WO2022054932A1 - 藻類の培養方法および藻類培養システム - Google Patents
藻類の培養方法および藻類培養システム Download PDFInfo
- Publication number
- WO2022054932A1 WO2022054932A1 PCT/JP2021/033429 JP2021033429W WO2022054932A1 WO 2022054932 A1 WO2022054932 A1 WO 2022054932A1 JP 2021033429 W JP2021033429 W JP 2021033429W WO 2022054932 A1 WO2022054932 A1 WO 2022054932A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- culture
- tank
- algae
- digestive juice
- membrane
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M29/00—Means for introduction, extraction or recirculation of materials, e.g. pumps
- C12M29/04—Filters; Permeable or porous membranes or plates, e.g. dialysis
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D61/00—Processes of separation using semi-permeable membranes, e.g. dialysis, osmosis or ultrafiltration; Apparatus, accessories or auxiliary operations specially adapted therefor
- B01D61/14—Ultrafiltration; Microfiltration
- B01D61/147—Microfiltration
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D61/00—Processes of separation using semi-permeable membranes, e.g. dialysis, osmosis or ultrafiltration; Apparatus, accessories or auxiliary operations specially adapted therefor
- B01D61/24—Dialysis ; Membrane extraction
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D61/00—Processes of separation using semi-permeable membranes, e.g. dialysis, osmosis or ultrafiltration; Apparatus, accessories or auxiliary operations specially adapted therefor
- B01D61/24—Dialysis ; Membrane extraction
- B01D61/243—Dialysis
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D69/00—Semi-permeable membranes for separation processes or apparatus characterised by their form, structure or properties; Manufacturing processes specially adapted therefor
- B01D69/02—Semi-permeable membranes for separation processes or apparatus characterised by their form, structure or properties; Manufacturing processes specially adapted therefor characterised by their properties
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M21/00—Bioreactors or fermenters specially adapted for specific uses
- C12M21/02—Photobioreactors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M23/00—Constructional details, e.g. recesses, hinges
- C12M23/34—Internal compartments or partitions
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M41/00—Means for regulation, monitoring, measurement or control, e.g. flow regulation
- C12M41/30—Means for regulation, monitoring, measurement or control, e.g. flow regulation of concentration
- C12M41/32—Means for regulation, monitoring, measurement or control, e.g. flow regulation of concentration of substances in solution
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/12—Unicellular algae; Culture media therefor
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2315/00—Details relating to the membrane module operation
- B01D2315/06—Submerged-type; Immersion type
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2325/00—Details relating to properties of membranes
- B01D2325/02—Details relating to pores or porosity of the membranes
- B01D2325/0283—Pore size
Definitions
- the present invention relates to an algae culturing method and an algae culturing system.
- HAP Hydroxyapatite
- MAP magnesium ammonium phosphate
- ammonia tripping method methods for recovering nutrient salts from wastewater containing high-concentration nutrient salts.
- Non-Patent Documents 1 to 3 The HAP method and the MAP method are methods for recovering phosphorus in sewage sludge or human waste as wastewater containing high-concentration nutrients. With these methods, phosphorus and nitrogen can be recovered as a precipitate (solid matter), but it is often used in the wastewater treatment process, and it is necessary to perform pretreatment such as removal of turbidity components in the wastewater. Met.
- the ammonia tripping method is a method of recovering ammonia in the gas by expelling ammoniacal nitrogen from the liquid phase to the gas phase as ammonia gas at a high concentration contained in the wastewater. This method is also often used in the wastewater treatment process, and the recovered ammonia is also treated by catalytic decomposition.
- Non-Patent Document 5 a method of recovering nutrient salts by combining processes of membrane separation, electrodialysis, and distillation is also known (Non-Patent Document 5).
- FO membrane forward osmosis
- RO membrane reverse osmosis membrane
- the use of membrane is known, but in order to utilize osmosis, it is necessary to set the salt concentration on the recovery side high (sodium hydroxide 3.5M), and the usage of the recovered nitrogen component is limited. It was an issue.
- the present invention provides a new method for supplying and recovering nutrient salts using a membrane, and by continuously culturing algae, it is possible to put into practical use the production of biofuel and bioenergy at low cost and in a small space.
- An object of the present invention is to provide a method for supplying algae from a high-concentration nutrient-containing digestive juice into a culture medium at a supply rate suitable for algae cultivation, and culturing algae in a large amount at low cost, and an algae culture system for that purpose. And.
- the present inventors prevented clogging of the membrane by installing a membrane having a pore size of 0.45 ⁇ m between the digestive juice tank and the culture tank in the reaction tank. Furthermore, by keeping the volume of the digestive juice tank and the culture tank the same, the high-concentration nutrients in the digestive juice are cultured through the membrane by diffusion using the concentration difference between the digestive juice tank and the culture tank. It has been found that algae can be continuously cultivated by repeating the cycle of consuming the nutrients supplied to the tank and supplied with the algae. The present invention has been completed based on these findings.
- [Item 1] A method for culturing algae using a digestive juice tank containing a digestive juice containing high-concentration nutrient salts, a membrane having a pore size of 0.45 ⁇ m or less, and a reaction tank provided with a culture medium and a culture tank containing algae.
