WO2012114349A1 - An improved process for the preparation of pentosan polysulfate sodium - Google Patents
An improved process for the preparation of pentosan polysulfate sodium Download PDFInfo
- Publication number
- WO2012114349A1 WO2012114349A1 PCT/IN2011/000393 IN2011000393W WO2012114349A1 WO 2012114349 A1 WO2012114349 A1 WO 2012114349A1 IN 2011000393 W IN2011000393 W IN 2011000393W WO 2012114349 A1 WO2012114349 A1 WO 2012114349A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- compound
- preparation
- pentosan polysulfate
- mixtures
- Prior art date
Links
- 238000000034 method Methods 0.000 title claims abstract description 46
- 229960003820 pentosan polysulfate sodium Drugs 0.000 title claims abstract description 42
- QUCDWLYKDRVKMI-UHFFFAOYSA-M sodium;3,4-dimethylbenzenesulfonate Chemical compound [Na+].CC1=CC=C(S([O-])(=O)=O)C=C1C QUCDWLYKDRVKMI-UHFFFAOYSA-M 0.000 title claims abstract description 42
- 238000002360 preparation method Methods 0.000 title claims abstract description 23
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 21
- 239000012528 membrane Substances 0.000 claims abstract description 17
- 239000007864 aqueous solution Substances 0.000 claims abstract description 9
- 238000011026 diafiltration Methods 0.000 claims abstract description 8
- 238000007865 diluting Methods 0.000 claims abstract description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 36
- 150000001875 compounds Chemical class 0.000 claims description 21
- 239000000203 mixture Substances 0.000 claims description 21
- 229920001221 xylan Polymers 0.000 claims description 18
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 14
- 150000003839 salts Chemical class 0.000 claims description 14
- 239000002904 solvent Substances 0.000 claims description 14
- 150000004823 xylans Chemical class 0.000 claims description 14
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 claims description 12
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 claims description 12
- 238000002955 isolation Methods 0.000 claims description 11
- -1 sulfuric acid ester Chemical class 0.000 claims description 11
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 claims description 10
- QAOWNCQODCNURD-UHFFFAOYSA-N sulfuric acid Substances OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 claims description 10
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims description 8
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 claims description 8
- 239000003513 alkali Substances 0.000 claims description 7
- 239000007800 oxidant agent Substances 0.000 claims description 7
- XTHPWXDJESJLNJ-UHFFFAOYSA-N sulfurochloridic acid Chemical compound OS(Cl)(=O)=O XTHPWXDJESJLNJ-UHFFFAOYSA-N 0.000 claims description 7
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 claims description 6
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 claims description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 claims description 6
- 238000001914 filtration Methods 0.000 claims description 6
- 238000000746 purification Methods 0.000 claims description 6
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 claims description 5
- 230000001476 alcoholic effect Effects 0.000 claims description 5
- 150000001298 alcohols Chemical class 0.000 claims description 5
- 230000001590 oxidative effect Effects 0.000 claims description 5
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 claims description 5
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 claims description 4
- KHIWWQKSHDUIBK-UHFFFAOYSA-N periodic acid Chemical class OI(=O)(=O)=O KHIWWQKSHDUIBK-UHFFFAOYSA-N 0.000 claims description 4
- 159000000000 sodium salts Chemical class 0.000 claims description 4
- 150000001408 amides Chemical class 0.000 claims description 3
- 229930195733 hydrocarbon Natural products 0.000 claims description 3
- 150000002430 hydrocarbons Chemical class 0.000 claims description 3
- 150000002576 ketones Chemical class 0.000 claims description 3
- 150000002825 nitriles Chemical class 0.000 claims description 3
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical class CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 claims description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical class OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 claims description 2
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical class CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 claims description 2
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical class CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 claims description 2
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 claims description 2
- 229910052783 alkali metal Inorganic materials 0.000 claims description 2
- 150000004649 carbonic acid derivatives Chemical class 0.000 claims description 2
- 239000003153 chemical reaction reagent Substances 0.000 claims description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 claims description 2
- DHAFIDRKDGCXLV-UHFFFAOYSA-N n,n-dimethylformamide;1-methylpyrrolidin-2-one Chemical compound CN(C)C=O.CN1CCCC1=O DHAFIDRKDGCXLV-UHFFFAOYSA-N 0.000 claims description 2
- 150000002978 peroxides Chemical class 0.000 claims description 2
- FVSKHRXBFJPNKK-UHFFFAOYSA-N propionitrile Chemical compound CCC#N FVSKHRXBFJPNKK-UHFFFAOYSA-N 0.000 claims description 2
- 239000008096 xylene Substances 0.000 claims description 2
- 239000000047 product Substances 0.000 description 20
- 239000000243 solution Substances 0.000 description 18
- 239000011541 reaction mixture Substances 0.000 description 15
- 239000007787 solid Substances 0.000 description 13
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 12
- 238000001223 reverse osmosis Methods 0.000 description 12
- 229940043138 pentosan polysulfate Drugs 0.000 description 8
- 239000012265 solid product Substances 0.000 description 7
- KEQGZUUPPQEDPF-UHFFFAOYSA-N 1,3-dichloro-5,5-dimethylimidazolidine-2,4-dione Chemical compound CC1(C)N(Cl)C(=O)N(Cl)C1=O KEQGZUUPPQEDPF-UHFFFAOYSA-N 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 3
- 238000000108 ultra-filtration Methods 0.000 description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 229920001903 high density polyethylene Polymers 0.000 description 2
- 239000004700 high-density polyethylene Substances 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- JQWHASGSAFIOCM-UHFFFAOYSA-M sodium periodate Chemical compound [Na+].[O-]I(=O)(=O)=O JQWHASGSAFIOCM-UHFFFAOYSA-M 0.000 description 2
