WO2012163822A1 - Polyester with styrene copolymers - Google Patents
Polyester with styrene copolymers Download PDFInfo
- Publication number
- WO2012163822A1 WO2012163822A1 PCT/EP2012/059823 EP2012059823W WO2012163822A1 WO 2012163822 A1 WO2012163822 A1 WO 2012163822A1 EP 2012059823 W EP2012059823 W EP 2012059823W WO 2012163822 A1 WO2012163822 A1 WO 2012163822A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- weight
- molding compositions
- thermoplastic molding
- compositions according
- structural units
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L67/00—Compositions of polyesters obtained by reactions forming a carboxylic ester link in the main chain; Compositions of derivatives of such polymers
- C08L67/02—Polyesters derived from dicarboxylic acids and dihydroxy compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L25/00—Compositions of, homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an aromatic carbocyclic ring; Compositions of derivatives of such polymers
- C08L25/02—Homopolymers or copolymers of hydrocarbons
- C08L25/04—Homopolymers or copolymers of styrene
- C08L25/08—Copolymers of styrene
- C08L25/12—Copolymers of styrene with unsaturated nitriles
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L51/00—Compositions of graft polymers in which the grafted component is obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers
- C08L51/04—Compositions of graft polymers in which the grafted component is obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers grafted on to rubbers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L69/00—Compositions of polycarbonates; Compositions of derivatives of polycarbonates
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2205/00—Polymer mixtures characterised by other features
- C08L2205/02—Polymer mixtures characterised by other features containing two or more polymers of the same C08L -group
Definitions
- the invention relates to thermoplastic molding compositions containing
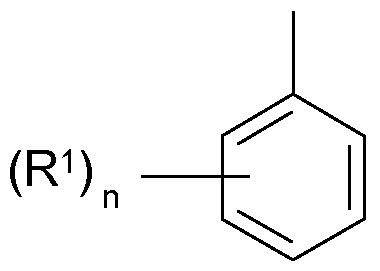
- R is an alkyl radical having 1 to 8 C atoms or a hydrogen atom and R 1 is an alkyl radical having 1 to 8 C atoms and n is 1, 2 or 3 and
- the invention relates to the use of the thermoplastic molding compositions for the production of fibers, films and moldings as well as fibers, films and moldings, which are obtainable from the thermoplastic molding compositions according to the invention.
- Polymer blends are finding increasing interest in the art because they offer tailored property combinations. Of particular interest are polymer blends of incompatible polymers that have unusual property combinations. Polymer blends based on polyesters and styrene copolymers have been known for years (DE 33 36 499, US Pat. No. 4,485,212, EP 135 677). Due to the incompatibility between polyesters and styrene copolymers, however, these products have insufficient mechanical properties.
- the subject of DE 37 33 829 are glass fiber-reinforced molding compositions based on polyesters and styrene copolymers, in which styrene-acrylonitrile-maleic anhydride terpolymers are used to improve the compatibility.
- the improvement in mechanical properties is offset by a significant deterioration in processing stability.
- the subject of DE 10 2009 055 403 are molding compositions based on polyesters, butadiene-containing stryol copolymers and reactive styrene copolymers as compatibilizers.
- the object of the present invention was therefore to provide blends of polyester with SAN and, if appropriate, rubbers which have good mechanics, in particular toughness, good long-term stability and high processing stability, especially in the case of the mixing temperatures required for fiber-reinforced molding compositions.
- the molding compositions of the invention contain 2 to 98.5, preferably 20 to 97.5 and in particular 20 to 80 wt .-% of at least one thermoplastic
- polyesters A) based on aromatic dicarboxylic acids and an aliphatic or aromatic dihydroxy compound are used.
- a first group of preferred polyesters are polyalkylene terephthalates, in particular those having 2 to 10 carbon atoms in the alcohol part.
- Dicarboxylic acid is derived.
- the aromatic ring may also be substituted, e.g. by halogen, such as chlorine and bromine, or by C 1 -C 4 -alkyl groups, such as methyl, ethyl, 25-i or n-propyl and n, i and t-butyl groups.
- polyalkylene terephthalates can be prepared by reacting aromatic dicarboxylic acids, their esters or other ester-forming derivatives with aliphatic dihydroxy compounds in a manner known per se.
- Preferred dicarboxylic acids are 2,6-naphthalenedicarboxylic acid, terephthalic acid and isophthalic acid or mixtures thereof. Up to 30 mol%, preferably not more than 10 mol% of the aromatic dicarboxylic acids can be replaced by aliphatic or cycloaliphatic dicarboxylic acids such as adipic acid, azelaic acid, sebacic acid, dodecanedioic acids and cyclohexanedicarboxylic acids.
- aliphatic dihydroxy compounds are diols having 2 to 6 carbon atoms, in particular 1, 2-ethanediol, 1, 3-propanediol, 1, 4-butanediol, 1, 6-hexanediol, 1, 4-hexanediol,
- polyesters (A) are polyalkylene terephthalates which are derived from alkanediols having 2 to 6 C atoms. Of these, in particular, polyethylene terephthalate, polypropylene terephthalate and polybutylene terephthalate or mixtures thereof are preferred. Also preferred are PET and / or PBT, which up to 1 wt .-%, preferably up to 0.75 wt .-% 1, 6-hexanediol and / or 2-methyl-1, 5-pentanediol as further monomer units.
- the viscosity number of the polyesters (A) is generally in the range from 50 to 220, preferably from 80 to 160 (measured in a 0.5% strength by weight solution in a phenol / o-dichlorobenzene mixture (wt. Ratio 1: 1 at 25 ° C) according to ISO 1628.
- polyesters whose carboxyl end group content is up to 100 meq / kg, preferably up to 50 meq / kg and in particular up to 40 meq / kg of polyester.
- Such polyesters can be prepared, for example, by the process of DE-A 44 01 055.
- the carboxyl end group content is usually determined by titration methods (eg potentiometry).
- Particularly preferred molding compositions contain as component A) a mixture of polyesters which are different from PBT, such as, for example, polyethylene terephthalate (PET).
- PBT polyethylene terephthalate
- the proportion e.g. of the polyethylene terephthalate is preferably in the mixture up to 50, in particular 1, 0 to 35 wt .-%, based on 100 wt .-% A).
- PET recyclates also termed scrap PET
- PBT polyalkylene terephthalates
- Post Industrial Recyclate these are production waste in polycondensation or in processing, e.g. Sprues in injection molding processing, start-up goods in injection molding or extrusion or edge sections of extruded sheets or foils.
- Both types of recycled material can be present either as regrind or in the form of granules. In the latter case, the slag cyclates after separation and purification are melted in an extruder and granulated. This usually facilitates the handling, the flowability and the metering for further processing steps.
- the maximum edge length should be 10 mm, preferably less than 8 mm. Due to the hydrolytic cleavage of polyesters during processing (due to traces of moisture) it is advisable to pre-dry the recyclate.
- the residual moisture content after drying is preferably ⁇ 0.2%, in particular ⁇ 0.05%.
- Another group to be mentioned are fully aromatic polyesters derived from aromatic dicarboxylic acids and aromatic dihydroxy compounds.
- Suitable aromatic dicarboxylic acids are the compounds already described for the polyalkylene terephthalates. Preference is given to using mixtures of 5 to 100 mol% of isophthalic acid and 0 to 95 mol% of terephthalic acid, in particular mixtures of about 80% of terephthalic acid with 20% of isophthalic acid to approximately equivalent mixtures of these two acids.
- the aromatic dihydroxy compounds preferably have the general formula
- Z represents an alkylene or cycloalkylene group having up to 8 C atoms, an arylene group having up to 12 C atoms, a carbonyl group, a sulfonyl group, an oxygen or sulfur atom or a chemical bond and in the m is the value 0 to 2 has.
- the compounds can also carry C 1 -C 6 -alkyl or alkoxy groups and fluorine, chlorine or bromine as substituents on the phenylene groups.
- 2.2- Di (3 ', 5'-dimethyl-4'-hydroxyphenyl) propane or mixtures thereof is preferred.
- mixtures of polyalkylene terephthalates and wholly aromatic polyesters are also possible. These generally contain from 20 to 98% by weight of the polyalkylene terephthalate and from 2 to 80% by weight of the wholly aromatic polyester.
- polyester block copolymers such as copolyetheresters may also be used.
- Such products are known per se and are known in the literature, e.g. in US Pat. No. 3,651,014. Also in the trade, corresponding products are available, e.g. Hytrel® (DuPont).
- halogen-free polycarbonates can also be used as component B) in amounts of from 0 to 70, preferably up to 60% by weight.
- Suitable halogen-free polycarbonates are, for example, those based on diphenols of the general formula
- Q is a single bond, a C 1 to C 5 alkylene, a C 2 to C 3 alkylidene, a C 3 to C 6 cycloalkylidene group, a C 6 to C 12 arylene group and also -O-, -S- or -SO 2 - and m is an integer from 0 to 2.
- the diphenols may also have substituents on the phenylene radicals, such as C 1 - to C 6 -alkyl or C 1 - to C 6 -alkoxy.
- Preferred diphenols of the abovementioned formula are, for example, hydroquinone, resorcinol, 4,4'-dihydroxydiphenyl, 2,2-bis (4-hydroxyphenyl) -propane, 2,4-bis (4-hydroxyphenyl) -
- the suitable polycarbonates may be branched in a known manner, preferably by the incorporation of from 0.05 to 2.0 mol%, based on the sum of the diphenols used, of at least trifunctional compounds, for example those having three or more than three phenolic compounds OH groups.
- the relative viscosities ⁇ ⁇ ⁇ ⁇ 1, 10 to 1, 50, in particular from 1, 25 to 1, 40 have. This corresponds to average molecular weights M w (weight average) of from 10,000 to 200,000, preferably from 20,000 to 80,000 g / mol.
- the diphenols of the general formula are known per se or can be prepared by known processes.
- the polycarbonates can be prepared, for example, by reacting the diphenols with phosgene by the phase boundary process or with phosgene by the homogeneous phase process (the so-called pyridine process), the molecular weight to be set in each case being achieved in a known manner by a corresponding amount of known chain terminators.
- phosgene by the phase boundary process or with phosgene by the homogeneous phase process (the so-called pyridine process)
- the molecular weight to be set in each case being achieved in a known manner by a corresponding amount of known chain terminators.
- Suitable chain terminators include phenol, pt-butylphenol but also long-chain alkylphenols such as 4- (1, 3-tetramethyl-butyl) -phenol, according to DE-OS 28 42 005 or monoalkylphenols or dialkylphenols having a total of 8 to 20 carbon atoms in the alkyl substituents according to DE-A 3506472, such as p-nonylphenyl, 3,5-di-t-butylphenol, pt-octylphenol, p-dodecylphenol, 2- (3,5-dimethyl-heptyl) -phenol and 4- (3, 5-dimethylheptyl) -phenol.
- alkylphenols such as 4- (1, 3-tetramethyl-butyl) -phenol, according to DE-OS 28 42 005 or monoalkylphenols or dialkylphenols having a total of 8 to 20 carbon atoms in the alkyl substituents according to DE-A 350
- Halogen-free polycarbonates in the context of the present invention means that the polycarbonates are composed of halogen-free diphenols, halogen-free chain terminators and optionally halogen-free branching agents, the content of minor ppm amounts of saponifiable chlorine, resulting, for example, from the preparation of the polycarbonates Phosgene according to the interfacial process, is not to be regarded as halogen-containing in the context of the invention.
- Such polycarbonates with ppm contents of saponifiable chlorine are halogen-free polycarbonates in the context of the present invention.
- suitable components A) may be mentioned amorphous polyester carbonates, wherein phosgene against aromatic dicarboxylic acid units such as isophthalic acid and / or terephthalic acid units, was replaced in the preparation.
- phosgene against aromatic dicarboxylic acid units such as isophthalic acid and / or terephthalic acid units
- bisphenol A can be replaced by bisphenol TMC.
- polycarbonates are available under the trademark APEC HT® from Bayer.
- the molding compositions according to the invention contain 1 to 97.5, preferably 1 to 80 and in particular 1 to 20 wt .-% of a copolymer
- R is an alkyl radical having 1 to 8 C atoms or a hydrogen atom and R 1 is an alkyl radical having 1 to 8 C atoms and n is 1, 2 or 3 and
- Preferred radicals R are methyl, ethyl or hydrogen and
- Preferred radicals R 1 are methyl, ethyl.
- Preferred components C1) are styrene or ⁇ -methylstyrene or mixtures thereof.
- Preferred components C2) are acrylonitrile or methacrylonitrile or mixtures thereof.
- the copolymers C are resinous, thermoplastic and rubber-free. Particularly preferred copolymers C are those of styrene and acrylonitrile, of ⁇ -methylstyrene and acrylonitrile or of styrene, ⁇ -methylstyrene and acrylonitrile. It is also possible to use several of the copolymers described at the same time.
- the Copolimerisate C are known per se and can be prepared by free-radical polymerization, in particular by emulsion, suspension, solution and bulk polymerization. They have viscosity numbers in the range of 40 to 160, which corresponds to average molecular weights Mw (weight average) of 40,000 to 2,000,000.
- the molding compositions according to the invention contain 0.5 to 30, preferably 1 to 20 and in particular 1 to 10 wt .-% (based on A to F) of a copolymer of di) 49.5 to 93.5 wt .-% Structural units derived from one or more vinylaromatic monomers,
- d 4) from 0 to 25 wt .-% which derives from other copolymerizable monomers structural units, wherein the wt .-% in each case on the total weight of itself), and d 4) structural units derived are obtained from the components di), d2), DSS and together 100% by weight.
- Suitable components di) are all vinylaromatic monomers known to the person skilled in the art and described in the prior art, for example DE 100 58 302 A1; Styrene, ⁇ -methylstyrene, p-methylstyrene, t-butylstyrene, vinylnaphthalene or mixtures thereof are preferably used; Styrene is particularly preferably used.
- Suitable components 2 are all vinyl cyanides known to the person skilled in the art and described in the prior art, for example DE 25 40 517 A1; acrylonitrile, methacrylonitrile or mixtures thereof are preferably used; Acrylonitrile is particularly preferably used.
- Suitable components d)) are all dicarboxylic anhydrides known to the person skilled in the art and described in the prior art; Preference is given to using maleic anhydride, methylmaleic anhydride, itaconic anhydride or mixtures thereof; Particular preference is given to using maleic anhydride.
- component d 4 ) of the copolymers D) according to the invention it is possible to use further monomers copolymerizable with the components di), d2) and 3) and different from these, which are familiar to the person skilled in the art.
- Particularly preferred are the copolymers D) of styrene-acrylonitrile-maleic anhydride copolymers.
- the preparation of the copolymers D) is carried out by bulk or solution polymerization, but preferably as a solution in the presence of an organic solvent, for example cyclohexane, ethylbenzene, toluene or dimethyl sulfoxide, preferably ethylbenzene.
- an organic solvent for example cyclohexane, ethylbenzene, toluene or dimethyl sulfoxide, preferably ethylbenzene.
- the initiation of the polymerization reaction can in principle be effected by adding chemical polymerization initiators, as described, for example, in DE 100 58 302 A1; Preferably, however, the initiation takes place purely thermally, ie without the addition of a polymerization initiator.
- the preparation can be carried out in a batch or semibatch process, but preferably a continuous process is carried out.
- the process is carried out continuously under steady-state conditions; under steady state conditions means: the concentrations of all the reactants and the composition of the copolymers A) formed remain virtually constant over the duration of the reaction (information on the relationship between monomer and polymer composition and for stationary reaction can be particularly EP 0 001 625 A1 and
- Suitable process parameters such as pressure, temperature, residence times, etc., suitable apparatuses for carrying out the process and suitable metered flow of the monomers, if any of the solvents, if any of the initiators and optionally further polymerization addition additives are known in the art and described in the prior art ,
- the workup of the polymerization mixture and the isolation of the copolymers D) can be carried out by methods known in the art and described in the prior art, for example by separation of low molecular weight compounds by applying a vacuum or stripping with inert gas.
- Preferred components D) have a nitrile content difference of less than 10% by weight to the nitrile content of component C).
- One or a mixture of different graft copolymers are used as component E) in the molding compositions according to the invention in amounts of from 0 to 50% by weight, based on the sum of components A to F.
- Preferred molding compositions according to the invention contain from 1 to 40, particularly preferably from 1 to 20,% by weight, of at least one graft copolymer E.
- the graft polymers E are composed of
- graft base egg polymers come into consideration whose glass transition temperature is below 10 ° C, preferably below 0 ° C, more preferably below -20 ° C.
- Elastomers based on C1- to C8-alkyl esters of acrylic acid which may optionally contain further comonomers.
- graft bases Ei which are composed of E11) 69.9 to 99.9% by weight, preferably 99% by weight, of at least one alkyl acrylate having 1 to 8 C atoms in the alkyl radical, preferably n-butyl acrylate and / or 2 Ethylhexyl acrylate, especially n-butyl acrylate as the sole alkyl acrylate
- Suitable bi- or polyfunctional crosslinking monomers E13) are monomers which preferably contain two, optionally also three or more, ethylenic double bonds which are capable of copolymerization and are not conjugated in the 1, 3-positions.
- Suitable crosslinking monomers are, for example, divinylbenzene, diallyl maleate, diallyl fumarate, dialyl phthalate, triallyl cyanurate or triallyl isocyanurate.
- a particularly favorable crosslinking monomer has proven to be the acrylic acid ester of tricyclodecenyl alcohol (see DE-A 12 60 135).
- graft stocks E2 preference is given to those in which E21 is styrene or ⁇ -methylstyrene or mixtures thereof and E22 is acrylonitrile or methacrylonitrile.
- the preferred monomer mixtures used are, in particular, styrene and acrylonitrile or .alpha.-methylstyrene and acrylonitrile.
- the grafts are obtainable by copolymerizing the components E21 and E 22nd
- the graft base Ei of the graft polymers E which is composed of the components En, optionally E12, and E13, is also referred to as ASA rubber.
- the preparation is known per se and, for example, in DE-A 28 26 925, DE-A 31 49 358 and
- the preparation of the graft polymers E can be carried out, for example, by the method described in DE-PS 12 60 135.
- the structure of the graft (graft) of the graft polymers can be carried out in one or two stages.
- the 1st stage generally constitutes from 20 to 70% by weight, preferably from 25 to 50% by weight, based on E2.
- the 1st stage preferably only styrene or substituted styrenes or mixtures thereof (E21) are used.
- the second stage of the graft shell generally accounts for 30 to 80 wt .-%, in particular 50 to 75 wt .-%, each based on E2, from.
- the conditions of the graft polymerization are preferably chosen so that particle sizes of 50 to 700 nm (d50 value of the integral mass distribution) result. Measures for this purpose are known and described for example in DE-OS 2826925.
- the seed latex process can be used to directly produce a coarse-particle rubber dispersion.
- the particles of the rubber are prepared in a known manner, e.g. by agglomeration, so that the latex is bimodal (50 to 180 nm and 200 to 700 nm).
- a mixture of two graft polymers having particle diameters (d 50 value of the integral mass distribution) of from 50 to 180 nm or from 200 to 700 nm in a weight ratio of from 70:30 to 30:70 is used.
- the chemical structure of the two graft polymers is preferably the same, although the shell of the coarse-particulate graft polymer can be constructed in two stages in particular.
- component E it is also possible to use ethylene copolymers, ethylene-propylene copolymers, polyester elastomers or thermoplastic polyurethanes.
- these are copolymers which are preferably composed of at least two of the following monomers: ethylene, propylene, isobutene, chloroprene, vinyl acetate, styrene, acrylonitrile and acrylic or methacrylic acid esters having 1 to 18 C atoms in the alcohol - component.
- the molding compositions of the invention may contain from 0 to 60, in particular up to 50% by weight of further additives.
- the molding compositions according to the invention may contain 0 to 5, preferably 0.05 to 3 and in particular 0.1 to 2% by weight of at least one ester or amide of saturated or unsaturated aliphatic carboxylic acids having 10 to 40, preferably 16 to 22 carbon atoms. Atoms containing aliphatic saturated alcohols or amines having 2 to 40, preferably 2 to 6 carbon atoms.
- the carboxylic acids can be 1- or 2-valent. Examples which may be mentioned are pelargonic acid, palmitic acid, lauric acid, margaric acid, dodecanedioic acid, behenic acid and particularly preferably stearic acid, capric acid and montanic acid (mixture of fatty acids having 30 to 40 carbon atoms).
- the aliphatic alcohols can be 1 - to 4-valent. Examples of alcohols are n-butanol, n-octanol, stearyl alcohol, ethylene glycol, propylene glycol, neopentyl glycol, pentaerythritol, with glycerol and pentaerythritol being preferred.
- the aliphatic amines can be 1 - to 3-valent. Examples of these are stearylamine, ethylenediamine, propylenediamine, hexamethylenediamine, di (6-aminohexyl) amine, with ethylenediamine and hexamethylenediamine being particularly preferred. Accordingly, preferred esters or amides are glycerin distearate, glycerol tristearate, ethylenediamine distearate, glycerin monopalmitate, glycerol trilaurate, glycerin monobehenate and pentaerythritol tetrastearate.
- Fibrous or particulate fillers F which may be mentioned are carbon fibers, glass fibers, glass spheres, amorphous silica, asbestos, calcium silicate, calcium metasilicate, magnesium carbonate, kaolin, chalk, powdered quartz, mica, barium sulfate and feldspar, in amounts of up to 60% by weight. , in particular up to 50%.
- Preferred fibrous fillers are carbon fibers, aramid fibers and potassium titanate fibers, glass fibers being particularly preferred as E glass. These can be used as rovings or cut glass in the commercial forms.
- the fibrous fillers can be surface-pretreated for better compatibility with the thermoplastics with a silane compound.
- Suitable silane compounds are those of the general formula
- X is NH 2 -, CH-CH-, HO-,
- O n is an integer from 2 to 10, preferably 3 to 4
- n is an integer from 1 to 5, preferably 1 to 2
- k is an integer from 1 to 3, preferably 1
- Preferred silane compounds are aminopropyltrimethoxysilane, aminobutyltrimethoxysilane, aminopropyltriethoxysilane, aminobutyltriethoxysilane and the corresponding silanes which contain a glycidyl group as substituent X.
- the silane compounds are generally used in amounts of 0.05 to 5, preferably 0.5 to 1, 5 and in particular 0.8 to 1 wt .-% (based on F) for surface coating. Also suitable are acicular mineral fillers.
- the term "needle-shaped mineral fillers” is understood to mean a mineral filler with a pronounced, needle-like character.
- An example is acicular wollastonite.
- the mineral has an L / D (length diameter) ratio of 8: 1 to 35: 1, preferably 8: 1 to 1: 1: 1.
- the mineral filler may optionally be pretreated with the silane compounds mentioned above; however, pretreatment is not essential.
- Kaolin, calcined kaolin, talc and chalk are mentioned as further fillers.
- the thermoplastic molding compositions according to the invention may contain conventional processing aids such as stabilizers, antioxidants, agents against thermal decomposition and decomposition by ultraviolet light, lubricants and mold release agents, colorants such as dyes and pigments, nucleating agents, plasticizers, etc.
- processing aids such as stabilizers, antioxidants, agents against thermal decomposition and decomposition by ultraviolet light, lubricants and mold release agents, colorants such as dyes and pigments, nucleating agents, plasticizers, etc.
- antioxidants and heat stabilizers sterically hindered phenols and / or phosphites, hydroquinones, aromatic secondary amines such as diphenylamines, various substituted representatives of these groups and mixtures thereof in concentrations up to 1 wt .-%, based on the weight of the thermoplastic molding compositions mentioned.
- UV stabilizers which are generally used in amounts of up to 2 wt .-%, based on the molding composition, various substituted resorcinols, salicylates, Benzotriazo- le and benzophenones may be mentioned.
- transesterification stabilizers mention may be made of Irgafos® PEPQ and phosphates (for example monozine phosphate).
- inorganic pigments such as titanium dioxide, ultramarine blue, iron oxide and carbon black, furthermore organic pigments such as phthalocyanines, quinacridones, perylenes and also dyes such as nigrosine and anthraquinones as colorants.
- sodium phenylphosphinate, alumina, silica and preferably talc may be used as nucleating agents.
- lubricants and mold release agents are usually used in amounts of up to 1% by weight.
- Preferred are long-chain fatty acids (e.g., stearic acid or behenic acid) whose
- Salts eg Ca or Zn stearate
- montan waxes mixtures of straight-chain, saturated th carboxylic acids with chain lengths of 28 to 32 carbon atoms
- Ca or Na montanate and low molecular weight polyethylene or polypropylene waxes eg Ca or Zn stearate
- montan waxes mixtures of straight-chain, saturated th carboxylic acids with chain lengths of 28 to 32 carbon atoms
- Ca or Na montanate and low molecular weight polyethylene or polypropylene waxes.
- plasticizers are dioctyl phthalate, dibenzyl phthalate, butyl benzyl phthalate, hydrocarbon oils, N- (n-butyl) benzenesulfonamide.
- the molding compositions according to the invention may still contain from 0 to 2% by weight of fluorine-containing ethylene polymer.
- fluorine-containing ethylene polymer are polymers of ethylene with a fluorine content of 55 to 76 wt .-%, preferably 70 to 76 wt .-%.
- PTFE polytetrafluoroethylene
- tetrafluoroethylene-hexafluoropropylene copolymers or tetrafluoroethylene copolymers with smaller amounts (generally up to 50% by weight) of copolymerizable ethylenically unsaturated monomers.
- PTFE polytetrafluoroethylene
- tetrafluoroethylene-hexafluoropropylene copolymers or tetrafluoroethylene copolymers with smaller amounts (generally up to 50% by weight) of copolymerizable ethylenically unsaturated monomers.
- fluorine-containing ethylene polymers are homogeneously distributed in the molding compositions and preferably have a particle size dso (number average) in the range of 0.05 to 1 ⁇ , in particular from 0.1 to 5 ⁇ . These small particle sizes can be achieved particularly preferably by using aqueous dispersions of fluorine-containing ethylene polymers and incorporating them into a polyester melt.
- thermoplastic molding compositions according to the invention can be prepared by processes known per se, in which the starting components are mixed in conventional mixing devices, such as screw extruders, Brabender mills or Banbury mills, and then extruded. After extrusion, the extrudate can be cooled and comminuted. It is also possible to premix individual components and then to add the remaining starting materials individually and / or likewise mixed.
- the mixing temperatures are usually 230 to 290 ° C.
- the components C) and D) and optionally E) and / or F) can be mixed with a polyester prepolymer, formulated and granulated.
- the resulting granules are then condensed in solid phase under inert gas continuously or discontinuously at a temperature below the melting point of component A) to the desired viscosity.
- thermoplastic molding compositions of the invention are characterized by good processing and good thermal stability with good mechanical properties.
- the toughness is improved and the processing stability at high temperatures.
- polyesters according to the invention can be used to produce plugs, plug parts, plug connectors, wiring harness components, circuit carriers, circuit carrier components, three-dimensionally injection-molded circuit carriers, electrical connection elements, mechatronic components or optoelectronic components.
- instrument panels In the car interior, use is possible for instrument panels, steering column switches, seat parts, headrests, center consoles, transmission components and door modules, in the car exterior for door handles, headlight components, exterior mirror components, windscreen wiper components, windscreen wiper protection housings, grille, roof rails, sunroof frames and exterior body parts.
- polyesters for the production of components for kitchen appliances, such. Fryers, irons, buttons, and garden leisure applications, e.g. Components for irrigation systems or garden tools possible.
- Component A is a compound having Component A:
- Polybutylene terephthalate with a viscosity number VZ of 130 ml / g and a carboxyl end group content of 34 meq / kg (Ultradur® B 4500 from BASF SE) (VZ measured in 0.5% strength by weight solution of phenol / o-dichlorobenzene, 1 : 1 mixture at 25 ° C).
- component D / V1 a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 75 / 24.6 / 0.4 (wt.%), Viscosity number: 66 ml / g.
- Component D / 1 a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 75 / 24.6 / 0.4 (wt.%), Viscosity number: 66 ml / g.
- Component D / 1 a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 75 / 24.6 / 0.4 (wt.%), Viscosity number: 66 ml / g.
- Component D / 1 a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 75 /
- styrene-acrylonitrile-maleic anhydride terpolymer As component D / 1, a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 74.5 / 24.5 / 1.0 (wt%), viscosity number: 66 ml / g.
- styrene-acrylonitrile-maleic anhydride terpolymer As component D / 2, a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 74.1 / 23.9 / 2.0 (wt%), viscosity number: 67 ml / g.
- styrene-acrylonitrile-maleic anhydride terpolymer As component D / V2, a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 74 / 23.5 / 2.5 (wt%), viscosity number: 68 ml / g.
- the emulsion was kept at 65 ° C for one hour.
- the polystyrene core had a mean particle diameter d 50 of 250 nm.
- the emulsion had a solids content of 38.5%.
- 6070 g of the emulsion thus obtained were diluted with 2600 g of water and 5 g of the sodium salt of a C12 to C18 paraffin sulfonic acid and 4.5 g of potassium peroxodisulfate were added.
- the graft polymer E was precipitated from the emulsion by means of calcium chloride solution at 95 ° C., washed with water and dried in a stream of warm air.
- the impact resistance of the products was determined on ISO bars according to ISO 179 1 eU.
- Tear strength and elongation at break were determined according to ISO 527.
- the thermal stability of the products was characterized by the storage of tensile bars at 120 ° C / 500h in a convection oven and subsequent tensile test.
- the molding compositions were also processed at 300 ° C melt temperature / 80 ° C mold temperature.
- the composition of the styrene copolymers used was determined by quantitative IR spectroscopy.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Compositions Of Macromolecular Compounds (AREA)
Abstract
The invention relates to thermoplastic moulding materials which contain: A) between 2 and 98.5 wt.% of at least one polyester; B) between 0 and 70 wt.% of at least one polycarbonate; C) between 1 and 97.5 wt.% of a copolymerisate consisting of c1) 60 to 95 wt.% of styrene or substituted styrenes of general formula (I) or mixtures thereof, R representing an alkyl group comprising between 1 and 8 C atoms or a hydrogen atom, R1 representing an alkyl group comprising between 1 and 8 C atoms, and n being the value 1, 2 or 3, and c2) 5 to 40 wt.% of at least one unsaturated nitrile; D) between 0.5 and 30 wt.% of a copolymer that consists of d1) 49.5 to 93.5 wt.% structural units derived from one or more vinyl-aromatic monomers, d2) 6 to 50 wt.% structural units derived from one or more vinyl cyanides, d3) 0.5 to 2.4 wt.% structural units derived from one or more dicarboxylic acid hydrides, and d4) 0 to 25 wt.% structural units derived from additional copolymerisable monomers, each of these weight per cents relating to the total weight of the structural units derived from components d1, d2, d3 and d4, and together producing 100 wt.%; E) between 0 and 50 wt.% of a diene-free rubber; and F) between 0 and 60 wt.% of additional additives, the sum of the weight per cents A) to F) equalling 100%.
Description
Polyester mit Styrolcopolymeren Polyester with styrene copolymers
Beschreibung Die Erfindung betrifft thermoplastische Formmassen, enthaltend Description The invention relates to thermoplastic molding compositions containing
A) 2 bis 98,5 Gew.-% mindestens eines Polyesters A) 2 to 98.5 wt .-% of at least one polyester
B) 0 bis 70 Gew.-% mindestens eines Polycarbonats B) 0 to 70 wt .-% of at least one polycarbonate
C) 1 bis 97,5 Gew.-% eines Copolymerisates aus C) 1 to 97.5 wt .-% of a copolymer
Ci) 60 bis 95 Gew.-% Styrol oder substituierten Styrolen der allgemeinen Formel I oder deren Mischungen Ci) 60 to 95 wt .-% of styrene or substituted styrenes of the general formula I or mixtures thereof
worin R einen Alkylrest mit 1 bis 8 C-Atomen oder ein Wasserstoffatom und R1 einen Al- kylrest mit 1 bis 8 C-Atomen darstellen und n den Wert 1 , 2 oder 3 hat und in which R is an alkyl radical having 1 to 8 C atoms or a hydrogen atom and R 1 is an alkyl radical having 1 to 8 C atoms and n is 1, 2 or 3 and
C2) 5 bis 40 Gew.-% mindestens eines ungesättigten Nitrils, C2) from 5 to 40% by weight of at least one unsaturated nitrile,
D) 0,5 bis 30 Gew.-% eines Copolymeren aus di) 49,5 bis 93,5 Gew.-% sich von einem oder mehreren vinylaromatischen Monomeren ableitende Struktureinheiten, d2) 6 bis 50 Gew.-% sich von einem oder mehreren Vinylcyaniden ableitende Struktureinheiten, D) 0.5 to 30% by weight of a copolymer of di) 49.5 to 93.5% by weight of structural units derived from one or more vinylaromatic monomers, d2) from 6 to 50% by weight of one or more several vinyl cyanide-derived structural units,
0,5 bis 2,4 Gew.-% sich von einem oder mehreren Dicarbonsäureanhydriden ableitende Struktureinheiten und From 0.5 to 2.4% by weight of structural units derived from one or more dicarboxylic acid anhydrides and
0 bis 25 Gew.-% sich von weiteren copolymerisierbaren Monomeren ableitende Struktureinheiten, wobei die Gew.-% jeweils auf das Gesamtgewicht der sich von den Komponenten di , d2, d3 und d4 ableitenden Struktureinheiten bezogen sind und zusammen 100 Gew.-% ergeben,
E) 0 bis 50 Gew.-% eines Kautschuks, welcher kein Dien enthält, 0 to 25 wt .-% which derives from other copolymerizable monomers structural units, wherein the wt .-% are each based on the total weight of the derived from the components di, d2, d3 and d4 structural units and the total is 100 wt .-% . E) 0 to 50% by weight of a rubber containing no diene,
F) 0 bis 60 Gew.-% weiterer Zusatzstoffe, wobei die Summe der Gewichtsprozente A) bis F) 100 % ergibt. F) 0 to 60 wt .-% of other additives, wherein the sum of the weight percent A) to F) gives 100%.
Weiterhin betrifft die Erfindung die Verwendung der thermoplastischen Formmassen zur Herstellung von Fasern, Folien und Formkörpern sowie Fasern, Folien und Formkörper, welche aus den erfindungsgemäßen thermoplastischen Formmassen erhältlich sind. Furthermore, the invention relates to the use of the thermoplastic molding compositions for the production of fibers, films and moldings as well as fibers, films and moldings, which are obtainable from the thermoplastic molding compositions according to the invention.
Polymermischungen finden in der Technik zunehmendes Interesse, da sie maßgeschneiderte Eigenschaftskombinationen bieten. Von besonderem Interesse sind dabei Polymermischungen aus unverträglichen Polymeren, die ungewöhnliche Eigenschaftskombinationen aufweisen. Polymermischungen auf Basis von Polyestern und Styrolcopolymeren sind seit Jahren bekannt (DE 33 36 499, US 4,485,212, EP 135 677). Aufgrund der Unverträglichkeit zwischen Polyestern und Styrolcopolymeren weisen diese Produkte jedoch ungenügende mechanische Eigenschaften auf. Polymer blends are finding increasing interest in the art because they offer tailored property combinations. Of particular interest are polymer blends of incompatible polymers that have unusual property combinations. Polymer blends based on polyesters and styrene copolymers have been known for years (DE 33 36 499, US Pat. No. 4,485,212, EP 135 677). Due to the incompatibility between polyesters and styrene copolymers, however, these products have insufficient mechanical properties.
In der Literatur werden daher verschiedene Ansätze diskutiert, die Verträglichkeit der Phasen zu verbessern, wobei vor allem funktionalisierte Styrolcopolymere (EP 284 086, US 4,902,749, US 5,310,793, Lee P.-C, Kuo W.-F., Chang F.-C, Polymer 1994, 35, 5641 , ) und reaktive Acry- latcopolymere (EP 573 680, US 4,352,904, Hage E., Haie W., Keskkula, Paul D.R. Polymer, 1997, 38, 3237) verwendet werden. Gegenstand der DE 37 33 829 sind glasfaserverstärkte Formmassen auf Basis von Polyestern und Styrolcopolymeren, bei denen Styrol-Acrylnitril-Maleinsäureanhydrid-Terpolymere zur Verbesserung der Verträglichkeit eingesetzt werden. Der Verbesserung der mechanischen Eigenschaften steht eine deutliche Verschlechterung der Verarbeitungsstabilität gegenüber. In the literature, therefore, various approaches are discussed to improve the compatibility of the phases, especially functionalized styrene copolymers (EP 284 086, US 4,902,749, US 5,310,793, Lee P.-C, Kuo W.-F., Chang F.-C , Polymer 1994, 35, 5641,) and reactive acrylate copolymers (EP 573,680, US 4,352,904, Hage E., Haie W., Keskkula, Paul DR Polymer, 1997, 38, 3237). The subject of DE 37 33 829 are glass fiber-reinforced molding compositions based on polyesters and styrene copolymers, in which styrene-acrylonitrile-maleic anhydride terpolymers are used to improve the compatibility. The improvement in mechanical properties is offset by a significant deterioration in processing stability.
Die US 2010/0152359 A1 beschreibt Mischungen aus Polyestern, Styrolcopolymeren und Pfropfcopolymeren, wobei als Polyester ein Recyclat verwendet wird. Die genannten Produkte weisen verbesserte Chemikalienbeständigkeit auf. Faserverstärkte Produkte werden jedoch nicht erwähnt. US 2010/0152359 A1 describes mixtures of polyesters, styrene copolymers and graft copolymers, wherein a recycled material is used as polyester. The products mentioned have improved chemical resistance. However fiber-reinforced products are not mentioned.
Gegenstand der DE 10 2009 055 403 sind Formmassen auf Basis von Polyestern, Butadien- haltigen Stryolcopolymeren und reaktiven Styrolcopolymeren als Verträglichkeitsvermittler.
Aufgabe der vorliegenden Erfindung war es daher, Blends aus Polyester mit SAN und gegebenenfalls Kautschuken zur Verfügung zu stellen, welche gute Mechanik, insbesondere Zähigkeit, gute Dauergebrauchsbeständigkeit und eine hohe Verarbeitungsstabilität besonders bei den für faserverstärkten Formmassen erforderlichen Mischungstemperaturen aufweisen. The subject of DE 10 2009 055 403 are molding compositions based on polyesters, butadiene-containing stryol copolymers and reactive styrene copolymers as compatibilizers. The object of the present invention was therefore to provide blends of polyester with SAN and, if appropriate, rubbers which have good mechanics, in particular toughness, good long-term stability and high processing stability, especially in the case of the mixing temperatures required for fiber-reinforced molding compositions.
Demgemäß wurden die eingangs definierten Formmassen gefunden. Bevorzugte Ausführungsformen sind den Unteransprüchen zu entnehmen. Accordingly, the molding compositions defined above were found. Preferred embodiments are given in the dependent claims.
Als Komponente (A) enthalten die erfindungsgemäßen Formmassen 2 bis 98,5, bevorzugt 20 bis 97,5 und insbesondere 20 bis 80 Gew.-% mindestens eines thermoplastischen As component (A), the molding compositions of the invention contain 2 to 98.5, preferably 20 to 97.5 and in particular 20 to 80 wt .-% of at least one thermoplastic
Polyesters. Polyester.
Allgemein werden Polyester A) auf Basis von aromatischen Dicarbonsäuren und einer aliphatischen oder aromatischen Dihydroxyverbindung verwendet. Eine erste Gruppe bevorzugter Polyester sind Polyalkylenterephthalate, insbesondere solche mit 2 bis 10 C-Atomen im Alkoholteil. Generally, polyesters A) based on aromatic dicarboxylic acids and an aliphatic or aromatic dihydroxy compound are used. A first group of preferred polyesters are polyalkylene terephthalates, in particular those having 2 to 10 carbon atoms in the alcohol part.
Derartige Polyalkylenterephthalate sind an sich bekannt und in der Literatur beschrieben. Such polyalkylene terephthalates are known per se and described in the literature.
Sie enthalten einen aromatischen Ring in der Hauptkette, der von der aromatischen They contain an aromatic ring in the main chain, that of the aromatic
Dicarbonsäure stammt. Der aromatische Ring kann auch substituiert sein, z.B. durch Halogen wie Chlor und Brom oder durch Ci-C4-Alkylgruppen wie Methyl-, Ethyl-, 25 i- bzw. n-Propyl- und n-, i- bzw. t-Butylgruppen. Dicarboxylic acid is derived. The aromatic ring may also be substituted, e.g. by halogen, such as chlorine and bromine, or by C 1 -C 4 -alkyl groups, such as methyl, ethyl, 25-i or n-propyl and n, i and t-butyl groups.
Diese Polyalkylenterephthalate können durch Umsetzung von aromatischen Dicarbonsäuren, deren Estern oder anderen esterbildenden Derivaten mit aliphatischen Dihydroxyverbindungen in an sich bekannter Weise hergestellt werden. These polyalkylene terephthalates can be prepared by reacting aromatic dicarboxylic acids, their esters or other ester-forming derivatives with aliphatic dihydroxy compounds in a manner known per se.
Als bevorzugte Dicarbonsäuren sind 2,6-Naphthalindicarbonsäure, Terephthalsäure und Isophthalsäure oder deren Mischungen zu nennen. Bis zu 30 mol-%, vorzugsweise nicht mehr als 10 mol-% der aromatischen Dicarbonsäuren können durch aliphatische oder cycloaliphati- sche Dicarbonsäuren wie Adipinsäure, Azelainsäure, Sebacinsäure, 35 Dodecandisäuren und Cyclohexandicarbonsäuren ersetzt werden. Preferred dicarboxylic acids are 2,6-naphthalenedicarboxylic acid, terephthalic acid and isophthalic acid or mixtures thereof. Up to 30 mol%, preferably not more than 10 mol% of the aromatic dicarboxylic acids can be replaced by aliphatic or cycloaliphatic dicarboxylic acids such as adipic acid, azelaic acid, sebacic acid, dodecanedioic acids and cyclohexanedicarboxylic acids.
Von den aliphatischen Dihydroxyverbindungen werden Diole mit 2 bis 6 Kohlenstoffatomen, insbesondere 1 ,2-Ethandiol, 1 ,3-Propandiol, 1 ,4-Butandiol, 1 ,6-Hexandiol, 1 ,4-Hexandiol,Of the aliphatic dihydroxy compounds are diols having 2 to 6 carbon atoms, in particular 1, 2-ethanediol, 1, 3-propanediol, 1, 4-butanediol, 1, 6-hexanediol, 1, 4-hexanediol,
1 ,4-Cyclohexandiol, 1 ,4-Cyclohexandimethanol und Neopentylglykol oder deren Mischungen bevorzugt. 1, 4-cyclohexanediol, 1, 4-cyclohexanedimethanol and neopentyl glycol or mixtures thereof.
Als besonders bevorzugte Polyester (A) sind Polyalkylenterephthalate, die sich von Alkandiolen mit 2 bis 6 C-Atomen ableiten, zu nennen. Von diesen werden insbesondere Polyethylente- rephthalat, Polypropylenterephthalat und Polybutylenterephthalat oder deren Mischungen bevorzugt. Weiterhin bevorzugt sind PET und/oder PBT, welche bis zu 1 Gew.-%, vorzugsweise
bis zu 0,75 Gew.-% 1 ,6-Hexandiol und/oder 2-Methyl-1 ,5-Pentandiol als weitere Monomereinheiten enthalten. Particularly preferred polyesters (A) are polyalkylene terephthalates which are derived from alkanediols having 2 to 6 C atoms. Of these, in particular, polyethylene terephthalate, polypropylene terephthalate and polybutylene terephthalate or mixtures thereof are preferred. Also preferred are PET and / or PBT, which up to 1 wt .-%, preferably up to 0.75 wt .-% 1, 6-hexanediol and / or 2-methyl-1, 5-pentanediol as further monomer units.
Die Viskositätszahl der Polyester (A) liegt im allgemeinen im Bereich von 50 bis 220, vorzugs- weise von 80 bis 160 (gemessen in einer 0,5 gew.-%igen Lösung in einem Phenol/o-Dichlor- benzolgemisch (Gew.-Verh. 1 :1 bei 25°C) gemäß ISO 1628. The viscosity number of the polyesters (A) is generally in the range from 50 to 220, preferably from 80 to 160 (measured in a 0.5% strength by weight solution in a phenol / o-dichlorobenzene mixture (wt. Ratio 1: 1 at 25 ° C) according to ISO 1628.
Insbesondere bevorzugt sind Polyester, deren Carboxylendgruppengehalt bis zu 100 mval/kg, bevorzugt bis zu 50 mval/kg und insbesondere bis zu 40 mval/kg Polyester beträgt. Derartige Polyester können beispielsweise nach dem Verfahren der DE-A 44 01 055 hergestellt werden. Der Carboxylendgruppengehalt wird üblicherweise durch Titrationsverfahren (z.8. Potentiometrie) bestimmt. Particular preference is given to polyesters whose carboxyl end group content is up to 100 meq / kg, preferably up to 50 meq / kg and in particular up to 40 meq / kg of polyester. Such polyesters can be prepared, for example, by the process of DE-A 44 01 055. The carboxyl end group content is usually determined by titration methods (eg potentiometry).
Insbesondere bevorzugte Formmassen enthalten als Komponente A) eine Mischung aus Poly- estern, welche verschieden von PBT sind, wie beispielsweise Polyethylenterephthalat (PET). Der Anteil z.B. des Polyethylenterephthalates beträgt vorzugsweise in der Mischung bis zu 50, insbesondere 1 ,0-bis 35 Gew.-%, bezogen auf 100 Gew.-% A). Particularly preferred molding compositions contain as component A) a mixture of polyesters which are different from PBT, such as, for example, polyethylene terephthalate (PET). The proportion e.g. of the polyethylene terephthalate is preferably in the mixture up to 50, in particular 1, 0 to 35 wt .-%, based on 100 wt .-% A).
Weiterhin ist es vorteilhaft PET Rezyklate (auch scrap-PET genannt) gegebenenfalls in Mi- schung mit Polyalkylenterephthalaten wie PBT einzusetzen. Furthermore, it is advantageous to use PET recyclates (also termed scrap PET) optionally in combination with polyalkylene terephthalates such as PBT.
Unter Rezyklaten versteht man im allgemeinen: Recyclates are generally understood as:
1 ) sog. Post Industrial Rezyklat: hierbei handelt es sich um Produktionsabfälle bei der Poly- kondensation oder bei der Verarbeitung z.B. Angüsse bei der Spritzgussverarbeitung, Anfahrware bei der Spritzgussverarbeitung oder Extrusion oder Randabschnitte von extru- dierten Platten oder Folien. 1) so-called Post Industrial Recyclate: these are production waste in polycondensation or in processing, e.g. Sprues in injection molding processing, start-up goods in injection molding or extrusion or edge sections of extruded sheets or foils.
2) Post Consumer Rezyklat: hierbei handelt es sich um Kunststoffartikel, die nach der Nut- zung durch den Endverbraucher gesammelt und aufbereitet werden. Der mengenmäßig bei weitem dominierende Artikel sind blasgeformte PET Flaschen für Mineralwasser, Softdrinks und Säfte. 2) Post Consumer Recyclate: These are plastic items that are collected and processed after use by the end user. By far the dominating items in terms of volume are blow-molded PET bottles for mineral water, soft drinks and juices.
Beide Arten von Rezyklat können entweder als Mahlgut oder in Form von Granulat vorliegen. Im letzteren Fall werden die Rohrezyklate nach der Auftrennung und Reinigung in einem Extruder aufgeschmolzen und granuliert. Hierdurch wird meist das Handling, die Rieselfähigkeit und die Dosierbarkeit für weitere Verarbeitungsschritte erleichtert. Both types of recycled material can be present either as regrind or in the form of granules. In the latter case, the slag cyclates after separation and purification are melted in an extruder and granulated. This usually facilitates the handling, the flowability and the metering for further processing steps.
Sowohl granulierte als auch als Mahlgut vorliegende Rezyklate können zum Einsatz kommen, wobei die maximale Kantenlänge 10 mm, vorzugsweise kleiner 8 mm betragen sollte.
Aufgrund der hydrolytischen Spaltung von Polyestern bei der Verarbeitung (durch Feuchtigkeitsspuren) empfiehlt es sich, das Rezyklat vorzutrocknen. Der Restfeuchtegehalt nach der Trocknung beträgt vorzugsweise <0,2 %, insbesondere <0,05 %. Als weitere Gruppe sind voll aromatische Polyester zu nennen, die sich von aromatischen Di- carbonsäuren und aromatischen Dihydroxyverbindungen ableiten. Both granulated and as regrind present recyclates can be used, the maximum edge length should be 10 mm, preferably less than 8 mm. Due to the hydrolytic cleavage of polyesters during processing (due to traces of moisture) it is advisable to pre-dry the recyclate. The residual moisture content after drying is preferably <0.2%, in particular <0.05%. Another group to be mentioned are fully aromatic polyesters derived from aromatic dicarboxylic acids and aromatic dihydroxy compounds.
Als aromatische Dicarbonsäuren eignen sich die bereits bei den Polyalkylenterephthalaten beschriebenen Verbindungen. Bevorzugt werden Mischungen aus 5 bis 100 mol-% Isophthalsäure und 0 bis 95 mol-% Terephthalsäure, insbesondere Mischungen von etwa 80 % Terephthalsäu- re mit 20 % Isophthalsäure bis etwa äquivalente Mischungen dieser beiden Säuren verwendet. Suitable aromatic dicarboxylic acids are the compounds already described for the polyalkylene terephthalates. Preference is given to using mixtures of 5 to 100 mol% of isophthalic acid and 0 to 95 mol% of terephthalic acid, in particular mixtures of about 80% of terephthalic acid with 20% of isophthalic acid to approximately equivalent mixtures of these two acids.
Die aromatischen Dihydroxyverbindungen haben vorzugsweise die allgemeine Formel The aromatic dihydroxy compounds preferably have the general formula
in der Z eine Alkylen- oder Cycloalkylengruppe mit bis zu 8 C-Atomen, eine Arylengruppe mit bis zu 12 C-Atomen, eine Carbonylgruppe, eine Sulfonylgruppe, ein Sauerstoff- oder Schwefelatom oder eine chemische Bindung darstellt und in der m den Wert 0 bis 2 hat. Die Verbin- düngen können an den Phenylengruppen auch Ci-C6-Alkyl- oder Alkoxygruppen und Fluor, Chlor oder Brom als Substituenten tragen. in which Z represents an alkylene or cycloalkylene group having up to 8 C atoms, an arylene group having up to 12 C atoms, a carbonyl group, a sulfonyl group, an oxygen or sulfur atom or a chemical bond and in the m is the value 0 to 2 has. The compounds can also carry C 1 -C 6 -alkyl or alkoxy groups and fluorine, chlorine or bromine as substituents on the phenylene groups.
Als Stammkörper dieser Verbindungen seinen beispielsweise Dihydroxydiphenyl, As a parent of these compounds its example, dihydroxydiphenyl,
Di-(hydroxyphenyl)alkan, Di (hydroxyphenyl) alkane,
Di-(hydroxyphenyl)cycloalkan, Di (hydroxyphenyl) cycloalkane,
Di-(hydroxyphenyl)sulfid, Di (hydroxyphenyl) sulfide,
Di-(hydroxyphenyl)ether, Di (hydroxyphenyl) ether,
Di-(hydroxyphenyl)keton, ketone di- (hydroxyphenyl),
di-(hydroxyphenyl)sulfoxid, di- (hydroxyphenyl) sulfoxide,
a,a'-Di-(hydroxyphenyl)-dialkylbenzol, a, a'-di- (hydroxyphenyl) -dialkylbenzol,
Di-(hydroxyphenyl)sulfon, Di-(hydroxybenzoyl)benzol Resorcin und Hydrochinon sowie deren kernalkylierte oder kernhalogenierte Derivate genannt.
Von diesen werden Di- (hydroxyphenyl) sulfone, di- (hydroxybenzoyl) benzene, resorcinol and hydroquinone and their nuclear alkylated or ring-halogenated derivatives. Of these will be
4,4'-Dihydroxydiphenyl, 4,4'-dihydroxydiphenyl,
2,4-Di-(4'-hydroxyphenyl)-2-methylbutan 2,4-di- (4'-hydroxyphenyl) -2-methylbutane
a,a'-Di-(4-hydroxyphenyl)-p-diisopropylbenzol, a, a'-di- (4-hydroxyphenyl) -p-diisopropylbenzene,
2,2-Di-(3'-methyl-4'-hydroxyphenyl)propan und 2,2-di- (3'-methyl-4'-hydroxyphenyl) propane and
2,2-Di-(3'-chlor-4'-hydroxyphenyl)propan, sowie insbesondere 2,2-di- (3'-chloro-4'-hydroxyphenyl) propane, and in particular
2,2-Di-(4'-hydroxyphenyl)propan 2,2-di- (4'-hydroxyphenyl) propane
2,2-Di-(3',5-dichlordihydroxyphenyl)propan, 2,2-di- (3 ', 5-dichlordihydroxyphenyl) propane,
1 .1 - Di-(4'-hydroxyphenyl)cyclohexan, 1: 1 - di- (4'-hydroxyphenyl) cyclohexane,
3,4'-Dihydroxybenzophenon, 3,4'-dihydroxybenzophenone,
4,4'-Dihydroxydiphenylsulfon und 4,4'-dihydroxydiphenylsulfone and
2.2- Di(3',5'-dimethyl-4'-hydroxyphenyl)propan oder deren Mischungen bevorzugt. Selbstverständlich kann man auch Mischungen von Polyalkylenterephthalaten und vollaromatischen Polyestern einsetzen. Diese enthalten im allgemeinen 20 bis 98 Gew.-% des Polyalkylen- terephthalates und 2 bis 80 Gew.-% des vollaromatischen Polyesters. 2.2- Di (3 ', 5'-dimethyl-4'-hydroxyphenyl) propane or mixtures thereof is preferred. Of course, it is also possible to use mixtures of polyalkylene terephthalates and wholly aromatic polyesters. These generally contain from 20 to 98% by weight of the polyalkylene terephthalate and from 2 to 80% by weight of the wholly aromatic polyester.
Selbstverständlich können auch Polyesterblockcopolymere wie Copolyetherester verwendet werden. Derartige Produkte sind an sich bekannt und in der Literatur, z.B. in der US-A 3651 014, beschrieben. Auch im Handel sind entsprechende Produkte erhältlich, z.B. Hytrel® (Du- Pont). Of course, polyester block copolymers such as copolyetheresters may also be used. Such products are known per se and are known in the literature, e.g. in US Pat. No. 3,651,014. Also in the trade, corresponding products are available, e.g. Hytrel® (DuPont).
Als Komponente B) können erfindungsgemäß auch halogenfreie Polycarbonate eingesetzt wer- den in Mengen von 0 bis 70, bevorzugt bis zu 60 Gew.-%. Geeignete halogenfreie Polycarbonate sind beispielsweise solche auf Basis von Diphenolen der allgemeinen Formel According to the invention, halogen-free polycarbonates can also be used as component B) in amounts of from 0 to 70, preferably up to 60% by weight. Suitable halogen-free polycarbonates are, for example, those based on diphenols of the general formula
worin Q eine Einfachbindung, eine d- bis Cs-Alkylen-, eine C2- bis C3-Alkyliden-, eine C3- bis C6-Cycloalkylidengruppe, eine C6- bis Ci2-Arylengruppe sowie -O-, -S- oder -SO2- bedeutet und m eine ganze Zahl von 0 bis 2 ist.
Die Diphenole können an den Phenylenresten auch Substituenten haben wie Ci - bis C6-Alkyl oder Ci - bis C6-Alkoxy. where Q is a single bond, a C 1 to C 5 alkylene, a C 2 to C 3 alkylidene, a C 3 to C 6 cycloalkylidene group, a C 6 to C 12 arylene group and also -O-, -S- or -SO 2 - and m is an integer from 0 to 2. The diphenols may also have substituents on the phenylene radicals, such as C 1 - to C 6 -alkyl or C 1 - to C 6 -alkoxy.
Bevorzugte Diphenole der oben genannten Formel sind beispielsweise Hydrochinon, Resorcin, 4,4'-Dihydroxydiphenyl, 2,2-Bis-(4-hydroxyphenyl)-propan, 2,4-Bis-(4-hydroxyphenyl)-Preferred diphenols of the abovementioned formula are, for example, hydroquinone, resorcinol, 4,4'-dihydroxydiphenyl, 2,2-bis (4-hydroxyphenyl) -propane, 2,4-bis (4-hydroxyphenyl) -
2methylbutan, 1 ,1 -Bis-(4-hydroxyphenyl)-cyclohexan. Besonders bevorzugt sind 2,2-Bis(4- hydroxyphenyl)-propan und 1 ,1 -Bis-(4-hydroxyphenyl)-cyclohexan, sowie 1 ,1 -bis-(4- hydroxyphenyl)-3,3,5-trimethylcyclohexan. Sowohl Homopolycarbonate als auch Copolycarbonate sind als Komponente B geeignet, bevorzugt sind neben dem Bisphenol A-Homopolymerisat die Copolycarbonate von Bisphenol A. 2methylbutane, 1,1-bis- (4-hydroxyphenyl) -cyclohexane. Particularly preferred are 2,2-bis (4-hydroxyphenyl) propane and 1, 1-bis (4-hydroxyphenyl) cyclohexane, and 1, 1-bis (4-hydroxyphenyl) -3,3,5-trimethylcyclohexane , Both homopolycarbonates and copolycarbonates are suitable as component B, the copolycarbonates of bisphenol A are preferred in addition to the bisphenol A homopolymer.
Die geeigneten Polycarbonate können in bekannter Weise verzweigt sein, und zwar vorzugsweise durch den Einbau von 0,05 bis 2,0 mol-%, bezogen auf die Summe der eingesetzten Diphenole, an mindestens trifunktionellen Verbindungen, beispielsweise solchen mit drei oder mehr als drei phenolischen OH-Gruppen. The suitable polycarbonates may be branched in a known manner, preferably by the incorporation of from 0.05 to 2.0 mol%, based on the sum of the diphenols used, of at least trifunctional compounds, for example those having three or more than three phenolic compounds OH groups.
Als besonders geeignet haben sich Polycarbonate erwiesen, die relative Viskositäten ηΓθι νοη 1 ,10 bis 1 ,50, insbesondere von 1 ,25 bis 1 ,40 aufweisen. Dies entspricht mittleren Molekular- gewichten Mw (Gewichtsmittelwert) von 10 000 bis 200 000, vorzugsweise von 20 000 bis 80 000 g/mol. Particularly suitable polycarbonates have proven, the relative viscosities η Γθ ι νοη 1, 10 to 1, 50, in particular from 1, 25 to 1, 40 have. This corresponds to average molecular weights M w (weight average) of from 10,000 to 200,000, preferably from 20,000 to 80,000 g / mol.
Die Diphenole der allgemeinen Formel sind an sich bekannt oder nach bekannten Verfahren herstellbar. The diphenols of the general formula are known per se or can be prepared by known processes.
Die Herstellung der Polycarbonate kann beispielsweise durch Umsetzung der Diphenole mit Phosgen nach dem Phasengrenzflächenverfahren oder mit Phosgen nach dem Verfahren in homogener Phase (dem sogenannten Pyridinverfahren) erfolgen, wobei das jeweils einzustellende Molekulargewicht in bekannter Weise durch eine entsprechende Menge an bekannten Kettenabbrechern erzielt wird. (Bezüglich polydiorganosiloxanhaltigen Polycarbonaten siehe beispielsweise DE-OS 33 34 782). The polycarbonates can be prepared, for example, by reacting the diphenols with phosgene by the phase boundary process or with phosgene by the homogeneous phase process (the so-called pyridine process), the molecular weight to be set in each case being achieved in a known manner by a corresponding amount of known chain terminators. (With respect polydiorganosiloxanhaltigen polycarbonates see, for example, DE-OS 33 34 782).
Geeignete Kettenabbrecher sind beispielsweise Phenol, p-t-Butylphenol aber auch langkettige Alkylphenole wie 4-(1 ,3-Tetramethyl-butyl)-phenol, gemäß DE-OS 28 42 005 oder Monoa- kylphenole oder Dialkylphenole mit insgesamt 8 bis 20 C-Atomen in den Alkylsubstituenten gemäß DE-A 3506472, wie p-Nonylphenyl, 3,5-di-t-Butylphenol, p-t-Octylphenol, p-Dodecylphenol, 2-(3,5-dimethyl-heptyl)-phenol und 4-(3,5-Dimethylheptyl)-phenol. Suitable chain terminators include phenol, pt-butylphenol but also long-chain alkylphenols such as 4- (1, 3-tetramethyl-butyl) -phenol, according to DE-OS 28 42 005 or monoalkylphenols or dialkylphenols having a total of 8 to 20 carbon atoms in the alkyl substituents according to DE-A 3506472, such as p-nonylphenyl, 3,5-di-t-butylphenol, pt-octylphenol, p-dodecylphenol, 2- (3,5-dimethyl-heptyl) -phenol and 4- (3, 5-dimethylheptyl) -phenol.
Halogenfreie Polycarbonate im Sinne der vorliegenden Erfindung bedeutet, dass die Polycar- bonate aus halogenfreien Diphenolen, halogenfreien Kettenabbrechern und gegebenenfalls halogenfreien Verzweigern aufgebaut sind, wobei der Gehalt an untergeordneten ppm-Mengen an verseifbarem Chlor, resultierend beispielsweise aus der Herstellung der Polycarbonate mit
Phosgen nach dem Phasengrenzflächenverfahren, nicht als halogenhaltig im Sinne der Erfindung anzusehen ist. Derartige Polycarbonate mit ppm-Gehalten an verseifbarem Chlor sind halogenfreie Polycarbonate im Sinne vorliegender Erfindung. Als weitere geeignete Komponenten A) seien amorphe Polyestercarbonate genannt, wobei Phosgen gegen aromatische Dicarbonsäureeinheiten wie Isophthalsäure und/oder Terephthal- säureeinheiten, bei der Herstellung ersetzt wurde. Für nähere Einzelheiten sei an dieser Stelle auf die EP-A 71 1 810 verwiesen. Halogen-free polycarbonates in the context of the present invention means that the polycarbonates are composed of halogen-free diphenols, halogen-free chain terminators and optionally halogen-free branching agents, the content of minor ppm amounts of saponifiable chlorine, resulting, for example, from the preparation of the polycarbonates Phosgene according to the interfacial process, is not to be regarded as halogen-containing in the context of the invention. Such polycarbonates with ppm contents of saponifiable chlorine are halogen-free polycarbonates in the context of the present invention. As further suitable components A) may be mentioned amorphous polyester carbonates, wherein phosgene against aromatic dicarboxylic acid units such as isophthalic acid and / or terephthalic acid units, was replaced in the preparation. For further details, reference is made at this point to EP-A 71 1 810.
Weitere geeignete Copolycarbonate mit Cycloalkylresten als Monomereinheiten sind Further suitable copolycarbonates with cycloalkyl radicals as monomer units are
EP-A 365 916 beschrieben. EP-A 365 916 described.
Weiterhin kann Bisphenol A durch Bisphenol TMC ersetzt werden. Derartige Polycarbonate sind unter dem Warenzeichen APEC HT® der Firma Bayer erhältlich. Furthermore, bisphenol A can be replaced by bisphenol TMC. Such polycarbonates are available under the trademark APEC HT® from Bayer.
Als Komponente C) enthalten die erfindungsgemäßen Formmassen 1 bis 97,5, vorzugsweise 1 bis 80 und insbesondere 1 bis 20 Gew.-% eines Copolymerisates aus As component C), the molding compositions according to the invention contain 1 to 97.5, preferably 1 to 80 and in particular 1 to 20 wt .-% of a copolymer
60 bis 95, vorzugsweise 70 bis 85 Gew.-% Styrol oder substituierten Styrolen der allgemeinen Formel I oder deren Mischungen 60 to 95, preferably 70 to 85 wt .-% of styrene or substituted styrenes of the general formula I or mixtures thereof
CK
worin R einen Alkylrest mit 1 bis 8 C-Atomen oder ein Wasserstoffatom und R1 einen Al- kylrest mit 1 bis 8 C-Atomen darstellen und n den Wert 1 , 2 oder 3 hat und CK in which R is an alkyl radical having 1 to 8 C atoms or a hydrogen atom and R 1 is an alkyl radical having 1 to 8 C atoms and n is 1, 2 or 3 and
C2) 5 bis 40, vorzugsweise 15 bis 30 Gew.-% mindestens eines ungesättigten Nitrils. C2) 5 to 40, preferably 15 to 30 wt .-% of at least one unsaturated nitrile.
Bevorzugte Reste R sind Methyl, Ethyl oder Wasserstoff und Preferred radicals R are methyl, ethyl or hydrogen and
Bevorzugte Reste R1 sind Methyl, Ethyl . Preferred radicals R 1 are methyl, ethyl.
Bevorzugte Komponenten C1) sind Styrol oder α-Methylstyrol oder deren Mischungen. Preferred components C1) are styrene or α-methylstyrene or mixtures thereof.
Bevorzugte Komponenten C2) sind Acrylnitril oder Methacrylnitril oder deren Mischungen. Preferred components C2) are acrylonitrile or methacrylonitrile or mixtures thereof.
Die Copolymerisate C sind harzartig, thermoplastisch und kautschukfrei. Besonders bevorzugte Copolymerisate C sind solche aus Styrol und Acrylnitril, aus α-Methylstyrol und Acrylnitril oder aus Styrol, α-Methylstyrol und Acrylnitril. Es können auch mehrere der beschriebenen Copoly- mere gleichzeitig eingesetzt werden.
Die Copolimerisate C sind an sich bekannt und lassen sich durch radikalische Polymerisation, insbesondere durch Emulsions-, Suspensions-, Lösungs- und Massepolymerisation herstellen. Sie weisen Viskositätszahlen im Bereich von 40 bis 160 auf, dies entspricht mittleren Molekulargewichten Mw (Gewichtsmittelwert) von 40 000 bis 2 000 000. The copolymers C are resinous, thermoplastic and rubber-free. Particularly preferred copolymers C are those of styrene and acrylonitrile, of α-methylstyrene and acrylonitrile or of styrene, α-methylstyrene and acrylonitrile. It is also possible to use several of the copolymers described at the same time. The Copolimerisate C are known per se and can be prepared by free-radical polymerization, in particular by emulsion, suspension, solution and bulk polymerization. They have viscosity numbers in the range of 40 to 160, which corresponds to average molecular weights Mw (weight average) of 40,000 to 2,000,000.
Als Komponente D) enthalten die erfindungsgemäßen Formmassen 0,5 bis 30, vorzugsweise 1 bis 20 und insbesondere 1 bis 10 Gew.-% (bezogen auf A bis F) eines Copolymeren aus di) 49,5 bis 93,5 Gew.-% sich von einem oder mehreren vinylaromatischen Monomeren ab- leitende Struktureinheiten, As component D), the molding compositions according to the invention contain 0.5 to 30, preferably 1 to 20 and in particular 1 to 10 wt .-% (based on A to F) of a copolymer of di) 49.5 to 93.5 wt .-% Structural units derived from one or more vinylaromatic monomers,
d2) 6 bis 50 Gew.-% sich von einem oder mehreren Vinylcyaniden ableitende Struktureinheiten, d2) from 6 to 50% by weight of structural units derived from one or more vinyl cyanides,
Ö3) 0,5 bis 2,4 Gew.-% sich von einem oder mehreren Dicarbonsäureanhydriden ableitende Struktureinheiten und Ö3) 0.5 to 2.4 wt .-% of one or more dicarboxylic anhydrides derived structural units and
d4) 0 bis 25 Gew.-% sich von weiteren copolymerisierbaren Monomeren ableitende Struktureinheiten, wobei die Gew.-% jeweils auf das Gesamtgewicht der sich von den Komponenten di), d2), dß) und d4) ableitenden Struktureinheiten bezogen sind und zusammen 100 Gew.-% ergeben. d 4) from 0 to 25 wt .-% which derives from other copolymerizable monomers structural units, wherein the wt .-% in each case on the total weight of itself), and d 4) structural units derived are obtained from the components di), d2), DSS and together 100% by weight.
Bevorzugte Komponenten D enthaltenPreferred components D are included
d2) 6 bis 50 Gew.-% d 2 ) 6 to 50% by weight
d3) 0,8 bis 2,2 Gew.-% d 3 ) 0.8 to 2.2% by weight
d4) 0 bis 25 Gew.-%. d 4 ) 0 to 25 wt .-%.
Als Komponente di) kommen alle dem Fachmann bekannten und im Stand der Technik, beispielsweise DE 100 58 302 A1 , beschriebenen vinylaromatischen Monomere in Betracht; bevorzugt werden Styrol, α-Methylstyrol, p-Methylstyrol, t-Butylstyrol, Vinylnaphthalin oder deren Mischungen eingesetzt; besonders bevorzugt wird Styrol eingesetzt. Suitable components di) are all vinylaromatic monomers known to the person skilled in the art and described in the prior art, for example DE 100 58 302 A1; Styrene, α-methylstyrene, p-methylstyrene, t-butylstyrene, vinylnaphthalene or mixtures thereof are preferably used; Styrene is particularly preferably used.
Als Komponente 2) kommen alle dem Fachmann bekannten und im Stand der Technik, beispielsweise DE 25 40 517 A1 , beschriebenen Vinylcyanide in Betracht; bevorzugt werden Acrylnitril, Methacrylnitril oder deren Mischungen eingesetzt; besonders bevorzugt wird Acryl- nitril eingesetzt. Suitable components 2) are all vinyl cyanides known to the person skilled in the art and described in the prior art, for example DE 25 40 517 A1; acrylonitrile, methacrylonitrile or mixtures thereof are preferably used; Acrylonitrile is particularly preferably used.
Als Komponente dß) kommen alle dem Fachmann bekannten und im Stand der Technik beschriebenen Dicarbonsäureanhydride in Betracht; bevorzugt werden Maleinsäureanhydrid, Methylmaleinsäureanhydrid, Itaconsäureanhydrid oder deren Mischungen eingesetzt; besonders bevorzugt wird Maleinsäureanhydrid eingesetzt.
Als Komponente d4) der erfindungsgemäßen Copolymere D) können weitere, mit den Komponenten di), d2) und 3) copolymerisierbare und von diesen verschiedene Monomere eingesetzt werden, welche dem Fachmann geläufig sind. Besonders bevorzugt aufgebaut sind die Copolymeren D) aus Styrol-Acrylnitril-Maleinsäure- anhydrid-Copolymeren. Suitable components d)) are all dicarboxylic anhydrides known to the person skilled in the art and described in the prior art; Preference is given to using maleic anhydride, methylmaleic anhydride, itaconic anhydride or mixtures thereof; Particular preference is given to using maleic anhydride. As component d 4 ) of the copolymers D) according to the invention, it is possible to use further monomers copolymerizable with the components di), d2) and 3) and different from these, which are familiar to the person skilled in the art. Particularly preferred are the copolymers D) of styrene-acrylonitrile-maleic anhydride copolymers.
Die Herstellung der Copolymeren D) erfolgt durch Masse- oder Lösungspolymerisation, bevorzugt jedoch als Lösungspolymerisation in Anwesenheit eines organischen Lösungsmittels, bei- spielsweise Cyclohexan, Ethylbenzol, Toluol oder Dimethylsulfoxid, bevorzugt Ethylbenzol. The preparation of the copolymers D) is carried out by bulk or solution polymerization, but preferably as a solution in the presence of an organic solvent, for example cyclohexane, ethylbenzene, toluene or dimethyl sulfoxide, preferably ethylbenzene.
Sowohl bei der Lösungs- als auch bei der Massepolymerisation kann die Initiierung der Polymerisationsreaktion grundsätzlich durch Zugabe chemischer Polymerisationsinitiatoren erfolgen, wie beispielsweise in DE 100 58 302 A1 beschrieben; bevorzugt erfolgt die Initiierung aber rein thermisch, also ohne Zusatz eines Polymerisationsinitiators. Die Herstellung kann in einem Batch- oder Semibatch-Verfahren erfolgen, bevorzugt wird jedoch eine kontinuierliche Verfahrensführung durchgeführt. In both the solution and the bulk polymerization, the initiation of the polymerization reaction can in principle be effected by adding chemical polymerization initiators, as described, for example, in DE 100 58 302 A1; Preferably, however, the initiation takes place purely thermally, ie without the addition of a polymerization initiator. The preparation can be carried out in a batch or semibatch process, but preferably a continuous process is carried out.
In einer insbesondere bevorzugten Ausführungsform der erfindungsgemäßen Verfahren erfolgt die Verfahrensführung kontinuierlich unter stationären Bedingungen; unter stationären Bedingungen bedeutet: die Konzentrationen sämtlicher Reaktionsteilnehmer und die Zusammensetzung der gebildeten Copolymere A) bleiben über die Zeitdauer der Reaktion praktisch konstant (Angaben zum Zusammenhang zwischen Monomer- und Polymerzusammensetzung sowie zur stationären Reaktionsführung können insbesondere EP 0 001 625 A1 und In a particularly preferred embodiment of the process according to the invention, the process is carried out continuously under steady-state conditions; under steady state conditions means: the concentrations of all the reactants and the composition of the copolymers A) formed remain virtually constant over the duration of the reaction (information on the relationship between monomer and polymer composition and for stationary reaction can be particularly EP 0 001 625 A1 and
DE 25 40 517 A1 entnommen werden). DE 25 40 517 A1).
Geeignete Verfahrensparameter, wie Druck, Temperatur, Verweilzeiten etc., geeignete Apparate zur Durchführung der Verfahren sowie geeignete Mengenstromdosierungen der Monomere, falls vorhanden der Lösungsmittel, falls vorhanden der Initiatoren und ggf. weiterer Polymerisa- tionszusätze sind dem Fachmann bekannt und im Stand der Technik beschrieben. Suitable process parameters, such as pressure, temperature, residence times, etc., suitable apparatuses for carrying out the process and suitable metered flow of the monomers, if any of the solvents, if any of the initiators and optionally further polymerization addition additives are known in the art and described in the prior art ,
Die Aufarbeitung der Polymerisationsmischung und die Isolierung der Copolymere D) kann nach dem Fachmann bekannten und im Stand der Technik beschriebenen Methoden erfolgen, beispielsweise durch Abtrennung niedermolekularer Verbindungen mittels Anlegen von Vakuum oder Strippen mit Inertgas. The workup of the polymerization mixture and the isolation of the copolymers D) can be carried out by methods known in the art and described in the prior art, for example by separation of low molecular weight compounds by applying a vacuum or stripping with inert gas.
Bevorzugte Komponenten D) weisen einen Unterschied des Nitrilgehaltes von weniger als 10 Gew.-% zum Nitrilgehalt der Komponente C) auf. Ein oder eine Mischung unterschiedlicher Pfropfcopolymerisate werden als Komponente E) in den erfindungsgemäßen Formmassen in Mengen von 0 bis 50 Gew.-%, bezogen auf die Summe der Komponenten A bis F, eingesetzt. Bevorzugte erfindungsgemäße Formmassen enthal-
ten von 1 bis 40, besonders bevorzugt von 1 bis 20 Gew.-%, mindestens eines Pfropfcopolyme- risates E. Preferred components D) have a nitrile content difference of less than 10% by weight to the nitrile content of component C). One or a mixture of different graft copolymers are used as component E) in the molding compositions according to the invention in amounts of from 0 to 50% by weight, based on the sum of components A to F. Preferred molding compositions according to the invention contain from 1 to 40, particularly preferably from 1 to 20,% by weight, of at least one graft copolymer E.
Die Pfropfpolymerisate E sind aufgebaut aus The graft polymers E are composed of
40 bis 80 Gew.-%, vorzugsweise 50 bis 70 Gew.-% einer Pfropfgrundlage aus einem kautschukelastischen Polymeren auf der Basis von Alkylacrylaten mit 1 bis 8 C-Atomen im Alkylrest und mit einer Glasübergangstemperatur von unter 0°C E2) 20 bis 60 Gew.-%, vorzugsweise 30 bis 50 Gew.-% einer Pfropfauflage aus 40 to 80 wt .-%, preferably 50 to 70 wt .-% of a graft base of a rubber-elastic polymers based on alkyl acrylates having 1 to 8 carbon atoms in the alkyl radical and having a glass transition temperature of below 0 ° C E2) 20 to 60 Wt .-%, preferably 30 to 50 wt .-% of a graft
E21) 60 bis 95 Gew.-%, bevorzugt 70 bis 85 Gew.-% Styrol oder substituierten Styrolen der allgemeinen Formel I E22) 5 bis 40 Gew.-%, vorzugsweise 15 bis 30 Gew.-% mindestens eines ungesättigten Nitrils, bevorzugt Acrylnitril oder Methacrylnitril oder deren Mischungen. E21) 60 to 95 wt .-%, preferably 70 to 85 wt .-% of styrene or substituted styrenes of the general formula I E22) 5 to 40 wt .-%, preferably 15 to 30 wt .-% of at least one unsaturated nitrile, preferably Acrylonitrile or methacrylonitrile or mixtures thereof.
Für die Pfropfgrundlage Ei kommen Polymerisate in Betracht, deren Glasübergangstemperatur unterhalb 10°C, vorzugsweise unterhalb von 0°C, besonders bevorzugt unterhalb von -20°C liegt. Dies sind z.B. Elastomere auf der Basis von C1- bis C8-Alkylestern der Acrylsäure, die gegebenenfalls weitere Comonomere enthalten können. For the graft base egg polymers come into consideration whose glass transition temperature is below 10 ° C, preferably below 0 ° C, more preferably below -20 ° C. These are e.g. Elastomers based on C1- to C8-alkyl esters of acrylic acid, which may optionally contain further comonomers.
Bevorzugt sind Pfropfgrundlagen Ei , die aufgebaut sind aus E11) 69,9 bis 99,9 Gew.-%, vorzugsweise 99 Gew.-% mindestens eines Alkylacrylates mit 1 bis 8 C-Atomen im Alkylrest, vorzugsweise n-Butylacrylat und/oder 2-Ethyl-hexylacrylat, insbesondere n-Butylacrylat als alleiniges Alkylacrylat Preference is given to graft bases Ei which are composed of E11) 69.9 to 99.9% by weight, preferably 99% by weight, of at least one alkyl acrylate having 1 to 8 C atoms in the alkyl radical, preferably n-butyl acrylate and / or 2 Ethylhexyl acrylate, especially n-butyl acrylate as the sole alkyl acrylate
E12) 0 bis 30 Gew.-%, insbesondere 20 bis 30 Gew.-% eines weiteren copolymerisierbaren monoethylenisch ungesättigten Monomeren, wie Styrol, Acrylnitril, Methylmethacrylat oder Vinylmethylether oder deren Mischungen E12) 0 to 30 wt .-%, in particular 20 to 30 wt .-% of a further copolymerizable monoethylenically unsaturated monomers such as styrene, acrylonitrile, methyl methacrylate or vinyl methyl ether or mixtures thereof
E13) 0,1 bis 5 Gew.-%, vorzugsweise 1 bis 4 Gew.-% eines copolymerisierbaren, polyfunktionellen, vorzugsweise bi- oder tri-funktionellen, die Vernetzung bewirkenden Monomeren. E13) 0.1 to 5 wt .-%, preferably 1 to 4 wt .-% of a copolymerizable, polyfunctional, preferably bi- or tri-functional, causing the crosslinking monomers.
Als solche bi- oder polyfunktionellen Vernetzungsmonomeren E13) eignen sich Monomere, die vorzugsweise zwei, gegebenenfalls auch drei oder mehr, zur Copolymerisation befähigte ethy- lenische Doppelbindungen enthalten, die nicht in den 1 ,3-Stellungen konjugiert sind. Geeignete Vernetzungsmonomere sind beispielsweise Divinylbenzol, Diallylmaleat, Diallylfumarat, Dial- lylphthalat, Triallylcyanurat oder Triallylisocyanurat. Als besonders günstiges Vernetzungsmo- nomeres hat sich der Acrylsäureester des Tricyclodecenylalkohols erwiesen
(vgl. DE-A 12 60 135). Suitable bi- or polyfunctional crosslinking monomers E13) are monomers which preferably contain two, optionally also three or more, ethylenic double bonds which are capable of copolymerization and are not conjugated in the 1, 3-positions. Suitable crosslinking monomers are, for example, divinylbenzene, diallyl maleate, diallyl fumarate, dialyl phthalate, triallyl cyanurate or triallyl isocyanurate. A particularly favorable crosslinking monomer has proven to be the acrylic acid ester of tricyclodecenyl alcohol (see DE-A 12 60 135).
Diese Art von Pfropfgrundlagen ist an sich bekannt und in der Literatur beschrieben, beispielsweise in der DE-A 31 49 358. This type of grafting is known per se and described in the literature, for example in DE-A 31 49 358.
Von den Pfropfauflagen E2 sind diejenigen bevorzugt, in denen E21 Styrol oder a-Methylstyrol oder deren Mischungen und bei E22 Acrylnitril oder Methacrylnitril bedeutet. Als bevorzugte Monomerengemische werden vor allem Styrol und Acrylnitril oder α-Methylstyrol und Acrylnitril eingesetzt. Die Pfropfauflagen sind erhältlich durch Copolymerisation der Komponenten E21 und E22. Of the graft stocks E2, preference is given to those in which E21 is styrene or α-methylstyrene or mixtures thereof and E22 is acrylonitrile or methacrylonitrile. The preferred monomer mixtures used are, in particular, styrene and acrylonitrile or .alpha.-methylstyrene and acrylonitrile. The grafts are obtainable by copolymerizing the components E21 and E 22nd
Die Pfropfgrundlage Ei der Pfropfpolymerisate E), die aus den Komponenten En , gegebenenfalls E12, und E13 aufgebaut ist, wird auch als ASA-Kautschuk bezeichnet. Die Herstellung ist an sich bekannt und beispielsweise in der DE-A 28 26 925, der DE-A 31 49 358 und der The graft base Ei of the graft polymers E), which is composed of the components En, optionally E12, and E13, is also referred to as ASA rubber. The preparation is known per se and, for example, in DE-A 28 26 925, DE-A 31 49 358 and
DE-A 3414 1 18 beschrieben. DE-A 3414 1 18 described.
Die Herstellung der Pfropfpolymerisate E kann beispielsweise nach der in der DE-PS 12 60 135 beschriebenen Methode erfolgen. Der Aufbau der Pfropfauflage (Pfropfhülle) der Pfropfpolymerisate kann ein- oder zweistufig erfolgen. The preparation of the graft polymers E can be carried out, for example, by the method described in DE-PS 12 60 135. The structure of the graft (graft) of the graft polymers can be carried out in one or two stages.
Im Falle des einstufigen Aufbaues der Pfropfhülle wird ein Gemisch der Monomeren E21 und E22 in dem gewünschten Gew.-Verhältnis im Bereich 95:5 bis 50:50, vorzugsweise von 90:10 bis 65:35 in Gegenwart des Elastomeren Ei , in an sich bekannter Weise (vgl. z.B. DE-OS 28 26 925), vorzugsweise in Emulsion, polymerisiert. In the case of the single-stage construction of the graft, a mixture of the monomers E21 and E22 in the desired weight ratio in the range 95: 5 to 50:50, preferably from 90:10 to 65:35 in the presence of the elastomer Ei, in per se known manner (cf., for example, DE-OS 28 26 925), preferably in emulsion, polymerized.
Im Falle eines zweistufigen Aufbaus der Pfropfhülle E2 macht die 1. Stufe im allgemeinen 20 bis 70 Gew.-%, vorzugsweise 25 bis 50 Gew.-%, bezogen auf E2, aus. Zu ihrer Herstellung werden vorzugsweise nur Styrol oder substituierte Styrole oder deren Mischungen (E21) verwendet. In the case of a two-stage structure of the graft shell E2, the 1st stage generally constitutes from 20 to 70% by weight, preferably from 25 to 50% by weight, based on E2. For their preparation, preferably only styrene or substituted styrenes or mixtures thereof (E21) are used.
Die 2. Stufe der Pfropfhülle macht im allgemeinen 30 bis 80 Gew.-%, insbesondere 50 bis 75 Gew.-%, jeweils bezogen auf E2, aus. Zu ihrer Herstellung werden Mischungen aus den Monomeren E21 und den Nitrilen E22 im Gewichtsverhältnis E21/E22 von im allgemeinen 90:10 bis 60:40, insbesondere 80:20 bis 70:30 angewendet. The second stage of the graft shell generally accounts for 30 to 80 wt .-%, in particular 50 to 75 wt .-%, each based on E2, from. For their preparation, mixtures of the monomers E21 and the nitriles E22 in weight ratio E21 / E22 of generally 90:10 to 60:40, in particular 80:20 to 70:30 applied.
Die Bedingungen der Pfropfpolymerisation werden vorzugsweise so gewählt, daß Teilchengrößen von 50 bis 700 nm (d50-Wert der integralen Massenverteilung) resultieren. Maßnahmen hierzu sind bekannt und z.B. in der DE-OS 2826925 beschrieben.
Durch das Saatlatex-Verfahren kann direkt eine grobteilige Kautschukdispersion hergestellt werden. The conditions of the graft polymerization are preferably chosen so that particle sizes of 50 to 700 nm (d50 value of the integral mass distribution) result. Measures for this purpose are known and described for example in DE-OS 2826925. The seed latex process can be used to directly produce a coarse-particle rubber dispersion.
Um möglichst zähe Produkte zu erzielen, ist es häufig von Vorteil, eine Mischung mindestens zweier Pfropfpolymerisate mit unterschiedlicher Teilchengröße zu verwenden. In order to obtain products which are as tough as possible, it is often advantageous to use a mixture of at least two graft polymers having different particle sizes.
Um dies zu erreichen, werden die Teilchen des Kautschuks in bekannter Weise, z.B. durch Agglomeration, vergrößert, so dass der Latex bimodal (50 bis 180 nm und 200 bis 700 nm) aufgebaut ist. To achieve this, the particles of the rubber are prepared in a known manner, e.g. by agglomeration, so that the latex is bimodal (50 to 180 nm and 200 to 700 nm).
In einer bevorzugten Ausführungsform wird eine Mischung aus zwei Pfropfpolymerisaten mit Teilchendurchmessern (d50-Wert der integralen Massenverteilung) von 50 bis 180 nm bzw. 200 bis 700 nm im Gewichtsverhältnis 70:30 bis 30:70 eingesetzt. Der chemische Aufbau der beiden Pfropfpolymerisate ist vorzugsweise derselbe, obwohl die Hülle des grobteiligen Pfropfpolymerisates insbesondere auch zweistufig aufgebaut werden kann. In a preferred embodiment, a mixture of two graft polymers having particle diameters (d 50 value of the integral mass distribution) of from 50 to 180 nm or from 200 to 700 nm in a weight ratio of from 70:30 to 30:70 is used. The chemical structure of the two graft polymers is preferably the same, although the shell of the coarse-particulate graft polymer can be constructed in two stages in particular.
Als Komponente E) können auch Ethylencopolymere, Ethylen-Propylencopolymere, Polyester- elastomere oder thermoplastische Polyurethane eingesetzt werden. As component E) it is also possible to use ethylene copolymers, ethylene-propylene copolymers, polyester elastomers or thermoplastic polyurethanes.
Ganz allgemein handelt es sich dabei um Copolymerisate die bevorzugt aus mindestens zwei der folgenden Monomeren aufgebaut sind: Ethylen, Propylen, Isobuten, Chloropren, Vinylace- tat, Styrol, Acrylnitril und Acryl- bzw. Methacrylsäureester mit 1 bis 18 C-Atomen in der Alkohol- komponente. In general, these are copolymers which are preferably composed of at least two of the following monomers: ethylene, propylene, isobutene, chloroprene, vinyl acetate, styrene, acrylonitrile and acrylic or methacrylic acid esters having 1 to 18 C atoms in the alcohol - component.
Derartige Polymere werden z.B. in Houben-Weyl, Methoden der organischen Chemie, Bd. 14/1 (Georg-Thieme-Verlag, Stuttgart, 1961 ), Seiten 392 bis 406 und in der Monographie von C.B. Bucknall, "Toughened Plastics" (Applied Science Publishers, London, 1977) beschrieben. Such polymers are e.g. in Houben-Weyl, Methods of Organic Chemistry, Vol. 14/1 (Georg Thieme Verlag, Stuttgart, 1961), pages 392 to 406 and in the monograph by C.B. Bucknall, "Toughened Plastics" (Applied Science Publishers, London, 1977).
Als Komponente F) können die erfindungsgemäßen Formmassen 0 bis 60, insbesondere bis zu 50 Gew.- % weiterer Zusatzstoffe enthalten. As component F), the molding compositions of the invention may contain from 0 to 60, in particular up to 50% by weight of further additives.
Als Komponente F) können die erfindungsgemäßen Formmassen 0 bis 5, vorzugsweise 0,05 bis 3 und insbesondere 0,1 bis 2 Gew.-% mindestens eines Esters oder Amids gesättigter oder ungesättigter aliphatischer Carbonsäuren mit 10 bis 40, bevorzugt 16 bis 22 C-Atomen mit a- liphatischen gesättigten Alkoholen oder Aminen mit 2 bis 40, vorzugsweise 2 bis 6 C-Atomen enthalten. As component F), the molding compositions according to the invention may contain 0 to 5, preferably 0.05 to 3 and in particular 0.1 to 2% by weight of at least one ester or amide of saturated or unsaturated aliphatic carboxylic acids having 10 to 40, preferably 16 to 22 carbon atoms. Atoms containing aliphatic saturated alcohols or amines having 2 to 40, preferably 2 to 6 carbon atoms.
Die Carbonsäuren können 1 - oder 2-wertig sein. Als Beispiele seien Pelargonsäure, Palmitin- säure, Laurinsäure, Margarinsäure, Dodecandisäure, Behensäure und besonders bevorzugt Stearinsäure, Caprinsäure sowie Montansäure (Mischung von Fettsäuren mit 30 bis 40 C- Atomen) genannt.
Die aliphatischen Alkohole können 1 - bis 4-wertig sein. Beispiele für Alkohole sind n-Butanol, n- Octanol, Stearylalkohol, Ethylenglykol, Propylenglykol, Neopentylglykol, Pentaerythrit, wobei Glycerin und Pentaerythrit bevorzugt sind. Die aliphatischen Amine können 1 - bis 3-wertig sein. Beispiele hierfür sind Stearylamin, Ethylendiamin, Propylendiamin, Hexamethylendiamin, Di(6-Aminohexyl)amin, wobei Ethylendiamin und Hexamethylendiamin besonders bevorzugt sind. Bevorzugte Ester oder Amide sind entsprechend Glycerindistearat, Glycerintristearat, Ethylendiamindistearat, Glycerinmonopalmitat, Glycerintrilaurat, Glycerinmonobehenat und Pentaerythrittetrastearat. The carboxylic acids can be 1- or 2-valent. Examples which may be mentioned are pelargonic acid, palmitic acid, lauric acid, margaric acid, dodecanedioic acid, behenic acid and particularly preferably stearic acid, capric acid and montanic acid (mixture of fatty acids having 30 to 40 carbon atoms). The aliphatic alcohols can be 1 - to 4-valent. Examples of alcohols are n-butanol, n-octanol, stearyl alcohol, ethylene glycol, propylene glycol, neopentyl glycol, pentaerythritol, with glycerol and pentaerythritol being preferred. The aliphatic amines can be 1 - to 3-valent. Examples of these are stearylamine, ethylenediamine, propylenediamine, hexamethylenediamine, di (6-aminohexyl) amine, with ethylenediamine and hexamethylenediamine being particularly preferred. Accordingly, preferred esters or amides are glycerin distearate, glycerol tristearate, ethylenediamine distearate, glycerin monopalmitate, glycerol trilaurate, glycerin monobehenate and pentaerythritol tetrastearate.
Es können auch Mischungen verschiedener Ester oder Amide oder Ester mit Amiden in Kombination eingesetzt werden, wobei das Mischungsverhältnis beliebig ist. It is also possible to use mixtures of different esters or amides or esters with amides in combination, the mixing ratio being arbitrary.
Als faser- oder teilchenförmige Füllstoffe F) seien Kohlenstofffasern, Glasfasern, Glaskugeln, amorphe Kieselsäure, Asbest, Calciumsilicat, Calciummetasilicat, Magnesiumcarbonat, Kaolin, Kreide, gepulverter Quarz, Glimmer, Bariumsulfat und Feldspat genannt, die in Mengen bis zu 60 Gew.-%, insbesondere bis zu 50 % eingesetzt werden. Fibrous or particulate fillers F) which may be mentioned are carbon fibers, glass fibers, glass spheres, amorphous silica, asbestos, calcium silicate, calcium metasilicate, magnesium carbonate, kaolin, chalk, powdered quartz, mica, barium sulfate and feldspar, in amounts of up to 60% by weight. , in particular up to 50%.
Als bevorzugte faserförmige Füllstoffe seien Kohlenstofffasern, Aramid-Fasern und Kaliumtita- nat-Fasern genannt, wobei Glasfasern als E-Glas besonders bevorzugt sind. Diese können als Rovings oder Schnittglas in den handelsüblichen Formen eingesetzt werden. Preferred fibrous fillers are carbon fibers, aramid fibers and potassium titanate fibers, glass fibers being particularly preferred as E glass. These can be used as rovings or cut glass in the commercial forms.
Die faserförmigen Füllstoffe können zur besseren Verträglichkeit mit den Thermoplasten mit einer Silanverbindung oberflächlich vorbehandelt sein. The fibrous fillers can be surface-pretreated for better compatibility with the thermoplastics with a silane compound.
Geeignete Silanverbindungen sind solche der allgemeinen Formel Suitable silane compounds are those of the general formula
(X-(C H 2) n) k-S i-(0-Cm H 2m+ 1 )4-k in der die Substituenten folgende Bedeutung haben: (X- (CH 2) n) kS i- (O-C m H 2m + 1) 4 -k in which the substituents have the following meanings:
X NH2- , CH— CH-, HO-, X is NH 2 -, CH-CH-, HO-,
\ / \ /
O n eine ganze Zahl von 2 bis 10, bevorzugt 3 bis 4 O n is an integer from 2 to 10, preferably 3 to 4
m eine ganze Zahl von 1 bis 5, bevorzugt 1 bis 2 m is an integer from 1 to 5, preferably 1 to 2
k eine ganze Zahl von 1 bis 3, bevorzugt 1 k is an integer from 1 to 3, preferably 1
Bevorzugte Silanverbindungen sind Aminopropyltrimethoxysilan, Aminobutyltrimethoxysilan, Aminopropyltriethoxysilan, Aminobutyltriethoxysilan sowie die entsprechenden Silane, welche als Substituent X eine Glycidylgruppe enthalten.
Die Silanverbindungen werden im allgemeinen in Mengen von 0,05 bis 5, vorzugsweise 0,5 bis 1 ,5 und insbesondere 0,8 bis 1 Gew.-% (bezogen auf F) zur Oberflächenbeschichtung eingesetzt. Geeignet sind auch nadeiförmige mineralische Füllstoffe. Preferred silane compounds are aminopropyltrimethoxysilane, aminobutyltrimethoxysilane, aminopropyltriethoxysilane, aminobutyltriethoxysilane and the corresponding silanes which contain a glycidyl group as substituent X. The silane compounds are generally used in amounts of 0.05 to 5, preferably 0.5 to 1, 5 and in particular 0.8 to 1 wt .-% (based on F) for surface coating. Also suitable are acicular mineral fillers.
Unter nadeiförmigen mineralischen Füllstoffen wird im Sinne der Erfindung ein mineralischer Füllstoff mit stark ausgeprägtem nadeiförmigen Charakter verstanden. Als Beispiel sei nadeiförmiger Wollastonit genannt. Vorzugsweise weist das Mineral ein L/D (Länge Durchmesser)- Verhältnis von 8 : 1 bis 35 : 1 , bevorzugt von 8 : 1 bis 1 1 : 1 auf. Der mineralische Füllstoff kann gegebenenfalls mit den vorstehend genannten Silanverbindungen vorbehandelt sein; die Vorbehandlung ist jedoch nicht unbedingt erforderlich. In the context of the invention, the term "needle-shaped mineral fillers" is understood to mean a mineral filler with a pronounced, needle-like character. An example is acicular wollastonite. Preferably, the mineral has an L / D (length diameter) ratio of 8: 1 to 35: 1, preferably 8: 1 to 1: 1: 1. The mineral filler may optionally be pretreated with the silane compounds mentioned above; however, pretreatment is not essential.
Als weitere Füllstoffe seien Kaolin, calciniertes Kaolin, Talkum und Kreide genannt. Kaolin, calcined kaolin, talc and chalk are mentioned as further fillers.
Als Komponente F können die erfindungsgemäßen thermoplastischen Formmassen übliche Verarbeitungshilfsmittel wie Stabilisatoren, Oxidationsverzögerer, Mittel gegen Wärmezersetzung und Zersetzung durch ultraviolettes Licht, Gleit- und Entformungsmittel, Färbemittel wie Farbstoffe und Pigmente, Keimbildungsmittel, Weichmacher usw. enthalten. Als Beispiele für Oxidationsverzögerer und Wärmestabilisatoren sind sterisch gehinderte Phenole und/oder Phosphite, Hydrochinone, aromatische sekundäre Amine wie Diphenylamine, verschiedene substituierte Vertreter dieser Gruppen und deren Mischungen in Konzentrationen bis zu 1 Gew.-%, bezogen auf das Gewicht der thermoplastischen Formmassen genannt. Als UV-Stabilisatoren, die im allgemeinen in Mengen bis zu 2 Gew.-%, bezogen auf die Formmasse, verwendet werden, seien verschiedene substituierte Resorcine, Salicylate, Benzotriazo- le und Benzophenone genannt. As component F, the thermoplastic molding compositions according to the invention may contain conventional processing aids such as stabilizers, antioxidants, agents against thermal decomposition and decomposition by ultraviolet light, lubricants and mold release agents, colorants such as dyes and pigments, nucleating agents, plasticizers, etc. Examples of antioxidants and heat stabilizers sterically hindered phenols and / or phosphites, hydroquinones, aromatic secondary amines such as diphenylamines, various substituted representatives of these groups and mixtures thereof in concentrations up to 1 wt .-%, based on the weight of the thermoplastic molding compositions mentioned. As UV stabilizers, which are generally used in amounts of up to 2 wt .-%, based on the molding composition, various substituted resorcinols, salicylates, Benzotriazo- le and benzophenones may be mentioned.
Als Umesterungsstabilisatoren seien Irgafos® PEPQ sowie Phosphate (z.B. Monozinkphos- phat) genannt. As transesterification stabilizers, mention may be made of Irgafos® PEPQ and phosphates (for example monozine phosphate).
Es können anorganische Pigmente, wie Titandioxid, Ultramarinblau, Eisenoxid und Ruß, weiterhin organische Pigmente, wie Phthalocyanine, Chinacridone, Perylene sowie Farbstoffe, wie Nigrosin und Anthrachinone als Farbmittel zugesetzt werden. It is possible to add inorganic pigments such as titanium dioxide, ultramarine blue, iron oxide and carbon black, furthermore organic pigments such as phthalocyanines, quinacridones, perylenes and also dyes such as nigrosine and anthraquinones as colorants.
Als Keimbildungsmittel können Natriumphenylphosphinat, Aluminiumoxid, Siliziumdioxid sowie bevorzugt Talkum eingesetzt werden. As nucleating agents, sodium phenylphosphinate, alumina, silica and preferably talc may be used.
Weitere Gleit- und Entformungsmittel werden üblicherweise in Mengen bis zu 1 Gew.-% einge- setzt. Es sind bevorzugt langkettige Fettsäuren (z.B. Stearinsäure oder Behensäure), derenOther lubricants and mold release agents are usually used in amounts of up to 1% by weight. Preferred are long-chain fatty acids (e.g., stearic acid or behenic acid) whose
Salze (z.B. Ca- oder Zn-Stearat) oder Montanwachse (Mischungen aus geradkettigen, gesättig-
ten Carbonsäuren mit Kettenlängen von 28 bis 32 C-Atomen) sowie Ca- oder Na-Montanat sowie niedermolekulare Polyethylen- bzw. Polypropylenwachse. Salts (eg Ca or Zn stearate) or montan waxes (mixtures of straight-chain, saturated th carboxylic acids with chain lengths of 28 to 32 carbon atoms) and Ca or Na montanate and low molecular weight polyethylene or polypropylene waxes.
Als Beispiele für Weichmacher seien Phthalsäuredioctylester, Phthalsäuredibenzylester, Phthalsäurebutylbenzylester, Kohlenwasserstofföle, N-(n-Butyl)benzolsulfonamid genannt. Examples of plasticizers are dioctyl phthalate, dibenzyl phthalate, butyl benzyl phthalate, hydrocarbon oils, N- (n-butyl) benzenesulfonamide.
Die erfindungsgemäßen Formmassen können noch 0 bis 2 Gew.-% fluorhaltige Ethylenpolyme- risate enthalten. Hierbei handelt es sich um Polymerisate des Ethylens mit einem Fluorgehalt von 55 bis 76 Gew.-%, vorzugsweise 70 bis 76 Gew.-%. The molding compositions according to the invention may still contain from 0 to 2% by weight of fluorine-containing ethylene polymer. These are polymers of ethylene with a fluorine content of 55 to 76 wt .-%, preferably 70 to 76 wt .-%.
Beispiele hierfür sind Polytetrafluorethylen (PTFE), Tetrafluorethylenhexafluorpropylen- Copolymere oder Tetrafluorethylen-Copolymerisate mit kleineren Anteilen (in der Regel bis zu 50 Gew.-%) copolymerisierbarer ethylenisch ungesättigter Monomerer. Diese werden z.B. von Schildknecht in "Vinyl and Related Polymers", Wiley-Verlag, 1952, Seite 484 bis 494 und von Wall in "Fluorpolymers" (Wiley Interscience, 1972) beschrieben. Examples of these are polytetrafluoroethylene (PTFE), tetrafluoroethylene-hexafluoropropylene copolymers or tetrafluoroethylene copolymers with smaller amounts (generally up to 50% by weight) of copolymerizable ethylenically unsaturated monomers. These are e.g. by Schildknecht in "Vinyl and Related Polymers", Wiley-Verlag, 1952, pages 484 to 494 and by Wall in "Fluoropolymers" (Wiley Interscience, 1972).
Diese fluorhaltigen Ethylenpolymerisate liegen homogen verteilt in den Formmassen vor und weisen bevorzugt eine Teilchengröße dso (Zahlenmittelwert) im Bereich von 0,05 bis 1 μηη, insbesondere von 0,1 bis 5 μηη auf. Diese geringen Teilchengrößen lassen sich besonders bevor- zugt durch Verwendung von wässrigen Dispersionen von fluorhaltigen Ethylenpolymerisaten und deren Einarbeitung in eine Polyesterschmelze erzielen. These fluorine-containing ethylene polymers are homogeneously distributed in the molding compositions and preferably have a particle size dso (number average) in the range of 0.05 to 1 μηη, in particular from 0.1 to 5 μηη. These small particle sizes can be achieved particularly preferably by using aqueous dispersions of fluorine-containing ethylene polymers and incorporating them into a polyester melt.
Die erfindungsgemäßen thermoplastischen Formmassen können nach an sich bekannten Verfahren hergestellt werden, in dem man die Ausgangskomponenten in üblichen Mischvorrichtun- gen wie Schneckenextrudern, Brabender-Mühlen oder Banbury-Mühlen mischt und anschließend extrudiert. Nach der Extrusion kann das Extrudat abgekühlt und zerkleinert werden. Es können auch einzelne Komponenten vorgemischt werden und dann die restlichen Ausgangsstoffe einzeln und/oder ebenfalls gemischt hinzugegeben werden. Die Mischtemperaturen liegen in der Regel bei 230 bis 290°C. The thermoplastic molding compositions according to the invention can be prepared by processes known per se, in which the starting components are mixed in conventional mixing devices, such as screw extruders, Brabender mills or Banbury mills, and then extruded. After extrusion, the extrudate can be cooled and comminuted. It is also possible to premix individual components and then to add the remaining starting materials individually and / or likewise mixed. The mixing temperatures are usually 230 to 290 ° C.
Nach einer weiteren bevorzugten Arbeitsweise können die Komponenten C) und D) sowie gegebenenfalls E) und/oder F) mit einem Polyesterpräpolymeren gemischt, konfektioniert und granuliert werden. Das erhaltene Granulat wird in fester Phase anschließend unter Inertgas kontinuierlich oder diskontinuierlich bei einer Temperatur unterhalb des Schmelzpunktes der Komponente A) bis zur gewünschten Viskosität kondensiert. According to a further preferred procedure, the components C) and D) and optionally E) and / or F) can be mixed with a polyester prepolymer, formulated and granulated. The resulting granules are then condensed in solid phase under inert gas continuously or discontinuously at a temperature below the melting point of component A) to the desired viscosity.
Die erfindungsgemäßen thermoplastischen Formmassen zeichnen sich durch eine gute Verarbeitung und gute thermische Stabilität bei gleichzeitig guter Mechanik aus. Insbesondere die Zähigkeit ist verbessert sowie die Verarbeitungsstabilität bei hohen Temperaturen. The thermoplastic molding compositions of the invention are characterized by good processing and good thermal stability with good mechanical properties. In particular, the toughness is improved and the processing stability at high temperatures.
Diese eignen sich zur Herstellung von Fasern, Folien und Formkörpern jeglicher Art, insbesondere für Anwendungen als Stecker, Schalter, Gehäuseteile, Gehäusedeckel, Scheinwerferhin-
tergrund (Bezel), Brausenkopf, Armaturen, Bügeleisen, Drehschalter, Herdknöpfe, Friteusen- deckel, Türgriffe, (Rück-)spiegelgehäuse, (Heck-)scheibenwischer, Lichtwellenleiterummante- lungen. Im E/E-Bereich können mit den erfindungsgemäßen Polyestern Stecker, Steckerteile, Steckverbinder, Kabelbaumkomponenten, Schaltungsträger, Schaltungsträgerkomponenten, dreidimensional spritzgegossene Schaltungsträger, elektrische Verbindungselemente, mechatronische Komponenten oder optoelektronische Bauelemente hergestellt werden. Im Auto-Innenraum ist eine Verwendung für Armaturentafeln, Lenkstockschalter, Sitzteile, Kopfstützen, Mittelkonsolen, Getriebe-Komponenten und Türmodule, im Auto-Außenraum für Türgriffe, Frontscheinwerferkomponenten, Außenspiegelkomponenten, Scheibenwischerkomponenten, Scheibenwischerschutzgehäuse, Ziergitter, Dachreling, Schiebedachrahmen sowie Karosserieaußenteile möglich. These are suitable for the production of fibers, films and moldings of any kind, in particular for applications as plugs, switches, housing parts, housing cover, headlights. background (bezel), shower head, fittings, irons, rotary switches, stove knobs, fryer covers, door handles, (rear) mirror housings, (rear) windscreen wipers, fiber optic sheaths. In the E / E range, the polyesters according to the invention can be used to produce plugs, plug parts, plug connectors, wiring harness components, circuit carriers, circuit carrier components, three-dimensionally injection-molded circuit carriers, electrical connection elements, mechatronic components or optoelectronic components. In the car interior, use is possible for instrument panels, steering column switches, seat parts, headrests, center consoles, transmission components and door modules, in the car exterior for door handles, headlight components, exterior mirror components, windscreen wiper components, windscreen wiper protection housings, grille, roof rails, sunroof frames and exterior body parts.
Für den Küchen- und Haushaltsbereich ist der Einsatz der Polyester zur Herstellung von Komponenten für Küchengeräte, wie z.B. Friteusen, Bügeleisen, Knöpfe, sowie Anwendungen im Garten-Freizeitbereich, z.B. Komponenten für Bewässerungssysteme oder Gartengeräte möglich. For the kitchen and household sector, the use of the polyesters for the production of components for kitchen appliances, such. Fryers, irons, buttons, and garden leisure applications, e.g. Components for irrigation systems or garden tools possible.
Beispiele Examples
Komponente A: Component A:
Polybutylenterephthalat mit einer Viskositätszahl VZ von 130 ml/g und einem Carboxylendgrup- pengehalt von 34 mval/kg (Ultradur® B 4500 der BASF SE) (VZ gemessen in 0,5 gew.-%iger Lösung aus Phenol/o-Dichlorbenzol, 1 :1 -Mischung bei 25°C). Polybutylene terephthalate with a viscosity number VZ of 130 ml / g and a carboxyl end group content of 34 meq / kg (Ultradur® B 4500 from BASF SE) (VZ measured in 0.5% strength by weight solution of phenol / o-dichlorobenzene, 1 : 1 mixture at 25 ° C).
Komponente C/1 Component C / 1
SAN mit einem AN-Anteil von 19 Gew.% und einer Viskositätszahl von 70 ml/g (gemessen in DMF, 0,5 gew.-%ige Lösung). SAN with an AN content of 19% by weight and a viscosity number of 70 ml / g (measured in DMF, 0.5% strength by weight solution).
Komponente C/2 Component C / 2
SAN mit einem AN-Anteil von 35 Gew.% und einer Viskositätszahl vom 65 ml/g (gemessen in DMF, 0,5 gew.-%ige Lösung). SAN with an AN content of 35% by weight and a viscosity number of 65 ml / g (measured in DMF, 0.5% strength by weight solution).
Komponente DA/1 Component DA / 1
Als Komponente D/V1 wurde ein Styrol-Acrylnitril-Maleinsäureanhydrid-Terpolymer verwendet, welches eine Zusammensetzung von 75/24,6/0,4 (Gew.-%) hatte, Viskositätszahl: 66 ml/g.
Komponente D/1 As component D / V1, a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 75 / 24.6 / 0.4 (wt.%), Viscosity number: 66 ml / g. Component D / 1
Als Komponente D/1 wurde ein Styrol-Acrylnitril-Maleinsäureanhydrid-Terpolymer verwendet, welches eine Zusammensetzung von 74,5/24,5/1 ,0 (Gew.-%) hatte, Viskositätszahl: 66 ml/g. As component D / 1, a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 74.5 / 24.5 / 1.0 (wt%), viscosity number: 66 ml / g.
Komponente D/2 Component D / 2
Als Komponente D/2 wurde ein Styrol-Acrylnitril-Maleinsäureanhydrid-Terpolymer verwendet, welches eine Zusammensetzung von 74,1/23,9/2,0 (Gew.-%) hatte, Viskositätszahl: 67 ml/g. As component D / 2, a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 74.1 / 23.9 / 2.0 (wt%), viscosity number: 67 ml / g.
Komponente DA/2 Component DA / 2
Als Komponente D/V2 wurde ein Styrol-Acrylnitril-Maleinsäureanhydrid-Terpolymer verwendet, welches eine Zusammensetzung von 74/23,5/2,5 (Gew.-%) hatte, Viskositätszahl: 68 ml/g. As component D / V2, a styrene-acrylonitrile-maleic anhydride terpolymer was used which had a composition of 74 / 23.5 / 2.5 (wt%), viscosity number: 68 ml / g.
Herstellung des Pfropfcopolymerisates E a) Herstellung des Polystyrol-Kerns Preparation of the Graft Copolymer E a) Preparation of the Polystyrene Core
700 g Wasser, 3,4 g des Natriumsalzes einer C12- bis C18-Paraffinsulfonsäure, 1 ,75 g Kaliumperoxodisulfat, 2,5 g Natriumhydrogencarbonat, 1 g Natriumpyrophosphat und 50 g eines Polystyrol-Saatlatexes (Feststoffgehalt = 38,7 %, d50 = 85 nm) wurden auf 70 °C erwärmt. Anschließend wurde ein Gemisch aus 550 g Styrol, 1 1 ,5 g DCPA und 10 g Divinylbenzol innerhalb von 2 Stunden zugegeben. 700 g of water, 3.4 g of the sodium salt of a C12 to C18 paraffin sulfonic acid, 1.75 g of potassium peroxodisulfate, 2.5 g of sodium bicarbonate, 1 g of sodium pyrophosphate and 50 g of a polystyrene seed latex (solids content = 38.7%, d50 = 85 nm) were heated to 70 ° C. Subsequently, a mixture of 550 g of styrene, 1 1, 5 g of DCPA and 10 g of divinylbenzene was added within 2 hours.
Nach Beendigung der Monomerzugabe wurde die Emulsion noch eine Stunde bei 65°C gehalten. Der Polystyrol-Kern hatte einen mittleren Teilchendurchmesser d 50 von 250 nm. Die Emulsion hatte einen Feststoffgehalt von 38,5 %. b) Herstellung der Polybutylacrylat-Schale After completion of the addition of monomer, the emulsion was kept at 65 ° C for one hour. The polystyrene core had a mean particle diameter d 50 of 250 nm. The emulsion had a solids content of 38.5%. b) Preparation of the polybutyl acrylate shell
Zur erhaltenen Emulsion wurden 10 g des Natriumsalzes einer C12- bis C18-Paraffin- sulfonsäure, 6,5 g Kaliumperoxodisulfat, 5 g Natriumhydrogencarbonat und 2 g Natriumpyrophosphat sowie 2490 g Wasser gegeben. Bei 65°C wurden 1730 g Butylacrylat und 35 g DCPA innerhalb von 3,5 h zugegeben und danach weitere 2 h bei 65°C gerührt. (d50 = 420 nm, Feststoffgehalt = 38,6 %). c) Herstellung der Polystyrol/Acrylnitril-Pfropfhülle To the emulsion obtained, 10 g of the sodium salt of a C12 to C18 paraffin sulfonic acid, 6.5 g of potassium peroxodisulfate, 5 g of sodium bicarbonate and 2 g of sodium pyrophosphate and 2490 g of water were added. At 65 ° C 1730 g of butyl acrylate and 35 g of DCPA were added within 3.5 h and then stirred at 65 ° C for a further 2 h. (d50 = 420 nm, solids = 38.6%). c) Preparation of the polystyrene / acrylonitrile graft shell
6070 g der so erhaltenen Emulsion wurden mit 2600 g Wasser verdünnt und 5 g des Natriumsalzes einer C12- bis C18-Paraffinsulfonsäure und 4,5 g Kaliumperoxodisulfat zugegeben. Bei 65°C wurde ein Gemisch aus 790 g Styrol und 260 g Acrylnitril innerhalb von 2 h zugetropft und weitere 2 h bei 65°C nachgerührt. (d50 = 500 nm, Feststoffgehalt = 34,8 %).
Das Pfropfpolymerisat E wurde mittels Calciumchloridlösung bei 95°C aus der Emulsion gefällt, mit Wasser gewaschen und im warmen Luftstrom getrocknet. 6070 g of the emulsion thus obtained were diluted with 2600 g of water and 5 g of the sodium salt of a C12 to C18 paraffin sulfonic acid and 4.5 g of potassium peroxodisulfate were added. At 65 ° C, a mixture of 790 g of styrene and 260 g of acrylonitrile was added dropwise within 2 h and stirred at 65 ° C for a further 2 h. (d50 = 500 nm, solids = 34.8%). The graft polymer E was precipitated from the emulsion by means of calcium chloride solution at 95 ° C., washed with water and dried in a stream of warm air.
Komponente F Component F
Glasfaser, die mit einer Epoxy-Schlichte ausgerüstet war, Faserdurchmesser 10 μηη. Herstellung und Prüfung der Formmassen Zum Mischen der Komponenten wurde ein Zweiwellenextruder verwendet, die Massetemperatur betrug 260°C. Die Schmelze wurde durch ein Wasserbad geleitet und granuliert. Glass fiber, which was equipped with an epoxy size, fiber diameter 10 μηη. Preparation and Testing of Molding Compounds A twin-screw extruder was used to mix the components, the melt temperature was 260 ° C. The melt was passed through a water bath and granulated.
Des Weiteren wurden die mechanischen Eigenschaften an mittels Spritzguss hergestellten Proben (Massetemperatur: 250°C/Werkzeugtemp. 80°C) bestimmt. Furthermore, the mechanical properties of injection molded samples (melt temperature: 250 ° C / tool temp. 80 ° C) were determined.
Die Schlagzähigkeit der Produkte wurde an ISO-Stäben nach ISO 179 1 eU bestimmt. The impact resistance of the products was determined on ISO bars according to ISO 179 1 eU.
Reißfestigkeit und Reißdehnung wurden nach ISO 527 bestimmt. Die thermische Beständigkeit der Produkte wurde durch Lagerung von Zugstäben bei 120°C/500h im Umluftschrank und nachfolgendem Zugversuch charakterisiert. Zur Beurteilung der Verarbeitungsstabilität wurden die Formmassen auch bei 300°C Massetemperatur/80°C Werkzeugtemperatur verarbeitet. Die Zusammensetzung der verwendeten Styrolcopolymere wurde durch quantitative IR- Spektroskopie ermittelt. Tear strength and elongation at break were determined according to ISO 527. The thermal stability of the products was characterized by the storage of tensile bars at 120 ° C / 500h in a convection oven and subsequent tensile test. To assess the processing stability, the molding compositions were also processed at 300 ° C melt temperature / 80 ° C mold temperature. The composition of the styrene copolymers used was determined by quantitative IR spectroscopy.
Die Zusammensetzungen der Formmassen und die Ergebnisse der Prüfungen sind in Tabelle 1 aufgeführt.
The compositions of the molding compositions and the results of the tests are listed in Table 1.
Tabelle 1: Table 1:
Formmasse Nr. V1 V2 V3 4 5 V6 V7Molding compound no. V1 V2 V3 4 5 V6 V7
Komponente [Gew.-%] Component [% by weight]
C/1 10,7 - 6,7 6,7 6,7 6,7 - C / 1 10.7 - 6.7 6.7 6.7 6.7 -
C/2 - 10,7 - - - - 6,7C / 2 - 10.7 - - - - 6.7
A 58,2 58,2 58,2 58,2 58,2 58,2 58,2A 58.2 58.2 58.2 58.2 58.2 58.2 58.2
DA/1 - - 4 - - - -DA / 1 - - 4 - - - -
D1 - - - 4 - - 4D1 - - - 4 - - 4
D2 - - - - 4 - -D2 - - - - 4 - -
DA/2 - - - - - 4 -DA / 2 - - - - - 4 -
E 11,1 11,1 11,1 11,1 11,1 11,1 11,1E 11.1 11.1 11.1 11.1 11.1 11.1 11.1
F 20 20 20 20 20 20 20 an [kJ/m2] 52,9 49,7 53,3 62,6 61,7 53,5 53,7F 20 20 20 20 20 20 20 a n [kJ / m 2 ] 52.9 49.7 53.3 62.6 61.7 53.5 53.7
Reißfestigkeit [N/mm2] 103 99 104 114 114 110 104Tensile strength [N / mm 2 ] 103 99 104 114 114 110 104
Reißdehnung [%] 2,7 2,6 2,7 3,1 3,0 2,9 2,5Elongation at break [%] 2.7 2.6 2.7 3.1 3.0 2.9 2.5
Reißfestigkeit nach 100 97 102 113 112 101 100 500h/120°C [MPa] Tensile strength according to 100 97 102 113 112 101 100 500h / 120 ° C [MPa]
an Verarbeitung bei 42,3 40,4 41,4 58,7 56,7 37,4 45,3 300°C/80°C [kJ/m2]
a n Processing at 42.3 40.4 41.4 58.7 56.7 37.4 45.3 300 ° C / 80 ° C [kJ / m 2 ]
Claims
Patentansprüche claims
1 . Thermoplastische Formmassen, enthaltend 1 . Thermoplastic molding compositions containing
2 bis 98,5 Gew.-% mindestens eines Polyesters 2 to 98.5% by weight of at least one polyester
0 bis 70 Gew.-% mindestens eines Polycarbonats 0 to 70 wt .-% of at least one polycarbonate
1 bis 97,5 Gew.-% eines Copolymerisates aus 1 to 97.5 wt .-% of a copolymer of
60 bis 95 Gew.-% Styrol oder substituierten Styrolen der allgemeinen Formel I oder deren Mischungen 60 to 95 wt .-% of styrene or substituted styrenes of the general formula I or mixtures thereof
worin R einen Alkylrest mit 1 bis 8 C-Atomen oder ein Wasserstoffatom und R1 Alkylrest mit 1 bis 8 C-Atomen darstellen und n den Wert 1 , 2 oder 3 hat und wherein R is an alkyl group having 1 to 8 carbon atoms or a hydrogen atom and R 1 is alkyl of 1 to 8 carbon atoms and n is 1, 2 or 3 and
5 bis 40 Gew.-% mindestens eines ungesättigten Nitrils, From 5 to 40% by weight of at least one unsaturated nitrile,
D) 0,5 bis 30 Gew.-% eines Copolymeren aus D) 0.5 to 30 wt .-% of a copolymer
49,5 bis 93,5 Gew.-% sich von einem oder mehreren vinylaromatischen Monomeren ableitende Struktureinheiten, From 49.5 to 93.5% by weight of structural units derived from one or more vinylaromatic monomers,
6 bis 50 Gew.-% sich von einem oder mehreren Vinylcyaniden ableitende Struktureinheiten, From 6 to 50% by weight of structural units derived from one or more vinyl cyanides,
0,5 bis 2,4 Gew.-% sich von einem oder mehreren Dicarbonsäureanhydriden ableitende Struktureinheiten und From 0.5 to 2.4% by weight of structural units derived from one or more dicarboxylic acid anhydrides and
0 bis 25 Gew.-% sich von weiteren copolymerisierbaren Monomeren ableitende Struktureinheiten, wobei die Gew.-% jeweils auf das Gesamtgewicht der sich von den Komponenten di , d2, d3 und d4 ableitenden Struktureinheiten bezogen sind und zusammen 100 Gew.-% ergeben, 0 to 25 wt .-% which derives from other copolymerizable monomers structural units, wherein the wt .-% are each based on the total weight of the derived from the components di, d2, d3 and d4 structural units and the total is 100 wt .-% .
0 bis 50 Gew.-% eines Kautschuks, welcher kein Dien enthält,
F) O bis 60 Gew.-% weiterer Zusatzstoffe, wobei die Summe der Gewichtsprozente A) bis F) 100 % ergibt. 0 to 50% by weight of a rubber containing no diene, F) O to 60 wt .-% of other additives, wherein the sum of the weight percent A) to F) gives 100%.
Thermoplastische Formmassen nach Anspruch 1 , enthaltend Thermoplastic molding compositions according to claim 1, comprising
10 bis 97,5 Gew.-% 10 to 97.5% by weight
0 bis 60 Gew.-% 0 to 60% by weight
1 bis 97,5 Gew.-% From 1 to 97.5% by weight
0,5 bis 30 Gew.-% 0.5 to 30% by weight
1 bis 40 Gew.-% 1 to 40% by weight
0 bis 60 Gew.-%, wobei die Summe der Gewichtsprozente A) bis F) 100 % ergibt. 0 to 60 wt .-%, wherein the sum of the weight percent A) to F) gives 100%.
Thermoplastische Formmassen nach den Ansprüchen 1 oder 2, enthaltend als Komponente E) ein Pfropfpolymerisat aufgebaut aus Thermoplastic molding compositions according to claims 1 or 2, comprising as component E) a graft polymer composed of
Ei) 40 bis 80 Gew.-% einer Pfropfgrundlage aus einem kautschukelastischen Polymeren auf Basis von Alkylacrylaten mit 1 bis 8 C-Atomen im Alkylrest und mit einer Glasübergangstemperatur von unter 10°C, Ei) 40 to 80 wt .-% of a graft of a rubber-elastic polymers based on alkyl acrylates having 1 to 8 carbon atoms in the alkyl radical and having a glass transition temperature of less than 10 ° C,
E2) 20 bis 60 Gew.-% einer Pfropfauflage aus E2) from 20 to 60% by weight of a grafting pad
E21) 60 bis 95 Gew.-% Styrol oder substituierten Styrolen der allgemeinen Formel I oder deren Mischungen, E21) from 60 to 95% by weight of styrene or substituted styrenes of the general formula I or mixtures thereof,
E22) 5 bis 40 Gew.-% mindestens eines ungesättigten Nitrils. E22) from 5 to 40% by weight of at least one unsaturated nitrile.
Thermoplastische Formmassen nach den Ansprüchen 1 bis 3, enthaltend als Komponente D ein Copolymer aus
Thermoplastic molding compositions according to claims 1 to 3, containing as component D a copolymer
d2) 6 bis 50 Gew.-% d 2 ) 6 to 50% by weight
d3) 0,8 bis 2,2 Gew.-% d 3 ) 0.8 to 2.2% by weight
d4) 0 bis 25 Gew.-% d 4 ) 0 to 25% by weight
Thermoplastische Formmassen nach den Ansprüchen 1 bis 4, enthaltend als Komponente D) ein Terpolymerisat aus di) Styrol, α-Methylstyrol, p-Methylstyrol, t-Butylstyrol, Vinylnaphthalin oder ein Gemisch aus zwei oder mehr dieser Monomeren,
Komponente c ) Acrylnitril, Methacrylnitril oder ein Gemisch dieser Monomeren und Thermoplastic molding compositions according to claims 1 to 4, containing as component D) a terpolymer of di) styrene, α-methylstyrene, p-methylstyrene, t-butylstyrene, vinylnaphthalene or a mixture of two or more of these monomers, Component c) acrylonitrile, methacrylonitrile or a mixture of these monomers and
Komponente dß) Maleinsäureanhydrid, Methylmaleinsäureanhydrid, Itaconsäureanhydrid oder ein Gemisch aus zwei oder mehr dieser Monomeren. Component dβ) maleic anhydride, methylmaleic anhydride, itaconic anhydride or a mixture of two or more of these monomers.
6. Thermoplastische Formmassen nach den Ansprüchen 1 bis 5, in welchen das Copolymer D) ein Styrol-Acrylnitril-Maleinsäureanhydrid-Copolymer ist. 6. Thermoplastic molding compositions according to claims 1 to 5, in which the copolymer D) is a styrene-acrylonitrile-maleic anhydride copolymer.
7. Thermoplastische Formmassen nach den Ansprüchen 1 bis 6, in denen die Komponente D) erhältlich ist durch Masse- oder Lösungspolymerisation. 7. Thermoplastic molding compositions according to claims 1 to 6, in which component D) is obtainable by mass or solution polymerization.
8. Thermoplastische Formmassen nach den Ansprüchen 1 bis 7, in denen die Komponente D) durch thermische Polymerisation ohne Zusatz eines Polymerisationsinitiators hergestellt wird. 8. Thermoplastic molding compositions according to claims 1 to 7, in which component D) is prepared by thermal polymerization without the addition of a polymerization initiator.
9. Thermoplastische Formmassen nach den Ansprüchen 1 bis 8, wobei der Unterschied des Nitrilgehaltes der Komponenten C) und D) weniger als 10 Gew.-% beträgt. 9. Thermoplastic molding compositions according to claims 1 to 8, wherein the difference of the nitrile content of the components C) and D) is less than 10 wt .-%.
10. Verwendung der thermoplastischen Formmassen gemäß den Ansprüchen 1 bis 9 zur Herstellung von Fasern, Folien und Formkörpern jeglicher Art. 10. Use of the thermoplastic molding compositions according to claims 1 to 9 for the production of fibers, films and moldings of any kind.
1 1 . Formkörper jeglicher Art, Fasern und Folien erhältlich aus den thermoplastischen Formmassen gemäß den Ansprüchen 1 bis 9.
1 1. Shaped body of any kind, fibers and films obtainable from the thermoplastic molding compositions according to claims 1 to 9.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11168350.4 | 2011-06-01 | ||
| EP11168350 | 2011-06-01 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012163822A1 true WO2012163822A1 (en) | 2012-12-06 |
Family
ID=46317342
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2012/059823 WO2012163822A1 (en) | 2011-06-01 | 2012-05-25 | Polyester with styrene copolymers |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2012163822A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110408225A (en) * | 2019-06-21 | 2019-11-05 | 涂民强 | A kind of preparation method of composite plant fiber buffer packaging material |
Citations (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1260135B (en) | 1965-01-05 | 1968-02-01 | Basf Ag | Impact-resistant thermoplastic molding compounds |
| US3651014A (en) | 1969-07-18 | 1972-03-21 | Du Pont | Segmented thermoplastic copolyester elastomers |
| DE2540517A1 (en) | 1975-09-11 | 1977-03-31 | Bayer Ag | Continuous bulk polymn. process - for moulding compsns., made from styrene plus other monomers of different reactivity |
| EP0001625A1 (en) | 1977-10-26 | 1979-05-02 | Bayer Ag | Thermoplastic moulding materials |
| DE2826925A1 (en) | 1978-06-20 | 1980-01-17 | Basf Ag | WEATHER-RESISTANT, IMPACT THERMOPLASTIC MATERIALS WITH GOOD SIMPLABILITY |
| DE2842005A1 (en) | 1978-09-27 | 1980-04-10 | Bayer Ag | POLYCARBONATES WITH ALKYLPHENYL END GROUPS, THEIR PRODUCTION AND THEIR USE |
| US4352904A (en) | 1978-02-28 | 1982-10-05 | E. I. Du Pont De Nemours And Company | Molding resins |
| DE3149358A1 (en) | 1981-12-12 | 1983-06-16 | Basf Ag, 6700 Ludwigshafen | THERMOPLASTIC MOLDING |
| DE3334782A1 (en) | 1983-04-19 | 1984-10-25 | Bayer Ag, 5090 Leverkusen | METHOD FOR PRODUCING POLYDIORGANOSILOXANES WITH HYDROXYARYLOXY END GROUPS |
| US4485212A (en) | 1983-05-27 | 1984-11-27 | Uniroyal, Inc. | Impact resistant blend of polybutylene terephthalate resin and OSA graft copolymer |
| EP0135677A1 (en) | 1983-07-05 | 1985-04-03 | General Electric Company | Composition of polyester resin |
| DE3336499A1 (en) | 1983-10-07 | 1985-04-25 | Bayer Ag, 5090 Leverkusen | MOLDING MATERIALS FROM THERMOPLASTIC POLYESTER AND GRAFT RUBBER, METHOD FOR THE PRODUCTION THEREOF AND THEIR USE FOR THE PRODUCTION OF MOLDED BODIES |
| DE3414118A1 (en) | 1984-04-14 | 1985-10-24 | Basf Ag, 6700 Ludwigshafen | THERMOPLASTIC MOLDS |
| DE3506472A1 (en) | 1985-02-23 | 1986-08-28 | Bayer Ag, 5090 Leverkusen | NEW POLYDIORGANOSILOXANE POLYCARBONATE BLOCK COPOLYMERS |
| EP0284086A2 (en) | 1987-03-27 | 1988-09-28 | Sumitomo Dow Limited | Molding resin composition |
| DE3733829A1 (en) | 1987-10-07 | 1989-04-20 | Basf Ag | Glass-fibre-reinforced thermoplastic moulding compositions based on polyesters and graft polymers |
| US4902749A (en) | 1987-08-24 | 1990-02-20 | Allied-Signal Inc. | High impact, styrenic polymer/thermoplastic polymer grafted blends |
| EP0365916A1 (en) | 1988-10-24 | 1990-05-02 | Bayer Ag | Polymer mixtures |
| EP0573680A1 (en) | 1992-06-05 | 1993-12-15 | General Electric Company | Impact modified polyester and/or polycarbonate |
| US5310793A (en) | 1989-02-02 | 1994-05-10 | Bayer Aktiengesellschaft | Mixtures of polyalkylene terephthalates, new polycarbonates and/or polyester-carbonates and elastomers |
| DE4401055A1 (en) | 1994-01-15 | 1995-07-20 | Basf Ag | Process for the preparation of thermoplastic polyesters with a low carboxyl end group content |
| EP0711810A1 (en) | 1994-11-14 | 1996-05-15 | General Electric Company | Autoclave resistant blends of poly(ester-carbonate) and polyetherimide resins |
| DE10058302A1 (en) | 1999-12-02 | 2001-06-07 | Basf Ag | Stabilized styrenic copolymers, useful for the production of molded articles, film, fiber and foam, are prepared by the addition of an antioxidant during a continuous polymerization process. |
| WO2004055107A1 (en) * | 2002-12-17 | 2004-07-01 | Basf Aktiengesellschaft | Thermoplastic molding compositions |
| US20100152359A1 (en) | 2008-12-11 | 2010-06-17 | Cheil Industries Inc. | Environmentally Sound Thermoplastic Resin Composition Using Recycled Polyester Resin |
| DE102009055403A1 (en) | 2008-12-30 | 2010-07-08 | Cheil Industries Inc., Uiwang | Thermoplastic resin composition with high flowability, excellent chemical resistance, impact resistance and excellent gloss |
| WO2010130621A1 (en) * | 2009-05-11 | 2010-11-18 | Basf Se | Reinforced styrene copolymers |
-
2012
- 2012-05-25 WO PCT/EP2012/059823 patent/WO2012163822A1/en active Application Filing
Patent Citations (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1260135B (en) | 1965-01-05 | 1968-02-01 | Basf Ag | Impact-resistant thermoplastic molding compounds |
| US3651014A (en) | 1969-07-18 | 1972-03-21 | Du Pont | Segmented thermoplastic copolyester elastomers |
| DE2540517A1 (en) | 1975-09-11 | 1977-03-31 | Bayer Ag | Continuous bulk polymn. process - for moulding compsns., made from styrene plus other monomers of different reactivity |
| EP0001625A1 (en) | 1977-10-26 | 1979-05-02 | Bayer Ag | Thermoplastic moulding materials |
| US4352904A (en) | 1978-02-28 | 1982-10-05 | E. I. Du Pont De Nemours And Company | Molding resins |
| DE2826925A1 (en) | 1978-06-20 | 1980-01-17 | Basf Ag | WEATHER-RESISTANT, IMPACT THERMOPLASTIC MATERIALS WITH GOOD SIMPLABILITY |
| DE2842005A1 (en) | 1978-09-27 | 1980-04-10 | Bayer Ag | POLYCARBONATES WITH ALKYLPHENYL END GROUPS, THEIR PRODUCTION AND THEIR USE |
| DE3149358A1 (en) | 1981-12-12 | 1983-06-16 | Basf Ag, 6700 Ludwigshafen | THERMOPLASTIC MOLDING |
| DE3334782A1 (en) | 1983-04-19 | 1984-10-25 | Bayer Ag, 5090 Leverkusen | METHOD FOR PRODUCING POLYDIORGANOSILOXANES WITH HYDROXYARYLOXY END GROUPS |
| US4485212A (en) | 1983-05-27 | 1984-11-27 | Uniroyal, Inc. | Impact resistant blend of polybutylene terephthalate resin and OSA graft copolymer |
| EP0135677A1 (en) | 1983-07-05 | 1985-04-03 | General Electric Company | Composition of polyester resin |
| DE3336499A1 (en) | 1983-10-07 | 1985-04-25 | Bayer Ag, 5090 Leverkusen | MOLDING MATERIALS FROM THERMOPLASTIC POLYESTER AND GRAFT RUBBER, METHOD FOR THE PRODUCTION THEREOF AND THEIR USE FOR THE PRODUCTION OF MOLDED BODIES |
| DE3414118A1 (en) | 1984-04-14 | 1985-10-24 | Basf Ag, 6700 Ludwigshafen | THERMOPLASTIC MOLDS |
| DE3506472A1 (en) | 1985-02-23 | 1986-08-28 | Bayer Ag, 5090 Leverkusen | NEW POLYDIORGANOSILOXANE POLYCARBONATE BLOCK COPOLYMERS |
| EP0284086A2 (en) | 1987-03-27 | 1988-09-28 | Sumitomo Dow Limited | Molding resin composition |
| US4902749A (en) | 1987-08-24 | 1990-02-20 | Allied-Signal Inc. | High impact, styrenic polymer/thermoplastic polymer grafted blends |
| DE3733829A1 (en) | 1987-10-07 | 1989-04-20 | Basf Ag | Glass-fibre-reinforced thermoplastic moulding compositions based on polyesters and graft polymers |
| EP0365916A1 (en) | 1988-10-24 | 1990-05-02 | Bayer Ag | Polymer mixtures |
| US5310793A (en) | 1989-02-02 | 1994-05-10 | Bayer Aktiengesellschaft | Mixtures of polyalkylene terephthalates, new polycarbonates and/or polyester-carbonates and elastomers |
| EP0573680A1 (en) | 1992-06-05 | 1993-12-15 | General Electric Company | Impact modified polyester and/or polycarbonate |
| DE4401055A1 (en) | 1994-01-15 | 1995-07-20 | Basf Ag | Process for the preparation of thermoplastic polyesters with a low carboxyl end group content |
| EP0711810A1 (en) | 1994-11-14 | 1996-05-15 | General Electric Company | Autoclave resistant blends of poly(ester-carbonate) and polyetherimide resins |
| DE10058302A1 (en) | 1999-12-02 | 2001-06-07 | Basf Ag | Stabilized styrenic copolymers, useful for the production of molded articles, film, fiber and foam, are prepared by the addition of an antioxidant during a continuous polymerization process. |
| WO2004055107A1 (en) * | 2002-12-17 | 2004-07-01 | Basf Aktiengesellschaft | Thermoplastic molding compositions |
| US20100152359A1 (en) | 2008-12-11 | 2010-06-17 | Cheil Industries Inc. | Environmentally Sound Thermoplastic Resin Composition Using Recycled Polyester Resin |
| DE102009055403A1 (en) | 2008-12-30 | 2010-07-08 | Cheil Industries Inc., Uiwang | Thermoplastic resin composition with high flowability, excellent chemical resistance, impact resistance and excellent gloss |
| WO2010130621A1 (en) * | 2009-05-11 | 2010-11-18 | Basf Se | Reinforced styrene copolymers |
Non-Patent Citations (6)
| Title |
|---|
| C.B. BUCKNALL: "Toughened Plastics", 1977, APPLIED SCIENCE PUBLISHERS |
| HAGE E.; HALE W.; KESKKULA, PAUL D.R., POLYMER, vol. 38, 1997, pages 3237 |
| HOUBEN-WEYL: "Methoden der organischen Chemie", vol. 14/1, 1961, GEORG-THIEME-VERLAG, pages: 392 - 406 |
| LEE P.-C.; KUO W.-F.; CHANG F.-C., POLYMER, vol. 35, 1994, pages 5641 |
| SCHILDKNECHT: "Vinyl and Related Polymers", 1952, WILEY-VERLAG, pages: 484 - 494 |
| WALL: "Fluorpolymers", 1972, WILEY INTERSCIENCE |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110408225A (en) * | 2019-06-21 | 2019-11-05 | 涂民强 | A kind of preparation method of composite plant fiber buffer packaging material |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE19956539A1 (en) | A heat resistant thermoplastic molding material useful for production of fibers, films and profiled bodies, especially in electronics, domestic and medical equipment has long life, ductility, flowability, and melt stability | |
| EP1240250B1 (en) | Filled thermoplastic moulding materials on the basis of polycarbonate and styrene copolymers | |
| DE19929301A1 (en) | Welded composite material, e.g. for moulded or laminated housings and other parts in cars, has at least one moulded part consisting of polyester material reinforced with aminosilane- and epoxy resin-treated fibres | |
| EP0158931A1 (en) | Thermoplastic moulding compositions based on polycarbonate-graft polymer mixtures | |
| DE102004038976A1 (en) | Flowable polyester molding compounds with ASA / ABS and SAN | |
| EP2723813B1 (en) | Weather-resistant polyester moulding compounds comprising styrene copolymers | |
| EP2841501B1 (en) | Pc/abs compounds with good thermal and chemical resistance | |
| EP1192221B1 (en) | Polyester/polycarbonate blends | |
| WO2006050872A1 (en) | Polymer blends consisting of polyesters and linear oligomer polycarbonates | |
| EP0482451A2 (en) | Stable to aging-by-light, flame-retardant polycarbonate, polyestercarbonate or polyarylate moulding compositions | |
| DE19545289A1 (en) | Thermoplastic materials with improved X-ray contrast | |
| EP3044255B1 (en) | Flame-resistant polyester | |
| WO2012163822A1 (en) | Polyester with styrene copolymers | |
| EP1917308B1 (en) | Parts of headlights made from polyester | |
| EP2861665A1 (en) | Flame-proof polyester comprising polyacrylonitrile homopolymerisates | |
| WO2012022669A2 (en) | Nanocomposite blends with polyesters | |
| EP3697845A1 (en) | Flame-resistant polycarbonate-acrylate-rubber composition having a reduced bisphenol-a content | |
| WO2018050426A1 (en) | Polyester for profile extrusion and/or pipe extrusion | |
| WO2001042361A2 (en) | Isotropic thermoplastic moulding materials based on polycarbonates and styrene copolymers | |
| WO2023088806A1 (en) | Thermoplastic mixtures | |
| EP4192912A1 (en) | Thermoplastic mixtures | |
| WO2000041470A2 (en) | Method for producing polyester blends | |
| WO2007088125A1 (en) | Polyester molding compositions comprising maleimide copolymers | |
| EP3697846A1 (en) | Flame-resistant polycarbonate composition having a reduced bisphenol-a content | |
| DE4005118A1 (en) | FUEL-RESISTANT THERMOPLASTIC MOLDING |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12727807 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12727807 Country of ref document: EP Kind code of ref document: A1 |