- a method for culturing algae which comprises a step of supplying the nutrient salts contained in the digestive juice to the culture broth through a membrane by diffusion using the difference in the concentration of nutrient salts between the digestive juice tank and the culture tank.
- Item 3 The method according to Item 1 or Item 2, wherein the film has an area of 0.0193 m 2 or more.
- Items 1 to 3 further include a step in which the digestive juice tank and the culture tank are further provided with a pump, and each liquid is circulated in the same tank by the pump to keep the liquid amounts of the digestive liquid and the culture liquid the same.
- Items 6 The method according to any one of Items 1 to 5, further comprising a step of supplying CO 2 to the culture tank.
- [Item 7] The method according to any one of Items 1 to 6, further comprising a step of supplying phosphate ions ( PO 4-3- ) to the culture tank.
- [Item 8] The method according to any one of Items 1 to 7, wherein the digestive liquid is a methane fermentation digestive liquid.
- the culture solution is tap water without descaling, groundwater, or river / lake water.
- [Item 10] The method according to any one of Items 1 to 9, wherein the alga is a microalgae.
- the membrane is a microfiltration membrane (MF membrane).
- the nutrient salts include at least one selected from the group consisting of ammonia nitrogen, nitrate nitrogen, phosphoric acid phosphorus, orthosilicic acid, potassium, calcium, magnesium and sulfur, items 1 to 11.
- An algae culture system including a digestive juice tank, a membrane having a pore size of 0.45 ⁇ m or less, and a culture tank, wherein the digestive juice tank contains digestive juice containing high-concentration nutrient salts, and the culture tank is used for culturing.
- An algae culture system comprising liquids and algae, wherein the membrane is placed as a partition between the digestive juice tank and the culture tank.
- the algae in the culture tank consume the nutrient salts to maintain the difference in concentration between the digestive juice tank and the culture tank, and the nutrient salts contained in the digestive juice are supplied to the culture solution by concentration diffusion.
- Item 13. The algae culture system according to Item 13.
- Item 15. The algae culture system according to Item 13 or Item 14, wherein the digestive juice tank, the membrane, and the culture tank are installed in a horizontal row in this order.
- Item 16. The algae culture system according to Item 13 or Item 14, wherein the digestive juice tank, the membrane, and the culture tank are installed in a vertical row in this order.
- the algae in the culture tank consume the nutrient salts to maintain the difference in the concentration of the nutrient salts between the digestive juice tank and the culture tank, and the nutrient salts contained in the digestive juice are supplied to the culture solution through the membrane by concentration diffusion.
- a method for supplying nutrient salts which is characterized by the fact that.
- nutrient salts can be supplied from the digestive juice at a supply rate suitable for culturing algae and in a required amount.
- nutrient salts can be supplied without the need for pretreatment such as removal of turbidity components, it is possible to realize low-cost culture of algae.
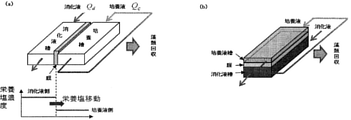
- FIG. 1 It is a schematic block diagram which shows an example of the algae culture system which concerns on this invention.
- (A) is a horizontal type, and (b) is a vertical type.
- Q d indicates the amount of inflow to the digestive juice tank
- Q c indicates the amount of inflow to the culture tank.
- the change with time of the fluorescence intensity of each culture medium prepared from a 20-fold diluted digestive solution, a 50-fold diluted digestive solution, a 100-fold diluted digestive solution and a CSi medium is shown.
- CO 2 gas mixed gas
- the experimental device for culturing by adding mixed gas is an aluminum gas bag (400 mL) containing a gas in which CO 2 gas and air are mixed and the CO 2 gas concentration is adjusted to about 10%.
- a vial (volume 228 mL) of a butyl rubber aluminum seal stopper containing a diluted digestive solution (100 mL) and a microalgae solution (20 mL) the experimental device for culturing by atmospheric addition is a diluted digestive solution (100 mL) and a fine gas.
- FIG. 4 It is a schematic diagram of an experimental device for separating turbidity components and nutrient salts in digestive juice.
- H represents the amount of liquid (water level), and ⁇ 40 represents a diameter of 40 mm.
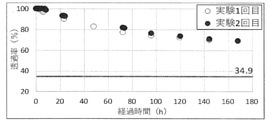
- the time course of the light transmittance (%) of the culture solution obtained by using the experimental apparatus shown in FIG. 4 is shown.
- ⁇ indicates the time course of the light transmittance of the culture solution in the first experiment, and ⁇ indicates the time course of the light transmittance of the culture solution in the second experiment.
- the line with a transmittance of 34.9% indicates the light transmittance of the 50-fold diluted digestive juice shown in Example 1, which is optimal for culturing microalgae.
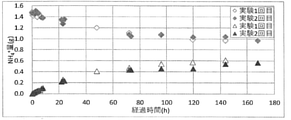
- the time course of ammonium ion (NH 4+ ) (g) in the digestive juice tank and the culture tank is shown.
- ⁇ indicates the change over time of ammonium ion (NH 4 + ) in the digestive juice tank in the first experiment, and ⁇ indicates the change over time in the ammonium ion (NH 4 + ) in the digestive juice tank in the second experiment.
