WO2014192830A1 - Chemical and physical phenomena detection method and device - Google Patents
Chemical and physical phenomena detection method and device Download PDFInfo
- Publication number
- WO2014192830A1 WO2014192830A1 PCT/JP2014/064177 JP2014064177W WO2014192830A1 WO 2014192830 A1 WO2014192830 A1 WO 2014192830A1 JP 2014064177 W JP2014064177 W JP 2014064177W WO 2014192830 A1 WO2014192830 A1 WO 2014192830A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- sensing unit
- microparticles
- recess
- detection method
- detection
- Prior art date
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/403—Cells and electrode assemblies
- G01N27/414—Ion-sensitive or chemical field-effect transistors, i.e. ISFETS or CHEMFETS
- G01N27/4145—Ion-sensitive or chemical field-effect transistors, i.e. ISFETS or CHEMFETS specially adapted for biomolecules, e.g. gate electrode with immobilised receptors
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5027—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip
- B01L3/502761—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip specially adapted for handling suspended solids or molecules independently from the bulk fluid flow, e.g. for trapping or sorting beads, for physically stretching molecules
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54313—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals the carrier being characterised by its particulate form
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54313—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals the carrier being characterised by its particulate form
- G01N33/54326—Magnetic particles
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54366—Apparatus specially adapted for solid-phase testing
- G01N33/54373—Apparatus specially adapted for solid-phase testing involving physiochemical end-point determination, e.g. wave-guides, FETS, gratings
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2200/00—Solutions for specific problems relating to chemical or physical laboratory apparatus
- B01L2200/06—Fluid handling related problems
- B01L2200/0647—Handling flowable solids, e.g. microscopic beads, cells, particles
- B01L2200/0668—Trapping microscopic beads
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/06—Auxiliary integrated devices, integrated components
- B01L2300/0627—Sensor or part of a sensor is integrated
- B01L2300/0645—Electrodes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0809—Geometry, shape and general structure rectangular shaped
- B01L2300/0819—Microarrays; Biochips
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0848—Specific forms of parts of containers
- B01L2300/0851—Bottom walls
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0893—Geometry, shape and general structure having a very large number of wells, microfabricated wells
Definitions
- the present invention relates to a chemical / physical phenomenon detection method and improvement of the apparatus.
- a cumulative chemical / physical phenomenon detection device described in Patent Document 1 is known.
- the potential change of the sensing unit when the sample is brought into contact with the sensing unit is converted into a change in the amount of charge, and the change in the amount of charge is accumulated in the floating diffusion unit to detect a highly sensitive ion concentration.
- the sensing unit of this detection device has a structure in which a silicon nitride film (ion sensitive film) is laminated on a silicon substrate via a silicon oxide film (insulating film).
- sensing is performed to convert this potential change into a change in charge.
- a charge supply (ID) part, a charge injection control (ICG) part, a gate (TG) part, and a floating diffusion (FD) part are attached around the part. Because of these electrode materials, the periphery of the sensing part is raised, in other words, the sensing part is present on the bottom surface of the recess.
- ISFET ion-sensitive field effect semiconductor sensor
- the detection target In order to measure the chemical phenomenon or physical phenomenon of the detection target contained in the sample or those phenomena caused by the detection target with higher sensitivity, the detection target must be brought into contact with or close to the sensing unit. However, depending on the detection target, it may be difficult to stably contact or approach the sensing unit. For example, when the material of the detection target and the material of the sensing unit are chemically repelled, or when the detection target is fine and lightweight and affected by the molecular motion of the surroundings (dispersion medium, etc.), in these cases, The action of the detection target on the sensing unit becomes unstable.
- the opening area of the recess becomes very small, so that the sensing object can be stabilized to the sensing part located on the bottom surface. Thus, it becomes more difficult to make contact or proximity.
- one of the molecules (for example, antibody) is used in the sensing part.
- the sensing unit may not respond to it.
- the antibody is longer than the Debye length of the sensing unit, there is a possibility that no potential change occurs in the ion exchange membrane even if an antigen is bound to the antibody.
- the first aspect of the present invention solves at least one of the above-described problems, and is defined as follows. That is, A chemical / physical phenomenon detection method for measuring a potential change of the sensing unit by bringing a detection target into contact with or in proximity to the sensing unit, A chemical / physical phenomenon detection method for measuring a potential change of the sensing unit in a state in which the fine particles captured by the detection target are fixed to the sensing unit.
- the detection target is trapped by the microparticles, and thus the detection target cannot be stably brought into contact with and close to the sensing unit by itself.
- the detection method according to the first aspect includes a filling step in which the sensing portion is present on the bottom surface of the recess in the sensor surface, and the microparticles are filled into the recess.
- a force that is, an acceleration so that the fine particles approach the bottom surface of the recess, that is, the sensing unit (third aspect).
- the method of applying acceleration to the fine particles is not particularly limited, such as the use of gravity, the use of centrifugal force, the use of magnetic force, etc., but it is an apparatus that uses the magnetic force by forming the fine particles with a magnetic material. This is preferable from the viewpoint of simplifying the configuration (fourth aspect). If a force in the direction of the bottom of the recess, that is, the sensing portion is applied to the microparticles, the microparticles may be stacked on the peripheral wall surface of the recess.
- the microparticles stacked on the surface of the peripheral wall interfere with the microparticles to be filled in the recess and prevent the microparticles from being filled in the recess.
- the recesses may be filled with the microparticles and overflow from there. It is preferable to remove such extra fine particles from the sensor surface of the sensor chip (fifth aspect).
- the removal method is not particularly limited, but can be performed by scraping the sensor surface with a blade or the like.
- the microparticles filled in the recess are separated from the recess.
- a force (gravity, centrifugal force, magnetic force, etc.) is applied to the microparticles in a direction away from the sensing unit.
- a magnet is disposed above the opening of the recess to suck out the fine particles from the recess.
- a force is applied after breaking the chemical bonds.
- the method of decomposing the chemical bond is not particularly limited, but by adding an acid, alkali or enzyme that decomposes the detection target itself.
- the detection target is not particularly limited as long as the detection target is captured by the microparticles and acts on the sensing unit directly or indirectly to change the potential of the sensing unit.
- the detection target reflects the chemical characteristics and physical characteristics of the sample. For example, when an ion species is selected as the detection target, the ion concentration of the detection target is specified by measuring the amount.
- the antigen when one molecule (for example, antigen) of a pair of molecules constituting an intermolecular interaction is to be detected, the antigen itself becomes an ionic species or itself chemically changes to generate an ionic species. Or react with other drugs to produce ionic species.
- detection targets include (1) virus, bacteria, parasites, protozoa (malaria, etc.) pathogens, (2) cancer cells, iPS cells, leukocytes, eggs, sperm, stem cells (bone marrow, nerves, etc.) ) Cells, (3) enzymes, cytokines, hormones (insulin, etc.), snake venom, proteins / peptides such as A ⁇ 40, (4) nucleic acids such as DNA, mRNA, miRNA, (5) synthetic compounds, drugs, nucleic acid metabolites (8OHdG etc.), amino acid metabolites, lipid metabolites, sugar metabolites, natural compounds, vitamins, toxins (tetrodotoxin etc.) compounds, (6) mercury, lead, zinc, chromium, cobalt, gold, cadmium, tin, palladium And metals such as amalgam.
- virus bacteria, parasites, protozoa (malaria, etc.) pathogens
- cancer cells iPS cells, leukocytes, eggs,
- exosomes When exosomes are used as detection targets, antibodies that capture exosomes themselves can be planted in microparticles, and DNA that hybridizes with miRNA released from degraded exosomes can be planted in microparticles. preferable. When this DNA binds to miRNA, miRNA is also detected. Since exosomes are captured in the vicinity of DAN by microparticles, high detection sensitivity for exosome-derived miRNA can be achieved.
- the capsule (the exosome) containing the detection target is captured by the first capturing body (the exosome antibody) bound to the microparticle, and the capsule Is released, and the detection target contained therein is released, and then captured by a second capturing body (same as that of DNA) bound to the microparticles.
- the capsule in the sample solution is captured by the first capturing body of the fine particles and concentrated in the sample solution.
- the detection target released from the capsule thus concentrated is also captured by the second capture body that is coupled to the microparticles (that is, present in the vicinity of the capsule).
- the detection target can be detected with high sensitivity.
- the detection device is not particularly limited as long as the detection device includes a sensing unit that can temporarily or permanently fix the microparticles.
- the effect of using the microparticles becomes significant. This is because it is difficult to selectively plant one of the molecular pairs constituting the intermolecular interaction (for example, an antibody) on the bottom surface of the recess.
- this type of detection device there are a CCD type and an ISFET type disclosed in Patent Document 1.
- the material, shape, size, and capturing method of the microparticles are appropriately selected according to the detection target and the sensing unit.
- the material of the microparticles may be any material that is stable with respect to the sample, and inorganic materials such as metals and ceramics, organic materials such as plastics, or hybrid materials thereof can be used.
- a magnetic field a magnetic material such as iron that can be moved under the influence of an external magnetic field is used.
- the shape of the fine particles is preferably spherical or elliptical from the viewpoint of securing a surface area and reducing interference between the fine particles.
- the fine particles may be primary particles, secondary particles obtained by aggregating them, or other multi-particles.
- the size (diameter) of the fine particles is sufficiently larger than the detection target to be captured.
- one of the molecular pairs is implanted in the microparticle, so that the microparticle is sufficiently larger than the antibody.
- a sensing part exists in the bottom face of a recess, it makes it sufficiently smaller than the opening part of a recess.
- the method of capturing the detection target by the microparticles can be arbitrarily selected according to the characteristics of the detection target. As described above, when the detection target is an antigen, it can be indirectly captured via an antibody on the surface of the microparticle.
- the surface of the microparticle can be chemically modified, and the microparticle can be captured by a method such as inclusion or chelation. Furthermore, a physical capturing method such as providing fine pores in the microparticles and adsorbing the detection target to the fine pores can also be adopted.
- the timing at which such a microparticle captures the detection target can be arbitrarily selected.
- the detection target can be captured in advance by a fine particle and then fixed to the sensing unit, or the fine particle can be fixed in advance to the sensing unit and contacted with the sample to form a fine particle.
- the detection target may be captured by the body.
- “fixed” refers to a state in which the position of the microparticle does not vary with respect to the sensing unit. In other words, “fixed” in this specification refers to a state in which no potential change is caused in the sensing unit due to a change in position of the microparticles themselves.
- FIG. 1 shows the basic configuration of a chemical / physical phenomenon detection apparatus according to an embodiment of the present invention.
- This detection device has the same principle structure as the detection device described in Patent Document 1, and as shown in FIG. 1, n + -type doped regions 11 and 13 and a p-type doped region 15 are formed on a silicon substrate 10.
- a silicon oxide film 19 is stacked in the p-type doped region 15 as a gate insulating film.
- Two gate electrodes 22 and 24 are provided on the silicon oxide film 19.
- the upper side surface is a sensor surface, and the portion of the ion sensitive film 23 in contact with the silicon oxide film 19 becomes the sensing unit 3. Needless to say, the potential of the sample is kept constant by a reference electrode (not shown).
