WO2013028441A2 - New poly(heteroarylene vinylene)s based on diketopyrrolopyrrole - Google Patents
New poly(heteroarylene vinylene)s based on diketopyrrolopyrrole Download PDFInfo
- Publication number
- WO2013028441A2 WO2013028441A2 PCT/US2012/051027 US2012051027W WO2013028441A2 WO 2013028441 A2 WO2013028441 A2 WO 2013028441A2 US 2012051027 W US2012051027 W US 2012051027W WO 2013028441 A2 WO2013028441 A2 WO 2013028441A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- har
- repeating units
- independently selected
- diketopyrrolopyrrole
- mol
- Prior art date
Links
- 0 CCCCC(CC)=CN(C(C)(C12)C3[S+]C(*C)=CC3)C1=C(C1SC(C=C*(C=C3C(C)(C4)C=CC(C)(C)*3C)=C4O)=CC1)[Tm]2O[C@](CCC1)[C@]2[C@](*)[C@@](C)C[C@]1(C)CCC(CC)C2 Chemical compound CCCCC(CC)=CN(C(C)(C12)C3[S+]C(*C)=CC3)C1=C(C1SC(C=C*(C=C3C(C)(C4)C=CC(C)(C)*3C)=C4O)=CC1)[Tm]2O[C@](CCC1)[C@]2[C@](*)[C@@](C)C[C@]1(C)CCC(CC)C2 0.000 description 15
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G61/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G61/12—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule
- C08G61/122—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule derived from five- or six-membered heterocyclic compounds, other than imides
- C08G61/123—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule derived from five- or six-membered heterocyclic compounds, other than imides derived from five-membered heterocyclic compounds
- C08G61/124—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule derived from five- or six-membered heterocyclic compounds, other than imides derived from five-membered heterocyclic compounds with a five-membered ring containing one nitrogen atom in the ring
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G61/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G61/12—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule
- C08G61/122—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule derived from five- or six-membered heterocyclic compounds, other than imides
- C08G61/123—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule derived from five- or six-membered heterocyclic compounds, other than imides derived from five-membered heterocyclic compounds
- C08G61/126—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule derived from five- or six-membered heterocyclic compounds, other than imides derived from five-membered heterocyclic compounds with a five-membered ring containing one sulfur atom in the ring
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B57/00—Other synthetic dyes of known constitution
- C09B57/004—Diketopyrrolopyrrole dyes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B69/00—Dyes not provided for by a single group of this subclass
- C09B69/008—Dyes containing a substituent, which contains a silicium atom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B69/00—Dyes not provided for by a single group of this subclass
- C09B69/10—Polymeric dyes; Reaction products of dyes with monomers or with macromolecular compounds
- C09B69/109—Polymeric dyes; Reaction products of dyes with monomers or with macromolecular compounds containing other specific dyes
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/12—Light sources with substantially two-dimensional radiating surfaces
- H05B33/14—Light sources with substantially two-dimensional radiating surfaces characterised by the chemical or physical composition or the arrangement of the electroluminescent material, or by the simultaneous addition of the electroluminescent material in or onto the light source
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/10—Definition of the polymer structure
- C08G2261/12—Copolymers
- C08G2261/124—Copolymers alternating
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/30—Monomer units or repeat units incorporating structural elements in the main chain
- C08G2261/32—Monomer units or repeat units incorporating structural elements in the main chain incorporating heteroaromatic structural elements in the main chain
- C08G2261/322—Monomer units or repeat units incorporating structural elements in the main chain incorporating heteroaromatic structural elements in the main chain non-condensed
- C08G2261/3223—Monomer units or repeat units incorporating structural elements in the main chain incorporating heteroaromatic structural elements in the main chain non-condensed containing one or more sulfur atoms as the only heteroatom, e.g. thiophene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/30—Monomer units or repeat units incorporating structural elements in the main chain
- C08G2261/33—Monomer units or repeat units incorporating structural elements in the main chain incorporating non-aromatic structural elements in the main chain
- C08G2261/332—Monomer units or repeat units incorporating structural elements in the main chain incorporating non-aromatic structural elements in the main chain containing only carbon atoms
- C08G2261/3327—Monomer units or repeat units incorporating structural elements in the main chain incorporating non-aromatic structural elements in the main chain containing only carbon atoms alkene-based
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/30—Monomer units or repeat units incorporating structural elements in the main chain
- C08G2261/34—Monomer units or repeat units incorporating structural elements in the main chain incorporating partially-aromatic structural elements in the main chain
- C08G2261/344—Monomer units or repeat units incorporating structural elements in the main chain incorporating partially-aromatic structural elements in the main chain containing heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/40—Polymerisation processes
- C08G2261/41—Organometallic coupling reactions
- C08G2261/414—Stille reactions
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/90—Applications
- C08G2261/91—Photovoltaic applications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2261/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G2261/90—Applications
- C08G2261/92—TFT applications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/14—Macromolecular compounds
- C09K2211/1441—Heterocyclic
- C09K2211/1466—Heterocyclic containing nitrogen as the only heteroatom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/621—Aromatic anhydride or imide compounds, e.g. perylene tetra-carboxylic dianhydride or perylene tetracarboxylic di-imide
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
- Y02E10/549—Organic PV cells
Definitions
- OLEDs organic light emitting diodes
- solar cells solar cells and/or transistors.
- Some such polymers and/or copolymers known in the prior art have achieved reasonable current carrying capabilities, in the form of either reasonably good hole mobilities, reasonably good electron mobilities, or in a few cases a combination of both hole mobility and high electron mobility, i.e. "ambipolar" properties.
- Some of the prior art polymers and/or copolymers can be used to produce reasonably good performance when used to make transistors, or encouraging efficiencies in solar cells for converting solar radiation to electrical energy (3-5%).
- the relatively few known ambipolar polymers and/or copolymers have not yet achieved a reasonably good and matched level of both hole and electron mobility that would allow the fabrication of high efficiency solar cells, or transistors and derived devices comprising single copolymer wherein the devices have sufficiently high on/off current ratios to enable practical and competitive end use applications.
- Conjugated donor-acceptor (D-A) polymers and/or copolymers have received attention in the art for tailoring the electronic structure, highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), the associated bandgap energy and intensive photon absorption in a broad region of the UV, visible, and infrared wavelengths.
- the selection of the electron donor and acceptor subunits in these conjugated polymers and/or copolymers can modulate the HOMO/ LUMO energy levels, so as to modulate the light absorption and charge transport properties.
- DPP dihydropyrrolo[3,4-c]pyrrole
- PCT Patent Application WO 2008/000664 contemplated a very broad class of such DPP polymers as organic semiconductors potentially useful for making transistors and solar cells (the suggested generic structure is shown below).
- the '664 PCT broadly contemplated the possible use of a very wide variety of potential combinations of the various Ar subunits, including a wide variety of aryl, heteroaryl, fused heteroaryl, ethylene, acetylene, and other potential Ar subunits described therein. More specifically, the '664 PCT reported the actual synthesis of a DPP polymer having the structure
- DPP diketopyrrolopyrrole
- homopolymers and/or copolymers comprising a plurality of repeating units, more than 50 mol. % and up to 100 mol. % of the repeating units are repeating units RU of one or more structural formula(e) complying with general structural formula (I)
- each R 1 and R 1 is independently selected from normal, branched, or cyclic organic group, such as for example alkyls or fluorinated derivatives thereof;
- each hAr 1 and hAr 1 is independently selected from heteroaryls.
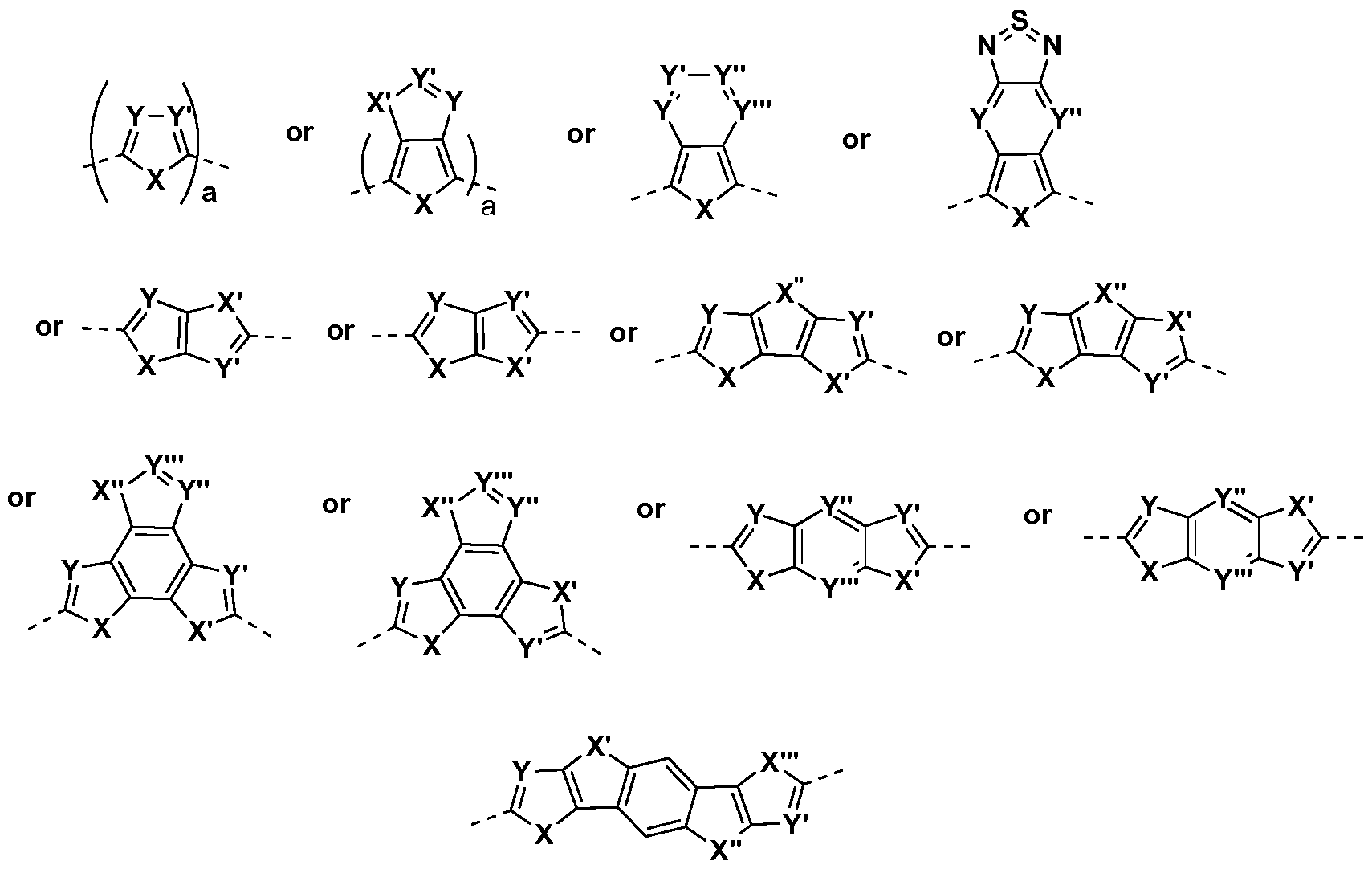
- heteroaryls examples include the structures
- a is an integer equal to 1 , 2, 3, or 4;
- each X, X', X or X' " is independently selected from O, S, Se,
- R is a normal, branched, or cyclic organic group, such as an alkyl group
- each Y, Y', Y" and Y" ' is independently selected from N, and CR 4 , where R 4 is hydrogen, halogen, cyano, or a normal, branched, or cyclic organic group, such as an alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; and
- each R and R is independently selected from hydrogen, cyano, or
- organic group such as an alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy group.
- the DPP polymers defined above can be homopolymers.
- the DPP polymers can be various types of copolymers, comprising both the repeating units RU having general structural formula (I) shown above, as well as other types of repeating units, as further described below.
- these ones are copolymers the repeating units RU of which are a mix comprising or consisting of repeating units RU1 of specific structural formula (II)
- each R , R , R and R is independently selected from normal, branched, or cyclic alkyls or fluorinated derivatives thereof;
- each hAr , hAr , hAr and hAr is independently selected from Ci-
- the DPP copolymers are block copolymers wherein
- R 1 1 2 and R 2"2 are different from each other and/or R 12' and R 22' are different from each other.
- Examples of preferred hAr heteroaryls for use in connection with the DPP copolymers comprising a mix of repeat units of formula (II) and formula (III) include the structures
- a is an integer equal to 1 , 2, 3, or 4;
- each X, X', X or X' " is independently selected from O, S, Se,
- each Y, Y', Y" and Y" ' is independently selected from N, and CR 4 , where R 4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; b) each R 12 , R 12' , R 22 and R 22' is independently selected from hydrogen, cyano, or C1-C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl,
- the DPP copolymers are random copolymers wherein R 12 and R 22 are identical to each other and R 12' and R 22' are identical to each other.
- the DPP polymers comprising repeat units of Formula (I), as well as other repeat units having Formulas (II), and (III), and their various subgenera and subspecies, are useful for making electronic devices such as transistors and solar cells, and logic circuits such as inverters and NAND or NOR circuits, and can have an unexpectedly superior ability to conduct electrical current in the form of holes, electrons, or both (i.e. "ambipolar" characteristics).
- the particular combination of hAr and vinylidene polymer subunits of these DPP polymers are related to the unexpectedly superior properties and utilities.
- FIGURE 1 shows Differential Scanning Calorimeter scans of the HD-PPTV
- HD-PPV, and PPTPV polymers at a heating rate of 10 °C/min under N 2 , as described in Example 4.
- Figure 2a shows optical absorption spectra of HD-PPTV, HD-PPPV, and PPTPV polymers in dilute toluene solutions.
- Figure 2b shows optical absorption spectra of HD-PPTV, HD-PPPV, and PPTPV as polymer thin films.
- Figure 3a shows optical absorption spectra of HD-PPPV, in dilute toluene solutions and as a thin film.
- Figure 3b shows optical absorption spectra of PPTPV polymer in dilute toluene solution and as a thin film
- Figures 4a, 4b, and 4c show cyclic voltammograms of HD-PPTV (A), HD-PPPV (B), and PPTPV (C).
- Figure 5 shows X-ray diffraction patterns of drop-cast films of the three polymers HD-PPTV, HD-PPPV, and PPTPV.
- Figure 6a shows output characteristics of the field-effect transistor based on HD-PPTV, after annealing at 150 °C.
- Figure 7 shows the structure and results obtained from inverter circuits fabricated using HD-PPTV as an active ambipolar semiconductor.
- Figure 8 shows the structure and results obtained from NAND circuits fabricated using HD-PPTV as an active ambipolar semiconductor.
- Figure 9 shows the structure and results obtained from NOR circuits fabricated using HD-PPTV as an active ambipolar semiconductor.
- DPP diketopyrrolopyrrole
- the diketopyrrolopyrrole (DPP) polymers of the invention include homopolymers and/or copolymers that comprise a plurality of repeating units wherein more than 50 mol. % and up to 100 mol. % of the repeating units are repeating units RU of one or more structural formula(e) com lying with general structural formula (I)
- each R 1 and R 1 is independently selected from C 1 -C30 normal, branched, or cyclic alkyls or fluorinated derivatives thereof;
- each hAr 1 and hAr 1 is independently selected from Ci-C 6 o heteroaryls having the structure
- a is an integer equal to 1 , 2, 3, or 4;
- each X, X', X or X' " is independently selected from O, S, Se,
- Si(R ) 2 , and NR where R is a C 1 -C30 normal, branched, or cyclic alkyl group; iii) each Y, Y', Y" and Y" ' is independently selected from N, and CR 4 , where R 4 is hydrogen, halogen, cyano, or a C 1 -C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; and
- R and R is independently selected from hydrogen, cyano, or Ci-
- the number of repeat units of the DPP polymers can be described by an integer n, which can be any positive integer of 5 or greater, but can also be from 5-10,000, or from about 10-1 ,000. It should be noted however that in most embodiments of the DPP polymers described herein, a plurality of the repeating units, i.e. more than 50 mol. %, or more than 60%, 70%, 80%, 90%, 95%, 98%, or 99%), or up to and/or including 100 mol. % of the repeating units are repeating units RU of one or more structural formula(e) complying with general structural formula (I) described above.
- more than 50 mol. %, up to 100 mol. % (preferably, between 90 mol. % and 99 mol. %) of the repeating units are repeating units RU of one and only one specific structural formula complying with general structural formula (I).
- more than 50 mol. %, up to less than 100 mol. % of the repeating units are repeating units RU of one and only one specific structural formula complying with general structural formula (I), and between 0 mol. % and 50 mol. % (preferably, between 1 mol. % and 10 mol. %) of the repeating units are repeating units RU* of one or more specific structural formula(e) not complying with general structural formula (I).
- the DPP polymers are homopolymers.
- homopolymer means that all the repeating units of the polymer are repeating units RU, and that the repeating units RU are of one and only one structural formula complying with general structural formula (I).
- the inventions relate to diketopyrrolopyrrole polymers wherein more than 50 mol. % (preferably, more than 90 mol. %), up to 100 mol. % of the repeating units are repeating units RU of at least two distinct specific structural formulae complying with general structural formula (I).
- copolymers comprising more than one type of multi-subunit substructure, such as for example diketopyrrolopyrrole copolymers wherein the repeating units RU are a mix consisting of repeating units RU1 of specific structural formula II)
- each R , R , R and R is independently selected from C1-C30 normal, branched, or cyclic alkyls or fluorinated derivatives thereof;
- each hAr 11 , hAr 11' , hAr 21 and hAr 21' is independently selected from Ci- C 6 o heteroaryls having the structure
- a is an integer equal to 1 , 2, 3, or 4;
- each X, X', X or X' " is independently selected from O, S, Se,
- each Y, Y', Y" and Y" ' is independently selected from N, and CR 4 , where R 4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; b) each R 12 , R 12' , R 22 and R 22' is independently selected from hydrogen, cyano, or Ci- C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl,
- Some embodiments of the "mixed" copolymers that comprise repeating subunits of formula (II) and formula (III) can be copolymers in which all the repeating units are repeating units RU, said repeating units RU being a mix consisting of repeating units RUl of specific formula (II) and repeating units RU2 of specific formula (III).
- repeating units RU consisting of repeating units RU1 of specific structural formula (II) and repeating units RU2 of specific formula (III), and between 0 mol. % and about 50 mol. % of the repeating units are repeating units RU* of one or more structural formula(e) not complying with general structural formula (I).
- the mole ratio of the repeating units RU1 and RU2 can range from about 5 % to about 95 % (preferably, from about 30 % to about 70 %).
- copolymers that comprise repeating subunits of formula (II) and formula (III), it should be understood that the copolymers can be either “block” or random copolymers.
- copolymers of formula (II) allow, for example, for the incorporation of more than one kind of "R" substituent on the nitrogen atoms of the DPP subunits, which can beneficially modify the solubility and/or physical characteristics of the polymers for certain applications, in either the solid or solution state.
- Such "mixed" copolymers can also allow for the presence of multiple types of DPP or hAr groups, which can be beneficial in some applications such as solar cells, where the incorporation of differing hAr groups can be used to tune and/or widen the optical absorption bands of the copolymers, so as to better absorb the entire solar spectrum and thereby increase the efficiency of collection of solar energy.
- the DPP polymers and/or copolymers comprise at least one or more, types of l,4-Diketo-2,5-dihydropyrrolo[3,4-c]pyrrole ("DPP”) electron accepting subunits having the generic structure
- DPP DPP subunits provide useful properties because the fused lactam group is strongly electron-withdrawing, planar and highly conjugated, and can readily conjugate with the hAr and vinylidene subunits of the polymers, which can result in small bandgaps that are highly beneficial to charge transport properties for applications in transistors and solar cells, and provide broad and intensive absorption of light in the visible to near-IR wavelengths, which is beneficial in applications in solar cells.
- Varying the substituent at the 2,5- amide nitrogen positions of the DPP subunits can be used to tune the solubility, co-planarity, hydrogen bonding interactions, crystallinity, self-assembly, conjugation length, and electronic structure of the DPP polymers and copolymers.
- the terminal "R" groups at the 2,5-positions of the DPP subunits can be independently selected from R 1 , R 1' , R 11 , R 11' , R 21 or R 21' groups, which can potentially be selected from a wide variety of organic groups, including C1-C30, C1-C20, or C1-C12, organic groups.
- R , R 1 or R 1 groups are selected from groups that are expected to be thermally and air stable to temperatures up to about 300 °C or higher, and are expected to be stable to oxidation by holes or reduction by electrons at the operating conditions of the electronic devices made therefrom, such as optionally substituted alkyls, perfluoroalkyls, alkoxy, perfluoroalkoxy, aryls, heteroaryls, alkylaryls, alkyheteroaryls, and the like.
- R 11' , R 21 or R 21 ' is independently selected from normal, branched, or cyclic alkyls or fluorinated derivatives thereof.
- R 11 , R 11' , R 21 or R 21' group is a normal or branched alkyl or perfluoroalkyl. In some embodiments, each R 1 , R 1 , R 11 , R 11 , R 21 or R 21 group is the same group.
- the DPP polymers and/or copolymers also comprise independently selected hAr 1 , hAr 1' hAr 11 , hAr 11' , hAr 21 and hAr 21' "heteroaryl" subunits in the copolymer backbone, each of which comprise five membered heteroaryl rings directly bonded to the DPP subunit.
- This arrangement is believed to present fewer unfavorable steric interactions (related to possible substituents in neighboring a-positions) with the neighboring DPP subunits than would a comparable six -membered aryl or heteroaryl, ring.
- binding less sterically demanding five membered heteroaryl rings to the DPP group increase the probability of co-planarity and/or ⁇ conjugation of the hAr 1 , hAr 1 , hAr 11 , hAr 11 , hAr 21 and hAr 21 groups relative to the DPP groups bonded thereto, at least as compared to the higher steric demands that could be presented by potentially six -membered aryl or heteroaryl groups bonded to DPP.
- the improved potential for co-planarity is also believed increase the potential for improved intermolecular ⁇ - ⁇ stacking between the copolymer chains in the solid state.
- the hAr heteroaryl subunits and/or rings comprise at least one carbon atom and at least one heteroatom selected from O, N, S, Se, and Si, as part of a ⁇ - conjugated aromatic ring or ring system.
- hAr 1 , hAr 1 , hAr 11 , hAr 11 , hAr 21 and hAr 21 "heteroaryl" subunits are Ci-C 6 o, C1-C30, C1-C20, or C1-C12 subunits.
- the hAr 1 , hAr 1 ' , hAr 11 , hAr 11 ' , hAr 21 and hAr 21 ' "heteroaryl" subunits can nevertheless optionally comprise one or more peripheral substituent groups such as for example cyano, alkyl, alkoxy, thioalkyl, or thioalkoxy substituent groups, so as to provide the potential to vary the electronic characteristics and/or solubility of the resulting copolymers.
- peripheral substituent groups such as for example cyano, alkyl, alkoxy, thioalkyl, or thioalkoxy substituent groups
- any such optional substitutent groups for the hAr 1 , hAr 1 ' , hAr 11 , hAr 11 ' , hAr 21 and hAr 21 ' "heteroaryl" subunits are not bonded to the five membered heteroaryl ring that is directly bonded to the DPP group, so as to minimize possible steric interactions with the DPP group.
- the hAr 1 , hAr 1 ' , hAr 11 , hAr 11 ' , hAr 21 and hAr 21 ' "heteroaryl" groups can be independently selected from one or all of the heteroaryls having the structure wherein
- a is an integer equal to 1, 2, 3, or 4, or in some embodiments 1 or 2; ii) each X, X', X or X' " is independently selected from O, S, Se,
- each Y, Y', Y"and Y' " can be independently selected from N, and CR 4 , where R 4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group.
- the resulting heterocycles can have a variety of physical, solubility, and electronic characteristics (from electron donating to electron withdrawing) that can be used to tune the characteristics, hole or electron transporting characteristics, and/or optical bandgaps of the resulting polymers and copolymers.
- hAr , hAr , hAr and hAr heteroaryl groups whose structures are shown generically above can be exemplified by one of the heterocyclic structures shown below:
- R 3 is a C1-C30 normal, branched, or cyclic alkyl group
- R 4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group.
- each hAr 1 and hAr 1 is independently selected from
- R 4 and R 4 are hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group groups.
- each hAr 1 and hAr 1 is independently selected from
- each hAr 1 and hAr 1 is independently selected
- R and/or R is a C1-C30 normal, branched, or cyclic alkyl group
- R and/or R 4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group.
- each hAr 1 and hAr 1 is independently selected from
- R 4 and R 4 are hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group groups.
- each hAr 1 and hAr 1 is independently selected
- alkyl perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group groups.
- eac 11 and hAr 11 can be independently selected from
- each hAr 21 and hAr 21' can be independently selected from
- each X, X', X or X' " and each Y, Y', Y" and Y" ' can have any of the meanings already detailed above.
- each hAr 11 and hAr 11 can be independently selected from
- each hAr 21 and hAr 21' can be independently selected from
- R and/or R is a C1-C30 normal, branched, or cyclic alkyl group
- R and/or R 4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group.
- the DPP polymers described herein also comprise an optionally substituted vinylidene subunit having the structure:
- each R and R group is independently selected from hydrogen, cyano, or C1-C30 alkyl, perfluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy
- each R and R group is hydrogen.
- Such ethylene subunits are believed to play an important role in contributing to the electronic and physical properties of the DPP polymers comprising repeat units of formulas (I), and copolymers comprising repeat units of formulae (II) and (III), because they can allow for extended conjugation along the polymer or copolymer backbone in a very sterically non-demanding fashion, which allows for high co-planarity among the subunits of the DPP polymers and copolymers, which allows for a high degree of intermolecular ⁇ - ⁇ stacking in the solid state, which can lead to dramatic increases in hole or electron mobility in the solid state.
- the ethylene subunits can also function to increase the solubility and/or improve the solution processing characteristics of the DPP polymers or copolymers.
- repeating units RU may be present in the DPP polymers or copolymers described herein other than the repeating units of general structural formulae (I), (II), and/or (III), in amounts that are less than about 50 (or 40, or 30 or 20 or 10 or 5 or 2 or 1) mol. %.
- Such other repeating units often comprise a wide variety of difunctional heteroaryl groups.
- the DPP polymers and/or copolymers of the invention can optionally comprise between 0 mol. % and 50 mol. % of the repeating units are repeating units RU* of one or more of the following general formulae
- R 4 and R 4 can be independently selected from hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group groups.
- those DPP polymers or copolymers can in some embodiments have a number average degree of polymerization of at least 10, as determined by gel permeation chromatography using polystyrene calibration standards.
- the DPP polymers or copolymers described herein can in some embodiments have a number average degree of polymerization of at most 100, as determined by gel permeation chromatography using polystyrene calibration standards.
- the various DPP polymers and/or copolymers described herein can have unexpectedly high solubility in common organic solvents, which can be of great benefit to adjust ink formulation to a large variety of solution printing methods (such as ink jet printing, etc) for the preparation of desirable organic electronic devices, such as photovoltaic cells and/or transistors.
- the DPP polymers and/or copolymers can have solubility at one or more temperature(s) selected in the range from 25 °C to 50 °C, such as 35°C, in at least one solvent selected from the group consisting of toluene, xylene, mesitylene, tetrahydrofuran, chloroform, chlorobenzene, dichlorobenzene and mixtures thereof, of at least 10 mg/ml, preferably at least 20 mg/ml.
- a solvent selected from the group consisting of toluene, xylene, mesitylene, tetrahydrofuran, chloroform, chlorobenzene, dichlorobenzene and mixtures thereof, of at least 10 mg/ml, preferably at least 20 mg/ml.
- the DPP polymers and/or copolymers can have solubility at 25 °C and/or at
- the DPP polymers and/or copolymers can have solubility at 25 °C and/or at
- the DPP polymers and/or copolymers can have solubility at 25 °C and/or 50 °C in a mixture consisting of chloroform and chlorobenzene in a 50:50 wt. ratio of at least 10 mg/ml.
- the starting heteroaryl nitriles can often be made by many methods known to those of ordinary skill in the art, such as for example from the corresponding heteroaryl aldehydes or brominated hetereoaryl aldehydes, by condensation with hydroxylamine, or by condensation of cyanides with heteroaryl diazonium salts (Mowry, D. T. Chem. Rev. 1948, 42, 189-283.; Barclay, R. M.; Cordes, A. W.; MacKinnon, C. D.; Oakley, R. T.; Reed, R. W. Chem. Mater. 1997, 9, 981-990.),
- Polymerizable brominated bis-heteroaryl substituted diketopyrrolopyrrole comonomers such as ml and m2 in the diagram above can each be condensed (optionally in the presence of one or more additional dibrominated hetereroaryl comonomers) with trans- 1 ,2-bis(tributylstannyl)ethylenes via the well known palladium catalyzed Stille condensation, to yield the polymers comprising the repeat units of formula (I).
- the inventions described herein relate to methods for making the polymers described herein, comprising the steps of a) obtaining or providing at least one bis-heteroaryl substituted
- LG is a leaving group such a bromine atom, an iodide atom, an organic sulonic acid group, or the like ;
- LG is a leaving group capable of being eliminated by reacting with LG 1 , such as SnR 2" 2"
- R is an alkyl or aryl group
- Such transition metal catalyzed couplings/polymerization reactions s are well known in the art, especially in the presence of nickel or palladium catalysts.
- Typical soluble palladium catalyst complexes useful in such catalytic coupling/polymerization reactions include palladium acetate, tetrakis
- ferrocene]palladium(II) dichloride or bis(triphenylphosphine)palladium(II) dichloride, and Tris(dibenzylideneacetone)dipalladium(0) (iePd 2 (dba)3), typically at a concentration of about 0.1 to about 20 wt%, and optionally in the presence of additional and/or excess phosphine ligands, such as triarylphosphines.
- reaction mixture is substantially free of any monomer other than the at least one first bis-heteroaryl substituted
- the reaction mixture comprises susbtantially equimolar amounts of the at least one bis-heteroaryl substituted diketopyrrolopyrrole monomer(s) and the at least one bis-leaving group substituted ethylene monomer(s).
- other co-monomers can be present in the reaction mixture, to make copolymers.
- the reaction mixture further comprises at least one self-reacting monomer having the structure
- Ci-C 6 o heteroaryl wherein hAr* is a Ci-C 6 o heteroaryl group, and LG and LG are leaving groups as described above.
- suitable Ci-C 6 o heteroaryls include the structures
- the self-reacting monomer can, to at least some extent, self-condense in the presence of the transition metal catalysts, to form copolymer blocks (within the DPP copolymers of the invention) having the structure
- x is an integer greater than one, and preferably in the range from 2 to 100.
- mixtures of DPP/hAr comonomers such as ml or m2 shown below can be condensed with bis(tributylstannyl)ethylenes to yield random or block copolymers comprising at least some repeat units comprising formulae (II) and/or (III).
- the inventions described herein relate to methods for making random copolymers, comprising the steps of a) obtaining or providing at least a first bis-heteroaryl substituted
- LG is a leaving group
- such DPP copolymers are random copolymers wherein R 11 and R 11' are identical to each other and R 21 and R 21' are identical to each other. In other embodiments, such DPP copolymers are block copolymers wherein R 11 and R 21 are different from each other and/or R 11' and R 21' are different from each other.
- copolymers prepared by such methods can be random or nearly random in the sense that the sequence of DPP/hAr repeat units can be random or near random, it is also possible to intersperse into such copolymers additional random hAr* repeat units, by including in the reaction mixture at least one co monomer having the structure
- Some aspects of the present inventions relate to novel organic electronic devices comprising the polymers of Formulas (I) or copolymers comprising repeat units having Formulas (II) and (III) described herein, including transistors and various logic circuits comprising transistors, and solar cells.
- Each of those end use applications typically require the formation of a film of the copolymers of the invention on a substrate.
- Organic films of the polymers and copolymers of the present invention can be prepared by known methods such as spin coating methods, casting methods, dip coating methods, inkjet methods, doctor blade coating methods, screen printing methods, and spray coating methods. By using such methods, it becomes possible to prepare organic films having good properties such as mechanical strength, toughness, and durability without forming cracks in the films. Therefore, the organic films can be preferably used for organic electronic devices such as photovoltaic cells, and OFET elements.
- Films of the polymers comprising repeat units of Formulas I or copolymers comprising repeat units of formulae (II), (III), (IV) and (V) are typically prepared by coating a coating liquid, which is prepared by dissolving the copolymer (and in the case of solar cells, typically an electron accepting material such as a fullerene derivative such as PCBM) in a solvent such as dichloromethane, tetrahydrofuran, chloroform, toluene, chlorobenzene, dichlorobenzene, or xylene, on a substrate.
- a coating liquid which is prepared by dissolving the copolymer (and in the case of solar cells, typically an electron accepting material such as a fullerene derivative such as PCBM) in a solvent such as dichloromethane, tetrahydrofuran, chloroform, toluene, chlorobenzene, dichlorobenzene, or xylene, on a substrate.
- Suitable materials for use as the substrate on which a film of the polymer of the present invention is formed include inorganic substrates such as glass plates, silicon plates, ITO plates, and FTO plates, and organic substrates such as plastic plates (e.g., PET films, polyimide films, and polystyrene films) , which can be optionally subjected to a surface treatment. It is preferable that the substrate has a smooth surface.
- the thickness of the organic film and the organic semiconductor layer of the organic thin film transistor of the present inventions are not particularly limited. However, the thickness is determined such that the resultant film or layer is a uniform thin layer (i. e., the film or layer does not include gaps or holes adversely affecting the carrier transport property thereof).
- the thickness of the organic semiconductor layer is generally not greater than 1 micron, and preferably from 5 to 200 nm.
- the organic thin film transistors of the present inventions typically have a configuration such that an organic semiconductor layer including the polymer comprising repeat units of formula (I) or copolymers comprising repeat units of formulae (II), (III), (IV), and/or (V) can be formed on a substrate, while also contacting the source electrode, drain electrode, gate electrode and insulating layer of the transistor.
- the organic film prepared above is typically annealed. Annealing is performed while the film is set on a substrate, and is believed to allow for improved ordering of the copolymer molecules in the solid state.
- the annealing temperature is determined depending on the property of the material constituting the substrate, but is preferably from room temperature to 300 °C, and more preferably from 50 to 300 °C.
- the transistors and/or circuits comprising them are thermally annealed at temperatures between about 100 and 250 °C. When the annealing temperature is too low, the organic solvent remaining in the organic film cannot be well removed therefrom. In contrast, when the annealing temperature is too high, the organic film tends to be thermally decomposed.
- Annealing is preferably performed in a vacuum, nitrogen, argon or air atmosphere. It is also possible to perform annealing in an atmosphere including a gas of an organic solvent capable of dissolving the polymer because the molecular motion of the polymer is accelerated, and thereby a good organic thin film can be prepared.
- the annealing time is properly determined depending on the aggregation speed of the polymer.
- An insulating layer is used for the gate of the organic thin film transistors comprising the polymers and/or copolymers of the present invention.
- Various insulating materials can be used for the insulating layer.
- Specific examples of the insulating materials include inorganic insulating materials such as silicon oxide, silicon nitride, aluminum oxide, aluminum nitride, titanium oxide, tantalum oxide, tin oxide, vanadium oxide, barium strontium titanate, barium zirconate titanate, lead zirconium titanate, lead lanthanum titanate, strontium titanate, barium titanate, barium magnesium fluoride, bismuth tantalate niobate, hafnium oxide, and trioxide yttrium; organic insulating materials such as polymer materials, e.g., polyimide, polyvinyl alcohol, polyvinyl phenol, polystyrene, polyester, polyethylene, polyphenylene sulfide, unsubstituted or
- Suitable methods for forming such an insulating layer include dry processes such as CVD methods, plasma CVD methods, plasma polymerization methods, and vapor deposition methods; wet processes such as spray coating methods, spin coating methods, dip coating methods, inkjet coating methods, cast coating methods, blade coating methods, and bar coating methods; etc.
- an organic thin film (intermediate layer) can be formed between the insulating layer and organic semiconductor layer.
- the materials for use in the intermediate layer are not particularly limited as long as the materials do not chemically affect the properties of the organic semiconductor layer, and for example, molecular films of organic materials, and thin films of polymers can be used therefor.
- the materials for use in preparing the molecular films include coupling agents such as octadecyltrichlorosilane, octyltrichlorosilane, octyltrimethoxysilane, hexamethyldisilazane (HMDS), and octadecylphosphonic acid.
- Specific examples of the polymers for use in preparing the polymer films include the polymers mentioned above for use in the insulating layer. Such polymer films can serve as the insulating layer as well as the intermediate layer.
- the materials of the electrodes (such as gate electrodes, source electrodes and drain electrodes) of the organic thin film transistor of the present invention are not particularly limited as long as the materials are electrically conductive.
- Specific examples of the materials include metals such as platinum, gold, silver, nickel, chromium, copper, iron, tin, antimony, lead, tantalum, indium, aluminum, zinc, tungsten, titanium, calcium, and magnesium; alloys of these metals;
- electrically conductive metal oxides such as indium tin oxide (ITO); inorganic or organic semiconductors, whose electroconductivity is improved by doping or the like, such as silicon single crystal, polysilicon, amorphous silicon, germanium, graphite, carbon nanotube, polyacetylene, polyparaphenylene, polythiophene, polypyrrole, polyaniline, polythienylenevinylene,
- ITO indium tin oxide
- polyparaphenylenevinylene and complexes of polyethylenedioxythiophene (PEDOT) and polystyrene sulfonic acid.
