WO2018229580A1 - An improved process for the preparation of cefovecin sodium - Google Patents
An improved process for the preparation of cefovecin sodium Download PDFInfo
- Publication number
- WO2018229580A1 WO2018229580A1 PCT/IB2018/053773 IB2018053773W WO2018229580A1 WO 2018229580 A1 WO2018229580 A1 WO 2018229580A1 IB 2018053773 W IB2018053773 W IB 2018053773W WO 2018229580 A1 WO2018229580 A1 WO 2018229580A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- compound
- sodium
- mixtures
- cefovecin
- Prior art date
Links
- 0 *OC(C(N([C@]([C@@]1N)SC2)C1=O)=C2[C@]1OCCC1)=* Chemical compound *OC(C(N([C@]([C@@]1N)SC2)C1=O)=C2[C@]1OCCC1)=* 0.000 description 4
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D501/00—Heterocyclic compounds containing 5-thia-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. cephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D501/02—Preparation
- C07D501/04—Preparation from compounds already containing the ring or condensed ring systems, e.g. by dehydrogenation of the ring, by introduction, elimination or modification of substituents
- C07D501/06—Acylation of 7-aminocephalosporanic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/54—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame
- A61K31/542—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame ortho- or peri-condensed with heterocyclic ring systems
- A61K31/545—Compounds containing 5-thia-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. cephalosporins, cefaclor, or cephalexine
- A61K31/546—Compounds containing 5-thia-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. cephalosporins, cefaclor, or cephalexine containing further heterocyclic rings, e.g. cephalothin
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D501/00—Heterocyclic compounds containing 5-thia-1-azabicyclo [4.2.0] octane ring systems, i.e. compounds containing a ring system of the formula:, e.g. cephalosporins; Such ring systems being further condensed, e.g. 2,3-condensed with an oxygen-, nitrogen- or sulfur-containing hetero ring
- C07D501/02—Preparation
- C07D501/12—Separation; Purification
Definitions
- the present invention relates to an improved process for the preparation of Cefovecin sodium of a Com ound of Formula (1).
- Cefovecin sodium is indicated for the treatment of skin infections (secondary superficial pyoderma, abscesses, and wounds) in dogs caused by susceptible strains of Staphylococcus intermedius and Streptococcus canis (Group G). Cefovecin sodium is indicated for the treatment of skin infections (wounds and abscesses) in cats caused by susceptible strains of Pasteurella multocida. Cefovecin sodium is marketed under the brand name CONVENIA®.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Cephalosporin Compounds (AREA)
Abstract
The present invention relates to an improved process for the preparation of Cefovecin sodium of a Compound of Formula (1), by reacting THF Cephem Compound of Formula (6) with MAEM Compound of Formula (8).
Description
AN IMPROVED PROCESS FOR THE PREPARATION OF CEFOVECIN SODIUM
FIELD OF THE INVENTION
The present invention relates to an improved process for the preparation of Cefovecin sodium of a Com ound of Formula (1).
BACKGROUND OF THE INVENTION
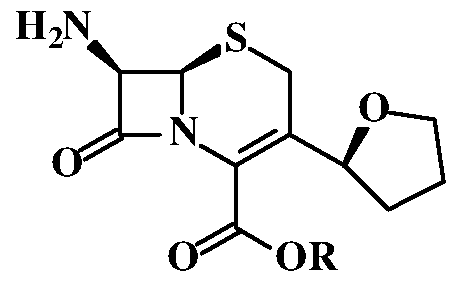
Cefovecin sodium is chemically known as (6R,7R)-7-[[(2Z)-(2-amino-4- thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-3-[(2S)-tetrahydro-2-furanyl]-5- thia-l-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, monosodium salt. Cefovecin sodium is a semi- synthetic broad- spectrum antibacterial agent from the cephalosporin class of chemo therapeutic agents. Cefovecin sodium is indicated for the treatment of skin infections (secondary superficial pyoderma, abscesses, and wounds) in dogs caused by susceptible strains of Staphylococcus intermedius and Streptococcus canis (Group G). Cefovecin sodium is indicated for the treatment of skin infections (wounds and abscesses) in cats caused by susceptible strains of Pasteurella multocida. Cefovecin sodium is marketed under the brand name CONVENIA®.
