WO2013144979A1 - Process for the preparation of aliskiren - Google Patents
Process for the preparation of aliskiren Download PDFInfo
- Publication number
- WO2013144979A1 WO2013144979A1 PCT/IN2013/000201 IN2013000201W WO2013144979A1 WO 2013144979 A1 WO2013144979 A1 WO 2013144979A1 IN 2013000201 W IN2013000201 W IN 2013000201W WO 2013144979 A1 WO2013144979 A1 WO 2013144979A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- aliskiren
- ammonia
- formula
- compound
- preparation
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C231/00—Preparation of carboxylic acid amides
- C07C231/12—Preparation of carboxylic acid amides by reactions not involving the formation of carboxamide groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C237/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups
- C07C237/02—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups having the carbon atoms of the carboxamide groups bound to acyclic carbon atoms of the carbon skeleton
- C07C237/22—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by amino groups having the carbon atoms of the carboxamide groups bound to acyclic carbon atoms of the carbon skeleton having nitrogen atoms of amino groups bound to the carbon skeleton of the acid part, further acylated
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C51/00—Preparation of carboxylic acids or their salts, halides or anhydrides
- C07C51/347—Preparation of carboxylic acids or their salts, halides or anhydrides by reactions not involving formation of carboxyl groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C51/00—Preparation of carboxylic acids or their salts, halides or anhydrides
- C07C51/41—Preparation of salts of carboxylic acids
- C07C51/412—Preparation of salts of carboxylic acids by conversion of the acids, their salts, esters or anhydrides with the same carboxylic acid part
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C57/00—Unsaturated compounds having carboxyl groups bound to acyclic carbon atoms
- C07C57/02—Unsaturated compounds having carboxyl groups bound to acyclic carbon atoms with only carbon-to-carbon double bonds as unsaturation
- C07C57/13—Dicarboxylic acids
- C07C57/15—Fumaric acid
Definitions
- the present invention relates to an improved process for the preparation of renin inhibitor Aliskiren and its pharmaceutically acceptable salts.
- Aliskiren is marketed by Novartis as TEKTURNA® in the form of its hemifumarate salt in a once-daily formulation.
- the present invention provides an improved process for the preparation of Aliskiren and its pharmaceutically acceptable salts thereof.
- Present invention further provides, Aliskiren hemifumarate having substantially free of diastereomeric impurities and process for the preparation of the same.
- Principle object of the present invention is to provide an improved process for the preparation of Aliskiren and its pharmaceutically acceptable salts thereof.
- One aspect of the present invention provides, an improved process for the preparation of Aliskiren and its pharmaceutically acceptable salts comprising reducing the compound of Formula-Z in presence of catalyst and ammonia.
- Formula-Z Another object of the present invention is to provide pure Aliskiren hemifumarate having substantially free of diastereomeric impurities.
- One aspect of the present invention is to provide a process for the preparation of Aliskiren hemifumarate having substantially free of diastereomeric impurities.
- Another aspect of the present invention provides Aliskiren hemifumarate of the following
- the present invention relates to an improved process for the preparation of renin inhibitors like Aliskiren and its pharmaceutically acceptable salts.
- the main aspect of the present invention provides an improved process for the preparation of Aliskiren and its pharmaceutically acceptable salts comprising reducing the compound of Formula-Z in presence of catalyst and ammonia.
- the catalyst used in the reduction of compound of Formula-Z is selected from Pd/C, Raney nickel or Pd(OH) 2 in presence of H 2 gas.
- the ammonia used is in the form of non-aqueous ammonia such as alcoholic ammonia.
- the alcoholic ammonia is selected from ethanolic ammonia and methanolic ammonia, preferably ethanolic ammonia.
- the reduction is carried out in presence of alcoholic solvent like ethanol, methanol, isopropanol and n-propanol; preferably ethanol.
- alcoholic solvent like ethanol, methanol, isopropanol and n-propanol; preferably ethanol.
- the compound of Formula-Z is prepared by the conventional processes disclosed in prior art for example as disclosed in US 7009078 or in our co pending patent application IN 3087/CHE/2010.
- the compound of Formula-Z is hydrogenated in the presence of 10% Pd/C and ethanolic ammonia solution in alcoholic solvent like ethanol.
- fumaric acid is added to get Aliskiren Hemifumarate.
- the present invention also relates to Aliskiren hemifumarate having substantially free of diastereomeric impurities.
- the present invention further relates to a process for the preparation of Aliskiren hemifumarate having substantially free of diastereomeric impurities.
- Another aspect of the present invention is to provide Aliskiren hemifumarate of the following structure:
- One embodiment of the present invention is to provide Aliskiren hemifumarate having diastereomeric impurity less than 0.18%, as measured by area percentage HPLC.
- One more embodiment of the present invention is to provide Aliskiren hemifumarate having diastereomeric impurity less than 0.15%, as measured by area percentage HPLC.
- One more aspect of the present invention provides, Aliskiren hemifumarate diastereomeric impurity of the following structure ALK-I.
- the intermediate compound of Formula- Y obtained by the above process is in oil form.
- the intermediate compounds play a vital role in the preparation of Aliskiren and its pharmaceutically acceptable salts thereof. Considering the lengthy synthesis of Aliskiren which involves almost all the intermediates as oil, it is highly beneficial if any of the intermediate could be obtained as a crystallisable solid which can give the intermediate of good purity as well as Aliskiren of good purity.
- the solid compound of Formula-Y is prepared by reacting compound-X with 3-Amino-2,2-dimethyl-propionitrile, characterized in that after completion of the reaction, reaction mixture containing compound of Formula-Y in water immiscible solvent selected from hydrocarbons such as toluene, xylene, pentane, hexane, preferably toluene; is washed with aqueous base such as sodium bicarbonate, potassium bicarbonate, sodium carbonate, potassium carbonate, preferably sodium bicarbonate to give solid compound of Formula-Y.
- water immiscible solvent selected from hydrocarbons such as toluene, xylene, pentane, hexane, preferably toluene
- aqueous base such as sodium bicarbonate, potassium bicarbonate, sodium carbonate, potassium carbonate, preferably sodium bicarbonate
- cyano group of solid compound of Formula-Y is converted into compound of Formula-Z in the known manner as disclosed in co-pending Indian patent application IN 3087/CHE/2010.
- reduction of compound of Formula-Z is carried out in the known manner as disclosed in co-pending Indian patent application IN 3087/CHE/2010.
- the compound of Formula-Y is prepared by reacting compound of Formula-X with 3-amino-2,2-dimethyl-propionitrile in presence of a base like organic or inorganic base, preferably organic base such as Triethylamine, Tripropylamine, diisopropylethylamine, etc., more preferably Triethylamine and a catalyst like 2-hydroxypyridine. After completion of the reaction the reaction mass is diluted with hydrocarbon solvent such as toluene, Xylene and chlorobenzene, preferably toluene and stirred with alkali solution to remove catalyst as an alkali salt.
- a base like organic or inorganic base
- organic base such as Triethylamine, Tripropylamine, diisopropylethylamine, etc., more preferably Triethylamine and a catalyst like 2-hydroxypyridine.
- hydrocarbon solvent such as toluene, Xylene and chlorobenzene, preferably toluene and stir
- 3-amino-2,2- dimethyl-propionitrile and Triethylamine are extracted from hydrocarbon solvent layer using an organic acid like acetic acid.
- the hydrocarbon solvent layer is stirred with 1- 10% aqueous base solution like sodium bicarbonate, sodium carbonate, potassium bicarbonate and potassium carbonate, preferably 3-5% sodium bicarbonate solution to crystallize compound of Formula-Y.
- the obtained product is filtered and dried under vacuum to get the compound of Formula-Y as white solid.
- Example-3 Process for the preparation of Aliskiren Hemifumarate
- the compound of Formula-Z (50g) was hydrogenated for 5-6 hours in the presence of 10% Pd/C (5 g) and ethanolic ammonia solution (10% w/w ammonia in ethanol ⁇ 29.4g) in ethanol (400 ml) at ambient temperature and 7 Kg/cm 2 pressure.
- the reaction mixture was filtered and the catalyst was washed with ethanol (50 ml) and distilled to get residue.
- the obtained residue was co-distilled with acetonitrile (50 ml) and re-dissolved in ethanol and acetonitrile at 35-40°C.
- fumaric acid (4.5g) was added and stirred to get clear solution and filtered at 35-40°C to remove any insoluble particles.
- Acetonitrile 150 ml was added to the above clear filtrate at 35-40°C and inoculated with 200mg of Aliskiren hemifumarate and agitated for 3hours to get precipitation of the product.
- acetonitrile was added and agitated for 17 hours at ambient temperature. The suspension was cooled to 0 °C and filtered off by suction after 2 hours. The product cake was washed with acetonitrile and then dried under vacuum at 35°C to yield 47g of Aliskiren hemifumarate as white crystals.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
The present invention relates to an improved process for the preparation of Aliskiren and its pharmaceutically acceptable salts comprising reducing the compound of Formula-Z in presence of catalyst and ammonia. The present invention further relates to Aliskiren hemifumarate having diastereomeric impurity less than 0.2%.(F) H3C CH3 NH2 H3C 0 H3C CH3 Formula-Z
Description
PROCESS FOR THE PREPARATION OF ALISKIREN
This application claims priority to this Indian patent application numbers 1 173/CHE/2012 filed on March 28, 2012 and 1876/CHE/ 2012 filed on May 11, 2012
FIELD OF THE INVENTION:
The present invention relates to an improved process for the preparation of renin inhibitor Aliskiren and its pharmaceutically acceptable salts.
BACKGROUND OF THE INVENTION:
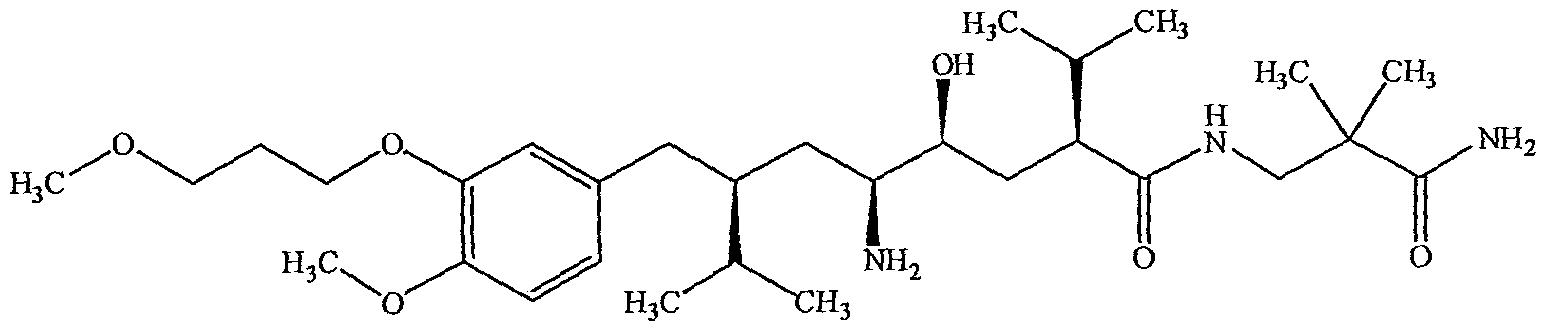
Aliskiren, (2S, 4S, 5S, 7S)-N-(2-carbamoyl-2-methyIpropyl)-5-amino-4-hydroxy-2,7- diisopropyl-8-[4-methoxy-3-(3-methoxypropoxy)phenyl] octanamide having the Formula-I, a new antihypertensive has been developed which interferes with the renin- angiotensin system at the beginning of angiotensin II biosynthesis.
Formula-I
Aliskiren is marketed by Novartis as TEKTURNA® in the form of its hemifumarate salt in a once-daily formulation.
US 5,559,111 discloses Aliskiren and related compounds along with the synthesis of Aliskiren. Aliskiren hemifumarate is having 4 chiral carbon atoms; hence the synthesis for the pure Aliskiren hemifumarate substantially free of diastereomeric impurities is quite difficult and demanding.
Further US 7132569, US 7009078, US 6730798 and US 6800769 claims novel intermediates used in the preparation of Aliskiren and process for the preparation of Aliskiren, which are incorporated here for reference.
In prior art US 7009078 the reduction of Azide compound (Formula-Z) is carried out in presence of ethanol amine. After completion of reaction the reaction mixture is filtered and the catalyst is washed with tert-butyl methyl ether. The filtrate is washed with sodium hydroxide and brine. To remove the ethanol amine repeated washing with water/aq. NaOH is required. The aqueous phases are extracted with tert-butyl methyl ether. The aqueous work-up decreases the yield of the final product..To overcome this problem the present inventors surprisingly found that usage of ammonia will increase the yield and purity of Aliskiren.
The present invention provides an improved process for the preparation of Aliskiren and its pharmaceutically acceptable salts thereof. Present invention further provides, Aliskiren hemifumarate having substantially free of diastereomeric impurities and process for the preparation of the same.
OBJECT AND SUMMARY OF THE INVENTION: Principle object of the present invention is to provide an improved process for the preparation of Aliskiren and its pharmaceutically acceptable salts thereof.
One aspect of the present invention provides, an improved process for the preparation of Aliskiren and its pharmaceutically acceptable salts comprising reducing the compound of Formula-Z in presence of catalyst and ammonia.
Formula-Z
Another object of the present invention is to provide pure Aliskiren hemifumarate having substantially free of diastereomeric impurities.
One aspect of the present invention is to provide a process for the preparation of Aliskiren hemifumarate having substantially free of diastereomeric impurities.
Another aspect of the present invention provides Aliskiren hemifumarate of the following
having diastereomeric impurity of less than 0.2%, as measured by area percentage HPLC.
DETAILED DESCRIPTION OF THE INVENTION:
The present invention relates to an improved process for the preparation of renin inhibitors like Aliskiren and its pharmaceutically acceptable salts.
The main aspect of the present invention provides an improved process for the preparation of Aliskiren and its pharmaceutically acceptable salts comprising reducing the compound of Formula-Z in presence of catalyst and ammonia.
In prior art US 7009078 the reduction of compound of Formula-Z is carried out in presence of ethanol amine. After completion of reaction the reaction mixture is filtered and the catalyst is washed with tert-butyl methyl ether. The filtrate is washed with sodium hydroxide and brine. To remove the ethanol amine repeated washing with water/aq. NaOH is required. The aqueous phases are extracted with tert-butyl methyl ether. The aqueous work-up decreases the yield of the final product. To overcome this problem the present inventors surprisingly found that usage of ammonia will increase the yield and purity of Aliskiren. Accordingly, present invention provides an improved process for the preparation of Aliskiren and its pharmaceutically acceptable salts comprising reducing the compound of Formula-Z in presence of catalyst and ammonia in an alcoholic solvent.
In one embodiment, the catalyst used in the reduction of compound of Formula-Z is selected from Pd/C, Raney nickel or Pd(OH)2 in presence of H2 gas.
In another embodiment, the ammonia used is in the form of non-aqueous ammonia such as alcoholic ammonia. The alcoholic ammonia is selected from ethanolic ammonia and methanolic ammonia, preferably ethanolic ammonia.
In one more embodiment, the reduction is carried out in presence of alcoholic solvent like ethanol, methanol, isopropanol and n-propanol; preferably ethanol.
In one more embodiment, the compound of Formula-Z is prepared by the conventional processes disclosed in prior art for example as disclosed in US 7009078 or in our co pending patent application IN 3087/CHE/2010.
As per the present invention, the compound of Formula-Z is hydrogenated in the presence of 10% Pd/C and ethanolic ammonia solution in alcoholic solvent like ethanol. To the obtained compound, fumaric acid is added to get Aliskiren Hemifumarate.
The present invention also relates to Aliskiren hemifumarate having substantially free of diastereomeric impurities. The present invention further relates to a process for the preparation of Aliskiren hemifumarate having substantially free of diastereomeric impurities.
Another aspect of the present invention is to provide Aliskiren hemifumarate of the following structure:
having diastereomeric impurity less than 0.2%, as measured by area percentage HPLC.
One embodiment of the present invention is to provide Aliskiren hemifumarate having diastereomeric impurity less than 0.18%, as measured by area percentage HPLC.
One more embodiment of the present invention is to provide Aliskiren hemifumarate having diastereomeric impurity less than 0.15%, as measured by area percentage HPLC.
One more aspect of the present invention provides, Aliskiren hemifumarate diastereomeric impurity of the following structure ALK-I.
Waters HPLC system having alliance 2695 model pump and 2487 (UV) detector with Empower chromatography software or its equivalent.
Chromatographic parameters:
Column YMC Pro CI 8, 250 x 4.6 mm, 5.0 μηι
Detector UV at 230 nm
Flow rate 1.0 mL / min.
Injection volume 10 μL
Column oven temp. 27 °C
Run time 65 minutes
Diluent Acetonitrile and water in the ratio 1 : l%v/v
Mobile Phase-A:
Weight and transfer about 1.0 g of Tetrabutyl ammonium hydrogen sulphate in 1000 mL of water and add 0.5 mL of Ortho phosphoric acid. Filter through 0.45 μιη or finer porosity membrane and degas.
Mobile Phase-B:
Prepare a degassed mixture of Acetonitrile: Methanol 55:45%v/v.
Gradient ro ram:
Standard solution
Weigh and transfer about 25.0 mg of each of standard into a 25 mL volumetric flask, dissolve in and dilute to volume with diluent.
Sample solution
Weigh and transfer about 25.0 mg of each of sample into a 25 mL volumetric flask, dissolve in and dilute to volume with diluent.
Procedure:
Inject sample solution in to the chromatograph and record the chromatograms. Disregard the peaks due to blank and peak responses which are 0.05% and below apart from known impurities. Typical retention time and relative retention time are as follows (for information). It was found that Aliskiren in TKETURNA tablet (Batch No: 1205809U12TA) is having purity 99.76 at RT 19.51 and diastereomeric impurity of Formula ALK-I is 0.24% at RT 18.64.
Our co-pending Indian patent application IN 3087/CHE/2010 discloses novel process for the preparation of Aliskiren as disclosed in scheme-I.
Compound-Z
Aliskiren
SCHEME-I
The intermediate compound of Formula- Y obtained by the above process is in oil form.
The intermediate compounds play a vital role in the preparation of Aliskiren and its pharmaceutically acceptable salts thereof. Considering the lengthy synthesis of Aliskiren which involves almost all the intermediates as oil, it is highly beneficial if any of the intermediate could be obtained as a crystallisable solid which can give the intermediate of good purity as well as Aliskiren of good purity. To avoid that problem and to improve the purity of Aliskiren, The present inventors tried to open the lactone compound (Formula- X) with 3-amino-2,2-dimethyl-propionitrile and not only succeeded in getting fruitful aminolysis reaction, but also isolating the corresponding amide compound of Formula-Y as pure crystalline compound of chromatographic purity greater than or equal to 99%, which is substantially free of its corresponding diastereomer. This results increase in the purity of Aliskiren.
Thus another aspect of the present invention provides process for the preparation of Aliskiren substantially free of diastereomeric impurity comprising the steps of:
a) converting the cyano group of solid compound of Formula-Y into amide group to give compound of Formula-Z;
b) reducing the azide group of compound of Formula-Z with suitable reducing agent to give Aliskiren; and
c) optionally converting Aliskiren into Aliskiren hemifumarate.
In one embodiment, the solid compound of Formula-Y is prepared by reacting compound-X with 3-Amino-2,2-dimethyl-propionitrile, characterized in that after completion of the reaction, reaction mixture containing compound of Formula-Y in water immiscible solvent selected from hydrocarbons such as toluene, xylene, pentane, hexane, preferably toluene; is washed with aqueous base such as sodium bicarbonate, potassium bicarbonate, sodium carbonate, potassium carbonate, preferably sodium bicarbonate to give solid compound of Formula-Y.
In another embodiment, cyano group of solid compound of Formula-Y is converted into compound of Formula-Z in the known manner as disclosed in co-pending Indian patent application IN 3087/CHE/2010.
In one more embodiment, reduction of compound of Formula-Z is carried out in the known manner as disclosed in co-pending Indian patent application IN 3087/CHE/2010.
According to the present invention, the compound of Formula-Y is prepared by reacting compound of Formula-X with 3-amino-2,2-dimethyl-propionitrile in presence of a base like organic or inorganic base, preferably organic base such as Triethylamine, Tripropylamine, diisopropylethylamine, etc., more preferably Triethylamine and a catalyst like 2-hydroxypyridine. After completion of the reaction the reaction mass is diluted with hydrocarbon solvent such as toluene, Xylene and chlorobenzene, preferably toluene and stirred with alkali solution to remove catalyst as an alkali salt. 3-amino-2,2- dimethyl-propionitrile and Triethylamine are extracted from hydrocarbon solvent layer using an organic acid like acetic acid. The hydrocarbon solvent layer is stirred with 1- 10% aqueous base solution like sodium bicarbonate, sodium carbonate, potassium bicarbonate and potassium carbonate, preferably 3-5% sodium bicarbonate solution to crystallize compound of Formula-Y. The obtained product is filtered and dried under vacuum to get the compound of Formula-Y as white solid.
All patents, patent applications, and non-patent publications cited herein by reference should be considered in their entirety. The following examples are provided to illustrate the process of the present invention. They, are however, not intended to limiting the scope of the present invention in any way and several variants of these examples would be evident to person ordinarily skilled in the art.
Experimental procedure:
Example-l:
A mixture of compound-X (100 g), 3-Amino-2,2-dimethyl-propionitrile (74.5 g), and 2- hydroxypyridine (22.5 g) in triethylamine was stirred for about 24 hours at 60-70°C. The progress of the reaction was monitored by HPLC analysis. After completion of the reaction the reaction mass was diluted with toluene and stirred with aq NaOH solution to precipitate and remove 2-hydroxypyridine as sodium salt. The organic layer was washed with aqueous acetic acid. The organic layer was stirred with aqueous sodium bicarbonate solution to crystallize out the desired product. The product was filtered, washed with DM water followed by prechilled toluene and dried under vacuum to yield compound of Formula-Y as white solid. HPLC purity: >99%. Melting point: 62 °C.
ExampIe-2: Process for the preparation of compound-Z
Formula-Z
To a mixture of Compound-Y (13g) and ethanol (65 ml) aqueous NaOH (5g dissolved in 45 ml of DM water) and 35% Hydrogen peroxide (20 ml) was added at room temperature and stirred the at 30°- 40 °C for 2-4h. The progress of the reaction was monitored by
HPLC analysis. After completion of the reaction the peroxides were quenched by stirring with sodium bisulfite solution. Thereafter product was extracted in toluene. The toluene extract was washed with ethanolamine and water. The solvent was distilled off completely under vacuum to obtain compound Z.
Example-3: Process for the preparation of Aliskiren Hemifumarate
The compound of Formula-Z (50g) was hydrogenated for 5-6 hours in the presence of 10% Pd/C (5 g) and ethanolic ammonia solution (10% w/w ammonia in ethanol ~29.4g) in ethanol (400 ml) at ambient temperature and 7 Kg/cm2 pressure. The reaction mixture was filtered and the catalyst was washed with ethanol (50 ml) and distilled to get residue. The obtained residue was co-distilled with acetonitrile (50 ml) and re-dissolved in ethanol and acetonitrile at 35-40°C. To this fumaric acid (4.5g) was added and stirred to get clear solution and filtered at 35-40°C to remove any insoluble particles. Acetonitrile (150 ml) was added to the above clear filtrate at 35-40°C and inoculated with 200mg of Aliskiren hemifumarate and agitated for 3hours to get precipitation of the product. To the above slurry acetonitrile was added and agitated for 17 hours at ambient temperature. The suspension was cooled to 0 °C and filtered off by suction after 2 hours. The product cake was washed with acetonitrile and then dried under vacuum at 35°C to yield 47g of Aliskiren hemifumarate as white crystals.
Claims
1. A process for the preparation of Aliskiren or its pharmaceutically acceptable salts comprises reducing the compound of Formula-Z in presence of catalyst and ammonia.
Formula-Z
2. The process according to claim 1, wherein the catalyst is selected from Pd/C, Raney nickel or Pd(OH)2.
3. The process according to claim 1, wherein the ammonia is in the form of nonaqueous ammonia.
4. The process according to claim 1, wherein the ammonia is in the form of alcoholic ammonia.
5. The process according to claim 4, wherein alcoholic ammonia is selected from ethanolic ammonia and methanolic ammonia.
6. The process according to claim 1, wherein the reaction is carried out in presence of an alcoholic solvent.
7. The process according to claim 6, wherein alcoholic solvent is selected from ethanol, methanol, isopropanol and n-propanol.
8. The process according to claim 1, wherein pharmaceutically acceptable salt is Fumarate salt.
9. Aliskiren hemifumarate having diastereomeric impurity less than 0.2%.
10. Aliskiren hemifumarate according to claim 9, wherein diastereomeric impurity less than 0.15%.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN1173/CHE/2012 | 2012-03-28 | ||
| IN1173CH2012 | 2012-03-28 | ||
| IN1876CH2012 | 2012-05-11 | ||
| IN1876/CHE/2012 | 2012-05-11 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013144979A1 true WO2013144979A1 (en) | 2013-10-03 |
Family
ID=48699906
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IN2013/000201 WO2013144979A1 (en) | 2012-03-28 | 2013-03-27 | Process for the preparation of aliskiren |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2013144979A1 (en) |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5559111A (en) | 1994-04-18 | 1996-09-24 | Ciba-Geigy Corporation | δ-amino-γ-hydroxy-ω-aryl-alkanoic acid amides |
| US6730798B2 (en) | 2000-07-05 | 2004-05-04 | Speedel Pharma Ag | Process for the preparation of substituted octanoyl amides |
| US6800769B2 (en) | 2000-07-25 | 2004-10-05 | Speedel Pharma Ag | Process for the preparation of substituted octanoyl amides |
| US7009078B1 (en) | 1999-07-29 | 2006-03-07 | Speedel Pharma Ag | Production of N-substituted 2,7-dialkyl-4-hydroxy-5-amino-8-arly-octanoylamides |
| US20060154926A1 (en) * | 2002-06-11 | 2006-07-13 | Elan Pharmaceuticals, Inc. | Methods of treating alzheimer's disease using aryl alkanoic acid amides |
| EP2062874A1 (en) * | 2007-11-20 | 2009-05-27 | KRKA, tovarna zdravil, d.d., Novo mesto | Process and intermediates for the preparation of aliskiren |
| US20100124550A1 (en) * | 2008-11-20 | 2010-05-20 | Auspex Pharmaceuticals, Inc. | Amide inhibitors of renin |
| EP2189442A1 (en) * | 2008-11-20 | 2010-05-26 | Krka Tovarna Zdravil, D.D., Novo Mesto | Process and intermediates for the preparation of aliskiren |
| WO2011148392A1 (en) * | 2010-05-28 | 2011-12-01 | Msn Laboratories Limited | Process for the preparation of (2s,4s,5s,7s)-n-(2-carbamyl-2- methylpropyl)-5-amino-4-hydroxy-2,7-diisopropyl-8-[4-methoxy-3-(3- methoxypropoxy)phenyl]-octanamide hemifumarate and its intermediates thereof |
-
2013
- 2013-03-27 WO PCT/IN2013/000201 patent/WO2013144979A1/en active Application Filing
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5559111A (en) | 1994-04-18 | 1996-09-24 | Ciba-Geigy Corporation | δ-amino-γ-hydroxy-ω-aryl-alkanoic acid amides |
| US7009078B1 (en) | 1999-07-29 | 2006-03-07 | Speedel Pharma Ag | Production of N-substituted 2,7-dialkyl-4-hydroxy-5-amino-8-arly-octanoylamides |
| US7132569B2 (en) | 1999-07-29 | 2006-11-07 | Speedel Pharma Ag | Preparation of N-substituted 2,7-dialkyl-4-hydroxy-5-amino-8-aryl-octanoyl amides |
| US6730798B2 (en) | 2000-07-05 | 2004-05-04 | Speedel Pharma Ag | Process for the preparation of substituted octanoyl amides |
| US6800769B2 (en) | 2000-07-25 | 2004-10-05 | Speedel Pharma Ag | Process for the preparation of substituted octanoyl amides |
| US20060154926A1 (en) * | 2002-06-11 | 2006-07-13 | Elan Pharmaceuticals, Inc. | Methods of treating alzheimer's disease using aryl alkanoic acid amides |
| EP2062874A1 (en) * | 2007-11-20 | 2009-05-27 | KRKA, tovarna zdravil, d.d., Novo mesto | Process and intermediates for the preparation of aliskiren |
| US20100124550A1 (en) * | 2008-11-20 | 2010-05-20 | Auspex Pharmaceuticals, Inc. | Amide inhibitors of renin |
| EP2189442A1 (en) * | 2008-11-20 | 2010-05-26 | Krka Tovarna Zdravil, D.D., Novo Mesto | Process and intermediates for the preparation of aliskiren |
| WO2011148392A1 (en) * | 2010-05-28 | 2011-12-01 | Msn Laboratories Limited | Process for the preparation of (2s,4s,5s,7s)-n-(2-carbamyl-2- methylpropyl)-5-amino-4-hydroxy-2,7-diisopropyl-8-[4-methoxy-3-(3- methoxypropoxy)phenyl]-octanamide hemifumarate and its intermediates thereof |
Non-Patent Citations (1)
| Title |
|---|
| DAMIEN BARBARAS ET AL: "Removal of Heavy Metals from Organic Reaction Mixtures: Preparation and Application of Functionalized Resins (1)", ORGANIC PROCESS RESEARCH & DEVELOPMENT, vol. 13, no. 6, 20 October 2009 (2009-10-20), pages 1068 - 1079, XP002712155, DOI: 10.1021/op900102a * |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3778568B1 (en) | Method of preparing high chiral purity lactam intermediate and brivaracetam | |
| US10421717B2 (en) | Process for preparing brivaracetam | |
| CN105198775B (en) | A kind of preparation method of chiral N Boc biphenyl Propanolamines | |
| EP2609099A2 (en) | Sitagliptin, salts and polymorphs thereof | |
| EP2980090A1 (en) | Method for producing (1s,4s,5s)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one | |
| EP2462107A1 (en) | Solid forms of an n-(phenylmethyl)propanamide derivative and processes of preparation | |
| CA2570833C (en) | Process for producing (z)-1-phenyl-1-diethylaminocarbonyl-2-aminomethylcyclopropane hydrochloride | |
| CN102746288B (en) | Preparation methods of anticoagulant and key intermediate of anticoagulant | |
| CN106699595B (en) | A kind of scheme for lacosamide preparation method | |
| WO2019036441A1 (en) | Processes for the preparation of niraparib and intermediates thereof | |
| EP2630118B1 (en) | Synthesis of aliskiren | |
| CN110028461A (en) | A kind of Preparation Method And Their Intermediate of candesartan Cilexetil | |
| US9469593B2 (en) | Process for preparing cinacalcet hydrochloride | |
| EP3063135A1 (en) | Process for the preparation of enzalutamide | |
| WO2013144979A1 (en) | Process for the preparation of aliskiren | |
| CN105418477B (en) | The method for reducing diastereoisomer impurity content in Lei Dipawei intermediate | |
| EP4144724A1 (en) | Brivaracetam and method for preparing intermediate thereof | |
| KR100880623B1 (en) | Method of Preparing Valsartan | |
| WO2019180547A1 (en) | A process for the preparation of vigabatrin | |
| ES2288376B1 (en) | PROCEDURE FOR OBTAINING USEFUL INTERMEDIATES IN OBTAINING A PHARMACEUTICALLY ACTIVE COMPOUND. | |
| EP2736875B1 (en) | Chemical process for opening ring compounds | |
| CN104649948B (en) | Cilastatin calcium crystal, preparation method and application thereof | |
| WO2013124868A2 (en) | Solid form of aliskiren intermediate | |
| SK286425B6 (en) | Process for preparation of R-(+)-6-carboxamido-3-N-methylamino- 1,2,3,4-tetrahydrocarbazole and use of L-pyroglutamic acid | |
| WO2013121443A1 (en) | An improved process for the preparation of aliskiren |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13731970 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13731970 Country of ref document: EP Kind code of ref document: A1 |