[Title established by the ISA under Rule 37.2] NOVEL FORM OF SULFENTRAZONE, PROCESS FOR ITS PREPARATION AND USE THEREOF
Cross Reference of related applications
The present application claims the priority of UK Patent Application No. 1608830.4, filed on May 19, 2016, the contents of which are incorporated herein by reference in its entirety.
Technical Field
The present disclosure describes a crystalline form of N- [2, 4-dichloro-5- [4- (difluoromethyl) -4, 5-dihydro-3-methyl-5-oxo-1H-1, 2, 4-triazol-1-yl] ph enyl] methanesulfonamide (sulfentrazone) , to its preparation processes and to its use in agrochemical preparations.
Background
N-[2, 4-dichloro-5- [4- (difluoromethyl) -4, 5-dihydro-3-methyl-5-oxo-1H-1, 2, 4-triazol-1-yl] phenyl] methanesulfonamide (sulfentrazone) , is a member of the aryl triazolinone group of chemicals. Sulfentrazone is a pre-emergent and post-emergent herbicide and very effective on sedges in turfgrass. It controls the weeds by a generally known mode of action called protoporphyrinogen oxidase (PPO) inhibition. The active ingredient sulfentrazone is mainly taken up by the roots, which can be identified by shoot-root soil placement studies, of the soil treated plants. As a result, the soil treated plants turn necrotic and die after exposure to sunlight, while the foliar contacted plant tissues results in desiccation and necrosis. Sulfentrazone controls annual and perennial sedges, cool season grasses and also broadleaf weeds that are in established, warm season, perennial grasses. In addition to this, it is also used to remove tall fescue and sedges from warm season turfgrasses and Kentucky bluegrass.
Sulfentrazone has molecular formula of C11H10Cl2F2N4O3S. Its chemical structure is
The commercially available sulfentrazone, which is usually manufactured by the process described in US PAT. NO. 4,818,275, which is incorporated herein by reference for all purposes, is present in an amorphous state having a melting point of 75℃ to 78℃. It has been found that sulfentrazone in an amorphous state is not suitable for being prepared as compositions or formulations as it is extremely susceptible to direct photolysis in aqueous solution. Direct photolysis of sulfentrazone aqueous solution exposed to simulated sunlight occurs rapidly with half-lives of about 1-12 hours. Therefore, there is a need to develop a novel form of sulfentrazone exhibiting an improved photolysis stability in aqueous solution.
Statements of Invention
In attempt to resolve some or all of the problems with existing amorphous form of sulfentrazone, a new and stable crystalline form of sulfentrazone has been prepared.
In a first aspect, the invention provides a novel crystalline form of N- [2, 4-dichloro-5- [4- (difluoromethyl) -4, 5-dihydro-3 -methyl-5-oxo-iH-1, 2, 4-triazol-1 -yl] ph enyl] methanesulfonamide (sulfentrazone) , termed “crystalline modification I” , exhibiting at least 3 of the following reflexes, in any combination, as 2θ ± 0.2 degree in an X-ray powder diffractogram (X-RPD) recorded using Cu-Kα radiation at 25℃:
2θ = 6.3 ± 0.2 (1)
2θ = 6.8 ± 0.2 (2)
2θ = 12.1 ± 0.2 (3)
2θ = 17.0 ± 0.2 (4)
2θ = 17.6 ± 0.2 (5)
2θ = 18.5 ± 0.2 (6)
2θ = 19.1 ± 0.2 (7)
2θ = 19.9 ± 0.2 (8)
2θ = 21.6 ± 0.2 (9)
2θ = 22.6 ± 0.2 (10)
2θ = 24.3 ± 0.2 (11)
2θ = 24.6 ± 0.2 (12)
2θ = 28.5 ± 0.2 (13)
2θ = 29.0 ± 0.2 (14)
2θ = 32.2 ± 0.2 (15)
2θ = 34.4 ± 0.2 (16) .
In an embodiment, the crystalline modification according to the first aspect of the invention, exhibits at least 3, 4, 5, 6, 7, 8 or all of the reflexes, in any combination, from the following:
2θ = 6.3 ± 0.2 (1)
2θ = 6.8 ± 0.2 (2)
2θ = 12.1 ± 0.2 (3)
2θ = 17.0 ± 0.2 (4)
2θ = 18.5 ± 0.2 (6)
2θ = 19.1 ± 0.2 (7)
2θ = 21.6 ± 0.2 (9)
2θ = 24.6 ± 0.2 (12)
2θ = 29.0 ± 0.2 (14) .
In a second aspect, the present invention provides a crystalline modification I of sulfentrazone, optionally according to the first aspect of the invention, exhibiting an infrared (IR) spectrum with characteristic functional group vibration peaks at wavenumbers (cm-1, ± 0.2%) of one or more of about 3236, 1741, 1613, 1483, 1394, 1318, 1145, 1096 and 1056cm-1.
In a third aspect, the present invention provides a crystalline modification I of sulfentrazone, optionally according to the first or second aspect of the invention, exhibiting a melting point 126-130℃, optionally 127-129℃, further optionally 128℃.
In a fourth aspect, the present invention provides a crystalline modification I of sulfentrazone, optionally according to any one of the first to third aspects of the invention, exhibiting a differential scanning calorimetry (DSC) profile having an endothermic melting peak with onset at 125℃ and peak maximum at 128℃, further optionally with a melting enthalpy of 73 J/g.
In a fifth aspect, the present invention provides a crystalline modification I of sulfentrazone, optionally according to any one of the first to fourth aspects of the invention, characterized by X-ray powder diffraction pattern as substantially shown in Figure 2, and/or characterized by an IR spectrum as substantially shown in Figure 1, and/or characterized by a DSC thermogram as substantially shown in Figure 3.
In a sixth aspect, the present invention provides a crystalline modification I of sulfentrazone, optionally according to any one of the first to fifth aspects of the invention, obtainable by the process as substantially as described in Example 2 or 3.
In a seventh aspect, the present invention provides a crystalline modification I of sulfentrazone, optionally according to any one of the first to sixth aspects of the invention, obtainable by the process of the eighth aspect of the invention.
It has been found that the crystalline modification I of sulfentrazone has a significant improvement in its photolysis stability. In addition, it is found that the crystalline modification I of sulfentrazone is easier to filter, comminute or grind, compared to amorphous sulfentrazone prepared in accordance with the disclosure of US PAT. NO. 4,818,275 and that formulations prepared using this crystalline modification I are stable after prolonged storage. In addition, the crystalline modification I has a lower tendency to degrade after exposure to light (photolysis) compared to the amorphous state described in US PAT. NO. 4,818,275. This allows the preparation of commercial formulations such as suspension concentrates (SC) , and water-dispersible granules (WG) . For these reasons, the crystalline modification I is more suitable for preparing commercial formulations. By virtue of its high photolysis stability, the crystalline modification I of sulfentrazone gives the desired long storage period to its formulations. Hence, it is possible to prepare any formulations of sulfentrazone of crystalline modification I, which will be disclosed
hereinafter.
In an eighth aspect, the present invention provides a process of preparing a crystalline modification I of sulfentrazone comprising steps of:
i) dissolving sulfentrazone in a solvent, or mixture of solvents;
ii) precipitating the dissolved compound into crystalline modification I of sulfentrazone; and
iii) isolating the precipitated crystalline modification I.
In an embodiment of the eighth aspect of the invention, the sulfentrazone in step i) is amorphous sulfentrazone.
Methods for preparing amorphous sulfentrazone are known in the art. Amorphous sulfentrazone is manufactured and available on a commercial scale. A particularly suitable method for preparing amorphous sulfentrazone is described in US PAT. NO. 4,818,275.
In an embodiment of the eighth aspect of the invention, the solvent is selected from the group consisting of halogenated hydrocarbons (for example, chlorobenzene, bromobenzene, dichlorobenzene, trifluoro methyl benzene and trichlorobenzene) , ethers (for example, diethyl ether, ethyl propyl ether, n-butyl ether, anisole, phenetole, cyclohexyl methyl ether, dimethyl ether, dimethyl glycol, diphenyl ether, dipropyl ether, diisopropyl ether, di-n-butyl ether, diisobutyl ether, diisoamyl ether, ethylene glycol dimethyl ether, isopropyl ethyl ether, methyl tert-butyl ether, tetrahydrofuran, methyltetrahydrofuran, dioxane, dichlorodiethyl ether, methyl-tetrahydrofuran, polyethers of ethylene oxide and/or propylene oxide) , nitrated hydrocarbons (for example, nitromethane, nitroethane, nitropropane, nitrobenzene, chloronitrobenzene and ethyl benzene) , aliphatic, cycloaliphatic or aromatic hydrocarbons (for example, pentane, n-hexane, n-heptane, n-octane, nonane) , cymene, petroleum fractions having a boiling range of from 70 ℃ to 190 ℃, cyclohexane, methylcyclohexane, petroleum ether, ligroin, octane, benzene and xylene) , esters (for example, malonates, acetic acid n-butyl ester (n-butyl acetate) , methyl acetate, ethyl acetate, isobutyl acetate, dimethyl carbonate, diethyl carbonate, dibutyl carbonate and ethylene carbonate) , and aliphatic alcohols (for example, methanol, ethanol, n-propanol, isopropanol, n-butanol and tert-amyl alcohol) , mesitylene, diethyl ketone, methyl ethyl ketone, acetonitrile and mixtures thereof.
In an embodiment of the eighth aspect of the invention, the solvent is selected from
the group consisting of xylene, benzene, chlorobenzene, dichlorobenzene, ethyl benzene, trifluoro methyl benzene, mesitylene, nitrobenzene, ether, diethyl ketone, methyl ethyl ketone, methanol, ethanol, isopropanol, acetonitrile or mixture of THF-hexane, ethyl acetate-hexane, dichloromethane-hexane, dichloromethane-methanol, THF-water and methanol-water. Solvent mixtures of more than 2, 3 or 4 components are also envisaged by embodiments of the invention.
In an embodiment of the eighth aspect of the present invention, the solvent is selected from the group consisting of diethyl ketone, xylene or a mixture thereof.
According to an embodiment of the eighth aspect of the present invention, crystalline modification I of sulfentrazone is prepared by dissolving amorphous sulfentrazone in a solvent or a solvent mixture as a concentrated solution by heating from ambient temperature to a temperature at or below the reflux temperature of the solvent or the solvent mixture. Optionally, the concentrated solutions can be prepared at the reflux temperature of the solvents. The concentration of the solution depends on the solubility of sulfentrazone in the corresponding solvent or solvent mixture.
In an embodiment of the eighth aspect of the invention, the concentrated homogeneous solution thus prepared as in step (i) is then cooled to room temperature or to a temperature of about 0℃ to 20℃ to crystallize the desired crystalline form from the solvent. The crystalline modification I of sulfentrazone can also be crystallized out by concentrating the homogeneous solution by removing the solvent or solvent mixture to a certain volume with or without applying vacuum and cooling to below the reflux temperature of the solvent or the solvent mixture.
In an embodiment of the eighth aspect of the invention, crystalline modification I of sulfentrazone can also be effected by adding seed crystals of the desired crystalline form during crystallization into a solution prepared in step (i) , which can promote or accelerate the crystallization.
The seed crystal amount added to the concentrated solution is typically in the range of 0.001%to 10%by weight, optionally 0.001%to 2.5%by weight, further optionally 0.005 to 0.5%by weight based on the weight of sulfentrazone used for the preparation of concentrated solution in step (i) . Optionally, the seed crystals are added to the concentrated solution at the temperature below the boiling point of the corresponding solvent or the solvent mixture.
In an embodiment of the eighth aspect of the invention, the precipitated crystalline modification I of sulfentrazone obtained from step (ii) is isolated by the usual solid component separation techniques from solutions, such as filtration, centrifugation or decantation. Then, the isolated solid is washed with solvent one or more times. Optionally, the solvent employed in the washing stage consists of one or more components of the solvent or solvent mixture employed for preparation of concentrated solution in step (i) , as described hereinbefore. The washing is usually carried out using the corresponding solvent or solvent mixture between room temperature and 0℃, depending on the solubility of the crystal, in order to minimize or avoid the loss of crystalline material in the corresponding washing solvent as much as possible. In an embodiment of the eighth aspect of the invention, crystalline modification I of sulfentrazone is dissolved and recrystallized. The washings and/or the solvent of crystallization in any of the methods may be concentrated to obtain solid sulfentrazone which may be recycled.