- ⁇ indicates the change over time of ammonium ion (NH 4 + ) in the culture tank in the first experiment, and ⁇ indicates the change over time in the ammonium ion (NH 4 + ) in the culture tank in the second experiment.
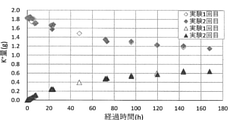
- the time course of potassium ion (K + ) (g) in the digestive juice tank and the culture tank is shown.
- ⁇ indicates the change over time of potassium ion (K + ) in the digestive juice tank in the first experiment
- ⁇ indicates the change over time in the potassium ion (K + ) in the digestive juice tank in the second experiment
- ⁇ indicates the change over time.
- the time course of potassium ion (K +) in the culture tank in the first experiment is shown
- ⁇ shows the time course of the potassium ion (K + ) in the culture tank in the second experiment.
- the amount of movement per unit area per unit time from the digestive juice tank to the culture tank (separation flux) and the amount of NH 4 + movement (movement flux) associated with the movement of water from the culture tank to the digestive juice tank are shown.
- the amount of microalgae in the culture tank from the 1st day to the 28th day is shown.
- the concentration on the 8th to 10th days was not measured.
- ⁇ indicates the day when distilled water was added
- ⁇ indicates the day when the digestive juice was replaced
- ⁇ indicates the day when the nutrient salt was added.
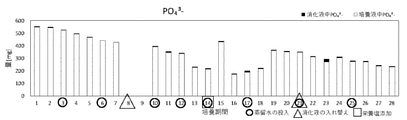
- the amount of PO 43 in the digestive juice / culture solution from the 1st day to the 28th day is shown.
- the amount of PO 433 on the 8th and 9th days was not measured.
- ⁇ indicates the amount of PO 43 in the digestive juice
- ⁇ indicates the amount of PO 4 3 in the culture solution
- ⁇ indicates the day when distilled water was added, ⁇ .
- ⁇ indicates the day when the nutrient salt was added.
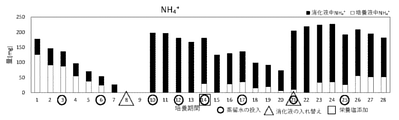
- the amount of NH 4+ in the digestive solution / culture solution from the 1st day to the 28th day is shown. The amount of NH 4+ on the 8th and 9th days was not measured .
- ⁇ indicates the amount of NH 4 + in the digestive juice
- ⁇ indicates the amount of NH 4 + in the culture solution
- in the culture period ⁇ indicates the day when distilled water was added
- ⁇ indicates the date when distilled water was added.
- the day when the digestive juice was replaced and ⁇ indicate the day when the nutrient salt was added.
- the present invention provides a method for culturing algae using a digestive juice tank containing a digestive juice containing high-concentration nutrient salts, a membrane having a pore size of 0.45 ⁇ m or less, and a reaction tank provided with a culture solution and a culture tank containing algae. It is a thing.
- the nutrient salts contained in the digestive juice are filmed by diffusion using the difference in the concentration of the nutrient salts between the digestive juice tank and the culture tank, which is generated by the algae in the culture tank consuming the nutrient salts. It is characterized in that it is supplied to the culture solution via.
- algae refers to organisms that perform oxygen-evolving photosynthesis, excluding moss plants, fern plants, and seed plants that mainly inhabit the ground.
- the algae of the present invention may be microorganisms that biosynthesize, such as Euglena.
- the algae are not particularly limited and can be appropriately selected according to the purpose.
- the algae of the present invention are preferably microalgae (for example, indigenous microalgae).
- the microalgae means microalgae whose individual existence cannot be recognized by the human naked eye.
- Microalgaes may be prokaryotes or eukaryotes.
- microalgae examples include green algae (Chlorophyta), gray algae (Glaucophyta), red algae (Rhodophyta), chloralachniophyta, Euglena (Euglenophyta), euglena (Euglenophyta), and crypta (crypta). Examples thereof include microalgae belonging to any one of Haptophyta, Heteromonyphyta, Dinophyta, Chromarida, and Cyanobacteria. Microalgaes may have uncertain taxa, and may be molecularly phylogenetically included in these taxa or shown to be closely related.
- one type of algae can be used alone or in combination of two or more types. If it has a symbiotic relationship with another organism, it may be used together with that organism.
- the method for obtaining microalgae is not particularly limited and may be appropriately selected depending on the purpose. For example, a method of collecting from the natural world, a method of using a commercially available product, a method of obtaining from a storage organization or a depository organization, etc. Can be mentioned
- the algae cultivated in the method for culturing algae of the present invention can be recovered from the culture solution by a commonly used method such as centrifugation, sedimentation with a flocculant, or membrane separation. It is also possible to deposit and recover the biofilm formed on the surface of the culture solution.
- the "digestive liquid” is obtained after being fermented in a biomass plant (BGP) using livestock excrement, food processing residue, waste cooking oil, swill, sewage sludge, human waste, septic tank sludge, etc. as raw materials. It refers to the residue that has been obtained.
- the digestive juice of the present invention is, for example, a methane fermentation digestive juice. Further, the digestive juice of the present invention is preferably derived from livestock excrement (for example, cow dung) because it is easy to secure a large amount of raw materials.
- the "nutrient salts” refer to salts necessary for nutrition of algae (for example, microalgae).
