WO2020202666A1 - 歯科補綴物用ブロック体の製造方法、歯科補綴物の製造方法 - Google Patents
歯科補綴物用ブロック体の製造方法、歯科補綴物の製造方法 Download PDFInfo
- Publication number
- WO2020202666A1 WO2020202666A1 PCT/JP2019/050162 JP2019050162W WO2020202666A1 WO 2020202666 A1 WO2020202666 A1 WO 2020202666A1 JP 2019050162 W JP2019050162 W JP 2019050162W WO 2020202666 A1 WO2020202666 A1 WO 2020202666A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- block body
- dental prosthesis
- manufacturing
- mass
- less
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61C—DENTISTRY; APPARATUS OR METHODS FOR ORAL OR DENTAL HYGIENE
- A61C13/00—Dental prostheses; Making same
- A61C13/08—Artificial teeth; Making same
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61C—DENTISTRY; APPARATUS OR METHODS FOR ORAL OR DENTAL HYGIENE
- A61C13/00—Dental prostheses; Making same
- A61C13/0003—Making bridge-work, inlays, implants or the like
- A61C13/0006—Production methods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61C—DENTISTRY; APPARATUS OR METHODS FOR ORAL OR DENTAL HYGIENE
- A61C13/00—Dental prostheses; Making same
- A61C13/0003—Making bridge-work, inlays, implants or the like
- A61C13/0022—Blanks or green, unfinished dental restoration parts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K6/00—Preparations for dentistry
- A61K6/80—Preparations for artificial teeth, for filling teeth or for capping teeth
- A61K6/831—Preparations for artificial teeth, for filling teeth or for capping teeth comprising non-metallic elements or compounds thereof, e.g. carbon
- A61K6/833—Glass-ceramic composites
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03B—MANUFACTURE, SHAPING, OR SUPPLEMENTARY PROCESSES
- C03B32/00—Thermal after-treatment of glass products not provided for in groups C03B19/00, C03B25/00 - C03B31/00 or C03B37/00, e.g. crystallisation, eliminating gas inclusions or other impurities; Hot-pressing vitrified, non-porous, shaped glass products
- C03B32/02—Thermal crystallisation, e.g. for crystallising glass bodies into glass-ceramic articles
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C10/00—Devitrified glass ceramics, i.e. glass ceramics having a crystalline phase dispersed in a glassy phase and constituting at least 50% by weight of the total composition
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C10/00—Devitrified glass ceramics, i.e. glass ceramics having a crystalline phase dispersed in a glassy phase and constituting at least 50% by weight of the total composition
- C03C10/0018—Devitrified glass ceramics, i.e. glass ceramics having a crystalline phase dispersed in a glassy phase and constituting at least 50% by weight of the total composition containing SiO2, Al2O3 and monovalent metal oxide as main constituents
- C03C10/0027—Devitrified glass ceramics, i.e. glass ceramics having a crystalline phase dispersed in a glassy phase and constituting at least 50% by weight of the total composition containing SiO2, Al2O3 and monovalent metal oxide as main constituents containing SiO2, Al2O3, Li2O as main constituents
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/076—Glass compositions containing silica with 40% to 90% silica, by weight
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/076—Glass compositions containing silica with 40% to 90% silica, by weight
- C03C3/083—Glass compositions containing silica with 40% to 90% silica, by weight containing aluminium oxide or an iron compound
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/076—Glass compositions containing silica with 40% to 90% silica, by weight
- C03C3/097—Glass compositions containing silica with 40% to 90% silica, by weight containing phosphorus, niobium or tantalum
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C4/00—Compositions for glass with special properties
- C03C4/0007—Compositions for glass with special properties for biologically-compatible glass
- C03C4/0021—Compositions for glass with special properties for biologically-compatible glass for dental use
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/01—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/01—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics
- C04B35/16—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics based on silicates other than clay
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/622—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/653—Processes involving a melting step
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C2205/00—Compositions applicable for the manufacture of vitreous enamels or glazes
- C03C2205/06—Compositions applicable for the manufacture of vitreous enamels or glazes for dental use
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/32—Metal oxides, mixed metal oxides, or oxide-forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3201—Alkali metal oxides or oxide-forming salts thereof
- C04B2235/3203—Lithium oxide or oxide-forming salts thereof
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/32—Metal oxides, mixed metal oxides, or oxide-forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3217—Aluminum oxide or oxide forming salts thereof, e.g. bauxite, alpha-alumina
Definitions
- This disclosure relates to a method for manufacturing a block body for a dental prosthesis and a method for manufacturing a dental prosthesis.
