WO2019150125A1 - Co-crystals comprising 2,4-d and a triazine herbicide - Google Patents
Co-crystals comprising 2,4-d and a triazine herbicide Download PDFInfo
- Publication number
- WO2019150125A1 WO2019150125A1 PCT/GB2019/050277 GB2019050277W WO2019150125A1 WO 2019150125 A1 WO2019150125 A1 WO 2019150125A1 GB 2019050277 W GB2019050277 W GB 2019050277W WO 2019150125 A1 WO2019150125 A1 WO 2019150125A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- crystals
- crystals according
- atrazine
- triazine herbicide
- triazine
- Prior art date
Links
- 0 *c1nc(N*)nc(N*)n1 Chemical compound *c1nc(N*)nc(N*)n1 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N39/00—Biocides, pest repellants or attractants, or plant growth regulators containing aryloxy- or arylthio-aliphatic or cycloaliphatic compounds, containing the group or, e.g. phenoxyethylamine, phenylthio-acetonitrile, phenoxyacetone
- A01N39/02—Aryloxy-carboxylic acids; Derivatives thereof
- A01N39/04—Aryloxy-acetic acids; Derivatives thereof
Definitions
- This invention relates to co-crystals of herbicide compounds.
- this invention relates to co-crystals comprising 2,4-dichlorophenoxyacetic acid and a triazine herbicide, and to agrochemical compositions comprising such co-crystals.
- 2.4-dichlorophenoxyacetic acid is a widely used systemic herbicide which is a member of the phenoxy family of herbicides.
- 2,4-D acts as a synthetic auxin which is a plant hormone which is absorbed by the leaves of plants. 2,4-D is used to control broadleaf weeds, in particular for agricultural applications.
- W02012/009395A1 (Dow Agrosciences LLC) describes a mixture for use as a herbicide containing the combination of the herbicides 2,4-D, aminopyralid, and atrazine.
- Kamath et al Journal of Dispersion Science and Technology, 29:1304-1310, 2008 describes the structural characterisation of composite particles of atrazine and 2,4- dichlorophenoxy acetic acid.
- the composite particles are not co-crystals of atrazine and
- triazine herbicides may be used to form co-crystals with 2,4-dichlorophenoxyacetic acid (2,4-D).
- Such co-crystals have been found to have high stability, a high melting point, and provide 2,4-D in a form which has reduced volatility.
- co-crystals of 2,4- dichlorophenoxyacetic acid (2,4-D) and a compound selected from the group of triazine herbicides are provided.
- Triazine herbicides of particular utility may be represented by formula (I):
- the triazine herbicide is selected from atrazine, simazine, cyanazine, propazine, terbutryn, prometryn or ametryn. More preferably, the triazine herbicide is cyanazine, simazine or atrazine. Typically, the molar ratio of 2,4-D: triazine herbicide is from 2:1 to 1 :1.
- the co-crystals may be formed by solution or slurry crystallisation processes. Therefore, in a second aspect of the invention there is provided a method for the preparation of co crystals as described herein which comprises combining 2,4-D and a triazine herbicide in a suitable solvent.
- the co-crystals may also be formed in a solvent-free method. Therefore, in a third aspect of the invention there is provided a solvent-free method for the preparation of co crystals as described herein which comprises the step of applying dual asymmetric centrifugal forces to a mixture of 2,4-D and a triazine herbicide, and optionally in the presence of milling or grinding media.
- a herbicidal composition comprising co-crystals as described herein and at least one agriculturally acceptable carrier.
- compositions may be an aqueous suspension or in the form of granules.
- a method for controlling undesired vegetation comprising contacting the vegetation with a composition comprising co crystals as described herein and at least one agriculturally acceptable carrier.
- Figure 1 shows the x-ray powder diffraction pattern of the co-crystals of Example 1.
- FIG. 1 shows the differential scanning calorimetry (DSC) curve of the co-crystals of Example 1.
- Figure 3 shows the x-ray powder diffraction pattern of the co-crystals of Example 2.
- FIG. 4 shows the differential scanning calorimetry (DSC) curve of the co-crystals of Example 2.
- Figure 5 shows the x-ray powder diffraction pattern of the co-crystals of Example 3.
- Figure 6 shows the differential scanning calorimetry (DSC) curve of the co-crystals of Example 3.
- Figure 7 shows the x-ray powder diffraction pattern of the co-crystals of Example 4.
- Figure 8 shows the differential scanning calorimetry (DSC) curve of the co-crystals of Example 4.
- Figure 9 shows a view of an atrazine: 2,4-D co-crystal from the crystal structure of Example 2.
- Figures 10A-C illustrate how centrifugal forces are applied to materials in a SpeedMixerTM.
- Figure 10A is a view from above showing the base plate and basket.
- the base plate rotates in a clockwise direction.
- Figure 10B is a side view of the base plate and basket.
- Figure 10C is a view from above along line A in Figure 10B.
- the basket rotates in an anti clockwise direction.
- the present invention relates to co-crystals comprising 2,4-dichlorophenoxyacetic acid (2,4-D) and a compound selected from the group of triazine herbicides.
- Co-crystals are multi-component crystalline materials in which at least two organic compounds form the crystalline material which has a defined single phase crystal structure. The at least two organic compounds interact by non-covalent bonding, such as hydrogen bonding, van der Waals interactions, P-P interactions, etc., and are not simple salts.
- the co-crystals may be distinguished from mixtures of 2,4-D and the selected triazine herbicide by standard analytical means which are well known to those skilled in the art, for example x-ray powder diffraction (XRPD), single crystal x-ray diffraction, or differential scanning calorimetry (DSC).
- XRPD x-ray powder diffraction
- DSC differential scanning calorimetry
- the molar ratio of the components of the co-crystals may be determined using, for example, HPLC or 1 H NMR.
- the co-crystals comprise 2,4-dichlorophenoxyacetic acid (2,4-D) and a triazine herbicide.
- T riazine herbicides are well known to those skilled in the art and comprise a 1 ,3,5- triazine core with amine substituents at the 2- and 4- positions.
- Triazine herbicides of particular utility may be represented by formula (I):
- X Cl, -OCH 3 , or -SCH 3 ;
- R1 ethyl or isopropyl;
- R 2 ethyl, iso-propyl, cyclopropyl, tert-butyl, or -C(CH3)2CN.
- the triazine herbicide is selected from atrazine (6-chloro-4-N-ethyl-2-N- (propan-2-yl)-1 , 3, 5-triazine-2, 4-diamine), simazine (6-chloro-2-N,4-N-diethyl-1 ,3,5- triazine-2, 4-diamine), cyanazine (2-[[4-chloro-6-(ethylamino)-1 ,3,5-triazin-2-yl]amino]-2- methylpropanenitrile), propazine (6-chloro-2-N,4-N-di(propan-2-yl)-1 ,3,5-triazine-2,4- diamine), terbutryn (2-N-tert-butyl-4-N-ethyl-6-methylsulfanyl-1 , 3, 5-triazine-2, 4-diamine), prometryn (6-methylsulfanyl-2-N,4-N
- the triazine herbicide is simazine, cyanazine, or atrazine.
- the present invention relates to co-crystals comprising, or consisting of, 2-4-D and atrazine (6-chloro-4-N-ethyl-2-N-(propan-2-yl)-1 , 3, 5-triazine-2, 4-diamine).
- co-crystals show significantly reduced volatility of 2,4-D.
- the molar ratio of 2,4-D and atrazine is generally in the range from 1 :1 to 2:1 , such as 1 :1 or 2:1. However, variations are possible which typically do not exceed 20 mol% and preferably do not exceed 10 mol%.
- the current inventors have identified a crystalline form which exhibits an x-ray powder diffraction pattern (Cu Ka radiation) substantially as shown in Figure 1.
- the XRPD 2-theta (2Q) values are shown in Table 1.
- the co-crystal shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 1 as 2-Theta (2Q) values (+/- 0.2 degrees 2Q).
- the crystalline form may also be identified by its differential scanning calorimetry (DSC) curve as shown in Figure 2, in particular by the melting point temperature of about 129.8 °C as determined by DSC.
- DSC differential scanning calorimetry
- the current inventors have identified a crystalline form which exhibits an x-ray powder diffraction pattern (Cu Ka radiation) substantially as shown in Figure 3.
- the XRPD 2-theta (2Q) values are shown in Table 2.
- the co-crystal shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 2 as 2-Theta (2Q) values (+/- 0.2 degrees 2Q).
- the crystalline form may also be identified by its differential scanning calorimetry (DSC) curve as shown in Figure 4, in particular by the melting point temperature of about 138.2 °C as determined by DSC.
- DSC differential scanning calorimetry
- the present invention relates to co-crystals comprising, or consisting of, 2-4-D and cyanazine ((2-[[4-chloro-6-(ethylamino)-1 ,3,5-triazin-2-yl]amino]- 2-methylpropanenitrile).
- the molar ratio of 2,4-D and cyanazine is generally in the range from 1 :1 to 2:1 , such as 1 :1. However, variations are possible which typically do not exceed 20 mol% and preferably do not exceed 10 mol%.
- the current inventors have identified a crystalline form which exhibits an x-ray powder diffraction pattern (Cu Ka radiation) substantially as shown in Figure 5.
- the XRPD 2-theta (2Q) values are shown in Table 5.
- the co-crystal shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 5 as 2-Theta (2Q) values (+/- 0.2 degrees 2Q).
- the crystalline form may also be identified by its differential scanning calorimetry (DSC) curve as shown in Figure 6, in particular by the melting point temperature of about 139.0 °C as determined by DSC.
- DSC differential scanning calorimetry
- the present invention relates to co-crystals comprising, or consisting essentially of, 2-4-D and simazine ((6-chloro-2-N,4-N-diethyl-1 ,3,5-triazine- 2, 4-diamine).
- the molar ratio of 2,4-D and simazine is generally in the range from 1 :1 to 2:1 , such as 2:1. However, variations are possible which typically do not exceed 20 mol% and preferably do not exceed 10 mol%.
- the current inventors have identified a crystalline form which exhibits an x-ray powder diffraction pattern (Cu Ka radiation) substantially as shown in Figure 7.
- the XRPD 2-theta (2Q) values are shown in Table 6.
- the co-crystal shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 6 as 2-Theta (2Q) values (+/- 0.2 degrees 2Q).
- the crystalline form may also be identified by its differential scanning calorimetry (DSC) curve as shown in Figure 8, in particular by the melting point temperature of about 146.3 °C as determined by DSC.
- the co-crystals may be formulated into a herbicidal composition with at least one agriculturally acceptable carrier.
- the compositions may be solids, for example powders, granules, or water-dispersable powders, or may be liquids, such as suspensions of co crystal particles.
- Suitable agriculturally acceptable carriers are well known to those skilled in the art. Such carriers should not be phytotoxic to crops, in particular at the concentrations employed for the control of undesirable plants in the presence of crops, and should not react chemically with the compounds of the co-crystals or other composition components.
- the compositions may be applied directly, or may be formulations or concentrates which are diluted, for example with water, prior to application.
- Liquid carriers that may be employed include water and organic solvents, although it is typically preferred that water is used.
- Solid carriers include mineral earths, such as clays, silicates, diatomaceous earths, or kaolin, fertilisers, and organic products such as woodmeal and cellulose carriers.
- compositions may also include further components, such as surfactants, viscosity modifiers, anti-freeze agents, agents for pH control, stabilisers and anti-caking agents.
- surfactants such as surfactants, viscosity modifiers, anti-freeze agents, agents for pH control, stabilisers and anti-caking agents.
- the concentration of active ingredients in the composition is generally between 1 and 99 wt%, such as between 5 and 95 wt% or 10 and 90 wt%. In compositions which are designed to be diluted prior to use the concentration of active ingredients is typically between about 10 and 90 wt%. Such compositions are then diluted, typically with water, to compositions which typically contain 0.001 and 1 wt% of active material.
- compositions as described herein may be used for controlling undesirable vegetation.
- Undesirable vegetation is understood to mean plants considered undesirable in a particular location, e.g. in an area of crops, and may be known as weeds.
- Control may be achieved by a method comprising contacting the vegetation with the herbicidal composition. It will be understood by the skilled person that the composition at the point of application should contain a herbicidally effective amount of the co-crystals.
- a herbicidally effective amount is an amount of the active ingredients which causes an adverse deviation of the natural development of the undesired vegetation.
- compositions have utility for controlling undesirable vegetation in a culture of crop plants, especially crop plants which are tolerant to 2,4-D and / or triazine herbicides, for example through genetic modification of the crop plants.
- compositions may also have particular utility for the control of undesirable vegetation which is resistant to either 2,4-D or the selected triazine herbicide.
- the present invention also comprises a method for the preparation of co-crystals according to the present invention, the method comprising combining 2,4-D and the selected triazine herbicide in a suitable solvent.
- 2,4-D and the triazine herbicide are dissolved in at least one solvent to form a solution, optionally with heating, and then co-crystallisation is induced, for example, by cooling the solution, evaporation of the solvent, or by precipitation. Precipitation may be induced, for example, by the addition of an anti solvent.
- co-crystals comprise 2,4-D and atrazine
- crystallisation may be achieved by the formation of a solution of 2,4-D and atrazine in a suitable solvent, such as chloroform or methyl ethyl ketone, at an elevated temperature, such as between 40 and 60 °C, and then cooling the solution to induce precipitation of the co-crystals.
- a suitable solvent such as chloroform or methyl ethyl ketone
- co crystallisation may be achieved by the formation a solution of 2,4-D and cyanazine in a suitable solvent, such as acetonitrile, at an elevated temperature, such as between 40 and 60 °C, and then cooling the solution to induce precipitation of the co-crystals.
- 2,4-D and the triazine herbicide are combined with a suitable solvent but the compounds are not dissolved so that solid material remains.
- suitable solvents include but are not limited to tetrahydrofuran and water (e.g. deionised water).
- the mixture is then subjected to milling or grinding.
- the amount of time required for co-crystal formation can be readily determined by one skilled in the art and depends on factors including the temperature and the level of energy input.
- co crystallisation may be achieved by the combination of 2,4-D and simazine with a suitable solvent, such as tetrahydrofuran, in an amount such that the compounds are not dissolved. The mixture is then ground until co-crystals are obtained.
- a suitable solvent such as tetrahydrofuran
- co-crystals comprise 2,4-D and a triazine herbicide selected from the group consisting of atrazine, simazine and cyanazine
- co-crystallisation may be achieved by the combination of 2,4-D and the triazine herbicide with water in an amount such that the compounds are not dissolved.
- the mixture is then ground (e.g. using low energy ball milling or low energy grinding) until the co-crystals are obtained.
- co-crystals may be formed in a method comprising the step of applying dual asymmetric centrifugal forces to a mixture of 2,4-D and a triazine herbicide as described herein, and optionally in the presence of milling or grinding media to form the co-crystals.
- the method is carried out in a substantially dry environment i.e. in the absence of a solvent.
- the co-crystals are formed using dual asymmetric centrifugal forces.
- dual asymmetric centrifugal forces we mean that two centrifugal forces, at an angle to each other, are simultaneously applied to the particles.
- the centrifugal forces preferably rotate in opposite directions.
- SpeedmixerTM by Hauschild (http://www.speedmixer.co.uk/index.php) utilises this dual rotation method whereby the motor of the SpeedmixerTM rotates the base plate of the mixing unit in a clockwise direction (see Figure 10A) and the basket is spun in an anti clockwise direction (see Figures 10B and 10C).
- the method may be controlled by various parameters including the rotation speed at which the method takes place, the length of processing time, the level to which the mixing container is filled, the use of milling media and/or the control of the temperature of the components within the milling pot.
- the dual asymmetric centrifugal forces may be applied for a continuous period of time.
- the period of time may be from about 1 second to about 30 minutes, such as about 5 seconds to about 27 minutes, for example, about 10 seconds to about 25 minutes. In one embodiment, the period of time may be about 20 minutes.
- the dual asymmetric centrifugal forces may be applied for an aggregate period of time.
- aggregate we mean the sum or total of more than one periods of time (e.g. 2, 3, 4, 5, 6, 7, 8, 9, 10 or more times).
- the advantage of applying the centrifugal forces in a stepwise manner is that excessive heating of the particles can be avoided.
- the dual asymmetric centrifugal forces may be applied for an aggregate period of about 1 second to about 30 minutes, for example about 5 seconds to about 27 minutes, such as about 10 seconds to about 25 minutes.
- the period of time may be 10 periods of time of two minutes each i.e. an aggregate period of time of about 20 minutes.
- the dual asymmetric centrifugal forces are applied in a stepwise manner with periods of cooling therebetween.
- the dual asymmetric centrifugal forces may be applied in a stepwise manner at one or more different speeds.
- the speed of the dual asymmetric centrifugal forces may be from about 200 rpm to about 4000 rpm. In one embodiment, the speed may be from about 300 rpm to about 3000 rpm, for example about 500 rpm to about 2500 rpm. In one embodiment, the speed may be about 1900 rpm.
- the level to which the mixing container is filled is determined by various factors which will be apparent to the skilled person. These factors include the apparent density of the 2,4-D and triazine herbicide, the volume of the mixing container and the weight restrictions imposed on the mixer itself.
- Milling or grinding media may be used to assist the reaction.
- the incorporation of hard, non-contaminating media can additionally assist in the breakdown of particles where agglomeration has occurred, for example, as a result of the
- milling/grinding media is well-known within the field of powder processing and materials such as stabilised zirconia and other ceramics are suitable provided they are sufficiently hard or ball bearings, such as stainless steel beads.
- Atrazine (98 mg) (Tokyo Chemical Industry) and 2,4-D (182 mg, 0.5 mol eq.) (Tokyo Chemical Industry) were dissolved in chloroform (7 ml) at 50°C with stirring. The clear solution was cooled to 5°C at 0.2°C min 1 to give a slurry. This slurry was filtered, then dried under suction to yield a crystalline product (69% yield).
- the co-crystals were also analysed by 1 H NMR which confirmed a molar ratio of 2,4-D: atrazine of 2:1.
- the co-crystals were also analysed by XRPD which showed a crystalline material and yielded a diffractogram as provided in Figure 1.
- the co-crystal of 2,4-D and atrazine shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 1 as 2-Theta (2Q) values.
- Example 2 - 2,4-D Atrazine (1 :1) co-crystal Atrazine (200 mg) (Tokyo Chemical Industry) and of 2,4-D (190 mg, 1 mol eq.) were dissolved in methyl ethyl ketone (3 ml) at 50°C with stirring. The clear solution was cooled to 5 °C at 0.2°C min 1 to give a slurry. This slurry was filtered, then dried under suction to yield a crystalline product. The co-crystals were also analysed by 1 H NMR which confirmed a molar ratio of 2,4-D: atrazine of 1 :1.
- the co-crystals were also analysed by XRPD which showed a crystalline material and yielded a diffractogram as provided in Figure 3.
- the co-crystal of 2,4-D and atrazine shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 2 as 2-Theta (2Q) values.
- the sample was further analysed by single crystal x-ray crystallography to determine the crystal structure as follows:
- Weighting scheme w 1 / [a 2 (F O 2 )+( 0.0641 P) 2 +0.2677P]
- the asymmetric unit contains a single, fully ordered molecule of Atrazine and 2,4- dichlorophenoxyacetic acid co-crystal, (designated Form A).
- Figure 9 shows a view of a molecule of Atrazine/2,4-D cocrystal, Form A from the crystal structure. Anisotropic atomic displacement ellipsoids for the non-hydrogen atoms are shown at the 50% probability level. Hydrogen atoms are displayed with an arbitrarily small radius.
- the co-crystals were analysed by 1 H NMR which confirmed a molar ratio of 2,4-D: cyanazine of 1 :1.
- co-crystals were also analysed by XRPD which showed a crystalline material and yielded a diffractogram as provided in Figure 5.
- This co-crystal of 2,4-D and cyanazine shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 5 as 2-Theta (2Q) values.
- co-crystals were also analysed by XRPD which showed a crystalline material and yielded a diffractogram as provided in Figure 7.
- This co-crystal of 2,4-D and simazine shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 6 as 2-Theta (2Q) values.
- Atrazine (Tokyo chemical industry) (20 mg), 2,4-D (20.5 mg; 1 mol eq) (Tokyo chemical industry) was placed in a 2 ml HPLC vial with two grinding beads. The mixture was initially wetted with 20 pi of deionised water, and ground for 2 h at 500 rpm using a
- Brucker D8 uses Cu Ka radiation (40 kV, 40 mA) and a Q-2Q goniometer fitted with a Ge monochromator.
- the incident beam passes through a 2.0 mm divergence slit followed by a 0.2 mm anti-scatter slit and knife edge.
- the diffracted beam passes through an 8.0 mm receiving slit with 2.5° Soller slits followed by the Lynxeye Detector.
- the software used for data collection and analysis was Diffrac Plus XRD Commander and Diffrac Plus EVA respectively. Samples were run under ambient conditions as flat plate specimens using powder as received. The sample was prepared on a polished, zero-background (510) silicon wafer by gently pressing onto the flat surface or packed into a cut cavity. The sample was rotated in its own plane. Data collection method is:
- Stability in water The stability in water over a period of three weeks was assessed by storing 50 mg of the compound with agitation in 1 ml water for 3 weeks at 25 °C then tested by XRPD.
- Aqueous solubility was determined by suspending sufficient amount of compound in deionised water to give a maximum final concentration of 310 mg/ml of the compound.
- the suspension was equilibrated at 25 °C, on a Heidolph plate shaker set to 750 rpm for 24 hours.
- the pH of the saturated solution was then measured and the suspension filtered through a glass fibre C filter (particle retention 1.2 pm) and diluted appropriately. Quantitation was by HPLC with reference to a standard solution of approximately 0.15 mg/ml in DMSO. Different volumes of the standard, diluted and undiluted sample solutions were injected.
- the solubility was calculated using the peak areas determined by integration of the peak found at the same retention time as the principal peak in the standard injection.
- Volatility data were collected on a TA Instruments Discovery TGA, equipped with a 25 position auto-sampler. Typically, >5 mg, to cover the surface of the pan of each sample was loaded onto a pre-tared aluminium pan (6.5 mm diameter) and heated at 10 °C/min from ambient temperature to 70 °C then held for 12 hours. A nitrogen purge at 25 ml/min was maintained over the sample. Volatility is calculated by measuring the weight loss between two time points once a steady state has been achieved, typically separated by 300-400 minutes, then dividing the weight loss by the time difference to get mg/min. Differential Scanning calorimetry (DSC)
- TGA was assessed using either a TA Instruments Q500 TGA or TA Instruments
- the humidity was controlled by mixing streams of dry and wet nitrogen, with a total flow rate of 200 ml/min
- the relative humidity was measured by a calibrated Rotronic probe (dynamic range of 1.0 - 100 %RH), located near the sample.
- the weight change, (mass relaxation) of the sample as a function of %RH was constantly monitored by a microbalance (accuracy ⁇ 0.005 mg).
- Example 1 The sample was recovered after completion of the isotherm and re-analysed by XRPD.
- Example 2 The sample was recovered after completion of the isotherm and re-analysed by XRPD.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
Abstract
Co-crystals comprising 2,4-dichlorophenoxyacetic acid (2,4-D) and a compound selected from the group of triazine herbicides are described, together with compositions comprising such co-crystals, and methods of their preparation.
Description
CO-CRYSTALS COMPRISING 2,4-D AND A TRIAZINE HERBICIDE
FIELD OF THE INVENTION
This invention relates to co-crystals of herbicide compounds. In particular, this invention relates to co-crystals comprising 2,4-dichlorophenoxyacetic acid and a triazine herbicide, and to agrochemical compositions comprising such co-crystals.
BACKGROUND OF THE INVENTION
2.4-dichlorophenoxyacetic acid (2,4-D) is a widely used systemic herbicide which is a member of the phenoxy family of herbicides. 2,4-D acts as a synthetic auxin which is a plant hormone which is absorbed by the leaves of plants. 2,4-D is used to control broadleaf weeds, in particular for agricultural applications.
An increasing number of weed populations have been identified which show resistance to 2,4-D, and the spread of these resistant populations could have a significant impact on the effectiveness of this herbicide. For example, it has been found that intensive use of
2.4-D in southern Europe has led to the evolution of 2,4-D resistant corn poppy biotypes (Pesticide Biochemistry and Physiology, 133, Oct 2016, p 67-72).
One strategy to overcome such issues is the use of 2,4-D in combination with one or more additional herbicide compounds with alternative modes of action. For example, a herbicide product (Enlist Duo (RTM)) containing the combination of 2,4-D and glyphosate has recently been registered for use on genetically engineered crops, and
W02012/009395A1 (Dow Agrosciences LLC) describes a mixture for use as a herbicide containing the combination of the herbicides 2,4-D, aminopyralid, and atrazine.
Kamath et al (Journal of Dispersion Science and Technology, 29:1304-1310, 2008) describes the structural characterisation of composite particles of atrazine and 2,4- dichlorophenoxy acetic acid. The composite particles are not co-crystals of atrazine and
2.4-dichlorophenoxy acetic acid but rather are an admixture of some sort. It is evident from the powder XRD patterns and DSC thermograms of the composite particles that co crystals are not produced.
The development of a single formulation containing each of the herbicide compounds to be applied in combination is attractive. For example, the development of such formulations can reduce the need for multiple applications of herbicide, can help to ensure consistent relative dosing amounts of each herbicide compound, and can reduce delivery costs.
However, the development of such formulations incorporating 2,4-D is challenging due to the physicochemical properties of 2,4-D. In particular, the high volatility of 2,4-D can lead to a number of issues. For example, the evaporation of 2,4-D from a formulation after application can lead to environmental issues, such as a strong odour and movement off the application site (herbicide drift). High volatility can also lead to a reduction in efficacy of the applied formulation, and a change from the desired ratio of actives in multi-component formulations.
In addition, if synergistic effects are desired, the relatively high aqueous solubility of 2,4- D can lead to potential problems in the development of combination formulations with less water soluble active agents, due to the relatively rapid release of 2,4-D after application.
There remains a need to develop new stable herbicide formulations containing 2,4-D which overcome one or more of the issues identified above.
SUMMARY OF THE INVENTION
The present inventors have surprisingly found that triazine herbicides may be used to form co-crystals with 2,4-dichlorophenoxyacetic acid (2,4-D). Such co-crystals have been found to have high stability, a high melting point, and provide 2,4-D in a form which has reduced volatility.
Therefore, in a first aspect of the invention there are provided co-crystals of 2,4- dichlorophenoxyacetic acid (2,4-D) and a compound selected from the group of triazine herbicides.
Triazine herbicides of particular utility may be represented by formula (I):
wherein X = Cl, -OCH3, or -SCH3; R1 = ethyl or isopropyl; R2 = ethyl, iso-propyl, cyclopropyl, tert-buty, or -C(CH3)2CN.
Preferably, the triazine herbicide is selected from atrazine, simazine, cyanazine, propazine, terbutryn, prometryn or ametryn. More preferably, the triazine herbicide is cyanazine, simazine or atrazine. Typically, the molar ratio of 2,4-D: triazine herbicide is from 2:1 to 1 :1.
The co-crystals may be formed by solution or slurry crystallisation processes. Therefore, in a second aspect of the invention there is provided a method for the preparation of co crystals as described herein which comprises combining 2,4-D and a triazine herbicide in a suitable solvent.
The co-crystals may also be formed in a solvent-free method. Therefore, in a third aspect of the invention there is provided a solvent-free method for the preparation of co crystals as described herein which comprises the step of applying dual asymmetric centrifugal forces to a mixture of 2,4-D and a triazine herbicide, and optionally in the presence of milling or grinding media.
In a fourth aspect of the invention there is provided a herbicidal composition comprising co-crystals as described herein and at least one agriculturally acceptable carrier.
Typically, such compositions may be an aqueous suspension or in the form of granules.
In a fifth aspect of the invention, there is provided the use such herbicidal compositions for controlling undesired vegetation, for example during crop cultivation.
In a sixth aspect of the invention there is provided a method for controlling undesired vegetation comprising contacting the vegetation with a composition comprising co crystals as described herein and at least one agriculturally acceptable carrier.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 shows the x-ray powder diffraction pattern of the co-crystals of Example 1.
Figure 2 shows the differential scanning calorimetry (DSC) curve of the co-crystals of Example 1.
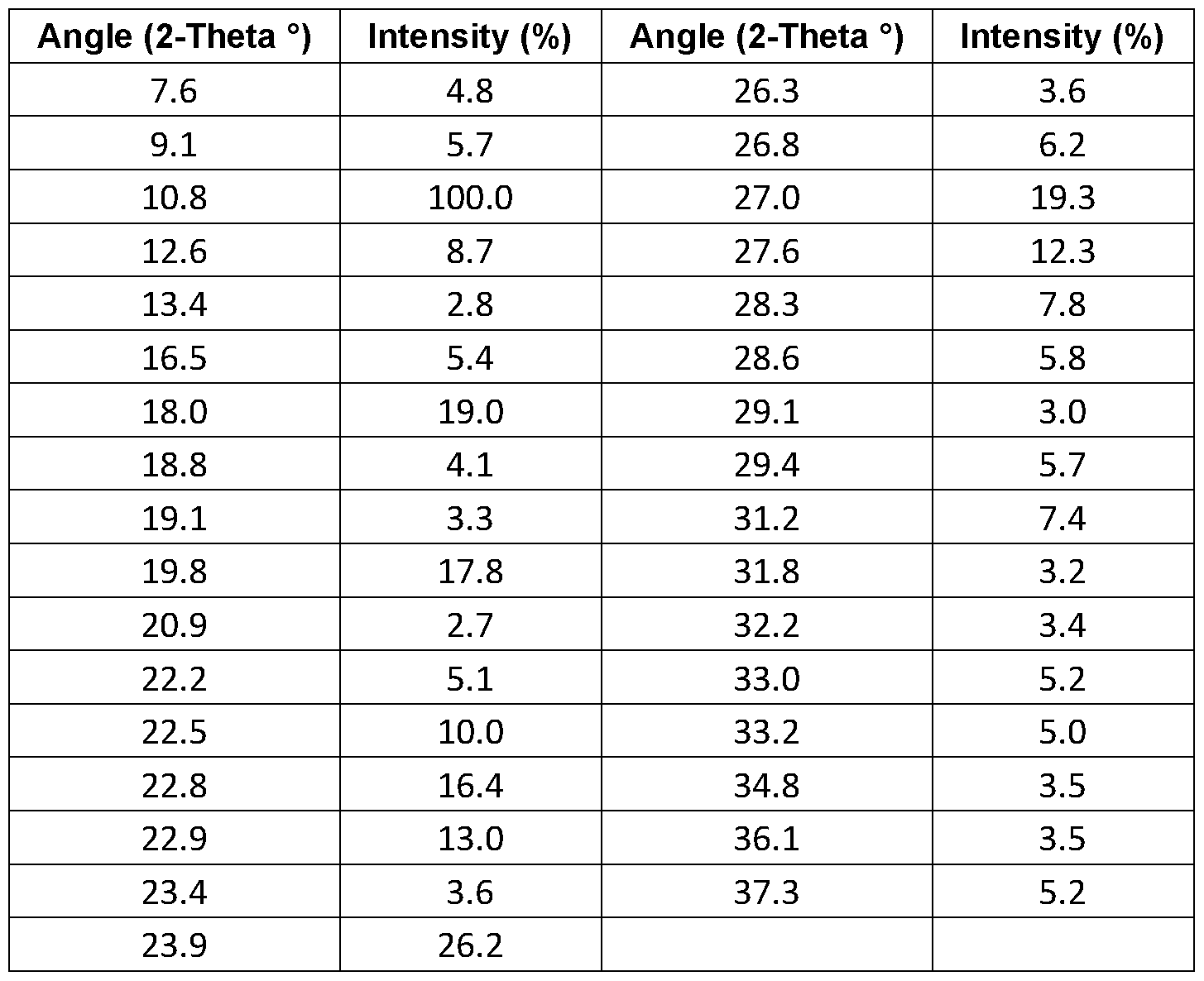
Figure 3 shows the x-ray powder diffraction pattern of the co-crystals of Example 2.
Figure 4 shows the differential scanning calorimetry (DSC) curve of the co-crystals of Example 2.
Figure 5 shows the x-ray powder diffraction pattern of the co-crystals of Example 3.
Figure 6 shows the differential scanning calorimetry (DSC) curve of the co-crystals of Example 3.
Figure 7 shows the x-ray powder diffraction pattern of the co-crystals of Example 4.
Figure 8 shows the differential scanning calorimetry (DSC) curve of the co-crystals of Example 4.
Figure 9 shows a view of an atrazine: 2,4-D co-crystal from the crystal structure of Example 2.
Figures 10A-C illustrate how centrifugal forces are applied to materials in a SpeedMixer™.
Figure 10A is a view from above showing the base plate and basket. The base plate rotates in a clockwise direction.
Figure 10B is a side view of the base plate and basket. Figure 10C is a view from above along line A in Figure 10B. The basket rotates in an anti clockwise direction.
DETAILED DESCRIPTION OF THE INVENTION
Preferred and/or optional features of the invention will now be set out. Any aspect of the invention may be combined with any other aspect of the invention unless the context demands otherwise. Any of the preferred and/or optional features of any aspect may be combined, either singly or in combination, with any aspect of the invention unless the context demands otherwise.
The present invention relates to co-crystals comprising 2,4-dichlorophenoxyacetic acid (2,4-D) and a compound selected from the group of triazine herbicides. Co-crystals are multi-component crystalline materials in which at least two organic compounds form the crystalline material which has a defined single phase crystal structure. The at least two organic compounds interact by non-covalent bonding, such as hydrogen bonding, van der Waals interactions, P-P interactions, etc., and are not simple salts. The co-crystals may be distinguished from mixtures of 2,4-D and the selected triazine herbicide by standard analytical means which are well known to those skilled in the art, for example x-ray powder diffraction (XRPD), single crystal x-ray diffraction, or differential scanning calorimetry (DSC). The molar ratio of the components of the co-crystals may be determined using, for example, HPLC or 1H NMR.
The co-crystals comprise 2,4-dichlorophenoxyacetic acid (2,4-D) and a triazine herbicide. T riazine herbicides are well known to those skilled in the art and comprise a 1 ,3,5- triazine core with amine substituents at the 2- and 4- positions.
Triazine herbicides of particular utility may be represented by formula (I):
wherein X = Cl, -OCH3, or -SCH3; R1 = ethyl or isopropyl; R2 = ethyl, iso-propyl, cyclopropyl, tert-butyl, or -C(CH3)2CN.
Preferably, X = Cl or -SCH3, more preferably X = Cl. Further preferred compounds have X = -OCH3, R1 = isopropyl, and R2 = isopropyl; or X = -SCH3, R1 = ethyl or iso-propyl, and R2 = iso-propyl or tert-butyl.
Preferably, the triazine herbicide is selected from atrazine (6-chloro-4-N-ethyl-2-N- (propan-2-yl)-1 , 3, 5-triazine-2, 4-diamine), simazine (6-chloro-2-N,4-N-diethyl-1 ,3,5- triazine-2, 4-diamine), cyanazine (2-[[4-chloro-6-(ethylamino)-1 ,3,5-triazin-2-yl]amino]-2- methylpropanenitrile), propazine (6-chloro-2-N,4-N-di(propan-2-yl)-1 ,3,5-triazine-2,4- diamine), terbutryn (2-N-tert-butyl-4-N-ethyl-6-methylsulfanyl-1 , 3, 5-triazine-2, 4-diamine), prometryn (6-methylsulfanyl-2-N,4-N-di(propan-2-yl)-1 , 3, 5-triazine-2, 4-diamine) or ametryn (4-N-ethyl-6-methylsulfanyl-2-N-propan-2-yl-1 , 3, 5-triazine-2, 4-diamine). More preferably, the triazine herbicide is simazine, cyanazine, or atrazine. In one embodiment, the present invention relates to co-crystals comprising, or consisting of, 2-4-D and atrazine (6-chloro-4-N-ethyl-2-N-(propan-2-yl)-1 , 3, 5-triazine-2, 4-diamine). Such co-crystals show significantly reduced volatility of 2,4-D. The molar ratio of 2,4-D and atrazine is generally in the range from 1 :1 to 2:1 , such as 1 :1 or 2:1. However, variations are possible which typically do not exceed 20 mol% and preferably do not exceed 10 mol%.
In the case that the molar ratio of 2,4-D and atrazine is 2:1 , the current inventors have identified a crystalline form which exhibits an x-ray powder diffraction pattern (Cu Ka radiation) substantially as shown in Figure 1. The XRPD 2-theta (2Q) values are shown
in Table 1. Preferably, the co-crystal shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 1 as 2-Theta (2Q) values (+/- 0.2 degrees 2Q). The crystalline form may also be identified by its differential scanning calorimetry (DSC) curve as shown in Figure 2, in particular by the melting point temperature of about 129.8 °C as determined by DSC.
In the case that the molar ratio of 2,4-D and atrazine is 1 :1 , the current inventors have identified a crystalline form which exhibits an x-ray powder diffraction pattern (Cu Ka radiation) substantially as shown in Figure 3. The XRPD 2-theta (2Q) values are shown in Table 2. Preferably, the co-crystal shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 2 as 2-Theta (2Q) values (+/- 0.2 degrees 2Q). The crystalline form may also be identified by its differential scanning calorimetry (DSC) curve as shown in Figure 4, in particular by the melting point temperature of about 138.2 °C as determined by DSC. The crystalline form may also be identified by the unit cell dimensions, as determined by crystal x-ray crystallography, of a = 4.251 (18) A; b = 9.889 (3) A; c = 23.442 (7) A; a= 98.748 (3)°; b= 93.047 (3)°; g = 90.288 (3)°.
In a further embodiment, the present invention relates to co-crystals comprising, or consisting of, 2-4-D and cyanazine ((2-[[4-chloro-6-(ethylamino)-1 ,3,5-triazin-2-yl]amino]- 2-methylpropanenitrile). The molar ratio of 2,4-D and cyanazine is generally in the range from 1 :1 to 2:1 , such as 1 :1. However, variations are possible which typically do not exceed 20 mol% and preferably do not exceed 10 mol%.
In the case that the molar ratio of 2,4-D and cyanazine is 1 :1 , the current inventors have identified a crystalline form which exhibits an x-ray powder diffraction pattern (Cu Ka radiation) substantially as shown in Figure 5. The XRPD 2-theta (2Q) values are shown in Table 5. Preferably, the co-crystal shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 5 as 2-Theta (2Q) values (+/- 0.2 degrees 2Q). The crystalline form may also be identified by its differential scanning calorimetry (DSC) curve as shown in Figure 6, in particular by the melting point temperature of about 139.0 °C as determined by DSC.
In another embodiment, the present invention relates to co-crystals comprising, or consisting essentially of, 2-4-D and simazine ((6-chloro-2-N,4-N-diethyl-1 ,3,5-triazine- 2, 4-diamine). The molar ratio of 2,4-D and simazine is generally in the range from 1 :1 to 2:1 , such as 2:1. However, variations are possible which typically do not exceed 20 mol% and preferably do not exceed 10 mol%.
In the case that the molar ratio of 2,4-D and simazine is 2:1 , the current inventors have identified a crystalline form which exhibits an x-ray powder diffraction pattern (Cu Ka radiation) substantially as shown in Figure 7. The XRPD 2-theta (2Q) values are shown in Table 6. Preferably, the co-crystal shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 6 as 2-Theta (2Q) values (+/- 0.2 degrees 2Q). The crystalline form may also be identified by its differential scanning calorimetry (DSC) curve as shown in Figure 8, in particular by the melting point temperature of about 146.3 °C as determined by DSC.
The co-crystals may be formulated into a herbicidal composition with at least one agriculturally acceptable carrier. The compositions may be solids, for example powders, granules, or water-dispersable powders, or may be liquids, such as suspensions of co crystal particles.
Suitable agriculturally acceptable carriers are well known to those skilled in the art. Such carriers should not be phytotoxic to crops, in particular at the concentrations employed for the control of undesirable plants in the presence of crops, and should not react chemically with the compounds of the co-crystals or other composition components. The compositions may be applied directly, or may be formulations or concentrates which are diluted, for example with water, prior to application.
Liquid carriers that may be employed include water and organic solvents, although it is typically preferred that water is used. Solid carriers include mineral earths, such as clays, silicates, diatomaceous earths, or kaolin, fertilisers, and organic products such as woodmeal and cellulose carriers.
It will be understood by the skilled person that the compositions may also include further components, such as surfactants, viscosity modifiers, anti-freeze agents, agents for pH control, stabilisers and anti-caking agents.
The concentration of active ingredients in the composition is generally between 1 and 99 wt%, such as between 5 and 95 wt% or 10 and 90 wt%. In compositions which are designed to be diluted prior to use the concentration of active ingredients is typically between about 10 and 90 wt%. Such compositions are then diluted, typically with water, to compositions which typically contain 0.001 and 1 wt% of active material.
The compositions as described herein may be used for controlling undesirable vegetation. Undesirable vegetation is understood to mean plants considered undesirable in a particular location, e.g. in an area of crops, and may be known as weeds.
Control may be achieved by a method comprising contacting the vegetation with the herbicidal composition. It will be understood by the skilled person that the composition at the point of application should contain a herbicidally effective amount of the co-crystals.
A herbicidally effective amount is an amount of the active ingredients which causes an adverse deviation of the natural development of the undesired vegetation.
In particular, the compositions have utility for controlling undesirable vegetation in a culture of crop plants, especially crop plants which are tolerant to 2,4-D and / or triazine herbicides, for example through genetic modification of the crop plants. The
compositions may also have particular utility for the control of undesirable vegetation which is resistant to either 2,4-D or the selected triazine herbicide.
The present invention also comprises a method for the preparation of co-crystals according to the present invention, the method comprising combining 2,4-D and the selected triazine herbicide in a suitable solvent.
In one embodiment of the process, 2,4-D and the triazine herbicide are dissolved in at least one solvent to form a solution, optionally with heating, and then co-crystallisation is induced, for example, by cooling the solution, evaporation of the solvent, or by precipitation. Precipitation may be induced, for example, by the addition of an anti solvent.
For example, in the case that the co-crystals comprise 2,4-D and atrazine, co
crystallisation may be achieved by the formation of a solution of 2,4-D and atrazine in a suitable solvent, such as chloroform or methyl ethyl ketone, at an elevated temperature, such as between 40 and 60 °C, and then cooling the solution to induce precipitation of the co-crystals. In the case that the co-crystals comprise 2,4-D and cyanazine, co crystallisation may be achieved by the formation a solution of 2,4-D and cyanazine in a suitable solvent, such as acetonitrile, at an elevated temperature, such as between 40 and 60 °C, and then cooling the solution to induce precipitation of the co-crystals.
In a further embodiment of the process, 2,4-D and the triazine herbicide are combined with a suitable solvent but the compounds are not dissolved so that solid material remains. Suitable solvents include but are not limited to tetrahydrofuran and water (e.g. deionised water). The mixture is then subjected to milling or grinding. The amount of time required for co-crystal formation can be readily determined by one skilled in the art and depends on factors including the temperature and the level of energy input.
For example, in the case that the co-crystals comprise 2,4-D and simazine, co crystallisation may be achieved by the combination of 2,4-D and simazine with a suitable solvent, such as tetrahydrofuran, in an amount such that the compounds are not dissolved. The mixture is then ground until co-crystals are obtained.
In the case that the co-crystals comprise 2,4-D and a triazine herbicide selected from the group consisting of atrazine, simazine and cyanazine, co-crystallisation may be achieved by the combination of 2,4-D and the triazine herbicide with water in an amount such that the compounds are not dissolved. The mixture is then ground (e.g. using low energy ball milling or low energy grinding) until the co-crystals are obtained.
Alternatively, co-crystals may be formed in a method comprising the step of applying dual asymmetric centrifugal forces to a mixture of 2,4-D and a triazine herbicide as described herein, and optionally in the presence of milling or grinding media to form the co-crystals. The method is carried out in a substantially dry environment i.e. in the absence of a solvent.
The co-crystals are formed using dual asymmetric centrifugal forces. By“dual asymmetric centrifugal forces” we mean that two centrifugal forces, at an angle to each other, are simultaneously applied to the particles. In order to create an efficient mixing environment, the centrifugal forces preferably rotate in opposite directions. The
Speedmixer™ by Hauschild (http://www.speedmixer.co.uk/index.php) utilises this dual rotation method whereby the motor of the Speedmixer™ rotates the base plate of the mixing unit in a clockwise direction (see Figure 10A) and the basket is spun in an anti clockwise direction (see Figures 10B and 10C).
The method may be controlled by various parameters including the rotation speed at which the method takes place, the length of processing time, the level to which the mixing container is filled, the use of milling media and/or the control of the temperature of the components within the milling pot.
The dual asymmetric centrifugal forces may be applied for a continuous period of time.
By“continuous” we mean a period of time without interruption. The period of time may be from about 1 second to about 30 minutes, such as about 5 seconds to about 27 minutes, for example, about 10 seconds to about 25 minutes. In one embodiment, the period of time may be about 20 minutes.
Alternatively, the dual asymmetric centrifugal forces may be applied for an aggregate period of time. By“aggregate” we mean the sum or total of more than one periods of
time (e.g. 2, 3, 4, 5, 6, 7, 8, 9, 10 or more times). The advantage of applying the centrifugal forces in a stepwise manner is that excessive heating of the particles can be avoided. The dual asymmetric centrifugal forces may be applied for an aggregate period of about 1 second to about 30 minutes, for example about 5 seconds to about 27 minutes, such as about 10 seconds to about 25 minutes. In one embodiment, the period of time may be 10 periods of time of two minutes each i.e. an aggregate period of time of about 20 minutes. In one embodiment, the dual asymmetric centrifugal forces are applied in a stepwise manner with periods of cooling therebetween. In another embodiment, the dual asymmetric centrifugal forces may be applied in a stepwise manner at one or more different speeds.
The speed of the dual asymmetric centrifugal forces may be from about 200 rpm to about 4000 rpm. In one embodiment, the speed may be from about 300 rpm to about 3000 rpm, for example about 500 rpm to about 2500 rpm. In one embodiment, the speed may be about 1900 rpm.
The level to which the mixing container is filled is determined by various factors which will be apparent to the skilled person. These factors include the apparent density of the 2,4-D and triazine herbicide, the volume of the mixing container and the weight restrictions imposed on the mixer itself.
Milling or grinding media may be used to assist the reaction. In this instance, the incorporation of hard, non-contaminating media can additionally assist in the breakdown of particles where agglomeration has occurred, for example, as a result of the
manufacturing process or during transit. Such breakdown of the agglomerates further enhances the reaction of 2,4-D and triazine herbicide. The use of milling/grinding media is well-known within the field of powder processing and materials such as stabilised zirconia and other ceramics are suitable provided they are sufficiently hard or ball bearings, such as stainless steel beads.
The present invention will now be described with reference to the following examples, which are provided to assist with understanding the present invention, and are not intended to limit its scope.
Examples
Example 1 - 2,4-D: Atrazine (2:1) co-crystal
Atrazine (98 mg) (Tokyo Chemical Industry) and 2,4-D (182 mg, 0.5 mol eq.) (Tokyo Chemical Industry) were dissolved in chloroform (7 ml) at 50°C with stirring. The clear
solution was cooled to 5°C at 0.2°C min 1 to give a slurry. This slurry was filtered, then dried under suction to yield a crystalline product (69% yield).
The co-crystals were also analysed by 1H NMR which confirmed a molar ratio of 2,4-D: atrazine of 2:1. The co-crystals were also analysed by XRPD which showed a crystalline material and yielded a diffractogram as provided in Figure 1.
The co-crystal of 2,4-D and atrazine shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 1 as 2-Theta (2Q) values.
Table 1 - 2,4-D: Atrazine (2:1) co-crystal XRPD data
DSC analysis (Figure 2) indicated a melting point of 129.8 °C.
Example 2 - 2,4-D: Atrazine (1 :1) co-crystal Atrazine (200 mg) (Tokyo Chemical Industry) and of 2,4-D (190 mg, 1 mol eq.) were dissolved in methyl ethyl ketone (3 ml) at 50°C with stirring. The clear solution was cooled to 5 °C at 0.2°C min 1 to give a slurry. This slurry was filtered, then dried under suction to yield a crystalline product.
The co-crystals were also analysed by 1H NMR which confirmed a molar ratio of 2,4-D: atrazine of 1 :1.
The co-crystals were also analysed by XRPD which showed a crystalline material and yielded a diffractogram as provided in Figure 3. The co-crystal of 2,4-D and atrazine shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 2 as 2-Theta (2Q) values.
Table 2 - 2,4-D: Atrazine (1 :1) co-crystal XRPD data
The sample was further analysed by single crystal x-ray crystallography to determine the crystal structure as follows:
Table 3. Data collection and structure refinement. Diffractometer SuperNova, Dual, Cu at zero, Atlas
Radiation source SuperNova (Cu) X-ray Source, CuKa Data collection method omega scans
Theta range for data collection 3.821 to 74.942°
Index ranges -4 < h £ 5, -12 < k £ 12, -28 < / < 29 Reflections collected 18533
Independent reflections 3940 [R(int) = 0.0529]
Coverage of independent reflections 100.0 %
Variation in check reflections n/a
Absorption correction Semi-empirical from equivalents
Max. and min. transmission 1.00000 and 0.63795
Structure solution technique Direct Methods
Structure solution program SHELXTL (Sheldrick, 2013)
Refinement technique Full-matrix least-squares on F^
Refinement program SHELXL-2014/6 (Sheldrick, 2014)
Data / restraints / parameters 3940 / 0 / 259
Goodness-of-fit on F^ 1.064
D/< 3c 0.001
Final R indices
3391 data; l>2o(l) R1 = 0.0374, U)R2 = 0.1006
all data R1 = 0.0455, U)R2 = 0.1077
Weighting scheme w =1 / [a2 (FO 2)+( 0.0641 P)2+0.2677P] where
P=(FO 2+2Fc 2)/3
Extinction coefficient n/a
Largest diff. peak and hole 0.551 and -0.377 eA 3
Refinement summary:
Ordered Non-H atoms, XYZ Freely refining
Ordered Non-H atoms, U Anisotropic
H atoms (on carbon), XYZ Idealised positions riding on attached atoms
H atoms (on carbon), U Appropriate multiple of U(eq) for bonded atom
H atoms (on heteroatoms), XYZ Freely refined on all hetero atoms
H atoms (on heteroatoms), U isotropic
Disordered atoms, OCC No disorder
Disordered atoms, XYZ No disorder
Disordered atoms, U No disorder
Table 4: Sample and crystal data for Example 2:
Compound number Atrazine/2,4-D Form A
Crystallisation solvents Acetone
Crystallisation method Slow cooling
Empirical formula C16H20CI3N5O3
Formula weight 436.72
Temperature 100(2) K
Wavelength 1.54184 A
Crystal size 0.700 x 0.050 x 0.040 mm
Crystal habit colourless rod
Crystal system Triclinic
Space group P-1
Unit cell dimensions a = 4.25112(18) A 0= 98.748(3)°
b = 9.8887(3) A b= 93.047(3)° c = 23.4421(7) A g = 90.288(3)°
Volume 972.53(6) A3
Z 2
Density (calculated) 1.491 Mg/m3
Absorption coefficient 4.514 mm 1
F(000) 452
The asymmetric unit contains a single, fully ordered molecule of Atrazine and 2,4- dichlorophenoxyacetic acid co-crystal, (designated Form A).
Final R1 [I>2s(I)] = 3.74 %.
Figure 9 shows a view of a molecule of Atrazine/2,4-D cocrystal, Form A from the crystal structure. Anisotropic atomic displacement ellipsoids for the non-hydrogen atoms are shown at the 50% probability level. Hydrogen atoms are displayed with an arbitrarily small radius.
Example 3 - 2,4-D: Cyanazine (1 :1 ) co-crystal
217 mg of cyanazine (Bond Chemicals Ltd) and 200 mg of 2,4-D (1 mol eq.) (Tokyo Chemical Industry) were dissolved in 10 ml acetonitrile at 50 °C with stirring. The clear solution was cooled to 5 °C at 0.2 °C min 1 to give a slurry. This slurry was filtered, then dried in a vacuum oven for a few days (Yield 52%).
The co-crystals were analysed by 1H NMR which confirmed a molar ratio of 2,4-D: cyanazine of 1 :1.
The co-crystals were also analysed by XRPD which showed a crystalline material and yielded a diffractogram as provided in Figure 5. This co-crystal of 2,4-D and cyanazine shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 5 as 2-Theta (2Q) values.
Table 5 - 2,4-D: Cyanazine (1 :1 ) co-crystal XRPD data
Example 4 - 2,4-D: Simazine (2:1 ) co-crystal
A mixture of 91 mg of simazine (Atlantic Research Chemicals Ltd) and 200 mg of 2,4-D (2 mol eq) (Tokyo chemical industry) was placed in a stainless steel jar lined with zirconium with a zirconium grinding bead. The mixture was initially wetted with 200 pi of tetrahydrofuran and shaken for 30 mins at 30 Hz using a Retsch Mixer Miller MM300. Samples obtained after this grinding procedure were air dried for 5 min, and analysed by XRPD. (Yield 68%)
The co-crystals were analysed by 1H NMR which confirmed a molar ratio of 2,4-D: simazine of 2:1.
The co-crystals were also analysed by XRPD which showed a crystalline material and yielded a diffractogram as provided in Figure 7. This co-crystal of 2,4-D and simazine shows at least 5, preferably at least 8 or at least 12, more preferably at least 15, even more preferably at least 20, or all of the reflexes shown in Table 6 as 2-Theta (2Q) values.
Table 6 - 2,4-D: Simazine (2:1) co-crystal XRPD data
Example 5 - Water assisted grinding
Atrazine:2,4-D
Atrazine (Tokyo chemical industry) (20 mg), 2,4-D (20.5 mg; 1 mol eq) (Tokyo chemical industry) was placed in a 2 ml HPLC vial with two grinding beads. The mixture was initially wetted with 20 pi of deionised water, and ground for 2 h at 500 rpm using a
Fritsch milling system with an Automaxion adapter. Samples obtained after this grinding procedure were air dried for 5 min, and analysed by XRPD.
Example 6 - Water assisted grinding
Simazine:2,4-D
Simazine (Atlantic research chemicals Ltd) (20 mg), 2,4-D (21.9 mg; 1 mol eq) (Tokyo chemical industry) was placed in a 2 ml HPLC vial with two grinding beads. The mixture was initially wetted with 20 pi of deionised water, and ground for 2 h at 500 rpm using a Fritsch milling system with an Automaxion adapter. Samples obtained after this grinding procedure were air dried for 5 min, and analysed by XRPD.
Example 7 - Water assisted grinding
Cvanazine:2,4-D
Cyanazine (Bond chemical Ltd) (20 mg), 2,4-D (Tokyo chemical industry) (18.9 mg; 1 mol eq) was placed in a 2 ml HPLC vial with two grinding beads. The mixture was initially wetted with 20 mI of deionised water, and ground for 2 h at 500 rpm using a Fritsch milling system with an Automaxion adapter. Samples obtained after this grinding procedure were air dried for 5 min, and analysed by XRPD.
Example 8 - solvent-free process
A mixture of atrazine (245 mg) (Tokyo chemical industry) and 2,4-D (500 mg, 2 mol eq.) (Tokyo chemical industry) was placed in a 40 ml plastic beaker with 3 mm stainless steel beads. The dry mixture was milled at 1900 rpm for 20 min in a DAC 150-FVZK
Speedmixer™ Samples obtained after this milling procedure were air-dried overnight before analysis. XRPD data and DSC analysis indicated a co-crystal consistent with Example 1 and Figures 1 and 2.
Co-crystal testing
X-Ray Powder Diffraction (XRPD)
XRPD diffractograms were collected on a Bruker D8 diffractometer.
Brucker D8 uses Cu Ka radiation (40 kV, 40 mA) and a Q-2Q goniometer fitted with a Ge monochromator. The incident beam passes through a 2.0 mm divergence slit followed by a 0.2 mm anti-scatter slit and knife edge. The diffracted beam passes through an 8.0 mm receiving slit with 2.5° Soller slits followed by the Lynxeye Detector. The software used for data collection and analysis was Diffrac Plus XRD Commander and Diffrac Plus EVA respectively.
Samples were run under ambient conditions as flat plate specimens using powder as received. The sample was prepared on a polished, zero-background (510) silicon wafer by gently pressing onto the flat surface or packed into a cut cavity. The sample was rotated in its own plane. Data collection method is:
• Angular range: 2 to 42° 2Q
• Step size: 0.05° 2Q
• Collection time: 0.5 s/step (total collection time: 6.40 min)
Stability in water The stability in water over a period of three weeks was assessed by storing 50 mg of the compound with agitation in 1 ml water for 3 weeks at 25 °C then tested by XRPD.
Thermodynamic solubility in water
Aqueous solubility was determined by suspending sufficient amount of compound in deionised water to give a maximum final concentration of ³10 mg/ml of the compound. The suspension was equilibrated at 25 °C, on a Heidolph plate shaker set to 750 rpm for 24 hours. The pH of the saturated solution was then measured and the suspension filtered through a glass fibre C filter (particle retention 1.2 pm) and diluted appropriately. Quantitation was by HPLC with reference to a standard solution of approximately 0.15 mg/ml in DMSO. Different volumes of the standard, diluted and undiluted sample solutions were injected. The solubility was calculated using the peak areas determined by integration of the peak found at the same retention time as the principal peak in the standard injection.
Analysis was performed on an Agilent HP1100 series system equipped with a diode array detector and using ChemStation software. Table 7 - HPLC method for solubility measurements
Volatility
Volatility data were collected on a TA Instruments Discovery TGA, equipped with a 25 position auto-sampler. Typically, >5 mg, to cover the surface of the pan of each sample was loaded onto a pre-tared aluminium pan (6.5 mm diameter) and heated at 10 °C/min from ambient temperature to 70 °C then held for 12 hours. A nitrogen purge at 25 ml/min was maintained over the sample. Volatility is calculated by measuring the weight loss between two time points once a steady state has been achieved, typically separated by 300-400 minutes, then dividing the weight loss by the time difference to get mg/min. Differential Scanning calorimetry (DSC)
DSC (melting point) was assessed using either a TA Instruments Q2000 or TA
Instruments Discovery DSC. Typically, 0.5 - 3 mg of each sample, in a pin-holed aluminium pan, was heated at 10 °C/min from 25 °C to 300 °C. A purge of dry nitrogen at 50 ml/min was maintained over the sample. Thermogravimetric analysis (TGA)
TGA was assessed using either a TA Instruments Q500 TGA or TA Instruments
Discovery TGA. Typically, 5 - 10 mg of each sample was loaded onto a pre-tared aluminium DSC pan and heated at 10 °C/min from ambient temperature to 350 °C. A nitrogen purge at 60 ml/min was maintained over the sample. Gravimetric Vapour Sorption
Hygroscopicity was assessed by Gravimetric vapour sorption. Sorption isotherms were obtained using a SMS DVS Intrinsic moisture sorption analyser, controlled by DVS Intrinsic Control software. The sample temperature was maintained at 25 °C by the instrument controls. The humidity was controlled by mixing streams of dry and wet nitrogen, with a total flow rate of 200 ml/min The relative humidity was measured by a calibrated Rotronic probe (dynamic range of 1.0 - 100 %RH), located near the sample. The weight change, (mass relaxation) of the sample as a function of %RH was constantly monitored by a microbalance (accuracy ±0.005 mg).
Typically, 5 - 30 mg of sample was placed in a fared mesh stainless steel basket under ambient conditions. The sample was loaded and unloaded at 40 %RH and 25 °C (typical room conditions). A moisture sorption isotherm was performed as outlined below (2 scans per complete cycle). The standard isotherm was performed at 25 °C at 10 %RH intervals over a 0 - 90 %RH range. Typically, a double cycle (4 scans) was carried out. Data analysis was carried out within Microsoft Excel using the DVS Analysis Suite. Table 8 - Method for SMS DVS Intrinsic experiments
The sample was recovered after completion of the isotherm and re-analysed by XRPD.
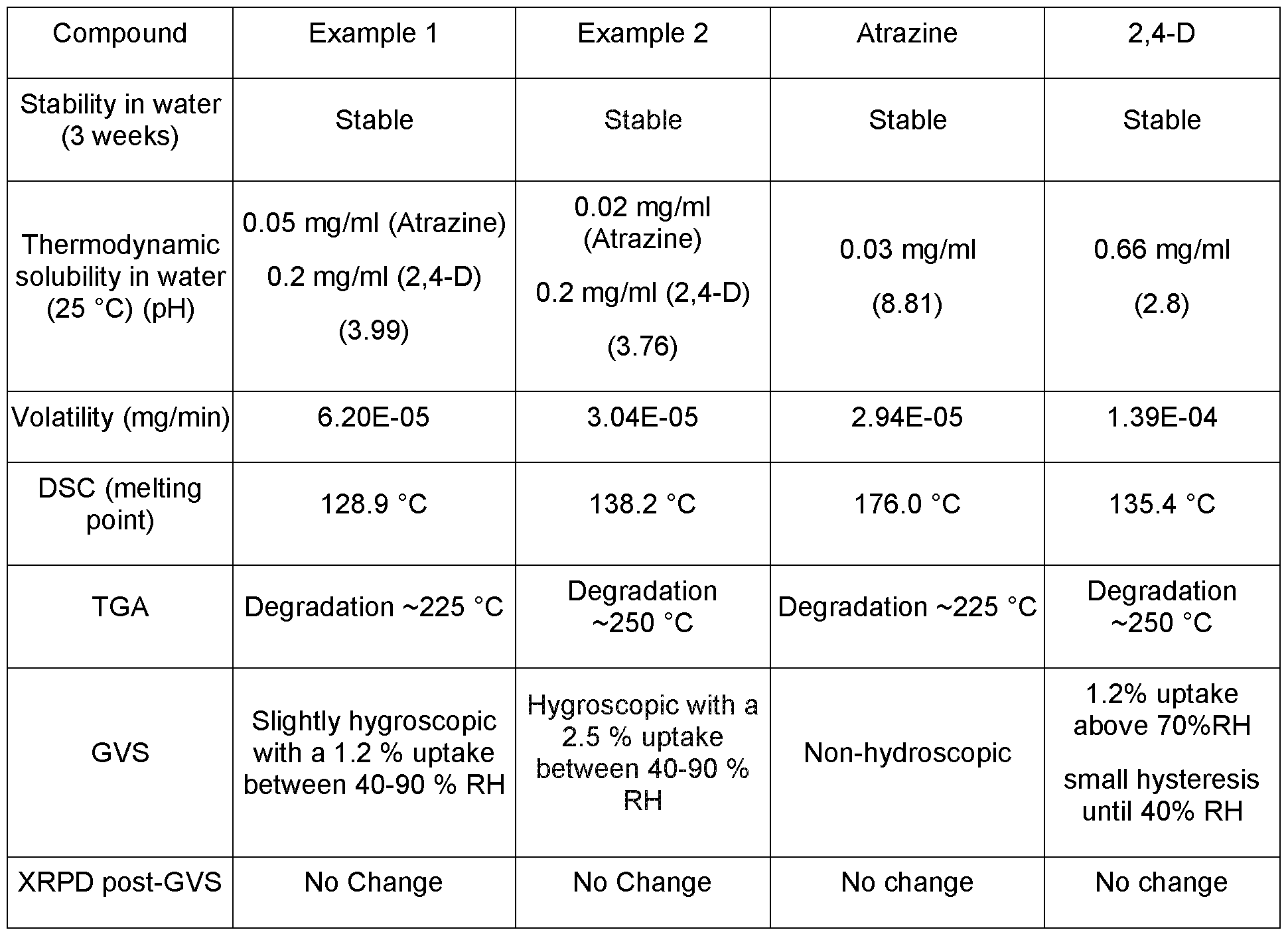
Example 1 and Example 2
The physicochemical properties of the 2,4-D: atrazine co-crystals formed in Example 1 and 2 were tested alongside the individual compounds 2,4-D and atrazine. The results are provided in Table 9. Table 9 - Results of the testing of physicochemical properties of co-crystals formed in Example 1 and Example 2.
Example 3
The physicochemical properties of the 2,4-D:cyanazine co-crystals formed in Example 3 were tested alongside the individual compounds 2,4-D and cyanazine. The results are provided in Table 10.
Table 10 - Results of the testing of physicochemical properties of co-crystals formed in Example 3.
Example 4
The physicochemical properties of the 2,4-D: simazine co-crystals formed in Example 4 were tested alongside the individual compounds 2,4-D and simazine. The results are provided in Table 11.
Table 11 - Results of the testing of physicochemical properties of co-crystals formed in Example 4.
The results of the testing of the physicochemical properties testing of the co-crystals generated in Examples 1-4 indicates that the 2,4-D / triazine co-crystals show good stability and a high melting point. In each case the volatility of the co-crystals is significantly lower than the volatility of 2,4-D alone. In addition, the solubility of the active agents is closely matched.
Claims
1. Co-crystals comprising:
2,4-dichlorophenoxyacetic acid (2,4-D); and a compound selected from the group of triazine herbicides. 2. Co-crystals according to claim 1 wherein the triazine herbicide is of formula (I):
wherein X = Cl, -OCH3, or -SCH3; R1 = ethyl or isopropyl; R2 = ethyl, iso-propyl, cyclopropyl, tert-butyl, or -C(CH ) CN.
3. Co-crystals according to claim 1 or claim 2 wherein the molar ratio of 2,4-D to the triazine herbicide is from 2:1 to 1 :1.
4. Co-crystals according to any one of the preceding claims wherein the triazine herbicide is selected from atrazine, simazine, cyanazine, propazine, terbutryn, prometryn or ametryn.
5. Co-crystals according to any one of the preceding claims wherein the triazine herbicide is atrazine
6. Co-crystals according to claim 5 wherein the molar ratio of 2,4-D: atrazine is 2:1.
7. Co-crystals according to claim 5 or claim 6 wherein an X-ray powder
diffractogram shows at least five of the following diffraction lines, given as 2Q values: 4.6, 6.8, 10.8, 11.7, 13.6, 15.0, 15.8, 16.8, 17.9, 18.8, 19.4, 20.5, 21.2, 21.9, 23.5, 24.0, 24.8,
25.0, 25.9, 28.0, 28.9, 30.7, 32.0, 33.3, 33.9, 36.4, 38.1 , 39.9.
8. Co-crystals according to claim 5 wherein the molar ratio of 2,4-D: atrazine is 1 : 1.
9. Co-crystals according to claim 5 or claim 8 wherein an X-ray powder
diffractogram shows at least five of the following diffraction lines, given as 2Q values: 7.6, 9.1 , 10.8, 12.6, 13.4, 16.5, 18.0, 18.8, 19.1 , 19.8, 20.9, 22.2, 22.5, 22.8, 22.9, 23.4, 23.9,
26.3, 26.8, 27.0, 27.6, 28.3, 28.6, 29.1 , 29.4, 31.2, 31.8, 32.2, 33.0, 33.2, 34.8, 36.1 ,
37.3.
10. Co-crystals according to claim 5 or claim 8 wherein the crystalline form has a unit cell, as determined by crystal x-ray crystallography, of the following dimensions: a = 4.251 (18) A; b = 9.889 (3) A; c = 23.442 (7) A; a= 98.748 (3)°; b= 93.047 (3)°; g = 90.288 (3)°.
11. Co-crystals according to any one of claims 1 to 4 wherein the triazine herbicide is cyanazine.
12. Co-crystals according to claim 11 wherein the molar ratio of 2,4-D: cyanazine is 1 :1.
13. Co-crystals according to claim 12 wherein an X-ray powder diffractogram shows at least five of the following diffraction lines, given as 2Q values: 8.9, 10.1 , 11.3, 12.1 ,
12.3, 14.3, 15.5, 16.5, 19.6, 20.3, 20.8, 21.1 , 21.6, 22.8, 24.8, 26.1 , 26.9, 27.5, 31.8,
37.4, 38.8, 39.8.
14. Co-crystals according to any one of claims 1 to 4 wherein the triazine herbicide is simazine.
15. Co-crystals according to claim 14 wherein the molar ratio of 2,4-D: simazine is 2: 1.
16. Co-crystals according to claim 15 wherein an X-ray powder diffractogram shows at least five of the following diffraction lines, given as 2Q values: 4.5, 7.2, 10.7, 12.0,
14.5, 16.7, 17.3, 19.1 , 20.7, 21.3, 21.7, 22.5, 22.8, 23.5, 24.3, 25.3, 25.8, 26.3, 26.7, 27.3, 28.1 , 28.9, 29.7, 30.5, 31.7, 33.9, 35.1 , 35.7.
17. A method of preparing co-crystals according to any one of the preceding claims, which comprises combining 2,4-D and a triazine herbicide in a suitable solvent.
18. A method for preparing co-crystals according to any one of claims 1 to 16 comprising the step of applying dual asymmetric centrifugal forces to a mixture of 2,4-D and a triazine herbicide, and optionally in the presence of milling or grinding media to form the co-crystals.
19. An herbicidal composition comprising co-crystals according to any one of claims 1 to 16 and at least one agriculturally acceptable carrier.
20. A herbicidal composition according to claim 19 wherein the composition is an aqueous suspension or granules.
21. The use of an herbicidal composition according to claim 19 or claim 20 for controlling undesired vegetation.
22. A method for controlling undesired vegetation comprising contacting the vegetation with a herbicidal composition according to claim 19 or claim 20.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB1801544.6 | 2018-01-31 | ||
| GBGB1801544.6A GB201801544D0 (en) | 2018-01-31 | 2018-01-31 | Co-crystals |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019150125A1 true WO2019150125A1 (en) | 2019-08-08 |
Family
ID=61558095
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB2019/050277 WO2019150125A1 (en) | 2018-01-31 | 2019-01-31 | Co-crystals comprising 2,4-d and a triazine herbicide |
Country Status (2)
| Country | Link |
|---|---|
| GB (1) | GB201801544D0 (en) |
| WO (1) | WO2019150125A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117550967A (en) * | 2023-11-10 | 2024-02-13 | 青岛科技大学 | 2, 4-dichlorophenoxyacetic acid eutectic and preparation method and application thereof |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3077392A (en) * | 1959-01-08 | 1963-02-12 | Chipman Chemical Company Inc | Plant growth control and herbicidal composition and process of using the same |
| FR1329306A (en) * | 1962-07-19 | 1963-06-07 | Geigy Ag J R | 2.4.6-substituted s-triazines salts and their applications |
| WO2009047043A1 (en) * | 2007-09-07 | 2009-04-16 | Basf Se | Co-crystals of pyrimethanil and dithianon |
| WO2012009395A1 (en) | 2010-07-14 | 2012-01-19 | Dow Agrosciences Llc | Synergistic herbicidal compositions containing aminopyralid, 2,4-dichlorophenoxyacetic acid and atrazine |
-
2018
- 2018-01-31 GB GBGB1801544.6A patent/GB201801544D0/en not_active Ceased
-
2019
- 2019-01-31 WO PCT/GB2019/050277 patent/WO2019150125A1/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3077392A (en) * | 1959-01-08 | 1963-02-12 | Chipman Chemical Company Inc | Plant growth control and herbicidal composition and process of using the same |
| FR1329306A (en) * | 1962-07-19 | 1963-06-07 | Geigy Ag J R | 2.4.6-substituted s-triazines salts and their applications |
| WO2009047043A1 (en) * | 2007-09-07 | 2009-04-16 | Basf Se | Co-crystals of pyrimethanil and dithianon |
| WO2012009395A1 (en) | 2010-07-14 | 2012-01-19 | Dow Agrosciences Llc | Synergistic herbicidal compositions containing aminopyralid, 2,4-dichlorophenoxyacetic acid and atrazine |
Non-Patent Citations (7)
| Title |
|---|
| ANDREW D. BOND: "What is a co-crystal?", CRYSTENGCOMM, vol. 9, no. 9, 1 January 2007 (2007-01-01), pages 833 - 834, XP055567443, DOI: 10.1039/b708112j * |
| BHUPINDER SINGH SEKHON: "Co-crystals of agrochemical actives", INTERNATIONAL JOURNAL OF AGRICULTURAL SCIENCES, 1 May 2015 (2015-05-01), India, pages 2167 - 447, XP055567453, Retrieved from the Internet <URL:http://www.internationalscholarsjournals.org/download.php?id=904273700268786541.pdf&type=application/pdf&op=1> [retrieved on 20190312] * |
| CHILDS S L ET AL: "The Salt-Cocrystal Continuum: The Influence of Crystal Structure on Ionization State", MOLECULAR PHARMACEUTICS, AMERICAN CHEMICAL SOCIETY, US, vol. 4, no. 3, 1 January 2007 (2007-01-01), pages 323 - 338, XP002483371, ISSN: 1543-8384, [retrieved on 20070427], DOI: 10.1021/MP0601345 * |
| GRAHAM SMITH ET AL: "Herbicides. Part 1. Crystal Structure of 2,4-D (2,4-Dichlorophenoxyacetic Acid)", J. CHEM. SOC., PERKIN TRANS. 2, 1 January 1976 (1976-01-01), U.K., pages 791 - 792, XP055567441, Retrieved from the Internet <URL:https://pubs.rsc.org/en/content/articlepdf/1976/p2/p29760000791> [retrieved on 20190312], DOI: 10.1039/P29760000791 * |
| KAMATH ET AL., JOURNAL OF DISPERSION SCIENCE AND TECHNOLOGY, vol. 29, 2008, pages 1304 - 1310 |
| KAMATH ET AL.: "Structural Characterisation of Composite Particles of Two Commonly Used Herbicides", JOURNAL OF DISPERSION SCIENCE AND TECHNOLOGY, vol. 29, 2008, pages 1304 - 1310, XP055567151 * |
| PESTICIDE BIOCHEMISTRY AND PHYSIOLOGY, vol. 133, October 2016 (2016-10-01), pages 67 - 72 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117550967A (en) * | 2023-11-10 | 2024-02-13 | 青岛科技大学 | 2, 4-dichlorophenoxyacetic acid eutectic and preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| GB201801544D0 (en) | 2018-03-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| BRPI1008770B1 (en) | 6- (substituted phenyl) -4-aminopicolinates and 2- (substituted phenyl) -6-amino-4-pyrimidinecarboxylates n-alkoxyamides, their production processes, herbicidal composition as well as undesirable vegetation control methods | |
| JP2008521850A (en) | 1-Alkynyl-2-aryloxyalkylamides and their use as fungicides. | |
| JP2005507402A (en) | Photoactive (R) -phenoxypropionic acid-N-methyl-N-2-fluorophenylamide compound having herbicidal activity | |
| JP2008503529A (en) | Pyridopyrazine for combating phytopathogenic fungi | |
| JPS6335605B2 (en) | ||
| US4979982A (en) | Herbicidal cinnamic ester uracils | |
| WO2019150125A1 (en) | Co-crystals comprising 2,4-d and a triazine herbicide | |
| JPS60123475A (en) | Imidazolinones, manufacture, herbicide and plant growth regulator | |
| KR900003659B1 (en) | Substitutied 2,6-sustitutied pyridine compounds | |
| EA018640B1 (en) | Crystalline complexes of pendimethalin and metazachlor | |
| CN105801513B (en) | N- with activity of weeding replaces alkyl virtue phenoxy base Propionamides compound and its preparation and application | |
| WO2020074852A1 (en) | Molecular complexes comprising mcpa and a triazine herbicide | |
| JP2004002324A (en) | Herbicide composition | |
| JP2006514029A (en) | Fungicide | |
| WO2020095017A1 (en) | Molecular complexes comprising dicamba and a triazine herbicide | |
| JP2006507338A (en) | Fungicide | |
| CA1246073A (en) | Triazine derivatives and herbicides | |
| KR910006988B1 (en) | Process for production 2-alkoxy aminosulfonyl benzene sulfonylurea | |
| JP2006516131A (en) | Fungicide | |
| JPS585161B2 (en) | All the best | |
| US20220369641A1 (en) | Molecular complexes | |
| KR20060006977A (en) | N-alkynyl-2-heteroaryloxyalkylamides for use as fungicides | |
| JPS62148479A (en) | Composition for controlling nematode and insect | |
| JP5860030B2 (en) | Pyrimethanyl or cyprodinil eutectic | |
| PL144076B1 (en) | Herbicide |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19704420 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19704420 Country of ref document: EP Kind code of ref document: A1 |