WO2018009891A1 - Conductive conformal coatings - Google Patents
Conductive conformal coatings Download PDFInfo
- Publication number
- WO2018009891A1 WO2018009891A1 PCT/US2017/041236 US2017041236W WO2018009891A1 WO 2018009891 A1 WO2018009891 A1 WO 2018009891A1 US 2017041236 W US2017041236 W US 2017041236W WO 2018009891 A1 WO2018009891 A1 WO 2018009891A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- blend
- less
- ppm
- conductive polymer
- matrix material
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/24—Electrically-conducting paints
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C1/00—Tyres characterised by the chemical composition or the physical arrangement or mixture of the composition
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C1/00—Tyres characterised by the chemical composition or the physical arrangement or mixture of the composition
- B60C1/0016—Compositions of the tread
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C1/00—Tyres characterised by the chemical composition or the physical arrangement or mixture of the composition
- B60C1/0025—Compositions of the sidewalls
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J11/00—Features of adhesives not provided for in group C09J9/00, e.g. additives
- C09J11/08—Macromolecular additives
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J9/00—Adhesives characterised by their physical nature or the effects produced, e.g. glue sticks
- C09J9/02—Electrically-conducting adhesives
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/20—Conductive material dispersed in non-conductive organic material
- H01B1/24—Conductive material dispersed in non-conductive organic material the conductive material comprising carbon-silicon compounds, carbon or silicon
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C19/00—Tyre parts or constructions not otherwise provided for
- B60C19/08—Electric-charge-dissipating arrangements
Definitions
- Embodiments of the present invention generally relate to modification of resins, adhesives, varnishes and other conformal coating substances with a conductive polymer.
- a conductive polymer is mixed with a resin to create a top coat or composite that can conduct electricity for charge dissipation.
- the polymer is complexed with an appropriate molecule to promote good adhesion to a substrate surface.
- Metals have long been used for increasing the conductivity of materials. When added to a matrix material, such as paints, resins, and adhesives, the matrix material will be able to easily transport electrons. Electronic conductivity is required across a range of applications, including electrostatic dissipation for flooring and airplanes.
- Ni a common additive
- many conformal coating applications require a small particle size for the additives to be incorporated properly.
- Nano-metals and nano-carbons can have an increased health risk due to inhalation.
- embodiments of the current invention are directed towards compositions comprising physical blends comprising of conductive polymers, which may be used, for example, as coatings (e.g., conformal coating) for devices used in consumer electronics and other products.
- the blends comprise conductive polymers and a matrix material and exhibit desirable physical and electrical properties suitable for use in electronics devices.
- the matrix material may be any suitable material.
- the matrix material is a plastic, and in other embodiments the matrix material is a UV cured resin.
- the matrix material may be itself comprised of multiple materials.
- the matrix material is a paint or varnish that comprises nickel particles.
- the conductive polymer may be any suitable conductive polymer material.
- the conductive polymers comprise 3,4- ethylenedioxythiophene (EDOT), and in other embodiments the conductive polymers comprise a D50 of less than 1 um.
- EDOT 3,4- ethylenedioxythiophene
- the blend may comprise substantially the matrix material.
- the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 1% to 5%, and in other embodiments, the electrical conductivity of the blend is below 10 2 S/cm.
- the invention provides an electronic device that comprises a conformal coating or surface mount adhesive.
- Fig. 1 illustrates an exemplary consumer electronic article on which a conformal coating is applied according to certain embodiments.
- Conducting polymer refers to a polymeric substance which is capable of conducting electrons.
- the substance may be a conjugated polymer, such as PEDOT:PSS.
- the substance may be a polymer comprising conductive particles or dopants.
- “Matrix” or “matrix material” refers to any substance which may be combined with a conductive polymer.
- the matrix material may be a resin or a liquid solvent, such as water.
- the matrix comprises a solid, such as a powder.
- the matrix material comprises water, benzene, xylene, toluene, ethanol, methanol, ethyl-methyl-ketone, isopropanol, acetone or a combination thereof.
- “Spray polymer” refers to a substance which includes a polymer dispersed in a solvent.
- spray does not limit the substance for use in spraying for application.
- the substance may be applied to a surface through alternative, non-spray techniques such as spin coating and cloth transfer.
- PEDOT refers to a polymer comprising poly (3,4- ethylenedioxythiophene). In some embodiments, PEDOT consists of poly(3,4- ethylenedioxythiophene). Alternate varieties of PEDOT may be achieved through modification using dopants.
- Polymer refers to a macromolecule comprised of one or more structural repeating units.
- “Monomer” is a molecule that can be combined with itself or other molecules to form a polymer.
- Dopant is an element, molecule or compound that is inserted into a substance to purposefully modify physical, chemical, or performance characteristics.
- Impurity or “impurity element” refers to an undesired foreign substance (e.g., a chemical element) within a material which differs from the chemical composition of the base material.
- an impurity in a conductive polymer refers to any element or combination of elements which are present in the material itself, excluding dopants.
- a “powder” or “powder material” refers to a solid form of discreet particles.
- “Skeletal density” refers to the mass of a substance that occupies one cubic centimeter of volume, as measured using helium pycnometry.
- binding molecule is a chemical or compound that strongly attaches to another chemical or compound.
- EDOT refers to the compound 3,4-ethylenedioxythiophene.
- PEDOT:PSS refers to the polymer PEDOT, namely polype- ethyl enedioxythiophene), that is associated to the binding polymer molecule polystyrene- sulfonate (PSS).
- a “salt” is a neutral molecule or compound comprising a positively charged ion and a negatively charged ion.
- Oxide refers to a molecule or compound comprising an element or molecule that is bound to oxygen.
- Silane is a saturated chemical compounds comprising one or multiple silicon atoms linked to each other and/or to one or multiple atoms of other chemical elements.
- Chlorate refers to the C10 3 " anion.
- Chlorite refers to the C10 2 " anion.
- an “acid” is a molecule or compound capable of donating a proton to another molecule or compound.
- the definition can also include molecules or compounds capable of accepting a pair of electrons from another chemical or compound.
- the definition refers to any substance that is capable of lowering the pH of a solution. Acids include Arrhenius, Bransted and Lewis acids.
- a “solid acid” refers to a dried or granular compound that yields an acidic solution when dissolved in a solvent.
- the term “acidic” means having the properties of an acid.
- Frctional groups are the chemically reactive portions of a molecule.
- Hydroxyl refers to the -OH substituent.
- “Functionality” refers to the specific functional group present on a molecule or compound.
- solvent refers to a substance which dissolves, disperses or suspends materials. The materials may or may not undergo further reaction within the solvent.
- the present disclosure uses solvents both for the synthesis of the polymer material as well as the dispersion of the polymer for application as a film.
- Example solvents include, but are not limited to, acetone, ethanol, water, methanol, isopropanol, toluene, xylene, methyl ethyl ketone and benzene.
- Preferred Carrier Fluid or “PCF” refers to the chosen solvent for the application of the spray polymer material. The polymer is dispersed within the solvent.
- Frm or “coating” is a thin layer of material layered onto the surface of another.
- the material may or may not be chemically adhered to the surface of another.
- Substrate is a surface to which a coating is applied.
- the substrate can be modified prior to coating to increase mechanical properties such as adhesion.
- Dispossion is a mixture of a solid or polymer material within a solvent.
- the solvent can be aqueous or non-aqueous.
- “Amine” refers to a substituent of the formula -N(R) 2 , where each R is independently H, alkyl or aryl as defined herein.
- an “alcohol” is a compound including a -OH moiety.
- Ether refers to a compound of the formula ROR, where each R is independently H, alkyl or aryl as defined herein.
- Aliphatic is a family of compounds which contain open carbon chains. Examples include alkanes, alkenes, and alkynes.
- a "phenyl” is a compound which contains an aromatic, cyclic group of carbon atoms with alternating single and double bonds, C 6 H 5 . Examples include chlorobenzene and biphenyl.
- Anionic refers to a chemical species which has either gained an electron or lost a proton to form a negatively charged ion.
- a “surfactant” is a substance which reduces the surface tension between two materials and thus allows them to interact more intimately.
- a “fluoride” is a compound comprising at least one fluorine atom.
- An “ionic liquid” is a salt wherein the ions are poorly packed and thus the material is a liquid below 100 °C.
- “Monovalent” refers to an atom or ion that is capable of forming just one chemical bond.
- Niro refers to the -N0 2 substituent.
- polyol is a molecule or compound comprising more than one hydroxyl group available for reaction; these materials often serve as the precursor monomer of polyol polymers.
- Alkyl refers to a straight or branched hydrocarbon chain radical consisting solely of carbon and hydrogen atoms, which is saturated or unsaturated (i.e., contains one or more double and/or triple bonds), having from one to twelve carbon atoms (Ci-Ci 2 alkyl), preferably one to eight carbon atoms (Ci-C 8 alkyl) or one to six carbon atoms (Ci- C 6 alkyl), and which is attached to the rest of the molecule by a single bond, e.g., methyl, ethyl, ⁇ -propyl, 1-methylethyl (z ' so-propyl), «-butyl, «-pentyl, 1, 1-dimethylethyl (t-butyl), 3-methylhexyl, 2-methylhexyl, ethenyl, prop-l-enyl, but-l-enyl, pent-l-enyl,
- Alkyl includes alkenyls (one or more carbon-carbon double bonds) and alkynyls (one or more carbon-carbon triple bonds such as ethynyl and the like).
- Fluoroalkyl refers to an alkyl group comprising at least one fluoro substituent.
- Aliphatic refers to an alkyl group optionally containing one or more carbon-carbon double bond or carbon-carbon triple bond. Unless stated otherwise specifically in the specification, an alkyl and/or fluoroalkyl group is optionally substituted.
- Alkylene or “alkylene chain” refers to a straight or branched divalent hydrocarbon chain linking the rest of the molecule to a radical group, consisting solely of carbon and hydrogen, which is saturated or unsaturated (i.e., contains one or more double and/or triple bonds), and having from one to twelve carbon atoms, e.g., methylene, ethylene, propylene, «-butylene, ethenylene, propenylene, «-butenylene, propynylene, «-butynylene, and the like.
- the alkylene chain is attached to the rest of the molecule through a single or double bond and to the radical group through a single or double bond.
- the points of attachment of the alkylene chain to the rest of the molecule and to the radical group can be through one carbon or any two carbons within the chain. Unless stated otherwise specifically in the specification, an alkylene chain is optionally substituted.
- Alkoxy refers to a radical of the formula -OR a where R a is an alkyl radical as defined above containing one to twelve carbon atoms. Unless stated otherwise specifically in the specification, an alkoxy group is optionally substituted.
- Aryl refers to a carbocyclic ring system radical comprising hydrogen, 6 to 18 carbon atoms and at least one aromatic ring.
- the aryl radical is a monocyclic, bicyclic, tricyclic or tetracyclic ring system, which may include fused or bridged ring systems.
- Aryl radicals include, but are not limited to, aryl radicals derived from aceanthrylene, acenaphthylene, acephenanthrylene, anthracene, azulene, benzene, chrysene, fluoranthene, fluorene, as-indacene, s-indacene, indane, indene, naphthalene, phenalene, phenanthrene, pleiadene, pyrene, and triphenylene.
- substituted used herein means any of the above groups (e.g.., alkyl, alkylene, alkoxy and/or aryl) wherein at least one hydrogen atom (e.g., 1, 2, 3 or all hydrogen atoms) is replaced by a bond to a non-hydrogen atom such as, but not limited to: a halogen atom such as F, CI, Br, and I; an oxygen atom in groups such as hydroxyl groups, alkoxy groups, and ester groups; a sulfur atom in groups such as thiol groups, thioalkyl groups, sulfone groups, sulfonyl groups, and sulfoxide groups; a nitrogen atom in groups such as amines, amides, alkylamines, dialkylamines, arylamines, alkylarylamines, diarylamines, N-oxides, imides, and enamines; a silicon atom in groups such as trialkylsily
- “Substituted” also means any of the above groups in which one or more hydrogen atoms are replaced by a higher-order bond (e.g., a double- or triple- bond) to a heteroatom such as oxygen in oxo, carbonyl, carboxyl, and ester groups; and nitrogen in groups such as imines, oximes, hydrazones, and nitriles.
- a higher-order bond e.g., a double- or triple- bond
- nitrogen in groups such as imines, oximes, hydrazones, and nitriles.
- R g and R h are the same or different and
- alkyl independently hydrogen, alkyl, alkoxy, alkylaminyl, thioalkyl, aryl, aralkyl, cycloalkyl, cycloalkylalkyl, haloalkyl, heterocyclyl, N-heterocyclyl, heterocyclylalkyl, heteroaryl, N- heteroaryl and/or heteroarylalkyl.
- Substituted further means any of the above groups in which one or more hydrogen atoms are replaced by a bond to an aminyl, cyano, hydroxyl, imino, nitro, oxo, thioxo, halo, alkyl, alkoxy, alkylaminyl, thioalkyl, aryl, aralkyl, cycloalkyl, cycloalkylalkyl, haloalkyl, heterocyclyl, N-heterocyclyl, heterocyclylalkyl, heteroaryl, N-heteroaryl and/or heteroarylalkyl group.
- each of the foregoing substituents may also be optionally substituted with one or more of the above substituents.
- a " solvent” is a compound that contains at least one carbon and is liquid at room temperature.
- Example solvents include, but are not limited to, acetic acid, acetone, acetonitrile, benzene, chloroform, ethanol, methanol, N-methyl-2-pyrrolidinone, pentane, toluene, xylene, and butanol. solvents may be used as either a solvent for spray polymer dispersion or for film removal.
- An “acidic solution” is solution which has a pH less than 7.
- a “basic solution” is a solution which has a pH greater than 7.
- “Surface area” refers to the total specific surface area of a substance measurable by the BET technique. Surface area is typically expressed in units of m 2 /g.
- the BET (Brunauer/Emmett/Teller) technique employs an inert gas, for example nitrogen, to measure the amount of gas adsorbed on a material and is commonly used in the art to determine the accessible surface area of materials
- D(50) or “Dv50” or “average particle size” refers to the size of a particle as measured through methods known in the art, such as laser diffraction, wherein 50% of the volume of particles has a smaller particle size.
- Neutralizing agent is a substance which modifies the pH of a material or solution towards 7. In the instance of a material or solution that is acidic, the substance is basic. In the instance of a material or solution that is basic, the substance is acidic.
- “Haze” is defined as the percentage of incident light that is scattered away from a normally incident beam by the window.
- Color rendering index is a measurement of the degree to which light is the same color before and after passing through a medium.
- U-factor is a measurement of the rate of heat loss through the center of a transparent material. It is not relevant to non-transparent portions of windows, such as sashes and frames.
- Center of glass refers to the middle of a transparent material.
- the material does not need to be glass in composition and may include non-glass substances such as polymers.
- Ultraviolet refers to radiation with a wavelength less than 350 nm.
- the source of ultraviolet radiation may be natural (sunlight) or synthetically generated (light source).
- Infrared refers to radiation with a wavelength greater than 750 nm.
- the source of infrared radiation may be natural (sunlight) or synthetically generated (light source).
- Visible or “visible light” refers to radiation with a wavelength between 350 nm and 750 nm.
- the source of infrared radiation may be natural (sunlight) or synthetically generated (light source).
- a “semi-transparent" substrate is one which allows for the transmission of at least 5% of incoming visible light. It only refers to radiation in the visible spectrum.
- a “transparent” substrate is one which allows for the transmission of at least 50% of incoming visible light. It only refers to radiation in the visible spectrum.
- Electrode conductivity refers to the ability for a material to allow for electron flow.
- the conductive polymers can be designed with the purpose mixing with a matrix material to increase the electron flow of the blend.
- Viscosity refers to the resistance for a liquid substance to flow.
- the viscosity of a liquid may change with the addition of a conductive polymer, forming a blend.
- the present disclosure is directed to blends comprising conductive polymers and matrix materials.
- the blends result from physically mixing conductive polymers and matrix powders and thus have different properties than solid matrix materials, such as a formed plastic, embedded with conductive polymer.
- Embodiments of the disclosed blend comprise conductive polymers and matrix materials.
- the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material can be varied from 0.01% to 99.9%. In other various embodiments the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 0.01% to 20%, for example from 0.1% to 10% or from 1.0% to 2.0%. In other embodiments, the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 0.01% to 2%, from 0.5% to 2.5% or from 0.75% to 2.25%.
- the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 0.9% to 1.1%, from 1.1% to 1.3%, from 1.3% to 1.5%, from 1.5% to 1.7%, from 1.7% to 1.9% or from 1.9% to 2.1 %. In some embodiments, the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material is about 50%.
- the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 0.1% to 50%, from 0.1% to 10%, from 1% to 10%, from 1% to 5% or 1% to 3%). In still other embodiments, the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 50% to 99.9%, from 90% to 99.9% or from 90% to 99%.
