WO2017046694A1 - Process for preparing a pharmaceutical formulation of gadoterate meglumine - Google Patents
Process for preparing a pharmaceutical formulation of gadoterate meglumine Download PDFInfo
- Publication number
- WO2017046694A1 WO2017046694A1 PCT/IB2016/055436 IB2016055436W WO2017046694A1 WO 2017046694 A1 WO2017046694 A1 WO 2017046694A1 IB 2016055436 W IB2016055436 W IB 2016055436W WO 2017046694 A1 WO2017046694 A1 WO 2017046694A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- acid
- dota
- meglumine
- free
- gadolinium
- Prior art date
Links
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 21
- RYHQMKVRYNEBNJ-BMWGJIJESA-K gadoterate meglumine Chemical compound [Gd+3].CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.OC(=O)CN1CCN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC([O-])=O)CC1 RYHQMKVRYNEBNJ-BMWGJIJESA-K 0.000 title claims abstract description 18
- 229940016115 gadoterate meglumine Drugs 0.000 title claims abstract description 15
- 239000008194 pharmaceutical composition Substances 0.000 title claims description 12
- 239000000203 mixture Substances 0.000 claims abstract description 15
- 238000009472 formulation Methods 0.000 claims abstract description 11
- 239000007788 liquid Substances 0.000 claims abstract description 8
- WDLRUFUQRNWCPK-UHFFFAOYSA-N Tetraxetan Chemical compound OC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1 WDLRUFUQRNWCPK-UHFFFAOYSA-N 0.000 claims description 49
- 229910052688 Gadolinium Inorganic materials 0.000 claims description 20
- CMIHHWBVHJVIGI-UHFFFAOYSA-N gadolinium(iii) oxide Chemical compound [O-2].[O-2].[O-2].[Gd+3].[Gd+3] CMIHHWBVHJVIGI-UHFFFAOYSA-N 0.000 claims description 19
- UIWYJDYFSGRHKR-UHFFFAOYSA-N gadolinium atom Chemical compound [Gd] UIWYJDYFSGRHKR-UHFFFAOYSA-N 0.000 claims description 18
- 229910001938 gadolinium oxide Inorganic materials 0.000 claims description 14
- 229940075613 gadolinium oxide Drugs 0.000 claims description 14
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 claims description 12
- 229960003194 meglumine Drugs 0.000 claims description 11
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 10
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 9
- 239000002535 acidifier Substances 0.000 claims description 8
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 6
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 claims description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 6
- 238000002156 mixing Methods 0.000 claims description 5
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 claims description 4
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 claims description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 claims description 4
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 claims description 4
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 claims description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 claims description 4
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 claims description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims description 4
- 229910000147 aluminium phosphate Inorganic materials 0.000 claims description 3
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 claims description 2
- 239000005711 Benzoic acid Substances 0.000 claims description 2
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 claims description 2
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 claims description 2
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 claims description 2
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 claims description 2
- 229940092714 benzenesulfonic acid Drugs 0.000 claims description 2
- 235000010233 benzoic acid Nutrition 0.000 claims description 2
- 229960004365 benzoic acid Drugs 0.000 claims description 2
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 claims description 2
- 239000004327 boric acid Substances 0.000 claims description 2
- IJKVHSBPTUYDLN-UHFFFAOYSA-N dihydroxy(oxo)silane Chemical compound O[Si](O)=O IJKVHSBPTUYDLN-UHFFFAOYSA-N 0.000 claims description 2
- 235000019253 formic acid Nutrition 0.000 claims description 2
- 239000001530 fumaric acid Substances 0.000 claims description 2
- 229960002598 fumaric acid Drugs 0.000 claims description 2
- 235000011087 fumaric acid Nutrition 0.000 claims description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 claims description 2
- 239000011976 maleic acid Substances 0.000 claims description 2
- 229940098779 methanesulfonic acid Drugs 0.000 claims description 2
- 239000011975 tartaric acid Substances 0.000 claims description 2
