WO2012118092A1 - Fusion protein - Google Patents
Fusion protein Download PDFInfo
- Publication number
- WO2012118092A1 WO2012118092A1 PCT/JP2012/054967 JP2012054967W WO2012118092A1 WO 2012118092 A1 WO2012118092 A1 WO 2012118092A1 JP 2012054967 W JP2012054967 W JP 2012054967W WO 2012118092 A1 WO2012118092 A1 WO 2012118092A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- fusion protein
- rna
- virus
- mazf
- vector
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/16—Hydrolases (3) acting on ester bonds (3.1)
- C12N9/22—Ribonucleases RNAses, DNAses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/775—Apolipopeptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/20—Fusion polypeptide containing a tag with affinity for a non-protein ligand
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16033—Use of viral protein as therapeutic agent other than vaccine, e.g. apoptosis inducing or anti-inflammatory
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16211—Human Immunodeficiency Virus, HIV concerning HIV gagpol
- C12N2740/16222—New viral proteins or individual genes, new structural or functional aspects of known viral proteins or genes
Definitions
- the present invention relates to a fusion protein in which a polypeptide having affinity for viral RNA is fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner.
- HIV human immunodeficiency virus
- HCV hepatitis C virus
- influenza virus SARS virus
- HIV is rapidly spreading in developing countries and has become a social problem.
- HIV infects and destroys cells that express CD4 molecules.
- CD4 positive helper T cells and macrophages which are central cells that control immunity, are reduced in the human body that is infected with HIV, and finally it becomes a severe immunodeficiency state and opportunistic infection such as carini pneumonia. Develops symptoms. This condition is referred to as acquired immunodeficiency syndrome (AIDS).
- AIDS acquired immunodeficiency syndrome
- antiviral agents reverse transcriptase inhibitors, protease inhibitors, etc.
- some antiviral agents have already been put into practical use. Yes.
- mutants resistant to antiviral agents may appear in individuals infected with HIV.
- gene therapy drugs that inhibit the growth of HIV using nucleic acids such as RNA decoys and ribozymes, proteins such as transdominant mutant proteins and intracellular antibodies as active ingredients. Is not reached.
- Patent Document 1 a technique for expressing a single-stranded RNA-specific ribonuclease specifically for HIV-infected cells.
- Patent Document 2 a technique for expressing a single-stranded RNA-specific ribonuclease specifically for HIV-infected cells.
- the expression of single-stranded RNA-specific endoribonuclease is induced in a manner dependent on the Tat protein expressed with HIV infection, and the single-stranded RNA in the cell containing the HIV genome is degraded.
- HIV replication and budding are prevented in the cells.
- the HIV-derived RNA is degraded to stop the expression of Tat protein, and the expressed Tat protein disappears from the cell, the expression of endoribonuclease is also stopped.
- Patent Document 1 is advantageous in comparison with a method of inducing cell death specifically for HIV-infected cells in that it does not cause an excessive decrease in CD4-positive T cells.
- CTVI Capsid-Targeted Viral Activation
- the present invention has been made in view of the above prior art, and an object thereof is to provide a fusion protein useful for the treatment and prevention of RNA virus infection.
- the present inventors have developed an RNA virus particle from an RNA virus-infected cell using a fusion protein in which a polypeptide having affinity for viral RNA of an RNA virus is fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner. Has been found to be effectively suppressed, and further, the formation of provirus in the infected cells when the RNA virus is infected with the cells is effectively suppressed, and the present invention has been completed. It was.
- the present invention [1] A fusion protein in which a polypeptide having affinity for viral RNA of an RNA virus is fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner, [2] The fusion protein according to [1], wherein a polypeptide having affinity for viral RNA of an RNA virus is fused to an endoribonuclease that specifically cleaves single-stranded RNA via a linker peptide, [3] The fusion protein according to [1], further having a lipid modification signal in the N-terminal region, [4] The fusion protein according to [1], wherein the polypeptide showing affinity for viral RNA of RNA virus is a polypeptide showing affinity for packaging signal of RNA virus, [5] The fusion protein according to [4], wherein the polypeptide having affinity for the packaging signal of RNA virus is a polypeptide having affinity for the packaging signal of retrovirus, [6] The fusion protein according to [5],
- a fusion protein useful for treatment or prevention of RNA virus infection a nucleic acid encoding the fusion protein, a vector containing the nucleic acid, a pharmaceutical composition containing the vector, a cell into which the nucleic acid has been introduced,
- a method for treating or preventing an RNA virus infection comprising a step of introducing a vector into a cell, and a method for suppressing the budding of an RNA virus, comprising the step of introducing a fusion protein, nucleic acid or vector selected from the present invention into a cell.
- FIG. 1 is a diagram schematically showing an expression system of a vector constructed in Example 1.
- FIG. In the figure, 1 to 6 schematically show the expression systems of the vectors constructed in Example 1 (1) to (6), respectively. It is a figure which shows the observation result by the fluorescence microscope in Example 2.
- FIG. A in the figure is a cell prepared by using 0.025 ⁇ g of pBApo-CMV pur DNA, pBApo-MazF, or pBApo-Gag-MazF for DNA introduction (shown as Mock, MazF, Gag-MazF from the left in FIG. 2A).
- FIG. 4 is a diagram showing the measurement results by qRT-PCR in Example 2.
- A shows the result of qRT-PCR using total RNA extracted from cells prepared using 0.025 ⁇ g of pBApo-CMV pur DNA, pBApo-MazF, or pBApo-Gag-MazF for DNA introduction as a template ( Fig.
- 3A shows Mock, MazF, and Gag-MazF from the left), and B in the figure is prepared using 0.4 ⁇ g of pBApo-CMV pur DNA, pBApo-MazF, or pBApo-Gag-MazF for DNA introduction.
- the results of qRT-PCR using the total RNA extracted from the obtained cells as a template are shown. It is a figure which shows the result of the western blotting in Example 3. It is a figure which shows the result of the western blotting in Example 4. It is a figure which shows the result of the western blotting in Example 5.
- RNA virus is a generic term for viruses whose genome is composed of RNA.
- RNA virus includes single-stranded RNA viruses and double-stranded RNA viruses.
- double-stranded RNA virus is a generic term for viruses whose genome is composed of double-stranded RNA.
- double-stranded RNA virus includes viruses belonging to the family Reoviridae such as rotavirus.
- single-stranded RNA virus is a generic term for viruses whose genome is composed of single-stranded RNA.
- single-stranded RNA virus includes single-stranded (+) RNA viruses, single-stranded ( ⁇ ) RNA viruses, and retroviruses.
- single-stranded (+) RNA virus is a generic term for a single-stranded RNA virus in which genomic RNA itself that does not have a DNA stage in its life cycle can function as mRNA.
- the “single-stranded (+) RNA virus” in the present specification includes viruses belonging to the Picornaviridae family such as hepatitis A virus and foot-and-mouth disease virus, viruses belonging to the Caliciviridae family, viruses belonging to the Astroviridae family, and SARS viruses. Viruses belonging to the family Coronaviridae, such as West Nile virus, yellow fever virus, Japanese encephalitis virus, viruses belonging to Flaviviridae such as HCV, and viruses belonging to Togaviridae such as rubella virus.
- single-stranded ( ⁇ ) RNA virus is a general term for single-stranded RNA viruses having a life cycle in which genomic RNA is transcribed by RNA-dependent RNA polymerase.
- single-stranded ( ⁇ ) RNA virus includes viruses belonging to the Rhabdoviridae family such as rabies virus, viruses belonging to the Filoviridae family such as Ebola virus, and paramyxos such as epidemic parotitis virus.
- viruses belonging to the family Viridae viruses belonging to the Orthomyxoviridae family such as influenza virus, viruses belonging to the Bunyaviridae family, and viruses belonging to the Arenaviridae family such as Lassa virus and hepatitis D virus are included.
- retrovirus is a generic term for RNA viruses belonging to the Retroviridae family, whose genome is composed of RNA and has a life cycle that converts genomic RNA into DNA.
- retrovirus includes oncorretroviruses such as human T lymphophilic virus (HTLV) and Moloney murine leukemia virus (MMLV), human immunodeficiency virus (HIV), and simian immunodeficiency virus (SIV). Such as lentivirus.
- retroviral vector refers to a virus particle produced by genetic recombination technology based on oncorretrovirus, lentivirus, etc. belonging to the family Retroviridae, and includes oncoretrovirus vector, lentivirus vector, pseudo Contains a type vector.
- a pseudotype vector refers to a recombinant retroviral vector having an Env protein whose origin is different from that of a Gag protein or a Pol protein.
- virus RNA of an RNA virus refers to RNA derived from an RNA virus.
- viral viral RNA refers to genomic RNA of RNA virus, RNA generated by splicing the genomic RNA (for example, RNA that contributes to the expression of RNA viral accessory proteins), and Includes RNA transcribed from genomic RNA.
- RNA virus packaging signal refers to a cis-factor that is present on the genome of an RNA virus and is required for the incorporation of genomic RNA into a viral particle.
- the packaging signal ( ⁇ / Psi) is a region of about 800 bases from the 5 ′ end of the genomic RNA.
- endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner refers to an endo-type ribonuclease that hydrolyzes a diester bond of single-stranded RNA in a base sequence-specific manner.
- lipid modification signal refers to an amino acid sequence that directs modification of a protein by addition of lipids such as fatty acids and isoprenoids.
- lipid modification signals for example, myristoylation signals, palmitoylation signals, myristoylation and palmitoylation double lipid modification signals, O-acylation signals, and isoprenylation signals are known.
- the fusion protein of the present invention comprises a polypeptide having affinity for viral RNA of an RNA virus fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner.
- the fusion protein of the present invention recognizes a viral RNA such as a viral genomic RNA incorporated into a viral particle by a domain derived from a polypeptide having an affinity for the viral RNA of the RNA virus, and further sequences the single-stranded RNA in a sequence-specific manner.
- Viral RNA can be cleaved at a specific nucleotide sequence and decomposed by the endoribonuclease activity that cleaves it.
- the fusion protein of the present invention can suppress the formation of a provirus when an RNA virus infects a cell, and also cleaves the viral RNA in the cell infected with the RNA virus, thereby budding (production) RNA virus particles. Can be suppressed. Furthermore, the infectivity of the virus particles can be reduced by degrading the viral genome of the RNA virus even in the budding (produced) virus particles. Therefore, the fusion protein of the present invention is useful for the treatment or prevention of RNA virus infection.
- the polypeptide having affinity for viral RNA of RNA virus is not particularly limited to the present invention, but a polypeptide having affinity for packaging signal of RNA virus is exemplified.
- a polypeptide showing affinity for a single-stranded RNA virus packaging signal is more preferred, and a polypeptide showing affinity for a retroviral packaging signal is even more preferred.
- the amino acid sequence constituting the polypeptide having an affinity for the retroviral packaging signal is preferably an amino acid sequence derived from a retrovirus Gag protein, for example, an amino acid sequence derived from a human immunodeficiency virus Gag protein. Can be used.
- the amino acid sequence derived from the Gag protein include nucleocapsid.
- the nucleocapsid is one of the polypeptides constituting the retroviral Gag protein, and refers to a polypeptide involved in specific recognition of the packaging signal by the Gag protein. Nucleocapsid recognizes the secondary structure of the packaging signal through two zinc finger motifs.
- the full-length amino acid sequence constituting the natural nucleocapsid may be used, or a fragment or mutation thereof.
- the amino acid sequence constituting the body may be used.
- the amino acid sequence constituting the natural nucleocapsid include the amino acid sequence of a nucleocapsid derived from human immunodeficiency virus type 1 (HIV-1) (RefSeq Acc. No. NP_057850.1).
- the nucleocapsid fragment or mutant that can be used in the present invention is not particularly limited as long as it has an affinity for the packaging signal of retrovirus, but a fragment or mutation containing two zinc finger motifs derived from nucleocapsid.
- a polypeptide comprising an amino acid sequence in which one to several amino acid residues are substituted, deleted, and / or inserted in an amino acid sequence constituting a body or a natural nucleocapsid.
- a polypeptide comprising an amino acid sequence having a sequence identity of 90% or more, preferably 95% or more, more preferably 98% or more with an amino acid sequence constituting a natural nucleocapsid is exemplified.
- the fusion protein of the present invention may further have a lipid modification signal such as a myristoylation signal in the N-terminal region.
- a lipid modification signal such as a myristoylation signal in the N-terminal region.
- the fusion protein of the present invention in which the myristoylation signal of the matrix domain (MA), which is considered important for targeting the Gag protein to the cell membrane, is located in the N-terminal region can be localized on the cell membrane.
- the fusion protein of the present invention is localized in the cell membrane of an RNA virus-infected cell, the fusion protein is incorporated into the RNA virus particle as the RNA virus emerges. By doing so, even if RNA virus particles emerge from RNA virus-infected cells, the infectivity of the RNA virus particles can be reduced by the fusion protein of the present invention incorporated therein.
- the fusion protein of the present invention may have a basic region in the vicinity of the N-terminus of the Gag protein that is considered to contribute to the targeting of the Gag protein to the cell membrane, in addition to the lipid modification signal described above.
- a fusion protein in which a Gag protein is fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner is one of the preferred embodiments of the present invention.
- the fusion protein of the present invention can be used for treatment or prevention of RNA virus infection.
- the polypeptide having affinity for the viral RNA of the RNA virus can be appropriately selected according to the type of RNA virus that causes the disease to be treated or prevented.
- the present invention is not particularly limited, when the fusion protein of the present invention is used for the treatment or prevention of HIV infection, for example, a nucleocapsid of HIV is used as a polypeptide having an affinity for a retroviral packaging signal. That's fine.
- the endoribonuclease that specifically cleaves single-stranded RNA in the fusion protein of the present invention is not particularly limited as long as it is a ribonuclease having the above activity.
- examples thereof include ribonucleases that cannot cleave double-stranded nucleic acids such as double-stranded RNA and RNA-DNA hybrids.
- Endoribonucleases that specifically degrade single-stranded RNA in a ribosome-independent manner are particularly preferred for the present invention.
- the present invention includes a microorganism-derived endoribonuclease such as MazF, PemK, MqsR, which is an endoribonuclease constituting a toxin-antitoxin-type toxin, NE1181 polypeptide derived from Nitromononas europaea ATCC 19718, Deinococcus radioduran R2 polypeptide DR0, etc. Can be used in the fusion protein of the present invention.
- MazF is an enzyme that cleaves 5′-A / CA-3 ′ sequences in single-stranded RNA
- PemK is an enzyme that mainly cleaves 5′-U / A (C, A or U) -3 ′.
- MqsR is 5'-GCU-3 'sequence
- NE1181 polypeptide is 5'-AAAU-3' sequence
- DR0662 polypeptide is 5'-UUCCUUU-3 'sequence.
- a polypeptide having high homology for example, 50% or more, preferably 70% or more, more preferably 90% or more homology
- the amino acid sequence of the polypeptide may be used for the fusion protein of the present invention.
- a polypeptide having an endoribonuclease activity that cleaves single-stranded RNA in a sequence-specific manner has already been found from many microorganisms including cyanobacteria and archaea (International Publication No. 2006/123537, International Publication No. 1). 2007/010740 pamphlet, WO 2007/013264 pamphlet, WO 2007/013265 pamphlet, etc.).
- the endoribonuclease that constitutes the toxin-antitoxin toxin is not inhibited by cytoplasmic ribonuclease inhibitors such as human placenta-derived ribonuclease inhibitors, so the single-stranded RNA used in the fusion protein of the present invention is sequence-specific. It is suitable as an endoribonuclease that cleaves automatically.
- an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner the full length of the amino acid sequence constituting the natural endoribonuclease having the above-mentioned activity may be used, or a fragment or variant thereof is constructed.
- An amino acid sequence may be used.
- the endoribonuclease fragment or variant whose amino acid sequence can be used in the present invention is not particularly limited as long as it has an activity of specifically cleaving single-stranded RNA. Examples thereof include polypeptides comprising an amino acid sequence in which one to several amino acid residues are substituted, deleted, and / or inserted in the amino acid sequence to be prepared.
- fragment or mutant examples include an amino acid sequence constituting a natural endoribonuclease [for example, an amino acid sequence constituting a natural MazF (RefSec Acc. No. NP_417262)] and 90% or more, preferably 95% or more, and more preferably. Is exemplified by amino acid sequences having 98% or more sequence identity.
- the polypeptide showing affinity for the viral RNA of the RNA virus may be located on the C-terminal side with respect to the endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner. May be arranged on both sides thereof.
- the fusion protein of the present invention may have a linker peptide that connects a polypeptide having affinity for viral RNA of an RNA virus and an endoribonuclease that specifically cleaves single-stranded RNA.
- a fusion protein obtained by fusing a retrovirus-derived nucleocapsid with an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner via a linker peptide is one of the preferred embodiments of the present invention.
- the linker peptide can be appropriately selected in consideration of the effect on the function of both polypeptides fused via the linker peptide, and is not particularly limited.
- linker peptides used in the present invention include alanine linkers, serine-glycine linkers, and linker peptides having a protease recognition sequence.
- linker peptides having a protease recognition sequence For example, when an HIV-derived Gag protein is used as a polypeptide having affinity for the RNA virus packaging signal in the fusion protein of the present invention, a polypeptide having an HIV protease recognition sequence may be used as a linker peptide. . By doing so, when the fusion protein of the present invention is encapsulated in the virus particle, free endoribonuclease can be generated in the virus particle.
- nucleic acid of the present invention includes a gene encoding the fusion protein of the present invention.
- Cells into which the nucleic acid of the present invention has been introduced can be used for the production of the fusion protein of the present invention.
- budding (production) of RNA virus particles from RNA virus-infected cells can be suppressed by introducing the nucleic acid of the present invention into RNA virus-infected cells such as retrovirus-infected cells using an appropriate vector.
- the infectivity of RNA virus particles sprouting from RNA virus-infected cells can be reduced.
- provirus formation when cells infected with the nucleic acid of the present invention are infected with RNA viruses is suppressed.
- the nucleic acid sequence of the nucleic acid of the present invention is not particularly limited as long as it contains a gene encoding the fusion protein of the present invention, but the nucleic acid sequence cleaved by the endoribonuclease activity of the fusion protein expressed from the nucleic acid of the present invention. It is preferable to design so that it does not contain.
- the nucleic acid of the present invention encodes a transcriptional regulatory sequence whose transcription is induced by an RNA virus trans-acting factor, and a fusion protein of the present invention arranged in a form in which expression can be controlled by the sequence. It is composed of genes.
- a cell into which the nucleic acid of the present invention has been introduced or a cell differentiated from the cell is downstream of the transcriptional regulatory sequence when an RNA virus-derived trans-acting factor is generated in the cell after being infected with the RNA virus.
- Expression of the gene encoding the fusion protein of the present invention located in is induced, and the fusion protein of the present invention is expressed in the cell.
- Viral RNA of the RNA virus is degraded by the expressed fusion protein of the present invention.
- provirus formation, virus genome replication, virus protein synthesis, and budding (production) of RNA virus particles from virus-infected cells are suppressed in RNA virus-infected cells. Furthermore, even when RNA virus particles emerge from RNA virus-infected cells, the infectivity of RNA virus particles is reduced.
- Transcriptional regulatory sequences whose transcription is induced by RNA virus trans-acting factors are not particularly limited, but are transcribed by retroviral trans-acting factors such as transcriptional regulatory sequences whose transcription is induced by HIV Tat protein.
- Transcriptional regulatory sequences from which can be derived can be used.
- the Tat protein binds to a TAR (trans-activation response element) sequence present in RNA whose transcription has been initiated by the action of the HIV LTR promoter, and activates transcription downstream from it.
- a transcriptional regulatory sequence having a TAR region base sequence downstream can be used for the nucleic acid of the present invention.
- an HIV LTR or a transcriptional regulatory sequence obtained by appropriately modifying the LTR is used.
- Examples of the modification include deletion of a binding site with a host cell-derived transcription factor present in the promoter and deletion of a region unnecessary for Tat-specific transcription.
- the former modification can reduce the level of transcription unrelated to HIV infection by host-derived transcription factors.
- Examples of the latter modification include deletion of the LTR U5 region and a region downstream of the TAR sequence (part of R region and U5 region).
- the nucleic acid of the present invention having such a deleted LTR is advantageous in preparing a high-titer retroviral vector that retains the nucleic acid.
- RRE Rev-responsible element
- the fusion protein of the present invention is dependent on Rev protein, that is, HIV infection-dependent. Can be expressed.
- the sequence that interacts with the RNA virus-derived trans-acting factor may be used in combination with a functional sequence in which the sequence is inherently incorporated, for example, a promoter, or in combination with a heterologous functional sequence. Also good.
- a sequence constructed by combining a promoter that is not derived from an RNA virus and a sequence that interacts with a trans-acting factor derived from the RNA virus, which can initiate transcription of mRNA in a cell in which introduction of the nucleic acid of the present invention is desired. are included in the “transcriptional regulatory sequence” used in the present invention.
- the vector of the present invention is a vector containing the nucleic acid of the present invention.
- the vector of the present invention has a promoter and transcription initiation site for achieving transcription of RNA from the nucleic acid of the present invention, and other regulatory elements that cooperate with these factors, such as enhancer and operator sequences. Also good.
- a terminator sequence can be contained downstream of the nucleic acid of the present invention.
- the vector of the present invention may have an appropriate marker gene that enables selection of the gene-introduced cell.
- the marker gene for example, a drug resistance gene that confers resistance to antibiotics on a cell, a gene that encodes a fluorescent protein, or a reporter gene that can distinguish a cell into which a gene has been introduced by detecting enzyme activity can be used.
- the vector of the present invention may be loaded with a suicide gene for the purpose of excluding the transgenic cell from the living body when the treatment with the transgenic cell is completed or when some side effect occurs.
- the type of the vector of the present invention is not particularly limited, and examples thereof include plasmid vectors and virus vectors.
- virus vectors include retrovirus vectors, adenovirus vectors, adeno-associated virus vectors, and herpes virus vectors.
- retroviral vector having the ability to incorporate the nucleic acid of the present invention onto a chromosome is suitable as the vector of the present invention.
- retrovirus vector examples include oncorretrovirus vectors such as vectors based on Moloney leukemia virus (MMLV), vectors based on human immunodeficiency virus type 1 (HIV-1), and simian immunodeficiency.
- Lentiviral vectors such as vectors based on viruses (SIV) are exemplified. These vectors may be pseudotyped by using an envelope derived from a different virus as the envelope.
- pseudotype vectors include Env such as vesicular stomatitis virus (VSV), gibbon leukemia virus (GaLV), feline endogenous virus RD114, murine leukemia virus (Ecotropic-env, amphotropic-env, 10A1-env, etc.) Examples include oncoretrovirus vectors and lentivirus vectors having proteins.
- a replication-deficient recombinant retrovirus vector is one of the preferred embodiments of the vector of the present invention. The vector is non-pathogenic, deficient in replication so that it cannot replicate in infected cells.
- Known replication-defective retrovirus vectors include MFG vector (ATCC No. 68754), ⁇ -SGC vector (ATCC No.
- RNA virus-infected cells a retrovirus vector that can be restrictedly replicated in RNA virus-infected cells is also a preferred embodiment of the vector of the present invention.
- the vector selectively replicates in cells infected with the RNA virus and propagates a therapeutically effective fusion protein gene.
- a retrovirus packaging cell in which a gene encoding a retrovirus structural protein such as a gag-pol gene or an env gene has been incorporated into a chromosome in advance is packaged with a foreign gene.
- a method of producing by introducing a transfer vector carrying a signal, and a packaging plasmid having a gene encoding a retrovirus structural protein such as a gag-pol gene or an env gene in a cell having no retroviral structural protein At the same time, there can be mentioned a method of producing the aforementioned transfer vector by transfection.
- Examples of the vector of the present invention include those used as transfer vectors for retrovirus vector production.
- a plasmid vector is preferably exemplified as the vector of the present invention used as a transfer vector.
- the unit for transcription of the nucleic acid of the present invention is reverse to the transcription direction of the RNA genome of the retroviral vector, that is, the direction of transcription initiated by the transcriptional regulatory sequence of the nucleic acid of the present invention. It may be arranged in the retroviral vector so as to be opposite to the direction of transcription initiated from the 5′-LTR of the retroviral vector. If this configuration is adopted, the retroviral mRNA transcribed from the vector of the present invention corresponds to the antisense strand of the gene encoding the fusion protein of the present invention. Therefore, the expression of the fusion protein of the present invention can be expressed from this retroviral mRNA. It can be suppressed.
- the retroviral mRNA functions as an antisense RNA, so that the fusion protein of the present invention Expression is suppressed.
- a high titer retrovirus vector can be obtained by using the vector of this embodiment as a transfer vector for producing a retrovirus vector.
- the endoribonuclease that specifically cleaves single-stranded RNA in the fusion protein of the present invention is a toxin-antitoxin toxin
- an antitoxin producing strain is used as a cell that produces the retroviral vector. By doing so, a high titer retrovirus vector can be obtained.
- the retroviral vector of the present invention is a polypeptide having an affinity for the viral RNA of the RNA virus in the fusion protein of the present invention so that RNA transcribed from the vector is not recognized by the fusion protein of the present invention. It may have packaging signals with different origins.
- a retroviral vector comprising a gene encoding a fusion protein consisting of a nucleocapsid and endoribonuclease from a lentivirus (eg, HIV) and a packaging signal from an oncoretrovirus (eg, MMLV) is suitable for the vector of the present invention. This is one of the embodiments.
- the method of treating or preventing the disease of the present invention comprises the step of introducing the vector of the present invention into a cell, and treating or preventing an RNA virus infection such as a retrovirus infection.
- an RNA virus infection such as a retrovirus infection.
- RNA virus infections for which the treatment or prevention method of the present invention is effective include hepatitis A, foot-and-mouth disease, severe acute respiratory syndrome (SARS), West Nile fever, yellow fever, Japanese encephalitis, hepatitis C, rubella, Rabies, Ebola hemorrhagic fever, mumps, influenza, Lassa fever, hepatitis D, HIV infection including AIDS, and adult T-cell leukemia.
- SARS severe acute respiratory syndrome
- SARS severe acute respiratory syndrome
- West Nile fever West Nile fever
- yellow fever yellow fever
- Japanese encephalitis Japanese encephalitis
- hepatitis C rubella
- Rabies Ebola hemorrhagic fever
- mumps influenza
- the vector of the present invention is a cell that can be infected by HIV, that is, a cell containing a CD4 positive cell ( It is desired to be introduced into a cell group). Therefore, in the treatment or prevention method of the present invention, gene transfer is performed using CD4 positive cells (for example, T cells), progenitor cells that can differentiate into CD4 positive cells (for example, hematopoietic stem cells), or a cell population containing the cells as target cells. Is implemented.
- CD4 positive cells for example, T cells
- progenitor cells that can differentiate into CD4 positive cells
- hematopoietic stem cells for example, hematopoietic stem cells
- hematopoietic stem cells or a cell population containing the cells from the viewpoint of comprehensively introducing the nucleic acid construct into cells that may be infected with HIV.
- the cells are not particularly limited as long as they contain CD4 positive cells and their progenitor cells. Blood cells collected from individuals (peripheral blood cells, umbilical cord blood cells), bone marrow cells, and the aforementioned cells can be obtained by known methods. Examples include fractionated CD4-positive cells, progenitor cells of CD4-positive cells, hematopoietic stem cells, and the like.
- the method for introducing the vector of the present invention into a cell is not particularly limited.
- a plasmid vector is used as the vector of the present invention
- gene transfer methods such as the calcium phosphate method, the cationic lipid method, the liposome method, and the electroporation method can be used.
- a viral vector such as a retrovirus vector, an adenovirus vector, an adeno-associated virus vector, or a herpes virus vector
- the target cell may be infected under conditions suitable for each virus.
- a cell into which the nucleic acid of the present invention has been introduced by the vector of the present invention is also an embodiment of the present invention.
- ex vivo gene introduction is exemplified in which a vector is introduced into a cell collected from an individual organism in vitro.
- a viral vector the target cells collected from the living body and a viral vector, for example, a culture supernatant of a virus-producing cell or a viral vector purified from the supernatant are mixed and incubated under appropriate conditions.
- a viral vector for example, a culture supernatant of a virus-producing cell or a viral vector purified from the supernatant are mixed and incubated under appropriate conditions.