- the volume percent of conductive polymers as a percentage of the total volume of conductive polymers and matrix material can be varied from 0.1% to 99.9%. In various embodiments the volume percent of conductive polymers as a percentage of the total volume of conductive polymers and matrix material ranges from 1% to 99%, from 2% to 99%, from 3% to 99%, from 4% to 99%, from 5% to 99%, from 6% to 99%, from 7% to 99%, from 8% to 99%, from 9% to 99%, from 10% to 90%, from 20% to 80%, from 20% to 40%, from 1% to 20%, from 40% to 80% or from 40% to 60%. In some certain embodiment the volume percent of conductive polymers as a percentage of the total volume of conductive polymers and matrix material is about 50%.
- the volume percent of conductive polymers as a percentage of the total volume of conductive polymers and matrix material ranges from 0.1 % to 50%, from 0.1 % to 10% or from 1 % to 10%. In other embodiments, the volume percent of conductive polymers as a percentage of the total volume of conductive polymers and matrix material ranges from 50% to 99.9%, from 90% to 99.9% or from 90% to 99%.
- the blend skeletal density is assessed using helium pycnometry, known to those skilled in the art. The blend skeletal density may range from 0.1 g/cc to 10 g/cc.
- the skeletal density of the blend is below 0.2 g/cc, below 0.3 g/cc, below 0.4 g/cc, below 0.5 g/cc, below 0.6 g/cc, below 0.7 g/cc, below 0.8 g/cc, below 0.9 g/cc, below 1.0 g/cc, below 1.1 g/cc, below 1.2 g/cc, below 1.3 g/cc, below 1.4 g/cc, below 1.5 g/cc, below 1.6 g/cc, below 1.7 g/cc, below 1.8 g/cc, below 1.9 g/cc, below 2.0 g/cc, below 2.1 g/cc, below 2.2 g/cc.
- the skeletal density of the blend is below 2.5 g/cc, below 3.0 g/cc, below 3.5 g/cc, below 4.0 g/cc, below 5.0 g/cc, below 7 g/cc, below 10 g/cc.
- the matrix material may be any material with a measurable viscosity. After the addition of conductive polymers to the matrix material, the resulting blend may have different rheology than the starting matrix material.
- the viscosity of the blend measured at 25°C can vary between 0.1 cP and 100,000 cP. In some embodiments, the viscosity of the blend measured at 25°C ranges from 0.1 cP to 1,000 cP, from 0.5 cP to 500 cP, from 1 cP to 500 cP, from 1 cP to 200 cP, from 1 cP to 100 cP, from 5 cP to 50 cP.
- the viscosity of the blend measured at 25°C ranges from 1 cP to 10,000 cP, from 1,000 cP to 10,000 cP, from 2,000 cP to 5,000 cP, or from 3,000 cP to 4,000 cP. In certain embodiments, the viscosity of the blend measured at 25°C is greater than 10,000 cP or less than 100,000 cP.
- the purity may directly influence the performance properties of the blend.
- the amount of individual trace elements can be determined using inductively coupled plasma optical emission spectrometry (ICP-OES).
- ICP-OES inductively coupled plasma optical emission spectrometry
- the level of scandium present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm.
- the level of titanium present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm.
- the level of vanadium present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm.
- the level of chromium present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm.
- the level of manganese present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of iron present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm.
- the level of cobalt present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of nickel present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm.
- the level of copper present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm.
- the level of zinc present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm.
- the level of silver present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of molybdenum present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm.
- the level of platinum present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of cadmium present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm.
- the sum of all ICP-OES impurities, excluding dopants, present in the blends is less than 100,000 ppm, less than 20,000 ppm, less than 10,000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, or less than 10 ppm.
- Embodiments of the blend comprise a population of conductive polymer particles and matrix material particles.
- the blend comprises conductive polymer powders suspended or dispersed in a liquid-form matrix material.
- the blend comprises a solid form embedded with conductive polymers.
- the solid form of matrix material includes, but is not limited to UV cured resin, thermoset resins, thermoset plastics, PVC, epoxy, or polyurethane, or combinations therein.
- the blend comprises a solid material. Accordingly, in one embodiment the blend comprises a BET surface area of at least 1 m 2 /g, at least 2
- the blend comprises a BET surface area of at least 50 m 2 /g, at least 100 m 2 /g, at least 150 m 2 /g, at least 200 m 2 /g,
- the blend comprises a BET surface area of at least 1000 m 2 /g.
- the conductive polymers are designed to optimize the conductivity of the matrix material as a blend.
- the electronic conductivity of the blend may range between 10 ' 4 S/cm to 10 3 S/cm. In one embodiment the electronic conductivity of the blend is between 1 S/cm and 10 3 S/cm, 1 S/cm and 10 2 S/cm, 50 S/cm and 100 S/cm. In another embodiment
- the electronic conductivity of the blend is between 10 "4 S/cm and 10 S/cm, 10 " 3 S/cm and 1 S/cm, 10 "2 S/cm and 1 S/cm.
- the electrical properties of the blend may also be measured using surface techniques, known to those skilled in the art.
- the surface resistance is particularly important as many of the potential applications involve films and coatings.
- the surface resistance of the blend may range between 1 and 1E9 ohms/sq.
- the application requires electrostatic dissipative-level resistances, between 1E5 and 1E9 ohms/sq.
- the application requires moderate current flowing resistances, between 1E3 and 1E5 ohms/sq.
- the application requires very low levels of resistance, between 1 and 1E3 ohms/sq for applications such as conductive inks.
- the electronic device is a CPU or motherboard.
- the electronic device is an airplane, automobile, bicycle, or motorcycle.
- the electronic device is a computer, tablet, or faceplate.
- the properties of the conductive polymer comprised within matrix materials can be varied in order to obtain desired physical properties, for example adhesion, and performance properties, for example conductivity.
- conductive polymers can be any type of polymer whose conductivity is achieved with organic compounds.
- the conductive polymers comprise EDOT, aniline, pyrrole, fluorene, thiophene or combinations thereof.
- the conductive polymers can comprise EDOT. In other embodiments, the conductive polymers can comprise pyrrole, such as polypyrrole.
- impurities or unreacted precursors could impact conductive properties of the conductive polymer.
- concentration of impurities or unreacted precursors is between 0 and 1 wt%, 0 and 2 wt%, 0 and 3 wt%, 0 and 4 wt%, 0 and 5 wt%.
- concentration of impurities or unreacted precursors is approximately 0.5 wt%.
- concentration of impurities or unreacted precursors is between 1 and 5 wt%, 2 and 4 wt%, 2 and 3 wt%.
- the concentration of impurities or unreacted precursors is less than 1 wt% or greater than 5 wt%. In still another embodiment the concentration of impurities or unreacted precursors is much higher, between 5 and 30%, 5 and 20%, 10 and 15%.
- the conductive polymers may be organic in nature, comprising oxygen, nitrogen, carbon, sulfur, or combinations therein.
- the conductive polymers comprise at least 30%> carbon, at least 40% carbon, at least 50% carbon, at least 60%) carbon, at least 70% carbon, at least 80%> carbon, or at least 90% carbon as measured by CHNO.
- the conductive polymers comprise at least 30% oxygen, at least 40% oxygen, at least 50% oxygen, at least 60% oxygen, at least 70% oxygen, at least 80% oxygen, or at least 90% oxygen as measured by CHNO.
- the conductive polymers comprise at least 1% nitrogen, at least 3% nitrogen, at least 5% nitrogen, at least 10% nitrogen, at least 15% nitrogen, at least 20% nitrogen, or at least 25% nitrogen as measured by CHNO.
- the conductive polymers comprise at least 1% sulfur, at least 3% sulfur, at least 5% sulfur, at least 10% sulfur, at least 15% sulfur, at least 20% sulfur, or at least 25% sulfur as measured by CHNO.
- the purity of the material may directly influence the performance properties of the conductive polymer. It is desired to minimize the amount of trace metal elements, excluding dopants.
- the amount of individual trace elements can be determined using inductively coupled plasma optical emission spectrometry (ICP-OES).
- ICP-OES inductively coupled plasma optical emission spectrometry
- the level of scandium present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the level of titanium present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the level of vanadium present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the level of chromium present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the level of manganese present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the level of iron present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of cobalt present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of nickel present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of iron present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some
- the level of copper present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the level of zinc present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the level of silver present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the level of molybdenum present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the level of platinum present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the level of cadmium present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm.
- the sum of all ICP-OES impurities, excluding dopants, present in the conductive polymers is less than 5,000 ppm, less than 2,000 ppm, less than 1,000 ppm, less than 500 ppm, less than 400 ppm, less than 300 ppm, less than 100 ppm, or less than 10 ppm.
- Conductive polymers may be purposefully doped to alter the performance properties, such as doping with nickel to increase the conductivity.
- the mass percent of dopant as a percentage of the total mass of the conductive polymer and dopant ranges from 0.01% to 50%.
- the mass percent of dopant as a percentage of the total mass of the conductive polymer and dopant is less than 0.1%, less than 0.5%), less than 1%, less than 1.5%, less than 2%, less than 2.5%, less than 3%, less than 3.5%), less than 4%, less than 5%, less than 6%, less than 7%, less than 8%, less than 10%, less than 15%, less than 20%, less than 30%, less than 40%, less than 50%.
- the dopant comprises nickel. In other embodiments, the dopant comprises, nickel, aluminum, tin, silver, copper, iron, zinc, cobalt, titanium, vanadium, lead, phosphorus, sulfur, platinum, or any combinations herein.
- the surface area of conductive polymers is directly related to the number of reactive sites in contact with matrix materials.
- the number of reactive sites between the conductive polymers and the matrix materials will change the physical and chemical properties of the blend.
- the conductive polymers comprise a BET surface area of at least 1 m 2 /g, at least 2 m 2 /g, at
- the conductive polymers comprise a BET surface area of at least 50 m 2 /g, at least 100 m 2 /g, at least 150 m 2 /g, at least 200 m 2 /g, at least 250 m 2 /g, at least 300 m 2 /g, at least 350 m 2 /g, at least 400 m 2 /g, at least 450 m 2 /g, or at least 500 m 2 /g.
- the conductive polymers comprise a BET surface area of at least 1000 m 2 /g.
- the performance of the blend may be impacted by the molecular weight of the conductive polymers.
- a lower molecular weight of the conductive polymers as measured by gel permeation chromatography (GPC) may yield lower blend conductivity when compared to higher molecular weight conductive polymers.
- the molecular weight of the conductive polymers as measured by GPC may vary between 100 g/mol and 100,000,000 g/mol. In one embodiment, the molecular weight of the conductive polymers as measured by GPC ranges from 100 g/mol to 100,000 g/mol, from 500 g/mol to 50,000 g/mol, or from 1,000 g/mol to 10,000 g/mol.
- the molecular weight of the conductive polymers as measured by GPC ranges from 100,000 g/mol to 1,000,000 g/mol, from 200,000 g/mol to 900,000 g/mol, from 200,000 g/mol to 500,000 g/mol, or from 500,000 g/mol to 900,000 g/mol. In still another embodiment, the molecular weight of the conductive polymers as measured by GPC ranges from 1,000,000 g/mol to 100,000,000 g/mol, from 1,000,000 g/mol to 50,000,000 g/mol, or from 2,000,000 g/mol to 10,000,000 g/mol.
- Dispersing the conductive polymers into water and measuring the pH of the resultant dispersion can determine the pH of a solid.
- the pH of the conductive polymers dispersed in water is between 6 and 8.
- the pH of the conductive polymers is less than 1, less than 2, less than 3, less than 4, less than 5, or less than 6.
- the pH of the conductive polymers is greater than 8, greater than 9, greater than 10, or greater than 11.
- the electronic conductivity may range between 10 "4 S/cm to 10 3 S/cm. In one embodiment the electronic conductivity of the conductive polymers is between 1 S/cm and 10 3 S/cm, 1 S/cm and 10 2 S/cm, 50 S/cm and 100 S/cm. In another embodiment the electronic conductivity of the conductive polymers is between 10 "4 S/cm and 10 S/cm, 10 "3 S/cm and 1 S/cm, 10 "2 S/cm and 1 S/cm.
- the average diameter, or D(50), of conductive polymers can be measured using methods known in the art, such as laser scattering techniques.
- the D(50) of conductive polymers is between 10 and 1000 nm, 10 and 500 nm, 20 and 300 nm, 20 and 50 nm, 50 and 200 nm, 100 and 150 nm.
- the D(50) of conductive polymers is approximately 200 nm.
- the D(50) of conductive polymers is between 200 and 1000 nm, 200 and 500 nm, 200 and 400 nm, 250 and 300 nm.
- the D(50) of the complex particle is less than 10 nm or greater than 1000 nm.
- the conductive polymers may be purposefully functionalized in order to preferentially bond to various substrates.
- the conductive polymer functionality determines the physical and chemical adhesion and reaction to substrate surfaces.
- the conductive polymers can be optimized to bind with specific substrate materials.
- the functionality of the conductive polymers can be identified using infrared spectroscopy, a method known to those of skill in the art. In one
- the functional group may contain silicon, such as silyl and disilanyl.
- the matrix material can have any form factor.
- the matrix material may be a resin or a liquid solvent, such as water.
- the matrix comprises a solid, such as a powder.
- the matrix material comprises water, benzene, xylene, toluene, ethanol, methanol, methyl-ethyl-ketone, isopropanol, acetone or combination thereof.
- the matrix material comprises a surfactant such as Span 20, Span 40, Span 60, Span 80, Span 83, Span 85, Span 120, Tween 20, Tween 21, Tween 40, Tween 60, Tween 61, Tween 65, Tween 80.
- the matrix material comprises a phenolic, polyester, epoxy, nitrile, latex, lacquer, polyurethane, polyether, polyethylene terephthalate, acrylonitrile butadiene styrene, polystyrene, polypropylene, polyethylene, polycarbonate, nylon, polyurethane, thermoplastic polyester, okra gum, pitch, galbanum, amino resins or combinations thereof.
- the matrix polymer comprises a gum resin, a synthetic resin, a thermoplastic resin, or a thermoset resin.
- the matrix material comprises zinc, aluminum, titanium, silver, nickel, chromium, copper, tin, or combinations therein.
- the matrix material comprises a combination of substances listed above.
- the matrix material is a powder.
- D(50), of particles of the matrix powder material can be measured using methods known in the art, such as laser scattering techniques.
- the D(50) of matrix powder material is between 10 and 1000 nm, 10 and 500 nm, 20 and 300 nm, 20 and 50 nm, 50 and 200 nm, 100 and 150 nm.
- the D(50) of matrix powder material is approximately 200 nm.
- the D(50) of matrix powder material is between 200 and 1000 nm, 200 and 500 nm, 200 and 400 nm, 250 and 300 nm.
- the D(50) of matrix powder material is less than 10 nm or greater than 1000 nm.
- the electronic conductivity of the matrix material may range between 10 "4 S/cm to 10 3 S/cm. In one embodiment the electronic conductivity of the matrix material is between 1 S/cm and 10 3 S/cm, 1 S/cm and 10 2 S/cm, 50 S/cm and 100 S/cm. In another embodiment the electronic conductivity of the matrix material is between 10 "4 S/cm and 10 S/cm, 10 "3 S/cm and 1 S/cm, 10 "2 S/cm and 1 S/cm.
- the matrix material may be doped with an additive to further improve the performance characteristics.
- the level of nickel doped into the matrix material is greater than 1 wt. %, greater than 2 wt.%, greater than 5 wt.%, greater than 10 wt.%, greater than 20 wt.%, or greater than 30 wt.%.
- the level of nickel doped into the matrix material is between 3 wt.% and 7 wt.%.
- the level of nickel doped into the matrix material is ⁇ 6 wt.%.
- the level of aluminum doped into the matrix material is greater than 1 wt.
- the level of titanium doped into the matrix material is greater than 1 wt. %, greater than 2 wt.%, greater than 5 wt.%, greater than 10 wt.%, greater than 20 wt.%, or greater than 30 wt.%. In certain embodiments, the level of titanium doped into the matrix material is greater than 1 wt. %, greater than 2 wt.%, greater than 5 wt.%, greater than 10 wt.%, greater than 20 wt.%, or greater than 30 wt.%. In certain
- the level of silver doped into the matrix material is greater than 1 wt. %, greater than 2 wt.%, greater than 5 wt.%, greater than 10 wt.%, greater than 20 wt.%, or greater than 30 wt.%. In still another embodiment, the level of silver doped into the matrix material is between 1 wt.% and 5 wt.%. In yet another embodiment, the level of silver doped into the matrix material is ⁇ 3 wt.%. In another embodiment, the dopant comprises a powder form.
