WO2014204856A1 - Fatty acid anticancer derivatives and their uses - Google Patents
Fatty acid anticancer derivatives and their uses Download PDFInfo
- Publication number
- WO2014204856A1 WO2014204856A1 PCT/US2014/042542 US2014042542W WO2014204856A1 WO 2014204856 A1 WO2014204856 A1 WO 2014204856A1 US 2014042542 W US2014042542 W US 2014042542W WO 2014204856 A1 WO2014204856 A1 WO 2014204856A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- independently
- ethyl
- cancer
- compound
- Prior art date
Links
- 0 CCCC(C1(C(CC)C(C)(CCC)C(C(C)(CC)*CC)(N)N)C(CC)*1)N Chemical compound CCCC(C1(C(CC)C(C)(CCC)C(C(C)(CC)*CC)(N)N)C(CC)*1)N 0.000 description 6
- STMYNQSHAXVRMK-AAQCHOMXSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCSSCCNC(c(ccc(Nc1nc(-c(c(C(c(c(F)ccc2)c2OC)=NC2)c3)ccc3Cl)c2cn1)c1)c1OC)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCSSCCNC(c(ccc(Nc1nc(-c(c(C(c(c(F)ccc2)c2OC)=NC2)c3)ccc3Cl)c2cn1)c1)c1OC)=O)=O STMYNQSHAXVRMK-AAQCHOMXSA-N 0.000 description 2
- DGXROCKRBSATGS-SECBINFHSA-N CC(CCCC[C@H]1NNCC1)=O Chemical compound CC(CCCC[C@H]1NNCC1)=O DGXROCKRBSATGS-SECBINFHSA-N 0.000 description 1
- YJDAOHJWLUNFLX-UHFFFAOYSA-N CC(N1)=Nc(c(O)ccc2)c2C1=O Chemical compound CC(N1)=Nc(c(O)ccc2)c2C1=O YJDAOHJWLUNFLX-UHFFFAOYSA-N 0.000 description 1
- KTMORYSCENUIBC-UEFRZVIPSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCC(N[C@H](C1CCCCC1)C(Nc1cc([nH]c(-c2c[n](C)nc2)c2C=NNC3=O)c2c3c1)=O)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCC(N[C@H](C1CCCCC1)C(Nc1cc([nH]c(-c2c[n](C)nc2)c2C=NNC3=O)c2c3c1)=O)=O)=O KTMORYSCENUIBC-UEFRZVIPSA-N 0.000 description 1
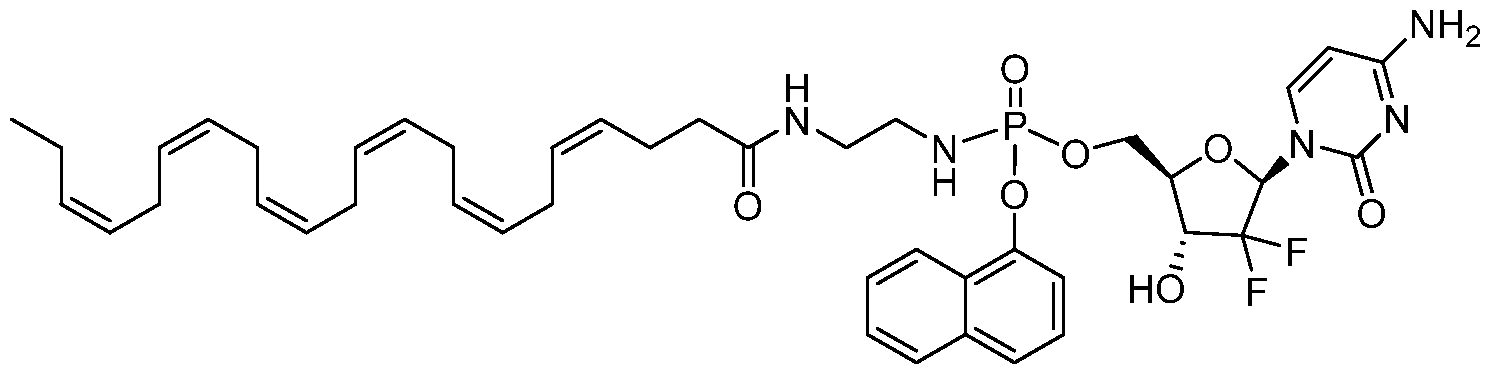
- JGGTUCCJPDBOJQ-RSGSBFICSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCN(C)CCNC(OC[C@H]([C@H](C1(F)F)O)O[C@H]1N(C=CC(N)=N1)C1=O)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCN(C)CCNC(OC[C@H]([C@H](C1(F)F)O)O[C@H]1N(C=CC(N)=N1)C1=O)=O)=O JGGTUCCJPDBOJQ-RSGSBFICSA-N 0.000 description 1
- KXYZLRBMAZLHEC-WFSQCCPVSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNC(CCc1c(C)[nH]c(/C=C(/c2ccccc2N2)\C2=O)c1C)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNC(CCc1c(C)[nH]c(/C=C(/c2ccccc2N2)\C2=O)c1C)=O)=O KXYZLRBMAZLHEC-WFSQCCPVSA-N 0.000 description 1
- LCAGDLVIGKHBSJ-JVVIBBLFSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNC(O[C@H](C)COc(c(C)c12)c[n]1ncnc2Oc1ccc2[nH]c(C)cc2c1F)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNC(O[C@H](C)COc(c(C)c12)c[n]1ncnc2Oc1ccc2[nH]c(C)cc2c1F)=O)=O LCAGDLVIGKHBSJ-JVVIBBLFSA-N 0.000 description 1
- OACBBQBWNODDJY-YFOSKJSOSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNP(OCC([C@H]([C@H](C1N(C=CC(N)=N2)C2=O)O)O)=C1F)(Oc1ccccc1)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNP(OCC([C@H]([C@H](C1N(C=CC(N)=N2)C2=O)O)O)=C1F)(Oc1ccccc1)=O)=O OACBBQBWNODDJY-YFOSKJSOSA-N 0.000 description 1
- BRINGIOCKIUOLA-RSJYGBFJSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNP(OC[C@H]([C@H](C1(F)F)O)O[C@H]1N(C=CC(N)=N1)C1=O)(Oc(cc1)ccc1Cl)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNP(OC[C@H]([C@H](C1(F)F)O)O[C@H]1N(C=CC(N)=N1)C1=O)(Oc(cc1)ccc1Cl)=O)=O BRINGIOCKIUOLA-RSJYGBFJSA-N 0.000 description 1
- AWTIUIKLFKGERI-WRWGKPEJSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNP(OC[C@H]([C@H](C1(F)F)O)O[C@H]1N(C=CC(N)=N1)C1=O)(Oc1ccccc1)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNP(OC[C@H]([C@H](C1(F)F)O)O[C@H]1N(C=CC(N)=N1)C1=O)(Oc1ccccc1)=O)=O AWTIUIKLFKGERI-WRWGKPEJSA-N 0.000 description 1
- SYDCSCGVTHSVFT-LSOAGRHRSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNP(OC[C@H]([C@H]([C@H]1O)O)OC1N(C=NC(N)=N1)C1=O)(Oc1cccc2ccccc12)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCNP(OC[C@H]([C@H]([C@H]1O)O)OC1N(C=NC(N)=N1)C1=O)(Oc1cccc2ccccc12)=O)=O SYDCSCGVTHSVFT-LSOAGRHRSA-N 0.000 description 1
- RYJLZDMKUUUDME-OFXBVLEGSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCOCCNC(OC[C@H]([C@H](C1(F)F)O)O[C@H]1N(C=CC(N)=N1)C1=O)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCOCCNC(OC[C@H]([C@H](C1(F)F)O)O[C@H]1N(C=CC(N)=N1)C1=O)=O)=O RYJLZDMKUUUDME-OFXBVLEGSA-N 0.000 description 1
- FHIGJQZXGSLHQR-MTEUQOPKSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCOCCNC(OC[C@H]([C@H]([C@H]1O)O)OC1[n]1c(Cl)nc2c(N)ncnc12)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCOCCNC(OC[C@H]([C@H]([C@H]1O)O)OC1[n]1c(Cl)nc2c(N)ncnc12)=O)=O FHIGJQZXGSLHQR-MTEUQOPKSA-N 0.000 description 1
- SIVVYNIWQFGXDE-AAQCHOMXSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCSSCCNC(OCCN(CC1)CCN1c1cc(Nc2ncc(C(Nc3c(C)cccc3Cl)=O)[s]2)nc(C)n1)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCSSCCNC(OCCN(CC1)CCN1c1cc(Nc2ncc(C(Nc3c(C)cccc3Cl)=O)[s]2)nc(C)n1)=O)=O SIVVYNIWQFGXDE-AAQCHOMXSA-N 0.000 description 1
- NTQZVYLQQIWVRK-JEBPEJKESA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCSSCCNC(Oc1cccc2c1N=C(C)NC2=O)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCSSCCNC(Oc1cccc2c1N=C(C)NC2=O)=O)=O NTQZVYLQQIWVRK-JEBPEJKESA-N 0.000 description 1
- ONHOFQMTTVSXBC-RTLYQEMRSA-N CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCSSCCNP(OC[C@H]([C@H](C1)O)OC1N(C=C(C(N1)=O)F)C1=O)(Oc1cccc2ccccc12)=O)=O Chemical compound CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCSSCCNP(OC[C@H]([C@H](C1)O)OC1N(C=C(C(N1)=O)F)C1=O)(Oc1cccc2ccccc12)=O)=O ONHOFQMTTVSXBC-RTLYQEMRSA-N 0.000 description 1
- LJWHVHBCONNTJV-LJAQVGFWSA-N CC[C@@](C(CC1)=O)(C(C=C2N3Cc4cc(c(CC)c(cc5)OC(N6CCNCC6)=O)c5cc24)=C1C3=O)O Chemical compound CC[C@@](C(CC1)=O)(C(C=C2N3Cc4cc(c(CC)c(cc5)OC(N6CCNCC6)=O)c5cc24)=C1C3=O)O LJWHVHBCONNTJV-LJAQVGFWSA-N 0.000 description 1
- SWBRQVLIMYOFCE-YNYNYLDESA-N CC[C@@](C(CC1)=O)(C(C=C2N3Cc4cc(c(CN(C)C)c(cc5)OC(N6CCN(Cc(cc7)ccc7NC(CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CC)=O)CC6)=O)c5cc24)=C1C3=O)O Chemical compound CC[C@@](C(CC1)=O)(C(C=C2N3Cc4cc(c(CN(C)C)c(cc5)OC(N6CCN(Cc(cc7)ccc7NC(CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CC)=O)CC6)=O)c5cc24)=C1C3=O)O SWBRQVLIMYOFCE-YNYNYLDESA-N 0.000 description 1
- XLIGPCNSMMFTCE-UMSFTDKQSA-N CC[C@@](C(CC1)=O)(C(C=C2N3Cc4cc(cc(cc5)OC(N6CCN(Cc(cc7)ccc7N)CC6)=O)c5cc24)=C1C3=O)O Chemical compound CC[C@@](C(CC1)=O)(C(C=C2N3Cc4cc(cc(cc5)OC(N6CCN(Cc(cc7)ccc7N)CC6)=O)c5cc24)=C1C3=O)O XLIGPCNSMMFTCE-UMSFTDKQSA-N 0.000 description 1
- FNYXRZVCGNPYBA-CHVHDLNMSA-N CC[C@H](COC(NCCNC(CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CC)=O)=O)Nc(nc1NCc2ccccc2)nc2c1nc[n]2C(C)C Chemical compound CC[C@H](COC(NCCNC(CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CC)=O)=O)Nc(nc1NCc2ccccc2)nc2c1nc[n]2C(C)C FNYXRZVCGNPYBA-CHVHDLNMSA-N 0.000 description 1
- NCBXZVLOVKDCMW-UHFFFAOYSA-N CN(C)Cc(cc1)ccc1C(c1c2cccc1O)=CNC2=O Chemical compound CN(C)Cc(cc1)ccc1C(c1c2cccc1O)=CNC2=O NCBXZVLOVKDCMW-UHFFFAOYSA-N 0.000 description 1
- HMABYWSNWIZPAG-UHFFFAOYSA-N CNCc(cc1)ccc1-c1c(CCNC(c2cc(F)c3)=O)c2c3[nH]1 Chemical compound CNCc(cc1)ccc1-c1c(CCNC(c2cc(F)c3)=O)c2c3[nH]1 HMABYWSNWIZPAG-UHFFFAOYSA-N 0.000 description 1
- AFSXGZYGBBFNKI-NVXWUHKLSA-N COc1ccc([C@H](N2)N(CC[C@H](c3ccc4)N)c3c4C2=O)cc1 Chemical compound COc1ccc([C@H](N2)N(CC[C@H](c3ccc4)N)c3c4C2=O)cc1 AFSXGZYGBBFNKI-NVXWUHKLSA-N 0.000 description 1
- JNAHVYVRKWKWKQ-CYBMUJFWSA-N C[C@@]1(c2nc(c(C(N)=O)ccc3)c3[nH]2)NCCC1 Chemical compound C[C@@]1(c2nc(c(C(N)=O)ccc3)c3[nH]2)NCCC1 JNAHVYVRKWKWKQ-CYBMUJFWSA-N 0.000 description 1
- NHFDRBXTEDBWCZ-ZROIWOOFSA-N Cc1c(/C=C(/c(cccc2)c2N2)\C2=O)[nH]c(C)c1CCC(O)=O Chemical compound Cc1c(/C=C(/c(cccc2)c2N2)\C2=O)[nH]c(C)c1CCC(O)=O NHFDRBXTEDBWCZ-ZROIWOOFSA-N 0.000 description 1
- KOUGHMOSYJCJLE-UHFFFAOYSA-N NC(c1c2nc(-c(cc3)ccc3O)[nH]c2ccc1)=O Chemical compound NC(c1c2nc(-c(cc3)ccc3O)[nH]c2ccc1)=O KOUGHMOSYJCJLE-UHFFFAOYSA-N 0.000 description 1
- GSCPDZHWVNUUFI-UHFFFAOYSA-N NC(c1cc(N)ccc1)=O Chemical compound NC(c1cc(N)ccc1)=O GSCPDZHWVNUUFI-UHFFFAOYSA-N 0.000 description 1
- PCHKPVIQAHNQLW-CQSZACIVSA-N NC(c1cccc2c[n](-c3ccc([C@H]4CNCCC4)cc3)nc12)=O Chemical compound NC(c1cccc2c[n](-c3ccc([C@H]4CNCCC4)cc3)nc12)=O PCHKPVIQAHNQLW-CQSZACIVSA-N 0.000 description 1
- XTPZRMNYIRIKMJ-UHFFFAOYSA-N O=C(CCCCC1NNCC1)I Chemical compound O=C(CCCCC1NNCC1)I XTPZRMNYIRIKMJ-UHFFFAOYSA-N 0.000 description 1
- CMNQIVHHHBBVSC-UHFFFAOYSA-N Oc1cccc2c1CCNC2=O Chemical compound Oc1cccc2c1CCNC2=O CMNQIVHHHBBVSC-UHFFFAOYSA-N 0.000 description 1
- MQDRJIKLAZSSLF-UHFFFAOYSA-N Oc1cccc2c1N=CNC2=O Chemical compound Oc1cccc2c1N=CNC2=O MQDRJIKLAZSSLF-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/21—Esters, e.g. nitroglycerine, selenocyanates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
Definitions
- the invention relates to fatty acid anticancer derivatives; compositions comprising an effective amount of a fatty acid anticancer derivative; and methods for treating or preventing a cancer comprising the administration of an effective amount of a fatty acid anticancer derivative.
- Oily cold water fish such as salmon, trout, herring, and tuna are the source of dietary marine omega-3 fatty acids, with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) being the key marine derived omega-3 fatty acids.
- Omega-3 fatty acids have previously been shown to improve insulin sensitivity and glucose tolerance in
- Omega-3 fatty acids have also been shown to improve insulin resistance in obese and non-obese patients with an inflammatory phenotype. Lipid, glucose, and insulin metabolism have been shown to improve in overweight hypertensive subjects through treatment with omega-3 fatty acids. Omega-3 fatty acids (EPA/DHA) have also been shown to decrease triglycerides and to reduce the risk for sudden death caused by cardiac arrhythmias in addition to improve mortality in patients at risk of a cardiovascular event. Omega-3 fatty acids have also been taken as part of the dietary supplement portion of therapy used to treat dyslipidemia. Last, but not least, omega-3 fatty acids have been known to have a number of anti-inflammatory properties.
- omega-3 fatty acids lower levels of circulating TNF-a and IL-6, two of the cytokines that are markedly increased during inflammation processes (Chapkin et al, Prostaglandins, Leukot Essent Fatty Acids 2009, 81, p. 187-191; Duda et al, Cardiovasc Res 2009, 84, p. 33-41).
- a higher intake of omega-3 fatty acids has been shown to increase levels of the well-characterized anti-inflammatory cytokine IL-10 (Bradley et al, Obesity (Silver Spring) 2008, 16, p. 938-944).
- Both DHA and EPA are characterized as long chain fatty acids (aliphatic portion between 12-22 carbons).
- Medium chain fatty acids are characterized as those having the aliphatic portion between 6-12 carbons.
- Lipoic acid is a medium chain fatty acid found naturally in the body. It plays many important roles such as free radical scavenger, chelator to heavy metals and signal transduction mediator in various inflammatory and metabolic pathways, including the NF- ⁇ pathway (Shay, K. P. et al. Biochim. Biophys. Acta 2009, 1790, 1149-1160). Lipoic acid has been found to be useful in a number of chronic diseases that are associated with oxidative stress (for a review see Smith, A. R. et al Curr. Med.
- Lipoic acid has now been evaluated in the clinic for the treatment of diabetes (Morcos, M. et al Diabetes Res. Clin. Pract. 2001, 52, p. 175-183) and diabetic neuropathy (Mijnhout, G. S. et al Neth. J. Med. 2010, 110, p. 158-162). Lipoic acid has also been found to be potentially useful in treating cardiovascular diseases (Ghibu, S. et al, J. Cardiovasc. Pharmacol. 2009, 54, p. 391-8), Alzheimer's disease (Maczurek, A. et al, Adv. Drug Deliv. Rev. 2008, 60, p.
- Omega-3 fatty acids have also been shown to affect numerous biological targets that are relevant to cancer inhibition.
- Omega-3 fatty acid has been shown to inhibit the expression of PD-Ll via inhibition of STATl, STAT3 and NF- ⁇ (Romberg et al, J. Allergy Clin. Immunol. 2013, 132, p. 1460; Marzec et al, PNAS 2013, 105, p. 20852; Loke and Allison, PNAS 2003, 100, p. 5336).
- Programmed death 1 (PD 1) and its ligands (PD-Ll and PD-L2) are important in regulating the balance between T cell activation, tolerance and immunopathology.
- EGFR epidermal growth factor receptor
- omega-3 fatty acids has been demonstrated to decrease PTEN, PARP (Poly-ADP-ribose polymerase), NF-KB and VEGF and thereby activate apoptosis, diminish DNA damage and reduce inflammation signaling to inhibit the progression of colon cancer (Kansal et al PLOS One 2014, 9, e84627).
- Cancer cell growth is controlled by the coordinated activation of numerous regulatory proteins including cyclins and catalytic cyclin-dependent kinases (CDK).
- CDK catalytic cyclin-dependent kinases
- Oxidized metabolites of omega-3 fatty acids have recently been shown to decrease cancer cell proliferation by down-regulating the cyclin D1/CDK4 pathway (Cui et al, Brit. J.Pharm. 2011, 162, p. l 143).
- a covalent conjugate of omega-3 fatty acid with an anticancer agent allows the delivery of both of these components simultaneously to an intracellular component with matched kinetic. Because of the ability of omega-3 fatty acids to impact multiple pathways that are critical for the proliferation of cancer cells, a covalent conjugate of omega-3 fatty acid with an anticancer agent is expected to have synergistic activity that cannot be reproduced by the individual components or a combination of the individual components (i.e. omega-3 fatty acid and anticancer agent).
- Non-limiting examples of an anticancer agent that can be used in a covalent conjugate with an omega-3 fatty acid include a cytotoxic agent, a nucleoside agent, a DNA intercalator, a proteasome inhibitor, a microtubule-targeting agent, an agent that causes crosslinking of DNA, an apoptotic agent, a PARP inhibitor, a histone deacetylase inhibitor, a topoisomerase inhibitor, a heat shock protein inhibitor, a histone methyltransferase inhibitor, a matrix metalloprotease inhibitor, an isocitrate dehydrogenase 1 or 2 (IDH 1 or IDH 2) inhibitor, an indoleamine-2,3- dioxygenase inhibitor (IDO), an inhibitor of the nuclear export protein Exportin 1 (XPO 1), a protein tyrosine kinase inhibitor or protein serine/threonine kinase inhibitor.
- a cytotoxic agent a nucleoside agent, a DNA intercal
- rapamycin is a serine/threonine protein kinase that can act as a master switch for cellular anabolic and catabolic processes, which in turn regulates the rate of cell growth and proliferation.
- Dysregulation of mTOR signaling pathway occurs frequently in a variety of human tumors. Rapamycin and a number of rapamycin analogs are some mTOR inhibitors that have shown effectiveness as anticancer agents. (Laplante et al Cell 2012, 149, p. 274-293).
- TGF transforming growth factor ⁇
- Ras-MAPK Ras- mytogen activated protein kinase
- Gastric cancer is a highly lethal malignancy, with a low 5-year survival rate.
- Aberration activation of the protein kinase B (AKT) has now been shown to be one of the most common molecular findings in gastric cancer. Therefore, agents designed to specifically target AKT are currently being developed for the treatment of gastric cancer (Almhanna et al Anticancer Research 2011, 31, p. 4387-4392).
- the extracellular signal- regulated kinase 1 (ERKl) / 2 mytogen-activated protein (MAP) kinase module is yet another signaling pathway that has been shown to have a major role in the control of cell
- Aurora kinases can play an important role in the control of cell cycle and have been implicated in acute myeloid leukemia, chronic myeloid leukemia, acute lymphoblastic leukemia, multiple myeloma, aggressive non- Hodgkin lymphoma and Hodgkin lymphoma (Farag, S. British Journal of Haematology
- AMPK adenosine monophosphate activated protein kinase
- Activation of AMPK not only affects gastrointestinal cancer cell growth and proliferation, but also promotes cancer cell cycle arrest and apoptosis of cancer cells via activation of caspase-9.
- Activation of AMPK can potentially reprogram cellular metabolism and enforce metabolic checkpoints by acting on mTOR, p53, fatty synthase and other molecules for regulating cell growth and metabolism (Luo et al Future Oncology 2010, 6, p. 457-470).
- a fatty acid anticancer derivative represents a covalently linked anticancer agent and an omega-3 fatty acid such as DHA or EPA or a fatty acid that can be metabolized in vivo to an omega-3 fatty acid.
- a fatty acid anticancer derivative is designed to be stable in the plasma; and once inside target cells can undergo hydrolysis to release the individual components (i.e. anticancer agent and omega-3 fatty acid as defined herein). Because the anticancer agent is released only inside target cells, the fatty acid anticancer derivative exhibits less side effects than the corresponding unconjugated anticancer agents.
- the fatty acid anticancer derivatives can be designed to target certain cancer tissue types. Selective targeting to certain tissue types can enhance the overall efficacy, as well as reduced the side effects. Selective tissue targeting is possible since the fatty acid component can strongly bind to circulating albumin (Ren et al, J. Nanomed.
- the invention is based in part on the discovery of fatty acid anticancer derivatives and their demonstrated effects in achieving improved treatment that cannot be achieved by administering fatty acids or anticancer, alone, or in simple (non covalently linked)
- a molecular conjugate which comprises an anticancer agent and a fatty acid covalently linked directly, or indirectly through a linker, wherein the linkage is through a free hydroxyl, amine, thiol, carboxylate, phosphate, or the like, on the anticancer agent and the fatty acid, wherein the fatty acid is selected from the group consisting of omega-3 fatty acids, fatty acids that are metabolized in vivo to omega-3 fatty acids, and lipoic acid, and the conjugate is stable in the plasma and capable of hydrolysis to produce free anticancer and free fatty acid, with the proviso that the molecular conjugate is not
- the fatty acid is selected from the group consisting of all- cz ' s-7,10,13-hexadecatrienoic acid, a-linolenic acid, stearidonic acid, eicosatrienoic acid, eicosatetraenoic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid, docosahexaenoic acid (DHA), tetracosapentaenoic acid, tetracosahexaenoic acid and lipoic acid.
- the fatty acid is selected from eicosapentaenoic acid, docosahexaenoic acid and lipoic acid.
- the anticancer agent is selected from the group consisting of non-nucleotide anticancer agents that include, but are not limited to, Epirubicin, Lonidamine, Pirarubicin, Idarubicin, Placlitaxel, Irinotecan, Docetaxel, Raltitrexed,
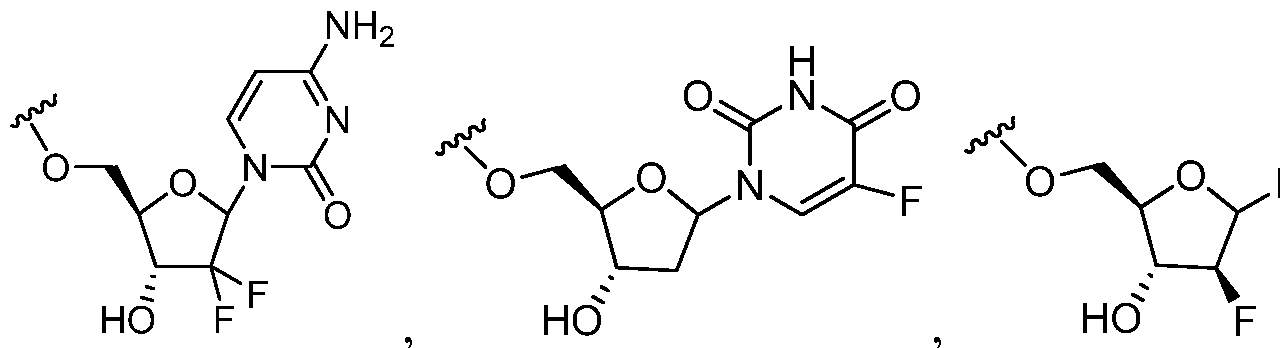
- the anticancer agent is selected from the group consisting of nucleoside anticancer agents that include, but are not limited to, Fludarabine, Pentostatin, Cladribine, Cytarabine, Gemcitabine, Azacitidine, Nelarabine, and Decitabine.
- the nucleoside anticancer agent is selected from a group of agents in which the ribose or deoxyribose part of the nucleoside has been replaced with a different functional group.
- nucleosides in which the ribose or deoxyribose moiety has been replaced with amino acids N-vinyl-2-pyrrolidinone, dihydroxy acyclic systems, tetrahydrofuranyl, tetrahydropyranyl, butyrolactones, pyrrolidine, cyclopentanes and cyclopentenes can be found in Koomen's "Synthesis and Biological Properties of Selected Nucleoside Analogs" Recueil des Travaux Chimiques des Pay-Bas 1993, 112, p.51-65.
- the linker comprises two amines. In other embodiments, the linker comprises a hydroxyl and an amine. In some embodiments, the linker amine is attached to a phosphate group of the anticancer agent.
- the hydrolysis is enzymatic.
- Fatty acid anticancer derivatives are inactive until they enter the cell and are hydrolyzed into the individual components to produce free anticancer agent and free fatty acid. Thus, the side effects of many anticancer agents are minimized.
- the fatty acid anticancer derivatives are targeted preferentially to certain cancer tissues over normal healthy tissues.
- the nucleoside anticancer agents may undergo phosphorylation in cells and targeted tissues to generate the corresponding monophosphate, diphosphate and triphosphate species.
- the triphosphate species is the more active metabolite.
- the fatty acid anticancer conjugates are created by covalently joining the nucleoside moiety to the omega-3 fatty acid portion via a phosphoramidate functionality or a phosphorodiamidate functionality at the 5 ' position of the nucleoside. With this type of phosphoramidate or phosphorodiamidate functionality, enzymatic degradation in targeted tissues can generate the corresponding nucleoside monophosphate and the omega-3 fatty acid.
- the nucleoside monophosphate in turn, can be phosphorylated further to the corresponding triphosphate species.
- the fatty acid portion in the phosphoramidate or phosphorodiamidate conjugate with the anticancer agent can deliver a synergistic activity that cannot be duplicated with the individual components or even with a combination of the individual components (i.e. the fatty acid and the nucleoside anticancer agent).
- R n i is a nucleoside anticancer agent
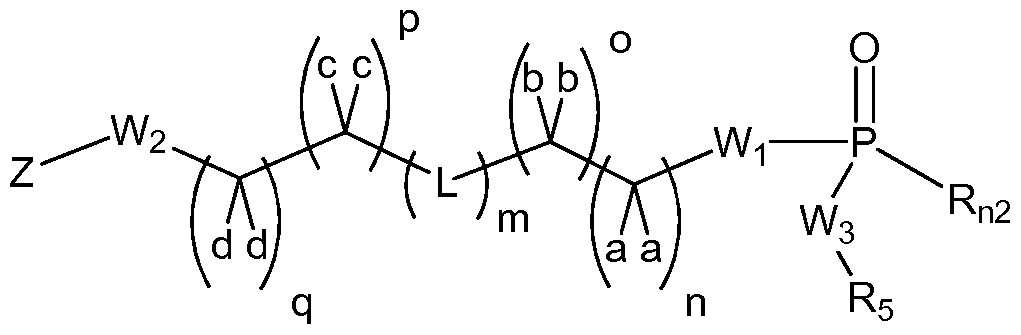
- Wi and W 2 are each independently null, O, S, NH, NR, or Wi and W 2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W 2 can not be O simultaneously;
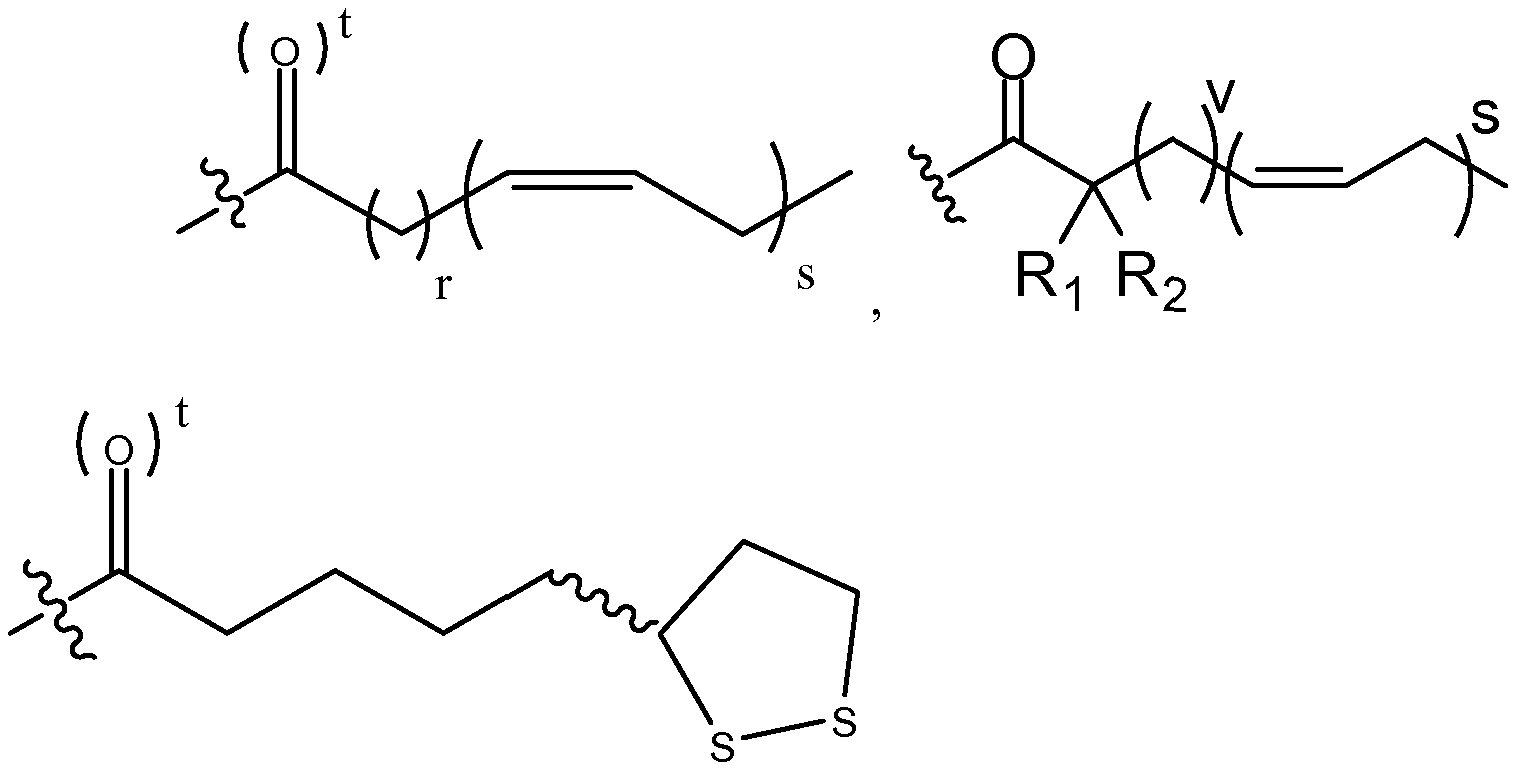
- W 3 is each independently O or NR, each a, b, c and d is independently -H, -D, -CH 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0) 2 -, -S-S-, -(Ci-C 6 alkyl)-, -(C 3 - C 6 cycloalkyl)-, a heterocycle, a heteroaryl,
- L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula I;
- R6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C 4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)Ci-C 3 alkyl, -S(0) 2 Ci-C 3 alkyl;
- each r is independently 2, 3, or 7;
- each s is independently 3, 5, or 6;
- each t is independently 0 or 1 ;
- each v is independently 1, 2, or 6;
- Ri and R 2 are each independently hydrogen, deuterium, -C 1 -C4 alkyl, -halogen, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C 4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(C C 3 alkyl), -S(0)C C 3 alkyl, -S(0) 2 Ci-C 3 alkyl; and each R is independently -H, -C 1 -C3 alkyl, or straight or
- Wi and W 2 are each independently null, O, S, NH, NR, or Wi and W 2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W 2 can not be O simultaneously;
- W 3 is each independently O or NR, each a, b, c and d is independently -H, -D, -CH 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0) 2 -, -S-S-, -(Ci-C 6 alkyl)-, -(C 3 - Cecycloalkyl)-, a heterocycle, a heteroaryl,
- L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula II;
- R6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C 4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)Ci-C 3 alkyl, -S(0) 2 Ci-C 3 alkyl;
- each r is independently 2, 3, or 7;
- each s is independently 3, 5, or 6;
- each t is independently 0 or 1 ;
- each v is independently 1, 2, or 6;
- Ri and R 2 are each independently hydrogen, deuterium, -C 1 -C4 alkyl, -halogen, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C 4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(C C 3 alkyl), -S(0)C C 3 alkyl, -S(0) 2 Ci-C 3 alkyl; and each R is independently -H, -C 1 -C3 alkyl, or straight or
- Formula III and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers, and stereoisomers thereof; wherein R n 3 is an anticancer agent;
- Wi and W 2 are each independently null, O, S, NH, NR, or Wi and W 2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W 2 can not be O simultaneously; each a, b, c and d is independently -H, -D, -CH 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0) 2 -, -S-S-, -(Ci-C 6 alkyl)-, -(C 3 - Cecycloalkyl)
- R6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C 4 alkyl, -C C 3 alkene, -C C 3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)Ci-C 3 alkyl, -S(0) 2 Ci-C 3 alkyl;
- each Z is independently -H
- each r is independently 2, 3, or 7;
- Ri and R 2 are each independently hydrogen, deuterium, -C 1 -C4 alkyl, -halogen, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C 4 alkyl, -C C 3 alkene, -C C 3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)Ci-C 3 alkyl, -S(0) 2 Ci-C 3 alkyl; and
- each R is independently -H, -Ci-C 3 alkyl, phenyl or straight or branched C 1 -C 4 alkyl optionally substituted with OH, or halogen; provided that when m, n, o, p, and q are each 0, Wi and W 2 are each null, and Z is
- Wi and W 2 are each independently null, O, S, NH, NR, or Wi and W 2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W 2 can not be O simultaneously; each a, b, c and d is independently -H, -D, -CH 3 , -OCH 3 , -OCH 2 CH 3 , -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0) 2 -, -S-S-, -(Ci-C 6 alkyl)-, -(C 3 - Cecycloalkyl)
- R6 is independently -H, -D, -C 1 -C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C 4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(Ci-C 3 alkyl), -S(0)Ci-C 3 alkyl, -S(0) 2 Ci-C 3 alkyl;
- each r is independently 2, 3, or each s is independently 3, 5, or each t is independently 0 or 1 ;
- each v is independently 1, 2, or Ri and R 2 are each independently hydrogen, deuterium, -C 1 -C4 alkyl, -halogen, -OH, -C(0)Ci-C 4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C 4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C 4 alkyl, -NH 2 , -NH(Ci-C 3 alkyl), -N(Ci-C 3 alkyl) 2 , -NH(C(0)Ci-C 3 alkyl), -N(C(0)Ci-C 3 alkyl) 2 , -SH, -S(C C 3 alkyl), -S(0)C C 3 alkyl, -S(0) 2 Ci-C 3 alkyl; and each R is independently -H, -C 1 -C3 alkyl, phenyl or straight or
- any one or more of H may be substituted with a deuterium. It is also understood in Formulae I, II, III and IV, that a methyl substituent can be substituted with a Ci-C 6 alkyl.