- 230000019635 sulfation Effects 0.000 description 2
- 238000005670 sulfation reaction Methods 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 239000011593 sulfur Substances 0.000 description 2
- BIIBYWQGRFWQKM-JVVROLKMSA-N (2S)-N-[4-(cyclopropylamino)-3,4-dioxo-1-[(3S)-2-oxopyrrolidin-3-yl]butan-2-yl]-2-[[(E)-3-(2,4-dichlorophenyl)prop-2-enoyl]amino]-4,4-dimethylpentanamide Chemical compound CC(C)(C)C[C@@H](C(NC(C[C@H](CCN1)C1=O)C(C(NC1CC1)=O)=O)=O)NC(/C=C/C(C=CC(Cl)=C1)=C1Cl)=O BIIBYWQGRFWQKM-JVVROLKMSA-N 0.000 description 1
- QIVUCLWGARAQIO-OLIXTKCUSA-N (3s)-n-[(3s,5s,6r)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl]-2-oxospiro[1h-pyrrolo[2,3-b]pyridine-3,6'-5,7-dihydrocyclopenta[b]pyridine]-3'-carboxamide Chemical compound C1([C@H]2[C@H](N(C(=O)[C@@H](NC(=O)C=3C=C4C[C@]5(CC4=NC=3)C3=CC=CN=C3NC5=O)C2)CC(F)(F)F)C)=C(F)C=CC(F)=C1F QIVUCLWGARAQIO-OLIXTKCUSA-N 0.000 description 1
- OFUMROLKEGKJMS-UHFFFAOYSA-N 2-[2-(1,3-benzodioxol-5-yl)-3-[2-(cyclohexylamino)pyrimidin-4-yl]imidazol-4-yl]acetonitrile Chemical compound O1COC2=C1C=CC(=C2)C=1N(C(=CN=1)CC#N)C1=NC(=NC=C1)NC1CCCCC1 OFUMROLKEGKJMS-UHFFFAOYSA-N 0.000 description 1
- DWKNOLCXIFYNFV-HSZRJFAPSA-N 2-[[(2r)-1-[1-[(4-chloro-3-methylphenyl)methyl]piperidin-4-yl]-5-oxopyrrolidine-2-carbonyl]amino]-n,n,6-trimethylpyridine-4-carboxamide Chemical compound CN(C)C(=O)C1=CC(C)=NC(NC(=O)[C@@H]2N(C(=O)CC2)C2CCN(CC=3C=C(C)C(Cl)=CC=3)CC2)=C1 DWKNOLCXIFYNFV-HSZRJFAPSA-N 0.000 description 1
- BSKHPKMHTQYZBB-UHFFFAOYSA-N 2-methylpyridine Chemical compound CC1=CC=CC=N1 BSKHPKMHTQYZBB-UHFFFAOYSA-N 0.000 description 1
- SJVGFKBLUYAEOK-SFHVURJKSA-N 6-[4-[(3S)-3-(3,5-difluorophenyl)-3,4-dihydropyrazole-2-carbonyl]piperidin-1-yl]pyrimidine-4-carbonitrile Chemical compound FC=1C=C(C=C(C=1)F)[C@@H]1CC=NN1C(=O)C1CCN(CC1)C1=CC(=NC=N1)C#N SJVGFKBLUYAEOK-SFHVURJKSA-N 0.000 description 1
- 101100243951 Caenorhabditis elegans pie-1 gene Proteins 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 240000000731 Fagus sylvatica Species 0.000 description 1
- 235000010099 Fagus sylvatica Nutrition 0.000 description 1
- 208000001034 Frostbite Diseases 0.000 description 1
- 229920002683 Glycosaminoglycan Polymers 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000005615 Interstitial Cystitis Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 239000005708 Sodium hypochlorite Substances 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 230000002429 anti-coagulating effect Effects 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 229940127219 anticoagulant drug Drugs 0.000 description 1
- 150000001719 carbohydrate derivatives Chemical class 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- ZCDOYSPFYFSLEW-UHFFFAOYSA-N chromate(2-) Chemical class [O-][Cr]([O-])(=O)=O ZCDOYSPFYFSLEW-UHFFFAOYSA-N 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 229940113088 dimethylacetamide Drugs 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 230000003480 fibrinolytic effect Effects 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 150000004680 hydrogen peroxides Chemical class 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- VOVZXURTCKPRDQ-CQSZACIVSA-N n-[4-[chloro(difluoro)methoxy]phenyl]-6-[(3r)-3-hydroxypyrrolidin-1-yl]-5-(1h-pyrazol-5-yl)pyridine-3-carboxamide Chemical compound C1[C@H](O)CCN1C1=NC=C(C(=O)NC=2C=CC(OC(F)(F)Cl)=CC=2)C=C1C1=CC=NN1 VOVZXURTCKPRDQ-CQSZACIVSA-N 0.000 description 1
- XULSCZPZVQIMFM-IPZQJPLYSA-N odevixibat Chemical compound C12=CC(SC)=C(OCC(=O)N[C@@H](C(=O)N[C@@H](CC)C(O)=O)C=3C=CC(O)=CC=3)C=C2S(=O)(=O)NC(CCCC)(CCCC)CN1C1=CC=CC=C1 XULSCZPZVQIMFM-IPZQJPLYSA-N 0.000 description 1
- 230000009965 odorless effect Effects 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 229910000489 osmium tetroxide Inorganic materials 0.000 description 1
- 201000008482 osteoarthritis Diseases 0.000 description 1
- 239000012466 permeate Substances 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 150000004804 polysaccharides Chemical class 0.000 description 1
- FJVZDOGVDJCCCR-UHFFFAOYSA-M potassium periodate Chemical compound [K+].[O-]I(=O)(=O)=O FJVZDOGVDJCCCR-UHFFFAOYSA-M 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- WBHQBSYUUJJSRZ-UHFFFAOYSA-M sodium bisulfate Chemical compound [Na+].OS([O-])(=O)=O WBHQBSYUUJJSRZ-UHFFFAOYSA-M 0.000 description 1
- UKLNMMHNWFDKNT-UHFFFAOYSA-M sodium chlorite Chemical compound [Na+].[O-]Cl=O UKLNMMHNWFDKNT-UHFFFAOYSA-M 0.000 description 1
- 229960002218 sodium chlorite Drugs 0.000 description 1
- SUKJFIGYRHOWBL-UHFFFAOYSA-N sodium hypochlorite Chemical compound [Na+].Cl[O-] SUKJFIGYRHOWBL-UHFFFAOYSA-N 0.000 description 1
- CRWJEUDFKNYSBX-UHFFFAOYSA-N sodium;hypobromite Chemical compound [Na+].Br[O-] CRWJEUDFKNYSBX-UHFFFAOYSA-N 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000001180 sulfating effect Effects 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- YBBRCQOCSYXUOC-UHFFFAOYSA-N sulfuryl dichloride Chemical compound ClS(Cl)(=O)=O YBBRCQOCSYXUOC-UHFFFAOYSA-N 0.000 description 1
- 239000001117 sulphuric acid Substances 0.000 description 1
- 235000011149 sulphuric acid Nutrition 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- KMIOJWCYOHBUJS-HAKPAVFJSA-N vorolanib Chemical compound C1N(C(=O)N(C)C)CC[C@@H]1NC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C KMIOJWCYOHBUJS-HAKPAVFJSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08B—POLYSACCHARIDES; DERIVATIVES THEREOF
- C08B37/00—Preparation of polysaccharides not provided for in groups C08B1/00 - C08B35/00; Derivatives thereof
- C08B37/0006—Homoglycans, i.e. polysaccharides having a main chain consisting of one single sugar, e.g. colominic acid
- C08B37/0057—Homoglycans, i.e. polysaccharides having a main chain consisting of one single sugar, e.g. colominic acid beta-D-Xylans, i.e. xylosaccharide, e.g. arabinoxylan, arabinofuronan, pentosans; (beta-1,3)(beta-1,4)-D-Xylans, e.g. rhodymenans; Hemicellulose; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08B—POLYSACCHARIDES; DERIVATIVES THEREOF
- C08B37/00—Preparation of polysaccharides not provided for in groups C08B1/00 - C08B35/00; Derivatives thereof
- C08B37/0003—General processes for their isolation or fractionation, e.g. purification or extraction from biomass
Definitions
- the present invention discloses an improved process for the preparation of
- Pentosan Polysulfate Sodium and also disclosed an improved process for the isolation of compound of Formula I.