- the dopant is carbon, such as carbon black, carbon nanotubes, activated carbon, graphite, graphene,
- the dopant comprises a metal oxide, such as zinc oxide, nickel (II) oxide, copper (IV) oxide, or molybdenum (III) oxide.
- the conductive polymers may be made from a polymer comprising a conjugated polymer base.
- the conjugated polymer can be synthesized by mixing a monomer (Component 1), a counter-ion (Component 2) and an oxidizing agent (Component 3).
- the conjugated polymer can be purchased from common commercial agencies and further modified.
- the monomer (Component 1), used to synthesize the conjugated polymer can be chosen from a range of materials known in the art. Not wishing to be bound by theory, the monomer can be important to tailoring the optically transparent properties of the spray on film.
- the initial monomer used to synthesize the conjugated polymer is EDOT, aniline, pyrrole, fluorene, thiophene or combinations thereof.
- the monomer can contain EDOT. In other embodiments, the monomer can contain pyrrole.
- Component 1 can be uniquely characterized through independent polymerization and measurement of molecular weight.
- the molecular weight of Component 1 after polymerization is above 1,000 g/mol, above 2,000 g/mol, above 5,000 g/mol, above 10,000 g/mol, above 20,000 g/mol, above 50,000 g/mol, above 150,000 g/mol, above 400,000 g/mol, above 1,000,000 g/mol, above 2,000,000 g/mol.
- the molecular weight of Component 1 after polymerization is below 5,000,000 g/mol.
- the counter-ion (Component 2), or dopant, when present, can be tailored for increased adhesion.
- the counter-ion itself comprises two
- Functionality A acts as an acidic functional group that complexes strongly with Component 1 while Functionality B is a glass-interactive functional group.
- Functionality A is sulfonic acid and Functionality B is maleic acid.
- the possible combinations of Functionality A and Functionality B are listed but not limited to the materials in Table 1.
- Component 2 includes polystyrene sulfonic acid- co-maleic acid. In other embodiments, Component 2 includes polyacrylic acid, polymaleic acid, polystyrene sulfonate, polyacrylic-co-maleic acid, polystyrene sulfonic acid-co- maleic acid, polyacrylic acid-co-polystyrene, polystyrene sulfonate-co-polyethylene oxide, and polystyrene sulfonate-co-polypropylene oxide.
- the weight ratio between Component 1 and Component 2 has been found to impact the final performance properties of the polymer in addition to altering the reaction kinetics.
- the weight ratio of Component 1 to Component 2 is between 2: 1 and 1 : 1000.
- the weight ratio of Component 1 to Component 2 is between 1 : 1 and 1 :500, 1 :2 and 1 : 100, 1 :3 and 1 :20, 1 :4 and 1 : 10.
- the weight ratio of Component 1 to Component 2 is between 2: 1 and 1 : 1, 1.8: 1 and 1.1 : 1, and 1 : 7 and 1.5: 1.
- the weight ratio of Component 1 to Component 2 is 1 :4.
- the solvent for the reaction can be altered for safety for both the user and the environment.
- the solvent used for synthesis is water.
- the solvents are water, ethanol, acetone, methanol, toluene, isopropanol, benzene, xylene, tetrahydrofuran, dichlorobenzene, chlorobenzene, methyl- ethyl-ketone, acetonitrile, chloroform, dichloromethane, or combinations thereof.
- the concentration of Component 1 when mixed with Component 2 in solution is chosen to facilitate the reaction with Component 3.
- the concentration of Component 1 when mixed with Component 2 in solution is between 0.05M and 5M.
- the concentration of Component 1 when mixed with Component 2 in solution is between 0.05M and 1M, 0.1M and 0.9M, 0.5M and 0.9M, 1M and 4M, 2M and 3M.
- the concentration of Component 1 when mixed with Component 2 in solution is less than 0.05M or greater than 5M.
- the concentration of Component 1 when mixed with Component 2 in solution is 0.08M.
- an oxidization agent To facilitate the synthesis of the polymeric material, an oxidization agent
- the oxidizing agent is sodium persulfate (NaPS), potassium persulfate (KPS), ferric chloride, sodium persulfate, or combinations thereof.
- NaPS sodium persulfate
- KPS potassium persulfate
- ferric chloride sodium persulfate
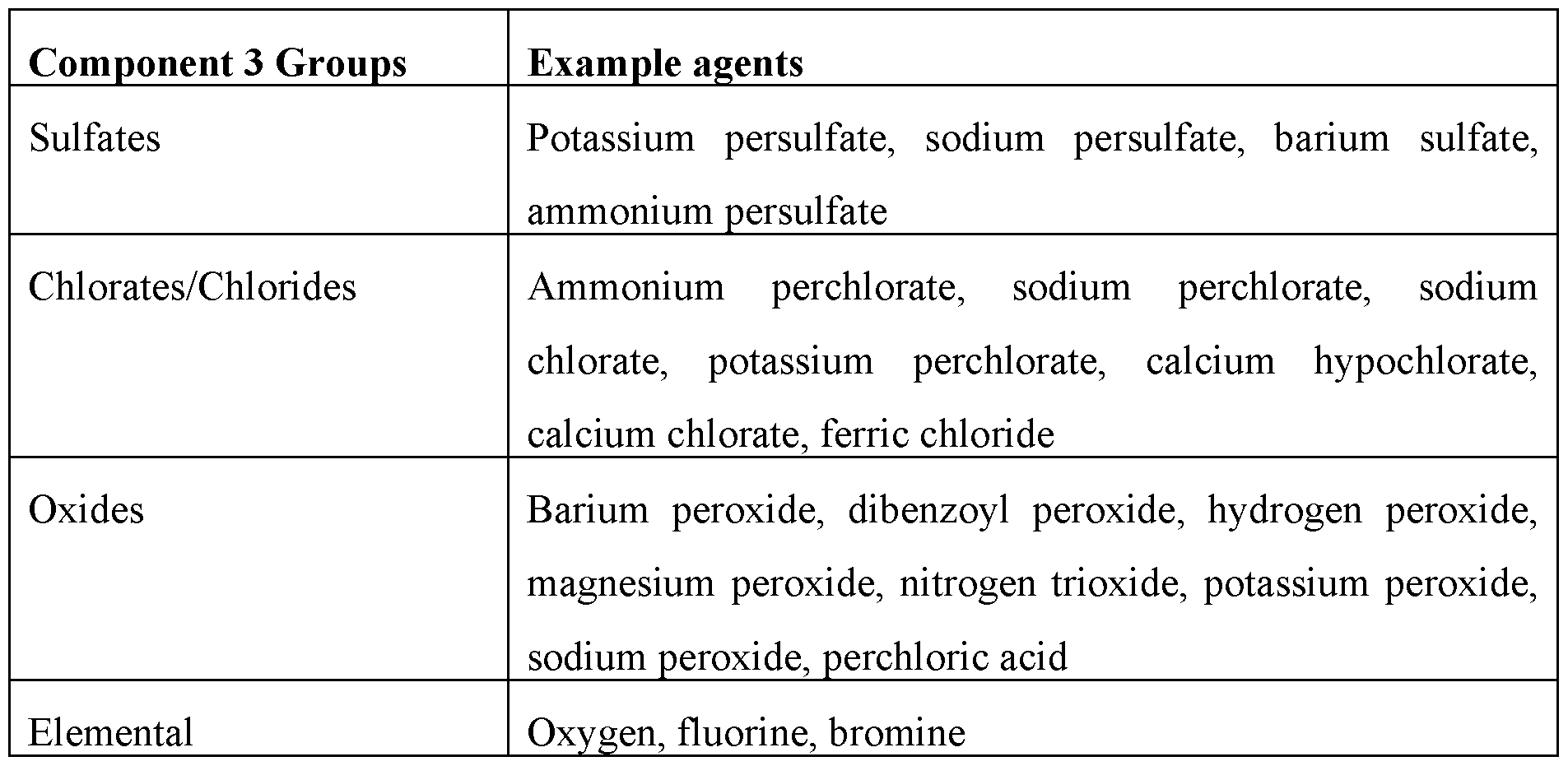
- the oxidizing agents refer to a large class of materials and are listed in Table 2. The oxidizing agent may be chosen for its reaction rate, solubility, and cost. In an additional embodiment, no oxidizing agent is used. Table 2.
- the ratio between Component 1 and Component 3 directly impacts the rate and extent of reaction.
- the molar ratio of Component 1 to Component 3 is between 5: 1 and 1 :5, 1 : 1 and 5: 1, 2: 1 and 3 : 1, 1 : 1 and 1 :5, 1 :2 and 1 :3.
- the molar ratio of Component 1 to Component 3 is—1 : 1.
- the combination of Component 1, Component 2 and Component 3 can be heated or cooled to directly impact the rate of reaction.
- the polymerization temperature of Mixture 1 is increased above 30 °C, above 40 °C, above 50 °C, above 60 °C, above 70 °C, above 100 °C, above 150 °C, above 200 °C, above 300 °C.
- the polymerization temperature of Mixture 1 is decreased below 30 °C, below 20 °C, below 10°C, below 0 °C.
- the temperature is held constant throughout the polymerization process.
- the temperature of Mixture 1 is dynamic.
- the time allowed for polymerization of Mixture 1 can be controlled to obtain ideal particle size.
- the time allowed for polymerization of Mixture 1 is between 5 minutes and 48 hours.
- the polymerization time for Mixture 1 is between 5 minutes and 10 hours, 30 minutes and 8 hours, 1 hour and 5 hours, 2 hours and 4 hours.
- the polymerization time for Mixture 1 is
- the polymerization time for Mixture 1 is between 10 hours and 48 hours, 12 hours and 24 hours, 16 hours and 20 hours. In still another embodiment the polymerization time for Mixture 1 is greater than 48 hours.
- Dopant 1 an extra dopant can be included in Mixture 1 prior to or after polymerization in order to supplement the performance characteristics and mechanical properties such as adhesion.
- Dopant 1 is a material with suitable electron withdrawing capacity such as ferric chloride, methyl sulfonic acid or tosylate.
- Dopant 1 is a combination of multiple materials as listed above. Possible dopants are listed but not limited to the materials in Table 3.
- Dopant 1 is typically added into solution after the combination of
- the concentration of Dopant 1 as a measurement of the total mass of Component 1, Component 2, Component 3, and solvent is between 0.001 and 1 wt%, 0.01 and 0.1 wt%, 0.05 and 0.1 wt%. In another embodiment the concentration of Dopant 1 is between 0.1 and 5%, 1 and 10%, 5 and 20%, 10 and 30%.
- the total concentration of dopants, as defined by the sum of the mass of all dopants, in Mixture 1 is between 0.001 and 1 wt%, 0.01 and 0.1 wt%, 0.05 and 0.1 wt%. In another embodiment the concentration of Dopant 1 is between 0.1 and 5%, 1 and 10%, 5 and 20%, 10 and 30%.
- the polymerized product after the reaction is acidic.
- the pH after the polymerization is between 1 and 6, 2 and 5, 2 and 4.
- Additive 1 can be a strong base such as, but not limited to, sodium hydroxide, potassium hydroxide, lithium hydroxide, calcium hydroxide, or barium hydroxide.
- Additive 1 can be a weak base such as, but not limited to, ammonia, ammonium hydroxide, pyridine, and trimethyl ammonia.
- the intermediate product may be dried. In one embodiment the intermediate product is dried using vacuum filtration, centrifuged, air dried, oven dried, or freeze dried. In another embodiment the intermediate product may never undergo a drying phase. Instead, the polymer dispersion may undergo a continuous solvent exchange.
- the complex can be commercially purchased or synthesized using methods known to those in the art.
- the commercially available material used as the complex is PEDOT:PSS, poly(p-phenylene vinylene), poly(3- hexylthiophene), poly(pyrrole), poly(fluorene), poly(aniline), and poly(acetylene).
- the commercially available complexes may be further modified using Dopant 1 or Additive 1 to yield a novel material.
- the methods of incorporation to form the blend involve mechanical mixing solids. In other embodiments, the methods of incorporation to form the blend involve dispersion within a liquid.
- the methods to form the blend depend on the initial form factor of the conductive polymers and the matrix material.
- the conductive polymers and the matrix material are both powder.
- the powders can be combined through mechanical mixing including hand mixing in a mortar and pestle, grinding, ball milling, or jet milling. Alternatively, the powders may be added together with no further mixing or post-processing.
- the conductive polymer is a powder and the matrix material is a liquid.
- additional processing such as dispersion, high shear mixing, stir bar mixing, shaking, or wet milling may be used to achieve a uniform consistency.
- the conductive polymer powder and liquid matrix material are combined with no further processing.
- the conductive polymer is a solid and the matrix material is a solid.
- the conductive polymer in a powder form is added to an epoxy resin under high shear to form the blend.
- the blend is then cured under thermal or UV conditions to create a solid, comprised of both the matrix material and conductive polymers.
- the matrix material is a monomer that undergoes cross-linking.
- the matrix material is fibrous. The conductive polymers are woven into the fabric threads to create a conductive fiber.
- the disclosed blends can be used as an electron transport material in any number of electronics devices.
- One such device is a computer part, for example a circuit board or CPU.
- the electrical conductivity of the blend allows for electrostatic dissipation across the surface of the device, though prevents shorting from occurring between terminals and parts.
- Figure 1 provides an illustration of a consumer electronic article.
- the consumer electronic article may include a surface mount adhesive 1, a thermal interface material 2, low pressure molding materials 3, a flip chip on board underfill 4, liquid encapsulant glob top 5, a silicone encapsulant 6, gasket compounds 7, chip scale package 8, flip chip air package underfill 9, a coating powder 10, mechanic molding compound 11, a potting compound 12, an optoelectronic 13, die attach 14, a conformal coating 15, photonic components 16, semiconductor mold compound 17, and solder 18.
- the disclosed blend may be used as the surface mount adhesive 1, thermal interface material 2, low pressure molding materials 3, a coating powder 10, a potting compound 12, a conformal coating 15, or a semiconductor mold compound 17.
- the blend When used as a conformal coating 15, the blend may be a flowable material that is poured, pasted, or sprayed onto the consumer electronics article. The coated flowable blend may then undergo further curing or drying creating a solid blend form.
- the blend as a solid conformal coating 15 may vary in both thickness and color, depending on the application. In one embodiment, the blend as a conformal coating 15 on circuit boards is less than 10 mils thick. In another embodiment, the blend as a conformal coating 15 on circuit boards is blue in color.
- the blend may be used as a surface mount adhesive 1, which provides binding function as well as an electrostatic dissipation function.
- the surface mount adhesive 1 will be able to conduct static charge build upon the surface of chips and parts while being insulative to continuous electron flow and shorting.
- Alternative uses of the blend may be for electrostatic dissipation (ESD), for transportation fields such as aerospace and automotive, for corrosion protection and inhibition, for flooring, for windows and transparent coatings, for EMI/RFI shielding, for conductive inks, for 3D printing, or for top coats.
- ESD electrostatic dissipation
- the matrix material of the blend is a rubber composition.
- any of the aforementioned conductive polymers, such as a conjugated polymer, or any of the aforementioned blends may be used in tires or other elastomer devices. It is sometimes desired to provide a tire with a rubber tread to promote reduced rolling resistance for the tire itself and thereby improved fuel economy for an associated vehicle, as well as reduced heat buildup in the tire tread during operation of the tire which, in turn, is expected to promote improved tire tread durability. To promote one or more of such desirable properties, it is sometimes desired to promote a reduction in the hysteretic property of the tread rubber.
- the tread rubber e.g. to promote a reduction in the rubber's physical rebound property
- a tire having a rubber composition comprising at least one diene-based elastomer, precipitated silica, and a conductive polymer, such as a conjugated polymer.
- a conductive polymer such as a conjugated polymer
- the composition has a surface resistivity ranging between 1 and 1 x 10 9 ohms/sq and the D50 particle size of the polymer is less than 1000 nm.
- the conductive polymer comprises a conjugated polymer.
- Tires with a tread made with a rubber composition including the conductive and/or conjugated polymers show low volume resistivity, indicating good ability to conduct static electricity.
- the composition has a volume resistivity that is less than 1 x 10 9 ohm-cm as measured by ASTM D257-98.
- the rubber composition and tread has a volume resistivity that is less than 1 x 10 " ohm-cm as measured by ASTM D257-98.
- the rubber composition can be tested (e.g., volume resistivity) and/or processed (e.g., compounded, vulcanized, mixed) using methods generally known in the art. For example, such methods are described in U.S. Pat. No. 9, 162,530 and U.S. Pat. App, 201 1/0146859, which are herein incorporated by reference in their entirety.
- the rubber composition can be compounded by methods such as mixing the various sulfur-vulcanizabie constituent rubbers with various commonly used additive materials such as, for example, curing aids, such as sulfur, activators, retarders and accelerators, processing additives, such as oils, resins including tackifying resins, silicas, and piasticizers, fillers, pigments, fatty acid, zinc oxide, waxes, antioxidants and antiozonants, peptizing agents and reinforcing fillers materials such as, for example, the aforementioned rubber reinforcing carbon black and precipitated silica.