In a ninth aspect, the present invention provides a crystalline material comprising a crystalline modification I of sulfentrazone obtained according to the eighth aspect of the invention, having a content of a crystalline modification I of sulfentrazone of at least 98%by weight.
In a tenth aspect, the present invention provides a composition comprising the crystalline modification I of sulfentrazone according to any one of the first to seventh and ninth aspects of the invention, and at least one auxiliary.
In an eleventh aspect, the present invention provides a use of the crystalline modification I of sulfentrazone according to any one of the first to seventh and ninth aspects of the invention, or a composition according to the tenth aspect of the invention, for weed control.
In an embodiment of the tenth aspect of the invention, the amount of the crystalline modification I of sulfentrazone is less than 90%by weight of the composition, optionally less than 75%by weight of the composition, further optionally less than 60%by weight of the composition, still further optionally about 50%by weight of the composition.
The use of amorphous sulfentrazone as an herbicide is known in the art and is used on a commercial scale. It has been found that the crystalline modification I of sulfentrazone is also active in controlling undesirable plants, such as weeds. As a result, the techniques of formulating and applying sulfentrazone known in the art with respect to amorphous
sulfentrazone, for example as disclosed in the prior art documents described hereinbefore, can also be applied in an analogous manner to sulfentrazone in the crystalline modification I of the invention.
Accordingly, the invention provides a herbicidal composition comprising sulfentrazone in the crystalline modification I as defined hereinbefore.
The invention furthermore provides processes for preparing compositions for controlling undesirable plants, such as weeds using the crystalline modification I of sulfentrazone.
The invention also provides a method for controlling unwanted plant growth, comprising applying to the plant, plant part, or surroundings of the plant, a herbicidally effective amount of crystalline modification I of sulfentrazone according to any one of the first to seventh and ninth aspects of the invention, or a composition according to the tenth aspect of the invention. Accordingly, this provides for controlling undesirable plants in plants, plant parts, and/or their surroundings, comprising applying to the foliage or fruit of the plant, plant part, or surroundings of the plant, a herbicidally effective amount of crystalline modification I of sulfentrazone.
In an embodiment of the tenth aspect of the invention, the composition is in the form of a suspension concentrate (SC) , an oil-based suspension concentrate (OD) , a water-soluble granule (SG) , a dispersible concentrate (DC) , an emulsifiable concentrate (EC) , an emulsion seed dressing, a suspension seed dressing, a granule (GR) , a microgranule (MG) , a suspoemulsion (SE) or a water-dispersible granule (WG) . Crystalline modification I of sulfentrazone can be included into these customary formulations in a known manner using suitable auxiliaries, carriers and solvents and the like, in a manner analogous to that known for amorphous sulfentrazone.
In an embodiment of the tenth aspect of the invention, the composition is in the form of a suspension concentrate (SC) .
In an embodiment of the tenth aspect of the invention, the composition is in the form of a water-dispersible granule (WG) .
In an embodiment of the tenth aspect of the invention, the crystalline modification I of sulfentrazone may be present in a concentration sufficient to achieve the required dosage when applied to plants or the loci thereof, desirably in a concentration of about 1 to about 75%by weight of the total mixture. The formulations are prepared, for example, by
extending the crystalline modification I of sulfentrazone with water, solvents and carriers, using, if appropriate, emulsifiers and/or dispersants, and/or other auxiliaries.
These formulations are prepared by mixing the crystalline modification I of sulfentrazone with at least one herbicidally acceptable auxiliary, for example, surfactants, liquid diluents, solid diluents, wetting agents, dispersants, thickening agents, antifoaming agents, anti-freezing agents, preservatives, antioxidants, solid adherents, inert fillers and other formulation ingredients.
Surfactants can be an emulsifier, dispersant or wetting agent of ionic or nonionic type. Examples which may be used include, but are not limited to, salts of polyacrylic acids, salts of lignosulphonic acid, salts of phenylsulphonic or naphthalenesulphonic acids, polycondensates of ethylene oxide with fatty alcohols or with fatty acids or with fatty amines, substituted phenols, especially alkylphenols, sulphosuccinic ester salts, taurine derivatives, especially alkyltaurates, or phosphoric esters of polyethoxylated phenols or alcohols.
Liquid diluents include, but are not limited to, water, N, N-dimethylmamide, dimethyl sulfoxide, N-alkylpyrrolidone, ethylene glycol, polypropylene glycol, propylene carbonate, dibasic esters, paraffines, alkylbenzenes, alkyl naphthalenes, glycerine, triacetine, oils of olive, castor, linseed, sesame, corn, peanut, cotton-seed, soybean, rape-seed and coconut, ketones such as cyclohexanone, 2-heptanone, isophorone and 4-hydroxy-4-methyl-2-pentanone, acetates such as hexyl acetate, heptyl acetate and octyl acetate, and alcohols such methanol, cyclohexanol, decanol, benzyl and tetrahydrofurfuryl alcohol, and mixtures thereof.
Solid diluents can be water-soluble or water-insoluble. Water-soluble solid diluents include, but are not limited to, salts such as alkali metal phosphates (e.g., sodium dihydrogen phosphate) , alkaline earth phosphates, sulfates of sodium, potassium, magnesium and zinc, sodium and potassium chloride, sodium acetate, sodium carbonate and sodium benzoate, and sugars and sugar derivatives such as sorbitol, lactose, sucrose and mannitol. Examples of water-insoluble solid diluents include, but are not limited to clays, synthetic and diatomaceous silicas, calcium and magnesium silicates, titanium dioxide, aluminum, calcium and zinc oxide, and mixtures thereof.