- nitrogen such as ammonia nitrogen, nitrate nitrogen, nitrite nitrogen, organic nitrogen, phosphoric acid phosphorus, phosphorus such as organic phosphorus, silicon such as orthosilicic acid, potassium, calcium, magnesium
- sulfur examples include sulfur.
- nutrient salts can be used as a nutrient source for the growth of algae.
- the "culture solution” refers to a solution having a high light transmittance.
- examples of the culture solution include tap water without descaling, groundwater, river / lake water, and the like. It is desirable that the culture solution of the present invention has a light transmittance of 34.9% or more.
- the culture solution of the present invention used distilled water, and the bottom water of the pond on the campus of Hokkaido University was planted as early indigenous microalgae.
- nutrient salts are supplied from the digestive juice tank, and the supplied nutrient salts are consumed by indigenous microalgaes and repeated in a cycle in which the nutrient salts are maintained at a low concentration. ..
- the "membrane (filter)" is used as a partition between the digestive juice tank and the culture tank.
- the membrane of the present invention include a microfiltration membrane (MF membrane), an ultrafiltration membrane (UF membrane), and a nanofiltration membrane (NF membrane).
- the film of the present invention preferably has a pore size of 0.45 ⁇ m or less. When the pore size of the membrane exceeds 0.45 ⁇ m, the turbidity component in the digestive juice also moves into the culture broth, so that the light transmittance of the culture broth decreases and the biosynthesis of algae is inhibited.
- the membrane of the present invention has an area of 0.0193 m 2 or more, preferably 0.0256 m 2 or more, per 1 m 3 of the volume of the tank.
- the "turbidity component” is a substance that causes turbidity in a liquid and has a particle size of more than 0.45 ⁇ m.
- the turbidity component of the present invention include particulate organic substances, plankton and other microorganisms, suspended solids and the like.
- the method for culturing algae of the present invention can supply nutrient salts at a supply rate of 177 to 188 g-N / m 2 / d.
- the method for culturing algae of the present invention can cultivate algae at a culture rate (growth rate) of 49 to 234 g / m 3 / d.
- the culture rates are 49 to 73 g / m 3 / d, 49 to 78 g / m 3 / d, 49 to 93 g / m 3 / d, 49 to 126 g / m 3 / d, 73 to 93 g / m.
- the method for culturing algae of the present invention is to cultivate algae by supplying a phosphorus source, preferably phosphate ion ( PO 43- ) , to the culture tank at an appropriate ratio according to the amount of nitrogen supplied in the digestive juice. You can increase the speed. For example, the ratio of the nitrogen supply amount in the digestive juice to the phosphate ion supply amount in the culture tank is 7: 1.
- the digestive solution and the culture solution are circulated in the digestive solution tank and the culture solution, respectively, by a stirrer, preferably a pump, to keep the amounts of the digestive solution and the culture solution at the same water level for digestion.
- a stirrer preferably a pump
- the culture of algae can be enhanced by supplying a carbon dioxide source, preferably CO 2 to the culture tank.
- the method for culturing algae of the present invention is to cultivate algae by supplying a phosphorus source, preferably phosphate ion ( PO 43- ) , to the culture tank at an appropriate ratio according to the amount of nitrogen supplied in the digestive juice. Can be enhanced.
- a phosphorus source preferably phosphate ion ( PO 43- )
- the ratio of the nitrogen supply amount in the digestive juice to the phosphate ion supply amount in the culture tank is 7: 1.
- the culture rate of the algae is increased and the culture of the algae is enhanced.
- the "algae culture system” in the present invention includes a digestive juice tank, a membrane (filter), and a culture tank.
- the digestive juice tank contains digestive juice containing high-concentration salts
- the culture tank contains culture medium and algae.
- the digestive juice tank and the culture tank may have one or more devices such as a stirrer, a temperature control device, a pH control device, a turbidity measuring device, an optical control device, and a specific gas concentration measuring device such as CO 2 . ..
- the membrane is installed between the digestive juice tank and the culture tank, and the pore size thereof is preferably 0.45 ⁇ m or less.
- the algae culture system in the present invention may be installed in a horizontal row in the order of a digestive juice tank, a membrane, and a culture tank, or a vertical row. It may be installed in.
- a transparent pipe made of vinyl chloride having a diameter of 40 mm is sandwiched between a packing and a filter of 0.45 ⁇ m and connected to each other, and the digestive juice and distillation are placed in the digestive liquid tank and the culture tank separated by the filter, respectively. Since 600 mL of water is added at the same water level and the inside is stirred, the liquid can be circulated from the lower part to the upper part of the tank at 400 mL / min for use.
- the algae culture system in the present invention maintains the difference in concentration between the digestive juice tank and the culture tank by consuming the nutrient salts by the algae in the culture tank, and supplies the nutrient salts contained in the digestive juice to the culture solution by concentration diffusion. can do.
- the algae culture system in the present invention can supply nutrient salts to the culture tank at a supply rate suitable for algae culture, and can realize low cost.
- the algae culture system of the present invention can minimize the movement of the turbidity component even when a digestive juice containing a high concentration nutrient salt containing a large amount of the turbidity component is used.
- the change in the concentration of nutrient salts in the digestive juice tank in the algae culture system of the present invention shown in FIG. 1 can be calculated by the formula (1).
