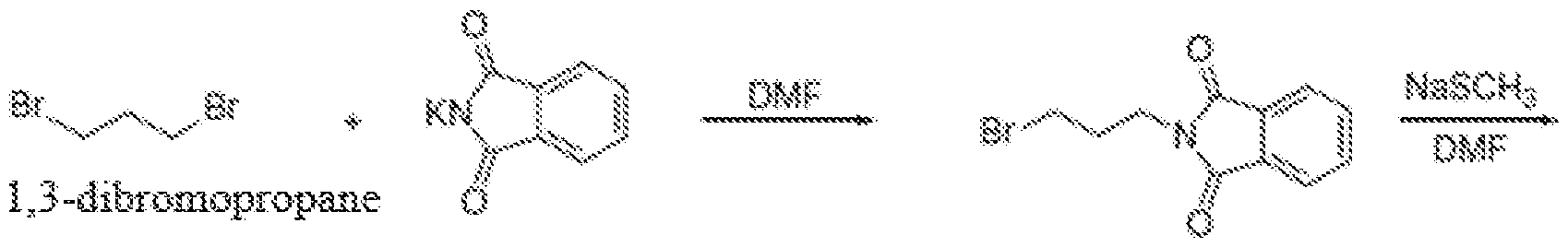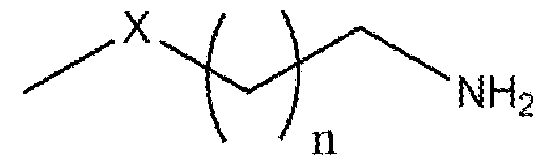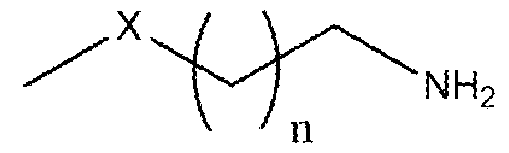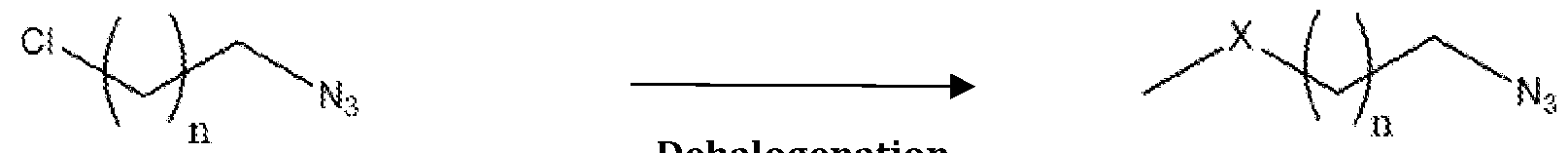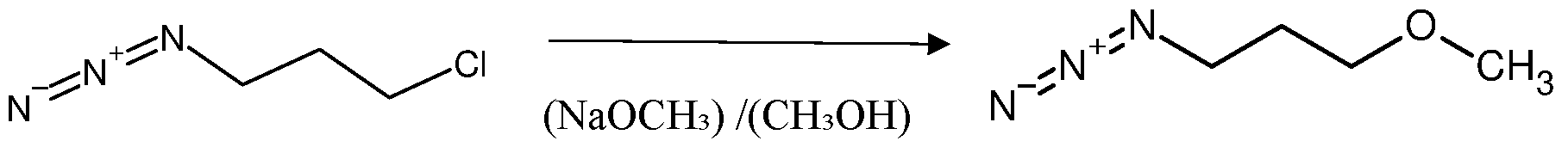TITLE OF THE INVENTION An in situ process for the preparation of primary amine compounds TECHNICAL FIELD: The present invention relates to an in situ process for the preparation of primary amine compounds. Particularly, it relates to the preparation of primary amine compounds having general Formula (I).
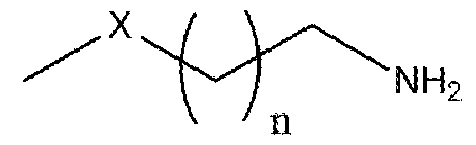
Formula (I) wherein n is 1 to 9 and X is -S, -N- or -O. More particularly, the present invention relates to the preparation of primary amine compounds with high yield and purity which is further used as intermediate in the synthesis of isothiocyanates (ITCs) BACKGROUND OF THE INVENTION: Isothiocyanates (ITCs) are an important functional group in natural products as well as in synthetic products which have the structural formula SCN-R. Isothiocyanates (ITCs) are the most studied glucosinolate-derived bioactive food components in terms of bioactivity. ITCs has good medicinal properties, more than few decades there are many research on the use of cruciferous vegetable containing isothiocyanate compounds in the chemoprevention. Multiple epidemiological trials have shown benefit of ITCs in lung cancer, breast cancer, prostate cancer, bladder cancer, stomach cancer, colorectal cancer and kidney cancer. Currently, ITCs have been approved for clinical use in the United States and China in the prevention of cancer in healthy people. ITCs also has good neuroprotective properties, for the prevention and treatment of disorders related to the central nervous system. Particularly, in the neurodegenerative disorders such as Alzheimer’s, Parkinson’s, or Hunginton’s disease, multiple sclerosis, amyotrophic lateral sclerosis, or ischemic brain injury. ITCs also can act as an antioxidant and provide protection against neurodegenerative disorders by activating Nrf2/ARE pathway. Furthermore, ITCs are active in the nervous system via other mechanisms such as reduction in the activation of cell death by apoptosis [S. Giacoppo et. al., Oxidative Med. Cell. Longev. (2013) 1-10]. ITCs have received growing attention as therapeutic compounds to be used in medicine as a best alternative to synthetic drugs, since ITCs have low or no toxicity as well as side effects. ITCs are easily available in the market and can be taken continuously without any concern.
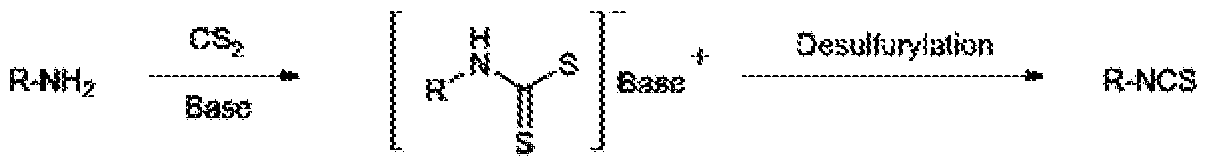
Generally, ITCs are manufactured by extracting components contained in cruciferous plants. However, there are various methods in prior arts which have been proposed for synthetic preparation of ITCs and there are various starting materials or intermediates used in the preparation of ITCs, such as use of isocyanides, organic halides, olefins, aldoximes, or isonitriles as starting materials, but the most used methodology is that of starting from amines. [ R. Recio et al. Biologically active isothicynates chapter 6, 2017] The first synthesis of an ITC compound from amine was published by Ratke in 1872 wherein an amine is treated with thiophosgene. This process reported as a scheme 1. Scheme 1 However, thiophosgene CSCl2 is highly toxic and thioureas can be formed as a by- product. To avoid this drawback, the new method was developed which involves the conversion of an amine into the corresponding dithiocarbamate by reacting with CS2 in the presence of an organic or inorganic base. A subsequent desulfurylation led to the ITC by using a proper desulfurylation reagent. The said process is reported as scheme 2.