- curing aids such as sulfur, activators, retarders and accelerators
- processing additives such as oils, resins including tackifying resins, silicas, and piasticizers
- fillers pigments, fatty acid, zinc oxide, waxes, antioxidants and antiozonants
- peptizing agents and reinforcing fillers materials such as, for example, the aforementioned rubber reinforcing carbon black and precipitated silica.
- Typical amounts of tackifier resins may, for example, comprise about 0.5 to about 10 phr, usually about 1 to about 5 phr.
- processing aids if used, may comprise, for example from about 1 to about 50 phr.
- processing aids can include, for example and where appropriate, aromatic, napthenic, and/or paraffinic processing oils.
- Typical amounts of antioxidants where used may comprise, for example, about 1 to about 5 phr. Representative antioxidants may be, for example, diphenyl-p- phenylenediamine and others, such as, for example, those disclosed in The Vcmderhik Rubber Handbook (1978), Pages 344 through 346.
- Typical amounts of antiozonants may comprise for example about 1 to 5 phr.
- Typical amounts of zinc oxide may comprise, for example, from about 1 to about 10 phr.
- Typical amounts of waxes, such as for example microcrystalline waxes, where used, may comprise, for example, from about 1 to about 5 phr.
- Typical amounts of peptizers, where used, may comprise, for example, from about 0.1 to about 1 phr.
- the vulcanization is conducted in the presence of a sulfur vulcanizing agent.
- suitable sulfur vulcanizing agents include elemental sulfur (free sulfur) or sulfur donating vulcanizing agents, for example, an amine disulfide, polymeric polysulfide or sulfur olefin adducts.
- the sulfur vulcanizing agent is elemental sulfur.
- sulfur vulcanizing agents may be used, for example, in an amount ranging from about 0.5 to about 4 phr, or even, in some circumstances, up to about 8 phr.
- Sulfur vulcanization accelerators are used to control the time and/or temperature required for vulcanization and to improve the properties of the vulcanizate.
- a single accelerator system may be used, i.e., primary accelerator.
- a primary acceierator(s) is used in total amounts ranging, for example, from about 0,5 to about 4, alternately about 0,8 to about 1.5 phr.
- combinations of a primary and a secondary accelerator might be used with the secondary accelerator, where used, being usually used in smaller amounts (for example about 0.05 to about 3 phr) in order to activate and to improve the properties of the vulcanizate.
- Combinations of these accelerators might be expected to produce a synergistic effect on the final properties and are somewhat better than those produced by use of either accelerator alone.
- delayed action accelerators may be used, for example, which are not affected by normal processing temperatures but produce a satisfactory cure at ordinary vulcanization temperatures.
- Vulcanization retarders might also be used, where desired or appropriate.
- Suitable types of accelerators that may be used in the present invention may be, for example, amines, disulfides, guanidines, thioureas, thiazoles, thiurams, sulfenamides, dithiocarbamates and xanthates.
- the primary accelerator is a sulfanamide.
- the secondary accelerator may be, for example, a guanidine, dithiocarbamate or thiuram compound.
- the mixing of the rubber composition can be accomplished by methods known to those having skill in the rubber mixing art.
- the ingredients are typically mixed in at least two stages, namely, at least one non-productive stage followed by a productive mix stage.
- the final curatives are typically mixed in the final stage which is conventionally called the "productive" mix stage in which the mixing typically occurs at a temperature, or ultimate temperature, lower than the mix temperature(s) than the preceding non-productive mix stage(s).
- the rubber, and reinforcing fillers, including the conjugated polymers and alternative additional reinforcing fillers such as, for example precipitated silica and rubber reinforcing carbon black mixed in one or more non- productive mix stages.
- the terms “non-productive” and “productive” mix stages are well known to those having skill in the rubber mixing art.
- the thermomechanical mixing step generally comprises a mechanical working in a mixer or extruder for a period of time suitable in order to produce a rubber temperature between 140° C. and 190° C.
- the appropriate duration of the thermomechanical working varies as a function of the operating conditions, and the volume and nature of the components. For example, the
- thermomechanical working may be from 1 to 20 minutes.
- the rubber composition may be incorporated in a variety of rubber components of the tire.
- the rubber component may be a tread (including tread cap and tread base), sidewall, apex, chafer, sidewall insert, wirecoat or innerliner.
- Vulcanization of the pneumatic tire of the present invention is generally carried out at conventional temperatures ranging from about 100° C. to 200° C. In one embodiment, the vulcanization is conducted at temperatures ranging from about 1 10° C. to 180° C. Any of the usual vulcanization processes may be used such as heating in a press or mold, heating with superheated steam or hot air.
- Such tires can be built, shaped, molded and cured by various methods which are known and will be readily apparent to those having skill in such art.
- the conjugated polymer dispersion was synthesized by mixing poly(4- styrenesulfonic acid-co-maleic acid), PSSA-co-MA, and water to create a 4.8 wt% solution.
- EDOT 3,4-ethylenedioxythiophene monomer
- PSSA-co-MA:EDOT weight ratio of 4: 1
- KPS potassium persulfate
- the mixture was heated to 50 °C and allowed to polymerize to completion (approximately 3-24 hours). The polymerization was deemed complete by a drastic change in color to dark blue.
- Table 4 shows various combinations of Component 1, Component 2 and Component 3 to prepare the conjugated polymer.
- the PEDOT dispersion can be further modified through additional dopants.
- Ferric chloride (FeCl 3 ) was added to the PEDOT dispersion in the amount of 0.1 wt% at room temperature. The dispersion and dopant were allowed to mix for 4 hours.
- the pH of the dispersion from Example 2 was measured, typically falling between 2 and 4. To raise the pH of the dispersion for safe handling and application, aqueous sodium hydroxide was slowly titrated into the dispersion while constantly stirring until the pH is between 6 and 8. Alternatively, other strong and weak bases can be used to achieve a similar outcome.
- the dispersion from Examples 1-3 can be further post-processed into the spray polymer solution product.
- 500 mL of dispersion was poured onto a vacuum filtration system using Whatman 602 H paper .
- the vacuum filtration was allowed to continue until complete solvent removal. Further drying was performed in air on a hot plate or oven at 50 °C.
- the dispersion from Examples 1-3 can also be left as a liquid dispersion or a thick paste. Approximately 1 gram of dispersion or paste was weighed and placed onto a hot plate overnight. The sample was heated to at least 50 °C. After the sample was cooled and fully dried, it was weighed and the amount of solids was calculated from the percent weight loss.
- polyester was added to a beaker of 0.5 grams of conductive powder.
- a high shear mixer was used for 10-30 minutes until the coating was free from larger particles.
- a Hegman grind gauge was used to assess the particle size and dispersion quality.
- the powder may be dispersed first in xylene or a compatible solvent to assist in powder dispersion within the matrix.
- the conductive coating may be made from a paste or dispersion, from
- Example X A SkyDrop Resistant Clear Polyurethane Topcoat was chosen as the matrix material. A paste was added at a loading of 2.6 wt% and mixed in the presence of 30 wt% IPA.
- Example 6 The coating was applied from Example 6 using a doctor blade at a wet height of 8 mils on polyethylene or PET. The coating was allowed to cure at room temperature for greater than 15 hours or until no longer tacky. Length of cure and dry conditions may vary on the matrix material manufacturer requirements.
- EXAMPLE 8 The coating was applied from Example 6 using a doctor blade at a wet height of 8 mils on polyethylene or PET. The coating was allowed to cure at room temperature for greater than 15 hours or until no longer tacky. Length of cure and dry conditions may vary on the matrix material manufacturer requirements.
- exemplary coatings are depicted in Table 5.
- the conductive polymer powder is capable of reaching ESD-performance sheet resistivity at only 3.2 wt%. Further performance can be achieved through higher loadings of powder to 5 wt%.
- Properties of exemplary coatings in polyurethane are listed in Table 6.
- Exemplary coatings in additional matrices are shown in Table 7.
- V35 a PEDOT:DBSA:NaPS conductive polymer
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Mechanical Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Materials Engineering (AREA)
- Wood Science & Technology (AREA)
- Physics & Mathematics (AREA)
- Dispersion Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Paints Or Removers (AREA)
Abstract
Blends comprising a conductive polymer and a matrix material are provided. The blends find utility in a number of applications, including use as a conformal coating on consumer electronic articles.
Description
CONDUCTIVE CONFORMAL COATINGS
BACKGROUND
Field
Embodiments of the present invention generally relate to modification of resins, adhesives, varnishes and other conformal coating substances with a conductive polymer. In embodiments of the present invention, a conductive polymer is mixed with a resin to create a top coat or composite that can conduct electricity for charge dissipation. In some embodiments the polymer is complexed with an appropriate molecule to promote good adhesion to a substrate surface. Description of the Related Art
Metals have long been used for increasing the conductivity of materials. When added to a matrix material, such as paints, resins, and adhesives, the matrix material will be able to easily transport electrons. Electronic conductivity is required across a range of applications, including electrostatic dissipation for flooring and airplanes.
Unfortunately, the use of metals has fallen out of favor due to health hazards and manufacturing dangers. For example, nickel, a common additive, is also a known carcinogen. In addition, many conformal coating applications require a small particle size for the additives to be incorporated properly. Nano-metals and nano-carbons can have an increased health risk due to inhalation.
As metals begin to be phased out of products, a new alternative will be required to maintain the physical and chemical properties of resins and other materials, while meeting more rigorous regulations and standards. Embodiments of the present invention provide this and related advantages.
BRIEF SUMMARY
In general terms, embodiments of the current invention are directed towards compositions comprising physical blends comprising of conductive polymers, which may be used, for example, as coatings (e.g., conformal coating) for devices used in consumer electronics and other products. In some embodiments, the blends comprise conductive polymers and a matrix material and exhibit desirable physical and electrical properties suitable for use in electronics devices. The matrix material may be any suitable material. For example, in some embodiments, the matrix material is a plastic, and in other embodiments the matrix material is a UV cured resin. The matrix material may be itself comprised of multiple materials. For example, in one embodiment, the matrix material is a paint or varnish that comprises nickel particles.
The conductive polymer may be any suitable conductive polymer material. For example, in some embodiments the conductive polymers comprise 3,4- ethylenedioxythiophene (EDOT), and in other embodiments the conductive polymers comprise a D50 of less than 1 um.
The blend may comprise substantially the matrix material. For example, in another embodiment, the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 1% to 5%, and in other embodiments, the electrical conductivity of the blend is below 102 S/cm.
In still other embodiments, the invention provides an electronic device that comprises a conformal coating or surface mount adhesive.
These and other aspects of the invention will be apparent upon reference to the following detailed description. To this end, various references are set forth herein which describe in more detail certain background information, procedures, compounds and/or compositions, and are each hereby incorporated by reference in their entirety.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 illustrates an exemplary consumer electronic article on which a conformal coating is applied according to certain embodiments.
DETAILED DESCRIPTION
In the following description, certain specific details are set forth in order to provide a thorough understanding of various embodiments. However, one skilled in the art will understand that the invention may be practiced without these details. In other instances, well-known structures have not been shown or described in detail to avoid unnecessarily obscuring descriptions of the embodiments. Unless the context requires otherwise, throughout the specification and claims which follow, the word "comprise" and variations thereof, such as, "comprises" and "comprising" are to be construed in an open, inclusive sense, that is, as "including, but not limited to."
Further, headings provided herein are for convenience only and do not interpret the scope or meaning of the claimed invention.
Reference throughout this specification to "one embodiment" or "an embodiment" means that a particular feature, structure or characteristic described in connection with the embodiment is included in at least one embodiment. Thus, the appearances of the phrases "in one embodiment" or "in an embodiment" in various places throughout this specification are not necessarily all referring to the same embodiment. Furthermore, the particular features, structures, or characteristics may be combined in any suitable manner in one or more embodiments. Also, as used in this specification and the appended claims, the singular forms "a," "an," and "the" include plural referents unless the content clearly dictates otherwise. It should also be noted that the term "or" is generally employed in its sense including "and/or" unless the content clearly dictates otherwise.
Definitions
As used herein, and unless the context dictates otherwise, the following terms have the meanings as specified below.
"Conducting polymer" refers to a polymeric substance which is capable of conducting electrons. For example the substance may be a conjugated polymer, such as PEDOT:PSS. Alternatively, the substance may be a polymer comprising conductive particles or dopants.
"Matrix" or "matrix material" refers to any substance which may be combined with a conductive polymer. For example, the matrix material may be a resin or a liquid solvent, such as water. In some embodiments the matrix comprises a solid, such as a powder. In certain embodiments, the matrix material comprises water, benzene, xylene, toluene, ethanol, methanol, ethyl-methyl-ketone, isopropanol, acetone or a combination thereof.
"Spray polymer" refers to a substance which includes a polymer dispersed in a solvent. The term "spray" does not limit the substance for use in spraying for application. The substance may be applied to a surface through alternative, non-spray techniques such as spin coating and cloth transfer.
"PEDOT" refers to a polymer comprising poly (3,4- ethylenedioxythiophene). In some embodiments, PEDOT consists of poly(3,4- ethylenedioxythiophene). Alternate varieties of PEDOT may be achieved through modification using dopants.
"Polymer" refers to a macromolecule comprised of one or more structural repeating units.
"Monomer" is a molecule that can be combined with itself or other molecules to form a polymer.
"Dopant" is an element, molecule or compound that is inserted into a substance to purposefully modify physical, chemical, or performance characteristics.
"Impurity" or "impurity element" refers to an undesired foreign substance (e.g., a chemical element) within a material which differs from the chemical composition of the base material. For example, an impurity in a conductive polymer refers to any element or combination of elements which are present in the material itself, excluding dopants.
A "powder" or "powder material" refers to a solid form of discreet particles.
"Skeletal density" refers to the mass of a substance that occupies one cubic centimeter of volume, as measured using helium pycnometry.
A "binding molecule" is a chemical or compound that strongly attaches to another chemical or compound.
"EDOT" refers to the compound 3,4-ethylenedioxythiophene.
"PEDOT:PSS" refers to the polymer PEDOT, namely polype- ethyl enedioxythiophene), that is associated to the binding polymer molecule polystyrene- sulfonate (PSS).
A "salt" is a neutral molecule or compound comprising a positively charged ion and a negatively charged ion.
An "oxide" refers to a molecule or compound comprising an element or molecule that is bound to oxygen.
"Silane" is a saturated chemical compounds comprising one or multiple silicon atoms linked to each other and/or to one or multiple atoms of other chemical elements.
"Sulfate" refers to the -OSO3H substituent.
"Sulfonate" refers to the -SO3H substituent.
"Amide" refers to a substituent of the formula -C(=0)N(R)2 or -NRC(=0)R, where each R is independently H, alkyl or aryl as defined herein.
"Ester" refers to a substituent of the formula -OC(=0)R or -C(=0)OR, where R is alkyl or aryl as defined herein.
"Chlorate" refers to the C103 " anion.
"Chlorite" refers to the C102 " anion.
An "acid" is a molecule or compound capable of donating a proton to another molecule or compound. The definition can also include molecules or compounds capable of accepting a pair of electrons from another chemical or compound. The definition refers to any substance that is capable of lowering the pH of a solution. Acids include Arrhenius, Bransted and Lewis acids. A "solid acid" refers to a dried or granular compound that yields an acidic solution when dissolved in a solvent. The term "acidic" means having the properties of an acid.
"Functional groups" are the chemically reactive portions of a molecule.
"Hydroxyl" refers to the -OH substituent.
"Functionality" refers to the specific functional group present on a molecule or compound.
"Solvent" refers to a substance which dissolves, disperses or suspends materials. The materials may or may not undergo further reaction within the solvent. The present disclosure uses solvents both for the synthesis of the polymer material as well as the dispersion of the polymer for application as a film. Example solvents include, but are not limited to, acetone, ethanol, water, methanol, isopropanol, toluene, xylene, methyl ethyl ketone and benzene.
"Preferred Carrier Fluid" or "PCF" refers to the chosen solvent for the application of the spray polymer material. The polymer is dispersed within the solvent.
"Film" or "coating" is a thin layer of material layered onto the surface of another. The material may or may not be chemically adhered to the surface of another.
"Substrate" is a surface to which a coating is applied. The substrate can be modified prior to coating to increase mechanical properties such as adhesion.
"Dispersion" is a mixture of a solid or polymer material within a solvent.
The solvent can be aqueous or non-aqueous.
"Amine" refers to a substituent of the formula -N(R)2, where each R is independently H, alkyl or aryl as defined herein.
An "alcohol" is a compound including a -OH moiety.
