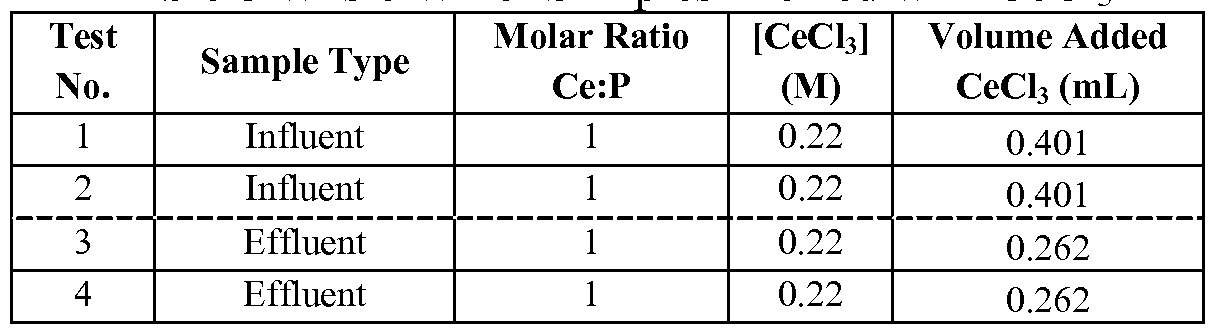
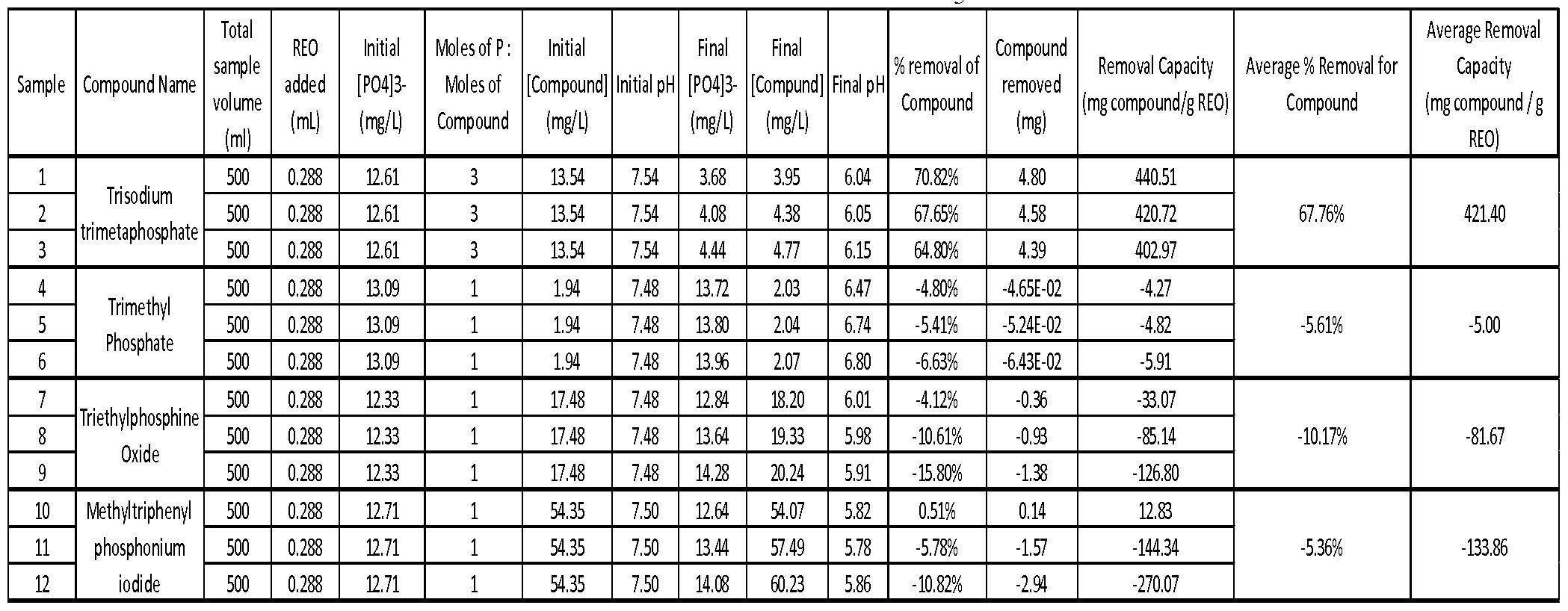
WO2012141895A2 - Rare earth removal of phosphorus-containing materials - Google Patents
Rare earth removal of phosphorus-containing materials Download PDFInfo
- Publication number
- WO2012141895A2 WO2012141895A2 PCT/US2012/030974 US2012030974W WO2012141895A2 WO 2012141895 A2 WO2012141895 A2 WO 2012141895A2 US 2012030974 W US2012030974 W US 2012030974W WO 2012141895 A2 WO2012141895 A2 WO 2012141895A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- phosphorus
- rare earth
- target material
- commonly
- cerium
- Prior art date
Links
- 229910052761 rare earth metal Inorganic materials 0.000 title claims abstract description 385
- 150000002910 rare earth metals Chemical class 0.000 title claims abstract description 377
- 229910052698 phosphorus Inorganic materials 0.000 title claims abstract description 309
- 239000011574 phosphorus Substances 0.000 title claims abstract description 306
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 title claims abstract description 302
- 239000000463 material Substances 0.000 title claims description 56
- 239000013077 target material Substances 0.000 claims abstract description 285
- 238000000034 method Methods 0.000 claims abstract description 153
- 239000000654 additive Substances 0.000 claims abstract description 146
- 239000000203 mixture Substances 0.000 claims description 269
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 166
- 229910001868 water Inorganic materials 0.000 claims description 163
- 230000000996 additive effect Effects 0.000 claims description 140
- 239000012736 aqueous medium Substances 0.000 claims description 128
- 230000008569 process Effects 0.000 claims description 110
- XQTIWNLDFPPCIU-UHFFFAOYSA-N cerium(3+) Chemical compound [Ce+3] XQTIWNLDFPPCIU-UHFFFAOYSA-N 0.000 claims description 75
- ITZXULOAYIAYNU-UHFFFAOYSA-N cerium(4+) Chemical compound [Ce+4] ITZXULOAYIAYNU-UHFFFAOYSA-N 0.000 claims description 62
- 229910019142 PO4 Inorganic materials 0.000 claims description 56
- 235000021317 phosphate Nutrition 0.000 claims description 53
- 229910052684 Cerium Inorganic materials 0.000 claims description 45
- GWXLDORMOJMVQZ-UHFFFAOYSA-N cerium Chemical compound [Ce] GWXLDORMOJMVQZ-UHFFFAOYSA-N 0.000 claims description 44
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical class OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 claims description 40
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 37
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 claims description 34
- 150000002148 esters Chemical class 0.000 claims description 33
- 239000010452 phosphate Substances 0.000 claims description 33
- 239000002253 acid Substances 0.000 claims description 28
- 239000007800 oxidant agent Substances 0.000 claims description 26
- 150000001408 amides Chemical class 0.000 claims description 24
- 238000006243 chemical reaction Methods 0.000 claims description 21
- 150000003013 phosphoric acid derivatives Chemical class 0.000 claims description 21
- 230000002829 reductive effect Effects 0.000 claims description 21
- 239000003638 chemical reducing agent Substances 0.000 claims description 17
- 229910000147 aluminium phosphate Inorganic materials 0.000 claims description 16
- ACVYVLVWPXVTIT-UHFFFAOYSA-M phosphinate Chemical compound [O-][PH2]=O ACVYVLVWPXVTIT-UHFFFAOYSA-M 0.000 claims description 14
- 125000005538 phosphinite group Chemical group 0.000 claims description 14
- XRBCRPZXSCBRTK-UHFFFAOYSA-N phosphonous acid Chemical compound OPO XRBCRPZXSCBRTK-UHFFFAOYSA-N 0.000 claims description 14
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 claims description 14
- 239000000084 colloidal system Substances 0.000 claims description 13
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 claims description 13
- ACVYVLVWPXVTIT-UHFFFAOYSA-N phosphinic acid Chemical compound O[PH2]=O ACVYVLVWPXVTIT-UHFFFAOYSA-N 0.000 claims description 13
- 238000001179 sorption measurement Methods 0.000 claims description 13
- 239000000725 suspension Substances 0.000 claims description 13
- 150000001450 anions Chemical class 0.000 claims description 12
- 230000007935 neutral effect Effects 0.000 claims description 7
- 150000001768 cations Chemical class 0.000 claims description 6
- -1 cyclic phosphate salt Chemical class 0.000 description 115
- 239000002245 particle Substances 0.000 description 67
- 239000003643 water by type Substances 0.000 description 61
- 239000007787 solid Substances 0.000 description 57
- 150000001875 compounds Chemical class 0.000 description 56
- 238000001914 filtration Methods 0.000 description 51
- 239000007788 liquid Substances 0.000 description 46
- 241000894007 species Species 0.000 description 46
- 239000000126 substance Substances 0.000 description 43
- 239000000243 solution Substances 0.000 description 42
- 229910000422 cerium(IV) oxide Inorganic materials 0.000 description 41
- 230000003647 oxidation Effects 0.000 description 41
- 238000007254 oxidation reaction Methods 0.000 description 41
- CETPSERCERDGAM-UHFFFAOYSA-N ceric oxide Chemical compound O=[Ce]=O CETPSERCERDGAM-UHFFFAOYSA-N 0.000 description 37
- 239000002609 medium Substances 0.000 description 35
- 238000009472 formulation Methods 0.000 description 32
- 230000000249 desinfective effect Effects 0.000 description 31
- 230000001112 coagulating effect Effects 0.000 description 30
- VYLVYHXQOHJDJL-UHFFFAOYSA-K cerium trichloride Chemical compound Cl[Ce](Cl)Cl VYLVYHXQOHJDJL-UHFFFAOYSA-K 0.000 description 29
- 230000029087 digestion Effects 0.000 description 26
- 229910052746 lanthanum Inorganic materials 0.000 description 23
- 229910004664 Cerium(III) chloride Inorganic materials 0.000 description 21
- XYFCBTPGUUZFHI-UHFFFAOYSA-N Phosphine Chemical compound P XYFCBTPGUUZFHI-UHFFFAOYSA-N 0.000 description 20
- 229910052777 Praseodymium Inorganic materials 0.000 description 20
- 238000004659 sterilization and disinfection Methods 0.000 description 20
- 229910052772 Samarium Inorganic materials 0.000 description 19
- 238000007517 polishing process Methods 0.000 description 19
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 18
- 229910052779 Neodymium Inorganic materials 0.000 description 18
- 150000003839 salts Chemical class 0.000 description 17
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 16
- 239000012530 fluid Substances 0.000 description 15
- 229910052760 oxygen Inorganic materials 0.000 description 15
- 239000001301 oxygen Substances 0.000 description 15
- 239000000758 substrate Substances 0.000 description 15
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 14
- 239000011230 binding agent Substances 0.000 description 14
- 229910052717 sulfur Inorganic materials 0.000 description 14
- 150000007970 thio esters Chemical class 0.000 description 14
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 13
- 235000011007 phosphoric acid Nutrition 0.000 description 13
- 238000005498 polishing Methods 0.000 description 13
- 125000000217 alkyl group Chemical group 0.000 description 12
- 239000000356 contaminant Substances 0.000 description 12
- 229910052500 inorganic mineral Inorganic materials 0.000 description 12
- 239000011707 mineral Substances 0.000 description 12
- 239000011148 porous material Substances 0.000 description 12
- 239000011593 sulfur Substances 0.000 description 12
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 11
- 239000007864 aqueous solution Substances 0.000 description 11
- 229910052799 carbon Inorganic materials 0.000 description 11
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 11
- 239000000460 chlorine Substances 0.000 description 11
- 229910052801 chlorine Inorganic materials 0.000 description 11
- 238000010438 heat treatment Methods 0.000 description 11
- 229910052739 hydrogen Inorganic materials 0.000 description 11
- 235000015097 nutrients Nutrition 0.000 description 11
- 238000012545 processing Methods 0.000 description 11
- 239000002351 wastewater Substances 0.000 description 11
- 125000004429 atom Chemical group 0.000 description 10
- 239000002585 base Substances 0.000 description 10
- 238000005352 clarification Methods 0.000 description 10
- 238000005345 coagulation Methods 0.000 description 10
- 230000015271 coagulation Effects 0.000 description 10
- 239000001257 hydrogen Substances 0.000 description 10
- FZLIPJUXYLNCLC-UHFFFAOYSA-N lanthanum atom Chemical compound [La] FZLIPJUXYLNCLC-UHFFFAOYSA-N 0.000 description 10
- 229910052757 nitrogen Inorganic materials 0.000 description 10
- 239000000843 powder Substances 0.000 description 10
- 238000000926 separation method Methods 0.000 description 10
- 238000005273 aeration Methods 0.000 description 9
- 238000001816 cooling Methods 0.000 description 9
- 125000004122 cyclic group Chemical group 0.000 description 9
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 9
- 229910052711 selenium Inorganic materials 0.000 description 9
- 239000011669 selenium Substances 0.000 description 9
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 8
- 230000000670 limiting effect Effects 0.000 description 8
- QEFYFXOXNSNQGX-UHFFFAOYSA-N neodymium atom Chemical compound [Nd] QEFYFXOXNSNQGX-UHFFFAOYSA-N 0.000 description 8
- PUDIUYLPXJFUGB-UHFFFAOYSA-N praseodymium atom Chemical compound [Pr] PUDIUYLPXJFUGB-UHFFFAOYSA-N 0.000 description 8
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical group [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 7
- 150000007513 acids Chemical class 0.000 description 7
- AQLMHYSWFMLWBS-UHFFFAOYSA-N arsenite(1-) Chemical compound O[As](O)[O-] AQLMHYSWFMLWBS-UHFFFAOYSA-N 0.000 description 7
- 125000003118 aryl group Chemical group 0.000 description 7
- 229910052794 bromium Inorganic materials 0.000 description 7
- 150000007942 carboxylates Chemical class 0.000 description 7
- 238000002474 experimental method Methods 0.000 description 7
- 150000002500 ions Chemical class 0.000 description 7
- 229920000642 polymer Polymers 0.000 description 7
- 238000001556 precipitation Methods 0.000 description 7
- 150000003254 radicals Chemical class 0.000 description 7
- 238000006722 reduction reaction Methods 0.000 description 7
- KZUNJOHGWZRPMI-UHFFFAOYSA-N samarium atom Chemical compound [Sm] KZUNJOHGWZRPMI-UHFFFAOYSA-N 0.000 description 7
- 239000011550 stock solution Substances 0.000 description 7
- 229910052714 tellurium Inorganic materials 0.000 description 7
- 238000012546 transfer Methods 0.000 description 7
- DJHGAFSJWGLOIV-UHFFFAOYSA-K Arsenate3- Chemical compound [O-][As]([O-])([O-])=O DJHGAFSJWGLOIV-UHFFFAOYSA-K 0.000 description 6
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 229910021578 Iron(III) chloride Inorganic materials 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 150000001298 alcohols Chemical class 0.000 description 6
- 229940000489 arsenate Drugs 0.000 description 6
- 150000004770 chalcogenides Chemical class 0.000 description 6
- 238000009826 distribution Methods 0.000 description 6
- 229910052736 halogen Inorganic materials 0.000 description 6
- 150000002367 halogens Chemical class 0.000 description 6
- RBTARNINKXHZNM-UHFFFAOYSA-K iron trichloride Chemical compound Cl[Fe](Cl)Cl RBTARNINKXHZNM-UHFFFAOYSA-K 0.000 description 6
- CZMAIROVPAYCMU-UHFFFAOYSA-N lanthanum(3+) Chemical compound [La+3] CZMAIROVPAYCMU-UHFFFAOYSA-N 0.000 description 6
- 239000000575 pesticide Substances 0.000 description 6
- 150000008301 phosphite esters Chemical class 0.000 description 6
- 150000004714 phosphonium salts Chemical class 0.000 description 6
- ZJAOAACCNHFJAH-UHFFFAOYSA-N phosphonoformic acid Chemical compound OC(=O)P(O)(O)=O ZJAOAACCNHFJAH-UHFFFAOYSA-N 0.000 description 6
- 229910000073 phosphorus hydride Inorganic materials 0.000 description 6
- 238000002203 pretreatment Methods 0.000 description 6
- 230000009467 reduction Effects 0.000 description 6
- UGTZMIPZNRIWHX-UHFFFAOYSA-K sodium trimetaphosphate Chemical compound [Na+].[Na+].[Na+].[O-]P1(=O)OP([O-])(=O)OP([O-])(=O)O1 UGTZMIPZNRIWHX-UHFFFAOYSA-K 0.000 description 6
- PORWMNRCUJJQNO-UHFFFAOYSA-N tellurium atom Chemical group [Te] PORWMNRCUJJQNO-UHFFFAOYSA-N 0.000 description 6
- 238000010977 unit operation Methods 0.000 description 6
- 241000894006 Bacteria Species 0.000 description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 5
- 239000005958 Fenamiphos (aka phenamiphos) Substances 0.000 description 5
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 5
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 5
- 239000004599 antimicrobial Substances 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- ZCJPOPBZHLUFHF-UHFFFAOYSA-N fenamiphos Chemical compound CCOP(=O)(NC(C)C)OC1=CC=C(SC)C(C)=C1 ZCJPOPBZHLUFHF-UHFFFAOYSA-N 0.000 description 5
- 229910052731 fluorine Inorganic materials 0.000 description 5
- 239000008187 granular material Substances 0.000 description 5
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 5
- 239000002198 insoluble material Substances 0.000 description 5
- 239000007791 liquid phase Substances 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 150000004766 phosphaalkenes Chemical class 0.000 description 5
- YSWYYGKGAYSAOJ-UHFFFAOYSA-N phosphane Chemical compound P.P YSWYYGKGAYSAOJ-UHFFFAOYSA-N 0.000 description 5
- 238000003756 stirring Methods 0.000 description 5
- WVLBCYQITXONBZ-UHFFFAOYSA-N trimethyl phosphate Chemical compound COP(=O)(OC)OC WVLBCYQITXONBZ-UHFFFAOYSA-N 0.000 description 5
- ZSSWXNPRLJLCDU-UHFFFAOYSA-N 1-diethylphosphorylethane Chemical compound CCP(=O)(CC)CC ZSSWXNPRLJLCDU-UHFFFAOYSA-N 0.000 description 4
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 4
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 4
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 4
- XTEGARKTQYYJKE-UHFFFAOYSA-M Chlorate Chemical compound [O-]Cl(=O)=O XTEGARKTQYYJKE-UHFFFAOYSA-M 0.000 description 4
- 241001507939 Cormus domestica Species 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 4
- 229920002274 Nalgene Polymers 0.000 description 4
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 229910052770 Uranium Inorganic materials 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 150000001413 amino acids Chemical class 0.000 description 4
- CKMXBZGNNVIXHC-UHFFFAOYSA-L ammonium magnesium phosphate hexahydrate Chemical compound [NH4+].O.O.O.O.O.O.[Mg+2].[O-]P([O-])([O-])=O CKMXBZGNNVIXHC-UHFFFAOYSA-L 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 4
- 150000001735 carboxylic acids Chemical class 0.000 description 4
- OSVXSBDYLRYLIG-UHFFFAOYSA-N dioxidochlorine(.) Chemical compound O=Cl=O OSVXSBDYLRYLIG-UHFFFAOYSA-N 0.000 description 4
- SWJAOBXRZSMKNS-UHFFFAOYSA-N diphosphene Chemical compound P=P SWJAOBXRZSMKNS-UHFFFAOYSA-N 0.000 description 4
- 229910000075 diphosphene Inorganic materials 0.000 description 4
- 239000007789 gas Substances 0.000 description 4
- 229930195733 hydrocarbon Natural products 0.000 description 4
- 229910052740 iodine Inorganic materials 0.000 description 4
- 239000012528 membrane Substances 0.000 description 4
- 244000005700 microbiome Species 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 4
- 150000002894 organic compounds Chemical class 0.000 description 4
- 239000011368 organic material Substances 0.000 description 4
- 150000002903 organophosphorus compounds Chemical class 0.000 description 4
- 150000003003 phosphines Chemical class 0.000 description 4
- AQSJGOWTSHOLKH-UHFFFAOYSA-N phosphite(3-) Chemical class [O-]P([O-])[O-] AQSJGOWTSHOLKH-UHFFFAOYSA-N 0.000 description 4
- 238000004062 sedimentation Methods 0.000 description 4
- 229910052567 struvite Inorganic materials 0.000 description 4
- UNXRWKVEANCORM-UHFFFAOYSA-N triphosphoric acid Chemical class OP(O)(=O)OP(O)(=O)OP(O)(O)=O UNXRWKVEANCORM-UHFFFAOYSA-N 0.000 description 4
- JFALSRSLKYAFGM-UHFFFAOYSA-N uranium(0) Chemical compound [U] JFALSRSLKYAFGM-UHFFFAOYSA-N 0.000 description 4
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 3
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical class [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 3
- 229910052692 Dysprosium Inorganic materials 0.000 description 3
- 229910052693 Europium Inorganic materials 0.000 description 3
- 229910052688 Gadolinium Inorganic materials 0.000 description 3
- 239000007995 HEPES buffer Substances 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 3
- 229910052765 Lutetium Inorganic materials 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- DYAHQFWOVKZOOW-UHFFFAOYSA-N Sarin Chemical compound CC(C)OP(C)(F)=O DYAHQFWOVKZOOW-UHFFFAOYSA-N 0.000 description 3
- 229910052771 Terbium Inorganic materials 0.000 description 3
- 229910052775 Thulium Inorganic materials 0.000 description 3
- 229910052769 Ytterbium Inorganic materials 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 229910052785 arsenic Inorganic materials 0.000 description 3
- RQNWIZPPADIBDY-UHFFFAOYSA-N arsenic atom Chemical compound [As] RQNWIZPPADIBDY-UHFFFAOYSA-N 0.000 description 3
- 235000010323 ascorbic acid Nutrition 0.000 description 3
- 239000011668 ascorbic acid Substances 0.000 description 3
- 229960005070 ascorbic acid Drugs 0.000 description 3
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 229910000420 cerium oxide Inorganic materials 0.000 description 3
- ZMZNLKYXLARXFY-UHFFFAOYSA-H cerium(3+);oxalate Chemical compound [Ce+3].[Ce+3].[O-]C(=O)C([O-])=O.[O-]C(=O)C([O-])=O.[O-]C(=O)C([O-])=O ZMZNLKYXLARXFY-UHFFFAOYSA-H 0.000 description 3
- HSJPMRKMPBAUAU-UHFFFAOYSA-N cerium(3+);trinitrate Chemical compound [Ce+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O HSJPMRKMPBAUAU-UHFFFAOYSA-N 0.000 description 3
- OZECDDHOAMNMQI-UHFFFAOYSA-H cerium(3+);trisulfate Chemical compound [Ce+3].[Ce+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O OZECDDHOAMNMQI-UHFFFAOYSA-H 0.000 description 3
- 229910000333 cerium(III) sulfate Inorganic materials 0.000 description 3
- 238000004891 communication Methods 0.000 description 3
- 239000000470 constituent Substances 0.000 description 3
- AZSFNUJOCKMOGB-UHFFFAOYSA-K cyclotriphosphate(3-) Chemical compound [O-]P1(=O)OP([O-])(=O)OP([O-])(=O)O1 AZSFNUJOCKMOGB-UHFFFAOYSA-K 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- VEENJGZXVHKXNB-VOTSOKGWSA-N dicrotophos Chemical compound COP(=O)(OC)O\C(C)=C\C(=O)N(C)C VEENJGZXVHKXNB-VOTSOKGWSA-N 0.000 description 3
- 239000003651 drinking water Substances 0.000 description 3
- 235000020188 drinking water Nutrition 0.000 description 3
- KBQHZAAAGSGFKK-UHFFFAOYSA-N dysprosium atom Chemical compound [Dy] KBQHZAAAGSGFKK-UHFFFAOYSA-N 0.000 description 3
- OGPBJKLSAFTDLK-UHFFFAOYSA-N europium atom Chemical compound [Eu] OGPBJKLSAFTDLK-UHFFFAOYSA-N 0.000 description 3
- UIWYJDYFSGRHKR-UHFFFAOYSA-N gadolinium atom Chemical compound [Gd] UIWYJDYFSGRHKR-UHFFFAOYSA-N 0.000 description 3
- 230000005484 gravity Effects 0.000 description 3
- WQYVRQLZKVEZGA-UHFFFAOYSA-N hypochlorite Chemical compound Cl[O-] WQYVRQLZKVEZGA-UHFFFAOYSA-N 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- OHSVLFRHMCKCQY-UHFFFAOYSA-N lutetium atom Chemical compound [Lu] OHSVLFRHMCKCQY-UHFFFAOYSA-N 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- NNKVPIKMPCQWCG-UHFFFAOYSA-N methamidophos Chemical compound COP(N)(=O)SC NNKVPIKMPCQWCG-UHFFFAOYSA-N 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- MPQXHAGKBWFSNV-UHFFFAOYSA-N oxidophosphanium Chemical class [PH3]=O MPQXHAGKBWFSNV-UHFFFAOYSA-N 0.000 description 3
- BMMGVYCKOGBVEV-UHFFFAOYSA-N oxo(oxoceriooxy)cerium Chemical compound [Ce]=O.O=[Ce]=O BMMGVYCKOGBVEV-UHFFFAOYSA-N 0.000 description 3
- 230000000737 periodic effect Effects 0.000 description 3
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 3
- 239000002244 precipitate Substances 0.000 description 3
- 230000035484 reaction time Effects 0.000 description 3
- 238000005096 rolling process Methods 0.000 description 3
- 229910052706 scandium Inorganic materials 0.000 description 3
- SIXSYDAISGFNSX-UHFFFAOYSA-N scandium atom Chemical compound [Sc] SIXSYDAISGFNSX-UHFFFAOYSA-N 0.000 description 3
- 229910052709 silver Inorganic materials 0.000 description 3
- 239000004332 silver Substances 0.000 description 3
- 239000002002 slurry Substances 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- GZCRRIHWUXGPOV-UHFFFAOYSA-N terbium atom Chemical compound [Tb] GZCRRIHWUXGPOV-UHFFFAOYSA-N 0.000 description 3
- MDDUHVRJJAFRAU-YZNNVMRBSA-N tert-butyl-[(1r,3s,5z)-3-[tert-butyl(dimethyl)silyl]oxy-5-(2-diphenylphosphorylethylidene)-4-methylidenecyclohexyl]oxy-dimethylsilane Chemical compound C1[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H](O[Si](C)(C)C(C)(C)C)C(=C)\C1=C/CP(=O)(C=1C=CC=CC=1)C1=CC=CC=C1 MDDUHVRJJAFRAU-YZNNVMRBSA-N 0.000 description 3
- 238000004065 wastewater treatment Methods 0.000 description 3
- 239000002349 well water Substances 0.000 description 3
- 235000020681 well water Nutrition 0.000 description 3
- NAWDYIZEMPQZHO-UHFFFAOYSA-N ytterbium Chemical compound [Yb] NAWDYIZEMPQZHO-UHFFFAOYSA-N 0.000 description 3
- 229910052727 yttrium Inorganic materials 0.000 description 3
- VWQVUPCCIRVNHF-UHFFFAOYSA-N yttrium atom Chemical compound [Y] VWQVUPCCIRVNHF-UHFFFAOYSA-N 0.000 description 3
- 125000004400 (C1-C12) alkyl group Chemical group 0.000 description 2
- PJISLFCKHOHLLP-UHFFFAOYSA-N 2-diethoxyphosphorylsulfanyl-n,n-diethylethanamine Chemical group CCOP(=O)(OCC)SCCN(CC)CC PJISLFCKHOHLLP-UHFFFAOYSA-N 0.000 description 2
- XTWYTFMLZFPYCI-KQYNXXCUSA-N 5'-adenylphosphoric acid Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O XTWYTFMLZFPYCI-KQYNXXCUSA-N 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- 229910004755 Cerium(III) bromide Inorganic materials 0.000 description 2
- 239000004155 Chlorine dioxide Substances 0.000 description 2
- 235000008733 Citrus aurantifolia Nutrition 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- 241000195493 Cryptophyta Species 0.000 description 2
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- 239000005949 Malathion Substances 0.000 description 2
- AMQJEAYHLZJPGS-UHFFFAOYSA-N N-Pentanol Chemical compound CCCCCO AMQJEAYHLZJPGS-UHFFFAOYSA-N 0.000 description 2
- 229910002651 NO3 Inorganic materials 0.000 description 2
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 2
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 2
- YGYAWVDWMABLBF-UHFFFAOYSA-N Phosgene Chemical compound ClC(Cl)=O YGYAWVDWMABLBF-UHFFFAOYSA-N 0.000 description 2
- 229920000388 Polyphosphate Polymers 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- FOIXSVOLVBLSDH-UHFFFAOYSA-N Silver ion Chemical compound [Ag+] FOIXSVOLVBLSDH-UHFFFAOYSA-N 0.000 description 2
- RAHZWNYVWXNFOC-UHFFFAOYSA-N Sulphur dioxide Chemical compound O=S=O RAHZWNYVWXNFOC-UHFFFAOYSA-N 0.000 description 2
- 235000011941 Tilia x europaea Nutrition 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 150000001299 aldehydes Chemical class 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 229910021529 ammonia Inorganic materials 0.000 description 2
- 229910052788 barium Inorganic materials 0.000 description 2
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 2
- 235000012206 bottled water Nutrition 0.000 description 2
- CODNYICXDISAEA-UHFFFAOYSA-N bromine monochloride Chemical compound BrCl CODNYICXDISAEA-UHFFFAOYSA-N 0.000 description 2
- 229910052792 caesium Inorganic materials 0.000 description 2
- TVFDJXOCXUVLDH-UHFFFAOYSA-N caesium atom Chemical compound [Cs] TVFDJXOCXUVLDH-UHFFFAOYSA-N 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 150000001721 carbon Chemical group 0.000 description 2
- JJWKPURADFRFRB-UHFFFAOYSA-N carbonyl sulfide Chemical compound O=C=S JJWKPURADFRFRB-UHFFFAOYSA-N 0.000 description 2
- 125000002843 carboxylic acid group Chemical group 0.000 description 2
- 239000000919 ceramic Substances 0.000 description 2
- DRVWBEJJZZTIGJ-UHFFFAOYSA-N cerium(3+);oxygen(2-) Chemical class [O-2].[O-2].[O-2].[Ce+3].[Ce+3] DRVWBEJJZZTIGJ-UHFFFAOYSA-N 0.000 description 2
- ZEDZJUDTPVFRNB-UHFFFAOYSA-K cerium(3+);triiodide Chemical compound I[Ce](I)I ZEDZJUDTPVFRNB-UHFFFAOYSA-K 0.000 description 2
- MOOUSOJAOQPDEH-UHFFFAOYSA-K cerium(iii) bromide Chemical compound [Br-].[Br-].[Br-].[Ce+3] MOOUSOJAOQPDEH-UHFFFAOYSA-K 0.000 description 2
- CQGVSILDZJUINE-UHFFFAOYSA-N cerium;hydrate Chemical compound O.[Ce] CQGVSILDZJUINE-UHFFFAOYSA-N 0.000 description 2
- 150000001793 charged compounds Polymers 0.000 description 2
- 235000019398 chlorine dioxide Nutrition 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 238000001784 detoxification Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- JXSJBGJIGXNWCI-UHFFFAOYSA-N diethyl 2-[(dimethoxyphosphorothioyl)thio]succinate Chemical compound CCOC(=O)CC(SP(=S)(OC)OC)C(=O)OCC JXSJBGJIGXNWCI-UHFFFAOYSA-N 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 239000012153 distilled water Substances 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- SHJJIRWILPHYGV-UHFFFAOYSA-N erbium holmium Chemical compound [Ho].[Er] SHJJIRWILPHYGV-UHFFFAOYSA-N 0.000 description 2
- UFZOPKFMKMAWLU-UHFFFAOYSA-N ethoxy(methyl)phosphinic acid Chemical compound CCOP(C)(O)=O UFZOPKFMKMAWLU-UHFFFAOYSA-N 0.000 description 2
- 239000011737 fluorine Substances 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- 238000012812 general test Methods 0.000 description 2
- 239000003673 groundwater Substances 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- 150000002431 hydrogen Chemical class 0.000 description 2
- JGJLWPGRMCADHB-UHFFFAOYSA-N hypobromite Chemical compound Br[O-] JGJLWPGRMCADHB-UHFFFAOYSA-N 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 description 2
- 238000005342 ion exchange Methods 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 230000002262 irrigation Effects 0.000 description 2
- 238000003973 irrigation Methods 0.000 description 2
- 229910052747 lanthanoid Inorganic materials 0.000 description 2
- 150000002602 lanthanoids Chemical class 0.000 description 2
- MRELNEQAGSRDBK-UHFFFAOYSA-N lanthanum(3+);oxygen(2-) Chemical class [O-2].[O-2].[O-2].[La+3].[La+3] MRELNEQAGSRDBK-UHFFFAOYSA-N 0.000 description 2
- 239000004571 lime Substances 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 229960000453 malathion Drugs 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 238000005065 mining Methods 0.000 description 2
- MEFBJEMVZONFCJ-UHFFFAOYSA-N molybdate Chemical compound [O-][Mo]([O-])(=O)=O MEFBJEMVZONFCJ-UHFFFAOYSA-N 0.000 description 2
- 239000002105 nanoparticle Substances 0.000 description 2
- 229910052755 nonmetal Inorganic materials 0.000 description 2
- SBOJXQVPLKSXOG-UHFFFAOYSA-N o-amino-hydroxylamine Chemical compound NON SBOJXQVPLKSXOG-UHFFFAOYSA-N 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- AUONHKJOIZSQGR-UHFFFAOYSA-N oxophosphane Chemical compound P=O AUONHKJOIZSQGR-UHFFFAOYSA-N 0.000 description 2
- 125000004430 oxygen atom Chemical group O* 0.000 description 2
- 239000013618 particulate matter Substances 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- 239000003208 petroleum Substances 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- 150000002989 phenols Chemical class 0.000 description 2
- LSMAIBOZUPTNBR-UHFFFAOYSA-N phosphanium;iodide Chemical compound [PH4+].[I-] LSMAIBOZUPTNBR-UHFFFAOYSA-N 0.000 description 2
- UEZVMMHDMIWARA-UHFFFAOYSA-M phosphonate Chemical compound [O-]P(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-M 0.000 description 2
- VBQCHPIMZGQLAZ-UHFFFAOYSA-N phosphorane Chemical class [PH5] VBQCHPIMZGQLAZ-UHFFFAOYSA-N 0.000 description 2
- 150000003017 phosphorus Chemical class 0.000 description 2
- 229910052699 polonium Inorganic materials 0.000 description 2
- HZEBHPIOVYHPMT-UHFFFAOYSA-N polonium atom Chemical compound [Po] HZEBHPIOVYHPMT-UHFFFAOYSA-N 0.000 description 2
- 239000001205 polyphosphate Substances 0.000 description 2
- 235000011176 polyphosphates Nutrition 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 230000001376 precipitating effect Effects 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 150000003141 primary amines Chemical class 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- QYMMJNLHFKGANY-UHFFFAOYSA-N profenofos Chemical compound CCCSP(=O)(OCC)OC1=CC=C(Br)C=C1Cl QYMMJNLHFKGANY-UHFFFAOYSA-N 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 230000002285 radioactive effect Effects 0.000 description 2
- 230000008929 regeneration Effects 0.000 description 2
- 238000011069 regeneration method Methods 0.000 description 2
- CPRMKOQKXYSDML-UHFFFAOYSA-M rubidium hydroxide Chemical compound [OH-].[Rb+] CPRMKOQKXYSDML-UHFFFAOYSA-M 0.000 description 2
- 238000009287 sand filtration Methods 0.000 description 2
- 150000003335 secondary amines Chemical class 0.000 description 2
- 239000010865 sewage Substances 0.000 description 2
- 150000004760 silicates Chemical class 0.000 description 2
- 239000010802 sludge Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- RDZTWEVXRGYCFV-UHFFFAOYSA-M sodium 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonate Chemical compound [Na+].OCCN1CCN(CCS([O-])(=O)=O)CC1 RDZTWEVXRGYCFV-UHFFFAOYSA-M 0.000 description 2
- 239000011343 solid material Substances 0.000 description 2
- 239000002195 soluble material Substances 0.000 description 2
- 229910052712 strontium Inorganic materials 0.000 description 2
- CIOAGBVUUVVLOB-UHFFFAOYSA-N strontium atom Chemical compound [Sr] CIOAGBVUUVVLOB-UHFFFAOYSA-N 0.000 description 2
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 2
- 150000003512 tertiary amines Chemical class 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- DQWPFSLDHJDLRL-UHFFFAOYSA-N triethyl phosphate Chemical compound CCOP(=O)(OCC)OCC DQWPFSLDHJDLRL-UHFFFAOYSA-N 0.000 description 2
- YWWDBCBWQNCYNR-UHFFFAOYSA-N trimethylphosphine Chemical compound CP(C)C YWWDBCBWQNCYNR-UHFFFAOYSA-N 0.000 description 2
- SOBHUZYZLFQYFK-UHFFFAOYSA-K trisodium;hydroxy-[[phosphonatomethyl(phosphonomethyl)amino]methyl]phosphinate Chemical compound [Na+].[Na+].[Na+].OP(O)(=O)CN(CP(O)([O-])=O)CP([O-])([O-])=O SOBHUZYZLFQYFK-UHFFFAOYSA-K 0.000 description 2
- JGFMNFBZRAISGQ-UHFFFAOYSA-N (propan-2-yloxy)phosphinic acid Chemical group CC(C)OP(O)=O JGFMNFBZRAISGQ-UHFFFAOYSA-N 0.000 description 1
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- YRIZYWQGELRKNT-UHFFFAOYSA-N 1,3,5-trichloro-1,3,5-triazinane-2,4,6-trione Chemical compound ClN1C(=O)N(Cl)C(=O)N(Cl)C1=O YRIZYWQGELRKNT-UHFFFAOYSA-N 0.000 description 1
- TUSDEZXZIZRFGC-UHFFFAOYSA-N 1-O-galloyl-3,6-(R)-HHDP-beta-D-glucose Natural products OC1C(O2)COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC1C(O)C2OC(=O)C1=CC(O)=C(O)C(O)=C1 TUSDEZXZIZRFGC-UHFFFAOYSA-N 0.000 description 1
- MFGOFGRYDNHJTA-UHFFFAOYSA-N 2-amino-1-(2-fluorophenyl)ethanol Chemical compound NCC(O)C1=CC=CC=C1F MFGOFGRYDNHJTA-UHFFFAOYSA-N 0.000 description 1
- IAJOBQBIJHVGMQ-UHFFFAOYSA-N 2-amino-4-[hydroxy(methyl)phosphoryl]butanoic acid Chemical compound CP(O)(=O)CCC(N)C(O)=O IAJOBQBIJHVGMQ-UHFFFAOYSA-N 0.000 description 1
- 125000000022 2-aminoethyl group Chemical group [H]C([*])([H])C([H])([H])N([H])[H] 0.000 description 1
- YTVQIZRDLKWECQ-UHFFFAOYSA-N 2-benzoylcyclohexan-1-one Chemical group C=1C=CC=CC=1C(=O)C1CCCCC1=O YTVQIZRDLKWECQ-UHFFFAOYSA-N 0.000 description 1
- XVNBZVNXJMOQEX-UHFFFAOYSA-N 3-[chloro(methyl)phosphoryl]oxy-2,2-dimethylbutane Chemical compound CC(C)(C)C(C)OP(C)(Cl)=O XVNBZVNXJMOQEX-UHFFFAOYSA-N 0.000 description 1
- QJZYHAIUNVAGQP-UHFFFAOYSA-N 3-nitrobicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid Chemical compound C1C2C=CC1C(C(=O)O)C2(C(O)=O)[N+]([O-])=O QJZYHAIUNVAGQP-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 229910000497 Amalgam Inorganic materials 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 239000004135 Bone phosphate Substances 0.000 description 1
- 101100283604 Caenorhabditis elegans pigk-1 gene Proteins 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical class [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- 150000000703 Cerium Chemical class 0.000 description 1
- 229910008069 Cerium(III) iodide Inorganic materials 0.000 description 1
- QDHHCQZDFGDHMP-UHFFFAOYSA-N Chloramine Chemical class ClN QDHHCQZDFGDHMP-UHFFFAOYSA-N 0.000 description 1
- JPVYNHNXODAKFH-UHFFFAOYSA-N Cu2+ Chemical compound [Cu+2] JPVYNHNXODAKFH-UHFFFAOYSA-N 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- 241000406799 Deto Species 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 229910052691 Erbium Inorganic materials 0.000 description 1
- 239000001263 FEMA 3042 Substances 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- QGWNDRXFNXRZMB-UUOKFMHZSA-K GDP(3-) Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)[C@H]1O QGWNDRXFNXRZMB-UUOKFMHZSA-K 0.000 description 1
- 239000005561 Glufosinate Substances 0.000 description 1
- 239000005562 Glyphosate Substances 0.000 description 1
- 239000008053 Hepes sodium buffer Substances 0.000 description 1
- 229910052689 Holmium Inorganic materials 0.000 description 1
- 229910016859 Lanthanum iodide Inorganic materials 0.000 description 1
- 229920001732 Lignosulfonate Polymers 0.000 description 1
- 239000004117 Lignosulphonate Substances 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- 101000706020 Nicotiana tabacum Pathogenesis-related protein R minor form Proteins 0.000 description 1
- IOVCWXUNBOPUCH-UHFFFAOYSA-M Nitrite anion Chemical compound [O-]N=O IOVCWXUNBOPUCH-UHFFFAOYSA-M 0.000 description 1
- CAXMORXQKRVSHV-UHFFFAOYSA-N O(O)O.[Ce+4] Chemical compound O(O)O.[Ce+4] CAXMORXQKRVSHV-UHFFFAOYSA-N 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- LRBQNJMCXXYXIU-PPKXGCFTSA-N Penta-digallate-beta-D-glucose Natural products OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@@H]2[C@H]([C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-PPKXGCFTSA-N 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 229910021607 Silver chloride Inorganic materials 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical class OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- RYYWUUFWQRZTIU-UHFFFAOYSA-N Thiophosphoric acid Chemical class OP(O)(S)=O RYYWUUFWQRZTIU-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- 229910021536 Zeolite Inorganic materials 0.000 description 1
- DBXHFBVMRQOQKW-UHFFFAOYSA-N [4-[3-oxo-1-(4-phosphonooxyphenyl)-2-benzofuran-1-yl]phenyl] dihydrogen phosphate;pyridine Chemical compound C1=CC=NC=C1.C1=CC(OP(O)(=O)O)=CC=C1C1(C=2C=CC(OP(O)(O)=O)=CC=2)C2=CC=CC=C2C(=O)O1 DBXHFBVMRQOQKW-UHFFFAOYSA-N 0.000 description 1
- BUFLLCUFNHESEH-UHFFFAOYSA-N [5-(2-amino-6-oxo-3h-purin-9-yl)-4-hydroxy-2-[[hydroxy(phosphonooxy)phosphoryl]oxymethyl]oxolan-3-yl] phosphono hydrogen phosphate Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1C1OC(COP(O)(=O)OP(O)(O)=O)C(OP(O)(=O)OP(O)(O)=O)C1O BUFLLCUFNHESEH-UHFFFAOYSA-N 0.000 description 1
- GDTKCPOMNLECHU-UHFFFAOYSA-N [Se](Cl)Cl.[P] Chemical compound [Se](Cl)Cl.[P] GDTKCPOMNLECHU-UHFFFAOYSA-N 0.000 description 1
- 150000008065 acid anhydrides Chemical class 0.000 description 1
- 229920000122 acrylonitrile butadiene styrene Polymers 0.000 description 1
- 150000001266 acyl halides Chemical class 0.000 description 1
- 239000003463 adsorbent Substances 0.000 description 1
- 238000005276 aerator Methods 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 150000004703 alkoxides Chemical class 0.000 description 1
- 229910052873 allanite Inorganic materials 0.000 description 1
- DIZPMCHEQGEION-UHFFFAOYSA-H aluminium sulfate (anhydrous) Chemical compound [Al+3].[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O DIZPMCHEQGEION-UHFFFAOYSA-H 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 239000011959 amorphous silica alumina Substances 0.000 description 1
- 239000012491 analyte Substances 0.000 description 1
- 239000003957 anion exchange resin Substances 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 229910052586 apatite Inorganic materials 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- LULLIKNODDLMDQ-UHFFFAOYSA-N arsenic(3+) Chemical compound [As+3] LULLIKNODDLMDQ-UHFFFAOYSA-N 0.000 description 1
- HAYXDMNJJFVXCI-UHFFFAOYSA-N arsenic(5+) Chemical compound [As+5] HAYXDMNJJFVXCI-UHFFFAOYSA-N 0.000 description 1
- GCPXMJHSNVMWNM-UHFFFAOYSA-N arsenous acid Chemical class O[As](O)O GCPXMJHSNVMWNM-UHFFFAOYSA-N 0.000 description 1
- 239000010426 asphalt Substances 0.000 description 1
- 229910052789 astatine Inorganic materials 0.000 description 1
- RYXHOMYVWAEKHL-UHFFFAOYSA-N astatine atom Chemical compound [At] RYXHOMYVWAEKHL-UHFFFAOYSA-N 0.000 description 1
- 229910052826 autunite Inorganic materials 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- RQPZNWPYLFFXCP-UHFFFAOYSA-L barium dihydroxide Chemical compound [OH-].[OH-].[Ba+2] RQPZNWPYLFFXCP-UHFFFAOYSA-L 0.000 description 1
- 229910001863 barium hydroxide Inorganic materials 0.000 description 1
- 235000013405 beer Nutrition 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- SIPUZPBQZHNSDW-UHFFFAOYSA-N bis(2-methylpropyl)aluminum Chemical compound CC(C)C[Al]CC(C)C SIPUZPBQZHNSDW-UHFFFAOYSA-N 0.000 description 1