- a viral vector for example, a culture supernatant of a virus-producing cell or a viral vector purified from the supernatant are mixed and incubated under appropriate conditions.
- gene transfer is achieved.
- a vector capable of gene transfer in vivo such as an adenovirus vector or an adeno-associated virus vector
- a vector containing the nucleic acid of the present invention may be directly administered to an individual.
- a target cell When a retroviral vector is used for ex vivo gene introduction, a target cell can be infected with a retroviral vector with high efficiency in the presence of a functional substance having a retroviral binding activity.
- a fibronectin fragment having both a cell adhesion domain and a heparin binding domain is preferably exemplified.
- the fibronectin fragment can be prepared from fibronectin purified from a living body by means such as protease digestion or can be prepared by recombinant DNA technology.
- a recombinant fibronectin fragment sold by Takara Bio Inc. as RetroNectin (registered trademark) can be suitably used in a gene introduction method using a functional substance having a retrovirus binding activity.
- the fusion protein of the present invention to be administered may be a purified protein itself, a protein encapsulated with an adjuvant, or a virus-like particle.
- the treatment or prevention method of the present invention may be carried out alone or in combination with multi-drug combination therapy (HAART) in which 3 to 4 types of antiviral drugs (reverse transcriptase inhibitor, protease inhibitor, etc.) are used in combination. May be implemented.
- HAART multi-drug combination therapy
- the fusion protein of the present invention, the vector, and the cell into which the vector is introduced can be used for the treatment and prevention of RNA virus infection. That is, the present invention provides a pharmaceutical composition for the treatment and prevention of RNA virus infection.
- the pharmaceutical composition is not particularly limited as long as it contains the vector of the present invention or cells into which the nucleic acid of the present invention has been introduced by the vector as an active ingredient. It can take any form such as a preparation prepared by combining an active ingredient with a pharmaceutically acceptable carrier, a kit combining the vector and a reagent for gene transfer into cells ex vivo.
- Example 1 Construction of Expression Plasmid (1) Construction of pBApo-Gag-MazF A DNA fragment comprising the nucleic acid sequence described in SEQ ID NO: 1 in the Sequence Listing is a mammalian vector pBApo-CMV pur DNA (manufactured by Takara Bio Inc.). A plasmid pBApo-Gag-MazF inserted between the BamHI site and the HindIII site was constructed.
- pBApo-Gag-MazF is a fusion protein obtained by fusing HIV-1 Gag protein and single-stranded RNA sequence-specific endoribonuclease MazF via a linker peptide containing a recognition sequence of HIV protease.
- a plasmid that can be expressed under the control of a promoter / enhancer (FIG. 1).
- the amino acid sequence of the fusion protein expressed from pBApo-Gag-MazF is shown in SEQ ID NO: 2 in the sequence listing.
- SEQ ID NO: 1 shows the base sequence containing the BamHI site on the 5 ′ end side and the HindIII site on the 3 ′ end side as the nucleic acid sequence of the inserted fragment in pBApo-Gag-MazF.
- the nucleotide sequence encoding HIV-1 Gag in the nucleic acid sequence shown in SEQ ID NO: 1 is an optimized sequence of the natural HIV-1 Gag gene for human codons.
- the base sequence encoding MazF is an ACA that is a recognition sequence of MazF present in the base sequence of the natural mazF gene (the complementary strand sequence of the 2908778th to 2909113th nucleic acid sequences in RefSec Acc. No. NC_000913). This is an artificially synthesized sequence modified to other sequence so that amino acid substitution does not occur (ACA-less mazF gene; SEQ ID NO: 5 in the sequence listing).
- pBApo-Gag (G2A) -MazF expresses a fusion protein of Gag (G2A) and MazF in which the second glycine residue from the N-terminus of the Gag protein is mutated to an alanine residue under the control of the CMV promoter / enhancer A possible plasmid (FIG. 1).
- the second glycine residue from the N-terminus of the Gag protein is an amino acid residue that undergoes myristoylation modification, which is considered important for targeting Gag to the cell membrane.
- Gag (G2A) is a mutant lacking this myristoylation modification site.
- pBApo-Gag ( ⁇ NC) -MazF is a plasmid capable of expressing a fusion protein of Gag ( ⁇ NC) and MazF, which lacks the amino acid sequence of the nucleocapsid protein (NC) region of the Gag protein, under the control of the CMV promoter / enhancer. (FIG. 1).
- pBApo-Gag ( ⁇ 2-31) -MazF is a fusion of Gaz ( ⁇ 2-31) and MazF, which lacks the amino acid sequence from the 2nd glycine residue to the 31st leucine residue from the N-terminus of the Gag protein.
- Gag ( ⁇ 2-31) is a mutant lacking the myristoylation modification site and the basic region near the N-terminus, which are thought to be important for targeting Gag to the cell membrane.
- RNA sequence (ACA-less mazF gene; SEQ ID NO: 5 in the sequence listing) converted to another base sequence without changing the amino acid sequence of natural MazF as a template
- primer mazF (HindIII) _R (arrangement) PCR amplification was performed using SEQ ID NO: 3) in the column table and primer mazF (BamHI) _F (SEQ ID NO: 4 in the sequence table).
- the obtained PCR product was digested with restriction enzymes BamHI and HindIII, and then inserted into the plasmid pBApo-CMV pur DNA digested with the same enzymes.
- the thus constructed plasmid was designated as pBApo-MazF.
- pBApo-MazF is a plasmid capable of expressing MazF protein under the control of a CMV promoter / enhancer (FIG. 1).
- the plasmid is a plasmid capable of expressing a fusion protein of NC and MazF (NC-MazF) under the control of a CMV promoter / enhancer (FIG. 1).
- Example 2 Degradation of viral RNA with fusion protein of the present invention
- DMEM Dulbecco's modified Eagle medium
- GOBCO 10% fetal bovine serum
- lentiviral packaging mix (manufactured by Invitrogen) is inserted into each dish cell, and plasmids pLenti-ZsGreen and pBApo-CMV pur DNA constructed by inserting the ZsGreen1 gene into the pLenti6.3 / V5 vector (manufactured by Invitrogen).
- PBApo-Tat and pBApo-CMV pur DNA constructed by inserting the HIV-1-derived Tat gene were introduced using TransIT-293 (manufactured by Milas) as described in the instructions for the product.
- the cells thus prepared were used as control cells (Mock cells). Two types of Mock cells were prepared, one using 0.025 ⁇ g of pBApo-CMV pur DNA for introduction and one using 0.4 ⁇ g for introduction.
- Example 1 preparation of the above Mock cell except that pBApo-MazF constructed in Example 1 (5) or pBApo-Gag-MazF constructed in Example 1 (1) was introduced instead of pBApo-CMV pur DNA.
- Cells were prepared by the same method, and the introduced / expressed cells were designated as MazF cells and Gag-MazF cells.
- MazF cells and Gag-MazF cells used 0.025 ⁇ g of pBApo-MazF or pBApo-Gag-MazF for introduction and 0.4 ⁇ g of pBApo-MazF or pBApo-Gag-MazF for introduction. Two types of each were prepared.
- Example 2 Confirmation of expression of ZsGreen1
- a fluorescence microscope In control cells (Mock cells), a strong fluorescent signal derived from ZsGreen1 protein was detected, whereas in MazF cells, a fluorescent signal with a lower intensity was detected compared to control cells.
- the intensity of the fluorescent signal derived from ZsGreen1 decreased dramatically. This tendency was dependent on the amount of plasmid expressing MazF and Gag-MazF introduced into the cells.
- RNA encoding ZsGreen1 and RNA encoding ⁇ -actin in each cell was calculated by calculating a relative value with respect to the 18S rRNA amount by real-time RT-PCR (qRT-PCR).
- Primer human_18S_Fw (SEQ ID NO: 9 in the sequence listing) and primer human_18S_rev (SEQ ID NO: 10 in the sequence listing) are used for qRT-PCR of 18S rRNA
- primer ZsG_F2 sequence listing is used for qRT-PCR of RNA encoding ZsGreen1).
- the expression level of ZsGreen1 RNA was significantly decreased in MazF cells, and the expression level of ZsGreen1 RNA was significantly decreased in Gag-MazF cells.
- the expression level of ⁇ -actin mRNA which is a housekeeping gene, did not differ greatly between cells. This indicates that the remarkable decrease in the fluorescence signal intensity derived from intracellular ZsGreen1 due to Gag-MazF expression observed in Example 3 (1) is due to the degradation of ZsGreen1 RNA by the Gag-MazF fusion protein.
- the results of this example indicate that the Gag-MazF fusion protein effectively degrades RNA containing the RNA virus packaging signal, and the Gag-MazF fusion protein is effective in treating viral RNA in RNA virus-infected cells. It can be decomposed automatically.
- Example 3 Identification of a region necessary for RNA virus particle budding inhibitory effect and RNA virus particle budding inhibitory effect of the fusion protein of the present invention
- Preparation of fusion protein expression vector-introduced cells In the same manner as in Example 2 (1) Mock cells, MazF cells, and Gag-MazF cells were prepared.
- pBApo-CMV pur DNA pBApo-Gag (G2A) -MazF constructed in Example 1 (2), pBApo-Gag ( ⁇ NC) -MazF constructed in Example 1 (3), or Example 1 Cells were prepared in the same manner as the preparation of Mock cells in Example 2 (1) except that pBApo-Gag ( ⁇ 2-31) -MazF constructed in (4) was introduced.
- (G2A) -MazF cells, Gag ( ⁇ NC) -MazF cells, and Gag ( ⁇ 2-31) -MazF cells were used.
- pBApo-CMV pur DNA 0.25 ⁇ g of pBApo-CMV pur DNA, pBApo-MazF, pBApo-Gag-MazF, pBApo-Gag (G2A) -MazF, pBApo-Gag ( ⁇ NC) -MazF, Alternatively, pBApo-Gag ( ⁇ 2-31) -MazF was used.
- Example 3 (1) Preparation of concentrated virus Each cell prepared in Example 3 (1) was cultured for 24 hours under conditions of 37 ° C. and 5% CO 2 , and then further cultured for 24 hours after changing the medium. After completion of the culture, the culture supernatant was collected and filtered through a 0.45 ⁇ m filter, and the filtrate was subjected to low-speed centrifugation (6000 ⁇ g, 16 h, 4 ° C.) to pellet the virus. Subsequently, the pelleted virus was suspended in PBS buffer to obtain a 20-fold concentrated virus.
- low-speed centrifugation 6000 ⁇ g, 16 h, 4 ° C.
- the Gag-MazF fusion protein Viral budding (production) was significantly inhibited to the same extent as the expressing cells.
- results of this example show that the effect of inhibiting the budding (production) of virus particles by the Gag-MazF fusion protein has a polypeptide and single-stranded RNA sequence-specific end that has an affinity for the packaging signal of RNA virus in the fusion protein. It shows that it is caused by ribonuclease.
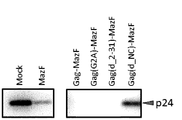
- Example 4 Inhibitory effect of virus particle budding by NC-MazF fusion protein Mock cells, MazF cells, and Gag-MazF cells were prepared in the same manner as in Example 2 (1). Further, human 293T / 17 cells were prepared in the same manner as the preparation of Mock cells in Example 2 (1) except that pBApo-NC-MazF constructed in Example 1 (6) was introduced instead of pBApo-CMV pur DNA. Plasmids were introduced into the cells to obtain NC-MazF cells. Next, concentrated virus was prepared from each cell by the same method as in Example 3 (2), and analyzed by Western blotting in the same manner as in Example 3 (3). The results are shown in FIG. As shown in FIG.
- NC-MazF cells showed the same virus particle budding (production) inhibitory effect as Gag-MazF-introduced cells (denoted as Gag-MazF in the figure).
- the above results indicate that a fusion protein consisting of a single-stranded RNA sequence-specific endoribonuclease fused with a polypeptide that has an affinity for RNA virus packaging signals significantly suppresses RNA virus particle budding (production). Indicates to do.
- RNA virus particle inactivation effect of the fusion protein of the present invention (1) Preparation of fusion protein expression vector-introduced cells Dulbecco's modified Eagle medium containing 10% fetal calf serum (GIBCO) in a 6 cm dish for cell culture ( 4 mL of DMEM (manufactured by Sigma) was added, and 1.8 ⁇ 10 6 human Lenti-X 293T / 17 cells (manufactured by Clontech) were further added, and cultured for 24 hours.
- DMEM fetal calf serum
- lBA virus packaging mix (Clontech), pLVX-AcGFP1-C1 vector (Clontech), and pBApo-Tat constructed by inserting HIV-1-derived Tat gene into pBApo-CMV pur DNA into each dish cell.
- pBApo-CMV pur DNA was introduced using TransIT-293 (manufactured by Milas) as described in the instructions for the product.
- the cells thus prepared were used as control cells (Mock cells). In the preparation of Mock cells, 0.025 ⁇ g of pBApo-CMV pur DNA was introduced.
- Example 1 preparation of the above Mock cell except that pBApo-MazF constructed in Example 1 (5) or pBApo-Gag-MazF constructed in Example 1 (1) was introduced instead of pBApo-CMV pur DNA.
- Cells were prepared by the same method, and the introduced / expressed cells were designated as MazF cells and Gag-MazF cells.
- Example 5 (2) Preparation of concentrated virus Each cell prepared in Example 5 (1) was cultured under the conditions of 37 ° C and 5% CO 2 for 24 hours, and then the medium was changed and further cultured for 24 hours. After completion of the culture, the culture supernatant was collected and filtered through a 0.45 ⁇ m filter, and the filtrate was subjected to low-speed centrifugation (6000 ⁇ g, 16 h, 4 ° C.) to pellet the virus. Subsequently, the pelleted virus was suspended in PBS buffer to obtain a 10-fold concentrated virus.
- low-speed centrifugation 6000 ⁇ g, 16 h, 4 ° C.
- Example 5 Western blotting analysis of concentrated virus Among the concentrated viruses obtained in Example 5 (2), the concentrated virus obtained from MazF cells and the concentrated virus obtained from Gag-MazF cells were respectively used as a sample buffer containing SDS. The mixture was heat-treated and subjected to Western blotting using an anti-MazF antibody. The results are shown in FIG. As shown in FIG. 6, MazF protein could not be detected from a virus produced from MazF cells (indicated as MazF in the figure). On the other hand, free virus and a small amount of Gag-MazF protein were detected from virus produced from Gag-MazF cells (denoted as Gag-MazF in the figure).
- Gag-MazF was effectively encapsulated in virus particles in Gag-MazF cells expressing Gag-MazF protein. Furthermore, in the virus particle, it is considered that Gag-MazF was divided into Gag protein and MazF protein by activated HIV protease, and free MazF protein was accumulated. Since this free MazF protein produced in the virus particle is not degraded by activated HIV protease, it is reasonable to assume that the ribonuclease activity is maintained in the particle.
- Example 5 (4) ELISA analysis of concentrated virus sample The amount of virus particles of each concentrated virus obtained in Example 5 (2) was quantified with a p24 antigen ELISA kit (manufactured by Zeptomerix) that detects the structural protein p24 of HIV-1 particles. did.
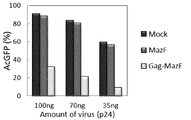
- the amount of virus particles corresponding to 100 ng, 70 ng, or 35 ng in terms of the amount of p24 protein was used for the addition of virus particles based on the quantitative value obtained in Example 5 (4).
- 1 mL of medium was added to each well and further cultured for 24 hours.
- the cells were further cultured for 2 days. The cultured cells were washed with PBS buffer, and then collected by trypsin treatment. The cells were subjected to flow cytometer analysis.
- FIG. 7 shows that the virus-prepared virus from the Gag-MazF cells, whereas the virus-prepared virus particles prepared from the MazF cells have the same AcGFP1-positive rate as the cells infected with the virus particles prepared from the Mock cells. It was found that the AcGFP1-positive rate was remarkably low in the cells infected with the particles.
- a fusion protein comprising a polypeptide having an affinity for RNA virus packaging signal and a single-stranded RNA sequence-specific endoribonuclease is fused with viral particles in cells infected with RNA virus ( Not only significantly suppresses production), but also significantly degrades the infectivity of the virus particles (remarkably inactivates the virus particles) by degrading the viral genome of the RNA virus within the budding (produced) virus particles. It shows that.
- Example 6 Virus infection inhibitory effect of cells expressing Gag-MazF and NC-MazF (1) Preparation of fusion protein expression vector-introduced cells Dulbecco modification containing 10% fetal calf serum (GIBCO) in 6-well plate for cell culture 2 mL of Eagle's medium (DMEM; manufactured by Sigma) was added, and 6 ⁇ 10 5 human Lenti-X 293T / 17 cells (manufactured by Clontech) were added and cultured for 24 hours.
- DMEM fetal calf serum
- Example 1 2 ⁇ g of pBApo-MazF constructed in Example 1 (5), pBApo-Gag-MazF constructed in Example 1 (1), or pBApo-NC constructed in Example 1 (6) was added to each well cell.
- -MazF was introduced using TransIT-293 (Mirras) as described in the product instructions. After culturing for 5.5 hours, the medium was changed and cultured for 18 hours. The cells thus prepared were designated as MazF cells, Gag-MazF cells, and NC-MazF cells, respectively.
- lentiviral vector AcGFP1 was used in the virus infection experiment of Example 6 (3).
- Example 6 (3) Lentiviral infection experiment using cells expressing the fusion protein
- the lentiviral vector prepared in Example 6 (2) was diluted with a medium to which polybrene was added to a final concentration of 8 ⁇ g / mL. After removing the medium from the suspension of each cell prepared in Example 6 (1), 1 mL of this diluted virus was added. After 24 hours, the medium was changed and further cultured for 24 hours. Cells in each well were collected by trypsinization, and the cell pellet was washed with PBS.
- the amount of proviral DNA was determined by the primer Lenti-copy1-F (SEQ ID NO: 15 in the sequence listing), the primer Lenti-copy1-R (SEQ ID NO: 16 in the sequence listing), and the probe P1S (SEQ ID NO: 16 in the sequence listing). It was determined by qPCR using 17). The number of copies of proviral DNA per host cell was calculated from the amount of host cells and the amount of proviral DNA determined by the above qPCR. The results are shown in FIG.
- FIG. 8 shows that the copy number of proviral DNA per cell in Gag-MazF cells and NC-MazF cells is significantly lower than that in MazF cells. From this result, it is found that expression of the fusion protein of the present invention in cells not only suppresses budding of RNA viruses, but can also effectively suppress provirus formation when RNA viruses infect cells. Indicated. The suppression of provirus formation is presumed to be due to the fact that the viral genome RNA of the RNA virus infecting the cells is cleaved before the reverse transcription reaction by the fusion protein of the present invention having an affinity for lentiviral RNA.
- the present invention is particularly useful for the treatment or prevention of RNA virus infections.
- SEQ ID NO: 1 Insert DNA comprising a gene encoding a Gag_mazF fusion protein.
- SEQ ID NO: 2 Gag_MazF fusion protein.
- SEQ ID NO: 3 Oligonucleotide primer named mazF (HindIII) _R.
- SEQ ID NO: 4 Oligonucleotide primer named mazF (BamHI) _F.
- SEQ ID NO: 5 Gene encoding an ACA-less-mazF.
- SEQ ID NO: 7 Oligonucleotide primer named NC-FWD.
- SEQ ID NO: 8 Oligonucleotide primer named NC-Rev3.
- SEQ ID NO: 9 Oligonucleotide primer named human_18S_fw.
Abstract
The purpose of the present invention is to provide a fusion protein which is useful for the treatment or prevention of RNA virus infections. Provided are: a fusion protein produced by fusing a polypeptide having an affinity for viral RNA of an RNA virus to an endoribonuclease capable of cleaving single-stranded RNA in a sequence-specific manner; a nucleic acid which encodes the fusion protein; a vector which carries the nucleic acid; a method of treating or preventing retrovirus infections, comprising a step of introducing the vector into a cell; and a method of inhibiting the budding of a retrovirus using a cell having the nucleic acid introduced therein and the fusion protein. This fusion protein can inhibit the budding (production) of retrovirus particles from a retrovirus-infected cell effectively.
Description
本発明は、ウイルスRNAに親和性を示すポリペプチドが一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合されてなる融合タンパク質に関する。
The present invention relates to a fusion protein in which a polypeptide having affinity for viral RNA is fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner.
ヒト免疫不全ウイルス(Human Immunodeficiency Virus;HIV)、C型肝炎ウイルス(HCV)、インフルエンザウイルス、SARSウイルス等の病原性が高いRNAウイルスによる感染症が問題となっている。特にHIVは開発途上国において急速に感染が拡大しており、社会問題化している。
Infectious diseases caused by highly pathogenic RNA viruses such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), influenza virus, SARS virus, and the like are problematic. In particular, HIV is rapidly spreading in developing countries and has become a social problem.
HIVは、CD4分子を発現する細胞に感染し、破壊する。このため、HIVの感染を受けたヒトの体内では免疫を統御する中枢細胞であるCD4陽性のヘルパーT細胞やマクロファージが減少し、ついには重度の免疫不全状態に陥ってカリニ肺炎のような日和見感染症を発症する。この状態は後天性免疫不全症候群(acquired immunodeficiency syndrome;AIDS)と称されている。
HIV infects and destroys cells that express CD4 molecules. For this reason, CD4 positive helper T cells and macrophages, which are central cells that control immunity, are reduced in the human body that is infected with HIV, and finally it becomes a severe immunodeficiency state and opportunistic infection such as carini pneumonia. Develops symptoms. This condition is referred to as acquired immunodeficiency syndrome (AIDS).
HIV感染症の治療方法として、HIVの生活環を遮断する抗ウイルス剤(逆転写酵素阻害剤、プロテアーゼ阻害剤等)の開発が行われており、いくつかの抗ウイルス剤はすでに実用化されている。しかし、HIVは変異頻度が高いため、HIVが感染した個体内で抗ウイルス剤に耐性を持つ変異体が出現することがある。また、RNAデコイやリボザイムのような核酸、トランスドミナント変異タンパクや細胞内抗体のようなタンパク質を有効成分としてHIVの増殖を阻止する遺伝子治療薬を開発する試みもなされているが、いまだ完成の域には達していない。また、既存の抗ウイルス薬により治療効果が得られていた感染患者であっても、高齢化に伴うT細胞の再構築能の低下等のために、抗ウイルス薬の効果が低下する可能性が懸念されている。特に米国でのHIV感染患者の高齢化が進む中、新たな治療法の開発が急務である。別のアプローチとして、HIV感染細胞特異的に細胞死を誘導する方法が考案されている。この方法は、HIV由来のLTRプロモーターの下流に細胞毒性を示す産物をコードする遺伝子を接続するものであるが、これまでのところ臨床的に応用された例は知られていない。
As a method of treating HIV infection, antiviral agents (reverse transcriptase inhibitors, protease inhibitors, etc.) that block the HIV life cycle have been developed, and some antiviral agents have already been put into practical use. Yes. However, since HIV has a high mutation frequency, mutants resistant to antiviral agents may appear in individuals infected with HIV. Attempts have also been made to develop gene therapy drugs that inhibit the growth of HIV using nucleic acids such as RNA decoys and ribozymes, proteins such as transdominant mutant proteins and intracellular antibodies as active ingredients. Is not reached. In addition, even in an infected patient who has obtained a therapeutic effect with an existing antiviral drug, the effect of the antiviral drug may decrease due to a decrease in the ability to reconstruct T cells accompanying aging. There are concerns. In particular, with the aging of HIV-infected patients in the United States, the development of new therapies is urgent. As another approach, a method for inducing cell death specifically for HIV-infected cells has been devised. In this method, a gene encoding a cytotoxic product is connected downstream of the LTR promoter derived from HIV, but no clinically applied examples are known so far.
近年、HIV感染細胞特異的に一本鎖RNA特異的リボヌクレアーゼを発現させる技術が考案された(例えば特許文献1、非特許文献2)。この方法では、HIVの感染にともなって発現されるTatタンパク質依存的に一本鎖RNA特異的エンドリボヌクレアーゼの発現が誘導され、HIVゲノムを含む細胞内の一本鎖RNAが分解される。この結果、当該細胞ではHIVの複製、出芽が阻止される。HIV由来のRNAが分解されてTatタンパク質の発現が停止し、かつ発現されていたTatタンパク質が細胞から消失するとエンドリボヌクレア-ゼの発現も停止する。この間、細胞内のリボソームやtRNAは破壊されず、前記エンドリボヌクレア-ゼの発現の停止とともに通常のタンパク質合成が再開されるため、この時点で破壊されていない細胞は増殖を再開する。特許文献1の技術は、過度にCD4陽性T細胞の減少を招かない点で、HIV感染細胞特異的に細胞死を誘導する方法と比較して有利である。
Recently, a technique for expressing a single-stranded RNA-specific ribonuclease specifically for HIV-infected cells has been devised (for example, Patent Document 1 and Non-Patent Document 2). In this method, the expression of single-stranded RNA-specific endoribonuclease is induced in a manner dependent on the Tat protein expressed with HIV infection, and the single-stranded RNA in the cell containing the HIV genome is degraded. As a result, HIV replication and budding are prevented in the cells. When the HIV-derived RNA is degraded to stop the expression of Tat protein, and the expressed Tat protein disappears from the cell, the expression of endoribonuclease is also stopped. During this time, ribosomes and tRNA in the cell are not destroyed, and normal protein synthesis is resumed with the termination of the expression of the endoribonuclease. At this point, cells that are not destroyed resume proliferation. The technique of Patent Document 1 is advantageous in comparison with a method of inducing cell death specifically for HIV-infected cells in that it does not cause an excessive decrease in CD4-positive T cells.
一方、ウイルスの増殖を抑制する技術として、Capsid-Targeted Viral Inactivation(以下、CTVIと記載する)と呼ばれる技術が開発されている。これは、ウイルスのカプシドタンパク質とカルシウム依存性ヌクレアーゼとの融合タンパク質を利用して、当該融合タンパク質が取り込まれたウイルス粒子中でウイルスゲノムを分解し、ウイルス粒子の感染性を低減させる技術である。この技術は、レトロウイルスの増殖抑制にも有効であることが示されている(非特許文献1)。しかしながら、CTVIでは、ウイルス感染細胞から出芽したウイルス粒子の感染性を低減させることはできるが、ウイルス感染細胞からのウイルス粒子の出芽およびプロウイルス形成を抑制することはできない。
On the other hand, a technique called Capsid-Targeted Viral Activation (hereinafter referred to as CTVI) has been developed as a technique for suppressing virus growth. This is a technique that uses a fusion protein of a capsid protein of a virus and a calcium-dependent nuclease to degrade the virus genome in the virus particle in which the fusion protein is incorporated, thereby reducing the infectivity of the virus particle. This technique has been shown to be effective in suppressing the growth of retroviruses (Non-Patent Document 1). However, CTVI can reduce the infectivity of virus particles sprouting from virus-infected cells, but cannot suppress budding of virus particles and virus formation from virus-infected cells.
本発明は上記の従来技術を鑑みて行われたものであり、その目的は、RNAウイルス感染症の治療・予防に有用な融合タンパク質を提供することにある。
The present invention has been made in view of the above prior art, and an object thereof is to provide a fusion protein useful for the treatment and prevention of RNA virus infection.
本発明者らは、RNAウイルスのウイルスRNAに親和性を示すポリペプチドが一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合されてなる融合タンパク質により、RNAウイルス感染細胞からのRNAウイルス粒子の出芽(産生)が効果的に抑制されること、さらにはRNAウイルスが細胞に感染した際の感染細胞内でのプロウイルスの形成が効果的に抑制されることを見出し、本発明を完成させた。
The present inventors have developed an RNA virus particle from an RNA virus-infected cell using a fusion protein in which a polypeptide having affinity for viral RNA of an RNA virus is fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner. Has been found to be effectively suppressed, and further, the formation of provirus in the infected cells when the RNA virus is infected with the cells is effectively suppressed, and the present invention has been completed. It was.