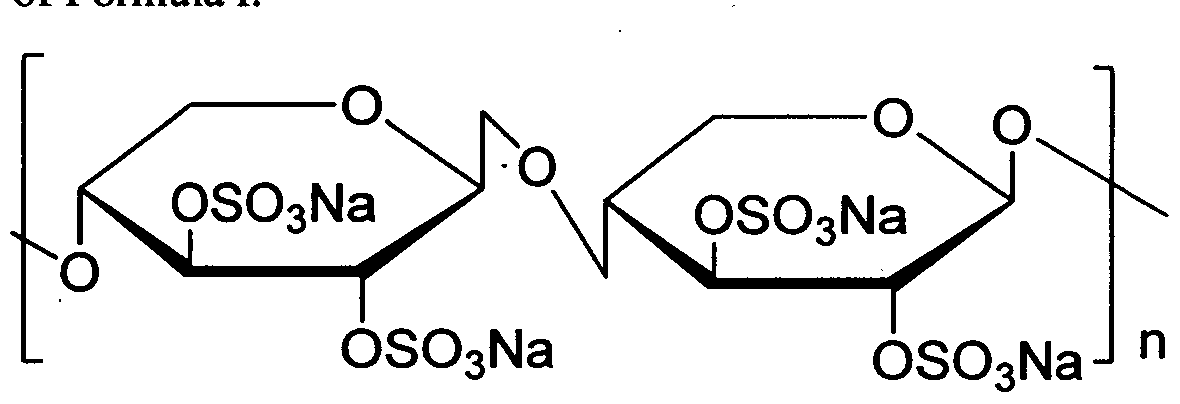
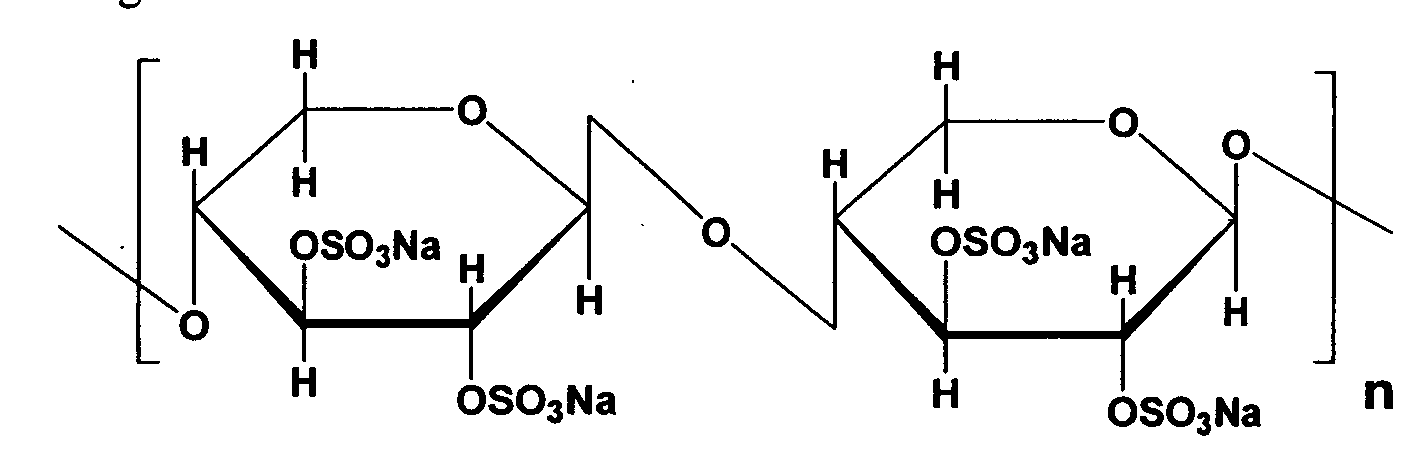
- Pentosan Polysulfate Sodium is chemically known as ⁇ -D-Xylan, (1-4), 2,3 -bis (hydrogen Sulfate) sodium salt having molecular range of 4000-6000 Dalton and has the following structural formula:
- Pentosan Polysulfate Sodium is a semi-synthetically produced heparin like macromolecular carbohydrate derivative which chemically and structurally resembles glycosaminoglycans. It has anticoagulant and fibrinolytic effects. It is a white odorless powder, slightly hygroscopic and soluble in water upto 50% at pH-6.
- Pentosan Polysulfate is produced from a chemical solution of polysaccharides (e.g. Xylan) extracted from the bark of the beech tree or other plant sources which is then treated with sulfating agents such as chlorosulfonic acid or sulfuryl chloride and an acid. After sulfation, Pentosan Polysulfate is usually treated with sodium hydroxide to yield the sodium salt.
- Pentosan Polysulfate is most commonly used as an oral formulation to treat interstitial cystitis in humans and as an injectable drug to treat osteoarthritis in companion animals. It has also been used for treatment of hematomes, hemarrohoids, frostbites, burns and multiparameter illnesses such as thrombosis and athereosclerosis.
- Pentosan Polysulfate is disclosed in US patent no. 2689848.
- the same patent disclosed the process for the production of salts of sulfuric ester of xylan, the steps comprising oxidizing the aqueous solution of the a salt of sulfuric acid ester of highly polymeric xylan in an aqueous solution of H 2 0 2 and H 2 S0 4 to depolymerize such highly polymeric xylan ester partially, dialyzing the depolymerized product and fractioning an aqueous mixture of the dialyzate with an organic water miscible solvent to obtain a fraction having a ZD value between 0.0030 and 0.015 (The value of ZD is proportional to the degree of polymerization and consequently, serves as an index of the molecular weight or length of the chain of the xylan sulphuric acid esters) and sulfur content of 13.5 to 17%.
- the process as disclosed therein does not describe about the molecular weight of product after getting step-2 or even of final
- WO2008107906 disclosed process for the preparation of Pentosan Polysulfate and an amorphous form of Pentosan Polysulfate sodium with molecular weight of about 3000 to 10000 Dalton.
- WO 2009047699 also described a process for the preparation of an amorphous form of Pentosan Polysulfate Sodium comprising steps of dissolving crude pentosan polysulfate sodium having a molecular weight of 4000 to 6000 Dalton in water (step I) followed by spray drying the solution to obtain amorphous Pentosan Polysulfate Sodium.
- Pentosan Polysulfate Sodium Formula (I) by diluting the aqueous solution with excess of water followed by DiaAlteration through membrane system and isolation of resulting mixture provides high yield with desired molecular weight of 4000-6000 Dalton.
- An object of the present invention is to provide an improved process for preparing Pentosan Polysulfate Sodium.
- It a further object of the present invention to provide a process for purification of Pentosan Polysulfate Sodium using suitable technique.
- It a further object of the present invention to provide an improved process for isolation of Pentosan Polysulfate Sodium using suitable solvents.
- an improved process for preparing Pentosan Polysulfate Sodium of Formula (I), comprising steps of: i) reacting xylan with chlorosulfonic acid in the presence of pyridine followed by addition of alkali in the presence of alcoholic solvent to obtain sodium salt of sulfuric acid ester of xylan. ii) depolymerizing the salt of sulfuric acid ester of xylan with the mixture of H 2 S0 4 and an oxidizing agent to obtain depolymerized crude Pentosan Polysulfate Sodium of Formula (IV)
- the invention provides a process for the preparation of Pentosan Polysulfate sodium of Formula (I) as depicted following steps:
- the steps comprises:
- the suitable solvent used in step-(i) may be selected from (Q-C5) alcohols such as methanol, ethanol, propanol, isopropanol, butanol or mixtures thereof.
- the suitable alkali used in step-(i) is selected from alkali metal salt of carbonates, bicarbonates, hydroxide or mixtures thereof.
- the suitable oxidizing agent used in step-(ii) may be selected from peroxides such as hydrogen peroxides and the like; halogenated oxidizing agents such as sodium hypochlorite, sodiumhypobromide & the like; metallic oxidizing reagents such as osmium tetraoxide, chromates, permanganates, tungestates & the like; periodic acids or salts of periodic acids such as sodium metaperiodate, potassium metaperiodate & the likes either independently or in a suitable mixtures thereof.
- peroxides such as hydrogen peroxides and the like
- halogenated oxidizing agents such as sodium hypochlorite, sodiumhypobromide & the like
- metallic oxidizing reagents such as osmium tetraoxide, chromates, permanganates, tungestates & the like
- periodic acids or salts of periodic acids such as sodium metaperiodate, potassium metaperiodate & the likes
- Suitable diafiltration membranes as are known in the art may be used.
- the diafiltration can be done continuously or discontinuously. Such techniques are well within the ability of a skilled person.
- the suitable membrane system in the filtration step as discussed herein is carried out by using pure flow-U30S ultra filtration membrane.
- This advanced thin film composite ultra filtration membrane has a filtration range of less than 4 KD, preferably 1 D - 4 KD and more preferably 2.8 KD nominal MWCO (Molecular wt. cut off). It removes most of bivalent ions, inorganic salts and unreacted low molecules of solute.
- the suitable solvent for isolation used in step-(iv) may be selected from hydrocarbons such as toluene, xylene, n-heptane, cyclohexane, n-hexane; nitriles such as acetonitrile, propionitrile; (Q to C 5 ) alcohols such as methanol, ethanol, isopropanol and the like; ketones such as acetone, methyl ethyl ketones, methyl isobutyl ketones & the like; amides such as ⁇ , ⁇ -dimethyl acetamides, dimethyl formamide N-methyl-2- pyrrolidinone and the likes either independently or suitable mixtures thereof.
- hydrocarbons such as toluene, xylene, n-heptane, cyclohexane, n-hexane
- nitriles such as acetonitrile, propionitrile
- (Q to C 5 ) alcohols
- the present invention also encompasses a process for the purification of compound of formula (IV).
- Purification of compound of the Formula (IV) followed by Diafilteration through suitable membrane system removes most of bivalent ions, inorganic salts and unreacted low molecules of solute. So, one of the significance of the present process by using the purification technique is to get the desired molecular weight of pure compound of Formula (I) in a pure form.
- Chlorosulfonic acid (350 g) was added slowly in pyridine (870 g) at 25 to 60°C.
- reaction mixture was stirred for next 4-6 hours, adjust pH 6 to 7 with dilute acetic acid.
- the mass was filtered and collected aqueous solution for next step (Diafilteration) without isolation of product.
- GPC average molecular weight 3500 to 6000 D.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Materials Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Sustainable Development (AREA)
- Polysaccharides And Polysaccharide Derivatives (AREA)
Abstract
The present invention relates to provide an improved process of preparation of Pentosan Polysulfate Sodium of Formula (I) comprising a step of purifying the depolymerized aqueous solution by diluting with excess of water followed by diafiltration through membrane system.
Description
AN IMPROVED PROCESS FOR THE PREPARATION OF PENTOSAN
POLYSULFATE SODIUM
FIELD OF INVENTION
The present invention discloses an improved process for the preparation of
Pentosan Polysulfate Sodium and also disclosed an improved process for the isolation of compound of Formula I.
Pentosan Polysulfate Sodium (Formula I)
BACKGROUND OF THE INVENTION
Pentosan Polysulfate Sodium is chemically known as β-D-Xylan, (1-4), 2,3 -bis (hydrogen Sulfate) sodium salt having molecular range of 4000-6000 Dalton and has the following structural formula:
Pentosan Polysulfate Sodium
Pentosan Polysulfate Sodium is a semi-synthetically produced heparin like macromolecular carbohydrate derivative which chemically and structurally resembles glycosaminoglycans. It has anticoagulant and fibrinolytic effects. It is a white odorless powder, slightly hygroscopic and soluble in water upto 50% at pH-6.
Pentosan Polysulfate is produced from a chemical solution of polysaccharides (e.g. Xylan) extracted from the bark of the beech tree or other plant sources which is then treated with sulfating agents such as chlorosulfonic acid or sulfuryl chloride and an acid. After sulfation, Pentosan Polysulfate is usually treated with sodium hydroxide to yield the sodium salt. Pentosan Polysulfate is most commonly used as an oral formulation to treat interstitial cystitis in humans and as an injectable drug to treat osteoarthritis in companion animals. It has also been used for treatment of hematomes,
hemarrohoids, frostbites, burns and multiparameter illnesses such as thrombosis and athereosclerosis.
Pentosan Polysulfate is disclosed in US patent no. 2689848. The same patent disclosed the process for the production of salts of sulfuric ester of xylan, the steps comprising oxidizing the aqueous solution of the a salt of sulfuric acid ester of highly polymeric xylan in an aqueous solution of H202 and H2S04 to depolymerize such highly polymeric xylan ester partially, dialyzing the depolymerized product and fractioning an aqueous mixture of the dialyzate with an organic water miscible solvent to obtain a fraction having a ZD value between 0.0030 and 0.015 (The value of ZD is proportional to the degree of polymerization and consequently, serves as an index of the molecular weight or length of the chain of the xylan sulphuric acid esters) and sulfur content of 13.5 to 17%. The process as disclosed therein does not describe about the molecular weight of product after getting step-2 or even of final product. The patent only characterized the final product by ZD value and sulfur content.
US 4713373 disclosed novel xylan sulfates having a sulfation degree between
1.5 and 2.0 and an apparent molecular weight from about 2000-5000.The process disclosed the ultra filtration technique for the preparation of above claimed compound. Moreover the process does not provide the desired pentosan polysulfate sodium with molecular weight of 4000 to 6000 Dalton specifically.
WO2008107906 disclosed process for the preparation of Pentosan Polysulfate and an amorphous form of Pentosan Polysulfate sodium with molecular weight of about 3000 to 10000 Dalton. In the process xylan treated with chlorosulfonic acid in the presence of pyridine followed by addition of alkali in the presence of alcoholic solvent to obtain salt of sulfuric acid ester of xylan. Oxidizing the salt with the mixture of H2S04 and 30% ¾02 to obtain depolymerized crude pentosan polysulfate Sodium of Formula (I). Purifying depolymerized crude pentosan polysulfate sodium by filtration through NF membrane system. Lyophilizing the product to obtain amorphous form of pentosan polysulfate sodium. Moreover the process does not provide the desired pentosan polysulfate sodium with molecular weight of 4000 to 6000 Dalton specifically WO2009087581 disclosed process for the preparation of Pentosan Polysulfate or salt thereof comprising a step of treating xylan with chlorosulfonic acid in the presence of picoline followed by addition of alkali in the presence of alcoholic solvent to obtain salt of sulfuric acid ester of xylan. Oxidizing the salt with the mixture of
H2S04 and 30% H202 to obtain depolymerized crude pentosan polysulfate of Formula (I) or its salt, purifying depolymerized crude pentosan polysulfate of Formula (I) or its salt by filtration through NF membrane system.
WO 2009047699 also described a process for the preparation of an amorphous form of Pentosan Polysulfate Sodium comprising steps of dissolving crude pentosan polysulfate sodium having a molecular weight of 4000 to 6000 Dalton in water (step I) followed by spray drying the solution to obtain amorphous Pentosan Polysulfate Sodium.
Thus the prior processes described above for the preparation of Pentosan Polysulfate Sodium is tedious and costly. So, there is a need in the art to develop a process for preparation of Pentosan Polysulfate Sodium which provides good yield, simple, convenient, economical and easily applicable at an industrial scale.
We herein disclosed a purification method of Pentosan Polysulfate Sodium Formula (I) by diluting the aqueous solution with excess of water followed by DiaAlteration through membrane system and isolation of resulting mixture provides high yield with desired molecular weight of 4000-6000 Dalton.
OBJECTS OF THE INVENTION
An object of the present invention is to provide an improved process for preparing Pentosan Polysulfate Sodium.
It a further object of the present invention to provide a process for purification of Pentosan Polysulfate Sodium using suitable technique.
It a further object of the present invention to provide an improved process for isolation of Pentosan Polysulfate Sodium using suitable solvents.
The above and other embodiments are further described in the following paragraphs.
DETAILED DESCRD7TION
The above and other objects of the present invention are achieved by an improved process for preparing Pentosan Polysulfate Sodium of Formula (I), comprising steps of: i) reacting xylan with chlorosulfonic acid in the presence of pyridine followed by addition of alkali in the presence of alcoholic solvent to obtain sodium salt of sulfuric acid ester of xylan.
ii) depolymerizing the salt of sulfuric acid ester of xylan with the mixture of H2S04 and an oxidizing agent to obtain depolymerized crude Pentosan Polysulfate Sodium of Formula (IV)
iii) purifying the depolymerized aqueous solution of Formula (IV) by diluting with excess of water followed by diafiltration through suitable membrane system
iv) isolation of the product obtained in the step-(iii) from suitable solvents to obtain pure Pentosan Polysulfate Sodium of Formula (I)
Accordingly, the invention provides a process for the preparation of Pentosan Polysulfate sodium of Formula (I) as depicted following steps:
The steps comprises:
i) reacting xylan with chlorosulfonic acid in the presence of pyridine followed by addition of suitable alkali in the presence of suitable alcoholic solvent to obtain sodium salt of sulfuric acid e
Formula-Ill
The suitable solvent used in step-(i) may be selected from (Q-C5) alcohols such as methanol, ethanol, propanol, isopropanol, butanol or mixtures thereof. The most preferably is methanol.
The suitable alkali used in step-(i) is selected from alkali metal salt of carbonates, bicarbonates, hydroxide or mixtures thereof. The most preferably is sodium hydroxide.
ii) reacting the compound of Formula (III) with a mixture of suitable oxidizing agents and H2S04 to obtain the depolymerized compound of Formula (IV)
Formula-IV
Average M.W. 3500-6000 D
The suitable oxidizing agent used in step-(ii) may be selected from peroxides such as hydrogen peroxides and the like; halogenated oxidizing agents such as sodium hypochlorite, sodiumhypobromide & the like; metallic oxidizing reagents such as osmium tetraoxide, chromates, permanganates, tungestates & the like; periodic acids or salts of periodic acids such as sodium metaperiodate, potassium metaperiodate & the likes either independently or in a suitable mixtures thereof.
iii) purifying the depolymerized aqueous solution of Formula (IV) obtained in step-(ii) by diluting with excess of water to 0.25 - 2.5 % followed by Dia-filteration through suitable membrane system to obtain compound of formula (V)
Average M.W. 3500-6000 D
Formula-V
Average M.W. 4000-6000 D
Suitable diafiltration membranes as are known in the art may be used. The diafiltration can be done continuously or discontinuously. Such techniques are well within the ability of a skilled person. For the present invention the suitable membrane
system in the filtration step as discussed herein is carried out by using pure flow-U30S ultra filtration membrane. This advanced thin film composite ultra filtration membrane has a filtration range of less than 4 KD, preferably 1 D - 4 KD and more preferably 2.8 KD nominal MWCO (Molecular wt. cut off). It removes most of bivalent ions, inorganic salts and unreacted low molecules of solute.
iv) isolation of the compound of Formula (V) in suitable solvent to obtain compound of Formula (I)
Formula-I
Average M.W. 4000-6000 D
The suitable solvent for isolation used in step-(iv) may be selected from hydrocarbons such as toluene, xylene, n-heptane, cyclohexane, n-hexane; nitriles such as acetonitrile, propionitrile; (Q to C5) alcohols such as methanol, ethanol, isopropanol and the like; ketones such as acetone, methyl ethyl ketones, methyl isobutyl ketones & the like; amides such as Ν,Ν-dimethyl acetamides, dimethyl formamide N-methyl-2- pyrrolidinone and the likes either independently or suitable mixtures thereof.
Thus, the present invention also encompasses a process for the purification of compound of formula (IV). Purification of compound of the Formula (IV) followed by Diafilteration through suitable membrane system removes most of bivalent ions, inorganic salts and unreacted low molecules of solute. So, one of the significance of the present process by using the purification technique is to get the desired molecular weight of pure compound of Formula (I) in a pure form.
The process of the present invention is further described by the following non- limiting examples, which provides the preferred mode of carrying out the process of the present invention. It is to be appreciated that several alterations, modifications, optimizations of the processes described herein are well within the scope of a person
skilled in the art should be construed to be within the scope of the present inventive concept as is disclosed anywhere in the specification.
Exam pie- 1
Process for the preparation of Pentosan Polysulfate Sodium (Formula III)
Chlorosulfonic acid (350 g) was added slowly in pyridine (870 g) at 25 to 60°C.
The solution was maintained for 15 minutes. Xylan (100 g) was added at 25-60 °C. The reaction mixture was heated at 80-85°C. The reaction mixture was stirred for next 2-4 hours at 80-85°C. The reaction mixture was cool down to 55-60 °C. Methanol (1600 mL) was slowly added. The mass was cooled at 25-35°C and stirred for 1-2 hours. The solid mass was filtered and washed with methanol. The wet cake was taken into RO (Reverse Osmosis) water and stirred to get clear solution. Previously prepared sodium chlorite (28 g) and Cone, hydrochloric acid (22 mL) solution was added into above solution in 30 minutes and stirred for 4-6 hours and quenched into methanolic sodium hydroxide solution and was stirred for 1-3 hours at 25-35°C. To the reaction mixture acetic acid was added to get pH 6 to 7 and stirred with 1 hour. The solid mass was filtered and washed with methanol. The wet cake was taken into methanol (500 mL) and stirred for next 1 hour. The solid mass was filtered and washed with methanol. The wet solid was dried at 50-55°C under vacuum to get 180 to 240 g product). GPC average molecular weight. 30000 to 50000D (Yield: 70 to 94 %).
Examp e-2
Process for the preparation of Pentosan Polysulfate Sodium (Formula IV)
In a 2-lit R. B. Flask water (500 mL) and Pentosan Polysulfate Sodium (Formula III) (200 g) were added. The solution was heated to 95-100°C. The solution of 25 to 33% hydrogen peroxide (15.85 g), sulfuric acid (0.9 g) and RO water (100 mL) was added in 30 minutes at 95-100 °C. The reaction mixture was further stirred at 95- 100°C next 4 to 7 hours (till one achieves average M.W. 3500 to 6000 D). The reaction mixture was cooled to 15 to 20°C. Activated carbon (5.0 g) and 30% sodium hydroxide solution was added in to reaction mass below 30°C. The reaction mixture was stirred for next 4-6 hours, adjust pH 6 to 7 with dilute acetic acid. The mass was filtered and collected aqueous solution for next step (Diafilteration) without isolation of product. GPC average molecular weight 3500 to 6000 D.
Example-3
Process for the preparation of Pentosan Polysulfate Sodium (Formula V)
In a 50-lit HDPE Tank equipped with mechanical stirrer, RO water (40 L) and Pentosan Polysulfate Sodium (Formula IV) solution obtained in example-2 above, (approx 1200 mL) were charged. The reaction mixture was stirred for 15 minutes. The solution was passed through 2.8 D membrane and removed free sulfate and lower molecular weight product were collected in permeate HDPE Tank. After removing sulfate salt and lower molecular weight product, solution was passed through RO (Reverse Osmosis) membrane to get concentrated solution by removing the excess of water from the solution. Finally the product in retanate (approx. 7 to 8% solution, 500 to 550 mL) was; used for next step without isolation of product. GPC average molecular weight 4000 to 6000 D.
Example-4
Process for the preparation of Pentosan Polysulfate Sodium (Formula I)
In a 2-lit R. B. Flask Pentosan Polysulfate Sodium (Formula V) obtained in example-3 above (550 mL) was added. Water was distilled out below 65°C under vacuum. Finally leaving behind approximately 130 to 150 mL residual product. The reaction mixture was cooled to 25 to 30°C. Methanol (800 mL) was added slowly over 3-4 hours. The solid mass was stirred for next 3 hours. The solid was filtered and washed with methanol. The product was dried at 60 to 65°C for 10 hours to get 60 to 70 g pure product. GPC average molecular weight 4000 to 6000 D. (Yield: 90 to 98 %) Further aspect of the present invention is to provide an improved process of isolation method for preparation of Pentosan polysulfate Sodium of Formula (I)
Example-5
To a 2-lit R. B. Flask Pentosan Polysulfate Sodium (80 g) and Cyclohexane (800 mL) was charged. The solution was heated at 80°C for 1 hour. Cyclohexane (400 mL) was distilled at 80 to 85°C. Solution was cooled down to 25-35°C and stirred for 30 min. The solid product was filtered and washed with Cyclohexane. The wet solid was dried at 65 to 75°C to get product 72 to 80 g (Yield: 90 to 100 %).
Example -6
To a 50 mL R. B. flask Pentosan Polysulfate Sodium (2 g) was dissolved in RO water under stirring at 25-35°C. Acetonitrile was added dropwise in 1 hour. The reaction mixture was stirred at 25-35°C for next 2 hours. The solid product was filtered and washed with Acetonitrile. The wet solid was dried at 65 to75°C to get product 1.70 g (Yield: 85 %).
Example - 7
To a 50 mL R. B. flask Pentosan Polysulfate Sodium (2 g) was dissolved in RO water under stirring at 25-35°C. Acetone was added dropwise in 1 hour. The reaction mixture was stirred at 25-35°C for next 2 hours. The solid product was filtered and washed with Acetone. The wet solid was dried at 65 to75°C to get product 1.78 g. (Yield: 89 %).
Example - 8
To a 50 mL R. B. flask Pentosan Polysulfate Sodium (2 g) was dissolved in RO water under stirring at 25-35°C. Ν,Ν-dimethyl acetamide was added dropwise in 1 hour. The reaction mixture was stirred at 25-35°C for next 2 hours. The solid product was filtered and washed with N, N-dimethyl acetamide. The wet solid was dried at 65 to75°C to get product 1.62 g (Yield: 81 %).
Example - 9
To a 50 mL R. B. Flask Pentosan Polysulfate Sodium (2 g) was dissolved in RO water under stirring at 25-35°C. Ethanol was added dropwise in 1 hour. The reaction mixture was stirred at 25-35°C for next 2 hours. The solid product was filtered and washed with ethanol. The wet solid was dried at 65 to75°C to get product 1.82 g. (Yield: 91 %).
Example - 10
To a 50 mL R. B. flask Pentosan Polysulfate Sodium (2 g) was dissolved in RO water under stirring at 25-35°C. Isopropanol was added dropwise in 1 hour. The reaction mixture was stirred at 25-35°C for next 2 hours. The solid product was filtered and washed with isopropanol. The wet solid was dried at 65 to75°C to get product 1.69 g (Yield: 84.5 %).
Example - 11
To a 50 mL R. B. flask Pentosan Polysulfate Sodium (2 g) was dissolved in RO water under stirring at 25-35°C. 1-propanol was added dropwise in 1 hour. The reaction mixture was stirred at 25-35°C for next 2 hours. The solid product was filtered and washed with 1-propanol. The wet solid was dried at 65 to75°C to get product 1.83 g (Yield: 91.5 %).
Claims
laim:
A process for the preparation of Pentosan Polysulfate Sodium of Formula (I) comprising steps of:
i) reacting xylan with chiorosulfonic acid in the presence of pyridine followed by addition of suitable alkali in the presence of suitable alcoholic solvent to obtain sodium salt of sulfuric acid ester of xylan Formula (III)
Formula-Ill
ϋ) reacting the compound of Formula (III) with a mixture of suitable oxidizing agents and H2S04 to obtain the depolymerized compound of Formula (IV)
Formula-IV
Average M.W. 3500-6000 D
iii) purifying the depolymerized aqueous solution of Formula (IV) obtained in step (ii) by diluting with excess of water followed by diafiltration through suitable membrane system to obtain compound of Formula (V)
Average M.W. 3500-6000 D
Formula-V
Average M.W. 4000-6000 D
iv) isolation of the compound of Formula (V) in suitable solvent to obtain compound of Formula (I)
Formula-I
Average M.W. 4000-6000 D
2. The process for the preparation of compound of Formula (I) as claimed in claim 1 step (i), wherein the suitable alkali is selected from alkali metal salt of carbonates, bicarbonates, hydroxide or mixtures thereof.