"Ether" refers to a compound of the formula ROR, where each R is independently H, alkyl or aryl as defined herein.
"Aliphatic" is a family of compounds which contain open carbon chains. Examples include alkanes, alkenes, and alkynes.
A "phenyl" is a compound which contains an aromatic, cyclic group of carbon atoms with alternating single and double bonds, C6H5. Examples include chlorobenzene and biphenyl.
"Anionic" refers to a chemical species which has either gained an electron or lost a proton to form a negatively charged ion.
A "surfactant" is a substance which reduces the surface tension between two materials and thus allows them to interact more intimately.
A "fluoride" is a compound comprising at least one fluorine atom.
"Imide" refers to substituent of the formula -C(=0) RC(=0)R' substituent, where R is H, alkyl or aryl, and R' is alkyl or aryl as defined herein.
An "ionic liquid" is a salt wherein the ions are poorly packed and thus the material is a liquid below 100 °C.
"Ketone" refers to the -C(=0)R substituent, where R is alkyl or aryl as defined herein.
"Monovalent" refers to an atom or ion that is capable of forming just one chemical bond.
"Nitrile" refers to the -CN substituent.
"Nitro" refers to the -N02 substituent.
A "polyol" is a molecule or compound comprising more than one hydroxyl group available for reaction; these materials often serve as the precursor monomer of polyol polymers.
"Sulfoxide" refers to the -S(=0)R substituent, where R is alkyl or aryl as defined herein.
"Alkyl" refers to a straight or branched hydrocarbon chain radical consisting solely of carbon and hydrogen atoms, which is saturated or unsaturated (i.e., contains one or more double and/or triple bonds), having from one to twelve carbon atoms (Ci-Ci2 alkyl), preferably one to eight carbon atoms (Ci-C8 alkyl) or one to six carbon atoms (Ci- C6 alkyl), and which is attached to the rest of the molecule by a single bond, e.g., methyl, ethyl, ^-propyl, 1-methylethyl (z'so-propyl), «-butyl, «-pentyl, 1, 1-dimethylethyl (t-butyl), 3-methylhexyl, 2-methylhexyl, ethenyl, prop-l-enyl, but-l-enyl, pent-l-enyl,
penta-l,4-dienyl, ethynyl, propynyl, butynyl, pentynyl, hexynyl, and the like. Alkyl includes alkenyls (one or more carbon-carbon double bonds) and alkynyls (one or more carbon-carbon triple bonds such as ethynyl and the like). "Fluoroalkyl" refers to an alkyl group comprising at least one fluoro substituent. "Aliphatic" refers to an alkyl group
optionally containing one or more carbon-carbon double bond or carbon-carbon triple bond. Unless stated otherwise specifically in the specification, an alkyl and/or fluoroalkyl group is optionally substituted.
"Alkylene" or "alkylene chain" refers to a straight or branched divalent hydrocarbon chain linking the rest of the molecule to a radical group, consisting solely of carbon and hydrogen, which is saturated or unsaturated (i.e., contains one or more double and/or triple bonds), and having from one to twelve carbon atoms, e.g., methylene, ethylene, propylene, «-butylene, ethenylene, propenylene, «-butenylene, propynylene, «-butynylene, and the like. The alkylene chain is attached to the rest of the molecule through a single or double bond and to the radical group through a single or double bond. The points of attachment of the alkylene chain to the rest of the molecule and to the radical group can be through one carbon or any two carbons within the chain. Unless stated otherwise specifically in the specification, an alkylene chain is optionally substituted.
"Alkoxy" refers to a radical of the formula -ORa where Ra is an alkyl radical as defined above containing one to twelve carbon atoms. Unless stated otherwise specifically in the specification, an alkoxy group is optionally substituted.
"Aryl" refers to a carbocyclic ring system radical comprising hydrogen, 6 to 18 carbon atoms and at least one aromatic ring. For purposes of this invention, the aryl radical is a monocyclic, bicyclic, tricyclic or tetracyclic ring system, which may include fused or bridged ring systems. Aryl radicals include, but are not limited to, aryl radicals derived from aceanthrylene, acenaphthylene, acephenanthrylene, anthracene, azulene, benzene, chrysene, fluoranthene, fluorene, as-indacene, s-indacene, indane, indene, naphthalene, phenalene, phenanthrene, pleiadene, pyrene, and triphenylene. Unless stated otherwise specifically in the specification, the term "aryl" or the prefix "ar-" (such as in "aralkyl") is meant to include aryl radicals that are optionally substituted.
The term "substituted" used herein means any of the above groups (e.g.., alkyl, alkylene, alkoxy and/or aryl) wherein at least one hydrogen atom (e.g., 1, 2, 3 or all hydrogen atoms) is replaced by a bond to a non-hydrogen atom such as, but not limited to: a halogen atom such as F, CI, Br, and I; an oxygen atom in groups such as hydroxyl groups,
alkoxy groups, and ester groups; a sulfur atom in groups such as thiol groups, thioalkyl groups, sulfone groups, sulfonyl groups, and sulfoxide groups; a nitrogen atom in groups such as amines, amides, alkylamines, dialkylamines, arylamines, alkylarylamines, diarylamines, N-oxides, imides, and enamines; a silicon atom in groups such as trialkylsilyl groups, dialkylarylsilyl groups, alkyldiarylsilyl groups, and triarylsilyl groups; and other heteroatoms in various other groups. In some embodiments, "substituted" means that at least one hydrogen atom is replaced with a bond to -OH, -SH, -C02H, -OPO3H, -PO3H, -OSO3H, -SO3H or -C(=N)OH. "Substituted" also means any of the above groups in which one or more hydrogen atoms are replaced by a higher-order bond (e.g., a double- or triple- bond) to a heteroatom such as oxygen in oxo, carbonyl, carboxyl, and ester groups; and nitrogen in groups such as imines, oximes, hydrazones, and nitriles. For example,
"substituted" includes any of the above groups in which one or more hydrogen atoms are replaced with - RgRh, - RgC(=0)Rh, - RgC(=0) RgRh, - RgC(=0)ORh, - RgS02Rh, -OC(=0) RgRh, -ORg, -SRg, -SORg, -S02Rg, -OS02Rg, -S02ORg, =NS02Rg,
or -S02NRgRh. "Substituted also means any of the above groups in which one or more hydrogen atoms are replaced with -C(=0)Rg, -C(=0)ORg, -C(=0) RgRh, -CH2S02Rg, or -CH2S02NRgRh. In the foregoing, Rg and Rh are the same or different and
independently hydrogen, alkyl, alkoxy, alkylaminyl, thioalkyl, aryl, aralkyl, cycloalkyl, cycloalkylalkyl, haloalkyl, heterocyclyl, N-heterocyclyl, heterocyclylalkyl, heteroaryl, N- heteroaryl and/or heteroarylalkyl. "Substituted" further means any of the above groups in which one or more hydrogen atoms are replaced by a bond to an aminyl, cyano, hydroxyl, imino, nitro, oxo, thioxo, halo, alkyl, alkoxy, alkylaminyl, thioalkyl, aryl, aralkyl, cycloalkyl, cycloalkylalkyl, haloalkyl, heterocyclyl, N-heterocyclyl, heterocyclylalkyl, heteroaryl, N-heteroaryl and/or heteroarylalkyl group. In addition, each of the foregoing substituents may also be optionally substituted with one or more of the above substituents.
A " solvent" is a compound that contains at least one carbon and is liquid at room temperature. Example solvents include, but are not limited to, acetic acid, acetone, acetonitrile, benzene, chloroform, ethanol, methanol, N-methyl-2-pyrrolidinone, pentane,
toluene, xylene, and butanol. solvents may be used as either a solvent for spray polymer dispersion or for film removal.
An "acidic solution" is solution which has a pH less than 7.
A "basic solution" is a solution which has a pH greater than 7. "Surface area" refers to the total specific surface area of a substance measurable by the BET technique. Surface area is typically expressed in units of m2/g. The BET (Brunauer/Emmett/Teller) technique employs an inert gas, for example nitrogen, to measure the amount of gas adsorbed on a material and is commonly used in the art to determine the accessible surface area of materials
"D(50)" or "Dv50" or "average particle size" refers to the size of a particle as measured through methods known in the art, such as laser diffraction, wherein 50% of the volume of particles has a smaller particle size.
"Neutralizing agent" is a substance which modifies the pH of a material or solution towards 7. In the instance of a material or solution that is acidic, the substance is basic. In the instance of a material or solution that is basic, the substance is acidic.
"Haze" is defined as the percentage of incident light that is scattered away from a normally incident beam by the window.
"Color rendering index" is a measurement of the degree to which light is the same color before and after passing through a medium.
"U-factor" is a measurement of the rate of heat loss through the center of a transparent material. It is not relevant to non-transparent portions of windows, such as sashes and frames.
"Center of glass" or "COG" refers to the middle of a transparent material. The material does not need to be glass in composition and may include non-glass substances such as polymers.
"Ultraviolet" refers to radiation with a wavelength less than 350 nm. The source of ultraviolet radiation may be natural (sunlight) or synthetically generated (light source).
"Infrared" refers to radiation with a wavelength greater than 750 nm. The source of infrared radiation may be natural (sunlight) or synthetically generated (light source).
"Visible" or "visible light" refers to radiation with a wavelength between 350 nm and 750 nm. The source of infrared radiation may be natural (sunlight) or synthetically generated (light source).
A "semi-transparent" substrate is one which allows for the transmission of at least 5% of incoming visible light. It only refers to radiation in the visible spectrum.
A "transparent" substrate is one which allows for the transmission of at least 50% of incoming visible light. It only refers to radiation in the visible spectrum.
"Electronic conductivity" refers to the ability for a material to allow for electron flow. The conductive polymers can be designed with the purpose mixing with a matrix material to increase the electron flow of the blend.
"Viscosity" refers to the resistance for a liquid substance to flow. The viscosity of a liquid may change with the addition of a conductive polymer, forming a blend.
A. Blends of Conductive Polymer and Matrix Materials
In one embodiment, the present disclosure is directed to blends comprising conductive polymers and matrix materials. In certain embodiments, the blends result from physically mixing conductive polymers and matrix powders and thus have different properties than solid matrix materials, such as a formed plastic, embedded with conductive polymer.
Embodiments of the disclosed blend comprise conductive polymers and matrix materials. The mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material can be varied from 0.01% to 99.9%. In other various embodiments the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 0.01% to 20%, for example from 0.1% to 10% or from 1.0% to 2.0%. In other embodiments, the mass percent
of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 0.01% to 2%, from 0.5% to 2.5% or from 0.75% to 2.25%. In some other embodiments, the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 0.9% to 1.1%, from 1.1% to 1.3%, from 1.3% to 1.5%, from 1.5% to 1.7%, from 1.7% to 1.9% or from 1.9% to 2.1 %. In some embodiments, the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material is about 50%.
Alternatively, in other embodiments the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 0.1% to 50%, from 0.1% to 10%, from 1% to 10%, from 1% to 5% or 1% to 3%). In still other embodiments, the mass percent of conductive polymers as a percentage of the total mass of conductive polymers and matrix material ranges from 50% to 99.9%, from 90% to 99.9% or from 90% to 99%.
The volume percent of conductive polymers as a percentage of the total volume of conductive polymers and matrix material can be varied from 0.1% to 99.9%. In various embodiments the volume percent of conductive polymers as a percentage of the total volume of conductive polymers and matrix material ranges from 1% to 99%, from 2% to 99%, from 3% to 99%, from 4% to 99%, from 5% to 99%, from 6% to 99%, from 7% to 99%, from 8% to 99%, from 9% to 99%, from 10% to 90%, from 20% to 80%, from 20% to 40%, from 1% to 20%, from 40% to 80% or from 40% to 60%. In some certain embodiment the volume percent of conductive polymers as a percentage of the total volume of conductive polymers and matrix material is about 50%.
In other alternative embodiments, the volume percent of conductive polymers as a percentage of the total volume of conductive polymers and matrix material ranges from 0.1 % to 50%, from 0.1 % to 10% or from 1 % to 10%. In other embodiments, the volume percent of conductive polymers as a percentage of the total volume of conductive polymers and matrix material ranges from 50% to 99.9%, from 90% to 99.9% or from 90% to 99%.
The blend skeletal density is assessed using helium pycnometry, known to those skilled in the art. The blend skeletal density may range from 0.1 g/cc to 10 g/cc. In certain embodiments, the skeletal density of the blend is below 0.2 g/cc, below 0.3 g/cc, below 0.4 g/cc, below 0.5 g/cc, below 0.6 g/cc, below 0.7 g/cc, below 0.8 g/cc, below 0.9 g/cc, below 1.0 g/cc, below 1.1 g/cc, below 1.2 g/cc, below 1.3 g/cc, below 1.4 g/cc, below 1.5 g/cc, below 1.6 g/cc, below 1.7 g/cc, below 1.8 g/cc, below 1.9 g/cc, below 2.0 g/cc, below 2.1 g/cc, below 2.2 g/cc. In still other embodiments the skeletal density of the blend is below 2.5 g/cc, below 3.0 g/cc, below 3.5 g/cc, below 4.0 g/cc, below 5.0 g/cc, below 7 g/cc, below 10 g/cc.
The matrix material may be any material with a measurable viscosity. After the addition of conductive polymers to the matrix material, the resulting blend may have different rheology than the starting matrix material. The viscosity of the blend measured at 25°C can vary between 0.1 cP and 100,000 cP. In some embodiments, the viscosity of the blend measured at 25°C ranges from 0.1 cP to 1,000 cP, from 0.5 cP to 500 cP, from 1 cP to 500 cP, from 1 cP to 200 cP, from 1 cP to 100 cP, from 5 cP to 50 cP. In another embodiment, the viscosity of the blend measured at 25°C ranges from 1 cP to 10,000 cP, from 1,000 cP to 10,000 cP, from 2,000 cP to 5,000 cP, or from 3,000 cP to 4,000 cP. In certain embodiments, the viscosity of the blend measured at 25°C is greater than 10,000 cP or less than 100,000 cP.
The purity may directly influence the performance properties of the blend.
The amount of individual trace elements can be determined using inductively coupled plasma optical emission spectrometry (ICP-OES). In some embodiments, the level of scandium present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of titanium present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of vanadium present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of chromium
present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of manganese present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of iron present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of cobalt present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of nickel present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of copper present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of zinc present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of silver present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of molybdenum present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of platinum present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of cadmium present in the blends is less than 10000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 10 ppm, or less than 1 ppm. In yet other embodiments, the sum of all ICP-OES impurities, excluding dopants, present in the blends is less than 100,000 ppm, less than 20,000 ppm, less than 10,000 ppm, less than 5000 ppm, less than 1000 ppm, less than 500 ppm, less than 100 ppm, or less than 10 ppm.
Embodiments of the blend comprise a population of conductive polymer particles and matrix material particles. Alternatively, in another embodiment, the blend comprises conductive polymer powders suspended or dispersed in a liquid-form matrix material. In certain embodiments, the blend comprises a solid form embedded with conductive polymers. The solid form of matrix material includes, but is not limited to UV cured resin, thermoset resins, thermoset plastics, PVC, epoxy, or polyurethane, or combinations therein.
In some embodiments, the blend comprises a solid material. Accordingly, in one embodiment the blend comprises a BET surface area of at least 1 m2/g, at least 2
2 2 2 2 2 2 m /g, at least 3 m /g, at least 4 m /g, at least 5 m /g, at least 6 m /g, at least 7 m /g, at least 8 m2/g, at least 9 m2/g, or at least 10 m2/g. In other embodiments, the blend comprises a BET surface area of at least 50 m2/g, at least 100 m2/g, at least 150 m2/g, at least 200 m2/g,
2 2 2 2 2 at least 250 m /g, at least 300 m /g, at least 350 m /g, at least 400 m /g, at least 450 m /g, or at least 500 m2/g. In yet another embodiment, the blend comprises a BET surface area of at least 1000 m2/g.
The conductive polymers are designed to optimize the conductivity of the matrix material as a blend. The electronic conductivity of the blend may range between 10' 4 S/cm to 103 S/cm. In one embodiment the electronic conductivity of the blend is between 1 S/cm and 103 S/cm, 1 S/cm and 102 S/cm, 50 S/cm and 100 S/cm. In another
embodiment the electronic conductivity of the blend is between 10"4 S/cm and 10 S/cm, 10" 3 S/cm and 1 S/cm, 10"2 S/cm and 1 S/cm.
The electrical properties of the blend may also be measured using surface techniques, known to those skilled in the art. The surface resistance is particularly important as many of the potential applications involve films and coatings. The surface resistance of the blend may range between 1 and 1E9 ohms/sq. In one embodiment, the application requires electrostatic dissipative-level resistances, between 1E5 and 1E9 ohms/sq. In another embodiment, the application requires moderate current flowing resistances, between 1E3 and 1E5 ohms/sq. In yet another embodiment, the application
requires very low levels of resistance, between 1 and 1E3 ohms/sq for applications such as conductive inks.
Other embodiments of the present invention include the use of the disclosed polymer-matrix blends in an electronic device, for example as a coating on an electronic device. In some embodiments, the electronic device is a CPU or motherboard. In other embodiments, the electronic device is an airplane, automobile, bicycle, or motorcycle. In still other embodiments, the electronic device is a computer, tablet, or faceplate.
B. Conductive Polymers
The properties of the conductive polymer comprised within matrix materials can be varied in order to obtain desired physical properties, for example adhesion, and performance properties, for example conductivity.
The conductive polymers can be any type of polymer whose conductivity is achieved with organic compounds. For example, in some embodiments conductive polymers comprise PEDOT:PSS, PPy:PSS, PPy:DBSA (DBS A = dodecylbenzene sulphonic acid), PEDOT:DBSA, PEDOT:PSS:DBSA, PAN: DBS A, PAN:PSS,
PEDOT:poly(vinyl alcohol (PVA), PPy:PVA, PAN:PVA, PEDOT:toluenesulfonic acid (Tos), PPy:Tos, and PAN:Tos . In some embodiments the conductive polymers comprise EDOT, aniline, pyrrole, fluorene, thiophene or combinations thereof. In certain
embodiments, the conductive polymers can comprise EDOT. In other embodiments, the conductive polymers can comprise pyrrole, such as polypyrrole.
The inclusion of impurities or unreacted precursors could impact conductive properties of the conductive polymer. The presence and concentration of such impurities can be determined with thermal gravimetric analysis, as known by those skilled in the art. In one embodiment the concentration of impurities or unreacted precursors is between 0 and 1 wt%, 0 and 2 wt%, 0 and 3 wt%, 0 and 4 wt%, 0 and 5 wt%. In another embodiment the concentration of impurities or unreacted precursors is approximately 0.5 wt%. In yet another embodiment the concentration of impurities or unreacted precursors is between 1 and 5 wt%, 2 and 4 wt%, 2 and 3 wt%. In still another embodiment the concentration of
impurities or unreacted precursors is less than 1 wt% or greater than 5 wt%. In still another embodiment the concentration of impurities or unreacted precursors is much higher, between 5 and 30%, 5 and 20%, 10 and 15%.
The conductive polymers may be organic in nature, comprising oxygen, nitrogen, carbon, sulfur, or combinations therein. In one embodiment, the conductive polymers comprise at least 30%> carbon, at least 40% carbon, at least 50% carbon, at least 60%) carbon, at least 70% carbon, at least 80%> carbon, or at least 90% carbon as measured by CHNO. In another embodiment, the conductive polymers comprise at least 30% oxygen, at least 40% oxygen, at least 50% oxygen, at least 60% oxygen, at least 70% oxygen, at least 80% oxygen, or at least 90% oxygen as measured by CHNO. In still another embodiment, the conductive polymers comprise at least 1% nitrogen, at least 3% nitrogen, at least 5% nitrogen, at least 10% nitrogen, at least 15% nitrogen, at least 20% nitrogen, or at least 25% nitrogen as measured by CHNO. In yet another embodiment, the conductive polymers comprise at least 1% sulfur, at least 3% sulfur, at least 5% sulfur, at least 10% sulfur, at least 15% sulfur, at least 20% sulfur, or at least 25% sulfur as measured by CHNO.
The purity of the material may directly influence the performance properties of the conductive polymer. It is desired to minimize the amount of trace metal elements, excluding dopants. The amount of individual trace elements can be determined using inductively coupled plasma optical emission spectrometry (ICP-OES). In some embodiments, the level of scandium present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of titanium present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of vanadium present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of chromium present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some
embodiments, the level of manganese present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of iron present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of cobalt present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of nickel present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some
embodiments, the level of copper present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of zinc present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of silver present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of molybdenum present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of platinum present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In some embodiments, the level of cadmium present in the conductive polymers is less than 1000 ppm, less than 500 ppm, less than 100 ppm, less than 50 ppm, less than 10 ppm, or less than 1 ppm. In yet other embodiments, the sum of all ICP-OES impurities, excluding dopants, present in the conductive polymers is less than 5,000 ppm, less than 2,000 ppm, less than 1,000 ppm, less than 500 ppm, less than 400 ppm, less than 300 ppm, less than 100 ppm, or less than 10 ppm.
Conductive polymers may be purposefully doped to alter the performance properties, such as doping with nickel to increase the conductivity. In one embodiment, the mass percent of dopant as a percentage of the total mass of the conductive polymer and
dopant ranges from 0.01% to 50%. In another embodiment the mass percent of dopant as a percentage of the total mass of the conductive polymer and dopant is less than 0.1%, less than 0.5%), less than 1%, less than 1.5%, less than 2%, less than 2.5%, less than 3%, less than 3.5%), less than 4%, less than 5%, less than 6%, less than 7%, less than 8%, less than 10%, less than 15%, less than 20%, less than 30%, less than 40%, less than 50%. In certain embodiments, the dopant comprises nickel. In other embodiments, the dopant comprises, nickel, aluminum, tin, silver, copper, iron, zinc, cobalt, titanium, vanadium, lead, phosphorus, sulfur, platinum, or any combinations herein.
Not wanting to be bound by theory, the surface area of conductive polymers is directly related to the number of reactive sites in contact with matrix materials. The number of reactive sites between the conductive polymers and the matrix materials will change the physical and chemical properties of the blend. Accordingly, in one embodiment the conductive polymers comprise a BET surface area of at least 1 m2/g, at least 2 m2/g, at
2 2 2 2 2 2 least 3 m /g, at least 4 m /g, at least 5 m /g, at least 6 m /g, at least 7 m /g, at least 8 m /g, at least 9 m2/g, or at least 10 m2/g. In other embodiments, the conductive polymers comprise a BET surface area of at least 50 m2/g, at least 100 m2/g, at least 150 m2/g, at least 200 m2/g, at least 250 m2/g, at least 300 m2/g, at least 350 m2/g, at least 400 m2/g, at least 450 m2/g, or at least 500 m2/g. In yet another embodiment, the conductive polymers comprise a BET surface area of at least 1000 m2/g.
The performance of the blend may be impacted by the molecular weight of the conductive polymers. For example, a lower molecular weight of the conductive polymers, as measured by gel permeation chromatography (GPC), may yield lower blend conductivity when compared to higher molecular weight conductive polymers. The molecular weight of the conductive polymers as measured by GPC may vary between 100 g/mol and 100,000,000 g/mol. In one embodiment, the molecular weight of the conductive polymers as measured by GPC ranges from 100 g/mol to 100,000 g/mol, from 500 g/mol to 50,000 g/mol, or from 1,000 g/mol to 10,000 g/mol. In another embodiment, the molecular weight of the conductive polymers as measured by GPC ranges from 100,000 g/mol to 1,000,000 g/mol, from 200,000 g/mol to 900,000 g/mol, from 200,000 g/mol to 500,000
g/mol, or from 500,000 g/mol to 900,000 g/mol. In still another embodiment, the molecular weight of the conductive polymers as measured by GPC ranges from 1,000,000 g/mol to 100,000,000 g/mol, from 1,000,000 g/mol to 50,000,000 g/mol, or from 2,000,000 g/mol to 10,000,000 g/mol.
Dispersing the conductive polymers into water and measuring the pH of the resultant dispersion, a method known to those skilled in the art, can determine the pH of a solid. In certain embodiments, the pH of the conductive polymers dispersed in water is between 6 and 8. In another embodiment the pH of the conductive polymers is less than 1, less than 2, less than 3, less than 4, less than 5, or less than 6. In still another embodiment, the pH of the conductive polymers is greater than 8, greater than 9, greater than 10, or greater than 11.
Another characterization is of the electronic conductivity of the conductive polymers. The electronic conductivity may range between 10"4 S/cm to 103 S/cm. In one embodiment the electronic conductivity of the conductive polymers is between 1 S/cm and 103 S/cm, 1 S/cm and 102 S/cm, 50 S/cm and 100 S/cm. In another embodiment the electronic conductivity of the conductive polymers is between 10"4 S/cm and 10 S/cm, 10"3 S/cm and 1 S/cm, 10"2 S/cm and 1 S/cm.
The average diameter, or D(50), of conductive polymers can be measured using methods known in the art, such as laser scattering techniques. In one embodiment the D(50) of conductive polymers is between 10 and 1000 nm, 10 and 500 nm, 20 and 300 nm, 20 and 50 nm, 50 and 200 nm, 100 and 150 nm. In another embodiment the D(50) of conductive polymers is approximately 200 nm. In yet another embodiment the D(50) of conductive polymers is between 200 and 1000 nm, 200 and 500 nm, 200 and 400 nm, 250 and 300 nm. In still another embodiment the D(50) of the complex particle is less than 10 nm or greater than 1000 nm.
The conductive polymers may be purposefully functionalized in order to preferentially bond to various substrates. Not wanting to be bound by theory, the conductive polymer functionality determines the physical and chemical adhesion and reaction to substrate surfaces. The conductive polymers can be optimized to bind with
specific substrate materials. The functionality of the conductive polymers can be identified using infrared spectroscopy, a method known to those of skill in the art. In one
embodiment, the functional group may be hydroxyl (R-OH), carbonyl (R-CO-R), aldehyde (R-CHO), carbonate (R-OCOO-R), carboxylate (R-COO"), carboxylic acid (R-COOH), sulfonate (R-SOx-R), thiol (R-SH), ester (R-COO-R), amide (R-CON-R2), amine (Ry- HX), imine (R-C= H-R), or fluoroalkane (R-CHF-R). In another embodiment the functional group may contain silicon, such as silyl and disilanyl.
C. Matrix Material
The matrix material can have any form factor. For example, the matrix material may be a resin or a liquid solvent, such as water. In some embodiments the matrix comprises a solid, such as a powder. In certain embodiments, the matrix material comprises water, benzene, xylene, toluene, ethanol, methanol, methyl-ethyl-ketone, isopropanol, acetone or combination thereof. In another embodiment the matrix material comprises a surfactant such as Span 20, Span 40, Span 60, Span 80, Span 83, Span 85, Span 120, Tween 20, Tween 21, Tween 40, Tween 60, Tween 61, Tween 65, Tween 80. In other embodiments, the matrix material comprises a phenolic, polyester, epoxy, nitrile, latex, lacquer, polyurethane, polyether, polyethylene terephthalate, acrylonitrile butadiene styrene, polystyrene, polypropylene, polyethylene, polycarbonate, nylon, polyurethane, thermoplastic polyester, okra gum, pitch, galbanum, amino resins or combinations thereof. In another embodiment, the matrix polymer comprises a gum resin, a synthetic resin, a thermoplastic resin, or a thermoset resin. In yet other embodiments the matrix material comprises zinc, aluminum, titanium, silver, nickel, chromium, copper, tin, or combinations therein. Alternatively, the matrix material comprises a combination of substances listed above.
In one embodiment the matrix material is a powder. The diameter, or
D(50), of particles of the matrix powder material can be measured using methods known in the art, such as laser scattering techniques. In one embodiment the D(50) of matrix powder material is between 10 and 1000 nm, 10 and 500 nm, 20 and 300 nm, 20 and 50 nm, 50 and
200 nm, 100 and 150 nm. In another embodiment the D(50) of matrix powder material is approximately 200 nm. In yet another embodiment the D(50) of matrix powder material is between 200 and 1000 nm, 200 and 500 nm, 200 and 400 nm, 250 and 300 nm. In still another embodiment the D(50) of matrix powder material is less than 10 nm or greater than 1000 nm.
The electronic conductivity of the matrix material may range between 10"4 S/cm to 103 S/cm. In one embodiment the electronic conductivity of the matrix material is between 1 S/cm and 103 S/cm, 1 S/cm and 102 S/cm, 50 S/cm and 100 S/cm. In another embodiment the electronic conductivity of the matrix material is between 10"4 S/cm and 10 S/cm, 10"3 S/cm and 1 S/cm, 10"2 S/cm and 1 S/cm.
The matrix material may be doped with an additive to further improve the performance characteristics. In certain embodiments, the level of nickel doped into the matrix material is greater than 1 wt. %, greater than 2 wt.%, greater than 5 wt.%, greater than 10 wt.%, greater than 20 wt.%, or greater than 30 wt.%. In still another embodiment, the level of nickel doped into the matrix material is between 3 wt.% and 7 wt.%. In yet another embodiment, the level of nickel doped into the matrix material is ~6 wt.%. In other embodiments, the level of aluminum doped into the matrix material is greater than 1 wt. %, greater than 2 wt.%, greater than 5 wt.%, greater than 10 wt.%, greater than 20 wt.%), or greater than 30 wt.%. In certain embodiments, the level of titanium doped into the matrix material is greater than 1 wt. %, greater than 2 wt.%, greater than 5 wt.%, greater than 10 wt.%, greater than 20 wt.%, or greater than 30 wt.%. In certain
embodiments, the level of silver doped into the matrix material is greater than 1 wt. %, greater than 2 wt.%, greater than 5 wt.%, greater than 10 wt.%, greater than 20 wt.%, or greater than 30 wt.%. In still another embodiment, the level of silver doped into the matrix material is between 1 wt.% and 5 wt.%. In yet another embodiment, the level of silver doped into the matrix material is ~3 wt.%. In another embodiment, the dopant comprises a powder form. In yet another embodiment, the dopant is carbon, such as carbon black, carbon nanotubes, activated carbon, graphite, graphene, In still another embodiment, the
dopant comprises a metal oxide, such as zinc oxide, nickel (II) oxide, copper (IV) oxide, or molybdenum (III) oxide.
D. Preparation of the Blend
1. Preparation of conductive polymers
The conductive polymers may be made from a polymer comprising a conjugated polymer base. In one embodiment, the conjugated polymer can be synthesized by mixing a monomer (Component 1), a counter-ion (Component 2) and an oxidizing agent (Component 3). In another embodiment, the conjugated polymer can be purchased from common commercial agencies and further modified.
The monomer (Component 1), used to synthesize the conjugated polymer, can be chosen from a range of materials known in the art. Not wishing to be bound by theory, the monomer can be important to tailoring the optically transparent properties of the spray on film. In some embodiments the initial monomer used to synthesize the conjugated polymer is EDOT, aniline, pyrrole, fluorene, thiophene or combinations thereof. In certain embodiments, the monomer can contain EDOT. In other embodiments, the monomer can contain pyrrole. Component 1 can be uniquely characterized through independent polymerization and measurement of molecular weight. For example, the molecular weight of Component 1 after polymerization is above 1,000 g/mol, above 2,000 g/mol, above 5,000 g/mol, above 10,000 g/mol, above 20,000 g/mol, above 50,000 g/mol, above 150,000 g/mol, above 400,000 g/mol, above 1,000,000 g/mol, above 2,000,000 g/mol. In another example the molecular weight of Component 1 after polymerization is below 5,000,000 g/mol.
The counter-ion (Component 2), or dopant, when present, can be tailored for increased adhesion. In some embodiments, the counter-ion itself comprises two
functionalities, Functionality A and Functionality B. Not to be bound by theory, in some embodiments Functionality A acts as an acidic functional group that complexes strongly with Component 1 while Functionality B is a glass-interactive functional group. In certain
embodiments, Functionality A is sulfonic acid and Functionality B is maleic acid. The possible combinations of Functionality A and Functionality B are listed but not limited to the materials in Table 1.
Table 1
In certain embodiments, Component 2 includes polystyrene sulfonic acid- co-maleic acid. In other embodiments, Component 2 includes polyacrylic acid, polymaleic acid, polystyrene sulfonate, polyacrylic-co-maleic acid, polystyrene sulfonic acid-co- maleic acid, polyacrylic acid-co-polystyrene, polystyrene sulfonate-co-polyethylene oxide, and polystyrene sulfonate-co-polypropylene oxide.
The weight ratio between Component 1 and Component 2 has been found to impact the final performance properties of the polymer in addition to altering the reaction kinetics. In one embodiment the weight ratio of Component 1 to Component 2 is between
2: 1 and 1 : 1000. In another embodiment the weight ratio of Component 1 to Component 2 is between 1 : 1 and 1 :500, 1 :2 and 1 : 100, 1 :3 and 1 :20, 1 :4 and 1 : 10. In yet another embodiment the weight ratio of Component 1 to Component 2 is between 2: 1 and 1 : 1, 1.8: 1 and 1.1 : 1, and 1 : 7 and 1.5: 1. In yet still another embodiment the weight ratio of Component 1 to Component 2 is 1 :4.