すなわち、本発明は、
[1]RNAウイルスのウイルスRNAに親和性を示すポリペプチドが一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合されてなる融合タンパク質、
[2]RNAウイルスのウイルスRNAに親和性を示すポリペプチドがリンカーペプチドを介して一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合されてなる、[1]に記載の融合タンパク質、
[3]さらに、脂質修飾シグナルをN末端領域に有する[1]に記載の融合タンパク質、
[4]RNAウイルスのウイルスRNAに親和性を示すポリペプチドが、RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドである、[1]に記載の融合タンパク質、
[5]RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドが、レトロウイルスのパッケージングシグナルに親和性を示すポリペプチドである[4]に記載の融合タンパク質、
[6]レトロウイルスのパッケージングシグナルに親和性を示すポリペプチドがレトロウイルスのGagタンパク質に由来する、[5]に記載の融合タンパク質、
[7]レトロウイルスのGagタンパク質が、ヒト免疫不全ウイルスに由来するGagタンパク質である[6]に記載の融合タンパク質、
[8]Gagタンパク質に由来するレトロウイルスのパッケージングシグナルに親和性を示すポリペプチドがヌクレオカプシドである[4]に記載の融合タンパク質、
[9]一本鎖RNAを配列特異的に切断するエンドリボヌクレア-ゼがMazFタンパク質である[1]に記載の融合タンパク質、
[10][1]~[9]のいずれか一に記載の融合タンパク質をコードする遺伝子を含む核酸、
[11]さらに、RNAウイルスのトランス作用因子により転写が誘導される転写調節配列を有し、ここで、前記の融合タンパク質をコードする遺伝子は前記の転写調節配列により発現の制御が可能な形態に配置されている[10]に記載の核酸、
[12]転写調節配列が、Tatタンパク質および/またはRevタンパク質により転写が誘導される転写調節配列である[11]に記載の核酸、
[13][10]に記載の核酸を含むベクター、
[14]レトロウイルスベクターである[13]に記載のベクター、
[15][13]に記載のベクターを含む医薬組成物、
[16][10]に記載の核酸が導入されてなる細胞、
[17][13]に記載のベクターを細胞に接触する工程を含むRNAウイルス感染症の治療又は予防方法、並びに
[18][1]に記載の融合タンパク質、[10]に記載の核酸、及び[13]に記載のベクターからなる群より選択された少なくとも1種を細胞に導入する工程を含む、RNAウイルスの出芽およびプロウイルス形成の抑制方法、
に関する。 That is, the present invention
[1] A fusion protein in which a polypeptide having affinity for viral RNA of an RNA virus is fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner,
[2] The fusion protein according to [1], wherein a polypeptide having affinity for viral RNA of an RNA virus is fused to an endoribonuclease that specifically cleaves single-stranded RNA via a linker peptide,
[3] The fusion protein according to [1], further having a lipid modification signal in the N-terminal region,
[4] The fusion protein according to [1], wherein the polypeptide showing affinity for viral RNA of RNA virus is a polypeptide showing affinity for packaging signal of RNA virus,
[5] The fusion protein according to [4], wherein the polypeptide having affinity for the packaging signal of RNA virus is a polypeptide having affinity for the packaging signal of retrovirus,
[6] The fusion protein according to [5], wherein the polypeptide having affinity for a retroviral packaging signal is derived from a retroviral Gag protein,
[7] The fusion protein according to [6], wherein the retrovirus Gag protein is a Gag protein derived from a human immunodeficiency virus,
[8] The fusion protein according to [4], wherein the polypeptide having affinity for a retroviral packaging signal derived from a Gag protein is a nucleocapsid;
[9] The fusion protein according to [1], wherein the endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner is MazF protein,
[10] A nucleic acid comprising a gene encoding the fusion protein according to any one of [1] to [9],
[11] It further has a transcriptional regulatory sequence whose transcription is induced by a trans-acting factor of RNA virus, wherein the gene encoding the fusion protein is in a form in which the expression can be controlled by the transcriptional regulatory sequence. The nucleic acid according to [10], which is arranged;
[12] The nucleic acid according to [11], wherein the transcription regulatory sequence is a transcription regulatory sequence whose transcription is induced by Tat protein and / or Rev protein,
[13] A vector comprising the nucleic acid according to [10],
[14] The vector according to [13], which is a retroviral vector,
[15] A pharmaceutical composition comprising the vector according to [13],
[16] A cell into which the nucleic acid according to [10] is introduced,
[17] A method for treating or preventing an RNA virus infection comprising a step of contacting a vector according to [13] with a cell, a fusion protein according to [18] [1], a nucleic acid according to [10], and A method of suppressing RNA virus budding and provirus formation, which comprises introducing at least one selected from the group consisting of the vector of [13] into a cell;
About.
[1]RNAウイルスのウイルスRNAに親和性を示すポリペプチドが一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合されてなる融合タンパク質、
[2]RNAウイルスのウイルスRNAに親和性を示すポリペプチドがリンカーペプチドを介して一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合されてなる、[1]に記載の融合タンパク質、
[3]さらに、脂質修飾シグナルをN末端領域に有する[1]に記載の融合タンパク質、
[4]RNAウイルスのウイルスRNAに親和性を示すポリペプチドが、RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドである、[1]に記載の融合タンパク質、
[5]RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドが、レトロウイルスのパッケージングシグナルに親和性を示すポリペプチドである[4]に記載の融合タンパク質、
[6]レトロウイルスのパッケージングシグナルに親和性を示すポリペプチドがレトロウイルスのGagタンパク質に由来する、[5]に記載の融合タンパク質、
[7]レトロウイルスのGagタンパク質が、ヒト免疫不全ウイルスに由来するGagタンパク質である[6]に記載の融合タンパク質、
[8]Gagタンパク質に由来するレトロウイルスのパッケージングシグナルに親和性を示すポリペプチドがヌクレオカプシドである[4]に記載の融合タンパク質、
[9]一本鎖RNAを配列特異的に切断するエンドリボヌクレア-ゼがMazFタンパク質である[1]に記載の融合タンパク質、
[10][1]~[9]のいずれか一に記載の融合タンパク質をコードする遺伝子を含む核酸、
[11]さらに、RNAウイルスのトランス作用因子により転写が誘導される転写調節配列を有し、ここで、前記の融合タンパク質をコードする遺伝子は前記の転写調節配列により発現の制御が可能な形態に配置されている[10]に記載の核酸、
[12]転写調節配列が、Tatタンパク質および/またはRevタンパク質により転写が誘導される転写調節配列である[11]に記載の核酸、
[13][10]に記載の核酸を含むベクター、
[14]レトロウイルスベクターである[13]に記載のベクター、
[15][13]に記載のベクターを含む医薬組成物、
[16][10]に記載の核酸が導入されてなる細胞、
[17][13]に記載のベクターを細胞に接触する工程を含むRNAウイルス感染症の治療又は予防方法、並びに
[18][1]に記載の融合タンパク質、[10]に記載の核酸、及び[13]に記載のベクターからなる群より選択された少なくとも1種を細胞に導入する工程を含む、RNAウイルスの出芽およびプロウイルス形成の抑制方法、
に関する。 That is, the present invention
[1] A fusion protein in which a polypeptide having affinity for viral RNA of an RNA virus is fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner,
[2] The fusion protein according to [1], wherein a polypeptide having affinity for viral RNA of an RNA virus is fused to an endoribonuclease that specifically cleaves single-stranded RNA via a linker peptide,
[3] The fusion protein according to [1], further having a lipid modification signal in the N-terminal region,
[4] The fusion protein according to [1], wherein the polypeptide showing affinity for viral RNA of RNA virus is a polypeptide showing affinity for packaging signal of RNA virus,
[5] The fusion protein according to [4], wherein the polypeptide having affinity for the packaging signal of RNA virus is a polypeptide having affinity for the packaging signal of retrovirus,
[6] The fusion protein according to [5], wherein the polypeptide having affinity for a retroviral packaging signal is derived from a retroviral Gag protein,
[7] The fusion protein according to [6], wherein the retrovirus Gag protein is a Gag protein derived from a human immunodeficiency virus,
[8] The fusion protein according to [4], wherein the polypeptide having affinity for a retroviral packaging signal derived from a Gag protein is a nucleocapsid;
[9] The fusion protein according to [1], wherein the endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner is MazF protein,
[10] A nucleic acid comprising a gene encoding the fusion protein according to any one of [1] to [9],
[11] It further has a transcriptional regulatory sequence whose transcription is induced by a trans-acting factor of RNA virus, wherein the gene encoding the fusion protein is in a form in which the expression can be controlled by the transcriptional regulatory sequence. The nucleic acid according to [10], which is arranged;
[12] The nucleic acid according to [11], wherein the transcription regulatory sequence is a transcription regulatory sequence whose transcription is induced by Tat protein and / or Rev protein,
[13] A vector comprising the nucleic acid according to [10],
[14] The vector according to [13], which is a retroviral vector,
[15] A pharmaceutical composition comprising the vector according to [13],
[16] A cell into which the nucleic acid according to [10] is introduced,
[17] A method for treating or preventing an RNA virus infection comprising a step of contacting a vector according to [13] with a cell, a fusion protein according to [18] [1], a nucleic acid according to [10], and A method of suppressing RNA virus budding and provirus formation, which comprises introducing at least one selected from the group consisting of the vector of [13] into a cell;
About.
本発明により、RNAウイルス感染症の治療又は予防に有用な融合タンパク質、当該融合タンパク質をコードする核酸、当該核酸を含むベクター、当該ベクターを含む医薬組成物、前記核酸が導入されてなる細胞、当該ベクターを細胞に導入する工程を含むRNAウイルス感染症の治療又は予防方法、及び本発明の融合タンパク質、核酸、ベクターから選択されるものを細胞に導入する工程を含む、RNAウイルスの出芽抑制方法が提供される。
According to the present invention, a fusion protein useful for treatment or prevention of RNA virus infection, a nucleic acid encoding the fusion protein, a vector containing the nucleic acid, a pharmaceutical composition containing the vector, a cell into which the nucleic acid has been introduced, A method for treating or preventing an RNA virus infection comprising a step of introducing a vector into a cell, and a method for suppressing the budding of an RNA virus, comprising the step of introducing a fusion protein, nucleic acid or vector selected from the present invention into a cell. Provided.
本明細書において「RNAウイルス」とは、ゲノムがRNAで構成されるウイルスの総称を示す。本明細書における「RNAウイルス」には、一本鎖RNAウイルス及び二本鎖RNAウイルスが含まれる。
In this specification, “RNA virus” is a generic term for viruses whose genome is composed of RNA. As used herein, “RNA virus” includes single-stranded RNA viruses and double-stranded RNA viruses.
本明細書において「二本鎖RNAウイルス」とは、ゲノムが二本鎖RNAで構成されるウイルスの総称を示す。本明細書における「二本鎖RNAウイルス」には、ロタウイルス等のレオウイルス科に属するウイルスが含まれる。
In this specification, “double-stranded RNA virus” is a generic term for viruses whose genome is composed of double-stranded RNA. As used herein, “double-stranded RNA virus” includes viruses belonging to the family Reoviridae such as rotavirus.
本明細書において「一本鎖RNAウイルス」とは、ゲノムが一本鎖RNAで構成されるウイルスの総称を示す。本明細書における「一本鎖RNAウイルス」には、一本鎖(+)RNAウイルス、一本鎖(-)RNAウイルス、及びレトロウイルスが含まれる。
As used herein, “single-stranded RNA virus” is a generic term for viruses whose genome is composed of single-stranded RNA. As used herein, “single-stranded RNA virus” includes single-stranded (+) RNA viruses, single-stranded (−) RNA viruses, and retroviruses.
本明細書において「一本鎖(+)RNAウイルス」とは、生活環にDNAステージを有さないゲノムRNA自体がmRNAとして機能し得る一本鎖RNAウイルスの総称を示す。本明細書における「一本鎖(+)RNAウイルス」には、A型肝炎ウイルスや口蹄疫ウイルス等のピコルナウイルス科に属するウイルス、カリシウイルス科に属するウイルス、アストロウイルス科に属するウイルス、SARSウイルス等のコロナウイルス科に属するウイルス、ウエストナイルウイルス、黄熱ウイルス、日本脳炎ウイルス及びHCV等のフラビウイルス科に属するウイルス、並びに風疹ウイルス等のトガウイルス科に属するウイルスが含まれる。
As used herein, “single-stranded (+) RNA virus” is a generic term for a single-stranded RNA virus in which genomic RNA itself that does not have a DNA stage in its life cycle can function as mRNA. The “single-stranded (+) RNA virus” in the present specification includes viruses belonging to the Picornaviridae family such as hepatitis A virus and foot-and-mouth disease virus, viruses belonging to the Caliciviridae family, viruses belonging to the Astroviridae family, and SARS viruses. Viruses belonging to the family Coronaviridae, such as West Nile virus, yellow fever virus, Japanese encephalitis virus, viruses belonging to Flaviviridae such as HCV, and viruses belonging to Togaviridae such as rubella virus.
本明細書において「一本鎖(-)RNAウイルス」とは、RNA依存性RNAポリメラーゼによってゲノムRNAを転写する生活環を有する一本鎖RNAウイルスの総称を示す。本明細書における「一本鎖(-)RNAウイルス」には、狂犬病ウイルス等のラブドウイルス科に属するウイルス、エボラウイルス等のフィロウイルス科に属するウイルス、流行性耳下腺炎ウイルス等のパラミクソウイルス科に属するウイルス、インフルエンザウイルス等のオルトミクソウイルス科に属するウイルス、ブニヤウイルス科に属するウイルス、並びにラッサウイルスやD型肝炎ウイルス等のアレナウイルス科に属するウイルスが含まれる。
As used herein, “single-stranded (−) RNA virus” is a general term for single-stranded RNA viruses having a life cycle in which genomic RNA is transcribed by RNA-dependent RNA polymerase. In the present specification, “single-stranded (−) RNA virus” includes viruses belonging to the Rhabdoviridae family such as rabies virus, viruses belonging to the Filoviridae family such as Ebola virus, and paramyxos such as epidemic parotitis virus. Viruses belonging to the family Viridae, viruses belonging to the Orthomyxoviridae family such as influenza virus, viruses belonging to the Bunyaviridae family, and viruses belonging to the Arenaviridae family such as Lassa virus and hepatitis D virus are included.
本明細書において「レトロウイルス」とは、ゲノムがRNAで構成され、ゲノムRNAをDNAに変換する生活環を有するレトロウイルス科に属するRNAウイルスの総称を示す。本明細書における「レトロウイルス」には、ヒトTリンパ好性ウイルス(HTLV)やモロニーマウス白血病ウイルス(MMLV)のようなオンコレトロウイルス及びヒト免疫不全ウイルス(HIV)やサル免疫不全ウイルス(SIV)のようなレンチウイルスが含まれる。
As used herein, “retrovirus” is a generic term for RNA viruses belonging to the Retroviridae family, whose genome is composed of RNA and has a life cycle that converts genomic RNA into DNA. As used herein, “retrovirus” includes oncorretroviruses such as human T lymphophilic virus (HTLV) and Moloney murine leukemia virus (MMLV), human immunodeficiency virus (HIV), and simian immunodeficiency virus (SIV). Such as lentivirus.
本明細書において「レトロウイルスベクター」とは、レトロウイルス科に属するオンコレトロウイルスやレンチウイルス等を基に遺伝子組換え技術により作製されたウイルス粒子を示し、オンコレトロウイルスベクター、レンチウイルスベクター、シュードタイプベクターが含まれる。シュードタイプベクターとは、Gagタンパク質やPolタンパク質とは由来を異にするEnvタンパク質を有する組換えレトロウイルスベクターのことを言う。
As used herein, “retroviral vector” refers to a virus particle produced by genetic recombination technology based on oncorretrovirus, lentivirus, etc. belonging to the family Retroviridae, and includes oncoretrovirus vector, lentivirus vector, pseudo Contains a type vector. A pseudotype vector refers to a recombinant retroviral vector having an Env protein whose origin is different from that of a Gag protein or a Pol protein.
本明細書において「RNAウイルスのウイルスRNA」とは、RNAウイルスに由来するRNAのことを示す。本明細書における「RNAウイルスのウイルスRNA」には、RNAウイルスのゲノムRNA、当該ゲノムRNAがスプライシングを受けて生成したRNA(例えば、RNAウイルスのアクセサリータンパク質の発現に寄与するRNA)、及び前記のゲノムRNAから転写されたRNAを含む。
In the present specification, “virus RNA of an RNA virus” refers to RNA derived from an RNA virus. In the present specification, “viral viral RNA” refers to genomic RNA of RNA virus, RNA generated by splicing the genomic RNA (for example, RNA that contributes to the expression of RNA viral accessory proteins), and Includes RNA transcribed from genomic RNA.
本明細書において「RNAウイルスのパッケージングシグナル」とは、RNAウイルスのゲノム上に存在する、ゲノムRNAのウイルス粒子への取り込みに必要なシス因子のことを示す。HIVの場合、パッケージングシグナル(Ψ/Psi)はゲノムRNAの5´末端から約800塩基分の領域とされている。
As used herein, “RNA virus packaging signal” refers to a cis-factor that is present on the genome of an RNA virus and is required for the incorporation of genomic RNA into a viral particle. In the case of HIV, the packaging signal (Ψ / Psi) is a region of about 800 bases from the 5 ′ end of the genomic RNA.
本明細書において「一本鎖RNAを配列特異的に切断するエンドリボヌクレア-ゼ」とは、一本鎖RNAのジエステル結合をその塩基配列特異的に加水分解するエンド型のリボヌクレアーゼのことを言う。
As used herein, “endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner” refers to an endo-type ribonuclease that hydrolyzes a diester bond of single-stranded RNA in a base sequence-specific manner. .
本明細書において「脂質修飾シグナル」(lipidation signal)とは、脂肪酸やイソプレノイド等の脂質の付加によるタンパク質の修飾を指令するアミノ酸配列を指す。脂質修飾シグナルとしては、例えば、ミリストイル化シグナル、パルミトイル化シグナル、ミリストイル化とパルミトイル化の二重脂質修飾シグナル、O-アシル化シグナル、並びにイソプレニル化シグナルが知られている。
As used herein, the term “lipid modification signal” refers to an amino acid sequence that directs modification of a protein by addition of lipids such as fatty acids and isoprenoids. As lipid modification signals, for example, myristoylation signals, palmitoylation signals, myristoylation and palmitoylation double lipid modification signals, O-acylation signals, and isoprenylation signals are known.
(1)本発明の融合タンパク質
本発明の融合タンパク質は、RNAウイルスのウイルスRNAに親和性を示すポリペプチドが一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合されてなる。本発明の融合タンパク質は、そのRNAウイルスのウイルスRNAに親和性を示すポリペプチドに由来するドメインにより、ウイルス粒子に取り込まれるウイルスゲノムRNA等のウイルスRNAを認識し、さらに一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼ活性によってウイルスRNAを特定の塩基配列の箇所で切断し、分解することができる。本発明の融合タンパク質は、RNAウイルスが細胞に感染した際のプロウイルスの形成を抑制することができるうえ、RNAウイルスが感染した細胞内でウイルスRNAを切断し、RNAウイルス粒子の出芽(産生)を抑制することができる。さらには、出芽(産生)したウイルス粒子内においてもRNAウイルスのウイルスゲノムを分解することにより、ウイルス粒子の感染性を低下させることができる。このため、本発明の融合タンパク質は、RNAウイルス感染症の治療又は予防に有用である。 (1) Fusion protein of the present invention The fusion protein of the present invention comprises a polypeptide having affinity for viral RNA of an RNA virus fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner. The fusion protein of the present invention recognizes a viral RNA such as a viral genomic RNA incorporated into a viral particle by a domain derived from a polypeptide having an affinity for the viral RNA of the RNA virus, and further sequences the single-stranded RNA in a sequence-specific manner. Viral RNA can be cleaved at a specific nucleotide sequence and decomposed by the endoribonuclease activity that cleaves it. The fusion protein of the present invention can suppress the formation of a provirus when an RNA virus infects a cell, and also cleaves the viral RNA in the cell infected with the RNA virus, thereby budding (production) RNA virus particles. Can be suppressed. Furthermore, the infectivity of the virus particles can be reduced by degrading the viral genome of the RNA virus even in the budding (produced) virus particles. Therefore, the fusion protein of the present invention is useful for the treatment or prevention of RNA virus infection.
本発明の融合タンパク質は、RNAウイルスのウイルスRNAに親和性を示すポリペプチドが一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合されてなる。本発明の融合タンパク質は、そのRNAウイルスのウイルスRNAに親和性を示すポリペプチドに由来するドメインにより、ウイルス粒子に取り込まれるウイルスゲノムRNA等のウイルスRNAを認識し、さらに一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼ活性によってウイルスRNAを特定の塩基配列の箇所で切断し、分解することができる。本発明の融合タンパク質は、RNAウイルスが細胞に感染した際のプロウイルスの形成を抑制することができるうえ、RNAウイルスが感染した細胞内でウイルスRNAを切断し、RNAウイルス粒子の出芽(産生)を抑制することができる。さらには、出芽(産生)したウイルス粒子内においてもRNAウイルスのウイルスゲノムを分解することにより、ウイルス粒子の感染性を低下させることができる。このため、本発明の融合タンパク質は、RNAウイルス感染症の治療又は予防に有用である。 (1) Fusion protein of the present invention The fusion protein of the present invention comprises a polypeptide having affinity for viral RNA of an RNA virus fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner. The fusion protein of the present invention recognizes a viral RNA such as a viral genomic RNA incorporated into a viral particle by a domain derived from a polypeptide having an affinity for the viral RNA of the RNA virus, and further sequences the single-stranded RNA in a sequence-specific manner. Viral RNA can be cleaved at a specific nucleotide sequence and decomposed by the endoribonuclease activity that cleaves it. The fusion protein of the present invention can suppress the formation of a provirus when an RNA virus infects a cell, and also cleaves the viral RNA in the cell infected with the RNA virus, thereby budding (production) RNA virus particles. Can be suppressed. Furthermore, the infectivity of the virus particles can be reduced by degrading the viral genome of the RNA virus even in the budding (produced) virus particles. Therefore, the fusion protein of the present invention is useful for the treatment or prevention of RNA virus infection.
RNAウイルスのウイルスRNAに親和性を示すポリペプチドとしては、本発明を特に限定するものではないが、RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドが例示される。本発明には、一本鎖RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドがより好適であり、レトロウイルスのパッケージングシグナルに親和性を示すポリペプチドがさらにより好適である。
The polypeptide having affinity for viral RNA of RNA virus is not particularly limited to the present invention, but a polypeptide having affinity for packaging signal of RNA virus is exemplified. In the present invention, a polypeptide showing affinity for a single-stranded RNA virus packaging signal is more preferred, and a polypeptide showing affinity for a retroviral packaging signal is even more preferred.
上記のレトロウイルスのパッケージングシグナルに親和性を示すポリペプチドを構成するアミノ酸配列としては、レトロウイルスのGagタンパク質に由来するアミノ酸配列、例えばヒト免疫不全ウイルスのGagタンパク質に由来するアミノ酸配列を好適に使用することができる。当該Gagタンパク質に由来するアミノ酸配列としては、ヌクレオカプシドが例示される。本明細書においてヌクレオカプシドとは、レトロウイルスのGagタンパク質を構成するポリペプチドの一つであり、Gagタンパク質によるパッケージングシグナルの特異的認識に関与するポリペプチドのことを指す。ヌクレオカプシドは、2つのジンクフィンガーモチーフによりパッケージングシグナルの二次構造を認識する。
The amino acid sequence constituting the polypeptide having an affinity for the retroviral packaging signal is preferably an amino acid sequence derived from a retrovirus Gag protein, for example, an amino acid sequence derived from a human immunodeficiency virus Gag protein. Can be used. Examples of the amino acid sequence derived from the Gag protein include nucleocapsid. In this specification, the nucleocapsid is one of the polypeptides constituting the retroviral Gag protein, and refers to a polypeptide involved in specific recognition of the packaging signal by the Gag protein. Nucleocapsid recognizes the secondary structure of the packaging signal through two zinc finger motifs.
RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドのアミノ酸配列としてヌクレオカプシドに由来するアミノ酸配列を使用する場合、天然のヌクレオカプシドを構成するアミノ酸配列の全長を利用してもよいし、その断片や変異体を構成するアミノ酸配列を利用してもよい。天然のヌクレオカプシドを構成するアミノ酸配列としては、例えば、ヒト免疫不全ウイルス1型(HIV-1)由来のヌクレオカプシドのアミノ酸配列(RefSeq Acc. No. NP_057850.1)が挙げられる。本発明に利用可能なヌクレオカプシドの断片や変異体としては、レトロウイルスのパッケージングシグナルに親和性を示すものであれば特に限定はないが、ヌクレオカプシドに由来する2つのジンクフィンガーモチーフを含む断片や変異体、あるいは天然のヌクレオカプシドを構成するアミノ酸配列において1~数個のアミノ酸残基が置換、欠失、及び/又は挿入されてなるアミノ酸配列からなるポリペプチドが例示される。例えば、天然のヌクレオカプシドを構成するアミノ酸配列と90%以上、好ましくは95%以上、さらに好ましくは98%以上の配列同一性を有するアミノ酸配列からなるポリペプチドが例示される。
When using an amino acid sequence derived from a nucleocapsid as the amino acid sequence of a polypeptide that has an affinity for an RNA virus packaging signal, the full-length amino acid sequence constituting the natural nucleocapsid may be used, or a fragment or mutation thereof. The amino acid sequence constituting the body may be used. Examples of the amino acid sequence constituting the natural nucleocapsid include the amino acid sequence of a nucleocapsid derived from human immunodeficiency virus type 1 (HIV-1) (RefSeq Acc. No. NP_057850.1). The nucleocapsid fragment or mutant that can be used in the present invention is not particularly limited as long as it has an affinity for the packaging signal of retrovirus, but a fragment or mutation containing two zinc finger motifs derived from nucleocapsid. And a polypeptide comprising an amino acid sequence in which one to several amino acid residues are substituted, deleted, and / or inserted in an amino acid sequence constituting a body or a natural nucleocapsid. For example, a polypeptide comprising an amino acid sequence having a sequence identity of 90% or more, preferably 95% or more, more preferably 98% or more with an amino acid sequence constituting a natural nucleocapsid is exemplified.
本発明の融合タンパク質は、さらにミリストイル化シグナル等の脂質修飾シグナルをN末端領域に有していてもよい。例えば、Gagタンパク質の細胞膜へのターゲッティングに重要と考えられているマトリックスドメイン(MA)のミリストイル化シグナルをN末端領域に配置した本発明の融合タンパク質は、細胞膜に局在することができる。RNAウイルス感染細胞の細胞膜に本発明の融合タンパク質が局在した場合、RNAウイルスの出芽とともに前記融合タンパク質がRNAウイルス粒子内に取り込まれる。こうすることにより、仮にRNAウイルス感染細胞からRNAウイルス粒子が出芽しても、そこに取り込まれた本発明の融合タンパク質により、当該RNAウイルス粒子の感染性を低減させることができる。さらに、本発明の融合タンパク質は、上記の脂質修飾シグナルに加えて、Gagタンパク質の細胞膜へのターゲッティングに寄与すると考えられているGagタンパク質のN末端近傍の塩基性領域を有していてもよい。Gagタンパク質が一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合してなる融合タンパク質は、本発明の好適な態様の一つである。
The fusion protein of the present invention may further have a lipid modification signal such as a myristoylation signal in the N-terminal region. For example, the fusion protein of the present invention in which the myristoylation signal of the matrix domain (MA), which is considered important for targeting the Gag protein to the cell membrane, is located in the N-terminal region can be localized on the cell membrane. When the fusion protein of the present invention is localized in the cell membrane of an RNA virus-infected cell, the fusion protein is incorporated into the RNA virus particle as the RNA virus emerges. By doing so, even if RNA virus particles emerge from RNA virus-infected cells, the infectivity of the RNA virus particles can be reduced by the fusion protein of the present invention incorporated therein. Furthermore, the fusion protein of the present invention may have a basic region in the vicinity of the N-terminus of the Gag protein that is considered to contribute to the targeting of the Gag protein to the cell membrane, in addition to the lipid modification signal described above. A fusion protein in which a Gag protein is fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner is one of the preferred embodiments of the present invention.
本発明の融合タンパク質は、RNAウイルス感染症の治療又は予防に使用することができる。この場合、RNAウイルスのウイルスRNAに親和性を示すポリペプチドは、治療又は予防の対象とする疾患の原因となるRNAウイルスの種類に応じて適宜選択することができる。本発明を特に限定するものではないが、本発明の融合タンパク質をHIV感染症の治療又は予防に使用する場合、例えばHIVのヌクレオカプシドをレトロウイルスのパッケージングシグナルに親和性を示すポリペプチドとして利用すればよい。
The fusion protein of the present invention can be used for treatment or prevention of RNA virus infection. In this case, the polypeptide having affinity for the viral RNA of the RNA virus can be appropriately selected according to the type of RNA virus that causes the disease to be treated or prevented. Although the present invention is not particularly limited, when the fusion protein of the present invention is used for the treatment or prevention of HIV infection, for example, a nucleocapsid of HIV is used as a polypeptide having an affinity for a retroviral packaging signal. That's fine.
本発明の融合タンパク質における一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼは、前記活性を有するリボヌクレアーゼであれば特に限定はない。例えば、二本鎖RNAやRNA-DNAハイブリッド等の二本鎖核酸を切断できないリボヌクレアーゼが例示される。リボソーム非依存的に一本鎖RNAを配列特異的に分解するエンドリボヌクレアーゼは本発明に特に好ましい。本発明には微生物由来のエンドリボヌクレアーゼ、例えば、トキシン-アンチトキシン系のトキシンを構成するエンドリボヌクレアーゼであるMazF、PemK、MqsR、Nitrosomonas europaea ATCC19718株由来NE1181ポリペプチド、Deinococcus radioduran R1株由来DR0662ポリペプチド等を本発明の融合タンパク質に使用することができる。MazFは一本鎖RNA中の5´-A/CA-3´の配列を、PemKは主に5´-U/A(C,A or U)-3´を切断する酵素である。また、MqsRは5´-GCU-3´の配列、NE1181ポリペプチドは5´-AAAU-3´の配列、DR0662ポリペプチドは5´-UUCCUUU-3´の配列で、それぞれ一本鎖RNAを切断する。さらに、公知のデータベースから前記のMazFやPemKのアミノ酸配列との間に高い相同性(例えば50%以上、好ましくは70%以上、より好ましくは90%以上の相同性)を有するポリペプチドを選択し、そこから一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼを有するポリペプチドを見出したうえで、当該ポリペプチドのアミノ酸配列を本発明の融合タンパク質に利用してもよい。既に藍藻類や古細菌を含む多数の微生物から、一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼ活性を有するポリペプチドが見出されている(国際公開第2006/123537号パンフレット、国際公開第2007/010740号パンフレット、国際公開第2007/013264号パンフレット、国際公開第2007/013265号パンフレット等)。
The endoribonuclease that specifically cleaves single-stranded RNA in the fusion protein of the present invention is not particularly limited as long as it is a ribonuclease having the above activity. Examples thereof include ribonucleases that cannot cleave double-stranded nucleic acids such as double-stranded RNA and RNA-DNA hybrids. Endoribonucleases that specifically degrade single-stranded RNA in a ribosome-independent manner are particularly preferred for the present invention. The present invention includes a microorganism-derived endoribonuclease such as MazF, PemK, MqsR, which is an endoribonuclease constituting a toxin-antitoxin-type toxin, NE1181 polypeptide derived from Nitromononas europaea ATCC 19718, Deinococcus radioduran R2 polypeptide DR0, etc. Can be used in the fusion protein of the present invention. MazF is an enzyme that cleaves 5′-A / CA-3 ′ sequences in single-stranded RNA, and PemK is an enzyme that mainly cleaves 5′-U / A (C, A or U) -3 ′. MqsR is 5'-GCU-3 'sequence, NE1181 polypeptide is 5'-AAAU-3' sequence, and DR0662 polypeptide is 5'-UUCCUUU-3 'sequence. To do. Furthermore, a polypeptide having high homology (for example, 50% or more, preferably 70% or more, more preferably 90% or more homology) between the amino acid sequences of MazF and PemK is selected from a known database. Then, after finding a polypeptide having an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner, the amino acid sequence of the polypeptide may be used for the fusion protein of the present invention. A polypeptide having an endoribonuclease activity that cleaves single-stranded RNA in a sequence-specific manner has already been found from many microorganisms including cyanobacteria and archaea (International Publication No. 2006/123537, International Publication No. 1). 2007/010740 pamphlet, WO 2007/013264 pamphlet, WO 2007/013265 pamphlet, etc.).
トキシン-アンチトキシン系のトキシンを構成するエンドリボヌクレアーゼは、ヒト胎盤由来リボヌクレアーゼインヒビターのような細胞質リボヌクレアーゼインヒビターによって阻害されることがないので、本発明の融合タンパク質に利用される一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼとして適している。
The endoribonuclease that constitutes the toxin-antitoxin toxin is not inhibited by cytoplasmic ribonuclease inhibitors such as human placenta-derived ribonuclease inhibitors, so the single-stranded RNA used in the fusion protein of the present invention is sequence-specific. It is suitable as an endoribonuclease that cleaves automatically.
本発明では、一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼとして、前記の活性を有する天然のエンドリボヌクレアーゼを構成するアミノ酸配列の全長を利用してもよいし、その断片や変異体を構成するアミノ酸配列を利用してもよい。本発明にそのアミノ酸配列が利用可能なエンドリボヌクレアーゼの断片や変異体としては、一本鎖RNAを配列特異的に切断する活性を有するものであれば特に限定はないが、天然のエンドリボヌクレアーゼを構成するアミノ酸配列において1~数個のアミノ酸残基が置換、欠失、及び/又は挿入されてなるアミノ酸配列からなるポリペプチドが例示される。前記の断片や変異体としては、天然のエンドリボヌクレアーゼを構成するアミノ酸配列〔例えば天然のMazFを構成するアミノ酸配列(RefSec Acc. No. NP_417262)〕と90%以上、好ましくは95%以上、さらに好ましくは98%以上の配列同一性を有するアミノ酸配列のものが例示される。
In the present invention, as an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner, the full length of the amino acid sequence constituting the natural endoribonuclease having the above-mentioned activity may be used, or a fragment or variant thereof is constructed. An amino acid sequence may be used. The endoribonuclease fragment or variant whose amino acid sequence can be used in the present invention is not particularly limited as long as it has an activity of specifically cleaving single-stranded RNA. Examples thereof include polypeptides comprising an amino acid sequence in which one to several amino acid residues are substituted, deleted, and / or inserted in the amino acid sequence to be prepared. Examples of the fragment or mutant include an amino acid sequence constituting a natural endoribonuclease [for example, an amino acid sequence constituting a natural MazF (RefSec Acc. No. NP_417262)] and 90% or more, preferably 95% or more, and more preferably. Is exemplified by amino acid sequences having 98% or more sequence identity.
本発明の融合タンパク質において、RNAウイルスのウイルスRNAに親和性を示すポリペプチドは、一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに対してC末端側に配置されていても、N末端側に配置されていてもよく、その両側に配置されていてもよい。
In the fusion protein of the present invention, the polypeptide showing affinity for the viral RNA of the RNA virus may be located on the C-terminal side with respect to the endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner. May be arranged on both sides thereof.
本発明の融合タンパク質は、RNAウイルスのウイルスRNAに親和性を示すポリペプチドと一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼとをつなぐリンカーペプチドを有していてもよい。例えば、レトロウイルス由来のヌクレオカプシドがリンカーペプチドを介して一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合してなる融合タンパク質は、本発明の好適な態様の一つである。
The fusion protein of the present invention may have a linker peptide that connects a polypeptide having affinity for viral RNA of an RNA virus and an endoribonuclease that specifically cleaves single-stranded RNA. For example, a fusion protein obtained by fusing a retrovirus-derived nucleocapsid with an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner via a linker peptide is one of the preferred embodiments of the present invention.
リンカーペプチドは、それを介して融合される両ポリペプチドの機能発揮への影響を考慮して適宜選択可能であり、特に限定はない。本発明に使用されるリンカーペプチドとして例えばアラニンリンカー、セリン-グリシンリンカー、及びプロテアーゼ認識配列を有するリンカーペプチドが例示される。例えば、本発明の融合タンパク質におけるRNAウイルスのパッケージングシグナルに親和性を示すポリペプチドとしてHIV由来のGagタンパク質を利用する場合、リンカーペプチドとしてHIVプロテアーゼの認識配列を有するポリペプチドを利用してもよい。こうすることにより、本発明の融合たんぱく質がウイルス粒子に封入された際に、ウイルス粒子内で遊離のエンドリボヌクレアーゼを生成させることができる。
The linker peptide can be appropriately selected in consideration of the effect on the function of both polypeptides fused via the linker peptide, and is not particularly limited. Examples of linker peptides used in the present invention include alanine linkers, serine-glycine linkers, and linker peptides having a protease recognition sequence. For example, when an HIV-derived Gag protein is used as a polypeptide having affinity for the RNA virus packaging signal in the fusion protein of the present invention, a polypeptide having an HIV protease recognition sequence may be used as a linker peptide. . By doing so, when the fusion protein of the present invention is encapsulated in the virus particle, free endoribonuclease can be generated in the virus particle.
(2)本発明の核酸
本発明の核酸は、本発明の融合タンパク質をコードする遺伝子を含む。本発明の核酸を導入した細胞は、本発明の融合タンパク質の製造に使用することができる。また、本発明の核酸を適切なベクターによってレトロウイルス感染細胞等のRNAウイルス感染細胞内に導入することにより、RNAウイルス感染細胞からのRNAウイルス粒子の出芽(産生)を抑制することができる。また、RNAウイルス感染細胞より出芽するRNAウイルス粒子の感染性を低下させることができる。さらには、本発明の核酸が導入された細胞は、RNAウイルスが感染した際のプロウイルスの形成が抑制される。 (2) Nucleic acid of the present invention The nucleic acid of the present invention includes a gene encoding the fusion protein of the present invention. Cells into which the nucleic acid of the present invention has been introduced can be used for the production of the fusion protein of the present invention. Moreover, budding (production) of RNA virus particles from RNA virus-infected cells can be suppressed by introducing the nucleic acid of the present invention into RNA virus-infected cells such as retrovirus-infected cells using an appropriate vector. In addition, the infectivity of RNA virus particles sprouting from RNA virus-infected cells can be reduced. Furthermore, provirus formation when cells infected with the nucleic acid of the present invention are infected with RNA viruses is suppressed.
本発明の核酸は、本発明の融合タンパク質をコードする遺伝子を含む。本発明の核酸を導入した細胞は、本発明の融合タンパク質の製造に使用することができる。また、本発明の核酸を適切なベクターによってレトロウイルス感染細胞等のRNAウイルス感染細胞内に導入することにより、RNAウイルス感染細胞からのRNAウイルス粒子の出芽(産生)を抑制することができる。また、RNAウイルス感染細胞より出芽するRNAウイルス粒子の感染性を低下させることができる。さらには、本発明の核酸が導入された細胞は、RNAウイルスが感染した際のプロウイルスの形成が抑制される。 (2) Nucleic acid of the present invention The nucleic acid of the present invention includes a gene encoding the fusion protein of the present invention. Cells into which the nucleic acid of the present invention has been introduced can be used for the production of the fusion protein of the present invention. Moreover, budding (production) of RNA virus particles from RNA virus-infected cells can be suppressed by introducing the nucleic acid of the present invention into RNA virus-infected cells such as retrovirus-infected cells using an appropriate vector. In addition, the infectivity of RNA virus particles sprouting from RNA virus-infected cells can be reduced. Furthermore, provirus formation when cells infected with the nucleic acid of the present invention are infected with RNA viruses is suppressed.
本発明の核酸の核酸配列は、本発明の融合タンパク質をコードする遺伝子を含むものであれば特に限定はないが、本発明の核酸から発現される融合タンパク質のエンドリボヌクレアーゼ活性によって切断される核酸配列を含まないように設計することが好ましい。
The nucleic acid sequence of the nucleic acid of the present invention is not particularly limited as long as it contains a gene encoding the fusion protein of the present invention, but the nucleic acid sequence cleaved by the endoribonuclease activity of the fusion protein expressed from the nucleic acid of the present invention. It is preferable to design so that it does not contain.
好適な態様において、本発明の核酸は、RNAウイルスのトランス作用因子により転写が誘導される転写調節配列と、前記配列により発現の制御が可能な形態に配置された本発明の融合タンパク質をコードする遺伝子とにより構成される。
In a preferred embodiment, the nucleic acid of the present invention encodes a transcriptional regulatory sequence whose transcription is induced by an RNA virus trans-acting factor, and a fusion protein of the present invention arranged in a form in which expression can be controlled by the sequence. It is composed of genes.
この態様において、本発明の核酸が導入された細胞や当該細胞から分化した細胞は、RNAウイルスの感染を受けて細胞内にRNAウイルス由来のトランス作用因子が生成した際に、転写調節配列の下流に位置する本発明の融合タンパク質をコードする遺伝子の発現が誘導され、細胞内に本発明の融合タンパク質が発現する。発現した本発明の融合タンパク質によりRNAウイルスのウイルスRNAは分解される。その結果、RNAウイルス感染細胞内でのプロウイルスの形成、ウイルスゲノムの複製、ウイルスタンパク質の合成、及びウイルス感染細胞からのRNAウイルス粒子の出芽(産生)が抑制される。さらには、RNAウイルス感染細胞からRNAウイルス粒子が出芽した場合においても、RNAウイルス粒子の感染性が低減される。
In this embodiment, a cell into which the nucleic acid of the present invention has been introduced or a cell differentiated from the cell is downstream of the transcriptional regulatory sequence when an RNA virus-derived trans-acting factor is generated in the cell after being infected with the RNA virus. Expression of the gene encoding the fusion protein of the present invention located in is induced, and the fusion protein of the present invention is expressed in the cell. Viral RNA of the RNA virus is degraded by the expressed fusion protein of the present invention. As a result, provirus formation, virus genome replication, virus protein synthesis, and budding (production) of RNA virus particles from virus-infected cells are suppressed in RNA virus-infected cells. Furthermore, even when RNA virus particles emerge from RNA virus-infected cells, the infectivity of RNA virus particles is reduced.
RNAウイルスのトランス作用因子により転写が誘導される転写調節配列には特に限定はないが、例えば、HIVのTatタンパク質により転写が誘導される転写調節配列のような、レトロウイルスのトランス作用因子により転写が誘導される転写調節配列を使用することができる。Tatタンパク質は、HIVのLTRプロモーターの作用により転写が開始されたRNA内に存在するTAR(trans-activation responsive element)配列に結合し、それより下流の転写を活性化することから、転写開始点の下流にTAR領域の塩基配列を有する転写調節配列を本発明の核酸に使用することができる。特に好適には、HIVのLTRや、前記LTRに適切な改変を施した転写調節配列が使用される。前記の改変としては、例えばプロモーター内に存在する宿主細胞由来の転写因子との結合部位の欠失やTat特異的な転写に不要な領域の欠失が例示される。前者の改変により、宿主由来転写因子による、HIV感染とは無関係な転写のレベルを低下させることが可能である。また、後者の改変としてLTRのU5領域やTAR配列より下流の領域(R領域の一部及びU5領域)の欠失が例示される。このような欠失LTRを有する本発明の核酸は、当該核酸を保持する高タイターのレトロウイルスベクターを作製する際に有利である。
Transcriptional regulatory sequences whose transcription is induced by RNA virus trans-acting factors are not particularly limited, but are transcribed by retroviral trans-acting factors such as transcriptional regulatory sequences whose transcription is induced by HIV Tat protein. Transcriptional regulatory sequences from which can be derived can be used. The Tat protein binds to a TAR (trans-activation response element) sequence present in RNA whose transcription has been initiated by the action of the HIV LTR promoter, and activates transcription downstream from it. A transcriptional regulatory sequence having a TAR region base sequence downstream can be used for the nucleic acid of the present invention. Particularly preferably, an HIV LTR or a transcriptional regulatory sequence obtained by appropriately modifying the LTR is used. Examples of the modification include deletion of a binding site with a host cell-derived transcription factor present in the promoter and deletion of a region unnecessary for Tat-specific transcription. The former modification can reduce the level of transcription unrelated to HIV infection by host-derived transcription factors. Examples of the latter modification include deletion of the LTR U5 region and a region downstream of the TAR sequence (part of R region and U5 region). The nucleic acid of the present invention having such a deleted LTR is advantageous in preparing a high-titer retroviral vector that retains the nucleic acid.
また、HIVゲノムに存在するRRE(Rev-responsible element)は、HIV由来のトランス作用因子であるRevタンパク質との相互作用によってタンパク質の発現を促進することが知られている。さらに、gag、pol遺伝子内に存在するINSと呼ばれる領域は、Revタンパク質の非存在下ではHIVのmRNAの転写を抑制しているが、前記のRRE及びRevタンパク質との相互作用によってこの抑制作用が解除されることが知られている[ジャーナル・オブ・ウイロロジー(J. Virol.)、第66巻、p7176-7182(1992)]。したがって、本発明の核酸において、本発明の融合タンパク質をコードする遺伝子の3´側にこれらの転写調節配列を組み込むことにより、本発明の融合タンパク質をRevタンパク質依存的に、すなわちHIV感染依存的に発現させることができる。
Also, it is known that RRE (Rev-responsible element) present in the HIV genome promotes protein expression through interaction with Rev protein, which is a trans-acting factor derived from HIV. Furthermore, a region called INS present in the gag and pol genes suppresses transcription of HIV mRNA in the absence of Rev protein, but this inhibitory action is caused by the interaction with RRE and Rev protein. [Journal of virology (J. Virol.), Vol. 66, p7176-7182 (1992)]. Therefore, by incorporating these transcriptional regulatory sequences into the 3 ′ side of the gene encoding the fusion protein of the present invention in the nucleic acid of the present invention, the fusion protein of the present invention is dependent on Rev protein, that is, HIV infection-dependent. Can be expressed.
前記のRNAウイルス由来のトランス作用因子と相互作用する配列は、当該配列が本来的に組み込まれている機能的配列、例えばプロモーターとともに使用してもよく、異種の機能的配列と組み合わせて使用してもよい。例えば、本発明の核酸の導入が望まれる細胞においてmRNAの転写を開始しうる、RNAウイルス由来ではないプロモーター及び前記のRNAウイルス由来のトランス作用因子と相互作用する配列を組み合わせて構築された配列も、本発明に使用される「転写調節配列」に包含される。
The sequence that interacts with the RNA virus-derived trans-acting factor may be used in combination with a functional sequence in which the sequence is inherently incorporated, for example, a promoter, or in combination with a heterologous functional sequence. Also good. For example, a sequence constructed by combining a promoter that is not derived from an RNA virus and a sequence that interacts with a trans-acting factor derived from the RNA virus, which can initiate transcription of mRNA in a cell in which introduction of the nucleic acid of the present invention is desired. Are included in the “transcriptional regulatory sequence” used in the present invention.
(3)本発明のベクター
本発明のベクターは、本発明の核酸を含むベクターである。本発明のベクターは、本発明の核酸からのRNAの転写を達成するためのプロモーター及び転写開始部位、ならびにこれらの因子と協働する他の調節要素、例えば、エンハンサーやオペレーター配列を有していても良い。さらに、本発明の核酸の下流にターミネーター配列を含有することができる。また、本発明のベクターは、遺伝子導入された細胞の選択を可能にする適当なマーカー遺伝子を有していてもよい。マーカー遺伝子としては、例えば、細胞に抗生物質に対する耐性を付与する薬剤耐性遺伝子や、蛍光タンパク質をコードする遺伝子、酵素活性の検出によって遺伝子導入された細胞を見分けることができるレポーター遺伝子が利用できる。さらに、本発明のベクターは、遺伝子導入細胞による治療が完遂した場合、あるいは何らかの副作用が生じた場合に生体内から遺伝子導入細胞を排除する目的で、自殺遺伝子を搭載していても良い。 (3) Vector of the present invention The vector of the present invention is a vector containing the nucleic acid of the present invention. The vector of the present invention has a promoter and transcription initiation site for achieving transcription of RNA from the nucleic acid of the present invention, and other regulatory elements that cooperate with these factors, such as enhancer and operator sequences. Also good. Furthermore, a terminator sequence can be contained downstream of the nucleic acid of the present invention. Further, the vector of the present invention may have an appropriate marker gene that enables selection of the gene-introduced cell. As the marker gene, for example, a drug resistance gene that confers resistance to antibiotics on a cell, a gene that encodes a fluorescent protein, or a reporter gene that can distinguish a cell into which a gene has been introduced by detecting enzyme activity can be used. Furthermore, the vector of the present invention may be loaded with a suicide gene for the purpose of excluding the transgenic cell from the living body when the treatment with the transgenic cell is completed or when some side effect occurs.
本発明のベクターは、本発明の核酸を含むベクターである。本発明のベクターは、本発明の核酸からのRNAの転写を達成するためのプロモーター及び転写開始部位、ならびにこれらの因子と協働する他の調節要素、例えば、エンハンサーやオペレーター配列を有していても良い。さらに、本発明の核酸の下流にターミネーター配列を含有することができる。また、本発明のベクターは、遺伝子導入された細胞の選択を可能にする適当なマーカー遺伝子を有していてもよい。マーカー遺伝子としては、例えば、細胞に抗生物質に対する耐性を付与する薬剤耐性遺伝子や、蛍光タンパク質をコードする遺伝子、酵素活性の検出によって遺伝子導入された細胞を見分けることができるレポーター遺伝子が利用できる。さらに、本発明のベクターは、遺伝子導入細胞による治療が完遂した場合、あるいは何らかの副作用が生じた場合に生体内から遺伝子導入細胞を排除する目的で、自殺遺伝子を搭載していても良い。 (3) Vector of the present invention The vector of the present invention is a vector containing the nucleic acid of the present invention. The vector of the present invention has a promoter and transcription initiation site for achieving transcription of RNA from the nucleic acid of the present invention, and other regulatory elements that cooperate with these factors, such as enhancer and operator sequences. Also good. Furthermore, a terminator sequence can be contained downstream of the nucleic acid of the present invention. Further, the vector of the present invention may have an appropriate marker gene that enables selection of the gene-introduced cell. As the marker gene, for example, a drug resistance gene that confers resistance to antibiotics on a cell, a gene that encodes a fluorescent protein, or a reporter gene that can distinguish a cell into which a gene has been introduced by detecting enzyme activity can be used. Furthermore, the vector of the present invention may be loaded with a suicide gene for the purpose of excluding the transgenic cell from the living body when the treatment with the transgenic cell is completed or when some side effect occurs.
本発明のベクターの種類に特に限定はないが、プラスミドベクターやウイルスベクターが例示される。ウイルスベクターとしては、レトロウイルスベクター、アデノウイルスベクター、アデノ随伴ウイルスベクター、ヘルペスウイルスベクターが例示される。本発明の核酸を染色体上に組み込む能力を有するレトロウイルスベクターは、本発明のベクターとして好適である。
The type of the vector of the present invention is not particularly limited, and examples thereof include plasmid vectors and virus vectors. Examples of virus vectors include retrovirus vectors, adenovirus vectors, adeno-associated virus vectors, and herpes virus vectors. A retroviral vector having the ability to incorporate the nucleic acid of the present invention onto a chromosome is suitable as the vector of the present invention.
本発明のベクターとして利用可能なレトロウイルスベクターとしては、例えば、モロニー白血病ウイルス(MMLV)に基づくベクター等のオンコレトロウイルスベクター、ヒト免疫不全ウイルス1型(HIV-1)に基づくベクターやサル免疫不全ウイルス(SIV)に基づくベクター等のレンチウイルスベクターが例示される。これらベクターは、エンベロープとして異種のウイルス由来のエンベロープを用いることによってシュードタイプ化しても良い。ここで、シュードタイプベクターとしては、水泡性口内炎ウイルス(VSV)、テナガザル白血病ウイルス(GaLV)、ネコ内因性ウイルスRD114、マウス白血病ウイルス(Ecotropic-env、amphotropic-env、10A1-env等)等のEnvタンパク質を有するオンコレトロウイルスベクターやレンチウイルスベクターが例示される。また、複製能欠損組換えレトロウイルスベクターは、本発明のベクターの好適な態様の一つである。該ベクターは、感染した細胞中で自己複製できないように複製能を欠損させてあり、非病原性である。公知の複製能欠損レトロウイルスベクターとしては、MFGベクター(ATCC No.68754)、α-SGCベクター(ATCC No.68755)、LXSNベクター[バイオテクニクス(BioTechniques)、第7巻、第980~990頁(1989年)]、タカラバイオ社製のDON-5、DON-AI-2、MEI-5レトロウイルスベクター、クロンテック社製のRetro-X Qベクターシリーズ、Lenti-Xベクターシリーズ等が例示される。また、RNAウイルス感染細胞において制限的に複製できるレトロウイルスベクターも本発明のベクターの好適な態様の一つである。該ベクターは、RNAウイルスが感染した細胞中で選択的に複製し、治療効果のある融合タンパク質遺伝子が伝搬される。
Examples of the retrovirus vector that can be used as the vector of the present invention include oncorretrovirus vectors such as vectors based on Moloney leukemia virus (MMLV), vectors based on human immunodeficiency virus type 1 (HIV-1), and simian immunodeficiency. Lentiviral vectors such as vectors based on viruses (SIV) are exemplified. These vectors may be pseudotyped by using an envelope derived from a different virus as the envelope. Here, pseudotype vectors include Env such as vesicular stomatitis virus (VSV), gibbon leukemia virus (GaLV), feline endogenous virus RD114, murine leukemia virus (Ecotropic-env, amphotropic-env, 10A1-env, etc.) Examples include oncoretrovirus vectors and lentivirus vectors having proteins. A replication-deficient recombinant retrovirus vector is one of the preferred embodiments of the vector of the present invention. The vector is non-pathogenic, deficient in replication so that it cannot replicate in infected cells. Known replication-defective retrovirus vectors include MFG vector (ATCC No. 68754), α-SGC vector (ATCC No. 68755), LXSN vector [BioTechniques, Volume 7, pages 980-990 ( 1989)], DON-5, DON-AI-2, MEI-5 retroviral vectors manufactured by Takara Bio Inc., Retro-X Q vector series, Lenti-X vector series manufactured by Clontech. In addition, a retrovirus vector that can be restrictedly replicated in RNA virus-infected cells is also a preferred embodiment of the vector of the present invention. The vector selectively replicates in cells infected with the RNA virus and propagates a therapeutically effective fusion protein gene.
一般的なレトロウイルスベクターの産生方法としては、あらかじめgag-pol遺伝子、env遺伝子等のレトロウイルスの構造タンパク質をコードする遺伝子が染色体上に組み込まれたレトロウイルスパッケージング細胞に、外来遺伝子とパッケージングシグナルを搭載したトランスファーベクターを導入することによって産生させる方法、及びレトロウイルスの構造タンパク質を有さない細胞に、gag-pol遺伝子やenv遺伝子等のレトロウイルス構造タンパク質をコードする遺伝子を有するパッケージングプラスミドと同時に、前記のトランスファーベクターをトランスフェクションして産生させる方法が挙げられる。
As a general method for producing a retrovirus vector, a retrovirus packaging cell in which a gene encoding a retrovirus structural protein such as a gag-pol gene or an env gene has been incorporated into a chromosome in advance is packaged with a foreign gene. A method of producing by introducing a transfer vector carrying a signal, and a packaging plasmid having a gene encoding a retrovirus structural protein such as a gag-pol gene or an env gene in a cell having no retroviral structural protein At the same time, there can be mentioned a method of producing the aforementioned transfer vector by transfection.
本発明のベクターとしては、レトロウイルスベクター産生用のトランスファーベクターとして使用されるものも例示される。トランスファーベクターとして使用される本発明のベクターとしては、プラスミドベクターが好適に例示される。
Examples of the vector of the present invention include those used as transfer vectors for retrovirus vector production. A plasmid vector is preferably exemplified as the vector of the present invention used as a transfer vector.
