WO2012113967A1 - Gsk-3 inhibitors that can be used in neurodegenerative and inflammatory diseases, cancer, diabetes and in regenerative processes - Google Patents
Gsk-3 inhibitors that can be used in neurodegenerative and inflammatory diseases, cancer, diabetes and in regenerative processes Download PDFInfo
- Publication number
- WO2012113967A1 WO2012113967A1 PCT/ES2012/070119 ES2012070119W WO2012113967A1 WO 2012113967 A1 WO2012113967 A1 WO 2012113967A1 ES 2012070119 W ES2012070119 W ES 2012070119W WO 2012113967 A1 WO2012113967 A1 WO 2012113967A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- disease
- formula
- diabetes
- cancer
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
- A61K31/404—Indoles, e.g. pindolol
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/44—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having three double bonds between ring members or between ring members and non-ring members
- C07D207/444—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having three double bonds between ring members or between ring members and non-ring members having two doubly-bound oxygen atoms directly attached in positions 2 and 5
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/04—Indoles; Hydrogenated indoles
- C07D209/10—Indoles; Hydrogenated indoles with substituted hydrocarbon radicals attached to carbon atoms of the hetero ring
- C07D209/14—Radicals substituted by nitrogen atoms, not forming part of a nitro radical
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
Definitions
- GSK-3 inhibitors useful in neurodegenerative, inflammatory diseases, cancer, diabetes and in reqenerative processes.
- the present invention relates to a series of compounds that are derived from maleimides and that are capable of inhibiting the GSK-3 enzyme in micro and nanomolar ranges reversibly or irreversibly. These compounds, therefore, are useful for the manufacture of a medicament for the treatment and / or prevention of diseases in which GSK-3 is involved, such as neurodegenerative diseases, inflammatory diseases, cancer, diabetes, as well as promoting various processes. regenerative diseases, inflammatory diseases, cancer, diabetes, as well as promoting various processes.
- Glycogen synthase kinase 3 is an enzyme in the kinase family that catalyzes phosphorylation of serine or threonine residues in various substrates. It was originally discovered for its role in glycogen biosynthesis, to which it owes its name [Rylatt, DB, Aitken, A., Bilham, T., Condom, GD, Embi, N., Cohen, P. "Glycogen Synthase Kinase 3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase ". Eur J Biochem. 1980 107, 519-527.].
- the enzyme is involved in the regulation of several cell signaling pathways, including Wnt pathways, the cell division cycle, DNA damage response, cell death and survival, and neuronal differentiation among others [ Van Waue, J., Haefner, B. "Glycogen Synthase Kinase-3 as drug target: from wallflower to center of attention”. Drug News Perspect. 2003 16, 557-565]. Recent studies show that overexpression of GSK-3 is sufficient to induce neuronal death [Hetmán, M., Cavanaugh, JE, Kimelman, D., Xia, Z. "Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal ". J Neuroso.
- GSK-3 plays an important role in cell proliferation and differentiation signals because of its essential role in the signaling pathways of RTK, Wnt and Shh.
- MSC Mesemchymal stem cells
- GSK-3 promotes cardiomyocyte proliferation in the heart of adults
- inhibiting GSK3 could be a strategy to promote cardiac regeneration in pathological states [Woulfe KC, Gao E, Lal H , Harris D, Fan Q, Vagnozzi R, DeCaul M, Shang X, Patel S, Woodgett JR, Forcé T, Zhou J. "Glycogen synthase kinase-3beta regulates post-myocardial infarction remodeling and stress-induced cardiomyocyte proliferation in vivo" Circ Res. 2010 May 28; 1 06 (10): 1 635-45].
- the present invention presents a family of four compounds, and their mode of production, which possess the ability to inhibit the GSK-3 enzyme in micro and nanomolar order.
- some of these compounds according to a model adapted to the laboratory, are capable of crossing the blood-brain barrier, a key feature for compounds that can serve as drugs for diseases of the central nervous system.
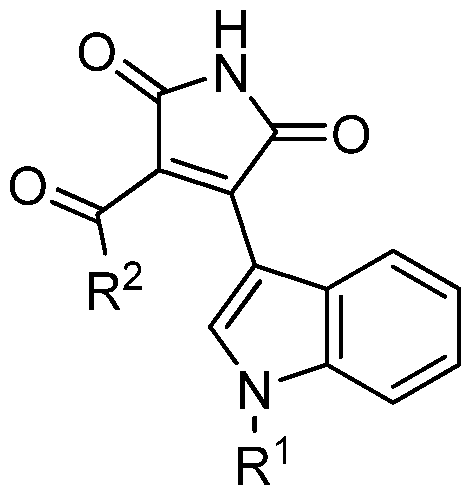
- the present invention relates to a compound of formula (I):
- R 1 is selected from H or C C 0 alkyl and R 2 is selected from dC-io O alkyl Crdo alkenyl, optionally substituted by halogen, or its pharmaceutically acceptable salts, solvates or isomers.
- alkyl refers, in the present invention, to radicals of hydrocarbon chains, linear or branched, having 1 to 10 carbon atoms, preferably 1 to 4, and which are attached to the rest of the molecule by a single bond, for example, methyl, ethyl, n-propyl, / -propyl, n-butyl, tere-butyl, sec-butyl, n-pentyl, n-hexyl, etc.
- the alkyl groups may be optionally substituted by one or more halogen atoms, that is, fluorine, chlorine, bromine and iodine, preferably bromine.
- alkenyl refers to radicals of linear or branched hydrocarbon chains, having 1 to 10 carbon atoms, preferably from 1 to 4 and containing one or more double carbon-carbon bonds, for example, vinyl, 1-propenyl, allyl, isoprenyl, 2-butenyl, 1, 3- butadienyl, etc. Alkenyl radicals may be optionally substituted by one or more halogen atoms.
- R 1 is H.
- R 1 is CC 4 alkyl and more preferably methyl.
- R 2 is CrC 4 alkyl and more preferably methyl.
- R 2 is -CH 2 Br.
- the compound of formula (I) is selected from the list comprising:
- the compounds of the present invention represented by formula (I) may include isomers, depending on the presence of multiple bonds (eg, Z, E), including optical isomers or enantiomers, depending on the presence of chiral centers.
- the individual isomers, enantiomers or diastereoisomers and mixtures thereof fall within the scope of the present invention, that is, the term isomer also refers to any mixture of isomers, such as diastereomers, racemic, etc., even their optically isomers. assets or mixtures in different proportions thereof.
- the individual enantiomers or diastereoisomers, as well as mixtures thereof, can be separated by conventional techniques.
- the compounds of the invention may be in crystalline form as free compounds or as solvates.
- solvate includes both pharmaceutically acceptable solvates, that is, solvates of the compound of formula (I) that can be used in the manufacture of a medicament, as pharmaceutically acceptable solvates, which may be useful in the preparation of pharmaceutically acceptable solvates or salts.
- pharmaceutically acceptable solvate is not critical as long as it is pharmaceutically acceptable.
- the solvate is a hydrate. Solvates can be obtained by conventional solvation methods known to those skilled in the art.
- Another aspect of the invention relates to the use of a compound of formula (I) for the manufacture of a medicament.
- Another aspect of the invention relates to the use of a compound of formula (I) for the manufacture of a medicament for the treatment and / or prevention of a disease that is selected from neurodegenerative diseases, inflammatory diseases, cancer or diabetes.
- the neurodegenerative disease is selected from Alzheimer's disease, Parkinson 's disease, amyotrophic lateral sclerosis, cerebral ischemia, post-encephalitic Parkinsonism, dystonia, Tourette 's, pathologies limb movements newspapers, restless leg syndrome, deficit disorders of attention with hyperactivity, Hungtinton's disease, progressive supranuclear paralysis, Pick's disease, frontotemporal dementia, neuromuscular diseases.
- the inflammatory disease is selected from Crohn's disease, ulcerative colitis, arthritis, atherosclerosis, vasculitis, multiple sclerosis.
- the cancer is selected from glioblastoma, leukemia, lymphoma, lung, breast, prostate or colon cancer and in general from any cancerous or metastatic process in which GSK-3 is involved.
- diabetes is selected as non-dependent insulin type II diabetes.
- Another aspect of the invention is the use of a compound of formula (I) for the manufacture of a medicament to promote regenerative processes.
- the regenerative processes are selected from those that promote the differentiation of the stem cells of the nervous system, the hematopoietic system, the bone system, the myocardium.
- the compounds of formula (I), their salts, solvates or isomers will preferably be found in a pharmaceutically acceptable or substantially pure form, that is, having a pharmaceutically acceptable level of purity excluding pharmaceutical additives. normal such as diluents and carriers, and not including material considered toxic at normal dosage levels.
- the purity levels for the active ingredient are preferably greater than 50%, more preferably greater than 70%, and still more preferably greater than 90%. In a preferred embodiment, they are greater than 95% of the compound of formula (I), or of its salts, solvates or isomers.
- the compounds of the present invention of formula (I) can be obtained according to the procedure described in the document [Faul, M.M., Winneroski, L.L., Brussee, J., Krumrich, C.A. "A new one step synthesis of maleimides by condensation of glyoxylate esters with acetamides". Tetrahedron Letters 1999 40, 1 109-1 1 12].
- the present invention also relates to pharmaceutical compositions comprising at least one compound of the invention, or an isomer, a pharmaceutically acceptable salt, a derivative or a prodrug. thereof, together with a pharmaceutically acceptable carrier or carrier, an excipient or a vehicle, for administration to a patient.
- the pharmaceutical composition further comprises another active ingredient.
- compositions are the adjuvants and vehicles known to those skilled in the art and commonly used in the elaboration of therapeutic compositions.
- Another aspect of the invention relates to a compound of formula (I) for use as a medicament and particularly as a medicament for treating and / or preventing neurodegenerative diseases, inflammatory diseases, cancer or diabetes or for promoting regenerative processes.
- Another aspect of the invention is a method of treating a neurodegenerative disease, an inflammatory disease, cancer or diabetes, as well as a method of promoting cell regeneration, which comprises administering to a patient a therapeutically effective amount of a compound of formula (I) or of a pharmaceutical composition comprising it,
- the term "therapeutically effective amount” refers to the amount of the agent or compound capable of developing the therapeutic action determined by its pharmacological properties, calculated to produce the desired effect and, in general, will be determined, among other causes, due to the characteristics of the compounds, including the age, condition of the patient, the severity of the alteration or disorder, and the route and frequency of administration.
- the compounds described in the present invention, their salts, prodrugs and / or solvates as well as the pharmaceutical compositions containing them can be used together with other drugs, or active ingredients, additional to provide a combination therapy.
- Said additional drugs may be part of the same pharmaceutical composition or, alternatively, they may be provided in the form of a separate composition for simultaneous or non-simultaneous administration to the pharmaceutical composition comprising a compound of formula (I), or a salt, prodrug or solvate thereof.
- said therapeutic composition is prepared in the form of a solid form or aqueous suspension, in a pharmaceutically acceptable diluent.
- the therapeutic composition provided by this invention may be administered by any appropriate route of administration, for which said composition will be formulated in the pharmaceutical form appropriate to the route of administration chosen.
- the administration of the therapeutic composition provided by this invention is performed orally, topically, rectally or parenterally (including subcutaneously, intraperitoneally, intradermally, intramuscularly, intravenously, etc.).
- the pharmaceutical compositions are suitable for oral administration, in solid or liquid form.
- Possible forms for oral administration are tablets, capsules, syrups or solutions and may contain conventional excipients known in the pharmaceutical field, as aggregating agents (eg syrup, acacia, gelatin, sorbitol, tragacanth or polyvinyl pyrrolidone), fillers (eg lactose, sugar, corn starch, calcium phosphate, sorbitol or glycine), disintegrants (eg starch, polyvinyl pyrrolidone or microcrystalline cellulose) or a pharmaceutically acceptable surfactant such as sodium lauryl sulfate.