Cefovecin sodium (1) was disclosed first time in US 6,020,329. This patent discloses a process for the preparation of Cefovecin sodium (1) by treating 2-(Z)-methoxyimino-2-(2-tritylaminothiazol-4-yl)acetic acid hydrochloride (2) with mesyl chloride in Ν,Ν-diisopropylethylamine (DIPEA) to produce mesylate compound (3), which on further condensation with Cephem compound namely t- butyl (6R,7R)-7-amino-3-(tetrahydrofuran-2-yl)ceph-3-em-4-carboxylate (4) to
produce protected Cefovecin compound (5) followed by deprotection and hydrolysis to give Cefovecin sodium (1).
The process as shown in Scheme-I below:
Scheme-I
This process is not viable for large-scale production as it involves multiple steps with sensitive reagents such as mesyl chloride, Dimethylformamide (DMF), and DIPEA and this reduces the quality and yield of final product. Further, the deprotection requires the usage of Trifluoro acetic acid (TFA) and anisole. Also, the process involves tedious work-up procedure and chromatographic purification, which are difficult to operate on commercial scale.
US 7,129,350 also discloses variant process for the preparation of Cefovecin sodium (1). The process comprises, condensing THF (Tetrahydrofuran) Cephem compound (6) with (Z)-2-amino-a-(methoxyimino)-4-thiazoleacetic acid anhydride and 0,0-diethyl hydrogenphosphorothioate (7) to produce Cefovecin sodium (1).
Cefovecin sodium (1)
Scheme-II
Applicant found that the process is not viable for commercial scale. It involves expensive reagents such as 0,0-diethyl hydrogenphosphorothioate, increases cost of product at higher scale. The reported process involves the usage of very large volume of solvents, which impacts the productivity of final product with superior quality and better yield.
Considering the importance of Cefovecin sodium, there is always a need for an alternative preparative routes, which for example, involve fewer steps, use reagents that are less expensive and/or easier to handle, consume smaller amounts of reagents, provide a higher yield of product, have smaller and/or more eco- friendly waste products, and/or provide a product of higher purity. In view of this our scientists have developed the present invention, related to a process for the preparation of Cefovecin sodium (1) with good yield and high purity.
ABBREVIATIONS
THF : Tetrahydrofuran;
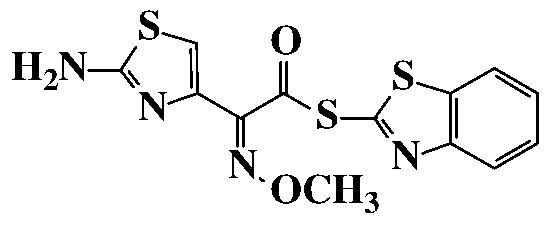
MAEM 2-Mercaptobenzothiazolyl [(Z)-2-(2-amino-thiazolyl)-2- methoxyimino] acetate OBJECTIVE OF THE INVENTION
The main objective of the present invention is to provide a simple and cost effective process for the preparation of Cefovecin sodium (1) with high purity and good yield on commercial scale. SUMMARY OF THE INVENTION
In one embodiment, the present invention provides an improved process for the reparation of Cefovecin sodium of a Compound of Formula (1):
which comprises;
(a) step of reactin THF Cephem Compound of Formula (6a);
wherein R is selected from hydrogen or carboxy protecting group;
with Com ound of formula (8);
Formula 8
wherein Rl is substituted or unsubstituted mercaptobenzothiazole, substituted or unsubstituted mercaptobenzimidazole group;
to roduce a Compound of formula (la);
(b) optionally deprotecting the Compound of formula (la);
(c) isolating Cefovecin sodium of a Compound of Formula (1).
In another embodiment, the present invention provides an improved process for the preparation of Cephalosporin Compound of Formula (lb):
wherein the group C02R' is a carboxylic acid or a carboxylate salt; which comprises step of a) reacting THF Ce hem Compound of Formula (6);
wherein the group C02R' is a carboxylic acid or a carboxylate salt; with Compound of formula (8);
Formula 8
wherein Rl is substituted or unsubstituted mercaptobenzothiazole substituted or unsubstituted mercaptobenzimidazole group; and b) isolating Cephalosporin Compound of Formula (lb).
In another embodiment, the present invention provides an improved process for the preparation of Cefovecin sodium of a Compound of Formula (1):
(a) step of reacting THF Cephem Compound of Formula (6);
with MAEM Compound of formula (8);
(b) optionally, purifying Cefovecin sodium of a Compound of Formula (1); and (c) isolating Cefovecin sodium of a Compound of Formula (1).
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 - PXRD of Cefovecin sodium obtained from Example 1.
DETAILED DESCRIPTION OF THE INVENTION In one embodiment, the present invention provides an improved process for the preparation of Cefovecin sodium of a Compound of Formula (1):
which comprises;
(a) step of reacting THF Cephem Compound of Formula (6a);
wherein R is selected from hydrogen or carboxy protecting group; with Compound of formula (8);
wherein Rl is substituted or unsubstituted mercaptobenzothiazole, substituted or unsubstituted mercaptobenzimidazole group; to produce a Compound of formula (la);
Formula la
(b) optionally deprotecting the Compound of formula (la);
(c) isolating Cefovecin sodium of a Compound of Formula (1).
In an embodiment of the invention, Compound of formula (8) is selected from 2-mercaptobenzothiazolyl [(Z)-2-(2-amino-thiazolyl)-2- methoxyimino] acetate; shown below.
In still another embodiment, in a Compound of Formula (6), R is selected from hydrogen or carboxy protecting group.
In still another embodiment, carboxy protecting group is selected from para-nitrobenzyl or allyl.
In still another embodiment, the reaction of a Compound of Formula (6) with Compound of formula (8) in step (a) is carried out in the presence of a base and a solvent.
The base is organic or inorganic base. The inorganic base comprises potassium carbonate, lithium carbonate, sodium carbonate, sodium ethoxide, sodium bicarbonate, potassium bicarbonate, sodium hydroxide, potassium hydroxide, ammonium hydroxide, and mixtures thereof; the organic base comprises diisopropylamine, diisopropylethylamine triethylamine, dimethylamine, trimethyl amine, pyridine, l,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and mixtures thereof.
The solvent used in the reaction step comprises polar protic solvent or polar aprotic solvent or non-polar solvents and/or mixtures thereof.
In another embodiment, polar protic solvent comprises water, methanol, ethanol, isopropanol n-butanol, and/or mixtures thereof; polar aprotic solvent comprises dimethylformamide (DMF), dimethylsulfoxide (DMSO), tetrahydrofuran (THF), acetonitrile, acetone, ethyl acetate, N- methylpyrrolidoneand/or mixtures thereof; and non-polar solvents comprises hexane, benzene, toluene, 1,4-dioxane, chloroform, diethyl ether, methylene chloride (CH2C12) and/or mixtures thereof. In still another embodiment of the invention, wherein said deprotection in step (b), deprotecting agent is sodium dithionite or a catalytic hydrogenating agent.
Catalytic hydrogenating agent such as hydrogen gas over 10% palladium over carbon or tetrakis triphenyl phosphine palladium (0). In still another embodiment of the invention, wherein said deprotection in step (b), is carried out in the presence of a solvent.
The solvent used in the step (b) acetone, water, tetrahydrofuran and/or mixtures thereof.
In another embodiment, the present invention provides an improved process for the preparation of Cephalosporin Compound of Formula (lb):
wherein the group C02R' is a carboxylic acid or a carboxylate salt; which comprises step of
a) reacting THF Ce hem Compound of Formula (6);
wherein the group C02R' is a carboxylic acid or a carboxylate salt; with Compound of formula (8);
wherein Ri is substituted or unsubstituted mercaptobenzothiazole group, substituted or unsubstituted mercaptobenzimidazole group; and b) isolating Cephalosporin Compound of Formula (lb).
In another embodiment, wherein the carboxylate salt is sodium salt or potassium salt.
In still another embodiment, the reaction is carried out in the presence of a base and a solvent.
In still another embodiment, Base and a solvent are described here in above.
In another embodiment, the present invention provides an improved process for the preparation of Cefovecin sodium of a Compound of Formula (1):
(a) step of reacting THF Cephem Compound of Formula (6);
with MAEM Compound of formula (8);
(b) optionally, purifying Cefovecin sodium of a Compound of Formula (1); and
(c) isolating Cefovecin sodium of a Compound of Formula (1).
In still another embodiment, the reaction is carried out in the presence of a base and a solvent. In still another embodiment, base and a solvent are described here in above.
In one more embodiment, the reaction is carried out at a temperature of OoC to 50°C preferably 0 to 20°C more preferably 0 to 5°C for a time period of 1- 5 hours. In one more embodiment, the pH of the reaction is adjusted to 2-3 with an acid.
In one more embodiment, acid comprises HC1 or sulfuric acid.
In another embodiment, after completion of the reaction, Compound (1) is isolated by conventional methods such as by removing the solvent under reduced pressure.
In another embodiment, Compound (1) is optionally purified by conventional methods.
In another embodiment, purification is carried out by crystallization using a solvent or mixture of solvents. The solvent used in the purification comprising, acetone, water, tetrahydrofuran and/or mixtures thereof.
In still another embodiment, the THF Cephem Compound of Formula (6) is prepared by the methods known in the literature.
The following examples illustrate the nature of the invention and are provided for illustrative purposes only and should not be construed to limit the scope of the invention.
EXAMPLES:
EXAMPLE 1:
PREPARATION OF SODIUM (6R,7R)-3-[(2S)-TETRAHYDRO-2- FURANYL]-7-[[(Z)-2-(2-AMINOTHIAZOL-4-YL)-2-(METHOXYIMINO)- ACETYL]AMINO]-8-OXO-5-THIA-l-AZABICYCLO [4.2.0]OCT-2-ENE- 2-CARBOXYLATE (CEFOVECIN SODIUM) (Compound 1)
In a 2L RBF, (6R,7R)-7-Amino-8-oxo-3-[(2S)-tetrahydro-2-furanyl]-5- thia-l-azabicylco[4.2.0] oct-2-ene-2-carboxylic acid [THF Cephem Compound of Formula (6)] (62 g); 2-mercaptobenzothiozolyl [(Z)-2-(2-amino-4-thiazolyl)-2- methoxyimino] acetate [MAEM Compound of Formula (8)] (93.3 g) were suspended in a mixture of tetrahydrofuran (372 ml) and DM water (372 ml) at 0- 5°C. Tetrahydrofuran solution of triethylamine (26 g dissolved in 50 ml tetrahydrofuran) was added to the above mixture, over a period of 30 min at 0- 5°C. The reaction mass was stirred at 0-5°C for 4 h while maintaining pH at 7.8- 8.2. After completion of the reaction, the mass was washed with methylene chloride (1 x 630 ml, 2 x 330 ml) and the combined methylene chloride layer was
extracted with DM water (180 ml). The combined aqueous layer containing the product was degassed and diluted with tetrahydrofuran (372 ml). Sodium chloride (136 g) was added to this solution and pH was adjusted to 2.85 by using hydrochloric acid (20 ml) at 15-18°C. The organic layer was separated and aqueous layer was extracted with tetrahydrofuran (125 ml). The combined organic layer was treated with activated carbon (5 g) at 15-20°C for 30 min., filtered through Hyflo and the residue was washed with tetrahydrofuran (70 ml).
Tetrahydrofuran solution of sodium-2-ethylhexanoate (45.90 g dissolved in 200 ml tetrahydrofuran) was added to the above filtrate at 15-20°C. The resulting slurry mass was cooled to 2°C and diluted with tetrahydrofuran (240 ml) and stirred for 1 h at 5-10°C. The product was filtered, washed with pre-cooled tetrahydrofuran (120 ml), followed by acetone (2 x 120 ml) under nitrogen atmosphere. The wet product was dried at 40-45°C under reduced pressure (<50 mm Hg) to obtain Cefovecin sodium. Yield: 86.5 g.
Chromatographic purity (By HPLC): 97.64%.