- CAD / CAM Computer Aided Design / Computer Aided Manufacturing
- the shape of the designed dental prosthesis is handled as digital data converted into a predetermined format, and the data is processed by a processing device.
- the processing apparatus automatically performs machining such as cutting and grinding based on the data to produce a dental prosthesis. This makes it possible to quickly provide dental prostheses.
- Such a dental prosthesis shall have strength, hardness, chemical durability against the oral environment, and aesthetics (hue, texture) similar to those of natural teeth, which are the basic functions of a dental prosthesis. Is required.
- the dental prosthesis has complicated irregularities, and it is important to machine the complicated shape in a short time without causing problems such as chipping. By using a material that can be processed in such a short time, it becomes possible to produce a dental prosthesis more quickly.
- Patent Document 1 discloses a material for dental prostheses containing a predetermined component, thereby improving the above basic functions and machinability.
- An object of the present disclosure is to provide a method for manufacturing a block body for a dental prosthesis having good machinability.
- One aspect of the present disclosure is a method of producing a block body before machining to obtain a dental prosthesis, in which a glass blank having a temperature lower than the formation temperature of lithium metasilicate crystals is used as a lithium disilicate crystal.
- This is a method for producing a block body for a dental prosthesis, which comprises a step of exposing to an atmosphere above the formation temperature and below the melting point and heating the main crystal phase to lithium disilicate.
- It may have a step of slowly cooling after the step of heating.
- the temperature of the atmosphere may be 750 ° C or higher and 900 ° C or lower.
- Exposure to the above atmosphere may be to put the glass blank in the heating device.
- SiO 2 is 60% by mass or more and 80% by mass or less
- Li 2 O is 10% by mass or more and 20% by mass or less
- Al 2 O 3 is 3% by mass or more and 15% by mass or less. It may be included in.
- Another aspect of the present disclosure is a method for manufacturing a dental prosthesis, which comprises a step of manufacturing a block body for a dental prosthesis by the above manufacturing method and machining the block body for a dental prosthesis. This machining may be cutting.
- a block body for a dental prosthesis having good machinability can be obtained.
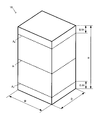
- FIG. 1 is an external perspective view of the block body 10 for a dental prosthesis.
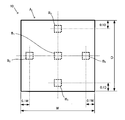
- FIG. 2 is a diagram showing a part of the cut surface enlarged so that crystals can be seen.
- FIG. 3 is a diagram illustrating a method of measuring the ratio.
- FIG. 4 is another diagram illustrating a method of measuring the ratio.
- This block body for dental prostheses (hereinafter, may be referred to as "block body”) is a square pillar, a cylinder, or a plate shape (disc shape), and is deformed from here by machining such as cutting or grinding. And carve it out to make a dental prosthesis.
- a dental prosthesis when produced by cutting, it can be formed into a prism shape or a plate shape (disc shape).
- the prismatic block body is often used mainly for carving out a single dental prosthesis, and the plate-shaped block body may be used for carving out a plurality of dental prostheses from one block body. ..
- FIG. 1 shows an external perspective view of the block body 10 which is a prism.
- the width W, the depth D, and the height H can each be in the range of 10 mm or more and 35 mm or less.
- the thickness can be configured to be in the range of 10 mm or more and 35 mm or less.
- the material can be composed of the following components.
- SiO 2 is 60% by mass or more and 80% by mass or less
- Al 2 O 3 is 3% by mass or more and 15% by mass or less.
- each of the above components is as follows. If the content of SiO 2 is less than 60% by mass or more than 80% by mass, it becomes difficult to obtain a homogeneous block body. More preferably, it is 65% by mass or more and 75% by mass or less. If the content of Li 2 O is less than 10% by mass or more than 20% by mass, it becomes difficult to obtain a homogeneous block body, and the machinability tends to decrease. More preferably, it is 12% by mass or more and 18% by mass or less. If the content of Al 2 O 3 is less than 3% by mass, lithium disilicate is precipitated as the main crystal phase, but the machinability tends to decrease. On the other hand, if it exceeds 15% by mass, the main crystal phase is not lithium disilicate, and the strength tends to decrease. More preferably, it is 3% by mass or more and 7% by mass or less.