- compositions comprising at least one fatty acid anticancer derivative.
- the invention also includes pharmaceutical compositions that comprise an effective amount of a fatty acid anticancer derivative and a pharmaceutically acceptable carrier.
- the compositions are useful for treating or preventing a metabolic disease.
- the invention includes a fatty acid anticancer derivative provided as a pharmaceutically acceptable prodrug, a hydrate, a salt, such as a pharmaceutically acceptable salt, enantiomer, stereoisomer, or mixtures thereof.
- Figure 1A, IB and 1C The effect of test compounds on the expression of IL- ⁇ (Figure 1A), TNF-a ( Figure IB) and PD-L1 ( Figure 1C) in THP-1 cells.
- Figure 2A, 2B, 2C and 2D show relative PD-L1 expression in three tumor cell lines treated with test compounds.
- Figure 2B shows relative IL- ⁇ expression in three tumor cell lines treated with test compounds.
- Figure 2C shows relative Fltl expression in three tumor cell lines treated with test compounds.
- Figure 2D shows relative Myc expression in three tumor cell lines treated with test compounds.
- Figure 3A, 3B, 3C and 3D show relative TERT expression in MiaPaCa-2 cells treated with test compounds.
- Figure 3B shows relative CCNDl expression in MiaPaCa-2 cells treated with test compounds.
- Figure 3C shows relative Bcl-2 expression in MiaPaCa-2 cells treated with test compounds.
- Figure 3D shows relative Flt-1 expression in MiaPaCa-2 cells treated with test compounds.
- Figure 4 A and 4B Figure 4 A shows relative Actin protein expression in
- FIG. 4B shows relative cleaved PARP protein in MiaPaCa-2 cells treated with test compounds.
- the fatty acid anticancer derivatives have been designed to bring together at least one fatty acid and an anticancer agent into a single molecular conjugate.
- the activity of the fatty acid anticancer derivatives is greater than the sum of the individual components of the molecular conjugate, suggesting that the activity induced by the fatty acid derivative is synergistic.
- fatty acid anticancer derivatives includes any and all possible isomers, stereoisomers, enantiomers, diastereomers, tautomers, pharmaceutically acceptable salts, hydrates, solvates, and prodrugs of the fatty acid anticancer derivatives described herein.
- aryl refers to cyclic, aromatic hydrocarbon groups that have 1 to 2 aromatic rings, including monocyclic or bicyclic groups such as phenyl, biphenyl or naphthyl. Where containing two aromatic rings (bicyclic, etc.), the aromatic rings of the aryl group may be joined at a single point (e.g., biphenyl), or fused (e.g., naphthyl).
- the aryl group may be optionally substituted by one or more substituents, e.g., 1 to 5 substituents, at any point of attachment. The substituents can themselves be optionally substituted.
- C 1 -C 3 alkyl refers to a straight or branched chain saturated hydrocarbon containing 1-3 carbon atoms.
- Examples of a C 1 -C 3 alkyl group include, but are not limited to, methyl, ethyl, propyl and isopropyl.
- C 1 -C 4 alkyl refers to a straight or branched chain saturated hydrocarbon containing 1-4 carbon atoms.
- Examples of a C 1 -C 4 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, isopropyl, isobutyl, sec-butyl and tert-butyl.
- C 1 -C 5 alkyl refers to a straight or branched chain saturated hydrocarbon containing 1-5 carbon atoms.
- Examples of a C 1 -C 5 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, pentyl, isopropyl, isobutyl, sec-butyl and tert-butyl, isopentyl and neopentyl.
- Ci-C 6 alkyl refers to a straight or branched chain saturated hydrocarbon containing 1-6 carbon atoms. Examples of a Ci-C 6 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, pentyl, hexyl, isopropyl, isobutyl, sec-butyl, tert-butyl, isopentyl, and neopentyl.
- cycloalkyl refers to a cyclic hydrocarbon containing 3-6 carbon atoms.
- cycloalkyl group examples include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. It is understood that any of the substitutable hydrogens on a cycloalkyl can be substituted with halogen, C 1 -C 3 alkyl, hydroxyl, alkoxy and cyano groups.
- heterocycle refers to a cyclic hydrocarbon containing 3- 6 atoms wherein at least one of the atoms is an O, N, or S.
- heterocycles include, but are not limited to, aziridine, oxirane, thiirane, azetidine, oxetane, thietane, pyrrolidine, tetrahydrofuran, tetrahydrothiophene, piperidine, tetrahydropyran, thiane, imidazolidine, oxazolidine, thiazolidine, dioxolane, dithiolane, piperazine, oxazine, dithiane, and dioxane.
- heteroaryl refers to a monocyclic or bicyclic ring structure having 5 to 12 ring atoms wherein one or more of the ring atoms is a heteroatom, e.g. N, O or S and wherein one or more rings of the bicyclic ring structure is aromatic.
- heteroaryl are pyridyl, furyl, pyrrolyl, thienyl, thiazolyl, oxazolyl, imidazolyl, indolyl, tetrazolyl, benzofuryl, xanthenes and dihydroindole. It is understood that any of the substitutable hydrogens on a heteroaryl can be substituted with halogen, C 1 -C 3 alkyl, hydroxyl, alkoxy and cyano groups.
- any one of the side chains of the naturally occurring amino acids means a side chain of any one of the following amino acids: Isoleucine, Alanine, Leucine, Asparagine, Lysine, Aspartate, Methionine, Cysteine, Phenylalanine, Glutamate, Threonine, Glutamine, Tryptophan, Glycine, Valine, Proline, Arginine, Serine, Histidine, and Tyrosine.
- fatty acid as used herein means an omega-3 fatty acid and fatty acids that are metabolized in vivo to omega-3 fatty acids.
- Non- limiting examples of fatty acids are a//-cz ' s-7,10,13-hexadecatrienoic acid, a-linolenic acid (ALA or all-cis-9, 12,15- octadecatrienoic acid), stearidonic acid (STD or a/7-cz ' s-6,9,12,15-octadecatetraenoic acid), eicosatrienoic acid (ETE or all-cis- 11,14,17-eicosatrienoic acid), eicosatetraenoic acid (ETA or all-cis-%,11,14,17-eicosatetraenoic acid), eicosapentaenoic acid (EPA or all-cis- 5,8,11,14, 17-eicosapentaenoic acid
- fatty acid can also refer to medium chain fatty acids such as lipoic acid.
- anticancer agent as used herein means any of the class of compounds known as either non-nucleotide anticancer agents or nucleotide anticancer agents, and any derivatives thereof.
- non-nucleotide anticancer agents include, but are not limited to Epirubicin, Lonidamine, Pirarubicin, Idarubicin, Placlitaxel, Irinotecan, Docetaxel, Raltitrexed, Topotecan, Capecitabine, Alitretinoin, Bexarotene, Fulvestrant, Bortezomib, Pemetrexed, Ixabepilone, Pralatrexate, Eribulin, Tivantinib, Alisertib, Imatinib, Sorafenib, and Dasatinib.
- XPO 1 nuclear export protein Exportin 1
- XPO 1 nuclear export protein Exportin 1
- the protein kinase inhibitors are selected from a class consisting of ATP-competitive tyrosine kinase inhibitors, the type I kinase inhibitors. In some embodiments, the protein kinase inhibitors are selected from a class consisting of non- ATP competitive inhibitors, the type II and type III kinase inhibitors. In some embodiments, the protein kinase inhibitors are selected from a class of irreversible kinase inhibitors.
- Non- limiting examples of kinases which have been found to be therapeutically relevant in the oncology field include: Aurora kinases, anaplastic lymphoma kinase (ALK), the cyclin dependent kinases (CDK 1, CDK2, CDK4, CDK5, CDK6, CDK 7), cMet, epidermal growth factor receptor (EGFR), fibroblast growth factor receptor (FGFRl , FGFR2, FGFR3, FGFR4), vascular endothelial growth factor receptor (VEGFRl, VEGFR2, VEGFR3), platelet-derived growth factor receptor (PDGFRa, PDGFR ), checkpoint kinases (Chkl, Chk2), break point cluster- Abelson (Bcr-Abl), Src protein tyrosine kinase, spleen tyrosine kinase (Syk), Rho- associated coiled-coil containing kinase (ROCK1), polo-like kina
- the anticancer agent is selected from the group consisting of nucleoside anticancer agents that include, but are not limited to, Fludarabine, Pentostatin, Cladribine, Cytarabine, Gemcitabine, Azacitidine, Nelarabine, and Decitabine.
- the nucleoside anticancer agent is selected from a group of agents in which the ribose or deoxyribose part of the nucleoside has been replaced with a different functional group.
- Non-limiting examples of nucleosides in which the ribose or deoxyribose moiety has been replaced with amino acids N-vinyl-2-pyrrolidinone, dihydroxy acyclic systems, tetrahydrofuranyl, tetrahydropyranyl, butyrolactones, pyrrolidine, cyclopentanes and cyclopentenes can be found in Koomen's "Synthesis and Biological Properties of Selected Nucleoside Analogs" Recueil des Travaux Chimiques des Pay-Bas 1993, 112, p.51-65.
- Examples of non-ribose nucleosides are Aristeromycin, Neplanocin A, Fluoroneplanocin A. Additional non- limiting examples of 1- fluorocyclopent-l-ene analogs that can be used as anticancer nucleosides can be found in US 200502221 as illustrated with RX-3117.
- a "subject” is a mammal, e.g., a human, mouse, rat, guinea pig, dog, cat, horse, cow, pig, or non-human primate, such as a monkey, chimpanzee, baboon or rhesus, and the terms “subject” and “patient” are used interchangeably herein.
- the invention also includes pharmaceutical compositions comprising an effective amount of a fatty acid anticancer derivative and a pharmaceutically acceptable carrier.
- the invention includes a fatty acid anticancer derivative provided as a pharmaceutically acceptable prodrug, hydrate, salt, such as a pharmaceutically acceptable salt, enantiomers, stereoisomers, or mixtures thereof.
- Representative "pharmaceutically acceptable salts” include, e.g., water-soluble and water-insoluble salts, such as the acetate, amsonate (4,4-diaminostilbene-2, 2 - disulfonate), benzenesulfonate, benzonate, bicarbonate, bisulfate, bitartrate, borate, bromide, butyrate, calcium, calcium edetate, camsylate, carbonate, chloride, citrate, clavulariate, dihydrochloride, edetate, edisylate, estolate, esylate, fiunarate, gluceptate, gluconate, glutamate, glycollylarsanilate, hexafluorophosphate, hexylresorcinate, hydrabamine, hydrobromide, hydrochloride, hydroxynaphthoate, iodide, isothionate, lactate, lactobionate, la
- metabolic disease refers to disorders, diseases and syndromes involving dyslipidemia, and the terms metabolic disorder, metabolic disease, and metabolic syndrome are used interchangeably herein.
- an "effective amount" when used in connection with a fatty acid anticancer derivative is an amount effective for treating or preventing a metabolic disease.
- carrier encompasses carriers, excipients, and diluents and means a material, composition or vehicle, such as a liquid or solid filler, diluent, excipient, solvent or encapsulating material, involved in carrying or transporting a pharmaceutical agent from one organ, or portion of the body, to another organ, or portion of the body.
- treating refers to improving at least one symptom of the subject's disorder. Treating can be curing, improving, or at least partially ameliorating the disorder.
- disorder is used in this disclosure to mean, and is used interchangeably with, the terms disease, condition, or illness, unless otherwise indicated.
- administer refers to either directly administering a compound or pharmaceutically acceptable salt of the compound or a composition to a subject, or administering a prodrug derivative or analog of the compound or pharmaceutically acceptable salt of the compound or composition to the subject, which can form an equivalent amount of active compound within the subject's body.
- prodrug means a compound which is convertible in vivo by metabolic means ⁇ e.g., by hydrolysis) to a fatty acid anticancer derivative.
- Boc and BOC are tert-butoxycarbonyl, Boc 2 0 is di-tert-butyl dicarbonate, CDI is ⁇ , ⁇ - carbonyldiimidazole, DCC is N,N'-dicyclohexylcarbodiimide, DIEA is N,N- diisopropylethylamine, DMAP is 4-dimethylaminopyridine, DOSS is sodium dioctyl sulfosuccinate, EDC and EDO are l-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, EtOAc is ethyl acetate, h is hour, HATU is 2-(7-aza-lH-benzotriazole-l-yl)- 1,1,3,3-tetramethyluronium hexafluorophosphate, HPMC is hydroxypropyl methylcellulose, min is minutes, Pd/C is palladium on carbon, TFA
- a molecular conjugate which comprises an anticancer agent and a fatty acid covalently linked directly, or indirectly through a linker, wherein the linkage is through a free hydroxyl, amine, thiol, carboxylate, phosphate, or the like, on the anticancer agent and the fatty acid, wherein the fatty acid is selected from the group consisting of omega-3 fatty acids, fatty acids that are metabolized in vivo to omega-3 fatty acids, and lipoic acid, and the conjugate is stable in the plasma and capable of hydrolysis to produce free anticancer and free fatty acid, with the proviso that the molecular conjugate is not
- the anticancer agent is selected from the group consisting of non-nucleoside anticancer agents that include, but are not limited to, Epirubicin,
- the anticancer agent is selected from the group consisting of nucleoside anticancer agents that include, but are not limited to, Fludarabine, Pentostatin, Cladribine, Cytarabine, Gemcitabine, Azacitidine, Nelarabine, and Decitabine.
- the nucleoside anticancer agent is selected from a group of agents in which the ribose or deoxyribose part of the nucleoside has been replaced with a different functional group.
- nucleosides in which the ribose or deoxyribose moiety has been replaced with amino acids N-vinyl-2-pyrrolidinone, dihydroxy acyclic systems, tetrahydrofuranyl, tetrahydropyranyl, butyrolactones, pyrrolidine, cyclopentanes and cyclopentenes can be found in Koomen's "Synthesis and Biological Properties of Selected Nucleoside Analogs" Recueil des Travaux Chimiques des Pay-Bas 1993, 112, p.51-65.
- non-ribose nucleosides examples include Aristeromycin, Neplanocin A, Fluoroneplanocin A. Additional non-limiting examples of 1-fluorocyclopent-l-ene analogs that can be used as anticancer n leosides can be found in US 20050222185, as illustrated with RX-3117.
- the fatty acid is selected from the group consisting of all- cz ' s-7,10,13-hexadecatrienoic acid, a-linolenic acid, stearidonic acid, eicosatrienoic acid, eicosatetraenoic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid, docosahexaenoic acid (DHA), tetracosapentaenoic acid, tetracosahexaenoic acid, and lipoic acid.
- the fatty acid is selected from eicosapentaenoic acid and docosahexaenoic acid.
- the hydrolysis is enzymatic.
- the anticancer agent is selected from the group consisting of PARP inhibitors.
- PARP inhibitors Non- limiting examples of PARP are listed below. Additional PARP inhibitors can also be found in the following review: Dana V. Ferraris "Evolution of Poly(ADP-ribose)polymerase (PARP-1) inhibitors. From concept to clinic" J. Med.Chem. 2010, 53, p. 4561.
- the anticancer agent is selected from the group consisting of indoleamine-2,3-dioxygenase (IDO) inhibitors.
- IDO inhibitors include the following:
- the present invention provides fatty acid anticancer derivatives according to Formulae I, II, III and IV:
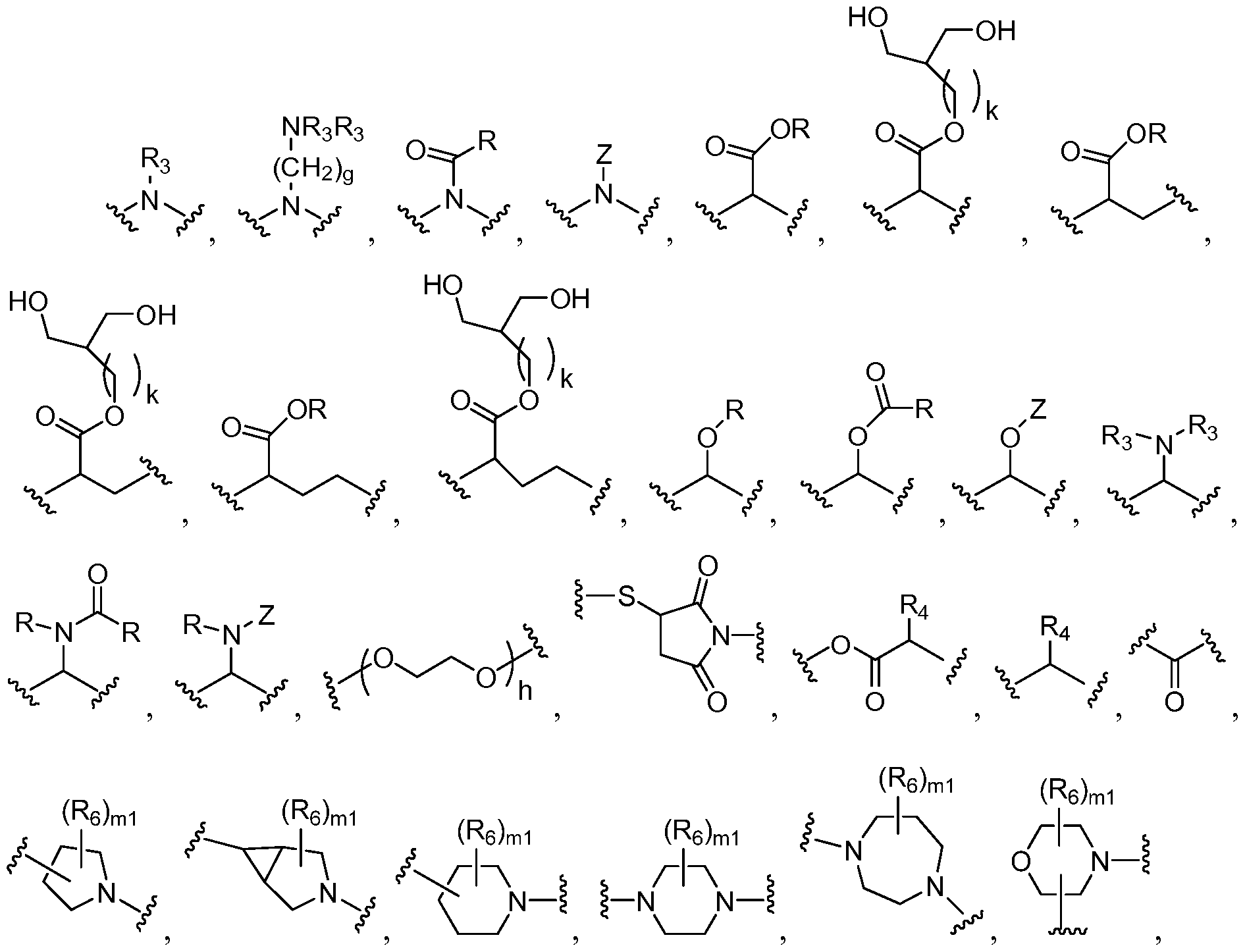
- Wi, W 2 , a, c, b, d, e, j, k, m, ml , n, o, p, q, L, Z, Z', r, s, t, v, z, R nl , R n2 , R n3 , R n4 , Ri, R 2 , R 3 , R4, R and R 6 are as defined above for Formula I-IV, with the proviso that there is at least one of
- one Z is
- one Z is
- one Z is
- one Z is
- Z is
- Wi is NH.
- W 2 is NH
- Wi is O.
- W 2 is O.
- Wi is null.
- W 2 is null.
- Wi and W 2 are each NH.
- Wi and W 2 are each null.
- Wi is O and W 2 is NH.
- Wi and W 2 are each NR, and R is CH 3
- m is 0.
- m is 1.
- m is 2.
- L is -S- or -S-S-.
- L is -0-.
- L is -C(O)-.
- L is heteroaryl
- L is heterocycle
- L is N
- L is N
- L is N
- L is N
- L is N
- L is N
- L is N
- L is N
- L is
- L is
- L is
- L is
- one of n, o, p, and q is 1.
- two of n, o, p, and q are each 1.
- n, o, p, and q are each 1.
- n, o, p, and q are each 1.
- one d is C(0)OR.
- r is 2 and s is 6.
- r is 3 and s is 5.
- t is 1.
- r is 2
- s is 6
- t is 1.
- r is 3, s is 5 and t is 1.
- j 0.
- j is 1.
- W 3 is O.
- W 3 is NH
- R 5 is ethyl
- R 5 is methyl
- R 5 is phenyl
- R 5 is naphthol
- R5 is phenyl that is optionally substituted at the meta position with CONH 2 .
- R 5 is e that is optionally substituted C0 2 R wherein e is the side chain of a naturally occurring amino acid.
- R n2 is [0132] In some embodiments, R n2 is
- R n2 is
- R n2 is
- R n2 is
- R n2 is
- R n4 is
- R n4 is
- R n4 is .
- R n4 is
- R n4 is [0144] In some embodiments, R n4 is
- R n4 is
- R n4 is
- R n4 is
- R n4 is
- R n4 is 1.
- R n4 is 1.
- R n4 is .
- R n4 and j 1. [0156] In some embodiments, R n4 is
- R n4 is
- R n4 is
- R n4 is
- R n4 is
- R n4 is
- R n4 is [0163] In some embodiments, R n4 i
- R n4 is
- R n4 andj 0. [0170] In some embodiments, R n4 is
- R n4 1.
- R n is 1.
- R n4 d j 0.
- R n4 is .
- R n4 is
- R n4 is
- R n4 is 1.
- R n4 is
- R n4 1
- R n4 [0191] In some embodiments, R n4
- R n4 is
- R n4 is
- R n4 is
- R die4 is
- R n4 is
- R n4 is
- R n4 is
- R n4 is [0205] In some embodiments, R n4 is 1.
- R n4 1.
- R n4 is
- R n4 is 1.
- R n4 is 0.
- R n4 is 1.
- R n4 is
- R n4 is
- R n4 is
- R n4 is
- R n4 is
- R n4 is andj
- R n4 is andj
- R n4 is
- R n4 is
- R n4 is .
- R n4 is l .
- R n4 is 0.
- R n4 is .
- R n4 is 1.
- R n4 is 0.
- R n4 is
- R n4 is
- any one or more of H may be substituted with a deuterium. It is also understood in Formulae I, II, III and IV, that a methyl substituent can be substituted with a Ci-C 6 alkyl.
- ALL Acute Lymphoblastic Leukemia
- AML Acute Myeloid Leukemia
- JMML Myelomonocytic Leukemia
- MDS Myelodysplastic syndrome
- Adrenocortical Carcinoma AIDS-Related Cancers (including Kaposi Sarcoma, Lymphoma) Anal Cancer, Appendix Cancer, Astrocytomas, Atypical Teratoid/Rhabdoid Tumor, Basal Cell Carcinoma, Bile Duct Cancer, Bladder Cancer, Bone Cancer, Osteosarcoma and Malignant Fibrous Histiocytoma, Brain Stem Glioma, Brain Tumor (including Astrocytomas, Brain and Spinal Cord Tumors, Brain Stem Glioma, Central Nervous System Atypical Teratoid/Rhabdoid Tumor, Central Nervous System Embryonal Tumors, Central Nervous System Germ Cell Tumors, Craniopharyngioma, Ependymoblastoma, Ependymoma, Medulloblastoma, Medulloe
- Gastrointestinal Carcinoma of Unknown Primary, Cardiac (Heart) Tumors, Central Nervous System (including Atypical Teratoid/Rhabdoid Tumor, Embryonal Tumors, Germ Cell Tumor, Childhood, Lymphoma, Primary), Cervical Cancer, Childhood Cancers, Chordoma, cholangiocarcinoma (or cancer that originates in the bile ducts), biliary tract cancer
- pancreatic cancer including pancreatic cancer, gall bladder cancer, and cancer of the ampulla of Vater
- Chronic Myeloproliferative Disorders including Colon Cancer, Colorectal Cancer
- Ependymoblastoma Ependymoblastoma, Ependymoma, Esophageal Cancer, Esthesioneuroblastoma, Childhood, Ewing Sarcoma Family of Tumors, Extracranial Germ Cell Tumor, Extragonadal Germ Cell Tumor, Extrahepatic Bile Duct Cancer, Eye Cancer, Gallbladder Cancer, Gastric (Stomach) Cancer, Gastrointestinal Carcinoid Tumor, Gastrointestinal Stromal Tumors (GIST), Germ Cell Tumor (including Central Nervous System, Extracranial, Extragonadal, Ovarian, Testicular), Gestational Trophoblastic Tumor, Glioma, Hairy Cell Leukemia, Head and Neck Cancer, Heart Cancer, Hepatocellular (Liver) Cancer, Histiocytosis, Langerhans Cell, Hodgkin Lymphoma, Hypopharyngeal Cancer, Intraocular Melanoma, Islet Cell Tumors, Pancreatic Neuroendocrine Tu
- Macroglobulinemia Waldenstrom, Male Breast Cancer, Malignant Fibrous Histiocytoma of Bone and Osteosarcoma, Medulloblastoma, Medulloepithelioma, Melanoma (including Childhood, Intraocular), Merkel Cell Carcinoma, Mesothelioma, Malignant,Metastatic Squamous Neck Cancer with Occult Primary, Midline Tract Carcinoma Involving NUT Gene, Mouth Cancer, Multiple Endocrine Neoplasia Syndromes, Multiple Myeloma/Plasma Cell Neoplasm, Mycosis Fungoides, Myelodysplasia Syndromes,
- AML Acute Myeloid Leukemia
- Multiple Myeloma Multiple Myeloma
- Myeloproliferative Disorders Nasal
- breast cancers there are also multiple sub-types. For instance, certain types of breast cancer are more sensitive to hormone-based treatments; and these include the estrogen receptor positive (ER), the progesterone receptor positive (PR). The hormone receptor (HR) negative type of breast cancer, on the other, does not respond to hormone- based therapy.
- ER estrogen receptor positive
- PR progesterone receptor positive
- HR hormone receptor negative type of breast cancer
- HER-2 positive breast cancer is a type of breast cancer that tests positive for the human epidermal growth factor receptor 2 (HER-2) gene.
- breast cancers are also divided into four different groups: Group 1 (luminal A) includes tumors that are ER and PR positive, but negative for HER-2; Group 2 (luminal B) includes tumors that are ER positive, PR negative and HER-2 positive; Group 3 (HER-2 positive) includes tumors that are ER and PR negative, but HER-2 positive; Group 4 (basal-like) includes tumors that are ER, PR and HER-2 negative. Group 4 breast cancers are also referred to as triple-negative breast cancers.
- the invention also includes pharmaceutical compositions useful for treating or preventing a cancer.
- the compositions are suitable for internal use and comprise an effective amount of a fatty acid anticancer derivative and a pharmaceutically acceptable carrier.
- the fatty acid anticancer derivatives are especially useful in that they demonstrate very low peripheral toxicity or no peripheral toxicity.
- the subject is administered an effective amount of a fatty acid anticancer derivative.
- the fatty acid anticancer derivatives can each be administered in amounts that are sufficient to treat a cancer. In other embodiments, the fatty acid anticancer derivatives can each be administered in amounts that are sufficient to prevent the development of a cancer in a subject.
- Administration of the fatty acid anticancer derivatives can be accomplished via any mode of administration for therapeutic agents. These modes include systemic or local administration such as oral, nasal, parenteral, transdermal, subcutaneous, vaginal, buccal, rectal or topical administration modes.
- compositions can be in solid, semi-solid or liquid dosage form, such as, for example, injectables, tablets,
- suppositories pills, time-release capsules, elixirs, tinctures, emulsions, syrups, powders, liquids, suspensions, or the like, sometimes in unit dosages and consistent with conventional pharmaceutical practices.
- they can also be administered in intravenous (both bolus and infusion), intraperitoneal, subcutaneous or intramuscular form, all using forms well known to those skilled in the pharmaceutical arts.
- Illustrative pharmaceutical compositions are tablets and gelatin capsules comprising a fatty acid anticancer derivative and a pharmaceutically acceptable carrier, such as: a) a diluent, e.g., purified water, triglyceride oils, such as hydrogenated or partially hydrogenated vegetable oil, or mixtures thereof, corn oil, olive oil, sunflower oil, safflower oil, fish oils, such as EPA or DHA, or their esters or triglycerides or mixtures thereof, omega- 3 fatty acids or derivatives thereof, lactose, dextrose, sucrose, mannitol, sorbitol, cellulose, sodium, saccharin, glucose and/or glycine; b) a lubricant, e.g., silica, talcum, stearic acid, its magnesium or calcium salt, sodium oleate, sodium stearate, magnesium stearate, sodium benzoate, sodium acetate, sodium chloride and/or polyethylene glyco
- Liquid, particularly injectable, compositions can, for example, be prepared by dissolution, dispersion, etc.
- the fatty acid anticancer derivative is dissolved in or mixed with a pharmaceutically acceptable solvent such as, for example, water, saline, aqueous dextrose, glycerol, ethanol, and the like, to thereby form an injectable isotonic solution or suspension.
- a pharmaceutically acceptable solvent such as, for example, water, saline, aqueous dextrose, glycerol, ethanol, and the like.
- Proteins such as albumin, chylomicron particles, or serum proteins can be used to solubilize the fatty acid anticancer derivatives.
- the fatty acid anticancer derivatives can be also formulated as a suppository that can be prepared from fatty emulsions or suspensions; using polyalkylene glycols such as propylene glycol, as the carrier.
- the fatty acid anticancer derivatives can also be administered in the form of liposome delivery systems, such as small unilamellar vesicles, large unilamellar vesicles and multilamellar vesicles.
- Liposomes can be formed from a variety of phospholipids, containing cholesterol, stearylamine or phosphatidylcholines.
- a film of lipid components is hydrated with an aqueous solution of drug to a form lipid layer encapsulating the drug, as described in United States Patent No. 5,262,564, the contents of which are herein incorporated by reference in their entirety.
- Fatty acid anticancer derivatives can also be delivered by the use of monoclonal antibodies as individual carriers to which the fatty acid anticancer derivatives are coupled.
- the fatty acid anticancer derivatives can also be coupled with soluble polymers as targetable drug carriers.
- soluble polymers can include polyvinylpyrrolidone, pyran copolymer, polyhydroxypropylmethacrylamide-phenol, polyhydroxyethylaspanamidephenol, or polyethyleneoxidepolylysine substituted with palmitoyl residues.
- the fatty acid anticancer derivatives can be coupled to a class of biodegradable polymers useful in achieving controlled release of a drug, for example, polylactic acid, polyepsilon caprolactone, polyhydroxy butyric acid, polyorthoesters, polyacetals, polydihydropyrans,
- fatty acid anticancer derivatives are not covalently bound to a polymer, e.g. , a polycarboxylic acid polymer, or a polyacrylate.
- Parenteral injectable administration is generally used for subcutaneous, intramuscular or intravenous injections and infusions.
- Injectables can be prepared in conventional forms, either as liquid solutions or suspensions or solid forms suitable for dissolving in liquid prior to injection.
- compositions can be prepared according to conventional mixing, granulating or coating methods, respectively, and the present pharmaceutical compositions can contain from about 0.1 % to about 90 %, from about 10 % to about 90 %, or from about 30 % to about 90 % of the fatty acid anticancer derivative by weight or volume.
- the dosage regimen utilizing the fatty acid anticancer derivative is selected in accordance with a variety of factors including type, species, age, weight, sex and medical condition of the patient; the severity of the condition to be treated; the route of
- Effective dosage amounts of the present invention when used for the indicated effects, range from about 20 mg to about 5,000 mg of the fatty acid anticancer derivative per day.
- Compositions for in vivo or in vitro use can contain about 20, 50, 75, 100, 150, 250, 500, 750, 1,000, 1,250, 2,500, 3,500, or 5,000 mg of the fatty acid anticancer derivative.
- the compositions are in the form of a tablet that can be scored.
- Effective plasma levels of the fatty acid anticancer derivative can range from about 5 ng/mL to about 5,000 ng/mL.
- Appropriate dosages of the fatty acid anticancer derivatives can be determined as set forth in Goodman, L. S.; Gilman, A. The Pharmacological Basis of Therapeutics, 5th ed.; MacMillan: New York, 1975, pp. 201-226.
- Fatty acid anticancer derivatives can be administered in a single daily dose, or the total daily dosage can be administered in divided doses of two, three or four times daily. Furthermore, fatty acid anticancer derivatives can be administered in intranasal form via topical use of suitable intranasal vehicles, or via transdermal routes, using those forms of transdermal skin patches well known to those of ordinary skill in that art. To be administered in the form of a transdermal delivery system, the dosage administration can be continuous rather than intermittent throughout the dosage regimen.
- Topical preparations include creams, ointments, lotions, aerosol sprays and gels, wherein the concentration of the fatty acid anticancer derivative ranges from about 0.1 % to about 15 %, w/w or w/v.
- anticancer agents that can be used in combination with any of the fatty acid anticancer conjugates of this invention include carboplatin, cisplatin, oxaliplatin, paclitaxel, cyclosphosphamide, nitrogen mustard, melphalan, chlorambucil, busulphan, Temozolamide, nitrosoureas, tegafur, raltitrexed, hydroxyurea, Adriamycin, Combretastatin A4, Daunomycin, Mytocin-C, Mythramycin, Abraxane, Velcade,
- Mitoxantrone Vinblastine, Vincristine, Vindesine, Vinorelbine, Taxol, Docetaxel,
- Panitunumab, ranibizumab and Ipilimumab an antibody drug conjugate (such as Moxetumomab, Brentuximab vedotin, Trastuzumab emtansine), a PD-1 antibody (such as Lambrolizumab, Nivolumab, and MEDI 4736), a PD-Ll antibody (such as MEDI 0680 and RG 7446), an antisense therapy (such as ISIS-2503, an anti-ras antisense or G3139, an anti- Bcl2 antisense), a gene therapy approach (such as the one replacing aberrant genes that include p53, BRCA1 or BRCA2, and GDEPT), and an immunotherapy approach (examples of which include ex vivo and in vivo approaches to increase the immunogenicity of patient tumor cells, transfection with cytokines such as IL-2, IL-4 or granulocyte macrophage colony stimulating factor, approaches to decrease T cell
- the agent that can be used in combination with the compounds of the invention is itself a combination of approved anticancer drugs.
- Examples of commonly used combination of anticancer drugs include CVP (cyclophosphamide + vincristine + prednisone), ACVBP (doxorubicin + cyclophosphamide + vindesine + bleomycin + prednisone), CHOP (cyclophosphamide + doxorubicin + vincristine + prednisone), CNOP (cyclophosphamide + mitoxantrone + vincristine + prednisone), m- BACOD (methotrexate + bleomycin + doxorubicin + cyclophosphamide + vincristine + dexamethasone + leucovorin), MACOP-B (methotrexate + doxorubicin + cyclophosphamide + vincristine + prednisone fixed dose + ble
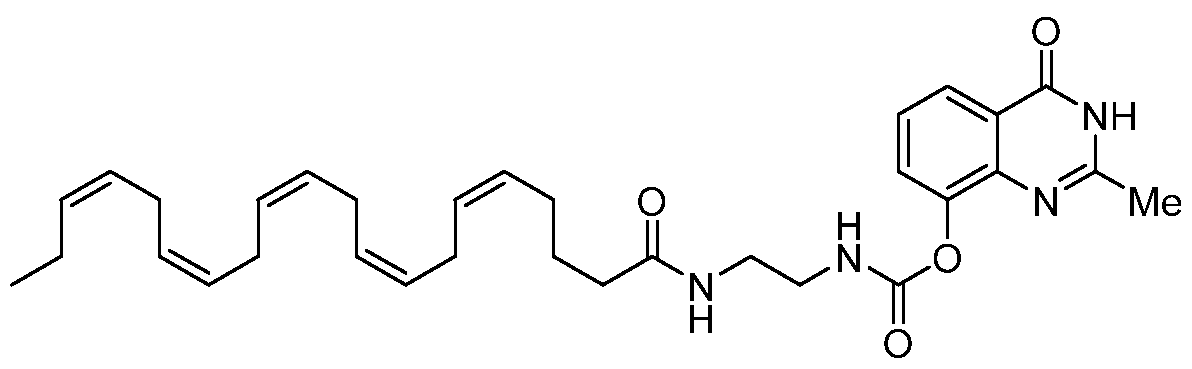
- compound A represents Bexarotene.
- other anticancer agents with a carboxylic acid group can also be subjected to the same chemistry in order to prepare the appropriate fatty acid anticancer agents.
- anticancer agents that have a carboxylic acid group include, but are not limited to,
- the mono-BOC protected amine of the formula C can be obtained from commercial sources or prepared according to known procedures, depending on the group X (wherein X can be -NPv 4 -, - NC(0)R- -0-, -S-, -CH(OH)-, -OCH 2 CH 2 O-).
- OCH 2 CH 2 0 OCH 2 CH 2 0
- the amine derivative B is then coupled with the compound A using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, followed by deprotection of the BOC group with acids such as TFA or HC1 in a solvent such as CH 2 C1 2 or dioxane to produce compound C.
- a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, followed by deprotection of the BOC group with acids such as TFA or HC1 in a solvent such as CH 2 C1 2 or dioxane to produce compound C.
- Compound C can be coupled with a fatty acid of formula D using HATU in the presence of a tertiary amine such as DIEA to afford compounds of the formula E.
- the fatty acid D can also be substituted with lip
- Compound A (Bexarotene) is coupled with a BOC-protected diamine of the general formula DA using either EDCI or HATU to obtain the BOC-protected amide derivative of the general formula F.
- the resulting amine can be coupled with a fatty acid of the formula E.
- BOC-protected diamines are commercially available. Examples of which include, but are not limited to, tert-butyl (2- aminoethyl)carbamate and tert-butyl piperazine-l-carboxylate.
- the following diamines can be prepared according to the rocedures outlined in the corresponding references:
- compound H represents Dasatinib.
- anticancer agents with a free hydroxyl group can also be subjected to the same chemistry in order to prepare the appropriate fatty acid anticancer agents.
- anticancer agents that have a free hydroxyl group include, but are not limited to, Fludarabine, Pentostatin, Cladribine, Cytarabine, Gemcitabine, Azacidine, TAK-733 and TAK-285.
- the mono-BOC protected amine of the formula C can be obtained from commercial sources or prepared according to known procedures, depending on the group X (wherein X can be - NR 4 -, -NC(0)R- -0-, -S-, -CH(OH)-, -OCH 2 CH 2 0-).
- OCH 2 CH 2 0 OCH 2 CH 2 0
- Compound H can be reacted first with 4-nitrochloroformate, in the presence of a tertiary amine such as triethylamine, followed by the reaction with a mono-Boc protected amine of the formula B in order to obtain compounds of the formula I.
- the Boc protecting group can be removed by treatment with HC1, and the resulting amine can be coupled with a fatty acid of the formula D using HATU in the presence of DIEA to obtain compounds of the general formula J.
- R, r and s are as defined above.
- compound M represents gemcitabine that has been protected at the 3' position according to the procedure outlined in Guo et al J. Org. Chem. 2014, 64, p. 8319.
- any other suitably protected nucleosides can be used for this coupling reaction to form the desired phosphoramidate. Such protection is necessary in order to favor the proposed reaction at the 5' position of the nucleoside.
- the diamine B as described above, is coupled with the fatty acid derivative D in the presence of EDC/HOBT and a tertiary amine such as triethylamine in order to afford the amine derivative K.
- the amine B used in Scheme 4 can be substituted with a diamine of the general formula DA, described previously in Scheme 2.
- the alcohol ROH can also be substituted with an amine of the formula RNH 2 .
- RNH 2 is 3- aminobenzamide
- the phosphorodiaminate derivative that is generated is shown in formula O.
- the IC 50 of fatty acid anticancer conjugates against a number of tumor cell lines were determined in an antiproliferative assay using standard protocols at Charles River Discovery Research Services. Briefly, the desired cell lines (2000-4000 cells/well, see below for list of tumor cells) were seeded in a 96-well microculture plate (Costar flat bottom # 3997) in a total volume of 100 uL/well. After 24 hours, a 2 x drug master plate in growth medium from 10 ⁇ of stock drug was prepared.
- the fatty acid anticancer conjugates were first solubilized in protein-rich buffers as follows: The 100% Fetal Bovine Serum (FBS, Gemini Benchmark Lot # A45B00Z)) or a 10% BSA solution in PBS (Sigma # A1595) was pre-warmed to 37 °C in a water bath. The fatty acid anticancer conjugates were dissolved in ethanol with vigorous vortexing to form a 100 mM 1000 x ethanol solution. A lOx stock solution of the fatty acid anticancer conjugates in protein buffer was then prepared by transferring 5 of the 1000 x ethanol solution into 495 mL of protein solution (either 100% FBS or 10%) BSA solution). The resulting mixture was vortexed vigorously.
- This 10 x (1 mM) solution was used for the assay by further diluting the stock 1 : 10 in the desired buffer and then serially diluted to the desired concentrations.
- 100 of the serially diluted fatty acid anticancer conjugate was added to cells. After 72 hours, relative cell number was estimated using Promega Cell Titer Glo® assay (Promega # G7571). This was done by first bringing the Cell Titer Glo® reagents to room temperature. Next, 100 of the growth medium was removed and 50 ⁇ , of Cell Titer Glo® reagent was added to each well. The plate was shaken for 10 min and then to equilibrate for 2 min before transferring to a white plate. Luminescence was read on the Tecan GNEios microplate reader.
- B is the maximal % of control luminescence
- A is the minimal % of control luminescence at the highest agent concentration
- C is the IC 50
- D is the slope factor.
- IC 50 is the concentration of agent that inhibits cell growth by 50% compared to the control cells. For NF (Nullfit), a meaningful IC 50 was not generated from the available data.
- NSLC non small cell lung cancer
- H460 H460
- H522 LL
- pancreatic cancer BxPc-3
- MIAPaCa-2 pancreatic cancer
- PANC-1 pancreatic cancer
- PAN02 pancreatic cancer
- PC3 prostate cancer: DU145, PC3
- Inflammation is a hallmark of cancer and a driving force for tumor progression.
- a fatty acid anticancer conjugate can exert a significant anti-inflammatory response and therefore be of utility in the treatment of various cancers.
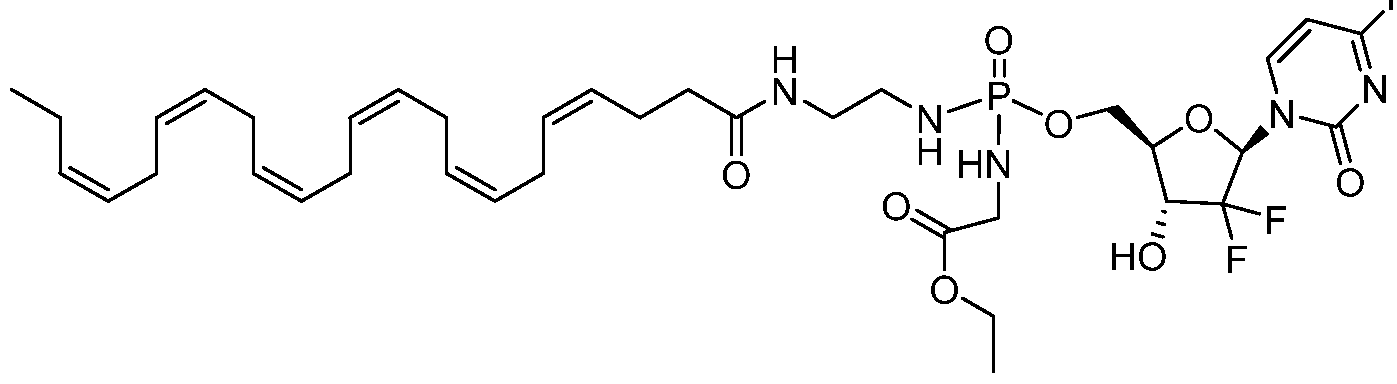
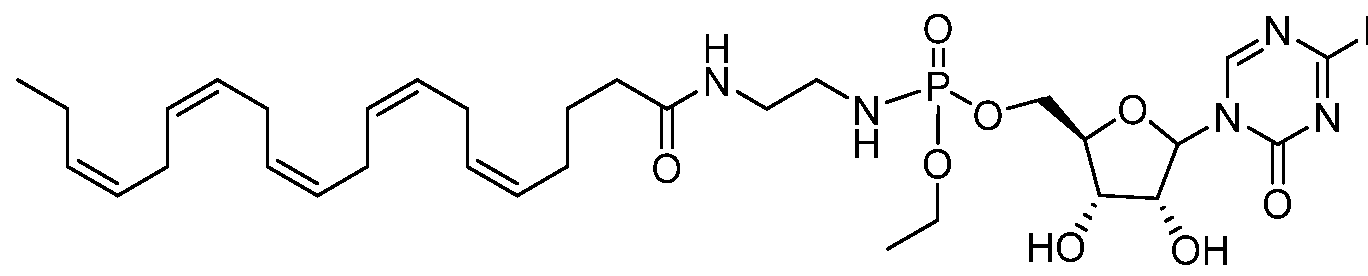
- Gemcitabine is a nucleoside anticancer agent commonly used in treatment of certain forms of breast, colorectal and pancreatic cancer.
- the phosphoramidate NUC-1031 derivative (abbreviated here and in subsequent examples as NUC) represents a pro-drug form of gemcitabine (Slucarczyk et al J. Med. Chem. 2014, 57, p. 1531).
- the fatty acid gemcitabine conjugates II-3 and II-4 are used in the direct comparison with gemcitabine and its phosphoramidate pro-drug form.
- the effects of II-3 and II-4 relative to GEM and NUC on basal NF- ⁇ activity were examined first in the human monocytic l
- FIGS 1A, IB and 1C summarized the effect of the test compounds in THP-1 cells.
- the basal expression of two classical NF- ⁇ target genes, IL- ⁇ and TNF-a are increased following a 6 hour treatment with 50 ⁇ of gemcitabine (GEM) and its pro-drug form NUC ( Figures 1A and IB).
- GEM gemcitabine
- NUC pro-drug form NUC
- II-3 and II-4 had little effect on basal NF- ⁇ driven expression.
- II-4 and II-3 treatment produced an unexpected reduction in the expression of PD-L1, which is known to contain NF- ⁇ binding sites in its promoter ( Figure 1C).
- the fatty acid anticancer conjugates of this invention allow the simultaneous delivery of an omega-3 fatty acid and anticancer agent to a cellular compartment to achieve unexpected and synergistic activity against certain biological pathways.
- Programmed death 1 (PD 1) and its ligands (PD-L1 and PD-L2) are important in regulating the balance between T cell activation, tolerance and immunopathology.
- the PD-1 and PD-L1 pathway has recently been validated clinically as an important therapeutic target against certain cancers. Cancer cells are also pro-inflammatory; and agents that can exert anti-inflammatory activity can potentially be synergistic and beneficial as anticancer agents.
- Gemcitabine is used here as an example of an anticancer agent that cannot inhibit the PD-L1 pathway or the inflammatory IL- ⁇ pathway as a standalone agent.
- fatty acid gemcitabine conjugates as shown in II-3 and II-4, display unexpected inhibitory activity against both the PD-L1 and IL- ⁇ pathways.
- the compounds of the invention are produced by covalently linking an anticancer agent with an omega-3 fatty acid.
- the resulting fatty acid anticancer conjugates demonstrated an unexpected synergistic activity that cannot be reproduced by using a combination of the individual components.
- three different tumor cell lines DU-145, MiaPaCa-2 and PC3 were treated for 24 h with either the control or 3.16 ⁇ each of gemcitabine (GEM), its phosphoramidate prodrug NUC- 1031 (NUC), a combination of the omega-3 fatty acid EPA and gemcitabine (abbreviated as E/G), or the fatty acid gemcitabine conjugates II-3 and II-4.
- RNA was harvested, purified and analyzed by qRT-PCR in the same manner as described in example 2.
- Figures 2A, 2B, 2C, and 2D summarize the results for the 6 different treatment groups across the three tumor cell lines.
- Figures 2A and 2B show the RNA expression of PD-L1 and IL- ⁇ , respectively, across the three tumor cell lines.
- gemcitabine GEM
- NUC treatment as well as the combination of EPA and gemcitabine (E/G)
- E/G increased PD-L1 and IL- ⁇ RNA expression while the fatty acid gemcitabine conjugates II-4 and II-3, at the same concentration, had a lesser effect.
- FIG. 2C shows the RNA expression of VEGFR1 (also known as Fltl).
- the MiaPaCa-2 cells were more sensitive to the induction of this gene; the GEM, the NUC, as well as the combination of EPA and gemcitabine (E/G) treatment groups all markedly increased the expression of this critical receptor. In sharp contrast, this induction was suppressed with the fatty acid gemcitabine conjugates II-4 and II-3.
- FIG. 2D summarizes the RNA expression of Myc, a target gene that is activated upon various mitogenic signaling and is capable of driving tumor cell proliferation by regulating apoptosis through the up-regulation of the anti-apoptotic protein Bcl-2.
- Myc a target gene that is activated upon various mitogenic signaling and is capable of driving tumor cell proliferation by regulating apoptosis through the up-regulation of the anti-apoptotic protein Bcl-2.
- the tumor cell line DU-145 was most sensitive.
- Treatment with GEM or NUC resulted in an up-regulation of Myc.
- the fatty acid gemcitabine conjugates II-4 and II-3 both suppressed the up-regulation of this target gene.
- this synergistic effect on Myc could not be reproduced by using the combination of EPA and gemcitabine (E/G treatment group).
- MiaPaCa-2 tumor cells were treated for 24 h with either the control group or a higher concentration (31.6 ⁇ ) of the omega-3 fatty acid EPA, gemcitabine (GEM), a combination of EPA and gemcitabine (abbreviated as E/G), the fatty acid gemcitabine conjugates II-3 and II-4.
- RNA was harvested, purified by analyzed by qRT- PCR in the same manner as described in example 2.
- Figures 3A, 3B, 3C and 3D summarize the results of the six treatment groups in this tumor cell line.
- telomerase (TERT) expression in cancer is required for replicative immortality, and its expression is upregulated in many human cancers.
- An inhibition of telomerase activity in cancer cells can cause senescence and apoptosis without affecting normal human cells.
- CCND1 is the target gene of the cyclin Dl; amplification or overexpression of which can alter cell cycle progression and contribute to tumorgenesis.
- Bcl-2 is an anti-apoptotic member of the Bcl-2 family that regulates programmed cell death. Cancer cells overexpress Bcl-2 as a means to escape apoptosis.
- VEGFR1 (Fltl) was described earlier in example 3.
- compound II-3 showed better inhibitory activity against these 4 target genes that compound II-4 ( Figures 3A-3D). More importantly, the fatty acid gemcitabine conjugate II-3 showed an unexpected and synergistic activity on these 4 target genes and this effect could not be reproduced by using either the individual components (i.e. the treatment groups E, GEM) or a combination of the individual components (i.e. the treatment group E/G).
- MiaPaCa-2 tumor cells were treated for 48 h with 31.6 ⁇ of the omega-3 fatty acid EPA, gemcitabine (GEM), a combination of EPA and gemcitabine (abbreviated as E/G), or the fatty acid gemcitabine conjugates II-3 and II-4.
- GEM gemcitabine
- E/G gemcitabine conjugates II-3 and II-4.
- Figures 4 A and 4B summarize the results of the 6 different treatment groups. Keeping this concentration of the test compounds on the cells for 48 h revealed differential killing of the fatty acid gemcitabine conjugates II-4 and II-3 relative to GEM, EPA, or the combination of GEM and EPA. This was assessed as the expression of B-Actin in equal volumes of total cell lysates. Greater cell killing was presumably achieved by a greater induction of apoptosis, an increase in caspase-3 activity and greater cleavage of PARP.
- RAW 264.7 cells stably expressing a 3x NFkB response elemement-driven luciferase reporter were seeded into 96 well plates in sera- free medium (Optimem) 18 hours prior to compound application.
- Compounds of the invention were prepared by first making 100 mM stock solutions in EtOH. Stock solutions were then diluted 1 : 100 in low LPS FBS (Gemini BenchMark 100-106), mixed vigorously and allowed to incubate at room
- the MTD assay can be performed using female Balb/c nude mice, 6-8 weeks old. Animals, in groups of 6-8, are administered with the test compound or the vehicle control group over a period of 2 weeks.
- the formulation that is needed to appropriately dissolve the test compound for oral dosing can be a mixture of tween, peceol and PEG400 in water.
- the test compound can be dissolved in DMSO, N-methylpyrrolidone or 40% captisol solution in water. Animals are dosed i.p. either 2 x a week or orally once a day over a period of 2 weeks.
- the dose to be used can range from 0.05 mmol/kg to 0.5 mmol/kg, depending on the test compound. Mice are monitored daily for body weight and clinical symptoms for 2 weeks. The results can be expressed as means ⁇ SEM.
- the in vivo xenograft mouse model can be performed using standard protocols that have been described in E.A. Sausville and A. M. Burger's "Contributions of Human Tumor Xenografts to Anticancer Drug Development” Cancer Res. 2006, 66, p. 3351-4.
- mice from an immune compromised strain (such as NOD.CB17- r c scld /J, CBySmn.CB17-iWc scld /J, NOD.Cg- r c scld I/2rg tmlWjI /SzJ, B6A29Sl-Ragl tmlMom / NU/J, all commercially available from JAX labs) are used for the xenotransplantation. Animals are housed and allowed ad libitum access to standard chow and water. After the acclimation period, mice are subcutaneously injected in the flank with the desired tumor cells (see below for representative tumor cell lines).
- an immune compromised strain such as NOD.CB17- r c scld /J, CBySmn.CB17-iWc scld /J, NOD.Cg- r c scld I/2
- the cohorts are dosed with the appropriate test compound or control vehicle by using either the oral or i.p. route of administration.
- the formulation can be a mixture of tween, peceol and PEG400 in water.
- the formulation can be DMSO, N- methylpyrrolidone or 40% captisol solution in water.
- the dose can range from 0.05 mmol/kg to 0.5 mmol/kg, depending on the test compound.
- a dose of 0.15-0.2 mmol/kg i.p. is typically used.
- Animals are administered at the indicated dose 2 x a week for a period of 3 weeks. Mice are then allowed to grow for one week without the drug treatment. Tumor volume is measured by digital caliper, and mice are weighed 3 times a week until the conclusion of the study. The results can be expressed as means ⁇ SEM. Data can be analyzed by Student's t test. Significant differences are considered to exist for those probabilities below 5% (p ⁇ 0.05).
- the following cell lines can be used for xenotransplantation using the above general protocol: PC3 (prostate), DU145 (prostate), LNCaP (prostate), MCF7 (breast), MDA- MB-231 (breast), T-47D (breast), HT-29 (colon), HCT 116 (colon), SK-OV-3 (ovary), NIH: OVCAR-3 (ovary), A549 (lung), NCI-H460 (lung), MSTO-211H (lung), Caki - 1 (kidney), Caki - 2 (kidney), A-375 (skin), SK-MEL-2 (skin), PANC-1 (pancreas), BxPC-3 (pancreas), RPM8226 (blood), HL-60 (blood).
- Group 4 breast cancer also referred to triple negative breast cancer, accounts for 15% of all breast cancers and frequently harbors defects in the DNA double-strand break repair through homologous recombination, such as BRCAl dysfunction. This type of DNA- repair defect is sensitive to PARP inhibition. Because of the presence of the omega-3 fatty acid component, fatty acid anticancer conjugates do exhibit PARP inhibition and therefore can also be evaluated in the appropriate xenograft model bearing BRCAl -deficient breast tumor.
- PD 1 and PD-L2 are important in regulating the balance between T cell activation, tolerance and immunopathology. Inhibition of PD-1 and PD-Ll via the use of monoclonal antibodies is currently being investigated as potential anticancer therapeutics. Because the simultaneous delivery of the omega-3 fatty acid into cells along with the anticancer agent, fatty acid anticancer conjugates can inhibit STAT1, STAT3 and NF-kB, which in turn, inhibit the expression of PD-Ll . Fatty acid anticancer conjugates can be evaluated in the appropriate murine pancreatic adenocarcinoma using the tumor cell line PAN02.
- the PD-Ll pathway represents a validated therapeutic target against certain cancer types.
- the compounds of this invention which display inhibitory PD-Ll activity, can also be evaluated in the xenograft mouse model using immunocompromised NOD/SCID (non-obese diabetic/severe combined immunodeficiency) mice.
- NOD/SCID non-obese diabetic/severe combined immunodeficiency mice.
- the mice can be engrafted subcutaneous ly with human cancer cell lines expressing human PD-Ll and human CD4+ and CD8+ T cells that were previously isolated from peripheral blood mononuclear cells of healthy donors and cultured to enrich for alloreactive effector T cells.
- the cancer cell lines that can be used in this type of xenograft include the human pancreatic cell line HPAC and the human melanoma cell line A375. Detailed protocols to carry out this type of xenograft studies can be found in WO 2011/066389 and in Yan et al Cancer Lett. 2013, 336, p. 253.
- naphthalen-l-yl (4-nitrophenyl) (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate as a light yellow wax.
- gemcitabine was suitably protected at the 3 ' position as the tert-butyl carbonate according to the procedures outlined in Journal of Organic Chemistry 1999, 43, p. 8319-8322.
- the resulting reaction mixture was stirred at room temperature for 16 h.
- the reaction mixture was then diluted with CH 2 CI 2 (100 mL).
- the organic layer was washed with aq.NH 4 Cl (3 x 100 mL), brine (3 x 100 mL), dried over anhydrous Na 2 S0 4 and concentrated under reduced pressure.
- the reaction mixture was diluted with DCM (100 mL). The organic layer was washed with aq.NH 4 Cl (100 mL*3) and brine (100 mL*3). The organic layer was dried over anhydrous Na 2 S0 4 and concentrated under reduced pressure.
- the resulting reaction mixture was stirred at room temperature for 18 h.
- the reaction mixture was then diluted with CH 2 C1 2 (20 mL).
- the organic layer was washed with aq.NH 4 Cl (3 x 10 mL), brine (3 x 10 mL), dried over anhydrous Na 2 S0 4 and concentrated under reduced pressure.
Abstract
The invention relates to fatty acid anticancer derivatives; compositions comprising an effective amount of a fatty acid anticancer derivative; and methods for treating or preventing cancer comprising the administration of an effective amount of a fatty acid anticancer derivative.
Description
FATTY ACID ANTICANCER DERIVATIVES AND THEIR USES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Patent Application No. 61/835,869, entitled "FATTY ACID ANTICANCER DERIVATIVES AND THEIR USES," filed on June 17, 2013, the contents of which are incorporated herein in their entirety.
FIELD OF THE INVENTION
[0002] The invention relates to fatty acid anticancer derivatives; compositions comprising an effective amount of a fatty acid anticancer derivative; and methods for treating or preventing a cancer comprising the administration of an effective amount of a fatty acid anticancer derivative. All patents, patent applications, and publications cited herein are hereby incorporated by reference in their entireties.
BACKGROUND OF THE INVENTION
[0003] Oily cold water fish, such as salmon, trout, herring, and tuna are the source of dietary marine omega-3 fatty acids, with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) being the key marine derived omega-3 fatty acids. Omega-3 fatty acids have previously been shown to improve insulin sensitivity and glucose tolerance in
normoglycemic men and in obese individuals. Omega-3 fatty acids have also been shown to improve insulin resistance in obese and non-obese patients with an inflammatory phenotype. Lipid, glucose, and insulin metabolism have been shown to improve in overweight hypertensive subjects through treatment with omega-3 fatty acids. Omega-3 fatty acids (EPA/DHA) have also been shown to decrease triglycerides and to reduce the risk for sudden death caused by cardiac arrhythmias in addition to improve mortality in patients at risk of a cardiovascular event. Omega-3 fatty acids have also been taken as part of the dietary supplement portion of therapy used to treat dyslipidemia. Last, but not least, omega-3 fatty acids have been known to have a number of anti-inflammatory properties. For instance, a higher intake of omega-3 fatty acids lower levels of circulating TNF-a and IL-6, two of the cytokines that are markedly increased during inflammation processes (Chapkin et al, Prostaglandins, Leukot Essent Fatty Acids 2009, 81, p. 187-191; Duda et al, Cardiovasc Res
2009, 84, p. 33-41). In addition, a higher intake of omega-3 fatty acids has been shown to increase levels of the well-characterized anti-inflammatory cytokine IL-10 (Bradley et al, Obesity (Silver Spring) 2008, 16, p. 938-944).
[0004] Both DHA and EPA are characterized as long chain fatty acids (aliphatic portion between 12-22 carbons). Medium chain fatty acids are characterized as those having the aliphatic portion between 6-12 carbons. Lipoic acid is a medium chain fatty acid found naturally in the body. It plays many important roles such as free radical scavenger, chelator to heavy metals and signal transduction mediator in various inflammatory and metabolic pathways, including the NF-κΒ pathway (Shay, K. P. et al. Biochim. Biophys. Acta 2009, 1790, 1149-1160). Lipoic acid has been found to be useful in a number of chronic diseases that are associated with oxidative stress (for a review see Smith, A. R. et al Curr. Med. Chem. 2004, 11, p. 1135-46). Lipoic acid has now been evaluated in the clinic for the treatment of diabetes (Morcos, M. et al Diabetes Res. Clin. Pract. 2001, 52, p. 175-183) and diabetic neuropathy (Mijnhout, G. S. et al Neth. J. Med. 2010, 110, p. 158-162). Lipoic acid has also been found to be potentially useful in treating cardiovascular diseases (Ghibu, S. et al, J. Cardiovasc. Pharmacol. 2009, 54, p. 391-8), Alzheimer's disease (Maczurek, A. et al, Adv. Drug Deliv. Rev. 2008, 60, p. 1463-70) and multiple sclerosis (Yadav, V. Multiple Sclerosis 2005, 11, p. 159-65; Salinthone, S. et al, Endocr. Metab. Immune Disord. Drug Targets 2008, S, p. 132-42).
[0005] Omega-3 fatty acids have also been shown to affect numerous biological targets that are relevant to cancer inhibition. Omega-3 fatty acid, for instance, has been shown to inhibit the expression of PD-Ll via inhibition of STATl, STAT3 and NF-κΒ (Romberg et al, J. Allergy Clin. Immunol. 2013, 132, p. 1460; Marzec et al, PNAS 2013, 105, p. 20852; Loke and Allison, PNAS 2003, 100, p. 5336). Programmed death 1 (PD 1) and its ligands (PD-Ll and PD-L2) are important in regulating the balance between T cell activation, tolerance and immunopathology. Antibodies to PD 1 and PD-Ll are currently being developed as new immunotherapies against certain types of cancer (for a review see: Keir et al Annu. Rev. Immunol. 2008, 26, p. 677). Studies have also shown that omega-3 fatty acids could reduce the proliferation of prostate and breast cancer cells through the inhibition of the sterol regulatory element binding protein 1 (SREBP 1) and SREBP 2 (Griffiths et al Cancer Metabolism 2013, 1, p. 3; Krycer et al Biochem. 2012, 446, p. 191). Furthermore, omega-3 fatty acids could inhibit the proliferation of certain cancer cell lines by pre-disposing them to apoptosis. This was achieved through the downregulation of the anti-apoptotic protein Bcl-2
and by the attenuation of the epidermal growth factor receptor (EGFR) signaling (Corsetto et al, Lipids and Health Disease 2011, 10, p. 73). The tumor suppressor PTEN (phosphatase and tensin homo log deleted on chromosome 10) plays a functional role in cell cycle arrest and apoptosis. NF-κΒ and its downstream regulators (such as VEGF) can also prevent apoptosis and further promote inflammation and tumor growth. Administration of omega-3 fatty acids has been demonstrated to decrease PTEN, PARP (Poly-ADP-ribose polymerase), NF-KB and VEGF and thereby activate apoptosis, diminish DNA damage and reduce inflammation signaling to inhibit the progression of colon cancer (Kansal et al PLOS One 2014, 9, e84627). Cancer cell growth is controlled by the coordinated activation of numerous regulatory proteins including cyclins and catalytic cyclin-dependent kinases (CDK). The cyclin D1/CDK4 complex is important in activating genes that enable the progression of the cell cycle toward mitogenesis. Oxidized metabolites of omega-3 fatty acids have recently been shown to decrease cancer cell proliferation by down-regulating the cyclin D1/CDK4 pathway (Cui et al, Brit. J.Pharm. 2011, 162, p. l 143). A covalent conjugate of omega-3 fatty acid with an anticancer agent allows the delivery of both of these components simultaneously to an intracellular component with matched kinetic. Because of the ability of omega-3 fatty acids to impact multiple pathways that are critical for the proliferation of cancer cells, a covalent conjugate of omega-3 fatty acid with an anticancer agent is expected to have synergistic activity that cannot be reproduced by the individual components or a combination of the individual components (i.e. omega-3 fatty acid and anticancer agent). Non-limiting examples of an anticancer agent that can be used in a covalent conjugate with an omega-3 fatty acid include a cytotoxic agent, a nucleoside agent, a DNA intercalator, a proteasome inhibitor, a microtubule-targeting agent, an agent that causes crosslinking of DNA, an apoptotic agent, a PARP inhibitor, a histone deacetylase inhibitor, a topoisomerase inhibitor, a heat shock protein inhibitor, a histone methyltransferase inhibitor, a matrix metalloprotease inhibitor, an isocitrate dehydrogenase 1 or 2 (IDH 1 or IDH 2) inhibitor, an indoleamine-2,3- dioxygenase inhibitor (IDO), an inhibitor of the nuclear export protein Exportin 1 (XPO 1), a protein tyrosine kinase inhibitor or protein serine/threonine kinase inhibitor.
[0006] In recent years, certain protein tyrosine kinases or protein serine/threonine kinases have emerged as important therapeutic targets to treat a variety of cancers. For instance, the mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that can act as a master switch for cellular anabolic and catabolic processes, which in turn regulates the rate of cell growth and proliferation. Dysregulation of mTOR signaling pathway occurs frequently
in a variety of human tumors. Rapamycin and a number of rapamycin analogs are some mTOR inhibitors that have shown effectiveness as anticancer agents. (Laplante et al Cell 2012, 149, p. 274-293). Signaling along the transforming growth factor β (TGF ) and Ras- mytogen activated protein kinase (Ras-MAPK) pathways are critical for cell development and cell cycle regulation, as well as in tumor formation and metastasis. In the absence of cellular transformation, these two pathways operate in opposition to one another. However, in colorectal and pancreatic cancers, TGF and Ras-MAPK are simultaneously activated to further promote cancer progression and metastasis (Chapnick et al Cell & Bioscience 2011, 1, p. 42). Inhibition of the epidermal growth factor receptor (EGFR) has become an important target in the treatment of advanced non-small cell lung cancer (Wang et al
Therapeutic Advances in Medical Oncology 2012, 4, p. 19-29; Han et al Cancer Letters 2012, 318, p. 124-134). Gastric cancer is a highly lethal malignancy, with a low 5-year survival rate. Aberration activation of the protein kinase B (AKT) has now been shown to be one of the most common molecular findings in gastric cancer. Therefore, agents designed to specifically target AKT are currently being developed for the treatment of gastric cancer (Almhanna et al Anticancer Research 2011, 31, p. 4387-4392). The extracellular signal- regulated kinase 1 (ERKl) / 2 mytogen-activated protein (MAP) kinase module is yet another signaling pathway that has been shown to have a major role in the control of cell
proliferation, survival and differentiation. Upon engagement of growth factor receptors, this pathway is turned on, which in turn, leads to activation of Ras and to the sequential phosphorylation/activation of Raf, MEK1/MEK2 and ERK1/ERK2 protein kinases. It is believed that activation along this pathway can contribute to the increased motility, invasiveness and dissemination of tumor cells (Voisin et al Cancer Metastasis 2010, 15, p. 25-40). The cyclin-dependent kinases (CDKs) have also been known as important regulators of cell growth and proliferation. Impaired regulation of their activity can lead diseases such as heart hypertrophy, chronic inflammation and even cancer (Idowu, M. Biotechnology & Biotechnology Equipment 2011, 25, p. 2583-2586). Aurora kinases can play an important role in the control of cell cycle and have been implicated in acute myeloid leukemia, chronic myeloid leukemia, acute lymphoblastic leukemia, multiple myeloma, aggressive non- Hodgkin lymphoma and Hodgkin lymphoma (Farag, S. British Journal of Haematology
2011, 155, p. 561-579). The adenosine monophosphate activated protein kinase (AMPK) is an evolutionarily conserved fuel-sensing enzyme that has been shown to have a role in linking metabolic syndrome and cancer. Activation of AMPK not only affects
gastrointestinal cancer cell growth and proliferation, but also promotes cancer cell cycle arrest and apoptosis of cancer cells via activation of caspase-9. Activation of AMPK can potentially reprogram cellular metabolism and enforce metabolic checkpoints by acting on mTOR, p53, fatty synthase and other molecules for regulating cell growth and metabolism (Luo et al Future Oncology 2010, 6, p. 457-470).
[0007] In addition to the large number of tyrosine kinase inhibitors that are currently in development, there are a number of other agents that have been extensively in the clinic as anticancer agents. These include Epirubicin, Lonidamine, Pirarubicin, Idarubicin, Placlitaxel, Irinotecan, Docetaxel, Raltitrexed, Topotecan, Capecitabine, Alitretinoin, Bexarotene, Fulvestrant, Bortezomib, Pemetrexed, Ixabepilone, Pralatrexate, Eribulin, Fludarabine, Pentostatin, Cladribine, Cytarabine, Gemcitabine, Azacitidine, Nelarabine, and Decitabine. A fatty acid anticancer derivative represents a covalently linked anticancer agent and an omega-3 fatty acid such as DHA or EPA or a fatty acid that can be metabolized in vivo to an omega-3 fatty acid. A fatty acid anticancer derivative is designed to be stable in the plasma; and once inside target cells can undergo hydrolysis to release the individual components (i.e. anticancer agent and omega-3 fatty acid as defined herein). Because the anticancer agent is released only inside target cells, the fatty acid anticancer derivative exhibits less side effects than the corresponding unconjugated anticancer agents. Because the overall physical properties of the fatty acid anticancer derivatives are different than the corresponding free anticancer agents, the fatty acid anticancer derivatives can be designed to target certain cancer tissue types. Selective targeting to certain tissue types can enhance the overall efficacy, as well as reduced the side effects. Selective tissue targeting is possible since the fatty acid component can strongly bind to circulating albumin (Ren et al, J. Nanomed.
Nanotech. 2013, 4, p. 4). Albumin, in turn, is taken up more readily by a variety of tumor cells over normal healthy tissues (Ho et al, Brit. J. of Rad. 1997, 70, p. 823). Therefore, fatty acid anticancer derivatives that are described herein offer new treatment options for a variety of cancers.
SUMMARY OF THE INVENTION
[0008] The invention is based in part on the discovery of fatty acid anticancer derivatives and their demonstrated effects in achieving improved treatment that cannot be achieved by administering fatty acids or anticancer, alone, or in simple (non covalently linked)
combination. These novel compounds are useful to treat or prevent a cancer.
[0009] Accordingly in one aspect, a molecular conjugate is described which comprises an anticancer agent and a fatty acid covalently linked directly, or indirectly through a linker, wherein the linkage is through a free hydroxyl, amine, thiol, carboxylate, phosphate, or the like, on the anticancer agent and the fatty acid, wherein the fatty acid is selected from the group consisting of omega-3 fatty acids, fatty acids that are metabolized in vivo to omega-3 fatty acids, and lipoic acid, and the conjugate is stable in the plasma and capable of hydrolysis to produce free anticancer and free fatty acid, with the proviso that the molecular conjugate is not
[0010] In some embodiments, the fatty acid is selected from the group consisting of all- cz's-7,10,13-hexadecatrienoic acid, a-linolenic acid, stearidonic acid, eicosatrienoic acid,
eicosatetraenoic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid, docosahexaenoic acid (DHA), tetracosapentaenoic acid, tetracosahexaenoic acid and lipoic acid. In other embodiments, the fatty acid is selected from eicosapentaenoic acid, docosahexaenoic acid and lipoic acid. In some embodiments, the anticancer agent is selected from the group consisting of non-nucleotide anticancer agents that include, but are not limited to, Epirubicin, Lonidamine, Pirarubicin, Idarubicin, Placlitaxel, Irinotecan, Docetaxel, Raltitrexed,
Topotecan, Capecitabine, Alitretinoin, Bexarotene, Fulvestrant, Bortezomib, Pemetrexed, Ixabepilone, Pralatrexate, Eribulin, Tivantinib, Alisertib, Imatinib, Sorafenib, and Dasatinib. In some embodiments, the anticancer agent is selected from the group consisting of nucleoside anticancer agents that include, but are not limited to, Fludarabine, Pentostatin, Cladribine, Cytarabine, Gemcitabine, Azacitidine, Nelarabine, and Decitabine. In some embodiments, the nucleoside anticancer agent is selected from a group of agents in which the ribose or deoxyribose part of the nucleoside has been replaced with a different functional group. Non- limiting examples of nucleosides in which the ribose or deoxyribose moiety has been replaced with amino acids, N-vinyl-2-pyrrolidinone, dihydroxy acyclic systems, tetrahydrofuranyl, tetrahydropyranyl, butyrolactones, pyrrolidine, cyclopentanes and cyclopentenes can be found in Koomen's "Synthesis and Biological Properties of Selected Nucleoside Analogs" Recueil des Travaux Chimiques des Pay-Bas 1993, 112, p.51-65.
Additional non-limiting examples of 1-fluorocyclopent-l-ene analogs that can be used as anticancer nucleosides can be found in US 20050222185, as illustrated with RX-3117. In some embodiments, the linker comprises two amines. In other embodiments, the linker comprises a hydroxyl and an amine. In some embodiments, the linker amine is attached to a phosphate group of the anticancer agent.
[0011] In some embodiments, the hydrolysis is enzymatic. Fatty acid anticancer derivatives are inactive until they enter the cell and are hydrolyzed into the individual components to produce free anticancer agent and free fatty acid. Thus, the side effects of many anticancer agents are minimized. In some embodiments, the fatty acid anticancer derivatives are targeted preferentially to certain cancer tissues over normal healthy tissues.
In the present invention, the nucleoside anticancer agents may undergo phosphorylation in cells and targeted tissues to generate the corresponding monophosphate, diphosphate and triphosphate species. For many of these nucleoside anticancer agents, the triphosphate species is the more active metabolite. In some embodiments, the fatty acid anticancer conjugates are created by covalently joining the nucleoside moiety to the omega-3 fatty acid portion via a phosphoramidate functionality or a phosphorodiamidate functionality at the 5 '
position of the nucleoside. With this type of phosphoramidate or phosphorodiamidate functionality, enzymatic degradation in targeted tissues can generate the corresponding nucleoside monophosphate and the omega-3 fatty acid. The nucleoside monophosphate, in turn, can be phosphorylated further to the corresponding triphosphate species. In the present invention, the fatty acid portion in the phosphoramidate or phosphorodiamidate conjugate with the anticancer agent can deliver a synergistic activity that cannot be duplicated with the individual components or even with a combination of the individual components (i.e. the fatty acid and the nucleoside anticancer agent).
[0012] In one aspect, compounds of Formula I are described:
Formula I and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers, and stereoisomers thereof; wherein
Rni is a nucleoside anticancer agent;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W2 can not be O simultaneously;
W3 is each independently O or NR, each a, b, c and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
- 10-
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula I;
R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl;
each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; m is 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; ml is 0, 1, 2 or 3; k is 0, 1, 2, or 3; z is 1 , 2, or 3; each R3 is independently H or Ci-C6 alkyl, or both R3 groups, when taken together with the nitrogen to which they are attached, can form a heterocycle; each R4 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R5 is independently H, aryl, heteroaryl, heterocyclic, straight or branched C1-C10 alkyl which can be optionally substituted with one or two groups selected from halogen e, OH, NH2, C02R, CONH2, CONR2, phenyl, C6H4OH, imidazole or arginine;
each Z is independently -H,
with the proviso that there is at least one
in the compound;
each r is independently 2, 3, or 7;
each s is independently 3, 5, or 6;
each t is independently 0 or 1 ;
each v is independently 1, 2, or 6;
Ri and R2 are each independently hydrogen, deuterium, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(C C3 alkyl), -S(0)C C3 alkyl, -S(0)2Ci-C3 alkyl; and each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with OH, or halogen; provided that when m, n, o, p, and q are each 0, Wi and W2 are each null, and Z is
then t must be 0; and when m, n, o, p, and q are each 0, and Wi and W2 are each null, then Z must not be
[0013] In another aspect, compounds of Formula II are described:
Formula II and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers, and stereoisomers thereof; wherein Rn2 is independently
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W2 can not be O simultaneously;
W3 is each independently O or NR, each a, b, c and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- Cecycloalkyl)-, a heterocycle, a heteroaryl,
- 15 -
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula II;
R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl;
each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; m is 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; ml is 0, 1, 2 or 3; k is 0, 1, 2, or 3; z is 1 , 2, or 3; each R3 is independently H or Ci-C6 alkyl, or both R3 groups, when taken together with the nitrogen to which they are attached, can form a heterocycle; each R4 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R5 is independently H, aryl, heteroaryl, heterocyclic^, straight or branched Ci- C10 alkyl which can be optionally substituted with one or two groups selected from halogen OH, NH2, C02R, CONH2, CONR2, phenyl, C6H4OH, imidazole or arginine;
each Z is independently -H,
with the proviso that there is at least one
in the compound;
each r is independently 2, 3, or 7;
each s is independently 3, 5, or 6;
each t is independently 0 or 1 ;
each v is independently 1, 2, or 6;
Ri and R2 are each independently hydrogen, deuterium, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(C C3 alkyl), -S(0)C C3 alkyl, -S(0)2Ci-C3 alkyl; and each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with OH, or halogen; provided that when m, n, o, p, and q are each 0, Wi and W2 are each null, and Z is
then t must be 0; and when m, n, o, p, and q are each 0, and Wi and W2 are each null, then Z must not be
[0014] In another aspect, compounds of Formula III are described:
Formula III and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers, and stereoisomers thereof; wherein
Rn3 is an anticancer agent;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W2 can not be O simultaneously; each a, b, c and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- Cecycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula III;
R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C4 alkyl, -C C3 alkene, -C C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl;
each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; m is 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; ml is 0, 1, 2 or 3; j is 0 or 1 ; k is 0, 1, 2, or 3; z is 1 , 2, or 3; each R3 is independently H or Ci-C6 alkyl, or both R3 groups, when taken together with the nitrogen to which they are attached, can form a heterocycle; each R4 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine;
each e is independently H or any one of the side chains of the naturally occurring amino acids;
each Z is independently -H,
in the compound;
each r is independently 2, 3, or 7;
each s is independently 3, 5, or 6;
each t is independently 0 or 1 ; each v is independently 1, 2, or 6;
Ri and R2 are each independently hydrogen, deuterium, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)C C4 alkyl, -C C3 alkene, -C C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl; and
each R is independently -H, -Ci-C3 alkyl, phenyl or straight or branched C1-C4 alkyl optionally substituted with OH, or halogen; provided that when m, n, o, p, and q are each 0, Wi and W2 are each null, and Z is
then t must be 0; and when m, n, o, p, and q are each 0, and Wi and W2 are each null, then Z must not be
(2aR,4S,4aS,6R,9S,l lS,12S,12aR,12bS)-9-(((2R,3S)-3-benzamido-2- ((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenoyloxy)-3-phenylpropanoyl)oxy)- 12-(benzoyloxy)-4, 11 -dihydroxy-4a,8 , 13,13 -tetramethyl-5 -oxo-
2a,3,4,4a,5,6,9,10,l l,12,12a,12b-dodecahydro-lH-7,l l-methanocyclodeca[3,4]benzo[l,2- b]oxete-6,12b-diyl diacetate;
l-(chloromethyl)-3-(5-(5-fluoro-lH-indole-2-carboxamido)-lH-indole-2-carbonyl)-2,3- dihydro- 1 H-benzo[e]indol-5-yl 4-(4-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)benzyl)piperazine- 1 -carboxylate;
(S)-9-((dimethylamino)methyl)- 1 -ethyl- 1 -hydroxy-2,5-dioxo- 1 ,2,3 ,4,5,7- hexahydrobenzo[5 ,6]isoindolo[2, 1 -b]isoquinolin- 10-yl 4-(4-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)- docosa-4,7, 10,13,16,19-hexaenamido)benzyl)piperazine- 1 -carboxylate;
(S)-l ,9-diethyl-l -hydroxy-2,5-dioxo-l ,2,3,4,5, 7-hexahydrobenzo[5, 6]isoindolo[2, 1- b]isoquinolin- 10-yl 4-(4-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)benzyl)piperazine- 1 -carboxylate;
(S)- 1 -ethyl- 1 -hydroxy-2,5-dioxo- 1 ,2,3 ,4,5 ,7-hexahydrobenzo[5 ,6]isoindolo[2, 1 - b]isoquinolin- 10-yl 4-(4-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)benzyl)piperazine- 1 -carboxylate;
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Ζ,ΝΈ)-Ν*-( 1 -((2S,4S)-4-(((2R,4S,5 S,6S)-4-amino-5-hydroxy-6- methyltetrahydro-2H-pyran-2-yl)oxy)-2,5 , 12-trihydroxy-7-methoxy-6, 11 -dioxo- 1,2,3,4,6,11- hexahydrotetracen-2-yl)-2-hydroxyethylidene)docosa-4,7, 10,13,16,19-hexaenehydrazide;
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-((S)- 1 -(((S)- 1 -(((2S,3 S,4S,6R)-3-hydroxy-2-methyl-6- (((lS,3S)-3,5,12-trihydroxy-3-(2-hydroxyacetyl)-10-methoxy-6,l l-dioxo-l,2,3,4,6,l l- hexahydrotetracen- 1 -yl)oxy)tetrahydro-2H-pyran-4-yl)amino)-3 -methyl- 1 -oxobutan-2- yl)amino)- 1 -oxopropan-2-yl)docosa-4,7, 10,13,16,19-hexaenamide;
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-((S)- 1 -(((S)- 1 -(((2S,3R,4S,6R)-3 -hydroxy-2-methyl-6- (((lS,3S)-3,5,12-trihydroxy-3-(2-hydroxyacetyl)-10-methoxy-6,l l-dioxo-l,2,3,4,6,l l- hexahydrotetracen- 1 -yl)oxy)tetrahydro-2H-pyran-4-yl)amino)-3 -methyl- 1 -oxobutan-2- yl)amino)- 1 -oxopropan-2-yl)docosa-4,7, 10,13,16,19-hexaenamide.
[0015] In another aspect, fatty acid anticancer derivatives of Formula IV are described:
Formula IV and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers, and stereoisomers thereof; wherein R„4 is
wherein
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W2 can not be O simultaneously; each a, b, c and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle;
each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- Cecycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula IV;
R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl;
each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; m is 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; ml is 0, 1, 2 or 3; j is 0 or 1 ; k is 0, 1, 2, or 3; z is 1 , 2, or 3; each R3 is independently H or Ci-C6 alkyl, or both R3 groups, when taken together with the nitrogen to which they are attached, can form a heterocycle; each R4 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids;
each Z is independently -H,
in the compound;
each r is independently 2, 3, or each s is independently 3, 5, or each t is independently 0 or 1 ;
each v is independently 1, 2, or
Ri and R2 are each independently hydrogen, deuterium, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(C C3 alkyl), -S(0)C C3 alkyl, -S(0)2Ci-C3 alkyl; and each R is independently -H, -C1-C3 alkyl, phenyl or straight or branched C1-C4 alkyl optionally substituted with OH, or halogen; provided that when m, n, o, p, and q are each 0, Wi and W2 are each null, and Z is
then t must be 0; and when m, n, o, p, and q are each 0, and Wi and W2 are each null, then Z must not be
(2aR,4S,4aS,6R,9S,l lS,12S,12aR,12bS)-9-(((2R,3S)-3-benzamido-2- ((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenoyloxy)-3-phenylpropanoyl)oxy)- 12-(benzoyloxy)-4, 11 -dihydroxy-4a,8 , 13,13 -tetramethyl-5 -oxo-
2a,3,4,4a,5,6,9,10,l l ,12,12a,12b-dodecahydro-lH-7,l l-methanocyclodeca[3,4]benzo[l,2- b]oxete-6,12b-diyl diacetate;
l-(chloromethyl)-3-(5-(5-fluoro-lH-indole-2-carboxamido)-lH-indole-2-carbonyl)-2,3- dihydro- 1 H-benzo[e]indol-5-yl 4-(4-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)benzyl)piperazine- 1 -carboxylate;
(S)-9-((dimethylamino)methyl)-l-ethyl-l-hydroxy-2,5-dioxo-l, 2,3,4, 5,7- hexahydrobenzo[5 ,6]isoindolo[2, 1 -b]isoquinolin- 10-yl 4-(4-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)- docosa-4,7, 10,13,16,19-hexaenamido)benzyl)piperazine- 1 -carboxylate;
(S)-l,9-diethyl-l-hydroxy-2,5-dioxo-l,2,3,4,5,7-hexahydrobenzo[5,6]isoindolo[2,l- b]isoquinolin- 10-yl 4-(4-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)benzyl)piperazine- 1 -carboxylate;
(S)- 1 -ethyl- 1 -hydroxy-2,5-dioxo- 1 ,2,3 ,4,5 ,7-hexahydrobenzo[5 ,6]isoindolo[2, 1 - b]isoquinolin- 10-yl 4-(4-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)benzyl)piperazine- 1 -carboxylate;
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Ζ,ΝΈ)-Ν*-( 1 -((2S,4S)-4-(((2R,4S,5 S,6S)-4-amino-5-hydroxy-6- methyltetrahydro-2H-pyran-2-yl)oxy)-2,5 , 12-trihydroxy-7-methoxy-6, 11 -dioxo- 1,2,3,4,6,11- hexahydrotetracen-2-yl)-2-hydroxyethylidene)docosa-4,7, 10,13,16,19-hexaenehydrazide;
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-((S)- 1 -(((S)- 1 -(((2S,3 S,4S,6R)-3-hydroxy-2-methyl-6- (((lS,3S)-3,5,12-trihydroxy-3-(2-hydroxyacetyl)-10-methoxy-6,l l-dioxo-l,2,3,4,6,l l- hexahydrotetracen- 1 -yl)oxy)tetrahydro-2H-pyran-4-yl)amino)-3 -methyl- 1 -oxobutan-2- yl)amino)- 1 -oxopropan-2-yl)docosa-4,7, 10,13,16,19-hexaenamide;
(4Z,7Z, 10Z, 13Z, 16Z, 19Z)-N-((S)- 1 -(((S)- 1 -(((2S,3R,4S,6R)-3 -hydroxy-2-methyl-6- (((lS,3S)-3,5,12-trihydroxy-3-(2-hydroxyacetyl)-10-methoxy-6,l l-dioxo-l,2,3,4,6,l l- hexahydrotetracen- 1 -yl)oxy)tetrahydro-2H-pyran-4-yl)amino)-3 -methyl- 1 -oxobutan-2- yl)amino)- 1 -oxopropan-2-yl)docosa-4,7, 10,13,16,19-hexaenamide.
[0016] In Formulae I, II, III and IV, any one or more of H may be substituted with a deuterium. It is also understood in Formulae I, II, III and IV, that a methyl substituent can be substituted with a Ci-C6 alkyl.
[0017] Also described are pharmaceutical formulations comprising at least one fatty acid anticancer derivative.
[0018] Also described herein are methods of treating a disease susceptible to treatment with a fatty acid anticancer derivative in a patient in need thereof by administering to the patient an effective amount of a fatty acid anticancer derivative.
[0019] Also described herein are methods of treating or preventing a cancer by administering to a patient in need thereof an effective amount of a fatty acid anticancer derivative.
[0020] The invention also includes pharmaceutical compositions that comprise an effective amount of a fatty acid anticancer derivative and a pharmaceutically acceptable carrier. The compositions are useful for treating or preventing a metabolic disease. The invention includes a fatty acid anticancer derivative provided as a pharmaceutically acceptable prodrug, a hydrate, a salt, such as a pharmaceutically acceptable salt, enantiomer, stereoisomer, or mixtures thereof.
[0021] The details of the invention are set forth in the accompanying description below. Although methods and materials similar or equivalent to those described herein can be used
in the practice or testing of the present invention, illustrative methods and materials are now described. Other features, objects, and advantages of the invention will be apparent from the description and from the claims. In the specification and the appended claims, the singular forms also include the plural unless the context clearly dictates otherwise. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. All patents and publications cited in this specification are incorporated herein by reference in their entireties.
BRIEF DESCRIPTION OF THE FIGURES
[0022] Figure 1A, IB and 1C. The effect of test compounds on the expression of IL-Ιβ (Figure 1A), TNF-a (Figure IB) and PD-L1 (Figure 1C) in THP-1 cells.
[0023] Figure 2A, 2B, 2C and 2D. Figure 2A shows relative PD-L1 expression in three tumor cell lines treated with test compounds. Figure 2B shows relative IL-Ιβ expression in three tumor cell lines treated with test compounds. Figure 2C shows relative Fltl expression in three tumor cell lines treated with test compounds. Figure 2D shows relative Myc expression in three tumor cell lines treated with test compounds.
[0024] Figure 3A, 3B, 3C and 3D. Figure 3A shows relative TERT expression in MiaPaCa-2 cells treated with test compounds. Figure 3B shows relative CCNDl expression in MiaPaCa-2 cells treated with test compounds. Figure 3C shows relative Bcl-2 expression in MiaPaCa-2 cells treated with test compounds. Figure 3D shows relative Flt-1 expression in MiaPaCa-2 cells treated with test compounds.
[0025] Figure 4 A and 4B. Figure 4 A shows relative Actin protein expression in
MiaPaCa-2 cells treated with test compounds. Figure 4B shows relative cleaved PARP protein in MiaPaCa-2 cells treated with test compounds.
DETAILED DESCRIPTION OF THE INVENTION
[0026] The fatty acid anticancer derivatives have been designed to bring together at least one fatty acid and an anticancer agent into a single molecular conjugate. The activity of the fatty acid anticancer derivatives is greater than the sum of the individual components of the molecular conjugate, suggesting that the activity induced by the fatty acid derivative is synergistic.
DEFINITIONS
[0027] The following definitions are used in connection with the fatty acid anticancer derivatives:
[0028] The term "fatty acid anticancer derivatives" includes any and all possible isomers, stereoisomers, enantiomers, diastereomers, tautomers, pharmaceutically acceptable salts, hydrates, solvates, and prodrugs of the fatty acid anticancer derivatives described herein.
[0029] The articles "a" and "an" are used in this disclosure to refer to one or more than one (i.e., to at least one) of the grammatical object of the article. By way of example, "an element" means one element or more than one element.
[0030] The term "and/or" is used in this disclosure to mean either "and" or "or" unless indicated otherwise.
[0031] Unless otherwise specifically defined, the term "aryl" refers to cyclic, aromatic hydrocarbon groups that have 1 to 2 aromatic rings, including monocyclic or bicyclic groups such as phenyl, biphenyl or naphthyl. Where containing two aromatic rings (bicyclic, etc.), the aromatic rings of the aryl group may be joined at a single point (e.g., biphenyl), or fused (e.g., naphthyl). The aryl group may be optionally substituted by one or more substituents, e.g., 1 to 5 substituents, at any point of attachment. The substituents can themselves be optionally substituted.
[0032] "C1-C3 alkyl" refers to a straight or branched chain saturated hydrocarbon containing 1-3 carbon atoms. Examples of a C1-C3 alkyl group include, but are not limited to, methyl, ethyl, propyl and isopropyl.
[0033] "C1-C4 alkyl" refers to a straight or branched chain saturated hydrocarbon containing 1-4 carbon atoms. Examples of a C1-C4 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, isopropyl, isobutyl, sec-butyl and tert-butyl.
[0034] "C1-C5 alkyl" refers to a straight or branched chain saturated hydrocarbon containing 1-5 carbon atoms. Examples of a C1-C5 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, pentyl, isopropyl, isobutyl, sec-butyl and tert-butyl, isopentyl and neopentyl.
[0035] "Ci-C6 alkyl" refers to a straight or branched chain saturated hydrocarbon containing 1-6 carbon atoms. Examples of a Ci-C6 alkyl group include, but are not limited to, methyl, ethyl, propyl, butyl, pentyl, hexyl, isopropyl, isobutyl, sec-butyl, tert-butyl, isopentyl, and neopentyl.
[0036] The term "cycloalkyl" refers to a cyclic hydrocarbon containing 3-6 carbon atoms. Examples of a cycloalkyl group include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. It is understood that any of the substitutable hydrogens on a cycloalkyl can be substituted with halogen, C1-C3 alkyl, hydroxyl, alkoxy and cyano groups.
[0037] The term "heterocycle" as used herein refers to a cyclic hydrocarbon containing 3- 6 atoms wherein at least one of the atoms is an O, N, or S. Examples of heterocycles include, but are not limited to, aziridine, oxirane, thiirane, azetidine, oxetane, thietane, pyrrolidine, tetrahydrofuran, tetrahydrothiophene, piperidine, tetrahydropyran, thiane, imidazolidine, oxazolidine, thiazolidine, dioxolane, dithiolane, piperazine, oxazine, dithiane, and dioxane.
[0038] The term "heteroaryl" as used herein refers to a monocyclic or bicyclic ring structure having 5 to 12 ring atoms wherein one or more of the ring atoms is a heteroatom, e.g. N, O or S and wherein one or more rings of the bicyclic ring structure is aromatic. Some examples of heteroaryl are pyridyl, furyl, pyrrolyl, thienyl, thiazolyl, oxazolyl, imidazolyl, indolyl, tetrazolyl, benzofuryl, xanthenes and dihydroindole. It is understood that any of the substitutable hydrogens on a heteroaryl can be substituted with halogen, C1-C3 alkyl, hydroxyl, alkoxy and cyano groups.
[0039] The term "any one of the side chains of the naturally occurring amino acids" as used herein means a side chain of any one of the following amino acids: Isoleucine, Alanine, Leucine, Asparagine, Lysine, Aspartate, Methionine, Cysteine, Phenylalanine, Glutamate, Threonine, Glutamine, Tryptophan, Glycine, Valine, Proline, Arginine, Serine, Histidine, and Tyrosine.
[0040] The term "fatty acid" as used herein means an omega-3 fatty acid and fatty acids that are metabolized in vivo to omega-3 fatty acids. Non- limiting examples of fatty acids are a//-cz's-7,10,13-hexadecatrienoic acid, a-linolenic acid (ALA or all-cis-9, 12,15- octadecatrienoic acid), stearidonic acid (STD or a/7-cz's-6,9,12,15-octadecatetraenoic acid), eicosatrienoic acid (ETE or all-cis- 11,14,17-eicosatrienoic acid), eicosatetraenoic acid (ETA or all-cis-%,11,14,17-eicosatetraenoic acid), eicosapentaenoic acid (EPA or all-cis- 5,8,11,14, 17-eicosapentaenoic acid), docosapentaenoic acid (DP A, clupanodonic acid or all- cz's-7,10,13,16,19-docosapentaenoic acid), docosahexaenoic acid (DHA or all-cis- 4,7,10,13,16,19-docosahexaenoic acid), tetracosapentaenoic acid (a/7-cz's-9,12,15,18,21- docosahexaenoic acid), or tetracosahexaenoic acid (nisinic acid or a/7-cz's-6,9,12,15,18,21- tetracosenoic acid). In addition, the term "fatty acid" can also refer to medium chain fatty acids such as lipoic acid.
[0041] The term "anticancer agent" as used herein means any of the class of compounds known as either non-nucleotide anticancer agents or nucleotide anticancer agents, and any derivatives thereof. Examples of non-nucleotide anticancer agents include, but are not limited to Epirubicin, Lonidamine, Pirarubicin, Idarubicin, Placlitaxel, Irinotecan, Docetaxel, Raltitrexed, Topotecan, Capecitabine, Alitretinoin, Bexarotene, Fulvestrant, Bortezomib, Pemetrexed, Ixabepilone, Pralatrexate, Eribulin, Tivantinib, Alisertib, Imatinib, Sorafenib, and Dasatinib. Additional non- limiting examples of an anticancer agent that can be used in a covalent conjugate with an omega-3 fatty acid include a cytotoxic agent, a DNA intercalator, a proteasome inhibitor, a microtubule-targeting agent, an agent that causes crosslinking of DNA, an apoptotic agent, a PARP inhibitor, a histone deacetylase inhibitor, a topoisomerase inhibitor, a heat shock protein inhibitor, a histone methyltransferase inhibitor, a matrix metalloprotease inhibitor, an isocitrate dehydrogenase 1 or 2 (IDH 1 or IDH 2) inhibitor, an indoleamine-2,3-dioxygenase (IDO) inhibitor, an inhibitor of the nuclear export protein Exportin 1 (XPO 1), a protein serine/threonine kinase inhibitor or a protein tyrosine kinase inhibitor. In some embodiments, the protein kinase inhibitors are selected from a class consisting of ATP-competitive tyrosine kinase inhibitors, the type I kinase inhibitors. In some embodiments, the protein kinase inhibitors are selected from a class consisting of non- ATP competitive inhibitors, the type II and type III kinase inhibitors. In some embodiments, the protein kinase inhibitors are selected from a class of irreversible kinase inhibitors. Non- limiting examples of kinases which have been found to be therapeutically relevant in the oncology field include: Aurora kinases, anaplastic lymphoma kinase (ALK), the cyclin dependent kinases (CDK 1, CDK2, CDK4, CDK5, CDK6, CDK 7), cMet, epidermal growth factor receptor (EGFR), fibroblast growth factor receptor (FGFRl , FGFR2, FGFR3, FGFR4), vascular endothelial growth factor receptor (VEGFRl, VEGFR2, VEGFR3), platelet-derived growth factor receptor (PDGFRa, PDGFR ), checkpoint kinases (Chkl, Chk2), break point cluster- Abelson (Bcr-Abl), Src protein tyrosine kinase, spleen tyrosine kinase (Syk), Rho- associated coiled-coil containing kinase (ROCK1), polo-like kinase (PLK1), keratinocyte growth factor receptor (KGFR), Bruton's tyrosine kinase (BTK), mammalian target of rapamycin (mTor), v-raf murine sarcoma viral oncogene homolog 1 (BRAF), mitogen activated protein kinase (MAPK, MEK), phosphatidylinositol-4,5-bisphosphate 3kinase (PI3K), protein kinase B (PKB, also known as Akt).. In some embodiments, the anticancer agent is selected from the group consisting of nucleoside anticancer agents that include, but are not limited to, Fludarabine, Pentostatin, Cladribine, Cytarabine, Gemcitabine,
Azacitidine, Nelarabine, and Decitabine. In some embodiments, the nucleoside anticancer agent is selected from a group of agents in which the ribose or deoxyribose part of the nucleoside has been replaced with a different functional group. Non-limiting examples of nucleosides in which the ribose or deoxyribose moiety has been replaced with amino acids, N-vinyl-2-pyrrolidinone, dihydroxy acyclic systems, tetrahydrofuranyl, tetrahydropyranyl, butyrolactones, pyrrolidine, cyclopentanes and cyclopentenes can be found in Koomen's "Synthesis and Biological Properties of Selected Nucleoside Analogs" Recueil des Travaux Chimiques des Pay-Bas 1993, 112, p.51-65. Examples of non-ribose nucleosides are Aristeromycin, Neplanocin A, Fluoroneplanocin A. Additional non- limiting examples of 1- fluorocyclopent-l-ene analogs that can be used as anticancer nucleosides can be found in US 200502221 as illustrated with RX-3117.
[0042] A "subject" is a mammal, e.g., a human, mouse, rat, guinea pig, dog, cat, horse, cow, pig, or non-human primate, such as a monkey, chimpanzee, baboon or rhesus, and the terms "subject" and "patient" are used interchangeably herein.
[0043] The invention also includes pharmaceutical compositions comprising an effective amount of a fatty acid anticancer derivative and a pharmaceutically acceptable carrier. The invention includes a fatty acid anticancer derivative provided as a pharmaceutically acceptable prodrug, hydrate, salt, such as a pharmaceutically acceptable salt, enantiomers, stereoisomers, or mixtures thereof.
[0044] Representative "pharmaceutically acceptable salts" include, e.g., water-soluble and water-insoluble salts, such as the acetate, amsonate (4,4-diaminostilbene-2, 2 - disulfonate), benzenesulfonate, benzonate, bicarbonate, bisulfate, bitartrate, borate, bromide, butyrate, calcium, calcium edetate, camsylate, carbonate, chloride, citrate, clavulariate, dihydrochloride, edetate, edisylate, estolate, esylate, fiunarate, gluceptate, gluconate, glutamate, glycollylarsanilate, hexafluorophosphate, hexylresorcinate, hydrabamine, hydrobromide, hydrochloride, hydroxynaphthoate, iodide, isothionate, lactate, lactobionate, laurate, magnesium, malate, maleate, mandelate, mesylate, methylbromide, methylnitrate, methylsulfate, mucate, napsylate, nitrate, N-methylglucamine ammonium salt, 3-hydroxy-2-
naphthoate, oleate, oxalate, palmitate, pamoate (l,l-methene-bis-2-hydroxy-3-naphthoate, einbonate), pantothenate, phosphate/diphosphate, picrate, polygalacturonate, propionate, p-toluenesulfonate, salicylate, stearate, subacetate, succinate, sulfate, sulfosalicylate, suramate, tannate, tartrate, teoclate, tosylate, triethiodide, and valerate salts.
[0045] The term "metabolic disease" as used herein refers to disorders, diseases and syndromes involving dyslipidemia, and the terms metabolic disorder, metabolic disease, and metabolic syndrome are used interchangeably herein.
[0046] An "effective amount" when used in connection with a fatty acid anticancer derivative is an amount effective for treating or preventing a metabolic disease.
[0047] The term "carrier", as used in this disclosure, encompasses carriers, excipients, and diluents and means a material, composition or vehicle, such as a liquid or solid filler, diluent, excipient, solvent or encapsulating material, involved in carrying or transporting a pharmaceutical agent from one organ, or portion of the body, to another organ, or portion of the body.
[0048] The term "treating", with regard to a subject, refers to improving at least one symptom of the subject's disorder. Treating can be curing, improving, or at least partially ameliorating the disorder.
[0049] The term "disorder" is used in this disclosure to mean, and is used interchangeably with, the terms disease, condition, or illness, unless otherwise indicated.
[0050] The term "administer", "administering", or "administration" as used in this disclosure refers to either directly administering a compound or pharmaceutically acceptable salt of the compound or a composition to a subject, or administering a prodrug derivative or analog of the compound or pharmaceutically acceptable salt of the compound or composition to the subject, which can form an equivalent amount of active compound within the subject's body.
[0051] The term "prodrug," as used in this disclosure, means a compound which is convertible in vivo by metabolic means {e.g., by hydrolysis) to a fatty acid anticancer derivative.
[0052] The following abbreviations are used herein and have the indicated definitions:
Boc and BOC are tert-butoxycarbonyl, Boc20 is di-tert-butyl dicarbonate, CDI is Ι,Γ- carbonyldiimidazole, DCC is N,N'-dicyclohexylcarbodiimide, DIEA is N,N- diisopropylethylamine, DMAP is 4-dimethylaminopyridine, DOSS is sodium dioctyl sulfosuccinate, EDC and EDO are l-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, EtOAc is ethyl acetate, h is hour, HATU is 2-(7-aza-lH-benzotriazole-l-yl)-
1,1,3,3-tetramethyluronium hexafluorophosphate, HPMC is hydroxypropyl methylcellulose, min is minutes, Pd/C is palladium on carbon, TFA is trifluoroacetic acid, TGPS is tocopherol propylene glycol succinate, THF is tetrahydrofuran, and TNF is tumor necrosis factor.
COMPOUNDS
[0053] Accordingly in one aspect, a molecular conjugate is described which comprises an anticancer agent and a fatty acid covalently linked directly, or indirectly through a linker, wherein the linkage is through a free hydroxyl, amine, thiol, carboxylate, phosphate, or the like, on the anticancer agent and the fatty acid, wherein the fatty acid is selected from the group consisting of omega-3 fatty acids, fatty acids that are metabolized in vivo to omega-3 fatty acids, and lipoic acid, and the conjugate is stable in the plasma and capable of hydrolysis to produce free anticancer and free fatty acid, with the proviso that the molecular conjugate is not
[0054] In some embodiments, the anticancer agent is selected from the group consisting of non-nucleoside anticancer agents that include, but are not limited to, Epirubicin,
Lonidamine, Pirarubicin, Idarubicin, Placlitaxel, Irinotecan, Docetaxel, Raltitrexed,
Topotecan, Capecitabine, Alitretinoin, Bexarotene, Fulvestrant, Bortezomib, Pemetrexed, Ixabepilone, Pralatrexate, Eribulin, Tivantinib, Alisertib, Imatinib, Sorafenib, and Dasatinib. In some embodiments, the anticancer agent is selected from the group consisting of nucleoside anticancer agents that include, but are not limited to, Fludarabine, Pentostatin, Cladribine, Cytarabine, Gemcitabine, Azacitidine, Nelarabine, and Decitabine. In some embodiments, the nucleoside anticancer agent is selected from a group of agents in which the ribose or deoxyribose part of the nucleoside has been replaced with a different functional group. Non- limiting examples of nucleosides in which the ribose or deoxyribose moiety has been replaced with amino acids, N-vinyl-2-pyrrolidinone, dihydroxy acyclic systems, tetrahydrofuranyl, tetrahydropyranyl, butyrolactones, pyrrolidine, cyclopentanes and cyclopentenes can be found in Koomen's "Synthesis and Biological Properties of Selected Nucleoside Analogs" Recueil des Travaux Chimiques des Pay-Bas 1993, 112, p.51-65. Examples of non-ribose nucleosides are Aristeromycin, Neplanocin A, Fluoroneplanocin A. Additional non-limiting examples of 1-fluorocyclopent-l-ene analogs that can be used as anticancer n leosides can be found in US 20050222185, as illustrated with RX-3117.
[0055] In some embodiments, the fatty acid is selected from the group consisting of all- cz's-7,10,13-hexadecatrienoic acid, a-linolenic acid, stearidonic acid, eicosatrienoic acid, eicosatetraenoic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid, docosahexaenoic acid (DHA), tetracosapentaenoic acid, tetracosahexaenoic acid, and lipoic acid. In other embodiments, the fatty acid is selected from eicosapentaenoic acid and docosahexaenoic acid. In some embodiments, the hydrolysis is enzymatic.
[0056] In some embodiments, the anticancer agent is selected from the group consisting of PARP inhibitors. Non- limiting examples of PARP are listed below. Additional PARP inhibitors can also be found in the following review: Dana V. Ferraris "Evolution of Poly(ADP-ribose)polymerase (PARP-1) inhibitors. From concept to clinic" J. Med.Chem. 2010, 53, p. 4561.
PARP inhibitors:
[0057] In some embodiments, the anticancer agent is selected from the group consisting of indoleamine-2,3-dioxygenase (IDO) inhibitors. Non-limiting examples of IDO inhibitors include the following:
[0058] Additional non-limiting examples of IDO inhibitors can be found in US
20060258719, US2007023140, US 20070185165, US 20070173524, US20070105907, WO 2004/094409, US 2004005154, WO 2006/005185 and in the following references: MuUer et al Expert Opin. Thr Targets 2005, 9, p.831; Gaspari et al J Med.Chem. 2006, 49, p.684; MuUer et al, Nat. Med. 2005, 11, p. 312; Peterson et al Med. Chem. Res. 1993, 3, p. 473; Sono et al Biochemistry 1989, 28, p. 5392; and Votero et al Biotechnol. J. 2006, 1, p. 282.
[0059] In some embodiments, the present invention provides fatty acid anticancer derivatives according to Formulae I, II, III and IV:
Formula IV and pharmaceutically acceptable salts, hydrates, solvates, prodrugs, enantiomers and stereoisomers thereof; wherein
Wi, W2 , a, c, b, d, e, j, k, m, ml , n, o, p, q, L, Z, Z', r, s, t, v, z, Rnl, Rn2, Rn3, Rn4, Ri, R2, R3, R4, R and R6 are as defined above for Formula I-IV, with the proviso that there is at least one of
(2aR,4S,4aS,6R,9S,l lS,12S,12aR,12bS)-9-(((2R,3S)-3-benzamido-2- ((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenoyloxy)-3-phenylpropanoyl)oxy)- 12-(benzoyloxy)-4, 11 -dihydroxy-4a,8, 13, 13-tetramethyl-5-oxo-
2a,3 ,4,4a,5 ,6,9, 10, 11 , 12, 12a, 12b-dodecahydro- 1H-7, 1 1 -methanocyclodeca[3 ,4]benzo[ 1 ,2- b]oxete-6,12b-diyl diacetate;
l-(chloromethyl)-3-(5-(5-fluoro-lH-indole-2-carboxamido)-lH-indole-2-carbonyl)-2,3- dihydro- 1 H-benzo[e]indol-5-yl 4-(4-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16, 19- hexaenamido)benzyl)piperazine- 1 -carboxylate;
(S)-9-((dimethylamino)methyl)-l -ethyl-l-hydroxy-2,5-dioxo-l , 2,3,4,5, 7- hexahydrobenzo[5 ,6]isoindolo[2, 1 -b]isoquinolin- 10-yl 4-(4-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)- docosa-4,7, 10,13,16,19-hexaenamido)benzyl)piperazine- 1 -carboxylate;
(S)-l ,9-diethyl-l -hydroxy-2,5-dioxo-l ,2,3,4,5, 7-hexahydrobenzo[5, 6]isoindolo[2, 1- b]isoquinolin- 10-yl 4-(4-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)benzyl)piperazine- 1 -carboxylate;
(S)- 1 -ethyl- 1 -hydroxy-2,5-dioxo- 1 ,2,3 ,4,5 ,7-hexahydrobenzo[5 ,6]isoindolo[2, 1 - b]isoquinolin- 10-yl 4-(4-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)benzyl)piperazine- 1 -carboxylate;
(4Z,7Z, 10Z, 13Z, 16Z, 19Ζ,ΝΈ)-Ν*-( 1 -((2S,4S)-4-(((2R,4S,5 S,6S)-4-amino-5-hydroxy-6- methyltetrahydro-2H-pyran-2-yl)oxy)-2,5 , 12-trihydroxy-7-methoxy-6, 11 -dioxo- 1,2,3,4,6,11- hexahydrotetracen-2-yl)-2-hydroxyethylidene)docosa-4,7, 10,13,16,19-hexaenehydrazide;
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-((S)- 1 -(((S)- 1 -(((2S,3 S,4S,6R)-3-hydroxy-2-methyl-6- ((( 1 S,3 S)-3 ,5 , 12-trihydroxy-3-(2-hydroxyacetyl)- 10-methoxy-6, 11 -dioxo- 1 ,2,3 ,4,6, 11 - hexahydrotetracen- 1 -yl)oxy)tetrahydro-2H-pyran-4-yl)amino)-3 -methyl- 1 -oxobutan-2- yl)amino)- 1 -oxopropan-2-yl)docosa-4,7, 10,13,16,19-hexaenamide;
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-((S)- 1 -(((S)- 1 -(((2S,3R,4S,6R)-3 -hydroxy-2-methyl-6- ((( 1 S,3 S)-3 ,5 , 12-trihydroxy-3-(2-hydroxyacetyl)- 10-methoxy-6, 11 -dioxo- 1 ,2,3 ,4,6, 11 - hexahydrotetracen- 1 -yl)oxy)tetrahydro-2H-pyran-4-yl)amino)-3 -methyl- 1 -oxobutan-2- yl)amino)- 1 -oxopropan-2-yl)docosa-4,7, 10,13,16,19-hexaenamide.
[0060] In some embodiments, one Z is
and r is 3.
[0062] In some embodiments,
and r is 7.
[0063] In other embodiments,
and s is 3.
[0064] In some embodiments,
[0065] In some embodiments,
[0066] In some embodiments, one Z is
[0067] In other embodiments,
[0068] In some embodiments,
[0069] In some embodiments,
[0070] In some embodiments, one Z is
[0071] In other embodiments,
and t is 1.
[0074] In some embodiments, Wi is NH.
[0075] In some embodiments, W2 is NH.
[0076] In some embodiments, Wi is O.
[0077] In some embodiments, W2 is O.
[0078] In some embodiments, Wi is null.
[0079] In some embodiments, W2 is null.
[0080] In some embodiments, Wi and W2 are each NH.
[0081] In some embodiments, Wi and W2 are each null.
[0082] In some embodiments, Wi is O and W2 is NH.
[0083] In some embodiments, Wi and W2 are each NR, and R is CH3
[0084] In some embodiments, m is 0.
[0085] In other embodiments, m is 1.
[0086] In other embodiments, m is 2.
[0087] In some embodiments, L is -S- or -S-S-.
[0088] In some embodiments, L is -0-.
[0089] In some embodiments, L is -C(O)-.
[0090] In some embodiments, L is heteroaryl.
[0091] In some embodiments, L is heterocycle.
[0093] In some embodiments, L is
[0096] In some embodiments, L is
[0103] In some embodiments, L is
[0104] In other embodiments, one of n, o, p, and q is 1.
[0105] In some embodiments, two of n, o, p, and q are each 1.
[0106] In other embodiments, three of n, o, p, and q are each 1.
[0107] In some embodiments n, o, p, and q are each 1.
[0108] In some embodiments, one d is C(0)OR.
[0109] In some embodiments, r is 2 and s is 6.
[0110] In some embodiments, r is 3 and s is 5.
[0111] In some embodiments, t is 1.
[0112] In some of the foregoing embodiments, r is 2, s is 6 and t is 1.
[0113] In some of the foregoing embodiments, r is 3, s is 5 and t is 1.
[0114] In some embodiments, j is 0.
[0115] In some embodiments, j is 1.
[0116] In some embodiments, W3 is O.
[0117] In some embodiments, W3 is NH.
[0118] In some embodiments, R5 is ethyl.
[0119] In some embodiments, R5 is methyl.
[0120] In some embodiments, R5 is phenyl.
[0121] In some embodiments, R5 is naphthol.
[0122] In some embodiments, R5 is phenyl that is optionally substituted at the meta position with CONH2.
[0123] In some embodiments, R5 is e that is optionally substituted C02R wherein e is the side chain of a naturally occurring amino acid.
[
0131] In some embodiments, Rn2 is
[0132] In some embodiments, Rn2 is
[0133] In some embodiments, Rn2 is
[0134] In some embodiments, Rn2 is
[0135] In some embodiments, Rn2 is
[0139] In some embodiments, Rn4 is
[0140] In some embodiments, Rn4 is
[0141] In some embodiments, Rn4 is .
[0142] In some embodiments, Rn4 is
[0145] In some embodiments, Rn4 is
[0146] In some embodiments, Rn4 is
[0147] In some embodiments, Rn4 is
[0148] In some embodiments, Rn4 is
1.
[0151] In some embodiments, Rn4 is 1.
[0152] In some embodiments, Rn4 is 1.
[0153] In some embodiments, Rn4 is .
[0154] In some embodiments, Rn4 is and j = 1.
[0157] In some embodiments, Rn4 is
[0158] In some embodiments, Rn4 is
[0159] In some embodiments, Rn4 is
[0160] In some embodiments, Rn4 is
[0161] In some embodiments, Rn4 is
[0164] In some embodimens, Rn4 is
[0165] In some embodiments, Rn4 i
[0166] In some embodiments, Rn4 i
[0167] In some embodiments, Rn4 i
[0168] In some embodiments, Rn4 i
[0171] In some embodiments, Rn4 = 1.
[0172] In some embodiments, Rn4 is and j = 1.
[0173] In some embodiments, Rn is and j = 0.
[0174] In some embodiments, Rn is 1.
[0175] In some embodiments, Rn4 is j = 1.
[0178] In some embodiments, Rn4 is d j = 1.
[0179] In some embodiments, Rn4 d j = 0.
[0180] In some embodiments, Rn4 is .
[0181] In some embodiments, Rn4 is
[0182] In some embodiments, Rn4 is
[0185] In some embodiments, Rn4 is 1.
[0186] In some embodiments, Rn4 is and j = 1.
[0187] In some embodiments, Rn4 is
[0188] In some embodiments, Rn4 is = 1
[0191] In some embodiments, Rn4
[0192] In some embodiments, Rn4 is
[0193] In some embodiments, Rn4 is
[0194] In some embodiments, Rn4 is
[0195] In some embodiments, R„4 is
[0198] In some embodiments, Rn4 is = 1.
[0199] In some embodiments, Rn4 is j = 1.
[0200] In some embodiments, Rn4 is
[0201] In some embodiments, Rn4 is
[0202] In some embodiments, Rn4 is
[0203] In some embodiments, Rn4 is and j = 1.
[0206] In some embodiments, Rn4 = 1.
[0207] In some embodiments, Rn4 is and j = 1.
[0208] In some embodiments, Rn4 is andj = 0.
[0209] In some embodiments, Rn4 is
[0210] In some embodiments, Rn4 is 1.
[0211] In some embodiments, Rn4 is 0.
[0214] In some embodiments, Rn4 is 1.
[0215] In some embodiments, Rn4 is
[0216] In some embodiments, Rn4 is
[0217] In some embodiments, Rn4 is
[0218] In some embodiments, Rn4 is
[0219] In some embodiments, Rn4 is
[0222] In some embodiments, Rn4 is ndj = 0.
[0223] In some embodiments, Rn4 is andj
[0224] In some embodiments, Rn4 is andj
[0225] In some embodiments, Rn4 is
[0226] In some embodiments, Rn4 is
[0229] In some embodiments, R„4 is dj = 0.
[0230] In some embodiments, R„4 is andj = l.
[0231] In some embodiments, Rn4 is andj = l.
[0237] In some embodiments, Rn4 is l .
[0238] In some embodiments, Rn4 is 0.
[0239] In some embodiments, Rn4 is .
[0240] In some embodiments, Rn4 is 1.
[0241] In some embodiments, Rn4 is 0.
[0242] In some embodiments, Rn4 is
[0249] In some embodiments, Rn4 is j=l.
[0250] In some embodiments, Rn4 is j = 1.
[0254] In Formula I, II, Illand IV, any one or more of H may be substituted with a deuterium. It is also understood in Formulae I, II, III and IV, that a methyl substituent can be substituted with a Ci-C6 alkyl.
[0255] In other illustrative embodiments, compounds of Formulae I, II, III and IV are as set forth below:
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl methyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (II-l).
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl ethyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (II-2) .
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydroi i^ 2-yl)methyl phenyl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)phosphoramidate (II-3) .
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl naphthalen- 1 -yl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 1 1,14,17- pentaenamido)ethyl)phosphoramidate (Π-4).
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl (4-fluorophenyl) (2-((5Z,8Z,l lZ, 14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (II-5) .
2-yl)methyl (4-chlorophenyl) (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (II-6) .
ethyl 3-(((((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3- hydroxytetrahydrofuran-2-yl)methoxy)(2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethoxy)phosphoryl)amino)propanoate (II-8).
Compound 11-10.
Compound 11-11.
2-yl)methyl phenyl (2-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)phosphoramidate (11-13).
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydroi iran- 2-yl)methyl naphthalen- 1 -yl (2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)phosphoramidate (11-14).
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydroi iran- 2-yl)methyl (4-fluorophenyl) (2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)phosphoramidate (II- 15) .
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydroi iran- 2-yl)methyl (4-chlorophenyl) (2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)phosphoramidate (II- 16) .
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin- 1 (2H^
2-yl)methyl phenyl (2-((2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethyl)amino)-2-oxoethyl)phosphoramidate (11-17).
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydroi iran- 2-yl)methyl phenyl (2-(2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethoxy)ethyl)phosphoramidate (11-18).
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl phenyl (2-((2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)phosphoramidate (11-19).
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl phenyl (2-((2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)phosphoramidate (11-20) .
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin- 1 (2H^
2-yl)methyl phenyl (2-((2-((4Z,7Z, 10Z, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)disulfanyl)ethyl)phosphoramidate (11-21).
Compound 11-24.
ethyl (((2R,3S)-5-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-l(2H)-yl)-3- hydroxytetrahydrofuran-2-yl)methyl) (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (11-25) .
((2R,3 S)-5 -(5 -fluoro-2,4-dioxo-3 ,4-dihydropyrimidin- 1 (2H)-yl)-3 -hydroxytetrahydrofuran-2- yl)methyl phenyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (11-26) .
((2R,3S)-5-(5-fluoro-2,4-dioxo-3,4-dihydropyrimi
yl)methyl naphthalen- 1 -yl (2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8 , 11,14,17- pentaenamido)ethyl)phosphoramidate (11-27) .
ethyl (((2R,3S)-5-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-l(2H)-yl)-3- hydroxytetrahydrofuran-2-yl)methyl) (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-;
((2R,3 S)-5 -(5 -fluoro-2,4-dioxo-3 ,4-dihydropyrimidin- 1 (2H)-yl)-3 -hydroxytetrahydrofuran-2- yl)methyl phenyl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)phosphoramidate (11-30).
((2R,3 S)-5 -(5 -fluoro-2,4-dioxo-3 ,4-dihydropyrimidin- 1 (2H)-yl)-3 -hydroxytetrahydrofuran-2- yl)methyl naphthalen-l-yl (2-((2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)disulfanyl)ethyl)phosphoramidate (11-31).
((2R,3S,4R)-5-(4-amino-2-oxo-l,3,5-triazin-l(2H)-yl)-3,4-dihydroxytetrahydrofuran-2- yl)methyl ethyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (11-33) .
((2R,3S,4R)-5-(4-amino-2-oxo-l,3,5-triazin-l(2H)-yl)-3,4-dihydroxytetrahydrofuran-2- yl)methyl phenyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (11-34) .
((2R,3S,4R)-5-(4-amino-2-oxo-l,3,5-triazin-l(2H)-yl)-3,4-dihydroxytetrahydrofuran-2- yl)methyl naphthalen- 1 -yl (2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8 , 11,14,17- pentaenamido)ethyl)phosphoramidate (11-35).
((2R,3S,4R)-5-(4-amino-2-oxo-l,3,5-triazin-l(2H)-yl)-3,4-dihydroxytetrahydrofuran-2- yl)methyl ethyl (2-((2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)disulfanyl)ethyl)phosphoramidate (11-37).
((2R,3S,4R)-5-(4-amino-2 -oxo-1 , 3,5-triazin-l(2H)-yl)-3,4-dihydroxytetrahydrofuran-2- yl)methyl phenyl (2-((2-((5Z,8Z,l 1 Z, 14Z,17Z)-icosa-5, 8,1 1 ,14,17- pentaenamido)ethyl)disulfanyl)ethyl)phosphoramidate (11-38).
((2R,3S,4R)-5-(4-amino-2-oxo- 1 ,3,5-triazin- 1 (2H)-yl)-3,4-dihydroxytetrahydroftiran-2- yl)methyl naphthalen- 1 -yl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 1 1,14,17- pentaenamido)ethyl)disulfanyl)ethyl)phosphoramidate (11-39).
((2R,3R,4S)-5-(6-amino-2-chloro-9H-purin-9-yl)-4-fluoro-3-hydroxytetrahydrofuran-2- yl)methyl ethyl (2-((5Z,8Z, l lZ, 14Z, 17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (11-41).
((2R,3R,4S)-5-(6-amino-2-chloro-9H-purin-9-yl)-4-fluoro-3-hydroxytetrahydrofuran-2- yl)methyl phenyl (2-((5Z,8Z, 1 1 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)phosphoramidate (11-42) .
((2R,3R,4S)-5-(6-amino-2-chloro-9H-purin-9-yl)-4-fluoro-3-hydroxytetrahydrofuran-2- yl)methyl naphthalen- 1 -yl (2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8 , 11,14,17- pentaenamido)ethyl)phosphoramidate (11-43) .
((2R,3S,4S)-5-(6-amino-2-fluoro-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl ethyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)phosphoramidate (II- 45).
((2R,3S,4S)-5-(6-amino-2-fluoro-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl phenyl (2-((5Z,8Z,l 1Z,14Z, 17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)phosphoramidate (11-46).
((2R,3S,4S)-5-(6-amino-2-fluoro-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl naphthalen- 1 -yl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)phosphoramidate (11-47) .
((2R,3S)-5-(6-amino-2-chloro-9H-purin-9-yl)-3-hydroxytetrahydroi iran-2-yl)methyl ethyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl)phosphoramidate (11-49).
((2R,3S)-5-(6-amino-2-chloro-9H-purin-9-yl)-3-hydroxytetrahydrofuran-2-yl)methyl phenyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)phosphoramidate (11-50).
((2R,3 S)-5 -(6-amino-2-chloro-9H-purin-9-yl)-3 -hydroxytetrahydrofuran-2-yl)methyl naphthalen- 1 -yl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)phosphoramidate (11-51).
((2R,3S,4R)-5-(6-amino-8-chloro-9H-purin-9-yl)-3,4-dihydroxyte1xahydrofuran-2-yl)methyl ethyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)phosphoramidate (II- 53).
((2R,3S,4R)-5-(6-amino-8-chloro-9H-purin-9-yl)-3,4-dihydroxytetrahydroi iran-2-yl)methyl phenyl (2-((5Z,8Z,l 1Z,14Z, 17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)phosphoramidate (11-54).
((2R,3S,4R)-5-(6-amino-8-chloro-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl naphthalen- 1 -yl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)phosphoramidate (11-55) .
((2R,3 S)-5 -(4-amino-2-oxo- 1 ,3 ,5 -triazin- 1 (2H)-yl)-3 -hydroxytetrahydrofuran-2-yl)methyl ethyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)phosphoramidate (II- 57).
((2R,3 S)-5 -(4-amino-2-oxo- 1 ,3 ,5 -triazin- 1 (2H)-yl)-3 -hydroxytetrahydrofuran-2-yl)methyl phenyl (2-((5Z,8Z,l 1Z,14Z, 17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)phosphoramidate (11-58).
((2R,3 S)-5 -(4-amino-2-oxo- 1 ,3 ,5 -triazin- 1 (2H)-yl)-3 -hydroxytetrahydrofuran-2-yl)methyl naphthalen- 1 -yl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)phosphoramidate (11-59) .
((4S,5R)-3-(4-amino-2-oxopyrimidin-l(2H)-yl)-2-fluoro-4,5-dihydroxycyclopent-l-en-l- yl)methyl phenyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (11-61).
((4S,5R)-3-(4-amino-2-oxopyrimidin-l(2H)-yl)-2-fluoro-4,5-dihydroxycyclopent-l-en-l- yl)methyl naphthalen- 1 -yl (2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8 , 11,14,17- pentaenamido)ethyl)phosphoramidate (11-62) .
4-((9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[c]pyrimido[4,5-e]azepin-2-yl)amino)-
N-(2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl)-2-methoxybenzamide
4-((9-chloro-7-(2-fluoro-6-methoxyphenyl)-^
N-(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)-2-methoxybenzamide (IV-2).
4-((9-chloro-7-(2-fluoro-6-methoxyphenyl)-^
N-(2-(2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethoxy)ethyl)-2- methoxybenzamide (IV-3).
4-((9-chloro-7-(2-fluoro-6-methoxyphenyl)-^
N-(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenamido)ethyl)disulfanyl)ethyl)-2- methoxybenzamide (IV-4).
4-((4-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenoyl)piperazin- 1 -yl)methyl)-N-(4- methyl-3 -((4-(pyridin-3 -y l)pyrimidin-2-yl)amino)phenyl)benzamide (IV-5) .
4-((4-(((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenoyl)glycyl)piperazin- 1 -yl)methyl)- N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)benzamide (IV-6).
2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl 4-(4-((4-methyl-3-((4- (pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)carbamoyl)benzyl)piperazine- 1 -carboxylate (IV- 7)·
2-(4-(6-((5-((2-chloro-6-methylphenyl)carbam
yl)piperazin- 1 -yl)ethyl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)carbamate (IV-8).
2-(4-(6-((5-((2-chloro-6-methylphenyl)carbamoyl)thiazol-2-yl)amino)-2-methylpyrim yl)piperazin- 1 -yl)ethyl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)carbamate (IV-9) .
2-(4-(6-((5-((2-chloro-6-methylphenyl)carbamoyl)thiazol-2-yl)amino)-2-methylpyrim yl)piperazin- 1 -yl)ethyl (2-(2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethoxy)ethyl)carbamate (IV-10).
2-(4-(6-((5-((2-chloro-6-methylphenyl)carbamoyl)thiazol-2-yl)amino)-2-methylpyrim yl)piperazin- 1 -yl)ethyl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)carbamate (IV-11).
(R)-2-((6-(benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butyl (2-((5Z,8Z,l 1Z,14Z,17Z)- icosa-5, 8,11,14, 17-pentaenamido)ethyl)carbamate (IV-12).
(R)-2-((6-(benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butyl (2-((2- ((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)carbamate (IV-13).
(R)-2-((6-(benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butyl (2-(2-
((5Z,8Z,11Z,14Z, 17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethoxy)ethyl)carbamate (IV-14).
(R)-2-((6-(benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butyl (2-((2-
((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11 , 14, 17-pentaenamido)ethyl)disulfanyl)ethyl)carbamate
(IV-15).
((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethyl)carbamate (IV-16).
2-(4-(6-((5-((2-chloro-6-methylphenyl)carbamoyl)thiazol-2-yl)amino)-2-methylpyrimidm yl)piperazin- 1 -yl)ethyl (2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)carbamate (IV-17).
(5Z,8Z,1 lZ,14Z,17Z)-N-(4-methyl-5-(2-((4-morpholinophenyl)amino)pyrimidin-4- yl)thiazol-2 -yl)icosa-5, 8,11,14, 17-pentaenamide (IV-18).
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-(4-methyl-5-(2-((4-morpholinophenyl)amino)pyrimidin-4- yl)thiazol-2-yl)docosa-4,7, 10,13,16,19-hexaenamide (IV-19).
(5Z,8Z,l lZ,14Z,17Z)-N-(2-((4-methyl-5-(2 (4-morpholinophenyl)amino)pyrimidin-4- yl)thiazol-2-yl)amino)-2-oxoethyl)icosa-5, 8,11,14, 17-pentaenamide (IV-20).
(5Z,8Z,l lZ,14Z,17Z)-N-(5-(2,6-dimorpholm^
yl)icosa-5, 8,11,14, 17-pentaenamide (IV-21).
(4Z,7Z, 10Z, 13Z, 16Z, 19Z)-N-(5-(2,6-dimorpholinopyrimidin-4-yl)-4- (trifluoromethyl)pyridin-2-yl)docosa-4,7, 10,13,16,19-hexaenamide (IV-22).
(5Z,8Z,l lZ,14Z,17Z)-N-(2-((5-(2,6-dimorpholm^
2-yl)amino)-2-oxoethyl)icosa-5, 8, 11, 14, 17-pentaenamide (IV-23).
N-(2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenamido)ethyl)-2-methyl- 1 -(2-methyl-
N-(2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethyl)-2-methyl- 1 -(2- methyl-3-(trifluoromethyl)benzyl)-6-morpholino-lH-benzo[d]imidazole-4-carbox (IV- 25).
N-(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)-2 -methyl- 1 -(2-methyl-3 -(trifluoromethyl)benzyl)- 6-morpholino-lH-benzo[d]imidazole-4-carboxamide (IV-26).
N-(2-(2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenam
(2-methyl-3-(trifluoromethyl)benz
N-(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenamido)ethyl)disulfanyl)ethyl)-2- methyl- 1 -(2-methyl-3 -(trifluoromethyl)benzyl)-6-morpholino- 1 H-benzo [d]imidazole-4- carboxamide (IV-28).
(5Z,8Z,l lZ,14Z,17Z)-N-((S)-l-((2-(3-methyl-lH-indazol-5-yl)pyridin-4-yl)oxy)-3- phenylpropan-2-yl)icosa-5, 8,11,14, 17-pentaenamide (IV-29).
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-((S)- 1 -((2-(3 -methyl- 1 H-indazol-5-yl)pyridin-4-yl)oxy)-3- phenylpropan-2-yl)docosa-4,7, 10,13,16,19-hexaenamide (IV-30).
(5Z,8Z,l lZ,14Z,17Z)-N-(2-(((S)-l-((2-(3-methyl-lH-indazol-5-yl)pyridin-4-yl)oxy)-3- phenylpropan-2-yl)amino)-2-oxoethyl)icosa-5 ,8, 11,14,17-pentaenamide (IV-31).
(5Z,8Z,l lZ,14Z,17Z)-N-(l-(4-(3-oxo-9-phenyl-2,3-dihydro-[l,2,4]triazolo[3,4- f][l,6]naphthyridin-8-yl)phenyl)cyclobutyl)icosa-5, 8, 11, 14, 17-pentaenamide (IV-32).
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-( 1 -(4-(3-oxo-9-phenyl-2,3-dihydro-[ 1 ,2,4]triazolo[3 ,4- f] [ 1 ,6]naphthyridin-8-yl)phenyl)cyclobutyl)docosa-4,7, 10,13,16,19-hexaenamide (IV-33).
(5Z,8Z,l lZ,14Z,17Z)-N-(2-oxo-2-((l-(4-(3-oxo-9-phenyl-2,3-dihydro-[l,2,4]triazolo[3,4- f] [ 1 ,6]naphthyridin-8-yl)phenyl)cyclobutyl)amino)ethyl)icosa-5 ,8, 11,14,17-pentaenamide (IV-34).
(Z)-N-(2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8 , 11,14,17-pentaenamido)ethyl)-3 -(((4-(N-methyl-2- (4-methylpiperazin-l-yl)acetamido)phenyl)amino)(phenyl)methylene)-2-oxoindoline-6- carboxamide (IV-35).
(Z)-N-(2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)ethyl)-3-(((4-(N- methyl-2-(4-methylpiperazin-l-yl)acetam
oxoindoline-6-carboxamide (IV-36).
(Z)-N-(2-((2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)-3 -(((4-(N-methyl-2-(4-methylpiperazin- 1 - yl)acetamido)phenyl)amino)(phenyl)methylene)-2-oxoindoline-6-carboxamide (IV-37).
(Z)-N-(2-(2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenamido)ethoxy)ethyl)-3-(((4- (N-methyl-2-(4-methylpiperazin-l-yl)acetamido)phenyl)amino)(phenyl)methylene)-2 oxoindoline-6-carboxamide (IV-38).
(Z)-N-(2-((2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)-3 -(((4-(N-methyl-2-(4-methylpiperazin- 1 - yl)acetamido)phenyl)amino)(phenyl)methylene)-2-oxoindoline-6-carboxamide (IV-39).
(5Z,8Z,l lZ,14Z,17Z)-N-(2-(3-(2,4-dimethyl-5-(((Z)-2-oxoindolin-3-ylidene)methyl)-lH- pyrrol-3-yl)propanamido)ethyl)icosa-5,8,l l,14,17-pentaenamide (IV-40).
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-(2-(3-(2,4-dimethyl-5-(((Z)-2-oxoindolin-3-ylidene)methyl)- 1 H-pyrrol-3-yl)propanamido)ethyl)docosa-4,7, 10,13,16,19-hexaenamide (IV-41).
(5Z,8Z,l lZ,14Z,17Z)-N-(2-((2-(3-(2,4-dimethyl-5-(((Z)-2-oxoindolin-3-ylidene)methyl)-lH- pyrrol-3 -yl)propanamido)ethyl)(methyl)amino)ethyl)icosa-5 ,8,11,14,17-pentaenamide (IV- 42).
(5Z,8Z,l lZ,14Z,17Z)-N-(2-(2-(3-(2,4-dimethyl-5-(((Z)-2-oxoindolin-3-ylidene)methyl)-lH- pyrrol-3 -yl)propanamido)ethoxy)ethyl)icosa-5 ,8 , 11,14,17-pentaenamide (IV-43) .
(4Z,7Z, 10Z, 13Z, 16Z, 19Z)-N-(2-((2-(3 -(2,4-dimethyl-5-(((Z)-2-oxoindolin-3- ylidene)methyl)- 1 H-pyrrol-3-yl)propanamido)ethyl)disulfanyl)ethyl)docosa-4,7, 10,13,16,19- hexaenamide (IV-44).
(5Z, 8Z, 11 Z, 14Z, 17Z)-N-((R)- 1 -cyclohexyl-2-((5 -( 1 -methyl- 1 H-pyrazol-4-yl)- 1 -oxo-2,6- dihydro-lH-[l,2]diazepino[4,5,6-cd]indol-8-yl)amino)-2-oxoethyl)icosa-5,8,l l,14,17- pentaenamide (IV-45).
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-((R)- 1 -cyclohexyl-2-((5-( 1 -methyl- 1 H-pyrazol-4-yl)- 1 -oxo-2,6- dihydro- 1 H-[ 1 ,2]diazepino[4,5 ,6-cd]indol-8-yl)amino)-2-oxoethyl)docosa-4,7, 10,13,16,19- hexaenamide (IV-46).
(5Z, 8Z, 11 Z, 14Z, 17Z)-N-(2-(((R)- 1 -cyclohexyl-2-((5 -( 1 -methyl- 1 H-pyrazol-4-yl)- 1 -oxo-2,6- dihydro-lH-[l,2]diazepino[4,5,6-cd]indol-8-yl)amino)-2-oxoethyl)amino)-2-oxoethyl)icosa- 5,8,11,14,17-pentaenamide (IV-47).
3-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19-hexaenamido)benzamide (IV-49).
2-(4-((S)-l-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenoyl)piperidin-3-yl)phenyl)-2H- indazole-7-carboxamide (IV-51).
2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl (S)-3-(4-(7-carbamoyl-2H- indazol-2-yl)phenyl)piperidine-l-carboxylate (IV-52).
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydroi iran- 2-yl)methyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)carbamate (IV-53).
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydroi iran- 2-yl)methyl (2-((2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)carbamate (IV-54) .
2-yl)methyl (2-(2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethoxy)ethyl)carbamate (IV-55).
((2R,3S)-5-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-l(2H)-yl)-3-hydroxytetrahydrofuran-2- yl)methyl (2-((5Z,8Z,l 1Z,14Z, 17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)carbamate (IV- 56).
((2R,3S)-5-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-l(2H)-yl)-3-hydroxytetrahydrofuran-2- yl)methyl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)carbamate (IV-57) .
((2R,3S)-5-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-l(2H)-yl)-3-hydroxytetrahydrofuran-2- yl)methyl (2-(2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethoxy)ethyl)carbamate (IV-58).
((2R,3S,4R)-5-(6-amino-8-chloro-9H-purin-9-yl)-3,4-dihydroxytetrahydroi iran-2-yl)methy (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl)carbamate (IV-59).
((2R,3S,4R)-5-(6-amino-8-chloro-9H-purin-9-yl)-3,4-dihy
(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)carbamate (IV-60) .
((2R,3S,4R)-5-(6-amino-8-chloro-9H-purin-9-yl)-3,4-dihy
(2-(2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethoxy)ethyl)carbamate (IV- 61).
((2R,3S,4R)-5-(4-amino-2-oxo-l,3,5-triazin-l(2H)-yl)-3,4-dihydroxytetrahydroi iran-2- yl)methyl (2-((5Z,8Z,l 1Z,14Z, 17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)carbamate (IV- 62).
((2R,3S,4R)-5-(4-amino-2-oxo-l,3,5-triazin-l(2H)-yl)-3,4-dihydroxytetrahydroi iran-2- yl)methyl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)carbamate (IV-63) .
((2R,3S,4R)-5-(4-amino-2-oxo-l,3,5-triazin-l(2H)-yl)-3,4-dihydroxytetrahydroi iran-2 yl)methyl (2-(2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethoxy)ethyl)carbamate (IV-64).
(R)-l-((4-((4-fluoro-2-methyl H-indol-5-yl)oxy)-5-methylpyrrolo[2,l-q[l,2,4]triazin-6- yl)oxy)propan-2-yl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)carbamate (IV-65).
(R)-l-((4-((4-fluoro-2-methyl H-indol-5-yl)oxy)-5-methylpyrrolo[2,l-q[l,2,4]triazin-6- yl)oxy)propan-2-yl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)carbamate (IV-66).
(2R,3R)-3-((2-((4-(cyclopropanesulfonimidoyl)phenyl)amino)-5-(trifluoromethyl)pyrim 4-yl)oxy)butan-2-yl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)carbamate (IV-67).
(2R,3R)-3-((2-((4-(cyclopropanesulfonimidoyl)phenyl)amino)-5-(trifluoromethyl)pyrim 4-yl)oxy)butan-2-yl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)carbamate (IV-68).
4-((9-chloro-7-(2,6-difluorophenyl)-5H-benzo[c]pyrimido[4,5-e]azepin-2-yl)amino)-N-(2- ((5Z,8Z,1 lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl)benzamide (IV-69).
4-((9-chloro-7-(2,6-difluorophenyl)-5H-benzo[c]pyrimido[4,5-e]azepin-2-yl)amino)-N-(2- ((2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)(methyl)amino)ethyl)benzamide (IV-70) .
4-((9-chloro-7-(2,6-difluorophenyl)-5H-benzo[c]pyrimido[4,5-e]azepin-2-yl)amino)-N-(2- ((2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)disulfanyl)ethyl)benzamide (IV-71).
2-(ethyl(3-((4-((3-(2-((3-fluorophenyl)amino)-2-oxoethyl)-lH-pyrazol-5- yl)amino)quinazolin-7-yl)oxy)propyl)amino)ethyl (2-((5Z,8Z, 11Z, 14Z, 17Z)- 5,8,11,14, 17-pentaenamido)ethyl)carbamate (IV- 72).
2-(ethyl(3-((4-((3-(2-((3-fluorophenyl)amino)-2-oxoethyl)-lH-pyrazol-5- yl)amino)quinazolin-7-yl)oxy)propyl)amino)ethyl (2-((2-((5Z,8Z,l lZ,14Z,17Z)-icosa- 5,8,11,14,17-pentaenamido)ethyl)disulfanyl)ethyl)carbamate (IV-73) .
3-(((3-ethyl-5-((S)-2-(2-(((2-((5Z,8Z, 11Z, 14Z, 17Z)-icosa-5,8, 11,14,17- pentaenamido)ethyl)carbamoyl)oxy)ethyl)piperidin- 1 -yl)pyrazolo[ 1 ,5-a]pyrimidin-7- yl)amino)methyl)pyridine 1 -oxide (IV- 74).
3-(((5-((S)-2-((17Z,20Z,23Z,26Z,29Z)-4,13-dioxo-3-oxa-8,9-dithia-5,12-diazadotriaconta- 17,20,23, 26,29-pentaen- 1 -yl)piperidin-l -yl)-3-ethylpyrazolo[ 1 ,5-a]pyrimidin-7- yl)amino)methyl)pyridine 1 -oxide (IV-75).
(5Z,8Z,l lZ,14Z,17Z)-N-(l-(4-((5-chloro-4 lH-indol-3-yl)pyrimidin-2-yl)amino)-3- methoxyphenyl)piperidin-4-yl)icosa-5,8,l l,14,17-pentaenamide (IV-76).
(5Z,8Z,l lZ,14Z,17Z)-N-(2-((l-(4-((5-chloro-4-(lH-indol-3-yl)pyrimidin-2-yl)amino)-3- methoxyphenyl)piperidin-4-yl)amino)-2-oxoethyl)icosa-5,8,l l,14,17-pentaenamide (IV-77).
2-(4-(l-(quinolin-6-ylmethyl)-lH-[l,2,3]triazolo[4,5-b]pyrazin-6-yl)-lH-pyrazol-l-yl)ethyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl)carbamate (IV-78).
2-(4-(l-(quinolin-6-ylmethyl)-lH-[l,2,3]triazolo[4,5-b]pyrazin-6-yl)-lH-pyrazol-l-yl)ethyl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)carbamate (IV-79).
4-(4-carbamoyl-lH-benzo[d]imidazol-2-yl)phenyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa- 5,8,11,14, 17-pentaenamido)ethyl)carbamate (IV-80).
4-(4-carbamoyl-lH-benzo[d]imidazol-2-yl)phenyl (2-((2-((5Z,8Z,l lZ,14Z,17Z)- 5,8,11,14,17-pentaenamido)ethyl)disulfanyl)ethyl)carbamate (IV-81) .
2-methyl-4-oxo-3,4-dihydroquinazolin-8-yl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)carbamate (IV-82).
2-methyl-4-oxo-3,4-dihydroquinazolin-8-yl (2-((2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)disulfanyl)ethyl)carbamate (IV-83).
-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenoyl)- 1 -methyl-D-tryptophan
(5Z,8Z,l lZ,14Z,17Z)-N-(4-(N*-(3-chloro-4-fluorophenyl)-N-hydroxycarbamimidoyl)-l,2,5- oxadiazol-3-yl)icosa-5, 8,11,14, 17-pentaenamide (IV-85).
Methods for using fatty acid anticancer derivatives
[0256] Provided in the invention is a method for treating or preventing the following cancers:
Acute Lymphoblastic Leukemia (ALL), Acute Myeloid Leukemia (AML), Juvenile
Myelomonocytic Leukemia (JMML), Myelodysplastic syndrome (MDS), , Adrenocortical Carcinoma, AIDS-Related Cancers (including Kaposi Sarcoma, Lymphoma) Anal Cancer, Appendix Cancer, Astrocytomas, Atypical Teratoid/Rhabdoid Tumor, Basal Cell Carcinoma, Bile Duct Cancer, Bladder Cancer, Bone Cancer, Osteosarcoma and Malignant Fibrous Histiocytoma, Brain Stem Glioma, Brain Tumor (including Astrocytomas, Brain and Spinal Cord Tumors, Brain Stem Glioma, Central Nervous System Atypical Teratoid/Rhabdoid Tumor, Central Nervous System Embryonal Tumors, Central Nervous System Germ Cell Tumors, Craniopharyngioma, Ependymoblastoma, Ependymoma, Medulloblastoma,
Medulloepithelioma, Pineal Parenchymal Tumors of Intermediate Differentiation,
Supratentorial Primitive Neuroectodermal Tumors and Pineoblastoma), Breast Cancer, Bronchial Tumors, Burkitt Lymphoma, Carcinoid Tumor (Including Childhood,
Gastrointestinal), Carcinoma of Unknown Primary, Cardiac (Heart) Tumors, Central Nervous System (including Atypical Teratoid/Rhabdoid Tumor, Embryonal Tumors, Germ Cell Tumor, Childhood, Lymphoma, Primary), Cervical Cancer, Childhood Cancers, Chordoma, cholangiocarcinoma (or cancer that originates in the bile ducts), biliary tract cancer
(including pancreatic cancer, gall bladder cancer, and cancer of the ampulla of Vater), Chronic Myeloproliferative Disorders, Colon Cancer, Colorectal Cancer,
Craniopharyngioma, Cutaneous T-Cell Lymphoma, Duct, Bile,Ductal Carcinoma In Situ (DCIS) Embryonal Tumors, Central Nervous System, Endometrial Cancer,
Ependymoblastoma, Ependymoma, Esophageal Cancer, Esthesioneuroblastoma, Childhood, Ewing Sarcoma Family of Tumors, Extracranial Germ Cell Tumor, Extragonadal Germ Cell Tumor, Extrahepatic Bile Duct Cancer, Eye Cancer, Gallbladder Cancer, Gastric (Stomach) Cancer, Gastrointestinal Carcinoid Tumor, Gastrointestinal Stromal Tumors (GIST), Germ Cell Tumor (including Central Nervous System, Extracranial, Extragonadal, Ovarian, Testicular), Gestational Trophoblastic Tumor, Glioma, Hairy Cell Leukemia, Head and Neck Cancer, Heart Cancer, Hepatocellular (Liver) Cancer, Histiocytosis, Langerhans Cell, Hodgkin Lymphoma, Hypopharyngeal Cancer, Intraocular Melanoma, Islet Cell Tumors, Pancreatic Neuroendocrine Tumors, Kaposi Sarcoma, Kidney, Langerhans Cell Histiocytosis, Laryngeal Cancer, Leukemia (including Acute Lymphoblastic, Acute Myeloid, Chronic Lymphocytic, Chronic Myelogenous, Hairy Cell), Lip and Oral Cavity Cancer, Liver Cancer (Primary), Lobular Carcinoma In Situ (LCIS), Lung Cancer (including Non-Small Cell, Small Cell), Lymphoma (including AIDS-Related, Burkitt - see Non-Hodgkin Lymphoma, Cutaneous T-Cell, Hodgkin, Non-Hodgkin, Primary Central Nervous System),
Macroglobulinemia, Waldenstrom, Male Breast Cancer, Malignant Fibrous Histiocytoma of Bone and Osteosarcoma, Medulloblastoma, Medulloepithelioma, Melanoma (including Childhood, Intraocular), Merkel Cell Carcinoma, Mesothelioma, Malignant,Metastatic Squamous Neck Cancer with Occult Primary, Midline Tract Carcinoma Involving NUT Gene, Mouth Cancer, Multiple Endocrine Neoplasia Syndromes, Multiple Myeloma/Plasma Cell Neoplasm, Mycosis Fungoides, Myelodysplasia Syndromes,
Myelodysplastic/Myeloproliferative Neoplasms, Chronic Myelogenous Leukemia (CML),
Acute Myeloid Leukemia (AML), Multiple Myeloma, Myeloproliferative Disorders, Nasal
Cavity and Paranasal Sinus Cancer, Nasopharyngeal Cancer, Neuroblastoma, Non-Hodgkin
Lymphoma, Non-Small Cell Lung Cancer, Oral Cancer, Oropharyngeal Cancer, Osteosarcoma and Malignant Fibrous Histiocytoma of Bone, Ovarian Cancer (including Childhood, surface epithelial, Germ Cell Tumor, stromal cell tumor and Low Malignant Potential Tumor), Pancreatic Cancer, Papillomatosis, Paraganglioma, Paranasal Sinus and Nasal Cavity Cancer, Parathyroid Cancer, Penile Cancer, Pharyngeal Cancer,
Pheochromocytoma, Pineal Parenchymal Tumors of Intermediate Differentiation,
Pineoblastoma and Supratentorial Primitive Neuroectodermal Tumors, Pituitary Tumor, Plasma Cell Neoplasm/Multiple Myeloma, Pleuropulmonary Blastoma, Pregnancy and Breast Cancer, Primary Central Nervous System (CNS) Lymphoma (including precursor T cell lymphoma, follicular lymphoma, difuse large B cell lymphoma, Mantle cell lymphoma, B cell chronic lymphoma, MALT lymphoma, Burkitt lymphoma, Mycosis fungoides, peripheral T cell lymphoma, nodular sclerosis form of Hodgkin, mixed cellularity subtype of Hodgkin lymphoma), myelofibrosis, myelodysplastic syndrome (MDS), Prostate Cancer (including acinar adenocarcinoma, ductal adenocarcinoma, transitional cell or urothelial cancer, squamous cell cancer, carcinoid, small cell cancer, sarcomas and sarcomatoid cancers), Rectal Cancer, Renal Cell (Kidney) Cancer, Renal Pelvis and Ureter, Transitional Cell Cancer, Retinoblastoma, Rhabdomyosarcoma, Salivary Gland Cancer, Sarcoma (including Ewing Sarcoma Family of Tumors, Kaposi, Osteosarcoma, Rhadomyosarcoma, Soft Tissue, Uterine), Sezary Syndrome, Skin Cancer (Including Childhood, Melanoma, Merkel Cell Carcinoma, Nonmelanoma), Small Cell Lung Cancer, Small Intestine Cancer, Soft Tissue Sarcoma, Squamous Cell Carcinoma, Squamous Neck Cancer, Stomach (Gastric) Cancer, T- Cell Lymphoma, Cutaneous - see Mycosis Fungoides and Sezary Syndrome, Testicular Cancer, Throat Cancer, Thymoma and Thymic Carcinoma, Thyroid Cancer, Transitional Cell Cancer of the Renal Pelvis and Ureter, Trophoblastic Tumor, Gestational, Unusual Cancers of Childhood, Ureter and Renal Pelvis, Transitional Cell Cancer, Urethral Cancer, Uterine Cancer, Endometrial, Uterine Sarcoma, Vaginal Cancer, Vulvar Cancer, Waldenstrom
Macroglobulinemia, Wilms Tumor.
[0257] With certain cancers, there are also multiple sub-types. For instance, certain types of breast cancer are more sensitive to hormone-based treatments; and these include the estrogen receptor positive (ER), the progesterone receptor positive (PR). The hormone receptor (HR) negative type of breast cancer, on the other, does not respond to hormone- based therapy. Breast cancers are also categorized according to their genetic makeup. HER-2 positive breast cancer is a type of breast cancer that tests positive for the human epidermal
growth factor receptor 2 (HER-2) gene. Along with the previously mentioned hormone- fueled breast cancers and the presence of HER-2, breast cancers are also divided into four different groups: Group 1 (luminal A) includes tumors that are ER and PR positive, but negative for HER-2; Group 2 (luminal B) includes tumors that are ER positive, PR negative and HER-2 positive; Group 3 (HER-2 positive) includes tumors that are ER and PR negative, but HER-2 positive; Group 4 (basal-like) includes tumors that are ER, PR and HER-2 negative. Group 4 breast cancers are also referred to as triple-negative breast cancers.
[0258] The invention also includes pharmaceutical compositions useful for treating or preventing a cancer. The compositions are suitable for internal use and comprise an effective amount of a fatty acid anticancer derivative and a pharmaceutically acceptable carrier. The fatty acid anticancer derivatives are especially useful in that they demonstrate very low peripheral toxicity or no peripheral toxicity.
[0259] In some embodiments, the subject is administered an effective amount of a fatty acid anticancer derivative.
[0260] In some embodiments, the fatty acid anticancer derivatives can each be administered in amounts that are sufficient to treat a cancer. In other embodiments, the fatty acid anticancer derivatives can each be administered in amounts that are sufficient to prevent the development of a cancer in a subject.
[0261] Administration of the fatty acid anticancer derivatives can be accomplished via any mode of administration for therapeutic agents. These modes include systemic or local administration such as oral, nasal, parenteral, transdermal, subcutaneous, vaginal, buccal, rectal or topical administration modes.
[0262] Depending on the intended mode of administration, the compositions can be in solid, semi-solid or liquid dosage form, such as, for example, injectables, tablets,
suppositories, pills, time-release capsules, elixirs, tinctures, emulsions, syrups, powders, liquids, suspensions, or the like, sometimes in unit dosages and consistent with conventional pharmaceutical practices. Likewise, they can also be administered in intravenous (both bolus and infusion), intraperitoneal, subcutaneous or intramuscular form, all using forms well known to those skilled in the pharmaceutical arts.
[0263] Illustrative pharmaceutical compositions are tablets and gelatin capsules comprising a fatty acid anticancer derivative and a pharmaceutically acceptable carrier, such as: a) a diluent, e.g., purified water, triglyceride oils, such as hydrogenated or partially hydrogenated vegetable oil, or mixtures thereof, corn oil, olive oil, sunflower oil, safflower oil, fish oils, such as EPA or DHA, or their esters or triglycerides or mixtures thereof, omega-
3 fatty acids or derivatives thereof, lactose, dextrose, sucrose, mannitol, sorbitol, cellulose, sodium, saccharin, glucose and/or glycine; b) a lubricant, e.g., silica, talcum, stearic acid, its magnesium or calcium salt, sodium oleate, sodium stearate, magnesium stearate, sodium benzoate, sodium acetate, sodium chloride and/or polyethylene glycol; for tablets also; c) a binder, e.g., magnesium aluminum silicate, starch paste, gelatin, tragacanth, methylcellulose, sodium carboxymethylcellulose, magnesium carbonate, natural sugars such as glucose or beta-lactose, corn sweeteners, natural and synthetic gums such as acacia, tragacanth or sodium alginate, waxes and/or polyvinylpyrrolidone, if desired; d) a disintegrant, e.g., starches, agar, methyl cellulose, bentonite, xanthan gum, alginic acid or its sodium salt, or effervescent mixtures; e) absorbent, colorant, flavorant and sweetener; f) an emulsifier or dispersing agent, such as Tween 80, Labrasol, HPMC, DOSS, caproyl 909, labrafac, labrafil, peceol, transcutol, capmul MCM, capmul PG-12, captex 355, gelucire, vitamin E TGPS or other acceptable emulsifier; and/or g) an agent that enhances absorption of the compound such as cyclodextrin, hydroxypropyl-cyclodextrin, PEG400, PEG200.
[0264] Liquid, particularly injectable, compositions can, for example, be prepared by dissolution, dispersion, etc. For example, the fatty acid anticancer derivative is dissolved in or mixed with a pharmaceutically acceptable solvent such as, for example, water, saline, aqueous dextrose, glycerol, ethanol, and the like, to thereby form an injectable isotonic solution or suspension. Proteins such as albumin, chylomicron particles, or serum proteins can be used to solubilize the fatty acid anticancer derivatives.
[0265] The fatty acid anticancer derivatives can be also formulated as a suppository that can be prepared from fatty emulsions or suspensions; using polyalkylene glycols such as propylene glycol, as the carrier.
[0266] The fatty acid anticancer derivatives can also be administered in the form of liposome delivery systems, such as small unilamellar vesicles, large unilamellar vesicles and multilamellar vesicles. Liposomes can be formed from a variety of phospholipids, containing cholesterol, stearylamine or phosphatidylcholines. In some embodiments, a film of lipid components is hydrated with an aqueous solution of drug to a form lipid layer encapsulating the drug, as described in United States Patent No. 5,262,564, the contents of which are herein incorporated by reference in their entirety.
[0267] Fatty acid anticancer derivatives can also be delivered by the use of monoclonal antibodies as individual carriers to which the fatty acid anticancer derivatives are coupled.
The fatty acid anticancer derivatives can also be coupled with soluble polymers as targetable drug carriers. Such polymers can include polyvinylpyrrolidone, pyran copolymer,
polyhydroxypropylmethacrylamide-phenol, polyhydroxyethylaspanamidephenol, or polyethyleneoxidepolylysine substituted with palmitoyl residues. Furthermore, the fatty acid anticancer derivatives can be coupled to a class of biodegradable polymers useful in achieving controlled release of a drug, for example, polylactic acid, polyepsilon caprolactone, polyhydroxy butyric acid, polyorthoesters, polyacetals, polydihydropyrans,
polycyanoacrylates and cross-linked or amphipathic block copolymers of hydrogels. In one embodiment, fatty acid anticancer derivatives are not covalently bound to a polymer, e.g. , a polycarboxylic acid polymer, or a polyacrylate.
[0268] Parenteral injectable administration is generally used for subcutaneous, intramuscular or intravenous injections and infusions. Injectables can be prepared in conventional forms, either as liquid solutions or suspensions or solid forms suitable for dissolving in liquid prior to injection.
[0269] Compositions can be prepared according to conventional mixing, granulating or coating methods, respectively, and the present pharmaceutical compositions can contain from about 0.1 % to about 90 %, from about 10 % to about 90 %, or from about 30 % to about 90 % of the fatty acid anticancer derivative by weight or volume.
[0270] The dosage regimen utilizing the fatty acid anticancer derivative is selected in accordance with a variety of factors including type, species, age, weight, sex and medical condition of the patient; the severity of the condition to be treated; the route of
administration; the renal or hepatic function of the patient; and the particular fatty acid anticancer derivative employed. A physician or veterinarian of ordinary skill in the art can readily determine and prescribe the effective amount of the drug required to prevent, counter or arrest the progress of the condition.
[0271] Effective dosage amounts of the present invention, when used for the indicated effects, range from about 20 mg to about 5,000 mg of the fatty acid anticancer derivative per day. Compositions for in vivo or in vitro use can contain about 20, 50, 75, 100, 150, 250, 500, 750, 1,000, 1,250, 2,500, 3,500, or 5,000 mg of the fatty acid anticancer derivative. In one embodiment, the compositions are in the form of a tablet that can be scored. Effective plasma levels of the fatty acid anticancer derivative can range from about 5 ng/mL to about 5,000 ng/mL. Appropriate dosages of the fatty acid anticancer derivatives can be determined as set forth in Goodman, L. S.; Gilman, A. The Pharmacological Basis of Therapeutics, 5th ed.; MacMillan: New York, 1975, pp. 201-226.
[0272] Fatty acid anticancer derivatives can be administered in a single daily dose, or the total daily dosage can be administered in divided doses of two, three or four times daily.
Furthermore, fatty acid anticancer derivatives can be administered in intranasal form via topical use of suitable intranasal vehicles, or via transdermal routes, using those forms of transdermal skin patches well known to those of ordinary skill in that art. To be administered in the form of a transdermal delivery system, the dosage administration can be continuous rather than intermittent throughout the dosage regimen. Other illustrative topical preparations include creams, ointments, lotions, aerosol sprays and gels, wherein the concentration of the fatty acid anticancer derivative ranges from about 0.1 % to about 15 %, w/w or w/v.
Combination therapy:
[0273] In certain cancer treatment, it is a common practice to sometimes use a
combination of two or more anticancer agents in order to achieve the most effective treatment. Non-limiting examples of anticancer agents that can be used in combination with any of the fatty acid anticancer conjugates of this invention include carboplatin, cisplatin, oxaliplatin, paclitaxel, cyclosphosphamide, nitrogen mustard, melphalan, chlorambucil, busulphan, Temozolamide, nitrosoureas, tegafur, raltitrexed, hydroxyurea, Adriamycin, Combretastatin A4, Daunomycin, Mytocin-C, Mythramycin, Abraxane, Velcade,
Procarbazine, Dacarbazine, Daunorubicin, Doxorubicin, Epirubicin, Idarubicin,
Mitoxantrone, Vinblastine, Vincristine, Vindesine, Vinorelbine, Taxol, Docetaxel,
Topotecan, Camptosar, Methotrexate, Tamoxifen, Flutamide, Dactinomycin, Bleomycin, Amsacrine, Topotecan, Etoposide, Teniposide, an antiandrogen (such as Bicalutamide, Flutamide, Nilutamide and cyproterone acetate), a Luteinizing-hormone-releasing hormone (LHRH) antagonist or LHRH agonist (such as Goserelin, Leuprolin and Buserelin), a progestogen (such as gegestrol acetate), an aromatase inhibitor (such as Anastrozole, Letrozole, Vorazole and Exemestane), an 5-a-reductase inhibitor (such as finasteride), a metalloproteinase inhibitor (such as Marimastat), a kinase inhibitor (such as Afatinib, Axitinib, Bosutinib, Crizotinib, Dasutinib, Erlotinib, Gefitinib, Ibrutinib, Imatinib, Lapatinib, Lenvatinib, Mubritinib, Nilotinib, Pazopanib, Pegaptamib, Ponatinib, Ruxolitinib, Sorafenib, Sunitinib, SU6656, Tofacitinib, Vandetanib, and Vemurafenib), a PARP inhibitor (such as iniparib, BMN-673, Olaparib, Rucaparib, Veliparib, CEP 9722 and MK 4827), an inhibitor of p53 and mouse double minute 2 homolog (MDM2) and mouse double minute 4 protein (MDM4 or MDMX) (such as the stapled peptide ATSP-7041), a monoclonal antibody (such as Trastuzumab, Urelumab, Lirlumab, Elotuzumab, Cetuximab, Rituximab, Daclizumab, Alemtuzumab, Avastin, Panitumumab, Ofatumumab, Obinutuzumab, Bevacizumab,
Panitunumab, ranibizumab and Ipilimumab), an antibody drug conjugate (such as
Moxetumomab, Brentuximab vedotin, Trastuzumab emtansine), a PD-1 antibody (such as Lambrolizumab, Nivolumab, and MEDI 4736),a PD-Ll antibody (such as MEDI 0680 and RG 7446), an antisense therapy (such as ISIS-2503, an anti-ras antisense or G3139, an anti- Bcl2 antisense), a gene therapy approach (such as the one replacing aberrant genes that include p53, BRCA1 or BRCA2, and GDEPT), and an immunotherapy approach (examples of which include ex vivo and in vivo approaches to increase the immunogenicity of patient tumor cells, transfection with cytokines such as IL-2, IL-4 or granulocyte macrophage colony stimulating factor, approaches to decrease T cell anergy, approaches using transfected immune cells such as cytokine transfected dendritic cells, approaches using cytokine transfected tumor cell lines and approaches using anti idiotypic antibodies, adoptive T-cell transfer using T cells that have been non-specifically activated or targeted to a specific antigen of interest ex vivo).
[0274] In some embodiments, the agent that can be used in combination with the compounds of the invention is itself a combination of approved anticancer drugs. Examples of commonly used combination of anticancer drugs include CVP (cyclophosphamide + vincristine + prednisone), ACVBP (doxorubicin + cyclophosphamide + vindesine + bleomycin + prednisone), CHOP (cyclophosphamide + doxorubicin + vincristine + prednisone), CNOP (cyclophosphamide + mitoxantrone + vincristine + prednisone), m- BACOD (methotrexate + bleomycin + doxorubicin + cyclophosphamide + vincristine + dexamethasone + leucovorin), MACOP-B (methotrexate + doxorubicin + cyclophosphamide + vincristine + prednisone fixed dose + bleomycin + leucovorin), ProMace CytaBOM (prednisone + doxorubicin + cyclophosphamide + etoposide + cytarabine + bleomycin + vincristine + methotrexate + leucovorin)
Methods for making the fatty acid anticancer derivatives
[0275] Examples of synthetic pathways useful for making fatty acid anticancer derivatives of Formula I-IV are set forth in the Examples below and generalized in Schemes 1-3.
Scheme 1
wherein r, and s are as defined above.
[0276] In scheme 1, compound A represents Bexarotene. To those familiar in the art, other anticancer agents with a carboxylic acid group can also be subjected to the same chemistry in order to prepare the appropriate fatty acid anticancer agents. Examples of anticancer agents that have a carboxylic acid group include, but are not limited to,
Lonidamine, Raltitrexed, Pemetrexed, and Pralatrexate. In Scheme 1, the mono-BOC protected amine of the formula C can be obtained from commercial sources or prepared according to known procedures, depending on the group X (wherein X can be -NPv4-, - NC(0)R- -0-, -S-, -CH(OH)-, -OCH2CH2O-). The mono-BOC protected amine C (wherein X = -NR4-) can be prepared according to the procedures outlined in Krapcho et al. Synthetic Commun. 1990, 20, 2559-2564. The mono-BOC protected amine C (wherein X = NC(0)R,) can be prepared according to the procedures outlined in Andruszkiewicz et al. Synthetic Commun. 2008, 38, 905-913. The mono-BOC protected amine C (wherein X = O or CH(OH)) can be prepared according to the procedures outlined in Dahan et al. J. Org. Chem. 2007, 72, 2289-2296. The mono-BOC protected amine C (wherein X = S or
OCH2CH20) can be obtained from commercial sources.
[0277] The amine derivative B is then coupled with the compound A using a coupling reagent such as DCC, CDI, EDC, or optionally with a tertiary amine base and/or catalyst, e.g., DMAP, followed by deprotection of the BOC group with acids such as TFA or HC1 in a solvent such as CH2C12 or dioxane to produce compound C. Compound C can be coupled with a fatty acid of formula D using HATU in the presence of a tertiary amine such as DIEA
to afford compounds of the formula E. To those familiar in the art, the fatty acid D can also be substituted with lipoic acid in this scheme and in the subsequent schemes.
Scheme 2
[0278] Compound A (Bexarotene) is coupled with a BOC-protected diamine of the general formula DA using either EDCI or HATU to obtain the BOC-protected amide derivative of the general formula F. After treatment with HCl in dioxane, the resulting amine can be coupled with a fatty acid of the formula E. A variety of BOC-protected diamines are commercially available. Examples of which include, but are not limited to, tert-butyl (2- aminoethyl)carbamate and tert-butyl piperazine-l-carboxylate. The following diamines can be prepared according to the rocedures outlined in the corresponding references:
DA1 DA2 DA3 DA4
diamine DAI, Stocks et al, Bioorganic and Medicinal Chemistry Letters 2010, p. 7458; diamine DA2, Fritch et al, Bioorganic and Medicinal Chemistry Letters 2010, p. 6375;
diamine DA3 and DA4, Moffat et al, J. Med. Chem. 2010, 53, p.8663-8678). To those familiar in the art, detailed procedures to prepare a variety of mono-protected diamines can
also be found in the following references: WO 2004092172, WO 2004092171, and WO 2004092173.
Scheme 3
wherein r and s are as defined above.
[0279] In scheme 3, compound H represents Dasatinib. To those familiar in the art, other anticancer agents with a free hydroxyl group can also be subjected to the same chemistry in order to prepare the appropriate fatty acid anticancer agents. Examples of anticancer agents that have a free hydroxyl group include, but are not limited to, Fludarabine, Pentostatin, Cladribine, Cytarabine, Gemcitabine, Azacidine, TAK-733 and TAK-285. In Scheme 3, the mono-BOC protected amine of the formula C can be obtained from commercial sources or prepared according to known procedures, depending on the group X (wherein X can be - NR4-, -NC(0)R- -0-, -S-, -CH(OH)-, -OCH2CH20-). The mono-BOC protected amine B (wherein X = -NR4-) can be prepared according to the procedures outlined in Krapcho et al. Synthetic Commun. 1990, 20, 2559-2564. The mono-BOC protected amine C (wherein X = NC(0)R,) can be prepared according to the procedures outlined in Andruszkiewicz et al. Synthetic Commun. 2008, 38, 905-913. The mono-BOC protected amine C (wherein X = O or CH(OH)) can be prepared according to the procedures outlined in Dahan et al. J. Org. Chem. 2007, 72, 2289-2296. The mono-BOC protected amine C (wherein X = S or
OCH2CH20) can be obtained from commercial sources.
[0280] Compound H can be reacted first with 4-nitrochloroformate, in the presence of a tertiary amine such as triethylamine, followed by the reaction with a mono-Boc protected amine of the formula B in order to obtain compounds of the formula I. The Boc protecting group can be removed by treatment with HC1, and the resulting amine can be coupled with a fatty acid of the formula D using HATU in the presence of DIEA to obtain compounds of the general formula J.
Scheme 4
wherein R, r and s are as defined above.
[0281] In Scheme 4, compound M represents gemcitabine that has been protected at the 3' position according to the procedure outlined in Guo et al J. Org. Chem. 2014, 64, p. 8319. To those familiar in the art, any other suitably protected nucleosides can be used for this coupling reaction to form the desired phosphoramidate. Such protection is necessary in order to favor the proposed reaction at the 5' position of the nucleoside. The diamine B, as described above, is coupled with the fatty acid derivative D in the presence of EDC/HOBT and a tertiary amine such as triethylamine in order to afford the amine derivative K. 4- Nitrophenyl phosphorodichloridate is reacted with the alcohol ROH in the presence of Et3N, followed by the addition of the amine derivative K in order to form the intermediate phosphoramidate L. This is then reacted with the nucleoside M, followed by treatment with acid, such as HC1 or TFA, in order to obtain the fatty acid phosphoramidate derivative N.
[0282] To those familiar in the art, the amine B used in Scheme 4 can be substituted with a diamine of the general formula DA, described previously in Scheme 2. Also, the alcohol
ROH can also be substituted with an amine of the formula RNH2. When RNH2 is 3- aminobenzamide, the phosphorodiaminate derivative that is generated is shown in formula O.
EXAMPLES
[0283] The disclosure is further illustrated by the following examples, which are not to be construed as limiting this disclosure in scope or spirit to the specific procedures herein described. It is to be understood that the examples are provided to illustrate certain embodiments and that no limitation to the scope of the disclosure is intended thereby. It is to be further understood that resort may be had to various other embodiments, modifications, and equivalents thereof which may suggest themselves to those skilled in the art without departing from the spirit of the present disclosure and/or scope of the appended claims.
Example 1
Assay for determination of antiproliferative activity of the compounds of the invention against tumor cell lines
[0284] The IC50 of fatty acid anticancer conjugates against a number of tumor cell lines were determined in an antiproliferative assay using standard protocols at Charles River Discovery Research Services. Briefly, the desired cell lines (2000-4000 cells/well, see below for list of tumor cells) were seeded in a 96-well microculture plate (Costar flat bottom # 3997) in a total volume of 100 uL/well. After 24 hours, a 2 x drug master plate in growth medium from 10 μΜ of stock drug was prepared. The fatty acid anticancer conjugates were first solubilized in protein-rich buffers as follows: The 100% Fetal Bovine Serum (FBS, Gemini Benchmark Lot # A45B00Z)) or a 10% BSA solution in PBS (Sigma # A1595) was pre-warmed to 37 °C in a water bath. The fatty acid anticancer conjugates were dissolved in ethanol with vigorous vortexing to form a 100 mM 1000 x ethanol solution. A lOx stock
solution of the fatty acid anticancer conjugates in protein buffer was then prepared by transferring 5 of the 1000 x ethanol solution into 495 mL of protein solution (either 100% FBS or 10%) BSA solution). The resulting mixture was vortexed vigorously. This 10 x (1 mM) solution was used for the assay by further diluting the stock 1 : 10 in the desired buffer and then serially diluted to the desired concentrations. For the assay, 100 of the serially diluted fatty acid anticancer conjugate was added to cells. After 72 hours, relative cell number was estimated using Promega Cell Titer Glo® assay (Promega # G7571). This was done by first bringing the Cell Titer Glo® reagents to room temperature. Next, 100 of the growth medium was removed and 50 μΐ, of Cell Titer Glo® reagent was added to each well. The plate was shaken for 10 min and then to equilibrate for 2 min before transferring to a white plate. Luminescence was read on the Tecan GNEios microplate reader.
[0285] Percent of control cell growth was calculated relative to untreated control wells. All tests were performed in quadruplicate at each concentration level for test agents. The IC50 value for the test agents was estimated by curve-fitting the data using the following four parameter logistical equation:
where B is the maximal % of control luminescence, A is the minimal % of control luminescence at the highest agent concentration, C is the IC50, and D is the slope factor. IC50 is the concentration of agent that inhibits cell growth by 50% compared to the control cells. For NF (Nullfit), a meaningful IC50 was not generated from the available data.
[0286] The following human tumor cell lines (unless otherwise indicated) from Charles River Lab were used in the assay:
For non small cell lung cancer (NSLC) A549, H460, H522, LL (mouse) For pancreatic cancer: BxPc-3, MIAPaCa-2, PANC-1, PAN02 (mouse) For prostate cancer: DU145, PC3
For breast cancer: MDA-MB-231 , MCF-7, 4T1 (mouse)
For liver cancer: HepG2
For ovarian cancer: A2780 Table 1.
NF - nullfit
Example 2
Effect of the compounds of the invention on NF-κΒ in THP cells
[0287] Inflammation is a hallmark of cancer and a driving force for tumor progression. A fatty acid anticancer conjugate can exert a significant anti-inflammatory response and therefore be of utility in the treatment of various cancers. Gemcitabine (GEM) is a nucleoside anticancer agent commonly used in treatment of certain forms of breast, colorectal and pancreatic cancer. The phosphoramidate NUC-1031 derivative (abbreviated here and in subsequent examples as NUC) represents a pro-drug form of gemcitabine (Slucarczyk et al J.
Med. Chem. 2014, 57, p. 1531). The fatty acid gemcitabine conjugates II-3 and II-4 are used in the direct comparison with gemcitabine and its phosphoramidate pro-drug form. The effects of II-3 and II-4 relative to GEM and NUC on basal NF-κΒ activity were examined first in the human monocytic l
Gemcitibine (GEM)
Phosphoramidate NUC- 1031 (NUC)
-3
Compound II-4
[0288] Briefly, cells were washed in sera free RPMI and brought up at a concentration of lmillion cells per mL before the addition of the test compounds, mixed with FBS such that, when added to the cells, a final concentration of 50 μΜ compound in 10% FBS was achieved. THP-1 cells were incubated for 6 h separately with gemcitabine (GEM), NUC- 1031 (NUC), compound II-3 or compound II-4. Total RNA was collected using RNeasy Plus Mini Kit (Qiagen # 74136) and cDNA generated using Superscript III (Invitrogen # 18080-044) with random hexamers following the manufacturer's protocol. Relative mRNA
expression levels were determined using the appropriate TaqMan probes (Applied Biosystems, using the recommended best primer pairs) with HPRT as internal control.
Figures 1A, IB and 1C summarized the effect of the test compounds in THP-1 cells. The basal expression of two classical NF-κΒ target genes, IL-Ιβ and TNF-a, are increased following a 6 hour treatment with 50 μΜ of gemcitabine (GEM) and its pro-drug form NUC (Figures 1A and IB). In sharp contrast, equivalent concentrations of II-3 and II-4, however, had little effect on basal NF-κΒ driven expression. Importantly, unlike GEM and NUC, II-4 and II-3 treatment produced an unexpected reduction in the expression of PD-L1, which is known to contain NF-κΒ binding sites in its promoter (Figure 1C).
[0289] The fatty acid anticancer conjugates of this invention allow the simultaneous delivery of an omega-3 fatty acid and anticancer agent to a cellular compartment to achieve unexpected and synergistic activity against certain biological pathways. Programmed death 1 (PD 1) and its ligands (PD-L1 and PD-L2) are important in regulating the balance between T cell activation, tolerance and immunopathology. The PD-1 and PD-L1 pathway has recently been validated clinically as an important therapeutic target against certain cancers. Cancer cells are also pro-inflammatory; and agents that can exert anti-inflammatory activity can potentially be synergistic and beneficial as anticancer agents. Gemcitabine is used here as an example of an anticancer agent that cannot inhibit the PD-L1 pathway or the inflammatory IL-Ιβ pathway as a standalone agent. On the other hand, fatty acid gemcitabine conjugates, as shown in II-3 and II-4, display unexpected inhibitory activity against both the PD-L1 and IL-Ιβ pathways.
Example 3
The synergistic effect of the compounds of the invention on the target genes PD-L1, IL-
1β, Flt-1 and Myc in three different tumor cell lines
[0290] The compounds of the invention are produced by covalently linking an anticancer agent with an omega-3 fatty acid. The resulting fatty acid anticancer conjugates demonstrated an unexpected synergistic activity that cannot be reproduced by using a combination of the individual components. In this example, three different tumor cell lines DU-145, MiaPaCa-2 and PC3 were treated for 24 h with either the control or 3.16 μΜ each of gemcitabine (GEM), its phosphoramidate prodrug NUC- 1031 (NUC), a combination of the omega-3 fatty acid EPA and gemcitabine (abbreviated as E/G), or the fatty acid gemcitabine conjugates II-3 and II-4. RNA was harvested, purified and analyzed by qRT-PCR in the same manner as
described in example 2. Figures 2A, 2B, 2C, and 2D summarize the results for the 6 different treatment groups across the three tumor cell lines.
[0291] Figures 2A and 2B show the RNA expression of PD-L1 and IL-Ιβ, respectively, across the three tumor cell lines. As with the non-adherent THP-1 cells, gemcitabine (GEM) and NUC treatment, as well as the combination of EPA and gemcitabine (E/G), increased PD-L1 and IL-Ιβ RNA expression while the fatty acid gemcitabine conjugates II-4 and II-3, at the same concentration, had a lesser effect.
[0292] One of the many effects that inflammation has on tumor development is that it drives angiogenesis. One established mechanism by which this occurs is via the NF-KB- driven expression of VEGFR1 (vascular epidermal growth factor receptor 1). Figure 2C shows the RNA expression of VEGFR1 (also known as Fltl). The MiaPaCa-2 cells were more sensitive to the induction of this gene; the GEM, the NUC, as well as the combination of EPA and gemcitabine (E/G) treatment groups all markedly increased the expression of this critical receptor. In sharp contrast, this induction was suppressed with the fatty acid gemcitabine conjugates II-4 and II-3. This suggests that not only will compounds II-4 and II-3 help prevent immune evasion by down regulation of PD-L1, but that it will also help prevent tumor vascularization. More importantly, the effect is synergistic and cannot be reproduced by using a combination of the individual components, i.e. EPA and gemcitabine.
[0293] Figure 2D summarizes the RNA expression of Myc, a target gene that is activated upon various mitogenic signaling and is capable of driving tumor cell proliferation by regulating apoptosis through the up-regulation of the anti-apoptotic protein Bcl-2. With this target gene, the tumor cell line DU-145 was most sensitive. Treatment with GEM or NUC resulted in an up-regulation of Myc. In sharp contrast, the fatty acid gemcitabine conjugates II-4 and II-3 both suppressed the up-regulation of this target gene. Again, this synergistic effect on Myc could not be reproduced by using the combination of EPA and gemcitabine (E/G treatment group).
Example 4
The synergistic effect of the compounds of the invention on the target genes TERT,
CCND1, Bcl-2 and Fltl in the MiaPaCa-2 tumor cell line.
[0294] In this assay, MiaPaCa-2 tumor cells were treated for 24 h with either the control group or a higher concentration (31.6 μΜ) of the omega-3 fatty acid EPA, gemcitabine (GEM), a combination of EPA and gemcitabine (abbreviated as E/G), the fatty acid gemcitabine conjugates II-3 and II-4. RNA was harvested, purified by analyzed by qRT-
PCR in the same manner as described in example 2. Figures 3A, 3B, 3C and 3D summarize the results of the six treatment groups in this tumor cell line.
[0295] Telomerase (TERT) expression in cancer is required for replicative immortality, and its expression is upregulated in many human cancers. An inhibition of telomerase activity in cancer cells can cause senescence and apoptosis without affecting normal human cells. CCND1 is the target gene of the cyclin Dl; amplification or overexpression of which can alter cell cycle progression and contribute to tumorgenesis. Bcl-2 is an anti-apoptotic member of the Bcl-2 family that regulates programmed cell death. Cancer cells overexpress Bcl-2 as a means to escape apoptosis. VEGFR1 (Fltl) was described earlier in example 3.
[0296] Under the test conditions, compound II-3 showed better inhibitory activity against these 4 target genes that compound II-4 (Figures 3A-3D). More importantly, the fatty acid gemcitabine conjugate II-3 showed an unexpected and synergistic activity on these 4 target genes and this effect could not be reproduced by using either the individual components (i.e. the treatment groups E, GEM) or a combination of the individual components (i.e. the treatment group E/G).
Example 5
The effect of the compounds of the invention in a Western blot assay using the
MiaPaCa-2 tumor cell line
[0297] In this Western blot assay, MiaPaCa-2 tumor cells were treated for 48 h with 31.6 μΜ of the omega-3 fatty acid EPA, gemcitabine (GEM), a combination of EPA and gemcitabine (abbreviated as E/G), or the fatty acid gemcitabine conjugates II-3 and II-4. Following this incubation period, cells were washed twice with cold PBS, and lysed in RIPA buffer with protease and phosphatase inhibitors (20 μg/mL Trypsin inhibitor, 10 μg/mL Leupeptin, 0.2 mM NaOvanadate, 5 μg/mL Pepstatin A, 10 μg/mL Aprotinin, 100 μg/mL PMSF, 0.1M NaFl). Gels were transferred using a BioRad semi-dry blot apparatus and densitometry was performed using an Odyssey Infrared Imaging system (Application Software Version 3.0.25) using standard protocols. Anti-cleaved PARP rabbit monoclonal antibody was Cell Signaling (#5625). Anti-PAR rabbit polyclonal was Trevigen (#4336- APC-050). Anti B-Actin mouse monoclonal antibody was Abeam (#Ab8226).
[0298] Figures 4 A and 4B summarize the results of the 6 different treatment groups. Keeping this concentration of the test compounds on the cells for 48 h revealed differential killing of the fatty acid gemcitabine conjugates II-4 and II-3 relative to GEM, EPA, or the
combination of GEM and EPA. This was assessed as the expression of B-Actin in equal volumes of total cell lysates. Greater cell killing was presumably achieved by a greater induction of apoptosis, an increase in caspase-3 activity and greater cleavage of PARP. The densitometry shown in Figures 4B clearly showed a greater level of apoptosis with the fatty acid gemcitabine conjugates II-4 and II-3, as indicated by a respective 5 and 7-fold increase in cleaved PARP, relative to the individual components or a combination of the individual components.
Example 6
Effects of compounds of the invention on NF-κΒ Levels in RAW 264.7 Macrophages
[0299] RAW 264.7 cells stably expressing a 3x NFkB response elemement-driven luciferase reporter were seeded into 96 well plates in sera- free medium (Optimem) 18 hours prior to compound application. Compounds of the invention were prepared by first making 100 mM stock solutions in EtOH. Stock solutions were then diluted 1 : 100 in low LPS FBS (Gemini BenchMark 100-106), mixed vigorously and allowed to incubate at room
temperature for 30 minutes. 1 :2 serial dilutions were then made in FBS supplemented with 1% EtOH, mixed vigorously, and again allowed to incubate at room temperature for 30 minutes before adding to RAW 264.7 reporter cells (final concentrations: 10% FBS, lOOuM highest compound dilution, 0.1% EtOH) for a 2 hour pretreatment prior to stimulation with LPS. Cells were then stimulated with 200 ng/ml LPS or vehicle control for 3 hours in the presence of the compounds of the invention. A set of six vehicles was left unstimulated with LPS in order to measure the assay floor. AlamarBlue viability dye (Invitrogen) was added to cells simultaneously with the delivery of LPS (final AlamarBlue concentration of 10%). After the 3 h incubation period with LPS, cell viability was measured by reading fluorescence (excitation 550 nm, emission 595 nm) with a Perkin Elmer Victor V plate reader. Then cell media was aspirated from each well. Luciferase signal was then developed by addition of the Britelite Plus reagent (Perkin Elmer). Luciferase activity was measured with the Perkin Elmer Victor V plate reader. NF-κΒ activity was expressed as a percent of the vehicle control wells (stimulated with LPS). Compounds were tested at 6 dose point titrations in triplicate to determine IC50 values. As an illustrative example, compound IV-21 was evaluated in this NF-kB reporter assay and its IC50 was determined to be 75 μΜ.
Example 7
Maximum tolerated dose (MTD) assay
[0300] The MTD assay can be performed using female Balb/c nude mice, 6-8 weeks old. Animals, in groups of 6-8, are administered with the test compound or the vehicle control group over a period of 2 weeks. With the fatty acid anticancer conjugates described in this invention, the formulation that is needed to appropriately dissolve the test compound for oral dosing can be a mixture of tween, peceol and PEG400 in water. For intraperitoneal (i.p.) administration, the test compound can be dissolved in DMSO, N-methylpyrrolidone or 40% captisol solution in water. Animals are dosed i.p. either 2 x a week or orally once a day over a period of 2 weeks. The dose to be used can range from 0.05 mmol/kg to 0.5 mmol/kg, depending on the test compound. Mice are monitored daily for body weight and clinical symptoms for 2 weeks. The results can be expressed as means ± SEM.
Example 8
In vivo xenograft models of tumor bearing mice assay
[0301] The in vivo xenograft mouse model can be performed using standard protocols that have been described in E.A. Sausville and A. M. Burger's "Contributions of Human Tumor Xenografts to Anticancer Drug Development" Cancer Res. 2006, 66, p. 3351-4.
Typically, mice from an immune compromised strain (such as NOD.CB17- r cscld/J, CBySmn.CB17-iWcscld/J, NOD.Cg- r cscldI/2rgtmlWjI/SzJ, B6A29Sl-RagltmlMom/ NU/J, all commercially available from JAX labs) are used for the xenotransplantation. Animals are housed and allowed ad libitum access to standard chow and water. After the acclimation period, mice are subcutaneously injected in the flank with the desired tumor cells (see below for representative tumor cell lines). The tumor site is palpated up to 3 times weekly until the tumor is established. Once the tumor is sufficiently large, the mice are stratified by tumor size and randomly assigned to various cohorts (n = 8). The cohorts are dosed with the appropriate test compound or control vehicle by using either the oral or i.p. route of administration. For oral dosing the formulation can be a mixture of tween, peceol and PEG400 in water. For intraperitoneal (i.p.) administration, the formulation can be DMSO, N- methylpyrrolidone or 40% captisol solution in water. The dose can range from 0.05 mmol/kg to 0.5 mmol/kg, depending on the test compound. For example, with gemcitabine or the fatty acid gemcitabine conjugates, a dose of 0.15-0.2 mmol/kg i.p. is typically used. Animals are administered at the indicated dose 2 x a week for a period of 3 weeks. Mice are then allowed
to grow for one week without the drug treatment. Tumor volume is measured by digital caliper, and mice are weighed 3 times a week until the conclusion of the study. The results can be expressed as means ± SEM. Data can be analyzed by Student's t test. Significant differences are considered to exist for those probabilities below 5% (p < 0.05).
[0302] The following cell lines can be used for xenotransplantation using the above general protocol: PC3 (prostate), DU145 (prostate), LNCaP (prostate), MCF7 (breast), MDA- MB-231 (breast), T-47D (breast), HT-29 (colon), HCT 116 (colon), SK-OV-3 (ovary), NIH: OVCAR-3 (ovary), A549 (lung), NCI-H460 (lung), MSTO-211H (lung), Caki - 1 (kidney), Caki - 2 (kidney), A-375 (skin), SK-MEL-2 (skin), PANC-1 (pancreas), BxPC-3 (pancreas), RPM8226 (blood), HL-60 (blood).
Example 9
In vivo xenograft model bearing Brc ;p53' breast tumor
[0303] Group 4 breast cancer, also referred to triple negative breast cancer, accounts for 15% of all breast cancers and frequently harbors defects in the DNA double-strand break repair through homologous recombination, such as BRCAl dysfunction. This type of DNA- repair defect is sensitive to PARP inhibition. Because of the presence of the omega-3 fatty acid component, fatty acid anticancer conjugates do exhibit PARP inhibition and therefore can also be evaluated in the appropriate xenograft model bearing BRCAl -deficient breast tumor. Protocols to carry out in vivo studies using the appropriate PARP inhibitor in combination with cisplatin or cyclophosphamide using BRCAl -deficient MX-1 xenografts have been described in Donawho et al "ABT-888, an orally active poly(ADP- ribose)polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models" Clin. Cancer Res. 2007, 13, p. 2728. An alternative xenograft study using ^ BrcaV1' ;p53~'~ breast tumor in the K14cre;BrcalF/F;p53F/F mouse has also been reported in Rottenberg et al "High sensitivity of BRCAl -deficient mammary tumors to the PARP inhibitor
AZD2281 alone and in combination with platinum drugs" PNAS 2008, 105,p.17079.
Example 10
Evaluation of fatty acid anticancer conjugates in a murine pancreatic adenocarcinoma model
[0304] Programmed death 1 (PD 1) and its ligands (PD-L1 and PD-L2) are important in regulating the balance between T cell activation, tolerance and immunopathology. Inhibition
of PD-1 and PD-Ll via the use of monoclonal antibodies is currently being investigated as potential anticancer therapeutics. Because the simultaneous delivery of the omega-3 fatty acid into cells along with the anticancer agent, fatty acid anticancer conjugates can inhibit STAT1, STAT3 and NF-kB, which in turn, inhibit the expression of PD-Ll . Fatty acid anticancer conjugates can be evaluated in the appropriate murine pancreatic adenocarcinoma using the tumor cell line PAN02. Detailed protocols for this in vivo model can be found in Nomi et al "Clinical significance and therapeutic potential of the programmed death- 1 ligand/programmed death- 1 pathway in human pancreatic cancer" Clin. Cancer Res. 2007, 75, p. 2151.
Example 11
Evaluation of fatty acid anticancer conjugates in a xenograft model using
immunocompromised NOD/SCID mice
[0305] The PD-Ll pathway represents a validated therapeutic target against certain cancer types. The compounds of this invention, which display inhibitory PD-Ll activity, can also be evaluated in the xenograft mouse model using immunocompromised NOD/SCID (non-obese diabetic/severe combined immunodeficiency) mice. Here, the mice can be engrafted subcutaneous ly with human cancer cell lines expressing human PD-Ll and human CD4+ and CD8+ T cells that were previously isolated from peripheral blood mononuclear cells of healthy donors and cultured to enrich for alloreactive effector T cells. The cancer cell lines that can be used in this type of xenograft include the human pancreatic cell line HPAC and the human melanoma cell line A375. Detailed protocols to carry out this type of xenograft studies can be found in WO 2011/066389 and in Yan et al Cancer Lett. 2013, 336, p. 253.
Compounds
[0306] The following non-limiting compound examples serve to illustrate further embodiments of the fatty acid anticancer derivatives. It is to be understood that any embodiments listed in the Examples section are embodiments of the fatty acid anticancer derivatives and, as such, are suitable for use in the methods and compositions described above.
Example 12
Preparation of ((2R,3R,5R)-5-(4-amino-2-oxopyrimidin- 1 (2H)-yl)-4,4-difluoro-3- hydroxytetrahydrofuran-2-yl)methyl naphthalen-l-yl (2-((5Z,8Z,HZ,14Z,17Z)-icosa- 5,8,1 l,14,17-pentaenamido)ethyl)phosphoramidate (II-4):
[0307] The entire reaction sequence was carried out in flame-dried flasks under an inert atmosphere of argon. A solution containing 4-nitrophenyl phosphorodichloridate (1.8 g, 7.0 mmol) in 20 mL of CH2CI2 was cooled to -78 °C. A solution containing naphthalen-l-ol (1.0 g, 7.0 mmol) and Et3N (1 mL, 7.7 mmol) in 20 mL CH2CI2 was then added dropwise at -78 °C over a period of 15 min. The resulting reaction mixture was stirred vigorously for 1 hour at -78 °C and then slowly transferred to a solution of ((5Z,8Z,11Ζ,14Ζ,17Ζ)-Ν-(2- aminoethyl)icosa-5,8,l 1,14,17-pentaenamide (2.3 g, 1.0 equivalent) in CH2C12 (20 mL) at 0 °C. Next, Et3N (2.4 mL, 2.5 equivalents) was added and the resulting reaction mixture was stirred at 0 °C for 2 h. ((5Z,8Z,1 lZ,14Z,17Z)-N-(2-Aminoethyl)icosa-5,8,l 1,14,17- pentaenamide, in turn, was prepared according to the procedures outlined in WO
2012115695. Upon completion, the crude reaction was concentrated under reduced pressure. The resulting residue was taken up in 40 mL of EtOAc and the white solids were removed by filtration. The filtrate was concentrated under reduced pressure. The resulting residue was purified by silica chromatography (0-10% gradient of MeOH in CH2CI2) to afford
naphthalen-l-yl (4-nitrophenyl) (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate as a light yellow wax. Next, gemcitabine was suitably protected at the 3 ' position as the tert-butyl carbonate according to the procedures outlined in
Journal of Organic Chemistry 1999, 43, p. 8319-8322. This material, (2R,3R,5R)-5-(4- amino-2-oxopyrimidin- 1 (2H)-yl)-4,4-difluoro-2-(hydroxymethyl)tetrahydrofuran-3-yl tert- butyl carbonate (1.5g, 4.13mmol) was stirred vigorously in 16 mL anhydrous DMF at rt. tBuMgCl (1 M in THF, 4.2 mL, 1 equivalent) was then slowly added and the resulting reaction mixture was stirred at rt for 1 h. This mixture was then slowly added to a solution containing naphthalen-l-yl (4-nitrophenyl) (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (5.54 g, 2 equivalents) in 16 mL of anhydrous DMF at rt. The resulting reaction mixture was stirred at rt for 16 h. The next day, the reaction was quenched with 5mL H20. The resulting crude mixture was extracted EtOAc. The combined organic layers were washed with brine, dried (Na2S04), and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (a mixture of CH2Cl2/MeOH containing 0.2% Et3N) to afford 628 mg of (2R,3R,5R)-5-(4-amino-2- oxopyrimidin- 1 (2H)-yl)-4,4-difluoro-2-(((((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)amino)(naphthalen- 1 -yloxy)phosphoryl)oxy)methyl)tetrahydrofuran-3 -yl tert-butyl carbonate as a light brown oil (26% yield). This material was taken up in 8 mL of a 1 : 1 mixture of TFA/CH2C12. The resulting reaction mixture was stirred at rt for 2h. The crude reaction was diluted with EtOAc and concentrated under reduced pressure. The resulting residue was taken up in EtOAc. The organic layer was washed with saturated aq NaHC03, dried (Na2S04) and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (a mixture of CH2Cl2/MeOH containing 0.2% Et3N) to afford ((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl naphthalen- 1 -yl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 1 1,14,17- pentaenamido)ethyl)phosphoramidate as a light brown wax. MS (EI) calculated for
C4iH52F2N507P: 795.36; Found: 796 [M+H]+
[0308]
Example 13
Preparation of ((2R,3R,5R)-5-(4-amino-2-oxopyrimidin- 1 (2H)-yl)-4,4-difluoro-3- hydroxytetrahydrofur an-2-yl)methyl phenyl (2-((5Z,8Z, 11Z,14Z, 17Z)-icosa- 5,8,1 l,14,17-pentaenamido)ethyl)phosphoramidate (II-3):
[0309] The same experimental procedure outlined in example 12 was used, substituting phenol instead for naphthalen-l-ol. The resulting product, namely ((2R,3R,5R)-5-(4-amino- 2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran-2-yl)methyl phenyl (2- ((5Z,8Z,11Z,14Z, 17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)phosphoramidate, was obtained after purification by silica gel chromatography. MS (EI) calculated for
C37H5oF2N507P: 745.34; Found: 746 [M+H]+
Example 14
Preparation of ((2R,3R,5R)-5-(4-amino-2-oxopyrimidin- 1 (2H)-yl)-4,4-difluoro-3- hydroxytetrahydrofuran-2-yl)methyl phenyl (2-((4Z,7Z,10Z,13Z,16Z,19Z)-docosa- 4,7,10,13, 16,19-hexaenamido)ethyl)phosphoramidate (11-13):
[0310] The same experimental procedure outlined in examples 12 and 13 was used, substituting (4Z,7Z, 10Z, 13Z, 16Z, 19Z)-N-(2-aminoethyl)docosa-4,7, 10,13,16,19- hexaenamide as the desired amine component during the generation of the phosphoramidate intermediate.
Example 15
Preparation of 4-((4-((5Z,8Z,llZ,14Z,17Z)-icosa-5,8,ll,14,17-pentaenoyl)piperazin-l- yl)methyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)benzamide
(IV-5):
[0311 ] The commercially available 6-methyl-N 1 -(4-(pyridin-3 -yl)pyrimidin-2- yl)benzene-l,3-diamine (10.0 g, 36.0 mmol) and Et3N (10.0 ml, 72.2 mmol) were taken up in THF (100 mL). The resulting solution was cooled to 0 °C with stirring and maintained for 10 min. A solution of 4-(chloromethyl)benzoyl chloride (7.8 g, 41.4 mmol) in THF (50 mL) was added dropwise. After stirring at 0 °C for four hours, water (500 ml) was added dropwise to the reaction mixture, and a light-yellow precipitate appeared. The resulting precipitate was collected by suction filtration, washed with water ( 2 x 500 ml), and dried under reduced pressure to afford 4-(chloromethyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2- yl)amino)phenyl)benzamide (15.1 g, yield: 97.4 %) as a light-yellow solid. This material (4 g, 93 mmol) and tert - butyl piperazinecarboxylate (8.8 g, 465 mmol) were dissolved in N- methyl-2-pyrrolidone (20 mL). The solution was reacted under microwave conditions at 120 °C for 1 h. After cooling to room temperature, CH2CI2 (50 mL) was added to the reaction mixture. The resulting mixture was extracted with 1M HC1 (20 mL). The acidic aqueous phase was neutralized with sodium carbonate, and then extracted with CH2CI2 (2 x 50 mL). The combined organic layers were dried over anhydrous Na2S04, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography
(pentane/EtOAc) to give 4 g of tert-butyl 4-(4-((4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2- yl)amino)phenyl)carbamoyl)benzyl)piperazine-l-carboxylate (72% yield) as a yellow solid. This material (580 mg, 1 mmol) was dissolved in a solution of HC1 in EtOAc (5 mL, 4 M). The resulting reaction mixture was stirred at room temperature for 2 h and then concentrated
under reduced pressure to afford the HC1 salt of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin- 2-yl)amino)phenyl)-4-(piperazin-l-ylmethyl)benzamide.MS (EI) calculated for C28H29N7O: 479.58; Found: 480 [M+H]+(5Z,8Z,l lZ,14Z,17Z)-Eicosa-5,8,l l,14,17-pentaenoic acid (EPA, 0.27 g, 0.92 mmol) was taken up in 15 mL of CH2C12 along with HATU (0.47 g, 1.24 mmol), Et3N (0.25 g, 2.49 mmol) and the HC1 salt of N-(4-methyl-3-((4-(pyridin-3- yl)pyrimidin-2-yl)amino)phenyl)-4-(piperazin-l-ylmethyl)benzamide (0.4 g, 0.83 mmol). The resulting reaction mixture was stirred at room temperature for 16 h. The reaction mixture was then diluted with CH2CI2 (100 mL). The organic layer was washed with aq.NH4Cl (3 x 100 mL), brine (3 x 100 mL), dried over anhydrous Na2S04 and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (Gradient elution, 2: 1 pentane/EtOAc to 100% EtOAc) to afford 0.4 g of 4-((4-((5Z,8Z,l lZ,14Z,17Z)-icosa- 5,8,11,14,17-pentaenoyl)piperazin- 1 -yl)methyl)-N-(4-methyl-3 -((4-(pyridin-3 -yl)pyrimidin- 2-yl)amino)phenyl)benzamide (62% yield). MS (EI) calculated for C48H57N702: 764.01; Found: 765.05 [M+H] +
Example 16
Preparation of 4-((4-(((5Z,8Z,llZ,14Z,17Z)-icosa-5,8,ll,14,17- pentaenoyl)glycyl)piperazin-l-yl)methyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2- yl)amino)phenyl)benzamide (I V-6) :
[0312] In a typical run, EPA (1.5 g, 4.9 mmol) was taken up in 100 mL of CH2CI2 along with HOBt (1.0 g, 7.4 mmol), EDCI (1.4 g, 7.4 mmol), glycine methyl ester HC1 (0.68 g, 5.5 mmol) and Et3N (1.5 g, 14.9 mmol). The resulting reaction mixture was stirred at room temperature for 16 h and then diluted with CH2CI2. The organic layer was washed with aq.NH4Cl (3 x 100 mL), brine (3 x 100 mL), dried over anhydrous Na2S04 and concentrated
under reduced pressure. The resulting residue was purified by silica gel chromatography (pentane/EtOAc) to afford 1.6 g of methyl ((5Z,8Z,1 lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenoyl)glycinate (86% yield). This material (1.6 g, 4.3 mmol) was taken up in 10 mL of THF and 3.5 mL of a 5 M aq. NaOH was added. The resulting reaction mixture was stirred at room temperature for 2 h. The reaction mixture was concentrated under reduced pressure to remove most of the THF. It was then cooled in ice and acidified to pH 2 with 6 N HC1. The resulting aqueous mixture was extracted with EtOAc (3 x 100 mL). The combined organic layers were washed with water (4 x 100 mL), brine (100 mL), dried over anhydrous Na2S04 and concentrated under reduced pressure to afford a total of 1.3 g of ((5Z,8Z,11Z,14Z,17Z)- icosa-5,8,1 l,14,17-pentaenoyl)glycine (80% yield). ((5Z,8Z,1 lZ,14Z,17Z)-Icosa- 5,8,11,14, 17-pentaenoyl)glycine (0.33 g, 0.92 mmol) was taken up in 15 mL of CH2C12 along with HATU (0.47 g, 1.24 mmol), TEA (0.25 g, 2.49 mmol) and the HC1 salt of N-(4-methyl- 3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)-4-(piperazin- 1 -ylmethyl)benzamide (0.4 g, 0.83 mmol). The resulting reaction mixture was stirred at room temperature overnight. The reaction mixture was diluted with DCM (100 mL). The organic layer was washed with aq.NH4Cl (100 mL*3) and brine (100 mL*3). The organic layer was dried over anhydrous Na2S04 and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (pentane/EtOAc) to afford 0.3 g of 4-((4-(((5Z,8Z,l lZ,14Z,17Z)-icosa- 5,8,11,14,17-pentaenoyl)glycyl)piperazin- 1 -yl)methyl)-N-(4-methyl-3 -((4-(pyridin-3 - yl)pyrimidin-2-yl)amino)phenyl)benzamide (44%> yield). MS (EI) calculated for C50H60N8O3: 821.06; Found: 822.1 [M+H] +
Example 17
Preparation of 2-((5Z,8Z,llZ,14Z,17Z)-icosa-5,8,ll,14,17-pentaenamido)ethyl 4-(4-((4- methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)carbamoyl)benzyl)piperazine- 1-carboxylate (IV- 7):
[0313] In a typical run, tert-butyl (2-hydroxyethyl)carbamate (1.61 g, 10 mmol) and 4- nitrophenyl carbonochloridate (3.02 g, 15 mmol) were taken up in CH2CI2 (50 mL) and cooled to 0 °C. Et3N (3 g, 30 mml) was then added and the resulting reaction mixture was stirred at room temperature for overnight. The organic layer was washed with aq.NH4Cl (3 x 20 mL) and brine (3 x 20 mL), dried over anhydrous Na2S04 and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (pentane/EtOAc) to afford 1.7 g of tert-butyl (2-(((4-nitrophenoxy)carbonyl)oxy)ethyl)carbamate (52 %). The HCI salt of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)-4-(piperazin- 1 - ylmethyl)benzamide (480 mg, 1 mmol) was taken up in 20 mL of CH2C12 along with HOBT (160 mg, 1.2 mmol), EDCI (230 mg, 1.2 mmol), tert-butyl (2-(((4- nitrophenoxy)carbonyl)oxy)ethyl)carbamate (330 mg, 1 mmol) and Et3N (253 mg, 2.5 mmol). The resulting reaction mixture was stirred at room temperature for 16 h and then diluted with CH2C12 (10 mL). The organic layer was washed with aq.NH4Cl (3 x 10 mL), brine (3 x 10 mL), dried over anhydrous Na2S04 and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (pentane/EtOAc) to afford
550 mg of 2-((tert-butoxycarbonyl)amino)ethyl 4-(4-((4-methyl-3-((4-(pyridin-3- yl)pyrimidin-2-yl)amino)phenyl)carbamoyl)benzyl)piperazine-l-carboxylate (70 % yield). This material (550 mg, 0.7 mmol) was taken up in 5 mL of 4 N HC1 in EtOAc. The resulting reaction mixture was stirred at room temperature for 2 h and then concentrated under reduced pressure to afford 480 mg of the HC1 salt of 2-aminoethyl 4-(4-((4-methyl-3-((4-(pyridin-3- yl)pyrimidin-2-yl)amino)phenyl)carbamoyl)benzyl)piperazine- 1 -carboxylate. This material (480 mg, 0.7 mmol), EPA (237 mg, 0.7 mmol) and HATU (405 mg, 10 mmol) were taken up in 10 mL of CH2CI2 and cooled to 0 °C. DIEA (529 mg, 3.5 mmol) was added and the resulting reaction mixture was stirred at room temperature for 16 h. The reaction mixture was then diluted with 10 mL of CH2CI2 and washed with aq.NH4Cl (3 x 5 mL), brine (3 x 5 mL), dried over anhydrous Na2S04 and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (pentane/EtOAc) to afford 240 mg of 2- ((5Z,8Z,1 lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl 4-(4-((4-methyl-3-((4- (pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)carbamoyl)benzyl)piperazine-l -carboxylate (34 % yield). MS (EI) calculated for C5iH62N804: 851.09; Found: 852.4 [M+H] +
Example 18
Preparation of (5Z,8Z,1 lZ,14Z,17Z)-N-(5-(2,6-dimorpholinopyrimidin-4-yl)-4- (trifluoromethyl)pyridin-2-yl)icosa-5,8,ll,14,17-pentaenamide (IV-21):
[0314] (5Z,8Z, 11 Z, 14Z, 17Z)-Eicosa-5 ,8, 11,14,17-pentaenoic acid (EPA, 0.14 g, 0.45 mmol) was taken up in 5 mL of DMF along with HATU (0.23 g, 0.60 mmol), N,N- diisopropylethylamine (0.22 mL, 0.13 mmol) and 5-(2,6-dimorpholinopyrimidin-4-yl)-4- (trifluoromethyl)pyridin-2-amine (0.21 g, 0.50 mmol). The resulting reaction mixture was stirred at room temperature for 18 h and then at 50 °C for an additional 6 h. The solvent was removed under reduced pressure and the residue was dissolved in CH2CI2 (25 mL). The
organic layer was washed with aq.NH4Cl (3 x 10 mL), brine (3 x 10 mL), dried over anhydrous Na2S04 and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (Gradient elution, dichloromethane to 19: 1
dichloromethane methanol) to afford 0.024 g of (5Z,8Z,11Ζ,14Ζ,17Ζ)-Ν-(5-(2,6- dimorpholinopyrimidin-4-yl)-4-(trifluoromethyl)pyridin-2-yl)icosa-5 ,8, 11,14,17- pentaenamide (7% yield). MS (EI) calculated for C38H49F3N603: 694.38; Found: 695.4
[M+H] +
Example 19
Preparation of (5Z,8Z,llZ,14Z,17Z)-N-(2-(3-(2,4-dimethyl-5-((Z)-(2-oxoindolin-3- ylidene)methyl)-lH-pyrrol-3-yl)propanamido)ethyl)icosa-5,8,ll,14,17-pentaenamide
(IV-40):
[0315] (5Z,8Z,1 lZ,14Z,17Z)-N-(2-aminoethyl)icosa-5,8,l 1,14,17-pentaenamide (0.040 g, 0.12 mmol) was taken up in 2 mL of 1 : 1 DMF:CH2C12 along with HATU (0.060 g, 0.16 mmol), Et3N (0.055 mL, 0.39 mmol) and (Z)-3-(2,4-dimethyl-5-((2-oxoindolin-3- ylidene)methyl)-lH-pyrrol-3-yl)propanoic acid (0.040 g, 0.13 mmol). The resulting reaction mixture was stirred at room temperature for 18 h. The reaction mixture was then diluted with CH2C12 (20 mL). The organic layer was washed with aq.NH4Cl (3 x 10 mL), brine (3 x 10 mL), dried over anhydrous Na2S04 and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (Gradient elution, dichloromethane to 19: 1 dichloromethane methanol) to afford 0.050 g of (5Z,8Z,1 lZ,14Z,17Z)-N-(2-(3-(2,4-dimethyl- 5-((Z)-(2-oxoindolin-3-ylidene)methyl)-lH-pyrrol-3-yl)propanamido)ethyl)icosa- 5,8,11,14,17-pentaenamide (68% yield). MS (EI) calculated for C40H52N4O3: 636.40; Found: 637.4 [M+H] +
Example 20
Preparation of (R)-2-((6-(benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butyl (2- -icosa-5,8,ll,14,17-pentaenamido)ethyl)carbamate (IV-16):
[0316] In a typical run, (5Z,8Z,1 lZ,14Z,17Z)-N-(2-aminoethyl)icosa-5,8,l 1,14,17- pentaenamide (69 mg, 0.2 mmol) was taken up in 5 mL anhydrous of CH2CI2 along with 4- nitrophenyl chloroformate (0.26 mmol). Triethylamine (56 μί, 0.4 mmol) was then added dropwise at room temperature. The resulting reaction mixture was stirred at rt for 16 h. (R)- 2-((6-(Benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butan-l-ol (42 mg, 0.12 mmol) was then added in a single portion at rt to this freshly prepared mixture of 4-nitrophenyl carbamate derivative. Once DIEA (200 μί, 1.2 mmol) was added, the reaction mixture was stirred at a gentle reflux for 24 h. The crude reaction mixture was diluted with 25 mL of EtOAc. The resulting organic layer was washed with brine (3 x 5 mL), dried over Na2S04 and concentrated under reduced pressure. The crude material was purified over a silica gel chromatography (gradient elution: 0-10% MeOH in 0.2% triethylamine spiked
Preparation of make 3-((5Z,8Z,llZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)benzamide (IV-48 ):
[0317] In a typical run, (5Z,8Z,1 lZ,14Z,17Z)-icosa-5, 8,11,14, 17-pentaenoic acid (1.5 g, 5 mmol) was taken up in 50 mL of anhydrous DMF . Oxalyl chloride (468 μί, 5.5 mmol) was then added dropwise under Argon. The resulting reaction mixture was stirred at rt for 2 h. 3- Aminobenzamide (675 mg, 5 mmol) was added in a single portion at rt, followed by dropwise addition of triethylamine (2 mL, 15 mmol). The resulting reaction mixture was stirred at room temperature for 4 h and then diluted with EtOAc (200 mL). The organic layer was washed brine (3 x 10 mL), dried (Na2S04) and then concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (gradient elution: CH2C12 to 19: CH2Cl2:MeOH)) to afford 1.2 g of 5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)benzamide as a pink waxy solid. MS (EI) calculated for C27H36N202: 420.59; Found: 421.2[M+H] +.
EQUIVALENTS
[0318] Those skilled in the art will recognize, or be able to ascertain, using no more than routine experimentation, numerous equivalents to the specific embodiments described specifically herein. Such equivalents are intended to be encompassed in the scope of the following claims.
Claims
1. A molecular conjugate comprising an anticancer agent and a fatty acid covalently linked directly, or indirectly through a linker, wherein the link is through a hydroxyl, amine, thiol, carboxylate, phosphate, or the like, on the anticancer agent and the fatty acid, wherein the fatty acid is selected from the group consisting of omega-3 fatty acids, fatty acids that are metabolized in vivo to omega-3 fatty acids, and lipoic acid, and the conjugate is stable in the plasma and capable of hydrolysis to produce free anticancer and free fatty acid, with the proviso that the molecular conjugate is not
2. A compound of Formula I:
Formula I or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer, or stereoisomer thereof; wherein
Rni is a nucleoside anticancer agent;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W2 can not be O simultaneously;
W3 is each independently O or NR, each a, b, c and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- C6cycloalkyl)-, a heterocycle, a heteroaryl,
- 149-
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula I;
R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl;
each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; m is 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; ml is 0, 1, 2 or 3; k is 0, 1, 2, or 3; z is 1 , 2, or 3; each R3 is independently H or Ci-C6 alkyl, or both R3 groups, when taken together with the nitrogen to which they are attached, can form a heterocycle; each R4 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R5 is independently H, aryl, heteroaryl, heterocyclic, straight or branched C1-C10 alkyl which can be optionally substituted with one or two groups selected from halogen e, OH, NH2, C02R, CONH2, CONR2, phenyl, C6H4OH, imidazole or arginine;
each Z is independently -H,
with the proviso that there is at least one
in the compound;
each r is independently 2, 3, or 7;
each s is independently 3, 5, or 6;
each t is independently 0 or 1 ;
each v is independently 1, 2, or 6;
Ri and R2 are each independently hydrogen, deuterium, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(C C3 alkyl), -S(0)C C3 alkyl, -S(0)2Ci-C3 alkyl; and each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with OH, or halogen; provided that when m, n, o, p, and q are each 0, Wi and W2 are each null, and Z is
then t must be 0; and when m, n, o, p, and q are each 0, and Wi and W2 are each null, then Z must not be
3. A compound of Formula II:
Formula II or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer, or stereoisomer thereof;
wherein Rn2 is independently
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W2 can not be O simultaneously;
W3 is each independently O or NR, each a, b, c and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- Cecycloalkyl)-, a heterocycle, a heteroaryl,
- 154-
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula II;
R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl;
each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; m is 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; ml is 0, 1, 2 or 3; k is 0, 1, 2, or 3; z is 1 , 2, or 3; each R3 is independently H or Ci-C6 alkyl, or both R3 groups, when taken together with the nitrogen to which they are attached, can form a heterocycle; each R4 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each R5 is independently H, aryl, heteroaryl, heterocyclic^, straight or branched Ci- C10 alkyl which can be optionally substituted with one or two groups selected from halogen OH, NH2, C02R, CONH2, CONR2, phenyl, C6H4OH, imidazole or arginine;
each Z is independently -H,
with the proviso that there is at least one
in the compound;
each r is independently 2, 3, or 7;
each s is independently 3, 5, or 6;
each t is independently 0 or 1 ;
each v is independently 1, 2, or 6;
Ri and R2 are each independently hydrogen, deuterium, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(C C3 alkyl), -S(0)C C3 alkyl, -S(0)2Ci-C3 alkyl; and each R is independently -H, -C1-C3 alkyl, or straight or branched C1-C4 alkyl optionally substituted with OH, or halogen; provided that when m, n, o, p, and q are each 0, Wi and W2 are each null, and Z is
then t must be 0; and when m, n, o, p, and q are each 0, and Wi and W2 are each null, then Z must not be
4. A compound of Formula III:
Formula III or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer, or stereoisomer thereof; wherein
Rn3 is an anticancer agent;
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W2 can not be O simultaneously; each a, b, c and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- Cecycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula III;
R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(C C3 alkyl), -N(C C3 alkyl)2, -NH(C(0)C C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(Ci-C3 alkyl), -S(0)Ci-C3 alkyl, -S(0)2Ci-C3 alkyl;
each g is independently 2, 3 or 4; each h is independently 1, 2, 3 or 4; m is 0, 1, 2, or 3; if m is more than 1, then L can be the same or different; ml is 0, 1, 2 or 3; j is 0 or 1 ; k is 0, 1, 2, or 3; z is 1 , 2, or 3; each R3 is independently H or Ci-C6 alkyl, or both R3 groups, when taken together with the nitrogen to which they are attached, can form a heterocycle;
each R4 is independently e, H or straight or branched C1-C10 alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each Z is independently -H,
with the proviso that there is at least one
in the compound; each r is independently 2, 3, or 7; each s is independently 3, 5, or 6; each t is independently 0 or 1 ; each v is independently 1, 2, or 6;
Ri and R2 are each independently hydrogen, deuterium, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(C C3 alkyl), -S(0)C C3 alkyl, -S(0)2Ci-C3 alkyl; and each R is independently -H, -C1-C3 alkyl, phenyl or straight or branched C1-C4 alkyl optionally substituted with OH, or halogen; provided that when m, n, o, p, and q are each 0, Wi and W2 are each null, and Z is
then t must be 0; and
when m, n, o, p, and q are each 0, and Wi and W2 are each null, then Z must not be
with the proviso that the compound is not
5. A compound of Formula IV:
Formula IV or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, enantiomer, or stereoisomer thereof; wherein R„4 is
- 167-
wherein
Wi and W2 are each independently null, O, S, NH, NR, or Wi and W2 can be taken together can form an imidazolidine or piperazine group, with the proviso that Wi and W2 can not be O simultaneously; each a, b, c and d is independently -H, -D, -CH3, -OCH3, -OCH2CH3, -C(0)OR, or -O-Z, or benzyl, or two of a, b, c, and d can be taken together, along with the single carbon to which they are bound, to form a cycloalkyl or heterocycle; each n, o, p, and q is independently 0, 1 or 2; each L is independently null, -0-, -S-, -S(O)-, -S(0)2-, -S-S-, -(Ci-C6alkyl)-, -(C3- Cecycloalkyl)-, a heterocycle, a heteroaryl,
wherein the representation of L is not limited directionally left to right as is depicted, rather either the left side or the right side of L can be bound to the Wi side of the compound of Formula IV;
R6 is independently -H, -D, -C1-C4 alkyl, -halogen, cyano, oxo, thiooxo, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(C C3 alkyl), -S(0)C C3 alkyl, -S(0)2Ci-C3 alkyl;
each g is independently 2, 3 or 4;
each h is independently 1, 2, 3 or 4;
m is 0, 1, 2, or 3; if m is more than 1, then L can be the same or different;
ml is 0, 1, 2 or 3;
j is 0 or 1 ;
k is 0, 1, 2, or 3; z is 1 , 2, or 3; each R3 is independently H or Ci-C6 alkyl, or both R3 groups, when taken together with the nitrogen to which they are attached, can form a heterocycle; each R4 is independently e, H or straight or branched Ci-Cio alkyl which can be optionally substituted with OH, NH2, C02R, CONH2, phenyl, C6H4OH, imidazole or arginine; each e is independently H or any one of the side chains of the naturally occurring amino acids; each Z is independently -H,
with the proviso that there is at least one
in the compound; each r is independently 2, 3, or 7; each s is independently 3, 5, or 6; each t is independently 0 or 1 ; each v is independently 1, 2, or 6;
Ri and R2 are each independently hydrogen, deuterium, -C1-C4 alkyl, -halogen, -OH, -C(0)Ci-C4 alkyl, -O-aryl, -O-benzyl, -OC(0)Ci-C4 alkyl, -C1-C3 alkene, -C1-C3 alkyne, -C(0)Ci-C4 alkyl, -NH2, -NH(Ci-C3 alkyl), -N(Ci-C3 alkyl)2, -NH(C(0)Ci-C3 alkyl), -N(C(0)Ci-C3 alkyl)2, -SH, -S(C C3 alkyl), -S(0)C C3 alkyl, -S(0)2Ci-C3 alkyl; and each R is independently -H, -C1-C3 alkyl, phenyl or straight or branched C1-C4 alkyl optionally substituted with OH, or halogen; provided that when m, n, o, p, and q are each 0, Wi and W2 are each null, and Z is
then t must be 0; and
when m, n, o, p, and q are each 0, and Wi and W2 are each null, then Z must not be
with the proviso that the compound is not
6. A pharmaceutical composition comprising a molecular conjugate of Claim 1 and a pharmaceutically acceptable carrier.
7. A pharmaceutical composition comprising a compound of Claim 2 and a pharmaceutically acceptable carrier.
8. A pharmaceutical composition comprising a compound of Claim 3 and a pharmaceutically acceptable carrier.
9. A pharmaceutical composition comprising a compound of Claim 4 and a pharmaceutically acceptable carrier.
10. A pharmaceutical composition comprising a compound of Claim 5 and a pharmaceutically acceptable carrier.
11. A compound of claim 3 wherein Rn2 is
13. A compound of claim 11 wherein the compound is selected from a group consisting of
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl methyl (2-((5Z,8Z, l lZ, 14Z, 17Z)-icosa-5,8, l 1,14, 17- pentaenamido)ethyl)phosphoramidate (II-l),
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl ethyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1 ,14,17- pentaenamido)ethyl)phosphoramidate (II-2),
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl phenyl (2-((5Z,8Z,l lZ, 14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (II-3),
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difl^
2-yl)methyl naphthalen- 1 -yl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 1 1,14,17- pentaenamido)ethyl)phosphoramidate (II-4),
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl phenyl (2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa-4,7, 10,13,16,19- hexaenamido)ethyl)phosphoramidate (11-13),
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3- hydroxytetrahydrofuran-2-yl)methyl naphthalen- 1 -yl (2-((4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-docosa- 4,7,10,13,16,19-hexaenamido)ethyl)phosphoramidate (11-14), and
((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-l(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran- 2-yl)methyl phenyl (2-((2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8,11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)phosphoramidate (11-20) .
14. A compound of claim 11 wherein the compound is selected from a group consisting of
((2R,3 S)-5 -(5 -fluoro-2,4-dioxo-3 ,4-dihydropyrimidin- 1 (2H)-yl)-3 -hydroxytetrahydrofuran-2- yl)methyl phenyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (11-26),
((2R,3 S)-5 -(5 -fluoro-2,4-dioxo-3 ,4-dihydropyrimidin- 1 (2H)-yl)-3 -hydroxytetrahydrofuran-2- yl)methyl naphthalen- 1 -yl (2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8 , 11,14,17- pentaenamido)ethyl)phosphoramidate (11-27),
((2R,3 S)-5 -(5 -fluoro-2,4-dioxo-3 ,4-dihydropyrimidin- 1 (2H)-yl)-3 -hydroxytetrahydrofuran-2- yl)methyl phenyl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)phosphoramidate (11-30), and
15. A compound of claim 11 wherein the compound is selected from a group consisting of
((2R,3R,4S)-5-(6-amino-2-chloro-9H-purin-9-yl)-4-fluoro-3-hydroxytetrahydrofuran-2- yl)methyl phenyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (11-42) and
((2R,3R,4S)-5-(6-amino-2-chloro-9H-purin-9-yl)-4-fluoro-3-hydroxytetrahydrofuran-2- yl)methyl naphthalen- 1 -yl (2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8 , 11,14,17- pentaenamido)ethyl)phosphoramidate (11-43) .
16. A compound of claim 11 wherein the compound is selected from a group consisting of
((2R,3S,4S)-5-(6-amino-2-fluoro-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl phenyl (2-((5Z,8Z,l 1Z,14Z, 17Z)-icosa-5, 8,11,14, 17-pentaenamido)ethyl)phosphoramidate (11-46) and
((2R,3S,4S)-5-(6-amino-2-fluoro-9H-purin-9-^
naphthalen- 1 -yl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)phosphoramidate (11-47) .
17. A compound of claim 16 wherein the compound is selected from a group consisting of
((2R,3S,4R)-5-(4-amino-2-oxo-l,3,5-triazin-l(2H)-yl)-3,4-dihydroxytetrahydrofuran-2- yl)methyl phenyl (2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l 1,14,17- pentaenamido)ethyl)phosphoramidate (11-34) and
((2R,3S,4R)-5-(4-amino-2-oxo-l,3,5-triazin-l(2H)-yl)-3,4-dihydroxytetrahydrofuran-2- yl)methyl naphthalen- 1 -yl (2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8 , 11,14,17- pentaenamido)ethyl)phosphoramidate (11-35).
4-((9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[c]pyrimido[4,5-e]azepin-2-yl)amm^ N-(2-((5Z,8Z,l lZ,14Z,17Z)-icosa-5,8,l l,14,17-pentaenamido)ethyl)-2-methoxybenzamide (IV-1),
4-((9-chloro-7-(2-fluoro-6-methoxyphenyl^
N-(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenamido)ethyl)disulfanyl)ethyl)-2- methoxybenzamide (IV-4),
2-(4-(6-((5-((2-chloro-6-methylphenyl)carbamoyl)thiazol-2-yl)amino)-2-methylpyrim yl)piperazin- 1 -yl)ethyl (2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)carbamate (IV-8),
2-(4-(6-((5-((2-chloro-6-methylphenyl)carbamoyl)thiazol-2-yl)amino)-2-methylpyrim yl)piperazin- 1 -yl)ethyl (2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17- pentaenamido)ethyl)disulfanyl)ethyl)carbamate (IV-11),
(R)-2-((6-(benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butyl (2-((5Z,8Z,l 1Z,14Z,17Z)- icosa-5, 8,11,14, 17-pentaenamido)ethyl)carbamate (IV-12),
(R)-2-((6-(benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butyl (2-((2-
((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11 , 14, 17-pentaenamido)ethyl)disulfanyl)ethyl)carbamate -15),
(5Z,8Z,1 lZ,14Z,17Z)-N-(4-methyl-5-(2-((4-morpholinophenyl)amino)pyrimidin-4- yl)thiazol-2 -yl)icosa-5, 8,11,14, 17-pentaenamide (IV-18),
(5Z,8Z,l lZ,14Z,17Z)-N-(2-((4-methyl-5-(2 (4-morpholinophenyl)amino)pyrimidin-4- l)thiazol-2-yl)amino)-2-oxoethyl)icosa-5 ,8, 11,14,17-pentaenamide (IV-20),
(5Z,8Z,l lZ,14Z,17Z)-N-(5-(2,6-dimorpholinopyrm^
yl)icosa-5, 8,11,14, 17-pentaenamide (IV-21),
(5Z,8Z,l lZ,14Z,17Z)-N-(2-((5-(2,6-dimorpholinopyrimidin-4-yl)-4-(trifluoromethy 2-yl)amino)-2-oxoethyl)icosa-5,8,l l,14,17-pentaenamide (IV-23),
N-(2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenamido)ethyl)-2-methyl- 1 -(2-methyl- 3-(trifluoromethyl)benzyl)-6-morpholino-lH-benzo[d]imidazole-4-carboxamide (IV-24),
N-(2-((2-((5Z,8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8, 11,14,17-pentaenamido)ethyl)disulfanyl)ethyl)-2- methyl- 1 -(2 -methyl-3 -(trifluoromethyl)benzyl)-6-morpho lino- 1 H-benzo [d]imidazole-4- carboxamide (IV-28),
(5Z,8Z,l lZ,14Z,17Z)-N-(l-(4-(3-oxo-9-phenyl-2,3-dihydro-[l,2,4]triazolo[3,4- f [ 1 ,6]naphthyridin-8-yl)phenyl)cyclobutyl)icosa-5 ,8, 11,14,17-pentaenamide (IV-32),
(5Z,8Z,l lZ,14Z,17Z)-N-(2-oxo-2-((l-(4-(3-oxo-9-phenyl-2,3-dihydro-[l,2,4]triazolo[3,4- f] [ 1 ,6]naphthyridin-8-yl)phenyl)cyclobutyl)amino)ethyl)icosa-5 ,8, 11,14,17-pentaenamide (IV-34),
(Z)-N-(2-((5Z, 8Z, 11 Z, 14Z, 17Z)-icosa-5 ,8 , 11,14,17-pentaenamido)ethyl)-3 -(((4-(N-methyl-2- (4-methylpiperazin-l-yl)acetamido)phenyl)amino)(phenyl)methylene)-2-oxoindoline-6- carboxamide (IV-35),
(4Z,7Z, 1 OZ, 13Z, 16Z, 19Z)-N-(2-((2-(3 -(2,4-dimethyl-5-(((Z)-2-oxoindolin-3- ylidene)methyl)- 1 H-pyrrol-3-yl)propanamido)ethyl)disulfanyl)ethyl)docosa-4,7, 10,13,16,19- hexaenamide (IV-44).
19. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a molecular conjugate of Claim 1.
20. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 2.
21. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 3.
22. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 4.
23. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 5.
24. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 11.
25. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 12.
26. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 13.
27. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 14.
28. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 15.
29. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 16.
30. A method of treating or preventing a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 17.
31. A method of treating a cancer, the method comprising administering to a patient in need thereof an effective amount of a compound of Claim 18.
32. The method of claim 26 wherein the cancer is selected from breast cancer (including group 1, 2, 3 and 4 breast cancer), colorectal cancer, cholangiocarcinoma, ovarian cancer, and pancreatic cancer.
33. The method of claim 27 wherein the cancer is selected from anal cancer, breast cancer (including group 1, 2, 3, and 4 breast cancer), colorectal cancer, oesaphageal cancer, stomach cancer, pancreatic cancer, skin cancer, head and neck cancer.
34. The method of claims 28, 29 or 30 wherein the cancer is selected from lymphoma, which includes including precursor T cell lymphoma, follicular lymphoma, difuse large B cell lymphoma, Mantle cell lymphoma, B cell chronic lymphoma, MALT lymphoma, Burkitt lymphoma, Mycosis fungoides, peripheral T cell lymphoma, nodular sclerosis form of Hodgkin, mixed cellularity subtype of Hodgkin lymphoma), myelofibrosis, myelodysplastic syndrome (MDS), non-Hodgkins lymphoma and leukemia which includes acute myelogenous
leukemia (AML), acute lymphoblastic leukemia (ALL) and juvenile myeolomonocytic leukemia (JMML).
35. The method of claim 31 wherein the cancer is selected from from anal cancer, breast cancer, colorectal cancer, oesaphageal cancer, stomach cancer, pancreatic cancer, skin cancer, head and neck cancer, lymphoma and leukemia.
36. The method of claim 26 wherein the compound can be used in combination with another therapeutic agent selected from the group consisting of carboplatin, cisplatin, paclitaxel, cyclosphosphamide, Abraxane, Daunorubicin, Doxorubicin, Epirubicin, Idarubicin,
Mitoxantrone, Vinblastine, Vincristine, Topotecan, Camptosar, Methotrexate, a kinase inhibitor, a PARP inhibitor, an inhibitor of p53, a mouse double minute 2 homolog (MDM2), a mouse double minute 4 protein (MDM4 or MDMX), a monoclonal antibody, an antibody drug conjugate, a PD-1 antibody, and a PD-L1 antibody.
37. The method of claim 36 wherein the kinase inhibitor is selecetd from the group consisting of Afatinib, Axitinib, Bosutinib, Crizotinib, Dasutinib, Erlotinib, Gefitinib, Ibrutinib,
Imatinib, Lapatinib, Lenvatinib, Mubritinib, Nilotinib, Pazopanib, Pegaptamib, Ponatinib, Ruxolitinib, Sorafenib, Sunitinib, SU6656, Tofacitinib, Vandetanib, and Vemurafenib.
38. The method of claim 36 wherein the PARP inhibitor is selected from iniparib, BMN-673, Olaparib, Rucaparib, Veliparib, CEP 9722 and MK 4827
39. The method of claim 36 wherein the mouse double minute 4 protein (MDM4 or MDMX) is the stapled peptide ATSP-7041.
40. The method of claim 36 wherein the monoclonal antibody is selected from the group consisting of Trastuzumab, Urelumab, Lirlumab, Elotuzumab, Cetuximab, Rituximab, Daclizumab, Alemtuzumab, Avastin, Panitumumab, Ofatumumab, Obinutuzumab,
Bevacizumab, Panitunumab, ranibizumab and Ipilimumab.
41. The method of claim 36 wherein the antibody drug conjugate is selected from the group consisting of Moxetumomab, Brentuximab vedotin, Trastuzumab emtansine.
42. The method of claim 36 wherein the PD-1 antibody is selected from the group consisting of Lambrolizumab, Nivolumab, and MEDI 4736.
43. The method of claim 36 wherein the PD-Ll antibody is selected from the group consisting of MEDI 0680 and RG 7446.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361835869P | 2013-06-17 | 2013-06-17 | |
| US61/835,869 | 2013-06-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014204856A1 true WO2014204856A1 (en) | 2014-12-24 |
Family
ID=52105161
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2014/042542 WO2014204856A1 (en) | 2013-06-17 | 2014-06-16 | Fatty acid anticancer derivatives and their uses |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2014204856A1 (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104945437A (en) * | 2015-05-19 | 2015-09-30 | 广州诺威生物技术有限公司 | New pyrimidine compound |
| US10251845B2 (en) | 2014-11-26 | 2019-04-09 | Catabasis Pharmaceuticals, Inc. | Fatty acid cysteamine conjugates and their use as activators of autophagy |
| US10669300B2 (en) | 2014-10-06 | 2020-06-02 | NuCana plc | Methods of separating gemcitabine-phosphate diastereoisomers |
| CN111529716A (en) * | 2020-06-02 | 2020-08-14 | 南方医科大学 | Polypeptide-paclitaxel conjugate and application thereof |
| US11040027B2 (en) | 2017-01-17 | 2021-06-22 | Heparegenix Gmbh | Protein kinase inhibitors for promoting liver regeneration or reducing or preventing hepatocyte death |
| US11377467B2 (en) | 2015-12-23 | 2022-07-05 | NuCana plc | Crystalline form of gemcitabine |
| WO2023079428A1 (en) | 2021-11-03 | 2023-05-11 | Pfizer Inc. | Combination therapies using tlr7/8 agonist |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080153899A1 (en) * | 2001-03-23 | 2008-06-26 | Luitpold Pharmaceuticals, Inc. | Fatty amine drug conjugates |
| US20080221132A1 (en) * | 2006-09-11 | 2008-09-11 | Xiong Cai | Multi-Functional Small Molecules as Anti-Proliferative Agents |
| US20100215729A1 (en) * | 2005-09-29 | 2010-08-26 | Supergen, Inc. | Oligonucleotide analogues incorporating 5-aza-cytosine therein |
| US20110263525A1 (en) * | 2008-12-01 | 2011-10-27 | James Turkson | Drug composition cytotoxic for pancreatic cancer cells |
| US20120070411A1 (en) * | 2010-09-22 | 2012-03-22 | Alios Biopharma, Inc. | Substituted nucleotide analogs |
| US20120207708A1 (en) * | 2011-02-14 | 2012-08-16 | Services | Biomarkers for cancer-related fatigue and use thereof |
| WO2012142093A2 (en) * | 2011-04-13 | 2012-10-18 | Merck Sharp & Dohme Corp. | 2'-cyano substituted nucleoside derivatives and methods of use thereof for the treatment of viral diseases |
| WO2012154554A1 (en) * | 2011-05-06 | 2012-11-15 | Catabasis Pharmaceuticals, Inc. | Fatty acid triterpene derivatives and their uses |
| WO2013019906A1 (en) * | 2011-08-01 | 2013-02-07 | Genentech, Inc. | Methods of treating cancer using pd-1 axis binding antagonists and mek inhibitors |
-
2014
- 2014-06-16 WO PCT/US2014/042542 patent/WO2014204856A1/en active Application Filing
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080153899A1 (en) * | 2001-03-23 | 2008-06-26 | Luitpold Pharmaceuticals, Inc. | Fatty amine drug conjugates |
| US20100215729A1 (en) * | 2005-09-29 | 2010-08-26 | Supergen, Inc. | Oligonucleotide analogues incorporating 5-aza-cytosine therein |
| US20080221132A1 (en) * | 2006-09-11 | 2008-09-11 | Xiong Cai | Multi-Functional Small Molecules as Anti-Proliferative Agents |
| US20110263525A1 (en) * | 2008-12-01 | 2011-10-27 | James Turkson | Drug composition cytotoxic for pancreatic cancer cells |
| US20120070411A1 (en) * | 2010-09-22 | 2012-03-22 | Alios Biopharma, Inc. | Substituted nucleotide analogs |
| US20120207708A1 (en) * | 2011-02-14 | 2012-08-16 | Services | Biomarkers for cancer-related fatigue and use thereof |
| WO2012142093A2 (en) * | 2011-04-13 | 2012-10-18 | Merck Sharp & Dohme Corp. | 2'-cyano substituted nucleoside derivatives and methods of use thereof for the treatment of viral diseases |
| WO2012154554A1 (en) * | 2011-05-06 | 2012-11-15 | Catabasis Pharmaceuticals, Inc. | Fatty acid triterpene derivatives and their uses |
| WO2013019906A1 (en) * | 2011-08-01 | 2013-02-07 | Genentech, Inc. | Methods of treating cancer using pd-1 axis binding antagonists and mek inhibitors |
Non-Patent Citations (3)
| Title |
|---|
| "Stapled Peptide Oncology Drug Candidate Exhibits Robust Efficacy in Xenograft Cancer Models", NEWS-MEDICAL, 8 November 2012 (2012-11-08), pages 1, Retrieved from the Internet <URL:http://www.news-medical.net/news/20121108/Stapled-Peptide-oncology-drug-candidate-exhibits-robust-efficacy-in-xenograft-cancer-models.aspx> [retrieved on 20140825] * |
| HURVITZ, SA ET AL.: "Phase II Randomized Study of Trastuzumab Emtansine Versus Trastuzumab Plus Docetaxel in Patients With Human Epidermal Growth Factor Receptor 2- Positive Metastatic Breast Cancer.", JOUMAL OF CLINICAL ONCOLOGY, vol. 31, no. 9, 20 March 2013 (2013-03-20), pages 1157 - 1163, Retrieved from the Internet <URL:http://jco.ascopubs.org/content/31/23/2977.1.full.pdf+html> [retrieved on 20140825] * |
| SZNOL, M ET AL.: "Antagonist Antibodies to PD-1 and B7-H1 (PD-L1) in the Treatment of Advanced Human Cancer.", CLINICAL CANCER RESEARCH, vol. 19, no. 5, 1 March 2013 (2013-03-01), pages 1021 - 1034, XP055123957, Retrieved from the Internet <URL:http://clincancerres.aacrjournals.org/content/19/5/1021.full.pdf+html> [retrieved on 20140825], DOI: doi:10.1158/1078-0432.CCR-12-2063 * |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10669300B2 (en) | 2014-10-06 | 2020-06-02 | NuCana plc | Methods of separating gemcitabine-phosphate diastereoisomers |
| US10251845B2 (en) | 2014-11-26 | 2019-04-09 | Catabasis Pharmaceuticals, Inc. | Fatty acid cysteamine conjugates and their use as activators of autophagy |
| CN104945437A (en) * | 2015-05-19 | 2015-09-30 | 广州诺威生物技术有限公司 | New pyrimidine compound |
| US11377467B2 (en) | 2015-12-23 | 2022-07-05 | NuCana plc | Crystalline form of gemcitabine |
| US11040027B2 (en) | 2017-01-17 | 2021-06-22 | Heparegenix Gmbh | Protein kinase inhibitors for promoting liver regeneration or reducing or preventing hepatocyte death |
| CN111529716A (en) * | 2020-06-02 | 2020-08-14 | 南方医科大学 | Polypeptide-paclitaxel conjugate and application thereof |
| CN111529716B (en) * | 2020-06-02 | 2021-05-28 | 南方医科大学 | Polypeptide-paclitaxel conjugate and application thereof |
| WO2023079428A1 (en) | 2021-11-03 | 2023-05-11 | Pfizer Inc. | Combination therapies using tlr7/8 agonist |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2014204856A1 (en) | Fatty acid anticancer derivatives and their uses | |
| ES2909612T3 (en) | Aminothiazole compounds and use thereof | |
| US9278136B2 (en) | Fatty acid niacin conjugates and their uses | |
| ES2637266T3 (en) | Fatty acid fumarate derivatives and their uses | |
| BR102018007822A2 (en) | compound, methods for inhibiting pd-1, pd-11 and / or interaction of pd-1 / pd-11 and for cancer treatment, pharmaceutical composition, and kit for treating or preventing cancer or a disease or condition | |
| JP2018529713A (en) | Method for treating cancer using a combination of a DNA damaging agent and an ATR inhibitor | |
| WO2015106292A1 (en) | Bcr-abl tyrosine-kinase ligands capable of dimerizing in an aqueous solution, and methods of using same | |
| Kang et al. | Repositioning of the antipsychotic trifluoperazine: Synthesis, biological evaluation and in silico study of trifluoperazine analogs as anti-glioblastoma agents | |
| ES2922898T3 (en) | Multifunctional inhibitors of the MEK/PI3K and mTOR/MEK/PI3K biological pathways and therapeutic methods using the same | |
| KR101238452B1 (en) | Conjugated anti-psychotic drugs and uses thereof | |
| US20210000966A1 (en) | Hsp90-targeting conjugates and formulations thereof | |
| CN102083829A (en) | Improved Raf inhibitors | |
| WO2020014541A2 (en) | Compounds, compositions, methods, and uses for treating cancer and immunological disorders | |
| JP6557253B2 (en) | Sigma-2 receptor ligand drug conjugates as antitumor compounds, their synthesis and use | |
| CA2891900C (en) | Derivatives of indole for the treatment of cancer, viral infections and lung diseases | |
| Chamariya et al. | Role of KSP inhibitors as anti-cancer therapeutics: an update | |
| JP2014515026A (en) | Acadesine derivatives, formulations and compositions containing them, their therapeutic use, and their synthesis | |
| USRE46608E1 (en) | Fatty acid niacin conjugates and their uses |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14813228 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14813228 Country of ref document: EP Kind code of ref document: A1 |