3. The process for the preparation of compound of Formula (I) as claimed in claim 1 step (i), wherein the suitable solvent selected from alcohols such as methanol, ethanol, propanol, isopropanol, butanol or mixtures thereof.
4. The process for the preparation of compound of Formula (I) as claimed in claim 1 step (ii), wherein the suitable oxidizing agent selected from peroxides, halogenated oxidizing agents, metallic oxidizing reagents, periodic acids or salts of periodic acids or in suitable mixtures thereof.
5. The process for the preparation of compound of Formula (I) as claimed in claim 1 step (iii), wherein the purification of depolymerized aqueous solution of formula (IV) obtain in step-2 by diluting with excess of water to 0.25 - 2.5 % followed by diafiltration membrane system.
6. The process for the preparation of compound of Formula (I) as claimed in claim 1 step (iii), wherein the diafiltration membrane is having a filtration capacity of less than 4 KD, preferably 1 KD - 4 KD.
7. The process as claimed in claim 6, wherein the diafiltration membrane having a filtration capacity of preferably 2.8 KD.
8. The process for the preparation of compound of Formula (I) as claimed in claim 1 step (iv), wherein the suitable solvent for isolation is selected from hydrocarbons, nitriles, (Ci to C5) alcohols, ketones, amides and suitable mixtures thereof.
9. The process as claimed in claim 8, wherein the hydrocarbons are selected from toluene, xylene, n-heptane, cyclohexane, n-hexane and suitable mixtures thereof.
10. The process as claimed in claim 8, wherein the nitriles are selected from acetonitrile, propionitrile and suitable mixtures thereof.
11. The process as claimed in claim 8, wherein the alcohols are selected from methanol, ethanol, isopropanol, butanol and suitable mixtures thereof.
12. The process as claimed in claim 8, wherein the ketones are selected from acetone, methyl ethyl ketones, methyl isobutyl ketones and suitable mixtures thereof.
13. The process as claimed in claim 8, wherein the amides are selected from N,N- dimethyl acetamides, dimethylformamide N-methyl-2-pyrrolidinone and suitable mixtures thereof.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN499/MUM/2011 | 2011-02-23 | ||
| IN499MU2011 | 2011-02-23 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012114349A1 true WO2012114349A1 (en) | 2012-08-30 |
Family
ID=44532997
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IN2011/000393 WO2012114349A1 (en) | 2011-02-23 | 2011-06-13 | An improved process for the preparation of pentosan polysulfate sodium |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2012114349A1 (en) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103130917A (en) * | 2013-03-21 | 2013-06-05 | 苏州鸿洋医药科技有限公司 | Preparation method of pentosan polysulfuric acid and salt thereof |
| ITMI20130112A1 (en) * | 2013-01-24 | 2014-07-25 | Chemi Spa | QUALIFICATION METHOD OF PENTOSAN POLYPHOSPHATE PREPARATIONS, ITS RAW MATERIALS AND PRODUCTION PROCESSES |
| WO2016191698A1 (en) * | 2015-05-27 | 2016-12-01 | Vanguard Therapeutics, Inc. | Pentosan polysulfate sodium for the treatment of sickle cell disease |
| WO2018159580A1 (en) * | 2017-02-28 | 2018-09-07 | 王子ホールディングス株式会社 | Pentosan polysulfate, pharmaceutical composition and anticoagulant |
| CN109793751A (en) * | 2019-01-15 | 2019-05-24 | 广西壮族自治区中国科学院广西植物研究所 | Application of arabinoxylan sulfonate in preparation of medicine for treating osteoarthritis |
| WO2021050320A1 (en) * | 2019-09-09 | 2021-03-18 | Harrow Ip, Llc | Pharmaceutical compositions comprising heparinoids and methods for preparing thereof |
| US11278485B2 (en) | 2017-05-31 | 2022-03-22 | Oji Holdings Corporation | Moisturizing topical preparation |
| US11286272B2 (en) | 2016-08-31 | 2022-03-29 | Oji Holdings Corporation | Production method for acidic xylooligosaccharide, and acidic xylooligosaccharide |
| US11312790B2 (en) | 2016-08-31 | 2022-04-26 | Oji Holdings Corporation | Production method for pentosan polysulfate |
| US11344570B2 (en) | 2017-12-20 | 2022-05-31 | Oji Holdings Corporation | Pentosan polysulfate and medicine containing pentosan polysulfate |
| US11390693B2 (en) | 2017-09-12 | 2022-07-19 | Oji Holdings Corporation | Pentosan polysulfate and method for producing pentosan polysulfate |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2689848A (en) | 1951-02-06 | 1954-09-21 | Wander Ag Dr A | Salts of sulfuric acid esters of xylan |
| US4699900A (en) * | 1983-03-24 | 1987-10-13 | Sanofi S.A. | Xylane sulfates, process for their preparation, and anti-thrombosis and hypolipemic activity thereof |
| US4713373A (en) | 1984-11-07 | 1987-12-15 | Sanofi S.A. | Xylan sulfates of low molecular weight, process for their preparation and medicaments containing them |
| US5145841A (en) * | 1987-03-19 | 1992-09-08 | Arthropharm Pty. Limited | Anti-inflammatory compounds and compositions |
| WO2008107906A1 (en) | 2007-03-06 | 2008-09-12 | Alembic Limited | Process for the preparation of pentosan polysulfate or salts thereof |
| WO2009047699A1 (en) | 2007-10-10 | 2009-04-16 | Alembic Limited | An improved process for the preparation of an amorphous form of pentosan polysulfate or salts thereof |
| WO2009087581A1 (en) | 2008-01-04 | 2009-07-16 | Alembic Limited | An improved process for the preparation of pentosan polysulfate or salts thereof |
| WO2010000013A1 (en) * | 2008-07-04 | 2010-01-07 | Parnell Laboratories (Aust) Pty Ltd | A sulfated polysaccharide compound and the preparation and use thereof |
-
2011
- 2011-06-13 WO PCT/IN2011/000393 patent/WO2012114349A1/en active Application Filing
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2689848A (en) | 1951-02-06 | 1954-09-21 | Wander Ag Dr A | Salts of sulfuric acid esters of xylan |
| US4699900A (en) * | 1983-03-24 | 1987-10-13 | Sanofi S.A. | Xylane sulfates, process for their preparation, and anti-thrombosis and hypolipemic activity thereof |
| US4713373A (en) | 1984-11-07 | 1987-12-15 | Sanofi S.A. | Xylan sulfates of low molecular weight, process for their preparation and medicaments containing them |
| US5145841A (en) * | 1987-03-19 | 1992-09-08 | Arthropharm Pty. Limited | Anti-inflammatory compounds and compositions |