Scheme 2 US20200079731 discloses high-purity isothiocyanate compound preparation method for industrial production, reported as scheme 3.
Scheme 3 Hence, it is well known that amine compounds, more particularly primary amine compounds are key intermediate for the preparation of ITCs. There are various processes known in art for the preparation of this key intermediate. In chemical industry there are three common well known methods used for the preparation of primary amines, such as 1. Gabriel Synthesis:
2. Curtius Rearrangement:
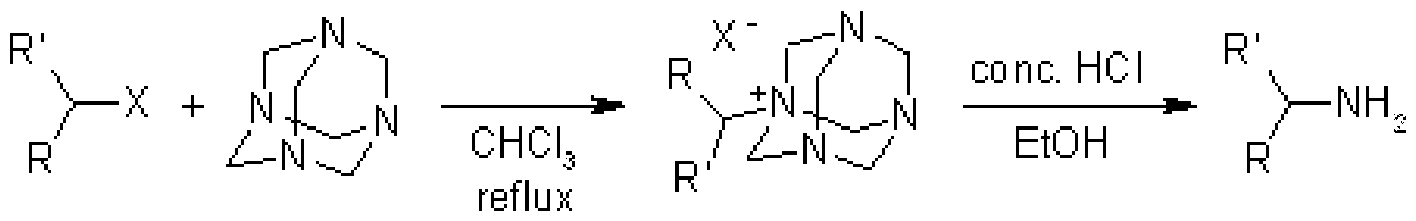
3. Delépine Reaction:
Primary amine can also be prepared by optimizing the metal hydride/ammonia mediated reductive amination of aldehydes and hemiacetals, primary amines were selectively prepared with no or minimal formation of the usual secondary and tertiary amine byproduct. The methodology was performed on a range of functionalized aldehyde substrates, including in situ formed aldehydes from a Vasella reaction. [E. M. Dangerfield et al., J. Org. Chem., 2010, 75, 5470-5477]. Another process wherein metal and base-free, chemo-selective reaction of boronic acids with cyanamidyl/arylcyanamidyl radicals provides primary aryl-, heteroaryl-, and alkyl amines at ambient temperature. The reaction is mediated by Phenyliodine bis(trifluoroacetate) and N-Bromosuccinimide. [N. Chatterjee, J. Org. Chem., 2016, 81, 5120-5127]. The processes for preparation of amines as intermediates for ITCs have been disclosed in various literature like R. Recio et. al., Studies in Natural Products Chemistry, Vol.53. which is reported hereinbelow as scheme 5 (based on Gabriel Synthesis) and scheme 6.
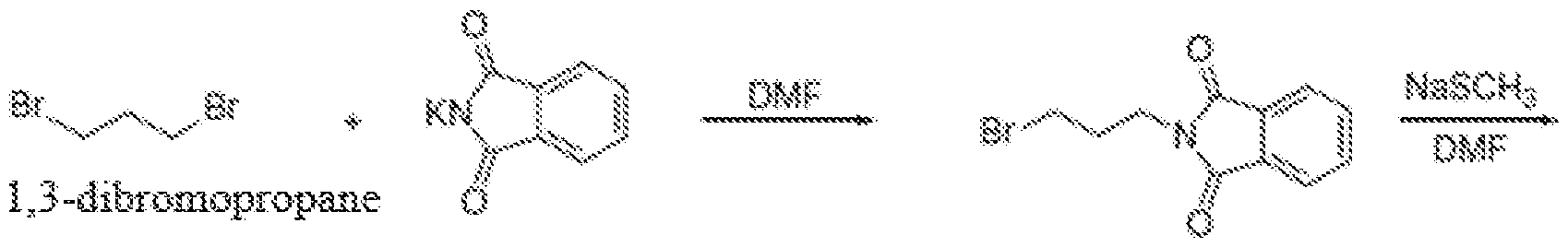
Scheme 5: 3-methylthiopropylamine obtained from 1,3-dibromopropane
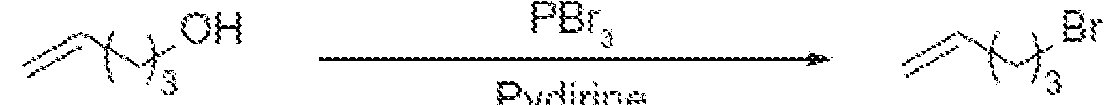

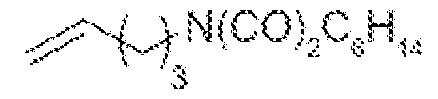
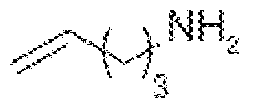
Scheme 6: Primary amines obtained from 4-penten-1-ol All prior art processes for the preparation of the primary amine compound require longer duration for conversion reaction . Most prior art processes go through multiple stages with expensive reagents. Due to the products high decomposition rate formation of unrelated impurities are also high which sacrifice the final yield and purity. The prior art processes are economically very high to produce on industrial scale. It is also observed that conventional ITC end products such as methyl sulfane, methyl sulfinyl, sulfonyl are very unstable not only in aqueous but also in polar aprotic environments. Some products are unstable to heat and temperature. The known process for preparation of ITC involves bulky leaving group which is expensive and need high temperature to react, further few processes involve inflammable, harmful and corrosive solvents which are harmful to the skin and eyes. Further it is observed that high decomposition rate of the end products, the production of unrelated impurities is also high which sacrifice the final yield and purity. To overcome the existing expensive, time-consuming cumbersome process for the preparation of ITCs, there is a need to develop novel, specific, high-quality intermediate chemicals for the preparation of ITCs, where the desired product can be obtained with high yield and purity and better stability.
OBJECTIVE OF THE INVENTION: An object of the present invention is to provide an improved process for preparation of ITC intermediates i.e. primary amine compounds of a Formula (I).
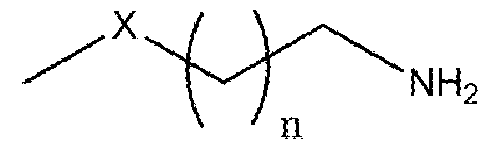
Formula (I) wherein n is 1 to 9 and X is -S, -N- or -O. Another object of the present invention is to provide an improved in situ process for preparation of primary amine compounds of the Formula I. Yet another object of the present invention is to provide a green process that avoids production of unwanted or harmful by-products through the build-up of reliable, sustainable, and eco-friendly synthesis procedures. Yet another object of the present invention is to provide industrially viable process for the preparation of primary amine compounds of the Formula I with high purity and yield. SUMMARY OF THE INVENTION: The inventors of the present invention have developed cost effective, feasible and prompt process for preparation of compounds of formula I, the potent precursors or intermediates of isothiocyanates ITCs. In a particular aspect, the present invention relates to a process for the preparation of primary amine compound of formula (I) which can be further converted into therapeutically active ITCs. In one aspect of the present invention provides a process for the preparation of primary amine compound of formula (I),
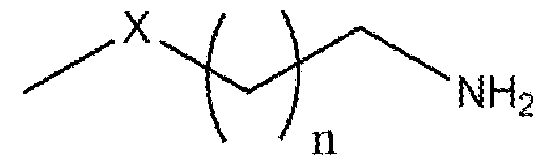
Formula (I) wherein n is 1 to 9 and X is -S, -N- or -O. which is prepared from azido-chloroalkane compound of formula (III) and azido-alkyl compound of formula (II) in the presence of suitable reagents, catalysts, and solvents.
Formula (III) Formula (II) In another aspect, the process of present invention avoids excessive use of solvents thereby making this process industrially feasible and environmentally safe. In yet another aspect, the product obtained from the process of the present invention shows purity in a range of about 90-99% thus, the process of present invention avoids complex purification by using expensive and hazardous catalyst or reagents. In further aspect, the process of the present invention avoids lengthy steps, avoid use of large amounts of solvents and hazardous catalysts required for purification thereby avoiding yield loss thereof and making process of the present invention substantially simple, green, safe and cost-effective. DETAILED DESCRIPTION OF THE INVENTION: The invention will now be described in connection with certain preferred and optional embodiments, so that various aspects thereof may be more fully interpreted and comprehended. However, any skilled person or artisan will appreciate the extent to which such embodiments could be generalized in practice. It is further to be understood that all terminology used herein is for the purpose of describing particular embodiment only and is not intended to be limiting in any manner or scope. Unless defined otherwise, all technical and scientific expressions used herein have the same meaning as commonly understood by one of ordinary skill in the art to which embodiments of the invention pertain. In describing and claiming the embodiments of the present invention, the following terminology will be used in accordance with the definitions set out below which are known in the state of art. As used in the specification the singular forms "a" "an" and "the" include plural references unless the context clearly dictates otherwise. Thus, for example, reference to "a solvent" includes mixtures of solvents, reference to "an agent" includes mixtures of two or more such agents, and the like.
The term “pharmaceutically/ nutraceutically acceptable salt,” as use herein, represents those salts which are within the scope of sound medical judgment, are suitable for use in contact with the tissues of humans and animals without undue toxicity, irritation, allergic response and the like and are commensurate with a reasonable benefit/risk ratio. Particularly, the term “pharmaceutically-acceptable salts” refers to the relatively non-toxic, inorganic and organic acid addition salts of compounds, alkali or alkaline earth metal salts, as well as solvates, co- crystals, polymorphs and the like of the salts. The term “catalyst” as used herein, represents a compound which alter the rate of reaction by changing the path of reaction. It is used to speed up or increase the rate of the reaction. In essence, catalysts encourage molecules to react and make the whole reaction process easier and more efficient. The term “solvents” as used herein, represents a compound or mixture or substance, ordinarily a liquid, in which other materials dissolve to form a solution. The role of a solvent in a chemical reaction can either be non-participatory or participatory which depends on the type of solvent and its strength. Some solvents do not participate in chemical reactions. They simply serve as the reaction medium to enable chemical reactions to occur more rapidly. All modifications and substitutions that come within the meaning of the description and the range of their legal equivalents are to be embraced within their scope. A description using the transitional phrase “comprising” allows the inclusion of other elements to be within the scope of the invention. In preferred embodiment, the present invention relates to an improved process for the preparation of primary amine compounds of Formula (I), which is a key intermediate in the preparation of ITCs.
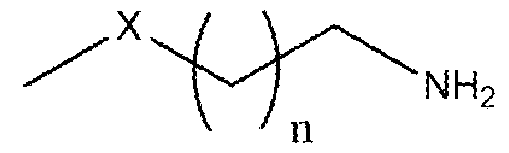
Formula (I) wherein n is 1 to 9 and X is -S, -N- or -O. In another embodiment, the invention provides cost-effective, viable process for the preparation of primary amine compounds of Formula (I) with high purity and good yield.
In yet another embodiment, the present invention provides a process for the preparation of primary amine compounds of formula (I),
Formula (I) wherein n is 1 to 9 and X is -S, -N- or -O. which is prepared from azido-chloro compounds of formula (III) and azido-hetero alkyl compounds of formula (II) in presence of suitable reagents, catalysts and solvents.
Formula (III) Formula (II) In one preferred embodiment, the present invention provides an improved in situ process for the preparation of primary amine compounds of Formula (I).
