WO2016206663A1 - A pharmaceutical formulation of sofosbuvir - Google Patents
A pharmaceutical formulation of sofosbuvir Download PDFInfo
- Publication number
- WO2016206663A1 WO2016206663A1 PCT/CZ2016/000073 CZ2016000073W WO2016206663A1 WO 2016206663 A1 WO2016206663 A1 WO 2016206663A1 CZ 2016000073 W CZ2016000073 W CZ 2016000073W WO 2016206663 A1 WO2016206663 A1 WO 2016206663A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- pharmaceutical formulation
- sofosbuvir
- weight
- formulation according
- microcrystalline cellulose
- Prior art date
Links
- 0 CC(C)OC([C@](C)N[P@@](*)(OC[C@]([C@@]([C@@]1(C)F)O)O[C@]1N(C=CC(N1)=O)C1=O)=O)=O Chemical compound CC(C)OC([C@](C)N[P@@](*)(OC[C@]([C@@]([C@@]1(C)F)O)O[C@]1N(C=CC(N1)=O)C1=O)=O)=O 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
- A61K31/7072—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid having two oxo groups directly attached to the pyrimidine ring, e.g. uridine, uridylic acid, thymidine, zidovudine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
Definitions
- the invention relates to a new pharmaceutical formulation of sofosbuvir of formula i.
- Sofosbuvir is a substance designed for treatment of hepatitis type C.
- the substance was first described in the patent application WO2008121634; a general preparation procedure of this substance and a number of other substances with a similar composition are included in the above mentioned application on pages 668 to 671.
- the synthesis is further documented in examples 13 to 66. Reproduction of these procedures has provided sofosbuvir in an amorphous form (foam or oil form).
- SOFOSBUVIR is a prodrug, which is easily enzymatically transformed directly in the liver to the active substance 2'-deoxy-2'-a-fluoro ⁇ -C-methyluridine-5 :riphosphate, which serves as an inhibitor of RNA polymerase (NS5B protein), thus inhibiting the synthesis of viral RNA.
- Sofosbuvir represents the first non-interferon treatment of chronic hepatitis C with the cure rate of up to 90%.
- Form VI is prepared by crystallization from an aqueous solution, but it is surprisingly non-hygroscopic and stable when exposed to the air.
- Forms V and IV are prepared by crystallization from anisole and acetonitrile. However, both of them pass to Form I already by mere filtration.
- Form III is described as a 1:1 solvate with chloroform, while Form II is described as a 1:1 solvate with dichloromethane.
- Form I represents a non-solvated form of Sofosbuvir.
- the patent further describes single crystal X-ray crystallographic data of Forms I, II, III.
- PV 2014-502 we described another crystalline Form X (referred to as Z-l in PV 2014-502).
- Form X exhibits extraordinary stability even at elevated humidity and it is predestined for a successful pharmaceutical formulation by its characteristics.
- Form X was characterized by the diffraction peaks 8.0; 12.3; 17.1; 19.9 and 20.8 ⁇ 0.2° with the use of CuKoc radiation.
- a particular pharmaceutical formulation was described in the patent application WO 2013/082003.
- the formulation according to that document contained 25 to 35% by weight of sofosbuvir in a crystalline form, preferably in Forms I or VI.
- Form VI was especially preferred.
- the formulation described there can contain 400 mg of sofosbuvir, 360 mg of mannitol, 356 mg of microcrystalline cellulose, 60 mg of sodium croscarmellose, 6 mg of colloidal silica and 18 mg of magnesium stearate. It means that the particular composition described there contains 33% of sofosbuvir and about 60% of the filler consisting of mannitol and MCC. Such a composition containing 33% of sofosbuvir was only arrived at after complicated optimization of the tablet.
- the present invention provides a pharmaceutical formulation of sofosbuvir, having the content of the active ingredient of 40% by weight or higher.
- the formulation according to the invention contains 40 to 60% by weight of sofosbuvir.
- the formulation with this high content of sofosbuvir contains one or more fillers, a disintegrant, a binder, a lubricant and a glidant. It preferably comprises the Prosolv SMCC90 selection of fillers.
- the invention also relates to a method for preparing the new formulation, which may be performed by means of wet granulation, or dry granulation, or direct tabletting.
- wet granulation the PVP K35 binder is preferably selected.
- the invention provides a formulation of the therapeutic agent Sofosbuvir, which has not been described in the literature yet.
- This dosage form is characterized by a higher content of the active ingredient, which, at the therapeutical dose of 400 mg/tablet, makes the administration of Sofosbuvir significantly easier and simpler for the patient.
- the described dosage form is prepared by means of common unit operations.
- Sofosbuvir meets these requirements with difficulties.
- a pharmaceutical tablet was described in the application WO 2013/082003. According to that patent application one tablet of Sofosbuvir contains 25 - 35% by weight of the active ingredient. With regard to the total therapeutic dose of Sofosbuvir of 400 mg, the size of the final tablet is about 1150 - 1600 mg. Such large tablets are unpleasant and difficult to swallow for patients.
- the problem solved by this invention was to find such procedures and such formulations that would make it possible to obtain a tablet with the content of the active ingredient of at least 40% by weight.
- the pharmaceutical composition especially contains a filler, a disintegrant, a glidant and a lubricant. It may also contain a binder.
- the amounts of individual components are selected with respect to the required amount of the active ingredient, requirement for tablet size, character of individual formulation components and finally the processing method.
- Preferred amounts for the formulation according to the invention are 20 to 45% by weight of the filler, 5 to 12% by weight of the disintegrant and 4 to 15% by weight of other substances that may fulfil the functions of a binder, glidant or lubricant.
- compositions enable production by means of the process of wet granulation (granulation by kneading as well as fluid granulation), dry granulation and direct tabletting.
- Each of the procedures requires an optimized composition of the mixture and preparation process. Therefore, preparation methods of the dosage form represent another aspect of the invention.
- wet granulation appears to be the most suitable technology in terms of weight reduction of the final dosage form. Using this technology, the content of the active ingredient has successfully been increased to up to 60%.
- Sofosbuvir (crystalline Form I) was water granulated together with microcrystalline cellulose, crospovidone and Klucel. The obtained granulate was dried in a fluidization drier and sieved. Lactose monohydrate, the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate.
- the tabletting matter prepared this way was tabletted in a rotary tabletting machine into oblong tablets with the size of 1000 mg. The tabletting matter did not have optimum flow properties, which caused problems during the tabletting process. During the tabletting, parts of the tablets also got torn off. This phenomenon could be caused by selection of an unsuitable filler.
- Sofosbuvir (crystalline Form I) was water granulated together with microcrystalline cellulose, crospovidone and Klucel. The granulate was dried in a fluidization drier and sieved. Mannitol, the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 1000 mg/tablet.
- Sofosbuvir (crystalline Form X) was water granulated together with microcrystalline cellulose, crospovidone and Klucel. The granulate was dried in a fluidization drier and sieved. Mannitol, the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 1000 mg/tablet.
- Magnesium stearate Lubricant 1.5 Sofosbuvir (crystalline Form X) was water granulated together with microcrystalline cellulose, crospovidone and Klucel. The granulate was dried in a fluidization drier and sieved. Prosolv (microcrystalline cellulose with a colloidal silica layer), the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 1000 mg/tablet.
- Sofosbuvir (crystalline Form X) was water granulated together with microcrystalline cellulose, crospovidone and povidone. The granulate was dried in a fluidization drier and sieved. Prosolv (microcrystalline cellulose with a colloidal silica layer), the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 1000 mg/tablet. The cores produced this way showed suitable parameters as abrasion, disintegration and dissolution rate of the active ingredient during analytic tests. Further experimental steps focused on increasing the share of the active ingredient in the tablet. In the first experiments, there was an effort to reduce the tablet weight by reducing the amount of microcrystalline cellulose. In the final stage, microcrystalline cellulose was completely eliminated from the granulation process. Thus, the share of 60% of the active ingredient in one tablet was achieved.
- Sofosbuvir (crystalline Form X) was water granulated together with crospovidone and povidone. The granulate was dried in a fluidization drier and sieved through a sieve with the mesh size of 0.8 mm. Prosolv (microcrystalline cellulose with a colloidal silica layer), microcrystalline cellulose, the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 667 mg/tablet. The flow properties of the material met the tabletting parameters. Suitable disintegration, abrasion and dissolution and stability characteristics of the resulting tablets were also achieved.
- Dry granulation (also compaction) was another process that was selected as suitable for processing of sofosbuvir.
- a similar composition as in the case of wet granulation was used.
- Sofosbuvir (crystalline Form X) was compacted together with microcrystalline cellulose, colloidal silica, crospovidone and magnesium stearate.
- Prosolv microcrystalline cellulose with a colloidal silica layer
- the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the compacted matter.
- the tablets were tabletted in a rotary tabletting machine.
- the weight of the cores was 1000 mg/tablet.
- Sofosbuvir (crystalline Form I) was compacted together with microcrystalline cellulose, colloidal silica, crospovidone and magnesium stearate.
- Prosolv microcrystalline cellulose with a colloidal silica layer
- the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the compacted matter.
- the tablets were tabletted in a rotary tabletting machine.
- the weight of the cores was 1000 mg/tablet.
- Sofosbuvir (crystalline Form X) was compacted together with microcrystalline cellulose, Prosolv, crospovidone and magnesium stearate. The rest of crospovidone, magnesium stearate and colloidal silica were admixed to the compacted matter.
- the tablets were tabletted in a rotary tabletting machine. The weight of the cores was 800 mg/tablet. During the tabletting, the material adhered to the punches. This phenomenon could only be prevented by addition of 1% of a lubricant or use of external lubrication of the punches during tabletting. Thus, the final content of the active ingredient was 49.5% by weight.
- Sofosbuvir (crystalline Form X) was mixed together with microcrystalline cellulose, Prosolv, crospovidone, pre-gelatinized starch, colloidal silica and magnesium stearate.
- the tablets were tabletted in a rotary tabletting machine.
- the weight of the cores was 800 mg/tablet.
- the final content of the active ingredient was thus about 49.5% by weight.
Abstract
The present invention provides a pharmaceutical formulation of sofosbuvir of formula (I), having the content of the active substance of 40% by weight or higher. The pharmaceutical formulation preferably contains 40 to 60% by weight of sofosbuvir. The formulation with this high content of sofosbuvir contains one or more fillers, a disintegrant, a binder, a lubricant and a glidant. It preferably comprises the Prosolv SMCC90 selection of fillers.
Description
A PHARMACEUTICAL FORMULATION OF SOFOSBUVIR
Technical Field
The invention relates to a new pharmaceutical formulation of sofosbuvir of formula i.
Background Art
Sofosbuvir is a substance designed for treatment of hepatitis type C. The substance was first described in the patent application WO2008121634; a general preparation procedure of this substance and a number of other substances with a similar composition are included in the above mentioned application on pages 668 to 671. The synthesis is further documented in examples 13 to 66. Reproduction of these procedures has provided sofosbuvir in an amorphous form (foam or oil form).
SOFOSBUVIR is a prodrug, which is easily enzymatically transformed directly in the liver to the active substance 2'-deoxy-2'-a-fluoro^-C-methyluridine-5 :riphosphate, which serves as an inhibitor of RNA polymerase (NS5B protein), thus inhibiting the synthesis of viral RNA. Sofosbuvir represents the first non-interferon treatment of chronic hepatitis C with the cure rate of up to 90%.
The patent application WO2010/135569 describes preparation and characterization of five polymorphs and an amorphous form of Sofosbuvir, while the application WO2011/123645 adds Form VI to this list. Form VI is prepared by crystallization from an aqueous solution, but it is surprisingly non-hygroscopic and stable when exposed to the air. Forms V and IV are prepared by crystallization from anisole and acetonitrile. However, both of them pass to Form I already by mere filtration. Form III is described as a 1:1 solvate with chloroform, while Form II is described as a 1:1 solvate with dichloromethane. Form I represents a non-solvated form of Sofosbuvir. The patent further describes single crystal X-ray crystallographic data of Forms I, II, III.
In the patent application PV 2014-502 we described another crystalline Form X (referred to as Z-l in PV 2014-502). Form X exhibits extraordinary stability even at elevated humidity and it is predestined for a successful pharmaceutical formulation by its characteristics. Form X was characterized by the diffraction peaks 8.0; 12.3; 17.1; 19.9 and 20.8 ± 0.2° with the use of CuKoc radiation.
A particular pharmaceutical formulation was described in the patent application WO 2013/082003. The formulation according to that document contained 25 to 35% by weight of sofosbuvir in a crystalline form, preferably in Forms I or VI. Form VI was especially preferred. In particular, the formulation described there can contain 400 mg of sofosbuvir, 360 mg of mannitol, 356 mg of microcrystalline cellulose, 60 mg of sodium croscarmellose, 6 mg of colloidal silica and 18 mg of magnesium stearate. It means that the particular composition described there contains 33% of sofosbuvir and about 60% of the filler consisting of mannitol and MCC. Such a composition containing 33% of sofosbuvir was only arrived at after complicated optimization of the tablet.
Disclosure of Invention
The present invention provides a pharmaceutical formulation of sofosbuvir, having the content of the active ingredient of 40% by weight or higher. In a preferred embodiment, the formulation according to the invention contains 40 to 60% by weight of sofosbuvir. The formulation with this high content of sofosbuvir contains one or more fillers, a disintegrant, a binder, a lubricant and a glidant. It preferably comprises the Prosolv SMCC90 selection of fillers.
The invention also relates to a method for preparing the new formulation, which may be performed by means of wet granulation, or dry granulation, or direct tabletting. In the case of wet granulation the PVP K35 binder is preferably selected.
Detailed description of the invention
The invention provides a formulation of the therapeutic agent Sofosbuvir, which has not been described in the literature yet. This dosage form is characterized by a higher content of the active ingredient, which, at the therapeutical dose of 400 mg/tablet, makes the
administration of Sofosbuvir significantly easier and simpler for the patient. The described dosage form is prepared by means of common unit operations.
The proposed production procedures and the composition of the final dosage form meet all requirements for the parameters of the processed material for all the production steps and the subsequent stability of the resulting product. Sofosbuvir meets these requirements with difficulties. A pharmaceutical tablet was described in the application WO 2013/082003. According to that patent application one tablet of Sofosbuvir contains 25 - 35% by weight of the active ingredient. With regard to the total therapeutic dose of Sofosbuvir of 400 mg, the size of the final tablet is about 1150 - 1600 mg. Such large tablets are unpleasant and difficult to swallow for patients.
The problem solved by this invention was to find such procedures and such formulations that would make it possible to obtain a tablet with the content of the active ingredient of at least 40% by weight.
With regard to the character of the input material it was necessary to optimize the composition of the dosage form and the production process. Several technologies have been tested, including wet granulation, compaction, fluid granulation and others. The selection of suitable fillers has turned out to be very important. During the experiments, different characteristics of various crystalline forms have been found to substantially influence the amount of the active ingredient in the dosage form. For the formulation experiments, crystalline Forms I and X were used.
This has resulted in a dosage form containing 40% by weight or more of the active ingredient in one tablet, preferably 40 to 60% by weight. This reduced its weight to 670 to 1000 mg, which significantly facilitates administration of the drug to the patient, as well as the treatment.
Besides the active ingredient, the pharmaceutical composition especially contains a filler, a disintegrant, a glidant and a lubricant. It may also contain a binder.
The amounts of individual components are selected with respect to the required amount of the active ingredient, requirement for tablet size, character of individual formulation components and finally the processing method.
Preferred amounts for the formulation according to the invention are 20 to 45% by weight of the filler, 5 to 12% by weight of the disintegrant and 4 to 15% by weight of other substances that may fulfil the functions of a binder, glidant or lubricant.
The proposed compositions enable production by means of the process of wet granulation (granulation by kneading as well as fluid granulation), dry granulation and direct tabletting. Each of the procedures requires an optimized composition of the mixture and preparation process. Therefore, preparation methods of the dosage form represent another aspect of the invention.
The use of wet granulation appears to be the most suitable technology in terms of weight reduction of the final dosage form. Using this technology, the content of the active ingredient has successfully been increased to up to 60%.
Experimental part
Example 1
Sofosbuvir (crystalline Form I) was water granulated together with microcrystalline cellulose, crospovidone and Klucel. The obtained granulate was dried in a fluidization drier and sieved. Lactose monohydrate, the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tabletting matter prepared this way was tabletted in a rotary tabletting machine into oblong tablets with the size of 1000 mg.
The tabletting matter did not have optimum flow properties, which caused problems during the tabletting process. During the tabletting, parts of the tablets also got torn off. This phenomenon could be caused by selection of an unsuitable filler.
To avoid the problems of the first experiment more tests were caried out. The problem of selecting a suitable filler was solved first. Microcrystalline cellulose, mannitol, lactose, sorbitol, and magnesium carbonate and more were tested as replacement fillers for the preparation of the granulate.
Example 2
Sofosbuvir (crystalline Form I) was water granulated together with microcrystalline cellulose, crospovidone and Klucel. The granulate was dried in a fluidization drier and sieved. Mannitol, the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 1000 mg/tablet.
During this experiment parts of the cores did not get torn off during tabletting any more. However, the problem with the flow properties of the material was not successfully solved. The disintegration and abrasion of the tablets were also unsuitable.
Example 3
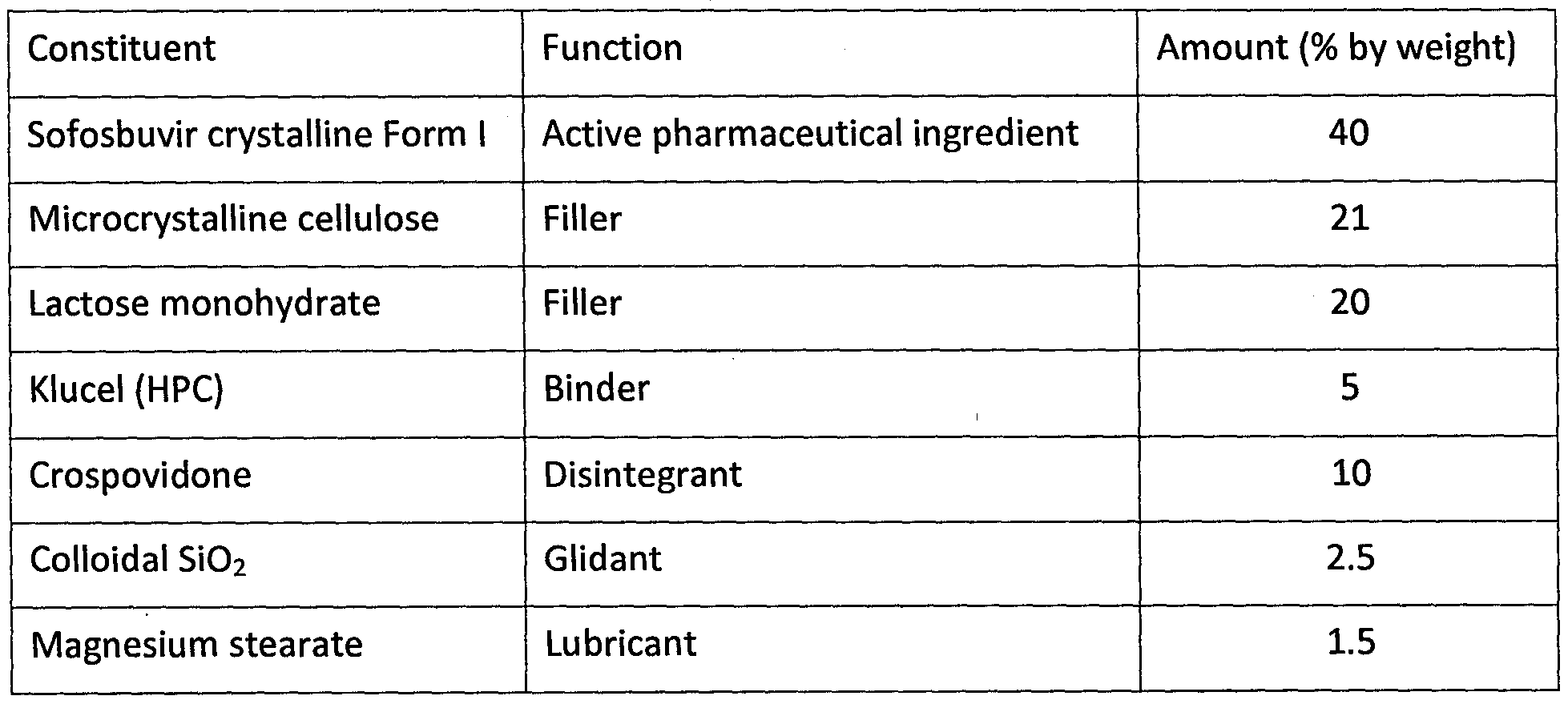
Constituent Function Amount (% by weight)
Sofosbuvir crystalline Form X Active pharmaceutical ingredient 40
Microcrystalline cellulose Filler 25
Mannitol Filler 16
Klucel (HPC) Binder 5
Crospovidone Disintegrant 10
Colloidal Si02 Glidant 2.5
Magnesium stearate Lubricant 1.5
Sofosbuvir (crystalline Form X) was water granulated together with microcrystalline cellulose, crospovidone and Klucel. The granulate was dried in a fluidization drier and sieved. Mannitol, the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 1000 mg/tablet.
During the experiment, the material behaved in the same way as in the case of using the crystalline Form I. The same problem with flow properties of the material was registered.
Example 4
Constituent Function Amount (% by weight)
Sofosbuvir crystalline Form X Active pharmaceutical ingredient 40
Microcrystalline cellulose Filler 15
Prosolv SMCC90 Filler 26
Klucel (HPC) Binder 5
Crospovidone Disintegrant 10
Colloidal Si02 Glidant 2.5
Magnesium stearate Lubricant 1.5
Sofosbuvir (crystalline Form X) was water granulated together with microcrystalline cellulose, crospovidone and Klucel. The granulate was dried in a fluidization drier and sieved. Prosolv (microcrystalline cellulose with a colloidal silica layer), the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 1000 mg/tablet.
During this experiment parts of the cores did not get torn off during tabletting any more. The flow properties of the material met the tabletting parameters in this case. Suitable disintegration and abrasion of the final tablets was also achieved. During the dissolution tests a problem occurred concerning disintegration of the granules caused by an unsuitable type of the binder used for the granulation. Tests with various binders were carried out - povidone, starch, methocel and more. Povidone was selected as the most suitable binder.
Example 5
Sofosbuvir (crystalline Form X) was water granulated together with microcrystalline cellulose, crospovidone and povidone. The granulate was dried in a fluidization drier and sieved. Prosolv (microcrystalline cellulose with a colloidal silica layer), the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 1000 mg/tablet. The cores produced this way showed suitable parameters as abrasion, disintegration and dissolution rate of the active ingredient during analytic tests.
Further experimental steps focused on increasing the share of the active ingredient in the tablet. In the first experiments, there was an effort to reduce the tablet weight by reducing the amount of microcrystalline cellulose. In the final stage, microcrystalline cellulose was completely eliminated from the granulation process. Thus, the share of 60% of the active ingredient in one tablet was achieved.
Example 6
Sofosbuvir (crystalline Form X) was water granulated together with crospovidone and povidone. The granulate was dried in a fluidization drier and sieved through a sieve with the mesh size of 0.8 mm. Prosolv (microcrystalline cellulose with a colloidal silica layer), microcrystalline cellulose, the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the granulate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 667 mg/tablet. The flow properties of the material met the tabletting parameters. Suitable disintegration, abrasion and dissolution and stability characteristics of the resulting tablets were also achieved.
Dry granulation (also compaction) was another process that was selected as suitable for processing of sofosbuvir. In the first experiments, a similar composition as in the case of wet granulation was used.
Example 7
Sofosbuvir (crystalline Form X) was compacted together with microcrystalline cellulose, colloidal silica, crospovidone and magnesium stearate. Prosolv (microcrystalline cellulose with a colloidal silica layer), the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the compacted matter. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 1000 mg/tablet.
No process problems occurred during this experiment. The flow properties of the material met the tabletting parameters in this case. Suitable disintegration and abrasion of the final tablets was also achieved. During the dissolution tests a problem occurred concerning releasing of the active ingredient during dissolution. With regard to the possible influence of magnesium stearate on the dissolution this excipient was replaced with sodium stearyl fumarate.
Example 8
Constituent Function Amount (% by weight)
Sofosbuvir crystalline Form 1 Active pharmaceutical ingredient 40
Microcrystalline cellulose Filler 30
Prosolv SMCC90 Filler 11
Klucel (HPC) Binder 5
Crospovidone Disintegrant 10
Colloidal Si02 Glidant 2.5
Sodium stearyl fumarate Lubricant 1.5
Sofosbuvir (crystalline Form I) was compacted together with microcrystalline cellulose, colloidal silica, crospovidone and magnesium stearate. Prosolv (microcrystalline cellulose with a colloidal silica layer), the rest of crospovidone, colloidal silica and magnesium stearate were admixed to the compacted matter. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 1000 mg/tablet.
In this case, the problem concerning dissolution of the active ingredient was eliminated. The next step consisted in increasing the share of the active ingredient in the tablet over 40%. The experiments were conducted both with crystalline Form I and with crystalline Form X.
Example 9
Sofosbuvir (crystalline Form X) was compacted together with microcrystalline cellulose, Prosolv, crospovidone and magnesium stearate. The rest of crospovidone, magnesium stearate and colloidal silica were admixed to the compacted matter. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 800 mg/tablet. During the tabletting, the material adhered to the punches. This phenomenon could only be prevented by addition of 1% of a lubricant or use of external lubrication of the punches during tabletting. Thus, the final content of the active ingredient was 49.5% by weight.
Example 10
Sofosbuvir (crystalline Form X) was mixed together with microcrystalline cellulose, Prosolv, crospovidone, pre-gelatinized starch, colloidal silica and magnesium stearate. The tablets were tabletted in a rotary tabletting machine. The weight of the cores was 800 mg/tablet. During the tabletting, the material adhered to the punches. This phenomenon could only be prevented by addition of 1% by weight of a lubricant or use of external lubrication of the punches during tabletting. The final content of the active ingredient was thus about 49.5% by weight.
Claims
1. A pharmaceutical formulation containing the active ingredient sofosbuvir, characterized in that the concentration of the active ingredient is 40% by weight or higher.
2. The pharmaceutical formulation according to claim 1, characterized in that the concentration of the active ingredient is in the range of 40 to 60% by weight.
3. The pharmaceutical formulation according to any one of the preceding claims, characterized in that it comprises other pharmaceutically acceptable materials, including a filler, a disintegrant, a glidant and a lubricant.
4. The pharmaceutical formulation according to claim 3, characterized in that it comprises 20 to 45% by weight of the filler or fillers, 5 to 12% by weight of the disintegrant and 4 to 15% by weight of the other materials.
5. The pharmaceutical formulation according to claims 3 to 4, characterized in that as it comprises Prosolv as the filler, i.e. microcrystalline cellulose with a colloidal silica layer in combination with microcrystalline cellulose.
6. The pharmaceutical formulation according to claims 3 to 4, characterized in that the filler comprises a sugar alcohol in combination with microcrystalline cellulose.
7. The pharmaceutical formulation according to claims 3 to 6, characterized in that the lubricant is selected from magnesium stearate and sodium stearyl fumarate.
8. The pharmaceutical formulation according to claims 3 to 7, characterized in that it further comprises a binder.
9. The pharmaceutical formulation according to claim 8, characterized in that the binder is selected from polyvinyl pyrrolidone and starch.
10. The pharmaceutical formulation according to any one of the preceding claims,
characterized in that it comprises sofosbuvir of a crystalline form characterized by the diffraction peaks 8.0; 12.3; 17.1; 19.9 and 20.8 ± 0.2° with the use of CuKa radiation.
11. A tablet comprising the pharmaceutical formulation as defined in claims 1 to 10, characterized in that it contains 400 mg of sofosbuvir.
12. A method for producing the pharmaceutical formulation as defined in claims 1 to 10, characterized in that it comprises mixing of sofosbuvir with one or more pharmaceutically acceptable materials and possible further processing by compression into tablet cores.
13. The method according to claim 12, characterized in that it comprises water granulation of a free flowing mixture of sofosbuvir with one or more pharmaceutically acceptable materials.
14. The method according to claim 12, characterized in that it comprises compaction of sofosbuvir with one or more pharmaceutically acceptable materials and subsequent grinding of the compacted matter into granules.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CZ2015-443A CZ2015443A3 (en) | 2015-06-26 | 2015-06-26 | Sofosbuvir pharmaceutical formulation |
| CZPV2015-443 | 2015-06-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016206663A1 true WO2016206663A1 (en) | 2016-12-29 |
Family
ID=56617961
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CZ2016/000073 WO2016206663A1 (en) | 2015-06-26 | 2016-06-27 | A pharmaceutical formulation of sofosbuvir |
Country Status (2)
| Country | Link |
|---|---|
| CZ (1) | CZ2015443A3 (en) |
| WO (1) | WO2016206663A1 (en) |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008121634A2 (en) | 2007-03-30 | 2008-10-09 | Pharmasset, Inc. | Nucleoside phosphoramidate prodrugs |
| WO2010135569A1 (en) | 2009-05-20 | 2010-11-25 | Pharmasset, Inc. | N- [ (2 ' r) -2 ' -deoxy-2 ' -fluoro-2 ' -methyl-p-phenyl-5 ' -uridylyl] -l-alanine 1-methylethyl ester and process for its production |
| WO2011123645A2 (en) | 2010-03-31 | 2011-10-06 | Pharmasset, Inc. | Nucleoside phosphoramidates |
| WO2013066748A1 (en) * | 2011-10-31 | 2013-05-10 | Gilead Pharmasset Llc | Methods and compositions for treating hepatitis c virus |
| WO2013082003A1 (en) | 2011-11-29 | 2013-06-06 | Gilead Pharmasset Llc | Compositions and methods for treating hepatitis c virus |
| WO2015150561A2 (en) * | 2014-04-03 | 2015-10-08 | Sandoz Ag | Solid composition comprising amorphous sofosbuvir |
| WO2015166071A1 (en) * | 2014-05-02 | 2015-11-05 | Boehringer Ingelheim International Gmbh | Combination therapy for treating hcv infection |
| WO2015189386A1 (en) * | 2014-06-13 | 2015-12-17 | Teva Pharmaceuticals International Gmbh | Composition with a high drug load of sofosbuvir |
| EP2959901A1 (en) * | 2014-06-23 | 2015-12-30 | Sanovel Ilac Sanayi ve Ticaret A.S. | Pharmaceutical combinations of sofosbuvir and ribavirin |
| EP2959888A1 (en) * | 2014-06-23 | 2015-12-30 | Sanovel Ilac Sanayi ve Ticaret A.S. | A novel pharmaceutical composition of sofosbuvir and ribavirin |
| EP2959891A1 (en) * | 2014-06-23 | 2015-12-30 | Sanovel Ilac Sanayi ve Ticaret A.S. | Modified release pharmaceutical compositions of sofosbuvir and ribavirin |
| CN105287424A (en) * | 2015-12-04 | 2016-02-03 | 石家庄四药有限公司 | Sofosbuvir tablet and preparation method thereof |
| WO2016035006A1 (en) * | 2014-09-01 | 2016-03-10 | Dr. Reddy’S Laboratories Limited | Novel nucleotide analogs, process for the preparation of sofosbuvir and its analogs, novel forms of sofosbuvir and solid dispersion of sofosbuvir |
-
2015
- 2015-06-26 CZ CZ2015-443A patent/CZ2015443A3/en unknown
-
2016
- 2016-06-27 WO PCT/CZ2016/000073 patent/WO2016206663A1/en active Application Filing
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008121634A2 (en) | 2007-03-30 | 2008-10-09 | Pharmasset, Inc. | Nucleoside phosphoramidate prodrugs |
| WO2010135569A1 (en) | 2009-05-20 | 2010-11-25 | Pharmasset, Inc. | N- [ (2 ' r) -2 ' -deoxy-2 ' -fluoro-2 ' -methyl-p-phenyl-5 ' -uridylyl] -l-alanine 1-methylethyl ester and process for its production |
| WO2011123645A2 (en) | 2010-03-31 | 2011-10-06 | Pharmasset, Inc. | Nucleoside phosphoramidates |
| WO2013066748A1 (en) * | 2011-10-31 | 2013-05-10 | Gilead Pharmasset Llc | Methods and compositions for treating hepatitis c virus |
| WO2013082003A1 (en) | 2011-11-29 | 2013-06-06 | Gilead Pharmasset Llc | Compositions and methods for treating hepatitis c virus |
| WO2015150561A2 (en) * | 2014-04-03 | 2015-10-08 | Sandoz Ag | Solid composition comprising amorphous sofosbuvir |
| WO2015166071A1 (en) * | 2014-05-02 | 2015-11-05 | Boehringer Ingelheim International Gmbh | Combination therapy for treating hcv infection |
| WO2015189386A1 (en) * | 2014-06-13 | 2015-12-17 | Teva Pharmaceuticals International Gmbh | Composition with a high drug load of sofosbuvir |
| EP2959901A1 (en) * | 2014-06-23 | 2015-12-30 | Sanovel Ilac Sanayi ve Ticaret A.S. | Pharmaceutical combinations of sofosbuvir and ribavirin |
| EP2959888A1 (en) * | 2014-06-23 | 2015-12-30 | Sanovel Ilac Sanayi ve Ticaret A.S. | A novel pharmaceutical composition of sofosbuvir and ribavirin |
| EP2959891A1 (en) * | 2014-06-23 | 2015-12-30 | Sanovel Ilac Sanayi ve Ticaret A.S. | Modified release pharmaceutical compositions of sofosbuvir and ribavirin |
| WO2016035006A1 (en) * | 2014-09-01 | 2016-03-10 | Dr. Reddy’S Laboratories Limited | Novel nucleotide analogs, process for the preparation of sofosbuvir and its analogs, novel forms of sofosbuvir and solid dispersion of sofosbuvir |
| CN105287424A (en) * | 2015-12-04 | 2016-02-03 | 石家庄四药有限公司 | Sofosbuvir tablet and preparation method thereof |
Non-Patent Citations (1)
| Title |
|---|
| MICHAEL J. SOFIA ET AL: "Discovery of a beta-D-2'-Deoxy-2'-alpha-fluoro-2'-beta-C-methyluridine Nucleotide Prodrug (PSI-7977) for the Treatment of Hepatitis C Virus", JOURNAL OF MEDICINAL CHEMISTRY, vol. 53, no. 19, 16 September 2010 (2010-09-16), pages 7202 - 7218, XP055004442, ISSN: 0022-2623, DOI: 10.1021/jm100863x * |
Also Published As
| Publication number | Publication date |
|---|---|
| CZ2015443A3 (en) | 2017-01-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1762230A1 (en) | Filmtablet or granulate comprising a pyridylpyrimidine | |
| KR101977785B1 (en) | Composite formulation for oral administration comprising ezetimibe and rosuvastatin and a process for the preparation thereof | |
| KR20180109992A (en) | A pharmaceutical composition comprising a JAK kinase inhibitor or a pharmaceutically acceptable salt thereof | |
| TW201040142A (en) | Tablets for combination therapy | |
| TW201206447A (en) | Pharmaceutical formulations | |
| WO2012164578A1 (en) | Compositions and methods for preparing immediate release formulations of nilotinib | |
| WO2007086891A1 (en) | Levetiracetam formulations and methods for their manufacture | |
| JP6895779B2 (en) | Azilsartan-containing solid pharmaceutical composition | |
| CN113925833A (en) | Dosage form compositions comprising tyrosine protein kinase inhibitors | |
| EA029416B1 (en) | Tablet comprising imatinib or a pharmaceutically acceptable salt thereof and process for the preparation thereof | |
| WO2015110952A1 (en) | Solid oral pharmaceutical compositions comprising ticagrelor or salt thereof | |
| CN109692164A (en) | Compound A or the pharmaceutical composition of its salt and preparation method thereof | |
| EP2359816B1 (en) | Aripiprazole formulations | |
| AU2009243734B2 (en) | Granulate comprising escitalopram oxalate | |
| WO2013128467A1 (en) | Ritonavir compositions | |
| EP3860606B1 (en) | Pharmaceutical composition comprising lenvatinib esylate or tosylate | |
| WO2016206663A1 (en) | A pharmaceutical formulation of sofosbuvir | |
| KR20160014619A (en) | Agomelatine formulations comprising agomelatine in the form of co-crystals | |
| JP5755382B2 (en) | Orally disintegrating tablets | |
| KR101938872B1 (en) | Composition comprising complex for prevention and treatment of dementia and cognitive impairment | |
| EP2644196B1 (en) | Pharmaceutical compositions of Entecavir | |
| KR20190007370A (en) | Combination formulation having improved stability and dissolution rate comprising Bazedoxifene or its pharmaceutically acceptable salt, and Cholecalciferol or its pharmaceutically acceptable salt | |
| JP7362302B2 (en) | Tablets containing levetiracetam and their manufacturing method | |
| KR20120134545A (en) | Method for preparing immediate release pharmaceutical composition having improved stability and content uniformity | |
| JP7115825B2 (en) | Oral formulation containing ezetimibe and its manufacturing method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16750086 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 16750086 Country of ref document: EP Kind code of ref document: A1 |