| WO2008107906A1 (en) | 2007-03-06 | 2008-09-12 | Alembic Limited | Process for the preparation of pentosan polysulfate or salts thereof |
| WO2009047699A1 (en) | 2007-10-10 | 2009-04-16 | Alembic Limited | An improved process for the preparation of an amorphous form of pentosan polysulfate or salts thereof |
| WO2009087581A1 (en) | 2008-01-04 | 2009-07-16 | Alembic Limited | An improved process for the preparation of pentosan polysulfate or salts thereof |
| WO2010000013A1 (en) * | 2008-07-04 | 2010-01-07 | Parnell Laboratories (Aust) Pty Ltd | A sulfated polysaccharide compound and the preparation and use thereof |
Cited By (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11242412B2 (en) | 2013-01-24 | 2022-02-08 | Chemi S.P.A. | Method for the qualification of preparations of pentosan polysulfate, raw materials and production processes thereof |
| ITMI20130112A1 (en) * | 2013-01-24 | 2014-07-25 | Chemi Spa | QUALIFICATION METHOD OF PENTOSAN POLYPHOSPHATE PREPARATIONS, ITS RAW MATERIALS AND PRODUCTION PROCESSES |
| WO2014114723A1 (en) * | 2013-01-24 | 2014-07-31 | Chemi S.P.A. | Method for the qualification of preparations of pentosan polysulfate, raw materials and production processes thereof |
| US10407515B2 (en) | 2013-01-24 | 2019-09-10 | Chemi S.P.A. | Method for the qualification of preparations of pentosan polysulfate, raw materials and production processes thereof |
| CN103130917A (en) * | 2013-03-21 | 2013-06-05 | 苏州鸿洋医药科技有限公司 | Preparation method of pentosan polysulfuric acid and salt thereof |
| WO2016191698A1 (en) * | 2015-05-27 | 2016-12-01 | Vanguard Therapeutics, Inc. | Pentosan polysulfate sodium for the treatment of sickle cell disease |
| US11312790B2 (en) | 2016-08-31 | 2022-04-26 | Oji Holdings Corporation | Production method for pentosan polysulfate |
| US11286272B2 (en) | 2016-08-31 | 2022-03-29 | Oji Holdings Corporation | Production method for acidic xylooligosaccharide, and acidic xylooligosaccharide |
| WO2018159580A1 (en) * | 2017-02-28 | 2018-09-07 | 王子ホールディングス株式会社 | Pentosan polysulfate, pharmaceutical composition and anticoagulant |
| KR102520754B1 (en) | 2017-02-28 | 2023-04-11 | 오지 홀딩스 가부시키가이샤 | Polypentoic acid, pharmaceutical composition and anticoagulant |
| CN110325553B (en) * | 2017-02-28 | 2021-10-08 | 王子控股株式会社 | Pentosan polysulfate, pharmaceutical composition and anticoagulant |
| KR20190120216A (en) * | 2017-02-28 | 2019-10-23 | 오지 홀딩스 가부시키가이샤 | Polysulfate pentosan, pharmaceutical compositions and anticoagulants |
| US11274165B2 (en) | 2017-02-28 | 2022-03-15 | Oji Holdings Corporation | Pentosan polysulfate, pharmaceutical composition, and anticoagulant |
| CN110325553A (en) * | 2017-02-28 | 2019-10-11 | 王子控股株式会社 | Pentosulfate, pharmaceutical composition and anti-coagulants |
| US11278485B2 (en) | 2017-05-31 | 2022-03-22 | Oji Holdings Corporation | Moisturizing topical preparation |
| US11390693B2 (en) | 2017-09-12 | 2022-07-19 | Oji Holdings Corporation | Pentosan polysulfate and method for producing pentosan polysulfate |
| US11344570B2 (en) | 2017-12-20 | 2022-05-31 | Oji Holdings Corporation | Pentosan polysulfate and medicine containing pentosan polysulfate |
| CN109793751A (en) * | 2019-01-15 | 2019-05-24 | 广西壮族自治区中国科学院广西植物研究所 | Application of arabinoxylan sulfonate in preparation of medicine for treating osteoarthritis |
| CN109793751B (en) * | 2019-01-15 | 2021-02-26 | 广西壮族自治区中国科学院广西植物研究所 | Application of arabinoxylan sulfonate in preparation of medicine for treating osteoarthritis |
| WO2021050320A1 (en) * | 2019-09-09 | 2021-03-18 | Harrow Ip, Llc | Pharmaceutical compositions comprising heparinoids and methods for preparing thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2012114349A1 (en) | An improved process for the preparation of pentosan polysulfate sodium | |
| JPH0558002B2 (en) | ||
| EP0076279B1 (en) | Method for obtaining biologically active mucopolysaccharides of high purity by controlled depolymerization of heparin | |
| CA2501546C (en) | Heparin-derived polysaccharide mixtures, preparation thereof and pharmaceutical compositions containing same | |
| WO2008107906A1 (en) | Process for the preparation of pentosan polysulfate or salts thereof | |
| CA1183132A (en) | Heparin esters used for the preparation of drugs and preparation process | |
| JP2005507438A (en) | Extraction of polysaccharides from vegetables and microbial substances | |
| WO2009087581A1 (en) | An improved process for the preparation of pentosan polysulfate or salts thereof | |
| FR2593801A1 (en) | Process for the preparation of basic zinc carbonate | |
| OA12340A (en) | Heparin - derived polysaccharide mixtures, their preparation and pharmaceutical compositions containing them. | |
| CN103554303B (en) | A kind of method of purifying cm-chitosan | |
| EP3724235B1 (en) | Process for the preparation of low molecular weight heparin | |
| EP0116251B1 (en) | Process for the depolymerization and sulfation of polysaccharides | |
| JPH04117401A (en) | Production of sulfonated chitosan | |
| WO2003046014A1 (en) | Method for sulphonation of compounds comprising free hydroxyl (oh) groups or primary or secondary amines | |
| EP0148057B1 (en) | Chitosan-6-sulphate and process for its preparation | |
| US10889654B2 (en) | Acylation process | |
| RU2441025C1 (en) | Method of producing low-molecular pectin | |
| JP7674376B2 (en) | Method for the direct sulfation of polysaccharides in an ecologically acceptable solvent | |
| US7345165B2 (en) | Method for preparing water-soluble free amine chitosan | |
| Heeres et al. | Synthesis and reduction of 2-nitroalkyl polysaccharide ethers | |
| RU2546965C1 (en) | Method of obtaining sulphated derivatives of arabinogalactan | |
| RU2441024C1 (en) | Method of producing low-molecular pectin | |
| EP0253740A1 (en) | Process for the preparation of an aqueous solution of the sodium salt of methionine | |
| FR3079831A1 (en) | PROCESS FOR EXTRACTING OLIGOFUCANS AND ITS APPLICATIONS |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11749557 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11749557 Country of ref document: EP Kind code of ref document: A1 |