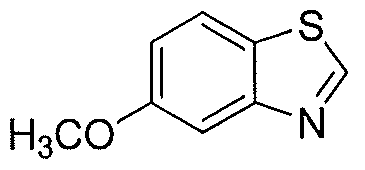
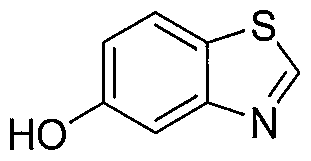
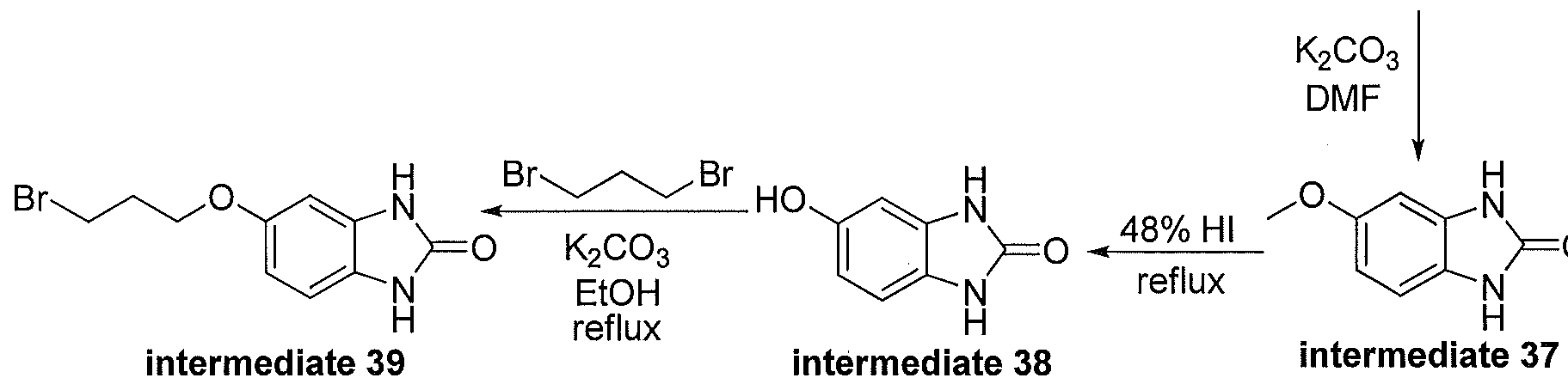
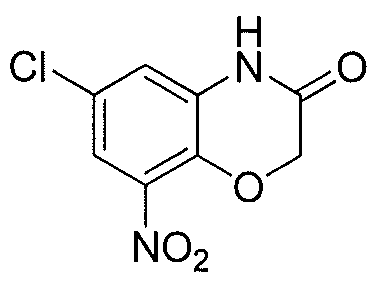
WO2012003418A2 - Functionally selective ligands of dopamine d2 receptors - Google Patents
Functionally selective ligands of dopamine d2 receptors Download PDFInfo
- Publication number
- WO2012003418A2 WO2012003418A2 PCT/US2011/042734 US2011042734W WO2012003418A2 WO 2012003418 A2 WO2012003418 A2 WO 2012003418A2 US 2011042734 W US2011042734 W US 2011042734W WO 2012003418 A2 WO2012003418 A2 WO 2012003418A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- butoxy
- benzo
- diazepan
- compound
- dichlorophenyl
- Prior art date
Links
- 239000003446 ligand Substances 0.000 title abstract description 24
- 102000004980 Dopamine D2 Receptors Human genes 0.000 title 1
- 108090001111 Dopamine D2 Receptors Proteins 0.000 title 1
- 150000001875 compounds Chemical class 0.000 claims abstract description 212
- 208000015114 central nervous system disease Diseases 0.000 claims abstract description 14
- 230000000694 effects Effects 0.000 claims description 45
- 102000005962 receptors Human genes 0.000 claims description 43
- 108020003175 receptors Proteins 0.000 claims description 43
- 238000000034 method Methods 0.000 claims description 32
- 239000003814 drug Substances 0.000 claims description 30
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 28
- 125000001072 heteroaryl group Chemical group 0.000 claims description 25
- 108050004812 Dopamine receptor Proteins 0.000 claims description 24
- 102000015554 Dopamine receptor Human genes 0.000 claims description 24
- 229940002612 prodrug Drugs 0.000 claims description 24
- 239000000651 prodrug Substances 0.000 claims description 24
- 150000003839 salts Chemical class 0.000 claims description 23
- 208000035475 disorder Diseases 0.000 claims description 22
- 229910052760 oxygen Inorganic materials 0.000 claims description 19
- 208000028017 Psychotic disease Diseases 0.000 claims description 17
- 108010080367 beta-Arrestins Proteins 0.000 claims description 17
- 102000000072 beta-Arrestins Human genes 0.000 claims description 17
- 230000003287 optical effect Effects 0.000 claims description 16
- 125000003118 aryl group Chemical group 0.000 claims description 15
- 125000002950 monocyclic group Chemical group 0.000 claims description 15
- 125000004106 butoxy group Chemical group [*]OC([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 claims description 14
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 14
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 claims description 13
- 208000012902 Nervous system disease Diseases 0.000 claims description 12
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical compound C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 claims description 12
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 claims description 10
- 125000004188 dichlorophenyl group Chemical group 0.000 claims description 10
- TZOYXRMEFDYWDQ-UHFFFAOYSA-N 3,4-dihydro-1h-quinolin-2-one Chemical compound C1=CC=C2NC(=O)CCC2=C1 TZOYXRMEFDYWDQ-UHFFFAOYSA-N 0.000 claims description 9
- 239000008194 pharmaceutical composition Substances 0.000 claims description 8
- 125000002572 propoxy group Chemical group [*]OC([H])([H])C(C([H])([H])[H])([H])[H] 0.000 claims description 8
- LISFMEBWQUVKPJ-UHFFFAOYSA-N quinolin-2-ol Chemical compound C1=CC=C2NC(=O)C=CC2=C1 LISFMEBWQUVKPJ-UHFFFAOYSA-N 0.000 claims description 8
- 125000005605 benzo group Chemical group 0.000 claims description 7
- 229940124597 therapeutic agent Drugs 0.000 claims description 7
- ASSKVPFEZFQQNQ-UHFFFAOYSA-N 2-benzoxazolinone Chemical compound C1=CC=C2OC(O)=NC2=C1 ASSKVPFEZFQQNQ-UHFFFAOYSA-N 0.000 claims description 6
- FBXGQDUVJBKEAJ-UHFFFAOYSA-N 4h-oxazin-3-one Chemical compound O=C1CC=CON1 FBXGQDUVJBKEAJ-UHFFFAOYSA-N 0.000 claims description 6
- 239000003937 drug carrier Substances 0.000 claims description 6
- 208000020016 psychiatric disease Diseases 0.000 claims description 6
- 229930185107 quinolinone Natural products 0.000 claims description 6
- 208000024827 Alzheimer disease Diseases 0.000 claims description 5
- 208000020925 Bipolar disease Diseases 0.000 claims description 5
- 208000014993 Pituitary disease Diseases 0.000 claims description 5
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 5
- VHSIAYLBCLUAFT-UHFFFAOYSA-N n-[3-acetyl-6-(4-chlorophenyl)-7-(2,4-dichlorophenyl)-1-methyl-2-oxo-1,8-naphthyridin-4-yl]acetamide Chemical group C=1C=C(Cl)C=CC=1C=1C=C2C(NC(=O)C)=C(C(C)=O)C(=O)N(C)C2=NC=1C1=CC=C(Cl)C=C1Cl VHSIAYLBCLUAFT-UHFFFAOYSA-N 0.000 claims description 5
- 201000000980 schizophrenia Diseases 0.000 claims description 5
- 208000017701 Endocrine disease Diseases 0.000 claims description 4
- 208000025282 Hypothalamo-pituitary disease Diseases 0.000 claims description 4
- 206010026749 Mania Diseases 0.000 claims description 4
- 208000030172 endocrine system disease Diseases 0.000 claims description 4
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 claims description 4
- FZVBQNQGKGJASF-UHFFFAOYSA-N 1,3,4,4a-tetrahydropyrido[3,2-b]azepin-2-one Chemical compound N1=CC=CC=C2NC(=O)CCC21 FZVBQNQGKGJASF-UHFFFAOYSA-N 0.000 claims description 3
- AICIYIDUYNFPRY-UHFFFAOYSA-N 1,3-dihydro-2H-imidazol-2-one Chemical compound O=C1NC=CN1 AICIYIDUYNFPRY-UHFFFAOYSA-N 0.000 claims description 3
- VHMICKWLTGFITH-UHFFFAOYSA-N 2H-isoindole Chemical compound C1=CC=CC2=CNC=C21 VHMICKWLTGFITH-UHFFFAOYSA-N 0.000 claims description 3
- KQJVDEBWHJRJBT-UHFFFAOYSA-N 3,4-dihydro-1h-1,8-naphthyridin-2-one Chemical compound C1=CN=C2NC(=O)CCC2=C1 KQJVDEBWHJRJBT-UHFFFAOYSA-N 0.000 claims description 3
- NKDQNOFCLUVBIY-UHFFFAOYSA-N 5-[3-[4-(2,3-dichlorophenyl)piperazin-1-yl]propoxy]-1,3-dihydrobenzimidazol-2-one Chemical compound ClC1=CC=CC(N2CCN(CCCOC=3C=C4NC(=O)NC4=CC=3)CC2)=C1Cl NKDQNOFCLUVBIY-UHFFFAOYSA-N 0.000 claims description 3
- WOXYKTMEOIXZAQ-UHFFFAOYSA-N 6-[3-[4-(2,3-dichlorophenyl)piperazin-1-yl]propoxy]-1h-indazole Chemical compound ClC1=CC=CC(N2CCN(CCCOC=3C=C4NN=CC4=CC=3)CC2)=C1Cl WOXYKTMEOIXZAQ-UHFFFAOYSA-N 0.000 claims description 3
- MCIWPVPYVLZFFO-UHFFFAOYSA-N 7-[4-[[4-(2,3-dichlorophenyl)piperazin-1-yl]methyl]phenoxy]-3,4-dihydro-1h-quinolin-2-one Chemical compound ClC1=CC=CC(N2CCN(CC=3C=CC(OC=4C=C5NC(=O)CCC5=CC=4)=CC=3)CC2)=C1Cl MCIWPVPYVLZFFO-UHFFFAOYSA-N 0.000 claims description 3
- 208000019901 Anxiety disease Diseases 0.000 claims description 3
- 208000020401 Depressive disease Diseases 0.000 claims description 3
- 208000023105 Huntington disease Diseases 0.000 claims description 3
- VBHBKXBUHHJVBZ-UHFFFAOYSA-N 5-[3-[4-(2,3-dichlorophenyl)piperazin-1-yl]propoxy]-1,3-benzothiazole Chemical compound ClC1=CC=CC(N2CCN(CCCOC=3C=C4N=CSC4=CC=3)CC2)=C1Cl VBHBKXBUHHJVBZ-UHFFFAOYSA-N 0.000 claims description 2
- URYZTMIOHYGTNJ-UHFFFAOYSA-N 5-[3-[4-(2,3-dichlorophenyl)piperidin-1-yl]propoxy]-1,3-benzothiazole Chemical compound ClC1=CC=CC(C2CCN(CCCOC=3C=C4N=CSC4=CC=3)CC2)=C1Cl URYZTMIOHYGTNJ-UHFFFAOYSA-N 0.000 claims description 2
- JBKITGSPOFFGJB-UHFFFAOYSA-N 5-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-1,3-benzothiazole Chemical compound ClC1=CC=CC(N2CCN(CCCCOC=3C=C4N=CSC4=CC=3)CC2)=C1Cl JBKITGSPOFFGJB-UHFFFAOYSA-N 0.000 claims description 2
- DXXXGJIMFKZHRL-UHFFFAOYSA-N 5-[4-[4-(2,3-dichlorophenyl)piperidin-1-yl]butoxy]-1,3-benzothiazole Chemical compound ClC1=CC=CC(C2CCN(CCCCOC=3C=C4N=CSC4=CC=3)CC2)=C1Cl DXXXGJIMFKZHRL-UHFFFAOYSA-N 0.000 claims description 2
- JEZQFKLGYQTDDK-UHFFFAOYSA-N 5-[4-[4-(2,3-dichlorophenyl)piperidin-1-yl]butoxy]-1,3-dihydrobenzimidazol-2-one Chemical compound ClC1=CC=CC(C2CCN(CCCCOC=3C=C4NC(=O)NC4=CC=3)CC2)=C1Cl JEZQFKLGYQTDDK-UHFFFAOYSA-N 0.000 claims description 2
- PSNCKHBBWMUIGZ-UHFFFAOYSA-N 6-[3-[4-(2,3-dichlorophenyl)piperidin-1-yl]propoxy]-1h-indazole Chemical compound ClC1=CC=CC(C2CCN(CCCOC=3C=C4NN=CC4=CC=3)CC2)=C1Cl PSNCKHBBWMUIGZ-UHFFFAOYSA-N 0.000 claims description 2
- FZUOKOWBQHNFMZ-UHFFFAOYSA-N 6-[3-[4-(2-methoxyphenyl)piperazin-1-yl]propoxy]-1h-indazole Chemical compound COC1=CC=CC=C1N1CCN(CCCOC=2C=C3NN=CC3=CC=2)CC1 FZUOKOWBQHNFMZ-UHFFFAOYSA-N 0.000 claims description 2
- IKOXHMZXTCWMQE-UHFFFAOYSA-N 6-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-1h-indazole Chemical compound ClC1=CC=CC(N2CCN(CCCCOC=3C=C4NN=CC4=CC=3)CC2)=C1Cl IKOXHMZXTCWMQE-UHFFFAOYSA-N 0.000 claims description 2
- JURMNSZLXFFVBL-UHFFFAOYSA-N 6-[4-[4-(2,3-dichlorophenyl)piperidin-1-yl]butoxy]-1h-indazole Chemical compound ClC1=CC=CC(C2CCN(CCCCOC=3C=C4NN=CC4=CC=3)CC2)=C1Cl JURMNSZLXFFVBL-UHFFFAOYSA-N 0.000 claims description 2
- PGUULVJMJLJLQW-UHFFFAOYSA-N 6-[4-[4-(2-methoxyphenyl)piperazin-1-yl]butoxy]-1h-indazole Chemical compound COC1=CC=CC=C1N1CCN(CCCCOC=2C=C3NN=CC3=CC=2)CC1 PGUULVJMJLJLQW-UHFFFAOYSA-N 0.000 claims description 2
- SKGKYEPRBPJYRW-OWOJBTEDSA-N 7-[(e)-4-[4-(2,3-dichlorophenyl)piperazin-1-yl]but-2-enoxy]-3,4-dihydro-1h-quinolin-2-one Chemical compound ClC1=CC=CC(N2CCN(C\C=C\COC=3C=C4NC(=O)CCC4=CC=3)CC2)=C1Cl SKGKYEPRBPJYRW-OWOJBTEDSA-N 0.000 claims description 2
- PULGLQOWTRCVQB-UHFFFAOYSA-N 7-[3-[[4-(2,3-dichlorophenyl)piperazin-1-yl]methyl]phenoxy]-3,4-dihydro-1h-quinolin-2-one Chemical compound ClC1=CC=CC(N2CCN(CC=3C=C(OC=4C=C5NC(=O)CCC5=CC=4)C=CC=3)CC2)=C1Cl PULGLQOWTRCVQB-UHFFFAOYSA-N 0.000 claims description 2
- UEDWVSDCSLRXEQ-UHFFFAOYSA-N 7-[4-[3-(1h-indazol-6-yloxy)propyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2C=NNC2=CC(OCCCN2CCN(CC2)C=2C=CC=C3NC(OC3=2)=O)=C1 UEDWVSDCSLRXEQ-UHFFFAOYSA-N 0.000 claims description 2
- LFJXYNBEMDCXPW-UHFFFAOYSA-N 7-[4-[4-(1h-indazol-6-yloxy)butyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2C=NNC2=CC(OCCCCN2CCN(CC2)C=2C=CC=C3NC(OC3=2)=O)=C1 LFJXYNBEMDCXPW-UHFFFAOYSA-N 0.000 claims description 2
- FNZOOYYZKQCPEG-UHFFFAOYSA-N 7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]but-2-ynoxy]-3,4-dihydro-1h-quinolin-2-one Chemical compound ClC1=CC=CC(N2CCN(CC#CCOC=3C=C4NC(=O)CCC4=CC=3)CC2)=C1Cl FNZOOYYZKQCPEG-UHFFFAOYSA-N 0.000 claims description 2
- HVSUPPZVIPTMEM-UHFFFAOYSA-N 7-[4-[4-(2-methoxyphenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1h-quinolin-2-one Chemical compound COC1=CC=CC=C1N1CCN(CCCCOC=2C=C3NC(=O)CCC3=CC=2)CC1 HVSUPPZVIPTMEM-UHFFFAOYSA-N 0.000 claims description 2
- 208000020706 Autistic disease Diseases 0.000 claims description 2
- 208000001287 Galactorrhea Diseases 0.000 claims description 2
- 206010017600 Galactorrhoea Diseases 0.000 claims description 2
- 208000021384 Obsessive-Compulsive disease Diseases 0.000 claims description 2
- 208000007913 Pituitary Neoplasms Diseases 0.000 claims description 2
- 208000020186 Schizophreniform disease Diseases 0.000 claims description 2
- 208000000323 Tourette Syndrome Diseases 0.000 claims description 2
- 208000016620 Tourette disease Diseases 0.000 claims description 2
- 208000029560 autism spectrum disease Diseases 0.000 claims description 2
- 208000028683 bipolar I disease Diseases 0.000 claims description 2
- 208000025307 bipolar depression Diseases 0.000 claims description 2
- 201000003104 endogenous depression Diseases 0.000 claims description 2
- 208000024714 major depressive disease Diseases 0.000 claims description 2
- PYODBBFAXCRGHN-UHFFFAOYSA-N n-[5-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-2-methylphenyl]acetamide Chemical compound C1=C(C)C(NC(=O)C)=CC(OCCCCN2CCN(CC2)C=2C(=C(Cl)C=CC=2)Cl)=C1 PYODBBFAXCRGHN-UHFFFAOYSA-N 0.000 claims description 2
- 208000030153 prolactin-producing pituitary gland adenoma Diseases 0.000 claims description 2
- 208000022610 schizoaffective disease Diseases 0.000 claims description 2
- 208000019022 Mood disease Diseases 0.000 claims 2
- UJZYHMZRXGNDFB-UHFFFAOYSA-N 1,3-benzothiazol-5-amine Chemical compound NC1=CC=C2SC=NC2=C1 UJZYHMZRXGNDFB-UHFFFAOYSA-N 0.000 claims 1
- BXZAIRAIQWJAOE-UHFFFAOYSA-N 2-[4-[4-[(7-oxo-6,8-dihydro-5h-1,8-naphthyridin-2-yl)oxy]butyl]-1,4-diazepan-1-yl]benzonitrile Chemical compound N1=C2NC(=O)CCC2=CC=C1OCCCCN(CC1)CCCN1C1=CC=CC=C1C#N BXZAIRAIQWJAOE-UHFFFAOYSA-N 0.000 claims 1
- RIBSVWFGPDTXOJ-UHFFFAOYSA-N 5-[3-[4-(2,3-dichlorophenyl)-1,4-diazepan-1-yl]propylamino]-1,3-dihydrobenzimidazol-2-one Chemical compound ClC1=CC=CC(N2CCN(CCCNC=3C=C4NC(=O)NC4=CC=3)CCC2)=C1Cl RIBSVWFGPDTXOJ-UHFFFAOYSA-N 0.000 claims 1
- NSIDSQRHPHVVHK-UHFFFAOYSA-N 5-[3-[4-(2,3-dichlorophenyl)piperazin-1-yl]propylamino]-1,3-dihydrobenzimidazol-2-one Chemical compound ClC1=CC=CC(N2CCN(CCCNC=3C=C4NC(=O)NC4=CC=3)CC2)=C1Cl NSIDSQRHPHVVHK-UHFFFAOYSA-N 0.000 claims 1
- CXEVWLGSLGMVQX-UHFFFAOYSA-N 5-[3-[4-(2,3-dichlorophenyl)piperidin-1-yl]propylamino]-1,3-dihydrobenzimidazol-2-one Chemical compound ClC1=CC=CC(C2CCN(CCCNC=3C=C4NC(=O)NC4=CC=3)CC2)=C1Cl CXEVWLGSLGMVQX-UHFFFAOYSA-N 0.000 claims 1
- FESUJISSANOILM-UHFFFAOYSA-N 5-[3-[4-(2-methoxyphenyl)piperazin-1-yl]propoxy]-1,3-benzothiazole Chemical compound COC1=CC=CC=C1N1CCN(CCCOC=2C=C3N=CSC3=CC=2)CC1 FESUJISSANOILM-UHFFFAOYSA-N 0.000 claims 1
- AGEKQJSUYGOWJW-UHFFFAOYSA-N 5-[3-[4-(2-methoxyphenyl)piperazin-1-yl]propoxy]-1,3-dihydrobenzimidazol-2-one Chemical compound COC1=CC=CC=C1N1CCN(CCCOC=2C=C3NC(=O)NC3=CC=2)CC1 AGEKQJSUYGOWJW-UHFFFAOYSA-N 0.000 claims 1
- BTASWECXNYOPIX-UHFFFAOYSA-N 5-[3-[4-(2-methoxyphenyl)piperazin-1-yl]propylamino]-1,3-dihydrobenzimidazol-2-one Chemical compound COC1=CC=CC=C1N1CCN(CCCNC=2C=C3NC(=O)NC3=CC=2)CC1 BTASWECXNYOPIX-UHFFFAOYSA-N 0.000 claims 1
- LROJLXGQKUBROL-UHFFFAOYSA-N 5-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-1,3-dihydrobenzimidazol-2-one Chemical compound ClC1=CC=CC(N2CCN(CCCCOC=3C=C4NC(=O)NC4=CC=3)CC2)=C1Cl LROJLXGQKUBROL-UHFFFAOYSA-N 0.000 claims 1
- QUUNWCRHYBNHMD-UHFFFAOYSA-N 5-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butylamino]-1,3-dihydrobenzimidazol-2-one Chemical compound ClC1=CC=CC(N2CCN(CCCCNC=3C=C4NC(=O)NC4=CC=3)CC2)=C1Cl QUUNWCRHYBNHMD-UHFFFAOYSA-N 0.000 claims 1
- KORUCWZQIREKRN-UHFFFAOYSA-N 5-[4-[4-(2,3-dichlorophenyl)piperidin-1-yl]butylamino]-1,3-dihydrobenzimidazol-2-one Chemical compound ClC1=CC=CC(C2CCN(CCCCNC=3C=C4NC(=O)NC4=CC=3)CC2)=C1Cl KORUCWZQIREKRN-UHFFFAOYSA-N 0.000 claims 1
- SZTHJRLWJXYLME-UHFFFAOYSA-N 5-[4-[4-(2-methoxyphenyl)piperazin-1-yl]butoxy]-1,3-benzothiazole Chemical compound COC1=CC=CC=C1N1CCN(CCCCOC=2C=C3N=CSC3=CC=2)CC1 SZTHJRLWJXYLME-UHFFFAOYSA-N 0.000 claims 1
- VJAMRKAYPJCOMN-UHFFFAOYSA-N 5-[4-[4-(2-methoxyphenyl)piperazin-1-yl]butoxy]-1,3-dihydrobenzimidazol-2-one Chemical compound COC1=CC=CC=C1N1CCN(CCCCOC=2C=C3NC(=O)NC3=CC=2)CC1 VJAMRKAYPJCOMN-UHFFFAOYSA-N 0.000 claims 1
- WJUPJEUQFMKVQX-UHFFFAOYSA-N 5-[4-[4-(2-methoxyphenyl)piperazin-1-yl]butylamino]-1,3-dihydrobenzimidazol-2-one Chemical compound COC1=CC=CC=C1N1CCN(CCCCNC=2C=C3NC(=O)NC3=CC=2)CC1 WJUPJEUQFMKVQX-UHFFFAOYSA-N 0.000 claims 1
- OAJIHQXBHTUCIQ-UHFFFAOYSA-N 7-[4-(4-naphthalen-1-yl-1,4-diazepan-1-yl)butoxy]-3,4-dihydro-1h-1,8-naphthyridin-2-one Chemical compound N1=C2NC(=O)CCC2=CC=C1OCCCCN(CC1)CCCN1C1=CC=CC2=CC=CC=C12 OAJIHQXBHTUCIQ-UHFFFAOYSA-N 0.000 claims 1
- BWLXMOCVRICSHU-UHFFFAOYSA-N 7-[4-(4-quinolin-5-yl-1,4-diazepan-1-yl)butoxy]-3,4-dihydro-1h-1,8-naphthyridin-2-one Chemical compound N1=C2NC(=O)CCC2=CC=C1OCCCCN(CC1)CCCN1C1=CC=CC2=NC=CC=C12 BWLXMOCVRICSHU-UHFFFAOYSA-N 0.000 claims 1
- DIUUVWWDGSQEHZ-UHFFFAOYSA-N 7-[4-(4-quinolin-8-yl-1,4-diazepan-1-yl)butoxy]-3,4-dihydro-1h-1,8-naphthyridin-2-one Chemical compound N1=C2NC(=O)CCC2=CC=C1OCCCCN(CC1)CCCN1C1=CC=CC2=CC=CN=C12 DIUUVWWDGSQEHZ-UHFFFAOYSA-N 0.000 claims 1
- RKEIZCBTDHGTBU-UHFFFAOYSA-N 7-[4-[3-(1,3-benzothiazol-5-ylamino)propyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2SC=NC2=CC(NCCCN2CCN(CC2)C=2C=CC=C3NC(OC3=2)=O)=C1 RKEIZCBTDHGTBU-UHFFFAOYSA-N 0.000 claims 1
- MYNMYINEXUJHGK-UHFFFAOYSA-N 7-[4-[3-(1,3-benzothiazol-5-yloxy)propyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2SC=NC2=CC(OCCCN2CCN(CC2)C=2C=CC=C3NC(OC3=2)=O)=C1 MYNMYINEXUJHGK-UHFFFAOYSA-N 0.000 claims 1
- SZQYGDINWOXAEX-UHFFFAOYSA-N 7-[4-[3-(1h-indazol-6-ylamino)propyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2C=NNC2=CC(NCCCN2CCN(CC2)C=2C=CC=C3NC(OC3=2)=O)=C1 SZQYGDINWOXAEX-UHFFFAOYSA-N 0.000 claims 1
- QGJIMWAIPFTZPH-UHFFFAOYSA-N 7-[4-[3-[(2-oxo-1,3-dihydrobenzimidazol-5-yl)amino]propyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2NC(=O)NC2=CC=C1NCCCN(CC1)CCN1C1=CC=CC2=C1OC(=O)N2 QGJIMWAIPFTZPH-UHFFFAOYSA-N 0.000 claims 1
- GAXQLEYQSLKQOL-UHFFFAOYSA-N 7-[4-[3-[(2-oxo-1,3-dihydrobenzimidazol-5-yl)oxy]propyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2NC(=O)NC2=CC=C1OCCCN(CC1)CCN1C1=CC=CC2=C1OC(=O)N2 GAXQLEYQSLKQOL-UHFFFAOYSA-N 0.000 claims 1