- aggregating agents eg syrup, acacia, gelatin, sorbitol, tragacanth or polyvinyl pyrrolidone
- fillers eg lactose, sugar, corn starch, calcium phosphate, sorbitol or glycine
- disintegrants eg starch, polyviny
- compositions for oral administration can be prepared by conventional methods of Galenic Pharmacy, as mixing and dispersion.
- the tablets can be coated following methods known in the pharmaceutical industry.
- the pharmaceutical compositions can be adapted for parenteral administration, as sterile solutions, suspensions, or lyophilized products of the invention, using the appropriate dose. Suitable excipients, such as pH buffering agents or surfactants, can be used.
- the aforementioned formulations can be prepared using conventional methods, such as those described in the Pharmacopoeias of different countries and in other reference texts.
- the administration of the compounds or compositions of the present invention can be performed by any suitable method, such as intravenous infusion and oral, intraperitoneal or intravenous routes. Oral administration is preferred for the convenience of patients and for the chronic nature of the diseases to be treated.
- the amount administered of a compound of the present invention will depend on the relative efficacy of the compound chosen, the severity of the disease to be treated and the weight of the patient. However, the compounds of this invention will be administered one or more times a day, for example 1, 2, 3 or 4 times daily, with a total dose between 0.1 and 1000 mg / kg / day. It is important to keep in mind that it may be necessary to introduce variations in the dose, depending on the age and condition of the patient, as well as modifications in the route of administration.
- the compounds and compositions of the present invention can be used together with other medicaments in combination therapies.
- the other drugs may be part of the same composition or of a different composition, for administration at the same time or at different times.
- Figure 1 Shows the linear correlation between described and experimental permeability of 10 commercial compounds using the PAMPA-Blood-brain barrier methodology.
- Enzyme inhibition assays were performed using the kinasa-glo® luminometric method methodology.
- Recombinant human enzyme GSK3 (n Q catalog 14-306) was purchased from Upstate (Dundee, UK).
- the prefosphorylated polypeptide was synthesized by American Peptide Inc (Sunnyvale, CA).
- the luminescent kinase kit (n Q catalog V671 1) was obtained from Promega. ATP and other reagents were purchased in Sigma-Aldrich (St. Louis, MO)
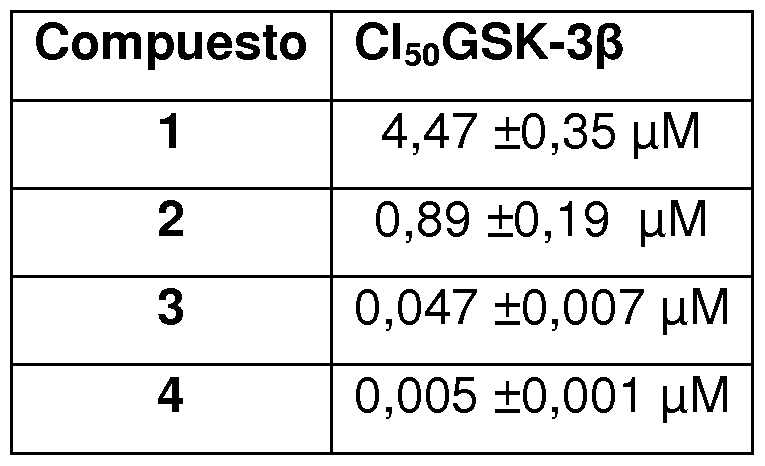
- the tests were performed in buffer using 96-well plates. 10 ⁇ of the compound to be tested (dissolved in dimethisulfoxide at a concentration of 1 mM, and in turn dissolved in buffer until the concentration necessary for the experiment) and 10 ⁇ (20ng) of the enzyme are added to each well followed by 20 ⁇ of buffer containing 25 ⁇ of the substrate and 1 ⁇ of ATP. The final concentration of DMSO in the experiment did not exceed 1%. After incubation for half an hour at 30 Q C for enzymatic reaction with the reagent 40 ⁇ -Glo kinase. The luminescence is measured after ten minutes using a POLARstar Optima multimode reader. The activity is proportional to the difference between total and consumed ATP. Inhibition activities were calculated based on maximum activity, measured in the absence of inhibitor. Table 1. Inhibitory concentration of the compounds.
- the four compounds evaluated show a very potent enzyme inhibition, with IC 5 values or that vary from the low micromolar to low nanomolar range, irreversible inhibitors (3 and 4) being more potent than reversible ones (1 and 2).
- the step from reversible inhibitor (1 and 2) to irreversible (3 and 4) implies an increase in the inhibition of GSK-3 by an order of magnitude, respectively.
- Central nervous system (CNS) penetration Experiment One vitro) determination of permeability using parallel artificial membranes (PAMPA) of blood brain penetration.
- PAMPA parallel artificial membranes
- the prediction of the permeability of various compounds on the central nervous system was determined using the methodology of parallel artificial membranes (PAMPA) [Di, L., Kerns, EH, Fan, K., McConnell, OJ, Carter, GT "High throughput artificial membrane permeability assay for blood-brain barrier” Eur. J. Med. Chem., 2003, 38, 223-232].
- PAMPA parallel artificial membranes
- Commercial compounds, pH 7.4 phosphate buffer (PBS), ethanol and dodecane were obtained from the Sigma, Acros organics, Merck, Aldrich and Fluka commercial houses.
- the porcine brain lipid was purchased from Avanti Polar Lipids.
- 96-well donor plate Multiscreen® IP Sterile P ⁇ ate membrane PDVF, pore size 0.45 ⁇ , reference catalog MAIPS4510
- 96-well acceptor plate Multiscreen®, reference catalog MAMCS9610
- PDVF membrane filters (30 mm in diameter, pore size 0.45 ⁇ ) from the Symta commercial house were used.
- He The equipment used to perform ultraviolet absorbance measurements in 96-well plates was a Thermoscientific Multiskan spectrum.
- the donor plate was impregnated with 4 ⁇ _ of a solution of the porcine brain lipid dissolved in dodecane (20 mg mi “1 ). After 5 minutes, 180 ⁇ _ of solution of each compound was added on this plate. To assess their penetration into the central nervous system, they were taken between 1-2 mg and dissolved in 1500 ⁇ _ of EtOH and 3500 ⁇ _ of phosphate buffer PBS (pH 7.4) buffer, filtered and added to the donor plate 96 Wells, the donor plate was then placed on the acceptor forming a kind of "sandwich” and left to incubate for 2h at 25 ° C. The compounds will pass from the donor plate through the porcine brain lipid to the acceptor plate.
- Desipramine 12 12.4 ⁇ 1.0
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
Abstract
The present invention relates to a compound of formula (I) derived from
maleimide and that has the ability to reversibly or irreversibly inhibit the GSK-3 enzyme in the micro- and nanomolar ranges. The present invention likewise relates to the use of these compounds for producing a drug for treating and/or preventing diseases in which GSK-3 is implicated, such as neurodegenerative diseases, inflammatory diseases, cancer or diabetes. These compounds can also be used to promote regenerative processes in various tissues.
Description
Inhibidores de GSK-3 útiles en enfermedades neurodegenerativas, inflamatorias, cáncer, diabetes y en procesos reqenerativos. GSK-3 inhibitors useful in neurodegenerative, inflammatory diseases, cancer, diabetes and in reqenerative processes.
La presente invención se refiere a una serie de compuestos que son derivados de maleimidas y que son capaces de inhibir la enzima GSK-3 en rangos micro y nanomolar de forma reversible o irreversible. Estos compuestos, por tanto, son útiles para la fabricación de un medicamento para el tratamiento y/o prevención de enfermedades en las que GSK-3 esté implicada, tales como, enfermedades neurodegenerativas, enfermedades inflamatorias, cáncer, diabetes, así como promover diversos procesos regenerativos. The present invention relates to a series of compounds that are derived from maleimides and that are capable of inhibiting the GSK-3 enzyme in micro and nanomolar ranges reversibly or irreversibly. These compounds, therefore, are useful for the manufacture of a medicament for the treatment and / or prevention of diseases in which GSK-3 is involved, such as neurodegenerative diseases, inflammatory diseases, cancer, diabetes, as well as promoting various processes. regenerative
ESTADO DE LA TÉCNICA ANTERIOR STATE OF THE PREVIOUS TECHNIQUE
La glucógeno sintasa quinasa 3, GSK-3, es una enzima de la familia de las quinasas que cataliza la fosforilación de residuos de serina o treonina en diversos sustratos. Originariamente fue descubierta por su papel en la biosíntesis del glucógeno, al cual debe su nombre [Rylatt, D.B., Aitken, A., Bilham, T., Condón, G. D., Embi, N., Cohén, P. "Glycogen Synthase Kinase 3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase". Eur J Biochem. 1980 107, 519-527.]. La enzima está implicada en la regulación de varias rutas de señalización celular, entre las que se encuentran las rutas de Wnt, el ciclo de división celular, la respuesta de daño en el ADN, la muerte y supervivencia celular y la diferenciación neuronal entre otras [Van Waue, J., Haefner, B. "Glycogen Synthase Kinase-3 as drug target: from wallflower to center of attention". Drug News Perspect. 2003 16, 557-565]. Estudios recientes demuestran que una sobreexpresión de GSK-3 es suficiente para inducir la muerte neuronal [Hetmán, M., Cavanaugh, J. E., Kimelman, D., Xia, Z. "Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal". J Neuroso. 2000 20, 2567-2574], relacionándose con diversas patologías tales como desórdenes bipolares, enfermedades neurodegenerativas, en especial la enfermedad de Alzheimer, diabetes de tipo I I y enfermedades inflamatorias crónicas.
