1; m/z found, 447.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.73 (dd, J = 2.3, 1.6 Hz, 1H), 7.61 (dd, J = 2.7, 1.6 Hz, 1H), 7.50 (s, 1H), 7.46 – 7.40 (m, 1H), 7.40 – 7.34 (m, 1H), 7.24 – 7.13 (m, 3H), 6.90 (ddd, J = 15.1, 11.9, 4.9 Hz, 1H), 6.56 – 6.36 (m, 2H), 5.91 (dd, J = 8.3, 1.6 Hz, 1H), 5.16 – 5.07 (m, 1H), 4.99 – 4.89 (m, 1H), 2.90 (d, J = 17.7 Hz, 3H), 1.67 (d, J = 6.7 Hz, 3H), 1.41 (dd, J = 12.2, 7.1 Hz, 3H). 148 QB\184200.00081\93036187.2
VVID-747PC Example 58: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3- (oxazol-2-yl)benzamide. O O N N (R) S 3- of 1H J = J = J = 2-
(2- Chlorophenyl)ethan-1-amine hydrochloride (80 mg, 0.420 mmol, 1.0 eq) and methyl 5-chloropyrazine-2- carboxylate (77 mg, 0.440 mmol, 1.05 eq) were taken up in 1,4-dioxane (2.0 mL, 0.2 M). To this was added N,N-diisopropylethylamine (0.15 mL, 0.880 mmol, 2.1 eq) and the reaction was heated to 100°C for 16 h. After cooling to rt, the reaction was quenched with water and extracted with EtOAc. The combined organic layers were dried over Na
2SO
4, filtered, and concentrated under reduced pressure. Purification by FCC on silica (0-75% EtOAc in heptanes) provided methyl (S)-5-((1-(2- chlorophenyl)ethyl)amino)pyrazine-2-carboxylate (117 mg, 96% yield). MS (ESI): mass calcd. for C
14H
14ClN
3O
2, 291.1; m/z found, 292.0 [M+H]
+. [00371] Step B: (S)-5-((1-(2-Chlorophenyl)ethyl)amino)pyrazine-2-carboxylic acid. Methyl (S)-5-((1-(2- chlorophenyl)ethyl)amino)pyrazine-2-carboxylate (117 mg, 0.400 mmol, 1.0 eq) was taken up in methanol (2.0 mL, 0.2 M). To this was added lithium hydroxide (2N in water, 1.6 mL, 3.20 mmol, 8.0 eq) and the reaction was stirred at 60°C for 3 hours. After cooling to rt, the reaction was adjusted to pH ∼2 with 1N HCl. The precipitated product was collected by filtration to provide (S)-5-((1-(2- 149 QB\184200.00081\93036187.2
VVID-747PC chlorophenyl)ethyl)amino)pyrazine-2-carboxylic acid (41 mg, 37% yield) as a white solid. MS (ESI): mass calcd. for C
13H
12ClN
3O
2, 277.1; m/z found, 278.0 [M+H]
+. [00372] Step C: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide. (S)-5-((1-(2-Chlorophenyl)ethyl)amino)pyrazine-2-carboxylic acid (39 mg, 0.140 mmol, 1.0 eq) and HATU (64 mg, 0.170 mmol, 1.2 eq) were taken up in DMF (0.7 mL, 0.2 M). N,N- Diisopropylethylamine (73 µL, 0.420 mmol, 3.0 eq) was added and the reaction was allowed to stir for 5 minutes. (R,E)-4-(Methylsulfonyl)but-3-en-2-amine, 4-methylbenzenesulfonic acid (Intermediate 1, 45 mg, 0.140 mmol, 1.0 eq) was added and the reaction was stirred for 30 min at rt. The crude mixture was filtered through a PTFE filter and purified by RP-HPLC (20-95% ACN in 0.1% aq. HCOOH) to provide 5- (((S)-1-(2-chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (48 mg, 84% yield). MS (ESI): mass calcd. for C
18H
21ClN
4O
3S, 408.1; m/z found, 409.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.77 (d, J = 1.4 Hz, 1H), 7.59 (s, 1H), 7.49 (d, J = 8.4 Hz, 1H), 7.45 – 7.31 (m, 2H), 7.26 – 7.14 (m, 2H), 6.93 (dd, J = 15.1, 4.5 Hz, 1H), 6.47 (dd, J = 15.1, 1.7 Hz, 1H), 5.78 (d, J = 6.6 Hz, 1H), 5.34 (p, J = 6.6 Hz, 1H), 5.05 – 4.83 (m, 1H), 2.92 (s, 3H), 1.62 (d, J = 6.8 Hz, 3H), 1.40 (d, J = 7.1 Hz, 3H). Example 60: 5-(((S)-1-(2-Chloro-4-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide.
prepared in a manner analogous to Example 59 using (S)-1-(2-chloro-4- fluorophenyl)ethan-1-amine hydrochloride instead of (S)-1-(2-chlorophenyl)ethan-1-amine in Step A. MS (ESI): mass calcd. for C
18H
20ClFN
4O
3S, 426.1; m/z found, 427.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.77 (d, J = 1.4 Hz, 1H), 7.58 (d, J = 1.4 Hz, 1H), 7.47 (d, J = 8.4 Hz, 1H), 7.35 (dd, J = 8.7, 5.9 Hz, 1H), 7.15 (dd, J = 8.4, 2.6 Hz, 1H), 7.02 – 6.85 (m, 2H), 6.46 (dd, J = 15.1, 1.8 Hz, 1H), 5.57 (d, J = 6.5 Hz, 1H), 5.30 (p, J = 6.7 Hz, 1H), 5.01 – 4.86 (m, 1H), 2.92 (s, 3H), 1.61 (s, 3H), 1.41 (d, J = 7.1 Hz, 3H). 150 QB\184200.00081\93036187.2
VVID-747PC Example 61: 5-((*S)-1-(2-Chloro-3,6-difluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 59 using 1-(2-chloro-3,6-
of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-fluoropyrimidine-2- carboxylate instead of methyl 5-chloropyrazine-2-carboxylate, and NaH instead of DIPEA in Step A. Separation of 5-(1-(2-chloro-3,6-difluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 8.24 min (second eluting product)) provided the title compound. MS (ESI): mass calcd. for C
18H
18ClF
2N
3O
4S, 445.1; m/z found, 446.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.97 (d, J = 8.4 Hz, 1H), 8.49 - 8.62 (m, 2H), 7.51 (td, J = 8.8, 4.6 Hz, 1H), 7.35 (td, J = 9.8, 4.3 Hz, 1H), 6.64 - 6.87 (m, 2H), 6.23 (q, J = 6.3 Hz, 1H), 4.69 - 4.84 (m, 1H), 2.98 (s, 3H), 1.79 (d, J = 6.5 Hz, 3H), 1.30 (d, J = 7.0 Hz, 3H). Example 62: 7-((1-(2-Chlorophenyl)ethyl)amino)-1-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)- 1H-indazole-4-carboxamide.
was prepared in a manner analogous to Example 59 using 1-(2- chlorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 7-fluoro-1-methyl- 1H-indazole-4-carboxylate (Intermediate 14) instead of methyl 5-chloropyrazine-2-carboxylate, and pyridine instead of DIPEA in Step A. MS (ESI): mass calcd. for C
22H
25ClN
4O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 1.8 Hz, 1H), 7.46 – 7.37 (m, 2H), 7.24 – 7.14 (m, 3H), 6.91 (ddd, J = 15.1, 5.4, 4.6 Hz, 1H), 6.46 (td, J = 15.0, 1.7 Hz, 1H), 6.06 (dd, J = 8.0, 3.8 Hz, 1H), 5.99 (t, J = 7.0 Hz, 1H), 5.11 – 4.90 (m, 3H), 4.52 (s, 3H), 2.90 (d, J = 9.0 Hz, 3H), 1.66 (d, J = 6.6 Hz, 3H), 1.40 (t, J = 7.5 Hz, 3H). 151 QB\184200.00081\93036187.2
VVID-747PC Example 63: 7-((1-(2-Chlorophenyl)ethyl)amino)-2-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)- 2H-indazole-4-carboxamide. was prepared in a manner analogous to Example 59 using 1-(2-
of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 7-fluoro-2-methyl- 2H-indazole-4-carboxylate (Intermediate 15) instead of methyl 5-chloropyrazine-2-carboxylate, and pyridine instead of DIPEA in Step A. MS (ESI): mass calcd. for C
22H
25ClN
4O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.41 (s, 1H), 7.46 – 7.41 (m, 1H), 7.41 – 7.35 (m, 1H), 7.22 – 7.11 (m, 3H), 6.93 (ddd, J = 15.1, 7.8, 4.5 Hz, 1H), 6.47 (ddd, J = 17.1, 15.1, 1.7 Hz, 1H), 5.95 (dd, J = 7.7, 2.9 Hz, 1H), 5.83 (dd, J = 7.9, 3.2 Hz, 1H), 5.76 (s, 1H), 5.17 – 5.12 (m, 1H), 4.97 (s, 1H), 4.23 (s, 3H), 2.90 (d, J = 11.1 Hz, 3H), 1.65 (d, J = 6.7 Hz, 3H), 1.39 (dd, J = 10.1, 7.1 Hz, 3H). Example 64: 5-(((*R)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide.
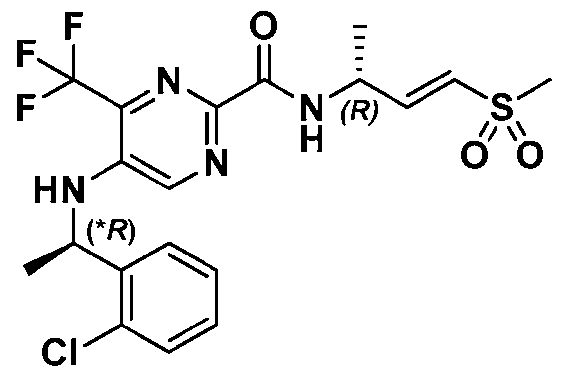
was prepared in a manner analogous to Example 59 using 1-(2- chlorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-chloro-4- (trifluoromethyl)pyrimidine-2-carboxylate (Intermediate 17) instead of methyl 5-chloropyrazine-2- carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-((1-(2- chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4-(trifluoromethyl)pyrimidine-2- carboxamide (Step C) via SFC (Stationary phase: AD (3x25 cm); Mobile phase: 15% MeOH/CO
2; Rt = 5.00 min) provided the title compound. MS (ESI): mass calcd. for C
19H
20ClF
3N
4O
3S, 476.1; m/z found, 477.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.79 (d, J = 8.5 Hz, 1H), 8.07 (s, 1H), 7.50 (ddd, J = 7.4, 5.5, 1.8 Hz, 2H), 7.25 - 7.39 (m, 2H), 6.99 (d, J = 6.8 Hz, 1H), 6.64 - 6.83 (m, 2H), 5.19 (t, J = 6.8 Hz, 1H), 4.70 - 4.83 (m, 1H), 2.98 (s, 3H), 1.60 (d, J = 6.6 Hz, 3H), 1.28 (d, J = 7.0 Hz, 3H). 152 QB\184200.00081\93036187.2
VVID-747PC Example 65: 5-(((*S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide. was prepared in a manner analogous to Example 59 using 1-(2-
of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-chloro-4- (trifluoromethyl)pyrimidine-2-carboxylate (Intermediate 17) instead of methyl 5-chloropyrazine-2- carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-((1-(2- chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4-(trifluoromethyl)pyrimidine-2- carboxamide (Step C) via SFC (Stationary phase: AD (3x25 cm); Mobile phase: 15% MeOH/CO
2; Rt = 6.60 min) provided the title compound. MS (ESI): mass calcd. for C
19H
20ClF
3N
4O
3S, 476.1; m/z found, 477.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.81 (d, J = 8.4 Hz, 1H), 8.08 (s, 1H), 7.51 (td, J = 7.4, 1.7 Hz, 2H), 7.25 - 7.41 (m, 2H), 6.99 (d, J = 6.8 Hz, 1H), 6.64 - 6.80 (m, 2H), 5.20 (quin, J = 6.6 Hz, 1H), 4.69 - 4.82 (m, 1H), 2.95 (s, 3H), 1.60 (d, J = 6.8 Hz, 3H), 1.30 (d, J = 7.0 Hz, 3H). Example 66: 5-((*S)-1-(2-Chloro-6-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 59 using 1-(2-chloro-6- fluorophenyl)ethan-1-ol instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-fluoropyrimidine-2- carboxylate instead of methyl 5-chloropyrazine-2-carboxylate, and NaH instead of DIPEA in Step A. Separation of 5-(1-(2-chloro-6-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 9.15 min (second eluting product)) provided the title compound. MS (ESI): mass calcd. for C
18H
19ClFN
3O
4S, 427.1; m/z found, 428.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.95 (d, J = 8.5 Hz, 1H), 8.53 (s, 2H), 7.34 - 7.49 (m, 2H), 7.25 (ddd, J = 1.4, 8.0, 10.9 Hz, 1H), 6.66 - 6.84 (m, 2H), 6.21 (q, J = 6.5 Hz, 1H), 4.72 - 4.84 (m, 1H), 2.98 (s, 3H), 1.78 (d, J = 6.6 Hz, 3H), 1.30 (d, J = 7.0 Hz, 3H). 153 QB\184200.00081\93036187.2
VVID-747PC Example 67: 5-(((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide. in a manner analogous to Example 59 using (*R)-(2-chloro-3-
(Intermediate 7) instead of (S)-1-(2-chlorophenyl)ethan-1-amine and DMF instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C
20H
22ClFN
4O
3S, 452.1; m/z found, 453.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.51 (d, J = 8.5 Hz, 1H), 8.35 - 8.46 (m, 2H), 8.00 (s, 1H), 7.24 - 7.46 (m, 3H), 6.74 - 6.89 (m, 1H), 6.63 - 6.73 (m, 1H), 5.02 (br t, J = 7.7 Hz, 1H), 4.71 - 4.86 (m, 1H), 2.99 (s, 3H), 1.25 - 1.39 (m, 4H), 0.44 - 0.66 (m, 2H), 0.38 (br d, J = 3.9 Hz, 2H). Example 68: 5-(((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide.
in a manner analogous to Example 59 using (*S)-(2-chloro-3- fluorophenyl)(cyclopropyl)methanamine (Intermediate 6) instead of (S)-1-(2-chlorophenyl)ethan-1-amine and DMF instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C20H22ClFN4O3S, 452.1; m/z found, 453.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.50 (d, J = 8.6 Hz, 1H), 8.35 - 8.44 (m, 2H), 8.01 (s, 1H), 7.26 - 7.54 (m, 3H), 6.73 - 6.88 (m, 1H), 6.58 - 6.71 (m, 1H), 5.02 (br t, J = 7.7 Hz, 1H), 4.72 - 4.85 (m, 1H), 2.97 (s, 3H), 1.21 - 1.35 (m, 4H), 0.54 - 0.64 (m, 1H), 0.46 - 0.53 (m, 1H), 0.35 - 0.45 (m, 2H). Example 69: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. 154 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 59 using methyl 5-fluoro-4-
(Intermediate 18) instead of methyl 5-chloropyrazine-2-carboxylate and DMSO instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C
19H
23ClN
4O
3S, 422.1; m/z found, 423.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.62 (d, J = 8.5 Hz, 1H), 7.42 - 7.52 (m, 3H), 7.23 - 7.35 (m, 2H), 6.73 - 6.82 (m, 1H), 6.63 - 6.70 (m, 1H), 6.54 (d, J = 6.8 Hz, 1H), 4.97 (q, J = 6.6 Hz, 1H), 4.67 - 4.80 (m, 1H), 2.98 (s, 3H), 2.54 (s, 3H), 1.56 (d, J = 6.8 Hz, 3H), 1.27 (d, J = 7.0 Hz, 3H). Example 70: 5-(((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
in a manner analogous to Example 59 using (*R)-(2-chloro-3- fluorophenyl)(cyclopropyl)methanamine (Intermediate 7) instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-fluoropyrimidine-2-carboxylate instead of methyl 5-chloropyrazine-2-carboxylate, and pyridine instead of DIPEA in Step A. MS (ESI): mass calcd. for C
20H
22ClFN
4O
3S, 452.1; m/z found, 453.1 [M+H]
+. 1H NMR (400 MHz, DMSO-d
6) δ 8.66 (d, J = 8.6 Hz, 1H), 8.02 (s, 2H), 7.61 (d, J = 6.9 Hz, 1H), 7.46 - 7.26 (m, 3H), 6.83 - 6.72 (m, 1H), 6.71 - 6.60 (m, 1H), 4.85 - 4.65 (m, 1H), 4.44 (t, J = 7.6 Hz, 1H), 2.97 (s, 3H), 1.41 - 1.25 (m, 4H), 0.74 - 0.59 (m, 1H), 0.57 - 0.33 (m, 3H). Example 71: 5-(((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
VVID-747PC [00384] The title compound was prepared in a manner analogous to Example 59 using (*S)-(2-chloro-3- fluorophenyl)(cyclopropyl)methanamine (Intermediate 6) instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-fluoropyrimidine-2-carboxylate instead of methyl 5-chloropyrazine-2-carboxylate, and pyridine instead of DIPEA in Step A. MS (ESI): mass calcd. for C
20H
22ClFN
4O
3S, 452.1; m/z found, 453.1 [M+H]
+. 1H NMR (400 MHz, DMSO-d
6) δ 8.66 (d, J = 8.6 Hz, 1H), 8.02 (s, 2H), 7.61 (d, J = 6.8 Hz, 1H), 7.46 - 7.25 (m, 3H), 6.80 - 6.72 (m, 1H), 6.70 - 6.63 (m, 1H), 4.82 - 4.67 (m, 1H), 4.44 (t, J = 7.6 Hz, 1H), 2.97 (s, 3H), 1.28 (d, J = 7.0 Hz, 4H), 0.70 - 0.60 (m, 1H), 0.57 - 0.39 (m, 3H). Example 72: 6-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)nicotinamide. (1-(2-chlorophenyl)ethyl)amino)nicotinate. Methyl 6-fluoronicotinate (200
mg, 1.30 mmol, 1.0 eq), (S)-1-(2-chlorophenyl)ethan-1-amine hydrochloride (275 mg, 1.40 mmol, 1.1 eq), and K
2CO
3 (437 mg, 3.20 mmol, 2.5 eq) were taken up in DMF (6.3 mL, 0.2 M). The reaction was heated to 100°C for 24 h. After cooling to rt, the reaction was quenched with water and extracted with EtOAc. The combined organic extracts were dried over Na
2SO
4, filtered, and concentrated under reduced pressure. Purification by FCC on silica (0-100% EtOAc in heptanes) provided methyl (S)-6-((1-(2- chlorophenyl)ethyl)amino)nicotinate (155 mg, 42% yield). MS (ESI): mass calcd. for C
15H
15ClN
2O
2, 290.1; m/z found, 291.0 [M+H]
+. [00386] Step B: (S)-6-((1-(2-Chlorophenyl)ethyl)amino)nicotinic acid. Methyl (S)-6-((1-(2- chlorophenyl)ethyl)amino)nicotinate (155 mg, 0.530 mmol, 1.0 eq) was taken up in methanol (2.7 mL, 0.2 M). To this was added lithium hydroxide (2N in water, 2.1 mL, 4.30 mmol, 8.0 eq) and the reaction was stirred at 60°C for 3 hours. After cooling to rt, the reaction was adjusted to pH ∼2 with 1N HCl. The precipitated product was collected by filtration to provide (S)-6-((1-(2-chlorophenyl)ethyl)amino)nicotinic acid (145 mg, 98% yield) as a white solid. MS (ESI): mass calcd. for C
14H
13ClN
2O
2, 276.1; m/z found, 277.0 [M+H]
+. [00387] Step C: 6-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)nicotinamide. (S)-6-((1-(2-Chlorophenyl)ethyl)amino)nicotinic acid (177 mg, 0.640 mmol, 1.0 eq) and HATU (291 mg, 0.770 mmol, 1.2 eq) were taken up in DMF (3.0 mL, 0.2 M). DIPEA (0.33 mL, 1.90 mmol, 3.0 eq) was added and the reaction was allowed to stir for 5 min. (R,E)-4-(Methylsulfonyl)but-3- en-2-amine, 4-methylbenzenesulfonic acid (Intermediate 1, 205 mg, 0.640 mmol, 1.0 eq) was added and the reaction was stirred for 30 min at rt. The crude mixture was filtered through a PTFE filter and purified by RP-HPLC (20-95% ACN in 0.1% aq. HCOOH) to provide 6-(((S)-1-(2-chlorophenyl)ethyl)amino)-N- 156 QB\184200.00081\93036187.2
VVID-747PC ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)nicotinamide (146 mg, 56% yield). MS (ESI): mass calcd. for C
19H
22ClN
3O
3S, 407.1; m/z found, 408.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.60 – 8.45 (m, 1H), 7.77 (dd, J = 8.8, 2.4 Hz, 1H), 7.40 – 7.30 (m, 2H), 7.16 (dd, J = 5.9, 3.5 Hz, 2H), 6.98 – 6.76 (m, 2H), 6.50 (dd, J = 15.2, 1.7 Hz, 1H), 6.12 (d, J = 8.8 Hz, 1H), 5.78 (d, J = 6.5 Hz, 1H), 5.13 (p, J = 6.6 Hz, 1H), 5.04 – 4.88 (m, 1H), 2.87 (s, 3H), 1.51 (s, 3H), 1.35 (d, J = 7.1 Hz, 3H). Example 73: 6-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)nicotinamide. prepared in a manner analogous to Example 72 using methyl 6-chloro-4-
(trifluoromethyl)nicotinate instead of methyl 6-fluoronicotinate in Step A. MS (ESI): mass calcd. for C
20H
21ClF
3N
3O
3S, 475.1; m/z found, 476.0 [M+H]
+.
1H NMR (499 MHz, CDCl
3) δ 8.28 (s, 1H), 7.40 – 7.37 (m, 1H), 7.36 – 7.32 (m, 1H), 7.24 – 7.18 (m, 2H), 6.86 (dd, J = 15.1, 5.0 Hz, 1H), 6.52 – 6.44 (m, 2H), 5.92 (d, J = 7.9 Hz, 1H), 5.56 (d, J = 6.6 Hz, 1H), 5.27 (t, J = 6.8 Hz, 1H), 4.92 – 4.84 (m, 1H), 2.91 (s, 3H), 1.58 (d, J = 6.8 Hz, 3H), 1.38 (d, J = 7.1 Hz, 3H). 157 QB\184200.00081\93036187.2
VVID-747PC Example 74: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-3,5-difluoro-N-((R, E)-4-(methylsulfonyl)but-3- en-2-yl)benzamide. prepared in a manner analogous to Example 72 using methyl 3,4,5-
methyl 6-fluoronicotinate in Step A. MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.39 (br d, J = 7.9 Hz, 1H), 7.34 - 7.49 (m, 4H), 7.13 - 7.32 (m, 2H), 6.70 - 6.80 (m, 2H), 6.19 (br d, J = 9.3 Hz, 1H), 5.25 -
5.37 (m, 1H), 4.76 (td, J = 7.3, 2.7 Hz, 1H), 2.99 , 1.48 (d, J = 6.8 Hz, 3H), 1.26 (d, J = 7.0 Hz, 3H). Example 75: 5-((S)-1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide.
was prepared in a manner analogous to Example 72 using (S)-1-(2- chlorophenyl)ethan-1-ol instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-fluoropyrimidine-2- carboxylate instead of methyl 6-fluoronicotinate, and potassium t-butoxide instead of K
2CO
3 in Step A. MS (ESI): mass calcd. for C
18H
20ClN
3O
4S, 409.1; m/z found, 410.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.37 (s, 2H), 7.83 (d, J = 8.3 Hz, 1H), 7.48 – 7.35 (m, 2H), 7.30 – 7.15 (m, 2H), 6.91 (dd, J = 15.1, 4.5 Hz, 1H), 6.47 (dd, J = 15.1, 1.7 Hz, 1H), 5.88 (q, J = 6.3 Hz, 1H), 5.04 – 4.91 (m, 1H), 2.91 (s, 3H), 1.73 (d, J = 6.3 Hz, 3H), 1.44 (s, 3H). 158 QB\184200.00081\93036187.2
VVID-747PC Example 76: 5-((S)-1-(2-Chloro-4-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 72 using (S)-1-(2-chloro-4-
of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-fluoropyrimidine-2- carboxylate instead of methyl 6-fluoronicotinate, and potassium tert-butoxide instead of potassium carbonate in Step A. MS (ESI): mass calcd. for C
18H
19ClFN
3O
4S, 427.1; m/z found, 428.1 [M+H]
+.
1H NMR (499 MHz, CDCl
3) δ 8.36 (s, 2H), 7.82 (d, J = 8.3 Hz, 1H), 7.39 (dd, J = 8.8, 5.9 Hz, 1H), 7.17 (dd, J = 8.3, 2.6 Hz, 1H), 6.99 (ddd, J = 8.8, 7.8, 2.6 Hz, 1H), 6.91 (dd, J = 15.1, 4.6 Hz, 1H), 6.47 (dd, J = 15.1, 1.7 Hz, 1H), 5.84 (q, J = 6.3 Hz, 1H), 5.05 – 4.90 (m, 1H), 2.92 (s, 3H), 1.71 (d, J = 6.4 Hz, 3H), 1.44 (d, J = 7.1 Hz, 3H). Example 77: 5-((1-(2-Chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide.
prepared in a manner analogous to Example 72 using 1-(2-chloro-3- fluorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 5-chloropyrazine- 2-carboxylate instead of methyl 6-fluoronicotinate in Step A. MS (ESI): mass calcd. for C
18H
20ClFN
4O
3S, 426.1; m/z found, 427.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.52 (dd, J = 8.6, 1.7 Hz, 1H), 8.44 (s, 1H), 8.35 - 8.40 (m, 1H), 8.00 (br s, 1H), 7.24 - 7.38 (m, 3H), 6.75 - 6.83 (m, 1H), 6.63 - 6.72 (m, 1H), 5.38 (br t, J = 6.9 Hz, 1H), 4.69 - 4.87 (m, 1H), 2.98 (d, J = 6.3 Hz, 3H), 1.48 (d, J = 6.8 Hz, 3H), 1.30 (dd, J = 7.0, 4.0 Hz, 3H). 159 QB\184200.00081\93036187.2
VVID-747PC Example 78: 5-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide. prepared via separation of 5-((1-(2-chloro-3-fluorophenyl)ethyl)amino)-
- 3-en-2-yl)pyrazine-2-carboxamide (Example 77) by SFC (Stationary phase: IG (3x25 cm); Mobile phase: 40% MeOH/CO
2; Rt = 8.67 min (second eluting product)). MS (ESI): mass calcd. for C
18H
20ClFN
4O
3S, 426.1; m/z found, 427.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.52 (br d, J = 8.4 Hz, 1H), 8.31 - 8.46 (m, 2H), 8.01 (br s, 1H), 7.20 - 7.40 (m, 3H), 6.60 - 6.86 (m, 2H), 5.28 - 5.44 (m, 1H), 4.79 (br d, J = 4.9 Hz, 1H), 2.91 - 3.01 (m, 3H), 1.48 (br d, J = 6.4 Hz, 3H), 1.31 (br d, J = 6.5 Hz, 3H). Example 79: 5-(((S)-1-(2-Chloro-4-methoxyphenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrazine-2-carboxamide.
prepared in a manner analogous to Example 72 using methyl 5- chloropyrazine-2-carboxylate instead of methyl 6-fluoronicotinate and (S)-1-(2-chloro-4- methoxyphenyl)ethan-1-amine hydrochloride instead of (S)-1-(2-chlorophenyl)ethan-1-amine in Step A. MS (ESI): mass calcd. for C
19H
23ClN
4O
4S, 438.1; m/z found, 439.1 [M+H]
+.
1H NMR (400 MHz, DMSO- d
6) δ 8.50 (d, J = 8.6 Hz, 1H), 8.45 (s, 1H), 8.25 (d, J = 7.4 Hz, 1H) 7.96 (br s, 1H), 7.36 (d, J = 8.6 Hz, 1H), 7.01 (d, J = 2.5 Hz, 1H), 6.89 (dd, J = 8.7, 2.6 Hz, 1H), 6.75 - 6.83 (m, 1H), 6.63 - 6.71 (m, 1H), 5.31 (br t, J = 6.9 Hz, 1H), 4.72 - 4.85 (m, 1H), 3.74 (s, 3H), 2.98 (s, 3H), 1.44 (d, J = 6.9 Hz, 3H), 1.31 (d, J = 7.0 Hz, 3H). 160 QB\184200.00081\93036187.2
VVID-747PC Example 80: 5-((2-Chlorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 72 using (2-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 5- fluoropyrimidine-2-carboxylate instead of methyl 6-fluoronicotinate in Step A. MS (ESI): mass calcd. for C
20H
22ClN
3O
4S, 435.1; m/z found, 436.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.92 (d, J = 8.5 Hz, 1H), 8.48 (s, 2H), 7.56 - 7.64 (m, 1H), 7.46 - 7.55 (m, 1H), 7.31 - 7.41 (m, 2H), 6.62 - 6.84 (m, 2H), 5.46 (d, J = 8.1 Hz, 1H), 4.77 (br dd, J = 12.8, 7.1 Hz, 1H), 2.97 (d, J = 3.4 Hz, 3H), 1.52 (dt, J = 7.8, 5.1 Hz, 1H), 1.29 (dd, J = 7.0, 2.1 Hz, 3H), 0.51 - 0.76 (m, 4H). Example 81: 5-((*R)-(2-Chlorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
via separation of 5-((2-chlorophenyl)(cyclopropyl)methoxy)-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 80) by SFC (Stationary phase: IH (3x25 cm); Mobile phase: 40% iPrOH/CO
2; Rt = 8.40 min). MS (ESI): mass calcd. for C
20H
22ClN
3O
4S, 435.1; m/z found, 436.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.92 (d, J = 8.5 Hz, 1H), 8.48 (s, 2H), 7.55 - 7.61 (m, 1H), 7.47 - 7.53 (m, 1H), 7.31 - 7.40 (m, 2H), 6.63 - 6.86 (m, 2H), 5.46 (d, J = 8.1 Hz, 1H), 4.69 - 4.84 (m, 1H), 2.98 (s, 3H), 1.45 - 1.57 (m, 1H), 1.29 (d, J = 7.0 Hz, 3H), 0.51 - 0.78 (m, 4H). 161 QB\184200.00081\93036187.2
VVID-747PC Example 82: 5-((*S)-(2-Chlorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. via separation of 5-((2-chlorophenyl)(cyclopropyl)methoxy)-N-
- 2-yl)pyrimidine-2-carboxamide (Example 80) by SFC (Stationary phase: IH (3x25 cm); Mobile phase: 40% iPrOH/CO
2; Rt = 11.3 min). MS (ESI): mass calcd. for C
20H
22ClN
3O
4S, 435.1; m/z found, 436.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.4 Hz, 1H), 8.48 (s, 2H), 7.56 - 7.61 (m, 1H), 7.48 - 7.54 (m, 1H), 7.31 - 7.43 (m, 2H), 6.65 - 6.83 (m, 2H), 5.46 (d, J = 8.1 Hz, 1H), 4.70 - 4.83 (m, 1H), 2.97 (s, 3H), 1.46 - 1.58 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H), 0.52 - 0.76 (m, 4H). Example 83: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-3-cyano-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide.
prepared in a manner analogous to Example 72 using methyl 3-cyano-4- fluorobenzoate instead of methyl 6-fluoronicotinate in Step A. MS (ESI): mass calcd. for C
21H
22ClN
3O
3S, 431.1; m/z found, 432.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.37 (br d, J = 7.8 Hz, 1H), 8.09 (d, J = 1.9 Hz, 1H), 7.81 (dd, J = 1.8, 9.0 Hz, 1H), 7.53 (dd, J = 1.6, 7.4 Hz, 1H), 7.46 (dd, J = 1.1, 7.6 Hz, 1H), 7.20 - 7.36 (m, 2H), 7.10 (br d, J = 7.0 Hz, 1H), 6.64 - 6.84 (m, 2H), 6.37 (d, J = 9.0 Hz, 1H), 4.99 (br t, J = 6.8 Hz, 1H), 4.76 (dt, J = 3.4, 7.2 Hz, 1H), 2.98 (s, 3H), 1.56 (d, J = 6.8 Hz, 3H), 1.26 (d, J = 7.1 Hz, 3H). 162 QB\184200.00081\93036187.2
VVID-747PC Example 84: 5-(2-Chloro-3-fluorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 72 using methyl 5-
instead of methyl 6-fluoronicotinate and (2-chloro-3- fluorophenyl)(cyclopropyl)methanol (Intermediate 10) instead of (S)-1-(2-chlorophenyl)ethan-1-amine in Step A. MS (ESI): mass calcd. for C
20H
21ClFN
3O
4S, 453.1; m/z found, 454.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d6) δ 8.93 (d, J = 8.5 Hz, 1H), 8.50 (s, 2H), 7.31 - 7.50 (m, 3H), 6.62 - 6.85 (m, 2H), 5.50 (d, J = 8.3 Hz, 1H), 4.77 (sext, J = 6.7 Hz, 1H), 2.98 (d, J = 3.0 Hz, 3H), 1.52 (qt, J = 8.0, 5.0 Hz, 1H), 1.30 (dd, J = 7.0, 2.0 Hz, 3H), 0.52 - 0.74 (m, 4H). Example 85: 5-((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide.