- SVZAZOBJUSLUSE-UHFFFAOYSA-N 7-[4-[4-(1,3-benzothiazol-5-ylamino)butyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2SC=NC2=CC(NCCCCN2CCN(CC2)C=2C=CC=C3NC(OC3=2)=O)=C1 SVZAZOBJUSLUSE-UHFFFAOYSA-N 0.000 claims 1
- ZVTUAOSTEZURAR-UHFFFAOYSA-N 7-[4-[4-(1,3-benzothiazol-5-yloxy)butyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2SC=NC2=CC(OCCCCN2CCN(CC2)C=2C=CC=C3NC(OC3=2)=O)=C1 ZVTUAOSTEZURAR-UHFFFAOYSA-N 0.000 claims 1
- PAICQCIOTVXKKZ-UHFFFAOYSA-N 7-[4-[4-(1-benzofuran-7-yl)-1,4-diazepan-1-yl]butoxy]-3,4-dihydro-1h-1,8-naphthyridin-2-one Chemical compound N1=C2NC(=O)CCC2=CC=C1OCCCCN(CC1)CCCN1C1=CC=CC2=C1OC=C2 PAICQCIOTVXKKZ-UHFFFAOYSA-N 0.000 claims 1
- PMUKGRGVMMWLFG-UHFFFAOYSA-N 7-[4-[4-(1-benzothiophen-4-yl)-1,4-diazepan-1-yl]butoxy]-3,4-dihydro-1h-1,8-naphthyridin-2-one Chemical compound N1=C2NC(=O)CCC2=CC=C1OCCCCN(CC1)CCCN1C1=CC=CC2=C1C=CS2 PMUKGRGVMMWLFG-UHFFFAOYSA-N 0.000 claims 1
- MWKJXVLZUBCMBR-UHFFFAOYSA-N 7-[4-[4-(1h-indazol-6-ylamino)butyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2C=NNC2=CC(NCCCCN2CCN(CC2)C=2C=CC=C3NC(OC3=2)=O)=C1 MWKJXVLZUBCMBR-UHFFFAOYSA-N 0.000 claims 1
- UIFQUEVOXOYWNK-UHFFFAOYSA-N 7-[4-[4-(1h-indol-4-yl)-1,4-diazepan-1-yl]butoxy]-3,4-dihydro-1h-1,8-naphthyridin-2-one Chemical compound N1=C2NC(=O)CCC2=CC=C1OCCCCN(CC1)CCCN1C1=CC=CC2=C1C=CN2 UIFQUEVOXOYWNK-UHFFFAOYSA-N 0.000 claims 1
- UAILOOIRJOOOAV-UHFFFAOYSA-N 7-[4-[4-(2-ethoxyphenyl)-1,4-diazepan-1-yl]butoxy]-1h-quinolin-2-one Chemical compound CCOC1=CC=CC=C1N1CCN(CCCCOC=2C=C3NC(=O)C=CC3=CC=2)CCC1 UAILOOIRJOOOAV-UHFFFAOYSA-N 0.000 claims 1
- PWSAFPBCVGJSKB-UHFFFAOYSA-N 7-[4-[4-[(2-oxo-1,3-dihydrobenzimidazol-5-yl)amino]butyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2NC(=O)NC2=CC=C1NCCCCN(CC1)CCN1C1=CC=CC2=C1OC(=O)N2 PWSAFPBCVGJSKB-UHFFFAOYSA-N 0.000 claims 1
- DSSHKWPSVNXFQY-UHFFFAOYSA-N 7-[4-[4-[(2-oxo-1,3-dihydrobenzimidazol-5-yl)oxy]butyl]piperazin-1-yl]-3h-1,3-benzoxazol-2-one Chemical compound C1=C2NC(=O)NC2=CC=C1OCCCCN(CC1)CCN1C1=CC=CC2=C1OC(=O)N2 DSSHKWPSVNXFQY-UHFFFAOYSA-N 0.000 claims 1
- 201000005746 Pituitary adenoma Diseases 0.000 claims 1
- 206010061538 Pituitary tumour benign Diseases 0.000 claims 1
- 125000006309 butyl amino group Chemical group 0.000 claims 1
- AGZLUJZSDWPKAI-UHFFFAOYSA-N n-[3-[4-(2,3-dichlorophenyl)piperazin-1-yl]propyl]-1,3-benzothiazol-5-amine Chemical compound ClC1=CC=CC(N2CCN(CCCNC=3C=C4N=CSC4=CC=3)CC2)=C1Cl AGZLUJZSDWPKAI-UHFFFAOYSA-N 0.000 claims 1
- ZHRUARTXLSHCKF-UHFFFAOYSA-N n-[3-[4-(2,3-dichlorophenyl)piperazin-1-yl]propyl]-1h-indazol-6-amine Chemical compound ClC1=CC=CC(N2CCN(CCCNC=3C=C4NN=CC4=CC=3)CC2)=C1Cl ZHRUARTXLSHCKF-UHFFFAOYSA-N 0.000 claims 1
- WZHKKEBDRZMEPG-UHFFFAOYSA-N n-[3-[4-(2,3-dichlorophenyl)piperidin-1-yl]propyl]-1,3-benzothiazol-5-amine Chemical compound ClC1=CC=CC(C2CCN(CCCNC=3C=C4N=CSC4=CC=3)CC2)=C1Cl WZHKKEBDRZMEPG-UHFFFAOYSA-N 0.000 claims 1
- NAIFOGPVMCOHIN-UHFFFAOYSA-N n-[3-[4-(2,3-dichlorophenyl)piperidin-1-yl]propyl]-1h-indazol-6-amine Chemical compound ClC1=CC=CC(C2CCN(CCCNC=3C=C4NN=CC4=CC=3)CC2)=C1Cl NAIFOGPVMCOHIN-UHFFFAOYSA-N 0.000 claims 1
- WVQWCVMJVXAUMN-UHFFFAOYSA-N n-[3-[4-(2-methoxyphenyl)piperazin-1-yl]propyl]-1,3-benzothiazol-5-amine Chemical compound COC1=CC=CC=C1N1CCN(CCCNC=2C=C3N=CSC3=CC=2)CC1 WVQWCVMJVXAUMN-UHFFFAOYSA-N 0.000 claims 1
- OOZUWVFPAJVDPL-UHFFFAOYSA-N n-[3-[4-(2-methoxyphenyl)piperazin-1-yl]propyl]-1h-indazol-6-amine Chemical compound COC1=CC=CC=C1N1CCN(CCCNC=2C=C3NN=CC3=CC=2)CC1 OOZUWVFPAJVDPL-UHFFFAOYSA-N 0.000 claims 1
- VZJBQKJPCQPLCF-UHFFFAOYSA-N n-[4-[4-(2,3-dichlorophenyl)-1,4-diazepan-1-yl]butyl]-1,3-benzothiazol-5-amine Chemical compound ClC1=CC=CC(N2CCN(CCCCNC=3C=C4N=CSC4=CC=3)CCC2)=C1Cl VZJBQKJPCQPLCF-UHFFFAOYSA-N 0.000 claims 1
- LQEXMSLWVOJMFB-UHFFFAOYSA-N n-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl]-1,3-benzothiazol-5-amine Chemical compound ClC1=CC=CC(N2CCN(CCCCNC=3C=C4N=CSC4=CC=3)CC2)=C1Cl LQEXMSLWVOJMFB-UHFFFAOYSA-N 0.000 claims 1
- MUZUJTOLDGGAMM-UHFFFAOYSA-N n-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl]-1h-indazol-6-amine Chemical compound ClC1=CC=CC(N2CCN(CCCCNC=3C=C4NN=CC4=CC=3)CC2)=C1Cl MUZUJTOLDGGAMM-UHFFFAOYSA-N 0.000 claims 1
- PGBANLLJTTUPNK-UHFFFAOYSA-N n-[4-[4-(2,3-dichlorophenyl)piperidin-1-yl]butyl]-1,3-benzothiazol-5-amine Chemical compound ClC1=CC=CC(C2CCN(CCCCNC=3C=C4N=CSC4=CC=3)CC2)=C1Cl PGBANLLJTTUPNK-UHFFFAOYSA-N 0.000 claims 1
- RDSZSNGJGNJKBW-UHFFFAOYSA-N n-[4-[4-(2,3-dichlorophenyl)piperidin-1-yl]butyl]-1h-indazol-6-amine Chemical compound ClC1=CC=CC(C2CCN(CCCCNC=3C=C4NN=CC4=CC=3)CC2)=C1Cl RDSZSNGJGNJKBW-UHFFFAOYSA-N 0.000 claims 1
- CCLZJEZFLACAQE-UHFFFAOYSA-N n-[4-[4-(2-methoxyphenyl)piperazin-1-yl]butyl]-1,3-benzothiazol-5-amine Chemical compound COC1=CC=CC=C1N1CCN(CCCCNC=2C=C3N=CSC3=CC=2)CC1 CCLZJEZFLACAQE-UHFFFAOYSA-N 0.000 claims 1
- RYRULYWQNMKTJI-UHFFFAOYSA-N n-[4-[4-(2-methoxyphenyl)piperazin-1-yl]butyl]-1h-indazol-6-amine Chemical compound COC1=CC=CC=C1N1CCN(CCCCNC=2C=C3NN=CC3=CC=2)CC1 RYRULYWQNMKTJI-UHFFFAOYSA-N 0.000 claims 1
- 208000021310 pituitary gland adenoma Diseases 0.000 claims 1
- 239000000556 agonist Substances 0.000 abstract description 38
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 abstract description 27
- 239000005557 antagonist Substances 0.000 abstract description 23
- 229960003638 dopamine Drugs 0.000 abstract description 14
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 359
- 239000000543 intermediate Substances 0.000 description 325
- 239000000203 mixture Substances 0.000 description 261
- 235000019439 ethyl acetate Nutrition 0.000 description 179
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 162
- 238000010992 reflux Methods 0.000 description 128
- 230000015572 biosynthetic process Effects 0.000 description 108
- 238000003786 synthesis reaction Methods 0.000 description 107
- 239000000243 solution Substances 0.000 description 100
- 239000012267 brine Substances 0.000 description 84
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 84
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 83
- 239000011734 sodium Substances 0.000 description 83
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 80
- 239000000741 silica gel Substances 0.000 description 77
- 229910002027 silica gel Inorganic materials 0.000 description 77
- 238000010828 elution Methods 0.000 description 75
- 238000003818 flash chromatography Methods 0.000 description 75
- 238000001819 mass spectrum Methods 0.000 description 71
- 238000004128 high performance liquid chromatography Methods 0.000 description 70
- 239000010410 layer Substances 0.000 description 61
- 229910000027 potassium carbonate Inorganic materials 0.000 description 59
- 239000007787 solid Substances 0.000 description 58
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 53
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 48
- -1 morpholino, piperazinyl Chemical group 0.000 description 48
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 42
- 239000011541 reaction mixture Substances 0.000 description 42
- 239000000706 filtrate Substances 0.000 description 41
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 39
- 230000002829 reductive effect Effects 0.000 description 37
- 239000013078 crystal Substances 0.000 description 32
- 238000006243 chemical reaction Methods 0.000 description 30
- 238000005481 NMR spectroscopy Methods 0.000 description 29
- 239000004698 Polyethylene Substances 0.000 description 29
- 239000012044 organic layer Substances 0.000 description 29
- 210000004027 cell Anatomy 0.000 description 28
- 229940079593 drug Drugs 0.000 description 23
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 22
- 238000009472 formulation Methods 0.000 description 21
- 229920006395 saturated elastomer Polymers 0.000 description 19
- JQSRFMXTGAVHIR-UHFFFAOYSA-N 7-[4-[4-(2,3-dichlorophenyl)-1,4-diazepan-1-yl]butoxy]-3,4-dihydro-1h-1,8-naphthyridin-2-one Chemical compound ClC1=CC=CC(N2CCN(CCCCOC=3N=C4NC(=O)CCC4=CC=3)CCC2)=C1Cl JQSRFMXTGAVHIR-UHFFFAOYSA-N 0.000 description 18
- 230000037361 pathway Effects 0.000 description 17
- CEUORZQYGODEFX-UHFFFAOYSA-N Aripirazole Chemical compound ClC1=CC=CC(N2CCN(CCCCOC=3C=C4NC(=O)CCC4=CC=3)CC2)=C1Cl CEUORZQYGODEFX-UHFFFAOYSA-N 0.000 description 16
- 241000699670 Mus sp. Species 0.000 description 16
- 229960004372 aripiprazole Drugs 0.000 description 16
- 239000003112 inhibitor Substances 0.000 description 15
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 15
- 238000007792 addition Methods 0.000 description 14
- 230000000561 anti-psychotic effect Effects 0.000 description 14
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 14
- 238000001727 in vivo Methods 0.000 description 14
- 208000009132 Catalepsy Diseases 0.000 description 13
- 206010047853 Waxy flexibility Diseases 0.000 description 13
- 238000012360 testing method Methods 0.000 description 13
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 12
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 12
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 12
- JTJMJGYZQZDUJJ-UHFFFAOYSA-N phencyclidine Chemical compound C1CCCCN1C1(C=2C=CC=CC=2)CCCCC1 JTJMJGYZQZDUJJ-UHFFFAOYSA-N 0.000 description 12
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 11
- 238000003556 assay Methods 0.000 description 11
- 239000003981 vehicle Substances 0.000 description 11
- 0 C*(C**)*(*)*(*1C)[C@](C)*1N(CC1)CCC(C)(C)C**1[Al] Chemical compound C*(C**)*(*)*(*1C)[C@](C)*1N(CC1)CCC(C)(C)C**1[Al] 0.000 description 10
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 10
- 239000003795 chemical substances by application Substances 0.000 description 10
- 238000001914 filtration Methods 0.000 description 10
- 239000003921 oil Substances 0.000 description 10
- 230000019491 signal transduction Effects 0.000 description 10
- 238000011282 treatment Methods 0.000 description 10
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 9
- 125000000217 alkyl group Chemical group 0.000 description 9
- 230000003389 potentiating effect Effects 0.000 description 9
- 239000000843 powder Substances 0.000 description 9
- 239000002562 thickening agent Substances 0.000 description 9
- KWTSXDURSIMDCE-QMMMGPOBSA-N (S)-amphetamine Chemical compound C[C@H](N)CC1=CC=CC=C1 KWTSXDURSIMDCE-QMMMGPOBSA-N 0.000 description 8
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 8
- 239000003693 atypical antipsychotic agent Substances 0.000 description 8
- 229940127236 atypical antipsychotics Drugs 0.000 description 8
- 239000000872 buffer Substances 0.000 description 8
- 238000004440 column chromatography Methods 0.000 description 8
- UEXQBEVWFZKHNB-UHFFFAOYSA-N intermediate 29 Natural products C1=CC(N)=CC=C1NC1=NC=CC=N1 UEXQBEVWFZKHNB-UHFFFAOYSA-N 0.000 description 8
- 239000004031 partial agonist Substances 0.000 description 8
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 8
- 239000002904 solvent Substances 0.000 description 8
- 125000001424 substituent group Chemical group 0.000 description 8
- 239000000725 suspension Substances 0.000 description 8
- NGCKUAWDNUFNBB-UHFFFAOYSA-N 7-[4-[4-(2,3-dichlorophenyl)-1,4-diazepan-1-yl]butoxy]-3,4-dihydro-1h-quinolin-2-one Chemical compound ClC1=CC=CC(N2CCN(CCCCOC=3C=C4NC(=O)CCC4=CC=3)CCC2)=C1Cl NGCKUAWDNUFNBB-UHFFFAOYSA-N 0.000 description 7
- LKLSFDWYIBUGNT-UHFFFAOYSA-N 7-hydroxy-3,4-dihydro-1h-quinolin-2-one Chemical compound C1CC(=O)NC2=CC(O)=CC=C21 LKLSFDWYIBUGNT-UHFFFAOYSA-N 0.000 description 7
- 241001465754 Metazoa Species 0.000 description 7
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium on carbon Substances [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 7
- 125000004429 atom Chemical group 0.000 description 7
- 239000002585 base Substances 0.000 description 7
- 210000004556 brain Anatomy 0.000 description 7
- 239000011575 calcium Substances 0.000 description 7
- 229960000632 dexamfetamine Drugs 0.000 description 7
- 229960003878 haloperidol Drugs 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 229910052757 nitrogen Inorganic materials 0.000 description 7
- CYPYTURSJDMMMP-WVCUSYJESA-N (1e,4e)-1,5-diphenylpenta-1,4-dien-3-one;palladium Chemical compound [Pd].[Pd].C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1.C=1C=CC=CC=1\C=C\C(=O)\C=C\C1=CC=CC=C1 CYPYTURSJDMMMP-WVCUSYJESA-N 0.000 description 6
- VEFLKXRACNJHOV-UHFFFAOYSA-N 1,3-dibromopropane Chemical compound BrCCCBr VEFLKXRACNJHOV-UHFFFAOYSA-N 0.000 description 6
- ULTHEAFYOOPTTB-UHFFFAOYSA-N 1,4-dibromobutane Chemical compound BrCCCCBr ULTHEAFYOOPTTB-UHFFFAOYSA-N 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 6
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 6
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 6
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 6
- 230000009471 action Effects 0.000 description 6
- 238000005576 amination reaction Methods 0.000 description 6
- MUALRAIOVNYAIW-UHFFFAOYSA-N binap Chemical compound C1=CC=CC=C1P(C=1C(=C2C=CC=CC2=CC=1)C=1C2=CC=CC=C2C=CC=1P(C=1C=CC=CC=1)C=1C=CC=CC=1)C1=CC=CC=C1 MUALRAIOVNYAIW-UHFFFAOYSA-N 0.000 description 6
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 6
- 201000010099 disease Diseases 0.000 description 6
- 239000012091 fetal bovine serum Substances 0.000 description 6
- 230000035863 hyperlocomotion Effects 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 238000011813 knockout mouse model Methods 0.000 description 6
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 6
- 230000007935 neutral effect Effects 0.000 description 6
- 229950010883 phencyclidine Drugs 0.000 description 6
- 230000011664 signaling Effects 0.000 description 6
- 239000002002 slurry Substances 0.000 description 6
- 230000001225 therapeutic effect Effects 0.000 description 6
- 238000010792 warming Methods 0.000 description 6
- FQUYSHZXSKYCSY-UHFFFAOYSA-N 1,4-diazepane Chemical compound C1CNCCNC1 FQUYSHZXSKYCSY-UHFFFAOYSA-N 0.000 description 5
- 238000005160 1H NMR spectroscopy Methods 0.000 description 5
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 5
- 239000007995 HEPES buffer Substances 0.000 description 5
- 108010050904 Interferons Proteins 0.000 description 5
- 102000014150 Interferons Human genes 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 239000000654 additive Substances 0.000 description 5
- 230000008484 agonism Effects 0.000 description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 125000004432 carbon atom Chemical group C* 0.000 description 5
- 229920002678 cellulose Polymers 0.000 description 5
- 238000002825 functional assay Methods 0.000 description 5
- 239000005457 ice water Substances 0.000 description 5
- 239000002502 liposome Substances 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 239000002609 medium Substances 0.000 description 5
- 239000012528 membrane Substances 0.000 description 5
- 239000001301 oxygen Substances 0.000 description 5
- 238000011160 research Methods 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 230000000699 topical effect Effects 0.000 description 5
- ASGMFNBUXDJWJJ-JLCFBVMHSA-N (1R,3R)-3-[[3-bromo-1-[4-(5-methyl-1,3,4-thiadiazol-2-yl)phenyl]pyrazolo[3,4-d]pyrimidin-6-yl]amino]-N,1-dimethylcyclopentane-1-carboxamide Chemical compound BrC1=NN(C2=NC(=NC=C21)N[C@H]1C[C@@](CC1)(C(=O)NC)C)C1=CC=C(C=C1)C=1SC(=NN=1)C ASGMFNBUXDJWJJ-JLCFBVMHSA-N 0.000 description 4
- UAOUIVVJBYDFKD-XKCDOFEDSA-N (1R,9R,10S,11R,12R,15S,18S,21R)-10,11,21-trihydroxy-8,8-dimethyl-14-methylidene-4-(prop-2-enylamino)-20-oxa-5-thia-3-azahexacyclo[9.7.2.112,15.01,9.02,6.012,18]henicosa-2(6),3-dien-13-one Chemical compound C([C@@H]1[C@@H](O)[C@@]23C(C1=C)=O)C[C@H]2[C@]12C(N=C(NCC=C)S4)=C4CC(C)(C)[C@H]1[C@H](O)[C@]3(O)OC2 UAOUIVVJBYDFKD-XKCDOFEDSA-N 0.000 description 4
- AOSZTAHDEDLTLQ-AZKQZHLXSA-N (1S,2S,4R,8S,9S,11S,12R,13S,19S)-6-[(3-chlorophenyl)methyl]-12,19-difluoro-11-hydroxy-8-(2-hydroxyacetyl)-9,13-dimethyl-6-azapentacyclo[10.8.0.02,9.04,8.013,18]icosa-14,17-dien-16-one Chemical compound C([C@@H]1C[C@H]2[C@H]3[C@]([C@]4(C=CC(=O)C=C4[C@@H](F)C3)C)(F)[C@@H](O)C[C@@]2([C@@]1(C1)C(=O)CO)C)N1CC1=CC=CC(Cl)=C1 AOSZTAHDEDLTLQ-AZKQZHLXSA-N 0.000 description 4
- ABJSOROVZZKJGI-OCYUSGCXSA-N (1r,2r,4r)-2-(4-bromophenyl)-n-[(4-chlorophenyl)-(2-fluoropyridin-4-yl)methyl]-4-morpholin-4-ylcyclohexane-1-carboxamide Chemical compound C1=NC(F)=CC(C(NC(=O)[C@H]2[C@@H](C[C@@H](CC2)N2CCOCC2)C=2C=CC(Br)=CC=2)C=2C=CC(Cl)=CC=2)=C1 ABJSOROVZZKJGI-OCYUSGCXSA-N 0.000 description 4
- GLGNXYJARSMNGJ-VKTIVEEGSA-N (1s,2s,3r,4r)-3-[[5-chloro-2-[(1-ethyl-6-methoxy-2-oxo-4,5-dihydro-3h-1-benzazepin-7-yl)amino]pyrimidin-4-yl]amino]bicyclo[2.2.1]hept-5-ene-2-carboxamide Chemical compound CCN1C(=O)CCCC2=C(OC)C(NC=3N=C(C(=CN=3)Cl)N[C@H]3[C@H]([C@@]4([H])C[C@@]3(C=C4)[H])C(N)=O)=CC=C21 GLGNXYJARSMNGJ-VKTIVEEGSA-N 0.000 description 4