The solvent for the reaction can be altered for safety for both the user and the environment. In one embodiment, the solvent used for synthesis is water. However, in yet other embodiments the solvents are water, ethanol, acetone, methanol, toluene, isopropanol, benzene, xylene, tetrahydrofuran, dichlorobenzene, chlorobenzene, methyl- ethyl-ketone, acetonitrile, chloroform, dichloromethane, or combinations thereof.
The concentration of Component 1 when mixed with Component 2 in solution is chosen to facilitate the reaction with Component 3. In one embodiment the concentration of Component 1 when mixed with Component 2 in solution is between 0.05M and 5M. In another embodiment the concentration of Component 1 when mixed with Component 2 in solution is between 0.05M and 1M, 0.1M and 0.9M, 0.5M and 0.9M, 1M and 4M, 2M and 3M. In yet another embodiment the concentration of Component 1 when mixed with Component 2 in solution is less than 0.05M or greater than 5M. In still yet another embodiment, the concentration of Component 1 when mixed with Component 2 in solution is 0.08M.
To facilitate the synthesis of the polymeric material, an oxidization agent
(Component 3) may be used. In one embodiment, the oxidizing agent is sodium persulfate (NaPS), potassium persulfate (KPS), ferric chloride, sodium persulfate, or combinations thereof. Broadly speaking, the oxidizing agents refer to a large class of materials and are listed in Table 2. The oxidizing agent may be chosen for its reaction rate, solubility, and cost. In an additional embodiment, no oxidizing agent is used.
Table 2.
The ratio between Component 1 and Component 3 directly impacts the rate and extent of reaction. For example, the molar ratio of Component 1 to Component 3 is between 5: 1 and 1 :5, 1 : 1 and 5: 1, 2: 1 and 3 : 1, 1 : 1 and 1 :5, 1 :2 and 1 :3. In another example the molar ratio of Component 1 to Component 3 is—1 : 1.
The combination of Component 1, Component 2 and Component 3 (Mixture 1), can be heated or cooled to directly impact the rate of reaction. In one embodiment the polymerization temperature of Mixture 1 is increased above 30 °C, above 40 °C, above 50 °C, above 60 °C, above 70 °C, above 100 °C, above 150 °C, above 200 °C, above 300 °C. In another embodiment the polymerization temperature of Mixture 1 is decreased below 30 °C, below 20 °C, below 10°C, below 0 °C. In still another embodiment, the temperature is held constant throughout the polymerization process. In yet another embodiment the temperature of Mixture 1 is dynamic.
The time allowed for polymerization of Mixture 1 can be controlled to obtain ideal particle size. For example, the time allowed for polymerization of Mixture 1 is between 5 minutes and 48 hours. In one embodiment, the polymerization time for Mixture 1 is between 5 minutes and 10 hours, 30 minutes and 8 hours, 1 hour and 5 hours, 2 hours
and 4 hours. In another embodiment the polymerization time for Mixture 1 is
approximately 3 hours. In yet another embodiment, the polymerization time for Mixture 1 is between 10 hours and 48 hours, 12 hours and 24 hours, 16 hours and 20 hours. In still another embodiment the polymerization time for Mixture 1 is greater than 48 hours.
In addition to Component 1, Component 2 and Component 3, an extra dopant (Dopant 1) can be included in Mixture 1 prior to or after polymerization in order to supplement the performance characteristics and mechanical properties such as adhesion. In one example, Dopant 1 is a material with suitable electron withdrawing capacity such as ferric chloride, methyl sulfonic acid or tosylate. In another example, Dopant 1 is a combination of multiple materials as listed above. Possible dopants are listed but not limited to the materials in Table 3.
Table 3.
Nitriles acetonitrile
Nitro compounds nitromethane
Polyols glycerol, ethylene glycol, sorbitol, weso-erythritol
Salts copper (II) chloride, ferric chloride, gold chloride, auric chloride, sodium naphthalenide
Sulfoxides dimethyl sulfoxide
Dopant 1 is typically added into solution after the combination of
Component 1, Component 2 and Component 3, though it can also be added after the combination and the polymerization of Component 1, Component 2 and Component 3. In one embodiment, the concentration of Dopant 1 as a measurement of the total mass of Component 1, Component 2, Component 3, and solvent is between 0.001 and 1 wt%, 0.01 and 0.1 wt%, 0.05 and 0.1 wt%. In another embodiment the concentration of Dopant 1 is between 0.1 and 5%, 1 and 10%, 5 and 20%, 10 and 30%.
Multiple dopants may be used to create unique physical and chemical properties of the material. In one embodiment, the total concentration of dopants, as defined by the sum of the mass of all dopants, in Mixture 1 is between 0.001 and 1 wt%, 0.01 and 0.1 wt%, 0.05 and 0.1 wt%. In another embodiment the concentration of Dopant 1 is between 0.1 and 5%, 1 and 10%, 5 and 20%, 10 and 30%.
Typically the polymerized product after the reaction is acidic. In one embodiment the pH after the polymerization is between 1 and 6, 2 and 5, 2 and 4.
Depending on the application, such as corrosion, a neutral or basic product may be desired. It has been found that the addition of a neutralizing agent (Additive 1) can be used to alter the pH of the final product without harm to the performance characteristics. For example, Additive 1 can be a strong base such as, but not limited to, sodium hydroxide, potassium hydroxide, lithium hydroxide, calcium hydroxide, or barium hydroxide. In yet another example, Additive 1 can be a weak base such as, but not limited to, ammonia, ammonium hydroxide, pyridine, and trimethyl ammonia.
After polymerization of Mixture 1 and neutralization using Additive 1, the intermediate product may be dried. In one embodiment the intermediate product is dried using vacuum filtration, centrifuged, air dried, oven dried, or freeze dried. In another embodiment the intermediate product may never undergo a drying phase. Instead, the polymer dispersion may undergo a continuous solvent exchange.
Alternatively, the complex can be commercially purchased or synthesized using methods known to those in the art. In one embodiment, the commercially available material used as the complex is PEDOT:PSS, poly(p-phenylene vinylene), poly(3- hexylthiophene), poly(pyrrole), poly(fluorene), poly(aniline), and poly(acetylene). The commercially available complexes may be further modified using Dopant 1 or Additive 1 to yield a novel material.
2. Incorporation of Conductive Polymer and Matrix Material
This disclosure describes, but is not limited to, a number of techniques one skilled in the art will know to incorporate the conductive polymer and the matrix material to form the blend. In some embodiments, the methods of incorporation to form the blend involve mechanical mixing solids. In other embodiments, the methods of incorporation to form the blend involve dispersion within a liquid.
The methods to form the blend depend on the initial form factor of the conductive polymers and the matrix material. In one embodiment, the conductive polymers and the matrix material are both powder. The powders can be combined through mechanical mixing including hand mixing in a mortar and pestle, grinding, ball milling, or jet milling. Alternatively, the powders may be added together with no further mixing or post-processing.
In another embodiment, the conductive polymer is a powder and the matrix material is a liquid. Known by those skilled in the art, additional processing such as dispersion, high shear mixing, stir bar mixing, shaking, or wet milling may be used to achieve a uniform consistency. Alternatively, the conductive polymer powder and liquid matrix material are combined with no further processing.
In yet another embodiment, the conductive polymer is a solid and the matrix material is a solid. For example, the conductive polymer in a powder form is added to an epoxy resin under high shear to form the blend. In one embodiment, the blend is then cured under thermal or UV conditions to create a solid, comprised of both the matrix material and conductive polymers. In another embodiment, the matrix material is a monomer that undergoes cross-linking. In certain embodiments, the matrix material is fibrous. The conductive polymers are woven into the fabric threads to create a conductive fiber.
E. Devices Comprising the Blends
The disclosed blends can be used as an electron transport material in any number of electronics devices. One such device is a computer part, for example a circuit board or CPU. The electrical conductivity of the blend allows for electrostatic dissipation across the surface of the device, though prevents shorting from occurring between terminals and parts.
Figure 1 provides an illustration of a consumer electronic article. The consumer electronic article may include a surface mount adhesive 1, a thermal interface material 2, low pressure molding materials 3, a flip chip on board underfill 4, liquid encapsulant glob top 5, a silicone encapsulant 6, gasket compounds 7, chip scale package 8, flip chip air package underfill 9, a coating powder 10, mechanic molding compound 11, a potting compound 12, an optoelectronic 13, die attach 14, a conformal coating 15, photonic components 16, semiconductor mold compound 17, and solder 18.
The disclosed blend may be used as the surface mount adhesive 1, thermal interface material 2, low pressure molding materials 3, a coating powder 10, a potting compound 12, a conformal coating 15, or a semiconductor mold compound 17.
When used as a conformal coating 15, the blend may be a flowable material that is poured, pasted, or sprayed onto the consumer electronics article. The coated flowable blend may then undergo further curing or drying creating a solid blend form. The blend as a solid conformal coating 15 may vary in both thickness and color, depending on
the application. In one embodiment, the blend as a conformal coating 15 on circuit boards is less than 10 mils thick. In another embodiment, the blend as a conformal coating 15 on circuit boards is blue in color.
Alternatively, the blend may be used as a surface mount adhesive 1, which provides binding function as well as an electrostatic dissipation function. Using a blend with low levels of electron transport, the surface mount adhesive 1 will be able to conduct static charge build upon the surface of chips and parts while being insulative to continuous electron flow and shorting.
Alternative uses of the blend may be for electrostatic dissipation (ESD), for transportation fields such as aerospace and automotive, for corrosion protection and inhibition, for flooring, for windows and transparent coatings, for EMI/RFI shielding, for conductive inks, for 3D printing, or for top coats.
In some embodiments, the matrix material of the blend is a rubber composition. For example, any of the aforementioned conductive polymers, such as a conjugated polymer, or any of the aforementioned blends may be used in tires or other elastomer devices. It is sometimes desired to provide a tire with a rubber tread to promote reduced rolling resistance for the tire itself and thereby improved fuel economy for an associated vehicle, as well as reduced heat buildup in the tire tread during operation of the tire which, in turn, is expected to promote improved tire tread durability. To promote one or more of such desirable properties, it is sometimes desired to promote a reduction in the hysteretic property of the tread rubber.
To promote a reduction in the hysteresis of the tread rubber (e.g. to promote a reduction in the rubber's physical rebound property) it may desired to significantly reduce its reinforcing carbon black content and increase its precipitated silica content.
Accordingly, in one embodiment, a tire having a rubber composition comprising at least one diene-based elastomer, precipitated silica, and a conductive polymer, such as a conjugated polymer, is provided. Inclusion of electrically conductive polymers, particularly conjugated polymers, in such a rubber composition can aid in discharging static electricity through the electrically non-conductive tread to its running surface. In more specific
embodiments, the composition has a surface resistivity ranging between 1 and 1 x 109 ohms/sq and the D50 particle size of the polymer is less than 1000 nm. In another embodiment, the conductive polymer comprises a conjugated polymer.
Tires with a tread made with a rubber composition including the conductive and/or conjugated polymers show low volume resistivity, indicating good ability to conduct static electricity. In one embodiment, the composition has a volume resistivity that is less than 1 x 109 ohm-cm as measured by ASTM D257-98. In one embodiment, the rubber composition and tread has a volume resistivity that is less than 1 x 10" ohm-cm as measured by ASTM D257-98.
It is readily understood by those having skill in the art that the rubber composition can be tested (e.g., volume resistivity) and/or processed (e.g., compounded, vulcanized, mixed) using methods generally known in the art. For example, such methods are described in U.S. Pat. No. 9, 162,530 and U.S. Pat. App, 201 1/0146859, which are herein incorporated by reference in their entirety.
The rubber composition can be compounded by methods such as mixing the various sulfur-vulcanizabie constituent rubbers with various commonly used additive materials such as, for example, curing aids, such as sulfur, activators, retarders and accelerators, processing additives, such as oils, resins including tackifying resins, silicas, and piasticizers, fillers, pigments, fatty acid, zinc oxide, waxes, antioxidants and antiozonants, peptizing agents and reinforcing fillers materials such as, for example, the aforementioned rubber reinforcing carbon black and precipitated silica. As known to those skilled in the art, depending on the intended use of the sulfur vulcanizable and sulfur vulcanized material (rubbers), the additives mentioned above are selected and commonly used in conventional amounts.
Typical amounts of tackifier resins, if used, may, for example, comprise about 0.5 to about 10 phr, usually about 1 to about 5 phr. Typical amounts of processing aids, if used, may comprise, for example from about 1 to about 50 phr. Such processing aids can include, for example and where appropriate, aromatic, napthenic, and/or paraffinic processing oils. Typical amounts of antioxidants where used may comprise, for example,
about 1 to about 5 phr. Representative antioxidants may be, for example, diphenyl-p- phenylenediamine and others, such as, for example, those disclosed in The Vcmderhik Rubber Handbook (1978), Pages 344 through 346. Typical amounts of antiozonants, where used, may comprise for example about 1 to 5 phr. Typical amounts of fatty acids, if used, which can include stearic acid and combinations of stearic acid with one or more of palmitic acid oleic acid and may comprise, for example, from about 0.5 to about 3 phr. Typical amounts of zinc oxide may comprise, for example, from about 1 to about 10 phr. Typical amounts of waxes, such as for example microcrystalline waxes, where used, may comprise, for example, from about 1 to about 5 phr. Typical amounts of peptizers, where used, may comprise, for example, from about 0.1 to about 1 phr.
The vulcanization is conducted in the presence of a sulfur vulcanizing agent. Examples of suitable sulfur vulcanizing agents include elemental sulfur (free sulfur) or sulfur donating vulcanizing agents, for example, an amine disulfide, polymeric polysulfide or sulfur olefin adducts. Preferably, the sulfur vulcanizing agent is elemental sulfur. As known to those skilled in the art, sulfur vulcanizing agents may be used, for example, in an amount ranging from about 0.5 to about 4 phr, or even, in some circumstances, up to about 8 phr.
Sulfur vulcanization accelerators are used to control the time and/or temperature required for vulcanization and to improve the properties of the vulcanizate. In one embodiment, a single accelerator system may be used, i.e., primary accelerator.
Conventionally and preferably, a primary acceierator(s) is used in total amounts ranging, for example, from about 0,5 to about 4, alternately about 0,8 to about 1.5 phr. In another embodiment, combinations of a primary and a secondary accelerator might be used with the secondary accelerator, where used, being usually used in smaller amounts (for example about 0.05 to about 3 phr) in order to activate and to improve the properties of the vulcanizate. Combinations of these accelerators might be expected to produce a synergistic effect on the final properties and are somewhat better than those produced by use of either accelerator alone. In addition, delayed action accelerators may be used, for example, which are not affected by normal processing temperatures but produce a satisfactory cure at
ordinary vulcanization temperatures. Vulcanization retarders might also be used, where desired or appropriate. Suitable types of accelerators that may be used in the present invention may be, for example, amines, disulfides, guanidines, thioureas, thiazoles, thiurams, sulfenamides, dithiocarbamates and xanthates. Preferably, the primary accelerator is a sulfanamide. If a second accelerator is used, the secondary accelerator may be, for example, a guanidine, dithiocarbamate or thiuram compound.
The presence and relative amounts of the above additives are not required features of the present invention, unless otherwise indicated herein, which in some embodiments is directed to the utilization of conductive and/or conjugated polymers in a rubber composition (matrix material), for example in a tire.
The mixing of the rubber composition can be accomplished by methods known to those having skill in the rubber mixing art. For example, the ingredients are typically mixed in at least two stages, namely, at least one non-productive stage followed by a productive mix stage. The final curatives are typically mixed in the final stage which is conventionally called the "productive" mix stage in which the mixing typically occurs at a temperature, or ultimate temperature, lower than the mix temperature(s) than the preceding non-productive mix stage(s). The rubber, and reinforcing fillers, including the conjugated polymers and alternative additional reinforcing fillers such as, for example precipitated silica and rubber reinforcing carbon black mixed in one or more non- productive mix stages. The terms "non-productive" and "productive" mix stages are well known to those having skill in the rubber mixing art. The thermomechanical mixing step generally comprises a mechanical working in a mixer or extruder for a period of time suitable in order to produce a rubber temperature between 140° C. and 190° C. The appropriate duration of the thermomechanical working varies as a function of the operating conditions, and the volume and nature of the components. For example, the
thermomechanical working may be from 1 to 20 minutes. The rubber composition may be incorporated in a variety of rubber components of the tire. For example, the rubber component may be a tread (including tread cap and tread base), sidewall, apex, chafer, sidewall insert, wirecoat or innerliner.
Vulcanization of the pneumatic tire of the present invention is generally carried out at conventional temperatures ranging from about 100° C. to 200° C. In one embodiment, the vulcanization is conducted at temperatures ranging from about 1 10° C. to 180° C. Any of the usual vulcanization processes may be used such as heating in a press or mold, heating with superheated steam or hot air. Such tires can be built, shaped, molded and cured by various methods which are known and will be readily apparent to those having skill in such art.
While various embodiments are disclosed herein for practicing embodiments of the invention, it will be apparent to those skilled in this art that various changes and modifications may be made therein without departing from the spirit or scope of the invention.
The various embodiments described above can be combined to provide further embodiments. To the extent that they are not inconsistent with the specific teachings and definitions herein, U.S. Provisional Application 62/360,047, filed July 8, 2016; U.S. Provisional Application 62/475,646, filed March 23, 2017; ail of the U.S. patents, U.S. patent application publications, U.S. patent applications, foreign patents, foreign patent applications and non-patent publications referred to in this specification and/or listed in the Application Data Sheet are incorporated herein by reference, in their entirety.
EXAMPLES
The blends disclosed were made using the methods in the following Examples. Chemicals were obtained through commercial sources and were used without further processing unless otherwise stated.
EXAMPLE 1
PREPARATION OF CONDUCTIVE POLYMER DISPERSION BLEND
The conjugated polymer dispersion was synthesized by mixing poly(4- styrenesulfonic acid-co-maleic acid), PSSA-co-MA, and water to create a 4.8 wt% solution. EDOT, 3,4-ethylenedioxythiophene monomer, was added to the PSSA-co-MA solution at a weight ratio of 4: 1 (PSSA-co-MA:EDOT) and allowed to mix for 1 hour. Potassium persulfate (KPS) was added at a molar ratio of 1 : 1 (KPS:EDOT) to the PSSA- co-MA solution. The mixture was heated to 50 °C and allowed to polymerize to completion (approximately 3-24 hours). The polymerization was deemed complete by a drastic change in color to dark blue.
Table 4 shows various combinations of Component 1, Component 2 and Component 3 to prepare the conjugated polymer.
Table 4
EXAMPLE 2
MODIFICATION OF DISPERSION BLEND
The PEDOT dispersion can be further modified through additional dopants. Ferric chloride (FeCl3) was added to the PEDOT dispersion in the amount of 0.1 wt% at room temperature. The dispersion and dopant were allowed to mix for 4 hours.
EXAMPLE 3
NEUTRALIZATION OF DISPERSION BLEND
The pH of the dispersion from Example 2 was measured, typically falling between 2 and 4. To raise the pH of the dispersion for safe handling and application, aqueous sodium hydroxide was slowly titrated into the dispersion while constantly stirring until the pH is between 6 and 8. Alternatively, other strong and weak bases can be used to achieve a similar outcome.
EXAMPLE 4
SYNTHESIS OF CONDUCTIVE POLYMER POWDER
The dispersion from Examples 1-3 can be further post-processed into the spray polymer solution product. 500 mL of dispersion was poured onto a vacuum filtration system using Whatman 602 H paper . The vacuum filtration was allowed to continue until complete solvent removal. Further drying was performed in air on a hot plate or oven at 50 °C.
EXAMPLE 5
DETERMINATION OF SOLIDS WEIGHT IN PASTES
The dispersion from Examples 1-3 can also be left as a liquid dispersion or a thick paste. Approximately 1 gram of dispersion or paste was weighed and placed onto a hot plate overnight. The sample was heated to at least 50 °C. After the sample was cooled
and fully dried, it was weighed and the amount of solids was calculated from the percent weight loss.
EXAMPLE 6
INCORPORATION OF CONDUCTIVE POLYMER POWDER INTO MATRIX MATERIAL
To create a conductive coating, 5 grams of polyester was added to a beaker of 0.5 grams of conductive powder. A high shear mixer was used for 10-30 minutes until the coating was free from larger particles. A Hegman grind gauge was used to assess the particle size and dispersion quality. Alternatively, the powder may be dispersed first in xylene or a compatible solvent to assist in powder dispersion within the matrix.
Alternatively, the conductive coating may be made from a paste or dispersion, from
Example X. A SkyDrop Resistant Clear Polyurethane Topcoat was chosen as the matrix material. A paste was added at a loading of 2.6 wt% and mixed in the presence of 30 wt% IPA.
EXAMPLE 7
PROCESSING OF THE COATING
The coating was applied from Example 6 using a doctor blade at a wet height of 8 mils on polyethylene or PET. The coating was allowed to cure at room temperature for greater than 15 hours or until no longer tacky. Length of cure and dry conditions may vary on the matrix material manufacturer requirements. EXAMPLE 8
CHARACTERIZATION OF THE COATING
Properties of exemplary coatings are depicted in Table 5. In polyester, the conductive polymer powder is capable of reaching ESD-performance sheet resistivity at only 3.2 wt%. Further performance can be achieved through higher loadings of powder to 5 wt%. Properties of exemplary coatings in polyurethane are listed in Table 6. Exemplary coatings in additional matrices are shown in Table 7.
Once the coating from Example 7 was cured, it is electrically tested using a model 272 A Monroe Surface Resistivity Meter. All measurements were recorded at 10V. The meter was placed in the center of the coating and allowed to come to equilibrium for 10 seconds before recording the value.
Table 5
V35 = a PEDOT:DBSA:NaPS conductive polymer
Table 6
Table 7
Claims
1. A blend comprising a conductive polymer and a matrix material, wherein the matrix material comprises a paint, resin, plastic, elastomer, or adhesive wherein the surface resistivity of the blend ranges between 1 and 1E9 ohms/sq and the D50 particle size of the polymer is less than 10,000nm.
2. The blend of claim 1, wherein the mass percent of conductive polymer to the total mass of conductive polymer and matrix material ranges from 0.01% to 50%.
3. The blend of any of the preceding claims, wherein the mass percent of conductive polymer to the total mass of conductive polymer and matrix material is less than 10%.
4. The blend of any of the preceding claims, wherein the mass percent of conductive polymer to the total mass of conductive polymer and matrix material is less than 3%.
5. The blend of any of the preceding claims, wherein the electrical conductivity of the blend ranges from 10"2 S/cm to 1 S/cm.
6. The blend of any of the preceding claims, wherein the electrical conductivity of the blend ranges from 10 S/cm to 1000 S/cm.
7. The blend of any of the preceding claims, wherein the surface conductivity of the blend ranges from 1E3 to 1E8 ohms/sq.
8. The blend of any of the preceding claims, wherein the surface conductivity of the blend is below 1E6 ohms/sq.
9. The blend of any of the preceding claims, wherein the matrix material is a liquid.
10. The blend of any of the preceding claims, wherein the matrix material is a solid.
11. The blend of any of the preceding claims, wherein the matrix material is a powder.
12. The blend of claim 11, wherein the D50 of the matrix material ranges from 1 um to 50 um.
13. The blend of claim 1, wherein the matrix material is a thermoset plastic.
14. The blend of any of the preceding claims, wherein the matrix material comprises a metal dopant.
15. The blend of claim 14, wherein the metal dopant comprises zinc.
16. The blend of claim 14, wherein the metal dopant comprises silver.
17. The blend of claim 14, wherein the metal dopant comprises tin.
18. The blend of any of the preceding claims, wherein the conductive polymer comprises a poly 3,4-ethylenedioxythiophene.
19. The blend of any of the preceding claims, wherein the conductive polymer comprises a polypyrrole.
20. The blend of any of the preceding claims, wherein the conductive polymer comprises a polyfluorene.
21. The blend of any of the preceding claims, wherein the conductive polymer comprises one or more dopants, wherein the sum of the concentrations of all dopants in the conductive polymer ranges from 0.01 and 5% of the total mass of the blend.
22. The blend of any of the preceding claims, wherein the conductive polymer comprises a carboxylic acid counter ion.
23. The blend of any of the preceding claims wherein the conductive polymer comprises an amine counter ion.
24. The blend of any of the preceding claims, wherein the weight ratio between the monomer and the counter ion of the conductive polymer ranges from 1 to 1000.
25. The blend of any of the preceding claims, wherein the conductive polymer comprises a persulfate oxidizing agent.
26. The blend of any of the preceding claims, wherein the conductive polymer comprises a chlorate oxidizing agent.
27. The blend of any of the preceding claims, wherein the conductive polymer comprises an oxide oxidizing agent.
28. The blend of any of the preceding claims, wherein the conductive polymer comprises a chloride oxidizing agent.
29. The blend of any of the preceding claims, wherein the conductive polymer comprises an oxidizing agent, and the oxidizing agent is in its elemental form.
30. The blend of any of the preceding claims, wherein the conductive polymer comprises a ferric chloride dopant.
31. The blend of any of the preceding claims, wherein the conductive polymer comprises a methyl sulfonic acid dopant.
32. The blend of any of the preceding claims, wherein the blend comprises a pH between 6 and 8.
33. The blend of any of the preceding claims, wherein the electronic conductivity of the blend ranges from 0.001 to 1000 S/cm.
34. The blend of any of the preceding claims, wherein the matrix material comprises a phenolic, polyester, epoxy, nitrile, latex, lacquer, polyurethane, polyether, polyethylene terephthalate, acrylonitrile butadiene styrene, polystyrene, polypropylene, polyethylene, polycarbonate, nylon, polyurethane, thermoplastic polyester, okra gum, pitch, galbanum, amino resins or combinations thereof.
35. The blend of any of the preceding claims, wherein the matrix material comprises a polyester or polyurethane.
A substrate comprising the blend of any of the preceding claims on a surface thereof.
37. The substrate of claim 36, wherein the surface comprises a film of the blend.
39. A rubber composition comprising at least one diene-based elastomer, precipitated silica, and a conductive polymer.
40. The rubber composition of claim 39, wherein the composition has a surface resistivity ranging between 1 and 1 x 109 ohms/sq and the D50 particle size of the polymer is less than 1000 nm.
41. The rubber composition of any one of claims 39-40, wherein the conductive polymer comprises a conjugated polymer.
42. The rubber composition of any one of claims 39-41, wherein the composition has a volume resistivity less than 1 x 109 ohm-cm as measured by ASTM D257-98.
43. The rubber composition of any one of claims 39-42, wherein the rubber composition is in the form of a tire.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662360047P | 2016-07-08 | 2016-07-08 | |
| US62/360,047 | 2016-07-08 | ||
| US201762475646P | 2017-03-23 | 2017-03-23 | |
| US62/475,646 | 2017-03-23 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018009891A1 true WO2018009891A1 (en) | 2018-01-11 |
Family
ID=60901621
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2017/041236 Ceased WO2018009891A1 (en) | 2016-07-08 | 2017-07-07 | Conductive conformal coatings |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2018009891A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10087320B2 (en) | 2017-02-17 | 2018-10-02 | Polydrop, Llc | Conductive polymer-matrix compositions and uses thereof |
| CN109266263A (en) * | 2018-08-31 | 2019-01-25 | 王成 | A kind of preparation method of high viscosity Laminating adhesive |
| CN110305603A (en) * | 2018-03-20 | 2019-10-08 | 拓自达电线株式会社 | conductive adhesive layer |
| CN112898541A (en) * | 2019-12-04 | 2021-06-04 | 波音公司 | Organic soluble conductive polymers |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4705645A (en) * | 1983-11-23 | 1987-11-10 | Gte Laboratories Incorporated | Method for preparing an electrically conductive polymer blend |
| US5494609A (en) * | 1992-04-15 | 1996-02-27 | Kulkarni; Vaman G. | Electrically conductive coating compositions and method for the preparation thereof |
| US6331586B1 (en) * | 1998-02-12 | 2001-12-18 | Cabot Corporation | Conductive polymer blends with finely divided conductive material selectively localized in continuous polymer phase or continuous interface |
| US20030207094A1 (en) * | 2002-04-30 | 2003-11-06 | 3M Innovative Properties Company | Resistivity-controlled image recording sheet |
| US20110233450A1 (en) * | 2010-03-25 | 2011-09-29 | Nec Tokin Corporation | Conductive polymer and method for producing the same, conductive polymer dispersion, and solid electrolytic capacitor and method for producing the same |
| US8080177B2 (en) * | 2008-08-19 | 2011-12-20 | The Boeing Company | Low RF loss static dissipative adhesive |
-
2017
- 2017-07-07 WO PCT/US2017/041236 patent/WO2018009891A1/en not_active Ceased
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4705645A (en) * | 1983-11-23 | 1987-11-10 | Gte Laboratories Incorporated | Method for preparing an electrically conductive polymer blend |
| US5494609A (en) * | 1992-04-15 | 1996-02-27 | Kulkarni; Vaman G. | Electrically conductive coating compositions and method for the preparation thereof |
| US6331586B1 (en) * | 1998-02-12 | 2001-12-18 | Cabot Corporation | Conductive polymer blends with finely divided conductive material selectively localized in continuous polymer phase or continuous interface |
| US20030207094A1 (en) * | 2002-04-30 | 2003-11-06 | 3M Innovative Properties Company | Resistivity-controlled image recording sheet |
| US8080177B2 (en) * | 2008-08-19 | 2011-12-20 | The Boeing Company | Low RF loss static dissipative adhesive |
| US20110233450A1 (en) * | 2010-03-25 | 2011-09-29 | Nec Tokin Corporation | Conductive polymer and method for producing the same, conductive polymer dispersion, and solid electrolytic capacitor and method for producing the same |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10087320B2 (en) | 2017-02-17 | 2018-10-02 | Polydrop, Llc | Conductive polymer-matrix compositions and uses thereof |
| CN110305603A (en) * | 2018-03-20 | 2019-10-08 | 拓自达电线株式会社 | conductive adhesive layer |
| CN109266263A (en) * | 2018-08-31 | 2019-01-25 | 王成 | A kind of preparation method of high viscosity Laminating adhesive |
| CN112898541A (en) * | 2019-12-04 | 2021-06-04 | 波音公司 | Organic soluble conductive polymers |
| EP3831896A1 (en) * | 2019-12-04 | 2021-06-09 | The Boeing Company | Organically soluble conductive polymers |
| US11352509B2 (en) | 2019-12-04 | 2022-06-07 | The Boeing Company | Organically soluble conductive polymers |
| US11787954B2 (en) | 2019-12-04 | 2023-10-17 | The Boeing Company | Organically soluble conductive polymers |
| CN112898541B (en) * | 2019-12-04 | 2024-07-16 | 波音公司 | Organic soluble conductive polymers |
| US12065586B2 (en) | 2019-12-04 | 2024-08-20 | The Boeing Company | Organically soluble conductive polymers |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10087320B2 (en) | Conductive polymer-matrix compositions and uses thereof | |
| WO2018009891A1 (en) | Conductive conformal coatings | |
| CN1239576C (en) | Polythiophene | |
| Senthilkumar et al. | Synthesis, characterization and electrical properties of nano metal and metal-oxide doped with conducting polymer composites by in-situ chemical polymerization | |
| KR20100126261A (en) | Particles with Core-Shell Structure for Conductive Layer | |
| CN101979444B (en) | Methods for preparing quaternary phosphonium salt ionic liquid pillaring-modified montmorillonite and polymer/montmorillonite nanocomposite | |
| CN100523005C (en) | Dispersion of intrinsically conductive polymer and its preparation method | |
| TW201437300A (en) | Formulations comprising washed silver nanowires and PEDOT | |
| EP2947126A1 (en) | Conductive polymer composite and substrate | |
| JP6998962B2 (en) | Compositions useful for forming antistatic layers or electromagnetic radiation shields | |
| TW201734056A (en) | Hollow particle and use thereof | |
| CN102089348A (en) | Organic solvent-dispersible conductive polymer and method for manufacture thereof | |
| JP6905744B2 (en) | A conductive surface-treated powder filler and a resin composition containing the filler. | |
| US9321863B2 (en) | Polyvinyl sulfonic acid, production method thereof, and use thereof | |
| JPWO2017094809A1 (en) | Sealing composition | |
| WO2014163059A1 (en) | Electrically conductive coating material, and adherend using same | |
| JP4501030B2 (en) | Conductive fine particles and method for producing the same | |
| JP7485279B2 (en) | Conductive surface-treated powder filler, its manufacturing method, and conductive composition containing said filler | |
| KR102581426B1 (en) | Liquid composition comprising particles of a conductive polymer and an organic solvent forming an azeotrope with water | |
| WO2017218516A1 (en) | Compositions comprising conjugated polymers and uses thereof | |
| KR101398514B1 (en) | Coating composition comprising aniline terminated waterborne polyurethane and poly(3,4-ethylenedioxythiophene)/polystyrene sulfonate | |
| CN106459487B (en) | Electroconductive polymer aqueous dispersions and antistatic coating fluid | |
| KR102059972B1 (en) | Anti-static release film for in-mold injection molding process | |
| CN119213052A (en) | Novel polythiophene/polyanion composite |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17825044 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 32PN | Ep: public notification in the ep bulletin as address of the adressee cannot be established |
Free format text: NOTING OF LOSS OF RIGHTS PURSUANT TO RULE 112(1) EPC |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17825044 Country of ref document: EP Kind code of ref document: A1 |