- 235000002906 tartaric acid Nutrition 0.000 claims description 2
- 229960001367 tartaric acid Drugs 0.000 claims description 2
- YNJBWRMUSHSURL-UHFFFAOYSA-N trichloroacetic acid Chemical compound OC(=O)C(Cl)(Cl)Cl YNJBWRMUSHSURL-UHFFFAOYSA-N 0.000 claims description 2
- 229960004319 trichloroacetic acid Drugs 0.000 claims 1
- 239000002872 contrast media Substances 0.000 abstract description 2
- 238000002595 magnetic resonance imaging Methods 0.000 abstract description 2
- 239000000243 solution Substances 0.000 description 30
- 238000000034 method Methods 0.000 description 12
- GFSTXYOTEVLASN-UHFFFAOYSA-K gadoteric acid Chemical compound [Gd+3].OC(=O)CN1CCN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC([O-])=O)CC1 GFSTXYOTEVLASN-UHFFFAOYSA-K 0.000 description 9
- 239000008215 water for injection Substances 0.000 description 7
- 229960003823 gadoteric acid Drugs 0.000 description 6
- 238000011049 filling Methods 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 4
- 239000013522 chelant Substances 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 239000003446 ligand Substances 0.000 description 2
- 238000010979 pH adjustment Methods 0.000 description 2
- ZPDFIIGFYAHNSK-CTHHTMFSSA-K 2-[4,10-bis(carboxylatomethyl)-7-[(2r,3s)-1,3,4-trihydroxybutan-2-yl]-1,4,7,10-tetrazacyclododec-1-yl]acetate;gadolinium(3+) Chemical compound [Gd+3].OC[C@@H](O)[C@@H](CO)N1CCN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC([O-])=O)CC1 ZPDFIIGFYAHNSK-CTHHTMFSSA-K 0.000 description 1
- PCZHWPSNPWAQNF-LMOVPXPDSA-K 2-[[(2s)-2-[bis(carboxylatomethyl)amino]-3-(4-ethoxyphenyl)propyl]-[2-[bis(carboxylatomethyl)amino]ethyl]amino]acetate;gadolinium(3+);hydron Chemical compound [Gd+3].CCOC1=CC=C(C[C@@H](CN(CCN(CC(O)=O)CC([O-])=O)CC([O-])=O)N(CC(O)=O)CC([O-])=O)C=C1 PCZHWPSNPWAQNF-LMOVPXPDSA-K 0.000 description 1
- 150000000921 Gadolinium Chemical class 0.000 description 1
- -1 Gadolinium ions Chemical class 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 125000002057 carboxymethyl group Chemical group [H]OC(=O)C([H])([H])[*] 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- OCDAWJYGVOLXGZ-VPVMAENOSA-K gadobenate dimeglumine Chemical compound [Gd+3].CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.OC(=O)CN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC(O)=O)C(C([O-])=O)COCC1=CC=CC=C1 OCDAWJYGVOLXGZ-VPVMAENOSA-K 0.000 description 1
- 229960004455 gadobenic acid Drugs 0.000 description 1
- 229960003411 gadobutrol Drugs 0.000 description 1
- RJOJUSXNYCILHH-UHFFFAOYSA-N gadolinium(3+) Chemical compound [Gd+3] RJOJUSXNYCILHH-UHFFFAOYSA-N 0.000 description 1
- IZOOGPBRAOKZFK-UHFFFAOYSA-K gadopentetate Chemical compound [Gd+3].OC(=O)CN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC(O)=O)CC([O-])=O IZOOGPBRAOKZFK-UHFFFAOYSA-K 0.000 description 1
- 229960003460 gadopentetic acid Drugs 0.000 description 1
- 229960005451 gadoteridol Drugs 0.000 description 1
- DPNNNPAKRZOSMO-UHFFFAOYSA-K gadoteridol Chemical compound [Gd+3].CC(O)CN1CCN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC([O-])=O)CC1 DPNNNPAKRZOSMO-UHFFFAOYSA-K 0.000 description 1
- 229960001547 gadoxetic acid Drugs 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910052747 lanthanoid Inorganic materials 0.000 description 1
- 150000002602 lanthanoids Chemical class 0.000 description 1
- 239000012669 liquid formulation Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 150000003460 sulfonic acids Chemical class 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000925 very toxic Toxicity 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/06—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations
- A61K49/08—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by the carrier
- A61K49/10—Organic compounds
- A61K49/101—Organic compounds the carrier being a complex-forming compound able to form MRI-active complexes with paramagnetic metals
- A61K49/106—Organic compounds the carrier being a complex-forming compound able to form MRI-active complexes with paramagnetic metals the complex-forming compound being cyclic, e.g. DOTA
- A61K49/108—Organic compounds the carrier being a complex-forming compound able to form MRI-active complexes with paramagnetic metals the complex-forming compound being cyclic, e.g. DOTA the metal complex being Gd-DOTA
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/06—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations
- A61K49/18—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by a special physical form, e.g. emulsions, microcapsules, liposomes
Definitions
- Gadoteric acid is a drug that is approved as a contrast agent for magnetic resonance imaging.
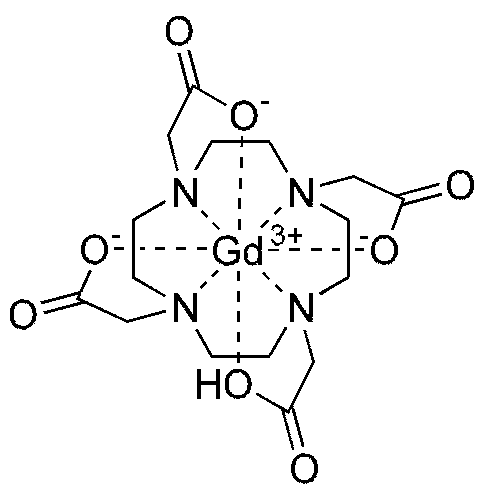
- the chemical name of Gadoteric acid is 2-[4,7,10- tris(carboxymethyl)-l,4,7,10-tetraazacyclododec-l-yl]acetate.
- the compound has the following structure:
- Gadoterate Meglumine is marketed by Guerbet under the brand name DOTAREM .
- Each molecule of Gadoteric acid contains one gadolinium ion, which is complexed by l,4,7,10-tetraazacyclododecane-l,4,7,10-tetraacetic acid (DOTA).
- DOTA l,4,7,10-tetraazacyclododecane-l,4,7,10-tetraacetic acid
- Gadoteric acid is the most stable gadolinium complex.
- WO 2013/076743 to Jagadeesh et al. discloses process for the synthesis and purification of DOTA.
- EP 0270483 to Heinz et al., describes pharmaceutical metal- containing complex compounds and processes for their preparation
- Free ions of Gadolinium i.e. non-complexed Gadolinium
- DOTA is believed to form the most thermodynamically and kinetically stable complexes with Gadolinium.
- Gd-DOTA very stable complexes
- WO 2009/103744 to Meyer et al. suggests measuring the concentration of free macrocyclic chelate and/or free Gadolinium oxide in the liquid pharmaceutical formulation after mixing of initial amounts of Gadolinium oxide and DOTA, and then adjusting the concentrations of free DOTA and/or free Gadolinium ions in order to obtain free chelate within the desired concentration range.
- WO 2014/161925 to Diederik et al. describes a process for producing a complex of a lanthanide or similar compound with a macrocyclic ligand, based on the measurement of the moisture content of the material comprising a macrocyclic ligand.
- the present invention relates to a process of preparing a liquid parenteral pharmaceutical formulation containing Gadoterate meglumine.
- the invention further provides a process for preparing a liquid parenteral pharmaceutical formulation containing Gadoterate meglumine comprising of the following steps:
- the amount of DOTA present in the free form is in the range of 0.002% to 0.5% and the content of free Gadolinium is 0.02% or less.
- the inventors of the present invention have found that an accurate level of free DOTA in the finished dosage form may be easily obtained by titrating up a solution of Gadoterate Meglumine with DOTA or an acidifying agent until a desired pH-value is reached. This process is simple unlike the prior art time consuming in-process measurements of Gadolinium and/or free DOTA.
- free DOTA means any DOTA not complexed with Gadolinium or with other metal ions.
- free Gadolinium means any Gadolinium not complexed with chelate DOTA.
- Gadoterate meglumine comprises of complex of Gadolinium oxide (Gd 2 03) and 1, 4,7, 10-tetraazacyclododecane- 1,4,7, 10- tetraacetic acid (DOTA) used in form of the meglumine salt i.e., DOTA-Gd meglumine.
- Gd 2 03 Gadolinium oxide
- DOTA 10-tetraazacyclododecane- 1,4,7, 10- tetraacetic acid
- the process of preparing a liquid pharmaceutical formulation of Gadoterate meglumine encompasses the following steps: a) mixing a pre-determined amount of Gadolinium oxide, DOTA and Meglumine in water.
- the amount of free DOTA present is in the range of 0.002% to 0.5% and the content of free Gadolinium is 0.02% or less.
- the amounts of free DOTA and Gd 2 0 3 added are such that not all the DOTA is complexed with the Gd 2 0 3 or such that not all the Gd 2 0 3 is complexed with the DOTA. Consequently, after the step b), the pharmaceutical formulation will typically comprise DOTA-gadolinium complex and either free DOTA, or free gadolinium.
- the pharmaceutical formulation according to the present invention is characterized in that the formulation contains from about 0.002 to 0.5% of free DOTA and 0.02% or less of free Gadolinium.
- Formulations of the present invention have a pH value of about 6.5 to 8.2.
- the pH of the formulation is adjusted to desired range using DOTA or any suitable acidifying agent such as mineral acids, organic carboxylic acids, sulfonic acids and the like.
- suitable acidifying agents include, but not limited to hydrochloric acid, sulfuric acid, boric acid, silica acid, acetic acid, phosphoric acid, formic acid, maleic acid, citric acid, trichloroacetic acid, trifluoroacetic acid, benzoic acid, fumaric acid, tartaric acid, methanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid and the like.
- the manufacturing process for preparing liquid parenteral pharmaceutical formulation of Gadoterate meglumine according to the present invention comprises of the following steps:
- Water for injection is heated in a manufacturing vessel to about 80+5°C.
- Gadolinium oxide (Gd 2 0 3 ) is added to the above solution and stirred till it dissolves completely, maintaining the temperature of solution at 80+5 °C. iv.
- the solution is cooled to 25+2°C and meglumine is added to the solution and stirred.
- the pH of the solution is adjusted to around 6.5 to 8.2, by the addition of DOTA or acidifying agent.
- the solution is filtered and filled into suitable containers or vials, stoppered and sealed followed by terminal sterilization.
- Formulations prepared according to the invention are tested for various parameters such as pH, Assay, free Gd and free DOTA content and the results are tabulated in table 1.
- the amount of free Gd present in the formulation is analysed by Agilent Fluorescence detector.
- the amount of free DOTA in the formulation is analysed by HPLC method.
Landscapes
- Health & Medical Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Radiology & Medical Imaging (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Abstract
The present invention relates to a process for preparing a liquid parenteral formulation of Gadoterate meglumine which is used as a contrast agent for magnetic resonance imaging.
Description
PROCESS FOR PREPARING A PHARMACEUTICAL FORMULATION OF
GADOTERATE MEGLUMINE
Background of the invention
Gadoteric acid (Gd-DOTA) is a drug that is approved as a contrast agent for magnetic resonance imaging. The chemical name of Gadoteric acid is 2-[4,7,10- tris(carboxymethyl)-l,4,7,10-tetraazacyclododec-l-yl]acetate. The compound has the following structure:
Gadoterate Meglumine is marketed by Guerbet under the brand name DOTAREM .
Each molecule of Gadoteric acid contains one gadolinium ion, which is complexed by l,4,7,10-tetraazacyclododecane-l,4,7,10-tetraacetic acid (DOTA). Compared with other Gadolinium complexes such as Gadopentetic acid, Gadobenic acid, Gadoxetic acid, Gadoteridol or Gadobutrol, Gadoteric acid is the most stable gadolinium complex.
WO 2013/076743 to Jagadeesh et al., discloses process for the synthesis and purification of DOTA.
European patent No. EP 0270483 to Heinz et al., describes pharmaceutical metal- containing complex compounds and processes for their preparation
Free ions of Gadolinium, i.e. non-complexed Gadolinium, are very toxic. Hence it is essential to select a proper chelating agent to form a complex with the Gadolinium. Amongst several related compounds that are used to complex Gadolinium, DOTA is
believed to form the most thermodynamically and kinetically stable complexes with Gadolinium. However, even very stable complexes such as Gd-DOTA tend to release Gadolinium. In order to prevent the undesired release of Gadolinium, it is common practice to use an excess of DOTA. Since free DOTA is also toxic, the excess quantity has to be kept within a certain range.
Prior art disclosures suggest that it is not possible to obtain Gadoteric acid solutions with an excess of DOTA in the desired target range by simply weighing and mixing together Gadolinium oxide, DOTA and Meglumine in water for injection.
To overcome this obstacle, WO 2009/103744 to Meyer et al., suggests measuring the concentration of free macrocyclic chelate and/or free Gadolinium oxide in the liquid pharmaceutical formulation after mixing of initial amounts of Gadolinium oxide and DOTA, and then adjusting the concentrations of free DOTA and/or free Gadolinium ions in order to obtain free chelate within the desired concentration range.
WO 2014/161925 to Diederik et al., describes a process for producing a complex of a lanthanide or similar compound with a macrocyclic ligand, based on the measurement of the moisture content of the material comprising a macrocyclic ligand.
In order to obtain a final composition with the concentration of free DOTA within the specifications, both processes known in the art require several additional process steps such as the measurement of free Gadolinium oxide and the moisture content in the DOTA.
It is thus desirable to obtain an accurate, but simpler process for the manufacture of liquid formulations of Gadoteric acid for the pharmaceutical use on an industrial scale.
Summary of the invention
The present invention relates to a process of preparing a liquid parenteral pharmaceutical formulation containing Gadoterate meglumine.
The invention further provides a process for preparing a liquid parenteral pharmaceutical formulation containing Gadoterate meglumine comprising of the following steps:
a) mixing a pre-determined amount of Gadolinium oxide, DOTA and Meglumine in water.
b) adjusting the pH-value of the mixture in the range of about 6.5 to 8.2, by adding DOTA or any suitable acidifying agent,
wherein the amount of DOTA present in the free form is in the range of 0.002% to 0.5% and the content of free Gadolinium is 0.02% or less.
Detailed description of the invention
The inventors of the present invention have found that an accurate level of free DOTA in the finished dosage form may be easily obtained by titrating up a solution of Gadoterate Meglumine with DOTA or an acidifying agent until a desired pH-value is reached. This process is simple unlike the prior art time consuming in-process measurements of Gadolinium and/or free DOTA.
In the context of the present invention the term "free DOTA" means any DOTA not complexed with Gadolinium or with other metal ions.
In the context of the present invention the term "free Gadolinium" means any Gadolinium not complexed with chelate DOTA.
In the context of the present invention the term "Gadoterate meglumine" comprises of complex of Gadolinium oxide (Gd203) and 1, 4,7, 10-tetraazacyclododecane- 1,4,7, 10- tetraacetic acid (DOTA) used in form of the meglumine salt i.e., DOTA-Gd meglumine.
According to the present invention, the process of preparing a liquid pharmaceutical formulation of Gadoterate meglumine encompasses the following steps:
a) mixing a pre-determined amount of Gadolinium oxide, DOTA and Meglumine in water.
b) adjusting the pH-value of the mixture in the range of about 6.5 to 8.2, by adding DOTA or any suitable acidifying agent,
wherein, the amount of free DOTA present is in the range of 0.002% to 0.5% and the content of free Gadolinium is 0.02% or less.
Thus, in one advantageous embodiment, the amounts of free DOTA and Gd203 added are such that not all the DOTA is complexed with the Gd203 or such that not all the Gd203 is complexed with the DOTA. Consequently, after the step b), the pharmaceutical formulation will typically comprise DOTA-gadolinium complex and either free DOTA, or free gadolinium.
Advantageously, the pharmaceutical formulation according to the present invention is characterized in that the formulation contains from about 0.002 to 0.5% of free DOTA and 0.02% or less of free Gadolinium.
Formulations of the present invention have a pH value of about 6.5 to 8.2. The pH of the formulation is adjusted to desired range using DOTA or any suitable acidifying agent such as mineral acids, organic carboxylic acids, sulfonic acids and the like. Suitable acidifying agents include, but not limited to hydrochloric acid, sulfuric acid, boric acid, silica acid, acetic acid, phosphoric acid, formic acid, maleic acid, citric acid, trichloroacetic acid, trifluoroacetic acid, benzoic acid, fumaric acid, tartaric acid, methanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid and the like.
The manufacturing process for preparing liquid parenteral pharmaceutical formulation of Gadoterate meglumine according to the present invention comprises of the following steps:
i. Water for injection is heated in a manufacturing vessel to about 80+5°C.
ii. DOTA is added to the above solution and stirred well.
iii. Gadolinium oxide (Gd203) is added to the above solution and stirred till it dissolves completely, maintaining the temperature of solution at 80+5 °C.
iv. The solution is cooled to 25+2°C and meglumine is added to the solution and stirred.
v. The pH of the solution is adjusted to around 6.5 to 8.2, by the addition of DOTA or acidifying agent.
vi. The solution is filtered and filled into suitable containers or vials, stoppered and sealed followed by terminal sterilization.
Experiments were carried out in order to limit the amount of free DOTA and free gadolinium (Gd), and thus to reduce the toxicity of the product. Formulations prepared according to the invention are tested for various parameters such as pH, Assay, free Gd and free DOTA content and the results are tabulated in table 1. The amount of free Gd present in the formulation is analysed by Agilent Fluorescence detector. The amount of free DOTA in the formulation is analysed by HPLC method.
Table 1: pH-adjustment with DOTA
From the above results it can be concluded that the manufacturing process by the pH- adjustment method is suitable and meets the specifications for gadoterate meglumine injection.
The formulation prepared according to the invention is compared with commercially available Gadoterate meglumine injection i.e., Dotarem Injection (Batch No: 12GD100A, 11GD089A), the results are tabulated in table 2.
Table 2: Comparison with Dotarem1
From table 2 it is evident that the product prepared according to the invention is comparable to that of the commercially available product.
The following examples further describe certain specific aspects and embodiments of the present invention and demonstrate the practice and advantages thereof. It is to be understood that the examples are given by way of illustration only and are not intended to limit the scope of the invention in any manner.
Example 1
Manufacturing process
Water for injection was taken in a manufacturing vessel and heated to 80+5°C. DOTA was added and stirred, followed by the addition of Gadolinium oxide while maintaining the temperature at 80+5°C. The solution was cooled to 25+2°C, then meglumine was added and stirred till a homogenous solution was obtained. The pH of the solution was adjusted to around 7.5 to 8.0 with hydrochloric acid. The final pH of the solution was adjusted using DOTA to around 7.3+0.2. The obtained solution was filtered followed by filling into the vials.
Example 2
Manufacturing process
Water for injection was taken in a manufacturing vessel and heated to 80+5°C. DOTA was added and stirred, followed by the addition of Gadolinium oxide while maintaining the temperature at 80+5°C. The solution was cooled to 25+2°C, then meglumine was added and stirred till a homogenous solution was obtained. The pH of the solution was adjusted using DOTA to around 7.3+0.2. The obtained solution was filtered followed by filling into the vials.
Example 3
Manufacturing process
Water for injection was taken in a manufacturing vessel and heated to 80+5°C. DOTA was added and stirred, followed by the addition of Gadolinium oxide while maintaining the temperature at 80+5°C. The solution was cooled to 25+2°C, then meglumine was added and stirred till a homogenous solution was obtained. The pH of the solution was adjusted using DOTA to around 7.3+0.2. The obtained solution was filtered followed by filling into the vials.
Example 4
Manufacturing process
Water for injection was taken in a manufacturing vessel and heated to 80+5°C. DOTA was added and stirred, followed by the addition of Gadolinium oxide while maintaining the temperature at 80+5°C. The solution was cooled to 25+2°C, then meglumine was added and stirred till a homogenous solution was obtained. The pH of the solution was adjusted to around 7.4 using acetic acid. The obtained solution was filtered followed by filling into the vials.
Example 5
Manufacturing process
Water for injection was taken in a manufacturing vessel and heated to 80+5°C. DOTA was added and stirred, followed by the addition of Gadolinium oxide while maintaining the temperature at 80+5°C. The solution was cooled to 25+2°C, then meglumine was added and stirred till a homogenous solution was obtained. The pH of the solution was adjusted to around 7.4 using phosphoric acid and/or DOTA. The obtained solution was filtered followed by filling into the vials.
Claims
We Claim:
Claim 1: Process for preparing a liquid parenteral pharmaceutical formulation of Gadoterate meglumine comprising of the following steps:
a) mixing a pre-determined amount of gadolinium oxide, DOTA and meglumine in water.
b) adjusting the pH-value of the mixture in the range of about 6.5 to 8.2, by adding DOTA or any suitable acidifying agent.
Claim 2: Process for preparing a liquid parenteral pharmaceutical formulation containing Gadoterate meglumine of claim 1, wherein the amount of free DOTA in the formulation ranges from about 0.002% to 0.5% and the content of free Gadolinium is 0.02% or less.
Claim 3: A process for preparing formulation according to claim 1, wherein the pH of the formulation ranges from about 7.0 and 8.0.
Claim 4: The parenteral pharmaceutical formulation of Gadoterate meglumine of claiml, wherein the acidifying agent is selected from the group comprising of hydrochloric acid, sulfuric acid, boric acid, silica acid, acetic acid, phosphoric acid, formic acid, maleic acid, citric acid, tri-chloroacetic acid, trifluoroacetic acid, benzoic acid, fumaric acid, tartaric acid, methanesulfonic acid, benzenesulfonic acid and p- toluenesulfonic acid.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN4896/CHE/2015 | 2015-09-15 | ||
| IN4896CH2015 | 2015-09-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017046694A1 true WO2017046694A1 (en) | 2017-03-23 |
Family
ID=58160323
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2016/055436 WO2017046694A1 (en) | 2015-09-15 | 2016-09-13 | Process for preparing a pharmaceutical formulation of gadoterate meglumine |
Country Status (2)
| Country | Link |
|---|---|
| DE (1) | DE102015013939A1 (en) |
| WO (1) | WO2017046694A1 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018125916A1 (en) * | 2016-12-29 | 2018-07-05 | Inventure, LLC | Solvent-free gadolinium contrast agents |
| WO2019020662A1 (en) * | 2017-07-27 | 2019-01-31 | Sanochemia Pharmazeutika Ag | Preparation containing a contrast agent, and method for the production thereof |
| US10653804B2 (en) | 2016-12-29 | 2020-05-19 | Inventure, LLC | Solvent-free gadolinium contrast agents |
| EP3315141B1 (en) * | 2016-10-28 | 2020-10-21 | B.E. Imaging GmbH | Method for the manufacture of pharmaceutical compositions comprising gadolinium chelate complexes with reduced toxic contamination |
| CN111989124A (en) * | 2018-02-23 | 2020-11-24 | 萨诺化学药物有限公司 | Method for preparing contrast medium |
| CN113527222A (en) * | 2020-04-21 | 2021-10-22 | 威智医药有限公司 | Preparation method of meglumine gadoterate |
| CN113801071A (en) * | 2021-09-14 | 2021-12-17 | 安徽普利药业有限公司 | Refining method of meglumine gadoterate |
| CN115779105A (en) * | 2022-11-17 | 2023-03-14 | 华润双鹤药业股份有限公司 | Gadobutrol injection and preparation method thereof |
| CN115869426A (en) * | 2022-12-28 | 2023-03-31 | 华润双鹤药业股份有限公司 | A kind of gadoterate meglumine injection and preparation method thereof |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009103744A2 (en) * | 2008-02-19 | 2009-08-27 | Guerbet | Process for preparing a pharmaceutical formulation of contrast agents |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3640708C2 (en) | 1986-11-28 | 1995-05-18 | Schering Ag | Improved pharmaceuticals containing metals |
| CN104169252B (en) | 2011-11-25 | 2017-03-22 | 拜欧弗印度制药有限公司 | For the technique of purifying polyaminocarboxylic acid ester |
| EP2786768A1 (en) | 2013-04-04 | 2014-10-08 | Agfa Healthcare | Process for preparing a material comprising a macrocyclic ligand and for producing a pharmaceutical formulation comprising said ligand with a lanthanide |
-
2015
- 2015-10-28 DE DE102015013939.8A patent/DE102015013939A1/en not_active Withdrawn
-
2016
- 2016-09-13 WO PCT/IB2016/055436 patent/WO2017046694A1/en active Application Filing
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009103744A2 (en) * | 2008-02-19 | 2009-08-27 | Guerbet | Process for preparing a pharmaceutical formulation of contrast agents |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3315141B1 (en) * | 2016-10-28 | 2020-10-21 | B.E. Imaging GmbH | Method for the manufacture of pharmaceutical compositions comprising gadolinium chelate complexes with reduced toxic contamination |
| EP3789044A1 (en) * | 2016-10-28 | 2021-03-10 | B.E. Imaging GmbH | Method for the manufacture of pharmaceutical compositions comprising gadolinium chelate complexes with reduced toxic contamination |
| WO2018125916A1 (en) * | 2016-12-29 | 2018-07-05 | Inventure, LLC | Solvent-free gadolinium contrast agents |
| US10653804B2 (en) | 2016-12-29 | 2020-05-19 | Inventure, LLC | Solvent-free gadolinium contrast agents |
| WO2019020662A1 (en) * | 2017-07-27 | 2019-01-31 | Sanochemia Pharmazeutika Ag | Preparation containing a contrast agent, and method for the production thereof |
| CN111989124A (en) * | 2018-02-23 | 2020-11-24 | 萨诺化学药物有限公司 | Method for preparing contrast medium |
| CN113527222A (en) * | 2020-04-21 | 2021-10-22 | 威智医药有限公司 | Preparation method of meglumine gadoterate |
| CN113527222B (en) * | 2020-04-21 | 2023-06-13 | 威智医药股份有限公司 | Preparation method of gadoteric acid meglumine |
| CN113801071A (en) * | 2021-09-14 | 2021-12-17 | 安徽普利药业有限公司 | Refining method of meglumine gadoterate |
| CN113801071B (en) * | 2021-09-14 | 2023-04-07 | 安徽普利药业有限公司 | Refining method of meglumine gadoterate |
| CN115779105A (en) * | 2022-11-17 | 2023-03-14 | 华润双鹤药业股份有限公司 | Gadobutrol injection and preparation method thereof |
| CN115869426A (en) * | 2022-12-28 | 2023-03-31 | 华润双鹤药业股份有限公司 | A kind of gadoterate meglumine injection and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| DE102015013939A1 (en) | 2017-03-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2017046694A1 (en) | Process for preparing a pharmaceutical formulation of gadoterate meglumine | |
| EP3223863B1 (en) | Formulations comprising a meglumine salt of the metal complex gd-dota | |
| KR102456908B1 (en) | Metal complex formulations | |
| Loncin et al. | Coordination of lanthanides by two polyamino polycarboxylic macrocycles: formation of highly stable lanthanide complexes | |
| HRP20161312T2 (en) | Process for preparing a pharmaceutical formulation of contrast agents | |
| JP6846347B2 (en) | Lantanide complex preparation | |
| CN105073144A (en) | Process for producing a complex of a lanthanide with a macrocyclic ligand | |
| KR20170037659A (en) | Method for producing a liquid pharmaceutical preparation | |
| JPH01259850A (en) | NMR imaging using Mn(II) coordination compositions | |
| CN111989124A (en) | Method for preparing contrast medium | |
| EP4284443A1 (en) | Synthesis methods and compositions of low intermediate and low dichelate intermediate contrast agents | |
| HK40038504A (en) | Process for producing a contrast agent | |
| WO2013144604A1 (en) | Biotinidase resistant biotinyl compounds | |
| HUP0202873A2 (en) | Calcium complex of ethylene diamine derivatives containing phosphorus | |
| HK1242189A1 (en) | Formulations comprising a meglumine salt of the metal complex gd-dota | |
| HK1242189B (en) | Formulations comprising a meglumine salt of the metal complex gd-dota | |
| HK1242188A1 (en) | Metal complex formulations |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16845803 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 16845803 Country of ref document: EP Kind code of ref document: A1 |