- Reference numeral 23 in the figure denotes an ion sensitive film, and a silicon nitride film is adopted when amyloid protein A ⁇ 40 as an ionic species is to be detected.
- the n + region 11, the first gate electrode 22, the second gate electrode 24, and the n + region 13 of the substrate are connected to the terminals ID, ICG, TG, and FD, respectively, and a predetermined potential is applied at a predetermined timing.
- the n + region 11 of the substrate serves as the charge supply unit 1
- the part corresponding to the first gate electrode 22 serves as the charge injection control unit 2
- the part corresponding to the ion sensitive film 23 serves as the sensing unit 3.
- the portion corresponding to the gate electrode 24 becomes the barrier portion 4, and the n + -type region 13 becomes the floating diffusion portion 5.
- FIG. 2 shows a sensor chip in which the detection device 1 (element) shown in FIG. 1 is integrated.
- the sensor surface of the element is integrated in the central rectangular portion that occupies the widest area, and becomes the sensor surface of the sensor chip.
- the gate electrodes 22 and 24 exist as shown in FIG. 1, it turns out that the sensing part 3 exists in the bottom face of a recess in the upper surface of an element.
- a control wiring is attached in addition to the gate electrode, so that the periphery of the sensing unit 3 is surrounded by a wall. More specifically, in the sensor chip of FIG.
- the sensing unit 3 is a rectangle of 13.5 ⁇ m ⁇ 24.5 ⁇ m, and the height of the surrounding wall is 3.0 ⁇ m. is there.
- the surface of the wall around the sensing unit 3 is exposed by the same ion sensitive film 23 as the sensing unit 3.
- an anti-A ⁇ antibody is selectively placed on the sensing unit 3 on the bottom surface of a micron order and is fixed (planted). ), But no such method is known.
- general-purpose 6E10 is employed as the anti-amyloid protein A ⁇ 40 antibody, its molecular length is about 10 nm.
- the sensing unit 3 since the sensing unit 3 has a Debye length (sensitive area) of 10 to 20 nm due to the apparatus configuration, even if the antibody can be selectively planted on the surface of the sensing unit 3, it is captured by the antibody.
- the amyloid protein A ⁇ 40 exists outside the Debye length. Therefore, sufficient sensitivity for amyloid protein A ⁇ 40 cannot be ensured.
- the antibody 101 was implanted on the surface of the microparticle 100. Then, as shown in FIG. 4, the microparticles 100 were filled in the recesses 110 on the sensor surface. FG beads (manufactured by Tamagawa Seiki Co., Ltd .: particle size: about 0.2 ⁇ m) were used as the microparticles 100, and Covance (molecular length: about 10 nm) was used for the antibody 101 of the amyloid protein A ⁇ 40. . The antibody 101 was planted on the microparticles 100 as follows.
- a microparticle 100 having a carboxyl group on the surface as a linker (COOH amount: 100 to 200 nmol / mg) was added to ethyl (dimethylaminopropyl) carbodiimide as a coupling reagent and N-hydroxysuccinimide as an activating reagent for carboxylic acid ( NHS) is mixed to form an NHS body having an unstable ester bond.
- the antibody 101 is added thereto to react with the amino group of the antibody, and the antibody is implanted around the microparticle by an amide bond.
- the width of the wall section that divides the sensing section 3 of one element and the sensing section 3 of another element of the detection device 1 is 10 to 20 ⁇ m.
- the microparticles 100 in which the antibodies are planted are dispersed in water, and the sensor chip is immersed in this so that the sensing unit 3 faces upward (FIG. 4A).
- a magnet 120 residual magnetic flux density: 1.17 to 1.38 Tesla, size: 1 cm 3
- the microparticles 100 floating in water are attracted to the sensing unit 3 side (FIG. 4B).
- the magnet is drawn so as to be opposed to each element, but this is conceptually drawn as means for applying a magnetic field, and the sensor of FIG. 2 whose one side is less than 10 mm.
- the chip one magnet is disposed on the back surface of the sensor chip to apply a magnetic field to the microparticles.
- the sensor chip is immersed in the sample (FIG. 4C).
- This sample contains the amyloid protein A ⁇ 40 as the detection target 130, and this protein binds to the antibody 101 implanted in the microparticle 100. That is, it is indirectly captured by the microparticles 100.
- some of the antibodies 101 planted in the microparticles 100 face the surface of the sensing unit 3, so that the amyloid protein A ⁇ 40 bound thereto contacts the sensing unit 3 or within its Debye length.
- FIG. 4D Thereby, the sensitivity with respect to amyloid protein A (beta) 40 improves.
- the sensor chip is operated in the state shown in FIG. 4D. The output from each element integrated in the sensor chip was added to obtain the output of the sensor chip.
- the concentration of amyloid protein A ⁇ 40 in the sample can be specified more accurately by statistically processing the output from each element.
- the sample is taken out from the sample or immersed in pure water, and a magnetic field is applied so that the microparticles 100 are separated from the sensing unit 3 (the previously used magnet is moved closer to the upper surface of the sensor chip). Thereby, the microparticles 100 are removed from the recess 110.
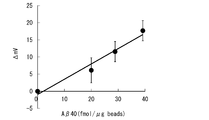
- FIG. 5 shows the output of the sensor chip obtained when the microparticles 100 having different amyloid protein A ⁇ 40 binding amounts are prepared and the above method is executed.
- the vertical axis represents the output of the sensor chip
- the horizontal axis represents the amount of amyloid protein A ⁇ 40 bound.
- Sensing unit 100 microparticles 101, antibody 110 recess, 130 detection target
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Physics & Mathematics (AREA)
- Urology & Nephrology (AREA)
- Biomedical Technology (AREA)
- Analytical Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Microbiology (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Cell Biology (AREA)
- Biotechnology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Clinical Laboratory Science (AREA)
- Dispersion Chemistry (AREA)
- Fluid Mechanics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Electrochemistry (AREA)
- Investigating Or Analyzing Materials By The Use Of Magnetic Means (AREA)
Abstract
A detection method suitable for CCD and ISFET sensors is provided. A chemical and physical phenomena detection method for bringing an object to be detected into contact with or close to a sensing unit and measuring the potential variation of the sensing unit, wherein the potential variation of the sensing unit is measured in a state in which a microparticle body on which the object to be detected is captured is fixed to the sensing unit. A microparticle body having the object to be detected captured thereon may be fixed to the sensing unit, or the microparticle body may capture the object to be detected after the microparticle body is fixed to the sensing unit.
Description
本発明は、化学・物理現象検出方法及びその装置の改良に関する。
The present invention relates to a chemical / physical phenomenon detection method and improvement of the apparatus.
センシング部にイオン感応膜を備えた検出装置の例として、特許文献1に記載の累積型化学・物理現象検出装置が知られている。
この検出装置では、試料をセンシング部に接触させたときのセンシング部の電位変化を電荷量の変化に変換し、この電荷量の変化をフローティングディフュージョン部に蓄積して高感度なイオン濃度の検出を達成している。
この検出装置のセンシング部は、シリコン基板上にシリコン酸化膜(絶縁膜)を介してシリコン窒化膜(イオン感応膜)を積層した構造である。このセンシング部に接触する試料のイオン濃度に応じてシリコン窒化膜の電位が変化し、その変化に応じてシリコン基板表面の電位が変化するので、この電位変化量を電荷量変化に変換するためセンシング部の周囲に電荷供給(ID)部、電荷注入調節(ICG)部、ゲート(TG)部及びフローティングディフュージョン(FD)部が付設される。これらの電極材料のためセンシング部の周囲は盛り上がっており、換言すれば、センシング部は凹所の底面に存在している。 As an example of a detection device provided with an ion-sensitive film in a sensing unit, a cumulative chemical / physical phenomenon detection device described inPatent Document 1 is known.
In this detection device, the potential change of the sensing unit when the sample is brought into contact with the sensing unit is converted into a change in the amount of charge, and the change in the amount of charge is accumulated in the floating diffusion unit to detect a highly sensitive ion concentration. Have achieved.
The sensing unit of this detection device has a structure in which a silicon nitride film (ion sensitive film) is laminated on a silicon substrate via a silicon oxide film (insulating film). Since the potential of the silicon nitride film changes according to the ion concentration of the sample in contact with the sensing unit, and the potential of the silicon substrate surface changes according to the change, sensing is performed to convert this potential change into a change in charge. A charge supply (ID) part, a charge injection control (ICG) part, a gate (TG) part, and a floating diffusion (FD) part are attached around the part. Because of these electrode materials, the periphery of the sensing part is raised, in other words, the sensing part is present on the bottom surface of the recess.
この検出装置では、試料をセンシング部に接触させたときのセンシング部の電位変化を電荷量の変化に変換し、この電荷量の変化をフローティングディフュージョン部に蓄積して高感度なイオン濃度の検出を達成している。
この検出装置のセンシング部は、シリコン基板上にシリコン酸化膜(絶縁膜)を介してシリコン窒化膜(イオン感応膜)を積層した構造である。このセンシング部に接触する試料のイオン濃度に応じてシリコン窒化膜の電位が変化し、その変化に応じてシリコン基板表面の電位が変化するので、この電位変化量を電荷量変化に変換するためセンシング部の周囲に電荷供給(ID)部、電荷注入調節(ICG)部、ゲート(TG)部及びフローティングディフュージョン(FD)部が付設される。これらの電極材料のためセンシング部の周囲は盛り上がっており、換言すれば、センシング部は凹所の底面に存在している。 As an example of a detection device provided with an ion-sensitive film in a sensing unit, a cumulative chemical / physical phenomenon detection device described in
In this detection device, the potential change of the sensing unit when the sample is brought into contact with the sensing unit is converted into a change in the amount of charge, and the change in the amount of charge is accumulated in the floating diffusion unit to detect a highly sensitive ion concentration. Have achieved.
The sensing unit of this detection device has a structure in which a silicon nitride film (ion sensitive film) is laminated on a silicon substrate via a silicon oxide film (insulating film). Since the potential of the silicon nitride film changes according to the ion concentration of the sample in contact with the sensing unit, and the potential of the silicon substrate surface changes according to the change, sensing is performed to convert this potential change into a change in charge. A charge supply (ID) part, a charge injection control (ICG) part, a gate (TG) part, and a floating diffusion (FD) part are attached around the part. Because of these electrode materials, the periphery of the sensing part is raised, in other words, the sensing part is present on the bottom surface of the recess.
同様の構造は、イオン感応型電解効果半導体センサ(ISFET)のセンサ面にも見られる。
A similar structure can also be seen on the sensor surface of an ion-sensitive field effect semiconductor sensor (ISFET).
試料に含まれる検出対象自体の化学現象や物理現象若しくは検出対象が引き起こすそれらの現象をより感度よく測定するには、検出対象をセンシング部に接触させるか若しくは近接させなければならない。
しかしながら、検出対象によってはこれをセンシング部に安定して接触ないし近接させることが困難な場合がある。例えば、検出対象の材料とセンシング部の材料とが化学的に反発する場合や、検出対象が微細かつ軽量で周囲(分散媒等)の分子運動の影響を受ける場合などであり、これらの場合、センシング部に対する検出対象の作用が不安定になる。
また、センシング部が凹所の底面に存在する構造のセンサ面を有するタイプの検出装置を集積した場合、凹所の開口面積が微小になるので、その底面に存在するセンシング部へ検出対象を安定して接触ないし近接させることがより困難になる。 In order to measure the chemical phenomenon or physical phenomenon of the detection target contained in the sample or those phenomena caused by the detection target with higher sensitivity, the detection target must be brought into contact with or close to the sensing unit.
