Composition and method to treat, ameliorate or prevent a psychological, neurological or inflammatory disorder, and method for preparing composition Field of the Invention The present invention relates to a composition including at least one psychedelic substance, more particularly, a composition used to treat, ameliorate, or prevent a psychological, neurological, or inflammatory disorder. The present invention also relates to a method for preparing compositions, wherein the composition includes at least one psychedelic substance. Background of the Invention Psychological disorders may present with a diverse range of signs and/or symptoms. Similarly, such disorders present a clinician with a range of recommended treatment options. For example, the common sleep disorder, insomnia, is characterised by a difficulty with one or more of the following: falling asleep, staying asleep, and returning to sleep if awakened. The disorder may present on its own, or as a symptom of a coexisting condition (e.g., depression, bipolar disorder, an anxiety disorder, post- traumatic stress disorder, or a personality disorder). As with other psychological disorders, the treatment strategy will depend on several factors, namely, frequency and severity of the disorder, patient age and tolerance to medications, and the potential presence of co-morbidities, particularly coexisting psychological disorders. Additionally, while prescription medications are available, and often recommended for managing psychological disorders, treatment response can be inadequate for up to half of patients, and unwanted side effects can also occur in some patients which may negatively impact patient compliance and thus drug efficacy. For example, antidepressants can be associated with emotional numbing, detachment, reduced positive affect, suicidal tendencies, sexual difficulties, weight gain, and negative withdrawal effects. Meanwhile, antipsychotics used in the treatment of schizophrenia and other severe mental illnesses may cause movement-related side effects such as tremors and dystonia. Similarly, mood stabilizers used to manage bipolar disorders may
cause tremors, hair loss, weight gain, sexual problems, as well as liver and/or kidney damage, thereby limiting long-term patient compliance. The side effect profile of a given medication should be carefully considered when designing a treatment plan for a patient with one or more coexisting psychological disorder(s), as the side effects from a medication used to treat one disorder may exacerbate the second. For example, selective serotonin reuptake inhibitors (SSRIs) used in the treatment of depression may cause loss of appetite or insomnia, making the drug undesirable for a patient who presents with an eating disorder (i.e., anorexia nervosa or bulimia nervosa) or pre-existing insomnia. Additionally, since psychological disorders often present as a suite of conditions, such as chronic pain with depression and anxiety, the treatment plan may include administering one or more pharmaceutical agent(s) in conjunction with a psychological based therapy. It is an object of the present invention to overcome, or at least alleviate, one or more of the difficulties or deficiencies associated with the prior art. Summary of the Invention In one aspect, the present invention provides a composition including at least one psychedelic substance or a pharmaceutically acceptable salt thereof, wherein the composition treats, ameliorates, or prevents a psychological disorder, a neurological disorder, an inflammatory disorder, or provides other therapeutic effects. In a preferred embodiment, invention provides a composition including at least one psychedelic substance and at least one harmala alkaloid, or a pharmaceutically acceptable salt thereof, wherein the composition treats, ameliorates, or prevents a psychological disorder, a neurological disorder, or an inflammatory disorder. By the term ‘psychedelic substance’ as used herein, is meant a chemical compound which may produce changes in the user’s perception (e.g., distorted vision, illusions, or hallucinations), mood (e.g., euphoria, elation, panic, or fear), or cognitive processes (e.g., memory, attention, creativity, language patterns or word associations).
By the term ‘harmala alkaloid’ as used herein, is meant a chemical compound which may reversibly inhibit monoamine oxidase A (MAO-A) and/or monoamine oxidase B (MAO-B). By the term ‘pharmaceutically acceptable salt’ as used herein, is meant any salt preparation that is appropriate for use in a pharmaceutical application. Pharmaceutically acceptable salts include, but are not limited to, amine salts; alkali metal salts, such as lithium, potassium, sodium and the like; alkali earth metal salts, such as barium, calcium, magnesium and the like; transition metal salts, such as zinc, aluminium and the like; other metal salts, such as sodium hydrogen phosphate, disodium phosphate and the like; mineral acids, such as hydrochlorides, sulfates and the like; and salts of organic acids, such as acetates, lactates, malates, tartrates, citrates, ascorbates, succinates, fumarates and the like. By the term ‘psychological disorder’ as used herein, we mean one or more condition(s) wherein a pattern of behavioural, psychological, or physical symptoms impact multiple areas of life. For example, such psychological disorders may include attention deficit hyperactivity disorder, bipolar disorder, borderline personality disorder, chronic or persistent pain or inflammation (e.g., fibromyalgia, rheumatologic pain, and headache), depression, an eating disorder or obesity, generalised anxiety disorder, insomnia, mixed anxiety, obsessive-compulsive disorder, panic disorder, post-traumatic stress disorder, schizophrenia or other severe mental illness (e.g., schizoaffective disorder, manic depressive disorder, or autism), social phobia or other specific phobias (e.g., animals, heights, blood, needles, or public speaking), or substance and alcohol use disorders. By the term ‘neurological disorder’, as used herein, we mean a condition characterized by a structural, biochemical, or electrical abnormality in the brain, spinal cord, or nerves. For example, a neurological disorder may include headaches and migraines, stroke, seizures, Parkinson’s Disease, dementia, Alzheimer’s Disease, epilepsy, aphasia, multiple sclerosis, concussion, and neck and lower back pain. By the term ‘inflammatory disorder’, as used herein, we mean a condition characterized by acute or prolonged inflammation subsequent to an autoimmune disorder (e.g., rheumatoid arthritis, lupus, psoriasis, ankylosing spondylitis, or type I diabetes), the disease process of a co-morbidity (e.g., Alzheimer’s disease, asthma, cancer, heart
disease, or type 2 diabetes), or from a contributing lifestyle factor (e.g., excessive alcohol consumption, obesity or an elevated body mass index (BMI), chronic stress, excessive strenuous exercise, or habitual smoking). By the term " therapeutic effects" as used herein, we mean effects that contribute positively to the overall health, quality of life, or mental state of an individual. These benefits may include but are not limited to mood enhancement, cognitive function improvement, increased self-awareness, stress reduction, better sleep quality, general discomfort alleviation, enhancement of life satisfaction, contentment, or resilience against daily stresses. In a preferred embodiment, the composition may include a psychedelic substance(s) selected from the group consisting of phenethylamine, N,N-dimethyltryptamine, b- methyl-phenethylamine, N-methyl-b-phenethylamine, 5-methoxy- N, N-dimethyltrypt amine, 4-phosphoryloxy-N,N-dimethyltryptamine, methyl (2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(furan-3-yl)-6a,10b-dimethyl-4,10- dioxo-dodecahydro-1H-naphtho [2,1-c]pyran-7-carboxylate (i.e., Salvinorin A), 2-(3,4,5- trimethoxyphenyl) ethanamine (i.e., mescaline), 9,10-didehydro-N,N-diethyl-6- methylergoline-8b-carboxamide (i.e., LSD), 3-[2-(dimethylaminoethyl]-1H-indol-4-yl dihydrogen phosphate (i.e., psilocybin), 3-(2-dimethylaminoethyl)-1H-indol-4-ol (i.e., psilocin), or a pharmaceutically acceptable salt thereof, or any combination thereof. More preferably, the psychedelic substance may be N,N-dimethyltryptamine or a pharmaceutically acceptable salt thereof. Even more preferably, the psychedelic substance is N,N-dimethyltryptamine succinate, N,N-dimethyltryptamine fumarate or N,N-dimethyltryptamine hemifumarate. Preferably, these psychedelic substances may be isolated from Acacia spp., Phalaris spp., Diplopterys spp., Desmodium spp., Mimosa spp., Virola spp., Psilocybe spp., Salvia spp., or Echinopsis spp. plant species. More preferably, the psychedelic substance may be isolated from Acacia acuminata, Acacia courtii, Acacia obtusifolia, or Diplopterys cabrerana plant species. In a preferred embodiment, the composition may include at least one beta-carboline (β- carboline).
In a preferred embodiment, the composition may include at least one harmala alkaloid. Preferably, the composition may include at least two harmala alkaloids, more preferably, three harmala alkaloids. In a preferred embodiment, the composition may include at least one harmala alkaloid(s) selected from the group consisting of harmane, harmalol, harmaline, harman, harmine, norharman, tetrahydroharmine or any pharmaceutically acceptable salt thereof, or a combination thereof. More preferably, at least one harmala alkaloid may be selected from the group consisting of harmine, harmaline, tetrahydroharmine or any pharmaceutically acceptable salt thereof. Even more preferably, the combination of harmala alkaloids may include harmine, harmaline and/or tetrahydroharmine or pharmaceutically acceptable salts thereof. In a preferred embodiment, the composition may include at least one harmala alkaloid(s) selected from the group consisting of harmane, harmalol, harman, harmine, norharman, tetrahydroharmine or any pharmaceutically acceptable salt thereof, or a combination thereof. More preferably, at least one harmala alkaloid may be selected from the group consisting of harmine, tetrahydroharmine or any pharmaceutically acceptable salt thereof. Even more preferably, the combination of harmala alkaloids may include harmine and/or tetrahydroharmine or pharmaceutically acceptable salts thereof. That is, in a preferred embodiment, the composition does not include harmaline or any pharmaceutically acceptable salt thereof. The omission of harmaline may improve the safety profile of the composition. Harmaline may have a narrower therapeutic index than other harmala alkaloids in areas such as genotoxicity, cardiovascular effects, and tremorgenic/convulsant effects. Preferably, the harmala alkaloid may be isolated from a Banisteriopsis spp., Peganum spp., Acacia spp., or Passiflora spp. plant species. More preferably, the bioactive compound(s) may include an harmala alkaloid isolated from a Banisteriopsis caapi or Peganum harmala plant species. The psychedelic substance(s) may be produced by a plant species and is pharmacologically active, whether isolated from a plant species or synthesised. As previously described, preferably, the psychedelic substance is isolated from a plant species.
The psychedelic substance(s) may be isolated from any part of the plant, e.g., an organ. In preferred embodiments, the psychedelic substance(s) is purified or isolated from a flower, flower bract, seed, phyllode, leaf, berry, petiole, stem, bark, root, soil, or rhizosphere of the plant, more preferably a bark, leaf or phyllode, seed, or stem. The harmala alkaloid(s) may be produced by a plant species and is pharmacologically active, whether isolated from a plant species or synthesised. As previously described, preferably, the harmala alkaloid(s) is isolated from a plant species. The harmala alkaloid(s) may be isolated from any part of the plant, e.g., an organ. In preferred embodiments, the harmala alkaloid(s) is purified or isolated from a flower, flower bract, seed, phyllode, leaf, berry, petiole, stem, bark, root, soil, or rhizosphere of the plant, more preferably a bark, leaf or phyllode, seed, or stem. Alternatively, or in addition, the harmala alkaloid(s) may be isolated from a plant species, and may then undergo chemical conversion. For example, harmaline may be isolated from a Banisteriopsis caapi or Peganum harmala plant species and may then undergo chemical conversion to yield tetrahydroharmine. In this context, the term ‘isolated’ means that psychedelic substance(s) and/or the harmala alkaloid(s) is removed from its original environment (e.g., the natural environment if it is naturally occurring). For example, a naturally occurring psychedelic substance(s) or harmala alkaloid(s) present in a living organism, such as a plant, is not isolated, but the same psychedelic substance(s) or harmala alkaloid(s) separated or extracted from some or all of the coexisting materials of the organism, being purified from said coexisting material, is isolated. Alternatively, once the psychedelic substance(s) and/or the harmala alkaloid(s) has been isolated and characterised, it may be prepared using standard synthetic techniques well known in the art. By ‘purified’ as used in the context of a psychedelic substance(s) is meant that the psychedelic substance(s) is free, including substantially free, of other compounds and/or plant components. Preferably the psychedelic substance(s) is at least approximately 50% pure, more preferably at least approximately 60% pure, even more preferably at least approximately 70% pure. In some embodiments, ‘pure’ in the context of a psychedelic substance(s) is meant that the psychedelic substance(s) is free, including
substantially free, of at least one plant component, for example, free of foreign organic matter, ash, residual pesticides, aflatoxins, or microbial impurities, or any combination thereof. In some embodiments, the psychedelic substance may be at least approximately 80% pure, more preferably at least approximately 90% pure, even more preferably at least approximately 95% pure. In some embodiments, the psychedelic substance is at least about 82%, at least about 84%, at least about 86%, at least about 88%, at least about 90%, at least about 92%, at least about 94%, at least about 96%, at least about 98%, at least about 99%, at least about 99.5%, or at least about 99.9% pure. In some further embodiments the psychedelic substance may be approximately 50% pure, preferably approximately 60% pure, more preferably approximately 70% pure, even more preferably approximately 80% pure. In a still further embodiment, the psychedelic substance may be approximately 90% pure, more preferably approximately 95% pure, even more preferably 97% pure. In some embodiments, the psychedelic substance is approximately 50%, approximately 55%, approximately 60%, approximately 65%, approximately 70%, approximately 75%, approximately 80%, approximately 82%, approximately 84%, approximately 86%, approximately 88%, approximately 90%, approximately 91%, approximately 92%, approximately 93%, approximately 94%, approximately 95%, approximately 96%, approximately 97%, approximately 98%, approximately 99%, approximately 99.5%, or approximately 99.9% pure. In some embodiments the purity of the psychedelic substance may be not less than (NLT) approximately 50% pure, preferably NLT approximately 60% pure, more preferably NLT approximately 70% pure, even more preferably NLT approximately 80% pure. In a further embodiment, the psychedelic substance may be NLT approximately 90% pure, preferably NLT approximately 95% pure, even more preferably NLT approximately 99% pure. In some embodiments, the psychedelic substance is NLT approximately 99.9%, approximately 99.8%, approximately 99.7%, approximately 99.6%, approximately 99.5%, approximately 99.4%, approximately 99.3%, approximately 99.2%, approximately 99.1%, approximately 99%, approximately 98%, approximately 97%, approximately 96%, approximately 95%, approximately 92%,
approximately 90%, approximately 85%, approximately 80%, approximately 75% or approximately 70% pure. In some embodiments the purity of the psychedelic substance may be within the range of approximately 50% to approximately 99% pure, preferably approximately 70% to approximately 99% pure, more preferably approximately 80% to approximately 99% pure, even more preferably approximately 90% to approximately 99% pure. In some embodiments the purity of the psychedelic substance may be within the range of approximately 60% to approximately 99% pure, approximately 65% to approximately 99.9% pure, approximately 70% to approximately 99.9% pure, or approximately 85% to approximately 95% pure. In some embodiments, the purity of the psychedelic substance may be less than approximately 100%, less than approximately 99%, less than approximately 98%, or less than approximately 97%. For example, the purity may be less than approximately 96%. In a further example it may have a purity less than approximately 90%. In a still further example, it may have a purity less than approximately 85%. By ‘purified’ as used in the context of a harmala alkaloid(s) is meant that the harmala alkaloid(s) is free, including substantially free, of other compounds and/or plant components. Preferably the harmala alkaloid(s) is at least approximately 50% pure, more preferably at least approximately 60% pure, even more preferably at least approximately 70% pure. In some embodiments, ‘purified’ as used herein in the context of harmala alkaloid(s) is meant that the harmala alkaloid is free, including substantially free, of at least one plant component, for example, free of foreign organic matter, ash, residual pesticides, aflatoxins, or microbial impurities, or any combination thereof. In some embodiments, the harmala alkaloid(s) may be at least approximately 80% pure, more preferably at least approximately 90% pure, even more preferably at least approximately 95% pure. In some embodiments, the harmala alkaloid(s) are at least about 82%, at least about 84%, at least about 86%, at least about 88%, at least about 90%, at least about 92%, at least about 94%, at least about 96%, at least about 98%, at least about 99%, at least about 99.5%, or at least about 99.9% pure.
In some embodiments the harmala alkaloid(s) substance may be approximately 50% pure, preferably approximately 60% pure, more preferably approximately 70% pure, even more preferably approximately 80% pure. In a still further embodiment the harmala alkaloid(s) may be approximately 90% pure, more preferably approximately 95% pure. In some embodiments, the harmala alkaloid(s) are approximately 50%, approximately 55%, approximately 60%, approximately 65%, approximately 70%, approximately 75%, approximately 80%, approximately 82%, approximately 84%, approximately 86%, approximately 88%, approximately 90%, approximately 91%, approximately 92%, approximately 93%, approximately 94%, approximately 95%, approximately 96%, approximately 97%, approximately 98%, approximately 99%, approximately 99.5%, or approximately 99.9% pure. In some embodiments the purity of the harmala alkaloid(s) substance may be NLT approximately 50%, preferably NLT approximately 60%, more preferably NLT than approximately 70%, even more preferably NLT approximately 80%. In a further embodiment, the harmala alkaloid(s) may be NLT approximately 90% pure, more preferably NLT approximately 95% pure, even more preferably NLT approximately 99% pure. In some embodiments, the purity of the harmala alkaloid(s) substance may be NLT than approximately 99.9%, 99.8%, 99.5%, 99%, 97%, 95%, 90%, 85%, 80%, 75%, or 70% pure. In some embodiments the purity of the harmala alkaloid(s) substance may be within the range of approximately 50% to approximately 99%, preferably approximately 70% to approximately 99%, more preferably approximately 80% to approximately 99%, even more preferably approximately 90% to approximately 99%. In some embodiments, the purity of the harmala alkaloid(s) substance may be less than approximately 100%. For example, the purity may be less than approximately 99%, less than approximately 98%, less than approximately 97%, less than approximately 96% or less than approximately 90%. In a further example, the harmala alkaloid(s) substance may have a purity of less than approximately 85%. In a further preferred embodiment, the composition may include a therapeutically effective amount of the psychedelic substance(s) and/or the harmala alkaloid(s), wherein the therapeutically effective amount is selected to improve pharmacokinetic
activity and/or pharmacodynamic activity of the composition. Preferably the psychedelic substance to the harmala alkaloid(s) may be present in a weight ratio of about 1:1 to about 1:10, more preferably about 1:2 to about 1:8, even more preferably about 1:3 to about 1:6. In some embodiments, the psychedelic substance to the harmala alkaloid(s) may be present in a weight ratio of about 1:1 to 1:6, 1:2 to 1:5, 1:1 to 1:5, 1:3 to 1:5, or 1:3 to 1:6. For example, the psychedelic substance to the harmala alkaloid(s) may be present in a weight ratio of about 1:4. In some embodiments, the psychedelic substance to the harmala alkaloid(s) may be present in a weight ratio of about 1:3.2, 1:3.5, 1:3.8, 1:3.9, 2:1, 2.5:1, 2.75:1, 3:1, 3.25:1, 3.5:1, 3.75:1, 3.9:1, 4:1, 4.1:1, 4.25:1, 4.5:1, 5:1, 6:1, 7:1, or about 8:1. By ‘pure’ as used in the context of a composition provided herein (e.g., a pharmaceutical composition provided herein) is meant that a composition is free, including substantially free, of certain other compounds and/or plant components. In some embodiments, ‘pure’ as used herein in the context of a composition provided herein is meant that the composition is free, including substantially free, of at least one plant component, for example, free of foreign organic matter, ash, residual pesticides, aflatoxins, or microbial impurities, or any combination thereof. Preferably the composition is at least approximately 50% pure, more preferably at least approximately 60% pure, even more preferably at least approximately 70% pure. In some embodiments, the composition is at least approximately 55% pure, at least approximately 65% pure, at least approximately 75% pure, at least approximately 80% pure, at least approximately 85% pure, at least approximately 90% pure, at least approximately 91% pure, at least approximately 92% pure, at least approximately 93% pure, at least approximately 94% pure, at least approximately 95% pure, at least approximately 96% pure, at least approximately 97% pure, at least approximately 98% pure, or at least approximately 99% pure. In some embodiments, the composition is less than approximately 100% pure, less than approximately 99.9% pure, less than approximately 99.5% pure, less than approximately 99.0% pure, less than approximately 95% pure, or less than approximately 90% pure. In a preferred embodiment, the composition may include the psychedelic substance as a percentage by weight (%w/w) of between approximately 10% w/w and approximately
30% w/w. Preferably the psychedelic substance is present at between approximately 15% w/w and approximately 25% w/w, more preferably between approximately 17% w/w and approximately 23% w/w, even more preferably between approximately 18% w/w and approximately 22% w/w. In some embodiments the composition may include the psychedelic substance at between approximately 90.91% w/w and approximately 9.09% w/w, preferably between approximately 88.89% w/w and approximately 11.11% w/w, more preferably between approximately 83.33% w/w and approximately 16.67% w/w, even more preferably between approximately 75.5% w/w and approximately 17.2% w/w. In a preferred embodiment, the composition may include the harmala alkaloid(s) at between approximately 70% w/w and approximately 90% w/w. Preferably, the harmala alkaloid may be present at between approximately 75% w/w and approximately 85% w/w, more preferably between approximately 78% w/w and approximately 83% w/w, even more preferably, between approximately 79% w/w and approximately 82% w/w. In some embodiments the composition may include the harmala alkaloid(s) at between approximately 9.09% w/w and approximately 90.91% w/w, preferably between approximately 11.11% w/w and approximately 88.89% w/w, more preferably between approximately 16.67% w/w and approximately 83.33% w/w, even more preferably between approximately 53.5% w/w and approximately 82.5% w/w. In a further preferred embodiment, the composition may include the harmala alkaloid(s) at between approximately 43.05% w/w and approximately 90.91% w/w, preferably between approximately 64.11% w/w and approximately 90.15% w/w, more preferably between approximately 76.67% w/w and approximately 83.33% w/w. For example, the composition may include approximately 20% w/w of a psychedelic substance and approximately 80% w/w harmala alkaloid(s). In another example, a composition may include approximately 25% w/w of a psychedelic substance (as a free base) and approximately 75% w/w harmala alkaloid(s) (as a free base). In a further example, a composition may include approximately 21% w/w of a psychedelic substance (as a free base) and approximately 79% w/w harmala alkaloid(s) (as a free base).
