WO2014034754A1 - Psf1由来ペプチド - Google Patents
Psf1由来ペプチド Download PDFInfo
- Publication number
- WO2014034754A1 WO2014034754A1 PCT/JP2013/073084 JP2013073084W WO2014034754A1 WO 2014034754 A1 WO2014034754 A1 WO 2014034754A1 JP 2013073084 W JP2013073084 W JP 2013073084W WO 2014034754 A1 WO2014034754 A1 WO 2014034754A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- peptide
- cells
- ctl
- cell
- psf1
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4702—Regulators; Modulating activity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/06—Linear peptides containing only normal peptide links having 5 to 11 amino acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/08—Linear peptides containing only normal peptide links having 12 to 20 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/53—DNA (RNA) vaccination
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/57—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2
- A61K2039/572—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2 cytotoxic response
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
Definitions
- the present invention relates to a PSF1-derived peptide useful for specific immunotherapy for HLA-A * 02 positive cancer patients.
- PSF1 forms a tetramer (GINS complex) composed of SLD5, PSF2 and PSF3, and is associated with MCM (mini-chromosome maintenance complex) and cdc45 and is involved in the initiation and elongation of DNA replication. It is known (Non-Patent Document 1-2). Regarding the relationship with cancer, increased expression of PSF1 was observed in human breast cancer cell lines, and when PSF1 expression was decreased, significant growth suppression was observed. Furthermore, it has been shown that a patient group having a low PSF1 expression level in a cancer tissue derived from a human breast cancer patient has a significantly higher overall survival rate than a high patient group (Non-patent Document 3).
- Non-patent Documents 4-5 and Patent Document 1 the expression of lung cancer and esophageal cancer in cancer tissues has also been reported.
- Patent Document 5 the relationship between PSF1 and cancer stem cells has also been reported.
- An object of the present invention is to provide a peptide useful for specific immunotherapy for cancer patients.
- the present inventors have identified a peptide derived from PSF1 that binds to the extracellular region of the HLA-A * 02 molecule in a human breast cancer cell line. Furthermore, the present invention was completed by confirming that these peptides induce peptide-specific CTL (cytotoxic T cells).
- the present invention (1) is a part of a polypeptide consisting of the amino acid sequence of SEQ ID NO: 1, a peptide having CTL inducing ability, (2) a peptide having a sequence of 8 to 14 residues contained in positions 70 to 110 of SEQ ID NO: 1 and having the ability to induce CTL, (3) a peptide comprising the amino acid sequence shown in any of SEQ ID NOs: 3 to 5 and having a CTL-inducing ability, (4) a peptide comprising the amino acid sequence shown in any one of SEQ ID NOs: 3 to 5, (5) a peptide capable of inducing CTL, wherein one, two, or several amino acids are substituted, deleted, and / or inserted in the amino acid sequence shown in any of SEQ ID NOs: 3 to 5, (6) a nucleic acid molecule encoding the peptide according to any one of (1) to (5), (7) a vector comprising the nucleic acid molecule according to (6), (8) A pharmaceutical composition comprising the
- a peptide having CTL-inducing ability wherein one or two amino acids are substituted in the amino acid sequence shown in any of SEQ ID NOs: 3 to 5, wherein the substitution is Substitution of isoleucine, leucine, valine, methionine, alanine or threonine of the second amino acid from the N-terminal in the amino acid sequence shown in any of SEQ ID NOs: 3 to 5, A peptide selected from the substitution of the C-terminal amino acid in the amino acid sequence shown in any of SEQ ID NOs: 3 to 5 with isoleucine, leucine, valine, methionine, alanine or threonine, (12) In the peptide comprising the amino acid sequence shown in SEQ ID NO: 3, an alanine added to the N-terminal side in the amino acid sequence shown in SEQ ID NO: 3, or arginine on the C-terminal side in the amino acid sequence shown in SEQ ID NO: 3 A peptide to which is added, (13)
- a peptide to which is added (14) A method for inducing antigen-presenting cells by contacting antigen-presenting cells collected from a living body with the peptide according to any one of (1) to (5), (15) An antigen-presenting cell comprising a complex formed between an HLA antigen and the peptide according to any one of (1) to (5), (16) A method for inducing cytotoxic T cells by contacting T cells collected from a living body with the peptide according to any one of (1) to (5), (17) A cytotoxic T cell induced by contacting a T cell collected from a living body with the peptide according to any one of (1) to (5), (18) The cancer of a patient is treated by administering to the patient the peptide according to any one of (1) to (5), the nucleic acid molecule according to (6), or the vector according to (7).
- a PSF1-derived peptide capable of inducing CTL that can damage cancer cells in HLA-A * 02 positive cancer patients was provided.
- the PSF1-derived peptide of the present invention enables specific immunotherapy for HLA-A * 02 positive cancer patients.
- CTL cells obtained by culture were double-stained with PE-labeled tetramer and APC-H7-labeled anti-CD8 antibody, and the fluorescence intensity was measured with FACS Aria.
- the cell fraction stained with both labels was detected at the position surrounded by a line (upper side of each panel). Positive cells were confirmed in the two types of CTL lines (0209-01 H2, 0209-02 D2), which revealed that CTLs that recognize YLYDRLLRI (SEQ ID NO: 3) were obtained.
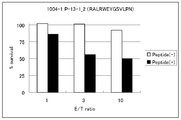
- the cytotoxic activity by a peptide-specific CTL line is shown.
- CTL E; effector cell
- T2 cells T2 cells
- E T ratio
- the survival rate of the target cell was calculated on the next day, and the survival rate was lower in the target cell that was pulsed with the peptide than in the non-pulsed target cell.
- the survival rate decreased according to the ratio of the co-cultured cells, the cytotoxic activity by peptide-specific CTL was shown.
- the cytotoxic activity by a peptide-specific CTL line is shown.
- CTL E; effector cell
- T2 cells T2 cells
- E T2 cells
- T target cell
- the survival rate of the target cell was calculated on the next day, and the survival rate was lower in the target cell that was pulsed with the peptide than in the non-pulsed target cell.
- the survival rate decreased according to the ratio of the co-cultured cells, the cytotoxic activity by peptide-specific CTL was shown.
- the cytotoxic activity by a peptide-specific CTL line is shown.
- CTL E; effector cell
- T2 cells T2 cells
- E T2 cells
- T target cell
- the survival rate of the target cell was calculated on the next day, and the survival rate was lower in the target cell that was pulsed with the peptide than in the non-pulsed target cell.
- the survival rate decreased according to the ratio of the co-cultured cells, the cytotoxic activity by peptide-specific CTL was shown.
- Peptides and polypeptides The present invention provides peptides having the ability to induce CTL derived from PSF1 (hereinafter referred to as peptides of the present invention).
- PSF1-derived peptide means a peptide fragment consisting of a part of the amino acid sequence of PSF1.
- the amino acid sequence (SEQ ID NO: 1) and nucleic acid sequence (SEQ ID NO: 2) of PSF1 are disclosed in GeneBank (NP_066545 and NM_021067, respectively).
- the peptide of the present invention was found as a peptide having high binding affinity with the HLA-A * 02 antigen. This means that the peptide can be complexed with HLA-A * 02 and presented on the cell surface.
- “having the ability to induce CTL” means that the peptide is recognized by a specific CTL, in other words, has the ability to induce a peptide-specific CTL.
- Peptides with high binding affinity for HLA antigens as described above are expected to be highly effective as cancer vaccines, but candidate peptides selected as pharmaceutical active ingredients are about the presence of actual CTL inducibility. It is necessary to investigate. Whether or not it has the ability to induce peptide-specific CTL is determined by, for example, interferon- ⁇ (IFN- ⁇ ) in response to peptide-stimulated peripheral blood mononuclear cells (PBMC) in response to antigen-presenting cells pulsed with the corresponding peptide.
- IFN- ⁇ interferon- ⁇
- PBMC peripheral blood mononuclear cells
- the number of amino acid residues of the peptide of the present invention is preferably within the range of 8 to 14, more preferably 8 to 11, particularly preferably 9 or 10.
- peptides having high binding affinity for HLA antigens do not necessarily have high inducing ability.
- peptides selected from peptides comprising the amino acid sequence shown by YLYDRLLRI (SEQ ID NO: 3), RALRWEYGSVLPN (SEQ ID NO: 4) and ALRWEYGSV (SEQ ID NO: 5) showed particularly high CTL inducing ability and cytotoxic activity. . It can be seen that all of these three types of peptides belong to the region of positions 79 to 100 of PSF1 (see FIG. 3).
- YLYDRLLRI® SEQ ID NO: 3
- AYLYDRLLRI® SEQ ID NO: 6
- YLYDRLLRIR® SEQ ID NO: 7
- ALRWEYGSV SEQ ID NO: 5
- ALRWEYGSVL SEQ ID NO: 8
- RALRWEYGSV SEQ ID NO: 9
- the present invention also provides derivatives of PSF1-derived peptides (hereinafter referred to as derivatives of the present invention).
- derivatives of the present invention refers to a peptide having an CTL-inducing ability consisting of an amino acid sequence in which 1 or 2 amino acid substitutions, deletions and / or insertions are made in the amino acid sequence of the PSF1-derived peptide. means. Whether the derivative has a desired property can be examined by the above-described method.
- Amino acid substitution should be performed between homologous amino acids (polar amino acids, nonpolar amino acids, hydrophobic amino acids, hydrophilic amino acids, positively charged amino acids, negatively charged amino acids, aromatic amino acids, etc.) from the viewpoint of not changing the properties of the peptide. Is preferred. Deletion and insertion of amino acids are preferably performed so that the number of amino acid residues of the derivative is 8-14.
- peptides that bind to HLA have a regular amino acid sequence that depends on the type of HLA.
- the regular amino acid sequence is called a binding motif.
- the binding motif for HLA-A * 02 is the second amino acid from the N-terminus is isoleucine, leucine, valine, methionine, alanine or threonine, and the C-terminal amino acid is isoleucine, leucine, valine, methionine, alanine or threonine. It refers to a sequence (Current Pharmaceutical Design 2010, 16, 3149-3157).
- Binding of peptides having an HLA-A02 binding motif to HLA-A * 02 can be determined by computer analysis such as Bioinformatics and Molecular Analysis Section (NIH, Bethesda, MD) (Parker KC, et al., J. Immunol., 152: 163-175, 1994).
- amino acid substitutions, deletions and / or insertions are preferably allowed on the HLA-binding motif.
- the second amino acid from the N-terminal of the amino acid sequence of the derivative is isoleucine, leucine, valine, methionine, alanine or threonine
- the C-terminal amino acid is isoleucine, leucine, It is preferable to carry out so that it becomes valine, methionine, alanine or threonine.
- the amino acids constituting the peptides and derivatives of the present invention may be natural amino acids or amino acid analogs.
- amino acid analogs include N-acylated products, O-acylated products, esterified products, acid amidated products, and alkylated products of amino acids. Is mentioned.
- the peptides and derivatives of the present invention may be modified in their constituent amino acids or carboxyl groups as long as the functions are not significantly impaired. Modifications include N-terminal and free amino groups bound to formyl, acetyl, t-butoxycarbonyl, etc., and C-terminal and free carboxyl groups to methyl, ethyl, t-butyl, and benzyl groups. And the like. Others include, for example, the introduction of D-amino acids and other amino acid mimetics that can be used to increase the serum half-life of the peptides.
- the peptides and derivatives of the present invention can be produced by conventional peptide synthesis.
- such peptides can be prepared synthetically using either recombinant DNA techniques or chemical synthesis. Examples of such methods include the methods described in Peptide Synthesis, Interscience, New York, 1966; 'The Proteins, Vol2, Academic Press Inc., New York, 1976.
- Nucleic acid / vector The present invention further provides a nucleic acid molecule encoding the peptide or derivative of the present invention and a vector comprising the nucleic acid molecule.
- the vector containing the nucleic acid molecule of the present invention expresses the peptide or derivative of the present invention and presents them on the cell surface as a complex with HLA. This antigen-presenting cell can efficiently proliferate CTL that damages cancer cells in a peptide-specific manner.
- vectors incorporating the nucleic acid molecule of the present invention include various plasmids and viral vectors such as adenovirus, adeno-associated virus, retrovirus, vaccinia virus (Liu M, Acres B, Balloul JM, Bizouarne N, Paul S, Slos. P, Squiban P.eneGene-based vaccines and immunotherapeutics. Proc Natl Acad Sci USA 101 Suppl, 56714567-71, 2004). Methods for preparing vectors are well known in the art (Molecular Cloning: A laboraroy manual, 2nd edn. New York, Cold Spring Harbor Laboratory).
- the vector of the present invention can be administered to a patient in order to express the peptide or derivative of the present invention in an antigen-presenting cell in the patient.
- the vector of the present invention may be introduced into a patient-derived dendritic cell outside the patient's body, and the cell expressing the peptide or derivative of the present invention may be returned to the patient.
- These methods are well known in the art (Hrouda D, Dalgleish AG. Gene therapy for prostate cancer. Gene Ther 3: 845-52, 1996).
- the dosage varies depending on the disease state, the age, weight, etc. of each individual patient.
- the DNA amount is 0.1 ⁇ g to 100 mg, preferably 1 ⁇ g to 50 mg.
- the administration method include intravenous injection, subcutaneous administration, intradermal administration and the like.
- compositions further provides a pharmaceutical composition comprising a peptide or derivative of the present invention, a nucleic acid molecule encoding said peptide or derivative, or a vector comprising said nucleic acid molecule.
- the pharmaceutical composition of the present invention is useful in treating or preventing cancer.
- PSF1 is associated with the regeneration of cancer stem cells, it is also useful in the treatment or prevention of cancers that are resistant to chemotherapy or radiation therapy, relapsed, or metastatic.
- the pharmaceutical composition of the present invention may contain one type of peptide or derivative, or may contain a combination of two or more types of peptides and / or derivatives.
- CTLs in cancer patients are a collection of cells that recognize different cancer antigen peptides, it is more effective to use a combination of multiple types of peptides and / or derivatives. You may combine with cancer antigen peptides other than the peptide of this invention.
- the pharmaceutical composition of the present invention can contain a pharmaceutically acceptable carrier and the like in addition to the peptide or derivative.
- a pharmaceutically acceptable carrier cellulose, polymerized amino acid, albumin and the like can be used.
- the pharmaceutical composition of the present invention may be a liposome preparation, a particulate preparation bound to beads having a diameter of several ⁇ m, a preparation bound to a lipid, and the like.
- it can also administer with the adjuvant conventionally known to be used for vaccine administration so that an immune response may be effective. Suitable adjuvants are described in the literature (Johnson AG. (1994) Clin. Microbiol. Rev.,. 7: 277-89).
- Exemplary adjuvants include, but are not limited to, aluminum phosphate, aluminum hydroxide, and alum.
- the administration method is, for example, intradermal administration or subcutaneous administration.
- the pharmaceutical composition of the present invention can be used as a cancer vaccine.
- the dose can be appropriately adjusted depending on the disease state, the age, weight, etc. of the individual patient.
- the amount of the peptide or derivative in the pharmaceutical composition is 0.0001 to 1000 mg, preferably 0.001 to mg to 100. mg, more preferably 0.01 to 10 mg, even more preferably 0.1 to 5 mg or 0.5 to 30 mg. This is preferably administered daily, weekly, or once every few days, weeks or months for 1-3 years.
- the present invention further provides a method for inducing CTL.
- CTL has cytotoxicity against HLA-A * 02 positive cancer cells.
- Cytotoxic means to recognize a complex of a cancer antigen peptide and HLA on a cancer cell and to have the ability to damage the cell.
- CTL can be obtained, for example, by culturing PBMC collected from an HLA-A * 02 positive cancer patient in vitro in the presence of the peptide or derivative of the present invention.
- the CTL induction method of the present invention is useful for adoptive immunotherapy in which CTL induced in the body of a patient from whom PBMC has been collected is returned to damage cancer cells.
- the present invention further provides a method for inducing antigen-presenting cells.
- the method of the present invention induces antigen-presenting cells that can induce CTLs that damage HLA-A * 02-positive cancer cells.
- the method of the present invention includes, for example, culturing a cell having antigen-presenting ability derived from a patient having HLA-A * 02-positive cancer together with the peptide or derivative of the present invention, binding the peptide or derivative to HLA-A * 02, and We do by letting you present.
- a vector capable of expressing such a peptide may be introduced into a cell having antigen-presenting ability derived from a cancer patient who is positive for HLA-A * 02.
- a cell having an antigen presenting ability is, for example, a dendritic cell.
- the patient-derived dendritic cells can be obtained, for example, by separating cultured plate adherent cells from PBMC collected from the patient and culturing the cells in the presence of IL-4 and GM-CSF for about 1 week.
- Antigen-presenting cells induced by the method of the present invention can induce CTLs that specifically recognize complexes of peptides or derivatives presented on the cell surface and HLA-A * 02, and are administered to cancer patients. Induction of cancer-reactive CTL in the patient can be promoted.
- the present invention further provides a method for treating or preventing cancer, comprising administering to a patient a peptide, nucleic acid, or vector of the present invention.
- the present invention also provides a peptide, nucleic acid, or vector of the present invention for use in treating or preventing cancer.
- Example 1 Direct epitope discovery Cytotoxic T cells (CTLs) induced by cancer vaccines are thought to recognize and attack complexes of HLA-A * 02 molecules and peptides expressed on the surface of cancer cells. Therefore, the protein from which the peptide is derived may be a cancer antigen. Therefore, we searched for a PSF1 sequence peptide that binds to HLA-A * 02 molecules in cancer cells, and investigated the possibility that PSF1 becomes a cancer antigen.
- CTLs Cytotoxic T cells
- the extracted peptides were ultrafiltered using Amicon Ultra 10K (Millipore), and the peptides were fractionated by SCX (GL Sciences). For each fraction, the peptide was purified with a C18 column (GL Sciences), and centrifuged with Speed Vac. The sample after centrifugal concentration is redissolved in 0.1% TFA / 2% acetonitrile, measured using LTQ Orbitrap XL (Thermo Fisher Scientific), and then searched for the peptide sequence derived from PSF1 by MASCOT algorithm (Matrix Science) did.
- Example 2 In silico prediction In Example 1, PSF1 was shown to be a cancer antigen, and a plurality of cancer vaccine candidate peptides were identified. On the other hand, in reverse immunology, peptides that can bind to HLA-A * 02 molecules are obtained using software (Immunology and Cell Biology (2006) 84, 318-330). Therefore, a software search was performed to obtain PSF1 peptide candidates other than Example 1.
- Example 3 Mouse CTL induction experiment (ELISPOT assay) It is confirmed whether the PSF1 peptide obtained by the above example induces a peptide-specific CTL via HLA-A * 02.
- mice obtained CB6F1-Tg (HLA-A * 0201 / H2-Kb) A * 0201 from Taconic.
- a PBS solution was prepared by mixing up to 5 types of each peptide solution dissolved in DMSO, and mixed with the same amount of MOTANIDE ISA 51VG (SEPPIC) to prepare an emulsion antigen solution.
- Stimulator cell T2 cells used for ELISPOT assay were prepared by overnight culture in AIM-V medium supplemented with each immunized evaluation peptide (30 ⁇ g / mL) or negative control peptide (ELAGIGILTV) .
- the ELISPOT assay was performed by detecting IFN- ⁇ produced from the responder cell. The method followed the attachment of Mouse IFN- ⁇ ELISpot PLUS (MABTECH), a measurement kit. Pre-culture for IFN- ⁇ production was performed by adding cells to the 96-wel plate included in the measurement kit. Each stimulator cell was seeded at 5 ⁇ 10 4 cell / 100 ⁇ L / well in duplicate or single well, and then a responder cell was added at 2 ⁇ 10 6 cell / 100 ⁇ L / well. After overnight co-culture, the cells were removed, the wells were washed, and the primary antibody (biotin-anti-mouse IFN- ⁇ antibody) included in the measurement kit was added.
- the primary antibody biotin-anti-mouse IFN- ⁇ antibody
- the number of blue spots seen at the bottom of the well was measured with an ImmunoSpot® S5 Verse Analyzer (Cellular Technology Limited). In addition, the measured value when the spot was unclear due to being colored in a wide range was not adopted. In duplicate well, an average value was obtained, and the difference from the negative control was calculated to obtain the number of peptide-specific spots. The average value and SD were calculated using the values for each mouse, and when the difference between the average value and the SD value was a positive number, it was judged positive.
- Example 4 Human CTL induction experiment (ELISPOT assay) It is confirmed whether the PSF1 peptide obtained by Examples 1 and 2 induces a peptide-specific human CTL.
- HBSS (-) (Wako Pure Chemicals), to which HEPES (SIGMA) was added to 5 mM, was added to the cell pellet, suspended, and overlaid on Ficoll-paque PREMIUM (GE Healthcare). Centrifugation was carried out at 400 g for 40 minutes to collect peripheral blood monocyte cells (PBMC) separated in the intermediate layer.
- PBMC peripheral blood monocyte cells
- CD14 microbeads (Miltenyi Biotec) were added to PBMC and reacted at 4 ° C. for 15 minutes while rotating. Cells were passed through an LS column (Miltenyi Biotec), and CD14-positive cells were obtained using QuadroMACS (TM) Separator (Miltenyi Biotec). The remaining cells were collected and washed, and CD8 microbeads (Miltenyi Biotec) were added and reacted at 4 ° C. for 15 minutes. Thereafter, the same treatment was performed to obtain CD8 positive cells. The CD14 positive cells were subsequently induced to differentiate into monocyte-derived dendritic cells (Mo-DC), and the CD8 positive cells were once stored frozen.
- TM QuadroMACS Separator
- OK-432 (Chugai Pharmaceutical Co., Ltd.) was added to one plate at a concentration of 0.1 KE / ml, and the cells were collected the next day and seeded on a 96-well U bottom plate.
- One type of evaluation peptide was added at 20 ⁇ g / ml per half surface (48 wells) of the plate and cultured in a 37 ° C., 5% CO 2 incubator for one day to produce peptide-presenting cells.
- the second preparation was performed in the same manner on the other plate 12-14 days after the start of culture.
- peptide-presenting cells in the 96-well plate were irradiated with X-rays (30 Gy), and then cryopreserved CD8 positive cells were added to all wells for the first antigen stimulation.
- the culture solution used was an AIM-V medium containing antibiotics and serum supplemented with 10 ng / ml IL-7 (R & D Systems). After culturing for 7 days, the cells contained in the supernatant were collected, and co-cultured with the peptide-presented cells (X-irradiated) prepared in the second round, followed by second antigen stimulation in a 96-well plate.
- the stimulator cell T2 cells used in the ELISPOT assay were prepared by culturing overnight in AIM-V medium supplemented with the evaluation peptide (20 ⁇ g / mL). As a negative control stimulator cell, only T2 cells were used (no peptide). Each cell was irradiated with X-rays (30 Gy), washed with an AIM-V medium containing antibiotics and serum, and adjusted to a concentration of 2 ⁇ 10 5 cells / ml.
- the ELISPOT assay was performed by detecting IFN- ⁇ produced from the responder cell. The method followed the attachment of ELISpotPRO for Human IFN- ⁇ (MABTECH), which is a measurement kit. Responder cells were added to each 96-well plate of the measurement kit at 100 ⁇ L / well for 2 wells, and each cell was seeded with 100 ⁇ L / well of a stimulator cell to which the evaluation peptide was added or a negative control stimulator cell (no peptide). After overnight culture, cells in each well were removed and the wells were washed, and ALP-labeled anti-IFN- ⁇ antibody included in the measurement kit was added. After 2 hours of reaction at room temperature, the plate was thoroughly washed, and the substrate solution was added and left at room temperature for 5 minutes. Thorough washing with running water was performed to complete the color reaction, and the plate was air-dried.
- ELISpotPRO for Human IFN- ⁇ MABTECH
- the number of blue spots seen at the bottom of the well was measured with an ImmunoSpot® S5 Verse Analyzer.
- the number of peptide-specific spots was calculated by subtracting the number of spots from the negative control stimulator cell (no peptide) from the number of spots from the stimulator cell to which the peptide was added. When the difference was 50 or more, the following 7 peptides were positive in the examination for a plurality of donors.
- Tetramer assay Human CTL induction experiment (Tetramer assay) Tetramer assay was performed to confirm that PSF1 peptide-specific CTL was induced by another method.
- Tetramer assay A tetramer (MBL) for PSF1 peptide (YLYDRLLR) was prepared.
- CTL line cells were collected, stained with tetramer and APC H7-labeled anti-CD8 antibody (SK-1) (BD), and measured with FACS Aria (BD).
- Two types of CTL lines (0209-01 H2, 0209-02 D2) were used.
- TM cytotoxicity testing CellTracker
- the effector cells used for the cytotoxicity test were the three types of CTL lines (0209-1 H2; YLYDRLLRI, 1004-1 P-13-) obtained by the culture after the ELISPOT assay described above. 1_2; RALRWEYGSVLPN, 1004-1 P-10-9_1; ALRWEYGSVL). Cells were prepared to a concentration of 50, 15, 5 ⁇ 10 4 cells / ml after washing with AIM-V. The preparation was performed in AIM-V medium containing antibiotics and serum.
- E Effector cell
- cell ratio E / T ratio 10: 1, 3 : 1 and 1: 1 and co-cultured overnight.
- MACS Quant Miltenyi Biotec
- FACS Aria 0.1 ⁇ g / ml DAPI was added to separate live and dead cells.
- the percentage of DAPI negative cells (live cells) in the target cells labeled with CMFDA was determined, and the survival rate of the target cells was determined with the culture conditions (only target cells) that were not co-cultured with the effector cells as 100%.
- a peptide capable of significantly inducing CTL a DNA encoding the peptide, a pharmaceutical composition containing these peptides or DNA, and a cancer vaccine using these peptides or DNA. It was.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Mycology (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DK13833212.7T DK2891663T3 (en) | 2012-08-31 | 2013-08-29 | PSF1-Derived Peptide |
| EP13833212.7A EP2891663B8 (en) | 2012-08-31 | 2013-08-29 | Psf1-derived peptide |
| ES13833212.7T ES2662808T3 (es) | 2012-08-31 | 2013-08-29 | Péptido obtenido de PSF1 |
| US14/424,981 US9683016B2 (en) | 2012-08-31 | 2013-08-29 | PSF1-derived peptide |
| JP2014533063A JP5920742B2 (ja) | 2012-08-31 | 2013-08-29 | Psf1由来ペプチド |
| NO13833212A NO2891663T3 (enExample) | 2012-08-31 | 2013-08-29 | |
| US15/413,343 US9919039B2 (en) | 2012-08-31 | 2017-01-23 | Partner of SLD five 1 (PSF1)-derived peptide |
| US15/891,238 US10617750B2 (en) | 2012-08-31 | 2018-02-07 | Partner of SLD five 1 (PSF1)-derived peptide |
| US16/815,068 US20200206330A1 (en) | 2012-08-31 | 2020-03-11 | Partner of sld five 1 (psf1)-derived peptide |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012191050 | 2012-08-31 | ||
| JP2012-191050 | 2012-08-31 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/424,981 A-371-Of-International US9683016B2 (en) | 2012-08-31 | 2013-08-29 | PSF1-derived peptide |
| US15/413,343 Continuation US9919039B2 (en) | 2012-08-31 | 2017-01-23 | Partner of SLD five 1 (PSF1)-derived peptide |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014034754A1 true WO2014034754A1 (ja) | 2014-03-06 |
Family
ID=50183559
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/073084 Ceased WO2014034754A1 (ja) | 2012-08-31 | 2013-08-29 | Psf1由来ペプチド |
Country Status (9)
| Country | Link |
|---|---|
| US (4) | US9683016B2 (enExample) |
| EP (1) | EP2891663B8 (enExample) |
| JP (1) | JP5920742B2 (enExample) |
| DK (1) | DK2891663T3 (enExample) |
| ES (1) | ES2662808T3 (enExample) |
| HU (1) | HUE036243T2 (enExample) |
| NO (1) | NO2891663T3 (enExample) |
| PT (1) | PT2891663T (enExample) |
| WO (1) | WO2014034754A1 (enExample) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111518216B (zh) * | 2019-02-02 | 2024-02-02 | 上海细胞治疗集团有限公司 | 多肽、含有多肽的组合物及其在肿瘤免疫中的应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003042661A2 (en) | 2001-11-13 | 2003-05-22 | Protein Design Labs, Inc. | Methods of diagnosis of cancer, compositions and methods of screening for modulators of cancer |
| WO2007119515A1 (ja) * | 2006-03-28 | 2007-10-25 | Dainippon Sumitomo Pharma Co., Ltd. | 新規腫瘍抗原ペプチド |
| WO2008102557A1 (en) * | 2007-02-21 | 2008-08-28 | Oncotherapy Science, Inc. | Peptide vaccines for cancers expressing tumor-associated antigens |
| WO2011034128A1 (ja) | 2009-09-16 | 2011-03-24 | 塩野義製薬株式会社 | コラーゲンネオエピトープ抗体 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4966848A (en) * | 1988-02-08 | 1990-10-30 | The General Hospital Corporation | Isolation, purification, characterization, cloning and sequencing of N α-acetyltransferase |
| US5223421A (en) * | 1989-10-25 | 1993-06-29 | The General Hospital Corporation | Identification of methionine Nα-acetyltransferase |
| US5595756A (en) * | 1993-12-22 | 1997-01-21 | Inex Pharmaceuticals Corporation | Liposomal compositions for enhanced retention of bioactive agents |
| US5688489A (en) * | 1995-09-15 | 1997-11-18 | Resolution Pharmaceuticals, Inc. | Non-receptor mediated imaging agents |
| AU2002359281A1 (en) * | 2001-10-19 | 2003-04-28 | Dgi Bio Technologies, Inc. | Identification of binding partners for specific proteins |
| GB0518877D0 (en) * | 2005-09-15 | 2005-10-26 | Medical Res Council | Markers and methods |
-
2013
- 2013-08-29 WO PCT/JP2013/073084 patent/WO2014034754A1/ja not_active Ceased
- 2013-08-29 PT PT138332127T patent/PT2891663T/pt unknown
- 2013-08-29 JP JP2014533063A patent/JP5920742B2/ja not_active Expired - Fee Related
- 2013-08-29 US US14/424,981 patent/US9683016B2/en not_active Expired - Fee Related
- 2013-08-29 DK DK13833212.7T patent/DK2891663T3/en active
- 2013-08-29 HU HUE13833212A patent/HUE036243T2/hu unknown
- 2013-08-29 NO NO13833212A patent/NO2891663T3/no unknown
- 2013-08-29 ES ES13833212.7T patent/ES2662808T3/es active Active
- 2013-08-29 EP EP13833212.7A patent/EP2891663B8/en active Active
-
2017
- 2017-01-23 US US15/413,343 patent/US9919039B2/en not_active Expired - Fee Related
-
2018
- 2018-02-07 US US15/891,238 patent/US10617750B2/en not_active Expired - Fee Related
-
2020
- 2020-03-11 US US16/815,068 patent/US20200206330A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003042661A2 (en) | 2001-11-13 | 2003-05-22 | Protein Design Labs, Inc. | Methods of diagnosis of cancer, compositions and methods of screening for modulators of cancer |
| WO2007119515A1 (ja) * | 2006-03-28 | 2007-10-25 | Dainippon Sumitomo Pharma Co., Ltd. | 新規腫瘍抗原ペプチド |
| WO2008102557A1 (en) * | 2007-02-21 | 2008-08-28 | Oncotherapy Science, Inc. | Peptide vaccines for cancers expressing tumor-associated antigens |
| WO2011034128A1 (ja) | 2009-09-16 | 2011-03-24 | 塩野義製薬株式会社 | コラーゲンネオエピトープ抗体 |
Non-Patent Citations (21)
| Title |
|---|
| "Molecular Cloning: A laboratory manual", COLD SPRING HARBOR LABORATORY |
| "Peptide Synthesis", 1966, INTERSCIENCE |
| "The Proteins", vol. 2, 1976, ACADEMIC PRESS INC. |
| APARICIO ET AL., NUCLEIC ACID RES, vol. 37, 2009, pages 2087 - 2095 |
| CURRENT PHARMACEUTICAL DESIGN, vol. 16, 2010, pages 3149 - 3157 |
| HARANO ET AL., INT. J. CANCER, vol. 123, 2008, pages 2616 - 2625 |
| HAWKINS ET AL., J. PROTEOME. RES, vol. 7, 2008, pages 1445 - 1457 |
| HROUDA D; DALGLEISH A G.: "Gene therapy for prostate cancer", GENE THER, vol. 3, 1996, pages 845 - 52, XP000616512 |
| IMMUNOLOGY AND CELL BIOLOGY, vol. 84, 2006, pages 318 - 330 |
| IZUMI ET AL., GENES TO CELLS, vol. 15, 2010, pages 1025 - 1024 |
| IZUMI NAKAHARA ET AL.: "PSF1 Idenshi no Kajo Hatsugen wa Nyugan Saibo no Zoshoku o Sokushin suru", 32ND ANNUAL MEETING OF THE MOLECULAR BIOLOGY SOCIETY OF JAPAN YOSHISHU, 2009, pages 1P-0755, XP008176884 * |
| JEDEMA I. ET AL.: "New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population", BLOOD, vol. 103, 2004, pages 2677 - 2682 |
| JOHNSON A G., CLIN. MICROBIOL. REV., vol. 7, 1994, pages 277 - 89 |
| KAMADA ET AL., NAT STRUCT MOL BIOL, vol. 14, 2007, pages 388 - 396 |
| LIU M; ACRES B; BALLOUL J M; BIZOUARNE N; PAUL S; SLOS P; SQUIBAN P.: "Gene-based vaccines and immunotherapeutics", PROC NATL ACAD SCI USA, vol. 101, 2004, pages 14567 - 71, XP002384828, DOI: doi:10.1073/pnas.0404845101 |
| NAGAHAMA Y. ET AL., CANCER RES., vol. 70, 2010, pages 1215 - 24 |
| NAKAHARA, I. ET AL.: "Up-regulation of PSF1 promotes the growth of breast cancer cells", GENES TO CELLS, vol. 15, 2010, pages 1015 - 1024, XP055196485 * |
| NATURE, vol. 351, 1991, pages 290 - 296 |
| PARKER K C ET AL., J. IMMUNOL., vol. 152, 1994, pages 153 - 175 |
| RYU B. ET AL., PROSONE, vol. 7, 2007, pages E594 |
| See also references of EP2891663A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2891663A1 (en) | 2015-07-08 |
| DK2891663T3 (en) | 2018-03-26 |
| EP2891663B1 (en) | 2017-12-20 |
| US20200206330A1 (en) | 2020-07-02 |
| JP5920742B2 (ja) | 2016-05-18 |
| US10617750B2 (en) | 2020-04-14 |
| US20170173133A1 (en) | 2017-06-22 |
| EP2891663B8 (en) | 2018-04-04 |
| US9919039B2 (en) | 2018-03-20 |
| HUE036243T2 (hu) | 2018-06-28 |
| ES2662808T3 (es) | 2018-04-09 |
| PT2891663T (pt) | 2018-03-20 |
| US20150218216A1 (en) | 2015-08-06 |
| US20180169203A1 (en) | 2018-06-21 |
| NO2891663T3 (enExample) | 2018-05-19 |
| JPWO2014034754A1 (ja) | 2016-08-08 |
| EP2891663A4 (en) | 2016-04-13 |
| US9683016B2 (en) | 2017-06-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11707512B2 (en) | Cancer vaccine composition | |
| RU2464275C2 (ru) | Пептидные вакцины для раков, экспрессирующих опухолеспецифические антигены | |
| JP5065273B2 (ja) | Hla−a24分子結合性kif由来ペプチド | |
| JP5920742B2 (ja) | Psf1由来ペプチド | |
| HK1261028A1 (en) | Cancer vaccine composition | |
| HK1261028B (en) | Cancer vaccine composition | |
| HK1143552B (en) | Cancer vaccine composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13833212 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14424981 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 2014533063 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |