WO2015193779A1 - Barium humate production method - Google Patents
Barium humate production method Download PDFInfo
- Publication number
- WO2015193779A1 WO2015193779A1 PCT/IB2015/054461 IB2015054461W WO2015193779A1 WO 2015193779 A1 WO2015193779 A1 WO 2015193779A1 IB 2015054461 W IB2015054461 W IB 2015054461W WO 2015193779 A1 WO2015193779 A1 WO 2015193779A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- humate
- barium
- humic acid
- coal
- humic
- Prior art date
Links
- 229910052788 barium Inorganic materials 0.000 title claims abstract description 51
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 title claims abstract description 51
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 26
- 239000004021 humic acid Substances 0.000 claims abstract description 52
- QJZYHAIUNVAGQP-UHFFFAOYSA-N 3-nitrobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid Chemical compound C1C2C=CC1C(C(=O)O)C2(C(O)=O)[N+]([O-])=O QJZYHAIUNVAGQP-UHFFFAOYSA-N 0.000 claims abstract description 51
- 239000003245 coal Substances 0.000 claims abstract description 32
- 238000010438 heat treatment Methods 0.000 claims abstract description 20
- 238000001354 calcination Methods 0.000 claims abstract description 7
- 238000000034 method Methods 0.000 claims description 26
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 15
- 239000011734 sodium Substances 0.000 claims description 13
- 229910052708 sodium Inorganic materials 0.000 claims description 13
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 12
- 159000000009 barium salts Chemical class 0.000 claims description 8
- 238000010908 decantation Methods 0.000 claims description 6
- 238000000746 purification Methods 0.000 claims description 6
- 238000000926 separation method Methods 0.000 claims description 6
- 238000005406 washing Methods 0.000 claims description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 claims description 4
- 238000005342 ion exchange Methods 0.000 claims description 4
- WDIHJSXYQDMJHN-UHFFFAOYSA-L barium chloride Chemical compound [Cl-].[Cl-].[Ba+2] WDIHJSXYQDMJHN-UHFFFAOYSA-L 0.000 claims description 3
- 229910001626 barium chloride Inorganic materials 0.000 claims description 3
- 229910052799 carbon Inorganic materials 0.000 claims description 3
- 239000003077 lignite Substances 0.000 claims description 3
- 239000007791 liquid phase Substances 0.000 claims description 3
- RQPZNWPYLFFXCP-UHFFFAOYSA-L barium dihydroxide Chemical compound [OH-].[OH-].[Ba+2] RQPZNWPYLFFXCP-UHFFFAOYSA-L 0.000 claims description 2
- IWOUKMZUPDVPGQ-UHFFFAOYSA-N barium nitrate Inorganic materials [Ba+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O IWOUKMZUPDVPGQ-UHFFFAOYSA-N 0.000 claims description 2
- 238000010981 drying operation Methods 0.000 claims description 2
- 239000007790 solid phase Substances 0.000 claims description 2
- 238000000605 extraction Methods 0.000 abstract description 6
- 229910010272 inorganic material Inorganic materials 0.000 abstract description 5
- 239000011147 inorganic material Substances 0.000 abstract description 5
- 230000004048 modification Effects 0.000 abstract description 4
- 238000012986 modification Methods 0.000 abstract description 4
- 238000001179 sorption measurement Methods 0.000 description 20
- 239000000126 substance Substances 0.000 description 18
- 239000003463 adsorbent Substances 0.000 description 14
- 229920001971 elastomer Polymers 0.000 description 10
- 239000005060 rubber Substances 0.000 description 10
- 239000000243 solution Substances 0.000 description 10
- 229910052802 copper Inorganic materials 0.000 description 9
- 239000010949 copper Substances 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 8
- 239000000919 ceramic Substances 0.000 description 7
- 239000002270 dispersing agent Substances 0.000 description 7
- 229910001385 heavy metal Inorganic materials 0.000 description 7
- 239000011133 lead Substances 0.000 description 7
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 6
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 6
- 230000002378 acidificating effect Effects 0.000 description 6
- 239000000945 filler Substances 0.000 description 6
- 229910052753 mercury Inorganic materials 0.000 description 6
- 229910021645 metal ion Inorganic materials 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 5
- 229910052793 cadmium Inorganic materials 0.000 description 5
- 229910052804 chromium Inorganic materials 0.000 description 5
- 239000011651 chromium Substances 0.000 description 5
- 229910052742 iron Inorganic materials 0.000 description 5
- 229910052745 lead Inorganic materials 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 229910052759 nickel Inorganic materials 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 238000010092 rubber production Methods 0.000 description 5
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 4
- 229910052783 alkali metal Inorganic materials 0.000 description 4
- 125000003118 aryl group Chemical group 0.000 description 4
- 239000006229 carbon black Substances 0.000 description 4
- 235000019241 carbon black Nutrition 0.000 description 4
- 238000004132 cross linking Methods 0.000 description 4
- 150000002500 ions Chemical class 0.000 description 4
- 239000002957 persistent organic pollutant Substances 0.000 description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N phenol group Chemical group C1(=CC=CC=C1)O ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 4
- 229910052700 potassium Inorganic materials 0.000 description 4
- -1 turbo Substances 0.000 description 4
- 229910052725 zinc Inorganic materials 0.000 description 4
- 239000011701 zinc Substances 0.000 description 4
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 3
- 229940072056 alginate Drugs 0.000 description 3
- 235000010443 alginic acid Nutrition 0.000 description 3
- 229920000615 alginic acid Polymers 0.000 description 3
- 229910052785 arsenic Inorganic materials 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 239000003337 fertilizer Substances 0.000 description 3
- 239000002509 fulvic acid Substances 0.000 description 3
- 150000002430 hydrocarbons Chemical group 0.000 description 3
- 229910052748 manganese Inorganic materials 0.000 description 3
- 239000011572 manganese Substances 0.000 description 3
- 239000011591 potassium Substances 0.000 description 3
- 239000002689 soil Substances 0.000 description 3
- 229920003048 styrene butadiene rubber Polymers 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- PUKLDDOGISCFCP-JSQCKWNTSA-N 21-Deoxycortisone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2=O PUKLDDOGISCFCP-JSQCKWNTSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- FCYKAQOGGFGCMD-UHFFFAOYSA-N Fulvic acid Natural products O1C2=CC(O)=C(O)C(C(O)=O)=C2C(=O)C2=C1CC(C)(O)OC2 FCYKAQOGGFGCMD-UHFFFAOYSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 239000002174 Styrene-butadiene Substances 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 125000001931 aliphatic group Chemical group 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 150000001340 alkali metals Chemical class 0.000 description 2
- 239000012670 alkaline solution Substances 0.000 description 2
- 239000002956 ash Substances 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- MTAZNLWOLGHBHU-UHFFFAOYSA-N butadiene-styrene rubber Chemical compound C=CC=C.C=CC1=CC=CC=C1 MTAZNLWOLGHBHU-UHFFFAOYSA-N 0.000 description 2
- 239000002738 chelating agent Substances 0.000 description 2
- 239000013065 commercial product Substances 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 229940095100 fulvic acid Drugs 0.000 description 2
- 239000002663 humin Substances 0.000 description 2
- 239000003864 humus Substances 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 239000011785 micronutrient Substances 0.000 description 2