Wetting agents include, but are not limited to, alkyl sulfosuccinates, laureates, alkyl sulfates, phosphate esters, acetylenic diols, ethoxyfluornated alcohols, ethoxylated silicones,
alkyl phenol ethyoxylates, benzene sulfonates, alkyl-substituted benzene sulfonates, alkyl a-olefin sulfonates, naphthalene sulfonates, alkyl-substituted napthalene sulfonates, condensates of naphthalene sulfonates and alkyl-substituted naphthalene sulfonates with formaldehyde, and alcohol ethoxylates, and mixtures thereof. Alkyl naphthalene sulphonates, sodium salts are particularly useful for the composition of the invention
Dispersants include, but are not limited to, sodium, calcium and ammonium salts of ligninsulfonates (optionally polyethoxylated) ; sodium and ammonium salts of maleic anhydride copolymers; sodium salts of condensed phenolsulfonic acid; and naphthalene sulfonate-formaldehyde condensates. Ligninsulfonates such as sodium ligninsulfonates are particularly useful for the composition of the invention. Naphthalene sulfonate-formaldehyde condensates such as naphthalenesulfonic acid, polymers with formaldehyde, and sodium salts are particularly useful for the composition of the invention
Thickening agents include, but are not limited to, guar gum, pectin, casein, carrageenan, xanthan gum, alginates, methylcellulose, hydroxyethylcellulo se, hydroxypropylcellulose, and carboxymethylcellulose, and mixtures thereof. Synthetic thickening agents include derivatives of the former categories, and also polyvinyl alcohols, polyacrylamides, polyvinylpyrrolidones, various polyethers, their copolymers as well as polyacrylic acids and their salts, and mixtures thereof. Alkylpolyvinylpyrrolidones are particularly useful for the composition of the invention.
Antifoaming agents include all substances which can normally be used for this purpose in agrochemical compositions. Suitable anti-foam agents are known in the art and are available commercially. Particularly preferred antifoam agents are mixtures of polydimethylsiloxanes and perfluroalkylphosphonic acids, such as the silicone antifoaming agents available from GE or Compton.
Preservatives include all substances which can normally be used for this purpose in agrochemical compositions of this type and again are well known in the art. Suitable examples that may be mentioned include
(from Bayer AG) and
(from Bayer AG) .
Antioxidants include all substances which can normally be used for this purpose in agrochemical compositions, as is known in the art. Preference is given to butylated hydroxytoluene.
Solid adherents include organic adhesives, including tackifiers, such as celluloses or
substituted celluloses, natural and synthetic polymers in the form of powders, granules, or lattices, and inorganic adhesives such as gypsum, silica or cement.
Inert fillers include but are not limited to, natural ground minerals, such as kaolins, aluminas, talc, chalk, quartz, attapulgite, montmorillonite, and diatomaceous earth, or synthetic ground minerals, such as highly dispersed silicic acid, aluminum oxide, silicates, and calcium phosphates and calcium hydrogen phosphates. Suitable inert fillers for granules include, for example, crushed and fractionated natural minerals, such as calcite, marble, pumice, sepiolite, and dolomite, or synthetic granules of inorganic and organic ground materials, as well as granules of organic materials, such as sawdust, coconut husks, corn cobs, and tobacco stalks.
Other formulation ingredients can also be used in the present invention such as dyes, drying agents, and the like. These ingredients are known to one skilled in the art.
In an embodiment of the tenth aspect of the invention, the crystalline modification I of sulfentrazone can be present in formulations and in other forms that are prepared from these formulations, and as a mixture with other active compounds (such as attractants, sterilizing agents, bactericides, acaricides, nematicides, fungicides, growth-regulating substances, herbicides, safeners, fertilizers, semiochemicals and other insecticides) or with agents for improving plant properties.
All plants, plant parts, and their surroundings can be treated with the crystalline modification I of sulfentrazone in accordance with any aspect or embodiment of the invention. In the present context, plants are to be understood as meaning all plants and plant populations such as desired and undesired wild plants or crop plants (including naturally occurring crop plants) . Crop plants can be plants which can be obtained by conventional breeding and optimization methods, by biotechnological and genetic engineering methods, or by combinations of these methods, including the transgenic plants and the plant cultivars which can or cannot be protected by plant breeders′rights. Plant parts are to be understood as meaning all parts and organs of plants above and below the ground, such as shoot, leaves, needles, stalks, stems, flowers, fruit bodies, fruits, seeds, roots, tubers and rhizomes. Harvested materials, and vegetative and generative propagation materials, for example, cutting, tubers, meristem tissue, rhizomes, offsets, seeds, single and multiple plant cells and any other plant tissues, are also included.
Treatment of the plants, plant parts, and/or their surroundings, with the compositions
or formulations of the invention can be carried out directly or by allowing the compositions or formulations to act on their surroundings, habitat or storage space by the customary treatment methods. Examples of these customary treatment methods include dipping, spraying, vaporizing, fogging, broadcasting, painting on in the case of propagation material, and applying one or more coats particularly in the case of seed.
The benefits of the invention are seen most when the herbicidal composition is applied to kill weeds in growing crops of useful plants: leguminous plants (such as soybean) , oil plants such as sunflower) , maize (corn) including field corns, pop corns and sweet corns, cotton, cereal, barley, wheat, rice, oats, potatoes, sugar beets, plantation crops (such as bananas, fruit trees, rubber trees, tree nurseries) , vines, citrus, olive, amenity, vegetables (such as spinach, lettuce, asparagus, cabbages, carrots, onions, tomatoes, potatoes, paprika, garlic and leeks) , bushberries (such as blueberries) , caneberries, cranberries, flax, grain sorghum, okra, peppermint, rhubarb, peanut spearmint, turf grass, grapevine and sugarcane. In this invention, treatment of soybean, sunflower peanut and sugarcane are particularly beneficial.
Throughout the description and claims of this specification, the words “comprise” and variations of the words, for example “comprising” and “comprises” , mean “including but not limited to” , and do not exclude other moieties, additives, components, integers or steps. Moreover the singular encompasses the plural unless the context otherwise requires: in particular, where the indefinite article is used, the specification is to be understood as contemplating plurality as well as singularity, unless the context requires otherwise.