Formula (I) wherein the steps involve; a. dehalogenation of azido-choro compounds of a Formula (III) in presence of polar solvent to obtain azido-heteroalkyl compounds of Formula (II);
Formula (II)
b. catalytic hydrogenation of azido-heteroalkyl compounds of Formula (II) in the presence of polar solvent to obtain primary amine compounds of Formula (I) with high yield and purity. hydrogenation
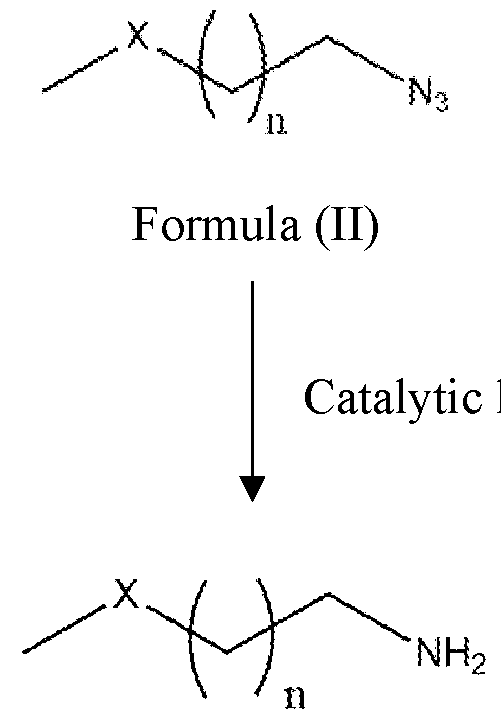
Formula (I) In another embodiment the invention provides advanced process for the preparation of azido-heteroalkyl compounds of Formula (II) by the dehalogenation of a compound of a Formula (III) in presence of low molecular weight polar solvents. The azido-heteroalkyl compounds of Formula (II) include but not limited to azido- alkoxy, azido- thioalkyl or azido amino-alkyl compounds. In another embodiment, the dehalogenating agents are selected from the group consisting of sodium methyl mercaptan, sodium methoxide, ethanolic ammonia with chloromethane at temperature ranges from 20°C to 60°C. Optionally temperature can be maintained below 20°C based on the rate of reaction. In yet another embodiment, the dilution of dehalogenating agents ranges from 10% to 50% in polar solvent, additionally lower alcohols are added to expedite the process. In some embodiment the dehalogenating agent when X is sulfur is sodium methyl mercaptan with 20% aqueous solution. The reduction of azides to amines is an important transformation in organic synthesis. Readily prepared in regio- and stereoselective processes, azides are widely used as amine precursors. In another embodiment, the invention provides simple green process for preparation of compounds of formula I comprising catalytic hydrogenation of compound of formula II where organic azides are reduced to primary amines, liberating N2 in the process.The catalytic hydrogenation is carried out in presence of homogeneous or heterogeneous catalyst.
Homogeneous catalysts for hydrogenation are usually metal-complexes with rubidium or rhodium such as Ru-MACHO or Wilkinson's catalyst. A heterogeneous catalyst is not in the same state of matter and usually is a solid metal catalyst in hydrogenations. In another embodiment, the heterogeneous catalyst metals are selected from nickel, copper, palladium, and platinum or combination thereof. These metals are typically finely divided and adsorbed on the surface of a high surface-area material such as activated carbon or alumina (Al2O3), Since these do not dissolve in solution, they are called heterogeneous catalysts. In yet another embodiment the catalytic hydrogenation of azide compounds of Formula II is carried out in the presence of heterogeneous catalyst i.e. palladium metal is supported on activated carbon to maximize its surface area and activity. Pd is deposited by an ultrasonic-assisted method, leading to the formation of highly dispersed Pd nanoparticles. The fresh hydrogen pressure is maintained in the range of 10-20 kg/cm
2. In some embodiment the suitable metal catalysts are platinum, titanium, activated charcoal, carbon, palladium, nickel, cobalt, manganese, silver, mercury, antimony trioxide, copper chloride, iron chloride, iron oxide, ziegler-natta, zeolite, magnesium oxide, aluminoxane, triethylaluminium, chromia alumina, boron, molybdenum, titanium, tin, zinc nitrate, lead oxide, ruthenium, iridium, chromium (vi) oxide, vanadium etc. In further embodiment, the solvent used in the catalytic hydrogenation is a polar solvent such as water, acetone, acetonitrile, ethyl acetate, THF, acetic acid, dimethylformamide (DMF), dimelthylsulfoxide (DMSO), isopropanol, propanol, butanol, ethanol, and methanol. In some embodiment, the green solvent used is lower alcohol like ethanol or methanol which can be easily recycled on completion of the reaction by distillation. In another embodiment, the temperature maintains for catalytic hydrogenation where reduction of azide is performed in situ under flow of H
2. The catalytic hydrogenation temperature is maintained not more than 60°C. In another embodiment, the azido-choro compounds of Formula III are selected from the group consisting of 1-azido 2-chloroethane; 1-azido 3-chloropropane; 1-azido 4- chlorobutane; 1-azido 5-chloropentane. In yet another embodiment, the azido- heteroalkyl compounds of Formula II is selected from the group consisting of 1-azido-4-(methylsulfanyl) ethane; 1-azido-4-(methylsulfanyl)
propane; 1-azido-4-(methylsulfanyl) butane; 1-azido-4-(methylsulfanyl) pentane; 1-azido-3- methoxypropane; 3-azido-N-methylpropan-1-amine; 4-azido-N-methylbutan-1-amine. In another embodiment, the presently improved in situ process provides compounds of Formula II with high purity and yield, wherein the yield and purity is not less than 90% after drying. In yet another embodiment, the primary amine compounds of Formula I is selected from the group consisting of 2-(methylsulfanyl) ethan-1-amine; 3-(methylsulfanyl) propan-1-amine; 4-(methylsulfanyl) butan-1-amine; 5-(methylsulfanyl) pentan-1-amine; 3-methoxypropan-1- amine; N1-methylpropane-1,3-diamine; N1-methylbutane-1,4-diamine. In another embodiment, the presently improved in situ process provides compounds of Formula- I with high purity and yield, wherein the yield and purity is not less than 80%. Preferably, the practical yield is in the range of 80% to 100%, where molar yield is in the range of 65 % to 100%. The term “reagents” as used herein, represents a compound or mixture added to a system to start or test a chemical reaction or a type of chemical ingredient that is added to an organic mixture or solution in order to transform it into another type of substance. A reagent can be used to determine the presence or absence of a specific chemical substance as certain reactions are triggered by the binding of reagents to the substance or other related substances. In another embodiment the examples of suitable reagents and solvents are selected from water, acetic acid, acetone, acetylene, DMF, DMSO, methanol, ethanol, propanol, butanol, acetonitrile, diethyl ether, ethyl acetate, carbon tetrachloride, methylene chloride, chloromethane, ammonia, chloromethane, sodium methyl mercaptan, sodium ethyl mercaptan, sodium methoxide sodium ethoxide, ammonium hydroxide, hexane, butanone (methyl ethyl ketone), butylated hydroxytoluene, n-butyllithium, carbon disulfide, carbon tetrachloride, chloroform, chromic acid, diethyl ether, dihydropyran, diisobutylaluminium hydride, dimethyl ether, dimethylformamide, dimethylsulfide, dimethyl sulfoxide, dioxane, ethanol, formaldenyde, formic acid, Grignard reagents, , hydrazine, hydrazoic acid, hydrochloric acid, hydrofluoric acid, hydrogen peroxide, imidazole, isopropyl alcohol, lime, limestone, lithium aluminium hydride, manganese dioxide, methyl tert-butyl ether, methyl mercaptan sodium nitric acid, perchloric acid, phosphoric acid, phosphorus pentachloride, phosphorus tribromide, phosphorus trichloride, phosphoryl chloride, potassium dichromate, potassium hydroxide, potassium permanganate, raney nickel, silver oxide, silver nitrate, sodium amide, sodium azide, sodium bis(trimethylsilyl)amide, sodium borohydride, sodium chlorite, sodium hydride,