- 235000013369 micronutrients Nutrition 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 239000011368 organic material Substances 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- SQGYOTSLMSWVJD-UHFFFAOYSA-N silver(1+) nitrate Chemical compound [Ag+].[O-]N(=O)=O SQGYOTSLMSWVJD-UHFFFAOYSA-N 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000011115 styrene butadiene Substances 0.000 description 2
- 239000011573 trace mineral Substances 0.000 description 2
- 235000013619 trace mineral Nutrition 0.000 description 2
- 239000002351 wastewater Substances 0.000 description 2
- KPZGRMZPZLOPBS-UHFFFAOYSA-N 1,3-dichloro-2,2-bis(chloromethyl)propane Chemical compound ClCC(CCl)(CCl)CCl KPZGRMZPZLOPBS-UHFFFAOYSA-N 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 229910014033 C-OH Inorganic materials 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 241000195493 Cryptophyta Species 0.000 description 1
- 229910014570 C—OH Inorganic materials 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 1
- XSTXAVWGXDQKEL-UHFFFAOYSA-N Trichloroethylene Chemical group ClC=C(Cl)Cl XSTXAVWGXDQKEL-UHFFFAOYSA-N 0.000 description 1
- 229910021536 Zeolite Inorganic materials 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 238000003916 acid precipitation Methods 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 150000001447 alkali salts Chemical class 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 239000000908 ammonium hydroxide Substances 0.000 description 1
- 229910001863 barium hydroxide Inorganic materials 0.000 description 1
- 229910001422 barium ion Inorganic materials 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 238000005842 biochemical reaction Methods 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- BDOSMKKIYDKNTQ-UHFFFAOYSA-N cadmium atom Chemical compound [Cd] BDOSMKKIYDKNTQ-UHFFFAOYSA-N 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 235000010410 calcium alginate Nutrition 0.000 description 1
- 239000000648 calcium alginate Chemical class 0.000 description 1
- 229960002681 calcium alginate Drugs 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- OKHHGHGGPDJQHR-YMOPUZKJSA-L calcium;(2s,3s,4s,5s,6r)-6-[(2r,3s,4r,5s,6r)-2-carboxy-6-[(2r,3s,4r,5s,6r)-2-carboxylato-4,5,6-trihydroxyoxan-3-yl]oxy-4,5-dihydroxyoxan-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylate Chemical class [Ca+2].O[C@@H]1[C@H](O)[C@H](O)O[C@@H](C([O-])=O)[C@H]1O[C@H]1[C@@H](O)[C@@H](O)[C@H](O[C@H]2[C@H]([C@@H](O)[C@H](O)[C@H](O2)C([O-])=O)O)[C@H](C(O)=O)O1 OKHHGHGGPDJQHR-YMOPUZKJSA-L 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000005341 cation exchange Methods 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 229910010293 ceramic material Inorganic materials 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 239000002274 desiccant Substances 0.000 description 1
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000005553 drilling Methods 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000010292 electrical insulation Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000010881 fly ash Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 239000002440 industrial waste Substances 0.000 description 1
- 239000000976 ink Substances 0.000 description 1
- 239000013067 intermediate product Substances 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 239000010985 leather Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 244000005706 microflora Species 0.000 description 1
- 238000005065 mining Methods 0.000 description 1
- 125000000896 monocarboxylic acid group Chemical group 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 239000011028 pyrite Substances 0.000 description 1
- NIFIFKQPDTWWGU-UHFFFAOYSA-N pyrite Chemical compound [Fe+2].[S-][S-] NIFIFKQPDTWWGU-UHFFFAOYSA-N 0.000 description 1
- 229910052683 pyrite Inorganic materials 0.000 description 1
- 238000006722 reduction reaction Methods 0.000 description 1
- 238000000518 rheometry Methods 0.000 description 1
- 238000010058 rubber compounding Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 230000007226 seed germination Effects 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 229910001961 silver nitrate Inorganic materials 0.000 description 1
- 239000010802 sludge Substances 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000003516 soil conditioner Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- 239000003476 subbituminous coal Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000004073 vulcanization Methods 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 239000010920 waste tyre Substances 0.000 description 1
- 238000004065 wastewater treatment Methods 0.000 description 1
- 239000010457 zeolite Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F3/00—Compounds containing elements of Groups 2 or 12 of the Periodic Table
- C07F3/003—Compounds containing elements of Groups 2 or 12 of the Periodic Table without C-Metal linkages
Definitions
- This invention relates to the production of the insoluble barium humate in alkaline conditions by the directly use of coal having low heating value, and high content of inorganic materials or by using humic acid obtained from the alkaline extraction of coal, and the modification of barium humate by applying heat treatment (calcination).
- industrial wastes such as waste tires, fly ash or waste sludge, agricultural products such as starch, tree bark, compressed palm fiber or iignin, or byproducts, natural materials such as coal, turbo, humic acid, pyrite, verrnicuiite or natural zeolite and other biosorbents such as biornass, algae, starch, chitosan or alginate beads have been used as low cost adsorbents.
- biosorbents such as biornass, algae, starch, chitosan or alginate beads
- humic substances such as molecular weight, size, structure and the number of functional groups vary according to the source of humic substances, extraction methods and natural conditions during formation. Humic substances according to their solubility in water are divided into three major groups called as humic acid, fulvic acid and humin. Humic substances have usually amorphous, brown or black colors, acidic and polydisperse properties, and the molecular weights vary from a few hundred to ten thousands.
- Humin is the fraction of humic substances that is not soluble in water at any pH value and in alkali. The molecular structure is too large. It is the most resistant to disintegration between humic substances [1-3].
- Humic acid is obtained by acid precipitation of alkali humates which are produced as a result of the extraction with alkaline solution (sodium hydroxide, potassium hydroxide, or ammonium hydroxide) of coal sources such as lignite, leonardite with high humic acid content.
- Alkali metal humates Na and K are defined as the water soluble salts of humic acid.
- Humic acid used as a soil conditioner in agriculture is not a fertilizer, but is a complement to fertilizer. The benefits are given as follows;
- humic acid and derivatives have been used in various industries such as in the ceramic industry as dispersants, in wastewater treatment as adsorbents, in drilling fluids as loss prevention additives, in the lead acid batteries as a surfactant material, in black or dark colored inks as a rheology improver, in medicine for treatment of various diseases.
- Humic acid produced with alkaline extraction of coal with low heating value and high content of inorganic materials, sent by Turkey Coal Enterprise General Directorate (TKI) has 11-12% percent humidity and more than 30% inorganic content. ⁇ 4/ ⁇ 6 ratio was found as 3.26 and this value indicates that the aromatic groups in the sample of TKI humic acid are higher.
- the cation exchange capacity was determined as 900 meq/IGO g.
- the total acidity and exchange capacity in the humic substances show the existence of dissociable proton (or H +) in aromatic and aliphatic carboxyi or phenolic hydroxy! groups.
- the cross-linking takes place via the carboxylic groups and the phenolic OH groups present in the structure of humic acid.
- Humates obtained from divalent metals such as calcium, magnesium and barium have water insoluble property on the contrary of sodium and potassium humates.
- the compounds obtained with polyvalent metals such as iron, zinc, copper, etc. are called as chelate.
- coordination bonds are also formed. It was shown in the U.S. Patent No. 4,746,442 [10] that alkali salt of humic acids obtained from sub-bituminous coal at 120 to 350°C under oxygen pressure by the dry oxidation could be used in heavy metal adsorption in the range of pH: 1.5-5 .5.
- adsorbent material coded as Humasorb-L was effective in the removal of metal ions like Fe, Al, Cr, Pb, Cu, Zn, Co, Hg, Cd, Ni, Mn and organic pollutants like trichlorethylene, tetrakioraetil and benzene. Because it is expressed that huniic acid became insoluble as a result of the adsorption of heavy metal ions. It was indicated that humic acid-based adsorbents containing Fe, Cu, Zn, Mg and Mn can be used as fertilizer with micronutrients in agriculture area.
- alkali metal salts of humic acid potassium or sodium humate
- calcium alginate humate and iron humate have been used for the removal of metal ions and various organic substances causing pollution in the wastewater.
- Rubber which is multi-purpose and has the potential for widespread use is a unique raw material for vital rubber materials in every aspect of life. It is known that rubber is indispensable product for automotive industry (fuel and brake hoses, windshield wipers, suspension parts, etc.), for white ware sector (rubber bellows, gaskets, hoses, etc.), for conveyor belt production, for footwear manufacture, for the industries of food, medical and electronics.
- the fillers added to the rubber are used for rubber strengthening, for improving workability, for formation of economical mixtures and for coloring.
- Carbon blacks take the most important place within fillers. The consumption of carbon black in the rubber industry throughout the world is three times more than the consumption of other fillers. Respectively, kaolin, calcium carbonate and silica follow the consumption of carbon black.
- Humic substances are substances in the organic structure having hydrophobic and hydrophilic moieties and defined as amphiphilic substances. Hydrophobic part comprises the aliphatic and aromatic based hydrocarbons structures. Hydrophilic groups include polar or charged groups. As a result, humic acid as well has polar end groups with a hydrocarbon chain like surfactant.
- Hydrocarbon chain of the humic substances forms micelles surrounded by polar groups dissolved in water.
- the source of negative charges are partially dissociated carboxylic groups (-COOH), phenolic hydroxyl group (-OH) and phenolic hydroxyl (-OH) groups.
- Humic acid is a natural surfactant, because it causes the decrease in the surface tension of the solution and naturally serves as a dispersant when placed in an aqueous alkaline solution.
- USA patent documents numbered as US2007/0149383 and US2008/0300129 it was shown that sodium salt of humic acid, sodium humate, was used as dispersants in the ceramics industry [ 16-17],
- the object of the invention is related to the production of the insoluble barium humate in alkaline conditions by the directly use of coal having low heating value and high content of inorganic materials or by using humic acid obtained from the alkaline extraction of coal, and after that, the modification of barium humate by applying heat treatment (calcination) to increase the surface area.
- Another object of the invention is to realize the production method obtaining low cost barium humate from coal having low heating value and high content of inorganic materials in order to decrease the solubility of humic acid which is soluble under the alkaline conditions, namely at high pH values (>7).
- Another object of the invention is to realize a production method realizing the production of the adsorbent material that is humic acid based and inexpensive, and that has the reduced solubility in alkaline conditions and the increased adsorption capacity with the increase of surface area.
- Another object of the invention is to realize the production method for the product, used as an adsorbent for the removal of heavy metals and organic contaminants; used as filler in the rubber production; and used as a dispersant in ceramic production.
- Another object of the invention is to obtain the production method providing the production of barium humate insoluble in alkaline and acidic medium, without using any agent such as alginate, and the use of it as adsorbent to remove organic pollutants and heavy metals.
- Another object of the invention is to realize a production method providing the use of alkaline insoluble barium humate in the styrene butadiene rubber production and ensuring better electrical insulation property due to the showing higher resistance of the products containing it.
- the inventive production method used to obtain barium humate comprises the following steps;
- coal is directly reacted with barium hydroxide when coal is used.
- the mentioned reaction temperature changes between 80-95°C.
- barium humate is obtained and then separated from the solution with decantation and centrifuge and then it is dried. Drying is made between 70-100 °C.
- humic acid extracted from coal when humic acid extracted from coal is used, humic acid without any purification is reacted with sodium hydroxide and sodium humate is obtained.
- the ion exchange of the obtained sodium humate with barium salt is applied.
- the mentioned process is realized at the temperature of 25-60 °C, in the concentration of 1-5 % and in the time of 4-24 hours.
- the barium humate present in the solid phase is separated with decantation or centrifuge operations. After the separation, the chlorine ion from barium humate is removed by washing with water and then barium humate is obtained.
- barium humate is dried. Drying is realized between 70-100 °C.
- humic acid derived from coal is obtained from lignite based coal which has 43.04 % high ash content and 31.4 % low humic acid content.
- the coal is mined in Konya Hgin region.
- humic acid is used directly without any purification process.
- the pH value of humic acid solution prepared in the concentration of 1-5 % is adjusted with sodium hydroxide granules.
- BaCl 2 and/or Ba(N0 3 ) 2 as barium salts are added to the solution.
- the studies for the humic acid solutions prepared in the different concentrations (1-5 %) were performed at the different temperatures (25-60 C) and different times (4-24 hours).
- the reason to use sodium hydroxide instead of potassium hydroxide in the conversion of humic acid to humate is that the diameter of potassium atom is higher than sodium.
- Barium humate is separated from liquid phase by using decantation and centrifuge operations. The ion exchange of the obtained sodium humate with BaC12 as barium salt is applied.
- the washing with distilled water is carried out until the chlorine ion is removed.
- the presence of chlorine ion is controlled with silver nitrate solution.
- Barium humate after separation is dried in the oven at the temperatures between 70-100 °C. Barium content is determined with ICP.
- Barium humate that is humic acid salt, obtained by the inventive method is insoluble in acidic and basic conditions and it does not permit the passing of the barium ion confined in its structure to the water phase.
- Barium humate obtained in the inventive method is subjected to direct or gradual heating process.
- direct heating it is calcined at the temperature of 50-440 °C and in the time of 0-4 hours.
- gradual calcination is applied by heating to 50-250 C for 0-4 hours in the first stage and by heating to 150-400°C for 0-4 hours in the second stage.
- the barium humate which is the barium salt of humic acid, the highest surface area is obtained.
- Calcined barium humate is used in the adsorption of As, Cd, Co, Cr, Cu, Hg, Ni and Pb metal ions for different amounts and different times.
- the surface area which is an important property affecting the adsorption capacity of the adsorbents, of the synthesized barium humate is obtained as 0.8 m 2 / g , the barium humate subjected directly and gradually to heat treatment are obtained about 16 m 2 /g and about 22 m 2 /g , respectively.
- surface area increased 30 fold of untreated synthesized barium humate. While the obtained barium humate is used as filler in the rubber production and as a dispersing agent in the production of ceramics and is used as a desiccant, the calcined barium humate is used as adsorbents for the removal of metal ions and organic pollutants in the water.
- Graphic 1 shows the adsorption percentage after the adsorption process for As, Cd, Co, Cr, Cu, Hg, Ni and Pb metal ions for 2 and 24 hours adsorption time, by using barium humate obtained in the inventive method in the mass of 0.5 and 1 gram.
- Adsorption percentage is obtained by using the following equation.
- AD % 1 - C(t)/C(i) * 100
- humic acid and eight different metal humates in the rubber were investigated and they were used in SBR (styrene butadiene) based rubber as an alternative to carbon black.
- SBR styrene butadiene
- the curing was realized with the use of ammonium humate, barium humate, potassium humate and sodium humate among 8 different humates.
- the tensile strength of the samples was obtained 8-9 fold lower than original sample, but elongation and shore strength values were similar. As seen from Table 1, according to the electrical values the samples containing barium humate can be provide better electrical isolation due to the high resistance.
- humic acid and 6 different humate mixture were investigated.
- Humic acid and barium humate mixture containing 6 different humates showed better property than that of alone sodium humate.
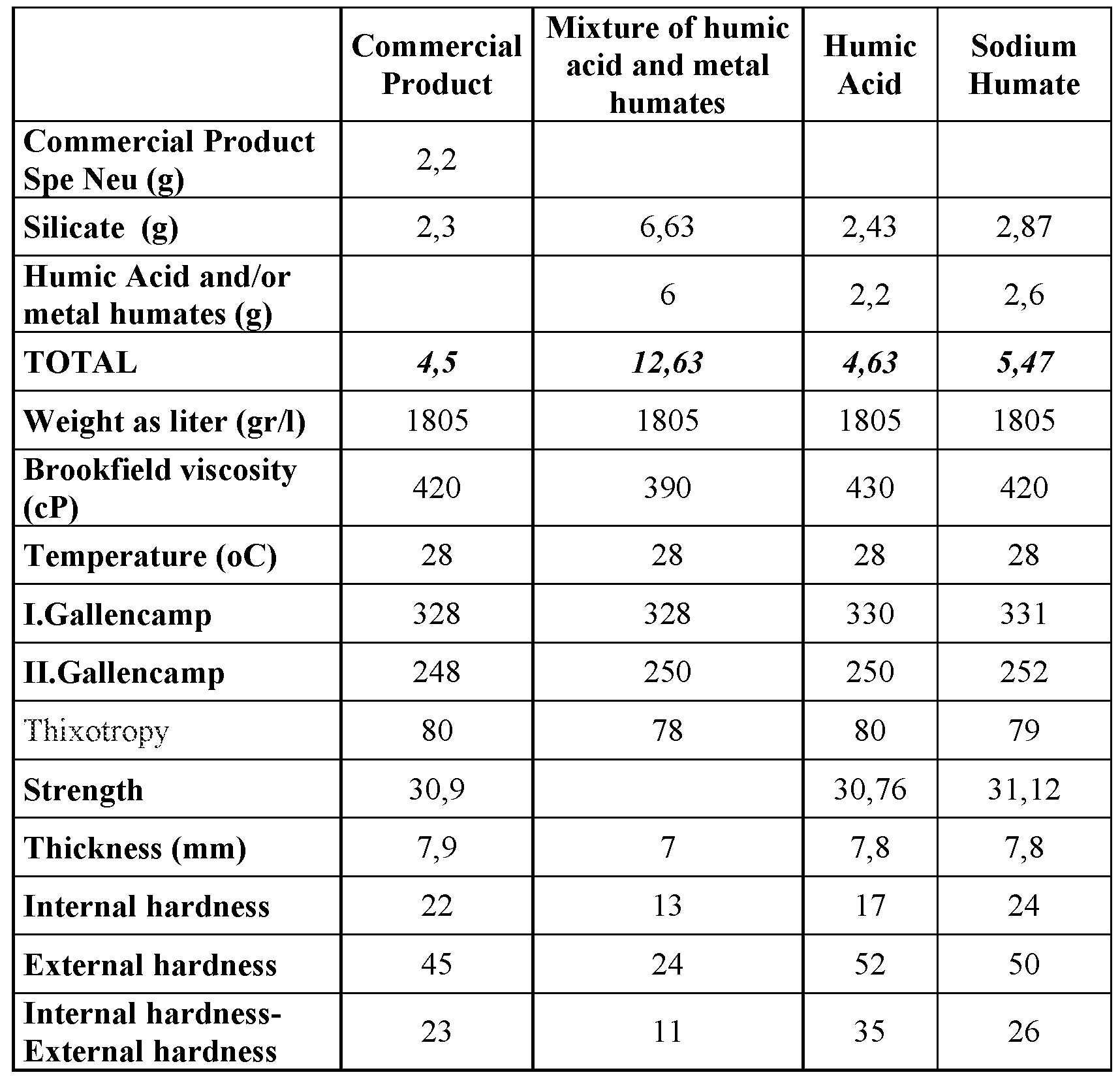
- Internal and external hardness of the mixture was obtained as 1 ⁇ 2 of the value of the commercial product (reference), humic acid and sodium humate.
- Barium salt of humic acid obtained in the inventive method is produced from humic acid derived from Konya Ilgm coal which have high ash content and low humic acid content, without applying any purification, and may be used as a low cost adsorbent material which is insoluble in alkaline conditions.
- humic acid is directly used without any purification step.
- After applying the modification such as heat treatment to the barium salt of humic acid it is used in the adsorption of metal ions (As, Cd, Co, Cr, Cu, Hg, Ni, Pb) prepared as synthetically.
- the adsorption capacity of the polyvalent humate obtained in the inventive method is increased by applying different treatment such as calcination and it was observed that in the adsortion of heavy metal and organic pollutants it gave better result than the commercial humic acid based products. In addition, it was shown that it can be used as filler in the rubber production and as a dispersant in the ceramic production.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
Abstract
This invention relates to the production of the insoluble barium humate in alkaline conditions by the directly use of coal having low heating value, and high content of inorganic materials or by using humic acid obtained from the alkaline extraction of coal, and the modification of barium humate by applying heat treatment (calcination).
Description
DESCRIPTION
BARIUM HUMATE PRODUCTION METHOD Technical Field of the Invention
This invention relates to the production of the insoluble barium humate in alkaline conditions by the directly use of coal having low heating value, and high content of inorganic materials or by using humic acid obtained from the alkaline extraction of coal, and the modification of barium humate by applying heat treatment (calcination).
Background of the Invention Various methods such as coagulation, chemical oxidation and reduction, solvent extraction, liquid membrane separation, adsorption and ion exchange have been used in the removal of heavy metals such as copper, cadmium, mercury, lead, nickel, etc. present in the wastewater of various industrial sectors like mining, metal plating and cleaning, battery and paint manufacturing, leather, chior-alkaii, electronic device manufacturing facilities etc.. Because of the use of low cost adsorbent materials, adsorption process is considered to be most promising method. In this context, industrial wastes such as waste tires, fly ash or waste sludge, agricultural products such as starch, tree bark, compressed palm fiber or iignin, or byproducts, natural materials such as coal, turbo, humic acid, pyrite, verrnicuiite or natural zeolite and other biosorbents such as biornass, algae, starch, chitosan or alginate beads have been used as low cost adsorbents. Hence the development of the natural adsorbents according to the present natural resources of the countries should be given weight to.
Humic substances constituting the most important part of the coal that is one of the adsorbent materials, occur as a result of chemical and biological (with microorganisms) degradation of the plants. Coal is the formation of advanced stage of decomposed plant residues. Coal represents the many types of humus because the humic substances are intermediate products during coal formation. Coal formation starts with the accumulation of organic material in low oxygen environment, namely the raw material of humic substances.
The chemical and physical properties of humic substances such as molecular weight, size, structure and the number of functional groups vary according to the source of humic substances, extraction methods and natural conditions during formation. Humic substances according to their solubility in water are divided into three major groups called as humic acid, fulvic acid and humin. Humic substances have usually amorphous, brown or black colors, acidic and polydisperse properties, and the molecular weights vary from a few hundred to ten thousands.
Humic acids are organic materials which have high molecular weight, and are soluble in alkaline and insoluble in acidic medium. It has 4 aromatic rings and there are C = O bonds between rings. It also includes O-C-OH functional side groups.
Fulvic acid is a chemical compound soluble in all pH values (acidic or basic) of water and solvents, having short-chain molecules and low molecular weight. Fulvic acids are light yellow to yellow-brown in color. Rather, it is a polycyclic compound and rich with respect to C=0.
Humin is the fraction of humic substances that is not soluble in water at any pH value and in alkali. The molecular structure is too large. It is the most resistant to disintegration between humic substances [1-3].
Humic acid is obtained by acid precipitation of alkali humates which are produced as a result of the extraction with alkaline solution (sodium hydroxide, potassium hydroxide, or ammonium hydroxide) of coal sources such as lignite, leonardite with high humic acid content. Alkali metal humates (Na and K) are defined as the water soluble salts of humic acid. Humic acid used as a soil conditioner in agriculture is not a fertilizer, but is a complement to fertilizer. The benefits are given as follows;
It plays role as a chelating agent for trace elements and nutrient in the soil and it accelerates the absorption of these elements and nutrients by plants. In the absence of chelating agents, iron, copper, zinc, manganese and other trace elements are transformed into insoluble hydroxides. Humic acid causes to keep in the solution phase by chelating of these ions providing biochemical reaction.
Support the transfer of micronutrients from the soil to the plant.
Enhances the retention of water;
Increase the seed germination speed and percentage.
Contributes to the development of microflora in the soil. Except for agricultural uses, humic acid and derivatives have been used in various industries such as in the ceramic industry as dispersants, in wastewater treatment as adsorbents, in drilling fluids as loss prevention additives, in the lead acid batteries as a surfactant material, in black or dark colored inks as a rheology improver, in medicine for treatment of various diseases.
Humic acid produced with alkaline extraction of coal with low heating value and high content of inorganic materials, sent by Turkey Coal Enterprise General Directorate (TKI) has 11-12% percent humidity and more than 30% inorganic content. Ε4/Έ6 ratio was found as 3.26 and this value indicates that the aromatic groups in the sample of TKI humic acid are higher. The cation exchange capacity was determined as 900 meq/IGO g.
The total acidity and exchange capacity in the humic substances show the existence of dissociable proton (or H +) in aromatic and aliphatic carboxyi or phenolic hydroxy! groups. The presence of the high amount of carboxyi (COOH) and carbonyl (C = O) groups and the low amount of phenolic groups can be expressed that bumification degree of humic acid is fairly good [1 -7].
Two methods have been used in the production of humic acid based materials which are insoluble in acidic and alkaline media. 1. Cross-linking with aldehydes
2. Cross-linking with polyvalent cations
The cross-linking takes place via the carboxylic groups and the phenolic OH groups present in the structure of humic acid. Humates obtained from divalent metals such as calcium, magnesium and barium have water insoluble property on the contrary of sodium and potassium humates. The compounds obtained with polyvalent metals such as iron, zinc, copper, etc. are called as chelate. In addition to the valence bonding, coordination bonds are also formed. It was shown in the U.S. Patent No. 4,746,442 [10] that alkali salt of humic acids obtained from sub-bituminous coal at 120 to 350°C under oxygen pressure by the dry oxidation could be used in heavy metal adsorption in the range of pH: 1.5-5 .5.
In the patent documents numbered as US 5906960 and US 6143692 applied by Arctech Inc., liquid alkali metal humic acid salt (Humasorb-L) extracted from the coal, and humic acid in a solid form (Humasorb-S) soluble at higher pH values and obtained by precipitation of Humosorb-L with HCl and then by purification were developed. Solid form of humic matter having low solubility at high pH values was obtained by cross-linking of Humasorb-L/Humosorb-S with glutaraldehyde and then immobilization with alginate and shown as Humasorb- CS.
It was found that adsorbent material coded as Humasorb-L was effective in the removal of metal ions like Fe, Al, Cr, Pb, Cu, Zn, Co, Hg, Cd, Ni, Mn and organic pollutants like trichlorethylene, tetrakioraetil and benzene. Because it is expressed that huniic acid became insoluble as a result of the adsorption of heavy metal ions. It was indicated that humic acid-based adsorbents containing Fe, Cu, Zn, Mg and Mn can be used as fertilizer with micronutrients in agriculture area.
In the study of the use of the barium humate in the adsorption of organic contaminants, it was observed that 10 and 40 ppm phenol and tetrachloride were adsorbed at a value close to 100%.
As a result of the literature study, alkali metal salts of humic acid (potassium or sodium humate), calcium alginate humate and iron humate have been used for the removal of metal ions and various organic substances causing pollution in the wastewater.
Rubber Production: Rubber which is multi-purpose and has the potential for widespread use is a unique raw material for vital rubber materials in every aspect of life. It is known that rubber is indispensable product for automotive industry (fuel and brake hoses, windshield wipers, suspension parts, etc.), for white ware sector (rubber bellows, gaskets, hoses, etc.), for conveyor belt production, for footwear manufacture, for the industries of food, medical and electronics. The fillers added to the rubber are used for rubber strengthening, for improving workability, for formation of economical mixtures and for coloring. Carbon blacks take the most important place within fillers. The consumption of carbon black in the rubber industry throughout the world is three times more than the consumption of other fillers. Respectively, kaolin, calcium carbonate and silica follow the consumption of carbon black.
In the USA patent documents numbered as US3075931, US3356623, US3533988, US4532260 and US4600728, it has been mentioned that alkali metal humate is
added to the rubber formulation and the shaping is performed after vulcanization process [1 1-15].
In the production of ceramic materials: Humic substances are substances in the organic structure having hydrophobic and hydrophilic moieties and defined as amphiphilic substances. Hydrophobic part comprises the aliphatic and aromatic based hydrocarbons structures. Hydrophilic groups include polar or charged groups. As a result, humic acid as well has polar end groups with a hydrocarbon chain like surfactant.
Hydrocarbon chain of the humic substances forms micelles surrounded by polar groups dissolved in water. The source of negative charges are partially dissociated carboxylic groups (-COOH), phenolic hydroxyl group (-OH) and phenolic hydroxyl (-OH) groups.
Humic acid is a natural surfactant, because it causes the decrease in the surface tension of the solution and naturally serves as a dispersant when placed in an aqueous alkaline solution. In the USA patent documents numbered as US2007/0149383 and US2008/0300129, it was shown that sodium salt of humic acid, sodium humate, was used as dispersants in the ceramics industry [ 16-17],
The documents cited in the prior technical section are listed below.
1. Stevenson, F.J., Humus Chemistry genesis, Compositions, Reactions, 2nd edition, Wiley, (1984).
2. Ghabbour, E.A., Davies, G., Understanding humic substances: Advanced methods, properties and applications, Northeastern University, USA (1999).
3. Tipping, E., Cation binding by humic substances, Cambridge University Pres, (2002) Sayfa: 8,
Klavins, M., Eglite, L., Immobilization of humic substances, Colloids and Surfaces A: Physicochemical and Engineering Aspects 203, 47-54, (2002). Kahalili, F. "Preparation and Characterization of Selected Metal -Humate Complexes", Soil Science, 150, 3, 565-570, (1990).
Seki, H. and Suzuki, A. , Adsorption of Heavy Metal Ions onto Insolubilized Humic Acid, Journal of Colloid and Interface Science, 171, 490-494, (1995).
Havelcova, M., Mizera, J., Sykorova, I, Pekar M., Sorption of metal ions on lignite and the derived humic substances, Journal of Hazardous Materials, 161, 559-564, (2009).
Sanjay, H.G., Srivastava, K.C., Walia, D.S., Adsorbent, US5906960, 1999 Sanjay, H.G., Srivastava, K.C., Walia, D.S., Adsorbent, US6143692, 2000 Davidson, W.L., Levesque, P.E., latourette, H.K., Vulcanizable rubber composition containing as a reinforcing agent, a partial polyvalent metal salt of coal derived humic acids, US3075931, 1963.
Schwartz, N.N., Process for using humic acids and lignin in vulcanizable rubber, US3356623, 1967.
Morris, D.C., Maassen, G.C., Waterman, R.R., Rubber masterbatch containing humic acid, US3533988, 1970.
MacKeighen, H.R., Cortesi, V.T., Elastomer compositions containing humates US4532260, 1985.
MacKeighen, H.R., Cortesi, V.T., Elastomer compositions containing humates US4600728 1986.
Calemma, V., Menicagli, R., Rausa, R., Process for the removal of metals from waters containing them, US4746442 , 1988.
Saleh, E., Ceramic material, compositions and methods for manufacture thereof, US2007/0149383, 2007.
Saleh, E., Ceramic material, compositions and methods for manufacture thereof US2008/0300129, 2008.
The citations for aforementioned documents are given with numbers in the previous technique.
Brief Description of the Invention
The object of the invention is related to the production of the insoluble barium humate in alkaline conditions by the directly use of coal having low heating value and high content of inorganic materials or by using humic acid obtained from the alkaline extraction of coal, and after that, the modification of barium humate by applying heat treatment (calcination) to increase the surface area.
Another object of the invention is to realize the production method obtaining low cost barium humate from coal having low heating value and high content of inorganic materials in order to decrease the solubility of humic acid which is soluble under the alkaline conditions, namely at high pH values (>7).
Another object of the invention is to realize a production method realizing the production of the adsorbent material that is humic acid based and inexpensive, and that has the reduced solubility in alkaline conditions and the increased adsorption capacity with the increase of surface area.
Another object of the invention is to realize the production method for the product, used as an adsorbent for the removal of heavy metals and organic contaminants; used as filler in the rubber production; and used as a dispersant in ceramic production.
Another object of the invention is to obtain the production method providing the production of barium humate insoluble in alkaline and acidic medium, without using any agent such as alginate, and the use of it as adsorbent to remove organic pollutants and heavy metals.
Another object of the invention is to realize a production method providing the use of alkaline insoluble barium humate in the styrene butadiene rubber production and ensuring better electrical insulation property due to the showing higher resistance of the products containing it.
Detailed Description of the Invention
The inventive production method used to obtain barium humate comprises the following steps;
- Obtaining of barium humate solution from coal or humic acid extracted from coal
- Application of decantation and/or centrifuge operations for the solution
- Washing and drying operations after the separation of barium humate from liquid phase
In the inventive methods, coal is directly reacted with barium hydroxide when coal is used. The mentioned reaction temperature changes between 80-95°C. At the end of the treatment, barium humate is obtained and then separated from the solution with decantation and centrifuge and then it is dried. Drying is made between 70-100 °C.
In the inventive methods, when humic acid extracted from coal is used, humic acid without any purification is reacted with sodium hydroxide and sodium humate is obtained. The ion exchange of the obtained sodium humate with barium salt is applied. The mentioned process is realized at the temperature of 25-60 °C, in the concentration of 1-5 % and in the time of 4-24 hours. Then, the barium humate present in the solid phase is separated with decantation or centrifuge operations. After the separation, the chlorine ion from barium humate is removed by washing with water and then barium humate is obtained. As a last step, barium humate is dried. Drying is realized between 70-100 °C.
In the inventive method, humic acid derived from coal is obtained from lignite based coal which has 43.04 % high ash content and 31.4 % low humic acid content. The coal is mined in Konya Hgin region. In the preferred application, humic acid is used directly without any purification process.
In the inventive method, the pH value of humic acid solution prepared in the concentration of 1-5 % is adjusted with sodium hydroxide granules. After the necessary adjustment, BaCl2 and/or Ba(N03)2 as barium salts are added to the solution. The studies for the humic acid solutions prepared in the different concentrations (1-5 %) were performed at the different temperatures (25-60 C) and different times (4-24 hours). The reason to use sodium hydroxide instead of potassium hydroxide in the conversion of humic acid to humate is that the diameter of potassium atom is higher than sodium. Barium humate is separated from liquid phase by using decantation and centrifuge operations. The ion exchange of the obtained sodium humate with BaC12 as barium salt is applied. When using BaCl2, the washing with distilled water is carried out until the chlorine ion is removed. In the preferred application, the presence of chlorine ion is controlled with silver nitrate solution. Barium humate after separation is dried in the oven at the temperatures between 70-100 °C. Barium content is determined with ICP. Barium humate that is humic acid salt, obtained by the inventive method is insoluble in acidic and basic conditions and it does not permit the passing of the barium ion confined in its structure to the water phase.
Barium humate obtained in the inventive method is subjected to direct or gradual heating process. In the direct heating, it is calcined at the temperature of 50-440 °C and in the time of 0-4 hours.
In the preferred another application, gradual calcination is applied by heating to 50-250 C for 0-4 hours in the first stage and by heating to 150-400°C for 0-4 hours in the second stage. With the application of heat treatment to the barium humate which is the barium salt of humic acid, the highest surface area is obtained. Calcined barium humate is used in the adsorption of As, Cd, Co, Cr, Cu, Hg, Ni and Pb metal ions for different amounts and different times.
The surface area which is an important property affecting the adsorption capacity of the adsorbents, of the synthesized barium humate is obtained as 0.8 m2/ g , the barium humate subjected directly and gradually to heat treatment are obtained about 16 m2/g and about 22 m2/g , respectively. By applying gradually heat treatment to barium humate, surface area increased 30 fold of untreated synthesized barium humate. While the obtained barium humate is used as filler in the rubber production and as a dispersing agent in the production of ceramics and is used as a desiccant, the calcined barium humate is used as adsorbents for the removal of metal ions and organic pollutants in the water. Graphic 1 shows the adsorption percentage after the adsorption process for As, Cd, Co, Cr, Cu, Hg, Ni and Pb metal ions for 2 and 24 hours adsorption time, by using barium humate obtained in the inventive method in the mass of 0.5 and 1 gram.
Graphic 1. Adsorption percentage for barium humate
Adsorption percentage is obtained by using the following equation.
AD % = 1 - C(t)/C(i) * 100
Where Q andCt are the initial and final concentrations, respectively, of metal in solution.
100 % of adsorption percentage is obtained for the adsorption of Cu, Cr, Hg and Pb ions by using 1 g adsorbent obtained with the calcination of the barium humate obtained in the inventive method for 24 hours adsorption time. These values were compared with the commercial product called as Humasorb-CS and the results are given in Graphic 2. As seen from graphic, it is understood that the barium humate
obtained by the inventive production method have superior adsorption percentage for a lot of heavy metal ions adsorption.
Graphic 2. Comparative results of Barium Humate with Humasorb-CS
The use of humic acid and eight different metal humates in the rubber was investigated and they were used in SBR (styrene butadiene) based rubber as an alternative to carbon black. The curing was realized with the use of ammonium humate, barium humate, potassium humate and sodium humate among 8 different humates. The tensile strength of the samples was obtained 8-9 fold lower than original sample, but elongation and shore strength values were similar. As seen from Table 1, according to the electrical values the samples containing barium humate can be provide better electrical isolation due to the high resistance.
Table 1. Electrical and mechanical properties of the rubber samples
The use of humic acid and 6 different humate mixture in the ceramic production was investigated. Humic acid and barium humate mixture containing 6 different humates showed better property than that of alone sodium humate. Internal and external hardness of the mixture was obtained as ½ of the value of the commercial product (reference), humic acid and sodium humate.
Table 2. Effect of humic acid-based compounds used as dispersant on the ceramic properties
Barium salt of humic acid obtained in the inventive method is produced from humic acid derived from Konya Ilgm coal which have high ash content and low humic acid content, without applying any purification, and may be used as a low cost adsorbent material which is insoluble in alkaline conditions. In this study, humic acid is directly used without any purification step. After applying the modification such as heat treatment to the barium salt of humic acid, it is used in
the adsorption of metal ions (As, Cd, Co, Cr, Cu, Hg, Ni, Pb) prepared as synthetically.
The adsorption capacity of the polyvalent humate obtained in the inventive method is increased by applying different treatment such as calcination and it was observed that in the adsortion of heavy metal and organic pollutants it gave better result than the commercial humic acid based products. In addition, it was shown that it can be used as filler in the rubber production and as a dispersant in the ceramic production. Within the basic concepts, it is possible to develop various applications related to the production method of barium humate in the invention and the invention is not limited to the examples described herein and is essentially according to the claims.
Claims
A method of manufacturing barium humate, characterized in that the method comprises the following steps;
- Obtaining of barium humate solution from coal or humic acid extracted from coal
- Application of decantation and/or centrifuge operations for the solution and
- Washing and drying operations after the separation of barium humate from liquid phase,
A method according to claim 1 characterized in that when humic acid extracted from coal is used, humic acid without any purification is reacted with sodium hydroxide and sodium humate is obtained.
A method according to claim 2 characterized in that the ion-exchange of the obtained sodium humate with barium salt is applied.
A method according to claim 3 characterized in that BaCl2 and/or Ba(N03)2 as barium salts are added.
A method according to claim 3 or 4 characterized in that the barium humate present in the solid phase is separated with decantation or centrifuge operations, after the separation, the chlorine ion from barium humate is removed by washing with water and then barium humate is obtained.
6. A method according to any claim 1 to 5 characterized in that humic acid derived from coal is obtained from lignite based coal which has 43.04 % high ash content and 31.4 % low humic acid content.
7. A method according to claim 1 characterized in that coal is directly treated with Ba(OH)2 and barium humate is obtained.
8. A method according to any of the claims given above characterized in that obtained barium humate is exposed to heating process as directly or gradually.
9. A method according to claim 8 characterized in that it is calcined at the temperature of 50-440°C and in the time of 0-4 hours in the direct heating,
10. A method according to claim 9 characterized in that gradual calcination is applied by heating to 50-250°C for 0-4 hours in the first stage and by heating to 150-400°C for 0-4 hours in the second stage.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| TR2014/07025 | 2014-06-17 | ||
| TR201407025 | 2014-06-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015193779A1 true WO2015193779A1 (en) | 2015-12-23 |
Family
ID=53674221
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2015/054461 WO2015193779A1 (en) | 2014-06-17 | 2015-06-12 | Barium humate production method |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2015193779A1 (en) |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3075931A (en) | 1959-10-02 | 1963-01-29 | Fmc Corp | Vulcanizable rubber composition containing as a reinforcing agent, a partial polyvalent metal salt of coal derived humic acids |
| US3356623A (en) | 1964-03-16 | 1967-12-05 | Fmc Corp | Process for using humic acids and lignin in vulcanizable rubber |
| US3533988A (en) | 1967-06-02 | 1970-10-13 | Vanderbilt Co R T | Rubber masterbatch containing humic acids |
| US4532260A (en) | 1984-10-18 | 1985-07-30 | Alfred D. Lobo Co., L.P.A. | Elastomer compositions containing humates |
| US4600728A (en) | 1984-10-18 | 1986-07-15 | Alfred D. Lobo Co., L.P.A. | Elastomer compositions containing humates |
| US4746442A (en) | 1986-03-14 | 1988-05-24 | Eniricerche S.P.A. | Process for the removal of metals from waters containing them |
| US5906960A (en) | 1995-08-15 | 1999-05-25 | Arctech, Inc. | Adsorbent |
| US20070149383A1 (en) | 2005-12-28 | 2007-06-28 | Caroma Insdustries Limited | Ceramic material, compositions and methods for manufacture thereof |
| US20080300129A1 (en) | 2005-12-28 | 2008-12-04 | Caroma Insdustries Limited | Ceramic material, compositions and methods for manufacture thereof |
-
2015
- 2015-06-12 WO PCT/IB2015/054461 patent/WO2015193779A1/en active Application Filing
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3075931A (en) | 1959-10-02 | 1963-01-29 | Fmc Corp | Vulcanizable rubber composition containing as a reinforcing agent, a partial polyvalent metal salt of coal derived humic acids |
| US3356623A (en) | 1964-03-16 | 1967-12-05 | Fmc Corp | Process for using humic acids and lignin in vulcanizable rubber |
| US3533988A (en) | 1967-06-02 | 1970-10-13 | Vanderbilt Co R T | Rubber masterbatch containing humic acids |
| US4532260A (en) | 1984-10-18 | 1985-07-30 | Alfred D. Lobo Co., L.P.A. | Elastomer compositions containing humates |
| US4600728A (en) | 1984-10-18 | 1986-07-15 | Alfred D. Lobo Co., L.P.A. | Elastomer compositions containing humates |
| US4746442A (en) | 1986-03-14 | 1988-05-24 | Eniricerche S.P.A. | Process for the removal of metals from waters containing them |
| US5906960A (en) | 1995-08-15 | 1999-05-25 | Arctech, Inc. | Adsorbent |
| US6143692A (en) | 1995-08-15 | 2000-11-07 | Arctech, Inc. | Adsorbent |
| US20070149383A1 (en) | 2005-12-28 | 2007-06-28 | Caroma Insdustries Limited | Ceramic material, compositions and methods for manufacture thereof |
| US20080300129A1 (en) | 2005-12-28 | 2008-12-04 | Caroma Insdustries Limited | Ceramic material, compositions and methods for manufacture thereof |
Non-Patent Citations (9)
| Title |
|---|
| BORCAKL M., BABAN A.: "Laboratory tests of treatment and decontamination technologies for process water in fruit juice industries", 31 March 2014 (2014-03-31), pages 1 - 47, XP002743466, Retrieved from the Internet <URL:http://www.resfood.eu/web/wp-content/uploads/D3.3-PU-Lab-tests-treatment-technologies-fruit-juice-industries.pdf> [retrieved on 20150818] * |
| ELHAM A. GHABBOUR, ET AL.: "Humic Substances : Structures, Models and Functions", 10 October 2001, ROYAL SOCIETY OF CHEMISTRY, ISBN: 978-1-84755-108-5, article GHABBOUR E. A. ET AL.: "A comparative evaluation of known liquid humic acid analysis methods", pages: 337 - 342, XP008177236, DOI: 10.1039/9781847551085-00337 * |
| GHABBOUR, E.A.; DAVIES, G.: "Understanding humic substances: Advanced methods, properties and applications", 1999, NORTHEASTERN UNIVERSITY |
| HAVELCOVA, M.; MIZERA, J.; SYKOROVA, I.; PEKAR M.: "Sorption of metal ions on lignite and the derived humic substances", JOURNAL OF HAZARDOUS MATERIALS, vol. 161, 2009, pages 559 - 564, XP025673056, DOI: doi:10.1016/j.jhazmat.2008.03.136 |
| KAHALILI, F.: "Preparation and Characterization of Selected Metal-Humate Complexes", SOIL SCIENCE, vol. 150, no. 3, 1990, pages 565 - 570 |
| KLAVINS, M.; EGLITE, L.: "Immobilization of humic substances", COLLOIDS AND SURFACES A: PHYSICOCHEMICAL AND ENGINEERING ASPECTS, vol. 203, 2002, pages 47 - 54 |
| SEKI, H.; SUZUKI, A.: "Adsorption of Heavy Metal Ions onto Insolubilized Humic Acid", JOURNAL OF COLLOID AND INTERFACE SCIENCE, vol. 171, 1995, pages 490 - 494 |
| STEVENSON, F.J.: "Humus Chemistry genesis, Compositions, Reactions, 2ND ED.", 1984, WILEY |
| TIPPING, E.: "Cation binding by humic substances", 2002, CAMBRIDGE UNIVERSITY PRES |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Mariana et al. | Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption | |
| Park et al. | Removing mercury from aqueous solution using sulfurized biochar and associated mechanisms | |
| Ince et al. | An overview of adsorption technique for heavy metal removal from water/wastewater: a critical review | |
| Mohan et al. | Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water | |
| Ali | The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater | |
| Kim et al. | Biosorption of cationic basic dye and cadmium by the novel biosorbent Bacillus catenulatus JB-022 strain | |
| Rathod et al. | Efficient removal of phosphate from aqueous solutions by red seaweed, Kappaphycus alverezii | |
| Rafique et al. | Immobilization and mitigation of chromium toxicity in aqueous solutions and tannery waste-contaminated soil using biochar and polymer-modified biochar | |
| Calugaru et al. | Metals and metalloids treatment in contaminated neutral effluents using modified materials | |
| Bakshi et al. | Capture and release of orthophosphate by Fe-modified biochars: mechanisms and environmental applications | |
| Kumar et al. | Removal of lead and copper metal ions in single and binary systems using biopolymer modified spinel ferrite | |
| Ghaedi et al. | Removal of heavy metal ions from polluted waters by using of low cost adsorbents | |
| Tay et al. | Pleurotus ostreatus spent mushroom compost as green biosorbent for nickel (II) biosorption | |
| Gupta et al. | Adsorbents for water treatment: development of low-cost alternatives to carbon | |
| Wajima | A new carbonaceous adsorbent for heavy metal removal from aqueous solution prepared from paper sludge by sulfur-impregnation and pyrolysis | |
| Chai et al. | Enhanced removal of Hg (II) from acidic aqueous solution using thiol-functionalized biomass | |
| Chen et al. | Biosorption of V (V) onto Lantana camara biochar modified by H3PO4: Characteristics, mechanism, and regenerative capacity | |
| Ouafi et al. | SAWDUST IN THE TREATMENT OF HEAVY METALS-CONTAMINATED WASTEWATE. | |
| Rahim et al. | Conversion of coconut waste into cost effective adsorbent for Cu (II) and Ni (II) removal from aqueous solutions | |
| Anantha et al. | Bio-composites for the sorption of copper from aqueous solution: A comparative study | |
| Shaikh | Adsorption of Pb (II) from wastewater by natural and synthetic adsorbents | |
| CN114276817B (en) | Soil restoration agent and preparation method and application thereof | |
| Zhao et al. | Adsorption of congo red onto lignocellulose/montmorillonite nanocomposite | |
| Sorokhaibam et al. | Phenolic wastewater treatment: development and applications of new adsorbent materials | |
| CN104105532A (en) | Methods for treating waste waters using sulfidized red mud sorbents |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15738998 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2016/18803 Country of ref document: TR |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15738998 Country of ref document: EP Kind code of ref document: A1 |