was prepared via separation of 5-(2-chloro-3- fluorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide (Example 84) by SFC (Stationary phase: IH (3x25 cm); Mobile phase: 35% iPrOH/CO
2; Rt = 8.61 min). MS (ESI): mass calcd. for C
20H
21ClFN
3O
4S, 453.1; m/z found, 454.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.5 Hz, 1H), 8.50 (s, 2H), 7.34 - 7.50 (m, 3H), 6.66 - 6.83 (m, 2H), 5.50 (d, J = 8.3 Hz, 1H), 4.72 - 4.83 (m, 1H), 2.98 (s, 3H), 1.52 (qt, J = 8.1, 5.0 Hz, 1H), 1.30 (d, J = 7.0 Hz, 3H), 0.51 - 0.76 (m, 4H). 163 QB\184200.00081\93036187.2
VVID-747PC Example 86: 5-((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide. was prepared via separation of 5-(2-chloro-3-
-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide (Example 84) by SFC (Stationary phase: IH (3x25 cm); Mobile phase: 35% iPrOH/CO
2; Rt = 11.7 min). MS (ESI): mass calcd. for C
20H
21ClFN
3O
4S, 453.1; m/z found, 454.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d6) δ 8.93 (d, J = 8.5 Hz, 1H), 8.50 (s, 2H), 7.34 - 7.50 (m, 3H), 6.65 - 6.83 (m, 2H), 5.50 (d, J = 8.3 Hz, 1H), 4.70 - 4.84 (m, 1H), 2.97 (s, 3H), 1.46 - 1.59 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H), 0.51 - 0.76 (m, 4H). Example 87: 5-(((*S)-1-(2-Chlorophenyl)ethyl)(cyclopropyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 72 using methyl 5- (cyclopropylamino)pyrimidine-2-carboxylate (Intermediate 20) instead of methyl 6-fluoronicotinate and 2-chloro-α-methylbenzyl chloride instead of (S)-1-(2-chlorophenyl)ethan-1-amine in Step A. Separation of 5-((1-(2-chlorophenyl)ethyl)(cyclopropyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 8.59 min (second eluting product)) provided the title compound. MS (ESI): mass calcd. for C
21H
25ClN
4O
3S, 448.1; m/z found, 449.2 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.78 (d, J = 8.6 Hz, 1H), 8.52 (s, 2H), 7.45 - 7.59 (m, 2H), 7.32 -
, 6.78 - 6.86 (m, 1H), 6.69 - 6.76 (m, 1H), 5.23 (q, J = 6.9 Hz, 1H), 4.80 (dq, J = 13.2, 6.7 Hz, 1H), 3.00 (s, 3H), 2.24 - 2.32 (m, 1H), 1.70 (d, J = 7.0 Hz, 3H), 1.33 (d, J = 7.0 Hz, 3H), 0.82 - 0.93 (m, 1H), 0.67 - 0.81 (m, 2H), 0.28 - 0.41 (m, 1H). Example 88: 5-((2-Chlorophenyl)(tetrahydrofuran-3-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide. 164 QB\184200.00081\93036187.2
VVID-747PC in a manner analogous to Example 72 using methyl 5-
instead of methyl 6-fluoronicotinate and (2- chlorophenyl)(tetrahydrofuran-3-yl)methanol (Intermediate 11) instead of (S)-1-(2-chlorophenyl)ethan-1- amine in Step A. MS (ESI): mass calcd. for C
21H
24ClN
3O
5S, 465.1; m/z found, 466.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.94 (d, J = 8.5 Hz, 1H), 8.37 - 8.58 (m, 2H), 7.47 - 7.65 (m, 2H), 7.25 - 7.43 (m, 2H), 6.61 - 6.86 (m, 2H), 5.75 - 5.94 (m, 1H), 4.65 - 4.87 (m, 1H), 3.78 - 3.99 (m, 2H), 3.61 - 3.75 (m, 1H), 3.45 - 3.60 (m, 1H), 2.97 (d, J = 3.8 Hz, 4H), 2.00 - 2.16 (m, 1H), 1.52 - 1.79 (m, 1H), 1.29 (dd, J = 6.9, 2.3 Hz, 3H). Example 89: 5-((*S)-(2-Chlorophenyl)((*R)-tetrahydrofuran-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
via separation of methyl 5-((2-chlorophenyl)(tetrahydrofuran-3- yl)methoxy)pyrimidine-2-carboxylate (Example 88, Step A) by SFC (Stationary phase: IG (3x25 cm); Mobile phase: 45% EtOH/CO
2; Rt = 2.65 min (first co-eluting product) followed by Stationary phase: AD (3x25 cm); Mobile phase: 25% MeOH/CO
2; Rt = 6.43 min (second eluting product)). The isolated product was elaborated to the title compound. MS (ESI): mass calcd. for C
21H
24ClN
3O
5S, 465.1; m/z found, 466.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.94 (d, J = 8.5 Hz, 1H), 8.49 (s, 2H), 7.47 - 7.65 (m, 2H), 7.28 - 7.43 (m, 2H), 6.74 - 6.81 (m, 1H), 6.66 - 6.73 (m, 1H), 5.86 (d, J = 7.9 Hz, 1H), 4.77 (br d, J = 5.1 Hz, 1H), 3.83 - 3.92 (m, 1H), 3.67 - 3.74 (m, 1H), 3.53 - 3.61 (m, 1H), 3.45 - 3.52 (m, 1H), 2.90 - 3.01 (m, 4H), 2.01 - 2.16 (m, 2H), 1.29 (d, J = 7.0 Hz, 3H). 165 QB\184200.00081\93036187.2
VVID-747PC Example 90: 5-((*S)-(2-Chlorophenyl)((*S)-tetrahydrofuran-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. via separation of methyl 5-((2-chlorophenyl)(tetrahydrofuran-3-
(Example 88, Step A) by SFC (Stationary phase: IG (3x25 cm); Mobile phase: 45% EtOH/CO
2; Rt = 7.50 min (fourth eluting product)). The isolated product was elaborated to the title compound. MS (ESI): mass calcd. for C
21H
24ClN
3O
5S, 465.1; m/z found, 466.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.94 (d, J = 8.5 Hz, 1H), 8.48 (s, 2H), 7.56 - 7.62 (m, 1H), 7.49 - 7.55 (m, 1H), 7.32 - 7.41 (m, 2H), 6.74 - 6.82 (m, 1H), 6.64 - 6.73 (m, 1H), 5.83 (d, J = 9.1 Hz, 1H), 4.70 - 4.82 (m, 1H), 3.90 (d, J = 6.5 Hz, 2H), 3.84 (td, J = 8.0, 5.3 Hz, 1H), 3.65 (q, J = 7.4 Hz, 1H), 2.90 - 3.03 (m, 4H), 1.56 - 1.77 (m, 2H), 1.29 (d, J = 7.0 Hz, 3H). Example 91: 5-((*R)-(2-Chlorophenyl)((R)-tetrahydrofuran-2-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 72 using methyl 5- fluoropyrimidine-2-carboxylate instead of methyl 6-fluoronicotinate and (*R)-(2-chlorophenyl)((R)- tetrahydrofuran-2-yl)methanol (Intermediate 12) instead of (S)-1-(2-chlorophenyl)ethan-1-amine in Step A. MS (ESI): mass calcd. for C
21H
24ClN
3O
5S, 465.1; m/z found, 466.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.4 Hz, 1H), 8.47 - 8.57 (m, 2H), 7.48 - 7.61 (m, 2H), 7.33 - 7.43 (m, 2H), 6.64 - 6.86 (m, 2H), 5.82 (d, J = 6.5 Hz, 1H), 4.63 - 4.85 (m, 1H), 4.29 - 4.46 (m, 1H), 3.70 - 3.98 (m, 2H), 2.98 (s, 3H), 1.68 - 2.08 (m, 4H), 1.30 (d, J = 7.0 Hz, 3H). 166 QB\184200.00081\93036187.2
VVID-747PC Example 92: 5-((*R)-(2-Chlorophenyl)((S)-tetrahydrofuran-2-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 72 using methyl 5-
of methyl 6-fluoronicotinate and (*R)-(2-chlorophenyl)((S)- tetrahydrofuran-2-yl)methanol (Intermediate 13) instead of (S)-1-(2-chlorophenyl)ethan-1-amine in Step A. MS (ESI): mass calcd. for C
21H
24ClN
3O
5S, 465.1; m/z found, 466.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.94 (d, J = 8.4 Hz, 1H), 8.38 - 8.55 (m, 2H), 7.46 - 7.69 (m, 2H), 7.31 - 7.40 (m, 2H), 6.59 - 6.86 (m, 2H), 5.87 (d, J = 5.1 Hz, 1H), 4.75 - 4.90 (m, 1H), 4.34 (q, J = 6.1 Hz, 1H), 3.62 - 3.84 (m, 2H), 2.98 (s, 3H), 1.76 - 2.13 (m, 4H), 1.29 (d, J = 7.0 Hz, 3H). Example 93: 4-(1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide.
chlorophenyl)ethoxy)benzoate. Methyl 4-hydroxybenzoate (50 mg, 0.330 mmol, 1.0 eq) was taken up in THF (1.6 mL, 0.2 M) and placed under N
2. To this was added 1-(2- chlorophenyl)ethanol (38 µL, 0.330 mmol, 1.0 eq), triphenylphosphine (117 mg, 0.450 mmol, 1.4 eq), and DIAD (88 µL, 0.450 mmol, 1.4 eq). The reaction was stirred at rt overnight before being concentrated under reduced pressure. Purification by FCC on silica (0-20% EtOAc in heptanes) provided methyl 4-(1- (2-chlorophenyl)ethoxy)benzoate (73 mg, 77% yield). MS (ESI): mass calcd. for C
16H
15ClO
3, 290.1; m/z found, 291.0 [M+H]
+. [00409] Step B: 4-(1-(2-Chlorophenyl)ethoxy)benzoic acid. Methyl 4-(1-(2- chlorophenyl)ethoxy)benzoate (73 mg, 0.250 mmol, 1.0 eq) was taken up in MeOH (1.6 mL, 0.16 M). To this was added lithium hydroxide (2N in water, 1.0 mL, 2.00 mmol, 8.0 eq) and the reaction was stirred at 60°C for 3 hours. After cooling to rt, the reaction was adjusted to pH ∼2 with 1N HCl. The precipitate was collected by filtration to provide 4-(1-(2-chlorophenyl)ethoxy)benzoic acid (64 mg, 91% yield) as a white solid. MS (ESI): mass calcd. for C
15H
13ClO
3, 276.1; m/z found, 277.0 [M+H]
+. 167 QB\184200.00081\93036187.2
VVID-747PC [00410] Step C: 4-(1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide. 4- (1-(2-Chlorophenyl)ethoxy)benzoic acid (64 mg, 0.230 mmol, 1.0 eq) and HATU (105 mg, 0.280 mmol, 1.2 eq) were taken up in DMF (1.1 mL, 0.2 M). DIPEA (120 µL, 0.690 mmol, 3.0 eq) was added and the reaction was stirred for 5 min. (R,E)-4-(Methylsulfonyl)but-3-en-2-amine, 4-methylbenzenesulfonic acid (Intermediate 1, 74 mg, 0.230 mmol, 1.0 eq) was added and the reaction was stirred for 30 min at rt. Purification via RP-HPLC (5-95% ACN in 0.1% aq. HCOOH) provided 4-(1-(2-chlorophenyl)ethoxy)-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide (80 mg, 85% yield) as a white solid. MS (ESI): mass calcd. for C
20H
22ClNO
4S, 407.1; m/z found, 408.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.69 – 7.60 (m, 2H), 7.45 – 7.33 (m, 2H), 7.24 – 7.15 (m, 2H), 6.90 (ddd, J = 15.1, 4.6, 1.2 Hz, 1H), 6.87 – 6.80 (m, 2H), 6.46 (ddd, J = 15.1, 3.0, 1.7 Hz, 1H), 5.96 (d, J = 7.6 Hz, 1H), 5.76 (qd, J = 6.1, 1.0 Hz, 1H), 5.04 – 4.86 (m, 1H), 2.91 (d, J = 1.0 Hz, 3H), 1.66 (d, J = 6.4 Hz, 3H), 1.56 (s, 1H), 1.40 (dd, J = 7.1, 1.8 Hz, 3H). Example 94: 4-((*S)-1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide.
prepared via separation of 4-(1-(2-chlorophenyl)ethoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide (Example 93) by SFC (Stationary phase: A6-5 (3x25 cm); Mobile phase: 40% MeOH/CO
2; Rt = 4.63 min (second eluting product)). MS (ESI): mass calcd. for C
20H
22ClNO
4S, 407.1; m/z found, 408.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.70 – 7.57 (m, 2H), 7.46 – 7.31 (m, 2H), 7.24 – 7.11 (m, 2H), 6.95 – 6.76 (m, 3H), 6.45 (dd, J = 15.1, 1.7 Hz, 1H), 6.19 (d, J = 7.8 Hz, 1H), 5.75 (q, J = 6.4 Hz, 1H), 5.01 – 4.88 (m, 1H), 2.89 (s, 3H), 1.65 (d, J = 6.4 Hz, 3H), 1.38 (d, J = 7.1 Hz, 3H). Example 95: 4-(1-(2-Chlorophenyl)ethoxy)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide. O
prepared in a manner analogous to Example 93 using methyl 2-fluoro-4- hydroxybenzoate instead of methyl 4-hydroxybenzoate in Step A. MS (ESI): mass calcd. for C
20H
21ClFNO
4S, 425.1; m/z found, 426.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.89 (t, J = 9.1 Hz, 1H), 168 QB\184200.00081\93036187.2
VVID-747PC 7.46 – 7.30 (m, 2H), 7.24 – 7.15 (m, 2H), 6.89 (ddd, J = 15.1, 4.7, 1.1 Hz, 1H), 6.73 – 6.37 (m, 4H), 5.71 (qd, J = 6.3, 1.3 Hz, 1H), 5.03 – 4.89 (m, 1H), 2.89 (d, J = 0.9 Hz, 3H), 1.64 (d, J = 6.4 Hz, 3H), 1.38 (dd, J = 7.1, 2.0 Hz, 3H). Example 96: 4-((S)-1-(2-Chlorophenyl)ethoxy)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide. was prepared in a manner analogous to Example 93 using (S)-1-(2-
of 1-(2-chlorophenyl)ethan-1-ol and methyl 2-fluoro-4-hydroxybenzoate instead of methyl 4-hydroxybenzoate in Step A. MS (ESI): mass calcd. for C
20H
21ClFNO
4S, 425.1; m/z found, 426.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.91 (t, J = 9.1 Hz, 1H), 7.39 (ddt, J = 8.9, 6.3, 3.7 Hz, 2H), 7.26 – 7.17 (m, 2H), 6.91 (dd, J = 15.1, 4.6 Hz, 1H), 6.70 (dd, J = 8.8, 2.4 Hz, 1H), 6.65 – 6.42 (m, 3H), 5.73 (q, J = 6.3 Hz, 1H), 5.04 – 4.93 (m, 1H), 2.92 (s, 3H), 1.66 (d, J = 6.3 Hz, 3H), 1.40 (d, J = 7.1 Hz, 3H). Example 97: 4-((S)-1-(2-Chloro-4-fluorophenyl)ethoxy)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)benzamide.
prepared in a manner analogous to Example 93 using (S)-1-(2-chloro-4- fluorophenyl)ethan-1-ol instead of 1-(2-chlorophenyl)ethan-1-ol and methyl 2-fluoro-4-hydroxybenzoate instead of methyl 4-hydroxybenzoate in Step A. MS (ESI): mass calcd. for C
20H
20ClF
2NO
4S, 443.1; m/z found, 444.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.92 (t, J = 9.1 Hz, 1H), 7.38 (dd, J = 8.8, 6.0 Hz, 1H), 7.13 (dd, J = 8.3, 2.5 Hz, 1H), 7.00 – 6.85 (m, 2H), 6.68 (dt, J = 8.9, 2.6 Hz, 1H), 6.63 – 6.42 (m, 3H), 5.68 (q, J = 6.3 Hz, 1H), 5.04 – 4.91 (m, 1H), 2.91 (s, 3H), 1.63 (d, J = 6.3 Hz, 3H), 1.40 (dd, J = 7.1, 1.8 Hz, 3H). Example 98: 4-((S)-1-(2-Chlorophenyl)ethoxy)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide. 169 QB\184200.00081\93036187.2
VVID-747PC was prepared in a manner analogous to Example 93 using (S)-1-(2-
of 1-(2-chlorophenyl)ethan-1-ol and methyl 2,5-difluoro-4- hydroxybenzoate instead of methyl 4-hydroxybenzoate in Step A. MS (ESI): mass calcd. for C
20H
20ClF
2NO
4S, 443.1; m/z found, 444.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.76 (dd, J = 11.5, 7.3 Hz, 1H), 7.50 – 7.42 (m, 1H), 7.42 – 7.35 (m, 1H), 7.31 – 7.18 (m, 2H), 6.91 (dd, J = 15.1, 4.8 Hz, 1H), 6.58 (dd, J = 13.7, 7.6 Hz, 1H), 6.53 – 6.37 (m, 2H), 5.77 (q, J = 6.3 Hz, 1H), 5.03 – 4.91 (m, 1H), 2.94 (s, 3H), 1.71 (d, J = 6.3 Hz, 3H), 1.39 (d, J = 7.1 Hz, 3H). Example 99: 5-(((S)-1-(2-Chlorophenyl)ethyl)(methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
((1-(2-chlorophenyl)ethyl)amino)pyrimidine-2-carboxylate. Methyl 5- bromopyrimidine-2-carboxylate (200 mg, 0.903 mmol, 1.0 eq), (S)-1-(2-chlorophenyl)ethan-1-amine hydrochloride (192 mg, 0.948 mmol, 1.05 eq), RuPhos Pd G4 (77 mg, 0.090 mmol, 0.1 eq), and Cs
2CO
3 (901 mg, 2.71 mmol, 3.0 eq) were taken up in toluene (4.5 mL, 0.2 M) and placed under N
2. The reaction was heated to 100°C for 18 h. After cooling to rt, the reaction was quenched with water and extracted with EtOAc. The combined organic extracts were dried over Na
2SO
4, filtered, and concentrated under reduced pressure. Purification by FCC on silica (0-100% EtOAc in heptanes) provided methyl (S)-5-((1-(2- chlorophenyl)ethyl)amino)pyrimidine-2-carboxylate (91 mg, 35% yield). MS (ESI): mass calcd. for C
14H
14ClN
3O
2, 291.1; m/z found, 292.0 [M+H]
+. [00417] Step B: (S)-5-((1-(2-Chlorophenyl)ethyl)(methyl)amino)pyrimidine-2-carboxylic acid. In an oven-dried flask under N
2, NaH (18 mg, 0.442 mmol, 3.0 eq) was taken up in DMF (0.7 mL, 0.2 M) and cooled to 0°C. To this was added methyl (S)-5-((1-(2-chlorophenyl)ethyl)amino)pyrimidine-2-carboxylate (43 mg, 0.147 mmol, 1.0 eq) and the reaction was allowed to stir for 5 min. Finally, iodomethane (27 µL, 0.442 mmol, 3.0 eq) was added and the reaction was stirred at 0°C for 30 min. The reaction was quenched with minimal water, filtered through a PTFE filter, and purified by RP-HPLC (5-95% ACN in 0.1% aq. 170 QB\184200.00081\93036187.2
VVID-747PC HCOOH) to provide (S)-5-((1-(2-chlorophenyl)ethyl)(methyl)amino)pyrimidine-2-carboxylic acid (34 mg, 79% yield). MS (ESI): mass calcd. for C
14H
14ClN
3O
2, 291.1; m/z found, 292.0 [M+H]
+. [00418] Step C: 5-(((S)-1-(2-Chlorophenyl)ethyl)(methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. (S)-5-((1-(2-Chlorophenyl)ethyl)(methyl)amino)pyrimidine-2-carboxylic acid (17 mg, 0.058 mmol, 1.0 eq), (R,E)-4-(methylsulfonyl)but-3-en-2-amine, 4-methylbenzenesulfonic acid (Intermediate 1, 20 mg, 0.061 mmol, 1.05 eq), and HATU (26 mg, 0.064 mmol, 1.1 eq) were taken up in DMF (0.3 mL, 0.2 M). To this was added DIPEA (22 µL, 0.128 mmol, 2.2 eq) and the reaction was stirred at rt for 30 min. The crude material was filtered through a PTFE filter with MeOH. Purification by RP-HPLC (10-95% ACN in 0.1% aq. HCOOH) provided 5-(((S)-1-(2-chlorophenyl)ethyl)(methyl)amino)- N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (20 mg, 81% yield). MS (ESI): mass calcd. for C
19H
23ClN
4O
3S, 422.1; m/z found, 423.0 [M+H]
+.
1H NMR (499 MHz, CDCl
3) δ 8.29 (s, 2H), 7.81 (d, J = 8.3 Hz, 1H), 7.41 (dd, J = 7.5, 1.7 Hz, 1H), 7.34 (dd, J = 7.4, 2.2 Hz, 1H), 7.32 – 7.25 (m, 2H), 6.94 (dd, J = 15.1, 4.4 Hz, 1H), 6.50 (dd, J = 15.1, 1.8 Hz, 1H), 5.35 (q, J = 6.9 Hz, 1H), 5.05 – 4.98 (m, 1H), 2.92 (s, 3H), 2.91 (s, 3H), 1.66 (d, J = 7.0 Hz, 4H), 1.45 (d, J = 7.1 Hz, 3H). Example 100: 4-(((S)-1-(2-Chlorophenyl)ethyl)(methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide.
((1-(2-chlorophenyl)ethyl)amino)benzoate. Methyl 4-bromobenzoate (120 mg, 0.558 mmol, 1.0 eq) was taken up in toluene (2.8 mL, 0.2 M) and placed under N2. To this was added (S)-1-(2-chlorophenyl)ethan-1-amine hydrochloride (113 mg, 0.586 mmol, 1.05 eq), cesium carbonate (545 mg, 1.67 mmol, 3.0 eq), and SPhos Pd G4 (44 mg, 0.056 mmol, 0.1 eq). The reaction was heated to 100°C for 24 hours. After cooling to rt, the reaction was quenched with water and extracted with EtOAc. The combined organic extracts were dried over Na
2SO
4, filtered, and concentrated under reduced pressure. Purification by FCC on silica (0-25% EtOAc in heptanes) provided methyl (S)-4-((1-(2- chlorophenyl)ethyl)amino)benzoate (120 mg, 74% yield). MS (ESI): mass calcd. for C
16H
16ClNO
2, 289.1; m/z found, 290.0 [M+H]
+. [00420] Step B: Methyl (S)-4-((1-(2-chlorophenyl)ethyl)(methyl)amino)benzoate. Sodium hydride (6.2 mg, 0.155 mmol, 1.5 eq) was taken up in DMF (1.3 mL, 0.08 M), placed under N
2, and cooled to 0°C. Methyl (S)-4-((1-(2-chlorophenyl)ethyl)amino)benzoate (30 mg, 0.104 mmol, 1.0 eq) was added and the reaction was allowed to stir for 5 min. Iodomethane (10 µL, 0.155 mmol, 1.5 eq) was added and the reaction was stirred for 30 min at 0 ºC. The reaction was quenched with water and extracted with EtOAc. The combined organic extracts were dried over Na
2SO
4, filtered, and concentrated under reduced pressure. 171 QB\184200.00081\93036187.2
VVID-747PC Purification by RP-HPLC (20-95% ACN in 0.1% aq. HCOOH) provided methyl (S)-4-((1-(2- chlorophenyl)ethyl)(methyl)amino)benzoate (24 mg, 76% yield). MS (ESI): mass calcd. for C
17H
18ClNO
2, 303.1; m/z found, 304.1 [M+H]
+. [00421] Step C: (S)-4-((1-(2-Chlorophenyl)ethyl)(methyl)amino)benzoic acid. Methyl (S)-4-((1-(2- chlorophenyl)ethyl)(methyl)amino)benzoate (24 mg, 0.079 mmol, 1.0 eq) was taken up in methanol (0.4 mL, 0.2 M). To this was added lithium hydroxide (2N in water, 0.32 mL, 0.635 mmol, 8.0 eq) and the reaction was stirred at 60°C for 3 hours. After cooling to rt, the reaction was adjusted to pH ~2 with 1N HCl. The precipitate was collected by filtration to provide (S)-4-((1-(2- chlorophenyl)ethyl)(methyl)amino)benzoic acid (16 mg, 67% yield) as a white solid. MS (ESI): mass calcd. for C
16H
16ClNO
2, 289.1; m/z found, 290.0 [M+H]
+. [00422] Step D: 4-(((S)-1-(2-Chlorophenyl)ethyl)(methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide. (S)-4-((1-(2-Chlorophenyl)ethyl)(methyl)amino)benzoic acid (16 mg, 0.053 mmol, 1.0 eq) and HATU (24 mg, 0.064 mmol, 1.2 eq) were taken up in DMF (0.27 mL, 0.2 M). DIPEA (28 µL, 0.160 mmol, 3.0 eq) was added and the reaction was allowed to stir for 5 min. (R,E)-4-(Methylsulfonyl)but-3- en-2-amine, 4-methylbenzenesulfonic acid (Intermediate 1, 17 mg, 0.054 mmol, 1.0 eq) was added and the reaction was stirred for 30 minutes at rt. Purification via RP-HPLC (20-95% ACN in 0.1% aq. HCOOH) provided 4-(((S)-1-(2-chlorophenyl)ethyl)(methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide (17 mg, 74% yield) as a white solid. MS (ESI): mass calcd. for C
21H
25ClN
2O
3S, 420.1; m/z found, 421.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.73 – 7.62 (m, 2H), 7.42 – 7.35 (m, 1H), 7.31 (dd, J = 7.3, 2.1 Hz, 1H), 7.28 – 7.19 (m, 2H), 6.93 (dd, J = 15.1, 4.5 Hz, 1H), 6.80 – 6.68 (m, 2H), 6.49 (dd, J = 15.1, 1.8 Hz, 1H), 6.02 (d, J = 7.7 Hz, 1H), 5.33 (q, J = 7.0 Hz, 1H), 5.06 – 4.92 (m, 1H), 2.92 (s, 3H), 2.88 (s, 3H), 1.60 (d, J = 6.9 Hz, 3H), 1.40 (d, J = 7.1 Hz, 3H). 172 QB\184200.00081\93036187.2
VVID-747PC Example 101: 5-(((S)-1-(2-Chlorophenyl)ethyl)(ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. (2-chlorophenyl)ethyl)(ethyl)amino)pyrimidine-2-carboxylate. To a
- ethan-1-amine (1.0 g, 6.43 mmol, 1.0 eq) in DMSO (20 mL, 0.32 M) was added pyridine (1.0 mL, 12.9 mmol, 2.0 eq) and methyl 5-fluoropyrimidine-2-carboxylate (1.3 g, 8.35 mmol, 1.3 eq). The mixture was stirred at 100℃ for 16 h. After cooling to rt, the reaction mixture was poured into water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (0-20% EtOAc in PE) to give methyl (S)-5-((1-(2- chlorophenyl)ethyl)(ethyl)amino)pyrimidine-2-carboxylate (1.2 g, 64% yield) as a white solid. MS (ESI): mass calcd. for C
14H
14ClN
3O
2, 291.1; m/z found, 292.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.07 (s, 2H), 7.29 - 7.39 (m, 2H), 7.14 - 7.24 (m, 2H), 4.97 - 5.13 (m, 2H), 3.96 (s, 3H), 1.58 (d, J = 6.6 Hz, 3H). [00424] The title compound was prepared in a manner analogous to Example 100, Steps B-D using methyl (S)-5-((1-(2-chlorophenyl)ethyl)(ethyl)amino)pyrimidine-2-carboxylate instead of methyl (S)-4-((1-(2- chlorophenyl)ethyl)amino)benzoate and iodoethane instead of iodomethane in Step B. MS (ESI): mass calcd. for C
20H
25ClN
4O
3S, 436.1; m/z found, 437.2 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.69 (d, J = 8.5 Hz, 1H), 8.38 (s, 2H), 7.58 (dd, J = 7.5, 1.7 Hz, 1H), 7.50 (dd, J = 7.5, 1.7 Hz, 1H), 7.32 - 7.44 (m, 2H), 6.77 - 6.89 (m, 1H), 6.67 - 6.76 (m, 1H), 5.45 (q, J = 6.6 Hz, 1H), 4.80 (br dd, J = 6.7, 1.5 Hz, 1H), 3.36 - 3.44 (m, 2H), 3.00 (s, 3H), 1.57 (d, J = 6.8 Hz, 3H), 1.33 (d, J = 7.0 Hz, 3H), 0.84 (t, J = 6.9 Hz, 3H). Example 102: 4-(((*R)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide.
chloro-3-fluorophenyl)ethyl)amino)-2-fluorobenzoate. To a solution of 1-(2-chloro-3-fluorophenyl)ethanone (1.0 g, 5.79 mmol, 1.0 eq) and methyl 4-amino-2-fluorobenzoate (1.5 173 QB\184200.00081\93036187.2
VVID-747PC g, 8.69 mmol, 1.5 eq) in dimethoxyethane (10 mL, 0.58 M) was added TsOH ^H
2O (80 mg) and 4Å molecular sieves (800 mg) under N
2. The reaction was stirred at 120°C for 4 h under N
2. After cooling to rt, AcOH (10 drops) and NaBH
3CN (728 mg, 11.6 mmol, 2.0 eq) were added. The reaction was stirred at rt for 12 h under N
2 before being diluted with sat. aq. NaHCO
3 and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under vacuum. The resulting residue was purified by FCC on silica (PE:EtOAc = 10:1) to give methyl 4-((1-(2-chloro-3- fluorophenyl)ethyl)amino)-2-fluorobenzoate (340 mg, 18% yield) as a white solid.
1H NMR (400 MHz, CDCl
3) δ 7.71 (t, J = 8.5 Hz, 1H), 7.12 - 7.23 (m, 2H), 7.04 - 7.10 (m, 1H), 6.24 (dd, J = 8.8, 1.8 Hz, 1H), 6.01 - 6.13 (m, 1H), 4.94 (q, J = 6.6 Hz, 1H), 4.59 - 4.67 (m, 1H), 3.84 (s, 3H), 1.56 (d, J = 6.6 Hz, 3H). [00426] Step B: 4-((1-(2-Chloro-3-fluorophenyl)ethyl)amino)-2-fluorobenzoic acid. To a solution of methyl 4-((1-(2-chloro-3-fluorophenyl)ethyl)amino)-2-fluorobenzoate (200 mg, 0.610 mmol, 1.0 eq) in THF/MeOH/water (3:1:1 v/v, 1.5 mL, 0.41 M) was added LiOH ^H
2O (77 mg, 1.84 mmol, 3.0 eq). The reaction was stirred at 50°C for 5 h. After cooling to rt, the reaction was concentrated then diluted with water and neutralized with 1M HCl to pH ~5-6. The product was extracted with EtOAc and the combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated to give 4-((1-(2- chloro-3-fluorophenyl)ethyl)amino)-2-fluorobenzoic acid (150 mg, 78% yield) as a yellow solid.
1H NMR (400 MHz, CDCl
3) δ 7.68 (t, J = 8.6 Hz, 1H), 7.23 (s, 1H), 7.04 - 7.15 (m, 2H), 6.18 (dd, J = 8.8, 2.3 Hz, 1H), 5.96 - 6.07 (m, 1H), 4.88 (q, J = 6.7 Hz, 1H), 1.49 (d, J = 6.6 Hz, 3H). [00427] Step C: 4-(((*R)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. To a solution of 4-((1-(2-chloro-3-fluorophenyl)ethyl)amino)- 2-fluorobenzoic acid (150 mg, 0.481 mmol, 1.0 eq) in DCM (3.0 mL, 0.16 M) was added DIPEA (94 mg, 0.722 mmol, 1.5 eq) and HATU (274 mg, 0.722 mmol, 1.5 eq). The reaction was stirred at rt for 10 min before addition of (R,E)-4-(methylsulfonyl)but-3-en-2-amine, 4-methylbenzenesulfonic acid (Intermediate 1, 155 mg, 0.481 mmol, 1.0 eq). The reaction was stirred at rt for 2 h before being diluted with water and extracted with DCM. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under vacuum. The resulting residue was purified by prep-TLC (PE:EtOAc = 0:1) to give 4-(1-(2-chloro-3-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)benzamide (200 mg, 94% yield) as a colorless oil. Separation via SFC (Stationary phase: IH (3x25 cm); Mobile phase: 30% EtOH/CO
2; Rt = 5.15 min) provided the title compound. MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 7.92 (dd, J = 7.9, 3.1 Hz, 1H), 7.21 - 7.46 (m, 5H), 6.73 - 6.84 (m, 1H), 6.63 - 6.71 (m, 1H), 6.24 - 6.38 (m, 1H), 6.06 - 6.16 (m, 1H), 4.80 - 4.93 (m, 1H), 4.68 - 4.79 (m, 1H), 2.95 - 3.05 (m, 3H), 1.45 (d, J = 6.6 Hz, 3H), 1.15 - 1.29 (m, 3H). Example 103: 4-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-2-fluoro-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. 174 QB\184200.00081\93036187.2
VVID-747PC prepared by separation of 4-(1-(2-chloro-3-fluorophenyl)ethyl)amino)-2-
- but-3-en-2-yl)benzamide (Example 102, Step C) via SFC (Stationary phase: IH (3x25 cm); Mobile phase: 30% EtOH/CO
2; Rt = 7.17 min). MS (ESI): mass calcd. for C
20H
21ClF
2N
2O
3S, 442.1; m/z found, 443.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 7.91 (dd, J = 7.9, 3.3 Hz, 1H), 7.20 - 7.43 (m, 5H), 6.72 - 6.80 (m, 1H), 6.63 - 6.69 (m, 1H), 6.31 (dd, J = 8.6, 1.6 Hz, 1H), 6.04 - 6.19 (m, 1H), 4.80 - 4.92 (m, 1H), 4.68 - 4.78 (m, 1H), 2.98 (s, 3H), 1.45 (d, J = 6.7 Hz, 3H), 1.25 (d, J = 7.0 Hz, 3H). Example 104: 6-((1-(2-Chlorophenyl)ethyl)amino)-4-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)nicotinamide.
ethyl)-4-fluoro-5-iodopyridin-2-amine. A solution of 1-(2- chlorophenyl)ethan-1-amine (1.0 g, 6.43 mmol, 1.0 eq), 2,4-difluoro-5-iodopyridine (1.9 g, 7.71 mmol, 1.2 eq), and potassium carbonate (2.7 g, 19.3 mmol, 3.0 eq) in DMF (2.0 mL, 3.2 M) was stirred at 80°C for 16 h. After cooling to rt, the reaction mixture was poured into H
2O and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (0-25% EtOAc in PE) to provide N-(1-(2-chlorophenyl)ethyl)-4-fluoro-5-iodopyridin-2-amine (120 mg, 5% yield) as a colorless oil.
1H NMR (400 MHz, CDCl
3) δ 8.18 - 8.32 (m, 1H), 7.35 - 7.43 (m, 2H), 7.17 - 7.26 (m, 2H), 5.88 (br d, J = 10.0 Hz, 1H), 5.41 - 5.55 (m, 1H), 5.00 (quin, J = 6.6 Hz, 1H), 1.56 (d, J = 6.7 Hz, 3H). [00430] Step B: Methyl 6-((1-(2-chlorophenyl)ethyl)amino)-4-fluoronicotinate. To a solution of N-(1-(2- chlorophenyl)ethyl)-4-fluoro-5-iodopyridin-2-amine (120 mg, 0.319 mmol, 1.0 eq) in DMF/MeOH (1:1 v/v, 6.0 mL, 0.05 M) was added Pd(dppf)Cl
2 (40 mg) and TEA (64 mg, 0.637 mmol, 2.0 eq). The reaction was stirred at 70°C for 16 h under CO (50 psi). After cooling to rt, the mixture was concentrated in vacuo, poured into H
2O, and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by 175 QB\184200.00081\93036187.2
VVID-747PC prep-TLC (PE:EtOAc = 5:1) to provide methyl 6-((1-(2-chlorophenyl)ethyl)amino)-4-fluoronicotinate (50 mg, 51% yield) as a yellow oil. MS (ESI): mass calcd. for C
15H
14ClFN
2O
2, 308.1; m/z found, 309.1 [M+H]
+. [00431] Step C: 6-((1-(2-Chlorophenyl)ethyl)amino)-4-fluoronicotinic acid. A solution of methyl 6-((1- (2-chlorophenyl)ethyl)amino)-4-fluoronicotinate (40 mg, 0.130 mmol, 1.0 eq) and LiOH ^H
2O (16 mg, 0.389 mmol, 3.0 eq) in THF/water (3:1 v/v, 4.0 mL, 0.03 M) was stirred at 30°C for 16 h. The reaction mixture was poured into water and acidified with 1N HCl to pH ~3 before being extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure to give 6-((1-(2-chlorophenyl)ethyl)amino)-4-fluoronicotinic acid (38 mg, quant. yield) as a yellow oil. MS (ESI): mass calcd. for C
14H
12ClFN
2O
2, 294.1; m/z found, 295.1 [M+H]
+. [00432] Step D: 6-((1-(2-Chlorophenyl)ethyl)amino)-4-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)nicotinamide. A solution of 6-((1-(2-chlorophenyl)ethyl)amino)-4-fluoronicotinic acid (38 mg, 0.129 mmol, 1.0 eq), (R,E)-4-(methylsulfonyl)but-3-en-2-amine, 4-methylbenzenesulfonic acid (Intermediate 1, 46 mg, 0.142 mmol, 1.1 eq), HATU (74 mg, 0.193 mmol, 1.5 eq), and DIPEA (50 mg, 0.387 mmol, 3.0 eq) in DCM (1.0 mL, 0.13 M) was stirred at rt for 16 h. The reaction mixture was poured into H
2O and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by prep-TLC (PE:EtOAc = 0:1) to provide 6-((1-(2-chlorophenyl)ethyl)amino)-4-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)nicotinamide (51 mg, 90% yield) as a white solid. MS (ESI): mass calcd. for C
19H
21ClFN
3O
3S, 425.1; m/z found, 426.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.13 - 8.32 (m, 2H), 7.92 (br d, J = 7.3 Hz, (m, . 3-
(2- chlorophenyl)ethan-1-amine (350 mg, 2.25 mmol, 1.0 eq) in NMP (5.0 mL, 0.5 M) was added TEA (0.94 mL, 6.76 mmol, 3.0 eq) and 2,3,6-trifluoropyridine (300 mg, 2.25 mmol, 1.0 eq) under N
2. The reaction was stirred at 100°C for 3 h. After cooling to rt, the reaction mixture was poured into water and extracted with EtOAc. The combined organic layers were washed with water and brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (0-15% 176 QB\184200.00081\93036187.2
VVID-747PC EtOAc in PE) to give (S)-N-(1-(2-chlorophenyl)ethyl)-3,6-difluoropyridin-2-amine (320 mg, 53% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 7.34 - 7.44 (m, 2H), 7.15 - 7.26 (m, 3H), 6.01 (dt, J = 8.2, 2.6 Hz, 1H), 5.52 (quin, J = 6.7 Hz, 1H), 5.08 (br s, 1H), 1.59 (d, J = 6.8 Hz, 3H). [00434] Step B: (S)-5-Bromo-N-(1-(2-chlorophenyl)ethyl)-3,6-difluoropyridin-2-amine. To a solution of (S)-N-(1-(2-chlorophenyl)ethyl)-3,6-difluoropyridin-2-amine (320 mg, 1.19 mmol, 1.0 eq) in ACN (5.0 mL, 0.2 M) was added NBS (233 mg, 1.31 mmol, 1.1 eq) under N
2. The reaction was stirred at 80°C for 12 h. After cooling to rt, the reaction mixture was concentrated under reduced pressure. The resulting residue was purified by FCC on silica (0-10% EtOAc in PE) to give (S)-5-bromo-N-(1-(2- chlorophenyl)ethyl)-3,6-difluoropyridin-2-amine (370 mg, 89% yield) as a yellow oil. MS (ESI): mass calcd. for C
13H
10BrClF
2N
2, 346.0; m/z found, 347.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.34 - 7.42 (m, 3H), 7.17 - 7.26 (m, 2H), 5.47 (q, J = 7.0 Hz, 1H), 5.11 (br d, J = 4.2 Hz, 1H), 1.60 (d, J = 6.8 Hz, 3H). [00435] Step C: Methyl (S)-6-((1-(2-chlorophenyl)ethyl)amino)-2,5-difluoronicotinate. To a solution of (S)-5-bromo-N-(1-(2-chlorophenyl)ethyl)-3,6-difluoropyridin-2-amine (370 mg, 1.06 mmol, 1.0 eq) in DMF/MeOH (1:1 v/v, 6.0 mL, 0.17 M) was added TEA (0.30 mL, 2.13 mmol, 2.0 eq) and Pd(dppf)Cl
2 (77 mg, 0.106 mmol, 0.1 eq) under N
2. The reaction was stirred at 80°C for 12 h under CO (50 psi). After cooling to rt, the mixture was poured into water and extracted with EtOAc. The combined organic layers were washed with water and brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (0-15% EtOAc in PE) to give methyl (S)-6-((1-(2- chlorophenyl)ethyl)amino)-2,5-difluoronicotinate (300 mg, 86% yield) as a white solid.