However, depending on the detection target, it may be difficult to stably contact or approach the sensing unit. For example, when the material of the detection target and the material of the sensing unit are chemically repelled, or when the detection target is fine and lightweight and affected by the molecular motion of the surroundings (dispersion medium, etc.), in these cases, The action of the detection target on the sensing unit becomes unstable.
In addition, when a sensing device with a sensor surface with a structure where the sensing part is located on the bottom surface of the recess is integrated, the opening area of the recess becomes very small, so that the sensing object can be stabilized to the sensing part located on the bottom surface. Thus, it becomes more difficult to make contact or proximity.
しかしながら、検出対象によってはこれをセンシング部に安定して接触ないし近接させることが困難な場合がある。例えば、検出対象の材料とセンシング部の材料とが化学的に反発する場合や、検出対象が微細かつ軽量で周囲(分散媒等)の分子運動の影響を受ける場合などであり、これらの場合、センシング部に対する検出対象の作用が不安定になる。
また、センシング部が凹所の底面に存在する構造のセンサ面を有するタイプの検出装置を集積した場合、凹所の開口面積が微小になるので、その底面に存在するセンシング部へ検出対象を安定して接触ないし近接させることがより困難になる。 In order to measure the chemical phenomenon or physical phenomenon of the detection target contained in the sample or those phenomena caused by the detection target with higher sensitivity, the detection target must be brought into contact with or close to the sensing unit.
However, depending on the detection target, it may be difficult to stably contact or approach the sensing unit. For example, when the material of the detection target and the material of the sensing unit are chemically repelled, or when the detection target is fine and lightweight and affected by the molecular motion of the surroundings (dispersion medium, etc.), in these cases, The action of the detection target on the sensing unit becomes unstable.
In addition, when a sensing device with a sensor surface with a structure where the sensing part is located on the bottom surface of the recess is integrated, the opening area of the recess becomes very small, so that the sensing object can be stabilized to the sensing part located on the bottom surface. Thus, it becomes more difficult to make contact or proximity.
他方、抗原抗体反応、蛋白-蛋白反応、蛋白-低分子反応、DNA-DNAハイブリダイゼーション、DNA-RNAハイブリダイゼーションなどの分子間相互作用を利用した測定では、センシング部に分子の一方(例えば抗体)を植立しなければならない。この抗体が長いと、検出対象である抗原がこの抗体に結合してもセンシング部がそれに感応しないおそれがある。例えば、センシング部のデバイ長より抗体が長いとこの抗体に抗原が結合してもイオン交換膜に電位変化が生じないおそれがある。
分子間相互作用を利用した測定に関して言えば、微小な開口面積の凹所の底面に存在するセンシング部へ選択的に分子(例えば抗体)を植立することは極めて困難である。 On the other hand, in measurement using intermolecular interactions such as antigen-antibody reaction, protein-protein reaction, protein-small molecule reaction, DNA-DNA hybridization, DNA-RNA hybridization, etc., one of the molecules (for example, antibody) is used in the sensing part. Must be planted. If this antibody is long, even if an antigen to be detected binds to this antibody, the sensing unit may not respond to it. For example, if the antibody is longer than the Debye length of the sensing unit, there is a possibility that no potential change occurs in the ion exchange membrane even if an antigen is bound to the antibody.
As far as measurement using intermolecular interaction is concerned, it is extremely difficult to selectively implant molecules (for example, antibodies) in the sensing part present on the bottom surface of the recess having a small opening area.
分子間相互作用を利用した測定に関して言えば、微小な開口面積の凹所の底面に存在するセンシング部へ選択的に分子(例えば抗体)を植立することは極めて困難である。 On the other hand, in measurement using intermolecular interactions such as antigen-antibody reaction, protein-protein reaction, protein-small molecule reaction, DNA-DNA hybridization, DNA-RNA hybridization, etc., one of the molecules (for example, antibody) is used in the sensing part. Must be planted. If this antibody is long, even if an antigen to be detected binds to this antibody, the sensing unit may not respond to it. For example, if the antibody is longer than the Debye length of the sensing unit, there is a possibility that no potential change occurs in the ion exchange membrane even if an antigen is bound to the antibody.
As far as measurement using intermolecular interaction is concerned, it is extremely difficult to selectively implant molecules (for example, antibodies) in the sensing part present on the bottom surface of the recess having a small opening area.
この発明の第1の局面は上記課題の少なくとも1つを解決するものであり、次のように規定される。即ち、
検出対象をセンシング部に接触若しくは近接させて、前記センシング部の電位変化を測定する化学・物理現象検出方法であって、
前記検出対象が捕捉された微小粒体を前記センシング部に固定した状態で、前記センシング部の電位変化を測定する化学・物理現象検出方法。 The first aspect of the present invention solves at least one of the above-described problems, and is defined as follows. That is,
A chemical / physical phenomenon detection method for measuring a potential change of the sensing unit by bringing a detection target into contact with or in proximity to the sensing unit,
A chemical / physical phenomenon detection method for measuring a potential change of the sensing unit in a state in which the fine particles captured by the detection target are fixed to the sensing unit.
検出対象をセンシング部に接触若しくは近接させて、前記センシング部の電位変化を測定する化学・物理現象検出方法であって、
前記検出対象が捕捉された微小粒体を前記センシング部に固定した状態で、前記センシング部の電位変化を測定する化学・物理現象検出方法。 The first aspect of the present invention solves at least one of the above-described problems, and is defined as follows. That is,
A chemical / physical phenomenon detection method for measuring a potential change of the sensing unit by bringing a detection target into contact with or in proximity to the sensing unit,
A chemical / physical phenomenon detection method for measuring a potential change of the sensing unit in a state in which the fine particles captured by the detection target are fixed to the sensing unit.
このように規定される第1の局面の化学・物理現象検出方法では、検出対象を微小粒体に捕捉させているので、それ単体では安定してセンシング部に接触及び近接させることができない検出対象でも、微小粒体を適宜選択することにより、これを安定してセンシング部に接触若しくは近接させられる。
例えば、センシング部を構成するイオン感応膜のデバイ長より長い分子の抗体であっても、これを微小粒体の表面に植立(捕捉)してこれに抗原(検出対象)を反応させておいて、微小粒体をセンシング部へ固定すれば、抗体の分子長に関係なく、抗原をセンシング部へ接触ないし近接させられる。 In the chemical / physical phenomenon detection method of the first aspect defined as described above, the detection target is trapped by the microparticles, and thus the detection target cannot be stably brought into contact with and close to the sensing unit by itself. However, it is possible to stably contact or approach the sensing unit by appropriately selecting the fine particles.
For example, even an antibody having a molecule longer than the Debye length of the ion-sensitive membrane that constitutes the sensing unit is implanted (captured) on the surface of a microparticle and reacted with an antigen (detection target). If the microparticles are fixed to the sensing part, the antigen can be brought into contact with or close to the sensing part regardless of the molecular length of the antibody.
例えば、センシング部を構成するイオン感応膜のデバイ長より長い分子の抗体であっても、これを微小粒体の表面に植立(捕捉)してこれに抗原(検出対象)を反応させておいて、微小粒体をセンシング部へ固定すれば、抗体の分子長に関係なく、抗原をセンシング部へ接触ないし近接させられる。 In the chemical / physical phenomenon detection method of the first aspect defined as described above, the detection target is trapped by the microparticles, and thus the detection target cannot be stably brought into contact with and close to the sensing unit by itself. However, it is possible to stably contact or approach the sensing unit by appropriately selecting the fine particles.
For example, even an antibody having a molecule longer than the Debye length of the ion-sensitive membrane that constitutes the sensing unit is implanted (captured) on the surface of a microparticle and reacted with an antigen (detection target). If the microparticles are fixed to the sensing part, the antigen can be brought into contact with or close to the sensing part regardless of the molecular length of the antibody.
特にセンシング部がセンサ面の凹所の底面に存在するタイプでは、センシング部に対する抗体の植立が何ら要求されないので、センシング部自体を何ら変更する必要がない。換言すれば、異なる検出対象に対してこれを捕捉可能な微小粒子を適宜選択すれば、センシング部自体の構造は何ら変更を要しない(勿論、イオン交換膜の材料の選択は必要である)。
そこでこの発明の第2の局面は次のように規定される。
第1の局面の検出方法において、前記センサ面において前記センシング部が凹所の底面に存在し、前記微小粒体を前記凹所へ充填する充填ステップが含まれる。 In particular, in the type in which the sensing unit is located on the bottom surface of the recess of the sensor surface, no planting of the antibody to the sensing unit is required, and therefore there is no need to change the sensing unit itself. In other words, if the microparticles that can capture this are appropriately selected for different detection targets, the structure of the sensing unit itself does not require any change (of course, selection of the material for the ion exchange membrane is necessary).
Therefore, the second aspect of the present invention is defined as follows.
The detection method according to the first aspect includes a filling step in which the sensing portion is present on the bottom surface of the recess in the sensor surface, and the microparticles are filled into the recess.
そこでこの発明の第2の局面は次のように規定される。
第1の局面の検出方法において、前記センサ面において前記センシング部が凹所の底面に存在し、前記微小粒体を前記凹所へ充填する充填ステップが含まれる。 In particular, in the type in which the sensing unit is located on the bottom surface of the recess of the sensor surface, no planting of the antibody to the sensing unit is required, and therefore there is no need to change the sensing unit itself. In other words, if the microparticles that can capture this are appropriately selected for different detection targets, the structure of the sensing unit itself does not require any change (of course, selection of the material for the ion exchange membrane is necessary).
Therefore, the second aspect of the present invention is defined as follows.
The detection method according to the first aspect includes a filling step in which the sensing portion is present on the bottom surface of the recess in the sensor surface, and the microparticles are filled into the recess.
第2の局面において微小粒体を凹所に充填するには、微小粒体が凹所の底面、即ちセンシング部に近づくように力、即ち加速度を印加することが好ましい(第3の局面)。
微小粒体に加速度を印加する方法は、重力の利用、遠心力の利用、磁力の利用等特に限定されるものではないが、微小粒体を磁性材料で構成して磁力を利用することが装置構成を簡素化する見地から好ましい(第4の局面)。
凹所の底面、即ちセンシング部方向への力を微小粒体に加えると、微小粒体は凹所の周壁表面にも積層するおそれがある。この周壁表面に積層した微小粒体は、凹所内に充填されるべき微小粒体に干渉し、微小粒体が凹所に充填されることに妨げになる。
また、微小粒体に長い時間力を加えると、凹所が微小粒体で満杯になりこれからあふれ出るおそれがある。
このような余分な微小粒体はセンサチップのセンサ面から除去することが好ましい(第5の局面)。除去の方法は特に限定されるものではないが、センサ面をブレード等でスクレイプすることにより行える。 In order to fill the recess with the fine particles in the second aspect, it is preferable to apply a force, that is, an acceleration so that the fine particles approach the bottom surface of the recess, that is, the sensing unit (third aspect).
The method of applying acceleration to the fine particles is not particularly limited, such as the use of gravity, the use of centrifugal force, the use of magnetic force, etc., but it is an apparatus that uses the magnetic force by forming the fine particles with a magnetic material. This is preferable from the viewpoint of simplifying the configuration (fourth aspect).
If a force in the direction of the bottom of the recess, that is, the sensing portion is applied to the microparticles, the microparticles may be stacked on the peripheral wall surface of the recess. The microparticles stacked on the surface of the peripheral wall interfere with the microparticles to be filled in the recess and prevent the microparticles from being filled in the recess.
In addition, if a long time force is applied to the microparticles, the recesses may be filled with the microparticles and overflow from there.
It is preferable to remove such extra fine particles from the sensor surface of the sensor chip (fifth aspect). The removal method is not particularly limited, but can be performed by scraping the sensor surface with a blade or the like.
微小粒体に加速度を印加する方法は、重力の利用、遠心力の利用、磁力の利用等特に限定されるものではないが、微小粒体を磁性材料で構成して磁力を利用することが装置構成を簡素化する見地から好ましい(第4の局面)。
凹所の底面、即ちセンシング部方向への力を微小粒体に加えると、微小粒体は凹所の周壁表面にも積層するおそれがある。この周壁表面に積層した微小粒体は、凹所内に充填されるべき微小粒体に干渉し、微小粒体が凹所に充填されることに妨げになる。
また、微小粒体に長い時間力を加えると、凹所が微小粒体で満杯になりこれからあふれ出るおそれがある。
このような余分な微小粒体はセンサチップのセンサ面から除去することが好ましい(第5の局面)。除去の方法は特に限定されるものではないが、センサ面をブレード等でスクレイプすることにより行える。 In order to fill the recess with the fine particles in the second aspect, it is preferable to apply a force, that is, an acceleration so that the fine particles approach the bottom surface of the recess, that is, the sensing unit (third aspect).
The method of applying acceleration to the fine particles is not particularly limited, such as the use of gravity, the use of centrifugal force, the use of magnetic force, etc., but it is an apparatus that uses the magnetic force by forming the fine particles with a magnetic material. This is preferable from the viewpoint of simplifying the configuration (fourth aspect).
If a force in the direction of the bottom of the recess, that is, the sensing portion is applied to the microparticles, the microparticles may be stacked on the peripheral wall surface of the recess. The microparticles stacked on the surface of the peripheral wall interfere with the microparticles to be filled in the recess and prevent the microparticles from being filled in the recess.
In addition, if a long time force is applied to the microparticles, the recesses may be filled with the microparticles and overflow from there.
It is preferable to remove such extra fine particles from the sensor surface of the sensor chip (fifth aspect). The removal method is not particularly limited, but can be performed by scraping the sensor surface with a blade or the like.
検出装置を繰り返し利用するため、凹所に充填された微小粒体を当該凹所から離脱させることが好ましい。
凹所から微小粒体を離脱させるためには、微小粒体に対してそのセンシング部から離れる方向に力(重力、遠心力、磁力等)を加える。
微小粒体が磁性材料からなるときは、凹所の開口部の上方に磁石を配置して、凹所から微小粒体を吸い出す。
なお、微小粒体若しくは微小粒体に捕捉された検出対象がセンシング部に化学的に結合しているときは、当該化学結合を分解した後に力を加える。化学結合の分解の方法は特に限定されないが、検出対象自体を分解する酸、アルカリ又は酵素を加えることによる。 In order to use the detection device repeatedly, it is preferable that the microparticles filled in the recess are separated from the recess.
In order to detach the microparticles from the recess, a force (gravity, centrifugal force, magnetic force, etc.) is applied to the microparticles in a direction away from the sensing unit.
When the fine particles are made of a magnetic material, a magnet is disposed above the opening of the recess to suck out the fine particles from the recess.
When the microparticles or the detection target captured by the microparticles are chemically bonded to the sensing unit, a force is applied after breaking the chemical bonds. The method of decomposing the chemical bond is not particularly limited, but by adding an acid, alkali or enzyme that decomposes the detection target itself.
凹所から微小粒体を離脱させるためには、微小粒体に対してそのセンシング部から離れる方向に力(重力、遠心力、磁力等)を加える。
微小粒体が磁性材料からなるときは、凹所の開口部の上方に磁石を配置して、凹所から微小粒体を吸い出す。
なお、微小粒体若しくは微小粒体に捕捉された検出対象がセンシング部に化学的に結合しているときは、当該化学結合を分解した後に力を加える。化学結合の分解の方法は特に限定されないが、検出対象自体を分解する酸、アルカリ又は酵素を加えることによる。 In order to use the detection device repeatedly, it is preferable that the microparticles filled in the recess are separated from the recess.
In order to detach the microparticles from the recess, a force (gravity, centrifugal force, magnetic force, etc.) is applied to the microparticles in a direction away from the sensing unit.
When the fine particles are made of a magnetic material, a magnet is disposed above the opening of the recess to suck out the fine particles from the recess.
When the microparticles or the detection target captured by the microparticles are chemically bonded to the sensing unit, a force is applied after breaking the chemical bonds. The method of decomposing the chemical bond is not particularly limited, but by adding an acid, alkali or enzyme that decomposes the detection target itself.
上記において、
(検出対象)
検出対象は微小粒体に捕捉され、それ自体がセンシング部に対して直接的に若しくは間接的に作用して、センシング部の電位を変化させるものであれば特に制限されない。
検出対象は、試料の化学的な特性や物理的な特性を反映するものとし、例えば検出対象としてイオン種を選択したとき、その量を測定することより、検出対象のイオン濃度が特定される。
分子間相互作用を構成する一対の分子対の一方の分子(例えば抗原)を検出対象とするとき、抗原はそれ自体がイオン種となるか、又はそれ自体が化学変化してイオン種を生成するか、若しくは他の薬剤と反応してイオン種を生成するものとする。
より具体的には、検出対象として、(1)ウィルス、細菌、寄生虫、原虫(マラリヤ等)の病原体、(2)がん細胞、iPS細胞、白血球、卵子、精子、幹細胞(骨髄、神経等)の細胞、(3)酵素、サイトカイン、ホルモン(インシュリン等)、ヘビ毒、Aβ40等の蛋白/ペプチド、(4)DNA,mRNA,miRNA等の核酸、(5)合成化合物、薬物、核酸代謝産物(8OHdG等)、アミノ酸代謝産物、脂質代謝産物、糖代謝産物、天然化合物、ビタミン、毒素(テトロドトキシン等)の化合物、(6)水銀、鉛、亜鉛、クロム、コバルト、金、カドミウム、スズ、パラジウム、アマルガム等の金属を挙げることができる。 In the above,
(Detection target)
The detection target is not particularly limited as long as the detection target is captured by the microparticles and acts on the sensing unit directly or indirectly to change the potential of the sensing unit.
The detection target reflects the chemical characteristics and physical characteristics of the sample. For example, when an ion species is selected as the detection target, the ion concentration of the detection target is specified by measuring the amount.
When one molecule (for example, antigen) of a pair of molecules constituting an intermolecular interaction is to be detected, the antigen itself becomes an ionic species or itself chemically changes to generate an ionic species. Or react with other drugs to produce ionic species.
More specifically, detection targets include (1) virus, bacteria, parasites, protozoa (malaria, etc.) pathogens, (2) cancer cells, iPS cells, leukocytes, eggs, sperm, stem cells (bone marrow, nerves, etc.) ) Cells, (3) enzymes, cytokines, hormones (insulin, etc.), snake venom, proteins / peptides such as Aβ40, (4) nucleic acids such as DNA, mRNA, miRNA, (5) synthetic compounds, drugs, nucleic acid metabolites (8OHdG etc.), amino acid metabolites, lipid metabolites, sugar metabolites, natural compounds, vitamins, toxins (tetrodotoxin etc.) compounds, (6) mercury, lead, zinc, chromium, cobalt, gold, cadmium, tin, palladium And metals such as amalgam.
(検出対象)
検出対象は微小粒体に捕捉され、それ自体がセンシング部に対して直接的に若しくは間接的に作用して、センシング部の電位を変化させるものであれば特に制限されない。
検出対象は、試料の化学的な特性や物理的な特性を反映するものとし、例えば検出対象としてイオン種を選択したとき、その量を測定することより、検出対象のイオン濃度が特定される。
分子間相互作用を構成する一対の分子対の一方の分子(例えば抗原)を検出対象とするとき、抗原はそれ自体がイオン種となるか、又はそれ自体が化学変化してイオン種を生成するか、若しくは他の薬剤と反応してイオン種を生成するものとする。
より具体的には、検出対象として、(1)ウィルス、細菌、寄生虫、原虫(マラリヤ等)の病原体、(2)がん細胞、iPS細胞、白血球、卵子、精子、幹細胞(骨髄、神経等)の細胞、(3)酵素、サイトカイン、ホルモン(インシュリン等)、ヘビ毒、Aβ40等の蛋白/ペプチド、(4)DNA,mRNA,miRNA等の核酸、(5)合成化合物、薬物、核酸代謝産物(8OHdG等)、アミノ酸代謝産物、脂質代謝産物、糖代謝産物、天然化合物、ビタミン、毒素(テトロドトキシン等)の化合物、(6)水銀、鉛、亜鉛、クロム、コバルト、金、カドミウム、スズ、パラジウム、アマルガム等の金属を挙げることができる。 In the above,
(Detection target)
The detection target is not particularly limited as long as the detection target is captured by the microparticles and acts on the sensing unit directly or indirectly to change the potential of the sensing unit.
The detection target reflects the chemical characteristics and physical characteristics of the sample. For example, when an ion species is selected as the detection target, the ion concentration of the detection target is specified by measuring the amount.
When one molecule (for example, antigen) of a pair of molecules constituting an intermolecular interaction is to be detected, the antigen itself becomes an ionic species or itself chemically changes to generate an ionic species. Or react with other drugs to produce ionic species.
More specifically, detection targets include (1) virus, bacteria, parasites, protozoa (malaria, etc.) pathogens, (2) cancer cells, iPS cells, leukocytes, eggs, sperm, stem cells (bone marrow, nerves, etc.) ) Cells, (3) enzymes, cytokines, hormones (insulin, etc.), snake venom, proteins / peptides such as Aβ40, (4) nucleic acids such as DNA, mRNA, miRNA, (5) synthetic compounds, drugs, nucleic acid metabolites (8OHdG etc.), amino acid metabolites, lipid metabolites, sugar metabolites, natural compounds, vitamins, toxins (tetrodotoxin etc.) compounds, (6) mercury, lead, zinc, chromium, cobalt, gold, cadmium, tin, palladium And metals such as amalgam.
検出対象としてエクソソームを採用したときは、エクソソーム自体を捕捉する抗体を微小粒体に植立するとともに、分解エクソソームから放出されたmiRNAとハイブリダイズするDNAを微小粒体に植立しておくことが好ましい。このDNAがmiRNAと結合することにより、miRNAも検出対象となる。エクソソームが微小粒体によりDAN近傍に捕捉されるので、エクソソーム由来のmiRNAに対する高い検出感度を達成できる。
上記を敷衍すれば、検出対象(上記の例では、miRNA)を含んだカプセル(同、エクソソーム)を微小粒体に結合された第1の捕捉体(同、エクソソーム抗体)で捕捉し、該カプセルを分解してその中に含まれる検出対象を放出させ、同じく微小粒体に結合された第2の捕捉体(同、DNA)で捕捉する。これによれば、まず試料溶液中におけるカプセルが微小粒体の第1の捕捉体で捕捉されて、試料溶液中で濃縮される。このように濃縮されたカプセルから放出される検出対象は同じく微小粒体に結合された(即ち、カプセルの近傍に存在する)第2の捕捉体で捕捉されるので、検出対象の無駄な分散が防止され、もって、検出対象を高い感度で検出可能となる。 When exosomes are used as detection targets, antibodies that capture exosomes themselves can be planted in microparticles, and DNA that hybridizes with miRNA released from degraded exosomes can be planted in microparticles. preferable. When this DNA binds to miRNA, miRNA is also detected. Since exosomes are captured in the vicinity of DAN by microparticles, high detection sensitivity for exosome-derived miRNA can be achieved.
If the above is applied, the capsule (the exosome) containing the detection target (miRNA in the above example) is captured by the first capturing body (the exosome antibody) bound to the microparticle, and the capsule Is released, and the detection target contained therein is released, and then captured by a second capturing body (same as that of DNA) bound to the microparticles. According to this, first, the capsule in the sample solution is captured by the first capturing body of the fine particles and concentrated in the sample solution. The detection target released from the capsule thus concentrated is also captured by the second capture body that is coupled to the microparticles (that is, present in the vicinity of the capsule). Thus, the detection target can be detected with high sensitivity.
上記を敷衍すれば、検出対象(上記の例では、miRNA)を含んだカプセル(同、エクソソーム)を微小粒体に結合された第1の捕捉体(同、エクソソーム抗体)で捕捉し、該カプセルを分解してその中に含まれる検出対象を放出させ、同じく微小粒体に結合された第2の捕捉体(同、DNA)で捕捉する。これによれば、まず試料溶液中におけるカプセルが微小粒体の第1の捕捉体で捕捉されて、試料溶液中で濃縮される。このように濃縮されたカプセルから放出される検出対象は同じく微小粒体に結合された(即ち、カプセルの近傍に存在する)第2の捕捉体で捕捉されるので、検出対象の無駄な分散が防止され、もって、検出対象を高い感度で検出可能となる。 When exosomes are used as detection targets, antibodies that capture exosomes themselves can be planted in microparticles, and DNA that hybridizes with miRNA released from degraded exosomes can be planted in microparticles. preferable. When this DNA binds to miRNA, miRNA is also detected. Since exosomes are captured in the vicinity of DAN by microparticles, high detection sensitivity for exosome-derived miRNA can be achieved.
If the above is applied, the capsule (the exosome) containing the detection target (miRNA in the above example) is captured by the first capturing body (the exosome antibody) bound to the microparticle, and the capsule Is released, and the detection target contained therein is released, and then captured by a second capturing body (same as that of DNA) bound to the microparticles. According to this, first, the capsule in the sample solution is captured by the first capturing body of the fine particles and concentrated in the sample solution. The detection target released from the capsule thus concentrated is also captured by the second capture body that is coupled to the microparticles (that is, present in the vicinity of the capsule). Thus, the detection target can be detected with high sensitivity.
(検出装置)
微小粒体を一次的に若しくは恒久的に固定できるセンシング部を備える検出装置であれば特に限定されない。
そのセンシング部が凹所の底部に存在するタイプにおいて分子間相互作用の測定をする際に、微小粒体の利用効果が顕著になる。分子間相互作用を構成する分子対の一方(例えば抗体)を凹所の底面へ選択的に植立することは困難だからである。
かかるタイプの検出装置として、特許文献1に示すCCDタイプやISFETタイプがある。 (Detection device)
The detection device is not particularly limited as long as the detection device includes a sensing unit that can temporarily or permanently fix the microparticles.
When measuring the intermolecular interaction in the type in which the sensing part is present at the bottom of the recess, the effect of using the microparticles becomes significant. This is because it is difficult to selectively plant one of the molecular pairs constituting the intermolecular interaction (for example, an antibody) on the bottom surface of the recess.
As this type of detection device, there are a CCD type and an ISFET type disclosed inPatent Document 1.
微小粒体を一次的に若しくは恒久的に固定できるセンシング部を備える検出装置であれば特に限定されない。
そのセンシング部が凹所の底部に存在するタイプにおいて分子間相互作用の測定をする際に、微小粒体の利用効果が顕著になる。分子間相互作用を構成する分子対の一方(例えば抗体)を凹所の底面へ選択的に植立することは困難だからである。
かかるタイプの検出装置として、特許文献1に示すCCDタイプやISFETタイプがある。 (Detection device)
The detection device is not particularly limited as long as the detection device includes a sensing unit that can temporarily or permanently fix the microparticles.
When measuring the intermolecular interaction in the type in which the sensing part is present at the bottom of the recess, the effect of using the microparticles becomes significant. This is because it is difficult to selectively plant one of the molecular pairs constituting the intermolecular interaction (for example, an antibody) on the bottom surface of the recess.
As this type of detection device, there are a CCD type and an ISFET type disclosed in
(微小粒体)
微小粒体の材質、形状、大きさ、捕捉方法は検出対象やセンシング部に応じて適宜選択される。
微小粒体の材質は試料に対して安定なものであればよく、金属やセラミックス等の無機材料やプラスチック等の有機材料、またはこれらのハイブリッド材料を用いることができる。磁場を利用する場合には外部磁場に影響されて移動可能な鉄などの磁性材料を用いる。
微小粒子の形状は、表面積を確保しかつ微小粒子同士の干渉を低減する見地から球状、若しくは楕円球状のものが好ましい。微小粒子は一次粒子でもそれらが集合してなる二次粒子その他の多次粒子でもかまわない。
微小粒子の大きさ(径)は捕捉する検出対象より十分に大きいものとする。分子間相互作用を利用した測定に用いる場合は、微小粒体に分子対の一方(抗体)を植立するので、当該微小粒体は抗体より十分大きいものとする。また、凹所の底面にセンシング部が存在する場合は、凹所の開口部より十分小さくする。
微小粒子による検出対象の捕捉の方法は、検出対象の特性に応じて任意に選択できる。既述のように検出対象が抗原の場合、微小粒子の表面の抗体を介して、間接的にこれを捕捉することができる。微小粒体の表面を化学的に修飾して、包接やキレート化等の方法で微小粒子を捕捉することができる。更には微小粒子自体に細孔を設けて、この細孔へ検出対象を吸着させる等の物理的な捕捉方法も採用できる。 (Fine particles)
The material, shape, size, and capturing method of the microparticles are appropriately selected according to the detection target and the sensing unit.
The material of the microparticles may be any material that is stable with respect to the sample, and inorganic materials such as metals and ceramics, organic materials such as plastics, or hybrid materials thereof can be used. When a magnetic field is used, a magnetic material such as iron that can be moved under the influence of an external magnetic field is used.
The shape of the fine particles is preferably spherical or elliptical from the viewpoint of securing a surface area and reducing interference between the fine particles. The fine particles may be primary particles, secondary particles obtained by aggregating them, or other multi-particles.
The size (diameter) of the fine particles is sufficiently larger than the detection target to be captured. When used for the measurement utilizing the intermolecular interaction, one of the molecular pairs (antibody) is implanted in the microparticle, so that the microparticle is sufficiently larger than the antibody. Moreover, when a sensing part exists in the bottom face of a recess, it makes it sufficiently smaller than the opening part of a recess.
The method of capturing the detection target by the microparticles can be arbitrarily selected according to the characteristics of the detection target. As described above, when the detection target is an antigen, it can be indirectly captured via an antibody on the surface of the microparticle. The surface of the microparticle can be chemically modified, and the microparticle can be captured by a method such as inclusion or chelation. Furthermore, a physical capturing method such as providing fine pores in the microparticles and adsorbing the detection target to the fine pores can also be adopted.
微小粒体の材質、形状、大きさ、捕捉方法は検出対象やセンシング部に応じて適宜選択される。
微小粒体の材質は試料に対して安定なものであればよく、金属やセラミックス等の無機材料やプラスチック等の有機材料、またはこれらのハイブリッド材料を用いることができる。磁場を利用する場合には外部磁場に影響されて移動可能な鉄などの磁性材料を用いる。
微小粒子の形状は、表面積を確保しかつ微小粒子同士の干渉を低減する見地から球状、若しくは楕円球状のものが好ましい。微小粒子は一次粒子でもそれらが集合してなる二次粒子その他の多次粒子でもかまわない。
微小粒子の大きさ(径)は捕捉する検出対象より十分に大きいものとする。分子間相互作用を利用した測定に用いる場合は、微小粒体に分子対の一方(抗体)を植立するので、当該微小粒体は抗体より十分大きいものとする。また、凹所の底面にセンシング部が存在する場合は、凹所の開口部より十分小さくする。
微小粒子による検出対象の捕捉の方法は、検出対象の特性に応じて任意に選択できる。既述のように検出対象が抗原の場合、微小粒子の表面の抗体を介して、間接的にこれを捕捉することができる。微小粒体の表面を化学的に修飾して、包接やキレート化等の方法で微小粒子を捕捉することができる。更には微小粒子自体に細孔を設けて、この細孔へ検出対象を吸着させる等の物理的な捕捉方法も採用できる。 (Fine particles)
The material, shape, size, and capturing method of the microparticles are appropriately selected according to the detection target and the sensing unit.
The material of the microparticles may be any material that is stable with respect to the sample, and inorganic materials such as metals and ceramics, organic materials such as plastics, or hybrid materials thereof can be used. When a magnetic field is used, a magnetic material such as iron that can be moved under the influence of an external magnetic field is used.
The shape of the fine particles is preferably spherical or elliptical from the viewpoint of securing a surface area and reducing interference between the fine particles. The fine particles may be primary particles, secondary particles obtained by aggregating them, or other multi-particles.
The size (diameter) of the fine particles is sufficiently larger than the detection target to be captured. When used for the measurement utilizing the intermolecular interaction, one of the molecular pairs (antibody) is implanted in the microparticle, so that the microparticle is sufficiently larger than the antibody. Moreover, when a sensing part exists in the bottom face of a recess, it makes it sufficiently smaller than the opening part of a recess.
The method of capturing the detection target by the microparticles can be arbitrarily selected according to the characteristics of the detection target. As described above, when the detection target is an antigen, it can be indirectly captured via an antibody on the surface of the microparticle. The surface of the microparticle can be chemically modified, and the microparticle can be captured by a method such as inclusion or chelation. Furthermore, a physical capturing method such as providing fine pores in the microparticles and adsorbing the detection target to the fine pores can also be adopted.
かかる微小粒体に検出対象を捕捉させるタイミングも任意に選択できる。例えば、予め検出対象を微小粒体に捕捉させておいてこれをセンシング部へ固定することもできるし、予め微小粒体をセンシング部へ固定しておいて、これを試料に接触させて微小粒体へ検出対象を捕捉させてもよい。
ここにおいて固定とは、センシング部に対して微小粒体の位置が変動しない状態をいう。換言すれば、この明細書で固定とは、微小粒体自体の位置変化に起因してセンシング部に電位変化を生じさせない状態を指す。 The timing at which such a microparticle captures the detection target can be arbitrarily selected. For example, the detection target can be captured in advance by a fine particle and then fixed to the sensing unit, or the fine particle can be fixed in advance to the sensing unit and contacted with the sample to form a fine particle. The detection target may be captured by the body.
Here, “fixed” refers to a state in which the position of the microparticle does not vary with respect to the sensing unit. In other words, “fixed” in this specification refers to a state in which no potential change is caused in the sensing unit due to a change in position of the microparticles themselves.
ここにおいて固定とは、センシング部に対して微小粒体の位置が変動しない状態をいう。換言すれば、この明細書で固定とは、微小粒体自体の位置変化に起因してセンシング部に電位変化を生じさせない状態を指す。 The timing at which such a microparticle captures the detection target can be arbitrarily selected. For example, the detection target can be captured in advance by a fine particle and then fixed to the sensing unit, or the fine particle can be fixed in advance to the sensing unit and contacted with the sample to form a fine particle. The detection target may be captured by the body.
Here, “fixed” refers to a state in which the position of the microparticle does not vary with respect to the sensing unit. In other words, “fixed” in this specification refers to a state in which no potential change is caused in the sensing unit due to a change in position of the microparticles themselves.
(第1の実施形態)
この発明の実施例の化学・物理現象検出装置の原理的な構成を図1に示す。
この検出装置は特許文献1に記載の検出装置と同一の原理的な構造を備え、図1に示すように、シリコン基板10にはn+型ドープ領域11、13とp型ドープ領域15が形成されている。p型ドープ領域15にはゲート絶縁膜としてシリコン酸化膜19が積層されている。このシリコン酸化膜19の上に2つのゲート電極22及び24が設けられている。図1において上側面がセンサ面であり、シリコン酸化膜19に接するイオン感応膜23の部分がセンシング部3となる。なお、図示しない参照電極により試料の電位が一定に保たれることはいうまでもない。
図中の符号23はイオン感応膜であり、イオン種としてのアミロイド蛋白Aβ40を検出対象としたときには、シリコン窒化膜を採用した。
基板のn+領域11、第1のゲート電極22、第2のゲート電極24及びn+領域13はそれぞれ、端子ID、ICG、TG及びFDに接続され、所定の電位が所定のタイミングで印加される。その結果、基板のn+領域11が電荷供給部1となり、第1のゲート電極22に対応する部分が電荷注入調節部2となり、イオン感応膜23に対応する部分がセンシング部3となり、第2のゲート電極24に対応する部分が障壁部4となり、n+型領域13がフローティングディフュージョン部5となる。 (First embodiment)
FIG. 1 shows the basic configuration of a chemical / physical phenomenon detection apparatus according to an embodiment of the present invention.
This detection device has the same principle structure as the detection device described inPatent Document 1, and as shown in FIG. 1, n + -type doped regions 11 and 13 and a p-type doped region 15 are formed on a silicon substrate 10. Has been. A silicon oxide film 19 is stacked in the p-type doped region 15 as a gate insulating film. Two gate electrodes 22 and 24 are provided on the silicon oxide film 19. In FIG. 1, the upper side surface is a sensor surface, and the portion of the ion sensitive film 23 in contact with the silicon oxide film 19 becomes the sensing unit 3. Needless to say, the potential of the sample is kept constant by a reference electrode (not shown).
Reference numeral 23 in the figure denotes an ion sensitive film, and a silicon nitride film is adopted when amyloid protein Aβ40 as an ionic species is to be detected.
The n + region 11, thefirst gate electrode 22, the second gate electrode 24, and the n + region 13 of the substrate are connected to the terminals ID, ICG, TG, and FD, respectively, and a predetermined potential is applied at a predetermined timing. The As a result, the n + region 11 of the substrate serves as the charge supply unit 1, the part corresponding to the first gate electrode 22 serves as the charge injection control unit 2, and the part corresponding to the ion sensitive film 23 serves as the sensing unit 3. The portion corresponding to the gate electrode 24 becomes the barrier portion 4, and the n + -type region 13 becomes the floating diffusion portion 5.
この発明の実施例の化学・物理現象検出装置の原理的な構成を図1に示す。
この検出装置は特許文献1に記載の検出装置と同一の原理的な構造を備え、図1に示すように、シリコン基板10にはn+型ドープ領域11、13とp型ドープ領域15が形成されている。p型ドープ領域15にはゲート絶縁膜としてシリコン酸化膜19が積層されている。このシリコン酸化膜19の上に2つのゲート電極22及び24が設けられている。図1において上側面がセンサ面であり、シリコン酸化膜19に接するイオン感応膜23の部分がセンシング部3となる。なお、図示しない参照電極により試料の電位が一定に保たれることはいうまでもない。
図中の符号23はイオン感応膜であり、イオン種としてのアミロイド蛋白Aβ40を検出対象としたときには、シリコン窒化膜を採用した。
基板のn+領域11、第1のゲート電極22、第2のゲート電極24及びn+領域13はそれぞれ、端子ID、ICG、TG及びFDに接続され、所定の電位が所定のタイミングで印加される。その結果、基板のn+領域11が電荷供給部1となり、第1のゲート電極22に対応する部分が電荷注入調節部2となり、イオン感応膜23に対応する部分がセンシング部3となり、第2のゲート電極24に対応する部分が障壁部4となり、n+型領域13がフローティングディフュージョン部5となる。 (First embodiment)
FIG. 1 shows the basic configuration of a chemical / physical phenomenon detection apparatus according to an embodiment of the present invention.
This detection device has the same principle structure as the detection device described in
Reference numeral 23 in the figure denotes an ion sensitive film, and a silicon nitride film is adopted when amyloid protein Aβ40 as an ionic species is to be detected.
The n + region 11, the
図2には、図1に示した検出装置1(素子)を集積したセンサチップを示す。図2において最も広い面積を占める中央の矩形部分に素子のセンサ面が集積され、センサチップにおけるセンサ面となる。
検出装置1では、図1に示すようにゲート電極22、24が存在するため、素子の上面においてセンシング部3は凹所の底面に存在していることがわかる。素子を集積化した図2に示すセンサチップでは、ゲート電極に加えて制御用の配線が付設されるため、センシング部3の周りは壁に囲まれることとなる。より具体的には、32×32の素子が集積された図2のセンサチップにおいて、センシング部3は13.5μm×24.5μmの矩形であり、その周りの壁の高さは3.0μmである。
なお、センシング部3の周りの壁の表面は、センシング部3と同じイオン感応膜23が表出している。
図1の検出装置1をそのままの状態で使用してアミロイド蛋白Aβ40を検出するためには、ミクロンオーダの狭い底面のセンシング部3へ選択的に抗Aβ抗体を配置してそこに固定(植立)することが求められるが、そのような手法は知られていない。
更には、抗アミロイド蛋白Aβ40抗体として汎用的な6E10を採用した場合、その分子長は約10nmである。他方、装置構成上、センシング部3は10~20nmのデバイ長(感応域)を有するので、センシング部3の表面へ選択的に抗体を植立することができたとしても、その抗体に捕捉されたアミロイド蛋白Aβ40はデバイ長外に存在する。よって、アミロイド蛋白Aβ40に対して十分な感度が確保できない。 FIG. 2 shows a sensor chip in which the detection device 1 (element) shown in FIG. 1 is integrated. In FIG. 2, the sensor surface of the element is integrated in the central rectangular portion that occupies the widest area, and becomes the sensor surface of the sensor chip.
In thedetection apparatus 1, since the gate electrodes 22 and 24 exist as shown in FIG. 1, it turns out that the sensing part 3 exists in the bottom face of a recess in the upper surface of an element. In the sensor chip shown in FIG. 2 in which elements are integrated, a control wiring is attached in addition to the gate electrode, so that the periphery of the sensing unit 3 is surrounded by a wall. More specifically, in the sensor chip of FIG. 2 in which 32 × 32 elements are integrated, the sensing unit 3 is a rectangle of 13.5 μm × 24.5 μm, and the height of the surrounding wall is 3.0 μm. is there.
The surface of the wall around thesensing unit 3 is exposed by the same ion sensitive film 23 as the sensing unit 3.
In order to detect the amyloid protein Aβ40 using thedetection apparatus 1 of FIG. 1 as it is, an anti-Aβ antibody is selectively placed on the sensing unit 3 on the bottom surface of a micron order and is fixed (planted). ), But no such method is known.
Furthermore, when general-purpose 6E10 is employed as the anti-amyloid protein Aβ40 antibody, its molecular length is about 10 nm. On the other hand, since thesensing unit 3 has a Debye length (sensitive area) of 10 to 20 nm due to the apparatus configuration, even if the antibody can be selectively planted on the surface of the sensing unit 3, it is captured by the antibody. The amyloid protein Aβ40 exists outside the Debye length. Therefore, sufficient sensitivity for amyloid protein Aβ40 cannot be ensured.
検出装置1では、図1に示すようにゲート電極22、24が存在するため、素子の上面においてセンシング部3は凹所の底面に存在していることがわかる。素子を集積化した図2に示すセンサチップでは、ゲート電極に加えて制御用の配線が付設されるため、センシング部3の周りは壁に囲まれることとなる。より具体的には、32×32の素子が集積された図2のセンサチップにおいて、センシング部3は13.5μm×24.5μmの矩形であり、その周りの壁の高さは3.0μmである。
なお、センシング部3の周りの壁の表面は、センシング部3と同じイオン感応膜23が表出している。
図1の検出装置1をそのままの状態で使用してアミロイド蛋白Aβ40を検出するためには、ミクロンオーダの狭い底面のセンシング部3へ選択的に抗Aβ抗体を配置してそこに固定(植立)することが求められるが、そのような手法は知られていない。
更には、抗アミロイド蛋白Aβ40抗体として汎用的な6E10を採用した場合、その分子長は約10nmである。他方、装置構成上、センシング部3は10~20nmのデバイ長(感応域)を有するので、センシング部3の表面へ選択的に抗体を植立することができたとしても、その抗体に捕捉されたアミロイド蛋白Aβ40はデバイ長外に存在する。よって、アミロイド蛋白Aβ40に対して十分な感度が確保できない。 FIG. 2 shows a sensor chip in which the detection device 1 (element) shown in FIG. 1 is integrated. In FIG. 2, the sensor surface of the element is integrated in the central rectangular portion that occupies the widest area, and becomes the sensor surface of the sensor chip.
In the
The surface of the wall around the
In order to detect the amyloid protein Aβ40 using the
Furthermore, when general-purpose 6E10 is employed as the anti-amyloid protein Aβ40 antibody, its molecular length is about 10 nm. On the other hand, since the
そこで、この実施例では、図3に示すように、微小粒体100の表面へ抗体101を植立させた。そして、この微小粒体100を、図4に示すように、センサ面の凹所110に充填した。
微小粒体100として、多摩川精機社製のFG beads(鉄製:粒径:約0.2μm)を採用し、アミロイド蛋白Aβ40の抗体101にはCovance社製の(分子長:約10nm)を採用した。微小粒体100に抗体101を次のようにして植立させた。リンカーとしてカルボキシル基を表面に有する微小粒子体100(COOH量:100~200nmol/mg)に、カップリング試薬であるエチル(ジメチルアミノプロピル)カルボジイミドとカルボン酸の活性化試薬であるN-ヒドロキシスクシンイミド(NHS)を混合し、不安定なエステル結合を有するNHS体を形成させる。そこに抗体101を加えることで抗体のアミノ基と反応させ、アミド結合によって微小粒子体の周囲に抗体を植立させる。 Therefore, in this example, as shown in FIG. 3, theantibody 101 was implanted on the surface of the microparticle 100. Then, as shown in FIG. 4, the microparticles 100 were filled in the recesses 110 on the sensor surface.
FG beads (manufactured by Tamagawa Seiki Co., Ltd .: particle size: about 0.2 μm) were used as themicroparticles 100, and Covance (molecular length: about 10 nm) was used for the antibody 101 of the amyloid protein Aβ40. . The antibody 101 was planted on the microparticles 100 as follows. A microparticle 100 having a carboxyl group on the surface as a linker (COOH amount: 100 to 200 nmol / mg) was added to ethyl (dimethylaminopropyl) carbodiimide as a coupling reagent and N-hydroxysuccinimide as an activating reagent for carboxylic acid ( NHS) is mixed to form an NHS body having an unstable ester bond. The antibody 101 is added thereto to react with the amino group of the antibody, and the antibody is implanted around the microparticle by an amide bond.
微小粒体100として、多摩川精機社製のFG beads(鉄製:粒径:約0.2μm)を採用し、アミロイド蛋白Aβ40の抗体101にはCovance社製の(分子長:約10nm)を採用した。微小粒体100に抗体101を次のようにして植立させた。リンカーとしてカルボキシル基を表面に有する微小粒子体100(COOH量:100~200nmol/mg)に、カップリング試薬であるエチル(ジメチルアミノプロピル)カルボジイミドとカルボン酸の活性化試薬であるN-ヒドロキシスクシンイミド(NHS)を混合し、不安定なエステル結合を有するNHS体を形成させる。そこに抗体101を加えることで抗体のアミノ基と反応させ、アミド結合によって微小粒子体の周囲に抗体を植立させる。 Therefore, in this example, as shown in FIG. 3, the
FG beads (manufactured by Tamagawa Seiki Co., Ltd .: particle size: about 0.2 μm) were used as the
図4において、検出装置1の1つの素子のセンシング部3と他の素子のセンシング部3とを区画する壁部の幅は10~20μmである。
実施例では、抗体を植立させた微小粒体100を水に分散させ、これにセンシング部3が上向きとなるようにセンサチップを浸漬する(図4A)。その後、チップの裏面に磁石120(残留磁束密度:1.17~1.38テスラ、サイズ:1cm3)を近接させ、水中に浮遊する微小粒体100をセンシング部3側へ吸引する(図4B)。凹所110内のセンシング部3の表面へ微小粒体100をより稠密に充填するには、磁力線が変化するように磁石120を回転若しくは移動させることが好ましい。更には、凹所110の上面をスクレイプして、センシング部3上へ微小粒体100が過剰に積層することを防止することが好ましい。
尚、図4Bでは磁石が各素子に対して対向配置されるように描かれているが、これは磁場を与える手段として概念的に描いたものであり、一辺が10mmに満たない図2のセンサチップでは、一つの磁石をセンサチップの裏面に配置して微小粒体へ磁場をかけている。
このようにして一旦微小粒体100を凹所110内に充填すると、磁石を外しても微小粒体100は安定して凹所110内に存在した。 In FIG. 4, the width of the wall section that divides thesensing section 3 of one element and the sensing section 3 of another element of the detection device 1 is 10 to 20 μm.
In the example, themicroparticles 100 in which the antibodies are planted are dispersed in water, and the sensor chip is immersed in this so that the sensing unit 3 faces upward (FIG. 4A). Thereafter, a magnet 120 (residual magnetic flux density: 1.17 to 1.38 Tesla, size: 1 cm 3 ) is brought close to the back surface of the chip, and the microparticles 100 floating in water are attracted to the sensing unit 3 side (FIG. 4B). ). In order to more densely fill the microparticles 100 on the surface of the sensing unit 3 in the recess 110, it is preferable to rotate or move the magnet 120 so that the lines of magnetic force change. Furthermore, it is preferable to scrape the upper surface of the recess 110 to prevent the microparticles 100 from being excessively stacked on the sensing unit 3.
In FIG. 4B, the magnet is drawn so as to be opposed to each element, but this is conceptually drawn as means for applying a magnetic field, and the sensor of FIG. 2 whose one side is less than 10 mm. In the chip, one magnet is disposed on the back surface of the sensor chip to apply a magnetic field to the microparticles.
Once thefine particles 100 were filled in the recess 110 in this manner, the fine particles 100 were stably present in the recess 110 even when the magnet was removed.
実施例では、抗体を植立させた微小粒体100を水に分散させ、これにセンシング部3が上向きとなるようにセンサチップを浸漬する(図4A)。その後、チップの裏面に磁石120(残留磁束密度:1.17~1.38テスラ、サイズ:1cm3)を近接させ、水中に浮遊する微小粒体100をセンシング部3側へ吸引する(図4B)。凹所110内のセンシング部3の表面へ微小粒体100をより稠密に充填するには、磁力線が変化するように磁石120を回転若しくは移動させることが好ましい。更には、凹所110の上面をスクレイプして、センシング部3上へ微小粒体100が過剰に積層することを防止することが好ましい。
尚、図4Bでは磁石が各素子に対して対向配置されるように描かれているが、これは磁場を与える手段として概念的に描いたものであり、一辺が10mmに満たない図2のセンサチップでは、一つの磁石をセンサチップの裏面に配置して微小粒体へ磁場をかけている。
このようにして一旦微小粒体100を凹所110内に充填すると、磁石を外しても微小粒体100は安定して凹所110内に存在した。 In FIG. 4, the width of the wall section that divides the
In the example, the
In FIG. 4B, the magnet is drawn so as to be opposed to each element, but this is conceptually drawn as means for applying a magnetic field, and the sensor of FIG. 2 whose one side is less than 10 mm. In the chip, one magnet is disposed on the back surface of the sensor chip to apply a magnetic field to the microparticles.
Once the
その後、センサチップを試料に浸漬する(図4C)。この試料は検出対象130としてのアミロイド蛋白Aβ40を含み、この蛋白は微小粒体100に植立された抗体101に結合する。即ち、微小粒体100へ間接的に捕捉されたこととなる。ここに、微小粒体100に植立された抗体101の中には、センシング部3の表面に対向するものがあるので、これに結合したアミロイド蛋白Aβ40はセンシング部3へ接触ないしそのデバイ長内において近接している(図4D)。これにより、アミロイド蛋白Aβ40に対する感度が向上する。
図4Dの状態においてセンサチップを稼働させる。センサチップに集積された各素子からの出力を合算して、センサチップの出力とした。なお、各素子からの出力を統計処理することにより、試料中のアミロイド蛋白Aβ40の濃度をより正確に特定できる。
測定終了後は、試料中もしくはこれから取り出して純水中に浸漬して、センシング部3から微小粒体100が離れるように磁場を印加する(先に使用した磁石をセンサチップの上面から近づける)。これにより、凹所110から微小粒体100が除去される。 Thereafter, the sensor chip is immersed in the sample (FIG. 4C). This sample contains the amyloid protein Aβ40 as thedetection target 130, and this protein binds to the antibody 101 implanted in the microparticle 100. That is, it is indirectly captured by the microparticles 100. Here, some of the antibodies 101 planted in the microparticles 100 face the surface of the sensing unit 3, so that the amyloid protein Aβ40 bound thereto contacts the sensing unit 3 or within its Debye length. In FIG. 4D. Thereby, the sensitivity with respect to amyloid protein A (beta) 40 improves.
The sensor chip is operated in the state shown in FIG. 4D. The output from each element integrated in the sensor chip was added to obtain the output of the sensor chip. The concentration of amyloid protein Aβ40 in the sample can be specified more accurately by statistically processing the output from each element.
After completion of the measurement, the sample is taken out from the sample or immersed in pure water, and a magnetic field is applied so that themicroparticles 100 are separated from the sensing unit 3 (the previously used magnet is moved closer to the upper surface of the sensor chip). Thereby, the microparticles 100 are removed from the recess 110.
図4Dの状態においてセンサチップを稼働させる。センサチップに集積された各素子からの出力を合算して、センサチップの出力とした。なお、各素子からの出力を統計処理することにより、試料中のアミロイド蛋白Aβ40の濃度をより正確に特定できる。
測定終了後は、試料中もしくはこれから取り出して純水中に浸漬して、センシング部3から微小粒体100が離れるように磁場を印加する(先に使用した磁石をセンサチップの上面から近づける)。これにより、凹所110から微小粒体100が除去される。 Thereafter, the sensor chip is immersed in the sample (FIG. 4C). This sample contains the amyloid protein Aβ40 as the
The sensor chip is operated in the state shown in FIG. 4D. The output from each element integrated in the sensor chip was added to obtain the output of the sensor chip. The concentration of amyloid protein Aβ40 in the sample can be specified more accurately by statistically processing the output from each element.
After completion of the measurement, the sample is taken out from the sample or immersed in pure water, and a magnetic field is applied so that the
アミロイド蛋白Aβ40結合量の異なる微小粒子体100を準備して、上記の方法を実行したときに得られるセンサチップの出力を図5に示す。図5において、縦軸がセンサチップの出力、横軸がアミロイド蛋白Aβ40結合量をそれぞれ示す。
正常尿におけるアミロイド蛋白Aβ40の濃度が6.5pM=6.5fmol/mlであることに鑑みれば、尿中におけるアミロイド蛋白濃度の測定にこのセンサチップが利用可能であることがわかる。このとき、センサチップの全表面はわずか1mlの尿で被覆でき、更には、センシング面であれば一滴(20μl)で被覆できる。即ち、微量の試料の測定が可能である。
図5の結果では、mV単位の出力が得られている。出力単位をμVとしても、その値は正確に特定できることは言うまでもないので、センサチップのセンサ面に配置する検出装置のユニット数を10~数十個までに削減可能である。そうであれば、測定に要する試料の量も更に削減可能である。 FIG. 5 shows the output of the sensor chip obtained when themicroparticles 100 having different amyloid protein Aβ40 binding amounts are prepared and the above method is executed. In FIG. 5, the vertical axis represents the output of the sensor chip, and the horizontal axis represents the amount of amyloid protein Aβ40 bound.
Considering that the concentration of amyloid protein Aβ40 in normal urine is 6.5 pM = 6.5 fmol / ml, it can be seen that this sensor chip can be used for measuring the amyloid protein concentration in urine. At this time, the entire surface of the sensor chip can be covered with as little as 1 ml of urine, and further, with the sensing surface, it can be covered with a single drop (20 μl). That is, it is possible to measure a very small amount of sample.
In the result of FIG. 5, an output in mV is obtained. Even if the output unit is μV, it is needless to say that the value can be accurately specified, so that the number of detection device units arranged on the sensor surface of the sensor chip can be reduced to 10 to several tens. If so, the amount of sample required for measurement can be further reduced.
正常尿におけるアミロイド蛋白Aβ40の濃度が6.5pM=6.5fmol/mlであることに鑑みれば、尿中におけるアミロイド蛋白濃度の測定にこのセンサチップが利用可能であることがわかる。このとき、センサチップの全表面はわずか1mlの尿で被覆でき、更には、センシング面であれば一滴(20μl)で被覆できる。即ち、微量の試料の測定が可能である。
図5の結果では、mV単位の出力が得られている。出力単位をμVとしても、その値は正確に特定できることは言うまでもないので、センサチップのセンサ面に配置する検出装置のユニット数を10~数十個までに削減可能である。そうであれば、測定に要する試料の量も更に削減可能である。 FIG. 5 shows the output of the sensor chip obtained when the
Considering that the concentration of amyloid protein Aβ40 in normal urine is 6.5 pM = 6.5 fmol / ml, it can be seen that this sensor chip can be used for measuring the amyloid protein concentration in urine. At this time, the entire surface of the sensor chip can be covered with as little as 1 ml of urine, and further, with the sensing surface, it can be covered with a single drop (20 μl). That is, it is possible to measure a very small amount of sample.
In the result of FIG. 5, an output in mV is obtained. Even if the output unit is μV, it is needless to say that the value can be accurately specified, so that the number of detection device units arranged on the sensor surface of the sensor chip can be reduced to 10 to several tens. If so, the amount of sample required for measurement can be further reduced.
この発明は、上記発明の実施の形態及び実施例の説明に何ら限定されるものではない。特許請求に範囲の記載の趣旨を逸脱せず、当業者が容易に想到できる範囲で種々の変形態様もこの発明に含まれる。
The present invention is not limited to the description of the embodiments and examples of the above invention. Various modifications may be included in the present invention as long as those skilled in the art can easily conceive without departing from the spirit of the scope of the claims.
3 センシング部、100 微小粒体 101 抗体110 凹所、130検出対象
3. Sensing unit, 100 microparticles 101, antibody 110 recess, 130 detection target
Claims (10)
- 検出対象をセンシング部に接触若しくは近接させて、前記センシング部の電位変化を測定する化学・物理現象検出方法であって、
前記検出対象が捕捉された微小粒体を前記センシング部に固定した状態で、前記センシング部の電位変化を測定する化学・物理現象検出方法。 A chemical / physical phenomenon detection method for measuring a potential change of the sensing unit by bringing a detection target into contact with or in proximity to the sensing unit,
A chemical / physical phenomenon detection method for measuring a potential change of the sensing unit in a state in which the fine particles captured by the detection target are fixed to the sensing unit. - 前記センシング部は凹所の底面に存在し、前記微小粒体を前記凹所へ充填する充填ステップが含まれる請求項1に記載の検出方法。 The detection method according to claim 1, wherein the sensing unit exists on a bottom surface of the recess, and includes a filling step of filling the recess with the fine particles.
- 前記充填ステップは、前記センシング部を前記微小粒体が分散した分散液に接触させるステップと、前記分散液中の微小粒体が前記センシング部側へ移動する方向に前記微小粒体を加速させる固定ステップと、を含む請求項2に記載の検出方法。 The filling step includes contacting the sensing unit with a dispersion in which the microparticles are dispersed, and fixing the microparticles in a direction in which the microparticles in the dispersion move toward the sensing unit. And a detection method according to claim 2.
- 前記微小粒体は磁性材料を含み、前記固定ステップにおいて、前記分散液中の該微小粒体が前記センシング部側へ移動するように磁場を印加する請求項3に記載の検出方法。 The detection method according to claim 3, wherein the microparticle includes a magnetic material, and a magnetic field is applied in the fixing step so that the microparticle in the dispersion moves toward the sensing unit.
- 前記充填ステップは、更に、前記センサ面において前記凹所に充填されない前記微小粒体を除去するステップを含む、請求項3に記載の検出方法。 The detection method according to claim 3, wherein the filling step further includes a step of removing the fine particles that are not filled in the recesses on the sensor surface.
- 前記センシング部の電位変化測定後、前記凹所に充填された微小粒体を該凹所から脱離するステップが更に含まれる請求項2~5のいずれかに記載の検出方法。 6. The detection method according to claim 2, further comprising a step of detaching the fine particles filled in the recess from the recess after measuring the potential change of the sensing unit.
- 予め前記検出対象が捕捉された微小粒体を前記センシング部に固定するステップが含まれる、請求項1~5のいずれかに記載の検出方法。 The detection method according to any one of claims 1 to 5, further comprising a step of fixing a microparticle on which the detection target is captured in advance to the sensing unit.
- 前記センシング部はイオン感応膜を含み、前記微小粒体には分子間相互作用を構成する分子対のうち一方の分子が捕捉され、前記検出対象は前記分子対の他方の分子である、請求項1~5のいずれかに記載の検出方法。 The sensing unit includes an ion-sensitive film, and the microparticles capture one molecule of molecular pairs constituting an intermolecular interaction, and the detection target is the other molecule of the molecular pair. The detection method according to any one of 1 to 5.
- 検出対象をセンサ面の凹所に存在するセンシング部に接触若しくは近接させて、前記センシング部の電位変化を測定する化学・物理現象検出装置であって、
前記検出対象を捕捉可能な微小粒体が前記センサ面の凹所に充填されている、検出装置。 A chemical / physical phenomenon detection device for measuring a potential change of the sensing unit by bringing a detection target into contact with or in proximity to a sensing unit located in a recess of the sensor surface,
A detection device in which fine particles capable of capturing the detection target are filled in a recess of the sensor surface. - 前記微小粒体は磁性材料を含み、該微小粒体に対して、これを前記センシング部側へ移動させる磁場を印加する磁場発生装置が更に備えられる、請求項9に記載の検出装置。 The detection device according to claim 9, wherein the microparticle includes a magnetic material, and further includes a magnetic field generation device that applies a magnetic field that moves the microparticle to the sensing unit side.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013112444A JP2014232032A (en) | 2013-05-29 | 2013-05-29 | Chemical/physical phenomenon detection method, and device of the same |
| JP2013-112444 | 2013-05-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014192830A1 true WO2014192830A1 (en) | 2014-12-04 |
Family
ID=51988853
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/064177 WO2014192830A1 (en) | 2013-05-29 | 2014-05-28 | Chemical and physical phenomena detection method and device |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP2014232032A (en) |
| WO (1) | WO2014192830A1 (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017033922A1 (en) * | 2015-08-24 | 2017-03-02 | 国立大学法人 東京大学 | Method of detecting target substance using field-effect transistor |
| JP7130223B2 (en) * | 2017-10-18 | 2022-09-05 | 株式会社Provigate | Antigen-antibody reaction detector using charge transition difference |
| JP2020186971A (en) * | 2019-05-13 | 2020-11-19 | アイシン精機株式会社 | Method of detecting minute substance |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010243299A (en) * | 2009-04-03 | 2010-10-28 | Sharp Corp | Biosensor, charge transfer type sensor, and measuring method |
| JP2012080873A (en) * | 2010-09-17 | 2012-04-26 | Univ Of Tokyo | Apparatus and method for dna base sequence analysis |
| WO2012107717A1 (en) * | 2011-02-07 | 2012-08-16 | Multi-Sense Technologies Limited | Microfluidics based assay device |
| JP2013050426A (en) * | 2011-08-31 | 2013-03-14 | Chiba Univ | Method of detecting influenza virus rna using fet-type sensor |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4010172B2 (en) * | 2001-04-25 | 2007-11-21 | 株式会社ニコン | Organic molecule detection element, organic molecule detection device, and organic molecule detection method |
| JP2004361227A (en) * | 2003-06-04 | 2004-12-24 | Tohoku Univ | Method for identifying amyloid beta bond protein |
| JP5188091B2 (en) * | 2006-03-31 | 2013-04-24 | キヤノン株式会社 | Sensor element, magnetic particle detection method using the element, and target substance detection method |
| EP2263084B1 (en) * | 2008-03-17 | 2017-02-22 | Koninklijke Philips N.V. | Cartridge for assays with magnetic particles |
| JP2013505438A (en) * | 2009-09-18 | 2013-02-14 | プロビオドルグ エージー | A novel assay for the detection of amyloid β peptide |
| JP5517125B2 (en) * | 2010-02-05 | 2014-06-11 | 国立大学法人 東京大学 | Cell measuring device |
-
2013
- 2013-05-29 JP JP2013112444A patent/JP2014232032A/en active Pending
-
2014
- 2014-05-28 WO PCT/JP2014/064177 patent/WO2014192830A1/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010243299A (en) * | 2009-04-03 | 2010-10-28 | Sharp Corp | Biosensor, charge transfer type sensor, and measuring method |
| JP2012080873A (en) * | 2010-09-17 | 2012-04-26 | Univ Of Tokyo | Apparatus and method for dna base sequence analysis |
| WO2012107717A1 (en) * | 2011-02-07 | 2012-08-16 | Multi-Sense Technologies Limited | Microfluidics based assay device |
| JP2013050426A (en) * | 2011-08-31 | 2013-03-14 | Chiba Univ | Method of detecting influenza virus rna using fet-type sensor |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2014232032A (en) | 2014-12-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6150171B2 (en) | Device for detecting a target analyte | |
| JP6389003B2 (en) | System and method for detecting substances in body fluids | |
| JP5975220B2 (en) | Device for analyzing biological fluid specimens and method for analyzing | |
| US10274455B2 (en) | Nanoelectronic sensor pixel | |
| JP4777159B2 (en) | Dual gate type sensor | |
| Elnathan et al. | Biorecognition layer engineering: overcoming screening limitations of nanowire-based FET devices | |
| Gao et al. | Direct ultrasensitive electrical detection of prostate cancer biomarkers with CMOS-compatible n-and p-type silicon nanowire sensor arrays | |
| EP3684950B1 (en) | Diagnostic device and system | |
| CN108885191B (en) | Chemical sensor | |
| JP2011511936A (en) | Integrated magnetic field generation and detection platform | |
| JP7347847B2 (en) | Biosensor using magnetic nanoparticles, detection device and detection method using the same | |
| CN110192096B (en) | Device and detection apparatus for detecting biological substance, method for measuring ion current, and method for identifying biological substance | |
| US11313828B2 (en) | Ultra-highly sensitive electrochemical biosensor using beads and method for manufacturing the same | |
| WO2014192830A1 (en) | Chemical and physical phenomena detection method and device | |
| US8093667B2 (en) | Flexible gate electrode device for bio-sensing | |
| CN104737008A (en) | Integrated circuit with sensing transistor array, sensing apparatus and measuring method | |
| JP2019082446A5 (en) | ||
| JP2008082987A (en) | Detection device of insulin | |
| JP2009501930A (en) | Sensor chip for biosensor | |
| JP2006184250A (en) | Biosensor, method for measuring object, cartridge for biosensor, and nonwoven fabric | |
| JP2010243299A (en) | Biosensor, charge transfer type sensor, and measuring method | |
| WO2017033922A1 (en) | Method of detecting target substance using field-effect transistor | |
| CN106461656B (en) | Biosensor for detecting a target component in a sample | |
| JP2009501929A (en) | Microchip assembly with short range interaction | |
| JP2006215039A (en) | Sensing switch and detection method using it |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14803754 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14803754 Country of ref document: EP Kind code of ref document: A1 |