PURIFICATION OF CEFOVECIN SODIUM:
In a 1L RBF, 85 g of above Cefovecin sodium (105 g) was dissolved in a mixture of acetone (95 ml) and DM water (95 ml) at 10-20°C. This was diluted with acetone (126 ml) and treated with activated carbon (9.50 g) for 30 min at 15- 20°C. Carbon was removed by filtration through Hyflo and the residue was washed with aqueous acetone (prepared by mixing 28.5 ml of DM water with 66.5 ml of acetone). To the filtrate, acetone (2060 ml) was added at 15-20°C and the resulting slurry mass was stirred at 15-20°C for 30 min. The product was filtered, washed with acetone (2 x 190 ml) under nitrogen atmosphere and dried at 40- 45°C under reduced pressure (< 20 mm Hg) to obtain Cefovecin sodium.
Yield: 85.5 g.
Chromatographic purity (By HPLC): 98.65%.
Claims
AIM:
An improved process for the preparation of Cephalosporin Compound Formula (lb):
wherein the group C02R' is a carboxylic acid or a carboxylate salt; which comprises steps of a) reacting THF Cephem Compound of Formula (6);
wherein the group C02R' is a carboxylic acid or a carboxylate salt; with a Compound of formula (8);
wherein Rl is substituted or unsubstituted mercaptobenzothiazole group, substituted or unsubstituted mercaptobenzimidazole group; and b) isolating Cephalosporin Compound of Formula (lb).
2. The process according to claim 1, the reaction of a Compound of Formula (6) with Compound of formula (8) in step (a) is carried out in the presence of a base and a solvent.
The process according to claim 2, base comprises potassium carbonate, lithium carbonate, sodium carbonate, sodium ethoxide, sodium bicarbonate, potassium bicarbonate, sodium hydroxide, potassium hydroxide, ammonium hydroxide, diisopropylamine, diisopropylethylamine triethylamine, dimethylamine, trimethyl amine, pyridine, l,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and/or mixtures thereof; and solvent comprises water, methanol, ethanol, isopropanol, n- butanol, and/or mixtures thereof; polar aprotic solvent comprises dimethylformamide (DMF), dimethylsulfoxide (DMSO), tetrahydrofuran (THF), acetonitrile, acetone, ethyl acetate, N-methylpyrrolidone and non- polar solvents comprises hexane, benzene, toluene, 1,4-dioxane, chloroform, diethyl ether, dichloromethane (CH2CI2) and/or mixtures thereof.
An improved process for the preparation of Cefovecin sodium of a Compound of Formula (1):
wherein R is selected from hydrogen or carboxy protecting group; with a Compound of formula (8);
wherein Rl is substituted or unsubstituted mercaptobenzothiazole, substituted or unsubstituted mercaptobenzimidazole group; to produce a Compound of formula (la);
(b) optionally deprotecting the Compound of formula (la); and
(c) isolating Cefovecin sodium of a Compound of Formula (1). 5. The process according to claim 5, wherein said deprotection in step (b), is carried out using deprotecting agent and a solvent.
6. The process according to claim 6, deprotecting agent comprises sodium dithionite or a catalytic hydrogenating agent such as hydrogen gas over 10% palladium over carbon or tetrakis triphenyl phosphine palladium (0)
and solvent comprises acetone, water, tetrahydrofuran and/or mixtures thereof.
An improved process for the preparation of Cefovecin sodium of Compound of Formula (1):
(a) step of reacting THF Cephem Compound of Formula (6);
with MAEM Compound of Formula (8);
(b) optionally, purifying Cefovecin sodium of a Compound of Formula (1); and
(c) isolating Cefovecin sodium of a Compound of Formula (1).
8. The process according to preceding claims, the reaction of step (a) is carried out in the presence of a base and a solvent.
The process according to claim 8, base comprises potassium carbonate, lithium carbonate, sodium carbonate, sodium ethoxide, sodium bicarbonate, potassium bicarbonate, sodium hydroxide, potassium hydroxide, ammonium hydroxide, diisopropylamine, diisopropylethylamine triethylamine, dimethylamine, trimethyl amine, pyridine, l,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and/or mixtures thereof.