- the block body for dental prosthesis may contain the following components in addition to the above components.
- the component represented here contains 0% by mass, it does not necessarily have to be contained, and it means that any of them may be contained.
- a component for adjusting the melting temperature can be contained in an amount of 0% by mass or more and 15% by mass or less. This makes it possible to make the melting temperature appropriate in the production described later. Each may be contained in an amount of more than 15% by mass, but the improvement in the effect is limited.
- Specific examples of the material (melting temperature adjusting material) for the component that adjusts the melting temperature include oxides of Na, K, Ca, Sr, Ba, Mg, Rb, Cs, Fr, Be, and Ra. it can. More preferably, it is as follows.
- K 2 O 10% by mass or less
- CaO 3% by mass or less
- SrO 10% by mass or less
- BaO 10% by mass or less
- MgO 3% by mass or less
- Rb 2 O 2.8% by mass %
- Cs 2 O 2.8% by mass or less
- Fr 2 O 2.8% by mass or less
- BeO 3% by mass or less
- RaO 10% by mass or less
- the components for forming crystal nuclei can be contained in the range of 0% by mass or more and 10% by mass or less in total. As a result, nuclei forming lithium disilicate crystals are efficiently generated. However, even if a larger amount of the compound is contained, the improvement of the effect is limited, so the content is set to 10% by mass or less.
- examples of the compound that functions as a material (crystal nucleation forming material) for a component for forming a crystal nuclei include oxides of Zr, P, and Ti (ZrO 2 , P 2 O 5 , and TIO 2 ). It can. At that time, at least one selected from ZrO 2 , P 2 O 5 , and TiO 2 is included, and the total is preferably 0% by mass or more and 10% by mass or less.
- the material for the block body may further include a known coloring material from the viewpoint of enhancing aesthetics. This may include, for example, at least one selected from V 2 O 5 , CeO 2 , Er 2 O 3 , MnO, Fe 2 O 3 and Tb 4 O 7 .
- the manufacturing method of this embodiment includes a melting step, a glass blank manufacturing step, a heat treatment step, and a processing step.
- each component described above is melted at 1100 ° C. or higher and 1600 ° C. or lower. This makes it possible to obtain molten glass for the block body for dental prostheses. This melting is preferably carried out over several hours in order to obtain sufficiently uniform properties.
- the glass blank manufacturing process is a process of obtaining a glass blank having a shape close to the shape of the block body for dental prosthesis.
- a glass blank is obtained by pouring the molten glass obtained in the melting step into a mold and cooling it to a temperature lower than the formation temperature of lithium metasilicate crystals, preferably 400 ° C. or lower, more preferably 100 ° C. or lower, and even more preferably room temperature.
- the cooling is performed by a slow temperature change to prevent deterioration and cracking of the material.
- the heat treatment step is a step of heating the glass blank obtained in the glass blank manufacturing step at a temperature in the range of the formation temperature of lithium disilicate crystals or more and lower than the melting point, more preferably 750 ° C. or higher and 900 ° C. or lower. Since rapid heating is preferable in this step, the glass cooled to less than the formation temperature of lithium metasilicate crystals, preferably 400 ° C. or lower, more preferably 100 ° C. or lower, still more preferably room temperature in the glass blank manufacturing step.
- the blank is heated by being placed in a heating device such as a furnace in order to expose the blank to an atmosphere in the range of 750 ° C. or higher and 900 ° C.
- the heating time is until the main crystal phase in the glass blank becomes lithium disilicate. Therefore, it is not limited, but can be 20 minutes or more.
- the upper limit of the time is not particularly limited, but can be 6 hours or less. If the temperature is less than the formation temperature of lithium disilicate crystals, there is a risk that a lithium disilicate blank having a main crystal phase of lithium disilicate cannot be obtained. On the other hand, if the temperature is higher than the melting point of the lithium disilicate crystal, there is a risk of softening.
- the heating is stopped and cooled to room temperature to obtain a block body for a dental prosthesis whose main crystal phase is lithium disilicate.
- This cooling is preferably performed in the furnace by slow cooling due to a slow temperature change in the natural cooling in the furnace.
- the "main crystal phase” means the crystal phase having the largest crystal precipitation ratio among the crystal phases observed by the analysis by the X-ray diffractometer.
- the processing process is a process of machining the obtained block body for a dental prosthesis into the shape of a dental prosthesis.
- the machining method is not particularly limited, and examples thereof include cutting and grinding. This makes it possible to obtain a dental prosthesis.
- This processing can be performed under high productivity conditions. That is, until now, a block body for a dental prosthesis in which lithium disilicate is the main crystal phase has poor machinability, and therefore it has been difficult to perform efficient cutting. Therefore, an easy-to-process block body (for example, a block body containing lithium metasilicate as the main crystal phase) that does not have a crystal phase mainly composed of lithium disilicate is prepared, machined, and further heat-treated after processing. By changing the main crystal phase to lithium disilicate, it was necessary to go through a process of increasing the strength later.
- the main crystal phase is lithium disilicate
- the main crystal phase is lithium metasilicate, which is equal to or higher than the processing of the easy-to-process block body.
- Cutting and grinding are possible under the conditions of. Since no heat treatment is required after processing, the dental prosthesis can be made without changing the shape and maintaining the accuracy of machining.
- FIG. 2 shows an enlarged view of a part of the cut surface of the block body 10 cut along the dotted line indicated by reference numeral A1 in FIG.
- This figure is an enlarged view in a field of view of 5 ⁇ m in the vertical direction (width direction) and 5 ⁇ m in the horizontal direction (depth direction).
- SEM scanning electron microscope
- the main crystal phase of the block body 10 is lithium disilicate.
- the total area of the crystals having a length of 0.5 ⁇ m or more among the appearing crystals is the visual field area (5 ⁇ m ⁇ 5 ⁇ m) shown in FIG.
- the ratio is preferably 21% or less, but it is not always necessary to be 21% or less, and it is sufficient that this ratio can be reduced by the production method of this embodiment as compared with the conventional production method.
- the ratio is preferably 10% or less, and more preferably 1% or less.
- the crystals having a length of 0.5 ⁇ m or more to be extracted may be limited to those made of lithium disilicate.
- the block body whose main crystal phase is lithium disilicate is processed, it is equal to or higher than the processing of the conventional block body which is easy to process (for example, the block body whose main crystal phase is lithium metasilicate). Cutting and grinding are possible under the conditions. According to this, for example, since the post-processing heat treatment required for a block body in which the main crystal phase is lithium metasilicate is not required, the dental prosthesis does not change its shape and maintains the accuracy of machining. It can be a thing.
- Such a ratio is obtained as follows. Taking the block body 10 shown in FIG. 1 as an example, in the largest direction (height direction in the example of FIG. 1), the center A 1 and the two end portions A 2 at positions 10% from the end face with respect to the total height H. and obtain three cut surfaces at the ends a 3.
- the Figure 3 shows a cut surface in the central A 1 of the three cutting surfaces. Then, on the cut surfaces at the center A 1 , the end A 2 , and the end A 3 , the center B 1 shown by the dotted line is adjacent to the B 1 in the width W direction, and 10% from the end with respect to the total width W.
- FIG. 1 A scanning electron microscope image is obtained with a field of view of 5 ⁇ m ⁇ 5 ⁇ m as in 2. Therefore, a total of 15 images are obtained, 5 per cut surface. An example of the obtained image is shown at the top of FIG.
- the block body for the dental prosthesis it is preferable that no void is observed in the block body for the dental prosthesis.
- the area occupied by the voids is 2% on average in the observation range of 60 ⁇ m in the vertical direction (width direction) ⁇ 60 ⁇ m in the horizontal direction (depth direction) at the 15 locations where the above ratio is measured.
- the granules of the coloring material are not visually recognized in the micrograph at a magnification of 200 times at the 15 places where the above ratio is measured. These voids and granules form an interface with the base material and may affect machinability.
- the presence of particles of the coloring material may cause color unevenness of the dental prosthesis.
- Such a block body for a dental prosthesis can be surely realized by molding by melting the material as described above instead of powder molding.
- the basic functions of dental prostheses are strength, hardness, chemical durability against the oral environment, and the same as natural teeth. It can have aesthetics (hue, texture).
- the machinability is also improved, and despite having the strength that heat treatment after processing is not required, processing of the same level or more as the conventional block body for dental prostheses with ceramic for cutting It is possible to machine without causing any trouble under the conditions.
- a block body is prepared by the manufacturing method by the melt molding method described above by changing the contained components and the heat treatment temperature, and the dental prosthesis is processed by cutting. Was produced and the machinability at that time was evaluated.
- the block body is a rectangular parallelepiped having a width W of 14 mm, a depth D of 12 mm, and a height H of 18 mm.
- the block bodies according to Examples 1 to 7, Comparative Example 5, and Comparative Example 6 were produced by heat treatment with rapid heating as follows.
- the materials shown in Table 1 were mixed according to the ratio and melted at 1300 ° C. for 3 hours to obtain molten glass (melting step).
- the obtained molten glass was poured into a mold and cooled to room temperature to obtain a glass blank (glass blank manufacturing step).
- the glass blank was put into a furnace preheated to the heat treatment temperature shown in Table 1 and maintained at the same heat treatment temperature for 30 minutes (heat treatment by rapid heating). Then, it was slowly cooled to room temperature (cooling step) to obtain a block body.
- the block bodies according to Comparative Examples 1 to 4 were produced by performing conventional heat treatment as follows.
- the materials shown in Table 1 were mixed according to the ratio and melted at 1300 ° C. for 3 hours to obtain molten glass (melting step).
- the obtained molten glass was poured into a mold and cooled to room temperature to obtain a glass blank (glass blank manufacturing step).
- the obtained glass blank was heated and maintained at 650 ° C. for 60 minutes, then heated to 850 ° C. and maintained for 10 minutes (conventional heat treatment by heating). Then, it was slowly cooled to room temperature (cooling step) to obtain a block body.
- Table 1 shows the content of each component in mass%. As can be seen from Table 1, Examples 1 and Comparative Example 1, Example 2 and Comparative Example 2, Example 3 and Comparative Example 3, and Example 4 and Comparative Example 4 have the same components other than the coloring material. .. Table 1 shows the types of heat treatment, the heat treatment temperature in the example of rapid heating, the types of the main crystal phase of the obtained block (“LDS” is lithium disilicate, “LS” is lithium metasilicate), and the above. The ratio (%) occupied by crystals having a length of 0.5 ⁇ m or more obtained by the described method and the machinability were shown. The blanks in the component items in Table 1 represent 0% by mass.
- the main crystal is measured using an X-ray diffractometer (Empylean (registered trademark); manufactured by Spectris Co., Ltd.), and as a result of quantitative analysis by the Rietveld method, the crystal phase having the highest crystal precipitation ratio among the observed crystal phases. And said.
- the “ratio” is the ratio of crystals having a length of 0.5 ⁇ m or more as described above, and is the area ratio (%) obtained by the above method.
- Reference 1 As for "machinability", two types of conventional block bodies for machining were prepared as Reference 1 and Reference 2.
- Each is a block body as follows.
- (Reference 1) A block body containing lithium metasilicate as the main crystal phase, in which SiO 2 is 72.3% by mass, Li 2 O is 15.0% by mass, and Al 2 O 3 is 1.6% by mass.
- (Reference 2) a crystalline phase and a block body which is contained in about the same proportions crystalline phase and the lithium disilicate lithium metasilicate, SiO 2 is 56.3 wt%, Li 2 O 14.7 wt% , Al 2 O 3 is contained in a proportion of 2.1% by mass.
- the machinability is good even though the main crystal phase is lithium disilicate.
- the "ratio" was kept lower than the ratio in the comparative example.
- Example 1 and Comparative Example 1, Example 2 and Comparative Example 2, Example 3 and Comparative Example 3, and Example 4 and Comparative Example 4 have the same components other than the coloring material, the manufacturing process is the same. It can also be seen that the machinability and the ratio differ greatly depending on the difference. In Comparative Example 5, lithium disilicate could not be obtained as the main crystal phase, and in Comparative Example 6, softening occurred.