- PEDOT polyethylenedioxythiophene
- the average hole mobility as measured from exemplary transistors comprising HD-PPTV as described below in Examples 1 and 6 were in the narrow range of 0.091-0.17 cm 2 V - " 1 s - “ 1 and the average electron mobility was 0.012-0.019 cm 2 V "1 s "1 with current on/off ratios of 10 2 -10 3 .
- These values of hole and electron mobility and resulting ambipolar behavior in the transistors were unexpectedly high as compared with the prior art known to the inventors.
- the threshold voltage of hole and electron transport are rather positive, having - 9.0 - -1.6 V for p-channel operation and 22.7-32.1 V for n-channel mode.
- the DPP polymers and copolymers described herein can have unexpectedly superior hole mobility of about 0.1 cm 2 V - " 1 s - " 1 or greater, as measured from a thin film transistor having a bottom gate, bottom contact geometry, employing doped silicon as a gate material, silicon dioxide as a gate dielectric, using gold source and drain electrodes with a chromium adhesive layer at a channel width of 400-800 ⁇ and lengths of 20-40 ⁇ , and employing the copolymers as the active semiconductor.
- the DPP polymers and copolymers described herein can have unexpectedly superior hole mobility electron mobility of about 0.01 cm /Vsec or greater, as measured from a thin film transistor having a bottom gate, bottom contact geometry, employing doped silicon as a gate material, silicon dioxide as a gate dielectric, using gold source and drain electrodes with a chromium adhesive layer at a channel width of 400-800 ⁇ and lengths of 20-40 ⁇ , and employing the copolymers as the active semiconductor.
- Some DPP polymers and/or copolymers described above exhibit ambipolar characteristics, and can have both equilibrated hole mobility and electron mobility of about 0.01 cm 2 V - " 1 s - " 1 or greater, as measured from a thin film transistor having a bottom gate, bottom contact geometry, employing doped silicon as a gate material, silicon dioxide as a gate dielectric, using gold source and drain electrodes with a chromium adhesive layer at a channel width of 400- 800 ⁇ and lengths of 20-40 ⁇ , and employing the copolymers as the active semiconductor.
- transistors comprising ambipolar materials with such high hole and electron mobilities can be suitable and unexpectedly economically superior for use in certain low performance and low cost applications, such as RFID tags.
- inverter with two ambipolar transistors but also more complicated electronic logic gates consisting of 3- or more ambipolar transistors and having 2- or more input signals, such as NAND- and NOR-gates, can be constructed by implementing ambipolar polymer semiconductors such as HD-PPTV.
- the complementary integrated circuits such as inverter, NAND logic circuits, NOR logic circuits, and so on, comprising the copolymer of the invention typically have one or more transistors which may function as either n- channel or p-channel device.
- the constituent transistors of an integrated circuit are electrically connected on a common substrate or separated substrates.
- the constituent materials of a transistor such as electrodes, insulators, and DPP polymers and copolymers described herein are formed by photolithography, soft lithography, spin-coating, printing methods, vapor deposition, and/or self- assembly.
- Solar cells comprising the DPP polymers and/or copolymers described above, and typically also an electron accepting material such as a fullerene or fullerene derivative such as PCBM, are an aspect of the inventions described herein.
- Such cells can typically be fabricated by first spin-coating a PEDOT buffer layer on top of ITO-coated glass substrates at 1500 rpm for 60 s and dried at 150°C for 10 min under vacuum to form an anode.
- the thickness of PEDOT layer should be around 40 nm.
- the solvents used for dissolving the mixture of polymers and/or copolymers of the invention and the electron acceptors can be chloroform, chlorobenzene, 1 ,2-dichlorbenzene, etc.
- the solvents for copolymer/fullerene blend can be a single solvent such as chloroform, chlorobenzene, 1 ,2- dichlorbenzene or a mixture of two or three different solvents, the second (third) solvent can be 1,8-diiodooctane, 1,8-dibromoctane, 1,8-octanedithiol, etc.
- the active blend layer (copolymer and fullerene blend) can be spin-coated on top of the PEDOT layer, from a copolymer/fullerene blend solution at a speed of 1000 rpm for 30 s, and then annealed on a hot plate at 150 ⁇ 40 °C for 10 min in a glove box.
- the active layer can also be spin-coated in air and dried in a vacuum oven without thermal annealing.
- the copolymer coated substrates are taken out of the glove box and loaded in a thermal evaporator for the deposition of the cathode.
- the cathodes consisting of 1.0 nm LiF and 80 nm aluminum layers can be sequentially deposited through a shadow mask on top of the copolymer/electron acceptor active layers in a vacuum of 8x 10 ⁇ 7 torr.
- DSC Differential scanning calorimetry
- the Ag/Ag + (AgN0 3 ) reference electrode was calibrated at the beginning of the experiments by running cyclic voltammetry on ferrocene as the internal standard. The potential values obtained in reference to Ag/Ag + electrode were then converted to the saturated calomel electrode (SCE) scale. The films of the polymers were coated onto the working electrode by dipping a Pt wire into a 10 wt% solution in chloroform/chlorobenzene and drying for 30 min.
- Example 1 Synthesis of Poly[3,6-(2,5-bis(2-hexyldecyl)pyrrolo[3,4- c]pyrrole-l,4-dione)-a/t-l,2-bis-(2'-thienyl)vinyl-5',5''-diyl], HD-PPTV.
- HD-PPTV a. KOtBu, diethyl succinate, t-amyl alcohol, 120 °C.
- d- Bu,Sn ⁇ ⁇ > Pd 2 (dba) 3 , P( «-toyl) 3 , 120 °C.
- HD-PPPV Poly[3,6-(2,5-bis(2-hexyldecyl)pyrrolo[3,4-c]pyrrole-l,4-dione)-aZi-l,2- bisphenylvinyl-4',4"-diyl], HD-PPPV, which comprises six-membered phenyl rings at the positions of the"hAr" subunits, was prepared by the reaction above, in a manner analogous to that employed in Example 1. Purple solid, yield: 47%. 1H NMR (CDCI 3 ), (ppm): 7.63 (br, 8H), 7.09(br, 2H), 3.85 (br, 4H), 1.7-1.25 (br, 50H), 0.89 (br, 12H).
- HD-PPTV and PPTPV have good solubility in common organic solvents such as chloroform, chlorobenzene, and 1,2-dichlorobenzene at high concentrations (10-20 mg/mL) whereas HD-PPPV shows only poor solubility ( ⁇ 2 mg/mL).
- the number- average molecular weights ( n ) of three copolymers were in the range of 21 800 - 88 800 g/mol with polydispersity indices (PDI) of 2.32-3.60, as summarized in Table 1.
- lactam-containing DPP-based copolymers HD-PPTV, HD-PPPV, and PPTPV demonstrated good thermal stability, but no clear thermal transition between 0 to 350 °C was observed for the three poly(arylene vinylene)s by differential scanning calorimetry (See Figure 1).
- HD-PPPV has an optical bandgap of 2.00 eV, lower than the reported 2.09-2.13 eV for poly(l,4-diketo- 2,5-dialkyl-3,6-diphenylpyrrolo[3,4-c]pyrrole) (see Rabindranath, A. R.; Zhu, Y.; Heim, I.; Tieke, B. Macromolecules 2006, 39, 8250).
- HD-PPTV has an optical bandgap of 1.22 eV, slightly lower than the reported 1.25 eV for poly(l,4-diketo-2,5-diocty-3,6-bis(thiophen-5-yl)pyrrolo[3,4-c]pyrrole) (See Bijleveld, J. C; Zoombelt, A. P.; Mathijssen, S. G. J.; Wienk, M. M.; Turbiez, M.; de Leeuw, D. M.; Janssen, R. A. J. J. Am. Chem. Soc. 2009, 131, 16616).
- the photoluminescence (PL) emission spectra of HD-PPPV and PPTPV were shown in Figures 3a and 3b.
- the PL spectrum of HD-PPTV was not obtained, probably because of weakness beyond the detection limit, and/or quenching in the solid state.
- HD-PPPV is red-emitting with PL maxima at 647 and 653 nm in dilute solution and as thin film, respectively.
- This HD-PPPV polymer has large Stoke shift of 109 and 116 nm in dilute solution and as thin films similar to other DPP-based polymers.
- the random copolymer PPTPV has a weak PL peak centered at 651 nm in dilute solution, but the photoluminescence is almost completely quenched in the thin film.
- HD-PPPV has a low-lying HOMO energy level of -5.48 eV compared to HD-PPTV (-5.14 eV) and PPTPV (-5.21 eV). Also, HD-PPPV has a high-lying LUMO energy level of -3.13 eV compared to -3.34 and -3.31 eV in HD-PPTV and PPTPV. Thus, HD-PPPV shows a much larger electrochemical bandgap of 2.35 eV than thel .80-1.90 eV of HD-PPTV and PPTPV.
- HOMO/LUMO energy levels of PPTPV are similar to those of HD- PPTV, probably because they share the same Th-DPP-Th core and resulting strong intramolecular charge transfers.
- the current intensity for the reduction curves was much lower than that of oxidation curves, suggesting they may have a better capability for transporting holes rather than electrons.
- the dioo spacing is believed to correspond to a stacking distance in a lamellar packing structure, dictated by the bulky 2- hexyldecyl chains.
- HD-PPPV has no such peak, and the random copolymer PPTPV shows only a weak reflection peak at 4.89°, corresponding to a dioo spacing of 18.02 A, indicating that PPTPV may have a lamellar packing structure similar to HD-PPTV.
- HD-PPTV The broad peak for HD-PPTV centered at 22.67° is believed to be related to an intermolecular ⁇ - ⁇ stacking distance between the HD-PPTV polymer chains (3.92 A), and may be indicative of the relatively highly crystalline nature of the HD-PPTV polymer in the solid state. In contrast, HD-PPPV and HD-PPTV did not show any similar reflection peaks near 22 degrees.
- Example 5 Fabrication and Characterization of Field-Effect Transistors.
- ODS-8 octyltrichlorosilane
- OCS-8 octyltrichlorosilane
- Polymer solutions in 1 ,2-dichlorobenzene (ODCB) were deposited on the substrates by spin-coating.
- the devices were annealed at various temperatures in a nitrogen- filled dry box. Electrical characteristics of the devices were measured by using HP4145B semiconductor parameter analyzer under nitrogen atmosphere.
- Figures 6a and 6b shows representative current-voltage characteristics of the OFETs based on HD-PPTV, HD-PPPV, and PPTPV as the polymer semiconductors.
- the transistors employing HD-PPTV ( Figure 6a) showed both hole and electron charge transport with typical ambipolar features, such as current modulation and saturation observed in both positive and negative polarities of applied voltages.
- HD-PPTV 110 0.11 0.015 -5.9 24.5 10 -10
- HD-PPPV 150 4.9x10 - -9.8 - 10
- the threshold voltage of hole and electron transport are -9.0 - -1.6 V for p- channel operation and 22.7-32.1 V for n-channel mode.
- the hole mobility in HD-PPTV transistors is about one order of magnitude higher than the electron mobility, which may result from the larger injection barriers for electrons from the gold electrodes.
- the mobilities (0.012-0.17 cm V “1 s “1 ) are comparable to the reported values (0.01-0.65 cm 2 V “1 s “1 ) for ambipolar transistors based on one single conjugated polymer semiconductor and are higher than the reported values (0.01-0.05 cm 2 V - " 1 s - “ 1 ) obtained from ambipolar transistors with similar bottom-contact and bottom-gate geometry, see Bwrgi, L.; Turbiez, M.; Pfeiffer, R.; Bienewald, F.; Kirner, H.-J.; Winnewisser,
- NAND circuit is off-state if both input signals are on-state; otherwise the output is on-state.
- Devices comprising HD-PPTV to make NOR logic circuits and the resulting electrical characteristics are shown in Figure 9.
- Output signal of the NOR circuit is on-state if both input signals are off-state; otherwise the output is off-state.
- the constituent transistors of NAND and NOR circuits are identical, the function of a circuit can be switched from a NAND gate to a NOR gate, or from a NOR gate to a NAND gate, by swapping voltage biases of a power supply and a ground.
- a first embodiment (i.e., embodiment 1) provides for
- diketopyrrolopyrrole polymers comprising a plurality of repeating units, wherein more than 50 mol. %, up to 100 mol. % of the repeating units are repeating units RU of one or more structural formula(e) complying with general structural formula (I)
- each R 1 and R 1 is independently selected from C 1 -C30 normal, branched, or cyclic alkyls or fluorinated derivatives thereof;
- each hAr 1 and hAr 1 is independently selected from Ci-C 6 o heteroaryls having the structure
- a is an integer equal to 1 , 2, 3, or 4;
- each X, X', X or X' " is independently selected from O, S, Se,
- R an R is independently selected from hydrogen, cyano, or Ci- C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy groups.
- a second embodiment is the diketopyrrolopyrrole polymers according to embodiment 1, wherein more than 50 mol. %, up to 100 mol. % of the repeating units are repeating units RU of one and only one specific structural formula complying with general structural formula (I).
- a third embodiment is the diketopyrrolopyrrole polymers according to one or more other embodiments, which are homopolymers.
- a fourth embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, wherein more than 50 mol. %, up to less than 100 mol. % of the repeating units are repeating units RU of one and only one specific structural formula complying with general structural formula (I), and between 0 mol. % and 50 mol. % of the repeating units are repeating units RU* of one or more specific structural formula(e) not complying with general structural formula (I).
- a fifth embodiment is the diketopyrrolopyrrole polymers according to embodiment 1, wherein more than 50 mol. %, up to 100 mol. % of the repeating units are repeating units RU of at least two distinct specific structural formulae complying with general structural formula (I).
- a sixth embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, wherein the repeating units RU are a mix consisting of repeating units RU1 of specific structural formula (II)
- each R , R , R and R is independently selected from C1-C30 normal, branched, or cyclic alkyls or fiuorinated derivatives thereof;
- a) e ,, h aanndd hAr is independently selected from Ci-C 6 o heteroaryls having the structure
- a is an integer equal to 1 , 2, 3, or 4;
- each X, X', X or X' " is independently selected from O, S, Se,
- each Y, Y', Y" and Y" ' is independently selected from N, and CR 4 , where R 4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; b) each R 12 , R 12' , R 22 and R 22' is independently selected from hydrogen, cyano, or C1-C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy groups,
- a seventh embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, which are block copolymers wherein R 11 and R 21 are different from each other and/or R 11 ' and R 21 ' are different from each other.
- An eighth embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, which are random copolymers wherein R 11 and R 11 are identical to each other and R 21 and R 21 ' are identical to each other.
- a ninth embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, which are copolymers of which all the repeating units are repeating units RU, said repeating units RU being a mix consisting of repeating units RUl of specific formula (II) and repeating units RU2 of specific formula (III).
- diketopyrrolopyrrole polymers are provided according to one or more other embodiments, wherein more than 50 mol. %, up to less than 100 mol. % of the repeating units are repeating units RU consisting of repeating units RUl of specific structural formula (II) and repeating units RU2 of specific formula (III), and between 0 mol. % and 50 mol. % of the repeating units are repeating units RU* of one or more structural formula(e) not complying with general structural formula (I).
- An eleventh embodiment provides diketopyrrolopyrrole polymers according to one or more other embodiments, wherein the mole ratio of the repeating units RUl and RU2 (RUl :RU2) ranges from 5 % to 95 %.
- a twelfth embodiment provides for diketopyrrolopyrrole polymers according to anyone of the preceding embodiments, wherein each hAr 1 and hAr 1 is independently selected from
- R 3 is a C1-C30 normal, branched, or cyclic alkyl group
- R 4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group
- a thirteenth embodiment provides for diketopyrrolopyrrole polymers according to one or more other embodiments, wherein each hAr 1 and hAr 1 is independently selected from
- diketopyrrolopyrrole polymers according to one or more prior embodiments are provided, wherein each hAr 1 and hAr 1 is independently selected from
- diketopyrrolopyrrole polymers are provided according to one or more other embodiments, wherein each hAr 1 and hAr 1 is independently selected from
- a sixteenth embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, wherein each hAr 1 and hAr 1 is independently selected from
- diketopyrrolopyrrole polymers according to claim 12, wherein each hAr 1 and hAr 1 is independently selected from
- An eighteenth embodiment is diketopyrrolopyrrole polymers according to one or more prior embodiments, wherein each hAr 1 and hAr 1 is independently selected from
- diketopyrrolopyrrole polymers according to one or more prior embodiments, wherein each hAr 1 and hAr 1 is independently selected from
- a twentieth embodiment is diketopyrrolopyrrole polymers according to one or more prior embodiments, wherein each hAr 1 and hAr 1 is independently selected from
- a twentyfirst embodiment is diketopyrrolopyrrole polymers according to anyone of other embodiments, wherein each hAr 11 and hAr 11 is independently selected from
- each hAr 21 and hAr 21 ' is independently selected from
- a twentysecond embodiment is diketopyrrolopyrrole polymers according to anyone of the other embodiments, wherein between 0 mol. % and 50 mol. % of the repeating units are repeating units RU* of one or more of the following general formulae
- a twentythird embodiment provides for diketopyrrolopyrrole polymers of any of the preceding embodiments, which have a number average degree of polymerization of at least 10, as determined by gel permeation chromatography using polystyrene calibration standards.
- diketopyrrolopyrrole polymers of any of the preceding embodiments which have a number average degree of polymerization of at most 100, as determined by gel permeation chromatography using polystyrene calibration standards.
- diketopyrrolopyrrole polymers according to anyone of the preceding embodiments are provided, having solubility at one or more temperature(s) selected in the range from 25 °C to 50 °C in at least one solvent selected from the group consisting of toluene, xylene, mesitylene, tetrahydrofuran, chloroform, chlorobenzene, dichlorobenzene and mixtures thereof, of at least 10 mg/ml .
- an electronic device comprising at least one polymer chosen from the diketopyrrolopyrrole polymers according to anyone of the preceding embodiments.
- the electronic device of any of the prior embodiments which comprises at least one photovoltaic cell or at least one transistor comprising the at least one polymer.
- the electronic device comprises at least one transistor comprising the at least one polymer.
- a method for making the polymers of any or more of the prior embodiments comprising the steps of
- LG 1 is a leaving group, such a bromine atom ;
- the method is provided according to one or more other embodiments, wherein the mixture comprises susbtantially equimolar amounts of the at least one first bis-heteroaryl substituted diketopyrrolopyrrole monomer and the at least one bis-leaving group substituted ethylene monomer.
- a method according to one or more other embodiments wherein the mixture is substantially free of any monomer other than the at least one first bis-heteroaryl substituted
- a method is provided according to on or more other embodiments, wherein the mixture further comprises at least one self- reacting monomer having the structure LG 1 - hAr* - LG 2 wherein hAr* is selected from C -C heteroar ls havin the structure
- LG 1 ,LG2, X, X', X", X"', Y, Y', Y", and Y'" are as defined in the previous embodiment.
- a method is provided according to one or more other embodiments, wherein the mixture further comprises at least one self- reacting monomer having the structure
- LG 1 , X, X', X", X"', Y, Y', Y", and Y'" are as defined in the relevant previous claim.
- a method for making the random copolymers of one or more of the other embodiments comprising the steps of : a) obtaining or providing at least a first bis-heteroaryl substituted diketopyrrolopyrrole monomer having the structure
- LG is a leaving group such a bromine atom ; b) obtaining or providing at least a second bis-heteroaryl substituted diketopyrrolopyrrole monomer having the structure
- LG is a leaving group capable of being eliminated by reacting with LG 1 , such as SnR 2" 2"
- R is an alkyl or aryl group
Abstract
The inventions disclosed, described, and/or claimed herein relate to various genera and subgenera of diketopyrrolopyrrole (DPP) polymers, including homopolymers and/or copolymers, comprising a plurality of repeating units, more than 50 mol. % and up to 100 mol. % of the repeating units are repeating units RU of one or more structural formula(e) complying with general structural formula (I) (see application) wherein (a) each R1 and R1' is independently selected from normal, branched, or cyclic organic group, such as for example alkyls or fluorinated derivatives thereof; (b) each hAr1 and hAr1' is independently selected from heteroaryls. The polymers are readily soluble and particularly useful for solution manufacturing organic electronic devices, including transistors and solar cells. Methods for making the polymers and the derivative electronic devices are also described.
Description
New Poly(heteroarylene vinylene)s Based on
Diketopyrrolopyrrole
RELATED APPLICATIONS
This application claims priority to U.S. provisional application serial number 61/525,618 filed August 19, 2011, which is hereby incorporated by reference in its entirety.
STATEMENT OF GOVERNMENT LICENSE RIGHTS
The inventors received partial funding support through the National Science Foundation (DMR-0805259). The Federal Government retains certain license rights in the invention.
TECHNICAL FIELD OF THE INVENTION
The various inventions disclosed, described, and/or claimed herein relate to the field of semiconducting organic polymers and their uses, including the manufacture of organic electronic devices, such as transistors and solar cells.
BACKGROUND OF THE INVENTION
Solution-processable conjugated polymer and/or copolymer
semiconductors have attracted attention in the art due to their potential applications in making large area, flexible, and low-cost electronic devices, including organic light emitting diodes (OLEDs), solar cells and/or transistors.
Some such polymers and/or copolymers known in the prior art have achieved reasonable current carrying capabilities, in the form of either reasonably good hole mobilities, reasonably good electron mobilities, or in a few cases a combination of both hole mobility and high electron mobility, i.e. "ambipolar" properties. Some of the prior art polymers and/or copolymers can be used to produce reasonably good performance when used to make transistors, or encouraging efficiencies in solar cells for converting solar radiation to electrical
energy (3-5%). The relatively few known ambipolar polymers and/or copolymers have not yet achieved a reasonably good and matched level of both hole and electron mobility that would allow the fabrication of high efficiency solar cells, or transistors and derived devices comprising single copolymer wherein the devices have sufficiently high on/off current ratios to enable practical and competitive end use applications.
Conjugated donor-acceptor (D-A) polymers and/or copolymers have received attention in the art for tailoring the electronic structure, highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), the associated bandgap energy and intensive photon absorption in a broad region of the UV, visible, and infrared wavelengths. The selection of the electron donor and acceptor subunits in these conjugated polymers and/or copolymers can modulate the HOMO/ LUMO energy levels, so as to modulate the light absorption and charge transport properties.
One known class of such polymers comprise l,4-Diketo-2,5- dihydropyrrolo[3,4-c]pyrrole ("DPP") electron accepting subunits having the structure
DPP
For example, PCT Patent Application WO 2008/000664 contemplated a very broad class of such DPP polymers as organic semiconductors potentially useful for making transistors and solar cells (the suggested generic structure is shown below).
The '664 PCT broadly contemplated the possible use of a very wide variety of potential combinations of the various Ar subunits, including a wide variety of aryl, heteroaryl, fused heteroaryl, ethylene, acetylene, and other potential Ar subunits described therein. More specifically, the '664 PCT reported the actual synthesis of a DPP polymer having the structure
Thin-film, bottom gate field effect transistors made from this DPP polymer (4) were reported to give "clear p-type transistor behavior" with a field effect mobility of 0.15 cm /Vs, threshold voltage offsets of about 0-5 volts, and on/off
4 7 2
ratios of between 10 -10 , and ambipolar mobilities up to 0.1 cm /Vs, and the transistors were reported to be thermally stable and performance that was reasonably stable in air over two months. No mention was made of any n-type current carrying ability. Bulk-heterojunction solar cells constructed from the polymer were reported to convert solar energy at an efficiency of up to 3.06%.
Recently, Bijleveld et al (J. Amer. Chem. Soc. 2009, 131, 16616-16617) have reported a slightly different polymer having the structure shown below
A Mn= 10,000 g/mol version of PDPP3T polymer (wherein HD = 2- hexyldecyl) gave a bottom gate, bottom contact transistor with ambipolar behavior, with hole mobilities of 0.04 cm /Vs and electron mobilities of 0.01
2
cm / Vs. That polymer was used to make inverters, and to make bulk heterojunction solar cells (in combination with PCBM) that gave light
conversion in efficiencies up to 4.7%. Somewhat later, WO 2010/049321 reported that a DPP polymer with the same structural formula as that of Bijleveld, but a molecular weight of 39,500 gr/mole gave a bottom gate, bottom contact transistor with balanced ambipolar behavior, with hole mobilities of 0.43
2 2
cm /Vs and electron mobilities of 0.35 cm / Vs. It is not obvious why such a small structural changes between the '664 PCT polymer, the Bijleveld polymer, and the polymer of WO 2010/049321 gives such large differences in electrical performance.
Very recently, Bronstein et al reported (J. Am. Chem. Soc. 2011, 133, 3272-3275) two related DPP polymers and employed them in OFETs that gave maximum hole mobilities of up to 1.95 cm / Vs and organic photovoltaic devices with power conversion efficiencies of 5.4%.
However, also recently, Zhang et al {Synthetic Metals, 2010, 160, 1945- 1952) reported three other similar DPP polymers comprising vinylidene subunits in the pol mer chain having the structures
Solar cells comprising these DPP/vinylidene polymers gave OPV efficiencies of only 0.72, 0.16, and 0.16%. C6DPPDHPV had the highest reported hole mobility in an OFET, of only 5.4x10 -"4 cm 2 /Vs, with no report of electron mobility or ambipolar behavior. The reasons for these rather large variations in properties and performance as compared to other DPP polymers were not clear, but in view of the results reported by Zhang et al, one of ordinary skill in the art might be motivated against considering inclusion of vinylidene subunits in similar polymers.
Nevertheless, there remains a need in the art for new and improved polymeric and/or copolymeric materials and/or compositions derived therefrom that can provide high and reproducible mobility of either holes or electrons coupled with improved processability, enabling high solubility, control over molecular weight and/or film forming ability, low cost, and high thermal and oxidative stability for use in organic electronic devices, especially transistors, solar cells, and more complex organic electronic devices and/or circuits. In particular, there is still an unmet need in the art for ambipolar organic polymers and/or copolymers with improved levels of hole and electron mobility, lower offset voltages, and better on/off ratios, so as to enable the manufacture of complementary circuits and electronic devices from a single semicinductor polymer. It is toward solving such problems that the various embodiments of the various inventions described below are directed.
SUMMARY OF THE INVENTION
The various inventions disclosed, described, and/or claimed herein relate to various genera and subgenera of diketopyrrolopyrrole (DPP) polymers, including homopolymers and/or copolymers, comprising a plurality of repeating units, more than 50 mol. % and up to 100 mol. % of the repeating units are repeating units RU of one or more structural formula(e) complying with general structural formula (I)
(I)
wherein
a) each R1 and R1 is independently selected from normal, branched, or cyclic organic group, such as for example alkyls or fluorinated derivatives thereof;
b) each hAr1 and hAr1 is independently selected from heteroaryls.
Examples of such heteroaryls include the structures
i) a is an integer equal to 1 , 2, 3, or 4;
ii) each X, X', X or X' " is independently selected from O, S, Se,
3 3 3
Si(R )2, and NR , where R is a normal, branched, or cyclic organic group, such as an alkyl group;
iii) each Y, Y', Y" and Y" ' is independently selected from N, and CR4, where R4 is hydrogen, halogen, cyano, or a normal, branched, or
cyclic organic group, such as an alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; and
2 2'
c) each R and R is independently selected from hydrogen, cyano, or
organic group, such as an alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy group.
In some embodiments the DPP polymers defined above can be homopolymers. In other embodiments, the DPP polymers can be various types of copolymers, comprising both the repeating units RU having general structural formula (I) shown above, as well as other types of repeating units, as further described below.
In some embodiments of the DPP polymers, these ones are copolymers the repeating units RU of which are a mix comprising or consisting of repeating units RU1 of specific structural formula (II)
(II)
(ill) wherein
11 11' 21 21'
each R , R , R and R is independently selected from normal, branched, or cyclic alkyls or fluorinated derivatives thereof;
11 11' 21 21'
a) each hAr , hAr , hAr and hAr is independently selected from Ci-
C6o heteroaryls.
In some embodiments, the DPP copolymers are block copolymers wherein
R 112 and R 2"2 are different from each other and/or R 12' and R 22' are different from each other.
Examples of preferred hAr heteroaryls for use in connection with the DPP copolymers comprising a mix of repeat units of formula (II) and formula (III) include the structures
i) a is an integer equal to 1 , 2, 3, or 4;
ii) each X, X', X or X' " is independently selected from O, S, Se,
3 3 3
Si(R )2, and NR , where R is a C1-C30 normal, branched, or cyclic alkyl group;
iii) each Y, Y', Y" and Y" ' is independently selected from N, and CR4, where R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; b) each R 12 , R 12' , R 22 and R 22' is independently selected from hydrogen, cyano, or C1-C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl,
carboxyalkyl, or acetoxy groups,
c) with the proviso that at least one of the radicals R11, R11 , hAr11 and hAr11 included in formula (II) differs from its homologue included in formula (III), respectively R21, R21', hAr21 and hAr21'.
In other embodiments, the DPP copolymers are random copolymers wherein R 12 and R 22 are identical to each other and R 12' and R 22' are identical to each other.
The DPP polymers comprising repeat units of Formula (I), as well as other repeat units having Formulas (II), and (III), and their various subgenera and subspecies, are useful for making electronic devices such as transistors and solar cells, and logic circuits such as inverters and NAND or NOR circuits, and can have an unexpectedly superior ability to conduct electrical current in the form of holes, electrons, or both (i.e. "ambipolar" characteristics). The particular combination of hAr and vinylidene polymer subunits of these DPP polymers are related to the unexpectedly superior properties and utilities.
Further detailed description of various and/or preferred embodiments of the various inventions broadly outlined above will be provided below in the Detailed Description section provided below.
BRIEF DESCRIPTION OF THE FIGURES Figure 1 shows Differential Scanning Calorimeter scans of the HD-PPTV,
HD-PPV, and PPTPV polymers, at a heating rate of 10 °C/min under N2, as described in Example 4.
Figure 2a shows optical absorption spectra of HD-PPTV, HD-PPPV, and PPTPV polymers in dilute toluene solutions. Figure 2b shows optical absorption spectra of HD-PPTV, HD-PPPV, and PPTPV as polymer thin films.
Figure 3a shows optical absorption spectra of HD-PPPV, in dilute toluene solutions and as a thin film. Figure 3b shows optical absorption spectra of PPTPV polymer in dilute toluene solution and as a thin film
Figures 4a, 4b, and 4c show cyclic voltammograms of HD-PPTV (A), HD-PPPV (B), and PPTPV (C).
Figure 5 shows X-ray diffraction patterns of drop-cast films of the three polymers HD-PPTV, HD-PPPV, and PPTPV.
Figure 6a shows output characteristics of the field-effect transistor based on HD-PPTV, after annealing at 150 °C. Figure 6b shows overlays of representative transfer curves (Vds= ±80 V) of the transistors comprising HD- PPTV, HD-PPPV, and PPTPV. See Example 5.
Figure 7 shows the structure and results obtained from inverter circuits fabricated using HD-PPTV as an active ambipolar semiconductor.
Figure 8 shows the structure and results obtained from NAND circuits fabricated using HD-PPTV as an active ambipolar semiconductor.
Figure 9 shows the structure and results obtained from NOR circuits fabricated using HD-PPTV as an active ambipolar semiconductor.
DETAILED DESCRIPTION OF THE INVENTION
The various inventions disclosed, described, and/or claimed herein relate to various genera and subgenera of diketopyrrolopyrrole (DPP) polymers, are useful for making electronic devices such as transistors and solar cells, and logic circuits such as inverters and NAND or NOR circuits, and have an unexpectedly superior ability to conduct electrical current in the form of holes, or electrons, or in some cases both holes and electrons (i.e. "ambipolar" characteristics). It is believed that the particular combination of the DPP, hAr and vinylidene polymer subunits found in these polymers are related to and provide the unexpectedly superior properties, processing characteristics, and/or utilities.
It is also possible to "tune" the identity of the polymer subunits and their substituents so as to generate electronic and physical properties in the solid state to encourage intermolecular π-π stacking and/or long range order in the solid state, which can result in a high mobility of electrical current carriers such as holes, electrons, or both.
The DPP Polymers of Formulas I and Its Subgenera
The diketopyrrolopyrrole (DPP) polymers of the invention, and its various embodiments and subgenera, include homopolymers and/or copolymers that comprise a plurality of repeating units wherein more than 50 mol. % and up to
100 mol. % of the repeating units are repeating units RU of one or more structural formula(e) com lying with general structural formula (I)
wherein
a) each R1 and R1 is independently selected from C1-C30 normal, branched, or cyclic alkyls or fluorinated derivatives thereof;
b) each hAr1 and hAr1 is independently selected from Ci-C6o heteroaryls having the structure
i) a is an integer equal to 1 , 2, 3, or 4;
ii) each X, X', X or X' " is independently selected from O, S, Se,
3 3 3
Si(R )2, and NR , where R is a C1-C30 normal, branched, or cyclic alkyl group;
iii) each Y, Y', Y" and Y" ' is independently selected from N, and CR4, where R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; and
c) each 2 2'
R and R is independently selected from hydrogen, cyano, or Ci-
C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy groups.
The number of repeat units of the DPP polymers can be described by an integer n, which can be any positive integer of 5 or greater, but can also be from 5-10,000, or from about 10-1 ,000. It should be noted however that in most embodiments of the DPP polymers described herein, a plurality of the repeating units, i.e. more than 50 mol. %, or more than 60%, 70%, 80%, 90%, 95%, 98%, or 99%), or up to and/or including 100 mol. % of the repeating units are repeating units RU of one or more structural formula(e) complying with general structural formula (I) described above.
In some embodiments of the DPP polymers, more than 50 mol. %, up to 100 mol. % (preferably, between 90 mol. % and 99 mol. %) of the repeating units are repeating units RU of one and only one specific structural formula complying with general structural formula (I).
In some embodiments of the DPP polymers, more than 50 mol. %, up to less than 100 mol. % of the repeating units are repeating units RU of one and only one specific structural formula complying with general structural formula (I), and between 0 mol. % and 50 mol. % (preferably, between 1 mol. % and 10 mol. %) of the repeating units are repeating units RU* of one or more specific structural formula(e) not complying with general structural formula (I).
Some embodiments the DPP polymers are homopolymers. As used herein, "homopolymer" means that all the repeating units of the polymer are repeating units RU, and that the repeating units RU are of one and only one structural formula complying with general structural formula (I).
However, as will be detailed below, it is also possible to prepare copolymers with mixtures of two or more types of "R1 "substituent groups, and/or two or more types of "hAr" subunits. Accordingly, in some embodiments, the
inventions relate to diketopyrrolopyrrole polymers wherein more than 50 mol. % (preferably, more than 90 mol. %), up to 100 mol. % of the repeating units are repeating units RU of at least two distinct specific structural formulae complying with general structural formula (I).
For example, it is possible to prepare copolymers comprising more than one type of multi-subunit substructure, such as for example diketopyrrolopyrrole copolymers wherein the repeating units RU are a mix consisting of repeating units RU1 of specific structural formula II)
(I I)
and repeating units RU2 of s ecific structural formula (III)
wherein
11 11' 21 21'
each R , R , R and R is independently selected from C1-C30 normal, branched, or cyclic alkyls or fluorinated derivatives thereof;
a) each hAr 11 , hAr 11' , hAr 21 and hAr 21' is independently selected from Ci- C6o heteroaryls having the structure
wherein
i) a is an integer equal to 1 , 2, 3, or 4;
ii) each X, X', X or X' " is independently selected from O, S, Se,
3 3 3
Si(R )2, and NR , where R is a C1-C30 normal, branched, or cyclic alkyl group;
iii) each Y, Y', Y" and Y" ' is independently selected from N, and CR4, where R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; b) each R 12 , R 12' , R 22 and R 22' is independently selected from hydrogen, cyano, or Ci- C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl,
carboxyalkyl, or acetoxy groups,
with the proviso that at least one of the radicals R11, R11 , hAr11 and hAr11 included in formula (II) differs from its homologue included in formula (III), respectively R21, R21', hAr21 and hAr21'.
Some embodiments of the "mixed" copolymers that comprise repeating subunits of formula (II) and formula (III) can be copolymers in which all the repeating units are repeating units RU, said repeating units RU being a mix consisting of repeating units RUl of specific formula (II) and repeating units RU2 of specific formula (III). In some embodiments of the "mixed" copolymers
that comprise repeating subunits of formula II and formula (III), more than 50 mol. %, up to less than 100 mol. % (preferably, between 90 mol. % and 99 mol. %) of the repeating units are repeating units RU consisting of repeating units RU1 of specific structural formula (II) and repeating units RU2 of specific formula (III), and between 0 mol. % and about 50 mol. % of the repeating units are repeating units RU* of one or more structural formula(e) not complying with general structural formula (I). In such embodiments of the "mixed" copolymers that comprise repeating subunits of formula (II) and formula (III), the mole ratio of the repeating units RU1 and RU2 (RU1 :RU2) can range from about 5 % to about 95 % (preferably, from about 30 % to about 70 %).
In the "mixed" copolymers that comprise repeating subunits of formula (II) and formula (III), it should be understood that the copolymers can be either "block" or random copolymers.
The copolymers of formula (II) allow, for example, for the incorporation of more than one kind of "R" substituent on the nitrogen atoms of the DPP subunits, which can beneficially modify the solubility and/or physical characteristics of the polymers for certain applications, in either the solid or solution state. Such "mixed" copolymers can also allow for the presence of multiple types of DPP or hAr groups, which can be beneficial in some applications such as solar cells, where the incorporation of differing hAr groups can be used to tune and/or widen the optical absorption bands of the copolymers, so as to better absorb the entire solar spectrum and thereby increase the efficiency of collection of solar energy.
The DPP polymers and/or copolymers comprise at least one or more, types of l,4-Diketo-2,5-dihydropyrrolo[3,4-c]pyrrole ("DPP") electron accepting subunits having the generic structure
DPP
DPP subunits provide useful properties because the fused lactam group is strongly electron-withdrawing, planar and highly conjugated, and can readily conjugate with the hAr and vinylidene subunits of the polymers, which can result in small bandgaps that are highly beneficial to charge transport properties for applications in transistors and solar cells, and provide broad and intensive absorption of light in the visible to near-IR wavelengths, which is beneficial in applications in solar cells. Varying the substituent at the 2,5- amide nitrogen positions of the DPP subunits (or on the hAr or vinylidene subunits) can be used to tune the solubility, co-planarity, hydrogen bonding interactions, crystallinity, self-assembly, conjugation length, and electronic structure of the DPP polymers and copolymers.
In many embodiments, the terminal "R" groups at the 2,5-positions of the DPP subunits can be independently selected from R1, R1', R11, R11', R21 or R21' groups, which can potentially be selected from a wide variety of organic groups, including C1-C30, C1-C20, or C1-C12, organic groups. Preferably, the R1, R1 , R11,
11' 21 21 '
R , R 1 or R 1 groups are selected from groups that are expected to be thermally and air stable to temperatures up to about 300 °C or higher, and are expected to be stable to oxidation by holes or reduction by electrons at the operating conditions of the electronic devices made therefrom, such as optionally substituted alkyls, perfluoroalkyls, alkoxy, perfluoroalkoxy, aryls, heteroaryls, alkylaryls, alkyheteroaryls, and the like. In some embodiments, each R1, R1 , R11,
R 11' , R 21 or R 21 ' is independently selected from normal, branched, or cyclic alkyls or fluorinated derivatives thereof. In some preferred embodiments, each R1, R1 ,
R 11 , R 11' , R 21 or R 21' group is a normal or branched alkyl or perfluoroalkyl. In some embodiments, each R1, R1 , R11, R11 , R21 or R21 group is the same group.
The DPP polymers and/or copolymers also comprise independently selected hAr1, hAr1' hAr11, hAr11', hAr21 and hAr21' "heteroaryl" subunits in the copolymer backbone, each of which comprise five membered heteroaryl rings directly bonded to the DPP subunit. This arrangement is believed to present fewer unfavorable steric interactions (related to possible substituents in neighboring a-positions) with the neighboring DPP subunits than would a comparable six -membered aryl or heteroaryl, ring. Without wishing to be bound
by theory, it is believed that binding less sterically demanding five membered heteroaryl rings to the DPP group increase the probability of co-planarity and/or π conjugation of the hAr1, hAr1 , hAr11, hAr11 , hAr21 and hAr21 groups relative to the DPP groups bonded thereto, at least as compared to the higher steric demands that could be presented by potentially six -membered aryl or heteroaryl groups bonded to DPP. The improved potential for co-planarity is also believed increase the potential for improved intermolecular π-π stacking between the copolymer chains in the solid state.
The hAr heteroaryl subunits and/or rings comprise at least one carbon atom and at least one heteroatom selected from O, N, S, Se, and Si, as part of a π- conjugated aromatic ring or ring system. The hAr1, hAr1 hAr11, hAr11 , hAr21
21 '
and hAr "heteroaryl" subunits can also comprise one or more additional optionally substituted aryl or heteroaryl groups bonded or fused to the five membered heteroaryl ring that is directly bonded to the diketopyrrolopyrrole subunit, so as to form multi-ring or fused ring hAr subunits, as further discussed below. In many embodiments, the hAr1, hAr1 , hAr11, hAr11 , hAr21 and hAr21 "heteroaryl" subunits, including any optional substituents, are Ci-C6o, C1-C30, C1-C20, or C1-C12 subunits.
The hAr1, hAr1 ', hAr11, hAr11 ', hAr21 and hAr21 ' "heteroaryl" subunits can nevertheless optionally comprise one or more peripheral substituent groups such as for example cyano, alkyl, alkoxy, thioalkyl, or thioalkoxy substituent groups, so as to provide the potential to vary the electronic characteristics and/or solubility of the resulting copolymers. Preferably any such optional substitutent groups for the hAr1, hAr1 ', hAr11, hAr11 ', hAr21 and hAr21 ' "heteroaryl" subunits are not bonded to the five membered heteroaryl ring that is directly bonded to the DPP group, so as to minimize possible steric interactions with the DPP group.
In many embodiments, the hAr1, hAr1 ', hAr11, hAr11 ', hAr21 and hAr21 ' "heteroaryl" groups can be independently selected from one or all of the heteroaryls having the structure
wherein
i) a is an integer equal to 1, 2, 3, or 4, or in some embodiments 1 or 2; ii) each X, X', X or X' " is independently selected from O, S, Se,
3 3 3
Si(R )2, and NR , where R is a C1-C30 normal, branched, or cyclic alkyl group; and
iii) each Y, Y', Y"and Y' " can be independently selected from N, and CR4, where R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group.
It should be noted that for the hAr1, hAr1', hAr11, hAr11', hAr21 and hAr21' heteroaryl groups shown immediately above, depending on the selection of the X, X', X" or X' " groups, and Y, Y', Y"and Y' " groups, the resulting heterocycles can have a variety of physical, solubility, and electronic characteristics (from electron donating to electron withdrawing) that can be used to tune the characteristics, hole or electron transporting characteristics, and/or optical bandgaps of the resulting polymers and copolymers.
In many embodiments, examples of species of the various hAr1, hAr1',
11 11' 21 21'
hAr , hAr , hAr and hAr heteroaryl groups whose structures are shown
generically above can be exemplified by one of the heterocyclic structures shown below:
wherein R3 is a C1-C30 normal, branched, or cyclic alkyl group, R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group.
For example, in some embodiments, each hAr1 and hAr1 is independently selected from
wherein R4 and R4 are hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group groups.
In additional embodiments, each hAr1 and hAr1 is independently selected
3 3' 4 wherein R and/or R is a C1-C30 normal, branched, or cyclic alkyl group, and R and/or R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group.
In additional embodiments, each hAr1 and hAr1 is independently selected from
wherein R4 and R4 are hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group groups.
In additional embodiments, each hAr1 and hAr1 is independently selected
alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group groups.
In some embodiments of the copolymers comprising repeating units RUl of specific structural formula (II) and repeating units RU2 of specific structural formula (III), eac 11 and hAr11 can be independently selected from
and each hAr 21 and hAr 21' can be independently selected from
wherein each X, X', X or X' " and each Y, Y', Y" and Y" ' can have any of the meanings already detailed above.
In some preferred embodiments of the copolymers comprising repeating units RUl of specific structural formula (II) and repeating units RU2 of specific structural formula (III), each hAr11 and hAr11 can be independently selected from
and each hAr 21 and hAr 21' can be independently selected from
3 3' 4 wherein R and/or R is a C1-C30 normal, branched, or cyclic alkyl group, and R and/or R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group.
The DPP polymers described herein also comprise an optionally substituted vinylidene subunit having the structure:
2 2'
wherein each R and R group is independently selected from hydrogen, cyano, or C1-C30 alkyl, perfluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy
2 2'
groups. In many embodiments, each R and R group is hydrogen.
Such ethylene subunits are believed to play an important role in contributing to the electronic and physical properties of the DPP polymers comprising repeat units of formulas (I), and copolymers comprising repeat units of formulae (II) and (III), because they can allow for extended conjugation along the polymer or copolymer backbone in a very sterically non-demanding fashion, which allows for high co-planarity among the subunits of the DPP polymers and copolymers, which allows for a high degree of intermolecular π-π stacking in the solid state, which can lead to dramatic increases in hole or electron mobility in the solid state. The ethylene subunits can also function to increase the solubility and/or improve the solution processing characteristics of the DPP polymers or copolymers.
Further and additional types of repeating units RU may be present in the DPP polymers or copolymers described herein other than the repeating units of
general structural formulae (I), (II), and/or (III), in amounts that are less than about 50 (or 40, or 30 or 20 or 10 or 5 or 2 or 1) mol. %. Such other repeating units often comprise a wide variety of difunctional heteroaryl groups. For example, the DPP polymers and/or copolymers of the invention can optionally comprise between 0 mol. % and 50 mol. % of the repeating units are repeating units RU* of one or more of the following general formulae
Formula (IV)
Formula (V)
wherein a, and each X or X', and each Y or Y' can have any of the meanings already detailed above. Preferred examples of such repeat units of formulae (IV) and (V) can include heterocycles having the structures shown below.
wherein R4 and R4 can be independently selected from hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group groups.
With respect to the properties of the various embodiments of the DPP polymers or copolymers described herein, those DPP polymers or copolymers can in some embodiments have a number average degree of polymerization of at least 10, as determined by gel permeation chromatography using polystyrene calibration standards. Similarly, the DPP polymers or copolymers described herein can in some embodiments have a number average degree of polymerization of at most 100, as determined by gel permeation chromatography using polystyrene calibration standards.
In many embodiments, the various DPP polymers and/or copolymers described herein can have unexpectedly high solubility in common organic solvents, which can be of great benefit to adjust ink formulation to a large variety of solution printing methods (such as ink jet printing, etc) for the preparation of desirable organic electronic devices, such as photovoltaic cells and/or transistors. Thus, in some embodiments, the DPP polymers and/or copolymers can have solubility at one or more temperature(s) selected in the range from 25 °C to 50 °C, such as 35°C, in at least one solvent selected from the group consisting of toluene, xylene, mesitylene, tetrahydrofuran, chloroform, chlorobenzene, dichlorobenzene and mixtures thereof, of at least 10 mg/ml, preferably at least 20 mg/ml. For example:
- the DPP polymers and/or copolymers can have solubility at 25 °C and/or at
50 °C in toluene of at least 10 mg/ml;
- the DPP polymers and/or copolymers can have solubility at 25 °C and/or at
50 °C in tetrahydrofuran of at least 10 mg/ml;
and/or
- the DPP polymers and/or copolymers can have solubility at 25 °C and/or 50 °C in a mixture consisting of chloroform and chlorobenzene in a 50:50 wt. ratio of at least 10 mg/ml.
Synthesis of the Polymers and/or Copolymers
Generic synthetic schemes for making polymerizable comonomers required to synthesize the polymers and/or copolymers of the invention are presented below, and specific examples of such generic synthetic methods are also provided below in the "Examples" section of this disclosure.
The synthetic diagram below illustrates two general methods for making polymerizable brominated bis-heteroaryl substituted diketopyrrolopyrrole comonomers that are suitable for making the polymers of the inventions. The starting heteroaryl nitriles can often be made by many methods known to those of ordinary skill in the art, such as for example from the corresponding heteroaryl aldehydes or brominated hetereoaryl aldehydes, by condensation with hydroxylamine, or by condensation of cyanides with heteroaryl diazonium salts (Mowry, D. T. Chem. Rev. 1948, 42, 189-283.; Barclay, R. M.; Cordes, A. W.; MacKinnon, C. D.; Oakley, R. T.; Reed, R. W. Chem. Mater. 1997, 9, 981-990.),
a. KOtBu, diethyl succinate, t-amyl alcohol, 120 °C.
b. R^Br, K2C03, DMF, 130 °C.
c. NBS, DMF.CHCI3
Polymerizable brominated bis-heteroaryl substituted diketopyrrolopyrrole comonomers such as ml and m2 in the diagram above can each be condensed (optionally in the presence of one or more additional dibrominated hetereroaryl comonomers) with trans- 1 ,2-bis(tributylstannyl)ethylenes via the well known palladium catalyzed Stille condensation, to yield the polymers comprising the repeat units of formula (I).
Accordingly, in some embodiments the inventions described herein relate to methods for making the polymers described herein, comprising the steps of a) obtaining or providing at least one bis-heteroaryl substituted
wherein LG is a leaving group such a bromine atom, an iodide atom, an organic sulonic acid group, or the like ;
b) obtaining or providing at least one bis-leaving group substituted ethylene monomer having the structure
2
wherein LG is a leaving group capable of being eliminated by reacting with LG 1 , such as SnR 2" 2"
3 wherein R is an alkyl or aryl group; c) reacting a mixture comprising the at least one first bis-heteroaryl
substituted diketopyrrolopyrrole monomer and the at least one bis-leaving group substituted ethylene monomer in the presence of a transition metal catalyst complex, to produce the polymers comprising the repeat units of formula (I).
Such transition metal catalyzed couplings/polymerization reactions s are well known in the art, especially in the presence of nickel or palladium catalysts. For example, suitable palladium catalyzed coupling/polymerization methods include Stille couplings between aryl or heteroaryl halides (i.e. LG1 = bromide or iodide) with organotin compounds (LG 1 is SnR 2" 2"
3 wherein R is an alkyl or aryl group), or Suzuki couplings between aryl or heteroaryl halides (i.e. LG1 = bromide or iodide) with organo-boronic acid ester compounds (i.e. LG = B(OR)2 wherein R is an alkyl group, or Hyama couplings between aryl or heteroaryl halides (i.e. LG1 = bromide or iodide) with organo-silicons
2 2" 2"
compounds (i.e. LG = SiR 3 wherein R is an alkyl or aryl group.
Typical soluble palladium catalyst complexes useful in such catalytic coupling/polymerization reactions include palladium acetate, tetrakis
(triphenylphosphine)palladium(O), [1 ,1 '-bis(diphenylphosphino)
ferrocene]palladium(II) dichloride, or bis(triphenylphosphine)palladium(II) dichloride, and Tris(dibenzylideneacetone)dipalladium(0) (iePd2(dba)3), typically at a concentration of about 0.1 to about 20 wt%, and optionally in the presence of additional and/or excess phosphine ligands, such as triarylphosphines.
In some embodiments, the reaction mixture is substantially free of any monomer other than the at least one first bis-heteroaryl substituted
diketopyrrolopyrrole monomer and the at least one bis-leaving group substituted ethylene monomer.
In many embodiments of the methods for making the polymers of the invention, the reaction mixture comprises susbtantially equimolar amounts of the at least one bis-heteroaryl substituted diketopyrrolopyrrole monomer(s) and the at least one bis-leaving group substituted ethylene monomer(s). However, in other embodiments, other co-monomers can be present in the reaction mixture, to make copolymers. For example, in some embodiments, the reaction mixture further comprises at least one self-reacting monomer having the structure
LG1 - hAr* - LG2
1 2
wherein hAr* is a Ci-C6o heteroaryl group, and LG and LG are leaving groups as described above. Examples of suitable Ci-C6o heteroaryls include the structures
herein above.
In such embodiments, the self-reacting monomer can, to at least some extent, self-condense in the presence of the transition metal catalysts, to form copolymer blocks (within the DPP copolymers of the invention) having the structure
-( hAr* )x-.
wherein x is an integer greater than one, and preferably in the range from 2 to 100.
Alternatively, mixtures of DPP/hAr comonomers such as ml or m2 shown below can be condensed with bis(tributylstannyl)ethylenes to yield random or block copolymers comprising at least some repeat units comprising formulae (II) and/or (III). Accordingly, in some embodiments, the inventions described herein relate to methods for making random copolymers, comprising the steps of a) obtaining or providing at least a first bis-heteroaryl substituted
diketopyrrolopyrrole monomer having the structure
b) obtaining or providing at least a second bis-heteroaryl substituted
diketopyrrolopyrrole monomer having the structure
obtaining or providing at least one bis-leaving group substituted ethylene monomer having the structure
wherein LG is a leaving group capable of being eliminated by reacting with LG1 , d) reacting a mixture comprising the at least first and second bis-heteroaryl substituted diketopyrrolopyrrole monomers and the at least one bis- leaving group substituted ethylene monomer in the presence of a transition metal catalyst complex, to produce the copolymers.
In some embodiments, such DPP copolymers are random copolymers wherein R 11 and R 11' are identical to each other and R 21 and R 21' are identical to each other. In other embodiments, such DPP copolymers are block copolymers
wherein R 11 and R 21 are different from each other and/or R 11' and R 21' are different from each other.
While copolymers prepared by such methods can be random or nearly random in the sense that the sequence of DPP/hAr repeat units can be random or near random, it is also possible to intersperse into such copolymers additional random hAr* repeat units, by including in the reaction mixture at least one co monomer having the structure
LG1 - hAr* - LG1
wherein the possible structures of hAr* and LG1 have already been previously described above.
Specific examples of the synthesis of such polymers, and their properties are provided below in the Example section.
Organic Electronic Devices Comprising the Polymers and/or Copolymers
Some aspects of the present inventions relate to novel organic electronic devices comprising the polymers of Formulas (I) or copolymers comprising repeat units having Formulas (II) and (III) described herein, including transistors and various logic circuits comprising transistors, and solar cells. Each of those end use applications typically require the formation of a film of the copolymers of the invention on a substrate. Organic films of the polymers and copolymers of the present invention can be prepared by known methods such as spin coating methods, casting methods, dip coating methods, inkjet methods, doctor blade coating methods, screen printing methods, and spray coating methods. By using such methods, it becomes possible to prepare organic films having good properties such as mechanical strength, toughness, and durability without forming cracks in the films. Therefore, the organic films can be preferably used for organic electronic devices such as photovoltaic cells, and OFET elements.
Films of the polymers comprising repeat units of Formulas I or copolymers comprising repeat units of formulae (II), (III), (IV) and (V) are typically prepared by coating a coating liquid, which is prepared by dissolving the copolymer (and in the case of solar cells, typically an electron accepting material such as a fullerene derivative such as PCBM) in a solvent such as dichloromethane,
tetrahydrofuran, chloroform, toluene, chlorobenzene, dichlorobenzene, or xylene, on a substrate. Specific examples of the coating methods include spray coating methods, spin coating methods, blade coating methods, dip coating methods, cast coating methods, roll coating methods, bar coating methods, die coating methods, ink jet methods, dispense methods, etc. In this regard, a proper method and a proper solvent are selected in consideration of the properties of the polymer used. Suitable materials for use as the substrate on which a film of the polymer of the present invention is formed include inorganic substrates such as glass plates, silicon plates, ITO plates, and FTO plates, and organic substrates such as plastic plates (e.g., PET films, polyimide films, and polystyrene films) , which can be optionally subjected to a surface treatment. It is preferable that the substrate has a smooth surface.
The thickness of the organic film and the organic semiconductor layer of the organic thin film transistor of the present inventions are not particularly limited. However, the thickness is determined such that the resultant film or layer is a uniform thin layer (i. e., the film or layer does not include gaps or holes adversely affecting the carrier transport property thereof). The thickness of the organic semiconductor layer is generally not greater than 1 micron, and preferably from 5 to 200 nm.
Making Transistors Comprising the Polymers and/or Copolymers of the Invention.
The organic thin film transistors of the present inventions typically have a configuration such that an organic semiconductor layer including the polymer comprising repeat units of formula (I) or copolymers comprising repeat units of formulae (II), (III), (IV), and/or (V) can be formed on a substrate, while also contacting the source electrode, drain electrode, gate electrode and insulating layer of the transistor.
The organic film prepared above is typically annealed. Annealing is performed while the film is set on a substrate, and is believed to allow for improved ordering of the copolymer molecules in the solid state. The annealing temperature is determined depending on the property of the material constituting the substrate, but is preferably from room temperature to 300 °C, and more
preferably from 50 to 300 °C. In many embodiments, the transistors and/or circuits comprising them are thermally annealed at temperatures between about 100 and 250 °C. When the annealing temperature is too low, the organic solvent remaining in the organic film cannot be well removed therefrom. In contrast, when the annealing temperature is too high, the organic film tends to be thermally decomposed. Annealing is preferably performed in a vacuum, nitrogen, argon or air atmosphere. It is also possible to perform annealing in an atmosphere including a gas of an organic solvent capable of dissolving the polymer because the molecular motion of the polymer is accelerated, and thereby a good organic thin film can be prepared. The annealing time is properly determined depending on the aggregation speed of the polymer.
An insulating layer is used for the gate of the organic thin film transistors comprising the polymers and/or copolymers of the present invention. Various insulating materials can be used for the insulating layer. Specific examples of the insulating materials include inorganic insulating materials such as silicon oxide, silicon nitride, aluminum oxide, aluminum nitride, titanium oxide, tantalum oxide, tin oxide, vanadium oxide, barium strontium titanate, barium zirconate titanate, lead zirconium titanate, lead lanthanum titanate, strontium titanate, barium titanate, barium magnesium fluoride, bismuth tantalate niobate, hafnium oxide, and trioxide yttrium; organic insulating materials such as polymer materials, e.g., polyimide, polyvinyl alcohol, polyvinyl phenol, polystyrene, polyester, polyethylene, polyphenylene sulfide, unsubstituted or halogen-atom substituted polyparaxylylene, polyacrylonitrile, and cyanoethylpullulan; etc. These materials can be used alone or in combination. Among these materials, materials having a high dielectric constant and a low conductivity are preferably used.
Suitable methods for forming such an insulating layer include dry processes such as CVD methods, plasma CVD methods, plasma polymerization methods, and vapor deposition methods; wet processes such as spray coating methods, spin coating methods, dip coating methods, inkjet coating methods, cast coating methods, blade coating methods, and bar coating methods; etc.
In order to improve the adhesion between the insulating layer and organic semiconductor layer, to promote charge transport, and to reduce the gate voltage and leak current, an organic thin film (intermediate layer) can be formed between the insulating layer and organic semiconductor layer. The materials for use in the intermediate layer are not particularly limited as long as the materials do not chemically affect the properties of the organic semiconductor layer, and for example, molecular films of organic materials, and thin films of polymers can be used therefor. Specific examples of the materials for use in preparing the molecular films include coupling agents such as octadecyltrichlorosilane, octyltrichlorosilane, octyltrimethoxysilane, hexamethyldisilazane (HMDS), and octadecylphosphonic acid. Specific examples of the polymers for use in preparing the polymer films include the polymers mentioned above for use in the insulating layer. Such polymer films can serve as the insulating layer as well as the intermediate layer.
The materials of the electrodes (such as gate electrodes, source electrodes and drain electrodes) of the organic thin film transistor of the present invention are not particularly limited as long as the materials are electrically conductive. Specific examples of the materials include metals such as platinum, gold, silver, nickel, chromium, copper, iron, tin, antimony, lead, tantalum, indium, aluminum, zinc, tungsten, titanium, calcium, and magnesium; alloys of these metals;
electrically conductive metal oxides such as indium tin oxide (ITO); inorganic or organic semiconductors, whose electroconductivity is improved by doping or the like, such as silicon single crystal, polysilicon, amorphous silicon, germanium, graphite, carbon nanotube, polyacetylene, polyparaphenylene, polythiophene, polypyrrole, polyaniline, polythienylenevinylene,
polyparaphenylenevinylene, and complexes of polyethylenedioxythiophene (PEDOT) and polystyrene sulfonic acid.
The average hole mobility as measured from exemplary transistors comprising HD-PPTV as described below in Examples 1 and 6 were in the narrow range of 0.091-0.17 cm 2 V -"1 s -"1 and the average electron mobility was 0.012-0.019 cm2 V"1 s"1 with current on/off ratios of 102-103. These values of hole and electron mobility and resulting ambipolar behavior in the transistors
were unexpectedly high as compared with the prior art known to the inventors. The threshold voltage of hole and electron transport are rather positive, having - 9.0 - -1.6 V for p-channel operation and 22.7-32.1 V for n-channel mode.
The DPP polymers and copolymers described herein can have unexpectedly superior hole mobility of about 0.1 cm 2 V -"1 s -"1 or greater, as measured from a thin film transistor having a bottom gate, bottom contact geometry, employing doped silicon as a gate material, silicon dioxide as a gate dielectric, using gold source and drain electrodes with a chromium adhesive layer at a channel width of 400-800 μιη and lengths of 20-40 μιη, and employing the copolymers as the active semiconductor.
Similarly, the DPP polymers and copolymers described herein can have unexpectedly superior hole mobility electron mobility of about 0.01 cm /Vsec or greater, as measured from a thin film transistor having a bottom gate, bottom contact geometry, employing doped silicon as a gate material, silicon dioxide as a gate dielectric, using gold source and drain electrodes with a chromium adhesive layer at a channel width of 400-800 μιη and lengths of 20-40 μιη, and employing the copolymers as the active semiconductor.
Some DPP polymers and/or copolymers described above exhibit ambipolar characteristics, and can have both equilibrated hole mobility and electron mobility of about 0.01 cm 2 V -"1 s -"1 or greater, as measured from a thin film transistor having a bottom gate, bottom contact geometry, employing doped silicon as a gate material, silicon dioxide as a gate dielectric, using gold source and drain electrodes with a chromium adhesive layer at a channel width of 400- 800 μιη and lengths of 20-40 μιη, and employing the copolymers as the active semiconductor. While the on/off ratios and threshold voltages observed from ambipolar transistors comprising a single active layer of HD-PPTV as described in Example may not be optimum for some applications, transistors comprising ambipolar materials with such high hole and electron mobilities can be suitable and unexpectedly economically superior for use in certain low performance and low cost applications, such as RFID tags. We have clearly demonstrated that not only an inverter with two ambipolar transistors but also more complicated electronic logic gates consisting of 3- or more ambipolar transistors and having
2- or more input signals, such as NAND- and NOR-gates, can be constructed by implementing ambipolar polymer semiconductors such as HD-PPTV.
Making Integrated Circuits Comprising the Polymers and/or
Copolymers of the Invention.
The complementary integrated circuits, such as inverter, NAND logic circuits, NOR logic circuits, and so on, comprising the copolymer of the invention typically have one or more transistors which may function as either n- channel or p-channel device. The constituent transistors of an integrated circuit are electrically connected on a common substrate or separated substrates. The constituent materials of a transistor such as electrodes, insulators, and DPP polymers and copolymers described herein are formed by photolithography, soft lithography, spin-coating, printing methods, vapor deposition, and/or self- assembly.
Making Solar Cells Comprising the DPP Polymers and/or
Copolymers.
Solar cells comprising the DPP polymers and/or copolymers described above, and typically also an electron accepting material such as a fullerene or fullerene derivative such as PCBM, are an aspect of the inventions described herein.
Such cells can typically be fabricated by first spin-coating a PEDOT buffer layer on top of ITO-coated glass substrates at 1500 rpm for 60 s and dried at 150°C for 10 min under vacuum to form an anode. The thickness of PEDOT layer should be around 40 nm.
The solvents used for dissolving the mixture of polymers and/or copolymers of the invention and the electron acceptors can be chloroform, chlorobenzene, 1 ,2-dichlorbenzene, etc. The solvents for copolymer/fullerene blend can be a single solvent such as chloroform, chlorobenzene, 1 ,2- dichlorbenzene or a mixture of two or three different solvents, the second (third) solvent can be 1,8-diiodooctane, 1,8-dibromoctane, 1,8-octanedithiol, etc. The active blend layer (copolymer and fullerene blend) can be spin-coated on top of the PEDOT layer, from a copolymer/fullerene blend solution at a speed of 1000 rpm for 30 s, and then annealed on a hot plate at 150 ± 40 °C for 10 min in a
glove box. The active layer can also be spin-coated in air and dried in a vacuum oven without thermal annealing.
After cooling down, the copolymer coated substrates are taken out of the glove box and loaded in a thermal evaporator for the deposition of the cathode. The cathodes consisting of 1.0 nm LiF and 80 nm aluminum layers can be sequentially deposited through a shadow mask on top of the copolymer/electron acceptor active layers in a vacuum of 8x 10~7 torr.
EXAMPLES
The various inventions described above are further illustrated by the following specific examples, which are not intended to be construed in any way as imposing limitations upon the scope of the invention disclosures or claims attached herewith. On the contrary, it is to be clearly understood that resort may be had to various other embodiments, modifications, and equivalents thereof which, after reading the description herein, may suggest themselves to one of ordinary skill in the art without departing from the spirit of the present invention or the scope of the appended claims.
Materials. Trans- l,2-bis(tributylstannyl)ethylene was purchased from
Alfa Aesar and used as received. Anhydrous chlorobenzene and other chemicals were purchased from Aldrich and used as received.
3,6-bis(5-bromo-thiophen-2-yl)-2,5-bis(2-hexyldecyl)pyrrolo[3,4- c]pyrrole-l,4-dione (ml) was prepared by the methods reported by Bwrgi, L.;
Turbiez, M.; Pfeiffer, R.; Bienewald, F.; Kirner, H.-J.; Winnewisser, C. Adv.
Mater. 2008, 20, 2217, and/or Bijleveld, J. C; Zoombelt, A. P.; Mathijssen, S. G.
J.; Wienk, M. M.; Turbiez, M.; de Leeuw, D. M.; Janssen, R. A. J. /. Am. Chem. Soc. 2009, 131, 16616. 3,6-bis(4-bromophenyl)-2,5-bis(2-hexyldecyl)- pyrrolo[3,4-c]pyrrole-l,4-dione (m2) was synthesized by the method of Fukuda,
M.; Kodama, K.; Yamamoto, H.; Mito, K. Dyes and Pigments 2004, 63, 115.
Characterization. 1H NMR spectra were recorded on a Bruker-AF300 spectrometer at 300 MHz. UV-visible absorption spectra were recorded on a Perkin-Elmer model Lambda 900 UV/vis/near-IR spectrophotometer. The photoluminescence (PL) emission spectra were obtained with a Photon
Technology International (PTI) Inc. model QM-2001-4 spectrofluorometer. The molecular weights reported for the polymers were determined on a Polymer Lab gel permeation chromatograph (GPC) Model 120 (DRI, PL-BV400HT Viscometer) against polystyrene standards in chlorobenzene at 60 °C. X-ray diffraction (XRD) patterns were obtained on a Bruker AXS D8 Focus diffractometer with Cu K beam (40 kV, 40 mA; =0.15418 nm). Data were obtained from 2 angles of 2-35° at a scan rate of 0.01°/s. The d-spacing was calculated from the equation, n = 2d sin . Differential scanning calorimetry (DSC) scans were obtained on TA Instrument Q20 at a heating rate of 10 °C/min. Cyclic voltammetry experiments were done on an EG&G Princeton Applied Research Potentiostat/Galvanostat (Model 273A) using 0.1 M tetrabutylammonium hexafluorophosphate (Bu4NPF6) in acetonitrile as electrolyte. A three-electrode cell was used in all experiments. Platinum wire electrodes were used as both counter and working electrodes and silver/silver ion (Ag in 0.1 M AgN03 solution, Bioanalytical System, Inc.) was used as a reference electrode. The Ag/Ag+ (AgN03) reference electrode was calibrated at the beginning of the experiments by running cyclic voltammetry on ferrocene as the internal standard. The potential values obtained in reference to Ag/Ag+ electrode were then converted to the saturated calomel electrode (SCE) scale. The films of the polymers were coated onto the working electrode by dipping a Pt wire into a 10 wt% solution in chloroform/chlorobenzene and drying for 30 min.
Example 1 - Synthesis of Poly[3,6-(2,5-bis(2-hexyldecyl)pyrrolo[3,4- c]pyrrole-l,4-dione)-a/t-l,2-bis-(2'-thienyl)vinyl-5',5''-diyl], HD-PPTV.
HD-PPTV a. KOtBu, diethyl succinate, t-amyl alcohol, 120 °C.
b. 2-hexyldecyl bromide, K2C03, DMF, 130 °C.
c. NBS, DMF.CHCI3
d- Bu,Sn^^ . Pd2 dba)3, P(„-toyl)3, 120 °C.
Poly[3,6-(2,5-bis(2-hexyldecyl)pyrrolo[3,4-c]pyrrole-l,4-dione)-a/t-l,2-bis- (2'-thienyl)vinyl-5',5"-diyl], HD-PPTV. 3,6-Bis(5-bromo-thiophen-2-yl)-2,5- bis(2-hexyldecyl)pyrrolo[3,4-c]pyrrole-l,4-dione (ml) (480 mg, 0.53 mmol) and trans- l,2-bis(tributylstannyl)ethylene (320.8 mg, 0.53 mmol) was dissolved in anhydrous chlorobenzene (18 rtiL) and purged with Ar. The catalyst of Pd2(dba)3 (9.7 mg, 0.01 mmol) and the ligand P(o-toyl)3 (12.9 mg, 0.04 mmol) were added and degassed. After purging with Ar for 5 min, the solution was refluxed at 120°C for 2 days. The solution was then quenched and precipitated into methanol. The solid was filtered and underwent Soxhlet extraction with acetone and dried in vacuum oven to afford a dark green solid (398 mg, yield: 95%). 1H NMR (CDC13), (ppm): 8.93 (br, 2H), 7.01 (br, 4H), 4.09 (br, 4H), 1.97 (br, 2H), 1.25 (48H), 0.87 (br, 12H).
Comparative Example 2 - Synthesis of Poly[3,6-(2,5-bis(2- hexyldecyl)pyrrolo[3,4-c]pyrrole-l,4-dione)-a/M,2-bisphenylvinyl-4',4"- -PPPV.
a. KOtBu, diethyl succinate, t-amyl alcohol, 120 °C.
b. 2-hexyldecyl bromide, K2C03, DMF, 130 °C.
c. BS, DMF.CHCI3.
d- Bu,Sn^=^ > Pd 2(dba)3, P(«-toyl)3, 120 °C.
Poly[3,6-(2,5-bis(2-hexyldecyl)pyrrolo[3,4-c]pyrrole-l,4-dione)-aZi-l,2- bisphenylvinyl-4',4"-diyl], HD-PPPV, which comprises six-membered phenyl rings at the positions of the"hAr" subunits, was prepared by the reaction above, in a manner analogous to that employed in Example 1. Purple solid, yield: 47%. 1H NMR (CDCI3), (ppm): 7.63 (br, 8H), 7.09(br, 2H), 3.85 (br, 4H), 1.7-1.25 (br, 50H), 0.89 (br, 12H).
Comparative Example 3 - Synthesis of Poly[3,6-(2,5-bis(2- hexyldecyl)pyrrolo[3,4-c]pyrrole-l,4-dione)-a/M,2-bisphenylvinyl-4',4"- diyl], HD-PPPV.
d. Pd2(dba , P(o-toyl , 120 oC.
Poly{ [3,6-(2,5-bis(2-hexyldecyl)pyrrolo[3,4-c]pyrrole- l,4-dione)-alt- l,2- bisphenylvinyl-4',4"-diyl]-co-3,6-(2,5-bis(2-hexyldecyl)pyrrolo[3,4-c]pyrrole- l,4-dione)-alt- l,2-bis-(2'-thienyl)vinyl-5',5"-diyl] }, PPTPV, which comprises six-membered phenyl rings at some of the positions of the "hAr" subunits, was synthesized as shown above. Black solid, yield: 33%. 1H NMR (CDCI3), (ppm): 8.92 (br, 1H), 7.84-6.74 (br, 7H), 4.07 (br, 2H), 3.82 (br, 2H), 1.94-1.25 (br, 49H), 0.87 (br, 12H). The actual composition (x) of the random copolymer was calculated to be 0.5 using integration of the -methylene proton resonance as follows: x= (4.07)/[ (4.07)+ (3.82)]..
Example 4 - Physical Properties of the HD-PPTV, HD-PPPV, and PPTPV Copolymers
Both HD-PPTV and PPTPV have good solubility in common organic solvents such as chloroform, chlorobenzene, and 1,2-dichlorobenzene at high concentrations (10-20 mg/mL) whereas HD-PPPV shows only poor solubility (< 2 mg/mL). The number- average molecular weights ( n) of three copolymers were in the range of 21 800 - 88 800 g/mol with polydispersity indices (PDI) of 2.32-3.60, as summarized in Table 1.
HD-PPPV 206 000 88 800 2.32 2.00 -3.13 -5.48 2.35
HD-PPTV 154 000 56 200 2.74 1.22 -3.34 -5.14 1.80
PPTPV 78 500 21 800 3.60 1.26 -3.31 -5.21 1.90
The lactam-containing DPP-based copolymers HD-PPTV, HD-PPPV, and PPTPV demonstrated good thermal stability, but no clear thermal transition between 0 to 350 °C was observed for the three poly(arylene vinylene)s by differential scanning calorimetry (See Figure 1).
Photophysical Properties. The optical absorption spectra of the three HD-PPTV, HD-PPPV, and PPTPV copolymers in dilute solution (Ixl0~6 -lxl0~5 M) and as thin films were shown in Figures 2a and 2b. In toluene solution (Figure 2a), HD-PPPV and HD-PPTV exhibit absorption maxima ( max) at 538 nm and 887 nm, respectively. In thin films (Figure 2b), HD-PPPV and HD- PPTV exhibit max at 537 nm and 814 nm, respectively. HD-PPPV has an optical bandgap of 2.00 eV, lower than the reported 2.09-2.13 eV for poly(l,4-diketo- 2,5-dialkyl-3,6-diphenylpyrrolo[3,4-c]pyrrole) (see Rabindranath, A. R.; Zhu, Y.; Heim, I.; Tieke, B. Macromolecules 2006, 39, 8250). Similarly, HD-PPTV has an optical bandgap of 1.22 eV, slightly lower than the reported 1.25 eV for poly(l,4-diketo-2,5-diocty-3,6-bis(thiophen-5-yl)pyrrolo[3,4-c]pyrrole) (See Bijleveld, J. C; Zoombelt, A. P.; Mathijssen, S. G. J.; Wienk, M. M.; Turbiez, M.; de Leeuw, D. M.; Janssen, R. A. J. J. Am. Chem. Soc. 2009, 131, 16616).
The photoluminescence (PL) emission spectra of HD-PPPV and PPTPV were shown in Figures 3a and 3b. The PL spectrum of HD-PPTV was not obtained, probably because of weakness beyond the detection limit, and/or quenching in the solid state. HD-PPPV is red-emitting with PL maxima at 647 and 653 nm in dilute solution and as thin film, respectively. This HD-PPPV polymer has large Stoke shift of 109 and 116 nm in dilute solution and as thin films similar to other DPP-based polymers. Similar to HD-PPPV, the random copolymer PPTPV has a weak PL peak centered at 651 nm in dilute solution, but the photoluminescence is almost completely quenched in the thin film.
Electrochemical Properties. Cyclic voltammetry (CV) was used to preliminarily evaluate the electronic structure and the energy levels of HD- PPTV, HD-PPPV, and PPTPV polymers. Both reduction and oxidation scans of
the three copolymers showed quasi-reversible process as shown in Figures 4a, 4b, and 4c.
The electron structure and energy levels extracted from of the CV scans are summarized in Table 1 above. HD-PPPV has a low-lying HOMO energy level of -5.48 eV compared to HD-PPTV (-5.14 eV) and PPTPV (-5.21 eV). Also, HD-PPPV has a high-lying LUMO energy level of -3.13 eV compared to -3.34 and -3.31 eV in HD-PPTV and PPTPV. Thus, HD-PPPV shows a much larger electrochemical bandgap of 2.35 eV than thel .80-1.90 eV of HD-PPTV and PPTPV. The HOMO/LUMO energy levels of PPTPV are similar to those of HD- PPTV, probably because they share the same Th-DPP-Th core and resulting strong intramolecular charge transfers. However, the current intensity for the reduction curves was much lower than that of oxidation curves, suggesting they may have a better capability for transporting holes rather than electrons.
Crystallinity of DPP-Based Poly(arylene vinylene)s. Each copolymer was dissolved in chloroform/chlorobenzene at 10-20 mg/mL and the solutions were drop-cast onto glass substrates. The films were then dried on a hot plate at 60 °C in air. The possible crystalline nature of the three poly(arylene vinylene)s HD-PPTV, HD-PPPV, and PPTPV were investigated by X-ray diffraction (XRD) of the drop-cast films, as shown in Figure 5. HD-PPTV has a (100) reflection peak at 4.78°, corresponding to a dioo spacing of 18.47 A. Without wishing to be bound by theory, the dioo spacing is believed to correspond to a stacking distance in a lamellar packing structure, dictated by the bulky 2- hexyldecyl chains. HD-PPPV has no such peak, and the random copolymer PPTPV shows only a weak reflection peak at 4.89°, corresponding to a dioo spacing of 18.02 A, indicating that PPTPV may have a lamellar packing structure similar to HD-PPTV. The broad peak for HD-PPTV centered at 22.67° is believed to be related to an intermolecular π-π stacking distance between the HD-PPTV polymer chains (3.92 A), and may be indicative of the relatively highly crystalline nature of the HD-PPTV polymer in the solid state. In contrast, HD-PPPV and HD-PPTV did not show any similar reflection peaks near 22 degrees.
Example 5 - Fabrication and Characterization of Field-Effect Transistors.
Field-effect transistors comprising HD-PPTV, HD-PPPV, and PPTPV polymers as active semiconductor layers were fabricated on heavily doped silicon substrates with thermally grown silicon dioxide gate insulator (200 nm). Gold electrodes (40 nm) with chromium adhesive layer (2 nm) acted as the source and drain electrodes in the bottom-contact/bottom-gate transistors, forming the channel widths (W) of 400-800 μιη and lengths (L) of 20-40 μιη (W/L = 20). The substrates were cleaned by ultrasonication with acetone and isopropyl alcohol and dried by flow of nitrogen. Surface of a silicon dioxide substrate was treated with octyltrichlorosilane (OTS-8) to form a hydrophobic self-assembled monolayer. Polymer solutions in 1 ,2-dichlorobenzene (ODCB) were deposited on the substrates by spin-coating. The devices were annealed at various temperatures in a nitrogen- filled dry box. Electrical characteristics of the devices were measured by using HP4145B semiconductor parameter analyzer under nitrogen atmosphere.
Figures 6a and 6b shows representative current-voltage characteristics of the OFETs based on HD-PPTV, HD-PPPV, and PPTPV as the polymer semiconductors. Figure 6b shows overlays of representative transfer curves (Vds= ±80 V) of the transistors comprising HD-PPTV, HD-PPPV, and PPTPV. The transistors employing HD-PPTV (Figure 6a) showed both hole and electron charge transport with typical ambipolar features, such as current modulation and saturation observed in both positive and negative polarities of applied voltages. The charge-carrier mobilities were calculated from transfer curves using the standard saturation equation of metal-oxide-semiconductor field-effect transistors: Ids=( WCo/2L)(Vg-Vt)2. Hole mobility as high as 0.20 cm2 V"1 s"1 and electron mobility as high as 0.03 cm 2 V -"1 s -"1 were obtained. The extracted electrical parameters are collected in Table 2.
HD-PPTV 110 0.11 0.015 -5.9 24.5 10 -10
2 3
150 0.17 0.017 -5.5 22.7 10 -10
2 3
200 0.14 0.019 -1.6 28.8 10 -10
2 3
250 0.091 0.012 -9.0 32.1 10 -10
1
HD-PPPV 150 4.9x10 - -9.8 - 10
2 3
PPTPV 110 3.9x10 2.9x10 3.9 17.9 10 -10
2 3
150 2.2xl0"3 2.3xl0"5 -2.6 16.4 10 -10
2 3
200 1.5xl0"3 1.9xl0"5 -9.3 24.2 10 -10 a The polymer film was annealed at Ta for 10 mins. Average of 5-6 devices. The average hole mobility as measured from exemplary transistors comprising HD-PPTV was in the narrow range of 0.091-0.17 cm 2 V -"1 s-"1 and the average electron mobility was 0.012-0.019 cm 2 V -"1 s-"1 with current on/off ratios of 102-103.
The threshold voltage of hole and electron transport are -9.0 - -1.6 V for p- channel operation and 22.7-32.1 V for n-channel mode.
In the case of HD-PPPV, a low hole mobility of 4.9xl0"7 cm2 V"1 s"1 was obtained without electron transport which is understandable because it has a large bandgap of 2.0 eV with a high-lying LUMO level, resulting in large injection barriers for electrons from the gold electrodes. PPTPV had a compromised OFET performance with hole and electron mobilities of 3.9xl0"4- 2.2xl0"3 and 2.9xl0"6-2.3xl0"5 cm2 V"1 s"1, respectively, after annealing at 110-200 °C. The highest average mobilities in PPTPV OFETs were observed after annealing at 150 °C (Table 2).
The hole mobility in HD-PPTV transistors is about one order of magnitude higher than the electron mobility, which may result from the larger injection barriers for electrons from the gold electrodes. The mobilities (0.012-0.17 cm V"1 s"1) are comparable to the reported values (0.01-0.65 cm2 V"1 s"1) for ambipolar transistors based on one single conjugated polymer semiconductor and are higher than the reported values (0.01-0.05 cm 2 V -"1 s -"1 ) obtained from ambipolar transistors with similar bottom-contact and bottom-gate geometry, see
Bwrgi, L.; Turbiez, M.; Pfeiffer, R.; Bienewald, F.; Kirner, H.-J.; Winnewisser,
C. Adv. Mater. 2008, 20, 2217; Bijleveld, J. C; Zoombelt, A. P.; Mathijssen, S.
G. J.; Wienk, M. M.; Turbiez, M.; de Leeuw, D. M.; Janssen, R. A. J. /. Am.
Chem. Soc. 2009, 131, 16616; Tsai, J.-H.; Lee, W.-Y.; Chen, W.-C; Yu, C.-Y.; Hwang, G.-H.; Ting, C. Chem. Mater. 2010, 22, 3290; Sonar, P.; Singh, S. P.; Li,
Y.; Soh, M. S.; Dodabalapur, A. Adv. Mater. 2010, 22, 5409.; and Ashraf, R. S.;
Chen, Z.; Leem, D. S.; Bronstein, H.; Zhang, W.; Schroeder, B.; Geerts, Y.;
Smith, J.; Watkins, S.; Anthopoulos, T. W.; Sirringhaus, H.; de Mello, J. C;
Heeney, M.; McCulloch, I. Chem. Mater. 2011, 23, 768.
Example 6 - Fabrication and Characterization of Devices Comprising
Inverters and NAND and NOR Circuits.
In light of the ambipolar feature of HD-PPTV, a complementary inverter and circuits were successfully fabricated and demonstrated by integrating two or four transistors. Devices comprising logic circuits comprising transistors comprising HD-PPTV as the active ambipolar semiconductor are shown in
Figures 8, 9, and 10. Complementary inverters having the structures shown in
Figure 7 were fabricated on a substrate with large channel dimensions (W=5000 m and L=100 m). Electrical characteristics of the inverter devices are also shown in Figure 7. The complementary nature of inverters based on the ambipolar HD-PPTV transistors resulted in sharp switching characteristics of the logic operation with the voltage gain (-dVout/dVin) of 15-27.
Devices comprising HD-PPTV to make NAND logic circuits and the resulting electrical characteristics are shown in Figure 8. As input signals switched between on-state (high-voltage) and off-state (low-voltage), output voltage switched according to the NAND logic operation. Output signal of the
NAND circuit is off-state if both input signals are on-state; otherwise the output is on-state.
Devices comprising HD-PPTV to make NOR logic circuits and the resulting electrical characteristics are shown in Figure 9. As input signals switched between on-state and off-state, output voltage switched according to the NOR logic operation. Output signal of the NOR circuit is on-state if both input signals are off-state; otherwise the output is off-state.
Because the constituent transistors of NAND and NOR circuits are identical, the function of a circuit can be switched from a NAND gate to a NOR gate, or from a NOR gate to a NAND gate, by swapping voltage biases of a power supply and a ground.
PRIORITY DOCUMENT CLAIMED EMBODIMENTS
The 34 claims of priority US provisional application 61/525,618 filed August 19, 2011 are provided hereinbelow as 34 embodiments and also incorporated by reference.
A first embodiment (i.e., embodiment 1) provides for
diketopyrrolopyrrole polymers comprising a plurality of repeating units, wherein more than 50 mol. %, up to 100 mol. % of the repeating units are repeating units RU of one or more structural formula(e) complying with general structural formula (I)
(I)
wherein
a) each R1 and R1 is independently selected from C1-C30 normal, branched, or cyclic alkyls or fluorinated derivatives thereof;
each hAr1 and hAr1 is independently selected from Ci-C6o heteroaryls having the structure
i) a is an integer equal to 1 , 2, 3, or 4;
ii) each X, X', X or X' " is independently selected from O, S, Se,
3 3 3
Si(R )2, and NR , where R is a C1-C30 normal, branched, or cyclic alkyl group;
iii) each Y, Y', Y"and Y' "is independently selected from N, and CR4, where R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; and
c) each 2 d 2'
R an R is independently selected from hydrogen, cyano, or Ci- C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy groups.
A second embodiment is the diketopyrrolopyrrole polymers according to embodiment 1, wherein more than 50 mol. %, up to 100 mol. % of the repeating units are repeating units RU of one and only one specific structural formula complying with general structural formula (I).
A third embodiment is the diketopyrrolopyrrole polymers according to one or more other embodiments, which are homopolymers.
A fourth embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, wherein more than 50 mol. %, up to less than 100 mol. % of the repeating units are repeating units RU of one and only one specific structural formula complying with general structural formula (I), and between 0 mol. % and 50 mol. % of the repeating units are repeating units RU* of one or more specific structural formula(e) not complying with general structural formula (I).
A fifth embodiment is the diketopyrrolopyrrole polymers according to embodiment 1, wherein more than 50 mol. %, up to 100 mol. % of the repeating units are repeating units RU of at least two distinct specific structural formulae complying with general structural formula (I).
A sixth embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, wherein the repeating units RU are a mix consisting of repeating units RU1 of specific structural formula (II)
(II) and repeating units RU2 of s ecific structural formula (III)
(Ml)
wherein
11 11' 21 21'
each R , R , R and R is independently selected from C1-C30 normal, branched, or cyclic alkyls or fiuorinated derivatives thereof;
a) each 1 2
ach hhAArr111 hAArr211 21'
i) a is an integer equal to 1 , 2, 3, or 4;
ii) each X, X', X or X' " is independently selected from O, S, Se,
3 3 3
Si(R )2, and NR , where R is a C1-C30 normal, branched, or cyclic alkyl group;
iii) each Y, Y', Y" and Y" ' is independently selected from N, and CR4, where R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; b) each R 12 , R 12' , R 22 and R 22' is independently selected from hydrogen, cyano, or C1-C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy groups,
with the proviso that at least one of the radicals R11, R11 , hAr11 and hAr11
included in formula (II) differs from its homologue included in formula (III), respectively R21, R21', hAr21 and hAr21'.
A seventh embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, which are block copolymers wherein R11 and R 21 are different from each other and/or R 11 ' and R 21 ' are different from each other.
An eighth embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, which are random copolymers wherein R11 and R11 are identical to each other and R 21 and R 21 ' are identical to each other.
A ninth embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, which are copolymers of which all the repeating units are repeating units RU, said repeating units RU being a mix consisting of repeating units RUl of specific formula (II) and repeating units RU2 of specific formula (III).
In a tenth embodiment, diketopyrrolopyrrole polymers are provided according to one or more other embodiments, wherein more than 50 mol. %, up to less than 100 mol. % of the repeating units are repeating units RU consisting of repeating units RUl of specific structural formula (II) and repeating units RU2 of specific formula (III), and between 0 mol. % and 50 mol. % of the repeating units are repeating units RU* of one or more structural formula(e) not complying with general structural formula (I).
An eleventh embodiment provides diketopyrrolopyrrole polymers according to one or more other embodiments, wherein the mole ratio of the repeating units RUl and RU2 (RUl :RU2) ranges from 5 % to 95 %.
A twelfth embodiment provides for diketopyrrolopyrrole polymers according to anyone of the preceding embodiments, wherein each hAr1 and hAr1 is independently selected from
wherein R3 is a C1-C30 normal, branched, or cyclic alkyl group, R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group
A thirteenth embodiment provides for diketopyrrolopyrrole polymers according to one or more other embodiments, wherein each hAr1 and hAr1 is independently selected from
In a fourteenth embodiment, diketopyrrolopyrrole polymers according to one or more prior embodiments are provided, wherein each hAr1 and hAr1 is independently selected from
In a fifteenth embodiment, diketopyrrolopyrrole polymers are provided according to one or more other embodiments, wherein each hAr1 and hAr1 is independently selected from
A sixteenth embodiment is diketopyrrolopyrrole polymers according to one or more other embodiments, wherein each hAr1 and hAr1 is independently selected from
In a seventeenth embodiment, diketopyrrolopyrrole polymers according to claim 12, wherein each hAr1 and hAr1 is independently selected from
An eighteenth embodiment is diketopyrrolopyrrole polymers according to one or more prior embodiments, wherein each hAr1 and hAr1 is independently selected from
In a nineteenth embodiment, diketopyrrolopyrrole polymers according to one or more prior embodiments, wherein each hAr1 and hAr1 is independently selected from
A twentieth embodiment is diketopyrrolopyrrole polymers according to one or more prior embodiments, wherein each hAr1 and hAr1 is independently selected from
A twentyfirst embodiment is diketopyrrolopyrrole polymers according to anyone of other embodiments, wherein each hAr11 and hAr11 is independently selected from
- each hAr 21 and hAr 21 ' is independently selected from
A twentysecond embodiment is diketopyrrolopyrrole polymers according to anyone of the other embodiments, wherein between 0 mol. % and 50 mol. % of the repeating units are repeating units RU* of one or more of the following general formulae
Formula (V)
A twentythird embodiment provides for diketopyrrolopyrrole polymers of any of the preceding embodiments, which have a number average degree of polymerization of at least 10, as determined by gel permeation chromatography using polystyrene calibration standards.
In a twentyfourth embodiment, diketopyrrolopyrrole polymers of any of the preceding embodiments is provided, which have a number average degree of polymerization of at most 100, as determined by gel permeation chromatography using polystyrene calibration standards.
In a twentyfifth embodiment, diketopyrrolopyrrole polymers according to anyone of the preceding embodiments are provided, having solubility at one or more temperature(s) selected in the range from 25 °C to 50 °C in at least one solvent selected from the group consisting of toluene, xylene, mesitylene, tetrahydrofuran, chloroform, chlorobenzene, dichlorobenzene and mixtures thereof, of at least 10 mg/ml .
In embodiment twentysix, an electronic device is provided comprising at least one polymer chosen from the diketopyrrolopyrrole polymers according to anyone of the preceding embodiments.
In a twentyseventh embodiment, the electronic device of any of the prior embodiments, which comprises at least one photovoltaic cell or at least one transistor comprising the at least one polymer.
In a twentyeighth embodiment, the electronic device comprises at least one transistor comprising the at least one polymer.
In a twentyninth embodiment, a method is provided for making the polymers of any or more of the prior embodiments, comprising the steps of
a) obtaining or providing at least one bis-heteroaryl substituted
(I) wherein LG1 is a leaving group, such a bromine atom ;
b) obtaining or providing at least one bis-leaving group substituted ethylene monomer having the structure
wherein LG is a leaving group capable of being eliminated by reacting with LG 1 , such as SnR 2" 3 wherein R 2" is an alkyl or aryl group; c) reacting a mixture comprising the at least one first bis-heteroaryl substituted diketopyrrolopyrrole monomer and the at least one bis-leaving group substituted ethylene monomer in the presence of a transition metal catalyst complex, to produce the copolymers of any one or more of the embodiments described herein.
In a thirtieth embodiment, the method is provided according to one or more other embodiments, wherein the mixture comprises susbtantially equimolar amounts of the at least one first bis-heteroaryl substituted diketopyrrolopyrrole monomer and the at least one bis-leaving group substituted ethylene monomer.
In a thirtyfirst embodiment, a method according to one or more other embodiments is provided, wherein the mixture is substantially free of any monomer other than the at least one first bis-heteroaryl substituted
diketopyrrolopyrrole monomer and the at least one bis-leaving group substituted ethylene monomer.
In a thirtysecond embodiment, a method is provided according to on or more other embodiments, wherein the mixture further comprises at least one self- reacting monomer having the structure
LG1 - hAr* - LG2 wherein hAr* is selected from C -C heteroar ls havin the structure
and a, LG1,LG2, X, X', X", X"', Y, Y', Y", and Y'" are as defined in the previous embodiment.
In a thirtythird embodiment, a method is provided according to one or more other embodiments, wherein the mixture further comprises at least one self- reacting monomer having the structure
LG1 - hAr* - LG1 wherein hAr* is selected from Ci-C6o heteroaryls having the structure
and a, LG1, X, X', X", X"', Y, Y', Y", and Y'" are as defined in the relevant previous claim.
In a thirty fourth embodiment, a method is provided for making the random copolymers of one or more of the other embodiments, comprising the steps of : a) obtaining or providing at least a first bis-heteroaryl substituted diketopyrrolopyrrole monomer having the structure
wherein LG is a leaving group such a bromine atom ;
b) obtaining or providing at least a second bis-heteroaryl substituted diketopyrrolopyrrole monomer having the structure
(III) c) obtaining or providing at least one bis-leaving group substituted ethylene monomer having the structure
2
wherein LG is a leaving group capable of being eliminated by reacting with LG 1 , such as SnR 2" 2"
3 wherein R is an alkyl or aryl group; d) reacting a mixture comprising the at least first and second bis-heteroaryl substituted diketopyrrolopyrrole monomers and the at least one bis-leaving group substituted ethylene monomer in the presence of a transition metal catalyst complex, to produce the copolymers of one or more of the other embodiments.
Conclusions
The above specification, examples and data provide exemplary description of the manufacture and use of the various compositions and devices of the inventions, and methods for their manufacture and use. In view of those disclosures, one of ordinary skill in the art will be able to envision many additional embodiments of the inventions disclosed and claimed herein to be obvious, and that they can be made without departing from the scope of the invention. The claims hereinafter appended define some of those embodiments.
Claims
1. Diketopyrrolopyrrole polymers comprising a plurality of repeating units, wherein more than 50 mol. %, up to 100 mol. % of the repeating units are repeating units RU of one or more structural formula(e) complying with general structural formula (I)
(I)
wherein
a) each R1 and R1 is independently selected from C1-C30 normal, branched, or cyclic alkyls or fluorinated derivatives thereof;
b) each hAr1 and hAr1 is independently selected from Ci-C6o heteroaryls having the structure
v) each X, X' , X or X" ' is independently selected from O, S, Se,
3 3 3
Si(R )2, and NR , where R is a C1-C30 normal, branched, or cyclic alkyl group;
vi) each Y, Y' , Y' 'and Y' "is independently selected from N, and CR4, where R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; and
c) each R 2 and R 2' is independently selected from hydrogen, cyano, or Ci- C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy groups.
2. Diketopyrrolopyrrole polymers according to claim 1, wherein more than 50 mol. %, up to 100 mol. % of the repeating units are repeating units RU of one and only one specific structural formula complying with general structural formula (I).
3. Diketopyrrolopyrrole polymers according to claims 1-2, which are homopolymers.
4. Diketopyrrolopyrrole polymers according to claim 2, wherein more than 50 mol. %, up to less than 100 mol. % of the repeating units are repeating units RU of one and only one specific structural formula complying with general structural formula (I), and between 0 mol. % and 50 mol. % of the repeating units are repeating units RU* of one or more specific structural formula(e) not complying with general structural formula (I).
5. Diketopyrrolopyrrole polymers according to claim 1, wherein the repeating units RU are a mix consisting of repeating units RU1 of specific structural formula (II)
and repeating units RU2 of s ecific structural formula (III)
11 11' 21 21'
each R , R , R and R is independently selected from C1-C30 normal, branched, or cyclic alkyls or fluorinated derivatives thereof;
a) each hAr 11 , hAr 11' , hAr 21 and hAr 21' is independently selected from Ci-C heteroaryls having the structure
i) a is an integer equal to 1, 2, 3, or 4;
ii) each X, X', X or X' " is independently selected from O, S, Se,
3 3 3
Si(R )2, and NR , where R is a C1-C30 normal, branched, or cyclic alkyl group;
iii) each Y, Y', Y" and Y' " is independently selected from N, and CR4, where R4 is hydrogen, halogen, cyano, or a C1-C30 normal, branched, or cyclic alkyl, perfluoroalkyl, alkoxy, thioalkyl, or thioalkoxy group; b) each R 12 , R 12' , R 22 and R 22' is independently selected from hydrogen, cyano, or C1-C30 alkyl, fluoroalkyl, alkoxy, aryl, heteroaryl, carboxyalkyl, or acetoxy groups,
with the proviso that at least one of the radicals R11, R11 , hAr11 and hAr11 included in formula (II) differs from its homologue included in formula (III), respectively R21, R21', hAr21 and hAr21'.
6. Diketopyrrolopyrrole polymers according to anyone of the preceding claims, wherein each hAr1 and hAr1 is independently selected from
Diketopyrrolopyrrole polymers according to claim 6, wherein each hAr hAr1 is independently selected from
Diketopyrrolopyrrole polymers according to claim 6, wherein each hAr hAr1 is independently selected from
Diketopyrrolopyrrole polymers according to claim 6, wherein each hAr hAr1 is independently selected from
10. Diketopyrrolopyrrole polymers according to anyone of the preceding claims but 3, wherein between 0 mol. % and 50 mol. % of the repeating units are repeating units RU* of one or more of the following general formulae
Formula (V)
11. Diketopyrrolopyrrole polymers of any of the preceding claims, which have a number average degree of polymerization of at least 10, as determined by gel permeation chromatography using polystyrene calibration standards.
12. Diketopyrrolopyrrole polymers according to anyone of the preceding claims, having solubility at one or more temperature(s) selected in the range from 25 °C to 50 °C in at least one solvent selected from the group consisting of toluene, xylene, mesitylene, tetrahydrofuran, chloroform, chlorobenzene, dichlorobenzene and mixtures thereof, of at least 10 mg/ml .
13. An electronic device comprising at least one polymer chosen from the diketopyrrolopyrrole polymers according to anyone of the preceding claims.
14. The electronic device of claim 26, which comprises at least one photovoltaic cell or at least one transistor comprising the at least one polymer.
15. A method for making one or more polymers of the preceding claims comprising the steps of a) obtaining or providing at least one bis-heteroaryl substituted
(I) wherein LG1 is a leaving group, such a bromine atom ;
b) obtaining or providing at least one bis-leaving group substituted ethylene monomer having the structure wherein LG is a leaving group capable of being eliminated by reacting with LG 1 , such as SnR 2" 3 wherein R 2" is an alkyl or aryl group; c) reacting a mixture comprising the at least one first bis-heteroaryl substituted diketopyrrolopyrrole monomer and the at least one bis-leaving group substituted ethylene monomer in the presence of a transition metal catalyst complex, to produce the copolymers of one or more of the preceding claims.
16. The method according to claim 15, wherein the mixture comprises susbtantially equimolar amounts of the at least one first bis-heteroaryl substituted diketopyrrolopyrrole monomer and the at least one bis-leaving group substituted ethylene monomer.
17. The method according to claim 15 or 16, wherein the mixture is substantially free of any monomer other than the at least one first bis-heteroaryl substituted diketopyrrolopyrrole monomer and the at least one bis-leaving group substituted ethylene monomer.
18. The method according to claim 15 or 16, wherein the mixture further comprises at least one self-reacting monomer having the structure LG1 - hAr* - LG2 wherein hAr* is selected from C -C heteroar ls havin the structure
and a, LG1,LG2, X, X', X", X"', Y, Y', Y", and Y'" are as defined in a relevant previous claim.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161525618P | 2011-08-19 | 2011-08-19 | |
| US61/525,618 | 2011-08-19 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2013028441A2 true WO2013028441A2 (en) | 2013-02-28 |
| WO2013028441A3 WO2013028441A3 (en) | 2013-06-06 |
Family
ID=46755131
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2012/051027 WO2013028441A2 (en) | 2011-08-19 | 2012-08-16 | New poly(heteroarylene vinylene)s based on diketopyrrolopyrrole |
Country Status (2)
| Country | Link |
|---|---|
| TW (1) | TW201321427A (en) |
| WO (1) | WO2013028441A2 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140131627A1 (en) * | 2011-06-28 | 2014-05-15 | Merck Patent Gmbh | Indaceno derivatives as organic semiconductors |
| WO2014181910A1 (en) * | 2013-05-07 | 2014-11-13 | 경상대학교산학협력단 | Diketopyrrolopyrrole polymer and organic electronic device containing same |
| WO2021118242A1 (en) * | 2019-12-10 | 2021-06-17 | 경상국립대학교산학협력단 | Novel polymer and organic electronic device including same |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104193971B (en) * | 2014-07-24 | 2016-08-17 | 合肥工业大学 | A kind of semiconductive conjugated polymer and synthetic method thereof |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008000664A1 (en) | 2006-06-30 | 2008-01-03 | Ciba Holding Inc. | Diketopyrrolopyrrole polymers as organic semiconductors |
| WO2010049321A1 (en) | 2008-10-31 | 2010-05-06 | Basf Se | Diketopyrrolopyrrole polymers for use in organic field effect transistors |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI290164B (en) * | 1999-08-26 | 2007-11-21 | Ciba Sc Holding Ag | DPP-containing conjugated polymers and electroluminescent devices |
| EP1078970B1 (en) * | 1999-08-26 | 2004-03-17 | Ciba SC Holding AG | DPP-containing conjugated polymers and electroluminescent devices |
| EP1087005B1 (en) * | 1999-09-27 | 2004-02-25 | Ciba SC Holding AG | Fluorescent diketopyrrolopyrroles |
| BR0307402A (en) * | 2002-02-01 | 2004-12-28 | Ciba Sc Holding Ag | Fluorescent compositions comprising diketopyrrolopyrols |
| CN100572419C (en) * | 2003-10-28 | 2009-12-23 | 西巴特殊化学品控股有限公司 | Novel diketopyrrolopyrrolepolymers polymers |
| US7932344B2 (en) * | 2007-09-06 | 2011-04-26 | Xerox Corporation | Diketopyrrolopyrrole-based polymers |
| US7910684B2 (en) * | 2007-09-06 | 2011-03-22 | Xerox Corporation | Diketopyrrolopyrrole-based derivatives for thin film transistors |
| EP2350160B1 (en) * | 2008-10-31 | 2018-04-11 | Basf Se | Diketopyrrolopyrrole polymers for use in organic semiconductor devices |
| JP5635070B2 (en) * | 2009-03-23 | 2014-12-03 | ビーエーエスエフ ソシエタス・ヨーロピアBasf Se | Diketopyrrolopyrrole polymers for use in organic semiconductor devices |
| CN102449030B (en) * | 2009-05-27 | 2014-06-25 | 巴斯夫欧洲公司 | Diketopyrrolopyrrole polymers for use in organic semiconductor devices |
| WO2011119446A1 (en) * | 2010-03-20 | 2011-09-29 | Polyera Corporation | Pyrrolo[3,2-b]pyrrole semiconducting compounds and devices incorporating same |
| CN102892807A (en) * | 2010-05-19 | 2013-01-23 | 巴斯夫欧洲公司 | Diketopyrrolopyrrole polymers for use in organic semiconductor devices |
| CN102959753B (en) * | 2010-06-24 | 2017-10-31 | 巴斯夫欧洲公司 | With improving ON/OFF electric current than the organic field effect tube that is moved with controllable threshold |
| KR101880777B1 (en) * | 2010-09-29 | 2018-08-17 | 바스프 에스이 | Semiconductors based on diketopyrrolopyrroles |
| KR102009846B1 (en) * | 2011-06-22 | 2019-08-12 | 바스프 에스이 | Diketopyrrolopyrrole oligomers for use in organic semiconductor devices |
| KR102030859B1 (en) * | 2011-09-02 | 2019-10-10 | 바스프 에스이 | Diketopyrrolopyrrole oligomers and compositions, comprising diketopyrrolopyrrole oligomers |
-
2012
- 2012-08-16 TW TW101129719A patent/TW201321427A/en unknown
- 2012-08-16 WO PCT/US2012/051027 patent/WO2013028441A2/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008000664A1 (en) | 2006-06-30 | 2008-01-03 | Ciba Holding Inc. | Diketopyrrolopyrrole polymers as organic semiconductors |
| WO2010049321A1 (en) | 2008-10-31 | 2010-05-06 | Basf Se | Diketopyrrolopyrrole polymers for use in organic field effect transistors |
Non-Patent Citations (13)
| Title |
|---|
| ASHRAF, R. S.; CHEN, Z.; LEEM, D. S.; BRONSTEIN, H.; ZHANG, W.; SCHROEDER, B.; GEERTS, Y.; SMITH, J.; WATKINS, S.; ANTHOPOULOS, T., CHEM. MATER., vol. 23, 2011, pages 768 |
| BARCLAY, R. M.; CORDES, A. W.; MACKINNON, C. D.; OAKLEY, R. T.; REED, R. W., CHEM. MATER., vol. 9, 1997, pages 981 - 990 |
| BIIRGI, L.; TURBIEZ, M.; PFEIFFER, R.; BIENEWALD, F.; KIRNER, H.-J.; WINNEWISSER, C., ADV. MATER., vol. 20, 2008, pages 2217 |
| BIJLEVELD ET AL., J. AMER. CHEM. SOC., vol. 131, 2009, pages 16616 - 16617 |
| BIJLEVELD, J. C.; ZOOMBELT, A. P.; MATHIJSSEN, S. G. J.; WIENK, M. M.; TURBIEZ, M.; DE LEEUW, D. M.; JANSSEN, R. A. J. J., ANA. CHEM. SOC., vol. 131, 2009, pages 16616 |
| BIJLEVELD, J. C.; ZOOMBELT, A. P.; MATHIJSSEN, S. G. J.; WIENK, M. M.; TURBIEZ, M.; DE LEEUW, D. M.; JANSSEN, R. A. J., J. AM. CHEM. SOC., vol. 131, 2009, pages 16616 |
| BRONSTEIN ET AL., J. AM. CHEM. SOC., vol. 133, 2011, pages 3272 - 3275 |
| FUKUDA, M.; KODAMA, K.; YAMAMOTO, H.; MITO, K., DYES AND PIGMENTS, vol. 63, 2004, pages 115 |
| MOWRY, D. T., CHEM. REV., vol. 42, 1948, pages 189 - 283 |
| RABINDRANATH, A. R.; ZHU, Y.; HEIM, L; TIEKE, B., MACROMOLECULES, vol. 39, 2006, pages 8250 |
| SONAR, P.; SINGH, S. P.; LI, Y.; SOH, M. S.; DODABALAPUR, A., ADV. MATER., vol. 22, 2010, pages 5409 |
| TSAI, J.-H.; LEE, W.-Y.; CHEN, W.-C.; YU, C.-Y.; HWANG, G.-H.; TING, C., CHERN. MATER., vol. 22, no. 3290, 2010 |
| ZHANG ET AL., SYNTHETIC METALS, vol. 160, 2010, pages 1945 - 1952 |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140131627A1 (en) * | 2011-06-28 | 2014-05-15 | Merck Patent Gmbh | Indaceno derivatives as organic semiconductors |
| US9520565B2 (en) * | 2011-06-28 | 2016-12-13 | Merck Patent Gmbh | Indaceno derivatives as organic semiconductors |
| WO2014181910A1 (en) * | 2013-05-07 | 2014-11-13 | 경상대학교산학협력단 | Diketopyrrolopyrrole polymer and organic electronic device containing same |
| KR101485398B1 (en) * | 2013-05-07 | 2015-01-26 | 경상대학교산학협력단 | Diketopyrrolopyrrole polymer and organic electronic device using the same |
| US10090471B2 (en) | 2013-05-07 | 2018-10-02 | Industry-Academic Cooperation Foundation Gyeongsang National University | Diketopyrrolopyrrole polymer and organic electronic device containing same |
| WO2021118242A1 (en) * | 2019-12-10 | 2021-06-17 | 경상국립대학교산학협력단 | Novel polymer and organic electronic device including same |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201321427A (en) | 2013-06-01 |
| WO2013028441A3 (en) | 2013-06-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8871884B2 (en) | Copolymer semiconductors comprising thiazolothiazole or benzobisthiazole, or benzobisoxazole electron acceptor subunits, and electron donor subunits, and their uses in transistors and solar cells | |
| Wang et al. | Rational Design of High‐Mobility Semicrystalline Conjugated Polymers with Tunable Charge Polarity: Beyond Benzobisthiadiazole‐Based Polymers | |
| Yang et al. | Synthesis and optical and electroluminescent properties of novel conjugated copolymers derived from fluorene and benzoselenadiazole | |
| Gao et al. | High mobility ambipolar diketopyrrolopyrrole-based conjugated polymers synthesized via direct arylation polycondensation: Influence of thiophene moieties and side chains | |
| Sonar et al. | Furan containing diketopyrrolopyrrole copolymers: synthesis, characterization, organic field effect transistor performance and photovoltaic properties | |
| US7495251B2 (en) | Electronic devices containing acene-thiophene copolymers with silylethynyl groups | |
| KR101102079B1 (en) | Polymers incorporating carbazole derivatives and organic photovoltaic devices using them | |
| US7667230B2 (en) | Electronic devices containing acene-thiophene copolymers | |
| Fei et al. | Conjugated copolymers of vinylene flanked naphthalene diimide | |
| Hwang et al. | New thienothiadiazole-based conjugated copolymers for electronics and optoelectronics | |
| Shi et al. | Bichalcogenophene imide-based homopolymers: chalcogen-atom effects on the optoelectronic property and device performance in organic thin-film transistors | |
| KR20130090736A (en) | Heteroaromatic compound and organic solar cell comprising the same | |
| Li et al. | Benzodifuran‐containing Well‐defined π‐conjugated Polymers for Photovoltaic Cells | |
| Khim et al. | Highly stable printed polymer field-effect transistors and inverters via polyselenophene conjugated polymers | |
| Jang et al. | Synthesis and characterization of low-band-gap poly (thienylenevinylene) derivatives for polymer solar cells | |
| US9508937B2 (en) | Acenaphthylene imide-derived semiconductors | |
| Kim et al. | D–A copolymer with high ambipolar mobilities based on dithienothiophene and diketopyrrolopyrrole for polymer solar cells and organic field-effect transistors | |
| Liao et al. | Progress in the synthesis of imide-based N-type polymer semiconductor materials | |
| Lim et al. | Solution-processable oligothiophenes with solubilizing β-alkyl groups for organic photovoltaic cells | |
| Kim et al. | Molecular design and ordering effects of alkoxy aromatic donor in a DPP copolymer on OTFTs and OPVs | |
| WO2013028441A2 (en) | New poly(heteroarylene vinylene)s based on diketopyrrolopyrrole | |
| Piyakulawat et al. | Effect of thiophene donor units on the optical and photovoltaic behavior of fluorene-based copolymers | |
| Zhang et al. | Synthesis, characterization and comparative photovoltaic behavior of diketopyrrolopyrrole (DPP)-containing poly (phenylene vinylene) s varying at N-alkyl or 3, 6-aryl substituents in DPP units | |
| WO2013102035A1 (en) | Thienothiadiazole based polymer semiconductors and uses in electronics and optoelectronics | |
| Kim et al. | A diketopyrrolopyrrole-based regular terpolymer bearing two different π-extended donor units and its application in solar cells |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12751690 Country of ref document: EP Kind code of ref document: A2 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12751690 Country of ref document: EP Kind code of ref document: A2 |