- SZUVGFMDDVSKSI-WIFOCOSTSA-N (1s,2s,3s,5r)-1-(carboxymethyl)-3,5-bis[(4-phenoxyphenyl)methyl-propylcarbamoyl]cyclopentane-1,2-dicarboxylic acid Chemical compound O=C([C@@H]1[C@@H]([C@](CC(O)=O)([C@H](C(=O)N(CCC)CC=2C=CC(OC=3C=CC=CC=3)=CC=2)C1)C(O)=O)C(O)=O)N(CCC)CC(C=C1)=CC=C1OC1=CC=CC=C1 SZUVGFMDDVSKSI-WIFOCOSTSA-N 0.000 description 4
- GHYOCDFICYLMRF-UTIIJYGPSA-N (2S,3R)-N-[(2S)-3-(cyclopenten-1-yl)-1-[(2R)-2-methyloxiran-2-yl]-1-oxopropan-2-yl]-3-hydroxy-3-(4-methoxyphenyl)-2-[[(2S)-2-[(2-morpholin-4-ylacetyl)amino]propanoyl]amino]propanamide Chemical compound C1(=CCCC1)C[C@@H](C(=O)[C@@]1(OC1)C)NC([C@H]([C@@H](C1=CC=C(C=C1)OC)O)NC([C@H](C)NC(CN1CCOCC1)=O)=O)=O GHYOCDFICYLMRF-UTIIJYGPSA-N 0.000 description 4
- YJLIKUSWRSEPSM-WGQQHEPDSA-N (2r,3r,4s,5r)-2-[6-amino-8-[(4-phenylphenyl)methylamino]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C=1C=C(C=2C=CC=CC=2)C=CC=1CNC1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O YJLIKUSWRSEPSM-WGQQHEPDSA-N 0.000 description 4
- VIJSPAIQWVPKQZ-BLECARSGSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-acetamido-5-(diaminomethylideneamino)pentanoyl]amino]-4-methylpentanoyl]amino]-4,4-dimethylpentanoyl]amino]-4-methylpentanoyl]amino]propanoyl]amino]-5-(diaminomethylideneamino)pentanoic acid Chemical compound NC(=N)NCCC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O VIJSPAIQWVPKQZ-BLECARSGSA-N 0.000 description 4
- IWZSHWBGHQBIML-ZGGLMWTQSA-N (3S,8S,10R,13S,14S,17S)-17-isoquinolin-7-yl-N,N,10,13-tetramethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-amine Chemical compound CN(C)[C@H]1CC[C@]2(C)C3CC[C@@]4(C)[C@@H](CC[C@@H]4c4ccc5ccncc5c4)[C@@H]3CC=C2C1 IWZSHWBGHQBIML-ZGGLMWTQSA-N 0.000 description 4
- MPDDTAJMJCESGV-CTUHWIOQSA-M (3r,5r)-7-[2-(4-fluorophenyl)-5-[methyl-[(1r)-1-phenylethyl]carbamoyl]-4-propan-2-ylpyrazol-3-yl]-3,5-dihydroxyheptanoate Chemical compound C1([C@@H](C)N(C)C(=O)C2=NN(C(CC[C@@H](O)C[C@@H](O)CC([O-])=O)=C2C(C)C)C=2C=CC(F)=CC=2)=CC=CC=C1 MPDDTAJMJCESGV-CTUHWIOQSA-M 0.000 description 4
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 4
- WZZBNLYBHUDSHF-DHLKQENFSA-N 1-[(3s,4s)-4-[8-(2-chloro-4-pyrimidin-2-yloxyphenyl)-7-fluoro-2-methylimidazo[4,5-c]quinolin-1-yl]-3-fluoropiperidin-1-yl]-2-hydroxyethanone Chemical compound CC1=NC2=CN=C3C=C(F)C(C=4C(=CC(OC=5N=CC=CN=5)=CC=4)Cl)=CC3=C2N1[C@H]1CCN(C(=O)CO)C[C@@H]1F WZZBNLYBHUDSHF-DHLKQENFSA-N 0.000 description 4
- ONBQEOIKXPHGMB-VBSBHUPXSA-N 1-[2-[(2s,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1OC1=CC(O)=CC(O)=C1C(=O)CCC1=CC=C(O)C=C1 ONBQEOIKXPHGMB-VBSBHUPXSA-N 0.000 description 4
- UNILWMWFPHPYOR-KXEYIPSPSA-M 1-[6-[2-[3-[3-[3-[2-[2-[3-[[2-[2-[[(2r)-1-[[2-[[(2r)-1-[3-[2-[2-[3-[[2-(2-amino-2-oxoethoxy)acetyl]amino]propoxy]ethoxy]ethoxy]propylamino]-3-hydroxy-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-[(2r)-2,3-di(hexadecanoyloxy)propyl]sulfanyl-1-oxopropan-2-yl Chemical compound O=C1C(SCCC(=O)NCCCOCCOCCOCCCNC(=O)COCC(=O)N[C@@H](CSC[C@@H](COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC)C(=O)NCC(=O)N[C@H](CO)C(=O)NCCCOCCOCCOCCCNC(=O)COCC(N)=O)CC(=O)N1CCNC(=O)CCCCCN\1C2=CC=C(S([O-])(=O)=O)C=C2CC/1=C/C=C/C=C/C1=[N+](CC)C2=CC=C(S([O-])(=O)=O)C=C2C1 UNILWMWFPHPYOR-KXEYIPSPSA-M 0.000 description 4
- FQMZXMVHHKXGTM-UHFFFAOYSA-N 2-(1-adamantyl)-n-[2-[2-(2-hydroxyethylamino)ethylamino]quinolin-5-yl]acetamide Chemical compound C1C(C2)CC(C3)CC2CC13CC(=O)NC1=CC=CC2=NC(NCCNCCO)=CC=C21 FQMZXMVHHKXGTM-UHFFFAOYSA-N 0.000 description 4
- YSUIQYOGTINQIN-UZFYAQMZSA-N 2-amino-9-[(1S,6R,8R,9S,10R,15R,17R,18R)-8-(6-aminopurin-9-yl)-9,18-difluoro-3,12-dihydroxy-3,12-bis(sulfanylidene)-2,4,7,11,13,16-hexaoxa-3lambda5,12lambda5-diphosphatricyclo[13.2.1.06,10]octadecan-17-yl]-1H-purin-6-one Chemical compound NC1=NC2=C(N=CN2[C@@H]2O[C@@H]3COP(S)(=O)O[C@@H]4[C@@H](COP(S)(=O)O[C@@H]2[C@@H]3F)O[C@H]([C@H]4F)N2C=NC3=C2N=CN=C3N)C(=O)N1 YSUIQYOGTINQIN-UZFYAQMZSA-N 0.000 description 4
- TVTJUIAKQFIXCE-HUKYDQBMSA-N 2-amino-9-[(2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-7-prop-2-ynyl-1H-purine-6,8-dione Chemical compound NC=1NC(C=2N(C(N(C=2N=1)[C@@H]1O[C@@H]([C@H]([C@H]1O)F)CO)=O)CC#C)=O TVTJUIAKQFIXCE-HUKYDQBMSA-N 0.000 description 4
- QBWKPGNFQQJGFY-QLFBSQMISA-N 3-[(1r)-1-[(2r,6s)-2,6-dimethylmorpholin-4-yl]ethyl]-n-[6-methyl-3-(1h-pyrazol-4-yl)imidazo[1,2-a]pyrazin-8-yl]-1,2-thiazol-5-amine Chemical compound N1([C@H](C)C2=NSC(NC=3C4=NC=C(N4C=C(C)N=3)C3=CNN=C3)=C2)C[C@H](C)O[C@H](C)C1 QBWKPGNFQQJGFY-QLFBSQMISA-N 0.000 description 4
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 4
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 4
- 229940126657 Compound 17 Drugs 0.000 description 4
- 229940126639 Compound 33 Drugs 0.000 description 4
- 229940127007 Compound 39 Drugs 0.000 description 4
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 4
- 241000699666 Mus <mouse, genus> Species 0.000 description 4
- OPFJDXRVMFKJJO-ZHHKINOHSA-N N-{[3-(2-benzamido-4-methyl-1,3-thiazol-5-yl)-pyrazol-5-yl]carbonyl}-G-dR-G-dD-dD-dD-NH2 Chemical compound S1C(C=2NN=C(C=2)C(=O)NCC(=O)N[C@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC(O)=O)C(N)=O)=C(C)N=C1NC(=O)C1=CC=CC=C1 OPFJDXRVMFKJJO-ZHHKINOHSA-N 0.000 description 4
- KYYIDSXMWOZKMP-UHFFFAOYSA-N O-desmethylvenlafaxine Chemical compound C1CCCCC1(O)C(CN(C)C)C1=CC=C(O)C=C1 KYYIDSXMWOZKMP-UHFFFAOYSA-N 0.000 description 4
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 4
- PNUZDKCDAWUEGK-CYZMBNFOSA-N Sitafloxacin Chemical compound C([C@H]1N)N(C=2C(=C3C(C(C(C(O)=O)=CN3[C@H]3[C@H](C3)F)=O)=CC=2F)Cl)CC11CC1 PNUZDKCDAWUEGK-CYZMBNFOSA-N 0.000 description 4
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 4
- LJOOWESTVASNOG-UFJKPHDISA-N [(1s,3r,4ar,7s,8s,8as)-3-hydroxy-8-[2-[(4r)-4-hydroxy-6-oxooxan-2-yl]ethyl]-7-methyl-1,2,3,4,4a,7,8,8a-octahydronaphthalen-1-yl] (2s)-2-methylbutanoate Chemical compound C([C@H]1[C@@H](C)C=C[C@H]2C[C@@H](O)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)CC1C[C@@H](O)CC(=O)O1 LJOOWESTVASNOG-UFJKPHDISA-N 0.000 description 4
- SPXSEZMVRJLHQG-XMMPIXPASA-N [(2R)-1-[[4-[(3-phenylmethoxyphenoxy)methyl]phenyl]methyl]pyrrolidin-2-yl]methanol Chemical compound C(C1=CC=CC=C1)OC=1C=C(OCC2=CC=C(CN3[C@H](CCC3)CO)C=C2)C=CC=1 SPXSEZMVRJLHQG-XMMPIXPASA-N 0.000 description 4
- LNUFLCYMSVYYNW-ZPJMAFJPSA-N [(2r,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[[(3s,5s,8r,9s,10s,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-disulfo Chemical compound O([C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1[C@@H](COS(O)(=O)=O)O[C@H]([C@@H]([C@H]1OS(O)(=O)=O)OS(O)(=O)=O)O[C@@H]1C[C@@H]2CC[C@H]3[C@@H]4CC[C@@H]([C@]4(CC[C@@H]3[C@@]2(C)CC1)C)[C@H](C)CCCC(C)C)[C@H]1O[C@H](COS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@H](OS(O)(=O)=O)[C@H]1OS(O)(=O)=O LNUFLCYMSVYYNW-ZPJMAFJPSA-N 0.000 description 4
- 125000003277 amino group Chemical group 0.000 description 4
- 239000002246 antineoplastic agent Substances 0.000 description 4
- XRWSZZJLZRKHHD-WVWIJVSJSA-N asunaprevir Chemical compound O=C([C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)OC1=NC=C(C2=CC=C(Cl)C=C21)OC)N[C@]1(C(=O)NS(=O)(=O)C2CC2)C[C@H]1C=C XRWSZZJLZRKHHD-WVWIJVSJSA-N 0.000 description 4
- KGNDCEVUMONOKF-UGPLYTSKSA-N benzyl n-[(2r)-1-[(2s,4r)-2-[[(2s)-6-amino-1-(1,3-benzoxazol-2-yl)-1,1-dihydroxyhexan-2-yl]carbamoyl]-4-[(4-methylphenyl)methoxy]pyrrolidin-1-yl]-1-oxo-4-phenylbutan-2-yl]carbamate Chemical compound C1=CC(C)=CC=C1CO[C@H]1CN(C(=O)[C@@H](CCC=2C=CC=CC=2)NC(=O)OCC=2C=CC=CC=2)[C@H](C(=O)N[C@@H](CCCCN)C(O)(O)C=2OC3=CC=CC=C3N=2)C1 KGNDCEVUMONOKF-UGPLYTSKSA-N 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- 229940125904 compound 1 Drugs 0.000 description 4
- 229940125797 compound 12 Drugs 0.000 description 4
- 229940126543 compound 14 Drugs 0.000 description 4
- 229940125758 compound 15 Drugs 0.000 description 4
- 229940126142 compound 16 Drugs 0.000 description 4
- 229940126086 compound 21 Drugs 0.000 description 4
- 229940125833 compound 23 Drugs 0.000 description 4
- 229940125961 compound 24 Drugs 0.000 description 4
- 229940125846 compound 25 Drugs 0.000 description 4
- 229940125851 compound 27 Drugs 0.000 description 4
- 229940127204 compound 29 Drugs 0.000 description 4
- 229940127573 compound 38 Drugs 0.000 description 4
- 229940127271 compound 49 Drugs 0.000 description 4
- 229960001623 desvenlafaxine Drugs 0.000 description 4
- 231100000673 dose–response relationship Toxicity 0.000 description 4
- 239000003995 emulsifying agent Substances 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- 239000000284 extract Substances 0.000 description 4
- 238000011049 filling Methods 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- 125000005843 halogen group Chemical group 0.000 description 4
- 125000005842 heteroatom Chemical group 0.000 description 4
- 239000004615 ingredient Substances 0.000 description 4
- 229940079322 interferon Drugs 0.000 description 4
- 230000006742 locomotor activity Effects 0.000 description 4
- QARBMVPHQWIHKH-UHFFFAOYSA-N methanesulfonyl chloride Chemical compound CS(Cl)(=O)=O QARBMVPHQWIHKH-UHFFFAOYSA-N 0.000 description 4
- IOMMMLWIABWRKL-WUTDNEBXSA-N nazartinib Chemical compound C1N(C(=O)/C=C/CN(C)C)CCCC[C@H]1N1C2=C(Cl)C=CC=C2N=C1NC(=O)C1=CC=NC(C)=C1 IOMMMLWIABWRKL-WUTDNEBXSA-N 0.000 description 4
- 229960005017 olanzapine Drugs 0.000 description 4
- KVWDHTXUZHCGIO-UHFFFAOYSA-N olanzapine Chemical compound C1CN(C)CCN1C1=NC2=CC=CC=C2NC2=C1C=C(C)S2 KVWDHTXUZHCGIO-UHFFFAOYSA-N 0.000 description 4
- PIDFDZJZLOTZTM-KHVQSSSXSA-N ombitasvir Chemical compound COC(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)NC1=CC=C([C@H]2N([C@@H](CC2)C=2C=CC(NC(=O)[C@H]3N(CCC3)C(=O)[C@@H](NC(=O)OC)C(C)C)=CC=2)C=2C=CC(=CC=2)C(C)(C)C)C=C1 PIDFDZJZLOTZTM-KHVQSSSXSA-N 0.000 description 4
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 4
- 239000002244 precipitate Substances 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 239000003755 preservative agent Substances 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 230000007115 recruitment Effects 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 229960001534 risperidone Drugs 0.000 description 4
- RAPZEAPATHNIPO-UHFFFAOYSA-N risperidone Chemical compound FC1=CC=C2C(C3CCN(CC3)CCC=3C(=O)N4CCCCC4=NC=3C)=NOC2=C1 RAPZEAPATHNIPO-UHFFFAOYSA-N 0.000 description 4
- 229910052708 sodium Inorganic materials 0.000 description 4
- 229940083542 sodium Drugs 0.000 description 4
- MFRIHAYPQRLWNB-UHFFFAOYSA-N sodium tert-butoxide Chemical compound [Na+].CC(C)(C)[O-] MFRIHAYPQRLWNB-UHFFFAOYSA-N 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- 229910052717 sulfur Inorganic materials 0.000 description 4
- 239000011593 sulfur Substances 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 239000003826 tablet Substances 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 4
- JWZZKOKVBUJMES-UHFFFAOYSA-N (+-)-Isoprenaline Chemical compound CC(C)NCC(O)C1=CC=C(O)C(O)=C1 JWZZKOKVBUJMES-UHFFFAOYSA-N 0.000 description 3
- IUSARDYWEPUTPN-OZBXUNDUSA-N (2r)-n-[(2s,3r)-4-[[(4s)-6-(2,2-dimethylpropyl)spiro[3,4-dihydropyrano[2,3-b]pyridine-2,1'-cyclobutane]-4-yl]amino]-3-hydroxy-1-[3-(1,3-thiazol-2-yl)phenyl]butan-2-yl]-2-methoxypropanamide Chemical compound C([C@H](NC(=O)[C@@H](C)OC)[C@H](O)CN[C@@H]1C2=CC(CC(C)(C)C)=CN=C2OC2(CCC2)C1)C(C=1)=CC=CC=1C1=NC=CS1 IUSARDYWEPUTPN-OZBXUNDUSA-N 0.000 description 3
- WWTBZEKOSBFBEM-SPWPXUSOSA-N (2s)-2-[[2-benzyl-3-[hydroxy-[(1r)-2-phenyl-1-(phenylmethoxycarbonylamino)ethyl]phosphoryl]propanoyl]amino]-3-(1h-indol-3-yl)propanoic acid Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)O)C(=O)C(CP(O)(=O)[C@H](CC=1C=CC=CC=1)NC(=O)OCC=1C=CC=CC=1)CC1=CC=CC=C1 WWTBZEKOSBFBEM-SPWPXUSOSA-N 0.000 description 3
- STBLNCCBQMHSRC-BATDWUPUSA-N (2s)-n-[(3s,4s)-5-acetyl-7-cyano-4-methyl-1-[(2-methylnaphthalen-1-yl)methyl]-2-oxo-3,4-dihydro-1,5-benzodiazepin-3-yl]-2-(methylamino)propanamide Chemical compound O=C1[C@@H](NC(=O)[C@H](C)NC)[C@H](C)N(C(C)=O)C2=CC(C#N)=CC=C2N1CC1=C(C)C=CC2=CC=CC=C12 STBLNCCBQMHSRC-BATDWUPUSA-N 0.000 description 3
- QFLWZFQWSBQYPS-AWRAUJHKSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[5-[(3aS,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-[1-bis(4-chlorophenoxy)phosphorylbutylamino]-4-oxobutanoic acid Chemical compound CCCC(NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CCCCC1SC[C@@H]2NC(=O)N[C@H]12)C(C)C)P(=O)(Oc1ccc(Cl)cc1)Oc1ccc(Cl)cc1 QFLWZFQWSBQYPS-AWRAUJHKSA-N 0.000 description 3
- UDQTXCHQKHIQMH-KYGLGHNPSA-N (3ar,5s,6s,7r,7ar)-5-(difluoromethyl)-2-(ethylamino)-5,6,7,7a-tetrahydro-3ah-pyrano[3,2-d][1,3]thiazole-6,7-diol Chemical compound S1C(NCC)=N[C@H]2[C@@H]1O[C@H](C(F)F)[C@@H](O)[C@@H]2O UDQTXCHQKHIQMH-KYGLGHNPSA-N 0.000 description 3
- HUWSZNZAROKDRZ-RRLWZMAJSA-N (3r,4r)-3-azaniumyl-5-[[(2s,3r)-1-[(2s)-2,3-dicarboxypyrrolidin-1-yl]-3-methyl-1-oxopentan-2-yl]amino]-5-oxo-4-sulfanylpentane-1-sulfonate Chemical compound OS(=O)(=O)CC[C@@H](N)[C@@H](S)C(=O)N[C@@H]([C@H](C)CC)C(=O)N1CCC(C(O)=O)[C@H]1C(O)=O HUWSZNZAROKDRZ-RRLWZMAJSA-N 0.000 description 3
- YQOLEILXOBUDMU-KRWDZBQOSA-N (4R)-5-[(6-bromo-3-methyl-2-pyrrolidin-1-ylquinoline-4-carbonyl)amino]-4-(2-chlorophenyl)pentanoic acid Chemical compound CC1=C(C2=C(C=CC(=C2)Br)N=C1N3CCCC3)C(=O)NC[C@H](CCC(=O)O)C4=CC=CC=C4Cl YQOLEILXOBUDMU-KRWDZBQOSA-N 0.000 description 3
- RTHCYVBBDHJXIQ-MRXNPFEDSA-N (R)-fluoxetine Chemical compound O([C@H](CCNC)C=1C=CC=CC=1)C1=CC=C(C(F)(F)F)C=C1 RTHCYVBBDHJXIQ-MRXNPFEDSA-N 0.000 description 3
- ZEUITGRIYCTCEM-KRWDZBQOSA-N (S)-duloxetine Chemical compound C1([C@@H](OC=2C3=CC=CC=C3C=CC=2)CCNC)=CC=CS1 ZEUITGRIYCTCEM-KRWDZBQOSA-N 0.000 description 3
- KQZLRWGGWXJPOS-NLFPWZOASA-N 1-[(1R)-1-(2,4-dichlorophenyl)ethyl]-6-[(4S,5R)-4-[(2S)-2-(hydroxymethyl)pyrrolidin-1-yl]-5-methylcyclohexen-1-yl]pyrazolo[3,4-b]pyrazine-3-carbonitrile Chemical compound ClC1=C(C=CC(=C1)Cl)[C@@H](C)N1N=C(C=2C1=NC(=CN=2)C1=CC[C@@H]([C@@H](C1)C)N1[C@@H](CCC1)CO)C#N KQZLRWGGWXJPOS-NLFPWZOASA-N 0.000 description 3
- PYRKKGOKRMZEIT-UHFFFAOYSA-N 2-[6-(2-cyclopropylethoxy)-9-(2-hydroxy-2-methylpropyl)-1h-phenanthro[9,10-d]imidazol-2-yl]-5-fluorobenzene-1,3-dicarbonitrile Chemical compound C1=C2C3=CC(CC(C)(O)C)=CC=C3C=3NC(C=4C(=CC(F)=CC=4C#N)C#N)=NC=3C2=CC=C1OCCC1CC1 PYRKKGOKRMZEIT-UHFFFAOYSA-N 0.000 description 3
- PMXMIIMHBWHSKN-UHFFFAOYSA-N 3-{2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl}-9-hydroxy-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one Chemical compound FC1=CC=C2C(C3CCN(CC3)CCC=3C(=O)N4CCCC(O)C4=NC=3C)=NOC2=C1 PMXMIIMHBWHSKN-UHFFFAOYSA-N 0.000 description 3
- 102000040125 5-hydroxytryptamine receptor family Human genes 0.000 description 3
- 108091032151 5-hydroxytryptamine receptor family Proteins 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- BQXUPNKLZNSUMC-YUQWMIPFSA-N CCN(CCCCCOCC(=O)N[C@H](C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](C)c1ccc(cc1)-c1scnc1C)C(C)(C)C)CCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Chemical compound CCN(CCCCCOCC(=O)N[C@H](C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](C)c1ccc(cc1)-c1scnc1C)C(C)(C)C)CCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 BQXUPNKLZNSUMC-YUQWMIPFSA-N 0.000 description 3
- 241000283707 Capra Species 0.000 description 3
- 206010012289 Dementia Diseases 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 108091006027 G proteins Proteins 0.000 description 3
- 102000030782 GTP binding Human genes 0.000 description 3
- 108091000058 GTP-Binding Proteins 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 108010002350 Interleukin-2 Proteins 0.000 description 3
- 102000000588 Interleukin-2 Human genes 0.000 description 3
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 3
- 108010000817 Leuprolide Proteins 0.000 description 3
- 208000025966 Neurological disease Diseases 0.000 description 3
- PHVGLTMQBUFIQQ-UHFFFAOYSA-N Nortryptiline Chemical compound C1CC2=CC=CC=C2C(=CCCNC)C2=CC=CC=C21 PHVGLTMQBUFIQQ-UHFFFAOYSA-N 0.000 description 3
- RGCVKNLCSQQDEP-UHFFFAOYSA-N Perphenazine Chemical compound C1CN(CCO)CCN1CCCN1C2=CC(Cl)=CC=C2SC2=CC=CC=C21 RGCVKNLCSQQDEP-UHFFFAOYSA-N 0.000 description 3
- OTKJDMGTUTTYMP-ROUUACIJSA-N Safingol ( L-threo-sphinganine) Chemical compound CCCCCCCCCCCCCCC[C@H](O)[C@@H](N)CO OTKJDMGTUTTYMP-ROUUACIJSA-N 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- KLBQZWRITKRQQV-UHFFFAOYSA-N Thioridazine Chemical compound C12=CC(SC)=CC=C2SC2=CC=CC=C2N1CCC1CCCCN1C KLBQZWRITKRQQV-UHFFFAOYSA-N 0.000 description 3
- 229920004890 Triton X-100 Polymers 0.000 description 3
- 239000013504 Triton X-100 Substances 0.000 description 3
- PSLUFJFHTBIXMW-WYEYVKMPSA-N [(3r,4ar,5s,6s,6as,10s,10ar,10bs)-3-ethenyl-10,10b-dihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-6-(2-pyridin-2-ylethylcarbamoyloxy)-5,6,6a,8,9,10-hexahydro-2h-benzo[f]chromen-5-yl] acetate Chemical compound O([C@@H]1[C@@H]([C@]2(O[C@](C)(CC(=O)[C@]2(O)[C@@]2(C)[C@@H](O)CCC(C)(C)[C@@H]21)C=C)C)OC(=O)C)C(=O)NCCC1=CC=CC=N1 PSLUFJFHTBIXMW-WYEYVKMPSA-N 0.000 description 3
- SMNRFWMNPDABKZ-WVALLCKVSA-N [[(2R,3S,4R,5S)-5-(2,6-dioxo-3H-pyridin-3-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [[[(2R,3S,4S,5R,6R)-4-fluoro-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl] hydrogen phosphate Chemical compound OC[C@H]1O[C@H](OP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)C2C=CC(=O)NC2=O)[C@H](O)[C@@H](F)[C@@H]1O SMNRFWMNPDABKZ-WVALLCKVSA-N 0.000 description 3
- 235000010443 alginic acid Nutrition 0.000 description 3
- 229920000615 alginic acid Polymers 0.000 description 3
- 229960000836 amitriptyline Drugs 0.000 description 3
- KRMDCWKBEZIMAB-UHFFFAOYSA-N amitriptyline Chemical compound C1CC2=CC=CC=C2C(=CCCN(C)C)C2=CC=CC=C21 KRMDCWKBEZIMAB-UHFFFAOYSA-N 0.000 description 3
- 238000010171 animal model Methods 0.000 description 3
- 239000003963 antioxidant agent Substances 0.000 description 3
- 235000006708 antioxidants Nutrition 0.000 description 3
- 239000000164 antipsychotic agent Substances 0.000 description 3
- 239000012298 atmosphere Substances 0.000 description 3
- 230000002238 attenuated effect Effects 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 230000000903 blocking effect Effects 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 235000010980 cellulose Nutrition 0.000 description 3
- 210000003169 central nervous system Anatomy 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 229960004782 chlordiazepoxide Drugs 0.000 description 3
- ANTSCNMPPGJYLG-UHFFFAOYSA-N chlordiazepoxide Chemical compound O=N=1CC(NC)=NC2=CC=C(Cl)C=C2C=1C1=CC=CC=C1 ANTSCNMPPGJYLG-UHFFFAOYSA-N 0.000 description 3
- 229960001076 chlorpromazine Drugs 0.000 description 3
- ZPEIMTDSQAKGNT-UHFFFAOYSA-N chlorpromazine Chemical compound C1=C(Cl)C=C2N(CCCN(C)C)C3=CC=CC=C3SC2=C1 ZPEIMTDSQAKGNT-UHFFFAOYSA-N 0.000 description 3
- 229940125810 compound 20 Drugs 0.000 description 3
- 229940126208 compound 22 Drugs 0.000 description 3
- 229940125877 compound 31 Drugs 0.000 description 3
- 229940125878 compound 36 Drugs 0.000 description 3
- 229940125807 compound 37 Drugs 0.000 description 3
- 229940126540 compound 41 Drugs 0.000 description 3
- 229940125936 compound 42 Drugs 0.000 description 3
- 229940125844 compound 46 Drugs 0.000 description 3
- 239000012043 crude product Substances 0.000 description 3
- 229940127089 cytotoxic agent Drugs 0.000 description 3
- 230000002939 deleterious effect Effects 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 125000005433 dihydrobenzodioxinyl group Chemical group O1C(COC2=C1C=CC=C2)* 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- 229960002866 duloxetine Drugs 0.000 description 3
- WSEQXVZVJXJVFP-FQEVSTJZSA-N escitalopram Chemical compound C1([C@]2(C3=CC=C(C=C3CO2)C#N)CCCN(C)C)=CC=C(F)C=C1 WSEQXVZVJXJVFP-FQEVSTJZSA-N 0.000 description 3
- 229960004341 escitalopram Drugs 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 239000012065 filter cake Substances 0.000 description 3
- 229960002464 fluoxetine Drugs 0.000 description 3
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 3
- JAXFJECJQZDFJS-XHEPKHHKSA-N gtpl8555 Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)N[C@H](B1O[C@@]2(C)[C@H]3C[C@H](C3(C)C)C[C@H]2O1)CCC1=CC=C(F)C=C1 JAXFJECJQZDFJS-XHEPKHHKSA-N 0.000 description 3
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 229940125425 inverse agonist Drugs 0.000 description 3
- 229940039009 isoproterenol Drugs 0.000 description 3
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 3
- 229960004338 leuprorelin Drugs 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 150000002632 lipids Chemical class 0.000 description 3
- RENRQMCACQEWFC-UGKGYDQZSA-N lnp023 Chemical compound C1([C@H]2N(CC=3C=4C=CNC=4C(C)=CC=3OC)CC[C@@H](C2)OCC)=CC=C(C(O)=O)C=C1 RENRQMCACQEWFC-UGKGYDQZSA-N 0.000 description 3
- 230000033001 locomotion Effects 0.000 description 3
- 238000002483 medication Methods 0.000 description 3
- UZKWTJUDCOPSNM-UHFFFAOYSA-N methoxybenzene Substances CCCCOC=C UZKWTJUDCOPSNM-UHFFFAOYSA-N 0.000 description 3
- 230000000813 microbial effect Effects 0.000 description 3
- 230000001095 motoneuron effect Effects 0.000 description 3
- 229960001158 nortriptyline Drugs 0.000 description 3
- 229960001057 paliperidone Drugs 0.000 description 3
- 229960000762 perphenazine Drugs 0.000 description 3
- 108090000765 processed proteins & peptides Proteins 0.000 description 3
- 238000000159 protein binding assay Methods 0.000 description 3
- 229960004431 quetiapine Drugs 0.000 description 3
- URKOMYMAXPYINW-UHFFFAOYSA-N quetiapine Chemical compound C1CN(CCOCCO)CCN1C1=NC2=CC=CC=C2SC2=CC=CC=C12 URKOMYMAXPYINW-UHFFFAOYSA-N 0.000 description 3
- FTSUPYGMFAPCFZ-ZWNOBZJWSA-N quinpirole Chemical compound C([C@H]1CCCN([C@@H]1C1)CCC)C2=C1C=NN2 FTSUPYGMFAPCFZ-ZWNOBZJWSA-N 0.000 description 3
- 229950001037 quinpirole Drugs 0.000 description 3
- 238000003653 radioligand binding assay Methods 0.000 description 3
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 description 3
- VGKDLMBJGBXTGI-SJCJKPOMSA-N sertraline Chemical compound C1([C@@H]2CC[C@@H](C3=CC=CC=C32)NC)=CC=C(Cl)C(Cl)=C1 VGKDLMBJGBXTGI-SJCJKPOMSA-N 0.000 description 3
- 229960002073 sertraline Drugs 0.000 description 3
- 239000012679 serum free medium Substances 0.000 description 3
- 210000003491 skin Anatomy 0.000 description 3
- 229950006050 spiromustine Drugs 0.000 description 3
- 125000000547 substituted alkyl group Chemical group 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 3
- 229960002784 thioridazine Drugs 0.000 description 3
- PNVNVHUZROJLTJ-UHFFFAOYSA-N venlafaxine Chemical compound C1=CC(OC)=CC=C1C(CN(C)C)C1(O)CCCCC1 PNVNVHUZROJLTJ-UHFFFAOYSA-N 0.000 description 3
- 229960004688 venlafaxine Drugs 0.000 description 3
- AHOUBRCZNHFOSL-YOEHRIQHSA-N (+)-Casbol Chemical compound C1=CC(F)=CC=C1[C@H]1[C@H](COC=2C=C3OCOC3=CC=2)CNCC1 AHOUBRCZNHFOSL-YOEHRIQHSA-N 0.000 description 2
- HZSBSRAVNBUZRA-RQDPQJJXSA-J (1r,2r)-cyclohexane-1,2-diamine;tetrachloroplatinum(2+) Chemical compound Cl[Pt+2](Cl)(Cl)Cl.N[C@@H]1CCCC[C@H]1N HZSBSRAVNBUZRA-RQDPQJJXSA-J 0.000 description 2
- YYZAUJKMIKXWMD-ZDUSSCGKSA-N (2S)-2-[[4-[(2-amino-1-methyl-4-oxopteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid Chemical compound C=1N=C2N(C)C(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 YYZAUJKMIKXWMD-ZDUSSCGKSA-N 0.000 description 2
- SWXOGPJRIDTIRL-DOUNNPEJSA-N (4r,7s,10s,13r,16s,19r)-10-(4-aminobutyl)-n-[(2s)-1-amino-3-(1h-indol-3-yl)-1-oxopropan-2-yl]-19-[[(2r)-2-amino-3-phenylpropanoyl]amino]-16-[(4-hydroxyphenyl)methyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-7-propan-2-yl-1,2-dithia-5,8,11,14,17-pent Chemical compound C([C@H]1C(=O)N[C@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](N)CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(N)=O)=O)C(C)C)C1=CC=C(O)C=C1 SWXOGPJRIDTIRL-DOUNNPEJSA-N 0.000 description 2
- UVNPEUJXKZFWSJ-LMTQTHQJSA-N (R)-N-[(4S)-8-[6-amino-5-[(3,3-difluoro-2-oxo-1H-pyrrolo[2,3-b]pyridin-4-yl)sulfanyl]pyrazin-2-yl]-2-oxa-8-azaspiro[4.5]decan-4-yl]-2-methylpropane-2-sulfinamide Chemical compound CC(C)(C)[S@@](=O)N[C@@H]1COCC11CCN(CC1)c1cnc(Sc2ccnc3NC(=O)C(F)(F)c23)c(N)n1 UVNPEUJXKZFWSJ-LMTQTHQJSA-N 0.000 description 2
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 description 2
- VSWBSWWIRNCQIJ-GJZGRUSLSA-N (R,R)-asenapine Chemical compound O1C2=CC=CC=C2[C@@H]2CN(C)C[C@H]2C2=CC(Cl)=CC=C21 VSWBSWWIRNCQIJ-GJZGRUSLSA-N 0.000 description 2
- FONKWHRXTPJODV-DNQXCXABSA-N 1,3-bis[2-[(8s)-8-(chloromethyl)-4-hydroxy-1-methyl-7,8-dihydro-3h-pyrrolo[3,2-e]indole-6-carbonyl]-1h-indol-5-yl]urea Chemical compound C1([C@H](CCl)CN2C(=O)C=3NC4=CC=C(C=C4C=3)NC(=O)NC=3C=C4C=C(NC4=CC=3)C(=O)N3C4=CC(O)=C5NC=C(C5=C4[C@H](CCl)C3)C)=C2C=C(O)C2=C1C(C)=CN2 FONKWHRXTPJODV-DNQXCXABSA-N 0.000 description 2
- BAXOFTOLAUCFNW-UHFFFAOYSA-N 1H-indazole Chemical compound C1=CC=C2C=NNC2=C1 BAXOFTOLAUCFNW-UHFFFAOYSA-N 0.000 description 2
- YRAJNWYBUCUFBD-UHFFFAOYSA-N 2,2,6,6-tetramethylheptane-3,5-dione Chemical compound CC(C)(C)C(=O)CC(=O)C(C)(C)C YRAJNWYBUCUFBD-UHFFFAOYSA-N 0.000 description 2
- OOMDVERDMZLRFX-UHFFFAOYSA-N 2,2-bis(aminomethyl)propane-1,3-diol;cyclobutane-1,1-dicarboxylic acid;platinum Chemical compound [Pt].NCC(CN)(CO)CO.OC(=O)C1(C(O)=O)CCC1 OOMDVERDMZLRFX-UHFFFAOYSA-N 0.000 description 2
- QXLQZLBNPTZMRK-UHFFFAOYSA-N 2-[(dimethylamino)methyl]-1-(2,4-dimethylphenyl)prop-2-en-1-one Chemical compound CN(C)CC(=C)C(=O)C1=CC=C(C)C=C1C QXLQZLBNPTZMRK-UHFFFAOYSA-N 0.000 description 2
- YMDZDFSUDFLGMX-UHFFFAOYSA-N 2-chloro-n-(2-chloroethyl)ethanamine;hydron;chloride Chemical compound [Cl-].ClCC[NH2+]CCCl YMDZDFSUDFLGMX-UHFFFAOYSA-N 0.000 description 2
- UZFPOOOQHWICKY-UHFFFAOYSA-N 3-[13-[1-[1-[8,12-bis(2-carboxyethyl)-17-(1-hydroxyethyl)-3,7,13,18-tetramethyl-21,24-dihydroporphyrin-2-yl]ethoxy]ethyl]-18-(2-carboxyethyl)-8-(1-hydroxyethyl)-3,7,12,17-tetramethyl-22,23-dihydroporphyrin-2-yl]propanoic acid Chemical compound N1C(C=C2C(=C(CCC(O)=O)C(C=C3C(=C(C)C(C=C4N5)=N3)CCC(O)=O)=N2)C)=C(C)C(C(C)O)=C1C=C5C(C)=C4C(C)OC(C)C1=C(N2)C=C(N3)C(C)=C(C(O)C)C3=CC(C(C)=C3CCC(O)=O)=NC3=CC(C(CCC(O)=O)=C3C)=NC3=CC2=C1C UZFPOOOQHWICKY-UHFFFAOYSA-N 0.000 description 2
- QNKJFXARIMSDBR-UHFFFAOYSA-N 3-[2-[bis(2-chloroethyl)amino]ethyl]-1,3-diazaspiro[4.5]decane-2,4-dione Chemical compound O=C1N(CCN(CCCl)CCCl)C(=O)NC11CCCCC1 QNKJFXARIMSDBR-UHFFFAOYSA-N 0.000 description 2
- WDBQJSCPCGTAFG-QHCPKHFHSA-N 4,4-difluoro-N-[(1S)-3-[4-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)piperidin-1-yl]-1-pyridin-3-ylpropyl]cyclohexane-1-carboxamide Chemical compound FC1(CCC(CC1)C(=O)N[C@@H](CCN1CCC(CC1)N1C(=NN=C1C)C(C)C)C=1C=NC=CC=1)F WDBQJSCPCGTAFG-QHCPKHFHSA-N 0.000 description 2
- BWGRDBSNKQABCB-UHFFFAOYSA-N 4,4-difluoro-N-[3-[3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-thiophen-2-ylpropyl]cyclohexane-1-carboxamide Chemical compound CC(C)C1=NN=C(C)N1C1CC2CCC(C1)N2CCC(NC(=O)C1CCC(F)(F)CC1)C1=CC=CS1 BWGRDBSNKQABCB-UHFFFAOYSA-N 0.000 description 2
- CLPFFLWZZBQMAO-UHFFFAOYSA-N 4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-yl)benzonitrile Chemical compound C1=CC(C#N)=CC=C1C1N2C=NC=C2CCC1 CLPFFLWZZBQMAO-UHFFFAOYSA-N 0.000 description 2
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 2
- AKJHMTWEGVYYSE-AIRMAKDCSA-N 4-HPR Chemical compound C=1C=C(O)C=CC=1NC(=O)/C=C(\C)/C=C/C=C(C)C=CC1=C(C)CCCC1(C)C AKJHMTWEGVYYSE-AIRMAKDCSA-N 0.000 description 2
- RJYQLMILDVERHH-UHFFFAOYSA-N 4-Ipomeanol Chemical compound CC(O)CCC(=O)C=1C=COC=1 RJYQLMILDVERHH-UHFFFAOYSA-N 0.000 description 2
- QFMJFXFXQAFGBO-UHFFFAOYSA-N 4-methoxy-2-nitroaniline Chemical compound COC1=CC=C(N)C([N+]([O-])=O)=C1 QFMJFXFXQAFGBO-UHFFFAOYSA-N 0.000 description 2
- VFXVJEKXKLUDSR-UHFFFAOYSA-N 5-(3-bromopropoxy)-1,3-benzothiazole Chemical compound BrCCCOC1=CC=C2SC=NC2=C1 VFXVJEKXKLUDSR-UHFFFAOYSA-N 0.000 description 2
- LWJPMQCMGHWZSH-UHFFFAOYSA-N 5-(3-bromopropoxy)-1,3-dihydrobenzimidazol-2-one Chemical compound BrCCCOC1=CC=C2NC(=O)NC2=C1 LWJPMQCMGHWZSH-UHFFFAOYSA-N 0.000 description 2
- XAUDJQYHKZQPEU-KVQBGUIXSA-N 5-aza-2'-deoxycytidine Chemical compound O=C1N=C(N)N=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 XAUDJQYHKZQPEU-KVQBGUIXSA-N 0.000 description 2
- LDCYZAJDBXYCGN-VIFPVBQESA-N 5-hydroxy-L-tryptophan Chemical compound C1=C(O)C=C2C(C[C@H](N)C(O)=O)=CNC2=C1 LDCYZAJDBXYCGN-VIFPVBQESA-N 0.000 description 2
- 229940000681 5-hydroxytryptophan Drugs 0.000 description 2
- MUAMSDRVZKCSPE-UHFFFAOYSA-N 6-(3-bromopropoxy)-1h-indazole Chemical compound BrCCCOC1=CC=C2C=NNC2=C1 MUAMSDRVZKCSPE-UHFFFAOYSA-N 0.000 description 2
- CYEQSOYROKGJDA-UHFFFAOYSA-N 6-methoxy-1h-indazole Chemical compound COC1=CC=C2C=NNC2=C1 CYEQSOYROKGJDA-UHFFFAOYSA-N 0.000 description 2
- MBOHAVAGDOGRBS-UHFFFAOYSA-N 7-(4-bromobutoxy)-1h-quinolin-2-one Chemical compound C1=CC(=O)NC2=CC(OCCCCBr)=CC=C21 MBOHAVAGDOGRBS-UHFFFAOYSA-N 0.000 description 2
- URHLNHVYMNBPEO-UHFFFAOYSA-N 7-(4-bromobutoxy)-3,4-dihydro-1h-quinolin-2-one Chemical compound C1CC(=O)NC2=CC(OCCCCBr)=CC=C21 URHLNHVYMNBPEO-UHFFFAOYSA-N 0.000 description 2
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 2
- RTHKPHCVZVYDFN-UHFFFAOYSA-N 9-amino-5-(2-aminopyrimidin-4-yl)pyrido[3',2':4,5]pyrrolo[1,2-c]pyrimidin-4-ol Chemical compound NC1=NC=CC(C=2C3=C(O)C=CN=C3N3C(N)=NC=CC3=2)=N1 RTHKPHCVZVYDFN-UHFFFAOYSA-N 0.000 description 2
- 102100033830 Amphiphysin Human genes 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 241000826760 Barnea Species 0.000 description 2
- 102100029648 Beta-arrestin-2 Human genes 0.000 description 2
- QQPMHFWUHJUHEJ-UHFFFAOYSA-N BrCCCCOc1ccc(cn[nH]2)c2c1 Chemical compound BrCCCCOc1ccc(cn[nH]2)c2c1 QQPMHFWUHJUHEJ-UHFFFAOYSA-N 0.000 description 2
- CIUUIPMOFZIWIZ-UHFFFAOYSA-N Bropirimine Chemical compound NC1=NC(O)=C(Br)C(C=2C=CC=CC=2)=N1 CIUUIPMOFZIWIZ-UHFFFAOYSA-N 0.000 description 2
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 2
- LDZJNMJIPNOYGA-UHFFFAOYSA-N C1=C(OC(C)=O)C(OC)=CC=C1C1=C2C3=CC(OC)=C(OC(C)=O)C=C3C=CN2C2=C1C(C=C(OC)C(OC(C)=O)=C1)=C1OC2=O Chemical compound C1=C(OC(C)=O)C(OC)=CC=C1C1=C2C3=CC(OC)=C(OC(C)=O)C=C3C=CN2C2=C1C(C=C(OC)C(OC(C)=O)=C1)=C1OC2=O LDZJNMJIPNOYGA-UHFFFAOYSA-N 0.000 description 2
- 238000011740 C57BL/6 mouse Methods 0.000 description 2
- FAKVJGDVZFHZPG-UHFFFAOYSA-N COc(cccc1)c1N1CCN(CCCCOc2ccc(CCC(N3)=O)c3c2)CCC1 Chemical compound COc(cccc1)c1N1CCN(CCCCOc2ccc(CCC(N3)=O)c3c2)CCC1 FAKVJGDVZFHZPG-UHFFFAOYSA-N 0.000 description 2
- FVLVBPDQNARYJU-XAHDHGMMSA-N C[C@H]1CCC(CC1)NC(=O)N(CCCl)N=O Chemical compound C[C@H]1CCC(CC1)NC(=O)N(CCCl)N=O FVLVBPDQNARYJU-XAHDHGMMSA-N 0.000 description 2
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 2
- PTOAARAWEBMLNO-KVQBGUIXSA-N Cladribine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 PTOAARAWEBMLNO-KVQBGUIXSA-N 0.000 description 2
- GJSURZIOUXUGAL-UHFFFAOYSA-N Clonidine Chemical compound ClC1=CC=CC(Cl)=C1NC1=NCCN1 GJSURZIOUXUGAL-UHFFFAOYSA-N 0.000 description 2
- PHEDXBVPIONUQT-UHFFFAOYSA-N Cocarcinogen A1 Natural products CCCCCCCCCCCCCC(=O)OC1C(C)C2(O)C3C=C(C)C(=O)C3(O)CC(CO)=CC2C2C1(OC(C)=O)C2(C)C PHEDXBVPIONUQT-UHFFFAOYSA-N 0.000 description 2
- 229910021591 Copper(I) chloride Inorganic materials 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- ZQZFYGIXNQKOAV-OCEACIFDSA-N Droloxifene Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=C(O)C=CC=1)\C1=CC=C(OCCN(C)C)C=C1 ZQZFYGIXNQKOAV-OCEACIFDSA-N 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- UGJMXCAKCUNAIE-UHFFFAOYSA-N Gabapentin Chemical compound OC(=O)CC1(CN)CCCCC1 UGJMXCAKCUNAIE-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 102100039619 Granulocyte colony-stimulating factor Human genes 0.000 description 2
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 2
- 239000012981 Hank's balanced salt solution Substances 0.000 description 2
- 101000779845 Homo sapiens Amphiphysin Proteins 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- WTDHULULXKLSOZ-UHFFFAOYSA-N Hydroxylamine hydrochloride Chemical compound Cl.ON WTDHULULXKLSOZ-UHFFFAOYSA-N 0.000 description 2
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 2
- 108010078049 Interferon alpha-2 Proteins 0.000 description 2
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 2
- 240000007472 Leucaena leucocephala Species 0.000 description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- 229930126263 Maytansine Natural products 0.000 description 2
- NPPQSCRMBWNHMW-UHFFFAOYSA-N Meprobamate Chemical compound NC(=O)OCC(C)(CCC)COC(N)=O NPPQSCRMBWNHMW-UHFFFAOYSA-N 0.000 description 2
- HRHKSTOGXBBQCB-UHFFFAOYSA-N Mitomycin E Natural products O=C1C(N)=C(C)C(=O)C2=C1C(COC(N)=O)C1(OC)C3N(C)C3CN12 HRHKSTOGXBBQCB-UHFFFAOYSA-N 0.000 description 2
- KLPWJLBORRMFGK-UHFFFAOYSA-N Molindone Chemical compound O=C1C=2C(CC)=C(C)NC=2CCC1CN1CCOCC1 KLPWJLBORRMFGK-UHFFFAOYSA-N 0.000 description 2
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 2
- QHJLPOSPWKZACG-UHFFFAOYSA-N N-Methylspiperone Chemical compound C1CN(CCCC(=O)C=2C=CC(F)=CC=2)CCC21C(=O)N(C)CN2C1=CC=CC=C1 QHJLPOSPWKZACG-UHFFFAOYSA-N 0.000 description 2
- NUGPIZCTELGDOS-QHCPKHFHSA-N N-[(1S)-3-[4-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)piperidin-1-yl]-1-pyridin-3-ylpropyl]cyclopentanecarboxamide Chemical compound C(C)(C)C1=NN=C(N1C1CCN(CC1)CC[C@@H](C=1C=NC=CC=1)NC(=O)C1CCCC1)C NUGPIZCTELGDOS-QHCPKHFHSA-N 0.000 description 2
- LFZAGIJXANFPFN-UHFFFAOYSA-N N-[3-[4-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)piperidin-1-yl]-1-thiophen-2-ylpropyl]acetamide Chemical compound C(C)(C)C1=NN=C(N1C1CCN(CC1)CCC(C=1SC=CC=1)NC(C)=O)C LFZAGIJXANFPFN-UHFFFAOYSA-N 0.000 description 2
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 2
- LYPFDBRUNKHDGX-SOGSVHMOSA-N N1C2=CC=C1\C(=C1\C=CC(=N1)\C(=C1\C=C/C(/N1)=C(/C1=N/C(/CC1)=C2/C1=CC(O)=CC=C1)C1=CC(O)=CC=C1)\C1=CC(O)=CC=C1)C1=CC(O)=CC=C1 Chemical compound N1C2=CC=C1\C(=C1\C=CC(=N1)\C(=C1\C=C/C(/N1)=C(/C1=N/C(/CC1)=C2/C1=CC(O)=CC=C1)C1=CC(O)=CC=C1)\C1=CC(O)=CC=C1)C1=CC(O)=CC=C1 LYPFDBRUNKHDGX-SOGSVHMOSA-N 0.000 description 2
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 2
- NUYZVDBIVNOTSC-UHFFFAOYSA-N Oc1ccc(cn[nH]2)c2c1 Chemical compound Oc1ccc(cn[nH]2)c2c1 NUYZVDBIVNOTSC-UHFFFAOYSA-N 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- 229930012538 Paclitaxel Natural products 0.000 description 2
- AHOUBRCZNHFOSL-UHFFFAOYSA-N Paroxetine hydrochloride Natural products C1=CC(F)=CC=C1C1C(COC=2C=C3OCOC3=CC=2)CNCC1 AHOUBRCZNHFOSL-UHFFFAOYSA-N 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 2
- 229940123924 Protein kinase C inhibitor Drugs 0.000 description 2
- NPXOKRUENSOPAO-UHFFFAOYSA-N Raney nickel Chemical compound [Al].[Ni] NPXOKRUENSOPAO-UHFFFAOYSA-N 0.000 description 2
- XSVMFMHYUFZWBK-NSHDSACASA-N Rivastigmine Chemical compound CCN(C)C(=O)OC1=CC=CC([C@H](C)N(C)C)=C1 XSVMFMHYUFZWBK-NSHDSACASA-N 0.000 description 2
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 238000003639 Student–Newman–Keuls (SNK) method Methods 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- 102000036693 Thrombopoietin Human genes 0.000 description 2
- 108010041111 Thrombopoietin Proteins 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 108010050144 Triptorelin Pamoate Proteins 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 229930003427 Vitamin E Natural products 0.000 description 2
- ODEDPKNSRBCSDO-UHFFFAOYSA-N [2-(hexadecylsulfanylmethyl)-3-methoxypropyl] 2-(trimethylazaniumyl)ethyl phosphate Chemical compound CCCCCCCCCCCCCCCCSCC(COC)COP([O-])(=O)OCC[N+](C)(C)C ODEDPKNSRBCSDO-UHFFFAOYSA-N 0.000 description 2
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 2
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Natural products CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 2
- 150000001241 acetals Chemical class 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- USZYSDMBJDPRIF-SVEJIMAYSA-N aclacinomycin A Chemical compound O([C@H]1[C@@H](O)C[C@@H](O[C@H]1C)O[C@H]1[C@H](C[C@@H](O[C@H]1C)O[C@H]1C[C@]([C@@H](C2=CC=3C(=O)C4=CC=CC(O)=C4C(=O)C=3C(O)=C21)C(=O)OC)(O)CC)N(C)C)[C@H]1CCC(=O)[C@H](C)O1 USZYSDMBJDPRIF-SVEJIMAYSA-N 0.000 description 2
- 229960004176 aclarubicin Drugs 0.000 description 2
- SMPZPKRDRQOOHT-UHFFFAOYSA-N acronycine Chemical compound CN1C2=CC=CC=C2C(=O)C2=C1C(C=CC(C)(C)O1)=C1C=C2OC SMPZPKRDRQOOHT-UHFFFAOYSA-N 0.000 description 2
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 229950004955 adozelesin Drugs 0.000 description 2
- BYRVKDUQDLJUBX-JJCDCTGGSA-N adozelesin Chemical compound C1=CC=C2OC(C(=O)NC=3C=C4C=C(NC4=CC=3)C(=O)N3C[C@H]4C[C@]44C5=C(C(C=C43)=O)NC=C5C)=CC2=C1 BYRVKDUQDLJUBX-JJCDCTGGSA-N 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 108700025316 aldesleukin Proteins 0.000 description 2
- 229960005310 aldesleukin Drugs 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 229960004538 alprazolam Drugs 0.000 description 2
- VREFGVBLTWBCJP-UHFFFAOYSA-N alprazolam Chemical compound C12=CC(Cl)=CC=C2N2C(C)=NN=C2CN=C1C1=CC=CC=C1 VREFGVBLTWBCJP-UHFFFAOYSA-N 0.000 description 2
- 229960000473 altretamine Drugs 0.000 description 2
- 229960003036 amisulpride Drugs 0.000 description 2
- NTJOBXMMWNYJFB-UHFFFAOYSA-N amisulpride Chemical compound CCN1CCCC1CNC(=O)C1=CC(S(=O)(=O)CC)=C(N)C=C1OC NTJOBXMMWNYJFB-UHFFFAOYSA-N 0.000 description 2
- 235000019270 ammonium chloride Nutrition 0.000 description 2
- 229960001220 amsacrine Drugs 0.000 description 2
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 2
- 229960002932 anastrozole Drugs 0.000 description 2
- YBBLVLTVTVSKRW-UHFFFAOYSA-N anastrozole Chemical compound N#CC(C)(C)C1=CC(C(C)(C#N)C)=CC(CN2N=CN=C2)=C1 YBBLVLTVTVSKRW-UHFFFAOYSA-N 0.000 description 2
- 239000000935 antidepressant agent Substances 0.000 description 2
- 229940005513 antidepressants Drugs 0.000 description 2
- 229940045985 antineoplastic platinum compound Drugs 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 229960005245 asenapine Drugs 0.000 description 2
- XFILPEOLDIKJHX-QYZOEREBSA-N batimastat Chemical compound C([C@@H](C(=O)NC)NC(=O)[C@H](CC(C)C)[C@H](CSC=1SC=CC=1)C(=O)NO)C1=CC=CC=C1 XFILPEOLDIKJHX-QYZOEREBSA-N 0.000 description 2
- 229950001858 batimastat Drugs 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- MYONAGGJKCJOBT-UHFFFAOYSA-N benzimidazol-2-one Chemical compound C1=CC=CC2=NC(=O)N=C21 MYONAGGJKCJOBT-UHFFFAOYSA-N 0.000 description 2
- 125000000499 benzofuranyl group Chemical group O1C(=CC2=C1C=CC=C2)* 0.000 description 2
- 229960000997 bicalutamide Drugs 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 229950008548 bisantrene Drugs 0.000 description 2
- 229950006844 bizelesin Drugs 0.000 description 2
- ILAHWRKJUDSMFH-UHFFFAOYSA-N boron tribromide Chemical compound BrB(Br)Br ILAHWRKJUDSMFH-UHFFFAOYSA-N 0.000 description 2
- 229950009494 bropirimine Drugs 0.000 description 2
- CZBZUDVBLSSABA-UHFFFAOYSA-N butylated hydroxyanisole Chemical compound COC1=CC=C(O)C(C(C)(C)C)=C1.COC1=CC=C(O)C=C1C(C)(C)C CZBZUDVBLSSABA-UHFFFAOYSA-N 0.000 description 2
- 229910000024 caesium carbonate Inorganic materials 0.000 description 2
- 239000001110 calcium chloride Substances 0.000 description 2
- 229910001628 calcium chloride Inorganic materials 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- 125000002843 carboxylic acid group Chemical group 0.000 description 2
- BBZDXMBRAFTCAA-AREMUKBSSA-N carzelesin Chemical compound C1=2NC=C(C)C=2C([C@H](CCl)CN2C(=O)C=3NC4=CC=C(C=C4C=3)NC(=O)C3=CC4=CC=C(C=C4O3)N(CC)CC)=C2C=C1OC(=O)NC1=CC=CC=C1 BBZDXMBRAFTCAA-AREMUKBSSA-N 0.000 description 2
- 229950007509 carzelesin Drugs 0.000 description 2
- 210000002421 cell wall Anatomy 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 230000036755 cellular response Effects 0.000 description 2
- NQGMIPUYCWIEAW-OVCLIPMQSA-N chembl1834105 Chemical compound O/N=C/C1=C(SC)C(OC)=CC(C=2N=CC=CC=2)=N1 NQGMIPUYCWIEAW-OVCLIPMQSA-N 0.000 description 2
- PBKVEOSEPXMKDN-LZHUFOCISA-N chembl2311030 Chemical class CS(O)(=O)=O.CS(O)(=O)=O.CS(O)(=O)=O.CS(O)(=O)=O.C1=CC([C@H]2C[C@H](CN(C)[C@@H]2C2)C(=O)N[C@]3(C(=O)N4[C@H](C(N5CCC[C@H]5[C@]4(O)O3)=O)C(C)C)C(C)C)=C3C2=CNC3=C1.C1=CC([C@H]2C[C@H](CN(C)[C@@H]2C2)C(=O)N[C@]3(C(=O)N4[C@H](C(N5CCC[C@H]5[C@]4(O)O3)=O)C(C)CC)C(C)C)=C3C2=CNC3=C1.C1=CC([C@H]2C[C@H](CN(C)[C@@H]2C2)C(=O)N[C@]3(C(=O)N4[C@H](C(N5CCC[C@H]5[C@]4(O)O3)=O)CC(C)C)C(C)C)=C3C2=CNC3=C1.C([C@H]1C(=O)N2CCC[C@H]2[C@]2(O)O[C@](C(N21)=O)(NC(=O)[C@H]1CN(C)[C@H]2[C@@H](C=3C=CC=C4NC=C(C=34)C2)C1)C(C)C)C1=CC=CC=C1 PBKVEOSEPXMKDN-LZHUFOCISA-N 0.000 description 2
- 239000000460 chlorine Substances 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 229960002436 cladribine Drugs 0.000 description 2
- 229960002896 clonidine Drugs 0.000 description 2
- 229960004170 clozapine Drugs 0.000 description 2
- QZUDBNBUXVUHMW-UHFFFAOYSA-N clozapine Chemical compound C1CN(C)CCN1C1=NC2=CC(Cl)=CC=C2NC2=CC=CC=C12 QZUDBNBUXVUHMW-UHFFFAOYSA-N 0.000 description 2
- 229940125773 compound 10 Drugs 0.000 description 2
- 229940125782 compound 2 Drugs 0.000 description 2
- 229940126214 compound 3 Drugs 0.000 description 2
- 229940125898 compound 5 Drugs 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- OXBLHERUFWYNTN-UHFFFAOYSA-M copper(I) chloride Chemical compound [Cu]Cl OXBLHERUFWYNTN-UHFFFAOYSA-M 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 229960003603 decitabine Drugs 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- WVYXNIXAMZOZFK-UHFFFAOYSA-N diaziquone Chemical compound O=C1C(NC(=O)OCC)=C(N2CC2)C(=O)C(NC(=O)OCC)=C1N1CC1 WVYXNIXAMZOZFK-UHFFFAOYSA-N 0.000 description 2
- 229950002389 diaziquone Drugs 0.000 description 2
- SBZXBUIDTXKZTM-UHFFFAOYSA-N diglyme Chemical compound COCCOCCOC SBZXBUIDTXKZTM-UHFFFAOYSA-N 0.000 description 2
- OTKJDMGTUTTYMP-UHFFFAOYSA-N dihydrosphingosine Natural products CCCCCCCCCCCCCCCC(O)C(N)CO OTKJDMGTUTTYMP-UHFFFAOYSA-N 0.000 description 2
- 229960003668 docetaxel Drugs 0.000 description 2
- NOPFSRXAKWQILS-UHFFFAOYSA-N docosan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCCCCCO NOPFSRXAKWQILS-UHFFFAOYSA-N 0.000 description 2
- ADEBPBSSDYVVLD-UHFFFAOYSA-N donepezil Chemical compound O=C1C=2C=C(OC)C(OC)=CC=2CC1CC(CC1)CCN1CC1=CC=CC=C1 ADEBPBSSDYVVLD-UHFFFAOYSA-N 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 229960005426 doxepin Drugs 0.000 description 2
- ODQWQRRAPPTVAG-GZTJUZNOSA-N doxepin Chemical compound C1OC2=CC=CC=C2C(=C/CCN(C)C)/C2=CC=CC=C21 ODQWQRRAPPTVAG-GZTJUZNOSA-N 0.000 description 2
- 229950004203 droloxifene Drugs 0.000 description 2
- 238000009510 drug design Methods 0.000 description 2
- 229940040520 ergoloid mesylates Drugs 0.000 description 2
- HCZKYJDFEPMADG-UHFFFAOYSA-N erythro-nordihydroguaiaretic acid Natural products C=1C=C(O)C(O)=CC=1CC(C)C(C)CC1=CC=C(O)C(O)=C1 HCZKYJDFEPMADG-UHFFFAOYSA-N 0.000 description 2
- FRPJXPJMRWBBIH-RBRWEJTLSA-N estramustine Chemical compound ClCCN(CCCl)C(=O)OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 FRPJXPJMRWBBIH-RBRWEJTLSA-N 0.000 description 2
- 229940011871 estrogen Drugs 0.000 description 2
- 239000000262 estrogen Substances 0.000 description 2
- 239000000328 estrogen antagonist Substances 0.000 description 2
- 229950006566 etanidazole Drugs 0.000 description 2
- WCDWBPCFGJXFJZ-UHFFFAOYSA-N etanidazole Chemical compound OCCNC(=O)CN1C=CN=C1[N+]([O-])=O WCDWBPCFGJXFJZ-UHFFFAOYSA-N 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- DEFVIWRASFVYLL-UHFFFAOYSA-N ethylene glycol bis(2-aminoethyl)tetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=O DEFVIWRASFVYLL-UHFFFAOYSA-N 0.000 description 2
- 229960000752 etoposide phosphate Drugs 0.000 description 2
- LIQODXNTTZAGID-OCBXBXKTSA-N etoposide phosphate Chemical compound COC1=C(OP(O)(O)=O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 LIQODXNTTZAGID-OCBXBXKTSA-N 0.000 description 2
- 229950011548 fadrozole Drugs 0.000 description 2
- NMUSYJAQQFHJEW-ARQDHWQXSA-N fazarabine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-ARQDHWQXSA-N 0.000 description 2
- 229950005096 fazarabine Drugs 0.000 description 2
- 229950003662 fenretinide Drugs 0.000 description 2
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 2
- CJOFXWAVKWHTFT-XSFVSMFZSA-N fluvoxamine Chemical compound COCCCC\C(=N/OCCN)C1=CC=C(C(F)(F)F)C=C1 CJOFXWAVKWHTFT-XSFVSMFZSA-N 0.000 description 2
- 229960004038 fluvoxamine Drugs 0.000 description 2
- 125000002541 furyl group Chemical group 0.000 description 2
- ASUTZQLVASHGKV-JDFRZJQESA-N galanthamine Chemical compound O1C(=C23)C(OC)=CC=C2CN(C)CC[C@]23[C@@H]1C[C@@H](O)C=C2 ASUTZQLVASHGKV-JDFRZJQESA-N 0.000 description 2
- CHPZKNULDCNCBW-UHFFFAOYSA-N gallium nitrate Chemical compound [Ga+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O CHPZKNULDCNCBW-UHFFFAOYSA-N 0.000 description 2
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 229960005277 gemcitabine Drugs 0.000 description 2
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 2
- UUVWYPNAQBNQJQ-UHFFFAOYSA-N hexamethylmelamine Chemical compound CN(C)C1=NC(N(C)C)=NC(N(C)C)=N1 UUVWYPNAQBNQJQ-UHFFFAOYSA-N 0.000 description 2
- 229960001340 histamine Drugs 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- 229950006905 ilmofosine Drugs 0.000 description 2
- 125000002883 imidazolyl group Chemical group 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 230000001965 increasing effect Effects 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 239000011630 iodine Substances 0.000 description 2
- 229910052740 iodine Inorganic materials 0.000 description 2
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 2
- ZLVXBBHTMQJRSX-VMGNSXQWSA-N jdtic Chemical compound C1([C@]2(C)CCN(C[C@@H]2C)C[C@H](C(C)C)NC(=O)[C@@H]2NCC3=CC(O)=CC=C3C2)=CC=CC(O)=C1 ZLVXBBHTMQJRSX-VMGNSXQWSA-N 0.000 description 2
- 230000008466 key signal transduction pathway Effects 0.000 description 2
- 108010021336 lanreotide Proteins 0.000 description 2
- 229960003881 letrozole Drugs 0.000 description 2
- HPJKCIUCZWXJDR-UHFFFAOYSA-N letrozole Chemical compound C1=CC(C#N)=CC=C1C(N1N=CN=C1)C1=CC=C(C#N)C=C1 HPJKCIUCZWXJDR-UHFFFAOYSA-N 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- 239000012280 lithium aluminium hydride Substances 0.000 description 2
- 230000003137 locomotive effect Effects 0.000 description 2
- 239000007937 lozenge Substances 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 238000004020 luminiscence type Methods 0.000 description 2
- 229960003951 masoprocol Drugs 0.000 description 2
- HCZKYJDFEPMADG-TXEJJXNPSA-N masoprocol Chemical compound C([C@H](C)[C@H](C)CC=1C=C(O)C(O)=CC=1)C1=CC=C(O)C(O)=C1 HCZKYJDFEPMADG-TXEJJXNPSA-N 0.000 description 2
- WKPWGQKGSOKKOO-RSFHAFMBSA-N maytansine Chemical compound CO[C@@H]([C@@]1(O)C[C@](OC(=O)N1)([C@H]([C@@H]1O[C@@]1(C)[C@@H](OC(=O)[C@H](C)N(C)C(C)=O)CC(=O)N1C)C)[H])\C=C\C=C(C)\CC2=CC(OC)=C(Cl)C1=C2 WKPWGQKGSOKKOO-RSFHAFMBSA-N 0.000 description 2
- LWYJUZBXGAFFLP-OCNCTQISSA-N menogaril Chemical compound O1[C@@]2(C)[C@H](O)[C@@H](N(C)C)[C@H](O)[C@@H]1OC1=C3C(=O)C(C=C4C[C@@](C)(O)C[C@H](C4=C4O)OC)=C4C(=O)C3=C(O)C=C12 LWYJUZBXGAFFLP-OCNCTQISSA-N 0.000 description 2
- 229950002676 menogaril Drugs 0.000 description 2
- 229960004815 meprobamate Drugs 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- VRQVVMDWGGWHTJ-CQSZACIVSA-N methotrimeprazine Chemical compound C1=CC=C2N(C[C@H](C)CN(C)C)C3=CC(OC)=CC=C3SC2=C1 VRQVVMDWGGWHTJ-CQSZACIVSA-N 0.000 description 2
- 229940042053 methotrimeprazine Drugs 0.000 description 2
- HRHKSTOGXBBQCB-VFWICMBZSA-N methylmitomycin Chemical compound O=C1C(N)=C(C)C(=O)C2=C1[C@@H](COC(N)=O)[C@@]1(OC)[C@H]3N(C)[C@H]3CN12 HRHKSTOGXBBQCB-VFWICMBZSA-N 0.000 description 2
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 2
- RONZAEMNMFQXRA-UHFFFAOYSA-N mirtazapine Chemical compound C1C2=CC=CN=C2N2CCN(C)CC2C2=CC=CC=C21 RONZAEMNMFQXRA-UHFFFAOYSA-N 0.000 description 2
- 229960001785 mirtazapine Drugs 0.000 description 2
- 229960004938 molindone Drugs 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 238000010172 mouse model Methods 0.000 description 2
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical class CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 2
- NJSMWLQOCQIOPE-OCHFTUDZSA-N n-[(e)-[10-[(e)-(4,5-dihydro-1h-imidazol-2-ylhydrazinylidene)methyl]anthracen-9-yl]methylideneamino]-4,5-dihydro-1h-imidazol-2-amine Chemical compound N1CCN=C1N\N=C\C(C1=CC=CC=C11)=C(C=CC=C2)C2=C1\C=N\NC1=NCCN1 NJSMWLQOCQIOPE-OCHFTUDZSA-N 0.000 description 2
- VWPOSFSPZNDTMJ-UCWKZMIHSA-N nadolol Chemical compound C1[C@@H](O)[C@@H](O)CC2=C1C=CC=C2OCC(O)CNC(C)(C)C VWPOSFSPZNDTMJ-UCWKZMIHSA-N 0.000 description 2
- 229960004255 nadolol Drugs 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 230000000926 neurological effect Effects 0.000 description 2
- 235000001968 nicotinic acid Nutrition 0.000 description 2
- 239000011664 nicotinic acid Substances 0.000 description 2
- 238000001543 one-way ANOVA Methods 0.000 description 2
- 229950008017 ormaplatin Drugs 0.000 description 2
- 229960001816 oxcarbazepine Drugs 0.000 description 2
- CTRLABGOLIVAIY-UHFFFAOYSA-N oxcarbazepine Chemical compound C1C(=O)C2=CC=CC=C2N(C(=O)N)C2=CC=CC=C21 CTRLABGOLIVAIY-UHFFFAOYSA-N 0.000 description 2
- 150000002923 oximes Chemical class 0.000 description 2
- LDCYZAJDBXYCGN-UHFFFAOYSA-N oxitriptan Natural products C1=C(O)C=C2C(CC(N)C(O)=O)=CNC2=C1 LDCYZAJDBXYCGN-UHFFFAOYSA-N 0.000 description 2
- 229960001592 paclitaxel Drugs 0.000 description 2
- 229960002296 paroxetine Drugs 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 229960001744 pegaspargase Drugs 0.000 description 2
- 108010001564 pegaspargase Proteins 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- VPAWVRUHMJVRHU-VGDKGRGNSA-N perfosfamide Chemical compound OO[C@@H]1CCO[P@@](=O)(N(CCCl)CCCl)N1 VPAWVRUHMJVRHU-VGDKGRGNSA-N 0.000 description 2
- 229950009351 perfosfamide Drugs 0.000 description 2
- NDTYTMIUWGWIMO-UHFFFAOYSA-N perillyl alcohol Chemical compound CC(=C)C1CCC(CO)=CC1 NDTYTMIUWGWIMO-UHFFFAOYSA-N 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- PHEDXBVPIONUQT-RGYGYFBISA-N phorbol 13-acetate 12-myristate Chemical compound C([C@]1(O)C(=O)C(C)=C[C@H]1[C@@]1(O)[C@H](C)[C@H]2OC(=O)CCCCCCCCCCCCC)C(CO)=C[C@H]1[C@H]1[C@]2(OC(C)=O)C1(C)C PHEDXBVPIONUQT-RGYGYFBISA-N 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 229960003634 pimozide Drugs 0.000 description 2