本発明のベクターは、本発明の核酸を転写するためのユニットをレトロウイルスベクターのRNAゲノムの転写方向に対して逆向き、すなわち、本発明の核酸の転写調節配列より開始される転写の向きをレトロウイルスベクターの5’-LTRより開始される転写の向きと反対となるようにレトロウイルスベクター内に配置してもよい。この構成を採用すれば、本発明のベクターから転写されるレトロウイルスmRNAは本発明の融合タンパク質をコードする遺伝子のアンチセンス鎖に相当するため、このレトロウイルスmRNAから本発明の融合タンパク質の発現が抑えられる。また、前記転写調節配列の特性に起因する、本発明の融合タンパク質をコードする遺伝子からの微弱な転写があったとしても、レトロウイルスmRNAがアンチセンスRNAとして機能することで本発明の融合タンパク質の発現が抑制される。このため、本態様のベクターをレトロウイルスベクター産生用のトランスファーベクターとして使用することにより、高い力価のレトロウイルスベクターを得ることができる。さらに、本発明の融合タンパク質における一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼが、トキシン-アンチトキシン系のトキシンである場合、当該レトロウイルスベクターを産生する細胞として、アンチトキシン産生株を使用することにより、高い力価のレトロウイルスベクターを得ることができる。
In the vector of the present invention, the unit for transcription of the nucleic acid of the present invention is reverse to the transcription direction of the RNA genome of the retroviral vector, that is, the direction of transcription initiated by the transcriptional regulatory sequence of the nucleic acid of the present invention. It may be arranged in the retroviral vector so as to be opposite to the direction of transcription initiated from the 5′-LTR of the retroviral vector. If this configuration is adopted, the retroviral mRNA transcribed from the vector of the present invention corresponds to the antisense strand of the gene encoding the fusion protein of the present invention. Therefore, the expression of the fusion protein of the present invention can be expressed from this retroviral mRNA. It can be suppressed. In addition, even if there is weak transcription from the gene encoding the fusion protein of the present invention due to the characteristics of the transcription regulatory sequence, the retroviral mRNA functions as an antisense RNA, so that the fusion protein of the present invention Expression is suppressed. For this reason, a high titer retrovirus vector can be obtained by using the vector of this embodiment as a transfer vector for producing a retrovirus vector. Furthermore, when the endoribonuclease that specifically cleaves single-stranded RNA in the fusion protein of the present invention is a toxin-antitoxin toxin, an antitoxin producing strain is used as a cell that produces the retroviral vector. By doing so, a high titer retrovirus vector can be obtained.
また、本発明のレトロウイルスベクターは、ベクターから転写されるRNAが本発明の融合タンパク質によって認識されないようにするため、本発明の融合タンパク質におけるRNAウイルスのウイルスRNAに親和性を示すポリペプチドとは由来が異なるパッケージングシグナルを有していてもよい。例えば、レンチウイルス(例えばHIV)由来のヌクレオカプシド及びエンドリボヌクレアーゼからなる融合タンパク質をコードする遺伝子、並びにオンコレトロウイルス(例えばMMLV)由来のパッケージングシグナルを含むレトロウイルスベクターは、本発明のベクターの好適な態様の一つである。
The retroviral vector of the present invention is a polypeptide having an affinity for the viral RNA of the RNA virus in the fusion protein of the present invention so that RNA transcribed from the vector is not recognized by the fusion protein of the present invention. It may have packaging signals with different origins. For example, a retroviral vector comprising a gene encoding a fusion protein consisting of a nucleocapsid and endoribonuclease from a lentivirus (eg, HIV) and a packaging signal from an oncoretrovirus (eg, MMLV) is suitable for the vector of the present invention. This is one of the embodiments.
(4)本発明の疾病の治療又は予防方法
本発明の疾病の治療又は予防方法は、本発明のベクターを細胞に導入する工程を含み、レトロウイルス感染症等のRNAウイルス感染症の治療又は予防に有用である。本発明の治療又は予防方法が有効なRNAウイルス感染症としては、例えば、A型肝炎、口蹄疫、重症急性呼吸器症候群(SARS)、ウエストナイル熱、黄熱病、日本脳炎、C型肝炎、風疹、狂犬病、エボラ出血熱、流行性耳下腺炎、インフルエンザ、ラッサ熱、D型肝炎、AIDSを含むHIV感染症、及び成人T細胞白血病が挙げられる。なお、これらの疾患等の治療又は予防のための本発明のベクターを含む医薬組成物は、本発明の一態様である。 (4) Method of treating or preventing the disease of the present invention The method of treating or preventing the disease of the present invention comprises the step of introducing the vector of the present invention into a cell, and treating or preventing an RNA virus infection such as a retrovirus infection. Useful for. Examples of RNA virus infections for which the treatment or prevention method of the present invention is effective include hepatitis A, foot-and-mouth disease, severe acute respiratory syndrome (SARS), West Nile fever, yellow fever, Japanese encephalitis, hepatitis C, rubella, Rabies, Ebola hemorrhagic fever, mumps, influenza, Lassa fever, hepatitis D, HIV infection including AIDS, and adult T-cell leukemia. Note that a pharmaceutical composition containing the vector of the present invention for treatment or prevention of these diseases and the like is one embodiment of the present invention.
本発明の疾病の治療又は予防方法は、本発明のベクターを細胞に導入する工程を含み、レトロウイルス感染症等のRNAウイルス感染症の治療又は予防に有用である。本発明の治療又は予防方法が有効なRNAウイルス感染症としては、例えば、A型肝炎、口蹄疫、重症急性呼吸器症候群(SARS)、ウエストナイル熱、黄熱病、日本脳炎、C型肝炎、風疹、狂犬病、エボラ出血熱、流行性耳下腺炎、インフルエンザ、ラッサ熱、D型肝炎、AIDSを含むHIV感染症、及び成人T細胞白血病が挙げられる。なお、これらの疾患等の治療又は予防のための本発明のベクターを含む医薬組成物は、本発明の一態様である。 (4) Method of treating or preventing the disease of the present invention The method of treating or preventing the disease of the present invention comprises the step of introducing the vector of the present invention into a cell, and treating or preventing an RNA virus infection such as a retrovirus infection. Useful for. Examples of RNA virus infections for which the treatment or prevention method of the present invention is effective include hepatitis A, foot-and-mouth disease, severe acute respiratory syndrome (SARS), West Nile fever, yellow fever, Japanese encephalitis, hepatitis C, rubella, Rabies, Ebola hemorrhagic fever, mumps, influenza, Lassa fever, hepatitis D, HIV infection including AIDS, and adult T-cell leukemia. Note that a pharmaceutical composition containing the vector of the present invention for treatment or prevention of these diseases and the like is one embodiment of the present invention.
本発明の疾病の治療又は予防方法により、例えばヒト免疫不全ウイルス感染症の治療又は予防を行う場合、本発明のベクターは、HIVが感染する可能性のある細胞、すなわちCD4陽性細胞を含む細胞(細胞群)に導入されることが望まれる。したがって、本発明の治療又は予防方法では、CD4陽性細胞(例えばT細胞)やCD4陽性細胞に分化しうる前駆細胞(例えば造血幹細胞)、あるいは前記の細胞を含有する細胞集団を標的細胞として遺伝子導入が実施される。HIVの感染を受ける可能性のある細胞に網羅的に前記の核酸構築物を導入する観点から、本発明では造血幹細胞又は当該細胞を含有する細胞集団を標的とすることが好ましい。前記の細胞は、CD4陽性細胞やその前駆細胞を含有するものであれば特に限定はなく、個体より採取された血液細胞(末梢血細胞、臍帯血細胞)、骨髄細胞や、前記の細胞から公知方法により分画されたCD4陽性細胞、CD4陽性細胞の前駆細胞、造血幹細胞等が例示される。
When treating or preventing a human immunodeficiency virus infection, for example, by the method of treating or preventing a disease of the present invention, the vector of the present invention is a cell that can be infected by HIV, that is, a cell containing a CD4 positive cell ( It is desired to be introduced into a cell group). Therefore, in the treatment or prevention method of the present invention, gene transfer is performed using CD4 positive cells (for example, T cells), progenitor cells that can differentiate into CD4 positive cells (for example, hematopoietic stem cells), or a cell population containing the cells as target cells. Is implemented. In the present invention, it is preferable to target hematopoietic stem cells or a cell population containing the cells from the viewpoint of comprehensively introducing the nucleic acid construct into cells that may be infected with HIV. The cells are not particularly limited as long as they contain CD4 positive cells and their progenitor cells. Blood cells collected from individuals (peripheral blood cells, umbilical cord blood cells), bone marrow cells, and the aforementioned cells can be obtained by known methods. Examples include fractionated CD4-positive cells, progenitor cells of CD4-positive cells, hematopoietic stem cells, and the like.
本発明のベクターを細胞に導入する方法には特に限定はない。例えば、本発明のベクターとしてプラスミドベクターを使用する場合は、リン酸カルシウム法、カチオニック・リピド法、リポソーム法、エレクトロポーレーション法等の遺伝子導入法が使用できる。本発明のベクターとして、レトロウイルスベクター、アデノウイルスベクター、アデノ随伴ウイルスベクター、ヘルペスウイルスベクター等のウイルスベクターを用いる場合には、各ウイルスに適した条件で目的の細胞に感染させればよい。こうして本発明のベクターにより本発明の核酸が導入されてなる細胞も、本発明の一態様である。
The method for introducing the vector of the present invention into a cell is not particularly limited. For example, when a plasmid vector is used as the vector of the present invention, gene transfer methods such as the calcium phosphate method, the cationic lipid method, the liposome method, and the electroporation method can be used. When a viral vector such as a retrovirus vector, an adenovirus vector, an adeno-associated virus vector, or a herpes virus vector is used as the vector of the present invention, the target cell may be infected under conditions suitable for each virus. Thus, a cell into which the nucleic acid of the present invention has been introduced by the vector of the present invention is also an embodiment of the present invention.
本発明の治療又は予防方法の一態様としては、生物個体より採取された細胞に生体外でベクターを導入するエクス・ビボ(ex vivo)遺伝子導入が例示される。ウイルスベクターを使用する場合、生体より採取された前記の標的細胞とウイルスベクター、例えばウイルス産生細胞の培養液上清や前記上清から精製されたウイルスベクターとを混合し、適切な条件でインキュベートすることにより、遺伝子の導入が達成される。こうして遺伝子導入された細胞を生物個体に投与することにより、当該生物個体の免疫不全ウイルス感染症等の治療又は予防を行うことができる。イン・ビボ(in vivo)で遺伝子導入可能なベクター、例えばアデノウイルスベクターやアデノ随伴ウイルスベクターを使用する場合には、本発明の核酸を含有するベクターを個体に直接投与してもよい。
As one aspect of the treatment or prevention method of the present invention, ex vivo gene introduction is exemplified in which a vector is introduced into a cell collected from an individual organism in vitro. When using a viral vector, the target cells collected from the living body and a viral vector, for example, a culture supernatant of a virus-producing cell or a viral vector purified from the supernatant are mixed and incubated under appropriate conditions. Thus, gene transfer is achieved. By administering the gene-introduced cells to an individual organism, it is possible to treat or prevent immunodeficiency virus infection or the like of the individual organism. When using a vector capable of gene transfer in vivo, such as an adenovirus vector or an adeno-associated virus vector, a vector containing the nucleic acid of the present invention may be directly administered to an individual.
レトロウイルスベクターをエクス・ビボ遺伝子導入に使用する場合、レトロウイルス結合活性を有する機能性物質の存在下に標的細胞にレトロウイルスベクターを高効率で感染させることができる。
When a retroviral vector is used for ex vivo gene introduction, a target cell can be infected with a retroviral vector with high efficiency in the presence of a functional substance having a retroviral binding activity.
レトロウイルス結合活性を有する機能性物質を使用する遺伝子導入方法は、例えばWO 95/26200号国際公開パンフレット、WO 97/18318号国際公開パンフレット、Nature Medicine、第2巻、第876-882頁(1996)等に記載されている。当該方法には、レトロウイルス結合部位と標的細胞結合部位の両方を同一分子上に有する機能性物質を使用する方法、レトロウイルス結合部位を有する機能性物質と標的細胞結合部位とを有する機能性物質との混合物を使用する方法とがあり、いずれの方法も本発明に使用することができる。
For example, WO 95/26200 International Publication Pamphlet, WO 97/18318 International Publication Pamphlet, Nature Medicine, Vol. 2, pages 876-882 (1996). ) Etc. In the method, a method using a functional substance having both a retrovirus binding site and a target cell binding site on the same molecule, a functional substance having a retrovirus binding site and a target cell binding site Any of these methods can be used in the present invention.
前記のレトロウイルス結合活性を有する機能性物質としては、細胞接着ドメインとヘパリン結合ドメインの両者を有するフィブロネクチンフラグメントが好適に例示される。当該フィブロネクチンフラグメントは、生体より精製されたフィブロネクチンからプロテアーゼ消化などの手段で調製することができ、また、組換えDNA技術により作製することができる。例えば、レトロネクチン(登録商標)としてタカラバイオ社から発売されている組換えフィブロネクチンフラグメントは、レトロウイルス結合活性を有する機能性物質を使用する遺伝子導入方法に好適に使用できる。
As the functional substance having the retrovirus binding activity, a fibronectin fragment having both a cell adhesion domain and a heparin binding domain is preferably exemplified. The fibronectin fragment can be prepared from fibronectin purified from a living body by means such as protease digestion or can be prepared by recombinant DNA technology. For example, a recombinant fibronectin fragment sold by Takara Bio Inc. as RetroNectin (registered trademark) can be suitably used in a gene introduction method using a functional substance having a retrovirus binding activity.
本発明の治療又は予防方法の上記とは異なる態様としては、本発明の融合タンパク質を直接生物個体に投与する方法が例示される。このとき、投与される本発明の融合タンパク質は、精製されたタンパク質そのものや、アジュバント等により封入されたもの、ウイルス様粒子に封入されたものであっても良い。
As an aspect different from the above of the treatment or prevention method of the present invention, a method of directly administering the fusion protein of the present invention to an individual organism is exemplified. In this case, the fusion protein of the present invention to be administered may be a purified protein itself, a protein encapsulated with an adjuvant, or a virus-like particle.
本発明の治療又は予防方法は、単独で実施してもよいが、3~4種類の抗ウイルス薬(逆転写酵素阻害剤、プロテアーゼ阻害剤等)を併用する多剤併用療法(HAART)と組み合わせて実施しても良い。
The treatment or prevention method of the present invention may be carried out alone or in combination with multi-drug combination therapy (HAART) in which 3 to 4 types of antiviral drugs (reverse transcriptase inhibitor, protease inhibitor, etc.) are used in combination. May be implemented.
上記のとおり、本発明の融合タンパク質、ベクター、ならびに当該ベクターが導入された細胞はRNAウイルス感染症の治療、予防のために使用することができる。すなわち、本発明はRNAウイルス感染症の治療、予防のための医薬組成物を提供する。前記の医薬組成物は、本発明のベクターまたは当該ベクターにより本発明の核酸が導入された細胞を有効成分として含有するものであれば特に限定するものではないが、当該有効成分自体の他、当該有効成分を薬学的に許容される担体と組み合わせて調製される製剤、当該ベクターとエクス・ビボで細胞へ遺伝子導入を行うための試薬を組み合わせたキット等、任意の形態をとることができる。
As described above, the fusion protein of the present invention, the vector, and the cell into which the vector is introduced can be used for the treatment and prevention of RNA virus infection. That is, the present invention provides a pharmaceutical composition for the treatment and prevention of RNA virus infection. The pharmaceutical composition is not particularly limited as long as it contains the vector of the present invention or cells into which the nucleic acid of the present invention has been introduced by the vector as an active ingredient. It can take any form such as a preparation prepared by combining an active ingredient with a pharmaceutically acceptable carrier, a kit combining the vector and a reagent for gene transfer into cells ex vivo.
以下、実施例により本発明を更に具体的に説明するが、本発明はこれら実施例に限定されるものではない。
Hereinafter, the present invention will be described more specifically by way of examples. However, the present invention is not limited to these examples.
実施例1 発現プラスミドの構築
(1)pBApo-Gag-MazFの構築
配列表の配列番号:1に記載の核酸配列からなるDNA断片が哺乳動物用ベクターpBApo-CMV pur DNA(タカラバイオ社製)のBamHIサイトとHindIIIサイトの間に挿入されてなるプラスミドpBApo-Gag-MazFを構築した。pBApo-Gag-MazFは、HIV-1のGagタンパク質と一本鎖RNA配列特異的なエンドリボヌクレアーゼであるMazFとがHIVプロテアーゼの認識配列を含むリンカーペプチドを介して融合されてなる融合タンパク質を、CMVプロモーター/エンハンサーの制御下に発現可能なプラスミドである(図1)。pBApo-Gag-MazFより発現される融合タンパク質のアミノ酸配列を配列表の配列番号:2に示す。なお、配列番号:1は、5´末端側のBamHIサイトと3´末端側のHindIIIサイトを含む塩基配列を、pBApo-Gag-MazFにおける挿入断片の核酸配列として示している。配列番号:1に記載の核酸配列におけるHIV-1のGagをコードする塩基配列は、天然のHIV-1のGag遺伝子の配列をヒトのコドンに最適化したものである。また、MazFをコードする塩基配列は、天然のmazF遺伝子(RefSec Acc. No. NC_000913中、2908778番目~2909113番目の核酸配列の相補鎖配列)の塩基配列に存在するMazFの認識配列であるACAをアミノ酸置換が生じないように他の配列に改変した人工合成配列である(ACA-less mazF遺伝子;配列表の配列番号:5)。 Example 1 Construction of Expression Plasmid (1) Construction of pBApo-Gag-MazF A DNA fragment comprising the nucleic acid sequence described in SEQ ID NO: 1 in the Sequence Listing is a mammalian vector pBApo-CMV pur DNA (manufactured by Takara Bio Inc.). A plasmid pBApo-Gag-MazF inserted between the BamHI site and the HindIII site was constructed. pBApo-Gag-MazF is a fusion protein obtained by fusing HIV-1 Gag protein and single-stranded RNA sequence-specific endoribonuclease MazF via a linker peptide containing a recognition sequence of HIV protease. A plasmid that can be expressed under the control of a promoter / enhancer (FIG. 1). The amino acid sequence of the fusion protein expressed from pBApo-Gag-MazF is shown in SEQ ID NO: 2 in the sequence listing. SEQ ID NO: 1 shows the base sequence containing the BamHI site on the 5 ′ end side and the HindIII site on the 3 ′ end side as the nucleic acid sequence of the inserted fragment in pBApo-Gag-MazF. The nucleotide sequence encoding HIV-1 Gag in the nucleic acid sequence shown in SEQ ID NO: 1 is an optimized sequence of the natural HIV-1 Gag gene for human codons. The base sequence encoding MazF is an ACA that is a recognition sequence of MazF present in the base sequence of the natural mazF gene (the complementary strand sequence of the 2908778th to 2909113th nucleic acid sequences in RefSec Acc. No. NC_000913). This is an artificially synthesized sequence modified to other sequence so that amino acid substitution does not occur (ACA-less mazF gene; SEQ ID NO: 5 in the sequence listing).
(1)pBApo-Gag-MazFの構築
配列表の配列番号:1に記載の核酸配列からなるDNA断片が哺乳動物用ベクターpBApo-CMV pur DNA(タカラバイオ社製)のBamHIサイトとHindIIIサイトの間に挿入されてなるプラスミドpBApo-Gag-MazFを構築した。pBApo-Gag-MazFは、HIV-1のGagタンパク質と一本鎖RNA配列特異的なエンドリボヌクレアーゼであるMazFとがHIVプロテアーゼの認識配列を含むリンカーペプチドを介して融合されてなる融合タンパク質を、CMVプロモーター/エンハンサーの制御下に発現可能なプラスミドである(図1)。pBApo-Gag-MazFより発現される融合タンパク質のアミノ酸配列を配列表の配列番号:2に示す。なお、配列番号:1は、5´末端側のBamHIサイトと3´末端側のHindIIIサイトを含む塩基配列を、pBApo-Gag-MazFにおける挿入断片の核酸配列として示している。配列番号:1に記載の核酸配列におけるHIV-1のGagをコードする塩基配列は、天然のHIV-1のGag遺伝子の配列をヒトのコドンに最適化したものである。また、MazFをコードする塩基配列は、天然のmazF遺伝子(RefSec Acc. No. NC_000913中、2908778番目~2909113番目の核酸配列の相補鎖配列)の塩基配列に存在するMazFの認識配列であるACAをアミノ酸置換が生じないように他の配列に改変した人工合成配列である(ACA-less mazF遺伝子;配列表の配列番号:5)。 Example 1 Construction of Expression Plasmid (1) Construction of pBApo-Gag-MazF A DNA fragment comprising the nucleic acid sequence described in SEQ ID NO: 1 in the Sequence Listing is a mammalian vector pBApo-CMV pur DNA (manufactured by Takara Bio Inc.). A plasmid pBApo-Gag-MazF inserted between the BamHI site and the HindIII site was constructed. pBApo-Gag-MazF is a fusion protein obtained by fusing HIV-1 Gag protein and single-stranded RNA sequence-specific endoribonuclease MazF via a linker peptide containing a recognition sequence of HIV protease. A plasmid that can be expressed under the control of a promoter / enhancer (FIG. 1). The amino acid sequence of the fusion protein expressed from pBApo-Gag-MazF is shown in SEQ ID NO: 2 in the sequence listing. SEQ ID NO: 1 shows the base sequence containing the BamHI site on the 5 ′ end side and the HindIII site on the 3 ′ end side as the nucleic acid sequence of the inserted fragment in pBApo-Gag-MazF. The nucleotide sequence encoding HIV-1 Gag in the nucleic acid sequence shown in SEQ ID NO: 1 is an optimized sequence of the natural HIV-1 Gag gene for human codons. The base sequence encoding MazF is an ACA that is a recognition sequence of MazF present in the base sequence of the natural mazF gene (the complementary strand sequence of the 2908778th to 2909113th nucleic acid sequences in RefSec Acc. No. NC_000913). This is an artificially synthesized sequence modified to other sequence so that amino acid substitution does not occur (ACA-less mazF gene; SEQ ID NO: 5 in the sequence listing).
(2)pBApo-Gag(G2A)-MazFの構築
pBApo-Gag-MazFにおける配列番号:1に記載の核酸配列の5´末端から17番目のグアニンに相当する塩基がシトシンであることを除いてpBApo-Gag-MazFと同じ核酸配列からなるプラスミドpBApo-Gag(G2A)-MazFを構築した。pBApo-Gag(G2A)-MazFは、Gagタンパク質のN末端から2番目のグリシン残基がアラニン残基に変異したGag(G2A)とMazFとの融合タンパク質を、CMVプロモーター/エンハンサーの制御下に発現可能なプラスミドである(図1)。Gagタンパク質のN末端から2番目のグリシン残基は、Gagの細胞膜へのターゲッティングに重要と考えられているミリストイル化修飾を受けるアミノ酸残基である。Gag(G2A)は、このミリストイル化修飾部位を欠損させた変異体である。 (2) Construction of pBApo-Gag (G2A) -MazF pBApo except that the base corresponding to the 17th guanine from the 5 ′ end of the nucleic acid sequence described in SEQ ID NO: 1 in pBApo-Gag-MazF is cytosine. A plasmid pBApo-Gag (G2A) -MazF consisting of the same nucleic acid sequence as -Gag-MazF was constructed. pBApo-Gag (G2A) -MazF expresses a fusion protein of Gag (G2A) and MazF in which the second glycine residue from the N-terminus of the Gag protein is mutated to an alanine residue under the control of the CMV promoter / enhancer A possible plasmid (FIG. 1). The second glycine residue from the N-terminus of the Gag protein is an amino acid residue that undergoes myristoylation modification, which is considered important for targeting Gag to the cell membrane. Gag (G2A) is a mutant lacking this myristoylation modification site.
pBApo-Gag-MazFにおける配列番号:1に記載の核酸配列の5´末端から17番目のグアニンに相当する塩基がシトシンであることを除いてpBApo-Gag-MazFと同じ核酸配列からなるプラスミドpBApo-Gag(G2A)-MazFを構築した。pBApo-Gag(G2A)-MazFは、Gagタンパク質のN末端から2番目のグリシン残基がアラニン残基に変異したGag(G2A)とMazFとの融合タンパク質を、CMVプロモーター/エンハンサーの制御下に発現可能なプラスミドである(図1)。Gagタンパク質のN末端から2番目のグリシン残基は、Gagの細胞膜へのターゲッティングに重要と考えられているミリストイル化修飾を受けるアミノ酸残基である。Gag(G2A)は、このミリストイル化修飾部位を欠損させた変異体である。 (2) Construction of pBApo-Gag (G2A) -MazF pBApo except that the base corresponding to the 17th guanine from the 5 ′ end of the nucleic acid sequence described in SEQ ID NO: 1 in pBApo-Gag-MazF is cytosine. A plasmid pBApo-Gag (G2A) -MazF consisting of the same nucleic acid sequence as -Gag-MazF was constructed. pBApo-Gag (G2A) -MazF expresses a fusion protein of Gag (G2A) and MazF in which the second glycine residue from the N-terminus of the Gag protein is mutated to an alanine residue under the control of the CMV promoter / enhancer A possible plasmid (FIG. 1). The second glycine residue from the N-terminus of the Gag protein is an amino acid residue that undergoes myristoylation modification, which is considered important for targeting Gag to the cell membrane. Gag (G2A) is a mutant lacking this myristoylation modification site.
(3)pBApo-Gag(ΔNC)-MazFの構築
pBApo-Gag-MazFにおける配列番号:1に記載の核酸配列の5´末端から1156番目から1299番目に相当する核酸配列が欠失していることを除いてpBApo-Gag-MazFと同じ核酸配列からなるプラスミドpBApo-Gag(ΔNC)-MazFを構築した。pBApo-Gag(ΔNC)-MazFは、Gagタンパク質のヌクレオカプシドタンパク質(NC)の領域のアミノ酸配列が欠失したGag(ΔNC)とMazFとの融合タンパク質をCMVプロモーター/エンハンサーの制御下に発現可能なプラスミドである(図1)。 (3) Construction of pBApo-Gag (ΔNC) -MazF The nucleic acid sequence corresponding to the 1156th to 1299th positions from the 5 ′ end of the nucleic acid sequence described in SEQ ID NO: 1 in pBApo-Gag-MazF is deleted. A plasmid pBApo-Gag (ΔNC) -MazF having the same nucleic acid sequence as that of pBApo-Gag-MazF was constructed. pBApo-Gag (ΔNC) -MazF is a plasmid capable of expressing a fusion protein of Gag (ΔNC) and MazF, which lacks the amino acid sequence of the nucleocapsid protein (NC) region of the Gag protein, under the control of the CMV promoter / enhancer. (FIG. 1).
pBApo-Gag-MazFにおける配列番号:1に記載の核酸配列の5´末端から1156番目から1299番目に相当する核酸配列が欠失していることを除いてpBApo-Gag-MazFと同じ核酸配列からなるプラスミドpBApo-Gag(ΔNC)-MazFを構築した。pBApo-Gag(ΔNC)-MazFは、Gagタンパク質のヌクレオカプシドタンパク質(NC)の領域のアミノ酸配列が欠失したGag(ΔNC)とMazFとの融合タンパク質をCMVプロモーター/エンハンサーの制御下に発現可能なプラスミドである(図1)。 (3) Construction of pBApo-Gag (ΔNC) -MazF The nucleic acid sequence corresponding to the 1156th to 1299th positions from the 5 ′ end of the nucleic acid sequence described in SEQ ID NO: 1 in pBApo-Gag-MazF is deleted. A plasmid pBApo-Gag (ΔNC) -MazF having the same nucleic acid sequence as that of pBApo-Gag-MazF was constructed. pBApo-Gag (ΔNC) -MazF is a plasmid capable of expressing a fusion protein of Gag (ΔNC) and MazF, which lacks the amino acid sequence of the nucleocapsid protein (NC) region of the Gag protein, under the control of the CMV promoter / enhancer. (FIG. 1).
(4)pBApo-Gag(Δ2-31)-MazFの構築
pBApo-Gag-MazFにおける配列番号:1に記載の核酸配列の5´末端から16番目から105番目に相当する核酸配列が欠失していることを除いてpBApo-Gag-MazFと同じ核酸配列からなるプラスミドpBApo-Gag(Δ2-31)-MazFを構築した。pBApo-Gag(Δ2-31)-MazFは、Gagタンパク質のN末端から2番目のグリシン残基から31番目のロイシン残基までのアミノ酸配列が欠失したGag(Δ2-31)とMazFとの融合タンパク質をCMVプロモーター/エンハンサーの制御下に発現可能なプラスミドである。Gag(Δ2-31)は、Gagの細胞膜へのターゲッティングに重要と考えられているミリストイル化修飾部位及びN末端近傍の塩基性領域を欠損させた変異体である。 (4) Construction of pBApo-Gag (Δ2-31) -MazF The nucleic acid sequence corresponding to the 16th to 105th positions from the 5 ′ end of the nucleic acid sequence described in SEQ ID NO: 1 in pBApo-Gag-MazF was deleted. A plasmid pBApo-Gag (Δ2-31) -MazF having the same nucleic acid sequence as that of pBApo-Gag-MazF was constructed. pBApo-Gag (Δ2-31) -MazF is a fusion of Gaz (Δ2-31) and MazF, which lacks the amino acid sequence from the 2nd glycine residue to the 31st leucine residue from the N-terminus of the Gag protein. A plasmid capable of expressing a protein under the control of a CMV promoter / enhancer. Gag (Δ2-31) is a mutant lacking the myristoylation modification site and the basic region near the N-terminus, which are thought to be important for targeting Gag to the cell membrane.
pBApo-Gag-MazFにおける配列番号:1に記載の核酸配列の5´末端から16番目から105番目に相当する核酸配列が欠失していることを除いてpBApo-Gag-MazFと同じ核酸配列からなるプラスミドpBApo-Gag(Δ2-31)-MazFを構築した。pBApo-Gag(Δ2-31)-MazFは、Gagタンパク質のN末端から2番目のグリシン残基から31番目のロイシン残基までのアミノ酸配列が欠失したGag(Δ2-31)とMazFとの融合タンパク質をCMVプロモーター/エンハンサーの制御下に発現可能なプラスミドである。Gag(Δ2-31)は、Gagの細胞膜へのターゲッティングに重要と考えられているミリストイル化修飾部位及びN末端近傍の塩基性領域を欠損させた変異体である。 (4) Construction of pBApo-Gag (Δ2-31) -MazF The nucleic acid sequence corresponding to the 16th to 105th positions from the 5 ′ end of the nucleic acid sequence described in SEQ ID NO: 1 in pBApo-Gag-MazF was deleted. A plasmid pBApo-Gag (Δ2-31) -MazF having the same nucleic acid sequence as that of pBApo-Gag-MazF was constructed. pBApo-Gag (Δ2-31) -MazF is a fusion of Gaz (Δ2-31) and MazF, which lacks the amino acid sequence from the 2nd glycine residue to the 31st leucine residue from the N-terminus of the Gag protein. A plasmid capable of expressing a protein under the control of a CMV promoter / enhancer. Gag (Δ2-31) is a mutant lacking the myristoylation modification site and the basic region near the N-terminus, which are thought to be important for targeting Gag to the cell membrane.
(5)pBApo-MazFの構築
大腸菌由来のmazF遺伝子(RefSec Acc. No. RefSec Acc. No. NC_000913中、2908778番目~2909113番目の核酸配列の相補鎖配列)中に存在するACAの塩基配列を、天然のMazFのアミノ酸配列を変化させることなしに他の塩基配列に変換した人口合成配列(ACA-less mazF遺伝子;配列表の配列番号:5)を鋳型にして、プライマーmazF(HindIII)_R(配列表の配列番号:3)とプライマーmazF(BamHI)_F(配列表の配列番号:4)とを用いてPCR増幅した。得られたPCR産物を制限酵素BamHIとHindIIIで消化後、同酵素で消化した前記プラスミドpBApo-CMV pur DNAに挿入した。こうして構築されたプラスミドをpBApo-MazFと命名した。pBApo-MazFは、CMVプロモーター/エンハンサーの制御下にMazFタンパク質を発現することができるプラスミドである(図1)。 (5) Construction of pBApo-MazF The base sequence of ACA present in the mazF gene derived from E. coli (complementary strand sequence of the 2908778th to 2909113th nucleic acid sequences in RefSec Acc. No. RefSec Acc. No. NC_000913) Using a synthetic synthetic sequence (ACA-less mazF gene; SEQ ID NO: 5 in the sequence listing) converted to another base sequence without changing the amino acid sequence of natural MazF as a template, primer mazF (HindIII) _R (arrangement) PCR amplification was performed using SEQ ID NO: 3) in the column table and primer mazF (BamHI) _F (SEQ ID NO: 4 in the sequence table). The obtained PCR product was digested with restriction enzymes BamHI and HindIII, and then inserted into the plasmid pBApo-CMV pur DNA digested with the same enzymes. The thus constructed plasmid was designated as pBApo-MazF. pBApo-MazF is a plasmid capable of expressing MazF protein under the control of a CMV promoter / enhancer (FIG. 1).
大腸菌由来のmazF遺伝子(RefSec Acc. No. RefSec Acc. No. NC_000913中、2908778番目~2909113番目の核酸配列の相補鎖配列)中に存在するACAの塩基配列を、天然のMazFのアミノ酸配列を変化させることなしに他の塩基配列に変換した人口合成配列(ACA-less mazF遺伝子;配列表の配列番号:5)を鋳型にして、プライマーmazF(HindIII)_R(配列表の配列番号:3)とプライマーmazF(BamHI)_F(配列表の配列番号:4)とを用いてPCR増幅した。得られたPCR産物を制限酵素BamHIとHindIIIで消化後、同酵素で消化した前記プラスミドpBApo-CMV pur DNAに挿入した。こうして構築されたプラスミドをpBApo-MazFと命名した。pBApo-MazFは、CMVプロモーター/エンハンサーの制御下にMazFタンパク質を発現することができるプラスミドである(図1)。 (5) Construction of pBApo-MazF The base sequence of ACA present in the mazF gene derived from E. coli (complementary strand sequence of the 2908778th to 2909113th nucleic acid sequences in RefSec Acc. No. RefSec Acc. No. NC_000913) Using a synthetic synthetic sequence (ACA-less mazF gene; SEQ ID NO: 5 in the sequence listing) converted to another base sequence without changing the amino acid sequence of natural MazF as a template, primer mazF (HindIII) _R (arrangement) PCR amplification was performed using SEQ ID NO: 3) in the column table and primer mazF (BamHI) _F (SEQ ID NO: 4 in the sequence table). The obtained PCR product was digested with restriction enzymes BamHI and HindIII, and then inserted into the plasmid pBApo-CMV pur DNA digested with the same enzymes. The thus constructed plasmid was designated as pBApo-MazF. pBApo-MazF is a plasmid capable of expressing MazF protein under the control of a CMV promoter / enhancer (FIG. 1).
(6)pBApo-NC-MazFの構築
実施例1(5)で得られたPCR産物を制限酵素BamHIとHindIIIで消化後、同酵素で消化したpUC19プラスミドDNAに挿入した。こうして構築されたプラスミドをpUC―MazFと命名した。次に、Gag遺伝子のNC領域のアミノ酸配列(配列表の配列番号:6)をコードする塩基配列をプライマーNC-Fw(配列表の配列番号:7)とプライマーNC-Rev3(配列表の配列番号:8)とを用いてPCR増幅後、制限酵素BamHIとNcoIで消化し、さらに同酵素で消化したpUC-MazFプラスミドに挿入した。得られたプラスミドを制限酵素BamHIとHindIIIで消化することによってNC-MazF DNA断片を切り出し、同酵素で処理したpBApo-CMV purプラスミドに挿入した。こうして構築されたプラスミドをpBApo-NC-MazFと命名した。前記プラスミドは、CMVプロモーター/エンハンサーの制御下にNCとMazFの融合タンパク質(NC-MazF)を発現することができるプラスミドである(図1)。 (6) Construction of pBApo-NC-MazF The PCR product obtained in Example 1 (5) was digested with restriction enzymes BamHI and HindIII, and then inserted into pUC19 plasmid DNA digested with the same enzymes. The plasmid constructed in this way was named pUC-MazF. Next, the base sequence encoding the amino acid sequence of the NC region of the Gag gene (SEQ ID NO: 6 in the sequence listing) is expressed as primer NC-Fw (SEQ ID NO: 7 in the sequence listing) and primer NC-Rev3 (SEQ ID NO: in the sequence listing). : 8), and then digested with restriction enzymes BamHI and NcoI, and further inserted into the pUC-MazF plasmid digested with the same enzymes. The obtained plasmid was digested with restriction enzymes BamHI and HindIII to excise the NC-MazF DNA fragment and inserted into the pBApo-CMV pur plasmid treated with the enzyme. The thus constructed plasmid was designated as pBApo-NC-MazF. The plasmid is a plasmid capable of expressing a fusion protein of NC and MazF (NC-MazF) under the control of a CMV promoter / enhancer (FIG. 1).
実施例1(5)で得られたPCR産物を制限酵素BamHIとHindIIIで消化後、同酵素で消化したpUC19プラスミドDNAに挿入した。こうして構築されたプラスミドをpUC―MazFと命名した。次に、Gag遺伝子のNC領域のアミノ酸配列(配列表の配列番号:6)をコードする塩基配列をプライマーNC-Fw(配列表の配列番号:7)とプライマーNC-Rev3(配列表の配列番号:8)とを用いてPCR増幅後、制限酵素BamHIとNcoIで消化し、さらに同酵素で消化したpUC-MazFプラスミドに挿入した。得られたプラスミドを制限酵素BamHIとHindIIIで消化することによってNC-MazF DNA断片を切り出し、同酵素で処理したpBApo-CMV purプラスミドに挿入した。こうして構築されたプラスミドをpBApo-NC-MazFと命名した。前記プラスミドは、CMVプロモーター/エンハンサーの制御下にNCとMazFの融合タンパク質(NC-MazF)を発現することができるプラスミドである(図1)。 (6) Construction of pBApo-NC-MazF The PCR product obtained in Example 1 (5) was digested with restriction enzymes BamHI and HindIII, and then inserted into pUC19 plasmid DNA digested with the same enzymes. The plasmid constructed in this way was named pUC-MazF. Next, the base sequence encoding the amino acid sequence of the NC region of the Gag gene (SEQ ID NO: 6 in the sequence listing) is expressed as primer NC-Fw (SEQ ID NO: 7 in the sequence listing) and primer NC-Rev3 (SEQ ID NO: in the sequence listing). : 8), and then digested with restriction enzymes BamHI and NcoI, and further inserted into the pUC-MazF plasmid digested with the same enzymes. The obtained plasmid was digested with restriction enzymes BamHI and HindIII to excise the NC-MazF DNA fragment and inserted into the pBApo-CMV pur plasmid treated with the enzyme. The thus constructed plasmid was designated as pBApo-NC-MazF. The plasmid is a plasmid capable of expressing a fusion protein of NC and MazF (NC-MazF) under the control of a CMV promoter / enhancer (FIG. 1).
実施例2 本発明の融合タンパク質によるウイルスRNAの分解
(1)融合タンパク質発現ベクター導入細胞の調製
コラーゲンコート処理した6cmディッシュに10%ウシ胎仔血清(GIBCO社製)を含有するダルベッコ改変イーグル培地(DMEM;シグマ社製) 4mLを添加し、さらに2.2×106個のヒト293T/17細胞(ATCC CRL-11268)を添加し、24時間培養した。次いで各ディッシュの細胞にレンチウイルスパッケージングミックス(インビトロジェン社製)、pLenti6.3/V5ベクター(インビトロジェン社製)にZsGreen1遺伝子を挿入することにより構築されたプラスミドpLenti-ZsGreen、pBApo-CMV pur DNAにHIV-1由来Tat遺伝子を挿入することにより構築されたpBApo-Tat、及びpBApo-CMV pur DNAを、TransIT-293(ミラス社製)を用いて当該製品の説明書の記載に従って導入した。こうして調製した細胞をコントロール細胞(Mock細胞)とした。なお、Mock細胞は、0.025μgのpBApo-CMV pur DNAを導入に使用したものと、0.4μgを導入に使用したものの2種類を調製した。 Example 2 Degradation of viral RNA with fusion protein of the present invention (1) Preparation of fusion protein expression vector-introduced cell Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (GIBCO) in a collagen-coated 6 cm dish A product of Sigma) 4 mL was added, and further 2.2 × 10 6 human 293T / 17 cells (ATCC CRL-11268) were added and cultured for 24 hours. Next, lentiviral packaging mix (manufactured by Invitrogen) is inserted into each dish cell, and plasmids pLenti-ZsGreen and pBApo-CMV pur DNA constructed by inserting the ZsGreen1 gene into the pLenti6.3 / V5 vector (manufactured by Invitrogen). PBApo-Tat and pBApo-CMV pur DNA constructed by inserting the HIV-1-derived Tat gene were introduced using TransIT-293 (manufactured by Milas) as described in the instructions for the product. The cells thus prepared were used as control cells (Mock cells). Two types of Mock cells were prepared, one using 0.025 μg of pBApo-CMV pur DNA for introduction and one using 0.4 μg for introduction.
(1)融合タンパク質発現ベクター導入細胞の調製
コラーゲンコート処理した6cmディッシュに10%ウシ胎仔血清(GIBCO社製)を含有するダルベッコ改変イーグル培地(DMEM;シグマ社製) 4mLを添加し、さらに2.2×106個のヒト293T/17細胞(ATCC CRL-11268)を添加し、24時間培養した。次いで各ディッシュの細胞にレンチウイルスパッケージングミックス(インビトロジェン社製)、pLenti6.3/V5ベクター(インビトロジェン社製)にZsGreen1遺伝子を挿入することにより構築されたプラスミドpLenti-ZsGreen、pBApo-CMV pur DNAにHIV-1由来Tat遺伝子を挿入することにより構築されたpBApo-Tat、及びpBApo-CMV pur DNAを、TransIT-293(ミラス社製)を用いて当該製品の説明書の記載に従って導入した。こうして調製した細胞をコントロール細胞(Mock細胞)とした。なお、Mock細胞は、0.025μgのpBApo-CMV pur DNAを導入に使用したものと、0.4μgを導入に使用したものの2種類を調製した。 Example 2 Degradation of viral RNA with fusion protein of the present invention (1) Preparation of fusion protein expression vector-introduced cell Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (GIBCO) in a collagen-coated 6 cm dish A product of Sigma) 4 mL was added, and further 2.2 × 10 6 human 293T / 17 cells (ATCC CRL-11268) were added and cultured for 24 hours. Next, lentiviral packaging mix (manufactured by Invitrogen) is inserted into each dish cell, and plasmids pLenti-ZsGreen and pBApo-CMV pur DNA constructed by inserting the ZsGreen1 gene into the pLenti6.3 / V5 vector (manufactured by Invitrogen). PBApo-Tat and pBApo-CMV pur DNA constructed by inserting the HIV-1-derived Tat gene were introduced using TransIT-293 (manufactured by Milas) as described in the instructions for the product. The cells thus prepared were used as control cells (Mock cells). Two types of Mock cells were prepared, one using 0.025 μg of pBApo-CMV pur DNA for introduction and one using 0.4 μg for introduction.
また、pBApo-CMV pur DNAの代わりに実施例1(5)で構築したpBApo-MazF、又は実施例1(1)で構築したpBApo-Gag-MazFを導入する以外は上記のMock細胞の調製と同様の方法で細胞を調製し、それぞれの導入・発現細胞をMazF細胞、及びGag-MazF細胞とした。なお、MazF細胞及びGag-MazF細胞は、0.025μgのpBApo-MazF又はpBApo-Gag-MazFを導入に使用したものと、0.4μgのpBApo-MazF又はpBApo-Gag-MazFを導入に使用したものの2種類をそれぞれ調製した。
In addition, preparation of the above Mock cell except that pBApo-MazF constructed in Example 1 (5) or pBApo-Gag-MazF constructed in Example 1 (1) was introduced instead of pBApo-CMV pur DNA. Cells were prepared by the same method, and the introduced / expressed cells were designated as MazF cells and Gag-MazF cells. MazF cells and Gag-MazF cells used 0.025 μg of pBApo-MazF or pBApo-Gag-MazF for introduction and 0.4 μg of pBApo-MazF or pBApo-Gag-MazF for introduction. Two types of each were prepared.
(2)ZsGreen1の発現確認
実施例2(1)で調製した各細胞を蛍光顕微鏡で観察した(図2)。コントロール細胞(Mock細胞)では、強いZsGreen1タンパク質由来の蛍光シグナルを検出したのに対し、MazF細胞ではコントロール細胞と比較して低い強度の蛍光シグナルを検出した。一方、Gag-MazF細胞では、ZsGreen1由来の蛍光シグナルの強度が劇的に低下した。この傾向は、細胞に導入するMazFおよびGag-MazFを発現するプラスミド量に依存していた。 (2) Confirmation of expression of ZsGreen1 Each cell prepared in Example 2 (1) was observed with a fluorescence microscope (FIG. 2). In control cells (Mock cells), a strong fluorescent signal derived from ZsGreen1 protein was detected, whereas in MazF cells, a fluorescent signal with a lower intensity was detected compared to control cells. On the other hand, in the Gag-MazF cells, the intensity of the fluorescent signal derived from ZsGreen1 decreased dramatically. This tendency was dependent on the amount of plasmid expressing MazF and Gag-MazF introduced into the cells.
実施例2(1)で調製した各細胞を蛍光顕微鏡で観察した(図2)。コントロール細胞(Mock細胞)では、強いZsGreen1タンパク質由来の蛍光シグナルを検出したのに対し、MazF細胞ではコントロール細胞と比較して低い強度の蛍光シグナルを検出した。一方、Gag-MazF細胞では、ZsGreen1由来の蛍光シグナルの強度が劇的に低下した。この傾向は、細胞に導入するMazFおよびGag-MazFを発現するプラスミド量に依存していた。 (2) Confirmation of expression of ZsGreen1 Each cell prepared in Example 2 (1) was observed with a fluorescence microscope (FIG. 2). In control cells (Mock cells), a strong fluorescent signal derived from ZsGreen1 protein was detected, whereas in MazF cells, a fluorescent signal with a lower intensity was detected compared to control cells. On the other hand, in the Gag-MazF cells, the intensity of the fluorescent signal derived from ZsGreen1 decreased dramatically. This tendency was dependent on the amount of plasmid expressing MazF and Gag-MazF introduced into the cells.
(3)細胞内ZsGreen1 RNAのqRT-PCRによる測定
実施例2(1)で調製した各細胞より常法に従って全RNAを抽出し、各細胞におけるZsGreen1をコードするRNA及びβ-actinをコードするRNAの発現量を、リアルタイムRT-PCR(qRT-PCR)により18S rRNA量に対する相対値を算出することにより求めた。18S rRNAのqRT-PCRにはプライマーhuman_18S_Fw(配列表の配列番号:9)及びプライマーhuman_18S_rev(配列表の配列番号:10)を、ZsGreen1をコードするRNAのqRT-PCRにはプライマーZsG_F2(配列表の配列番号:11)及びプライマーZsG_R2(配列表の配列番号:12)を、β-actinをコードするRNAのqRT-PCRにはプライマーbeta_actin_fw(配列表の配列番号:13)及びプライマーbeta_actin_rev(配列表の配列番号:14)をそれぞれ使用した。結果を表1及び図3に示す。 (3) Measurement of intracellular ZsGreen1 RNA by qRT-PCR Extracting total RNA from each cell prepared in Example 2 (1) according to a conventional method, RNA encoding ZsGreen1 and RNA encoding β-actin in each cell Was calculated by calculating a relative value with respect to the 18S rRNA amount by real-time RT-PCR (qRT-PCR). Primer human_18S_Fw (SEQ ID NO: 9 in the sequence listing) and primer human_18S_rev (SEQ ID NO: 10 in the sequence listing) are used for qRT-PCR of 18S rRNA, and primer ZsG_F2 (sequence listing is used for qRT-PCR of RNA encoding ZsGreen1). SEQ ID NO: 11) and primer ZsG_R2 (SEQ ID NO: 12 in the sequence listing), primer beta_actin_fw (SEQ ID NO: 13 in the sequence listing) and primer beta_actin_rev (SEQ ID NO: 13 in the sequence listing) for qRT-PCR of RNA encoding β-actin SEQ ID NO: 14) was used respectively. The results are shown in Table 1 and FIG.
実施例2(1)で調製した各細胞より常法に従って全RNAを抽出し、各細胞におけるZsGreen1をコードするRNA及びβ-actinをコードするRNAの発現量を、リアルタイムRT-PCR(qRT-PCR)により18S rRNA量に対する相対値を算出することにより求めた。18S rRNAのqRT-PCRにはプライマーhuman_18S_Fw(配列表の配列番号:9)及びプライマーhuman_18S_rev(配列表の配列番号:10)を、ZsGreen1をコードするRNAのqRT-PCRにはプライマーZsG_F2(配列表の配列番号:11)及びプライマーZsG_R2(配列表の配列番号:12)を、β-actinをコードするRNAのqRT-PCRにはプライマーbeta_actin_fw(配列表の配列番号:13)及びプライマーbeta_actin_rev(配列表の配列番号:14)をそれぞれ使用した。結果を表1及び図3に示す。 (3) Measurement of intracellular ZsGreen1 RNA by qRT-PCR Extracting total RNA from each cell prepared in Example 2 (1) according to a conventional method, RNA encoding ZsGreen1 and RNA encoding β-actin in each cell Was calculated by calculating a relative value with respect to the 18S rRNA amount by real-time RT-PCR (qRT-PCR). Primer human_18S_Fw (SEQ ID NO: 9 in the sequence listing) and primer human_18S_rev (SEQ ID NO: 10 in the sequence listing) are used for qRT-PCR of 18S rRNA, and primer ZsG_F2 (sequence listing is used for qRT-PCR of RNA encoding ZsGreen1). SEQ ID NO: 11) and primer ZsG_R2 (SEQ ID NO: 12 in the sequence listing), primer beta_actin_fw (SEQ ID NO: 13 in the sequence listing) and primer beta_actin_rev (SEQ ID NO: 13 in the sequence listing) for qRT-PCR of RNA encoding β-actin SEQ ID NO: 14) was used respectively. The results are shown in Table 1 and FIG.
表1及び図3に示すとおり、MazF細胞ではZsGreen1 RNAの発現量が顕著に低下しており、Gag-MazF細胞ではZsGreen1 RNAの発現量がより顕著に低下していた。ハウスキーピング遺伝子であるβ-actin mRNAの発現量は、各細胞間において大きな違いはなかった。このことは、実施例3(1)で観察したGag-MazF発現による細胞内ZsGreen1由来の蛍光シグナル強度の著しい低下は、Gag-MazF融合タンパク質によるZsGreen1 RNAの分解に由来することを示している。
As shown in Table 1 and FIG. 3, the expression level of ZsGreen1 RNA was significantly decreased in MazF cells, and the expression level of ZsGreen1 RNA was significantly decreased in Gag-MazF cells. The expression level of β-actin mRNA, which is a housekeeping gene, did not differ greatly between cells. This indicates that the remarkable decrease in the fluorescence signal intensity derived from intracellular ZsGreen1 due to Gag-MazF expression observed in Example 3 (1) is due to the degradation of ZsGreen1 RNA by the Gag-MazF fusion protein.
本実施例の結果は、Gag-MazF融合タンパク質がRNAウイルスのパッケージングシグナルを含むRNAを効果的に分解することを示しており、Gag-MazF融合タンパク質がRNAウイルス感染細胞内においてウイルスRNAを効果的に分解できることを示している。
The results of this example indicate that the Gag-MazF fusion protein effectively degrades RNA containing the RNA virus packaging signal, and the Gag-MazF fusion protein is effective in treating viral RNA in RNA virus-infected cells. It can be decomposed automatically.
実施例3 本発明の融合タンパク質のRNAウイルス粒子出芽阻害効果及びRNAウイルス粒子出芽阻害効果に必要な領域の同定
(1)融合タンパク質発現ベクター導入細胞の調製
実施例2(1)と同様の方法にて、Mock細胞、MazF細胞、及びGag-MazF細胞を調製した。また、pBApo-CMV pur DNAの代わりに実施例1(2)で構築したpBApo-Gag(G2A)-MazF、実施例1(3)で構築したpBApo-Gag(ΔNC)-MazF、又は実施例1(4)で構築したpBApo-Gag(Δ2-31)-MazFを導入する以外は実施例2(1)のMock細胞の調製と同様の方法で細胞を調製し、それぞれの導入・発現細胞をGag(G2A)-MazF細胞、Gag(ΔNC)-MazF細胞、及びGag(Δ2-31)-MazF細胞とした。なお、細胞へのベクター導入の際には、0.25μgのpBApo-CMV pur DNA、pBApo-MazF、pBApo-Gag-MazF、pBApo-Gag(G2A)-MazF、pBApo-Gag(ΔNC)-MazF、又はpBApo-Gag(Δ2-31)-MazFを使用した。 Example 3 Identification of a region necessary for RNA virus particle budding inhibitory effect and RNA virus particle budding inhibitory effect of the fusion protein of the present invention (1) Preparation of fusion protein expression vector-introduced cells In the same manner as in Example 2 (1) Mock cells, MazF cells, and Gag-MazF cells were prepared. Further, instead of pBApo-CMV pur DNA, pBApo-Gag (G2A) -MazF constructed in Example 1 (2), pBApo-Gag (ΔNC) -MazF constructed in Example 1 (3), or Example 1 Cells were prepared in the same manner as the preparation of Mock cells in Example 2 (1) except that pBApo-Gag (Δ2-31) -MazF constructed in (4) was introduced. (G2A) -MazF cells, Gag (ΔNC) -MazF cells, and Gag (Δ2-31) -MazF cells were used. When introducing the vector into the cells, 0.25 μg of pBApo-CMV pur DNA, pBApo-MazF, pBApo-Gag-MazF, pBApo-Gag (G2A) -MazF, pBApo-Gag (ΔNC) -MazF, Alternatively, pBApo-Gag (Δ2-31) -MazF was used.
(1)融合タンパク質発現ベクター導入細胞の調製
実施例2(1)と同様の方法にて、Mock細胞、MazF細胞、及びGag-MazF細胞を調製した。また、pBApo-CMV pur DNAの代わりに実施例1(2)で構築したpBApo-Gag(G2A)-MazF、実施例1(3)で構築したpBApo-Gag(ΔNC)-MazF、又は実施例1(4)で構築したpBApo-Gag(Δ2-31)-MazFを導入する以外は実施例2(1)のMock細胞の調製と同様の方法で細胞を調製し、それぞれの導入・発現細胞をGag(G2A)-MazF細胞、Gag(ΔNC)-MazF細胞、及びGag(Δ2-31)-MazF細胞とした。なお、細胞へのベクター導入の際には、0.25μgのpBApo-CMV pur DNA、pBApo-MazF、pBApo-Gag-MazF、pBApo-Gag(G2A)-MazF、pBApo-Gag(ΔNC)-MazF、又はpBApo-Gag(Δ2-31)-MazFを使用した。 Example 3 Identification of a region necessary for RNA virus particle budding inhibitory effect and RNA virus particle budding inhibitory effect of the fusion protein of the present invention (1) Preparation of fusion protein expression vector-introduced cells In the same manner as in Example 2 (1) Mock cells, MazF cells, and Gag-MazF cells were prepared. Further, instead of pBApo-CMV pur DNA, pBApo-Gag (G2A) -MazF constructed in Example 1 (2), pBApo-Gag (ΔNC) -MazF constructed in Example 1 (3), or Example 1 Cells were prepared in the same manner as the preparation of Mock cells in Example 2 (1) except that pBApo-Gag (Δ2-31) -MazF constructed in (4) was introduced. (G2A) -MazF cells, Gag (ΔNC) -MazF cells, and Gag (Δ2-31) -MazF cells were used. When introducing the vector into the cells, 0.25 μg of pBApo-CMV pur DNA, pBApo-MazF, pBApo-Gag-MazF, pBApo-Gag (G2A) -MazF, pBApo-Gag (ΔNC) -MazF, Alternatively, pBApo-Gag (Δ2-31) -MazF was used.
(2)濃縮ウイルスの調製
実施例3(1)で調製した各細胞を37℃、5%CO2の条件下で24時間培養後、培地交換を行ってさらに24時間培養した。培養終了後、培養上清を集めて0.45μmのフィルターでろ過し、このろ液を低速遠心(6000xg、16 h、4℃)することにより、ウイルスをペレット化した。続いて、ペレット化したウイルスをPBSバッファーで懸濁し、20倍濃縮ウイルスを得た。 (2) Preparation of concentrated virus Each cell prepared in Example 3 (1) was cultured for 24 hours under conditions of 37 ° C. and 5% CO 2 , and then further cultured for 24 hours after changing the medium. After completion of the culture, the culture supernatant was collected and filtered through a 0.45 μm filter, and the filtrate was subjected to low-speed centrifugation (6000 × g, 16 h, 4 ° C.) to pellet the virus. Subsequently, the pelleted virus was suspended in PBS buffer to obtain a 20-fold concentrated virus.
実施例3(1)で調製した各細胞を37℃、5%CO2の条件下で24時間培養後、培地交換を行ってさらに24時間培養した。培養終了後、培養上清を集めて0.45μmのフィルターでろ過し、このろ液を低速遠心(6000xg、16 h、4℃)することにより、ウイルスをペレット化した。続いて、ペレット化したウイルスをPBSバッファーで懸濁し、20倍濃縮ウイルスを得た。 (2) Preparation of concentrated virus Each cell prepared in Example 3 (1) was cultured for 24 hours under conditions of 37 ° C. and 5% CO 2 , and then further cultured for 24 hours after changing the medium. After completion of the culture, the culture supernatant was collected and filtered through a 0.45 μm filter, and the filtrate was subjected to low-speed centrifugation (6000 × g, 16 h, 4 ° C.) to pellet the virus. Subsequently, the pelleted virus was suspended in PBS buffer to obtain a 20-fold concentrated virus.
(3)濃縮ウイルスサンプルのウェスタンブロッティング解析
実施例3(2)で得た濃縮ウイルスをSDS入りサンプルバッファーと混合、熱処理したものを、レンチウイルス粒子の構成成分であるp24タンパク質に対する抗p24抗体(アブカム社製)を用いたウェスタンブロッティングに供した。結果を図4に示す。図4に示すとおり、MazFを発現する細胞(以下図中MazFと表記)では、コントロール細胞(Mock細胞:以下図中Mockと表記)と比べてウイルス粒子量が低下し、Gag-MazF細胞(図中Gag-MazFと表記)ではウイルス粒子量がさらに著しく低下していた。この結果から、Gag-MazF融合タンパク質には、MazFと比較してもウイルス粒子出芽(産生)の著しい阻害作用があることが分かった。 (3) Western Blotting Analysis of Concentrated Virus Sample Anti-p24 antibody against p24 protein (abcam), which is obtained by mixing the concentrated virus obtained in Example 3 (2) with a sample buffer containing SDS and heat-treating it. The product was subjected to Western blotting using The results are shown in FIG. As shown in FIG. 4, in the cells expressing MazF (hereinafter referred to as “MazF” in the figure), the amount of virus particles is reduced compared to control cells (Mock cells: hereinafter referred to as “Mock” in the figure), and Gag-MazF cells (FIG. 4). In the middle Gag-MazF), the amount of virus particles was further significantly reduced. From these results, it was found that the Gag-MazF fusion protein has a significant inhibitory effect on budding (production) of virus particles even compared with MazF.
実施例3(2)で得た濃縮ウイルスをSDS入りサンプルバッファーと混合、熱処理したものを、レンチウイルス粒子の構成成分であるp24タンパク質に対する抗p24抗体(アブカム社製)を用いたウェスタンブロッティングに供した。結果を図4に示す。図4に示すとおり、MazFを発現する細胞(以下図中MazFと表記)では、コントロール細胞(Mock細胞:以下図中Mockと表記)と比べてウイルス粒子量が低下し、Gag-MazF細胞(図中Gag-MazFと表記)ではウイルス粒子量がさらに著しく低下していた。この結果から、Gag-MazF融合タンパク質には、MazFと比較してもウイルス粒子出芽(産生)の著しい阻害作用があることが分かった。 (3) Western Blotting Analysis of Concentrated Virus Sample Anti-p24 antibody against p24 protein (abcam), which is obtained by mixing the concentrated virus obtained in Example 3 (2) with a sample buffer containing SDS and heat-treating it. The product was subjected to Western blotting using The results are shown in FIG. As shown in FIG. 4, in the cells expressing MazF (hereinafter referred to as “MazF” in the figure), the amount of virus particles is reduced compared to control cells (Mock cells: hereinafter referred to as “Mock” in the figure), and Gag-MazF cells (FIG. 4). In the middle Gag-MazF), the amount of virus particles was further significantly reduced. From these results, it was found that the Gag-MazF fusion protein has a significant inhibitory effect on budding (production) of virus particles even compared with MazF.
また、Gagの細胞膜への局在化に必要なミリストイル化修飾部位を欠損したGag(G2A)-MazF融合タンパク質を発現する細胞[図中Gag(G2A)-MazFと表記]やミリストイル化修飾部位、ミリストイル化シグナル及びN末端近傍の塩基性領域を欠損したGag(Δ2-31)-MazF融合タンパク質を発現する細胞[図中Gag(d_2-31)-MazFと表記]では、Gag-MazF融合タンパク質を発現する細胞と同程度にウイルス粒子の出芽(産生)が著しく阻害されていた。一方、NC領域のアミノ酸配列を欠損したGag(ΔNC)-MazF融合タンパク質を発現する細胞[図中Gag(d_NC)mazFと表記]では、ウイルス粒子の出芽(産生)の著しい阻害は認められなかった。
In addition, a cell expressing a Gag (G2A) -MazF fusion protein lacking a myristoylation modification site necessary for localization of Gag to the cell membrane [denoted as Gag (G2A) -MazF in the figure], myristoylation modification site, In cells expressing a myristoylation signal and a Gag (Δ2-31) -MazF fusion protein lacking the basic region near the N-terminus [denoted as Gag (d_2-31) -MazF in the figure], the Gag-MazF fusion protein Viral budding (production) was significantly inhibited to the same extent as the expressing cells. On the other hand, in cells expressing a Gag (ΔNC) -MazF fusion protein lacking the amino acid sequence of the NC region [denoted as Gag (d_NC) mazF in the figure], no significant inhibition of budding (production) of virus particles was observed. .
本実施例の結果は、Gag-MazF融合タンパク質によるウイルス粒子の出芽(産生)阻害効果が、融合タンパク質中のRNAウイルスのパッケージングシグナルに親和性を示すポリペプチドと一本鎖RNA配列特異的エンドリボヌクレアーゼによりもたらされていることを示す。
The results of this example show that the effect of inhibiting the budding (production) of virus particles by the Gag-MazF fusion protein has a polypeptide and single-stranded RNA sequence-specific end that has an affinity for the packaging signal of RNA virus in the fusion protein. It shows that it is caused by ribonuclease.
実施例4 NC-MazF融合タンパク質によるウイルス粒子出芽阻害効果
実施例2(1)と同様の方法にて、Mock細胞、MazF細胞、及びGag-MazF細胞を調製した。また、pBApo-CMV pur DNAの代わりに実施例1(6)で構築したpBApo-NC-MazFを導入する以外は実施例2(1)のMock細胞の調製と同様の方法でヒト293T/17細胞へのプラスミドの導入を行い、NC-MazF細胞を得た。次に、各細胞より実施例3(2)と同様の方法で濃縮ウイルスを調製し、実施例3(3)と同様の方法にてウェスタンブロッティングにより解析した。結果を図5に示す。図5に示すとおり、NC-MazF細胞(図中NC-MazFと表記)では、Gag-MazF導入細胞(図中Gag-MazFと表記)と同等のウイルス粒子出芽(産生)阻害効果が認められた。以上の結果は、RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドと一本鎖RNA配列特異的エンドリボヌクレアーゼとが融合してなる融合タンパク質が、RNAウイルス粒子の出芽(産生)を顕著に抑制することを示す。 Example 4 Inhibitory effect of virus particle budding by NC-MazF fusion protein Mock cells, MazF cells, and Gag-MazF cells were prepared in the same manner as in Example 2 (1). Further, human 293T / 17 cells were prepared in the same manner as the preparation of Mock cells in Example 2 (1) except that pBApo-NC-MazF constructed in Example 1 (6) was introduced instead of pBApo-CMV pur DNA. Plasmids were introduced into the cells to obtain NC-MazF cells. Next, concentrated virus was prepared from each cell by the same method as in Example 3 (2), and analyzed by Western blotting in the same manner as in Example 3 (3). The results are shown in FIG. As shown in FIG. 5, NC-MazF cells (denoted as NC-MazF in the figure) showed the same virus particle budding (production) inhibitory effect as Gag-MazF-introduced cells (denoted as Gag-MazF in the figure). . The above results indicate that a fusion protein consisting of a single-stranded RNA sequence-specific endoribonuclease fused with a polypeptide that has an affinity for RNA virus packaging signals significantly suppresses RNA virus particle budding (production). Indicates to do.
実施例2(1)と同様の方法にて、Mock細胞、MazF細胞、及びGag-MazF細胞を調製した。また、pBApo-CMV pur DNAの代わりに実施例1(6)で構築したpBApo-NC-MazFを導入する以外は実施例2(1)のMock細胞の調製と同様の方法でヒト293T/17細胞へのプラスミドの導入を行い、NC-MazF細胞を得た。次に、各細胞より実施例3(2)と同様の方法で濃縮ウイルスを調製し、実施例3(3)と同様の方法にてウェスタンブロッティングにより解析した。結果を図5に示す。図5に示すとおり、NC-MazF細胞(図中NC-MazFと表記)では、Gag-MazF導入細胞(図中Gag-MazFと表記)と同等のウイルス粒子出芽(産生)阻害効果が認められた。以上の結果は、RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドと一本鎖RNA配列特異的エンドリボヌクレアーゼとが融合してなる融合タンパク質が、RNAウイルス粒子の出芽(産生)を顕著に抑制することを示す。 Example 4 Inhibitory effect of virus particle budding by NC-MazF fusion protein Mock cells, MazF cells, and Gag-MazF cells were prepared in the same manner as in Example 2 (1). Further, human 293T / 17 cells were prepared in the same manner as the preparation of Mock cells in Example 2 (1) except that pBApo-NC-MazF constructed in Example 1 (6) was introduced instead of pBApo-CMV pur DNA. Plasmids were introduced into the cells to obtain NC-MazF cells. Next, concentrated virus was prepared from each cell by the same method as in Example 3 (2), and analyzed by Western blotting in the same manner as in Example 3 (3). The results are shown in FIG. As shown in FIG. 5, NC-MazF cells (denoted as NC-MazF in the figure) showed the same virus particle budding (production) inhibitory effect as Gag-MazF-introduced cells (denoted as Gag-MazF in the figure). . The above results indicate that a fusion protein consisting of a single-stranded RNA sequence-specific endoribonuclease fused with a polypeptide that has an affinity for RNA virus packaging signals significantly suppresses RNA virus particle budding (production). Indicates to do.
実施例5 本発明の融合タンパク質のRNAウイルス粒子不活化効果
(1)融合タンパク質発現ベクター導入細胞の調製
細胞培養用6cmディッシュに10%ウシ胎仔血清(GIBCO社製)を含有するダルベッコ改変イーグル培地(DMEM;シグマ社製) 4mLを添加し、さらに1.8×106個のヒトLenti-X 293T/17細胞(クロンテック社製)を添加し、24時間培養した。次いで各ディッシュの細胞にレンチウイルスパッケージングミックス(クロンテック社)、pLVX-AcGFP1-C1ベクター(クロンテック社)、pBApo-CMV pur DNAにHIV-1由来Tat遺伝子を挿入することにより構築されたpBApo-Tat、及びpBApo-CMV pur DNAを、TransIT-293(ミラス社製)を用いて当該製品の説明書の記載に従って導入した。こうして調製した細胞をコントロール細胞(Mock細胞)とした。なお、Mock細胞の調製の際には、0.025μgのpBApo-CMV pur DNAを導入した。 Example 5 RNA virus particle inactivation effect of the fusion protein of the present invention (1) Preparation of fusion protein expression vector-introduced cells Dulbecco's modified Eagle medium containing 10% fetal calf serum (GIBCO) in a 6 cm dish for cell culture ( 4 mL of DMEM (manufactured by Sigma) was added, and 1.8 × 10 6 human Lenti-X 293T / 17 cells (manufactured by Clontech) were further added, and cultured for 24 hours. Next, lBA virus packaging mix (Clontech), pLVX-AcGFP1-C1 vector (Clontech), and pBApo-Tat constructed by inserting HIV-1-derived Tat gene into pBApo-CMV pur DNA into each dish cell. , And pBApo-CMV pur DNA was introduced using TransIT-293 (manufactured by Milas) as described in the instructions for the product. The cells thus prepared were used as control cells (Mock cells). In the preparation of Mock cells, 0.025 μg of pBApo-CMV pur DNA was introduced.
(1)融合タンパク質発現ベクター導入細胞の調製
細胞培養用6cmディッシュに10%ウシ胎仔血清(GIBCO社製)を含有するダルベッコ改変イーグル培地(DMEM;シグマ社製) 4mLを添加し、さらに1.8×106個のヒトLenti-X 293T/17細胞(クロンテック社製)を添加し、24時間培養した。次いで各ディッシュの細胞にレンチウイルスパッケージングミックス(クロンテック社)、pLVX-AcGFP1-C1ベクター(クロンテック社)、pBApo-CMV pur DNAにHIV-1由来Tat遺伝子を挿入することにより構築されたpBApo-Tat、及びpBApo-CMV pur DNAを、TransIT-293(ミラス社製)を用いて当該製品の説明書の記載に従って導入した。こうして調製した細胞をコントロール細胞(Mock細胞)とした。なお、Mock細胞の調製の際には、0.025μgのpBApo-CMV pur DNAを導入した。 Example 5 RNA virus particle inactivation effect of the fusion protein of the present invention (1) Preparation of fusion protein expression vector-introduced cells Dulbecco's modified Eagle medium containing 10% fetal calf serum (GIBCO) in a 6 cm dish for cell culture ( 4 mL of DMEM (manufactured by Sigma) was added, and 1.8 × 10 6 human Lenti-X 293T / 17 cells (manufactured by Clontech) were further added, and cultured for 24 hours. Next, lBA virus packaging mix (Clontech), pLVX-AcGFP1-C1 vector (Clontech), and pBApo-Tat constructed by inserting HIV-1-derived Tat gene into pBApo-CMV pur DNA into each dish cell. , And pBApo-CMV pur DNA was introduced using TransIT-293 (manufactured by Milas) as described in the instructions for the product. The cells thus prepared were used as control cells (Mock cells). In the preparation of Mock cells, 0.025 μg of pBApo-CMV pur DNA was introduced.
また、pBApo-CMV pur DNAの代わりに実施例1(5)で構築したpBApo-MazF、又は実施例1(1)で構築したpBApo-Gag-MazFを導入する以外は上記のMock細胞の調製と同様の方法で細胞を調製し、それぞれの導入・発現細胞をMazF細胞、及びGag-MazF細胞とした。
In addition, preparation of the above Mock cell except that pBApo-MazF constructed in Example 1 (5) or pBApo-Gag-MazF constructed in Example 1 (1) was introduced instead of pBApo-CMV pur DNA. Cells were prepared by the same method, and the introduced / expressed cells were designated as MazF cells and Gag-MazF cells.
(2)濃縮ウイルスの調製
実施例5(1)で調製した各細胞を37℃、5%CO2の条件下で24時間培養後、培地交換を行ってさらに24時間培養した。培養終了後、培養上清を集めて0.45μmのフィルターでろ過し、このろ液を低速遠心(6000xg、16 h、4℃)することにより、ウイルスをペレット化した。続いて、ペレット化したウイルスをPBSバッファーで懸濁し、10倍濃縮ウイルスを得た。 (2) Preparation of concentrated virus Each cell prepared in Example 5 (1) was cultured under the conditions of 37 ° C and 5% CO 2 for 24 hours, and then the medium was changed and further cultured for 24 hours. After completion of the culture, the culture supernatant was collected and filtered through a 0.45 μm filter, and the filtrate was subjected to low-speed centrifugation (6000 × g, 16 h, 4 ° C.) to pellet the virus. Subsequently, the pelleted virus was suspended in PBS buffer to obtain a 10-fold concentrated virus.
実施例5(1)で調製した各細胞を37℃、5%CO2の条件下で24時間培養後、培地交換を行ってさらに24時間培養した。培養終了後、培養上清を集めて0.45μmのフィルターでろ過し、このろ液を低速遠心(6000xg、16 h、4℃)することにより、ウイルスをペレット化した。続いて、ペレット化したウイルスをPBSバッファーで懸濁し、10倍濃縮ウイルスを得た。 (2) Preparation of concentrated virus Each cell prepared in Example 5 (1) was cultured under the conditions of 37 ° C and 5% CO 2 for 24 hours, and then the medium was changed and further cultured for 24 hours. After completion of the culture, the culture supernatant was collected and filtered through a 0.45 μm filter, and the filtrate was subjected to low-speed centrifugation (6000 × g, 16 h, 4 ° C.) to pellet the virus. Subsequently, the pelleted virus was suspended in PBS buffer to obtain a 10-fold concentrated virus.
(3)濃縮ウイルスのウェスタンブロッティング解析
実施例5(2)で得た濃縮ウイルスのうち、MazF細胞より得られた濃縮ウイルス、及びGag-MazF細胞より得られた濃縮ウイルスをそれぞれSDS入りサンプルバッファーと混合・熱処理し、抗MazF抗体を用いたウェスタンブロッティングに供した。結果を図6に示す。図6に示すとおり、MazF細胞より産生したウイルス(図中MazFと表記)からは、MazFタンパク質を検出することはできなかった。一方、Gag-MazF細胞より産生したウイルス(図中Gag-MazFと表記)からは、遊離の状態のMazFタンパク質と少量のGag-MazFタンパク質を検出した。この結果から、Gag-MazFタンパク質を発現するGag-MazF細胞では、ウイルス粒子内にGag-MazFが効果的に封入されたと考えられる。さらに、ウイルス粒子内では、活性化したHIVプロテアーゼによりGag-MazFがGagタンパク質とMazFタンパク質とに分断され、遊離型MazFタンパク質が蓄積したと考えられる。ウイルス粒子内で生成したこの遊離型MazFタンパク質は、活性化したHIVプロテアーゼによる分解が認められないことから、粒子内においてリボヌクレアーゼ活性を維持していると考えるのが妥当である。 (3) Western blotting analysis of concentrated virus Among the concentrated viruses obtained in Example 5 (2), the concentrated virus obtained from MazF cells and the concentrated virus obtained from Gag-MazF cells were respectively used as a sample buffer containing SDS. The mixture was heat-treated and subjected to Western blotting using an anti-MazF antibody. The results are shown in FIG. As shown in FIG. 6, MazF protein could not be detected from a virus produced from MazF cells (indicated as MazF in the figure). On the other hand, free virus and a small amount of Gag-MazF protein were detected from virus produced from Gag-MazF cells (denoted as Gag-MazF in the figure). From this result, it is considered that Gag-MazF was effectively encapsulated in virus particles in Gag-MazF cells expressing Gag-MazF protein. Furthermore, in the virus particle, it is considered that Gag-MazF was divided into Gag protein and MazF protein by activated HIV protease, and free MazF protein was accumulated. Since this free MazF protein produced in the virus particle is not degraded by activated HIV protease, it is reasonable to assume that the ribonuclease activity is maintained in the particle.
実施例5(2)で得た濃縮ウイルスのうち、MazF細胞より得られた濃縮ウイルス、及びGag-MazF細胞より得られた濃縮ウイルスをそれぞれSDS入りサンプルバッファーと混合・熱処理し、抗MazF抗体を用いたウェスタンブロッティングに供した。結果を図6に示す。図6に示すとおり、MazF細胞より産生したウイルス(図中MazFと表記)からは、MazFタンパク質を検出することはできなかった。一方、Gag-MazF細胞より産生したウイルス(図中Gag-MazFと表記)からは、遊離の状態のMazFタンパク質と少量のGag-MazFタンパク質を検出した。この結果から、Gag-MazFタンパク質を発現するGag-MazF細胞では、ウイルス粒子内にGag-MazFが効果的に封入されたと考えられる。さらに、ウイルス粒子内では、活性化したHIVプロテアーゼによりGag-MazFがGagタンパク質とMazFタンパク質とに分断され、遊離型MazFタンパク質が蓄積したと考えられる。ウイルス粒子内で生成したこの遊離型MazFタンパク質は、活性化したHIVプロテアーゼによる分解が認められないことから、粒子内においてリボヌクレアーゼ活性を維持していると考えるのが妥当である。 (3) Western blotting analysis of concentrated virus Among the concentrated viruses obtained in Example 5 (2), the concentrated virus obtained from MazF cells and the concentrated virus obtained from Gag-MazF cells were respectively used as a sample buffer containing SDS. The mixture was heat-treated and subjected to Western blotting using an anti-MazF antibody. The results are shown in FIG. As shown in FIG. 6, MazF protein could not be detected from a virus produced from MazF cells (indicated as MazF in the figure). On the other hand, free virus and a small amount of Gag-MazF protein were detected from virus produced from Gag-MazF cells (denoted as Gag-MazF in the figure). From this result, it is considered that Gag-MazF was effectively encapsulated in virus particles in Gag-MazF cells expressing Gag-MazF protein. Furthermore, in the virus particle, it is considered that Gag-MazF was divided into Gag protein and MazF protein by activated HIV protease, and free MazF protein was accumulated. Since this free MazF protein produced in the virus particle is not degraded by activated HIV protease, it is reasonable to assume that the ribonuclease activity is maintained in the particle.
(4)濃縮ウイルスサンプルのELISA解析
実施例5(2)で得た各濃縮ウイルスのウイルス粒子量を、HIV-1粒子の構造タンパク質p24を検出するp24 アンチジェンELISAキット(Zeptomerix社製)により定量した。 (4) ELISA analysis of concentrated virus sample The amount of virus particles of each concentrated virus obtained in Example 5 (2) was quantified with a p24 antigen ELISA kit (manufactured by Zeptomerix) that detects the structural protein p24 of HIV-1 particles. did.
実施例5(2)で得た各濃縮ウイルスのウイルス粒子量を、HIV-1粒子の構造タンパク質p24を検出するp24 アンチジェンELISAキット(Zeptomerix社製)により定量した。 (4) ELISA analysis of concentrated virus sample The amount of virus particles of each concentrated virus obtained in Example 5 (2) was quantified with a p24 antigen ELISA kit (manufactured by Zeptomerix) that detects the structural protein p24 of HIV-1 particles. did.
(5)ウイルスの感染性
細胞培養用6ウェルプレートに10%ウシ胎仔血清(GIBCO社製)を含有するダルベッコ改変イーグル培地(DMEM;シグマ社製) 2mLを添加し、さらに5×104個のヒトHT1080細胞(ATCC No.CCL-121)を添加し、24時間培養した。次いで終濃度8μg/mLのポリブレン添加培地0.9mLを用いて培地交換した。実施例5(2)で調製した濃縮ウイルス粒子を各ウェルに添加した。この際、ウイルス粒子の添加には、実施例5(4)で得た定量値に基づき、p24タンパク質量で100ng、70ng、もしくは35ngに相当するウイルス粒子量を使用した。7時間の培養後、各ウェルに1mLの培地を添加し、さらに24時間培養した。2mLの培地で培地交換後、さらに2日間培養した。培養後の細胞をPBSバッファーで洗浄した後、トリプシン処理により細胞を回収した。この細胞をフローサイトメーター解析に供した。 (5) Infectivity ofvirus 2 mL of Dulbecco's modified Eagle medium (DMEM; Sigma) containing 10% fetal calf serum (GIBCO) was added to a 6-well plate for cell culture, and 5 × 10 4 Human HT1080 cells (ATCC No. CCL-121) were added and cultured for 24 hours. Subsequently, the medium was changed using 0.9 mL of polybrene-added medium having a final concentration of 8 μg / mL. The concentrated virus particles prepared in Example 5 (2) were added to each well. At this time, the amount of virus particles corresponding to 100 ng, 70 ng, or 35 ng in terms of the amount of p24 protein was used for the addition of virus particles based on the quantitative value obtained in Example 5 (4). After culturing for 7 hours, 1 mL of medium was added to each well and further cultured for 24 hours. After changing the medium with 2 mL of medium, the cells were further cultured for 2 days. The cultured cells were washed with PBS buffer, and then collected by trypsin treatment. The cells were subjected to flow cytometer analysis.
細胞培養用6ウェルプレートに10%ウシ胎仔血清(GIBCO社製)を含有するダルベッコ改変イーグル培地(DMEM;シグマ社製) 2mLを添加し、さらに5×104個のヒトHT1080細胞(ATCC No.CCL-121)を添加し、24時間培養した。次いで終濃度8μg/mLのポリブレン添加培地0.9mLを用いて培地交換した。実施例5(2)で調製した濃縮ウイルス粒子を各ウェルに添加した。この際、ウイルス粒子の添加には、実施例5(4)で得た定量値に基づき、p24タンパク質量で100ng、70ng、もしくは35ngに相当するウイルス粒子量を使用した。7時間の培養後、各ウェルに1mLの培地を添加し、さらに24時間培養した。2mLの培地で培地交換後、さらに2日間培養した。培養後の細胞をPBSバッファーで洗浄した後、トリプシン処理により細胞を回収した。この細胞をフローサイトメーター解析に供した。 (5) Infectivity of
フローサイトメーター解析の結果から算出したAcGFP1陽性率(全細胞中の、ウイルスにより遺伝子が導入されて細胞内でAcGFP1が発現している細胞の割合)と、濃縮ウイルスの由来やウイルス粒子添加量との関係を図7に示す。図7より、MazF細胞から調製したウイルス粒子を感染させた細胞のAcGFP1陽性率がMock細胞から調製したウイルス粒子を感染させた細胞と同程度であるのに対し、Gag-MazF細胞から調製したウイルス粒子を感染させた細胞では、AcGFP1陽性率が著しく低いことが分かった。
AcGFP1 positive rate calculated from the results of flow cytometer analysis (the ratio of cells in which the gene is introduced by the virus and expressing AcGFP1 in the cells), the origin of the concentrated virus, and the amount of virus particles added The relationship is shown in FIG. FIG. 7 shows that the virus-prepared virus from the Gag-MazF cells, whereas the virus-prepared virus particles prepared from the MazF cells have the same AcGFP1-positive rate as the cells infected with the virus particles prepared from the Mock cells. It was found that the AcGFP1-positive rate was remarkably low in the cells infected with the particles.
以上の結果は、RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドと一本鎖RNA配列特異的エンドリボヌクレアーゼとが融合してなる融合タンパク質が、RNAウイルスが感染した細胞においてウイルス粒子の出芽(産生)を著しく抑制するだけではなく、出芽(産生)したウイルス粒子内においてもRNAウイルスのウイルスゲノムを分解することにより、ウイルス粒子の感染性を顕著に低下(ウイルス粒子を顕著に不活化)させることを示す。
The above results indicate that a fusion protein comprising a polypeptide having an affinity for RNA virus packaging signal and a single-stranded RNA sequence-specific endoribonuclease is fused with viral particles in cells infected with RNA virus ( Not only significantly suppresses production), but also significantly degrades the infectivity of the virus particles (remarkably inactivates the virus particles) by degrading the viral genome of the RNA virus within the budding (produced) virus particles. It shows that.
実施例6 Gag-MazF及びNC-MazF発現細胞のウイルス感染阻害効果
(1)融合タンパク質発現ベクター導入細胞の調製
細胞培養用6ウェルプレートに10%ウシ胎仔血清(GIBCO社製)を含有するダルベッコ改変イーグル培地(DMEM;シグマ社製)2mLを添加し、さらに6×105個のヒトLenti-X 293T/17細胞(クロンテック社製)を添加し、24時間培養した。次いで各ウェルの細胞に、2μgの実施例1(5)で構築したpBApo-MazF、実施例1(1)で構築したpBApo-Gag-MazF、又は実施例1(6)で構築したpBApo-NC-MazFを、TransIT-293(ミラス社製)を用いて当該製品の説明書の記載に従って導入した。5.5時間培養後、培地交換し18時間培養した。こうして調製した細胞を、それぞれ、MazF細胞、Gag-MazF細胞、及びNC-MazF細胞とした。 Example 6 Virus infection inhibitory effect of cells expressing Gag-MazF and NC-MazF (1) Preparation of fusion protein expression vector-introduced cells Dulbecco modification containing 10% fetal calf serum (GIBCO) in 6-well plate forcell culture 2 mL of Eagle's medium (DMEM; manufactured by Sigma) was added, and 6 × 10 5 human Lenti-X 293T / 17 cells (manufactured by Clontech) were added and cultured for 24 hours. Next, 2 μg of pBApo-MazF constructed in Example 1 (5), pBApo-Gag-MazF constructed in Example 1 (1), or pBApo-NC constructed in Example 1 (6) was added to each well cell. -MazF was introduced using TransIT-293 (Mirras) as described in the product instructions. After culturing for 5.5 hours, the medium was changed and cultured for 18 hours. The cells thus prepared were designated as MazF cells, Gag-MazF cells, and NC-MazF cells, respectively.