sodium hydroxide, sodium hypochlorite, sodium methanethiolate, sodium mercaptides, sodium nitrite, sulfuric acid, tert-butyl hydroperoxide, tetramethylammonium hydroxide, tetramethylsilane, thionyl chloride, thiophenol and etc in combination thereof. In certain embodiments, the compounds of formula I are used for the preparation of isothiocyanates (ITCs) where the end products can be formulated in different dosage forms. Pharmaceutically acceptable carriers and excipients are chosen such that side effects from the pharmaceutical compound(s) are minimized. In some embodiment, the compounds of Formula I are useful intermediate for the synthesis of 4-(Methylthio)butyl isothiocyanate, 5-methylsulfinylpentyl isothiocyanate,1- isothiocyanato-4-methylsulfonylbutane, 4-(Methylsulfinyl) butyl isothiocyanate, 3- (Methylthio)propyl Isothiocyanate, 3-Methylsulfinylpropyl isothiocyanate, 5- Methylthiopentyl isothiocyanate 3-(Methylsulfonyl)-propyl isothiocyanate, 1-Methylsulfinylbutenyl isothiocyante. In yet another embodiment, the invention provides compounds of Formula-I, a potent intermediate useful in the preparation of isothiocyanates which are used in the treatment of certain neuropsychiatric disorders, neurological disorders, metabolic disorders, neurocognition disorder, neurodevelopmental disorders. Generally, neuropsychiatric disorders include but are not limited to schizophrenia, schizophrenia, schizophreniform disorder, brief psychotic disorder, delusional disorder, schizotypal personality disorder, major depressive disorder, bipolar disorder, chronic hallucinatory psychosis, dissociative disorders, obsessive compulsive disorder, induced delusional disorder, posttraumatic stress disorder, menstrual psychosis, cycloid psychosis depression, mania (Bipolar disorder), visual hallucination, auditory hallucination, eating disorder, attention deficit hyperactivity disorder, Tourette's syndrome, other movement disorders, substance dependence (alcohol, cocaine), bipolar affective disorders, or unipolar affective disorder, adolescent conduct disorder. Further, the term ‘neurological disorders’ relates to any disorder of the nervous system (central and peripheral nervous system), structural, biochemical or electrical abnormalities in the brain, spinal cord or other nerves like cranial nerves, peripheral nerves, nerve roots, autism (ASD), autonomic nervous system, neuromuscular junction, and muscles that can result in a range of symptoms. These disorders include epilepsy, Alzheimer’s disease, dementia, cerebrovascular diseases including stroke, migraine and other headache disorders, multiple
sclerosis, Parkinson's disease, neuroinfectious, brain tumours, traumatic disorders of the nervous system due to head trauma. Further, the term neurocognition disorder, defined as a significant decline in cognitive abilities that is severe enough to interfere with the individual’s everyday activities, dementia, ADHD, memory loss, Alzheimer, Lewy body disease, HIV, Huntington disease, Parkinson disease, Lewy body disease, Creutzfeldt–Jakob disease, Traumatic brain injury, Frontotemporal Degeneration, Multiple sclerosis, Normal pressure hydrocephalus. Additionally, the term metabolic disorder refers to any disorder which occurs when abnormal chemical reactions in human body disrupt the metabolic processes. Metabolic syndrome is a cluster of conditions that occur together, increasing risk of heart disease, stroke, type 2 diabetes, high blood pressure, high blood sugar, abdominal obesity, liver health, NASH, ASH and abnormal cholesterol or triglyceride levels. In some embodiment, the compounds represented by Formula I, or pharmaceutically acceptable salts thereof, are formulated for ITCs medicaments, which preferably take the form of therapeutically effective individual doses adjusted to the form of administration. In some embodiment, the daily dose of a pharmaceutical composition disclosed herein varies over a wide range from about 0.1 mg to about 5000 mg; preferably, the dose is in the range of about 1 mg to about 1000 mg per day for an average human. The term "therapeutically effective amount " denotes an amount that reduces the risk, potential, possibility or occurrence of a disease or disorder, or provides advanced alleviation, mitigation, and/or reduction or restoration or modulation, regulation of at least one indicator/biomarker (e.g., blood or serum CRP level), and/or minimize at least one clinical symptom related to neurological disorders like ASD. A "therapeutically effective amount" means the amount of the compound that, when administered to a subject to treat a disease or condition referred to herein, is sufficient to perform such treatment for the disease or condition. The "therapeutically effective amount" will vary depending on the form of the compound (for example, the form of the salt), the disease or condition in question and its severity, as well as the age, weight, etc., of the subject to be treated. The term ‘subject in need thereof’ pertains to a subject preferably mammal, more preferably a human suffering or suspected with neuropsychiatric disorder.
In the context of the present invention, the term “treatment” refers to alleviate, mitigate, prophylaxis, attenuate, manage, regulate, modulate, control, minimize, lessen, decrease, down regulate, up regulate, moderate, inhibit, restore, suppress, limit, block, decrease, modulate, prevent, inhibit, stabilize, ameliorate or cure, heal neurological disorders observed in the subject in need thereof. Certain compounds of the present invention exist in unsolvated forms as well as solvated forms, including hydrated forms. Further, some compounds of the present invention exist in multiple crystalline or amorphous forms (“polymorphs”). Compounds of the invention are formulated in geometric or, enantiomeric or stereoisomeric forms. In general, all physical forms are of use in the methods contemplated by the present invention and are intended to be within the scope of the invention. Compound or pharmaceutically acceptable salts, hydrates, polymorphs or solvates of a compound intends the inclusive meaning of “or”, in those materials meeting more than one of the stated criteria are included, e.g., a material that is both a salt and a solvate is encompassed. Some of the crystalline forms of the compound exist as polymorphs and as such are intended to be included in the present disclosure. In addition, some of the compounds may form solvates with water (i.e., hydrates) or common organic solvents, and such solvates are intended to be encompassed by some embodiments. In some embodiment, the invention provides medicinal compositions comprising compounds of Formula I present in effective amount along with pharmaceutically acceptable excipients. As used herein, the term “pharmaceutically acceptable carriers, diluents or excipients” is purported to mean, without limitation, any adjuvant, carrier, excipient, sweetening agent, diluents, preservative, dye/colorant, flavor enhancer, surfactant, wetting agent, dispersing agent, suspending agent, stabilizer, isotonic agent, solvent, emulsifier, or encapsulating agent, encapsulating polymeric delivery systems or polyethyleneglycol matrix, which is acceptable for use in the subject, preferably humans. Excipients also include, for example: antiadherents, antioxidants, binders, coatings, compression aids, disintegrants, dyes (colors), emollients, emulsifiers, fillers (diluents), film formers or coatings, fragrances, glidants (flow enhancers), lubricants, preservatives, sorbents, suspending or dispersing agents, sweeteners, surfactant, anticaking agent, food additives, or waters of hydration, salts.
In another embodiment, the present invention provides compounds of Formula I useful in the preparation of the medicinal composition comprising ITCs compounds which can be prepared in a manner well known in the pharmaceutical art and administered by a variety of routes, depending upon whether local or systemic treatment is desired and upon the area to be treated. The preferable route of administration includes but is not limited to sublingual, rectal, topical, parenteral, nasal or oral. In some embodiment, the present invention provides compounds of Formula I useful in the preparation of the solid pharmaceutical or medicinal composition comprising fine particles or crystalline form of compounds of ITCs. In some embodiment, the present invention provides compounds of Formula I useful in the preparation of medicinal composition comprising ITC compounds administered to a subject in need thereof, in the form which is suitable for oral use, such as a tablet, capsule (in the form of delayed release, extended release, sustained release, enteric coated release); hard gelatin capsules, soft gelatin capsules in an oily vehicle, veg capsule, hard or soft cellulose capsule, granulate for sublingual use, effervescent or carbon tablets, aqueous or oily solution, suspension or emulsion, encapsulate, matrix, coat, beadlets, nanoparticles, caplet, granule, particulate, agglomerate, spansule, chewable tablet, lozenge, troche, solution, suspension, rapidly dissolving film, elixir, gel, tablets, pellets, granules, capsules, lozenges, aqueous or oily solutions, suspensions, emulsions, sprays or reconstituted dry powdered form with a liquid medium or syrup; for topical use including transmucosal and transdermal use, such as a cream, ointment, gel, aqueous or oil solution or suspension, salve, parch or plaster; for nasal use, such as a snuff nasal spray or nasal drops; for vaginal or rectal use, such as a suppository; for administration by inhalation, such as a finely divided powder or a liquid aerosol; for sub-lingual or buccal use, such as a tablet, capsule, film, spray. In another embodiment, the composition is formulated for parenteral use including intravenous, subcutaneous, intramuscular, intravascular, infusion, intraperitoneal, intracerebral, intracerebroventricular, or intradermal routes of administration. In a further embodiment, the present composition is formulated in the form of age- appropriate paediatric oral dosage forms such as syrup, minitablets, chewable formulations, orodispersible films, orodispersible tablets.
Further, the present composition can be formulated in the form of age-appropriate pediatric oral dosage forms such as syrup, minitablets, chewable formulations, orodispersible films orodispersible tablets. It can also be prepared in the form of known food products. Notably, the present composition is stable, non-hazardous, non-toxic and safe for human consumption without any side effects, therefore the present nutritional composition can also be used under preventive therapy/adjuvant therapy/add-on therapy/ combination therapy in a subject in need thereof. In another embodiment, the compounds of Formula I of the present invention and end products thereof are non-toxic, cost effective, enriched with nutrients or biomolecules and provides safeguard against problems associated with neurotransmission without any adverse effect. In some embodiments, the compounds of Formula-I are useful for the formulation of ITCs medicaments by using pharmaceutically acceptable excipients such as diluents, binders, lubricants, solubilizing agents, surfactants, stabilizers, colors, flavoring agents, sweeteners, glidants, plasticizers, and other additives. In yet another embodiment, the invention provides a medicinal composition comprising compounds of Formula I along with pharmaceutical excipients, wherein the pharmaceutical excipients are selected from a diluent, a binder, a lubricant, a glidant, an additive, a surfactant, a stabilizer or mixtures thereof. In another embodiment, the present invention provides a method for treating neuropsychiatric disorders in a subject in need thereof. The method comprises administering an oral dose of a therapeutically effective amount of a medicinal composition comprising compounds of Formula-I along with pharmaceutically acceptable excipients. In certain embodiments, the invention provides the potent synergistic medicinal composition wherein the effective unit dose for an oral administration is formulated in a range of 1 to 1000 mg. It is further recommended that children, patients over 60 years old, initially receive low doses and that the dosage be titrated based on individual physiological responses and/or pharmacokinetics. It can be necessary to use dosages outside these ranges in some cases, as will be apparent to those in the art. The present composition can be used as infant formula as well as adult formula by varying the concentration of active ingredients. Further, it is noted that the dietician or nutritionist or certified physician knows how and when to interrupt, adjust or terminate therapy in conjunction with an individual patient's response.
The use of any and all examples, or exemplary language (e.g., such as) provided herein, is intended merely to better illuminate the invention and does not pose a limitation on the scope of the invention unless otherwise claimed. While in the foregoing specification this invention has been described in relation to certain embodiments thereof, and many details have been put forth for the purpose of illustration, it will be apparent to those skilled in the art that the invention is susceptible to additional embodiments and that certain of the details described herein can be varied considerably without departing from the basic principles of the invention. The present invention is not to be limited in terms of the particular embodiments described in this application, which are intended as single illustrations of individual aspects of the invention. The invention may be further be illustrated by the following examples, which are for illustrative purposes only and should not be construed as limiting the scope of the invention in anyway. The present disclosure is therefore to be considered as in all respects illustrative and not restrictive, the scope of the invention being indicated by the appended claims and examples, and all changes or alterations which come within the ambit of equivalency are intended to be encompassed therein. EXAMPLES: Having described the basic aspects of the present invention, the following non-limiting examples illustrate specific embodiments thereof. Those skilled in the art will appreciate that many modifications may be made in the invention without changing the essence of invention. Example-1: Dehalogenation of compounds of Formula III to obtain compounds of Formula II.
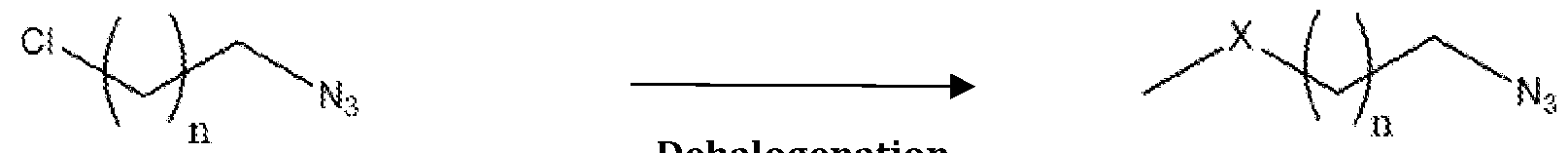
Formula III Formula-II (Azido Chloro compounds ) (Azido-Heteroalkyl compounds) Catalytic Hydrogenation
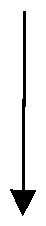
Formula (I) (Primary amine compounds) Table -1: Compounds of formula -III Sr. No. Compound Of Formula -III n M.WT 1 1-azido -2 chloroethane 1 105.52 2 1 azido- 3 chloropropane 2 119.55 3 1 azido 4-chlorobutane 3 133.58 4 1 azido 5 chloropentane 4 147.60 Table -2: Compounds of formula -II Sr. Compound Of Formula -II n X % Purity % Yield No. 1 1-azido-4-(methylsulfanyl)ethane 1 S 95 93 2 1-azido-4-(methylsulfanyl)propane 2 S 92 91 3 1-azido-4-(methylsulfanyl)butane 3 S 97 100 4 1-azido-4-(methylsulfanyl)pentane 4 S 95 90 5 1-azido-3-methoxypropane 2 O 92 89 6 3-azido-N-methylpropan-1-amine 2 N 95 92 7 4-azido-N-methylbutan-1-amine 3 N 93 90 Table -3: Compounds of formula-I Sr. Compound Of Formula -I n X % Purity % Yield No. 1 2-(methylsulfanyl)ethan-1-amine 1 S 90 85 2 3-(methylsulfanyl)propan-1-amine 2 S 91 80 3 4-(methylsulfanyl)butan-1-amine 3 S 94 82 4 5-(methylsulfanyl)pentan-1-amine 4 S 94 88 5 3-methoxypropan-1-amine 2 O 93 81 6 N1-methylpropane-1,3-diamine 2 N 93 90 7 N1-methylbutane-1,4-diamine 3 N 95 92
Example 2: Synthesis of 1-azido-4-(methylsulfanyl)butane from 1-azido-4-chlorobutane.
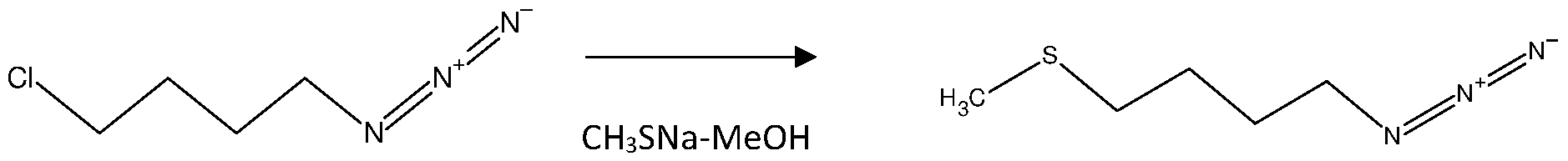
The synthesis of 1-azido-4-(methylsulfanyl)butane from azidochlorobutane involves the substitution of the chlorine atom in azidochlorobutane with a sulfur-containing nucleophile. Sodium methyl mercaptan (NaSCH3) can be used as the nucleophile, resulting in the formation of 1-azido-4-(methylsulfanyl)butane. Set up of the reaction was done under inert atmosphere conditions (e.g., nitrogen atmosphere) to prevent the interference of moisture and oxygen. In round bottom flask with overhead stirrer, thermometer pockets on tube bath, 700 gm of 1-azido-4-chlorobutane was charged in situ. Further 1400 ml of methanol was added, stirred the reaction mass for 10 to 15 minutes at temperature of 25 to 35°C. After that 20% aqueous solution of 5.7 moles of methyl mercaptan sodium salt was added with constant stirring for 24 hrs. The settled layer was separated, lower layer charged with 3000 ml of toluene and equal quantity of potable water and continued the stirring. The obtained organic layer was charged with 4000 ml of 10% sodium chloride solution, stirred and separated the organic layer and distilled out the solvent under vacuum and degas. The obtained oil mass weight was 680 to 761 gm with practical yield (0.97 to 1.2 w/w wrt starting material). The theoretical yield ratio was 1.087 and Molar yield corresponds to 89 to 100%. The HPLC purity was in the range of 90-97%, after purification the purity was enhanced upto 98-99%. Example-3: Synthesis of 4-(methylsulfanyl)butan-1-amine from 1-azido-4- (methylsulfanyl)butane.
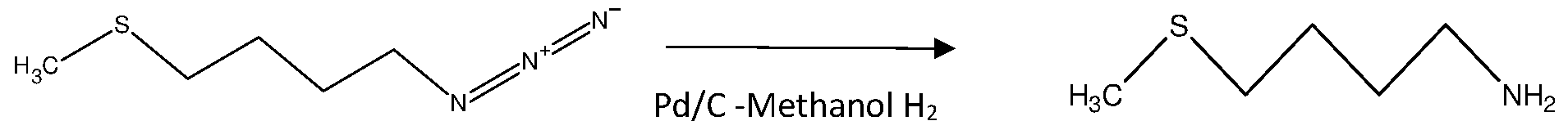
750 gm of oil mass obtained from previous stage charged with 1500 ml of methanol in situ, stirred the reaction mass; Subsequently charged 37.5 gm of 10% palladium on activated carbon, stirred and filtered the mass through hyflowbed, washed the hyflowbed using 750 ml of methanol. The filtrate was collected and charged in autoclave pressure reactor, flushed the autoclave using nitrogen, the hydrogen gas applied with pressure 12-15 kg/cm
2.Further cooled
the reaction mass and filtered through hyflowbed, washed the mass in hyflowbed using 375 ml methanol. The filtrate was collected in RBF and distilled out solvent under vacuum. The obtained oil mass weight was 650 to 740 gm with practical yield (0.866 to 0.986 w/w wrt starting material). The theoretical yield ratio was 0.9866 and molar yield corresponds to 80 to 100%. The HPLC purity was in the range of 90-96%, after purification the purity was enhanced to 99%. Example 4: Synthesis of 1-azido-3-methoxypropane from 1-azido-3-chloropropane.