- the change in the concentration of nutrient salts in the culture tank in the algae culture system of the present invention shown in FIG. 1 can be calculated by the formula (2).
- the concentration change of the algae concentration in the culture tank can be calculated by the formula (3).
- the nutrient salt separation flux (the amount of nutrients transferred from the digestive juice tank to the culture tank per unit time unit area) in the algae culture system of the present invention shown in FIG. 1 can be calculated by the formula (4). ..
- V is the volume of the tank (V d is the volume of the digestive juice tank, V c is the volume of the culture tank), C s is the nutrient concentration (C s d is the nutrient concentration in the digestive juice tank, C cd ).
- C x is the concentration of algae
- Q is the amount of water flowing into the tank (Q d is the amount of water flowing into the digestive juice tank, Q c is the amount of water flowing into the culture tank)
- r x is fine.
- the growth rate of algae Y xs is the nutrient consumption per microalga
- F is the nutrient separation flux
- a f is the filter area
- k is the movement rate coefficient of the membrane.
- the nutrient concentration in the digestive juice tank should be calculated by the formula (5). Can be done.
- the nutrient concentration in the culture tank can be calculated by the formula (6). can.
- the concentration of algae can be calculated by the formula (7).
- the present invention is a method for supplying nutrient salts using a digestive juice tank containing a digestive juice containing high-concentration nutrient salts, a membrane having a pore size of 0.45 ⁇ m or less, and a reaction tank provided with a culture solution and a culture tank containing algae.
- a method of supplying nutrient salts which is characterized by supplying.
- Example 1 Examination of digestive juice useful for algae culture To 50 mL of medium (cattle manure methane fermentation digestive juice and standard medium (CSi)), 10 mL of environmental water (collected from the bottom layer of a pond on the premises of Hokkaido University) was added. The culture medium was prepared. In addition, the bovine manure methane fermentation digestive juice was centrifuged and diluted 20-fold, 50-fold and 100-fold with distilled water to prepare a 20-fold diluted digestive solution, a 50-fold diluted digestive solution and a 100-fold diluted digestive solution. ..
- Figure 2 shows the change over time in the fluorescence intensity of each culture solution.
- the fluorescence intensity tends to increase as in the CSi medium
- the number of days in which the fluorescence intensity increases is the other digestive solution and the CSi medium.
- the increase in fluorescence intensity was similar.
- microalgae can be cultivated if the digestive juice has a light transmittance of 34.9% or more. Further, it is considered that the 50-fold diluted digestive juice having the highest fluorescence intensity is most suitable for culturing microalgae.
- Example 2 Examination of factors that inhibit light transmission
- the digestive juice is filtered using a membrane filter having a pore size of 1 ⁇ M or 0.45 ⁇ M, and the light transmittance of the digestive juice stock solution and the filtrate filtered by each filter is determined. , Measured using a spectrophotometer (U-1800, Hitachi High-Tech Science). Further, 1 g or 0.25 g of granular activated carbon was added to 50 mL of digestive juice, shaken at 200 rpm for 0.5 hours or more, and then filtered by the same method as described above to measure the light transmittance of the filtrate. .. The wavelength of the light was 684 ⁇ m, and the transmittance of the 1 cm quartz cell in which distilled water was placed was 100%.
- Table 2 shows the light transmittances of the digestive juice stock solution and the filtrate.
- the light transmittance of the digestive juice stock solution was zero, and the light transmittance did not change in the filtrate of the 1 ⁇ m filter regardless of the amount of activated carbon.
- the light transmittance was greatly improved by adding activated carbon as compared with the digestive juice stock solution. This suggests that the coloring component (0.45 ⁇ m or less) is adsorbed on the granular active sputum.
- the light transmittance was not improved by the filtrate of the 1 ⁇ m filter, it was shown that the light transmittance was not improved in the presence of particles of 1 ⁇ m or less even if the coloring component was removed.
- the turbidity component having a larger particle size than the coloring component inhibits the transmission of light, and it is necessary to separate the turbidity component and the nutrient salts in the culture of algae. Shown. It was also shown that it is useful to use a filter having a pore size of 0.45 ⁇ m or less for the separation of turbidity components and nutrient salts.
- Example 3 Effect of addition of mixed gas (CO 2 gas) in culturing indigenous microalgae Centrifugation of digestive juice collected from a bovine manure biomass plant (BGP) is centrifuged, diluted 50-fold with distilled water, and has a wavelength of 684 nm.
- a diluted digestive solution was prepared by setting the transmittance to 28% and adding KH 2 PO 4 as a phosphorus source to a concentration of 60 mg / L.
- KH 2 PO 4 as a phosphorus source
- the concentration of indigenous microalgae in the CO 2 gas culture with the mixed gas was higher than the concentration of the indigenous microalgae in the air culture. It is considered that this is because the CO 2 gas supply by the mixed gas is larger than the CO 2 gas supply from the atmosphere. Therefore, it was suggested that the culture of algae was activated by supplying CO 2 gas.
- Example 4 Separation test of turbidity component and nutrient salts in digestive juice Using the experimental device shown in FIG. 4, the light transmission rate of the culture solution and the ammonium ion (NH 4+ ) and ammonium ion (NH 4+ ) of the digestive solution and the culture solution and The potassium ion (K + ) concentration was measured to confirm that the turbidity component and nutrient salts were separated. Specifically, a transparent pipe made of vinyl chloride with a diameter of 40 mm is sandwiched between a packing and a 0.45 ⁇ m microfiltration (MF) membrane with a flange and connected, and the membrane is separated to form a digestive juice tank and a culture tank, respectively.
- MF microfiltration
- each of digestive juice and distilled water were added to make the water level the same.
- the liquid was circulated from the bottom to the top of the tank at 400 mL / min by a pump.
- the test period is 7 days.
- FIG. 5 shows changes in the light transmittance of the culture solution over time. Over time, the light transmittance decreased, but the change gradually slowed down. It is considered that this is because the coloring component of less than 0.45 ⁇ m permeates the film and the moving speed decreases as the concentration difference becomes smaller. Since the light transmittance of the culture solution is higher than the light transmittance (34.9%) of the 50-fold diluted digestive solution considered to be optimal for culturing microalgae shown in Example 1, the algae have a light transmittance. It was shown that the movement of the turbidity component, which hinders the culture, could be suppressed to a minimum.
- FIG. 8 shows the amount of movement per unit area (separation flux) from the digestive juice tank to the culture tank and the NH 4 + movement amount (movement flux) associated with the movement of water from the culture tank to the digestive juice tank.
- the separation flux was calculated from the concentration difference between the two tanks and the concentration change in the culture tank, and the movement due to the concentration difference was observed.
- the transfer flux was calculated by obtaining the amount of water transfer from the difference in head between the two tanks and multiplying by the concentration in the culture tank. It was confirmed that the head tends to be higher on the culture tank side over time and reaches 3 to 4 cm.
- Example 5 Examination of mass culture of microalgae
- the unit volume is one unit
- the volume of the tank is 1 m 3
- the parameters related to the generation of digestive juice shown in Table 5 are used.
- the growth rate of microalgae was predicted when the digestive juice obtained from the scale of 100 dairy cows was used.
- the amount of manure generated and the water content of the digestive juice are described in the New Energy Foundation: Biomass Technology Handbook, p.240 (2008), Ohmsha and Heinz Schulz, Barbara Eder: Biogas Practical Technology p.135 (2002), respectively. I used a parameter.
- the parameters of the NH4 concentration of the digestive juice used the experimental values obtained by using an ion chromatography analyzer (DIONEX DX --120, Thermo Fisher Scientific KK).
- the NH4 concentration is 272 or 387 g / 387 g / to obtain the same NH4 amount per microalgae as the initial amount. It becomes m 3 .
- the area of the membrane is as follows: When calculated based on, it becomes 0.0193 or 0.0256m 2 .
- the moving speed coefficient of the film was 0.087 m / s (slope of the approximate straight line in FIG. 5).
- the supply rate of nutrient salts can be realized while achieving the general algae culturing rate, so that continuous algae culturing becomes possible, and a large amount of algae can be cultivated. Enables culture and recovery.
- Example 6 Effect of nutrient salts on the cultivation of microalgae
- a device equipped with a digestive juice tank, a precision filtration membrane having a pore size of 0.45 ⁇ m, and a 10 L tub (culture tank) was used as an algae culture system.
- the concentrations of nutrient salts and microalgae were measured and analyzed, and the effects of nutrient salts on the culture of microalgae were investigated. Specifically, the concentration of microalgae in the culture tank and the concentration of NH 4+ and PO 433 in the digestive juice / culture medium were measured according to the following procedure. In this experiment, indigenous microalgae were used as microalgae.
- the NH 4 + concentration in the digestive juice and culture medium was measured, and it was examined whether microalgae were cultured using the nutrient salts in the digestive juice.
- the microalgae concentration is calculated from the weight obtained by collecting the microalgae in the culture solution using a microfiltration membrane of 0.45 ⁇ m, drying at 105 ° C. for 24 hours, and measuring the PO 433 and NH 4 + concentration. , Ion chromatography (DIONEX DX-120, Thermo Fisher Scientific KK) or ion chromatograph (IC-2010, Tokyo Kaken Co., Ltd.). It should be noted that the addition of distilled water on the 3, 6, 10, 12, 14, 17, 21 and 25 days, the replacement of the digestive juice in the apparatus on the 8 and 21 days, and the KH 3 PO 4 on the 14th day. Addition was performed.
- the amount of microalgae in the culture tank from the 1st day to the 28th day is shown in FIG.
- the concentration on the 8th to 10th days was not measured. No growth of microalgae was observed until about 9 days after seeding of microalgae, but the culture solution turned green from about 10th day, and growth of microalgae could be confirmed visually.
- On the 12th day 1 L of the culture solution (amount of microalgae: 0.16 g) was recovered, and thereafter, the amount of microalgae gradually decreased, and it was confirmed visually that the color of the culture solution became lighter.
- the membrane separation device When the membrane separation device was disassembled after the measurement of the amount of microalgae on the 28th day, the microalgae entered into the gap between the flanges of the device, and the microalgae were proliferating.
- the amount of microalgae that had entered the inside of the device was calculated by dropping them into the culture solution with a brush and found to be 0.5 g.
- the amount of PO 4-3- in the digestive solution / culture solution from the 1st day to the 28th day is shown in Table 7 and FIG.
- the amount of PO 433 on the 8th and 9th days was not measured.
- the digestive juice contains almost no PO 4-3- . Therefore, it was shown that PO 433 in the culture broth was derived from the added KH 3 PO 4 , and that PO 433 was hardly transferred between the digestive broth and the culture broth. In addition, since PO 43- was consumed at a substantially constant rate in the culture solution, it was shown that microalgae could be cultured using PO 43 .
- the growth rate of microalgae was calculated at regular intervals (1st to 6th days, 10th to 14th days, 15th to 19th days and 19th to 28th days).
- the calculated growth rate of microalgae was 78 to 234 g / m 3 / d, which was larger than the growth rates of air culture and CO 2 gas culture.
- the growth rate of microalgae in each period is (PO 43 - amount (mg) in the culture solution on the first day - PO 43 - amount (mg) in the culture solution on the last day) x phosphoric acid consumption of the microalgae. It was calculated by (0.044 mg) / liquid volume (L) / number of days (day).
- the growth rate of microalgae on the 1st to 6th days is (PO 4-3 -amount ( 547.63 mg) in the culture solution on the 1st day-63 - amount ( 440.74 mg) in the culture solution on the 6th day).
- x Phosphoric acid consumption of microalgae (0.044 mg) / liquid volume (3.8648 L) / number of days (5 day) 125.71 mg / L / day (g / m 3 / day).
- the amount of NH 4+ in the digestive solution / culture solution from the 1st day to the 28th day is shown in Table 8 and FIG.
- the amount of NH 4+ on the 8th and 9th days was not measured .
- the total amount of NH 4+ decreased from the 10th day to the 13th day, but NH 4+ could not be confirmed in the culture medium . It is considered that this is because the consumption of NH 4+ exceeded the supply amount due to the grown microalgae .
- nutrient salts can be supplied from the digestive juice at a supply rate suitable for culturing algae and in a required amount. Further, since the present invention can supply nutrient salts without requiring pretreatment such as removal of turbidity components, it is possible to realize low-cost culture and recovery of algae. Furthermore, it will be possible to cultivate algae in large quantities, and it is expected that the production of biofuels and bioenergy on a commercial scale will be put into practical use.
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Water Supply & Treatment (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Sustainable Development (AREA)
- Urology & Nephrology (AREA)
- Botany (AREA)
- Cell Biology (AREA)
- Medicinal Chemistry (AREA)
- Tropical Medicine & Parasitology (AREA)
- Virology (AREA)
- Analytical Chemistry (AREA)
- Molecular Biology (AREA)
- Clinical Laboratory Science (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US18/025,769 US20230348835A1 (en) | 2020-09-11 | 2021-09-10 | Method of Culturing Alga and Alga Culture System |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020153276 | 2020-09-11 | ||
| JP2020-153276 | 2020-09-11 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022054932A1 true WO2022054932A1 (ja) | 2022-03-17 |
Family
ID=80631787
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/033429 Ceased WO2022054932A1 (ja) | 2020-09-11 | 2021-09-10 | 藻類の培養方法および藻類培養システム |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US20230348835A1 (enExample) |
| JP (1) | JP2022047532A (enExample) |
| WO (1) | WO2022054932A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023146888A (ja) * | 2022-03-29 | 2023-10-12 | 太平洋セメント株式会社 | Co2溶解液を用いた藻類培養装置 |
| TWI891220B (zh) * | 2024-01-24 | 2025-07-21 | 索瑪沛思生物科技股份有限公司 | 藻類培養設備 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007330215A (ja) * | 2006-06-19 | 2007-12-27 | Electric Power Dev Co Ltd | 微細藻類培養器具 |
| US20130052719A1 (en) * | 2010-02-22 | 2013-02-28 | Inha-Industry Partnership Institute | Photobioreactor for mass culture of microalgae, and method for culturing microalgae by using same |
| US20160075981A1 (en) * | 2013-04-05 | 2016-03-17 | Inha-Industry Partnership Institute | Photobioreactor for mass culturing of photosynthetic microorganism |
| CN106219871A (zh) * | 2016-08-09 | 2016-12-14 | 重庆大学 | 一种畜禽养殖废水处理方法 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL46022A (en) * | 1974-11-08 | 1977-06-30 | Dor I | Process for promotion of algae growth in a sewage medium |
| WO2017169713A1 (ja) * | 2016-03-29 | 2017-10-05 | 富士フイルム株式会社 | 微細藻類の培養方法、及び藻類バイオマスの製造方法 |
-
2021
- 2021-09-10 JP JP2021148180A patent/JP2022047532A/ja active Pending
- 2021-09-10 US US18/025,769 patent/US20230348835A1/en active Pending
- 2021-09-10 WO PCT/JP2021/033429 patent/WO2022054932A1/ja not_active Ceased
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007330215A (ja) * | 2006-06-19 | 2007-12-27 | Electric Power Dev Co Ltd | 微細藻類培養器具 |
| US20130052719A1 (en) * | 2010-02-22 | 2013-02-28 | Inha-Industry Partnership Institute | Photobioreactor for mass culture of microalgae, and method for culturing microalgae by using same |
| US20160075981A1 (en) * | 2013-04-05 | 2016-03-17 | Inha-Industry Partnership Institute | Photobioreactor for mass culturing of photosynthetic microorganism |
| CN106219871A (zh) * | 2016-08-09 | 2016-12-14 | 重庆大学 | 一种畜禽养殖废水处理方法 |
Non-Patent Citations (3)
| Title |
|---|
| LIU, XIN ET AL: "P-H03: Microalgae cultivation by anaerobic fermentation digestion liquid with nitrification processing and membrane filtration.", LECTURES OF THE 52ND JAPAN SOCIETY ON WATER ENVIRONMENT, JAPAN SOCIETY ON WATER ENVIRONMENT., JP, vol. 52, 9 March 2018 (2018-03-09), JP, pages 593, XP009534999 * |
| TAKUMI NAKAJIMA ET AL.: "Study on separation of color components and nutrients of bovine manure methane fermentation digestive juice using microfiltration membrane for indigenous microalgae culture", THE 31ST SOCIETY FOR WASTE RESOURCE RECYCLING RESEARCH PRESENTATION, 16-18 AUGUST 2020, JP., 21 August 2020 (2020-08-21) - 18 September 2020 (2020-09-18), JP, pages 215 - 216, XP009535001 * |
| TAKUMI NAKAJIMA: "Study on separation of color components and nutrients of bovine manure methane fermentation digestive juice using microfiltration membrane for indigenous microalgae culture", PROCEEDINGS OF THE 48TH ENVIRONMENTAL SYSTEMS RESEARCH PAPER PRESENTATION, 17 OCTOBER 2020, vol. 48, 17 October 2020 (2020-10-17), pages 23 - 27, XP055911195, DOI: PROCEEDINGS OF THE 48TH ENVIRONMENTAL SYSTEMS RESEARCH PAPER PRESENTATION, 17 OCTOBER 202 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023146888A (ja) * | 2022-03-29 | 2023-10-12 | 太平洋セメント株式会社 | Co2溶解液を用いた藻類培養装置 |
| TWI891220B (zh) * | 2024-01-24 | 2025-07-21 | 索瑪沛思生物科技股份有限公司 | 藻類培養設備 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2022047532A (ja) | 2022-03-24 |
| US20230348835A1 (en) | 2023-11-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Sahle-Demessie et al. | Bio-desalination of brackish and seawater using halophytic algae | |
| Xu et al. | Roles of SRT and HRT of an algal membrane bioreactor system with a tanks-in-series configuration for secondary wastewater effluent polishing | |
| Ruiz-Martinez et al. | Microalgae cultivation in wastewater: nutrient removal from anaerobic membrane bioreactor effluent | |
| Larsdotter | Wastewater treatment with microalgae-a literature review | |
| Nwoba et al. | Growth comparison of microalgae in tubular photobioreactor and open pond for treating anaerobic digestion piggery effluent | |
| Michels et al. | Growth of Tetraselmis suecica in a tubular photobioreactor on wastewater from a fish farm | |
| US8940340B2 (en) | Systems and methods for maintaining the dominance of Nannochloropsis in an algae cultivation system | |
| de Mattos et al. | COD and nitrogen removal from sugarcane vinasse by heterotrophic green algae Desmodesmus sp. | |
| US20120214198A1 (en) | Algaculture method | |
| González et al. | Wastewater nutrient recovery using twin-layer microalgae technology for biofertilizer production | |
| Olguín et al. | Anaerobic digestates from vinasse promote growth and lipid enrichment in Neochloris oleoabundans cultures | |
| US9113605B2 (en) | Methods and compositions to aggregate algae | |
| Mohan et al. | A sustainable process train for a marine microalga-mediated biomass production and CO2 capture: A pilot-scale cultivation of Nannochloropsis salina in open raceway ponds and harvesting through electropreciflocculation | |
| Hønsvall et al. | Continuous harvesting of microalgae by new microfluidic technology for particle separation | |
| CN109844094A (zh) | 具有凝集能的新微细藻类 | |
| El Nadi et al. | Desalination using algae ponds under nature Egyptian conditions | |
| Shirazi et al. | Simultaneous biomass production and water desalination concentrate treatment by using microalgae | |
| Podevin et al. | Detailing the start-up and microalgal growth performance of a full-scale photobioreactor operated with bioindustrial wastewater | |
| Romero-Villegas et al. | Utilization of centrate for the outdoor production of marine microalgae at pilot-scale in flat-panel photobioreactors | |
| Lee et al. | Semi-continuous operation and fouling characteristics of submerged membrane photobioreactor (SMPBR) for tertiary treatment of livestock wastewater | |
| CN104245916A (zh) | 以增强的光合效率大规模培养微藻的方法和系统 | |
| WO2022054932A1 (ja) | 藻類の培養方法および藻類培養システム | |
| Böpple et al. | Water treatment of recirculating aquaculture system (RAS) effluent water through microalgal biofilms | |
| Han et al. | Cycling of iodine by microalgae: Iodine uptake and release by a microalgae biofilm in a groundwater holding pond | |
| Zhuang et al. | Comparison of microalgae cultivation modes for advanced wastewater purification and pollutant-resource conversion: a pilot study |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21866889 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 21866889 Country of ref document: EP Kind code of ref document: A1 |