10. The process according to claim 8, solvent comprises water, methanol, ethanol, isopropanol, n-butanol, and/or mixtures thereof; polar aprotic solvent comprises dimethylformamide (DMF), dimethylsulfoxide (DMSO), tetrahydrofuran (THF), acetonitrile, acetone, ethyl acetate, N- methylpyrrolidone and non-polar solvents comprises hexane, benzene, toluene, 1,4-dioxane, chloroform, diethyl ether, dichloro methane (CH2CI2) and/or mixtures thereof.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN201741020734 | 2017-06-14 | ||
| IN201741020734 | 2017-06-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018229580A1 true WO2018229580A1 (en) | 2018-12-20 |
Family
ID=64659473
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2018/053773 WO2018229580A1 (en) | 2017-06-14 | 2018-05-28 | An improved process for the preparation of cefovecin sodium |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2018229580A1 (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002046198A1 (en) * | 2000-12-04 | 2002-06-13 | Pfizer Products Inc. | Coupling process and intermediates useful for preparing cephalosphorins |
| CN105254648A (en) * | 2015-11-13 | 2016-01-20 | 广东温氏大华农生物科技有限公司 | Convenia synthetic method and Convenia sodium salt synthetic method |
-
2018
- 2018-05-28 WO PCT/IB2018/053773 patent/WO2018229580A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002046198A1 (en) * | 2000-12-04 | 2002-06-13 | Pfizer Products Inc. | Coupling process and intermediates useful for preparing cephalosphorins |
| CN105254648A (en) * | 2015-11-13 | 2016-01-20 | 广东温氏大华农生物科技有限公司 | Convenia synthetic method and Convenia sodium salt synthetic method |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7345169B2 (en) | Process for the preparation of cephalosporin antibiotic | |
| US20040242557A1 (en) | Process for preparing cefdinir | |
| CN102372728B (en) | Synthesizing method for cephalosporin compound | |
| WO2008155615A2 (en) | An improved process for the preparation of cephalosporin antibiotic | |
| WO2002053563A1 (en) | Novel thioester derivatives of thiazolyl acetic acid and their use in the preparation of cephalosporin compounds | |
| WO2011042776A1 (en) | Process for preparation of cefotaxime acid and pharmaceutically acceptable salt thereof | |
| JP2006501305A (en) | Cefdinir intermediate salt | |
| US20040087786A1 (en) | Process for the preparation of 3-propenyl cephalosporin DMF solvate | |
| US9139597B2 (en) | Method for the production of ceftobiprole medocaril | |
| EP1546154A1 (en) | Process for the preparation of cefdinir | |
| EP1016665B1 (en) | Process for the selective preparation of z-isomers of 3-(2-substituted vinyl)cephalosporins | |
| EP1068211B1 (en) | Process for purification of a cephalosporin derivative | |
| US20180338981A1 (en) | Process for Preparing Ceftolozane from 7-Aminocephalosporanic Acid (7-ACA) | |
| WO2007013043A2 (en) | Processes for the preparation of 7-amino-3-vinyl cephalosporanic acid | |
| WO2006067803A1 (en) | A novel intermediate for the preparation of cefepime | |
| González et al. | An alternative procedure for preparation of cefdinir | |
| WO2011077217A1 (en) | An improved process for the preparation of cefpodoxime acid | |
| WO2008041100A1 (en) | Improved process for the preparation of cephalosporin antibiotics | |
| US6919449B2 (en) | Process for the preparation of cephalosporin intermediate and its use for the manufacture of cephalosporin compounds | |
| WO2018229580A1 (en) | An improved process for the preparation of cefovecin sodium | |
| WO2004037833A1 (en) | Process for the preparation of cephalosporin antibiotics | |
| US20060149055A1 (en) | Process for the manufacture of cefpodoxime proxetil | |
| US20050043531A1 (en) | Process for preparing cefepime | |
| WO2011042775A1 (en) | Process for preparation of cefotaxime acid | |
| Negi et al. | Studies on orally active cephalosporins I. Synthesis and structure-activity relationships of new 3-substituted carbamoyloxymethyl cephalosporins |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18818349 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 18818349 Country of ref document: EP Kind code of ref document: A1 |