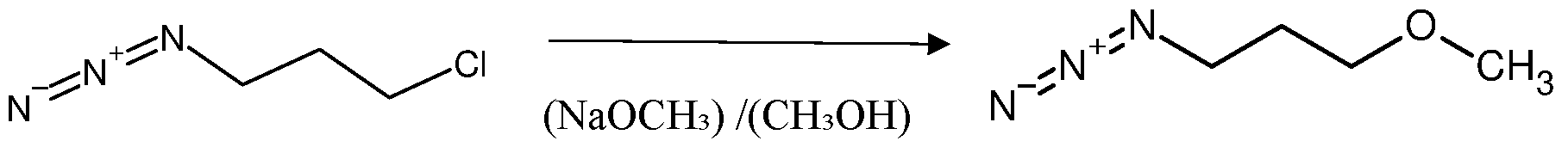
The synthesis of 1-azido-3-methoxypropane from 1-azido-3-chloropropane involves the substitution of the chlorine atom with a methoxy (OCH₃) group. In the round bottom flask 700 gm of 1-azido-3-chloropropane was added with 1500 methanol in situ , Subsequently sodium methoxide methanolic solution 500 ml added in the reaction vessel. The reaction carried on at atmospheric pressure at temperature of 50 °C in a MeOH (methanol) solution. The reaction mixture was stirred and separated from the aqueous layer. The organic solvent distilled out under vacuum to obtain oil mass with weight 600-670 gm (with practical yield (0.85 to 0.96 w/w wrt starting material). The theoretical yield ratio was 0.957 and molar yield corresponds to 80 to 100%. The HPLC purity was in the range of 90- 96%, after purification the purity was enhanced to 99%. Example 5: Synthesis of 3-methoxypropan-1-amine from 1-azido-3-methoxypropane.
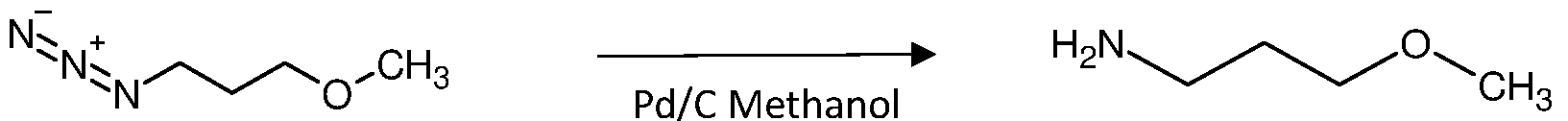
The 650 gm of oil mass obtained from previous stage charged with 1200 ml of methanol in situ, stirred the reaction mass; Subsequently charged 30.5 gm of 10% palladium on carbon, stirred and filtered the mass through hyflowbed, washed the hyflowbed using 500 ml of methanol. The filtrate was collected and charged in autoclave pressure reactor, flushed the autoclave using nitrogen, the hydrogen gas applied with pressure 12-15 kg/cm
2.Further cooled the reaction mass and filtered through hyflowbed, washed the mass in hyflowbed using 300 ml methanol. The filtrate was collected in RBF and distilled out solvent under vacuum. The obtained oil mass weight was 550 to 630 gm with practical yield (0.846 to 0.969 w/w wrt starting material). The theoretical yield ratio was 0.9699 and molar yield corresponds to 80 to
100%. The HPLC purity was in the range of 90-96%, after purification the purity was enhanced to 99%. Example-6: Process for the preparation of ITC from compounds of Formula I. Stage (i): Synthesis of tert-butyl [4-(methylthio) butyl] carbamate from 4 -(methylthio) butan-1-amine
(Boc)2 O In the (tert-butyloxycarbonyl) BOC-protected amine, the nitrogen atom is temporarily capped with a BOC group, providing protection from undesired reactions. This protection can later be removed using acid-catalyzed hydrolysis to regenerate the free amine. 1.0 mmol of 4 -(methylthio) butan-1-amine was treated with (Boc)
2O 260 mg, 1 mmol 10 mL of water: acetone 9.5: 0.5 at room temperature. The reaction was monitored by TLC. The reaction mixture was extracted with ethyl acetate (3×5mL), the organic layer was separated and dried with anhydrous Na2SO4, and the solvent was eliminated in vacuum. The product was purified in a silica gel column (hexane: diethylether 3:1) to get tert-butyl [4-(methylthio) butyl] carbamate in pure form with 95% yield. Stage-ii: Synthesis of tert-butyl [4-(methylsulfinyl) butyl] carbamate from tert-butyl [4- (methylthio) butyl] carbamate

Hydrogen peroxide (10 mmol, 30%) was slowly added to the tert-butyl [4-(methylthio) butyl] carbamate (4 mmol) in glacial acetic acid (2 mL). The reaction mixture was then stirred at room temperature until thin layer chromatography indicated the reaction was complete. The resulting solution was neutralized with aqueous NaOH (4 M) and the product was extracted with dicloromethane. The organic layer was dried over anhydrous Na2SO4 and then concentrated under reduced pressure to yield analytically pure product.
Stage- iii: Synthesis of 4-(methanesulfinyl)butan-1-amine from tert-butyl [4- (methylsulfinyl) butyl] carbamate
The obtained sulfoxide [tert-butyl [4-(methylsulfinyl) butyl] carbamate] was heated in a mixture of aqueous hydrochloric acid and toluene at 65 °C to obtain 4-(methanesulfinyl) butan- 1-amine with high yield and purity [ Yield -NLT 85%, Purity NLT-90%]. Stage-iv: Synthesis of racemic 4-(Methylsulfinyl)butyl isothiocyanate from 4- (methanesulfinyl) butan-1-amine
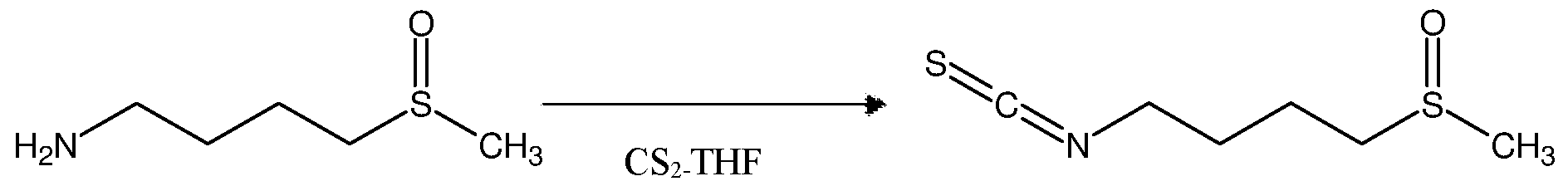
In a multi-neck round bottom flask, 4-(methanesulfinyl) butan-1-amine, 1.0 equiv. and triethylamine (1.0 equiv.) were added, and the solution was further stirred until it had cooled below -10° C in presence of 10 volume of THF. Carbon disulfide (3.35 mole 1.0 equiv.) was added dropwise over 2 hours while keeping the internal temperature below -3° C and later warmed to 10° C. Hydrogen peroxide (35% aq, 1.0 equiv.) was added slowly while keeping the internal temperature below 20° C. (bath temperature was 0° C.), further on extraction with ethyl acetate followed by treatment with 10% sodium chloride solution and activated charcoal. The reaction mass was filtered and washed with organic solvent to obtain yellowish 4- (Methylsulfinyl)butyl isothiocyanate (94% yield) material at 98% purity.