- YVUQSNJEYSNKRX-UHFFFAOYSA-N pimozide Chemical compound C1=CC(F)=CC=C1C(C=1C=CC(F)=CC=1)CCCN1CCC(N2C(NC3=CC=CC=C32)=O)CC1 YVUQSNJEYSNKRX-UHFFFAOYSA-N 0.000 description 2
- 230000001817 pituitary effect Effects 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 150000003058 platinum compounds Chemical class 0.000 description 2
- 229920000058 polyacrylate Polymers 0.000 description 2
- 239000004584 polyacrylic acid Substances 0.000 description 2
- 229960004293 porfimer sodium Drugs 0.000 description 2
- 229950004406 porfiromycin Drugs 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 102000004196 processed proteins & peptides Human genes 0.000 description 2
- WIKYUJGCLQQFNW-UHFFFAOYSA-N prochlorperazine Chemical compound C1CN(C)CCN1CCCN1C2=CC(Cl)=CC=C2SC2=CC=CC=C21 WIKYUJGCLQQFNW-UHFFFAOYSA-N 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 239000003881 protein kinase C inhibitor Substances 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 2
- 125000004076 pyridyl group Chemical group 0.000 description 2
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 2
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 2
- 239000002287 radioligand Substances 0.000 description 2
- 238000001953 recrystallisation Methods 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000005316 response function Methods 0.000 description 2
- 229960004136 rivastigmine Drugs 0.000 description 2
- QXKJWHWUDVQATH-UHFFFAOYSA-N rogletimide Chemical compound C=1C=NC=CC=1C1(CC)CCC(=O)NC1=O QXKJWHWUDVQATH-UHFFFAOYSA-N 0.000 description 2
- 229950005230 rogletimide Drugs 0.000 description 2
- MOCVYVBNJQIVOV-TVQRCGJNSA-N rohitukine Chemical compound O[C@@H]1CN(C)CC[C@@H]1C1=C(O)C=C(O)C2=C1OC(C)=CC2=O MOCVYVBNJQIVOV-TVQRCGJNSA-N 0.000 description 2
- 229950008902 safingol Drugs 0.000 description 2
- CGFVUVWMYIHGHS-UHFFFAOYSA-N saintopin Chemical compound C1=C(O)C=C2C=C(C(=O)C=3C(=C(O)C=C(C=3)O)C3=O)C3=C(O)C2=C1O CGFVUVWMYIHGHS-UHFFFAOYSA-N 0.000 description 2
- 229960003440 semustine Drugs 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 238000007493 shaping process Methods 0.000 description 2
- 238000010898 silica gel chromatography Methods 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- 239000012312 sodium hydride Substances 0.000 description 2
- 229910000104 sodium hydride Inorganic materials 0.000 description 2
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 2
- XBUIKNRVGYFSHL-IAVQPKKASA-M sodium;[(1e,3r,4r,6r,7z,9z,11e)-3,6,13-trihydroxy-3-methyl-1-[(2r)-6-oxo-2,3-dihydropyran-2-yl]trideca-1,7,9,11-tetraen-4-yl] hydrogen phosphate Chemical compound [Na+].OC/C=C/C=C\C=C/[C@H](O)C[C@@H](OP(O)([O-])=O)[C@@](O)(C)\C=C\[C@H]1CC=CC(=O)O1 XBUIKNRVGYFSHL-IAVQPKKASA-M 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- PVYJZLYGTZKPJE-UHFFFAOYSA-N streptonigrin Chemical compound C=1C=C2C(=O)C(OC)=C(N)C(=O)C2=NC=1C(C=1N)=NC(C(O)=O)=C(C)C=1C1=CC=C(OC)C(OC)=C1O PVYJZLYGTZKPJE-UHFFFAOYSA-N 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 229960001674 tegafur Drugs 0.000 description 2
- WFWLQNSHRPWKFK-ZCFIWIBFSA-N tegafur Chemical compound O=C1NC(=O)C(F)=CN1[C@@H]1OCCC1 WFWLQNSHRPWKFK-ZCFIWIBFSA-N 0.000 description 2
- LMBFAGIMSUYTBN-MPZNNTNKSA-N teixobactin Chemical compound C([C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H]1C(N[C@@H](C)C(=O)N[C@@H](C[C@@H]2NC(=N)NC2)C(=O)N[C@H](C(=O)O[C@H]1C)[C@@H](C)CC)=O)NC)C1=CC=CC=C1 LMBFAGIMSUYTBN-MPZNNTNKSA-N 0.000 description 2
- 229960002197 temoporfin Drugs 0.000 description 2
- 229960001278 teniposide Drugs 0.000 description 2
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 2
- DJWNFMMYIQIDBH-UHFFFAOYSA-N tert-butyl 4-(2-methoxyphenyl)piperazine-1-carboxylate Chemical compound COC1=CC=CC=C1N1CCN(C(=O)OC(C)(C)C)CC1 DJWNFMMYIQIDBH-UHFFFAOYSA-N 0.000 description 2
- KYDRBEKKUJYDDV-UHFFFAOYSA-N tert-butyl 4-(2-propan-2-yloxyphenyl)-1,4-diazepane-1-carboxylate Chemical compound CC(C)OC1=CC=CC=C1N1CCN(C(=O)OC(C)(C)C)CCC1 KYDRBEKKUJYDDV-UHFFFAOYSA-N 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 125000001544 thienyl group Chemical group 0.000 description 2
- 125000005309 thioalkoxy group Chemical group 0.000 description 2
- 229950002376 tirapazamine Drugs 0.000 description 2
- QVMPZNRFXAKISM-UHFFFAOYSA-N tirapazamine Chemical compound C1=CC=C2[N+]([O-])=NC(=N)N(O)C2=C1 QVMPZNRFXAKISM-UHFFFAOYSA-N 0.000 description 2
- TVPNFKRGOFJQOO-UHFFFAOYSA-N topsentin b1 Chemical compound C1=CC=C2C(C3=CN=C(N3)C(=O)C=3C4=CC=C(C=C4NC=3)O)=CNC2=C1 TVPNFKRGOFJQOO-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 230000032258 transport Effects 0.000 description 2
- 229960001099 trimetrexate Drugs 0.000 description 2
- NOYPYLRCIDNJJB-UHFFFAOYSA-N trimetrexate Chemical compound COC1=C(OC)C(OC)=CC(NCC=2C(=C3C(N)=NC(N)=NC3=CC=2)C)=C1 NOYPYLRCIDNJJB-UHFFFAOYSA-N 0.000 description 2
- VXKHXGOKWPXYNA-PGBVPBMZSA-N triptorelin Chemical compound C([C@@H](C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 VXKHXGOKWPXYNA-PGBVPBMZSA-N 0.000 description 2
- 229960004824 triptorelin Drugs 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 2
- MSRILKIQRXUYCT-UHFFFAOYSA-M valproate semisodium Chemical compound [Na+].CCCC(C(O)=O)CCC.CCCC(C([O-])=O)CCC MSRILKIQRXUYCT-UHFFFAOYSA-M 0.000 description 2
- 229960000604 valproic acid Drugs 0.000 description 2
- 229960002730 vapreotide Drugs 0.000 description 2
- 108700029852 vapreotide Proteins 0.000 description 2
- ZQFGRJWRSLZCSQ-ZSFNYQMMSA-N verteporfin Chemical compound C=1C([C@@]2([C@H](C(=O)OC)C(=CC=C22)C(=O)OC)C)=NC2=CC(C(=C2C=C)C)=NC2=CC(C(=C2CCC(O)=O)C)=NC2=CC2=NC=1C(C)=C2CCC(=O)OC ZQFGRJWRSLZCSQ-ZSFNYQMMSA-N 0.000 description 2
- 229960003895 verteporfin Drugs 0.000 description 2
- 235000019165 vitamin E Nutrition 0.000 description 2
- 239000011709 vitamin E Substances 0.000 description 2
- 229940046009 vitamin E Drugs 0.000 description 2
- 229960001771 vorozole Drugs 0.000 description 2
- XLMPPFTZALNBFS-INIZCTEOSA-N vorozole Chemical compound C1([C@@H](C2=CC=C3N=NN(C3=C2)C)N2N=CN=C2)=CC=C(Cl)C=C1 XLMPPFTZALNBFS-INIZCTEOSA-N 0.000 description 2
- 239000011534 wash buffer Substances 0.000 description 2
- 229950003017 zeniplatin Drugs 0.000 description 2
- MVWVFYHBGMAFLY-UHFFFAOYSA-N ziprasidone Chemical compound C1=CC=C2C(N3CCN(CC3)CCC3=CC=4CC(=O)NC=4C=C3Cl)=NSC2=C1 MVWVFYHBGMAFLY-UHFFFAOYSA-N 0.000 description 2
- 229960000607 ziprasidone Drugs 0.000 description 2
- XOFLBQFBSOEHOG-UUOKFMHZSA-N γS-GTP Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=S)[C@@H](O)[C@H]1O XOFLBQFBSOEHOG-UUOKFMHZSA-N 0.000 description 2
- OPFTUNCRGUEPRZ-UHFFFAOYSA-N (+)-beta-Elemen Natural products CC(=C)C1CCC(C)(C=C)C(C(C)=C)C1 OPFTUNCRGUEPRZ-UHFFFAOYSA-N 0.000 description 1
- BMKDZUISNHGIBY-ZETCQYMHSA-N (+)-dexrazoxane Chemical compound C([C@H](C)N1CC(=O)NC(=O)C1)N1CC(=O)NC(=O)C1 BMKDZUISNHGIBY-ZETCQYMHSA-N 0.000 description 1
- OPFTUNCRGUEPRZ-QLFBSQMISA-N (-)-beta-elemene Chemical compound CC(=C)[C@@H]1CC[C@@](C)(C=C)[C@H](C(C)=C)C1 OPFTUNCRGUEPRZ-QLFBSQMISA-N 0.000 description 1
- 229930007631 (-)-perillyl alcohol Natural products 0.000 description 1
- OTWVIYXCRFLDJW-QMVMUTFZSA-N (1-hydroxy-1-phosphonooxyethyl) dihydrogen phosphate;rhenium-186 Chemical compound [186Re].OP(=O)(O)OC(O)(C)OP(O)(O)=O OTWVIYXCRFLDJW-QMVMUTFZSA-N 0.000 description 1
- IGLYMJRIWWIQQE-QUOODJBBSA-N (1S,2R)-2-phenylcyclopropan-1-amine (1R,2S)-2-phenylcyclopropan-1-amine Chemical compound N[C@H]1C[C@@H]1C1=CC=CC=C1.N[C@@H]1C[C@H]1C1=CC=CC=C1 IGLYMJRIWWIQQE-QUOODJBBSA-N 0.000 description 1
- WYDUSKDSKCASEF-LJQANCHMSA-N (1s)-1-cyclohexyl-1-phenyl-3-pyrrolidin-1-ylpropan-1-ol Chemical compound C([C@](O)(C1CCCCC1)C=1C=CC=CC=1)CN1CCCC1 WYDUSKDSKCASEF-LJQANCHMSA-N 0.000 description 1
- MNHVIVWFCMBFCV-AVGNSLFASA-N (2S)-2-[[(2S)-2-[[(4S)-4-amino-4-carboxybutanoyl]amino]-6-diazo-5-oxohexanoyl]amino]-6-diazo-5-oxohexanoic acid Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CCC(=O)C=[N+]=[N-])C(=O)N[C@@H](CCC(=O)C=[N+]=[N-])C(O)=O MNHVIVWFCMBFCV-AVGNSLFASA-N 0.000 description 1
- MXABZXILAJGOTL-AUYMZICSSA-N (2S)-N-[(2S)-1-[(2S)-1-[(2S,3S)-1-[(2S)-1-[2-[(2S)-1,3-dihydroxy-1-[(E)-1-hydroxy-1-[(2S,3S)-1-hydroxy-3-methyl-1-[[(2Z,6S,9S,12R)-5,8,11-trihydroxy-9-(2-methylpropyl)-6-propan-2-yl-1-thia-4,7,10-triazacyclotrideca-2,4,7,10-tetraen-12-yl]imino]pentan-2-yl]iminobut-2-en-2-yl]iminopropan-2-yl]imino-2-hydroxyethyl]imino-1,5-dihydroxy-5-iminopentan-2-yl]imino-1-hydroxy-3-methylpentan-2-yl]imino-1-hydroxy-3-methylbutan-2-yl]imino-1-hydroxy-3-phenylpropan-2-yl]-2-[[(2S)-2-[[(2S)-2-[[(Z)-2-[[(2S)-2-[[(Z)-2-[[(2S)-2-[[[(2S)-1-[(Z)-2-[[(2S)-2-(dimethylamino)-1-hydroxypropylidene]amino]but-2-enoyl]pyrrolidin-2-yl]-hydroxymethylidene]amino]-1-hydroxypropylidene]amino]-1-hydroxybut-2-enylidene]amino]-1-hydroxy-3-phenylpropylidene]amino]-1-hydroxybut-2-enylidene]amino]-1-hydroxy-3-methylbutylidene]amino]-1-hydroxypropylidene]amino]pentanediimidic acid Chemical compound CC[C@H](C)[C@H](\N=C(/O)[C@@H](\N=C(/O)[C@H](Cc1ccccc1)\N=C(/O)[C@H](CCC(O)=N)\N=C(/O)[C@H](C)\N=C(/O)[C@@H](\N=C(/O)\C(=C\C)\N=C(/O)[C@H](Cc1ccccc1)\N=C(/O)\C(=C\C)\N=C(/O)[C@H](C)\N=C(/O)[C@@H]1CCCN1C(=O)\C(=C\C)\N=C(/O)[C@H](C)N(C)C)C(C)C)C(C)C)C(\O)=N\[C@@H](CCC(O)=N)C(\O)=N\C\C(O)=N\[C@@H](CO)C(\O)=N\C(=C\C)\C(\O)=N\[C@@H]([C@@H](C)CC)C(\O)=N\[C@H]1CS\C=C/N=C(O)\[C@@H](\N=C(O)/[C@H](CC(C)C)\N=C1\O)C(C)C MXABZXILAJGOTL-AUYMZICSSA-N 0.000 description 1
- BUSGWUFLNHIBPT-XYBORKQMSA-N (2e,4e,6e)-7-[(1r,5r,6s)-3-[[(2e,4e)-5-cyclohexylpenta-2,4-dienoyl]amino]-5-hydroxy-2-oxo-7-oxabicyclo[4.1.0]hept-3-en-5-yl]hepta-2,4,6-trienoic acid Chemical compound C([C@]([C@H]1O[C@H]1C1=O)(O)/C=C/C=C/C=C/C(=O)O)=C1NC(=O)\C=C\C=C\C1CCCCC1 BUSGWUFLNHIBPT-XYBORKQMSA-N 0.000 description 1
- LCADVYTXPLBAGB-AUQKUMLUSA-N (2e,4e,6z,8e,10e,14e)-13-hydroxy-n-(1-hydroxypropan-2-yl)-2,10,12,14,16-pentamethyl-18-phenyloctadeca-2,4,6,8,10,14-hexaenamide Chemical compound OCC(C)NC(=O)C(\C)=C\C=C\C=C/C=C/C(/C)=C/C(C)C(O)C(\C)=C\C(C)CCC1=CC=CC=C1 LCADVYTXPLBAGB-AUQKUMLUSA-N 0.000 description 1
- FKHUGQZRBPETJR-RXSRXONKSA-N (2r)-2-[[(4r)-4-[[(2s)-2-[[(2r)-2-[(3r,4r,5s,6r)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxypropanoyl]amino]propanoyl]amino]-5-amino-5-oxopentanoyl]amino]-6-(octadecanoylamino)hexanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(=O)NCCCC[C@H](C(O)=O)NC(=O)CC[C@H](C(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](C)O[C@H]1[C@H](O)[C@@H](CO)OC(O)[C@@H]1NC(C)=O FKHUGQZRBPETJR-RXSRXONKSA-N 0.000 description 1
- SWTGJCNCBUCXSS-ISUZDFFFSA-N (2r)-3,4-dihydroxy-2-[(4s)-2-phenyl-1,3-dioxolan-4-yl]-2h-furan-5-one Chemical compound OC1=C(O)C(=O)O[C@@H]1[C@H]1OC(C=2C=CC=CC=2)OC1 SWTGJCNCBUCXSS-ISUZDFFFSA-N 0.000 description 1
- RCGXNDQKCXNWLO-WLEIXIPESA-N (2r)-n-[(2s)-5-amino-1-[[(2r,3r)-1-[[(3s,6z,9s,12r,15r,18r,19s)-9-benzyl-15-[(2r)-butan-2-yl]-6-ethylidene-19-methyl-2,5,8,11,14,17-hexaoxo-3,12-di(propan-2-yl)-1-oxa-4,7,10,13,16-pentazacyclononadec-18-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxopent Chemical compound N([C@@H](CCCN)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H]1C(N[C@@H](C(=O)N[C@@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)NC(/C(=O)N[C@H](C(=O)O[C@H]1C)C(C)C)=C\C)C(C)C)[C@H](C)CC)=O)C(=O)[C@H]1CCCN1C(=O)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](NC(=O)CCCC(C)C)C(C)C)[C@@H](C)O)C(C)C)C(C)C RCGXNDQKCXNWLO-WLEIXIPESA-N 0.000 description 1
- PAYBYKKERMGTSS-MNCSTQPFSA-N (2r,3r,3as,9ar)-7-fluoro-2-(hydroxymethyl)-6-imino-2,3,3a,9a-tetrahydrofuro[1,2][1,3]oxazolo[3,4-a]pyrimidin-3-ol Chemical compound N=C1C(F)=CN2[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3OC2=N1 PAYBYKKERMGTSS-MNCSTQPFSA-N 0.000 description 1
- WDQLRUYAYXDIFW-RWKIJVEZSA-N (2r,3r,4s,5r,6r)-4-[(2s,3r,4s,5r,6r)-3,5-dihydroxy-4-[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-[[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxy-6-(hydroxymethyl)oxane-2,3,5-triol Chemical compound O[C@@H]1[C@@H](CO)O[C@@H](O)[C@H](O)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)[C@H](O)[C@@H](CO[C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)O1 WDQLRUYAYXDIFW-RWKIJVEZSA-N 0.000 description 1
- NOENHWMKHNSHGX-IZOOSHNJSA-N (2s)-1-[(2s)-2-[[(2s)-2-[[(2r)-2-[[(2r)-2-[[(2s)-2-[[(2r)-2-[[(2s)-2-[[(2r)-2-acetamido-3-naphthalen-2-ylpropanoyl]amino]-3-(4-chlorophenyl)propanoyl]amino]-3-pyridin-3-ylpropanoyl]amino]-3-hydroxypropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-6-(ca Chemical compound C([C@H](C(=O)N[C@H](CCCCNC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCNC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@H](C)C(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](CC=1C=NC=CC=1)NC(=O)[C@H](CC=1C=CC(Cl)=CC=1)NC(=O)[C@@H](CC=1C=C2C=CC=CC2=CC=1)NC(C)=O)C1=CC=C(O)C=C1 NOENHWMKHNSHGX-IZOOSHNJSA-N 0.000 description 1
- ZZKNRXZVGOYGJT-VKHMYHEASA-N (2s)-2-[(2-phosphonoacetyl)amino]butanedioic acid Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)CP(O)(O)=O ZZKNRXZVGOYGJT-VKHMYHEASA-N 0.000 description 1
- XDZGQQRZJDKPTG-HBNQUELISA-N (2s)-2-[(3s,6s)-6-[2-[(1r,2r,4as,8as)-1-hydroxy-2,4a,5,5,8a-pentamethyl-2,3,4,6,7,8-hexahydronaphthalen-1-yl]ethyl]-6-methyldioxan-3-yl]propanoic acid Chemical compound O1O[C@H]([C@H](C)C(O)=O)CC[C@@]1(C)CC[C@]1(O)[C@@]2(C)CCCC(C)(C)[C@]2(C)CC[C@H]1C XDZGQQRZJDKPTG-HBNQUELISA-N 0.000 description 1
- CUCSSYAUKKIDJV-FAXBSAIASA-N (2s)-2-[[(2r)-2-[[(2s)-2-[[(2r)-2-[[(2s)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-3-(1h-indol-3-yl)propanoyl]-methylamino]-3-phenylpropanoyl]amino]-3-(1h-indol-3-yl)propanoyl]amino]-n-[(2s)-1-amino-4-methylsulfanyl-1-oxobutan-2-yl]-4-methylpent Chemical compound C([C@@H](C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O)N(C)C(=O)[C@@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@@H](N)CCCN=C(N)N)C1=CC=CC=C1 CUCSSYAUKKIDJV-FAXBSAIASA-N 0.000 description 1
- ZUQBAQVRAURMCL-DOMZBBRYSA-N (2s)-2-[[4-[2-[(6r)-2-amino-4-oxo-5,6,7,8-tetrahydro-1h-pyrido[2,3-d]pyrimidin-6-yl]ethyl]benzoyl]amino]pentanedioic acid Chemical compound C([C@@H]1CC=2C(=O)N=C(NC=2NC1)N)CC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 ZUQBAQVRAURMCL-DOMZBBRYSA-N 0.000 description 1
- JRBXPUUAYKCCLQ-QMMMGPOBSA-N (2s)-2-amino-2-[3-hydroxy-4-(hydroxymethyl)phenyl]acetic acid Chemical compound OC(=O)[C@@H](N)C1=CC=C(CO)C(O)=C1 JRBXPUUAYKCCLQ-QMMMGPOBSA-N 0.000 description 1
- HJNZCKLMRAOTMA-BRBGIFQRSA-N (2s)-n-[(2s)-1-[[(2s)-1-[[(2s)-1-[[(2s)-1-[[(2r)-1-[[(2s)-1-[[(2s)-5-(diaminomethylideneamino)-1-[(2s)-2-(ethylcarbamoyl)pyrrolidin-1-yl]-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(2-methyl-1h-indol-3-yl)-1-oxopropan-2-yl]amino]-3-(4-hydr Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=C(C)NC2=CC=CC=C12 HJNZCKLMRAOTMA-BRBGIFQRSA-N 0.000 description 1
- HWMMBHOXHRVLCU-QOUANJGESA-N (2s,4s,5s)-4-[(1e,3e,5e)-7-[(2r,6r)-6-[(2r,3s,4ar,12bs)-2,3,4a,8,12b-pentahydroxy-3-methyl-1,7,12-trioxo-2,4-dihydrobenzo[a]anthracen-9-yl]-2-methyloxan-3-yl]oxy-7-oxohepta-1,3,5-trienyl]-2,5-dimethyl-1,3-dioxolane-2-carboxylic acid Chemical compound C[C@@H]1O[C@](C)(C(O)=O)O[C@H]1\C=C\C=C\C=C\C(=O)OC1[C@@H](C)O[C@@H](C=2C(=C3C(=O)C4=C([C@]5(C(=O)[C@H](O)[C@@](C)(O)C[C@@]5(O)C=C4)O)C(=O)C3=CC=2)O)CC1 HWMMBHOXHRVLCU-QOUANJGESA-N 0.000 description 1
- NAALWFYYHHJEFQ-ZASNTINBSA-N (2s,5r,6r)-6-[[(2r)-2-[[6-[4-[bis(2-hydroxyethyl)sulfamoyl]phenyl]-2-oxo-1h-pyridine-3-carbonyl]amino]-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Chemical compound N([C@@H](C(=O)N[C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C=1C=CC(O)=CC=1)C(=O)C(C(N1)=O)=CC=C1C1=CC=C(S(=O)(=O)N(CCO)CCO)C=C1 NAALWFYYHHJEFQ-ZASNTINBSA-N 0.000 description 1
- RDIMTXDFGHNINN-UHFFFAOYSA-N (3R,9R,10R)-1-heptadecen-4,6-diyne-3,9,10-triol Natural products CCCCCCCC(O)C(O)CC#CC#CC(O)C=C RDIMTXDFGHNINN-UHFFFAOYSA-N 0.000 description 1
- MKJIEFSOBYUXJB-HOCLYGCPSA-N (3S,11bS)-9,10-dimethoxy-3-isobutyl-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-one Chemical compound C1CN2C[C@H](CC(C)C)C(=O)C[C@H]2C2=C1C=C(OC)C(OC)=C2 MKJIEFSOBYUXJB-HOCLYGCPSA-N 0.000 description 1
- DNXIKVLOVZVMQF-UHFFFAOYSA-N (3beta,16beta,17alpha,18beta,20alpha)-17-hydroxy-11-methoxy-18-[(3,4,5-trimethoxybenzoyl)oxy]-yohimban-16-carboxylic acid, methyl ester Natural products C1C2CN3CCC(C4=CC=C(OC)C=C4N4)=C4C3CC2C(C(=O)OC)C(O)C1OC(=O)C1=CC(OC)=C(OC)C(OC)=C1 DNXIKVLOVZVMQF-UHFFFAOYSA-N 0.000 description 1
- DIWRORZWFLOCLC-HNNXBMFYSA-N (3s)-7-chloro-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-1,4-benzodiazepin-2-one Chemical compound N([C@H](C(NC1=CC=C(Cl)C=C11)=O)O)=C1C1=CC=CC=C1Cl DIWRORZWFLOCLC-HNNXBMFYSA-N 0.000 description 1
- TVIRNGFXQVMMGB-OFWIHYRESA-N (3s,6r,10r,13e,16s)-16-[(2r,3r,4s)-4-chloro-3-hydroxy-4-phenylbutan-2-yl]-10-[(3-chloro-4-methoxyphenyl)methyl]-6-methyl-3-(2-methylpropyl)-1,4-dioxa-8,11-diazacyclohexadec-13-ene-2,5,9,12-tetrone Chemical compound C1=C(Cl)C(OC)=CC=C1C[C@@H]1C(=O)NC[C@@H](C)C(=O)O[C@@H](CC(C)C)C(=O)O[C@H]([C@H](C)[C@@H](O)[C@@H](Cl)C=2C=CC=CC=2)C/C=C/C(=O)N1 TVIRNGFXQVMMGB-OFWIHYRESA-N 0.000 description 1