In some embodiments, the psychedelic substance may be present in an amount of at least 10% w/w, preferably at least 15% w/w, more preferably at least 18% w/w, even more preferably at least 19% w/w. In some embodiments, the psychedelic substance may be present in an amount of at most 30% w/w, preferably at most 28% w/w, more preferably at most 25% w/w. In some embodiments, the psychedelic substance may be present in an amount of about 18% w/w. In some embodiments, the psychedelic substance may be present in an amount of approximately 20% w/w. In some embodiments the psychedelic substance may be present in an amount of approximately 22 % w/w. In some embodiments, the psychedelic substance may be present in an amount of at least 9% w/w, 10% w/w, 12% w/w, 14% w/w, 16% w/w, 17% w/w, 18% w/w, 19% w/w, 20% w/w, 21% w/w, 22% w/w, 23% w/w, 24% w/w, 25% w/w, 27% w/w, or 30% w/w. In some embodiments, the psychedelic substance may be present in an amount of at most 35% w/w, 30% w/w, 28% w/w, 27% w/w, 26% w/w, 25% w/w, 24% w/w, 23% w/w, 22% w/w, 21% w/w, 20% w/w, 19% w/w, 18% w/w, 16% w/w, 14% w/w, 12% w/w, or at most 10% w/w. In some embodiments, the psychedelic substance may be present in an amount of about 35% w/w, about 30% w/w, about 28% w/w, about 27% w/w, about 26% w/w, about 25% w/w, about 24% w/w, about 23% w/w, about 22% w/w, about 21% w/w, about 20% w/w, about 19% w/w, about 18% w/w, about 16% w/w, about 14% w/w, about 12% w/w, or about 10% w/w. In some embodiments, the harmala alkaloid(s) substance may be present in an amount of at least 70% w/w, preferably at least 75% w/w, more preferably at least 78% w/w. In some embodiments, the harmala alkaloid(s) substance may be present in an amount of at most 90% w/w, preferably at most 85% w/w, more preferably at most 84% w/w, even more preferably at most 83% w/w. In some embodiments, the harmala alkaloid(s) substance may be present in an amount of approximately 78% w/w. In some embodiments, the harmala alkaloid(s) substance may be present in an amount of approximately 80% w/w. In some embodiments the psychedelic substance may be present in an amount of approximately 82% w/w. In some embodiments, the harmala alkaloid(s) substance may be present in an amount of at least 50% w/w, 55% w/w, 60% w/w, 65% w/w, 70% w/w, 72% w/w, 74% w/w, 75%
w/w, 76% w/w, 77% w/w, 78% w/w, 79% w/w, 80% w/w, 81% w/w, 82% w/w, 83% w/w, or at least 85% w/w. In some embodiments, the harmala alkaloid(s) substance may be present in an amount of at most 95% w/w, 90% w/w, 87% w/w, 85% w/w, 84% w/w, 83% w/w, 82% w/w, 81% w/w, 80% w/w, 79% w/w, 78% w/w, 77% w/w, 76% w/w, 75% w/w, 72% w/w, 70% w/w, or at most 65% w/w. In some embodiments, the harmala alkaloid(s) substance may be present in an amount of about 55% w/w, about 60% w/w, about 65% w/w, about 70% w/w, about 72% w/w, about 75% w/w, about 76% w/w, about 77% w/w, about 78% w/w, about 79% w/w, about 80% w/w, about 81% w/w, about 82% w/w, about 85% w/w, about 87% w/w, about 90% w/w, or about 95% w/w. In a further preferred embodiment, the composition may include impurities. The impurities may be present in an amount of not more than (NMT) 10% w/w and not less than (NLT) 0.01% w/w relative to the amount of the psychedelic substance(s) and/or the harmala alkaloid(s). Preferably, the impurities may be present in an amount of NMT 8% w/w and NLT 0.01% w/w relative to the amount of the psychedelic substance(s) and/or the harmala alkaloid(s). More preferably, NMT 6% w/w and NLT 0.01% w/w. Even more preferably, NMT 4% w/w and NLT 0.01% w/w and even more preferably NMT 1% w/w and not less than 0.01% w/w. In some embodiments, impurities may be present in an amount of at least approximately 0.01% w/w. For example, impurities may be present in an amount of at least approximately 1% w/w, at least approximately 2% w/w, or at least approximately 4% w/w. In some instances, impurities may be present in an amount of at least 0.01% w/w, 0.05% w/w, 0.1% w/w, 0.2% w/w, 0.3% w/w, 0.4% w/w, 0.5% w/w, 0.7% w/w, 1% w/w, 2% w/w, 3% w/w, 4% w/w, 5% w/w, 6% w/w, 7% w/w, 8% w/w, or at least 9% w/w. In some embodiments, impurities may be present in an amount of at most approximately 10% w/w, at most approximately 8% w/w, at more approximately 6% w/w, or at most approximately 4% w/w. In some instances, impurities may be present in an amount of at most 10% w/w, 9% w/w, 8% w/w, 7% w/w, 6% w/w, 5% w/w, 4% w/w, 3.5% w/w, 3% w/w, 2.5% w/w, 2% w/w, 1.5% w/w, 1% w/w, 0.75% w/w, 0.5% w/w, 0.1% w/w, or at most 0.01% w/w. In some embodiments, impurities may be present in an amount of about 0.01% w/w, about 0.05% w/w, about 1% w/w, about 1.5% w/w, about 2% w/w, about 2.5% w/w,
about 3% w/w, about 4% w/w, about 5% w/w, about 6% w/w, about 7% w/w, about 8% w/w, about 9% w/w, or about 10% w/w. The impurities may be derived from the plant source as per the extraction process of the psychedelic substance and/or the harmala alkaloid(s). For example, the compound palmitoleamide may be present is the composition in an amount of between approximately 0.05% w/w and approximately 1% w/w relative to the amount of the psychedelic substance(s) and/or the harmala alkaloid(s). Palmitoleamide has beneficial properties in respect of the biological processes including immune response, pain perception, neuronal survival, and/or enhancing the expression of anti-inflammatory cytokines. It may therefore be beneficial to include palmitoleamide in the composition. In a further embodiment, the composition may include other impurities derived from the plant source such as, for example, tryptamine, methyltetrahydro-β-carboline, N-methyl- phenethylamine, N-methyltryptamine. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 3:1 to about 9:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 3:1 to about 9:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N- Dimethyltryptamine in the composition is about 3:1 to about 9:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the
harmala alkaloids comprise harmine and harmaline, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 3:1 to about 9:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 3:1 to about 9:1. Preferably the weight ratio of harmala alkaloid(s) to N,N-Dimethyltryptamine may be about 3.25:1 to about 9:1, more preferably about 3.25:1 to about 6:1, even more preferably about 3.25:1 to about 5:1. In a further preferred embodiment, the weight ratio of harmala alkaloid(s) to N,N-Dimethyltryptamine may be about 3.5:1 to about 9:1, more preferably about 3.7:1 to 6:1. In a further embodiment, the weight ratio of harmala alkaloid(s) to N,N-Dimethyltryptamine may be about 3.5:1 to 5:1, 3.6:1 to 4.8:1, 3.5:1 to 4.5:1, or 3.7:1 to 4.4:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 4:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 4:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N- Dimethyltryptamine in the composition is about 4:1
In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 4:1 In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 4:1. For example, the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 3.96:1. In a further example, the weight ratio of harmala alkaloid(s) to N,N-Dimethyltryptamine in the composition is about 3.56:1. In a still further example, the weight ratio of harmala alkaloid(s) to N,N-Dimethyltryptamine in the composition is about 3.75:1. In a further embodiment, the weight ratio of harmala alkaloid(s) to N,N- Dimethyltryptamine is about 2:1, about 3:1, about 3.2:1, about 3.4:1, about 3.5:1, about 3.6:1, about 3.7:1, about 3.8:1, about 3.9:1, about 4:1, about 4.1:1, about 4.2:1, or about 4.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at least about 3:1, preferably at least about 3.25:1, even more preferably about 3.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at least about 3:1, preferably at least about 3.25:1, even more preferably about 3.5:1.
In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at least about 3:1, preferably at least about 3.25:1, even more preferably about 3.5:1. For example, the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at is at least about 1.5:1, at least about 1.6:1 at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.3:1, at least about 2.4:1, at least about 2.5:1, at least about 2.6:1, at least about 2.7:1, at least about 2.8:1, at least about 2.9:1, at least about 3.0:1, at least about 3.1:1, at least about 3.2:1, at least about 3.3:1, at least about 3.4:1, at least about 3.5:1, at least about 3.6:1, at least about 3.7:1, at least about 3.8:1 at least about 3.9:1, at least about 4.0:1, at least about 4.1:1, at least about 4.2:1, at least about 4.3:1, at least about 4.4:1, at least about 4.5:1, at least about 4.6:1, at least about 4.7:1, at least about 4.8:1, at least about 4.9:1, at least about 5.0:1, at least about 5.1:1, at least about 5.2:1, at least about 5.3:1, at least about 5.4:1, at least about 5.5:1, at least about 5.6:1, at least about 5.7:1, at least about 5.8:1, at least about 5.9:1, or at least about 6.0:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at least about 3:1, preferably at least about 3.25:1, even more preferably about 3.5:1. In some embodiments, the composition may include
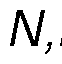
Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.3:1, at least about 2.4:1, at least about 2.5:1, at least about 2.6:1, at least about 2.7:1, at least about 2.8:1, at least about 2.9:1,
at least about 3.0:1, at least about 3.1:1, at least about 3.2:1, at least about 3.3:1, at least about 3.4:1, at least about 3.5:1, at least about 3.6:1, at least about 3.7:1, at least about 3.8:1 or at least about 3.9:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at least about 3:1, preferably at least about 3.25:1, even more preferably about 3.5:1. In some embodiments, the composition may include
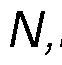
Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.3:1, at least about 2.4:1, at least about 2.5:1, at least about 2.6:1, at least about 2.7:1, at least about 2.8:1, at least about 2.9:1, at least about 3.0:1, at least about 3.1:1, at least about 3.2:1, at least about 3.3:1, at least about 3.4:1, at least about 3.5:1, at least about 3.6:1, at least about 3.7:1, at least about 3.8:1 or at least about 3.9:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N- Dimethyltryptamine in the composition is at least about 3:1, preferably at least about 3.25:1, even more preferably about 3.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.3:1, at least about 2.4:1, at least about 2.5:1, at least about 2.6:1, at least about 2.7:1, at least about
2.8:1, at least about 2.9:1, at least about 3.0:1, at least about 3.1:1, at least about 3.2:1, at least about 3.3:1, at least about 3.4:1, at least about 3.5:1, at least about 3.6:1, at least about 3.7:1, at least about 3.8:1 or at least about 3.9:1. In some embodiments, the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at most about 9:1, preferably at most about 6:1 and even more preferably at most about 5:1. In some embodiments, the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 4:1. In some embodiments, the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 3.8:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmala alkaloids to N,N- Dimethyltryptamine in the composition is at most about 3.5:1, at most about 3.6:1, at most about 3.7:1, at most about 3.8:1, at most about 3.85:1, at most about 3.9:1, at most about 3.91:1, at most about 3.92:1, at most about 3.93:1, at most about 3.94:1, at most about 3.95:1, at most about 3.96:1, at most about 3.97:1, at most about 3.98:1, at most about 3.99:1, at most about 4.00:1, at most about 4.05:1, at most about 4.10:1, at most about 4.16:1, at most about 4.20:1, at most about 4.30:1, at most about 4.42:1, at most about 4.48:1at most about 4.51:1, at most about 5:1, at most about 5.50:1, at most about 6.01:1, at most about 6.50:1, at most about 7.05:1, at most about 7.50:1, at most about 8.0:1, at most about 8.05:1, at most about 8.50:1, at most about 9.0:1, at most about 10:1, at most about 11:1, or at most about 12:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmala alkaloids to N,N- Dimethyltryptamine in the composition is at most about 3.5:1, at most about 3.6:1, at most about 3.7:1, at most about 3.8:1, at most about 3.85:1, at most about 3.9:1, at most about 3.91:1, at most about 3.92:1, at most about 3.93:1, at most about 3.94:1, at most about 3.95:1, at most about 3.96:1, at most about 3.97:1, at most about 3.98:1, at most
about 3.99:1, at most about 4.00:1, at most about 4.05:1, at most about 4.10:1, at most about 4.16:1, at most about 4.20:1, at most about 4.30:1, at most about 4.42:1, at most about 4.48:1at most about 4.51:1, at most about 5:1, at most about 5.50:1, at most about 6.01:1, at most about 6.50:1, at most about 7.05:1, at most about 7.50:1, at most about 8.0:1, at most about 8.05:1, at most about 8.50:1, at most about 9.0:1, at most about 10:1, at most about 11:1, or at most about 12:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at most about 3.5:1, at most about 3.6:1, at most about 3.7:1, at most about 3.8:1, at most about 3.85:1, at most about 3.9:1, at most about 3.91:1, at most about 3.92:1, at most about 3.93:1, at most about 3.94:1, at most about 3.95:1, at most about 3.96:1, at most about 3.97:1, at most about 3.98:1, at most about 3.99:1, at most about 4.00:1, at most about 4.05:1, at most about 4.10:1, at most about 4.16:1, at most about 4.20:1, at most about 4.30:1, at most about 4.42:1, at most about 4.48:1at most about 4.51:1, at most about 5:1, at most about 5.50:1, at most about 6.01:1, at most about 6.50:1, at most about 7.05:1, at most about 7.50:1, at most about 8.0:1, at most about 8.05:1, at most about 8.50:1, at most about 9.0:1, at most about 10:1, at most about 11:1, or at most about 12:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmala alkaloids to N,N- Dimethyltryptamine in the composition is at most about 3.5:1, at most about 3.6:1, at most about 3.7:1, at most about 3.8:1, at most about 3.85:1, at most about 3.9:1, at most about 3.91:1, at most about 3.92:1, at most about 3.93:1, at most about 3.94:1, at most about 3.95:1, at most about 3.96:1, at most about 3.97:1, at most about 3.98:1, at most about 3.99:1, at most about 4.00:1, at most about 4.05:1, at most about 4.10:1, at most about 4.16:1, at most about 4.20:1, at most about 4.30:1, at most about 4.42:1, at most about 4.48:1at most about 4.51:1, at most about 5:1, at most about 5.50:1, at most about 6.01:1, at most about 6.50:1, at most about 7.05:1, at most about 7.50:1, at most about
8.0:1, at most about 8.05:1, at most about 8.50:1, at most about 9.0:1, at most about 10:1, at most about 11:1, or at most about 12:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is at most about 3.5:1, at most about 3.6:1, at most about 3.7:1, at most about 3.8:1, at most about 3.85:1, at most about 3.9:1, at most about 3.91:1, at most about 3.92:1, at most about 3.93:1, at most about 3.94:1, at most about 3.95:1, at most about 3.96:1, at most about 3.97:1, at most about 3.98:1, at most about 3.99:1, at most about 4.00:1, at most about 4.05:1, at most about 4.10:1, at most about 4.16:1, at most about 4.20:1, at most about 4.30:1, at most about 4.42:1, at most about 4.48:1at most about 4.51:1, at most about 5:1, at most about 5.50:1, at most about 6.01:1, at most about 6.50:1, at most about 7.05:1, at most about 7.50:1, at most about 8.0:1, at most about 8.05:1, at most about 8.50:1, at most about 9.0:1, at most about 10:1, at most about 11:1, or at most about 12:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 3.25:1 to about 7.5:1. Preferably the weight ratio of N,N-Dimethyltryptamine to harmaline is about 4:1 to about 7:1, or about 4.5:1 to about 7:1, or about 5:1 to about 7.5:1, or more preferably about 5:1 to about 7:1, even more preferably about 6:1 to about 7:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of N,N- Dimethyltryptamine to harmaline in the composition is about 6.15:1 to about 6.52:1; about 6.2:1 to about 6.5:1, or about 6:24 to about 6.6:1.
For example the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 6:1. In a further example the weight ratio of N,N- Dimethyltryptamine to harmaline in the composition is about 6.3:1. In a still further example the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 6.4:1. In another embodiment, the weight ratio of N,N-Dimethyltryptamine to harmaline is about 5.5:1, about 5.6:1, about 5.63:1, about 5.64:1, about 5.7:1, about 5.8:1, about 5.9:1, about 6:1, about 6.1:1, about 6.2:1, about 6.23:1, about 6.3:1, about 6.31:1, about 6.32:1, about 6.4:1, about 6.5:1, or about 6.6:1, or about 6.8:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 6.6:1. In a further embodiment, the weight ratio of N,N-Dimethyltryptamine to harmaline to in the composition is about.6.8:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is at least about 3:1, at least about 3.5:1, at least about 4:1, at least about 4.5:1, at least about 4.75:1, at least about 5:1, at least about 5.25:1, at least about 5.5:1, at least about 5.75:1, at least about 5.8:1, at least about 5.9:1, at least about 6:1, at least about 6.1:1, at least about 6.25:1, at least about 6.5:1, or at least about 7:1. In some embodiments, the composition may include N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is at least about 5.10:1, at least about 5.20:1, at least about 5.24:1, at least about 5.31:1, at least about 5.34:1, at least about 5.60:1, at least about 5.70:1, at least about 6.15:1, at least about 6.17:1,
at least about 6.20:1, at least about 6.24:1, at least about 6.26:1, at least about 6.27:1, at least about 6.28:1, at least about 6.29:1, at least about 6.30:1, at least about 6.31:1, at least about 6.32:1, at least about 6.33:1, at least about 6.34:1, at least about 6.35:1, at least about 6.37:1, at least about 6.44:1, at least about 6.54:1, at least about 6.62:1, or at least about 6.70:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof(in any combination thereof), wherein the weight ratio of N,N- Dimethyltryptamine to harmaline in the composition is at most about 12:1, at most about 11:1, at most about 10:1, at most about 9:1, at most about 8:1, at most about 7:1, at most about 7.5:1, at most about 7:1, at most about 6.8:1, at most about 6.6:1, at most about 6.4:1, at most about 6.2:1, at most about 6:1, at most about 5.5:1, at most about 5:1, or at most about 4.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of N,N- Dimethyltryptamine to harmaline in the composition is at least about 3.25:1. In some embodiments, the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is at most about 7.5:1. In some embodiments, the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is at least about 4:1. In some embodiments the weight ratio of N,N- Dimethyltryptamine to harmaline in the composition is at most about 7:1. In some embodiments, the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is at least about 5:1, preferably at least about 6:1. In some embodiments the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is at most about 6.8:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the
harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is about 1:1 to about 7:1. In some embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine is about 1:1 to about 6:1, about 1:1 to about 5.5:1, or about 1:1 to about 5:1. Preferably the weight ratio of harmine to N,N-Dimethyltryptamine may be about 2:1 to about 5:1. More preferably, about 2.5:1 to about 5:1:, even more preferably about 3:1 to about 5:1. In further preferred embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine is about 1:1 to about 3:1. Preferably about 1.5:1 to about 2.5:1,more preferably about 1.6:1 to about 2.2:1, even more preferably about 1.8:1 to about 2:1, even more preferably about 1.85:1. For example the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 4.5:1. In a further example the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 4:1. In a still further example the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is about 3.5:1. For example, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 3.2:1, 3.4:1, 3.6:1, 3.8:1, 4.2:1, 4.4:1, 4.6:1 or 4.8:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1:1 to about 7:1. In some embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine is about 1:1 to about 6:1, about 1:1 to about 5.5:1, or about 1:1 to about 5:1. Preferably the weight ratio of harmine to N,N-Dimethyltryptamine may be about 2:1 to about 5:1. More preferably, about 2.5:1 to about 5:1:, even more preferably about 3:1 to about 5:1. In further preferred embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine is about 1:1 to about 3:1. Preferably about 1.5:1 to about 2.5:1,more preferably about 1.6:1 to about 2.2:1, even more preferably about 1.8:1 to about 2:1, even more preferably about 1.85:1.
For example the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1. In a further example the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is about 1.84:1. In a still further example the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.9:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is about 1.5:1 to about 3:1. In some embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine is about 1.5:1 to about 2.5:1, about 1.5:1 to about 2.25:1, or about 1.6:1 to about 2:1. Preferably the weight ratio of harmine to N,N-Dimethyltryptamine may be about 1.6:1 to about 2:1. More preferably, about 1.75:1 to about 1.9:1:, even more preferably about 1.75:1 to about 1.88:1. For example the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1. In a further example the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.84:1. In a still further example the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.9:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.5:1 to about 3:1. In some embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine is about 1.5:1 to about 2.5:1, about 1.5:1 to about 2.25:1, or about 1.6:1 to about 2:1.
Preferably the weight ratio of harmine to N,N-Dimethyltryptamine may be about 1.6:1 to about 2:1. More preferably, about 1.75:1 to about 1.9:1:, even more preferably about 1.75:1 to about 1.88:1. For example the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1. In a further example the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.85:1. In a still further example the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.9:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.82:1. In a further embodiment, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1. In a further embodiment, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 0.1:1, about 0.4:1, about 0.6:1, about 0.8:1, about 1:1, about 1.2:1, about 1.4:1, about 1.5:1, about 1.7:1, about 1.8:1, about 1.9:1, about 2:1, about 2.1:1, about 2.2:1, about 2.5:1, about 3:1, about 3.5:1, about 4:1, about 4.5:1 or about 5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is about 1.82:1. In a further embodiment, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1. In a further embodiment, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 0.1:1, about 0.4:1, about 0.6:1, about 0.8:1, about 1:1, about 1.2:1, about 1.4:1, about 1.5:1, about 1.7:1, about 1.8:1, about 1.9:1, about 2:1, about 2.1:1, about 2.2:1, about 2.5:1, about 3:1, about 3.5:1, about 4:1, about 4.5:1 or about 5:1.
In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is about 1.82:1. In a further embodiment, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1. In a further embodiment, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 0.1:1, about 0.4:1, about 0.6:1, about 0.8:1, about 1:1, about 1.2:1, about 1.4:1, about 1.5:1, about 1.7:1, about 1.8:1, about 1.9:1, about 2:1, about 2.1:1, about 2.2:1, about 2.5:1, about 3:1, about 3.5:1, about 4:1, about 4.5:1 or about 5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.82:1. In a further embodiment, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1. In a further embodiment, the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is about 0.1:1, about 0.4:1, about 0.6:1, about 0.8:1, about 1:1, about 1.2:1, about 1.4:1, about 1.5:1, about 1.7:1, about 1.8:1, about 1.9:1, about 2:1, about 2.1:1, about 2.2:1, about 2.5:1, about 3:1, about 3.5:1, about 4:1, about 4.5:1 or about 5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at least about 0.1:1, at least about 0.5:1, at least about 0.75:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.3:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.5:1, at least about 2.8:1, at least about 3:1, at least about 3.2:1, at least about 3.5:1 or at least about 4:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala
alkaloids, wherein the harmala alkaloids comprise harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of N,N-Dimethyltryptamine to harmine in the composition is at least about 0.10:1, at least about 0.15:1, at least about 0.20:1, at least about 0.25:1, at least about 0.30:1, at least about 0.35:1, at least about 0.40:1, at least about 0.45:1, at least about 0.50:1, at least about 0.55:1, at least about 0.60:1, at least about 0.65:1, at least about 0.70:1, at least about 0.75:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.3:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.5:1, at least about 2.8:1, at least about 3:1, at least about 3.2:1, at least about 3.5:1 or at least about 4:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is at least about 0.1:1, at least about 0.5:1, at least about 0.75:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.3:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.3:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.5:1, at least about 2.8:1, at least about 3:1, at least about 3.2:1, at least about 3.5:1 or at least about 4:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of N,N-Dimethyltryptamine to harmine in the composition is at least about 0.10:1, at least about 0.15:1, at least about 0.20:1, at least about 0.25:1, at least about 0.30:1, at least about 0.35:1, at least about 0.40:1, at least about 0.45:1, at least about 0.50:1, at least about 0.55:1, at least about 0.60:1, at least about 0.65:1, at least about 0.70:1, or at least about 0.75:, at least about 1:1, at least
about 1.1:1, at least about 1.2:1, at least about 1.3:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.5:1, at least about 2.8:1, at least about 3:1, at least about 3.2:1, at least about 3.5:1 or at least about 4:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is at least about 0.1:1, at least about 0.5:1, at least about 0.75:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.3:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.3:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.5:1, at least about 2.8:1, at least about 3:1, at least about 3.2:1, at least about 3.5:1 or at least about 4:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of N,N-Dimethyltryptamine to harmine in the composition is at least about 0.10:1, at least about 0.15:1, at least about 0.20:1, at least about 0.25:1, at least about 0.30:1, at least about 0.35:1, at least about 0.40:1, at least about 0.45:1, at least about 0.50:1, at least about 0.55:1, at least about 0.60:1, at least about 0.65:1, at least about 0.70:1, at least about 0.75:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.3:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.5:1, at least about 2.8:1, at least about 3:1, at least about 3.2:1, at least about 3.5:1 or at least about 4:1.
In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at least about 0.1:1, at least about 0.5:1, at least about 0.75:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.3:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2:1, at least about 2.1:1, at least about 2.2:1, at least about 2.5:1, at least about 2.8:1, at least about 3:1, at least about 3.2:1, at least about 3.5:1 or at least about 4:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of N,N-Dimethyltryptamine to harmine in the composition is at least about 0.10:1, at least about 0.15:1, at least about 0.20:1, at least about 0.25:1, at least about 0.30:1, at least about 0.35:1, at least about 0.40:1, at least about 0.45:1, at least about 0.50:1, at least about 0.55:1, at least about 0.60:1, at least about 0.65:1, at least about 0.70:1, at least about 0.75:1, at least about 2.5:1, at least about 2.8:1, at least about 3:1, at least about 3.2:1, at least about 3.5:1 or at least about 4:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at most about 5.7:1, at most about 5.5:1, at most about 5:1, at most about 4.5:1, at most about 4:1, at most about 3.5:1, at most about 3:1, at most about 2.5:1, at most about 2.4:1, at most about 2.2:1, at most about 2.1:1, at most about 1.9:1, at most about 1.8:1, at most about 1.7:1, at most about 1.6:1, or at most about 1.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N-
Dimethyltryptamine in the composition is at most about 5.7:1, at most about 5.5:1, at most about 5:1, at most about 4.5:1, at most about 4:1, at most about 3.5:1, at most about 3:1, at most about 2.5:1, at most about 2.4:1, at most about 2.2:1, at most about 2.1:1, at most about 1.9:1, at most about 1.8:1, at most about 1.7:1, at most about 1.6:1, or at most about 1.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is at most about 5.7:1, at most about 5.5:1, at most about 5:1, at most about 4.5:1, at most about 4:1, at most about 3.5:1, at most about 3:1, at most about 2.5:1, at most about 2.4:1, at most about 2.2:1, at most about 2.1:1, at most about 1.9:1, at most about 1.8:1, at most about 1.7:1, at most about 1.6:1, or at most about 1.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at most about 5.7:1, at most about 5.5:1, at most about 5:1, at most about 4.5:1, at most about 4:1, at most about 3.5:1, at most about 3:1, at most about 2.5:1, at most about 2.4:1, at most about 2.2:1, at most about 2.1:1, at most about 1.9:1, at most about 1.8:1, at most about 1.7:1, at most about 1.6:1, or at most about 1.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at least about 1.5:1. In some embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at most about 5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala
alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at least about 1.5:1. In some embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at most about 5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at least about 1.5:1. In some embodiments, the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is at most about 5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at least about 1.5:1. In some embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at most about 5:1. In some embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at least about 1.7:1. In some embodiments the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at most about 4:1. In some embodiments, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at least about 1.8:1, preferably at least about 1.81:1. In some embodiments the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is at most about 1.95:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1:1 to about 7:1, about 1:1 to about 6:1, about 1:1 to about 5.5:1, about 1:1 to about 5:1, about 2:1 to about 5:1, or about 2.5:1 to about 5:1. Preferably the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine may be about 3:1 to
about 5:1. More preferably, about 3.5:1 to about 5:1, even more preferably about 3.5:1 to about 4.5:1. For example the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 4:1. In a further example the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 3.8:1. In a still further example, the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 4.2:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N- Dimethyltryptamine in the composition is about 1:1 to about 3:1, about 1:1 to about 4:1, about 1:1 to about 2.5:1, about 1.5:1 to about 2.5:1, about 1.5:1 to about 2.25:1, or about 1.75:1 to about 2:1. Preferably the weight ratio of tetrahydroharmine to N,N- Dimethyltryptamine may be about 1.5:1 to about 2.8:1. More preferably, about 1.6:1 to about 2.6:1, even more preferably about 1.7:1 to about 2.2:1. For example the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.8:1. In a further example the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.9:1. In a still further example, the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 2:1. In a further preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine
to N,N-Dimethyltryptamine in the composition is about 1:1 to about 3:1, about 1:1 to about 4:1, about 1:1 to about 2.5:1, about 1.5:1 to about 2.5:1, about 1.5:1 to about 2.25:1, or about 1.75:1 to about 2:1. Preferably the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine may be about 1.5:1 to about 2.8:1. More preferably, about 1.6:1 to about 2.6:1, even more preferably about 1.7:1 to about 2.2:1. For example the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N- Dimethyltryptamine in the composition is about 1.8:1. In a further example the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.9:1. In a still further example, the weight ratio of tetrahydroharmine to N,N- Dimethyltryptamine in the composition is about 2:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.95:1. In some embodiments, the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 0.1:1, about 0.4:1, about 0.6:1, about 0.8:1, about 1:1, about 1.2:1, about 1.4:1, about 1.5:1, about 1.7:1, about 1.8:1, about 1.9:1, about 2:1, about 2.1:1, about 2.2:1, about 2.5:1, about 3:1, about 3.5:1, about 3.7:1, about 3.9:1, about 4:1, about 4.2:1, about 4.3:1 or about 4.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.95:1. In some embodiments, the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 0.1:1, about 0.4:1, about 0.6:1, about 0.8:1, about 1:1, about 1.2:1, about 1.4:1, about 1.5:1, about 1.7:1, about 1.8:1, about 1.9:1, about 2:1, about 2.1:1, about 2.2:1, about 2.5:1 or about
3:1.In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N- Dimethyltryptamine in the composition is about 1.95:1. In some embodiments, the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 0.1:1, about 0.4:1, about 0.6:1, about 0.8:1, about 1:1, about 1.2:1, about 1.4:1, about 1.5:1, about 1.7:1, about 1.8:1, about 1.9:1, about 2:1, about 2.1:1, about 2.2:1, about 2.5:1 or about 3:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of tetrahydroharmine to N,N- Dimethyltryptamine in the composition is at least about 0.1:1, at least about 0.2:1, at least about 0.3:1, at least about 0.5:1, at least about 0.7:1, at least about 0.9:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, , at least about 2:1, at least about 2.5:1, at least about 2.7:1, at least about 3:1, at least about 3.2:1, at least about 3.3:1, at least about 3.5:1, at least about 3.8:1 or at least about 4:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of N,N-Dimethyltryptamine to tetrahydroharmine in the composition is at least about 0.1:1, at least about 0.2:1, at least about 0.3:1, at least about 0.5:1, at least about 0.7:1, at least about 0.9:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2.0:1, at least about 2.1:1, at least about 2.2:1, at least about 2.3:1, at least about 2.4:1, at least about 2.5:1, at least about 2.6:1, at least about 2.7:1, at least about 2.8:1, at least about 2.9:1, at least about 3..0:1, at least about 3.1:1, at least about 3.2:1, at least about 3.3:1, at least about 3.4:1, at least about 3.5:1, at least about 3.6:1, at
least about 3.7:1, at least about 3.8:1, at least about 3.9:1, at least about 4.0:1, at least about 4.1:1, at least about 4.2:1, at least about 4.3:1, at least about 4.4:1, at least about 4.5:1, at least about 4.6:1, at least about 4.7:1, at least about 4.8:1, at least about 4.9:1, or at least about 5.0:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at least about 0.1:1, at least about 0.2:1, at least about 0.3:1, at least about 0.5:1, at least about 0.7:1, at least about 0.9:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, at least about 2.0:1, at least about 2.1:1, at least about 2.2:1, at least about 2.3:1, at least about 2.4:1, at least about 2.5:1, at least about 2.6:1, at least about 2.7:1, at least about 2.8:1, at least about 2.9:1, or at least about 3:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of N,N-Dimethyltryptamine to tetrahydroharmine in the composition is at least about 0.1:1, at least about 0.2:1, at least about 0.3:1, at least about 0.5:1, at least about 0.7:1, at least about 0.9:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, or at least about 2:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at least about 0.1:1, at least about 0.2:1, at least about 0.3:1, at least about 0.5:1, at least about 0.7:1, at least about 0.9:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, or at least about 2:1. In some embodiments, the composition
may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of N,N-Dimethyltryptamine to tetrahydroharmine in the composition is at least about 0.1:1, at least about 0.2:1, at least about 0.3:1, at least about 0.5:1, at least about 0.7:1, at least about 0.9:1, at least about 1:1, at least about 1.1:1, at least about 1.2:1, at least about 1.4:1, at least about 1.5:1, at least about 1.6:1, at least about 1.7:1, at least about 1.8:1, at least about 1.9:1, or at least about 2:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of tetrahydroharmine to N,N- Dimethyltryptamine in the composition is at most about 5.7:1, at most about 5.5:1, at most about 5:1, at most about 4.5:1, at most about 4:1, at most about 3.5:1, at most about 3:1, at most about 2.5:1, at most about 2.4:1, at most about 2.2:1, at most about 2.1:1, at most about 1.9:1, at most about 1.8:1, at most about 1.7:1, at most about 1.6:1, or at most about 1.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at most about 5.7:1, at most about 5.5:1, at most about 5:1, at most about 4.5:1, at most about 4:1, at most about 3.5:1, at most about 3:1, at most about 2.5:1, at most about 2.4:1, at most about 2.2:1, at most about 2.1:1, at most about 1.9:1, at most about 1.8:1, at most about 1.7:1, at most about 1.6:1, or at most about 1.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof (in any combination thereof), wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at most about 5.7:1,
at most about 5.5:1, at most about 5:1, at most about 4.5:1, at most about 4:1, at most about 3.5:1, at most about 3:1, at most about 2.5:1, at most about 2.4:1, at most about 2.2:1, at most about 2.1:1, at most about 1.9:1, at most about 1.8:1, at most about 1.7:1, at most about 1.6:1, or at most about 1.5:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at least about 1.5:1. In some embodiments, the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at most about 6:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at least about 1.5:1. In some embodiments, the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at most about 4:1. In some embodiments, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N- Dimethyltryptamine in the composition is at least about 1.5:1. In some embodiments, the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at most about 4:1. In some embodiments, the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at least about 1.6:1. In some embodiments the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at most about 3:1. In some embodiments, the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at least about 1.8:1. In some embodiments the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is at most about 2:1.
In a still further preferred embodiment, the composition may include N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 3.25:1 to about 7.5:1; and the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.5:1 to about 3:1. Preferably, the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 4:1 to about 7:1; and the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.6:1 to about 2:1. More preferably, the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 5:1 to about 7:1; and the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1 to about 1.9:1. Even more preferably, the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 6:1 to about 7:1; and the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1 to about 1.88:1. In a preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.5:1 to about 3:1. In a preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.5:1 to about 3:1.; and the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1:1 to about 3:1. Preferably, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.6:1 to about 2:1 and the weight ratio of tetrahydroharmine to N,N- Dimethyltryptamine in the composition is about 1.5:1 to about 2.8:1.
More preferably, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1 to about 1.9:1 and the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.6:1 to about 2.6:1. Even more preferably, the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1 to about 1.88:1 and the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.7:1 to about 2.2:1. In a preferred embodiment, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 3.25:1 to about 7.5:1; the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.5:1 to about 3:1.; and the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1:1 to about 3:1. Preferably, the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 4:1 to about 7:1; the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.6:1 to about 2:1 and the weight ratio of tetrahydroharmine to N,N- Dimethyltryptamine in the composition is about 1.5:1 to about 2.8:1. More preferably, the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 5:1 to about 7:1; the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is about 1.75:1 to about 1.9:1 and the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.6:1 to about 2.6:1. Even more preferably, the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 6:1 to about 7:1; the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is about 1.75:1 to about 1.88:1 and the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.7:1 to about 2.2:1. In a further example, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala
alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof: wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1 to about 1.88:1; and the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.6:1 to about 2.2:1. In a further example, the composition may include N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof: wherein the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 3.25:1 to about 7.5:1; the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.75:1 to about 1.88:1; and the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1.6:1 to about 2.2:1. In a further embodiment, the composition may include between approximately 10% w/w and approximately 30% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, preferably approximately 15% w/w to approximately 25% w/w, more preferably approximately 17% w/w to approximately 23% w/w, even more preferably approximately 18% w/w to approximately 22% w/w. In some embodiments, the composition may include between approximately 19% w/w to approximately 21% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof. In some embodiments, the composition comprises at least about 5% w/w, at least about 10% w/w, at least about 11% w/w, at least about 12% w/w, at least about 13% w/w, at least about 14% w/w, at least about 15% w/w, at least about 16% w/w, at least about 17% w/w, at least about 18% w/w, at least about 19% w/w, at least about 20% w/w, at least about 21% w/w, at least about 22% w/w, at least about 23% w/w, at least about 24% w/w, at least about 25% w/w, at least about 26% w/w, at least about 27% w/w, at least about 28% w/w, at least about 29% w/w or at least about 30% w/w of N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof. In some embodiments, the composition comprises at most about 40% w/w, at most about 35% w/w, at most about 30% w/w, at most about 28% w/w, at most about 26% w/w, at most about 24% w/w, at most about 22% w/w, at most about 21% w/w, at most about 20% w/w, at most about 19% w/w, at most about 18% w/w, at most about 17% w/w, at most
about 16% w/w, at most about 14% w/w, at most about 12% w/w, or at most about 10% w/w of N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof. In some embodiments, the composition comprises about 10% w/w, about 12% w/w, about 14% w/w, about 16% w/w, about 17% w/w, about 18% w/w, about 19% w/w, about 20% w/w, about 21% w/w, about 22% w/w, about 23% w/w, about 24% w/w, about 25% w/w, about 26% w/w, about 28% w/w, or about 30% w/w of N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof. In a further embodiment, the composition may include between approximately 30% w/w and approximately 50% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof, preferably approximately 35% w/w to approx. 45% w/w, more preferably approximately 37% w/w to approximately 43% w/w, even more preferably approximately 38% w/w to approximately 42% w/w. In some embodiments, the composition may include between approximately 39% w/w to approximately 41% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof. In some embodiments, the composition comprises tetrahydroharmine or a pharmaceutically acceptable salt thereof, in an amount of at least about 20% w/w, at least about 22% w/w, at least about 24% w/w, at least about 26% w/w, at least about 28% w/w, at least about 30% w/w, at least about 32% w/w, at least about 34% w/w, at least about 36% w/w, at least about 37% w/w, at least about 38% w/w, at least about 39% w/w, at least about 40% w/w, at least about 41% w/w, at least about 42% w/w, or at least about 43% w/w. In some embodiments, the composition comprises tetrahydroharmine or a pharmaceutically acceptable salt thereof in an amount of at most about 55% w/w, at most about 50% w/w, at most about 48% w/w, at most about 46% w/w, at most about 45% w/w, at most about 44% w/w, at most about 43% w/w, at most about 42% w/w, at most about 41% w/w, at most about 40% w/w, at most about 39% w/w, or at most about 38% w/w. In some embodiments, the composition comprises tetrahydroharmine or a pharmaceutically acceptable salt thereof in an amount of about 30% w/w, about 32% w/w, about 34% w/w, about 35% w/w, about 36% w/w, about 37% w/w, about 38% w/w, about 39% w/w, about 40% w/w, about 41% w/w, about 42% w/w, about 43% w/w, about 45% w/w, about 48% w/w, or about 50% w/w.
In a further embodiment, the composition may include between approximately 25% w/w and approximately 45% w/w harmine or a pharmaceutically acceptable salt thereof, preferably approximately 30% w/w to approximately 40% w/w, more preferably approximately 32% w/w to approx.39% w/w, even more preferably approximately 35% w/w to approximately 38% w/w. In some embodiments, the composition may include between 36% w/w to approximately 38% w/w harmine or a pharmaceutically acceptable salt thereof. In some embodiments, the composition comprises harmine or a pharmaceutically acceptable salt thereof in an amount of at least about 20% w/w, at least about 22% w/w, at least about 24% w/w, at least about 26% w/w, at least about 28% w/w, at least about 30% w/w, at least about 32% w/w, at least about 34% w/w, at least about 36% w/w, at least about 37% w/w, at least about 38% w/w, at least about 39% w/w, at least about 40% w/w, at least about 41% w/w, at least about 42% w/w, or at least about 43% w/w. In some embodiments, the composition comprises harmine or a pharmaceutically acceptable salt thereof, in an amount of at most about 55% w/w, at most about 50% w/w, at most about 48% w/w, at most about 46% w/w, at most about 45% w/w, at most about 44% w/w, at most about 43% w/w, at most about 42% w/w, at most about 41% w/w, at most about 40% w/w, at most about 39% w/w, or at most about 38% w/w. In some embodiments, the composition comprises harmine or a pharmaceutically acceptable salt thereof in an amount of about 30% w/w, about 32% w/w, about 34% w/w, about 35% w/w, about 36% w/w, about 37% w/w, about 38% w/w, about 39% w/w, about 40% w/w, about 41% w/w, about 42% w/w, about 43% w/w, about 45% w/w, about 48% w/w, or about 50% w/w. In a further embodiment, the composition may include between approximately 0.5% w/w and approximately 15% w/w harmaline or a pharmaceutically acceptable salt thereof, preferably between approximately 1% w/w to approximately 10% w/w, more preferably between approximately 1% w/w to approximately 5% w/w, even more preferably between approximately 2% w/w to approximately 4% w/w. In some embodiments, the composition may include approximately 3% w/w to approximately 4% w/w harmaline or a pharmaceutically acceptable salt thereof.
In some embodiments, the composition comprises harmaline or a pharmaceutically acceptable salt thereof in an amount of at least about 0.1% w/w, at least about 0.5% w/w, at least about 0.75% w/w, at least about 1% w/w, at least about 1.25% w/w, at least about 1.5% w/w, at least about 1.75% w/w, at least about 2% w/w, at least about 2.25% w/w, at least about 2.5% w/w, at least about 2.75% w/w, at least about 3% w/w, at least about 3.25% w/w, at least about 3.5% w/w, or at least about 3.75% w/w. In some embodiments, the composition comprises harmaline or a pharmaceutically acceptable salt thereof in an amount of at most about 15% w/w, at most about 12% w/w, at most about 10% w/w, at most about 8% w/w, at most about 7% w/w, at most about 6% w/w, at most about 5% w/w, at most about 5.5% w/w, at most about 5% w/w, at most about 4.5% w/w, at most about 4% w/w, at most about 3.5% w/w, at most about 3% w/w, at most about 2.5% w/w, or at most about 2% w/w. In some embodiments, the composition comprises harmaline or a pharmaceutically acceptable salt thereof in an amount of about 2% w/w, about 2.5% w/w, about 2.6% w/w, about 2.7% w/w, about 2.8% w/w, about 2.9% w/w, about 3% w/w, about 3.1% w/w, about 3.2% w/w, about 3.3% w/w, about 3.4% w/w, about 3.5% w/w, about 4% w/w, about 5% w/w, or about 6% w/w. In a particularly preferred embodiment, the composition may include approximately 10% to approximately 30% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof and approximately 30% w/w to approximately 50% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof. In a particularly preferred embodiment, the composition may include approximately 10% to approximately 30% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof and approximately 25% w/w to approximately 45% w/w harmine or a pharmaceutically acceptable salt thereof. In a particularly preferred embodiment, the composition may include approximately 10% to approximately 30% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, approximately 30% w/w to approximately 50% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof, and approximately 25% w/w to approximately 45% w/w harmine or a pharmaceutically acceptable salt thereof. In a particularly preferred embodiment, the composition may include approximately 10% to approximately 30% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable
salt thereof, approximately 30% w/w to approximately 50% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof, approximately 25% w/w to approximately 45% w/w harmine or a pharmaceutically acceptable salt thereof, and approximately 0.5 % w/w to approximately 15% w/w harmaline or a pharmaceutically acceptable salt thereof. In a further particularly preferred embodiment, the composition may include approximately 18% to approximately 22% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof and approximately 35% w/w to approximately 38% w/w harmine or a pharmaceutically acceptable salt thereof. In a further particularly preferred embodiment, the composition may include approximately 18% to approximately 22% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof and approximately 40% w/w to approximately 44% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof. In a further particularly preferred embodiment, the composition may include approximately 18% to approximately 22% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, approximately 40% w/w to approximately 44% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof and approximately 35% w/w to approximately 38% w/w harmine or a pharmaceutically acceptable salt thereof. In a further particularly preferred embodiment, the composition may include approximately 18% to approximately 22% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, approximately 40% w/w to approximately 44% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof, approximately 35% w/w to approximately 38% w/w harmine or a pharmaceutically acceptable salt thereof and approximately 2% w/w to approximately 4% w/w harmaline or a pharmaceutically acceptable salt thereof. In some embodiments, the compositions provided herein comprise N,N- Dimethyltryptamine in an amount (free-base equivalent) of at least about 1 mg, at least about 5 mg, at least about 10 mg, at least about 15 mg, at least about 20 mg, at least about 25 mg, at least about 30 mg, at least about 35 mg, at least about 40 mg, at least
about 45 mg, at least about 50 mg, at least about 55 mg, at least about 60 mg, at least about 65 mg, or at least about 70 mg. In some embodiments, the compositions provided herein comprise
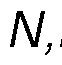
Dimethyltryptamine in an amount (free-base equivalent) of at most about 200 mg, at most about 150 mg, at most about 140 mg, at most about 120 mg, at most about 110 mg, at most about 100 mg, at most about 90 mg, at most about 80 mg, at most about 70 mg, at most about 60 mg, at most about 50 mg, at most about 40 mg, at most about 30 mg, at most about 20 mg, or at most about 10 mg. In some embodiments, the compositions provided herein comprise
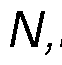
Dimethyltryptamine in an amount (free-base equivalent) of about 5 mg, about 10 mg, about 15 mg, about 20 mg, about 25 mg, about 30 mg, about 35 mg, about 40 mg, about 45 mg, about 50 mg, about 55 mg, about 60 mg, about 65 mg, about 70 mg, about 80 mg, about 90 mg, or about 100 mg. In some embodiments, the compositions provided herein comprise N,N-Dimethyltryptamine in an amount (free-base equivalent) of about 20 mg or about 60 mg or about 80 mg. In some embodiments, the compositions provided herein comprise harmala alkaloid(s) in an amount (free-base equivalent) of at least about 40 mg, at least about 60 mg, at least about 80 mg, at least about 100 mg, at least about 120 mg, at least about 140 mg, at least about 160 mg, at least about 180 mg, at least about 200 mg, at least about 220 mg, at least about 240 mg, at least about 260 mg, at least about 280 mg, at least about 300 mg, at least about 320 mg, at least about 340 mg, or at least about 360 mg. In some embodiments, the compositions provided herein comprise harmala alkaloid(s) in an amount (free-base equivalent) of at most about 500 mg, at most about 450 mg, at most about 400 mg, at most about 375 mg, at most about 350 mg, at most about 325 mg, at most about 300 mg, at most about 275 mg, at most about 250 mg, at most about 225 mg, at most about 200 mg, at most about 175 mg, at most about 150 mg, at most about 125 mg, or at most about 100 mg. In some embodiments, the compositions provided herein comprise harmala alkaloid(s) in an amount (free-base equivalent) of about 400 mg, about 380 mg, about 360 mg, about 340 mg, about 320 mg, about 300 mg, about 280 mg, about 260 mg, about 230 mg, about 210 mg, about 190 mg, about 170 mg, about 150 mg, about 120 mg, about 100 mg, about 80 mg, or about 60 mg. In some
embodiments, the compositions provided herein comprise harmala alkaloid(s) in an amount of about 80 mg, 150 mg, or 300 mg. In some embodiments, the compositions provided herein comprise harmaline in an amount (free-base equivalent) of at least about 0.5 mg, at least about 1 mg, at least about 2 mg, at least about 3 mg, at least about 5 mg, at least about 7 mg, at least about 9 mg, at least about 12 mg, at least about 14 mg, or at least about 16 mg. In some embodiments, the compositions provided herein comprise harmaline in an amount (free- base equivalent) of at most about 25 mg, at most about 23 mg, at most about 21 mg, at most about 20 mg, at most about 19 mg, at most about 18 mg, at most about 17 mg, at most about 16 mg, at most about 15 mg, at most about 14 mg, at most about 13 mg, at most about 12 mg, at most about 11 mg, at most about 10 mg, at most about 9 mg, at most about 8 mg, at most about 7 mg, at most about 6 mg, at most about 5 mg, or at most about 4 mg. In some embodiments, the compositions provided herein comprise harmaline in an amount (free-base equivalent) of about 1 mg, about 2 mg, about 3 mg, about 4 mg, about 5 mg, about 6 mg, about 7 mg, about 8 mg, about 9 mg, about 10 mg, about 11 mg, about 12 mg, about 13 mg, about 14 mg, about 15 mg, about 16 mg, or about 17 mg. In some embodiments, the compositions provided herein comprise tetrahydroharmine in an amount (free-base equivalent) of at least about 10 mg, at least about 20 mg, at least about 30 mg, at least about 40 mg, at least about 50 mg, at least about 60 mg, at least about 70 mg, at least about 80 mg, at least about 90 mg, at least about 100 mg, at least about 110 mg, at least about 120 mg, at least about 140 mg, or at least about 160 mg. In some embodiments, the compositions provided herein comprise tetrahydroharmine in an amount (free-base equivalent) of at most about 200 mg, at most about 180 mg, at most about 170 mg, at most about 150 mg, at most about 130 mg, at most about 120 mg, at most about 110 mg, at most about 100 mg, at most about 90 mg, at most about 80 mg, at most about 70 mg, at most about 60 mg, at most about 50 mg, at most about 40 mg, at most about 30 mg, at most about 20 mg, at most about 15 mg, at most about 10 mg, or at most about 5 mg. In some embodiments, the compositions provided herein comprise tetrahydroharmine in an amount (free-base equivalent) of about 10 mg, 20 mg, about 30 mg, about 40 mg, about 50 mg, about 60 mg, about 70 mg, about 80 mg, about 90 mg, about 100 mg, about 110 mg, 120 mg, 140 mg, or about
160 mg. In some embodiments, the compositions provided herein comprise tetrahydroharmine in an amount of about 120 mg, 100 mg, or 40 mg. In some embodiments, the compositions provided herein comprise harmine in an amount (free-base equivalent) of at least about 8 mg, at least about 16 mg, at least about 24 mg, at least about 32 mg, at least about 36 mg, at least about 44 mg, at least about 52 mg, at least about 60 mg, at least about 70 mg, at least about 80 mg, at least about 90 mg, at least about 100 mg, at least about 105 mg, at least about 110 mg or at least about 120 mg. In some embodiments, the compositions provided herein comprise harmine in an amount (free-base equivalent) of at most about 150 mg, at most about 145 mg, at most about 140 mg, at most about 135 mg, at most about 130 mg, at most about 125 mg, at most about 120 mg, at most about 115 mg, at most about 110 mg, at most about 100 mg, at most about 90 mg, at most about 80 mg, at most about 70 mg, at most about 60 mg, at most about 50 mg, at most about 40 mg, at most about 36 mg, at most about 30 mg, at most about 20 mg, at most about 15 mg, at most about 10 mg, or at most about 8 mg. In some embodiments, the compositions provided herein comprise harmine in an amount (free-base equivalent) of about 8 mg, 16 mg, about 24 mg, about 32 mg, about 36 mg, about 44 mg, about 52 mg, about 60 mg, about 70 mg, about 80 mg, about 90 mg, about 100 mg, about 105 mg, 110 mg, 115 mg, or about 120 mg. In some embodiments, the compositions provided herein comprise harmine in an amount of about 110 mg, 60 mg, or 36 mg. By ‘pharmacokinetic activity’ as used herein, is meant the biological response on a composition administered to a user. As such, pharmacokinetic activity may include the absorption, distribution, metabolism, and excretion of the bioactive compound(s) within a composition following its administration. By ‘pharmacodynamic activity’ as used herein, is meant the biological response of a user to a composition administered thereto. As such, pharmacodynamic activity may include the effect of the bioactive compound(s) in relation to the concentration of said bioactive compound(s) in a body fluid. In an alternatively preferred embodiment, the composition may further include one or more pharmaceutically acceptable excipient(s) selected from diluents, lubricants, glidants, disintegrants, and binders. Examples of suitable diluents include lactose,
dextrin, glucose, sucrose, sorbitol, silicates, calcium and/or magnesium salts, sodium chloride, and potassium chloride. Examples of suitable lubricants include stearic acid and salts thereof (e.g., magnesium stearate and calcium stearate), talc, paraffin, sodium lauryl sulphate, sodium benzoate, and poly(ethylene glycol). Examples of suitable disintegrants include starch, cellulose derivatives, alginates, croscarmellose sodium and crospovidone. Examples of suitable glidants include colloidal anhydrous silicon and other silica compounds (e.g., silica gel), corn starch, and talc. Examples of suitable binders include gelatin, glucose, lactose, povidone, sodium alginate. In yet another preferred embodiment, the composition may be a solid formulation, preferably a capsule or tablet for oral administration. In an alternative preferred embodiment, the composition may be a liquid formulation, dry powder, gel, or aerosol formulation. For example, the composition may be for oral, transdermal, intranasal, sublingual, or intravenous administration. In a preferred embodiment, the psychological disorder to be treated may be selected from the group consisting of depression (e.g., major depressive disorder, treatment- resistant major depressive disorder, bipolar disorder depression), attention deficit hyperactivity disorder, neurological disorders (e.g. dementia, multiple sclerosis, motor neurone disease), chronic or persistent inflammation or pain (e.g., fibromyalgia, rheumatologic pain, and headache), eating disorders or obesity, generalised anxiety disorder, mixed anxiety, insomnia, panic disorder, post-traumatic stress disorder, obsessive-compulsive disorder, social phobia or other specific phobias (e.g., animals, heights, blood, needles, or public speaking), or substance and alcohol use disorders. In a more preferred embodiment, the psychological disorder is depression (e.g., major depressive disorder, treatment-resistant major depressive disorder, bipolar disorder depression), anxiety disorders, post-traumatic stress disorder, substance and alcohol use disorder, or eating disorders. The present invention arises, in part, from the discovery that the method by which a composition is formulated may direct the range and clinical efficacy of the composition. In particular, the range and efficacy of the composition may be directed by adjusting the relative amounts of the psychedelic substance(s) and the harmala alkaloid(s) included in the composition.
By ‘range’ as used in respect to psychological disorders, is meant the number of disorders which present with distinct symptoms. For example, chronic pain and social anxiety may be considered disorders with distinct presentations in that neither disorder may routinely be classified as a symptom of the other, as with, for example, depression and insomnia. Therefore, in a second aspect of the present invention, there is provided a method for preparing a composition to treat, ameliorate, or prevent a psychological disorder, wherein the method includes: • providing at least one psychedelic substance and/or at least one harmala alkaloid, or pharmaceutically acceptable salt(s) thereof; and • formulating into a composition for administering to a patient The step of providing the psychedelic substance and the harmala alkaloid, may include isolating the psychedelic substance and/or the harmala alkaloid from a plant source according to routine techniques known in the trade. The formulating step may include recovering isolated psychedelic substance(s) and/or the harmala alkaloid from an extraction medium by, for example, removing the extraction solvent via rotary evaporation. Preferably, the isolated psychedelic substance and the isolated harmala alkaloid are, respectively, at least approximately 50% pure, more preferably at least approximately 70% pure, more preferably at least approximately 90% pure, even more preferably at least approximately 95% pure. Alternatively, or in addition, one or more of the psychedelic substance(s) and/or the harmala alkaloid(s) may be synthesised utilising standard synthetic techniques. In some embodiments, the formulating step may comprise selecting therapeutically effective amounts of the psychedelic substance and harmala alkaloid(s), such as amounts provided in the compositions elsewhere herein. In a further preferred embodiment, the formulating step may further include selecting therapeutically effective amounts of the psychedelic substance and the harmala alkaloid/s to improve the clinical efficacy of the bioactive composition. Preferably, the psychedelic substance and the harmala alkaloid(s) may be present in a weight ratio of about 1:1 to about 1:10, more preferably about 1:2 to about 1.8, even more preferably
about 1.3 to about 1:6. For example, the psychedelic substance and the harmala alkaloid(s) may be present in a weight ratio of about 1:4. In a further preferred embodiment, the formulating step may include selecting
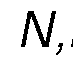
Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N- Dimethyltryptamine in the composition is about 3:1 to about 9:1. In a further preferred embodiment, the formulating step may include selecting N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 3:1 to about 9:1. In a further preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 3:1 to about 9:1. Preferably the weight ratio of harmala alkaloid(s) to N,N-Dimethyltryptamine may be about 3.25:1 to about 9.1:1, more preferably about 3.25:1 to about 6:1. Even more preferably about 3.25:1 to about 5:1. In a still further preferred embodiment the weight ratio of harmala alkaloid(s) to N,N-Dimethyltryptamine may be about 3.5 to about 9:1, more preferably more preferably about 3.7 to about 6:1. In a further preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 3:1 to about 9:1. Preferably the weight ratio of harmala alkaloid(s) to N,N-Dimethyltryptamine may be about 3.25:1 to about 9.1:1, more preferably about 3.25:1 to about 6:1. Even more preferably about 3.25:1 to about 5:1. In a still further preferred embodiment the weight ratio of harmala
alkaloid(s) to N,N-Dimethyltryptamine may be about 3.5 to about 9:1, more preferably more preferably about 3.7 to about 6:1. In a further preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmala alkaloids to N,N-Dimethyltryptamine in the composition is about 3:1 to about 9:1. Preferably the weight ratio of harmala alkaloid(s) to N,N-Dimethyltryptamine may be about 3.25:1 to about 9.1:1, more preferably about 3.25:1 to about 6:1. Even more preferably about 3.25:1 to about 5:1. In a still further preferred embodiment the weight ratio of harmala alkaloid(s) to N,N-Dimethyltryptamine may be about 3.5 to about 9:1, more preferably more preferably about 3.7 to about 6:1. In a further preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 3.25:1 to about 7.5:1. Preferably the weight ratio of N,N-Dimethyltryptamine to harmaline may be about 4:1 to about 7:1, more preferably, about 5:1 to about 7:1, even more preferably about 6:1 to about 7:1. In a further preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.5:1 to about 4:1. In a further preferred embodiment, the formulating step may include selecting N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.5:1 to about 3:1. Preferably the weight ratio of harmine to N,N-Dimethyltryptamine may be
about 1.6:1 to about 2:1. More preferably, about 1.75:1 to about 1.9:1, even more preferably about 1.75:1 to about 1.88:1. In a further preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N- Dimethyltryptamine in the composition is about 1.5:1 to about 4:1. In a further preferred embodiment, the formulating step may include selecting N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and harmaline or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.5:1 to about 3:1. Preferably the weight ratio of harmine to N,N- Dimethyltryptamine may be about 1.6:1 to about 2:1. More preferably, about 1.75:1 to about 1.9:1, even more preferably about 1.75:1 to about 1.88:1. In a further preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.5:1 to about 3:1. Preferably the weight ratio of harmine to N,N-Dimethyltryptamine may be about 1.6:1 to about 2:1. More preferably, about 1.75:1 to about 1.9:1, even more preferably about 1.75:1 to about 1.88:1. In a further preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise tetrahydroharmine or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1:1 to about 7:1. In a further preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmine and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine
to N,N-Dimethyltryptamine in the composition is about 1:4 to about 3:1. Preferably the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine is about 1.5:1 to about 2.8:1, more preferably about 1.6:1 to about 2.6:1, even more preferably about 1.7:1 to about 2.2:1. In a further preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof, wherein the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1:4 to about 3:1. Preferably the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine is about 1.5:1 to about 2.8:1, more preferably about 1.6:1 to about 2.6:1, even more preferably about 1.7:1 to about 2.2:1. In a preferred embodiment, the formulating step may include selecting approximately 10% w/w to approximately 30% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof and approximately 25% w/w to approximately 45% w/w harmine or a pharmaceutically acceptable salt thereof. In a preferred embodiment, the formulating step may include selecting approximately 10% w/w to approximately 30% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof and approximately 30% w/w to approximately 50% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof. In a preferred embodiment, the formulating step may include selecting approximately 10% w/w to approximately 30% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, approximately 30% w/w to approximately 50% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof and approximately 25% w/w to approximately 45% w/w harmine or a pharmaceutically acceptable salt thereof. In a preferred embodiment, the formulating step may include selecting approximately 10% w/w to approximately 30% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, approximately 30% w/w to approximately 50% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof, approximately 25% w/w to approximately 45% w/w harmine or a pharmaceutically acceptable salt thereof
and approximately 0.5% w/w to approximately 15% w/w harmaline or a pharmaceutically acceptable salt thereof. In a preferred embodiment, the formulating step may include selecting approximately 18% to approximately 22% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof and approximately 35% w/w to approximately 38% w/w harmine or a pharmaceutically acceptable salt thereof. In a preferred embodiment, the formulating step may include selecting approximately 18% to approximately 22% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof and approximately 40% w/w to approximately 44% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof. In a preferred embodiment, the formulating step may include selecting approximately 18% to approximately 22% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, approximately 40% w/w to approximately 44% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof and approximately 35% w/w to approximately 38% w/w harmine or a pharmaceutically acceptable salt thereof. In a preferred embodiment, the formulating step may include selecting approximately 18% to approximately 22% w/w N,N-Dimethyltryptamine or a pharmaceutically acceptable salt thereof, approximately 38% w/w to approximately 42% w/w tetrahydroharmine or a pharmaceutically acceptable salt thereof, approximately 35% w/w to approximately 38% w/w harmine or a pharmaceutically acceptable salt thereof and approximately 2% w/w to approximately 4% w/w harmaline or a pharmaceutically acceptable salt thereof. In a particularly preferred embodiment, the formulating step may include selecting N,N- Dimethyltryptamine or a pharmaceutically acceptable salt thereof, and harmala alkaloids, wherein the harmala alkaloids comprise harmaline, harmine, and tetrahydroharmine, or pharmaceutically acceptable salts thereof: wherein the weight ratio of N,N-Dimethyltryptamine to harmaline in the composition is about 3.25:1 to about 7.5:1; the weight ratio of harmine to N,N-Dimethyltryptamine in the composition is about 1.5:1 to about 3:1.; and the weight ratio of tetrahydroharmine to N,N-Dimethyltryptamine in the composition is about 1:4 to about 3:1.
The present invention also arises from the discovery that the composition as described herein may be formulated such that the ability of the composition to treat, ameliorate, or prevent a range of psychological disorders may be improved through adjunct psychedelic-assisted therapy (PAP). In particular, the present invention arises from the surprising discovery that PAP may directly improve the clinical efficacy of a composition formulated according to the methods described herein. By ‘psychedelic-assisted therapy’ as used herein, is meant one or more sessions with a mental health professional in a controlled setting following the ingestion of a psychedelic substance. In this context, ‘controlled setting’ means an environment structured to optimise patient comfort by, for example, removing or otherwise disguising medical equipment when sessions are completed in, e.g., a hospital room. By ‘mental health professional’ is meant an individual, such as a psychiatrist, psychologist, mental health nurse, or social worker, who has been formally trained to diagnose and/or treat psychological disorders. In a further aspect, the present invention provides a method to treat, ameliorate, or prevent a psychological disorder, wherein the method includes conducting one or more preparatory therapy sessions with a patient; administering a composition as hereinbefore described to the patient; and conducting one or more integration sessions with the patient. In a preferred embodiment, the composition includes at least one psychedelic substance and at least one harmala alkaloid. In yet another aspect, the present invention provides the use of a composition as hereinbefore described for the manufacture of a medicament for the treatment, amelioration, or prevention of a psychological disorder. Preferably, the medicament includes at least one psychedelic substance and at least one harmala alkaloid. In this specification, the term ‘comprises’ and its variants are not intended to exclude the presence of other integers, components, or steps. In the present disclosure, wherever aspects are described herein with the language ‘comprising’ otherwise analogous aspects described in terms of ‘consisting of’ and/or ‘consisting essentially of’ are also provided. All definitions herein described whether
specifically mentioned or not, should be construed to refer to definitions as used throughout the specification and attached claims. In this specification, reference to any prior art in the specification is not and should not be taken as an acknowledgement or any form of suggestion that this prior art forms part of the common general knowledge in Australia or any other jurisdiction or that this prior art could reasonably expected to be combined by a person skilled in the art. Numeric ranges are inclusive of the numbers defining the range. The term ‘about’ or ‘approximately’ as used herein generally mean plus or minus ten percent (10%) of a value, inclusive of the value, unless otherwise indicated by the context of the usage. For example, ‘about 100’ or ‘approximately 100’ refers to any number from 90 to 110. Whenever a term such as ‘at least,’ ‘greater than,’ or ‘greater than or equal to’ precedes the first numerical value in a series of two or more numerical values, the term ‘at least,’ ‘greater than’ or ‘greater than or equal to’ applies to each of the numerical values in that series of numerical values. For example, greater than or equal to 1, 2, or 3 is equivalent to greater than or equal to 1, greater than or equal to 2, or greater than or equal to 3. Whenever a term such as ‘no more than,’ ‘no less than,’ ‘less than or equal to,’ or ‘at most’ precedes the first numerical value in a series of two or more numerical values, the term ‘no more than,’ ‘no less than,’ ‘less than or equal to,’ or ‘at most’ applies to each of the numerical values in that series of numerical values. For example, less than or equal to 3, 2, or 1 is equivalent to less than or equal to 3, less than or equal to 2, or less than or equal to 1. As used herein, the term ‘or’ is used to refer to a nonexclusive or, such as ‘A or B’ includes ‘A but not B,’ ‘B but not A,’ and ‘A and B,’ unless otherwise indicated. As used herein, ‘a’, ‘an’, and ‘the’ can include plural referents unless otherwise limited expressly or by context. The present invention will now be more fully described with reference to the accompanying Examples and Figures. It should be understood, however, that the description following is illustrative only and should not be taken in any way as a restriction on the generality of the invention described above.
Brief Description of the Drawings/Figures In the Figures: Figure 1 shows a UHPLC trace of purified N,N-dimethyltryptamine extracted from Acacia courtii leaves. Figure 2 shows the N,N-dimethyltryptamine extraction process using Acacia acuminata leaves, where (a) the leaves are washed with acetic acid, and (b) the aqueous extracts are filtered, (c) extracted into methyl tert-butyl ether (MTBE), and (d) the MTBE fractions containing N,N-dimethyltryptamine are combined. Figure 3 shows a UHPLC trace of N,N-dimethyltryptamine extracted from Acacia acuminata leaves. Figure 4 shows a UHPLC trace of N,N-dimethyltryptamine extracted from Acacia acuminata leaves and purified by acetylation. Figure 5 shows an
1H NMR (500 MHz, CDCl
3) spectrum of N,N-dimethyltryptamine extracted from Acacia acuminata leaves and purified by acetylation. Figure 6 shows a schematic of the extraction and purification process for recovering N,N-dimethyltryptamine from Acacia acuminata leaves. Figure 7 shows a UHPLC trace of the N,N-dimethyltryptamine hemifumarate salt obtained from purified Acacia acuminata extract. Figure 8 shows an
1H NMR (500 MHz, d
6-DMSO) spectrum of N,N-dimethyltryptamine hemifumarate salt from Acacia acuminata extract. Figure 9 shows a UHPLC trace of triturated Peganum harmala extract. Figure 10 shows a schematic of the pH-based harmala alkaloid separation procedure. Figure 11 shows a GC-MS analysis of a recrystallised tetrahydroharmine free bases. Figure 12 shows a UHPLC trace of a mixture of harmaline and harmine hydrochloride salts.
Figure 13 shows an
1H NMR (400 MHz, d6-DMSO) spectrum of ~1:1 mixture of harmaline hydrochloride and harmine hydrochloride. Figure 14 shows a UHPLC trace of a harmine-enriched fraction extracted from Peganum harmala seeds. Figure 15 shows a UHPLC trace of a harmaline-enriched fraction extracted from Peganum harmala seeds. Figure 16 shows a UHPLC trace of a tetrahydroharmine-rich, borohydride-reduced harmaline-enriched fraction extracted from Peganum harmala seeds. Figure 17 shows GC MS analysis of recrystallised tetrahydroharmine free base. Figure 18 shows
1H NMR (400 MHz, d
6-DMSO) spectrum of tetrahydroharmine hydrochloride. Figure 19 shows a UHPLC trace of purified tetrahydroharmine hydrochloride. Figure 20 shows a UHPLC trace of a harmine-rich, borohydride-reduced harmine- enriched fraction extracted from Peganum harmala seeds. Figure 21 shows GC-MS analysis of recrystallised harmine free base. Figure 22 shows an
1H NMR (400 MHz, d6-DMSO) spectrum of purified harmine hydrocholoride. Figure 23 shows a UHPLC trace of a harmine hydrochloride salt. Figure 24 shows strength of the psychedelic experience by formulation Figure 25 shows quality of the psychedelic experience compared to ayahuasca by formulation Figure 26 shows perceived therapeutic effect of the psychedelic experience, relative to ayahuasca Figure 27 shows average scores for MEQ subscales and MEQ total score for different investigational formulas (A, B, C (low), C (high)) and a comparison with a naturalistic ayahuasca use
Figure 28 shows short Index of Mystical Orientation (SIMO) average scores Figure 29 shows Personal Insights Questionnaire (PIQ) average insights by formulation Detailed Description of the Embodiments Example 1 – Evaluation of techniques for extracting N,N-dimethyltryptamine (DMT) from Acacia spp. Plants Plant material processing Plant material was harvested in Australia during the afternoon in spring. The plant material harvested from mature Acacia spp. Plants (i.e., Acacia courtii and Acacia acuminata) were dried in a drying oven (40 °C , 24 h), freeze dried (-55 to -60 °C , 0.5 to 0.1 bar for 24 h), and stored at -20 °C for further use. The dried leaves were then ground to a fine consistent powder in the absence of heat (900W NutriBullet blender). This raw plant extract was stored with extreme care. The weight fraction of DMT in the raw plant extract was evaluated with respect to the harvesting site (see Table 1) and with respect to the type of plant material. In general, a higher concentration of DMT was observed in extracts prepared from the leaf of a plant than from the stem of said plant (see Table 2). Table 1: Concentration of DMT within harvested Acacia spp. Plants
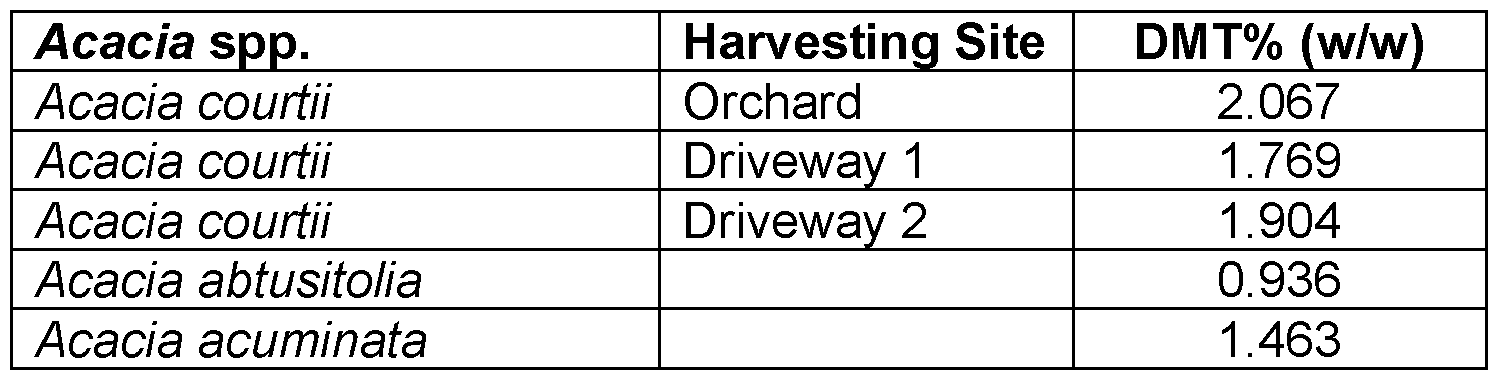
Table 2: Concentration of DMT within the leaves and stem harvested from Acacia courtii plant species.
Lipids, oily substances, and steroidal compounds were selectively removed from the plant material via solid-liquid extraction by subjecting processed plant material to 30 min of ultrasonic agitation in the presence of a nonpolar solvent (e.g., heptane, hexane, benzene, toluene, etc.) used at a ratio of 1 g plant material to 5 mL solvent. The Acacia residue was then filtered (11 μm pore size), and the defatting protocol was repeated. The defatted Acacia residue was then dried to remove any trace of nonpolar solvent. In this manner, potentially toxic hydrophobic compounds comprising about 1% (w/w) to 2% (w/w) of the total plant mass were selectively removed from the plant material. As shown in Table 3, the defatting step did not significantly reduce the weight fraction of DMT relative to that of the raw plant extract. Table 3. DMT loss resulting from the defatting step.
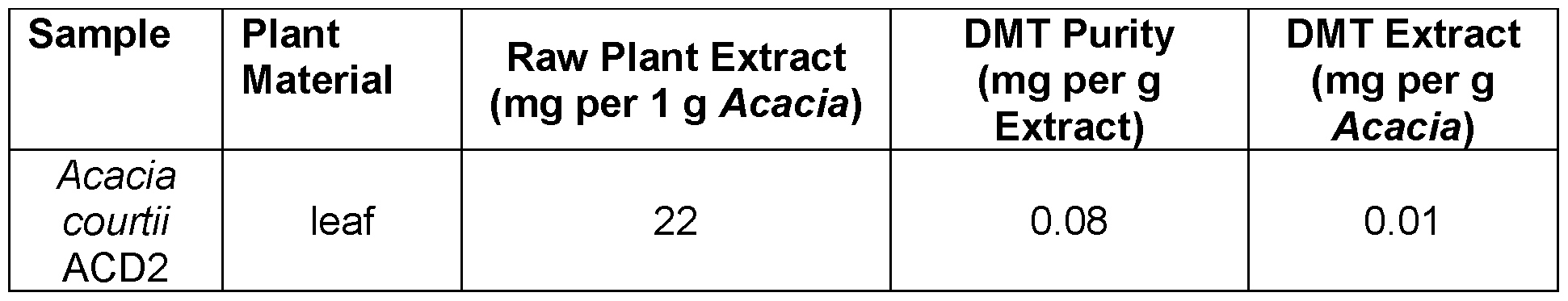
Hydrocarbon extraction techniques A. Sonication-assisted extraction A solid-liquid extraction was employed to isolate DMT from the defatted Acacia residue. Specifically, the Acacia residue was submerged in a solvent (e.g., ethanol, methanol, isopropanol, dimethylformamide, acetonitrile, acetone, or ethyl acetate), sonicated (400 W Thermoline UB-410, maximum intensity), and then filtered (11 μm pore size). The resulting crude ethanoic filtrates were subjected to rotary evaporation and dried to completeness with a stream of nitrogen to provide an Acacia crude ethanol extract.
Experimental factors including the solvent, solution pH, and sonication conditions (e.g., temperature and duration) were varied, and the impact of such factors on overall yield and DMT concentration therein were assessed. For example, solid-liquid extraction of DMT from Acacia residue harvested from plant leaves was performed using an excess of one of a selection of solvents. As shown in Table 4, the highest extract yields and DMT concentrations were observed for extractions performed with methanol, ethanol, and dimethylformamide (DMF). Table 4. Influence of Solvent on Extract Yield and DMT Concentration.
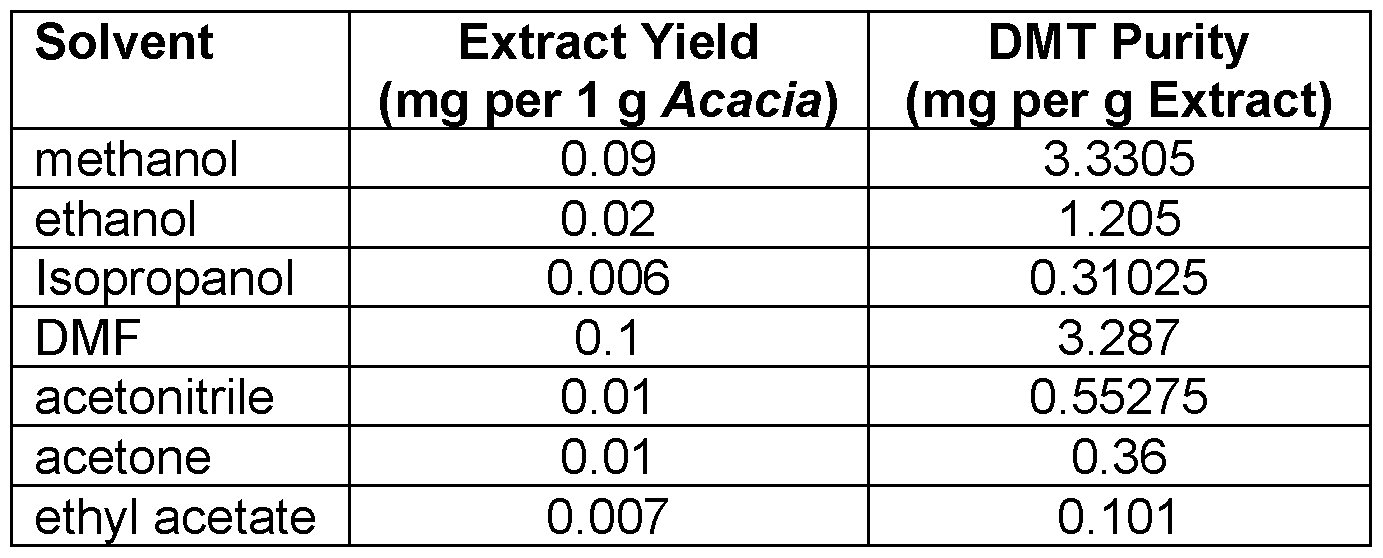
Likewise, the effect of solution pH, sonication temperature, and sonication duration (see Table 7) on experimental outcomes were evaluated Acacia acuminata residue in ethanol. DMT concentration was found to be highest when the solid-liquid extraction is performed at a neutral pH (i.e., without the addition of an acid or base; see Table 5) and at a temperature of 40 °C or less (see Table 6). Additionally, an extended sonication time did not provide a significantly different DMT concentration (see Table 7). Table 5. pH Dependence of Solid-Liquid Extraction of DMT from Acacia acuminata
Table 6. Temperature Dependence of Solid-Liquid Extraction of DMT from Acacia acuminata
Table 7. Time Dependence of Solid-Liquid Extraction of DMT from Acacia acuminata
The relationship between the number solid-liquid extraction cycles and the concentration of DMT in the final extract was also evaluated. For each cycle, Acacia courtii residue (ACD2 leaf) was submerged in 10 mL ethanol per 1 g Acacia and sonicated (400 W Thermoline UB-410, maximum intensity,) for a 30 min. As shown in Table 8, repeating the solid-liquid extraction beyond 2 cycles had diminishing rates of return. Table 8. Total DMT isolated from defatted Acacia residue following repeated rounds of ethanol extraction
When 2 ethanol-based extraction cycles were completed using 30 min of sonication (400W Thermoline UB-410, maximum intensity) in the absence of heat a final extract yield of around 10% (~6% DMT) was obtained.
B. Reflux extraction Table 9. Comparative Yield and DMT Concentration Obtained via Sonication and Reflux Extraction Techniques
Selective aqueous purification The ethanol extract was then dissolved in water at a ratio of 1 g per 200 mL. This suspension was subjected to ice cold sonication (400W Thermoline UB-410, 0
°C, 30 min). This step solubilises the DMT which is then collected by filtering (11 μm pore size) off the less polar compounds leaving the DMT in solution. The solution was then reduced to ~10% volume using rotary evaporation. Stabilising final formulation The resulting aqueous extract was stabilised using a combination of microcrystalline cellulose, mannitol, silica gel, and magnesium stearate. The aqueous extract is combined with the stabilising agent at a 1:1 ratio. The ratio is estimated from the yield of aqueous extract obtained from the starting plant material. Specifically, for every 100 g (dry) of starting material, 3 g of aqueous extract was recovered and dried by rotary evaporation (40
°C) to yield a dry extract powder (overall yield ~6% of dried material, purity ~5% DMT).
Example 2 – Extraction of N,N-dimethyltryptamine (DMT) from Acacia spp. Plants Acacia courtii leaves Dried Acacia courtii leaves (30.26 g) were milled in a 900W NutriBullet to produce a fine green powder. The resultant green powder was transferred to a conical flask with a magnetic stir bar. N-heptane was (150 mL) was added, and the suspension was stirred (250 rpm) for 40 minutes at room temperature then filtered. The solid was then returned to a conical flask and stirred with a second volume of n-heptane (150 mL) for 40 min before filtering again. The filtrates were discarded, and the leaf material was extracted with 0.1 M aq. Acetic acid (AcOH, 150 mL) stirring at room temperature for 90 min. The suspension was filtered, giving a clear, bright orange solution. The leaf material was extracted a second time with 0.1 M aq. AcOH (150 mL) at room temperature for 70 min, then filtered. The spent plant material was discarded, and the combined filtrates (pH~3- 4) were washed with ethyl acetate (EtOAc, 2 x 300 mL). The pale yellow ethyl acetate phase was discarded. The separated, clear, bright orange aqueous solution was cooled in an ice-water bath and basified by adding 10 M aq. Sodium hydroxide (NaOH) dropwise (~170 drops) until the pH of the solution was ~11. The resulting cloudy, brown mixture was transferred to a separating funnel and extracted with EtOAc (3 x 300 mL). The aqueous solution (pH~10, after extraction) was discarded. The EtOAc combined extracts (clear, bright yellow) were dried over anhydrous MgSO
4, then the solvent was evaporated via rotary evaporation (40°C bath). The residue was freeze-dried to give a pale greenish-yellow solid (474.7 mg). UHPLC analysis showed 67.4% DMT (see Figure 1). Acacia acuminata leaves A.acuminata (1 kg phyllodes and twigs and 2 small bags desiccant) were harvested 13 April 2023 near Coolgardie, Western Australia on a sunny day (30°C, sunny, 1PM). The leaves were immediately dried in the oven at 40°C for 24 h, double bagged in plastic bags with paper towels, and stored in the freezer (-20°C) until use. The dried A. Acuminata leaf material (127.06 g, AAC01) was milled in a 900W Nutribullet blender, and the resultant shredded green leaves was transferred to a 2 L conical flask fitted with a magnetic stirrer and n-heptane (750 mL) was added. The suspension was
stirred for 20 minutes at room temperature then filtered. The solid was returned to the conical flask and stirred with fresh n-heptane (750 mL) a second time at room temperature for 20 minutes, and then filtered. The n-heptane washings were discarded. The washed leaf material (see Figure 2a) was transferred back to the conical flask and extracted with 5% (v/v) aq. AcOH (700 mL) by stirring vigorously at room temperature for 20 minutes before filtering. The filtrate was reserved. The solid was transferred back to the conical flask and stirred vigorously with a second portion of 5% aq. AcOH (700 mL) at room temperature for 30 minutes, then filtered. The spent leaf material was discarded. The clear, bright orange aqueous extracts (see Figure 2b) were combined in a 5 L separating funnel (pH 3) and washed with EtOAc (2 x 140 mL). The EtOAc washes were discarded, and the separated aqueous solution was cooled in an ice-water bath with stirring while adding 10M aqueous NaOH slowly over 20 min until the mixture had a pH of 12 (60 mL). The resultant murky, light brown mixture (see Figure 2c) was transferred to a 5 L separating funnel and extracted with methyl tert butyl ether (MTBE, 2 x 1.5 L, plus 1 x 1L). The MTBE extracts (see Figure 2d) were combined, washed with 2M NaOH (3 x 1L) followed by brine (1L), and then dried over anhydrous sodium sulfate (Na2SO4) and filtered. The solvent was evaporated under reduced pressure to yield 3.37 g of crude extract (26.5 mg of crude extract per gram of leaves). The crude extract was analysed by UHPLC which showed 43.8% DMT in the extract (see Figure 3). Purification of crude extracts. The crude A. acuminata extract (3.34 g) was dissolved in 2-propanol (25 mL) and transferred to a 250 mL round-bottom flask fitted with a magnetic stirrer. The flask was placed in an ice bath, and 3.3 M aqueous sodium acetate solution (40 mL) was added. The mixture was stirred vigorously while acetic anhydride (19 mL, 20.5 g, 0.20 mol) was added dropwise over 20 minutes. The reaction mixture was stirred in the ice bath for 30 minutes. The bath was then removed, and the mixture was stirred at room temperature for a further 30 minutes. The mixture was acidified to pH 1 by addition of 2 M aqueous hydrochloric acid (90 mL). The mixture was washed three times with EtOAc (180 mL, 120 mL, 105 mL), and the EtOAc washings were discarded. The separated aqueous solution was cooled in an ice bath, and 10 M aqueous NaOH was added slowly until the mixture had a pH of 13 (~25 mL). The basified mixture was transferred to a 250 mL separating funnel and extracted with MTBE (3 x 110 mL). The MTBE layers were
combined, dried over anhydrous Na2SO4, filtered, and the solvent was evaporated under reduced pressure to yield 0.616 g of a light orange solid. The product was analysed by UHPLC (see Figure 4), which showed a purity of N,N-dimethyltryptamine of 87.7% (0.53 g of DMT from 127.1 g of leaves). In order to remove residual hordenine, this material was dissolved in MTBE (30 mL) and washed with 2M NaOH (3 x 30 mL). The aqueous washings were discarded and the organic phase dried (Na2SO4) and evaporated to give N,N-dimethyltryptamine as a pale yellow solid. The purity of N,N-dimethyltryptamine was further confirmed by proton NMR (see Figure 5). A schematic of the N,N-dimethyltryptamine extraction and purification process using A. acuminata leaves is provided in Figure 6. Alternatively, the step of removing hordenine could be completed before the final purification step. Optimisation of acetylation yields After the residual acetic anhydride was quenched, the acidified mixture was rotary evaporated to remove 2-propanol whilst retaining aqueous solvent. As the amount of 2- propanol decreased, many of the acetylated impurities fell out of solution. Acetylated impurities may be removed by filtration and then washing the filtrate with ethyl acetate. Then, the aqueous solution was basified and DMT extracted into MTBE. Alternatively, the yield may also be increased using the following steps: After the residual acetic anhydride was quenched, the acidified mixture was extracted with ethyl acetate to remove the acetylated impurities and (depending upon the quantity of 2-propanol used) some of the DMT. Any remaining DMT in the aqueous solution was recovered by basifying the solution and then extraction with MTBE. Ethyl acetate and MTBE solutions were rotary evaporated and the products pooled. The dried product was extracted with 0.1 M HCl, which dissolved the DMT but left most of the acetylated impurities. Any sparingly acid soluble impurities were removed by washing with ethyl acetate. Ultimately, the aqueous solution was basified and DMT was extracted into MTBE.
Formation of fumarate salts A sample of purified Acacia acuminata leaf extract (255.4 mg, 93.03 wt.% DMT, i.e., 237.6 mg DMT / 1.26 mmol DMT) was dissolved in acetone (3 mL). This solution was mixed with a solution of fumaric acid (73.7 mg, 0.635 mmol, 0.50 equivalents) in acetone (20 mL) at room temperature with stirring. After 5 minutes a solid precipitated. The solid was isolated by filtration, washed with acetone, and dried under reduced pressure at room temperature to yield 233.1 mg of a pale-yellow solid. UHPLC analysis (Figure 7) showed a purity of 70.37% DMT (w/w) in the product which corresponds to a 92.06% (w/w) purity of the DMT-hemifumarate salt. The
1H NMR spectrum (Figure 8) in deuterated DMSO was consistent with >90% purity. Example 3 – DMT manufacturing process The N,N,dimethyltryptamine (DMT) is extracted from the leaves of Acacia acuminata and the extract is concentrated and further purified by chemical purification to produce DMT with NLT 90% assay. The DMT manufacturing process includes the following steps. 1. Drying and milling of leaves 2. Extraction of DMT 3. Isolation and purification of DMT by acetylation and removal of other alkaloids 4. Conversion of DMT freebase to the hemifumarate salt form Drying and milling Harvested Acacia acuminata leaves (phyllodes) are dried and ground using a common grinder. Dried A. acuminata plant material (265.5 g) was milled to produce 263.2 g of milled material. The milled Acacia acuminata leaves were then stirred with heptane. The heptane wash was performed by stirring the plant material in heptane at room temperature mins before removing the solids by vacuum filtration.. The plant material was left to dry on the filter, under vacuum in the presence of air until no heptane could be seen leaving the funnel. Extraction of DMT
The heptane washed plant material was then extracted with 10% aqueous acetic acid . The acetic extraction was performed by stirring the plant material in 10% Acetic acid for NLT 1 hour at room temperature, before removing the solids by vacuum filtration.. The filtration was complete when no liquid could be seen leaving the funnel. Both acetic acid extracts where then combined and extracted with Ethyl acetate. The ethyl acetate extract is discarded and the aqueous phase is collected. The pH of the aqueous phase is adjusted to pH 12.. A dark brown solid precipitates out of solution at pH 12 which is removed by filtration before proceeding with the next extraction. The filtered, alkaline solution is then extracted with tert-Butyl Methyl Ether. The pH of the aqueous phase must be maintained at 12 during the extraction. The tert-Butyl Methyl Ether extracts are combined, washed with 2M Sodium Hydroxide ,dried over Sodium Sulfate, filtered and evaporated to dryness to obtain the crude N,N-DMT extract (4.22 – 5.12 g) as a yellow-orange viscous liquid. Alternatively, after the sodium hydroxide wash, the combined tert-Butyl Methyl Ether extract can be solvent swapped with Isopropanol while removing the residual water by azeotropic distillation. The extract would then remain in isopropanol for the next step. Isolation and purification of DMT by acetylation and removal of other alkaloids The crude A. Acuminata extract (4.22 – 5.12 g) is dissolved in isopropanol. The isopropanol solution is then cooled below 10 °C. Aqueous sodium acetate solution is then added in one portion followed by a dropwise addition of acetic anhydride. The resulting reaction mixture is then stirred at temperatures below 10 °C (internal)... The internal temperature is then increased to within 20 – 25 °C and the reaction mixture was allowed to stir at this temperature for NLT 10 mins. The reaction is then quenched by the addition of 2 M hydrochloric acid in one portion. The isopropanol is then removed by vacuum distillation. The aqueous suspension (pH 1) is then extracted with ethyl acetate. The ethyl acetate extract is discarded. The pH of the aqueous phase is then adjusted to pH 13 by the addition of aqueous NaOH solution.. The alkaline aqueous phase is then extracted with tert-butyl methyl ether.. The tert-butyl methyl ether extract is the dried over sodium sulfate, filtered and evaporated to dryness to afford N,N-DMT as a viscous yellow oil (0.4188 – 1.09 g, 10 – 22%). The yellow oil can solidify upon standing at room temperature.
Conversion of DMT freebase to the hemifumarate salt form The enriched N,N-DMT extract is dissolved in Isopropanol at ambient temperature to form a yellow solution. Fumaric acid is dissolved in isopropanol to form a colourless solution. While stirring the DMT/isopropanol solution, the fumaric acid/isopropanol solution is added in one portion. The fumaric acid/isopropanol vessel is then rinsed with additional isopropanol, the rinse is then added to the mixture in one portion. The reaction mixture is then stirred for NLT 12 hours at ambient temperature. The DMT- Hemifumarate salt precipitates out of solution. The suspension is filtered and the isolated salt is washed with cold isopropanol.. The isolated salt in then dried in a vacuum oven set to 20 – 25 °C for NLT 12 hours. The final product is isolated as a faint yellow to orange solid (0.548 – 0.811, 61 – 68%). Example 4 – Isolation of Harmala alkaloids from Peganum Harmala Milling and extraction of P. Harmala seeds (1) P. Harmala seeds (100.60 g) are milled to produce 97.42 g of milled material. The milled material is stirred with Ethanol containing 1% Acetic acid at room temperature for 1 hour. The solid are then removed by vacuum filtration. The solids are recovered from the funnel and the ethanol/acetic acid extraction is repeated. =. The extraction vessel is then rinsed with Ethanol containing 1% Acetic acid, passed through the same filter and combined with extraction volume. After the rinse the exhausted plant material is discarded. The combined ethanol/acetic acid extracts are then distilled under vacuum. Upon reaching a concentrated extract , Toluene (0.20 vols) is added. Vacuum distillation is continued until a concentrated residue is obtained. The residue is then dissolved with agitation in aqueous 5% Acetic acid and extracted with Ethyl Acetate. The ethyl acetate extracts are combined and back extracted with aqueous 5% Acetic acid. The ethyl acetate extracts are then discarded. The combined aqueous phase is then extracted with Heptane (. The heptane extract is discarded. The pH of the aqueous phase is then adjusted to pH 10 by the addition of aqueous Ammonia. A brown solid starts to precipitate out of solution. The formed suspension is then cooled below 10 °C and stirred at 5 – 10 °C for NMT 1 hour. The solid is then isolated by vacuum filtration on a glass frit, washed with Water and dried. The solid is then collected and
stirred with 1:1 Ethyl acetate/Acetone for NLT 10 mins. The solid is then isolated by vacuum filtration and dried.. The crude extract is isolated as a tan coloured solid (1.16 g). Milling and extraction of P. Harmala seeds (2) P. Harmala seeds (611.60 g) are milled to produce 589.91 g of milled material. The milled material is stirred with Ethanol containing 1% (v/v) Acetic acid at room temperature for 1 hour. The solid are then removed by vacuum filtration.. The solids are recovered from the funnel and the ethanol/acetic acid extraction is repeated. The extraction vessel is then rinsed with Ethanol containing 1% (v/v) Acetic acid. After the rinse the exhausted plant material is discarded. The combined ethanol/acetic acid extracts are then distilled under vacuum to obtain a dark red oil (71.32 g) The crude red oil is then dissolved with agitation in aqueous 5% Acetic acid (v/v) and extracted with Ethyl Acetate .The ethyl acetate extracts are combined and back extracted with aqueous 5% Acetic acid (v/v). The ethyl acetate extracts are then discarded. The combined aqueous phase is then extracted with Heptane. The heptane extract is discarded. The pH of the aqueous phase is then adjusted to pH 10 by the addition of 25% aqueous Ammonia The formed suspension is then stirred at room temperature for NMT 1 hour. The solid is then isolated by vacuum filtration,, washed with Water, and dried. . The solid is then collected and stirred with 1:1 Ethyl acetate/Acetone (1 vol) for NLT 10 mins. The solid is then isolated by vacuum filtration and dried under vacuum (in the presence of air) for NLT 2 hours. The crude extract is isolated as a tan coloured solid (8.5 g) Separation of Harmala alkaloids (1) The crude extract (1.30 g) is dissolved in aqueous 5% acetic acid. A calibrated pH probe was immersed into the solution and the starting pH was determined to be 4.45. The pH of the aqueous solution was adjusted to around 7via dropwise addition of aqueous 28 – 30% ammonia solution. A brown solid precipitated out of solution. The solid was isolated by vacuum filtration,, washed with water and dried under vacuum in the presence of air. A total of 0.483 g of solid is isolated.
The aqueous solution is transferred to an appropriate vessel and the pH is adjusted to around 9via the dropwise addition of aqueous 28-30% ammonia solution. A brown solid precipitated out of solution. The solid was isolated by vacuum filtration, washed with water and dried under vacuum in the presence of air. A total of 0.639 g of a pale brown/pink coloured solid is isolated. Separation of Harmala alkaloids (2) The crude solid (8.5 g) is dissolved in 1:1 Toluene/Methanol and loaded onto the column as a solution. The target compounds (Harmine and Harmaline) are eluted with 1:1 Toluene/Methanol containing 0.1% aqueous Ammonia. Column fractions are collected and the progress of the purification is monitored by TLC.. Once the Harmine is confirmed to have eluted from the column, column fractions are collected until completion. All column fractions are then analysed by TLC.. Fractions containing a single constituent are combined and evaporated to yield each component. Harmine and Harmaline are isolated as brown solids (Harmine 3.08 g, Harmaline 3.08 g). Fractions containing a mixture of both components are combined separately (Mix fraction 2.53 g). The mixture can either be re-chromatographed or recrystallized from Ethanol to recover additional Harmaline. Harmine (3.08 g) is recrystallised from Ethanol to obtain a light brown solid (1.56 g). Harmaline (3.08 g) is recrystallised from Ethanol to obtain a light brown solid (2.11 g). Synthesis of Tetrahydroharmine from Harmaline HCl Harmaline HCl (2 g) is dissolved in Ethanol and Water. The formed solution is cooled to less than 10 °C. Sodium Borohydride (0.2 vols) is then added in portions, as a solid. The reaction mixture is then stirred at 5 – 10 °C for NMT 10 minutes, before being stirred at room temperature overnight. The reaction mixture is then quenched by the addition of 2M Hydrochloric acid. The solution is then stirred at room temperature for NMT 10 minutes. The pH of the reaction mixture is then adjusted to around 10 by the addition of 25% aqueous Ammonia. A white precipitate forms and the resulting suspension is cooled.. The precipitate is then isolated by vacuum filtration, washed with Water and dried under vacuum for NLT 1 hour. Tetrahydroharmine is isolated as a white solid (1.52 g, 87%).
Example 5 – Extraction of harmala alkaloids from Banisteriopsis spp. Plants Plant material processing Branches harvested from mature Banisteriopsis caapi plants were dried in a drying oven (40
°C, 24 h). The semi dried branchers were then freeze dried (-55 to -60
°C, 0.5 to 0.1 bar for 24 h), and stored at -20
°C for further use. The dried branches were ground to a fine consistent powder without heat. The raw material is approximately 1% (w/w) THH, 0.1% (w/w) HL, 0.5% (w/w) H. Defatting The lipids, oily substances and steroidal compounds were selectively extracted from the plant material at a ratio of 1 g plant material to 5 mL heptane, using ultrasonic agitation in the absence of heat (400 W Thermoline UB-410, maximum intensity, 30 min). The heptane was filtered from the Banisteriopsis residue (11 μm pore size), and the defatting protocol was repeated, thereby removing water and insoluble and potentially toxic compounds comprising about 0.1% (w/w) of the total plant mass. The defatted Banisteriopsis residue is dried to remove any trace of heptane. Reflux and acid assisted hydrocarbon extraction The target compounds (i.e., THH, HL, and H) were then extracted from the defatted Banisteriopsis residue via two rounds of acidic ethanol reflux extraction. For each extraction round, the Banisteriopsis residue was refluxed (30 min) in ethanol using a ratio of 1 g Banisteriopsis to 10 mL ethanol with 0.1% formic acid and filtered (11 μm pore size). The extraction process had a final yield of approximately 6% THH, approximately 0.1% HL, and approximately 8% H. Crude ethanoic filtrates were evaporated using rotary evaporation and finished to dryness using a stream of nitrogen to provide a Banisteriopsis crude ethanol extract. The drying is conducted to completion to remove traces of ethanol that would otherwise influence the effectiveness of aqueous purification. Selective aqueous purification The ethanol extract was then dissolved in water at a ratio of 1 g per 200 mL. This suspension was subjected to ice cold sonication (400W Thermoline UB-410, 0
°C, 30
min). The harmala alkaloids are soluble in water so the non-soluble components are filtered out (11 μm pore size). This leaves the harmala alkaloids solution. The solution is then reduced to approximately 10% volume using rotary evaporation. Stabilising final formulation The resulting aqueous extract was stabilised using a combination of microcrystalline cellulose, mannitol, silica gel, and magnesium stearate. The aqueous extract is combined with the stabilising agent at a 1:1 ratio. The ratio is estimated based on a yield of 3% achieved from the starting material. For every (Dry) 100 g of starting material 3g of and dried by rotary evaporation (40 °C) to yield the dry extract powder (overall yield ~4% to 8% of dried material, purity ~3.5% THH, 0.5% HL, and 3.5% H). Example 6 – Ethanol Extraction of harmala alkaloids from Peganum spp. Plants Plant material processing Peganum harmala seeds sourced from the Middle-Eastern region were ground to a fine consistent powder with no heat. The raw material was approximately 0% (w/w) THH, 0.4% (w/w) HL, and approximately 0.4% (w/w) H. Defatting The lipids, oily substances and steroidal compounds were selectively extracted from the plant material using a ratio of 1g plant material to 5 ml heptane, using ultrasonic agitation (400 W Thermoline UB-410, maximum intensity with no heat) for 30minutes. The heptane was filtered from the Peganum residue (11 μm pore size), and the defatting protocol was repeated, thereby removing water insoluble and potentially toxic compounds comprising about 5-7% of the total seed mass. The defatted Peganum residue was dried to remove any trace of heptane. Ultrasonication and assisted hydrocarbon extraction The target compounds (i.e., THH, HL, and H) were then extracted from the defatted Peganum residue via two rounds of ethanol sonication extraction. For each extraction round, the Peganum residue was sonicated (30 min) in ethanol using a ratio of 1 g Peganum to 10 mL ethanol and filtered (11 μm pore size). The extraction process had
a final yield of around 5% to 10% (0% THH, 15% HL, 12% H). Crude ethanoic filtrates were evaporated using rotary evaporation and finished to dryness using a stream of nitrogen to provide a Peganum crude ethanol extract. The drying is conducted to completion to remove traces of ethanol that would otherwise influence the effectiveness of aqueous purification. Selective aqueous purification The ethanol extract was then dissolved in water at a ratio of 1 g per 200 mL. This suspension was subjected to ice cold sonication (400W Thermoline UB-410, 0
°C, 30 min). The harmala alkaloids are soluble in water so the non-soluble components are filtered out (11 μm pore size). This leaves the harmala alkaloids solution. The solution was then reduced to approximately 10% volume using rotor evaporation to yield the dry extract powder (overall yield ~4% to 8% of dried material, purity ~0% THH, 12% HL, and 12% H). Stabilising final formulation The resulting aqueous extract was stabilised using a combination of microcrystalline cellulose, mannitol, silica gel, and magnesium stearate. The aqueous extract was combined with the stabilising agent at a 1:1 ratio. The ratio is estimated based on a yield of 6% achieved from the starting material. For every (Dry) 100 g of starting material 6g was dried by rotary evaporation (40
°C) to yield the dry extract powder (overall yield ~4% to 8% of dried material, purity ~0% THH, 12% HL, and 12% H). Conversion of HL to THH Harmaline recovered from Peganum seeds was converted to THH by combining the harmaline (6 g) with 5 vol% aqueous acetic acid (150 mL) and zinc powder (2.8 g activated by rinsing with 1M HCl and collected by filtration and aqueous rinsing). The mixture was refluxed for 1 h, then the zinc was removed by filtration (11 μm pore size). The filtrate was slowly neutralised by adding 10 vol% aqueous ammonium hydroxide. Precipitate formed in successive neutralization rounds were sequentially collected via filtration. The first precipitate was predominantly impurity from the Peganum extract; however, the second precipitate was predominantly unreacted harmine (60% purity). The remainder was THH.
Recombination of Peganum extract with converted Peganum extract The neutralised filtrate was freeze-dried. THH was recovered from the resultant dry mass at a ratio of 95 parts THH to 5 parts H, and the product was desalted. Note that THH can be potentially converted back to HL to allow for an additional process to achieve the desired ratio. A target ratio of H:HL:THH was set at 1.0: 0.1:0.9. Example 7 – Acetic Acid Extraction of harmala alkaloids from Peganum spp. Plants Peganum harmala seed (10.09 g) was processed in NutriBullet until a fine powder was obtained (3 x 1 min). The powder was transferred to a 150 mL flask equipped with a large stir bar, n-heptane (50 mL) was added, and the mixture was stirred vigorously (1100 rpm) for 1h. The mixture was then filtered through a Büchner funnel with Whatman #1 filter paper. The seed residue was returned to the flask, and the washing procedure was repeated with another 50 mL of n-heptane. Seed residue was returned to the flask, and 5% aqueous AcOH (50 mL) was added. The mixture was stirred vigorously (1100 rpm) for 1h. Next, the mixture was divided between two 50 mL Falcon Tubes and centrifuged. A clear brown supernatant was observed above a solid pellet. A cloudy brown layer, containing visible particulates, was present on the surface. The supernatant was filtered through a fritted glass filter very slowly. The pellet was then re- suspended in 5% aqueous AcOH (50 mL) and stirred vigorously (1100 rpm) for another 1h. The resulting clear supernatant was combined with the filtrate and washed with EtOAc (50 mL). Some emulsion was observed. Using 25% aqueous ammonia, the pH was gradually increased to pH ~11, whereupon a pale precipitate was observed. The product was then extracted into 1:1 EtOAc:2-methyltetrahydrofuran mixture (80 mL) (most of brown colour remained in aqueous layer). Finally, the organic layer was washed with brine, dried (Na
2SO
4), and evaporated to give a pale yellow solid (0.393 g). UHPLC analysis showed: harmaline 51%, harmine 22% (See Figure 5). Example 8 – Ethanol/Triethylamine Extraction of harmala alkaloids from Peganum spp. Plants
Titration of crude extract P. harmala seed (49.3 g) was ground finely in a NutriBullet (3 x 1 min). The resultant powder was stirred with ethanol (200 mL) and triethylamine (1 mL) vigorously for 1h then filtered through a Buchner funnel with Whatman #1 filter paper. The seed residue was returned to the round bottom flask and re-extracted with ethanol (200 mL) and triethylamine (1 mL) for a further 1h then filtered. The filtrates (clear brown) were combined and evaporated to a dark reddish-brown oily residue. The residue was partitioned between 5% aqueous acetic acid (200 mL) and ethyl acetate (200 mL). The layers were separated, and the organic phase extracted again with 5% aqueous acetic acid (200 mL). The combined aqueous phase was washed with ethyl acetate (200 mL). The combined ethyl acetate washes (dark brown) were disposed of to Schedule 9 waste. The combined aqueous phase (yellow-brown) was adjusted to pH ~10 with 25% aq. Ammonia and extracted with 1:1 ethyl acetate/2-methyltetrahydrofuran (3 x 100 mL). The combined extracts were dried (Na2SO4) and evaporated to give a reddish-brown solid (3.60 g). UHPLC analysis showed 48% harmaline, 24% harmine. This extract was slurried with acetone/methanol (1:1) (2 x 10 mL) and filtered to give a tan powder (2.33 g). UHPLC analysis showed: 59% harmaline, 24% harmine (see Figure 9). This material was subjected to pH-based fractionation (see Figure 10). The acetone/ethanol filtrate was evaporated, and the red residue was triturated with acetone/ethanol to give a pale brown solid (0.424 g). This was recrystallised from ethanol and dried under high vacuum to give a pale tan powder (0.154 g). GC-MS analysis showed 48.06% harmaline and 51.30% harmine (see Figure 11). The only other compound detected was palmitoleamide at 0.63%. This material was used in preparation of a harmaline hydrochloride-containing fraction (see below). Alternatively, it is contemplated that the harmaline-rich fraction obtained from the pH-based fractionation could be purified by recrystallisation then converted to a hydrochloride salt according to methods commonly used in the art.
Harmaline hydrochloride (mixture with harmine hydrochloride) The purified mixture of harmine and harmaline free bases (0.150 g) was suspended in ethanol (5 mL) and 37% aq. HCl (11.6 M, 0.091 mL, 1.5 eq.) was added with stirring. The mixture became clear before a precipitate formed. After stirring at room temperature for 30 min, the yellow suspension was evaporated to give a mixture of harmaline hydrochloride and harmine hydrochloride. UHPLC analysis, corrected for 1 equivalent of HCl, showed 43.4% harmaline and 41.4% harmine (see Figure 12). The presence of harmaline hydrochloride and harmine hydrochloride was confirmed via
1H NMR (see Figure 13). Separation of alkaloids by pH-based fractionation Harmaline and harmine were separated according to known procedures (Van Der Sypt 2016) (see Figure 10). The harmala seed extract obtained above (2.23 g, 59 wt% harmaline, 24 wt% harmine) was added to a 100 mL beaker and dissolved in 5% aqueous acetic acid (~20 mL). A pH electrode (calibrated with pH 7.0 and pH 10.0 buffers) was immersed in the solution and 25% aqueous ammonia solution was added slowly with magnetic stirring and ice-cooling to maintain the temperature around 20 °C. As the pH approached 7, time was allowed for the pH to stabilise after addition of each drop. When the pH remained steady at 7.5, for 2-3 min. the precipitate was collected by filtration and dried under high vacuum overnight to give a pale brown powder (1.11 g). UHPLC analysis showed: 29% harmaline, 45% harmine (see Figure 14). Bright yellow filtrate returned to beaker and addition of concentrated. Aqueous ammonia continued dropwise to pH 7.71. The newly precipitated solid was collected by filtration and dried under high vacuum to give a pinkish-tan powder (0.768 g). UHPLC analysis showed: 81% harmaline, 4.4% harmine. The filtrate (bright fluorescent yellow) was returned to the beaker and addition of 25% aqueous NH
3 continued to pH ~10. The precipitate was collected by filtration and dried under high vacuum to give a tan powder (0.170 g). UHPLC analysis showed: 86% harmaline, 2.5% harmine (see Figure 15).
Harmaline-rich fraction: Reduction of harmaline in harmaline/harmine mixture with sodium borohydride A modified procedure was used to reduce harmaline (Schönenberger et al. 1986). Harmaline-enriched P. harmala extract (0.746 g) containing harmaline (0.606 mg, 2.83 mmol) was suspended with stirring in ethanol (6 mL) and water (3 mL), and the resultant suspension cooled to 0°C. 6M HCl (2 drops) was added, followed by a solution of sodium borohydride (118 mg, 3.11 mmol) in H2O (4 mL). The sodium borohydride solution was added dropwise over 5 min. The mixture was stirred at room temperature for 3 h then quenched by a slow addition of 2M HCl (5 mL over 5 min). The mixture was then made alkaline to pH 13 by careful addition of 10% aq. NaOH (a pale precipitate was observed at this stage). The mixture was extracted with 2-methyltetrahydrofuran (3 x 20 ml). The aqueous phase was discarded. The combined organic extracts (pale yellow solution) were washed with brine, dried (Na
2SO
4), evaporated, and the residue dried under high vacuum overnight to give a pale tan solid (0.749 g). UHPLC analysis showed: 86% tetrahydroharmine, 0.2% harmaline, 6.6% harmine (total 93% harmala alkaloids) (see Figure 16). A sample (0.288 g) of this material was recrystallised from ethanol to give an off-white solid (0.192 g), which GC-MS analysis showed to be 98% tetrahydroharmine (see Figure 17). This material (0.190 g, 0.877 mmol) was suspended in ethanol, 37% aq. HCl (11.6 M, 0.114 mL, 1.5 eq.) was added, and the mixture was stirred at room temperature for 30 min, then evaporated to give tetrahydroharmine hydrochloride (see Figure 18). UHPLC analysis, corrected for 1 equivalent of HCl, showed 105% tetrahydroharmine and 0.77% harmine (see Figure 19). Harmine-rich fraction: Reduction of harmaline in harmaline/harmine mixture with sodium borohydride Harmine-enriched P. harmala extract (0.984 g) containing 29 wt% harmaline (0.285 g, 1.33 mmol) was suspended in EtOH (3 mL) and H2O (1.5 mL) and cooled to 0°C. 6M HCl (1 drop) was added, followed by a solution of sodium borohydride (55 mg, 1.46 mmol) in H
2O (2 mL). The mixture was removed from the ice bath and stirred at room temperature for 3h, then quenched by addition of 2M HCl (5 mL). The mixture was then made alkaline to pH 13 by addition of 10% aq. NaOH and extracted with 2-
methyltetrahydrofuran (3 x 20 mL). The aqueous phase was discarded. The combined organic extracts (clear brown solution) were washed with brine, dried (Na
2SO
4) and evaporated under reduced pressure. The pale tan solid was dried under high vacuum (0.962 g). UHPLC analysis shows: 23% tetrahydroharmine, 5.2% harmaline, 47% harmine (total 75% harmala alkaloids) (see Figure 20). Purification of harmine by acetylation and removal of tetrahydroharmine Sodium borohydride-treated P. harmala extract (0.860 g) containing harmine (47 wt%), tetrahydroharmine (23 wt%), and harmaline (5.2% wt %), was suspended in isopropanol (5 mL), and a mixture of sodium acetate (1.40 g) in water (4 mL) was added. The mixture was cooled in an ice bath and stirred vigorously while acetic anhydride (2 mL) was added dropwise over 5 min. Stirring was continued at 0°C for 30 min, then the ice bath was removed and stirring continued at room temperature for 30 min. The mixture was diluted with water (30 mL), and 2M HCl (30 mL) was added (mixture had pH~1). This mixture was extracted with EtOAc (50 mL). At this stage the biphasic mixture was filtered to remove an insoluble pale yellow precipitate which interfered with phase separation. The yellow precipitate was washed with 25% aqueous NH
3 and dried under high vacuum to give an off-white powder (0.399 g).
1H NMR analysis showed this material to consist of >90% harmine. The EtOAc phase was dried (Na2SO4) and evaporated to give a pale yellow solid (0.160 g), which was also mostly harmine. The two solids were combined and stirred vigorously with a mixture of distilled water (5 mL) and EtOAc (5 mL) for 10 min before the solid was collected by filtration and washed thoroughly with distilled water. The solid was dried under suction then recrystallised from ethanol to give an off- white powder (0.194 g). GC-MS analysis (Figure 21) showed 99.1% harmine, with harmaline (0.19%) and palmitoleamide (0.71%) as impurities. Formation of hydrochloride salts This purified harmine (0.192 g, 0.903 mmol) was suspended in ethanol, 37% aq. HCl (11.6 M, 0.117 mL, 1.5 eq.) was added, and the mixture was stirred at room temperature for 30 min. The resultant pale yellow suspension was evaporated in vacuo to give harmine hydrochloride. The presence of the hydrochloride salt was confirmed by 1H NMR (see Figure 22). UHPLC analysis, corrected for 1 equivalent of HCl, showed 86.5% harmine and 1.1% harmaline (see Figure 23).
It is contemplated that purified hydrochloride salts of harmine, harmaline, and tetrahydroharmine could be combined in varying ratios with an overall purity equivalent to the weight average of the purity of each component provided in the foregoing examples. Example 9 – harmala alkaloid manufacturing process The harmala alkaloid drug substance (i.e. tetrahydroharmine, harmine, and harmaline in a specified ratio) is produced by extracting Peganum harmala seeds, isolating harmine and harmaline, and converting excess quantities of harmaline into tetrahydroharmine. All three purified compounds are then combined in the specified ratio. The final drug substance has NLT 90% assay for each compound. The harmala alkaloid drug substance manufacturing process includes the following steps. 1. Milling and extraction of Peganum harmala seeds 2. Isolation of harmine and harmaline 3. Separation of harmine and harmaline 4. Conversion of excess harmaline into tetrahydroharmine 5. Conversion of all three purified compounds into HCl salt form 6. Blending of three compounds (tetrahydroharmine, harmine, and harmaline ) in the specified ratio Milling and extraction of Peganum harmala seeds The Peganum harmala seeds are powdered using a common grinder. The milled seeds are extracted in ethanol containing acetic acid. Isolation of Harmine and Harmaline The ethanol extract is filtered, evaporated and dissolved in acetic acid. The resulting solution is then washed with ethyl acetate. The acidic aqueous phase is then basified using ammonia and filtered. The filtrate is discarded and the precipitate slurry is washed in acetone and ethanol to produce a solid mixture of harmine and harmaline free bases.
Separation of Harmine and Harmaline Harmine and harmaline are separated by means of pH-dependent sequential precipitation from aqueous solution. The material is dissolved in acetic acid solution and the pH of the solution is gradually raised by addition of aqueous ammonia. The bulk of harmine free-base precipitated before harmaline free base began to precipitate due to the difference in the pKa of the protonated forms of harmine and harmaline. Ammonia is added gradually while monitoring the pH of the solution. The initial precipitate of harmine is collected by filtration. The remaining harmaline was then precipitated from the filtrate by further addition of ammonia. Freebase harmine and harmaline are recrystallised in ethanol to obtain NLT 90% purity. Conversion of excess Harmaline into tetrahydroharmine The tetrahydroharmine required to attain the intended harmala alkaloid ratio is obtained from surplus harmaline by reduction of the imine to the secondary amine. Harmaline hydrochloride is rapidly and efficiently reduced to racemic tetrahydroharmine using sodium borohydride in ethanol solution. Following the addition of aqueous hydrochloric acid to destroy excess borohydride, the reaction mixture is made alkaline to precipitate racemic tetrahydroharmine freebase, which is collected by filtration, washed with water and dried under vacuum to give a colourless, free-flowing powder Conversion of all three purified compounds into HCl salt form Harmine, harmaline and tetrahydroharmine freebases are separately converted to their respective hydrochloride salts by adding aqueous HCl to suspensions of the alkaloid free-bases in ethanol. The hydrochloride salts of harmine and harmaline precipitates from solution and are collected by filtration. In the case of THH, the mixture is evaporated to recover the salt. All three compounds are dried to produce harmine, harmaline and tetrahydroharmine HCl salt showing NLT 90% assay. Blending of two or three compounds (tetrahydroharmine, harmine, and harmaline or tetrahydroharmine and harmine)
Purified tetrahydroharmine HCl, harmine HCl, and harmaline HCl are blended in the specified ratio using a common pharmaceutical grade blender. The ratio of the two or three compounds is confirmed via HPLC analysis. Example 10 –Compositions Drug Product weight ratio relative to DMT
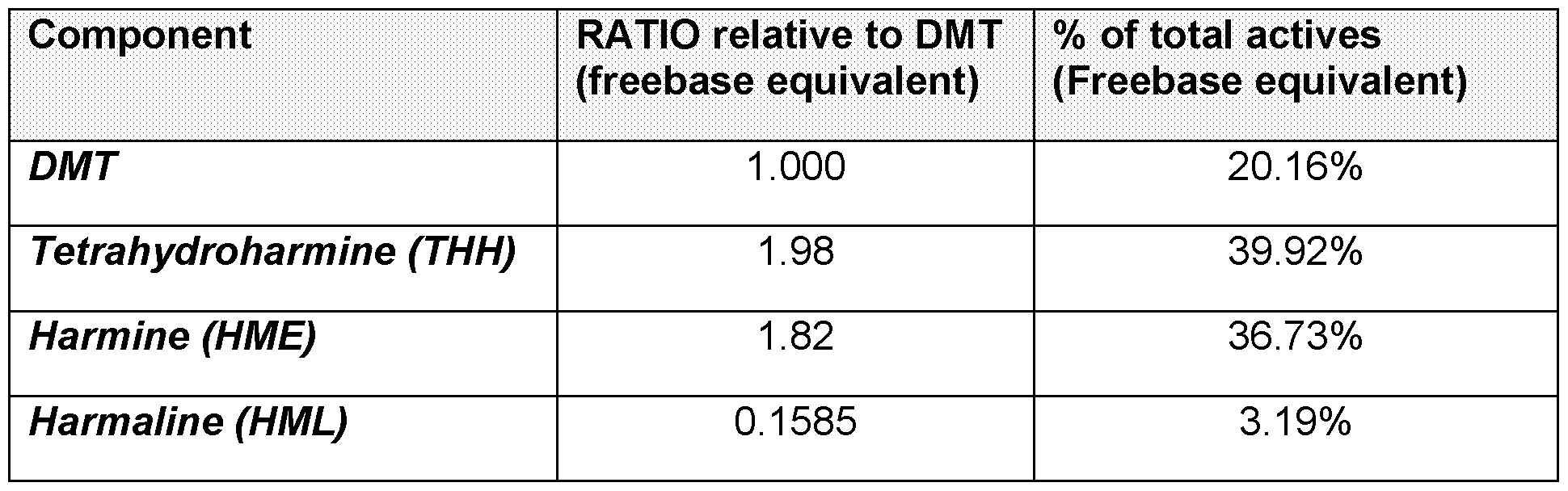
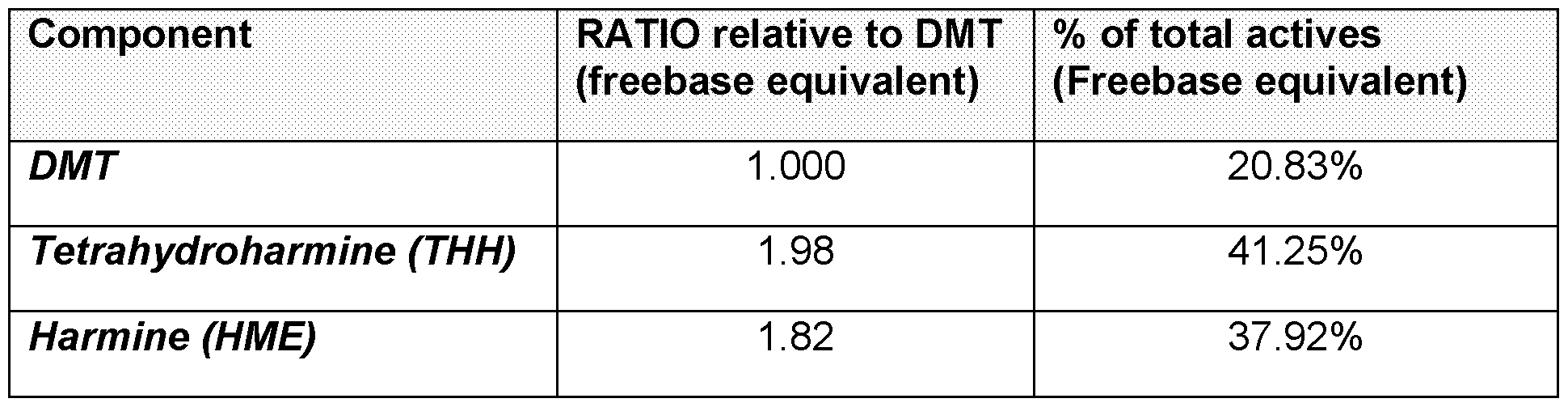
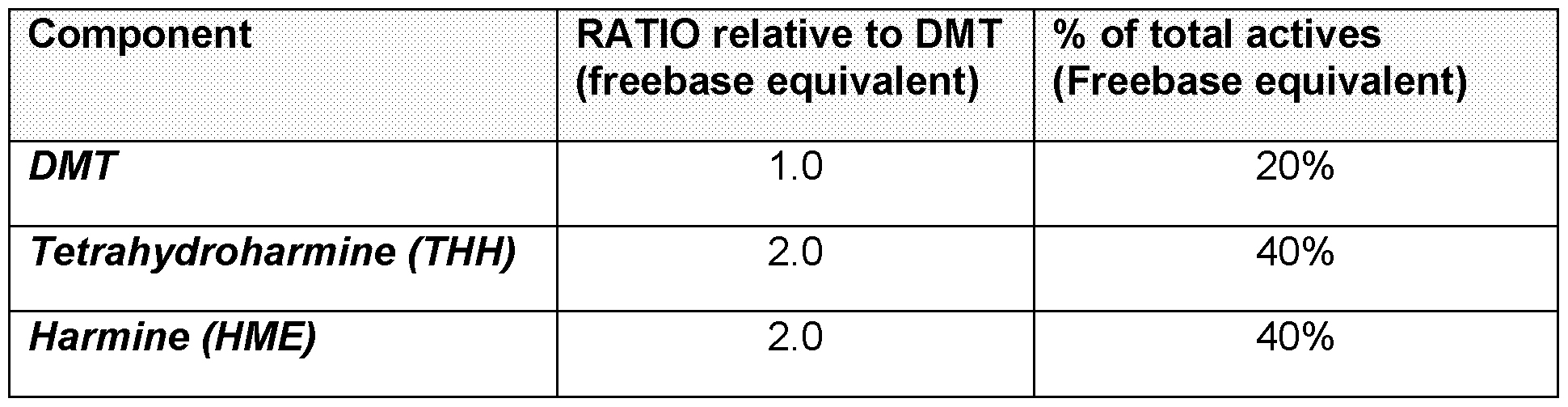
Freebase:Salt ratios
Capsules
Harmala Drug substance
Drug Product
5
Example 11 – Prophetic Compositions (Drug product weight ratio relative to DMT) Drug product 1
Drug product 2
Drug product 3
Drug product 4
Drug product 5
Drug product 6
Drug product 7
Drug product 8
Drug product 9
Drug product 10
Drug product 11
Drug product 12
Drug product 13
Drug product 14
Drug product 15
Drug product 16
Drug product 17
Drug product 18
Drug product 19
Drug product 20
Drug product 21
Drug product 22
Drug product 23
Drug product 24
Drug product 25
Drug product 26
Drug product 27
Drug product 28
Drug product 29
Drug product 30
Drug product 31
Drug product 32
Drug product 33
Drug product 34
Drug product 35
Drug product 36
Drug product 37
Drug product 38
Drug product 39
Drug product 40
Drug product 41
Drug product 42
Drug product 43
Drug product 44
Drug product 45
Drug product 46
Drug product 47
Drug product 48
Drug product 49
Drug product 50
Drug product 51
Drug product 52
Drug product 53
Drug product 54
Drug product 55
Drug product 56
Drug product 57
Drug product 58
Drug product 59
Drug product 60
Drug product 61
Drug product 62
Drug product 63
Drug product 64
Drug product 65
Drug product 66
Drug product 67
Drug product 68
Drug product 69
Drug product 70
Drug product 71
Drug product 72
Drug product 73
Drug product 74
Drug product 75
Drug product 76
Drug product 77
Drug product 78
Drug product 79
Drug product 80
Drug product 81
Drug product 82
Drug product 83
Drug product 84
Drug product 85
Drug product 86
Drug product 87
Drug product 88
Drug product 89
Drug product 90
Drug product 91
Drug product 92
Drug product 93
Drug product 94
Drug product 95
Drug product 96
Drug product 97
Drug product 98
Drug product 99
Drug product 100
Drug product 101
Drug product 102
Drug product 103
Drug product 104
Drug product 105
Drug product 106
Drug product 107
Drug product 108
Drug product 109
Drug product 110
Capsules Capsule 1
Capsule 2
Capsule 3
Capsule 4
Capsule 5
Capsule 6
Capsule 7
Capsule 8
Harmala Drug Substance
5
Drug Product
5
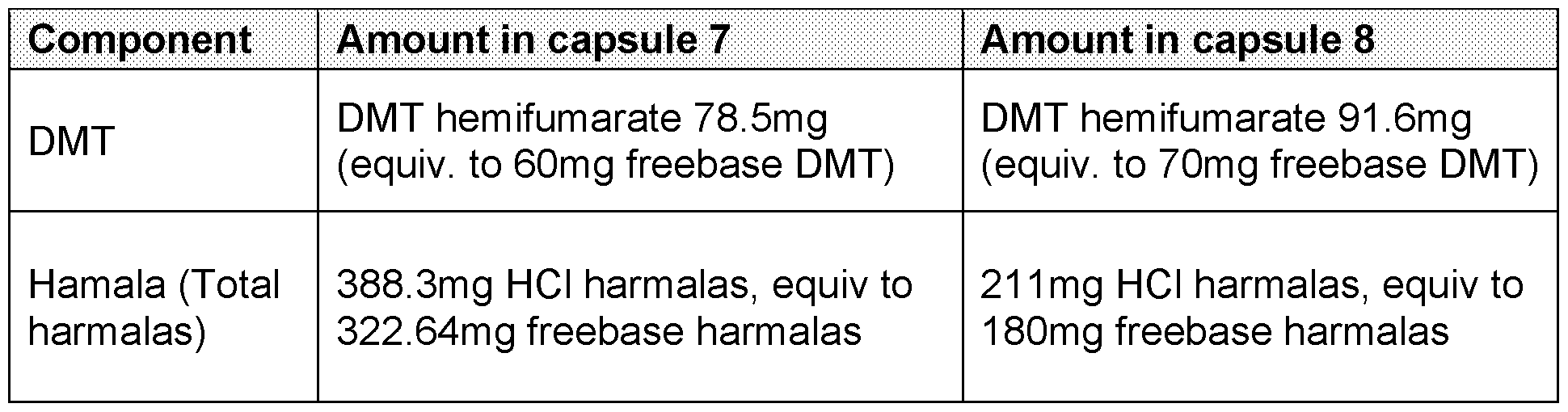
Example 12 - Evaluating the safety, tolerability, and subjective effects of three DMT-harmaloid formulations in healthy volunteers The study evaluated the safety, tolerability, and subjective effects of three formulations of N,N-Dimethyltryptamine (DMT) and harmala alkaloids (tetrahydro harmine, harmine, and harmaline), on a small sample of healthy volunteers. Participants had prior experience consuming ayahuasca in naturalistic settings. The study involving two different formulations (A and B). Each formulation contained the same dose of DMT and harmala alkaloids, but with DMT in formulation A being extracted from Acacia courtii and the DMT in formulation B being extracted from Acacia acuminata. The purity of the DMT and harmala plant extracts used in this formulation was approximately 60%. Other plant constituents were present in each formulation. Additional alkaloids present in the acuminata extract included NMT (N- Methyltryptamine), MTHBC (N-Methyl-1,2,3,4-tetrahydro-β-carboline) and tryptamine. Four participants received a single dose of Formulation A followed by a single dose of Formulation B at minimum two-week washout. The dosages were set at 1.0 mg/kg for DMT and 4 mg/kg harmalas for both formulations. A third formulation, labelled as C, was introduced for the second half of the study. Formulation C was derived from Acacia acuminata as the source of DMT and Peganum harmala as the source of the Harmala alkaloids. However, much higher purity extracts (>90% pure) were obtained and converted to specific salt forms: hemifumarate in the case of DMT and hydrochloride in the case of the three harmala alkaloids. Formulation C was administered twice to four participants. The initial dose (Formulation C (low)) was at the same level as had been used for formulations A and B (1mg/kg DMT
plus 4mg/kg harmalas). For the second administration (Formulation C (high)) the dosage was escalated to 1.4 mg/kg for DMT and 5.6 mg/kg harmalas, if the initial dose was well tolerated. All four participants tolerated first dose well and increased to the higher dose for the second administration. Formulations Formulation A DMT (extracted from Acacia courtii; purity approx.60%) – administered at 1.0 mg/kg Harmala alkaloids (purity approx.60%) – administered at 4 mg/kg Harmala alkaloids include tetrahydroharmine (approx. 50 % w/w), harmine (approx.46 % w/w) and harmaline (approx.4 % w/w) Formulation B DMT (extracted from Acacia acuminata; purity approx.60%) – administered at 1.0 mg/kg Harmala alkaloids (purity approx.60%) – administered at 4 mg/kg Harmala alkaloids include tetrahydroharmine (approx. 50 % w/w), harmine (approx.46 % w/w) and harmaline (approx.4 % w/w) Other plant constitutes include N-Methyltryptamine, N-Methyl-1,2,3,4-tetrahydro-β- carboline and tryptamine. Formulation C Two administrations; initial dose (Formulation C (low)), followed by a second administration (Formulation C (high)) Formulation C (low) DMT as hemifumarate salt - (extracted from Acacia acuminata, purity approx. >90%) – administered at 1.0 mg/kg Harmala alkaloids all present as hydrochloride salts (extracted from Peganum harmala, purity approx. >90%) – administered at 4 mg/kg
Harmala alkaloids include tetrahydroharmine (approx.50 % w/w per free base), harmine (approx.46 % w/w per free base) and harmaline (approx. 4 % w/w per free base) Formulation C (high) DMT as hemifumarate salt - (extracted from Acacia acuminata, purity approx. >90%) – administered at 1.4 mg/kg Harmala alkaloids all present as hydrochloride salts (extracted from Peganum harmala, purity approx. >90%) – administered at 5.6 mg/kg Harmala alkaloids include tetrahydroharmine (approx.50 % w/w per free base ), harmine (approx.46 % w/w per free base) and harmaline (approx. 4 % w/w per free base) Safety Monitoring and Adverse Events There were no serious adverse events in this study. All formulations were well tolerated with other adverse effects being transient and consistent with those reported in other psychedelic research. The most commonly reported adverse events were gastrointestinal issues such as feelings of nausea (due to the serotonergic activity of these substances), headache and dizziness, and temporary psychological or perceptual disturbances, which are commonly reported in psychedelic research and reflect the profound impact of psychedelics on the nervous system. These symptoms are manageable with supportive care and typically resolve shortly after the effects of the medication wear off. Relative strength of the psychedelic experience The two graphs shown in Figure 24 compare the strength of the psychedelic experience across the four formulations (A, B, C (low), and C (high)) using both ratings and average scores. The ratings graph shows that most participants rated formula A as "Weaker" compared to previous ayahuasca experiences, while responses to formula B were mixed. For formula C (low), most participants found the experience similar to ayahuasca, while one participant rated it as stronger. In contrast, all participants rated the high dose
of formula C as "Stronger," highlighting its potency. The average score graph provides a numerical comparison, where 1 = Weaker, 2 = Similar, and 3 = Stronger and confirms a linear relationship between formulations and the strength of the psychedelic experience. Relative quality of the psychedelic experience In relation to the quality of the psychedelic experience, Figure 25 shows that it was seen as similar to ayahuasca by 75% of respondents for formula C (low) and C (high) and around 50% of for formulas A and B, indicating the improved profile of the more highly purified Formulation C. Relative therapeutic effect of the psychedelic experience As with the strength of the psychedelic experience, the perceived therapeutic effect of the psychedelic experience, relative to ayahuasca see Figure 26, followed a linear relationship increasing from formula A to formula C (high), with 3 of 4 subjects reporting formula C-high to be “far more beneficial” and one similarly beneficial. The four C (low) administrations were reported as “similar” or “more” beneficial. Formula B elicited mixed responses and all formula A experiences were rated as less therapeutically beneficial than ayahuasca. Nature of the acute psychedelic experience Several psychometric instruments were utilised to measure the strength and nature of the psychedelic experience. These included: • Mystical Experience Questionnaire (MEQ-30) The Mystical Experience Questionnaire (MEQ) is a psychometric instrument designed to assess the intensity and quality of mystical experiences. • Short Index of Mystical Orientation (SIMO) An adapted experience focused version of this scale was used. The SIMO items are informed by Happold's seven characteristic components of mysticism. • 5 Dimensions of Altered States of Consciousness (5D-ASC) The 5D-ASC is a psychometric instrument commonly used in psychedelic research to measure various dimensions of altered states of consciousness.
Mystical Experience Questionnaire (MEQ-30) The graph in Figure 27 shows the average scores of different investigational formulas (A, B, C (low), C (high)) and a comparison with a naturalistic ayahuasca use sample (Nat) across the MEQ-30 subscales: Mystical experience, Positive mood, Transcendence, Ineffability, and MEQ total score. The Formula C (high) stands out with the highest overall scores across all subscales, closely followed by Formula C (low). Formula B showed moderate scores across the subscales, whereas Formula A consistently had the lowest scores. The natural Ayahuasca group (Nat) displayed relatively high scores: the total MEQ score was slightly below Formula C (high) but slightly above Formulation C (low). The naturalistic ayahuasca group scored higher on the Mystical and Mood than Formulation C(high), but lower on Transcendence and Ineffability. Overall, the comparison highlights that formulas C (high) and C (low) were both relatively close the level of mystical experience experienced by naturalistic ayahuasca drinkers. Short Index of Mystical Orientation (SIMO) Like with the MEQ, within the SIMO scores Formula C (high) again stood out with the highest average score (Figure 28), surpassing even the naturalistic ayahuasca group (data from a different study). The Formula C (low) also achieved a high score, while Formulas A and B had notably lower scores. These patterns across both the MEQ and SIMO measures reinforce the potency of Formula C, particularly at higher doses, in producing an intense and spiritually significant experience compared to other investigational formulas, which is comparable with experiences achieved with Ayahuasca in naturalistic settings. 5 Dimensions of Altered States of Consciousness (5D-ASC) Scores reported on the 5D-ASC (Figure 29) generally followed a linear pattern with increasing effects from Formula A to Formula C (high), which had the highest ratings on 8 of the 11 dimensions. Audio-visual synaesthesia was marginally higher for Formulation C(low), and Formulations B and A were somewhat higher for Blissful, and lowest for Formulation C(low). The Disembodiment average score was approximately the same for all of the four formulations. It is also notable the Anxiety dimension displayed a linear
relationship with the highest score obtained for Formula C (high), which is not unexpected given its stronger psychedelic effects. Other Participant Experiences Personal Insights Questionnaire (PIQ) The PIQ measures the personal insights gained during psychedelic experiences, focusing on changes in self-perception, life perspective, and existential reflections. The number of personal insights obtained during a psychedelic experience has been associated with various mental health and wellbeing outcomes. Figure 29 shows all formulas provided between 3.6 and 4.2 personal insights when reported 1-week post consumption, with this number increasing to between 4 and 4.7 by 4-weeks post consumption, suggesting a deepening of the experience or further integration over time. The Formula C (high) achieved the highest average number of insights at both time points. However, differences between the formulas were smaller than on the measures presented above. Persisting Effects Questionnaire The Persisting Effects Questionnaire (PEQ) is a self-report instrument designed to assess the long-term psychological, behavioural, and spiritual effects of a significant psychedelic experience. Change in personal wellbeing or life satisfaction. All formulations were reported to have an average beneficial effect on wellbeing or life satisfaction, and no individual participants reported any reduction in these areas. Formula A showed the lowest increase, representing only a “slight” improvement in wellbeing/satisfaction. Formula B demonstrated a greater improvement, representing a perceived “slight” to “moderate” increase. Formula C (low) resulted in close to a “moderate” perceived increase reported at 1-week, while Formula C (high) displayed the highest overall improvement, maintaining a score of 2 at both time points, representing a “moderate” perceived increase in wellbeing/life satisfaction.
In relation to the extent to which the experiences were perceived as psychologically insightful, a similar patter emerged with scores being lowest for Formula A, representing experiences similar to those that occur once a month, followed by B (similar to experiences that occur one a year to once every 5 years). Formula C (high) was reported to produce profound experiences of insight, which were perceived as among the 5 most insightful experiences of a person’s life. All formulations were reported to have some level of spiritual effect, with these effects increasing across the formulations as suggested by the MEQ and SIMO instruments. Formula A was the least spiritually significant (equivalent to experiences that occur once a month to once a year), Formula B increased from the same level at week 1 to a higher level of spiritual significance at week 4 (equivalent to experiences that occur every 5 years). Formula C (low) was only marginally above A and B at week-1, while Formula C (high) was substantially higher with an average score of 7, equivalent to among the 5 most spiritually significant experiences of a person’s life. The ratings of personal meaningfulness by formulation were similar to those provided spiritual meaningfulness, with Formula C (high) perceived to produce experiences that were among the 5 most personally meaningful of a person’s life, at weeks 1 and 4. Formula C (low) was also highly rated, equivalent to just below the most 10 personally meaningful experiences of a person’s life. Ratings were again lower for formulas B and A. The level of psychological challenge associated with the experiences corresponded closely to the level of spiritual significance and personal meaningfulness, suggesting that these concepts are closely intertwined. Formula C (high) was on average reported to provide the most challenging experiences, equivalent to the most 5 to 10 most difficult or challenging experiences of a person’s life. Formula C (low) was on average equivalent to difficult or challenging experiences encountered slightly less than every 5 years. Formulas B and A produced very minimal psychological challenge, equivalent to experiences occurring weekly at week 1, and monthly at week 4.
Summary Data obtained in this study indicate that Formulation C, a highly purified DMT-harmala product derived from Acacia acuminata and Peganum harmala, outperformed other tested formulations across various measures. Effects of Formulation C, particularly at the higher dose, closely align with or surpass experiences reported by naturalistic ayahuasca users. Participants consistently rated Formulation C as producing a stronger, more beneficial, and more meaningful psychedelic experiences than both Formulations A and B. The subjective effects, assessed through several psychometric instruments, identified Formulation C (high) as able to achieve the highest scores on mystical and transformative experiences. These results were comparable to those reported by naturalistic ayahuasca users, suggesting that the present purified DMT-harmala formulation can closely replicate and even exceed the transformative effects and therapeutic potential of traditional ayahuasca brews. Formulation C not only produced more profound mystical and psychological insights than the other formulations, but also proved to be well tolerated, with no serious adverse events reported and other common adverse effects limited to the pharmacological duration of action. Participants experienced a range of positive mental health outcomes, including increased life satisfaction, personal insights, and enhanced psychological wellbeing. The Formulation C (high) was particularly impactful, providing profound therapeutic benefits comparable to those of naturalistic ayahuasca use, while also being rated among the five most insightful and spiritually significant experiences of participants' lives. Although, as is often the case with traditional ayahuasca, the experiences could also be psychologically challenging. In summary, this study found Formulation C to perform favourably across all measures. Data suggests that it may be offer a pharmaceutical grade DMT-harmala drug product that is capable of inducing transformative therapeutic experiences.
References Van Der Sypt, F.A.P. A harm-reduction approach to the isolation of harmine and its hydrogenated derivatives from Peganum Harmala seed in low-tech settings, Zenodo 2016; 10.5281/zenodo.2553922. Schönenberger, B and Brossi A. Fragmentation of Optically Active (1-Phenylethyl)- and (1-Naphthylethyl)ureas in Refluxing Alcohols: Easy Preparation of Optically Active Amines of High Optical Purity, Helv. Chim. Acta.1986, 69, 1486-1497.



















































































































