Recientemente, algunos grupos han sugerido que la GSK-3 presenta una función importante en señales de proliferación y diferenciación celular por su papel esencial en las vías de señalización de RTK, Wnt y Shh. Las células madre mesemquimales (MSC) tienen la capacidad de diferenciarse en varios tipos celulares, incluidos osteoblastos. La activación de la señalización Wnt mediante la inhibición de GSK-3 provoca la diferenciación de las MSC en osteoblastos y el consiguiente incremento de masa ósea [Gambardella A, Nagaraju CK, O'Shea PJ, Mohanty ST, Kottam L, Pilling J, Sullivan M, Djerbi M, Koopmann W, Croucher Pl, Bellantuono I "Glycogen synthase kinase-3a/p inhibition promotes in vivo amplification of endogenous mesenchymal progenitors with osteogenic and adipogenic potential and their differentiation to the osteogenic lineage" . J Bone Miner Res. 2010 Oct 1 1 ]. Asimismo, también se ha demostrado que la inhibición de GSK-3 promueve la proliferación de cardiomiocitos en el corazón de adultos, por lo que inhibir GSK3 podría ser una estrategia para promover la regeneración cardíaca en estados patológicos [Woulfe KC, Gao E, Lal H, Harris D, Fan Q, Vagnozzi R, DeCaul M, Shang X, Patel S, Woodgett JR, Forcé T, Zhou J. "Glycogen synthase kinase-3beta regulates post-myocardial infarction remodeling and stress-induced cardiomyocyte proliferation in vivo" Circ Res. 2010 May 28;1 06(10) :1 635-45]. Glycogen synthase kinase 3, GSK-3, is an enzyme in the kinase family that catalyzes phosphorylation of serine or threonine residues in various substrates. It was originally discovered for its role in glycogen biosynthesis, to which it owes its name [Rylatt, DB, Aitken, A., Bilham, T., Condom, GD, Embi, N., Cohen, P. "Glycogen Synthase Kinase 3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase ". Eur J Biochem. 1980 107, 519-527.]. The enzyme is involved in the regulation of several cell signaling pathways, including Wnt pathways, the cell division cycle, DNA damage response, cell death and survival, and neuronal differentiation among others [ Van Waue, J., Haefner, B. "Glycogen Synthase Kinase-3 as drug target: from wallflower to center of attention". Drug News Perspect. 2003 16, 557-565]. Recent studies show that overexpression of GSK-3 is sufficient to induce neuronal death [Hetmán, M., Cavanaugh, JE, Kimelman, D., Xia, Z. "Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal ". J Neuroso. 2000 20, 2567-2574], relating to various pathologies such as bipolar disorders, neurodegenerative diseases, especially Alzheimer's disease, type II diabetes and chronic inflammatory diseases. Recently, some groups have suggested that GSK-3 plays an important role in cell proliferation and differentiation signals because of its essential role in the signaling pathways of RTK, Wnt and Shh. Mesemchymal stem cells (MSC) have the ability to differentiate into several cell types, including osteoblasts. Activation of Wnt signaling by inhibition of GSK-3 causes the differentiation of MSCs into osteoblasts and the consequent increase in bone mass [Gambardella A, Nagaraju CK, O'Shea PJ, Mohanty ST, Kottam L, Pilling J, Sullivan M, Djerbi M, Koopmann W, Croucher Pl, Bellantuono I "Glycogen synthase kinase-3a / p inhibition promotes in vivo amplification of endogenous mesenchymal progenitors with osteogenic and adipogenic potential and their differentiation to the osteogenic lineage". J Bone Miner Res. 2010 Oct 1 1]. Likewise, it has also been shown that inhibition of GSK-3 promotes cardiomyocyte proliferation in the heart of adults, so inhibiting GSK3 could be a strategy to promote cardiac regeneration in pathological states [Woulfe KC, Gao E, Lal H , Harris D, Fan Q, Vagnozzi R, DeCaul M, Shang X, Patel S, Woodgett JR, Forcé T, Zhou J. "Glycogen synthase kinase-3beta regulates post-myocardial infarction remodeling and stress-induced cardiomyocyte proliferation in vivo" Circ Res. 2010 May 28; 1 06 (10): 1 635-45].
En los últimos años se han sintetizado numerosos inhibidores de GSK-3, que resultan moléculas prometedoras para el tratamiento de enfermedades diversas, como la diabetes, cáncer y enfermedades neurodegenerativas [Martínez, A. "Preclinical efficacy on GSK-3 inhibitors: towards a future generation of powerful drugs". Med. Res. Rev. 2008 28, 773-796]. Además, la inhibición de quinasas que inhiben la proteína tau podría ser beneficioso para enfermedades neurodegenerativas [Martínez, A., Castro, A. "Inhibition of tau phosphorylation : A new therapeutical strategy for the treatment of Alzheimer's disease and other neurodegenerative disorders" Expert Opin. Ther. Pal 2000 10, 1 51 9-1 527]. En concreto en este tipo de enfermedades, estos inhibidores están demostrando su eficacia, y a día de hoy existe un inhibidor de GSK-3 que está en fases clínicas para el tratamiento del Alzheimer, y para la parálisis supranuclear progresiva.
DESCRIPCIÓN DE LA INVENCIÓN In recent years, numerous GSK-3 inhibitors have been synthesized, resulting promising molecules for the treatment of various diseases, such as diabetes, cancer and neurodegenerative diseases [Martínez, A. "Preclinical efficacy on GSK-3 inhibitors: towards a future generation of powerful drugs. " Med. Res. Rev. 2008 28, 773-796]. In addition, the inhibition of kinases that inhibit tau protein could be beneficial for neurodegenerative diseases [Martínez, A., Castro, A. "Inhibition of tau phosphorylation: A new therapeutical strategy for the treatment of Alzheimer's disease and other neurodegenerative disorders" Expert Opinion . Ther. Pal 2000 10, 1 51 9-1 527]. Specifically in these types of diseases, these inhibitors are demonstrating their effectiveness, and today there is a GSK-3 inhibitor that is in clinical phases for the treatment of Alzheimer's disease, and for progressive supranuclear paralysis. DESCRIPTION OF THE INVENTION
La presente invención presenta una familia de cuatro compuestos, y su modo de obtención, que poseen la capacidad de inhibir la enzima GSK-3 en orden micro y nanomolar. Además algunos de estos compuestos, según un modelo adaptado al laboratorio, son capaces de atravesar la barrera hematoencefálica, característica clave para los compuestos susceptibles de servir como fármacos para enfermedades del sistema nervioso central. En un primer aspecto, la presente invención se refiere a un compuesto de fórmula (I): The present invention presents a family of four compounds, and their mode of production, which possess the ability to inhibit the GSK-3 enzyme in micro and nanomolar order. In addition, some of these compounds, according to a model adapted to the laboratory, are capable of crossing the blood-brain barrier, a key feature for compounds that can serve as drugs for diseases of the central nervous system. In a first aspect, the present invention relates to a compound of formula (I):
(I) (I)
donde R1 se selecciona entre H o alquilo C Ci0 y R2 se selecciona entre alquilo d-C-io O alquenilo Crdo, opcionalmente sustituidos por halógeno, o sus sales, solvatos o isómeros farmacéuticamente aceptables. where R 1 is selected from H or C C 0 alkyl and R 2 is selected from dC-io O alkyl Crdo alkenyl, optionally substituted by halogen, or its pharmaceutically acceptable salts, solvates or isomers.
El término "alquilo" se refiere, en la presente invención, a radicales de cadenas hidrocarbonadas, lineales o ramificadas, que tienen de 1 a 10 átomos de carbono, preferiblemente de 1 a 4, y que se unen al resto de la molécula mediante un enlace sencillo, por ejemplo, metilo, etilo, n-propilo, /-propilo, n- butilo, tere-butilo, sec-butilo, n-pentilo, n-hexilo, etc. Los grupos alquilo pueden estar opcionalmente sustituidos por uno o más átomos de halógeno, es decir, flúor, cloro, bromo y yodo, preferiblemente bromo. El término "alquenilo" se refiere a radicales de cadenas hidrocarbonadas lineales o ramificadas, que tienen de 1 a 10 átomos de carbono,
preferiblemente de 1 a 4 y que contienen uno o más enlaces carbono-carbono dobles, por ejemplo, vinilo, 1 -propenilo, alilo, isoprenilo, 2-butenilo, 1 ,3- butadienilo, etc. Los radicales alquenilos pueden estar opcionalmente sustituidos por uno o más átomos de halógeno. The term "alkyl" refers, in the present invention, to radicals of hydrocarbon chains, linear or branched, having 1 to 10 carbon atoms, preferably 1 to 4, and which are attached to the rest of the molecule by a single bond, for example, methyl, ethyl, n-propyl, / -propyl, n-butyl, tere-butyl, sec-butyl, n-pentyl, n-hexyl, etc. The alkyl groups may be optionally substituted by one or more halogen atoms, that is, fluorine, chlorine, bromine and iodine, preferably bromine. The term "alkenyl" refers to radicals of linear or branched hydrocarbon chains, having 1 to 10 carbon atoms, preferably from 1 to 4 and containing one or more double carbon-carbon bonds, for example, vinyl, 1-propenyl, allyl, isoprenyl, 2-butenyl, 1, 3- butadienyl, etc. Alkenyl radicals may be optionally substituted by one or more halogen atoms.
En una realización preferida, R1 es H. In a preferred embodiment, R 1 is H.
En otra realización preferida, R1 es alquilo C C4 y más preferiblemente metilo. En una realización más preferida, R2 es alquilo CrC4 y más preferiblemente metilo. In another preferred embodiment, R 1 is CC 4 alkyl and more preferably methyl. In a more preferred embodiment, R 2 is CrC 4 alkyl and more preferably methyl.
En otra realización más preferida, R2 es -CH2Br. En una realización preferida, el compuesto de fórmula (I) se selecciona de la lista que comprende: In another more preferred embodiment, R 2 is -CH 2 Br. In a preferred embodiment, the compound of formula (I) is selected from the list comprising:
■ 3-acetil-4-(1 H-indol-3-il)-1 H-pirrol-2,5-diona ■ 3-acetyl-4- (1 H-indole-3-yl) -1 H-pyrrole-2,5-dione
■ 3-acetil-4-(1 -metil-1 H-indol-3-il)-1 H-pirrol-2,5-diona ■ 3-Acetyl-4- (1-methyl-1 H-indole-3-yl) -1 H-pyrrole-2,5-dione
■ 3-(2-bromoacetil)-4-(1 H-indol-3-il)-1 H-pirrol-2,5-diona ■ 3- (2-Bromoacetyl) -4- (1 H-indole-3-yl) -1 H-pyrrole-2,5-dione
■ 3-(2-bromoacetil)-4-(1 H-indol-3-il)-1 H-pirrol-2,5-diona ■ 3- (2-Bromoacetyl) -4- (1 H-indole-3-yl) -1 H-pyrrole-2,5-dione
o de sus sales, solvatos o isómeros farmacéuticamente aceptables. or of its pharmaceutically acceptable salts, solvates or isomers.
Los compuestos de la presente invención representados por la fórmula (I) pueden incluir isómeros, dependiendo de la presencia de enlaces múltiples (por ejemplo, Z, E), incluyendo isómeros ópticos o enantiomeros, dependiendo de la presencia de centros quirales. Los isómeros, enantiomeros o diastereoisómeros individuales y las mezclas de los mismos caen dentro del alcance de la presente invención, es decir, el término isómero también se refiere a cualquier mezcla de isómeros, como diastereómeros, racémicos, etc., incluso a sus isómeros ópticamente activos o las mezclas en distintas proporciones de los mismos. Los enantiomeros o diastereoisómeros individuales, así como sus mezclas, pueden separarse mediante técnicas convencionales.
Los compuestos de la invención pueden estar en forma cristalina como compuestos libres o como solvatos. En este sentido, el término "solvato", tal como aquí se utiliza, incluye tanto solvatos farmacéuticamente aceptables, es decir, solvatos del compuesto de fórmula (I) que pueden ser utilizados en la elaboración de un medicamento, como solvatos farmacéuticamente no aceptables, los cuales pueden ser útiles en la preparación de solvatos o sales farmacéuticamente aceptables. La naturaleza del solvato farmacéuticamente aceptable no es crítica siempre y cuando sea farmacéuticamente aceptable. En una realización particular, el solvato es un hidrato. Los solvatos pueden obtenerse por métodos convencionales de solvatación conocidos por los expertos en la materia. The compounds of the present invention represented by formula (I) may include isomers, depending on the presence of multiple bonds (eg, Z, E), including optical isomers or enantiomers, depending on the presence of chiral centers. The individual isomers, enantiomers or diastereoisomers and mixtures thereof fall within the scope of the present invention, that is, the term isomer also refers to any mixture of isomers, such as diastereomers, racemic, etc., even their optically isomers. assets or mixtures in different proportions thereof. The individual enantiomers or diastereoisomers, as well as mixtures thereof, can be separated by conventional techniques. The compounds of the invention may be in crystalline form as free compounds or as solvates. In this sense, the term "solvate", as used herein, includes both pharmaceutically acceptable solvates, that is, solvates of the compound of formula (I) that can be used in the manufacture of a medicament, as pharmaceutically acceptable solvates, which may be useful in the preparation of pharmaceutically acceptable solvates or salts. The nature of the pharmaceutically acceptable solvate is not critical as long as it is pharmaceutically acceptable. In a particular embodiment, the solvate is a hydrate. Solvates can be obtained by conventional solvation methods known to those skilled in the art.