Preferred features of each aspect of the invention may be as described in connection with any of the other aspects. Other features of the invention will become apparent from the following examples. Generally speaking the invention extends to any novel one, or any novel combination, of the features disclosed in this specification (including any accompanying claims and drawings) . Thus features, integers, characteristics, compounds, chemical moieties or groups described in conjunction with a particular aspect, embodiment or example of the invention are to be understood to be applicable to any other aspect, embodiment or example described herein unless incompatible therewith. Moreover unless stated otherwise, any feature disclosed herein may be replaced by an alternative feature serving the same or a similar purpose.
Where upper and lower limits are quoted for a property then a range of values
defined by a combination of any of the upper limits with any of the lower limits may also be implied.
In this specification, references to properties are -unless stated otherwise -to properties measured under ambient conditions, i.e. at atmospheric pressure and at a temperature of about 20℃.
As used herein, the term “about” or ″around″ when used in connection with a numerical amount or range, means somewhat more or somewhat less than the stated numerical amount or range, and for example to a deviation of ± 10%of the stated numerical amount or endpoint of the range.
“Surrounding, ” as used herein, refers to the place on which the plants are growing, the place on which the plant propagation materials of the plants are sown or the place on which the plant propagation materials of the plants will be sown.
″Precipitation″ as used herein, refers to the sedimentation of a solid material (a precipitate) , including the sedimentation of a crystalline material, from a liquid solution in which the solid material is present in amounts greater than its solubility in the amount of liquid solution.
The term “herbicidally effective amount” as used herein, refers to the quantity of such a compound or combination of such compounds that is capable of producing a controlling effect on the growth of plants. The controlling effects include all deviation from the natural development of the target plants, for example killing, retardation of one or more aspects of the development and growth of the plant, leaf burn, albinism, dwarfing and the like.
All percentages are given in weight %unless otherwise indicated.
Brief Description of Drawings
Various features and aspects of the embodiments of the invention disclosed herein can be more clearly understood by reference to the drawings, which are intended to exemplify and illustrate, but not to limit, the scope of the invention, and wherein:
FIG. 1 is an infrared (IR) spectrum of crystalline modification I of sulfentrazone;
FIG. 2 is a X-ray powder diffractogram (X-RPD) of crystalline modification I of sulfentrazone;
FIG. 3 is a Differential Scanning Calorimetry (DSC) thermogram of crystalline modification I of sulfentrazone;
FIG. 4 is a X-ray powder diffractogram of amorphous sulfentrazone.
Detailed Description
The present invention will now be described by the following examples, and in which the following measurement techniques have been employed, and which the examples are provided for illustrative purposes only, and not intended to limit the scope of the disclosure.
All X-ray diffractograms were determined using powder diffractometer in reflection geometry at 25℃, using the following acquisition parameters:
| X’Pert Pro MPD from PANalytical B.V. |
| Theta compensating slit and graphite monochromator |
| Copper (K-alpha) radiation, 40 kV, 40 mA |
| Step size: 0.03 degree 2-theta |
| Count time: 1.0 second |
| Maximum peak intensity: 1705 counts per second |
| Scan range: 3-60 degrees 2-theta |
The IR spectrum was measured with the resolution of 4 cm-1 and with the number of scans of 16 for the crystallized samples. The crystalline modification I of sulfentrazone can be identified by its characteristic functional group vibration peaks at wavenumbers (cm-1, ± 0.2%) of one or more of about 3236, 1741, 1613, 1483, 1394, 1318, 1145, 1096 and 1056 cm-1 as shown in Figure 1.
All IR spectra were obtained using the following acquisition parameters:
| FT-IR spectrometer |
NicoletTM iS 5 |
| Diamond ATR unit |
Thermo ScientificTM iD5 ATR |
| Wavelength range |
550-4000 cm-1
|
| Resolution |
4 cm-1
|
| Number of scans |
16 |
All DSC thermograms were obtained using the following acquisition parameters:
Examples
Example 1: Preparation of amorphous sulfentrazone in accordance with the disclosure of US PAT. NO. 4,818,275 (Example 13, Example 14 and Example 15) :
1. Preparation of 1- (5-amino-2, 4-dichlorophenyl) -3-methyl-4-difluoromethyl-Δ2-
1, 2, 4-triazolin-5-one as an intermediate (Example 13)
Step A -Synthesis of pyruvic acid, 2, 4-dichlorophenylhydrazone as an intermediate
To a stirred solution of 16.2 g (0.07 mole) of commercially available 2, 4-dichlorophenylhydrazine hydrochloride in 100 ml of ethanol was added in one portion 9.2 g (0.11 mole) ofpyruvic acid in 100 ml of water. The reaction mixture was stirred for 10 minutes and the resultant solid collected by filtration to yield when dried 13.5 g of pyruvic acid, 2, 4-dichlorophenylhydrazone, m.p. 193℃-194℃. The reaction was repeated several times.
Step B -Synthesis of 1- (2, 4-dichlorophenyl) -3-methyl-Δ2-1, 2, 4-triazolin-5-one as an intermediate
To a stirred suspension of 13.6 g (0.054 mole) of pyruvic acid, 2, 4-dichlorophenylhydrazone in 100 ml of toluene, 5.5 g (0.054 mole) of triethylamine was added. The reaction mixture became homogeneous and 14.9 g (0.054 mole) of triphenylphosphoryl azide was added. Upon completion of addition the reaction mixture was heated to reflux where it stirred for two hours. The reaction mixture was cooled to ambient temperature and extracted with 300 ml of aqueous 1N sodium hydroxide. The extract was neutralized with concentrated hydrochloric acid and a solid precipitate collected by filtration. The solid was washed with water and dried. The yield of 1- (2, 4-dichlorophenyl) -3-methyl-Δ2-1, 2, 4-triazolin-5-one was 13.0 g; m.p. 174℃-175℃. The reaction was repeated several times.
Step C -synthesis of 1- (2, 4-dichlorophenyl) -3-methyl-4-difluoromethyl-Δ2 -1, 2, 4-
triazolin-5-one as an Intermediate
A stirred solution of 16.0 g (0.065 mole) of1- (2, 4-dichlorophenyl) -3-methyl-Δ2-1, 2, 4-triazolin-5-one, 7.3 g (0.13 mole) of potassium hydroxide, and 10.5 g (0.03 mole) of tetrabutylammonium bromide in 150 ml of tetrahydrofuran was cooled in an ice bath and chlorodifluoromethane was bubbled into the reaction mixture. The ice bath was removed and chlorodifluoromethane continued to bubble into the reaction mixture until condensation of it was observed on a dry ice condenser attached to the reaction vessel. Upon completion of addition the reaction mixture stirred at ambient temperature for 16 hours. An additional 6.7 g (0.12 mole) of powdered potassium hydroxide was added to the reaction mixture and it was again saturated with chlorodifluoromethane. The reaction mixture was stirred for two hours then diluted with water. The mixture was extracted with diethyl ether and the combined extracts washed with water. The organic layer was dried with sodium sulfate and filtered. The filtrate was
concentrated under reduced pressure to a residue. The residue was dissolved in methylene chloride and passed through a pad of silica gel. The eluate was concentrated under reduced pressure to a residual solid. The solid was precipitated from methylene chloride-heptane. The yield of 1 - (2, 4-dichlorophenyl) -3 -methyl-4-difluoromethyl-Δ2-1, 2, 4-triazolin-5-one was 4.1 g; m.p. 108℃-110℃. The reaction was repeated several times.
Step D -Synthesis of 1- (2, 4-dichloro-5-nitrophenyl) -3-methyl-4-difluoromethyl-Δ2-
1, 2, 4-triazolin-5-one as an intermediate
To a stirred solution of 4.0 g (0.013 mole) of 1- (2, 4-dichlorophenyl) -3-methyl-4-difluoromethyl-Δ2 -1, 2, 4-triazolin-5-one in 20 ml of concentrated sulfuric acid was slowly added 1.2 ml (0.015 mole) of 70%nitric acid, while maintaining the reaction mixture temperature at 25℃. Upon completion of addition the reaction mixture was stirred at 25℃. for 30 minutes, then poured into ice water. The resultant solid was collected by filtration. The solid was dissolved in methylene chloride and passed through a pad of silica gel. The eluate was subjected to column chromatography on silica gel. Elution was completed using 1∶1-petroleum ether: methylene chloride. The appropriate fractions were combined and concentrated under reduced pressure. The yield of 1- (2, 4-dichloro-5-nitrophenyl) -3-methyl-4-difluoromethyl-Δ2-1, 2, 4-triazolin-5-one was one 3.0 g; m.p. 95℃-97℃. The reaction was repeated several times.
Step E -Synthesis of 1- (5-amino-2, 4-dichlorophenyl) -3-methyl-4-difluoromethyl-Δ2
-1,2, 4-triazolin-5-one as an intermediate
To a stirred solution of 2.5 g (0.007 mole) of 1- (2, 4-dichloro-5-nitrophenyl) -3-methyl-4-difluoromethyl-Δ2-1, 2, 4-triazolin-5-one in 6 ml of acetic acid and 60 ml of water was added portionwise 2.5 g (0.045 mole) of powdered iron at a rate to maintain the reaction mixture temperature below 35℃. Upon completion of addition the reaction mixture was stirred at 25℃-30℃ for two hours. The reaction mixture was diluted with diethyl ether with stirring, then was filtered through diatomaceous earth. The stirred filtrate was made basic with aqueous 10%sodium bicarbonate solution and solid potassium carbonate. The organic layer was separated, washed with three portions of water, then dried with sodium sulfate. The mixture was filtered and the filtrate was concentrated under reduced pressure to a residue. The residue was purified by column chromatography on silica gel using methylene chloride: acetone as an eluent. The appropriate fractions were combined and concentrated under reduced pressure. The yield of 1- (5-amino-2, 4-dichlorophenyl) -3-methyl-4-difluoromethyl-Δ2-1, 2, 4-triazolin-5-one was 2.0 g; m.p. 133℃-135℃. The reaction was repeated several times.
2. Preparation of 1- [2, 4-dichloro-5- [bis (N-methylsulfonyl) amino] phenyl] -3-methyl -4-difluoromethyl-Δ2 -1, 2, 4-triazolin-5-one (Example 14)
Stir the solution of 1.2 g (0.004 mole) of 1- (5-amino-2, 4-dichlorophenyl) -3-methyl-4-difluoromethyl-1, 2, 4-triazolin-5-one, and 0.95 g (0.009 mole) of triethylamine in 15 ml of methylene chloride which was cooled in an ice/acetone bath. 0.97 g (0.009 mole) of methanesulfonyl chloride was added dropwise at a rate to maintain the reaction mixture temperature below 0℃. The complete addition required five minutes. Upon completion of addition the reaction mixture was allowed to warm to ambient temperature where it stirred for 16 hours. After this time the reaction mixture was concentrated under reduced pressure to a residue. The residue was purified by column chromatography on silica gel using 50∶1-methylene chloride-acetone as an eluent. The appropriate fractions were combined and concentrated under reduced pressure to a solid. The solid was precipitated from acetone/heptane. The yield of 1- [2, 4-dichloro-5- [bis (N-methylsulfonyl) amino] phenyl] -3 -methyl-4-difluoromethyl-1, 2, 4-tri azolin-5-one was 1.3 g; m.p. 213°-214℃.
3. Preparation of sulfentrazone (Example 15)
Stir the solution of 0.8 g (0.002 mole) of 1- [2, 4-dichloro-5- [bis (N-methylsulfonyl) amino] phenyl] -3 -methyl-4-difluoromethyl-1, 2, 4-tri azolin-5-one in 10 ml ethanol and 0.14 g (0.003 mole) of sodium hydroxide in 0.3 ml of water was added. Upon completion of addition the reaction mixture was stirred for 15 minutes then poured into 100 ml of water. The mixture was neutralized with concentrated hydrochloric acid and the solid precipitate collected by filtration. The yield of N- [2, 4-dichloro-5- [4- (difluoromethyl) -4, 5-dihydro-3 -methyl-5-oxo-1H-1, 2, 4-triazol-1 -yl] ph enyl] methanesulfonamide (sulfentrazone) was 0.5 g; m.p. 75℃-78℃.
Preparation of crystalline modification I of sulfentrazone
Example 2 -Crystallization from xylene
Amorphous sulfentrazone sample (10 g) as prepared in Example 1 was taken in a 3-neck round bottom flask along with xylene (60 ml) . The resulting slurry was heated to 90℃ to get a homogeneous solution. The homogeneous solution was stirred at 90℃ for 2h and the insoluble particles, if any, were filtered and the solution was slowly cooled to 20-25℃. Upon cooling, fine crystals were formed and the resulting heterogeneous mixture was stirred at 20℃ for 2h. Then, the slurry was filtered and washed with xylene (3 ml) at 20℃. The filtered crystals were dried under vacuum at 60℃. The crystalline product obtained had a purity of about 98%and the recovered product as crystal was found to be about 85%yield.
The obtained crystals were analyzed by IR spectrometry, X-RPD and DSC and found out to be crystalline modification I of sulfentrazone as shown in Figure 1, Figure 2 and Figure 3, respectively.
The IR spectrum of sulfentrazone exhibited the functional group characteristic vibrations peaks at wavenumbers of one or more of about 3235.67, 1740.65, 1613.45, 1482.73, 1393.54, 1318.30, 1145.46, 1095.85 and 1056.05 cm-1 as shown in Figure 1.
The DSC thermogram of sulfentrazone exhibited an endothermic melting peak with onset at 125.2℃ and peak maximum at 128.3℃, further optionally with a melting enthalpy of 73.42 J/g as shown in Figure 3.
The X-ray powder diffractogram of the crystals exhibited the reflexes in Figure 2 and the values are summarized in Table 1.
Table 1. X-ray powder diffractogram reflexes of crystalline modification I of sulfentrazone
Example 3 -Crystallization from diethyl ketone
Sulfentrazone (5 g) sample as prepared in Example 1 was taken in a 3-neck round bottom flask along with diethyl ketone (35 mi) . The resulting slurry was heated to 80℃ to get a homogeneous solution. The resultant hot solution was stirred at 80℃ for 2h and the insoluble particles, if any, were filtered and the solution was slowly cooled to 20℃. Upon cooling, fine crystals were formed and the resulting heterogeneous mixture was stirred at 20℃ for 2h. Then, the slurry was filtered, washed with diethyl ketone (3 mi) at 20℃ and
dried under vacuum at 50℃. The crystalline product obtained had a purity of about 98%and the recovered yield was found to be about 85%.
The crystals were characterized as being sulfentrazone crystalline modification I using IR spectrometry, X-ray powder diffraction and DSC, as described in Example 2.
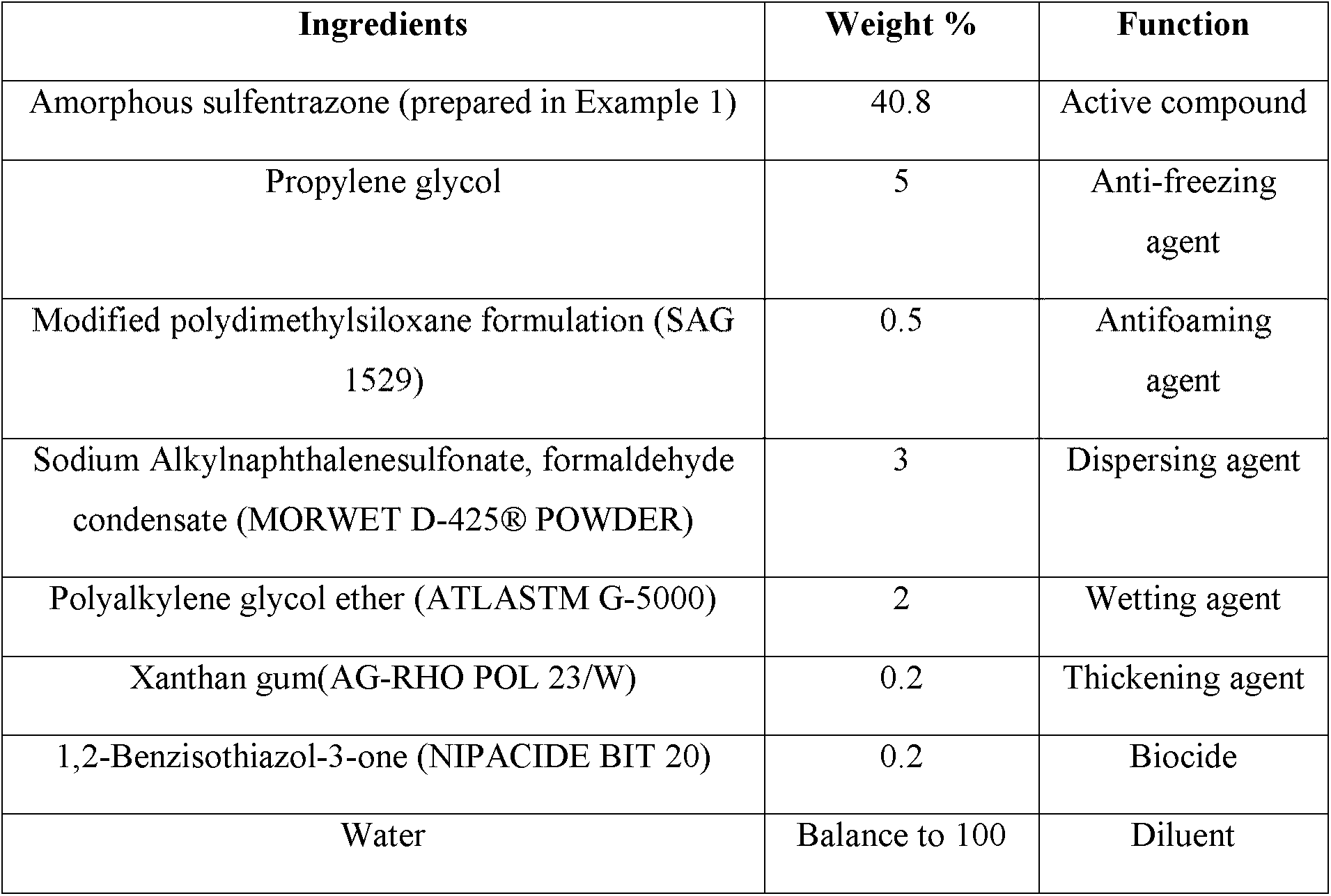
Example 4 -Preparation of suspension concentrate (SC) of amorphous sulfentrazone
All the components listed in Table 2 below were mixed uniformly and the resulting mixture was ground with a Dyno-Mill (manufactured by Willy A. Bachofen AG) to obtain a suspension concentrate.
Table 2 -SC formulations
Example 5 -Preparation of suspension concentrate (SC) of the crystalline modification I of sulfentrazone
All the components listed in Table 3 below were mixed uniformly and the resulting mixture was ground with a Dyno-Mill (manufactured by Willy A. Bachofen AG) to obtain a suspension concentrate.
Table 3 -SC formulations
Example 6 -Preparation of water dispersible granules (WG) of amorphous sulfentrazone
All the components listed in Table 4 below were mixed, blended and milled in a high-speed rotary mill. Sufficient water was added to obtain an extrudable paste. The paste was extruded through a die or screen to form an extrudate. The wet extrudate was dried at 70℃ in a vacuum oven and then sifted through 0.71 mm -2 mm screens to obtain the product granules.
Table 4 -WG formulations
Example 7 -Preparation of water dispersible granules (WG) of the crystalline modification I of sulfentrazone
All the components listed in Table 5 below were mixed, blended and milled in a high-speed rotary mill. Sufficient water was added to obtain an extrudable paste. The paste was extruded through a die or screen to form an extrudate. The wet extrudate was dried at 70℃ in a vacuum oven and then sifted through 0.71 mm -2 mm screens to obtain the product granules.
Table 5 -WG formulations
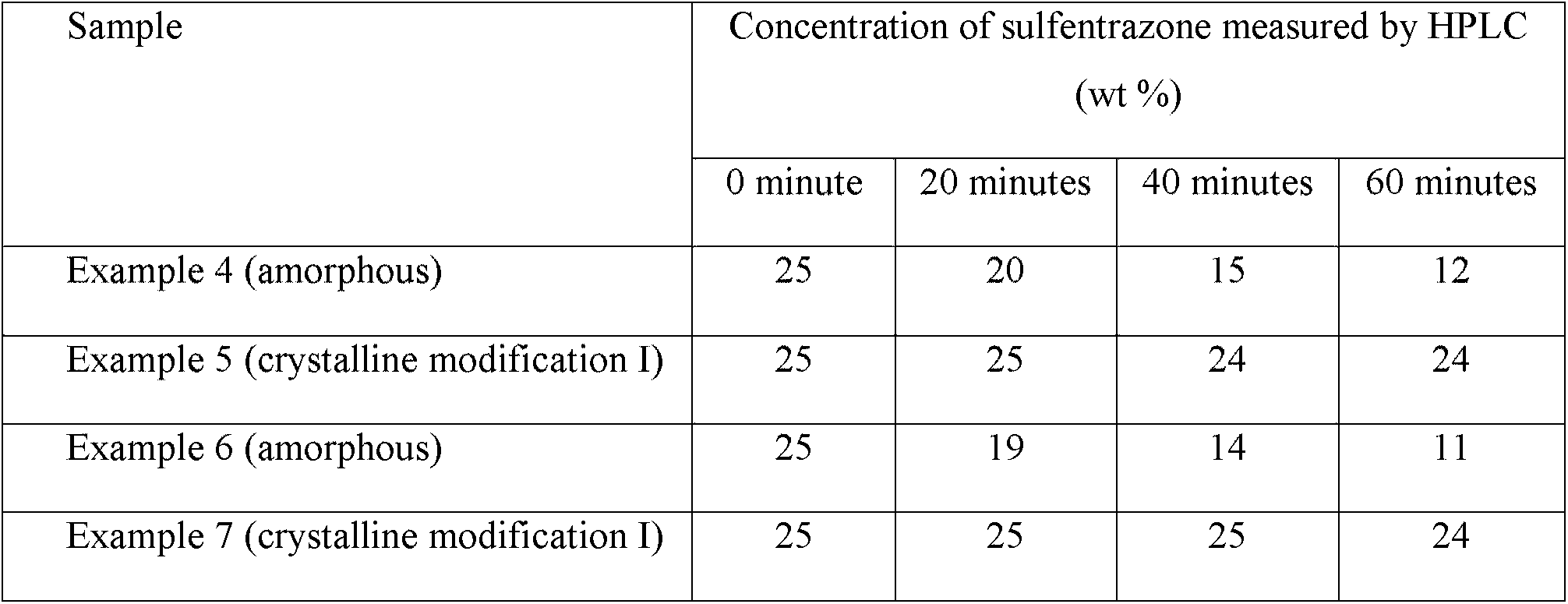
Example 8 -Photolysis Stability Test
The test was conducted by dispersing in water the samples of sulfentrazone prepared in Examples 4 to 7 to produce a concentration of 25%by weight of sulfentrazone. 20 mL of the resulting sulfentrazone suspension of each sample was added to a quartz tube. The tube was continuously irradiated with UV light from a UV lamp at room temperature. An aliquot of each suspension was removed from the tube for analysis at intervals of 20 minutes. The concentration of sulfentrazone in each aliquot was determined by a high pressure liquid chromatography (HPLC) with a reversed phase chromatography column and UV detection. The test was replicated 5 times to ensure accuracy.
The results are summarized in Table 6 below.
Table 6 -Photolysis Stability Test Result
It is surprisingly found that the crystalline modification I of the crystalline sulfentrazone obtained in Examples 5 and 7 exhibited a very high photolysis stability compared with the amorphous sulfentrazone product of Examples 4 and 6. That is, after 60 minutes under these strenuous test conditions, Examples 5 and 7 (containing the crystalline modification I of sulfentrazone) , showed very little decomposition. By contrast, the concentration of the sulfentrazone in Examples 4 and 6 (containing the amorphous sulfentrazone) had halved.