- 125000005340 bisphosphate group Chemical group 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M bisulphate group Chemical group S([O-])(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 239000012267 brine Substances 0.000 description 1
- 229910000182 britholite Inorganic materials 0.000 description 1
- FNXLCIKXHOPCKH-UHFFFAOYSA-N bromamine Chemical class BrN FNXLCIKXHOPCKH-UHFFFAOYSA-N 0.000 description 1
- 230000031709 bromination Effects 0.000 description 1
- 238000005893 bromination reaction Methods 0.000 description 1
- RDKFCARAWNLFDR-UHFFFAOYSA-N butyl(ethoxy)phosphinic acid Chemical compound CCCCP(O)(=O)OCC RDKFCARAWNLFDR-UHFFFAOYSA-N 0.000 description 1
- HUCVOHYBFXVBRW-UHFFFAOYSA-M caesium hydroxide Inorganic materials [OH-].[Cs+] HUCVOHYBFXVBRW-UHFFFAOYSA-M 0.000 description 1
- WUKWITHWXAAZEY-UHFFFAOYSA-L calcium difluoride Chemical compound [F-].[F-].[Ca+2] WUKWITHWXAAZEY-UHFFFAOYSA-L 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- QGJOPFRUJISHPQ-NJFSPNSNSA-N carbon disulfide-14c Chemical compound S=[14C]=S QGJOPFRUJISHPQ-NJFSPNSNSA-N 0.000 description 1
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 1
- 150000001723 carbon free-radicals Chemical class 0.000 description 1
- 229910002090 carbon oxide Inorganic materials 0.000 description 1
- BCZZCSPWPQMEQS-UHFFFAOYSA-N carbonic acid hydrofluoride Chemical compound C(O)(O)=O.F BCZZCSPWPQMEQS-UHFFFAOYSA-N 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 239000004568 cement Substances 0.000 description 1
- 229940044927 ceric oxide Drugs 0.000 description 1
- 229910000175 cerite Inorganic materials 0.000 description 1
- IKNAJTLCCWPIQD-UHFFFAOYSA-K cerium(3+);lanthanum(3+);neodymium(3+);oxygen(2-);phosphate Chemical compound [O-2].[La+3].[Ce+3].[Nd+3].[O-]P([O-])([O-])=O IKNAJTLCCWPIQD-UHFFFAOYSA-K 0.000 description 1
- GHLITDDQOMIBFS-UHFFFAOYSA-H cerium(3+);tricarbonate Chemical compound [Ce+3].[Ce+3].[O-]C([O-])=O.[O-]C([O-])=O.[O-]C([O-])=O GHLITDDQOMIBFS-UHFFFAOYSA-H 0.000 description 1
- KHSBAWXKALEJFR-UHFFFAOYSA-H cerium(3+);tricarbonate;hydrate Chemical compound O.[Ce+3].[Ce+3].[O-]C([O-])=O.[O-]C([O-])=O.[O-]C([O-])=O KHSBAWXKALEJFR-UHFFFAOYSA-H 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000013626 chemical specie Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000005660 chlorination reaction Methods 0.000 description 1
- RHAHLPAWJVKXAZ-UHFFFAOYSA-N chlorosarin Chemical compound CC(C)OP(C)(Cl)=O RHAHLPAWJVKXAZ-UHFFFAOYSA-N 0.000 description 1
- ZCDOYSPFYFSLEW-UHFFFAOYSA-N chromate(2-) Chemical class [O-][Cr]([O-])(=O)=O ZCDOYSPFYFSLEW-UHFFFAOYSA-N 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 238000000975 co-precipitation Methods 0.000 description 1
- 239000000701 coagulant Substances 0.000 description 1
- 229910000169 coffinite Inorganic materials 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 239000000498 cooling water Substances 0.000 description 1
- 150000004696 coordination complex Chemical class 0.000 description 1
- 229910000365 copper sulfate Inorganic materials 0.000 description 1
- ARUVKPQLZAKDPS-UHFFFAOYSA-L copper(II) sulfate Chemical compound [Cu+2].[O-][S+2]([O-])([O-])[O-] ARUVKPQLZAKDPS-UHFFFAOYSA-L 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 150000003950 cyclic amides Chemical class 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 239000000645 desinfectant Substances 0.000 description 1
- 238000003795 desorption Methods 0.000 description 1
- 230000001687 destabilization Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- FHIVAFMUCKRCQO-UHFFFAOYSA-N diazinon Chemical compound CCOP(=S)(OCC)OC1=CC(C)=NC(C(C)C)=N1 FHIVAFMUCKRCQO-UHFFFAOYSA-N 0.000 description 1
- KSHDLNQYVGBYHZ-UHFFFAOYSA-N dibutylphosphinic acid Chemical compound CCCCP(O)(=O)CCCC KSHDLNQYVGBYHZ-UHFFFAOYSA-N 0.000 description 1
- KTLIMPGQZDZPSB-UHFFFAOYSA-N diethylphosphinic acid Chemical compound CCP(O)(=O)CC KTLIMPGQZDZPSB-UHFFFAOYSA-N 0.000 description 1
- NGKCHGKFHQDOPZ-UHFFFAOYSA-N dihexylphosphinic acid Chemical compound CCCCCCP(O)(=O)CCCCCC NGKCHGKFHQDOPZ-UHFFFAOYSA-N 0.000 description 1
- WOAFDHWYKSOANX-UHFFFAOYSA-N diisopropyl methylphosphonate Chemical class CC(C)OP(C)(=O)OC(C)C WOAFDHWYKSOANX-UHFFFAOYSA-N 0.000 description 1
- VONWDASPFIQPDY-UHFFFAOYSA-N dimethyl methylphosphonate Chemical class COP(C)(=O)OC VONWDASPFIQPDY-UHFFFAOYSA-N 0.000 description 1
- 125000002147 dimethylamino group Chemical group [H]C([H])([H])N(*)C([H])([H])[H] 0.000 description 1
- GOJNABIZVJCYFL-UHFFFAOYSA-N dimethylphosphinic acid Chemical compound CP(C)(O)=O GOJNABIZVJCYFL-UHFFFAOYSA-N 0.000 description 1
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 1
- 229910001882 dioxygen Inorganic materials 0.000 description 1
- KUFDFOXPFQCUST-UHFFFAOYSA-N dipentylphosphinic acid Chemical compound CCCCCP(O)(=O)CCCCC KUFDFOXPFQCUST-UHFFFAOYSA-N 0.000 description 1
- WMDPJKZHARKRQI-UHFFFAOYSA-N dipropylphosphinic acid Chemical compound CCCP(O)(=O)CCC WMDPJKZHARKRQI-UHFFFAOYSA-N 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000009300 dissolved air flotation Methods 0.000 description 1
- 230000009189 diving Effects 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000000909 electrodialysis Methods 0.000 description 1
- 238000005868 electrolysis reaction Methods 0.000 description 1
- 239000003344 environmental pollutant Substances 0.000 description 1
- UYAHIZSMUZPPFV-UHFFFAOYSA-N erbium Chemical compound [Er] UYAHIZSMUZPPFV-UHFFFAOYSA-N 0.000 description 1
- YJCXKNNURGCXFQ-UHFFFAOYSA-N ethoxy(ethyl)phosphinic acid Chemical compound CCOP(O)(=O)CC YJCXKNNURGCXFQ-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- SMNZHEHSKZSQLM-UHFFFAOYSA-N ethyl(methoxy)phosphinic acid Chemical compound CCP(O)(=O)OC SMNZHEHSKZSQLM-UHFFFAOYSA-N 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 230000005281 excited state Effects 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000008394 flocculating agent Substances 0.000 description 1
- 230000003311 flocculating effect Effects 0.000 description 1
- 238000005189 flocculation Methods 0.000 description 1
- 230000016615 flocculation Effects 0.000 description 1
- 238000003682 fluorination reaction Methods 0.000 description 1
- 239000010436 fluorite Substances 0.000 description 1
- NBVXSUQYWXRMNV-UHFFFAOYSA-N fluoromethane Chemical compound FC NBVXSUQYWXRMNV-UHFFFAOYSA-N 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000013505 freshwater Substances 0.000 description 1
- 229910000199 gadolinite Inorganic materials 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 238000001879 gelation Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 229940097068 glyphosate Drugs 0.000 description 1
- XDDAORKBJWWYJS-UHFFFAOYSA-M glyphosate(1-) Chemical compound OP(O)(=O)CNCC([O-])=O XDDAORKBJWWYJS-UHFFFAOYSA-M 0.000 description 1
- QGWNDRXFNXRZMB-UHFFFAOYSA-N guanidine diphosphate Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1OC(COP(O)(=O)OP(O)(O)=O)C(O)C1O QGWNDRXFNXRZMB-UHFFFAOYSA-N 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 239000008241 heterogeneous mixture Substances 0.000 description 1
- 150000004687 hexahydrates Chemical class 0.000 description 1
- 229940005740 hexametaphosphate Drugs 0.000 description 1
- KJZYNXUDTRRSPN-UHFFFAOYSA-N holmium atom Chemical compound [Ho] KJZYNXUDTRRSPN-UHFFFAOYSA-N 0.000 description 1
- 239000004021 humic acid Substances 0.000 description 1
- 150000001469 hydantoins Chemical class 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- TUJKJAMUKRIRHC-UHFFFAOYSA-N hydroxyl Chemical class [OH] TUJKJAMUKRIRHC-UHFFFAOYSA-N 0.000 description 1
- CUILPNURFADTPE-UHFFFAOYSA-N hypobromous acid Chemical compound BrO CUILPNURFADTPE-UHFFFAOYSA-N 0.000 description 1
- QWPPOHNGKGFGJK-UHFFFAOYSA-N hypochlorous acid Chemical compound ClO QWPPOHNGKGFGJK-UHFFFAOYSA-N 0.000 description 1
- 238000007654 immersion Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 238000001095 inductively coupled plasma mass spectrometry Methods 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 239000011147 inorganic material Substances 0.000 description 1
- 229910052816 inorganic phosphate Inorganic materials 0.000 description 1
- 230000026045 iodination Effects 0.000 description 1
- 238000006192 iodination reaction Methods 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- RUTXIHLAWFEWGM-UHFFFAOYSA-H iron(3+) sulfate Chemical compound [Fe+3].[Fe+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O RUTXIHLAWFEWGM-UHFFFAOYSA-H 0.000 description 1
- 229910000360 iron(III) sulfate Inorganic materials 0.000 description 1
- 239000012948 isocyanate Substances 0.000 description 1
- 150000002513 isocyanates Chemical class 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 150000003951 lactams Chemical class 0.000 description 1
- 150000002596 lactones Chemical class 0.000 description 1
- 150000002603 lanthanum Chemical class 0.000 description 1
- MCYSFVUDODFQPV-UHFFFAOYSA-K lanthanum(3+) trichlorate Chemical compound [La+3].[O-][Cl](=O)=O.[O-][Cl](=O)=O.[O-][Cl](=O)=O MCYSFVUDODFQPV-UHFFFAOYSA-K 0.000 description 1
- OXHNIMPTBAKYRS-UHFFFAOYSA-H lanthanum(3+);oxalate Chemical compound [La+3].[La+3].[O-]C(=O)C([O-])=O.[O-]C(=O)C([O-])=O.[O-]C(=O)C([O-])=O OXHNIMPTBAKYRS-UHFFFAOYSA-H 0.000 description 1
- KYKBXWMMXCGRBA-UHFFFAOYSA-K lanthanum(3+);triiodide Chemical compound I[La](I)I KYKBXWMMXCGRBA-UHFFFAOYSA-K 0.000 description 1
- FYDKNKUEBJQCCN-UHFFFAOYSA-N lanthanum(3+);trinitrate Chemical compound [La+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O FYDKNKUEBJQCCN-UHFFFAOYSA-N 0.000 description 1
- VQEHIYWBGOJJDM-UHFFFAOYSA-H lanthanum(3+);trisulfate Chemical compound [La+3].[La+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O VQEHIYWBGOJJDM-UHFFFAOYSA-H 0.000 description 1
- XKUYOJZZLGFZTC-UHFFFAOYSA-K lanthanum(iii) bromide Chemical compound Br[La](Br)Br XKUYOJZZLGFZTC-UHFFFAOYSA-K 0.000 description 1
- ICAKDTKJOYSXGC-UHFFFAOYSA-K lanthanum(iii) chloride Chemical compound Cl[La](Cl)Cl ICAKDTKJOYSXGC-UHFFFAOYSA-K 0.000 description 1
- 235000019357 lignosulphonate Nutrition 0.000 description 1
- 239000011981 lindlar catalyst Substances 0.000 description 1
- 239000012669 liquid formulation Substances 0.000 description 1
- 239000012280 lithium aluminium hydride Substances 0.000 description 1
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 1
- 239000000347 magnesium hydroxide Substances 0.000 description 1
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 238000013327 media filtration Methods 0.000 description 1
- 238000011177 media preparation Methods 0.000 description 1
- MJGFBOZCAJSGQW-UHFFFAOYSA-N mercury sodium Chemical compound [Na].[Hg] MJGFBOZCAJSGQW-UHFFFAOYSA-N 0.000 description 1
- YVUZUKYBUMROPQ-UHFFFAOYSA-N mercury zinc Chemical compound [Zn].[Hg] YVUZUKYBUMROPQ-UHFFFAOYSA-N 0.000 description 1
- 150000001247 metal acetylides Chemical class 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- DWHMMGGJCLDORC-UHFFFAOYSA-N methoxy(methyl)phosphinic acid Chemical compound COP(C)(O)=O DWHMMGGJCLDORC-UHFFFAOYSA-N 0.000 description 1
- RLDXWNAZJDJGCX-UHFFFAOYSA-N methoxy(propyl)phosphinic acid Chemical compound CCCP(O)(=O)OC RLDXWNAZJDJGCX-UHFFFAOYSA-N 0.000 description 1
- JNMIXMFEVJHFNY-UHFFFAOYSA-M methyl(triphenyl)phosphanium;iodide Chemical compound [I-].C=1C=CC=CC=1[P+](C=1C=CC=CC=1)(C)C1=CC=CC=C1 JNMIXMFEVJHFNY-UHFFFAOYSA-M 0.000 description 1
- XYDYWTJEGDZLTH-UHFFFAOYSA-N methylenetriphenylphosphorane Chemical compound C=1C=CC=CC=1P(C=1C=CC=CC=1)(=C)C1=CC=CC=C1 XYDYWTJEGDZLTH-UHFFFAOYSA-N 0.000 description 1
- SCLFRABIDYGTAZ-UHFFFAOYSA-N methylphosphonic acid dichloride Chemical compound CP(Cl)(Cl)=O SCLFRABIDYGTAZ-UHFFFAOYSA-N 0.000 description 1
- 238000001471 micro-filtration Methods 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 235000013379 molasses Nutrition 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 229910052590 monazite Inorganic materials 0.000 description 1
- KRTSDMXIXPKRQR-AATRIKPKSA-N monocrotophos Chemical compound CNC(=O)\C=C(/C)OP(=O)(OC)OC KRTSDMXIXPKRQR-AATRIKPKSA-N 0.000 description 1
- 150000007518 monoprotic acids Chemical class 0.000 description 1
- XVDBWWRIXBMVJV-UHFFFAOYSA-N n-[bis(dimethylamino)phosphanyl]-n-methylmethanamine Chemical compound CN(C)P(N(C)C)N(C)C XVDBWWRIXBMVJV-UHFFFAOYSA-N 0.000 description 1
- 238000001728 nano-filtration Methods 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 230000001546 nitrifying effect Effects 0.000 description 1
- 150000002825 nitriles Chemical class 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 150000002843 nonmetals Chemical class 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- 239000005416 organic matter Substances 0.000 description 1
- 150000003891 oxalate salts Chemical class 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 238000006385 ozonation reaction Methods 0.000 description 1
- 239000011236 particulate material Substances 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- VSIIXMUUUJUKCM-UHFFFAOYSA-D pentacalcium;fluoride;triphosphate Chemical compound [F-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O VSIIXMUUUJUKCM-UHFFFAOYSA-D 0.000 description 1
- VLTRZXGMWDSKGL-UHFFFAOYSA-M perchlorate Inorganic materials [O-]Cl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-M 0.000 description 1
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 1
- JRKICGRDRMAZLK-UHFFFAOYSA-L peroxydisulfate Chemical compound [O-]S(=O)(=O)OOS([O-])(=O)=O JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 1
- 229940085991 phosphate ion Drugs 0.000 description 1
- 150000008105 phosphatidylcholines Chemical class 0.000 description 1
- 150000003008 phosphonic acid esters Chemical class 0.000 description 1
- 150000003009 phosphonic acids Chemical class 0.000 description 1
- PTMHPRAIXMAOOB-UHFFFAOYSA-L phosphoramidate Chemical group NP([O-])([O-])=O PTMHPRAIXMAOOB-UHFFFAOYSA-L 0.000 description 1
- UXBZSSBXGPYSIL-UHFFFAOYSA-N phosphoric acid;yttrium(3+) Chemical compound [Y+3].OP(O)(O)=O UXBZSSBXGPYSIL-UHFFFAOYSA-N 0.000 description 1
- UHZYTMXLRWXGPK-UHFFFAOYSA-N phosphorus pentachloride Chemical compound ClP(Cl)(Cl)(Cl)Cl UHZYTMXLRWXGPK-UHFFFAOYSA-N 0.000 description 1
- FAIAAWCVCHQXDN-UHFFFAOYSA-N phosphorus trichloride Chemical compound ClP(Cl)Cl FAIAAWCVCHQXDN-UHFFFAOYSA-N 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000011295 pitch Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 231100000719 pollutant Toxicity 0.000 description 1
- 229920000867 polyelectrolyte Polymers 0.000 description 1
- 235000013824 polyphenols Nutrition 0.000 description 1
- 229920000137 polyphosphoric acid Polymers 0.000 description 1
- 150000007519 polyprotic acids Chemical class 0.000 description 1
- USHAGKDGDHPEEY-UHFFFAOYSA-L potassium persulfate Chemical compound [K+].[K+].[O-]S(=O)(=O)OOS([O-])(=O)=O USHAGKDGDHPEEY-UHFFFAOYSA-L 0.000 description 1
- 239000013630 prepared media Substances 0.000 description 1
- 238000011085 pressure filtration Methods 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000005588 protonation Effects 0.000 description 1
- 238000005086 pumping Methods 0.000 description 1
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 1
- 229910001404 rare earth metal oxide Inorganic materials 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000005057 refrigeration Methods 0.000 description 1
- 238000005067 remediation Methods 0.000 description 1
- 238000009877 rendering Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000001223 reverse osmosis Methods 0.000 description 1
- 238000009288 screen filtration Methods 0.000 description 1
- 150000003346 selenoethers Chemical class 0.000 description 1
- HKZLPVFGJNLROG-UHFFFAOYSA-M silver monochloride Chemical compound [Cl-].[Ag+] HKZLPVFGJNLROG-UHFFFAOYSA-M 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 229910001023 sodium amalgam Inorganic materials 0.000 description 1
- 229910000033 sodium borohydride Inorganic materials 0.000 description 1
- 239000012279 sodium borohydride Substances 0.000 description 1
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 1
- BWGBIGLMQJPOEE-UHFFFAOYSA-M sodium;phosphonoformate Chemical compound [Na+].OP(O)(=O)C([O-])=O BWGBIGLMQJPOEE-UHFFFAOYSA-M 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- UUCCCPNEFXQJEL-UHFFFAOYSA-L strontium dihydroxide Chemical compound [OH-].[OH-].[Sr+2] UUCCCPNEFXQJEL-UHFFFAOYSA-L 0.000 description 1
- 229910001866 strontium hydroxide Inorganic materials 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 150000003460 sulfonic acids Chemical class 0.000 description 1
- 239000004032 superbase Substances 0.000 description 1
- 150000007525 superbases Chemical class 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000009182 swimming Effects 0.000 description 1
- 229950009390 symclosene Drugs 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- LRBQNJMCXXYXIU-NRMVVENXSA-N tannic acid Chemical compound OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@@H]2[C@H]([C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-NRMVVENXSA-N 0.000 description 1
- 229940033123 tannic acid Drugs 0.000 description 1
- 235000015523 tannic acid Nutrition 0.000 description 1
- 229920002258 tannic acid Polymers 0.000 description 1
- 229920001864 tannin Polymers 0.000 description 1
- 239000001648 tannin Substances 0.000 description 1
- 235000018553 tannin Nutrition 0.000 description 1
- 239000011269 tar Substances 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 239000004753 textile Substances 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 238000005979 thermal decomposition reaction Methods 0.000 description 1
- 229920001169 thermoplastic Polymers 0.000 description 1
- 229920001187 thermosetting polymer Polymers 0.000 description 1
- 239000004634 thermosetting polymer Substances 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- WQYSXVGEZYESBR-UHFFFAOYSA-N thiophosphoryl chloride Chemical compound ClP(Cl)(Cl)=S WQYSXVGEZYESBR-UHFFFAOYSA-N 0.000 description 1
- 229910001432 tin ion Inorganic materials 0.000 description 1
- 229910052861 titanite Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- STCOOQWBFONSKY-UHFFFAOYSA-N tributyl phosphate Chemical compound CCCCOP(=O)(OCCCC)OCCCC STCOOQWBFONSKY-UHFFFAOYSA-N 0.000 description 1
- RXJKFRMDXUJTEX-UHFFFAOYSA-N triethylphosphine Chemical compound CCP(CC)CC RXJKFRMDXUJTEX-UHFFFAOYSA-N 0.000 description 1
- FIQMHBFVRAXMOP-UHFFFAOYSA-N triphenylphosphane oxide Chemical compound C=1C=CC=CC=1P(C=1C=CC=CC=1)(=O)C1=CC=CC=C1 FIQMHBFVRAXMOP-UHFFFAOYSA-N 0.000 description 1
- 239000001226 triphosphate Substances 0.000 description 1
- 235000011178 triphosphate Nutrition 0.000 description 1
- RXPQRKFMDQNODS-UHFFFAOYSA-N tripropyl phosphate Chemical compound CCCOP(=O)(OCCC)OCCC RXPQRKFMDQNODS-UHFFFAOYSA-N 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 229910000442 triuranium octoxide Inorganic materials 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 229910001727 uranium mineral Inorganic materials 0.000 description 1
- YIIYNAOHYJJBHT-UHFFFAOYSA-N uranium;dihydrate Chemical compound O.O.[U] YIIYNAOHYJJBHT-UHFFFAOYSA-N 0.000 description 1
- 229910000189 uranophane Inorganic materials 0.000 description 1
- 238000003828 vacuum filtration Methods 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
- 229910000164 yttrium(III) phosphate Inorganic materials 0.000 description 1
- 239000010457 zeolite Substances 0.000 description 1
- 229910052845 zircon Inorganic materials 0.000 description 1
- GFQYVLUOOAAOGM-UHFFFAOYSA-N zirconium(iv) silicate Chemical compound [Zr+4].[O-][Si]([O-])([O-])[O-] GFQYVLUOOAAOGM-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
- C02F1/281—Treatment of water, waste water, or sewage by sorption using inorganic sorbents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/0203—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising compounds of metals not provided for in B01J20/04
- B01J20/0207—Compounds of Sc, Y or Lanthanides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/0203—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising compounds of metals not provided for in B01J20/04
- B01J20/0274—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising compounds of metals not provided for in B01J20/04 characterised by the type of anion
- B01J20/0281—Sulfates of compounds other than those provided for in B01J20/045
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/0203—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising compounds of metals not provided for in B01J20/04
- B01J20/0274—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising compounds of metals not provided for in B01J20/04 characterised by the type of anion
- B01J20/0288—Halides of compounds other than those provided for in B01J20/046
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/0203—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising compounds of metals not provided for in B01J20/04
- B01J20/0274—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising compounds of metals not provided for in B01J20/04 characterised by the type of anion
- B01J20/0296—Nitrates of compounds other than those provided for in B01J20/04
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/06—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising oxides or hydroxides of metals not provided for in group B01J20/04
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/30—Processes for preparing, regenerating, or reactivating
- B01J20/32—Impregnating or coating ; Solid sorbent compositions obtained from processes involving impregnating or coating
- B01J20/3231—Impregnating or coating ; Solid sorbent compositions obtained from processes involving impregnating or coating characterised by the coating or impregnating layer
- B01J20/3234—Inorganic material layers
- B01J20/3236—Inorganic material layers containing metal, other than zeolites, e.g. oxides, hydroxides, sulphides or salts
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/001—Processes for the treatment of water whereby the filtration technique is of importance
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/02—Treatment of water, waste water, or sewage by heating
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/24—Treatment of water, waste water, or sewage by flotation
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
- C02F1/283—Treatment of water, waste water, or sewage by sorption using coal, charred products, or inorganic mixtures containing them
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
- C02F1/288—Treatment of water, waste water, or sewage by sorption using composite sorbents, e.g. coated, impregnated, multi-layered
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/30—Treatment of water, waste water, or sewage by irradiation
- C02F1/302—Treatment of water, waste water, or sewage by irradiation with microwaves
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/30—Treatment of water, waste water, or sewage by irradiation
- C02F1/307—Treatment of water, waste water, or sewage by irradiation with X-rays or gamma radiation
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/30—Treatment of water, waste water, or sewage by irradiation
- C02F1/32—Treatment of water, waste water, or sewage by irradiation with ultraviolet light
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/34—Treatment of water, waste water, or sewage with mechanical oscillations
- C02F1/36—Treatment of water, waste water, or sewage with mechanical oscillations ultrasonic vibrations
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/38—Treatment of water, waste water, or sewage by centrifugal separation
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/42—Treatment of water, waste water, or sewage by ion-exchange
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/44—Treatment of water, waste water, or sewage by dialysis, osmosis or reverse osmosis
- C02F1/441—Treatment of water, waste water, or sewage by dialysis, osmosis or reverse osmosis by reverse osmosis
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/44—Treatment of water, waste water, or sewage by dialysis, osmosis or reverse osmosis
- C02F1/444—Treatment of water, waste water, or sewage by dialysis, osmosis or reverse osmosis by ultrafiltration or microfiltration
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/46—Treatment of water, waste water, or sewage by electrochemical methods
- C02F1/461—Treatment of water, waste water, or sewage by electrochemical methods by electrolysis
- C02F1/467—Treatment of water, waste water, or sewage by electrochemical methods by electrolysis by electrochemical disinfection; by electrooxydation or by electroreduction
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/48—Treatment of water, waste water, or sewage with magnetic or electric fields
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/50—Treatment of water, waste water, or sewage by addition or application of a germicide or by oligodynamic treatment
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/52—Treatment of water, waste water, or sewage by flocculation or precipitation of suspended impurities
- C02F1/5236—Treatment of water, waste water, or sewage by flocculation or precipitation of suspended impurities using inorganic agents
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/52—Treatment of water, waste water, or sewage by flocculation or precipitation of suspended impurities
- C02F1/5236—Treatment of water, waste water, or sewage by flocculation or precipitation of suspended impurities using inorganic agents
- C02F1/5245—Treatment of water, waste water, or sewage by flocculation or precipitation of suspended impurities using inorganic agents using basic salts, e.g. of aluminium and iron
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/52—Treatment of water, waste water, or sewage by flocculation or precipitation of suspended impurities
- C02F1/54—Treatment of water, waste water, or sewage by flocculation or precipitation of suspended impurities using organic material
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/66—Treatment of water, waste water, or sewage by neutralisation; pH adjustment
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/68—Treatment of water, waste water, or sewage by addition of specified substances, e.g. trace elements, for ameliorating potable water
- C02F1/683—Treatment of water, waste water, or sewage by addition of specified substances, e.g. trace elements, for ameliorating potable water by addition of complex-forming compounds
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/70—Treatment of water, waste water, or sewage by reduction
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/72—Treatment of water, waste water, or sewage by oxidation
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/72—Treatment of water, waste water, or sewage by oxidation
- C02F1/722—Oxidation by peroxides
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/72—Treatment of water, waste water, or sewage by oxidation
- C02F1/76—Treatment of water, waste water, or sewage by oxidation with halogens or compounds of halogens
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/72—Treatment of water, waste water, or sewage by oxidation
- C02F1/78—Treatment of water, waste water, or sewage by oxidation with ozone
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2101/00—Nature of the contaminant
- C02F2101/10—Inorganic compounds
- C02F2101/103—Arsenic compounds
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2101/00—Nature of the contaminant
- C02F2101/10—Inorganic compounds
- C02F2101/105—Phosphorus compounds
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2103/00—Nature of the water, waste water, sewage or sludge to be treated
- C02F2103/003—Wastewater from hospitals, laboratories and the like, heavily contaminated by pathogenic microorganisms
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2103/00—Nature of the water, waste water, sewage or sludge to be treated
- C02F2103/005—Black water originating from toilets
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2103/00—Nature of the water, waste water, sewage or sludge to be treated
- C02F2103/02—Non-contaminated water, e.g. for industrial water supply
- C02F2103/023—Water in cooling circuits
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2103/00—Nature of the water, waste water, sewage or sludge to be treated
- C02F2103/06—Contaminated groundwater or leachate
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2103/00—Nature of the water, waste water, sewage or sludge to be treated
- C02F2103/10—Nature of the water, waste water, sewage or sludge to be treated from quarries or from mining activities
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2103/00—Nature of the water, waste water, sewage or sludge to be treated
- C02F2103/34—Nature of the water, waste water, sewage or sludge to be treated from industrial activities not provided for in groups C02F2103/12 - C02F2103/32
- C02F2103/36—Nature of the water, waste water, sewage or sludge to be treated from industrial activities not provided for in groups C02F2103/12 - C02F2103/32 from the manufacture of organic compounds
- C02F2103/365—Nature of the water, waste water, sewage or sludge to be treated from industrial activities not provided for in groups C02F2103/12 - C02F2103/32 from the manufacture of organic compounds from petrochemical industry (e.g. refineries)
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2103/00—Nature of the water, waste water, sewage or sludge to be treated
- C02F2103/42—Nature of the water, waste water, sewage or sludge to be treated from bathing facilities, e.g. swimming pools
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2303/00—Specific treatment goals
- C02F2303/04—Disinfection
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2303/00—Specific treatment goals
- C02F2303/16—Regeneration of sorbents, filters
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2305/00—Use of specific compounds during water treatment
- C02F2305/08—Nanoparticles or nanotubes
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F3/00—Biological treatment of water, waste water, or sewage
- C02F3/30—Aerobic and anaerobic processes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02W—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO WASTEWATER TREATMENT OR WASTE MANAGEMENT
- Y02W10/00—Technologies for wastewater treatment
- Y02W10/30—Wastewater or sewage treatment systems using renewable energies
- Y02W10/37—Wastewater or sewage treatment systems using renewable energies using solar energy
Definitions
- the present disclosure is related generally to removal of phosphorus-containing target materials from a fluid stream using a rare earth, more particularly to removal of phosphorus-containing materials from an aqueous stream using a rare earth.
- This disclosure relates generally to methods and rare earth-containing additives for removing inorganic and organic phosphorus-containing target materials.
- a method includes the step of contacting an aqueous medium comprising a phosphorus-containing target material with a rare earth-containing additive to remove at least a portion of the phosphorus-containing target material from the aqueous medium to form a treated aqueous medium.
- the phosphorus-containing target material includes one or more of phosphoric acid, phosphorus acid, polyphosphoric acids, inorganic phosphite, organic phosphite, phosphorus oxyhalide, phosphorus trihalide, phosphorus pentahalide, cyclic or non-cyclic phosphate salt, organophosphite,
- chalcogenide diphosphene, phospho-imide, phosphaalkene, phosphaalkyne, phosphine oxide, phosphinate, phosphine, phosphinite, non-fluoridated phosphonates, o-alkyl phosphoramideocyanidate, phosphonic acid, phosphinic acid, phospone oxide,
- phosphatidylcholine P-N amino compound, and an alklylated or protonated ester and salt thereof.
- a process includes the steps of:
- the reactive phosphate is in the form of an anion
- the non-reactive phosphate in the form of an anion, cation, neutral species, and/or colloid or suspension.
- a portion of the non-reactive phosphate can be converted into reactive phosphate or vice versa.
- the method and process can include further steps in other configurations.
- the method includes the sub-steps of:
- the method and process include the sub-steps of:
- the reduced phosphprous-containing target material having one of a different structure and/or composition than the phosphorus-containing target material
- the method and process include the sub-steps of:
- the method and process include the sub-steps of:
- a composition includes:
- a phosphorus-containing target material comprising one or more of phosphoric acid, phosphorus acid, inorganic phosphite, organic phosphite, phosphorus oxyhalide, phosphorus trihalide, phosphorus pentahalide, cyclic or non-cyclic phosphate salt, organophosphite, chalcogenide, diphosphene, phospho-imide, phosphaalkene,
- phosphaalkyne phosphine oxide, phosphinate, phosphine, phosphinite, non-fluoridated phosphonates, o-alkyl phosphoramideocyanidate, phosphonic acid, phosphinic acid, phospone oxide, phosphonite, phosphonium salt, alkyl phosphonyldihalide, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, phosphorane, phosphono carboxylate, halosoman, amiton, alkyl phosphite, DNA backbone phosphate group, DNA backbone phosphite groups thiophosphate and thiophosphyoryl ester, thioester and/or amide, phosphatidylcholine, P-N amino compound, and an alklylated or protonated ester and salt thereof, and
- a target-laden rare earth composition formed by the rare earth-containing additive and the phosphorus-containing target material.
- the rare earth-containing additive comprises primarily cerium
- the target-laden rare earth composition is a complex formed by complexion of the rare earth-containing additive and target material.
- the rare earth-containing additive comprises primarily cerium
- the target-laden rare earth composition is formed by sorption of the phosphorus- containing target material by the rare earth-containing additive.
- the rare earth-containing additive comprises primarily cerium
- the target-laden rare earth composition is formed by a chemical reaction between the phosphorus-containing target material and the rare earth-containing additive.
- the rare earth-containing additive can be water soluble, partially soluble, partially insoluble, and/or insoluble.
- the phosphorus-containing target material is selected from the group consisting essentially of phosphoric acid, phosphorus acid, phosphonic acid, phosphinic acid, and alklylated or protonated esters and salts thereof.
- the phosphorus-containing target material comprises an organic and/or inorganic phosphite.
- the phosphorus-containing target material comprises one or more of a phosphorus oxyhalide, phosphorus trihalide, alkyl phosphorus oxyhalide, phosphorus pentahalide, cyclic phosphate, chalcogenide, diphosphene, phospho-imide, phosphaalkene, and phosphaalkyne.
- the phosphorus-containing target material comprises one or more of a phosphine oxide, phosphinate, phosphine, phosphinite, non-fluoridated phosphonate, o-alkyl phosphoramideocyanidate, phospone oxide, phosphonite, phosphonium salt, alkyl phosphonyldihalide, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, phosphorane, phosphono carboxylate, halosoman, amiton, and alkyl phosphite.
- a process includes the steps of:
- the non-reactive phosphate is in the form of an anion, cation, neutral species, and/or colloid or suspension.
- a portion of the anqueous media may contain reactive phosphates, the reacative phosphates being converted to non-reactive phosphates prior to the contacting step.
- the reactive phosphate is in the form of an anion and may be in the form of colloid or suspension.
- a process includes the steps of:
- aqueous medium may contain non-reactive phosphates, the non-reacative phosphates being converted to reactive phosphates prior to the contacting step.
- the non-reactive phosphate is in the form of an anion, cation, neutral species, and/or colloid or suspension.
- the phosphorus-containing target material comprises one or more of a DNA backbone phosphate group, DNA backbone phosphite group,
- the rare earth- containing composition can remove effectively a large number of phosphorus-containing target materials, whether in the form of dissolved or undissolved species.
- the rare earth-containing composition can remove organophosphates and organophosphites.
- the pH and/or Eh can be adjusted to produce a selected primary target material species, which is removed more effectively by the rare earth composition than other target material species. High levels of removal of the primary target material can be therefore realized.
- the term “a” or “an” entity refers to one or more of that entity. As such, the terms “a” (or “an”), “one or more” and “at least one” can be used
- ABS refers to the penetration of one substance into the inner structure of another substance, as distinguished from adsorption.
- Adsorption refers to the adherence of atoms, ions, molecules, polyatomic ions, or other substances to the surface of another substance, called the adsorbent.
- the attractive force for adsorption can be in the form of a bond and/or force, such as covalent bonds, metallic bonds, coordination bonds, ionic bonds, hydrogen bonds, electrostatic forces (e.g., van der Waal's forces and London's forces), and the like.
- At least one means A alone, B alone, C alone, A and B together, A and C together, B and C together, or A, B and C together.
- water refers to any aqueous stream.
- the water may originate from any aqueous stream may be derived from any natural and/or industrial source.
- Non-limiting examples of such aqueous streams and/or waters are drinking waters, potable waters, recreational waters, waters derived from manufacturing processes, wastewaters, pool waters, spa waters, cooling waters, boiler waters, process waters, municipal waters, sewage waters, agricultural waters, ground waters, power plant waters, remediation waters, co-mingled water and combinations thereof.
- amalgamate and “aggregate” refer to a composition formed by gathering one or more materials into a mass.
- amino acid are organic materials containing an amine group, a carboxylic acid group, and a side-chain that is specific to each amino acid.
- the key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen.
- a "binder” generally refers to one or more substances that bind together a material being agglomerated. Binders are typically solids, semi-solids, or liquids. Non-limiting examples of binders are polymeric materials, tar, pitch, asphalt, wax, cement water, solutions, dispersions, powders, silicates, gels, oils, alcohols, clays, starch, silicates, acids, molasses, lime, lignosulphonate oils, hydrocarbons, glycerin, stearate, or combinations thereof. The binder may or may not chemically react with the material being
- Non-liming examples of chemical reactions include hydration/dehydration, metal ion reactions, precipitation/gelation reactions, and surface charge modification.
- coagulation refers to the destabilization of colloids by neutralizing the forces that keep colloidal materials suspended. Cationic coagulants provide positive electrical charge to reduce the negative charge (zeta potential) of the colloids. The colloids thereby form larger particles (known as floes).
- composition generally refers to one or more chemical units composed of one or more atoms, such as a molecule, polyatomic ion, chemical compound, coordination complex, coordination compound, and the like.
- bonds and/or forces such as covalent bonds, metallic bonds, coordination bonds, ionic bonds, hydrogen bonds, electrostatic forces (e.g., van der Waal's forces and London's forces), and the like.
- “Chemical species” or “species” are atoms, elements, molecules, molecular fragments, ions, compounds, and other chemical structures. “Chemical transformation” refers to process where at least some of a material has had its chemical composition transformed by a chemical reaction.
- a “chemical transformation” differs from “a physical transformation”.
- a physical transformation refers to a process where the chemical composition has not been chemically transformed but a physical property, such as size or shape, has been transformed.
- the term "contained within the water” generally refers to materials suspended and/or dissolved within the water.
- Water is typically a solvent for dissolved materials and water-soluble material.
- water is typically not a solvent for water-insoluble materials.
- Suspended materials are substantially insoluble in water and dissolved materials are substantially soluble in water.
- the suspended materials have a particle size.
- De-toxify or “de-toxification” includes rendering a phosphorus-containing target material non-toxic or non-harmful to a living organism, such as, for example, a human or other animal.
- the phosphorus-containing target material may be rendered non-toxic by converting the phosphorus-containing target material into a non-toxic or non-harmful form and/or species.
- digest or “digestion” refers to the use of microorganisms, particularly bacteria, to digest target materials. This is commonly established by mixing forcefully contaminated water with bacteria and molecularly oxygen.
- diphosphene generally refers to a phosphorus-containing compound having the general structure R2P2.
- infectious refers to the use of an antimicrobial agent to kill or inhibit the growth of microorganisms, such as bacteria, fungi, protozoans, and viruses.
- antimicrobial agents include, oxidants, reductants, alchohols, aldehydes, halogens, phenolics, quaternary ammonium compounds, silver, copper, ultraviolet light, and other materials.
- flocculation refers to a process using a flocculant, which is typically a polymer, to form a bridge between floes and bind the particles into large agglomerates or clumps. Bridging occurs when segments of the polymer chain adsorb on different particles and help particles aggregate.
- fluid refers to a liquid, gas or both.
- halogen is a nonmetal element from Group 17 IUPAC Style (formerly: VII,
- the artificially created element 117 may also be a halogen.
- a "halide compound” is a compound having as one part of the compound at least one halogen atom and the other part the compound is an element or radical that is less electronegative (or more electropositive) than the halogen.
- the halide compound is typically a fluoride, chloride, bromide, iodide, or astatide compound.
- Many salts are halides having a halide anion.
- a halide anion is a halogen atom bearing a negative charge.
- the halide anions are fluoride (F ), chloride (CI ), bromide (Br ), iodide (T) and astatide (At ).
- phospho-imide generally refers to a phosphorus-containing compound having the general formula R 3 PNR' .
- organic material generally refers to a chemical compound or other species that is not organic.
- insoluble refers to materials that are intended to be and/or remain as solids in water. Insoluble materials are able to be retained in a device, such as a column, or be readily recovered from a batch reaction using physical means, such as filtration. Insoluble materials should be capable of prolonged exposure to water, over weeks or months, with little loss of mass. Typically, a little loss of mass refers to less than about 5% mass loss of the insoluble material after a prolonged exposure to water.
- An “ion” generally refers to an atom or group of atoms having a charge.
- the charge on the ion may be negative or positive.
- Organic carbons or “organic material” generally refer to any compound of carbon except such binary compounds as carbon oxides, the carbides, carbon disulfide, etc.; such ternary compounds as the metallic cyanides, metallic carbonyls, phosgene, carbonyl sulfide, etc.; and the metallic carbonates, such as alkali and alkaline earth metal carbonates.
- Exemplary organic carbons include humic acid, tannins, and tannic acid, polymeric materials, alcohols, carbonyls, carboxylic acids, oxalates, amino acids, hydrocarbons, and mixtures thereof.
- the phosphorus-containing target material is an organic material as defined herein.
- An alcohol is any organic compound in which a hydroxyl functional group (-OH) is bound to a carbon atom, the carbon atom is usually connected to other carbon or hydrogen atoms.
- examples of alcohols include acyclic alcohols, isopropyl alcohol, ethanol, methanol, pentanol, polyhydric alcohols, unsaturated aliphatic alcohols, and alicyclic alcohols, and the like.
- R' vary independently and may be H or any organic radical, R and R' may be the same or differ).
- organic compounds containing a carbonyl group include aldehydes, ketones, esters, amides, enones, acyl halides, acid anhydrides, urea, and carbamates and derivatives thereof, and the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates.
- organic compounds containing a carboxyl group include carboxylic acid (R-COOH) and salts and esters (or carboxylates) and other derivatives thereof. It can be appreciated that organic compounds include alcohols, carbonyls, and carboxylic acids, where one or more oxygens are, respectively, replaced with sulfur, selenium and/or tellurium.
- organophorous generally refers to a phosphorus-containing compound containing one or more carbon-phosphorus bonds.
- organophosphorus compounds include: phosphate and thiophosphyoryl esters, thioesters and amides (such as, mono-, di- and tri- phosphate esters, thioesters and amides
- X can be one of oxygen, sulfur or nitrogen
- Y can be one of oxygen, sulfur, selenium or tellurium
- R can be a Ci-Ci 2 alkyl or aryl group
- trimeyl phosphate triethyl phosphate, tripropyl phosphate, tributyl phosphate, diazinon, phosphatidylcholine, malathion, cyclophosphamide, triphyenylphosphate and dithiophosphate
- phosphinites such as, P(OR)R' 2
- phosphines such as, PR R R , where R , R and R can be one of H, C -Cn alkyl, C -Cn aryl, C 3 -Cg cyclic, amino, ether, thio, and hydroxyl and where R 1 , R 2 and R 3 can be the same or differ
- organophosphate generally refers to an ester of phosphoric acid.
- organophosphite generally refers to an ester of phosphorus acid.
- oxidizing agent refers to one or both of a chemical substance and physical process that transfers and/or assists in removal of one or more electrons from a substance.
- the substance having the one or more electrons being removed is oxidized.
- the physical process may removal and/or may assist in the removal of one or more electrons from the substance being oxidized.
- the substance to be oxidized can be oxidized by electromagnetic energy when the interaction of the electromagnetic energy with the substance be oxidized is sufficient to substantially remove one or more electrons from the substance.
- the interaction of the electromagnetic energy with the substance being oxidized may not be sufficient to remove one or more electrons, but may be enough to excite electrons to higher energy state, were the electron in the excited state can be more easily removed by one or more of a chemical substance, thermal energy, or such.
- phosphaalkynes generally refers to a phosphorus-containing compound having the general structure RC ⁇ P.
- phosphate compound generally refers to phosphorus-containing compounds formed from a P0 4 3 ⁇ (phosphate) or a related anion or group, such as
- OPO(OH) 2 structural unit alone or linked together by sharing oxygen atoms to form a linear chain or cyclic ring structure.
- Common phosphates are salts or esters of phosphoric acid or a tertiary salt of orthophosphoric acid.
- Non- limiting examples of phosphates are:
- P0 4 3" (phosphate); P 3 0io 5 ⁇ (triphosphate); P n 0 3n (n+2)" (polyphosphate); P 3 0c> 3 ⁇ (cyclic trimethaphosphate); adenosine diphosphoric acid (ADPH); guanosine 5 '-diphosphate 3'- dipphosphate (ppGpp); trimetaphosphate; hexametaphosphate; HP0 3 2" (phosphate); or one or more of their salts, acids, esters, anionic and organophosphor o us forms; and mixtures thereof.
- Other examples include DNA backbone phosphate groups.
- An exemplary phosphine oxide includes for triphenylphosphine oxide.
- exemplary phosphinates include glufosinate.
- phosphine generally refers to a phosphorus-containing compound including PH 3 as a structure unit, where a hydrogen can be replaced by a carbon radical.
- exemplary phosphines include trimethylphosphine and diphosphines PR 1 R 2 R 3 , where R 1 , R 2 and R 3 can be one of H, C1-C12 alkyl, C1-C12 aryl, C 3 -C 8 cyclic, amino, ether, thio, and hydroxyl and where R 1 , R 2 and R 3 can be the same or differ).
- phosphinite generally refers to a phosphorus-containing compound having the general formula P(OR)R' 2 or R 2 (RO)P.
- phosphite compound generally refers to a phosphorus-containing compound formed from a P0 2 3" (phosphite) structural unit alone or linked together by sharing oxygen atoms to form a linear chain or cyclic ring structure.
- Common phosphites are salts or esters of phosphorus acid and include H 2 P 2 0 5 2" (pyrophosphites such as Phenolphthalein bisphosphate pyridine salt) and H 2 P0 2 " (hypophosphite or phosphinate).
- Phosphite esters have the general structure P(OR) 3 with oxidation state +3.
- Phosphite” compounds include not only phosphites but also phosphonites and phosphinites.
- phosponates include glyphospate, biphosponate, Sarin, dimethylmethylphosponate, and aminophophonate.
- An example of a phosphonic acid ester is methylethyl phosphonate.
- phosphonite generally refers to a phosphorus-containing compound having the general formula (P(OR) 2 R) or R(RO) 2 P.
- phosphonium salt generally refers to a phosphorus-containing compound having the general formula [ ⁇ ] ⁇ ⁇ with formal oxidation state V.
- X can be any anion. Non-limiting examples of X " are chloride, bromide, iodide, perchlorate, acetate, tetrfluoroborate, sulfate, nitrate, nitrite, to name a few.
- Exemplary phosponium salts include is tetrakis(hydroxymethyl)phosphonium chloride, [P(CH 2 0H) 4 ]C1.
- phosphorane generally refers to a phosphorus-containing compound having the general formula R 3 PR 2 .
- polish refers to any process, such as filtration, to remove small (usually microscopic) particulate material or very small low concentrations of dissolved target material from water.
- pore volume and "pore size”, respectively, refer to pore volume and pore size determinations made by any suite measure method.
- the pore size and pore volume are determined by any suitable Barret- Joyner-Halenda method for determining pore size and volume.
- pore size and pore diameter can used interchangeably.
- Precipitation generally refers to the removal of a dissolved target material in the form of a target-laden rare earth composition, preferably an insoluble target-laden rare earth composition.
- the target-laden rare earth composition can comprise a target-laden cerium composition, a target-laden cerium (IV) composition, a target-laden cerium ( M l ) composition, a target-laden lanthanium composition comprising a rare earth, a target-laden composition comprising a rare earth other than cerium or lanthium, or a combination thereof.
- the target-laden rare earth composition comprises a target-laden rare earth composition.
- precipitation includes processes, such as adsorption and absorption of the phosphorus-containing target material by one or more of the cerium (IV) composition, the rare earth-containing additive, or a rare earth other than cerium (IV).
- the target-laden rare earth composition can comprise a +3 rare earth, such as cerium (III), lanthanum (III) or other lanthanoid having a +3 oxidation state.
- R and R' in a chemical formula, generally refer to a carbon- containing radical.
- a “radical” generally refers to an atom or group of atoms that are joined together in some particular spatial structure and that commonly take part in chemical reactions as a single unit.
- a radical may have a net positive or negative charge or be neutral.
- a radical is an atom, molecule, or ion that has one or more unpaired electrons.
- Rare earth refers to one or more of yttrium, scandium, lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium erbium, thulium, ytterbium, and lutetium.
- lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium erbium, thulium, ytterbium, and lutetium are known as lanthanoids.
- rare earth refers to singular and plural forms of the terms.
- IR refers to a single rare earth and/or combination and/or mixture of rare earths and the term "rare earth-containing
- composition refers to a single composition comprising a single rare earth and/or a mixture of differing rare earth-containing compositions containing one or more rare earths and/or a single composition containing one or more rare earths.
- the terms "rare earth- containing additive” and “rare earth-containing particle” are additives or particles including a single composition comprising a single rare earth and/or a mixture of differing rare earth-containing compositions containing one or more rare earths and/or a single composition containing one or more rare earths.
- processed rare earth composition refers not only to any composition containing a rare earth other than non- compositionally altered rare earth-containing minerals.
- processed rare earth-containing composition excludes comminuted naturally occurring rare earth-containing minerals.
- processed rare earth-containing composition includes a rare earth-containing mineral where one or both of the chemical composition and chemical structure of the rare earth-containing portion of the mineral has been compositionally altered. More specifically, a comminuted naturally occurring bastnasite would not be considered a processed rare earth-containing composition and/or processed rare earth-containing additive. However, a synthetically prepared bastnasite or a rare earth-containing composition prepared by a chemical transformation of naturally occurring bastnasite would be considered a processed rare earth-containing composition and/or processed rare earth-containing additive.
- the processed rare earth and/or rare- containing composition and/or additive are, in one application, not a naturally occurring mineral but synthetically manufactured.
- Exemplary naturally occurring rare earth- containing minerals include bastnasite (a carbonate-fluoride mineral) and monazite.
- rare earth-containing minerals include aeschynite, allanite, apatite, britholite, brockite, cerite, fluorcerite, fluorite, gadolinite, parisite, stillwellite, synchisite, titanite, xenotime, zircon, and zirconolite.
- exemplary uranium minerals include uraninite (U0 2 ), pitchblende (a mixed oxide, usually UsOg), brannerite (a complex oxide of uranium, rare-earths, iron and titanium), coffinite (uranium silicate), carnotite, autunite, davidite, gummite, torbernite and uranophane.
- the rare earth- containing composition is substantially free of one or more elements in Group 1, 2, 4-15, or 17 of the Periodic Table, a radioactive species, such as uranium, sulfur, selenium, tellurium, and polonium.
- reducing agent refers to an element or compound that donates one or more electrons to another species or agent this is reduced.
- the reducing agent is oxidized and the other species, which accepts the one or more electrons, is reduced.
- removing includes the sorption, precipitation, conversion, detoxification, deactivation, and/or combination thereof of a target material contained in a water and/or water handling system.
- soluble refers to a material that readily dissolves in a fluid, such as water or other solvent.
- a fluid such as water or other solvent.
- dissolution of a soluble material would necessarily occur on a time scale of minutes rather than days.
- the material to be considered to be soluble it is necessary that the
- material/composition has a significant solubility in the fluid such that upwards of about 5 g of the material will dissolve in about one liter of the fluid and be stable in the fluid.
- sorb refers to adsorption, absorption or both adsorption and absorption.
- suspension refers to a heterogeneous mixture of a solid, typically in the form of particulates dispersed in a liquid. In a suspension, the solid particulates are in the form of a discontinuous phase dispersed in a continuous liquid phase.
- colloid refers to a suspension comprising solid particulates that typically do not settle-out from the continuous liquid phase due to gravitational forces.
- a "colloid” typically generally refers to a system having finely divided particles ranging from about 10 to 10,000 angstroms in size, dispersed within a continuous medium.
- the terms "suspension”, “colloid” or “slurry” will be used interchangeably to refer to one or more materials dispersed and/or suspended in a continuous liquid phase.
- the terms “particle”, “particles”, “particulate”, and “particulates” will be used interchangeably and the use of one over the other does not connotate a chemical and/or physical difference.
- surface area refers to surface area of a material and/or substance determined by any suitable surface area measurement method.
- the surface area is determined by any suitable Brunauer-Emmett-Teller (BET) analysis technique for determining the specific area of a material and/or substance.
- BET Brunauer-Emmett-Teller
- the state of protonation is usually not specified. They could be bound to as many as three protons for the neutral H 3 PS 4 - x O x species. Two protons correspond to the related monoanions, and one proton for the dianions.
- water handling system generally refers to any system containing, conveying, manipulating, physically transforming, chemically processing, mechanically processing, purifying, generating and/or forming the aqueous composition, treating, mixing and/or co-mingling the aqueous composition with one or more other waters and any combination thereof.
- a “water handling system component” generally refers to one or more unit operations and/or pieces of equipment that process and/or treat water (such as a holding tank, reactor, purifier, treatment vessel or unit, mixing vessel or element, wash circuit, precipitation vessel, separation vessel or unit, settling tank or vessel, reservoir, pump, aerator, cooling tower, heat exchanger, valve, boiler, filtration device, solid liquid and/or gas liquid separator, nozzle, tender, and such), conduits interconnecting the unit operations and/or equipment (such as piping, hoses, channels, aqua-ducts, ditches, and such) and the water conveyed by the conduits.
- the water handling system components and conduits are in fluid communication.
- water and "water handling system” will be used interchangeably. That is, the term “water” may used to refer to “a water handling system” and the term “water handling system” may be used to refer to the term “water”.
- Fig. 1 depicts a water handling system and method according to an embodiment
- Figs. 2A-2E are prior art Pourbaix or Eh-pH diagrams for phosphoric acid constructed using differing thermodynamic databases, the thermodynamic database constitutes are denoted in the respective Figs; and
- Fig. 3 is a plot of arsenic capacity (mg As/g Ce0 2 ) against various solution compositions.
- the present disclosure is directed to removal from and/or detoxitication of water, a water-handling system or other aqueous media, of a phosphorus- containing target material.
- the phosphorus-containing target material may be in the form of a pollutant or contaminant.
- the phosphorus-containing target material is removed and/or detoxified by a rare earth-containing composition, additive or particle.
- the rare earth-containing composition, additive or particle is a processed rare earth- containing additive or particle.
- the phosphorus-containing target material is removed and/or detoxified by forming a target-laden rare earth composition.
- the target-laden rare earth composition comprises a rare earth and one or more of the phosphorus-containing target material or a portion of the phosphorus-containing.
- the phosphorus-containing target material is commonly one or more of phosphoric acid, phosphorus acid, phosphonic acid, or a salt or ester or mixture thereof.
- the rare earth-containing composition, additive and/or particles may be water- soluble, water-insoluble, a combination of water-soluble and/or water-insoluble rare earth- containing compositions, additives and/or particles, a partially water-soluble rare earth- containing composition, additive and/or particles, and/or a partially water-insoluble rare earth-containing composition, additive and/or particles.
- the rare earth-containing composition, additive and/or particles comprise cerium, in the form of a cerium-containing compound and/or a dissociated ionic form of cerium, lanthanum, in the form of a lanthanum-containing compound and/or dissociated ionic form of lanthanum, or a mixture thereof.
- More common rare earth- containing compositions, additives and particles are cerium (IV) oxides, cerium (III) oxides, cerium (IV) salts, cerium (III) salts, lanthanum (III) oxides, lanthanum (III) salts, or mixtures and/or combinations thereof.
- the rare earth-containing composition, additive and/or particles may contain one or more rare earths, and be in any suitable form, such as a free-flowing powder, a liquid formulation, or other form.
- Examples of rare earth-containing compositions, additives, and particles include cerium (III) oxides, cerium (IV) oxides, eerie (IV) salts (such as eerie chloride, eerie bromide, eerie iodide, eerie sulfate, eerie nitrate, eerie chlorate, and eerie oxalate), cerium (III) salts (such as cerous chloride, cerous bromide, cerous iodide, cerous sulfate, cerous nitrate, cerous chlorate, and cerous oxalate), lanthanum (III) oxides, lanthanum (III) salts (such as lanthanum chloride, lanthanum bromide, lanthanum iodide, lanthan
- the rare earth and/or rare earth-containing composition in the rare earth-containing additive can be rare earths in elemental, ionic or compounded forms.
- the rare earth and/or rare earth-containing composition can be contained in a fluid, such as water, or in the form of nanoparticles, particles larger than nanoparticles, agglomerates, or aggregates or combinations and/or mixtures thereof.
- the rare earth and/or rare earth-containing composition can be supported or unsupported.
- the rare earth and/or rare earth-containing composition can comprise one or more rare earths.
- the rare earths may be of the same or different valence and/or oxidation states and/or numbers.
- the rare earths can be a mixture of different rare earths, such as two or more of yttrium, scandium, cerium, lanthanum, praseodymium, and neodymium.
- the rare earth and/or rare earth-containing composition is, in one application, a processed rare earth-containing composition and does not include, or is substantially free of, a naturally occurring and/or derived mineral.
- the rare earth and/or rare earth-containing composition is substantially free of one or more elements in Group 1 , 2, 4-15, or 17 of the Periodic Table, and is substantially free of a radioactive species, such as uranium, sulfur, selenium, tellurium, and polonium.
- the rare earth-containing composition comprises one or more rare earths. While not wanting to be limited by example, the rare earth-containing composition can comprise a first rare earth and a second rare earth. The first and second rare earths may have the same or differing atomic numbers.
- the first rare earth comprises cerium (III) and the second rare earth comprises a rare earth other than cerium (III).
- the rare earth other than cerium (III) can be one or more trivalent rare earths, cerium (IV), or any other rare other than trivalent cerium.
- a mixture of rare earth-containing compositions can comprise a first rare earth having a +3 oxidation state and a second rare earth having a +4 oxidation state.
- the first and second rare earths are the same and comprise cerium. More specifically, the first rare earth comprises cerium (III) and the second rare earth comprises cerium (IV).
- the cerium is primarily in the form of a water-soluble cerium (III) salt, with the remaining cerium being present as cerium oxide, a substantially water insoluble cerium composition.
- the cerium is primarily in the form of a dissociated cerium (III) salt, with the remaining cerium being present as cerium (IV) oxide.
- cerium (IV) oxide For rare earth- containing compositions having a mixture of +3 and +4 oxidations states commonly at least some of the rare earth has a +3 oxidation sate, more commonly at least most of the rare earth has a +3 oxidation state, more commonly at least about 75 wt% of the rare earth has a +3 oxidation state, even more commonly at least about 90 wt% of the rare earth has a +3 oxidation state, and yet even more commonly at least about 98 wt% of the rare earth has a +3 oxidation state.
- the rare earth-containing composition commonly includes at least about 1 ppm, more commonly at least about 10 ppm, and even more commonly at least about 100 ppm cerium (IV) oxide. While in some embodiments, the rare earth- containing composition includes at least about 0.0001 wt% cerium (IV), preferably at least about 0.001 wt% cerium (IV) and even more preferably at least about 0.01 wt% cerium (IV) calculated as cerium oxide. Moreover, in some embodiments, the rare earth composition-containing commonly has at least about 20,000 ppm cerium (III), more commonly at least about 100,000 ppm cerium (III) and even more commonly at least about 250,000 ppm cerium (III).
- the molar ratio of cerium (III) to cerium (IV) is about 1 to about 1X10 "6 , more commonly is about 1 to about 1X10 "5 , even more commonly is about 1 to about 1X10 ⁇ 4 , yet even more commonly is about 1 to about 1X10 ⁇ 3 , still yet even more commonly is about 1 to about 1X10 ⁇ 2 , still yet even more commonly is about 1 to about 1X10 "1 , or still yet even more commonly is about 1 to about 1.
- the molar ratio of cerium (IV) to cerium (III) is about 1 to about 1X10 ⁇ 6 , more commonly is about 1 to about 1X10 ⁇ 5 , even more commonly is about 1 to about 1X10 "4 , yet even more commonly is about 1 to about 1X10 ⁇ 3 , still yet even more commonly is about 1 to about 1X10 ⁇ 2 , still yet even more commonly is about 1 to about 1X10 "1 , or still yet even more commonly is about 1 to about 1. Further, these molar ratios apply for any combinations of soluble and insoluble forms of Ce (III) and soluble and insoluble forms of Ce (IV).
- the cerium is primarily in the form of a dissociated cerium (III) salt, with the remaining cerium being present as cerium (IV) oxide.
- cerium (IV) oxide For rare earth- containing compositions having a mixture of +3 and +4 oxidations states commonly at least some of the rare earth has a +3 oxidation sate, more commonly at least most of the rare earth has a +3 oxidation state, more commonly at least about 75 wt% of the rare earth has a +3 oxidation state, even more commonly at least about 90 wt% of the rare earth has a +3 oxidation state, and yet even more commonly at least about 98 wt% of the rare earth has a +3 oxidation state.
- the rare earth-containing composition commonly includes at least about 1 ppm, more commonly at least about 10 ppm, and even more commonly at least about 100 ppm cerium (IV) oxide. While in some embodiments, the rare earth- containing composition includes at least about 0.0001 wt% cerium (IV), preferably at least about 0.001 wt% cerium (IV) and even more preferably at least about 0.01 wt% cerium (IV) calculated as cerium oxide. Moreover, in some embodiments, the rare earth composition-containing commonly has at least about 20,000 ppm cerium (III), more commonly at least about 100,000 ppm cerium (III) and even more commonly at least about 250,000 ppm cerium (III).
- the molar ratio of cerium (III) to cerium (IV) is about 1 to about 1X10 "6 , more commonly is about 1 to about 1X10 "5 , even more commonly is about 1 to about 1X10 "4 , yet even more commonly is about 1 to about 1X10 "3 , still yet even more commonly is about 1 to about 1X10 ⁇ 2 , still yet even more commonly is about 1 to about
- 1X10 "1 or still yet even more commonly is about 1 to about 1.
- the molar ratio of cerium (IV) to cerium (III) is aboutl to about 1X10 "6 , more commonly is about 1 to about 1X10 "5 , even more commonly is about 1 to about
- 1X10 "4 yet even more commonly is about 1 to about 1X10 "3 , still yet even more commonly is about 1 to about 1X10 ⁇ 2 , still yet even more commonly is about 1 to about 1X10 "1 , or still yet even more commonly is about 1 to about 1. Further, these molar ratios apply for any combinations of soluble and insoluble forms of Ce(III) and soluble and insoluble forms of Ce(IV).
- cerium (IV) compositions are cerium (IV) dioxide, cerium (IV) oxide, cerium (IV) oxyhydroxide, cerium (IV) hydroxide, and hydrous cerium (IV) oxide.
- dissociated cerium (III) provides for the opportunity to take advantage of cerium (III) solution sorption and/or precipitation chemistries, such as, but not limited to, the formation of insoluble cerium (III) oxyanion compositions.
- cerium (IV) composition presents, provides for the opportunity to take advantage of sorption and oxidation/reduction chemistries of cerium (IV), such as, the strong interaction of cerium (IV) with compositions such as phosphorus-containing target materials having multiple oxidation states.
- cerium (IV) can oxidize phosphorus-containing target materials having a phosphorus oxidation state of (II) or (III) to phosphorus-containing target materials having a phosphorus oxidation state of (V).
- cerium (IV) is also referred to as cerium (+4) and/or eerie.
- the rare earth composition comprises a water-soluble rare earth composition having a +3 oxidation state.
- suitable water-soluble rare earth compositions include rare earth chlorides, rare earth bromides, rare earth iodides, rare earth astatides, rare earth nitrates, rare earth sulfates, rare earth oxalates, rare earth perchlorates, rare earth carbonates, and mixtures thereof.
- the rare earth-containing additive includes water-soluble cerium (III) and lanthanum (III) compositions.
- the water-soluble cerium composition comprises cerium (III) chloride, CeCl 3 . Commonly, cerium (III) is also referred to as cerium (+3) and/or cerous.
- the rare earth composition comprises a water-soluble cerium +3 composition.
- suitable water-soluble cerium +3 compositions are cerium (III) chloride, cerium (III) nitrate, cerium (III) sulfate, cerium (III) oxalate, and a mixture thereof.
- the water-soluble cerium (III) composition may comprise, in addition to cerium, one or more other water-soluble rare earths.
- the rare earths other than cerium include yttrium, scandium, lanthanum, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium.
- the other rare earths may and may not be water-soluble.
- the water-soluble cerium-containing additive contains water- soluble cerium (III) and one or more other water-soluble trivalent rare earths (such as, but not limited to, one or more of lanthanum, neodymium, praseodymium and samarium).
- the molar ratio of cerium (III) to the other trivalent rare earths is commonly at least about 1 : 1, more commonly at least about 10: 1, more commonly at least about 15: 1, more commonly at least about 20: 1, more commonly at least about 25: 1, more commonly at least about 30: 1, more commonly at least about 35: 1, more commonly at least about 40: 1, more commonly at least about 45 : 1 , and more commonly at least about 50: 1.
- the water-soluble cerium-containing additive contains cerium (III) and one or more of water-soluble lanthanum, neodymium, praseodymium and samarium.
- the water-soluble rare earth-containing additive commonly includes at least about 0.01 wt% of one or more of lanthanum, neodymium, praseodymium and samarium.
- the water-soluble rare earth-containing additive commonly has on a dry basis no more than about 10 wt% La, more commonly no more than about 9 wt% La, even more commonly no more than about 8 wt% La, even more commonly no more than about 7 wt% La, even more commonly no more than about 6 wt% La, even more commonly no more than about 5 wt% La, even more commonly no more than about 4 wt% La, even more commonly no more than about 3 wt% La, even more commonly no more than about 2 wt% La, even more commonly no more than about 1 wt% La, even more commonly no more than about 0.5 wt% La, and even more commonly no more than about 0.1 wt% La.
- the water-soluble rare earth-containing additive commonly has on a dry basis no more than about 8 wt% Nd, more commonly no more than about 7 wt% Nd, even more commonly no more than about 6 wt% Nd, even more commonly no more than about 5 wt% Nd, even more commonly no more than about 4 wt% Nd, even more commonly no more than about 3 wt% Nd, even more commonly no more than about 2 wt% Nd, even more commonly no more than about 1 wt% Nd, even more commonly no more than about 0.5 wt% Nd, and even more commonly no more than about 0.1 wt% Nd.
- the water- soluble rare earth-containing additive commonly has on a dry basis no more than about 5 wt% Pr, more commonly no more than about 4 wt% Pr, even more commonly no more than about 3 wt% Pr, even more commonly no more than about 2.5 wt% Pr, even more commonly no more than about 2.0 wt% Pr, even more commonly no more than about 1.5 wt% Pr, even more commonly no more than about 1.0 wt% Pr, even more commonly no more than about 0.5 wt% Pr, even more commonly no more than about 0.4 wt% Pr, even more commonly no more than about 0.3 wt% Pr, even more commonly no more than about 0.2 wt% Pr, and even more commonly no more than about 0.1 wt% Pr.
- the water- soluble rare earth-containing additive commonly has on a dr basis no more than about 3 wt% Sm, more commonly no more than about 2.5 wt% Sm, even more commonly no more than about 2.0 wt% Sm, even more commonly no more than about 1.5 wt% Sm, even more commonly no more than about 1.0 wt% Sm, even more commonly no more than about 0.5 wt% Sm, even more commonly no more than about 0.4 wt% Sm, even more commonly no more than about 0.3 wt% Sm, even more commonly no more than about 0.2 wt% Sm, even more commonly no more than about 0.1 wt% Sm, even more commonly no more than about 0.05 wt% Sm, and even more commonly no more than about 0.01 wt% Sm.
- the water-soluble cerium-containing additive contains water- soluble cerium (III) and one or more other water-soluble trivalent rare earths (such as one or more of lanthanum, neodymium, praseodymium and samarium).
- the molar ratio of cerium (III) to the other trivalent rare earths is commonly at least about 1 : 1, more commonly at least about 10: 1, more commonly at least about 15: 1, more commonly at least about 20: 1, more commonly at least about 25: 1, more commonly at least about 30: 1, more commonly at least about 35: 1, more commonly at least about 40: 1, more commonly at least about 45 : 1 , and more commonly at least about 50: 1.
- the rare earth-containing additive consists essentially of a water-soluble cerium (III) salt, such as a cerium (III) chloride, cerium (III) bromide, cerium (III) iodide, cerium (III) astatide, cerium perhalogenates, cerium (III) carbonate, cerium (III) nitrate, cerium (III) sulfate, cerium (III) oxalate and mixtures thereof.
- a water-soluble cerium (III) salt such as a cerium (III) chloride, cerium (III) bromide, cerium (III) iodide, cerium (III) astatide, cerium perhalogenates, cerium (III) carbonate, cerium (III) nitrate, cerium (III) sulfate, cerium (III) oxalate and mixtures thereof.
- the rare earth in this formulation commonly is primarily cerium (III), more commonly at least about 75 mole% of the rare earth content of the rare earth-containing additive is cerium (III), that is no more than about 25 mole% of the rare earth content of the rare earth- containing additive comprises rare earths other than cerium (III). Even more commonly, the rare earth in this formulation commonly is primarily at least about 80 mole% cerium (III), yet even more commonly at least about 85 mole% cerium (III), still yet even more commonly at least about 90 mole% cerium (III), and yet still even more commonly at least about 95 mole% cerium (III).
- the rare earth composition may comprise a water-insoluble composition, such as a water-insoluble rare earth oxide, oxyhydroxide, and/or hydrous oxide.
- the insoluble rare earth composition may be in the form of a dispersion, suspension or slurry of rare earth particulates.
- the rare earth particulates can have an average particle size ranging from the sub-micron, to micron or greater than micron.
- the insoluble rare earth composition may have a surface area of at least about 1 m 2 /g. Commonly, the insoluble rare earth has a surface area of at least about 70 m 2 /g. In another formulation, the insoluble rare earth composition may have a surface area from about 25 m 2 /g to about 500 m 2 /g.
- the rare earth composition may be agglomerated.
- the rare earth composition may be in the form of an agglomerate, the agglomerate comprising a polymeric binder and the rare earth composition.
- the rare earth-containing additive comprises a rare earth and/or rare earth-containing composition comprising at least some water-insoluble cerium (IV) and water-soluble cerium (III) and/or lanthanum (III).
- the rare earth and/or rare earth-containing composition comprise at least some water-soluble cerium (III), typically in the form of water-soluble cerium (III) salt.
- the rare earth-containing additive comprises more than about 1 wt% of a water-soluble cerium (III) composition, more commonly more than about 5 wt% of a water-soluble cerium (III) composition, even more commonly more than about 10 wt% of a water-soluble cerium (III) composition, yet even more commonly more than about 20 wt% of a water-soluble cerium (III) composition, still yet even more commonly more than about 30 wt% of a water-soluble cerium (III) composition, or still yet even more commonly more than about 40 wt% of a water-soluble cerium (III) composition.
- the rare earth-containing additive typically comprises more than about 50 wt% of a water-soluble cerium (III) composition, more typically the rare earth-containing additive comprises more than about 60 wt% of a water- soluble cerium (III) composition, even more typically the rare earth-containing additive comprises more than about 65 wt% of a water-soluble cerium (III) composition, yet even more typically the rare earth-containing additive comprises more than about 70 wt% of a water-soluble cerium (III) composition, still yet even more typically the rare earth- containing additive comprises more than about 75 wt% of a water-soluble cerium (III) composition, still yet even more typically the rare earth-containing additive comprises more than about 80 wt% of a water-soluble cerium (III) composition, still yet even more typically the rare earth-containing additive comprises more than about 85 wt% of a water- soluble cerium (III) composition, still yet even more typically the rare earth-containing additive comprises more than about 90 wt% of a
- the rare earth-containing additive comprises one or more nitrogen-containing materials.
- the one or more nitrogen-containing materials commonly, comprise one or more of ammonia, an ammonium-containing composition, a primary amine, a secondary amine, a tertiary amine, an amide, a cyclic amine, a cyclic amide, a polycyclic amine, a polycyclic amide, and combinations thereof.
- the nitrogen-containing materials are typically less than about 1 ppm, less than about 5 ppm, less than about 10 ppm, less than about 25 ppm, less than about 50 ppm, less about 100 ppm, less than about 200 ppm, less than about 500 ppm, less than about 750 ppm or less than about 1000 ppm of the water-soluble rare earth-containing additive.
- the rare earth-containing additive comprises a water-soluble cerium (III) and/or lanthanum (III) composition. More commonly, the rare earth-containing additive comprises cerium (III) chloride.
- the rare earth-containing additive is typically dissolved in a liquid.
- the liquid is the rare earth- containing additive is dissolved in is preferably water.
- the rare earth-containing additive is in the form of one or more of: an aqueous solution containing substantially dissociated, dissolved forms of the rare earths and/or rare earth-containing compositions; free flowing granules, powder, particles, and/or particulates of rare earths and/or rare earth-containing compositions containing at least some water-soluble cerium (III); free flowing aggregated granules, powder, particles, and/or particulates of rare earths and/or rare earth-containing compositions substantially free of a binder and containing at least some water-soluble cerium (III); free flowing agglomerated granules, powder, particles, and/or particulates comprising a binder and rare earths and/or rare earth-containing compositions one or both of in an aggregated and non-aggregated form and containing at least some water-soluble cerium (III); rare earths and/or rare earth-containing compositions containing at least some water-soluble cerium ( 1 11 ) and supported on substrate; and combinations
- the particles in one formulation, have a particle size may be from about 1 nanometer to about 1000 nanometers. In another embodiment the particles may have a particle size less than about 1 nanometer. In yet another embodiment the particles may have a particle size from about 1 micrometer to about 1,000 micrometers.
- suitable substrates can include porous and fluid permeable solids having a desired shape and physical dimensions.
- the substrate for example, can be a sintered ceramic, sintered metal, micro-porous carbon, glass fiber, cellulosic fiber, alumina, gamma-alumina, activated alumina, acidified alumina, a metal oxide containing labile anions, crystalline aiumino-siiicate such as a zeolite, amorphous silica-alumina, ion exchange resin, clay, ferric sulfate, porous ceramic, and the like.
- Such substrates can be in the form of mesh, such as screens, tubes, honeycomb structures, monoliths, and blocks of various shapes, including cylinders and toroids.
- the structure of the substrate will vary depending on the application. Suitable structural forms of the substrate can include a woven substrate, non-woven substrate, porous membrane, filter, fabric, textile, or other fluid permeable structure.
- the rare earth-containing additive can be incorporated into or coated onto a filter block or monolith for use as a filter, such as a cross-flow type filter.
- the rare earth and/or rare earth-containing additive can be in the form of particles coated on to or incorporated in the substrate. In some configurations, the rare earth and/or rare earth-containing additive can be ionically substituted for cations in the substrate.
- the rare earth-coated substrate comprises at least about 0.1% by weight, more typically 1% by weight, more typically at least about 5%> by weight, more typically at least about 10%) by weight, more typically at least about 15% by weight, more typically at least about 20% by weight, more typically at least about 25% by weight, more typically at least about 30%) by weight, more typically at least about 35%> by weight, more typically at least about 40% by weight, more typically at least about 45% by weight, and more typically at least about 50%> by weight rare earth and/or rare earth-containing composition.
- the rare earth-coated substrate includes no more than about 95% by weight, more typically no more than about 90%> by weight, more typically no more than about 85% by weight, more typically no more than about 80% by weight, more typically no more than about
- the rare earth-containing additive includes a rare earth-containing composition supported on, coated on, or incorporated into a substrate, preferably the rare earth-containing composition is in the form of particulates.
- the rare earth-containing particulates can, for example, be supported or coated on the substrate with or without a bi der.
- the binder may be any suitable binder, such as those set forth herein.
- formulations comprising the rare earth-containing additive comprising rare earth-containing granules, powder, particles, and/or particulates agglomerated and/or aggregated together with or without a binder
- such formulations commonly have a mean, median, or P90 particle size of at least about 1 um, more commonly at least about 5 um, more commonly at least about 10 iim, still more commonly at least about 25 iim.
- the rare earth-containing agglomerates or aggregates have a mean, median, or P90 particle size distribution from about 100 to about 5,000 microns; a mean, median, or P90 particle size distribution from about 200 to about 2,500 microns; a mean, median, or P 90 particle size distribution from about 250 to about 2,500 microns; or a mean, median, or P90 particle size distribution from about 300 to about 500 microns.
- the agglomerates and/or aggregates can have a mean, median, or P90 particle size distribution of at least about 100 nm, specifically at least about 250 nm, more specifical ly at least about 500 nm, even more specifically at least about 1 um and yet even more specifically at least about 0.5 nm, the mean, median, or P 90 particle size distribution of the agglomerates and/or aggregates can be up to about 1 micron or more.
- the rare earth-containing particulates individually and/or in the form of agglomerates and/or aggregates, can have in some cases a surface area of at least about 5 m 2 /g, in other cases at least about 10 m 2 /g, in other cases at least about 70 m 2 /g, in yet other cases at least about 85 m 2 /g, in still yet other cases at least about 100 m 2 /g, in still yet other cases at least about 1 15 m7g, in still yet other cases at least about 125 m 2 /g, in still yet other cases at least about 1 50 m /g, in still yet other cases at least 300 m 2 /g, and in still yet other cases at least about 400 m 2 /g.
- the rare earth-containing particulates individually and/or in the form of agglomerates or aggregates commonly can have a surface area from about 50 to about 500 m 2 /g, or more commonly from about 1 10 to about 250 m 2 /g.
- the rare earth- containing agglomerate includes more than 10.01wt%, more commonly more than about 85 wt%, even more commonly more than about 90 wt%, yet even more commonly more than about 92 wt% and still yet even more commonly from about 95 to about 96.5 wt% rare earth-containing particulates, with the balance being primarily the binder.
- the binder can be less than about 15% by weight of the agglomerate, in some cases less than about 10% by weight, in still other cases less than about 8% by weight, in still other cases less than about 5% by weight, and in still other cases less than about 3.5% by weight of the agglomerate.
- the rare earth-containing particulates are in the form of powder and have aggregated nano-crystalline domains.
- the binder can include one or more polymers selected from the group consisting of thermosetting polymers, thermoplastic polymers, elastomeric polymers, cellulosic polymers and glasses.
- the binder comprises a fluorocarbon-containing polymer and/or an acry ic- polymcr.
- the rare earth-containing composition is in the form of a colloid, suspension, or slurry of particulates.
- the particulates commonly can have a mean, median and/or P 90 particle size of less than about 1 nanometer, more commonly a mean, median and/or P90 particle size from about 1 nanometer to about 1,000 nanometers, even more commonly a mean, median and/or P90 particle size from about 1 micron to about 1,000 microns, or yet even more commonly a mean, median and/or P90 particle size of at least about 1,000 microns.
- the particulates have a mean, median and/or P90 particle size from about 0.1 to about 1,000 nm, more preferably from about 0.1 to about 500 nm.
- the cerium (IV) particulates have a mean, median and/or P90 particle size from about 0.2 to about 100 nm.
- the particulates may have a mean and/or median surface area of at least about 1 m 2 /g, preferably a mean and/or median surface area of at least about 70 m 2 /g. In other embodiments, the particulates may preferably have a mean and/or median surface area from about 25 m 2 /g to about 500 m 2 /g and more preferably, a mean and/or median surface area of about 100 to about 250 m 2 /g. In some embodiments, the particulates may be in the form of one or more of a granule, crystal, crystallite, and particle.
- the particulates comprise cerium (IV), typically as cerium (IV) oxide.
- the weight percent (wt%) cerium (IV) content based on the total rare earth content of the cerium (IV) particulates typically is at least about 50 wt% cerium (IV), more typically at least about 60 wt%> cerium (IV), even more typically at least about 70 wt%> cerium (IV), yet even more typically at least about 75 wt% cerium (IV), still yet even more typically at least about 80 wt% cerium (IV), still yet even more typically at least about 85 wt% cerium (IV), still yet even more typically at least about 90 wt% cerium (IV), still yet even more typically at least about 95 wt% cerium (IV), and even more typically at least about 99 wt% cerium (IV).
- the cerium (IV) particulate is substantially devoid of rare earths other than cerium (IV). More preferably, the weight percent (wt%) cerium (IV) content based on the total rare earth content of the cerium (IV) particulates is about 100 wt% cerium (IV) and comprises one or more of cerium (IV) oxide, cerium (IV) hydroxide, cerium (IV) oxyhydroxyl, cerium (IV) hydrous oxide, cerium (IV) hydrous oxyhydroxyl, Ce0 2 , and/or Ce(IV)(0) w (OH) x (OH) y zH 2 0, where w, x, y and can be zero or a positive, real number.
- the medium (or media) 104 can be any fluid stream.
- the fluid stream may be derived from any source containing one or more phosphorus-containing target materials.
- the medium (or media) 104 is derived from any aqueous source containing one or more phosphorus-containing target materials.
- suitable medium (or media) 104 are recreational waters, municipal waters (such as, sewage, waste, agricultural, or ground waters), industrial (such as cooling, boiler, or process waters), wastewaters, well waters, septic waters, drinking waters, naturally occurring waters, (such as a lake, pond, reservoir, river, or stream), and other waters and/or aqueous process streams.
- Non-limiting examples of recreational waters are swimming pool waters, brine pool waters, therapy pool waters, diving pool waters, sauna waters, spa waters, and hot tub waters.
- Non-limiting examples of municipal waters are drinking waters, waters for irrigation, well waters, waters for agricultural use, waters for architectural use, reflective pool waters, water-fountain waters, water-wall waters, use, non-potable waters for munici al use and other non-potable munici al waters.
- Wastewaters include without limitation, municipal and/or agricultural run-off waters, septic waters, waters formed and/or generated during an industrial and/or manufacturing process, waters formed and/or generated by a medical facility, waters associated with mining, mineral production, recovery and/or processing (including petroleum), evaporation pound waters, and non- potable disposal waters.
- Well waters include without limitation waters produced from a subsurface well for the purpose of human consumption, agricultural use (including consumption by a animal, irrigation of crops or consumption by domesticated farm animals), mineral-containing waters, waters associated with mining and petroleum, production.
- Non- limiting examples of naturally occurring waters include associated with rains, storms, streams, rivers, lakes, aquifers, estuaries, lagoons, and such.
- the medium (or media) 104 is typically obtained from one or more of the above sources and processed, conveyed and/or manipulated by a water handling system.
- the medium (or media) 104 can be primarily the water in a water handling system.
- the water handling system components and configuration can vary depending on the treatment process, water, and water source. While not wanting to limited by example, municipal and/or wastewater handling systems typically one or more of the following process units: clarifying, disinfecting, coagulating, flocculating, aerating, filtering, separating solids and liquids, digesting, and polishing.
- the number and ordering of the process units can vary. Furthermore, some process units may occur two or more times within a water handling system. It can be appreciated that the one or more process units are in fluid communication.
- the water handling system may or may not have a clarifier.
- Some water handling systems may have more than one clarifier, such as primary and final clarifiers.
- Clarifiers typically reduce cloudiness of the water by removing biological matter (such as bacteria and/or algae), suspended and/or dispersed chemicals and/or particulates from the water. Commonly a clarification process occurs before and/or after a filtration process.
- the water handling system may or may not contain a filtering process.
- the water handling system contains at least one filtering process.
- Non- limiting examples of common filtering processes include without limitation screen filtration, trickling filtration, particulate filtration, sand filtration, macro-filtration, micro-filtration, ultra - filtration, nano-filtration, reverse osmosis, carbon/activated carbon filtration, dual media filtration, gravity filtration and combinations thereof.
- a filtration process occurs before and/or after a disinfection process.
- a filtration process to remove solid debris, such as solid organic matter and grit from the water typically precedes the disinfection process.
- a filtration process such as an activated carbon and/or sand filtrations follows the disinfection process.
- the post- disinfection filtration process removes at least some of the chemical disinfectant remaining in the treated water.
- the water handling system may or may not include a disinfection process.
- the disinfection process may include without limitation treating the aqueous stream and/or water with one or more of fluorine, fluorination, chlorine, chlorination, bromine, bromination, iodine, iodination, ozone, ozonation, electromagnetic irradiation, ultra-violet light, gama rays, electrolysis, chlorine dioxide, hypochlorite, heat, ultrasound,
- the water handling system contains a single disinfection process, more preferably the water handling system contains two or more disinfection processes.
- Disinfection processes are typically provided to one of at least remove, kill and/or detoxify pathogenic material contained in the water.
- the pathogenic material comprises biological contaminants, in particular biological contaminants comprising phosphorus- containing target materials.
- the disinfection process converts the phosphorus-containing target material into a form of the phosphorus-containing target material that can be removed and/or deto i fied by the rare earth-containing composition, additive and/or particle or particulate.
- the water handl ing system may or may not include coagulation.
- the water handling system may contain one or more coagulation processes.
- the coagulation process includes adding a flocculent to the water in the water handling system.
- Typical flocculants include aluminum sulfate, polyelectrolytes, polymers, lime and ferric chloride.
- the flocculent aggregates the particulate matter suspended and/or dispersed in the water, the aggregated particulate matter forms a coagulum.
- the coagulation process may or may not include separating the coagulum from the liquid phase.
- coagulation may comprise part, or ail, the entire clarification process.
- the coagulation process is separate and distinct from the clarification process.
- the coagulation process occurs before the disinfection process.
- the water handl ing system may or may not include aeration.
- aeration comprises passing a stream of air and/or molecular oxygen through the water contained in the water handling system.
- the aeration process promotes oxidation of contaminants contained in the water being processed by the water handling system, preferably the aeration promotes the oxidation of biological contaminates, such as phosphorus-containing biological contaminates.
- the aeration process converts the phosphorus-containing target material into a form of the phosphorus- containing target material that can be removed and/or detoxified by the rare earth- containing composition, additive and/or particle or particulate.
- the water handl ing system may contain one or more aeration processes. Typically, the disinfection process occurs after the aeration process.
- the water handling system may or may not have one or more of a heater, a cooler, and a heat exchanger to heat and/or cool the water being processed by the water handling system.
- the heater may be any method suitable for heating the water.
- suitable heating processes are solar heating systems, electromagnetic heating systems (such as, induction heating, microwave heating and infrared), immersion heaters, and thermal transfer heating systems (such as, combustion, stream, hot oil, and such, where the thermal heating source has a higher temperature than the water and transfers heat to the water to increase the temperature of the water).
- the heat exchanger can be any process that transfers thermal energy to or from the water.
- the heat exchanger can remove thermal energy from the water to cool and/or decrease the temperature of the water.
- the heat exchanger can transfer thermal energy to the water to heat and/or increase the temperature of the water.
- the cooler may be any method suitable for cooling the water.
- suitable cooling process are refrigeration process, evaporative coolers, and thermal transfer cooling systems (such as, chillers and such where the thermal (cooling) source has a lower temperature than the water and removes heat from the water to decrease the temperature of the water).
- Any of the clarification, disinfection, coagulation, aeration, filtration, sludge treatment, digestion, nutrient control, solid/liquid separation, and/or polisher processes may further include before, after and/or during one or both of a heating and cooling process. It can be appreciated that a heat exchanger typically includes at least one of heating and cooling process.
- the water handling system may or may not include a digestion process.
- the digestion process is one of an anaerobic or aerobic digestion process.
- the digestion process may include one of an anaerobic or aerobic digestion process followed by the other of the anaerobic or aerobic digestion processes.
- one such configuration can be an aerobic digestion process followed by an anaerobic digestion process.
- the digestion process comprises microorganisms that breakdown the biodegradable material contained i the water, in some embodiments, the biodegrablc material includes a phosphorus-containing target material.
- the digestion process converts the phosphorus-containing target material into a form of the phosphorus-containing target material that can be removed and/or detoxified by the rare earth-containing composition, additive and/or particle or particulate.
- the anaerobic digestion of biodegradable material proceeds in th e absence of oxygen
- the aerobic digestion of biodegradable material proceeds in the presence of oxygen.
- the digestion process is typically referred to as biological stage/digester or biological treatment stage/digester.
- the disinfection process comprises a digestion process.
- the water handling system may or may not include a nutrient control process.
- the water handling system may include one or more nutrient control processes.
- the nutrient control process typically includes nitrogen and/or phosphorus control.
- nitrogen control commonly may include nitrifying bacteria.
- phosphorus control refers to biological phosphorus control, preferably controlling phosphorus that can be used as a nutrient for algae.
- Nutrient control typically includes processes associated with control of oxygen demand substances, which include in addition to nutrients, pathogens, and inorganic and synthetic organic compositions.
- the nutrient control process can occur before or after the disinfection process.
- the nutrient control converts the phosphorus-containing target material into a form of the phosphorus-containing target material that can be removed and/or detoxified by the rare earth-containing composition, additive and/or particle or particulate.
- the water handling system may or may not include a solid/liquid separation process.
- the water handling system includes one or more solid/liquid separation processes.
- the solid/liquid separation process can comprise any process for separating a solid phase from a liquid phase, such as water.
- suitable solid liquid separation processes are clarification (including trickling filtration), filtration (as described above), vacuum and/or pressure filtration, cyclone (including hydrocyclones), floatation, sedimentation (including gravity sedimentation ), coagulation (as described above), sedimentation (including, but not limited to grit chambers), and combinations thereof.
- the water handling system may or may not include a polisher.
- the polishing process can include one or more of removing fine particulates from the water, an ion- exchange process to soften the water, an adjustment to the pH value of the water, or a combination thereof.
- the polishing process is after the disinfection step.
- the water handling system may further include additional processing equipment.
- the additional processing equipment includes without limitation holding tanks, reactors, purifiers, treatment vessels or units, mixing vessels or elements, wash circuits, precipitation vessels, separation vessels or units, settling tanks or vessels, reservoirs, pumps, cooling towers, heat exchangers, valves, boilers, gas liquid separators, nozzles, tenders, and such.
- the water handling system includes conduit(s) interconnecting the unit operations and/or additional processing equipment.
- the conduits include without limitation piping, hoses, channels, aqua-ducts, ditches, and such. The water is conveyed to and from the unit operations and/or additional processing equipment by the conduit(s).
- each unit operations and/or additional processing equipment is in fluid communication with the other unit operations and/or additional processing equipment by the conduits.
- the water handling system includes sludge and Biological
- BOD Oxygen Demand
- the treated medium 124 can be further subjected to tertiary treatment to remove dissolved substances, such as colorants, metals, organic chemicals, and microorganism nutrients, particularly phosphorus and nitrogen.
- Tertiary treatment can include clarification, anaerobic, anoxic, and/or aerobic biological nutrient removal, disinfection, dissolved air flotation, fermentation, digestion, and the like. The ordering of the steps is not critical and may be changed, depending on the application.
- the rare earth-containing additive can be added to any of the first intermediate treated medium, second intermediate treated medium, third intermediate treated medium, and/or fourth intermediate treated medium and/or in any of the clarification, digestion, or disinfection stages and/or in any tertiary treatment stage of inter-stage effluent.
- the rare earth-containing additive can be an antimicrobial agent, it is more common for the rare earth-containing additive to be added downstream of digestion to avoid interference with bacterial digestion. Because the rare earth-containing additive can be an antimicrobial agent, one process configuration uses the rare earth-containing additive for both phosphate removal and microcrobial removal. In this configuration, other antimicrobial agents may not be employed. In one configuration, the process steps can change the relative concentrations and therefore ratios of reactive phosphate ("RP") and non-reactive phosphate (“NRP”), particularly digestion (due to aeration and/or microbial activity) and disinfection.
- RP reactive phosphate
- NRP non-reactive phosphate
- the process converts a portion of NRP to RP, thereby increasing the proportion of total phosphates (which includes both RP and NRP) that is RP. In other applications, the process converts a portion of RP to NRP, thereby increasing the proportion of total phosphates that is NRP.
- the aqueous medium that is treated by the rare earth-containing composition, additive and/or particles may contain one or more phosphorus-containing target materials.
- the one or more phosphorus-containing target materials may include one or both of inorganic and organic phosphorus-containing target materials.
- Inorganic phosphorus- containing target materials metals include without limitation inorganic phosphate compounds, phosphite compounds, phosphorus oxyhalide (e.g., phosphorus oxychloride), phosphorus thiohalide (e.g., phosphorus thiochloride), phosphorus selenohalide (e.g., phosphorus selenochloride), phosphorus trihalide (e.g., phosphorus trichloride), phosphorus pentahalide (e.g., phosphorus pentachloride), phosphoric acid, and phosphorus acid and salts thereof (e.g., cyclic phosphate and phosphite salts).
- phosphorus oxyhalide e.g., phosphorus oxychloride
- phosphorus thiohalide e.g., phosphorus thiochloride
- phosphorus selenohalide e.g., phosphorus selenochlor
- Organic phosphorus- containing target materials include without limitation organophosphor o us compounds (such as organophosphates (or organic phosphate compounds), organophosphites (or organic phosphite compounds), and organophosphines, (or organic phosphines)).
- organophosphor o us compounds such as organophosphates (or organic phosphate compounds), organophosphites (or organic phosphite compounds), and organophosphines, (or organic phosphines)).
- organophosphorus compounds include chalcogenides, diphosphenes, phosoamides, phosphaalkenes, phosphaalkynes, phosphine oxides, phosphinates, phosphines, phosphinites, phosphonates (e.g., o-alkyl phosphonohalogenates (such as alkyl phosphonofluoridates (such as sarin or isopropyl methylphosphonofluoridate), o-alkyl, s- 2-dialkyl aminoethyl alkylphosphonothiolates, dimethyl methylphosphonates, diisopropyl methylphosphonates, and alkylated or protonated salts thereof), o-alkyl
- phosphoramideocyanidates and alklylated or protonated salts thereof phosphonic acids and esters thereof, phosphinic acids and esters thereof, phospone oxides, phosphonites (e.g., alkyl phosphonites), phosphonium salts, alkyl phosphonyldihalides (e.g., alkyl phosphonyldifluoride and methylphosphonyl dichloride), dialkyl phosphoramidic dihalides, alkyl phosphoramidate, phosphoranes (e.g., methylenetriphenyl-phosphorane), phosphono carboxylates, halosarins (e.g., chlorosarin), halosomans (e.g., chlorosoman), amitons, alkyl phosphorus oxyhalides (e.g., alkyl phosphorus oxychloride), alkyl phosphites, phosphamidons, DNA
- the phosphorus-containing target material may occur as a primary or secondary phosphorus-containing species (vide infra).
- One or both of the primary or secondary phosphorus-containing species may be in the form of a solid, a dissolved species, a suspension, or a combination thereof.
- the phosphorus-containing target material comprises a phosphorus (V)-containing target material, a phosphorus (Ill)-containing target material, a phosphorus (Il)-containing target material, or a mixture comprising one or more phosphorus (V)-, phosphorus (III)-, and phosphorus (Il)-containing target materials.
- the phosphorus-containing target material comprises reactive and/or non-reactive phosphates.
- Reactive phosphorus [RP] refers to
- NRP non-reactive phosphorus
- NRP can be in the form of a colloid, or suspension. Furthermore, the NRP may be in the form of an anion, cation, and/or neutral species.
- the NRP to RP molar and/or weight ratios (in the in the medium (or media) 104 is commonly no more than about 0.1, more commonly no more than about 0.2, more commonly no more about 0.3, more commonly no more than about 0.4, more commonly no more than about 0.5, more commonly no more than about 0.6, more commonly no more than about 0.7, more commonly no more than about 0.8, more commonly no more than about 0.9, more commonly no more than about 1.0, more commonly no more than about 1.1, more commonly no more than about 1.2, more commonly no more than about 1.3, more commonly no more than about 1.4, more commonly no more than about 1.5, more commonly no more than about 1.6, more commonly no more than about 1.7, more commonly no more about 1.8, more commonly no more than about 1.9, more commonly no more than about 1.9, and even more commonly no more than about 2.0 at a pH value of no more than about pH -2, at a pH value of more than about pH -1, at a pH value of more than about pH
- the rare earth-containing composition, additive and/or particles removes and/or detoxifies the phosphorus-containing target material from the medium 104.
- the phosphorus-containing target material and/or a portion thereof may be one or more of sorbed, precipitated, complexed, ionically bound, inter- valance shell complexed (with any one or more hybridized or non-hybridized s, p, d or f orbitals), covalently bounded or a combination thereof with the rare earth-containing composition, additive and/or particles.
- the phosphorus-containing target material and/or a portion thereof may selectively interact with a face or an edge of rare earth- containing composition, additive and/or particulate to form a target-laden rare earth composition, preferably a substantially insoluble target-laden rare earth composition.
- the rare earth composition, additive and/or particulate may be in the form of a substantially water-soluble or substantially water-insoluble rare earth composition, additive and/or particulate.
- the rare earth-containing composition, additive and/or particulate chemically and/or physically strongly sorbs, binds, reacts or such with the phosphorus-containing target material to form a target-laden rare earth composition.
- the target-laden rare earth composition is an insoluble target-laden rare earth composition.
- the phosphorus-containing material and/or portion thereof is removed and/or detoxified by chemically and/or physically attaching, sorbing and/or bonding with: the phosphorus- containing material and/or portion thereof; oxygen and/or an oxygen-containing portion (such as, but not limited to an ether, alcohol, carbonyl, or carboxylate) of the phosphorus- containing material; sulfur and/or a sulfur-containing portion (such as, but not limited to a thiol-ether, thiol alcohol, thiol-carbonyl or thio-carboxylate) of the phosphorus-containing material; selenium and/or a selenium-containing portion (such as, but not limited to seleno-ether, seleno-alcohol, seleno-carbonyl, seleno-carboxylate) of the phosphorus- containing material; nitrogen and/or a nitrogen-containing portion (such as, but not limited to
- the rare earth- containing composition, additive and/or particulate comprises cerium (IV).
- the cerium (IV) comprises cerium (IV) oxide.
- the cerium (IV) may oxidize and/or decompose the phosphorus-containing target material.
- the cerium (IV) may oxidize the phosphorus-containing material in the form of a phosphorus (II)- and/or phosphorus (Ill)-containing material to a phosphorus (IV)- containing material.
- the phosphorus (IV)-containing target material being one or both of more easily and effectively removed and/or detoxified than either one or both of the phosphorus (II)- and phosphorus (Ill)-containing target materials by the rare earth- containing composition, additive and/or particulate.
- the cerium (IV) may oxidize an alcoholic portion (such as, an oxygen-, sulfur-, seleno- and/or telluro- alcohol) to a carbonyl and/or carboxylate portion, respectively.
- the contacting of the rare earth-containing oxidizing agent and the phosphorus-containing target material may one or both: a) chemically interact with the phosphorus-containing target material and b) form a reduced rare earth and/or rare earth-containing oxidizing agent and an oxidized target material and/or portion thereof.
- a cerium (IV) oxidizing agent may be formed by contacting a first cerium-containing composition having cerium in a +3 oxidation state with an oxidant ⁇ vide infra) to form a second cerium-containing composition having cerium in a +4 oxidation state (or cerium (IV) oxidizing agent).
- the second cerium- containing composition comprises Ce0 2 particles.
- the cerium (IV) oxidizing agent then oxidizes the phosphorus-containing target material forming the first (reduced) cerium (Ill)-containing composition.
- the target-laden rare earth composition comprises the rare earth- and the phosphorus-containing target material or a component thereof.
- the target-laden rare earth composition can be in the form of an insoluble solid material either contained within the water or an insoluble solid material phase separated from the water.
- the target-laden rare earth composition may be one or more of an insoluble precipitate, an insoluble solid particle suspended within the water, a flocculated solid particle, and a combination thereof.
- the target-laden rare earth composition can be in form of solubilized and/or dissolved in the aqueous phase.
- the primary phosphorus-containing species depends upon the pH and Eh of the aqueous solution.
- Figs. 2A-2E area Pourbaix diagrams for phosphate under different thermodynamic conditions.
- the form of phosphate species present in solution and therefore the efficacy of precipitating, sorbing, or otherwise removing the phosphate species from and/or detoxifying the aqueous medium by treatment with the rare earth-containing composition, additive and/or particle or particulate can be increased substantially by adjusting one or both of the pH and Eh of the aqueous solution.
- the phosphorus-containing target material is removed from the aqueous media having a selected pH value.
- the selected pH value of the aqueous media may be from about pH 0 to about pH 14, more commonly the pH of the aqueous media may be from about pH 1 to about pH 13, even more commonly the pH of the aqueous media may be from about pH 2 to about pH 12, even more commonly the pH of the aqueous media may be from about pH 3 to about pH 11 , yet even more commonly the pH of the aqueous media may be from about pH 4 to about pH 10, still yet even more commonly the pH of the aqueous media may be from about pH 5 to about pH 9, or still yet even more commonly the pH of the aqueous media may be from about pH 6 to about pH 8.
- the aqueous media typically has a selected pH value of from about pH 6 to about pH 9, and more typically the aqueous media has a pH of from about pH 6.5 to about pH 8.5
- the aqueous media may be substantially acidic having a selected pH of about pH 0, more commonly having a selected pH of about pH 1, even more commonly having a selected pH of about pH 2, yet even more commonly having a selected pH of about pH 3, or still yet even more commonly having a selected pH about pH 4.
- the aqueous media may have a selected pH of about pH 5, more commonly having a selected pH of about pH 6, even more commonly having a selected pH of about pH 7, yet even more commonly having a selected pH of about pH 8, or still yet even more commonly having a selected pH of about pH 9.
- the aqueous media may have a selected pH of about pH 10, more commonly having a selected pH of about pH 11, even more commonly having a selected pH of about pH 12, yet even more commonly having a selected pH of about pH 13, or still yet even more commonly having a selected pH about pH 14.
- the phosphorus-containing target material is removed from the aqueous medium having a selected Eh value with respect to standardized reference electrode, such as a standard hydrogen electrode (SHE).
- standardized reference electrode such as a standard hydrogen electrode (SHE).
- the selected Eh of the aqueous medium is at least about -0.5 V, more commonly at least about -0.4 V, more commonly at least about -0.3 V, more commonly at least about -0.2 V, more commonly at least about -0.1 V, more commonly at least about 0 V, more commonly at least about 0.1 V, more commonly at least about 0.2 V, more commonly at least about 0.3 V, and more commonly at least about 0.4 V, and more commonly at least about 0.5 V.
- the selected Eh of the aqueous medium is below the level at which water is not electrochemically stable, more commonly no more than about 1.7 V, more commonly no more than about 1.6 V, more commonly no more than about 1.5 V, more commonly no more than about 1.4 V, more commonly no more than about 1.3 V, more commonly no more than about 1.2 V, more commonly no more than about 1.1 V, more commonly no more than about 1.0 V, more commonly no more than about 0.9 V, more commonly no more than about 0.8 V, and more commonly no more than about 0.7 V.
- the rare earth to phosphorus (in the phosphorus-containing target material)/target material ratio can also vary depending on the solution pH and/or Eh value.
- rare earths having a rare earth to phosphorus/target material ratio less than 1 have a greater molar removal capacity of phosphorus/target material than rare earths having a rare earth to phosphorus/target material ratio of 1 or more than 1.
- the greater the pH value the greater the rare earth to phosphorus/target material ratio.
- the greater the pH value the smaller the rare earth to phosphorus/target material ratio.
- the rare earth to phosphorus/target material ratio is substantially unchanged over a range of pH values.
- the rare earth to phosphorus/target material ratio is no more than about 0.1
- the rare earth to phosphorus/target material ratio is no more than about 0.2
- the rare earth phosphorus/target material ratio is no more about 0.3, the rare earth : phosphorus/target material ratio is no more than about 0.4, the rare earth to phosphorus/target material ratio is no more than about 0.5, the rare earth to phosphorus/target material ratio is no more than about 0.6, the rare earth to phosphorus/target material ratio is no more than about 0.7, the rare earth to phosphorus/target material ratio is no more than about 0.8, the rare earth to phosphorus/target material ratio is no more than about 0.9, the rare earth to
- phosphorus/target material ratio is no more than about 1.0, the rare earth to
- phosphorus/target material ratio is no more than about 1.1, the rare earth to
- phosphorus/target material ratio is no more than about 1.2, the rare earth to
- phosphorus/target material ratio is no more than about 1.3, the rare earth to
- phosphorus/target material ratio is no more than about 1.4, the rare earth to
- phosphorus/target material ratio is no more than about 1.5, the rare earth to
- phosphorus/target material ratio is no more than about 1.6, the rare earth to
- phosphorus/target material ratio is no more than about 1.7, the rare earth to
- the rare earth to phosphorus/target material ratio is no more about 1.8, the rare earth to phosphorus/target material ratio is no more than about 1.9, the rare earth to phosphorus/target material ratio is no more than about 1.9, or the rare earth to phosphorus/target material ratio is more than about 2.0 at a pH value of no more than about pH -2, at a pH value of more than about pH - 1 , at a pH value of more than about pH 0, at a pH value of more than about pH 1 , at a pH value of more than about pH 2, at a pH value of more than about pH 3, at a pH value of more than about pH 4, at a pH value of more than about pH 5, at a pH value of more than about pH 6, at a pH value of more than about pH 7, at a pH value of more than about pH 8, at a pH value of more than about pH 9, at a pH value of more than about pH 10, at a pH value of more than about pH 11, at a pH value of more than about pH 12, at a pH value of more
- the rare earth to phosphorus/target material ratio is no more than about 0.1, the rare earth to phosphorus/target material ratio is no more than about 0.2, the rare earth to phosphorus/target material ratio is no more about 0.3, the rare earth to phosphorus/target material ratio is no more than about 0.4, the rare earth to
- phosphorus/target material ratio is no more than about 0.5, the rare earth to
- the rare earth to phosphorus/target material ratio is no more than about 0.6, the rare earth to phosphorus/target material ratio is no more than about 0.7, the rare earth to phosphorus/target material ratio is no more than about 0.8, the rare earth to phosphorus/- target material ratio is no more than about 0.9, the rare earth to phosphorus/target material ratio is no more than about 1.0, the rare earth to phosphorus/target material ratio is no more than about 1.1, the rare earth to phosphorus/target material ratio is no more than about 1.2, the rare earth to phosphorus/target material ratio is no more than about 1.3, the rare earth to phosphorus/-target material ratio is no more than about 1.4, the rare earth to phosphorus/target material ratio is no more than about 1.5, the rare earth to
- phosphorus/target material ratio is no more than about 1.6, the rare earth to
- phosphorus/target material ratio is no more than about 1.7, the rare earth to
- the rare earth to phosphorus/target material ratio is no more about 1.8, the rare earth to phosphorus/target material ratio is no more than about 1.9, the rare earth to phosphorus/target material ratio is no more than about 1.9, or the rare earth to phosphorus/target material ratio is more than about 2.0 at a water pH value of no more than about pH -2, at a water pH value of more than about pH - 1 , at a water pH value of more than about pH 0, at a water pH value of more than about pH 1 , at a water pH value of more than about pH 2, at a water pH value of more than about pH 3, at a water pH value of more than about pH 4, at a water pH value of more than about pH 5, at a water pH value of more than about pH 6, at a water pH value of more than about pH 7, at a water pH value of more than about pH 8, at a water pH value of more than about pH 9, at a water pH value of more than about pH 10, at a water pH value of more than about pH 11 , at
- the concentration of the phosphorus-containing target material can vary depending on a number of factors.
- the concentration of either or both can be, for example, commonly at least about 1 ppm, more commonly at least about 5 ppm, more commonly at least about 10 ppm, more commonly at least about 25 ppm, 50 ppm, more commonly at least about 100 ppm, more commonly at least about 500 ppm, more commonly at least about 1,000 ppm, more commonly at least about 5,000 ppm, more commonly at least about 10,000 ppm, and more commonly at least about 100,000 ppm.
- 0.1 mg target material/g REO e.g. Ce0 2
- These can have rare earth:target material ratios that are significantly larger than 2.
- the rare earth to target material ratio is commonly no more than about 50,000, the rare earth to target material ratio is more commonly no more than about 47,500, the rare earth to target material ratio is more commonly no more about 45,000, the rare earth to target material ratio is more commonly no more than about 42,500, the rare earth to target material ratio is more commonly no more than about 40,000, the rare earth to target material ratio is no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more than about
- the rare earth to target material ratio is more commonly no more about 20,000, at a water pH value of no more than about pH -2, at a water pH value of more than about pH -1 , at a water pH value of more than about pH 0, at a water pH value of more than about pH 1 , at a water pH value of more than about pH 2, at a water pH value of more than about pH 3, at a water pH value of more than about pH 4, at a water pH value of more than about pH 5, at a water pH value of more than about pH 6, at a water pH value of more than about pH 7, at a water pH value of more than about pH 8, at a water pH value of more than about pH 9, at a water pH value of more than about pH 10, at a water pH value of more than about pH 11 , at a water pH value of more than about pH 12, at a water pH value of more than about pH 13, or at a water pH value of more than about pH 14.
- the medium 104 is optionally pre-treated to produce a selected primary species of the phosphorus-containing target material.
- the selected primary species is generally more effectively removed by the rare earth-containing composition, additive, and/or particle than the primary species in the medium 104.
- one or more of the Eh and pH values may be altered for more effective removal and/or detoxification the phosphorus-containing target material from the medium 104.
- pH is a measure of the activity of hydrogen ions while Eh is a measure of the electrochemical (oxidation/reduction) potential.
- the type of pre-treatment employed can depend on the application.
- an acid, acid equivalent, base, or base equivalent is added to adjust the pH to a desired pH value.
- acids or acid equivalents include monoprotic acids and polyprotic acids, such as mineral acids, sulfonic acids, carboxylic acids, vinylogous carboxylic acids, nucleic acids, and mixtures thereof.
- bases and base equivalents include strong bases (such as potassium hydroxide, barium hydroxide, cesium hydroxide, sodium hydroxide, strontium hydroxide, calcium hydroxide, magnesium hydroxide, lithium hydroxide, and rubidium hydroxide), superbases, carbonates, ammonia, hydroxides, metal oxides (particularly alkoxides), and counter anions of weak acids.
- Eh is a measure of the oxidation or reduction potential of the medium 104.
- the oxidation or reduction potential is commonly referred to as electromotive force or EMF.
- the EMF is typically measured with respect to a standardized reference electrode.
- Non- limiting examples of standardized reference electrodes are hydrogen electrode (commonly referred to as SHE), copper/copper sulfate electrode, and silver/silver chloride to name a few.
- the phosphorus-containing target material is contacted with an oxidizing agent to oxidize the phosphorus-containing target material to form a primary species.
- the oxidizing agent may comprise a chemical oxidizing agent, an oxidation process, or combination of both.
- a chemical oxidizing agent comprises a chemical composition in elemental or compounded form.
- the chemical oxidizing agent accepts an electron from the
- the oxidizing agent is reduced to form a reduced form of the oxidizing agent.
- preferred chemical oxidizing agents are chlorine, chloroamines, chlorine dioxide, hypochlorites, trihalomethane, haloacetic acid, ozone, hydrogen peroxide, peroxygen compounds, hypobromous acid, bromoamines, hypobromite, hypochlorous acid, isocyanurates, tricholoro-s-triazinetriones, hydantoins, bromochloro-dimethyldantoins, 1- bromo-3-chloro-5,5-dimethyldantoin, l,3-dichloro-5,5-dimethyldantoin, sulfur dioxide, bisulfates, and combinations thereof.
- one or more the following chemical compositions may oxidize the phosphorus-containing target material: bromine, BrCl, permanganates, phenols, alcohols, oxyanions, arsenites, chromates, trichloroisocyanuric acid, and surfactants.
- the chemical oxidizing agent may further be referred to as an "oxidant” or an "oxidizer”.
- An oxidation process comprises a physical process that alone or in combination with a chemical oxidizing agent.
- the oxidation process removes and/or facilitates the removal an electron from the phosphorus-containing target material.
- Non-limiting examples of oxidation processes are electromagnetic energy, ultra violet light, thermal energy, ultrasonic energy, and gamma rays.
- the phosphorus-containing target material is contacted with a reducing agent to reduce the phosphorus-containing target material and form a primary species.
- the oxidizing agent may comprise a chemical oxidizing agent, an oxidation process, or combination of both.
- a chemical reducing agent comprises a chemical composition in elemental or compounded form.
- the chemical reducing agent donates an electron to the phosphorus- containing target material to form the primary species. In the donating the electron, the reducing agent is oxidized to form an oxidized form of the reducing agent.
- preferred chemical reducing agents are lithium aluminum hydride, nascent (atomic) hydrogen, sodium amalgam, sodium borohydride, compounds containing divalent tin ion, sulfite compounds, hydrazine, zinc-mercury amalgam, diisobutylaluminum hydride, Lindlar catalyst, oxalic acid, formic acid, ascorbic acid, phosphites,
- the chemical reducing agent may further be referred to as a "reductant” or a "reducer”.
- a redox process is a physical process that alone or in combination with a chemical oxidizing agent transfers electrons to or form a target material.
- oxidation processes are electromagnetic energy, ultra violet light, thermal energy, ultrasonic energy, gamma rays, and biological processes.
- the aqueous media is contacted with a halogenated species, such as chlorine, bromine, iodine, or an acid, base, or salt thereof.
- a halogenated species such as chlorine, bromine, iodine, or an acid, base, or salt thereof.
- halogens impact the Eh of the aqueous media.
- halogens can impact the pH value of the aqueous media.
- pre-treatment may be employed to remove species from the aqueous media that can impair removal of the phosphorus-containing target material and/or adjustment of the pH and/or Eh of the aqueous media.
- the pre-treatment can comprise one or more of clarifying, disinfecting,
- the pre-treatment process can commonly comprise one of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes, more commonly any two of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, even more commonly any three of clarifying,
- the optionally pre-treated medium 104 is contacted with the rare earth- containing composition, additive, or particle to form the target-laden rare earth composition.
- Contact can be added by any suitable technique, including not only adding the rare earth-containing composition, additive or particle (as a water soluble, partially soluble, or insoluble form) to the pre-treated medium 104 or vice versa but also forming the rare earth-containing composition, additive, or particle in situ in the pre-treated medium 104.
- the rare earth-containing composition, additive and/or particulate chemically and/or physically reacts with, sorbs, precipitates, transforms, or otherwise deactivates or binds with the phosphorus-containing target material and/or portion thereof.
- the rare earth-containing composition, additive and/or particulate chemically and/or physically reacts with, sorbs, precipitates, transforms, or otherwise deactivates or binds with at least about 25%, more commonly at least about 50%, more commonly more commonly more than about 50%, more commonly at least about 75%, and even more commonly at least about 95% of the phosphorus-containing target material or portion thereof.
- the target-laden rare earth composition includes the rare earth and one or both of the phosphorus-containing target material and one or more constituents, components or portion of the phosphorus-containing target material, and, in some cases, one or more other constituents, components or portion of the rare earth-containing composition, additive and/or particulate.
- the temperature of the medium 104 during the contacting step can vary.
- the temperature of the aqueous solution can vary during the contacting step.
- temperature of aqueous solution can vary depending on the source of the water.
- the temperature of the aqueous solution is ambient temperature.
- the aqueous solution temperature ranges from about -5 degrees Celsius to about 50 degrees Celsius, more typically from about 0 degrees Celsius to about 45 degrees Celsius, yet even more typically from about 5 degrees Celsius to about 40 degrees Celsius and still yet even more typically from about 10 degrees Celsius to about 35 degrees
- each of the waters comprising each of the clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes may include optional processing units and/or operations that heat and/or cool one or more of each of the waters.
- each of the waters may be heated to have a temperature of typically at least about 20 degrees Celsius, more typically at least about 25 degrees Celsius, even more typically at least about 30 degrees
- each of the waters comprising each of the clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes may be cooled to have a temperature of typically of no more than about 110 degrees Celsius, more typically of no more than about 100 degrees Celsius, even more typically of no more than about 90 degrees Celsius, yet even more typically of no more than about 80 degrees Celsius, still yet even more typically of no more than about 70 degrees Celsius, still yet even more typically of no more than about 60 degrees Celsius, still yet even more typically of no more than about 50 degrees Celsius, still yet even more typically of no more than about 45 degrees Celsius, still yet even more typically of no more than about 40 degrees Celsius, still yet even more typically of no more than about 35 degrees Celsius, still yet even more typically of no more than about 30 degrees Celsius, still yet even more typically of no more than about 25 degrees Celsius, still yet even more typically of no more than about 20 degrees Celsius, still yet even more typically of no more than about 15 degrees Celsius, still yet even more typically of no more than about 10 degrees
- the target-laden rare earth composition is removed from the aqueous medium (medium 104) to form a treated aqueous medium 124.
- aqueous medium medium 104
- the target-laden rare earth composition comprises an insoluble material.
- the target-laden rare earth composition may be removed by any suitable technique, such as by a liquid/solid separation system. Non- limiting examples of liquid/solid separation systems are filtration, floatation, sedimentation, cyclone, and centrifuging.
- the rare earth-containing additive is in the form of a particulate bed or supported porous and permeable matrix, such as a filter, through which the media passes.
- the target-laden rare earth composition dissolved in the water, may remain in the water in a de-activated form.
- de-activated target- laden rare earth composition that may remain dissolved are environmentally stable co- ordination complexes of a primary or secondary phosphorus-containing species and the rare earth-containing composition.
- the treated aqueous medium 124 has a lower content of at least one of the one or more phosphorus-containing target materials compared to the aqueous medium 104.
- the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.9 of that for the aqueous medium 104, more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.8 of that for the aqueous medium 104, even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus- containing target materials is at least about 0.7 of that for the aqueous medium 104, yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.6 of that for the aqueous medium 104, still yet even more commonly the treated aqueous media 124 content for
- the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.1 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus- containing target materials is at least about 0.05 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.01 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.005 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus- containing target materials is at least about 0.001 of that for the aqueous medium 104, still yet even more commonly the treated aque
- the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 100,000 ppm, more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 10,000 ppm, even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 1,000 ppm, yet even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 100 ppm, still yet even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 10 ppm, still yet even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 1 ppm, still yet even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 100 ppb, still yet even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is
- Step 116 can include optional treatment steps.
- the treatment can comprise one or more of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes. More specifically, the treatment process can commonly comprise one of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing, more commonly any two of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, even more commonly any three of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, yet even more commonly any four of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, still yet even more commonly any five of clarifying, disinfecting, coagulating, aerating, fi ltering, separating solids and liquids
- the separated product may be subjected to suitable processes for removal of the phosphorus-containing target material or portions thereof from the target-laden rare earth composition to enable the rare earth to be recycled to step 112.
- Regeneration processes include, for example, desorption, oxidation, reduction, thermal processes, irradiation, and the like.
- cerium (III) may refer to cerium (+3), and cerium (+3) may refer to cerium (III).
- cerium (IV) may refer to cerium (+4), and cerium (+4) may refer to cerium (IV).
- Some embodiments include a method comprising contacting an aqueous medium having a phosphorus-containing target material with a rare earth-containing additive to remove at least a portion of the phosphorus-containing target material from the aqueous medium to form a treated aqueous medium.
- the phosphorus-containing target material comprises one or more of phosphoric acid, phospho-imide, phosphinate, phosphinite, non-fluoridated phosphonates, o-alkyl phosphoramideocyanidate, phosphonic acid, phosphinic acid, phosphonite, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, DNA backbone phosphate group, thiophosphate, thiophosphyoryl ester, thiophosphyoryl thioester, thiophosphyoryl amide, phosphatidylcholine, P-N amino compound, alklylated P-N amino compound, and P-N amino ester.
- the rare earth-containing additive is primarily in the form of cerium (IV), cerium (III) or a mixture of cerium (IV) and cerium (III).
- the rare earth-containing additive is water-soluble.
- the rare earth-containing additive is water-insoluble.
- the contacting step may further include: contacting the aqueous medium with an oxidizing agent to oxidize the
- the oxidized phosphorus-containing target material to form an oxidized phosphorus-containing target material, the oxidized phosphorus-containing target mater having one or both of a different structure and composition than the phosphorus-containing target material; and thereafter contacting, in the aqueous medium, the rare earth-containing additive with the oxidized phosphorus-containing target material to remove the oxidized phosphorus-containing target material.
- the contacting step may further include: contacting the aqueous medium with a reducing agent to reduce the phosphorus- containing target material to form a reduced phosphorus-containing target material, the reduced phosphorus-containing target material having one or both of a different structure and composition than the phosphorus-containing target material; and thereafter contacting, in the aqueous medium, the rare earth-containing additive with the reduced target phosphorus-containing target material to remove the reduced phosphorus-containing target material.
- the contacting step may further include: contacting the aqueous medium with a base and/or base equivalent to convert the phosphorus-containing target material to a primary species different from the phosphorus- containing target material; and thereafter contacting, in the aqueous medium, the rare earth-containing additive with the primary species to remove the primary species.
- the contacting step may further include: contacting the aqueous medium with an acid and/or acid equivalent to convert the phosphorus- containing target material to a primary species different from the phosphorus-containing target material; and thereafter contacting, in the aqueous medium, the rare earth-containing additive with the primary species to remove the primary species.
- the phosphorus-containing target material is selected from the group consisting essentially of phosphoric acid, phosphinate, phosphinite, non-fluoridated phosphonates, phosphonic acid, phosphinic acid, phosphonite, thiophosphate,
- the phosphorus-containing target material comprises phosphatidylcholine.
- the phosphorus-containing target material comprises one or more of a P-N amino compound, alklylated P-N amino compound, and P-N amino ester.
- the phosphorus-containing target material comprises one or more of a phospho-imide, o-alkyl
- the phosphorus-containing target material comprises a DNA backbone phosphate group.
- Some embodiments include a composition comprising an aqueous medium, a rare earth-containing additive, a phosphorus-containing target material, and a target-laden rare earth composition formed by the rare earth-containing additive and the phosphorus- containing target material.
- the phosphorus-containing target material comprises one or more of one or more of phosphoric acid, phospho-imide, phosphinate, phosphinite, non-fluoridated phosphonates, o-alkyl phosphoramideocyanidate, phosphonic acid, phosphinic acid, phosphonite, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, DNA backbone phosphate group, thiophosphate, thiophosphyoryl ester, thiophosphyoryl thioester, thiophosphyoryl amide, phosphatidylcholine, P-N amino compound, alklylated P-N amino compound, and P-N amino ester.
- the rare earth- containing additive preferably comprises primarily cerium and wherein the target-laden rare earth composition is a complex formed by the rare earth-containing additive and phosphorus-containing target material.
- the rare earth-containing additive may comprise primarily cerium and the target-laden rare earth composition may be formed by sorption of the phosphorus-containing target material by the rare earth-containing additive.
- the rare earth- containing additive may comprise primarily cerium and the target-laden rare earth composition may be formed by a chemical reaction between the phosphorus-containing target material and the rare earth-containing additive.
- the rare earth-containing additive is water-soluble.
- the rare earth-containing additive is water-insoluble.
- the phosphorus-containing target material may be selected from the group consisting essentially of phosphoric acid, phosphinate,
- the phosphorus-containing target material may comprise phosphatidylcholine.
- the phosphorus-containing target material may comprise one or more of a P-N amino compound, alklylated P-N amino compound, and P-N amino ester.
- the phosphorus-containing target material may comprise one or more of a phospho-imide, o-alkyl phosphoramideocyanidate, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, and thiophosphyoryl amide.
- the phosphorus- containing target material may comprise a DNA backbone phosphate group.
- ABS plastic filter housings (1.25 inches in diameter and 2.0 inches in length) were packed with ceric oxide (Ce0 2 ) that was prepared from the thermal decomposition of 99% cerium carbonate.
- the housings were sealed and attached to pumps for pumping an aqueous solution through the housings.
- the aqueous solutions were pumped through the material at flow rates of 50 and 75 ml/min.
- a gas chromatograph was used to measure the final content of the chemical contaminant.
- the chemical contaminants tested, their initial concentration in the aqueous solutions, and the percentage removed from solution are presented in Table 1.
- NSF general test water 2
- Influent Preparation The 30 mL stock solutions were prepared from solid or liquid reagents for each of the reagents in question. Influents were prepared from the stock solutions in 2.5 L batches for each reagent in question. 2.5 L of DI was measured out gravimetrically into a 4 L bottle. HEPES sodium buffer was added to the DI water followed by 2.5 mL of the stock solutions. The pH was adjusted to 7.5 ⁇ 0.25 using 1 N HC1 and 1 N NaOH.
- 500 mL of influent was measured out gravimetrically into four 500 mL bottles. Three bottles were labeled as a samples and the last was labeled as a control. The previously prepared media was poured into each sample bottle. Bottles were capped and sealed with electrical tape. Each bottle was then placed within a rolling container that could hold up to 10 bottles. The containers were then sealed with duct tape and placed on the rolling apparatus. Samples and controls were rolled for 24 hours. After 24 hours, the rolling containers were removed from the apparatus and the bottles were retrieved from the containers. A 10-45 mL sample of each solution was taken and filtered with a 0.2 ⁇ filter. Samples were analyzed either by a third party laboratory or with a HACH® colorimeter.
- Total phosphorus was analyzed with a HACH® DR/890 colorimeter according to the HACH® Method 8190 for total phosphorus as phosphate. Briefly, the sample is pretreated with sulfuric acid and persulfate under heat to hydrolyze organic and inorganic phosphorus to orthophosphate, and then reacted with molybdate in an acid medium to produce a phosphomolybdate complex. The sample is then reduced with ascorbic acid, resulting in a blue-colored compound, which is measured spectroscopically.
- cerium (IV) oxide The abilities of cerium (IV) oxide to remove phosphate and various combinations thereof
- organophosphorus target materials were also tested.
- Table 5 presents the removal capacities of cerium (IV) oxide for phosphate.
- This example demonstrates the successful removal of sulfate-containing compounds, halogenated compounds, carbonate-containing compounds, and phosphate- containing compounds, using a cerium (IV) dioxide powder.
- a cerium powder having a 400 ppb arsenic removal capacity, was contacted with various solutions containing arsenic (III) as arsenite and arsenic (V) as arsenate and elevated concentrations of the compounds that compete for the known binding affinity between arsenic and cerium.
- the competing compounds included sulfate ions, fluoride ions, chloride ions, carbonate ions, silicate ions, and phosphate ions at concentrations of approximately 500% of the corresponding NSF concentration for the ion.
- the cerium dioxide powder was further contacted with arsenic- contaminated distilled and NSF P231 "general test water 2" (“NSF”) water (Tables 3-5). Distilled water was used for the baseline measurement.
- NSF general test water 2
- Example 4 is a control having about 0.2 g of struvite, NFLiMgPC eFkO, particles mixed in about 0.1L of a 0.15 mol/L acidic ferric chloride, FeCl 3 , solution.
- the molar ratio of ferric chloride to struvite was about 1.0 and the initial pH of the solution was about pH 2.5.
- the initial pH of the control solution was low enough to dissolve the struvite without the presence of ferric chloride.
- a magnetic stir-bar was used to stir the control solution. After stirring for at least about 16 hours, the solids were filtered from the control solution. The filtered solids were analyzed by x-ray diffraction and the control solution was analyzed by ICP-MS. Final solution pH value was about pH 2.3. The results are summarized in Table 6.
- Example 4 shows that struvite can be more effectively removed with rare earth-containing compositions than with other removal materials such as ferric chloride.
- Example 5 shows that struvite can be more effectively removed with rare earth-containing compositions than with other removal materials such as ferric chloride.
- Phosphorus is commonly found in waste water systems as both reactive and non- reactive phosphorus.
- CeCl 3 To determine the abilities of CeCl 3 to remove both reactive and non- reactive phosphorus species, reactions were performed by the addition of CeCl 3 to both influent and effluent samples of waste water from a representative water treatment facility. Cerium (III) chloride was added in an equimolar ratio with the total phosphorus measured in solution, to ensure the ability of the reagent to react with most, if not all, phosphorus- containing compound(s) in solution. Following the addition of CeCl 3 , the samples were allowed to mix for 16 hours via magnetic stir before being filtered and analyzed for reactive and total phosphorus.
- Reactive phosphorus was measured (in terms of P) using a HACH® DR/890 Colorimeter.
- the analysis involves the reaction between orthophosphate and molybdate in an acidic medium to produce heteropolyacids which are then reduced using ascorbic acid. This reduced form of phosphomolybdate produces an intense molybdenum blue color which may be analyzed in accordance with Beer's Law.
- orthophosphate but first required a pretreatment to digest the compound and oxidize the non-reactive phosphorus to reactive orthophosphate. This digestion involved heating the solution to 150°C for 30 minutes in a sulfuric acid medium in the presence of potassium persulfate. The difference between reactive and total phosphorus is then calculated to determine the concentration of non-reactive phosphorus in solution.
- cerium (III) chloride demonstrated the ability to remove both reactive and non-reactive phosphorus with over 90% removal rate on an equimolar addition. This resulted in a removal capacity of approximately 176 mg P/ g theoretical rare earth oxie, which is near the theoretical removal rate of 180 mg P/g Ce0 2 . This demonstrates a high specificity of CeCl 3 for phosphorus, both reactive and non- reactive, in a waste water stream.
- the goal of this experiment was to remove organophosphate from solution by using CeCl 3 and Ce0 2
- the parameters for the experiment are as follows:
- trimetaphosphate Na 3 P 3 0g
- trimethyl phosphate ((CH 3 ) 3 PC"4)
- trimetaphosphate, trimethyl phosphate, tryelthylphophine and a solid compound of methyltriphenyl iodide to 4 2000 mL solutions of HEPES in D.I water and buffered to 7.5 pH with a (PO 4 ) 3" concentration of 12 mg/L.
- the methyltriphenyl iodide was added directly to the solution because of its low solubility.
- the solutions were then separated into 500 mL Nalgene® bottles. 500 mg of Ce0 2 from bucket 14 was weighed and added to 3 of the 4 500 mL Nalgene® bottles. They were then placed in the tumbler and tumbled for 24h. All 16 samples were then syringe filtered with 0.2 ⁇ filters.
- the samples were diluted by a factor of 4 since the test method used had a detection limit of 0.00 to 3.50 mg/L (PO 4 ) 3" .
- a digestion was then performed and tested using the HACH® colorimeter. The control for each compound was tested in triplicate.
- CeCl 3 batch reactions showed a definite removal of trisodium trimetaphosphate, removing trisodium trimetaphosphate from all three samples, with CeCl 3 removing an average of 67.76% trisodium trimetaphosphate from solution. The remaining
- Triethylphosphine oxide exhibited a loss in solution with the addition of Ce0 2 , with an average removal of 5.97%. These two organophosphate compounds exhibited removal in all of the three samples tested. Trimethyl phosphate and methyltriphenol phosphonium iodide showed no removal with the addition of Ce0 2 to the solutions.
- the goal of this experiment was to remove organophosphate from solution by using CeCl 3 and Ce0 2
- HEPES sodium salt D.I. sater, Ce0 2 (bucket 14), fenamiphos
- Methamidophos expressed a definite removal of organophosphates in the solution when using the Ce0 2 .
- Dicrotophos had an apparent removal of organophosphate removing only one of the three samples.
- Two of the four pesticides tested had to be heated to 75° C and fenamiphos stirred in solution for over 16h. There may have been a degeneration of the pesticide fenamiphos because the concentrations of the samples and the control came in much lower than the expected 3.5mg/L.
- the present disclosure includes providing devices and processes in the absence of items not depicted and/or described herein or in various embodiments, configurations, or aspects hereof, including in the absence of such items as may have been used in previous devices or processes, e.g., for improving performance, achieving ease and ⁇ or reducing cost of implementation.
Abstract
This disclosure relates generally to methods and rare earth-containing additives for removing inorganic and organic phosphorus-containing target materials.
Description
RARE EARTH REMOVAL OF PHOSPHORUS-CONTAINING MATERIALS
CROSS REFERENCE TO RELATED APPLICATIONS
The present application is a Continuation-in-Part of U.S. Application No.
13/356,574 filed January 23, 2012 and claims the benefit of U.S. Provisional Application Serial Nos.: 61/474,902, with a filing date of April 13, 2011, entitled "Process for Treating Waters and Water Handling Systems Using Rare Earth Metals";
61/475,155, with a filing date of April 13, 2011, entitled "Methods and Devices for Removing Oxyanions Using Reduction/Oxidation and Soluble and Insoluble Rare Earths";
61/539,780, with a filing date of September 27, 2011, entitled "Method for Removing Target Materials From a Fluid Stream using Rare Earths and/or a Rare Earth- Containing Additive";
61/476,667, with a filing date of April 18, 2011, entitled "Process for Treating Waters and Water Handling Systems Using Rare Earth Metals";
61/553,809, with a filing date of October 31, 2011, entitled "Process for Treating Waters and Water Handling Systems Using Rare Earth Metals";
61/558,887, with a filing date of November 11, 2011, entitled "Process for Treating Waters and Water Handling Systems Using Rare Earth Metals",
61/564,132, with a filing date of November 28, 2011, entitled "Process for
Treating Waters and Water Handling Systems Using Rare Earth Metals";
61/614,427, with a filing date of March 22, 2012, entitled "Rare Earth Removal of Hydrated and Hydroxyl Species";
61/613,857, with a filing date of March 21, 2012, entitled "Non-Metal Containing Oxyanion Removal from Waters Using Rare Earths";
61/538,634, with a filing date of September 23, 2011, entitled "Rare Earth Contaminant Removal in Pools, Hot Tubs, and Spas";
61/613,883, with a filing date of March 21, 2012, entitled "Rare Earth Removal of Phosphorus-Containing Materials";
61/614,418, with a filing date of March 22, 2012, entitled "Rare Earth Removal of
Phosphorus-Containing Materials";
each of which is incorporated in its entirety herein by this reference.
Cross reference is made to U.S. Patent Application Serial No. 13/244,092 filed September 23, 2011, entitled "PROCESS FOR TREATING WATERS AND WATER HANDLING SYSTEMS TO REMOVE SCALES AND REDUCE THE SCALING TENDENCY" having attorney docket no. 6062-89-3;
Cross reference is made to U.S. Patent Application Serial No. 13/410,081, filed
March 1, 2012, entitled "Contaminant Removal from Waters Using Rare Earths" having attorney docket no. 6062-89-1;
Cross reference is made to U.S. Patent Application Serial No. 13/356,581, filed January 23, 2012, entitled "Rare Earth Removal of Hydrated and Hydroxyl
Species"having attorney docket no. 6062-89-6;
Cross reference is made to U.S. Patent Application Serial No. 13/244,117, filed September 23, 2011, entitled "Particulate Cerium Dioxide and an In Situ Method for Making and Using the Same" having attorney docket no. 6062-89-4;
Cross reference is made to U.S. Patent Application Serial No.
filed March 28, 2012, entitled "NON-METAL-CONTAINING OXYANION REMOVAL FROM WATERS USING RARE EARTHS" having attorney docket no. 6062-89-2, which is incorporated herein by this reference in its entirety.
Cross reference is made to U.S. Patent Application Serial No.
filed March 28, 2012, entitled "RARE EARTH REMOVAL OF HYDRATED AND HYDROXYL SPECIES" having attorney docket no. 6062-89-6-CIP, which is incorporated herein by this reference in its entirety.
FIELD OF INVENTION
The present disclosure is related generally to removal of phosphorus-containing target materials from a fluid stream using a rare earth, more particularly to removal of phosphorus-containing materials from an aqueous stream using a rare earth.
BACKGROUND OF THE INVENTION
As fresh water resources grow increasingly scarce, water quality is rapidly becoming a major global concern. Various technologies have been used to remove contaminants from municipal, industrial, and recreational waters. Examples of such techniques include adsorption on high surface area materials, such as alumina and activated carbon, ion exchange with anion exchange resins, co-precipitation and electrodialysis. However, most technologies for contaminant removal are hindered by the
difficulty of removing problematic contaminants, more particularly the difficulty of removing phosphorus-containing contaminants, such as inorganic and/or inorganic phosphorus-containing materials.
SUMMARY OF THE INVENTION These and other needs are addressed by the various embodiments and
configurations of this disclosure. This disclosure relates generally to methods and rare earth-containing additives for removing inorganic and organic phosphorus-containing target materials.
In one embodiment, a method includes the step of contacting an aqueous medium comprising a phosphorus-containing target material with a rare earth-containing additive to remove at least a portion of the phosphorus-containing target material from the aqueous medium to form a treated aqueous medium. The phosphorus-containing target material includes one or more of phosphoric acid, phosphorus acid, polyphosphoric acids, inorganic phosphite, organic phosphite, phosphorus oxyhalide, phosphorus trihalide, phosphorus pentahalide, cyclic or non-cyclic phosphate salt, organophosphite,
chalcogenide, diphosphene, phospho-imide, phosphaalkene, phosphaalkyne, phosphine oxide, phosphinate, phosphine, phosphinite, non-fluoridated phosphonates, o-alkyl phosphoramideocyanidate, phosphonic acid, phosphinic acid, phospone oxide,
phosphonite, phosphonium salt, alkyl phosphonyldihalide, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, phosphorane, phosphono carboxylate, halosoman, amiton™, alkyl phosphite, DNA backbone phosphate group, DNA backbone phosphite groups thiophosphate and thiophosphyoryl ester, thioester and/or amide,
phosphatidylcholine, P-N amino compound, and an alklylated or protonated ester and salt thereof.
In one embodiment, a process includes the steps of:
(a) receiving an aqueous medium comprising both reactive and non-reactive phosphates; and
(b) contacting the aqueous medium with a (water soluble, partly soluble, and/or insoluble) rare earth-containing additive to remove most, if not all, of the reactive and non-reactive phosphates. In one configuration, the reactive phosphate is in the form of an anion, and the non-reactive phosphate in the form of an anion, cation, neutral species, and/or colloid or suspension. Before the contacting step, a portion of the non-reactive phosphate can be converted into reactive phosphate or vice versa.
The method and process can include further steps in other configurations.
In one configuration, the method includes the sub-steps of:
contacting the aqueous medium with an oxidizing agent with the phosphorus- containing target material to form an oxidized phosphorus-containing target material, the oxidized phosphorus-containing target material having one of a different structure and/or composition than the phosphorus-containing target material; and
thereafter contacting, in the aqueous medium, the rare earth-containing additive with the oxidized target material to remove the oxidized target material.
In one configuration, the method and process include the sub-steps of:
contacting the aqueous medium with a reducing agent to reduce the phosphorus- containing target material to form a reduced phosphorus-containing target material, the reduced phosphprous-containing target material having one of a different structure and/or composition than the phosphorus-containing target material; and
thereafter contacting, in the aqueous medium, the rare earth-containing additive with the reduced phosphorus-containing target material to remove the reduced
phosphorus-containing target material from the aqueous medium.
In one configuration, the method and process include the sub-steps of:
contacting the aqueous medium with a base and/or base equivalent to convert the phosphorus-containing target material to a primary species different from the phosphorus- containing target material; and
thereafter contacting, in the aqueous medium, the rare earth-containing additive with the primary species to remove the primary species.
In one configuration, the method and process include the sub-steps of:
contacting the aqueous medium with an acid and/or acid equivalent to convert the phosphorus-containing target material to a primary species different from the phosphorus- containing target material; and
thereafter contacting, in the aqueous medium, the rare earth-containing additive with the primary species to remove the primary species.
In an embodiment, a composition includes:
an aqueous medium,
a rare earth-containing additive,
a phosphorus-containing target material comprising one or more of phosphoric acid, phosphorus acid, inorganic phosphite, organic phosphite, phosphorus oxyhalide, phosphorus trihalide, phosphorus pentahalide, cyclic or non-cyclic phosphate salt,
organophosphite, chalcogenide, diphosphene, phospho-imide, phosphaalkene,
phosphaalkyne, phosphine oxide, phosphinate, phosphine, phosphinite, non-fluoridated phosphonates, o-alkyl phosphoramideocyanidate, phosphonic acid, phosphinic acid, phospone oxide, phosphonite, phosphonium salt, alkyl phosphonyldihalide, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, phosphorane, phosphono carboxylate, halosoman, amiton, alkyl phosphite, DNA backbone phosphate group, DNA backbone phosphite groups thiophosphate and thiophosphyoryl ester, thioester and/or amide, phosphatidylcholine, P-N amino compound, and an alklylated or protonated ester and salt thereof, and
a target-laden rare earth composition formed by the rare earth-containing additive and the phosphorus-containing target material.
In one application, the rare earth-containing additive comprises primarily cerium, and the target-laden rare earth composition is a complex formed by complexion of the rare earth-containing additive and target material.
In one application, the rare earth-containing additive comprises primarily cerium, and the target-laden rare earth composition is formed by sorption of the phosphorus- containing target material by the rare earth-containing additive.
In one application, the rare earth-containing additive comprises primarily cerium, and the target-laden rare earth composition is formed by a chemical reaction between the phosphorus-containing target material and the rare earth-containing additive.
In the above embodiments, the rare earth-containing additive can be water soluble, partially soluble, partially insoluble, and/or insoluble.
In some applications, the phosphorus-containing target material is selected from the group consisting essentially of phosphoric acid, phosphorus acid, phosphonic acid, phosphinic acid, and alklylated or protonated esters and salts thereof.
In some applications, the phosphorus-containing target material comprises an organic and/or inorganic phosphite.
In some applications, the phosphorus-containing target material comprises one or more of a phosphorus oxyhalide, phosphorus trihalide, alkyl phosphorus oxyhalide, phosphorus pentahalide, cyclic phosphate, chalcogenide, diphosphene, phospho-imide, phosphaalkene, and phosphaalkyne.
In some applications, the phosphorus-containing target material comprises one or more of a phosphine oxide, phosphinate, phosphine, phosphinite, non-fluoridated phosphonate, o-alkyl phosphoramideocyanidate, phospone oxide, phosphonite,
phosphonium salt, alkyl phosphonyldihalide, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, phosphorane, phosphono carboxylate, halosoman, amiton, and alkyl phosphite.
In one embodiment, a process includes the steps of:
(a) receiving an aqueous medium comprising non-reactive phosphates; and
(b) contacting the aqueous medium with a (water soluble, partly soluble, and/or insoluble) rare earth-containing additive to remove most, if not all, of the non-reactive phosphates. In one configuration, the non-reactive phosphate is in the form of an anion, cation, neutral species, and/or colloid or suspension. In some configurations, before the contacting step, a portion of the anqueous media may contain reactive phosphates, the reacative phosphates being converted to non-reactive phosphates prior to the contacting step. Preferably, the reactive phosphate is in the form of an anion and may be in the form of colloid or suspension.
In one embodiment, a process includes the steps of:
(a) receiving an aqueous medium comprising reactive phosphates; and
(b) contacting the aqueous medium with a (water soluble, partly soluble, and/or insoluble) rare earth-containing additive to remove most, if not all, of the reactive phosphates. In some configurations, before the contacting step, a portion of the anqueous media may contain non-reactive phosphates, the non-reacative phosphates being converted to reactive phosphates prior to the contacting step. Preferably, the non-reactive phosphate is in the form of an anion, cation, neutral species, and/or colloid or suspension.
In some applications, the phosphorus-containing target material comprises one or more of a DNA backbone phosphate group, DNA backbone phosphite group,
thiophosphate and thiophosphyoryl ester, thioester and amide, phosphatidylcholine, and P- N amino compound.
The disclosure can have a number of advantages. For example, the rare earth- containing composition can remove effectively a large number of phosphorus-containing target materials, whether in the form of dissolved or undissolved species. As an illustration, the rare earth-containing composition can remove organophosphates and organophosphites. The pH and/or Eh can be adjusted to produce a selected primary target material species, which is removed more effectively by the rare earth composition than other target material species. High levels of removal of the primary target material can be therefore realized.
These and other advantages will be apparent from the disclosure.
As used herein, the term "a" or "an" entity refers to one or more of that entity. As such, the terms "a" (or "an"), "one or more" and "at least one" can be used
interchangeably herein. It is also to be noted that the terms "comprising", "including", and "having" can be used interchangeably.
"Absorption" refers to the penetration of one substance into the inner structure of another substance, as distinguished from adsorption.
"Adsorption" refers to the adherence of atoms, ions, molecules, polyatomic ions, or other substances to the surface of another substance, called the adsorbent. Typically, the attractive force for adsorption can be in the form of a bond and/or force, such as covalent bonds, metallic bonds, coordination bonds, ionic bonds, hydrogen bonds, electrostatic forces (e.g., van der Waal's forces and London's forces), and the like.
"At least one", "one or more", and "and/or" are open-ended expressions that are both conjunctive and disjunctive in operation. For example, each of the expressions "at least one of A, B and C", "at least one of A, B, or C", "one or more of A, B, and C", "one or more of A, B, or C" and "A, B, and/or C" means A alone, B alone, C alone, A and B together, A and C together, B and C together, or A, B and C together.
The term "water" refers to any aqueous stream. The water may originate from any aqueous stream may be derived from any natural and/or industrial source. Non-limiting examples of such aqueous streams and/or waters are drinking waters, potable waters, recreational waters, waters derived from manufacturing processes, wastewaters, pool waters, spa waters, cooling waters, boiler waters, process waters, municipal waters, sewage waters, agricultural waters, ground waters, power plant waters, remediation waters, co-mingled water and combinations thereof.
The terms "agglomerate" and "aggregate" refer to a composition formed by gathering one or more materials into a mass.
The term "amide" generally refers to an organic material containing the functional group consisting of a carbonyl group (R-C=0) linked to a nitrogen atom (N).
The term "amino acid" are organic materials containing an amine group, a carboxylic acid group, and a side-chain that is specific to each amino acid. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen.
A "binder" generally refers to one or more substances that bind together a material being agglomerated. Binders are typically solids, semi-solids, or liquids. Non-limiting examples of binders are polymeric materials, tar, pitch, asphalt, wax, cement water, solutions, dispersions, powders, silicates, gels, oils, alcohols, clays, starch, silicates, acids,
molasses, lime, lignosulphonate oils, hydrocarbons, glycerin, stearate, or combinations thereof. The binder may or may not chemically react with the material being
agglomerated. Non-liming examples of chemical reactions include hydration/dehydration, metal ion reactions, precipitation/gelation reactions, and surface charge modification.
A "carbon-containing radical", such as R1, R2, R3 or such, generally refers to one or more of: an alkyl group; an aryl group; a Ci to C25 straight-chain, branched aliphatic hydrocarbon radical; a C5 to C30 cycloaliphatic hydrocarbon radical; a C6 to C30 aromatic hydrocarbon radical; a C7 to C40 alkylaryl radical; a C2 to C25 linear or branched aliphatic hydrocarbon radical having interruption by one or more heteroatoms, such as, oxygen, nitrogen or sulfur; a C2 to C25 linear or branced aliphatic hydrocarbon radical having interruption by one or more functionalities selected from the group consisting essentially of a carbonyl (-C(=0)-), an ester (-C(=0)0-), an amide (-C(=O)NH0_2-), a C2 to C25 linear or branced aliphatic hydrocarbon radical functionalized with one or more of CI, Br, F, I, NH(lor 2), OH, and SH; a C5 to C30 cycloaliphatic hydrocarbon radical functionalized with one or more of CI, Br, F, I, NH(lor 2), OH, and SH; and a C7 to C40 alkylaryl radical functionalized with one or more of CI, Br, F, I, NH(0, 1 or 2), OH, and SH.
The term "chalcogenide" generally refers to a phosphorus-containing compound having the general formula R3PE, wherein E = S, Se or Te.
The term "clarification" or "clarify" refers to the removal of suspended and, possibly, colloidal solids by gravitational settling techniques.
The term "coagulation" refers to the destabilization of colloids by neutralizing the forces that keep colloidal materials suspended. Cationic coagulants provide positive electrical charge to reduce the negative charge (zeta potential) of the colloids. The colloids thereby form larger particles (known as floes).
The term "composition" generally refers to one or more chemical units composed of one or more atoms, such as a molecule, polyatomic ion, chemical compound, coordination complex, coordination compound, and the like. As will be appreciated, a composition can be held together by various types of bonds and/or forces, such as covalent bonds, metallic bonds, coordination bonds, ionic bonds, hydrogen bonds, electrostatic forces (e.g., van der Waal's forces and London's forces), and the like.
"Chemical species" or "species" are atoms, elements, molecules, molecular fragments, ions, compounds, and other chemical structures. "Chemical transformation"
refers to process where at least some of a material has had its chemical composition transformed by a chemical reaction.
A "chemical transformation" differs from "a physical transformation". A physical transformation refers to a process where the chemical composition has not been chemically transformed but a physical property, such as size or shape, has been transformed.
The term "contained within the water" generally refers to materials suspended and/or dissolved within the water. Water is typically a solvent for dissolved materials and water-soluble material. Furthermore, water is typically not a solvent for water-insoluble materials. Suspended materials are substantially insoluble in water and dissolved materials are substantially soluble in water. The suspended materials have a particle size.
"De-toxify" or "de-toxification" includes rendering a phosphorus-containing target material non-toxic or non-harmful to a living organism, such as, for example, a human or other animal. The phosphorus-containing target material may be rendered non-toxic by converting the phosphorus-containing target material into a non-toxic or non-harmful form and/or species.
The term "digest" or "digestion" refers to the use of microorganisms, particularly bacteria, to digest target materials. This is commonly established by mixing forcefully contaminated water with bacteria and molecularly oxygen.
The term "diphosphene" generally refers to a phosphorus-containing compound having the general structure R2P2.
The term "disinfect" or "disinfecting" refers to the use of an antimicrobial agent to kill or inhibit the growth of microorganisms, such as bacteria, fungi, protozoans, and viruses. Common antimicrobial agents include, oxidants, reductants, alchohols, aldehydes, halogens, phenolics, quaternary ammonium compounds, silver, copper, ultraviolet light, and other materials.
The term "flocculation" refers to a process using a flocculant, which is typically a polymer, to form a bridge between floes and bind the particles into large agglomerates or clumps. Bridging occurs when segments of the polymer chain adsorb on different particles and help particles aggregate.
The term "fluid" refers to a liquid, gas or both.
A "halogen" is a nonmetal element from Group 17 IUPAC Style (formerly: VII,
VIIA) of the periodic table, comprising fluorine (F), chlorine (CI), bromine (Br), iodine
(I), and astatine (At). The artificially created element 117, provisionally referred to by the
systematic name ununseptium, may also be a halogen. A "halide compound" is a compound having as one part of the compound at least one halogen atom and the other part the compound is an element or radical that is less electronegative (or more electropositive) than the halogen. The halide compound is typically a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides having a halide anion. A halide anion is a halogen atom bearing a negative charge. The halide anions are fluoride (F ), chloride (CI ), bromide (Br ), iodide (T) and astatide (At ).
The term "phospho-imide" generally refers to a phosphorus-containing compound having the general formula R3PNR' .
The term "inorganic material" generally refers to a chemical compound or other species that is not organic.
The term "insoluble" refers to materials that are intended to be and/or remain as solids in water. Insoluble materials are able to be retained in a device, such as a column, or be readily recovered from a batch reaction using physical means, such as filtration. Insoluble materials should be capable of prolonged exposure to water, over weeks or months, with little loss of mass. Typically, a little loss of mass refers to less than about 5% mass loss of the insoluble material after a prolonged exposure to water.
An "ion" generally refers to an atom or group of atoms having a charge. The charge on the ion may be negative or positive.
"Organic carbons" or "organic material" generally refer to any compound of carbon except such binary compounds as carbon oxides, the carbides, carbon disulfide, etc.; such ternary compounds as the metallic cyanides, metallic carbonyls, phosgene, carbonyl sulfide, etc.; and the metallic carbonates, such as alkali and alkaline earth metal carbonates. Exemplary organic carbons include humic acid, tannins, and tannic acid, polymeric materials, alcohols, carbonyls, carboxylic acids, oxalates, amino acids, hydrocarbons, and mixtures thereof. In some embodiments, the phosphorus-containing target material is an organic material as defined herein. An alcohol is any organic compound in which a hydroxyl functional group (-OH) is bound to a carbon atom, the carbon atom is usually connected to other carbon or hydrogen atoms. Examples of alcohols include acyclic alcohols, isopropyl alcohol, ethanol, methanol, pentanol, polyhydric alcohols, unsaturated aliphatic alcohols, and alicyclic alcohols, and the like.
The carbonyl group is a functional group consisting of a carbonyl (RR'C=0) (where R and
R' vary independently and may be H or any organic radical, R and R' may be the same or differ). Examples of organic compounds containing a carbonyl group include aldehydes,
ketones, esters, amides, enones, acyl halides, acid anhydrides, urea, and carbamates and derivatives thereof, and the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Preferably, the carbonyl group comprises a carboxylic acid group, which has the formula -C(=0)OH, usually written as -COOH or -C02H. Examples of organic compounds containing a carboxyl group include carboxylic acid (R-COOH) and salts and esters (or carboxylates) and other derivatives thereof. It can be appreciated that organic compounds include alcohols, carbonyls, and carboxylic acids, where one or more oxygens are, respectively, replaced with sulfur, selenium and/or tellurium.
The term "organophorous" generally refers to a phosphorus-containing compound containing one or more carbon-phosphorus bonds. Non-limiting examples of
organophosphorus compounds include: phosphate and thiophosphyoryl esters, thioesters and amides (such as, mono-, di- and tri- phosphate esters, thioesters and amides
(P(=Y)(OH)2(XR), P(=Y)(OH)(XR)2, and P(=Y)(XR)3, where X can be one of oxygen, sulfur or nitrogen, Y can be one of oxygen, sulfur, selenium or tellurium and R can be a Ci-Ci2 alkyl or aryl group), trimeyl phosphate, triethyl phosphate, tripropyl phosphate, tributyl phosphate, diazinon, phosphatidylcholine, malathion, cyclophosphamide, triphyenylphosphate and dithiophosphate); phosphonic and phosphinic acids and their esters (such as, phosphonate esters (R1P(=0)(OR2)(OR3) and R1R2P(=0)(OR3), where R may be hydrogen or a C -Cn alkyl or aryl group), dimethyl phosphinic acid, diethyl phosphinic acid, dipropyl phosphinic acid, dibutyl phosphinic acid, dipentyl phosphinic acid, dihexyl phosphinic acid, methyl methylphosphonate, methyl ethylphosphonate, methyl propylphosphonate, ethyl methylphosphonate, ethyl ethylphosphonate, ethyl propylphsophonate, ethyl butylphosphonate, glyphosates, bisphosphates, and phosphinates (R2P(=0)(OR')); phosphine oxides (such as triethylphosphine oxide, phosphine oxides (R3P=0) and related P-N amino compounds, phospho-imides (R3PNR'), chalcogenides (such as, R3PE, where E=S, Se, or Te); phosphonium salts and phosphoranes (such as, PP +, and ylides), phosphites (such as, P(OR)3), phosphonites (such as, P(OR)2R'),
1 2 3 1 2 3 phosphinites (such as, P(OR)R'2), phosphines (such as, PR R R , where R , R and R can be one of H, C -Cn alkyl, C -Cn aryl, C3-Cg cyclic, amino, ether, thio, and hydroxyl and where R1, R2 and R3 can be the same or differ), tris(dimethylamino)phosphine, a phosonocarboxylic acid or one of its ester, carboxylate or alkali and alkaline earth phosphonocarboxylates (such as X02P(=0)(02R) where X is one or more of sodium, potassium, cesium, magnesium, calcium, strontium or barium and where R is a
carboxylate derived from a C1-C12 carboxylic acid), phosphonoformate and its esters and salts, and mixtures thereof. Other examples include thiophosphate and thiophosphyoryl esters, thioesters and amides (such as, mono-, di- and tri- phosphate esters, thioesters and amides (P(=Y)(OH)2(XR), P(=Y)(OH)(XR)2, and P(=Y)(XR)3,where X can be one of oxygen, sulfur or nitrogen, Y can be one of oxygen and sulfur and R can be a
alkyl or aryl group).
The term "organophosphate" generally refers to an ester of phosphoric acid.
The term "organophosphite" generally refers to an ester of phosphorus acid.
The term "oxidizing agent" refers to one or both of a chemical substance and physical process that transfers and/or assists in removal of one or more electrons from a substance. The substance having the one or more electrons being removed is oxidized. In regards to the physical process, the physical process may removal and/or may assist in the removal of one or more electrons from the substance being oxidized. For example, the substance to be oxidized can be oxidized by electromagnetic energy when the interaction of the electromagnetic energy with the substance be oxidized is sufficient to substantially remove one or more electrons from the substance. On the other hand, the interaction of the electromagnetic energy with the substance being oxidized may not be sufficient to remove one or more electrons, but may be enough to excite electrons to higher energy state, were the electron in the excited state can be more easily removed by one or more of a chemical substance, thermal energy, or such.
The term "phosphaalkene" generally refers to a phosphorus-containing compound having the general structure R2C=PR.
The term "phosphaalkynes" generally refers to a phosphorus-containing compound having the general structure RC≡P.
The term "phosphate compound" generally refers to phosphorus-containing compounds formed from a P04 3~ (phosphate) or a related anion or group, such as
OPO(OH)2, structural unit alone or linked together by sharing oxygen atoms to form a linear chain or cyclic ring structure. Common phosphates are salts or esters of phosphoric acid or a tertiary salt of orthophosphoric acid. Non- limiting examples of phosphates are:
P04 3" (phosphate); P30io5~(triphosphate); Pn03n (n+2)" (polyphosphate); P30c>3~ (cyclic trimethaphosphate); adenosine diphosphoric acid (ADPH); guanosine 5 '-diphosphate 3'- dipphosphate (ppGpp); trimetaphosphate; hexametaphosphate; HP03 2" (phosphate); or one or more of their salts, acids, esters, anionic and organophosphorous forms; and mixtures thereof. Other examples include DNA backbone phosphate groups.
The term "phosphinic acid" generally refers to a phosphorus-containing compound having the general structure C-P=0(OH)2 or C-P=0(OR)2.
The term "phosphine oxide" generally refers to a compound have the general structure R3P=0 with formal oxidation state V. An exemplary phosphine oxide includes for triphenylphosphine oxide.
The term "phosphinate" generally refers to a phosphorus-containing compound having the general formula R2P(=0)(OR') or R2P(OR)0. Exemplary phosphinates include glufosinate.
The term "phosphine" generally refers to a phosphorus-containing compound including PH3 as a structure unit, where a hydrogen can be replaced by a carbon radical. Exemplary phosphines include trimethylphosphine and diphosphines PR1R2R3, where R1, R2 and R3 can be one of H, C1-C12 alkyl, C1-C12 aryl, C3-C8 cyclic, amino, ether, thio, and hydroxyl and where R1, R2 and R3 can be the same or differ).
The term "phosphinite" generally refers to a phosphorus-containing compound having the general formula P(OR)R'2 or R2(RO)P.
The term "phosphite compound" generally refers to a phosphorus-containing compound formed from a P02 3" (phosphite) structural unit alone or linked together by sharing oxygen atoms to form a linear chain or cyclic ring structure. Common phosphites are salts or esters of phosphorus acid and include H2P205 2"(pyrophosphites such as Phenolphthalein bisphosphate pyridine salt) and H2P02 " (hypophosphite or phosphinate). Phosphite esters have the general structure P(OR)3 with oxidation state +3. "Phosphite" compounds include not only phosphites but also phosphonites and phosphinites.
The term "phosphonate" generally refers to an ester of phosphonic acid of the general form RP(=0)(OR')2 or RP(RO)20. Exemplary phosponates include glyphospate, biphosponate, Sarin, dimethylmethylphosponate, and aminophophonate. Phosphonate esters include phosphonate esters (R1P(=0)(OR2)(OR3) and R1R2P(=0)(OR3), where R may be hydrogen or a
alkyl or aryl group).
The term "phosphonic acid and esters thereof generally refers to a phosphorus- containing compound having the general structure C-P=0(OH)2 or C-P=0(OR)2 and R- P=0(OH)2. An example of a phosphonic acid ester is methylethyl phosphonate.
The term "phosphone oxide" generally refers to a phosphorus-containing compound having the general formula R3P=0.
The term "phosphonite" generally refers to a phosphorus-containing compound having the general formula (P(OR)2R) or R(RO)2P.
The term "phosphonium salt" generally refers to a phosphorus-containing compound having the general formula [ΡΡ ]Χ~ with formal oxidation state V. "X" can be any anion. Non-limiting examples of X" are chloride, bromide, iodide, perchlorate, acetate, tetrfluoroborate, sulfate, nitrate, nitrite, to name a few. Exemplary phosponium salts include is tetrakis(hydroxymethyl)phosphonium chloride, [P(CH20H)4]C1.
The term "phosphorane" generally refers to a phosphorus-containing compound having the general formula R3PR2.
The term "phosphono carboxylate" generally refers to a compound having a carboxylate functional group, such as X02P(=0)(02R) where X is one or more of sodium, potassium, cesium, magnesium, calcium, strontium or barium and where R is a
carboxylate derived from a C1-C12 carboxylic acid).
The term "polish" refers to any process, such as filtration, to remove small (usually microscopic) particulate material or very small low concentrations of dissolved target material from water.
The terms "pore volume" and "pore size", respectively, refer to pore volume and pore size determinations made by any suite measure method. Preferably, the pore size and pore volume are determined by any suitable Barret- Joyner-Halenda method for determining pore size and volume. Furthermore, it can be appreciated that as used herein pore size and pore diameter can used interchangeably.
"Precipitation" generally refers to the removal of a dissolved target material in the form of a target-laden rare earth composition, preferably an insoluble target-laden rare earth composition. The target-laden rare earth composition can comprise a target-laden cerium composition, a target-laden cerium (IV) composition, a target-laden cerium ( M l ) composition, a target-laden lanthanium composition comprising a rare earth, a target-laden composition comprising a rare earth other than cerium or lanthium, or a combination thereof. Typically, the target-laden rare earth composition comprises a target-laden rare earth composition. For example, "precipitation" includes processes, such as adsorption and absorption of the phosphorus-containing target material by one or more of the cerium (IV) composition, the rare earth-containing additive, or a rare earth other than cerium (IV). The target-laden rare earth composition can comprise a +3 rare earth, such as cerium (III), lanthanum (III) or other lanthanoid having a +3 oxidation state.
The symbols "R" and "R"', in a chemical formula, generally refer to a carbon- containing radical.
A "radical" generally refers to an atom or group of atoms that are joined together in some particular spatial structure and that commonly take part in chemical reactions as a single unit. A radical may have a net positive or negative charge or be neutral. A radical is an atom, molecule, or ion that has one or more unpaired electrons.
"Rare earth" refers to one or more of yttrium, scandium, lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium erbium, thulium, ytterbium, and lutetium. As will be appreciated, lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium erbium, thulium, ytterbium, and lutetium are known as lanthanoids.
The terms "rare earth", "rare earth-containing composition", "rare earth-containing additive" and "rare earth-containing particle" refer both to singular and plural forms of the terms. By way of example, the term "rare earth" refers to a single rare earth and/or combination and/or mixture of rare earths and the term "rare earth-containing
composition" refers to a single composition comprising a single rare earth and/or a mixture of differing rare earth-containing compositions containing one or more rare earths and/or a single composition containing one or more rare earths. The terms "rare earth- containing additive" and "rare earth-containing particle" are additives or particles including a single composition comprising a single rare earth and/or a mixture of differing rare earth-containing compositions containing one or more rare earths and/or a single composition containing one or more rare earths. The term "processed rare earth composition" refers not only to any composition containing a rare earth other than non- compositionally altered rare earth-containing minerals. In other words, as used herein "processed rare earth-containing composition" excludes comminuted naturally occurring rare earth-containing minerals. However, as used herein "processed rare earth-containing composition" includes a rare earth-containing mineral where one or both of the chemical composition and chemical structure of the rare earth-containing portion of the mineral has been compositionally altered. More specifically, a comminuted naturally occurring bastnasite would not be considered a processed rare earth-containing composition and/or processed rare earth-containing additive. However, a synthetically prepared bastnasite or a rare earth-containing composition prepared by a chemical transformation of naturally occurring bastnasite would be considered a processed rare earth-containing composition and/or processed rare earth-containing additive. The processed rare earth and/or rare- containing composition and/or additive are, in one application, not a naturally occurring mineral but synthetically manufactured. Exemplary naturally occurring rare earth-
containing minerals include bastnasite (a carbonate-fluoride mineral) and monazite.
Other naturally occurring rare earth-containing minerals include aeschynite, allanite, apatite, britholite, brockite, cerite, fluorcerite, fluorite, gadolinite, parisite, stillwellite, synchisite, titanite, xenotime, zircon, and zirconolite. Exemplary uranium minerals include uraninite (U02), pitchblende (a mixed oxide, usually UsOg), brannerite (a complex oxide of uranium, rare-earths, iron and titanium), coffinite (uranium silicate), carnotite, autunite, davidite, gummite, torbernite and uranophane. In one formulation, the rare earth- containing composition is substantially free of one or more elements in Group 1, 2, 4-15, or 17 of the Periodic Table, a radioactive species, such as uranium, sulfur, selenium, tellurium, and polonium.
The term "reducing agent", "reductant" or "reducer" refers to an element or compound that donates one or more electrons to another species or agent this is reduced. In the reducing process, the reducing agent is oxidized and the other species, which accepts the one or more electrons, is reduced.
The terminology "removal", "remove" or "removing" includes the sorption, precipitation, conversion, detoxification, deactivation, and/or combination thereof of a target material contained in a water and/or water handling system.
The term "soluble" refers to a material that readily dissolves in a fluid, such as water or other solvent. For purposes of this disclosure, it is anticipated that the dissolution of a soluble material would necessarily occur on a time scale of minutes rather than days. For the material to be considered to be soluble, it is necessary that the
material/composition has a significant solubility in the fluid such that upwards of about 5 g of the material will dissolve in about one liter of the fluid and be stable in the fluid.
The term "sorb" refers to adsorption, absorption or both adsorption and absorption. The term "suspension" refers to a heterogeneous mixture of a solid, typically in the form of particulates dispersed in a liquid. In a suspension, the solid particulates are in the form of a discontinuous phase dispersed in a continuous liquid phase. The term "colloid" refers to a suspension comprising solid particulates that typically do not settle-out from the continuous liquid phase due to gravitational forces. A "colloid" typically generally refers to a system having finely divided particles ranging from about 10 to 10,000 angstroms in size, dispersed within a continuous medium. As used hereinafter, the terms "suspension", "colloid" or "slurry" will be used interchangeably to refer to one or more materials dispersed and/or suspended in a continuous liquid phase. The terms "particle", "particles",
"particulate", and "particulates" will be used interchangeably and the use of one over the other does not connotate a chemical and/or physical difference.
The term "surface area" refers to surface area of a material and/or substance determined by any suitable surface area measurement method. Preferably, the surface area is determined by any suitable Brunauer-Emmett-Teller (BET) analysis technique for determining the specific area of a material and/or substance.
The term "thiophosphate" (or phosphorothioate) generally refers to a family of compounds and anions with the general chemical formula PS4_xOx 3~ (x = 0, 1, 2, or 3). The state of protonation is usually not specified. They could be bound to as many as three protons for the neutral H3PS4-xOx species. Two protons correspond to the related monoanions, and one proton for the dianions.
The term "water handling system" generally refers to any system containing, conveying, manipulating, physically transforming, chemically processing, mechanically processing, purifying, generating and/or forming the aqueous composition, treating, mixing and/or co-mingling the aqueous composition with one or more other waters and any combination thereof.
A "water handling system component" generally refers to one or more unit operations and/or pieces of equipment that process and/or treat water (such as a holding tank, reactor, purifier, treatment vessel or unit, mixing vessel or element, wash circuit, precipitation vessel, separation vessel or unit, settling tank or vessel, reservoir, pump, aerator, cooling tower, heat exchanger, valve, boiler, filtration device, solid liquid and/or gas liquid separator, nozzle, tender, and such), conduits interconnecting the unit operations and/or equipment (such as piping, hoses, channels, aqua-ducts, ditches, and such) and the water conveyed by the conduits. The water handling system components and conduits are in fluid communication.
The terms "water" and "water handling system" will be used interchangeably. That is, the term "water" may used to refer to "a water handling system" and the term "water handling system" may be used to refer to the term "water".
The preceding is a simplified summary of the disclosure to provide an
understanding of some aspects of the disclosure. This summary is neither an extensive nor exhaustive overview of the disclosure and its various embodiments. It is intended neither to identify key or critical elements of the disclosure nor to delineate the scope of the disclosure but to present selected concepts of the disclosure in a simplified form as an introduction to the more detailed description presented below. As will be appreciated,
other embodiments of the disclosure are possible utilizing, alone or in combination, one or more of the features set forth above or described in detail below.
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings, which are incorporated in and constitute a part of the specification, illustrates embodiments of the disclosure and together with the general description of the disclosure given above and the detailed description given below, serve to explain the principles of the disclosure.
Fig. 1 depicts a water handling system and method according to an embodiment;
Figs. 2A-2E are prior art Pourbaix or Eh-pH diagrams for phosphoric acid constructed using differing thermodynamic databases, the thermodynamic database constitutes are denoted in the respective Figs; and
Fig. 3 is a plot of arsenic capacity (mg As/g Ce02) against various solution compositions.
DETAILED DESCRIPTION
General Overview
As illustrated by Fig. 1, the present disclosure is directed to removal from and/or detoxitication of water, a water-handling system or other aqueous media, of a phosphorus- containing target material. The phosphorus-containing target material may be in the form of a pollutant or contaminant. The phosphorus-containing target material is removed and/or detoxified by a rare earth-containing composition, additive or particle. Preferably, the rare earth-containing composition, additive or particle is a processed rare earth- containing additive or particle. In some embodiments, the phosphorus-containing target material is removed and/or detoxified by forming a target-laden rare earth composition. The target-laden rare earth composition comprises a rare earth and one or more of the phosphorus-containing target material or a portion of the phosphorus-containing.
The phosphorus-containing target material is commonly one or more of phosphoric acid, phosphorus acid, phosphonic acid, or a salt or ester or mixture thereof.
Rare Earth-Containing Composition, Additive and/or Particle
The rare earth-containing composition, additive and/or particles may be water- soluble, water-insoluble, a combination of water-soluble and/or water-insoluble rare earth- containing compositions, additives and/or particles, a partially water-soluble rare earth-
containing composition, additive and/or particles, and/or a partially water-insoluble rare earth-containing composition, additive and/or particles.
Commonly, the rare earth-containing composition, additive and/or particles comprise cerium, in the form of a cerium-containing compound and/or a dissociated ionic form of cerium, lanthanum, in the form of a lanthanum-containing compound and/or dissociated ionic form of lanthanum, or a mixture thereof. More common rare earth- containing compositions, additives and particles are cerium (IV) oxides, cerium (III) oxides, cerium (IV) salts, cerium (III) salts, lanthanum (III) oxides, lanthanum (III) salts, or mixtures and/or combinations thereof.
The rare earth-containing composition, additive and/or particles may contain one or more rare earths, and be in any suitable form, such as a free-flowing powder, a liquid formulation, or other form. Examples of rare earth-containing compositions, additives, and particles include cerium (III) oxides, cerium (IV) oxides, eerie (IV) salts (such as eerie chloride, eerie bromide, eerie iodide, eerie sulfate, eerie nitrate, eerie chlorate, and eerie oxalate), cerium (III) salts (such as cerous chloride, cerous bromide, cerous iodide, cerous sulfate, cerous nitrate, cerous chlorate, and cerous oxalate), lanthanum (III) oxides, lanthanum (III) salts (such as lanthanum chloride, lanthanum bromide, lanthanum iodide, lanthanum chlorate, lanthanum sulfate, lanthanum oxalate, and lanthanum nitrate), and mixtures thereof.
The rare earth and/or rare earth-containing composition in the rare earth-containing additive can be rare earths in elemental, ionic or compounded forms. The rare earth and/or rare earth-containing composition can be contained in a fluid, such as water, or in the form of nanoparticles, particles larger than nanoparticles, agglomerates, or aggregates or combinations and/or mixtures thereof. The rare earth and/or rare earth-containing composition can be supported or unsupported. The rare earth and/or rare earth-containing composition can comprise one or more rare earths. The rare earths may be of the same or different valence and/or oxidation states and/or numbers. The rare earths can be a mixture of different rare earths, such as two or more of yttrium, scandium, cerium, lanthanum, praseodymium, and neodymium.
The rare earth and/or rare earth-containing composition is, in one application, a processed rare earth-containing composition and does not include, or is substantially free of, a naturally occurring and/or derived mineral. In one formulation, the rare earth and/or rare earth-containing composition is substantially free of one or more elements in Group 1 ,
2, 4-15, or 17 of the Periodic Table, and is substantially free of a radioactive species, such as uranium, sulfur, selenium, tellurium, and polonium.
In some formulations, the rare earth-containing composition comprises one or more rare earths. While not wanting to be limited by example, the rare earth-containing composition can comprise a first rare earth and a second rare earth. The first and second rare earths may have the same or differing atomic numbers. In some formulations, the first rare earth comprises cerium (III) and the second rare earth comprises a rare earth other than cerium (III). The rare earth other than cerium (III) can be one or more trivalent rare earths, cerium (IV), or any other rare other than trivalent cerium. For example, a mixture of rare earth-containing compositions can comprise a first rare earth having a +3 oxidation state and a second rare earth having a +4 oxidation state. In some embodiments, the first and second rare earths are the same and comprise cerium. More specifically, the first rare earth comprises cerium (III) and the second rare earth comprises cerium (IV). Preferably, the cerium is primarily in the form of a water-soluble cerium (III) salt, with the remaining cerium being present as cerium oxide, a substantially water insoluble cerium composition.
In one formulation, the cerium is primarily in the form of a dissociated cerium (III) salt, with the remaining cerium being present as cerium (IV) oxide. For rare earth- containing compositions having a mixture of +3 and +4 oxidations states commonly at least some of the rare earth has a +3 oxidation sate, more commonly at least most of the rare earth has a +3 oxidation state, more commonly at least about 75 wt% of the rare earth has a +3 oxidation state, even more commonly at least about 90 wt% of the rare earth has a +3 oxidation state, and yet even more commonly at least about 98 wt% of the rare earth has a +3 oxidation state. The rare earth-containing composition commonly includes at least about 1 ppm, more commonly at least about 10 ppm, and even more commonly at least about 100 ppm cerium (IV) oxide. While in some embodiments, the rare earth- containing composition includes at least about 0.0001 wt% cerium (IV), preferably at least about 0.001 wt% cerium (IV) and even more preferably at least about 0.01 wt% cerium (IV) calculated as cerium oxide. Moreover, in some embodiments, the rare earth composition-containing commonly has at least about 20,000 ppm cerium (III), more commonly at least about 100,000 ppm cerium (III) and even more commonly at least about 250,000 ppm cerium (III).
In some formulations, the molar ratio of cerium (III) to cerium (IV) is about 1 to about 1X10"6, more commonly is about 1 to about 1X10"5, even more commonly is about 1
to about 1X10~4, yet even more commonly is about 1 to about 1X10~3, still yet even more commonly is about 1 to about 1X10~2, still yet even more commonly is about 1 to about 1X10"1, or still yet even more commonly is about 1 to about 1. Moreover, in some formulations the molar ratio of cerium (IV) to cerium (III) is about 1 to about 1X10~6, more commonly is about 1 to about 1X10~5, even more commonly is about 1 to about 1X10"4, yet even more commonly is about 1 to about 1X10~3, still yet even more commonly is about 1 to about 1X10~2, still yet even more commonly is about 1 to about 1X10"1, or still yet even more commonly is about 1 to about 1. Further, these molar ratios apply for any combinations of soluble and insoluble forms of Ce (III) and soluble and insoluble forms of Ce (IV).
In one formulation, the cerium is primarily in the form of a dissociated cerium (III) salt, with the remaining cerium being present as cerium (IV) oxide. For rare earth- containing compositions having a mixture of +3 and +4 oxidations states commonly at least some of the rare earth has a +3 oxidation sate, more commonly at least most of the rare earth has a +3 oxidation state, more commonly at least about 75 wt% of the rare earth has a +3 oxidation state, even more commonly at least about 90 wt% of the rare earth has a +3 oxidation state, and yet even more commonly at least about 98 wt% of the rare earth has a +3 oxidation state. The rare earth-containing composition commonly includes at least about 1 ppm, more commonly at least about 10 ppm, and even more commonly at least about 100 ppm cerium (IV) oxide. While in some embodiments, the rare earth- containing composition includes at least about 0.0001 wt% cerium (IV), preferably at least about 0.001 wt% cerium (IV) and even more preferably at least about 0.01 wt% cerium (IV) calculated as cerium oxide. Moreover, in some embodiments, the rare earth composition-containing commonly has at least about 20,000 ppm cerium (III), more commonly at least about 100,000 ppm cerium (III) and even more commonly at least about 250,000 ppm cerium (III).
In some formulations, the molar ratio of cerium (III) to cerium (IV) is about 1 to about 1X10"6, more commonly is about 1 to about 1X10"5, even more commonly is about 1 to about 1X10"4, yet even more commonly is about 1 to about 1X10"3, still yet even more commonly is about 1 to about 1X10~2, still yet even more commonly is about 1 to about
1X10"1, or still yet even more commonly is about 1 to about 1. Moreover, in some formulations the molar ratio of cerium (IV) to cerium (III) is aboutl to about 1X10"6, more commonly is about 1 to about 1X10"5, even more commonly is about 1 to about
1X10"4, yet even more commonly is about 1 to about 1X10"3, still yet even more
commonly is about 1 to about 1X10~2, still yet even more commonly is about 1 to about 1X10"1, or still yet even more commonly is about 1 to about 1. Further, these molar ratios apply for any combinations of soluble and insoluble forms of Ce(III) and soluble and insoluble forms of Ce(IV).
Having a mixture of +3 and +4 cerium, preferably in the form of a dissociated cerium (III) salt and a cerium (IV) composition, can be advantageous. Preferred, non- limiting examples of cerium (IV) compositions are cerium (IV) dioxide, cerium (IV) oxide, cerium (IV) oxyhydroxide, cerium (IV) hydroxide, and hydrous cerium (IV) oxide. For example, having dissociated cerium (III) provides for the opportunity to take advantage of cerium (III) solution sorption and/or precipitation chemistries, such as, but not limited to, the formation of insoluble cerium (III) oxyanion compositions.
Furthermore, having a cerium (IV) composition presents, provides for the opportunity to take advantage of sorption and oxidation/reduction chemistries of cerium (IV), such as, the strong interaction of cerium (IV) with compositions such as phosphorus-containing target materials having multiple oxidation states. For example, common oxidation states of phosphorus are phosphours (II), phosphorus (III) and phosphours (V). More specifically, cerium (IV) can oxidize phosphorus-containing target materials having a phosphorus oxidation state of (II) or (III) to phosphorus-containing target materials having a phosphorus oxidation state of (V). Commonly, cerium (IV) is also referred to as cerium (+4) and/or eerie.
In one formulation, the rare earth composition comprises a water-soluble rare earth composition having a +3 oxidation state. Non-limiting examples of suitable water-soluble rare earth compositions include rare earth chlorides, rare earth bromides, rare earth iodides, rare earth astatides, rare earth nitrates, rare earth sulfates, rare earth oxalates, rare earth perchlorates, rare earth carbonates, and mixtures thereof. In one formulation, the rare earth-containing additive includes water-soluble cerium (III) and lanthanum (III) compositions. In some applications, the water-soluble cerium composition comprises cerium (III) chloride, CeCl3. Commonly, cerium (III) is also referred to as cerium (+3) and/or cerous.
More preferably, the rare earth composition comprises a water-soluble cerium +3 composition. Non-limiting examples of suitable water-soluble cerium +3 compositions are cerium (III) chloride, cerium (III) nitrate, cerium (III) sulfate, cerium (III) oxalate, and a mixture thereof.
In some formulations, the water-soluble cerium (III) composition may comprise, in addition to cerium, one or more other water-soluble rare earths. The rare earths other than cerium include yttrium, scandium, lanthanum, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium. The other rare earths may and may not be water-soluble.
In some formulations, the water-soluble cerium-containing additive contains water- soluble cerium (III) and one or more other water-soluble trivalent rare earths (such as, but not limited to, one or more of lanthanum, neodymium, praseodymium and samarium). The molar ratio of cerium (III) to the other trivalent rare earths is commonly at least about 1 : 1, more commonly at least about 10: 1, more commonly at least about 15: 1, more commonly at least about 20: 1, more commonly at least about 25: 1, more commonly at least about 30: 1, more commonly at least about 35: 1, more commonly at least about 40: 1, more commonly at least about 45 : 1 , and more commonly at least about 50: 1.
In some formulations, the water-soluble cerium-containing additive contains cerium (III) and one or more of water-soluble lanthanum, neodymium, praseodymium and samarium. The water-soluble rare earth-containing additive commonly includes at least about 0.01 wt% of one or more of lanthanum, neodymium, praseodymium and samarium. The water-soluble rare earth-containing additive commonly has on a dry basis no more than about 10 wt% La, more commonly no more than about 9 wt% La, even more commonly no more than about 8 wt% La, even more commonly no more than about 7 wt% La, even more commonly no more than about 6 wt% La, even more commonly no more than about 5 wt% La, even more commonly no more than about 4 wt% La, even more commonly no more than about 3 wt% La, even more commonly no more than about 2 wt% La, even more commonly no more than about 1 wt% La, even more commonly no more than about 0.5 wt% La, and even more commonly no more than about 0.1 wt% La. The water-soluble rare earth-containing additive commonly has on a dry basis no more than about 8 wt% Nd, more commonly no more than about 7 wt% Nd, even more commonly no more than about 6 wt% Nd, even more commonly no more than about 5 wt% Nd, even more commonly no more than about 4 wt% Nd, even more commonly no more than about 3 wt% Nd, even more commonly no more than about 2 wt% Nd, even more commonly no more than about 1 wt% Nd, even more commonly no more than about 0.5 wt% Nd, and even more commonly no more than about 0.1 wt% Nd. The water- soluble rare earth-containing additive commonly has on a dry basis no more than about 5 wt% Pr, more commonly no more than about 4 wt% Pr, even more commonly no more
than about 3 wt% Pr, even more commonly no more than about 2.5 wt% Pr, even more commonly no more than about 2.0 wt% Pr, even more commonly no more than about 1.5 wt% Pr, even more commonly no more than about 1.0 wt% Pr, even more commonly no more than about 0.5 wt% Pr, even more commonly no more than about 0.4 wt% Pr, even more commonly no more than about 0.3 wt% Pr, even more commonly no more than about 0.2 wt% Pr, and even more commonly no more than about 0.1 wt% Pr. The water- soluble rare earth-containing additive commonly has on a dr basis no more than about 3 wt% Sm, more commonly no more than about 2.5 wt% Sm, even more commonly no more than about 2.0 wt% Sm, even more commonly no more than about 1.5 wt% Sm, even more commonly no more than about 1.0 wt% Sm, even more commonly no more than about 0.5 wt% Sm, even more commonly no more than about 0.4 wt% Sm, even more commonly no more than about 0.3 wt% Sm, even more commonly no more than about 0.2 wt% Sm, even more commonly no more than about 0.1 wt% Sm, even more commonly no more than about 0.05 wt% Sm, and even more commonly no more than about 0.01 wt% Sm.
In some formulations, the water-soluble cerium-containing additive contains water- soluble cerium (III) and one or more other water-soluble trivalent rare earths (such as one or more of lanthanum, neodymium, praseodymium and samarium). The molar ratio of cerium (III) to the other trivalent rare earths is commonly at least about 1 : 1, more commonly at least about 10: 1, more commonly at least about 15: 1, more commonly at least about 20: 1, more commonly at least about 25: 1, more commonly at least about 30: 1, more commonly at least about 35: 1, more commonly at least about 40: 1, more commonly at least about 45 : 1 , and more commonly at least about 50: 1.
In one formulation, the rare earth-containing additive consists essentially of a water-soluble cerium (III) salt, such as a cerium (III) chloride, cerium (III) bromide, cerium (III) iodide, cerium (III) astatide, cerium perhalogenates, cerium (III) carbonate, cerium (III) nitrate, cerium (III) sulfate, cerium (III) oxalate and mixtures thereof. The rare earth in this formulation commonly is primarily cerium (III), more commonly at least about 75 mole% of the rare earth content of the rare earth-containing additive is cerium (III), that is no more than about 25 mole% of the rare earth content of the rare earth- containing additive comprises rare earths other than cerium (III). Even more commonly, the rare earth in this formulation commonly is primarily at least about 80 mole% cerium (III), yet even more commonly at least about 85 mole% cerium (III), still yet even more
commonly at least about 90 mole% cerium (III), and yet still even more commonly at least about 95 mole% cerium (III).
The rare earth composition may comprise a water-insoluble composition, such as a water-insoluble rare earth oxide, oxyhydroxide, and/or hydrous oxide. The insoluble rare earth composition may be in the form of a dispersion, suspension or slurry of rare earth particulates. The rare earth particulates can have an average particle size ranging from the sub-micron, to micron or greater than micron. The insoluble rare earth composition may have a surface area of at least about 1 m2/g. Commonly, the insoluble rare earth has a surface area of at least about 70 m2/g. In another formulation, the insoluble rare earth composition may have a surface area from about 25 m2/g to about 500 m2/g.
In some formulations, the rare earth composition may be agglomerated.
Commonly, the rare earth composition may be in the form of an agglomerate, the agglomerate comprising a polymeric binder and the rare earth composition.
In one formulation, the rare earth-containing additive comprises a rare earth and/or rare earth-containing composition comprising at least some water-insoluble cerium (IV) and water-soluble cerium (III) and/or lanthanum (III). The rare earth and/or rare earth- containing composition comprise at least some water-soluble cerium (III), typically in the form of water-soluble cerium (III) salt. Commonly, the rare earth-containing additive comprises more than about 1 wt% of a water-soluble cerium (III) composition, more commonly more than about 5 wt% of a water-soluble cerium (III) composition, even more commonly more than about 10 wt% of a water-soluble cerium (III) composition, yet even more commonly more than about 20 wt% of a water-soluble cerium (III) composition, still yet even more commonly more than about 30 wt% of a water-soluble cerium (III) composition, or still yet even more commonly more than about 40 wt% of a water-soluble cerium (III) composition.
In accordance with some formulations, the rare earth-containing additive typically comprises more than about 50 wt% of a water-soluble cerium (III) composition, more typically the rare earth-containing additive comprises more than about 60 wt% of a water- soluble cerium (III) composition, even more typically the rare earth-containing additive comprises more than about 65 wt% of a water-soluble cerium (III) composition, yet even more typically the rare earth-containing additive comprises more than about 70 wt% of a water-soluble cerium (III) composition, still yet even more typically the rare earth- containing additive comprises more than about 75 wt% of a water-soluble cerium (III) composition, still yet even more typically the rare earth-containing additive comprises
more than about 80 wt% of a water-soluble cerium (III) composition, still yet even more typically the rare earth-containing additive comprises more than about 85 wt% of a water- soluble cerium (III) composition, still yet even more typically the rare earth-containing additive comprises more than about 90 wt% of a water-soluble cerium (III) composition, still yet even more typically the rare earth-containing additive comprises more than about 95 wt% of a water-soluble cerium (III) composition, still yet even more typically the rare earth-containing additive comprises more than about 98 wt% of a water-soluble cerium (III) composition, still yet even more typically the rare earth-containing additive comprises more than about 99 wt% of a water-soluble cerium (III) composition, or yet still eve more typically comprises about 100 wt% of a water-soluble cerium (III) composition.
In some formulations, the rare earth-containing additive comprises one or more nitrogen-containing materials. The one or more nitrogen-containing materials, commonly, comprise one or more of ammonia, an ammonium-containing composition, a primary amine, a secondary amine, a tertiary amine, an amide, a cyclic amine, a cyclic amide, a polycyclic amine, a polycyclic amide, and combinations thereof. The nitrogen-containing materials are typically less than about 1 ppm, less than about 5 ppm, less than about 10 ppm, less than about 25 ppm, less than about 50 ppm, less about 100 ppm, less than about 200 ppm, less than about 500 ppm, less than about 750 ppm or less than about 1000 ppm of the water-soluble rare earth-containing additive. Commonly, the rare earth-containing additive comprises a water-soluble cerium (III) and/or lanthanum (III) composition. More commonly, the rare earth-containing additive comprises cerium (III) chloride. The rare earth-containing additive is typically dissolved in a liquid. The liquid is the rare earth- containing additive is dissolved in is preferably water.
In some formulations, the rare earth-containing additive is in the form of one or more of: an aqueous solution containing substantially dissociated, dissolved forms of the rare earths and/or rare earth-containing compositions; free flowing granules, powder, particles, and/or particulates of rare earths and/or rare earth-containing compositions containing at least some water-soluble cerium (III); free flowing aggregated granules, powder, particles, and/or particulates of rare earths and/or rare earth-containing compositions substantially free of a binder and containing at least some water-soluble cerium (III); free flowing agglomerated granules, powder, particles, and/or particulates comprising a binder and rare earths and/or rare earth-containing compositions one or both of in an aggregated and non-aggregated form and containing at least some water-soluble
cerium (III); rare earths and/or rare earth-containing compositions containing at least some water-soluble cerium ( 1 11 ) and supported on substrate; and combinations thereof.
Regarding particulate forms of rare earth-containing compositions, the particles, in one formulation, have a particle size may be from about 1 nanometer to about 1000 nanometers. In another embodiment the particles may have a particle size less than about 1 nanometer. In yet another embodiment the particles may have a particle size from about 1 micrometer to about 1,000 micrometers.
Regarding rare earths and/or rare earth-containing compositions supported on a substrate, suitable substrates can include porous and fluid permeable solids having a desired shape and physical dimensions. The substrate, for example, can be a sintered ceramic, sintered metal, micro-porous carbon, glass fiber, cellulosic fiber, alumina, gamma-alumina, activated alumina, acidified alumina, a metal oxide containing labile anions, crystalline aiumino-siiicate such as a zeolite, amorphous silica-alumina, ion exchange resin, clay, ferric sulfate, porous ceramic, and the like. Such substrates can be in the form of mesh, such as screens, tubes, honeycomb structures, monoliths, and blocks of various shapes, including cylinders and toroids. The structure of the substrate will vary depending on the application. Suitable structural forms of the substrate can include a woven substrate, non-woven substrate, porous membrane, filter, fabric, textile, or other fluid permeable structure. The rare earth-containing additive can be incorporated into or coated onto a filter block or monolith for use as a filter, such as a cross-flow type filter. The rare earth and/or rare earth-containing additive can be in the form of particles coated on to or incorporated in the substrate. In some configurations, the rare earth and/or rare earth-containing additive can be ionically substituted for cations in the substrate.
Typically, the rare earth-coated substrate comprises at least about 0.1% by weight, more typically 1% by weight, more typically at least about 5%> by weight, more typically at least about 10%) by weight, more typically at least about 15% by weight, more typically at least about 20% by weight, more typically at least about 25% by weight, more typically at least about 30%) by weight, more typically at least about 35%> by weight, more typically at least about 40% by weight, more typically at least about 45% by weight, and more typically at least about 50%> by weight rare earth and/or rare earth-containing composition. Typically, the rare earth-coated substrate includes no more than about 95% by weight, more typically no more than about 90%> by weight, more typically no more than about 85% by weight, more typically no more than about 80% by weight, more typically no more than about
75% by weight, more typically no more than about 70% by weight, and even more
typically no more than about 65% by weight rare earth and/or rare earth-containing composition.
In some formulations, the rare earth-containing additive includes a rare earth- containing composition supported on, coated on, or incorporated into a substrate, preferably the rare earth-containing composition is in the form of particulates. The rare earth-containing particulates can, for example, be supported or coated on the substrate with or without a bi der. The binder may be any suitable binder, such as those set forth herein.
Further regarding formulations comprising the rare earth-containing additive comprising rare earth-containing granules, powder, particles, and/or particulates agglomerated and/or aggregated together with or without a binder, such formulations commonly have a mean, median, or P90 particle size of at least about 1 um, more commonly at least about 5 um, more commonly at least about 10 iim, still more commonly at least about 25 iim. In some formulations, the rare earth-containing agglomerates or aggregates have a mean, median, or P90 particle size distribution from about 100 to about 5,000 microns; a mean, median, or P90 particle size distribution from about 200 to about 2,500 microns; a mean, median, or P90 particle size distribution from about 250 to about 2,500 microns; or a mean, median, or P90 particle size distribution from about 300 to about 500 microns. I n other formulations, the agglomerates and/or aggregates can have a mean, median, or P90 particle size distribution of at least about 100 nm, specifically at least about 250 nm, more specifical ly at least about 500 nm, even more specifically at least about 1 um and yet even more specifically at least about 0.5 nm, the mean, median, or P90 particle size distribution of the agglomerates and/or aggregates can be up to about 1 micron or more. Moreover, the rare earth-containing particulates, individually and/or in the form of agglomerates and/or aggregates, can have in some cases a surface area of at least about 5 m2/g, in other cases at least about 10 m2/g, in other cases at least about 70 m2/g, in yet other cases at least about 85 m2/g, in still yet other cases at least about 100 m2/g, in still yet other cases at least about 1 15 m7g, in still yet other cases at least about 125 m2/g, in still yet other cases at least about 1 50 m /g, in still yet other cases at least 300 m2/g, and in still yet other cases at least about 400 m2/g. In some configurations, the rare earth-containing particulates, individually and/or in the form of agglomerates or aggregates commonly can have a surface area from about 50 to about 500 m2/g, or more commonly from about 1 10 to about 250 m2/g. Commonly, the rare earth-
containing agglomerate includes more than 10.01wt%, more commonly more than about 85 wt%, even more commonly more than about 90 wt%, yet even more commonly more than about 92 wt% and still yet even more commonly from about 95 to about 96.5 wt% rare earth-containing particulates, with the balance being primarily the binder. Stated another way, the binder can be less than about 15% by weight of the agglomerate, in some cases less than about 10% by weight, in still other cases less than about 8% by weight, in still other cases less than about 5% by weight, and in still other cases less than about 3.5% by weight of the agglomerate. In some formulations, the rare earth-containing particulates are in the form of powder and have aggregated nano-crystalline domains. The binder can include one or more polymers selected from the group consisting of thermosetting polymers, thermoplastic polymers, elastomeric polymers, cellulosic polymers and glasses. Preferably, the binder comprises a fluorocarbon-containing polymer and/or an acry ic- polymcr.
In one embodiment, the rare earth-containing composition is in the form of a colloid, suspension, or slurry of particulates. The particulates commonly can have a mean, median and/or P90 particle size of less than about 1 nanometer, more commonly a mean, median and/or P90 particle size from about 1 nanometer to about 1,000 nanometers, even more commonly a mean, median and/or P90 particle size from about 1 micron to about 1,000 microns, or yet even more commonly a mean, median and/or P90 particle size of at least about 1,000 microns. Preferably, the particulates have a mean, median and/or P90 particle size from about 0.1 to about 1,000 nm, more preferably from about 0.1 to about 500 nm. Even more preferably, the cerium (IV) particulates have a mean, median and/or P90 particle size from about 0.2 to about 100 nm.
In some embodiments, the particulates may have a mean and/or median surface area of at least about 1 m2/g, preferably a mean and/or median surface area of at least about 70 m2/g. In other embodiments, the particulates may preferably have a mean and/or median surface area from about 25 m2/g to about 500 m2/g and more preferably, a mean and/or median surface area of about 100 to about 250 m2/g. In some embodiments, the particulates may be in the form of one or more of a granule, crystal, crystallite, and particle.
In one application, the particulates comprise cerium (IV), typically as cerium (IV) oxide. The weight percent (wt%) cerium (IV) content based on the total rare earth content of the cerium (IV) particulates typically is at least about 50 wt% cerium (IV), more typically at least about 60 wt%> cerium (IV), even more typically at least about 70 wt%>
cerium (IV), yet even more typically at least about 75 wt% cerium (IV), still yet even more typically at least about 80 wt% cerium (IV), still yet even more typically at least about 85 wt% cerium (IV), still yet even more typically at least about 90 wt% cerium (IV), still yet even more typically at least about 95 wt% cerium (IV), and even more typically at least about 99 wt% cerium (IV). Preferably, the cerium (IV) particulate is substantially devoid of rare earths other than cerium (IV). More preferably, the weight percent (wt%) cerium (IV) content based on the total rare earth content of the cerium (IV) particulates is about 100 wt% cerium (IV) and comprises one or more of cerium (IV) oxide, cerium (IV) hydroxide, cerium (IV) oxyhydroxyl, cerium (IV) hydrous oxide, cerium (IV) hydrous oxyhydroxyl, Ce02, and/or Ce(IV)(0)w(OH)x(OH)y zH20, where w, x, y and can be zero or a positive, real number.
The Medium (or Media) 104
The medium (or media) 104 can be any fluid stream. The fluid stream may be derived from any source containing one or more phosphorus-containing target materials. Preferably, the medium (or media) 104 is derived from any aqueous source containing one or more phosphorus-containing target materials. Non-limiting examples of suitable medium (or media) 104 are recreational waters, municipal waters (such as, sewage, waste, agricultural, or ground waters), industrial (such as cooling, boiler, or process waters), wastewaters, well waters, septic waters, drinking waters, naturally occurring waters, (such as a lake, pond, reservoir, river, or stream), and other waters and/or aqueous process streams.
Non-limiting examples of recreational waters are swimming pool waters, brine pool waters, therapy pool waters, diving pool waters, sauna waters, spa waters, and hot tub waters. Non-limiting examples of municipal waters are drinking waters, waters for irrigation, well waters, waters for agricultural use, waters for architectural use, reflective pool waters, water-fountain waters, water-wall waters, use, non-potable waters for munici al use and other non-potable munici al waters. Wastewaters include without limitation, municipal and/or agricultural run-off waters, septic waters, waters formed and/or generated during an industrial and/or manufacturing process, waters formed and/or generated by a medical facility, waters associated with mining, mineral production, recovery and/or processing (including petroleum), evaporation pound waters, and non- potable disposal waters. Well waters include without limitation waters produced from a subsurface well for the purpose of human consumption, agricultural use ( including
consumption by a animal, irrigation of crops or consumption by domesticated farm animals), mineral-containing waters, waters associated with mining and petroleum, production. Non- limiting examples of naturally occurring waters include associated with rains, storms, streams, rivers, lakes, aquifers, estuaries, lagoons, and such.
The medium (or media) 104 is typically obtained from one or more of the above sources and processed, conveyed and/or manipulated by a water handling system. The medium (or media) 104 can be primarily the water in a water handling system.
The water handling system components and configuration can vary depending on the treatment process, water, and water source. While not wanting to limited by example, municipal and/or wastewater handling systems typically one or more of the following process units: clarifying, disinfecting, coagulating, flocculating, aerating, filtering, separating solids and liquids, digesting, and polishing. The number and ordering of the process units can vary. Furthermore, some process units may occur two or more times within a water handling system. It can be appreciated that the one or more process units are in fluid communication.
The water handling system may or may not have a clarifier. Some water handling systems may have more than one clarifier, such as primary and final clarifiers. Clarifiers typically reduce cloudiness of the water by removing biological matter (such as bacteria and/or algae), suspended and/or dispersed chemicals and/or particulates from the water. Commonly a clarification process occurs before and/or after a filtration process.
The water handling system may or may not contain a filtering process. Typically, the water handling system contains at least one filtering process. Non- limiting examples of common filtering processes include without limitation screen filtration, trickling filtration, particulate filtration, sand filtration, macro-filtration, micro-filtration, ultra - filtration, nano-filtration, reverse osmosis, carbon/activated carbon filtration, dual media filtration, gravity filtration and combinations thereof. Commonly a filtration process occurs before and/or after a disinfection process. For example, a filtration process to remove solid debris, such as solid organic matter and grit from the water typically precedes the disinfection process. In some embodiments, a filtration process, such as an activated carbon and/or sand filtrations follows the disinfection process. The post- disinfection filtration process removes at least some of the chemical disinfectant remaining in the treated water.
The water handling system may or may not include a disinfection process. The disinfection process may include without limitation treating the aqueous stream and/or
water with one or more of fluorine, fluorination, chlorine, chlorination, bromine, bromination, iodine, iodination, ozone, ozonation, electromagnetic irradiation, ultra-violet light, gama rays, electrolysis, chlorine dioxide, hypochlorite, heat, ultrasound,
trich!o ro i so c y a n u r i c acid, soaps/detergents, alcohols, bromine chloride (BrCl), cupric ion (CU2T), silver, silver ion (Ag+), permanganate, phenols, and combinations thereof.
Preferably, the water handling system contains a single disinfection process, more preferably the water handling system contains two or more disinfection processes.
Disinfection processes are typically provided to one of at least remove, kill and/or detoxify pathogenic material contained in the water. Typically, the pathogenic material comprises biological contaminants, in particular biological contaminants comprising phosphorus- containing target materials. In some embodiments, the disinfection process converts the phosphorus-containing target material into a form of the phosphorus-containing target material that can be removed and/or deto i fied by the rare earth-containing composition, additive and/or particle or particulate.
The water handl ing system may or may not include coagulation. The water handling system may contain one or more coagulation processes. Typically, the coagulation process includes adding a flocculent to the water in the water handling system. Typical flocculants include aluminum sulfate, polyelectrolytes, polymers, lime and ferric chloride. The flocculent aggregates the particulate matter suspended and/or dispersed in the water, the aggregated particulate matter forms a coagulum. The coagulation process may or may not include separating the coagulum from the liquid phase. In some embodiments, coagulation may comprise part, or ail, the entire clarification process. In other embodiments, the coagulation process is separate and distinct from the clarification process. Typically, the coagulation process occurs before the disinfection process.
The water handl ing system may or may not include aeration. Within the water handing system, aeration comprises passing a stream of air and/or molecular oxygen through the water contained in the water handling system. The aeration process promotes oxidation of contaminants contained in the water being processed by the water handling system, preferably the aeration promotes the oxidation of biological contaminates, such as phosphorus-containing biological contaminates. In some embodiments, the aeration process converts the phosphorus-containing target material into a form of the phosphorus- containing target material that can be removed and/or detoxified by the rare earth- containing composition, additive and/or particle or particulate. The water handl ing system
may contain one or more aeration processes. Typically, the disinfection process occurs after the aeration process.
The water handling system may or may not have one or more of a heater, a cooler, and a heat exchanger to heat and/or cool the water being processed by the water handling system. The heater may be any method suitable for heating the water. Non-limiting examples of suitable heating processes are solar heating systems, electromagnetic heating systems (such as, induction heating, microwave heating and infrared), immersion heaters, and thermal transfer heating systems (such as, combustion, stream, hot oil, and such, where the thermal heating source has a higher temperature than the water and transfers heat to the water to increase the temperature of the water). The heat exchanger can be any process that transfers thermal energy to or from the water. The heat exchanger can remove thermal energy from the water to cool and/or decrease the temperature of the water. Or, the heat exchanger can transfer thermal energy to the water to heat and/or increase the temperature of the water. The cooler may be any method suitable for cooling the water. Non- limiting examples of suitable cooling process are refrigeration process, evaporative coolers, and thermal transfer cooling systems (such as, chillers and such where the thermal (cooling) source has a lower temperature than the water and removes heat from the water to decrease the temperature of the water). Any of the clarification, disinfection, coagulation, aeration, filtration, sludge treatment, digestion, nutrient control, solid/liquid separation, and/or polisher processes may further include before, after and/or during one or both of a heating and cooling process. It can be appreciated that a heat exchanger typically includes at least one of heating and cooling process.
The water handling system may or may not include a digestion process. Typically, the digestion process is one of an anaerobic or aerobic digestion process. In some configurations, the digestion process may include one of an anaerobic or aerobic digestion process followed by the other of the anaerobic or aerobic digestion processes. For example, one such configuration can be an aerobic digestion process followed by an anaerobic digestion process. Commonly, the digestion process comprises microorganisms that breakdown the biodegradable material contained i the water, in some embodiments, the biodegrablc material includes a phosphorus-containing target material. Furthermore, the digestion process converts the phosphorus-containing target material into a form of the phosphorus-containing target material that can be removed and/or detoxified by the rare earth-containing composition, additive and/or particle or particulate. The anaerobic digestion of biodegradable material proceeds in th e absence of oxygen , while the aerobic
digestion of biodegradable material proceeds in the presence of oxygen. In some water handling systems the digestion process is typically referred to as biological stage/digester or biological treatment stage/digester. Moreover, in some systems the disinfection process comprises a digestion process.
The water handling system may or may not include a nutrient control process.
Furthermore, the water handling system may include one or more nutrient control processes. The nutrient control process typically includes nitrogen and/or phosphorus control. Moreover, nitrogen control commonly may include nitrifying bacteria. Typically, phosphorus control refers to biological phosphorus control, preferably controlling phosphorus that can be used as a nutrient for algae. Nutrient control typically includes processes associated with control of oxygen demand substances, which include in addition to nutrients, pathogens, and inorganic and synthetic organic compositions. The nutrient control process can occur before or after the disinfection process. In some embodiments, the nutrient control converts the phosphorus-containing target material into a form of the phosphorus-containing target material that can be removed and/or detoxified by the rare earth-containing composition, additive and/or particle or particulate.
The water handling system may or may not include a solid/liquid separation process. Preferably, the water handling system includes one or more solid/liquid separation processes. The solid/liquid separation process can comprise any process for separating a solid phase from a liquid phase, such as water. Non- limiting examples of suitable solid liquid separation processes are clarification (including trickling filtration), filtration (as described above), vacuum and/or pressure filtration, cyclone (including hydrocyclones), floatation, sedimentation (including gravity sedimentation ), coagulation (as described above), sedimentation (including, but not limited to grit chambers), and combinations thereof.
The water handling system may or may not include a polisher. The polishing process can include one or more of removing fine particulates from the water, an ion- exchange process to soften the water, an adjustment to the pH value of the water, or a combination thereof. Typically, the polishing process is after the disinfection step.
While the water handling system typically includes one or more of a clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes, the water handling system may further include additional processing equipment. The additional processing equipment includes without limitation holding tanks, reactors, purifiers, treatment vessels or units, mixing vessels or elements, wash
circuits, precipitation vessels, separation vessels or units, settling tanks or vessels, reservoirs, pumps, cooling towers, heat exchangers, valves, boilers, gas liquid separators, nozzles, tenders, and such. Furthermore, the water handling system includes conduit(s) interconnecting the unit operations and/or additional processing equipment. The conduits include without limitation piping, hoses, channels, aqua-ducts, ditches, and such. The water is conveyed to and from the unit operations and/or additional processing equipment by the conduit(s). Moreover, each unit operations and/or additional processing equipment is in fluid communication with the other unit operations and/or additional processing equipment by the conduits.
In one configuration, the water handling system includes sludge and Biological
Oxygen Demand ("BOD") removal from an aqueous medium (or media) 104) by gravity settling techniques (by a settling tank), to form a first intermediate treated medium, clarification (by a clarifier vessel) of the first intermediate treated medium to form a second intermediate treated medium and remove more finely sized solids and BOD, digestion of the second intermediate treated medium to form a digested third intermediate treated medium, clarification (by a further clarifier vessel) of the digested third intermediate treated medium to form a fourth intermediate treated medium, and disinfection of the fourth intermediate treated medium to form the treated medium 124. The treated medium 124 can be further subjected to tertiary treatment to remove dissolved substances, such as colorants, metals, organic chemicals, and microorganism nutrients, particularly phosphorus and nitrogen. Tertiary treatment can include clarification, anaerobic, anoxic, and/or aerobic biological nutrient removal, disinfection, dissolved air flotation, fermentation, digestion, and the like. The ordering of the steps is not critical and may be changed, depending on the application. The rare earth-containing additive can be added to any of the first intermediate treated medium, second intermediate treated medium, third intermediate treated medium, and/or fourth intermediate treated medium and/or in any of the clarification, digestion, or disinfection stages and/or in any tertiary treatment stage of inter-stage effluent. Because the rare earth-containing additive can be an antimicrobial agent, it is more common for the rare earth-containing additive to be added downstream of digestion to avoid interference with bacterial digestion. Because the rare earth-containing additive can be an antimicrobial agent, one process configuration uses the rare earth-containing additive for both phosphate removal and microcrobial removal. In this configuration, other antimicrobial agents may not be employed. In one configuration, the process steps can change the relative concentrations and therefore ratios
of reactive phosphate ("RP") and non-reactive phosphate ("NRP"), particularly digestion (due to aeration and/or microbial activity) and disinfection. Typically, the process converts a portion of NRP to RP, thereby increasing the proportion of total phosphates (which includes both RP and NRP) that is RP. In other applications, the process converts a portion of RP to NRP, thereby increasing the proportion of total phosphates that is NRP.
The Phosphorus-Containing Target Material
The aqueous medium that is treated by the rare earth-containing composition, additive and/or particles may contain one or more phosphorus-containing target materials. The one or more phosphorus-containing target materials may include one or both of inorganic and organic phosphorus-containing target materials. Inorganic phosphorus- containing target materials metals include without limitation inorganic phosphate compounds, phosphite compounds, phosphorus oxyhalide (e.g., phosphorus oxychloride), phosphorus thiohalide (e.g., phosphorus thiochloride), phosphorus selenohalide (e.g., phosphorus selenochloride), phosphorus trihalide (e.g., phosphorus trichloride), phosphorus pentahalide (e.g., phosphorus pentachloride), phosphoric acid, and phosphorus acid and salts thereof (e.g., cyclic phosphate and phosphite salts). Organic phosphorus- containing target materials include without limitation organophosphorous compounds (such as organophosphates (or organic phosphate compounds), organophosphites (or organic phosphite compounds), and organophosphines, (or organic phosphines)). Examples of organophosphorus compounds include chalcogenides, diphosphenes, phosoamides, phosphaalkenes, phosphaalkynes, phosphine oxides, phosphinates, phosphines, phosphinites, phosphonates (e.g., o-alkyl phosphonohalogenates (such as alkyl phosphonofluoridates (such as sarin or isopropyl methylphosphonofluoridate), o-alkyl, s- 2-dialkyl aminoethyl alkylphosphonothiolates, dimethyl methylphosphonates, diisopropyl methylphosphonates, and alkylated or protonated salts thereof), o-alkyl
phosphoramideocyanidates and alklylated or protonated salts thereof, phosphonic acids and esters thereof, phosphinic acids and esters thereof, phospone oxides, phosphonites (e.g., alkyl phosphonites), phosphonium salts, alkyl phosphonyldihalides (e.g., alkyl phosphonyldifluoride and methylphosphonyl dichloride), dialkyl phosphoramidic dihalides, alkyl phosphoramidate, phosphoranes (e.g., methylenetriphenyl-phosphorane), phosphono carboxylates, halosarins (e.g., chlorosarin), halosomans (e.g., chlorosoman), amitons, alkyl phosphorus oxyhalides (e.g., alkyl phosphorus oxychloride), alkyl phosphites, phosphamidons, DNA backbone phosphate groups, DNA backbone phosphite
groups, thiophosphates, selenophosphates, thiophosphyoryl esters, selenophophyoryl esters, thioesters, selenoesters and amides (such as, mono-, di- and tri- phosphate esters, thioesters and amides (P(=Y)(OH)2(XR), P(=Y)(OH)(XR)2, and P(=Y)(XR)3, where X can be one of oxygen, sulfur or nitrogen, Y can be one of oxygen, sulfur and selenium and R can be a C1-C12 alkyl or aryl group), phosphatidylcholines, P-N amino compounds, monochrotophos, VX, methamidophos, dicrotophos, phosphamidons, and mixtures and combinations thereof. The phosphorus-containing target material may occur as a primary or secondary phosphorus-containing species (vide infra). One or both of the primary or secondary phosphorus-containing species may be in the form of a solid, a dissolved species, a suspension, or a combination thereof.
In some configurations, the phosphorus-containing target material comprises a phosphorus (V)-containing target material, a phosphorus (Ill)-containing target material, a phosphorus (Il)-containing target material, or a mixture comprising one or more phosphorus (V)-, phosphorus (III)-, and phosphorus (Il)-containing target materials.
In some configurations, the phosphorus-containing target material comprises reactive and/or non-reactive phosphates. "Reactive phosphorus" [RP] refers to
orthophosphate (Ρ04 3, HPO4"2, and H2P(V), while "non-reactive phosphorus" [NRP] refers to polyphosphates and organic phosphorus compounds. NRP can be in the form of a colloid, or suspension. Furthermore, the NRP may be in the form of an anion, cation, and/or neutral species. In some embodiments, the NRP to RP molar and/or weight ratios (in the in the medium (or media) 104 is commonly no more than about 0.1, more commonly no more than about 0.2, more commonly no more about 0.3, more commonly no more than about 0.4, more commonly no more than about 0.5, more commonly no more than about 0.6, more commonly no more than about 0.7, more commonly no more than about 0.8, more commonly no more than about 0.9, more commonly no more than about 1.0, more commonly no more than about 1.1, more commonly no more than about 1.2, more commonly no more than about 1.3, more commonly no more than about 1.4, more commonly no more than about 1.5, more commonly no more than about 1.6, more commonly no more than about 1.7, more commonly no more about 1.8, more commonly no more than about 1.9, more commonly no more than about 1.9, and even more commonly no more than about 2.0 at a pH value of no more than about pH -2, at a pH value of more than about pH -1, at a pH value of more than about pH 0, at a pH value of more than about pH 1 , at a pH value of more than about pH 2, at a pH value of more than about pH 3, at a pH value of more than about pH 4, at a pH value of more than about pH 5,
at a H value of more than about pH 6, at a pH value of more than about pH 7, at a pH value of more than about pH 8, at a pH value of more than about pH 9, at a pH value of more than about pH 10, at a pH value of more than about pH 11, at a pH value of more than about pH 12, at a pH value of more than about pH 13, or at a pH value of more than about pH 14.
It is not well understood how the rare earth-containing composition, additive and/or particles removes and/or detoxifies the phosphorus-containing target material from the medium 104. While not wishing to be bound by any theory, in some embodiments the phosphorus-containing target material and/or a portion thereof may be one or more of sorbed, precipitated, complexed, ionically bound, inter- valance shell complexed (with any one or more hybridized or non-hybridized s, p, d or f orbitals), covalently bounded or a combination thereof with the rare earth-containing composition, additive and/or particles. While not wishing to be bound by any theory, the phosphorus-containing target material and/or a portion thereof may selectively interact with a face or an edge of rare earth- containing composition, additive and/or particulate to form a target-laden rare earth composition, preferably a substantially insoluble target-laden rare earth composition. In some configurations, the rare earth composition, additive and/or particulate may be in the form of a substantially water-soluble or substantially water-insoluble rare earth composition, additive and/or particulate. In either instance, the rare earth-containing composition, additive and/or particulate chemically and/or physically strongly sorbs, binds, reacts or such with the phosphorus-containing target material to form a target-laden rare earth composition. In some embodiments, the target-laden rare earth composition is an insoluble target-laden rare earth composition.
While not wishing to be bound by any theory, in some embodiments the phosphorus-containing material and/or portion thereof is removed and/or detoxified by chemically and/or physically attaching, sorbing and/or bonding with: the phosphorus- containing material and/or portion thereof; oxygen and/or an oxygen-containing portion (such as, but not limited to an ether, alcohol, carbonyl, or carboxylate) of the phosphorus- containing material; sulfur and/or a sulfur-containing portion (such as, but not limited to a thiol-ether, thiol alcohol, thiol-carbonyl or thio-carboxylate) of the phosphorus-containing material; selenium and/or a selenium-containing portion (such as, but not limited to seleno-ether, seleno-alcohol, seleno-carbonyl, seleno-carboxylate) of the phosphorus- containing material; nitrogen and/or a nitrogen-containing portion (such as, but not limited to primary amine and/or amide, secondary amine or amide, tertiary amine or amide) of
phosphorus-containing material; tellurium and/or a tellurium-containing portion (such as, but not to telluro-ether, telluro-alcohol, telluro-carbonyl, telluro-carboxylate) of the phosphorus-containing material; chlorine and/or a chlorine-containing portion of the phosphorus-containing material; carbon and/or or a carbon-containing portion (such as, a ene (double bond, sp2 bond between two atoms) or yne (triple bond, sp bond between to atoms) portion) of the phosphorus-containing material; and/or a combination thereof to the rare earth or rare earth-containing composition, additive and/or particulate.
While not wishing to be bound by any theory, in some embodiments the rare earth- containing composition, additive and/or particulate comprises cerium (IV). Preferably, the cerium (IV) comprises cerium (IV) oxide. The cerium (IV) may oxidize and/or decompose the phosphorus-containing target material. While not wanting to be bound by example, the cerium (IV) may oxidize the phosphorus-containing material in the form of a phosphorus (II)- and/or phosphorus (Ill)-containing material to a phosphorus (IV)- containing material. The phosphorus (IV)-containing target material being one or both of more easily and effectively removed and/or detoxified than either one or both of the phosphorus (II)- and phosphorus (Ill)-containing target materials by the rare earth- containing composition, additive and/or particulate. While not wanting to be bound by example, the cerium (IV) may oxidize an alcoholic portion (such as, an oxygen-, sulfur-, seleno- and/or telluro- alcohol) to a carbonyl and/or carboxylate portion, respectively. The contacting of the rare earth-containing oxidizing agent and the phosphorus-containing target material may one or both: a) chemically interact with the phosphorus-containing target material and b) form a reduced rare earth and/or rare earth-containing oxidizing agent and an oxidized target material and/or portion thereof.
In some embodiments, a cerium (IV) oxidizing agent may be formed by contacting a first cerium-containing composition having cerium in a +3 oxidation state with an oxidant {vide infra) to form a second cerium-containing composition having cerium in a +4 oxidation state (or cerium (IV) oxidizing agent). Commonly, the second cerium- containing composition comprises Ce02 particles. The cerium (IV) oxidizing agent then oxidizes the phosphorus-containing target material forming the first (reduced) cerium (Ill)-containing composition.
Regardless of the precise mechanism, contact of the rare earth-containing composition, additive and/or particulate with the phosphorus-containing target material forms a target-laden rare earth composition. The target-laden rare earth composition comprises the rare earth- and the phosphorus-containing target material or a component
thereof. The target-laden rare earth composition can be in the form of an insoluble solid material either contained within the water or an insoluble solid material phase separated from the water. The target-laden rare earth composition may be one or more of an insoluble precipitate, an insoluble solid particle suspended within the water, a flocculated solid particle, and a combination thereof. In some configurations, the target-laden rare earth composition can be in form of solubilized and/or dissolved in the aqueous phase.
Regarding phosphorus-containing target materials in general and inorganic phosphorus-containing materials in particular, the primary phosphorus-containing species depends upon the pH and Eh of the aqueous solution. Figs. 2A-2E area Pourbaix diagrams for phosphate under different thermodynamic conditions. As discussed below, the form of phosphate species present in solution, and therefore the efficacy of precipitating, sorbing, or otherwise removing the phosphate species from and/or detoxifying the aqueous medium by treatment with the rare earth-containing composition, additive and/or particle or particulate can be increased substantially by adjusting one or both of the pH and Eh of the aqueous solution. It can be appreciated that while the efficacy of precipitating, sorbing, or removing the phosphorus-containing target material has been illustrated for various pH and Eh values for phosphate, the concept of adjusting one or both of pH and Eh is applicable for effectively removing and/or detoxifying an aqueous solution for
phosphorus-containing target materials other than phosphates.
In accordance with some embodiments, the phosphorus-containing target material is removed from the aqueous media having a selected pH value. Commonly, the selected pH value of the aqueous media may be from about pH 0 to about pH 14, more commonly the pH of the aqueous media may be from about pH 1 to about pH 13, even more commonly the pH of the aqueous media may be from about pH 2 to about pH 12, even more commonly the pH of the aqueous media may be from about pH 3 to about pH 11 , yet even more commonly the pH of the aqueous media may be from about pH 4 to about pH 10, still yet even more commonly the pH of the aqueous media may be from about pH 5 to about pH 9, or still yet even more commonly the pH of the aqueous media may be from about pH 6 to about pH 8.
In one embodiment, the aqueous media typically has a selected pH value of from about pH 6 to about pH 9, and more typically the aqueous media has a pH of from about pH 6.5 to about pH 8.5
Commonly in other embodiments, the aqueous media may be substantially acidic having a selected pH of about pH 0, more commonly having a selected pH of about pH 1,
even more commonly having a selected pH of about pH 2, yet even more commonly having a selected pH of about pH 3, or still yet even more commonly having a selected pH about pH 4. Even more commonly in other embodiments, the aqueous media may have a selected pH of about pH 5, more commonly having a selected pH of about pH 6, even more commonly having a selected pH of about pH 7, yet even more commonly having a selected pH of about pH 8, or still yet even more commonly having a selected pH of about pH 9. Commonly in other embodiments, the aqueous media may have a selected pH of about pH 10, more commonly having a selected pH of about pH 11, even more commonly having a selected pH of about pH 12, yet even more commonly having a selected pH of about pH 13, or still yet even more commonly having a selected pH about pH 14.
In accordance with some embodiments, the phosphorus-containing target material is removed from the aqueous medium having a selected Eh value with respect to standardized reference electrode, such as a standard hydrogen electrode (SHE).
Commonly, the selected Eh of the aqueous medium is at least about -0.5 V, more commonly at least about -0.4 V, more commonly at least about -0.3 V, more commonly at least about -0.2 V, more commonly at least about -0.1 V, more commonly at least about 0 V, more commonly at least about 0.1 V, more commonly at least about 0.2 V, more commonly at least about 0.3 V, and more commonly at least about 0.4 V, and more commonly at least about 0.5 V. Commonly, the selected Eh of the aqueous medium is below the level at which water is not electrochemically stable, more commonly no more than about 1.7 V, more commonly no more than about 1.6 V, more commonly no more than about 1.5 V, more commonly no more than about 1.4 V, more commonly no more than about 1.3 V, more commonly no more than about 1.2 V, more commonly no more than about 1.1 V, more commonly no more than about 1.0 V, more commonly no more than about 0.9 V, more commonly no more than about 0.8 V, and more commonly no more than about 0.7 V.
The rare earth to phosphorus (in the phosphorus-containing target material)/target material ratio can also vary depending on the solution pH and/or Eh value. In other words, rare earths having a rare earth to phosphorus/target material ratio less than 1 have a greater molar removal capacity of phosphorus/target material than rare earths having a rare earth to phosphorus/target material ratio of 1 or more than 1. In some embodiments, the greater the pH value the greater the rare earth to phosphorus/target material ratio. In other embodiments, the greater the pH value the smaller the rare earth to phosphorus/target material ratio. In yet other embodiment, the rare earth to phosphorus/target material ratio
is substantially unchanged over a range of pH values. In some embodiments, the rare earth to phosphorus/target material ratio is no more than about 0.1, the rare earth to phosphorus/target material ratio is no more than about 0.2, the rare earth to
phosphorus/target material ratio is no more about 0.3, the rare earth : phosphorus/target material ratio is no more than about 0.4, the rare earth to phosphorus/target material ratio is no more than about 0.5, the rare earth to phosphorus/target material ratio is no more than about 0.6, the rare earth to phosphorus/target material ratio is no more than about 0.7, the rare earth to phosphorus/target material ratio is no more than about 0.8, the rare earth to phosphorus/target material ratio is no more than about 0.9, the rare earth to
phosphorus/target material ratio is no more than about 1.0, the rare earth to
phosphorus/target material ratio is no more than about 1.1, the rare earth to
phosphorus/target material ratio is no more than about 1.2, the rare earth to
phosphorus/target material ratio is no more than about 1.3, the rare earth to
phosphorus/target material ratio is no more than about 1.4, the rare earth to
phosphorus/target material ratio is no more than about 1.5, the rare earth to
phosphorus/target material ratio is no more than about 1.6, the rare earth to
phosphorus/target material ratio is no more than about 1.7, the rare earth to
phosphorus/target material ratio is no more about 1.8, the rare earth to phosphorus/target material ratio is no more than about 1.9, the rare earth to phosphorus/target material ratio is no more than about 1.9, or the rare earth to phosphorus/target material ratio is more than about 2.0 at a pH value of no more than about pH -2, at a pH value of more than about pH - 1 , at a pH value of more than about pH 0, at a pH value of more than about pH 1 , at a pH value of more than about pH 2, at a pH value of more than about pH 3, at a pH value of more than about pH 4, at a pH value of more than about pH 5, at a pH value of more than about pH 6, at a pH value of more than about pH 7, at a pH value of more than about pH 8, at a pH value of more than about pH 9, at a pH value of more than about pH 10, at a pH value of more than about pH 11, at a pH value of more than about pH 12, at a pH value of more than about pH 13, or at a pH value of more than about pH 14.
In some embodiments, the rare earth to phosphorus/target material ratio is no more than about 0.1, the rare earth to phosphorus/target material ratio is no more than about 0.2, the rare earth to phosphorus/target material ratio is no more about 0.3, the rare earth to phosphorus/target material ratio is no more than about 0.4, the rare earth to
phosphorus/target material ratio is no more than about 0.5, the rare earth to
phosphorus/target material ratio is no more than about 0.6, the rare earth to
phosphorus/target material ratio is no more than about 0.7, the rare earth to phosphorus/target material ratio is no more than about 0.8, the rare earth to phosphorus/- target material ratio is no more than about 0.9, the rare earth to phosphorus/target material ratio is no more than about 1.0, the rare earth to phosphorus/target material ratio is no more than about 1.1, the rare earth to phosphorus/target material ratio is no more than about 1.2, the rare earth to phosphorus/target material ratio is no more than about 1.3, the rare earth to phosphorus/-target material ratio is no more than about 1.4, the rare earth to phosphorus/target material ratio is no more than about 1.5, the rare earth to
phosphorus/target material ratio is no more than about 1.6, the rare earth to
phosphorus/target material ratio is no more than about 1.7, the rare earth to
phosphorus/target material ratio is no more about 1.8, the rare earth to phosphorus/target material ratio is no more than about 1.9, the rare earth to phosphorus/target material ratio is no more than about 1.9, or the rare earth to phosphorus/target material ratio is more than about 2.0 at a water pH value of no more than about pH -2, at a water pH value of more than about pH - 1 , at a water pH value of more than about pH 0, at a water pH value of more than about pH 1 , at a water pH value of more than about pH 2, at a water pH value of more than about pH 3, at a water pH value of more than about pH 4, at a water pH value of more than about pH 5, at a water pH value of more than about pH 6, at a water pH value of more than about pH 7, at a water pH value of more than about pH 8, at a water pH value of more than about pH 9, at a water pH value of more than about pH 10, at a water pH value of more than about pH 11 , at a water pH value of more than about pH 12, at a water pH value of more than about pH 13, or at a water pH value of more than about pH 14.
The concentration of the phosphorus-containing target material can vary depending on a number of factors. The concentration of either or both can be, for example, commonly at least about 1 ppm, more commonly at least about 5 ppm, more commonly at least about 10 ppm, more commonly at least about 25 ppm, 50 ppm, more commonly at least about 100 ppm, more commonly at least about 500 ppm, more commonly at least about 1,000 ppm, more commonly at least about 5,000 ppm, more commonly at least about 10,000 ppm, and more commonly at least about 100,000 ppm.
For Ce02 as the rare earth-containing composition, additive, and/or particle or particulate, removal capacities of approximately 0.1 mg target material/g REO (e.g. Ce02) or less can be encountered. These can have rare earth:target material ratios that are significantly larger than 2. For example, 0.1 mg is 0.0001 g, so 1 g CeO2/0.0001 g target material = 10,000. In such embodiments, the rare earth to target material ratio is
commonly no more than about 50,000, the rare earth to target material ratio is more commonly no more than about 47,500, the rare earth to target material ratio is more commonly no more about 45,000, the rare earth to target material ratio is more commonly no more than about 42,500, the rare earth to target material ratio is more commonly no more than about 40,000, the rare earth to target material ratio is no more than about
37,500, the rare earth to target material ratio is more commonly no more than about
35,000, the rare earth to target material ratio is more commonly no more than about
35,000, the rare earth to target material ratio is more commonly no more than about
32,500, the rare earth to target material ratio is more commonly no more than about
30,000, the rare earth to target material ratio is more commonly no more than about
37,500, the rare earth to target material ratio is more commonly no more than about
35,000, the rare earth to target material ratio is more commonly no more than about
32,500, the rare earth to target material ratio is more commonly no more than about
30,000, the rare earth to target material ratio is more commonly no more than about
27,500, the rare earth to target material ratio is more commonly no more than about
25,000, the rare earth to target material ratio is more commonly no more than about
22,500, or the rare earth to target material ratio is more commonly no more about 20,000, at a water pH value of no more than about pH -2, at a water pH value of more than about pH -1 , at a water pH value of more than about pH 0, at a water pH value of more than about pH 1 , at a water pH value of more than about pH 2, at a water pH value of more than about pH 3, at a water pH value of more than about pH 4, at a water pH value of more than about pH 5, at a water pH value of more than about pH 6, at a water pH value of more than about pH 7, at a water pH value of more than about pH 8, at a water pH value of more than about pH 9, at a water pH value of more than about pH 10, at a water pH value of more than about pH 11 , at a water pH value of more than about pH 12, at a water pH value of more than about pH 13, or at a water pH value of more than about pH 14.
Aqueous Medium Pre-Treatment
In step 108, the medium 104 is optionally pre-treated to produce a selected primary species of the phosphorus-containing target material. The selected primary species is generally more effectively removed by the rare earth-containing composition, additive, and/or particle than the primary species in the medium 104. For example, one or more of the Eh and pH values may be altered for more effective removal and/or detoxification the phosphorus-containing target material from the medium 104. As will be appreciated, pH
is a measure of the activity of hydrogen ions while Eh is a measure of the electrochemical (oxidation/reduction) potential.
The type of pre-treatment employed can depend on the application.
In one application, an acid, acid equivalent, base, or base equivalent is added to adjust the pH to a desired pH value. Examples of acids or acid equivalents include monoprotic acids and polyprotic acids, such as mineral acids, sulfonic acids, carboxylic acids, vinylogous carboxylic acids, nucleic acids, and mixtures thereof. Examples of bases and base equivalents include strong bases (such as potassium hydroxide, barium hydroxide, cesium hydroxide, sodium hydroxide, strontium hydroxide, calcium hydroxide, magnesium hydroxide, lithium hydroxide, and rubidium hydroxide), superbases, carbonates, ammonia, hydroxides, metal oxides (particularly alkoxides), and counter anions of weak acids.
In one application, oxidation and reduction reactions can be used to adjust the Eh value. Eh is a measure of the oxidation or reduction potential of the medium 104. The oxidation or reduction potential is commonly referred to as electromotive force or EMF. The EMF is typically measured with respect to a standardized reference electrode. Non- limiting examples of standardized reference electrodes are hydrogen electrode (commonly referred to as SHE), copper/copper sulfate electrode, and silver/silver chloride to name a few.
In one variation, the phosphorus-containing target material is contacted with an oxidizing agent to oxidize the phosphorus-containing target material to form a primary species. The oxidizing agent may comprise a chemical oxidizing agent, an oxidation process, or combination of both.
A chemical oxidizing agent comprises a chemical composition in elemental or compounded form. The chemical oxidizing agent accepts an electron from the
phosphorus-containing target material. In the accepting of the electron, the oxidizing agent is reduced to form a reduced form of the oxidizing agent. Non-limiting examples of preferred chemical oxidizing agents are chlorine, chloroamines, chlorine dioxide, hypochlorites, trihalomethane, haloacetic acid, ozone, hydrogen peroxide, peroxygen compounds, hypobromous acid, bromoamines, hypobromite, hypochlorous acid, isocyanurates, tricholoro-s-triazinetriones, hydantoins, bromochloro-dimethyldantoins, 1- bromo-3-chloro-5,5-dimethyldantoin, l,3-dichloro-5,5-dimethyldantoin, sulfur dioxide, bisulfates, and combinations thereof. It is further believed that in some configurations one or more the following chemical compositions may oxidize the phosphorus-containing
target material: bromine, BrCl, permanganates, phenols, alcohols, oxyanions, arsenites, chromates, trichloroisocyanuric acid, and surfactants. The chemical oxidizing agent may further be referred to as an "oxidant" or an "oxidizer".
An oxidation process comprises a physical process that alone or in combination with a chemical oxidizing agent. The oxidation process removes and/or facilitates the removal an electron from the phosphorus-containing target material. Non-limiting examples of oxidation processes are electromagnetic energy, ultra violet light, thermal energy, ultrasonic energy, and gamma rays.
In another variation, the phosphorus-containing target material is contacted with a reducing agent to reduce the phosphorus-containing target material and form a primary species. The oxidizing agent may comprise a chemical oxidizing agent, an oxidation process, or combination of both.
A chemical reducing agent comprises a chemical composition in elemental or compounded form. The chemical reducing agent donates an electron to the phosphorus- containing target material to form the primary species. In the donating the electron, the reducing agent is oxidized to form an oxidized form of the reducing agent. Non-limiting examples of preferred chemical reducing agents are lithium aluminum hydride, nascent (atomic) hydrogen, sodium amalgam, sodium borohydride, compounds containing divalent tin ion, sulfite compounds, hydrazine, zinc-mercury amalgam, diisobutylaluminum hydride, Lindlar catalyst, oxalic acid, formic acid, ascorbic acid, phosphites,
hypophosphites, phosphorus acids, dithiothreitols, and compounds containing the divalent iron ion. The chemical reducing agent may further be referred to as a "reductant" or a "reducer".
A redox process is a physical process that alone or in combination with a chemical oxidizing agent transfers electrons to or form a target material. Non-limiting examples of oxidation processes are electromagnetic energy, ultra violet light, thermal energy, ultrasonic energy, gamma rays, and biological processes.
In one variation, the aqueous media is contacted with a halogenated species, such as chlorine, bromine, iodine, or an acid, base, or salt thereof. As will be appreciated, halogens impact the Eh of the aqueous media. In some configurations, halogens can impact the pH value of the aqueous media.
Other types of pre-treatment may be employed to remove species from the aqueous media that can impair removal of the phosphorus-containing target material and/or adjustment of the pH and/or Eh of the aqueous media.
The pre-treatment can comprise one or more of clarifying, disinfecting,
coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes. More specifically, the pre-treatment process can commonly comprise one of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes, more commonly any two of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, even more commonly any three of clarifying,
disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, yet even more commonly any four of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, still yet even more commonly any five of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, still yet even more commonly any six of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, still yet even more commonly any seven of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, still yet even more commonly any eight of clarifying, disinfecting, coagul ating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, still yet even more commonly any nine of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, still yet even more commonly any ten of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, still yet even more commonly any eleven of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes arranged in any order, and yet still even more commonly each of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing process arranged in any order. In some configurations, the pre-treatment may comprise or may further comprise processing by one or more of the additional process equipment of the water-handling system.
Contact of Aqueous Media with Rare Earth-Containing Additive
In step 112, the optionally pre-treated medium 104 is contacted with the rare earth- containing composition, additive, or particle to form the target-laden rare earth
composition. Contact can be added by any suitable technique, including not only adding the rare earth-containing composition, additive or particle (as a water soluble, partially soluble, or insoluble form) to the pre-treated medium 104 or vice versa but also forming the rare earth-containing composition, additive, or particle in situ in the pre-treated medium 104. As noted, the rare earth-containing composition, additive and/or particulate chemically and/or physically reacts with, sorbs, precipitates, transforms, or otherwise deactivates or binds with the phosphorus-containing target material and/or portion thereof. In one configuration, the rare earth-containing composition, additive and/or particulate chemically and/or physically reacts with, sorbs, precipitates, transforms, or otherwise deactivates or binds with at least about 25%, more commonly at least about 50%, more commonly more commonly more than about 50%, more commonly at least about 75%, and even more commonly at least about 95% of the phosphorus-containing target material or portion thereof. The target-laden rare earth composition includes the rare earth and one or both of the phosphorus-containing target material and one or more constituents, components or portion of the phosphorus-containing target material, and, in some cases, one or more other constituents, components or portion of the rare earth-containing composition, additive and/or particulate.
The temperature of the medium 104 during the contacting step can vary.
Typically, the temperature of the aqueous solution can vary during the contacting step. For example, temperature of aqueous solution can vary depending on the source of the water. Commonly, the temperature of the aqueous solution is ambient temperature.
Typically, the aqueous solution temperature ranges from about -5 degrees Celsius to about 50 degrees Celsius, more typically from about 0 degrees Celsius to about 45 degrees Celsius, yet even more typically from about 5 degrees Celsius to about 40 degrees Celsius and still yet even more typically from about 10 degrees Celsius to about 35 degrees
Celsius. It can be appreciated that each of the waters comprising each of the clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes may include optional processing units and/or operations that heat and/or cool one or more of each of the waters. In some configurations, each of the waters may be heated to have a temperature of typically at least about 20 degrees Celsius, more typically at least about 25 degrees Celsius, even more typically at least about 30 degrees
Celsius, yet even more typically of at least about 35 degrees Celsius, still yet even more typically of at least about 40 degrees Celsius, still yet even more typically of at least about
45 degrees Celsius, still yet even more typically of at least about 50 degrees Celsius, still
yet even more typically of at least about 60 degrees Celsius, still yet even more typically of at least about 70 degrees Celsius, still yet even more typically of at least about 80 degrees Celsius, still yet even more typically of at least about 90 degrees Celsius, still yet even more typically of at least about 100 degrees Celsius, still yet even more typically of at least about 110 degrees Celsius, still yet even more typically of at least about 120 degrees Celsius, still yet even more typically of at least about 140 degrees Celsius, still yet even more typically of at least about 150 degrees Celsius, or still yet even more typically of at least about 200 degrees Celsius. In some configurations, each of the waters comprising each of the clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes may be cooled to have a temperature of typically of no more than about 110 degrees Celsius, more typically of no more than about 100 degrees Celsius, even more typically of no more than about 90 degrees Celsius, yet even more typically of no more than about 80 degrees Celsius, still yet even more typically of no more than about 70 degrees Celsius, still yet even more typically of no more than about 60 degrees Celsius, still yet even more typically of no more than about 50 degrees Celsius, still yet even more typically of no more than about 45 degrees Celsius, still yet even more typically of no more than about 40 degrees Celsius, still yet even more typically of no more than about 35 degrees Celsius, still yet even more typically of no more than about 30 degrees Celsius, still yet even more typically of no more than about 25 degrees Celsius, still yet even more typically of no more than about 20 degrees Celsius, still yet even more typically of no more than about 15 degrees Celsius, still yet even more typically of no more than about 10 degrees Celsius, still yet even more typically of no more than about 5 degrees Celsius, or still yet even more typically of no more than about 0 degrees Celsius.
Separation of Target-Laden Rare Earth Composition from Aqueous Media
In optional step 116, the target-laden rare earth composition is removed from the aqueous medium (medium 104) to form a treated aqueous medium 124. In one configuration, commonly at least about 25%, more commonly at least about 50%, more commonly more commonly more than about 50%, more commonly at least about 75%, and even more commonly at least about 95% of the target-laden rare earth composition is removed from the aqueous media. It can be appreciated that in such instances, the target- laden rare earth composition comprises an insoluble material.
The target-laden rare earth composition may be removed by any suitable technique, such as by a liquid/solid separation system. Non- limiting examples of liquid/solid separation systems are filtration, floatation, sedimentation, cyclone, and centrifuging. Alternatively, the rare earth-containing additive is in the form of a particulate bed or supported porous and permeable matrix, such as a filter, through which the media passes.
Alternatively, the target-laden rare earth composition, dissolved in the water, may remain in the water in a de-activated form. Non-limiting examples of de-activated target- laden rare earth composition that may remain dissolved are environmentally stable co- ordination complexes of a primary or secondary phosphorus-containing species and the rare earth-containing composition.
In accordance with some embodiments, the treated aqueous medium 124 has a lower content of at least one of the one or more phosphorus-containing target materials compared to the aqueous medium 104. Commonly, the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.9 of that for the aqueous medium 104, more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.8 of that for the aqueous medium 104, even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus- containing target materials is at least about 0.7 of that for the aqueous medium 104, yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.6 of that for the aqueous medium 104, still yet even more commonly the treated aqueous media 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.5 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.4 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.3 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.2 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium
124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.1 of that for the aqueous medium 104, still yet even more commonly the
treated aqueous medium 124 content for at least one of the one or more phosphorus- containing target materials is at least about 0.05 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.01 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.005 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus- containing target materials is at least about 0.001 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.5 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 0.0005 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus- containing target materials is at least about 0.0001 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about 5x10~5 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about lxl 0"5 of that for the aqueous medium 104, still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus- containing target materials is at least about 5x10~6 of that for the aqueous medium 104, and still yet even more commonly the treated aqueous medium 124 content for at least one of the one or more phosphorus-containing target materials is at least about lxl 0"6 of that for the aqueous medium 104. Typically, the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 100,000 ppm, more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 10,000 ppm, even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 1,000 ppm, yet even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 100 ppm, still yet even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 10 ppm, still yet even more typically the phosphorus-containing target
material content in the treated aqueous medium 124 is no more than about 1 ppm, still yet even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 100 ppb, still yet even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 10 ppb, still yet even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 1 ppb, and yet still even more typically the phosphorus-containing target material content in the treated aqueous medium 124 is no more than about 0.1 ppb.
Step 116 can include optional treatment steps.
The treatment can comprise one or more of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing processes. More specifically, the treatment process can commonly comprise one of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing, more commonly any two of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, even more commonly any three of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, yet even more commonly any four of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, still yet even more commonly any five of clarifying, disinfecting, coagulating, aerating, fi ltering, separating solids and liquids, digesting, and polishing arranged in any order, still yet even more commonly any six of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, still yet even more commonly any seven of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, still yet even more commonly any eight of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, still yet even more commonly any nine of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, still yet even more commonly any ten of clarifying, disinfecting, coagulating, aerating, fi ltering, separating solids and liquids, digesting, and polishing arranged in any order, still yet even more commo ly any eleven of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order, and yet still
even more commonly each of clarifying, disinfecting, coagulating, aerating, filtering, separating solids and liquids, digesting, and polishing arranged in any order.
Regeneration of Rare Earth for Recycle
The separated product may be subjected to suitable processes for removal of the phosphorus-containing target material or portions thereof from the target-laden rare earth composition to enable the rare earth to be recycled to step 1 12. Regeneration processes include, for example, desorption, oxidation, reduction, thermal processes, irradiation, and the like.
As used herein cerium (III) may refer to cerium (+3), and cerium (+3) may refer to cerium (III). As used herein cerium (IV) may refer to cerium (+4), and cerium (+4) may refer to cerium (IV).
Some embodiments include a method comprising contacting an aqueous medium having a phosphorus-containing target material with a rare earth-containing additive to remove at least a portion of the phosphorus-containing target material from the aqueous medium to form a treated aqueous medium. In accordance with some embodiments, the phosphorus-containing target material comprises one or more of phosphoric acid, phospho-imide, phosphinate, phosphinite, non-fluoridated phosphonates, o-alkyl phosphoramideocyanidate, phosphonic acid, phosphinic acid, phosphonite, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, DNA backbone phosphate group, thiophosphate, thiophosphyoryl ester, thiophosphyoryl thioester, thiophosphyoryl amide, phosphatidylcholine, P-N amino compound, alklylated P-N amino compound, and P-N amino ester. Preferably, the rare earth-containing additive is primarily in the form of cerium (IV), cerium (III) or a mixture of cerium (IV) and cerium (III). Optionally, the rare earth-containing additive is water-soluble. Optionally, the rare earth-containing additive is water-insoluble. In accordance with some embodiments, the contacting step may further include: contacting the aqueous medium with an oxidizing agent to oxidize the
phosphorus-containing target material to form an oxidized phosphorus-containing target material, the oxidized phosphorus-containing target mater having one or both of a different structure and composition than the phosphorus-containing target material; and thereafter contacting, in the aqueous medium, the rare earth-containing additive with the oxidized phosphorus-containing target material to remove the oxidized phosphorus-containing target material. In accordance with some embodiments, the contacting step may further include: contacting the aqueous medium with a reducing agent to reduce the phosphorus-
containing target material to form a reduced phosphorus-containing target material, the reduced phosphorus-containing target material having one or both of a different structure and composition than the phosphorus-containing target material; and thereafter contacting, in the aqueous medium, the rare earth-containing additive with the reduced target phosphorus-containing target material to remove the reduced phosphorus-containing target material. In accordance with some embodiments, the contacting step may further include: contacting the aqueous medium with a base and/or base equivalent to convert the phosphorus-containing target material to a primary species different from the phosphorus- containing target material; and thereafter contacting, in the aqueous medium, the rare earth-containing additive with the primary species to remove the primary species. In accordance with some embodiments, the contacting step may further include: contacting the aqueous medium with an acid and/or acid equivalent to convert the phosphorus- containing target material to a primary species different from the phosphorus-containing target material; and thereafter contacting, in the aqueous medium, the rare earth-containing additive with the primary species to remove the primary species. In accordance with some embodiments, the phosphorus-containing target material is selected from the group consisting essentially of phosphoric acid, phosphinate, phosphinite, non-fluoridated phosphonates, phosphonic acid, phosphinic acid, phosphonite, thiophosphate,
thiophosphyoryl ester, and thiophosphyoryl thioester. In accordance with some embodiments, the phosphorus-containing target material comprises phosphatidylcholine. In accordance with some embodiments, the phosphorus-containing target material comprises one or more of a P-N amino compound, alklylated P-N amino compound, and P-N amino ester. In accordance with some embodiments, the phosphorus-containing target material comprises one or more of a phospho-imide, o-alkyl
phosphoramideocyanidate, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, and thiophosphyoryl amide. In accordance with embodiments, the phosphorus-containing target material comprises a DNA backbone phosphate group.
Some embodiments include a composition comprising an aqueous medium, a rare earth-containing additive, a phosphorus-containing target material, and a target-laden rare earth composition formed by the rare earth-containing additive and the phosphorus- containing target material. Preferably, the phosphorus-containing target material comprises one or more of one or more of phosphoric acid, phospho-imide, phosphinate, phosphinite, non-fluoridated phosphonates, o-alkyl phosphoramideocyanidate, phosphonic acid, phosphinic acid, phosphonite, dialkyl phosphoramidic dihalide, alkyl
phosphoramidate, DNA backbone phosphate group, thiophosphate, thiophosphyoryl ester, thiophosphyoryl thioester, thiophosphyoryl amide, phosphatidylcholine, P-N amino compound, alklylated P-N amino compound, and P-N amino ester. The rare earth- containing additive preferably comprises primarily cerium and wherein the target-laden rare earth composition is a complex formed by the rare earth-containing additive and phosphorus-containing target material. In accordance with some embodiments, the rare earth-containing additive may comprise primarily cerium and the target-laden rare earth composition may be formed by sorption of the phosphorus-containing target material by the rare earth-containing additive. In accordance with some embodiments, the rare earth- containing additive may comprise primarily cerium and the target-laden rare earth composition may be formed by a chemical reaction between the phosphorus-containing target material and the rare earth-containing additive. Optionally, the rare earth-containing additive is water-soluble. Optionally, the rare earth-containing additive is water-insoluble. In accordance with some embodiments, the phosphorus-containing target material may be selected from the group consisting essentially of phosphoric acid, phosphinate,
phosphinite, non-fluoridated phosphonates, phosphonic acid, phosphinic acid,
phosphonite, thiophosphate, thiophosphyoryl ester, and thiophosphyoryl thioester. In accordance with some embodiments, the phosphorus-containing target material may comprise phosphatidylcholine. In accordance with some embodiments, the phosphorus- containing target material may comprise one or more of a P-N amino compound, alklylated P-N amino compound, and P-N amino ester. In accordance with some embodiments, the phosphorus-containing target material may comprise one or more of a phospho-imide, o-alkyl phosphoramideocyanidate, dialkyl phosphoramidic dihalide, alkyl phosphoramidate, and thiophosphyoryl amide. In some embodiments, the phosphorus- containing target material may comprise a DNA backbone phosphate group.
EXAMPLES
The following examples are provided to illustrate certain embodiments and are not to be construed as limitations on the embodiments, as set forth in the appended claims. All parts and percentages are by weight unless otherwise specified.
Example 1
ABS plastic filter housings (1.25 inches in diameter and 2.0 inches in length) were packed with ceric oxide (Ce02) that was prepared from the thermal decomposition of 99%
cerium carbonate. The housings were sealed and attached to pumps for pumping an aqueous solution through the housings. The aqueous solutions were pumped through the material at flow rates of 50 and 75 ml/min. A gas chromatograph was used to measure the final content of the chemical contaminant. The chemical contaminants tested, their initial concentration in the aqueous solutions, and the percentage removed from solution are presented in Table 1.
Table 1
Example 2
Experiments were performed at ambient (room) temperature to remove various phosphorus-containing target materials from de-ionized and NSF standardized waters using cerium (IV) oxide.
The constituents of the NSF standardized solution, in accordance with NSF P231 "general test water 2" ("NSF"), are shown in Tables 2-5:
Table 2
Amount of Reagents Added
Table 3
Calculated Analyte Concentration
Media Preparation:
20 mg of Molycorp HSA cerium (IV) oxide was measured out in a plastic weigh boat for each sample to be tested. Approximately 10 mL of DI was added to the weigh boat and the media was allowed to wet for 30 minutes.
Influent Preparation:
The 30 mL stock solutions were prepared from solid or liquid reagents for each of the reagents in question. Influents were prepared from the stock solutions in 2.5 L batches for each reagent in question. 2.5 L of DI was measured out gravimetrically into a 4 L bottle. HEPES sodium buffer was added to the DI water followed by 2.5 mL of the stock solutions. The pH was adjusted to 7.5 ± 0.25 using 1 N HC1 and 1 N NaOH.
Isotherm Preparation:
500 mL of influent was measured out gravimetrically into four 500 mL bottles. Three bottles were labeled as a samples and the last was labeled as a control. The previously prepared media was poured into each sample bottle. Bottles were capped and sealed with electrical tape. Each bottle was then placed within a rolling container that could hold up to 10 bottles. The containers were then sealed with duct tape and placed on the rolling apparatus. Samples and controls were rolled for 24 hours. After 24 hours, the rolling containers were removed from the apparatus and the bottles were retrieved from the containers. A 10-45 mL sample of each solution was taken and filtered with a 0.2 μιη filter. Samples were analyzed either by a third party laboratory or with a HACH® colorimeter.
Total phosphorus was analyzed with a HACH® DR/890 colorimeter according to the HACH® Method 8190 for total phosphorus as phosphate. Briefly, the sample is pretreated with sulfuric acid and persulfate under heat to hydrolyze organic and inorganic phosphorus to orthophosphate, and then reacted with molybdate in an acid medium to produce a phosphomolybdate complex. The sample is then reduced with ascorbic acid, resulting in a blue-colored compound, which is measured spectroscopically.
The abilities of cerium (IV) oxide to remove phosphate and various
organophosphorus target materials were also tested.
The results are presented in Table 4:
Table 4
Glyphosate No Apparent Removal
Malathion No Apparent Removal
Phosphatidylcholine Removed
Sodium Phosphonoformate
Removed
tribasic hexahydrate
Triethyl phosphate No Apparent Removal
Tris(dimethylamino)
Removed
phosphine
Table 5 presents the removal capacities of cerium (IV) oxide for phosphate.
Table 5
This example demonstrates the successful removal of sulfate-containing compounds, halogenated compounds, carbonate-containing compounds, and phosphate- containing compounds, using a cerium (IV) dioxide powder. A cerium powder, having a 400 ppb arsenic removal capacity, was contacted with various solutions containing arsenic (III) as arsenite and arsenic (V) as arsenate and elevated concentrations of the compounds that compete for the known binding affinity between arsenic and cerium. The competing compounds included sulfate ions, fluoride ions, chloride ions, carbonate ions, silicate ions, and phosphate ions at concentrations of approximately 500% of the corresponding NSF concentration for the ion. The cerium dioxide powder was further contacted with arsenic- contaminated distilled and NSF P231 "general test water 2" ("NSF") water (Tables 3-5). Distilled water was used for the baseline measurement.
The results are presented in Fig. 3. As can be seen from Fig. 3, the ions in NSF water caused, relative to distilled water, a decreased cerium dioxide capacity for both
arsenite and arsenate, indicating a successful binding of these compounds to the rare earth metal. The presence of carbonate ion decreased the cerium dioxide removal capacity for arsenate more than arsenite. The presence of silicate ion decreased substantially cerium dioxide removal capacities for both arsenite and arsenate. Finally, phosphate ion caused the largest decrease in cerium dioxide removal capacities for arsenite (10X NSF concentration) and arsenate (50X NSF concentration), with the largest decrease in removal capacity being for arsenite. In other words, the preference of cerium dioxide for phosphate anion is greater than its preference for arsenite and arsenate.
Example 4
Example 4 is a control having about 0.2 g of struvite, NFLiMgPC eFkO, particles mixed in about 0.1L of a 0.15 mol/L acidic ferric chloride, FeCl3, solution. The molar ratio of ferric chloride to struvite was about 1.0 and the initial pH of the solution was about pH 2.5. The initial pH of the control solution was low enough to dissolve the struvite without the presence of ferric chloride. A magnetic stir-bar was used to stir the control solution. After stirring for at least about 16 hours, the solids were filtered from the control solution. The filtered solids were analyzed by x-ray diffraction and the control solution was analyzed by ICP-MS. Final solution pH value was about pH 2.3. The results are summarized in Table 6.
Table 6
Nominal Concentrations Residual Concentrations
Metal Struvite pH Mg P REE pH Mg P Metal P
Element (mg) Initial (ppm) (ppm) (ppm) Final (ppm) (ppm) (ppm) Removal
Fe 200 2.5 198 252 454 2.3 190 22 2.2 91.3%
The Example 4 shows that struvite can be more effectively removed with rare earth-containing compositions than with other removal materials such as ferric chloride. Example 5
An experiment was performed to remove reactive and non-f phosphorus from waste water utilizing cerium (III) chloride.
The parameters for the experiment were as follows:
Material: Waste Water (Influent, Effluent), Cerium Chloride (0.22 M or 37.8 g/L TREO)
Solutions:
Table 7 Waste Water Samples
Reaction Time: 16 hours
Reaction Volume: 250 mL
Analytical Instrument: HACH® DR/890 Colorimeter
Filtration:
Filter Type: Syringe Filter (Surfactant-Free Cellulose Acetate Membrane)
Pore Size: 0.2 μιη
Phosphorus is commonly found in waste water systems as both reactive and non- reactive phosphorus. To determine the abilities of CeCl3 to remove both reactive and non- reactive phosphorus species, reactions were performed by the addition of CeCl3 to both influent and effluent samples of waste water from a representative water treatment facility. Cerium (III) chloride was added in an equimolar ratio with the total phosphorus measured in solution, to ensure the ability of the reagent to react with most, if not all, phosphorus- containing compound(s) in solution. Following the addition of CeCl3, the samples were allowed to mix for 16 hours via magnetic stir before being filtered and analyzed for reactive and total phosphorus.
Reactive phosphorus was measured (in terms of P) using a HACH® DR/890 Colorimeter. The analysis involves the reaction between orthophosphate and molybdate in an acidic medium to produce heteropolyacids which are then reduced using ascorbic acid. This reduced form of phosphomolybdate produces an intense molybdenum blue color which may be analyzed in accordance with Beer's Law.
Total phosphorus was measured (in terms of P) using a HACH® DR/890
Colorimeter. This analysis was performed similar to the analysis for reactive
orthophosphate, but first required a pretreatment to digest the compound and oxidize the non-reactive phosphorus to reactive orthophosphate. This digestion involved heating the solution to 150°C for 30 minutes in a sulfuric acid medium in the presence of potassium
persulfate. The difference between reactive and total phosphorus is then calculated to determine the concentration of non-reactive phosphorus in solution.
Table 8 Waste Water Sam les Treated with CeCl
As can be seen from Tables 9-11, cerium (III) chloride demonstrated the ability to remove both reactive and non-reactive phosphorus with over 90% removal rate on an equimolar addition. This resulted in a removal capacity of approximately 176 mg P/ g theoretical rare earth oxie, which is near the theoretical removal rate of 180 mg P/g Ce02. This demonstrates a high specificity of CeCl3 for phosphorus, both reactive and non- reactive, in a waste water stream.
Table 9 Waste Water Treatment Results - Reactive Phosphorus
Table 10 Waste Water Treatment Results - Total Phos horus
Table 11 Waste Water Treatment Resu ts - Non-Reactive Phosphorus
Example 6
The goal of this experiment was to remove organophosphate from solution by using CeCl3 and Ce02
The parameters for the experiment are as follows:
Materials: HEPES Sodium Salt, D.I. Water, CeCl3, Ce02, trisodium
trimetaphosphate (Na3P30g), trimethyl phosphate ((CH3)3PC"4), triethylphosphine
((C2H5)3PO, methyltriphenyl phosphonium iodide (CH3P(C6H5)3I
Solutions:
Table 12 Stock Solutions
Reaction Volume: 500 mL per sample
Reaction Time: 24 hours
Filtration:
Filter Type: Membrane
Pore Size: 0.2 μιη
Analytical Instrument: HACH® DR/890 Colorimeter
Procedure:
For batch reactions, the procedure was to add stock solutions of Trisodium trimetaphosphate, trimethyl phosphate, tryelthylphophine and a solid compound of Methyltriphenyl Iodide to 4 2000 mL solutions of HEPES in D.I water buffered to 7.5 pH with a (PO4)3" concentration of 12 mg/L. The methyltriphenyl iodide was added directly to the solutions because of its low solubility. The solutions were then transferred to 4 separate 500 mL beakers. On a 1 : 1 molar ratio of Ce : P CeCl3 from the plant was added to 3 of the 4 samples and stirred for 24h using a stir bar. All 16 samples were then syringe filtered with 0.2 μιη filters. A digestion was then performed and tested using the HACH® colorimeter. The control for each compound was tested in triplicate.
For isotherms, the procedure was to add stock solutions of trisodium
trimetaphosphate, trimethyl phosphate, tryelthylphophine and a solid compound of methyltriphenyl iodide to 4 2000 mL solutions of HEPES in D.I water and buffered to 7.5 pH with a (PO4)3" concentration of 12 mg/L. The methyltriphenyl iodide was added directly to the solution because of its low solubility. The solutions were then separated into 500 mL Nalgene® bottles. 500 mg of Ce02 from bucket 14 was weighed and added to 3 of the 4 500 mL Nalgene® bottles. They were then placed in the tumbler and tumbled for 24h. All 16 samples were then syringe filtered with 0.2 μιη filters. The samples were
diluted by a factor of 4 since the test method used had a detection limit of 0.00 to 3.50 mg/L (PO4)3". A digestion was then performed and tested using the HACH® colorimeter. The control for each compound was tested in triplicate.
Results:
The results are presented in Tables 13-14 below.
Table 13 Batch Reactions with CeCl3
Table 14 Isotherms usin CeQ2
Conclusions:
CeCl3 batch reactions showed a definite removal of trisodium trimetaphosphate, removing trisodium trimetaphosphate from all three samples, with CeCl3 removing an average of 67.76% trisodium trimetaphosphate from solution. The remaining
organophosphates; trimethyl phosphate, triethylphosphine oxide and methyltriphenol phosphonium iodide, showed no removal from solution with the addition of CeCl3.
Ce02 isotherms showed a definite removal of trisodium trimetaphosphate. Ce02 removed an average of 72.96% trisodium trimetaphosphate from solution.
Triethylphosphine oxide exhibited a loss in solution with the addition of Ce02, with an average removal of 5.97%. These two organophosphate compounds exhibited removal in all of the three samples tested. Trimethyl phosphate and methyltriphenol phosphonium iodide showed no removal with the addition of Ce02 to the solutions.
Example 7
The goal of this experiment was to remove organophosphate from solution by using CeCl3 and Ce02
The parameters of the experiment were as follows:
Materials: HEPES sodium salt, D.I. sater, Ce02 (bucket 14), fenamiphos
(Ci3H22N03PS), profenofos (CnHi5BrC103PS), methamidophos (C2H8N02PS), dicrotophos (C8Hi6N05P)
Solutions:
Reaction Volume: 500 mL per sample
Reaction Time: 24 hours
Filtration:
Filter Type: Membrane
Pore Size: 0.2 μιη
Analytical Instrument: HACH® DR/890 Colorimeter
Procedure:
The procedure for isotherms was as follows:
According to a concentration of 3.5 mg/L [P04]3~ and using a molar ratio of organophosphates (pesticides) to [P04]3" an amount of compound was obtained and added to 2000 mL solutions of HEPES in D.I water and buffered to 7.5 pH. The fenamiphos remained stirring over night to allow the compound to dissolve in solution. The solutions were then separated into 4 500 mL Nalgene® bottles. 500 mg of Ce02 from bucket 14 was weighed and added to 3 of the 4 500 mL Nalgene® bottles. They were then placed in the tumbler and tumbled for 24h. All 16 samples were then syringe filtered with 0.2 μιη filters. A digestion was then performed on the pesticides and tested using the HACH® colorimeter. The control for each compound was tested in triplicate.
Results:
The results are presented in Tables 16-17 below:
Table 16 Addition of the pesticide compounds in solution
Table 17 Isotherms using CeO?
Conclusions:
After performing the isotherms of 4 different pesticides, it is concluded that fenamiphos and profenofos showed no removal with the addition of Ce02.
Methamidophos expressed a definite removal of organophosphates in the solution when using the Ce02. Dicrotophos had an apparent removal of organophosphate removing only one of the three samples. Two of the four pesticides tested had to be heated to 75° C and fenamiphos stirred in solution for over 16h. There may have been a degeneration of the pesticide fenamiphos because the concentrations of the samples and the control came in much lower than the expected 3.5mg/L.
A number of variations and modifications of the disclosure can be used. One of more embodiments of the disclosure can be used separately and i combination. That is, any embodiment alone can be used and all combinations and permutations thereof can be used. It would be possible to provide for some features of the disclosure without providing others.
The present disclosure, in various embodiments, configurations, or aspects, includes components, methods, processes, systems and/or apparatus substantially as depicted and described herein, including various embodiments, configurations, aspects, sub-combinations, and subsets thereof. Those of skill in the art will understand how to make and use the various embodiments, configurations, or aspects after understanding the present disclosure. The present disclosure, in various embodiments, configurations, and aspects, includes providing devices and processes in the absence of items not depicted and/or described herein or in various embodiments, configurations, or aspects hereof, including in the absence of such items as may have been used in previous devices or processes, e.g., for improving performance, achieving ease and\or reducing cost of implementation.
The foregoing discussion has been presented for purposes of illustration and description. The foregoing is not intended to limit the disclosure to the form or forms disclosed herein. In the foregoing Detailed Description for example, various features of the disclosure are grouped together in one or more embodiments, configurations, or aspects for the purpose of streamlining the disclosure. The features of the embodiments, configurations, or aspects of the disclosure may be combined in alternate embodiments, configurations, or aspects other than those discussed above. This method of disclosure is not to be interpreted as reflecting an intention that any claim and/or combination of claims require more features than are expressly recited in each claim. Rather, as the following claims reflect, inventive aspects lie in less than all features of a single foregoing disclosed embodiment, configuration, or aspect. Thus, the following claims are hereby incorporated into this Detailed Description, with each claim standing on its own as a separate preferred embodiment.
Moreover, though the description of the disclosure has included descriptions of one or more embodiments, configurations, or aspects and certain variations and modifications, other variations, combinations, and modifications are within the scope of the disclosure, e.g., as may be within the skill and knowledge of those in the art, after understanding the present disclosure. It is intended to obtain rights which include alternative embodiments, configurations, or aspects to the extent permitted, including alternate, interchangeable and/or equivalent structures, functions, ranges or steps to those claimed, whether or not such alternate, interchangeable and/or equivalent structures, functions, ranges or steps are disclosed herein, and without intending to publicly dedicate any patentable subject matter.
Claims
1. A method, comprising:
contacting an aqueous medium comprising a phosphorus-containing target material with a rare earth-containing additive to remove at least a portion of the phosphorus- containing target material from the aqueous medium to form a treated aqueous medium, wherein the phosphorus-containing target material comprises one or more of phosphoric acid, phosphinate, non-fluoridated phosphonates, phosphonic acid, phosphinic acid, phosphine oxide, metaphosphate, phosphonite, thiophosphate, thiophosphyoryl amide, and phosphatidylcholine .
2. The method of claim 1 , wherein the rare earth-containing additive is primarily in the form of cerium (IV), cerium (III) or a mixture of cerium (IV) and cerium (III).
3. The method of claim 1, wherein the contacting step comprises the sub- steps:
contacting the aqueous medium with an oxidizing agent to oxidize the phosphorus- containing target material to form an oxidized phosphorus-containing target material, the oxidized phosphorus-containing target mater having one or both of a different structure and composition than the phosphorus-containing target material; and
thereafter contacting, in the aqueous medium, the rare earth-containing additive with the oxidized phosphorus-containing target material to remove the oxidized phosphorus-containing target material.
4. The method of claim 1, wherein the contacting step comprises the sub- steps:
contacting the aqueous medium with a reducing agent to reduce the phosphorus- containing target material to form a reduced phosphorus-containing target material, the reduced phosphorus-containing target material having one or both of a different structure and composition than the phosphorus-containing target material; and
thereafter contacting, in the aqueous medium, the rare earth-containing additive with the reduced target phosphorus-containing target material to remove the reduced phosphorus-containing target material.
WO 2012/141895 PCT/US2012/030974 contacting the aqueous medium with a base and/or base equivalent to convert the phosphorus-containing target material to a primary species different from the phosphorus- containing target material; and
thereafter contacting, in the aqueous medium, the rare earth-containing additive with the primary species to remove the primary species.
6. The method of claim 1, wherein the contacting step comprises the sub- steps:
contacting the aqueous medium with an acid and/or acid equivalent to convert the phosphorus-containing target material to a primary species different from the phosphorus- containing target material; and
thereafter contacting, in the aqueous medium, the rare earth-containing additive with the primary species to remove the primary species.
7. The method of claim 1, wherein the rare earth-containing additive is water- soluble.
8. The method of claim 1, wherein the rare earth-containing additive is water- insoluble.
9. The method of claim 1 , wherein the phosphorus-containing target material is selected from the group consisting essentially of phosphoric acid, phosphinate, phosphinite, non-fluoridated phosphonates, phosphonic acid, phosphinic acid,
phosphonite, thiophosphate, and thiophosphyoryl ester.
10. The method of claim 1, wherein the phosphorus-containing target material comprises phosphatidylcholine.
11. The method of claim 1 , wherein the phosphorus-containing target material comprises a thiophosphyoryl amide.
12. A composition, comprising:
an aqueous medium,
a rare earth-containing additive,
a phosphorus-containing target material comprising one or more of one or more of phosphoric acid, phosphinate, phosphinite, non-fluoridated phosphonates, phosphonic acid, phosphinic acid, phosphine oxide, metaphosphate, phosphonite, thiophosphate, thiophosphyoryl ester, thiophosphyoryl amide, and phosphatidylcholine, and
a target-laden rare earth composition formed by the rare earth-containing additive and the phosphorus-containing target material.
WO 2012/141895 PCT/US2012/030974
13. The composition of claim 12, wherein the rare earth-containing additive comprises primarily cerium and wherein the target-laden rare earth composition is a complex formed by the rare earth-containing additive and phosphorus-containing target material.
14. The composition of claim 12, wherein the rare earth-containing additive comprises primarily cerium and wherein the target-laden rare earth composition is formed by sorption of the phosphorus-containing target material by the rare earth-containing additive.
15. The composition of claim 12, wherein the rare earth-containing additive comprises primarily cerium and wherein the target-laden rare earth composition is formed by a chemical reaction between the phosphorus-containing target material and the rare earth-containing additive.
16. The composition of claim 12, wherein the rare earth-containing additive is water-soluble.
17. The composition of claim 12, wherein the rare earth-containing additive is water-insoluble.
18. The composition of claim 12, wherein the phosphorus-containing target material is selected from the group consisting essentially of phosphoric acid, phosphinate, phosphinite, non-fluoridated phosphonates, phosphonic acid, phosphinic acid,
phosphonite, thiophosphate, and thiophosphyoryl ester.
19. The composition of claim 12, wherein the phosphorus-containing target material comprises phosphatidylcholine.
20. The composition of claim 12, wherein the phosphorus-containing target material comprises a thiophosphyoryl amide.
21. A process, comprising:
receiving an aqueous medium comprising a target material, the target material comprising both reactive and non-reactive phosphates; and
contacting the aqueous medium with a rare earth-containing additive to remove at least most of the reactive and non-reactive phosphates.
22. The process of claim 21 , wherein the reactive phosphate is in the form of an anion and the non-reactive phosphate is in the form of a cation, neutral species, and/or colloid or suspension.
23. The process of claim 22, wherein at least most of the rare earth-containing additive is water soluble.
WO 2012/141895 PCT/US2012/030974
24. The process of claim 22, wherein at least most of the rare earth-containing material is water insoluble.
25. The process of claim 21, further comprising before the contacting step: converting at least a portion of the non-reactive phosphate to reactive phosphate.
26. The process of claim 21, further comprising before the contacting step: converting at least a portion of the reactive phosphate to non-reactive phosphate.
Applications Claiming Priority (48)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161474902P | 2011-04-13 | 2011-04-13 | |
| US201161475155P | 2011-04-13 | 2011-04-13 | |
| US61/474,902 | 2011-04-13 | ||
| US61/475,155 | 2011-04-13 | ||
| US201161476667P | 2011-04-18 | 2011-04-18 | |
| US61/476,667 | 2011-04-18 | ||
| US201161484919P | 2011-05-11 | 2011-05-11 | |
| US61/484,919 | 2011-05-11 | ||
| US201161495731P | 2011-06-10 | 2011-06-10 | |
| US61/495,731 | 2011-06-10 | ||
| US201161496425P | 2011-06-13 | 2011-06-13 | |
| US61/496,425 | 2011-06-13 | ||
| US201161538634P | 2011-09-23 | 2011-09-23 | |
| US13/244,092 US20120074071A1 (en) | 2010-09-23 | 2011-09-23 | Process for treating waters and water handling systems to remove scales and reduce the scaling tendency |
| US13/244,117 US20120103909A1 (en) | 2010-09-23 | 2011-09-23 | Particulate cerium dioxide and an in situ method for making and using the same |
| US13/244,092 | 2011-09-23 | ||
| US61/538,634 | 2011-09-23 | ||
| US13/244,117 | 2011-09-23 | ||
| US201161539780P | 2011-09-27 | 2011-09-27 | |
| US61/539,780 | 2011-09-27 | ||
| US201161546803P | 2011-10-13 | 2011-10-13 | |
| US61/546,803 | 2011-10-13 | ||
| US201161553809P | 2011-10-31 | 2011-10-31 | |
| US61/553,809 | 2011-10-31 | ||
| US201161558887P | 2011-11-11 | 2011-11-11 | |
| US61/558,887 | 2011-11-11 | ||
| US201161564132P | 2011-11-28 | 2011-11-28 | |
| US61/564,132 | 2011-11-28 | ||
| US13/356,581 US20120187047A1 (en) | 2011-01-21 | 2012-01-23 | Rare earth removal of hydrated and hydroxyl species |
| US13/356,581 | 2012-01-23 | ||
| US13/356,574 | 2012-01-23 | ||
| US13/356,574 US20120187337A1 (en) | 2011-01-21 | 2012-01-23 | Rare earth removal of phosphorus-containing materials |
| US201261596851P | 2012-02-09 | 2012-02-09 | |
| US61/596,851 | 2012-02-09 | ||
| US13/410,081 | 2012-03-01 | ||
| US13/410,081 US20120223022A1 (en) | 2011-03-01 | 2012-03-01 | Contaminant removal from waters using rare earths |
| US201261613883P | 2012-03-21 | 2012-03-21 | |
| US201261613857P | 2012-03-21 | 2012-03-21 | |
| US61/613,857 | 2012-03-21 | ||
| US61/613,883 | 2012-03-21 | ||
| US201261614427P | 2012-03-22 | 2012-03-22 | |
| US201261614418P | 2012-03-22 | 2012-03-22 | |
| US61/614,418 | 2012-03-22 | ||
| US61/614,427 | 2012-03-22 | ||
| US13/432,987 | 2012-03-28 | ||
| US13/433,097 US20180251383A9 (en) | 2010-04-13 | 2012-03-28 | Non-metal-containing oxyanion removal from waters using rare earths |
| US13/433,097 | 2012-03-28 | ||
| US13/432,987 US9233863B2 (en) | 2011-04-13 | 2012-03-28 | Rare earth removal of hydrated and hydroxyl species |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2012141895A2 true WO2012141895A2 (en) | 2012-10-18 |
| WO2012141895A3 WO2012141895A3 (en) | 2013-06-13 |
Family
ID=47009911
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2012/030974 WO2012141895A2 (en) | 2011-04-13 | 2012-03-28 | Rare earth removal of phosphorus-containing materials |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2012141895A2 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016051329A1 (en) | 2014-10-02 | 2016-04-07 | Xylem Ip Management S.À R.L. | A method for treating wastewater |
| WO2016051328A1 (en) * | 2014-10-02 | 2016-04-07 | Xylem Ip Management S.À R.L. | Method for managing a wastewater treatment process |
| US10988395B2 (en) | 2018-09-25 | 2021-04-27 | Neo Chemicals & Oxides, LLC | Cerium-lanthanum treatment method for reduction of contaminants in wastewater membrane bioreactors |
| US11111161B2 (en) | 2017-11-01 | 2021-09-07 | Neo Water Treatment, Llc | Rare earth clarifying agent and method for use in primary treatment of wastewater |
| US11772054B2 (en) | 2020-03-23 | 2023-10-03 | Neo Water Treatment, Llc | Rare earth treatment of membranes to remove contaminants |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3617569A (en) * | 1970-07-31 | 1971-11-02 | Dow Chemical Co | Removal of phosphate from waste water |
| KR960000309B1 (en) * | 1993-05-07 | 1996-01-04 | 주식회사엘지금속 | Removing method of phosphotic acid desolved in waste water |
| US5573673A (en) * | 1994-05-06 | 1996-11-12 | Agency Of Industrial Science And Technology | Hydrous composite cerium-phosphorus oxide for immobilization of strontium ions in solution |
| JPH09141274A (en) * | 1995-11-15 | 1997-06-03 | Kankyo Eng Kk | High-degree treatment for waste water containing phosphorus |
| JP2004148289A (en) * | 2002-09-04 | 2004-05-27 | Japan Organo Co Ltd | Fluorine or phosphorus-containing water treatment equipment |
| US20050054077A1 (en) * | 2003-09-04 | 2005-03-10 | Tandem Labs. | Devices and methods for separating phospholipids from biological samples |
| US20050119497A1 (en) * | 2003-12-02 | 2005-06-02 | Jong-In Hong | Novel dinuclear metal complex and pyrophosphate assay using the same |
| WO2008010844A2 (en) * | 2005-12-09 | 2008-01-24 | Board Of Regents, The University Of Texas System | Compositions and methods for the detection of chemical warfare agents |
| US20080213906A1 (en) * | 2007-01-26 | 2008-09-04 | Sigma Aldrich Company | Compositions and methods for combining protein precipitation and solid phase extraction |
| US20100155330A1 (en) * | 2008-11-11 | 2010-06-24 | Molycorp Minerals, Llc | Target material removal using rare earth metals |
-
2012
- 2012-03-28 WO PCT/US2012/030974 patent/WO2012141895A2/en active Application Filing
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3617569A (en) * | 1970-07-31 | 1971-11-02 | Dow Chemical Co | Removal of phosphate from waste water |
| KR960000309B1 (en) * | 1993-05-07 | 1996-01-04 | 주식회사엘지금속 | Removing method of phosphotic acid desolved in waste water |
| US5573673A (en) * | 1994-05-06 | 1996-11-12 | Agency Of Industrial Science And Technology | Hydrous composite cerium-phosphorus oxide for immobilization of strontium ions in solution |
| JPH09141274A (en) * | 1995-11-15 | 1997-06-03 | Kankyo Eng Kk | High-degree treatment for waste water containing phosphorus |
| JP2004148289A (en) * | 2002-09-04 | 2004-05-27 | Japan Organo Co Ltd | Fluorine or phosphorus-containing water treatment equipment |
| US20050054077A1 (en) * | 2003-09-04 | 2005-03-10 | Tandem Labs. | Devices and methods for separating phospholipids from biological samples |
| US20050119497A1 (en) * | 2003-12-02 | 2005-06-02 | Jong-In Hong | Novel dinuclear metal complex and pyrophosphate assay using the same |
| WO2008010844A2 (en) * | 2005-12-09 | 2008-01-24 | Board Of Regents, The University Of Texas System | Compositions and methods for the detection of chemical warfare agents |
| US20080213906A1 (en) * | 2007-01-26 | 2008-09-04 | Sigma Aldrich Company | Compositions and methods for combining protein precipitation and solid phase extraction |
| US20100155330A1 (en) * | 2008-11-11 | 2010-06-24 | Molycorp Minerals, Llc | Target material removal using rare earth metals |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016051329A1 (en) | 2014-10-02 | 2016-04-07 | Xylem Ip Management S.À R.L. | A method for treating wastewater |
| WO2016051328A1 (en) * | 2014-10-02 | 2016-04-07 | Xylem Ip Management S.À R.L. | Method for managing a wastewater treatment process |
| US11111161B2 (en) | 2017-11-01 | 2021-09-07 | Neo Water Treatment, Llc | Rare earth clarifying agent and method for use in primary treatment of wastewater |
| US11713262B2 (en) | 2017-11-01 | 2023-08-01 | Neo Water Treatment, Llc | Rare earth clarifying agent and method for use in primary treatment of wastewater |
| US10988395B2 (en) | 2018-09-25 | 2021-04-27 | Neo Chemicals & Oxides, LLC | Cerium-lanthanum treatment method for reduction of contaminants in wastewater membrane bioreactors |
| US11772054B2 (en) | 2020-03-23 | 2023-10-03 | Neo Water Treatment, Llc | Rare earth treatment of membranes to remove contaminants |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2012141895A3 (en) | 2013-06-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20120187337A1 (en) | Rare earth removal of phosphorus-containing materials | |
| US20120261611A1 (en) | Rare earth removal of phosphorus-containing materials | |
| Saleh et al. | Water treatment technologies in removing heavy metal ions from wastewater: A review | |
| Khatri et al. | Recent strategies for the removal of iron from water: A review | |
| US20120223022A1 (en) | Contaminant removal from waters using rare earths | |
| US9233863B2 (en) | Rare earth removal of hydrated and hydroxyl species | |
| KR101613774B1 (en) | Method for processing toxic matter-containing water and processing device | |
| JP6295393B2 (en) | Method for removing lead compounds with additives containing rare earth elements | |
| WO2009063456A1 (en) | Method for adsorption of phosphate contaminants from water solutions and its recovery | |
| US20120074071A1 (en) | Process for treating waters and water handling systems to remove scales and reduce the scaling tendency | |
| WO2012141895A2 (en) | Rare earth removal of phosphorus-containing materials | |
| US20110278232A1 (en) | Heavy metal removal from waste streams | |
| US9890061B2 (en) | Compositions and methods for selective anion removal | |
| US20120187047A1 (en) | Rare earth removal of hydrated and hydroxyl species | |
| WO2012141897A2 (en) | Non-metal-containing oxyanion removal from waters using rare earths | |
| Ni et al. | Complexation-based selectivity of organic phosphonates adsorption from high-salinity water by neodymium-doped nanocomposite | |
| Zelmanov et al. | Phosphate removal from water and recovery using iron (Fe+ 3) oxide/hydroxide nanoparticles-based agglomerates suspension (AggFe) as adsorbent. | |
| Nir et al. | Removal of phosphorus from secondary effluents by coagulation and ultrafiltration | |
| Haldar et al. | Arsenic and fluoride problems of groundwater in West Bengal and available technologies for remediation | |
| Ngo et al. | A comparison of conventional and non-conventional treatment technologies on arsenic removal from water | |
| Ntwampea | Corrected Proof | |
| AU2022395767A1 (en) | Method for removing dissolved organic substances in liquids using a superfine adsorbent, and means for carrying out the method | |
| WO2022250612A2 (en) | Functionalized beads and their uses thereof | |
| Chelme‐Ayala et al. | Physico‐Chemical Processes | |
| Shomey et al. | Control of disinfection by-product (DBP) precursors |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12771854 Country of ref document: EP Kind code of ref document: A2 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12771854 Country of ref document: EP Kind code of ref document: A2 |