(1)融合タンパク質発現ベクター導入細胞の調製
細胞培養用6ウェルプレートに10%ウシ胎仔血清(GIBCO社製)を含有するダルベッコ改変イーグル培地(DMEM;シグマ社製)2mLを添加し、さらに6×105個のヒトLenti-X 293T/17細胞(クロンテック社製)を添加し、24時間培養した。次いで各ウェルの細胞に、2μgの実施例1(5)で構築したpBApo-MazF、実施例1(1)で構築したpBApo-Gag-MazF、又は実施例1(6)で構築したpBApo-NC-MazFを、TransIT-293(ミラス社製)を用いて当該製品の説明書の記載に従って導入した。5.5時間培養後、培地交換し18時間培養した。こうして調製した細胞を、それぞれ、MazF細胞、Gag-MazF細胞、及びNC-MazF細胞とした。 Example 6 Virus infection inhibitory effect of cells expressing Gag-MazF and NC-MazF (1) Preparation of fusion protein expression vector-introduced cells Dulbecco modification containing 10% fetal calf serum (GIBCO) in 6-well plate for
(2)レンチウイルスベクターの調製
コラーゲンコート処理した6cmディッシュに10%ウシ胎仔血清(GIBCO社製)を含有するダルベッコ改変イーグル培地(DMEM;シグマ社製) 4mLを添加し、さらに 2.5×106個のヒト293T/17細胞(ATCC CRL-11268)を添加し、24時間培養した。次いで各ディッシュの細胞にレンチウイルスパッケージングミックス(クロンテック社)、pLVX-AcGFP1-C1ベクター(クロンテック社)、pBApo-CMV pur DNAにHIV-1由来Tat遺伝子を挿入することにより構築されたpBApo-Tatを、TransIT-293(ミラス社製)を用いて当該製品の説明書の記載に従って導入した。各細胞を37℃、5%CO2の条件下で24時間培養後、培地交換を行ってさらに24時間培養した。培養終了後、培養上清を集めて0.45μmのフィルターでろ過した。得られたレンチウイルスベクター(AcGFP1)を、実施例6(3)のウイルス感染実験に使用した。 (2) Preparation oflentiviral vector 4 mL of Dulbecco's modified Eagle medium (DMEM; manufactured by Sigma) containing 10% fetal calf serum (GIBCO) was added to a collagen-coated 6 cm dish, and further 2.5 × 10 Six human 293T / 17 cells (ATCC CRL-11268) were added and cultured for 24 hours. Next, lBA virus packaging mix (Clontech), pLVX-AcGFP1-C1 vector (Clontech), and pBApo-Tat constructed by inserting HIV-1-derived Tat gene into pBApo-CMV pur DNA into each dish cell. Was introduced using TransIT-293 (manufactured by Milas) as described in the instructions for the product. Each cell was cultured under conditions of 37 ° C. and 5% CO 2 for 24 hours, and then the medium was changed and further cultured for 24 hours. After completion of the culture, the culture supernatant was collected and filtered through a 0.45 μm filter. The obtained lentiviral vector (AcGFP1) was used in the virus infection experiment of Example 6 (3).
コラーゲンコート処理した6cmディッシュに10%ウシ胎仔血清(GIBCO社製)を含有するダルベッコ改変イーグル培地(DMEM;シグマ社製) 4mLを添加し、さらに 2.5×106個のヒト293T/17細胞(ATCC CRL-11268)を添加し、24時間培養した。次いで各ディッシュの細胞にレンチウイルスパッケージングミックス(クロンテック社)、pLVX-AcGFP1-C1ベクター(クロンテック社)、pBApo-CMV pur DNAにHIV-1由来Tat遺伝子を挿入することにより構築されたpBApo-Tatを、TransIT-293(ミラス社製)を用いて当該製品の説明書の記載に従って導入した。各細胞を37℃、5%CO2の条件下で24時間培養後、培地交換を行ってさらに24時間培養した。培養終了後、培養上清を集めて0.45μmのフィルターでろ過した。得られたレンチウイルスベクター(AcGFP1)を、実施例6(3)のウイルス感染実験に使用した。 (2) Preparation of
(3)融合タンパク質を発現する細胞を用いたレンチウイルス感染実験
実施例6(2)で調製したレンチウイルスベクターを、終濃度8μg/mLになるようにポリブレンを添加した培地で希釈した。実施例6(1)で調製した各細胞の懸濁液より培地を除去後、この希釈ウイルス1mLを添加した。24時間後に培地交換し、さらに24時間培養した。各ウェルの細胞をトリプシン処理することにより回収し、PBSで細胞ペレットを洗浄した。 (3) Lentiviral infection experiment using cells expressing the fusion protein The lentiviral vector prepared in Example 6 (2) was diluted with a medium to which polybrene was added to a final concentration of 8 μg / mL. After removing the medium from the suspension of each cell prepared in Example 6 (1), 1 mL of this diluted virus was added. After 24 hours, the medium was changed and further cultured for 24 hours. Cells in each well were collected by trypsinization, and the cell pellet was washed with PBS.
実施例6(2)で調製したレンチウイルスベクターを、終濃度8μg/mLになるようにポリブレンを添加した培地で希釈した。実施例6(1)で調製した各細胞の懸濁液より培地を除去後、この希釈ウイルス1mLを添加した。24時間後に培地交換し、さらに24時間培養した。各ウェルの細胞をトリプシン処理することにより回収し、PBSで細胞ペレットを洗浄した。 (3) Lentiviral infection experiment using cells expressing the fusion protein The lentiviral vector prepared in Example 6 (2) was diluted with a medium to which polybrene was added to a final concentration of 8 μg / mL. After removing the medium from the suspension of each cell prepared in Example 6 (1), 1 mL of this diluted virus was added. After 24 hours, the medium was changed and further cultured for 24 hours. Cells in each well were collected by trypsinization, and the cell pellet was washed with PBS.
(4)宿主染色体に挿入されたレンチベクターのプロウイルスコピー数の測定
実施例6(3)で調製した各細胞ペレットより常法に従って全DNAを抽出した。この全DNAを鋳型として、CycleavePCR(登録商標) Core Kit(タカラバイオ社製)を用いたリアルタイムPCR(qPCR)により、宿主細胞当たりのレンチウイルスベクター由来のプロウイルスDNAのコピー数を算出した。宿主細胞量は、Provirus Copy Number Detection Primer Set, Human (for Real Time PCR)(タカラバイオ社製)のhIFNγ Primer Mix for ProvirusとhIFNγ Probe for Provirusをプライマー対、プローブとして用いたqPCRにより求めた。また、プロウイルスDNA量は、プライマーLenti-copy1-F(配列表の配列番号:15)、プライマーLenti-copy1-R(配列表の配列番号:16)、及びプローブP1S(配列表の配列番号:17)を用いたqPCRにより求めた。宿主細胞当たりのプロウイルスDNAのコピー数は、上記のqPCRにより求めた宿主細胞量とプロウイルスDNA量より算出した。結果を図8に示す。 (4) Measurement of proviral copy number of lentivector inserted into host chromosome Total DNA was extracted from each cell pellet prepared in Example 6 (3) according to a conventional method. Using this total DNA as a template, the copy number of proviral DNA derived from a lentiviral vector per host cell was calculated by real-time PCR (qPCR) using Cycle PCR (registered trademark) Core Kit (manufactured by Takara Bio Inc.). The amount of host cells was determined by using hIFNγ Primer Mix for Probe and hIFNγ Probe for Probe as a probe using hIFNγ Primer Mix for Probe from Probe Copy Number Detection Primer Set, Human (for Real Time PCR) (manufactured by Takara Bio Inc.). In addition, the amount of proviral DNA was determined by the primer Lenti-copy1-F (SEQ ID NO: 15 in the sequence listing), the primer Lenti-copy1-R (SEQ ID NO: 16 in the sequence listing), and the probe P1S (SEQ ID NO: 16 in the sequence listing). It was determined by qPCR using 17). The number of copies of proviral DNA per host cell was calculated from the amount of host cells and the amount of proviral DNA determined by the above qPCR. The results are shown in FIG.
実施例6(3)で調製した各細胞ペレットより常法に従って全DNAを抽出した。この全DNAを鋳型として、CycleavePCR(登録商標) Core Kit(タカラバイオ社製)を用いたリアルタイムPCR(qPCR)により、宿主細胞当たりのレンチウイルスベクター由来のプロウイルスDNAのコピー数を算出した。宿主細胞量は、Provirus Copy Number Detection Primer Set, Human (for Real Time PCR)(タカラバイオ社製)のhIFNγ Primer Mix for ProvirusとhIFNγ Probe for Provirusをプライマー対、プローブとして用いたqPCRにより求めた。また、プロウイルスDNA量は、プライマーLenti-copy1-F(配列表の配列番号:15)、プライマーLenti-copy1-R(配列表の配列番号:16)、及びプローブP1S(配列表の配列番号:17)を用いたqPCRにより求めた。宿主細胞当たりのプロウイルスDNAのコピー数は、上記のqPCRにより求めた宿主細胞量とプロウイルスDNA量より算出した。結果を図8に示す。 (4) Measurement of proviral copy number of lentivector inserted into host chromosome Total DNA was extracted from each cell pellet prepared in Example 6 (3) according to a conventional method. Using this total DNA as a template, the copy number of proviral DNA derived from a lentiviral vector per host cell was calculated by real-time PCR (qPCR) using Cycle PCR (registered trademark) Core Kit (manufactured by Takara Bio Inc.). The amount of host cells was determined by using hIFNγ Primer Mix for Probe and hIFNγ Probe for Probe as a probe using hIFNγ Primer Mix for Probe from Probe Copy Number Detection Primer Set, Human (for Real Time PCR) (manufactured by Takara Bio Inc.). In addition, the amount of proviral DNA was determined by the primer Lenti-copy1-F (SEQ ID NO: 15 in the sequence listing), the primer Lenti-copy1-R (SEQ ID NO: 16 in the sequence listing), and the probe P1S (SEQ ID NO: 16 in the sequence listing). It was determined by qPCR using 17). The number of copies of proviral DNA per host cell was calculated from the amount of host cells and the amount of proviral DNA determined by the above qPCR. The results are shown in FIG.
図8より、Gag-MazF細胞及びNC-MazF細胞では、細胞あたりのプロウイルスDNAのコピー数がMazF細胞のそれと比較して著しく低いことが分かる。この結果から、本発明の融合タンパク質を細胞内で発現させることにより、RNAウイルスの出芽を抑えるだけでなく、RNAウイルスが細胞に感染した際のプロウイルス形成も効果的に抑制可能であることが示された。プロウイルス形成の抑制は、レンチウイルスRNAと親和性のある本発明の融合タンパク質により、細胞に感染したRNAウイルスのウイルスゲノムRNAが逆転写反応前に切断されるためであると推察される。
FIG. 8 shows that the copy number of proviral DNA per cell in Gag-MazF cells and NC-MazF cells is significantly lower than that in MazF cells. From this result, it is found that expression of the fusion protein of the present invention in cells not only suppresses budding of RNA viruses, but can also effectively suppress provirus formation when RNA viruses infect cells. Indicated. The suppression of provirus formation is presumed to be due to the fact that the viral genome RNA of the RNA virus infecting the cells is cleaved before the reverse transcription reaction by the fusion protein of the present invention having an affinity for lentiviral RNA.
本発明は、特にRNAウイルス感染症の治療又は予防に有用である。
The present invention is particularly useful for the treatment or prevention of RNA virus infections.
SEQ ID NO:1 ; Insert DNA comprising a gene encoding a Gag_mazF fusion protein.
SEQ ID NO:2 ; Gag_MazF fusion protein.
SEQ ID NO:3 ; Oligonucleotide primer named mazF(HindIII)_R.
SEQ ID NO:4 ; Oligonucleotide primer named mazF(BamHI)_F.
SEQ ID NO:5 ; Gene encoding an ACA-less-mazF.
SEQ ID NO:7 ; Oligonucleotide primer named NC-FWD.
SEQ ID NO:8 ; Oligonucleotide primer named NC-Rev3.
SEQ ID NO:9 ; Oligonucleotide primer named human_18S_fw.
SEQ ID NO:10 ; Oligonucleotide primer named human_18S_rev.
SEQ ID NO:11 ; Oligonucleotide primer named ZsG_F2.
SEQ ID NO:12 ; Oligonucleotide primer named ZsG_R2.
SEQ ID NO:13 ; Oligonucleotide primer named beta_actin_fw.
SEQ ID NO:14 ; Oligonucleotide primer named beta_actin_rev.
SEQ ID NO:15 ; Oligonucleotide primer named Lenti-copy1-F.
SEQ ID NO:16 ; Oligonucleotide primer named Lenti-copy1-R.
SEQ ID NO:17 ; Chimeric oligonucleotide probe named P1S. SEQ ID NO: 1; Insert DNA comprising a gene encoding a Gag_mazF fusion protein.
SEQ ID NO: 2; Gag_MazF fusion protein.
SEQ ID NO: 3; Oligonucleotide primer named mazF (HindIII) _R.
SEQ ID NO: 4; Oligonucleotide primer named mazF (BamHI) _F.
SEQ ID NO: 5; Gene encoding an ACA-less-mazF.
SEQ ID NO: 7; Oligonucleotide primer named NC-FWD.
SEQ ID NO: 8; Oligonucleotide primer named NC-Rev3.
SEQ ID NO: 9; Oligonucleotide primer named human_18S_fw.
SEQ ID NO: 10; Oligonucleotide primer named human_18S_rev.
SEQ ID NO: 11; Oligonucleotide primer named ZsG_F2.
SEQ ID NO: 12; Oligonucleotide primer named ZsG_R2.
SEQ ID NO: 13; Oligonucleotide primer named beta_actin_fw.
SEQ ID NO: 14; Oligonucleotide primer named beta_actin_rev.
SEQ ID NO: 15; Oligonucleotide primer named Lenti-copy1-F.
SEQ ID NO: 16; Oligonucleotide primer named Lenti-copy1-R.
SEQ ID NO: 17; Chimeric oligonucleotide probe named P1S.
SEQ ID NO:2 ; Gag_MazF fusion protein.
SEQ ID NO:3 ; Oligonucleotide primer named mazF(HindIII)_R.
SEQ ID NO:4 ; Oligonucleotide primer named mazF(BamHI)_F.
SEQ ID NO:5 ; Gene encoding an ACA-less-mazF.
SEQ ID NO:7 ; Oligonucleotide primer named NC-FWD.
SEQ ID NO:8 ; Oligonucleotide primer named NC-Rev3.
SEQ ID NO:9 ; Oligonucleotide primer named human_18S_fw.
SEQ ID NO:10 ; Oligonucleotide primer named human_18S_rev.
SEQ ID NO:11 ; Oligonucleotide primer named ZsG_F2.
SEQ ID NO:12 ; Oligonucleotide primer named ZsG_R2.
SEQ ID NO:13 ; Oligonucleotide primer named beta_actin_fw.
SEQ ID NO:14 ; Oligonucleotide primer named beta_actin_rev.
SEQ ID NO:15 ; Oligonucleotide primer named Lenti-copy1-F.
SEQ ID NO:16 ; Oligonucleotide primer named Lenti-copy1-R.
SEQ ID NO:17 ; Chimeric oligonucleotide probe named P1S. SEQ ID NO: 1; Insert DNA comprising a gene encoding a Gag_mazF fusion protein.
SEQ ID NO: 2; Gag_MazF fusion protein.
SEQ ID NO: 3; Oligonucleotide primer named mazF (HindIII) _R.
SEQ ID NO: 4; Oligonucleotide primer named mazF (BamHI) _F.
SEQ ID NO: 5; Gene encoding an ACA-less-mazF.
SEQ ID NO: 7; Oligonucleotide primer named NC-FWD.
SEQ ID NO: 8; Oligonucleotide primer named NC-Rev3.
SEQ ID NO: 9; Oligonucleotide primer named human_18S_fw.
SEQ ID NO: 10; Oligonucleotide primer named human_18S_rev.
SEQ ID NO: 11; Oligonucleotide primer named ZsG_F2.
SEQ ID NO: 12; Oligonucleotide primer named ZsG_R2.
SEQ ID NO: 13; Oligonucleotide primer named beta_actin_fw.
SEQ ID NO: 14; Oligonucleotide primer named beta_actin_rev.
SEQ ID NO: 15; Oligonucleotide primer named Lenti-copy1-F.
SEQ ID NO: 16; Oligonucleotide primer named Lenti-copy1-R.
SEQ ID NO: 17; Chimeric oligonucleotide probe named P1S.
Claims (18)
- RNAウイルスのウイルスRNAに親和性を示すポリペプチドが一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合されてなる、融合タンパク質。 A fusion protein in which a polypeptide having affinity for viral RNA of an RNA virus is fused to an endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner.
- RNAウイルスのウイルスRNAに親和性を示すポリペプチドがリンカーペプチドを介して一本鎖RNAを配列特異的に切断するエンドリボヌクレアーゼに融合されてなる、請求項1に記載の融合タンパク質。 The fusion protein according to claim 1, wherein a polypeptide having affinity for viral RNA of an RNA virus is fused to an endoribonuclease that specifically cleaves single-stranded RNA via a linker peptide.
- さらに、脂質修飾シグナルをN末端領域に有する、請求項1に記載の融合タンパク質。 The fusion protein according to claim 1, further comprising a lipid modification signal in the N-terminal region.
- RNAウイルスのウイルスRNAに親和性を示すポリペプチドが、RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドである、請求項1に記載の融合タンパク質。 The fusion protein according to claim 1, wherein the polypeptide having affinity for viral RNA of RNA virus is a polypeptide having affinity for packaging signal of RNA virus.
- RNAウイルスのパッケージングシグナルに親和性を示すポリペプチドが、レトロウイルスのパッケージングシグナルに親和性を示すポリペプチドである、請求項4に記載の融合タンパク質。 The fusion protein according to claim 4, wherein the polypeptide having affinity for the packaging signal of RNA virus is a polypeptide having affinity for the packaging signal of retrovirus.
- レトロウイルスのパッケージングシグナルに親和性を示すポリペプチドがレトロウイルスのGagタンパク質に由来する、請求項5に記載の融合タンパク質。 The fusion protein according to claim 5, wherein the polypeptide having an affinity for a retroviral packaging signal is derived from a retroviral Gag protein.
- レトロウイルスのGagタンパク質が、ヒト免疫不全ウイルスに由来するGagタンパク質である、請求項6に記載の融合タンパク質。 The fusion protein according to claim 6, wherein the retrovirus Gag protein is a Gag protein derived from a human immunodeficiency virus.
- Gagタンパク質に由来するレトロウイルスのパッケージングシグナルに親和性を示すポリペプチドがヌクレオカプシドである、請求項6に記載の融合タンパク質。 The fusion protein according to claim 6, wherein the polypeptide having an affinity for a packaging signal of a retrovirus derived from a Gag protein is a nucleocapsid.
- 一本鎖RNAを配列特異的に切断するエンドリボヌクレア-ゼがMazFタンパク質である、請求項1に記載の融合タンパク質。 The fusion protein according to claim 1, wherein the endoribonuclease that cleaves single-stranded RNA in a sequence-specific manner is MazF protein.
- 請求項1~9のいずれか一項に記載の融合タンパク質をコードする遺伝子を含む核酸。 A nucleic acid comprising a gene encoding the fusion protein according to any one of claims 1 to 9.
- さらに、RNAウイルスのトランス作用因子により転写が誘導される転写調節配列を有し、ここで、前記の融合タンパク質をコードする遺伝子は前記の転写調節配列により発現の制御が可能な形態に配置されている、請求項10に記載の核酸。 Furthermore, it has a transcriptional regulatory sequence whose transcription is induced by a trans-acting factor of RNA virus, wherein the gene encoding the fusion protein is arranged in a form in which the expression can be controlled by the transcriptional regulatory sequence. The nucleic acid according to claim 10.
- 転写調節配列が、Tatタンパク質および/またはRevタンパク質により転写が誘導される転写調節配列である、請求項11に記載の核酸。 The nucleic acid according to claim 11, wherein the transcription regulatory sequence is a transcription regulatory sequence whose transcription is induced by Tat protein and / or Rev protein.
- 請求項10に記載の核酸を含むベクター。 A vector comprising the nucleic acid according to claim 10.
- レトロウイルスベクターである、請求項13に記載のベクター。 The vector according to claim 13, which is a retroviral vector.
- 請求項13に記載のベクターを含む医薬組成物。 A pharmaceutical composition comprising the vector according to claim 13.
- 請求項10に記載の核酸が導入されてなる細胞。 A cell into which the nucleic acid according to claim 10 has been introduced.
- 請求項13に記載のベクターを細胞に接触する工程を含む、RNAウイルス感染症の治療又は予防方法。 A method for treating or preventing RNA virus infection, comprising a step of contacting the vector according to claim 13 with a cell.
- 請求項1に記載の融合タンパク質、請求項10に記載の核酸、及び請求項13に記載のベクターからなる群より選択された少なくとも1種を細胞に導入する工程を含む、RNAウイルスの出芽およびプロウイルス形成の抑制方法。 Sprouting and prosthesis of an RNA virus, comprising the step of introducing into a cell at least one selected from the group consisting of the fusion protein according to claim 1, the nucleic acid according to claim 10, and the vector according to claim 13. Method for inhibiting virus formation.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011-044379 | 2011-03-01 | ||
| JP2011044379 | 2011-03-01 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012118092A1 true WO2012118092A1 (en) | 2012-09-07 |
Family
ID=46758023
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/054967 WO2012118092A1 (en) | 2011-03-01 | 2012-02-28 | Fusion protein |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2012118092A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110139870A (en) * | 2016-12-16 | 2019-08-16 | 爱维斯健有限公司 | Penetratin and intracellular delivery vehicles comprising it |
| US11208433B2 (en) | 2017-12-28 | 2021-12-28 | Avixgen Inc. | Peptide for inhibiting skin inflammation and composition for preventing or treating skin inflammation containing the same |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007020873A1 (en) * | 2005-08-16 | 2007-02-22 | Takara Bio Inc. | Nucleic acid for treatment or prevention of immunodeficiency virus infection |
| WO2008133137A1 (en) * | 2007-04-20 | 2008-11-06 | Takara Bio Inc. | Vector for gene therapy |
| WO2009051078A1 (en) * | 2007-10-15 | 2009-04-23 | Takara Bio Inc. | Therapeutic gene expression system |
-
2012
- 2012-02-28 WO PCT/JP2012/054967 patent/WO2012118092A1/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007020873A1 (en) * | 2005-08-16 | 2007-02-22 | Takara Bio Inc. | Nucleic acid for treatment or prevention of immunodeficiency virus infection |
| WO2008133137A1 (en) * | 2007-04-20 | 2008-11-06 | Takara Bio Inc. | Vector for gene therapy |
| WO2009051078A1 (en) * | 2007-10-15 | 2009-04-23 | Takara Bio Inc. | Therapeutic gene expression system |
Non-Patent Citations (7)
| Title |
|---|
| KOYANAGI, Y.: "Outline of the HIV Replication and its Cellular Factors: the Track of an Invader in Cell", VIRUS, vol. 55, no. 2, 2005, pages 251 - 257 * |
| MURAKAMI, T. ET AL.: "Regulation of GAG trafficking and functions", THE JOURNAL OF AIDS RESEARCH, vol. 9, no. 2, 2007, pages 102 - 107 * |
| NATSOULIS, G. ET AL.: "Targeting of a nuclease to murine leukemia virus capsids inhibits viral multiplication", PROC. NATL. ACAD. SCI. USA, vol. 92, January 1995 (1995-01-01), pages 364 - 368 * |
| OKAMOTO, M. ET AL.: "RNA Bunkai Koso MazF ga HIV LTR Ryoiki no Shihaika de Hatsugen suru Retrovirus Vector Donyu Saibo ni Okeru HIV-1 Teikosei no Kakutoku", THE JOURNAL OF AIDS RESEARCH, vol. 10, no. 4, 2008, pages P-090 * |
| SAKURAGI, J.: "Structure and Function of HIV Virion Hoso Shutsuga kara Kyuchaku Shinnyu made", VIRUS, vol. 52, no. 1, 2002, pages 95 - 102 * |
| SCHUMANN, G. ET AL.: "Antiretroviral effect of a gag-RNase HI fusion gene", GENE THERAPY, vol. 4, 1997, pages 593 - 599 * |
| SCHUMANN, G. ET AL.: "Therapeutic effect of a gag-nuclease fusion protein against retroviral infection in vivo", JOURNAL OF VIROLOGY, vol. 75, no. 15, August 2001 (2001-08-01), pages 7030 - 7041 * |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110139870A (en) * | 2016-12-16 | 2019-08-16 | 爱维斯健有限公司 | Penetratin and intracellular delivery vehicles comprising it |
| EP3556766A4 (en) * | 2016-12-16 | 2020-07-22 | Avixgen Inc. | Cytoplasmic transduction peptide and intracellular messenger comprising same |
| CN110139870B (en) * | 2016-12-16 | 2023-12-01 | 爱维斯健有限公司 | Cell membrane penetrating peptide and intracellular delivery vehicle comprising same |
| US11208433B2 (en) | 2017-12-28 | 2021-12-28 | Avixgen Inc. | Peptide for inhibiting skin inflammation and composition for preventing or treating skin inflammation containing the same |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2022001047A (en) | Delivery of construct of crispr/cas that cuts off gene necessary for hiv-1 infection and replication by lentivirus | |
| Rethwilm | The replication strategy of foamy viruses | |
| CA2328404C (en) | Novel lentiviral packaging cells | |
| KR20010033064A (en) | Method and means for producing high titer, safe, recombinant lentivirus vectors | |
| Antzin-Anduetza et al. | Increasing the CpG dinucleotide abundance in the HIV-1 genomic RNA inhibits viral replication | |
| JP5122957B2 (en) | Nucleic acids for the treatment or prevention of immunodeficiency virus infection | |
| US20140170709A1 (en) | Vector for gene therapy | |
| ES2886919T3 (en) | Particle for the encapsidation of a genome engineering system | |
| Goujon et al. | Heterologous human immunodeficiency virus type 1 lentiviral vectors packaging a simian immunodeficiency virus-derived genome display a specific postentry transduction defect in dendritic cells | |
| US7803582B2 (en) | Recombinant vector and use in gene therapy | |
| WO2012118092A1 (en) | Fusion protein | |
| Schüle et al. | Restriction of HIV-1 replication in monocytes is abolished by Vpx of SIVsmmPBj | |
| Trabalza et al. | Enhanced central nervous system transduction with lentiviral vectors pseudotyped with RVG/HIV-1gp41 chimeric envelope glycoproteins | |
| JP5324912B2 (en) | HIVVif mutant | |
| Poluri et al. | Genetic therapy for HIV/AIDS | |
| JP2010517555A (en) | Methods for incorporating proteins into lentiviral vectors | |
| US20120034693A1 (en) | Recombinant vector and use in gene therapy | |
| EP4326885A1 (en) | Packaging cells with targeted gene knockouts that improve retroviral vector titers | |
| Croyle et al. | 892. Purification of Recombinant Lentiviral V 892. Purification of Recombinant Lentiviral Vector by Anion Exchange Chromatography | |
| JP2001514517A (en) | Expression of modified foamy virus envelope protein | |
| August et al. | HIV: Molecular Biology and Pathogenesis: Viral Mechanisms | |
| Guo | Understanding the multiple roles of the CA-SP1 boundary region in HIV-1 Gag multimerization, gag-membrane binding and viral RNA packaging | |
| JP2016067306A (en) | Phylaxis method for human immunodeficiency virus |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12752591 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12752591 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |