WO2023036404A1 - Taste balancing botanical compounds - Google Patents
Taste balancing botanical compounds Download PDFInfo
- Publication number
- WO2023036404A1 WO2023036404A1 PCT/EP2021/074604 EP2021074604W WO2023036404A1 WO 2023036404 A1 WO2023036404 A1 WO 2023036404A1 EP 2021074604 W EP2021074604 W EP 2021074604W WO 2023036404 A1 WO2023036404 A1 WO 2023036404A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- mixture
- madecassoside
- terminoloside
- total weight
- Prior art date
Links
- 150000001875 compounds Chemical class 0.000 title claims abstract description 86
- 235000019640 taste Nutrition 0.000 title description 5
- 239000000203 mixture Substances 0.000 claims abstract description 100
- 238000000034 method Methods 0.000 claims abstract description 15
- 235000019605 sweet taste sensations Nutrition 0.000 claims abstract description 15
- 238000004519 manufacturing process Methods 0.000 claims abstract description 13
- 235000013305 food Nutrition 0.000 claims abstract description 8
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 5
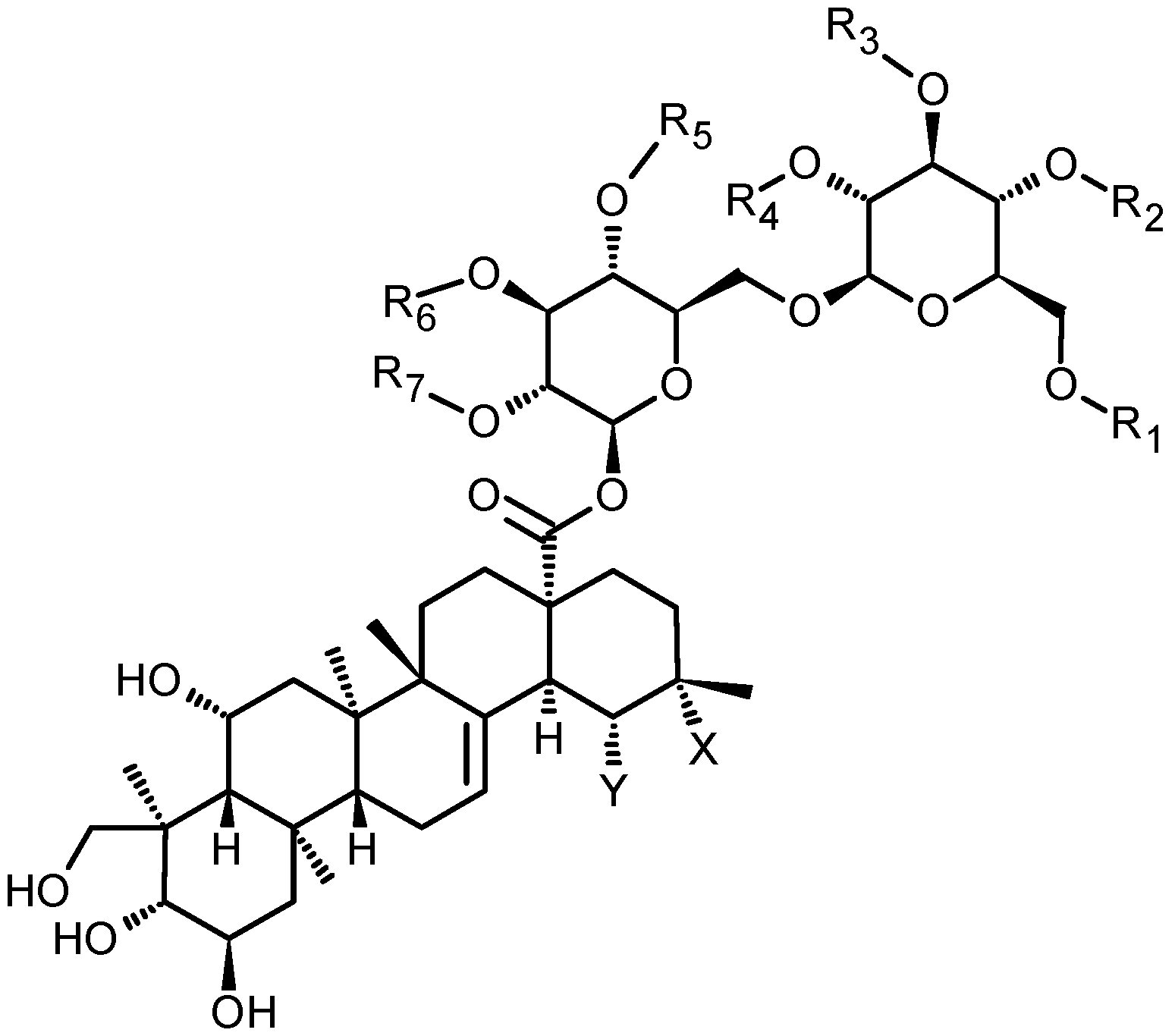
- NNWMHSNRRWMMBI-PJISEHJASA-N Asiaticoside B Chemical compound O[C@@H]1[C@H](O)[C@@H](O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](CO)O[C@@H](OC[C@@H]2[C@H]([C@H](O)[C@@H](O)[C@H](OC(=O)[C@@]34[C@@H](CC(C)(C)CC3)C=3[C@@]([C@]5(C)C[C@@H](O)[C@H]6[C@](C)(CO)[C@@H](O)[C@H](O)C[C@]6(C)[C@H]5CC=3)(C)CC4)O2)O)[C@H](O)[C@H]1O NNWMHSNRRWMMBI-PJISEHJASA-N 0.000 claims description 54
- BNMGUJRJUUDLHW-HCZMHFOYSA-N Madecassoside Chemical compound O([C@@H]1[C@@H](CO)O[C@H]([C@@H]([C@H]1O)O)OC[C@H]1O[C@H]([C@@H]([C@@H](O)[C@@H]1O)O)OC(=O)[C@]12CC[C@H]([C@@H]([C@H]1C=1[C@@]([C@@]3(C[C@@H](O)[C@H]4[C@](C)(CO)[C@@H](O)[C@H](O)C[C@]4(C)[C@H]3CC=1)C)(C)CC2)C)C)[C@@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O BNMGUJRJUUDLHW-HCZMHFOYSA-N 0.000 claims description 52
- QCYLIQBVLZBPNK-UHFFFAOYSA-N asiaticoside A Natural products O1C(C(=O)C(C)C)=CC(C)C(C2(C(OC(C)=O)CC34C5)C)C1CC2(C)C3CCC(C1(C)C)C45CCC1OC1OCC(O)C(O)C1O QCYLIQBVLZBPNK-UHFFFAOYSA-N 0.000 claims description 48
- BNMGUJRJUUDLHW-HLUHVYOBSA-N Madecassoside Natural products C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)C[C@@H](O)[C@H](O)[C@@](C)(CO)[C@@H]5[C@H](O)C[C@@]34C)[C@@H]2[C@H]1C)C(=O)O[C@@H]6O[C@H](CO[C@@H]7O[C@H](CO)[C@@H](O[C@@H]8O[C@H](C)[C@H](O)[C@@H](O)[C@H]8O)[C@H](O)[C@H]7O)[C@@H](O)[C@H](O)[C@H]6O BNMGUJRJUUDLHW-HLUHVYOBSA-N 0.000 claims description 44
- 229940090813 madecassoside Drugs 0.000 claims description 44
- 150000001720 carbohydrates Chemical class 0.000 claims description 31
- 239000000178 monomer Substances 0.000 claims description 23
- 150000003839 salts Chemical class 0.000 claims description 23
- 229930006000 Sucrose Natural products 0.000 claims description 21
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 21
- 229960004793 sucrose Drugs 0.000 claims description 21
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 20
- SHZGCJCMOBCMKK-UHFFFAOYSA-N D-mannomethylose Natural products CC1OC(O)C(O)C(O)C1O SHZGCJCMOBCMKK-UHFFFAOYSA-N 0.000 claims description 18
- PNNNRSAQSRJVSB-UHFFFAOYSA-N L-rhamnose Natural products CC(O)C(O)C(O)C(O)C=O PNNNRSAQSRJVSB-UHFFFAOYSA-N 0.000 claims description 18
- 235000013681 dietary sucrose Nutrition 0.000 claims description 17
- 108700023372 Glycosyltransferases Proteins 0.000 claims description 16
- 102000051366 Glycosyltransferases Human genes 0.000 claims description 16
- SHZGCJCMOBCMKK-JFNONXLTSA-N L-rhamnopyranose Chemical compound C[C@@H]1OC(O)[C@H](O)[C@H](O)[C@H]1O SHZGCJCMOBCMKK-JFNONXLTSA-N 0.000 claims description 16
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 claims description 16
- 239000008103 glucose Substances 0.000 claims description 15
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 claims description 14
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 claims description 13
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 claims description 12
- 244000146462 Centella asiatica Species 0.000 claims description 9
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 claims description 9
- 239000005913 Maltodextrin Substances 0.000 claims description 9
- 229920002774 Maltodextrin Polymers 0.000 claims description 9
- 229910052739 hydrogen Inorganic materials 0.000 claims description 9
- 229940035034 maltodextrin Drugs 0.000 claims description 9
- 239000000419 plant extract Substances 0.000 claims description 9
- 239000000126 substance Substances 0.000 claims description 9
- 235000004032 Centella asiatica Nutrition 0.000 claims description 8
- 239000005715 Fructose Substances 0.000 claims description 8
- 229930091371 Fructose Natural products 0.000 claims description 8
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 8
- 239000001257 hydrogen Substances 0.000 claims description 8
- 238000011534 incubation Methods 0.000 claims description 8
- 108010055629 Glucosyltransferases Proteins 0.000 claims description 7
- 102000000340 Glucosyltransferases Human genes 0.000 claims description 7
- 108010042194 dextransucrase Proteins 0.000 claims description 7
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 claims description 6
- IAJILQKETJEXLJ-QTBDOELSSA-N aldehydo-D-glucuronic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-QTBDOELSSA-N 0.000 claims description 6
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 claims description 6
- SHZGCJCMOBCMKK-DVKNGEFBSA-N alpha-D-quinovopyranose Chemical compound C[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@@H]1O SHZGCJCMOBCMKK-DVKNGEFBSA-N 0.000 claims description 6
- 108010048202 alternansucrase Proteins 0.000 claims description 6
- PYMYPHUHKUWMLA-WDCZJNDASA-N arabinose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)C=O PYMYPHUHKUWMLA-WDCZJNDASA-N 0.000 claims description 6
- 238000001035 drying Methods 0.000 claims description 6
- 238000004108 freeze drying Methods 0.000 claims description 6
- 229930182830 galactose Natural products 0.000 claims description 6
- 229940097043 glucuronic acid Drugs 0.000 claims description 6
- HSCJRCZFDFQWRP-JZMIEXBBSA-N UDP-alpha-D-glucose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OP(O)(=O)OP(O)(=O)OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C(NC(=O)C=C2)=O)O1 HSCJRCZFDFQWRP-JZMIEXBBSA-N 0.000 claims description 5
- HSCJRCZFDFQWRP-UHFFFAOYSA-N Uridindiphosphoglukose Natural products OC1C(O)C(O)C(CO)OC1OP(O)(=O)OP(O)(=O)OCC1C(O)C(O)C(N2C(NC(=O)C=C2)=O)O1 HSCJRCZFDFQWRP-UHFFFAOYSA-N 0.000 claims description 5
- 238000002156 mixing Methods 0.000 claims description 5
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 claims description 4
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 claims description 4
- 108010025880 Cyclomaltodextrin glucanotransferase Proteins 0.000 claims description 4
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 claims description 4
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 claims description 4
- 229920002472 Starch Polymers 0.000 claims description 4
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 claims description 4
- 239000008101 lactose Substances 0.000 claims description 4
- 239000008107 starch Substances 0.000 claims description 4
- 235000019698 starch Nutrition 0.000 claims description 4
- 239000005720 sucrose Substances 0.000 claims description 4
- 229920000858 Cyclodextrin Polymers 0.000 claims description 3
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 claims description 3
- 229920001503 Glucan Polymers 0.000 claims description 2
- 101710172282 Oleandomycin glycosyltransferase Proteins 0.000 claims description 2
- 238000001694 spray drying Methods 0.000 claims description 2
- IGVKWAAPMVVTFX-BUHFOSPRSA-N (e)-octadec-5-en-7,9-diynoic acid Chemical compound CCCCCCCCC#CC#C\C=C\CCCC(O)=O IGVKWAAPMVVTFX-BUHFOSPRSA-N 0.000 claims 1
- GVOISEJVFFIGQE-YCZSINBZSA-N n-[(1r,2s,5r)-5-[methyl(propan-2-yl)amino]-2-[(3s)-2-oxo-3-[[6-(trifluoromethyl)quinazolin-4-yl]amino]pyrrolidin-1-yl]cyclohexyl]acetamide Chemical compound CC(=O)N[C@@H]1C[C@H](N(C)C(C)C)CC[C@@H]1N1C(=O)[C@@H](NC=2C3=CC(=CC=C3N=CN=2)C(F)(F)F)CC1 GVOISEJVFFIGQE-YCZSINBZSA-N 0.000 claims 1
- -1 a-glucosidase Proteins 0.000 description 20
- 235000013361 beverage Nutrition 0.000 description 16
- 239000000047 product Substances 0.000 description 16
- 235000002639 sodium chloride Nutrition 0.000 description 16
- 150000002772 monosaccharides Chemical class 0.000 description 13
- 239000000284 extract Substances 0.000 description 11
- 235000003599 food sweetener Nutrition 0.000 description 11
- 235000000346 sugar Nutrition 0.000 description 11
- 239000003765 sweetening agent Substances 0.000 description 11
- IDQVFXZQPGAVAM-GGVBUJAJSA-N (4as,6ar,6as,6br,8r,8ar,9r,10r,11r,12ar,14bs)-8,10,11-trihydroxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid Chemical compound C1[C@@H](O)[C@H](O)[C@@](C)(CO)[C@@H]2[C@H](O)C[C@@]3(C)[C@]4(C)CC[C@@]5(C(O)=O)CCC(C)(C)C[C@H]5C4=CC[C@@H]3[C@]21C IDQVFXZQPGAVAM-GGVBUJAJSA-N 0.000 description 9
- 235000013399 edible fruits Nutrition 0.000 description 9
- PRAUVHZJPXOEIF-AOLYGAPISA-N madecassic acid Chemical compound C1[C@@H](O)[C@H](O)[C@@](C)(CO)[C@@H]2[C@H](O)C[C@@]3(C)[C@]4(C)CC[C@@]5(C(O)=O)CC[C@@H](C)[C@H](C)[C@H]5C4=CC[C@@H]3[C@]21C PRAUVHZJPXOEIF-AOLYGAPISA-N 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- IDQVFXZQPGAVAM-UHFFFAOYSA-N (2alpha,3beta,6beta)-2,3,6,23-Tetrahydroxy-12-oleanen-28-oic acid Natural products C1C(O)C(O)C(C)(CO)C2C(O)CC3(C)C4(C)CCC5(C(O)=O)CCC(C)(C)CC5C4=CCC3C21C IDQVFXZQPGAVAM-UHFFFAOYSA-N 0.000 description 8
- 210000004027 cell Anatomy 0.000 description 8
- 229940011656 madecassic acid Drugs 0.000 description 8
- BUWCHLVSSFQLPN-UHFFFAOYSA-N madecassic acid Natural products CC1CCC2(CCC3(C)C(=CCC4C5(C)CC(O)C(O)C(C)(C5CCC34C)C(=O)O)C2C1C)C(=O)OC6OC(COC7OC(CO)C(OC8OC(C)C(O)C(O)C8O)C(O)C7O)C(O)C(O)C6O BUWCHLVSSFQLPN-UHFFFAOYSA-N 0.000 description 8
- 235000014633 carbohydrates Nutrition 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- GWCRPYGYVRXVLI-UHFFFAOYSA-N 2-ethyl-4-hydroxy-5-methyl-3(2H)-furanone Chemical compound CCC1OC(C)=C(O)C1=O GWCRPYGYVRXVLI-UHFFFAOYSA-N 0.000 description 6
- 241000588724 Escherichia coli Species 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 229930193140 Neomycin Natural products 0.000 description 6
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 6
- 229960004927 neomycin Drugs 0.000 description 6
- 229920000642 polymer Polymers 0.000 description 6
- 229930182478 glucoside Natural products 0.000 description 5
- 150000008131 glucosides Chemical class 0.000 description 5
- 150000004676 glycans Chemical class 0.000 description 5
- 150000002482 oligosaccharides Polymers 0.000 description 5
- 229920001282 polysaccharide Polymers 0.000 description 5
- 239000005017 polysaccharide Substances 0.000 description 5
- 230000009466 transformation Effects 0.000 description 5
- 150000004043 trisaccharides Chemical class 0.000 description 5
- 235000013311 vegetables Nutrition 0.000 description 5
- WRMNZCZEMHIOCP-UHFFFAOYSA-N 2-phenylethanol Chemical compound OCCC1=CC=CC=C1 WRMNZCZEMHIOCP-UHFFFAOYSA-N 0.000 description 4
- GLZPCOQZEFWAFX-UHFFFAOYSA-N Geraniol Chemical compound CC(C)=CCCC(C)=CCO GLZPCOQZEFWAFX-UHFFFAOYSA-N 0.000 description 4
- WYQVAPGDARQUBT-FGWHUCSPSA-N Madecassol Chemical compound O([C@@H]1[C@@H](CO)O[C@H]([C@@H]([C@H]1O)O)OC[C@H]1O[C@H]([C@@H]([C@@H](O)[C@@H]1O)O)OC(=O)[C@]12CC[C@H]([C@@H]([C@H]1C=1[C@@]([C@@]3(CC[C@H]4[C@](C)(CO)[C@@H](O)[C@H](O)C[C@]4(C)[C@H]3CC=1)C)(C)CC2)C)C)[C@@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O WYQVAPGDARQUBT-FGWHUCSPSA-N 0.000 description 4
- 239000007983 Tris buffer Substances 0.000 description 4
- 229940022757 asiaticoside Drugs 0.000 description 4
- WYQVAPGDARQUBT-XCWYDTOWSA-N asiaticoside Natural products O=C(O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@H]2[C@H](O)[C@H](O)[C@H](O[C@H]3[C@H](O)[C@H](O)[C@@H](O)[C@H](C)O3)[C@@H](CO)O2)O1)[C@@]12[C@@H]([C@@H](C)[C@H](C)CC1)C=1[C@](C)([C@@]3(C)[C@@H]([C@@]4(C)[C@H]([C@@](CO)(C)[C@@H](O)[C@H](O)C4)CC3)CC=1)CC2 WYQVAPGDARQUBT-XCWYDTOWSA-N 0.000 description 4
- XUPYJHCZDLZNFP-UHFFFAOYSA-N butyl butanoate Chemical compound CCCCOC(=O)CCC XUPYJHCZDLZNFP-UHFFFAOYSA-N 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 239000008367 deionised water Substances 0.000 description 4
- 229940079919 digestives enzyme preparation Drugs 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- IFYYFLINQYPWGJ-UHFFFAOYSA-N gamma-decalactone Chemical compound CCCCCCC1CCC(=O)O1 IFYYFLINQYPWGJ-UHFFFAOYSA-N 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- KVWWIYGFBYDJQC-UHFFFAOYSA-N methyl dihydrojasmonate Chemical compound CCCCCC1C(CC(=O)OC)CCC1=O KVWWIYGFBYDJQC-UHFFFAOYSA-N 0.000 description 4
- 239000008188 pellet Substances 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- UNYNVICDCJHOPO-UHFFFAOYSA-N sotolone Chemical compound CC1OC(=O)C(O)=C1C UNYNVICDCJHOPO-UHFFFAOYSA-N 0.000 description 4
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 4
- PHXATPHONSXBIL-UHFFFAOYSA-N xi-gamma-Undecalactone Chemical compound CCCCCCCC1CCC(=O)O1 PHXATPHONSXBIL-UHFFFAOYSA-N 0.000 description 4
- PSQYTAPXSHCGMF-BQYQJAHWSA-N β-ionone Chemical compound CC(=O)\C=C\C1=C(C)CCCC1(C)C PSQYTAPXSHCGMF-BQYQJAHWSA-N 0.000 description 4
- XPCTZQVDEJYUGT-UHFFFAOYSA-N 3-hydroxy-2-methyl-4-pyrone Chemical compound CC=1OC=CC(=O)C=1O XPCTZQVDEJYUGT-UHFFFAOYSA-N 0.000 description 3
- INAXVXBDKKUCGI-UHFFFAOYSA-N 4-hydroxy-2,5-dimethylfuran-3-one Chemical compound CC1OC(C)=C(O)C1=O INAXVXBDKKUCGI-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 241000194108 Bacillus licheniformis Species 0.000 description 3
- 244000299461 Theobroma cacao Species 0.000 description 3
- 150000001299 aldehydes Chemical class 0.000 description 3
- JXSVIVRDWWRQRT-UYDOISQJSA-N asiatic acid Chemical compound C1[C@@H](O)[C@H](O)[C@@](C)(CO)[C@@H]2CC[C@@]3(C)[C@]4(C)CC[C@@]5(C(O)=O)CC[C@@H](C)[C@H](C)[C@H]5C4=CC[C@@H]3[C@]21C JXSVIVRDWWRQRT-UYDOISQJSA-N 0.000 description 3
- 229940011658 asiatic acid Drugs 0.000 description 3
- LBGFKBYMNRAMFC-PYSQTNCISA-N asiatic acid Natural products C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)C[C@@H](O)[C@H](O)[C@@](C)(CO)[C@@H]5CC[C@@]34C)[C@]2(C)[C@H]1C)C(=O)O LBGFKBYMNRAMFC-PYSQTNCISA-N 0.000 description 3
- 230000036983 biotransformation Effects 0.000 description 3
- 239000013592 cell lysate Substances 0.000 description 3
- 230000006037 cell lysis Effects 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 235000013601 eggs Nutrition 0.000 description 3
- 230000002255 enzymatic effect Effects 0.000 description 3
- CLXOLTFMHAXJST-UHFFFAOYSA-N esculentic acid Natural products C12CC=C3C4CC(C)(C(O)=O)CCC4(C(O)=O)CCC3(C)C1(C)CCC1C2(C)CCC(O)C1(CO)C CLXOLTFMHAXJST-UHFFFAOYSA-N 0.000 description 3
- 239000012634 fragment Substances 0.000 description 3
- HIGQPQRQIQDZMP-UHFFFAOYSA-N geranil acetate Natural products CC(C)=CCCC(C)=CCOC(C)=O HIGQPQRQIQDZMP-UHFFFAOYSA-N 0.000 description 3
- HIGQPQRQIQDZMP-DHZHZOJOSA-N geranyl acetate Chemical compound CC(C)=CCC\C(C)=C\COC(C)=O HIGQPQRQIQDZMP-DHZHZOJOSA-N 0.000 description 3
- 238000006206 glycosylation reaction Methods 0.000 description 3
- 238000004255 ion exchange chromatography Methods 0.000 description 3
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 3
- SATCULPHIDQDRE-UHFFFAOYSA-N piperonal Chemical compound O=CC1=CC=C2OCOC2=C1 SATCULPHIDQDRE-UHFFFAOYSA-N 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 235000014347 soups Nutrition 0.000 description 3
- 150000008163 sugars Chemical class 0.000 description 3
- 150000003648 triterpenes Chemical class 0.000 description 3
- 238000002604 ultrasonography Methods 0.000 description 3
- SFEOKXHPFMOVRM-UHFFFAOYSA-N (+)-(S)-gamma-ionone Natural products CC(=O)C=CC1C(=C)CCCC1(C)C SFEOKXHPFMOVRM-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-SVZMEOIVSA-N (+)-Galactose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-SVZMEOIVSA-N 0.000 description 2
- MBDOYVRWFFCFHM-SNAWJCMRSA-N (2E)-hexenal Chemical compound CCC\C=C\C=O MBDOYVRWFFCFHM-SNAWJCMRSA-N 0.000 description 2
- JZQKTMZYLHNFPL-BLHCBFLLSA-N (2E,4E)-deca-2,4-dienal Chemical compound CCCCC\C=C\C=C\C=O JZQKTMZYLHNFPL-BLHCBFLLSA-N 0.000 description 2
- ZHHYXNZJDGDGPJ-BSWSSELBSA-N (2e,4e)-nona-2,4-dienal Chemical compound CCCC\C=C\C=C\C=O ZHHYXNZJDGDGPJ-BSWSSELBSA-N 0.000 description 2
- BSAIUMLZVGUGKX-BQYQJAHWSA-N (E)-non-2-enal Chemical compound CCCCCC\C=C\C=O BSAIUMLZVGUGKX-BQYQJAHWSA-N 0.000 description 2
- LVBXEMGDVWVTGY-VOTSOKGWSA-N (E)-oct-2-enal Chemical compound CCCCC\C=C\C=O LVBXEMGDVWVTGY-VOTSOKGWSA-N 0.000 description 2
- AROCNZZBLCAOPH-UHFFFAOYSA-N 1-methyl-4-prop-2-enoxybenzene Chemical compound CC1=CC=C(OCC=C)C=C1 AROCNZZBLCAOPH-UHFFFAOYSA-N 0.000 description 2
- JLIDVCMBCGBIEY-UHFFFAOYSA-N 1-penten-3-one Chemical compound CCC(=O)C=C JLIDVCMBCGBIEY-UHFFFAOYSA-N 0.000 description 2
- NCPWFIVLKCFWSP-BSWSSELBSA-N 2,4-Nonadien-1-ol Chemical compound CCCC\C=C\C=C\CO NCPWFIVLKCFWSP-BSWSSELBSA-N 0.000 description 2
- FZOZFDAMVVEZSJ-UHFFFAOYSA-N 2-Acetyl-4,5-dihydrothiazole Chemical compound CC(=O)C1=NCCS1 FZOZFDAMVVEZSJ-UHFFFAOYSA-N 0.000 description 2
- JZBCTZLGKSYRSF-UHFFFAOYSA-N 2-Ethyl-3,5-dimethylpyrazine Chemical compound CCC1=NC=C(C)N=C1C JZBCTZLGKSYRSF-UHFFFAOYSA-N 0.000 description 2
- LNIMMWYNSBZESE-UHFFFAOYSA-N 2-Ethyl-3-methylpyrazine, 9CI Chemical compound CCC1=NC=CN=C1C LNIMMWYNSBZESE-UHFFFAOYSA-N 0.000 description 2
- DBZAKQWXICEWNW-UHFFFAOYSA-N 2-acetylpyrazine Chemical compound CC(=O)C1=CN=CC=N1 DBZAKQWXICEWNW-UHFFFAOYSA-N 0.000 description 2
- WHMWOHBXYIZFPF-UHFFFAOYSA-N 2-ethyl-3,(5 or 6)-dimethylpyrazine Chemical compound CCC1=NC(C)=CN=C1C WHMWOHBXYIZFPF-UHFFFAOYSA-N 0.000 description 2
- HCFAJYNVAYBARA-UHFFFAOYSA-N 4-heptanone Chemical compound CCCC(=O)CCC HCFAJYNVAYBARA-UHFFFAOYSA-N 0.000 description 2
- QJYOEDXNPLUUAR-UHFFFAOYSA-N 4-hydroxy-2-methyl-5-ethyl-3-oxo-2H-furan Natural products CCC1=C(O)C(=O)C(C)O1 QJYOEDXNPLUUAR-UHFFFAOYSA-N 0.000 description 2
- JOOXCMJARBKPKM-UHFFFAOYSA-N 4-oxopentanoic acid Chemical compound CC(=O)CCC(O)=O JOOXCMJARBKPKM-UHFFFAOYSA-N 0.000 description 2
- OALYTRUKMRCXNH-UHFFFAOYSA-N 5-pentyloxolan-2-one Chemical compound CCCCCC1CCC(=O)O1 OALYTRUKMRCXNH-UHFFFAOYSA-N 0.000 description 2
- GHBSPIPJMLAMEP-UHFFFAOYSA-N 6-pentyloxan-2-one Chemical compound CCCCCC1CCCC(=O)O1 GHBSPIPJMLAMEP-UHFFFAOYSA-N 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Natural products CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- KWOLFJPFCHCOCG-UHFFFAOYSA-N Acetophenone Chemical compound CC(=O)C1=CC=CC=C1 KWOLFJPFCHCOCG-UHFFFAOYSA-N 0.000 description 2
- SCCDQYPEOIRVGX-UHFFFAOYSA-N Acetyleugenol Chemical compound COC1=CC(CC=C)=CC=C1OC(C)=O SCCDQYPEOIRVGX-UHFFFAOYSA-N 0.000 description 2
- 244000223760 Cinnamomum zeylanicum Species 0.000 description 2
- 102000002322 Egg Proteins Human genes 0.000 description 2
- 108010000912 Egg Proteins Proteins 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 description 2
- 241001646716 Escherichia coli K-12 Species 0.000 description 2
- WEEGYLXZBRQIMU-UHFFFAOYSA-N Eucalyptol Chemical compound C1CC2CCC1(C)OC2(C)C WEEGYLXZBRQIMU-UHFFFAOYSA-N 0.000 description 2
- 239000001329 FEMA 3811 Substances 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 239000006391 Luria-Bertani Medium Substances 0.000 description 2
- PBILBHLAPJTJOT-CQSZACIVSA-N Phyllodulcin Chemical compound C1=C(O)C(OC)=CC=C1[C@@H]1OC(=O)C2=C(O)C=CC=C2C1 PBILBHLAPJTJOT-CQSZACIVSA-N 0.000 description 2
- NBBJYMSMWIIQGU-UHFFFAOYSA-N Propionic aldehyde Chemical compound CCC=O NBBJYMSMWIIQGU-UHFFFAOYSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 108010073771 Soybean Proteins Proteins 0.000 description 2
- 241000186988 Streptomyces antibioticus Species 0.000 description 2
- MOYAFQVGZZPNRA-UHFFFAOYSA-N Terpinolene Chemical compound CC(C)=C1CCC(C)=CC1 MOYAFQVGZZPNRA-UHFFFAOYSA-N 0.000 description 2
- 244000269722 Thea sinensis Species 0.000 description 2
- 244000185386 Thladiantha grosvenorii Species 0.000 description 2
- 235000011171 Thladiantha grosvenorii Nutrition 0.000 description 2
- 239000005862 Whey Substances 0.000 description 2
- 102000007544 Whey Proteins Human genes 0.000 description 2
- 108010046377 Whey Proteins Proteins 0.000 description 2
- PYMYPHUHKUWMLA-LMVFSUKVSA-N aldehydo-D-ribose Chemical compound OC[C@@H](O)[C@@H](O)[C@@H](O)C=O PYMYPHUHKUWMLA-LMVFSUKVSA-N 0.000 description 2
- PNNNRSAQSRJVSB-BXKVDMCESA-N aldehydo-L-rhamnose Chemical compound C[C@H](O)[C@H](O)[C@@H](O)[C@@H](O)C=O PNNNRSAQSRJVSB-BXKVDMCESA-N 0.000 description 2
- 229940024606 amino acid Drugs 0.000 description 2
- HUMNYLRZRPPJDN-UHFFFAOYSA-N benzaldehyde Chemical compound O=CC1=CC=CC=C1 HUMNYLRZRPPJDN-UHFFFAOYSA-N 0.000 description 2
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical compound C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 description 2
- QUKGYYKBILRGFE-UHFFFAOYSA-N benzyl acetate Chemical compound CC(=O)OCC1=CC=CC=C1 QUKGYYKBILRGFE-UHFFFAOYSA-N 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 235000015155 buttermilk Nutrition 0.000 description 2
- CRPUJAZIXJMDBK-UHFFFAOYSA-N camphene Chemical compound C1CC2C(=C)C(C)(C)C1C2 CRPUJAZIXJMDBK-UHFFFAOYSA-N 0.000 description 2
- ULDHMXUKGWMISQ-UHFFFAOYSA-N carvone Chemical compound CC(=C)C1CC=C(C)C(=O)C1 ULDHMXUKGWMISQ-UHFFFAOYSA-N 0.000 description 2
- 235000019219 chocolate Nutrition 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 229960005233 cineole Drugs 0.000 description 2
- 235000017803 cinnamon Nutrition 0.000 description 2
- NEHNMFOYXAPHSD-UHFFFAOYSA-N citronellal Chemical compound O=CCC(C)CCC=C(C)C NEHNMFOYXAPHSD-UHFFFAOYSA-N 0.000 description 2
- QMVPMAAFGQKVCJ-UHFFFAOYSA-N citronellol Chemical compound OCCC(C)CCC=C(C)C QMVPMAAFGQKVCJ-UHFFFAOYSA-N 0.000 description 2
- JOZKFWLRHCDGJA-UHFFFAOYSA-N citronellol acetate Chemical compound CC(=O)OCCC(C)CCC=C(C)C JOZKFWLRHCDGJA-UHFFFAOYSA-N 0.000 description 2
- 235000009508 confectionery Nutrition 0.000 description 2
- 239000002537 cosmetic Substances 0.000 description 2
- ZYGHJZDHTFUPRJ-UHFFFAOYSA-N coumarin Chemical compound C1=CC=C2OC(=O)C=CC2=C1 ZYGHJZDHTFUPRJ-UHFFFAOYSA-N 0.000 description 2
- 239000012043 crude product Substances 0.000 description 2
- 235000013365 dairy product Nutrition 0.000 description 2
- UDGKKUWYNITJRX-UHFFFAOYSA-N davidigenin Chemical compound C1=CC(O)=CC=C1CCC(=O)C1=CC=C(O)C=C1O UDGKKUWYNITJRX-UHFFFAOYSA-N 0.000 description 2
- 229910021641 deionized water Inorganic materials 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- LZCLXQDLBQLTDK-UHFFFAOYSA-N ethyl 2-hydroxypropanoate Chemical compound CCOC(=O)C(C)O LZCLXQDLBQLTDK-UHFFFAOYSA-N 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- TVQGDYNRXLTQAP-UHFFFAOYSA-N ethyl heptanoate Chemical compound CCCCCCC(=O)OCC TVQGDYNRXLTQAP-UHFFFAOYSA-N 0.000 description 2
- SHZIWNPUGXLXDT-UHFFFAOYSA-N ethyl hexanoate Chemical compound CCCCCC(=O)OCC SHZIWNPUGXLXDT-UHFFFAOYSA-N 0.000 description 2
- WDAXFOBOLVPGLV-UHFFFAOYSA-N ethyl isobutyrate Chemical compound CCOC(=O)C(C)C WDAXFOBOLVPGLV-UHFFFAOYSA-N 0.000 description 2
- FKRCODPIKNYEAC-UHFFFAOYSA-N ethyl propionate Chemical compound CCOC(=O)CC FKRCODPIKNYEAC-UHFFFAOYSA-N 0.000 description 2
- RRAFCDWBNXTKKO-UHFFFAOYSA-N eugenol Chemical compound COC1=CC(CC=C)=CC=C1O RRAFCDWBNXTKKO-UHFFFAOYSA-N 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 235000011389 fruit/vegetable juice Nutrition 0.000 description 2
- IFYYFLINQYPWGJ-VIFPVBQESA-N gamma-Decalactone Natural products CCCCCC[C@H]1CCC(=O)O1 IFYYFLINQYPWGJ-VIFPVBQESA-N 0.000 description 2
- PHXATPHONSXBIL-JTQLQIEISA-N gamma-Undecalactone Natural products CCCCCCC[C@H]1CCC(=O)O1 PHXATPHONSXBIL-JTQLQIEISA-N 0.000 description 2
- 229940020436 gamma-undecalactone Drugs 0.000 description 2
- 230000013595 glycosylation Effects 0.000 description 2
- LPLVUJXQOOQHMX-QWBHMCJMSA-N glycyrrhizinic acid Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@H](O[C@@H]1O[C@@H]1C([C@H]2[C@]([C@@H]3[C@@]([C@@]4(CC[C@@]5(C)CC[C@@](C)(C[C@H]5C4=CC3=O)C(O)=O)C)(C)CC2)(C)CC1)(C)C)C(O)=O)[C@@H]1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O LPLVUJXQOOQHMX-QWBHMCJMSA-N 0.000 description 2
- CATSNJVOTSVZJV-UHFFFAOYSA-N heptan-2-one Chemical compound CCCCCC(C)=O CATSNJVOTSVZJV-UHFFFAOYSA-N 0.000 description 2
- NGAZZOYFWWSOGK-UHFFFAOYSA-N heptan-3-one Chemical compound CCCCC(=O)CC NGAZZOYFWWSOGK-UHFFFAOYSA-N 0.000 description 2
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 2
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 2
- MLFHJEHSLIIPHL-UHFFFAOYSA-N isoamyl acetate Chemical compound CC(C)CCOC(C)=O MLFHJEHSLIIPHL-UHFFFAOYSA-N 0.000 description 2
- PQLMXFQTAMDXIZ-UHFFFAOYSA-N isoamyl butyrate Chemical compound CCCC(=O)OCCC(C)C PQLMXFQTAMDXIZ-UHFFFAOYSA-N 0.000 description 2
- PHTQWCKDNZKARW-UHFFFAOYSA-N isoamylol Chemical compound CC(C)CCO PHTQWCKDNZKARW-UHFFFAOYSA-N 0.000 description 2
- RGFNRWTWDWVHDD-UHFFFAOYSA-N isobutyl butyrate Chemical compound CCCC(=O)OCC(C)C RGFNRWTWDWVHDD-UHFFFAOYSA-N 0.000 description 2
- NTOPKICPEQUPPH-UHFFFAOYSA-N isopropyl methoxy pyrazine Chemical compound COC1=NC=CN=C1C(C)C NTOPKICPEQUPPH-UHFFFAOYSA-N 0.000 description 2
- ZYTMANIQRDEHIO-KXUCPTDWSA-N isopulegol Chemical compound C[C@@H]1CC[C@@H](C(C)=C)[C@H](O)C1 ZYTMANIQRDEHIO-KXUCPTDWSA-N 0.000 description 2
- CDOSHBSSFJOMGT-UHFFFAOYSA-N linalool Chemical compound CC(C)=CCCC(C)(O)C=C CDOSHBSSFJOMGT-UHFFFAOYSA-N 0.000 description 2
- UWKAYLJWKGQEPM-LBPRGKRZSA-N linalyl acetate Chemical compound CC(C)=CCC[C@](C)(C=C)OC(C)=O UWKAYLJWKGQEPM-LBPRGKRZSA-N 0.000 description 2
- 235000013622 meat product Nutrition 0.000 description 2
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- VAMXMNNIEUEQDV-UHFFFAOYSA-N methyl anthranilate Chemical compound COC(=O)C1=CC=CC=C1N VAMXMNNIEUEQDV-UHFFFAOYSA-N 0.000 description 2
- 229930189775 mogroside Natural products 0.000 description 2
- ITVGXXMINPYUHD-CUVHLRMHSA-N neohesperidin dihydrochalcone Chemical compound C1=C(O)C(OC)=CC=C1CCC(=O)C(C(=C1)O)=C(O)C=C1O[C@H]1[C@H](O[C@H]2[C@@H]([C@H](O)[C@@H](O)[C@H](C)O2)O)[C@@H](O)[C@H](O)[C@@H](CO)O1 ITVGXXMINPYUHD-CUVHLRMHSA-N 0.000 description 2
- 229940089953 neohesperidin dihydrochalcone Drugs 0.000 description 2
- 235000010434 neohesperidine DC Nutrition 0.000 description 2
- 235000019520 non-alcoholic beverage Nutrition 0.000 description 2
- SJWFXCIHNDVPSH-UHFFFAOYSA-N octan-2-ol Chemical compound CCCCCCC(C)O SJWFXCIHNDVPSH-UHFFFAOYSA-N 0.000 description 2
- NMRPBPVERJPACX-UHFFFAOYSA-N octan-3-ol Chemical compound CCCCCC(O)CC NMRPBPVERJPACX-UHFFFAOYSA-N 0.000 description 2
- YLYBTZIQSIBWLI-UHFFFAOYSA-N octyl acetate Chemical compound CCCCCCCCOC(C)=O YLYBTZIQSIBWLI-UHFFFAOYSA-N 0.000 description 2
- VWMVAQHMFFZQGD-UHFFFAOYSA-N p-Hydroxybenzyl acetone Natural products CC(=O)CC1=CC=C(O)C=C1 VWMVAQHMFFZQGD-UHFFFAOYSA-N 0.000 description 2
- JYVLIDXNZAXMDK-UHFFFAOYSA-N pentan-2-ol Chemical compound CCCC(C)O JYVLIDXNZAXMDK-UHFFFAOYSA-N 0.000 description 2
- MDHYEMXUFSJLGV-UHFFFAOYSA-N phenethyl acetate Chemical compound CC(=O)OCCC1=CC=CC=C1 MDHYEMXUFSJLGV-UHFFFAOYSA-N 0.000 description 2
- DTUQWGWMVIHBKE-UHFFFAOYSA-N phenylacetaldehyde Chemical compound O=CCC1=CC=CC=C1 DTUQWGWMVIHBKE-UHFFFAOYSA-N 0.000 description 2
- 229940067107 phenylethyl alcohol Drugs 0.000 description 2
- VGEREEWJJVICBM-UHFFFAOYSA-N phloretin Chemical compound C1=CC(O)=CC=C1CCC(=O)C1=C(O)C=C(O)C=C1O VGEREEWJJVICBM-UHFFFAOYSA-N 0.000 description 2
- 229920005990 polystyrene resin Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- HUAZGNHGCJGYNP-UHFFFAOYSA-N propyl butyrate Chemical compound CCCOC(=O)CCC HUAZGNHGCJGYNP-UHFFFAOYSA-N 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 239000012264 purified product Substances 0.000 description 2
- NJGBTKGETPDVIK-UHFFFAOYSA-N raspberry ketone Chemical compound CC(=O)CCC1=CC=C(O)C=C1 NJGBTKGETPDVIK-UHFFFAOYSA-N 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 235000015067 sauces Nutrition 0.000 description 2
- 235000013580 sausages Nutrition 0.000 description 2
- 235000011888 snacks Nutrition 0.000 description 2
- 239000001509 sodium citrate Substances 0.000 description 2
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 2
- VWDWKYIASSYTQR-UHFFFAOYSA-N sodium nitrate Chemical compound [Na+].[O-][N+]([O-])=O VWDWKYIASSYTQR-UHFFFAOYSA-N 0.000 description 2
- 229940001941 soy protein Drugs 0.000 description 2
- 235000013599 spices Nutrition 0.000 description 2
- DSAJORLEPQBKDA-AWEZNQCLSA-N sterubin Chemical compound C1([C@@H]2CC(=O)C3=C(O)C=C(C=C3O2)OC)=CC=C(O)C(O)=C1 DSAJORLEPQBKDA-AWEZNQCLSA-N 0.000 description 2
- DSAJORLEPQBKDA-UHFFFAOYSA-N sterubin Natural products O1C2=CC(OC)=CC(O)=C2C(=O)CC1C1=CC=C(O)C(O)=C1 DSAJORLEPQBKDA-UHFFFAOYSA-N 0.000 description 2
- 235000019202 steviosides Nutrition 0.000 description 2
- 125000001424 substituent group Chemical group 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 235000013616 tea Nutrition 0.000 description 2
- MGSRCZKZVOBKFT-UHFFFAOYSA-N thymol Chemical compound CC(C)C1=CC=C(C)C=C1O MGSRCZKZVOBKFT-UHFFFAOYSA-N 0.000 description 2
- 235000019583 umami taste Nutrition 0.000 description 2
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 2
- MWOOGOJBHIARFG-UHFFFAOYSA-N vanillin Chemical compound COC1=CC(C=O)=CC=C1O MWOOGOJBHIARFG-UHFFFAOYSA-N 0.000 description 2
- 239000003643 water by type Substances 0.000 description 2
- NPNUFJAVOOONJE-ZIAGYGMSSA-N β-(E)-Caryophyllene Chemical compound C1CC(C)=CCCC(=C)[C@H]2CC(C)(C)[C@@H]21 NPNUFJAVOOONJE-ZIAGYGMSSA-N 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- NOOLISFMXDJSKH-UTLUCORTSA-N (+)-Neomenthol Chemical compound CC(C)[C@@H]1CC[C@@H](C)C[C@@H]1O NOOLISFMXDJSKH-UTLUCORTSA-N 0.000 description 1
- MHXCIKYXNYCMHY-AUSJPIAWSA-N (+)-lariciresinol Chemical compound C1=C(O)C(OC)=CC(C[C@@H]2[C@@H]([C@H](OC2)C=2C=C(OC)C(O)=CC=2)CO)=C1 MHXCIKYXNYCMHY-AUSJPIAWSA-N 0.000 description 1
- YGWKXXYGDYYFJU-SSDOTTSWSA-N (+)-menthofuran Chemical compound C1[C@H](C)CCC2=C1OC=C2C YGWKXXYGDYYFJU-SSDOTTSWSA-N 0.000 description 1
- WTOYNNBCKUYIKC-JMSVASOKSA-N (+)-nootkatone Chemical compound C1C[C@@H](C(C)=C)C[C@@]2(C)[C@H](C)CC(=O)C=C21 WTOYNNBCKUYIKC-JMSVASOKSA-N 0.000 description 1
- NZGWDASTMWDZIW-MRVPVSSYSA-N (+)-pulegone Chemical compound C[C@@H]1CCC(=C(C)C)C(=O)C1 NZGWDASTMWDZIW-MRVPVSSYSA-N 0.000 description 1
- QEBNYNLSCGVZOH-NFAWXSAZSA-N (+)-valencene Chemical compound C1C[C@@H](C(C)=C)C[C@@]2(C)[C@H](C)CCC=C21 QEBNYNLSCGVZOH-NFAWXSAZSA-N 0.000 description 1
- MATGKVZWFZHCLI-LSDHHAIUSA-N (-)-matairesinol Chemical compound C1=C(O)C(OC)=CC(C[C@@H]2[C@H](C(=O)OC2)CC=2C=C(OC)C(O)=CC=2)=C1 MATGKVZWFZHCLI-LSDHHAIUSA-N 0.000 description 1
- GEWDNTWNSAZUDX-WQMVXFAESA-N (-)-methyl jasmonate Chemical compound CC\C=C/C[C@@H]1[C@@H](CC(=O)OC)CCC1=O GEWDNTWNSAZUDX-WQMVXFAESA-N 0.000 description 1
- RMLYXMMBIZLGAQ-UHFFFAOYSA-N (-)-monatin Natural products C1=CC=C2C(CC(O)(CC(N)C(O)=O)C(O)=O)=CNC2=C1 RMLYXMMBIZLGAQ-UHFFFAOYSA-N 0.000 description 1
- 239000001871 (1R,2R,5S)-5-methyl-2-prop-1-en-2-ylcyclohexan-1-ol Substances 0.000 description 1
- NIONDZDPPYHYKY-SNAWJCMRSA-N (2E)-hexenoic acid Chemical compound CCC\C=C\C(O)=O NIONDZDPPYHYKY-SNAWJCMRSA-N 0.000 description 1
- AMXYRHBJZOVHOL-UHFFFAOYSA-N (2E,6E)-2,6-Nonadien-1-ol Natural products CCC=CCCC=CCO AMXYRHBJZOVHOL-UHFFFAOYSA-N 0.000 description 1
- 239000001890 (2R)-8,8,8a-trimethyl-2-prop-1-en-2-yl-1,2,3,4,6,7-hexahydronaphthalene Substances 0.000 description 1
- AMXYRHBJZOVHOL-DYWGDJMRSA-N (2e,6e)-nona-2,6-dien-1-ol Chemical compound CC\C=C\CC\C=C\CO AMXYRHBJZOVHOL-DYWGDJMRSA-N 0.000 description 1
- VYPONAGZHAJHGT-ZBJFTSOASA-N (2r)-2-[(z)-pent-2-enyl]-2,3-dihydropyran-6-one Chemical compound CC\C=C/C[C@@H]1CC=CC(=O)O1 VYPONAGZHAJHGT-ZBJFTSOASA-N 0.000 description 1
- NEDIAPMWNCQWNW-SECBINFHSA-N (2r)-2-pentyl-2,3-dihydropyran-6-one Chemical compound CCCCC[C@@H]1CC=CC(=O)O1 NEDIAPMWNCQWNW-SECBINFHSA-N 0.000 description 1
- SDOFMBGMRVAJNF-KVTDHHQDSA-N (2r,3r,4r,5r)-6-aminohexane-1,2,3,4,5-pentol Chemical compound NC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO SDOFMBGMRVAJNF-KVTDHHQDSA-N 0.000 description 1
- RMLYXMMBIZLGAQ-HZMBPMFUSA-N (2s,4s)-4-amino-2-hydroxy-2-(1h-indol-3-ylmethyl)pentanedioic acid Chemical compound C1=CC=C2C(C[C@](O)(C[C@H](N)C(O)=O)C(O)=O)=CNC2=C1 RMLYXMMBIZLGAQ-HZMBPMFUSA-N 0.000 description 1
- 239000001490 (3R)-3,7-dimethylocta-1,6-dien-3-ol Substances 0.000 description 1
- 239000001082 (3R)-3-sulfanylpentan-2-one Substances 0.000 description 1
- NUFKRGBSZPCGQB-FLBSXDLDSA-N (3s)-3-amino-4-oxo-4-[[(2r)-1-oxo-1-[(2,2,4,4-tetramethylthietan-3-yl)amino]propan-2-yl]amino]butanoic acid;pentahydrate Chemical compound O.O.O.O.O.OC(=O)C[C@H](N)C(=O)N[C@H](C)C(=O)NC1C(C)(C)SC1(C)C.OC(=O)C[C@H](N)C(=O)N[C@H](C)C(=O)NC1C(C)(C)SC1(C)C NUFKRGBSZPCGQB-FLBSXDLDSA-N 0.000 description 1
- 229940098795 (3z)- 3-hexenyl acetate Drugs 0.000 description 1
- VUDZSIYXZUYWSC-DBRKOABJSA-N (4r)-1-[(2r,4r,5r)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-hydroxy-1,3-diazinan-2-one Chemical compound FC1(F)[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)N[C@H](O)CC1 VUDZSIYXZUYWSC-DBRKOABJSA-N 0.000 description 1
- 239000001730 (5R)-5-butyloxolan-2-one Substances 0.000 description 1
- 239000001745 (6R)-3,6-dimethyl-4,5,6,7-tetrahydro-1-benzofuran Substances 0.000 description 1
- ZWTDXYUDJYDHJR-UHFFFAOYSA-N (E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl)-2-propen-1-one Natural products OC1=CC(O)=CC=C1C=CC(=O)C1=CC=C(O)C=C1O ZWTDXYUDJYDHJR-UHFFFAOYSA-N 0.000 description 1
- YDXQPTHHAPCTPP-AATRIKPKSA-N (E)-3-Octen-1-ol Chemical compound CCCC\C=C\CCO YDXQPTHHAPCTPP-AATRIKPKSA-N 0.000 description 1
- NDFKTBCGKNOHPJ-AATRIKPKSA-N (E)-hept-2-enal Chemical compound CCCC\C=C\C=O NDFKTBCGKNOHPJ-AATRIKPKSA-N 0.000 description 1
- QMVPMAAFGQKVCJ-SNVBAGLBSA-N (R)-(+)-citronellol Natural products OCC[C@H](C)CCC=C(C)C QMVPMAAFGQKVCJ-SNVBAGLBSA-N 0.000 description 1
- CDOSHBSSFJOMGT-JTQLQIEISA-N (R)-linalool Natural products CC(C)=CCC[C@@](C)(O)C=C CDOSHBSSFJOMGT-JTQLQIEISA-N 0.000 description 1
- MIEKOFWWHVOKQX-UHFFFAOYSA-N (S)-2-(4-Methoxyphenoxy)propanoic acid Chemical class COC1=CC=C(OC(C)C(O)=O)C=C1 MIEKOFWWHVOKQX-UHFFFAOYSA-N 0.000 description 1
- VIQXICKUKPVFRK-UHFFFAOYSA-N (S)-3-Methylthiohexyl acetate Chemical compound CCCC(SC)CCOC(C)=O VIQXICKUKPVFRK-UHFFFAOYSA-N 0.000 description 1
- WMBOCUXXNSOQHM-FLIBITNWSA-N (Z)-3-butylidenephthalide Chemical compound C1=CC=C2C(=C/CCC)/OC(=O)C2=C1 WMBOCUXXNSOQHM-FLIBITNWSA-N 0.000 description 1
- UFLHIIWVXFIJGU-ARJAWSKDSA-N (Z)-hex-3-en-1-ol Chemical compound CC\C=C/CCO UFLHIIWVXFIJGU-ARJAWSKDSA-N 0.000 description 1
- RLWJIETUBNSFMD-VMPITWQZSA-N (e)-n-[2-(1,3-benzodioxol-5-yl)ethyl]-3-(3,4-dimethoxyphenyl)prop-2-enamide Chemical compound C1=C(OC)C(OC)=CC=C1\C=C\C(=O)NCCC1=CC=C(OCO2)C2=C1 RLWJIETUBNSFMD-VMPITWQZSA-N 0.000 description 1
- VVGOCOMZRGWHPI-ARJAWSKDSA-N (z)-4-heptenal Chemical compound CC\C=C/CCC=O VVGOCOMZRGWHPI-ARJAWSKDSA-N 0.000 description 1
- YGFGZTXGYTUXBA-UHFFFAOYSA-N (±)-2,6-dimethyl-5-heptenal Chemical compound O=CC(C)CCC=C(C)C YGFGZTXGYTUXBA-UHFFFAOYSA-N 0.000 description 1
- HNAGHMKIPMKKBB-UHFFFAOYSA-N 1-benzylpyrrolidine-3-carboxamide Chemical compound C1C(C(=O)N)CCN1CC1=CC=CC=C1 HNAGHMKIPMKKBB-UHFFFAOYSA-N 0.000 description 1
- PZNPLUBHRSSFHT-RRHRGVEJSA-N 1-hexadecanoyl-2-octadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[C@@H](COP([O-])(=O)OCC[N+](C)(C)C)COC(=O)CCCCCCCCCCCCCCC PZNPLUBHRSSFHT-RRHRGVEJSA-N 0.000 description 1
- 239000001169 1-methyl-4-propan-2-ylcyclohexa-1,4-diene Substances 0.000 description 1
- OQWNKUAZQSLNSR-UHFFFAOYSA-N 12-methyltridecanal Chemical compound CC(C)CCCCCCCCCCC=O OQWNKUAZQSLNSR-UHFFFAOYSA-N 0.000 description 1
- AEQDJSLRWYMAQI-UHFFFAOYSA-N 2,3,9,10-tetramethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[2,1-b]isoquinoline Chemical compound C1CN2CC(C(=C(OC)C=C3)OC)=C3CC2C2=C1C=C(OC)C(OC)=C2 AEQDJSLRWYMAQI-UHFFFAOYSA-N 0.000 description 1
- PSINWXIDJYEXLO-UHFFFAOYSA-N 2,3-Diethyl-5-methylpyrazine Chemical compound CCC1=NC=C(C)N=C1CC PSINWXIDJYEXLO-UHFFFAOYSA-N 0.000 description 1
- BAMPVSWRQZNDQC-UHFFFAOYSA-N 2,4,5-trimethylthiazole Chemical compound CC1=NC(C)=C(C)S1 BAMPVSWRQZNDQC-UHFFFAOYSA-N 0.000 description 1
- ZHHYXNZJDGDGPJ-UHFFFAOYSA-N 2,4-Nonadienal Natural products CCCCC=CC=CC=O ZHHYXNZJDGDGPJ-UHFFFAOYSA-N 0.000 description 1
- DBBHCZMXKBCICL-UHFFFAOYSA-N 2,5-dimethylfuran-3-thiol Chemical compound CC1=CC(S)=C(C)O1 DBBHCZMXKBCICL-UHFFFAOYSA-N 0.000 description 1
- 239000001278 2-(5-ethenyl-5-methyloxolan-2-yl)propan-2-ol Substances 0.000 description 1
- MOMFXATYAINJML-UHFFFAOYSA-N 2-Acetylthiazole Chemical compound CC(=O)C1=NC=CS1 MOMFXATYAINJML-UHFFFAOYSA-N 0.000 description 1
- OXQGTIUCKGYOAA-UHFFFAOYSA-N 2-Ethylbutanoic acid Chemical compound CCC(CC)C(O)=O OXQGTIUCKGYOAA-UHFFFAOYSA-N 0.000 description 1
- RUYNUXHHUVUINQ-UHFFFAOYSA-N 2-Methyl-3-furanthiol Chemical compound CC=1OC=CC=1S RUYNUXHHUVUINQ-UHFFFAOYSA-N 0.000 description 1
- HSDXVAOHEOSTFZ-UHFFFAOYSA-N 2-Pentylpyridine Chemical compound CCCCCC1=CC=CC=N1 HSDXVAOHEOSTFZ-UHFFFAOYSA-N 0.000 description 1
- RCSBILYQLVXLJG-UHFFFAOYSA-N 2-Propenyl hexanoate Chemical compound CCCCCC(=O)OCC=C RCSBILYQLVXLJG-UHFFFAOYSA-N 0.000 description 1
- QMIBAVZANYVPEF-UHFFFAOYSA-N 2-[[(benzhydrylamino)-(3,5-dichloroanilino)methylidene]amino]acetic acid Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)N=C(NCC(=O)O)NC1=CC(Cl)=CC(Cl)=C1 QMIBAVZANYVPEF-UHFFFAOYSA-N 0.000 description 1
- DQBQWWSFRPLIAX-UHFFFAOYSA-N 2-acetyl-1-pyrroline Chemical compound CC(=O)C1=NCCC1 DQBQWWSFRPLIAX-UHFFFAOYSA-N 0.000 description 1
- SVONRAPFKPVNKG-UHFFFAOYSA-N 2-ethoxyethyl acetate Chemical compound CCOCCOC(C)=O SVONRAPFKPVNKG-UHFFFAOYSA-N 0.000 description 1
- 239000001363 2-ethyl-3,5-dimethylpyrazine Substances 0.000 description 1
- KXYCNEIORCQEFU-UHFFFAOYSA-N 2-ethyl-4-(hydroxymethyl)furan-3-one Chemical compound CCC1OC=C(CO)C1=O KXYCNEIORCQEFU-UHFFFAOYSA-N 0.000 description 1
- 239000001487 2-ethyl-4-hydroxy-5-methylfuran-3-one Substances 0.000 description 1
- ABFVFIZSXKRBRL-UHFFFAOYSA-N 2-hydroxy-2-phenyl-3h-chromen-4-one Chemical class C1C(=O)C2=CC=CC=C2OC1(O)C1=CC=CC=C1 ABFVFIZSXKRBRL-UHFFFAOYSA-N 0.000 description 1
- CFAKWWQIUFSQFU-UHFFFAOYSA-N 2-hydroxy-3-methylcyclopent-2-en-1-one Chemical compound CC1=C(O)C(=O)CC1 CFAKWWQIUFSQFU-UHFFFAOYSA-N 0.000 description 1
- OFLXNHNYPQPQKW-UHFFFAOYSA-N 2-isopropyl-4-methylthiazole Chemical compound CC(C)C1=NC(C)=CS1 OFLXNHNYPQPQKW-UHFFFAOYSA-N 0.000 description 1
- OQVAOEIMSKZGAL-UHFFFAOYSA-N 2-methyl-3-methylsulfanylfuran Chemical compound CSC=1C=COC=1C OQVAOEIMSKZGAL-UHFFFAOYSA-N 0.000 description 1
- XHIUFYZDQBSEMF-UHFFFAOYSA-N 2-methylbutyl acetate Chemical compound CCC(C)COC(C)=O XHIUFYZDQBSEMF-UHFFFAOYSA-N 0.000 description 1
- WLAMNBDJUVNPJU-UHFFFAOYSA-N 2-methylbutyric acid Chemical compound CCC(C)C(O)=O WLAMNBDJUVNPJU-UHFFFAOYSA-N 0.000 description 1
- VMUXSMXIQBNMGZ-UHFFFAOYSA-N 3,4-dihydrocoumarin Chemical compound C1=CC=C2OC(=O)CCC2=C1 VMUXSMXIQBNMGZ-UHFFFAOYSA-N 0.000 description 1
- SLAOFBPSPQHAJS-UHFFFAOYSA-N 3-(4-hydroxycyclohexa-2,4-dien-1-yl)-1-phenylprop-2-en-1-one Chemical class C1=CC(O)=CCC1C=CC(=O)C1=CC=CC=C1 SLAOFBPSPQHAJS-UHFFFAOYSA-N 0.000 description 1
- SZECUQRKLXRGSJ-UHFFFAOYSA-N 3-Mercapto-2-pentanone Chemical compound CCC(S)C(C)=O SZECUQRKLXRGSJ-UHFFFAOYSA-N 0.000 description 1
- NMRPBPVERJPACX-QMMMGPOBSA-N 3-Octanol Natural products CCCCC[C@@H](O)CC NMRPBPVERJPACX-QMMMGPOBSA-N 0.000 description 1
- STZUZYMKSMSTOU-UHFFFAOYSA-N 3-Octyl acetate Chemical compound CCCCCC(CC)OC(C)=O STZUZYMKSMSTOU-UHFFFAOYSA-N 0.000 description 1
- RLPQPHISCURSNX-UHFFFAOYSA-N 3-ethylfuran-2-ol Chemical compound CCC=1C=COC=1O RLPQPHISCURSNX-UHFFFAOYSA-N 0.000 description 1
- CLUWOWRTHNNBBU-UHFFFAOYSA-N 3-methylthiopropanal Chemical compound CSCCC=O CLUWOWRTHNNBBU-UHFFFAOYSA-N 0.000 description 1
- PBILBHLAPJTJOT-UHFFFAOYSA-N 3S-phyllodulcin Natural products C1=C(O)C(OC)=CC=C1C1OC(=O)C2=C(O)C=CC=C2C1 PBILBHLAPJTJOT-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- CYDQOEWLBCCFJZ-UHFFFAOYSA-N 4-(4-fluorophenyl)oxane-4-carboxylic acid Chemical compound C=1C=C(F)C=CC=1C1(C(=O)O)CCOCC1 CYDQOEWLBCCFJZ-UHFFFAOYSA-N 0.000 description 1
- HFNGYHHRRMSKEU-UHFFFAOYSA-N 4-Methoxybenzyl acetate Chemical compound COC1=CC=C(COC(C)=O)C=C1 HFNGYHHRRMSKEU-UHFFFAOYSA-N 0.000 description 1
- XPDORSROGAZEGY-UHFFFAOYSA-N 4-Methoxybenzyl formate Chemical compound COC1=CC=C(COC=O)C=C1 XPDORSROGAZEGY-UHFFFAOYSA-N 0.000 description 1
- OSFGNTLIOUHOKN-UHFFFAOYSA-N 4-[benzyl(methyl)sulfamoyl]benzoic acid Chemical compound C=1C=C(C(O)=O)C=CC=1S(=O)(=O)N(C)CC1=CC=CC=C1 OSFGNTLIOUHOKN-UHFFFAOYSA-N 0.000 description 1
- QCVGEOXPDFCNHA-UHFFFAOYSA-N 5,5-dimethyl-2,4-dioxo-1,3-oxazolidine-3-carboxamide Chemical compound CC1(C)OC(=O)N(C(N)=O)C1=O QCVGEOXPDFCNHA-UHFFFAOYSA-N 0.000 description 1
- BKAWJIRCKVUVED-UHFFFAOYSA-N 5-(2-hydroxyethyl)-4-methylthiazole Chemical compound CC=1N=CSC=1CCO BKAWJIRCKVUVED-UHFFFAOYSA-N 0.000 description 1
- DALUCNUGMDNXET-UHFFFAOYSA-N 5-Ethyl-2,4-dimethylthiazole Chemical compound CCC=1SC(C)=NC=1C DALUCNUGMDNXET-UHFFFAOYSA-N 0.000 description 1
- IUFQZPBIRYFPFD-UHFFFAOYSA-N 5-ethyl-3-hydroxy-4-methyl-2(5H)-furanone Chemical compound CCC1OC(=O)C(O)=C1C IUFQZPBIRYFPFD-UHFFFAOYSA-N 0.000 description 1
- OUDFNZMQXZILJD-UHFFFAOYSA-N 5-methyl-2-furaldehyde Chemical compound CC1=CC=C(C=O)O1 OUDFNZMQXZILJD-UHFFFAOYSA-N 0.000 description 1
- JGVWYJDASSSGEK-UHFFFAOYSA-N 5-methyl-2-propan-2-ylidenecyclohexan-1-ol Chemical compound CC1CCC(=C(C)C)C(O)C1 JGVWYJDASSSGEK-UHFFFAOYSA-N 0.000 description 1
- YZRXRLLRSPQHDK-UHFFFAOYSA-N 6-Hexyltetrahydro-2H-pyran-2-one Chemical compound CCCCCCC1CCCC(=O)O1 YZRXRLLRSPQHDK-UHFFFAOYSA-N 0.000 description 1
- PVXPPJIGRGXGCY-DJHAAKORSA-N 6-O-alpha-D-glucopyranosyl-alpha-D-fructofuranose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@](O)(CO)O1 PVXPPJIGRGXGCY-DJHAAKORSA-N 0.000 description 1
- 239000001606 7-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-5-hydroxy-2-(4-hydroxyphenyl)chroman-4-one Substances 0.000 description 1
- LDIOUQIXNSSOGU-UHFFFAOYSA-N 8-(3-pentylamino)-2-methyl-3-(2-chloro-4-methoxyphenyl)-6,7-dihydro-5h-cyclopenta[d]pyrazolo[1,5-a]pyrimidine Chemical compound CC1=NN2C(NC(CC)CC)=C3CCCC3=NC2=C1C1=CC=C(OC)C=C1Cl LDIOUQIXNSSOGU-UHFFFAOYSA-N 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- NVEQFIOZRFFVFW-UHFFFAOYSA-N 9-epi-beta-caryophyllene oxide Natural products C=C1CCC2OC2(C)CCC2C(C)(C)CC21 NVEQFIOZRFFVFW-UHFFFAOYSA-N 0.000 description 1
- WBZFUFAFFUEMEI-UHFFFAOYSA-M Acesulfame k Chemical compound [K+].CC1=CC(=O)[N-]S(=O)(=O)O1 WBZFUFAFFUEMEI-UHFFFAOYSA-M 0.000 description 1
- ROWKJAVDOGWPAT-UHFFFAOYSA-N Acetoin Chemical compound CC(O)C(C)=O ROWKJAVDOGWPAT-UHFFFAOYSA-N 0.000 description 1
- 239000004377 Alitame Substances 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 108010011485 Aspartame Proteins 0.000 description 1
- 241000228215 Aspergillus aculeatus Species 0.000 description 1
- 241000228245 Aspergillus niger Species 0.000 description 1
- 240000006439 Aspergillus oryzae Species 0.000 description 1
- 241000228257 Aspergillus sp. Species 0.000 description 1
- 241000193752 Bacillus circulans Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 239000005973 Carvone Substances 0.000 description 1
- 102000005575 Cellulases Human genes 0.000 description 1
- 108010084185 Cellulases Proteins 0.000 description 1
- JSQCMNXZFPMWES-WRBXKWMASA-N Centellasaponin B Natural products O=C(O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@H]2[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O2)O1)[C@@]12[C@@H]([C@@H](C)[C@H](C)CC1)C=1[C@](C)([C@@]3(C)[C@@H]([C@]4(C)[C@@H]([C@H](O)C3)[C@@](CO)(C)[C@@H](O)[C@H](O)C4)CC=1)CC2 JSQCMNXZFPMWES-WRBXKWMASA-N 0.000 description 1
- JSQCMNXZFPMWES-UHFFFAOYSA-N Centellasaponin B Chemical compound C1CC(C2(CC(O)C3C(C)(CO)C(O)C(O)CC3(C)C2CC=2)C)(C)C=2C2C(C)C(C)CCC21C(=O)OC(C(C(O)C1O)O)OC1COC1OC(CO)C(O)C(O)C1O JSQCMNXZFPMWES-UHFFFAOYSA-N 0.000 description 1
- NPBVQXIMTZKSBA-UHFFFAOYSA-N Chavibetol Natural products COC1=CC=C(CC=C)C=C1O NPBVQXIMTZKSBA-UHFFFAOYSA-N 0.000 description 1
- WTEVQBCEXWBHNA-UHFFFAOYSA-N Citral Natural products CC(C)=CCCC(C)=CC=O WTEVQBCEXWBHNA-UHFFFAOYSA-N 0.000 description 1
- JOZKFWLRHCDGJA-LLVKDONJSA-N Citronellyl acetate Natural products CC(=O)OCC[C@H](C)CCC=C(C)C JOZKFWLRHCDGJA-LLVKDONJSA-N 0.000 description 1
- 240000000560 Citrus x paradisi Species 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- UDIPTWFVPPPURJ-UHFFFAOYSA-M Cyclamate Chemical compound [Na+].[O-]S(=O)(=O)NC1CCCCC1 UDIPTWFVPPPURJ-UHFFFAOYSA-M 0.000 description 1
- YYLLIJHXUHJATK-UHFFFAOYSA-N Cyclohexyl acetate Chemical compound CC(=O)OC1CCCCC1 YYLLIJHXUHJATK-UHFFFAOYSA-N 0.000 description 1
- DCXYFEDJOCDNAF-UWTATZPHSA-N D-Asparagine Chemical compound OC(=O)[C@H](N)CC(N)=O DCXYFEDJOCDNAF-UWTATZPHSA-N 0.000 description 1
- RFSUNEUAIZKAJO-VRPWFDPXSA-N D-Fructose Natural products OC[C@H]1OC(O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-VRPWFDPXSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- LKDRXBCSQODPBY-JDJSBBGDSA-N D-allulose Chemical compound OCC1(O)OC[C@@H](O)[C@@H](O)[C@H]1O LKDRXBCSQODPBY-JDJSBBGDSA-N 0.000 description 1
- HEBKCHPVOIAQTA-QWWZWVQMSA-N D-arabinitol Chemical compound OC[C@@H](O)C(O)[C@H](O)CO HEBKCHPVOIAQTA-QWWZWVQMSA-N 0.000 description 1
- 229930182846 D-asparagine Natural products 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- MNQZXJOMYWMBOU-VKHMYHEASA-N D-glyceraldehyde Chemical compound OC[C@@H](O)C=O MNQZXJOMYWMBOU-VKHMYHEASA-N 0.000 description 1
- ROHFNLRQFUQHCH-RXMQYKEDSA-N D-leucine Chemical compound CC(C)C[C@@H](N)C(O)=O ROHFNLRQFUQHCH-RXMQYKEDSA-N 0.000 description 1
- 229930182819 D-leucine Natural products 0.000 description 1
- COLNVLDHVKWLRT-MRVPVSSYSA-N D-phenylalanine Chemical compound OC(=O)[C@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-MRVPVSSYSA-N 0.000 description 1
- 229930182832 D-phenylalanine Natural products 0.000 description 1
- SRBFZHDQGSBBOR-SOOFDHNKSA-N D-ribopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@@H]1O SRBFZHDQGSBBOR-SOOFDHNKSA-N 0.000 description 1
- LKDRXBCSQODPBY-IANNHFEVSA-N D-sorbose Chemical compound OCC1(O)OC[C@@H](O)[C@H](O)[C@H]1O LKDRXBCSQODPBY-IANNHFEVSA-N 0.000 description 1
- LKDRXBCSQODPBY-OEXCPVAWSA-N D-tagatose Chemical compound OCC1(O)OC[C@@H](O)[C@H](O)[C@@H]1O LKDRXBCSQODPBY-OEXCPVAWSA-N 0.000 description 1
- UNXHWFMMPAWVPI-QWWZWVQMSA-N D-threitol Chemical compound OC[C@@H](O)[C@H](O)CO UNXHWFMMPAWVPI-QWWZWVQMSA-N 0.000 description 1
- AYFVYJQAPQTCCC-STHAYSLISA-N D-threonine Chemical compound C[C@H](O)[C@@H](N)C(O)=O AYFVYJQAPQTCCC-STHAYSLISA-N 0.000 description 1
- 229930182822 D-threonine Natural products 0.000 description 1
- 229930182827 D-tryptophan Natural products 0.000 description 1
- QIVBCDIJIAJPQS-SECBINFHSA-N D-tryptophane Chemical compound C1=CC=C2C(C[C@@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-SECBINFHSA-N 0.000 description 1
- NOOLISFMXDJSKH-UHFFFAOYSA-N DL-menthol Natural products CC(C)C1CCC(C)CC1O NOOLISFMXDJSKH-UHFFFAOYSA-N 0.000 description 1
- 206010013911 Dysgeusia Diseases 0.000 description 1
- 239000004278 EU approved seasoning Substances 0.000 description 1
- DWONJCNDULPHLV-HOTGVXAUSA-N Enterodiol Chemical compound C([C@@H](CO)[C@H](CO)CC=1C=C(O)C=CC=1)C1=CC=CC(O)=C1 DWONJCNDULPHLV-HOTGVXAUSA-N 0.000 description 1
- AOJXPBNHAJMETF-UHFFFAOYSA-N Enterodiol Natural products OCC(Cc1ccc(O)cc1)C(CO)Cc2ccc(O)cc2 AOJXPBNHAJMETF-UHFFFAOYSA-N 0.000 description 1
- 239000004386 Erythritol Substances 0.000 description 1
- ZFDIRQKJPRINOQ-HWKANZROSA-N Ethyl crotonate Chemical compound CCOC(=O)\C=C\C ZFDIRQKJPRINOQ-HWKANZROSA-N 0.000 description 1
- YIKYNHJUKRTCJL-UHFFFAOYSA-N Ethyl maltol Chemical compound CCC=1OC=CC(=O)C=1O YIKYNHJUKRTCJL-UHFFFAOYSA-N 0.000 description 1
- WEEGYLXZBRQIMU-WAAGHKOSSA-N Eucalyptol Chemical compound C1C[C@H]2CC[C@]1(C)OC2(C)C WEEGYLXZBRQIMU-WAAGHKOSSA-N 0.000 description 1
- 239000005770 Eugenol Substances 0.000 description 1
- 239000005792 Geraniol Substances 0.000 description 1
- GLZPCOQZEFWAFX-YFHOEESVSA-N Geraniol Natural products CC(C)=CCC\C(C)=C/CO GLZPCOQZEFWAFX-YFHOEESVSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 244000303040 Glycyrrhiza glabra Species 0.000 description 1
- 235000006200 Glycyrrhiza glabra Nutrition 0.000 description 1
- 239000004378 Glycyrrhizin Substances 0.000 description 1
- HYQNKKAJVPMBDR-HIFRSBDPSA-N Hernandulcin Chemical compound CC(C)=CCC[C@](C)(O)[C@@H]1CCC(C)=CC1=O HYQNKKAJVPMBDR-HIFRSBDPSA-N 0.000 description 1
- HYQNKKAJVPMBDR-UHFFFAOYSA-N Hernandulcin Natural products CC(C)=CCCC(C)(O)C1CCC(C)=CC1=O HYQNKKAJVPMBDR-UHFFFAOYSA-N 0.000 description 1
- 244000267823 Hydrangea macrophylla Species 0.000 description 1
- 235000014486 Hydrangea macrophylla Nutrition 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 206010022489 Insulin Resistance Diseases 0.000 description 1
- XINCECQTMHSORG-UHFFFAOYSA-N Isoamyl isovalerate Chemical compound CC(C)CCOC(=O)CC(C)C XINCECQTMHSORG-UHFFFAOYSA-N 0.000 description 1
- AMIMRNSIRUDHCM-UHFFFAOYSA-N Isopropylaldehyde Chemical compound CC(C)C=O AMIMRNSIRUDHCM-UHFFFAOYSA-N 0.000 description 1
- 241001138401 Kluyveromyces lactis Species 0.000 description 1
- 241000170280 Kluyveromyces sp. Species 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- 229930182821 L-proline Natural products 0.000 description 1
- SRBFZHDQGSBBOR-OWMBCFKOSA-N L-ribopyranose Chemical compound O[C@H]1COC(O)[C@@H](O)[C@H]1O SRBFZHDQGSBBOR-OWMBCFKOSA-N 0.000 description 1
- 241000186604 Lactobacillus reuteri Species 0.000 description 1
- YVRYZXAHRGGELT-UHFFFAOYSA-N Lariciresinol Natural products C1=C2OCOC2=CC(C2C(C)C3(OC)C=C(CC=C)C(=O)CC3(O2)OC)=C1 YVRYZXAHRGGELT-UHFFFAOYSA-N 0.000 description 1
- 239000005639 Lauric acid Substances 0.000 description 1
- BRHDDEIRQPDPMG-UHFFFAOYSA-N Linalyl oxide Chemical compound CC(C)(O)C1CCC(C)(C=C)O1 BRHDDEIRQPDPMG-UHFFFAOYSA-N 0.000 description 1
- 244000292467 Lippia dulcis Species 0.000 description 1
- 235000000144 Lippia dulcis Nutrition 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- HYMLWHLQFGRFIY-UHFFFAOYSA-N Maltol Natural products CC1OC=CC(=O)C1=O HYMLWHLQFGRFIY-UHFFFAOYSA-N 0.000 description 1
- 244000070406 Malus silvestris Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- MZSGWZGPESCJAN-MOBFUUNNSA-N Melitric acid A Natural products O([C@@H](C(=O)O)Cc1cc(O)c(O)cc1)C(=O)/C=C/c1cc(O)c(O/C(/C(=O)O)=C/c2cc(O)c(O)cc2)cc1 MZSGWZGPESCJAN-MOBFUUNNSA-N 0.000 description 1
- YGWKXXYGDYYFJU-UHFFFAOYSA-N Menthofuran Natural products C1C(C)CCC2=C1OC=C2C YGWKXXYGDYYFJU-UHFFFAOYSA-N 0.000 description 1
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical group OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 1
- KVWWIYGFBYDJQC-GHMZBOCLSA-N Methyl dihydrojasmonate Chemical compound CCCCC[C@@H]1[C@@H](CC(=O)OC)CCC1=O KVWWIYGFBYDJQC-GHMZBOCLSA-N 0.000 description 1
- OSWPMRLSEDHDFF-UHFFFAOYSA-N Methyl salicylate Natural products COC(=O)C1=CC=CC=C1O OSWPMRLSEDHDFF-UHFFFAOYSA-N 0.000 description 1
- KWKVAGQCDSHWFK-VNKDHWASSA-N Methyl sorbate Chemical compound COC(=O)\C=C\C=C\C KWKVAGQCDSHWFK-VNKDHWASSA-N 0.000 description 1
- 102000014171 Milk Proteins Human genes 0.000 description 1
- 108010011756 Milk Proteins Proteins 0.000 description 1
- 101710084933 Miraculin Proteins 0.000 description 1
- 235000009815 Momordica Nutrition 0.000 description 1
- 241000218984 Momordica Species 0.000 description 1
- 108050004114 Monellin Proteins 0.000 description 1
- 241000594464 Mycetia Species 0.000 description 1
- OZNUPWACHHUIKC-JXMROGBWSA-N N-[2-(3,4-Dimethoxyphenyl)ethyl]-3,4-dimethoxycinnamic acid amide Chemical compound C1=C(OC)C(OC)=CC=C1CCNC(=O)\C=C\C1=CC=C(OC)C(OC)=C1 OZNUPWACHHUIKC-JXMROGBWSA-N 0.000 description 1
- YQHMWTPYORBCMF-UHFFFAOYSA-N Naringenin chalcone Natural products C1=CC(O)=CC=C1C=CC(=O)C1=C(O)C=C(O)C=C1O YQHMWTPYORBCMF-UHFFFAOYSA-N 0.000 description 1
- 239000004384 Neotame Substances 0.000 description 1
- GLZPCOQZEFWAFX-JXMROGBWSA-N Nerol Natural products CC(C)=CCC\C(C)=C\CO GLZPCOQZEFWAFX-JXMROGBWSA-N 0.000 description 1
- CKMNPXWFAZLBNK-QOFYEYOWSA-N OC[C@H]1O[C@@H]2O[C@@H]3[C@@H](CO)O[C@H](O[C@@H]4[C@@H](CO)O[C@H](O[C@@H]5[C@@H](CO)O[C@H](O[C@H]1[C@H](O)[C@H]2O)[C@H](O)[C@H]5O)[C@H](O)[C@H]4O)[C@H](O)[C@H]3O Chemical compound OC[C@H]1O[C@@H]2O[C@@H]3[C@@H](CO)O[C@H](O[C@@H]4[C@@H](CO)O[C@H](O[C@@H]5[C@@H](CO)O[C@H](O[C@H]1[C@H](O)[C@H]2O)[C@H](O)[C@H]5O)[C@H](O)[C@H]4O)[C@H](O)[C@H]3O CKMNPXWFAZLBNK-QOFYEYOWSA-N 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- RZPAKFUAFGMUPI-UHFFFAOYSA-N Oleandomycin Natural products O1C(C)C(O)C(OC)CC1OC1C(C)C(=O)OC(C)C(C)C(O)C(C)C(=O)C2(OC2)CC(C)C(OC2C(C(CC(C)O2)N(C)C)O)C1C RZPAKFUAFGMUPI-UHFFFAOYSA-N 0.000 description 1
- 239000004104 Oleandomycin Substances 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 206010033307 Overweight Diseases 0.000 description 1
- 235000021314 Palmitic acid Nutrition 0.000 description 1
- 241000985535 Penicillium decumbens Species 0.000 description 1
- 101000865553 Pentadiplandra brazzeana Defensin-like protein Proteins 0.000 description 1
- LQKRYVGRPXFFAV-UHFFFAOYSA-N Phenylmethylglycidic ester Chemical compound CCOC(=O)C1OC1(C)C1=CC=CC=C1 LQKRYVGRPXFFAV-UHFFFAOYSA-N 0.000 description 1
- 240000004760 Pimpinella anisum Species 0.000 description 1
- 235000012550 Pimpinella anisum Nutrition 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- PXRCIOIWVGAZEP-UHFFFAOYSA-N Primaeres Camphenhydrat Natural products C1CC2C(O)(C)C(C)(C)C1C2 PXRCIOIWVGAZEP-UHFFFAOYSA-N 0.000 description 1
- UVMRYBDEERADNV-UHFFFAOYSA-N Pseudoeugenol Natural products COC1=CC(C(C)=C)=CC=C1O UVMRYBDEERADNV-UHFFFAOYSA-N 0.000 description 1
- 241000220324 Pyrus Species 0.000 description 1
- MUPFEKGTMRGPLJ-RMMQSMQOSA-N Raffinose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 MUPFEKGTMRGPLJ-RMMQSMQOSA-N 0.000 description 1
- OZNUPWACHHUIKC-UHFFFAOYSA-N Rubemamine Natural products C1=C(OC)C(OC)=CC=C1CCNC(=O)C=CC1=CC=C(OC)C(OC)=C1 OZNUPWACHHUIKC-UHFFFAOYSA-N 0.000 description 1
- RLWJIETUBNSFMD-UHFFFAOYSA-N Rubescenamine Natural products C1=C(OC)C(OC)=CC=C1C=CC(=O)NCCC1=CC=C(OCO2)C2=C1 RLWJIETUBNSFMD-UHFFFAOYSA-N 0.000 description 1
- 241001092459 Rubus Species 0.000 description 1
- GRLJIIJNZJVMGP-UHFFFAOYSA-N S-Methyl butanethioate Chemical compound CCCC(=O)SC GRLJIIJNZJVMGP-UHFFFAOYSA-N 0.000 description 1
- WINXNKPZLFISPD-UHFFFAOYSA-M Saccharin sodium Chemical compound [Na+].C1=CC=C2C(=O)[N-]S(=O)(=O)C2=C1 WINXNKPZLFISPD-UHFFFAOYSA-M 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 240000003768 Solanum lycopersicum Species 0.000 description 1
- 235000002560 Solanum lycopersicum Nutrition 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 244000228451 Stevia rebaudiana Species 0.000 description 1
- 235000006092 Stevia rebaudiana Nutrition 0.000 description 1
- UEDUENGHJMELGK-HYDKPPNVSA-N Stevioside Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@]12C(=C)C[C@@]3(C1)CC[C@@H]1[C@@](C)(CCC[C@]1([C@@H]3CC2)C)C(=O)O[C@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O UEDUENGHJMELGK-HYDKPPNVSA-N 0.000 description 1
- 239000004376 Sucralose Substances 0.000 description 1
- 235000009470 Theobroma cacao Nutrition 0.000 description 1
- 241001141092 Thermomicrobia Species 0.000 description 1
- 239000005844 Thymol Substances 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- VYPONAGZHAJHGT-UHFFFAOYSA-N Tuberolactone Natural products CCC=CCC1CC=CC(=O)O1 VYPONAGZHAJHGT-UHFFFAOYSA-N 0.000 description 1
- MUPFEKGTMRGPLJ-UHFFFAOYSA-N UNPD196149 Natural products OC1C(O)C(CO)OC1(CO)OC1C(O)C(O)C(O)C(COC2C(C(O)C(O)C(CO)O2)O)O1 MUPFEKGTMRGPLJ-UHFFFAOYSA-N 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- NIONDZDPPYHYKY-UHFFFAOYSA-N Z-hexenoic acid Natural products CCCC=CC(O)=O NIONDZDPPYHYKY-UHFFFAOYSA-N 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000016383 Zea mays subsp huehuetenangensis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- HRHOWZHRCRZVCU-WAYWQWQTSA-N [(z)-hex-2-enyl] acetate Chemical compound CCC\C=C/COC(C)=O HRHOWZHRCRZVCU-WAYWQWQTSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 235000013334 alcoholic beverage Nutrition 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- GZCGUPFRVQAUEE-KVTDHHQDSA-N aldehydo-D-mannose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)C=O GZCGUPFRVQAUEE-KVTDHHQDSA-N 0.000 description 1
- 235000019409 alitame Nutrition 0.000 description 1
- 108010009985 alitame Proteins 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- FAMPSKZZVDUYOS-UHFFFAOYSA-N alpha-Caryophyllene Natural products CC1=CCC(C)(C)C=CCC(C)=CCC1 FAMPSKZZVDUYOS-UHFFFAOYSA-N 0.000 description 1
- XCPQUQHBVVXMRQ-UHFFFAOYSA-N alpha-Fenchene Natural products C1CC2C(=C)CC1C2(C)C XCPQUQHBVVXMRQ-UHFFFAOYSA-N 0.000 description 1
- UZFLPKAIBPNNCA-BQYQJAHWSA-N alpha-ionone Chemical compound CC(=O)\C=C\C1C(C)=CCCC1(C)C UZFLPKAIBPNNCA-BQYQJAHWSA-N 0.000 description 1
- UZFLPKAIBPNNCA-UHFFFAOYSA-N alpha-ionone Natural products CC(=O)C=CC1C(C)=CCCC1(C)C UZFLPKAIBPNNCA-UHFFFAOYSA-N 0.000 description 1
- QUMXDOLUJCHOAY-UHFFFAOYSA-N alpha-methylbenzyl acetate Natural products CC(=O)OC(C)C1=CC=CC=C1 QUMXDOLUJCHOAY-UHFFFAOYSA-N 0.000 description 1
- WUOACPNHFRMFPN-UHFFFAOYSA-N alpha-terpineol Chemical compound CC1=CCC(C(C)(C)O)CC1 WUOACPNHFRMFPN-UHFFFAOYSA-N 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 235000021016 apples Nutrition 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000000605 aspartame Substances 0.000 description 1
- 235000010357 aspartame Nutrition 0.000 description 1
- IAOZJIPTCAWIRG-QWRGUYRKSA-N aspartame Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 IAOZJIPTCAWIRG-QWRGUYRKSA-N 0.000 description 1
- 229960003438 aspartame Drugs 0.000 description 1
- 235000015173 baked goods and baking mixes Nutrition 0.000 description 1
- 235000013527 bean curd Nutrition 0.000 description 1
- 235000013405 beer Nutrition 0.000 description 1
- 229940007550 benzyl acetate Drugs 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- NOPLRNXKHZRXHT-YFVJMOTDSA-N beta-Sinensal Chemical compound O=CC(/C)=C/CCC(/C)=C/CCC(=C)C=C NOPLRNXKHZRXHT-YFVJMOTDSA-N 0.000 description 1
- NPNUFJAVOOONJE-UHFFFAOYSA-N beta-cariophyllene Natural products C1CC(C)=CCCC(=C)C2CC(C)(C)C21 NPNUFJAVOOONJE-UHFFFAOYSA-N 0.000 description 1
- JGQFVRIQXUFPAH-UHFFFAOYSA-N beta-citronellol Natural products OCCC(C)CCCC(C)=C JGQFVRIQXUFPAH-UHFFFAOYSA-N 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- OHDFENKFSKIFBJ-UHFFFAOYSA-N bis(2-methyl-3-furyl)disulfide Chemical compound O1C=CC(SSC2=C(OC=C2)C)=C1C OHDFENKFSKIFBJ-UHFFFAOYSA-N 0.000 description 1
- 235000015895 biscuits Nutrition 0.000 description 1
- 235000019658 bitter taste Nutrition 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 235000013532 brandy Nutrition 0.000 description 1
- 235000008429 bread Nutrition 0.000 description 1
- 235000015496 breakfast cereal Nutrition 0.000 description 1
- OBNCKNCVKJNDBV-UHFFFAOYSA-N butanoic acid ethyl ester Natural products CCCC(=O)OCC OBNCKNCVKJNDBV-UHFFFAOYSA-N 0.000 description 1
- 235000014121 butter Nutrition 0.000 description 1
- RPRPDTXKGSIXMD-UHFFFAOYSA-N butyl hexanoate Chemical compound CCCCCC(=O)OCCCC RPRPDTXKGSIXMD-UHFFFAOYSA-N 0.000 description 1
- ZTQSAGDEMFDKMZ-UHFFFAOYSA-N butyric aldehyde Natural products CCCC=O ZTQSAGDEMFDKMZ-UHFFFAOYSA-N 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229930006739 camphene Natural products 0.000 description 1
- ZYPYEBYNXWUCEA-UHFFFAOYSA-N camphenilone Natural products C1CC2C(=O)C(C)(C)C1C2 ZYPYEBYNXWUCEA-UHFFFAOYSA-N 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 235000013736 caramel Nutrition 0.000 description 1
- NPNUFJAVOOONJE-UONOGXRCSA-N caryophyllene Natural products C1CC(C)=CCCC(=C)[C@@H]2CC(C)(C)[C@@H]21 NPNUFJAVOOONJE-UONOGXRCSA-N 0.000 description 1
- 229940117948 caryophyllene Drugs 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 235000013351 cheese Nutrition 0.000 description 1
- 229940112822 chewing gum Drugs 0.000 description 1
- 235000015218 chewing gum Nutrition 0.000 description 1
- 235000010675 chips/crisps Nutrition 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 229930007050 cineol Natural products 0.000 description 1
- CCRCUPLGCSFEDV-UHFFFAOYSA-N cinnamic acid methyl ester Natural products COC(=O)C=CC1=CC=CC=C1 CCRCUPLGCSFEDV-UHFFFAOYSA-N 0.000 description 1
- WJSDHUCWMSHDCR-VMPITWQZSA-N cinnamyl acetate Natural products CC(=O)OC\C=C\C1=CC=CC=C1 WJSDHUCWMSHDCR-VMPITWQZSA-N 0.000 description 1
- NPFVOOAXDOBMCE-PLNGDYQASA-N cis-3-Hexenyl acetate Natural products CC\C=C/CCOC(C)=O NPFVOOAXDOBMCE-PLNGDYQASA-N 0.000 description 1
- RRGOKSYVAZDNKR-ARJAWSKDSA-M cis-3-hexenylacetate Chemical compound CC\C=C/CCCC([O-])=O RRGOKSYVAZDNKR-ARJAWSKDSA-M 0.000 description 1
- NNWHUJCUHAELCL-UHFFFAOYSA-N cis-Methyl isoeugenol Natural products COC1=CC=C(C=CC)C=C1OC NNWHUJCUHAELCL-UHFFFAOYSA-N 0.000 description 1
- NNWHUJCUHAELCL-PLNGDYQASA-N cis-isomethyleugenol Chemical compound COC1=CC=C(\C=C/C)C=C1OC NNWHUJCUHAELCL-PLNGDYQASA-N 0.000 description 1
- 229940043350 citral Drugs 0.000 description 1
- 229930003633 citronellal Natural products 0.000 description 1
- 235000000983 citronellal Nutrition 0.000 description 1
- 235000000484 citronellol Nutrition 0.000 description 1
- 235000016213 coffee Nutrition 0.000 description 1
- 235000013353 coffee beverage Nutrition 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 235000008504 concentrate Nutrition 0.000 description 1
- 108010010165 curculin Proteins 0.000 description 1
- 239000000625 cyclamic acid and its Na and Ca salt Substances 0.000 description 1
- 125000000422 delta-lactone group Chemical group 0.000 description 1
- FYTRVXSHONWYNE-UHFFFAOYSA-N delta-octanolide Chemical compound CCCC1CCCC(=O)O1 FYTRVXSHONWYNE-UHFFFAOYSA-N 0.000 description 1
- SQIFACVGCPWBQZ-UHFFFAOYSA-N delta-terpineol Natural products CC(C)(O)C1CCC(=C)CC1 SQIFACVGCPWBQZ-UHFFFAOYSA-N 0.000 description 1
- 208000002925 dental caries Diseases 0.000 description 1
- 210000003298 dental enamel Anatomy 0.000 description 1
- 230000037123 dental health Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000000378 dietary effect Effects 0.000 description 1
- DMSHWWDRAYHEBS-UHFFFAOYSA-N dihydrocoumarin Natural products C1CC(=O)OC2=C1C=C(OC)C(OC)=C2 DMSHWWDRAYHEBS-UHFFFAOYSA-N 0.000 description 1
- 235000021186 dishes Nutrition 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- WTOYNNBCKUYIKC-UHFFFAOYSA-N dl-nootkatone Natural products C1CC(C(C)=C)CC2(C)C(C)CC(=O)C=C21 WTOYNNBCKUYIKC-UHFFFAOYSA-N 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 235000014103 egg white Nutrition 0.000 description 1
- 210000000969 egg white Anatomy 0.000 description 1
- 210000002969 egg yolk Anatomy 0.000 description 1
- 235000013345 egg yolk Nutrition 0.000 description 1
- SBHXYTNGIZCORC-ZDUSSCGKSA-N eriodictyol Chemical compound C1([C@@H]2CC(=O)C3=C(O)C=C(C=C3O2)O)=CC=C(O)C(O)=C1 SBHXYTNGIZCORC-ZDUSSCGKSA-N 0.000 description 1
- TUJPOVKMHCLXEL-UHFFFAOYSA-N eriodictyol Natural products C1C(=O)C2=CC(O)=CC(O)=C2OC1C1=CC=C(O)C(O)=C1 TUJPOVKMHCLXEL-UHFFFAOYSA-N 0.000 description 1
- 235000011797 eriodictyol Nutrition 0.000 description 1
- SBHXYTNGIZCORC-UHFFFAOYSA-N eriodyctiol Natural products O1C2=CC(O)=CC(O)=C2C(=O)CC1C1=CC=C(O)C(O)=C1 SBHXYTNGIZCORC-UHFFFAOYSA-N 0.000 description 1
- 235000019414 erythritol Nutrition 0.000 description 1
- 229940009714 erythritol Drugs 0.000 description 1
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 description 1
- OPCRGEVPIBLWAY-QNRZBPGKSA-N ethyl (2E,4Z)-deca-2,4-dienoate Chemical compound CCCCC\C=C/C=C/C(=O)OCC OPCRGEVPIBLWAY-QNRZBPGKSA-N 0.000 description 1
- RGXWDWUGBIJHDO-UHFFFAOYSA-N ethyl decanoate Chemical compound CCCCCCCCCC(=O)OCC RGXWDWUGBIJHDO-UHFFFAOYSA-N 0.000 description 1
- PPXUHEORWJQRHJ-UHFFFAOYSA-N ethyl isovalerate Chemical compound CCOC(=O)CC(C)C PPXUHEORWJQRHJ-UHFFFAOYSA-N 0.000 description 1
- 229940116333 ethyl lactate Drugs 0.000 description 1
- 229940093503 ethyl maltol Drugs 0.000 description 1
- BTCQMCOBMIXUCG-UHFFFAOYSA-N ethyl vanillin isobutyrate Chemical compound CCOC1=CC(C=O)=CC=C1OC(=O)C(C)C BTCQMCOBMIXUCG-UHFFFAOYSA-N 0.000 description 1
- 229960002217 eugenol Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 235000011194 food seasoning agent Nutrition 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 235000021433 fructose syrup Nutrition 0.000 description 1
- 235000015203 fruit juice Nutrition 0.000 description 1
- 235000012055 fruits and vegetables Nutrition 0.000 description 1
- FBPFZTCFMRRESA-GUCUJZIJSA-N galactitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-GUCUJZIJSA-N 0.000 description 1
- OALYTRUKMRCXNH-QMMMGPOBSA-N gamma-Nonalactone Natural products CCCCC[C@H]1CCC(=O)O1 OALYTRUKMRCXNH-QMMMGPOBSA-N 0.000 description 1
- 125000000457 gamma-lactone group Chemical group 0.000 description 1
- IPBFYZQJXZJBFQ-UHFFFAOYSA-N gamma-octalactone Chemical compound CCCCC1CCC(=O)O1 IPBFYZQJXZJBFQ-UHFFFAOYSA-N 0.000 description 1
- WTEVQBCEXWBHNA-JXMROGBWSA-N geranial Chemical compound CC(C)=CCC\C(C)=C\C=O WTEVQBCEXWBHNA-JXMROGBWSA-N 0.000 description 1
- 229940113087 geraniol Drugs 0.000 description 1
- 229930182470 glycoside Natural products 0.000 description 1
- 150000002338 glycosides Chemical class 0.000 description 1
- LPLVUJXQOOQHMX-UHFFFAOYSA-N glycyrrhetinic acid glycoside Natural products C1CC(C2C(C3(CCC4(C)CCC(C)(CC4C3=CC2=O)C(O)=O)C)(C)CC2)(C)C2C(C)(C)C1OC1OC(C(O)=O)C(O)C(O)C1OC1OC(C(O)=O)C(O)C(O)C1O LPLVUJXQOOQHMX-UHFFFAOYSA-N 0.000 description 1
- 229960004949 glycyrrhizic acid Drugs 0.000 description 1
- UYRUBYNTXSDKQT-UHFFFAOYSA-N glycyrrhizic acid Natural products CC1(C)C(CCC2(C)C1CCC3(C)C2C(=O)C=C4C5CC(C)(CCC5(C)CCC34C)C(=O)O)OC6OC(C(O)C(O)C6OC7OC(O)C(O)C(O)C7C(=O)O)C(=O)O UYRUBYNTXSDKQT-UHFFFAOYSA-N 0.000 description 1
- 235000019410 glycyrrhizin Nutrition 0.000 description 1
- 235000011868 grain product Nutrition 0.000 description 1
- 235000014168 granola/muesli bars Nutrition 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 235000011617 hard cheese Nutrition 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000005802 health problem Effects 0.000 description 1
- NDFKTBCGKNOHPJ-UHFFFAOYSA-N hex-2-enal Natural products CCCCC=CC=O NDFKTBCGKNOHPJ-UHFFFAOYSA-N 0.000 description 1
- UFLHIIWVXFIJGU-UHFFFAOYSA-N hex-3-en-1-ol Natural products CCC=CCCO UFLHIIWVXFIJGU-UHFFFAOYSA-N 0.000 description 1
- VLKZOEOYAKHREP-UHFFFAOYSA-N hexane Substances CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 238000004896 high resolution mass spectrometry Methods 0.000 description 1
- FTODBIPDTXRIGS-ZDUSSCGKSA-N homoeriodictyol Chemical compound C1=C(O)C(OC)=CC([C@H]2OC3=CC(O)=CC(O)=C3C(=O)C2)=C1 FTODBIPDTXRIGS-ZDUSSCGKSA-N 0.000 description 1
- FTODBIPDTXRIGS-UHFFFAOYSA-N homoeriodictyol Natural products C1=C(O)C(OC)=CC(C2OC3=CC(O)=CC(O)=C3C(=O)C2)=C1 FTODBIPDTXRIGS-UHFFFAOYSA-N 0.000 description 1
- 235000015243 ice cream Nutrition 0.000 description 1
- 235000021539 instant coffee Nutrition 0.000 description 1
- 235000014109 instant soup Nutrition 0.000 description 1
- 235000020344 instant tea Nutrition 0.000 description 1
- PANBRUWVURLWGY-UHFFFAOYSA-N intreleven aldehyde Natural products CCCCCCCCC=CC=O PANBRUWVURLWGY-UHFFFAOYSA-N 0.000 description 1
- 229960004903 invert sugar Drugs 0.000 description 1
- 229940117955 isoamyl acetate Drugs 0.000 description 1
- 229940094941 isoamyl butyrate Drugs 0.000 description 1
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 1
- JSLCOZYBKYHZNL-UHFFFAOYSA-N isobutyric acid butyl ester Natural products CCCCOC(=O)C(C)C JSLCOZYBKYHZNL-UHFFFAOYSA-N 0.000 description 1
- 229940095045 isopulegol Drugs 0.000 description 1
- 235000015141 kefir Nutrition 0.000 description 1
- 235000008960 ketchup Nutrition 0.000 description 1
- 239000000832 lactitol Substances 0.000 description 1
- 235000010448 lactitol Nutrition 0.000 description 1
- VQHSOMBJVWLPSR-JVCRWLNRSA-N lactitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-JVCRWLNRSA-N 0.000 description 1
- 229960003451 lactitol Drugs 0.000 description 1
- 229940001882 lactobacillus reuteri Drugs 0.000 description 1
- JCQLYHFGKNRPGE-FCVZTGTOSA-N lactulose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 JCQLYHFGKNRPGE-FCVZTGTOSA-N 0.000 description 1
- 229960000511 lactulose Drugs 0.000 description 1
- PFCRQPBOOFTZGQ-UHFFFAOYSA-N lactulose keto form Natural products OCC(=O)C(O)C(C(O)CO)OC1OC(CO)C(O)C(O)C1O PFCRQPBOOFTZGQ-UHFFFAOYSA-N 0.000 description 1
- 235000006826 lariciresinol Nutrition 0.000 description 1
- 239000007942 layered tablet Substances 0.000 description 1
- 229940040102 levulinic acid Drugs 0.000 description 1
- 229930013686 lignan Natural products 0.000 description 1
- 235000009408 lignans Nutrition 0.000 description 1
- 150000005692 lignans Chemical class 0.000 description 1
- 229930007744 linalool Natural products 0.000 description 1
- UWKAYLJWKGQEPM-UHFFFAOYSA-N linalool acetate Natural products CC(C)=CCCC(C)(C=C)OC(C)=O UWKAYLJWKGQEPM-UHFFFAOYSA-N 0.000 description 1
- 235000020094 liqueur Nutrition 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 235000011477 liquorice Nutrition 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- IHKGGKRBWGTNNG-UHFFFAOYSA-N lugduname Chemical compound C=1C=CC=2OCOC=2C=1C/N=C(/NCC(=O)O)NC1=CC=C(C#N)C=C1 IHKGGKRBWGTNNG-UHFFFAOYSA-N 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 235000009973 maize Nutrition 0.000 description 1
- 229940043353 maltol Drugs 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 235000000055 matairesinol Nutrition 0.000 description 1
- RNXYRAQIZQGUIK-UHFFFAOYSA-N matairesinol Natural products COc1cc(CC2OCC(=O)C2Cc3ccc(O)c(OC)c3)ccc1O RNXYRAQIZQGUIK-UHFFFAOYSA-N 0.000 description 1
- 239000008268 mayonnaise Substances 0.000 description 1
- 235000010746 mayonnaise Nutrition 0.000 description 1
- QWIZNVHXZXRPDR-WSCXOGSTSA-N melezitose Chemical compound O([C@@]1(O[C@@H]([C@H]([C@@H]1O[C@@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)O)CO)CO)[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O QWIZNVHXZXRPDR-WSCXOGSTSA-N 0.000 description 1
- 229940041616 menthol Drugs 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 239000001375 methyl (2E,4E)-hexa-2,4-dienoate Substances 0.000 description 1
- GEWDNTWNSAZUDX-UHFFFAOYSA-N methyl 7-epi-jasmonate Natural products CCC=CCC1C(CC(=O)OC)CCC1=O GEWDNTWNSAZUDX-UHFFFAOYSA-N 0.000 description 1
- 229940102398 methyl anthranilate Drugs 0.000 description 1
- NUKZAGXMHTUAFE-UHFFFAOYSA-N methyl hexanoate Chemical compound CCCCCC(=O)OC NUKZAGXMHTUAFE-UHFFFAOYSA-N 0.000 description 1
- 229960001047 methyl salicylate Drugs 0.000 description 1
- CCRCUPLGCSFEDV-BQYQJAHWSA-N methyl trans-cinnamate Chemical compound COC(=O)\C=C\C1=CC=CC=C1 CCRCUPLGCSFEDV-BQYQJAHWSA-N 0.000 description 1
- SBENKNZHVXGNTP-UHFFFAOYSA-N methylconiferyl ether Natural products COCC=CC1=CC=C(O)C(OC)=C1 SBENKNZHVXGNTP-UHFFFAOYSA-N 0.000 description 1
- 238000001471 micro-filtration Methods 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 235000021239 milk protein Nutrition 0.000 description 1
- 235000020124 milk-based beverage Nutrition 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 1
- WMBOCUXXNSOQHM-UHFFFAOYSA-N n-butylidenephthalide Natural products C1=CC=C2C(=CCCC)OC(=O)C2=C1 WMBOCUXXNSOQHM-UHFFFAOYSA-N 0.000 description 1
- 229930019673 naringin Natural products 0.000 description 1
- DFPMSGMNTNDNHN-ZPHOTFPESA-N naringin Chemical compound O[C@@H]1[C@H](O)[C@@H](O)[C@H](C)O[C@H]1O[C@H]1[C@H](OC=2C=C3O[C@@H](CC(=O)C3=C(O)C=2)C=2C=CC(O)=CC=2)O[C@H](CO)[C@@H](O)[C@@H]1O DFPMSGMNTNDNHN-ZPHOTFPESA-N 0.000 description 1
- 229940052490 naringin Drugs 0.000 description 1
- ZYTMANIQRDEHIO-UHFFFAOYSA-N neo-Isopulegol Natural products CC1CCC(C(C)=C)C(O)C1 ZYTMANIQRDEHIO-UHFFFAOYSA-N 0.000 description 1
- 235000019412 neotame Nutrition 0.000 description 1
- HLIAVLHNDJUHFG-HOTGVXAUSA-N neotame Chemical compound CC(C)(C)CCN[C@@H](CC(O)=O)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 HLIAVLHNDJUHFG-HOTGVXAUSA-N 0.000 description 1
- 108010070257 neotame Proteins 0.000 description 1
- HIGQPQRQIQDZMP-FLIBITNWSA-N neryl acetate Chemical compound CC(C)=CCC\C(C)=C/COC(C)=O HIGQPQRQIQDZMP-FLIBITNWSA-N 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- YYZUSRORWSJGET-UHFFFAOYSA-N octanoic acid ethyl ester Natural products CCCCCCCC(=O)OCC YYZUSRORWSJGET-UHFFFAOYSA-N 0.000 description 1
- RZPAKFUAFGMUPI-KGIGTXTPSA-N oleandomycin Chemical compound O1[C@@H](C)[C@H](O)[C@@H](OC)C[C@@H]1O[C@@H]1[C@@H](C)C(=O)O[C@H](C)[C@H](C)[C@H](O)[C@@H](C)C(=O)[C@]2(OC2)C[C@H](C)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C RZPAKFUAFGMUPI-KGIGTXTPSA-N 0.000 description 1
- 229960002351 oleandomycin Drugs 0.000 description 1
- 235000019367 oleandomycin Nutrition 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- HFPZCAJZSCWRBC-UHFFFAOYSA-N p-cymene Chemical compound CC(C)C1=CC=C(C)C=C1 HFPZCAJZSCWRBC-UHFFFAOYSA-N 0.000 description 1
- 229940098695 palmitic acid Drugs 0.000 description 1
- QNGNSVIICDLXHT-UHFFFAOYSA-N para-ethylbenzaldehyde Natural products CCC1=CC=C(C=O)C=C1 QNGNSVIICDLXHT-UHFFFAOYSA-N 0.000 description 1
- SQYNKIJPMDEDEG-UHFFFAOYSA-N paraldehyde Chemical compound CC1OC(C)OC(C)O1 SQYNKIJPMDEDEG-UHFFFAOYSA-N 0.000 description 1
- 229960003868 paraldehyde Drugs 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- 235000021017 pears Nutrition 0.000 description 1
- TZMFJUDUGYTVRY-UHFFFAOYSA-N pentane-2,3-dione Chemical compound CCC(=O)C(C)=O TZMFJUDUGYTVRY-UHFFFAOYSA-N 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 150000007875 phellandrene derivatives Chemical class 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 229940100595 phenylacetaldehyde Drugs 0.000 description 1
- 229940081310 piperonal Drugs 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 235000013606 potato chips Nutrition 0.000 description 1
- 235000008476 powdered milk Nutrition 0.000 description 1
- 230000003658 preventing hair loss Effects 0.000 description 1
- 229960002429 proline Drugs 0.000 description 1
- 235000021568 protein beverage Nutrition 0.000 description 1
- MUPFEKGTMRGPLJ-ZQSKZDJDSA-N raffinose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)O1 MUPFEKGTMRGPLJ-ZQSKZDJDSA-N 0.000 description 1
- 229930188195 rebaudioside Natural products 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 229930000308 sinensal Natural products 0.000 description 1
- 230000009759 skin aging Effects 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 229960004249 sodium acetate Drugs 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229960002668 sodium chloride Drugs 0.000 description 1
- 229960001462 sodium cyclamate Drugs 0.000 description 1
- 239000000176 sodium gluconate Substances 0.000 description 1
- 235000012207 sodium gluconate Nutrition 0.000 description 1
- 229940005574 sodium gluconate Drugs 0.000 description 1
- 239000001540 sodium lactate Substances 0.000 description 1
- 229940005581 sodium lactate Drugs 0.000 description 1
- 235000011088 sodium lactate Nutrition 0.000 description 1
- 239000004317 sodium nitrate Substances 0.000 description 1
- 235000010344 sodium nitrate Nutrition 0.000 description 1
- 229940001516 sodium nitrate Drugs 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 235000008983 soft cheese Nutrition 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 235000013322 soy milk Nutrition 0.000 description 1
- 239000008347 soybean phospholipid Substances 0.000 description 1
- 238000007655 standard test method Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 229940013618 stevioside Drugs 0.000 description 1
- OHHNJQXIOPOJSC-UHFFFAOYSA-N stevioside Natural products CC1(CCCC2(C)C3(C)CCC4(CC3(CCC12C)CC4=C)OC5OC(CO)C(O)C(O)C5OC6OC(CO)C(O)C(O)C6O)C(=O)OC7OC(CO)C(O)C(O)C7O OHHNJQXIOPOJSC-UHFFFAOYSA-N 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 235000019408 sucralose Nutrition 0.000 description 1
- BAQAVOSOZGMPRM-QBMZZYIRSA-N sucralose Chemical compound O[C@@H]1[C@@H](O)[C@@H](Cl)[C@@H](CO)O[C@@H]1O[C@@]1(CCl)[C@@H](O)[C@H](O)[C@@H](CCl)O1 BAQAVOSOZGMPRM-QBMZZYIRSA-N 0.000 description 1
- LBDVSPIQWSQRLB-UHFFFAOYSA-N sucrononic acid Chemical compound C=1C=C(C#N)C=CC=1\N=C(/NCC(=O)O)NC1CCCCCCCC1 LBDVSPIQWSQRLB-UHFFFAOYSA-N 0.000 description 1
- 150000005846 sugar alcohols Chemical class 0.000 description 1
- 235000019527 sweetened beverage Nutrition 0.000 description 1
- 229930006978 terpinene Natural products 0.000 description 1
- 150000003507 terpinene derivatives Chemical class 0.000 description 1
- 229940116411 terpineol Drugs 0.000 description 1
- 239000000892 thaumatin Substances 0.000 description 1
- 235000010436 thaumatin Nutrition 0.000 description 1
- 229960000790 thymol Drugs 0.000 description 1
- MBDOYVRWFFCFHM-UHFFFAOYSA-N trans-2-hexenal Natural products CCCC=CC=O MBDOYVRWFFCFHM-UHFFFAOYSA-N 0.000 description 1
- LVBXEMGDVWVTGY-UHFFFAOYSA-N trans-2-octenal Natural products CCCCCC=CC=O LVBXEMGDVWVTGY-UHFFFAOYSA-N 0.000 description 1
- NPFVOOAXDOBMCE-UHFFFAOYSA-N trans-3-hexenyl acetate Natural products CCC=CCCOC(C)=O NPFVOOAXDOBMCE-UHFFFAOYSA-N 0.000 description 1
- ZFDIRQKJPRINOQ-UHFFFAOYSA-N transbutenic acid ethyl ester Natural products CCOC(=O)C=CC ZFDIRQKJPRINOQ-UHFFFAOYSA-N 0.000 description 1
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 1
- 238000004704 ultra performance liquid chromatography Methods 0.000 description 1
- 238000000825 ultraviolet detection Methods 0.000 description 1
- WCTNXGFHEZQHDR-UHFFFAOYSA-N valencene Natural products C1CC(C)(C)C2(C)CC(C(=C)C)CCC2=C1 WCTNXGFHEZQHDR-UHFFFAOYSA-N 0.000 description 1
- 229940005605 valeric acid Drugs 0.000 description 1
- 235000015192 vegetable juice Nutrition 0.000 description 1
- 239000000052 vinegar Substances 0.000 description 1
- 235000021419 vinegar Nutrition 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
- 235000013618 yogurt Nutrition 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07J—STEROIDS
- C07J63/00—Steroids in which the cyclopenta(a)hydrophenanthrene skeleton has been modified by expansion of only one ring by one or two atoms
- C07J63/008—Expansion of ring D by one atom, e.g. D homo steroids
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L2/00—Non-alcoholic beverages; Dry compositions or concentrates therefor; Their preparation
- A23L2/52—Adding ingredients
- A23L2/60—Sweeteners
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L27/00—Spices; Flavouring agents or condiments; Artificial sweetening agents; Table salts; Dietetic salt substitutes; Preparation or treatment thereof
- A23L27/30—Artificial sweetening agents
- A23L27/33—Artificial sweetening agents containing sugars or derivatives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L27/00—Spices; Flavouring agents or condiments; Artificial sweetening agents; Table salts; Dietetic salt substitutes; Preparation or treatment thereof
- A23L27/88—Taste or flavour enhancing agents
Definitions
- the invention relates to novel compounds according to formula (I), a method for producing such a compound, a mixture comprising such a compound, a composition for food or pleasure or a pharmaceutical composition comprising one or more such compound(s) or a such a mixture, as well as to the use of such a compound or of such a mixture for imparting or modifying a sweet taste impression and to a method for imparting or modifying a sweet taste impression.
- the World Health Organization recommends a daily uptake of free sugars of at most 10 % of the daily total energy supply. Considering a daily total energy supply of 2000 kcal, the recommendation corresponds to approximately 50 g of free sugar such as glucose, fructose or saccharose (WHO, 2015, ISBN 9789241549028).
- Sweeteners are chemical compounds, which have no or only a small caloric value and, at the same time, provide a strong sweet taste impression. An overview of such sweeteners may, for example, be found in the Journal of the American Dietetic Association 2004, 104 (2), 255-275).
- these sweeteners may be added to foodstuff in low amounts. In this way, it is possible to replace large amounts of carbohydrates with low amounts of sweeteners.
- Such sweeteners include steviosides from Stevia rebaudiana, rubusosides from Rubus suavissimus and mogrosides from Siraitia grosvenorii.
- X and Y are selected from hydrogen or CH3, wherein only one of X and Y is CH3, wherein R 1 to R 7 are selected from hydrogen or a saccharide, wherein the or all monomer subunit(s) of the saccharide is I are monomer(s) selected from the group consisting of glucose, galactose, fructose, rhamnose, xylose, glucuronic acid, quinovose, arabinose and mixtures thereof, wherein R 1 to R 7 are selected such that the compound comprises a sum of at least two monomer subunits represented by one or more of R 1 to R 7 , or a salt thereof.
- this saccharide can be bound to the remaining formula (I) via an a- or via a p-glycosidic bond, which may be individual for each saccharide.
- the saccharide is bound to the remaining formula (I) via an a-1-2-, a-1-3-, a-1- 4-, a-1 -6-, p-1-2-, p-1-3-, p-1-4- or p-1 -6-glycosidic bond, which may be individual for each saccharide.
- saccharide as used herein describes a substituent structure.
- this substituent is obtained by removing the hemiacetal hydroxyl group from the monosaccharide or from the first monosaccharide subunit of the polymeric chain of monosaccharide subunits to form a glycosidic bond.
- saccharide as used herein describes mono-, di-, tri-, oligo- and/or polysaccharides.
- di-, tri-, oligo- and polysaccharides describes a polymeric chain of monosaccharide subunits, which are bound together by glycosidic bonds.
- the polymeric chain may be linear or branched at one or more elements of the chain, wherein “linear” describes that each monomer of the polymeric chain is connected to two other monomers, wherein the first and the last monomer of the chain are only connected to one other monomer.
- the polymeric chain is linear.
- the monosaccharide subunits of the polymeric chain of monosaccharide subunits may be the same monosaccharide subunits or may be different units, which is described by the term “mixtures thereof’ as used in the selection of monomers.
- monosaccharide subunit describes a monosaccharide, which is, in the di-, tri-, oligo- or polysaccharides, connected to at least one other monosaccharide to form the di-, tri-, oligo- or polysaccharides.
- the term describes a structure, which is based on the single monosaccharide molecule but which is connected to at least one further monosaccharide monomer to form the di-, tri-, oligo- or polysaccharides.
- R 1 to R 7 are selected such that the compound comprises a sum of at least two monomer subunits represented by one or more of R 1 to R 7 ” as used herein describes that even though R 1 to R 7 may be selected individually, formula (I) requires that in sum at least two monomer subunits need to be found among the residues R 1 to R 7 .
- the at least two monomer subunits can be present in the same residue of R 1 to R 7 or in different residues of R 1 to R 7 .
- R 1 may comprise one monomer subunit and e.g. R 2 may comprise another monomer subunit.
- R 1 may comprise two (or more) subunits.
- the salt of a compound according to formula (I), as described herein, may be any salt.
- the salt is a pharmaceutically acceptable salt.
- Madecassoside and terminoloside are known glucosides of madecassic acid or terminolic acid.
- Madecassoside (CAS registry number: 34540-22-2) has been known since the middle of the 20 th century.
- the sugar residue of madecassoside was described by Pinhas et al in 1967 as 2x D-glucose and 1x L-rhamnose (Pinhas et al., Bulletin de la Societe Chimique de France (1967), (6), 1888-90).
- No application regarding sweetness-modulation has been described for these glucosides.
- madecassic acid and terminolic acid or, respectively, madecassoside and terminoloside are rather used for cosmetic purposes.
- madecassic acid-28-monoglucoside (Centelloside C, CAS registry number 1361016-45-6) and madecassic acid-28-diglucoside (Centellasaponin B, CAS registry number 386223-75- 2) are known.
- Madecassic acid and terminolic acid are the main components synthesized by the plant Gotu Kola (Centella asiatica), in addition to the triterpenes asiaticoside and asiatic acid (Feng-Lun et al., Biomedical Chromatography (2008), 22(2), 119-124).
- EP 0867447 A1 describes the production of a water- soluble extract from Gotu Kola, which contains high amounts of asiaticoside and madecassoside.
- FR 2848117 A1 describes the production of an extract containing more than 95% of a mixture of madecassoside and terminoloside.
- Extracts from Gotu Kola are also mainly used for cosmetic purposes such as in skin care products for preventing skin aging (WO 97/39734 A1) or for preventing hair loss (WO 2005/123032 A2).
- sweetness-modulation has been described. It was thus surprisingly found that the compounds according to formula (I) provide sweetness-modulating attributes, which may improve the taste profile of sugar-reduced foodstuff or beverage. This was particularly surprising, since madecassic acid and terminolic acid provide a bitter taste. Moreover, this was also surprising, since it was found that e.g. madecassoside does not provide any sweetness-modulating attributes, as shown in example 5.1 below.
- the compounds according to the invention may be of any possible three-dimensional structure, i.e. formula (I) encompasses any possible stereoisomer.
- the compounds according to the invention are of formula (la): wherein X and Y are selected from hydrogen or CH3, wherein only one of X and Y is CH3, wherein R 1 to R 7 are selected from hydrogen or a saccharide, wherein the or all monomer subunit(s) of the saccharide is / are monomer(s) selected from the group consisting of glucose, galactose, fructose, rhamnose, xylose, glucuronic acid, quinovose, arabinose and mixtures thereof, wherein R 1 to R 7 are selected such that the compound comprises a sum of at least two monomer subunits represented by one or more of R 1 to R 7 , or a salt thereof.
- rhamnose or glucose if “Glc” is mentioned, which is bound to the remaining structure via an a-1 ,4 glycosidic bond.
- rhamnose or “glucose”, as used in this context, does not describe a non-connected, single rhamnose or glucose molecule. Instead, the term describes a structure, which is based on the single rhamnose or glucose molecule but which is connected to the remaining structure to form the compound according to the invention.
- the invention further relates to a method for producing a compound according to the invention or a salt thereof, comprising the following steps:
- madecassoside and/or terminoloside preferably providing a plant extract comprising madecassoside and/or terminoloside, particularly preferably providing a plant extract of Gotu Kola comprising madecassoside and/or terminoloside, further preferably providing a plant extract of Gotu Kola comprising 50 to 95 wt.-% madecassoside and/or terminoloside, based on the total weight of the plant extract,
- saccharide(s) preferably wherein the, one or more or all saccharide(s) is I are selected from the group consisting of glucose, galactose, fructose, rhamnose, xylose, saccharose, lactose, UDP-glucose, maltose, starch, maltodextrin, cyclodextrin, glucuronic acid, quinovose, arabinose and mixtures thereof,
- Gotu Kota mainly synthesizes the triterpenes asiaticoside, madecassoside, asiatic acid, madecassic acid, terminolic acid and terminoloside. These triterpenes contribute (in sum) to more than 1 % of the dried plant mass (Feng-Lun et al., Biomedical Chromatohraphy 2008, 22(2), 119-124). Therefore, it is preferred that extracts from Gotu Kota are used as starting material for providing madecassoside and/or terminoloside.
- such an extract comprises 1 to 100 wt.-% of asiaticoside, madecassoside, asiatic acid, madecassic acid, terminolic acid and terminoloside, particularly preferably such an extract comprises 50 to 95 wt.-% madecassoside and/or terminoloside, based on the total weight of the plant extract.
- the glycosyltransferase provided in step (iii) is a glycosyltransferase according to Gerwig et al., Advances in Carbohydrate Chemistry and Biochemistry, 2016, 73, or according to Zhao et al., J Agric. Food Chem., 2020, 68 or a mixture thereof.
- the glycosyltransferase provided in step (iii) is selected from the group consisting of glucanotransferases, glucosyltransferases and mixtures thereof, preferably wherein the glycosyltransferase is selected from the group consisting of a- glucosyltransferases (such as a-glucosidase, CGTase, glucan sucrase (preferably glucansucrase GTF 180) or dextransucrase), p-glucosyltransferases (such as 1 ,2-p- glucosyltransferase (OleD, preferably from Streptomyces antibioticus, Yjic, preferably from Bacillus licheniformis, or UGTSL2, preferably from Solanum lycopersicum), p-1 ,3- glucosyltransferases (such as laminarinase) or p-1 ,4-glucosyltransferase
- a-galactosidases such as enzyme preparations originating from A. oryzae, Kluyveromyces lactis, Kluyveromyces sp., B. licheniformis'
- p-galactosidases such as enzyme preparations originating from Kluyveromces lactis or B. circulans
- L- rhamnosidases such as enzyme preparations originating from Thermomicrobia sp.
- glycosyltransferase is selected from the group consisting of cyclomaltodextrin glucanotransferase, glucan sucrose, yjic glucosyltransferase, oleandomycin glycosyltransferase and mixtures thereof.
- the glycosyltransferase is obtained commercially or via a cell culture approach, in which cells, preferably E.coli cells, are transformed with a vector comprising the coding sequence of the glycosyltransferase of interest, e.g. as described in the below example section.
- monosaccharides from substrates as e.g. saccharose, lactose, UDP-glucose, maltose, starch, maltodextrin, cyclodextrin and mixtures thereof can be bound to formula (I), i.e. as one or more of R 1 to R 7 , via a a-1-2-, a-1-3-, a-1-4-, a-1-6-, p- 1-2-, p-1-3-, p-1-4- or p-1-6-glycosidic bond, which may be individual for each saccharide.
- formula (I) i.e. as one or more of R 1 to R 7 , via a a-1-2-, a-1-3-, a-1-4-, a-1-6-, p- 1-2-, p-1-3-, p-1-4- or p-1-6-glycosidic bond, which may be individual for each saccharide.
- the, one or more or all saccharide(s) provided in step (ii) is I are selected from the group consisting of maltodextrin, saccharose and mixtures thereof, and/or the glycosyltransferase is selected from the group consisting of CTGase, Glucansucrase GTF 180 and mixtures thereof.
- the saccharide provided in step (ii) comprises or consists of maltodextrin and saccharose and the glycosyltransferase provided in step (iii) comprises or consists of CTGase and Glucansucrase GTF 180.
- the saccharide provided in step (ii) consists of maltodextrin and saccharose and the glycosyltransferase provided in step (iii) consists of CTGase and Glucansucrase GTF 180.
- step (iv) is performed while shaking.
- step (iv) allows a glycosylation of the provided madecassoside and/or terminoloside to obtain a compound according to formula (I).
- the method additionally comprises the step of purifying the obtained glycosylated madecassoside and/or terminoloside, wherein the term “purifying” is broadly understood as increasing the concentration of the obtained glycosylated madecassoside and/or terminoloside, e.g. by removing other components of the obtained mixture.
- step (iv) is performed at a temperature in a range of from 30 to 60 °C, preferably in a range of from 35 to 55 °C, since particularly advantageous yields were obtained in these temperature ranges.
- step (iv) is performed for 5 to 48 h, preferably for 12 to 36 h, particularly preferably for 18 to 30 h, further preferably for 20 to 27 h.
- step (iv) is inactivated, preferably before the step of purification is performed, if present.
- the method according to the invention further comprises the step of purifying the mixture obtained after step (iv), preferably by chromatography, such as ion exchange chromatography, or by solid phase purification or microfiltration.
- step (iv) is inactivated, preferably by applying a temperature in the range of from 70 to 100 °C, preferably in the range of from 75 to 90 °C, particularly preferably in the range of from 75 to 85 °C, and/or that the mixture obtained in step (iv) is dried, preferably by a drying method selected from the group consisting of freeze-drying, spray drying, vacuum belt drying or heat drying, particularly preferably by freeze-drying.
- the invention further relates to a compound according to the invention, wherein the compound is obtainable or obtained by a method according to the invention, or a salt thereof.
- the invention relates to a mixture comprising
- the total weight of the compound(s) according to the invention and their salts is in a range of from 0.1 to 99 wt.-%, preferably in a range of from 1 to 50 wt.-%, particularly preferably in a range of from 3 to 10 wt.-%, based on the total weight of the mixture.
- the total weight of the compound(s) according to the invention and their salts refers in this case to the sum of all compounds according to the invention and all salts thereof, as far as present in the mixture.
- madecassoside and/or terminoloside is I are glycosylated.
- the mixture comprises madecassoside, wherein the total weight of madecassoside is at most 50 wt.-%, preferably at most 40 wt.-%, particularly preferably at most 30 wt.-%, further preferably at most 25 wt.- %, more preferably at most 20 wt.-, based on the total weight of the mixture.
- the mixture comprises terminoloside, wherein the total weight of terminoloside is at most 50 wt.-%, preferably at most 40 wt.-%, particularly preferably at most 30 wt.-%, further preferably at most 25 wt.-%, more preferably at most 20 wt.-, based on the total weight of the mixture.
- the mixture comprises madecassoside and terminoloside, wherein the total weight of madecassoside and terminoloside is at most 50 wt.-%, preferably at most 40 wt.-%, particularly preferably at most 30 wt.-%, further preferably at most 25 wt.-%, more preferably at most 20 wt.-, based on the total weight of the mixture.
- the present invention relates to a composition for food or pleasure or pharmaceutical composition
- a composition for food or pleasure or pharmaceutical composition comprising one or more compound(s) according the invention or a mixture according to the invention.
- the composition according to the invention may be present in a form selected from the group consisting of tablets (non-coated as well as coated tablets, single or multiple layered tablets), capsules, lozenges, granules, pellets, solid substance mixtures, dispersions in liquid phases, emulsions, powders, solutions, juices, pastes or other swallowable or chewable preparations.
- the composition according to the invention serving for food or pleasure may be selected from the group consisting of baked goods, for example, bread, dry biscuits, cakes, other baked products, confectionery (for example, chocolate, chocolate bar products, other bar products, fruit gum, hard and soft caramels, chewing gum), alcoholic or nonalcoholic beverages (for example, coffee, tea, iced tea, wine, wine-containing beverages, beer, beercontaining beverages, liqueurs, brandies, (carbonated) fruit-containing beverages, (carbonated) isotonic beverages, (carbonated) soft beverages, nectars, spritzers, fruit and vegetable juices, fruit or vegetable juice formulations, instant beverages (for example, instant cocoa beverages, instant tea beverages, instant coffee beverages, instant fruit beverages), meat products (for example, ham, fresh sausage or uncooked sausage formulations, seasoned or marinated fresh or salted meat products), eggs or egg products (dried egg, egg white, egg yolk), cereal products (for example, breakfast cereals, muesli bars, precooked ready-made rice products), dairy products (for example), dairy products
- sweets particularly preferred herein are sweets, dairy products and very particularly preferred are non-alcoholic beverages where sweetened beverages are particularly preferred.
- composition according to the invention may contain one or more further sweeteners and/or one or more further aroma substances.
- the, one or more or all further sweeteners may be selected from the group consisting of carbohydrates and specifically sugars such as, for example, sucrose/saccharose, trehalose, lactose, maltose, melizitose, raffinose, palatinose, lactulose, D-fructose, D-allulose, D-glucose, D-galactose, L-rhamnose, D-sorbose, D- mannose, D-tagatose, D-arabinose, L-arabinose, D-xylose, D-ribose, D-glyceraldehyde or maltodextrin; synthetic, i.e.
- sugars such as, for example, sucrose/saccharose, trehalose, lactose, maltose, melizitose, raffinose, palatinose, lactulose, D-fructose, D-allulose, D-
- sugar hydrolysates invert sugar, fructose syrup
- fruit concentrates e.g., on the basis of apples or pears
- sugar alcohols e.g., erythritol, threitol, arabitol, ribotol, xylitol, sorbitol, mannitol, dulcitol, lactitol
- proteins e.g., miraculin, monellin, thaumatin, curculin, brazzein
- sweeteners e.g., magap, sodium cyclamate, acesulfam K, neohesperidin dihydrochalcone, saccharine sodium salt, aspartame, superaspartame, neotame, alitame, sucralose, stevioside, rebaudioside, lugduname, carrelame, sucrononate, sucrooctate, monatin, phen
- Extracts comprising individual substances such as, for example, Momordica grosvenori [Luo Han Guo] and the mogrosides obtained thereof, Hydrangea dulcis or extracts, phyllodulcin; balansins of Mycetia balansae.
- the, one or more or all further aroma substances may be selected from the group consisting of acetophenone, allyl caproate, alpha-ionone, beta-ionone, aniseed aldehyde, anisyl acetate, anisyl formate, benzaldehyde, benzothiazole, benzyl acetate, benzyl alcohol, benzyl benzoate, beta-ionone, butyl butyrate, butyl capronate, butylidene phthalide, carvone, camphene, caryophyllene, cineol, cinnamyl acetate, citral, citronellol, citronellal, citronellyl acetate, cyclohexyl acetate, cymol, damascene, decalactone, dihydrocoumarin, dimethyl anthranilate, dimethyl anthranilate, dodecalactone, ethoxy ethyl
- the total weight of the compound(s) according to the invention is in a range of from 0.001 to 60 wt.-%, preferably in a range of from 0.01 to 40 wt.-%, particularly preferably in a range of from 0.1 to 30 wt.- %, further preferably in a range of from 0.5 to 20 wt.-%, more preferably in a range of from 1 to 10 wt.-%, based on the total weight of the composition.
- the invention further relates to the use of a compound according to the invention or a mixture according to the invention for imparting or modifying a sweet taste impression. Moreover, the invention also relates to a method for imparting and/or modifying a sweet taste impression, comprising the following steps:
- step (ii) mixing the compound or mixture provided in step (i) with a substance or product, of which a sweet taste impression shall be imparted and/or modified.
- modifying a sweet taste impression describes any kind of modification, such as optimizing or increasing a sweet taste impression.
- Example 1 Production of qlucosylated madecassoside with cyclomaltodextrin qlucanotransferase
- the reaction was inactivated by applying a temperature of 80 °C for 10 min.
- the composition of the obtained mixture was analysed via HPLC coupled to high resolution mass spectrometry, UV detection and Corona detection (LC-CAD).
- LC-CAD high resolution mass spectrometry, UV detection and Corona detection
- the chromatographic separation of the compounds according to the invention was performed at a Waters Acquity UPLC Systems (Waters, Eschborn, Germany) with a C-18 column (Kinetex, 100 mm x2.1 mm, 1 .7 pm; Phenomenex) at a temperature of 50 °C, Water/Acetonitrile with 0.1 % formic acid as mobile phase and a flow of 0.55 mL/min.
- the detection was performed with a Bruker microTOFQII mass spectrometer with ESI ionization at a scanning range of 50 to 2000 Da and a charged aerosol detector (Corona Veo, Thermo Scientific, Germany).
- the area ratios were generally used to calculate the amount ratios of the respective compounds, based on the educt concentration.
- a pET28a_gft180Q-1130E-A-N vector was synthesized and codon-optimized for E. coli K12.
- the expression plasmide was transformed in E-coli W3110 (ADE3).
- E.coli W3110 including pET28a_gtf180-Q1140E-AN was cultured in 15 mL Luria- Bertani Medium (Carl Roth) with 30 pg/mL Neomycin (Carl Roth) at 37 °C, 200 rpm over night in a laboratory shaker. Subsequently, 200 mL Terrific Broth Medium (Carl Roth) with 30 pg/mL Neomycin were inoculated at an OD of 0.1 (600 nm) and cultured at 37 °C while shaking. After obtaining an OD of 0.6 to 0-8 (600 nm), the culture was induced with 0.5 mM IPTG and further incubated at 20 °C for 16 to 18 h.
- the cells were harvested by centrifugation at 17,000 x g for 10 min. Subsequently, the pellet was resuspended in 25 mM sodium citrate buffer pH 4.8 + 1 mM CaCh, such that the concentration corresponded to 200 gBFM/L. The cell lysis was performed via ultrasound sonotrode. The cell fragments were separated at 17,000 x g for 10 min.
- Compounds 31 a to 64a correspond to compounds 31 a to 64a as in table 1.
- a pET28a_yjic vector was synthesized and codon-optimized for E. col 7K12.
- the expression plasmide was transformed in E-coli W3110 (ADE3).
- E.coli W3110 including pET28a_Yjic was cultured in 15 mL Luria-Bertani Medium (Carl Roth) with 30 pg/mL Neomycin (Carl Roth) at 37 °C, 200 rpm overnight in a laboratory shaker. Subsequently, 200 mL Terrific Broth Medium (Carl Roth) with 30 pg/mL Neomycin were inoculated at an OD of 0.1 (600 nm) and cultured at 37 °C while shaking. After obtaining an OD of 0.6 to 0-8 (600 nm), the culture was induced with 0.5 mM IPTG and further incubated at 20 °C for 16 to 18 h.
- the cells were harvested by centrifugation at 17,000 x g for 10 min. Subsequently, the pellet was resuspended in 100 mM Tris/HCI (pH 7.5) + 2 mM MgCh, such that the concentration corresponded to 200 gBFM/L. The cell lysis was performed via ultrasound sonotrode. The cell fragments were separated at 17,000 x g for 10 min. Biotransformation of madecassoside into glucosylated madecassoside with Yjic
- the mixture contained the following components
- Compounds 65a to 70a correspond to compounds 65a to 70a as in table 1 .
- a pET28a_SaOleDGtf vector was synthesized and codon-optimized for E. coli K12.
- the expression plasmide was transformed in E-coli BL21 (ADE3).
- E.coli BL21 (ADE3) including pET28a_SaOleDGtf was cultured in 15 mL Luria-Bertani
- the composition of the obtained mixture was analysed via LC-CAD.
- the mixture contained the following components
- Compounds 65a to 67a correspond to compounds 65a to 67a as in table 1 .
- Compounds 1 a to 64a correspond to compounds 1 a to 64a as in table 1 .
- the compounds according to the invention provide sweetnessmodulating attributes, even though the glucoside madecassoside did not show any effect on sweetness.
- the enzymatic transformation of madecassoside according to Example 1 provides compounds 1 a to 17a, which increase the sweetness of the 5 % saccharose solution (Test no. 3).
- compounds 6a to 10a increase the sweetness of the 5 % saccharose solution (Test no. 5).
- compounds 31 a to 33a increase the sweetness of the 5 % saccharose solution (Test no. 6).
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Nutrition Science (AREA)
- Engineering & Computer Science (AREA)
- Food Science & Technology (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Saccharide Compounds (AREA)
Abstract
The invention relates to novel compounds according to formula (I), a method for producing such a compound, a mixture comprising such a compound, a composition for food or pleasure or a pharmaceutical composition comprising one or more such compound(s) or a such a mixture, as well as to the use of such a compound or of such a mixture for imparting or modifying a sweet taste impression and to a method for imparting or modifying a sweet taste impression.
Description
Taste balancing botanical compounds
The invention relates to novel compounds according to formula (I), a method for producing such a compound, a mixture comprising such a compound, a composition for food or pleasure or a pharmaceutical composition comprising one or more such compound(s) or a such a mixture, as well as to the use of such a compound or of such a mixture for imparting or modifying a sweet taste impression and to a method for imparting or modifying a sweet taste impression.
Consumers generally have a strong preference for foodstuffs or indulgence foods, which have a large amount of high caloric sugar, in particular sucrose (saccharose), glucose, fructose or mixtures thereof, due to the pleasant sweetness and sweetness profile associated therewith.
However, it is generally known that a large content of readily metabolizable carbohydrates causes a steep rise in blood sugar levels, leads to the formation of fat deposits and ultimately can result in health problems such as overweight, obesity, insulin resistance, age-onset diabetes and complications thereof.
Furthermore, several of such carbohydrates may negatively affect dental health, as they can be metabolized by several bacteria, which are present in the oral cavity. The metabolites of these carbohydrates may in turn degrade the tooth enamel and finally lead to caries.
The World Health Organization (WHO) recommends a daily uptake of free sugars of at most 10 % of the daily total energy supply. Considering a daily total energy supply of 2000
kcal, the recommendation corresponds to approximately 50 g of free sugar such as glucose, fructose or saccharose (WHO, 2015, ISBN 9789241549028).
The actual daily uptake of free sugars of men and women in industrialized countries, e.g. in Germany, lies significantly higher than the recommendation of the WHO, i.e. 61 g/day (women) or, respectively, 78 g/day (men).
Therefore, it has long been an objective to reduce the high caloric sugar content of food or beverage products as far as possible, without significantly affecting the perceived sweetness.
One measure is to replace the carbohydrates by sweeteners. Sweeteners are chemical compounds, which have no or only a small caloric value and, at the same time, provide a strong sweet taste impression. An overview of such sweeteners may, for example, be found in the Journal of the American Dietetic Association 2004, 104 (2), 255-275).
With their high sweetening power, these sweeteners may be added to foodstuff in low amounts. In this way, it is possible to replace large amounts of carbohydrates with low amounts of sweeteners.
Such sweeteners include steviosides from Stevia rebaudiana, rubusosides from Rubus suavissimus and mogrosides from Siraitia grosvenorii.
However, one drawback of these sweeteners is their unpleasant aftertaste, including taste impressions such as lingering or bitter. The application of these sweeteners is thus limited.
It was thus an object of the present invention to provide novel substances with sweetnessmodulating attributes, which may improve the taste profile of sugar-reduced foodstuff or beverage.
The primary object of the present invention is solved by a compound according to formula (I):
wherein X and Y are selected from hydrogen or CH3, wherein only one of X and Y is CH3, wherein R1 to R7 are selected from hydrogen or a saccharide, wherein the or all monomer subunit(s) of the saccharide is I are monomer(s) selected from the group consisting of glucose, galactose, fructose, rhamnose, xylose, glucuronic acid, quinovose, arabinose and mixtures thereof, wherein R1 to R7 are selected such that the compound comprises a sum of at least two monomer subunits represented by one or more of R1 to R7, or a salt thereof. In case a residue of R1 to R7 is a saccharide, this saccharide can be bound to the remaining formula (I) via an a- or via a p-glycosidic bond, which may be individual for each saccharide.
Preferably, the saccharide is bound to the remaining formula (I) via an a-1-2-, a-1-3-, a-1- 4-, a-1 -6-, p-1-2-, p-1-3-, p-1-4- or p-1 -6-glycosidic bond, which may be individual for each saccharide.
The term “saccharide” as used herein describes a substituent structure. Preferably, this substituent is obtained by removing the hemiacetal hydroxyl group from the monosaccharide or from the first monosaccharide subunit of the polymeric chain of monosaccharide subunits to form a glycosidic bond.
Further, the term “saccharide” as used herein describes mono-, di-, tri-, oligo- and/or polysaccharides.
The terms “di-, tri-, oligo- and polysaccharides”, as used herein describes a polymeric chain of monosaccharide subunits, which are bound together by glycosidic bonds. The polymeric chain may be linear or branched at one or more elements of the chain, wherein “linear” describes that each monomer of the polymeric chain is connected to two other monomers, wherein the first and the last monomer of the chain are only connected to one other monomer. Preferably, the polymeric chain is linear. The monosaccharide subunits of the polymeric chain of monosaccharide subunits may be the same monosaccharide subunits or may be different units, which is described by the term “mixtures thereof’ as used in the selection of monomers.
The term “monosaccharide subunit”, as used herein describes a monosaccharide, which is, in the di-, tri-, oligo- or polysaccharides, connected to at least one other monosaccharide to form the di-, tri-, oligo- or polysaccharides. The term describes a structure, which is based on the single monosaccharide molecule but which is connected to at least one further monosaccharide monomer to form the di-, tri-, oligo- or polysaccharides.
The term “wherein R1 to R7 are selected such that the compound comprises a sum of at least two monomer subunits represented by one or more of R1 to R7” as used herein describes that even though R1 to R7 may be selected individually, formula (I) requires that in sum at least two monomer subunits need to be found among the residues R1 to R7. The at least two monomer subunits can be present in the same residue of R1 to R7 or in different residues of R1 to R7. Thus, e.g. R1 may comprise one monomer subunit and e.g. R2 may comprise another monomer subunit. Additionally or alternatively, e.g. R1 may comprise two (or more) subunits.
The salt of a compound according to formula (I), as described herein, may be any salt. Preferably, the salt is a pharmaceutically acceptable salt.
The compounds according to formula (I) are distinct glucosides of madecassic acid (CAS registry number: 18449-41-7; with X=H and Y=CH3) or of terminolic acid (CAS registry number: 564-13-6; with X=CH3 and Y=H).
Madecassoside and terminoloside are known glucosides of madecassic acid or terminolic acid.
Madecassoside (CAS registry number: 34540-22-2) has been known since the middle of the 20th century. The sugar residue of madecassoside was described by Pinhas et al in 1967 as 2x D-glucose and 1x L-rhamnose (Pinhas et al., Bulletin de la Societe Chimique de France (1967), (6), 1888-90). However, no application regarding sweetness-modulation has been described for these glucosides. Instead, madecassic acid and terminolic acid or, respectively, madecassoside and terminoloside are rather used for cosmetic purposes.
Little is known about further glucosides of madecassic acid or terminolic acid. Only madecassic acid-28-monoglucoside (Centelloside C, CAS registry number 1361016-45-6) and madecassic acid-28-diglucoside (Centellasaponin B, CAS registry number 386223-75- 2) are known.
Madecassic acid and terminolic acid are the main components synthesized by the plant Gotu Kola (Centella asiatica), in addition to the triterpenes asiaticoside and asiatic acid (Feng-Lun et al., Biomedical Chromatography (2008), 22(2), 119-124).
Several methods for obtaining an extract from Gotu Kola, which contains high amounts of madecassoside have been described. EP 0867447 A1 describes the production of a water- soluble extract from Gotu Kola, which contains high amounts of asiaticoside and madecassoside. Furthermore, FR 2848117 A1 describes the production of an extract containing more than 95% of a mixture of madecassoside and terminoloside.
Extracts from Gotu Kola are also mainly used for cosmetic purposes such as in skin care products for preventing skin aging (WO 97/39734 A1) or for preventing hair loss (WO 2005/123032 A2). However, no application regarding sweetness-modulation has been described.
It was thus surprisingly found that the compounds according to formula (I) provide sweetness-modulating attributes, which may improve the taste profile of sugar-reduced foodstuff or beverage. This was particularly surprising, since madecassic acid and terminolic acid provide a bitter taste. Moreover, this was also surprising, since it was found that e.g. madecassoside does not provide any sweetness-modulating attributes, as shown in example 5.1 below.
The compounds according to the invention may be of any possible three-dimensional structure, i.e. formula (I) encompasses any possible stereoisomer.
Preferably, the compounds according to the invention are of formula (la):
wherein X and Y are selected from hydrogen or CH3, wherein only one of X and Y is CH3, wherein R1 to R7 are selected from hydrogen or a saccharide, wherein the or all monomer subunit(s) of the saccharide is / are monomer(s) selected from the group consisting of
glucose, galactose, fructose, rhamnose, xylose, glucuronic acid, quinovose, arabinose and mixtures thereof, wherein R1 to R7 are selected such that the compound comprises a sum of at least two monomer subunits represented by one or more of R1 to R7, or a salt thereof.
Further preferably for a compound according to the invention, the compound is selected from compounds 1 a to 70b according to the following table 1 , with Rha = Rhamnose and Glc = Glucose:
The term “a-1->4 Rha”, and corresponding terms, in the above table 1 describes that the respective residue is rhamnose, or glucose if “Glc” is mentioned, which is bound to the remaining structure via an a-1 ,4 glycosidic bond. The same applies accordingly to the corresponding terms in the above table 1 . Thus, the term “rhamnose” or “glucose”, as used in this context, does not describe a non-connected, single rhamnose or glucose molecule. Instead, the term describes a structure, which is based on the single rhamnose or glucose molecule but which is connected to the remaining structure to form the compound according to the invention.
Compound 31a
Compound 7a
Compound 31b
Compound 7b or any stereoisomer or salt thereof.
The invention further relates to a method for producing a compound according to the invention or a salt thereof, comprising the following steps:
(i) providing madecassoside and/or terminoloside, preferably providing a plant extract comprising madecassoside and/or terminoloside, particularly preferably providing a plant extract of Gotu Kola comprising madecassoside and/or terminoloside, further preferably providing a plant extract of Gotu Kola comprising 50 to 95 wt.-% madecassoside and/or terminoloside, based on the total weight of the plant extract,
(ii) providing one or more saccharide(s), preferably wherein the, one or more or all saccharide(s) is I are selected from the group consisting of glucose, galactose, fructose, rhamnose, xylose, saccharose, lactose, UDP-glucose, maltose, starch, maltodextrin, cyclodextrin, glucuronic acid, quinovose, arabinose and mixtures thereof,
(iii) providing a glycosyltransferase
(iv) mixing the provided components and incubating the mixed components to obtain a mixture comprising a compound according to the invention.
It is known that Gotu Kota mainly synthesizes the triterpenes asiaticoside, madecassoside, asiatic acid, madecassic acid, terminolic acid and terminoloside. These triterpenes contribute (in sum) to more than 1 % of the dried plant mass (Feng-Lun et al., Biomedical Chromatohraphy 2008, 22(2), 119-124). Therefore, it is preferred that extracts from Gotu Kota are used as starting material for providing madecassoside and/or terminoloside.
Preferably, such an extract comprises 1 to 100 wt.-% of asiaticoside, madecassoside, asiatic acid, madecassic acid, terminolic acid and terminoloside, particularly preferably such an extract comprises 50 to 95 wt.-% madecassoside and/or terminoloside, based on the total weight of the plant extract.
Furthermore, it is preferred that the glycosyltransferase provided in step (iii) is a glycosyltransferase according to Gerwig et al., Advances in Carbohydrate Chemistry and Biochemistry, 2016, 73, or according to Zhao et al., J Agric. Food Chem., 2020, 68 or a mixture thereof.
Thus, it is preferred that the glycosyltransferase provided in step (iii) is selected from the group consisting of glucanotransferases, glucosyltransferases and mixtures thereof, preferably wherein the glycosyltransferase is selected from the group consisting of a- glucosyltransferases (such as a-glucosidase, CGTase, glucan sucrase (preferably glucansucrase GTF 180) or dextransucrase), p-glucosyltransferases (such as 1 ,2-p- glucosyltransferase (OleD, preferably from Streptomyces antibioticus, Yjic, preferably from Bacillus licheniformis, or UGTSL2, preferably from Solanum lycopersicum), p-1 ,3- glucosyltransferases (such as laminarinase) or p-1 ,4-glucosyltransferase), p-fructosidases (such as enzyme preparations originating from A. niger, Aspergillus sp., A. aculeatus or B. subtilis), a-galactosidases (such as enzyme preparations originating from A. oryzae, Kluyveromyces lactis, Kluyveromyces sp., B. licheniformis'), p-galactosidases (such as enzyme preparations originating from Kluyveromces lactis or B. circulans), L- rhamnosidases (such as enzyme preparations originating from Thermomicrobia sp. or Penicillium decumbens) and mixtures thereof, particularly preferably wherein the glycosyltransferase is selected from the group consisting of cyclomaltodextrin glucanotransferase, glucan sucrose, yjic glucosyltransferase, oleandomycin glycosyltransferase and mixtures thereof.
Preferably, the glycosyltransferase is obtained commercially or via a cell culture approach, in which cells, preferably E.coli cells, are transformed with a vector comprising the coding sequence of the glycosyltransferase of interest, e.g. as described in the below example section.
By enzymatic glycosylation, monosaccharides from substrates as e.g. saccharose, lactose, UDP-glucose, maltose, starch, maltodextrin, cyclodextrin and mixtures thereof can be bound to formula (I), i.e. as one or more of R1 to R7, via a a-1-2-, a-1-3-, a-1-4-, a-1-6-, p- 1-2-, p-1-3-, p-1-4- or p-1-6-glycosidic bond, which may be individual for each saccharide.
Further preferably, the, one or more or all saccharide(s) provided in step (ii) is I are selected from the group consisting of maltodextrin, saccharose and mixtures thereof, and/or the glycosyltransferase is selected from the group consisting of CTGase, Glucansucrase GTF 180 and mixtures thereof.
Particularly preferably, the saccharide provided in step (ii) comprises or consists of maltodextrin and saccharose and the glycosyltransferase provided in step (iii) comprises or consists of CTGase and Glucansucrase GTF 180.
Even further preferably, the saccharide provided in step (ii) consists of maltodextrin and saccharose and the glycosyltransferase provided in step (iii) consists of CTGase and Glucansucrase GTF 180.
It is preferred that in the method according to the invention the mixing and/or incubation in step (iv) is performed while shaking.
The incubation in step (iv) allows a glycosylation of the provided madecassoside and/or terminoloside to obtain a compound according to formula (I).
Preferably, the method additionally comprises the step of purifying the obtained glycosylated madecassoside and/or terminoloside, wherein the term “purifying” is broadly understood as increasing the concentration of the obtained glycosylated madecassoside and/or terminoloside, e.g. by removing other components of the obtained mixture.
It is preferred that the incubation in step (iv) is performed at a temperature in a range of from 30 to 60 °C, preferably in a range of from 35 to 55 °C, since particularly advantageous yields were obtained in these temperature ranges.
Additionally or alternatively, the incubation in step (iv) is performed for 5 to 48 h, preferably for 12 to 36 h, particularly preferably for 18 to 30 h, further preferably for 20 to 27 h.
Furthermore, it is preferred that the incubation in step (iv) is inactivated, preferably before the step of purification is performed, if present.
Additionally or alternatively, the method according to the invention further comprises the step of purifying the mixture obtained after step (iv), preferably by chromatography, such as ion exchange chromatography, or by solid phase purification or microfiltration.
Therefore, it is additionally or alternatively to the above temperature ranges preferred that the incubation in step (iv) is inactivated, preferably by applying a temperature in the range of from 70 to 100 °C, preferably in the range of from 75 to 90 °C, particularly preferably in the range of from 75 to 85 °C, and/or
that the mixture obtained in step (iv) is dried, preferably by a drying method selected from the group consisting of freeze-drying, spray drying, vacuum belt drying or heat drying, particularly preferably by freeze-drying.
The invention further relates to a compound according to the invention, wherein the compound is obtainable or obtained by a method according to the invention, or a salt thereof.
Furthermore, the invention relates to a mixture comprising
(i) one or more compounds according to the invention, or
(ii) one or more salts according to the invention, or
(iii) one or more compounds according to the invention and one or more salts according to the invention, preferably wherein the mixture is obtained or obtainable by a method according to the invention.
Preferably in the mixture according to the invention, the total weight of the compound(s) according to the invention and their salts is in a range of from 0.1 to 99 wt.-%, preferably in a range of from 1 to 50 wt.-%, particularly preferably in a range of from 3 to 10 wt.-%, based on the total weight of the mixture.
The term “the total weight of the compound(s) according to the invention and their salts” as used herein refers in this case to the sum of all compounds according to the invention and all salts thereof, as far as present in the mixture.
Typically, for obtaining a compound according to the invention, madecassoside and/or terminoloside is I are glycosylated. Thus, it is preferred that the mixture comprises madecassoside, wherein the total weight of madecassoside is at most 50 wt.-%, preferably at most 40 wt.-%, particularly preferably at most 30 wt.-%, further preferably at most 25 wt.- %, more preferably at most 20 wt.-, based on the total weight of the mixture.
Additionally or alternatively, the mixture comprises terminoloside, wherein the total weight of terminoloside is at most 50 wt.-%, preferably at most 40 wt.-%, particularly preferably at
most 30 wt.-%, further preferably at most 25 wt.-%, more preferably at most 20 wt.-, based on the total weight of the mixture.
Additionally or alternatively, the mixture comprises madecassoside and terminoloside, wherein the total weight of madecassoside and terminoloside is at most 50 wt.-%, preferably at most 40 wt.-%, particularly preferably at most 30 wt.-%, further preferably at most 25 wt.-%, more preferably at most 20 wt.-, based on the total weight of the mixture.
Moreover, the present invention relates to a composition for food or pleasure or pharmaceutical composition comprising one or more compound(s) according the invention or a mixture according to the invention.
Preferably, the composition according to the invention, preferably the pharmaceutical composition according to the invention, may be present in a form selected from the group consisting of tablets (non-coated as well as coated tablets, single or multiple layered tablets), capsules, lozenges, granules, pellets, solid substance mixtures, dispersions in liquid phases, emulsions, powders, solutions, juices, pastes or other swallowable or chewable preparations.
Preferably, the composition according to the invention serving for food or pleasure may be selected from the group consisting of baked goods, for example, bread, dry biscuits, cakes, other baked products, confectionery (for example, chocolate, chocolate bar products, other bar products, fruit gum, hard and soft caramels, chewing gum), alcoholic or nonalcoholic beverages (for example, coffee, tea, iced tea, wine, wine-containing beverages, beer, beercontaining beverages, liqueurs, brandies, (carbonated) fruit-containing beverages, (carbonated) isotonic beverages, (carbonated) soft beverages, nectars, spritzers, fruit and vegetable juices, fruit or vegetable juice formulations, instant beverages (for example, instant cocoa beverages, instant tea beverages, instant coffee beverages, instant fruit beverages), meat products (for example, ham, fresh sausage or uncooked sausage formulations, seasoned or marinated fresh or salted meat products), eggs or egg products (dried egg, egg white, egg yolk), cereal products (for example, breakfast cereals, muesli bars, precooked ready-made rice products), dairy products (for example, milk beverages, buttermilk beverages, milk ice, yoghurt, kefir, fresh cheese, soft cheese, hard cheese, dried milk powder, whey, whey beverages, butter, buttermilk, products containing partly or completely hydrolysed milk protein), products made of soy protein or other soybean fractions (for example, soy milk and products produced thereof, fruit beverages with soy protein, soy lecithin-containing formulations, fermented products such as tofu or tempe or
products produced thereof), products made of other plant-based protein sources, for example, oat protein beverages, fruit preparations (for example, preserves, fruit ice-cream, fruit sauces, fruit fillings), vegetable preparations (for example, ketchup, sauces, dried vegetables, frozen vegetables, precooked vegetables, vegetables preserved in vinegar), snacks (for example, baked or fried potato chips (crisps) or potato dough products, extrudates based on maize or peanuts), fat- and oil-based products or emulsions thereof (for example, mayonnaise, remoulade, dressings), other ready-made dishes and soups (for example dried soups, instant soups, precooked soups), spices or spice formulations and particularly seasonings, which are used, for example, in the snacks sector.
Particularly preferred herein are sweets, dairy products and very particularly preferred are non-alcoholic beverages where sweetened beverages are particularly preferred.
Particularly the composition according to the invention may contain one or more further sweeteners and/or one or more further aroma substances.
Preferably, the, one or more or all further sweeteners may be selected from the group consisting of carbohydrates and specifically sugars such as, for example, sucrose/saccharose, trehalose, lactose, maltose, melizitose, raffinose, palatinose, lactulose, D-fructose, D-allulose, D-glucose, D-galactose, L-rhamnose, D-sorbose, D- mannose, D-tagatose, D-arabinose, L-arabinose, D-xylose, D-ribose, D-glyceraldehyde or maltodextrin; synthetic, i.e. usually enzymatically produced, starch or sugar hydrolysates (invert sugar, fructose syrup); fruit concentrates (e.g., on the basis of apples or pears); sugar alcohols (e.g., erythritol, threitol, arabitol, ribotol, xylitol, sorbitol, mannitol, dulcitol, lactitol); proteins (e.g., miraculin, monellin, thaumatin, curculin, brazzein); sweeteners (e.g., magap, sodium cyclamate, acesulfam K, neohesperidin dihydrochalcone, saccharine sodium salt, aspartame, superaspartame, neotame, alitame, sucralose, stevioside, rebaudioside, lugduname, carrelame, sucrononate, sucrooctate, monatin, phenylodulcin); sweet-tasting amino acids (e.g., glycine, D-leucine, D-threonine, D-asparagine, D- phenylalanine, D-tryptophan, L-proline); further sweet-tasting low-molecular substances such as, e.g., hernandulcin, dihydrochalcon glycoside, particularly, neohesperidin dihydrochalcone, and naringin chaicone, glycyrrhizin, glycerrhetinic acid, their derivatives and salts, extracts of liquorice (Glycyrrhizza glabra ssp.), Lippia dulcis extracts, Momordica ssp. Extracts; individual substances such as, for example, Momordica grosvenori [Luo Han Guo] and the mogrosides obtained thereof, Hydrangea dulcis or extracts, phyllodulcin; balansins of Mycetia balansae.
Also preferably, the, one or more or all further aroma substances may be selected from the group consisting of acetophenone, allyl caproate, alpha-ionone, beta-ionone, aniseed aldehyde, anisyl acetate, anisyl formate, benzaldehyde, benzothiazole, benzyl acetate, benzyl alcohol, benzyl benzoate, beta-ionone, butyl butyrate, butyl capronate, butylidene phthalide, carvone, camphene, caryophyllene, cineol, cinnamyl acetate, citral, citronellol, citronellal, citronellyl acetate, cyclohexyl acetate, cymol, damascene, decalactone, dihydrocoumarin, dimethyl anthranilate, dimethyl anthranilate, dodecalactone, ethoxy ethyl acetate, ethylbutyric acid, ethyl butyrate, ethyl caprinate, ethyl capronate, ethyl crotonate, ethyl furaneol, ethylguaiakol, ethyl isobutyrate, ethyl isovalerianate, ethyl lactate, ethylmethyl butyrate, ethyl propionate, eucalyptol, eugenol, eugenyl acetate, ethyl heptylate, 4-(p-hydroxyphenyl)-2-butanone, gamma-decalactone, geraniol, geranyl acetate, geranyl acetate, grapefruit aldehyde, methyl dihydrojasmonate (e.g., 5 Hedion®), heliotropin, 2-heptanone, 3-heptanone, 4-heptanone, trans-2-heptenal, cis-4-heptenal, trans-2-hexenal, cis-3-hexenol, trans-2-hexenoic acid, trans-3-hexenoic acid, cis-2- hexenyl acetate, cis-3-hexenyl acetate, cis-3-hexenyl capronate, trans-2-hexenyl capronate, cis-3-hexenyl formiate, cis-2-hexyl acetate, cis-3-hexyl acetate, trans-2-hexyl acetate, cis-3-hexyl formiate, para-hydroxybenzyl acetone, isoamyl alcohol, isoamyl isovalerianate, isobutyl butyrate, isobutyl aldehyde, isoeugenol methyl ether, isopropyl methyl thiazole, lauric acid, levulinic acid, linalool, linalool oxide, linalyl acetate, menthol, menthofuran, methyl anthranilate, methyl butanol, methyl butyric acid, 2-methyl butyl acetate, methyl capronate, methyl cinnamate, 5-methyl furfural, 3, 2, 2-methyl cyclopentenolone, 6, 5, 2-methyl heptenone, methyl dihydrojasmonate, methyljasmonate, 2- methyl methylbutyrate, 2-methyl-2-pentanoic acid, methyl thiobutyrate, 3,1 -methyl thiohexanol, 3-methyl thiohexyl acetate, nerol, neryl acetate, trans, trans-2,4-nonadienal, 2,4-nonadienol, 2,6-nonadienol, 2,4-nonadienol, nootkatone, delta octalactone, gamma octalactone, 2-octanol, 3-octanol, 1 ,3-octenol, 1-octyl acetate, 3-octyl acetate, palmitic acid, paraldehyde, phellandrene, pentandione, phenylethyl acetate, phenylethyl alcohol, phenylethyl alcohol, phenylethyl isovalerianate, piperonal, propionaldehyde, propyl butyrate, pulegon, pulegol, sinensal, sulfurol, terpinene, terpineol, terpinolene, 8,3- thiomenthanone, 4,4,2-thiomethyl pentanone, thymol, delta-undecalactone, gammaundecalactone, valencene, valeric acid, vanilline, acetoine, ethyl vanilline, ethyl vanilline isobutyrate (= 3-ethoxy-4-isobutyryloxybenzaldehyde), 2,5-dimethyl-4-hydroxy-3(2H)- furanone and derivatives (here, preferably homofuraneol (= 2-ethyl-4-hydroxy-5-methyl- 3(2H)-furanone), homofuronol (= 2-ethyl-5-methyl-4-hydroxy-3(2H)-furanone and 5-ethyl- 2-methyl-4-hydroxy-3(2H)-furanone), maltol and maltol derivatives (here, preferably ethyl maltol), coumarine and coumarine derivatives, gamma-lactones (here, preferably gammaundecalactone, gamma-nonalactone, gamma-decalactone), delta-lactones (here,
preferably 4-methyldeltadecalactone, massoilactone, delta-decalactone, tubero lactone), methyl sorbate, divanilline, 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)furanone, 2- hydroxy-3-methyl-2-cyclopentenon, 3-hydroxy-4,5-dimethyl-2(5H)-furanone, isoamyl acetate, ethyl butyrate, butyl butyrate, isoamyl butyrate, methyl-3-ethyl butyrate, n- hexanoic acid allyl ester, n-hexanoic acid-n-butyl ester, n-ethyl octanoate, ethyl-3-methyl- 3-phenylglycidate, ethyl-2-trans-4-cis-decadienoate, 4-(p-hydroxyphenyl)-2-butanone, 1 ,1- dimethoxy-2,2,5-trimethyl-4-hexane, 2,6-dimethyl-5-hepten-1-al and phenylacetaldehyde, 2-methyl-3-(methylthio)furane, 2-methyl-3-furanthiol, bis(2-methyl-3-furyl)disulfide, furfuryl mercaptane, methional, 2-acetyl-2-thiazoline, 3-mercapto-2-pentanone, 2,5-dimethyl-3- furanthiol, 2,4,5-trimethylthiazol, 2-acetylthiazol, 2,4-dimethyl-5-ethylthiazol, 2-acetyl-1- pyrroline, 2-methyl-3-ethylpyrazine, 2-ethyl-3,5-dimethylpyrazine, 2-ethyl-3,6- dimethylpyrazine, 2,3-diethyl-5-methylpyrazine, 3-isopropyl-2-methoxypyrazine, 3- lsobutyl-2-methoxypyrazine, 2-acetylpyrazine, 2-pentylpyridine, (E,E)-2,4-decadienal, (E,E)-2,4-nonadienal, (E)-2-octenal, (E)-2-nonenal, 2-undecenal, 12-methyltridecanal, 1 - Penten-3-one, 4-hydroxy-2,5-dimethyl-3(2H)-furanone, guaiakol, 3-hydroxy-4,5-dimethyl- 2(5H)-furanone, 3-hydroxy-4-methyl-5-ethyl-2(5H)-furanone, cinnamon aldehyde, cinnamon alcohol, methyl salicylate, isopulegol; lactisoles, lactisol esters such as described in EP 2,292,224, sodium salts (e.g., sodium chloride, sodium lactate, sodium nitrate, sodium acetate, sodium gluconate), hydroxyflavanones, here, preferably eriodictyol, sterubin (eriodictyol-7-methyl ether), homoeriodictyol, and their sodium, potassium, calcium, magnesium or zinc salts (particularly those as described in EP 1258200 A2) divanillins (particularly those as described in WO 2004/078302) and 4- hydroxydihydrochalcones (preferably as described in US 2008/0227867 A1), here, particularly phloretin and davidigenin, umami compounds as described in WO 2008/046895 A1 and EP 1989944 A1 , umami compounds as described in EP 2064959 A1 , EP 2,529,632 B1 or EP 2135516 A1 , here particularly preferably rubemamine and rubescenamine, vanillyl lignans, particularly preferably lariciresinol or matairesinol as described in WO 2012/146584, enterodiol as described in DE 10 2012 214 560, alkamides, particularly pellitorines as described in EP 2,058,297, EP 1 ,977,655 B1 and EP 2,008,530 B1 , and N- decadienoyl amino acids as described in EP 2,597,082 and their mixtures, as well as stereoisomers, enantiomers, positional isomers, diastereomers, cis/trans-isomers and epimers of these substances.
Preferably in the composition according to the invention, the total weight of the compound(s) according to the invention is in a range of from 0.001 to 60 wt.-%, preferably in a range of from 0.01 to 40 wt.-%, particularly preferably in a range of from 0.1 to 30 wt.-
%, further preferably in a range of from 0.5 to 20 wt.-%, more preferably in a range of from 1 to 10 wt.-%, based on the total weight of the composition.
The invention further relates to the use of a compound according to the invention or a mixture according to the invention for imparting or modifying a sweet taste impression. Moreover, the invention also relates to a method for imparting and/or modifying a sweet taste impression, comprising the following steps:
(i) providing a compound according to the invention or a mixture according to the invention,
(ii) mixing the compound or mixture provided in step (i) with a substance or product, of which a sweet taste impression shall be imparted and/or modified.
The term “modifying a sweet taste impression” as used herein describes any kind of modification, such as optimizing or increasing a sweet taste impression.
Further aspects and advantages of the invention result from the subsequent description of preferred examples.
Examples
Example 1 : Production of qlucosylated madecassoside with cyclomaltodextrin qlucanotransferase
A mixture comprising 95 % of madecassoside (3 g), and maltodextrin (9 g) were dissolved in 15 mL water and incubated with 1.5 mL of a cyclomaltodextrin glucanotransferase (Novozymes, Denmark) at 50 °C for 6 h while shaking, for performing the glycosylation reaction. The reaction was inactivated by applying a temperature of 80 °C for 10 min.
After subsequent freeze-drying, the composition of the obtained mixture was analysed via HPLC coupled to high resolution mass spectrometry, UV detection and Corona detection (LC-CAD). The chromatographic separation of the compounds according to the invention was performed at a Waters Acquity UPLC Systems (Waters, Eschborn, Germany) with a C-18 column (Kinetex, 100 mm x2.1 mm, 1 .7 pm; Phenomenex) at a temperature of 50 °C, Water/Acetonitrile with 0.1 % formic acid as mobile phase and a flow of 0.55 mL/min. The detection was performed with a Bruker microTOFQII mass spectrometer with ESI ionization at a scanning range of 50 to 2000 Da and a charged aerosol detector (Corona Veo, Thermo Scientific, Germany). The area ratios were generally used to calculate the amount ratios of the respective compounds, based on the educt concentration.
In addition to non-reacted educt, several glucosylated compounds with R2=rhamnose and R1, R3 to R7 = 1 to 8 glucose subunits. The mixture contained the following components
Compounds 1 a to 30a correspond to compounds 1 a to 30a as in table 1 .
Example 2: Production of qlucosylated madecassoside with qlucansucrase GTF180- Q1140E-AN (AAU08001.1)
Transformation of gft180-Q1140E-AN of Lactobacillus reuteri in E. coli
A pET28a_gft180Q-1130E-A-N vector was synthesized and codon-optimized for E. coli K12. The expression plasmide was transformed in E-coli W3110 (ADE3).
Production of the recombinant GFT180-Q1140E-AN
E.coli W3110 (ADE3) including pET28a_gtf180-Q1140E-AN was cultured in 15 mL Luria- Bertani Medium (Carl Roth) with 30 pg/mL Neomycin (Carl Roth) at 37 °C, 200 rpm over night in a laboratory shaker. Subsequently, 200 mL Terrific Broth Medium (Carl Roth) with 30 pg/mL Neomycin were inoculated at an OD of 0.1 (600 nm) and cultured at 37 °C while shaking. After obtaining an OD of 0.6 to 0-8 (600 nm), the culture was induced with 0.5 mM IPTG and further incubated at 20 °C for 16 to 18 h. The cells were harvested by centrifugation at 17,000 x g for 10 min. Subsequently, the pellet was resuspended in 25 mM sodium citrate buffer pH 4.8 + 1 mM CaCh, such that the concentration corresponded to 200 gBFM/L. The cell lysis was performed via ultrasound sonotrode. The cell fragments were separated at 17,000 x g for 10 min.
Biotransformation of madecassoside into glucosylated madecassoside with GFT180-Q1140E-AN
10 g/L of a mixture comprising 91 % madecassoside, 70 g/L saccharose and 125 mL clarified cell lysate were filled up with 25 mM sodium citrate buffer pH 4.8 + 1 mM CaCh to 0.5 L and were incubated for 24 h at 37 °C and 200 rpm. The reaction was inactivated by applying a temperature of 80 °C for 30 min and the crude product was obtained.
Purification of glycosylated madecassoside with ion exchange chromatography
100 g polystyrene resin were added to the above reaction mixture and the mixture was incubated for 30 min and 200 rpm at 30 °C. The solids were filtered and it was washed three times with 200 mL deionized water. Subsequently, the mixture was eluted twice with 200 mL of 70 % ethanol and dried by evaporation under vacuum. The residue was dissolved in 100 mL deionised water and the purified product was obtained.
After subsequent freeze-drying, the composition of the obtained mixture was analysed via LC-CAD. The mixture contained the following components
Compounds 31 a to 64a correspond to compounds 31 a to 64a as in table 1.
Example 3: Production of qlucosylated madecassoside with qlycosyltransferase Yjic (AAU23479)
Transformation of Yjic of Bacillus licheniformis in E. coll
A pET28a_yjic vector was synthesized and codon-optimized for E. col 7K12. The expression plasmide was transformed in E-coli W3110 (ADE3).
Production of the recombinant Yjic
E.coli W3110 (ADE3) including pET28a_Yjic was cultured in 15 mL Luria-Bertani Medium (Carl Roth) with 30 pg/mL Neomycin (Carl Roth) at 37 °C, 200 rpm overnight in a laboratory shaker. Subsequently, 200 mL Terrific Broth Medium (Carl Roth) with 30 pg/mL Neomycin were inoculated at an OD of 0.1 (600 nm) and cultured at 37 °C while shaking. After obtaining an OD of 0.6 to 0-8 (600 nm), the culture was induced with 0.5 mM IPTG and further incubated at 20 °C for 16 to 18 h. The cells were harvested by centrifugation at 17,000 x g for 10 min. Subsequently, the pellet was resuspended in 100 mM Tris/HCI (pH 7.5) + 2 mM MgCh, such that the concentration corresponded to 200 gBFM/L. The cell lysis was performed via ultrasound sonotrode. The cell fragments were separated at 17,000 x g for 10 min.
Biotransformation of madecassoside into glucosylated madecassoside with Yjic
3 g/L of a mixture comprising 91 % madecassoside, 9.15 g/L UDP-glucose (UDP-GIc) and 35 mL clarified cell lysate were filled up with 100 mM Tris/HCI (pH 7.5) + 2 mM MgCh to 0.7 L and were incubated for 24 h at 37 °C and 200 rpm. The reaction was inactivated by applying a temperature of 80 °C for 30 min and the crude product was obtained.
Purification of glycosylated madecassoside with ion exchange chromatography
80 g polystyrene resin were added to the above reaction mixture and the mixture was incubated for 30 min and 200 rpm at 30 °C. The solids were filtered and it was washed three times with 160 mL deionized water. Subsequently, the mixture was eluted twice with 160 mL of 70 % ethanol and dried by evaporation under vacuum. The residue was dissolved in 100 mL deionised water and the purified product was obtained.
After subsequent freeze-drying, the composition of the obtained mixture was analysed via LC-CAD. The mixture contained the following components
Compounds 65a to 70a correspond to compounds 65a to 70a as in table 1 .
Example 4: Production of 8-1 ,2-qlucosylated madecassoside with oleandomycin qlycosyltransferase SaOleDGtf (CAA80301 .1)
Transformation of SaOleDGtf of Streptomyces antibioticus in E. coli
A pET28a_SaOleDGtf vector was synthesized and codon-optimized for E. coli K12. The expression plasmide was transformed in E-coli BL21 (ADE3).
Production of the recombinant SaOleDGtf
E.coli BL21 (ADE3) including pET28a_SaOleDGtf was cultured in 15 mL Luria-Bertani
Medium (Carl Roth) with 30 pg/mL Neomycin (Carl Roth) at 37 °C, 200 rpm overnight in a
laboratory shaker. Subsequently, 200 mL Terrific Broth Medium (Carl Roth) with 30 pg/mL Neomycin were inoculated at an OD of 0.1 (600 nm) and cultured at 37 °C while shaking. After obtaining an OD of 0.6 to 0-8 (600 nm), the culture was induced with 0.1 mM IPTG and further incubated at 16 °C for 16 to 18 h. The cells were harvested by centrifugation at 17,000 x g for 10 min. Subsequently, the pellet was resuspended in 100 mM Tris/HCI
(pH 8), such that the concentration corresponded to 200 gBFM/L. The cell lysis was performed via ultrasound sonotrode. The cell fragments were separated at 17,000 x g for 10 min.
Biotransformation of madecassoside into 0-1,2-glucosylated madecassoside with SaOleDGtf
3 g/L of a mixture comprising 91 % madecassoside, 9.15 g/L UDP-glucose (UDP-GIc) and 35 mL clarified cell lysate were filled up with 100 mM Tris/HCI (pH 8) to 0.7 L and were incubated for 24 h at 37 °C and 200 rpm. The reaction was inactivated by applying a temperature of 80 °C for 30 min.
The composition of the obtained mixture was analysed via LC-CAD. The mixture contained the following components
Compounds 65a to 67a correspond to compounds 65a to 67a as in table 1 .
Example 5: Sensory evaluation
Example 5.1 Sweetness modulation
Mixtures according to Examples 1 and 2, as well as fractions thereof were freeze-dried and tasted and sensorically described by flavorists (n=5). The following results were obtained:
Compounds 1 a to 64a correspond to compounds 1 a to 64a as in table 1 .
It was surprisingly found that the compounds according to the invention provide sweetnessmodulating attributes, even though the glucoside madecassoside did not show any effect on sweetness. The enzymatic transformation of madecassoside according to Example 1 provides compounds 1 a to 17a, which increase the sweetness of the 5 % saccharose solution (Test
no. 3). Particularly, compounds 6a to 10a increase the sweetness of the 5 % saccharose solution (Test no. 5).
It was surprisingly found that an enzymatic transformation of madecassoside according to Example 2 provides compounds 31 a to 64a, which particularly strongly (even stronger than according to Example 1) increase the sweetness of the 5 % saccharose solution (Test no.
4). Particularly, compounds 31 a to 33a increase the sweetness of the 5 % saccharose solution (Test no. 6).
Example 5.2: Intrinsic sweetness
For determining the sweetness of the compounds according to the invention, different concentrations of mixture no. 6 according to Example 5.1 were compared to 1.5 % saccharose solution in water in a 2-AFC test (2-Alternative Forced Choice Test; also known as Directional Difference Test or Paired Comparison Test). The determination according to ASTM Designation E2164-08: Standard Test Method for Directional Difference Test (n=30 panellists) revealed an intrinsic sweetness of 120 mg/kg in water. It was surprisingly found that the compounds provided a sweet taste. However, the sweet taste was not stronger than the 1 .5 % saccharose solution.
Claims
Claims Compound according to formula (I):
wherein X and Y are selected from hydrogen or CH3, wherein only one of X and Y is CH3, wherein R1 to R7 are selected from hydrogen or a saccharide, wherein the or all monomer subunit(s) of the saccharide is I are monomer(s) selected from the group consisting of glucose, galactose, fructose, rhamnose, xylose, glucuronic acid, quinovose, arabinose and mixtures thereof, wherein R1 to R7 are selected such that the compound comprises a sum of at least two monomer subunit(s) represented by one or more of R1 to R7, or a salt thereof.
Compound according to claim 1 , wherein the compound is of formula (la):
wherein X and Y are selected from hydrogen or CH3, wherein only one of X and Y is CH3, wherein R1 to R7 are selected from hydrogen or a saccharide, wherein the or all monomer subunit(s) of the saccharide is I are monomer(s) selected from the group consisting of glucose, galactose, fructose, rhamnose, xylose, glucuronic acid, quinovose, arabinose and mixtures thereof, wherein R1 to R7 are selected such that the compound comprises a sum of at least two monomer subunit(s) represented by one or more of R1 to R7, or a salt thereof.
. Compound according to any one of claims 1 or 2, wherein the compound is selected from compounds 1a to 70b according to the following table, with Rha = rhamnose and Glc = glucose:
-44-
-45-
Compound according to any one of the preceding claims, wherein the compound is selected from
Compound 1a
Compound 7b or any stereoisomer or salt thereof.
5. Method for producing a compound according to one of the preceding claims or a salt thereof, comprising the following steps:
(i) providing madecassoside and/or terminoloside, preferably providing a plant extract comprising madecassoside and/or terminoloside, particularly preferably providing a plant extract of Gotu Kola comprising madecassoside and/or terminoloside, further preferably providing a plant extract of Gotu Kola comprising 50 to 95 wt.-% madecassoside and/or terminoloside, based on the total weight of the plant extract,
(ii) providing one or more saccharide(s), preferably wherein the, one or more or all saccharide(s) is I are selected from the group consisting of glucose, galactose, fructose, rhamnose, xylose, saccharose, lactose, UDP-glucose, maltose, starch, maltodextrin, cyclodextrin, glucuronic acid, quinovose, arabinose and mixtures thereof,
(iii) providing a glycosyltransferase
- 53 -
(iv) mixing the provided components and incubating the mixed components to obtain a mixture comprising a compound according to one of the preceding claims. Method according to claim 5, wherein the glycosyltransferase provided in step (iii) is selected from the group consisting of glucanotransferases, glucosyltransferases and mixtures thereof, preferably wherein the glycosyltransferase is selected from the group consisting of a-glucosyltransferases, p-glucosyltransferases, p-1 ,3-glucosyltransferases, p- fructosidases, a-galactosidases, p-galactosidases, L-rhamnosidases and mixtures thereof, particularly preferably wherein the glycosyltransferase is selected from the group consisting of cyclomaltodextrin glucanotransferase, glucan sucrose, yjic glucosyltransferase, oleandomycin glycosyltransferase and mixtures thereof. Method according to any one of claims 5 or 6, wherein the, one or more or all saccharide(s) is I are selected from the group consisting of maltodextrin, saccharose and mixtures thereof, and/or wherein the glycosyltransferase is selected from the group consisting of CTGase, Glucansucrase GTF 180 and mixtures thereof. Method according to any one of claims 5 to 7, wherein the incubation in step (iv) is performed at a temperature in a range of from 30 to 60 °C, preferably in a range of from 35 to 55 °C, and/or wherein the incubation in step (iv) is inactivated, preferably by applying a temperature in the range of from 70 to 100 °C, preferably in the range of from 75 to 90 °C, particularly preferably in the range of from 75 to 85 °C, and/or
- 54 - wherein the mixture obtained in step (iv) is dried, preferably by a drying method selected from the group consisting of freeze-drying, spray drying, vacuum belt drying or heat drying. . Compound according to any one of claims 1 to 4, wherein the compound is obtainable or obtained by a method according to any one of claims 5 to 8, or a salt thereof. 0. Mixture comprising
(i) one or more compounds according to any one of claims 1 to 4 or 9, or
(ii) one or more salts according to any one of claims 1 to 4 or 9, or
(iii) one or more compounds according to any one of claims 1 to 4 or 9 and one or more salts according to any one of claims 1 to 4 or 9, preferably wherein the mixture is obtained or obtainable by a method according to any one of claims 5 to 8. 1 . Mixture according to claim 10, wherein the total weight of the compound(s) according to any one of claims 1 to 4 or 9 and their salts is in a range of from 0.1 to 99 wt.-%, preferably in a range of from 1 to 50 wt.-%, particularly preferably in a range of from 3 to 10 wt.-%, based on the total weight of the mixture. 2. Mixture according to any one of claims 10 or 1 1 , wherein the mixture comprises madecassoside, wherein the total weight of madecassoside is at most 50 wt.-%, preferably at most 40 wt.-%, particularly preferably at most 30 wt.-%, further preferably at most 25 wt.-%, more preferably at most 20 wt.-, based on the total weight of the mixture, and/or wherein the mixture comprises terminoloside, wherein the total weight of terminoloside is at most 50 wt.-%, preferably at most 40 wt.-%, particularly preferably at most 30 wt.-%, further preferably at most 25 wt.-%, more preferably at most 20 wt.-, based on the total weight of the mixture,
- 55 - and/or wherein the mixture comprises madecassoside and terminoloside, wherein the total weight of madecassoside and terminoloside is at most 50 wt.-%, preferably at most 40 wt.-%, particularly preferably at most 30 wt.-%, further preferably at most 25 wt.- %, more preferably at most 20 wt.-, based on the total weight of the mixture. Composition for food or pleasure or pharmaceutical composition comprising one or more compound(s) according to any one of claims 1 to 4 or 9 or a mixture according to any one of claims 10 to 12. Use of a compound according to any one of claims 1 to 4 or 9 or a mixture according to any one of claims 10 to 12 for imparting or modifying a sweet taste impression. Method for imparting and/or modifying a sweet taste impression, comprising the following steps:
(i) providing a compound according to any one of claims 1 to 4 or 9 or a mixture according to any one of claims 10 to 12,
(ii) mixing the compound or mixture provided in step (i) with a substance or product, of which a sweet taste impression shall be imparted and/or modified.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/EP2021/074604 WO2023036404A1 (en) | 2021-09-07 | 2021-09-07 | Taste balancing botanical compounds |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/EP2021/074604 WO2023036404A1 (en) | 2021-09-07 | 2021-09-07 | Taste balancing botanical compounds |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2023036404A1 true WO2023036404A1 (en) | 2023-03-16 |
Family
ID=77910744
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2021/074604 WO2023036404A1 (en) | 2021-09-07 | 2021-09-07 | Taste balancing botanical compounds |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2023036404A1 (en) |
Citations (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0383171A2 (en) * | 1989-02-11 | 1990-08-22 | Hoechst Aktiengesellschaft | 2,3,23-trihydroxy-urs-12-ene derivatives for treating cognitive disorders |
| WO1997039734A1 (en) | 1996-04-23 | 1997-10-30 | The Procter & Gamble Company | Methods of regulating skin condition with centella asiatica extract |
| EP0867447A1 (en) | 1997-03-24 | 1998-09-30 | Dong Kook Pharmaceutical Co., Ltd. | Water-soluble extract of asiaticoside and madecassoside from centella asiatica and isolating method thereof |
| EP1258200A2 (en) | 2001-05-11 | 2002-11-20 | Haarmann & Reimer Gmbh | Use of hydroxyflavones for masking of bitterness, and food and pharmaceutical compositions comprising an effective amount thereof |
| FR2848117A1 (en) | 2002-12-10 | 2004-06-11 | Roche Consumer Health Ltd | Antiiinflammatory Centella asiatica extracts useful in medicinal or cosmetic compositions, contain madecassoside and terminoloside, obtained by processing alcoholic extract |
| WO2004078302A1 (en) | 2003-03-08 | 2004-09-16 | Symrise Gmbh & Co. Kg | Use of divanillin as a flavouring agent |
| WO2005123032A2 (en) | 2004-06-15 | 2005-12-29 | The Boots Company Plc | Haircare compositions and methods |
| WO2008046895A1 (en) | 2006-10-18 | 2008-04-24 | Symrise Gmbh & Co. Kg | Substituted bicyclo[4.1.0]heptane-7-carboxylic acid amides and derivatives thereof as food flavor substances |
| US20080227867A1 (en) | 2007-03-13 | 2008-09-18 | Symrise Gmbh & Co. Kg | Use of 4-hydroxychalcone derivatives for masking an upleasant taste |
| EP1989944A1 (en) | 2007-05-08 | 2008-11-12 | Symrise GmbH & Co. KG | Substituted cyclopropane carbolic acid(3-methyl-cyclohexyl)amides as taste substances |
| EP2058297A1 (en) | 2007-11-08 | 2009-05-13 | Symrise GmbH & Co. KG | Use of alkamides for masking an unpleasant flavor |
| EP2064959A1 (en) | 2007-10-31 | 2009-06-03 | Symrise GmbH & Co. KG | Aromatic Neomenthylamides as flavouring agents |
| EP2135516A1 (en) | 2008-06-13 | 2009-12-23 | Symrise GmbH & Co. KG | Neo-menthyl derivatives as flavourings |
| EP2008530B1 (en) | 2007-06-19 | 2011-01-19 | Symrise AG | Aroma composition for reducing or suppressing unwanted bitter and astringent impressions |
| EP2292224A1 (en) | 2009-07-15 | 2011-03-09 | Symrise AG | Precursor compounds of sweet receptor antagonists for preventing or treating diabetes |
| EP1977655B1 (en) | 2007-03-29 | 2011-05-18 | Symrise AG | Aroma compositions of alkamides with hesperetin and/or 4-hydroxydihydrochalkones and their salts for reinforcing sweet sensory impressions |
| WO2012146584A2 (en) | 2011-04-29 | 2012-11-01 | Symrise Ag | Specific vanillyl lignans and use thereof as taste improvers |
| EP2597082A1 (en) | 2011-11-24 | 2013-05-29 | Symrise AG | Compounds for masking an unpleasant taste |
| DE102012214560A1 (en) | 2011-12-02 | 2013-06-06 | Symrise Ag | Use of 1-3 enterodiol compounds comprising e.g. (2R,3S)-2,3-bis-(3-hydroxy-benzyl)-butane-1,4-diol for sensorially changing unpleasant taste impression, preferably bitter-, astringent- and/or metallic taste impression of bitter substance |
| EP2529632B1 (en) | 2011-05-31 | 2013-08-28 | Symrise AG | Cinnamic acid amides as spicy flavour agents |
| US20180132514A1 (en) * | 2016-11-16 | 2018-05-17 | Sensorygen, Inc. | Natural sweetener compositions |
| CN110870870A (en) * | 2018-08-29 | 2020-03-10 | 好维股份有限公司 | Oral care composition and application thereof in inhibition of gingival bleeding |
-
2021
- 2021-09-07 WO PCT/EP2021/074604 patent/WO2023036404A1/en active Application Filing
Patent Citations (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0383171A2 (en) * | 1989-02-11 | 1990-08-22 | Hoechst Aktiengesellschaft | 2,3,23-trihydroxy-urs-12-ene derivatives for treating cognitive disorders |
| WO1997039734A1 (en) | 1996-04-23 | 1997-10-30 | The Procter & Gamble Company | Methods of regulating skin condition with centella asiatica extract |
| EP0867447A1 (en) | 1997-03-24 | 1998-09-30 | Dong Kook Pharmaceutical Co., Ltd. | Water-soluble extract of asiaticoside and madecassoside from centella asiatica and isolating method thereof |
| EP1258200A2 (en) | 2001-05-11 | 2002-11-20 | Haarmann & Reimer Gmbh | Use of hydroxyflavones for masking of bitterness, and food and pharmaceutical compositions comprising an effective amount thereof |
| FR2848117A1 (en) | 2002-12-10 | 2004-06-11 | Roche Consumer Health Ltd | Antiiinflammatory Centella asiatica extracts useful in medicinal or cosmetic compositions, contain madecassoside and terminoloside, obtained by processing alcoholic extract |
| WO2004078302A1 (en) | 2003-03-08 | 2004-09-16 | Symrise Gmbh & Co. Kg | Use of divanillin as a flavouring agent |
| WO2005123032A2 (en) | 2004-06-15 | 2005-12-29 | The Boots Company Plc | Haircare compositions and methods |
| WO2008046895A1 (en) | 2006-10-18 | 2008-04-24 | Symrise Gmbh & Co. Kg | Substituted bicyclo[4.1.0]heptane-7-carboxylic acid amides and derivatives thereof as food flavor substances |
| US20080227867A1 (en) | 2007-03-13 | 2008-09-18 | Symrise Gmbh & Co. Kg | Use of 4-hydroxychalcone derivatives for masking an upleasant taste |
| EP1977655B1 (en) | 2007-03-29 | 2011-05-18 | Symrise AG | Aroma compositions of alkamides with hesperetin and/or 4-hydroxydihydrochalkones and their salts for reinforcing sweet sensory impressions |
| EP1989944A1 (en) | 2007-05-08 | 2008-11-12 | Symrise GmbH & Co. KG | Substituted cyclopropane carbolic acid(3-methyl-cyclohexyl)amides as taste substances |
| EP2008530B1 (en) | 2007-06-19 | 2011-01-19 | Symrise AG | Aroma composition for reducing or suppressing unwanted bitter and astringent impressions |
| EP2064959A1 (en) | 2007-10-31 | 2009-06-03 | Symrise GmbH & Co. KG | Aromatic Neomenthylamides as flavouring agents |
| EP2058297A1 (en) | 2007-11-08 | 2009-05-13 | Symrise GmbH & Co. KG | Use of alkamides for masking an unpleasant flavor |
| EP2135516A1 (en) | 2008-06-13 | 2009-12-23 | Symrise GmbH & Co. KG | Neo-menthyl derivatives as flavourings |
| EP2292224A1 (en) | 2009-07-15 | 2011-03-09 | Symrise AG | Precursor compounds of sweet receptor antagonists for preventing or treating diabetes |
| WO2012146584A2 (en) | 2011-04-29 | 2012-11-01 | Symrise Ag | Specific vanillyl lignans and use thereof as taste improvers |
| EP2529632B1 (en) | 2011-05-31 | 2013-08-28 | Symrise AG | Cinnamic acid amides as spicy flavour agents |
| EP2597082A1 (en) | 2011-11-24 | 2013-05-29 | Symrise AG | Compounds for masking an unpleasant taste |
| DE102012214560A1 (en) | 2011-12-02 | 2013-06-06 | Symrise Ag | Use of 1-3 enterodiol compounds comprising e.g. (2R,3S)-2,3-bis-(3-hydroxy-benzyl)-butane-1,4-diol for sensorially changing unpleasant taste impression, preferably bitter-, astringent- and/or metallic taste impression of bitter substance |
| US20180132514A1 (en) * | 2016-11-16 | 2018-05-17 | Sensorygen, Inc. | Natural sweetener compositions |
| CN110870870A (en) * | 2018-08-29 | 2020-03-10 | 好维股份有限公司 | Oral care composition and application thereof in inhibition of gingival bleeding |
Non-Patent Citations (11)
| Title |
|---|
| CAS, no. 1361016-45-6 |
| FENG-LUN ET AL., BIOMEDICAL CHROMATOGRAPHY, vol. 22, no. 2, 2008, pages 119 - 124 |
| FENG-LUN ET AL., BIOMEDICAL CHROMATOHRAPHY, vol. 22, no. 2, 2008, pages 119 - 124 |
| GERWIG ET AL., ADVANCES IN CARBOHYDRATE CHEMISTRY AND BIOCHEMISTRY, 2016, pages 73 |
| JOURNAL OF THE AMERICAN DIETETIC ASSOCIATION, vol. 104, no. 2, 2004, pages 255 - 275 |
| KAI GUIGING ET AL: "Separation mechanism of oleanane and ursane pentacyclic triterpenoid isomers by coordination chromatography", SEPU, vol. 32, no. 3, 1 March 2014 (2014-03-01), pages 235 - 241, XP055928905, DOI: 10.3724/SP.J.1123.2013.09015 * |
| MANEENET JUTHAMART ET AL: "Merging the Multi-Target Effects of Kleeb Bua Daeng, a Thai Traditional Herbal Formula in Unpredictable Chronic Mild Stress-Induced Depression", PHARMACEUTICALS, vol. 14, no. 7, 9 July 2021 (2021-07-09), pages 659, XP055928910, DOI: 10.3390/ph14070659 * |
| PINHAS ET AL., BULLETIN DE LA SOCIETE CHIMIQUE DE FRANCE, vol. 6, 1967, pages 1888 - 90 |
| WANG CHANGHE ET AL: "The apparent formation constants of asiatic acid and its derivatives existing inwith cyclodextrins by HPLC", JOURNAL OF INCLUSION PHENOMENA AND MACROCYCLIC CHEMISTRY, KLUWER, DORDRECHT, NL, vol. 98, no. 3-4, 16 September 2020 (2020-09-16), pages 261 - 270, XP037278495, ISSN: 1388-3127, [retrieved on 20200916], DOI: 10.1007/S10847-020-01026-6 * |
| YAN SHAO ET AL: "Three New Pentacyclic Triterpenoids from Centella asiatica", HELVETICA CHIMICA ACTA, VERLAG HELVETICA CHIMICA ACTA, HOBOKEN, USA, vol. 97, no. 7, 15 July 2014 (2014-07-15), pages 992 - 998, XP071270801, ISSN: 0018-019X, DOI: 10.1002/HLCA.201300382 * |
| ZHAO ET AL., J AGRIC. FOOD CHEM., 2020, pages 68 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN103781367B (en) | The oral formulations that comprises some sweet taste triterpenes and triterpene glucoside | |
| US11154071B2 (en) | Sweetener iso-mogroside V | |
| US10538548B2 (en) | Material mixture containing rubusoside or alpha glycosylrubusoside, for enhancing sweet taste | |
| US10472385B2 (en) | Triterpene-glycosides as sweeteners or sweetener enhancers | |
| US20080220140A1 (en) | Use of propenylphenyl glycosides for enhancing sweet sensory impressions | |
| JP2017519812A5 (en) | ||
| MX2014006368A (en) | Taste-masking compositions, sweetener compositions and consumable product compositions containing the same. | |
| JP2022524024A (en) | Mixtures containing rare sugars and taste-modifying compounds | |
| JP2023522095A (en) | Composition | |
| WO2021198199A1 (en) | Flavor composition | |
| WO2006087370A1 (en) | Use of aroma glycosides as flavor or fragrance ingredient | |
| CN118215407A (en) | Dihydrochalcone derivatives | |
| WO2023036404A1 (en) | Taste balancing botanical compounds | |
| JPS647752B2 (en) | ||
| WO2023066457A1 (en) | Rubusoside glucosides | |
| CN113507843A (en) | Mixtures comprising rare sugars and taste modifying compounds | |
| WO2024213223A1 (en) | Acylated dihydrochalcones, methods of production and uses thereof | |
| CA2872990A1 (en) | Sweetener compositions comprising steviol glycosides and non-steviol secondary sweeteners | |
| JP2019004905A (en) | Novel tripelten glycoside used as sweetener or sweetness enhancer | |
| JP5027395B2 (en) | Carbohydrate derivative of cyclic maltosyl maltose, its production method and use | |
| WO2019162509A1 (en) | Composition comprising glucosylated terpene glycosides, terpene glycosides and cyclodextrine | |
| JPWO2019162509A5 (en) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21777211 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 21777211 Country of ref document: EP Kind code of ref document: A1 |