Otro aspecto de la invención se refiere al uso de un compuesto de fórmula (I) para la fabricación de un medicamento. Another aspect of the invention relates to the use of a compound of formula (I) for the manufacture of a medicament.
Otro aspecto de la invención se refiere al uso de un compuesto de fórmula (I) para la fabricación de un medicamento para el tratamiento y/o prevención de una enfermedad que se selecciona entre enfermedades neurodegenerativas, enfermedades inflamatorias, cáncer o diabetes. Another aspect of the invention relates to the use of a compound of formula (I) for the manufacture of a medicament for the treatment and / or prevention of a disease that is selected from neurodegenerative diseases, inflammatory diseases, cancer or diabetes.
Preferiblemente, la enfermedad neurodegenerativa se selecciona entre enfermedad de Alzheimer, enfermedad de Parkinson, esclerosis lateral amiotrófica, isquemia cerebral, parkinsonismos post-encefalítico, distonias, síndrome de Tourette's, patologías de movimientos límbicos periódicos, síndrome de piernas inquietas, trastornos de déficit de atención con hiperactividad, enfermedad de Hungtinton, parálisis supranuclear progresiva, enfermedad de Pick, demencia frontotemporal, enfermedades neuromusculares. Preferably, the neurodegenerative disease is selected from Alzheimer's disease, Parkinson 's disease, amyotrophic lateral sclerosis, cerebral ischemia, post-encephalitic Parkinsonism, dystonia, Tourette 's, pathologies limb movements newspapers, restless leg syndrome, deficit disorders of attention with hyperactivity, Hungtinton's disease, progressive supranuclear paralysis, Pick's disease, frontotemporal dementia, neuromuscular diseases.
Preferiblemente, la enfermedad inflamatoria se selecciona entre enfermedad de Crohn, colitis ulcerosa, artritis, aterosclerosis, vasculitis, esclerosis múltiple.
Preferiblemente, el cáncer se selecciona entre glioblastoma, leucemia, linfoma, cáncer de pulmón, de mama, de próstata o de colon y en general entre cualquier proceso canceroso o metastásico en el que esté implicada GSK-3. Preferiblemente, la diabetes se selecciona como diabetes tipo II insulino no dependiente. Preferably, the inflammatory disease is selected from Crohn's disease, ulcerative colitis, arthritis, atherosclerosis, vasculitis, multiple sclerosis. Preferably, the cancer is selected from glioblastoma, leukemia, lymphoma, lung, breast, prostate or colon cancer and in general from any cancerous or metastatic process in which GSK-3 is involved. Preferably, diabetes is selected as non-dependent insulin type II diabetes.
Otro aspecto de la invención es el uso de un compuesto de fórmula (I) para la fabricación de un medicamento para promover procesos regenerativos. Preferiblemente, los procesos regenerativos se seleccionan entre los que promueven la diferenciación de las células madres del sistema nervioso, del sistema hematopoyético, del sistema óseo, del miocardio. Another aspect of the invention is the use of a compound of formula (I) for the manufacture of a medicament to promote regenerative processes. Preferably, the regenerative processes are selected from those that promote the differentiation of the stem cells of the nervous system, the hematopoietic system, the bone system, the myocardium.
Para su aplicación en terapia, los compuestos de fórmula (I), sus sales, solvatos o isómeros, se encontrarán, preferentemente, en una forma farmacéuticamente aceptable o sustancialmente pura, es decir, que tiene un nivel de pureza farmacéuticamente aceptable excluyendo los aditivos farmacéuticos normales tales como diluyentes y portadores, y no incluyendo material considerado tóxico a niveles de dosificación normales. Los niveles de pureza para el principio activo son preferiblemente superiores al 50%, más preferiblemente superiores al 70%, y todavía más preferiblemente superiores al 90%. En una realización preferida, son superiores al 95% de compuesto de fórmula (I), o de sus sales, solvatos o isómeros. Los compuestos de la presente invención de formula (I) pueden ser obtenidos según el procedimiento descrito en el documento [Faul, M.M., Winneroski, L.L., Brussee, J., Krumrich, C.A. "A new one step synthesis of maleimides by condensation of glyoxylate esters with acetamides". Tetrahedron Letters. 1999 40, 1 109-1 1 12]. For their application in therapy, the compounds of formula (I), their salts, solvates or isomers, will preferably be found in a pharmaceutically acceptable or substantially pure form, that is, having a pharmaceutically acceptable level of purity excluding pharmaceutical additives. normal such as diluents and carriers, and not including material considered toxic at normal dosage levels. The purity levels for the active ingredient are preferably greater than 50%, more preferably greater than 70%, and still more preferably greater than 90%. In a preferred embodiment, they are greater than 95% of the compound of formula (I), or of its salts, solvates or isomers. The compounds of the present invention of formula (I) can be obtained according to the procedure described in the document [Faul, M.M., Winneroski, L.L., Brussee, J., Krumrich, C.A. "A new one step synthesis of maleimides by condensation of glyoxylate esters with acetamides". Tetrahedron Letters 1999 40, 1 109-1 1 12].
En otro aspecto, la presente invención también se refiere a las composiciones farmacéuticas que comprenden al menos un compuesto de la invención, o un isómero, una sal farmacéuticamente aceptable, un derivado o un profármaco
del mismo, junto con un transportador o "carrier" farmacéuticamente aceptable, un excipiente o un vehículo, para la administración a un paciente. In another aspect, the present invention also relates to pharmaceutical compositions comprising at least one compound of the invention, or an isomer, a pharmaceutically acceptable salt, a derivative or a prodrug. thereof, together with a pharmaceutically acceptable carrier or carrier, an excipient or a vehicle, for administration to a patient.
En una realización preferida, la composición farmacéutica comprende además otro principio activo. In a preferred embodiment, the pharmaceutical composition further comprises another active ingredient.
Los adyuvantes y vehículos farmacéuticamente aceptables que pueden ser utilizados en dichas composiciones son los adyuvantes y vehículos conocidos por los técnicos en la materia y utilizados habitualmente en la elaboración de composiciones terapéuticas. The pharmaceutically acceptable adjuvants and vehicles that can be used in said compositions are the adjuvants and vehicles known to those skilled in the art and commonly used in the elaboration of therapeutic compositions.
Otro aspecto de la invención se refiere a un compuesto de fórmula (I) para su uso como medicamento y particularmente, como medicamento para tratar y/o prevenir enfermedades neurodegenerativas, enfermedades inflamatorias, cáncer o diabetes o para promover procesos regenerativos. Another aspect of the invention relates to a compound of formula (I) for use as a medicament and particularly as a medicament for treating and / or preventing neurodegenerative diseases, inflammatory diseases, cancer or diabetes or for promoting regenerative processes.
Otro aspecto de la invención es un método de tratamiento de una enfermedad neurodegenerativa, una enfermedad inflamatoria, cáncer o diabetes, así como un método de promover la regeneración celular, que comprende la administración a un paciente de una cantidad terapéuticamente efectiva de un compuesto de fórmula (I) o de una composición farmacéutica que lo comprende, Another aspect of the invention is a method of treating a neurodegenerative disease, an inflammatory disease, cancer or diabetes, as well as a method of promoting cell regeneration, which comprises administering to a patient a therapeutically effective amount of a compound of formula (I) or of a pharmaceutical composition comprising it,
En el sentido utilizado en esta descripción, la expresión "cantidad terapéuticamente efectiva" se refiere a la cantidad del agente o compuesto capaz de desarrollar la acción terapéutica determinada por sus propiedades farmacológicas, calculada para producir el efecto deseado y, en general, vendrá determinada, entre otras causas, por las características propias de los compuestos, incluyendo la edad, estado del paciente, la severidad de la alteración o trastorno, y de la ruta y frecuencia de administración. In the sense used in this description, the term "therapeutically effective amount" refers to the amount of the agent or compound capable of developing the therapeutic action determined by its pharmacological properties, calculated to produce the desired effect and, in general, will be determined, among other causes, due to the characteristics of the compounds, including the age, condition of the patient, the severity of the alteration or disorder, and the route and frequency of administration.
Los compuestos descritos en la presente invención, sus sales, profármacos y/o solvatos así como las composiciones farmacéuticas que los contienen pueden
ser utilizados junto con otros fármacos, o principios activos, adicionales para proporcionar una terapia de combinación. Dichos fármacos adicionales pueden formar parte de la misma composición farmacéutica o, alternativamente, pueden ser proporcionados en forma de una composición separada para su administración simultánea o no a la de la composición farmacéutica que comprende un compuesto de fórmula (I), o una sal, profármaco o solvato del mismo. The compounds described in the present invention, their salts, prodrugs and / or solvates as well as the pharmaceutical compositions containing them can be used together with other drugs, or active ingredients, additional to provide a combination therapy. Said additional drugs may be part of the same pharmaceutical composition or, alternatively, they may be provided in the form of a separate composition for simultaneous or non-simultaneous administration to the pharmaceutical composition comprising a compound of formula (I), or a salt, prodrug or solvate thereof.
En otra realización particular, dicha composición terapéutica se prepara en forma de una forma sólida o suspensión acuosa, en un diluyente farmacéuticamente aceptable. La composición terapéutica proporcionada por esta invención puede ser administrada por cualquier vía de administración apropiada, para lo cual dicha composición se formulará en la forma farmacéutica adecuada a la vía de administración elegida. En una realización particular, la administración de la composición terapéutica proporcionada por esta invención se efectúa por vía oral, tópica, rectal o parenteral (incluyendo subcutánea, intraperitoneal, intradérmica, intramuscular, intravenosa, etc.). In another particular embodiment, said therapeutic composition is prepared in the form of a solid form or aqueous suspension, in a pharmaceutically acceptable diluent. The therapeutic composition provided by this invention may be administered by any appropriate route of administration, for which said composition will be formulated in the pharmaceutical form appropriate to the route of administration chosen. In a particular embodiment, the administration of the therapeutic composition provided by this invention is performed orally, topically, rectally or parenterally (including subcutaneously, intraperitoneally, intradermally, intramuscularly, intravenously, etc.).
En una realización preferida de la presente invención, las composiciones farmacéuticas son adecuadas para la administración oral, en forma sólida o líquida. Las posibles formas para la administración oral son tabletas, cápsulas, siropes o soluciones y pueden contener excipientes convencionales conocidos en el ámbito farmacéutico, como agentes agregantes (p.e. sirope, acacia, gelatina, sorbitol, tragacanto o polivinil pirrolidona), rellenos (p.e. lactosa, azúcar, almidón de maíz, fosfato de calcio, sorbitol o glicina), disgregantes (p.e. almidón, polivinil pirrolidona o celulosa microcristalina) o un surfactante farmacéuticamente aceptable como el lauril sulfato de sodio. In a preferred embodiment of the present invention, the pharmaceutical compositions are suitable for oral administration, in solid or liquid form. Possible forms for oral administration are tablets, capsules, syrups or solutions and may contain conventional excipients known in the pharmaceutical field, as aggregating agents (eg syrup, acacia, gelatin, sorbitol, tragacanth or polyvinyl pyrrolidone), fillers (eg lactose, sugar, corn starch, calcium phosphate, sorbitol or glycine), disintegrants (eg starch, polyvinyl pyrrolidone or microcrystalline cellulose) or a pharmaceutically acceptable surfactant such as sodium lauryl sulfate.
Las composiciones para administración oral pueden ser preparadas por métodos los convencionales de Farmacia Galénica, como mezcla y dispersión. Las tabletas se pueden recubrir siguiendo métodos conocidos en la industria farmacéutica.
Las composiciones farmacéuticas se pueden adaptar para la administración parenteral, como soluciones estériles, suspensiones, o liofilizados de los productos de la invención, empleando la dosis adecuada. Se pueden emplear excipientes adecuados, como agentes tamponadores del pH o surfactantes. Compositions for oral administration can be prepared by conventional methods of Galenic Pharmacy, as mixing and dispersion. The tablets can be coated following methods known in the pharmaceutical industry. The pharmaceutical compositions can be adapted for parenteral administration, as sterile solutions, suspensions, or lyophilized products of the invention, using the appropriate dose. Suitable excipients, such as pH buffering agents or surfactants, can be used.
Las formulaciones anteriormente mencionadas pueden ser preparadas usando métodos convencionales, como los descritos en las Farmacopeas de diferentes países y en otros textos de referencia. La administración de los compuestos o composiciones de la presente invención puede ser realizada mediante cualquier método adecuado, como la infusión intravenosa y las vías oral, intraperitoneal o intravenosa. La administración oral es la preferida por la conveniencia de los pacientes y por el carácter crónico de las enfermedades a tratar. The aforementioned formulations can be prepared using conventional methods, such as those described in the Pharmacopoeias of different countries and in other reference texts. The administration of the compounds or compositions of the present invention can be performed by any suitable method, such as intravenous infusion and oral, intraperitoneal or intravenous routes. Oral administration is preferred for the convenience of patients and for the chronic nature of the diseases to be treated.
La cantidad administrada de un compuesto de la presente invención dependerá de la relativa eficacia del compuesto elegido, la severidad de la enfermedad a tratar y el peso del paciente. Sin embargo, los compuestos de esta invención serán administrados una o más veces al día, por ejemplo 1 , 2, 3 ó 4 veces diarias, con una dosis total entre 0,1 y 1000 mg/Kg/día. Es importante tener en cuenta que puede ser necesario introducir variaciones en la dosis, dependiendo de la edad y de la condición del paciente, así como modificaciones en la vía de administración. Los compuestos y composiciones de la presente invención pueden ser empleados junto con otros medicamentos en terapias combinadas. Los otros fármacos pueden formar parte de la misma composición o de otra composición diferente, para su administración al mismo tiempo o en tiempos diferentes. El uso de los compuestos de la invención es compatible con su uso en protocolos en que los compuestos de la fórmula (I), o sus mezclas se usan por sí mismos o en combinaciones con otros tratamientos o cualquier procedimiento médico.
A lo largo de la descripción y las reivindicaciones la palabra "comprende" y sus variantes no pretenden excluir otras características técnicas, aditivos, componentes o pasos. Para los expertos en la materia, otros objetos, ventajas y características de la invención se desprenderán en parte de la descripción y en parte de la práctica de la invención. Los siguientes ejemplos y dibujos se proporcionan a modo de ilustración, y no se pretende que sean limitativos de la presente invención. DESCRIPCION DE LA FIGURA The amount administered of a compound of the present invention will depend on the relative efficacy of the compound chosen, the severity of the disease to be treated and the weight of the patient. However, the compounds of this invention will be administered one or more times a day, for example 1, 2, 3 or 4 times daily, with a total dose between 0.1 and 1000 mg / kg / day. It is important to keep in mind that it may be necessary to introduce variations in the dose, depending on the age and condition of the patient, as well as modifications in the route of administration. The compounds and compositions of the present invention can be used together with other medicaments in combination therapies. The other drugs may be part of the same composition or of a different composition, for administration at the same time or at different times. The use of the compounds of the invention is compatible with their use in protocols in which the compounds of the formula (I), or mixtures thereof are used by themselves or in combinations with other treatments or any medical procedure. Throughout the description and the claims the word "comprises" and its variants are not intended to exclude other technical characteristics, additives, components or steps. For those skilled in the art, other objects, advantages and features of the invention will be derived partly from the description and partly from the practice of the invention. The following examples and drawings are provided by way of illustration, and are not intended to be limiting of the present invention. DESCRIPTION OF THE FIGURE
Figura 1. Muestra la correlación linear entre permeabilidad descrita y experimental de 10 compuestos comerciales empleando la metodología PAMPA-Barrera hematoencefálica. Figure 1. Shows the linear correlation between described and experimental permeability of 10 commercial compounds using the PAMPA-Blood-brain barrier methodology.
EJEMPLOS EXAMPLES
A continuación se ilustrará la invención mediante unos ensayos realizados por los inventores, que ponen de manifiesto la especificidad y efectividad de los compuestos de la invención. The invention will now be illustrated by tests carried out by the inventors, which show the specificity and effectiveness of the compounds of the invention.
Síntesis de maleimidas 3,4-disustituidas Synthesis of 3,4-disubstituted maleimides
Comp. 1 R1 =H Comp. 3 R1=HComp. 1 R 1 = H Comp. 3 R 1 = H
Comp. 2 R1 =Me Comp.4 R1 =Me Comp. 2 R 1 = Me Comp. 4 R 1 = Me
3-acetil-4-(1 H-indol-3-il)-1 H-pirrolin-2,5-diona (Compuesto 1): Sobre una mezcla de acetoacetamida (1 equiv, 3,69 mmol, 372 mg) y fBuOK (disolución 1 M en THF) (3,5 equiv,12,9 mmol, 12,9 mi) disueltos en THF (20 mi) a -60 °-C
se añade 3-indolglioxilato de metilo (1 equiv, 3,69 mmol, 750 mg). La mezcla se agita hasta alcanzar temperatura ambiente y se añaden 1 1 ,5 ml de ácido clorhídrico concentrado, 30 ml de agua y 30 ml de diclorometano. A continuación, se separa la fase orgánica y se lava con una disolución saturada de NaHC03 (2 x 30 ml). Se seca con MgS04 y se evapora el disolvente a presión reducida. El sólido se purifica por recristalización en acetato de etilo/ pentano obteniéndose un sólido naranja, 0,65 g, rendimiento: 74%. 1H NMR (300 MHz, DMSO-cfe): δ 12,29 (sa, 1 H), 1 1 ,17 (sa, 1 H), 8,24 (s, 1 H), 7,50 (d, J = 7,01 Hz ,1 H), 7,27 - 7,17 (m, 1 H), 7,13 (s, 2H), 2,50 - 2,46 (s, 3H). 13C NMR (75 MHz, DMSO-cfe): δ 195,49, 171 ,74, 139,80, 137,50, 135,53, 125,57, 124,94, 123,51 , 122,67, 121 ,70, 1 13,36, 106,05, 31 ,72, HPLC/MS: Pureza >99%, t.r.=3,76 min, columna Sunfire C18, 3,5 μτη (50 X 4,6 mm) empleando como fase móvil acetonitrilo (0,08% ácido fórmico) y agua MiliQ (0,1 % ácido fórmico) y un gradiente de acetonitrilo (10% a 100%) durante 10 min con un flujo de 0,25 ml/min (m/z 255,237,1 65). P.f.= 225-226 QC. Análisis Elemental (Ci4Hi0N2O3) Calculado: C 66,14%; H 3,96%; N 1 1 ,02%. Hallado: C 65,89%; H 4,12%; N 10,74%. 3-acetyl-4- (1 H-indol-3-yl) -1 H-pyrrolin-2,5-dione (Compound 1): On a mixture of acetoacetamide (1 equiv, 3.69 mmol, 372 mg) and f BuOK (1 M solution in THF) (3.5 equiv, 12.9 mmol, 12.9 mi) dissolved in THF (20 mi) at -60 ° -C methyl 3-indolglioxylate (1 equiv, 3.69 mmol, 750 mg) is added. The mixture is stirred until room temperature is reached and 1.5 ml of concentrated hydrochloric acid, 30 ml of water and 30 ml of dichloromethane are added. Then, the organic phase is separated and washed with a saturated solution of NaHC0 3 (2 x 30 ml). It is dried with MgSO 4 and the solvent is evaporated under reduced pressure. The solid is purified by recrystallization from ethyl acetate / pentane to obtain an orange solid, 0.65 g, yield: 74%. 1 H NMR (300 MHz, DMSO-cfe): δ 12.29 (sa, 1 H), 1 1, 17 (sa, 1 H), 8.24 (s, 1 H), 7.50 (d, J = 7.01 Hz, 1 H), 7.27 - 7.17 (m, 1 H), 7.13 (s, 2H), 2.50 - 2.46 (s, 3H). 13 C NMR (75 MHz, DMSO-cfe): δ 195.49, 171, 74, 139.80, 137.50, 135.53, 125.57, 124.94, 123.51, 122.67, 121 , 70, 1 13.36, 106.05, 31, 72, HPLC / MS: Purity> 99%, tr = 3.76 min, Sunfire C18 column, 3.5 μτη (50 X 4.6 mm) using as mobile phase acetonitrile (0.08% formic acid) and MiliQ water (0.1% formic acid) and a gradient of acetonitrile (10% to 100%) for 10 min with a flow of 0.25 ml / min (m / z 255,237.1 65). Mp = 225-226 Q C. Elemental Analysis (Ci 4 Hi 0 N 2 O 3 ) Calculated: C 66.14%; H 3.96%; N 1 1, 02%. Found: C 65.89%; H 4.12%; N 10.74%.
3-acetil-4-(1-metil-indol-3-il)-1 H-pirrolin-2,5-diona (Compuesto 2): Sobre una mezcla de acetoacetamida (1 equiv, 3,69 mmol, 372 mg) y fBuOK (disolución 1 M en THF) (3,5 equiv, 12,9 mmol, 12,9 ml) disueltos en THF (20 ml) a -60 QC se añade (1 -metilindolil)-3-glioxilato de metilo (1 equiv, 3,69 mmol, 3,69 mmol, 800 mg). La mezcla se agita hasta alcanzar temperatura ambiente y se añaden 1 1 ,5 ml de ácido clorhídrico concentrado, 30 ml de agua y 30 ml de diclorometano. A continuación se separa la fase orgánica y se lava con una disolución saturada de NaHC03 (2 x 30 ml). Se seca con MgS04 y se evapora el disolvente a presión reducida. El sólido se purifica por recristalización en acetato de etilo/ pentano obteniéndose un sólido rojo, 0,55 g, rendimiento: 56%. 1H NMR (300 MHz, DMSO-cfe): δ 1 1 ,19 (s, 1 H), 8,27 (s, 1 H), 7,58 (d, J = 8,1 Hz, 1 H), 7,35 - 7,06 (m, 3H), 3,92 (s, 3H), 2,51 (s, 3H). 13C NMR (75 MHz, DMSO-cfe): δ 194,66, 170,97, 138,56, 138,14, 137,38, 125,28, 123,82, 122,84, 122,14, 121 ,31 , 1 1 1 ,08, 104,36, 33,30, 30,95. HPLC/MS: Pureza= 95%, t.r.=4,06 min, columna Sunfire C18, 3,5 μηπ (50 X 4,6 mm) empleando como
fase móvil acetonitrilo (0,08% ácido fórmico) y agua MiliQ (0,1 % ácido fórmico) y un gradiente de acetonitrilo (10% a 100%) durante 10 min con un flujo de 0,25 ml/min (m/z 269, 251 , 180). P.f.= 224-225 °-C. Análisis Elemental (Ci5H12N203) Calculado: C 66,60%; H 5,22%; N 10,36%. Hallado: C 66,89%; H 5,01 %; N 10,37%. 3-acetyl-4- (1-methyl-indole-3-yl) -1 H-pyrrolin-2,5-dione (Compound 2): On a mixture of acetoacetamide (1 equiv, 3.69 mmol, 372 mg) and f BuOK (1 M solution in THF) (3.5 equiv, 12.9 mmol, 12.9 ml) dissolved in THF (20 ml) at -60 Q C (1-methylindole) -3-glyoxylate is added methyl (1 equiv, 3.69 mmol, 3.69 mmol, 800 mg). The mixture is stirred until room temperature is reached and 1.5 ml of concentrated hydrochloric acid, 30 ml of water and 30 ml of dichloromethane are added. The organic phase is then separated and washed with a saturated solution of NaHC0 3 (2 x 30 ml). It is dried with MgSO 4 and the solvent is evaporated under reduced pressure. The solid is purified by recrystallization from ethyl acetate / pentane to obtain a red solid, 0.55 g, yield: 56%. 1 H NMR (300 MHz, DMSO-cfe): δ 1 1, 19 (s, 1 H), 8.27 (s, 1 H), 7.58 (d, J = 8.1 Hz, 1 H) , 7.35-7.06 (m, 3H), 3.92 (s, 3H), 2.51 (s, 3H). 13 C NMR (75 MHz, DMSO-cfe): δ 194.66, 170.97, 138.56, 138.14, 137.38, 125.28, 123.82, 122.84, 122.14, 121 , 31, 1 1 1, 08, 104.36, 33.30, 30.95. HPLC / MS: Purity = 95%, tr = 4.06 min, Sunfire C18 column, 3.5 μηπ (50 X 4.6 mm) using as mobile phase acetonitrile (0.08% formic acid) and MiliQ water (0.1% formic acid) and a gradient of acetonitrile (10% to 100%) for 10 min with a flow of 0.25 ml / min (m / z 269, 251, 180). Mp = 224-225 ° -C. Elemental Analysis (Ci 5 H 12 N 2 0 3 ) Calculated: C 66.60%; H 5.22%; N 10.36%. Found: C 66.89%; H 5.01%; N 10.37%.
3-(2-bromoacetil)-4-(1 H-indol-3-il)-1 H-pirrolin-2,5-diona (compuesto 3). El compuesto 1 (1 equiv, 2 mmol, 510 mg) se disuelve en ácido acético, y en condiciones de reflujo se añade el bromo (1 ,05 equiv, 2,1 mmol, 0,1 1 mi) disuelto en ácido acético. La mezcla se mantiene a reflujo durante 3 horas. Tras dejar enfriar la mezcla se añade agua y acetato de etilo y la fase orgánica se extrae y se lava con varias porciones de una disolución saturada de NaHC03. El disolvente se evapora a presión reducida y el sólido se purifica por recristalización en MeOH/H20 obteniéndose un sólido negro, 0,25 g, rendimiento: 39%. 1H NMR (300 MHz, DMSO-cfe): δ 12,54 (s, 1 H), 1 1 ,34 (s, 1 H), 8,39 (m, 1 H), 7,54 (d, J = 8,0 Hz, 1 H), 7,32 - 6,98 (m, 3H), 4,75 (s, 2H). 13C NMR (126 MHz, acetona-efe): δ 186,0, 1 69,6, 1 69,4, 142,1 , 135,9, 135,7, 124,7, 122,8, 121 ,0, 1 12,1 , 106,4, 35,0, HPLC/ MS: Pureza =95%, t.r.=4,35 min, columna Sunfire C18, 3,5 μτη (50 X 4,6 mm) empleando como fase móvil acetonitrilo (0,08% ácido fórmico) y agua MiliQ (0,1 % ácido fórmico) y un gradiente de acetonitrilo (10% a 100%) durante 10 min con un flujo de 0,25 ml/min (m/z 335, 333, 253,182). P.f.= 261 -262 °-C. Análisis Elemental (Ci4H9BrN203) Calculado: C 50,47%; H 2,72%; N 8,41 %; Br 23,99%. Hallado: C 50,68%; H 2,87%; N 8,61 %; Br 23,62. 3- (2-Bromoacetyl) -4- (1 H-indol-3-yl) -1 H-pyrrolin-2,5-dione (compound 3). Compound 1 (1 equiv, 2 mmol, 510 mg) is dissolved in acetic acid, and under reflux conditions bromine (1.05 equiv, 2.1 mmol, 0.1 1 mL) dissolved in acetic acid is added. The mixture is refluxed for 3 hours. After allowing the mixture to cool, water and ethyl acetate are added and the organic phase is extracted and washed with several portions of a saturated NaHC0 3 solution. The solvent is evaporated under reduced pressure and the solid is purified by recrystallization from MeOH / H 2 0 to obtain a black solid, 0.25 g, yield: 39%. 1 H NMR (300 MHz, DMSO-cfe): δ 12.54 (s, 1 H), 1 1, 34 (s, 1 H), 8.39 (m, 1 H), 7.54 (d, J = 8.0 Hz, 1 H), 7.32-6.98 (m, 3H), 4.75 (s, 2H). 13 C NMR (126 MHz, acetone-efe): δ 186.0, 1 69.6, 1 69.4, 142.1, 135.9, 135.7, 124.7, 122.8, 121, 0 , 1 12.1, 106.4, 35.0, HPLC / MS: Purity = 95%, tr = 4.35 min, Sunfire C18 column, 3.5 μτη (50 X 4.6 mm) using as mobile phase acetonitrile (0.08% formic acid) and MiliQ water (0.1% formic acid) and a gradient of acetonitrile (10% to 100%) for 10 min with a flow of 0.25 ml / min (m / z 335 , 333, 253,182). Mp = 261-262 ° -C. Elemental Analysis (Ci 4 H 9 BrN 2 0 3 ) Calculated: C 50.47%; H 2.72%; N 8.41%; Br 23.99%. Found: C 50.68%; H 2.87%; N 8.61%; Br 23.62.
3-(2-bromoacetil)-4-(1-metil-1 H-indol-3-il)-1 H-pirrolin-2,5-dione (compuesto3- (2-Bromoacetyl) -4- (1-methyl-1 H-indol-3-yl) -1 H-pyrrolin-2,5-dione (compound
4). El compuesto 2 (1 equiv, 2,25 mmol, 603 mg) se disuelve en ácido acético, y en condiciones de reflujo se añade el bromo (1 ,05 equiv, 2,36 mmol, 0,1 15 mi) disuelto en ácido acético. La mezcla se mantiene a reflujo durante 3 horas. Tras dejar enfriar la mezcla se añade agua y acetato de etilo y la fase orgánica se extrae y se lava con varias porciones de una disolución saturada de NaHC03. El disolvente se evapora a presión reducida y el sólido se purifica por cromatografía en columna en gel de sílice utilizando como eluyente
CH2CI2/MeOH (80:1 ) obteniéndose un sólido morado, 0,12 g, rendimiento: 15%. 1H NMR (300 MHz, CDCI3): δ 8,46 (s, 1 H), 7,38 (m, 4H), 5,31 (s, 1 H), 4,64 (s, 2H), 3,94 (s, 3H). 13C NMR (126 MHz, acetone-cfe): δ 185,80, 1 69,78, 1 69,55, 141 ,80, 139,52, 137,66, 125,37, 122,94, 122,88, 121 ,34, 1 19,28, 1 10,46, 105,53, 35,02, 32,71 , P.f.= 252-253 °-C. HPLC / MS: Pureza= 99%, t.r.=4,47 min, columna Sunfire C18, 3,5 μτη (50 X 4,6 mm) empleando como fase móvil acetonitrilo (0,08% ácido fórmico) y agua MiliQ (0,1 % ácido fórmico) y un gradiente de acetonitrilo (10% a 100%) durante 10 min con un flujo de 0,25 ml/min (m/z 347, 267, 196). Análisis Elemental (0Ι5ΗΙ Ι ΒΓΝ2Ο3) Calculado: C 51 ,90%; H 3,19%; N 8,07%; Br 23,02%. Hallado: C 52,03%; H 3,08%; N 7,77%; Br 22,67%. 4). Compound 2 (1 equiv, 2.25 mmol, 603 mg) is dissolved in acetic acid, and under reflux conditions bromine (1.05 equiv, 2.36 mmol, 0.1 15 mL) dissolved in acid is added acetic. The mixture is refluxed for 3 hours. After allowing the mixture to cool, water and ethyl acetate are added and the organic phase is extracted and washed with several portions of a saturated NaHC0 3 solution. The solvent is evaporated under reduced pressure and the solid is purified by column chromatography on silica gel using as eluent CH 2 CI 2 / MeOH (80: 1) obtaining a purple solid, 0.12 g, yield: 15%. 1 H NMR (300 MHz, CDCI 3 ): δ 8.46 (s, 1 H), 7.38 (m, 4H), 5.31 (s, 1 H), 4.64 (s, 2H), 3.94 (s, 3 H). 13 C NMR (126 MHz, acetone-cfe): δ 185.80, 1 69.78, 1 69.55, 141, 80, 139.52, 137.66, 125.37, 122.94, 122.88 , 121, 34, 1 19.28, 1 10.46, 105.53, 35.02, 32.71, Mp = 252-253 ° -C. HPLC / MS: Purity = 99%, tr = 4.47 min, Sunfire C18 column, 3.5 μτη (50 X 4.6 mm) using acetonitrile (0.08% formic acid) and MiliQ water (0 as mobile phase) , 1% formic acid) and a gradient of acetonitrile (10% to 100%) for 10 min with a flow of 0.25 ml / min (m / z 347, 267, 196). Elemental Analysis (0Ι 5 ΗΙ Ι ΒΓΝ 2 Ο 3 ) Calculated: C 51, 90%; H 3.19%; N 8.07%; Br 23.02%. Found: C 52.03%; H 3.08%; N 7.77%; Br 22.67%.
Medida de la inhibición de GSK-33 GSK-33 inhibition measure
Los ensayos de inhibición enzimática se realizaron utilizando la metodología del método luminométrico de kinasa-glo®. La enzima humana recombinante GSK3 (nQ catálogo 14-306) se adquirió de Upstate (Dundee, UK). El polipéptido prefosforilado se sintetizó por American Peptide Inc (Sunnyvale, CA). El kit de quinasa luminiscente (nQ catálogo V671 1 ) se obtuvo de Promega. El ATP y otros reactivos se compraron en Sigma-Aldrich (St. Louis, MO) Enzyme inhibition assays were performed using the kinasa-glo® luminometric method methodology. Recombinant human enzyme GSK3 (n Q catalog 14-306) was purchased from Upstate (Dundee, UK). The prefosphorylated polypeptide was synthesized by American Peptide Inc (Sunnyvale, CA). The luminescent kinase kit (n Q catalog V671 1) was obtained from Promega. ATP and other reagents were purchased in Sigma-Aldrich (St. Louis, MO)
Los ensayos fueron realizados en buffer utilizando placas de 96 pocilios. 10μΙ del compuesto a ensayar (disuelto en dimetiisulfoxido a una concentración de 1 mM, y a su vez disuelto en buffer hasta la concentración necesaria para el experimento) y 10μΙ (20ng) de la enzima se añaden a cada pocilio seguidos de 20μΙ de buffer que contiene 25μΜ del sustrato y 1 μΜ de ATP. La concentración final de DMSO en el experimento no excedió el 1 %. Tras una incubación de media hora a 30QC se para la reacción enzimática con 40μΙ del reactivo de kinasa-glo®. La luminiscencia se mide tras diez minutos usando un POLARstar Optima multimode reader. La actividad es proporcional a la diferencia entre el ATP total y el consumido. Las actividades de inhibición se calcularon en función de la actividad máxima, medida en ausencia de inhibidor.
Tabla 1. Concentración inhibitoria 50 de los compuestos. The tests were performed in buffer using 96-well plates. 10μΙ of the compound to be tested (dissolved in dimethisulfoxide at a concentration of 1 mM, and in turn dissolved in buffer until the concentration necessary for the experiment) and 10μΙ (20ng) of the enzyme are added to each well followed by 20μΙ of buffer containing 25μΜ of the substrate and 1 μΜ of ATP. The final concentration of DMSO in the experiment did not exceed 1%. After incubation for half an hour at 30 Q C for enzymatic reaction with the reagent 40μΙ-Glo kinase. The luminescence is measured after ten minutes using a POLARstar Optima multimode reader. The activity is proportional to the difference between total and consumed ATP. Inhibition activities were calculated based on maximum activity, measured in the absence of inhibitor. Table 1. Inhibitory concentration of the compounds.
Los cuatro compuestos evaluados muestran una inhibición enzimática muy potente, con valores de CI5o que varían del rango de bajo micromolar a bajo nanomolar, siendo más potentes los inhibidores irreversibles (3 y 4) que los reversibles (1 y 2). El paso de inhibidor reversible (1 y 2) a irreversible (3 y 4) supone un aumento de la inhibición de GSK-3 en un orden de magnitud, respectivamente. The four compounds evaluated show a very potent enzyme inhibition, with IC 5 values or that vary from the low micromolar to low nanomolar range, irreversible inhibitors (3 and 4) being more potent than reversible ones (1 and 2). The step from reversible inhibitor (1 and 2) to irreversible (3 and 4) implies an increase in the inhibition of GSK-3 by an order of magnitude, respectively.
Penetración sistema nervioso central (SNC): Experimento Un vitro) determinación de permeabilidad empleando membranas artificiales paralelas (PAMPA) de la penetración en la barrera hematoencefálica. Central nervous system (CNS) penetration: Experiment One vitro) determination of permeability using parallel artificial membranes (PAMPA) of blood brain penetration.
La predicción de la permeabilidad de diversos compuestos sobre el sistema nervioso central (SNC), fue determinada empleando la metodología de membranas artificiales paralelas (PAMPA) [Di, L., Kerns, E. H., Fan, K., McConnell, O. J., Cárter, G. T. "High throughput artificial membrane permeability assay for blood-brain barrier" Eur. J. Med. Chem., 2003, 38, 223-232]. Compuestos comerciales, tampón fosfato a pH 7,4 (PBS), etanol y dodecano fueron obtenidos de las casas comerciales Sigma, Acros organics, Merck, Aldrich y Fluka. El lípido de cerebro porcino (referencia catálogo 141 101 ) fue adquirido en Avanti Polar Lipids. Tanto la placa donadora de 96 pocilios (Multiscreen® IP Sterile Píate membrana PDVF, tamaño de poro 0,45 μΜ, referencia catálogo MAIPS4510) como la placa de 96 pocilios aceptora (Multiscreen®, referencia catálogo MAMCS9610) fueron adquiridas en Millipore. Con el fin de filtrar las muestras se emplearon los filtros de membrana PDVF (30 mm de diámetro, tamaño del poro 0,45 μηπ) de la casa comercial Symta. El
equipo empleado para realizar las medidas de absorbancia de ultravioleta en placas de 96 pocilios fue un Thermoscientific Multiskan spectrum. The prediction of the permeability of various compounds on the central nervous system (CNS) was determined using the methodology of parallel artificial membranes (PAMPA) [Di, L., Kerns, EH, Fan, K., McConnell, OJ, Carter, GT "High throughput artificial membrane permeability assay for blood-brain barrier" Eur. J. Med. Chem., 2003, 38, 223-232]. Commercial compounds, pH 7.4 phosphate buffer (PBS), ethanol and dodecane were obtained from the Sigma, Acros organics, Merck, Aldrich and Fluka commercial houses. The porcine brain lipid (reference catalog 141 101) was purchased from Avanti Polar Lipids. Both the 96-well donor plate (Multiscreen® IP Sterile Píate membrane PDVF, pore size 0.45 μΜ, reference catalog MAIPS4510) and the 96-well acceptor plate (Multiscreen®, reference catalog MAMCS9610) were purchased from Millipore. In order to filter the samples, PDVF membrane filters (30 mm in diameter, pore size 0.45 μηπ) from the Symta commercial house were used. He The equipment used to perform ultraviolet absorbance measurements in 96-well plates was a Thermoscientific Multiskan spectrum.
Diez compuestos fueron seleccionados con el fin de validar el experimento. Se tomaron distintas cantidades de los mismos [(3-5 mg de Cafeína, Enoxacino, Hidrocortisona, Desipramina, Ofloxacino, Piroxicam, Testosterona), (12 mg de Promazina) y 25 mg de Verapamilo y Atenolol] los cuales fueron disueltos en EtOH (1000 μΙ_). Se tomaron 100 microlitros de estas disoluciones y se añadieron 1400 μΙ_ de EtOH y 3500 μΙ_ de tampón fosfato PBS (pH 7,4) buffer, con el fin de alcanzar una concentración final de EtOH del 30% en la disolución. Se filtraron las disoluciones. 180 μΙ_ de una disolución de PBS/EtOH (70/30) fue añadida a cada pocilio de la placa aceptora de 96 pocilios. La placa donadora fue impregnada con 4 μΙ_ de una disolución del lípido de cerebro porcino disuelto en dodecano (20 mg mi"1). Una vez transcurridos 5 minutos, 180 μΙ_ de disolución de cada compuesto fue añadido sobre esta placa. De los compuestos a evaluar su penetración en el sistema nervioso central, se tomaron entre 1 -2 mg y fueron disueltos en 1500 μΙ_ de EtOH y 3500 μΙ_ de tampón fosfato PBS (pH 7,4) buffer, se filtraron y se añadieron a la placa donadora de 96 pocilios. A continuación la placa donadora se puso sobre la aceptora formando una especie de "sandwich" y se dejaron incubando durante 2h a 25 °C. Los compuestos irán pasando de la placa donadora a través del lípido de cerebro porcino a la placa aceptora. Transcurridas las 2h, se retira cuidadosamente la placa donadora. La concentración y absorbancia tanto de los compuestos comerciales como los derivados sintetizados que se evaluaron en las placas aceptoras y donadoras fueron determinadas empleando un lector de absorbancia de UV. Cada muestra fue analizada de 3 a 5 longitudes de onda, en 3 pocilios y en 2 experimentos independientes como mínimo. Los resultados son la media de las medidas [desviación estandard (SD)] de los distintos experimentos realizados. 10 compuestos comerciales (comentados previamente) cuya penetración en el sistema nervioso central es conocida, fueron utilizados en cada experimento con el fin de validar el método.
Tabla 2. Permeabilidad (Pe 10"6 cm s"1) en el experimento PAMPA-Barrera hematoencefálica para 10 compuestos comerciales (empleados para la validación del experimento) y distintos derivados sintetizados con su correspondiente predicción de penetración en el sistema nervioso central (SNC)). Ten compounds were selected in order to validate the experiment. Different amounts of them were taken [(3-5 mg of Caffeine, Enoxacin, Hydrocortisone, Desipramine, Ofloxacin, Piroxicam, Testosterone), (12 mg of Promazine) and 25 mg of Verapamil and Atenolol] which were dissolved in EtOH ( 1000 μΙ_). 100 microliters of these solutions were taken and 1400 μΙ_ of EtOH and 3500 μΙ_ of phosphate buffer PBS (pH 7.4) buffer were added, in order to reach a final concentration of EtOH of 30% in the solution. The solutions were filtered. 180 μΙ of a solution of PBS / EtOH (70/30) was added to each well of the 96-well acceptor plate. The donor plate was impregnated with 4 μΙ_ of a solution of the porcine brain lipid dissolved in dodecane (20 mg mi “1 ). After 5 minutes, 180 μΙ_ of solution of each compound was added on this plate. To assess their penetration into the central nervous system, they were taken between 1-2 mg and dissolved in 1500 μΙ_ of EtOH and 3500 μΙ_ of phosphate buffer PBS (pH 7.4) buffer, filtered and added to the donor plate 96 Wells, the donor plate was then placed on the acceptor forming a kind of "sandwich" and left to incubate for 2h at 25 ° C. The compounds will pass from the donor plate through the porcine brain lipid to the acceptor plate. After 2 hours, the donor plate is carefully removed.The concentration and absorbance of both commercial compounds and synthesized derivatives that were evaluated in the acceptor and donor plates were determined using or n UV absorbance reader. Each sample was analyzed for 3 to 5 wavelengths, in 3 wells and in at least 2 independent experiments. The results are the average of the measurements [standard deviation (SD)] of the different experiments performed. 10 commercial compounds (previously discussed) whose penetration into the central nervous system is known, were used in each experiment in order to validate the method. Table 2. Permeability (Pe 10 "6 cm s " 1 ) in the PAMPA-Blood-brain Barrier experiment for 10 commercial compounds (used for the validation of the experiment) and various derivatives synthesized with their corresponding prediction of central nervous system penetration (CNS) )).
Compuesto Pe (10 6 cm s 1)3 Pe (10 6 cm s 1)" Predicción Compound Pe (10 6 cm s 1 ) 3 Pe (10 6 cm s 1 ) "Prediction
Atenolol 0,8 0,2 ± 0,1 Atenolol 0.8 0.2 ± 0.1
Cafeína 1 ,3 0,9 ± 0,2 Caffeine 1, 3 0.9 ± 0.2
Desipramina 12 12,4 ± 1 ,0 Desipramine 12 12.4 ± 1.0
Enoxacino 0,9 0,3 ± 0,2 Enoxacin 0.9 0.3 ± 0.2
Hidrocortisona 1 ,9 0,6 ± 0,4 Hydrocortisone 1, 9 0.6 ± 0.4
Ofloxacino 0,8 0,7 ± 0,7 Ofloxacin 0.8 0.7 ± 0.7
Piroxicam 2,5 0,4 ± 0,4 Piroxicam 2.5 0.4 ± 0.4
Promazina 8,8 1 1 , 5 ± 0,9 Promazine 8.8 1 1, 5 ± 0.9
Testosterona 17 15,7 ± 1 ,2 Testosterone 17 15.7 ± 1, 2
Verapamilo 1 6 15,6 ± 1 ,2 Verapamil 1 6 15.6 ± 1, 2
Comp. 1 1 ,5 ± 0,5 SNC + / SNC - Comp. 1 1, 5 ± 0.5 SNC + / SNC -
Comp. 2 6,4 ± 1 ,5 SNC + Comp. 2 6.4 ± 1.5 SNC +
Comp. 3 1 ,1 ± 0,3 SNC - Comp. 3 1, 1 ± 0.3 SNC -
Comp. 4 3,9 ± 1 ,0 SNC + Comp. 4 3.9 ± 1.0 SNC +
PBS:EtOH (70:30) empleado como disolvente. a Datos estimados y presentados en el artículo Eur. J. Med. Chem., 2003, 38, 223-232. b Media de datos ± desviación estándar, de al menos 2 experimentos independientes. PBS: EtOH (70:30) used as solvent. a Data estimated and presented in Article Eur. J. Med. Chem., 2003, 38, 223-232. b Data average ± standard deviation of at least 2 independent experiments.
Estos datos sugieren que los compuestos 2 y 4 pueden resultar más útiles en patologías mediadas por GSK-3 que afecten al sitema nervisoso central y que posean la berrera hematoencefalica intacta. Mientras que los compuestos 1 y 3, al no pasar en principio la barrera hematoencefálica pueden ser de más utilidad en enfermedades mediadas por GSK-3 de sistema periférico.
These data suggest that compounds 2 and 4 may be more useful in pathologies mediated by GSK-3 that affect the central nervous system and that have the intact hematoencephalic barrier. While compounds 1 and 3, since the blood brain barrier does not pass in principle, they may be more useful in diseases mediated by GSK-3 peripheral system.
Claims
1 . Compuesto de fórmula (I): one . Compound of formula (I):
donde where
R1 se selecciona entre H, alquilo C-i-C-m o alquenilo C1-C10 y R2 se selecciona entre alquilo C-i-C-ιο θ alquenilo C1-C10 opcionalmente sustituidos por halógeno, R 1 is selected from H, CiCm alkyl or C1-C10 alkenyl and R 2 is selected from CiC-ιο θ C1-C10 alkenyl optionally substituted by halogen,
o sus sales, solvatos o isómeros farmacéuticamente aceptables. or its pharmaceutically acceptable salts, solvates or isomers.
2. Compuesto según la reivindicación 1 donde R1 es H. 2. Compound according to claim 1 wherein R 1 is H.
3. Compuesto según la reivindicación 1 donde R1 es alquilo C C4. 3. Compound according to claim 1 wherein R 1 is CC 4 alkyl.
4. Compuesto según la reivindicación 3 donde R1 es metilo. 4. Compound according to claim 3 wherein R 1 is methyl.
5. Compuesto según cualquiera de las reivindicaciones 1 a 4 donde R2 es alquilo d-C4. 5. Compound according to any of claims 1 to 4 wherein R 2 is dC 4 alkyl.
6. Compuesto según la reivindicación 5 donde R2 es metilo. 6. Compound according to claim 5 wherein R 2 is methyl.
7. Compuesto según cualquiera de las reivindicaciones 1 a 4 donde R2 es - CH2Br. 7. Compound according to any of claims 1 to 4 wherein R 2 is - CH 2 Br.
8. Compuesto de fórmula (I) que se selecciona de la lista que comprende: 8. Compound of formula (I) that is selected from the list comprising:
■ 3-acetil-4-(1 H-indol-3-il)-1 H-pirrol-2,5-diona ■ 3-acetil-4-(1 -metil-1 H-indol-3-il)-1 H-pirrol-2,5-diona■ 3-acetyl-4- (1 H-indole-3-yl) -1 H-pyrrole-2,5-dione ■ 3-Acetyl-4- (1-methyl-1 H-indole-3-yl) -1 H-pyrrole-2,5-dione
■ 3-(2-bromoacetil)-4-(1 H-indol-3-il)-1 H-pirrol-2,5-diona ■ 3- (2-Bromoacetyl) -4- (1 H-indole-3-yl) -1 H-pyrrole-2,5-dione
■ 3-(2-bromoacetil)-4-(1 H-indol-3-il)-1 H-pirrol-2,5-diona ■ 3- (2-Bromoacetyl) -4- (1 H-indole-3-yl) -1 H-pyrrole-2,5-dione
o sus sales, solvatos o isómeros farmacéuticamente aceptables. or its pharmaceutically acceptable salts, solvates or isomers.
9. Uso de un compuesto de fórmula (I) según cualquiera de las reivindicaciones 1 a 8 para la fabricación de un medicamento. 9. Use of a compound of formula (I) according to any of claims 1 to 8 for the manufacture of a medicament.
10. Uso de un compuesto de fórmula (I) según cualquiera de las reivindicaciones 1 a 8 para la fabricación de un medicamento para el tratamiento y/o prevención de una enfermedad que se selecciona entre enfermedades neurodegenerativas, enfermedades inflamatorias, cáncer, diabetes y procesos regenerativos. 10. Use of a compound of formula (I) according to any of claims 1 to 8 for the manufacture of a medicament for the treatment and / or prevention of a disease selected from neurodegenerative diseases, inflammatory diseases, cancer, diabetes and processes regenerative
1 1 . Uso según la reivindicación 10 donde la enfermedad neurodegenerativa se selecciona entre enfermedad de Alzheimer, enfermedad de Parkinson, esclerosis lateral amiotrófica, isquemia cerebral, parkinsonismos post- encefalítico, distonias, síndrome de Tourette's, patologías de movimientos límbicos periódicos, síndrome de piernas inquietas, trastornos de déficit de atención con hiperactividad, enfermedad de Hungtinton, parálisis supranuclear progresiva, enfermedad de Pick, demencia frontotemporal, enfermedades neuromusculares. eleven . Use according to claim 10 wherein the neurodegenerative disease is selected from Alzheimer's disease, Parkinson 's disease, amyotrophic lateral sclerosis, cerebral ischemia, Parkinsonism encephalitic post, dystonia, Tourette 's, periodic limb movements pathologies, restless leg syndrome , attention deficit hyperactivity disorders, Hungtinton's disease, progressive supranuclear palsy, Pick's disease, frontotemporal dementia, neuromuscular diseases.
12. Uso según la reivindicación 10 donde la enfermedad inflamatoria se selecciona entre enfermedad de Crohn, colitis ulcerosa, artritis reumatoide, aterosclerosis, vasculitis o esclerosis múltiple. 12. Use according to claim 10 wherein the inflammatory disease is selected from Crohn's disease, ulcerative colitis, rheumatoid arthritis, atherosclerosis, vasculitis or multiple sclerosis.
13. Uso según la reivindicación 10 donde el cáncer se selecciona entre glioblastoma, leucemias, linfomas, cáncer de pulmón, de mama, de próstata o de colon. 13. Use according to claim 10 wherein the cancer is selected from glioblastoma, leukemia, lymphomas, lung, breast, prostate or colon cancer.
14. Uso según la reivindicación 10 donde la diabetes se selecciona como diabetes tipo II, insulino no dependiente. 14. Use according to claim 10 wherein the diabetes is selected as non-dependent insulin type II diabetes.
1 5. Uso de un compuesto de fórmula (I) según cualquiera de las reivindicaciones 1 a 8 para la fabricación de un medicamento para promover procesos regenerativos. 1 5. Use of a compound of formula (I) according to any of claims 1 to 8 for the manufacture of a medicament to promote regenerative processes.
1 6. Uso según la reivindicación 15 donde en el proceso regenerativo está implicada la diferenciación de las células madres del sistema nervioso, del sistema hematopoyético, del sistema óseo o del miocardio. 1 6. Use according to claim 15 where differentiation of the stem cells of the nervous system, the hematopoietic system, the bone system or the myocardium is involved in the regenerative process.
17. Composición farmacéutica que comprende un compuesto de fórmula (I) según cualquiera de las reivindicaciones 1 a 8 y un vehículo o adyuvante farmacéuticamente aceptable. 17. Pharmaceutical composition comprising a compound of formula (I) according to any one of claims 1 to 8 and a pharmaceutically acceptable carrier or adjuvant.
1 8. Composición según la reivindicación 1 7 que además comprende otro principio activo. 1 8. Composition according to claim 1 7 further comprising another active ingredient.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES201130253A ES2387359B1 (en) | 2011-02-25 | 2011-02-25 | GSK-3 inhibitors useful in neurodegenerative, inflammatory diseases, cancer, diabetes and in regenerative processes |
| ESP201130253 | 2011-02-25 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012113967A1 true WO2012113967A1 (en) | 2012-08-30 |
Family
ID=46720141
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/ES2012/070119 WO2012113967A1 (en) | 2011-02-25 | 2012-02-27 | Gsk-3 inhibitors that can be used in neurodegenerative and inflammatory diseases, cancer, diabetes and in regenerative processes |
Country Status (2)
| Country | Link |
|---|---|
| ES (1) | ES2387359B1 (en) |
| WO (1) | WO2012113967A1 (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003104222A1 (en) * | 2002-06-05 | 2003-12-18 | Janssen Pharmaceutica N.V. | Bisindolyl-maleimid derivatives as kinase inhibitors |
| WO2005000836A1 (en) * | 2003-06-13 | 2005-01-06 | Janssen Pharmaceutica N.V. | Substituted indazolyl(indolyl)maleimide derivatives as kinase inhibitors |
-
2011
- 2011-02-25 ES ES201130253A patent/ES2387359B1/en not_active Withdrawn - After Issue
-
2012
- 2012-02-27 WO PCT/ES2012/070119 patent/WO2012113967A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003104222A1 (en) * | 2002-06-05 | 2003-12-18 | Janssen Pharmaceutica N.V. | Bisindolyl-maleimid derivatives as kinase inhibitors |
| WO2005000836A1 (en) * | 2003-06-13 | 2005-01-06 | Janssen Pharmaceutica N.V. | Substituted indazolyl(indolyl)maleimide derivatives as kinase inhibitors |
Non-Patent Citations (1)
| Title |
|---|
| M FAUL ET AL.: "A new one step synthesis of maleimides by condensation of glyoxylate esters with acetamides", TETRAHEDRON LETTERS, vol. 40, 1999, pages 1109 - 1112 * |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2387359A1 (en) | 2012-09-20 |
| ES2387359B1 (en) | 2013-08-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| ES2871949T3 (en) | N- [4-fluoro-5 - [[(2S, 4S) -2-methyl-4 - [(5-methyl-1,2,4-oxadiazol-3-yl) methoxy] -1-piperidylmethyl] thiazole- 2-yl] acetamide as an OGA inhibitor | |
| ES2446269T3 (en) | 3-Benzofuranyl-4-indolyl-maleimides as potent inhibitors of GSK-3 for neurodegenerative disorders | |
| JP6518270B2 (en) | Novel Thiadiazolidinediones as GSK-3 Inhibitors | |
| CA2686378C (en) | Pyrimidine derivatives and compositions as c-kit and pdgfr kinase inhibitors | |
| ES2754207T3 (en) | RNA polymerase I inhibitors and uses thereof | |
| ES2886910T3 (en) | Pyrrolopyrimidine crystal to prepare JAK inhibitor | |
| WO2011051535A1 (en) | Bis(aralkyl)amino and six membered (hetero)aromatic system derivatives and the use thereof in the treatment of neurodegenerative pathological conditions, including alzheimer's disease | |
| PT2099797E (en) | Compounds and compositions as protein kinase inhibitors | |
| BRPI0711080A2 (en) | use of a compound, compound, pharmaceutical composition and process for the preparation of this compound | |
| CN110240629A (en) | Protein degradation targets BCR-ABL compound and its antitumor application thereof | |
| WO2015058661A1 (en) | Bcr-abl kinase inhibitor and application thereof | |
| WO2014114825A1 (en) | Substituted benzothiazoles and therapeutic uses thereof for the treatment of human diseases | |
| BR112019018688A2 (en) | imidazo [4,5-c] quinoline derivatives as lrrk2 inhibitors | |
| CN110357905B (en) | Macrocyclic derivatives as protein kinase inhibitors, and preparation method and application thereof | |
| TW201712009A (en) | Pyrazolo[4,3-c]quinoline derivatives inhibition [beta]-glucuronidase | |
| ES2387359B1 (en) | GSK-3 inhibitors useful in neurodegenerative, inflammatory diseases, cancer, diabetes and in regenerative processes | |
| KR20210114377A (en) | Quaternary ammonium salts of nicotinic acid and nicotinamide mononucleotide and riboside as antioxidants | |
| US9757369B2 (en) | Heterocyclic GSK-3 allosteric modulators | |
| ES2710199T3 (en) | New inhibitors of PI3K / AKT / mTOR and pharmaceutical uses thereof | |
| ES2749743B2 (en) | PURINE DERIVATIVES INHIBITORS OF CDC7 AND THEIR USE FOR THE TREATMENT OF NEUROLOGICAL PATHOLOGIES | |
| ES2744304B2 (en) | LRRK2 inhibitor compounds and their use for the treatment of neurodegenerative diseases | |
| ES2686909A1 (en) | CDC-7 INHIBITORS COMPOUNDS AND THEIR USE FOR THE TREATMENT OF NEUROLOGICAL PATHOLOGIES (Machine-translation by Google Translate, not legally binding) | |
| TW201305171A (en) | Phthalazinone derivatives, preparation process and pharmaceutical use thereof | |
| WO2015150612A1 (en) | Substituted isatins and the therapeutic applications thereof for the treatment of neurodegenerative diseases |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12748880 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12748880 Country of ref document: EP Kind code of ref document: A1 |