WO2014160649A1 - Hydroxamic acid derivatives as lpxc inhibitors for the treatment of bacterial infections - Google Patents
Hydroxamic acid derivatives as lpxc inhibitors for the treatment of bacterial infections Download PDFInfo
- Publication number
- WO2014160649A1 WO2014160649A1 PCT/US2014/031612 US2014031612W WO2014160649A1 WO 2014160649 A1 WO2014160649 A1 WO 2014160649A1 US 2014031612 W US2014031612 W US 2014031612W WO 2014160649 A1 WO2014160649 A1 WO 2014160649A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- alkoxy
- crc
- optionally substituted
- group
- Prior art date
Links
- 238000011282 treatment Methods 0.000 title claims description 28
- 208000035143 Bacterial infection Diseases 0.000 title claims description 17
- 208000022362 bacterial infectious disease Diseases 0.000 title claims description 17
- 239000002253 acid Substances 0.000 title description 27
- 239000003112 inhibitor Substances 0.000 title description 12
- NEAQRZUHTPSBBM-UHFFFAOYSA-N 2-hydroxy-3,3-dimethyl-7-nitro-4h-isoquinolin-1-one Chemical compound C1=C([N+]([O-])=O)C=C2C(=O)N(O)C(C)(C)CC2=C1 NEAQRZUHTPSBBM-UHFFFAOYSA-N 0.000 title description 3
- 150000001875 compounds Chemical class 0.000 claims abstract description 265
- 239000000203 mixture Substances 0.000 claims abstract description 110
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 27
- 241000894006 Bacteria Species 0.000 claims abstract description 24
- 208000015181 infectious disease Diseases 0.000 claims abstract description 8
- 238000000034 method Methods 0.000 claims description 154
- 125000000217 alkyl group Chemical group 0.000 claims description 126
- -1 -OH Chemical group 0.000 claims description 114
- 229910052736 halogen Inorganic materials 0.000 claims description 97
- 150000002367 halogens Chemical group 0.000 claims description 97
- 125000003545 alkoxy group Chemical group 0.000 claims description 86
- 125000000623 heterocyclic group Chemical group 0.000 claims description 79
- 150000003839 salts Chemical class 0.000 claims description 74
- 125000001424 substituent group Chemical group 0.000 claims description 72
- 125000001072 heteroaryl group Chemical group 0.000 claims description 53
- 229910052739 hydrogen Inorganic materials 0.000 claims description 49
- 239000001257 hydrogen Substances 0.000 claims description 49
- 125000005842 heteroatom Chemical group 0.000 claims description 45
- 229910052757 nitrogen Inorganic materials 0.000 claims description 45
- 229910052760 oxygen Inorganic materials 0.000 claims description 45
- 229910052717 sulfur Inorganic materials 0.000 claims description 43
- 125000004438 haloalkoxy group Chemical group 0.000 claims description 41
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 39
- 125000001188 haloalkyl group Chemical group 0.000 claims description 38
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 29
- 229910052799 carbon Inorganic materials 0.000 claims description 27
- 239000003814 drug Substances 0.000 claims description 27
- 125000004429 atom Chemical group 0.000 claims description 24
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 20
- 125000003118 aryl group Chemical group 0.000 claims description 20
- 230000002401 inhibitory effect Effects 0.000 claims description 16
- 239000003937 drug carrier Substances 0.000 claims description 12
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 10
- 208000027096 gram-negative bacterial infections Diseases 0.000 claims description 10
- MYSWGUAQZAJSOK-UHFFFAOYSA-N ciprofloxacin Chemical compound C12=CC(N3CCNCC3)=C(F)C=C2C(=O)C(C(=O)O)=CN1C1CC1 MYSWGUAQZAJSOK-UHFFFAOYSA-N 0.000 claims description 8
- 241000588921 Enterobacteriaceae Species 0.000 claims description 7
- 241000589517 Pseudomonas aeruginosa Species 0.000 claims description 7
- 241000589291 Acinetobacter Species 0.000 claims description 6
- 241000588769 Proteus <enterobacteria> Species 0.000 claims description 6
- 241000607142 Salmonella Species 0.000 claims description 6
- 241001673062 Achromobacter xylosoxidans Species 0.000 claims description 5
- 241000588914 Enterobacter Species 0.000 claims description 5
- 241000588724 Escherichia coli Species 0.000 claims description 5
- 241000588653 Neisseria Species 0.000 claims description 5
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 claims description 5
- 241000607720 Serratia Species 0.000 claims description 5
- 241000122973 Stenotrophomonas maltophilia Species 0.000 claims description 5
- 238000002360 preparation method Methods 0.000 claims description 5
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 claims description 4
- 241000589513 Burkholderia cepacia Species 0.000 claims description 4
- 241000588923 Citrobacter Species 0.000 claims description 4
- 108090000790 Enzymes Proteins 0.000 claims description 4
- 102000004190 Enzymes Human genes 0.000 claims description 4
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 claims description 4
- 241000606790 Haemophilus Species 0.000 claims description 4
- 241000588748 Klebsiella Species 0.000 claims description 4
- 229960003405 ciprofloxacin Drugs 0.000 claims description 4
- 229940124597 therapeutic agent Drugs 0.000 claims description 4
- WZPBZJONDBGPKJ-UHFFFAOYSA-N Antibiotic SQ 26917 Natural products O=C1N(S(O)(=O)=O)C(C)C1NC(=O)C(=NOC(C)(C)C(O)=O)C1=CSC(N)=N1 WZPBZJONDBGPKJ-UHFFFAOYSA-N 0.000 claims description 3
- 241000588807 Bordetella Species 0.000 claims description 3
- 241000046135 Cedecea Species 0.000 claims description 3
- 241000607473 Edwardsiella <enterobacteria> Species 0.000 claims description 3
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 claims description 3
- 229930182566 Gentamicin Natural products 0.000 claims description 3
- 241000589248 Legionella Species 0.000 claims description 3
- 241000588771 Morganella <proteobacterium> Species 0.000 claims description 3
- 108010040201 Polymyxins Proteins 0.000 claims description 3
- 241000588768 Providencia Species 0.000 claims description 3
- 241000589516 Pseudomonas Species 0.000 claims description 3
- 241000607768 Shigella Species 0.000 claims description 3
- 239000004098 Tetracycline Substances 0.000 claims description 3
- 108010059993 Vancomycin Proteins 0.000 claims description 3
- 241000607734 Yersinia <bacteria> Species 0.000 claims description 3
- 229960004099 azithromycin Drugs 0.000 claims description 3
- MQTOSJVFKKJCRP-BICOPXKESA-N azithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)N(C)C[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 MQTOSJVFKKJCRP-BICOPXKESA-N 0.000 claims description 3
- 229960003644 aztreonam Drugs 0.000 claims description 3
- WZPBZJONDBGPKJ-VEHQQRBSSA-N aztreonam Chemical compound O=C1N(S([O-])(=O)=O)[C@@H](C)[C@@H]1NC(=O)C(=N/OC(C)(C)C(O)=O)\C1=CSC([NH3+])=N1 WZPBZJONDBGPKJ-VEHQQRBSSA-N 0.000 claims description 3
- 125000005843 halogen group Chemical group 0.000 claims description 3
- 229960001225 rifampicin Drugs 0.000 claims description 3
- 235000019364 tetracycline Nutrition 0.000 claims description 3
- 150000003522 tetracyclines Chemical class 0.000 claims description 3
- 229960000707 tobramycin Drugs 0.000 claims description 3
- NLVFBUXFDBBNBW-PBSUHMDJSA-N tobramycin Chemical compound N[C@@H]1C[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N NLVFBUXFDBBNBW-PBSUHMDJSA-N 0.000 claims description 3
- 229960003165 vancomycin Drugs 0.000 claims description 3
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 claims description 3
- SGKRLCUYIXIAHR-AKNGSSGZSA-N (4s,4ar,5s,5ar,6r,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1=CC=C2[C@H](C)[C@@H]([C@H](O)[C@@H]3[C@](C(O)=C(C(N)=O)C(=O)[C@H]3N(C)C)(O)C3=O)C3=C(O)C2=C1O SGKRLCUYIXIAHR-AKNGSSGZSA-N 0.000 claims description 2
- 125000006273 (C1-C3) alkyl group Chemical group 0.000 claims description 2
- 241001453380 Burkholderia Species 0.000 claims description 2
- 208000007764 Legionnaires' Disease Diseases 0.000 claims description 2
- GSDSWSVVBLHKDQ-JTQLQIEISA-N Levofloxacin Chemical compound C([C@@H](N1C2=C(C(C(C(O)=O)=C1)=O)C=C1F)C)OC2=C1N1CCN(C)CC1 GSDSWSVVBLHKDQ-JTQLQIEISA-N 0.000 claims description 2
- 241000588621 Moraxella Species 0.000 claims description 2
- WKDDRNSBRWANNC-UHFFFAOYSA-N Thienamycin Natural products C1C(SCCN)=C(C(O)=O)N2C(=O)C(C(O)C)C21 WKDDRNSBRWANNC-UHFFFAOYSA-N 0.000 claims description 2
- 241000607598 Vibrio Species 0.000 claims description 2
- 229960004821 amikacin Drugs 0.000 claims description 2
- LKCWBDHBTVXHDL-RMDFUYIESA-N amikacin Chemical compound O([C@@H]1[C@@H](N)C[C@H]([C@@H]([C@H]1O)O[C@@H]1[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O1)O)NC(=O)[C@@H](O)CCN)[C@H]1O[C@H](CN)[C@@H](O)[C@H](O)[C@H]1O LKCWBDHBTVXHDL-RMDFUYIESA-N 0.000 claims description 2
- 229960000723 ampicillin Drugs 0.000 claims description 2
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 claims description 2
- 229960002100 cefepime Drugs 0.000 claims description 2
- 229960004261 cefotaxime Drugs 0.000 claims description 2
- AZZMGZXNTDTSME-JUZDKLSSSA-M cefotaxime sodium Chemical compound [Na+].N([C@@H]1C(N2C(=C(COC(C)=O)CS[C@@H]21)C([O-])=O)=O)C(=O)\C(=N/OC)C1=CSC(N)=N1 AZZMGZXNTDTSME-JUZDKLSSSA-M 0.000 claims description 2
- 229960004755 ceftriaxone Drugs 0.000 claims description 2
- VAAUVRVFOQPIGI-SPQHTLEESA-N ceftriaxone Chemical compound S([C@@H]1[C@@H](C(N1C=1C(O)=O)=O)NC(=O)\C(=N/OC)C=2N=C(N)SC=2)CC=1CSC1=NC(=O)C(=O)NN1C VAAUVRVFOQPIGI-SPQHTLEESA-N 0.000 claims description 2
- 229960002227 clindamycin Drugs 0.000 claims description 2
- KDLRVYVGXIQJDK-AWPVFWJPSA-N clindamycin Chemical compound CN1C[C@H](CCC)C[C@H]1C(=O)N[C@H]([C@H](C)Cl)[C@@H]1[C@H](O)[C@H](O)[C@@H](O)[C@@H](SC)O1 KDLRVYVGXIQJDK-AWPVFWJPSA-N 0.000 claims description 2
- 229960003722 doxycycline Drugs 0.000 claims description 2
- 229960003276 erythromycin Drugs 0.000 claims description 2
- 229960002182 imipenem Drugs 0.000 claims description 2
- ZSKVGTPCRGIANV-ZXFLCMHBSA-N imipenem Chemical compound C1C(SCC\N=C\N)=C(C(O)=O)N2C(=O)[C@H]([C@H](O)C)[C@H]21 ZSKVGTPCRGIANV-ZXFLCMHBSA-N 0.000 claims description 2
- 229960003376 levofloxacin Drugs 0.000 claims description 2
- DMJNNHOOLUXYBV-PQTSNVLCSA-N meropenem Chemical compound C=1([C@H](C)[C@@H]2[C@H](C(N2C=1C(O)=O)=O)[C@H](O)C)S[C@@H]1CN[C@H](C(=O)N(C)C)C1 DMJNNHOOLUXYBV-PQTSNVLCSA-N 0.000 claims description 2
- 229960002260 meropenem Drugs 0.000 claims description 2
- 229940056360 penicillin g Drugs 0.000 claims description 2
- 229960002292 piperacillin Drugs 0.000 claims description 2
- WCMIIGXFCMNQDS-IDYPWDAWSA-M piperacillin sodium Chemical compound [Na+].O=C1C(=O)N(CC)CCN1C(=O)N[C@H](C=1C=CC=CC=1)C(=O)N[C@@H]1C(=O)N2[C@@H](C([O-])=O)C(C)(C)S[C@@H]21 WCMIIGXFCMNQDS-IDYPWDAWSA-M 0.000 claims description 2
- 229960002180 tetracycline Drugs 0.000 claims description 2
- 229930101283 tetracycline Natural products 0.000 claims description 2
- 229960004659 ticarcillin Drugs 0.000 claims description 2
- OHKOGUYZJXTSFX-KZFFXBSXSA-N ticarcillin Chemical compound C=1([C@@H](C(O)=O)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)C=CSC=1 OHKOGUYZJXTSFX-KZFFXBSXSA-N 0.000 claims description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims 13
- MMRINLZOZVAPDZ-LSGRDSQZSA-N (6r,7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-[(1-methylpyrrolidin-1-ium-1-yl)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;chloride Chemical compound Cl.S([C@@H]1[C@@H](C(N1C=1C([O-])=O)=O)NC(=O)\C(=N/OC)C=2N=C(N)SC=2)CC=1C[N+]1(C)CCCC1 MMRINLZOZVAPDZ-LSGRDSQZSA-N 0.000 claims 1
- JQXXHWHPUNPDRT-WLSIYKJHSA-N rifampicin Chemical compound O([C@](C1=O)(C)O/C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)\C=C\C=C(C)/C(=O)NC=2C(O)=C3C([O-])=C4C)C)OC)C4=C1C3=C(O)C=2\C=N\N1CC[NH+](C)CC1 JQXXHWHPUNPDRT-WLSIYKJHSA-N 0.000 claims 1
- MYPYJXKWCTUITO-LYRMYLQWSA-O vancomycin(1+) Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C([O-])=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)[NH2+]C)[C@H]1C[C@](C)([NH3+])[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-O 0.000 claims 1
- 230000000844 anti-bacterial effect Effects 0.000 abstract description 14
- 150000002894 organic compounds Chemical class 0.000 abstract 1
- 238000003786 synthesis reaction Methods 0.000 description 269
- 230000015572 biosynthetic process Effects 0.000 description 264
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 188
- 239000000243 solution Substances 0.000 description 137
- 238000005160 1H NMR spectroscopy Methods 0.000 description 107
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 100
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 100
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 100
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 93
- 235000019439 ethyl acetate Nutrition 0.000 description 87
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 85
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 73
- 239000011541 reaction mixture Substances 0.000 description 70
- AICOOMRHRUFYCM-ZRRPKQBOSA-N oxazine, 1 Chemical compound C([C@@H]1[C@H](C(C[C@]2(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@]21C)=O)CC1=CC2)C[C@H]1[C@@]1(C)[C@H]2N=C(C(C)C)OC1 AICOOMRHRUFYCM-ZRRPKQBOSA-N 0.000 description 67
- OKKJLVBELUTLKV-MZCSYVLQSA-N Deuterated methanol Chemical compound [2H]OC([2H])([2H])[2H] OKKJLVBELUTLKV-MZCSYVLQSA-N 0.000 description 62
- 238000006243 chemical reaction Methods 0.000 description 57
- 239000012044 organic layer Substances 0.000 description 56
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N DMSO Substances CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 53
- 238000010898 silica gel chromatography Methods 0.000 description 48
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 46
- 239000000047 product Substances 0.000 description 45
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 44
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 44
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 43
- 239000012267 brine Substances 0.000 description 42
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 40
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 39
- 239000002904 solvent Substances 0.000 description 37
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 36
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 36
- 150000002431 hydrogen Chemical group 0.000 description 34
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 33
- 239000003795 chemical substances by application Substances 0.000 description 32
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 32
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 30
- 238000003756 stirring Methods 0.000 description 29
- 229920006395 saturated elastomer Polymers 0.000 description 28
- 239000011734 sodium Substances 0.000 description 26
- HEMHJVSKTPXQMS-UHFFFAOYSA-M sodium hydroxide Inorganic materials [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 25
- 238000000746 purification Methods 0.000 description 22
- 239000007787 solid Substances 0.000 description 21
- 0 *I***C(NO)=O Chemical compound *I***C(NO)=O 0.000 description 20
- 239000007864 aqueous solution Substances 0.000 description 20
- 239000000463 material Substances 0.000 description 20
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 19
- 239000010410 layer Substances 0.000 description 19
- 239000002244 precipitate Substances 0.000 description 19
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 18
- 229910052805 deuterium Inorganic materials 0.000 description 18
- 235000019341 magnesium sulphate Nutrition 0.000 description 18
- 239000000741 silica gel Substances 0.000 description 18
- 229910002027 silica gel Inorganic materials 0.000 description 18
- 239000003242 anti bacterial agent Substances 0.000 description 17
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 17
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 16
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 16
- 229940079593 drug Drugs 0.000 description 16
- 230000008569 process Effects 0.000 description 16
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 15
- 125000006239 protecting group Chemical group 0.000 description 15
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 14
- 239000002585 base Substances 0.000 description 14
- 238000001914 filtration Methods 0.000 description 14
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 13
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 13
- 239000002552 dosage form Substances 0.000 description 13
- 150000002148 esters Chemical class 0.000 description 13
- 235000019441 ethanol Nutrition 0.000 description 13
- 229940093499 ethyl acetate Drugs 0.000 description 13
- 150000003254 radicals Chemical class 0.000 description 13
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 12
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 12
- 238000005481 NMR spectroscopy Methods 0.000 description 12
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 12
- 239000004480 active ingredient Substances 0.000 description 12
- 239000003153 chemical reaction reagent Substances 0.000 description 12
- 239000013078 crystal Substances 0.000 description 12
- 239000003921 oil Substances 0.000 description 12
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 12
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 11
- 230000001580 bacterial effect Effects 0.000 description 11
- 201000010099 disease Diseases 0.000 description 11
- 238000009472 formulation Methods 0.000 description 11
- 235000019198 oils Nutrition 0.000 description 11
- 239000000843 powder Substances 0.000 description 11
- 125000001743 benzylic group Chemical group 0.000 description 10
- 239000004305 biphenyl Substances 0.000 description 10
- 229960004132 diethyl ether Drugs 0.000 description 10
- 238000010348 incorporation Methods 0.000 description 10
- 239000007858 starting material Substances 0.000 description 10
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 9
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 9
- 125000004432 carbon atom Chemical group C* 0.000 description 9
- 238000004587 chromatography analysis Methods 0.000 description 9
- 230000000694 effects Effects 0.000 description 9
- 230000005764 inhibitory process Effects 0.000 description 9
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 9
- 230000002829 reductive effect Effects 0.000 description 9
- XJVCBQQIEWBUJL-UHFFFAOYSA-N 1,1-dioxothiane-4-carboxamide Chemical compound NC(=O)C1CCS(=O)(=O)CC1 XJVCBQQIEWBUJL-UHFFFAOYSA-N 0.000 description 8
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 8
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 8
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 8
- 239000000543 intermediate Substances 0.000 description 8
- 239000007788 liquid Substances 0.000 description 8
- 229910052938 sodium sulfate Inorganic materials 0.000 description 8
- 235000011152 sodium sulphate Nutrition 0.000 description 8
- HUHGPYXAVBJSJV-UHFFFAOYSA-N 2-[3,5-bis(2-hydroxyethyl)-1,3,5-triazinan-1-yl]ethanol Chemical compound OCCN1CN(CCO)CN(CCO)C1 HUHGPYXAVBJSJV-UHFFFAOYSA-N 0.000 description 7
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 7
- 150000001412 amines Chemical class 0.000 description 7
- 229940088710 antibiotic agent Drugs 0.000 description 7
- 238000002425 crystallisation Methods 0.000 description 7
- 230000008025 crystallization Effects 0.000 description 7
- 239000000706 filtrate Substances 0.000 description 7
- 238000010438 heat treatment Methods 0.000 description 7
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 7
- 239000000546 pharmaceutical excipient Substances 0.000 description 7
- 229920001223 polyethylene glycol Polymers 0.000 description 7
- 239000004576 sand Substances 0.000 description 7
- 239000003826 tablet Substances 0.000 description 7
- 230000001225 therapeutic effect Effects 0.000 description 7
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 7
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 6
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 6
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 6
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 6
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 6
- 238000010521 absorption reaction Methods 0.000 description 6
- 238000003556 assay Methods 0.000 description 6
- 239000002775 capsule Substances 0.000 description 6
- 239000000969 carrier Substances 0.000 description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 6
- 239000013058 crude material Substances 0.000 description 6
- 239000003085 diluting agent Substances 0.000 description 6
- 208000035475 disorder Diseases 0.000 description 6
- 239000002158 endotoxin Substances 0.000 description 6
- BDERNNFJNOPAEC-UHFFFAOYSA-N n-propyl alcohol Natural products CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 6
- CTSLXHKWHWQRSH-UHFFFAOYSA-N oxalyl chloride Chemical compound ClC(=O)C(Cl)=O CTSLXHKWHWQRSH-UHFFFAOYSA-N 0.000 description 6
- 239000012071 phase Substances 0.000 description 6
- HXITXNWTGFUOAU-UHFFFAOYSA-N phenylboronic acid Chemical compound OB(O)C1=CC=CC=C1 HXITXNWTGFUOAU-UHFFFAOYSA-N 0.000 description 6
- 108090000765 processed proteins & peptides Proteins 0.000 description 6
- KZPYGQFFRCFCPP-UHFFFAOYSA-N 1,1'-bis(diphenylphosphino)ferrocene Chemical compound [Fe+2].C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=C[C-]1P(C=1C=CC=CC=1)C1=CC=CC=C1 KZPYGQFFRCFCPP-UHFFFAOYSA-N 0.000 description 5
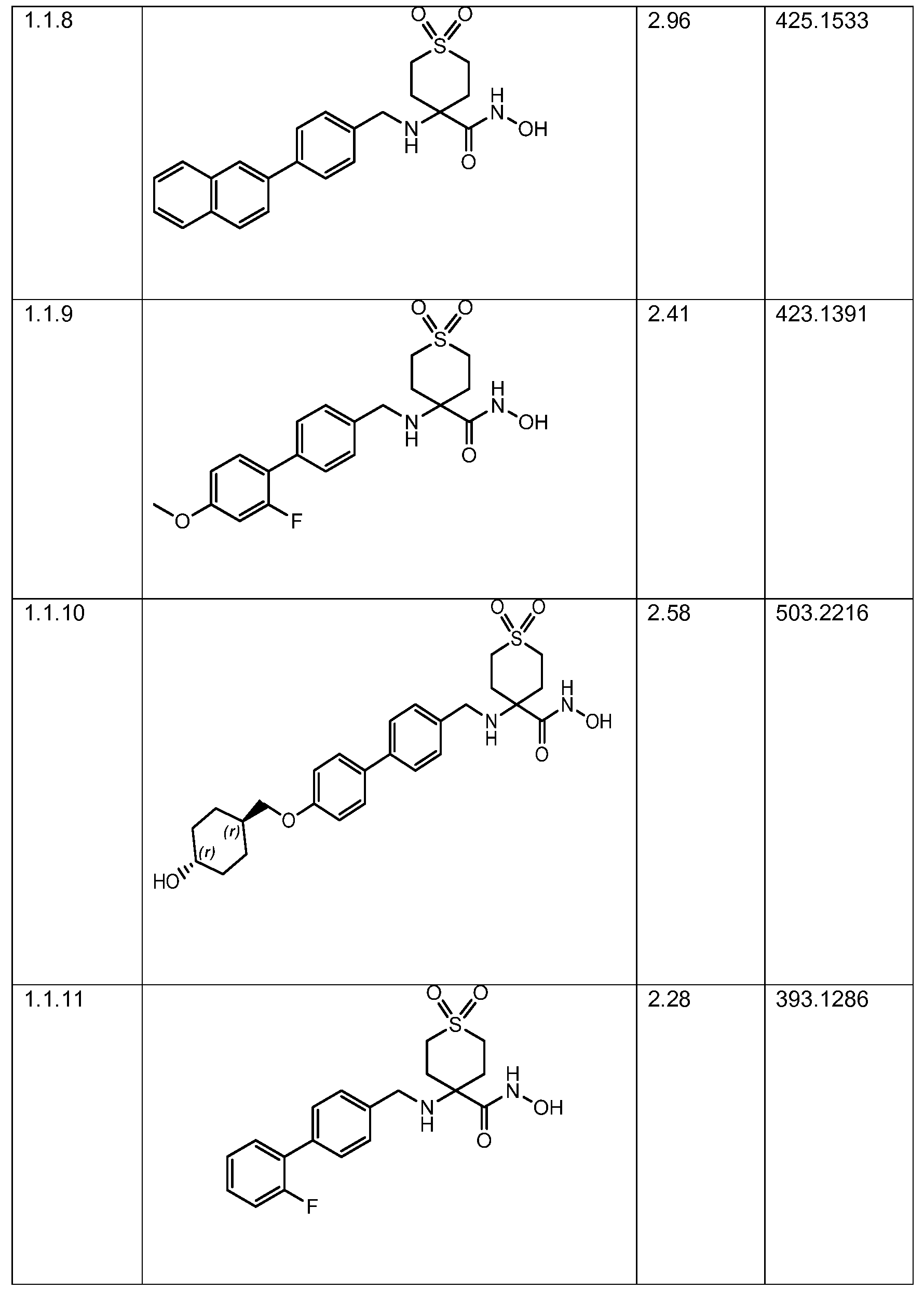
- NMDPZOIIBPOFAN-UHFFFAOYSA-N 2-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound OC1S(CCC(C1)C(=O)N)(=O)=O NMDPZOIIBPOFAN-UHFFFAOYSA-N 0.000 description 5
- 108010010803 Gelatin Proteins 0.000 description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 5
- 241000124008 Mammalia Species 0.000 description 5
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 5
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 5
- 229930006000 Sucrose Natural products 0.000 description 5
- 150000007513 acids Chemical class 0.000 description 5
- 125000005119 alkyl cycloalkyl group Chemical group 0.000 description 5
- 239000003963 antioxidant agent Substances 0.000 description 5
- 235000006708 antioxidants Nutrition 0.000 description 5
- QJKNFPXQHCBTMP-UHFFFAOYSA-N ethyl 5-(4-chloro-2-fluorophenyl)-1,2-oxazole-3-carboxylate Chemical compound CCOC(=O)c1cc(on1)-c1ccc(Cl)cc1F QJKNFPXQHCBTMP-UHFFFAOYSA-N 0.000 description 5
- 239000008273 gelatin Substances 0.000 description 5
- 229920000159 gelatin Polymers 0.000 description 5
- 235000019322 gelatine Nutrition 0.000 description 5
- 235000011852 gelatine desserts Nutrition 0.000 description 5
- 235000011187 glycerol Nutrition 0.000 description 5
- 230000012010 growth Effects 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- AJGGFIRFHVLLBM-UHFFFAOYSA-N methyl 4-[(4-bromo-3-fluorophenyl)methylamino]-1,1-dioxothiane-4-carboxylate Chemical compound BrC1=C(C=C(CNC2(CCS(CC2)(=O)=O)C(=O)OC)C=C1)F AJGGFIRFHVLLBM-UHFFFAOYSA-N 0.000 description 5
- 239000002674 ointment Substances 0.000 description 5
- 230000003287 optical effect Effects 0.000 description 5
- 239000003755 preservative agent Substances 0.000 description 5
- 229910052708 sodium Inorganic materials 0.000 description 5
- 239000012321 sodium triacetoxyborohydride Substances 0.000 description 5
- 239000007921 spray Substances 0.000 description 5
- 239000005720 sucrose Substances 0.000 description 5
- 239000000829 suppository Substances 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 5
- DGXMWHJISAVEOJ-UHFFFAOYSA-N 1-acetyl-N-hydroxy-4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]piperidine-4-carboxamide Chemical compound CC(=O)N1CCC(CCn2ccc(cc2=O)-c2ccccc2)(CC1)C(=O)NO DGXMWHJISAVEOJ-UHFFFAOYSA-N 0.000 description 4
- IBYHHJPAARCAIE-UHFFFAOYSA-N 1-bromo-2-chloroethane Chemical compound ClCCBr IBYHHJPAARCAIE-UHFFFAOYSA-N 0.000 description 4
- GQHTUMJGOHRCHB-UHFFFAOYSA-N 2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine Chemical compound C1CCCCN2CCCN=C21 GQHTUMJGOHRCHB-UHFFFAOYSA-N 0.000 description 4
- FDYFWQVFDMUAHA-UHFFFAOYSA-N 4-[(4-bromo-3-fluorophenyl)methylamino]-N-(oxan-2-yloxy)-1,1-dioxothiane-4-carboxamide Chemical compound BrC1=C(C=C(CNC2(CCS(CC2)(=O)=O)C(=O)NOC2OCCCC2)C=C1)F FDYFWQVFDMUAHA-UHFFFAOYSA-N 0.000 description 4
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 4
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 4
- 241000416162 Astragalus gummifer Species 0.000 description 4
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical compound OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 4
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- 108010078777 Colistin Proteins 0.000 description 4
- 206010011409 Cross infection Diseases 0.000 description 4
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 206010061218 Inflammation Diseases 0.000 description 4
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 4
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 4
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- 229920001615 Tragacanth Polymers 0.000 description 4
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical class OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 4
- WEVYAHXRMPXWCK-FIBGUPNXSA-N acetonitrile-d3 Chemical compound [2H]C([2H])([2H])C#N WEVYAHXRMPXWCK-FIBGUPNXSA-N 0.000 description 4
- 229910052783 alkali metal Inorganic materials 0.000 description 4
- 150000001408 amides Chemical class 0.000 description 4
- 235000019270 ammonium chloride Nutrition 0.000 description 4
- 239000003054 catalyst Substances 0.000 description 4
- 238000003776 cleavage reaction Methods 0.000 description 4
- 238000007796 conventional method Methods 0.000 description 4
- 125000004122 cyclic group Chemical group 0.000 description 4
- 239000003995 emulsifying agent Substances 0.000 description 4
- 229940052303 ethers for general anesthesia Drugs 0.000 description 4
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 4
- 238000001640 fractional crystallisation Methods 0.000 description 4
- 239000012634 fragment Substances 0.000 description 4
- 239000000499 gel Substances 0.000 description 4
- 238000004128 high performance liquid chromatography Methods 0.000 description 4
- 150000004677 hydrates Chemical class 0.000 description 4
- 229930195733 hydrocarbon Natural products 0.000 description 4
- 150000002430 hydrocarbons Chemical group 0.000 description 4
- 238000001727 in vivo Methods 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 150000002500 ions Chemical class 0.000 description 4
- QWTDNUCVQCZILF-UHFFFAOYSA-N isopentane Chemical compound CCC(C)C QWTDNUCVQCZILF-UHFFFAOYSA-N 0.000 description 4
- 230000000155 isotopic effect Effects 0.000 description 4
- 239000008101 lactose Substances 0.000 description 4
- YNESATAKKCNGOF-UHFFFAOYSA-N lithium bis(trimethylsilyl)amide Chemical compound [Li+].C[Si](C)(C)[N-][Si](C)(C)C YNESATAKKCNGOF-UHFFFAOYSA-N 0.000 description 4
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 4
- 230000002503 metabolic effect Effects 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- PRVCXEQAGFJWKX-UHFFFAOYSA-N methyl 4-amino-1,1-dioxothiane-4-carboxylate Chemical compound COC(=O)C1(N)CCS(=O)(=O)CC1 PRVCXEQAGFJWKX-UHFFFAOYSA-N 0.000 description 4
- 150000002772 monosaccharides Chemical class 0.000 description 4
- 229950006780 n-acetylglucosamine Drugs 0.000 description 4
- FFLCNTIJGRFDLX-UHFFFAOYSA-N n-hydroxy-1,1-dioxo-4-[[5-[4-(triazol-2-yl)phenyl]-1,2-oxazol-3-yl]methylamino]thiane-4-carboxamide Chemical compound C1=C(C=2C=CC(=CC=2)N2N=CC=N2)ON=C1CNC1(C(=O)NO)CCS(=O)(=O)CC1 FFLCNTIJGRFDLX-UHFFFAOYSA-N 0.000 description 4
- 239000006072 paste Substances 0.000 description 4
- 239000006187 pill Substances 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 239000002516 radical scavenger Substances 0.000 description 4
- 238000010992 reflux Methods 0.000 description 4
- 238000004007 reversed phase HPLC Methods 0.000 description 4
- 239000000377 silicon dioxide Substances 0.000 description 4
- 239000012279 sodium borohydride Substances 0.000 description 4
- 229910000033 sodium borohydride Inorganic materials 0.000 description 4
- 229910000029 sodium carbonate Inorganic materials 0.000 description 4
- 239000012453 solvate Substances 0.000 description 4
- 238000000638 solvent extraction Methods 0.000 description 4
- 239000011877 solvent mixture Substances 0.000 description 4
- 235000019698 starch Nutrition 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 239000000454 talc Substances 0.000 description 4
- 235000012222 talc Nutrition 0.000 description 4
- 229910052623 talc Inorganic materials 0.000 description 4
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 4
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 4
- 235000010487 tragacanth Nutrition 0.000 description 4
- 239000000196 tragacanth Substances 0.000 description 4
- 229940116362 tragacanth Drugs 0.000 description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 4
- 239000001993 wax Substances 0.000 description 4
- 239000000080 wetting agent Substances 0.000 description 4
- ULUIXJDBPYBAHS-UHFFFAOYSA-N (2-fluoro-4-methoxyphenyl)boronic acid Chemical compound COC1=CC=C(B(O)O)C(F)=C1 ULUIXJDBPYBAHS-UHFFFAOYSA-N 0.000 description 3
- QCSLIRFWJPOENV-UHFFFAOYSA-N (2-fluorophenyl)boronic acid Chemical compound OB(O)C1=CC=CC=C1F QCSLIRFWJPOENV-UHFFFAOYSA-N 0.000 description 3
- YBNDRTRLXPEWKQ-UHFFFAOYSA-N (4-chloro-2-fluorophenyl)boronic acid Chemical compound OB(O)C1=CC=C(Cl)C=C1F YBNDRTRLXPEWKQ-UHFFFAOYSA-N 0.000 description 3
- LFONTVSSNDSRJG-UHFFFAOYSA-N 1-[4-(triazol-2-yl)phenyl]ethanone Chemical compound C1=CC(C(=O)C)=CC=C1N1N=CC=N1 LFONTVSSNDSRJG-UHFFFAOYSA-N 0.000 description 3
- JPCXERIJFYEYBE-UHFFFAOYSA-N 1-[[4-(2-fluoro-4-methoxyphenyl)phenyl]methylamino]-N,3-dihydroxycyclobutane-1-carboxamide Chemical compound FC1=C(C=CC(=C1)OC)C1=CC=C(C=C1)CNC1(CC(C1)O)C(=O)NO JPCXERIJFYEYBE-UHFFFAOYSA-N 0.000 description 3
- LPMLXBQJYQBVPF-UHFFFAOYSA-N 2-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]triazole Chemical compound O1C(C)(C)C(C)(C)OB1C1=CC=C(N2N=CC=N2)C=C1 LPMLXBQJYQBVPF-UHFFFAOYSA-N 0.000 description 3
- SXZKMHPHJNIJDM-UHFFFAOYSA-N 3-(4-chloro-2-fluorophenyl)-1,2-oxazole-5-carbaldehyde Chemical compound Fc1cc(Cl)ccc1-c1cc(C=O)on1 SXZKMHPHJNIJDM-UHFFFAOYSA-N 0.000 description 3
- SYCFYQFCFHKYPI-UHFFFAOYSA-N 4-(2-phenylethynyl)benzaldehyde Chemical compound C1=CC(C=O)=CC=C1C#CC1=CC=CC=C1 SYCFYQFCFHKYPI-UHFFFAOYSA-N 0.000 description 3
- HNNNCQCIIXTYJT-UHFFFAOYSA-N 4-(4-chloro-2-fluorophenyl)benzaldehyde Chemical compound FC1=CC(Cl)=CC=C1C1=CC=C(C=O)C=C1 HNNNCQCIIXTYJT-UHFFFAOYSA-N 0.000 description 3
- JVXKJAOSMNOBSI-UHFFFAOYSA-N 4-[(3-fluoro-4-phenylphenyl)methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C=CC(=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NO)C1=CC=CC=C1 JVXKJAOSMNOBSI-UHFFFAOYSA-N 0.000 description 3
- AHUYTDOGRSJIRH-UHFFFAOYSA-N 4-[2-[4-(4-chlorophenyl)triazol-2-yl]ethyl]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ClC1=CC=C(C=C1)C1=NN(N=C1)CCC1(CCS(CC1)(=O)=O)C(=O)NO AHUYTDOGRSJIRH-UHFFFAOYSA-N 0.000 description 3
- CBBGSYBPCSDXQY-UHFFFAOYSA-N 4-[[4-chloro-5-(4-chloro-2-fluorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(=O)(=O)CC1)NCC1=NOC(=C1Cl)C1=C(F)C=C(Cl)C=C1 CBBGSYBPCSDXQY-UHFFFAOYSA-N 0.000 description 3
- DKMCTWOAEQUILK-UHFFFAOYSA-N 4-[[5-(4-chloro-2-fluorophenyl)-1,2-oxazol-3-yl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound C1=C(C=2C(=CC(Cl)=CC=2)F)ON=C1CNC1(C(=O)NO)CCS(=O)(=O)CC1 DKMCTWOAEQUILK-UHFFFAOYSA-N 0.000 description 3
- GBWYUPVVENXYRV-UHFFFAOYSA-N 4-[[5-(4-chloro-2-fluorophenyl)-4-fluoro-1,2-oxazol-3-yl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound N=1OC(C=2C(=CC(Cl)=CC=2)F)=C(F)C=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 GBWYUPVVENXYRV-UHFFFAOYSA-N 0.000 description 3
- BZGKESHJAZIBAJ-UHFFFAOYSA-N 4-[[5-[4-(difluoromethoxy)phenyl]-1,2-oxazol-3-yl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound C1=C(C=2C=CC(OC(F)F)=CC=2)ON=C1CNC1(C(=O)NO)CCS(=O)(=O)CC1 BZGKESHJAZIBAJ-UHFFFAOYSA-N 0.000 description 3
- GQHVAFIEAZLVJL-UHFFFAOYSA-N 5-(4-chloro-2-fluorophenyl)-1,2-oxazole-3-carbaldehyde Chemical compound FC1=CC(Cl)=CC=C1C1=CC(C=O)=NO1 GQHVAFIEAZLVJL-UHFFFAOYSA-N 0.000 description 3
- 229920001817 Agar Polymers 0.000 description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 3
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 3
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 3
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 3
- 229910004373 HOAc Inorganic materials 0.000 description 3
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 3
- 240000007472 Leucaena leucocephala Species 0.000 description 3
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 3
- 239000012359 Methanesulfonyl chloride Substances 0.000 description 3
- 229910017912 NH2OH Inorganic materials 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- 206010057190 Respiratory tract infections Diseases 0.000 description 3
- 206010040047 Sepsis Diseases 0.000 description 3
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 3
- DIVSUBTWKRTRPF-UHFFFAOYSA-N [3-(4-chloro-2-fluorophenyl)-1,2-oxazol-5-yl]methanol Chemical compound OCc1cc(no1)-c1ccc(Cl)cc1F DIVSUBTWKRTRPF-UHFFFAOYSA-N 0.000 description 3
- ZSOADVOHSMCRBY-UHFFFAOYSA-N [5-(4-chloro-2-fluorophenyl)-1,2-oxazol-3-yl]methanol Chemical compound OCc1cc(on1)-c1ccc(Cl)cc1F ZSOADVOHSMCRBY-UHFFFAOYSA-N 0.000 description 3
- 235000010419 agar Nutrition 0.000 description 3
- 235000010443 alginic acid Nutrition 0.000 description 3
- 229920000615 alginic acid Polymers 0.000 description 3
- 150000003863 ammonium salts Chemical class 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 235000012216 bentonite Nutrition 0.000 description 3
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 3
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 3
- 229910000024 caesium carbonate Inorganic materials 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 150000001768 cations Chemical class 0.000 description 3
- 239000007795 chemical reaction product Substances 0.000 description 3
- 238000004296 chiral HPLC Methods 0.000 description 3
- 230000001684 chronic effect Effects 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 239000003086 colorant Substances 0.000 description 3
- 239000000470 constituent Substances 0.000 description 3
- 238000001816 cooling Methods 0.000 description 3
- 239000006071 cream Substances 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- JGRIYNGMKOXSKB-UHFFFAOYSA-N ethyl 4-(4-chloro-2-fluorophenyl)-2,4-dioxobutanoate Chemical compound CCOC(=O)C(=O)CC(=O)c1ccc(Cl)cc1F JGRIYNGMKOXSKB-UHFFFAOYSA-N 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
- 229910052731 fluorine Inorganic materials 0.000 description 3
- 239000011737 fluorine Substances 0.000 description 3
- 239000008187 granular material Substances 0.000 description 3
- 238000011065 in-situ storage Methods 0.000 description 3
- 239000003701 inert diluent Substances 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 229920006008 lipopolysaccharide Polymers 0.000 description 3
- 238000001294 liquid chromatography-tandem mass spectrometry Methods 0.000 description 3
- 239000006210 lotion Substances 0.000 description 3
- 239000000314 lubricant Substances 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- QARBMVPHQWIHKH-UHFFFAOYSA-N methanesulfonyl chloride Chemical compound CS(Cl)(=O)=O QARBMVPHQWIHKH-UHFFFAOYSA-N 0.000 description 3
- OCBOIQWPCVXVKT-UHFFFAOYSA-N methyl thiane-4-carboxylate Chemical compound COC(=O)C1CCSCC1 OCBOIQWPCVXVKT-UHFFFAOYSA-N 0.000 description 3
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 3
- 239000004006 olive oil Substances 0.000 description 3
- 238000007911 parenteral administration Methods 0.000 description 3
- 230000004962 physiological condition Effects 0.000 description 3
- 235000018102 proteins Nutrition 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 3
- 230000002285 radioactive effect Effects 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 238000006722 reduction reaction Methods 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 3
- 235000012239 silicon dioxide Nutrition 0.000 description 3
- 239000002002 slurry Substances 0.000 description 3
- 235000010356 sorbitol Nutrition 0.000 description 3
- 239000000600 sorbitol Substances 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 238000006467 substitution reaction Methods 0.000 description 3
- 235000000346 sugar Nutrition 0.000 description 3
- 150000008163 sugars Chemical class 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- 239000000375 suspending agent Substances 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 235000002906 tartaric acid Nutrition 0.000 description 3
- 239000011975 tartaric acid Substances 0.000 description 3
- 230000000699 topical effect Effects 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 2
- OBETXYAYXDNJHR-SSDOTTSWSA-M (2r)-2-ethylhexanoate Chemical compound CCCC[C@@H](CC)C([O-])=O OBETXYAYXDNJHR-SSDOTTSWSA-M 0.000 description 2
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 2
- NQMRYYAAICMHPE-UHFFFAOYSA-N (4-methoxyphenyl)boron Chemical compound [B]C1=CC=C(OC)C=C1 NQMRYYAAICMHPE-UHFFFAOYSA-N 0.000 description 2
- SCYULBFZEHDVBN-UHFFFAOYSA-N 1,1-Dichloroethane Chemical compound CC(Cl)Cl SCYULBFZEHDVBN-UHFFFAOYSA-N 0.000 description 2
- WBIDJHKVRCDMFZ-UHFFFAOYSA-N 1,1-dioxothiolane-3-carboxamide Chemical compound NC(=O)C1CCS(=O)(=O)C1 WBIDJHKVRCDMFZ-UHFFFAOYSA-N 0.000 description 2
- HZQLUIZFUXNFHK-UHFFFAOYSA-N 1-(bromomethyl)-4-phenylbenzene Chemical group C1=CC(CBr)=CC=C1C1=CC=CC=C1 HZQLUIZFUXNFHK-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- MYHJCTUTPIKNAT-UHFFFAOYSA-N 1-o-tert-butyl 4-o-ethyl piperidine-1,4-dicarboxylate Chemical compound CCOC(=O)C1CCN(C(=O)OC(C)(C)C)CC1 MYHJCTUTPIKNAT-UHFFFAOYSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 2
- BAXOFTOLAUCFNW-UHFFFAOYSA-N 1H-indazole Chemical compound C1=CC=C2C=NNC2=C1 BAXOFTOLAUCFNW-UHFFFAOYSA-N 0.000 description 2
- UENGBOCGGKLVJJ-UHFFFAOYSA-N 2-chloro-1-(2,4-difluorophenyl)ethanone Chemical compound FC1=CC=C(C(=O)CCl)C(F)=C1 UENGBOCGGKLVJJ-UHFFFAOYSA-N 0.000 description 2
- SOOGPMALGBTTAU-UHFFFAOYSA-N 3-[[5-(4-chloro-2-fluorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiolane-3-carboxamide Chemical compound ClC1=CC(=C(C=C1)C1=CC(=NO1)CNC1(CS(CC1)(=O)=O)C(=O)NO)F SOOGPMALGBTTAU-UHFFFAOYSA-N 0.000 description 2
- SZXMDUKWIYIKKS-UHFFFAOYSA-N 3-phenyl-1,2-oxazole-5-carbaldehyde Chemical compound O1C(C=O)=CC(C=2C=CC=CC=2)=N1 SZXMDUKWIYIKKS-UHFFFAOYSA-N 0.000 description 2
- QVRMBOLVPWUJKK-UHFFFAOYSA-N 4-[2-[4-(2-fluoro-4-methoxyphenyl)-2-oxopyridin-1-yl]ethyl]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C=CC(=C1)OC)C1=CC(N(C=C1)CCC1(CCS(CC1)(=O)=O)C(=O)NO)=O QVRMBOLVPWUJKK-UHFFFAOYSA-N 0.000 description 2
- UNOBNJILYAZLKA-UHFFFAOYSA-N 4-[2-[4-(4-chloro-2-fluorophenyl)-2-oxopyridin-1-yl]ethyl]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ClC1=CC(=C(C=C1)C1=CC(N(C=C1)CCC1(CCS(CC1)(=O)=O)C(=O)NO)=O)F UNOBNJILYAZLKA-UHFFFAOYSA-N 0.000 description 2
- YJFZEGURBNVTAV-UHFFFAOYSA-N 4-[[5-(2,2-difluoro-1,3-benzodioxol-5-yl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1(OC2=C(O1)C=CC(=C2)C2=CC(=NO2)CNC2(CCS(CC2)(=O)=O)C(=O)NO)F YJFZEGURBNVTAV-UHFFFAOYSA-N 0.000 description 2
- RANVPUIVGPMODH-UHFFFAOYSA-N 4-[[5-(2-fluoro-4-methylphenyl)-1,2-oxazol-3-yl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=CC(C)=CC=C1C1=CC(CNC2(CCS(=O)(=O)CC2)C(=O)NO)=NO1 RANVPUIVGPMODH-UHFFFAOYSA-N 0.000 description 2
- CJYUPLLILBZFRV-UHFFFAOYSA-N 4-[[5-(2-fluorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C=CC=C1)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)NO CJYUPLLILBZFRV-UHFFFAOYSA-N 0.000 description 2
- RNARVQDPWAGOCR-UHFFFAOYSA-N 4-[[5-(3-chlorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(=O)(=O)CC1)NCC1=NOC(=C1)C1=CC(Cl)=CC=C1 RNARVQDPWAGOCR-UHFFFAOYSA-N 0.000 description 2
- YULKEPKWVMGDHR-UHFFFAOYSA-N 4-[[5-(3-fluoro-4-methoxyphenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC=1C=C(C=CC1OC)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)NO YULKEPKWVMGDHR-UHFFFAOYSA-N 0.000 description 2
- ILCBBGOPTBUOSD-UHFFFAOYSA-N 4-[[5-(4-cyanophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(=O)(=O)CC1)NCC1=NOC(=C1)C1=CC=C(C=C1)C#N ILCBBGOPTBUOSD-UHFFFAOYSA-N 0.000 description 2
- CMBNKKTVJHVZDE-UHFFFAOYSA-N 4-[[5-(4-fluoro-3-methoxyphenyl)-1,2-oxazol-3-yl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound C1=C(F)C(OC)=CC(C=2ON=C(CNC3(CCS(=O)(=O)CC3)C(=O)NO)C=2)=C1 CMBNKKTVJHVZDE-UHFFFAOYSA-N 0.000 description 2
- NKNJPVSXOAXONX-UHFFFAOYSA-N 4-[[5-[2-fluoro-4-(trifluoromethoxy)phenyl]-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(=O)(=O)CC1)NCC1=NOC(=C1)C1=C(F)C=C(OC(F)(F)F)C=C1 NKNJPVSXOAXONX-UHFFFAOYSA-N 0.000 description 2
- ALYNCZNDIQEVRV-UHFFFAOYSA-N 4-aminobenzoic acid Chemical compound NC1=CC=C(C(O)=O)C=C1 ALYNCZNDIQEVRV-UHFFFAOYSA-N 0.000 description 2
- ZRYZBQLXDKPBDU-UHFFFAOYSA-N 4-bromobenzaldehyde Chemical compound BrC1=CC=C(C=O)C=C1 ZRYZBQLXDKPBDU-UHFFFAOYSA-N 0.000 description 2
- POHJRJNCZQENQG-UHFFFAOYSA-N 4-iodo-1h-pyridin-2-one Chemical compound IC=1C=CNC(=O)C=1 POHJRJNCZQENQG-UHFFFAOYSA-N 0.000 description 2
- ISDBWOPVZKNQDW-UHFFFAOYSA-N 4-phenylbenzaldehyde Chemical compound C1=CC(C=O)=CC=C1C1=CC=CC=C1 ISDBWOPVZKNQDW-UHFFFAOYSA-N 0.000 description 2
- QRHAQXZGSWFBOK-UHFFFAOYSA-N 5-phenyl-1,2-oxazole-3-carbaldehyde Chemical compound O1N=C(C=O)C=C1C1=CC=CC=C1 QRHAQXZGSWFBOK-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 206010006458 Bronchitis chronic Diseases 0.000 description 2
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 2
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 2
- APXOQKJSQCYXIM-UHFFFAOYSA-N CCC(CC)(CC1)CCS1(=O)=O Chemical compound CCC(CC)(CC1)CCS1(=O)=O APXOQKJSQCYXIM-UHFFFAOYSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 229930186147 Cephalosporin Natural products 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 2
- UEXCJVNBTNXOEH-UHFFFAOYSA-N Ethynylbenzene Chemical compound C#CC1=CC=CC=C1 UEXCJVNBTNXOEH-UHFFFAOYSA-N 0.000 description 2
- 241000192125 Firmicutes Species 0.000 description 2
- 241000206672 Gelidium Species 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 241000588747 Klebsiella pneumoniae Species 0.000 description 2
- 229940123346 LpxC inhibitor Drugs 0.000 description 2
- 208000032376 Lung infection Diseases 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- 238000003820 Medium-pressure liquid chromatography Methods 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 2
- LKUQXDROMCQHBI-UHFFFAOYSA-N N-hydroxy-1,1-dioxo-3-[(5-phenyl-1,2-oxazol-3-yl)methylamino]thiolane-3-carboxamide Chemical compound ONC(=O)C1(CCS(=O)(=O)C1)NCc1cc(on1)-c1ccccc1 LKUQXDROMCQHBI-UHFFFAOYSA-N 0.000 description 2
- VYNGPZRBPXSXTH-UHFFFAOYSA-N N-hydroxy-1,1-dioxo-4-[(1-phenyltriazol-4-yl)methylamino]thiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(CC1)(=O)=O)NCC=1N=NN(C1)C1=CC=CC=C1 VYNGPZRBPXSXTH-UHFFFAOYSA-N 0.000 description 2
- FOZBSVNOFCLOBE-UHFFFAOYSA-N N-hydroxy-1,1-dioxo-4-[2-(4-phenylphenyl)ethyl]thiane-4-carboxamide Chemical compound C1(=CC=C(C=C1)CCC1(CCS(CC1)(=O)=O)C(=O)NO)C1=CC=CC=C1 FOZBSVNOFCLOBE-UHFFFAOYSA-N 0.000 description 2
- RAVLLWJDVRSEJO-UHFFFAOYSA-N N-hydroxy-1,1-dioxo-4-[2-(4-phenylpyrazol-1-yl)ethyl]thiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(CC1)(=O)=O)CCN1N=CC(=C1)C1=CC=CC=C1 RAVLLWJDVRSEJO-UHFFFAOYSA-N 0.000 description 2
- BHPSUYPKNUMMDW-UHFFFAOYSA-N N-hydroxy-1,1-dioxo-4-[[4-(2-phenylethynyl)phenyl]methylamino]thiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(=O)(=O)CC1)NCC1=CC=C(C=C1)C#CC1=CC=CC=C1 BHPSUYPKNUMMDW-UHFFFAOYSA-N 0.000 description 2
- SUTVMCZPQGTSJM-UHFFFAOYSA-N N-hydroxy-1-methylsulfonyl-4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]piperidine-4-carboxamide Chemical compound CS(=O)(=O)N1CCC(CCn2ccc(cc2=O)-c2ccccc2)(CC1)C(=O)NO SUTVMCZPQGTSJM-UHFFFAOYSA-N 0.000 description 2
- CCXFUASKVYUBCM-UHFFFAOYSA-N N-hydroxy-4-[(4-phenylphenyl)methylamino]oxane-4-carboxamide Chemical compound ONC(=O)C1(CCOCC1)NCc1ccc(cc1)-c1ccccc1 CCXFUASKVYUBCM-UHFFFAOYSA-N 0.000 description 2
- WCFAVQBABCJCRE-UHFFFAOYSA-N N-hydroxy-4-[2-(4-phenylphenyl)ethyl]oxane-4-carboxamide Chemical compound ONC(=O)C1(CCc2ccc(cc2)-c2ccccc2)CCOCC1 WCFAVQBABCJCRE-UHFFFAOYSA-N 0.000 description 2
- SVCPXAGTOKMKNG-UHFFFAOYSA-N N-hydroxy-4-[[4-(4-methoxyphenyl)phenyl]methylamino]-1,1-dioxothiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(CC1)(=O)=O)NCC1=CC=C(C=C1)C1=CC=C(C=C1)OC SVCPXAGTOKMKNG-UHFFFAOYSA-N 0.000 description 2
- NFHFRUOZVGFOOS-UHFFFAOYSA-N Pd(PPh3)4 Substances [Pd].C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1.C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 NFHFRUOZVGFOOS-UHFFFAOYSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 2
- ZTHYODDOHIVTJV-UHFFFAOYSA-N Propyl gallate Chemical compound CCCOC(=O)C1=CC(O)=C(O)C(O)=C1 ZTHYODDOHIVTJV-UHFFFAOYSA-N 0.000 description 2
- 241000588770 Proteus mirabilis Species 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 2
- 206010040070 Septic Shock Diseases 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- 150000008065 acid anhydrides Chemical class 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000009798 acute exacerbation Effects 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 150000001299 aldehydes Chemical class 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- 150000001340 alkali metals Chemical class 0.000 description 2
- 150000001341 alkaline earth metal compounds Chemical class 0.000 description 2
- 230000029936 alkylation Effects 0.000 description 2
- 238000005804 alkylation reaction Methods 0.000 description 2
- OBETXYAYXDNJHR-UHFFFAOYSA-N alpha-ethylcaproic acid Natural products CCCCC(CC)C(O)=O OBETXYAYXDNJHR-UHFFFAOYSA-N 0.000 description 2
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 2
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 2
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 2
- 239000012080 ambient air Substances 0.000 description 2
- 229940024606 amino acid Drugs 0.000 description 2
- 235000001014 amino acid Nutrition 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 229910021529 ammonia Inorganic materials 0.000 description 2
- 238000005349 anion exchange Methods 0.000 description 2
- 230000000845 anti-microbial effect Effects 0.000 description 2
- 229910052786 argon Inorganic materials 0.000 description 2
- 239000012300 argon atmosphere Substances 0.000 description 2
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- NBMKJKDGKREAPL-DVTGEIKXSA-N beclomethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O NBMKJKDGKREAPL-DVTGEIKXSA-N 0.000 description 2
- 229940092705 beclomethasone Drugs 0.000 description 2
- 239000000440 bentonite Substances 0.000 description 2
- 229910000278 bentonite Inorganic materials 0.000 description 2
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 102000006635 beta-lactamase Human genes 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 102000023732 binding proteins Human genes 0.000 description 2
- 108091008324 binding proteins Proteins 0.000 description 2
- 229920002988 biodegradable polymer Polymers 0.000 description 2
- 239000004621 biodegradable polymer Substances 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 206010006451 bronchitis Diseases 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 239000006172 buffering agent Substances 0.000 description 2
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 2
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 2
- 229940043430 calcium compound Drugs 0.000 description 2
- 150000001674 calcium compounds Chemical class 0.000 description 2
- 238000011088 calibration curve Methods 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- 150000001721 carbon Chemical group 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical class OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 2
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 2
- PFKFTWBEEFSNDU-UHFFFAOYSA-N carbonyldiimidazole Chemical compound C1=CN=CN1C(=O)N1C=CN=C1 PFKFTWBEEFSNDU-UHFFFAOYSA-N 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 150000001244 carboxylic acid anhydrides Chemical class 0.000 description 2
- 150000001735 carboxylic acids Chemical class 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229940124587 cephalosporin Drugs 0.000 description 2
- 150000001780 cephalosporins Chemical class 0.000 description 2
- JQXXHWHPUNPDRT-BQVAUQFYSA-N chembl1523493 Chemical compound O([C@](C1=O)(C)O\C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)/C=C\C=C(C)/C(=O)NC=2C(O)=C3C(O)=C4C)C)OC)C4=C1C3=C(O)C=2C=NN1CCN(C)CC1 JQXXHWHPUNPDRT-BQVAUQFYSA-N 0.000 description 2
- 239000000460 chlorine Substances 0.000 description 2
- 229910052801 chlorine Inorganic materials 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 238000013375 chromatographic separation Methods 0.000 description 2
- 208000007451 chronic bronchitis Diseases 0.000 description 2
- 235000015165 citric acid Nutrition 0.000 description 2
- 229960002626 clarithromycin Drugs 0.000 description 2
- AGOYDEPGAOXOCK-KCBOHYOISA-N clarithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@](C)([C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)OC)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 AGOYDEPGAOXOCK-KCBOHYOISA-N 0.000 description 2
- 238000011260 co-administration Methods 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 229940110456 cocoa butter Drugs 0.000 description 2
- 235000019868 cocoa butter Nutrition 0.000 description 2
- 229960003346 colistin Drugs 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000005494 condensation Effects 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 235000012343 cottonseed oil Nutrition 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 150000004292 cyclic ethers Chemical class 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 150000001975 deuterium Chemical group 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- AFABGHUZZDYHJO-UHFFFAOYSA-N dimethyl butane Natural products CCCC(C)C AFABGHUZZDYHJO-UHFFFAOYSA-N 0.000 description 2
- 229940113088 dimethylacetamide Drugs 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 239000008298 dragée Substances 0.000 description 2
- 238000000132 electrospray ionisation Methods 0.000 description 2
- 230000002255 enzymatic effect Effects 0.000 description 2
- 150000002170 ethers Chemical class 0.000 description 2
- MMXKVMNBHPAILY-UHFFFAOYSA-N ethyl laurate Chemical compound CCCCCCCCCCCC(=O)OCC MMXKVMNBHPAILY-UHFFFAOYSA-N 0.000 description 2
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 2
- 229940093471 ethyl oleate Drugs 0.000 description 2
- OJCSPXHYDFONPU-UHFFFAOYSA-N etoac etoac Chemical compound CCOC(C)=O.CCOC(C)=O OJCSPXHYDFONPU-UHFFFAOYSA-N 0.000 description 2
- 239000003172 expectorant agent Substances 0.000 description 2
- 238000003818 flash chromatography Methods 0.000 description 2
- 239000000796 flavoring agent Substances 0.000 description 2
- 230000004907 flux Effects 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 229960002518 gentamicin Drugs 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 229940093915 gynecological organic acid Drugs 0.000 description 2
- 150000008282 halocarbons Chemical class 0.000 description 2
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- 150000004679 hydroxides Chemical class 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 2
- 239000005457 ice water Substances 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 150000007529 inorganic bases Chemical class 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 235000010445 lecithin Nutrition 0.000 description 2
- 239000000787 lecithin Substances 0.000 description 2
- 229940067606 lecithin Drugs 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- GZQKNULLWNGMCW-PWQABINMSA-N lipid A (E. coli) Chemical compound O1[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@H](NC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)[C@@H]1OC[C@@H]1[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O1 GZQKNULLWNGMCW-PWQABINMSA-N 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 239000008297 liquid dosage form Substances 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 235000019359 magnesium stearate Nutrition 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 229960002510 mandelic acid Drugs 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 150000002736 metal compounds Chemical class 0.000 description 2
- ATEZPJXGOJUIJG-UHFFFAOYSA-N methyl 1,1-dioxo-4-[[4-(2-phenylethynyl)phenyl]methylamino]thiane-4-carboxylate Chemical compound C1(=CC=CC=C1)C#CC1=CC=C(CNC2(CCS(CC2)(=O)=O)C(=O)OC)C=C1 ATEZPJXGOJUIJG-UHFFFAOYSA-N 0.000 description 2
- OHHUWVUKFNDQPE-UHFFFAOYSA-N methyl 3-aminothiolane-3-carboxylate Chemical compound COC(=O)C1(N)CCSC1 OHHUWVUKFNDQPE-UHFFFAOYSA-N 0.000 description 2
- 239000004530 micro-emulsion Substances 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 230000000510 mucolytic effect Effects 0.000 description 2
- 229940066491 mucolytics Drugs 0.000 description 2
- JORAUNFTUVJTNG-BSTBCYLQSA-N n-[(2s)-4-amino-1-[[(2s,3r)-1-[[(2s)-4-amino-1-oxo-1-[[(3s,6s,9s,12s,15r,18s,21s)-6,9,18-tris(2-aminoethyl)-3-[(1r)-1-hydroxyethyl]-12,15-bis(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]amino]butan-2-yl]amino]-3-h Chemical compound CC(C)CCCCC(=O)N[C@@H](CCN)C(=O)N[C@H]([C@@H](C)O)CN[C@@H](CCN)C(=O)N[C@H]1CCNC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCN)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCN)NC1=O.CCC(C)CCCCC(=O)N[C@@H](CCN)C(=O)N[C@H]([C@@H](C)O)CN[C@@H](CCN)C(=O)N[C@H]1CCNC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCN)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCN)NC1=O JORAUNFTUVJTNG-BSTBCYLQSA-N 0.000 description 2
- ZESIAEVDVPWEKB-ORCFLVBFSA-N n-[(2s)-4-amino-1-[[(2s,3r)-1-[[(2s)-4-amino-1-oxo-1-[[(3s,6s,9s,12s,15r,18s,21s)-6,9,18-tris(2-aminoethyl)-3-[(1r)-1-hydroxyethyl]-12,15-bis(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]amino]butan-2-yl]amino]-3-h Chemical compound OS(O)(=O)=O.OS(O)(=O)=O.CC(C)CCCCC(=O)N[C@@H](CCN)C(=O)N[C@H]([C@@H](C)O)CN[C@@H](CCN)C(=O)N[C@H]1CCNC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCN)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCN)NC1=O.CCC(C)CCCCC(=O)N[C@@H](CCN)C(=O)N[C@H]([C@@H](C)O)CN[C@@H](CCN)C(=O)N[C@H]1CCNC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCN)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCN)NC1=O ZESIAEVDVPWEKB-ORCFLVBFSA-N 0.000 description 2
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- RZWKXWSREPGSOG-UHFFFAOYSA-N n-hydroxy-1,1-dioxo-4-[(4-phenylphenyl)methylamino]thiane-4-carboxamide Chemical compound C=1C=C(C=2C=CC=CC=2)C=CC=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 RZWKXWSREPGSOG-UHFFFAOYSA-N 0.000 description 2
- OFQKCVKKYABAEG-UHFFFAOYSA-N n-hydroxy-1,1-dioxo-4-[(5-phenyl-1,2-oxazol-3-yl)methylamino]thiane-4-carboxamide Chemical compound C1=C(C=2C=CC=CC=2)ON=C1CNC1(C(=O)NO)CCS(=O)(=O)CC1 OFQKCVKKYABAEG-UHFFFAOYSA-N 0.000 description 2
- IEKPDBUUDDNSEX-UHFFFAOYSA-N n-hydroxy-1,1-dioxo-4-[[4-[4-(triazol-2-yl)phenyl]phenyl]methylamino]thiane-4-carboxamide Chemical compound C=1C=C(C=2C=CC(=CC=2)N2N=CC=N2)C=CC=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 IEKPDBUUDDNSEX-UHFFFAOYSA-N 0.000 description 2
- RQTOOFIXOKYGAN-UHFFFAOYSA-N nedocromil Chemical compound CCN1C(C(O)=O)=CC(=O)C2=C1C(CCC)=C1OC(C(O)=O)=CC(=O)C1=C2 RQTOOFIXOKYGAN-UHFFFAOYSA-N 0.000 description 2
- 229960004398 nedocromil Drugs 0.000 description 2
- 238000006386 neutralization reaction Methods 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 150000002825 nitriles Chemical class 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- 239000012457 nonaqueous media Substances 0.000 description 2
- 235000008390 olive oil Nutrition 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 150000007530 organic bases Chemical class 0.000 description 2
- 239000001301 oxygen Chemical group 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- 239000002304 perfume Substances 0.000 description 2
- 235000011007 phosphoric acid Nutrition 0.000 description 2
- 238000006303 photolysis reaction Methods 0.000 description 2
- 230000015843 photosynthesis, light reaction Effects 0.000 description 2
- 229960005141 piperazine Drugs 0.000 description 2
- XDJYMJULXQKGMM-UHFFFAOYSA-N polymyxin E1 Natural products CCC(C)CCCCC(=O)NC(CCN)C(=O)NC(C(C)O)C(=O)NC(CCN)C(=O)NC1CCNC(=O)C(C(C)O)NC(=O)C(CCN)NC(=O)C(CCN)NC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(CCN)NC1=O XDJYMJULXQKGMM-UHFFFAOYSA-N 0.000 description 2
- KNIWPHSUTGNZST-UHFFFAOYSA-N polymyxin E2 Natural products CC(C)CCCCC(=O)NC(CCN)C(=O)NC(C(C)O)C(=O)NC(CCN)C(=O)NC1CCNC(=O)C(C(C)O)NC(=O)C(CCN)NC(=O)C(CCN)NC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)C(CCN)NC1=O KNIWPHSUTGNZST-UHFFFAOYSA-N 0.000 description 2
- 229920005862 polyol Polymers 0.000 description 2
- 150000003077 polyols Chemical class 0.000 description 2
- 238000002600 positron emission tomography Methods 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 229910000160 potassium phosphate Inorganic materials 0.000 description 2
- 235000011009 potassium phosphates Nutrition 0.000 description 2
- 239000000651 prodrug Substances 0.000 description 2
- 229940002612 prodrug Drugs 0.000 description 2
- 239000003380 propellant Substances 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 238000003908 quality control method Methods 0.000 description 2
- 239000000376 reactant Substances 0.000 description 2
- 238000001953 recrystallisation Methods 0.000 description 2
- 238000006268 reductive amination reaction Methods 0.000 description 2
- 208000020029 respiratory tract infectious disease Diseases 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 230000036303 septic shock Effects 0.000 description 2
- 239000008159 sesame oil Substances 0.000 description 2
- 235000011803 sesame oil Nutrition 0.000 description 2
- NDVLTYZPCACLMA-UHFFFAOYSA-N silver oxide Chemical compound [O-2].[Ag+].[Ag+] NDVLTYZPCACLMA-UHFFFAOYSA-N 0.000 description 2
- 238000002603 single-photon emission computed tomography Methods 0.000 description 2
- 235000017557 sodium bicarbonate Nutrition 0.000 description 2
- 229910000342 sodium bisulfate Inorganic materials 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 2
- 239000007909 solid dosage form Substances 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- 238000003797 solvolysis reaction Methods 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 229940032147 starch Drugs 0.000 description 2
- 239000008223 sterile water Substances 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 239000003765 sweetening agent Substances 0.000 description 2
- 230000002195 synergetic effect Effects 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- DYHSDKLCOJIUFX-UHFFFAOYSA-N tert-butoxycarbonyl anhydride Chemical compound CC(C)(C)OC(=O)OC(=O)OC(C)(C)C DYHSDKLCOJIUFX-UHFFFAOYSA-N 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- BSNQHVPRAPQLSW-UHFFFAOYSA-N thiolane-3-carboxylic acid Chemical compound OC(=O)C1CCSC1 BSNQHVPRAPQLSW-UHFFFAOYSA-N 0.000 description 2
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 229910000404 tripotassium phosphate Inorganic materials 0.000 description 2
- 238000010518 undesired secondary reaction Methods 0.000 description 2
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- 150000003952 β-lactams Chemical class 0.000 description 2
- QIJRTFXNRTXDIP-UHFFFAOYSA-N (1-carboxy-2-sulfanylethyl)azanium;chloride;hydrate Chemical compound O.Cl.SCC(N)C(O)=O QIJRTFXNRTXDIP-UHFFFAOYSA-N 0.000 description 1
- DNISEZBAYYIQFB-PHDIDXHHSA-N (2r,3r)-2,3-diacetyloxybutanedioic acid Chemical compound CC(=O)O[C@@H](C(O)=O)[C@H](C(O)=O)OC(C)=O DNISEZBAYYIQFB-PHDIDXHHSA-N 0.000 description 1
- CIDUJQMULVCIBT-MQDUPKMGSA-N (2r,3r,4r,5r)-2-[(1s,2s,3r,4s,6r)-4-amino-3-[[(2s,3r)-3-amino-6-(aminomethyl)-3,4-dihydro-2h-pyran-2-yl]oxy]-6-(ethylamino)-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol Chemical compound O([C@@H]1[C@@H](N)C[C@H]([C@@H]([C@H]1O)O[C@@H]1[C@@H]([C@@H](NC)[C@@](C)(O)CO1)O)NCC)[C@H]1OC(CN)=CC[C@H]1N CIDUJQMULVCIBT-MQDUPKMGSA-N 0.000 description 1
- YNLGFXOBRXSMJZ-UHFFFAOYSA-N (4-cyclopropylphenyl)boronic acid Chemical compound C1=CC(B(O)O)=CC=C1C1CC1 YNLGFXOBRXSMJZ-UHFFFAOYSA-N 0.000 description 1
- XIYOPDCBBDCGOE-IWVLMIASSA-N (4s,4ar,5s,5ar,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methylidene-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide Chemical compound C=C1C2=CC=CC(O)=C2C(O)=C2[C@@H]1[C@H](O)[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O XIYOPDCBBDCGOE-IWVLMIASSA-N 0.000 description 1
- FFTVPQUHLQBXQZ-KVUCHLLUSA-N (4s,4as,5ar,12ar)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1C2=C(N(C)C)C=CC(O)=C2C(O)=C2[C@@H]1C[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O FFTVPQUHLQBXQZ-KVUCHLLUSA-N 0.000 description 1
- GUXHBMASAHGULD-SEYHBJAFSA-N (4s,4as,5as,6s,12ar)-7-chloro-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1([C@H]2O)=C(Cl)C=CC(O)=C1C(O)=C1[C@@H]2C[C@H]2[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]2(O)C1=O GUXHBMASAHGULD-SEYHBJAFSA-N 0.000 description 1
- WDLWHQDACQUCJR-ZAMMOSSLSA-N (6r,7r)-7-[[(2r)-2-azaniumyl-2-(4-hydroxyphenyl)acetyl]amino]-8-oxo-3-[(e)-prop-1-enyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)/C=C/C)C(O)=O)=CC=C(O)C=C1 WDLWHQDACQUCJR-ZAMMOSSLSA-N 0.000 description 1
- GPYKKBAAPVOCIW-HSASPSRMSA-N (6r,7s)-7-[[(2r)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;hydrate Chemical compound O.C1([C@H](C(=O)N[C@@H]2C(N3C(=C(Cl)CC[C@@H]32)C(O)=O)=O)N)=CC=CC=C1 GPYKKBAAPVOCIW-HSASPSRMSA-N 0.000 description 1
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 description 1
- QKDHBVNJCZBTMR-LLVKDONJSA-N (R)-temafloxacin Chemical compound C1CN[C@H](C)CN1C(C(=C1)F)=CC2=C1C(=O)C(C(O)=O)=CN2C1=CC=C(F)C=C1F QKDHBVNJCZBTMR-LLVKDONJSA-N 0.000 description 1
- MIOPJNTWMNEORI-GMSGAONNSA-N (S)-camphorsulfonic acid Chemical compound C1C[C@@]2(CS(O)(=O)=O)C(=O)C[C@@H]1C2(C)C MIOPJNTWMNEORI-GMSGAONNSA-N 0.000 description 1
- XUBOMFCQGDBHNK-JTQLQIEISA-N (S)-gatifloxacin Chemical compound FC1=CC(C(C(C(O)=O)=CN2C3CC3)=O)=C2C(OC)=C1N1CCN[C@@H](C)C1 XUBOMFCQGDBHNK-JTQLQIEISA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- XLNHUSXTEMVHPB-ONNFQVAWSA-N (ne)-n-[(4-chloro-2-fluorophenyl)methylidene]hydroxylamine Chemical compound O\N=C\C1=CC=C(Cl)C=C1F XLNHUSXTEMVHPB-ONNFQVAWSA-N 0.000 description 1
- GGNUCYRMYZTWLX-UHFFFAOYSA-N 1,1-dioxo-4-[(4-phenylphenyl)methylamino]thiane-4-carboxylic acid Chemical compound OC(=O)C1(CCS(=O)(=O)CC1)NCc1ccc(cc1)-c1ccccc1 GGNUCYRMYZTWLX-UHFFFAOYSA-N 0.000 description 1
- BJAYWZWPKWATNB-UHFFFAOYSA-N 1,1-dioxo-4-[(5-phenyl-1,2-oxazol-3-yl)methylamino]thiane-4-carboxylic acid Chemical compound C1(=CC=CC=C1)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)O BJAYWZWPKWATNB-UHFFFAOYSA-N 0.000 description 1
- LWABQYZCEATIOG-UHFFFAOYSA-N 1,1-dioxo-4-[[4-(2-phenylethynyl)phenyl]methylamino]thiane-4-carboxylic acid Chemical compound OC(=O)C1(CCS(=O)(=O)CC1)NCc1ccc(cc1)C#Cc1ccccc1 LWABQYZCEATIOG-UHFFFAOYSA-N 0.000 description 1
- ASRQWTBGINUTIX-UHFFFAOYSA-N 1,1-dioxothiolane-3-carboxylic acid Chemical compound OC(=O)C1CCS(=O)(=O)C1 ASRQWTBGINUTIX-UHFFFAOYSA-N 0.000 description 1
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical compound C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 1
- 229940058015 1,3-butylene glycol Drugs 0.000 description 1
- JBYHSSAVUBIJMK-UHFFFAOYSA-N 1,4-oxathiane Chemical compound C1CSCCO1 JBYHSSAVUBIJMK-UHFFFAOYSA-N 0.000 description 1
- PIRRWUMTIBFCCW-UHFFFAOYSA-N 1-(2-fluoro-4-methoxyphenyl)ethanone Chemical compound COC1=CC=C(C(C)=O)C(F)=C1 PIRRWUMTIBFCCW-UHFFFAOYSA-N 0.000 description 1
- OIGMDNONQJGUCF-UHFFFAOYSA-N 1-(4-chloro-2-fluorophenyl)ethanone Chemical compound CC(=O)C1=CC=C(Cl)C=C1F OIGMDNONQJGUCF-UHFFFAOYSA-N 0.000 description 1
- BUZYGTVTZYSBCU-UHFFFAOYSA-N 1-(4-chlorophenyl)ethanone Chemical compound CC(=O)C1=CC=C(Cl)C=C1 BUZYGTVTZYSBCU-UHFFFAOYSA-N 0.000 description 1
- WRRDEQBYUYEIHN-UHFFFAOYSA-N 1-O-tert-butyl 3-O-ethyl 3-[(4-bromophenyl)methylamino]azetidine-1,3-dicarboxylate Chemical compound CCOC(=O)C1(CN(C1)C(=O)OC(C)(C)C)NCc1ccc(Br)cc1 WRRDEQBYUYEIHN-UHFFFAOYSA-N 0.000 description 1
- DMNDODZEOWTICB-UHFFFAOYSA-N 1-O-tert-butyl 4-O-ethyl 4-[(4-phenylphenyl)methyl]piperidine-1,4-dicarboxylate Chemical compound CCOC(=O)C1(Cc2ccc(cc2)-c2ccccc2)CCN(CC1)C(=O)OC(C)(C)C DMNDODZEOWTICB-UHFFFAOYSA-N 0.000 description 1
- LMXRHUMGZBFYCD-UHFFFAOYSA-N 1-O-tert-butyl 4-O-ethyl 4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]piperidine-1,4-dicarboxylate Chemical compound CCOC(=O)C1(CCn2ccc(cc2=O)-c2ccccc2)CCN(CC1)C(=O)OC(C)(C)C LMXRHUMGZBFYCD-UHFFFAOYSA-N 0.000 description 1
- RNFCZZWZXMSDTF-UHFFFAOYSA-N 1-O-tert-butyl 4-O-ethyl 4-[2-(4-iodo-2-oxopyridin-1-yl)ethyl]piperidine-1,4-dicarboxylate Chemical compound CCOC(=O)C1(CCn2ccc(I)cc2=O)CCN(CC1)C(=O)OC(C)(C)C RNFCZZWZXMSDTF-UHFFFAOYSA-N 0.000 description 1
- NEPYYKOEPRKPFR-UHFFFAOYSA-N 1-[(2-methylpropan-2-yl)oxycarbonyl]-4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]piperidine-4-carboxylic acid Chemical compound CC(C)(C)OC(=O)N1CCC(CCn2ccc(cc2=O)-c2ccccc2)(CC1)C(O)=O NEPYYKOEPRKPFR-UHFFFAOYSA-N 0.000 description 1
- YALBKQGVTSZQQO-UHFFFAOYSA-N 1-[[5-(4-chloro-2-fluorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-3-methylsulfonylcyclobutane-1-carboxamide Chemical compound CS(=O)(=O)C1CC(C1)(NCc1cc(on1)-c1ccc(Cl)cc1F)C(=O)NO YALBKQGVTSZQQO-UHFFFAOYSA-N 0.000 description 1
- JGWJLLIBGZNLJE-UHFFFAOYSA-N 1-acetyl-4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]piperidine-4-carboxylic acid Chemical compound CC(=O)N1CCC(CCn2ccc(cc2=O)-c2ccccc2)(CC1)C(O)=O JGWJLLIBGZNLJE-UHFFFAOYSA-N 0.000 description 1
- VNIMXISZSHTJOI-UHFFFAOYSA-N 1-acetyl-N-(oxan-2-yloxy)-4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]piperidine-4-carboxamide Chemical compound CC(=O)N1CCC(CCn2ccc(cc2=O)-c2ccccc2)(CC1)C(=O)NOC1CCCCO1 VNIMXISZSHTJOI-UHFFFAOYSA-N 0.000 description 1
- APTDRDYSJZQPPI-UHFFFAOYSA-N 1-bromo-4-(2-bromoethyl)benzene Chemical compound BrCCC1=CC=C(Br)C=C1 APTDRDYSJZQPPI-UHFFFAOYSA-N 0.000 description 1
- BSIMZHVOQZIAOY-SCSAIBSYSA-N 1-carbapenem-3-carboxylic acid Chemical compound OC(=O)C1=CC[C@@H]2CC(=O)N12 BSIMZHVOQZIAOY-SCSAIBSYSA-N 0.000 description 1
- LFZJRTMTKGYJRS-UHFFFAOYSA-N 1-chloro-4-ethynylbenzene Chemical compound ClC1=CC=C(C#C)C=C1 LFZJRTMTKGYJRS-UHFFFAOYSA-N 0.000 description 1
- FPIRBHDGWMWJEP-UHFFFAOYSA-N 1-hydroxy-7-azabenzotriazole Chemical compound C1=CN=C2N(O)N=NC2=C1 FPIRBHDGWMWJEP-UHFFFAOYSA-N 0.000 description 1
- DFPYXQYWILNVAU-UHFFFAOYSA-N 1-hydroxybenzotriazole Chemical compound C1=CC=C2N(O)N=NC2=C1.C1=CC=C2N(O)N=NC2=C1 DFPYXQYWILNVAU-UHFFFAOYSA-N 0.000 description 1
- XCBNHDSVRQZWLH-UHFFFAOYSA-N 1-hydroxycyclobutane-1-carboxylic acid Chemical compound OC(=O)C1(O)CCC1 XCBNHDSVRQZWLH-UHFFFAOYSA-N 0.000 description 1
- JKEJMMVGDVKQOG-UHFFFAOYSA-N 1-methylsulfonyl-4-[(4-phenylphenyl)methyl]piperidine-4-carboxylic acid Chemical compound CS(=O)(=O)N1CCC(Cc2ccc(cc2)-c2ccccc2)(CC1)C(O)=O JKEJMMVGDVKQOG-UHFFFAOYSA-N 0.000 description 1
- CMCRDNGKBRYBFU-UHFFFAOYSA-N 1-methylsulfonyl-4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]piperidine-4-carboxylic acid Chemical compound CS(=O)(=O)N1CCC(CCn2ccc(cc2=O)-c2ccccc2)(CC1)C(O)=O CMCRDNGKBRYBFU-UHFFFAOYSA-N 0.000 description 1
- LNETULKMXZVUST-UHFFFAOYSA-N 1-naphthoic acid Chemical compound C1=CC=C2C(C(=O)O)=CC=CC2=C1 LNETULKMXZVUST-UHFFFAOYSA-N 0.000 description 1
- MMEIVVYGFADKTL-UHFFFAOYSA-N 1-o-tert-butyl 3-o-ethyl 3-aminoazetidine-1,3-dicarboxylate Chemical compound CCOC(=O)C1(N)CN(C(=O)OC(C)(C)C)C1 MMEIVVYGFADKTL-UHFFFAOYSA-N 0.000 description 1
- KIBNEMIIQVFXBM-UHFFFAOYSA-N 1-o-tert-butyl 4-o-ethyl 4-(2-chloroethyl)piperidine-1,4-dicarboxylate Chemical compound CCOC(=O)C1(CCCl)CCN(C(=O)OC(C)(C)C)CC1 KIBNEMIIQVFXBM-UHFFFAOYSA-N 0.000 description 1
- AQBWYVZHTYJMES-UHFFFAOYSA-N 1-phenyltriazole-4-carbaldehyde Chemical compound N1=NC(C=O)=CN1C1=CC=CC=C1 AQBWYVZHTYJMES-UHFFFAOYSA-N 0.000 description 1
- NFTOEHBFQROATQ-UHFFFAOYSA-N 2,3-dihydrofuran-5-carboxylic acid Chemical compound OC(=O)C1=CCCO1 NFTOEHBFQROATQ-UHFFFAOYSA-N 0.000 description 1
- UKHJNJFJCGBKSF-UHFFFAOYSA-N 2,5-diazabicyclo[2.2.1]heptane Chemical compound C1NC2CNC1C2 UKHJNJFJCGBKSF-UHFFFAOYSA-N 0.000 description 1
- DUKDRWHFWAKSGZ-UHFFFAOYSA-N 2-(4-bromophenyl)triazole Chemical compound C1=CC(Br)=CC=C1N1N=CC=N1 DUKDRWHFWAKSGZ-UHFFFAOYSA-N 0.000 description 1
- WYEYFTIWQILPTE-UHFFFAOYSA-N 2-(4-cyclopropylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Chemical compound O1C(C)(C)C(C)(C)OB1C1=CC=C(C2CC2)C=C1 WYEYFTIWQILPTE-UHFFFAOYSA-N 0.000 description 1
- LBLYYCQCTBFVLH-UHFFFAOYSA-N 2-Methylbenzenesulfonic acid Chemical compound CC1=CC=CC=C1S(O)(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-N 0.000 description 1
- IZXIZTKNFFYFOF-UHFFFAOYSA-N 2-Oxazolidone Chemical class O=C1NCCO1 IZXIZTKNFFYFOF-UHFFFAOYSA-N 0.000 description 1
- WXHLLJAMBQLULT-UHFFFAOYSA-N 2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl]amino]-n-(2-methyl-6-sulfanylphenyl)-1,3-thiazole-5-carboxamide;hydrate Chemical compound O.C=1C(N2CCN(CCO)CC2)=NC(C)=NC=1NC(S1)=NC=C1C(=O)NC1=C(C)C=CC=C1S WXHLLJAMBQLULT-UHFFFAOYSA-N 0.000 description 1
- KMGUEILFFWDGFV-UHFFFAOYSA-N 2-benzoyl-2-benzoyloxy-3-hydroxybutanedioic acid Chemical compound C=1C=CC=CC=1C(=O)C(C(C(O)=O)O)(C(O)=O)OC(=O)C1=CC=CC=C1 KMGUEILFFWDGFV-UHFFFAOYSA-N 0.000 description 1
- KGHGZRVXCKCJGX-UHFFFAOYSA-N 2-bromo-1-(4-phenylphenyl)ethanone Chemical compound C1=CC(C(=O)CBr)=CC=C1C1=CC=CC=C1 KGHGZRVXCKCJGX-UHFFFAOYSA-N 0.000 description 1
- JBKINHFZTVLNEM-UHFFFAOYSA-N 2-bromoethoxy-tert-butyl-dimethylsilane Chemical compound CC(C)(C)[Si](C)(C)OCCBr JBKINHFZTVLNEM-UHFFFAOYSA-N 0.000 description 1
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- YEDUAINPPJYDJZ-UHFFFAOYSA-N 2-hydroxybenzothiazole Chemical compound C1=CC=C2SC(O)=NC2=C1 YEDUAINPPJYDJZ-UHFFFAOYSA-N 0.000 description 1
- JNODDICFTDYODH-UHFFFAOYSA-N 2-hydroxytetrahydrofuran Chemical compound OC1CCCO1 JNODDICFTDYODH-UHFFFAOYSA-N 0.000 description 1
- JWUJQDFVADABEY-UHFFFAOYSA-N 2-methyltetrahydrofuran Chemical compound CC1CCCO1 JWUJQDFVADABEY-UHFFFAOYSA-N 0.000 description 1
- DIQOUXNTSMWQSA-UHFFFAOYSA-N 2-oxa-5-azabicyclo[2.2.1]heptane Chemical compound C1OC2CNC1C2 DIQOUXNTSMWQSA-UHFFFAOYSA-N 0.000 description 1
- RJWXVIMWCSNIGW-UHFFFAOYSA-N 2-oxa-8-thiaspiro[4.5]decan-1-one Chemical compound O=C1OCCC11CCSCC1 RJWXVIMWCSNIGW-UHFFFAOYSA-N 0.000 description 1
- JYBWAYHAOLQZJX-UHFFFAOYSA-N 2-phenyltriazole Chemical compound N1=CC=NN1C1=CC=CC=C1 JYBWAYHAOLQZJX-UHFFFAOYSA-N 0.000 description 1
- 125000004105 2-pyridyl group Chemical group N1=C([*])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 125000000389 2-pyrrolyl group Chemical group [H]N1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000000175 2-thienyl group Chemical group S1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- LKDJYZBKCVSODK-UHFFFAOYSA-N 3,8-diazabicyclo[3.2.1]octane Chemical compound C1NCC2CCC1N2 LKDJYZBKCVSODK-UHFFFAOYSA-N 0.000 description 1
- NMFJGBBHWILXAQ-UHFFFAOYSA-N 3-[2-[4-(4-chloro-2-fluorophenyl)phenyl]ethyl]-N-hydroxy-1-methylsulfonylpyrrolidine-3-carboxamide Chemical compound CS(=O)(=O)N1CCC(CCc2ccc(cc2)-c2ccc(Cl)cc2F)(C1)C(=O)NO NMFJGBBHWILXAQ-UHFFFAOYSA-N 0.000 description 1
- KKIJPKCBCHNDPU-UHFFFAOYSA-N 3-[[4-(4-chloro-2-fluorophenyl)phenyl]methylamino]-N-hydroxy-1,1-dioxothiolane-3-carboxamide Chemical compound ClC1=CC(=C(C=C1)C1=CC=C(C=C1)CNC1(CS(CC1)(=O)=O)C(=O)NO)F KKIJPKCBCHNDPU-UHFFFAOYSA-N 0.000 description 1
- ALPARPOZUBHVIM-UHFFFAOYSA-N 3-[[4-(4-chloro-2-fluorophenyl)phenyl]methylamino]-N-hydroxy-1-methylsulfonylazetidine-3-carboxamide Chemical compound CS(=O)(=O)N1CC(C1)(NCc1ccc(cc1)-c1ccc(Cl)cc1F)C(=O)NO ALPARPOZUBHVIM-UHFFFAOYSA-N 0.000 description 1
- VBRBDAIOEASYAS-UHFFFAOYSA-N 3-[[4-(4-chloro-2-fluorophenyl)phenyl]methylamino]-N-hydroxyoxetane-3-carboxamide Chemical compound ONC(=O)C1(COC1)NCc1ccc(cc1)-c1ccc(Cl)cc1F VBRBDAIOEASYAS-UHFFFAOYSA-N 0.000 description 1
- DRNPRNLTBVUMSQ-UHFFFAOYSA-N 3-[[4-(4-chloro-2-fluorophenyl)phenyl]methylamino]-N-hydroxyoxolane-3-carboxamide Chemical compound ONC(=O)C1(CCOC1)NCc1ccc(cc1)-c1ccc(Cl)cc1F DRNPRNLTBVUMSQ-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 description 1
- 125000003682 3-furyl group Chemical group O1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- MNILDQSRDHCFJG-UHFFFAOYSA-N 3-oxa-8-azabicyclo[3.2.1]octane Chemical compound C1OCC2CCC1N2 MNILDQSRDHCFJG-UHFFFAOYSA-N 0.000 description 1
- 125000003349 3-pyridyl group Chemical group N1=C([H])C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000001397 3-pyrrolyl group Chemical group [H]N1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- 125000001541 3-thienyl group Chemical group S1C([H])=C([*])C([H])=C1[H] 0.000 description 1
- HETSDWRDICBRSQ-UHFFFAOYSA-N 3h-quinolin-4-one Chemical class C1=CC=C2C(=O)CC=NC2=C1 HETSDWRDICBRSQ-UHFFFAOYSA-N 0.000 description 1
- DPBWFNDFMCCGGJ-UHFFFAOYSA-N 4-Piperidine carboxamide Chemical compound NC(=O)C1CCNCC1 DPBWFNDFMCCGGJ-UHFFFAOYSA-N 0.000 description 1
- TWIYVUUHYHAAQA-UHFFFAOYSA-N 4-[(3-fluoro-4-phenylphenyl)methylamino]-N-(oxan-2-yloxy)-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C=CC(=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NOC1OCCCC1)C1=CC=CC=C1 TWIYVUUHYHAAQA-UHFFFAOYSA-N 0.000 description 1
- FGVXUGUHBQCKRA-UHFFFAOYSA-N 4-[(4-bromo-3-fluorophenyl)methylamino]-1,1-dioxothiane-4-carboxylic acid Chemical compound OC(=O)C1(CCS(=O)(=O)CC1)NCc1ccc(Br)c(F)c1 FGVXUGUHBQCKRA-UHFFFAOYSA-N 0.000 description 1
- YYWRMNUGCRXXOW-UHFFFAOYSA-N 4-[(4-bromophenyl)methylamino]-1,1-dioxothiane-4-carboxylic acid Chemical compound OC(=O)C1(CCS(=O)(=O)CC1)NCc1ccc(Br)cc1 YYWRMNUGCRXXOW-UHFFFAOYSA-N 0.000 description 1
- LZUXXDGNJFOCAB-UHFFFAOYSA-N 4-[(4-bromophenyl)methylamino]-N-(oxan-2-yloxy)-1,1-dioxothiane-4-carboxamide Chemical compound BrC1=CC=C(CNC2(CCS(CC2)(=O)=O)C(=O)NOC2OCCCC2)C=C1 LZUXXDGNJFOCAB-UHFFFAOYSA-N 0.000 description 1
- HLQJPQXGPBGJMG-UHFFFAOYSA-N 4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]piperidine-4-carboxylic acid Chemical compound OC(=O)C1(CCn2ccc(cc2=O)-c2ccccc2)CCNCC1 HLQJPQXGPBGJMG-UHFFFAOYSA-N 0.000 description 1
- VVGHVTUSEVHSRT-UHFFFAOYSA-N 4-[2-(4-iodo-2-oxopyridin-1-yl)ethyl]-N-(oxan-2-yloxy)-1,1-dioxothiane-4-carboxamide Chemical compound IC1=CC(N(C=C1)CCC1(CCS(CC1)(=O)=O)C(=O)NOC1OCCCC1)=O VVGHVTUSEVHSRT-UHFFFAOYSA-N 0.000 description 1
- LRBHDDHWXBACNO-UHFFFAOYSA-N 4-[2-(4-iodo-2-oxopyridin-1-yl)ethyl]-N-(oxan-2-yloxy)thiane-4-carboxamide Chemical compound Ic1ccn(CCC2(CCSCC2)C(=O)NOC2CCCCO2)c(=O)c1 LRBHDDHWXBACNO-UHFFFAOYSA-N 0.000 description 1
- VPLWVHWZRNRXAC-UHFFFAOYSA-N 4-[2-(4-iodo-2-oxopyridin-1-yl)ethyl]thiane-4-carboxylic acid Chemical compound OC(=O)C1(CCn2ccc(I)cc2=O)CCSCC1 VPLWVHWZRNRXAC-UHFFFAOYSA-N 0.000 description 1
- UKBHEMCXCNHDHZ-UHFFFAOYSA-N 4-[2-(4-phenylphenyl)ethyl]oxane-4-carboxylic acid Chemical compound OC(=O)C1(CCc2ccc(cc2)-c2ccccc2)CCOCC1 UKBHEMCXCNHDHZ-UHFFFAOYSA-N 0.000 description 1
- SEZICKRJQCVJLP-UHFFFAOYSA-N 4-[2-[4-(4-chloro-2-fluorophenyl)-2-oxopyridin-1-yl]ethyl]-N-(oxan-2-yloxy)-1,1-dioxothiane-4-carboxamide Chemical compound ClC1=CC(=C(C=C1)C1=CC(N(C=C1)CCC1(CCS(CC1)(=O)=O)C(=O)NOC1OCCCC1)=O)F SEZICKRJQCVJLP-UHFFFAOYSA-N 0.000 description 1
- UCPALIMHMYIZPZ-UHFFFAOYSA-N 4-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]morpholine Chemical compound O1C(C)(C)C(C)(C)OB1C1=CC=C(N2CCOCC2)C=C1 UCPALIMHMYIZPZ-UHFFFAOYSA-N 0.000 description 1
- KZIQYDIEFVJHKZ-UHFFFAOYSA-N 4-[[3-chloro-4-(2-fluoro-4-methoxyphenyl)phenyl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ClC1=C(C=CC(=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NO)C1=C(C=C(C=C1)OC)F KZIQYDIEFVJHKZ-UHFFFAOYSA-N 0.000 description 1
- QQQGIBHHWISKHL-UHFFFAOYSA-N 4-[[3-fluoro-4-(2-fluorophenyl)phenyl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound C=1C=C(C=2C(=CC=CC=2)F)C(F)=CC=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 QQQGIBHHWISKHL-UHFFFAOYSA-N 0.000 description 1
- QXLFGGHVKIALRG-UHFFFAOYSA-N 4-[[3-fluoro-4-(4-methoxyphenyl)phenyl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C=CC(=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NO)C1=CC=C(C=C1)OC QXLFGGHVKIALRG-UHFFFAOYSA-N 0.000 description 1
- ZVUNXUWTBJOJCG-UHFFFAOYSA-N 4-[[3-fluoro-4-[4-(triazol-2-yl)phenyl]phenyl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C=CC(=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NO)C1=CC=C(C=C1)N1N=CC=N1 ZVUNXUWTBJOJCG-UHFFFAOYSA-N 0.000 description 1
- UYBNVEHMKYNIAF-UHFFFAOYSA-N 4-[[4-(2-cyclopropylethynyl)phenyl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound C=1C=C(C#CC2CC2)C=CC=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 UYBNVEHMKYNIAF-UHFFFAOYSA-N 0.000 description 1
- KRTNGORJNACGNQ-UHFFFAOYSA-N 4-[[4-(2-fluoro-4-methoxyphenyl)-3-methylphenyl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C=CC(=C1)OC)C1=C(C=C(C=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NO)C KRTNGORJNACGNQ-UHFFFAOYSA-N 0.000 description 1
- NWTFANXEEJYXAX-UHFFFAOYSA-N 4-[[4-(2-fluoro-4-methoxyphenyl)phenyl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=CC(OC)=CC=C1C(C=C1)=CC=C1CNC1(C(=O)NO)CCS(=O)(=O)CC1 NWTFANXEEJYXAX-UHFFFAOYSA-N 0.000 description 1
- HXFRQHHORXSWPV-UHFFFAOYSA-N 4-[[4-(2-fluorophenyl)phenyl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound C=1C=C(C=2C(=CC=CC=2)F)C=CC=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 HXFRQHHORXSWPV-UHFFFAOYSA-N 0.000 description 1
- JOIXYIWXEYXHHG-UHFFFAOYSA-N 4-[[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]methyl]morpholine Chemical compound O1C(C)(C)C(C)(C)OB1C(C=C1)=CC=C1CN1CCOCC1 JOIXYIWXEYXHHG-UHFFFAOYSA-N 0.000 description 1
- GMLLXEISSGJEMC-UHFFFAOYSA-N 4-[[4-(4-chloro-2-fluorophenyl)-3-fluorophenyl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ClC1=CC(=C(C=C1)C1=C(C=C(C=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NO)F)F GMLLXEISSGJEMC-UHFFFAOYSA-N 0.000 description 1
- JEXODGKDDDGHPK-UHFFFAOYSA-N 4-[[4-(4-chloro-2-fluorophenyl)-3-methylphenyl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ClC1=CC(=C(C=C1)C1=C(C=C(C=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NO)C)F JEXODGKDDDGHPK-UHFFFAOYSA-N 0.000 description 1
- KIWFCHQGOWCLKA-UHFFFAOYSA-N 4-[[4-(4-chloro-2-fluorophenyl)phenyl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound C=1C=C(C=2C(=CC(Cl)=CC=2)F)C=CC=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 KIWFCHQGOWCLKA-UHFFFAOYSA-N 0.000 description 1
- PFHMLRBMUNBFJB-UHFFFAOYSA-N 4-[[4-(4-cyclopropylphenyl)-3-methylphenyl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound C1(CC1)C1=CC=C(C=C1)C1=C(C=C(C=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NO)C PFHMLRBMUNBFJB-UHFFFAOYSA-N 0.000 description 1
- RHUUTTUCPLYJNR-UHFFFAOYSA-N 4-[[4-(4-cyclopropylphenyl)phenyl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound C=1C=C(C=2C=CC(=CC=2)C2CC2)C=CC=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 RHUUTTUCPLYJNR-UHFFFAOYSA-N 0.000 description 1
- VXBIUWCDVAQCLS-UHFFFAOYSA-N 4-[[4-[4-(morpholin-4-ylmethyl)phenyl]phenyl]methylamino]-N-(oxan-2-yloxy)-1,1-dioxothiane-4-carboxamide Chemical compound O1CCN(CC1)CC1=CC=C(C=C1)C1=CC=C(C=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NOC1OCCCC1 VXBIUWCDVAQCLS-UHFFFAOYSA-N 0.000 description 1
- YEISDZWIQLMVKD-UHFFFAOYSA-N 4-[[5-(2,3-dichlorophenyl)-1,2-oxazol-3-yl]methylamino]-n-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound C1=C(C=2C(=C(Cl)C=CC=2)Cl)ON=C1CNC1(C(=O)NO)CCS(=O)(=O)CC1 YEISDZWIQLMVKD-UHFFFAOYSA-N 0.000 description 1
- XQFTZZZNJASYBM-UHFFFAOYSA-N 4-[[5-(2,4-difluorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C=CC(=C1)F)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)NO XQFTZZZNJASYBM-UHFFFAOYSA-N 0.000 description 1
- BALHXSDFGWSMKW-UHFFFAOYSA-N 4-[[5-(2,5-difluorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C=C(C=C1)F)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)NO BALHXSDFGWSMKW-UHFFFAOYSA-N 0.000 description 1
- SGSHAIHADOYYRM-UHFFFAOYSA-N 4-[[5-(2,6-difluorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C(=CC=C1)F)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)NO SGSHAIHADOYYRM-UHFFFAOYSA-N 0.000 description 1
- UMEOZXKENFMBRG-UHFFFAOYSA-N 4-[[5-(2-chloro-4-fluorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ClC1=C(C=CC(=C1)F)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)NO UMEOZXKENFMBRG-UHFFFAOYSA-N 0.000 description 1
- HEYXZNIAADKHRP-UHFFFAOYSA-N 4-[[5-(2-fluoro-4-methoxyphenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound FC1=C(C=CC(=C1)OC)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)NO HEYXZNIAADKHRP-UHFFFAOYSA-N 0.000 description 1
- HEHUYQKSQKUESO-UHFFFAOYSA-N 4-[[5-(3-chloro-5-fluorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ClC=1C=C(C=C(C1)F)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)NO HEHUYQKSQKUESO-UHFFFAOYSA-N 0.000 description 1
- NJIQPZOLXHQOHM-UHFFFAOYSA-N 4-[[5-(4-chloro-2,3-difluorophenyl)-1,2-oxazol-3-yl]methylamino]-N-hydroxy-1,1-dioxothiane-4-carboxamide Chemical compound ClC1=C(C(=C(C=C1)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)NO)F)F NJIQPZOLXHQOHM-UHFFFAOYSA-N 0.000 description 1
- SWHUROFMIMHWKS-UHFFFAOYSA-N 4-bromo-3-fluorobenzaldehyde Chemical compound FC1=CC(C=O)=CC=C1Br SWHUROFMIMHWKS-UHFFFAOYSA-N 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N 4-hydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- NIEBHDXUIJSHSL-UHFFFAOYSA-N 4-iodobenzaldehyde Chemical compound IC1=CC=C(C=O)C=C1 NIEBHDXUIJSHSL-UHFFFAOYSA-N 0.000 description 1
- GPGKNEKFDGOXPO-UHFFFAOYSA-N 4-phenyl-1h-pyrazole Chemical compound C1=NNC=C1C1=CC=CC=C1 GPGKNEKFDGOXPO-UHFFFAOYSA-N 0.000 description 1
- 125000000339 4-pyridyl group Chemical group N1=C([H])C([H])=C([*])C([H])=C1[H] 0.000 description 1
- KDDQRKBRJSGMQE-UHFFFAOYSA-N 4-thiazolyl Chemical compound [C]1=CSC=N1 KDDQRKBRJSGMQE-UHFFFAOYSA-N 0.000 description 1
- CWDWFSXUQODZGW-UHFFFAOYSA-N 5-thiazolyl Chemical group [C]1=CN=CS1 CWDWFSXUQODZGW-UHFFFAOYSA-N 0.000 description 1
- MPORYQCGWFQFLA-ONPDANIMSA-N 7-[(7s)-7-amino-5-azaspiro[2.4]heptan-5-yl]-8-chloro-6-fluoro-1-[(1r,2s)-2-fluorocyclopropyl]-4-oxoquinoline-3-carboxylic acid;trihydrate Chemical compound O.O.O.C([C@H]1N)N(C=2C(=C3C(C(C(C(O)=O)=CN3[C@H]3[C@H](C3)F)=O)=CC=2F)Cl)CC11CC1.C([C@H]1N)N(C=2C(=C3C(C(C(C(O)=O)=CN3[C@H]3[C@H](C3)F)=O)=CC=2F)Cl)CC11CC1 MPORYQCGWFQFLA-ONPDANIMSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- DGGKXQQCVPAUEA-UHFFFAOYSA-N 8-azabicyclo[3.2.1]octane Chemical compound C1CCC2CCC1N2 DGGKXQQCVPAUEA-UHFFFAOYSA-N 0.000 description 1
- POOPWPIOIMBTOH-UHFFFAOYSA-N 8-oxa-3-azabicyclo[3.2.1]octane Chemical compound C1NCC2CCC1O2 POOPWPIOIMBTOH-UHFFFAOYSA-N 0.000 description 1
- GSDSWSVVBLHKDQ-UHFFFAOYSA-N 9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid Chemical compound FC1=CC(C(C(C(O)=O)=C2)=O)=C3N2C(C)COC3=C1N1CCN(C)CC1 GSDSWSVVBLHKDQ-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 241000588626 Acinetobacter baumannii Species 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- QGZKDVFQNNGYKY-OUBTZVSYSA-N Ammonia-15N Chemical compound [15NH3] QGZKDVFQNNGYKY-OUBTZVSYSA-N 0.000 description 1
- 235000003276 Apios tuberosa Nutrition 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000010744 Arachis villosulicarpa Nutrition 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 108010001478 Bacitracin Proteins 0.000 description 1
- 241000606125 Bacteroides Species 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 108020004256 Beta-lactamase Proteins 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- 241000589562 Brucella Species 0.000 description 1
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 1
- ZRFZKLTVZNNDOF-UHFFFAOYSA-N CC(C)(C)C(C)(CC1)CS1(=O)=O Chemical compound CC(C)(C)C(C)(CC1)CS1(=O)=O ZRFZKLTVZNNDOF-UHFFFAOYSA-N 0.000 description 1
- LCDZQMQYKLQMCC-UHFFFAOYSA-N CC(C)(C)C(CC1)(CS1(=O)=O)C(C)(C)C Chemical compound CC(C)(C)C(CC1)(CS1(=O)=O)C(C)(C)C LCDZQMQYKLQMCC-UHFFFAOYSA-N 0.000 description 1
- VMYHDBYTGXFZMD-UHFFFAOYSA-N CCC(CC)(CC1)CC1S Chemical compound CCC(CC)(CC1)CC1S VMYHDBYTGXFZMD-UHFFFAOYSA-N 0.000 description 1
- OCXRYDVMQXVJHJ-UHFFFAOYSA-N CCCC(C)(CC1)CC1OC Chemical compound CCCC(C)(CC1)CC1OC OCXRYDVMQXVJHJ-UHFFFAOYSA-N 0.000 description 1
- YCSWTNOOIHXDLP-UHFFFAOYSA-N CCOC(C(C1)(CC1O)NC(OC(C)(C)C)=O)=O Chemical compound CCOC(C(C1)(CC1O)NC(OC(C)(C)C)=O)=O YCSWTNOOIHXDLP-UHFFFAOYSA-N 0.000 description 1
- ZRKSHRHJIFWXJG-UHFFFAOYSA-N CC[O](C)C(C1(CNC1)NCc(cc1)ccc1Br)=O Chemical compound CC[O](C)C(C1(CNC1)NCc(cc1)ccc1Br)=O ZRKSHRHJIFWXJG-UHFFFAOYSA-N 0.000 description 1
- JYUODXKBNUQKNV-UHFFFAOYSA-N COC(C(CCCl)(CC1)CCS1(=O)=O)=O Chemical compound COC(C(CCCl)(CC1)CCS1(=O)=O)=O JYUODXKBNUQKNV-UHFFFAOYSA-N 0.000 description 1
- VMPIEFBRLASOAN-UHFFFAOYSA-N COC1=CC=C(C=C1)C1=CC=C(C=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NOC1OCCCC1 Chemical compound COC1=CC=C(C=C1)C1=CC=C(C=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NOC1OCCCC1 VMPIEFBRLASOAN-UHFFFAOYSA-N 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- MKFCYQTVSDCXAQ-UHFFFAOYSA-N Cc(c(F)c1)ccc1Cl Chemical compound Cc(c(F)c1)ccc1Cl MKFCYQTVSDCXAQ-UHFFFAOYSA-N 0.000 description 1
- WQOHEWKWIJOAHQ-UHFFFAOYSA-N Cc(ccc(OC)c1)c1F Chemical compound Cc(ccc(OC)c1)c1F WQOHEWKWIJOAHQ-UHFFFAOYSA-N 0.000 description 1
- UQLLWWBDSUHNEB-CZUORRHYSA-N Cefaprin Chemical compound N([C@H]1[C@@H]2N(C1=O)C(=C(CS2)COC(=O)C)C(O)=O)C(=O)CSC1=CC=NC=C1 UQLLWWBDSUHNEB-CZUORRHYSA-N 0.000 description 1
- GNWUOVJNSFPWDD-XMZRARIVSA-M Cefoxitin sodium Chemical compound [Na+].N([C@]1(OC)C(N2C(=C(COC(N)=O)CS[C@@H]21)C([O-])=O)=O)C(=O)CC1=CC=CS1 GNWUOVJNSFPWDD-XMZRARIVSA-M 0.000 description 1
- 239000004099 Chlortetracycline Substances 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 241000588919 Citrobacter freundii Species 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- 201000003883 Cystic fibrosis Diseases 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- AEMOLEFTQBMNLQ-AQKNRBDQSA-N D-glucopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-AQKNRBDQSA-N 0.000 description 1
- FMTDIUIBLCQGJB-UHFFFAOYSA-N Demethylchlortetracyclin Natural products C1C2C(O)C3=C(Cl)C=CC(O)=C3C(=O)C2=C(O)C2(O)C1C(N(C)C)C(O)=C(C(N)=O)C2=O FMTDIUIBLCQGJB-UHFFFAOYSA-N 0.000 description 1
- 208000008960 Diabetic foot Diseases 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 108090000204 Dipeptidase 1 Proteins 0.000 description 1
- 241000589566 Elizabethkingia meningoseptica Species 0.000 description 1
- 241000588697 Enterobacter cloacae Species 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 241000589602 Francisella tularensis Species 0.000 description 1
- 108010015899 Glycopeptides Proteins 0.000 description 1
- 102000002068 Glycopeptides Human genes 0.000 description 1
- AIJTTZAVMXIJGM-UHFFFAOYSA-N Grepafloxacin Chemical compound C1CNC(C)CN1C(C(=C1C)F)=CC2=C1C(=O)C(C(O)=O)=CN2C1CC1 AIJTTZAVMXIJGM-UHFFFAOYSA-N 0.000 description 1
- 239000007821 HATU Substances 0.000 description 1
- 241000606768 Haemophilus influenzae Species 0.000 description 1
- 241000590002 Helicobacter pylori Species 0.000 description 1
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 241000588915 Klebsiella aerogenes Species 0.000 description 1
- 241000588749 Klebsiella oxytoca Species 0.000 description 1
- 239000011786 L-ascorbyl-6-palmitate Substances 0.000 description 1
- QAQJMLQRFWZOBN-LAUBAEHRSA-N L-ascorbyl-6-palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](O)[C@H]1OC(=O)C(O)=C1O QAQJMLQRFWZOBN-LAUBAEHRSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- XAGMUUZPGZWTRP-ZETCQYMHSA-N LSM-5745 Chemical compound C([C@@H](N1C2=C(C(C(C(O)=O)=C1)=O)C=C1F)C)OC2=C1C1(N)CC1 XAGMUUZPGZWTRP-ZETCQYMHSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- OJMMVQQUTAEWLP-UHFFFAOYSA-N Lincomycin Natural products CN1CC(CCC)CC1C(=O)NC(C(C)O)C1C(O)C(O)C(O)C(SC)O1 OJMMVQQUTAEWLP-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 108700041567 MDR Genes Proteins 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 1
- RJQXTJLFIWVMTO-TYNCELHUSA-N Methicillin Chemical compound COC1=CC=CC(OC)=C1C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 RJQXTJLFIWVMTO-TYNCELHUSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 241000588655 Moraxella catarrhalis Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 1
- QGWGUZTWCCQZLZ-UHFFFAOYSA-N N-(oxan-2-yloxy)-1,1-dioxo-4-[(4-phenylphenyl)methylamino]thiane-4-carboxamide Chemical compound C1(=CC=C(C=C1)CNC1(CCS(CC1)(=O)=O)C(=O)NOC1OCCCC1)C1=CC=CC=C1 QGWGUZTWCCQZLZ-UHFFFAOYSA-N 0.000 description 1
- CQRAELCWFQQYBW-UHFFFAOYSA-N N-(oxan-2-yloxy)-1,1-dioxo-4-[[4-(2-phenylethynyl)phenyl]methylamino]thiane-4-carboxamide Chemical compound C1(=CC=CC=C1)C#CC1=CC=C(CNC2(CCS(CC2)(=O)=O)C(=O)NOC2OCCCC2)C=C1 CQRAELCWFQQYBW-UHFFFAOYSA-N 0.000 description 1
- SYOPEYIDZSPDLC-UHFFFAOYSA-N N-(oxan-2-yloxy)-3-[(4-phenylphenyl)methylamino]thiolane-3-carboxamide Chemical compound O=C(NOC1CCCCO1)C1(CCSC1)NCc1ccc(cc1)-c1ccccc1 SYOPEYIDZSPDLC-UHFFFAOYSA-N 0.000 description 1
- GXCLVBGFBYZDAG-UHFFFAOYSA-N N-[2-(1H-indol-3-yl)ethyl]-N-methylprop-2-en-1-amine Chemical compound CN(CCC1=CNC2=C1C=CC=C2)CC=C GXCLVBGFBYZDAG-UHFFFAOYSA-N 0.000 description 1
- QIAFMBKCNZACKA-UHFFFAOYSA-N N-benzoylglycine Chemical compound OC(=O)CNC(=O)C1=CC=CC=C1 QIAFMBKCNZACKA-UHFFFAOYSA-N 0.000 description 1
- ANOYQNZUMDJZDP-UHFFFAOYSA-N N-hydroxy-1,1-dioxo-3-[(3-phenyl-1,2-oxazol-5-yl)methylamino]thiolane-3-carboxamide Chemical compound ONC(=O)C1(CS(CC1)(=O)=O)NCC1=CC(=NO1)C1=CC=CC=C1 ANOYQNZUMDJZDP-UHFFFAOYSA-N 0.000 description 1
- JWYNIVGMOWQHBE-UHFFFAOYSA-N N-hydroxy-1,1-dioxo-3-[[4-(2-phenylethynyl)phenyl]methylamino]thiolane-3-carboxamide Chemical compound ONC(=O)C1(CCS(=O)(=O)C1)NCc1ccc(cc1)C#Cc1ccccc1 JWYNIVGMOWQHBE-UHFFFAOYSA-N 0.000 description 1
- HXBVHOTUEDDHGA-UHFFFAOYSA-N N-hydroxy-1,1-dioxo-4-[(3-phenyl-1,2-oxazol-5-yl)methylamino]thiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(CC1)(=O)=O)NCC1=CC(=NO1)C1=CC=CC=C1 HXBVHOTUEDDHGA-UHFFFAOYSA-N 0.000 description 1
- ZOURMKNHPAULEF-UHFFFAOYSA-N N-hydroxy-1,1-dioxo-4-[[5-(2,3,4-trifluorophenyl)-1,2-oxazol-3-yl]methylamino]thiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(=O)(=O)CC1)NCC1=NOC(=C1)C1=C(F)C(F)=C(F)C=C1 ZOURMKNHPAULEF-UHFFFAOYSA-N 0.000 description 1
- CRQAOHWSMKGHHN-UHFFFAOYSA-N N-hydroxy-1-methylsulfonyl-4-[(4-phenylphenyl)methyl]piperidine-4-carboxamide Chemical compound CS(=O)(=O)N1CCC(Cc2ccc(cc2)-c2ccccc2)(CC1)C(=O)NO CRQAOHWSMKGHHN-UHFFFAOYSA-N 0.000 description 1
- OHDARYAWAAMLRR-UHFFFAOYSA-N N-hydroxy-3-[(4-phenylphenyl)methylamino]thiolane-3-carboxamide Chemical compound C1(=CC=C(C=C1)CNC1(CSCC1)C(=O)NO)C1=CC=CC=C1 OHDARYAWAAMLRR-UHFFFAOYSA-N 0.000 description 1
- NNQXONYWCILFOZ-UHFFFAOYSA-N N-hydroxy-4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]oxane-4-carboxamide Chemical compound ONC(=O)C1(CCn2ccc(cc2=O)-c2ccccc2)CCOCC1 NNQXONYWCILFOZ-UHFFFAOYSA-N 0.000 description 1
- LYAJCVLHWHIFJF-UHFFFAOYSA-N N-hydroxy-4-[[3-methyl-4-[4-(triazol-2-yl)phenyl]phenyl]methylamino]-1,1-dioxothiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(CC1)(=O)=O)NCC1=CC(=C(C=C1)C1=CC=C(C=C1)N1N=CC=N1)C LYAJCVLHWHIFJF-UHFFFAOYSA-N 0.000 description 1
- OLFODROZOXDMDB-UHFFFAOYSA-N N-hydroxy-4-[[5-(3-methoxyphenyl)-1,2-oxazol-3-yl]methylamino]-1,1-dioxothiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(CC1)(=O)=O)NCC1=NOC(=C1)C1=CC(=CC=C1)OC OLFODROZOXDMDB-UHFFFAOYSA-N 0.000 description 1
- AHLNWXSBHVBQAZ-UHFFFAOYSA-N N-hydroxy-4-[[5-(3-methylphenyl)-1,2-oxazol-3-yl]methylamino]-1,1-dioxothiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(CC1)(=O)=O)NCC1=NOC(=C1)C=1C=C(C=CC1)C AHLNWXSBHVBQAZ-UHFFFAOYSA-N 0.000 description 1
- ZGEUNTZLWPJJMM-UHFFFAOYSA-N N-hydroxy-4-[[5-(4-methylthiophen-2-yl)-1,2-oxazol-3-yl]methylamino]-1,1-dioxothiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(CC1)(=O)=O)NCC1=NOC(=C1)C=1SC=C(C1)C ZGEUNTZLWPJJMM-UHFFFAOYSA-N 0.000 description 1
- FCCNQAJVTGAJFP-UHFFFAOYSA-N N-hydroxy-4-[[5-(5-methylthiophen-2-yl)-1,2-oxazol-3-yl]methylamino]-1,1-dioxothiane-4-carboxamide Chemical compound ONC(=O)C1(CCS(CC1)(=O)=O)NCC1=NOC(=C1)C=1SC(=CC1)C FCCNQAJVTGAJFP-UHFFFAOYSA-N 0.000 description 1
- YLENZGGQERWYGS-UHFFFAOYSA-N N-hydroxy-4-methoxy-1-[(4-phenylphenyl)methylamino]cyclohexane-1-carboxamide Chemical compound COC1CCC(CC1)(NCc1ccc(cc1)-c1ccccc1)C(=O)NO YLENZGGQERWYGS-UHFFFAOYSA-N 0.000 description 1
- CXXQAFZGHOFVQH-UHFFFAOYSA-N N-hydroxy-N-methyl-3-[(4-phenylphenyl)methylamino]thiolane-3-carboxamide Chemical compound CN(O)C(=O)C1(CCSC1)NCc1ccc(cc1)-c1ccccc1 CXXQAFZGHOFVQH-UHFFFAOYSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- CMWTZPSULFXXJA-UHFFFAOYSA-N Naproxen Natural products C1=C(C(C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-UHFFFAOYSA-N 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- KTKOKZANTAIMCP-UHFFFAOYSA-N O=S1(CCC2(CCC2)CC1)=O Chemical compound O=S1(CCC2(CCC2)CC1)=O KTKOKZANTAIMCP-UHFFFAOYSA-N 0.000 description 1
- DXXYHEYUHAAQND-UHFFFAOYSA-N OC(C(CC1)(CS1(=O)=O)NCc1n[o]c(-c2ccccc2)c1)=O Chemical compound OC(C(CC1)(CS1(=O)=O)NCc1n[o]c(-c2ccccc2)c1)=O DXXYHEYUHAAQND-UHFFFAOYSA-N 0.000 description 1
- 240000007817 Olea europaea Species 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 239000004100 Oxytetracycline Substances 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- 229930195708 Penicillin V Natural products 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-L Phosphate ion(2-) Chemical compound OP([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-L 0.000 description 1
- VQDBNKDJNJQRDG-UHFFFAOYSA-N Pirbuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=N1 VQDBNKDJNJQRDG-UHFFFAOYSA-N 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 229920002732 Polyanhydride Polymers 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 241000588778 Providencia stuartii Species 0.000 description 1
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 235000004443 Ricinus communis Nutrition 0.000 description 1
- URWAJWIAIPFPJE-UHFFFAOYSA-N Rickamicin Natural products O1CC(O)(C)C(NC)C(O)C1OC1C(O)C(OC2C(CC=C(CN)O2)N)C(N)CC1N URWAJWIAIPFPJE-UHFFFAOYSA-N 0.000 description 1
- 229930189077 Rifamycin Natural products 0.000 description 1
- 235000019485 Safflower oil Nutrition 0.000 description 1
- 241000607715 Serratia marcescens Species 0.000 description 1
- 208000019802 Sexually transmitted disease Diseases 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 229930192786 Sisomicin Natural products 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 241000191967 Staphylococcus aureus Species 0.000 description 1
- SSZBUIDZHHWXNJ-UHFFFAOYSA-N Stearinsaeure-hexadecylester Natural products CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCC SSZBUIDZHHWXNJ-UHFFFAOYSA-N 0.000 description 1
- 241000122971 Stenotrophomonas Species 0.000 description 1
- 108010034396 Streptogramins Proteins 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- NHUHCSRWZMLRLA-UHFFFAOYSA-N Sulfisoxazole Chemical compound CC1=NOC(NS(=O)(=O)C=2C=CC(N)=CC=2)=C1C NHUHCSRWZMLRLA-UHFFFAOYSA-N 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 239000005864 Sulphur Chemical group 0.000 description 1
- 238000006069 Suzuki reaction reaction Methods 0.000 description 1
- 108010053950 Teicoplanin Proteins 0.000 description 1
- DHXVGJBLRPWPCS-UHFFFAOYSA-N Tetrahydropyran Chemical compound C1CCOCC1 DHXVGJBLRPWPCS-UHFFFAOYSA-N 0.000 description 1
- YPWFISCTZQNZAU-UHFFFAOYSA-N Thiane Chemical compound C1CCSCC1 YPWFISCTZQNZAU-UHFFFAOYSA-N 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- GCTFWCDSFPMHHS-UHFFFAOYSA-M Tributyltin chloride Chemical compound CCCC[Sn](Cl)(CCCC)CCCC GCTFWCDSFPMHHS-UHFFFAOYSA-M 0.000 description 1
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 1
- 108010003026 UDP-3-O-acyl-N-acetylglucosamine deacetylase Proteins 0.000 description 1
- 241000607493 Vibrionaceae Species 0.000 description 1
- 206010052428 Wound Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 241000607479 Yersinia pestis Species 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- ZWBTYMGEBZUQTK-PVLSIAFMSA-N [(7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,32-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-1'-(2-methylpropyl)-6,23-dioxospiro[8,33-dioxa-24,27,29-triazapentacyclo[23.6.1.14,7.05,31.026,30]tritriaconta-1(32),2,4,9,19,21,24,26,30-nonaene-28,4'-piperidine]-13-yl] acetate Chemical compound CO[C@H]1\C=C\O[C@@]2(C)Oc3c(C2=O)c2c4NC5(CCN(CC(C)C)CC5)N=c4c(=NC(=O)\C(C)=C/C=C/[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@@H]1C)c(O)c2c(O)c3C ZWBTYMGEBZUQTK-PVLSIAFMSA-N 0.000 description 1
- CDOGESOGFOQXSP-UHFFFAOYSA-N [5-(4-chloro-2-fluorophenyl)-4-fluoro-1,2-oxazol-3-yl]methanol Chemical compound ClC1=CC(=C(C=C1)C1=C(C(=NO1)CO)F)F CDOGESOGFOQXSP-UHFFFAOYSA-N 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000003655 absorption accelerator Substances 0.000 description 1
- 239000000370 acceptor Substances 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- CSCPPACGZOOCGX-WFGJKAKNSA-N acetone d6 Chemical compound [2H]C([2H])([2H])C(=O)C([2H])([2H])[2H] CSCPPACGZOOCGX-WFGJKAKNSA-N 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- YBCVMFKXIKNREZ-UHFFFAOYSA-N acoh acetic acid Chemical compound CC(O)=O.CC(O)=O YBCVMFKXIKNREZ-UHFFFAOYSA-N 0.000 description 1
- 239000003463 adsorbent Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 239000003570 air Substances 0.000 description 1
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- 229960003022 amoxicillin Drugs 0.000 description 1
- LSQZJLSUYDQPKJ-NJBDSQKTSA-N amoxicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 LSQZJLSUYDQPKJ-NJBDSQKTSA-N 0.000 description 1
- 230000000202 analgesic effect Effects 0.000 description 1
- 230000001088 anti-asthma Effects 0.000 description 1
- 230000001986 anti-endotoxic effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 239000000924 antiasthmatic agent Substances 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 235000010385 ascorbyl palmitate Nutrition 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- HONIICLYMWZJFZ-UHFFFAOYSA-N azetidine Chemical compound C1CNC1 HONIICLYMWZJFZ-UHFFFAOYSA-N 0.000 description 1
- 229960003623 azlocillin Drugs 0.000 description 1
- JTWOMNBEOCYFNV-NFFDBFGFSA-N azlocillin Chemical compound N([C@@H](C(=O)N[C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C=1C=CC=CC=1)C(=O)N1CCNC1=O JTWOMNBEOCYFNV-NFFDBFGFSA-N 0.000 description 1
- 229960003071 bacitracin Drugs 0.000 description 1
- 229930184125 bacitracin Natural products 0.000 description 1
- CLKOFPXJLQSYAH-ABRJDSQDSA-N bacitracin A Chemical compound C1SC([C@@H](N)[C@@H](C)CC)=N[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]1C(=O)N[C@H](CCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2N=CNC=2)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCCCC1 CLKOFPXJLQSYAH-ABRJDSQDSA-N 0.000 description 1
- 230000003385 bacteriostatic effect Effects 0.000 description 1
- 150000007514 bases Chemical class 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- JUHORIMYRDESRB-UHFFFAOYSA-N benzathine Chemical compound C=1C=CC=CC=1CNCCNCC1=CC=CC=C1 JUHORIMYRDESRB-UHFFFAOYSA-N 0.000 description 1
- HUMNYLRZRPPJDN-UHFFFAOYSA-N benzenecarboxaldehyde Natural products O=CC1=CC=CC=C1 HUMNYLRZRPPJDN-UHFFFAOYSA-N 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 229940066363 beractant Drugs 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000006696 biosynthetic metabolic pathway Effects 0.000 description 1
- SIPUZPBQZHNSDW-UHFFFAOYSA-N bis(2-methylpropyl)aluminum Chemical compound CC(C)C[Al]CC(C)C SIPUZPBQZHNSDW-UHFFFAOYSA-N 0.000 description 1
- IPWKHHSGDUIRAH-UHFFFAOYSA-N bis(pinacolato)diboron Chemical compound O1C(C)(C)C(C)(C)OB1B1OC(C)(C)C(C)(C)O1 IPWKHHSGDUIRAH-UHFFFAOYSA-N 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000006161 blood agar Substances 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 238000002815 broth microdilution Methods 0.000 description 1
- 229960004436 budesonide Drugs 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- 235000019437 butane-1,3-diol Nutrition 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 235000019282 butylated hydroxyanisole Nutrition 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 235000012241 calcium silicate Nutrition 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 229940026290 calfactant Drugs 0.000 description 1
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical compound C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 description 1
- 238000004850 capillary HPLC Methods 0.000 description 1
- 229940041011 carbapenems Drugs 0.000 description 1
- 229960003669 carbenicillin Drugs 0.000 description 1
- FPPNZSSZRUTDAP-UWFZAAFLSA-N carbenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)C(C(O)=O)C1=CC=CC=C1 FPPNZSSZRUTDAP-UWFZAAFLSA-N 0.000 description 1
- RBHJBMIOOPYDBQ-UHFFFAOYSA-N carbon dioxide;propan-2-one Chemical compound O=C=O.CC(C)=O RBHJBMIOOPYDBQ-UHFFFAOYSA-N 0.000 description 1
- 150000003857 carboxamides Chemical class 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000012876 carrier material Substances 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- QYIYFLOTGYLRGG-GPCCPHFNSA-N cefaclor Chemical compound C1([C@H](C(=O)N[C@@H]2C(N3C(=C(Cl)CS[C@@H]32)C(O)=O)=O)N)=CC=CC=C1 QYIYFLOTGYLRGG-GPCCPHFNSA-N 0.000 description 1
- 229960005361 cefaclor Drugs 0.000 description 1
- CZTQZXZIADLWOZ-CRAIPNDOSA-N cefaloridine Chemical compound O=C([C@@H](NC(=O)CC=1SC=CC=1)[C@H]1SC2)N1C(C(=O)[O-])=C2C[N+]1=CC=CC=C1 CZTQZXZIADLWOZ-CRAIPNDOSA-N 0.000 description 1
- 229960003866 cefaloridine Drugs 0.000 description 1
- OLVCFLKTBJRLHI-AXAPSJFSSA-N cefamandole Chemical compound CN1N=NN=C1SCC1=C(C(O)=O)N2C(=O)[C@@H](NC(=O)[C@H](O)C=3C=CC=CC=3)[C@H]2SC1 OLVCFLKTBJRLHI-AXAPSJFSSA-N 0.000 description 1
- 229960003012 cefamandole Drugs 0.000 description 1
- 229960004350 cefapirin Drugs 0.000 description 1
- 229960001139 cefazolin Drugs 0.000 description 1
- MLYYVTUWGNIJIB-BXKDBHETSA-N cefazolin Chemical compound S1C(C)=NN=C1SCC1=C(C(O)=O)N2C(=O)[C@@H](NC(=O)CN3N=NN=C3)[C@H]2SC1 MLYYVTUWGNIJIB-BXKDBHETSA-N 0.000 description 1
- RTXOFQZKPXMALH-GHXIOONMSA-N cefdinir Chemical compound S1C(N)=NC(C(=N\O)\C(=O)N[C@@H]2C(N3C(=C(C=C)CS[C@@H]32)C(O)=O)=O)=C1 RTXOFQZKPXMALH-GHXIOONMSA-N 0.000 description 1
- 229960003719 cefdinir Drugs 0.000 description 1
- HVFLCNVBZFFHBT-ZKDACBOMSA-O cefepime(1+) Chemical compound S([C@@H]1[C@@H](C(N1C=1C(O)=O)=O)NC(=O)\C(=N/OC)C=2N=C(N)SC=2)CC=1C[N+]1(C)CCCC1 HVFLCNVBZFFHBT-ZKDACBOMSA-O 0.000 description 1
- 229960002129 cefixime Drugs 0.000 description 1
- OKBVVJOGVLARMR-QSWIMTSFSA-N cefixime Chemical compound S1C(N)=NC(C(=N\OCC(O)=O)\C(=O)N[C@@H]2C(N3C(=C(C=C)CS[C@@H]32)C(O)=O)=O)=C1 OKBVVJOGVLARMR-QSWIMTSFSA-N 0.000 description 1
- GCFBRXLSHGKWDP-XCGNWRKASA-N cefoperazone Chemical compound O=C1C(=O)N(CC)CCN1C(=O)N[C@H](C=1C=CC(O)=CC=1)C(=O)N[C@@H]1C(=O)N2C(C(O)=O)=C(CSC=3N(N=NN=3)C)CS[C@@H]21 GCFBRXLSHGKWDP-XCGNWRKASA-N 0.000 description 1
- 229960004682 cefoperazone Drugs 0.000 description 1
- 229960002682 cefoxitin Drugs 0.000 description 1
- 229960000466 cefpirome Drugs 0.000 description 1
- DKOQGJHPHLTOJR-WHRDSVKCSA-N cefpirome Chemical compound N([C@@H]1C(N2C(=C(C[N+]=3C=4CCCC=4C=CC=3)CS[C@@H]21)C([O-])=O)=O)C(=O)\C(=N/OC)C1=CSC(N)=N1 DKOQGJHPHLTOJR-WHRDSVKCSA-N 0.000 description 1
- WYUSVOMTXWRGEK-HBWVYFAYSA-N cefpodoxime Chemical compound N([C@H]1[C@@H]2N(C1=O)C(=C(CS2)COC)C(O)=O)C(=O)C(=N/OC)\C1=CSC(N)=N1 WYUSVOMTXWRGEK-HBWVYFAYSA-N 0.000 description 1
- 229960005090 cefpodoxime Drugs 0.000 description 1
- 229960002580 cefprozil Drugs 0.000 description 1
- 229960002588 cefradine Drugs 0.000 description 1
- 229960000484 ceftazidime Drugs 0.000 description 1
- NMVPEQXCMGEDNH-TZVUEUGBSA-N ceftazidime pentahydrate Chemical compound O.O.O.O.O.S([C@@H]1[C@@H](C(N1C=1C([O-])=O)=O)NC(=O)\C(=N/OC(C)(C)C(O)=O)C=2N=C(N)SC=2)CC=1C[N+]1=CC=CC=C1 NMVPEQXCMGEDNH-TZVUEUGBSA-N 0.000 description 1
- 229960004086 ceftibuten Drugs 0.000 description 1
- UNJFKXSSGBWRBZ-BJCIPQKHSA-N ceftibuten Chemical compound S1C(N)=NC(C(=C\CC(O)=O)\C(=O)N[C@@H]2C(N3C(=CCS[C@@H]32)C(O)=O)=O)=C1 UNJFKXSSGBWRBZ-BJCIPQKHSA-N 0.000 description 1
- 229960001991 ceftizoxime Drugs 0.000 description 1
- NNULBSISHYWZJU-LLKWHZGFSA-N ceftizoxime Chemical compound N([C@@H]1C(N2C(=CCS[C@@H]21)C(O)=O)=O)C(=O)\C(=N/OC)C1=CSC(N)=N1 NNULBSISHYWZJU-LLKWHZGFSA-N 0.000 description 1
- 229960001668 cefuroxime Drugs 0.000 description 1
- JFPVXVDWJQMJEE-IZRZKJBUSA-N cefuroxime Chemical compound N([C@@H]1C(N2C(=C(COC(N)=O)CS[C@@H]21)C(O)=O)=O)C(=O)\C(=N/OC)C1=CC=CO1 JFPVXVDWJQMJEE-IZRZKJBUSA-N 0.000 description 1
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 1
- 229960000590 celecoxib Drugs 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 229940106164 cephalexin Drugs 0.000 description 1
- ZAIPMKNFIOOWCQ-UEKVPHQBSA-N cephalexin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C)C(O)=O)=CC=CC=C1 ZAIPMKNFIOOWCQ-UEKVPHQBSA-N 0.000 description 1
- RDLPVSKMFDYCOR-UEKVPHQBSA-N cephradine Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C)C(O)=O)=CCC=CC1 RDLPVSKMFDYCOR-UEKVPHQBSA-N 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- DDTDNCYHLGRFBM-YZEKDTGTSA-N chembl2367892 Chemical compound CC(=O)N[C@H]1[C@@H](O)[C@H](O)[C@H](CO)O[C@H]1O[C@@H]([C@H]1C(N[C@@H](C2=CC(O)=CC(O[C@@H]3[C@H]([C@H](O)[C@H](O)[C@@H](CO)O3)O)=C2C=2C(O)=CC=C(C=2)[C@@H](NC(=O)[C@@H]2NC(=O)[C@@H]3C=4C=C(O)C=C(C=4)OC=4C(O)=CC=C(C=4)[C@@H](N)C(=O)N[C@H](CC=4C=C(Cl)C(O5)=CC=4)C(=O)N3)C(=O)N1)C(O)=O)=O)C(C=C1Cl)=CC=C1OC1=C(O[C@H]3[C@H]([C@@H](O)[C@H](O)[C@H](CO)O3)NC(C)=O)C5=CC2=C1 DDTDNCYHLGRFBM-YZEKDTGTSA-N 0.000 description 1
- 239000012069 chiral reagent Substances 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 150000005827 chlorofluoro hydrocarbons Chemical class 0.000 description 1
- CYDMQBQPVICBEU-UHFFFAOYSA-N chlorotetracycline Natural products C1=CC(Cl)=C2C(O)(C)C3CC4C(N(C)C)C(O)=C(C(N)=O)C(=O)C4(O)C(O)=C3C(=O)C2=C1O CYDMQBQPVICBEU-UHFFFAOYSA-N 0.000 description 1
- 229960004475 chlortetracycline Drugs 0.000 description 1
- CYDMQBQPVICBEU-XRNKAMNCSA-N chlortetracycline Chemical compound C1=CC(Cl)=C2[C@](O)(C)[C@H]3C[C@H]4[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O CYDMQBQPVICBEU-XRNKAMNCSA-N 0.000 description 1
- 235000019365 chlortetracycline Nutrition 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- QGPKADBNRMWEQR-UHFFFAOYSA-N clinafloxacin Chemical compound C1C(N)CCN1C1=C(F)C=C2C(=O)C(C(O)=O)=CN(C3CC3)C2=C1Cl QGPKADBNRMWEQR-UHFFFAOYSA-N 0.000 description 1
- 229950001320 clinafloxacin Drugs 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 229960003326 cloxacillin Drugs 0.000 description 1
- LQOLIRLGBULYKD-JKIFEVAISA-N cloxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=CC=CC=C1Cl LQOLIRLGBULYKD-JKIFEVAISA-N 0.000 description 1
- 238000003181 co-melting Methods 0.000 description 1
- 230000001332 colony forming effect Effects 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 239000012230 colorless oil Substances 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 229940125904 compound 1 Drugs 0.000 description 1
- 239000007891 compressed tablet Substances 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 239000001767 crosslinked sodium carboxy methyl cellulose Substances 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 229960001305 cysteine hydrochloride Drugs 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 1
- DEZRYPDIMOWBDS-UHFFFAOYSA-N dcm dichloromethane Chemical compound ClCCl.ClCCl DEZRYPDIMOWBDS-UHFFFAOYSA-N 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 231100000517 death Toxicity 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 229960002398 demeclocycline Drugs 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- YFAGHNZHGGCZAX-JKIFEVAISA-N dicloxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=C(Cl)C=CC=C1Cl YFAGHNZHGGCZAX-JKIFEVAISA-N 0.000 description 1
- 229960001585 dicloxacillin Drugs 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- WYACBZDAHNBPPB-UHFFFAOYSA-N diethyl oxalate Chemical compound CCOC(=O)C(=O)OCC WYACBZDAHNBPPB-UHFFFAOYSA-N 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-M dihydrogenphosphate Chemical compound OP(O)([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-M 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- CETRZFQIITUQQL-UHFFFAOYSA-N dmso dimethylsulfoxide Chemical compound CS(C)=O.CS(C)=O CETRZFQIITUQQL-UHFFFAOYSA-N 0.000 description 1
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 1
- 229940043264 dodecyl sulfate Drugs 0.000 description 1
- 108010067396 dornase alfa Proteins 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 239000012039 electrophile Substances 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 108010072542 endotoxin binding proteins Proteins 0.000 description 1
- 108010027836 endotoxin receptor Proteins 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 229960002549 enoxacin Drugs 0.000 description 1
- IDYZIJYBMGIQMJ-UHFFFAOYSA-N enoxacin Chemical compound N1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNCC1 IDYZIJYBMGIQMJ-UHFFFAOYSA-N 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 229940092559 enterobacter aerogenes Drugs 0.000 description 1
- 238000001952 enzyme assay Methods 0.000 description 1
- 238000006345 epimerization reaction Methods 0.000 description 1
- LHWWETDBWVTKJO-UHFFFAOYSA-N et3n triethylamine Chemical compound CCN(CC)CC.CCN(CC)CC LHWWETDBWVTKJO-UHFFFAOYSA-N 0.000 description 1
- AFAXGSQYZLGZPG-UHFFFAOYSA-L ethanedisulfonate group Chemical group C(CS(=O)(=O)[O-])S(=O)(=O)[O-] AFAXGSQYZLGZPG-UHFFFAOYSA-L 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- OCLXJTCGWSSVOE-UHFFFAOYSA-N ethanol etoh Chemical compound CCO.CCO OCLXJTCGWSSVOE-UHFFFAOYSA-N 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- UXOLDCOJRAMLTQ-ZZXKWVIFSA-N ethyl (2e)-2-chloro-2-hydroxyiminoacetate Chemical compound CCOC(=O)C(\Cl)=N/O UXOLDCOJRAMLTQ-ZZXKWVIFSA-N 0.000 description 1
- IRDLHCXFDAEMEZ-UHFFFAOYSA-N ethyl 1,1-dioxo-4-[(4-phenylphenyl)methylamino]thiane-4-carboxylate Chemical compound C1(=CC=C(C=C1)CNC1(CCS(CC1)(=O)=O)C(=O)OCC)C1=CC=CC=C1 IRDLHCXFDAEMEZ-UHFFFAOYSA-N 0.000 description 1
- DFSYSSVVPOGHSO-UHFFFAOYSA-N ethyl 1-[(2-methylpropan-2-yl)oxycarbonylamino]-3-oxocyclobutane-1-carboxylate Chemical compound CC(C)(C)OC(=O)NC1(C(=O)OCC)CC(=O)C1 DFSYSSVVPOGHSO-UHFFFAOYSA-N 0.000 description 1
- YPXBMTZJTHRFSV-UHFFFAOYSA-N ethyl 1-methylsulfonyl-4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]piperidine-4-carboxylate Chemical compound CCOC(=O)C1(CCn2ccc(cc2=O)-c2ccccc2)CCN(CC1)S(C)(=O)=O YPXBMTZJTHRFSV-UHFFFAOYSA-N 0.000 description 1
- DILNLRSTMLPTFW-UHFFFAOYSA-N ethyl 3-[(4-bromophenyl)methylamino]-1-methylsulfonylazetidine-3-carboxylate Chemical compound CCOC(=O)C1(CN(C1)S(C)(=O)=O)NCc1ccc(Br)cc1 DILNLRSTMLPTFW-UHFFFAOYSA-N 0.000 description 1
- VUIVLEGVJANJIT-UHFFFAOYSA-N ethyl 3-[(4-bromophenyl)methylamino]azetidine-3-carboxylate Chemical compound CCOC(=O)C1(CNC1)NCc1ccc(Br)cc1 VUIVLEGVJANJIT-UHFFFAOYSA-N 0.000 description 1
- VJBNGWSLQUVGSR-UHFFFAOYSA-N ethyl 4-[(4-phenylphenyl)methyl]piperidine-4-carboxylate Chemical compound CCOC(=O)C1(Cc2ccc(cc2)-c2ccccc2)CCNCC1 VJBNGWSLQUVGSR-UHFFFAOYSA-N 0.000 description 1
- QIWNOCCZDGTCAZ-UHFFFAOYSA-N ethyl 4-[2-(2-oxo-4-phenylpyridin-1-yl)ethyl]piperidine-4-carboxylate Chemical compound CCOC(=O)C1(CCn2ccc(cc2=O)-c2ccccc2)CCNCC1 QIWNOCCZDGTCAZ-UHFFFAOYSA-N 0.000 description 1
- SDCYFJTYUOCLQL-UHFFFAOYSA-N ethyl 4-[2-(4-bromophenyl)ethyl]oxane-4-carboxylate Chemical compound CCOC(=O)C1(CCc2ccc(Br)cc2)CCOCC1 SDCYFJTYUOCLQL-UHFFFAOYSA-N 0.000 description 1
- XSQOLQZRTCYYGD-UHFFFAOYSA-N ethyl 4-[2-(4-phenylphenyl)ethyl]oxane-4-carboxylate Chemical compound CCOC(=O)C1(CCc2ccc(cc2)-c2ccccc2)CCOCC1 XSQOLQZRTCYYGD-UHFFFAOYSA-N 0.000 description 1
- WTIHPLZPMBKXSZ-UHFFFAOYSA-N ethyl 4-amino-1,1-dioxothiane-4-carboxylate Chemical compound CCOC(=O)C1(N)CCS(=O)(=O)CC1 WTIHPLZPMBKXSZ-UHFFFAOYSA-N 0.000 description 1
- GVOBYPBVOIYFSQ-UHFFFAOYSA-N ethyl 4-chloro-5-(4-chloro-2-fluorophenyl)-1,2-oxazole-3-carboxylate Chemical compound CCOC(=O)c1noc(c1Cl)-c1ccc(Cl)cc1F GVOBYPBVOIYFSQ-UHFFFAOYSA-N 0.000 description 1
- PNFSCLZYMKNOBW-UHFFFAOYSA-N ethyl 5-(4-chloro-2-fluorophenyl)-4-fluoro-1,2-oxazole-3-carboxylate Chemical compound CCOC(=O)c1noc(c1F)-c1ccc(Cl)cc1F PNFSCLZYMKNOBW-UHFFFAOYSA-N 0.000 description 1
- DUZOSQMZUNYJSO-UHFFFAOYSA-N ethyl 5-[4-(difluoromethoxy)phenyl]-1,2-oxazole-3-carboxylate Chemical compound CCOC(=O)c1cc(on1)-c1ccc(OC(F)F)cc1 DUZOSQMZUNYJSO-UHFFFAOYSA-N 0.000 description 1
- LDFBGRFJCWKQCA-UHFFFAOYSA-N ethyl 5-tributylstannyl-1,2-oxazole-3-carboxylate Chemical compound CCCC[Sn](CCCC)(CCCC)C1=CC(C(=O)OCC)=NO1 LDFBGRFJCWKQCA-UHFFFAOYSA-N 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- BCXINTIRMSZYKA-UHFFFAOYSA-N ethyl oxane-4-carboxylate Chemical compound CCOC(=O)C1CCOCC1 BCXINTIRMSZYKA-UHFFFAOYSA-N 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 239000003885 eye ointment Substances 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 229960003306 fleroxacin Drugs 0.000 description 1
- XBJBPGROQZJDOJ-UHFFFAOYSA-N fleroxacin Chemical compound C1CN(C)CCN1C1=C(F)C=C2C(=O)C(C(O)=O)=CN(CCF)C2=C1F XBJBPGROQZJDOJ-UHFFFAOYSA-N 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 229960000676 flunisolide Drugs 0.000 description 1
- 229940124307 fluoroquinolone Drugs 0.000 description 1
- 229960002714 fluticasone Drugs 0.000 description 1
- MGNNYOODZCAHBA-GQKYHHCASA-N fluticasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(O)[C@@]2(C)C[C@@H]1O MGNNYOODZCAHBA-GQKYHHCASA-N 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 229940118764 francisella tularensis Drugs 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 229940050411 fumarate Drugs 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 229960001430 garenoxacin Drugs 0.000 description 1
- NJDRXTDGYFKORP-LLVKDONJSA-N garenoxacin Chemical compound N([C@@H](C1=CC=2)C)CC1=CC=2C(C=1OC(F)F)=CC=C(C(C(C(O)=O)=C2)=O)C=1N2C1CC1 NJDRXTDGYFKORP-LLVKDONJSA-N 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 229960003923 gatifloxacin Drugs 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 229960003170 gemifloxacin Drugs 0.000 description 1
- ZRCVYEYHRGVLOC-HYARGMPZSA-N gemifloxacin Chemical compound C1C(CN)C(=N/OC)/CN1C(C(=C1)F)=NC2=C1C(=O)C(C(O)=O)=CN2C1CC1 ZRCVYEYHRGVLOC-HYARGMPZSA-N 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 230000008570 general process Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229960001731 gluceptate Drugs 0.000 description 1
- KWMLJOLKUYYJFJ-VFUOTHLCSA-N glucoheptonic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)C(O)=O KWMLJOLKUYYJFJ-VFUOTHLCSA-N 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 229960002442 glucosamine Drugs 0.000 description 1
- 229940097042 glucuronate Drugs 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- LEQAOMBKQFMDFZ-UHFFFAOYSA-N glyoxal Chemical compound O=CC=O LEQAOMBKQFMDFZ-UHFFFAOYSA-N 0.000 description 1
- 244000000058 gram-negative pathogen Species 0.000 description 1
- 229960000642 grepafloxacin Drugs 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 230000009036 growth inhibition Effects 0.000 description 1
- 229940047650 haemophilus influenzae Drugs 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 229940037467 helicobacter pylori Drugs 0.000 description 1
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-M hexadecanoate Chemical compound CCCCCCCCCCCCCCCC([O-])=O IPCSVZSSVZVIGE-UHFFFAOYSA-M 0.000 description 1
- FFUAGWLWBBFQJT-UHFFFAOYSA-N hexamethyldisilazane Chemical compound C[Si](C)(C)N[Si](C)(C)C FFUAGWLWBBFQJT-UHFFFAOYSA-N 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- BHEPBYXIRTUNPN-UHFFFAOYSA-N hydridophosphorus(.) (triplet) Chemical group [PH] BHEPBYXIRTUNPN-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 1
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 230000000266 injurious effect Effects 0.000 description 1
- 239000002054 inoculum Substances 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 239000013067 intermediate product Substances 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- OEXHQOGQTVQTAT-JRNQLAHRSA-N ipratropium Chemical compound O([C@H]1C[C@H]2CC[C@@H](C1)[N@@+]2(C)C(C)C)C(=O)C(CO)C1=CC=CC=C1 OEXHQOGQTVQTAT-JRNQLAHRSA-N 0.000 description 1
- 229960001888 ipratropium Drugs 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- UDIIBEDMEYAVNG-ZKFPOVNWSA-N isepamicin Chemical compound O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CN)O2)O)[C@@H](N)C[C@H]1NC(=O)[C@@H](O)CN UDIIBEDMEYAVNG-ZKFPOVNWSA-N 0.000 description 1
- 229960000798 isepamicin Drugs 0.000 description 1
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 125000004284 isoxazol-3-yl group Chemical group [H]C1=C([H])C(*)=NO1 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 239000003835 ketolide antibiotic agent Substances 0.000 description 1
- 229940001447 lactate Drugs 0.000 description 1
- 229940099584 lactobionate Drugs 0.000 description 1
- JYTUSYBCFIZPBE-AMTLMPIISA-N lactobionic acid Chemical compound OC(=O)[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O JYTUSYBCFIZPBE-AMTLMPIISA-N 0.000 description 1
- 229960005287 lincomycin Drugs 0.000 description 1
- OJMMVQQUTAEWLP-KIDUDLJLSA-N lincomycin Chemical compound CN1C[C@H](CCC)C[C@H]1C(=O)N[C@H]([C@@H](C)O)[C@@H]1[C@H](O)[C@H](O)[C@@H](O)[C@@H](SC)O1 OJMMVQQUTAEWLP-KIDUDLJLSA-N 0.000 description 1
- 229940041028 lincosamides Drugs 0.000 description 1
- 238000012417 linear regression Methods 0.000 description 1
- 229960003907 linezolid Drugs 0.000 description 1
- TYZROVQLWOKYKF-ZDUSSCGKSA-N linezolid Chemical compound O=C1O[C@@H](CNC(=O)C)CN1C(C=C1F)=CC=C1N1CCOCC1 TYZROVQLWOKYKF-ZDUSSCGKSA-N 0.000 description 1
- 239000006194 liquid suspension Substances 0.000 description 1
- 229960002422 lomefloxacin Drugs 0.000 description 1
- ZEKZLJVOYLTDKK-UHFFFAOYSA-N lomefloxacin Chemical compound FC1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNC(C)C1 ZEKZLJVOYLTDKK-UHFFFAOYSA-N 0.000 description 1
- 229960001977 loracarbef Drugs 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 229960003646 lysine Drugs 0.000 description 1
- 239000003120 macrolide antibiotic agent Substances 0.000 description 1
- 229940041033 macrolides Drugs 0.000 description 1
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 1
- 239000000347 magnesium hydroxide Substances 0.000 description 1
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- LROBJRRFCPYLIT-UHFFFAOYSA-M magnesium;ethyne;bromide Chemical compound [Mg+2].[Br-].[C-]#C LROBJRRFCPYLIT-UHFFFAOYSA-M 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 229940049920 malate Drugs 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-M mandelate Chemical compound [O-]C(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-M 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- BCVXHSPFUWZLGQ-UHFFFAOYSA-N mecn acetonitrile Chemical compound CC#N.CC#N BCVXHSPFUWZLGQ-UHFFFAOYSA-N 0.000 description 1
- 229960003194 meglumine Drugs 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- COTNUBDHGSIOTA-UHFFFAOYSA-N meoh methanol Chemical compound OC.OC COTNUBDHGSIOTA-UHFFFAOYSA-N 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- LMOINURANNBYCM-UHFFFAOYSA-N metaproterenol Chemical compound CC(C)NCC(O)C1=CC(O)=CC(O)=C1 LMOINURANNBYCM-UHFFFAOYSA-N 0.000 description 1
- 229940042016 methacycline Drugs 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- OFXSXYCSPVKZPF-UHFFFAOYSA-N methoxyperoxymethane Chemical compound COOOC OFXSXYCSPVKZPF-UHFFFAOYSA-N 0.000 description 1
- VHUHBRAJZOOOKJ-UHFFFAOYSA-N methyl 1,1-dioxo-4-[(5-phenyl-1,2-oxazol-3-yl)methylamino]thiane-4-carboxylate Chemical compound C1(=CC=CC=C1)C1=CC(=NO1)CNC1(CCS(CC1)(=O)=O)C(=O)OC VHUHBRAJZOOOKJ-UHFFFAOYSA-N 0.000 description 1
- HEKQCIAOOBLZJL-UHFFFAOYSA-N methyl 1,1-dioxo-4-[2-oxo-2-(4-phenylphenyl)ethyl]thiane-4-carboxylate Chemical compound COC(=O)C1(CC(=O)C2=CC=C(C=C2)C2=CC=CC=C2)CCS(=O)(=O)CC1 HEKQCIAOOBLZJL-UHFFFAOYSA-N 0.000 description 1
- CANDWIFYKYTEKB-UHFFFAOYSA-N methyl 1-[(2-methylpropan-2-yl)oxycarbonylamino]-4-oxocyclohexane-1-carboxylate Chemical compound CC(C)(C)OC(=O)NC1(C(=O)OC)CCC(=O)CC1 CANDWIFYKYTEKB-UHFFFAOYSA-N 0.000 description 1
- DUBUTYYRVIWPGR-UHFFFAOYSA-N methyl 1-amino-4-oxocyclohexane-1-carboxylate Chemical compound COC(=O)C1(N)CCC(=O)CC1 DUBUTYYRVIWPGR-UHFFFAOYSA-N 0.000 description 1
- PLGDWPZPJPOPHW-UHFFFAOYSA-N methyl 3-[(2-methylpropan-2-yl)oxycarbonylamino]-1,1-dioxothiolane-3-carboxylate Chemical compound COC(=O)C1(CCS(=O)(=O)C1)NC(=O)OC(C)(C)C PLGDWPZPJPOPHW-UHFFFAOYSA-N 0.000 description 1
- KIHNSFMAMJHMEE-UHFFFAOYSA-N methyl 3-[(2-methylpropan-2-yl)oxycarbonylamino]thiolane-3-carboxylate Chemical compound COC(=O)C1(CCSC1)NC(=O)OC(C)(C)C KIHNSFMAMJHMEE-UHFFFAOYSA-N 0.000 description 1
- VNEHUGQNNGOGCN-UHFFFAOYSA-N methyl 3-amino-1,1-dioxothiolane-3-carboxylate Chemical compound COC(=O)C1(N)CCS(=O)(=O)C1 VNEHUGQNNGOGCN-UHFFFAOYSA-N 0.000 description 1
- UPNLQRIRTAHAGA-UHFFFAOYSA-N methyl 3-aminooxetane-3-carboxylate Chemical compound COC(=O)C1(N)COC1 UPNLQRIRTAHAGA-UHFFFAOYSA-N 0.000 description 1
- WFJFGIAGOSGWMT-UHFFFAOYSA-N methyl 3-aminooxolane-3-carboxylate Chemical compound COC(=O)C1(N)CCOC1 WFJFGIAGOSGWMT-UHFFFAOYSA-N 0.000 description 1
- FBONBWXJVOFYBQ-UHFFFAOYSA-N methyl 4-[(4-bromophenyl)methylamino]-1,1-dioxothiane-4-carboxylate Chemical compound BrC1=CC=C(CNC2(CCS(CC2)(=O)=O)C(=O)OC)C=C1 FBONBWXJVOFYBQ-UHFFFAOYSA-N 0.000 description 1
- QAULBPGXKRTDCR-UHFFFAOYSA-N methyl 4-[(4-phenylphenyl)methylamino]oxane-4-carboxylate Chemical compound COC(=O)C1(CCOCC1)NCc1ccc(cc1)-c1ccccc1 QAULBPGXKRTDCR-UHFFFAOYSA-N 0.000 description 1
- XTBBTMPMZNPIMR-UHFFFAOYSA-N methyl 4-[2-[4-(4-chlorophenyl)triazol-2-yl]ethyl]-1,1-dioxothiane-4-carboxylate Chemical compound ClC1=CC=C(C=C1)C1=NN(N=C1)CCC1(CCS(CC1)(=O)=O)C(=O)OC XTBBTMPMZNPIMR-UHFFFAOYSA-N 0.000 description 1
- LQVYXRQSRYIYRT-UHFFFAOYSA-N methyl 4-[2-[tert-butyl(dimethyl)silyl]oxyethyl]thiane-4-carboxylate Chemical compound COC(=O)C1(CCO[Si](C)(C)C(C)(C)C)CCSCC1 LQVYXRQSRYIYRT-UHFFFAOYSA-N 0.000 description 1
- AQZUQDOYTWMQML-UHFFFAOYSA-N methyl 4-[2-oxo-2-(4-phenylphenyl)ethyl]thiane-4-carboxylate Chemical compound COC(=O)C1(CC(=O)c2ccc(cc2)-c2ccccc2)CCSCC1 AQZUQDOYTWMQML-UHFFFAOYSA-N 0.000 description 1
- AZFBPYNPITWILD-UHFFFAOYSA-N methyl 4-aminooxane-4-carboxylate Chemical compound COC(=O)C1(N)CCOCC1 AZFBPYNPITWILD-UHFFFAOYSA-N 0.000 description 1
- GJWRFJMQESSGPH-UHFFFAOYSA-N methyl 4-methoxy-1-[(4-phenylphenyl)methylamino]cyclohexane-1-carboxylate Chemical compound COC1CCC(CC1)(NCc1ccc(cc1)-c1ccccc1)C(=O)OC GJWRFJMQESSGPH-UHFFFAOYSA-N 0.000 description 1
- JZMJDSHXVKJFKW-UHFFFAOYSA-M methyl sulfate(1-) Chemical compound COS([O-])(=O)=O JZMJDSHXVKJFKW-UHFFFAOYSA-M 0.000 description 1
- UAEPNZWRGJTJPN-UHFFFAOYSA-N methylcyclohexane Chemical compound CC1CCCCC1 UAEPNZWRGJTJPN-UHFFFAOYSA-N 0.000 description 1
- 229960003085 meticillin Drugs 0.000 description 1
- 229960000198 mezlocillin Drugs 0.000 description 1
- YPBATNHYBCGSSN-VWPFQQQWSA-N mezlocillin Chemical compound N([C@@H](C(=O)N[C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C=1C=CC=CC=1)C(=O)N1CCN(S(C)(=O)=O)C1=O YPBATNHYBCGSSN-VWPFQQQWSA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 229960004023 minocycline Drugs 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 239000007932 molded tablet Substances 0.000 description 1
- 229940041009 monobactams Drugs 0.000 description 1
- 125000002950 monocyclic group Chemical group 0.000 description 1
- CQDGTJPVBWZJAZ-UHFFFAOYSA-N monoethyl carbonate Chemical compound CCOC(O)=O CQDGTJPVBWZJAZ-UHFFFAOYSA-N 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- 229960003702 moxifloxacin Drugs 0.000 description 1
- FABPRXSRWADJSP-MEDUHNTESA-N moxifloxacin Chemical compound COC1=C(N2C[C@H]3NCCC[C@H]3C2)C(F)=CC(C(C(C(O)=O)=C2)=O)=C1N2C1CC1 FABPRXSRWADJSP-MEDUHNTESA-N 0.000 description 1
- LLYKPZOWCPVRPD-UHFFFAOYSA-N n,n-dimethylpyridin-2-amine;n,n-dimethylpyridin-4-amine Chemical compound CN(C)C1=CC=NC=C1.CN(C)C1=CC=CC=N1 LLYKPZOWCPVRPD-UHFFFAOYSA-N 0.000 description 1
- YBSZEWLCECBDIP-UHFFFAOYSA-N n-[bis(dimethylamino)phosphoryl]-n-methylmethanamine Chemical compound CN(C)P(=O)(N(C)C)N(C)C.CN(C)P(=O)(N(C)C)N(C)C YBSZEWLCECBDIP-UHFFFAOYSA-N 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- WOOWBQQQJXZGIE-UHFFFAOYSA-N n-ethyl-n-propan-2-ylpropan-2-amine Chemical compound CCN(C(C)C)C(C)C.CCN(C(C)C)C(C)C WOOWBQQQJXZGIE-UHFFFAOYSA-N 0.000 description 1
- SJUPHKBFPMTTAD-UHFFFAOYSA-N n-hydroxy-4-[(4-naphthalen-2-ylphenyl)methylamino]-1,1-dioxothiane-4-carboxamide Chemical compound C=1C=C(C=2C=C3C=CC=CC3=CC=2)C=CC=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 SJUPHKBFPMTTAD-UHFFFAOYSA-N 0.000 description 1
- ZQVVUDXORMTCOR-UHFFFAOYSA-N n-hydroxy-4-[[4-(4-morpholin-4-ylphenyl)phenyl]methylamino]-1,1-dioxothiane-4-carboxamide Chemical compound C=1C=C(C=2C=CC(=CC=2)N2CCOCC2)C=CC=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 ZQVVUDXORMTCOR-UHFFFAOYSA-N 0.000 description 1
- VBOADQLQJONNRT-UHFFFAOYSA-N n-hydroxy-4-[[4-[4-(morpholin-4-ylmethyl)phenyl]phenyl]methylamino]-1,1-dioxothiane-4-carboxamide Chemical compound C=1C=C(C=2C=CC(CN3CCOCC3)=CC=2)C=CC=1CNC1(C(=O)NO)CCS(=O)(=O)CC1 VBOADQLQJONNRT-UHFFFAOYSA-N 0.000 description 1
- ZNCJJCBVTHOMSC-UHFFFAOYSA-N n-hydroxy-4-[[5-(4-methoxyphenyl)-1,2-oxazol-3-yl]methylamino]-1,1-dioxothiane-4-carboxamide Chemical compound C1=CC(OC)=CC=C1C1=CC(CNC2(CCS(=O)(=O)CC2)C(=O)NO)=NO1 ZNCJJCBVTHOMSC-UHFFFAOYSA-N 0.000 description 1
- GPXLMGHLHQJAGZ-JTDSTZFVSA-N nafcillin Chemical compound C1=CC=CC2=C(C(=O)N[C@@H]3C(N4[C@H](C(C)(C)S[C@@H]43)C(O)=O)=O)C(OCC)=CC=C21 GPXLMGHLHQJAGZ-JTDSTZFVSA-N 0.000 description 1
- 229960000515 nafcillin Drugs 0.000 description 1
- MHWLWQUZZRMNGJ-UHFFFAOYSA-N nalidixic acid Chemical compound C1=C(C)N=C2N(CC)C=C(C(O)=O)C(=O)C2=C1 MHWLWQUZZRMNGJ-UHFFFAOYSA-N 0.000 description 1
- 229960000210 nalidixic acid Drugs 0.000 description 1
- KPTRDYONBVUWPD-UHFFFAOYSA-N naphthalen-2-ylboronic acid Chemical compound C1=CC=CC2=CC(B(O)O)=CC=C21 KPTRDYONBVUWPD-UHFFFAOYSA-N 0.000 description 1
- CMWTZPSULFXXJA-VIFPVBQESA-N naproxen Chemical compound C1=C([C@H](C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-VIFPVBQESA-N 0.000 description 1
- 229960002009 naproxen Drugs 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 229960000808 netilmicin Drugs 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 229960001180 norfloxacin Drugs 0.000 description 1
- OGJPXUAPXNRGGI-UHFFFAOYSA-N norfloxacin Chemical compound C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNCC1 OGJPXUAPXNRGGI-UHFFFAOYSA-N 0.000 description 1
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 1
- NLXXVSKHVGDQAT-UHFFFAOYSA-N o-(oxan-2-yl)hydroxylamine Chemical compound NOC1CCCCO1 NLXXVSKHVGDQAT-UHFFFAOYSA-N 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-M octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC([O-])=O QIQXTHQIDYTFRH-UHFFFAOYSA-M 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OIPZNTLJVJGRCI-UHFFFAOYSA-M octadecanoyloxyaluminum;dihydrate Chemical compound O.O.CCCCCCCCCCCCCCCCCC(=O)O[Al] OIPZNTLJVJGRCI-UHFFFAOYSA-M 0.000 description 1
- 229960001699 ofloxacin Drugs 0.000 description 1
- 229940049964 oleate Drugs 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 229960002657 orciprenaline Drugs 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- UWYHMGVUTGAWSP-JKIFEVAISA-N oxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=CC=CC=C1 UWYHMGVUTGAWSP-JKIFEVAISA-N 0.000 description 1
- 229960001019 oxacillin Drugs 0.000 description 1
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical compound C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- DGOYLVBDCVINQZ-UHFFFAOYSA-N oxane-4-carboxamide Chemical compound NC(=O)C1CCOCC1 DGOYLVBDCVINQZ-UHFFFAOYSA-N 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 229960000321 oxolinic acid Drugs 0.000 description 1
- 229960000625 oxytetracycline Drugs 0.000 description 1
- IWVCMVBTMGNXQD-PXOLEDIWSA-N oxytetracycline Chemical compound C1=CC=C2[C@](O)(C)[C@H]3[C@H](O)[C@H]4[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-PXOLEDIWSA-N 0.000 description 1
- 235000019366 oxytetracycline Nutrition 0.000 description 1
- LSQZJLSUYDQPKJ-UHFFFAOYSA-N p-Hydroxyampicillin Natural products O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)C(N)C1=CC=C(O)C=C1 LSQZJLSUYDQPKJ-UHFFFAOYSA-N 0.000 description 1
- NXJCBFBQEVOTOW-UHFFFAOYSA-L palladium(2+);dihydroxide Chemical compound O[Pd]O NXJCBFBQEVOTOW-UHFFFAOYSA-L 0.000 description 1
- QNGNSVIICDLXHT-UHFFFAOYSA-N para-ethylbenzaldehyde Natural products CCC1=CC=C(C=O)C=C1 QNGNSVIICDLXHT-UHFFFAOYSA-N 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 235000010603 pastilles Nutrition 0.000 description 1
- 229960002625 pazufloxacin Drugs 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 229960004236 pefloxacin Drugs 0.000 description 1
- FHFYDNQZQSQIAI-UHFFFAOYSA-N pefloxacin Chemical compound C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCN(C)CC1 FHFYDNQZQSQIAI-UHFFFAOYSA-N 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 235000019371 penicillin G benzathine Nutrition 0.000 description 1
- 229940056367 penicillin v Drugs 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- BPLBGHOLXOTWMN-MBNYWOFBSA-N phenoxymethylpenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)COC1=CC=CC=C1 BPLBGHOLXOTWMN-MBNYWOFBSA-N 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- HKOOXMFOFWEVGF-UHFFFAOYSA-N phenylhydrazine Chemical compound NNC1=CC=CC=C1 HKOOXMFOFWEVGF-UHFFFAOYSA-N 0.000 description 1
- 229940067157 phenylhydrazine Drugs 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- PBMSWVPMRUJMPE-UHFFFAOYSA-N phthalylsulfathiazole Chemical compound OC(=O)C1=CC=CC=C1C(=O)NC1=CC=C(S(=O)(=O)\N=C\2SC=CN/2)C=C1 PBMSWVPMRUJMPE-UHFFFAOYSA-N 0.000 description 1
- 229960001106 phthalylsulfathiazole Drugs 0.000 description 1
- SRJOCJYGOFTFLH-UHFFFAOYSA-M piperidine-4-carboxylate Chemical compound [O-]C(=O)C1CCNCC1 SRJOCJYGOFTFLH-UHFFFAOYSA-M 0.000 description 1
- 229960005414 pirbuterol Drugs 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 229940061821 poractant alfa Drugs 0.000 description 1
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 238000002953 preparative HPLC Methods 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- TVDSBUOJIPERQY-UHFFFAOYSA-N prop-2-yn-1-ol Chemical compound OCC#C TVDSBUOJIPERQY-UHFFFAOYSA-N 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 239000000473 propyl gallate Substances 0.000 description 1
- 235000010388 propyl gallate Nutrition 0.000 description 1
- 229940075579 propyl gallate Drugs 0.000 description 1
- 229960004063 propylene glycol Drugs 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 229940107568 pulmozyme Drugs 0.000 description 1
- 125000004307 pyrazin-2-yl group Chemical group [H]C1=C([H])N=C(*)C([H])=N1 0.000 description 1
- 125000002206 pyridazin-3-yl group Chemical group [H]C1=C([H])C([H])=C(*)N=N1 0.000 description 1
- 125000004940 pyridazin-4-yl group Chemical group N1=NC=C(C=C1)* 0.000 description 1
- QLULGIRFKAWHOJ-UHFFFAOYSA-N pyridin-4-ylboronic acid Chemical compound OB(O)C1=CC=NC=C1 QLULGIRFKAWHOJ-UHFFFAOYSA-N 0.000 description 1
- 125000000246 pyrimidin-2-yl group Chemical group [H]C1=NC(*)=NC([H])=C1[H] 0.000 description 1
- 125000004527 pyrimidin-4-yl group Chemical group N1=CN=C(C=C1)* 0.000 description 1
- 125000004528 pyrimidin-5-yl group Chemical group N1=CN=CC(=C1)* 0.000 description 1
- HNJBEVLQSNELDL-UHFFFAOYSA-N pyrrolidin-2-one Chemical compound O=C1CCCN1 HNJBEVLQSNELDL-UHFFFAOYSA-N 0.000 description 1
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 description 1
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- WTHRRGMBUAHGNI-LCYNINFDSA-N quinupristin Chemical compound N([C@@H]1C(=O)N[C@@H](C(N2CCC[C@H]2C(=O)N(C)[C@@H](CC=2C=CC(=CC=2)N(C)C)C(=O)N2C[C@@H](CS[C@H]3C4CCN(CC4)C3)C(=O)C[C@H]2C(=O)N[C@H](C(=O)O[C@@H]1C)C=1C=CC=CC=1)=O)CC)C(=O)C1=NC=CC=C1O WTHRRGMBUAHGNI-LCYNINFDSA-N 0.000 description 1
- 229960005442 quinupristin Drugs 0.000 description 1
- 108700028429 quinupristin Proteins 0.000 description 1
- XFGOMLIRJYURLQ-GOKYHWASSA-N razupenem Chemical compound C=1([C@H](C)[C@@H]2[C@H](C(N2C=1C(O)=O)=O)[C@H](O)C)SC(SC=1)=NC=1C1=C[C@H](C)NC1 XFGOMLIRJYURLQ-GOKYHWASSA-N 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 239000003340 retarding agent Substances 0.000 description 1
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 1
- 229960000885 rifabutin Drugs 0.000 description 1
- BTVYFIMKUHNOBZ-QXMMDKDBSA-N rifamycin s Chemical class O=C1C(C(O)=C2C)=C3C(=O)C=C1NC(=O)\C(C)=C/C=C\C(C)C(O)C(C)C(O)C(C)C(OC(C)=O)C(C)C(OC)\C=C/OC1(C)OC2=C3C1=O BTVYFIMKUHNOBZ-QXMMDKDBSA-N 0.000 description 1
- 229940081192 rifamycins Drugs 0.000 description 1
- WDZCUPBHRAEYDL-GZAUEHORSA-N rifapentine Chemical compound O([C@](C1=O)(C)O/C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)\C=C\C=C(C)/C(=O)NC=2C(O)=C3C(O)=C4C)C)OC)C4=C1C3=C(O)C=2\C=N\N(CC1)CCN1C1CCCC1 WDZCUPBHRAEYDL-GZAUEHORSA-N 0.000 description 1
- 229960002599 rifapentine Drugs 0.000 description 1
- NZCRJKRKKOLAOJ-XRCRFVBUSA-N rifaximin Chemical compound OC1=C(C(O)=C2C)C3=C4N=C5C=C(C)C=CN5C4=C1NC(=O)\C(C)=C/C=C/[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@H](C)[C@@H](OC)\C=C\O[C@@]1(C)OC2=C3C1=O NZCRJKRKKOLAOJ-XRCRFVBUSA-N 0.000 description 1
- 229960003040 rifaximin Drugs 0.000 description 1
- RZJQGNCSTQAWON-UHFFFAOYSA-N rofecoxib Chemical compound C1=CC(S(=O)(=O)C)=CC=C1C1=C(C=2C=CC=CC=2)C(=O)OC1 RZJQGNCSTQAWON-UHFFFAOYSA-N 0.000 description 1
- 229960000371 rofecoxib Drugs 0.000 description 1
- 235000005713 safflower oil Nutrition 0.000 description 1
- 239000003813 safflower oil Substances 0.000 description 1
- 229960002052 salbutamol Drugs 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- 239000012047 saturated solution Substances 0.000 description 1
- 150000003335 secondary amines Chemical class 0.000 description 1
- 239000012363 selectfluor Substances 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 229910001923 silver oxide Inorganic materials 0.000 description 1
- 229910000367 silver sulfate Inorganic materials 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 229960005456 sisomicin Drugs 0.000 description 1
- URWAJWIAIPFPJE-YFMIWBNJSA-N sisomycin Chemical compound O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H](CC=C(CN)O2)N)[C@@H](N)C[C@H]1N URWAJWIAIPFPJE-YFMIWBNJSA-N 0.000 description 1
- 229960003177 sitafloxacin Drugs 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- WBHQBSYUUJJSRZ-UHFFFAOYSA-M sodium bisulfate Chemical compound [Na+].OS([O-])(=O)=O WBHQBSYUUJJSRZ-UHFFFAOYSA-M 0.000 description 1
- 229940100996 sodium bisulfate Drugs 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- HRZFUMHJMZEROT-UHFFFAOYSA-L sodium disulfite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])(=O)=O HRZFUMHJMZEROT-UHFFFAOYSA-L 0.000 description 1
- 229940001584 sodium metabisulfite Drugs 0.000 description 1
- 235000010262 sodium metabisulphite Nutrition 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 229940001482 sodium sulfite Drugs 0.000 description 1
- 235000010265 sodium sulphite Nutrition 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 229960004954 sparfloxacin Drugs 0.000 description 1
- DZZWHBIBMUVIIW-DTORHVGOSA-N sparfloxacin Chemical compound C1[C@@H](C)N[C@@H](C)CN1C1=C(F)C(N)=C2C(=O)C(C(O)=O)=CN(C3CC3)C2=C1F DZZWHBIBMUVIIW-DTORHVGOSA-N 0.000 description 1
- 229960000268 spectinomycin Drugs 0.000 description 1
- UNFWWIHTNXNPBV-WXKVUWSESA-N spectinomycin Chemical compound O([C@@H]1[C@@H](NC)[C@@H](O)[C@H]([C@@H]([C@H]1O1)O)NC)[C@]2(O)[C@H]1O[C@H](C)CC2=O UNFWWIHTNXNPBV-WXKVUWSESA-N 0.000 description 1
- 239000003206 sterilizing agent Substances 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 229940041030 streptogramins Drugs 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- SEEPANYCNGTZFQ-UHFFFAOYSA-N sulfadiazine Chemical compound C1=CC(N)=CC=C1S(=O)(=O)NC1=NC=CC=N1 SEEPANYCNGTZFQ-UHFFFAOYSA-N 0.000 description 1
- 229960004306 sulfadiazine Drugs 0.000 description 1
- 229960000654 sulfafurazole Drugs 0.000 description 1
- 229960005404 sulfamethoxazole Drugs 0.000 description 1
- FDDDEECHVMSUSB-UHFFFAOYSA-N sulfanilamide Chemical class NC1=CC=C(S(N)(=O)=O)C=C1 FDDDEECHVMSUSB-UHFFFAOYSA-N 0.000 description 1
- HXJUTPCZVOIRIF-UHFFFAOYSA-N sulfolane Chemical compound O=S1(=O)CCCC1 HXJUTPCZVOIRIF-UHFFFAOYSA-N 0.000 description 1
- JLKIGFTWXXRPMT-UHFFFAOYSA-N sulphamethoxazole Chemical compound O1C(C)=CC(NS(=O)(=O)C=2C=CC(N)=CC=2)=N1 JLKIGFTWXXRPMT-UHFFFAOYSA-N 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 229940080796 surfaxin Drugs 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 229960001608 teicoplanin Drugs 0.000 description 1
- LJVAJPDWBABPEJ-PNUFFHFMSA-N telithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)[C@@H](C)C(=O)O[C@@H]([C@]2(OC(=O)N(CCCCN3C=C(N=C3)C=3C=NC=CC=3)[C@@H]2[C@@H](C)C(=O)[C@H](C)C[C@@]1(C)OC)C)CC)[C@@H]1O[C@H](C)C[C@H](N(C)C)[C@H]1O LJVAJPDWBABPEJ-PNUFFHFMSA-N 0.000 description 1
- 229960003250 telithromycin Drugs 0.000 description 1
- 229960004576 temafloxacin Drugs 0.000 description 1
- 229960001114 temocillin Drugs 0.000 description 1
- BVCKFLJARNKCSS-DWPRYXJFSA-N temocillin Chemical compound N([C@]1(OC)C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C(C(O)=O)C=1C=CSC=1 BVCKFLJARNKCSS-DWPRYXJFSA-N 0.000 description 1
- IWVCMVBTMGNXQD-UHFFFAOYSA-N terramycin dehydrate Natural products C1=CC=C2C(O)(C)C3C(O)C4C(N(C)C)C(O)=C(C(N)=O)C(=O)C4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-UHFFFAOYSA-N 0.000 description 1
- 125000004213 tert-butoxy group Chemical group [H]C([H])([H])C(O*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- DZLFLBLQUQXARW-UHFFFAOYSA-N tetrabutylammonium Chemical compound CCCC[N+](CCCC)(CCCC)CCCC DZLFLBLQUQXARW-UHFFFAOYSA-N 0.000 description 1
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 1
- 229940040944 tetracyclines Drugs 0.000 description 1
- WHRNULOCNSKMGB-UHFFFAOYSA-N tetrahydrofuran thf Chemical compound C1CCOC1.C1CCOC1 WHRNULOCNSKMGB-UHFFFAOYSA-N 0.000 description 1
- RAOIDOHSFRTOEL-UHFFFAOYSA-N tetrahydrothiophene Chemical compound C1CCSC1 RAOIDOHSFRTOEL-UHFFFAOYSA-N 0.000 description 1
- 125000003831 tetrazolyl group Chemical group 0.000 description 1
- WROMPOXWARCANT-UHFFFAOYSA-N tfa trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F.OC(=O)C(F)(F)F WROMPOXWARCANT-UHFFFAOYSA-N 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- VLLMWSRANPNYQX-UHFFFAOYSA-N thiadiazole Chemical compound C1=CSN=N1.C1=CSN=N1 VLLMWSRANPNYQX-UHFFFAOYSA-N 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 238000003354 tissue distribution assay Methods 0.000 description 1
- 235000010384 tocopherol Nutrition 0.000 description 1
- 229960001295 tocopherol Drugs 0.000 description 1
- 229930003799 tocopherol Natural products 0.000 description 1
- 239000011732 tocopherol Substances 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 238000007832 transition metal-catalyzed coupling reaction Methods 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 229960005294 triamcinolone Drugs 0.000 description 1
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 description 1
- 150000003852 triazoles Chemical class 0.000 description 1
- YEMJHNYABQHWHL-UHFFFAOYSA-N tributyl(ethynyl)stannane Chemical compound CCCC[Sn](CCCC)(CCCC)C#C YEMJHNYABQHWHL-UHFFFAOYSA-N 0.000 description 1
- ITMCEJHCFYSIIV-UHFFFAOYSA-M triflate Chemical compound [O-]S(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-M 0.000 description 1
- 229960001082 trimethoprim Drugs 0.000 description 1
- IEDVJHCEMCRBQM-UHFFFAOYSA-N trimethoprim Chemical compound COC1=C(OC)C(OC)=CC(CC=2C(=NC(N)=NC=2)N)=C1 IEDVJHCEMCRBQM-UHFFFAOYSA-N 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 229960000281 trometamol Drugs 0.000 description 1
- 229960000497 trovafloxacin Drugs 0.000 description 1
- WVPSKSLAZQPAKQ-CDMJZVDBSA-N trovafloxacin Chemical compound C([C@H]1[C@@H]([C@H]1C1)N)N1C(C(=CC=1C(=O)C(C(O)=O)=C2)F)=NC=1N2C1=CC=C(F)C=C1F WVPSKSLAZQPAKQ-CDMJZVDBSA-N 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 238000004704 ultra performance liquid chromatography Methods 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 235000019871 vegetable fat Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 230000001018 virulence Effects 0.000 description 1
- 239000003039 volatile agent Substances 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 235000016804 zinc Nutrition 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
- 235000014692 zinc oxide Nutrition 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D335/00—Heterocyclic compounds containing six-membered rings having one sulfur atom as the only ring hetero atom
- C07D335/02—Heterocyclic compounds containing six-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C259/00—Compounds containing carboxyl groups, an oxygen atom of a carboxyl group being replaced by a nitrogen atom, this nitrogen atom being further bound to an oxygen atom and not being part of nitro or nitroso groups
- C07C259/04—Compounds containing carboxyl groups, an oxygen atom of a carboxyl group being replaced by a nitrogen atom, this nitrogen atom being further bound to an oxygen atom and not being part of nitro or nitroso groups without replacement of the other oxygen atom of the carboxyl group, e.g. hydroxamic acids
- C07C259/08—Compounds containing carboxyl groups, an oxygen atom of a carboxyl group being replaced by a nitrogen atom, this nitrogen atom being further bound to an oxygen atom and not being part of nitro or nitroso groups without replacement of the other oxygen atom of the carboxyl group, e.g. hydroxamic acids having carbon atoms of hydroxamic groups bound to carbon atoms of rings other than six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D205/00—Heterocyclic compounds containing four-membered rings with one nitrogen atom as the only ring hetero atom
- C07D205/02—Heterocyclic compounds containing four-membered rings with one nitrogen atom as the only ring hetero atom not condensed with other rings
- C07D205/04—Heterocyclic compounds containing four-membered rings with one nitrogen atom as the only ring hetero atom not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/46—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with hetero atoms directly attached to the ring nitrogen atom
- C07D207/48—Sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/92—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with a hetero atom directly attached to the ring nitrogen atom
- C07D211/96—Sulfur atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D305/00—Heterocyclic compounds containing four-membered rings having one oxygen atom as the only ring hetero atoms
- C07D305/02—Heterocyclic compounds containing four-membered rings having one oxygen atom as the only ring hetero atoms not condensed with other rings
- C07D305/04—Heterocyclic compounds containing four-membered rings having one oxygen atom as the only ring hetero atoms not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
- C07D305/08—Heterocyclic compounds containing four-membered rings having one oxygen atom as the only ring hetero atoms not condensed with other rings having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/04—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
- C07D307/18—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D307/24—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D309/00—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings
- C07D309/02—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
- C07D309/08—Heterocyclic compounds containing six-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/38—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/46—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings substituted on the ring sulfur atom
- C07D333/48—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings substituted on the ring sulfur atom by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/04—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/04—Systems containing only non-condensed rings with a four-membered ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2601/00—Systems containing only non-condensed rings
- C07C2601/12—Systems containing only non-condensed rings with a six-membered ring
- C07C2601/14—The ring being saturated
Definitions
- This invention pertains generally to treating bacterial infections.
- the invention pertains to treating infections caused by Gram- negative bacteria. More specifically, the invention described herein pertains to treating Gram-negative infections by inhibiting the activity of UDP-3-0-(R-3-hydroxydecanoyl)-N-acetylglucosamine deacetylase (LpxC).
- the present invention provides small molecule inhibitors of LpxC, pharmaceutical formulations containing such inhibitors, methods of treating patients with such
- the inhibitors can be used to treat Gram-negative infections of patients alone and in combination with other antibacterials.
- nosocomial infections occuring each year in the United States, 50 to 60% are caused by antimicrobial-resistant strains of bacteria. The high rate of resistance to commonly used antibacterial agents increases the morbidity, mortality, and costs associated with nosocomial infections. In the United States, nosocomial infections are thought to contribute to or cause more than 77,000 deaths per year and cost approximately $5 to $10 billion annually.
- the most important resistant pathogens are methicillin-(oxacillin-) resistant Staphylococcus aureus, ⁇ -lactam-resistant and multidrug-resistant pneumococci, and vancomycin-resistant enterococci.
- Gram-negative resistance Important causes of Gram-negative resistance include extended-spectrum ⁇ -lactamases (ESBLs) in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis, high-level third-generation cephalosporin (Amp C) ⁇ -lactamase resistance among Enterobacter species and Citrobacter freundii, and multidrug-resistance genes observed in Pseudomonas, Acinetobacter, and Stenotrophomonas.
- ESBLs extended-spectrum ⁇ -lactamases
- Amp C cephalosporin
- the problem of antibacterial resistance is compounded by the existence of bacterial strains resistant to multiple antibacterials.
- Pseudomonas aeruginosa isolates resistant to fluoroquinolones are virtually all resistant to additional antibacterial medicines.
- the present invention provides novel compounds, pharmaceutical formulations including the compounds, methods of inhibiting UDP-3-0-(R-3-hydroxydecanoyl)-N- acetylglucosamine deacetylase (LpxC), and methods of treating Gram-negative bacterial infections.
- the invention provides compounds of Formula I:
- A is a divalent radical selected from , and ;
- X is -(CH 2 ) n Y(CH 2 ) m -;
- Y is selected from the group consisting of -C(H,R 1 )-, -0-, -N(R 2 )-, and -S(0) 2 - n is 0 or 1 ;
- n 0 or 1 ;
- R is -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, d- C 4 haloalkyl, C 3 -C 7 cycloalkyl, CrC 4 alkoxy, CrC 4 haloalkoxy, C C alkyl optionally substituted with Ci-C alkoxy, CrC alkoxy optionally substituted with Ci-C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, C C alkoxy, C r C 4 haloalkoxy, CrC 4 haloalkyl or Ci-C 4 alkyl; or
- R is -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, Ci-C alkyl, CrC haloalkyl, Ci-C haloalkoxy or C C 4 alkoxy;
- R 1 is selected from the group consisting of -OH, C C 4 alkoxy and -S(0) 2 R 3 ;
- R 2 is selected from the group consisting of hydrogen, C C alkyl, -C(0)R 3 and - S(0) 2 R 3 ;
- R 3 is selected from the group consisting of C C 4 alkyl and C 3 -C 6 cycloalkyl
- Z is a divalent radical selected from
- R 5 is selected from the group consisting of hydrogen, halogen, -CN, d-C 4 alkyl, and C C 4 haloalkyl;
- R 6 , R 6a , R 6b or R 6c are independently selected from the group consisting of hydrogen, halogen, -CrC 4 alkyl, and CrC 4 haloalkyl;
- L is a divalent bond, -CH 2 -, -O- or
- the invention provides a method of inhibiting a deacetylase enzyme in Gram-negative bacteria, thereby affecting bacterial growth, comprising administering to a patient in need of such inhibition a compound of formula I.
- the invention provides a method of inhibiting LpxC, thereby modulating the virulence of a bacterial infection, comprising administering to a patient in need of such inhibition a compound of formula I.
- the invention provides a method for treating a subject with a Gram-negative bacterial infection comprising administering to the subject in need thereof an antibacterially effective amount of a compound of formula I with a pharmaceutically acceptable carrier.
- the subject is a mammal and in some other embodiments, the subject is a human.
- the invention provides a method of administering an inhibitory amount of a compound of formula I to fermentative or non-fermentative Gram-negative bacteria.
- the Gram-negative bacteria are selected from the group consisting of Pseudomonas aeruginosa and other Pseudomonas species, Stenotrophomonas maltophilia, Burkholderia cepacia and other Burkholderia species, Alcaligenes xylosoxidans, species of Acinetobacter,
- Campylobactor, Yersinia and Neisseria Campylobactor, Yersinia and Neisseria.
- the invention provides a method of administering an inhibitory amount of a compound of formula I to Gram-negative bacteria, such as
- Enterobacteriaceae which is selected from the group consisting of organisms such as Serratia, Proteus, Klebsiella, Enterobacter, Citrobacter, Salmonella, Providencia,
- Another embodiment of the invention provides a pharmaceutical composition comprising an effective amount of a compound of Formula I with a pharmaceutically acceptable carrier thereof.
- compositions according to the present invention are provided which include any of the compounds described above and a pharmaceutically acceptable carrier.
- the present invention provides novel compounds, methods for inhibiting LpxC in Gram-negative bacteria, and novel methods for treating bacterial infections.
- the compounds provided herein can be formulated into pharmaceutical formulations and medicaments that are useful in the methods of the invention.
- the invention also provides for the use of the compounds in preparing medicaments and pharmaceutical formulations, for use of the compounds in inhibiting LpxC, and for use of the compounds in treating bacterial infections in a subject.
- LpxC is an abbreviation that stands for UDP-3-0-(R-3-hydroxydecan- oyl)-N- acetylglucosamine deacetylase.
- This invention is directed to compounds of Formula l-V and subformulae thereof, and intermediates thereto, as well as pharmaceutical compositions containing the compounds for use in treatment of bacterial infections.
- This invention is also directed to the compounds of the invention or compositions thereof as LpxC inhibitors.
- the compounds are particularly useful in interfering with the life cycle of Gram-negative bacteria and in treating or preventing a Gram-negative bacterial infection or physiological conditions associated therewith.
- the present invention is also directed to methods of combination therapy for treating or preventing an Gram-negative bacterial infection in patients using the compounds of the invention or pharmaceutical compositions, or kits thereof in combination with at least one other therapeutic agent.
- the term "subject" refers to an animal.
- the animal is a mammal.
- a subject also refers to for example, primates (e.g., humans), cows, sheep, goats, horses, dogs, cats, rabbits, rats, mice, fish, birds and the like.
- primates e.g., humans
- the subject is a human.
- the term “inhibition” or “inhibiting” refers to the reduction or suppression of a given condition, symptom, or disorder, or disease, or a significant decrease in the baseline activity of a biological activity or process.
- treating refers in one embodiment, to ameliorating the disease or disorder (i.e., slowing or arresting or reducing the development of the disease or at least one of the clinical symptoms thereof).
- treating refers to alleviating or ameliorating at least one physical parameter including those which may not be discernible by the patient.
- treating or “treatment” refers to modulating the disease or disorder, either physically, (e.g., stabilization of a discernible symptom), physiologically, (e.g., stabilization of a physical parameter), or both.
- “treating” or “treatment” refers to preventing or delaying the onset or development or progression of the disease or disorder.
- antibacterial agent refers to agents synthesized or modified in the laboratory that have either bactericidal or bacteriostatic activity.
- An "active” agent in this context will inhibit the growth of P. aeruginosa and / or other Gram-negative bacteria.
- the term “inhibiting the growth” indicates that the rate of increase in the numbers of a population of a particular bacterium is reduced. Thus, the term includes situations in which the bacterial population increases but at a reduced rate, as well as situations where the growth of the population is stopped, as well as situations where the numbers of the bacteria in the population are reduced or the population even eliminated. If an enzyme activity assay is used to screen for inhibitors, one can make modifications in bacterial uptake/efflux, solubility, half-life, etc. to compounds in order to correlate enzyme inhibition with growth inhibition.
- Optionally substituted means the group referred to can be substituted at one or more positions by any one or any combination of the radicals listed thereafter.
- Halo or "halogen”, as used herein, may be fluorine, chlorine, bromine or iodine.
- CrC 6 -Alkyl denotes straight chain or branched alkyl having 1-8 carbon atoms. If a different number of carbon atoms is specified, such as Ce or C3, then the definition is to be amended accordingly, such as "CrC 4 -Alkyl” will represent methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl and tert-butyl.
- CrC 6 -Alkoxy denotes straight chain or branched alkoxy having 1-8 carbon atoms. If a different number of carbon atoms is specified, such as C 6 or C 3 , then the edefinition is to be amended accordingly, such as "CrC 4 -Alkoxy” will represent methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, sec-butoxy and tert-butoxy.
- CrC 4 -Haloalkyl denotes straight chain or branched alkyl having 1 - 4 carbon atoms with at least one hydrogen substituted with a halogen. If a different number of carbon atoms is specified, such as C 6 or C 3 , then the definition is to be amended accordingly, such as "Ci-C 4 -Haloalkyl” will represent methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl and tert-butyl that have at least one hydrogen substituted with halogen, such as where the halogen is fluorine: CF 3 CF 2 -, (CF 3 ) 2 CH-, CH 3 -CF 2 -, CF 3 CF 2 -, CF 3 , CF 2 H-, CF 3 CF 2 CHCF 3 or CF 3 CF 2 CF 2 CF 2 -.
- C 3 -C 8 -cycloalkyl refers to a saturated monocyclic hydrocarbon ring of 3 to 8 carbon atoms. Examples of such groups include cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. If a different number of carbon atoms is specified, such as C 3 -C 6 , then the definition is to be amended accordingly.
- heterocyclyl and "5- to 14-membered heterocyclyl”, refers, respectively, to 4- to 8- membered, 5- to 6-membered, 3- to 10-membered, 3- to 14-membered, 4- to 14-membered and 5- to 14-membered heterocyclic rings containing 1 to 7, 1 to 5 or 1 to 3 heteroatoms selected from the group consisting of nitrogen, oxygen and sulphur, which may be saturated, or partially saturated.
- the heterocyclic group can be attached at a heteroatom or a carbon atom.
- heterocyclyl includes single ring groups, fused ring groups and bridged groups.
- heterocyclyl examples include, but are not limited to pyrrolidine, piperidine, piperazine, pyrrolidine, pyrrolidinone, morpholine, tetrahydrofuran, tetrahydrothiophene, tetrahydrothiopyran, tetrahydropyran, 1 ,4-dioxane, 1 ,4-oxathiane, 8-aza- bicyclo[3.2.1 ]octane, 3,8-diazabicyclo[3.2.1 ]octane, 3-Oxa-8-aza-bicyclo[3.2.1 ]octane, 8- Oxa-3-aza-bicyclo[3.2.1 ]octane, 2-Oxa-5-aza-bicyclo[2.2.1 ]heptane, 2,5-Diaza- bicyclo[2.2.1 ]heptane, azetidine, ethylenedioxo, oxtane or thiazole.
- Heteroaryl is a completely unsaturated (aromatic) ring.
- the term “heteroaryl” refers to a 5-14 membered monocyclic- or bicyclic- or tricyclic-aromatic ring system, having 1 to 8 heteroatoms selected from N, O or S.
- the heteroaryl is a 5-10 membered ring system (e.g., 5-7 membered monocycle or an 8-10 membered bicycle) or a 5-7 membered ring system.
- Typical heteroaryl groups include furan, isotriazole, thiadiazole, oxadiazole, indazole, indazole, indole, quinoline, 2- or 3-thienyl, 2- or 3-furyl, 2- or 3-pyrrolyl, 2-, 4-, or 5- imidazolyl, 3-, 4-, or 5- pyrazolyl, 2-, 4-, or 5-thiazolyl, 3-, 4-, or 5-isothiazolyl, 2-, 4-, or 5- oxazolyl, 3-, 4-, or 5-isoxazolyl, 3- or 5-(1 ,2,4-triazolyl), 4- or 5-(1 ,2, 3-triazolyl), tetrazolyl, triazine, pyrimidine, 2-, 3-, or 4-pyridyl, 3- or 4-pyridazinyl, 3-, 4-, or 5-pyrazinyl, 2-pyrazinyl, and 2-, 4-, or 5-pyrimidinyl.
- the invention provides compounds of Formula I:
- A is a divalent radical selected from and
- X is -(CH 2 ) n Y(CH 2 ) m -;
- Y is selected from the group consisting of -C(H,R 1 )-, -0-, S, -N(R 2 )-, and -S(0) 2 - n is 0 or 1 ; m is 0 or 1 ;
- R is C 3 -6 cycloalkyi, -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said cycloalkyi, aryl and heteroaryl are each optionally substituted with up to three substituents selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, C C 4 haloalkyl, C 3 -C 7 cycloalkyl, C C 4 alkoxy, C C4haloalkoxy, C C4alkyl optionally substituted with Ci-C4alkoxy or a 5-6 membered heterocycle containing up to two heteroatoms selected from N, O and S as ring members and optionally substituted with R 10 , CrC 4 alkoxy optionally substituted with d-C 4 alkoxy or Ci_ 3 alkyl or C 3 -e cycloalkyi where the
- R is -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with one or two groups selected from halogen, Ci-C alkyl, d- C haloalkyl, C C haloalkoxy and CrC alkoxy;
- R 1 is selected from the group consisting of -OH, C C alkoxy and -S(0) 2 R 3 ;
- R 2 is selected from the group consisting of hydrogen, CrC 4 alkyl, -C(0)OR 3 , -C(0)R 3 and -S(0) 2 R 3 ;
- R 3 is selected from the group consisting of C C alkyl and C 3 -C 6 cycloalkyl
- Z is a divalent radical selected from
- R 5 is selected from the group consisting of hydrogen, halogen, -CN, d-dalkyl, and CrC 4 haloalkyl;
- R 6 , R 6a , R 6b or R 6c are independently selected from the group consisting of hydrogen, halogen, -CrC 4 alkyl, and CrC 4 haloalkyl;
- R 10 is selected from halo, C ⁇ alkyl, d-4 haloalkyl, d ⁇ alkoxy, -C(0)R 11 and -C(O)-
- R 11 is d ⁇ alkyl
- L is a divalent bond, -CH 2 -, -O- or
- A is a divalent radical selected from and
- X is -(CH 2 ) n Y(CH 2 ) m -;
- Y is selected from the group consisting of -C(H,R 1 )-, -0-, -N(R 2 )-, and -S(0) 2 - n is 0 or 1 ; m is 0 or 1 ;
- R is -C 6 -d 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said cycloalkyi, aryl and heteroaryl are each optionally substituted with substituents selected from the group consisting of halogen, -OH, -CN, - S(0) 2 (d-d)alkyl, d-dhaloalkyl, d-dcycloalkyl, d-dalkoxy, d-dhaloalkoxy, d-dalkyl optionally substituted with d-dalkoxy, d-dalkoxy optionally substituted with d-dalkoxy, and a 4 to 7 membered heterocycle wherein the 4 to 7 membered heterocycle contains 1 to 3 heteroatoms selected from N, S, and O as ring members and is optionally substituted with one or more halogen, d-C 4 alkoxy, CrC 4 haloalkoxy, Cr
- R is -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with groups selected from halogen, Ci-C alkyl, Ci-C haloalkyl, Ci- C haloalkoxy and C C alkoxy;
- R 1 is selected from the group consisting of -OH, C C alkoxy and -S(0) 2 R 3 ;
- R 2 is selected from the group consisting of hydrogen, CrC 4 alkyl, -C(0)R 3 and - S(0) 2 R 3 ;
- R 3 is selected from the group consisting of C C alkyl and C 3 -C 6 cycloalkyl
- Z is a divalent radical selected from
- R 5 is selected from the group consisting of hydrogen, halogen, -CN, d-C 4 alkyl, and CrC 4 haloalkyl;
- R 6 , R 6a , R 6b or R 6c are independently selected from the group consisting of hydrogen, halogen, -CrC 4 alkyl, and CrC 4 haloalkyl; and '
- L is a divalent bond, -CH 2 -, -O- or
- R is phenyl substituted with one or two substituents selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (Cr C 4 )alkyl, CrC 4 haloalkyl, C 3 -C 7 cycloalkyl, C C alkoxy, CrC haloalkoxy, and CrC alkyl optionally substituted with C C alkoxy.
- X is -CH 2 -SO 2 -CH 2 -.
- A is [Z]-CH 2 -NH-, where [Z] indicates the point where A attaches to Z in Formula I.
- R 5 is preferably
- R 6 , R 6a , R 6b and R 6c can all be H.
- L is a bond.
- R 2 is selected from the group consisting of hydrogen, C C4alkyl, -C(0)CR 3 and
- R 3 is selected from the group consisting of CrC 4 alkyl and C 3 -C 6 cycloalkyl.
- A is a divalent radical selected from , and ;
- R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, C C 4 haloalkyl, C 3 -C 7 cycloalkyl, C C 4 alkoxy, Ci-C 4 haloalkoxy, Ci-C 4 alkyl optionally substituted with Ci-C 4 alkoxy, Ci-C 4 alkoxy optionally substituted with C C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
- R is phenyl susbstituted by taking the substituents on adjacent atoms of the -C 6 - Ci 0 aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy;
- R 2 is selected from the group consisting of hydrogen, C C alkyl, -C(0)CR 3 and - S(0) 2 R 3 ;
- R 3 is selected from the group consisting of C C alkyl and C 3 -C 6 cycloalkyl
- Z is a divalent radical selected from
- R 5 is selected from the group consisting of hydrogen, halogen, -CN, Ci-C alkyl, and CrC 4 haloalkyl;
- R 6 , R 6a , R 6b or R 6c are independently selected from the group consisting of hydrogen, halogen, CrC 4 alkyl and CrC 4 haloalkyl;
- L is a divalent bond, -CH 2 -, -O- or
- R is phenyl substituted with one or two substituents selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, C C 4 haloalkyl, C 3 -C 7 cycloalkyl, C C 4 alkoxy, C C 4 haloalkoxy, and CrC alkyl optionally substituted with Ci-C alkoxy.
- Q is .
- A is [Z]-CH 2 -NH-, where [Z] indicates the point where A attaches to Z in Formula II.
- R 5 is preferably
- R 6 , R 6a , R 6b and R 6c can all be H.
- L is a bond.
- A is a divalent radical selected from , and
- R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, C C 4 haloalkyl, C 3 -C 7 cycloalkyl, d- C 4 alkoxy, CrC haloalkoxy, C C alkyl optionally substituted with C C alkoxy, C C alkoxy optionally substituted with C C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
- R is phenyl susbstituted by taking the substituents on adjacent atoms of the -C 6 - Ci 0 aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C 4 alkyl, CrC 4 haloalkyl, CrC 4 haloalkoxy or CrC 4 alkoxy;
- R 2 is selected from the group consisting of hydrogen, C C alkyl and -S(0) 2 R 3 ;
- R 3 is selected from the group consisting of C C alkyl and C 3 -C 6 cycloalkyl.
- Z is a divalent radical selected from
- R 5 is selected from the group consisting of hydrogen, halogen, C C alkyl, and C C haloalkyl;
- L is a divalent bond
- R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, C C 4 haloalkyl, C 3 -C 7 cycloalkyl, C C 4 alkoxy, Ci-C 4 haloalkoxy, Ci-C 4 alkyl optionally substituted with Ci-C 4 alkoxy, Ci-C 4 alkoxy optionally substituted with C C alkoxy, and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
- R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy;
- R 2 is selected from the group consisting of hydrogen, CrC 4 alkyl and -S(0)2R 3 ;
- R 3 is selected from the group consisting of C C alkyl and C 3 -C 6 cycloalkyl.
- R 5 is selected from the group consisting of hydrogen, halogen, CrC 4 alkyl, and C C 4 haloalkyl;
- L is a divalent bond
- A is ;
- R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, C C 4 haloalkyl, C 3 -C 7 cycloalkyl, C C 4 alkoxy, Ci-C 4 haloalkoxy, Ci-C 4 alkyl optionally substituted with Ci-C 4 alkoxy, Ci-C 4 alkoxy optionally substituted with C C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl;
- R 2 is selected from the group consisting of hydrogen, C C alkyl and -S(0) 2 R 3 ;
- R 3 is selected from the group consisting of C C alkyl and C 3 -C 6 cycloalkyl.
- Z is a divalent radical selected from
- R 5 is selected from the group consisting of hydrogen, halogen, -CN, d-C 4 alkyl, and CrC 4 haloalkyl;
- L is a divalent bond
- R 2 is selected from the group consisting of hydrogen, CrC 4 alkyl and -S(0) 2 R 3 ;
- R 3 is selected from the group consisting of CrC 4 alkyl and C 3 -C 6 cycloalkyl.
- Z is a divalent radical selected from
- R 6 , R 6a , R 6b or R 6c are independently selected from the group consisting of hydrogen, halogen, CrC 4 alkyl, CrC 4 haloalkyl; '
- L is a divalent bond
- R is -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, Ci- C 4 haloalkyl, C 3 -C 7 cycloalkyl, CrC 4 alkoxy, CrC 4 haloalkoxy, C C alkyl optionally substituted with Ci-C alkoxy, CrC alkoxy optionally substituted with Ci-C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, C C alkoxy, C r C 4 haloalkoxy, CrC 4 haloalkyl or Ci-C 4 alkyl; or
- R is -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, Ci-C alkyl, CrC haloalkyl, Ci-C haloalkoxy or C C 4 alkoxy;
- R 2 is selected from the group consisting of hydrogen, C C alkyl and -S(0) 2 R 3 ;
- R 3 is selected from the group consisting of C C alkyl and C 3 -C 6 cycloalkyl
- R 5 is selected from the group consisting of hydrogen, halogen, -CN, -OH, Ci-C alkyl, CrC haloalkyl, Ci-C haloalkoxy, CrC alkoxy and C 3 -C 7 cycloalkyl optionally substituted with halogen or Ci-C alkyl; '
- L is a direct bond, -CH 2 -, -O- or
- R is phenyl substituted with one or two substituents selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (C C )alkyl, Ci-C haloalkyl, C 3 -C 7 cycloalkyl, Ci-C alkoxy, Ci-C haloalkoxy, and Ci-C alkyl optionally substituted with C C alkoxy.
- L is a bond.
- R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, C C 4 haloalkyl, C 3 -C 7 cycloalkyl, C C 4 alkoxy, Ci-C 4 haloalkoxy, Ci-C 4 alkyl optionally substituted with Ci-C 4 alkoxy, Ci-C 4 alkoxy optionally substituted with C C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
- R is phenyl susbstituted by taking the substituents on adjacent atoms of the -C 6 - Ci 0 aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy.
- Q is selected from the group consisting of
- R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, C C 4 haloalkyl, C 3 -C 7 cycloalkyl, C C 4 alkoxy, CrC haloalkoxy, C C alkyl optionally substituted with C C alkoxy, C C alkoxy optionally substituted with C C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
- R is phenyl susbstituted by taking the substituents on adjacent atoms of the -C 6 - Ci 0 aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, Ci- C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy;
- R 2 is selected from the group consisting of hydrogen, C C alkyl and -S(0) 2 R 3 ;
- R 3 is selected from the group consisting of CrC 4 alkyl and Cs-Cecycloalkyl; '
- L is a direct bond, -CH 2 -, -O- or
- the compound or a pharmaceutically acceptable salt thereof wherein the compound is formula III
- R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, C C 4 haloalkyl, C 3 -C 7 cycloalkyl, C C 4 alkoxy, CrC haloalkoxy, C C alkyl optionally substituted with C C alkoxy, C C alkoxy optionally substituted with Ci-C 4 alkoxy, and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
- R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxyR 2 is selected from the group consisting of hydrogen, Ci-C alkyl and -S(0) 2 R 3 ;
- R 3 is selected from the group consisting of CrC 4 alkyl and C 3 -C 6 cycloalkyl
- L is a direct bond
- R is -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, Ci- C 4 haloalkyl, C 3 -C 7 cycloalkyl, CrC 4 alkoxy, CrC 4 haloalkoxy, C C alkyl optionally substituted with Ci-C alkoxy, CrC alkoxy optionally substituted with Ci-C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, C C alkoxy, C r C 4 haloalkoxy, CrC 4 haloalkyl or Ci-C 4 alkyl; or
- R is -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, Ci-C alkyl, CrC haloalkyl, Ci-C haloalkoxy or C C 4 alkoxy;
- R 2 is selected from the group consisting of hydrogen, C C alkyl, -C(0)CR 3 and - S(0) 2 R 3 ;
- R 3 is selected from the group consisting of C C alkyl and C 3 -C 6 cycloalkyl
- R 6 , R 6a , R 6b or R 6c are independently selected from the group consisting of hydrogen, halogen, -CN, C C alkyl, C 3 -C 7 cycloalkyl, C C alkoxy and CrC haloalkyl;
- L is a direct bond, -CH 2 -, -O- or
- R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, C C 4 haloalkyl, C 3 -C 7 cycloalkyl, C C 4 alkoxy, CrC haloalkoxy, C Calkyl optionally substituted with C C alkoxy, C Calkoxy optionally substituted with C Calkoxy, and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, d-C 4 alkoxy, CrC 4 haloalkoxy, CrC 4 haloalkyl or C C 4 alkyl; or
- R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxyR 2 is selected from the group consisting of hydrogen, Ci-C alkyl, -C(0)CR 3 and -S(0) 2 R 3 ;
- R 3 is selected from the group consisting of CrC 4 alkyl and Cs-Cecycloalkyl
- R 6 , R 6a , R 6b or R 6c are independently selected from the group consisting of hydrogen, halogen, C C alkyl, and CrC haloalkyl;
- L is a direct bond
- R is phenyl substituted with one or two substituents selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (Cr C )alkyl, Ci-C haloalkyl, C 3 -C 7 cycloalkyl, C C alkoxy, CrC haloalkoxy, and CrC alkyl optionally substituted with C C alkoxy.
- L is a bond.
- the compound is of formula V
- R is -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0) 2 (CrC 4 )alkyl, d- C 4 haloalkyl, C 3 -C 7 cycloalkyl, CrC 4 alkoxy, CrC 4 haloalkoxy, C C alkyl optionally substituted with Ci-C alkoxy, CrC alkoxy optionally substituted with Ci-C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, C C alkoxy, C r C 4 haloalkoxy, CrC 4 haloalkyl or Ci-C 4 alkyl; or
- R is -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C 6 -Ci 0 aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, d-C 4 alkyl, CrC 4 haloalkyl, CrC 4 haloalkoxy or C C 4 alkoxy;
- R 2 is selected from the group consisting of hydrogen, CrC 4 alkyl, -C(0)CR 3 and - S(0) 2 R 3 ;
- R 3 is selected from the group consisting of CrC 4 alkyl and C 3 -C 6 cycloalkyl.
- R is phenyl optionally substituted with halogen, Ci-4alkyl or Ci-C4alkoxy;
- R 2 is selected from the group consisting of hydrogen, CrC 4 alkyl, -C(0)CR 3 and - S(0) 2 R 3 ;
- R 3 is selected from the group consisting of C C 4 alkyl and Cs-Cecycloalkyl.
- R is selected from the group consisting of
- R is selected from the group consisting of
- the compound is of formula I to V, wherein L is a direct bond
- R is selected from the group consisting of
- the compounds as defined in embodiments may be synthesized by the general synthetic routes below, specific examples of which are described in more detail in the Examples.
- the invention further includes any variant of the present processes, in which an intermediate product obtainable at any stage thereof is used as starting material and the remaining steps are carried out, or in which the starting materials are formed in situ under the reaction conditions, or in which the reaction components are used in the form of their salts or optically pure material.
- protecting group a readily removable group that is not a constituent of the particular desired end product of the compounds of the present invention.
- the protection of functional groups by such protecting groups, the protecting groups themselves, and their cleavage reactions are described for example in standard reference works, such as J. F. W. McOmie, "Protective Groups in Organic Chemistry", Plenum Press, London and New York 1973, in T. W. Greene and P. G. M. Wuts, "Protective Groups in Organic Synthesis", Third edition, Wiley, New York 1999, in “The Peptides”; Volume 3 (editors: E. Gross and J.
- Salts of compounds of the present invention having at least one salt-forming group may be prepared in a manner known to those skilled in the art.
- salts of compounds of the present invention having acid groups may be formed, for example, by treating the compounds with metal compounds, such as alkali metal salts of suitable organic carboxylic acids, e.g. the sodium salt of 2-ethylhexanoic acid, with organic alkali metal or alkaline earth metal compounds, such as the corresponding hydroxides, carbonates or hydrogen carbonates, such as sodium or potassium hydroxide, carbonate or hydrogen carbonate, with corresponding calcium compounds or with ammonia or a suitable organic amine, stoichiometric amounts or only a small excess of the salt-forming agent preferably being used.
- metal compounds such as alkali metal salts of suitable organic carboxylic acids, e.g. the sodium salt of 2-ethylhexanoic acid
- organic alkali metal or alkaline earth metal compounds such as the corresponding hydroxides, carbonates or hydrogen carbonates
- Acid addition salts of compounds of the present invention are obtained in customary manner, e.g. by treating the compounds with an acid or a suitable anion exchange reagent.
- Internal salts of compounds of the present invention containing acid and basic salt-forming groups, e.g. a free carboxy group and a free amino group, may be formed, e.g. by the neutralisation of salts, such as acid addition salts, to the isoelectric point, e.g. with weak bases, or by treatment with ion exchangers.
- Salts can be converted into the free compounds in accordance with methods known to those skilled in the art.
- Metal and ammonium salts can be converted, for example, by treatment with suitable acids, and acid addition salts, for example, by treatment with a suitable basic agent.
- diastereo isomers can be separated, for example, by partitioning between polyphasic solvent mixtures, recrystallisation and/or chromatographic separation, for example over silica gel or by e.g. medium pressure liquid chromatography over a reversed phase column, and racemates can be separated, for example, by the formation of salts with optically pure salt-forming reagents and separation of the mixture of diastereoisomers so obtainable, for example by means of fractional crystallisation, or by chromatography over optically active column materials.
- Intermediates and final products can be worked up and/or purified according to standard methods, e.g. using chromatographic methods, distribution methods, (re-) crystallization, and the like.
- mixtures of isomers that are formed can be separated into the individual isomers, for example diastereo isomers or enantiomers, or into any desired mixtures of isomers, for example racemates or mixtures of diastereo isomers, for example analogously to the methods described under "Additional process steps”.
- solvents from which those solvents that are suitable for any particular reaction may be selected include those mentioned specifically or, for example, water, esters, such as lower alkyl-lower alkanoates, for example ethyl acetate, ethers, such as aliphatic ethers, for example diethyl ether, or cyclic ethers, for example tetrahydrofuran or dioxane, liquid aromatic hydrocarbons, such as benzene or toluene, alcohols, such as methanol, ethanol or 1- or 2-propanol, nitriles, such as acetonitrile, halogenated hydrocarbons, such as methylene chloride or chloroform, acid amides, such as dimethylformamide or dimethyl acetamide, bases, such as heterocyclic nitrogen bases, for example pyridine or N-methylpyrrolidin-2- one, carboxylic acid anhydrides, such as lower alkanoic acid anhydrides, for example acetic anhydride,
- the compounds of the present invention may also be obtained in the form of hydrates, or their crystals may, for example, include the solvent used for crystallization. Different crystalline forms may be present.
- the invention relates also to those forms of the process in which a compound obtainable as an intermediate at any stage of the process is used as starting material and the remaining process steps are carried out, or in which a starting material is formed under the reaction conditions or is used in the form of a derivative, for example in a protected form or in the form of a salt, or a compound obtainable by the process according to the invention is produced under the process conditions and processed further in situ.
- an optical isomer or "a stereoisomer” refers to any of the various stereoisomeric configurations which may exist for a given compound of the present invention and includes geometric isomers. It is understood that a substituent may be attached at a chiral center of a carbon atom.
- the term “chiral” refers to molecules which have the property of non-superimposability on their mirror image partner, while the term “achiral” refers to molecules which are superimposable on their mirror image partner. Therefore, the invention includes enantiomers, diastereomers or racemates of the compound.
- Enantiomers are a pair of stereoisomers that are non- superimposable mirror images of each other.
- a 1 : 1 mixture of a pair of enantiomers is a "racemic” mixture. The term is used to designate a racemic mixture where appropriate.
- Diastereoisomers are stereoisomers that have at least two asymmetric atoms, but which are not mirror-images of each other. The absolute stereochemistry is specified according to the Cahn- Ingold- Prelog R-S system. When a compound is a pure enantiomer the stereochemistry at each chiral carbon may be specified by either R or S.
- Resolved compounds whose absolute configuration is unknown can be designated (+) or (-) depending on the direction (dextro- or levorotatory) which they rotate plane polarized light at the wavelength of the sodium D line.
- Certain compounds described herein contain one or more asymmetric centers or axes and may thus give rise to enantiomers, diastereomers, and other stereoisomeric forms that may be defined, in terms of absolute stereochemistry, as (R)- or (S)-.
- the compounds can be present in the form of one of the possible isomers or as mixtures thereof, for example as pure optical isomers, or as isomer mixtures, such as racemates and diastereo isomer mixtures, depending on the number of asymmetric carbon atoms.
- the present invention is meant to include all such possible stereoisomers, including racemic mixtures, diasteriomeric mixtures and optically pure forms.
- Optically active (R)- and (S)- isomers may be prepared using chiral synthons or chiral reagents, or resolved using conventional techniques. If the compound contains a double bond, the substituent may be E or Z configuration. If the compound contains a disubstituted cycloalkyl, the cycloalkyl substituent may have a cis- or trans-configuration. All tautomeric forms are also intended to be included.
- any resulting racemates of final products or intermediates can be resolved into the optical antipodes by known methods, e.g., by separation of the diastereomeric salts thereof, obtained with an optically active acid or base, and liberating the optically active acidic or basic compound.
- a basic moiety may thus be employed to resolve the compounds of the present invention into their optical antipodes, e.g., by fractional crystallization of a salt formed with an optically active acid, e.g., tartaric acid, dibenzoyl tartaric acid, diacetyl tartaric acid, di-0,0-p-toluoyl tartaric acid, mandelic acid, malic acid or camphor-10-sulfonic acid.
- Racemic products can also be resolved by chiral
- HPLC high pressure liquid chromatography
- the compounds of the present invention can also be obtained in the form of their hydrates, or include other solvents used for their
- solvate refers to a molecular complex of a compound of the present invention (including pharmaceutically acceptable salts thereof) with one or more solvent molecules.
- solvent molecules are those commonly used in the pharmaceutical art, which are known to be innocuous to the recipient, e.g., water, ethanol, and the like.
- hydrate refers to the complex where the solvent molecule is water.
- the compounds of the present invention including salts, hydrates and solvates thereof, may inherently or by design form polymorphs.
- salt refers to an acid addition or base addition salt of a compound of the present invention.
- Salts include in particular “pharmaceutically acceptable salts”.
- pharmaceutically acceptable salts refers to salts that retain the biological effectiveness and properties of the compounds of this invention and, which typically are not biologically or otherwise undesirable.
- the compounds of the present invention are capable of forming acid and/or base salts by virtue of the presence of amino and/or carboxyl groups or groups similar thereto.
- Pharmaceutically acceptable acid addition salts can be formed with inorganic acids and organic acids, e.g., acetate, aspartate, benzoate, besylate, bromide/hydrobromide, bicarbonate/carbonate, bisulfate/sulfate, camphorsulfonate, chloride/hydrochloride, chlortheophyllonate, citrate, ethandisulfonate, fumarate, gluceptate, gluconate, glucuronate, hippurate, hydroiodide/iodide, isethionate, lactate, lactobionate, laurylsulfate, malate, maleate, malonate, mandelate, mesylate, methylsulphate, naphthoate, napsylate, nicotinate, nitrate, octadecanoate, oleate, oxalate, palmitate, pamoate, phosphate/hydrogen phosphate/dihydrogen
- Inorganic acids from which salts can be derived include, for example, hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, and the like.
- Organic acids from which salts can be derived include, for example, acetic acid, propionic acid, glycolic acid, oxalic acid, maleic acid, malonic acid, succinic acid, fumaric acid, tartaric acid, citric acid, benzoic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, toluenesulfonic acid, sulfosalicylic acid, and the like.
- Pharmaceutically acceptable base addition salts can be formed with inorganic and organic bases.
- Inorganic bases from which salts can be derived include, for example, ammonium salts and metals from columns I to XII of the periodic table.
- the salts are derived from sodium, potassium, ammonium, calcium, magnesium, iron, silver, zinc, and copper; particularly suitable salts include ammonium, potassium, sodium, calcium and magnesium salts.
- Organic bases from which salts can be derived include, for example, primary, secondary, and tertiary amines, substituted amines including naturally occurring substituted amines, cyclic amines, basic ion exchange resins, and the like.
- Certain organic amines include isopropylamine, benzathine, cholinate, diethanolamine, diethylamine, lysine, meglumine, piperazine and tromethamine.
- the pharmaceutically acceptable salts of the present invention can be synthesized from a basic or acidic moiety, by conventional chemical methods. Generally, such salts can be prepared by reacting free acid forms of these compounds with a stoichiometric amount of the appropriate base (such as Na, Ca, Mg, or K hydroxide, carbonate, bicarbonate or the like), or by reacting free base forms of these compounds with a stoichiometric amount of the appropriate acid. Such reactions are typically carried out in water or in an organic solvent, or in a mixture of the two. Generally, use of non-aqueous media like ether, ethyl acetate, ethanol, isopropanol, or acetonitrile is desirable, where practicable. Lists of additional suitable salts can be found, e.g., in "Remington's Pharmaceutical Sciences", 20th ed., Mack Publishing Company, Easton, Pa., (1985); and in "Handbook of Pharmaceutical Salts:
- any formula given herein is also intended to represent unlabeled forms as well as isotopically labeled forms of the compounds of the present invention.
- Isotopically labeled compounds have structures depicted by the formulas given herein except that one or more atoms are replaced by an atom having a selected atomic mass or mass number.
- isotopes that can be incorporated into compounds of the invention include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorous, fluorine, and chlorine, such as 2 H, 3 H, 11 C, 13 C, 14 C, 15 N, 18 F 31 P, 32 P, 35 S, 36 CI, 125 l respectively.
- the invention includes various isotopically labeled compounds of the present invention, for example those into which radioactive isotopes, such as 3 H and 14 C, or those into which non-radioactive isotopes, such as 2 H and 13 C are present.
- isotopically labelled compounds are useful in metabolic studies (with 14 C), reaction kinetic studies (with, for example 2 H or 3 H), detection or imaging techniques, such as positron emission tomography (PET) or single-photon emission computed tomography (SPECT) including drug or substrate tissue distribution assays, or in radioactive treatment of patients.
- PET positron emission tomography
- SPECT single-photon emission computed tomography
- an 18 F labeled compound of the present invention may be particularly desirable for PET or SPECT studies.
- Isotopically-labeled compounds of the present invention can generally be prepared by conventional techniques known to those skilled in the art or by processes analogous to those described in the accompanying Examples and Preparations using an appropriate isotopically-labeled reagent in place of the non-labeled reagent previously employed.
- isotopic enrichment factor means the ratio between the isotopic abundance and the natural abundance of a specified isotope.
- a substituent in a compound of this invention is denoted deuterium, such compound has an isotopic enrichment factor for each designated deuterium atom of at least 3500 (52.5% deuterium incorporation at each designated deuterium atom), at least 4000 (60% deuterium incorporation), at least 4500 (67.5% deuterium incorporation), at least 5000 (75% deuterium incorporation), at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), at least 6333.3 (95% deuterium incorporation), at least 6466.7 (97% deuterium incorporation), at least 6600 (99% deuterium incorporation), or at least 6633.3 (99.5% deuterium incorporation).
- solvates in accordance with the invention include those wherein the solvent of crystallization may be isotopically substituted, e.g. D 2 0, d 6 -acetone, de-DMSO.
- co-crystals may be capable of forming co-crystals with suitable co- crystal formers.
- These co-crystals may be prepared from compounds of the present invention by known co-crystal forming procedures. Such procedures include grinding, heating, co-subliming, co-melting, or contacting in solution compounds of the present invention with the co-crystal former under crystallization conditions and isolating co-crystals thereby formed.
- Suitable co-crystal formers include those described in WO 2004/078163. Hence the invention further provides co-crystals comprising a compound of the present invention.
- the present invention provides novel compounds, pharmaceutical formulations including the compounds, methods of inhibiting UDP-3-0-(R-3-hydroxydecanoyl)-N- acetylglucosamine deacetylase (LpxC), and methods of treating Gram-negative bacterial infections.
- substitution with heavier isotopes such as deuterium, i.e. 2 H may afford certain therapeutic advantages resulting from greater metabolic stability, for example, increased in vivo half-life or reduced dosage requirements, and hence may be preferred in some circumstances.
- deuterium substitution at non-exchangeable hydrocarbon bonds e.g., C-H
- Isotopically-labeled compounds of the invention i.e. compounds of formula (I)
- compounds of formula (I) can generally be prepared by conventional techniques known to those skilled in the art or by processes analogous to those described in the accompanying Examples and Preparations Sections using an appropriate isotopically-labeled reagent in place of the non-labeled reagent previously.
- the invention provides a method of inhibiting a deacetylase enzyme in a Gram-negative bacterium, the method comprising the step of contacting the Gram-negative bacteria with a compound of the invention, e.g., a compound of Formula I or salt thereof.
- the invention provides a method for treating a subject with a Gram-negative bacterial infection, the method comprising the step of administering to the subject in need thereof an antibacterially effective amount of a compound of the invention, e.g., a compound of Formula I or salt thereof with a pharmaceutically acceptable carrier.
- a compound of the invention e.g., a compound of Formula I or salt thereof with a pharmaceutically acceptable carrier.
- the compounds of the invention can be used for treating conditions caused by the bacterial production of endotoxin and, in particular, by Gram-negative bacteria and bacteria that use LpxC in the biosynthesis of lipopolysaccharide (LPS) or endotoxin.
- LPS lipopolysaccharide
- the compounds of the invention also are useful in the treatment of patients suffering from or susceptible to pneumonia, sepsis, cystic fibrosis, wound, complicated diabetic foot or complicated urinary track infections and sexually transmitted diseases caused by Gram- negative pathogens.
- the compounds of the invention also are useful in the conditions that are caused or exacerbated by the bacterial production of lipid A and LPS or endotoxin, such as sepsis, septic shock, systemic inflammation, localized inflammation, chronic obstructive pulmonary disease (COPD) and acute exacerbations of chronic bronchitis (AECB).
- treatment includes the administration of a compound of the invention, or a combination of compounds of the invention, optionally with a second agent wherein the second agent is a second antibacterial agent or a second non-antibacterial agent.
- a second agent is a second antibacterial agent or a second non-antibacterial agent.
- preferred second non-antibacterial agents include antiendotoxins including endotoxin receptor-binding antibodies, endotoxin-binding antibodies, antiCD14-binding protein antibodies antilipopolysaccharide-binding protein antibodies and tyrosine kinase inhibitors.
- the compounds of the present invention may also be used with second non-antibacterial agents administered via inhalation.
- Preferred non-antibacterial agents used in this treatment include antiinflammatory steroids, non-steroidal anti-inflammatory agents, bronchiodilators, mucolytics, anti-asthma therapeutics and lung fluid surfactants.
- the non-antibacterial agent may be selected from a group consisting of albuterol, salbuterol, budesonide,
- beclomethasone dexamethasone, nedocromil, beclomethasone, fluticasone, flunisolide, triamcinolone, ibuprofin, rofecoxib, naproxen, celecoxib, nedocromil, ipratropium, metaproterenol, pirbuterol, salneterol, bronchiodilators, mucolytics, calfactant, beractant, poractant alfa, surfaxin and pulmozyme (also called domase alfa).
- the compounds of the invention can be used, alone or in combination with a second antibacterial agent for the treatment of a serious or chronic respiratory tract infection including serious lung and nosocomial infections such as those caused by Enterobacter aerogenes, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Proteus mirabilis, Serratia marcescens, Stenotrophomonas maltophilia,
- Pseudomonas aeruginosa Burkholderia cepacia, Acinetobacter baumanii, Alcaligenes xylosoxidans, Flavobacterium meningosepticum, Providencia stuartii and Citrobacter freundi, community lung infections such as those caused by Haemophilus influenzae, Legionella species, Moraxella catarrhalis, Enterobacter species, Acinetobacter species, Klebsiella species, and Proteus species, and infections caused by other bacterial species such as Neisseria species, Shigella species, Salmonella species, Helicobacter pylori, Vibrionaceae and Bordetella species as well as the infections is caused by a Brucella species, Francisella tularensis and/or Yersinia Pestis.
- a compound of the present invention may also be used in combination with other agents, e.g., an additional antibiotic agent that is or is not of the formula I, for treatment of a bacterial infection in a subject.
- combination is meant either a fixed combination in one dosage unit form, or a kit of parts for the combined administration where a compound of the present invention and a combination partner may be administered independently at the same time or separately within time intervals that especially allow that the combination partners show a cooperative, e.g., synergistic, effect, or any combination thereof.
- the compounds of the present invention can be used to sensitize Gram-negative bacteria to the effects of a second agent.
- An embodiment of the present invention is compounds of the present invention used in combination with a second antibacterial agent, non-limiting examples of antibacterial agents may be selected from the following groups:
- Beta-lactams including penicillin such as penicillin G, penicillin V, methicillin, oxacillin, cloxacillin, dicloxacillin, nafcillin, ampicillin, amoxicillin, carbenicillin, ticarcillin, mezlocillin, piperacillin, azlocillin, temocillin, cephalosporin such as cepalothin, cephapirin, cephradine, cephaloridine, cefazolin, cefamandole, cefuroxime, cephalexin, cefprozil, cefaclor, loracarbef, cefoxitin, cefinetazole, cefotaxime, ceftizoxime, ceftriaxone,
- cefoperazone ceftazidime, cefixime, cefpodoxime, ceftibuten, cefdinir, cefpirome, cefepime, and carbapenems such as carbapenem, imipenem, meropenem and PZ-601 ;
- Quinolones such as nalidixic acid, oxolinic acid, norfloxacin, pefloxacin, enoxacin, ofloxacin, levofloxacin, ciprofloxacin, temafloxacin, lomefloxacin, fleroxacin, grepafloxacin, sparfloxacin, trovafloxacin, clinafloxacin, gatifloxacin, moxifloxacin, sitafloxacin,
- Antibacterial sulfonanmides and antibacterial sulphanilamides including para- aminobenzoic acid, sulfadiazine, sulfisoxazole, sulfamethoxazole and sulfathalidine;
- Aminoglycosides such as streptomycin, neomycin, kanamycin, paromycin, gentamicin, tobramycin, amikacin, netilmicin, spectinomycin, sisomicin, dibekalin and isepamicin;
- Tetracyclines such as tetracycline, chlortetracycline, demeclocycline, minocycline, oxytetracycline, methacycline, doxycycline, tegacycline;
- Rifamycins such as rifampicin (also called rifampin), rifapentine, rifabutin, bezoxazinorifamycin and rifaximin;
- Lincosamides such as lincomycin and clindamycin
- Glycopeptides such as vancomycin and teicoplanin
- the second antibacterial agent may be administered in combination with the compounds of the present inventions wherein the second antibacterial agent is administered prior to, simultaneously, or after the compound or compounds of the present invention.
- a compound of the invention may be formulated with a second agent into the same dosage form.
- An example of a dosage form containing a compound of the invention and a second agent is a tablet or a capsule.
- the compounds of the invention may be used alone or in combination with a second antibacterial agent administered via inhalation.
- a preferred second antibacterial agent is selected from a group consisting of tobramycin, gentamicin, aztreonam, ciprofloxacin, polymyxin, colistin, colymycin, azithromycin and clarithromycin.
- an effective amount of the compound is that amount necessary or sufficient to treat or prevent a bacterial infection and/or a disease or condition described herein.
- an effective amount of the LpxC inhibitor is the amount sufficient to treat bacterial infection in a subject.
- an effective amount of the LpxC inhibitor is an amount sufficient to treat a bacterial infection, such as, but not limited to Pseudomonas aeruginosa and the like in a subject.
- the effective amount can vary depending on such factors as the size and weight of the subject, the type of illness, or the particular compound of the invention. For example, the choice of the compound of the invention can affect what constitutes an "effective amount.”
- One of ordinary skill in the art would be able to study the factors contained herein and make the determination regarding the effective amount of the compounds of the invention without undue experimentation.
- the regimen of administration can affect what constitutes an effective amount.
- the compound of the invention can be administered to the subject either prior to or after the onset of a bacterial infection. Further, several divided dosages, as well as staggered dosages, can be administered daily or sequentially, or the dose can be continuously infused, or can be a bolus injection. Further, the dosages of the compound(s) of the invention can be proportionally increased or decreased as indicated by the exigencies of the therapeutic or prophylactic situation.
- Compounds of the invention may be used in the treatment of states, disorders or diseases as described herein, or for the manufacture of pharmaceutical compositions for use in the treatment of these diseases.
- the invention provides methods of use of compounds of the present invention in the treatment of these diseases or pharmaceutical preparations having compounds of the present invention for the treatment of these diseases.
- composition includes preparations suitable for administration to mammals, e.g., humans.
- pharmaceutical composition containing, for example, 0.1 to 99.5% (more preferably, 0.5 to 90%) of active ingredient in combination with a pharmaceutically acceptable carrier.
- phrases "pharmaceutically acceptable carrier” is art recognized and includes a pharmaceutically acceptable material, composition or vehicle, suitable for administering compounds of the present invention to mammals.
- the carriers include liquid or solid filler, diluent, excipient, solvent or encapsulating material, involved in carrying or transporting the subject agent from one organ, or portion of the body, to another organ, or portion of the body.
- Each carrier must be “acceptable” in the sense of being compatible with the other ingredients of the formulation and not injurious to the patient.
- materials which can serve as pharmaceutically acceptable carriers include: sugars, such as lactose, glucose and sucrose; starches, such as corn starch and potato starch; cellulose, and its derivatives, such as sodium carboxymethyl cellulose, ethyl cellulose and cellulose acetate; powdered tragacanth; malt; gelatin; talc; excipients, such as cocoa butter and suppository waxes; oils, such as peanut oil, cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and soybean oil; glycols, such as propylene glycol; polyols, such as glycerin, sorbitol, mannitol and polyethylene glycol; esters, such as ethyl oleate and ethyl laurate; agar; buffering agents, such as magnesium hydroxide and aluminum hydroxide; alginic acid; pyrogen-free water; isotonic saline; Ringer'
- wetting agents such as sodium lauryl sulfate and magnesium stearate, as well as coloring agents, release agents, coating agents, sweetening, flavoring and perfuming agents, preservatives and antioxidants can also be present in the compositions.
- antioxidants examples include: water soluble antioxidants, such as ascorbic acid, cysteine hydrochloride, sodium bisulfate, sodium metabisulfite, sodium sulfite and the like; oil-soluble antioxidants, such as ascorbyl palmitate, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), lecithin, propyl gallate, - tocopherol, and the like; and metal chelating agents, such as citric acid, ethylenediamine tetraacetic acid (EDTA), sorbitol, tartaric acid, phosphoric acid, and the like.
- water soluble antioxidants such as ascorbic acid, cysteine hydrochloride, sodium bisulfate, sodium metabisulfite, sodium sulfite and the like
- oil-soluble antioxidants such as ascorbyl palmitate, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), lecithin,
- Formulations of the present invention include those suitable for oral, nasal, inhalation, topical, transdermal, buccal, sublingual, rectal, vaginal and/or parenteral administration.
- the formulations may conveniently be presented in unit dosage form and may be prepared by any methods well known in the art of pharmacy.
- the amount of active ingredient that can be combined with a carrier material to produce a single dosage form will generally be that amount of the compound that produces a therapeutic effect. Generally, out of one hundred per cent, this amount will range from about 1 per cent to about ninety-nine percent of active ingredient, preferably from about 5 per cent to about 70 per cent, most preferably from about 10 per cent to about 30 per cent.
- Methods of preparing these formulations or compositions include the step of bringing into association a compound of the present invention with the carrier and, optionally, one or more accessory ingredients.
- the formulations are prepared by uniformly and intimately bringing into association a compound of the present invention with liquid carriers, or finely divided solid carriers, or both, and then, if necessary, shaping the product.
- Formulations of the invention suitable for oral administration may be in the form of capsules, cachets, pills, tablets, lozenges (using a flavored basis, usually sucrose and acacia or tragacanth), powders, granules, or as a solution or a suspension in an aqueous or non-aqueous liquid, or as an oil-in-water or water-in-oil liquid emulsion, or as an elixir or syrup, or as pastilles (using an inert base, such as gelatin and glycerin, or sucrose and acacia) and/or as mouth washes and the like, each containing a predetermined amount of a compound of the present invention as an active ingredient.
- a compound of the present invention may also be administered as a bolus, electuary or paste.
- the active ingredient is mixed with one or more pharmaceutically acceptable carriers, such as sodium citrate or dicalcium phosphate, and/or any of the following: fillers or extenders, such as starches, lactose, sucrose, glucose, mannitol, and/or silicic acid; binders, such as, for example, carboxymethylcellulose, alginates, gelatin, polyvinyl pyrrolidone, sucrose and/or acacia; humectants, such as glycerol; disintegrating agents, such as agar-agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and sodium carbonate; solution retarding agents, such as paraffin; absorption accelerators, such as quaternary ammonium compounds; wetting agents, such as, for example, cetyl alcohol and glycerol monostea
- compositions may also comprise buffering agents.
- Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugars, as well as high molecular weight polyethylene glycols and the like.
- a tablet may be made by compression or molding, optionally with one or more accessory ingredients.
- Compressed tablets may be prepared using binder (for example, gelatin or hydroxypropylmethyl cellulose), lubricant, inert diluent, preservative, disintegrant (for example, sodium starch glycolate or cross-linked sodium carboxymethyl cellulose), surface-active or dispersing agent.
- Molded tablets may be made by molding in a suitable machine a mixture of the powdered compound moistened with an inert liquid diluent.
- the tablets, and other solid dosage forms of the pharmaceutical compositions of the present invention may optionally be scored or prepared with coatings and shells, such as enteric coatings and other coatings well known in the pharmaceutical-formulating art. They may also be formulated so as to provide slow or controlled release of the active ingredient therein using, for example, hydroxypropylmethyl cellulose in varying proportions to provide the desired release profile, other polymer matrices, liposomes and/or microspheres.
- compositions may be sterilized by, for example, filtration through a bacteria-retaining filter, or by incorporating sterilizing agents in the form of sterile solid compositions that can be dissolved in sterile water, or some other sterile injectable medium immediately before use.
- These compositions may also optionally contain opacifying agents and may be of a composition that they release the active ingredient(s) only, or preferentially, in a certain portion of the gastrointestinal tract, optionally, in a delayed manner.
- embedding compositions that can be used include polymeric substances and waxes.
- the active ingredient can also be in micro-encapsulated form, if appropriate, with one or more of the above-described excipients.
- Liquid dosage forms for oral administration of the compounds of the invention include pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions, syrups and elixirs.
- the liquid dosage forms may contain inert diluent commonly used in the art, such as, for example, water or other solvents, solubilizing agents and emulsifiers, such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1 ,3-butylene glycol, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor and sesame oils), glycerol, tetrahydrofuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof.
- inert diluent commonly used in the art, such as, for example, water or other solvents, solubilizing agents and
- the oral compositions can also include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, coloring, perfuming and preservative agents.
- adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, coloring, perfuming and preservative agents.
- Suspensions in addition to the active compounds, may contain suspending agents as, for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar and tragacanth, and mixtures thereof.
- suspending agents as, for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar and tragacanth, and mixtures thereof.
- Formulations of the pharmaceutical compositions of the invention for rectal or vaginal administration may be presented as a suppository, which may be prepared by mixing one or more compounds of the invention with one or more suitable nonirritating excipients or carriers comprising, for example, cocoa butter, polyethylene glycol, a suppository wax or a salicylate, and which is solid at room temperature, but liquid at body temperature and, therefore, will melt in the rectum or vaginal cavity and release the active compound.
- suitable nonirritating excipients or carriers comprising, for example, cocoa butter, polyethylene glycol, a suppository wax or a salicylate, and which is solid at room temperature, but liquid at body temperature and, therefore, will melt in the rectum or vaginal cavity and release the active compound.
- Formulations of the present invention which are suitable for vaginal administration also include pessaries, tampons, creams, gels, pastes, foams or spray formulations containing such carriers as are known in the art to be appropriate.
- Dosage forms for the topical or transdermal administration of a compound of this invention include powders, sprays, ointments, pastes, creams, lotions, gels, solutions, patches and inhalants.
- the active compound may be mixed under sterile conditions with a pharmaceutically acceptable carrier, and with any preservatives, buffers, or propellants that may be required.
- the ointments, pastes, creams and gels may contain, in addition to an active compound of this invention, excipients, such as animal and vegetable fats, oils, waxes, paraffins, starch, tragacanth, cellulose derivatives, polyethylene glycols, silicones, bentonites, silicic acid, talc and zinc oxide, or mixtures thereof.
- excipients such as animal and vegetable fats, oils, waxes, paraffins, starch, tragacanth, cellulose derivatives, polyethylene glycols, silicones, bentonites, silicic acid, talc and zinc oxide, or mixtures thereof.
- Powders and sprays can contain, in addition to a compound of this invention, excipients such as lactose, talc, silicic acid, aluminum hydroxide, calcium silicates and polyamide powder, or mixtures of these substances.
- Sprays can additionally contain customary propellants, such as chlorofluorohydrocarbons and volatile unsubstituted hydrocarbons, such as butane and propane.
- Transdermal patches have the added advantage of providing controlled delivery of a compound of the present invention to the body.
- dosage forms can be made by dissolving or dispersing the compound in the proper medium.
- Absorption enhancers can also be used to increase the flux of the compound across the skin. The rate of such flux can be controlled by either providing a rate controlling membrane or dispersing the active compound in a polymer matrix or gel.
- Ophthalmic formulations are also contemplated as being within the scope of this invention.
- compositions of this invention suitable for parenteral administration comprise one or more compounds of the invention in combination with one or more pharmaceutically acceptable sterile isotonic aqueous or nonaqueous solutions, dispersions, suspensions or emulsions, or sterile powders which may be reconstituted into sterile injectable solutions or dispersions just prior to use, which may contain antioxidants, buffers, bacteriostats, solutes which render the formulation isotonic with the blood of the intended recipient or suspending or thickening agents.
- aqueous and nonaqueous carriers examples include water, ethanol, polyols (such as glycerol, propylene glycol, polyethylene glycol, and the like), and suitable mixtures thereof, vegetable oils, such as olive oil, and injectable organic esters, such as ethyl oleate.
- polyols such as glycerol, propylene glycol, polyethylene glycol, and the like
- vegetable oils such as olive oil
- injectable organic esters such as ethyl oleate.
- Proper fluidity can be maintained, for example, by the use of coating materials, such as lecithin, by the maintenance of the required particle size in the case of dispersions, and by the use of surfactants.
- compositions may also contain adjuvants such as preservatives, wetting agents, emulsifying agents and dispersing agents.
- microorganisms may be ensured by the inclusion of various antibacterial and antifungal agents, for example, paraben, chlorobutanol, phenol sorbic acid, and the like. It may also be desirable to include isotonic agents, such as sugars, sodium chloride, and the like into the compositions. In addition, prolonged absorption of the injectable pharmaceutical form may be brought about by the inclusion of agents that delay absorption such as aluminum monostearate and gelatin.
- various antibacterial and antifungal agents for example, paraben, chlorobutanol, phenol sorbic acid, and the like.
- isotonic agents such as sugars, sodium chloride, and the like into the compositions.
- prolonged absorption of the injectable pharmaceutical form may be brought about by the inclusion of agents that delay absorption such as aluminum monostearate and gelatin.
- the absorption of the drug in order to prolong the effect of a drug, it is desirable to slow the absorption of the drug from subcutaneous or intramuscular injection. This may be accomplished by the use of a liquid suspension of crystalline or amorphous material having poor water solubility. The rate of absorption of the drug then depends upon its rate of dissolution which, in turn, may depend upon crystal size and crystalline form. Alternatively, delayed absorption of a parenterally-administered drug form is accomplished by dissolving or suspending the drug in an oil vehicle.
- Injectable depot forms are made by forming microencapsule matrices of the subject compounds in biodegradable polymers such as polylactide-polyglycolide. Depending on the ratio of drug to polymer, and the nature of the particular polymer employed, the rate of drug release can be controlled. Examples of other biodegradable polymers include
- Depot injectable formulations are also prepared by entrapping the drug in liposomes or microemulsions that are compatible with body tissue.
- the preparations of the present invention may be given orally, parenterally, topically, or rectally. They are of course given by forms suitable for each administration route. For example, they are administered in tablets or capsule form, by injection, inhalation, eye lotion, ointment, suppository, etc., administration by injection, infusion or inhalation; topical by lotion or ointment; and rectal by suppositories. Oral administration is preferred.
- parenteral administration and “administered parenterally” as used herein means modes of administration other than enteral and topical administration, usually by injection, and includes, without limitation, intravenous, intramuscular, intraarterial, intrathecal, intracapsular, intraorbital, intracardiac, intradermal, intraperitoneal, transtracheal, subcutaneous, subcuticular, intraarticular, subcapsular, subarachnoid, intraspinal and intrasternal injection and infusion.
- systemic administration means the administration of a compound, drug or other material other than directly into the central nervous system, such that it enters the patient's system and, thus, is subject to metabolism and other like processes, for example, subcutaneous administration.
- These compounds may be administered to humans and other animals for therapy by any suitable route of administration, including orally, nasally, as by, for example, a spray, rectally, intravaginally, parenterally, intracisternally and topically, as by powders, ointments or drops, including buccally and sublingually.
- the compounds of the present invention which may be used in a suitable hydrated form, and/or the pharmaceutical compositions of the present invention, are formulated into pharmaceutically acceptable dosage forms by conventional methods known to those of skill in the art.
- Actual dosage levels of the active ingredients in the pharmaceutical compositions of this invention may be varied so as to obtain an amount of the active ingredient which is effective to achieve the desired therapeutic response for a particular patient, composition, and mode of administration, without being toxic to the patient.
- the selected dosage level will depend upon a variety of factors including the activity of the particular compound of the present invention employed, or the ester, salt or amide thereof, the route of administration, the time of administration, the rate of excretion of the particular compound being employed, the duration of the treatment, other drugs, compounds and/or materials used in combination with the particular compound employed, the age, sex, weight, condition, general health and prior medical history of the patient being treated, and like factors well known in the medical arts.
- a physician or veterinarian having ordinary skill in the art can readily determine and prescribe the effective amount of the pharmaceutical composition required.
- the physician or veterinarian could start doses of the compounds of the invention employed in the pharmaceutical composition at levels lower than that required in order to achieve the desired therapeutic effect and gradually increase the dosage until the desired effect is achieved.
- a suitable daily dose of a compound of the invention will be that amount of the compound that is the lowest dose effective to produce a therapeutic effect. Such an effective dose will generally depend upon the factors described above.
- intravenous and subcutaneous doses of the compounds of this invention for a patient when used for the indicated analgesic effects, will range from about 0.0001 to about 100 mg per kilogram of body weight per day, more preferably from about 0.01 to about 50 mg per kg per day, and still more preferably from about 1.0 to about 100 mg per kg per day.
- An effective amount is that amount treats a bacterial infection.
- the effective daily dose of the active compound may be administered as two, three, four, five, six or more sub-doses administered separately at appropriate intervals throughout the day, optionally, in unit dosage forms.
- a compound of the present invention While it is possible for a compound of the present invention to be administered alone, it is preferable to administer the compound as a pharmaceutical composition.
- the compounds as defined in embodiments may be synthesized by the general synthetic routes below, specific examples of which are described in more detail in the Examples.
- the compounds of general structure 1-1 can be synthesized by the Scheme 1 shown below.
- Compound 1 c can be prepared from amine 1 b either through reductive amination with aldehyde 1a or alkylation with appropriate electrophile 1 d.
- the ester in 1 c can be converted into hydroxamate in 1-1 in standard three step procedure.
- the ester was hydrolyzed under basic condition to provide carboxylic acid 1 e, which can then be coupled with protected hydroxylamine to give 1f. Finally the protecting group can be cleaved to generate the desired compound of formula 1-1.
- the synthetic route in Scheme 1 could be modified and the tail fragment represented by R-L could be coupled at late stage (as shown in Scheme 2).
- the initial step is to couple amine 1 b and aldehyde 2a by a reductive amination reaction.
- the product 2b can be hydrolyzed to acid 2c followed by amide coupling to provide protected hyroxamate 2d.
- the tail fragment can be attached by standard transition metal catalyzed coupling reaction to provide 2e.
- the protecting group can be cleaved to generate the desired compound of formula IV.
- ester 3a is a key intermediate, which can be converted to IV by standard three step procedures.
- the key step in the synthesis of 3a is attaching R-L-Z-CH 2 CH 2 moiety to ester 3c through alkylation.
- Step 2 couple with NH 2 0-PG
- protecting group a readily removable group that is not a constituent of the particular desired end product of the compounds of the present invention.
- the protection of functional groups by such protecting groups, the protecting groups themselves, and their cleavage reactions are described for example in standard reference works, such as e.g., Science of Synthesis: Houben-Weyl Methods of Molecular Transformation. Georg Thieme Verlag, Stuttgart, Germany. 2005. 41627 pp. (URL: http://www.science-of-synthesis.com (Electronic Version, 48 Volumes)); J. F. W. McOmie, "Protective Groups in Organic
- Salts of compounds of the present invention having at least one salt-forming group may be prepared in a manner known per se.
- salts of compounds of the present invention having acid groups may be formed, for example, by treating the compounds with metal compounds, such as alkali metal salts of suitable organic carboxylic acids, e.g., the sodium salt of 2-ethyl hexanoic acid, with organic alkali metal or alkaline earth metal compounds, such as the corresponding hydroxides, carbonates or hydrogen carbonates, such as sodium or potassium hydroxide, carbonate or hydrogen carbonate, with corresponding calcium compounds or with ammonia or a suitable organic amine, stoichiometric amounts or only a small excess of the salt-forming agent preferably being used.
- metal compounds such as alkali metal salts of suitable organic carboxylic acids, e.g., the sodium salt of 2-ethyl hexanoic acid
- organic alkali metal or alkaline earth metal compounds such as the corresponding hydroxides, carbonates or hydrogen carbonates
- Acid addition salts of compounds of the present invention are obtained in customary manner, e.g., by treating the compounds with an acid or a suitable anion exchange reagent.
- Internal salts of compounds of the present invention containing acid and basic salt-forming groups, e.g., a free carboxy group and a free amino group, may be formed, e.g., by the neutralisation of salts, such as acid addition salts, to the isoelectric point, e.g., with weak bases, or by treatment with ion exchangers.
- Salts can be converted in customary manner into the free compounds; metal and ammonium salts can be converted, for example, by treatment with suitable acids, and acid addition salts, for example, by treatment with a suitable basic agent.
- diastereoisomers can be separated in a manner known per se into the individual isomers; diastereoisomers can be separated, for example, by partitioning between polyphasic solvent mixtures, recrystallisation and/or chromatographic separation, for example over silica gel or by, e.g., medium pressure liquid chromatography over a reversed phase column, and racemates can be separated, for example, by the formation of salts with optically pure salt-forming reagents and separation of the mixture of diastereoisomers so obtainable, for example by means of fractional crystallisation, or by chromatography over optically active column materials.
- Intermediates and final products can be worked up and/or purified according to standard methods, e.g., using chromatographic methods, distribution methods, (re-) crystallization, and the like.
- the process steps to synthesize the compounds of the invention can be carried out under reaction conditions that are known per se, including those mentioned specifically, in the absence or, customarily, in the presence of solvents or diluents, including, for example, solvents or diluents that are inert towards the reagents used and dissolve them, in the absence or presence of catalysts, condensation or neutralizing agents, for example ion exchangers, such as cation exchangers, e.g., in the H + form, depending on the nature of the reaction and/or of the reactants at reduced, normal or elevated temperature, for example in a temperature range of from about -100 °C to about 190°C, including, for example, from approximately -80°C to approximately 150°C, for example at from -80 to -60°C, at room temperature, at from -20 to 40°C or at reflux temperature, under atmospheric pressure or in a closed vessel, where appropriate under pressure, and/or in an inert atmosphere, for example under an argon or
- mixtures of isomers that are formed can be separated into the individual isomers, for example diastereo isomers or enantiomers, or into any desired mixtures of isomers, for example racemates or mixtures of diastereo isomers, for example analogously to the methods described in Science of Synthesis: Houben-Weyl Methods of Molecular Transformation. Georg Thieme Verlag, Stuttgart, Germany. 2005.
- solvents from which those solvents that are suitable for any particular reaction may be selected include those mentioned specifically or, for example, water, esters, such as lower alkyl-lower alkanoates, for example ethyl acetate, ethers, such as aliphatic ethers, for example diethyl ether, or cyclic ethers, for example tetrahydrofurane or dioxane, liquid aromatic hydrocarbons, such as benzene or toluene, alcohols, such as methanol, ethanol or 1- or 2-propanol, nitriles, such as acetonitrile, halogenated hydrocarbons, such as methylene chloride or chloroform, acid amides, such as dimethylformamide or dimethyl acetamide, bases, such as heterocyclic nitrogen bases, for example pyridine or N-methylpyrrolidin-2- one, carboxylic acid anhydrides, such as lower alkanoic acid anhydrides, for example acetic anhydride,
- the compounds, including their salts, may also be obtained in the form of hydrates, or their crystals may, for example, include the solvent used for crystallization. Different crystalline forms may be present.
- the invention relates also to those forms of the process in which a compound obtainable as an intermediate at any stage of the process is used as starting material and the remaining process steps are carried out, or in which a starting material is formed under the reaction conditions or is used in the form of a derivative, for example in a protected form or in the form of a salt, or a compound obtainable by the process according to the invention is produced under the process conditions and processed further in situ.
- the present invention also relates to pro-drugs of a compound of the present invention that are converted in vivo to the compounds of the present invention as described herein. Any reference to a compound of the present invention is therefore to be understood as referring also to the corresponding pro-drugs of the compound of the present invention, as appropriate and expedient.
- a pharmaceutical combination comprising a) a first agent which is a compound of the invention, e.g. a compound of formula I or any subformulae thereof, and b) a co- agent, e.g. a second drug agent as defined above.
- a method as defined above comprising co-administration, e.g. concomitantly or in sequence, of a therapeutically effective amount of a compound of the invention, e.g. a compound of formula I or any subformulae thereof, and a co-agent, e.g. a second drug agent as defined above.
- a compound of the invention e.g. a compound of formula I or any subformulae thereof
- a co-agent e.g. a second drug agent as defined above.
- co-administration or “combined administration” or the like as utilized herein are meant to encompass administration of the selected therapeutic agents to a single patient, and are intended to include treatment regimens in which the agents are not necessarily administered by the same route of administration or at the same time. Fixed combinations are also within the scope of the present invention.
- the administration of a pharmaceutical combination of the invention results in a beneficial effect, e.g. a synergistic therapeutic effect, compared to a monotherapy applying only one of its pharmaceutically active ingredients.
- Each component of a combination according to this invention may be administered separately, together, or in any combination thereof.
- the compound of the invention and any additional agent may be formulated in separate dosage forms. Alternatively, to decrease the number of dosage forms
- the compound of the invention and any additional agent may be formulated together in any combination.
- the compound of the invention inhibitor may be formulated in one dosage form and the additional agent may be formulated together in another dosage form.
- Any separate dosage forms may be administered at the same time or different times.
- composition of this invention comprises an additional agent as described herein.
- Each component may be present in individual compositions, combination compositions, or in a single composition.
- the invention is further illustrated by the following examples, which should not be construed as further limiting.
- the assays used throughout the Examples are accepted. Demonstration of efficacy in these assays is predictive of efficacy in subjects.
- Mass spectra were run on LC-MS systems using electrospray ionization. These were WATERS Acquity Single Quard Detector. [M+H] + refers to mono-isotopic molecular weights.
- NMR spectra were run on open access Varian 400 NMR spectrometers. Spectra were measured at 298K and were referenced using the solvent peak.
- solvent A water with 0.1 % TFA
- solvent B CH 3 CN with 0.1 % TFA
- Compound 1.1.4 was prepared similarly to the synthesis for 1.1.2 changing the Suzuki coupling conditions in Step 4 as following.
- Pd(PPh 3 ) 4 (50.1 mg, 0.043 mmol) was added to a degassed mixture of 1.1.2c (100 mg, 0.217 mmol), 4-(4-(4,4,5,5-tetramethyl- 1 ,3,2-dioxaborolan-2-yl)benzyl)morpholine (99 mg, 0.325 mmol), Na 2 C0 3 (68.9 mg, 0.650 mmol), THF (1.1 mL) and Water (0.36 mL). The reaction mixture was heated to 70 °C for 18 hours.
- reaction mixture was diluted with DCM and neutralized with saturated aqueous NH 4 CI solution.
- the aqueous layer was extracted with DCM.
- the combined organic layer was dried over magnesium sulfate, stirred with Siliabond DMT pd. Scavenger, filtered and dried on to silica.
- Phenylhydrazine (1.8 ml_, 18.49 mmol) was dissolved in MeOH (30 ml_). To this solution, a solution of oxalaldehyde (1.0 ml_, 9.25 mmol) in MeOH (10.0 ml_) was added, followed by AcOH (0.032 ml_, 0.555 mmol). The mixture was then stirred at room temperature overnight. The precipitate was collected by filtration, washed with MeOH and dried in the air to afford 1.1.5a (1.37 g, 31.1 % yield) as a pale yellow solid. LCMS (m/z) 239.1 [M+H] +
- Compound 1.1.10 was prepared following the procedures described for the synthesis 1.1.04 using 4,4,5,5-tetramethyl-2-(4-(((1 r,4r)-4-((tetrahydro-2H-pyran-2- yl)oxy)cyclohexyl)methoxy)phenyl)-1 ,3,2-dioxaborolane (ref. WO2011/73845 A1 , 201 1 ) in step 4.
- Compound 1.1.17 was prepared following the procedures described for the synthesis of 1.1.13 using (2-(4-(4,4, 5, 5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenyl)-2H-1 ,2,3-triazole (prepared as described in Example 1.1.05d) in Step 4 (Step 4 was changed to heating at 80C for 20h in a sand bath using THF as solvent). MS m/z 460.2 [M+H] + .
- Compound 1.1.18 was prepared following the procedures described for the synthesis of 1.1.13 using 2-(4-(4,4, 5, 5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenyl)-2H-1 ,2,3-triazole (prepared as described in Example 1.1.05d) in Step 4. (Step 4 was changed to heating at 80C for 20h in a sand bath using THF as solvent). MS m/z 456.3 [M+H] + .
- carboxamide 1 , 1 -dioxide was prepared following the procedures described for the synthesis of 1.2.1 using 3-phenylisoxazole-5-carbaldehyde in step 1 .
- 1 H NMR 400 MHz, DMSO-d 6 ) ⁇ 10.98 (br-s, NH), 7.86 - 7.83 (m, 2H), 7.51 - 7.49 (m, 3H), 7.09 (s, 1 H), 3.92 (s, 2H), 3.37-3.33 (m. , 2H), 3.01 - 2.97 (m, 2H), 2.39-2.25 (m. , 4H).
- step 1 To the reaction mixture from step 1 was added propargyl alcohol (0.22 mL, 7.55 mmol, 5 equiv) followed by triethylamine (1.05 mL, 7.55 mmol, 5 equiv). After heated at reflux for 1 hour, the reaction mixture was cooled to room temperature and diluted with water and ethyl acetate. Aqueous layer was extracted with ethyl acetate. Combined organic layer was washed with brine, dried with Na 2 S0 4 , filtered and concentrated in vacuo.
- Step 1 Synthesis of ethyl 4-(4-chloro-2-fluorophenyl)-2,4-dioxobutanoate.
- Step 2 Synthesis of ethyl 5-(4-chloro-2-fluorophenyl)isoxazole-3-carboxylate.
- Step 5 Synthesis of 4-(((5-(4-chloro-2-fluorophenyl)isoxazol-3- yl)methyl)amino)-A -hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide.
- N-Cholorosuccinimide (636 mg, 4.76 mmol) was added to a solution of Ethyl 5-(4-chloro-2-fluorophenyl)isoxazole-3-carboxylate (214 mg, 0.794 mmol) in acetic acid (3.9 mL) and the mixture was heated at 95 °C for 17 hours. After cooling to room temperature, the reaction mixture was quenched by adding water (30 mL) and the mixture was extracted with DCM. The organic layers were combined and concentrated. The residue was purified by silica gel column chromatography (EtOAc/heptane) to afford product 1.2.10a (217 mg, 90% yield). MS m/z 304.0 [M+H] + .
- 1.2.11a (4 g, 12.7 mmol, 1.0 equiv) and ethyl (E)-2-chloro-2-(hydroxyimino)acetate (1.92 g, 12.7 mmol, 1.0 equiv) were dissoved in diethyl ether (40 ml_).
- TEA (6.41 g, 63.5 mmol, 5.0 equiv) was added dropwise and the reaction mixture was stirred at room temperature for 4 hours. The reaction mixture was quenched with water and extracted with EtOAc. The organic layer was washed with brine, dried over sodium sulfate and concentrated.
- 1.4.1a (967 mg, 3.04 mmol) was dissolved in 1.0 M HCI solution in MeOH (9.1 ml_, 9.1 mmol) and the reaction solution was stirred at room temperature for 40 minutes. The reaction mixture was concentrated in vacuo and dried under high vacuum to afford 1.4.1 b (523 mg, 100 % yield) as a white solid.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
This invention pertains generally to antibacterial organic compounds of Formula I as described herein, and pharmaceutical compositions containing such compounds. In certain aspects, the invention pertains to treating infections caused by Gram-negative bacteria using these compounds and compositions.
Description
HYDROXAMIC ACID DERIVATIVES AS LpxC INHIBITORS FOR THE TREATMENT OF
BACTERIAL INFECTIONS
FIELD OF THE INVENTION
This invention pertains generally to treating bacterial infections. In certain aspects, the invention pertains to treating infections caused by Gram- negative bacteria. More specifically, the invention described herein pertains to treating Gram-negative infections by inhibiting the activity of UDP-3-0-(R-3-hydroxydecanoyl)-N-acetylglucosamine deacetylase (LpxC). The present invention provides small molecule inhibitors of LpxC, pharmaceutical formulations containing such inhibitors, methods of treating patients with such
pharmaceutical formulations, and methods of preparing such pharmaceutical formulations and inhibitors. The inhibitors can be used to treat Gram-negative infections of patients alone and in combination with other antibacterials.
BACKGROUND OF THE INVENTION
Over the past several decades, the frequency of antimicrobial resistance and its association with serious infectious diseases have increased at alarming rates. The increasing prevalence of resistance among nosocomial pathogens is particularly
disconcerting. Of the over 2 million nosocomial infections occuring each year in the United States, 50 to 60% are caused by antimicrobial-resistant strains of bacteria. The high rate of resistance to commonly used antibacterial agents increases the morbidity, mortality, and costs associated with nosocomial infections. In the United States, nosocomial infections are thought to contribute to or cause more than 77,000 deaths per year and cost approximately $5 to $10 billion annually. Among Gram-positive organisms, the most important resistant pathogens are methicillin-(oxacillin-) resistant Staphylococcus aureus, β-lactam-resistant and multidrug-resistant pneumococci, and vancomycin-resistant enterococci. Important causes of Gram-negative resistance include extended-spectrum β-lactamases (ESBLs) in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis, high-level third-generation cephalosporin (Amp C) β-lactamase resistance among Enterobacter species and Citrobacter freundii, and multidrug-resistance genes observed in Pseudomonas, Acinetobacter, and Stenotrophomonas.
The problem of antibacterial resistance is compounded by the existence of bacterial strains resistant to multiple antibacterials. For example, Pseudomonas aeruginosa isolates resistant to fluoroquinolones are virtually all resistant to additional antibacterial medicines.
Thus there is a need for new antibacterials, particularly antibacterials with novel mechanisms of action. Most of the antibacterial discovery effort in the pharmaceutical industry is aimed at the development of drugs effective against Gram-positive bacteria.
However, there is also a need for new Gram-negative antibacterials. Gram-negative bacteria are in general more resistant to a large number of antibacterials and chemotherapeutic agents than are gram-positive bacteria.
SUMMARY OF THE INVENTION
The present invention provides novel compounds, pharmaceutical formulations including the compounds, methods of inhibiting UDP-3-0-(R-3-hydroxydecanoyl)-N- acetylglucosamine deacetylase (LpxC), and methods of treating Gram-negative bacterial infections.
In one aspect, the invention provides compounds of Formula I:
A is a divalent radical selected from , and ;
X is -(CH2)nY(CH2)m-;
Y is selected from the group consisting of -C(H,R1)-, -0-, -N(R2)-, and -S(0)2- n is 0 or 1 ;
m is 0 or 1 ;
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, d- C4haloalkyl, C3-C7cycloalkyl, CrC4alkoxy, CrC4haloalkoxy, C C alkyl optionally substituted with Ci-C alkoxy, CrC alkoxy optionally substituted with Ci-C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, C C alkoxy, Cr C4haloalkoxy, CrC4haloalkyl or Ci-C4 alkyl; or
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C6-Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, Ci-C alkyl, CrC haloalkyl, Ci-C haloalkoxy or C
C4alkoxy;
R1 is selected from the group consisting of -OH, C C4alkoxy and -S(0)2R3;
R2 is selected from the group consisting of hydrogen, C C alkyl, -C(0)R3 and - S(0)2R3;
R3 is selected from the group consisting of C C4alkyl and C3-C6cycloalkyl;
Z is a divalent radical selected from
Z is with the proviso that A is
R5 is selected from the group consisting of hydrogen, halogen, -CN, d-C4alkyl, and C C4haloalkyl;
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, -CrC4alkyl, and CrC4haloalkyl;
L is a divalent bond, -CH2-, -O- or
In one aspect, the invention provides a method of inhibiting a deacetylase enzyme in Gram-negative bacteria, thereby affecting bacterial growth, comprising administering to a patient in need of such inhibition a compound of formula I.
In another aspect, the invention provides a method of inhibiting LpxC, thereby modulating the virulence of a bacterial infection, comprising administering to a patient in need of such inhibition a compound of formula I.
In another aspect, the invention provides a method for treating a subject with a Gram-negative bacterial infection comprising administering to the subject in need thereof an antibacterially effective amount of a compound of formula I with a pharmaceutically acceptable carrier. In certain embodiments, the subject is a mammal and in some other embodiments, the subject is a human.
In another aspect, the invention provides a method of administering an inhibitory amount of a compound of formula I to fermentative or non-fermentative Gram-negative bacteria. In certain embodiment of the method of administering an inhibitory amount of a compound of formula I to fermentative or non-fermentative Gram-negative bacteria, the Gram-negative bacteria are selected from the group consisting of Pseudomonas aeruginosa and other Pseudomonas species, Stenotrophomonas maltophilia, Burkholderia cepacia and other Burkholderia species, Alcaligenes xylosoxidans, species of Acinetobacter,
Enterobacteriaceae, Haemophilus, Moraxella, Bacteroides, Fransicella, Shigella, Proteus, Vibrio, Salmonella, Bordetella, Helicobactor, Legionella, Citrobactor, Serratia,
Campylobactor, Yersinia and Neisseria.
In another embodiment, the invention provides a method of administering an inhibitory amount of a compound of formula I to Gram-negative bacteria, such as
Enterobacteriaceae which is selected from the group consisting of organisms such as Serratia, Proteus, Klebsiella, Enterobacter, Citrobacter, Salmonella, Providencia,
Morganella, Cedecea, Yersina and Edwardsiella species and Escherichia coli.
Another embodiment of the invention provides a pharmaceutical composition comprising an effective amount of a compound of Formula I with a pharmaceutically acceptable carrier thereof.
Pharmaceutical formulations according to the present invention are provided which include any of the compounds described above and a pharmaceutically acceptable carrier.
Other aspects of the invention are discussed infra.
The present invention provides novel compounds, methods for inhibiting LpxC in Gram-negative bacteria, and novel methods for treating bacterial infections. The compounds provided herein can be formulated into pharmaceutical formulations and medicaments that are useful in the methods of the invention. The invention also provides for the use of the compounds in preparing medicaments and pharmaceutical formulations, for use of the compounds in inhibiting LpxC, and for use of the compounds in treating bacterial infections in a subject.
The following abbreviations and definitions are used throughout this application: "LpxC" is an abbreviation that stands for UDP-3-0-(R-3-hydroxydecan- oyl)-N- acetylglucosamine deacetylase.
This invention is directed to compounds of Formula l-V and subformulae thereof, and intermediates thereto, as well as pharmaceutical compositions containing the compounds for use in treatment of bacterial infections. This invention is also directed to the compounds of the invention or compositions thereof as LpxC inhibitors. The compounds are particularly useful in interfering with the life cycle of Gram-negative bacteria and in treating or preventing a Gram-negative bacterial infection or physiological conditions associated therewith. The present invention is also directed to methods of combination therapy for treating or preventing an Gram-negative bacterial infection in patients using the compounds of the invention or pharmaceutical compositions, or kits thereof in combination with at least one other therapeutic agent.
DETAILED DESCRIPTION OF THE INVENTION
For purposes of interpreting this specification, the following definitions will apply unless specified otherwise and whenever appropriate, terms used in the singular will also include the plural and vice versa.
Definitions
Terms used in the specification have the following meanings:
As used herein, the term "subject" refers to an animal. In certain aspects, the animal is a mammal. A subject also refers to for example, primates (e.g., humans), cows, sheep, goats, horses, dogs, cats, rabbits, rats, mice, fish, birds and the like. In certain
embodiments, the subject is a human.
As used herein, the term "inhibition" or "inhibiting" refers to the reduction or suppression of a given condition, symptom, or disorder, or disease, or a significant decrease in the baseline activity of a biological activity or process.
As used herein, the term "treating" or "treatment" of any disease or disorder refers in one embodiment, to ameliorating the disease or disorder (i.e., slowing or arresting or
reducing the development of the disease or at least one of the clinical symptoms thereof). In another embodiment "treating" or "treatment" refers to alleviating or ameliorating at least one physical parameter including those which may not be discernible by the patient. In yet another embodiment, "treating" or "treatment" refers to modulating the disease or disorder, either physically, (e.g., stabilization of a discernible symptom), physiologically, (e.g., stabilization of a physical parameter), or both. In yet another embodiment, "treating" or "treatment" refers to preventing or delaying the onset or development or progression of the disease or disorder.
As used herein, the term "a," "an," "the" and similar terms used in the context of the present invention (especially in the context of the claims) are to be construed to cover both the singular and plural unless otherwise indicated herein or clearly contradicted by the context.
All methods described herein can be performed in any suitable order unless otherwise indicated herein or otherwise clearly contradicted by context. The use of any and all examples, or exemplary language (e.g. "such as") provided herein is intended merely to better illuminate the invention and does not pose a limitation on the scope of the invention otherwise claimed.
The term "antibacterial agent" refers to agents synthesized or modified in the laboratory that have either bactericidal or bacteriostatic activity. An "active" agent in this context will inhibit the growth of P. aeruginosa and / or other Gram-negative bacteria. The term "inhibiting the growth" indicates that the rate of increase in the numbers of a population of a particular bacterium is reduced. Thus, the term includes situations in which the bacterial population increases but at a reduced rate, as well as situations where the growth of the population is stopped, as well as situations where the numbers of the bacteria in the population are reduced or the population even eliminated. If an enzyme activity assay is used to screen for inhibitors, one can make modifications in bacterial uptake/efflux, solubility, half-life, etc. to compounds in order to correlate enzyme inhibition with growth inhibition.
"Optionally substituted" means the group referred to can be substituted at one or more positions by any one or any combination of the radicals listed thereafter.
"Halo" or "halogen", as used herein, may be fluorine, chlorine, bromine or iodine.
"CrC6-Alkyl", as used herein, denotes straight chain or branched alkyl having 1-8 carbon atoms. If a different number of carbon atoms is specified, such as Ce or C3, then the definition is to be amended accordingly, such as "CrC4-Alkyl" will represent methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl and tert-butyl.
"CrC6-Alkoxy", as used herein, denotes straight chain or branched alkoxy having 1-8 carbon atoms. If a different number of carbon atoms is specified, such as C6 or C3, then the
edefinition is to be amended accordingly, such as "CrC4-Alkoxy" will represent methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, sec-butoxy and tert-butoxy.
"CrC4-Haloalkyl", as used herein, denotes straight chain or branched alkyl having 1 - 4 carbon atoms with at least one hydrogen substituted with a halogen. If a different number of carbon atoms is specified, such as C6 or C3, then the definition is to be amended accordingly, such as "Ci-C4-Haloalkyl" will represent methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl and tert-butyl that have at least one hydrogen substituted with halogen, such as where the halogen is fluorine: CF3CF2-, (CF3)2CH-, CH3-CF2-, CF3CF2-, CF3, CF2H-, CF3CF2CHCF3 or CF3CF2CF2CF2-.
"C3-C8-cycloalkyl" as used herein refers to a saturated monocyclic hydrocarbon ring of 3 to 8 carbon atoms. Examples of such groups include cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. If a different number of carbon atoms is specified, such as C3-C6, then the definition is to be amended accordingly.
"4- to 8-Membered heterocyclyl", "5- to 6- membered heterocyclyl", "3- to 10- membered heterocyclyl", "3- to 14-membered heterocyclyl", "4- to 14-membered
heterocyclyl" and "5- to 14-membered heterocyclyl", refers, respectively, to 4- to 8- membered, 5- to 6-membered, 3- to 10-membered, 3- to 14-membered, 4- to 14-membered and 5- to 14-membered heterocyclic rings containing 1 to 7, 1 to 5 or 1 to 3 heteroatoms selected from the group consisting of nitrogen, oxygen and sulphur, which may be saturated, or partially saturated. The heterocyclic group can be attached at a heteroatom or a carbon atom. The term "heterocyclyl" includes single ring groups, fused ring groups and bridged groups. Examples of such heterocyclyl include, but are not limited to pyrrolidine, piperidine, piperazine, pyrrolidine, pyrrolidinone, morpholine, tetrahydrofuran, tetrahydrothiophene, tetrahydrothiopyran, tetrahydropyran, 1 ,4-dioxane, 1 ,4-oxathiane, 8-aza- bicyclo[3.2.1 ]octane, 3,8-diazabicyclo[3.2.1 ]octane, 3-Oxa-8-aza-bicyclo[3.2.1 ]octane, 8- Oxa-3-aza-bicyclo[3.2.1 ]octane, 2-Oxa-5-aza-bicyclo[2.2.1 ]heptane, 2,5-Diaza- bicyclo[2.2.1 ]heptane, azetidine, ethylenedioxo, oxtane or thiazole.
"Heteroaryl" is a completely unsaturated (aromatic) ring. The term "heteroaryl" refers to a 5-14 membered monocyclic- or bicyclic- or tricyclic-aromatic ring system, having 1 to 8 heteroatoms selected from N, O or S. Typically, the heteroaryl is a 5-10 membered ring system (e.g., 5-7 membered monocycle or an 8-10 membered bicycle) or a 5-7 membered ring system. Typical heteroaryl groups include furan, isotriazole, thiadiazole, oxadiazole, indazole, indazole, indole, quinoline, 2- or 3-thienyl, 2- or 3-furyl, 2- or 3-pyrrolyl, 2-, 4-, or 5- imidazolyl, 3-, 4-, or 5- pyrazolyl, 2-, 4-, or 5-thiazolyl, 3-, 4-, or 5-isothiazolyl, 2-, 4-, or 5- oxazolyl, 3-, 4-, or 5-isoxazolyl, 3- or 5-(1 ,2,4-triazolyl), 4- or 5-(1 ,2, 3-triazolyl), tetrazolyl, triazine, pyrimidine, 2-, 3-, or 4-pyridyl, 3- or 4-pyridazinyl, 3-, 4-, or 5-pyrazinyl, 2-pyrazinyl, and 2-, 4-, or 5-pyrimidinyl.
The term "hydroxy" or "hydroxyl" includes groups with an -OH.
The term "a," "an," "the" and similar terms used in the context of the present invention (especially in the context of the claims) are to be construed to cover both the singular and plural unless otherwise indicated herein or clearly contradicted by the context.
Various embodiments of the invention are described herein. It will be recognized that features specified in each embodiment may be combined with other specified features to provide further embodiments.
In one embodiment, the invention provides compounds of Formula I:
A is a divalent radical selected from and
X is -(CH2)nY(CH2)m-;
Y is selected from the group consisting of -C(H,R1)-, -0-, S, -N(R2)-, and -S(0)2- n is 0 or 1 ; m is 0 or 1 ;
R is C3-6 cycloalkyi, -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said cycloalkyi, aryl and heteroaryl are each optionally substituted with up to three substituents selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, C C4haloalkoxy, C C4alkyl optionally substituted with Ci-C4alkoxy or a 5-6 membered heterocycle containing up to two heteroatoms selected from N, O and S as ring members and optionally substituted with R10, CrC4alkoxy optionally substituted with d-C4alkoxy or Ci_ 3 alkyl or C3-e cycloalkyi where the C1-3 alkyl or C3-e cycloalkyi are each optionally substituted with hydroxy, methoxy, or methyl, and a 4 to 7 membered heterocycle or a 5 to 6 membered heteroaryl wherein the 4 to 7 membered heterocycle or 5 to 6 membered heteroaryl
contains 1 to 3 heteroatoms selected from N, S, and O as ring members and is optionally substituted with one or more halogen, CrC4alkoxy, CrC4haloalkoxy, CrC4haloalkyl or C C4 alkyl; or
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C6-Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with one or two groups selected from halogen, Ci-C alkyl, d- C haloalkyl, C C haloalkoxy and CrC alkoxy;
R1 is selected from the group consisting of -OH, C C alkoxy and -S(0)2R3;
R2 is selected from the group consisting of hydrogen, CrC4alkyl, -C(0)OR3 , -C(0)R3 and -S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl;
Z is a divalent radical selected from
with the proviso that A is
R5 is selected from the group consisting of hydrogen, halogen, -CN, d-dalkyl, and CrC4haloalkyl;
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, -CrC4alkyl, and CrC4haloalkyl;
R10 is selected from halo, C^ alkyl, d-4 haloalkyl, d^ alkoxy, -C(0)R11 and -C(O)-
OR11 ;
R11 is d^ alkyl; and '
L is a divalent bond, -CH2-, -O- or
A is a divalent radical selected from and
X is -(CH2)nY(CH2)m-;
Y is selected from the group consisting of -C(H,R1)-, -0-, -N(R2)-, and -S(0)2- n is 0 or 1 ; m is 0 or 1 ;
R is -C6-d0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said cycloalkyi, aryl and heteroaryl are each optionally substituted with substituents selected from the group consisting of halogen, -OH, -CN, - S(0)2(d-d)alkyl, d-dhaloalkyl, d-dcycloalkyl, d-dalkoxy, d-dhaloalkoxy, d-dalkyl optionally substituted with d-dalkoxy, d-dalkoxy optionally substituted with d-dalkoxy,
and a 4 to 7 membered heterocycle wherein the 4 to 7 membered heterocycle contains 1 to 3 heteroatoms selected from N, S, and O as ring members and is optionally substituted with one or more halogen, d-C4alkoxy, CrC4haloalkoxy, CrC4haloalkyl or C C4 alkyl; or
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C6-Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with groups selected from halogen, Ci-C alkyl, Ci-C haloalkyl, Ci- C haloalkoxy and C C alkoxy;
R1 is selected from the group consisting of -OH, C C alkoxy and -S(0)2R3;
R2 is selected from the group consisting of hydrogen, CrC4alkyl, -C(0)R3 and - S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl;
Z is a divalent radical selected from
Z is with the proviso that A is
R5 is selected from the group consisting of hydrogen, halogen, -CN, d-C4alkyl, and CrC4haloalkyl;
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, -CrC4alkyl, and CrC4haloalkyl; and '
L is a divalent bond, -CH2-, -O- or
In certain emodiments of the compounds of Formula I, R is phenyl substituted with one or two substituents selected from the group consisting of halogen, -OH, -CN, -S(0)2(Cr C4)alkyl, CrC4haloalkyl, C3-C7cycloalkyl, C C alkoxy, CrC haloalkoxy, and CrC alkyl optionally substituted with C C alkoxy.
In certain emodiments of the compounds of Formula I, X is -CH2-SO2-CH2-. In some of these embodiments, A is [Z]-CH2-NH-, where [Z] indicates the point where A attaches to Z in Formula I.
In certain emodiments of any of the compounds of Formula I as described above, Z
is or . In these embodiments, R5 is preferably
H, and R6, R6a, R6b and R6c can all be H.
In certain emodiments of any of the compounds of Formula I as described above, L is a bond.
In an embodiment of the invention, the compound or a pharmaceutically acceptable salt represented by formula II:
wherein Q is selected from the group consisting of
and
R2 is selected from the group consisting of hydrogen, C C4alkyl, -C(0)CR3 and
S(0)2R3;
R3 is selected from the group consisting of CrC4alkyl and C3-C6cycloalkyl.
In an embodiment of the invention, the compound or a pharmaceutically acceptable salt represented by formula II:
A is a divalent radical selected from , and ;
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, Ci-C4haloalkoxy, Ci-C4alkyl optionally substituted with Ci-C4alkoxy, Ci-C4alkoxy optionally substituted with C C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
R is phenyl susbstituted by taking the substituents on adjacent atoms of the -C6- Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy;
R2 is selected from the group consisting of hydrogen, C C alkyl, -C(0)CR3 and - S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl;
Z is a divalent radical selected from
R5 is selected from the group consisting of hydrogen, halogen, -CN, Ci-C alkyl, and
CrC4haloalkyl;
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, CrC4alkyl and CrC4haloalkyl;
L is a divalent bond, -CH2-, -O- or
In certain emodiments of the compounds of Formula II described above, R is phenyl substituted with one or two substituents selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, C C4haloalkoxy, and CrC alkyl optionally substituted with Ci-C alkoxy.
In certain emodiments of these compounds of Formula II, Q is . In some of these embodiments, A is [Z]-CH2-NH-, where [Z] indicates the point where A attaches to Z in Formula II.
In certain emodiments of any of the compounds of Formula II as described above, Z
is or In these embodiments, R5 is preferably
H, and R6, R6a, R6b and R6c can all be H.
In certain emodiments of any of the compounds of Formula II as described above, L is a bond.
In an embodiment of the invention, the compound or a pharmaceutically acceptable salt represented by formula II:
wherein Q is selected from the group consisting of
A is a divalent radical selected from , and
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, d- C4alkoxy, CrC haloalkoxy, C C alkyl optionally substituted with C C alkoxy, C C alkoxy optionally substituted with C C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
R is phenyl susbstituted by taking the substituents on adjacent atoms of the -C6-
Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C4alkyl, CrC4haloalkyl, CrC4haloalkoxy or CrC4alkoxy;
R2 is selected from the group consisting of hydrogen, C C alkyl and -S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl.
Z is a divalent radical selected from
, and ;
R5 is selected from the group consisting of hydrogen, halogen, C C alkyl, and C C haloalkyl;
L is a divalent bond.
In an embodiment of the invention, the compound or a pharmaceutically acceptable salt represented by formula II:
II
wherein Q is selected from the group consisting of
A is
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, Ci-C4haloalkoxy, Ci-C4alkyl optionally substituted with Ci-C4alkoxy, Ci-C4alkoxy optionally substituted with C C alkoxy, and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy;
R2 is selected from the group consisting of hydrogen, CrC4alkyl and -S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl.
, and
R5 is selected from the group consisting of hydrogen, halogen, CrC4alkyl, and C C4haloalkyl;
L is a divalent bond.
In an embodiment of the invention, the compound or a pharmaceutically acceptable salt represented by formula II:
II wherein Q is selected from the group consisting of
, and
A is ;
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, Ci-C4haloalkoxy, Ci-C4alkyl optionally substituted with Ci-C4alkoxy, Ci-C4alkoxy optionally substituted with C C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl;
R2 is selected from the group consisting of hydrogen, C C alkyl and -S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl.
Z is a divalent radical selected from
, and
R5 is selected from the group consisting of hydrogen, halogen, -CN, d-C4alkyl, and CrC4haloalkyl;
L is a divalent bond.
In an embodiment of the invention, the compound or a pharmaceutically acceptable salt represented by formula II:
R2 is selected from the group consisting of hydrogen, CrC4alkyl and -S(0)2R3; R3 is selected from the group consisting of CrC4alkyl and C3-C6cycloalkyl. Z is a divalent radical selected from
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, CrC4alkyl, CrC4haloalkyl; '
L is a divalent bond or
In an embodiment of the invention, the compound or a pharmaceutically acceptable salt thereof represented by formula III
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, Ci- C4haloalkyl, C3-C7cycloalkyl, CrC4alkoxy, CrC4haloalkoxy, C C alkyl optionally substituted with Ci-C alkoxy, CrC alkoxy optionally substituted with Ci-C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, C C alkoxy, Cr C4haloalkoxy, CrC4haloalkyl or Ci-C4 alkyl; or
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C6-Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, Ci-C alkyl, CrC haloalkyl, Ci-C haloalkoxy or C C4alkoxy;
R2 is selected from the group consisting of hydrogen, C C alkyl and -S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl;
R5 is selected from the group consisting of hydrogen, halogen, -CN, -OH, Ci-C alkyl, CrC haloalkyl, Ci-C haloalkoxy, CrC alkoxy and C3-C7cycloalkyl optionally substituted with halogen or Ci-C alkyl; '
L is a direct bond, -CH2-, -O- or
In certain emodiments of the compounds of Formula III, R is phenyl substituted with one or two substituents selected from the group consisting of halogen, -OH, -CN, -S(0)2(C C )alkyl, Ci-C haloalkyl, C3-C7cycloalkyl, Ci-C alkoxy, Ci-C haloalkoxy, and Ci-C alkyl optionally substituted with C C alkoxy.
In certain emodiments of these compounds of Formula III, Q is
In certain emodiments of any of the compounds of Formula III as described above, L is a bond.
In an embodiment of the invention, the compound or a pharmaceutically acceptable salt thereof according to any proceeding claim, wherein
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, Ci-C4haloalkoxy, Ci-C4alkyl optionally substituted with Ci-C4alkoxy, Ci-C4alkoxy optionally substituted with C C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
R is phenyl susbstituted by taking the substituents on adjacent atoms of the -C6- Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy.
In an embodiment of the invention, the compound or a pharmaceutically acceptable salt thereof according to any proceeding claim, wherein the compound is formula III
III
Q is selected from the group consisting of
and ;
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, CrC haloalkoxy, C C alkyl optionally substituted with C C alkoxy, C C alkoxy optionally substituted with C C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
R is phenyl susbstituted by taking the substituents on adjacent atoms of the -C6- Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, Ci- C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy;
R2 is selected from the group consisting of hydrogen, C C alkyl and -S(0)2R3;
R3 is selected from the group consisting of CrC4alkyl and Cs-Cecycloalkyl; '
L is a direct bond, -CH2-, -O- or
In an embodiment of the invention, the compound or a pharmaceutically acceptable salt thereof wherein the compound is formula III
, and
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, CrC haloalkoxy, C C alkyl optionally substituted with C C alkoxy, C C alkoxy optionally substituted with Ci-C4alkoxy, and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxyR2 is selected from the group consisting of hydrogen, Ci-C alkyl
and -S(0)2R3;
R3 is selected from the group consisting of CrC4alkyl and C3-C6cycloalkyl;
L is a direct bond or
In an embodiment of the invention, the compound represented by formula IV
, and
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, Ci- C4haloalkyl, C3-C7cycloalkyl, CrC4alkoxy, CrC4haloalkoxy, C C alkyl optionally substituted with Ci-C alkoxy, CrC alkoxy optionally substituted with Ci-C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, C C alkoxy, Cr C4haloalkoxy, CrC4haloalkyl or Ci-C4 alkyl; or
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C6-Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, Ci-C alkyl, CrC haloalkyl, Ci-C haloalkoxy or C C4alkoxy;
R2 is selected from the group consisting of hydrogen, C C alkyl, -C(0)CR3 and - S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl;
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, -CN, C C alkyl, C3-C7cycloalkyl, C C alkoxy and CrC haloalkyl;
L is a direct bond, -CH2-, -O- or
, and
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, CrC haloalkoxy, C Calkyl optionally substituted with C C alkoxy, C Calkoxy optionally substituted with C Calkoxy, and a 4 to 7 membered heterocycle containing 1 to 3
heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, d-C4alkoxy, CrC4haloalkoxy, CrC4haloalkyl or C C4 alkyl; or
R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxyR2 is selected from the group consisting of hydrogen, Ci-C alkyl, -C(0)CR3 and -S(0)2R3;
R3 is selected from the group consisting of CrC4alkyl and Cs-Cecycloalkyl;
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, C C alkyl, and CrC haloalkyl;
L is a direct bond, or
In certain emodiments of the compounds of Formula IV, R is phenyl substituted with one or two substituents selected from the group consisting of halogen, -OH, -CN, -S(0)2(Cr C )alkyl, Ci-C haloalkyl, C3-C7cycloalkyl, C C alkoxy, CrC haloalkoxy, and CrC alkyl optionally substituted with C C alkoxy.
In certain emodiments of these compounds of Formula III, Q is
In certain emodiments of any of the compounds of Formula IV as described above, L is a bond.
In an embodiment of the invention, the compound is of formula V
wherein Q is selected from the group consisting of
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, d- C4haloalkyl, C3-C7cycloalkyl, CrC4alkoxy, CrC4haloalkoxy, C C alkyl optionally substituted with Ci-C alkoxy, CrC alkoxy optionally substituted with Ci-C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, C C alkoxy, Cr C4haloalkoxy, CrC4haloalkyl or Ci-C4 alkyl; or
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C6-Ci0aryl, or 4 to 10 membered heteroaryl and
forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, d-C4alkyl, CrC4haloalkyl, CrC4haloalkoxy or C C4alkoxy;
R2 is selected from the group consisting of hydrogen, CrC4alkyl, -C(0)CR3 and - S(0)2R3;
R3 is selected from the group consisting of CrC4alkyl and C3-C6cycloalkyl.
In an embodiment of the invention, the compound, the compound of formula V
wherein Q is selected from the group consisting of
, and
R is phenyl optionally substituted with halogen, Ci-4alkyl or Ci-C4alkoxy;
R2 is selected from the group consisting of hydrogen, CrC4alkyl, -C(0)CR3 and - S(0)2R3;
R3 is selected from the group consisting of C C4alkyl and Cs-Cecycloalkyl.
In an embodiment of the invention, the compound of formula I to V, wherein L is a direct bond;
R is selected from the group consisting of
In an embodiment of the invention, the compound of formula I to V, wherein L is a direct bond;
R is selected from the group consisting of
In an embodiment of the invention, the compound is of formula I to V, wherein L is a direct bond;
R is selected from the group consisting of
, and
In another embodiment of the invention, the compound according to formula I to IV or a pharmaceutically acceptable salt thereof represented by
4-(([1 , 1 '-biphenyl]-4-ylmethyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4- carboxamide 1 , 1 -dioxide;
N-hydroxy-4-(((4'-methoxy-[1 , 1 '-biphenyl]-4-yl)methyl)amino)tetrahydro-2H-thiopyran- 4-carboxamide 1 , 1 -dioxide;
4-(((4'-chloro-2'-fluoro-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
N-hydroxy-4-(((4'-(morpholinomethyl)-[1 , 1 '-biphenyl]-4-yl)methyl)amino)tetrahydro- 2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((4'-(2H-1 ,2,3-triazol-2-yl)-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydro- 2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
N-hydroxy-4-(((4'-morpholino-[1 , 1 '-biphenyl]-4-yl)methyl)amino)tetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((4'-cyclopropyl-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
N-hydroxy-4-((4-(naphthalen-2-yl)benzyl)amino)tetrahydro-2H-thiopyran-4- carboxamide 1 ,1 -dioxide;
4-(((2'-fluoro-4'-methoxy-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 ,1 -dioxide;
N-hydroxy-4-(((4'-(((1 r,4r)-4-hydroxycyclohexyl)methoxy)-[1 ,1 '-biphenyl]-4- yl)methyl)amino)tetrahydro-2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((2'-fluoro-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4- carboxamide 1 ,1 -dioxide;
N-hydroxy-4-((4-(pyridin-4-yl)benzyl)amino)tetrahydro-2H hiopyran-4-carboxamide 1 ,1 -dioxide;
4-(((2-fluoro-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4- carboxamide 1 ,1 -dioxide;
4-(((2-fluoro-4'-methoxy-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 ,1 -dioxide;
4-(((2,2'-difluoro-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 ,1 -dioxide;
4-(((4'-chloro-2,2'-difluoro-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 ,1 -dioxide;
4-(((2-fluoro-4'-(2H-1 ,2,3-triazol-2-yl)-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide;
N-hydroxy-4-(((2-methyl-4'-(2H-1 ,2,3-triazol-2-yl)-[1 , 1 '-biphenyl]-4- yl)methyl)amino)tetrahydro-2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((4'-chloro-2'-fluoro-2-methyl-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide;
4-(((2'-fluoro-4'-methoxy-2-methyl-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide;
4-(((4'-cyclopropyl-2-methyl-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydro- 2H-thiopyran-4-carboxamide 1 ,1 -dioxide;
4-(((2-chloro-2'-fluoro-4'-methoxy-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide;
N-hydroxy-4-((4-(phenylethynyl)benzyl)amino)tetrahydro-2H-thiopyran-4- carboxamide 1 ,1 -dioxide;
N-hydroxy-4-(((5-phenylisoxazol-3-yl)methyl)amino)tetrahydro-2H-thiopyran-4- carboxamide 1 ,1 -dioxide;
N-hydroxy-4-(((3-phenylisoxazol-5-yl)methyl)amino)tetrahydro-2H-thiopyran-4- carboxamide 1 ,1 -dioxide;
4-(((5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(2-fluoro-4-methoxyphenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro- 2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(4-chloro-2-fluorophenyl)-4-fluoroisoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(4-(2H-1 ,2,3-triazol-2-yl)phenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((4-chloro-5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
N-hydroxy-4-((( 1 -phenyl-1 H-1 ,2,3-triazol-4-yl)methyl)amino)tetrahydro-2H-thiopyran- 4-carboxamide 1 , 1 -dioxide;
4-(2-(4-(4-chloro-2-fluorophenyl)-2-oxopyridin-1 (2H)-yl)ethyl)-N-hydroxytetrahydro- 2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
3-(([1 , 1 '-biphenyl]-4-ylmethyl)amino)-N-hydroxytetrahydrothiophene-3-carboxamide;
N-hydroxy-3-(((5-phenylisoxazol-3-yl)methyl)amino)tetrahydrothiophene-3- carboxamide 1 , 1 -dioxide;
N-hydroxy-3-(((3-phenylisoxazol-5-yl)methyl)amino)tetrahydrothiophene-3- carboxamide 1 , 1 -dioxide;
N-hydroxy-3-((4-(phenylethynyl)benzyl)amino)tetrahydrothiophene-3-carboxamide 1 , 1 -dioxide;
3- (((4'-chloro-2'-fluoro-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N- hydroxytetrahydrothiophene-3-carboxamide 1 , 1 -dioxide;
1 -acetyl-N-hydroxy-4-(2-(2-oxo-4-phenylpyridin-1 (2H)-yl)ethyl)piperidine-4- carboxamide;
N-hydroxy-1 -(methylsulfonyl)-4-(2-(2-oxo-4-phenylpyridin-1 (2H)-yl)ethyl)piperidine-4- carboxamide;
4- ([1 , 1 '-biphenyl]-4-ylmethyl)-N-hydroxy-1 -(methylsulfonyl)piperidine-4-carboxamide; (1 S,4S)-1 -(([1 , 1 '-biphenyl]-4-ylmethyl)amino)-N,4- dihydroxycyclohexanecarboxamide;
(1 R,4R)-1 -(([1 , 1 '-biphenyl]-4-ylmethyl)amino)-N,4- dihydroxycyclohexanecarboxamide;
(1 R,4R)-1 -(([1 , 1 '-biphenyl]-4-ylmethyl)amino)-N-hydroxy-4- methoxycyclohexanecarboxamide;
3-(((4'-chloro-2'-fluoro-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxytetrahydrofuran-3- carboxamide;
3- (((4'-chloro-2'-fluoro-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxyoxetane-3- carboxamide;
N-hydroxy-4-(2-(2-oxo-4-phenylpyridin-1 (2H)-yl)ethyl)tetrahydro-2H-pyran-4- carboxamide;
4- (2-([1 , 1 '-biphenyl]-4-yl)ethyl)-N-hydroxytetrahydro-2H-pyran-4-carboxamide; and 4-(([1 , 1 '-biphenyl]-4-ylmethyl)amino)-N-hydroxytetrahydro-2H-pyran-4-carboxamide.
Additional embodiments include:
4-(((5-(4-(difluoromethoxy)phenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro- 2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
N-hydroxy-4-(((5-(4-methoxyphenyl)isoxazol-3-yl)methyl)amino)tetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(4-fluoro-3-methoxyphenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro- 2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(2-fluoro-4-methylphenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(2,3-dichlorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(2,4-difluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
N-hydroxy-4-(((5-(m-tolyl)isoxazol-3-yl)methyl)amino)tetrahydro-2H-thiopyran-4- carboxamide 1 , 1 -dioxide;
N-hydroxy-4-(((5-(3-methoxyphenyl)isoxazol-3-yl)methyl)amino)tetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(2,2-difluorobenzo[d][1 ,3]dioxol-5-yl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(3-chloro-5-fluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(3-chlorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(4-chloro-2,3-difluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro- 2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(2-chloro-4-fluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(2,5-difluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(2-fluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(2-fluoro-4-(trifluoromethoxy)phenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(3-fluoro-4-methoxyphenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro- 2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
N-hydroxy-4-(((5-(2,3^-trifluorophenyl)isoxazol-3-yl)methyl)amino)tetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(4-cyanophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(2,6-difluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
N-hydroxy-4-(((5-(5-methylthiophen-2-yl)isoxazol-3-yl)methyl)amino)tetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
N-hydroxy-4-(((5-(4-methylthiophen-2-yl)isoxazol-3-yl)methyl)amino)tetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(((5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-((4-(cyclopropylethynyl)benzyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4- carboxamide 1 , 1 -dioxide;
4-(2-(4-(2-fluoro-4-methoxyphenyl)-2-oxopyridin-1 (2H)-yl)ethyl)-N-hydroxytetrahydro- 2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
N-hydroxy-4-(2-(4-phenyl-1 H-pyrazol-1 -yl)ethyl)tetrahydro-2H-thiopyran-4- carboxamide 1 , 1 -dioxide;
4-(2-(4-(4-chlorophenyl)-2H-1 ,2,3-triazol-2-yl)ethyl)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide;
4-(2-([1 , 1 '-biphenyl]-4-yl)ethyl)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 , 1 -dioxide;
3-(((5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydrothiophene-3-carboxamide 1 , 1 -dioxide;
3-(((4'-chloro-2'-fluoro-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-hydroxy-1 - (methylsulfonyl)azetidine-3-carboxamide;
1 -(((2'-fluoro-4'-methoxy-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N,3- dihydroxycyclobutanecarboxamide;
3-(2-(4'-chloro-2'-fluoro-[1 , 1 '-biphenyl]-4-yl)ethyl)-N-hydroxy-1 - (methylsulfonyl)pyrrolidine-3-carboxamide; and
1-(((5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methyl)amino)-N-hydroxy-3- (methylsulfonyl)cyclobutanecarboxamide.
Each of the compounds in Table 1 is a specific embodiment of the invention.
The compounds as defined in embodiments may be synthesized by the general synthetic routes below, specific examples of which are described in more detail in the Examples.
The invention further includes any variant of the present processes, in which an intermediate product obtainable at any stage thereof is used as starting material and the remaining steps are carried out, or in which the starting materials are formed in situ under the reaction conditions, or in which the reaction components are used in the form of their salts or optically pure material.
Compounds of the present invention and intermediates can also be converted into each other according to methods generally known to those skilled in the art.
Within the scope of this text, only a readily removable group that is not a constituent of the particular desired end product of the compounds of the present invention is designated a "protecting group", unless the context indicates otherwise. The protection of functional groups by such protecting groups, the protecting groups themselves, and their cleavage reactions are described for example in standard reference works, such as J. F. W. McOmie, "Protective Groups in Organic Chemistry", Plenum Press, London and New York 1973, in T. W. Greene and P. G. M. Wuts, "Protective Groups in Organic Synthesis", Third edition, Wiley, New York 1999, in "The Peptides"; Volume 3 (editors: E. Gross and J.
Meienhofer), Academic Press, London and New York 1981 , in "Methoden der organischen Chemie" (Methods of Organic Chemistry), Houben Weyl, 4th edition, Volume 15/1, Georg Thieme Verlag, Stuttgart 1974, in H.-D. Jakubke and H. Jeschkeit, "Aminosauren, Peptide, Proteine" (Amino acids, Peptides, Proteins), Verlag Chemie, Weinheim, Deerfield Beach, and Basel 1982, and in Jochen Lehmann, "Chemie der Kohlenhydrate: Monosaccharide und Derivate" (Chemistry of Carbohydrates: Monosaccharides and Derivatives), Georg Thieme Verlag, Stuttgart 1974. A characteristic of protecting groups is that they can be removed readily (i.e. without the occurrence of undesired secondary reactions) for example by solvolysis, reduction, photolysis or alternatively under physiological conditions (e.g. by enzymatic cleavage).
Salts of compounds of the present invention having at least one salt-forming group may be prepared in a manner known to those skilled in the art. For example, salts of compounds of the present invention having acid groups may be formed, for example, by
treating the compounds with metal compounds, such as alkali metal salts of suitable organic carboxylic acids, e.g. the sodium salt of 2-ethylhexanoic acid, with organic alkali metal or alkaline earth metal compounds, such as the corresponding hydroxides, carbonates or hydrogen carbonates, such as sodium or potassium hydroxide, carbonate or hydrogen carbonate, with corresponding calcium compounds or with ammonia or a suitable organic amine, stoichiometric amounts or only a small excess of the salt-forming agent preferably being used. Acid addition salts of compounds of the present invention are obtained in customary manner, e.g. by treating the compounds with an acid or a suitable anion exchange reagent. Internal salts of compounds of the present invention containing acid and basic salt-forming groups, e.g. a free carboxy group and a free amino group, may be formed, e.g. by the neutralisation of salts, such as acid addition salts, to the isoelectric point, e.g. with weak bases, or by treatment with ion exchangers.
Salts can be converted into the free compounds in accordance with methods known to those skilled in the art. Metal and ammonium salts can be converted, for example, by treatment with suitable acids, and acid addition salts, for example, by treatment with a suitable basic agent.
Mixtures of isomers obtainable according to the invention can be separated in a manner known to those skilled in the art into the individual isomers; diastereo isomers can be separated, for example, by partitioning between polyphasic solvent mixtures, recrystallisation and/or chromatographic separation, for example over silica gel or by e.g. medium pressure liquid chromatography over a reversed phase column, and racemates can be separated, for example, by the formation of salts with optically pure salt-forming reagents and separation of the mixture of diastereoisomers so obtainable, for example by means of fractional crystallisation, or by chromatography over optically active column materials.
Intermediates and final products can be worked up and/or purified according to standard methods, e.g. using chromatographic methods, distribution methods, (re-) crystallization, and the like.
The following applies in general to all processes mentioned herein before and hereinafter.
All the above-mentioned process steps can be carried out under reaction conditions that are known to those skilled in the art, including those mentioned specifically, in the absence or, customarily, in the presence of solvents or diluents, including, for example, solvents or diluents that are inert towards the reagents used and dissolve them, in the absence or presence of catalysts, condensation or neutralizing agents, for example ion exchangers, such as cation exchangers, e.g. in the H+ form, depending on the nature of the reaction and/or of the reactants at reduced, normal or elevated temperature, for example in a temperature range of from about -100 °C to about 190 °C, including, for example, from
approximately -80 °C to approximately 150 °C, for example at from -80 to -60 °C, at room temperature, at from -20 to 40 °C or at reflux temperature, under atmospheric pressure or in a closed vessel, where appropriate under pressure, and/or in an inert atmosphere, for example under an argon or nitrogen atmosphere.
At all stages of the reactions, mixtures of isomers that are formed can be separated into the individual isomers, for example diastereo isomers or enantiomers, or into any desired mixtures of isomers, for example racemates or mixtures of diastereo isomers, for example analogously to the methods described under "Additional process steps".
The solvents from which those solvents that are suitable for any particular reaction may be selected include those mentioned specifically or, for example, water, esters, such as lower alkyl-lower alkanoates, for example ethyl acetate, ethers, such as aliphatic ethers, for example diethyl ether, or cyclic ethers, for example tetrahydrofuran or dioxane, liquid aromatic hydrocarbons, such as benzene or toluene, alcohols, such as methanol, ethanol or 1- or 2-propanol, nitriles, such as acetonitrile, halogenated hydrocarbons, such as methylene chloride or chloroform, acid amides, such as dimethylformamide or dimethyl acetamide, bases, such as heterocyclic nitrogen bases, for example pyridine or N-methylpyrrolidin-2- one, carboxylic acid anhydrides, such as lower alkanoic acid anhydrides, for example acetic anhydride, cyclic, linear or branched hydrocarbons, such as cyclohexane, hexane or isopentane, methycyclohexane, or mixtures of those solvents, for example aqueous solutions, unless otherwise indicated in the description of the processes. Such solvent mixtures may also be used in working up, for example by chromatography or partitioning.
The compounds of the present invention, including their salts, may also be obtained in the form of hydrates, or their crystals may, for example, include the solvent used for crystallization. Different crystalline forms may be present.
The invention relates also to those forms of the process in which a compound obtainable as an intermediate at any stage of the process is used as starting material and the remaining process steps are carried out, or in which a starting material is formed under the reaction conditions or is used in the form of a derivative, for example in a protected form or in the form of a salt, or a compound obtainable by the process according to the invention is produced under the process conditions and processed further in situ.
All starting materials, building blocks, reagents, acids, bases, dehydrating agents, solvents and catalysts utilized to synthesize the compounds of the present invention are either commercially available or can be produced by organic synthesis methods known to one of ordinary skill in the art (Houben-Weyl 4th Ed. 1952, Methods of Organic Synthesis, Thieme, Volume 21 ).
The term "an optical isomer" or "a stereoisomer" refers to any of the various stereoisomeric configurations which may exist for a given compound of the present invention
and includes geometric isomers. It is understood that a substituent may be attached at a chiral center of a carbon atom. The term "chiral" refers to molecules which have the property of non-superimposability on their mirror image partner, while the term "achiral" refers to molecules which are superimposable on their mirror image partner. Therefore, the invention includes enantiomers, diastereomers or racemates of the compound.
"Enantiomers" are a pair of stereoisomers that are non- superimposable mirror images of each other. A 1 : 1 mixture of a pair of enantiomers is a "racemic" mixture. The term is used to designate a racemic mixture where appropriate. "Diastereoisomers" are stereoisomers that have at least two asymmetric atoms, but which are not mirror-images of each other. The absolute stereochemistry is specified according to the Cahn- Ingold- Prelog R-S system. When a compound is a pure enantiomer the stereochemistry at each chiral carbon may be specified by either R or S. Resolved compounds whose absolute configuration is unknown can be designated (+) or (-) depending on the direction (dextro- or levorotatory) which they rotate plane polarized light at the wavelength of the sodium D line. Certain compounds described herein contain one or more asymmetric centers or axes and may thus give rise to enantiomers, diastereomers, and other stereoisomeric forms that may be defined, in terms of absolute stereochemistry, as (R)- or (S)-.
Depending on the choice of the starting materials and procedures, the compounds can be present in the form of one of the possible isomers or as mixtures thereof, for example as pure optical isomers, or as isomer mixtures, such as racemates and diastereo isomer mixtures, depending on the number of asymmetric carbon atoms. The present invention is meant to include all such possible stereoisomers, including racemic mixtures, diasteriomeric mixtures and optically pure forms. Optically active (R)- and (S)- isomers may be prepared using chiral synthons or chiral reagents, or resolved using conventional techniques. If the compound contains a double bond, the substituent may be E or Z configuration. If the compound contains a disubstituted cycloalkyl, the cycloalkyl substituent may have a cis- or trans-configuration. All tautomeric forms are also intended to be included.
Any resulting mixtures of isomers can be separated on the basis of the
physicochemical differences of the constituents, into the pure or substantially pure geometric or optical isomers, diastereomers, racemates, for example, by chromatography and/or fractional crystallization.
Any resulting racemates of final products or intermediates can be resolved into the optical antipodes by known methods, e.g., by separation of the diastereomeric salts thereof, obtained with an optically active acid or base, and liberating the optically active acidic or basic compound. In particular, a basic moiety may thus be employed to resolve the compounds of the present invention into their optical antipodes, e.g., by fractional crystallization of a salt formed with an optically active acid, e.g., tartaric acid, dibenzoyl
tartaric acid, diacetyl tartaric acid, di-0,0-p-toluoyl tartaric acid, mandelic acid, malic acid or camphor-10-sulfonic acid. Racemic products can also be resolved by chiral
chromatography, e.g., high pressure liquid chromatography (HPLC) using a chiral adsorbent.
Furthermore, the compounds of the present invention, including their salts, can also be obtained in the form of their hydrates, or include other solvents used for their
crystallization. The compounds of the present invention may inherently or by design form solvates with pharmaceutically acceptable solvents (including water); therefore, it is intended that the invention embrace both solvated and unsolvated forms. The term "solvate" refers to a molecular complex of a compound of the present invention (including pharmaceutically acceptable salts thereof) with one or more solvent molecules. Such solvent molecules are those commonly used in the pharmaceutical art, which are known to be innocuous to the recipient, e.g., water, ethanol, and the like. The term "hydrate" refers to the complex where the solvent molecule is water.
The compounds of the present invention, including salts, hydrates and solvates thereof, may inherently or by design form polymorphs.
As used herein, the terms "salt" or "salts" refers to an acid addition or base addition salt of a compound of the present invention. "Salts" include in particular "pharmaceutically acceptable salts". The term "pharmaceutically acceptable salts" refers to salts that retain the biological effectiveness and properties of the compounds of this invention and, which typically are not biologically or otherwise undesirable. In many cases, the compounds of the present invention are capable of forming acid and/or base salts by virtue of the presence of amino and/or carboxyl groups or groups similar thereto.
Pharmaceutically acceptable acid addition salts can be formed with inorganic acids and organic acids, e.g., acetate, aspartate, benzoate, besylate, bromide/hydrobromide, bicarbonate/carbonate, bisulfate/sulfate, camphorsulfonate, chloride/hydrochloride, chlortheophyllonate, citrate, ethandisulfonate, fumarate, gluceptate, gluconate, glucuronate, hippurate, hydroiodide/iodide, isethionate, lactate, lactobionate, laurylsulfate, malate, maleate, malonate, mandelate, mesylate, methylsulphate, naphthoate, napsylate, nicotinate, nitrate, octadecanoate, oleate, oxalate, palmitate, pamoate, phosphate/hydrogen phosphate/dihydrogen phosphate, polygalacturonate, propionate, stearate, succinate, subsalicylate, tartrate, tosylate and trifluoroacetate salts.
Inorganic acids from which salts can be derived include, for example, hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, and the like.
Organic acids from which salts can be derived include, for example, acetic acid, propionic acid, glycolic acid, oxalic acid, maleic acid, malonic acid, succinic acid, fumaric acid, tartaric acid, citric acid, benzoic acid, mandelic acid, methanesulfonic acid,
ethanesulfonic acid, toluenesulfonic acid, sulfosalicylic acid, and the like. Pharmaceutically acceptable base addition salts can be formed with inorganic and organic bases.
Inorganic bases from which salts can be derived include, for example, ammonium salts and metals from columns I to XII of the periodic table. In certain embodiments, the salts are derived from sodium, potassium, ammonium, calcium, magnesium, iron, silver, zinc, and copper; particularly suitable salts include ammonium, potassium, sodium, calcium and magnesium salts.
Organic bases from which salts can be derived include, for example, primary, secondary, and tertiary amines, substituted amines including naturally occurring substituted amines, cyclic amines, basic ion exchange resins, and the like. Certain organic amines include isopropylamine, benzathine, cholinate, diethanolamine, diethylamine, lysine, meglumine, piperazine and tromethamine.
The pharmaceutically acceptable salts of the present invention can be synthesized from a basic or acidic moiety, by conventional chemical methods. Generally, such salts can be prepared by reacting free acid forms of these compounds with a stoichiometric amount of the appropriate base (such as Na, Ca, Mg, or K hydroxide, carbonate, bicarbonate or the like), or by reacting free base forms of these compounds with a stoichiometric amount of the appropriate acid. Such reactions are typically carried out in water or in an organic solvent, or in a mixture of the two. Generally, use of non-aqueous media like ether, ethyl acetate, ethanol, isopropanol, or acetonitrile is desirable, where practicable. Lists of additional suitable salts can be found, e.g., in "Remington's Pharmaceutical Sciences", 20th ed., Mack Publishing Company, Easton, Pa., (1985); and in "Handbook of Pharmaceutical Salts:
Properties, Selection, and Use" by Stahl and Wermuth (Wiley-VCH, Weinheim, Germany, 2002).
Any formula given herein is also intended to represent unlabeled forms as well as isotopically labeled forms of the compounds of the present invention. Isotopically labeled compounds have structures depicted by the formulas given herein except that one or more atoms are replaced by an atom having a selected atomic mass or mass number. Examples of isotopes that can be incorporated into compounds of the invention include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorous, fluorine, and chlorine, such as 2H, 3H, 11C, 13C, 14C, 15N, 18F 31P, 32P, 35S, 36CI, 125l respectively. The invention includes various isotopically labeled compounds of the present invention, for example those into which radioactive isotopes, such as 3H and 14C, or those into which non-radioactive isotopes, such as 2H and 13C are present. Such isotopically labelled compounds are useful in metabolic studies (with 14C), reaction kinetic studies (with, for example 2H or 3H), detection or imaging techniques, such as positron emission tomography (PET) or single-photon emission computed tomography (SPECT) including drug or substrate tissue distribution assays, or in
radioactive treatment of patients. In particular, an 18F labeled compound of the present invention may be particularly desirable for PET or SPECT studies. Isotopically-labeled compounds of the present invention can generally be prepared by conventional techniques known to those skilled in the art or by processes analogous to those described in the accompanying Examples and Preparations using an appropriate isotopically-labeled reagent in place of the non-labeled reagent previously employed.
Further, substitution with heavier isotopes, particularly deuterium (i.e., 2H or D) may afford certain therapeutic advantages resulting from greater metabolic stability, for example increased in vivo half-life or reduced dosage requirements or an improvement in therapeutic index. It is understood that deuterium in this context is regarded as a substituent of a compound of the present invention. The concentration of such a heavier isotope, specifically deuterium, may be defined by the isotopic enrichment factor. The term "isotopic enrichment factor" as used herein means the ratio between the isotopic abundance and the natural abundance of a specified isotope. If a substituent in a compound of this invention is denoted deuterium, such compound has an isotopic enrichment factor for each designated deuterium atom of at least 3500 (52.5% deuterium incorporation at each designated deuterium atom), at least 4000 (60% deuterium incorporation), at least 4500 (67.5% deuterium incorporation), at least 5000 (75% deuterium incorporation), at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), at least 6333.3 (95% deuterium incorporation), at least 6466.7 (97% deuterium incorporation), at least 6600 (99% deuterium incorporation), or at least 6633.3 (99.5% deuterium incorporation).
Pharmaceutically acceptable solvates in accordance with the invention include those wherein the solvent of crystallization may be isotopically substituted, e.g. D20, d6-acetone, de-DMSO.
Compounds of the present invention that contain groups capable of acting as donors and/or acceptors for hydrogen bonds may be capable of forming co-crystals with suitable co- crystal formers. These co-crystals may be prepared from compounds of the present invention by known co-crystal forming procedures. Such procedures include grinding, heating, co-subliming, co-melting, or contacting in solution compounds of the present invention with the co-crystal former under crystallization conditions and isolating co-crystals thereby formed. Suitable co-crystal formers include those described in WO 2004/078163. Hence the invention further provides co-crystals comprising a compound of the present invention.
All methods described herein can be performed in any suitable order unless otherwise indicated herein or otherwise clearly contradicted by context. The use of any and all examples, or exemplary language (e.g. "such as") provided herein is intended merely to
better illuminate the invention and does not pose a limitation on the scope of the invention otherwise claimed.
The present invention provides novel compounds, pharmaceutical formulations including the compounds, methods of inhibiting UDP-3-0-(R-3-hydroxydecanoyl)-N- acetylglucosamine deacetylase (LpxC), and methods of treating Gram-negative bacterial infections.
Substitution with heavier isotopes such as deuterium, i.e. 2H, may afford certain therapeutic advantages resulting from greater metabolic stability, for example, increased in vivo half-life or reduced dosage requirements, and hence may be preferred in some circumstances. For example, deuterium substitution at non-exchangeable hydrocarbon bonds (e.g., C-H) may retard epimerization and/or metabolic oxidation in vivo.
Isotopically-labeled compounds of the invention, i.e. compounds of formula (I), can generally be prepared by conventional techniques known to those skilled in the art or by processes analogous to those described in the accompanying Examples and Preparations Sections using an appropriate isotopically-labeled reagent in place of the non-labeled reagent previously.
In another aspect, the invention provides a method of inhibiting a deacetylase enzyme in a Gram-negative bacterium, the method comprising the step of contacting the Gram-negative bacteria with a compound of the invention, e.g., a compound of Formula I or salt thereof.
In still another aspect, the invention provides a method for treating a subject with a Gram-negative bacterial infection, the method comprising the step of administering to the subject in need thereof an antibacterially effective amount of a compound of the invention, e.g., a compound of Formula I or salt thereof with a pharmaceutically acceptable carrier.
The compounds of the invention can be used for treating conditions caused by the bacterial production of endotoxin and, in particular, by Gram-negative bacteria and bacteria that use LpxC in the biosynthesis of lipopolysaccharide (LPS) or endotoxin.
The compounds of the invention also are useful in the treatment of patients suffering from or susceptible to pneumonia, sepsis, cystic fibrosis, wound, complicated diabetic foot or complicated urinary track infections and sexually transmitted diseases caused by Gram- negative pathogens. The compounds of the invention also are useful in the conditions that are caused or exacerbated by the bacterial production of lipid A and LPS or endotoxin, such as sepsis, septic shock, systemic inflammation, localized inflammation, chronic obstructive pulmonary disease (COPD) and acute exacerbations of chronic bronchitis (AECB). For these conditions, treatment includes the administration of a compound of the invention, or a combination of compounds of the invention, optionally with a second agent wherein the second agent is a second antibacterial agent or a second non-antibacterial agent.
For sepsis, septic shock, systemic inflammation, localized inflammation, chronic obstructive pulmonary disease (COPD) and acute exacerbations of chronic bronchitis (AECB), preferred second non-antibacterial agents include antiendotoxins including endotoxin receptor-binding antibodies, endotoxin-binding antibodies, antiCD14-binding protein antibodies antilipopolysaccharide-binding protein antibodies and tyrosine kinase inhibitors.
In treatment of serious or chronic respiratory tract infections, the compounds of the present invention may also be used with second non-antibacterial agents administered via inhalation. Preferred non-antibacterial agents used in this treatment include antiinflammatory steroids, non-steroidal anti-inflammatory agents, bronchiodilators, mucolytics, anti-asthma therapeutics and lung fluid surfactants. In particular, the non-antibacterial agent may be selected from a group consisting of albuterol, salbuterol, budesonide,
beclomethasone, dexamethasone, nedocromil, beclomethasone, fluticasone, flunisolide, triamcinolone, ibuprofin, rofecoxib, naproxen, celecoxib, nedocromil, ipratropium, metaproterenol, pirbuterol, salneterol, bronchiodilators, mucolytics, calfactant, beractant, poractant alfa, surfaxin and pulmozyme (also called domase alfa).
The compounds of the invention can be used, alone or in combination with a second antibacterial agent for the treatment of a serious or chronic respiratory tract infection including serious lung and nosocomial infections such as those caused by Enterobacter aerogenes, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Proteus mirabilis, Serratia marcescens, Stenotrophomonas maltophilia,
Pseudomonas aeruginosa, Burkholderia cepacia, Acinetobacter baumanii, Alcaligenes xylosoxidans, Flavobacterium meningosepticum, Providencia stuartii and Citrobacter freundi, community lung infections such as those caused by Haemophilus influenzae, Legionella species, Moraxella catarrhalis, Enterobacter species, Acinetobacter species, Klebsiella species, and Proteus species, and infections caused by other bacterial species such as Neisseria species, Shigella species, Salmonella species, Helicobacter pylori, Vibrionaceae and Bordetella species as well as the infections is caused by a Brucella species, Francisella tularensis and/or Yersinia Pestis.
A compound of the present invention may also be used in combination with other agents, e.g., an additional antibiotic agent that is or is not of the formula I, for treatment of a bacterial infection in a subject.
By the term "combination", is meant either a fixed combination in one dosage unit form, or a kit of parts for the combined administration where a compound of the present invention and a combination partner may be administered independently at the same time or separately within time intervals that especially allow that the combination partners show a cooperative, e.g., synergistic, effect, or any combination thereof.
When used for treating Gram-negative bacteria, the compounds of the present invention can be used to sensitize Gram-negative bacteria to the effects of a second agent.
An embodiment of the present invention is compounds of the present invention used in combination with a second antibacterial agent, non-limiting examples of antibacterial agents may be selected from the following groups:
(1 ) Macrolides or ketolides such as erythromycin, azithromycin, clarithromycin, and telithromycin;
(2) Beta-lactams including penicillin such as penicillin G, penicillin V, methicillin, oxacillin, cloxacillin, dicloxacillin, nafcillin, ampicillin, amoxicillin, carbenicillin, ticarcillin, mezlocillin, piperacillin, azlocillin, temocillin, cephalosporin such as cepalothin, cephapirin, cephradine, cephaloridine, cefazolin, cefamandole, cefuroxime, cephalexin, cefprozil, cefaclor, loracarbef, cefoxitin, cefinetazole, cefotaxime, ceftizoxime, ceftriaxone,
cefoperazone, ceftazidime, cefixime, cefpodoxime, ceftibuten, cefdinir, cefpirome, cefepime, and carbapenems such as carbapenem, imipenem, meropenem and PZ-601 ;
(3) Monobactams such as aztreonam;
(4) Quinolones such as nalidixic acid, oxolinic acid, norfloxacin, pefloxacin, enoxacin, ofloxacin, levofloxacin, ciprofloxacin, temafloxacin, lomefloxacin, fleroxacin, grepafloxacin, sparfloxacin, trovafloxacin, clinafloxacin, gatifloxacin, moxifloxacin, sitafloxacin,
ganefloxacin, gemifloxacin and pazufloxacin;
(5) Antibacterial sulfonanmides and antibacterial sulphanilamides, including para- aminobenzoic acid, sulfadiazine, sulfisoxazole, sulfamethoxazole and sulfathalidine;
(6) Aminoglycosides such as streptomycin, neomycin, kanamycin, paromycin, gentamicin, tobramycin, amikacin, netilmicin, spectinomycin, sisomicin, dibekalin and isepamicin;
(7) Tetracyclines such as tetracycline, chlortetracycline, demeclocycline, minocycline, oxytetracycline, methacycline, doxycycline, tegacycline;
(8) Rifamycins such as rifampicin (also called rifampin), rifapentine, rifabutin, bezoxazinorifamycin and rifaximin;
(9) Lincosamides such as lincomycin and clindamycin;
(10) Glycopeptides such as vancomycin and teicoplanin;
(1 1 ) Streptogramins such as quinupristin and daflopristin;
(12) Oxazolidinones such as linezolid;
(13) Polymyxin, colistin and colymycin;
(14) Trimethoprim and bacitracin.
(15) Efflux pump inhibitors.
The second antibacterial agent may be administered in combination with the compounds of the present inventions wherein the second antibacterial agent is administered
prior to, simultaneously, or after the compound or compounds of the present invention. When simultaneous administration of a compound of the invention with a second agent is desired and the route of administration is the same, then a compound of the invention may be formulated with a second agent into the same dosage form. An example of a dosage form containing a compound of the invention and a second agent is a tablet or a capsule.
When used for treating serious or chronic respiratory tract infections, the compounds of the invention may be used alone or in combination with a second antibacterial agent administered via inhalation. In the case of inhalation, a preferred second antibacterial agent is selected from a group consisting of tobramycin, gentamicin, aztreonam, ciprofloxacin, polymyxin, colistin, colymycin, azithromycin and clarithromycin.
The language "effective amount" of the compound is that amount necessary or sufficient to treat or prevent a bacterial infection and/or a disease or condition described herein. In an example, an effective amount of the LpxC inhibitor is the amount sufficient to treat bacterial infection in a subject. In another example, an effective amount of the LpxC inhibitor is an amount sufficient to treat a bacterial infection, such as, but not limited to Pseudomonas aeruginosa and the like in a subject. The effective amount can vary depending on such factors as the size and weight of the subject, the type of illness, or the particular compound of the invention. For example, the choice of the compound of the invention can affect what constitutes an "effective amount." One of ordinary skill in the art would be able to study the factors contained herein and make the determination regarding the effective amount of the compounds of the invention without undue experimentation.
The regimen of administration can affect what constitutes an effective amount. The compound of the invention can be administered to the subject either prior to or after the onset of a bacterial infection. Further, several divided dosages, as well as staggered dosages, can be administered daily or sequentially, or the dose can be continuously infused, or can be a bolus injection. Further, the dosages of the compound(s) of the invention can be proportionally increased or decreased as indicated by the exigencies of the therapeutic or prophylactic situation.
Compounds of the invention may be used in the treatment of states, disorders or diseases as described herein, or for the manufacture of pharmaceutical compositions for use in the treatment of these diseases. The invention provides methods of use of compounds of the present invention in the treatment of these diseases or pharmaceutical preparations having compounds of the present invention for the treatment of these diseases.
The language "pharmaceutical composition" includes preparations suitable for administration to mammals, e.g., humans. When the compounds of the present invention are administered as pharmaceuticals to mammals, e.g., humans, they can be given per se or as a pharmaceutical composition containing, for example, 0.1 to 99.5% (more preferably, 0.5
to 90%) of active ingredient in combination with a pharmaceutically acceptable carrier.
The phrase "pharmaceutically acceptable carrier" is art recognized and includes a pharmaceutically acceptable material, composition or vehicle, suitable for administering compounds of the present invention to mammals. The carriers include liquid or solid filler, diluent, excipient, solvent or encapsulating material, involved in carrying or transporting the subject agent from one organ, or portion of the body, to another organ, or portion of the body. Each carrier must be "acceptable" in the sense of being compatible with the other ingredients of the formulation and not injurious to the patient. Some examples of materials which can serve as pharmaceutically acceptable carriers include: sugars, such as lactose, glucose and sucrose; starches, such as corn starch and potato starch; cellulose, and its derivatives, such as sodium carboxymethyl cellulose, ethyl cellulose and cellulose acetate; powdered tragacanth; malt; gelatin; talc; excipients, such as cocoa butter and suppository waxes; oils, such as peanut oil, cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and soybean oil; glycols, such as propylene glycol; polyols, such as glycerin, sorbitol, mannitol and polyethylene glycol; esters, such as ethyl oleate and ethyl laurate; agar; buffering agents, such as magnesium hydroxide and aluminum hydroxide; alginic acid; pyrogen-free water; isotonic saline; Ringer's solution; ethyl alcohol; phosphate buffer solutions; and other non-toxic compatible substances employed in pharmaceutical formulations.
Wetting agents, emulsifiers and lubricants, such as sodium lauryl sulfate and magnesium stearate, as well as coloring agents, release agents, coating agents, sweetening, flavoring and perfuming agents, preservatives and antioxidants can also be present in the compositions.
Examples of pharmaceutically acceptable antioxidants include: water soluble antioxidants, such as ascorbic acid, cysteine hydrochloride, sodium bisulfate, sodium metabisulfite, sodium sulfite and the like; oil-soluble antioxidants, such as ascorbyl palmitate, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), lecithin, propyl gallate, - tocopherol, and the like; and metal chelating agents, such as citric acid, ethylenediamine tetraacetic acid (EDTA), sorbitol, tartaric acid, phosphoric acid, and the like.
Formulations of the present invention include those suitable for oral, nasal, inhalation, topical, transdermal, buccal, sublingual, rectal, vaginal and/or parenteral administration. The formulations may conveniently be presented in unit dosage form and may be prepared by any methods well known in the art of pharmacy. The amount of active ingredient that can be combined with a carrier material to produce a single dosage form will generally be that amount of the compound that produces a therapeutic effect. Generally, out of one hundred per cent, this amount will range from about 1 per cent to about ninety-nine percent of active ingredient, preferably from about 5 per cent to about 70 per cent, most preferably from about 10 per cent to about 30 per cent.
Methods of preparing these formulations or compositions include the step of bringing into association a compound of the present invention with the carrier and, optionally, one or more accessory ingredients. In general, the formulations are prepared by uniformly and intimately bringing into association a compound of the present invention with liquid carriers, or finely divided solid carriers, or both, and then, if necessary, shaping the product.
Formulations of the invention suitable for oral administration may be in the form of capsules, cachets, pills, tablets, lozenges (using a flavored basis, usually sucrose and acacia or tragacanth), powders, granules, or as a solution or a suspension in an aqueous or non-aqueous liquid, or as an oil-in-water or water-in-oil liquid emulsion, or as an elixir or syrup, or as pastilles (using an inert base, such as gelatin and glycerin, or sucrose and acacia) and/or as mouth washes and the like, each containing a predetermined amount of a compound of the present invention as an active ingredient. A compound of the present invention may also be administered as a bolus, electuary or paste.
In solid dosage forms of the invention for oral administration (capsules, tablets, pills, dragees, powders, granules and the like), the active ingredient is mixed with one or more pharmaceutically acceptable carriers, such as sodium citrate or dicalcium phosphate, and/or any of the following: fillers or extenders, such as starches, lactose, sucrose, glucose, mannitol, and/or silicic acid; binders, such as, for example, carboxymethylcellulose, alginates, gelatin, polyvinyl pyrrolidone, sucrose and/or acacia; humectants, such as glycerol; disintegrating agents, such as agar-agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and sodium carbonate; solution retarding agents, such as paraffin; absorption accelerators, such as quaternary ammonium compounds; wetting agents, such as, for example, cetyl alcohol and glycerol monostearate; absorbents, such as kaolin and bentonite clay; lubricants, such a talc, calcium stearate, magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate, and mixtures thereof; and coloring agents. In the case of capsules, tablets and pills, the pharmaceutical compositions may also comprise buffering agents. Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugars, as well as high molecular weight polyethylene glycols and the like.
A tablet may be made by compression or molding, optionally with one or more accessory ingredients. Compressed tablets may be prepared using binder (for example, gelatin or hydroxypropylmethyl cellulose), lubricant, inert diluent, preservative, disintegrant (for example, sodium starch glycolate or cross-linked sodium carboxymethyl cellulose), surface-active or dispersing agent. Molded tablets may be made by molding in a suitable machine a mixture of the powdered compound moistened with an inert liquid diluent.
The tablets, and other solid dosage forms of the pharmaceutical compositions of the present invention, such as dragees, capsules, pills and granules, may optionally be scored
or prepared with coatings and shells, such as enteric coatings and other coatings well known in the pharmaceutical-formulating art. They may also be formulated so as to provide slow or controlled release of the active ingredient therein using, for example, hydroxypropylmethyl cellulose in varying proportions to provide the desired release profile, other polymer matrices, liposomes and/or microspheres. They may be sterilized by, for example, filtration through a bacteria-retaining filter, or by incorporating sterilizing agents in the form of sterile solid compositions that can be dissolved in sterile water, or some other sterile injectable medium immediately before use. These compositions may also optionally contain opacifying agents and may be of a composition that they release the active ingredient(s) only, or preferentially, in a certain portion of the gastrointestinal tract, optionally, in a delayed manner. Examples of embedding compositions that can be used include polymeric substances and waxes. The active ingredient can also be in micro-encapsulated form, if appropriate, with one or more of the above-described excipients.
Liquid dosage forms for oral administration of the compounds of the invention include pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions, syrups and elixirs. In addition to the active ingredient, the liquid dosage forms may contain inert diluent commonly used in the art, such as, for example, water or other solvents, solubilizing agents and emulsifiers, such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1 ,3-butylene glycol, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor and sesame oils), glycerol, tetrahydrofuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof.
Besides inert diluents, the oral compositions can also include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, coloring, perfuming and preservative agents.
Suspensions, in addition to the active compounds, may contain suspending agents as, for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar and tragacanth, and mixtures thereof.
Formulations of the pharmaceutical compositions of the invention for rectal or vaginal administration may be presented as a suppository, which may be prepared by mixing one or more compounds of the invention with one or more suitable nonirritating excipients or carriers comprising, for example, cocoa butter, polyethylene glycol, a suppository wax or a salicylate, and which is solid at room temperature, but liquid at body temperature and, therefore, will melt in the rectum or vaginal cavity and release the active compound.
Formulations of the present invention which are suitable for vaginal administration also include pessaries, tampons, creams, gels, pastes, foams or spray formulations containing such carriers as are known in the art to be appropriate.
Dosage forms for the topical or transdermal administration of a compound of this invention include powders, sprays, ointments, pastes, creams, lotions, gels, solutions, patches and inhalants. The active compound may be mixed under sterile conditions with a pharmaceutically acceptable carrier, and with any preservatives, buffers, or propellants that may be required.
The ointments, pastes, creams and gels may contain, in addition to an active compound of this invention, excipients, such as animal and vegetable fats, oils, waxes, paraffins, starch, tragacanth, cellulose derivatives, polyethylene glycols, silicones, bentonites, silicic acid, talc and zinc oxide, or mixtures thereof.
Powders and sprays can contain, in addition to a compound of this invention, excipients such as lactose, talc, silicic acid, aluminum hydroxide, calcium silicates and polyamide powder, or mixtures of these substances. Sprays can additionally contain customary propellants, such as chlorofluorohydrocarbons and volatile unsubstituted hydrocarbons, such as butane and propane.
Transdermal patches have the added advantage of providing controlled delivery of a compound of the present invention to the body. Such dosage forms can be made by dissolving or dispersing the compound in the proper medium. Absorption enhancers can also be used to increase the flux of the compound across the skin. The rate of such flux can be controlled by either providing a rate controlling membrane or dispersing the active compound in a polymer matrix or gel.
Ophthalmic formulations, eye ointments, powders, solutions and the like, are also contemplated as being within the scope of this invention.
Pharmaceutical compositions of this invention suitable for parenteral administration comprise one or more compounds of the invention in combination with one or more pharmaceutically acceptable sterile isotonic aqueous or nonaqueous solutions, dispersions, suspensions or emulsions, or sterile powders which may be reconstituted into sterile injectable solutions or dispersions just prior to use, which may contain antioxidants, buffers, bacteriostats, solutes which render the formulation isotonic with the blood of the intended recipient or suspending or thickening agents.
Examples of suitable aqueous and nonaqueous carriers that may be employed in the pharmaceutical compositions of the invention include water, ethanol, polyols (such as glycerol, propylene glycol, polyethylene glycol, and the like), and suitable mixtures thereof, vegetable oils, such as olive oil, and injectable organic esters, such as ethyl oleate. Proper fluidity can be maintained, for example, by the use of coating materials, such as lecithin, by the maintenance of the required particle size in the case of dispersions, and by the use of surfactants.
These compositions may also contain adjuvants such as preservatives, wetting
agents, emulsifying agents and dispersing agents. Prevention of the action of
microorganisms may be ensured by the inclusion of various antibacterial and antifungal agents, for example, paraben, chlorobutanol, phenol sorbic acid, and the like. It may also be desirable to include isotonic agents, such as sugars, sodium chloride, and the like into the compositions. In addition, prolonged absorption of the injectable pharmaceutical form may be brought about by the inclusion of agents that delay absorption such as aluminum monostearate and gelatin.
In some cases, in order to prolong the effect of a drug, it is desirable to slow the absorption of the drug from subcutaneous or intramuscular injection. This may be accomplished by the use of a liquid suspension of crystalline or amorphous material having poor water solubility. The rate of absorption of the drug then depends upon its rate of dissolution which, in turn, may depend upon crystal size and crystalline form. Alternatively, delayed absorption of a parenterally-administered drug form is accomplished by dissolving or suspending the drug in an oil vehicle.
Injectable depot forms are made by forming microencapsule matrices of the subject compounds in biodegradable polymers such as polylactide-polyglycolide. Depending on the ratio of drug to polymer, and the nature of the particular polymer employed, the rate of drug release can be controlled. Examples of other biodegradable polymers include
poly(orthoesters) and poly(anhydrides). Depot injectable formulations are also prepared by entrapping the drug in liposomes or microemulsions that are compatible with body tissue.
The preparations of the present invention may be given orally, parenterally, topically, or rectally. They are of course given by forms suitable for each administration route. For example, they are administered in tablets or capsule form, by injection, inhalation, eye lotion, ointment, suppository, etc., administration by injection, infusion or inhalation; topical by lotion or ointment; and rectal by suppositories. Oral administration is preferred.
The phrases "parenteral administration" and "administered parenterally" as used herein means modes of administration other than enteral and topical administration, usually by injection, and includes, without limitation, intravenous, intramuscular, intraarterial, intrathecal, intracapsular, intraorbital, intracardiac, intradermal, intraperitoneal, transtracheal, subcutaneous, subcuticular, intraarticular, subcapsular, subarachnoid, intraspinal and intrasternal injection and infusion.
The phrases "systemic administration," "administered systemically," "peripheral administration" and "administered peripherally" as used herein mean the administration of a compound, drug or other material other than directly into the central nervous system, such that it enters the patient's system and, thus, is subject to metabolism and other like processes, for example, subcutaneous administration.
These compounds may be administered to humans and other animals for therapy by
any suitable route of administration, including orally, nasally, as by, for example, a spray, rectally, intravaginally, parenterally, intracisternally and topically, as by powders, ointments or drops, including buccally and sublingually.
Regardless of the route of administration selected, the compounds of the present invention, which may be used in a suitable hydrated form, and/or the pharmaceutical compositions of the present invention, are formulated into pharmaceutically acceptable dosage forms by conventional methods known to those of skill in the art.
Actual dosage levels of the active ingredients in the pharmaceutical compositions of this invention may be varied so as to obtain an amount of the active ingredient which is effective to achieve the desired therapeutic response for a particular patient, composition, and mode of administration, without being toxic to the patient.
The selected dosage level will depend upon a variety of factors including the activity of the particular compound of the present invention employed, or the ester, salt or amide thereof, the route of administration, the time of administration, the rate of excretion of the particular compound being employed, the duration of the treatment, other drugs, compounds and/or materials used in combination with the particular compound employed, the age, sex, weight, condition, general health and prior medical history of the patient being treated, and like factors well known in the medical arts.
A physician or veterinarian having ordinary skill in the art can readily determine and prescribe the effective amount of the pharmaceutical composition required. For example, the physician or veterinarian could start doses of the compounds of the invention employed in the pharmaceutical composition at levels lower than that required in order to achieve the desired therapeutic effect and gradually increase the dosage until the desired effect is achieved.
In general, a suitable daily dose of a compound of the invention will be that amount of the compound that is the lowest dose effective to produce a therapeutic effect. Such an effective dose will generally depend upon the factors described above. Generally, intravenous and subcutaneous doses of the compounds of this invention for a patient, when used for the indicated analgesic effects, will range from about 0.0001 to about 100 mg per kilogram of body weight per day, more preferably from about 0.01 to about 50 mg per kg per day, and still more preferably from about 1.0 to about 100 mg per kg per day. An effective amount is that amount treats a bacterial infection.
If desired, the effective daily dose of the active compound may be administered as two, three, four, five, six or more sub-doses administered separately at appropriate intervals throughout the day, optionally, in unit dosage forms.
While it is possible for a compound of the present invention to be administered alone, it is preferable to administer the compound as a pharmaceutical composition.
The compounds as defined in embodiments may be synthesized by the general synthetic routes below, specific examples of which are described in more detail in the Examples.
General Synthetic Schemes
The compounds of general structure 1-1 can be synthesized by the Scheme 1 shown below. Compound 1 c can be prepared from amine 1 b either through reductive amination with aldehyde 1a or alkylation with appropriate electrophile 1 d. The ester in 1 c can be converted into hydroxamate in 1-1 in standard three step procedure. The ester was hydrolyzed under basic condition to provide carboxylic acid 1 e, which can then be coupled with protected hydroxylamine to give 1f. Finally the protecting group can be cleaved to generate the desired compound of formula 1-1.
Scheme 1
1-1
As is readily apparent to one skilled in the art, the synthetic route in Scheme 1 could be modified and the tail fragment represented by R-L could be coupled at late stage (as shown in Scheme 2). The initial step is to couple amine 1 b and aldehyde 2a by a reductive amination reaction. The product 2b can be hydrolyzed to acid 2c followed by amide coupling to provide protected hyroxamate 2d. The tail fragment can be attached by standard transition metal catalyzed coupling reaction to provide 2e. Finally the protecting group can be cleaved to generate the desired compound of formula IV.
Step 4
tail fragment coupling
The synthesis of compounds with formula I-2 was illustrated in Scheme 3. As shown in Scheme 1 and 2, ester 3a is a key intermediate, which can be converted to IV by standard three step procedures. The key step in the synthesis of 3a is attaching R-L-Z-CH2CH2 moiety to ester 3c through alkylation. There are a variety of methods to assemble individual [R-L-Z- A] fragments and attach to ester 3c. Such variations are feasible to one of skilled in the art.
Step 1. hydrolysis
Step 2. couple with NH20-PG
Step 3. deprotection
Synthetic Procedure
Compounds of the present invention are prepared from commonly available compounds using procedures known to those skilled in the art, including any one or more of the following conditions without limitation:
Within the scope of this text, only a readily removable group that is not a constituent of the particular desired end product of the compounds of the present invention is designated a "protecting group," unless the context indicates otherwise. The protection of functional groups by such protecting groups, the protecting groups themselves, and their cleavage reactions are described for example in standard reference works, such as e.g., Science of Synthesis: Houben-Weyl Methods of Molecular Transformation. Georg Thieme Verlag, Stuttgart, Germany. 2005. 41627 pp. (URL: http://www.science-of-synthesis.com (Electronic Version, 48 Volumes)); J. F. W. McOmie, "Protective Groups in Organic
Chemistry", Plenum Press, London and New York 1973, in T. W. Greene and P. G. M. Wuts, "Protective Groups in Organic Synthesis", Third edition, Wiley, New York 1999, in "The Peptides"; Volume 3 (editors: E. Gross and J. Meienhofer), Academic Press, London and New York 1981 , in "Methoden der organischen Chemie" (Methods of Organic Chemistry), Houben Weyl, 4th edition, Volume 15/1, Georg Thieme Verlag, Stuttgart 1974, in H.-D.
Jakubke and H. Jeschkeit, "Aminosauren, Peptide, Proteine" (Amino acids, Peptides,
Proteins), Verlag Chemie, Weinheim, Deerfield Beach, and Basel 1982, and in Jochen Lehmann, "Chemie der Kohlenhydrate: Monosaccharide und Derivate" (Chemistry of Carbohydrates: Monosaccharides and Derivatives), Georg Thieme Verlag, Stuttgart 1974. A characteristic of protecting groups is that they can be removed readily (i.e. , without the occurrence of undesired secondary reactions) for example by solvolysis, reduction, photolysis or alternatively under physiological conditions (e.g., by enzymatic cleavage).
Salts of compounds of the present invention having at least one salt-forming group may be prepared in a manner known per se. For example, salts of compounds of the present invention having acid groups may be formed, for example, by treating the compounds with metal compounds, such as alkali metal salts of suitable organic carboxylic acids, e.g., the sodium salt of 2-ethyl hexanoic acid, with organic alkali metal or alkaline earth metal compounds, such as the corresponding hydroxides, carbonates or hydrogen carbonates, such as sodium or potassium hydroxide, carbonate or hydrogen carbonate, with corresponding calcium compounds or with ammonia or a suitable organic amine, stoichiometric amounts or only a small excess of the salt-forming agent preferably being used. Acid addition salts of compounds of the present invention are obtained in customary manner, e.g., by treating the compounds with an acid or a suitable anion exchange reagent. Internal salts of compounds of the present invention containing acid and basic salt-forming groups, e.g., a free carboxy group and a free amino group, may be formed, e.g., by the neutralisation of salts, such as acid addition salts, to the isoelectric point, e.g., with weak bases, or by treatment with ion exchangers.
Salts can be converted in customary manner into the free compounds; metal and ammonium salts can be converted, for example, by treatment with suitable acids, and acid addition salts, for example, by treatment with a suitable basic agent.
Mixtures of isomers obtainable according to the invention can be separated in a manner known per se into the individual isomers; diastereoisomers can be separated, for example, by partitioning between polyphasic solvent mixtures, recrystallisation and/or chromatographic separation, for example over silica gel or by, e.g., medium pressure liquid chromatography over a reversed phase column, and racemates can be separated, for example, by the formation of salts with optically pure salt-forming reagents and separation of the mixture of diastereoisomers so obtainable, for example by means of fractional crystallisation, or by chromatography over optically active column materials.
Intermediates and final products can be worked up and/or purified according to standard methods, e.g., using chromatographic methods, distribution methods, (re-) crystallization, and the like.
General process conditions
The following applies in general to all processes mentioned throughout this disclosure.
The process steps to synthesize the compounds of the invention can be carried out under reaction conditions that are known per se, including those mentioned specifically, in the absence or, customarily, in the presence of solvents or diluents, including, for example, solvents or diluents that are inert towards the reagents used and dissolve them, in the absence or presence of catalysts, condensation or neutralizing agents, for example ion exchangers, such as cation exchangers, e.g., in the H+ form, depending on the nature of the reaction and/or of the reactants at reduced, normal or elevated temperature, for example in a temperature range of from about -100 °C to about 190°C, including, for example, from approximately -80°C to approximately 150°C, for example at from -80 to -60°C, at room temperature, at from -20 to 40°C or at reflux temperature, under atmospheric pressure or in a closed vessel, where appropriate under pressure, and/or in an inert atmosphere, for example under an argon or nitrogen atmosphere.
At all stages of the reactions, mixtures of isomers that are formed can be separated into the individual isomers, for example diastereo isomers or enantiomers, or into any desired mixtures of isomers, for example racemates or mixtures of diastereo isomers, for example analogously to the methods described in Science of Synthesis: Houben-Weyl Methods of Molecular Transformation. Georg Thieme Verlag, Stuttgart, Germany. 2005.
The solvents from which those solvents that are suitable for any particular reaction may be selected include those mentioned specifically or, for example, water, esters, such as lower alkyl-lower alkanoates, for example ethyl acetate, ethers, such as aliphatic ethers, for example diethyl ether, or cyclic ethers, for example tetrahydrofurane or dioxane, liquid aromatic hydrocarbons, such as benzene or toluene, alcohols, such as methanol, ethanol or 1- or 2-propanol, nitriles, such as acetonitrile, halogenated hydrocarbons, such as methylene chloride or chloroform, acid amides, such as dimethylformamide or dimethyl acetamide, bases, such as heterocyclic nitrogen bases, for example pyridine or N-methylpyrrolidin-2- one, carboxylic acid anhydrides, such as lower alkanoic acid anhydrides, for example acetic anhydride, cyclic, linear or branched hydrocarbons, such as cyclohexane, hexane or isopentane, or mixtures of those solvents, for example aqueous solutions, unless otherwise indicated in the description of the processes. Such solvent mixtures may also be used in working up, for example by chromatography or partitioning.
The compounds, including their salts, may also be obtained in the form of hydrates, or their crystals may, for example, include the solvent used for crystallization. Different crystalline forms may be present.
The invention relates also to those forms of the process in which a compound obtainable as an intermediate at any stage of the process is used as starting material and
the remaining process steps are carried out, or in which a starting material is formed under the reaction conditions or is used in the form of a derivative, for example in a protected form or in the form of a salt, or a compound obtainable by the process according to the invention is produced under the process conditions and processed further in situ.
The present invention also relates to pro-drugs of a compound of the present invention that are converted in vivo to the compounds of the present invention as described herein. Any reference to a compound of the present invention is therefore to be understood as referring also to the corresponding pro-drugs of the compound of the present invention, as appropriate and expedient.
In accordance with the foregoing the present invention provides in a yet further aspect:
• A pharmaceutical combination comprising a) a first agent which is a compound of the invention, e.g. a compound of formula I or any subformulae thereof, and b) a co- agent, e.g. a second drug agent as defined above.
• A method as defined above comprising co-administration, e.g. concomitantly or in sequence, of a therapeutically effective amount of a compound of the invention, e.g. a compound of formula I or any subformulae thereof, and a co-agent, e.g. a second drug agent as defined above.
The terms "co-administration" or "combined administration" or the like as utilized herein are meant to encompass administration of the selected therapeutic agents to a single patient, and are intended to include treatment regimens in which the agents are not necessarily administered by the same route of administration or at the same time. Fixed combinations are also within the scope of the present invention. The administration of a pharmaceutical combination of the invention results in a beneficial effect, e.g. a synergistic therapeutic effect, compared to a monotherapy applying only one of its pharmaceutically active ingredients.
Each component of a combination according to this invention may be administered separately, together, or in any combination thereof.
The compound of the invention and any additional agent may be formulated in separate dosage forms. Alternatively, to decrease the number of dosage forms
administered to a patient, the compound of the invention and any additional agent may be formulated together in any combination. For example, the compound of the invention inhibitor may be formulated in one dosage form and the additional agent may be formulated together in another dosage form. Any separate dosage forms may be administered at the same time or different times.
Alternatively, a composition of this invention comprises an additional agent as described herein. Each component may be present in individual compositions, combination
compositions, or in a single composition.
Exemplification of the Invention
The invention is further illustrated by the following examples, which should not be construed as further limiting. The assays used throughout the Examples are accepted. Demonstration of efficacy in these assays is predictive of efficacy in subjects.
GENERAL SYNTHESIS METHODS
All starting materials, building blocks, reagents, acids, bases, dehydrating agents, solvents, and catalysts utilized to synthesis the compounds of the present invention are either commercially available or can be produced by organic synthesis methods known to one of ordinary skill in the art (Houben-Weyl 4th Ed. 1952, Methods of Organic Synthesis, Thieme, Volume 21 ). Further, the compounds of the present invention can be produced by organic synthesis methods known to one of ordinary skill in the art as shown in the following examples.
LIST OF ABBREVIATIONS
Ac acetyl
ACN Acetonitrile
AcOEt / EtOAc Ethyl acetate
AcOH acetic acid
aq aqueous
Ar aryl
Bn benzyl
Bu butyl (nBu = n-butyl, tBu = tert-butyl)
CDI Carbonyldiimidazole
CH3CN Acetonitrile
DBU 1 ,8-Diazabicyclo[5.4.0]-undec-7-ene
Boc20 di-tert-butyl dicarbonate
DCE 1 ,2-Dichloroethane
DCM Dichloromethane
DiBAI-H Diisobutylaluminum Hydride
DIPEA N-Ethyldiisopropylamine
DMAP Dimethylaminopyridine
DMF N,N'-Dimethylformamide
DMSO Dimethylsulfoxide
El Electrospray ionisation
Et20 Diethylether
Et3N Triethylamine
Ether Diethylether
EtOAc Ethylacetate
EtOH Ethanol
FC Flash Chromatography
h hour(s)
HATU 0-(7-Azabenzotriazole-1-yl)-N,N,N'N'- tetramethyluronium hexafluorophosphate
HBTU 0-(Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate
HCI Hydrochloric acid
HMPA Hexamethylphosphoramide
HOBt 1-Hydroxybenzotriazole
HPLC High Performance Liquid Chromatography
H20 Water
L liter(s)
LC-MS Liquid Chromatography Mass Spectrometry
LiHMDS Lithium bis(trimethylsilyl)amide
MgS04 Magnesium Sulfate
Me methyl
Mel lodomethane
MeOH Methanol
mg milligram
min minute(s)
mL milliliter
MS Mass Spectrometry
NaHC03 Sodium Bicarbonate
Na2S04 Sodium Sulfate
NH2OH hydroxylamine
Pd/C palladium on charcoal
Pd(OH)2 palladium hydroxide
PG protecting group
Ph phenyl
Ph3P triphenyl phosphine
Prep Preparative
Rf ratio of fronts
RP reverse phase
Rt Retention time
rt Room temperature
Si02 Silica gel
SOCI2 Thionyl Chloride
TBAF Tetrabutylammonium
TEA Triethylamine
TFA Trifluoroacetic acid
THF Tetrahydrofuran
TLC Thin Layer Chromatography
General Conditions:
Mass spectra were run on LC-MS systems using electrospray ionization. These were WATERS Acquity Single Quard Detector. [M+H]+ refers to mono-isotopic molecular weights.
NMR spectra were run on open access Varian 400 NMR spectrometers. Spectra were measured at 298K and were referenced using the solvent peak.
If not indicated otherwise, the analytical UPLCconditions are as follows:
Method A
Column Phenonemax Kinetix C18 Column; 2.1 mm x 50 mm; 2.6 u core size
Column Temperature 50 °C
Eluents solvent A: water with 0.1 % TFA; solvent B: CH3CN with 0.1 % TFA
Flow Rate 1.2 mL/min
Gradient 2-88% solvent B in 9.5 mins
Synthesis of LpxC inhibitors
1.1.1 Synthesis of compound 1.1.1
Step 1. Synthesis of ethyl 4-(([1,1'-biphenyl]-4-ylmethyl)amino)tetrahydro-2H- thiopyran-4-carboxylate 1 ,1 -dioxide [1.1.1a]
1.1.1a
4-( Bromomethyl)biphenyl (150 mg, 0.607 mmol) and Ethyl 4-aminotetrahydro-2H- thiopyran-4-carboxylate 1 ,1-dioxide (178 mg, 0.728 mmol) were combined in ethanol (5.0 mL) in a microwave vial, and K2C03 (839 mg, 6.07 mmol) was added. The vial was sealed and heated to 60 °C for 24 hours. The mixture was filtered and the filtrate was evaporated. The residue was purified by silica gel column chromatography (EtOAc/heptane) to give product 1.1.1a (31.1 mg, 13 % yield). LCMS (m/z): 388.3 [M+H]+
Step 2. Synthesis of 4-(([1,1'-biphenyl]-4-ylmethyl)amino)tetrahydro-2H- thiopyran-4-carboxylic acid 1,1-dioxide [1.1.1 b]
To a mixture of 1.1.1a (22 mg, 0.057 mmol) in THF (0.4 mL), MeOH (0.1 mL,) and Water (0.1 mL) was added LiOH (7.15 mg, 0.170 mmol) and the resulting mixture was stirred at room temperature overnight. The reaction mixture was acidified by addition of 1 N HCI aqueous solution until pH=2 and then diluted with EtOAc. The organic layer was separated from the water layer and concentrated. Azeotroped 2x with toluene obtaining product
1.1.01 b (20.41 mg, 100 % yield). The crude material was used in the next step with no further purification. LCMS (m/z) 360.2 [M+H]+
Step 3. Synthesis of 4-(([1,1'-biphenyl]-4-ylmethyl)amino)-N-((tetrahydro-2H- pyran-2-yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide [1.1.1c]
To a solution of 1.1.01 b (20 mg, 0.056 mmol) in DCM (1 mL) was added Et3N (0.039 ml, 0.278 mmol), EDC.HCI (16.00 mg, 0.083 mmol), HOBT (15.34 mg, 0.100 mmol) and O- (tetrahydro-2H-pyran-2-yl)hydroxylamine (13.04 mg, 0.1 1 1 mmol). The reaction mixture was stirred at room temperature for 72 hours. The reaction mixture was quenched with water and extracted with EtOAc. The organic layer was washed with water and brine, dried over magnesium sulfate, filtered and concentrated. The residue was purified by silica gel column chromatography to afford product 1.1.1c (13.4 mg, 52.5 % yield). LCMS (m/z) 459.3 [M+H]+
Step 4. Synthesis of 4-(([1,1'-biphenyl]-4-ylmethyl)amino)-N-hydroxytetrahydro- 2H-thiopyran-4-carboxamide 1 ,1-dioxide [1.1.1]
1.1.1
To a solution of 1.1.1c (13.4 mg, 0.029 mmol) in Ethanol (2 mL) was added concentrated HCI aqueous solution (0.009 mL) was added. After stirring at room
temperature for 24 hours, aother 0.1 mL of Cone. HCI added, and the solution was stirred for another 96 hours. The reaction mixture was centrifuged and supernatant was removed. The remaining solid was triturated with Et20, centrifuged again. The solid residue was dried under vacuum to give 1.1.1 (10 mg, 81 % yield) as a white powder. LCMS (m/z) 375.2
[M+H]+ 1H NMR (400 MHz, CD3OD) δ ppm 2.36 - 2.71 (m, 4 H) 2.78 - 2.97 (m, 2 H) 3.25 (m, J=3.13, 1.57 Hz, 2 H) 4.15 (s, 2 H) 7.32 - 7.40 (m, 1 H) 7.42 - 7.49 (m, 2 H) 7.50 - 7.60 (m, 2 H) 7.60 - 7.67 (m, 2 H) 7.71 - 7.76 (m, 2 H)
Step 1. Synthesis of methyl 4-((4-bromobenzyl)amino)tetrahydro-2H-thiopyran- 4-carboxylate 1,1 -dioxide [1.1.2a]
Methyl 4-aminotetrahydro-2H-thiopyran-4-carboxylate 1 ,1-dioxide (1 g, 4.83 mmol) and 4-bromobenzaldehyde (0.893 g, 4.83 mmol) were dissolved in 1 ,2-dichloroethane (48.3 mL) and the resulting solution was stirred overnight at room temperature. To the solution was added acetic acid (0.829 mL, 14.48 mmol) and the reaction mixture was allowed to stir another 24 hours, at which time sodium triacetoxyborohydride (3.07 g, 14.48 mmol) was added and the reaction mixture was stirred for 72 hours. The reaction was quenched with water and extracted with diethyl ether. The organic layer was washed water, saturated NaHC03 aqueous solution and brine, then dried over MgS04 and concentrated to afford product 1.1.2a (1.8 g,100 % yield). The crude material was used in the next step without further purification. LCMS (m/z) 376.5 [M+H]+; 1H NMR (400 MHz, CDCI3) δ ppm 2.19 - 2.29 (m, 2 H) 2.49 - 2.60 (m, 2 H) 2.83 - 2.94 (m, 2 H) 3.41 (td, J=13.38, 3.42 Hz, 2 H) 3.53 (br. s, 2 H) 3.80 (s, 3 H) 7.18 - 7.22 (m, 2 H) 7.44 - 7.50 (m, 2 H)
Step 2. Synthesis of 4-((4-bromobenzyl)amino)tetrahydro-2H-thiopyran-4- carboxylic acid 1,1-dioxide [1.1.2b]
1.1.2b
To a solution of 1.1.2a (3.64 g, 9.67 mmol) in MeOH (55 ml.) and water (9 ml.) was added LiOH H20 (1.2 g, 29.0 mmol) and the resulting solution was stirred at room temperature for 24 hours. The mixture was then concentrated in vacuo to 1/3 of its volume, diluted with water and brought to a pH of 4 with 1.0 M HCI aqueous solution. The white precipitate was collected, washed with diethyl ether, dried on vacuum to afford 1.1.02b (2.83 g, 81 % yield). LCMS (m/z) 364.0 [M+H]+; 1H NMR (400 MHz, DMSO-d6) δ ppm 2.06 - 2.28 (m, 4 H) 2.95 (d, J=12.13 Hz, 2 H) 3.15 - 3.32 (m, 2 H) 3.56 (s, 2 H) 7.30 - 7.35 (m, 2 H) 7.48 - 7.53 (m, 2 H)
Step 3. Synthesis of 4-((4-bromobenzyl)amino)-N-((tetrahydro-2H-pyran-2- yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.1.2c]
1.1.2c
EDC HCI (2.097 g, 10.94 mmol) and aza-HOBt (1.914 g, 14.06 mmol) was added to a solution of 1.1.2b (2.83 g, 7.81 mmol), 0-(tetrahydro-2H-pyran-2-yl)hydroxylamine (1.373 g, 1 1.72 mmol) and Et3N (4.12 ml, 29.7 mmol) in DMF (39.1 ml_). After stirring at room temperature for 96 hours, the reaction mixture was quenched with water and saturated aqueous NH4CI solution. The mixture was extracted with diethyl ether and DCM until no more product was present in the aqueous layer. The combined organic layers were dried (MgS04), filtered and concentrated. Purification by silica gel column chromatography (EtOAc/heptane, 50-100%) afforded 1.1.2c (3.1 g, 86 % yield) as a white powder. LCMS (m/z) 463.1 [M+H]+; 1H NMR (400 MHz, CDCI3) δ ppm 1.63 - 2.03 (m, 6 H) 2.14 - 2.36 (m, 2 H) 2.41 - 2.68 (m, 2 H) 3.07 - 3.45 (m, 4 H) 3.48 - 3.73 (m, 3 H) 3.80 - 3.99 (m, 1 H) 4.96 (br. s., 1 H) 7.17 - 7.23 (m, 2 H) 7.42 - 7.52 (m, 2 H) 9.37 (br. s., 1 H)
Step 4. Synthesis of N-hydroxy-4-(((4'-methoxy-[1,1'-biphenyl]-4- yl)methyl)amino)tetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.1.2]
PdCI2(dppf).CH2CI2 (17.70 mg, 0.022 mmol) was added to a degassed solution of 1.1.2c (100 mg, 0.217 mmol), (4-methoxyphenyl) boronic acid (49.4 mg, 0.325 mmol) and Na2C03 (0.325 ml_, 0.650 mmol) in dimethoxy ether (1.1 ml_). The reaction was heated to 110 °C with microwave for 20 minutes, then was placed in a 100 °C sand bath for 1 hour. The reaction mixture was diluted with DCM and neutralized with saturated aqueous NH4CI solution. The aqueous layer was extracted with DCM. The organic was stirred with
Siliabond-DMT palladium-scavenger, then filtered and concentrated. Purification by silica gel column chromatography (EtO Ac/heptane 30-100%) afforded 4-(((4'-methoxy-[1 ,1 '-biphenyl]- 4-yl)methyl)amino)-N-((tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide 1 ,1-dioxide (15.8 mg, 15 % yield). The white solid was dissolved in HCI in EtOH (1.25 M.1.7 ml_, 2.2 mmol) and stirred for 1 hour. The white precipitate was collected by filtration to afford 1.1.2 (10.6 mg, 10 % yield). LCMS (m/z) 405.2 [M+H]+ 1H NMR (400 MHz, CD3OD) δ ppm 2.31 - 2.69 (m, 4 H) 2.91 (d, J=14.48 Hz, 2 H) 3.16 - 3.24 (m, 2 H) 3.75 - 3.90 (m, 3 H) 4.15 (s, 2 H) 6.95 - 7.07 (m, 2 H) 7.44 - 7.61 (m, 4 H) 7.69 (d, J=8.22 Hz, 2 H)
1.1.3 Synthesis of compound 1.1.3
Compound 1.1.3 was prepared following the procedures described for the synthesis of 1.1.2 using (4-chloro-2-fluorophenyl)boronic acid in Step 4. LCMS (m/z) 427.1 [M+H]+ 1H NMR (400 MHz, CD3OD) ppm 2.53 (d, J=7.43 Hz, 4 H) 2.88 (m, J=13.69 Hz, 4 H) 4.17 (s, 2 H) 7.25 - 7.36 (m, 2 H) 7.45 - 7.54 (m, 1 H) 7.55 - 7.70 (m, 4 H)
1.1.4 Synthesis of compound 1.1.4
Compound 1.1.4 was prepared similarly to the synthesis for 1.1.2 changing the Suzuki coupling conditions in Step 4 as following. Pd(PPh3)4 (50.1 mg, 0.043 mmol) was added to a degassed mixture of 1.1.2c (100 mg, 0.217 mmol), 4-(4-(4,4,5,5-tetramethyl- 1 ,3,2-dioxaborolan-2-yl)benzyl)morpholine (99 mg, 0.325 mmol), Na2C03 (68.9 mg, 0.650 mmol), THF (1.1 mL) and Water (0.36 mL). The reaction mixture was heated to 70 °C for 18 hours. The reaction mixture was diluted with DCM and neutralized with saturated aqueous NH4CI solution. The aqueous layer was extracted with DCM. The combined organic layer was dried over magnesium sulfate, stirred with Siliabond DMT pd. Scavenger, filtered and dried on to silica. Purification by silica gel column chromatography (MeOH/DCM, 1-10%) afforded 4-(((4'-(morpholinomethyl)-[1 , 1 '-biphenyl]-4-yl)methyl)amino)-N-((tetrahydro-2H- pyran-2-yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide 1 ,1-dioxide (23 mg, 19 % yield) 1H NMR (400 MHz, CDCI3) ppm 1.13 - 1.30 (m, 7 H) 1.64 - 1.89 (m, 6 H) 2.17 - 2.40 (m, 2 H) 2.48 (br. s., 7 H) 3.42 (br. s., 2 H) 3.55 (s, 2 H) 3.60 - 3.78 (m, 8 H) 7.39 (d, J=4.30 Hz, 2 H) 7.41 (d, J=3.91 Hz, 2 H) 7.54 (d, J=7.83 Hz, 2 H) 7.58 (d, J=8.22 Hz, 2 H). The product was dissolved in 1.25 M HCI in EtOH (3.5 mL, 4.33 mmol) and the resulting solution was stirred at room temperature for 3 hours. The precipitate was collected by filtration and washed with Et20 to afford product 1.1.4 (13.09 mg, 10 % yield) as a white solid. 1H NMR (400 MHz, CDCI3) δ ppm 3.16 - 3.28 (m, 5 H) 3.35 - 3.46 (m, 4 H) 3.75 (t, J=12.13 Hz, 2 H) 4.06 (d, J=12.13 Hz, 2 H) 4.14 (br. s., 2 H) 4.42 (s, 2 H) 7.64 (d, J=8.22 Hz, 4 H) 7.72 - 7.84 (m, 4 H) LCMS (m/z) 474.2 [M+H]+
StepL Synthesis of (1 E,2E)-1,2-bis(2-phenylhydrazono)ethane. [1.1.5a]
1.1.5a
Phenylhydrazine (1.8 ml_, 18.49 mmol) was dissolved in MeOH (30 ml_). To this solution, a solution of oxalaldehyde (1.0 ml_, 9.25 mmol) in MeOH (10.0 ml_) was added, followed by AcOH (0.032 ml_, 0.555 mmol). The mixture was then stirred at room temperature overnight. The precipitate was collected by filtration, washed with MeOH and dried in the air to afford 1.1.5a (1.37 g, 31.1 % yield) as a pale yellow solid. LCMS (m/z) 239.1 [M+H]+
Step2. Synthesis of 2-phenyl-2H-1,2,3-triazole. [1.1.5b]
1.1.5b
1.1.5a (2.9 g, 12.17 mmol) was suspended in ethylene glycol (50 ml_) in a glass pressure vessel and Copper(ll)Triflate (0.220 g, 0.609 mmol) was added. The mixture was stirred at 180 °C for 2 hours and at room temperature for 24 hours. The reaction mixture was partitioned between water and ethyl acetate. The phases were separated and the aqueous layer was extracted with ethyl acetate. The combined organic layer was washed with water, brine and dried over sodium sulfate. The organic layer was filtered, evaporated onto silica gel and purified by silica gel column chromatography to give product 1.1.5b (1.22 g, 69.1 % yield). LCMS (m/z) 146.2 [M+H]+ 1H NMR (400 MHz, CDCI3) 8.10 (d, J = 7.83 Hz, 2H), 7.82 (s, 2H), 7.50 (t, J = 7.83 Hz, 2H), 7.32 - 7.41 (m, 1 H)
Step3. Synthesis of 2-(4-bromophenyl)-2H-1,2,3-triazole. [1.1.5c]
1.1.5c
1.1.5b (68 mg, 0.468 mmol) was dissolved in concentrated H2S04 (2 mL) and Br2 (0.024 mL, 0.468 mmol) was added, followed by Ag2S04 (175 mg, 0.56 mmol). After stirring at room temperature for 40 minutes, the reaction mixture was poured into water and extracted with ethyl acetate. The organic layer was dried (Na2S04) and concentrated to afford 1.1.5c (40 mg, 38 % yield) as an off white solid. LCMS (m/z) 225.9 [M+H]+ 1H NMR (400 MHz, CDCI3) 7.99 (d, J = 8.61 Hz, 2H), 7.83 (s, 2H), 7.62 (d, J = 8.61 Hz, 2H)
Step 5. 2-(4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenyl)-2H-1 ,2,3- triazole. [1.1.5d]
A microwave vial was charged with 1.1.5c (1.66 g, 7.41 mmol) and 1 ,4-dioxane (60 mL). KOAc (2.254 g, 22.97 mmol) and bis(pinacolato)diboron (2.258 g, 8.89 mmol) were added and the mixture was purged with N2 for 5 minutes. PdCI2(dppf).CH2CI2 adduct (0.605 g, 0.741 mmol) was then added and the reaction mixture was stirred at 68 °C for 5 hours. The mixture was diluted with EtOAc and stirred with Siliabond DMT overnight, then washed with water, brine, dried filtered and evaporated onto silica gel. Purification by flash column chromatography on silica gel (EtO Ac/Heptane, 0 to 40%) afforded product 1.1.5d (1.38 g, 68.7 % yield) as an off white solid. LCMS (m/z) 272.2 [M+H]+ 1H NMR (400 MHz, CDCI3) δ 8.10 (d, J = 8.61 Hz, 2H), 7.94 (d, J = 8.22 Hz, 2H), 7.81 - 7.86 (m, 2H), 1.34 - 1.42 (m, 12H)
Step 6. Synthesis of 4-(((4,-(2H-1,2,3-triazol-2-yl)-[1,1,-biphenyl]-4- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide [1.1.5]
Compound 1.1.5 was prepared following the procedures described for the synthesis 1.1.04 using 1.1.5d in step 4.. LC/MS (m/z) 442.2 [M+H]+. 1H NMR (400 MHz, DMSO-d6) ppm 2.51 - 2.85 (m, 4 H) 2.96 - 3.23 (m, 2 H) 3.29 - 3.63 (m, 2 H) 3.70 - 4.27 (m, 2 H) 7.62 (br. s., 2 H) 7.80 (d, J=7.04 Hz, 2 H) 7.90 (d, J=8.61 Hz, 2 H) 8.02 - 8.27 (m, 4 H)
1.1.6 Synthesis of compound 1.1.6
Compound 1.1.6 was prepared following the procedures described for the synthesis 1.1.4 using 4-(4-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenyl)morpholine in Step 4. LC/MS (m/z) 460.2 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 2.34 (br. s., 2 H) 2.56 (s, 2 H) 3.04 (d, J=1.57 Hz, 2 H) 3.13 (d, J=4.70 Hz, 4 H) 3.23 - 3.30 (m, 2 H) 3.68 - 3.91 (m, 7 H) 6.98 (d, J=8.61 Hz, 2 H) 7.39 (d, J=8.22 Hz, 2 H) 7.44 - 7.49 (m, 2 H) 7.55 (d, J=7.43 Hz, 2
H)
1.1.7 Synthesis of compound 1.1.7
Compound 1.1.7 was prepared following the procedures described for the synthesis 1.1.4 using 2-(4-cyclopropylphenyl)-4, 4, 5, 5-tetramethyl-1 ,3,2-dioxaborolane in step 4.
LC/MS (m/z) 415.2 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 0.58 - 0.67 (m, 2 H) 0.86 - 0.96 (m, 2 H) 1.80 - 1.91 (m, 1 H) 2.28 - 2.44 (m, 4 H) 3.04 (br. s., 2 H) 3.25 - 3.34 (m, 2 H) 3.84 (br. s., 2 H) 7.06 (d, J=8.22 Hz, 2 H) 7.42 (dd, J=8.02, 3.33 Hz, 4 H) 7.56 (d, J=7.83 Hz, 2 H).
1.1.8 Synthesis of compound 1.1.8
Compound 1.1.8 was prepared following the procedures described for the synthesis 1.1.4 using naphthalen-2-ylboronic acid in step 4. LC/MS (m/z) 425.2 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm 3.00 - 3.23 (m, 2 H) 3.28 - 3.54 (m, 2 H) 3.58 - 4.26 (m, 7 H) 7.44 - 7.70 (m, 4 H) 7.80 - 7.97 (m, 3 H) 8.00 (t, J=8.02 Hz, 2 H) 8.24 (s, 1 H)
1.1.9 Synthesis of compound 1.1.9
Compound 1.1.9 was prepared following the procedures described for the synthesis 1.1.4 using (2-fluoro-4-methoxyphenyl)boronic acid in step 4. LC/MS (m/z) 423.2 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 2.50 (br. s., 4 H) 2.87 (br. s., 2 H) 3.14 - 3.27 (m, 3 H) 3.84 (s, 3 H) 4.1 1 (br. s., 2 H) 6.76 - 6.89 (m, 2 H) 7.40 (t, J=8.80 Hz, 1 H) 7.54 (d, J=7.04 Hz, 2 H) 7.57 - 7.63 (m, 2 H).
1.1.10 Synthesis of compound 1.1.10
Compound 1.1.10 was prepared following the procedures described for the synthesis 1.1.04 using 4,4,5,5-tetramethyl-2-(4-(((1 r,4r)-4-((tetrahydro-2H-pyran-2- yl)oxy)cyclohexyl)methoxy)phenyl)-1 ,3,2-dioxaborolane (ref. WO2011/73845 A1 , 201 1 ) in step 4. LC/MS (m/z) 503.2 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 1.02 - 1.30 (m, 4 H) 1.59 - 1.73 (m, 1 H) 1.81 - 1.98 (m, 4 H) 2.28 - 2.39 (m, 2 H) 2.47 - 2.65 (m, 2 H) 3.04 (dt, J=3.28, 1.64 Hz, 2 H) 3.25 (d, J=7.24 Hz, 2 H) 3.38 - 3.50 (m, 1 H) 3.67 - 3.90 (m, 4 H) 6.85 - 6.92 (m, 2 H) 7.39 (d, J=8.27 Hz, 2 H) 7.42 - 7.48 (m, 2 H) 7.53 (d, J=7.97 Hz, 2 H).
1.1.11 Synthesis of compound 1.1.11
Compound 1.1.11 was prepared following the procedures described for the synthesis 1.1.4 using (2-fluorophenyl)boronic acid in step 4. LC/MS (m/z) 393.2 [M+H]+. 1H NMR (400 MHz, <CD3CN>) δ ppm 2.62 (t, J=1 1.93 Hz, 2 H) 2.98 (br. s., 2 H) 3.15 - 3.29 (m, 2 H) 3.44 (d, J=1 1.74 Hz, 2 H) 4.16 (s, 2 H) 7.16 - 7.35 (m, 2 H) 7.39 - 7.48 (m, 1 H) 7.54 (td, J=7.92,
1.76 Hz, 1 H) 7.61 - 7.69 (m, 2 H) 7.74 (d, J=8.22 Hz, 2 H) 9.79 (br. s., 2 H) 1 1.71 (br. s., 1 H)
1.1.12 Synthesis of compound 1.1.12
Compound 1.1.12 was prepared following the procedures described for the synthesis 1.1.4 using pyridin-4-ylboronic acid in step 4. LC/MS (m/z) 276.2 [M+H]+, 1H NMR (400 MHz, <CD3OD>) δ ppm 2.26 - 2.53 (m, 4 H) 3.00 (d, J=12.91 Hz, 2 H) 3.35 - 3.46 (m, 3 H) 3.77 (br. s., 2 H) 7.68 (d, J=8.22 Hz, 2 H) 7.97 (d, J=8.22 Hz, 2 H) 8.31 (d, J=6.26 Hz, 2 H) 8.80 (d, J=6.65 Hz, 2 H).
1.1.13 Synthesis of 4-(((2-f luoro-[1 ,1 '-biphenyl]-4-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.1.13]
1.1.13
Step 1. Synthesis of Methyl 4-((4-bromo-3-fluorobenzyl)amino)tetrahydro-2H- thiopyran-4-carboxylate 1,1 -dioxide [1.1.13a]
1.1.13a
4-Bromo-3-fluorobenzaldehyde (1.39 g, 6.88 mmol, 1.9 equiv) was mixed with methyl 4-aminotetrahydro-2H-thiopyran-4-carboxylate 1 ,1-dioxide (0.750 g, 3.62 mmol) and DCE (18 mL) was added followed by AcOH (1.24 mL, 21.71 mmol, 6 equiv) and Na(AcO)3BH (2.15 g, 10.13mmol, 2.8 equiv). The mixture was stirred under nitrogen at ambient temperature for 48 hours. The reaction was quenched by slowly pouring it into saturated aqueous NaHC03 solution and then extracted with EtOAc. The combined organic layers were dried over sodium sulfate, filtered and concentrated. The crude material was purified by silica gel column chromatography, (EtOAc/heptane, 0-100%) to afford Methyl 4-((4-bromo-3- fluorobenzyl)amino)tetrahydro-2H-thiopyran-4-carboxylate 1 , 1-dioxide 1.1.13a as a white solid (1.25 g, 88%) MS m/z 394.1 [M+H]+
Step 2. Synthesis of 4-((4-bromo-3-fluorobenzyl)amino)tetrahydro-2H- thiopyran-4-carboxylic acid 1,1-dioxide [1.1.13b]
1.1.13b
Methyl 4-((4-bromo-3-fluorobenzyl)amino)tetrahydro-2H-thiopyran-4-carboxylate 1 ,1- dioxide 1.1.13a (1.25 g, 3.17 mmol) was dissolved in THF/MeOH (16mL, 4/1 ) and 2.0 M LiOH aqueous solution (4.76 mL, 9.51 mmol, 3 equiv) was added. The mixture was stirred at ambient temperature for 17 hours. The volatiles were removed under reduced pressure and 6.0 N HCI aqueous solution was added until the pH was ~1. The white precipitate was collected by filtration to afford Methyl 4-((4-bromo-3-fluorobenzyl)amino)tetrahydro-2H- thiopyran-4-carboxylate 1 ,1-dioxide 1.1.13b as a white solid (1.25g, 100%). MS m/z 380.0
[M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm 7.66 (t, J=7.80 Hz, 1 H) 7.43 (dd, J=10.10, 1.59 Hz, 1 H) 7.20 (dd, J=8.24, 1.49 Hz, 1 H) 3.65 (s, 2 H) 3.20 - 3.33 (m, 2 H) 2.97 (d, J=12.96 Hz, 2 H) 2.16 - 2.29 (m, 4 H)
Step 3. Synthesis of 4-((4-bromo-3-fluorobenzyl)amino)-N-((tetrahydro-2H- pyran-2-yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide [1.1.13c]
1.1.13c
Methyl 4-((4-bromo-3-fluorobenzyl)amino)tetrahydro-2H-thiopyran-4-carboxylate 1 ,1- dioxide 1.1.13b (1.20 g, 3.17 mmol), 0-(tetrahydro-2H-pyran-2-yl)hydroxylamine (0.557 g, 4.76 mmol, 1.5 equiv), EDC HCI (0.851 g, 4.44 mmol, 1.4 equiv) and aza-HOBt (0.777g, 5.71 mmol, 1.8 equiv) were dissolved in DMF (16 ml_) and triethylamine (0.795 ml_, 5.71 mmol, 1.8 equiv) was added. The solution was stirred at ambient temperature for 45 hours. The solution was poured into water and extracted with EtOAc. The combined organic layers were washed with brine, dried over sodium sulfate, filtered and concentrated. The crude material was purified by silica gel column chromatography (EtOAc/heptane 0 to 100%) to afford 4-((4-bromo-3-fluorobenzyl)amino)-N-((tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H- thiopyran-4-carboxamide 1 ,1-dioxide 1.1.13c as a white solid (1.24g, 82%). MS m/z 497.1 [M+H]+
Step 4. Synthesis of_ 4-(((2-fluoro-[1,1'-biphenyl]-4-yl)methyl)amino)-N- ((tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide
[1.1.13d]
1.1.13d
Na2C03 aqueous solution (2.0 M, 0.834ml_,1.66 mmol, 4.0 equiv) was added to a mixture of 1.1.13c (0.2 g, 0.417 mmol), phenylboronic acid (63.6 mg, 0.522 mmol, 1.25
equiv), DME (2.0 mL) and EtOH (2.0 mL). The mixture was purged with nitrogen 15 minutes and then Pd(PPh3)4 (14.4mg, 0.013 mmol, 0.03equiv) was added. The mixture was heated at 1 10 °C with microwave for 1 hour. The mixture was diluted with EtOAc, washed with water and brine, dried over sodium sulfate, filtered and concentrated. The residue was purified by silica gel column chromatography, (EtOAc/heptane, 0 to 100%) to afford 4-((4-bromo-3- fluorobenzyl)amino)-N-((tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-thiopyran-4- carboxamide 1 ,1-dioxide 1.1.13d as a yellow solid. (14mg, 7%). MS m/z 477.3 [M+H]+
Step 5. Synthesis of 4-(((2-fluoro-[1,1'-biphenyl]-4-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.1.13]
1.1.13
4-((4-bromo-3-fluorobenzyl)amino)-N-((tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H- thiopyran-4-carboxamide 1 ,1-dioxide 1.1.13c (14 mg, 0.029 mmol) was dissolved in
DCM/MeOH (0.294 mL,4/1 ) and 4.0 M HCI in dioxane (0.0734mL, 0.294 mmol) was added. The reaction was stirred at ambient temperature for 15 minutes. The solvents were removed under reduced pressure. The remaining material was purified by reverse phase HPLC to afford 1.1.13 as a white powder (4mg, 26%). MS m/z 393.1 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm 7.46 - 7.58 (m, 5 H) 7.37 - 7.44 (m, 2 H) 7.32 (d, J=7.83 Hz, 1 H) 3.26 - 3.38 (m, 3 H) 2.95 - 3.05 (m, 2 H) 2.16 - 2.30 (m, 3 H) Note: Benzylic protons were buried under the water peak.
1.1.14 Synthesis of 4-(((2-fluoro-4'-methoxy-[1,1,-biphenyl]-4-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide [1.1.14 ]
1.1.14
Compound 1.1.14 was prepared following the procedures described for the synthesis of 1.1.13 using (4-methoxyphenyl)boronic acid in Step 4. (The solvent used in Step 4 was changed to THF). MS m/z 423.1 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm 10.91 (br. s.,
1 H) 7.49 (dd, J=8.75, 1.52 Hz, 3 H) 7.26 - 7.42 (m, 2 H) 6.99 - 7.08 (m, 2 H) 3.78 - 3.83 (m, 5 H) 3.27 - 3.41 (m, 3 H) 3.05 (br. s., 2 H) 2.19 - 2.40 (m, 3 H) Note: Benzylic protons buried under water peak.
1.1.15 Synthesis of 4-(((2-fluoro-4'-methoxy-[1,1,-biphenyl]-4-yl)methyl)am
hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.1.15]
1.1.15
Compound 1.1.15 was prepared following the procedures described for the synthesis of 1.1.13 using (2-fluorophenyl)boronic acid in Step 4. (The solvent used in Step 4 was changed to THF) MS m/z 41 1.0 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm 7.40 - 7.54 (m, 4 H) 7.29 - 7.38 (m, 3 H) 3.34 (t, J=12.13 Hz, 3 H) 3.03 (br. s., 2 H) 2.32 (d, J=1.86 Hz, 3 H) Note: Benzylic protons buried under water peak
1.1.16 Synthesis of 4-(((4'-chloro-2,2'-difluoro-[1,1,-biphenyl]-4-yl)methyl)am
hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.1.16]
1.1.16
Compound 1.1.16 was prepared following the procedures described for the synthesis of 1.1.13 using (2-fluorophenyl)boronic acid in Step 4. (The solvent used in Step 4 was changed to THF). MS m/z 445.0 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm 10.81 (br. s., 1 H) 7.59 (dd, J=9.95, 2.03 Hz, 1 H) 7.41 - 7.53 (m, 4 H) 7.35 (d, J=7.58 Hz, 1 H) 3.33 (br. s., 3 H) 3.02 (br. s., 2 H) 2.18 - 2.33 (m, 3 H) Note: Benzylic protons buried under water peak
1.1.17 Synthesis of 4-(((2-fluoro-4,-(2H-1,2,3-triazol-2-yl)-[1,1,-biphenyl]-4- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide
1.1.17
Compound 1.1.17 was prepared following the procedures described for the synthesis of 1.1.13 using (2-(4-(4,4, 5, 5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenyl)-2H-1 ,2,3-triazole (prepared as described in Example 1.1.05d) in Step 4 (Step 4 was changed to heating at 80C for 20h in a sand bath using THF as solvent). MS m/z 460.2 [M+H]+. 1H NMR (400 MHz, DMSO-d6) 5 ppm 10.61 - 1 1.07 (m, 1 H) 8.06 - 8.12 (m, 4 H) 7.70 (dd, J=8.73, 1.44 Hz, 2 H) 7.55 (t, J=7.75 Hz, 1 H) 7.27 - 7.42 (m, 2 H) 3.28 (t, J=1 1.22 Hz, 3 H) 2.98 (br. s., 2 H) 2.14 - 2.29 (m, 3 H) Note: Benzylic protons buried under water peak
1.1.18 Synthesis of N-hydroxy^-^-methyl^'^H-l^^-triazol^-y -t r-biphenyl]^- yl)methyl)amino)tetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.1.18]
1.1.18
Compound 1.1.18 was prepared following the procedures described for the synthesis of 1.1.13 using 2-(4-(4,4, 5, 5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)phenyl)-2H-1 ,2,3-triazole (prepared as described in Example 1.1.05d) in Step 4. (Step 4 was changed to heating at 80C for 20h in a sand bath using THF as solvent). MS m/z 456.3 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm 8.16 (s, 2 H) 8.10 (d, J=8.66 Hz, 2 H) 7.54 (d, J=8.75 Hz, 2 H) 7.25 - 7.42 (m, 3 H) 3.30 - 3.45 (m, 3 H) 2.98 - 3.15 (m, 2 H) 2.31 - 2.41 (m, 3 H) 2.30 (s, 3 H) Note: Benzylic protons buried under water peak
1.1.19 Synthesis of 4-(((2-chloro-2'-fluoro-4'-methoxy-[1,1,-biphenyl]-4- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide
1.1.19
Compound 1.1.19 was prepared following the procedures described for the synthesis of 1.1.13 using (4-chloro-2-fluorophenyl)boronic acid in Step 4. (Step 4 was changed to heating at 80C for 18h in a sand bath using THF as solvent). MS m/z 441.2 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm 7.55 (dd, J=9.76, 1.98 Hz, 1 H) 7.30 - 7.42 (m, 4 H) 7.19 - 7.28 (m, 1 H) 3.36 (br. s., 3 H) 3.07 (br. s., 2 H) 2.25 - 2.42 (m, 3 H) 2.14 (s, 3 H) Note: Benzylic protons buried under water peak
1.1.20 Synthesis of 4-(((2'-fluoro-4,-methoxy-2-methyl-[1,1'-biphenyl]-4- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1- dioxidedioxide [1.1.20]
1.1.20
Compound 1.1.20 was prepared following the procedures described for the synthesis of 1.1.13 using (2-fluoro-4-methoxyphenyl)boronic acid in Step 4. (Step 4 was changed to heating at 80C for 17h in a sand bath using THF as solvent). MS m/z 437.2 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm 7.30 - 7.41 (m, 3 H) 7.20 (t, J=8.73 Hz, 3 H) 6.84 - 6.96 (m, 3 H) 3.82 (s, 5 H) 3.33 - 3.44 (m, 3 H) 3.03 - 3.14 (m, 2 H) 2.29 - 2.45 (m, 3 H) 2.15 (s, 3 H) Note: Benzylic protons were buried under the water peak
1.1.21 Synthesis of 4-(((4'-cyclopropyl-2-methyl-[1,1,-biphenyl]-4-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.1.21 ]
1.1.21
Compound 1.1.21 was prepared following the procedures described for the synthesis of 1.1.13 using (4-cyclopropylphenyl)boronic acid in Step 4. (Step 4 was changed to heating at 80C for 17h in a sand bath using THF as solvent). MS m/z 429.3 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm 7.27 - 7.36 (m, 3 H) 7.18 - 7.23 (m, 4 H) 7.12 - 7.16 (m, 3 H) 3.36 (d, J=9.24 Hz, 3 H) 3.08 (br. s., 2 H) 2.30 - 2.45 (m, 3 H) 2.24 (s, 3 H) 1.96 (tt, J=8.38, 5.03 Hz, 1 H) 0.95 - 1.02 (m, 2 H) 0.67 - 0.74 (m, 2 H) Note: Benzylic protons were buried under the water peak
1.1.22 Synthesis of 4 4-(((2-chloro-2'-fluoro-4'-methoxy-[1,1,-biphenyl]-4- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide
[1.1.22]
1.1.22
Compound 1.1.22 was prepared following the procedures described for the synthesis of 1.1.13 using (2-fluoro-4-methoxyphenyl)boronic acid in Step 4. (Step 4 was changed to heating at 80C for 17h in a sand bath using THF as solvent). MS m/z 457.1 [M+H]+. 1H NMR (400 MHz, DMSO-d6) ppm 10.99 (br. s., 1 H) 7.66 (s, 1 H) 7.36 - 7.51 (m, 2 H) 7.26 (t, J=8.66 Hz, 1 H) 6.84 - 7.01 (m, 2 H) 3.83 (s, 3 H) 3.36 (t, J=1 1.42 Hz, 3 H) 3.06 (d, J=10.91 Hz, 2 H) 2.32 (dd, J=3.69, 1.83 Hz, 3 H) Note: Benzylic protons were buried under the water peak
Step 1. Synthesis of 4-(phenylethynyl)benzaldehyde [1.1.23a]
1.1.23a
To a degassed solution of ethynylbenzene (0.3 ml_, 2.73 mmol), 4-iodobenzaldehyde (761 mg, 3.28 mmol), and Et3N (0.757 mL, 5.46 mmol) in THF (Volume: 21.6 ml_),
Pd(PPh3)2CI2 (96 mg, 0.137 mmol) and Cul (36.4 mg, 0.191 mmol) were added. After stirring at room temperature overnight, the reaction mixture was concentrated on to silica gel and purified by silica gel column chromatography (EtOAc/heptane, 0-50%). Fractions containing product were collected. Pale yellow crystals crashed out, which were washed with pentane and heptane to afford 4-(phenylethynyl)benzaldehyde [1.1.23a] (419 mg, 74.4 % yield). 1H NMR (400 MHz, CDCI3) δ ppm 7.30 - 7.44 (m, 3 H) 7.45 - 7.59 (m, 2 H) 7.62 - 7.73 (m, 2 H) 7.76 - 7.98 (m, 2 H) 10.02 (s, 1 H)
Step 2. Synthesis of methyl 4-((4-(phenylethynyl)benzyl)amino)tetrahydro-2H- thiopyran-4-carboxylate 1,1 -dioxide [1.1.23b]
Compound 1.1.23b was prepared following the procedures described for the synthesis 1.1. 2a using 1.1.23a in StepL 1H NMR (400 MHz,CDCI3) δ ppm 2.26 (d, J=14.48
Hz, 2 H) 2.46 - 2.65 (m, 2 H) 2.89 (d, J=12.91 Hz, 2 H) 3.34 - 3.52 (m, 2 H) 3.59 (s, 2 H) 3.80 (s, 3 H) 7.28 - 7.40 (m, 5 H) 7.47 - 7.58 (m, 4 H).
Step 3. Synthesis of 4-((4-(phenylethynyl)benzyl)amino)tetrahydro-2H- thiopyran-4-carboxylic acid 1,1 -dioxide [1.1.23c]
Compound 1.1.23c was prepared following the procedures described for the synthesis 1.1.2b in Step 2. LC/MS (m/z) 384.2 [M+H]+.
Step 4. Synthesis of 4-((4-(phenylethynyl)benzyl)amino)-N-((tetrahydro-2H- pyran-2-yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.1.23d]
Compound 1.1.23d was prepared following the procedures described for the synthesis 1.1.2c in Step 3. LC/MS (m/z) 384.2 [M+H]+.
Step 5. Synthesis of N-hydroxy-4-((4-(phenylethynyl)benzyl)amino)tetrahydro- 2H-thiopyran-4-carboxamide 1,1 -dioxide [1.1.23]
Compound 1.1.23d (148 mg) was dissolved in 4 M HCI in dioxane and the solution was stirred at room temperature of 1 hour, during which time, white precipitated crashed out.
The precipitate was collected by filtration to afford 1.1.23 (71 mg, 41.9 % yield). LC/MS (m/z) 399.1 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 2.53 (br. s., 2 H) 2.88 (d, J=12.13 Hz, 2 H) 3.16 - 3.29 (m, 4 H) 4.14 (s, 2 H) 7.32 - 7.42 (m, 3 H) 7.46 - 7.55 (m, 4 H) 7.57 - 7.68 (m, 2 H).
1.1.24 Synthesis of 4-((4-(cyclopropylethynyl)benzyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1,1 -dioxide 1.1.24]
Compound 1.1.24 was prepared following the procedures described for the synthesis 1.1.23. 1H NMR (CD3OD): 7.21-7.63 (m, 4H), 4.10 (br. s., 2H), 3.19 (d, J=1.6 Hz, 4H), 2.88 (br. s., 2H), 2.54 (br. s., 2H), 1.33-1.70 (m, 1 H), 0.84-1.01 (m, 2H), 0.66-0.81 (m, 2H). LCMS m/z 363.3 [M+H]+
1.2.1 Synthesis of A-hydroxy-4-(((5-phenylisoxazol-3-yl)methyl)amino)tetrahydro-2H- thiopyran-4-carboxamide 1,1 -dioxide [1.2.1]
Step 1. Synthesis of methyl 4-(((5-phenylisoxazol-3-yl)methyl)amino)tetrahydro- 2H-thiopyran-4-carboxylate 1,1-dioxide. [1.2.1a]
To a solution of methyl 4-aminotetrahydro-2H-thiopyran-4-carboxylate 1 ,1- dioxide (300 mg, 1.45 mmol, 1 equiv) and 5-phenylisoxazole-3-carbaldehyde (476 mg, 2.75 mmol, 1.9 equiv) in dichloroethane (7.2 mL) was added acetic acid (0.49 mL, 8.69 mmol, 6.0 equiv) followed by sodium triacetoxyborohydride (859 mg, 4.05 mmol, 2.8 equiv). After stirring at room temperature overnight, the reaction mixture was quenched with saturated aqueous sodium bicarbonate solution and extracted with ethyl acetate. Combined organic layer was washed with brine, dried with Na2S04, filtered and concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (EtO Ac/Heptane) to afford methyl 4-(((5-phenylisoxazol-3-yl)methyl)amino)tetrahydro-2/-/-thiopyran-4-carboxylate 1 ,1 -dioxide (455 mg, 86% yield). MS m/z 365.2 [M+H]+.
Step 2. Synthesis of 4-(((5-phenylisoxazol-3-yl)methyl)amino)tetrahydro-2H- thiopyran-4-carboxylic acid 1 ,1-dioxide. [1.2.1 b]
To a solution of methyl 4-(((5-phenylisoxazol-3-yl)methyl)amino)tetrahydro- 2/-/-thiopyran-4-carboxylate 1 ,1-dioxide (192 mg, 0.53 mmol, 1 equiv) in THF/methanol/water (4/1/1 , 2.6 mL) was added LiOH H20 as a solid (66.4 mg, 1.58 mmol, 3.0 equiv). After stirring at room temperature overnight, the reaction mixture was concentrated in vacuo and acidified to pH 1 by addition of 1 N HCI aqueous solution. The white precipitate was filtered and rinsed with ether to afford 4-(((5-phenylisoxazol-3-yl)methyl)amino)tetrahydro-2/-/- thiopyran-4-carboxylic acid 1 ,1-dioxide (144 mg, 78%). MS m/z 351.2 [M+H]+.
Step 3. Synthesis of 4-(((5-phenylisoxazol-3-yl)methyl)amino)-A -((tetrahydro- 2H-pyran-2-yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide. [1.2.1c]
To a solution of 4-(((5-phenylisoxazol-3-yl)methyl)amino)tetrahydro-2/-/-thiopyran-4- carboxylic acid 1 ,1-dioxide (184 mg, 0.53 mmol, 1 equiv) in DMF (2.1 mL) was added O- (tetrahydro-2/-/-pyran-2-yl)hydroxylamine (92 mg, 0.79 mmol, 1.5 equiv) followed by
triethylamine (0.28 mL, 1.99 mmol, 3.8 equiv). After stirring at room temperature for 5 minutes, EDC HCI (140 mg, 0.74 mmol, 1.5 equiv) was added followed by HOAt (129 mg, 0.95 mmol, 1.8 equiv). After stirring at room temperature overnight, the reaction mixture was diluted with saturated solution ammonium chloride solution and dichloromethane. Aqueous layer was extracted with dichloromethane. Combined organic layer was dried with Na2S04, filtered and concentrated in vacuo. The resulting residue was purified by silica gel column chromatorgraphy (acetone/heptane) to afford 4-(((5-phenylisoxazol-3-yl)methyl)amino)-/V- ((tetrahydro-2/-/-pyran-2-yl)oxy)tetrahydro-2/-/-thiopyran-4-carboxamide 1 , 1-dioxide (159 mg, 86%). MS m/z 450.2 [M+H]+.
Step 4. Synthesis of A -hydroxy-4-(((5-phenylisoxazol-3- yl)methyl)amino)tetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide. [1.2.1]
To a solution of 4-(((5-phenylisoxazol-3-yl)methyl)amino)-A/-((tetrahydro-2/-/-pyran-2- yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide 1 ,1-dioxide (159 mg, 0.35 mmol, 1 equiv) in dichloromethane/methanol (4/1 , 1.8 mL) was added HCI in dioxane (4M, 0.88 mL, 10 equiv). After stirring at room temperature for 1 h, the white precipitate was filtered to afford N- hydroxy-4-(((5-phenylisoxazol-3-yl)methyl)amino)tetrahydro-2/-/-thiopyran-4-carboxamide 1 ,1-dioxide (84 mg, 58%). MS m/z 366.1 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ 1 1.01 (br- s, 1 H), 7.86-7.84 (m, 2H), 7.56-7.48 (m, 3H), 7.17 (s, 1 H), 3.82 (s, 2H), 3.38-3.32 (m, 2H), 3.01-2.98 (m, 2H), 2.44-2.31 (m, 4H).
1.2.2 Synthesis of A -hydroxy-4-(((3-phenylisoxazol-5-yl)methyl)amino)tetrahydro-2H- thiopyran-4-carboxamide 1,1-dioxide [1.2.2]
N-hydroxy-4-(((3-phenylisoxazol-5-yl)methyl)amino)tetrahydro^
carboxamide 1 , 1 -dioxide was prepared following the procedures described for the synthesis of 1.2.1 using 3-phenylisoxazole-5-carbaldehyde in step 1 . MS m/z 366.1 [M+H]+. 1 H NMR (400 MHz, DMSO-d6) δ 10.98 (br-s, NH), 7.86 - 7.83 (m, 2H), 7.51 - 7.49 (m, 3H), 7.09 (s, 1 H), 3.92 (s, 2H), 3.37-3.33 (m. , 2H), 3.01 - 2.97 (m, 2H), 2.39-2.25 (m. , 4H).
1.2.3 Synthesis of 4-(((3-(4-chloro-2-fluorophenyl)isoxazol-5-yl)methyl)amino)-A - hydrox -carboxamide 1 ,1 -dioxide [1.2.3]
Step 1. Synthesis of (Z)-4-chloro-2-fluoro-A -hydroxybenzimidoyl chloride.
1.2.3a
To a solution of (E)-4-chloro-2-fluorobenzaldehyde oxime (262 mg, 1 .51 mmol, 1 equiv) in THF (2.9 mL) was added NCS (302 mg, 2.26 mmol, 1 .5 equiv) followed by pyridine ( 12 L, 0.15 mmol, 0.1 equiv). The mixture was stirred at 48 °C for 1 hour. The crude mixture was continued to the next step.
To the reaction mixture from step 1 was added propargyl alcohol (0.22 mL, 7.55 mmol, 5 equiv) followed by triethylamine (1.05 mL, 7.55 mmol, 5 equiv). After heated at reflux for 1 hour, the reaction mixture was cooled to room temperature and diluted with water and ethyl acetate. Aqueous layer was extracted with ethyl acetate. Combined organic layer was washed with brine, dried with Na2S04, filtered and concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (acetone/heptane) to afford (3-(4- chloro-2-fluorophenyl)isoxazol-5-yl)methanol (222 mg, 65% yield). MS m/z 228 [M+H]+.
Step 3. Synthesis of 3-(4-chloro-2-fluorophenyl)isoxazole-5-carbaldehyde.
[1.2.3c]
To a cooled solution of oxalyl chloride (0.19 mL, 2.2 mmol, 5.0 equiv) in dichloromethane (4.4 mL) at -78 °C was added DMSO (0.47 mL, 6.6 mmol, 15 equiv) dropwise. After stirring at -78 °C for 15 minutes, a solution of (3-(4-chloro-2- fluorophenyl)isoxazol-5-yl)methanol (100 mg, 0.44 mmol, 1 equiv) in dichloromethane (4.4 mL) was added to the reaction mixture followed by triethylamine (0.43 mL, 3.1 mmol, 7.0 equiv). After stirring at room temperature for 15 min, the reaction mixture was diluted with dichloromethane, washed with water, saturated sodium bicarbonate solution, brine, dried with Na2S04, filtered and concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (acetone/heptane) to afford 3-(4-chloro-2- fluorophenyl)isoxazole-5-carbaldehyde (66 mg, 66% yield). MS m/z 226.0 [M+H]+.
Step 4. Synthesis of 4-(((3-chloro-2-fluorophenyl)isoxazol-5-yl)methyl)amino)- A -hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide. [1.2.3]
4-(((3-chloro-2-fluorophenyl)isoxazol-5-yl)methyl)amino)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1 , 1 -dioxide was prepared following the procedures described for the synthesis of 1.2.1 using 3-(4-chloro-2-fluorophenyl)isoxazole-5-carbaldehyde in step 1 . HRMS m/z 418.0640 [M+H]+. 1H NMR (400 MHz, DMSO-d6) 10.72 (br-s., 1 H), 7.91 (t, J = 8.27 Hz, 1 H), 7.67 (dd, J = 2.05, 10.81 Hz, 1 H), 7.45 (dd, J = 2.20, 8.41 Hz, 1 H), 6.91 (d, J = 2.93 Hz, 1 H), 3.78 (s, 1 H), 3.22 - 3.29 (m, 2H), 2.93-2.96 (m, 2H), 2.14 - 2.31 (m, 4H).
1.2.4 Synthesis of 4-(((5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methyl)amino)-A - hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.2.4]
Step 1. Synthesis of ethyl 4-(4-chloro-2-fluorophenyl)-2,4-dioxobutanoate.
[1.2.4a]
1.2.4a
To a solution of LHMDS (1 .0 M in THF, 37.7 mL, 37.7 mmol) in ether (45 mL) at -78 °C was added a solution of 1 -(4-chloro-2-fluorophenyl)ethanone (5.0 g, 29 mmol, 1 equiv) in ether (15 mL). After stirring at -78 °C for 30 min, diethyl oxalate was added, and the reaction mixture was stirred at room temperature for 1 hour. The reaction mixture was
quenched with saturated aqueous NH4CI solution. The precipitate was collected by filtration and azeotroped with toluene to afford ethyl 4-(4-chloro-2-fluorophenyl)-2,4-dioxobutanoate which was used in the next step without further purification. MS m/z 273.1 [M+H]+.
Step 2. Synthesis of ethyl 5-(4-chloro-2-fluorophenyl)isoxazole-3-carboxylate.
[1.2.4b]
To a solution of ethyl 4-(4-chloro-2-fluorophenyl)-2,4-dioxobutanoate (7.9 g, 29 mmol, 1 equiv) in ethanol (145 mL) was added NH2OH HCI (6.7 g, 96 mmol, 3.3 equiv) followed by TsOH H20 (5.5 g, 29 mmol, 1 equiv). After refluxing overnight, the reaction mixture was cooled to room temperature and concentrated in vacuo. The resulting residue was partitioned between EtOAc and water. The aqueous layer was extracted with ethyl acetate. The combined organic layers were dried over Na2S04, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (EtOAc/heptane) to afford ethyl 5-(4-chloro-2-fluorophenyl)isoxazole-3-carboxylate (5.9 g, 76% yield). MS m/z 270.0 [M+H]+.
Step 3. Synthesis of (5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methanol. [1.2.4c]
To a solution of ethyl 5-(4-chloro-2-fluorophenyl)isoxazole-3-carboxylate (5.9 g, 22 mmol, 1 equiv) in THF/MeOH (10/1 , 1 10 mL) at 0 °C was added NaBH4 (2.5 g, 66 mmol, 3.0 equiv). After stirring at room temperature for 1 hour, the reaction mixture was quenched slowly with water, concentrated in vacuo and extracted with ethyl acetate. The combined organic layers were dried with Na2S04, filtered and concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (acetone/heptane) to afford (5-(4- chloro-2-fluorophenyl)isoxazol-3-yl)methanol (4.3 g, 85% yield). MS m/z 228.1 [M+H]+.
Step 4. Synthesis of 5-(4-chloro-2-fluorophenyl)isoxazole-3-carbaldehyde.
To a solution of oxalyl chloride (1 1.6 mL, 132 mmol, 7.0 equiv) in dichloromethane (94 mL) at -78 °C was added DMSO (26.8 mL, 378 mmol, 20 equiv) dropwise. After stirring at -78 °C for 15 min, a solution of (5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methanol (4.3 g, 18.9 mmol, 1 equiv) in dichloromethane (94 mL) was added to the reaction mixture followed by triethylamine (26 mL, 189 mmol, 10 equiv). After stirring at room temperature for 15 minutes, the reaction mixture was diluted with dichloromethane, washed with water, saturated aqueous NaHC03 solution, brine, dried over Na2S04, filtered, concentrated in vacuo. The resulting residue was azeotroped with toluene to afford 5-(4-chloro-2- fluorophenyl)isoxazole-3-carbaldehyde. The crude material was used in the next step without further purification. MS m/z 226.1 [M+H]+.
Step 5. Synthesis of 4-(((5-(4-chloro-2-fluorophenyl)isoxazol-3- yl)methyl)amino)-A -hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide.
[1.2.4]
4-(((5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methyl)amino)-/V-hydroxytetrahydro-2/-/- thiopyran-4-carboxamide 1 ,1 -dioxide was prepared following the procedures described for the synthesis of 1.2.1 using 5-(4-chloro-2-fluorophenyl)isoxazole-3-carbaldehyde in step 1. MS m/z (M+1 ) 418.0646 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ 10.80 (br-s., 1 H), 7.90 (t, J = 8.31 Hz, 1 H), 7.68 (dd, J = 1.96, 10.91 Hz, 1 H), 7.44 (dd, J = 1.96, 8.51 Hz, 1 H), 7.03 (d, J = 3.23 Hz, 1 H), 3.69 (br-s., 2H), 3.26-3.20 (m, 2H), 2.90-2.94 (m, 2H), 2.14-2.28 (m, 4H).
1.2.6 Synthesis of 4-(((5-(2-fluoro-4-methoxyphenyl)isoxazol-3-yl)methyl)am hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.2.6]
4-(((5-(2-fluoro-4-methoxyphenyl)isoxazol-3-yl)methyl)amino)-N-hydroxytetrahyd 2/-/-thiopyran-4-carboxamide 1 ,1-dioxide was prepared following the procedures described for the synthesis of 1.2.1 using 1-(2-fluoro-4-methoxyphenyl)ethanone in step 1. MS m/z 414.1 135 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ 10.91 (br-s., 1 H), 7.78 (t, J = 8.78 Hz, 1 H), 7.03 (dd, J = 2.40, 13.16 Hz, 1 H), 6.87 - 6.96 (m, 2H), 3.79 (s, 5H), 3.24 - 3.29 (m, 2H), 2.93-2.96 (m, 2H), 2.23-2.31 (m, 4H).
1.2.8. Synthesis of 4-(((5-(4-chloro-2-fluorophenyl)-4-fluoroisoxazol-3- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.2.8]
Step 1. Synthesis of Ethyl 5-(4-chloro-2-fluorophenyl)-4-fluoroisoxazole-3- carboxylate [1.2.8a]
To a solution of Ethyl 5-(4-chloro-2-fluorophenyl)isoxazole-3-carboxylate (297 mg, 1.101 mmol) in sulfolane (4 mL, melted in a warm water bath), Selectfluor (3902 mg, 11.01 mmol) was added and the resulting mixture was heated at 150 °C for 17 hours.
Saturated brine (10 mL) was added the mixture and the mixture was extracted with DCM. The organic layers were combined and concentrated. The residue was purified by silica gel column chromatography (EtO Ac/heptane) to give prodct (127 mg), which contained unreacted starting material. This mixture was continued to the next step. MS m/z 288.0
[M+H]+.
Step 2. Synthesis of (5-(4-chloro-2-fluorophenyl)-4-fluoroisoxazol-3-yl)methanol
[1.2.8b]
Sodium borohydride (50.1 mg, 1.325 mmol) was added to a solution of 1.2.8c (127 mg) in Methanol/THF (2 mL/0.2 mL) at 0 oC and the mixture was stirred at this temperature for 1 hour. Water (5 mL) was added and mixture was extracted with DCM. The orgnaic layers were combined and concentrated. The residue was purified by silica gel column chromatography (EtOAc/heptane) to give product 1.2.8b (36 mg,13.3 % yield over two steps).
Step 3. Synthesis of 4-(((5-(4-chloro-2-fluorophenyl)-4-fluoroisoxazol-3- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide [1.2.8]
Compound 1.2.8 was prepared following the procedures described for the synthesis of 1.2.4. HRMS m/z 436.0541 [M+H]+. 1H NMR (400 MHz, CD3OD) 7.77 (t, J=8.02 Hz, 1 H) 7.36 - 7.53 (m, 2 H) 3.87 (s, 2 H) 3.33 - 3.51 (m, 2 H) 2.96 (s, 1 H) 2.99 (s, 1 H) 2.37 - 2.49 (m, 2 H) 2.28 - 2.37 (m, 2 H)
1.2.9 Synthesis of 4-(((5-(4-(2H-1,2,3-triazol-2-yl)phenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.2.9]
Step 1. Synthesis of 1-(4-(2H-1,2,3-triazol-2-yl)phenyl)ethanone
An oven dried round bottom flask was charged with [Pd2(dba)3] (89 mg, 0.097 mmol, 0.75 mol%) and Me4tBuXPhos (1 12 mg, 0.233 mmol, 1.8 mol%), evacuated and backfilled with N2 (three times). Toluene (6.47 ml_) was added and heated to 120 °C for 3 minutes. K3PO4 (5.5 g, 25.9 mmol, 2.0 equiv), 4-chloroacetophenone (2.0 g, 12.94 mmol, 1 equiv), and triazole (1.1 g, 15.52 mmol, 1.2 equiv) were added and heated to 120 °C for 5 hours. The reaction mixture was cooled to room temperature, diluted with ethyl acetate, washed with brine, dried over Na2S04, concentrated in vacuo. The resulting residue was purified by silica gel column chromatography to give 1-(4-(2H-1 ,2,3-triazol-2-yl)phenyl)ethanone (1.4 g, 56 % yield). MS m/z 188.4 [M+H]+.
Step 2. Synthesis of 4-(((5-(4-(2H-1,2,3-triazol-2-yl)phenyl)isoxazol-3- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.2.9]
4-(((5-(4-(2H-1 ,2,3-triazol-2-yl)phenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide was prepared following the procedures described for the synthesis of 1.2.4 using 1.2.9a in step 1. HRMS m/z 433.1296 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ 8.14 (d., 4H), 8.01 (d, J = 8.61 Hz, 2H), 7.19 (br, s, 1 H), 3.73 (br, 2H), 3.29 (br, s, 2H), 2.94 (d, J = 13.69 Hz, 2H), 2.26 (m, 4H).
1.2.10. Synthesis of 4-(((4-chloro-5-(4-chloro-2-fluorophenyl)isoxazol-3- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide
[1.2.10]
Step 1. Synthesis of Ethyl 4-chloro-5-(4-chloro-2-fluorophenyl)isoxazole-3- carboxylate [1.2.10a]
N-Cholorosuccinimide (636 mg, 4.76 mmol) was added to a solution of Ethyl 5-(4-chloro-2-fluorophenyl)isoxazole-3-carboxylate (214 mg, 0.794 mmol) in acetic acid (3.9 mL) and the mixture was heated at 95 °C for 17 hours. After cooling to room temperature,
the reaction mixture was quenched by adding water (30 mL) and the mixture was extracted with DCM. The organic layers were combined and concentrated. The residue was purified by silica gel column chromatography (EtOAc/heptane) to afford product 1.2.10a (217 mg, 90% yield). MS m/z 304.0 [M+H]+.
Step 2. Synthesis of 4-(((4-chloro-5-(4-chloro-2-fluorophenyl)isoxazol-3- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide
[1.2.10]
Compound 1.2.10 was prepared following the procedures described for the synthesis of 1.2.4. HRMS m/z 452.0240 [M+H]+. 1H NMR (400 MHz, CD3OD) 7.68 (t, J=8.02 Hz, 1 H) 7.24 - 7.47 (m, 2 H) 3.78 (s, 2 H) 3.26 - 3.44 (m, 2 H) 2.89 (s, 1 H) 2.92 (s, 1 H) 2.31 - 2.45 (m, 2 H) 2.15 - 2.31 (m, 2 H)
1.2.11. Synthesis of 4-(((5-(4-(difluoromethoxy)phenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.2.11]
Step 1. Synthesis of tributyl(ethynyl)stannane [1.2.11a]
-= SnBu3
1.2.11a
A flask was charged with tributylchlorostannane (37.8 g, 1 16.1 mmol, 1.0 equiv) and THF (50 ml_). Ethynylmagnesium bromide (15 g, 1 16.1 mmol, 1.0 equiv) was added dropwise at -70 °C and the resulting solution was stirred at -70 °C for 1 hour and at room temperature for 3 hours. The reaction mixture was quenched with saturated aqueous ammonium chloride solution and extracted with EtOAc. The organic layer was washed with brine, dried over sodium sulfate and concentrated. The residue was purified by silica gel column chromatography (100 % Hexane) to afford product 1.2.11a (1 1 g, 30.1 % yield). 1H NMR (400 MHz, CDCI3) δ 4.50 (dq, J = 20.1 , 7.1 Hz, 2H), 1.84 - 1.50 (m, 5H), 1.51 - 1.16 (m, 13H), 1.04 - 0.77 (m, 7H).
1.2.11 b
1.2.11a (4 g, 12.7 mmol, 1.0 equiv) and ethyl (E)-2-chloro-2-(hydroxyimino)acetate (1.92 g, 12.7 mmol, 1.0 equiv) were dissoved in diethyl ether (40 ml_). TEA (6.41 g, 63.5 mmol, 5.0 equiv) was added dropwise and the reaction mixture was stirred at room temperature for 4 hours. The reaction mixture was quenched with water and extracted with EtOAc. The organic layer was washed with brine, dried over sodium sulfate and concentrated. The residue was purified by silica gel column chromatography (10-15 % EtOAc in Hexane) to afford product 1.2.11 b (2.5 g, 45.7 % yield). 1H NMR (400 MHz, CDCI3) δ 6.82 (s, 1 H), 4.46 (q, J = 7.1 Hz, 2H), 1.62 (d, J = 7.0 Hz, 2H), 1.58 - 1.53 (m, 2H), 1.44 (t, J = 7.1 Hz, 3H), 1.34 (ddd, J = 26.0, 16.7, 9.4 Hz, 8H), 1.26 - 1.14 (m, 6H), 0.92 (t, J = 7.3 Hz, 9H).
Step 3. Synthesis of ethyl 5-(4-(difluoromethoxy)phenyl)isoxazole-3- carboxylate [1.2.11c]
1.2.11c
A vial was charged with 1.2.11 b (0.76 g, 1.77 mmol, 1.0 equiv) and 1-bromo-4- uoromethoxy) benzene (0.5 g, 2.13 mmol, 1.2 equiv) were dissolved in 1 ,4-dioxane (10
ml_). Pd(PPh3)2CI2 (0.062 g, 0.085 mmol, 0.05 equiv) was added and the reaction mixture was stirred at 130 °C for 2 hours. The reaction mixture was quenched with water and extracted with EtOAc. The organic layer was washed with brine, dried over sodium sulfate and concentrated. The residue was purified by silica gel column chromatography (0-10 % EtOAc in Hexane) to afford product 1.2.11c (0.6 g, 94 % yield). LCMS (m/z): 284.2 [M+H]. 1H NMR (400 MHz, CDCI3) δ 7.89 - 7.81 (m, 2H), 7.28 - 7.24 (m, 2H), 6.93 (s, 1 H), 6.4 - 6.9 (3s, 1 H), 4.19 (q, J = 7.1 Hz, 2H), 1.31 - 1.29 (t, 3H).
Step 4. Synthesis of 4-(((5-(4-(difluoromethoxy)phenyl)isoxazol-3- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide
[1.2.11]
From ester 1.2.11c, hydroxamic acid was prepared following the procedures described for the synthesis of 1.2.4. LCMS (m/z): 432.3 [M+H]. 1 H NMR (400 MHz, DMSO) δ 10.72 (s, 1 H), 8.95 (s, 1 H), 7.94 (d, J = 8.7 Hz, 2H), 7.2-7.56 (3s, 1 H), 7.48 - 7.27 (m, 2H), 7.17 (d, J = 15.3 Hz, 1 H), 3.63 (d, J = 6.8 Hz, 2H), 3.40 (d, J = 7.0 Hz, 2H), 3.27 (d, J = 10.7 Hz, 2H), 2.96 (d, J = 12.7 Hz, 2H), 2.88 (s, 1 H), 2.35 - 2.08 (m, 4H).
Synthesis of N-hydroxy-4-(((5-(4-methoxyphenyl)isoxazol-3- pyran-4-carboxamide 1,1 -dioxide [1.2.12]
Compound 1.2.12 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.72 (s, 1 H), 8.95 (s, 1 H), 7.81 (d, J = 8.8 Hz, 2H), 7.10 (d, J = 8.9 Hz, 2H), 7.00 (s, 1 H), 3.83 (s, 3H), 3.61 (d, J = 6.9 Hz, 2H), 3.27 (d, J = 1 1.6
Hz, 2H), 2.96 (d, J = 12.6 Hz, 2H), 2.85 (s, 1 H), 2.29 - 2.13 (m, 4H). LCMS (m/z): 396.3 [M+H]+.
1.2.13 Synthesis of 4-(((5-(4-fluoro-3-methoxyphenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.2.13]
Compound 1.2.13 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.74 (s, 1 H), 8.96 (s, 1 H), 7.61 (dd, J = 8.2, 2.0 Hz, 1 H), 7.48 (ddd, J = 8.4, 4.5, 2.0 Hz, 1 H), 7.40 (dd, J = 1 1.2, 8.5 Hz, 1 H), 7.17 (s, 1 H), 3.95 (s, 3H), 3.64 (s, 2H), 3.27 (d, J = 1 1.0 Hz, 3H), 2.97 (d, J = 12.9 Hz, 2H), 2.88 (s, 1 H), 2.22 (dd, J = 34.3, 13.2 Hz, 4H). LCMS (m/z): 414.4 [M+H]+.
1.2.14 Synthesis of 4-(((5-(2-fluoro-4-methylphenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.2.14]
Compound 1.2.14 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.76 (s, 1 H), 8.94 (s, 1 H), 7.82 (t, J = 8.0 Hz, 1 H), 7.30 (d, J = 12.2 Hz, 1 H), 7.22 (d, J = 8.0 Hz, 1 H), 6.98 (d, J = 3.4 Hz, 1 H), 3.65 (s, 2H), 3.28 (dd, J = 18.5, 7.4 Hz, 2H), 2.97 (d, J = 12.9 Hz, 3H), 2.40 (s, 3H), 2.22 (dd, J = 35.4, 13.5 Hz, 4H). LCMS (m/z): 398.3 [M+H]+.
1.2.15 Synthesis of 4-(((5-(2,3-dichlorophenyl)isoxazol-3-yl)methyl)a hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.2.15]
Compound 1.2.15 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.78 (s, 1 H), 8.96 (s, 1 H), 7.86 (ddd, J = 1 1.1 , 8.0, 1.4 Hz, 2H), 7.57 (t, J = 8.0 Hz, 1 H), 7.27 (s, 1 H), 3.70 (s, 3H), 3.28 (t, J = 1 1.4 Hz, 2H), 2.97 (d, J = 13.1 Hz, 2H), 2.36 - 2.12 (m, 4H). LCMS (m/z): 434.2 [M+H]+.
1.2.16 Synthesis of 4-(((5-(2,4-difluorophenyl)isoxazol-3-yl)methyl)amino)-N- hydrox -4-carboxamide 1 ,1 -dioxide [1.2.16]
Compound 1.2.16 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 1 1.21 (s, 1 H), 8.02 (td, J = 8.7, 6.5 Hz, 1 H), 7.59 (ddd, J = 11.5, 9.3, 2.4 Hz, 1 H), 7.33 (td, J = 8.7, 2.5 Hz, 1 H), 7.14 (d, J = 25.6 Hz, 1 H), 3.93 (m, 2H), 3.41 (m, 2H), 3.05 (m, 2H), 2.49 - 2.42 (m, 2H), 2.38 (m, 2H). LCMS (m/z): 402.4 [M+H]+.
1.2.17 Synthesis of N-hydroxy-4-(((5-(m-tolyl)isoxazol-3-yl)methyl)amino)tetrahydro- 2H-thio ide [1.2.17]
1.2.17
Compound 1.2.17 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 7.69 (s, 1H), 7.66 (d, J = 7.5 Hz, 1H), 7.43 (t, J = 7.6 Hz, 1H), 7.33 (d, J = 7.3 Hz, 1H), 7.10 (s, 1H), 3.66 (s, 2H), 3.30 (t, J = 12.1 Hz, 2H), 2.97 (d, J= 13.1 Hz, 2H), 2.39 (s, 3H), 2.32-2.15 (m, 4H). LCMS (m/z): 380.3 [M+H]+.
1.2.18 Synthesis of N-hydroxy-4-(((5-(3-methoxyphenyl)isoxazol-3- yl)met ran-4-carboxamide 1 ,1 -dioxide [1.2.18]
Compound 1.2.18 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.74 (s, 1H), 8.96 (s, 1H), 7.50 - 7.43 (m, 2H), 7.42 - 7.38 (m, 1H), 7.19 (s, 1H), 7.09 (dt, J = 6.7, 2.6 Hz, 1H), 3.85 (s, 3H), 3.64 (s, 2H), 3.28 (d, J= 11.4 Hz, 2H), 2.97 (d, J= 13.3 Hz, 2H), 2.89 (s, 1H), 2.22 (dd, J= 34.5, 13.7 Hz, 4H). LCMS (m/z): 396.3 [M+H]+.
1.2.19 Synthesis of 4-(((5-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)isoxazol-3- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide
[1.2.19]
Compound 1.2.19 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.73 (s, 1H), 8.96 (s, 1H), 7.98 (d, J= 11.1 Hz, 1H), 7.77 (d, J= 8.4 Hz, 1H), 7.61 (d, J = 8.4 Hz, 1H), 7.20 (s, 1H), 3.63 (d, J = 6.1 Hz, 2H), 3.28 (t, J= 11.1 Hz, 2H), 2.96 (d, J= 12.9 Hz, 2H), 2.88 (s, 1H), 2.32-2.11 (m, 4H). LCMS (m/z): 446.5 [M+H]+.
1.2.20 Synthesis of 4-(((5-(3-chloro-5-fluorophenyl)isoxazol-3-yl)methyl)amino)-N- hydr rboxamide 1,1 -dioxide [1.2.20]
Compound 1.2.20 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.72 (s, 1 H), 8.97 (s, 1 H), 7.86 (s, 1 H), 7.78 (d, J = 9.1 Hz, 1 H), 7.64 (dd, J = 8.8, 1.9 Hz, 1 H), 7.39 (s, 1 H), 3.64 (d, J = 6.4 Hz, 2H), 3.27 (d, J = 11.4 Hz, 2H), 2.97 (d, J = 12.3 Hz, 2H), 2.89 (s, 1 H), 2.21 (dd, J = 32.6, 13.5 Hz, 4H). LCMS (m/z): 416.3 [M+H]+.
1.2.21 Synthesis of 4-(((5-(3-chlorophenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.2.21]
Compound 1.2.21 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.73 (s, 1 H), 8.97 (s, 1 H), 7.97 (d, J = 0.8 Hz, 1 H), 7.91 - 7.81 (m, 1 H), 7.59 (d, J = 5.2 Hz, 2H), 7.31 (s, 1 H), 3.64 (d, J = 5.8 Hz, 2H), 3.28 (d, J = 10.9 Hz, 2H), 2.97 (d, J = 12.9 Hz, 2H), 2.89 (s, 1 H), 2.31 - 2.12 (m, 4H). LCMS (m/z): 400.2 [M+H]+.
1.2.22 Synthesis of 4-(((5-(4-chloro-2,3-difluorophenyl)isoxazol-3-yl)methyl)amino)-N- hydr -carboxamide 1,1 -dioxide [1.2.22]
Compound 1.2.22 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 7.78 (t, J = 7.8 Hz, 1 H), 7.69 - 7.58 (m, 1 H), 7.17
(s, 1 H), 3.91 (s, 2H), 3.34 (t, J = 1 1.9 Hz, 2H), 3.03 (d, J = 12.7 Hz, 2H), 2.48 - 2.36 (m, 2H), 2.35 - 2.22 (m, 2H). LCMS (m/z): 436.4 [M+H]+.
1.2.23 Synthesis of 4-(((5-(2-chloro-4-fluorophenyl)isoxazol-3-yl)methyl)amino)-N- hydrox -4-carboxamide 1,1 -dioxide [1.2.23]
Compound 1.2.23 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.75 (s, 1 H), 8.95 (s, 1 H), 7.97 (dd, J = 8.8, 6.1 Hz, 1 H), 7.74 (dd, J = 8.9, 2.6 Hz, 1 H), 7.45 (td, J = 8.5, 2.6 Hz, 1 H), 7.19 (s, 1 H), 3.67 (d, J = 5.2 Hz, 2H), 3.27 (dd, J = 18.5, 7.1 Hz, 2H), 2.97 (d, J = 1 1.7 Hz, 3H), 2.31 - 2.10 (m, 4H). LCMS (m/z): 418.3 [M+H]+.
1.2.24 Synthesis of 4-(((5-(2,5-difluorophenyl)isoxazol-3-yl)methyl)amino)-N- hydrox arboxamide 1,1 -dioxide [1.2.24]
Compound 1.2.24 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.76 (s, 1 H), 8.94 (s, 1 H), 7.79 (ddd, J = 8.8, 5.7, 3.2 Hz, 1 H), 7.60 - 7.51 (m, 1 H), 7.47 (dt, J = 12.8, 6.3 Hz, 1 H), 7.14 (d, J = 3.3 Hz, 1 H), 3.66 (d, J = 7.1 Hz, 2H), 3.27 (t, J = 14.3 Hz, 1 H), 2.97 (d, J = 14.1 Hz, 2H), 2.21 (dd, J = 35.9, 13.4 Hz, 3H). LCMS (m/z): 402.2 [M+H]+.
1.2.25 Synthesis of 4-(((5-(2-fluorophenyl)isoxazol-3-yl)methyl)am hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.2.25]
Compound 1.2.25 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.92 (s, 1 H), 8.93 (s, 1 H), 7.94 (t, J = 6.9 Hz, 1 H), 7.59 (dd, J = 13.7, 5.7 Hz, 1 H), 7.44 (dt, J = 15.1 , 9.3 Hz, 2H), 7.07 (d, J = 3.1 Hz, 1 H), 3.65 (d, J = 6.6 Hz, 2H), 3.26 (t, J = 10.3 Hz, 2H), 3.08 (s, 1 H), 2.97 (d, J = 12.8 Hz, 2H), 2.30 - 2.1 1 (m, 4H). LCMS (m/z): 384.3 [M+H]+.
1.2.26 Synthesis of 4-(((5-(2-fluoro-4-(trifluoromethoxy)phenyl)isoxazol-3- yl)methyl)amino)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide
[1.2.26]
Compound 1.2.26 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.75 (s, 1 H), 8.93 (s, 1 H), 8.09 (t, J = 8.6 Hz, 1 H), 7.71 (d, J = 12.7 Hz, 1 H), 7.46 (d, J = 8.9 Hz, 1 H), 7.1 1 (d, J = 3.3 Hz, 1 H), 3.67 (d, J = 6.5 Hz, 2H), 3.28 (dd, J = 20.0, 9.1 Hz, 2H), 2.97 (d, J = 12.1 Hz, 3H), 2.22 (dd, J = 36.7, 13.6 Hz, 4H). LCMS (m/z): 468.2 [M+H]+.
1.2.27 Synthesis of 4-(((5-(3-fluoro-4-methoxyphenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.2.27]
NHOH
MeO F 1.2.27
Compound 1.2.27 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 7.72 (dd, J = 12.0, 2.0 Hz, 1 H), 7.66 (d, J = 8.6 Hz, 1 H), 7.32 (t, J = 8.8 Hz, 1 H), 7.01 (s, 1 H), 3.89 (s, 3H), 3.83 (s, 2H), 3.33 (t, J = 1 1.3 Hz, 2H), 3.02 (d, J = 14.5 Hz, 2H), 2.46 - 2.35 (m, 2H), 2.35 - 2.23 (m, 2H). LCMS (m/z): 414.2 [M+H]+.
1.2.28 Synthesis of N-hydroxy-4-(((5-(2,3,4-trifluorophenyl)isoxazol-3- yl)methyl)amino)tetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.2.28]
Compound 1.2.28 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.75 (s, 1 H), 8.93 (s, 1 H), 7.81 (d, J = 8.7 Hz, 1 H), 7.55 (dd, J = 16.8, 7.4 Hz, 1 H), 7.13 (d, J = 3.1 Hz, 1 H), 3.67 (d, J = 7.0 Hz, 2H), 3.28 (dd, J = 21.5, 10.7 Hz, 2H), 2.97 (d, J = 13.2 Hz, 3H), 2.31 - 2.10 (m, 3H). LCMS (m/z): 420.3 [M+H]+.
1.2.29 Synthesis of 4-(((5-(4-cyanophenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.2.29]
Compound 1.2.29 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.72 (s, 1 H), 8.94 (s, 1 H), 8.06 (q, J = 8.6 Hz, 4H), 7.40 (s, 1 H), 3.66 (d, J = 7.1 Hz, 2H), 3.33 - 3.22 (m, 2H), 3.02 - 2.87 (m, 3H), 2.32 - 2.12 (m, 4H). LCMS (m/z): 390.2 [M+H]+.
1.2.30 Synthesis of 4-(((5-(2,6-difluorophenyl)isoxazol-3-yl)methyl)amino)-N- hydr -carboxamide 1,1 -dioxide [1.2.30]
Compound 1.2.30 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, CD3CN) δ 9.76 (s, 1 H), 7.65 - 7.51 (m, 1 H), 7.20 (t, J = 8.8 Hz, 2H), 6.92 (s, 1 H), 3.80 (m, 2H), 3.30 (m, 3H), 3.00 (m, 2H), 2.40 (m, 4H). LCMS (m/z): 402.3 [M+H]+.
1.2.31 Synthesis of N-hydroxy-4-(((5-(5-methylthiophen-2-yl)isoxazol-3- yl)met hiopyran-4-carboxamide 1 ,1 -dioxide [1.2.31 ]
Compound 1.2.31 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, CD3CN) δ 7.40 (d, J = 3.6 Hz, 1 H), 6.89 (d, J = 3.7 Hz, 1 H),
6.62 (s, 1 H), 3.68 (s, 2H), 3.38 - 3.26 (m, 2H), 3.02 - 2.93 (m, 2H), 2.56 (d, J = 19.5 Hz, 3H), 2.36 (t, J = 13.2 Hz, 2H), 2.24 (d, J = 15.0 Hz, 2H). LCMS (m/z): 386.3 [M+H]+.
1.2.32 Synthesis of N-hydroxy-4-(((5-(4-methylthiophen-2-yl)isoxazol-3- yl)met opyran-4-carboxamide 1 ,1 -dioxide [1.2.32]
1.2.32
Compound 1.2.32 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, CD3CN) δ 7.69 (s, 1 H), 7.18 (s, 1 H), 6.64 (s, 1 H), 3.69 (s, 2H), 3.31 (t, J = 1 1.7 Hz, 2H), 2.97 (d, J = 13.6 Hz, 2H), 2.55 (d, J = 20.5 Hz, 3H), 2.36 (t, J = 12.3 Hz, 2H), 2.24 (d, J = 14.9 Hz, 2H). LCMS (m/z): 386.3 [M+H]+.
1.2.33 Synthesis of 4-(((5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methyl)amino)-N- hydrox -carboxamide 1,1 -dioxide [1.2.33]
Compound 1.2.33 was prepared following the procedures described for the synthesis of 1.2.11. 1H NMR (400 MHz, DMSO) δ 10.72 (s, 1 H), 8.95 (s, 1 H), 7.99 (dd, J = 10.1 , 1.6 Hz, 1 H), 7.88 - 7.71 (m, 2H), 7.31 (s, 1 H), 3.65 (s, 2H), 3.29 (t, J = 1 1.1 Hz, 2H), 2.97 (d, J = 12.7 Hz, 2H), 2.93 - 2.74 (m, 1 H), 2.32 - 2.10 (m, 4H). LCMS (m/z): 418.3 [M+H]+.
1.3.1. Synthesis of N-hydroxy-4-(((1-phenyl-1 H-1,2,3-triazol-4- yl)methyl)amino)tetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.3.1 ]
Compound 1.3.1 was prepared following the procedures described for the synthesis of 1.2.1 using 1-phenyl-1 H-1 ,2,3-triazole-4-carbaldehyde and methyl 4-aminotetrahydro-2H- thiopyran-4-carboxylate 1 ,1 -dioxide in Step 1. HRMS m/z 366.1230 [M+H]+. 1H NMR (400 MHz, CD3OD) 8.51 (s, 1 H) 7.85 (d, J=7.83 Hz, 2 H) 7.55 - 7.63 (m, 2 H) 7.47 - 7.54 (m, 1 H) 3.96 (br. s., 1 H) 3.48 (s, 2 H) 3.33 - 3.43 (m, 4 H) 3.08 (br. s., 2 H) 2.39 (br. s., 2 H)
1.4.1 Synthesis of compound 1.4.1
Step 1. Synthesis of methyl 4-(2-((tert-butyldimethylsilyl)oxy)ethyl)tetrahydro- 2H-thiopyran-4-carboxylate [1.4.1a]
1.4.1 a
To a solution of methyl tetrahydro-2H-thiopyran-4-carboxylate (817 mg, 5.1 mmol) in THF (10.2 mL) at -78 °C, LDA (3.06 mL, 6.12 mmol) was added dropwise over 15 minutes. The reaction was stirred for 15 minutes at which time a precipitate formed. A solution of (2- bromoethoxy)(tert-butyl)dimethylsilane (1.64 mL, 7.65 mmol) in THF (10.2 mL) was added and the reaction mixture was stirred at -78 °C for 30 minutes at which time the cooling bath was removed and the reaction was allowed to warm to room temperature. The reaction mixture was quenched with saturated aqueous NH4CI solution and extracted with EtOAc.
The organic ayer was washed with brine, dried over magnesium sulfate, filtered and concentrated. The residue was purified by silica gel column chromatography
(EtOAc/heptane, 0-50%) to afford 1.4.1a (967 mg, 59.5 % yield). 1H NMR (400 MHz, CDCI3) δ ppm 0.00 - 0.06 (m, 6 H) 0.87 (s, 9 H) 1.59 - 1.70 (m, 2 H) 1.77 (t, J=7.04 Hz, 2 H) 2.39 (d, J=12.91 Hz, 2 H) 2.52 (d, J=14.48 Hz, 2 H) 2.63 - 2.79 (m, 2 H) 3.60 (t, J=6.85 Hz, 2 H) 3.70 (s, 3 H).
Step 2. Synthesis of 2-oxa-8-thiaspiro[4.5]decan-1-one [1.4.01 b]
1.4.1b
1.4.1a (967 mg, 3.04 mmol) was dissolved in 1.0 M HCI solution in MeOH (9.1 ml_, 9.1 mmol) and the reaction solution was stirred at room temperature for 40 minutes. The reaction mixture was concentrated in vacuo and dried under high vacuum to afford 1.4.1 b (523 mg, 100 % yield) as a white solid. 1H NMR (400 MHz, CDCI3) δ ppm 1.83 (ddd, J=13.69, 7.04, 2.74 Hz, 2 H) 2.07 - 2.21 (m, 4 H) 2.57 - 2.69 (m, 2 H) 2.85 (td, J=6.65, 3.91 Hz, 2 H) 4.29 (t, J=7.04 Hz, 2 H).
Step 3. Synthesis of 4-(2-(4-iodo-2-oxopyridin-1(2H)-yl)ethyl)tetrahydro-2H- thiopyran-4-carboxylic acid [1.4.1c]
A slurry of 1.4.1 b (100 mg, 0.58 mmol), 4-iodopyridin-2(1 H)-one (257 mg, 1.16 mmol), Cs2C03 (378 mg, 1.16 mmol) and DMF (1.16 mL) was heated to 150 °C with miocrowave for 90 minutes. The reaction was quenched water and acidified with 1.0 M HCI aqueous to pH of 4. The aqueous layer was extracted with EtOAc. The organic layer was washed with brine, dried (MgS04), filtered and concentrated to afford a brown oil. The oil was dissolved in DMSO and purified by reverse phase HPLC to afford 1.4.1c (33 mg, 14 % yield) as a white crystalline solid. 1H NMR (400 MHz, CD3OD) ppm 1.61 - 1.74 (m, 2 H) 1.85 - 1.95 (m, 2 H) 2.30 - 2.42 (m, 2 H) 2.55 (d, J=14.48 Hz, 2 H) 2.64 - 2.78 (m, 2 H) 3.87 - 4.00 (m, 2 H) 6.69 (dd, J=7.04, 1.57 Hz, 1 H) 7.02 (d, J=1.17 Hz, 1 H) 7.31 (d, J=7.04 Hz, 1 H).
Step 4. Synthesis of 4-(2-(4-iodo-2-oxopyridin-1(2H)-yl)ethyl)-N-((tetrahydro-2H- pyran-2-yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide [1.4.1d]
1.4.1d
1.4.1c (33 mg, 0.084 mmol) was dissolved in DMF (0.559 ml_). To this solution, Et3N (46.5 μΙ, 0.336 mmol), 0-(tetrahydro-2H-pyran-2-yl)hydroxylamine (14.75 mg, 0.126 mmol), aza-HOBt (20.56 mg, 0.151 mmol) and aza-HOBt (20.56 mg, 0.151 mmol) were added. After stirring at room temperature for 24 hours, the reaction mixture was quenched by addition of saturated aqueous NH4CI solution and extracted with EtOAc. The organic layer was washed with brine, dried over MgS04 and concentrated on to silica gel. Purification by silica gel column chromatography (EtOac/heptane, 10-100%) afforded 1.4.1d (35 mg, 85 % yield). LC/MS (m/z) 408.98 [M+H]+
Step 5. Synthesis of 4-(2-(4-iodo-2-oxopyridin-1(2H)-yl)ethyl)-N-((tetrahydro-2H- pyran-2-yl)oxy)tetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.4.1e]
1.4.1e m-CPBA (35.0 mg, 0.142 mmol) was added to a solution of 1.4.1 d (35 mg, 0.07 mmol) in DCM (0.7 ml_) at -10 °C and the reaction was allowed to warm to 0°C for 2 hours. The mixture was quenched with water and extracted with DCM. The organic layer was dried over magnesium sulfate, filtered and concentrated on to silica gel. Purification by silica gel column chromatography (EtOAc/heptane,10-100%) afforded 1.4.1e (25 mg, 67 % yield). LC/MS (m/z) 440.98 [M+H]+.
Step 6. Synthesis of 4-(2-(4-(4-chloro-2-fluorophenyl)-2-oxopyridin-1(2H)- yl)ethyl)-N-hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.4.1]
A degassed mixture of 1.4.1e (25 mg, 0.048 mmol), (4-chloro-2-fluorophenyl)boronic acid (9.98 mg, 0.057 mmol), K3P04 (25.3 mg, 0.1 19 mmol) and PdCI2(dppf) CH2CI2 adduct (7.79 mg, 0.009 mmol) in THF (0.72 mL) and water (0.24 mL) was stirred at 60 °C for 3 hours. The reaction mixture was partitioned between EtOAc and saturated aqueous NH4CI solution. The organic layer was stirred with Siliabond DMT to remove the Pd residue, dried over magnesium sulfate, filtered and concentrated on to silica. Purification by silica gel column chromatography (EtOAc/heptane 10-100%) afforded 4-(2-(4-(4-chloro-2- fluorophenyl)-2-oxopyridin-1 (2H)-yl)ethyl)-N-((tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H- thiopyran-4-carboxamide 1 ,1 -dioxide (1 1.2 mg, 44.6 % yield). This material was then dissolved in 1.0 M HCI in MeOH and the solution was stirred at room temperature. The precipitate was collected by filtration to afford (2.8 mg, 13 % yield) LC/MS (m/z) 443.1
[M+H]+.1 H NMR (400 MHz, CD3OD) ppm 1.97 - 2.06 (m, 2 H) 2.07 - 2.22 (m, 2 H) 2.55 (d, J=14.87 Hz, 2 H) 3.00 - 3.26 (m, 4 H) 3.92 - 4.08 (m, 2 H) 6.60 (d, J=7.04 Hz, 1 H) 6.71 (s, 1 H) 7.27 - 7.40 (m, 2 H) 7.55 (t, J=8.41 Hz, 1 H) 7.68 (d, J=7.04 Hz, 1 H
1.4.2 Synthesis of 4-(2-(4-(2-fluoro-4-methoxyphenyl)-2-oxopyridin-1(2H)-yl)ethyl)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.4.2]
Compound 1.4.2 was synthesized following the procedure described for 1.4.1. 1H NMR (500 MHz, METHANOL-c4) δ ppm 2.03 - 2.1 1 (m, 2 H) 2.17 (t, J=12.30 Hz, 2 H) 2.58 (d, J=14.82 Hz, 2 H) 3.06 - 3.16 (m, 2 H) 3.16 - 3.27 (m, 2 H) 3.89 (s, 3 H) 4.04 - 4.12 (m, 2 H) 6.79 - 6.90 (m, 3 H) 6.92 (dd, J=8.67, 2.05 Hz, 1 H) 7.55 (t, J=8.67 Hz, 1 H) 7.79 (d,
J=6.94 Hz, 1 H). LC/MS (m/z) 439.1 [M+H]+
1.5.1 Synthesis of N-hydroxy-4-(2-(4-phenyl-1 H-pyrazol-1-yl)ethyl)tetrahydro-2H- thiopyran-4-carboxamide 1,1 -dioxide [1.5.1]
.1a
A solution of methyl tetrahydro-2H-thiopyran-4-carboxylate (1.0 g, 6.24 mmol) in THF (31.2 mL) was cooled at -78 °C. A solution of LDA (3.74 ml, 7.49 mmol, 2.0 M in heptane) was added over 15 min. After 30 mins, 1-bromo-2-chloroethane (1.039 ml, 12.48 mmol) was added over 5 min and the reaction was allowed to stir at -78 °C for 1 hour at which time the cooling bath was removed and the reaction was allowed to warm to room temperature. The reaction was quenched with saturated aqueous ammonium chloride and extracted with EtOAc. The organic layer was dried (MgS04), filtered and concentrated. The material was purified by silica gel column chromatography, EtOAc/heptane 0 to 30%, to afford 1.5.1a (667 mg, 48.0 % yield). 1H NMR (400 MHz, CDCI3) δ ppm 1.56 - 1.71 (m, 2 H) 1.94 - 2.08 (m, 2 H) 2.42 (d, J=12.52 Hz, 2 H) 2.53 (d, J=14.48 Hz, 2 H) 2.60 - 2.77 (m, 2 H) 3.39 - 3.53 (m, 2 H) 3.65 - 3.81 (m, 3 H)
Step 2. Synthesis of methyl 4-(2-chloroethyl)tetrahydro-2H-thiopyran-4- carb [1.5.1 b]
A solution of 1.5.1a (103 mg, 0.462 mmol) in DCM (4.6 ml_) was cooled in an ice water bath. mCPBA (239 mg, 0.971 mmol) was added and the reaction was stirred at 0 °C for 20 mins then at room temperature for 1 hour. The reaction was quenched with water and diluted with DCM. The organic layer was washed with saturated aqueous NaHC03 solution,
water and brine. The organic layer was dried over magnesium sulfate, filtered and concentrated to afford product 1.5.1b (1 18 mg, 100 % yield). 1H NMR (400 MHz, CDCI3) δ ppm 2.04 - 2.19 (m, 4 H) 2.56 (d, J=13.30 Hz, 2 H) 2.92 - 3.14 (m, 4 H) 3.46 (t, J=7.43 Hz, 2 H) 3.80 (s, 3 H)
Step 3. Synthesis of methyl 4-(2-(4-phenyl-1 H-pyrazol-1-yl)ethyl)tetrahydro-2H- thiopy oxide [1.5.1c]
A mixture of 1.5.1 b (1 18 mg, 0.463 mmol), 4-phenyl-1 H-pyrazole (55.7 mg, 0.386 mmol) and Cs2C03 (277 mg, 0.849 mmol) in DMF (Volume: 2.145 ml) was stirred at 45 °C for 24 hours. The reaction mixture was then partitioned between EtOAc and water. The organic layer was washed with brine, dried over Na2S04, filtered and concentrated to afford 1.5.1.C (124.3 mg, 89 % yield) as a white solid. 1H NMR (400 MHz, CDCI3) δ ppm 2.04 - 2.21 (m, 2 H) 2.29 (t, J=7.43 Hz, 2 H) 2.56 (d, J=14.09 Hz, 2 H) 2.91 - 3.15 (m, 6 H) 3.69 -
3.77 (m, 3 H) 4.15 (t, J=7.43 Hz, 2 H) 7.31 - 7.43 (m, 3 H) 7.44 - 7.49 (m, 2 H) 7.59 (s, 1 H)
7.78 (s, 1 H). LC/MS (m/z) 363.6 [M+H]+
Step 4. Synthesis of N-hydroxy-4-(2-(4-phenyl-1 H-pyrazol-1 -yl)ethyl)tetrahydro- 2H-thi -dioxide [1.5.1]
Compound 1.5.1 was prepared from ester 1.5.1c following the procedures described for the synthesis of 1.1.1 step 2-4. 1 H NMR (500 MHz, METHANOL-c 4) δ ppm 2.06 - 2.16 (m, 2 H) 2.16 - 2.23 (m, 2 H) 2.52 (d, J=15.13 Hz, 2 H) 3.02 - 3.1 1 (m, 2 H) 3.12 - 3.23 (m, 2 H) 4.17 - 4.27 (m, 2 H) 7.18 - 7.28 (m, 1 H) 7.37 (t, J=7.88 Hz, 2 H) 7.51 - 7.60 (m, 2 H) 7.89
- 7.95 (m, 1 H) 8.01 - 8.07 (m, 1 H). LC/MS (m/z) 364.2 [M+H]+.
1.5.2 Synthesis of 4-(2-(4-(4-chlorophenyl)-2H-1,2,3-triazol-2-yl)ethyl)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1 ,1 -dioxide [1.5.2]
Step 1. Synthesis of methyl 4-(2-(4-(4-chlorophenyl)-2H-1,2,3-triazol-2- yl)ethyl)tetrahydro-2H-thiopyran-4-carboxylate 1 ,1 -dioxide [1.5.2a]
A mixture of 1.5.1 b (100 mg, 0.393 mmol) and sodium azide (63.8 mg, 0.981 mmol) in DMF (0.491 mL) was stirred at 80 °C for 24 hours. The reaction mixture was cooled to room temperature. Cul (74.8 mg, 0.393 mmol) and 1-chloro-4-ethynylbenzene (53.6 mg, 0.393 mmol) were added and the reaction was then stirred at 80 °C for 72 hours. The reaction was quenched with water and then filtered. The filtrate was extracted with DCM. The combined organic layers were washed with brine, dried over Na2S04, filtered and concentrated. The residue was purified by silica gel column chromatography,
EtOAc/heptane 70% to 100%, to afford product 1.5.2a (55 mg, 35.2 % yield). 1H NMR (CDCI3) δ: 7.77 (d, J=8.5 Hz, 2H), 7.74 (s, 1 H), 7.43 (d, J=8.2 Hz, 2H), 4.42 (t, J=7.7 Hz, 2H), 3.77 (d, J=0.9 Hz, 3H), 3.06-3.16 (m, 2H), 2.97-3.06 (m, 2H), 2.62 (d, J=13.6 Hz, 2H), 2.35 (t, J=7.6 Hz, 2H), 2.14-2.25 (m, 2H). LC/MS (m/z) 398.2 [M+H]+,
Step 2. Synthesis of 4-(2-(4-(4-chlorophenyl)-2H-1,2,3-triazol-2-yl)ethyl)-N- hydroxytetrahydro-2H-thiopyran-4-carboxamide 1,1 -dioxide [1.5.2]
Compound 1.5.2 was prepared from ester 1.5.2a following the procedures described for the synthesis of 1.1.1 step 2-4. 1H NMR (500 MHz, DMSO-c/6) δ ppm 2.07 - 2.19 (m, 2 H) 2.22 - 2.33 (m, 2 H) 2.56 (d, J=14.82 Hz, 2 H) 3.03 - 3.1 1 (m, 2 H) 3.12 - 3.21 (m, 2 H) 4.43 - 4.54 (m, 2 H) 7.38 - 7.52 (m, 2 H) 7.80 (d, J=8.51 Hz, 2 H) 8.42 (d, J=3.15 Hz, 1 H). LC/MS
(m/z) 399.1 [M+H]+
1.6.1 Synthesis of 4-(2-([1,1'-biphenyl]-4-yl)ethyl)-N-hydroxytetrahydro-2H-thiopyran-4- carbo
Step 1. Synthesis of methyl 4-(2-([1,1'-biphenyl]-4-yl)-2-oxoethyl)tetrahydro-2H- thiopyran-4-carboxylate [1.6.1a]
A solution of methyl tetrahydro-2H-thiopyran-4-carboxylate (200 mg, 1.248 mmol) in THF (12.5 mL) was cooled in a dry ice acetone bath. LDA (0.749 ml_, 1.498 mmol, 2.0 M in heptane) was added dropwise. The reaction was allowed to stir for 30 min at which time a solution of 1-([1 ,1 '-biphenyl]-4-yl)-2-bromoethanone (515 mg, 1.872 mmol) in THF was added. The reaction was allowed to stir at -78 °C for 1 hour and then warmed to room temperature. The reaction was quenched with saturated aqueous ammonium chloride solution and extracted with Et20. The combined organic layers was washed with brine, dried over MgS04, filtered and concentrated. The residue was purified by silica gel column chromatography, EtOAc/Heptane 0-40% to afford product 1.6.1a (414 mg, 94 % yield). LC/MS (m/z) 355.3 [M+H]+
Step 2. Synthesis of methyl 4-(2-([1,1'-biphenyl]-4-yl)-2-oxoethyl)tetrahydro-2H- thiopyran-4-carboxylate 1,1-dioxide [1.6.1 b]
A solution of 1.6.1a (304 mg, 0.858 mmol) in THF (6.4 mL) and water (2.1 mL) was cooled at 0 °C. Oxone (1 160 mg, 1.887 mmol) was added and the mixture was stirred at 0 °C for 2 hours. The reaction mixture was brought to a neutral pH with 1.0 M aqueous NaOH solution and then extracted with EtOAc. The organic layer was washed with brine, dried over magnesium sulfate, filtered and concentrated. The residue was purified by silica gel column chromatography, EtOAc/heptane 20-80% to afford product 1.6.1 b (141 mg, 42.5 % yield). LC/MS (m/z) 387.2 [M+H]+
Step 3. Synthesis of methyl 4-(2-([1,1'-biphenyl]-4-yl)ethyl)tetrahydro-2H- thiopy e [1.6.1c]
A solution of 1.6.1 b (45 mg, 0.1 16 mmol) in MeOH (5.3 mL) and EtOAc (0.5 mL) was added to a mixture of Pd/C (10% on carbon, 18.59 mg, 0.017 mmol) in MeOH (0.3 mL). The mixture was stirred under 1 atm of hydrogen for 48 hours. The reaction mixture was then filtered through Celite and the filtrate was concentrated. The remaining oil was purified by silica gel column chromatorgaphy, EtOAc/heptane 10-100%, to give product 1.6.1 c (22 mg, 50.7 % yield). 1H NMR (400 MHz, CDCI3) δ ppm 1.37 (d, J=7.04 Hz, 3 H) 2.07 - 2.24 (m, 2 H) 2.48 (dd, J=14.28, 3.33 Hz, 1 H) 2.53 - 2.63 (m, 1 H) 2.89 - 3.01 (m, 3 H) 3.02 - 3.15 (m, 2 H) 3.73 (s, 3 H) 7.15 (d, J=7.83 Hz, 2 H) 7.31 - 7.38 (m, 1 H) 7.44 (t, J=7.63 Hz, 2 H) 7.53 (d, J=7.83 Hz, 2 H) 7.58 (d, J=7.43 Hz, 2 H). LC/MS (m/z) 372.3 [M+H]+
Step 4. Synthesis of 4-(2-([1,1'-biphenyl]-4-yl)ethyl)-N-hydroxytetrahydro-2H- thiopyran-4-carboxamide 1,1 -dioxide [1.6.1]
Compound 1.6.1 was prepared from ester 1.6.1c following the procedures described for the synthesis of 1.1.1 step 2-4. 1 H NMR (400 MHz, METHANOL-^) δ ppm 1.37 - 1.40 (m, 2 H) 1.95 - 2.12 (m, 2 H) 2.42 - 2.60 (m, 2 H) 2.88 - 3.08 (m, 4 H) 3.17 (t, J=14.09 Hz, 2 H) 7.24 - 7.35 (m, 3 H) 7.42 (t, J=7.63 Hz, 2 H) 7.59 (dd, J=1 1.93, 8.02 Hz, 4 H). LC/MS
(m/z) 374.2 [M+H]+
2.1.1 Synthesis of compound 2.1.1
Step 1. Synthesis of methyl 3-(([1,1'-biphenyl]-4-ylmethyl)amino)
tetrahydrothiophene-3-carboxylate [2.1.1a]
A slurry of methyl 3-aminotetrahydrothiophene-3-carboxylate (169 mg, 1.048 mmol), [1 ,1 '-biphenyl]-4-carbaldehyde (159 mg, 0.874 mmol) and acetic acid (0.250 ml, 4.37 mmol) in 1 ,2-dichloroethane (7.9 ml_) was stirred at room temperature overnight and then at 60 °C for 3 hours. The reaction was cooled to room temperature and sodium triacetoxyborohydnde (926 mg, 4.37 mmol) was added. After stirring at room temperature overnight, the reaction mixture was quenched with water and extracted with EtOAc. The organic layer was washed with 1.0 M NaOH aqueous solution and brine, dried over magnesium sulfate filtered and concentrated on to silica gel. Purification by silica gel column chromatography
(EtOAc/heptane, 0 to 50%) afforded 2.1.1a (132 mg, 46 % yield). LC/MS (m/z) 328.2 [M+H]+. 1H NMR (400 MHz, <CDCI3>) δ ppm 2.16 - 2.30 (m, 1 H) 2.42 (dt, J=12.91 , 7.83 Hz, 1 H) 2.84 - 3.00 (m, 2 H) 3.03 - 3.13 (m, 1 H) 3.29 (d, J=10.96 Hz, 1 H) 3.73 (s, 2 H) 3.75 - 3.82 (m, 3 H) 7.30 - 7.76 (m, 9 H)
Step 2. Synthesis of methyl 3-(([1,1'-biphenyl]-4-ylmethyl)amino)
A solution of 2.1.1a (132 mg, 0.403 mmol) and LiOH (2.0 M in water, 2.0 ml_, 4.0 mmol) in MeOH (0.8 ml_) was stirred overnight at room temperature. The reaction mixture was concentrated in vacuo. The remaining salt was dissolved in water and acidified to pH=4 by adding 6 M HCI aqueous solution. The white precipitate was collected and washed with diethyl ether to afford 2.1.1 b (66 mg, 52 % yield) LC/MS (m/z) 314.1 [M+H]+.
Step 3. Synthesis of methyl 3-(([1,1'-biphenyl]-4-ylmethyl)amino)-N- hydroxytetrahydrothiophene-3-carboxamide [2.1.1]
A solution of 2.1.1 b (1 18 mg, 0.38 mmol), 0-(tetrahydro-2H-pyran-2- yl)hydroxylamine (66 mg, 0.563 mmol), EDC HCI (101 mg, 0.526 mmol), aza-HOBt (92 mg, 0.676 mmol) and Et3N (94 μΙ, 0.676 mmol) in DMF (Volume: 3756 μΙ) was stirred for 72 hours. The reaction mixture was diluted with water and brought to a pH of 4 with NH4CI. The aqueous layer was extracted with EtOAc/Et20 (1/1 ). The organic layer was washed with water, brine and dried over MgS04. The organic layer was filtered and concentrated. The remaining material was purified by silica gel column chromatography (EtO Ac/heptane 0 to 50%) to afford 3-(([1 ,1'-biphenyl]-4-ylmethyl)amino)-N-((tetrahydro-2H-pyran-2- yl)oxy)tetrahydrothiophene-3-carboxamide (50 mg, 0.121 mmol, 32.3 % yield). 25 mg of this product was dissolved in a solution of HCI in EtOH (1.25 M, 3.0 ml_, 3.75 mmol) and the reaction solution was allowed to stir at room temperature for 2 hours. Et20 was added and the white precipitate was collected to afford 2.1.1 (6.7 mg, 4.84 % yield).
LC/MS (m/z) 329.1 [M+H]+. 1H NMR (400 MHz, <CD3OD>) δ ppm 2.40 - 2.68 (m, 2 H) 3.09 (t, J=6.85 Hz, 2 H) 3.36 - 3.45 (m, 1 H) 4.19 (br. s., 2 H) 7.30 - 7.40 (m, 1 H) 7.45 (t, J=7.63 Hz, 2 H) 7.57 - 7.68 (m, 4 H) 7.73 (d, J=8.22 Hz, 2 H)
Step 1. Synthesis of methyl 3-((tert- butoxycarbonyl)amino)tetrahydrothiophene-3-carboxylate [2.2.1a]
2.2.1a
Boc20 (3.46 ml, 14.92 mmol) was added to a solution of methyl 3- aminotetrahydrothiophene-3-carboxylate (2.186 g, 13.56 mmol) and Et3N (4.70 ml, 33.9 mmol) in THF (Volume: 67.8 ml) and the reaction was stirred at room temperature for 24 hours. The reaction was quenched with water and extracted with EtOAc. The EtOAc layer was dried (MgS04), filtered and concentrated to afford 2.2.1a (2.7 g, 76 % yield). 1H NMR (400 MHz, CDCI3) δ ppm 1.27 (s, 2 H) 1.43 (s, 9 H) 2.30 - 2.45 (m, 1 H) 2.50 - 2.67 (m, 1 H) 2.83 - 3.02 (m, 3 H) 3.20 - 3.35 (m, 1 H) 3.60 - 3.88 (m, 3 H)
Step 2. Synthesis of methyl 3-((tert- butoxycarbonyl)amino)tetrahydrothiophene-3-carboxylate 1,1 -dioxide [2.2.1 b]
2.2.1b
Oxone (14.0 g, 22.7 mmol) was added was added to a solution 2.2.1a (2.7 g, 10.33 mmol), THF (52 mL) and water (52 mL) at 0 °C. After stirring at 0 °C for 5 hours, the reaction mixture was diluted with water, neutralized by adding 1.0 M NaOH aqueous solution and extracted with EtOAc. The organic layer was dried on to silica gel and purified by silica gel
column chromatography (EtOAc/heptane 25-100%) to afford product 2.2.1 b (1.5 g, 50 % yield) as a white solid.
Step 3. Synthesis of methyl 3-aminotetrahydrothiophene-3-carboxylate 1 ,1- dioxide [2.2.1c]
2.2.1c
A solution of 2.2.1 b (1.4 g, 4.77 mmol) in 4.0 M HCI in dioxane (12.0 mL, 47.7 mmol) was stirred at room temperature for 72 hours. Et20 was added and the precipitate was collected by filtration to afford 2.2.1c as HCI salt (700 mg, 55 % yield). LC/MS (m/z) 194.0 [M+H]+.
Step 4. Synthesis of methyl 3-(((5-phenylisoxazol-3-yl)methyl)amino) tetrahydrothiophene-3-carboxylate 1,1 -dioxide [2.2.1 d]
A solution of 2.2.1c (100 mg, 0.376 mmol), acetic acid (0.064 mL, 1.127 mmol), Et3N (0.156 mL, 1.127 mmol) and 5-phenylisoxazole-3-carbaldehyde (65.1 mg, 0.376 mmol) was stirred at room temperature for 1 hour. Sodium triacetoxyborohydride (239 mg, 1.127 mmol) was added and the mixture was stirred overnight. The reaction was quenched with water, basified to a pH of 8 with 1.0 M aqueous NaOH solution and extracted with EtOAc. The organic layer was washed with water and brine, dried (MgS04), filtered and concentrated on to silica gel. Purification by silica gel column chromatography (EtOAc/heptane 0 to 50% afforded 2.2.1d (75 mg, 57 % yield). LC/MS (m/z) 351.1 [M+H]+.
Step 5. Synthesis of 3-(((5-phenylisoxazol-3-yl)methyl)amino)
A solution of 2.2.1d (72 mg, 0.205 mmol) and LiOH (2.0 M in water, 1.5 ml_, 3.0 mmol) in THF (4.1 ml_) was allowed to stir for 24 hours. The reaction mixture was concentrated and acidified to pH=4 with 1.0 N HCI aqueous solution. The precipitate was collected by filtration to afford 2.2.1e (55 mg, 80 % yield). LC/MS (m/z) 337.2 [M+H]+.
Step 6. Synthesis of N-hydroxy-3-(((5-phenylisoxazol-3-yl)methyl)amino) tetrahydrothiophene-3-carboxamide 1,1 -dioxide [2.2.1]
EDC HCI (43.9 mg, 0.229 mmol) and aza-HOBt (40.1 mg, 0.294 mmol) was added to a solution of 2.2.1e (55 mg, 0.164 mmol), 0-(tetrahydro-2H-pyran-2-yl)hydroxylamine (28.7 mg, 0.245 mmol) and Et3N (0.086 ml_, 0.621 mmol) in DMF (1.6 ml_). After stirring at room temperature over night, the reaction mixture was quenched with water, brought to pH=4 by adding saturated aqueous NH4CI solution. The mixture was extracted with Et20 and DCM, until no more product present in the aqueous layer. The combined organic layers were dried (MgS04), filtered and concentrated. The remaining oil was purified by silica gel column chromatography (EtOAc/heptane, 50-100%) to afford 3-(((5-phenylisoxazol-3- yl)methyl)amino)-N-((tetrahydro-2H-pyran-2-yl)oxy) tetrahydro-thiophene-3-carboxamide 1 ,1- dioxide (45 mg, 63.2 % yield). This material was dissolved in 4M HCI in dioxane and stirred for 1 hour. The precipitate was collected by filtration to afford 2.2.1 (32.2 mg, 50% yield). LC/MS (m/z) 352.1 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 2.49 - 2.62 (m, 1 H) 2.74 (dt, J=14.48, 7.24 Hz, 1 H) 3.24 - 3.29 (m, 1 H) 3.40 - 3.47 (m, 1 H) 3.50 (d, J=14.09 Hz, 1 H) 3.85 (d, J=14.48 Hz, 1 H) 4.00 - 4.16 (m, 2 H) 6.91 (s, 1 H) 7.43 - 7.57 (m, 3 H) 7.78 - 7.90 (m, 2 H)
2.2.2 Synthesis of compound 2.2.2
Compound 2.2.2 was prepared following the procedures described for the synthesis of 2.2.1 using 4'-chloro-2'-fluoro-[1 ,1'-biphenyl]-4-carbaldehyde (Ref. Tetrahedron Lett. 2005, 46, 7575-7579) in Step 4. LC/MS (m/z) 413.0 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 2.55 - 2.71 (m, 1 H) 2.90 (br. s., 1 H) 3.32 - 3.40 (m, 1 H) 3.51 (dd, J=13.69, 6.65 Hz, 1 H) 3.66 (s, 2 H) 3.98 (d, J=14.87 Hz, 1 H) 4.10 - 4.22 (m, 2 H) 7.28 - 7.36 (m, 2 H) 7.50 (t, J=8.41 Hz, 1 H) 7.58 - 7.68 (m, 4 H)
2.2.3 Synthesis of compound 2.2.3
Compound 2.2.3 was prepared following the procedures described for the synthesis of 2.2.1 using 3-phenylisoxazole-5-carbaldehyde in Step 4. LC/MS (m/z) 352.1 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 2.31 - 2.51 (m, 2 H) 3.08 - 3.18 (m, 1 H) 3.24 (br. s., 2 H) 3.60 (d, J=13.69 Hz, 1 H) 3.73 - 3.80 (m, 1 H) 3.82 - 3.90 (m, 1 H) 6.75 (s, 1 H) 7.33 - 7.42 (m, 3 H) 7.68 - 7.76 (m, 2 H).
Compound 2.2.4 was prepared following the procedures described for the synthesis of 2.2.1 using 4-(phenylethynyl)benzaldehyde in Step 4. LC/MS (m/z) 385.1 [M+H]+. 1 H NMR (400 MHz, CD3OD) δ ppm 2.51 - 2.68 (m, 1 H) 2.85 (br. s. , 1 H) 3.32 - 3.37 (m, 1 H) 3.43 - 3.53 (m, 1 H) 3.59 (d, J=14.48 Hz, 1 H) 3.93 (d, J=14.48 Hz, 1 H) 4.02 - 4.13 (m, 2 H) 7.35 - 7.41 (m, 3 H) 7.48 - 7.54 (m, 4 H) 7.57 - 7.62 (m, 2 H).
2.2.5 Synthesis of 3-(((5-(4-chloro-2-fluorophenyl)isoxazol-3-yl)methyl)amino)-N- hydroxytetrahydrothiophene-3-carboxamide 1 ,1 -dioxide [2.2.5]
Compound 2.2.5 was prepared following the procedures described for the synthesis of 2.2.1 . The racemic material was separated into two enantiomers 2.2.5a and 2.2.5b with chiral HPLC.
2.2.5a: 1 H NMR (400 MHz, DMSO-d6) δ ppm 2.22 - 2.43 (m, 2 H) 3.06 - 3.18 (m, 1 H) 3.23 (t, J=7.24 Hz, 1 H) 3.35 (s, 1 H) 3.52 (d, J=13.69 Hz, 1 H) 3.61 - 3.79 (m, 2 H) 7.02 (d, J=3.52 Hz, 1 H) 7.48 (dd, J=8.61 , 1 .57 Hz, 1 H) 7.72 (dd, J=10.96, 1 .96 Hz, 1 H) 7.93 (t, J=8.41 Hz, 1 H) 9.02 (s, 1 H) 10.82 (br. s. , 1 H). LC/MS (m/z) 404.1 [M+H]+. Rt 5.34 min, chiral HPLC, AD column, Heptane/EtOH 40/60, flow rate 1 .0 mL/min, run time 10 mins.
2.2.5b 1 H NMR (400 MHz, DMSO-d6) δ ppm 2.22 - 2.43 (m, 2 H) 3.06 - 3.18 (m, 1 H) 3.23 (t, J=7.24 Hz, 1 H) 3.35 (s, 1 H) 3.52 (d, J=13.69 Hz, 1 H) 3.61 - 3.79 (m, 2 H) 7.02 (d, J=3.52 Hz, 1 H) 7.48 (dd, J=8.61 , 1 .57 Hz, 1 H) 7.72 (dd, J=10.96, 1 .96 Hz, 1 H) 7.93 (t, J=8.41 Hz, 1 H) 9.02 (s, 1 H) 10.82 (br. s. , 1 H). LC/MS (m/z) 404.1 [M+H]+. Rt 7.67 min, chiral HPLC, AD column, Heptane/EtOH 40/60, flow rate 1 .0 mL/min, run time: 10 mins.
3.1.1 Synthesis of compound 3.1.1
Step 1. Synthesis of 1-tert-butyl 4-ethyl 4-(2-chloroethyl)piperidine-1,4- dicarboxylate [3.1.1a]
3.1.1a
A solution of 1-tert-butyl 4-ethyl piperidine-1 ,4-dicarboxylate (4.68 g, 18.19 mmol) in THF (91 mL) was added to LiHMDS solution in THF (1.0 M, 20.0 ml_, 20.0 mmol) at -78 °C over 50 minutes. 1-bromo-2-chloroethane (3.13 g, 21.8 mmol) was added and the reaction mixture was warmed to room temperature and stirred overnight. The reaction was quenched with saturated aqueous NH4CI solution and extracted with Et20. The organic layer was washed with water and brine, dried over magnesium sulfate, filtered and concentrated. The remaining oil was purified by silica gel column chromatography (EtOAc/heptane 0 to 20%) to afford 3.1.1a (2.0 g, 34.4 % yield) of a colorless oil. 1H NMR (400 MHz, CDCI3) δ ppm 1.29 (t, J=7.04 Hz, 3 H) 1.33 - 1.50 (m, 1 1 H) 1.97 - 2.06 (m, 2 H) 2.12 (d, J=13.30 Hz, 2 H) 2.79 - 2.99 (m, 2 H) 3.41 - 3.51 (m, 2 H) 3.80 - 3.98 (m, 2 H) 4.21 (q, J=7.04 Hz, 2 H).
Step 2. Synthesis of 1-tert-butyl 4-ethyl 4-(2-(4-iodo-2-oxopyridin-1(2H)- yl)ethyl)piperidine-1 ,4-dicarboxylate [3.1.1 b]
3.1.1a (796 mg, 2.489 mmol) was added to a slurry of 4-iodopyridin-2(1 H)-one (500 mg, 2.262 mmol) and Cs2C03 (1622 mg, 4.98 mmol) in DMF (Volume: 1 1.300 mL) and the reaction mixture was heated to 50 °C for 24 hours. The reaction mixture was quenched with water and extracted with Et20. The organic layer was washed with brine, dried over magnesium sulfate filtered and concentrated on to silica gel. Purification by silica gel column chromatography (EtOAc/heptane, 0-30%) afforded product 3.1.1 b (796 mg). 1H NMR (400 MHz, CDCI3) δ ppm 1.31 (t, J=7.24 Hz, 3 H) 1.35 - 1.52 (m, 1 1 H) 1.93 (t, J=8.02 Hz, 2 H) 2.12 (d, J=13.30 Hz, 2 H) 2.84 - 3.10 (m, 2 H) 3.67 - 3.95 (m, 4 H) 4.21 (q, J=7.04 Hz, 2 H) 6.47 (dd, J=7.04, 1.56 Hz, 1 H) 6.87 (d, J=7.04 Hz, 1 H) 7.06 (d, J=1.56 Hz, 1 H).
Step 3. Synthesis of 1-tert-butyl 4-ethyl 4-(2-(2-oxo-4-phenylpyridin-1(2H)- yl)ethyl)piperidine-1,4-dicarboxylate [3.1.1c]
PdCI2(dppf)-CH2CI2Adduct (34.3 mg, 0.042 mmol) was added to a degassed mixture of phenylboronic acid (154 mg, 1.261 mmol), 3.1.1b (424 mg, 0.841 mmol) and potassium phosphate (535 mg, 2.52 mmol) in 2-MeTHF (3.2 mL) and H20 (1.1 mL). The mixture was stirred at 65 °C for 1 hour, after which, the reaction mixture was diluted with water/Et20 and filtered through Celite. The aqueous layer was brought to a pH of 4 with 1.0 M aqueous NaHS04 solution and extracted with Et20. Then combined organic layer was washed with
water and brine, dried over magnesium sulfate, filtered. The filtrate was stirred with
Siliabond-DMT Pd scavenger for 2 hours. The Pd scavenger was then filtered off and the filtrate was concentrated to afford 3.1.1c (331 mg, 87 % yield). 1H NMR (400 MHz, CDCI3) δ ppm 1.32 (t, J=7.24 Hz, 3 H) 1.39 - 1.58 (m, 1 1 H) 2.02 (t, J=7.83 Hz, 2 H) 2.16 (d, J=13.30 Hz, 2 H) 3.00 (br. s., 2 H) 3.78 - 4.07 (m, 4 H) 4.24 (q, J=7.04 Hz, 2 H) 6.45 (d, J=6.26 Hz, 1 H) 6.77 (s, 1 H) 7.32 - 7.51 (m, 4 H) 7.53 - 7.59 (m, 2 H).
Step 4. Synthesis of 1-(tert-butoxycarbonyl)-4-(2-(2-oxo-4-phenylpyridin-1(2H)- yl)ethyl)piperidine-4-carboxylic acid [3.1.1d]
A solution of 3.1.1c (1 10 mg, 0.242 mmol) and LiOH.H20 (102 mg, 2.42 mmol) in MeOH (3.6 mL) and water (1.2 mL) was stirred at room temperature for one week and at 50°C for 48 hours. The reaction mixture was concentrated to 1/3 of its the volume and brought to a pH of 4 by adding 1.0 M HCI aqueous solution. The aqueous layer was extracted with EtOAc/Et20 (1/1 ). The combined organic layers were washed with brine, dried over magnesium sulfate, filtered and concentrated to afford 3.1.1d (66 mg, 64 % yield). 1H NMR (400 MHz, CDCI3) δ ppm 1.37 - 1.48 (m, 1 1 H) 2.05 (s, 1 H) 2.16 (s, 1 H) 2.89 - 3.19 (m, 2 H) 3.69 - 4.1 1 (m, 4 H) 6.48 (dd, J=7.24, 2.15 Hz, 1 H) 6.79 (d, J=1 .96 Hz, 1 H) 7.36 - 7.51 (m, 5 H) 7.37 - 7.37 (m, 1 H) 7.53 - 7.59 (m, 2 H).
Step 5. Synthesis of 4-(2-(2-oxo-4-phenylpyridin-1(2H)-yl)ethyl)piperidine-4- carboxylic acid [3.1.1e]
3.1.1d (66 mg, 0.155 mmol) was dissolved in HCI solution in EtOH (1.25 M, 2.5 mL, 3.1 mmol) and the resulting solution was stirred at room temperature overnight. The reaction mixture was concentrated in vacuo to afford 3.1.1e (56.1 mg, 100 % yield). LC/MS (m/z) 327.23 [M+H]+.
Step 6. Synthesis of 1-acetyl-4-(2-(2-oxo-4-phenylpyridin-1(2H)- yl)ethyl)piperidine-4-carboxylic acid [3.1.1f]
Ac20 (0.016 ml, 0.171 mmol) was added to a solution of 3.1.1e (0.054 g, 0.155 mmol) and Et3N (0.215 ml, 1.550 mmol) in DCM (3.1 mL). After stirring at room temperature for 30 minutes, the reaction mixture was concentrated in vacuo to afford 3.1.1f (quantitative yield). The crude product was continued to the next step without further purification. LC/MS (m/z) 369.2 [M+H]+.
Step 7. Synthesis of 1-acetyl-N-hydroxy-4-(2-(2-oxo-4-phenylpyridin-1(2H)- yl)ethyl)piperidine-4-carboxamide [3.1.1].
A solution of 3.1.1f (57.1 mg, 0.155 mmol), 0-(tetrahydro-2H-pyran-2- yl)hydroxylamine (27.2 mg, 0.233 mmol), EDC HCI (41.6 mg, 0.217 mmol), aza-HOBt (38.0 mg, 0.279 mmol) and Et3N (0.038 mL, 0.279 mmol) in DMF (1.5 mL) was stirred for 72 hours at room temperature. The reaction mixture was diluted with water and brought to a pH of 4 by adding saturated aqueous NH4CI solution. The aqueous layer was extracted with
EtOAc/Et20 (1/1 ). The organic layer was washed with water, brine, dried (MgS04) and concentrated. The remaining material was purified by silica gel column chromatography, (EtOAc/heptane 50-100%) to afford 1-acetyl-4-(2-(2-oxo-4-phenylpyridin-1 (2H)-yl)ethyl)-N- ((tetrahydro-2H-pyran-2-yl)oxy)piperidine-4-carboxamide (32 mg, 44.2 % yield). This material was dissolved in 1.0 M HCI ethanolic solution (1.25 M,. 1.2 mL, 1.75 mmol) and the reaction was stirred for 2 hours. Et20 was added to the reaction mixture was the white precipitate was collected by filtration to afford 3.1.1 (29.1 mg, 48.5 % yield). LC/MS (m/z) 384.2 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 1.47 - 1.68 (m, 2 H) 1.96 - 2.07 (m, 2 H) 2.09 - 2.30 (m, 5 H) 3.03 - 3.13 (m, 1 H) 3.33 - 3.40 (m, 1 H) 3.77 (d, J=13.99 Hz, 1 H) 4.03 - 4.20 (m, 4 H) 7.02 (br. s., 1 H) 7.10 (d, J=6.16 Hz, 1 H) 7.48 - 7.57 (m, 3 H) 7.73 (dd, J=4.23, 1.74 Hz, 2 H) 7.94 (d, J=6.99 Hz, 1 H).
3.1.2 Synthesis of compound 3.1.2
Step 1. Synthesis of ethyl 4-(2-(2-oxo-4-phenylpyridin-1(2H)-yl)ethyl)piperidine- 4-carboxylate [3.1.2a].
3.1.1c (220 mg, 0.484 mmol) was dissolved in HCI solution (4.0 M in dioxane, 4.84 mL, 19.36 mmol) and the resulting solution was stirred at room temperature for 5 days. The reaction mixture was diluted with water and neutralized by adding aqueous NaOH solution. The mixture was extracted with EtOAc. The organic layer was washed with brine, dried over magnesium sulfate, filtered and concentrated to afford 3.1.2a (150 mg, 87 % yield). LCMS (m/z) 355.2 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 1.31 (t, J=7.24 Hz, 3 H) 1 .49 - 1.65 (m, 2 H) 1.93 - 2.07 (m, 2 H) 2.23 (d, J=13.69 Hz, 2 H) 2.64 - 2.81 (m, 2 H) 3.03 (dt, J=13.1 1 , 3.62 Hz, 2 H) 3.94 - 4.05 (m, 2 H) 4.21 (q, J=7.04 Hz, 2 H) 6.70 (dd, J=7.04, 2.35 Hz, 1 H) 6.74 (d, J= .96 Hz, 1 H) 7.10 - 7.27 (m, 1 H) 7.28 - 7.73 (m, 6 H).
Step 2. Synthesis of ethyl 1-(methylsulfonyl)-4-(2-(2-oxo-4-phenylpyridin-1(2H)- yl)ethyl)piperidine-4-carboxylate [3.1.2b]
Methanesulfonyl chloride (36.3 μΙ, 0.466 mmol) was added to a solution of 3.1.2a (150 mg, 0.423 mmol) and Et3N (0.176 mL, 1.270 mmol) in DCM (4.2 mL) at 0 °C and the reaction mixture was allowed to warm to room temperature and stirred for 6 hours. The reaction mixture was quenched with water and extracted with Et20. The organic layer was washed with water and brine, dried over MgS04, filtered and concentrated on to silica gel. Purification by silica gel column chromatography (EtOAc/heptane 0-50%) afforded 3.1.2b
(152 mg, 83% yield). 1H NMR (400 MHz, CDCI3) δ ppm 1.34 (t, J=7.24 Hz, 3 H) 1.64 - 1.76 (m, 2 H) 1.97 - 2.13 (m, 2 H) 2.31 (d, J=13.69 Hz, 2 H) 2.77 (s, 3 H) 2.84 - 2.99 (m, 2 H) 3.54 - 3.69 (m, 2 H) 3.87 - 3.98 (m, 2 H) 4.25 (q, J=7.17 Hz, 2 H) 6.44 (dd, J=7.04, 1.96 Hz, 1 H) 6.76 (d, J=1.57 Hz, 1 H) 7.39 - 7.50 (m, 3 H) 7.56 (dd, J=7.43, 1.96 Hz, 2 H) 7.75 (d, J=6.65 Hz, 1 H).
Step 3. Synthesis of 1-(methylsulfonyl)-4-(2-(2-oxo-4-phenylpyridin-1(2H)- yl)ethyl)piperidine-4-carboxylic acid [3.1.2c]
A solution of 3.1.2b (152 mg, 0.351 mmol) and LiOH H20 (14.75 mg, 0.351 mmol) in MeOH (2.6 mL) and H20 (0.87 mL) was stirred at room temperature for 4 days, at 40 °C for 24 hours and at 60°C for 3 hours. The reaction mixture was concentrated to 1/3 of its volume then acidified by adding 1.0 M HCI aqueous solution. The precipitate was collected by filtration to afford 3.1.1c (142 mg, 100 % yield). LCMS (m/z) 405.2 [M+H]+.
Step 4. Synthesis of N-hydroxy-1-(methylsulfonyl)-4-(2-(2-oxo-4-phenylpyridin- 1 (2H)-yl)ethyl)piperidine-4-carboxamide [3.1.2]
Compound 3.1.2 was prepared following the procedure described for the synthesis of 3.1.1 using 3.1.2c in Step 7. LC/MS (m/z) 420.2 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 1.70 (ddd, J=13.89, 10.37, 3.91 Hz, 2 H) 1.94 - 2.08 (m, 2 H) 2.26 (d, J=14.09 Hz, 2 H) 2.82 (s, 3 H) 2.92 - 3.04 (m, 2 H) 3.48 - 3.56 (m, 2 H) 4.10 - 4.19 (m, 2 H) 7.03 (d, J=1.57 Hz, 1
H) 7.12 (dd, J=7.04, 1.96 Hz, 1 H) 7.49 - 7.57 (m, 3 H) 7.74 (dd, J=6.85, 2.93 Hz, 2 H) 7.95 (d, J=6.65 Hz, 1 H).
11-3.1.3 Synthesis of compound 3.1.3
3.1.3
Step 1. Synthesis 1-tert-butyl 4-ethyl 4-([1 ,1'-biphenyl]-4-ylmethyl)piperidine- 1,4-dicarboxylate [3.1.3a]
3.1.3a
□HMDS solution in THF (2.9 ml_, 1.0 M, 2.9 mmol) was added to a solution of 1-tert- butyl 4-ethyl piperidine-1 ,4-dicarboxylate (500 mg, 1.943 mmol) in THF (9.7 mL) at -78 °C and the reaction was stirred for 1 hour. To this solution, 4-(bromomethyl)-1 , 1 '-biphenyl (960 mg, 3.89 mmol) was added and the reaction mixture was warmed to room temperature overnight. The reaction was quenched with saturated aqueous NH4CI solution and extracted with EtOAc. The organic layer was washed with water and brine, dried over magnesium sulfate, filtered and concentrated on to silica. Purification by silica gel column
chromatography, (EtOAc/heptane 0-30%) afforded 3.1.3a (762 mg, 93 % yield). LCMS (m/z) 368.25 [M+H]+. 1H NMR (400 MHz, CDCI3) δ ppm 1.20 (t, J=7.24 Hz, 3 H) 1.37 - 1.51 (m, 12 H) 2.13 (d, J=13.30 Hz, 2 H) 2.73 - 2.92 (m, 5 H) 3.83 - 4.05 (m, 2 H) 4.13 (q, J=7.17 Hz, 2 H) 7.12 (d, J=7.83 Hz, 2 H) 7.34 (d, J=7.43 Hz, 1 H) 7.43 (t, J=7.63 Hz, 2 H) 7.49 (d, J=7.83 Hz, 2 H) 7.56 (d, J=7.43 Hz, 2 H).
Step 2. Synthesis of ethyl 4-([1,1'-biphenyl]-4-ylmethyl)piperidine-4-carboxylate
3.1.3b
In a flask charged with 3.1.3a (400 mg, 0.944 mmol), 4.0 M HCI solution in dioxane (4.72 mL, 18.89 mmol) was added and the reaction was stirred at room temperature for 8 hours. The reaction mixture was concentrated in vacuo to afford 3.1.3b (340 mg, 100 % yield). LCMS (m/z) 324.9 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 1.24 (t, J=7.24 Hz, 3 H) 1.72 - 1.86 (m, 2 H) 2.34 (d, J=14.09 Hz, 2 H) 2.87 - 3.05 (m, 4 H) 3.36 (d, J=13.30 Hz, 2 H) 4.19 (q, J=7.30 Hz, 2 H) 7.19 (d, J=8.22 Hz, 2 H) 7.28 - 7.36 (m, 1 H) 7.42 (t, J=7.63 Hz, 2 H) 7.56 (dd, J=10.37, 8.02 Hz, 4 H).
Step 3. Synthesis of ethyl 4-([1,1'-biphenyl]-4-ylmethyl)-1-(methylsulfonyl) piperidine-4-carboxylate [3.1.3c]
3.1.3c
To a solution of 3.1.3b (100 mg, 0.278 mmol) in DCM at 0 °C, methanesulfonyl chloride (0.22 mL, 0.306 mmol) was added and the reaction was stirred at 0 °C for 2 hours. The reaction was quenched with water and extracted with EtOAc. The organic layer was washed with water and brine, dried over magnesium sulfate and concentrated on to silica gel. Purification by silica gel column chromatography (EtO Ac/heptane, 0-50%) afforded
3.1.3c (81 mg, 72.6 % yield). LCMS (m/z) 402.2 [M+H]+. 1 H NMR (400 MHz, CDCI3) δ ppm 1.16 - 1.26 (m, 3 H) 1.67 (td, J=12.91 , 4.30 Hz, 2 H) 2.28 (d, J=12.52 Hz, 2 H) 2.64 - 2.78 (m, 5 H) 2.89 (s, 2 H) 3.71 (d, J=12.13 Hz, 2 H) 4.16 (q, J=7.17 Hz, 2 H) 7.12 (d, J=7.83 Hz, 2 H) 7.30 - 7.37 (m, 1 H) 7.43 (t, J=7.43 Hz, 2 H) 7.50 (d, J=7.83 Hz, 2 H) 7.57 (d, J=7.43 Hz, 2 H).
Step 4. Synthesis of 4-([1,1'-biphenyl]-4-ylmethyl)-1-(methylsulfonyl)piperidine- 4-carboxylic acid [3.1.3d]
3.1.3d
A solution of NaOH aqueous solution (1.0 M in water, 0.24 ml_, 0.24 mmol) was added to a solution of 3.1.3c (81 mg, 0.202 mmol) in THF (1.0 ml_) and the reaction was stirred at room temperature for 1 hour and at 50°C for 24 hours. The reaction mixture was diluted with water, acidified to pH=3 by adding 1.0 M HCI aqueous solution and extracted with Et20. The organic layer was washed with brine, dried over MgS04, filtered and concentrated to afford 3.1.3d (62 mg, 53.5 % yield). LCMS (m/z) 374.2 [M+H]+.
Step 5. Synthesis of 4-([1,1'-biphenyl]-4-ylmethyl)-N-hydroxy-1-(methylsulfonyl) piperidine-4-carboxamide [3.1.3]
3.1.3
Compound 3.1.3 was prepared following the procedure described for the synthesis of 3.1.1 using 3.1.3d in Step 7. LCMS (m/z) 389.2 [M+H]+. 1H NMR (400 MHz, CD3OD) ppm 1.59 - 1.75 (m, 2 H) 2.16 (d, J=14.09 Hz, 2 H) 2.72 - 2.95 (m, 7 H) 3.51 - 3.62 (m, 2 H) 7.21 (d, J=8.22 Hz, 2 H) 7.25 - 7.33 (m, 1 H) 7.41 (t, J=7.63 Hz, 2 H) 7.53 (d, J=7.83 Hz, 2 H) 7.58 (d, J=7.43 Hz, 2 H).
3.2.1 Synthesis of S-i^'-chloro^'-fluoro-n.l'-biphenylJ^-y methy aminoJ-N-hydroxy- 1-(methylsulfonyl)azetidine-3-carboxamide [3.2.1]
Step 1. Synthesis of 1-tert-butyl 3-ethyl 3-((4-bromobenzyl)amino)azetidine-1,3- dicarboxylate [3.2.1a]
A solution of 1-tert-butyl 3-ethyl 3-aminoazetidine-1 ,3-dicarboxylate (1.5 g, 6.14 mmol), 4-bromobenzaldehyde (1.250 g, 6.75 mmol) and acetic acid (1.055 ml, 18.42 mmol) in 1 ,2-Dichloroethane (Volume: 30.7 ml) was stirred at room temperature for 72 hours. Sodium triacetoxyborohydride (3.90 g, 18.42 mmol) was added and the resulting reaction mixture was allowed to stir for another 24 hours. The reaction was quenched with water and extracted with DCM. The organic layer was washed wtih saturated aqueous NaHC03 solution and brine. The organic layer was dried over magnesium sulfate, filtered and concentrated. The residue was was purified by silica gel column chromatography,
EtOAc/heptane 501-100% to afford product 3.2.1a (1.58 g, 62.3 % yield). 1H NMR (400 MHz, CDCI3) δ ppm 1.33 (t, J=7.04 Hz, 3 H) 1.45 (s, 9 H) 3.62 (s, 2 H) 3.85 (d, J=8.61 Hz, 2 H) 4.20 (d, J=9.00 Hz, 2 H) 4.26 (q, J=7.04 Hz, 2 H) 7.19 - 7.25 (m, 2 H) 7.45 (d, J=8.22 Hz, 2 H). LC/MS (m/z) 415.1 [M+H]+
Step 2. Synthesis of ethyl 3-((4-bromobenzyl)amino)azetidine-3-carboxylate
[3.2.1 b]
3.2.1a (1.58 g, 3.82 mmol) was dissolved in a solution of HCI in EtOH (1.0 M, 10 mL) and the resulting solution was stirred at room temperature for 18 hours. Diethyl ether was added to to the solution and after 48 hours a white precipitate crashed out. The solid was collected by filtration to afford amine HCI salt (1.3 g, 88 % yield). 1H NMR (400 MHz, CD3OD) 5 ppm 1.45 (t, J=7.24 Hz, 3 H) 4.19 (br. s., 2 H) 4.44 - 4.60 (m, 4 H) 4.67 (d, J=1 1.74 Hz, 2 H) 7.46 - 7.55 (m, 2 H) 7.63 (d, J=8.61 Hz, 2 H).
Step 3. Synthesis of ethyl 3-((4-bromobenzyl)amino)-1- (methylsulfonyl)azetidine-3-carboxylate [3.2.1c]
A solution of 3.2.1 b (500 mg, 1.295 mmol) and DIEA (0.905 ml_, 5.18 mmol) in DCM (6.5 ml_) was placed in an ice water bath. Methanesulfonyl chloride (0.131 ml_, 1.683 mmol) was slowly added and the resulting mixture was stirred at room temperature over 1 hour. The reaction was quenched with water and extracted with DCM. The DCM layer was washed with water and brine, dried over magnesium sulfate, filtered and concentrated. The remaining oil was purified by silica gel column chromatography, EtOAc/heptane 10-75% to afford product 3.2.1c (373 mg, 73.6 % yield). 1H NMR (500 MHz, CDCI3) δ ppm 1.37 (t, J=7.09 Hz, 3 H) 2.95 (s, 3 H) 3.68 (s, 2 H) 3.92 (d, J=8.51 Hz, 2 H) 4.25 (d, J=8.51 Hz, 2 H) 4.31 (q, J=7.25 Hz, 2 H) 7.24 (d, J=8.20 Hz, 2 H) 7.48 (d, J=8.51 Hz, 2 H). LC/MS (m/z) 393.0 [M+H]+.
Step 3. Synthesis of 3-(((4,-chloro-2'-fluoro-[1 ,1'-biphenyl]-4-yl)methyl)amino)- N-hydroxy-1-(methylsulfonyl)azetidine-3-carboxamide [3.2.1]
Compound 3.2.1 was prepared following the procedure described for the synthesis of 1.1.2. 1H NMR (400 MHz, CD3OD) δ ppm 2.91 (s, 4 H) 3.83 (s, 2 H) 3.96 (d, J=9.39 Hz, 2 H) 4.15 (d, J=9.39 Hz, 2 H) 7.1 1 - 7.29 (m, 2 H) 7.32 - 7.52 (m, 5 H). LC/MS (m/z) 428.1
[M+H]+
4.1.1. Synthesis of compound 4.1.1 and 4.1.2
Step 1. Synthesis of (1s,4s)-methyl 1-(([1,1'-biphenyl]-4-ylmethyl)amino)-4- hydroxycyclohexanecarboxylate [4.1.1a] and (1 r,4r)-methyl 1-(([1,1'-biphenyl]-4- ylmethyl)amino)-4-hydroxycyclohexanecarboxylate [4.1.2a]
To a solution of methyl 1 -amino-4-oxocyclohexanecarboxylate (100 mg, 0.584 mmol, 1.0 equiv) in DCE (2.0 mL) was added para-phenyl benzaldehyde (213 mg, 1 .2 mmol, 2.0 equiv) and HOAc (0.1 mL, 1 .75 mmol, 3.0 equiv). After stirring at room temperature for 10 minutes, sodium triacetoxy borohydride (371 mg, 1 .75 mmol, 3.0 equiv) was added and the resulting solution was stirred at room temperature for 18 hours. The reaction was then quenched by addition of saturated aqueous NaHC03 solution and extracted with EtOAc. The organic layer was washed with water and brine, dried over Na2SC>4 and concentrated. The residue was purified by silica gel column chromatography, (EtOAc/heptane 0 to 60%) to afford product 4.1.1a (50 mg, 25% yield, less polar diastereomer) and 4.1.2a (40 mg, 20% yield, more polar diastereomer). 4.1.1a MS (m/z) 340.6 [M+H]+; 4.1.2a MS (m/z) 340.3
[M+H]+.
Step 2. Synthesis of compound 4.1.1 and 4.1.2
Compounds 4.1.1 and 4.1.2 were synthesized from 4.1.1a and 4.1.2a following the procedures described for the synthesis of 1.2.1 step 2-4.
4.1.1 : MS (m/z) 341 .3 [M+H]+. 1 H NMR (400 MHz, DMSO-c/6) 1 .42 - 1 .74 (m, 4 H) 1 .93 - 2.26 (m, 4 H) 3.62 - 3.76 (m, 1 H) 3.86 - 4.14 (m, 2 H) 5.59 - 5.99 (m, 1 H) 7.38 (d, J=7.43 Hz, 1 H) 7.47 (t, J=7.63 Hz, 2 H) 7.58 - 7.76 (m, 6 H) 7.82 - 7.86 (m, 1 H) 9.06 - 9.46 (m, 2 H) 1 1 .10 - 1 1 .47 (m, 1 H)
4.1 .2: MS (m/z) 341 .3 [M+H]+. 1 H NMR (400 MHz, DMSO-c/6) 1 .07 - 1 .35 (m, 2 H) 1 .54 - 1 .91 (m, 4 H) 2.22 - 2.38 (m, 2 H) 3.39 - 3.51 (m, 1 H) 3.80 - 4.06 (m, 2 H) 7.34 (d, J=7.43 Hz, 1 H) 7.38 - 7.45 (m, 2 H) 7.53 (d, J=7.83 Hz, 2 H) 7.59 - 7.75 (m, 4 H) 9.09 - 9.54 (m, 2 H) 1 1 .13 - 1 1 .42 (m, 1 H)
4.1.3 Synthesis of compound 4.1.3
Step 1. Synthesis of 4.1.3a
4.1.3a
NaBH4 (84 mg, 2.21 1 mmol, 2.0 equiv) was added to a solution of methyl 1 -((tert- butoxycarbonyl)amino)-4-oxocyclohexanecarboxylate (300 mg, 1 .106 mmol) in THF (2 ml_)/MeOH (2 mL) at 0 °C. The mixture was stirred for 30 minutes and then was quenched with 5 mL saturated aqueous NH4CI solution. The aqueous phase was extracted with EtOAc. The organic layer was dried over Na2S04, concentrated. The remaining oil was purified by silica gel column chromatography (EtOAc/heptane 50-100%) to afford product 4.1.3a (309 mg, 100% yield). MS m/z [M+H]+ 274.2
Step 2. Synthesis of 4.1.3b
4.1.3b
Methyl iodide (0.869 mL, 13.90 mmol, 20 equiv) was added to a mixture of 4.1.3a (190 mg, 0.695 mmol, 1.0 equiv), silver oxide (1.289 g, 5.56 mmol, 8.0 equiv) in acetonitrile (9 mL) and the resulting mixture was stirred for 16 hours at room temperature. The mixture was diluted with EtOAc and filtered. The filtrate was concentrated and the residue was purified by silica gel column chromatography (EtOAc/heptane, 30-100%) to afford product 4.1.3b (146 mg, 73% yield). MS m/z [M+H]+ 288.2
Step 3. Synthesis of 4.1.3c
4.1.3c
TFA (0.196 mL, 2.54 mmol, 5.0 equiv) was added to a solution of 4.1.3b (146 mg, 0.508 mmol, 1.0 equiv) in DCM (2 mL) and the resulting solution was stirred at the room temperature for 4 hours. The solution was concentrated and the residue was dissolved in 20 mL DCM, washed with saturated aqueous NaHC03 solution, dried over Na2S04 and concentrated. The material was continued to the next step without further purification. MS m/z [M+H]+ 188.4
Step 4. Synthesis of methyl 1-(([1,1'-biphenyl]-4-ylmethyl)amino)-4- methoxycyclohexanecarboxylate [4.1.3d]
4.1.3d
[1 ,1'-biphenyl]-4-carbaldehyde (238 mg, 1.309 mmol, 2.5 equiv) and AcOH (0.090 mL, 1.570 mmol, 3.0 equiv) were added to a solution of 4.1.3c (98 mg, 0.523 mmol, 1.0 equiv) in dichloroethane (2 mL). After stirring at room temperature for 10 minutes,
NaBH(OAc)3 (333 mg, 1.570 mmol, 3.0 equiv) was added and the solution was stirred at room temperature for 16 hours. To the mixture, EtOAc and saturated aqueous NaHC03 solution were added. The phases were separated and the aqueous layer was extracted with 15 mL EtOAc. The combined organic layers were dried over Na2S04 and concentrated. The
residue was purified by silica gel column chromatography (EtOAc/heptane, 5-30%) to afford product 4.1.3c (123 mg, 66.5% yield). MS m/z [M+H]+ 354.3. 1H NMR (400 MHz,
CHLOROFORM-d) δ ppm 7.50 - 7.62 (m, 4 H) 7.40 - 7.4,9 (m, 4 H) 7.31 - 7.38 (m, 1 H) 3.71 - 3.77 (m, 3 H) 3.61 (s, 2 H) 3.32 - 3.41 (m, 3 H) 3.24 (m, J=3.91 Hz, 1 H) 1.73 - 1.95 (m, 8 H)
Step 5. Synthesis of 1-(([1,1'-biphenyl]-4-ylmethyl)amino)-N-hydroxy-4- methoxycyclohexanecarboxamide [4.1.3]
Compound 4.1.3d was converted to 4.1.3 following the procedure described for the synthesis of 1.2.1 (step2-4). MS m/z 355.3 [M+H]+. 1H NMR (500 MHz, CD3OD) δ ppm 7.74 (m, J=8.20 Hz, 2 H) 7.66 (dd, J=8.20, 0.95 Hz, 2 H) 7.59 (m, J=8.20 Hz, 2 H) 7.46 - 7.50 (m, 2 H) 7.37 - 7.42 (m, 1 H) 4.12 (s, 2 H) 3.52 (br. s., 1 H) 3.38 (s, 3 H) 2.28 (d, J=13.24 Hz, 2 H) 2.12 (br. s., 2 H) 1.91 - 2.03 (m, 2 H) 1.69 - 1.81 (m, 2 H)
5.1.1. Synthesis of 3 S-i^'-chloro^'-fluoro-tl.l'-biphenylJ^-y methy aminoJ-N- hydroxytetrahydrofuran-3-carboxamide [5.1.1]
Compound 5.1.1 was synthesized following the procedures described for the synthesis of 1.2.1 using 4'-chloro-2'-fluoro-[1 ,1 '-biphenyl]-4-carbaldehyde and methyl 3- aminotetrahydrofuran-3-carboxylate in step 1. HRMS m/z 365.1075 [M+H]+. 1H NMR (400 MHz, CD3OD) 7.63 (s, 4 H) 7.50 (t, J=8.41 Hz, 1 H) 7.33 (d, J=2.74 Hz, 1 H) 7.31 (s, 1 H) 3.99 - 4.29 (m, 6 H) 2.37 - 2.60 (m, 2 H).
4.2.1 Synthesis of l-i^'-fluoro^'-methoxy-n.l'-biphenyll^-y methy aminoJ-N^- dihydroxycyclobutanecarboxamide [4.2.1]
Step 1. Synthesis of ethyl 1-((tert-butoxycarbonyl)am
hydroxycyclobutanecarboxylate [4.2.1a]
4.2.1a
Sodium borohydride (0.29 g, 7.7 mmol) was added to a solution of ethyl 1 -((tert- butoxycarbonyl)amino)-3-oxocyclobutanecarboxylate (2.0 g, 7.7 mmol) in THF (26 ml_) at room temerature and the resulting mixture was stirred at room temperature for 2 hours. The reaction was then quenched by saturated aqueous NaHC03 solution. The mixture was extracted with EtOAc. The organic layers were washed with brine, dried over Na2S04 and concentrated. The residue was purified by silica gel column chromatography,
EtOAc/heptane 0-100% to afford product 4.2.1a 1.5 g. The product contained mixtures of diastereomers with 3/1 ratio. LC/MS (m/z) 260.2 [M+H]+.
Step 2. Synthesis of l-^'-fluoro^'-methoxy-II.I'-biphenyl]^- yl)methyl)amino)-N,3-dihydroxycyclobutanecarboxamide [4.2.1]
Compound 4.2.1 was prepared following the procedures described for the synthesis of 4.1.3 Steps 3-5. The product was purified by reverse phase HPLC to give two diastereomers.
4.2.1a: 1H NMR (400 MHz, DMSO-c/6) δ ppm 2.63 - 2.82 (m, 2 H) 3.47 - 3.66 (m, 1 H) 3.97 (br. s., 2 H) 4.51 (t, J=6.65 Hz, 1 H) 6.76 - 7.08 (m, 2 H) 7.37 - 7.67 (m, 5 H) 9.22 - 9.50 (m, 1 H) 9.76 (br. s., 2 H) 1 1.41 (br. s., 1 H). LC/MS (m/z) 361.1 [M+H]+
4.2.1b: 1H NMR (400 MHz, DMSO-c/6) δ ppm 2.16 - 2.38 (m, 2 H) 2.57 - 2.73 (m, 2 H) 3.83 - 3.94 (m, 2 H) 3.99 - 4.33 (m, 1 H) 5.35 - 5.85 (m, 1 H) 6.76 - 7.15 (m, 2 H) 7.48 (s, 6
H) 9.31 - 9.49 (m, 1 H) 9.50 - 9.69 (m, 1 H) 1 1.1 1 - 1 1.33 (m, 1 H). LC/MS (m/z) 361.1 [M+H]+
5.2.1. Synthesis of S-iii^-chloro^'-fluoro-n.l'-biphenyll^-y methy aminoJ-N- hydroxyoxetane-3-carboxamide [5.2.1]
Compound 5.2.1 was synthesized following the procedures described for the synthesis of 1.2.1 using 4'-chloro-2'-fluoro-[1 ,1 '-biphenyl]-4-carbaldehyde and methyl 3- aminooxetane-3-carboxylate in step 1. HRMS m/z 351.0916 [M+H]+. 1H NMR (500 MHz, CD3OD) 7.58 - 7.70 (m, 4 H) 7.53 (t, J=8.51 Hz, 1 H) 7.28 - 7.41 (m, 2 H) 4.93 (br. s., 2 H) 4.82 (d, J=8.20 Hz, 2 H) 4.14 (s, 2 H)
5.3.1. Synthesis of N-hydroxy-4-(2-(2-oxo-4-phenylpyridin-1(2H)-yl)ethyl)tetrahydro- 2H-pyran-4-carboxamide[5.3.1]
Compound 5.3.1 was prepared following the procedures described for the synthesis of 3.1.1. HRMS m/z 343.1654 [M+H]+. 1H NMR (400 MHz, CD3CI) 7.54 - 7.62 (m, 2 H) 7.49 (br. s., 1 H) 7.48 (d, J=1.96 Hz, 2 H) 7.43 (d, J=7.04 Hz, 1 H) 6.93 (s, 1 H) 6.69 (m, 1 H) 4.02 (d, J=8.61 Hz, 2 H) 3.76 (t, J=5.28 Hz, 4 H) 2.17 (d, J=3.13 Hz, 2 H) 2.15 (br. s., 2 H) 1.57 (m, 2 H)
5.3.2 Synthesis of compound 5.3.2
Step 1. Synthesis of ethyl 4-(4-bromophenethyl)tetrahydro-2H-pyran-4- carboxylate [5.3.2a]
5.3.2a
A solution of LDA (2.465 ml, 4.93 mmol) and THF (5.0 mL) was cooled at -78 oC. Ethyl tetrahydro-2H-pyran-4-carboxylate (600 mg, 3.79 mmol) in THF (2.0 mL) was added dropwise to above solution and the solution was stirred at -78 °C for 30 minutes. Then 1- bromo-4-(2-bromoethyl)benzene (0.873 ml, 5.69 mmol) was added. The mixture was stirred at -78 oC for 30 minutes and warmed to room temperature. The reaction was quenched with saturated aqueous NH4CI solution and extracted with EtOAc. The organic layer was washed with water and brine, dried over Na2S04, filtered and concentrated on to silica. Purification by silica gel column chromatography (EtO Ac/heptane, 0 to 70%) to afford 5.3.2a (85.4 mg, 6.6% yield). LCMS (m/z) 343.1 [M+H]+. 1H NMR (400 MHz, CDCI3) δ ppm 1.31 (t, J=7.24 Hz, 3 H) 1.50 - 1.62 (m, 2 H) 1.72 - 1.91 (m, 2 H) 2.15 (d, J=12.13 Hz, 2 H) 2.38 - 2.60 (m, 2H) 3.48 (td, J=1 1.74, 1.96 Hz, 2 H) 3.86 (dt, J=1 1.84, 3.47 Hz, 2 H) 4.21 (q, J=7.17 Hz, 2 H) 7.02 (d, J=8.22 Hz, 2 H) 7.40 (d, J=8.22 Hz, 2 H).
Step 2. Synthesis of ethyl 4-(2-([1,1'-biphenyl]-4-yl)ethyl)tetrahydro-2H-pyran-4- carboxylate [5.3.2b]
A microwave vial was charged with 5.3.2b (13 mg, 0.038 mmol), phenylboronic acid (9.29 mg, 0.076 mmol), potassium phosphate (24.26 mg, 0.1 14 mmol), THF (0.6 mL) and water (0.2 mL). The mixture was purged with N2 for 5 minutes and then PdCI2(dppf) CH2CI2 adduct (6.22 mg, 0.0076 mmol) was added. The reaction mixture was heated to 65 °C for 2 h. The reaction was diluted with EtOAc, stirred overnight with Siliabond DMT. The mixture was filtered. The filtrate was washed with water and brine, dried over Na2S04, filtered and concentrated afford 5.3.2b. The crude material was continued to the next step without further purification. LCMS (m/z) 339.3 [M+H]+.
Step 3. Synthesis of 4-(2-([1,1'-biphenyl]-4-yl)ethyl)tetrahydro-2H-pyran-4- carboxylic acid [5.3.2c]
LiOH (9.20 mg, 0.384 mmol) was added to a solution of 5.3.2b (13 mg, 0.038 mmol) in THF/MeOH/water (0.2/0.2/0.02 mL) and the solution was stirred at 85 °C for 16 hours. The reaction was concentrated, diluted with water and EtOAc. The aqueous layer was acidified to pH=1 by adding 6.0 N HCI aqueous solution and was extracted with EtOAc. The organic layer was dried over Na2S04 and concentrated to afford 5.3.2c (10 mg, 84 % yield). LCMS (m/z) 293.2 [M-OH]
Step 4. Synthesis 4-(2-([1,1'-biphenyl]-4-yl)ethyl)-N-hydroxytetrahydro-2H- pyran-4-carboxamide [5.3.2]
Oxalyl chloride (0.0028 mL, 0.032 mmol) and DMF (0.249 μΙ, 3.22 μιτιοΙ) was added to a solution of 5.3.2c (10 mg, 0.032 mmol) in DCM (0.4 mL). After stirred at room temperature fpr 1 hour the mixture was concentrated. The remaining material was dissolved in DCM (0.4 mL) and a solution of NH2OH HCI (4.48 mg, 0.064 mmol), Et3N (0.022 mL, 0.161 mmol) in DCM (0.4 mL) was added. The reaction mixture was stirred at room
temperature for 2 h and then was diluted with DCM, washed with water and brine. The organic layer was dried over Na2S04 and concentrated. The residue was purified by reverse phase prep HPLC to give product 5.3.2 (1.5 mg, 10 % yield). LCMS (m/z) 326.1 [M+H]+.
5.3.3 Synthesis of compound 5.3.3
Step 1. Synthesis methyl 4-(([1,1'-biphenyl]-4-ylmethyl)amino)tetrahydro-2H- pyran-4-carboxylate [5.3.3a]
Compound 5.3.3a was prepared following the procedure described for the synthesis of 1.1.1a using methyl 4-aminotetrahydro-2H-pyran-4-carboxylate in Step 1. LCMS (m/z) 326.2 [M+H]+.
Step 2. Synthesis of 4-(([1,1'-biphenyl]-4-ylmethyl)amino)-N-hydroxytetrahydro- 2H-pyran-4-carboxamide [5.3.3]
Hydroxylamine (50% in water, 1.5 mL, 25.6 mmol) and NaOH (1.0 M in water, 0.128 mL, 0.128 mmol) was to a solution of 5.3.3a (83.3 mg, 0.256 mmol) in MeOH (2.5 mL) and the reaction mixture was stirred at room temperature for 72 hours. The mixture was concentrated and the residue was purified by reverse phase HPLC to give product 5.3.3 (12.2 mg, 10.6 % yield). LCMS (m/z) 327.2 [M+H]+. 1H NMR (400 MHz, CD3OD) δ ppm 1.97
(d, J=16.04 Hz, 2 H) 2.46 (d, J=13.30 Hz, 2 H) 3.61 (t, J=10.76 Hz, 2 H) 3.91 - 4.08 (m, 2 H) 4.13 (s, 2H) 7.36 - 7.43 (m, 1 H) 7.47 (t, J=7.43 Hz, 2 H) 7.58 (d, J=8.22 Hz, 2 H) 7.62 - 7.69 (m, 2 H) 7.75 (d, J=7.83 Hz ,2H).
Table 1. UPLC retention time and HRMS
Pharmaceutical Activity
Example P. aeruginosa LpxC Inhibition Assay
The P. aeruginosa LpxC protein is produced according to the general method of Hyland et al (Journal of Bacteriology 1997 179, 2029-2037: Cloning, expression and purification of UDP-3-O-acyl-GlcNAc deacetylase from Pseudomonas aeruginosa: a metalloamidase of the lipid A biosynthesis pathway). The LC-MS/MS method for quantitation of LpxC product was developed using an Agilent 1200 Capillary HPLC system coupled to an Applied Biosystems MDS Sciex 4000QTRAP mass spectrometer. Both instruments are controlled using the Applied Biosystems MDS Sciex Analyst software. LpxC reaction product (UDP-3-0-(R-3-hydroxyacyl)-glucosamine) was produced by hydrolysis of LpxC substrate catalyzed by P. a. LpxC and purified using reversed phase chromatography on a
Phenomenex Luna C18(2) 4.6 x 50 mm column. An LpxC product calibration curve was generated to evaluate the sensitivity and dynamic range of the LC-MS/MS method. Briefly, compounds are pre-incubated with 1 nM P. aeruginosa LpxC for 30 min. at room
temperature. Reactions are initiated by the addition of 2 μΜ UDP-3-0-(R-3- hydroxydecanoyl)-GlcNAc. Reactions are conducted in a 384-well plate with a total volume
of 50 [it in each well containing 50 mM Sodium phosphate pH 7.5, 0.005% Trition X-100 for 20 min at room temperature. After quenching with 1 .8% HOAc (5 μΙ_ of a 20% HOAc added to each well), reaction mixtures are analyzed using the LC-MS/MS method and peak areas are transformed into product concentration using a LpxC product calibration curve. Total activity (0% inhibition control) is obtained from reactions with no inhibitors and 100% inhibition control is the background using quenched samples before reaction starts. For IC50 determinations, peak areas are converted to percent inhibition in Microsoft Excel. Percent inhibition values are plotted vs. log compound concentration using XLfit. Data is fit to the four-parameter logistic equation using the non-linear regression algorithm in XLfit to return the IC5o and hill slope values.
Bacterial Screens and Cultures
Bacterial isolates were cultivated from -70°C frozen stocks by two consecutive overnight passages at 35°C in ambient air on 5% blood agar (Remel, Lenexa, Kans.).
Quality control and P. aeruginosa ATCC 27853) is from the American Type Culture
Collection (ATCC; Rockville, Md.) and PA01 was received from Dr. K. Poole.
Susceptibility Testing
Minimal Inhibitory Concentrations (MICs) were determined by the broth microdilution method in accordance with Clinical and Laboratories Institute (CLSI) guidelines. In brief, fresh bacterial overnight cultures were resuspended in sterile saline, adjusted to a 0.5 McFarland turbidity standard and then diluted 20010-fold in cation adjusted Mueller-Hinton Broth II (MHB; Remel BBL) to yield a final inoculum of approximately 5x105 colony-forming units (CFU)/mL. Two-fold serial dilutions of compounds were prepared in 100% dimethyl sulfoxide (DMSO) at 100-fold the highest final assay concentration; the resulting dilution series of compounds were diluted 1 : 10 with sterile water. Ten μΙ of the drug dilution series in 10% DMSO was transferred to microtiter wells and 90 μΙ of bacterial suspension was inoculated into the wells. All inoculated microdilution trays were incubated in ambient air at 37 35°C for 20 hours. Following incubation, assay plates were read in a microtiter plate reader at 600 nm and visually inspected to confirm the MIC endpoint well with the OD value. Tthe lowest concentration of the compound that prevented visible growth was recorded as the MIC. Performance of the assay was monitored by testing ciprofloxacin against laboratory quality control strains in accordance with guidelines of the
CLSI. Compounds of Examples 1 -6, 8-19, 21 , 23-26 and 28-53 exhibit an MIC of 64 μg mL against at least one P. aeruginosa strain selected from PA01 and ATCC27853.
The LpxC inhibitory activity for the compounds of Examples is reported in Table A.
Table A: Biological data for the compounds above.
1.2.1 1 0.0003 1
1.2.12 <0.0005 0.75
1.2.13 0.0001 16
1.2.14 0.0003 0.75
1.2.15 0.0008 1
1.2.16 0.0008 2
1.2.17 0.0005 2
1.2.18 0.0004 8
1.2.19 0.002 4
1.2.20 0.0005 8
1.2.21 0.0002 4
1.2.22 0.0003 2
1.2.23 0.0006 2
1.2.24 0.0005 4
1.2.25 0.001 2
1.2.26 0.0002 2
1.2.27 <0.0005 8
1.2.28 <0.0005 4
1.2.29 0.003 16
1.2.30 0.001 8
1.2.31 0.005 2
1.2.32 0.005 8
1.2.33 0.0003 1
1.3.01 0.0051 64 32
1.4.01 0.0006 8 4
1.4.2 0.002 2
1.5.1 0.03 32
1.5.2 0.01 32
1.6.1 0.2 64
2.1.01 0.0019 16 4
2.2.01 0.002 4 2
2.2.02 0.002 16 8
2.2.03 0.001 8 4
2.2.4 0.001 2 1
2.2.5a 0.0005 1
2.2.5b 0.0005 0.5
3.1.01 0.12 > 64 > 64
3.1.02 0.005 32 16
3.1.03 0.054 > 64 > 64
3.2.1 0.0005 1
3.3.1 0.0005 8
4.1.01 < 0.0005 16 8
4.1.02 0.00175 64 16
4.1.03 0.042 64 32
4.2.1 a 0.0005 2
4.2.1 b 0.002 2
4.3.1 a 0.001 4
4.3.1 b 0.01 32
5.1.01 < 0.0005 4 1
5.2.01 < 0.0005 4 1
5.3.01 0.009 32 32
5.3.02 0.002
5.3.03 0.002 32 32
Those skilled in the art will recognize, or be able to ascertain using no more than routine experimentation, many equivalents to the specific embodiments and methods described herein. Such equivalents are intended to be encompassed by the scope of the following claims.
Claims
1. A compound of the formula I:
A is a divalent radical selected from , and ;
X is -(CH2)nY(CH2)m-;
Y is selected from the group consisting of -C(H,R1)-, -0-, S, -N(R2)-, and -S(0)2- n is 0 or 1 ; m is 0 or 1 ;
R is C3-6 cycloalkyi, -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said cycloalkyi, aryl and heteroaryl are each optionally substituted with up to three substituents selected from the group consisting of halogen, -OH, -CN , -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, C C4haloalkoxy, C C alkyl optionally substituted with C C alkoxy or a 5-6 membered heterocycle containing up to two heteroatoms selected from N, O and S as ring members and optionally substituted with R10, C C alkoxy optionally substituted with Ci-C alkoxy or Ci_ 3 alkyl or C3-e cycloalkyi where the C1-3 alkyl or C3-e cycloalkyi are each optionally substituted with hydroxy, methoxy, or methyl, and a 4 to 7 membered heterocycle or a 5 to 6 membered heteroaryl wherein the 4 to 7 membered heterocycle or 5 to 6 membered heteroaryl contains 1 to 3 heteroatoms selected from N, S, and O as ring members and is optionally substituted with one or more halogen, C C alkoxy, CrC haloalkoxy, CrC haloalkyl or Ci-C alkyl; or
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C6-Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with one or two groups selected from halogen, d-C4alkyl, c
C4haloalkyl, CrC4haloalkoxy and CrC4alkoxy;
R1 is selected from the group consisting of -OH, Ci-C4alkoxy and -S(0)2R3;
R2 is selected from the group consisting of hydrogen, C C alkyl, -C(0)OR3 , -C(0)R3 and -S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl; Z is a divalent radical selected from
Z is with the proviso that
R5 is selected from the group consisting of hydrogen, halogen, -CN, d-dalkyl, and Ci-C4haloalkyl;
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, -CrC4alkyl, and C C4haloalkyl;
R10 is selected from halo, d.4 alkyl, d-4 haloalkyl, d_4 alkoxy, -C(0)R11 and -C(O)-
OR11 ;
R11 is d.4 alkyl; and '
L is a divalent bond, -CH2-, -O- or
2. The compound or a pharmaceutically acceptable salt thereof according to claim 1 , represented by formula II:
II
A is a divalent radical selected from , and
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, d- C4haloalkyl, C3-C7cycloalkyl, CrC4alkoxy, CrC4haloalkoxy, C C alkyl optionally substituted with Ci-C alkoxy, CrC alkoxy optionally substituted with Ci-C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, C C alkoxy, Cr C haloalkoxy, CrC haloalkyl or C C alkyl; or
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C6-Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further
optionally substituted with halogen, d-C4alkyl, CrC4haloalkyl, CrC4haloalkoxy or C C4alkoxy;
R1 is selected from the group consisting of -OH, CrC4alkoxy and -S(0)2R3;
R2 is selected from the group consisting of hydrogen, CrC4alkyl, -C(0)CR3 and - S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl;
Z is a divalent radical selected from
Z is with the proviso that A is
R5 is selected from the group consisting of hydrogen, halogen, -CN, Ci-C alkyl, and CrC4haloalkyl;
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, CrC4alkyl, and CrC4haloalkyl; '
L is a divalent bond, -CH2-, -O- or
3. The compound or a pharmaceutically acceptable salt thereof according to claim 1 or 2, represented by formula III
, and
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, Ci- C4haloalkyl, C3-C7cycloalkyl, CrC4alkoxy, CrC4haloalkoxy, C C alkyl optionally substituted with Ci-C alkoxy, CrC alkoxy optionally substituted with Ci-C alkoxy and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, C C alkoxy, Cr C4haloalkoxy, CrC4haloalkyl or Ci-C4 alkyl; or
R is -C6-Ci0aryl, or 4 to 10 membered heteroaryl containing 1 to 3 heteroatoms selected from N, S and O, wherein said aryl and heteroaryl are susbstituted by taking the substituents on adjacent atoms of the -C6-Ci0aryl, or 4 to 10 membered heteroaryl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, Ci-C alkyl, CrC haloalkyl, Ci-C haloalkoxy or C C4alkoxy;
R2 is selected from the group consisting of hydrogen, C C alkyl, -C(0)CR3 and - S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl;
R5 is selected from the group consisting of hydrogen, halogen, -CN, Ci-C alkyl, and CrC haloalkyl; '
L is a direct bond or
4. The compound or a pharmaceutically acceptable salt thereof according to any any one of the proceeding claims, wherein
R is phenyl optionally substituted with a substituent selected from the group
consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, CrC haloalkoxy, C C alkyl optionally substituted with C C alkoxy, C C alkoxy optionally substituted with C C alkoxy, and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, Ci-C alkyl, CrC haloalkyl, d- C haloalkoxy or C C alkoxy.
5. The compound or a pharmaceutically acceptable salt thereof according to any any one of the proceeding claims, wherein the compound is formula III
and ;
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, Ci-C4haloalkoxy, Ci-C4alkyl optionally substituted with Ci-C4alkoxy, Ci-C4alkoxy optionally substituted with C C alkoxy, and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, Ci-C haloalkoxy, Ci-C haloalkyl or Ci-C alkyl; or
R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy;
R2 is selected from the group consisting of hydrogen, C C alkyl, C(0)CR3 and - S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl; '
L is a direct bond or
6. The compound or a pharmaceutically acceptable salt thereof according to any one of claims 1 to 4, wherein the compound is formula III
Q is selected from the group consisting of
, and
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, CrC haloalkoxy, C C alkyl optionally substituted with C C alkoxy, C C alkoxy optionally substituted with C C alkoxy, and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, C C haloalkoxy, Ci-C haloalkyl or C C alkyl; or
R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl;
; '
;' '
L is a direct bond or
7. The compound of claim 1 or 2, represented by formula IV
, and
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, CrC haloalkoxy, C Calkyl optionally substituted with C C alkoxy, C Calkoxy
optionally substituted with CrC4alkoxy, and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, d-C4alkoxy, CrC4haloalkoxy, CrC4haloalkyl or C C alkyl; or
R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C4alkyl, C C4haloalkyl, C C haloalkoxy or C C alkoxy;
R2 is selected from the group consisting of hydrogen, CrC4alkyl, -C(0)CR3 and - S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl;
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, C C alkyl and CrC haloalkyl; '
L is a direct bond, -CH2-, -O- or 7. The compound of claim 1 or 2, represented by formula III
and ;
R is phenyl optionally substituted with a substituent selected from the group consisting of halogen, -OH, -CN, -S(0)2(CrC4)alkyl, C C4haloalkyl, C3-C7cycloalkyl, C C4alkoxy, Ci-C4haloalkoxy, Ci-C4alkyl optionally substituted with Ci-C4alkoxy, Ci-C4alkoxy optionally substituted with C C alkoxy, and a 4 to 7 membered heterocycle containing 1 to 3 heteroatom selected from N, S, and O, wherein said heterocycle is optionally substituted with one or more halogen, Ci-C alkoxy, Ci-C haloalkoxy, Ci-C haloalkyl or Ci-C alkyl; or
R is phenyl optionally susbstituted by taking the substituents on adjacent atoms of the phenyl and forming a 3 to 7 membered heterocycle, wherein the formed bicycle substituent is further optionally substituted with halogen, C C alkyl, CrC haloalkyl, C C haloalkoxy or C C alkoxy;
R2 is selected from the group consisting of hydrogen, C C alkyl, -C(0)CR3 and - S(0)2R3;
R3 is selected from the group consisting of C C alkyl and C3-C6cycloalkyl;
R6, R6a, R6b or R6c are independently selected from the group consisting of hydrogen, halogen, C C alkyl, and CrC haloalkyl; '
L is a direct bond or
8. The compound according to any any one of the proceeding claims, wherein
L is a direct bond;
R is selected from the group consisting of
and
9. A pharmaceutical composition, comprising:
the compound according to claims 1 to 8 and
a pharmaceutically acceptable carrier.
10. A pharmaceutical combination composition, comprising:
the compound according to any one of claims 1 to 8,
an antibacterially effective amount of a second therapeutic agent, and
a pharmaceutically acceptable carrier.
11. The pharmaceutical combination composition according to claim 10, wherein the second therapeutic agent is selected from the group consisting of Ampicillin, Piperacillin, Penicillin G, Ticarcillin, Imipenem, Meropenem, Azithromycin, erythromycin, Aztreonam, Cefepime, Cefotaxime, Ceftriaxone, Cefatazidime, Ciprofloxacin, Levofloxacin, Clindamycin,
Doxycycline, Gentamycin, Amikacin, Tobramycin, Tetracycline, Tegacyclin, Rifampicin, Vancomycin and Polymyxin.
12. A method of inhibiting a deacetylase enzyme in a Gram-negative bacterium, comprising: contacting the Gram-negative bacteria with the compound according to any one of claims 1 to 8.
13. A method for treating a subject with a Gram-negative bacterial infection, comprising: administering to the subject in need thereof an antibacterially effective amount of the compound according to any one of claims 1 to 8 and a pharmaceutically acceptable carrier.
14. The method of claim 13, wherein the Ggram negative bacterial infection is an infection comprising at least one bacterium selected from the group consisting of Pseudomonas, Stenotrophomonas maltophila, Burkholderia, Alcaligenes xylosoxidans, Acinetobacter, Enterobacteriaceae, Haemophilus, Moraxella, Bacteroids, Fransicella, Shigella, Proteus, Vibrio, Salmonella, Bordetella, Helicobactor, Legionella, Citrobactor, Serratia,
Campylobactor, Yersinia and Neisseria.
15. The method of claim 14, wherein the bacterium is a Enterobacteriaceae which is selected from the group consisting of Serratia, Proteus, Klebsiella, Enterobacter, Citrobacter, Salmonella, Providencia, Morganella, Cedecea, Yersinia, and Edwardsiella species and Escherichia coli.
16. A compound according to any one of claims 1 to 8 or a pharmaceutically acceptable salt thereof, for use as a medicament.
17. A compound according to any one of claims 1 to 8 or a pharmaceutically acceptable salt thereof, for use in treatment of a Gram-negative bacterial infection.
18. A compound according to any one of claims 1 to 8 or a pharmaceutically acceptable salt thereof, for use in treatment of a Gram-negative bacterial infection, wherein the bacterial infection is selected from Pseudomonas aeruginosa, Stenotrophomonas maltophila, Burkholderia cepacia, Alcaligenes xylosoxidans, Acinetobacter, Enterobacteriaceae, Haemophilus, and Neisseria species.
19. Use of the compound according to any one of claims 1 to 8, for the preparation of a medicament for the treatment of a Gram-negative bacterial infection in a subject, wherein the bacterial infection is selected from Pseudomonas aeruginosa, Stenotrophomonas maltophila, Burkholderia cepacia, Alcaligenes xylosoxidans, Acinetobacter,
Enterobacteriaceae, Haemophilus, and Neisseria species.
20. The use of claim 19, wherein the bacterial infection is an Enterobacteriaceae selected from the group consisting of Serratia, Proteus, Klebsiella, Enterobacter, Citrobacter, Salmonella, Providencia, Morganella, Cedecea, and Edwardsiella species and Escherichia coli.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361806664P | 2013-03-29 | 2013-03-29 | |
| US61/806,664 | 2013-03-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014160649A1 true WO2014160649A1 (en) | 2014-10-02 |
Family
ID=50771589
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2014/031612 WO2014160649A1 (en) | 2013-03-29 | 2014-03-24 | Hydroxamic acid derivatives as lpxc inhibitors for the treatment of bacterial infections |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2014160649A1 (en) |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015066413A1 (en) | 2013-11-01 | 2015-05-07 | Novartis Ag | Oxazolidinone hydroxamic acid compounds for the treatment of bacterial infections |
| US9539305B1 (en) | 2014-03-14 | 2017-01-10 | Fleurir Abx Llc | Pristinamycin compositions, LpxC compositions, their improvements, and combinations thereof |
| US9549916B2 (en) | 2014-12-16 | 2017-01-24 | Novartis Ag | Isoxazole hydroxamic acid compounds as LpxC inhibitors |
| US9637482B2 (en) | 2014-04-22 | 2017-05-02 | Novartis Ag | Isoxazoline hydroxamic acid derivatives as LpxC inhibitors |
| WO2017083434A1 (en) | 2015-11-09 | 2017-05-18 | Forge Therapeutics, Inc. | Pyrone based compounds for treating bacterial infections |
| US10071973B2 (en) | 2016-06-14 | 2018-09-11 | Novartis Ag | Crystalline isoxazole hydroxamic acid compounds |
| US10414735B2 (en) | 2015-11-09 | 2019-09-17 | Forge Therapeutics, Inc. | Substituted hydroxypyrimidinones for treating bacterial infections |
| CN111867600A (en) * | 2018-03-15 | 2020-10-30 | 辉瑞大药厂 | Pyridone and pyrimidinone phosphates and borates used as antibacterial agents |
| US11021471B2 (en) | 2017-05-10 | 2021-06-01 | Forge Therapeutics, Inc. | Antibacterial compounds |
| CN113166077A (en) * | 2018-09-20 | 2021-07-23 | 福至治疗公司 | Antibacterial compounds |
| WO2023015236A3 (en) * | 2021-08-04 | 2023-03-02 | The Research Foundation For The State University Of New York | Composition and method for treatment of gram negative bacterial infection |
| US11731962B2 (en) | 2020-03-25 | 2023-08-22 | Blacksmith Medicines, Inc. | LpxC inhibitor and methods of making |
| US12187754B2 (en) | 2021-09-28 | 2025-01-07 | Blacksmith Medicines, Inc. | LpxC inhibitors and uses thereof |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004062601A2 (en) * | 2003-01-08 | 2004-07-29 | Chiron Corporation | Antibacterial agents |
| WO2004078163A2 (en) | 2003-02-28 | 2004-09-16 | Transform Pharmaceuticals, Inc. | Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen |
-
2014
- 2014-03-24 WO PCT/US2014/031612 patent/WO2014160649A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004062601A2 (en) * | 2003-01-08 | 2004-07-29 | Chiron Corporation | Antibacterial agents |
| WO2004078163A2 (en) | 2003-02-28 | 2004-09-16 | Transform Pharmaceuticals, Inc. | Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen |
Non-Patent Citations (17)
| Title |
|---|
| "Methoden der organischen Chemie", vol. 15/1, 1974, GEORG THIEME VERLAG |
| "Methods of Organic Synthesis", vol. 21, 1952, THIEME |
| "Remington's Pharmaceutical Sciences", 1985, MACK PUBLISHING COMPANY |
| "Science of Synthesis: Houben-Weyl Methods of Molecular Transformation", 2005, GEORG THIEME VERLAG |
| "Science of Synthesis: Houben-Weyl Methods of Molecular Transformation", 2005, GEORG THIEME VERLAG, pages: 41627 |
| "The Peptides", vol. 3, 1981, ACADEMIC PRESS |
| H.-D. JAKUBKE; H. JESCHKEIT: "Aminosauren, Peptide, Proteine" |
| H.-D. JAKUBKE; H. JESCHKEIT: "Aminosauren, Peptide, Proteine", 1982, VERLAG CHEMIE |
| HALE MICHAEL R ET AL: "Exploring the UDP pocket of LpxC through amino acid analogs", BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, PERGAMON, AMSTERDAM, NL, vol. 23, no. 8, 1 March 2013 (2013-03-01), pages 2362 - 2367, XP028525203, ISSN: 0960-894X, DOI: 10.1016/J.BMCL.2013.02.055 * |
| HYLAND ET AL., JOURNAL OF BACTERIOLOGY, vol. 179, 1997, pages 2029 - 2037 |
| J. F. W. MCOMIE: "Protective Groups in Organic Chemistry", 1973, PLENUM PRESS |
| JOCHEN LEHMANN: "Chemie der Kohlenhydrate: Monosaccharide und Derivate", 1974, GEORG THIEME VERLAG |
| JOCHEN LEHMANN: "Chemistry of Carbohydrates: Monosaccharides and Derivatives", 1974, GEORG THIEME VERLAG, article "Chemie der Kohlenhydrate: Monosaccharide und Derivate" |
| STAHL; WERMUTH: "Handbook of Pharmaceutical Salts: Properties, Selection, and Use", 2002, WILEY-VCH |
| T. W. GREENE; P. G. M. WUTS: "Protective Groups in Organic Synthesis", 1999, WILEY |
| T. W. GREENE; P. G. M. WUTS: "Protective Groups in Organic Synthesis", vol. 3, 1999, ACADEMIC PRESS, article "The Peptides" |
| TETRAHEDRON LETT., vol. 46, 2005, pages 7575 - 7579 |
Cited By (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015066413A1 (en) | 2013-11-01 | 2015-05-07 | Novartis Ag | Oxazolidinone hydroxamic acid compounds for the treatment of bacterial infections |
| US9539305B1 (en) | 2014-03-14 | 2017-01-10 | Fleurir Abx Llc | Pristinamycin compositions, LpxC compositions, their improvements, and combinations thereof |
| US11951148B1 (en) | 2014-03-14 | 2024-04-09 | Fleurir Abx, Llc | Pristinamycin compositions, LpxC compositions, their improvements, and combinations thereof |
| US9872884B1 (en) | 2014-03-14 | 2018-01-23 | Fleurir Abx Llc | Pristinamycin compositions, LPXC compositions, their improvements, and combinations thereof |
| US11141453B1 (en) | 2014-03-14 | 2021-10-12 | Fleurir Abx Llc | Pristinamycin compositions, LpxC compositions, their improvements, and combinations thereof |
| US10307457B1 (en) | 2014-03-14 | 2019-06-04 | Fleurir Abx Llc | Pristinamycin compositions, LpxC compositions, their improvements, and combinations thereof |
| US9637482B2 (en) | 2014-04-22 | 2017-05-02 | Novartis Ag | Isoxazoline hydroxamic acid derivatives as LpxC inhibitors |
| US9718792B2 (en) | 2014-04-22 | 2017-08-01 | Novartis Ag | Isoxazoline hydroxamic acid derivatives as LpxC inhibitors |
| US9549916B2 (en) | 2014-12-16 | 2017-01-24 | Novartis Ag | Isoxazole hydroxamic acid compounds as LpxC inhibitors |
| US9815804B2 (en) | 2014-12-16 | 2017-11-14 | Novartis Ag | Isoxazole hydroxamic acid compounds as LpxC inhibitors |
| US10029994B2 (en) | 2014-12-16 | 2018-07-24 | Novartis Ag | Isoxazole hydroxamic acid compounds as LpxC inhibitors |
| US10611747B2 (en) | 2015-11-09 | 2020-04-07 | Forge Therapeutics, Inc. | Pyrone based compounds for treating bacterial infections |
| US10414735B2 (en) | 2015-11-09 | 2019-09-17 | Forge Therapeutics, Inc. | Substituted hydroxypyrimidinones for treating bacterial infections |
| US10875832B2 (en) | 2015-11-09 | 2020-12-29 | Forge Therapeutics, Inc. | Substituted pyrimidines for treating bacterial infections |
| WO2017083434A1 (en) | 2015-11-09 | 2017-05-18 | Forge Therapeutics, Inc. | Pyrone based compounds for treating bacterial infections |
| US10071973B2 (en) | 2016-06-14 | 2018-09-11 | Novartis Ag | Crystalline isoxazole hydroxamic acid compounds |
| US11021471B2 (en) | 2017-05-10 | 2021-06-01 | Forge Therapeutics, Inc. | Antibacterial compounds |
| CN111867600A (en) * | 2018-03-15 | 2020-10-30 | 辉瑞大药厂 | Pyridone and pyrimidinone phosphates and borates used as antibacterial agents |
| CN113166077A (en) * | 2018-09-20 | 2021-07-23 | 福至治疗公司 | Antibacterial compounds |
| US11407740B2 (en) | 2018-09-20 | 2022-08-09 | Forge Therapeutics, Inc. | Antibacterial compounds |
| US12325699B2 (en) | 2018-09-20 | 2025-06-10 | Blacksmith Medicines, Inc. | Antibacterial compounds |
| US11731962B2 (en) | 2020-03-25 | 2023-08-22 | Blacksmith Medicines, Inc. | LpxC inhibitor and methods of making |
| WO2023015236A3 (en) * | 2021-08-04 | 2023-03-02 | The Research Foundation For The State University Of New York | Composition and method for treatment of gram negative bacterial infection |
| US12187754B2 (en) | 2021-09-28 | 2025-01-07 | Blacksmith Medicines, Inc. | LpxC inhibitors and uses thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2014160649A1 (en) | Hydroxamic acid derivatives as lpxc inhibitors for the treatment of bacterial infections | |
| US9718792B2 (en) | Isoxazoline hydroxamic acid derivatives as LpxC inhibitors | |
| AU2017294231B2 (en) | Aromatic acetylene or aromatic ethylene compound, intermediate, preparation method, pharmaceutical composition and use thereof | |
| CN107001295B (en) | Isoxazole hydroxamic acid compounds as LpxC inhibitors | |
| CN112074507B (en) | Compounds as Antibiotics | |
| US20220226298A1 (en) | Gpr40 agonists | |
| WO2016198691A1 (en) | Efflux-pump inhibitors and therapeutic uses thereof | |
| WO2015066413A1 (en) | Oxazolidinone hydroxamic acid compounds for the treatment of bacterial infections | |
| CN117279898A (en) | Antibacterial compounds | |
| KR102264248B1 (en) | Tetrahydropyridine derivatives and their use as antibacterial agents | |
| CN113677659A (en) | Substituted amide compounds useful as farnesoid X receptor modulators | |
| HK40043214A (en) | Compound acting as antibiotics | |
| HK40057175A (en) | Amino acid compounds and methods of use | |
| HK40008443A (en) | Imidazopyrrolopyridine as inhibitors of the jak family of kinases | |
| NZ751511B2 (en) | Tetrahydropyridine derivatives and their use as antibacterial agents |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14725829 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14725829 Country of ref document: EP Kind code of ref document: A1 |