- both the block bodies of Examples and Comparative Examples had the required strength. Further, the voids and granules also satisfy the above-mentioned preferable conditions.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Ceramic Engineering (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Geochemistry & Mineralogy (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Dentistry (AREA)
- Manufacturing & Machinery (AREA)
- Structural Engineering (AREA)
- Crystallography & Structural Chemistry (AREA)
- Plastic & Reconstructive Surgery (AREA)
- Molecular Biology (AREA)
- Dispersion Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Dental Preparations (AREA)
- Glass Compositions (AREA)
- Dental Prosthetics (AREA)
- Re-Forming, After-Treatment, Cutting And Transporting Of Glass Products (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201980094697.4A CN113677310B (zh) | 2019-03-29 | 2019-12-20 | 牙科修复体用块体的制造方法、牙科修复体的制造方法 |
| CN202311708323.1A CN117843241A (zh) | 2019-03-29 | 2019-12-20 | 牙科修复体用块体的制造方法、牙科修复体的制造方法 |
| JP2020503333A JP7478093B2 (ja) | 2019-03-29 | 2019-12-20 | 歯科補綴物用ブロック体の製造方法、歯科補綴物の製造方法 |
| EP19922438.7A EP3949938A4 (en) | 2019-03-29 | 2019-12-20 | METHOD FOR MANUFACTURING DENTAL BLOCK BODY, AND METHOD FOR MANUFACTURING DENTAL PROSTHESIS |
| US17/442,756 US20220183803A1 (en) | 2019-03-29 | 2019-12-20 | Method of producing block for dental prostheses, and method of producing dental prosthesis |
| JP2023010814A JP7665663B2 (ja) | 2019-03-29 | 2023-01-27 | 歯科補綴物用ブロック体の製造方法、歯科補綴物の製造方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019069330 | 2019-03-29 | ||
| JP2019-069330 | 2019-03-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020202666A1 true WO2020202666A1 (ja) | 2020-10-08 |
Family
ID=72668465
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/050162 Ceased WO2020202666A1 (ja) | 2019-03-29 | 2019-12-20 | 歯科補綴物用ブロック体の製造方法、歯科補綴物の製造方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20220183803A1 (enExample) |
| EP (1) | EP3949938A4 (enExample) |
| JP (2) | JP7478093B2 (enExample) |
| CN (2) | CN117843241A (enExample) |
| WO (1) | WO2020202666A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023535253A (ja) * | 2021-06-25 | 2023-08-17 | ハス カンパニー リミテッド | 歯科補綴物製造のためのバルクブロック |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2252660A1 (en) | 1997-11-10 | 1999-05-10 | Marcel Schweiger | A process for the preparation of shaped translucent lithium disilicate glass ceramic products |
| JP2013515659A (ja) * | 2009-12-23 | 2013-05-09 | フラオンホファー−ゲゼルシャフト・ツア・フェルデルング・デア・アンゲヴァンテン・フォルシュング・エー・ファオ | 二ケイ酸リチウムガラスセラミック、その製造方法およびその使用 |
| JP2014520061A (ja) * | 2011-06-22 | 2014-08-21 | ヴィタ ツァーンファブリク ハー.ラウター ゲーエムベーハー ウント コー.カーゲー | 歯科用修復材、その製造方法およびガラスセラミック |
| WO2016031399A1 (ja) * | 2014-08-27 | 2016-03-03 | 株式会社ジーシー | 歯科補綴物の製造方法、歯科補綴物用二ケイ酸リチウムブランクの製造方法、及び歯科補綴物用二ケイ酸リチウムブランク |
| US20160236971A1 (en) * | 2013-11-05 | 2016-08-18 | Ivoclar Vivadent Ag | Lithium disilicate-apatite glass ceramic with transition metal oxide |
| JP2017531607A (ja) * | 2015-08-26 | 2017-10-26 | ハス カンパニー リミテッドHass Co.,Ltd | 上部構造物が連結された歯科用結晶化ガラスブロック及びその製造方法 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6420288B2 (en) * | 1997-11-10 | 2002-07-16 | Ivoclar Ag | Process for the preparation of shaped translucent lithium disilicate glass ceramic products |
| PL2765974T3 (pl) * | 2011-10-14 | 2016-08-31 | Ivoclar Vivadent Ag | Litowokrzemianowa ceramika szklana i szkło zawierające tlenek metalu dwuwartościowego |
| WO2016190012A1 (ja) * | 2015-05-25 | 2016-12-01 | 株式会社ジーシー | 歯科補綴物用材料、歯科補綴物作製用ブロック体、及び歯科補綴物 |
| DE202015009943U1 (de) * | 2015-08-25 | 2021-10-12 | Ivoclar Vivadent Ag | Lithiumsilikat-Tiefquarz-Glaskeramik |
| JP6993093B2 (ja) | 2016-03-31 | 2022-01-13 | 株式会社松風 | Al2O3未含有のケイ酸リチウムガラス組成物 |
| DE102016119935A1 (de) | 2016-10-19 | 2018-04-19 | Degudent Gmbh | Verfahren zur Herstellung einer dentalen Restauration |
| KR101975548B1 (ko) | 2017-03-07 | 2019-05-08 | 주식회사 하스 | 열처리 온도의 변화로 가공성 또는 투광성 조절이 가능한 결정화 유리 제조 방법 |
-
2019
- 2019-12-20 CN CN202311708323.1A patent/CN117843241A/zh active Pending
- 2019-12-20 EP EP19922438.7A patent/EP3949938A4/en active Pending
- 2019-12-20 JP JP2020503333A patent/JP7478093B2/ja active Active
- 2019-12-20 CN CN201980094697.4A patent/CN113677310B/zh active Active
- 2019-12-20 US US17/442,756 patent/US20220183803A1/en active Pending
- 2019-12-20 WO PCT/JP2019/050162 patent/WO2020202666A1/ja not_active Ceased
-
2023
- 2023-01-27 JP JP2023010814A patent/JP7665663B2/ja active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2252660A1 (en) | 1997-11-10 | 1999-05-10 | Marcel Schweiger | A process for the preparation of shaped translucent lithium disilicate glass ceramic products |
| JP2013515659A (ja) * | 2009-12-23 | 2013-05-09 | フラオンホファー−ゲゼルシャフト・ツア・フェルデルング・デア・アンゲヴァンテン・フォルシュング・エー・ファオ | 二ケイ酸リチウムガラスセラミック、その製造方法およびその使用 |
| JP2014520061A (ja) * | 2011-06-22 | 2014-08-21 | ヴィタ ツァーンファブリク ハー.ラウター ゲーエムベーハー ウント コー.カーゲー | 歯科用修復材、その製造方法およびガラスセラミック |
| US20160236971A1 (en) * | 2013-11-05 | 2016-08-18 | Ivoclar Vivadent Ag | Lithium disilicate-apatite glass ceramic with transition metal oxide |
| WO2016031399A1 (ja) * | 2014-08-27 | 2016-03-03 | 株式会社ジーシー | 歯科補綴物の製造方法、歯科補綴物用二ケイ酸リチウムブランクの製造方法、及び歯科補綴物用二ケイ酸リチウムブランク |
| JP2017531607A (ja) * | 2015-08-26 | 2017-10-26 | ハス カンパニー リミテッドHass Co.,Ltd | 上部構造物が連結された歯科用結晶化ガラスブロック及びその製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3949938A4 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023535253A (ja) * | 2021-06-25 | 2023-08-17 | ハス カンパニー リミテッド | 歯科補綴物製造のためのバルクブロック |
| JP7517739B2 (ja) | 2021-06-25 | 2024-07-17 | ハス コーポレーション | 歯科補綴物製造のためのバルクブロック |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3949938A4 (en) | 2023-01-25 |
| JP7478093B2 (ja) | 2024-05-02 |
| CN113677310A (zh) | 2021-11-19 |
| JP2023058538A (ja) | 2023-04-25 |
| EP3949938A1 (en) | 2022-02-09 |
| CN117843241A (zh) | 2024-04-09 |
| CN113677310B (zh) | 2024-01-02 |
| US20220183803A1 (en) | 2022-06-16 |
| JP7665663B2 (ja) | 2025-04-21 |
| JPWO2020202666A1 (ja) | 2021-04-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6347792B2 (ja) | 歯科補綴物の製造方法、歯科補綴物用二ケイ酸リチウムブランクの製造方法、及び歯科補綴物用二ケイ酸リチウムブランク | |
| US20190177210A1 (en) | Zirconia-toughened glass ceramics | |
| CN106277798B (zh) | 一种偏硅酸锂玻璃陶瓷及其制备方法 | |
| JP6732868B2 (ja) | 歯科補綴物用材料、歯科補綴物作製用ブロック体、及び歯科補綴物 | |
| JP7665663B2 (ja) | 歯科補綴物用ブロック体の製造方法、歯科補綴物の製造方法 | |
| JP6903815B2 (ja) | 歯科補綴物用ブロック体 | |
| CN117279592A (zh) | 用于切削加工的牙科用大型块体及其制造方法 | |
| KR102818759B1 (ko) | 보철물 제작용 대면적 블랭크 및 그 제조방법 | |
| US11166795B2 (en) | Method for producing dental prosthesis, method for producing lithium disilicate blank for dental prosthesis and lithium disilicate blank for dental prosthesis | |
| JP2016108178A (ja) | ガラスブロック |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| ENP | Entry into the national phase |
Ref document number: 2020503333 Country of ref document: JP Kind code of ref document: A |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19922438 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2019922438 Country of ref document: EP Effective date: 20211029 |