1H NMR (400 MHz, CDCl
3) δ 7.78 (dd, J = 10.2, 7.0 Hz, 1H), 7.39 (ddd, J = 7.4, 4.1, 1.8 Hz, 2H), 7.24 (td, J = 7.3, 1.8 Hz, 2H), 5.49 - 5.66 (m, 2H), 3.86 (s, 3H), 1.64 (d, J = 6.8 Hz, 3H). [00436] Step D: (S)-6-((1-(2-Chlorophenyl)ethyl)amino)-2,5-difluoronicotinic acid. To a solution of (S)- methyl 6-((1-(2-chlorophenyl)ethyl)amino)-2,5-difluoronicotinate (100 mg, 0.306 mmol, 1.0 eq) in THF/MeOH/water (3:1:1 v/v, 5.0 mL, 0.06 M) was added LiOH ^H
2O (38 mg, 0.918 mmol, 3.0 eq). The reaction was stirred at rt for 12 h before being concentrated. The crude was diluted with water and neutralized with 1M HCl to pH ~5-6 then extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated to give (S)-6-((1-(2- chlorophenyl)ethyl)amino)-2,5-difluoronicotinic acid (90 mg, 94% yield) as a white solid.
1H NMR (400 MHz, CDCl
3) δ 7.80 (dd, J = 10.1, 6.9 Hz, 1H), 7.39 (ddd, J = 7.2, 5.0, 1.8 Hz, 2H), 7.21 - 7.27 (m, 2H), 5.57 - 5.66 (m, 2H), 1.65 (d, J = 6.4 Hz, 3H). [00437] Step E: 6-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2,5-difluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)nicotinamide. To a solution of (S)-6-((1-(2-chlorophenyl)ethyl)amino)-2,5-difluoronicotinic acid (90 mg, 0.287 mmol, 1.0 eq) in DCM (3.0 mL, 0.1 M) was added DIPEA (0.25 mL, 1.44 mmol, 5.0 eq), (R,E)-4-(methylsulfonyl)but-3-en-2-amine, 4-methylbenzenesulfonic acid (Intermediate 1, 93 mg, 0.287 mmol, 1.0 eq), and T
3P
® (518 mg, 0.719 mmol, 2.5 eq) under N
2. The reaction was stirred at rt for 2 h before being poured into H
2O and extracted with DCM. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under vacuum. The crude material was purified by 177 QB\184200.00081\93036187.2
VVID-747PC RP-HPLC (30-60% ACN in 10 mM aq. NH
4HCO
3) to give 6-(((S)-1-(2-chlorophenyl)ethyl)amino)-2,5- difluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)nicotinamide (73 mg, 56% yield) as a white solid. MS (ESI): mass calcd. for C
19H
20ClF
2N
3O
3S, 443.1; m/z found, 444.1 [M+H]
+.
1H NMR (400 MHz, DMSO- d
6) δ 8.09 - 8.25 (m, 2H), 7.75 (dd, J = 10.5, 7.2 Hz, 1H), 7.49 (dd, J = 7.7, 1.6 Hz, 1H), 7.41 (dd, J = 7.8, 1.3 Hz, 1H), 7.20 - 7.34 (m, 2H), 6.66 - 6.81 (m, 2H), 5.42 (quin, J = 7.0 Hz, 1H), 4.66 - 4.79 (m, 1H), 3.00 (s, 3H), 1.48 (d, J = 7.0 Hz, 3H), 1.25 (d, J = 7.1 Hz, 3H). Example 106: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-3,6-difluoro-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)picolinamide. ethyl)-2,5-difluoropyridin-3-amine. To a solution of 3-bromo-
2,5-difluoropyridine (800 mg, 4.12 mmol, 1.0 eq) in toluene (10 mL, 0.4 M) was added (S)-1-(2- chlorophenyl)ethanamine (706 mg, 4.53 mmol, 1.1 eq), RuPhos Pd G4 (175 mg, 0.206 mmol, 0.05 eq), and Cs
2CO
3 (2.7 g, 8.25 mmol, 2.0 eq). The reaction was stirred at 100°C for 16 h. After cooling to rt, the reaction mixture was concentrated then poured into H
2O and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under vacuum. The resulting residue was purified by prep-TLC (PE:EtOAc = 5:1) to give (S)-N-(1-(2-chlorophenyl)ethyl)-2,5- difluoropyridin-3-amine (300 mg, 27% yield) as a yellow solid. MS (ESI): mass calcd. for C
13H
11ClF
2N
2, 268.1; m/z found, 269.1 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.34 - 7.43 (m, 2H), 7.20 - 7.26 (m, 3H), 6.30 (td, J = 8.8, 2.6 Hz, 1H), 4.87 (q, J = 6.4 Hz, 1H), 4.55 (br s, 1H), 1.59 (d, J = 6.6 Hz, 3H). [00439] The title compound was prepared in a manner analogous to Example 105, Steps B-E using (S)-N- (1-(2-chlorophenyl)ethyl)-2,5-difluoropyridin-3-amine instead of (S)-N-(1-(2-chlorophenyl)ethyl)-3,6- difluoropyridin-2-amine in Step B. MS (ESI): mass calcd. for C
19H
20ClF
2N
3O
3S, 443.1; m/z found, 444.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.33 (d, J = 8.5 Hz, 1H), 7.42 - 7.60 (m, 3H), 7.25 - 7.39 (m, 2H), 6.62 - 6.82 (m, 2H), 6.40 (dd, J = 11.9, 7.8 Hz, 1H), 4.90 (q, J = 6.8 Hz, 1H), 4.67 - 4.79 (m, 1H), 2.98 (s, 3H), 1.52 (d, J = 6.8 Hz, 3H), 1.29 (d, J = 7.0 Hz, 3H). Example 107: 5-((*S)-1-(2-Chloro-3-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. 178 QB\184200.00081\93036187.2
VVID-747PC 3-fluorophenyl)ethoxy)pyrimidine-2-carboxylic acid. NaH (309 mg, 7.73
of 1-(2-chloro-3-fluorophenyl)ethanol (900 mg, 5.15 mmol, 1.0 eq) in THF (9.0 mL, 0.57 M) in portions at 0°C. The reaction was stirred at rt for 1 h before methyl 5- fluoropyrimidine-2-carboxylate (885 mg, 5.67 mmol, 1.1 eq) in THF (9.0 mL) was added slowly at 0°C. The reaction mixture was stirred at rt for 3 h before being poured into sat. aq. NH
4Cl. The aqueous phase was acidified with 1N HCl to pH ~5 and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure to give 5-(1-(2-chloro-3- fluorophenyl)ethoxy)pyrimidine-2-carboxylic acid (290 mg, 11% yield) as a yellow solid.
1H NMR (400 MHz, CDCl3) δ 8.44 (s, 2H), 7.23 - 7.27 (m, 1H), 7.18 - 7.22 (m, 1H), 7.14 (td, J = 8.3, 1.7 Hz, 1H), 5.90 (d, J = 6.4 Hz, 1H), 1.76 (d, J = 6.4 Hz, 3H). [00441] Step B: 5-((*S)-1-(2-Chloro-3-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. To a mixture of 5-(1-(2-chloro-3-fluorophenyl)ethoxy)pyrimidine-2- carboxylic acid (280 mg, 0.944 mmol, 1.0 eq) in DCM (5.0 mL, 0.19 M) was added DIPEA (366 mg, 2.83 mmol, 3.0 eq), HATU (538 mg, 1.46 mmol, 1.5 eq), and (R,E)-4-(methylsulfonyl)but-3-en-2-amine, 4- methylbenzenesulfonic acid (Intermediate 1, 334 mg, 1.04 mmol, 1.1 eq). The mixture was stirred at rt for 2 h before being diluted with H2O and extracted with DCM. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The crude product was purified by RP-HPLC (25-55% ACN in 10 mM aq. NH
4HCO
3) to give 5-(1-(2-chloro-3- fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (160 mg, 40% yield) as a yellow oil. Separation via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 7.57 min (second eluting product)) provided the title compound. MS (ESI): mass calcd. for C
18H
19ClFN
3O
4S, 427.1; m/z found, 428.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.94 (br d, J = 7.9 Hz, 1H), 8.54 (s, 2H), 7.41 (br d, J = 6.8 Hz, 3H), 6.76 - 6.84 (m, 1H), 6.68 - 6.74 (m, 1H), 6.08 (q, J = 5.9 Hz, 1H), 4.71 - 4.86 (m, 1H), 2.98 (s, 3H), 1.68 (br d, J = 6.3 Hz, 3H), 1.30 (br d, J = 7.1 Hz, 3H). Example 108: 5-((*S)-1-(2-Chloro-3,4-difluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. 179 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 107 using 1-(2-chloro-3,4-
of 1-(2-chloro-3-fluorophenyl)ethanol in Step A. Separation of 5-(1-(2- chloro-3,4-difluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step B) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 7.40 min (second eluting product)) provided the title compound. MS (ESI): mass calcd. for C
18H
18ClF
2N
3O
4S, 445.1; m/z found, 446.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.89 – 9.01 (m, 1H), 8.51 - 8.61 (m, 2H), 7.36 - 7.58 (m, 2H), 6.65 - 6.88 (m, 2H), 5.99 – 6.10 (m, 1H), 4.71 - 4.86 (m, 1H), 2.93 – 3.06 (m, 3H), 1.61 - 1.71 (m, 3H), 1.26 - 1.38 (m, 3H). Example 109: 5-(1-(2,3-Dichlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine- 2-carboxamide.
was prepared in a manner analogous to Example 107 using 1-(2,3- dichlorophenyl)ethan-1-ol instead of 1-(2-chloro-3-fluorophenyl)ethanol in Step A. MS (ESI): mass calcd. for C
18H
19Cl
2N
3O
4S, 443.1; m/z found, 444.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.37 (s, 2H), 7.85 (d, J = 8.2 Hz, 1H), 7.45 (ddd, J = 7.9, 3.1, 1.6 Hz, 1H), 7.33 (dd, J = 8.1, 1.6 Hz, 1H), 7.23 (td, J = 7.9, 1.1 Hz, 1H), 6.92 (ddd, J = 15.1, 4.5, 1.6 Hz, 1H), 6.49 (dt, J = 15.2, 1.4 Hz, 1H), 5.90 (qd, J = 6.3, 3.4 Hz, 1H), 5.06 – 4.91 (m, 1H), 2.93 (s, 3H), 1.75 (d, J = 6.3 Hz, 3H), 1.45 (d, J = 7.0 Hz, 3H). Example 110: 5-((S)-1-(2-Chlorophenyl)ethoxy)-3-fluoro-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)picolinamide. 180 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 107 using (S)-1-(2-
of 1-(2-chloro-3-fluorophenyl)ethanol and methyl 3,5-difluoropicolinate instead of methyl 5-fluoropyrimidine-2-carboxylate in Step A. MS (ESI): mass calcd. for C
19H
20ClFN
2O
4S, 426.1; m/z found, 427.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.04 (dd, J = 2.3, 0.8 Hz, 1H), 7.71 (d, J = 8.3 Hz, 1H), 7.48 – 7.35 (m, 2H), 7.32 – 7.21 (m, 2H), 6.99 – 6.79 (m, 2H), 6.50 (dd, J = 15.1, 1.7 Hz, 1H), 5.79 (q, J = 6.3 Hz, 1H), 5.01 – 4.87 (m, 1H), 2.94 (s, 3H), 1.72 (d, J = 6.3 Hz, 3H), 1.44 (d, J = 7.1 Hz, 3H). Example 111: 5-((*R)-1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide.
was prepared in a manner analogous to Example 107 using 1-(2- chlorophenyl)ethan-1-ol instead of 1-(2-chloro-3-fluorophenyl)ethanol and methyl 5-chloro-4- (trifluoromethyl)pyrimidine-2-carboxylate (Intermediate 17) instead of methyl 5-fluoropyrimidine-2- carboxylate in Step A. Separation of 5-(1-(2-chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)-4-(trifluoromethyl)pyrimidine-2-carboxamide (Step B) via SFC (Stationary phase: AD (3x25 cm); Mobile phase: 20% MeOH/CO
2; Rt = 4.16 min) provided the title compound. MS (ESI): mass calcd. for C
19H
19ClF
3N
3O
4S, 477.1; m/z found, 478.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.01 (d, J = 8.4 Hz, 1H), 8.71 (s, 1H), 7.58 - 7.52 (m, 1H), 7.50 - 7.45 (m, 1H), 7.44 - 7.36 (m, 2H), 6.86 - 6.78 (m, 1H), 6.77 - 6.71 (m, 1H), 6.30 (q, J = 6.1 Hz, 1H), 4.90 - 4.74 (m, 1H), 2.99 (s, 3H), 1.69 (d, J = 6.3 Hz, 3H), 1.32 (d, J = 7.0 Hz, 3H). Example 112: 5-((*S)-1-(2-Chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide. 181 QB\184200.00081\93036187.2
VVID-747PC was prepared in a manner analogous to Example 107 using 1-(2-
of 1-(2-chloro-3-fluorophenyl)ethanol and methyl 5-chloro-4- (trifluoromethyl)pyrimidine-2-carboxylate (Intermediate 17) instead of methyl 5-fluoropyrimidine-2- carboxylate in Step A. Separation of 5-(1-(2-chlorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)-4-(trifluoromethyl)pyrimidine-2-carboxamide (Step B) via SFC (Stationary phase: AD (3x25 cm); Mobile phase: 20% MeOH/CO
2; Rt = 6.33 min) provided the title compound. MS (ESI): mass calcd. for C
19H
19ClF
3N
3O
4S, 477.1; m/z found, 478.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.02 (br d, J = 8.4 Hz, 1H), 8.69 (s, 1H), 7.55 (dd, J = 1.8, 7.3 Hz, 1H), 7.51 - 7.45 (m, 1H), 7.44 - 7.36 (m, 2H), 6.82 - 6.76 (m, 1H), 6.76 - 6.71 (m, 1H), 6.30 (q, J = 6.2 Hz, 1H), 4.87 - 4.73 (m, 1H), 2.97 (s, 3H), 1.69 (d, J = 6.3 Hz, 3H), 1.33 (d, J = 7.1 Hz, 3H). Example 113: 5-((*R)-1-(2-Chloro-3-fluorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 107 using 1-(2-chloro-3- fluorophenyl)propan-1-ol (Intermediate 9) instead of 1-(2-chloro-3-fluorophenyl)ethanol in Step A. Separation of 5-(1-(2-chloro-3-fluorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Step B) via SFC (Stationary phase: IH (3x25 cm); Mobile phase: 30% EtOH/CO
2 with 0.1% NH
4OH; Rt = 4.50 min) provided the title compound. MS (ESI): mass calcd. for C
19H
21ClFN
3O
4S, 441.1; m/z found, 442.1[M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.5 Hz, 1H), 8.51 (s, 2H), 7.44 - 7.37 (m, 2H), 7.36 - 7.30 (m, 1H), 6.82 - 6.75 (m, 1H), 6.74 - 6.68 (m, 1H), 5.88 (t, J = 6.3 Hz, 1H), 4.84 - 4.72 (m, 1H), 3.00 - 2.96 (m, 3H), 2.12 - 1.93 (m, 2H), 1.30 (d, J = 7.0 Hz, 3H), 1.00 (t, J = 7.4 Hz, 3H). Example 114: 5-((*S)-1-(2-Chloro-3-fluorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. 182 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 107 using 1-(2-chloro-3-
9) instead of 1-(2-chloro-3-fluorophenyl)ethanol in Step A. Separation of 5-(1-(2-chloro-3-fluorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Step B) via SFC (Stationary phase: IH (3x25 cm); Mobile phase: 30% EtOH/CO
2 with 0.1% NH
4OH; Rt = 5.82 min) provided the title compound. MS (ESI): mass calcd. for C
19H
21ClFN
3O
4S, 441.1; m/z found, 442.1[M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.4 Hz, 1H), 8.51 (s, 2H), 7.37 (s, 2H), 7.36 - 7.31 (m, 1H), 6.82 - 6.74 (m, 1H), 6.72 (d, J = 0.9 Hz, 1H), 5.88 (t, J = 6.4 Hz, 1H), 4.85 - 4.69 (m, 1H), 2.97 (s, 3H), 2.11 - 1.95 (m, 2H), 1.30 (d, J = 7.0 Hz, 3H), 1.00 (t, J = 7.4 Hz, 3H). Example 115: 4-((S)-1-(2-Chlorophenyl)ethoxy)-3-(1H-imidazol-2-yl)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide.
(2-chlorophenyl)ethoxy)-3-formylbenzoate. To a solution of (S)-1-(2- chlorophenyl)ethanol (300 mg, 1.91 mmol, 1.0 eq) in DMSO (5.0 mL, 0.38 M) was added methyl 4-fluoro- 3-formyl-benzoate (349 mg, 1.91 mmol, 1.0 eq) and cesium carbonate (1.2 g, 3.83 mmol, 2.0 eq). The reaction was stirred at 60℃ for 4 h. After cooling to rt, the reaction mixture was poured into water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (0-20% EtOAc in PE) to give methyl (S)-4-(1-(2-chlorophenyl)ethoxy)-3-formylbenzoate (370 mg, 61% yield) as a yellow oil. [00450] Step B: Methyl (S)-4-(1-(2-chlorophenyl)ethoxy)-3-(1H-imidazol-2-yl)benzoate. To a solution of methyl (S)-4-(1-(2-chlorophenyl)ethoxy)-3-formylbenzoate (370 mg, 1.16 mmol, 1.0 eq) in ethanol (5.0 mL, 0.23 M) was added glyoxal (135 mg, 2.32 mmol, 2.0 eq) and ammonium acetate (894 mg, 11.6 mmol, 10 eq). The reaction was stirred at 20℃ for 16 h before being concentrated under reduced pressure. The resulting residue was poured into water and extracted with EtOAc. The combined organic layers were 183 QB\184200.00081\93036187.2
VVID-747PC washed with brine, dried over Na
2SO
4, and filtered. The filtrate was concentrated under reduced pressure to give methyl (S)-4-(1-(2-chlorophenyl)ethoxy)-3-(1H-imidazol-2-yl)benzoate (410 mg, quant. yield) as a yellow oil, which was used in the next step without purification. [00451] Step C: (S)-4-(1-(2-Chlorophenyl)ethoxy)-3-(1H-imidazol-2-yl)benzoic acid. To a solution of methyl (S)-4-(1-(2-chlorophenyl)ethoxy)-3-(1H-imidazol-2-yl)benzoate (410 mg, 1.15 mmol, 1.0 eq) in THF/water (3:1 v/v, 4.0 mL, 0.29 M) was added LiOH
.H2O (72 mg, 1.72 mmol, 1.5 eq). The mixture was stirred at 20℃ for 16 h before being poured into water and adjusted to pH ~4 with HCl. The mixture was extracted with EtOAc and the combined organic layers were washed with brine, dried over Na
2SO
4, and filtered. The filtrate was concentrated under reduced pressure to give (S)-4-(1-(2-chlorophenyl)ethoxy)-3- (1H-imidazol-2-yl)benzoic acid (390 mg, quant. yield) as a yellow oil, which was used in the next step without purification. MS (ESI): mass calcd. for C
18H
15ClN
2O
3, 342.1; m/z found, 343.1 [M+H]
+. [00452] Step D: 4-((S)-1-(2-Chlorophenyl)ethoxy)-3-(1H-imidazol-2-yl)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)benzamide. To a solution of (S)-4-(1-(2-chlorophenyl)ethoxy)-3-(1H-imidazol-2-yl)benzoic acid (240 mg, 0.700 mmol, 1.0 eq) in DCM (5.0 mL, 0.14 M) was added (R,E)-4-(methylsulfonyl)but-3- en-2-amine, 4-methylbenzenesulfonic acid (Intermediate 1, 270 mg, 0.840 mmol, 1.2 eq), T
3P
® (757 mg, 1.05 mmol, 1.5 eq), and DIPEA (0.37 mL, 2.10 mmol, 3.0 eq). The mixture was stirred at 20℃ for 1 h before being poured into water and extracted with DCM. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by RP-HPLC (15-45% ACN in 0.1% aq. TFA) to give 4-((S)-1-(2-chlorophenyl)ethoxy)-3-(1H- imidazol-2-yl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)benzamide (66 mg, 20% yield) as a white solid. MS (ESI): mass calcd. for C
23H
24ClN
3O
4S, 473.1; m/z found, 474.1 [M+H]
+.
1H NMR (400 MHz, DMSO- d
6) δ 14.23 - 14.62 (m, 1H), 8.60 (d, J = 7.9 Hz, 1H), 8.31 (d, J = 2.1 Hz, 1H), 8.02 (dd, J = 8.8, 2.0 Hz, 1H), 7.88 (s, 2H), 7.45 - 7.59 (m, 2H), 7.25 - 7.39 (m, 2H), 6.92 (d, J = 8.8 Hz, 1H), 6.68 - 6.85 (m, 2H), 5.91 (q, J = 6.21 Hz, 1H), 4.81 (td, J = 7.4, 3.3 Hz, 1H), 3.00 (s, 3H), 1.68 (d, J = 6.3 Hz, 3H), 1.30 (d, J = 7.0 Hz, 3H). Example 116: 5-((S)-1-(2-Chlorophenyl)ethoxy)-4-methoxy-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 1 using methyl 5-bromo-4- methylpicolinate instead of methyl 4-bromo-2-fluorobenzoate and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
20H
24ClN
3O
3S, 421.1; m/z found, 422.0 [M+H]
+.
1H NMR (400 184 QB\184200.00081\93036187.2
VVID-747PC MHz, CDCl
3) δ 7.84 (s, 1H), 7.75 (d, J = 8.5 Hz, 1H), 7.45 (s, 1H), 7.43 – 7.35 (m, 1H), 7.34 – 7.27 (m, 1H), 7.25 – 7.11 (m, 2H), 6.91 (dd, J = 15.1, 4.4 Hz, 1H), 6.45 (dd, J = 15.1, 1.8 Hz, 1H), 5.13 – 5.02 (m, 1H), 4.96 – 4.85 (m, 1H), 4.27 (d, J = 5.5 Hz, 1H), 2.90 (s, 3H), 2.29 (d, J = 0.8 Hz, 3H), 1.61 (d, J = 6.7 Hz, 3H), 1.36 (d, J = 7.1 Hz, 3H). Example 117: 8-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)imidazo[1,2-a]pyridine-5-carboxamide. prepared in a manner analogous to Example 1 using methyl 8-
bromoimidazo[1,5-a]pyridine-5-carboxylate instead of methyl 4-bromo-2-fluorobenzoate and RuPhos Pd G4 instead of SPhos Pd G4 in Step A. MS (ESI): mass calcd. for C
21H
23ClN
4O
3S, 446.1; m/z found, 447.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.64 (q, J = 1.3 Hz, 1H), 7.55 (dd, J = 2.3, 1.1 Hz, 1H), 7.41 – 7.33 (m, 2H), 7.21 – 7.11 (m, 2H), 7.07 (d, J = 8.0 Hz, 1H), 7.00 – 6.72 (m, 1H), 6.50 (dt, J = 15.2, 1.5 Hz, 1H), 6.45 – 6.24 (m, 1H), 6.18 (d, J = 6.8 Hz, 1H), 5.75 – 5.61 (m, 1H), 5.16 – 5.04 (m, 1H), 4.99 – 4.87 (m, 1H), 2.94 – 2.80 (m, 3H), 1.66 – 1.56 (m, 3H), 1.40 – 1.32 (m, 3H). Example 118: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N
2-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2,4-dicarboxamide. O O N
in a manner analogous to Example 1, Steps B-C, using ethyl (S)- 5-((1-(2-chlorophenyl)ethyl)amino)-4-cyanopyrimidine-2-carboxylate (Intermediate 55) instead of methyl (S)-4-((1-(2-chlorophenyl)ethyl)amino)benzoate in Step B. MS (ESI): mass calcd. for C
19H
22ClN
5O
4S, 451.1; m/z found, 452.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.26 - 9.00 (m, 3H), 8.07 (s, 2H), 7.54 - 7.48 (m, 1H), 7.44 - 7.37 (m, 1H), 7.35 - 7.24 (m, 2H), 6.86 - 6.67 (m, 2H), 5.17 (br t, J = 6.7 Hz, 1H), 4.92 - 4.79 (m, 1H), 2.98 (s, 3H), 1.56 (d, J = 6.6 Hz, 3H), 1.35 (d, J = 7.1 Hz, 3H). 185 QB\184200.00081\93036187.2
VVID-747PC Example 119: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-4-cyano-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. O N N N
(R) S prepared in a manner analogous to Example 1, Steps B-C, using ethyl (S)-
-4-cyanopyrimidine-2-carboxylate (Intermediate 55) at 0 °C instead of methyl (S)-4-((1-(2-chlorophenyl)ethyl)amino)benzoate in Step B. MS (ESI): mass calcd. for C
19H
20ClN
5O
3S, 433.1; m/z found, 434.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.91 - 8.81 (m, 1H), 8.17 (s, 1H), 8.07 - 8.00 (m, 1H), 7.60 - 7.46 (m, 2H), 7.42 - 7.27 (m, 2H), 6.83 - 6.64 (m, 2H), 5.28 - 5.13 (m, 1H), 4.83 - 4.67 (m, 1H), 2.98 (s, 3H), 1.58 (br d, J = 6.8 Hz, 3H), 1.28 (br d, J = 7.0 Hz, 3H). Example 120: 5-((S)-1-(2-Chlorophenyl)ethoxy)-4-methoxy-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
was prepared in a manner analogous to Example 59 using (S)-1-(2- chlorophenyl)ethan-1-ol instead of (S)-1-(2-chlorophenyl)ethan-1-amine, ethyl 5-fluoro-4- methoxypyrimidine-2-carboxylate (Intermediate 54) instead of methyl 5-chloropyrazine-2-carboxylate, and NaH instead of DIPEA in Step A. MS (ESI): mass calcd. for C
19H
22ClN
3O
5S, 439.1; m/z found, 440.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.82 (s, 1H), 7.73 (d, J = 8.3 Hz, 1H), 7.51 – 7.36 (m, 2H), 7.34 – 7.21 (m, 2H), 6.93 (dd, J = 15.1, 4.6 Hz, 1H), 6.50 (dd, J = 15.1, 1.7 Hz, 1H), 5.88 (q, J = 6.4 Hz, 1H), 5.01 – 4.89 (m, 1H), 4.21 (s, 3H), 2.94 (s, 3H), 1.76 (d, J = 6.4 Hz, 3H), 1.44 (d, J = 7.1 Hz, 3H). Example 121: 5-(((*S)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrazine-2-carboxamide. 186 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 59 using (2-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine and DMSO instead of 1,4-dioxane in Step A. Separation of 5-(((2-chlorophenyl)(cyclopropyl)methyl)amino)-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (Step C) via SFC (Stationary phase: A6 (3x25 cm); Mobile phase: 40% MeOH/CO
2 with 0.1% diethylamine; Rt = 3.58 min) provided the title compound. MS (ESI): mass calcd. for C
20H
23ClN
4O
3S, 434.1; m/z found, 435.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.75 (s, 1H), 7.59 (s, 1H), 7.50 (d, J = 8.4 Hz, 1H), 7.46 – 7.35 (m, 2H), 7.29 – 7.18 (m, 2H), 6.92 (dd, J = 15.1, 4.4 Hz, 1H), 6.47 (dd, J = 15.1, 1.7 Hz, 1H), 6.09 (d, J = 6.4 Hz, 1H), 5.01 – 4.88 (m, 1H), 4.85 (dd, J = 8.1, 6.2 Hz, 1H), 2.92 (s, 3H), 1.46 – 1.32 (m, 4H), 0.69 (tdd, J = 8.1, 6.3, 4.5 Hz, 1H), 0.62 – 0.49 (m, 2H), 0.42 (ddt, J = 9.3, 6.1, 4.7 Hz, 1H). Example 122: 5-(((*R)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrazine-2-carboxamide.
was isolated from the SFC separation of 5-(((2- chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2- carboxamide (Example 121, Step C, Rt = 4.40 min). MS (ESI): mass calcd. for C
20H
23ClN
4O
3S, 434.1; m/z found, 435.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.74 (s, 1H), 7.60 (s, 1H), 7.51 (d, J = 8.4 Hz, 1H), 7.47 – 7.33 (m, 2H), 7.23 (tt, J = 7.4, 5.4 Hz, 2H), 6.93 (dd, J = 15.1, 4.4 Hz, 1H), 6.48 (dd, J = 15.1, 1.7 Hz, 1H), 6.10 (d, J = 6.3 Hz, 1H), 4.95 (h, J = 6.8 Hz, 1H), 4.88 – 4.79 (m, 1H), 2.92 (s, 3H), 1.40 (d, J = 7.1 Hz, 4H), 0.75 – 0.62 (m, 1H), 0.55 (dt, J = 10.8, 6.5 Hz, 2H), 0.48 – 0.33 (m, 1H). Example 123: 5-(((*S)-1-(3-Fluoro-2-methylphenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrazine-2-carboxamide. 187 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 59 using 1-(3-fluoro-2-
of (S)-1-(2-chlorophenyl)ethan-1-amine and DMSO instead of 1,4- dioxane in Step A. Separation of 5-((1-(3-fluoro-2-methylphenyl)ethyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (Step C) via SFC (Stationary phase: A6 (3x25 cm); Mobile phase: 45% EtOH/CO
2; Rt = 2.39 min) provided the title compound. MS (ESI): mass calcd. for C
19H
23FN
4O
3S, 406.1; m/z found, 407.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.74 (s, 1H), 7.60 (s, 1H), 7.50 (d, J = 8.2 Hz, 1H), 7.12 (q, J = 3.2 Hz, 2H), 6.92 (ddd, J = 13.6, 6.6, 4.2 Hz, 2H), 6.46 (d, J = 14.9 Hz, 1H), 5.87 – 5.56 (m, 1H), 5.24 (q, J = 6.5 Hz, 1H), 5.01 – 4.81 (m, 1H), 2.91 (s, 3H), 2.31 (d, J = 2.1 Hz, 3H), 1.56 (d, J = 6.7 Hz, 3H), 1.40 (d, J = 7.0 Hz, 3H). Example 124: 5-(((*R)-1-(3-Fluoro-2-methylphenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrazine-2-carboxamide.
was isolated from the SFC separation of 5-(((2- chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2- carboxamide (Example 123, Step C, Rt = 3.57 min). MS (ESI): mass calcd. for C
19H
23FN
4O
3S, 406.1; m/z found, 407.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.72 (s, 1H), 7.71 – 7.39 (m, 2H), 7.11 (t, J = 5.4 Hz, 2H), 7.01 – 6.79 (m, 2H), 6.46 (d, J = 15.0 Hz, 1H), 5.82 (d, J = 6.6 Hz, 1H), 5.23 (p, J = 6.6 Hz, 1H), 5.00 – 4.87 (m, 1H), 2.90 (s, 3H), 2.31 (d, J = 2.1 Hz, 3H), 1.54 (s, 3H), 1.39 (d, J = 6.7 Hz, 3H). Example 125: 5-(((*S)-(2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 188 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 59 using (2-chloro-3-
methanamine (Intermediate 36) instead of (S)-1-(2- chlorophenyl)ethan-1-amine, methyl 5-fluoropyrimidine-2-carboxylate instead of methyl 5- chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-(((2-chloro-3- fluorophenyl)(1-fluorocyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine- 2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2, Rt = 4.33 min) provided the title compound. MS (ESI): mass calcd. for C
20H
21ClF
2N
4O
3S, 470.1; m/z found, 471.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.70 (d, J = 8.6 Hz, 1H), 8.15 (s, 2H), 7.82 (d, J = 7.6 Hz, 1H), 7.45 - 7.35 (m, 3H), 6.82 - 6.73 (m, 1H), 6.72 - 6.63 (m, 1H), 5.23 - 5.11 (m, 1H), 4.83 - 4.68 (m, 1H), 2.97 (s, 3H), 1.29 (d, J = 7.0 Hz, 3H), 1.25 - 1.06 (m, 2H), 0.98 - 0.87 (m, 2H). Example 126: 5-(((*R)-(2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
from the SFC separation of 5-(((2-chloro-3-fluorophenyl)(1- fluorocyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 125, Step C, Rt = 6.74 min). MS (ESI): mass calcd. for C
20H
21ClF
2N
4O
3S, 470.1; m/z found, 471.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.70 (d, J = 8.5 Hz, 1H), 8.15 (s, 2H), 7.82 (d, J = 7.5 Hz, 1H), 7.45 - 7.34 (m, 3H), 6.83 - 6.74 (m, 1H), 6.72 - 6.62 (m, 1H), 5.22 - 5.12 (m, 1H), 4.76 (td, J = 6.9, 5.9 Hz, 1H), 2.98 (s, 3H), 1.29 (d, J = 7.0 Hz, 3H), 1.25 - 1.04 (m, 2H), 0.98 - 0.87 (m, 2H). Example 127: 5-(((*S)-(2-Chloro-3-fluorophenyl)((*R)-2,2-difluorocyclopropyl)methyl)amino)-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide. 189 QB\184200.00081\93036187.2
VVID-747PC in a manner analogous to Example 59 using (2-chloro-3-
methanamine (Intermediate 37) instead of (S)-1-(2- chlorophenyl)ethan-1-amine and DMSO instead of 1,4-dioxane in Step A. Separation of methyl 5-(((2- chloro-3-fluorophenyl)(2,2-difluorocyclopropyl)methyl)amino)pyrazine-2-carboxylate (Step A) via SFC (Stationary phase: OZ (3x25 cm); Mobile phase: 40% MeOH/CO
2 with 0.1% NH
4OH; Rt = 6.90 min) provided methyl 5-(((*S)-(2-chloro-3-fluorophenyl)((*R)-2,2- difluorocyclopropyl)methyl)amino)pyrazine-2-carboxylate, which was elaborated to the title compound in a manner analogous to Example 59, Steps B-C. MS (ESI): mass calcd. for C
20H
20ClF
3N
4O
3S, 488.1; m/z found, 489.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.55 (d, J = 8.6 Hz, 1H), 8.50 (s, 1H), 8.47 (br d, J = 7.6 Hz, 1H), 8.01 (s, 1H), 7.55 - 7.51 (m, 1H), 7.46 (td, J = 7.9, 5.6 Hz, 1H), 7.41 - 7.34 (m, 1H), 6.82 - 6.76 (m, 1H), 6.71 - 6.64 (m, 1H), 5.38 (br t, J = 8.2 Hz, 1H), 4.85 - 4.76 (m, 1H), 2.98 (s, 3H), 2.47 - 2.38 (m, 1H), 1.87 - 1.77 (m, 1H), 1.73 - 1.64 (m, 1H), 1.31 (d, J = 7.0 Hz, 3H). Example 128: 5-(((*R)-(2-Chloro-3-fluorophenyl)((*S)-2,2-difluorocyclopropyl)methyl)amino)-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide.
in a manner analogous to Example 59 using methyl 5-(((*R)-(2- chloro-3-fluorophenyl)((*S)-2,2-difluorocyclopropyl)methyl)amino)pyrazine-2-carboxylate which was isolated from the SFC separation of methyl 5-(((2-chloro-3-fluorophenyl)(2,2- difluorocyclopropyl)methyl)amino)pyrazine-2-carboxylate (Example 127, Step A, Rt = 3.75 min). This intermediate was elaborated to the title compound in a manner analogous to Example 59, Steps B-C. MS (ESI): mass calcd. for C
20H
20ClF
3N
4O
3S, 488.1; m/z found, 489.0 [M+H]
+.
1H NMR (400 MHz, DMSO- d
6) δ 8.56 (d, J = 8.6 Hz, 1H), 8.51 (d, J = 1.1 Hz, 1H), 8.46 (br d, J = 7.8 Hz, 1H), 8.00 (d, J = 0.9 Hz, 1H), 7.56 - 7.51 (m, 1H), 7.46 (td, J = 8.0, 5.6 Hz, 1H), 7.41 - 7.34 (m, 1H), 6.84 - 6.76 (m, 1H), 6.73 - 190 QB\184200.00081\93036187.2
VVID-747PC 6.66 (m, 1H), 5.40 (br t, J = 8.5 Hz, 1H), 4.84 - 4.74 (m, 1H), 2.99 (s, 3H), 2.48 - 2.37 (m, 1H), 1.86 - 1.75 (m, 1H), 1.73 - 1.63 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H). Example 129: 5-(((*S)-(2-Chloro-3-fluorophenyl)((*S)-2,2-difluorocyclopropyl)methyl)amino)-N-((R,E)- 4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide. in a manner analogous to Example 59 using (2-chloro-3-
methanamine (Intermediate 37) instead of (S)-1-(2- chlorophenyl)ethan-1-amine and DMSO instead of 1,4-dioxane in Step A. Separation of methyl 5-(((2- chloro-3-fluorophenyl)(2,2-difluorocyclopropyl)methyl)amino)pyrazine-2-carboxylate (Step A) via SFC (Stationary phase: OJ (3x25 cm); Mobile phase: 20% MeOH/CO2 with 0.1% NH4OH; Rt = 5.84 min) provided methyl 5-(((*S)-(2-chloro-3-fluorophenyl)((*S)-2,2- difluorocyclopropyl)methyl)amino)pyrazine-2-carboxylate, which was elaborated to the title compound in a manner analogous to Example 59, Steps B-C. MS (ESI): mass calcd. for C
20H
20ClF
3N
4O
3S, 488.1; m/z found, 489.2 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.73 (br d, J = 7.3 Hz, 1H), 8.53 (d, J = 8.6 Hz, 1H), 8.44 (d, J = 1.2 Hz, 1H), 8.02 (s, 1H), 7.51 - 7.47 (m, 1H), 7.41 (td, J = 7.9, 5.6 Hz, 1H), 7.36 - 7.28 (m, 1H), 6.82 - 6.74 (m, 1H), 6.69 - 6.62 (m, 1H), 5.32 - 5.21 (m, 1H), 4.84 - 4.73 (m, 1H), 2.97 (s, 3H), 2.41 - 2.28 (m, 1H), 1.77 - 1.65 (m, 1H), 1.54 - 1.42 (m, 1H), 1.31 (d, J = 7.0 Hz, 3H). Example 130: 5-(((*R)-(2-chloro-3-fluorophenyl)((*R)-2,2-difluorocyclopropyl)methyl)amino)-N-((R,E)- 4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide.
in a manner analogous to Example 59 using methyl 5-(((*R)-(2- chloro-3-fluorophenyl)((*R)-2,2-difluorocyclopropyl)methyl)amino)pyrazine-2-carboxylate which was isolated from the SFC separation of methyl 5-(((2-chloro-3-fluorophenyl)(2,2- difluorocyclopropyl)methyl)amino)pyrazine-2-carboxylate (Example 129, Step A, Rt = 4.34 min). This intermediate was elaborated to the title compound in a manner analogous to Example 59, Steps B-C. MS 191 QB\184200.00081\93036187.2
VVID-747PC (ESI): mass calcd. for C
20H
20ClF
3N
4O
3S, 488.1; m/z found, 489.1 [M+H]
+.
1H NMR (400 MHz, DMSO- d
6) δ 8.72 (br d, J = 7.4 Hz, 1H), 8.54 (d, J = 8.6 Hz, 1H), 8.45 (s, 1H), 8.01 (s, 1H), 7.53 - 7.48 (m, 1H), 7.46 - 7.39 (m, 1H), 7.37 - 7.30 (m, 1H), 6.83 - 6.76 (m, 1H), 6.72 - 6.64 (m, 1H), 5.33 - 5.21 (m, 1H), 4.86 - 4.72 (m, 1H), 2.98 (s, 3H), 2.41 - 2.28 (m, 1H), 1.76 - 1.65 (m, 1H), 1.54 - 1.42 (m, 1H), 1.30 (d, J = 7.0 Hz, 3 H). Example 131: 5-(((*S)-1-(2-Chloro-3-fluorophenyl)-2-methylpropyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide. O N N
(R) S prepared in a manner analogous to Example 59 using 1-(2-chloro-3-
- (Intermediate 22) instead of (S)-1-(2-chlorophenyl)ethan-1-amine and DMSO instead of 1,4-dioxane in Step A. Separation of 5-((1-(2-chloro-3-fluorophenyl)-2- methylpropyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (Step C) via SFC (Stationary phase: OH (3x25 cm); Mobile phase: 35% IPA/CO
2, Rt = 13.0 min) provided the title compound. MS (ESI): mass calcd. for C
20H
24ClFN
4O
3S, 454.1; m/z found, 455.2 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.52 (d, J = 8.6 Hz, 1H), 8.44 (s, 1H), 8.21 (d, J = 8.4 Hz, 1H), 8.07 (br s, 1H), 7.36 (dd, J = 7.9, 5.5 Hz, 1H), 7.32 - 7.21 (m, 2H), 6.87 - 6.73 (m, 1H), 6.72 - 6.60 (m, 1H), 5.28 (br s, 1H), 4.78 (dt, J = 6.9, 5.8 Hz, 1H), 2.99 (s, 3H), 2.14 (qd, J = 13.7, 6.8 Hz, 1H), 1.29 (d, J = 7.0 Hz, 3H), 0.99 (d, J = 6.8 Hz, 3H), 0.87 (d, J = 6.9 Hz, 3H). Example 132: 5-(((*R)-1-(2-Chloro-3-fluorophenyl)-2-methylpropyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide.
isolated from the SFC separation of 5-((1-(2-chloro-3-fluorophenyl)-2- methylpropyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (Example 131, Step C, Rt = 10.2 min). MS (ESI): mass calcd. for C
20H
24ClFN
4O
3S, 454.1; m/z found, 455.2 [M+H]
+.
1H 192 QB\184200.00081\93036187.2
VVID-747PC NMR (400 MHz, DMSO-d
6) δ 8.52 (d, J = 8.5 Hz, 1H), 8.43 (s, 1H), 8.21 (d, J = 8.4 Hz, 1H), 8.08 (br s, 1H), 7.43 - 7.33 (m, 1H), 7.32 - 7.21 (m, 2H), 6.86 - 6.72 (m, 1H), 6.71 - 6.59 (m, 1H), 5.26 (br s, 1H), 4.87 - 4.71 (m, 1H), 2.97 (s, 3H), 2.23 - 2.02 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H), 1.08 - 0.95 (m, 3H), 0.88 (d, J = 6.9 Hz, 3H). Example 133: 5-(((*S)-1-(2-Chloro-3-fluorophenyl)propyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrazine-2-carboxamide. prepared in a manner analogous to Example 59 using 1-(2-chloro-3-
23) instead of (S)-1-(2-chlorophenyl)ethan-1-amine and DMSO instead of 1,4-dioxane in Step A. Separation of 5-((1-(2-chloro-3-fluorophenyl)propyl)amino)-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (Step C) via SFC (Stationary phase: OZ (3x25 cm); Mobile phase: 30% MeOH/CO
2, Rt = 2.14 min) provided the title compound. MS (ESI): mass calcd. for C
19H
22ClFN
4O
3S, 440.1; m/z found, 441.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.52 (d, J = 8.6 Hz, 1H), 8.43 (s, 1H), 8.34 (d, J = 7.6 Hz, 1H), 8.04 (br s, 1H), 7.41 - 7.31 (m, 1H), 7.31 - 7.22 (m, 2H), 6.84 - 6.74 (m, 1H), 6.72 - 6.61 (m, 1H), 5.34 - 5.20 (m, 1H), 4.86 - 4.71 (m, 1H), 2.97 (s, 3H), 1.89 - 1.71 (m, 2H), 1.31 (d, J = 7.0 Hz, 3H), 0.97 (t, J = 7.3 Hz, 3H). Example 134: 5-(((*R)-1-(2-Chloro-3-fluorophenyl)propyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrazine-2-carboxamide.
was isolated from the SFC separation of 5-((1-(2-chloro-3- fluorophenyl)propyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (Example 133, Step C, Rt = 1.94 min). MS (ESI): mass calcd. for C19H22ClFN4O3S, 440.1; m/z found, 441.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.52 (d, J = 8.6 Hz, 1H), 8.44 (s, 1H), 8.33 (d, J = 7.6 Hz, 1H), 8.03 (br s, 1H), 7.34 (dd, J = 7.8, 5.4 Hz, 1H), 7.31 - 7.23 (m, 2H), 6.85 - 6.74 (m, 1H), 6.73 - 193 QB\184200.00081\93036187.2
VVID-747PC 6.62 (m, 1H), 5.33 - 5.19 (m, 1H), 4.88 - 4.70 (m, 1H), 2.99 (s, 3H), 1.90 - 1.69 (m, 2H), 1.30 (d, J = 7.0 Hz, 3H), 0.97 (t, J = 7.3 Hz, 3H). Example 135: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-3-(1H-imidazol-2-yl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)benzamide. prepared in a manner analogous to Example 59 using methyl 4-fluoro-3-
41) instead of methyl 5-chloropyrazine-2-carboxylate and DMSO instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C
23H
25ClN
4O
3S, 472.1; m/z found, 473.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 12.88 - 12.41 (m, 1H), 9.92 (d, J = 6.4 Hz, 1H), 8.28 (d, J = 1.5 Hz, 1H), 8.13 (d, J = 7.9 Hz, 1H), 7.61 - 7.53 (m, 1H), 7.50 - 7.43 (m, 1H), 7.36 - 7.14 (m, 5H), 6.88 - 6.68 (m, 2H), 6.27 (d, J = 8.8 Hz, 1H), 5.05 (q, J = 6.5 Hz, 1H), 4.87 - 4.74 (m, 1H), 3.00 (s, 3H), 1.56 (d, J = 6.6 Hz, 3H), 1.29 (d, J = 7.0 Hz, 3H). Example 136: 5-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-4-(trifluoromethyl)pyrimidine-2-carboxamide.
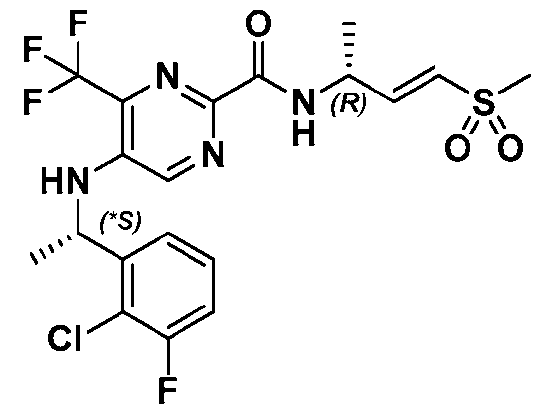
prepared in a manner analogous to Example 59 using 1-(2-chloro-3- fluorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-chloro-4- (trifluoromethyl)pyrimidine-2-carboxylate (Intermediate 17) instead of methyl 5-chloropyrazine-2- carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-((1-(2-chloro-3- fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4-(trifluoromethyl)pyrimidine-2- carboxamide (Step C) via SFC (Stationary phase: IC (3x25 cm); Mobile phase: 45% EtOH/CO
2; Rt = 12.3 min) provided the title compound. MS (ESI): mass calcd. for C
19H
19ClF
4N
4O3S, 494.1; m/z found, 495.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.80 (br d, J = 8.5 Hz, 1H), 8.08 (s, 1H), 7.44 - 7.28 (m, 3H), 7.02 (br d, J = 6.9 Hz, 1H), 6.81 - 6.73 (m, 1H), 6.72 - 6.66 (m, 1H), 5.32 - 5.18 (m, 1H), 4.83 - 4.70 (m, 1H), 2.96 (s, 3H), 1.61 (br d, J = 6.6 Hz, 3H), 1.30 (d, J = 7.0 Hz, 3H). 194 QB\184200.00081\93036187.2
VVID-747PC Example 137: 5-(((*R)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-4-(trifluoromethyl)pyrimidine-2-carboxamide. was isolated from the SFC separation of 5-((1-(2-chloro-3-
((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4-(trifluoromethyl)pyrimidine-2- carboxamide (Example 136, Step C, Rt = 9.09 min). MS (ESI): mass calcd. for C
19H
19ClF
4N
4O
3S, 494.1; m/z found, 495.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.79 (d, J = 8.4 Hz, 1H), 8.08 (s, 1H), 7.44 - 7.30 (m, 3H), 7.02 (br d, J = 6.8 Hz, 1H), 6.83 - 6.75 (m, 1H), 6.73 - 6.67 (m, 1H), 5.23 (br t, J = 6.8 Hz, 1H), 4.83 - 4.71 (m, 1H), 2.98 (s, 3H), 1.61 (d, J = 6.6 Hz, 3H), 1.28 (d, J = 7.0 Hz, 3H). Example 138: 8-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)imidazo[1,2-c]pyrimidine-5-carboxamide.
prepared in a manner analogous to Example 59 using methyl 8- fluoroimidazo[1,2-c]pyrimidine-5-carboxylate (Intermediate 47) instead of methyl 5-chloropyrazine-2- carboxylate and DMSO instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C
20H
22ClN
5O
3S, 447.1; m/z found, 448.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.99 (d, J = 8.6 Hz, 1H), 8.82 (d, J = 1.1 Hz, 1H), 7.73 (d, J = 1.0 Hz, 1H), 7.67 (br d, J = 6.8 Hz, 1H), 7.59 - 7.53 (m, 1H), 7.50 - 7.44 (m, 1H), 7.31 - 7.23 (m, 2H), 6.83 - 6.71 (m, 3H), 5.17 - 5.08 (m, 1H), 4.85 - 4.73 (m, 1H), 2.98 (s, 3H), 1.60 (d, J = 6.8 Hz, 3H), 1.30 (d, J = 7.0 Hz, 3H). Example 139: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-4-(difluoromethyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 195 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 59 using methyl 4-
2-carboxylate (Intermediate 44) instead of methyl 5-chloropyrazine- 2-carboxylate and DMSO instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C
19H
21ClF
2N
4O
3S, 458.1; m/z found, 459.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.72 (br d, J = 8.3 Hz, 1H), 7.87 (s, 1H), 7.51 - 7.43 (m, 2H), 7.36 - 7.28 (m, 2H), 7.13 - 7.00 (m, 1H), 6.85 - 6.66 (m, 2H), 5.13 - 5.08 (m, 1H), 4.80 - 4.72 (m, 2H), 2.97 (s, 3H), 1.57 (br d, J = 6.6 Hz, 3H), 1.28 (br d, J = 6.9 Hz, 3H). Example 140: 5-(((R)-1-(2-Chloro-6-fluorophenyl)ethyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 59 using methyl 5-fluoro-4-
methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 5-chloropyrazine-2-carboxylate, (R)- 1-(2-chloro-6-fluorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, and DMSO instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C
19H
22ClFN
4O
3S, 440.1; m/z found, 441.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.63 (br s, 1H), 7.65 (s, 1H), 7.36 - 7.30 (m, 2H), 7.23 - 7.13 (m, 1H), 6.79 - 6.73 (m, 1H), 6.70 - 6.63 (m, 1H), 6.33 (br d, J = 6.4 Hz, 1H), 5.24 - 5.13 (m, 1H), 4.78 (br d, J = 7.8 Hz, 1H), 2.97 (s, 3H), 2.48 - 2.48 (m, 3H), 1.69 (br d, J = 6.6 Hz, 3H), 1.31 - 1.26 (m, 3H). Example 141: 5-(((S)-1-(2-Chloro-6-fluorophenyl)ethyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
VVID-747PC [00478] The title compound was prepared in a manner analogous to Example 59 using methyl 5-fluoro-4- methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 5-chloropyrazine-2-carboxylate, (S)- 1-(2-chloro-6-fluorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, and DMSO instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C
19H
22ClFN
4O
3S, 440.1; m/z found, 441.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.64 (d, J = 8.8 Hz, 1H), 7.65 (s, 1H), 7.37 - 7.30 (m, 2H), 7.22 - 7.12 (m, 1H), 6.81 - 6.75 (m, 1H), 6.71 - 6.63 (m, 1H), 6.34 (br d, J = 6.9 Hz, 1H), 5.23 - 5.13 (m, 1H), 4.82 - 4.69 (m, 1H), 2.95 - 2.95 (m, 3H), 2.48 (br s, 3H), 1.69 (d, J = 6.8 Hz, 3H), 1.27 (d, J = 7.0 Hz, 3H). Example 142: 5-(((*R)-1-(2-Chlorophenyl)-2,2-difluoroethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 59 using 1-(2-chlorophenyl)-
38) instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5- fluoropyrimidine-2-carboxylate instead of methyl 5-chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-((1-(2-chlorophenyl)-2,2-difluoroethyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: OD (3x25 cm); Mobile phase: 38% MeOH/CO
2; Rt = 9.00 min, second eluting product) provided the title compound. MS (ESI): mass calcd. for C
18H
19ClF
2N
4O
3S, 444.1; m/z found, 445.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.73 (d, J = 8.5 Hz, 1H), 8.24 (s, 2H), 7.73 (d, J = 8.5 Hz, 1H), 7.63 - 7.51 (m, 2H), 7.41 (dd, J = 5.8, 3.5 Hz, 2H), 6.84 - 6.66 (m, 2H), 6.64 - 6.29 (m, 1H), 5.56 - 5.39 (m, 1H), 4.79 - 4.68 (m, 1H), 2.97 (s, 3H), 1.30 (d, J = 7.0 Hz, 3H). Example 143: 5-(((*R)-(2-Chloro-3-fluorophenyl)(1-cyanocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide.
in a manner analogous to Example 59 using 1-(amino(2-chloro- 3-fluorophenyl)methyl)cyclopropane-1-carbonitrile (Intermediate 39) instead of (S)-1-(2- 197 QB\184200.00081\93036187.2
VVID-747PC chlorophenyl)ethan-1-amine, methyl 5-fluoropyrazine-2-carboxylate instead of methyl 5-chloropyrazine- 2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-(((2-chloro-3-fluorophenyl)(1- cyanocyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (Step C) via SFC (Stationary phase: IG (3x25 cm); Mobile phase: 45% MeOH/CO
2; Rt = 4.17 min) provided the title compound. MS (ESI): mass calcd. for C
21H
21ClFN
5O
3S, 477.1; m/z found, 478.1 [M+H]
+. 1H NMR (400 MHz, DMSO-d
6) δ 8.62 (dd, J = 19.0, 8.1 Hz, 2H), 8.50 (d, J = 1.1 Hz, 1H), 8.13 (d, J = 1.1 Hz, 1H), 7.57 - 7.36 (m, 3H), 6.85 - 6.75 (m, 1H), 6.72 - 6.63 (m, 1H), 5.52 (d, J = 7.5 Hz, 1H), 4.87 - 4.74 (m, 1H), 2.98 (s, 3H), 1.48 - 1.35 (m, 2H), 1.32 (d, J = 7.0 Hz, 3H), 1.24 - 1.15 (m, 2H). Example 144: 5-(((*S)-(2-Chloro-3-fluorophenyl)(1-cyanocyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide. from the SFC separation of 5-(((2-chloro-3-fluorophenyl)(1-
- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (Example 143, Step C, Rt = 5.58 min). MS (ESI): mass calcd. for C
21H
21ClFN
5O
3S, 477.1; m/z found, 478.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.62 (dd, J = 14.0, 8.1 Hz, 2H), 8.51 (d, J = 1.0 Hz, 1H), 8.12 (d, J = 1.1 Hz, 1H), 7.59 - 7.38 (m, 3H), 6.85 - 6.76 (m, 1H), 6.74 - 6.66 (m, 1H), 5.53 (d, J = 7.5 Hz, 1H), 4.86 - 4.73 (m, 1H), 2.99 (s, 3H), 1.48 - 1.34 (m, 2H), 1.31 (d, J = 7.0 Hz, 3H), 1.22 - 1.14 (m, 2H). Example 145: 5-((*S)-(2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 59 using (2-chloro-3- fluorophenyl)(1-fluorocyclopropyl)methanol (Intermediate 35) instead of (S)-1-(2-chlorophenyl)ethan-1- amine, methyl 5-fluoropyrimidine-2-carboxylate instead of methyl 5-chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-((2-chloro-3-fluorophenyl)(1- 198 QB\184200.00081\93036187.2
VVID-747PC fluorocyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: IH (3x25 cm); Mobile phase: 40% IPA/CO
2; Rt = 5.06 min) provided the title compound. MS (ESI): mass calcd. for C
20H
20ClF
2N
3O
4S, 471.1; m/z found, 472.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.95 (d, J = 8.5 Hz, 1H), 8.54 (s, 2H), 7.52 - 7.34 (m, 3H), 6.85 - 6.64 (m, 2H), 6.22 - 6.07 (m, 1H), 4.85 - 4.71 (m, 1H), 2.97 (s, 3H), 1.30 (d, J = 7.0 Hz, 3H), 1.28 - 1.01 (m, 4H). Example 146: 5-((*R)-(2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. isolated from the SFC separation of 5-((2-chloro-3-fluorophenyl)(1-
- (R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 145, Step C, Rt = 7.99 min). MS (ESI): mass calcd. for C
20H
20ClF
2N
3O
4S, 471.1; m/z found, 472.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.96 (d, J = 8.5 Hz, 1H), 8.54 (s, 2H), 7.51 - 7.35 (m, 3H), 6.86 - 6.65 (m, 2H), 6.20 - 6.07 (m, 1H), 4.87 - 4.71 (m, 1H), 2.98 (s, 3H), 1.32 - 1.02 (m, 7H). Example 147: 5-(((*R)-(2-Chloro-6-fluorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 59 using methyl 5-fluoro-4- methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 5-chloropyrazine-2-carboxylate, (2- chloro-6-fluorophenyl)(cyclopropyl)methanamine (Intermediate 25) instead of (S)-1-(2- chlorophenyl)ethan-1-amine, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-(((2-chloro-6- fluorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 5.56 min) provided the title compound. MS (ESI): mass calcd. for C
21H
24ClFN
4O
3S, 466.1; m/z found, 467.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.65 (d, J = 8.7 Hz, 1H), 7.64 (s, 1H), 7.38 - 7.31 (m, 2H), 7.26 - 7.19 (m, 1H), 6.80 - 6.71 (m, 1H), 6.69 - 6.62 (m, 1H), 6.47 (d, J = 6.7 Hz, 1H), 199 QB\184200.00081\93036187.2
VVID-747PC 4.81 - 4.66 (m, 1H), 4.30 (br t, J = 7.9 Hz, 1H), 2.96 (s, 3H), 2.49 (s, 3H), 1.83 - 1.66 (m, 1H), 1.28 (d, J = 7.0 Hz, 3H), 0.78 - 0.67 (m, 1H), 0.65 - 0.49 (m, 2H), 0.48 - 0.35 (m, 1H). Example 148: 5-(((*S)-(2-Chloro-6-fluorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. was isolated from the SFC separation of 5-(((2-chloro-6-
amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Example 147, Step C, Rt = 9.97 min). MS (ESI): mass calcd. for C
21H
24ClFN
4O
3S, 466.1; m/z found, 467.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.64 (d, J = 8.7 Hz, 1H), 7.64 (s, 1H), 7.42 - 7.30 (m, 2H), 7.25 - 7.16 (m, 1H), 6.83 - 6.74 (m, 1H), 6.70 - 6.60 (m, 1H), 6.47 (d, J = 6.9 Hz, 1H), 4.80 - 4.68 (m, 1H), 4.36 - 4.23 (m, 1H), 2.97 (s, 3H), 2.49 (br s, 3H), 1.74 (br dd, J = 7.9, 4.4 Hz, 1H), 1.27 (d, J = 7.0 Hz, 3H), 0.80 - 0.65 (m, 1H), 0.64 - 0.49 (m, 2H), 0.42 (br dd, J = 9.5, 5.1 Hz, 1H). Example 149: 5-(((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-4-(trifluoromethyl)pyrimidine-2-carboxamide.
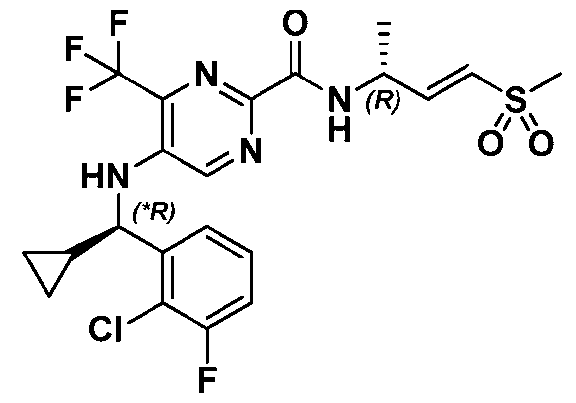
prepared in a manner analogous to Example 59 using (2-chloro-3- fluorophenyl)(cyclopropyl)methanamine (Intermediate 24) instead of (S)-1-(2-chlorophenyl)ethan-1- amine, methyl 5-chloro-4-(trifluoromethyl)pyrimidine-2-carboxylate (Intermediate 17) instead of methyl 5-chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-(((2-chloro- 3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: IC (3x25 cm); Mobile phase: 60% IPA/CO
2; Rt = 10.5 min) provided the title compound. MS (ESI): mass calcd. for C
21H
21ClF
4N
4O
3S, 520.1; m/z found, 521.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.80 (d, J = 8.5 Hz, 1H), 8.16 (s, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.48 - 7.31 (m, 2H), 7.02 (d, J = 6.8 Hz, 1H), 6.83 - 6.74 (m, 200 QB\184200.00081\93036187.2
VVID-747PC 1H), 6.73 - 6.66 (m, 1H), 4.84 - 4.70 (m, 1H), 4.52 (dd, J = 8.7, 7.2 Hz, 1H), 2.98 (s, 3H), 1.68 - 1.53 (m, 1H), 1.28 (d, J = 7.0 Hz, 3H), 0.70 - 0.61 (m, 1H), 0.59 - 0.48 (m, 3H). Example 150: 5-(((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-4-(trifluoromethyl)pyrimidine-2-carboxamide. was isolated from the SFC separation of 5-(((2-chloro-3-
amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide (Example 149, Step C, Rt = 16.6 min). MS (ESI): mass calcd. for C
21H
21ClF
4N
4O
3S, 520.1; m/z found, 521.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.81 (d, J = 8.5 Hz, 1H), 8.16 (s, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.48 - 7.30 (m, 2H), 7.02 (br d, J = 6.9 Hz, 1H), 6.83 - 6.64 (m, 2H), 4.84 - 4.70 (m, 1H), 4.52 (dd, J = 8.7, 7.2 Hz, 1H), 2.96 (s, 3H), 1.68 - 1.52 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H), 0.71 - 0.62 (m, 1H), 0.60 - 0.48 (m, 3H). Example 151: 8-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)- [1,2,4]triazolo[4,3-a]pyridine-5-carboxamide.
was prepared in a manner analogous to Example 59 using methyl 8-fluoro- [1,2,4]triazolo[4,3-a]pyridine-5-carboxylate (Intermediate 48) instead of methyl 5-chloropyrazine-2- carboxylate and DMSO instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C
20H
22ClN
5O
3S, 447.1; m/z found, 448.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.74 (s, 1H), 8.60 (d, J = 7.9 Hz, 1H), 8.04 (d, J = 7.3 Hz, 1H), 7.60 - 7.50 (m, 2H), 7.49 - 7.42 (m, 1H), 7.32 - 7.22 (m, 2H), 6.84 - 6.74 (m, 2H), 5.76 (d, J = 8.2 Hz, 1H), 5.16 - 5.05 (m, 1H), 4.87 - 4.74 (m, 1H), 3.00 (s, 3H), 1.61 (d, J = 6.9 Hz, 3H), 1.28 (d, J = 7.0 Hz, 3H). Example 152: 8-(((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)imidazo[1,2-c]pyrimidine-5-carboxamide. 201 QB\184200.00081\93036187.2
VVID-747PC was prepared in a manner analogous to Example 59 using (2-chloro-3-
(Intermediate 24) instead of (S)-1-(2-chlorophenyl)ethan-1- amine, methyl 8-fluoroimidazo[1,2-c]pyrimidine-5-carboxylate (Intermediate 47) instead of methyl 5- chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 8-(((2-chloro-3- fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)imidazo[1,2- c]pyrimidine-5-carboxamide (Step C) via RP-HPLC (35-75% ACN in 10 mM aq. NH
4HCO
3, first eluting product) provided the title compound. MS (ESI): mass calcd. for C
22H
23ClFN
5O
3S, 491.1; m/z found, 492.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.00 (br d, J = 8.7 Hz, 1H), 8.80 (s, 1H), 7.74 - 7.65 (m, 2H), 7.61 (br d, J = 7.4 Hz, 1H), 7.43 - 7.28 (m, 2H), 6.85 - 6.69 (m, 3H), 4.84 - 4.73 (m, 1H), 4.44 (br t, J = 8.0 Hz, 1H), 2.99 (s, 3H), 1.68 - 1.56 (m, 1H), 1.30 (br d, J = 6.8 Hz, 3H), 0.68 (br t, J = 8.2 Hz, 1H), 0.56 - 0.45 (m, 3H). Example 153: 8-(((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)imidazo[1,2-c]pyrimidine-5-carboxamide.
was isolated from the RP-HPLC separation of 8-(((2-chloro-3- fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)imidazo[1,2- c]pyrimidine-5-carboxamide (Example 152, Step C, second eluting product). MS (ESI): mass calcd. for C
22H
23ClFN
5O
3S, 491.1; m/z found, 492.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.99 (d, J = 8.5 Hz, 1H), 8.80 (s, 1H), 7.72 - 7.66 (m, 2H), 7.61 (d, J = 7.8 Hz, 1H), 7.43 - 7.27 (m, 2H), 6.83 - 6.77 (m, 1H), 6.76 - 6.67 (m, 2H), 4.78 (td, J = 7.7, 3.9 Hz, 1H), 4.43 (t, J = 8.3 Hz, 1H), 2.94 (s, 3H), 1.66 - 1.56 (m, 1H), 1.32 (d, J = 7.0 Hz, 3H), 0.73 - 0.64 (m, 1H), 0.57 - 0.46 (m, 3H). Example 154: 8-(((S)-1-(2-Chlorophenyl)ethyl)amino)-2-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)imidazo[1,2-c]pyrimidine-5-carboxamide. 202 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 59 using methyl 8-fluoro-2-
5-carboxylate (Intermediate 49) instead of methyl 5-chloropyrazine-2- carboxylate and DMSO instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C
21H
24ClN
5O
3S, 461.1; m/z found, 462.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.5 Hz, 1H), 8.57 (d, J = 0.8 Hz, 1H), 7.64 - 7.53 (m, 2H), 7.50 - 7.43 (m, 1H), 7.34 - 7.21 (m, 2H), 6.84 - 6.76 (m, 1H), 6.75 - 6.70 (m, 2H), 5.08 (q, J = 6.8 Hz, 1H), 4.82 - 4.72 (m, 1H), 2.99 (s, 3H), 2.42 (s, 3H), 1.59 (d, J = 6.8 Hz, 3H), 1.29 (d, J = 7.0 Hz, 3H). Example 155: 4-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazolo[1,5-a]pyrazine-7-carboxamide. was prepared in a manner analogous to Example 59 using methyl 7-
chloropyrazolo[1,5-a]pyrazine-4-carboxylate (Intermediate 50) instead of methyl 5-chloropyrazine-2- carboxylate and DMSO instead of 1,4-dioxane in Step A. MS (ESI): mass calcd. for C
20H
22ClN
5O
3S, 447.1; m/z found, 448.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.69 (d, J = 8.7 Hz, 1H), 8.54 (br d, J = 5.5 Hz, 1H), 8.30 (d, J = 2.3 Hz, 1H), 7.66 - 7.57 (m, 1H), 7.53 - 7.46 (m, 1H), 7.34 - 7.27 (m, 2H), 7.26 (d, J = 2.3 Hz, 1H), 6.90 (s, 1H), 6.86 - 6.77 (m, 1H), 6.74 - 6.66 (m, 1H), 5.27 (br t, J = 6.3 Hz, 1H), 4.87 - 4.74 (m, 1H), 2.99 (s, 3H), 1.67 (d, J = 6.8 Hz, 3H), 1.29 (d, J = 7.0 Hz, 3H). Example 156: 7-(((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-1-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-1H-pyrazolo[4,3-c]pyridine-4-carboxamide.
VVID-747PC [00493] The title compound was prepared in a manner analogous to Example 59 using (2-chloro-3- fluorophenyl)(cyclopropyl)methanamine (Intermediate 24) instead of (S)-1-(2-chlorophenyl)ethan-1- amine, methyl 7-fluoro-1-methyl-1H-pyrazolo[4,3-c]pyridine-4-carboxylate (Intermediate 51) instead of methyl 5-chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 7-(((2- chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-1-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)- 1H-pyrazolo[4,3-c]pyridine-4-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% EtOH/CO
2; Rt = 17.9 min, second eluting product) provided the title compound. MS (ESI): mass calcd. for C
23H
25ClFN
5O
3S, 505.1; m/z found, 506.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.76 (d, J = 8.8 Hz, 1H), 8.46 (s, 1H), 7.71 - 7.61 (m, 1H), 7.44 - 7.26 (m, 2H), 7.12 (s, 1H), 6.88 - 6.76 (m, 1H), 6.74 - 6.64 (m, 1H), 6.46 (d, J = 5.4 Hz, 1H), 4.80 (dt, J = 7.1, 5.9 Hz, 1H), 4.52 - 4.46 (m, 4H), 2.99 (s, 3H), 1.53 - 1.40 (m, 1H), 1.28 (d, J = 6.9 Hz, 3H), 0.68 (dt, J = 8.6, 3.7 Hz, 1H), 0.62 - 0.42 (m, 3H). Example 157: 7-(((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-2-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-2H-pyrazolo[4,3-c]pyridine-4-carboxamide. prepared in a manner analogous to Example 59 using (2-chloro-3-
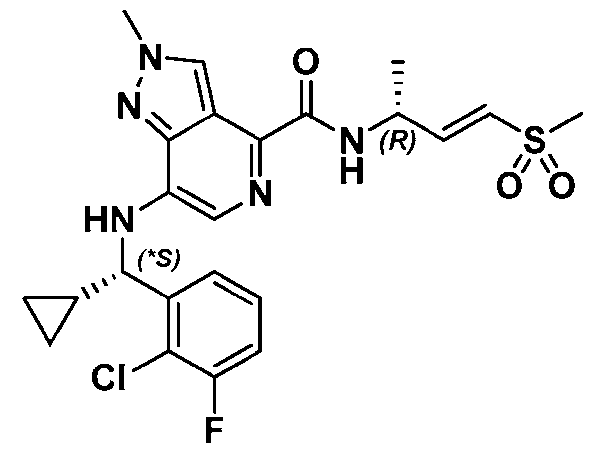
fluorophenyl)(cyclopropyl)methanamine (Intermediate 24) instead of (S)-1-(2-chlorophenyl)ethan-1- amine, methyl 7-fluoro-2-methyl-2H-pyrazolo[4,3-c]pyridine-4-carboxylate (Intermediate 52) instead of methyl 5-chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 7-(((2- chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-2-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)- 2H-pyrazolo[4,3-c]pyridine-4-carboxamide (Step C) via SFC (Stationary phase: AD (3x25 cm); Mobile phase: 27% EtOH/CO
2; Rt = 7.04 min, second eluting product) provided the title compound. MS (ESI): mass calcd. for C
23H
25ClFN
5O
3S, 505.1; m/z found, 506.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.74 (s, 1H), 8.58 (d, J = 8.8 Hz, 1H), 7.58 (d, J = 7.7 Hz, 1H), 7.39 - 7.23 (m, 3H), 7.06 (s, 1H), 6.85 - 6.76 (m, 1H), 6.72 - 6.63 (m, 1H), 4.85 - 4.73 (m, 1H), 4.52 (t, J = 8.1 Hz, 1H), 4.25 (s, 3H), 2.98 (s, 3H), 1.61 - 1.48 (m, 1H), 1.29 (d, J = 7.0 Hz, 3H), 0.72 - 0.61 (m, 1H), 0.57 - 0.40 (m, 3H). Example 158: 8-(((*R)-(2-Chloro-3-fluorophenyl)(oxetan-3-yl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)imidazo[1,2-c]pyrimidine-5-carboxamide. 204 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 59 using (2-chloro-3-
(Intermediate 40) instead of (S)-1-(2-chlorophenyl)ethan-1- amine, methyl 8-fluoroimidazo[1,2-c]pyrimidine-5-carboxylate (Intermediate 47) instead of methyl 5- chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 8-(((2-chloro-3- fluorophenyl)(oxetan-3-yl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)imidazo[1,2- c]pyrimidine-5-carboxamide (Step C) via SFC (Stationary phase: IC (3x25 cm); Mobile phase: 45% EtOH/CO
2 with 0.1% NH
4OH; Rt = 6.5 min) provided the title compound. MS (ESI): mass calcd. for C
22H
23ClFN
5O
4S, 507.1; m/z found, 508.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.04 (d, J = 8.5 Hz, 1H), 8.80 (d, J = 1.3 Hz, 1H), 7.77 (d, J = 8.0 Hz, 1H), 7.66 (d, J = 1.3 Hz, 1H), 7.51 (d, J = 7.6 Hz, 1H), 7.43 - 7.28 (m, 2H), 7.05 (s, 1H), 6.80 - 6.74 (m, 1H), 6.74 - 6.70 (m, 1H), 5.54 (dd, J = 10.4, 8.0 Hz, 1H), 4.86 - 4.75 (m, 2H), 4.55 (t, J = 6.0 Hz, 1H), 4.51 - 4.44 (m, 1H), 4.33 (t, J = 6.3 Hz, 1H), 3.77 (td, J = 9.6, 7.3 Hz, 1H), 2.94 (s, 3H), 1.34 (d, J = 7.0 Hz, 3H). Example 159: 8-(((*S)-(2-Chloro-3-fluorophenyl)(oxetan-3-yl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)imidazo[1,2-c]pyrimidine-5-carboxamide.
from the SFC separation of 8-(((2-chloro-3-fluorophenyl)(oxetan- 3-yl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)imidazo[1,2-c]pyrimidine-5-carboxamide (Example 158, Step C, Rt = 7.5 min). MS (ESI): mass calcd. for C22H23ClFN5O4S, 507.1; m/z found, 508.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.05 (d, J = 8.5 Hz, 1H), 8.80 (d, J = 1.3 Hz, 1H), 7.77 (d, J = 7.9 Hz, 1H), 7.66 (d, J = 1.3 Hz, 1H), 7.51 (d, J = 7.5 Hz, 1H), 7.43 - 7.29 (m, 2H), 7.05 (s, 1H), 6.85 - 6.77 (m, 1H), 6.77 - 6.72 (m, 1H), 5.54 (dd, J = 10.0, 8.3 Hz, 1H), 4.80 (br t, J = 6.8 Hz, 2H), 4.60 - 4.44 (m, 2H), 4.33 (t, J = 6.3 Hz, 1H), 3.85 - 3.67 (m, 1H), 2.99 (s, 3H), 1.31 (d, J = 7.0 Hz, 3H). Example 160: 8-(((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-[1,2,4]triazolo[4,3-c]pyrimidine-5-carboxamide. 205 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 59 using (2-chloro-3-
(Intermediate 24) instead of (S)-1-(2-chlorophenyl)ethan-1- amine, methyl 8-fluoro-[1,2,4]triazolo[4,3-c]pyrimidine-5-carboxylate (Intermediate 53) instead of methyl 5-chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 8-(((2-chloro- 3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)- [1,2,4]triazolo[4,3-c]pyrimidine-5-carboxamide (Step C) via SFC (Stationary phase: OD (3x25 cm); Mobile phase: 30% MeOH/CO
2 with 0.1% NH
4OH; Rt = 7.12 min) provided the title compound. MS (ESI): mass calcd. for C
21H
22ClFN
6O
3S, 492.1; m/z found, 493.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.84 (s, 1H), 9.08 (d, J = 8.6 Hz, 1H), 8.37 (d, J = 7.5 Hz, 1H), 7.63 (d, J = 7.8 Hz, 1H), 7.47 - 7.27 (m, 2H), 6.84 - 6.69 (m, 3H), 4.79 (dt, J = 7.6, 3.9 Hz, 1H), 4.48 (br t, J = 8.3 Hz, 1H), 2.98 (s, 3H), 1.67 - 1.54 (m, 1H), 1.31 (d, J = 7.0 Hz, 3H), 0.75 - 0.63 (m, 1H), 0.58 - 0.45 (m, 3H). Example 161: 8-(((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-[1,2,4]triazolo[4,3-c]pyrimidine-5-carboxamide.
was isolated from the SFC separation of 8-(((2-chloro-3- fluorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-[1,2,4]triazolo[4,3- c]pyrimidine-5-carboxamide (Example 160, Step C, Rt = 8.60 min). MS (ESI): mass calcd. for C
21H
22ClFN
6O
3S, 492.1; m/z found, 493.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.84 (s, 1H), 9.07 (d, J = 8.5 Hz, 1H), 8.38 (d, J = 7.5 Hz, 1H), 7.63 (d, J = 7.8 Hz, 1H), 7.46 - 7.27 (m, 2H), 6.84 - 6.68 (m, 3H), 4.87 - 4.73 (m, 1H), 4.47 (br t, J = 8.1 Hz, 1H), 2.94 (s, 3H), 1.67 - 1.54 (m, 1H), 1.33 (d, J = 7.1 Hz, 3H), 0.75 - 0.64 (m, 1H), 0.59 - 0.45 (m, 3H). Example 162: 5-(((*R)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 206 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 59 using (2-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5- fluoro-4-methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 5-chloropyrazine-2- carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-(((2- chlorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 5.62 min) provided the title compound. MS (ESI): mass calcd. for C
21H
25ClN
4O
3S, 448.1; m/z found, 449.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.62 (br d, J = 8.5 Hz, 1H), 7.66 (dd, J = 7.6, 1.5 Hz, 1H), 7.55 (s, 1H), 7.51 - 7.42 (m, 1H), 7.36 - 7.22 (m, 2H), 6.80 - 6.72 (m, 1H), 6.69 - 6.58 (m, 2H), 4.81 - 4.66 (m, 1H), 4.32 (br t, J = 7.8 Hz, 1H), 2.96 (s, 3H), 2.53 (s, 3H), 1.53 - 1.37 (m, 1H), 1.28 (d, J = 7.0 Hz, 3H), 0.70 - 0.59 (m, 1H), 0.56 - 0.39 (m, 3H). Example 163: 5-(((*S)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
was isolated from the SFC separation of 5-(((2- chlorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Example 162, Step C, Rt = 9.83 min). MS (ESI): mass calcd. for C
21H
25ClN
4O
3S, 448.1; m/z found, 449.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.63 (d, J = 8.5 Hz, 1H), 7.66 (dd, J = 7.7, 1.7 Hz, 1H), 7.55 (s, 1H), 7.46 (dd, J = 7.8, 1.3 Hz, 1H), 7.35 - 7.22 (m, 2H), 6.83 - 6.73 (m, 1H), 6.70 - 6.61 (m, 2H), 4.80 - 4.68 (m, 1H), 4.32 (t, J = 7.8 Hz, 1H), 2.97 (s, 3H), 2.53 (s, 3H), 1.51 - 1.40 (m, 1H), 1.27 (d, J = 7.0 Hz, 3H), 0.71 - 0.60 (m, 1H), 0.57 - 0.39 (m, 3H). Example 164: 5-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 207 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 59 using 1-(2-chloro-3-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-fluoro-4- methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 5-chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-(((2-chlorophenyl)(cyclopropyl)methyl)amino)- 4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 7.89 min, second eluting product) provided the title compound. MS (ESI): mass calcd. for C
19H
22ClFN
4O
3S, 440.1; m/z found, 441.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.62 (d, J = 8.6 Hz, 1H), 7.45 (s, 1H), 7.40 - 7.25 (m, 3H), 6.82 - 6.73 (m, 1H), 6.71 - 6.63 (m, 1H), 6.57 (d, J = 6.9 Hz, 1H), 5.01 (quin, J = 6.7 Hz, 1H), 4.81 - 4.68 (m, 1H), 2.98 (s, 3H), 2.54 (s, 3H), 1.57 (d, J = 6.8 Hz, 3H), 1.27 (d, J = 7.0 Hz, 3H). Example 165: 5-(((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 59 using (2-chloro-3- fluorophenyl)(cyclopropyl)methanamine (Intermediate 24) instead of (S)-1-(2-chlorophenyl)ethan-1- amine, methyl 5-fluoro-4-methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 5- chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-(((2-chloro-3- fluorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 5.55 min) provided the title compound. MS (ESI): mass calcd. for C
21H
24ClFN
4O
3S, 466.1; m/z found, 467.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.65 (d, J = 8.7 Hz, 1H), 7.56 - 7.49 (m, 2H), 7.42 - 7.28 (m, 2H), 6.81 - 6.61 (m, 3H), 4.73 (qd, J = 13.1, 6.6 Hz, 1H), 4.34 (t, J = 7.9 Hz, 1H), 2.96 (s, 3H), 2.53 (s, 3H), 1.51 - 1.41 (m, 1H), 1.28 (d, J = 7.0 Hz, 3H), 0.67 (br t, J = 8.7 Hz, 1H), 0.51 (br s, 3H). 208 QB\184200.00081\93036187.2
VVID-747PC Example 166: 5-(((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)amino)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. was isolated from the SFC separation of 5-(((2-chloro-3-
amino)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Example 165, Step C, Rt = 9.25 min). MS (ESI): mass calcd. for C
21H
24ClFN
4O
3S, 466.1; m/z found, 467.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.65 (d, J = 8.4 Hz, 1H), 7.55 - 7.50 (m, 2H), 7.41 - 7.28 (m, 2H), 6.80 - 6.63 (m, 3H), 4.80 - 4.68 (m, 1H), 4.34 (t, J = 7.9 Hz, 1H), 2.98 (s, 3H), 2.53 (s, 3H), 1.51 - 1.40 (m, 1H), 1.27 (d, J = 7.0 Hz, 3H), 0.67 (br t, J = 8.1 Hz, 1H), 0.50 (br d, J = 4.6 Hz, 3H). Example 167: 5-(((*S)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)-4-(trifluoromethyl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 59 using (2- chlorophenyl)(cyclopropyl)methanamine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5- chloro-4-(trifluoromethyl)pyrimidine-2-carboxylate (Intermediate 17) instead of methyl 5-chloropyrazine- 2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-(((2- chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (trifluoromethyl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 12.4 min, second eluting product) provided the title compound. MS (ESI): mass calcd. for C
21H
22ClF
3N
4O
3S, 502.1; m/z found, 503.0 [M+H]
+.
1H NMR (400 MHz, DMSO- d
6) δ 8.81 (d, J = 8.5 Hz, 1H), 8.15 (s, 1H), 7.72 (dd, J = 7.7, 1.6 Hz, 1H), 7.49 (dd, J = 7.9, 1.1 Hz, 1H), 7.37 (dt, J = 7.4, 1.1 Hz, 1H), 7.33 - 7.28 (m, 1H), 6.99 (d, J = 6.6 Hz, 1H), 6.82 - 6.75 (m, 1H), 6.72 - 6.66 (m, 1H), 4.83 - 4.69 (m, 1H), 4.47 (dd, J = 8.6, 7.0 Hz, 1H), 2.98 (s, 3H), 1.66 - 1.54 (m, 1H), 1.28 (d, J = 7.0 Hz, 3H), 0.68 - 0.61 (m, 1H), 0.56 - 0.48 (m, 3H). 209 QB\184200.00081\93036187.2
VVID-747PC Example 168: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-N-((R,E)-4-(cyclopropylsulfonyl)but-3-en-2-yl)- 4-methylpyrimidine-2-carboxamide. in a manner analogous to Example 59 using methyl 5-fluoro-4-
18) instead of methyl 5-chloropyrazine-2-carboxylate and DMSO instead of 1,4-dioxane in Step A and using (R,E)-4-(cyclopropylsulfonyl)but-3-en-2- amine (Intermediate 21) instead of (R,E)-4-(methylsulfonyl)but-3-en-2-amine in Step C. MS (ESI): mass calcd. for C
21H
25ClN
4O
3S, 448.1; m/z found, 449.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.65 (d, J = 8.63 Hz, 1H), 7.60 - 7.40 (m, 3H), 7.38 - 7.19 (m, 2H), 6.81 - 6.71 (m, 1H), 6.67 - 6.61 (m, 1H), 6.54 (d, J = 6.8 Hz, 1H), 4.98 (t, J = 6.8 Hz, 1H), 4.80 - 4.68 (m, 1H), 2.67 - 2.57 (m, 1H), 2.54 (s, 3H), 1.56 (d, J = 6.8 Hz, 3H), 1.27 (d, J = 7.0 Hz, 3H), 1.04 - 0.93 (m, 4H). Example 169: 5-(((*S)-1-(2-Chloro-3-fluorophenyl)ethyl)amino)-4-(difluoromethyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 59 using 1-(2-chloro-3- fluorophenyl)ethan-1-amine instead of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 4-(difluoromethyl)- 5-fluoropyrimidine-2-carboxylate (Intermediate 44) instead of methyl 5-chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-((1-(2-chloro-3-fluorophenyl)ethyl)amino)-4- (difluoromethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 70% MeOH/CO
2; Rt = 13.2 min, second eluting product) provided the title compound. MS (ESI): mass calcd. for C
19H
20ClF
3N
4O
3S, 476.1; m/z found, 477.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.73 (br d, J = 8.4 Hz, 1H), 7.88 (s, 1H), 7.50 - 7.19 (m, 4H), 7.10 (br d, J = 6.3 Hz, 1H), 6.84 - 6.73 (m, 1H), 6.73 - 6.64 (m, 1H), 5.15 (quin, J = 6.4 Hz, 1H), 4.84 - 4.65 (m, 1H), 2.97 (d, J = 8.0 Hz, 3H), 1.58 (d, J = 6.8 Hz, 3H), 1.38 - 1.22 (m, 3H). 210 QB\184200.00081\93036187.2
VVID-747PC Example 170: 5-(((*S)-1-(3-Fluoro-2-methylphenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 59 using 1-(3-fluoro-2-
of (S)-1-(2-chlorophenyl)ethan-1-amine, methyl 5-fluoropyrimidine- 2-carboxylate instead of methyl 5-chloropyrazine-2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-((1-(3-fluoro-2-methylphenyl)ethyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: C4 (3x25cm); Mobile phase: 40% EtOH/CO
2, Rt = 5.71 min, second eluting product) provided the title compound. MS (ESI): mass calcd. for C
19H
23FN
4O
3S, 406.2; m/z found, 407.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.00 (s, 2H), 7.76 (s, 1H), 7.15 – 7.06 (m, 2H), 6.98 – 6.85 (m, 2H), 6.48 (dd, J = 15.2, 1.6 Hz, 1H), 5.01 – 4.89 (m, 1H), 4.75 (q, J = 6.6 Hz, 1H), 2.91 (s, 3H), 2.34 (d, J = 2.0 Hz, 3H), 1.57 (d, J = 6.6 Hz, 3H), 1.41 (d, J = 7.0 Hz, 3H). Example 171: 5-(((*R)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (cyclopropylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide.
prepared in a manner analogous to Example 59 using (2- chlorophenyl)(cyclopropyl)methanamine instead of (S)-1-(2-chlorophenyl)ethan-1-amine and DMSO instead of 1,4-dioxane in Step A and using (R,E)-4-(cyclopropylsulfonyl)but-3-en-2-amine (Intermediate 21) instead of (R,E)-4-methylsulfonylbut-3-en-2-amine in Step C. Separation of 5-(((2- chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(cyclopropylsulfonyl)but-3-en-2-yl)pyrazine-2- carboxamide (Step C) via SFC (Stationary phase: C4 (3x25cm); Mobile phase: 30% EtOH/CO
2, Rt = 4.31 min) provided the title compound. MS (ESI): mass calcd. for C
22H
25ClN
4O
3S, 460.1; m/z found, 461.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.75 (d, J = 1.4 Hz, 1H), 7.56 (d, J = 1.3 Hz, 1H), 7.45 (d, J = 8.6 Hz, 1H), 7.43 – 7.36 (m, 2H), 7.25 – 7.19 (m, 2H), 6.84 (dd, J = 15.2, 4.5 Hz, 1H), 6.41 (dd, J = 15.2, 1.8 Hz, 1H), 5.92 (d, J = 6.3 Hz, 1H), 5.01 – 4.90 (m, 1H), 4.82 (dd, J = 8.2, 6.3 Hz, 1H), 2.32 (tt, J = 8.0, 4.8 211 QB\184200.00081\93036187.2
VVID-747PC Hz, 1H), 1.42 – 1.34 (m, 4H), 1.23 – 1.17 (m, 2H), 1.04 – 0.96 (m, 2H), 0.72 – 0.64 (m, 1H), 0.60 – 0.49 (m, 2H), 0.44 – 0.35 (m, 1H). Example 172: 5-(((*S)-(2-Chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4- (cyclopropylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide. was isolated from the SFC separation of 5-(((2-
-N-((R,E)-4-(cyclopropylsulfonyl)but-3-en-2-yl)pyrazine-2- carboxamide (Example 171, Step C, Rt = 5.10 min). MS (ESI): mass calcd. for C
22H
25ClN
4O
3S, 460.1; m/z found, 461.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.75 (d, J = 1.4 Hz, 1H), 7.57 (d, J = 1.4 Hz, 1H), 7.49 – 7.35 (m, 3H), 7.25 – 7.17 (m, 2H), 6.85 (dd, J = 15.2, 4.4 Hz, 1H), 6.42 (dd, J = 15.1, 1.8 Hz, 1H), 5.93 (d, J = 6.3 Hz, 1H), 5.02 – 4.90 (m, 1H), 4.82 (dd, J = 8.2, 6.3 Hz, 1H), 2.32 (tt, J = 8.0, 4.8 Hz, 1H), 1.42 – 1.33 (m, 4H), 1.23 – 1.17 (m, 2H), 1.05 – 0.97 (m, 2H), 0.72 – 0.64 (m, 1H), 0.61 – 0.48 (m, 2H), 0.44 – 0.36 (m, 1H). Example 173: 5-(((*S)-(2-Chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide.
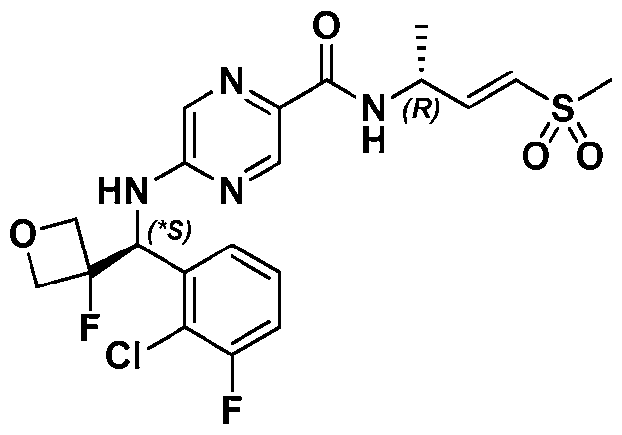
prepared in a manner analogous to Example 59 using (2-chloro-3- fluorophenyl)(3-fluorooxetan-3-yl)methanamine (Intermediate 26) instead of (S)-1-(2- chlorophenyl)ethan-1-amine, methyl 5-fluoropyrazine-2-carboxylate instead of methyl 5-chloropyrazine- 2-carboxylate, and DMSO instead of 1,4-dioxane in Step A. Separation of 5-(((2-chloro-3-fluorophenyl)(3- fluorooxetan-3-yl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (Step C) via SFC (Stationary phase: IG (3x25cm); Mobile phase: 35% EtOH/CO
2, Rt = 5.67 min) provided the title compound. MS (ESI): mass calcd. for C
20H
21ClF
2N
4O
4S, 486.1; m/z found, 487.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.60 (br dd, J = 12.3, 8.7 Hz, 2H), 8.53 (d, J = 1.3 Hz, 1H), 8.13 (d, J = 1.0 212 QB\184200.00081\93036187.2
VVID-747PC Hz, 1H), 7.49 - 7.33 (m, 3H), 6.84 - 6.72 (m, 1H), 6.71 - 6.61 (m, 1H), 6.35 - 6.17 (m, 1H), 4.93 - 4.53 (m, 5H), 2.97 (s, 3H), 1.31 (d, J = 7.0 Hz, 3H). Example 174: 5-(((*R)-(2-Chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methyl)amino)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide. O N N (R) S from the SFC separation of 5-(((2-chloro-3-fluorophenyl)(3-
- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrazine-2-carboxamide (Example 173, Step C, Rt = 7.59 min). MS (ESI): mass calcd. for C
20H
21ClF
2N
4O
4S, 486.1; m/z found, 487.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.60 (br t, J = 8.7 Hz, 2H), 8.54 (s, 1H), 8.12 (s, 1H), 7.53 - 7.33 (m, 3H), 6.86 - 6.62 (m, 2H), 6.41 - 6.16 (m, 1H), 4.92 - 4.45 (m, 5H), 2.99 (s, 3H), 1.31 (d, J = 7.0 Hz, 3H). Example 175: 5-((2-Chloro-3-fluorophenyl)(oxetan-3-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide.
in a manner analogous to Example 72 using (2-chloro-3- fluorophenyl)(oxetan-3-yl)methanol (Intermediate 30) instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 5-fluoropyrimidine-2-carboxylate instead of methyl 6-fluoronicotinate in Step A and using trimethyltin hydroxide/DCE instead of LiOH/MeOH in Step B. MS (ESI): mass calcd. for C20H21ClFN3O5S, 469.1; m/z found, 470.0 [M+H]
+.
1H NMR (400 MHz, CDCl3) δ 8.43 (d, J = 1.2 Hz, 2H), 7.85 (d, J = 8.3 Hz, 1H), 7.32 – 7.22 (m, 1H), 7.22 – 7.11 (m, 2H), 6.97 – 6.87 (m, 1H), 6.48 (dd, J = 15.2, 1.8 Hz, 1H), 6.09 (dd, J = 7.1, 2.7 Hz, 1H), 5.06 – 4.92 (m, 1H), 4.91 – 4.81 (m, 2H), 4.81 – 4.64 (m, 2H), 3.73 – 3.59 (m, 1H), 2.93 (s, 3H), 1.45 (d, J = 7.1 Hz, 3H). 213 QB\184200.00081\93036187.2
VVID-747PC Example 176: 5-((S)-1-(2-Chlorophenyl)ethoxy)-4-(hydroxymethyl)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 72 using methyl 4-
2-carboxylate (Intermediate 46) instead of methyl 6-fluoronicotinate and (S)-1-(2-chlorophenyl)ethan-1-ol instead of (S)-1-(2-chlorophenyl)ethan-1-amine in Step A. MS (ESI): mass calcd. for C
19H
22ClN
3O
5S, 439.1; m/z found, 440.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.09 (d, J = 8.5 Hz, 1H), 8.18 (s, 1H), 7.63 - 7.43 (m, 2H), 7.35 (dd, J = 5.9, 3.5 Hz, 2H), 6.88 - 6.64 (m, 2H), 6.04 (d, J = 6.5 Hz, 1H), 5.45 - 5.27 (m, 1H), 4.96 - 4.69 (m, 3H), 2.99 (s, 3H), 1.66 (d, J = 6.3 Hz, 3H), 1.33 (d, J = 7.1 Hz, 3H). Example 177: 5-((*S)-1-(2-Chloro-3-fluorophenyl)ethoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-4- (1-(trifluoromethyl)cyclopropyl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 72 using 1-(2-chloro-3- fluorophenyl)ethan-1-ol instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 5-fluoro-4-(1- (trifluoromethyl)cyclopropyl)pyrimidine-2-carboxylate (Intermediate 43) instead of methyl 6- fluoronicotinate in Step A. Separation of 5-(1-(2-chloro-3-fluorophenyl)ethoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-4-(1-(trifluoromethyl)cyclopropyl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% EtOH/CO
2, Rt = 8.8 min, second eluting product) provided the title compound. MS (ESI): mass calcd. for C
22H
22ClF
4N
3O
4S, 535.1; m/z found, 536.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.87 (br d, J = 8.4 Hz, 1H), 8.31 (s, 1H), 7.52 - 7.39 (m, 2H), 7.34 (br d, J = 7.4 Hz, 1H), 6.85 - 6.77 (m, 1H), 6.76 - 6.69 (m, 1H), 6.07 (q, J = 6.0 Hz, 1H), 4.84 - 4.75 (m, 1H), 3.00 (s, 3H), 1.67 (br d, J = 6.1 Hz, 3H), 1.53 (br s, 2H), 1.41 (br d, J = 8.5 Hz, 1H), 1.31 (br d, J = 6.9 Hz, 4H). 214 QB\184200.00081\93036187.2
VVID-747PC Example 178: 5-((*S)-(2-Chloro-3-fluorophenyl)(3-methyloxetan-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. in a manner analogous to Example 72 using (2-chlorophenyl)(3-
27) instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 5-fluoropyrimidine-2-carboxylate instead of methyl 6-fluoronicotinate in Step A. Separation of 5-((2- chlorophenyl)(3-methyloxetan-3-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide (Step C) via SFC (Stationary phase: IH (3x25 cm); Mobile phase: 25% MeOH/CO
2, Rt = 6.20 min) provided the title compound. MS (ESI): mass calcd. for C
21H
24ClN
3O
5S, 465.1; m/z found, 466.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d6) δ 8.96 (d, J = 8.5 Hz, 1H), 8.51 (s, 2H), 7.61 - 7.46 (m, 2H), 7.43 - 7.27 (m, 2H), 6.85 - 6.64 (m, 2H), 6.11 (s, 1H), 5.04 (d, J = 5.9 Hz, 1H), 4.87 (d, J = 6.0 Hz, 1H), 4.80 - 4.71 (m, 1H), 4.21 (t, J = 5.4 Hz, 2H), 2.96 (s, 3H), 1.38 (s, 3H), 1.30 (d, J = 7.0 Hz, 3H). Example 179: 5-((*R)-(2-Chloro-3-fluorophenyl)(3-methyloxetan-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
from the SFC separation of 5-((2-chlorophenyl)(3-methyloxetan- 3-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 178, Step C, Rt = 4.33 min). MS (ESI): mass calcd. for C
21H
24ClN
3O
5S, 465.1; m/z found, 466.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.96 (d, J = 8.5 Hz, 1H), 8.51 (s, 2H), 7.63 - 7.43 (m, 2H), 7.42 - 7.28 (m, 2H), 6.84 - 6.74 (m, 1H), 6.74 - 6.65 (m, 1H), 6.12 (s, 1H), 5.05 (d, J = 5.8 Hz, 1H), 4.87 (d, J = 6.0 Hz, 1H), 4.82 - 4.70 (m, 1H), 4.21 (t, J = 5.2 Hz, 2H), 2.98 (s, 3H), 1.38 (s, 3H), 1.29 (d, J = 7.0 Hz, 3H). Example 180: 5-((*R)-1-(2-Chloro-3-fluorophenyl)ethoxy)-4-((*S)-2,2-difluorocyclopropyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 215 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 72 using 1-(2-chloro-3-
of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 4-(2,2- difluorocyclopropyl)-5-fluoropyrimidine-2-carboxylate (Intermediate 42) instead of methyl 6- fluoronicotinate in Step A. Separation of 5-(1-(2-chloro-3-fluorophenyl)ethoxy)-4-(2,2- difluorocyclopropyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% EtOH/CO
2, Rt = 17.2 min) provided the title compound. MS (ESI): mass calcd. for C
21H
21ClF
3N
3O
4S, 503.0; m/z found, 504.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.85 (d, J = 8.5 Hz, 1H), 8.28 (s, 1H), 7.51 - 7.36 (m, 3H), 6.87 - 6.77 (m, 1H), 6.76 - 6.68 (m, 1H), 6.15 (q, J = 6.1 Hz, 1H), 4.87 - 4.71 (m, 1H), 3.65 - 3.48 (m, 1H), 2.99 (s, 3H), 2.80 - 2.66 (m, 1H), 2.22 - 2.09 (m, 1H), 1.71 (d, J = 6.3 Hz, 3H), 1.31 (d, J = 7.0 Hz, 3H). Example 181: 5-((*S)-1-(2-Chloro-3-fluorophenyl)ethoxy)-4-((*R)-2,2-difluorocyclopropyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
was isolated from the SFC separation of 5-(1-(2-chloro-3- fluorophenyl)ethoxy)-4-(2,2-difluorocyclopropyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine- 2-carboxamide (Example 180, Step C, Rt = 13.2 min). MS (ESI): mass calcd. for C
21H
21ClF
3N
3O
4S, 503.0; m/z found, 504.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.86 (d, J = 8.5 Hz, 1H), 8.34 (s, 1H), 7.49 - 7.32 (m, 3H), 6.85 - 6.77 (m, 1H), 6.76 - 6.66 (m, 1H), 6.20 - 6.08 (m, 1H), 4.87 - 4.73 (m, 1H), 3.55 - 3.42 (m, 1H), 2.99 (s, 3H), 2.78 - 2.65 (m, 1H), 2.23 - 2.09 (m, 1H), 1.72 (d, J = 6.3 Hz, 3H), 1.32 (d, J = 7.0 Hz, 3H). Example 182: 5-((*S)-1-(2-Chloro-3-fluorophenyl)ethoxy)-4-((*S)-2,2-difluorocyclopropyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 216 QB\184200.00081\93036187.2
VVID-747PC was isolated from the SFC separation of 5-(1-(2-chloro-3-
-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine- 2-carboxamide (Example 180, Step C, Rt = 6.99 min). MS (ESI): mass calcd. for C
21H
21ClF
3N
3O
4S, 503.0; m/z found, 504.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.86 (d, J = 8.5 Hz, 1H), 8.34 (s, 1H), 7.52 - 7.32 (m, 3H), 6.86 - 6.77 (m, 1H), 6.75 - 6.69 (m, 1H), 6.14 (q, J = 6.3 Hz, 1H), 4.87 - 4.72 (m, 1H), 3.55 - 3.40 (m, 1H), 2.98 (s, 3H), 2.79 - 2.65 (m, 1H), 2.22 - 2.09 (m, 1H), 1.72 (d, J = 6.3 Hz, 3H), 1.32 (d, J = 7.0 Hz, 3H). Example 183: 5-((S)-1-(2-Chlorophenyl)ethoxy)-4-(methoxymethyl)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 72 using (S)-1-(2- chlorophenyl)ethan-1-ol instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 5-fluoro-4- (methoxymethyl)pyrimidine-2-carboxylate (Intermediate 45) instead of methyl 6-fluoronicotinate in Step A. MS (ESI): mass calcd. for C
20H
24ClN
3O
5S, 453.1; m/z found, 454.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.85 (d, J = 8.5 Hz, 1H), 8.22 (s, 1H), 7.60 - 7.47 (m, 2H), 7.43 - 7.31 (m, 2H), 6.87 - 6.66 (m, 2H), 6.07 (q, J = 6.3 Hz, 1H), 4.86 - 4.74 (m, 1H), 4.73 - 4.61 (m, 2H), 3.42 (s, 3H), 2.99 (s, 3H), 1.67 (d, J = 6.3 Hz, 3H), 1.30 (d, J = 7.0 Hz, 3H). Example 184: 5-((*R)-(2-Chlorophenyl)(1-cyanocyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide. 217 QB\184200.00081\93036187.2
VVID-747PC in a manner analogous to Example 72 using 1-((2-
1-carbonitrile (Intermediate 33) instead of (S)-1-(2- chlorophenyl)ethan-1-amine and methyl 5-fluoropyrimidine-2-carboxylate instead of methyl 6- fluoronicotinate in Step A. Separation of 5-((2-chlorophenyl)(1-cyanocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: IH (3x25 cm); Mobile phase: 35% EtOH/CO
2, Rt = 5.7 min) provided the title compound. MS (ESI): mass calcd. for C
21H
21ClN
4O
4S, 460.1; m/z found, 461.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.97 (d, J = 8.5 Hz, 1H), 8.49 (s, 2H), 7.71 - 7.64 (m, 1H), 7.62 - 7.55 (m, 1H), 7.50 - 7.37 (m, 2H), 6.83 - 6.74 (m, 1H), 6.73 - 6.65 (m, 1H), 5.78 (s, 1H), 4.86 - 4.71 (m, 1H), 2.98 (s, 3H), 1.65 - 1.40 (m, 4H), 1.29 (d, J = 7.1 Hz, 3H). Example 185: 5-((*S)-(2-Chlorophenyl)(1-cyanocyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide. O N
isolated from the SFC separation of 5-((2-chlorophenyl)(1- cyanocyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 184, Step C, Rt = 7.8 min). MS (ESI): mass calcd. for C
21H
21ClN
4O
4S, 460.1; m/z found, 461.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 9.02 (d, J = 8.4 Hz, 1H), 8.55 (s, 2H), 7.75 - 7.69 (m, 1H), 7.69 - 7.63 (m, 1H), 7.54 - 7.46 (m, 2H), 6.89 - 6.79 (m, 1H), 6.79 - 6.73 (m, 1H), 5.84 (s, 1H), 4.93 - 4.75 (m, 1H), 3.03 (s, 3H), 1.76 - 1.45 (m, 4H), 1.36 (d, J = 7.0 Hz, 3H). Example 186: 5-((*R)-(2-Chlorophenyl)((*R)-2,2-difluorocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 218 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 72 using (2-
methanol (Intermediate 34) instead of (S)-1-(2- chlorophenyl)ethan-1-amine and methyl 5-fluoropyrimidine-2-carboxylate instead of methyl 6- fluoronicotinate in Step A. Separation of 5-((2-chlorophenyl)(2,2-difluorocyclopropyl)methoxy)-N-((R,E)- 4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK- O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2, Rt = 9.01 min) provided the title compound. MS (ESI): mass calcd. for C
20H
20ClF
2N
3O
4S, 471.1; m/z found, 472.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.96 (d, J = 8.5 Hz, 1H), 8.55 (s, 2H), 7.78 - 7.67 (m, 1H), 7.58 - 7.50 (m, 1H), 7.48 - 7.36 (m, 2H), 6.83 - 6.65 (m, 2H), 5.67 (d, J = 9.5 Hz, 1H), 4.82 - 4.71 (m, 1H), 2.97 (s, 3H), 2.75 - 2.59 (m, 1H), 1.92 - 1.76 (m, 1H), 1.69 - 1.54 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H). Example 187: 5-((*S)-(2-Chlorophenyl)((*S)-2,2-difluorocyclopropyl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
isolated from the SFC separation of 5-((2-chlorophenyl)(2,2- difluorocyclopropyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 186, Step C, Rt = 14.2 min). MS (ESI): mass calcd. for C
20H
20ClF
2N
3O
4S, 471.1; m/z found, 472.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.96 (d, J = 8.6 Hz, 1H), 8.55 (s, 2H), 7.77 - 7.68 (m, 1H), 7.58 - 7.51 (m, 1H), 7.46 - 7.38 (m, 2H), 6.84 - 6.74 (m, 1H), 6.74 - 6.67 (m, 1H), 5.67 (d, J = 9.5 Hz, 1H), 4.84 - 4.71 (m, 1H), 2.98 (s, 3H), 2.75 - 2.61 (m, 1H), 1.90 - 1.77 (m, 1H), 1.68 - 1.56 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H). Example 188: 5-((*R)-1-(2-Chlorophenyl)-2-methylpropoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide. 219 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 72 using 1-(2-chlorophenyl)-
of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 5-fluoro-4- methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 6-fluoronicotinate in Step A. Separation of 5-(1-(2-chlorophenyl)-2-methylpropoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2, Rt = 4.74 min) provided the title compound. MS (ESI): mass calcd. for C
21H
26ClN
3O
4S, 451.1; m/z found, 452.2 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.83 (d, J = 8.5 Hz, 1H), 7.95 (s, 1H), 7.59 - 7.40 (m, 2H), 7.38 - 7.31 (m, 2H), 6.84 - 6.64 (m, 2H), 5.59 (d, J = 6.6 Hz, 1H), 4.81 - 4.66 (m, 1H), 2.97 (s, 3H), 2.58 (s, 3H), 2.34 - 2.25 (m, 1H), 1.29 (d, J = 7.0 Hz, 3H), 1.12 (d, J = 6.6 Hz, 3H), 0.95 (d, J = 6.9 Hz, 3H). Example 189: 5-((*S)-1-(2-Chlorophenyl)-2-methylpropoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide.
