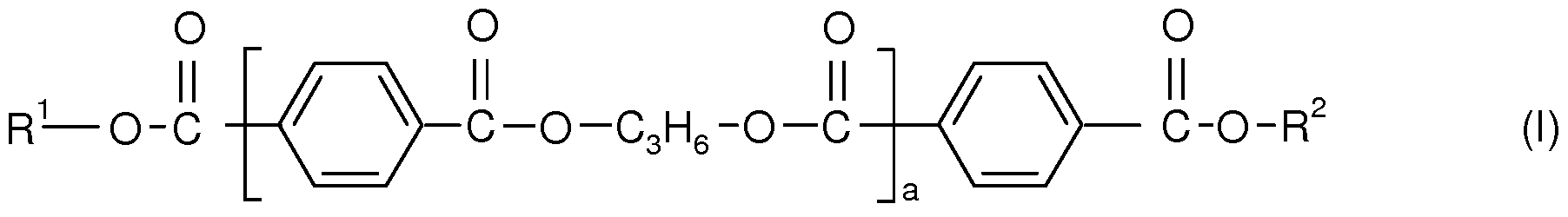WO2016005338A1 - Storage-stable compositions comprising soil release polymers - Google Patents
Storage-stable compositions comprising soil release polymers Download PDFInfo
- Publication number
- WO2016005338A1 WO2016005338A1 PCT/EP2015/065389 EP2015065389W WO2016005338A1 WO 2016005338 A1 WO2016005338 A1 WO 2016005338A1 EP 2015065389 W EP2015065389 W EP 2015065389W WO 2016005338 A1 WO2016005338 A1 WO 2016005338A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- component
- molar average
- polyesters
- groups
- weight
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/0005—Other compounding ingredients characterised by their effect
- C11D3/0036—Soil deposition preventing compositions; Antiredeposition agents
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/20—Organic compounds containing oxygen
- C11D3/2003—Alcohols; Phenols
- C11D3/2041—Dihydric alcohols
- C11D3/2044—Dihydric alcohols linear
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/20—Organic compounds containing oxygen
- C11D3/2068—Ethers
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/37—Polymers
- C11D3/3703—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- C11D3/3715—Polyesters or polycarbonates
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D2111/00—Cleaning compositions characterised by the objects to be cleaned; Cleaning compositions characterised by non-standard cleaning or washing processes
- C11D2111/10—Objects to be cleaned
- C11D2111/12—Soft surfaces, e.g. textile
Definitions
- compositions comprising soil release polymers
- the invention relates to compositions comprising polyesters.
- the polyesters are e.g. useful as soil release agents and the inventive compositions may be used in laundry detergent and fabric care products.
- soil release agent is applied to materials that modify the fabric surface minimizing the subsequent soiling and making the cleaning of the fabric easier on further washing cycles.
- Laundry detergent compositions containing polyesters have been widely disclosed in the art.
- EP 0 964 015 A1 discloses soil release oligoesters that may be used as soil release polymers in detergents and that are prepared using polyols comprising 3 to 6 hydroxyl groups.
- EP 1 661 933 A1 is directed to at room temperature flowable, amphiphilic and nonionic oligoesters prepared by reacting dicarboxylic acid compounds, polyol compounds and water-soluble alkylene oxide adducts and their use as additive in washing and cleaning compositions.
- many of the polyesters described in the prior art are in need of improved stability in an alkaline environment. Especially in alkaline heavy duty washing liquids polyesters often show turbidity upon incorporation and by alkaline hydrolysis thereby also losing soil release power.
- polyesters described in WO 2013/019658 A1 fulfill these requirements and possess an advantageous, increased stability against hydrolysis and an excellent soil-release-effect, but they are solids that melt at approximately 50 °C and therefore, their handling is not easy in practice due to the necessity of hot storage and handling.
- compositions of these polyesters that can be handled easily in practice and that are liquid and storage-stable.
- compositions comprising
- R and R 2 independently of one another are X-(OC2H 4 )n-(OC 3 H 6 )m wherein X is
- Ci- 4 alkyl and preferably methyl, the -(OC 2 H 4 ) groups and the -(OC 3 H 6 ) groups are arranged blockwise and the block consisting of the -(OC 3 H 6 ) groups is bound to a COO group or are HO-(C 3 H 6 ), and preferably are independently of one another X-(OC2H 4 )n-(OC 3 H 6 )m, n is based on a molar average a number of from 12 to 120 and
- m is based on a molar average a number of from 1 to 10 and preferably of from 1 to 7, and
- a is based on a molar average a number of from 4 to 9 and B) of from 10 to 30 % by weight of one or more alcohols selected from the group consisting of ethylene glycol, 1 ,2-propylene glycol, 1 ,3-propylene glycol, 1 ,2-butylene glycol, 1 ,3-butylene glycol, 1 ,4-butylene glycol and butyl glycol and C) of from 24 to 42 % by weight of water, the amounts in each case being based on the total weight of the composition.
- compositions comprising
- R and R 2 independently of one another are X-(OC2H 4 )n-(OC 3 H 6 )m wherein X is
- the -(OC 2 H 4 ) groups and the -(OC 3 H 6 ) groups are arranged blockwise and the block consisting of the -(OC 3 H 6 ) groups is bound to a COO group or are HO-(C 3 H 6 ), and preferably are independently of one another X-(OC2H 4 )n-(OC 3 H 6 )m, n is based on a molar average a number of from 12 to 120 and
- m is based on a molar average a number of from 1 to 10 and preferably of from 1 to 7, and
- a is based on a molar average a number of from 4 to 9 and
- Butyl glycol has the following structure: Ch ⁇ ChysOCHsCHsOH.
- Aqueous or aqueous-alcoholic solutions of the polyesters often possess a relatively good stability when stored at 5 °C.
- non-inventive compositions of the polyesters at first show a turbidity during storage that later results in massive precipitations. These precipitations cannot be dissolved again at 80 °C, meaning that the respective products may not be regarded as being storage-stable, and their properties are changed irreversibly by storage at elevated temperature.
- the inventive compositions furthermore possess the advantage that they are sufficiently storage-stable, also at elevated temperatures.
- inventive compositions preferably are solutions at 25 °C.
- group "X" is Ci -4 alkyl and preferably is methyl.
- the one or more polyesters of component A) of the inventive compositions are according to the following formula (I)
- R and R 2 independently of one another are H3C-(OC2H 4 )n-(OC 3 H6)m wherein the -(OC 2 H 4 ) groups and the -(OC 3 H 6 ) groups are arranged blockwise and the block consisting of the -(OC 3 H 6 ) groups is bound to a COO group or are HO-(C 3 H 6 ), and preferably are independently of one another H 3 C-(OC 2 H 4 )n-(OC 3 H 6 )m,
- n is based on a molar average a number of from 40 to 50
- m is based on a molar average a number of from 1 to 7, and
- a is based on a molar average a number of from 4 to 9.
- variable "a" based on a molar average preferably is a number of from 5 to 8 and more preferably is a number of from 6 to 7.
- variable "m" based on a molar average preferably is a number of from 2 to 5.
- variable "n" based on a molar average preferably is a number of from 43 to 47, more preferably is a number of from 44 to 46 and even more preferably is 45.
- the one or more polyesters of component A) of the inventive compositions are according to the following formula (I)
- R and R 2 independently of one another are H3C-(OC2H 4 )n-(OC 3 H6)m wherein the -(OC 2 H 4 ) groups and the -(OC 3 H 6 ) groups are arranged blockwise and the block consisting of the -(OC 3 H 6 ) groups is bound to a COO group,
- n is based on a molar average a number of from 44 to 46
- n based on a molar average
- a is based on a molar average a number of from 5 to 8.
- R and R 2 independently of one another are H3C-(OC2H 4 )n-(OC 3 H6)m wherein the -(OC 2 H 4 ) groups and the -(OC 3 H 6 ) groups are arranged blockwise and the block consisting of the -(OC 3 H 6 ) groups is bound to a COO group,
- n based on a molar average
- the one or more polyesters of component A) of the inventive compositions are according to the following formula (I)
- R and R 2 independently of one another are H3C-(OC2H 4 )n-(OC 3 H6)m wherein the -(OC 2 H 4 ) groups and the -(OC 3 H 6 ) groups are arranged blockwise and the block consisting of the -(OC 3 H 6 ) groups is bound to a COO group,
- H 3 C-(OC 2 H 4 ) n -(OC 3 H 6 )m are of the formula -O-CH 2 -CH 2 -.
- the groups -0-C 3 H 6 - in the structural units indexed with "a”, in the structural units "X-(OC 2 H 4 )n-(OC 3 H6)m” or "H 3 C-(OC2H 4 )n-(OC 3 H 6 )m” and in the structural units HO-(C 3 H 6 ) are of the formula -0-CH(CH 3 )-CH 2 - or -0-CH 2 -CH(CH 3 )-, i.e. are of the formula
- the polyesters of component A) of the inventive compositions may advantageously be prepared by a process which comprises heating dimethyl terephthalate (DMT), 1 ,2-propylene glycol (PG), and X-(OC 2 H 4 )n-(OC 3 H6)m-OH, wherein X is Ci -4 alkyl and preferably methyl, the -(OC 2 H 4 ) groups and
- polyesters of component A) of the inventive compositions are e.g. described in WO 2013/019658 A1 .
- the one or more alcohols of component B) of the inventive compositions are e.g. described in WO 2013/019658 A1 .
- the one or more alcohols of component B) of the inventive compositions are e.g. described in WO 2013/019658 A1 .
- the inventive compositions preferably comprise one or more additives (component D)), and in this case the amount of water of component C) in the inventive compositions preferably is of from 24 to 39.95 % by weight, the amounts in each case being based on the total weight of the inventive compositions.
- the one or more additives of component D) of the inventive compositions are preferably selected from the group consisting of sequestering agents, complexing agents, polymers different from the one or more polyesters of component A) and surfactants.
- Suitable sequestering agents e.g. are polyacrylic acid or acrylic acid / maleic acid copolymers (e.g. Sokalan ® CP 12S, BASF).
- Suitable complexing agents e.g. are EDTA (ethylene diamine tetraactetate), diethylene triamine pentaacetate, nitrilotriacetic acid salts or iminodisuccinic acid salts.
- the viscosity of the inventive compositions is of from 500 to 2 000 m Pa- s.
- the polyester synthesis is carried out by the reaction of dimethyl terephthalate (DMT), 1 ,2-propylene glycol (PG), and methyl polyalkyleneglycol using sodium acetate (NaOAc) and tetraisopropyl orthotitanate (IPT) as the catalyst system.
- the synthesis is a two-step procedure. The first step is a transesterification and the second step is a polycondensation. Transesterification
- the mixture is heated up to 170 °C for 1 h and then up to 210 °C for a further 1 h sparged by a nitrogen stream.
- the mixture is heated up to 230 °C. At 230 °C the pressure is reduced to 1 mbar over 160 min. Once the polycondenzation reaction has started, 1 ,2-propylene glycol is distilled out of the system. The mixture is stirred for 4 h at 230 °C and a pressure of 1 mbar. The reaction mixture is cooled down to 140 - 150 °C. Vacuum is released with nitrogen and the molten polymer is transferred into a glass bottle.
- n is based on a molar average 45
- m is based on a molar average 5
- R and R 2 are H 3 C-(OC 2 H 4 )n-(OC 3 H 6 )m wherein the -(OC 2 H 4 ) groups and
- a is based on a molar average a number of from 6 to 7. Stability tests
- Sokalan ® CP 12S (acrylic acid / maleic acid copolymer, BASF) has been used as the additive. From the table it can be seen that solutions of the soil release polyesters in water (Examples 1 - 4) become turbid at 45°C already after two weeks of storage.
- compositions comprising 1 ,2-propylene glycol or butyl glycol are still clear after 4 weeks of storage at 45°C.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Detergent Compositions (AREA)
- Polyesters Or Polycarbonates (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Abstract
Description
Claims
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15734373.2A EP3167032B1 (en) | 2014-07-09 | 2015-07-06 | Storage-stable compositions comprising soil release polymers |
| BR112016030988-0A BR112016030988B1 (en) | 2014-07-09 | 2015-07-06 | STABLE COMPOSITIONS FOR STORAGE INCLUDING POLYMERS FOR DIRT RELEASE |
| MX2017000323A MX375867B (en) | 2014-07-09 | 2015-07-06 | STORAGE-STABLE COMPOSITIONS COMPRISING DIRT-REMOVING POLYMERS. |
| ES15734373.2T ES2682984T3 (en) | 2014-07-09 | 2015-07-06 | Stable storage compositions comprising soil release polymers |
| CN201580036878.3A CN106536699B (en) | 2014-07-09 | 2015-07-06 | Storage stable compositions comprising soil release polymers |
| US15/324,143 US10087400B2 (en) | 2014-07-09 | 2015-07-06 | Storage-stable compositions comprising soil release polymers |
| JP2017500390A JP6505205B2 (en) | 2014-07-09 | 2015-07-06 | Storage-stable composition comprising soil release polymer |
| PL15734373T PL3167032T3 (en) | 2014-07-09 | 2015-07-06 | Storage-stable compositions comprising soil release polymers |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP14002349.0A EP2966160A1 (en) | 2014-07-09 | 2014-07-09 | Storage-stable compositions comprising soil release polymers |
| EP14002349.0 | 2014-07-09 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016005338A1 true WO2016005338A1 (en) | 2016-01-14 |
Family
ID=51167559
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2015/065389 Ceased WO2016005338A1 (en) | 2014-07-09 | 2015-07-06 | Storage-stable compositions comprising soil release polymers |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US10087400B2 (en) |
| EP (2) | EP2966160A1 (en) |
| JP (1) | JP6505205B2 (en) |
| CN (1) | CN106536699B (en) |
| BR (1) | BR112016030988B1 (en) |
| ES (1) | ES2682984T3 (en) |
| MX (1) | MX375867B (en) |
| PL (1) | PL3167032T3 (en) |
| WO (1) | WO2016005338A1 (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017202923A1 (en) | 2016-05-27 | 2017-11-30 | Unilever Plc | Laundry composition |
| WO2019105675A1 (en) | 2017-11-30 | 2019-06-06 | Unilever Plc | Detergent composition comprising protease |
| WO2019219531A1 (en) | 2018-05-17 | 2019-11-21 | Unilever Plc | Cleaning composition |
| WO2020058024A1 (en) | 2018-09-17 | 2020-03-26 | Unilever Plc | Detergent composition |
| WO2020104158A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104155A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104156A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104159A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104157A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020260006A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent compositions |
| WO2020259949A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020260040A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020260038A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020259947A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020259948A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2021043764A1 (en) | 2019-09-02 | 2021-03-11 | Unilever Global Ip Limited | Detergent composition |
| WO2021069516A1 (en) | 2019-10-07 | 2021-04-15 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2021185956A1 (en) | 2020-03-19 | 2021-09-23 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2021185870A1 (en) | 2020-03-19 | 2021-09-23 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2021249927A1 (en) | 2020-06-08 | 2021-12-16 | Unilever Ip Holdings B.V. | Method of improving protease activity |
| WO2022042989A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Surfactant and detergent composition |
| WO2022043138A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Surfactant and detergent composition |
| WO2022043042A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022042977A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022043045A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022122425A1 (en) | 2020-12-07 | 2022-06-16 | Unilever Ip Holdings B.V. | Composition |
| WO2022128781A1 (en) | 2020-12-17 | 2022-06-23 | Unilever Ip Holdings B.V. | Cleaning composition |
| WO2022128786A1 (en) | 2020-12-17 | 2022-06-23 | Unilever Ip Holdings B.V. | Use and cleaning composition |
| WO2023041694A1 (en) | 2021-09-20 | 2023-03-23 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2023067075A1 (en) | 2021-10-21 | 2023-04-27 | Unilever Ip Holdings B.V. | Detergent compositions |
| TWI818058B (en) * | 2018-08-10 | 2023-10-11 | 法商路易威登馬爾悌耶公司 | Method for creating luggage |
| WO2024115420A1 (en) | 2022-11-28 | 2024-06-06 | Unilever Ip Holdings B.V. | Detergent compositions |
| WO2024194190A1 (en) | 2023-03-17 | 2024-09-26 | Unilever Ip Holdings B.V. | Composition |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MX2017006153A (en) | 2014-11-11 | 2017-11-17 | Clariant Int Ltd | Laundry detergents containing soil release polymers. |
| US10240107B2 (en) | 2014-11-11 | 2019-03-26 | Clariant International Ltd. | Laundry detergents containing soil release polymers |
| EP3489340A1 (en) | 2017-11-28 | 2019-05-29 | Clariant International Ltd | Renewably sourced soil release polyesters |
| EP3802765B1 (en) * | 2018-05-24 | 2024-12-18 | Clariant International Ltd | Soil release polyesters for use in detergent compositions |
| BR112021022958A2 (en) * | 2019-05-28 | 2022-01-18 | Unilever Ip Holdings B V | Composition of oral care and method of minimizing or preventing tooth staining |
| WO2021116049A1 (en) * | 2019-12-09 | 2021-06-17 | Clariant International Ltd | Polyesters |
| DE102020006977A1 (en) | 2020-11-13 | 2022-05-19 | WeylChem Performance Products GmbH | Aqueous-alcoholic polyester compositions, detergents and cleaning agents containing these and their use |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102004056785A1 (en) * | 2004-11-24 | 2006-06-01 | Sasol Germany Gmbh | Flowable, amphiphilic and nonionic oligoesters |
| WO2014019658A1 (en) * | 2012-07-31 | 2014-02-06 | Clariant International Ltd | Polyesters |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4759876A (en) | 1985-03-19 | 1988-07-26 | Colgate-Palmolive Company | Stable soil release promoting enzymatic liquid detergent composition |
| DE19826356A1 (en) | 1998-06-12 | 1999-12-16 | Clariant Gmbh | Soil-removing oligoester |
| DE102005061058A1 (en) | 2005-12-21 | 2007-07-05 | Clariant Produkte (Deutschland) Gmbh | New polyester compounds useful in detergents and cleaning agents e.g. color detergents, bar soaps and dishwash detergents, as soil releasing agents, fabric care agents and means for the equipments of textiles |
| DE102007005532A1 (en) | 2007-02-03 | 2008-08-07 | Clariant International Limited | Aqueous oligo- and polyester preparations |
| DE102007013217A1 (en) | 2007-03-15 | 2008-09-18 | Clariant International Ltd. | Anionic Soil Release Polymers |
| DE102008023803A1 (en) | 2008-05-15 | 2009-11-26 | Clariant International Ltd. | Additives for detergents and cleaners |
| DE102008028409A1 (en) | 2008-06-17 | 2009-12-24 | Clariant International Ltd. | Process for the production of polyester granules |
| KR20140050698A (en) | 2011-07-29 | 2014-04-29 | 셀렉타 바이오사이언시즈, 인크. | Synthetic nanocarriers that generate humoral and cytotoxic t lymphocyte (ctl) immune responses |
| WO2014019659A1 (en) | 2012-07-31 | 2014-02-06 | Clariant International Ltd | Polyesters |
| BR112015001754B1 (en) | 2012-07-31 | 2021-03-16 | Unilever Ip Holdings B.V. | alkaline liquid detergent composition for washing |
| DE102012016444A1 (en) | 2012-08-18 | 2014-02-20 | Clariant International Ltd. | polyester |
| DE102012016462A1 (en) | 2012-08-18 | 2014-02-20 | Clariant International Ltd. | Use of polyesters in detergents and cleaners |
| MX2017006153A (en) | 2014-11-11 | 2017-11-17 | Clariant Int Ltd | Laundry detergents containing soil release polymers. |
| US10240107B2 (en) | 2014-11-11 | 2019-03-26 | Clariant International Ltd. | Laundry detergents containing soil release polymers |
-
2014
- 2014-07-09 EP EP14002349.0A patent/EP2966160A1/en not_active Withdrawn
-
2015
- 2015-07-06 CN CN201580036878.3A patent/CN106536699B/en active Active
- 2015-07-06 WO PCT/EP2015/065389 patent/WO2016005338A1/en not_active Ceased
- 2015-07-06 BR BR112016030988-0A patent/BR112016030988B1/en active IP Right Grant
- 2015-07-06 PL PL15734373T patent/PL3167032T3/en unknown
- 2015-07-06 MX MX2017000323A patent/MX375867B/en active IP Right Grant
- 2015-07-06 EP EP15734373.2A patent/EP3167032B1/en active Active
- 2015-07-06 ES ES15734373.2T patent/ES2682984T3/en active Active
- 2015-07-06 JP JP2017500390A patent/JP6505205B2/en active Active
- 2015-07-06 US US15/324,143 patent/US10087400B2/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102004056785A1 (en) * | 2004-11-24 | 2006-06-01 | Sasol Germany Gmbh | Flowable, amphiphilic and nonionic oligoesters |
| WO2014019658A1 (en) * | 2012-07-31 | 2014-02-06 | Clariant International Ltd | Polyesters |
Cited By (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017202923A1 (en) | 2016-05-27 | 2017-11-30 | Unilever Plc | Laundry composition |
| WO2019105675A1 (en) | 2017-11-30 | 2019-06-06 | Unilever Plc | Detergent composition comprising protease |
| WO2019219531A1 (en) | 2018-05-17 | 2019-11-21 | Unilever Plc | Cleaning composition |
| TWI818058B (en) * | 2018-08-10 | 2023-10-11 | 法商路易威登馬爾悌耶公司 | Method for creating luggage |
| WO2020058024A1 (en) | 2018-09-17 | 2020-03-26 | Unilever Plc | Detergent composition |
| WO2020104157A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104156A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104159A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104155A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104158A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020260006A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent compositions |
| WO2020259949A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020260040A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020260038A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020259947A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020259948A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2021043764A1 (en) | 2019-09-02 | 2021-03-11 | Unilever Global Ip Limited | Detergent composition |
| WO2021069516A1 (en) | 2019-10-07 | 2021-04-15 | Unilever Ip Holdings B.V. | Detergent composition |
| CN115298295A (en) * | 2020-03-19 | 2022-11-04 | 联合利华知识产权控股有限公司 | Detergent composition |
| WO2021185870A1 (en) | 2020-03-19 | 2021-09-23 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2021185956A1 (en) | 2020-03-19 | 2021-09-23 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2021249927A1 (en) | 2020-06-08 | 2021-12-16 | Unilever Ip Holdings B.V. | Method of improving protease activity |
| WO2022042989A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Surfactant and detergent composition |
| WO2022043138A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Surfactant and detergent composition |
| WO2022043042A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022042977A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022043045A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022122425A1 (en) | 2020-12-07 | 2022-06-16 | Unilever Ip Holdings B.V. | Composition |
| WO2022128781A1 (en) | 2020-12-17 | 2022-06-23 | Unilever Ip Holdings B.V. | Cleaning composition |
| WO2022128786A1 (en) | 2020-12-17 | 2022-06-23 | Unilever Ip Holdings B.V. | Use and cleaning composition |
| WO2023041694A1 (en) | 2021-09-20 | 2023-03-23 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2023067075A1 (en) | 2021-10-21 | 2023-04-27 | Unilever Ip Holdings B.V. | Detergent compositions |
| WO2023067073A1 (en) | 2021-10-21 | 2023-04-27 | Unilever Ip Holdings B.V. | Detergent compositions |
| WO2024115420A1 (en) | 2022-11-28 | 2024-06-06 | Unilever Ip Holdings B.V. | Detergent compositions |
| WO2024194190A1 (en) | 2023-03-17 | 2024-09-26 | Unilever Ip Holdings B.V. | Composition |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3167032A1 (en) | 2017-05-17 |
| EP2966160A1 (en) | 2016-01-13 |
| BR112016030988B1 (en) | 2022-05-03 |
| BR112016030988A2 (en) | 2017-08-22 |
| PL3167032T3 (en) | 2018-11-30 |
| JP6505205B2 (en) | 2019-04-24 |
| ES2682984T3 (en) | 2018-09-24 |
| EP3167032B1 (en) | 2018-06-27 |
| CN106536699B (en) | 2019-04-23 |
| US20170145348A1 (en) | 2017-05-25 |
| CN106536699A (en) | 2017-03-22 |
| JP2017523279A (en) | 2017-08-17 |
| US10087400B2 (en) | 2018-10-02 |
| MX375867B (en) | 2025-03-07 |
| MX2017000323A (en) | 2017-04-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10087400B2 (en) | Storage-stable compositions comprising soil release polymers | |
| CA2953273C (en) | Alkaline laundry liquid composition comprising polyesters | |
| EP2880076B1 (en) | Polyesters | |
| JP6475617B2 (en) | polyester | |
| EP3717613B1 (en) | Renewably sourced soil release polyesters | |
| CN104508000B (en) | Alkaline liquid laundry detergent composition comprising polyester | |
| WO2019105939A1 (en) | Detergent compositions containing renewably sourced soil release polyesters | |
| AU2013298728B9 (en) | Alkaline liquid laundry detergent compositions comprising polyesters |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15734373 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2017500390 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15324143 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2017/000323 Country of ref document: MX |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112016030988 Country of ref document: BR |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015734373 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015734373 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 112016030988 Country of ref document: BR Kind code of ref document: A2 Effective date: 20161229 |