isolated from the SFC separation of 5-(1-(2-chlorophenyl)-2- methylpropoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 188, Step C, Rt = 8.78 min). MS (ESI): mass calcd. for C
21H
26ClN
3O
4S, 451.1; m/z found, 452.2 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.83 (d, J = 8.5 Hz, 1H), 7.95 (s, 1H), 7.59 - 7.50 (m, 1H), 7.46 - 7.39 (m, 1H), 7.38 - 7.27 (m, 2H), 6.82 - 6.75 (m, 1H), 6.73 - 6.65 (m, 1H), 5.59 (d, J = 6.8 Hz, 1H), 4.83 - 4.72 (m, 1H), 2.98 (s, 3H), 2.58 (s, 3H), 2.36 - 2.23 (m, 1H), 1.28 (d, J = 7.0 Hz, 3H), 1.12 (d, J = 6.6 Hz, 3H), 0.95 (d, J = 6.9 Hz, 3H). Example 190: 5-((*R)-(2-Chlorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 220 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 72 using (2-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 5-fluoro- 4-methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 6-fluoronicotinate in Step A. Separation of 5-((2-chlorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2, Rt = 4.88 min) provided the title compound. MS (ESI): mass calcd. for C
21H
24ClN
3O
4S, 449.1; m/z found, 450.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.84 (d, J = 8.5 Hz, 1H), 8.05 (s, 1H), 7.60 - 7.47 (m, 2H), 7.39 - 7.31 (m, 2H), 6.80 - 6.73 (m, 1H), 6.72 - 6.65 (m, 1H), 5.45 (d, J = 7.9 Hz, 1H), 4.82 - 4.70 (m, 1H), 2.97 (s, 3H), 2.56 (s, 3H), 1.58 - 1.47 (m, 1H), 1.29 (d, J = 7.0 Hz, 3H), 0.74 - 0.53 (m, 4H). Example 191: 5-((*S)-(2-Chlorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
was isolated from the SFC separation of 5-((2- chlorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide (Example 190, Step C, Rt = 9.37 min). MS (ESI): mass calcd. for C
21H
24ClN
3O
4S, 449.1; m/z found, 450.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.85 (d, J = 8.5 Hz, 1H), 8.05 (s, 1H), 7.64 - 7.48 (m, 2H), 7.41 - 7.30 (m, 2H), 6.82 - 6.75 (m, 1H), 6.73 - 6.67 (m, 1H), 5.46 (d, J = 7.9 Hz, 1H), 4.82 - 4.70 (m, 1H), 2.99 (s, 3H), 2.57 (s, 3H), 1.60 - 1.48 (m, 1H), 1.29 (d, J = 7.0 Hz, 3H), 0.75 - 0.53 (m, 4H). Example 192: 5-((*R)-1-(2-Chloro-3-fluorophenyl)-2-methylpropoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 221 QB\184200.00081\93036187.2
VVID-747PC O N N (R) S prepared in a manner analogous to Example 72 using 1-(2-chloro-3-
ol (Intermediate 29) instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 5-fluoro-4-methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 6-fluoronicotinate in Step A. Separation of 5-(1-(2-chloro-3-fluorophenyl)-2-methylpropoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK- O1 (3x25 cm); Mobile phase: 60% MeOH/CO
2, Rt = 7.32 min) provided the title compound. MS (ESI): mass calcd. for C
21H
25ClFN
3O
4S, 469.1; m/z found, 470.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.84 (d, J = 8.5 Hz, 1H), 7.97 (s, 1H), 7.48 - 7.34 (m, 2H), 7.33 - 7.22 (m, 1H), 6.82 - 6.73 (m, 1H), 6.73 - 6.63 (m, 1H), 5.64 (d, J = 6.6 Hz, 1H), 4.83 - 4.69 (m, 1H), 2.97 (s, 3H), 2.58 (s, 3H), 2.30 (qd, J = 13.4, 6.7 Hz, 1H), 1.30 (d, J = 7.0 Hz, 3H), 1.11 (d, J = 6.6 Hz, 3H), 0.96 (d, J = 6.8 Hz, 3H). Example 193: 5-((*S)-1-(2-Chloro-3-fluorophenyl)-2-methylpropoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. O N
isolated from the SFC separation of 5-(1-(2-chloro-3-fluorophenyl)-2- methylpropoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 192, Step C, Rt = 12.4 min). MS (ESI): mass calcd. for C
21H
25ClFN
3O
4S, 469.1; m/z found, 470.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.84 (d, J = 8.5 Hz, 1H), 7.97 (s, 1H), 7.46 - 7.34 (m, 2H), 7.32 - 7.24 (m, 1H), 6.83 - 6.74 (m, 1H), 6.74 - 6.64 (m, 1H), 5.64 (d, J = 6.6 Hz, 1H), 4.76 (dt, J = 6.8, 5.7 Hz, 1H), 2.98 (s, 3H), 2.58 (s, 3H), 2.38 - 2.22 (m, 1H), 1.29 (d, J = 7.0 Hz, 3H), 1.11 (d, J = 6.6 Hz, 3H), 0.96 (d, J = 6.9 Hz, 3H). Example 194: 5-((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 222 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 72 using (2-chloro-3-
(Intermediate 10) instead of (S)-1-(2-chlorophenyl)ethan-1-amine and methyl 5-fluoro-4-methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 6- fluoronicotinate in Step A. Separation of 5-((2-chloro-3-fluorophenyl)(cyclopropyl)methoxy)-4-methyl-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 40% IPA/CO
2, Rt = 8.47 min) provided the title compound. MS (ESI): mass calcd. for C
21H
23ClFN
3O
4S, 467.1; m/z found, 468.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.85 (d, J = 8.5 Hz, 1H), 8.08 (s, 1H), 7.47 - 7.35 (m, 3H), 6.83 - 6.74 (m, 1H), 6.73 - 6.66 (m, 1H), 5.49 (d, J = 7.9 Hz, 1H), 4.84 - 4.67 (m, 1H), 2.98 (s, 3H), 2.56 (s, 3H), 1.59 - 1.50 (m, 1H), 1.29 (d, J = 7.0 Hz, 3H), 0.80 - 0.62 (m, 2H), 0.61 - 0.51 (m, 2H). Example 195: 5-((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
was isolated from the SFC separation of 5-((2-chloro-3- fluorophenyl)(cyclopropyl)methoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide (Example 194, Step C, Rt = 10.5 min). MS (ESI): mass calcd. for C
21H
23ClFN
3O
4S, 467.1; m/z found, 468.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.84 (d, J = 8.5 Hz, 1H), 8.08 (s, 1H), 7.48 - 7.35 (m, 3H), 6.81 - 6.73 (m, 1H), 6.73 - 6.63 (m, 1H), 5.49 (d, J = 7.9 Hz, 1H), 4.82 - 4.69 (m, 1H), 2.97 (s, 3H), 2.56 (s, 3H), 1.59 - 1.51 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H), 0.75 - 0.62 (m, 2H), 0.62 - 0.53 (m, 2H). Example 196: 5-((*S)-(2-Chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 223 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 72 using (2-chloro-3-
methanol (Intermediate 32) instead of (S)-1-(2-chlorophenyl)ethan-1- amine and methyl 5-fluoropyrimidine-2-carboxylate instead of methyl 6-fluoronicotinate in Step A. Separation of 5-((2-chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: IH (3x25 cm); Mobile phase: 30% EtOH/CO
2, Rt = 4.12 min) provided the title compound. MS (ESI): mass calcd. for C
20H
20ClF
2N
3O
5S, 487.1; m/z found, 488.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.98 (d, J = 8.4 Hz, 1H), 8.56 (s, 2H), 7.57 - 7.34 (m, 3H), 6.80 - 6.74 (m, 1H), 6.73 - 6.68 (m, 1H), 6.65 (d, J = 17.1 Hz, 1H), 5.17 - 5.03 (m, 1H), 4.97 - 4.83 (m, 1H), 4.82 - 4.61 (m, 3H), 2.97 (s, 3H), 1.30 (d, J = 7.0 Hz, 3H). Example 197: 5-((*R)-(2-Chloro-3-fluorophenyl)(3-fluorooxetan-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide.
from the SFC separation of 5-((2-chloro-3-fluorophenyl)(3- fluorooxetan-3-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 196, Step C, Rt = 6.53 min). MS (ESI): mass calcd. for C
20H
20ClF
2N
3O
5S, 487.1; m/z found, 488.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.98 (d, J = 8.5 Hz, 1H), 8.56 (s, 2H), 7.63 - 7.34 (m, 3H), 6.84 - 6.74 (m, 1H), 6.74 - 6.68 (m, 1H), 6.65 (d, J = 17.3 Hz, 1H), 5.16 - 5.04 (m, 1H), 4.96 - 4.83 (m, 1H), 4.82 - 4.62 (m, 3H), 2.98 (s, 3H), 1.30 (d, J = 7.0 Hz, 3H). Example 198: 5-(((S)-1-(2-Chlorophenyl)ethyl)(methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrazine-2-carboxamide. 224 QB\184200.00081\93036187.2
VVID-747PC was prepared in a manner analogous to Example 101 using methyl 5-
instead of methyl 5-fluoropyrimidine-2-carboxylate and triethylamine instead of pyridine in Step A and using iodomethane instead of iodoethane in Step B. MS (ESI): mass calcd. for C
19H
23ClN
4O
3S, 422.1; m/z found, 423.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.92 (d, J = 1.4 Hz, 1H), 7.89 (d, J = 1.4 Hz, 1H), 7.55 (d, J = 8.4 Hz, 1H), 7.44 (ddd, J = 13.5, 7.6, 1.8 Hz, 2H), 7.39 – 7.24 (m, 2H), 6.98 (dd, J = 15.1, 4.5 Hz, 1H), 6.53 (dd, J = 15.1, 1.8 Hz, 1H), 6.04 (q, J = 6.9 Hz, 1H), 5.06 – 4.95 (m, 1H), 2.97 (s, 2H), 2.89 (s, 3H), 1.65 (d, J = 6.9 Hz, 4H), 1.47 (d, J = 7.1 Hz, 3H). Example 199: 5-((S)-1-(2-Chlorophenyl)ethoxy)-4-methyl-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. O N N
(R) S was prepared in a manner analogous to Example 107 using (S)-1-(2-
chlorophenyl)ethan-1-ol instead of 1-(2-chloro-3-fluorophenyl)ethan-1-ol, methyl 5-fluoro-4- methylpyrimidine-2-carboxylate (Intermediate 18) instead of methyl 5-fluoropyrimidine-2-carboxylate, and Cs
2CO
3/DMF instead of NaH/THF in Step A. MS (ESI): mass calcd. for C
19H
22ClN
3O
4S, 423.1; m/z found, 424.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.82 (d, J = 8.4 Hz, 1H), 8.08 (s, 1H), 7.51 (dt, J = 7.1, 1.8 Hz, 2H), 7.39 - 7.31 (m, 2H), 6.82 - 6.76 (m, 1H), 6.74 - 6.66 (m, 1H), 6.01 (q, J = 6.3 Hz, 1H), 4.83 - 4.69 (m, 1H), 2.98 (s, 3H), 2.55 (s, 3H), 1.67 (d, J = 6.3 Hz, 3H), 1.29 (d, J = 7.0 Hz, 3H). Example 200: 5-((*R)-(2-Chlorophenyl)(cyclobutyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. O N
VVID-747PC [00537] The title compound was prepared in a manner analogous to Example 107 using (2- chlorophenyl)(cyclobutyl)methanol (Intermediate 28) instead of 1-(2-chloro-3-fluorophenyl)ethan-1-ol and Cs
2CO
3/DMF instead of NaH/THF in Step A. Separation of 5-((2-chlorophenyl)(cyclobutyl)methoxy)- N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: IH (3x25 cm); Mobile phase: 60% IPA/CO
2, Rt = 5.32 min) provided the title compound. MS (ESI): mass calcd. for C
21H
24ClN
3O
4S, 449.1; m/z found, 450. [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.4 Hz, 1H), 8.49 (s, 2H), 7.55 - 7.30 (m, 4H), 6.83 - 6.65 (m, 2H), 5.90 (d, J = 7.3 Hz, 1H), 4.84 - 4.71 (m, 1H), 2.98 (s, 3H), 2.95 - 2.89 (m, 1H), 2.24 - 2.12 (m, 1H), 2.06 - 1.94 (m, 2H), 1.91 - 1.80 (m, 3H), 1.29 (d, J = 7.0 Hz, 3H). Example 201: 5-((*S)-(2-Chlorophenyl)(cyclobutyl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. was isolated from the SFC separation of 5-((2-
-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2- carboxamide (Example 200, Step C, Rt = 8.81 min). MS (ESI): mass calcd. for C
21H
24ClN
3O
4S, 449.1; m/z found, 450.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.5 Hz, 1H), 8.48 (s, 2H), 7.57 - 7.30 (m, 4H), 6.84 - 6.65 (m, 2H), 5.90 (d, J = 7.4 Hz, 1H), 4.85 - 4.71 (m, 1H), 3.02 - 2.87 (m, 4H), 2.24 - 2.12 (m, 1H), 2.06 - 1.93 (m, 2H), 1.91 - 1.80 (m, 3H), 1.30 (d, J = 7.0 Hz, 3H). Example 202: 5-((*S)-1-(2-Chlorophenyl)-2-methylpropoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
in a manner analogous to Example 107 using 1-(2-chlorophenyl)- 2-methylpropan-1-ol instead of 1-(2-chloro-3-fluorophenyl)ethan-1-ol and Cs
2CO
3/DMF instead of NaH/THF in Step A. Separation of 5-(1-(2-chlorophenyl)-2-methylpropoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK- O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2, Rt = 14.8 min) provided the title compound. MS (ESI): 226 QB\184200.00081\93036187.2
VVID-747PC mass calcd. for C
20H
24ClN
3O
4S, 437.1; m/z found, 438.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.92 (d, J = 8.5 Hz, 1H), 8.46 (s, 2H), 7.59 - 7.31 (m, 4H), 6.85 - 6.64 (m, 2H), 5.62 (d, J =7.0 Hz, 1H), 4.87 - 4.68 (m, 1H), 2.98 (s, 3H), 2.37 - 2.21 (m, 1H), 1.29 (d, J = 7.0 Hz, 3H), 1.12 (d, J = 6.6 Hz, 3H), 0.92 (d, J = 6.9 Hz, 3H). Example 203: 5-((*R)-1-(2-Chlorophenyl)-2-methylpropoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. isolated from the SFC separation of 5-(1-(2-chlorophenyl)-2-
- - but-3-en-2-yl)pyrimidine-2-carboxamide (Example 202, Step C, Rt = 8.02 min). MS (ESI): mass calcd. for C
20H
24ClN
3O
4S, 437.1; m/z found, 438.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.92 (d, J = 8.5 Hz, 1H), 8.46 (s, 2H), 7.63 - 7.27 (m, 4H), 6.89 - 6.60 (m, 2H), 5.62 (d, J = 7.1 Hz, 1H), 4.85 - 4.67 (m, 1H), 2.97 (s, 3H), 2.36 - 2.20 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H), 1.12 (d, J = 6.6 Hz, 3H), 0.92 (d, J = 6.9 Hz, 3H). Example 204: 5-((*S)-1-(2-Chlorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 107 using 1-(2- chlorophenyl)propan-1-ol instead of 1-(2-chloro-3-fluorophenyl)ethan-1-ol and Cs
2CO
3/DMF instead of NaH/THF in Step A. Separation of 5-(1-(2-chlorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: IH (3x25 cm); Mobile phase: 22% EtOH/CO
2, Rt = 8.2 min) provided the title compound. MS (ESI): mass calcd. for C
19H
22ClN
3O
4S, 423.1; m/z found, 424.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.4 Hz, 1H), 8.48 (s, 2H), 7.60 - 7.43 (m, 2H), 7.40 - 7.31 (m, 2H), 6.82 - 6.64 (m, 2H), 5.84 (t, J = 6.4 Hz, 1H), 4.77 (br dd, J = 12.1, 7.1 Hz, 1H), 2.97 (s, 3H), 2.11 - 1.90 (m, 2H), 1.30 (d, J = 7.0 Hz, 3H), 1.00 (t, J = 7.4 Hz, 3H). 227 QB\184200.00081\93036187.2
VVID-747PC Example 205: 5-((*R)-1-(2-Chlorophenyl)propoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide. isolated from the SFC separation of 5-(1-(2-chlorophenyl)propoxy)-N-
2-yl)pyrimidine-2-carboxamide (Example 204, Step C, Rt = 5.9 min). MS (ESI): mass calcd. for C
19H
22ClN
3O
4S, 423.1; m/z found, 424.1 [M+H]
+.
1H NMR (400 MHz, DMSO- d
6) δ 8.92 (d, J = 8.4 Hz, 1H), 8.48 (s, 2H), 7.56 - 7.44 (m, 2H), 7.38 - 7.31 (m, 2H), 6.83 - 6.65 (m, 2H), 5.84 (t, J = 6.4 Hz, 1H), 4.89 - 4.70 (m, 1H), 2.98 (s, 3H), 2.14 - 1.89 (m, 2H), 1.29 (d, J = 7.0 Hz, 3H), 1.00 (t, J = 7.4 Hz, 3H). Example 206: 5-((*R)-1-(2-Chloro-3-fluorophenyl)-2-methylpropoxy)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide.
prepared in a manner analogous to Example 107 using 1-(2-chloro-3- fluorophenyl)-2-methylpropan-1-ol (Intermediate 29) instead of 1-(2-chloro-3-fluorophenyl)ethan-1-ol and Cs
2CO
3/DMSO instead of NaH/THF in Step A. Separation of 5-(1-(2-chloro-3-fluorophenyl)-2- methylpropoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Step C) via SFC (Stationary phase: WHELK-O1 (3x25 cm); Mobile phase: 50% MeOH/CO
2, Rt = 4.54 min) provided the title compound. MS (ESI): mass calcd. for C
20H
23ClFN
3O
4S, 455.1; m/z found, 456.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.4 Hz, 1H), 8.48 (s, 2H), 7.50 - 7.35 (m, 2H), 7.32 (br s, 1H), 6.82 - 6.74 (m, 1H), 6.74 - 6.66 (m, 1H), 5.66 (d, J = 6.9 Hz, 1H), 4.88 - 4.68 (m, 1H), 2.97 (s, 3H), 2.37 - 2.20 (m, 1H), 1.30 (d, J = 7.0 Hz, 3H), 1.11 (d, J = 6.6 Hz, 3H), 0.93 (d, J = 6.9 Hz, 3H). Example 207: 5-((*S)-1-(2-Chloro-3-fluorophenyl)-2-methylpropoxy)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide. 228 QB\184200.00081\93036187.2
VVID-747PC isolated from the SFC separation of 5-(1-(2-chloro-3-fluorophenyl)-2-
but-3-en-2-yl)pyrimidine-2-carboxamide (Example 206, Step C, Rt = 7.97 min). MS (ESI): mass calcd. for C
20H
23ClFN
3O
4S, 455.1; m/z found, 456.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.93 (d, J = 8.5 Hz, 1H), 8.48 (s, 2H), 7.45 - 7.35 (m, 2H), 7.31 (dd, J = 6.3, 3.1 Hz, 1H), 6.77 (d, J = 4.9 Hz, 1H), 6.74 - 6.64 (m, 1H), 5.67 (d, J = 6.9 Hz, 1H), 4.87 - 4.68 (m, 1H), 2.98 (s, 3H), 2.35 - 2.22 (m, 1H), 1.29 (d, J = 7.0 Hz, 3H), 1.11 (d, J = 6.6 Hz, 3H), 0.93 (d, J = 6.9 Hz, 3H). Example 208: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-4-cyclopropyl-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide. 5-fluoropyrimidine-2-carbonitrile.5-Fluoropyrimidine-2-carbonitrile (500
mg, 4.06 mmol, 1.0 eq) was taken up in ACN/water (v/v 1:1, 20 mL, 0.18 M) and placed under N
2. Silver nitrate (3.6 g, 21.2 mmol, 5.2 eq) and cyclopropanecarboxylic acid (1.0 g, 12.2 mmol, 3.0 eq) were added and the mixture was heated to 100 °C. A solution of ammonium persulfate (1.9 g, 8.12 mmol, 2.0 eq) in water (2.0 mL, 0.18 M) was added dropwise at 100 °C and the reaction was allowed to stir for 16 h. Upon cooling, the mixture was diluted with EtOAc and brine. The mixture was filtered and the filtrate was separated. The organic layers were washed with water and brine, dried over Na
2SO
4, filtered and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 1:0 - 5:1) and prep- TLC (PE:EtOAc = 5:1) to give 4-cyclopropyl-5-fluoropyrimidine-2-carbonitrile (200 mg, 30% yield) as a yellow solid.
1H NMR (400 MHz, CDCl
3) δ 8.44 (s, 1H), 2.42 - 2.32 (m, 1H), 1.36 - 1.28 (m, 4H). [00546] Step B: (S)-5-((1-(2-Chlorophenyl)ethyl)amino)-4-cyclopropylpyrimidine-2-carbonitrile. 4- Cyclopropyl-5-fluoropyrimidine-2-carbonitrile (100 mg, 0.613 mmol, 1.0 eq) was taken up in DMSO (2.0 mL, 0.31 M) and placed under N
2. (S)-1-(2-Chlorophenyl)ethan-1-amine (105 mg, 0.674 mmol, 1.1 eq) and DIPEA (158 mg, 1.23 mmol, 2.0 eq) were added and the reaction was stirred at 100 °C for 16 h. After cooling to rt, the mixture was diluted with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified 229 QB\184200.00081\93036187.2
VVID-747PC by prep-TLC (PE:EtOAc = 3:1) to give (S)-5-((1-(2-chlorophenyl)ethyl)amino)-4-cyclopropylpyrimidine- 2-carbonitrile (100 mg, 55% yield) as a yellow solid.
1H NMR (400 MHz, CDCl
3) δ 7.55 (s, 1H), 7.45 - 7.38 (m, 1H), 7.33 - 7.28 (m, 1H), 7.26 - 7.21 (m, 2H), 5.03 (q, J = 6.4 Hz, 1H), 4.84 (br d, J = 5.0 Hz, 1H), 1.95 - 1.84 (m, 1H), 1.66 (d, J = 6.8 Hz, 3H), 1.27 - 1.15 (m, 4H). [00547] Step C: (S)-5-((1-(2-Chlorophenyl)ethyl)amino)-4-cyclopropylpyrimidine-2-carboxylic acid. (S)- 5-((1-(2-Chlorophenyl)ethyl)amino)-4-cyclopropylpyrimidine-2-carbonitrile (50 mg, 0.167 mmol, 1.0 eq) was taken up in 10% aq. NaOH (2.0 mL) and stirred at 100 °C for 16 h. After cooling to rt, the mixture was diluted with water, adjusted to pH ~4 with 1N HCl, and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, and filtered. The filtrate was concentrated under reduced pressure to give (S)-5-((1-(2-chlorophenyl)ethyl)amino)-4-cyclopropylpyrimidine-2-carboxylic acid (50 mg, 94% yield) as a yellow solid, which was used in the next step without further purification. [00548] Step D: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-4-cyclopropyl-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)pyrimidine-2-carboxamide. (S)-5-((1-(2-Chlorophenyl)ethyl)amino)-4-cyclopropylpyrimidine- 2-carboxylic acid (50 mg, 0.157 mmol, 1.0 eq) was taken up in DCM (1.0 mL, 0.16 M). (R,E)-4- (Methylsulfonyl)but-3-en-2-amine (Intermediate 1, 61 mg, 0.189 mmol, 1.2 eq, TsOH salt), DIPEA (61 mg, 0.472 mmol, 3.0 eq) and T
4P (170 mg, 0.236 mmol, 1.5 eq) were added and the reaction was stirred at rt for 2 h. The mixture was diluted with water and extracted with DCM. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered and concentrated under reduced pressure. The residue was purified by prep-TLC (PE:EtOAc = 0:1) to give 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-4-cyclopropyl-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (10 mg, 15% yield) as a pale yellow solid. MS (ESI): mass calcd. for C
21H
25ClN
4O
3S, 448.1; m/z found, 449.2 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.51 (d, J = 8.4 Hz, 1H), 7.48 (dd, J = 1.6, 7.6 Hz, 2H), 7.41 (s, 1H), 7.36 - 7.21 (m, 2H), 6.88 - 6.73 (m, 2H), 6.71 - 6.61 (m, 1H), 4.98 (t, J = 6.8 Hz, 1H), 4.78 - 4.63 (m, 1H), 2.98 (s, 3H), 2.56 (br d, J = 6.8 Hz, 1H), 1.57 (d, J = 6.6 Hz, 3H), 1.29 - 1.20 (m, 4H), 1.13 - 1.04 (m, 3H). Example 209: 1-((*R)-1-(2-Chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-indazole- 5-carboxamide.
chlorophenyl)ethyl)-1H-indazole-5-carboxylate. Methyl 1H-indazole-5- carboxylate (150 mg, 0.851 mmol, 1.0 eq) was taken up in DMF (4.3 mL, 0.2M). To this was added, potassium carbonate (235 mg, 1.70 mmol, 2.0 eq) and the mixture was allowed to stir for 5 minutes. 1-Chloro-2-(1-chloroethyl)benzene (124 µL, 0.851 mmol, 1.0 eq) was added and the reaction was stirred at 100 ºC for 16 h. After cooling to rt, the reaction was quenched with water and extracted with EtOAc. 230 QB\184200.00081\93036187.2
VVID-747PC The combined organic extracts were dried over Na
2SO
4, filtered, and concentrated under reduced pressure. Purification via RP-HPLC (10-100% ACN in 0.1% HCOOH water) provided methyl 1-(1-(2- chlorophenyl)ethyl)-1H-indazole-5-carboxylate (95 mg, 35% yield). MS (ESI): mass calcd. for C
17H
15ClN
2O
2, 314.1; m/z found, 315.2 [M+H]
+. [00550] Step B: 1-(1-(2-Chlorophenyl)ethyl)-1H-indazole-5-carboxylic acid. Methyl 1-(1-(2- chlorophenyl)ethyl)-1H-indazole-5-carboxylate (95 mg, 0.302 mmol, 1.0 eq) was taken up in methanol (1.5 mL, 0.2M). To this was added LiOH (1.2 mL, 2.41 mmol, 8.0 eq, 2M in water) and the reaction was stirred at 60 °C for 3 hours. After cooling to rt, the reaction was adjusted to a pH∼2 with 1N HCl. The precipitated product was collected by filtration to provide 1-(1-(2-chlorophenyl)ethyl)-1H-indazole-5- carboxylic acid (66 mg, 72% yield) as a white solid. MS (ESI): mass calcd. for C
16H
13ClN
2O
2, 300.1; m/z found, 301.0 [M+H]
+. [00551] Step C: 1-((*R)-1-(2-Chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H- indazole-5-carboxamide. 1-(1-(2-Chlorophenyl)ethyl)-1H-indazole-5-carboxylic acid (66 mg, 0.218 mmol, 1.0 eq) and HATU (100 mg, 0.262 mmol, 1.2 eq) were taken up in DMF (1.1 mL, 0.2M). DIPEA (114 µL, 0.654 mmol, 3.0 eq) was added and the mixture was allowed to stir for 5 minutes. (R,E)-4- (Methylsulfonyl)but-3-en-2-amine (70 mg, 0.218 mmol, 1.0 eq, TsOH salt) was added and the reaction was allowed to stir for 30 min at rt. Purification via RP-HPLC (20-60% ACN in 0.1% HCOOH water) provided 1-(1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-indazole-5- carboxamide (57 mg, 60% yield). This product was separated via SFC (Stationary phase: IG (2x 25cm); Mobile phase: 35% EtOH/CO
2 with 0.1% diethylamine; Rt = 5.83 min) to provide the title compound. MS (ESI): mass calcd. for C
21H
22ClN
3O
3S, 431.1; m/z found, 432.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.18 – 8.06 (m, 2H), 7.66 (dd, J = 8.9, 1.6 Hz, 1H), 7.36 – 7.26 (m, 2H), 7.15 – 7.02 (m, 3H), 6.88 (dd, J = 15.1, 4.7 Hz, 1H), 6.44 (dd, J = 15.1, 1.7 Hz, 1H), 6.21 (dt, J = 14.3, 7.2 Hz, 2H), 5.01 – 4.88 (m, 1H), 2.86 (s, 3H), 1.97 (d, J = 6.9 Hz, 3H), 1.37 (d, J = 7.1 Hz, 3H). Example 210: 1-((*S)-1-(2-Chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-indazole- 5-carboxamide.
from the SFC separation of 1-(1-(2-chlorophenyl)ethyl)-N-((R,E)- 4-(methylsulfonyl)but-3-en-2-yl)-1H-indazole-5-carboxamide (Example 209, Step C, Rt = 4.11 min). MS (ESI): mass calcd. for C
21H
22ClN
3O
3S, 431.1; m/z found, 432.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.19 – 8.02 (m, 2H), 7.67 (dd, J = 8.8, 1.7 Hz, 1H), 7.36 – 7.25 (m, 2H), 7.15 – 7.02 (m, 3H), 6.88 (dd, J 231 QB\184200.00081\93036187.2
VVID-747PC = 15.1, 4.7 Hz, 1H), 6.44 (dd, J = 15.1, 1.7 Hz, 1H), 6.26 – 6.15 (m, 2H), 5.01 – 4.88 (m, 1H), 2.85 (s, 3H), 1.96 (d, J = 6.9 Hz, 3H), 1.37 (d, J = 7.1 Hz, 3H). Example 211: 3-((*R)-1-(2-Chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3H- imidazo[4,5-c]pyridine-6-carboxamide. prepared in a manner analogous to Example 209 using methyl 3H-
instead of methyl 1H-indazole-5-carboxylate in Step A. Separation of 3-(1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6- carboxamide (Step C) via SFC (Stationary phase: AD-H (3x25 cm); Mobile phase: 40% MeOH/CO
2 with 0.1% NH
4OH; Rt = 3.95 min) provided the title compound. MS (ESI): mass calcd. for C
20H
21ClN
4O
3S, 432.1; m/z found, 433.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.52 (d, J = 1.0 Hz, 1H), 8.42 (d, J = 1.0 Hz, 1H), 8.25 (s, 1H), 8.07 (d, J = 8.4 Hz, 1H), 7.39 (dd, J = 7.7, 1.6 Hz, 1H), 7.21 (dtd, J = 17.9, 7.4, 1.6 Hz, 2H), 7.03 (dd, J = 7.6, 1.9 Hz, 1H), 6.90 (dd, J = 15.1, 4.5 Hz, 1H), 6.45 (dd, J = 15.1, 1.8 Hz, 1H), 6.11 (q, J = 7.0 Hz, 1H), 4.98 – 4.87 (m, 1H), 2.85 (s, 3H), 2.01 (d, J = 7.0 Hz, 3H), 1.37 (d, J = 7.1 Hz, 3H). Example 212: 3-((*S)-1-(2-Chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3H- imidazo[4,5-c]pyridine-6-carboxamide.
from the SFC separation of 3-(1-(2-chlorophenyl)ethyl)-N-((R,E)- 4-(methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide (Example 211, Step C, Rt = 2.82 min). MS (ESI): mass calcd. for C
20H
21ClN
4O
3S, 432.1; m/z found, 433.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.52 (d, J = 1.0 Hz, 1H), 8.42 (d, J = 0.9 Hz, 1H), 8.26 (s, 1H), 8.10 (d, J = 8.4 Hz, 1H), 7.39 (dd, J = 7.7, 1.6 Hz, 1H), 7.22 (dtd, J = 17.6, 7.4, 1.6 Hz, 2H), 7.03 (dd, J = 7.6, 1.9 Hz, 1H), 6.87 (dd, J = 15.1, 4.5 Hz, 1H), 6.42 (dd, J = 15.1, 1.7 Hz, 1H), 6.10 (q, J = 7.0 Hz, 1H), 4.99 – 4.85 (m, 1H), 2.83 (s, 3H), 2.00 (d, J = 7.0 Hz, 3H), 1.38 (d, J = 7.1 Hz, 3H). 232 QB\184200.00081\93036187.2
VVID-747PC Example 213: 1-((*R)-1-(2-Chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H- pyrazolo[3,4-c]pyridine-5-carboxamide. prepared in a manner analogous to Example 209 using methyl 1H-
instead of methyl 1H-indazole-5-carboxylate in Step A. Separation of 3-(1-(2-chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6- carboxamide (Step C) via SFC (Stationary phase: C9 (2x25 cm); Mobile phase: 20% MeOH/CO
2; Rt = 7.62 min) provided the title compound. MS (ESI): mass calcd. for C
20H
21ClN
4O
3S, 432.1; m/z found, 433.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.78 (s, 1H), 8.57 (d, J = 1.2 Hz, 1H), 8.27 (s, 1H), 8.15 (d, J = 8.5 Hz, 1H), 7.46 – 7.36 (m, 1H), 7.33 – 7.13 (m, 3H), 6.98 (dd, J = 15.1, 4.4 Hz, 1H), 6.57 – 6.37 (m, 2H), 5.11 – 4.97 (m, 1H), 2.92 (s, 3H), 2.10 (d, J = 6.9 Hz, 3H), 1.49 (d, J = 7.1 Hz, 3H). Example 214: 1-((*S)-1-(2-Chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H- pyrazolo[3,4-c]pyridine-5-carboxamide.
from the SFC separation of 3-(1-(2-chlorophenyl)ethyl)-N-((R,E)- 4-(methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide (Example 213, Step C, Rt = 6.88 min). MS (ESI): mass calcd. for C
20H
21ClN
4O
3S, 432.1; m/z found, 433.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.78 (s, 1H), 8.55 (s, 1H), 8.35 – 8.00 (m, 2H), 7.42 (s, 1H), 7.24 (p, J
Hz, 3H), 7.00 (dd, J = 15.3, 4.5 Hz, 1H), 6.67 – 6.30 (m, 2H), 5.03 (q, J = 6.8 Hz, 1H), 2.94 (s, 3H), 2.09 (d, J = 7.0 Hz, 3H), 1.46 (d, J = 7.2 Hz, 3H). Example 215: 1-(1-(2-Chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)indoline-5- carboxamide. 233 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 209 using methyl indoline-
1H-indazole-5-carboxylate in Step A. MS (ESI): mass calcd. for C
22H
25ClN
2O
4S, 432.1; m/z found, 433.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.42 (s, 1H), 7.39 – 7.26 (m, 3H), 7.19 – 7.09 (m, 2H), 6.90 – 6.80 (m, 1H), 6.47 – 6.33 (m, 1H), 6.12 (d, J = 8.3 Hz, 1H), 5.79 (d, J = 7.7 Hz, 1H), 4.99 (q, J = 6.9 Hz, 1H), 4.90 (q, J = 6.6 Hz, 1H), 3.64 – 3.44 (m, 2H), 2.98 (t, J = 8.6 Hz, 2H), 2.90 – 2.80 (m, 3H), 1.50 (d, J = 6.9 Hz, 3H), 1.33 (dd, J = 7.1, 1.7 Hz, 3H). Example 216: 4-((*R)-1-(2-Chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3,4-dihydro- 2H-benzo[b][1,4]oxazine-7-carboxamide.
prepared in a manner analogous to Example 209 using methyl 3,4- dihydro-2H-1,4-benzoxazine-7-carboxylate instead of methyl 1H-indazole-5-carboxylate and cesium carbonate instead of potassium carbonate in Step A. Separation of 4-(1-(2-chlorophenyl)ethyl)-N-((R,E)- 4-(methylsulfonyl)but-3-en-2-yl)-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carboxamide (Step C) via SFC (Stationary phase: C9 (2x25 cm); Mobile phase: 25% EtOH/CO
2; Rt = 7.42 min) provided the title compound. MS (ESI): mass calcd. for C
22H
25ClN
2O
4S, 448.1; m/z found, 449.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.45 – 7.40 (m, 1H), 7.39 – 7.34 (m, 1H), 7.34 – 7.23 (m, 4H), 6.93 (dd, J = 15.1, 4.5 Hz, 1H), 6.73 – 6.64 (m, 1H), 6.48 (dd, J = 15.1, 1.7 Hz, 1H), 6.18 (d, J = 7.9 Hz, 1H), 5.31 (q, J = 6.9 Hz, 1H), 5.06 – 4.90 (m, 1H), 4.25 – 4.10 (m, 2H), 3.48 – 3.38 (m, 1H), 3.27 – 3.17 (m, 1H), 2.92 (s, 3H), 1.60 (d, J = 7.0 Hz, 3H), 1.41 (d, J = 7.1 Hz, 3H). Example 217: 4-((*S)-1-(2-Chlorophenyl)ethyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3,4-dihydro- 2H-benzo[b][1,4]oxazine-7-carboxamide. 234 QB\184200.00081\93036187.2
VVID-747PC isolated from the SFC separation of 4-(1-(2-chlorophenyl)ethyl)-N-((R,E)-
-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carboxamide (Example 216, Step C, Rt = 8.09 min). MS (ESI): mass calcd. for C
22H
25ClN
2O
4S, 448.1; m/z found, 449.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.35 – 7.30 (m, 1H), 7.30 – 7.25 (m, 1H), 7.23 – 7.13 (m, 4H), 6.84 (dd, J = 15.1, 4.5 Hz, 1H), 6.65 – 6.52 (m, 1H), 6.39 (dd, J = 15.1, 1.7 Hz, 1H), 5.96 (d, J = 7.8 Hz, 1H), 5.22 (q, J = 6.9 Hz, 1H), 4.96 – 4.81 (m, 1H), 4.15 – 4.01 (m, 2H), 3.38 – 3.28 (m, 1H), 3.17 – 3.08 (m, 1H), 2.84 (s, 3H), 1.51 (d, J = 6.9 Hz, 3H), 1.31 (d, J = 7.1 Hz, 3H). Example 218: 1-((*R)-(2-Chlorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-1H-pyrazolo[3,4-c]pyridine-5-carboxamide.
((2-chlorophenyl)(cyclopropyl)methyl)-1H-pyrazolo[3,4-c]pyridine-5- carboxylate. (2-Chlorophenyl)(cyclopropyl)methanol (491 mg, 2.69 mmol, 1.0 eq) was taken up in THF (25 mL, 0.1M). To this was added diisopropyl azodicarboxylate (0.72 mL, 3.65 mmol, 1.4 eq), triphenylphosphine (polymer bound ∼3 mmol/g, 1.2 g, 3.65 mmol, 1.4 eq), and methyl 1H-pyrazolo[3,4- c]pyridine-5-carboxylate (476 mg, 2.69 mmol, 1.0 eq). The reaction was purged with N
2 and stirred at rt for 16 h before being filtered and concentrated under reduced pressure. Purification via RP-HPLC (5-95% ACN in 0.1% HCOOH water) provided methyl 1-((2-chlorophenyl)(cyclopropyl)methyl)-1H- pyrazolo[3,4-c]pyridine-5-carboxylate (112 mg, 12% yield). MS (ESI): mass calcd. for C
18H
16ClN
3O
2, 341.1; m/z found, 342.0 [M+H]
+. [00561] Step B: 1-((2-Chlorophenyl)(cyclopropyl)methyl)-1H-pyrazolo[3,4-c]pyridine-5-carboxylic acid. Methyl 1-((2-chlorophenyl)(cyclopropyl)methyl)-1H-pyrazolo[3,4-c]pyridine-5-carboxylate (112 mg, 0.328 mmol, 1.0 eq) was taken up in methanol (1.6 mL, 0.2M). To this was added LiOH (1.3 mL, 2.62 mmol, 8.0 eq, 2M in water) and the reaction was stirred at 60 °C for 3 hours. After cooling to rt, the reaction was adjusted to a pH∼2 with 1N HCl then diluted with water and extracted with EtOAc. The combined organic extracts dried over Na
2SO
4, filtered, and concentrated under reduced pressure to provide 1-((2- 235 QB\184200.00081\93036187.2
VVID-747PC chlorophenyl)(cyclopropyl)methyl)-1H-pyrazolo[3,4-c]pyridine-5-carboxylic acid (61 mg, 57% yield). MS (ESI): mass calcd. for C
17H
14ClN
3O
2, 327.1; m/z found, 328.0 [M+H]
+. [00562] Step C: 1-((*R)-(2-Chlorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-1H-pyrazolo[3,4-c]pyridine-5-carboxamide. 1-((2-Chlorophenyl)(cyclopropyl)methyl)-1H- pyrazolo[3,4-c]pyridine-5-carboxylic acid (61 mg, 0.186 mmol, 1.0 eq) and HATU (85 mg, 0.223 mmol, 1.2 eq) were taken up in DMF (0.93 mL, 0.2M). DIPEA (97 µL, 0.558 mmol, 3.0 eq) was added and the mixture was stirred for 5 minutes. (R,E)-4-(Methylsulfonyl)but-3-en-2-amine (60 mg, 0.186 mmol, 1.0 eq, TsOH salt) was added and the reaction was stirred for 30 min at rt. Purification via RP-HPLC (10-100% ACN in 0.1% HCOOH water) provided 1-((2-chlorophenyl)(cyclopropyl)methyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-1H-pyrazolo[3,4-c]pyridine-5-carboxamide (31 mg, 36% yield). This product was separated via SFC (Stationary phase: IG (2x25 cm); Mobile phase: 35% EtOH/CO
2; Rt = 8.72 min) to provide the title compound. MS (ESI): mass calcd. for C
22H
23ClN
4O
3S, 458.1; m/z found, 459.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.74 (t, J = 1.0 Hz, 1H), 8.47 (d, J = 1.2 Hz, 1H), 8.16 (s, 1H), 8.06 (d, J = 8.5 Hz, 1H), 7.71 (dd, J = 7.7, 1.8 Hz, 1H), 7.34 – 7.14 (m, 3H), 6.92 (dd, J = 15.1, 4.4 Hz, 1H), 6.45 (dd, J = 15.1, 1.8 Hz, 1H), 5.49 (d, J = 9.3 Hz, 1H), 5.01 – 4.87 (m, 1H), 2.86 (s, 3H), 2.01 – 1.89 (m, 1H), 1.39 (d, J = 7.1 Hz, 3H), 0.74 (dt, J = 8.1, 2.0 Hz, 2H), 0.59 – 0.43 (m, 2H). Example 219: 1-((*S)-(2-Chlorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)- 1H-pyrazolo[3,4-c]pyridine-5-carboxamide.
was isolated from the SFC separation of 1-((2- chlorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-pyrazolo[3,4- c]pyridine-5-carboxamide (Example 218, Step C, Rt = 6.29 min). MS (ESI): mass calcd. for C
22H
23ClN
4O
3S, 458.1; m/z found, 459.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.81 (t, J = 1.0 Hz, 1H), 8.54 (d, J = 1.2 Hz, 1H), 8.23 (d, J = 0.8 Hz, 1H), 8.13 (d, J = 8.5 Hz, 1H), 7.76 (dd, J = 7.7, 1.8 Hz, 1H), 7.38 (dd, J = 7.7, 1.6 Hz, 1H), 7.36 – 7.24 (m, 2H), 6.97 (dd, J = 15.1, 4.4 Hz, 1H), 6.49 (dd, J = 15.1, 1.8 Hz, 1H), 5.55 (d, J = 9.3 Hz, 1H), 5.09 – 4.96 (m, 1H), 2.91 (s, 3H), 2.09 – 1.97 (m, 1H), 1.47 (d, J = 7.1 Hz, 3H), 0.87 – 0.74 (m, 2H), 0.67 – 0.49 (m, 2H). Example 220: 1-((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)-1H-pyrazolo[3,4-c]pyridine-5-carboxamide. 236 QB\184200.00081\93036187.2
VVID-747PC prepared in a manner analogous to Example 218 using (2-chloro-3-
(Intermediate 10) instead of (2-chlorophenyl)(cyclopropyl)methanol in Step A. Separation of 1-((2-chloro-3-fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-1H-pyrazolo[3,4-c]pyridine-5-carboxamide (Step C) via SFC (Stationary phase: OD-H (2x25 cm); Mobile phase: 25% EtOH/CO
2; Rt = 8.77 min) provided the title compound. MS (ESI): mass calcd. for C
22H
22ClFN
4O
3S, 476.1; m/z found, 477.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.74 (t, J = 1.0 Hz, 1H), 8.47 (d, J = 1.2 Hz, 1H), 8.16 (d, J = 0.7 Hz, 1H), 8.05 (d, J = 8.5 Hz, 1H), 7.50 (dt, J = 7.8, 1.3 Hz, 1H), 7.22 (td, J = 8.1, 5.3 Hz, 1H), 7.05 (td, J = 8.4, 1.5 Hz, 1H), 6.90 (dd, J = 15.1, 4.5 Hz, 1H), 6.42 (dd, J = 15.1, 1.8 Hz, 1H), 5.45 (d, J = 9.3 Hz, 1H), 5.01 – 4.88 (m, 1H), 2.83 (s, 3H), 2.01 – 1.87 (m, 1H), 1.40 (d, J = 7.1 Hz, 3H), 0.74 (dq, J = 8.1, 1.4 Hz, 2H), 0.58 – 0.42 (m, 2H). Example 221: 1-((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)-1H-pyrazolo[3,4-c]pyridine-5-carboxamide.
isolated from the SFC separation of 1-((2-chloro-3- fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-pyrazolo[3,4- c]pyridine-5-carboxamide (Example 220, Step C, Rt = 6.34 min). MS (ESI): mass calcd. for C
22H
22ClFN
4O
3S, 476.1; m/z found, 477.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.76 (t, J = 1.1 Hz, 1H), 8.48 (d, J = 1.2 Hz, 1H), 8.16 (d, J = 0.8 Hz, 1H), 8.06 (d, J = 8.5 Hz, 1H), 7.53 (dt, J = 8.0, 1.3 Hz, 1H), 7.23 (td, J = 8.1, 5.3 Hz, 1H), 7.06 (td, J = 8.5, 1.5 Hz, 1H), 6.92 (dd, J = 15.1, 4.5 Hz, 1H), 6.45 (dd, J = 15.1, 1.8 Hz, 1H), 5.47 (d, J = 9.3 Hz, 1H), 5.02 – 4.90 (m, 1H), 2.87 (s, 3H), 1.95 (dtt, J = 9.6, 8.0, 4.9 Hz, 1H), 1.40 (d, J = 7.1 Hz, 3H), 0.75 (dq, J = 8.1, 1.3 Hz, 2H), 0.58 – 0.47 (m, 2H). Example 222: 3-((*R)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but- 3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide. 237 QB\184200.00081\93036187.2
VVID-747PC in a manner analogous to Example 218 using methyl 3H-
instead of methyl 1H-pyrazolo[3,4-c]pyridine-5-carboxylate and (2- chloro-3-fluorophenyl)(cyclopropyl)methanol (Intermediate 10) instead of (2- chlorophenyl)(cyclopropyl)methanol in Step A. Separation of 3-((2-chloro-3- fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5- c]pyridine-6-carboxamide (Step C) via SFC (Stationary phase: C2 (2x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 11.3 min) provided the title compound. MS (ESI): mass calcd. for C
22H
22ClFN
4O
3S, 476.1; m/z found, 477.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.68 – 8.49 (m, 2H), 8.37 (s, 1H), 8.12 (d, J = 8.5 Hz, 1H), 7.28 (td, J = 8.0, 5.2 Hz, 1H), 7.19 (td, J = 8.4, 1.5 Hz, 1H), 7.09 (dt, J = 7.8, 1.4 Hz, 1H), 6.97 (dd, J = 15.1, 4.5 Hz, 1H), 6.52 (dd, J = 15.1, 1.8 Hz, 1H), 5.38 (d, J = 9.2 Hz, 1H), 5.07 – 4.90 (m, 1H), 2.93 (s, 3H), 1.84 – 1.71 (m, 1H), 1.44 (d, J = 7.1 Hz, 3H), 1.07 – 0.89 (m, 2H), 0.83 – 0.71 (m, 1H), 0.70 – 0.57 (m, 1H). Example 223: 3-((*S)-(2-Chloro-3-fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide.
isolated from the SFC separation of 1-((2-chloro-3- fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-pyrazolo[3,4- c]pyridine-5-carboxamide (Example 222, Step C, Rt = 6.71 min). MS (ESI): mass calcd. for C
22H
22ClFN
4O
3S, 476.1; m/z found, 477.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.61 (s, 2H), 8.35 (s, 1H), 8.13 (d, J = 8.5 Hz, 1H), 7.37 – 7.13 (m, 2H), 7.07 (d, J = 7.8 Hz, 1H), 6.95 (dd, J = 15.1, 4.4 Hz, 1H), 6.48 (dd, J = 14.9, 1.8 Hz, 1H), 5.38 (d, J = 9.2 Hz, 1H), 5.01 (h, J = 7.1 Hz, 1H), 2.91 (s, 3H), 1.77 (tq, J = 8.4, 4.2 Hz, 1H), 1.46 (d, J = 7.1 Hz, 3H), 1.07 – 0.87 (m, 2H), 0.78 (dq, J = 10.5, 5.2 Hz, 1H), 0.62 (dq, J = 10.3, 5.3 Hz, 1H). 238 QB\184200.00081\93036187.2
VVID-747PC Example 224: 3-((*R)-(2-Chloro-3-fluorophenyl)(oxetan-3-yl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide. in a manner analogous to Example 218 using methyl 3H-
instead of methyl 1H-pyrazolo[3,4-c]pyridine-5-carboxylate and (2- chloro-3-fluorophenyl)(oxetan-3-yl)methanol (Intermediate 30) instead of (2- chlorophenyl)(cyclopropyl)methanol in Step A and using trimethyltin hydroxide/DCE instead of LiOH/MeOH in Step B. Separation of 3-((2-chloro-3-fluorophenyl)(oxetan-3-yl)methyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide (Step C) via SFC (Stationary phase: AD-H (3x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 11.8 min) provided the title compound. MS (ESI): mass calcd. for C
22H
22ClFN
4O
4S, 492.1; m/z found, 493.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.63 (d, J = 24.3 Hz, 2H), 8.28 – 8.05 (m, 2H), 7.40 – 7.31 (m, 1H), 7.26 – 7.19 (m, 1H), 7.09 (d, J = 7.8 Hz, 1H), 6.97 (dd, J = 15.1, 4.4 Hz, 1H), 6.59 – 6.39 (m, 2H), 5.08 – 4.96 (m, 1H), 4.91 (q, J = 7.2 Hz, 2H), 4.58 (t, J = 6.1 Hz, 1H), 4.53 – 4.43 (m, 1H), 4.22 – 4.11 (m, 1H), 2.92 (s, 3H), 1.45 (d, J = 7.1 Hz, 3H). Example 225: 3-((*S)-(2-Chloro-3-fluorophenyl)(oxetan-3-yl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide.
isolated from the SFC separation of 1-((2-chloro-3- fluorophenyl)(cyclopropyl)methyl)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-1H-pyrazolo[3,4- c]pyridine-5-carboxamide (Example 224, Step C, Rt = 12.5 min). MS (ESI): mass calcd. for C
22H
22ClFN
4O
4S, 492.1; m/z found, 493.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.62 (d, J = 21.3 Hz, 2H), 8.22 – 8.04 (m, 2H), 7.35 (td, J = 8.0, 5.1 Hz, 1H), 7.26 – 7.18 (m, 1H), 7.07 (d, J = 7.8 Hz, 1H), 6.96 (dd, J = 15.1, 4.4 Hz, 1H), 6.55 – 6.41 (m, 2H), 5.07 – 4.96 (m, 1H), 4.91 (dt, J = 11.6, 7.1 Hz, 2H), 4.57 (t, J = 6.1 Hz, 1H), 4.50 (t, J = 6.2 Hz, 1H), 4.22 – 4.10 (m, 1H), 2.91 (s, 3H), 1.46 (d, J = 7.1 Hz, 3H). 239 QB\184200.00081\93036187.2
VVID-747PC Example 226: 3-((*S)-(2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide. prepared in a manner analogous to Example 218 using methyl 3H-
instead of methyl 1H-pyrazolo[3,4-c]pyridine-5-carboxylate and (2- chloro-3-fluorophenyl)(1-fluorocyclopropyl)methanol (Intermediate 35) instead of (2- chlorophenyl)(cyclopropyl)methanol in Step A and using trimethyltin hydroxide/DCE instead of LiOH/MeOH in Step B. Separation of 3-((2-chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide (Step C) via SFC (Stationary phase: OD (3x25 cm); Mobile phase: 42% IPA/CO
2, Rt = 3.21 min) provided the title compound. MS (ESI): mass calcd. for C
22H
21ClF
2N
4O
3S, 494.1; m/z found, 495.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.90 (d, J = 8.6 Hz, 1H), 8.73 (s, 1H), 8.55 (s, 1H), 8.31 (s, 1H), 7.79 (d, J = 7.5 Hz, 1H), 7.66 - 7.51 (m, 2H), 6.88 - 6.77 (m, 1H), 6.76 - 6.67 (m, 1H), 6.29 - 6.14 (m, 1H), 4.91 - 4.78 (m, 1H), 2.99 (s, 3H), 1.61 - 1.44 (m, 1H), 1.40 - 1.16 (m, 6H). Example 227: 3-(((*R)-(2-Chloro-3-fluorophenyl)(1-fluorocyclopropyl)methyl)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)-3H-imidazo[4,5-c]pyridine-6-carboxamide.
isolated from the SFC separation of 5-((2-chloro-3-fluorophenyl)(3- fluorooxetan-3-yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (Example 226, Step C, Rt = 5.05 min). MS (ESI): mass calcd. for C
22H
21ClF
2N
4O
3S, 494.1; m/z found, 495.0 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.90 (d, J = 8.5 Hz, 1H), 8.73 (s, 1H), 8.55 (s, 1H), 8.31 (s, 1H), 7.75 (br d, J = 7.4 Hz, 1H), 7.67 - 7.51 (m, 2H), 6.88 - 6.78 (m, 1H), 6.76 - 6.67 (m, 1H), 6.29 - 6.15 (m, 1H), 4.91 - 4.79 (m, 1H), 2.98 (s, 3H), 1.58 - 1.44 (m, 1H), 1.38 - 1.15 (m, 6H). 240 QB\184200.00081\93036187.2
VVID-747PC Example 228: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-4-methoxy-N-((R,E)-4-(methylsulfonyl)but-3-en- 2-yl)pyrimidine-2-carboxamide. ethyl)amino)-4-methoxypyrimidine-2-carbonitrile. 5-Bromo-
(350 mg, 1.64 mmol, 1.0 eq) was taken up in toluene (8.2 mL, 0.2M). To this was added (S)-1-(2-chlorophenyl)ethan-1-amine HCl (314 mg, 1.64 mmol, 1.0 eq), cesium carbonate (1.6 g, 4.91 mmol, 3.0 eq), and RuPhos Pd G4 (139 mg, 0.164 mmol, 0.1 eq). The reaction was placed under N
2 and heated to 100 °C for 36 hours. After cooling to rt, the reaction was quenched with water and extracted with EtOAc. The combined organic extracts were dried over Na
2SO
4, filtered, and concentrated under reduced pressure. Purification by FCC (0-100% EtOAc in heptanes) provided (S)-5- ((1-(2-chlorophenyl)ethyl)amino)-4-methoxypyrimidine-2-carbonitrile (277 mg, 59% yield). MS (ESI): mass calcd. for C
14H
13ClN
4O, 288.1; m/z found, 289.2 [M+H]
+. [00573] Step B: (S)-5-((1-(2-Chlorophenyl)ethyl)amino)-4-methoxypyrimidine-2-carboxylic acid. Sodium hydroxide (384 mg, 9.59 mmol, 10 eq) was taken up in water (2.5 mL) and stirred for 5 min. (S)-5-((1-(2- Chlorophenyl)ethyl)amino)-4-methoxypyrimidine-2-carbonitrile (277 mg, 0.959 mmol, 1.0 eq) was added followed by ACN (2.5 mL). The reaction was stirred at 110 °C for 16 h. Upon cooling to rt, the mixture was quenched with water and extracted with EtOAc. The aqueous layer was adjusted to pH∼2 with HCl and concentrated under reduced pressure before being extracted with 20% IPA in CHCl
3 to provide (S)-5- ((1-(2-chlorophenyl)ethyl)amino)-4-methoxypyrimidine-2-carboxylic acid (80 mg, 27% yield). MS (ESI): mass calcd. for C
14H
14ClN
3O
3, 307.1; m/z found, 308.0 [M+H]
+. [00574] Step C: 5-(((S)-1-(2-Chlorophenyl)ethyl)amino)-4-methoxy-N-((R,E)-4-(methylsulfonyl)but-3- en-2-yl)pyrimidine-2-carboxamide. (S)-5-((1-(2-Chlorophenyl)ethyl)amino)-4-methoxypyrimidine-2- carboxylic acid (80 mg, 0.260 mmol, 1.0 eq) and HATU (119 mg, 0.312 mmol, 1.2 eq) were taken up in DMF (1.3 mL, 0.2M). DIPEA (136 µL, 0.780 mmol, 3.0 eq) was added and the mixture was allowed to stir for 5 minutes. (R,E)-4-(Methylsulfonyl)but-3-en-2-amine (84 mg, 0.260 mmol, 1.0 eq, TsOH salt) was added and the reaction was stirred for 30 min at rt. Purification via RP-HPLC (10-100% ACN in 0.1% HCOOH water) provided 5-(((S)-1-(2-chlorophenyl)ethyl)amino)-4-methoxy-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (62 mg, 55% yield). MS (ESI): mass calcd. for C
19H
23ClN
4O
4S, 438.1; m/z found, 439.2 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.70 (d, J = 8.3 Hz, 1H), 7.43 – 7.36 (m, 2H), 7.35 – 7.29 (m, 1H), 7.26 – 7.17 (m, 2H), 6.93 (dd, J = 15.1, 4.5 Hz, 1H), 6.49 (dd, J = 15.1, 1.8 Hz, 1H), 5.08 – 4.80 (m, 3H), 4.20 (s, 3H), 2.93 (s, 3H), 1.63 (d, J = 6.6 Hz, 3H), 1.42 (d, J = 7.1 Hz, 3H). 241 QB\184200.00081\93036187.2
VVID-747PC Example 229: 5-(((2-Chlorophenyl)(cyclopropyl)methyl)amino)-4-methoxy-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. prepared in a manner analogous to Example 228 using (2-
instead of (S)-1-(2-chlorophenyl)ethan-1-amine in Step A. MS (ESI): mass calcd. for C
21H
25ClN
4O
4S, 464.1; m/z found, 465.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 7.70 (dd, J = 8.5, 3.9 Hz, 1H), 7.48 – 7.32 (m, 3H), 7.27 – 7.17 (m, 2H), 6.92 (dt, J = 15.1, 4.3 Hz, 1H), 6.52 – 6.43 (m, 1H), 5.14 (d, J = 4.9 Hz, 1H), 4.94 (q, J = 7.6 Hz, 1H), 4.52 (td, J = 5.1, 2.6 Hz, 1H), 4.20 (s, 3H), 2.92 (d, J = 2.9 Hz, 3H), 1.42 (dd, J = 7.1, 2.5 Hz, 3H), 1.38 – 1.26 (m, 1H), 0.77 – 0.68 (m, 1H), 0.68 – 0.53 (m, 2H), 0.42 – 0.31 (m, 1H). Example 230: 5-(((2-Chlorophenyl)(cyclopropyl)methyl)amino)-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)-6-oxo-1,6-dihydropyrimidine-2-carboxamide.
was isolated from the synthesis of 5-(((2- chlorophenyl)(cyclopropyl)methyl)amino)-4-methoxy-N-((R,E)-4-(methylsulfonyl)but-3-en-2- yl)pyrimidine-2-carboxamide (Example 229). MS (ESI): mass calcd. for C
20H
23ClN
4O
4S, 450.1; m/z found, 451.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 10.17 (s, 1H), 7.45 – 7.30 (m, 3H), 7.25 – 7.14 (m, 2H), 6.93 – 6.79 (m, 1H), 6.60 (d, J = 4.3 Hz, 1H), 6.49 – 6.37 (m, 1H), 5.81 (d, J = 5.3 Hz, 1H), 4.83 (d, J = 9.1 Hz, 1H), 4.48 – 4.40 (m, 1H), 2.92 (d, J = 12.7 Hz, 3H), 1.39 (dd, J = 10.8, 7.1 Hz, 3H), 1.31 – 1.17 (m, 1H), 0.75 – 0.62 (m, 1H), 0.62 – 0.48 (m, 2H), 0.38 – 0.24 (m, 1H). Example 231: 5-((*S)-(2-Chloro-3-fluorophenyl)(1-(2,2,2-trifluoroethyl)azetidin-3-yl)methoxy)-N- ((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 242 QB\184200.00081\93036187.2
VVID-747PC 5-((1-(tert-butoxycarbonyl)azetidin-3-yl)(2-chloro-3-
tert-Butyl 3-((2-chloro-3- fluorophenyl)(hydroxy)methyl)azetidine-1-carboxylate (Intermediate 31, 500 mg, 1.58 mmol, 1.0 eq) was taken up in DMSO (8.0 mL, 0.2 M). Methyl 5-fluoropyrimidine-2-carboxylate (272 mg, 1.74 mmol, 1.1 eq) and cesium carbonate (619 mg, 1.90 mmol, 1.2 eq) were added and the reaction was stirred at 60 ℃ for 2 h. After cooling to rt, the mixture was quenched with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (PE:EtOAc = 10:1 to 1:1) to give methyl 5-((1-(tert- butoxycarbonyl)azetidin-3-yl)(2-chloro-3-fluorophenyl)methoxy)pyrimidine-2-carboxylate (600 mg, 84% yield) as a colorless oil. MS (ESI): mass calcd. for C
21H
23ClFN
3O
5, 451.1; m/z found, 396.0 [M+2H-tBu]
+.
1H NMR (400 MHz, CDCl
3) δ 8.45 (s, 2H), 7.25 (dd, J = 7.9, 5.3 Hz, 1H), 7.19 - 7.13 (m, 2H), 5.91 (d, J = 6.8 Hz, 1H), 4.17 - 4.07 (m, 4H), 4.02 (s, 3H), 3.95 - 3.90 (m, 1H), 1.46 (s, 9H). [00578] Step B: Methyl 5-(azetidin-3-yl-(2-chloro-3-fluorophenyl)methoxy)pyrimidine-2-carboxylate. Methyl 5-((1-(tert-butoxycarbonyl)azetidin-3-yl)(2-chloro-3-fluorophenyl)methoxy)pyrimidine-2- carboxylate (600 mg, 1.33 mmol, 1.0 eq) was taken up in DCM (6.0 mL, 0.2 M). TFA (2.0 mL) was added and the reaction was stirred at rt for 1 h. The mixture was concentrated under reduced pressure then diluted with sat. aq. NaHCO3 and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4 and filtered. The filtrate was concentrated under reduced pressure to give methyl 5- (azetidin-3-yl-(2-chloro-3-fluorophenyl)methoxy)pyrimidine-2-carboxylate (360 mg, 95% yield) as a colorless oil, which was used in the next step directly. [00579] Step C: Methyl 5-((2-chloro-3-fluorophenyl)(1-(2,2,2-trifluoroethyl)azetidin-3- yl)methoxy)pyrimidine-2-carboxylate. Methyl 5-(azetidin-3-yl-(2-chloro-3- fluorophenyl)methoxy)pyrimidine-2-carboxylate (360 mg, 1.02 mmol, 1.0 eq) was taken up in toluene (6.0 mL, 0.17 M). TEA (311 mg, 3.07 mmol, 3.0 eq) and 2,2,2-trifluoroethyl trifluoromethanesulfonate (356 mg, 1.54 mmol, 1.5 eq) were added and the reaction was stirred at 80 ℃ for 3 h. After cooling to rt, the mixture was quenched with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified by FCC (40-60% EtOAc in PE) to give methyl 5-((2-chloro-3-fluorophenyl)(1-(2,2,2- trifluoroethyl)azetidin-3-yl)methoxy)pyrimidine-2-carboxylate (240 mg, 54% yield) as a colorless oil. MS (ESI): mass calcd. for C
18H
16ClF
4N
3O
3, 433.1; m/z found, 434.0 [M+H]
+.
1H NMR (400 MHz, CDCl
3) δ 8.44 (s, 2H), 7.26 - 7.20 (m, 1H), 7.17 - 7.09 (m, 2H), 5.97 (d, J = 7.5 Hz, 1H), 4.02 (s, 3H), 3.66 - 3.59 (m, 1H), 3.52 - 3.45 (m, 2H), 3.34 (t, J = 7.0 Hz, 1H), 3.22 - 3.14 (m, 1H), 3.05 (q, J = 9.3 Hz, 2H). 243 QB\184200.00081\93036187.2
VVID-747PC [00580] Step D: 5-((2-Chloro-3-fluorophenyl)(1-(2,2,2-trifluoroethyl)azetidin-3-yl)methoxy)pyrimidine- 2-carboxylic acid. Methyl 5-((2-chloro-3-fluorophenyl)(1-(2,2,2-trifluoroethyl)azetidin-3- yl)methoxy)pyrimidine-2-carboxylate (230 mg, 0.530 mmol, 1.0 eq) was taken up in THF/water (v/v 3:1, 4.0 mL, 0.13 M). LiOH ^H
2O (67 mg, 1.59 mmol, 3.0 eq) was added and the reaction was stirred at 25 ℃ for 2 h. The mixture was diluted with water, adjusted pH ~5 with 2M HCl, and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, and filtered. The filtrate was concentrated under reduced pressure to give 5-((2-chloro-3-fluorophenyl)(1-(2,2,2-trifluoroethyl)azetidin- 3-yl)methoxy)pyrimidine-2-carboxylic acid (220 mg, quant. yield) as a pale-yellow solid. MS (ESI): mass calcd. for C
17H
14ClF
4N
3O
3, 419.1; m/z found, 420.1 [M+H]
+. [00581] Step E: 5-((2-Chloro-3-fluorophenyl)(1-(2,2,2-trifluoroethyl)azetidin-3-yl)methoxy)-N-((R,E)-4- (methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide. 5-((2-Chloro-3-fluorophenyl)(1-(2,2,2- trifluoroethyl)azetidin-3-yl)methoxy)pyrimidine-2-carboxylic acid (24 mg, 0.057 mmol, 1.0 eq) was taken up in DMF (1.0 mL, 0.06 M). (R,E)-4-(Methylsulfonyl)but-3-en-2-amine (20 mg, 0.063 mmol, 1.1 eq, TsOH salt), HATU (33 mg, 0.086 mmol, 1.5 eq), and DIPEA (22 mg, 0.172 mmol, 3.0 eq) were added and the reaction was allowed to stir at 25 °C for 2 h. The crude mixture was purified by prep-HPLC (25-60% ACN in 10 mM aq. NH
4HCO
3) to give 5-((2-chloro-3-fluorophenyl)(1-(2,2,2-trifluoroethyl)azetidin-3- yl)methoxy)-N-((R,E)-4-(methylsulfonyl)but-3-en-2-yl)pyrimidine-2-carboxamide (10 mg, 33% yield). This product was separated via SFC (Stationary phase: WHELK-O1 (2x25 cm); Mobile phase: 50% MeOH/CO
2; Rt = 7.89 min, second eluting product) to provide the title compound. MS (ESI): mass calcd. for C
22H
23ClF
4N
4O
4S, 550.1; m/z found, 551.1 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.98 (d, J = 8.5 Hz, 1H), 8.60 - 8.47 (m, 2H), 7.48 - 7.34 (m, 3H), 6.87 - 6.66 (m, 2H), 6.26 - 6.12 (m, 1H), 4.93 - 4.70 (m, 1H), 3.61 - 3.48 (m, 2H), 3.44 - 3.38 (m, 1H), 3.31 - 3.14 (m, 4H), 3.04 - 2.95 (m, 3H), 1.36 - 1.26 (m, 3H). In vitro ELISA Target Engagement Assay for PIK3CA [00582] Engagement of compounds on PIK3CA were assessed in Jurkat cellular lysate via a sandwich ELISA. Synthesis of PIK3CA Biotin Probe (R,Z)-4-((4-(1,3-Dioxolan-2-yl)butyl)sulfonyl)but-3-en-2-amine
VVID-747PC [00583] Step A: tert-Butyl (R)-(4-((4-(1,3-dioxolan-2-yl)butyl)thio)but-3-yn-2-yl)carbamate. To a solution of tert-butyl (R)-but-3-yn-2-ylcarbamate (0.90 g, 5.32 mmol, 1.0 eq) in THF (50 mL, 0.1 M) under N
2 at -70 °C was slowly added 2.5M n-BuLi (4.7 mL, 11.7 mmol, 2.2 eq). The mixture was stirred for 1 h before sulfur (171 mg, 0.660 mmol, 0.12 eq) was added, causing the solution to become red. The reaction mixture was stirred 30 min at -70 °C then 30 min at 0 °C until consumption of sulfur was complete (dark red solution). 2-(4-Bromobutyl)-1,3-dioxolane (1.1 g, 5.32 mmol, 1.0 eq) in THF (10 mL) was added and the reaction mixture was stirred at 0 °C for 2 h. Sat. aq. ammonium chloride was added and the aqueous phase was extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by column to give tert-butyl (R)-(4-((4-(1,3-dioxolan-2-yl)butyl)thio)but-3-yn-2-yl)carbamate (1.0 g, 57% yield). [00584] Step B: tert-Butyl (R)-(4-((4-(1,3-dioxolan-2-yl)butyl)sulfonyl)but-3-yn-2-yl)carbamate. To a solution of tert-butyl (R)-(4-((4-(1,3-dioxolan-2-yl)butyl)thio)but-3-yn-2-yl)carbamate (200 mg, 0.610 mmol, 1.0 eq) in EtOAc (2.0 mL, 0.3 M) was added 3-chloroperbenzoic acid (262 mg, 1.52 mmol, 2.5 eq) at 0 ˚C. The mixture was allowed to warm to rt and stirred 16 h. The mixture was poured into sat. aq. Na
2SO
3 and extracted with EtOAc. The combined organic layers were washed with sat. aq. NaHCO
3, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The crude product tert-butyl (R)-(4-((4- (1,3-dioxolan-2-yl)butyl)sulfonyl)but-3-yn-2-yl)carbamate (250 mg, quant. yield) was used the next step directly without purification. [00585] Step C: tert-Butyl (R,Z)-(4-((4-(1,3-dioxolan-2-yl)butyl)sulfonyl)but-3-en-2-yl)carbamate. To a solution of tert-butyl (R)-(4-((4-(1,3-dioxolan-2-yl)butyl)sulfonyl)but-3-yn-2-yl)carbamate (250 mg, 0.690 mmol, 1.0 eq) in THF (3.0 mL, 0.23 M) was added Pd/C (250 mg) and the reaction was stirred at rt for 30 min under H
2 (50 psi). The solution was filtered to get the crude product which was purified by FCC (0-25% EtOAc in PE) to provide tert-butyl (R,Z)-(4-((4-(1,3-dioxolan-2-yl)butyl)sulfonyl)but-3-en- 2-yl)carbamate (130 mg, 52% yield) as a white solid. [00586] Step D: (R,Z)-4-((4-(1,3-Dioxolan-2-yl)butyl)sulfonyl)but-3-en-2-amine. To a solution of tert- butyl (R,Z)-(4-((4-(1,3-dioxolan-2-yl)butyl)sulfonyl)but-3-en-2-yl)carbamate (130 mg, 0.358 mmol, 1.0 eq) in acetonitrile (2.0 mL, 0.18 M) was added p-toluenesulfonic acid monohydrate (82 mg, 0.430 mmol, 1.2 eq) and the reaction was stirred at 50 ℃ for 2 h. The crude solution was evaporated to get the 4- methylbenzenesulfonic acid salt of (R,Z)-4-((4-(1,3-dioxolan-2-yl)butyl)sulfonyl)but-3-en-2-amine (160 mg, quant. yield). 1-((5-Bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoro-N-((R,Z)-4-((5-(4-(2-(2-(2-(5- ((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4- yl)pentanamido)ethoxy)ethoxy)acetyl)piperazin-1-yl)pentyl)sulfonyl)but-3-en-2-yl)piperidine-4- carboxamide (PIK3CA Biotin Probe) 245 QB\184200.00081\93036187.2
VVID-747PC
g, 3.36 mmol, 1.0 eq) in concentrated HCl (15 mL) and AcOH (5 mL, 0.09 M) was added NaNO
2 (254 mg, 1.1 eq) at 0 ℃. This mixture 1 was stirred at 0 ℃ for 1 h. In a separate flask, SO
2 was bubbled through AcOH (33 mL, 0.09 M) for 10 min at 0 ℃ before CuCl (99 mg, 0.3 eq) was added. SO
2 was bubbled through mixture 2 for 10 min. Mixture 1 was added to mixture 2 at 0 ℃ and this was stirred at 0-20 ℃ for 1 h. The reaction mixture was quenched with water and extracted with methyl tert-butyl ether. The combined organic layers were washed with sat. aq. sodium bicarbonate and brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The crude material was purified by FCC (PE:EtOAc 50:1) to give 4-bromo-2-iodo-benzenesulfonyl chloride (4.0 g, 62% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 7.72 (dd, J = 8.6, 1.9 Hz, 1H), 8.07 (d, J = 8.6 Hz, 1H), 8.37 (d, J = 1.9 Hz, 1H). [00588] Step 2: Ethyl 1-(4-bromo-2-iodo-phenyl)sulfonyl-4-fluoro-piperidine-4-carboxylate. To a solution of 4-bromo-2-iodo-benzenesulfonyl chloride (3.6 g, 9.44 mmol, 1.0 eq) and ethyl 4- fluoropiperidine-4-carboxylate hydrochloride (2.0 g, 9.44 mmol, 1.0 eq) in DCM (10 mL, 0.94 M) was added TEA (2.86 g, 28.3 mmol, 3.0 eq) dropwise at 0 °C. The reaction was stirred at rt for 1 hour before being quenched with water and extracted with DCM. The combined organic layers were washed 246 QB\184200.00081\93036187.2
VVID-747PC with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The crude material was purified by FCC to give ethyl 1-(4-bromo-2-iodo-phenyl)sulfonyl-4-fluoro-piperidine-4-carboxylate (4.3 g, 87% yield) as a yellow solid.
1H NMR (400 MHz, CDCl
3) δ 8.28 (d, J = 1.9 Hz, 1H), 7.99 (d, J = 8.5 Hz, 1H), 7.64 (dd, J = 8.4, 1.9 Hz, 1H), 4.27 (q, J = 7.1 Hz, 2H), 3.75 (dt, J = 13.0, 2.6 Hz, 2H), 3.18 (td, J = 12.8, 2.7 Hz, 2H), 2.12 - 2.31 (m, 2H), 1.99 - 2.08 (m, 2H), 1.32 (t, J = 7.1 Hz, 3H). [00589] Step 3: Ethyl 1-[4-bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-piperidine-4-carboxylate. To a solution of ethyl 1-(4-bromo-2-iodo-phenyl)sulfonyl-4-fluoro-piperidine-4-carboxylate (878 mg, 1.69 mmol, 1.0 eq) in 1,4-dioxane (9.0 mL, 0.19 M) and 1M aq. K
3PO
4 (2.7 mL) was added 2- chlorophenylboronic acid (290 mg, 1.86 mmol, 1.1 eq) and tetrakis(triphenylphosphine) (195 mg, 0.169 mmol, 0.1 eq). The mixture was stirred at 80 °C under N
2 for 16 h. After cooling to rt, the mixture was diluted with H
2O and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by prep-TLC (PE:EtOAc 3:1) to give ethyl 1-[4-bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro- piperidine-4-carboxylate (400 mg, 47% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 8.01 (d, J = 8.5 Hz, 1H), 7.67 (dd, J = 1.9, 8.5 Hz, 1H), 7.55 - 7.44 (m, 2H), 7.43 - 7.27 (m, 3H), 4.22 (q, J = 7.0 Hz, 2H), 3.24 (br d, J = 13.4 Hz, 1H), 3.07 (br d, J = 13.3 Hz, 1H), 2.98 - 2.85 (m, 1H), 2.72 - 2.58 (m, 1H), 2.04 - 1.79 (m, 4H), 1.34 - 1.27 (m, 3H). [00590] Step 4: 1-[4-Bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-piperidine-4-carboxylic acid. Ethyl 1-[4-bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-piperidine-4-carboxylate (460 mg, 0.911 mmol, 1.0 eq) and lithium hydroxide monohydrate (115 mg, 2.73 mmol, 3.0 eq) were taken up in THF/water (3:1 v/v, 8 mL, 0.11 M). The mixture was stirred at rt for 2 h before being concentrated and acidified with 6M HCl to adjust to pH 2-4. The resulting precipitate was collection by filtration to afford 1- [4-bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-piperidine-4-carboxylic acid (400 mg, 92% yield) as a white solid. [00591] Step 5: (R,Z)-N-(4-((4-(1,3-Dioxolan-2-yl)butyl)sulfonyl)but-3-en-2-yl)-1-((5-bromo-2'-chloro- [1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoropiperidine-4-carboxamide. To a solution of (R,Z)-4-((4-(1,3- dioxolan-2-yl)butyl)sulfonyl)but-3-en-2-amine, 4-methylbenzenesulfonic acid (153 mg, 0.352 mmol, 1.2 eq) in DCM (5.0 mL, 0.06 M) was added HATU (167 mg, 0.440 mmol, 1.5 eq) and DIPEA (0.13 mL, 0.734 mmol, 2.5 eq). After 5 mins, 1-[4-bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-piperidine-4- carboxylic acid (140 mg, 0.294 mmol, 1.0 eq) was added and the reaction was stirred at rt for 2 h. The reaction was quenched with sat. aq. NH
4Cl and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under vacuum. The resulting residue was purified by prep-TLC (SiO
2, PE:EtOAc = 1:1) to give (R,Z)-N-(4-((4-(1,3-dioxolan-2- yl)butyl)sulfonyl)but-3-en-2-yl)-1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoropiperidine- 4-carboxamide (210 mg, quant. yield). [00592] Step 6: (R,Z)-1-((5-Bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoro-N-(4-((5- oxopentyl)sulfonyl)but-3-en-2-yl)piperidine-4-carboxamide. To a solution of (R,Z)-N-(4-((4-(1,3- 247 QB\184200.00081\93036187.2
VVID-747PC dioxolan-2-yl)butyl)sulfonyl)but-3-en-2-yl)-1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4- fluoropiperidine-4-carboxamide (210 mg, 0.290 mmol, 1.0 eq) in TFA (3.0 mL, 0.02 M) was added concentrated HCl (0.6 mL) under nitrogen. The reaction was stirred at rt for 2 h before being filtered and the filtrate concentrated to provide (R,Z)-1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoro-N- (4-((5-oxopentyl)sulfonyl)but-3-en-2-yl)piperidine-4-carboxamide (200 mg, 96% yield). [00593] Step 7: tert-Butyl (R,Z)-4-(5-((3-(1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4- fluoropiperidine-4-carboxamido)but-1-en-1-yl)sulfonyl)pentyl)piperazine-1-carboxylate. To a solution of (R,Z)-1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoro-N-(4-((5-oxopentyl)sulfonyl)but-3- en-2-yl)piperidine-4-carboxamide (200 mg, 0.300 mmol, 1.0 eq) in THF/DCE (1:1 v/v, 4.0 mL, 0.07 M) was added tert-butyl piperazine-1-carboxylate (66 mg, 0.354 mmol, 1.2 eq). DIPEA was added until pH ~5 and the mixture was stirred for 5 min at rt. NaBH(OAc)
3 (248 mg, 4.0 eq) was added and the mixture was stirred at rt for 1 hour. The reaction was quenched with the water and extracted with DCM. The combined organic layers were dried over Na
2SO
4, filtered, and concentrated under vacuum. The resulting residue was purified by prep-HPLC to give tert-butyl (R,Z)-4-(5-((3-(1-((5-bromo-2'-chloro-[1,1'- biphenyl]-2-yl)sulfonyl)-4-fluoropiperidine-4-carboxamido)but-1-en-1-yl)sulfonyl)pentyl)piperazine-1- carboxylate (160 mg, 64% yield). [00594] Step 8: (R,Z)-1-((5-Bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoro-N-(4-((5-(piperazin- 1-yl)pentyl)sulfonyl)but-3-en-2-yl)piperidine-4-carboxamide. To a solution of tert-butyl (R,Z)-4-(5-((3- (1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoropiperidine-4-carboxamido)but-1-en-1- yl)sulfonyl)pentyl)piperazine-1-carboxylate (160 mg, 0.190 mmol, 1.0 eq) in acetonitrile (2.0 mL, 0.09 M) was added 4-methylbenzenesulfonic acid (39 mg, 0.230 mmol, 1.2 eq). The reaction was stirred at 50 ℃ for 30 min before the solution was evaporated to provide (R,Z)-1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2- yl)sulfonyl)-4-fluoro-N-(4-((5-(piperazin-1-yl)pentyl)sulfonyl)but-3-en-2-yl)piperidine-4-carboxamide (150 mg, quant. yield), which was used in the next step without further purification. [00595] Step 9: tert-Butyl (R,Z)-(2-(2-(2-(4-(5-((3-(1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)- 4-fluoropiperidine-4-carboxamido)but-1-en-1-yl)sulfonyl)pentyl)piperazin-1-yl)-2- oxoethoxy)ethoxy)ethyl)carbamate. To a solution of (R,Z)-1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2- yl)sulfonyl)-4-fluoro-N-(4-((5-(piperazin-1-yl)pentyl)sulfonyl)but-3-en-2-yl)piperidine-4-carboxamide (142 mg, 0.190 mmol, 1 eq) in DCM (5.0 mL, 0.04 M) was added HATU (108 mg, 0.280 mmol, 1.5 eq) and N,N-diisopropylethylamine (83 µL, 0.470 mmol, 2.5 eq). After 5 min, 2-[2-[2-(tert- butoxycarbonylamino)ethoxy]ethoxy]acetic acid (50 mg, 0.190 mmol, 1 eq) was added and the reaction mixture was stirred at rt for 2 h. The reaction was quenched with sat. aq. NH
4Cl extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under vacuum. The resulting residue was purified by prep-TLC to give tert-butyl (R,Z)-(2-(2-(2-(4-(5-((3-(1-((5- bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoropiperidine-4-carboxamido)but-1-en-1- yl)sulfonyl)pentyl)piperazin-1-yl)-2-oxoethoxy)ethoxy)ethyl)carbamate (110 mg, 58% yield). 248 QB\184200.00081\93036187.2
VVID-747PC [00596] Step 10: (R,Z)-N-(4-((5-(4-(2-(2-(2-Aminoethoxy)ethoxy)acetyl)piperazin-1- yl)pentyl)sulfonyl)but-3-en-2-yl)-1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4- fluoropiperidine-4-carboxamide. To a solution of tert-butyl (R,Z)-(2-(2-(2-(4-(5-((3-(1-((5-bromo-2'- chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoropiperidine-4-carboxamido)but-1-en-1- yl)sulfonyl)pentyl)piperazin-1-yl)-2-oxoethoxy)ethoxy)ethyl)carbamate (110 mg, 0.110 mmol, 1.0 eq) in ACN (20 mL, 0.006 M) was added 4-methylbenzenesulfonic acid (23 mg, 0.130 mmol, 1.2 eq). The reaction was stirred at 50 ℃ for 2 h before the solvent was evaporated to provide (R,Z)-N-(4-((5-(4-(2-(2- (2-aminoethoxy)ethoxy)acetyl)piperazin-1-yl)pentyl)sulfonyl)but-3-en-2-yl)-1-((5-bromo-2'-chloro-[1,1'- biphenyl]-2-yl)sulfonyl)-4-fluoropiperidine-4-carboxamide (90 mg, 91% yield), which was used in the next step without further purification. [00597] Step 11: 1-((5-Bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoro-N-((R,Z)-4-((5-(4-(2-(2- (2-(5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4- yl)pentanamido)ethoxy)ethoxy)acetyl)piperazin-1-yl)pentyl)sulfonyl)but-3-en-2-yl)piperidine-4- carboxamide. To a solution of (R,Z)-N-(4-((5-(4-(2-(2-(2-aminoethoxy)ethoxy)acetyl)piperazin-1- yl)pentyl)sulfonyl)but-3-en-2-yl)-1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4- fluoropiperidine-4-carboxamide (88 mg, 0.098 mmol, 1.2 eq) in DCM (5.0 mL, 0.02 M) was added HATU (47 mg, 0.120 mmol, 1.5 eq) and DIPEA (36 µL, 0.200 mmol, 2.5 eq). After 5 min, D-Biotin (20 mg, 0.082 mmol, 1.0 eq) was added and the reaction was stirred at rt for 2 h. The reaction was quenched with sat. aq. NH
4Cl and extracted with EtOAc. The combined organic layers wre washed with brine, dried over Na
2SO
4, filtered, and concentrated under vacuum. The resulting residue was purified by prep-TLC to give 1-((5-bromo-2'-chloro-[1,1'-biphenyl]-2-yl)sulfonyl)-4-fluoro-N-((R,Z)-4-((5-(4-(2-(2-(2-(5- ((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4- yl)pentanamido)ethoxy)ethoxy)acetyl)piperazin-1-yl)pentyl)sulfonyl)but-3-en-2-yl)piperidine-4- carboxamide (PIK3CA Biotin Probe) (11 mg, 12% yield). MS (ESI): mass calcd. for C
47H
66BrClFN
7O
10S
3, 1119; m/z found, 1120 [M+H]
+. Biochemical Assay [00598] A solution of PIK3CA Biotin Probe was generated at 10 mM and used in the biological testing. Table 2 Reagents Reagent working solutions Supplier and Stock conc catalo number
QB\184200.00081\93036187.2
VVID-747PC 500 mL-Pierce 20X PBS Tween Thermo Fisher 20 Buffer (PBST) #29352 Intercept (PBS) Blocking Buffer Fisher Scientific
re thawed on ice and resuspended in cold Dulbecco’s phosphate buffered saline (DPBS) (12 mL/300e
6 cells) then lysed by probe sonication (12x 3 second pulses). Jurkat lysate was plated (50 µl/well) in Armadillo 96 well PCR plates (Thermo Fisher catalog number AB2396). The lysate was treated with serial dilutions of test compounds up to 200 µM and incubated for 1 hour at room temperature. Following compound incubation, PIK3CA Biotin Probe was added to a final concentration of 500 nM, and lysate was mixed by pipetting gently and incubated for an additional 1 hour at room temperature. The mixture was then diluted in 75 µL Dilution buffer. The mixture was centrifuged for 5 minutes at 4122 g and stored at -80C until ELISA TE detection. [00600] ELISA TE Detection: Quantification of PIK3CA TE was initiated by coating of the ELISA plate (Thermo Scientific White 384-Well Immuno plates; Thermo Fisher catalog number 460372). The capture antibody was diluted in plating buffer (1:83) and added to the ELISA plate, 20 µl/well, overnight at 4°C. The plate was then washed twice with PBST, 100 µl/well, and blocked with addition of blocking buffer (100 µl/well, 1 hour at room temperature). During block, treated and probe labeled lysate was thawed. After Block, the block buffer was removed and the thawed lysate was added to the ELISA plate, 30 µl/well, for 1.5 hours at room temperature. Following lysate incubation, the assay plate was washed 3 times with PBST, 100 µl/well. The Neutravidin-HRP was then diluted (1:500 in dilution buffer) and added for 1 hour at room temperature, 20 µl/well. Following incubation, the assay plate was washed 4 times with PBST, 100 µl/well, HRP substrate was added to plate, 20 µl/well and luminescence signal was read (Clariostar Plate Reader). For each compound the potency of target engagement (TE
50) was determined using Graphpad Prism. [00601] Representative Elisa target engagement is presented in Table 3. Example Elisa Target I
UPAC N m En m nt )
QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
254 QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
255 QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
256 QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
258 QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
259 QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
261 QB\184200.00081\93036187.2
VVID-747PC Example Elisa Target N
o. IUPAC Name Engagement Assay (µM)
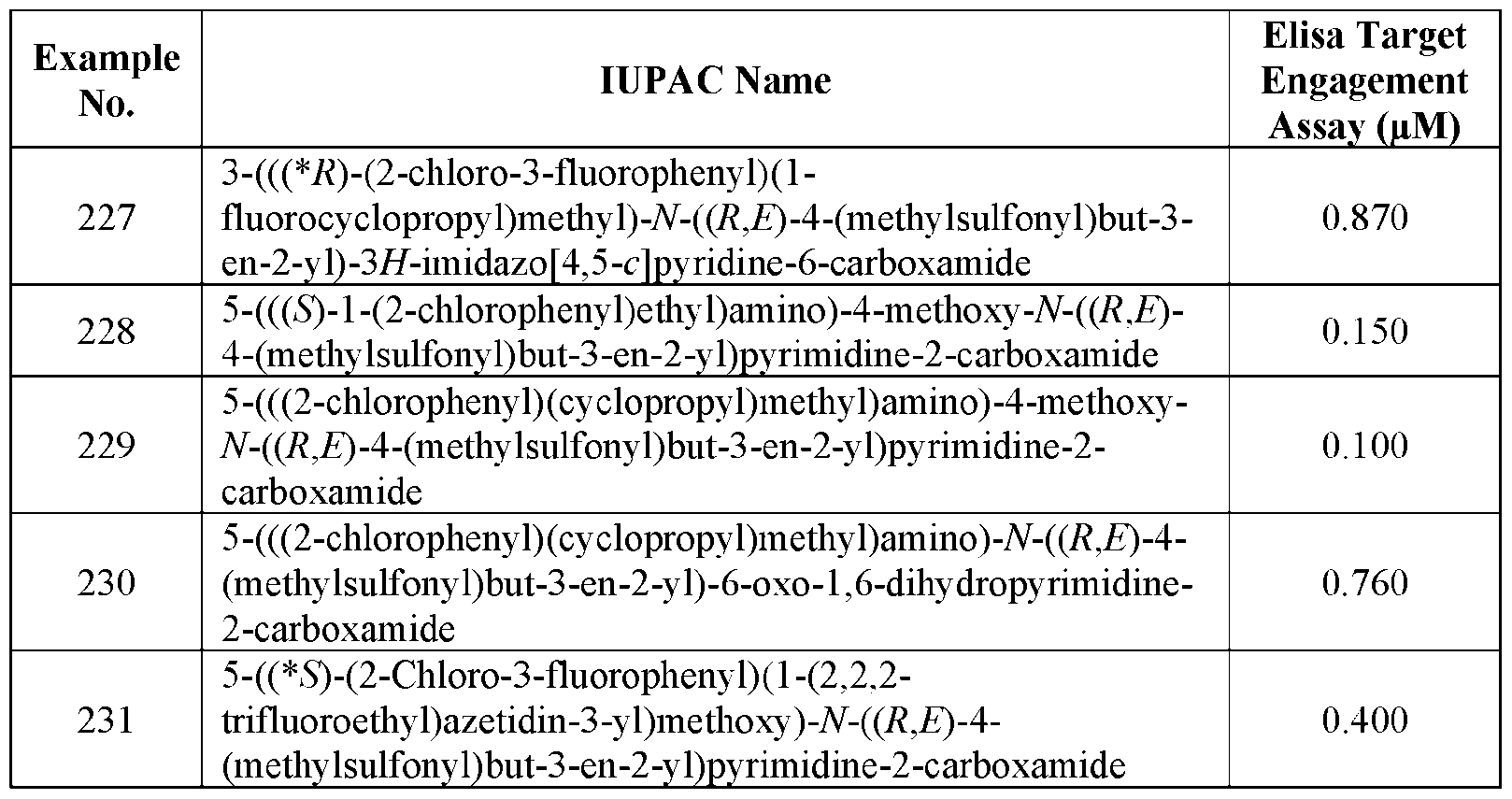
[00602] Functional activity of the compounds to inhibit pAKT were assessed in H358 cell lines as indicated below. Materials ^ Complete Media o FBS, Corning catalog #35-010-CV o Anti-Anti, Gibco catalog #15240-062 ^ 96 well plate, Corning catalog #3610 ^ TrypLE, Gibco catalog #12604013 ^ Phospho-AKT(S473) HTRF kit, Cisbio catalog #64AKSPET [00603] Cells were detached from the tissue culture flask with TrypLE. The cells were pelleted at 500 xg for 5 min, TrypLe was removed and then resuspended in the necessary volume of media + 1% FBS to give the appropriate cell density (17,500 cells/mL for FaDu; 20,000 cells/mL for H358). 100 µL of cell suspension/well was dispensed into the 96-well plate while keeping the outer wells of the plate free of cells and filled with PBS to prevent evaporation of interior wells. The plate was incubated at 37 °C and allowed to adhere overnight. The cells were treated with a dose response of compound and incubated at 37 °C for 30 minutes. The cells were then processed and pAKT(S473) levels were assessed following the specifications of the Cisbio HTRF kit. [00604] 1-[4-Bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-N-[(Z,1R)-1-methyl-3-methylsulfonyl- allyl]piperidine-4-carboxamide was used as an internal standard for the assay to normalize Imax and the absolute inhibition of pAKT for 1-[4-bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-N-[(Z,1R)-1- methyl-3-methylsulfonyl-allyl]piperidine-4-carboxamide varied from 40-89% for the H358 cell line. Synthesis of 1-[4-bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-N-[(Z,1R)-1-methyl-3- methylsulfonyl-allyl]piperidine-4-carboxamide 262 QB\184200.00081\93036187.2
VVID-747PC (R,Z)-4-(Methylsulfonyl)but-3-en-2-amine [00605] Butyllithium
solution - methylprop- 2-ynyl]carbamate (8.0 g, 47.3 mmol, 1.0 eq) and N,N,N,N-tetramethylethylenediamine (5.5 g, 47.3 mmol, 1.0 eq) in anhydrous THF (30 mL, 0.24 M) at 0 °C under N
2. After 30 minutes, 1-methyl-4- methylsulfanylsulfonyl-benzene (10 g, 49.6 mmol, 1.1 eq) in THF (80 mL, 0.6 M) was added and the mixture was stirred at 0 °C for 2 h. The reaction was quenched by addition of sat. aq. NH
4Cl at 0 °C then extracted with ethyl acetate. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated. The resulting residue was purified by FCC on silica (PE:EtOAc = 5:1) to afford tert-butyl N-[(R)-1-methyl-3-methylsulfanyl-prop-2-ynyl]carbamate (9.0 g, 88% yield) as a white solid.
1H NMR (400 MHz, CDCl
3) δ 4.65 - 4.76 (m, 1H) 4.50 - 4.63 (m, 1H) 2.37 (s, 3H) 1.46 (s, 9H) 1.39 (d, J = 6.9 Hz, 3H). [00606] Step 2: tert-Butyl N-[(Z,R)-1-methyl-3-methylsulfanyl-allyl]carbamate. tert-Butyl N-[(R)-1- methyl-3-methylsulfanyl-prop-2-ynyl]carbamate (3.0 g, 13.9 mmol, 1.0 eq), Lindlar catalyst (900 mg), and hexene (6 mL) were taken up in methanol (30 mL, 0.46 M). The mixture was stirred under H
2 (30 psi) at 25 °C for 3 h before being filtered. The filtrate was concentrated under vacuum and purified by FCC on silica (PE:EtOAc = 3:1) to afford tert-butyl N-[(Z,R)-1-methyl-3-methylsulfanyl-allyl]carbamate (2.6 g, 86% yield) as a white solid.
1H NMR (400 MHz, DMSO-d
6) δ 5.96 (d, J = 9.6 Hz, 1H), 5.43 (dd, J = 8.0, 9.5 Hz, 1H), 4.63 - 4.34 (m, 2H), 2.28 (s, 3H), 1.45 (s, 9H), 1.22 (d, J = 6.4 Hz, 3H). [00607] Step 3: tert-Butyl N-[(Z,R)-1-methyl-3-methylsulfonyl-allyl]carbamate. To a solution of tert-butyl N-[(Z,R)-1-methyl-3-methylsulfanyl-allyl]carbamate (2.6 g, 12.0 mmol, 1.0 eq) in DCM (10 mL, 1.2 M) was added 3-chloroperbenzoic acid (6.2 g, 35.9 mmol, 3.0 eq) in portions over 5 min. The mixture was stirred at 25 °C for 2 h before being diluted with sat. aq. Na
2SO
3 and extracted with DCM. The combined extracts were washed with sat. aq. NaHCO
3 and brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (PE:EtOAc = 1:1) to afford tert- butyl N-[(Z,R)-1-methyl-3-methylsulfonyl-allyl]carbamate (2.4 g, 79% yield) as a white solid.
1H NMR (400 MHz, CDCl
3) δ 6.31 - 6.22 (m, 1H), 6.21 - 6.11 (m, 1H), 4.63 (s, 1H), 3.19 (s, 3H), 1.42 (s, 9H), 1.31 (d, J = 6.8 Hz, 3H). [00608] Step 4: (R,Z)-4-(Methylsulfonyl)but-3-en-2-amine. tert-Butyl N-[(Z,R)-1-methyl-3- methylsulfonyl-allyl]carbamate (2.4 g, 9.47 mmol, 1.0 eq) and p-toluenesulfonic acid monohydrate (1.8 g, 9.47 mmol, 1.0 eq) were taken up in ACN (30 mL, 0.32 M). The mixture was stirred at 40 °C for 16 h. 263 QB\184200.00081\93036187.2
VVID-747PC After cooling to rt, the reaction was concentrated under vacuum to afford (R,Z)-4-(methylsulfonyl)but-3- en-2-amine (3.0 g, 99% yield) as a white solid, which was used without further purification.
1H NMR (400 MHz, CDCl
3) δ 8.13 (br s, 1H), 7.51 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 8.0 Hz, 2H), 6.75 (d, J = 11.2 Hz, 1H), 6.37 (dd, J = 9.6, 11.2 Hz, 1H), 5.09 (br s, 2H), 4.95 - 4.75 (m, 1H), 3.12 (s, 3H), 2.29 (s, 3H), 1.31 (d, J = 6.8 Hz, 3H). 1-[4-Bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-N-[(Z,1R)-1-methyl-3-methylsulfonyl- allyl]piperidine-4-carboxamide g,
3.36 1.0 v/v, 20 mL, 0.088 was NaNO
2 mg, 1.1 eq) at 0 ℃. This (mixture 1) was stirred at 0 ℃ for 1 hour. In a separate flask, SO
2 was bubbled through acetic acid (33 mL, 0.088 M) for 10 min at 0 ℃ before CuCl (99 mg, 0.3 eq) was added. SO
2 was bubbled through this (mixture 2) for 10 min. Mixture 1 was added to mixture 2 at 0 ℃ and the resulting solution was stirred at 0-20 ℃ for 1 hour. The reaction was quenched with water and extracted with methyl tert-butyl ether. The combined organic layers were washed with sat. aq. sodium bicarbonate and brine, dried over Na
2SO
4, filtered, and evaporated in vacuo. The crude material was purified by FCC on silica (PE:EtOAc 50:1) to give 4-bromo-2-iodo-benzenesulfonyl chloride (4.0 g, 62% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 7.72 (dd, J = 8.6, 1.9 Hz, 1H), 8.07 (d, J = 8.6 Hz, 1H), 8.37 (d, J = 1.9 Hz, 1H). [00610] Step 2: Ethyl 1-(4-bromo-2-iodo-phenyl)sulfonyl-4-fluoro-piperidine-4-carboxylate. To a solution of 4-bromo-2-iodo-benzenesulfonyl chloride (3.6 g, 9.44 mmol, 1.0 eq) and ethyl 4- fluoropiperidine-4-carboxylate hydrochloride (2.0 g, 9.44 mmol, 1.0 eq) in DCM (10 mL, 0.94 M) was added triethylamine (2.9 g, 28.3 mmol, 3.0 eq) dropwise at 0 °C. The resulting mixture was stirred at 20 °C for 1 hour before being quenched with water and extracted with DCM. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated in vacuo. The crude material was purified by FCC on silica to give ethyl 1-(4-bromo-2-iodo-phenyl)sulfonyl-4-fluoro-piperidine-4-carboxylate (4.3 g, 87% yield) as a yellow solid.
1H NMR (400 MHz, CDCl
3) δ 8.28 (d, J = 1.9 Hz, 1H), 7.99 (d, J = 8.5 Hz, 1H), 7.64 (dd, J = 8.4, 1.9 Hz, 1H), 4.27 (q, J = 7.1 Hz, 2H), 3.75 (dt, J = 13.0, 2.6 Hz, 2H), 3.18 (td, J = 12.8, 2.7 Hz, 2H), 2.12 - 2.31 (m, 2H), 1.99 - 2.08 (m, 2H), 1.32 (t, J = 7.1 Hz, 3H). 264 QB\184200.00081\93036187.2
VVID-747PC [00611] Step 3: Ethyl 1-[4-bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-piperidine-4-carboxylate. To a solution of ethyl 1-(4-bromo-2-iodo-phenyl)sulfonyl-4-fluoro-piperidine-4-carboxylate (878 mg, 1.69 mmol, 1.0 eq) in 1,4-dioxane (9.0 mL, 0.19 M) and 1 M aq. K
3PO
4 (2.7 mL) was added 2- chlorophenylboronic acid (290 mg, 1.86 mmol, 1.1 eq) and tetrakis(triphenylphosphine)palladium (195 mg, 0.169 mmol, 0.1 eq). The mixture was stirred at 80 °C under N
2 for 16 h. After cooling to rt, the mixture was diluted with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by prep-TLC (PE:EtOAc 3:1) to give ethyl 1-[4-bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4- fluoro-piperidine-4-carboxylate (400 mg, 47% yield) as a yellow oil.
1H NMR (400 MHz, CDCl
3) δ 8.01 (d, J = 8.5 Hz, 1H), 7.67 (dd, J = 1.9, 8.5 Hz, 1H), 7.55 - 7.44 (m, 2H), 7.43 - 7.27 (m, 3H), 4.22 (q, J = 7.0 Hz, 2H), 3.24 (br d, J = 13.4 Hz, 1H), 3.07 (br d, J = 13.3 Hz, 1H), 2.98 - 2.85 (m, 1H), 2.72 - 2.58 (m, 1H), 2.04 - 1.79 (m, 4H), 1.34 - 1.27 (m, 3H). MS (ESI): mass calcd. for C
20H
20BrClFNO
4S, 505; m/z found, 506 [M+H]
+. [00612] Step 4: 1-[4-Bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-piperidine-4-carboxylic acid. Ethyl 1-[4-bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-piperidine-4-carboxylate (460 mg, 0.911 mmol, 1.0 eq) and lithium hydroxide monohydrate (115 mg, 2.73 mmol, 3.0 eq) were taken up in THF/water (3:1 v/v, 8.0 mL, 0.11 M). The mixture was stirred at 25 °C for 2 h before being concentrated and acidified with 6 M HCl to pH 2~4. The resulting precipitate was collected by filtration to afford 1-[4- bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-piperidine-4-carboxylic acid (400 mg, 92% yield) as a white solid. [00613] Step 5: 1-[4-Bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4-fluoro-N-[(Z,1R)-1-methyl-3- methylsulfonyl-allyl]piperidine-4-carboxamide. 1-[4-Bromo-2-(2-chlorophenyl)phenyl]sulfonyl-4- fluoro-piperidine-4-carboxylic acid (500 mg, 1.05 mmol, 1.0 eq), (R,Z)-4-(methylsulfonyl)but-3-en-2- amine (438 mg, 1.36 mmol, 1.3 eq), T
3P
® (1.67 g, 2.62 mmol, 2.5 eq), and N,N-diisopropylethylamine (407 mg, 3.15 mmol, 3.0 eq) were combined in DCM (5.0 mL, 0.21 M). The mixture was stirred at 25 °C for 2 h before being diluted with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The resulting residue was purified by FCC on silica (0-50% EtOAc in PE) to afford 1-[4-bromo-2-(2- chlorophenyl)phenyl]sulfonyl-4-fluoro-N-[(Z,1R)-1-methyl-3-methylsulfonyl-allyl]piperidine-4- carboxamide (428 mg, 66% yield) as a white solid. MS (ESI): mass calcd. for C
23H
25BrClFN
2O
5S
2, 606; m/z found, 607 [M+H]
+.
1H NMR (400 MHz, DMSO-d
6) δ 8.37 (br d, J = 6.0 Hz, 1H), 7.99 - 7.92 (m, 1H), 7.92 - 7.86 (m, 1H), 7.60 (d, J = 2.0 Hz, 1H), 7.58 - 7.51 (m, 1H), 7.49 - 7.41 (m, 1H), 7.39 (d, J = 4.1 Hz, 2H), 6.45 (d, J = 11.3 Hz, 1H), 6.28 (dd, J = 9.5, 11.1 Hz, 1H), 5.44 - 5.27 (m, 1H), 3.21 - 3.05 (m, 5H), 2.79 - 2.69 (m, 1H), 2.65 - 2.56 (m, 1H), 2.02 - 1.61 (m, 4H), 1.20 (d, J = 6.9 Hz, 3H). Representative biochemical data for inhibition of pAKT is presented in Table 4. Example No. H358 pAKT IC
50 (µM) H358 pAKT Imax (%control)
QB\184200.00081\93036187.2
VVID-747PC Example No. H358 pAKT IC
50 (µM) H358 pAKT Imax (%control) 3 0.072 98.4 4 0.072 68.2
QB\184200.00081\93036187.2
VVID-747PC Example No. H358 pAKT IC
50 (µM) H358 pAKT Imax (%control) 57 0.195 92.7 58 0.328 90.8
QB\184200.00081\93036187.2
VVID-747PC Example No. H358 pAKT IC
50 (µM) H358 pAKT Imax (%control) 114 0.537 82.0 115 0.478 65.3
QB\184200.00081\93036187.2
VVID-747PC Example No. H358 pAKT IC
50 (µM) H358 pAKT Imax (%control) 167 0.112 94.2 168 0.055 103.9
QB\184200.00081\93036187.2
VVID-747PC Example No. H358 pAKT IC
50 (µM) H358 pAKT Imax (%control) 220 0.143 106.1 221 0.088 103.0
r illustrative purposes only and that various modifications or changes in light thereof will be suggested to persons skilled in the art and are to be included within the spirit and purview of this application and scope of the appended claims. All publications, patents, and patent applications cited herein are hereby incorporated by reference in their entirety for all purposes. 270 QB\184200.00081\93036187.2


















































































































































































































































































































































































