- NZBABHFYCIWRBJ-UHFFFAOYSA-N (4-methoxy-2-nitrophenyl) thiocyanate Chemical compound COC1=CC=C(SC#N)C([N+]([O-])=O)=C1 NZBABHFYCIWRBJ-UHFFFAOYSA-N 0.000 description 1
- FRCJDPPXHQGEKS-BCHFMIIMSA-N (4S,5R)-N-[4-[(2,3-dihydroxybenzoyl)amino]butyl]-N-[3-[(2,3-dihydroxybenzoyl)amino]propyl]-2-(2-hydroxyphenyl)-5-methyl-4,5-dihydro-1,3-oxazole-4-carboxamide Chemical compound C[C@H]1OC(=N[C@@H]1C(=O)N(CCCCNC(=O)c1cccc(O)c1O)CCCNC(=O)c1cccc(O)c1O)c1ccccc1O FRCJDPPXHQGEKS-BCHFMIIMSA-N 0.000 description 1
- GTEXXGIEZVKSLH-YPMHNXCESA-N (4as,12br)-8,10-dihydroxy-2,5,5,9-tetramethyl-3,4,4a,12b-tetrahydronaphtho[2,3-c]isochromene-7,12-dione Chemical compound O=C1C2=CC(O)=C(C)C(O)=C2C(=O)C2=C1[C@@H]1C=C(C)CC[C@@H]1C(C)(C)O2 GTEXXGIEZVKSLH-YPMHNXCESA-N 0.000 description 1
- DEQANNDTNATYII-OULOTJBUSA-N (4r,7s,10s,13r,16s,19r)-10-(4-aminobutyl)-19-[[(2r)-2-amino-3-phenylpropanoyl]amino]-16-benzyl-n-[(2r,3r)-1,3-dihydroxybutan-2-yl]-7-[(1r)-1-hydroxyethyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxa Chemical compound C([C@@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC=2C3=CC=CC=C3NC=2)NC(=O)[C@H](CC=2C=CC=CC=2)NC1=O)C(=O)N[C@H](CO)[C@H](O)C)C1=CC=CC=C1 DEQANNDTNATYII-OULOTJBUSA-N 0.000 description 1
- PUDHBTGHUJUUFI-SCTWWAJVSA-N (4r,7s,10s,13r,16s,19r)-10-(4-aminobutyl)-n-[(2s,3r)-1-amino-3-hydroxy-1-oxobutan-2-yl]-19-[[(2r)-2-amino-3-naphthalen-2-ylpropanoyl]amino]-16-[(4-hydroxyphenyl)methyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-7-propan-2-yl-1,2-dithia-5,8,11,14,17-p Chemical compound C([C@H]1C(=O)N[C@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](N)CC=1C=C2C=CC=CC2=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)=O)C(C)C)C1=CC=C(O)C=C1 PUDHBTGHUJUUFI-SCTWWAJVSA-N 0.000 description 1
- HLAKJNQXUARACO-ZDUSSCGKSA-N (5'r)-5'-hydroxy-2',5',7'-trimethylspiro[cyclopropane-1,6'-indene]-4'-one Chemical compound O=C([C@@]1(O)C)C2=CC(C)=CC2=C(C)C21CC2 HLAKJNQXUARACO-ZDUSSCGKSA-N 0.000 description 1
- WNHFUHQVIHNIMN-UHFFFAOYSA-N (5-methoxy-2-sulfanylphenyl)azanium;chloride Chemical compound Cl.COC1=CC=C(S)C(N)=C1 WNHFUHQVIHNIMN-UHFFFAOYSA-N 0.000 description 1
- WTSKMKRYHATLLL-UHFFFAOYSA-N (6-benzoyloxy-3-cyanopyridin-2-yl) 3-[3-(ethoxymethyl)-5-fluoro-2,6-dioxopyrimidine-1-carbonyl]benzoate Chemical compound O=C1N(COCC)C=C(F)C(=O)N1C(=O)C1=CC=CC(C(=O)OC=2C(=CC=C(OC(=O)C=3C=CC=CC=3)N=2)C#N)=C1 WTSKMKRYHATLLL-UHFFFAOYSA-N 0.000 description 1
- LKBBOPGQDRPCDS-YAOXHJNESA-N (7s,9r,10r)-7-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-9-ethyl-4,6,9,10,11-pentahydroxy-8,10-dihydro-7h-tetracene-5,12-dione Chemical compound O([C@H]1C[C@]([C@@H](C2=C(O)C=3C(=O)C4=CC=CC(O)=C4C(=O)C=3C(O)=C21)O)(O)CC)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 LKBBOPGQDRPCDS-YAOXHJNESA-N 0.000 description 1
- MWWSFMDVAYGXBV-FGBSZODSSA-N (7s,9s)-7-[(2r,4s,5r,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydron;chloride Chemical compound Cl.O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 MWWSFMDVAYGXBV-FGBSZODSSA-N 0.000 description 1
- GYPCWHHQAVLMKO-XXKQIVDLSA-N (7s,9s)-7-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-[(e)-n-[(1-hydroxy-2,2,6,6-tetramethylpiperidin-4-ylidene)amino]-c-methylcarbonimidoyl]-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydrochloride Chemical group Cl.O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(\C)=N\N=C1CC(C)(C)N(O)C(C)(C)C1)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 GYPCWHHQAVLMKO-XXKQIVDLSA-N 0.000 description 1
- RCFNNLSZHVHCEK-YGCMNLPTSA-N (7s,9s)-7-[(2s,4r,6s)-4-amino-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydrochloride Chemical compound Cl.O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)C[C@H](C)O1 RCFNNLSZHVHCEK-YGCMNLPTSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- VHZXNQKVFDBFIK-NBBHSKLNSA-N (8r,9s,10r,13s,14s,16r)-16-fluoro-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one Chemical compound C1CCC[C@]2(C)[C@H]3CC[C@](C)(C([C@H](F)C4)=O)[C@@H]4[C@@H]3CC=C21 VHZXNQKVFDBFIK-NBBHSKLNSA-N 0.000 description 1
- IEXUMDBQLIVNHZ-YOUGDJEHSA-N (8s,11r,13r,14s,17s)-11-[4-(dimethylamino)phenyl]-17-hydroxy-17-(3-hydroxypropyl)-13-methyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one Chemical compound C1=CC(N(C)C)=CC=C1[C@@H]1C2=C3CCC(=O)C=C3CC[C@H]2[C@H](CC[C@]2(O)CCCO)[C@@]2(C)C1 IEXUMDBQLIVNHZ-YOUGDJEHSA-N 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- METKIMKYRPQLGS-GFCCVEGCSA-N (R)-atenolol Chemical compound CC(C)NC[C@@H](O)COC1=CC=C(CC(N)=O)C=C1 METKIMKYRPQLGS-GFCCVEGCSA-N 0.000 description 1
- WSEQXVZVJXJVFP-HXUWFJFHSA-N (R)-citalopram Chemical compound C1([C@@]2(C3=CC=C(C=C3CO2)C#N)CCCN(C)C)=CC=C(F)C=C1 WSEQXVZVJXJVFP-HXUWFJFHSA-N 0.000 description 1
- MHFRGQHAERHWKZ-HHHXNRCGSA-N (R)-edelfosine Chemical compound CCCCCCCCCCCCCCCCCCOC[C@@H](OC)COP([O-])(=O)OCC[N+](C)(C)C MHFRGQHAERHWKZ-HHHXNRCGSA-N 0.000 description 1
- WSPOMRSOLSGNFJ-AUWJEWJLSA-N (Z)-chlorprothixene Chemical compound C1=C(Cl)C=C2C(=C/CCN(C)C)\C3=CC=CC=C3SC2=C1 WSPOMRSOLSGNFJ-AUWJEWJLSA-N 0.000 description 1
- WOGITNXCNOTRLK-VOTSOKGWSA-N (e)-3-phenylprop-2-enoyl chloride Chemical compound ClC(=O)\C=C\C1=CC=CC=C1 WOGITNXCNOTRLK-VOTSOKGWSA-N 0.000 description 1
- GVTLFGJNTIRUEG-ZHACJKMWSA-N (e)-n-(3-methoxyphenyl)-3-phenylprop-2-enamide Chemical compound COC1=CC=CC(NC(=O)\C=C\C=2C=CC=CC=2)=C1 GVTLFGJNTIRUEG-ZHACJKMWSA-N 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 1
- OJRZEKJECRTBPJ-NGAMADIESA-N (z,5s)-5-acetamido-1-diazonio-6-hydroxy-6-oxohex-1-en-2-olate Chemical compound CC(=O)N[C@H](C(O)=O)CC\C([O-])=C\[N+]#N OJRZEKJECRTBPJ-NGAMADIESA-N 0.000 description 1
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 1
- GMRQFYUYWCNGIN-UHFFFAOYSA-N 1,25-Dihydroxy-vitamin D3' Natural products C1CCC2(C)C(C(CCCC(C)(C)O)C)CCC2C1=CC=C1CC(O)CC(O)C1=C GMRQFYUYWCNGIN-UHFFFAOYSA-N 0.000 description 1
- MLPAIIATLGUTEE-UHFFFAOYSA-N 1,3-thiazol-5-ol Chemical compound OC1=CN=CS1 MLPAIIATLGUTEE-UHFFFAOYSA-N 0.000 description 1
- OGYGFUAIIOPWQD-UHFFFAOYSA-N 1,3-thiazolidine Chemical compound C1CSCN1 OGYGFUAIIOPWQD-UHFFFAOYSA-N 0.000 description 1
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 1
- MYALOPJJAPLQSJ-UHFFFAOYSA-N 1,4-bis(bromomethyl)cyclohexane Chemical compound BrCC1CCC(CBr)CC1 MYALOPJJAPLQSJ-UHFFFAOYSA-N 0.000 description 1
- RCHDLEVSZBOHOS-UHFFFAOYSA-N 1,4-dichlorobut-2-yne Chemical compound ClCC#CCCl RCHDLEVSZBOHOS-UHFFFAOYSA-N 0.000 description 1
- OUPZKGBUJRBPGC-HLTSFMKQSA-N 1,5-bis[[(2r)-oxiran-2-yl]methyl]-3-[[(2s)-oxiran-2-yl]methyl]-1,3,5-triazinane-2,4,6-trione Chemical compound O=C1N(C[C@H]2OC2)C(=O)N(C[C@H]2OC2)C(=O)N1C[C@H]1CO1 OUPZKGBUJRBPGC-HLTSFMKQSA-N 0.000 description 1
- CYQFNNSFAGXCEC-UHFFFAOYSA-N 1-(2,3-dichlorophenyl)piperazine;hydrochloride Chemical compound [Cl-].ClC1=CC=CC(N2CC[NH2+]CC2)=C1Cl CYQFNNSFAGXCEC-UHFFFAOYSA-N 0.000 description 1
- UOAFGUOASVSLPK-UHFFFAOYSA-N 1-(2-chloroethyl)-3-(2,2-dimethylpropyl)-1-nitrosourea Chemical compound CC(C)(C)CNC(=O)N(N=O)CCCl UOAFGUOASVSLPK-UHFFFAOYSA-N 0.000 description 1
- YQYBWJPESSJLTK-HXFLIBJXSA-N 1-(2-chloroethyl)-3-[(2r,3s,4r,6s)-3-hydroxy-2-(hydroxymethyl)-6-methoxyoxan-4-yl]-1-nitrosourea Chemical compound CO[C@@H]1C[C@@H](NC(=O)N(CCCl)N=O)[C@H](O)[C@@H](CO)O1 YQYBWJPESSJLTK-HXFLIBJXSA-N 0.000 description 1
- RCLLNBVPCJDIPX-UHFFFAOYSA-N 1-(2-chloroethyl)-3-[2-(dimethylsulfamoyl)ethyl]-1-nitrosourea Chemical compound CN(C)S(=O)(=O)CCNC(=O)N(N=O)CCCl RCLLNBVPCJDIPX-UHFFFAOYSA-N 0.000 description 1
- VNZLQLYBRIOLFZ-UHFFFAOYSA-N 1-(2-methoxyphenyl)piperazine Chemical compound COC1=CC=CC=C1N1CCNCC1 VNZLQLYBRIOLFZ-UHFFFAOYSA-N 0.000 description 1
- CADVXCVVJWMUQJ-UHFFFAOYSA-N 1-(2-propan-2-yloxyphenyl)-1,4-diazepane;hydrochloride Chemical compound Cl.CC(C)OC1=CC=CC=C1N1CCNCCC1 CADVXCVVJWMUQJ-UHFFFAOYSA-N 0.000 description 1
- JQJSFAJISYZPER-UHFFFAOYSA-N 1-(4-chlorophenyl)-3-(2,3-dihydro-1h-inden-5-ylsulfonyl)urea Chemical compound C1=CC(Cl)=CC=C1NC(=O)NS(=O)(=O)C1=CC=C(CCC2)C2=C1 JQJSFAJISYZPER-UHFFFAOYSA-N 0.000 description 1
- SNYUHPPZINRDSG-UHFFFAOYSA-N 1-(oxiran-2-ylmethyl)-4-[1-(oxiran-2-ylmethyl)piperidin-4-yl]piperidine Chemical compound C1CC(C2CCN(CC3OC3)CC2)CCN1CC1CO1 SNYUHPPZINRDSG-UHFFFAOYSA-N 0.000 description 1
- ZKFNOUUKULVDOB-UHFFFAOYSA-N 1-amino-1-phenylmethyl phosphonic acid Chemical compound OP(=O)(O)C(N)C1=CC=CC=C1 ZKFNOUUKULVDOB-UHFFFAOYSA-N 0.000 description 1
- VSNHCAURESNICA-NJFSPNSNSA-N 1-oxidanylurea Chemical compound N[14C](=O)NO VSNHCAURESNICA-NJFSPNSNSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- 101710175516 14 kDa zinc-binding protein Proteins 0.000 description 1
- PRDFBSVERLRRMY-UHFFFAOYSA-N 2'-(4-ethoxyphenyl)-5-(4-methylpiperazin-1-yl)-2,5'-bibenzimidazole Chemical compound C1=CC(OCC)=CC=C1C1=NC2=CC=C(C=3NC4=CC(=CC=C4N=3)N3CCN(C)CC3)C=C2N1 PRDFBSVERLRRMY-UHFFFAOYSA-N 0.000 description 1
- JVSFQJZRHXAUGT-UHFFFAOYSA-N 2,2-dimethylpropanoyl chloride Chemical compound CC(C)(C)C(Cl)=O JVSFQJZRHXAUGT-UHFFFAOYSA-N 0.000 description 1
- AEQDJSLRWYMAQI-UHFFFAOYSA-N 2,3,9,10-tetramethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[2,1-b]isoquinoline Chemical compound C1CN2CC(C(=C(OC)C=C3)OC)=C3CC2C2=C1C=C(OC)C(OC)=C2 AEQDJSLRWYMAQI-UHFFFAOYSA-N 0.000 description 1
- VKDGNNYJFSHYKD-UHFFFAOYSA-N 2,5-diamino-2-(difluoromethyl)pentanoic acid;hydron;chloride Chemical compound Cl.NCCCC(N)(C(F)F)C(O)=O VKDGNNYJFSHYKD-UHFFFAOYSA-N 0.000 description 1
- AZWFNQKHHGQCET-UHFFFAOYSA-N 2-(2-methylphenyl)acetamide Chemical compound CC1=CC=CC=C1CC(N)=O AZWFNQKHHGQCET-UHFFFAOYSA-N 0.000 description 1
- SGTNSNPWRIOYBX-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-(propan-2-yl)pentanenitrile Chemical compound C1=C(OC)C(OC)=CC=C1CCN(C)CCCC(C#N)(C(C)C)C1=CC=C(OC)C(OC)=C1 SGTNSNPWRIOYBX-UHFFFAOYSA-N 0.000 description 1
- NJWBUDCAWGTQAS-UHFFFAOYSA-N 2-(chrysen-6-ylmethylamino)-2-methylpropane-1,3-diol;methanesulfonic acid Chemical compound CS(O)(=O)=O.C1=CC=C2C(CNC(CO)(CO)C)=CC3=C(C=CC=C4)C4=CC=C3C2=C1 NJWBUDCAWGTQAS-UHFFFAOYSA-N 0.000 description 1
- 125000003821 2-(trimethylsilyl)ethoxymethyl group Chemical group [H]C([H])([H])[Si](C([H])([H])[H])(C([H])([H])[H])C([H])([H])C(OC([H])([H])[*])([H])[H] 0.000 description 1
- PDWUPXJEEYOOTR-UHFFFAOYSA-N 2-[(3-iodophenyl)methyl]guanidine Chemical compound NC(=N)NCC1=CC=CC(I)=C1 PDWUPXJEEYOOTR-UHFFFAOYSA-N 0.000 description 1
- YFGHCGITMMYXAQ-UHFFFAOYSA-N 2-[(diphenylmethyl)sulfinyl]acetamide Chemical compound C=1C=CC=CC=1C(S(=O)CC(=O)N)C1=CC=CC=C1 YFGHCGITMMYXAQ-UHFFFAOYSA-N 0.000 description 1
- KPRFMAZESAKTEJ-UHFFFAOYSA-N 2-[1-amino-4-[2,5-dioxo-4-(1-phenylethyl)pyrrolidin-3-yl]-1-oxobutan-2-yl]-5-carbamoylheptanedioic acid;azane Chemical compound [NH4+].[NH4+].C=1C=CC=CC=1C(C)C1C(CCC(C(CCC(CC([O-])=O)C(N)=O)C([O-])=O)C(N)=O)C(=O)NC1=O KPRFMAZESAKTEJ-UHFFFAOYSA-N 0.000 description 1
- XXVLKDRPHSFIIB-UHFFFAOYSA-N 2-[2-(dimethylamino)ethyl]-5-nitrobenzo[de]isoquinoline-1,3-dione Chemical compound [O-][N+](=O)C1=CC(C(N(CCN(C)C)C2=O)=O)=C3C2=CC=CC3=C1 XXVLKDRPHSFIIB-UHFFFAOYSA-N 0.000 description 1
- MHXVDXXARZCVRK-WCWDXBQESA-N 2-[2-[4-[(e)-3,3,3-trifluoro-1,2-diphenylprop-1-enyl]phenoxy]ethylamino]ethanol Chemical compound C1=CC(OCCNCCO)=CC=C1C(\C=1C=CC=CC=1)=C(C(F)(F)F)/C1=CC=CC=C1 MHXVDXXARZCVRK-WCWDXBQESA-N 0.000 description 1
- PXJJOGITBQXZEQ-JTHROIFXSA-M 2-[4-[(z)-1,2-diphenylbut-1-enyl]phenoxy]ethyl-trimethylazanium;iodide Chemical compound [I-].C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCC[N+](C)(C)C)=CC=1)/C1=CC=CC=C1 PXJJOGITBQXZEQ-JTHROIFXSA-M 0.000 description 1
- HYHJFNXFVPGMBI-UHFFFAOYSA-N 2-[[2-chloroethyl(nitroso)carbamoyl]-methylamino]acetamide Chemical compound NC(=O)CN(C)C(=O)N(CCCl)N=O HYHJFNXFVPGMBI-UHFFFAOYSA-N 0.000 description 1
- QCXJFISCRQIYID-IAEPZHFASA-N 2-amino-1-n-[(3s,6s,7r,10s,16s)-3-[(2s)-butan-2-yl]-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-10-propan-2-yl-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-4,6-dimethyl-3-oxo-9-n-[(3s,6s,7r,10s,16s)-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propa Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N=C2C(C(=O)N[C@@H]3C(=O)N[C@H](C(N4CCC[C@H]4C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]3C)=O)[C@@H](C)CC)=C(N)C(=O)C(C)=C2O2)C2=C(C)C=C1 QCXJFISCRQIYID-IAEPZHFASA-N 0.000 description 1
- SWFNPENEBHAHEB-UHFFFAOYSA-N 2-amino-4-chlorophenol Chemical compound NC1=CC(Cl)=CC=C1O SWFNPENEBHAHEB-UHFFFAOYSA-N 0.000 description 1
- VDCRFBBZFHHYGT-IOSLPCCCSA-N 2-amino-9-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-7-prop-2-enyl-3h-purine-6,8-dione Chemical compound O=C1N(CC=C)C=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O VDCRFBBZFHHYGT-IOSLPCCCSA-N 0.000 description 1
- NIXVOFULDIFBLB-QVRNUERCSA-N 2-amino-9-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]purine-6-sulfinamide Chemical compound C12=NC(N)=NC(S(N)=O)=C2N=CN1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O NIXVOFULDIFBLB-QVRNUERCSA-N 0.000 description 1
- VTOWGURRHAVIBF-UHFFFAOYSA-N 2-chloro-n-(5-chloro-2-hydroxyphenyl)acetamide Chemical compound OC1=CC=C(Cl)C=C1NC(=O)CCl VTOWGURRHAVIBF-UHFFFAOYSA-N 0.000 description 1
- UNWQNFJBBWXFBG-UHFFFAOYSA-N 2-fluoro-4-methoxybenzaldehyde Chemical compound COC1=CC=C(C=O)C(F)=C1 UNWQNFJBBWXFBG-UHFFFAOYSA-N 0.000 description 1
- 125000004204 2-methoxyphenyl group Chemical group [H]C1=C([H])C(*)=C(OC([H])([H])[H])C([H])=C1[H] 0.000 description 1
- DSWLRNLRVBAVFC-UHFFFAOYSA-N 2-methylsulfinyl-1-pyridin-2-ylethanone Chemical compound CS(=O)CC(=O)C1=CC=CC=N1 DSWLRNLRVBAVFC-UHFFFAOYSA-N 0.000 description 1
- NDMPLJNOPCLANR-UHFFFAOYSA-N 3,4-dihydroxy-15-(4-hydroxy-18-methoxycarbonyl-5,18-seco-ibogamin-18-yl)-16-methoxy-1-methyl-6,7-didehydro-aspidospermidine-3-carboxylic acid methyl ester Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 NDMPLJNOPCLANR-UHFFFAOYSA-N 0.000 description 1
- GRLUHXSUZYFZCW-UHFFFAOYSA-N 3-(8,8-diethyl-2-aza-8-germaspiro[4.5]decan-2-yl)-n,n-dimethylpropan-1-amine;dihydrochloride Chemical compound Cl.Cl.C1C[Ge](CC)(CC)CCC11CN(CCCN(C)C)CC1 GRLUHXSUZYFZCW-UHFFFAOYSA-N 0.000 description 1
- QGJZLNKBHJESQX-UHFFFAOYSA-N 3-Epi-Betulin-Saeure Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C(O)=O)CCC(C(=C)C)C5C4CCC3C21C QGJZLNKBHJESQX-UHFFFAOYSA-N 0.000 description 1
- GTJXPMSTODOYNP-BTKVJIOYSA-N 3-[(e)-1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-phenylbut-1-enyl]phenol;2-hydroxypropane-1,2,3-tricarboxylic acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C=1C=CC=CC=1C(/CC)=C(C=1C=C(O)C=CC=1)\C1=CC=C(OCCN(C)C)C=C1 GTJXPMSTODOYNP-BTKVJIOYSA-N 0.000 description 1
- WUIABRMSWOKTOF-OYALTWQYSA-N 3-[[2-[2-[2-[[(2s,3r)-2-[[(2s,3s,4r)-4-[[(2s,3r)-2-[[6-amino-2-[(1s)-3-amino-1-[[(2s)-2,3-diamino-3-oxopropyl]amino]-3-oxopropyl]-5-methylpyrimidine-4-carbonyl]amino]-3-[(2r,3s,4s,5s,6s)-3-[(2r,3s,4s,5r,6r)-4-carbamoyloxy-3,5-dihydroxy-6-(hydroxymethyl)ox Chemical compound OS([O-])(=O)=O.N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1NC=NC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C WUIABRMSWOKTOF-OYALTWQYSA-N 0.000 description 1
- WELIVEBWRWAGOM-UHFFFAOYSA-N 3-amino-n-[2-[2-(3-aminopropanoylamino)ethyldisulfanyl]ethyl]propanamide Chemical compound NCCC(=O)NCCSSCCNC(=O)CCN WELIVEBWRWAGOM-UHFFFAOYSA-N 0.000 description 1
- CLOUCVRNYSHRCF-UHFFFAOYSA-N 3beta-Hydroxy-20(29)-Lupen-3,27-oic acid Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C(O)=O)CCC5(C)CCC(C(=C)C)C5C4CCC3C21C CLOUCVRNYSHRCF-UHFFFAOYSA-N 0.000 description 1
- PDQGEKGUTOTUNV-TZSSRYMLSA-N 4'-deoxy-4'-iododoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](I)[C@H](C)O1 PDQGEKGUTOTUNV-TZSSRYMLSA-N 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- LIETVYHJBSLSSW-UHFFFAOYSA-N 4,6,9-trihydroxy-8-methyl-3,4-dihydro-2h-anthracen-1-one Chemical compound OC1CCC(=O)C2=C1C=C1C=C(O)C=C(C)C1=C2O LIETVYHJBSLSSW-UHFFFAOYSA-N 0.000 description 1
- JARCFMKMOFFIGZ-UHFFFAOYSA-N 4,6-dioxo-n-phenyl-2-sulfanylidene-1,3-diazinane-5-carboxamide Chemical compound O=C1NC(=S)NC(=O)C1C(=O)NC1=CC=CC=C1 JARCFMKMOFFIGZ-UHFFFAOYSA-N 0.000 description 1
- YODQESWJUQGRPE-UHFFFAOYSA-N 4-(2,3-dichlorophenyl)piperidine Chemical compound ClC1=CC=CC(C2CCNCC2)=C1Cl YODQESWJUQGRPE-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- FPPNTMAHFOKUFK-UHFFFAOYSA-N 4-(4-bromobutoxy)-1-methyl-2-nitrobenzene Chemical compound CC1=CC=C(OCCCCBr)C=C1[N+]([O-])=O FPPNTMAHFOKUFK-UHFFFAOYSA-N 0.000 description 1
- CYDQOEWLBCCFJZ-UHFFFAOYSA-N 4-(4-fluorophenyl)oxane-4-carboxylic acid Chemical compound C=1C=C(F)C=CC=1C1(C(=O)O)CCOCC1 CYDQOEWLBCCFJZ-UHFFFAOYSA-N 0.000 description 1
- HQFSNUYUXXPVKL-UHFFFAOYSA-N 4-[(4-fluorophenyl)methyl]-2-[1-(2-phenylethyl)azepan-4-yl]phthalazin-1-one Chemical compound C1=CC(F)=CC=C1CC(C1=CC=CC=C1C1=O)=NN1C1CCN(CCC=2C=CC=CC=2)CCC1 HQFSNUYUXXPVKL-UHFFFAOYSA-N 0.000 description 1
- OUQPTBCOEKUHBH-LSDHQDQOSA-N 4-[2-[4-[(e)-2-(5,5,8,8-tetramethyl-6,7-dihydronaphthalen-2-yl)prop-1-enyl]phenoxy]ethyl]morpholine Chemical compound C=1C=C(C(CCC2(C)C)(C)C)C2=CC=1C(/C)=C/C(C=C1)=CC=C1OCCN1CCOCC1 OUQPTBCOEKUHBH-LSDHQDQOSA-N 0.000 description 1
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical compound N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 description 1
- CTSNHMQGVWXIEG-UHFFFAOYSA-N 4-amino-n-(5-chloroquinoxalin-2-yl)benzenesulfonamide Chemical compound C1=CC(N)=CC=C1S(=O)(=O)NC1=CN=C(C(Cl)=CC=C2)C2=N1 CTSNHMQGVWXIEG-UHFFFAOYSA-N 0.000 description 1
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 1
- SGOOQMRIPALTEL-UHFFFAOYSA-N 4-hydroxy-N,1-dimethyl-2-oxo-N-phenyl-3-quinolinecarboxamide Chemical compound OC=1C2=CC=CC=C2N(C)C(=O)C=1C(=O)N(C)C1=CC=CC=C1 SGOOQMRIPALTEL-UHFFFAOYSA-N 0.000 description 1
- TYROJDFHUXSBHC-UHFFFAOYSA-N 4-phenylmethoxybutan-1-ol Chemical compound OCCCCOCC1=CC=CC=C1 TYROJDFHUXSBHC-UHFFFAOYSA-N 0.000 description 1
- NSUDGNLOXMLAEB-UHFFFAOYSA-N 5-(2-formyl-3-hydroxyphenoxy)pentanoic acid Chemical compound OC(=O)CCCCOC1=CC=CC(O)=C1C=O NSUDGNLOXMLAEB-UHFFFAOYSA-N 0.000 description 1
- PXLPCZJACKUXGP-UHFFFAOYSA-N 5-(3,4-dichlorophenyl)-6-ethylpyrimidine-2,4-diamine Chemical compound CCC1=NC(N)=NC(N)=C1C1=CC=C(Cl)C(Cl)=C1 PXLPCZJACKUXGP-UHFFFAOYSA-N 0.000 description 1
- APNRZHLOPQFNMR-WEIUTZTHSA-N 5-[(e)-5-[(1s)-2,2-dimethyl-6-methylidenecyclohexyl]-3-methylpent-2-enyl]phenazin-1-one Chemical compound C12=CC=CC=C2N=C(C(C=CC=2)=O)C=2N1C\C=C(/C)CC[C@@H]1C(=C)CCCC1(C)C APNRZHLOPQFNMR-WEIUTZTHSA-N 0.000 description 1
- XIJCYTQIQKGNHJ-UHFFFAOYSA-N 5-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-2-methylaniline Chemical compound C1=C(N)C(C)=CC=C1OCCCCN1CCN(C=2C(=C(Cl)C=CC=2)Cl)CC1 XIJCYTQIQKGNHJ-UHFFFAOYSA-N 0.000 description 1
- IDPUKCWIGUEADI-UHFFFAOYSA-N 5-[bis(2-chloroethyl)amino]uracil Chemical compound ClCCN(CCCl)C1=CNC(=O)NC1=O IDPUKCWIGUEADI-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- DQOGWKZQQBYYMW-LQGIGNHCSA-N 5-methyl-6-[(3,4,5-trimethoxyanilino)methyl]quinazoline-2,4-diamine;(2s,3s,4s,5r,6s)-3,4,5,6-tetrahydroxyoxane-2-carboxylic acid Chemical compound O[C@H]1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O.COC1=C(OC)C(OC)=CC(NCC=2C(=C3C(N)=NC(N)=NC3=CC=2)C)=C1 DQOGWKZQQBYYMW-LQGIGNHCSA-N 0.000 description 1
- XKFPYPQQHFEXRZ-UHFFFAOYSA-N 5-methyl-N'-(phenylmethyl)-3-isoxazolecarbohydrazide Chemical compound O1C(C)=CC(C(=O)NNCC=2C=CC=CC=2)=N1 XKFPYPQQHFEXRZ-UHFFFAOYSA-N 0.000 description 1
- PXBZKHOQHTVCSQ-QZTJIDSGSA-N 5-nitro-2-[(2r)-1-[2-[[(2r)-2-(5-nitro-1,3-dioxobenzo[de]isoquinolin-2-yl)propyl]amino]ethylamino]propan-2-yl]benzo[de]isoquinoline-1,3-dione Chemical compound [O-][N+](=O)C1=CC(C(N([C@@H](CNCCNC[C@@H](C)N2C(C=3C=C(C=C4C=CC=C(C=34)C2=O)[N+]([O-])=O)=O)C)C2=O)=O)=C3C2=CC=CC3=C1 PXBZKHOQHTVCSQ-QZTJIDSGSA-N 0.000 description 1
- ATCGGEJZONJOCL-UHFFFAOYSA-N 6-(2,5-dichlorophenyl)-1,3,5-triazine-2,4-diamine Chemical compound NC1=NC(N)=NC(C=2C(=CC=C(Cl)C=2)Cl)=N1 ATCGGEJZONJOCL-UHFFFAOYSA-N 0.000 description 1
- VJXSSYDSOJBUAV-UHFFFAOYSA-N 6-(2,5-dimethoxy-benzyl)-5-methyl-pyrido[2,3-d]pyrimidine-2,4-diamine Chemical compound COC1=CC=C(OC)C(CC=2C(=C3C(N)=NC(N)=NC3=NC=2)C)=C1 VJXSSYDSOJBUAV-UHFFFAOYSA-N 0.000 description 1
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 1
- OTSZCHORPMQCBZ-UHFFFAOYSA-N 6-[(3-chlorophenyl)-imidazol-1-ylmethyl]-1h-benzimidazole;hydron;chloride Chemical compound Cl.ClC1=CC=CC(C(C=2C=C3NC=NC3=CC=2)N2C=NC=C2)=C1 OTSZCHORPMQCBZ-UHFFFAOYSA-N 0.000 description 1
- LRHPCRBOMKRVOA-UHFFFAOYSA-N 6-[2-(2-hydroxyethylamino)ethyl]indeno[1,2-c]isoquinoline-5,11-dione Chemical compound C12=CC=CC=C2C(=O)N(CCNCCO)C2=C1C(=O)C1=CC=CC=C12 LRHPCRBOMKRVOA-UHFFFAOYSA-N 0.000 description 1
- KXBCLNRMQPRVTP-UHFFFAOYSA-N 6-amino-1,5-dihydroimidazo[4,5-c]pyridin-4-one Chemical compound O=C1NC(N)=CC2=C1N=CN2 KXBCLNRMQPRVTP-UHFFFAOYSA-N 0.000 description 1
- ZNTIXVYOBQDFFV-UHFFFAOYSA-N 6-amino-1,5-dihydroimidazo[4,5-c]pyridin-4-one;methanesulfonic acid Chemical compound CS(O)(=O)=O.O=C1NC(N)=CC2=C1N=CN2 ZNTIXVYOBQDFFV-UHFFFAOYSA-N 0.000 description 1
- LJIRBXZDQGQUOO-KVTDHHQDSA-N 6-amino-3-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,4-dihydro-1,3,5-triazin-2-one Chemical compound C1NC(N)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 LJIRBXZDQGQUOO-KVTDHHQDSA-N 0.000 description 1
- OBYJTLDIQBWBHM-UHFFFAOYSA-N 6-chloropyridin-2-amine Chemical compound NC1=CC=CC(Cl)=N1 OBYJTLDIQBWBHM-UHFFFAOYSA-N 0.000 description 1
- UJGDLLGKMWVCPT-UHFFFAOYSA-N 6-methoxy-2,3-dihydroinden-1-one Chemical compound COC1=CC=C2CCC(=O)C2=C1 UJGDLLGKMWVCPT-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- RHJNUFWFQIAFTD-UHFFFAOYSA-N 7-(3-bromopropoxy)-1h-quinolin-2-one Chemical compound C1=CC(=O)NC2=CC(OCCCBr)=CC=C21 RHJNUFWFQIAFTD-UHFFFAOYSA-N 0.000 description 1
- GRBHZWUWWCMUNU-UHFFFAOYSA-N 7-(3-bromopropoxy)-3,4-dihydro-1h-quinolin-2-one Chemical compound C1CC(=O)NC2=CC(OCCCBr)=CC=C21 GRBHZWUWWCMUNU-UHFFFAOYSA-N 0.000 description 1
- IYLVNEGCKIAUII-UHFFFAOYSA-N 7-(4-chlorobut-2-ynoxy)-3,4-dihydro-1h-quinolin-2-one Chemical compound C1CC(=O)NC2=CC(OCC#CCCl)=CC=C21 IYLVNEGCKIAUII-UHFFFAOYSA-N 0.000 description 1
- GOYNNCPGHOBFCK-UHFFFAOYSA-N 7-[4-(dimethylamino)-5-[(2,9-dimethyl-3-oxo-4,4a,5a,6,7,9,9a,10a-octahydrodipyrano[4,2-a:4',3'-e][1,4]dioxin-7-yl)oxy]-6-methyloxan-2-yl]oxy-9-ethyl-4,6,9,10,11-pentahydroxy-8,10-dihydro-7h-tetracene-5,12-dione Chemical compound O=C1C2=C(O)C=CC=C2C(=O)C2=C1C(O)=C1C(OC3OC(C)C(OC4OC(C)C5OC6OC(C)C(=O)CC6OC5C4)C(C3)N(C)C)CC(CC)(O)C(O)C1=C2O GOYNNCPGHOBFCK-UHFFFAOYSA-N 0.000 description 1
- YPZLXCVTFSPQSS-UHFFFAOYSA-N 7-[5-[4-(2-methoxyphenyl)piperazin-1-yl]pentoxy]-3,4-dihydro-1h-quinolin-2-one Chemical compound COC1=CC=CC=C1N1CCN(CCCCCOC=2C=C3NC(=O)CCC3=CC=2)CC1 YPZLXCVTFSPQSS-UHFFFAOYSA-N 0.000 description 1
- NOURCINYSYTUCH-UHFFFAOYSA-N 7-[[4-(bromomethyl)cyclohexyl]methoxy]-3,4-dihydro-1h-quinolin-2-one Chemical compound C1CC(CBr)CCC1COC1=CC=C(CCC(=O)N2)C2=C1 NOURCINYSYTUCH-UHFFFAOYSA-N 0.000 description 1
- XTKPMSAPQLUOSA-UHFFFAOYSA-N 7-[[4-[[4-(2,3-dichlorophenyl)piperazin-1-yl]methyl]cyclohexyl]methoxy]-3,4-dihydroquinoline Chemical compound ClC1=C(C=CC=C1Cl)N1CCN(CC1)CC1CCC(CC1)COC1=CC=C2CCC=NC2=C1 XTKPMSAPQLUOSA-UHFFFAOYSA-N 0.000 description 1
- KABRXLINDSPGDF-UHFFFAOYSA-N 7-bromoisoquinoline Chemical compound C1=CN=CC2=CC(Br)=CC=C21 KABRXLINDSPGDF-UHFFFAOYSA-N 0.000 description 1
- DBSPUDKBNOZFMX-UHFFFAOYSA-N 7-hydroxyquinolin-2(1H)-one Chemical compound C1=CC(=O)NC2=CC(O)=CC=C21 DBSPUDKBNOZFMX-UHFFFAOYSA-N 0.000 description 1
- GOJJWDOZNKBUSR-UHFFFAOYSA-N 7-sulfamoyloxyheptyl sulfamate Chemical compound NS(=O)(=O)OCCCCCCCOS(N)(=O)=O GOJJWDOZNKBUSR-UHFFFAOYSA-N 0.000 description 1
- LPDLEICKXUVJHW-QJILNLRNSA-N 78nz2pmp25 Chemical compound OS(O)(=O)=O.O([C@]12[C@H](OC(C)=O)[C@]3(CC)C=CCN4CC[C@@]5([C@H]34)[C@H]1N(C)C1=C5C=C(C(=C1)OC)[C@]1(C(=O)OC)C3=C(C4=CC=CC=C4N3)CCN3C[C@H](C1)C[C@@](C3)(O)CC)C(=O)N(CCCl)C2=O LPDLEICKXUVJHW-QJILNLRNSA-N 0.000 description 1
- JPASRFGVACYSJG-UHFFFAOYSA-N 8,10-dihydroimidazo[4,5-a]acridin-9-one Chemical class N1=C2C=CC3=NC=NC3=C2C=C2C1=CCC(=O)C2 JPASRFGVACYSJG-UHFFFAOYSA-N 0.000 description 1
- ZGXJTSGNIOSYLO-UHFFFAOYSA-N 88755TAZ87 Chemical compound NCC(=O)CCC(O)=O ZGXJTSGNIOSYLO-UHFFFAOYSA-N 0.000 description 1
- 229930000680 A04AD01 - Scopolamine Natural products 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- ITPDYQOUSLNIHG-UHFFFAOYSA-N Amiodarone hydrochloride Chemical compound [Cl-].CCCCC=1OC2=CC=CC=C2C=1C(=O)C1=CC(I)=C(OCC[NH+](CC)CC)C(I)=C1 ITPDYQOUSLNIHG-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- BOJKULTULYSRAS-OTESTREVSA-N Andrographolide Chemical compound C([C@H]1[C@]2(C)CC[C@@H](O)[C@]([C@H]2CCC1=C)(CO)C)\C=C1/[C@H](O)COC1=O BOJKULTULYSRAS-OTESTREVSA-N 0.000 description 1
- NQGMIPUYCWIEAW-UHFFFAOYSA-N Antibiotic SF 2738 Natural products COc1cc(nc(C=NO)c1SC)-c1ccccn1 NQGMIPUYCWIEAW-UHFFFAOYSA-N 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- MJINRRBEMOLJAK-DCAQKATOSA-N Arg-Lys-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCN=C(N)N MJINRRBEMOLJAK-DCAQKATOSA-N 0.000 description 1
- DRCNRVYVCHHIJP-AQBORDMYSA-N Arg-Lys-Glu-Val-Tyr Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 DRCNRVYVCHHIJP-AQBORDMYSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- BFYIZQONLCFLEV-DAELLWKTSA-N Aromasine Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC(=C)C2=C1 BFYIZQONLCFLEV-DAELLWKTSA-N 0.000 description 1
- 108010024976 Asparaginase Proteins 0.000 description 1
- 102000015790 Asparaginase Human genes 0.000 description 1
- 108700032558 Aspergillus restrictus MITF Proteins 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 241001106067 Atropa Species 0.000 description 1
- 241001263178 Auriparus Species 0.000 description 1
- YOZSEGPJAXTSFZ-ZETCQYMHSA-N Azatyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=N1 YOZSEGPJAXTSFZ-ZETCQYMHSA-N 0.000 description 1
- 229910015845 BBr3 Inorganic materials 0.000 description 1
- KPYSYYIEGFHWSV-UHFFFAOYSA-N Baclofen Chemical compound OC(=O)CC(CN)C1=CC=C(Cl)C=C1 KPYSYYIEGFHWSV-UHFFFAOYSA-N 0.000 description 1
- VGGGPCQERPFHOB-MCIONIFRSA-N Bestatin Chemical compound CC(C)C[C@H](C(O)=O)NC(=O)[C@@H](O)[C@H](N)CC1=CC=CC=C1 VGGGPCQERPFHOB-MCIONIFRSA-N 0.000 description 1
- DIZWSDNSTNAYHK-XGWVBXMLSA-N Betulinic acid Natural products CC(=C)[C@@H]1C[C@H]([C@H]2CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(=O)O DIZWSDNSTNAYHK-XGWVBXMLSA-N 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 238000009010 Bradford assay Methods 0.000 description 1
- 241000219198 Brassica Species 0.000 description 1
- 235000003351 Brassica cretica Nutrition 0.000 description 1
- 235000003343 Brassica rupestris Nutrition 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 1
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 1
- MFYCJTHPHMDXBY-UHFFFAOYSA-N C=[Br]CC1CCC(COc2cc(NC(CC3)=O)c3cc2)CC1 Chemical compound C=[Br]CC1CCC(COc2cc(NC(CC3)=O)c3cc2)CC1 MFYCJTHPHMDXBY-UHFFFAOYSA-N 0.000 description 1
- MMORVPBHAHXAHH-UHFFFAOYSA-N CC(C)Oc(cccc1)c1Br Chemical compound CC(C)Oc(cccc1)c1Br MMORVPBHAHXAHH-UHFFFAOYSA-N 0.000 description 1
- RGIQUYNIXHNFIV-UHFFFAOYSA-N CCOc(cccc1)c1N(CCC1)CCN1C(OC(C)(C)C)=O Chemical compound CCOc(cccc1)c1N(CCC1)CCN1C(OC(C)(C)C)=O RGIQUYNIXHNFIV-UHFFFAOYSA-N 0.000 description 1
- LVVVBEVBRWMYII-UHFFFAOYSA-N CCOc(cccc1)c1N1CCN(CCCCOc2nc(NC(CC3)=O)c3cc2)CCC1 Chemical compound CCOc(cccc1)c1N1CCN(CCCCOc2nc(NC(CC3)=O)c3cc2)CCC1 LVVVBEVBRWMYII-UHFFFAOYSA-N 0.000 description 1
- 101150041968 CDC13 gene Proteins 0.000 description 1
- TWDYMKMXJSOFGT-UHFFFAOYSA-N CN(c(cc(cc1)OCCCCBr)c1C=C1)C1=O Chemical compound CN(c(cc(cc1)OCCCCBr)c1C=C1)C1=O TWDYMKMXJSOFGT-UHFFFAOYSA-N 0.000 description 1
- DTVAIMZRGJKGPN-UHFFFAOYSA-N COc(cccc1)c1N1CCN(CCCCOc2nc(NC(CC3)=O)c3cc2)CC1 Chemical compound COc(cccc1)c1N1CCN(CCCCOc2nc(NC(CC3)=O)c3cc2)CC1 DTVAIMZRGJKGPN-UHFFFAOYSA-N 0.000 description 1
- HHLQCJATVSNEIJ-UHFFFAOYSA-N C[BrH]CCCCBr Chemical compound C[BrH]CCCCBr HHLQCJATVSNEIJ-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 1
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 102000005403 Casein Kinases Human genes 0.000 description 1
- 108010031425 Casein Kinases Proteins 0.000 description 1
- JDVVGAQPNNXQDW-WCMLQCRESA-N Castanospermine Natural products O[C@H]1[C@@H](O)[C@H]2[C@@H](O)CCN2C[C@H]1O JDVVGAQPNNXQDW-WCMLQCRESA-N 0.000 description 1
- JDVVGAQPNNXQDW-TVNFTVLESA-N Castinospermine Chemical compound C1[C@H](O)[C@@H](O)[C@H](O)[C@H]2[C@@H](O)CCN21 JDVVGAQPNNXQDW-TVNFTVLESA-N 0.000 description 1
- 102000053642 Catalytic RNA Human genes 0.000 description 1
- 108090000994 Catalytic RNA Proteins 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- VGCXGMAHQTYDJK-UHFFFAOYSA-N Chloroacetyl chloride Chemical compound ClCC(Cl)=O VGCXGMAHQTYDJK-UHFFFAOYSA-N 0.000 description 1
- PPASFTRHCXASPY-UHFFFAOYSA-N Cl.Cl.NCCCNc1ccc2c3c(nn2CCNCCO)c4c(O)ccc(O)c4C(=O)c13 Chemical compound Cl.Cl.NCCCNc1ccc2c3c(nn2CCNCCO)c4c(O)ccc(O)c4C(=O)c13 PPASFTRHCXASPY-UHFFFAOYSA-N 0.000 description 1
- WYMJRTXYUOPUMF-UHFFFAOYSA-N Clc1cccc(N2CCN(CCCCOc3ccc4[s]cnc4c3)CCC2)c1Cl Chemical compound Clc1cccc(N2CCN(CCCCOc3ccc4[s]cnc4c3)CCC2)c1Cl WYMJRTXYUOPUMF-UHFFFAOYSA-N 0.000 description 1
- GDLIGKIOYRNHDA-UHFFFAOYSA-N Clomipramine Chemical compound C1CC2=CC=C(Cl)C=C2N(CCCN(C)C)C2=CC=CC=C21 GDLIGKIOYRNHDA-UHFFFAOYSA-N 0.000 description 1
- 208000028698 Cognitive impairment Diseases 0.000 description 1
- HVXBOLULGPECHP-WAYWQWQTSA-N Combretastatin A4 Chemical compound C1=C(O)C(OC)=CC=C1\C=C/C1=CC(OC)=C(OC)C(OC)=C1 HVXBOLULGPECHP-WAYWQWQTSA-N 0.000 description 1
- DFDTZECTHJFPHE-UHFFFAOYSA-N Crambescidin 816 Natural products C1CC=CC(CC)OC11NC(N23)=NC4(OC(C)CCC4)C(C(=O)OCCCCCCCCCCCCCCCC(=O)N(CCCN)CC(O)CCN)C3(O)CCC2C1 DFDTZECTHJFPHE-UHFFFAOYSA-N 0.000 description 1
- 241000699802 Cricetulus griseus Species 0.000 description 1
- LUEYTMPPCOCKBX-UHFFFAOYSA-N Curacin A Natural products C=CCC(OC)CCC(C)=CC=CCCC=CC1CSC(C2C(C2)C)=N1 LUEYTMPPCOCKBX-UHFFFAOYSA-N 0.000 description 1
- LUEYTMPPCOCKBX-KWYHTCOPSA-N Curacin A Chemical compound C=CC[C@H](OC)CC\C(C)=C\C=C\CC\C=C/[C@@H]1CSC([C@H]2[C@H](C2)C)=N1 LUEYTMPPCOCKBX-KWYHTCOPSA-N 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- PQNNIEWMPIULRS-UHFFFAOYSA-N Cytostatin Natural products CC=CC=CC=CC(O)C(C)C(OP(O)(O)=O)CCC(C)C1OC(=O)C=CC1C PQNNIEWMPIULRS-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- SPKNARKFCOPTSY-UHFFFAOYSA-N D-asperlin Natural products CC1OC1C1C(OC(C)=O)C=CC(=O)O1 SPKNARKFCOPTSY-UHFFFAOYSA-N 0.000 description 1
- 108010092160 Dactinomycin Proteins 0.000 description 1
- HCYAFALTSJYZDH-UHFFFAOYSA-N Desimpramine Chemical compound C1CC2=CC=CC=C2N(CCCNC)C2=CC=CC=C21 HCYAFALTSJYZDH-UHFFFAOYSA-N 0.000 description 1
- GJKXGJCSJWBJEZ-XRSSZCMZSA-N Deslorelin Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CNC2=CC=CC=C12 GJKXGJCSJWBJEZ-XRSSZCMZSA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- KYHUYMLIVQFXRI-SJPGYWQQSA-N Didemnin B Chemical compound CN([C@H](CC(C)C)C(=O)N[C@@H]1C(=O)N[C@@H]([C@H](CC(=O)O[C@H](C(=O)[C@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N2CCC[C@H]2C(=O)N(C)[C@@H](CC=2C=CC(OC)=CC=2)C(=O)O[C@@H]1C)C(C)C)O)[C@@H](C)CC)C(=O)[C@@H]1CCCN1C(=O)[C@H](C)O KYHUYMLIVQFXRI-SJPGYWQQSA-N 0.000 description 1
- HWMMBHOXHRVLCU-UHFFFAOYSA-N Dioxamycin Natural products CC1OC(C)(C(O)=O)OC1C=CC=CC=CC(=O)OC1C(C)OC(C=2C(=C3C(=O)C4=C(C5(C(=O)C(O)C(C)(O)CC5(O)C=C4)O)C(=O)C3=CC=2)O)CC1 HWMMBHOXHRVLCU-UHFFFAOYSA-N 0.000 description 1
- MWWSFMDVAYGXBV-RUELKSSGSA-N Doxorubicin hydrochloride Chemical compound Cl.O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 MWWSFMDVAYGXBV-RUELKSSGSA-N 0.000 description 1
- 101150097070 Drd3 gene Proteins 0.000 description 1
- CYQFCXCEBYINGO-DLBZAZTESA-N Dronabinol Natural products C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@H]21 CYQFCXCEBYINGO-DLBZAZTESA-N 0.000 description 1
- VQNATVDKACXKTF-UHFFFAOYSA-N Duocarmycin SA Natural products COC1=C(OC)C(OC)=C2NC(C(=O)N3C4=CC(=O)C5=C(C64CC6C3)C=C(N5)C(=O)OC)=CC2=C1 VQNATVDKACXKTF-UHFFFAOYSA-N 0.000 description 1
- DYEFUKCXAQOFHX-UHFFFAOYSA-N Ebselen Chemical compound [se]1C2=CC=CC=C2C(=O)N1C1=CC=CC=C1 DYEFUKCXAQOFHX-UHFFFAOYSA-N 0.000 description 1
- NBEALWAVEGMZQY-UHFFFAOYSA-N Enpromate Chemical compound C=1C=CC=CC=1C(C#C)(C=1C=CC=CC=1)OC(=O)NC1CCCCC1 NBEALWAVEGMZQY-UHFFFAOYSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- VAPSMQAHNAZRKC-PQWRYPMOSA-N Epristeride Chemical compound C1C=C2C=C(C(O)=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)NC(C)(C)C)[C@@]1(C)CC2 VAPSMQAHNAZRKC-PQWRYPMOSA-N 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- ITIONVBQFUNVJV-UHFFFAOYSA-N Etomidoline Chemical compound C12=CC=CC=C2C(=O)N(CC)C1NC(C=C1)=CC=C1OCCN1CCCCC1 ITIONVBQFUNVJV-UHFFFAOYSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 102100024785 Fibroblast growth factor 2 Human genes 0.000 description 1
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 1
- 108010029961 Filgrastim Proteins 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- PLDUPXSUYLZYBN-UHFFFAOYSA-N Fluphenazine Chemical compound C1CN(CCO)CCN1CCCN1C2=CC(C(F)(F)F)=CC=C2SC2=CC=CC=C21 PLDUPXSUYLZYBN-UHFFFAOYSA-N 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 108010024636 Glutathione Proteins 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-M Glycolate Chemical compound OCC([O-])=O AEMRFAOFKBGASW-UHFFFAOYSA-M 0.000 description 1
- 229920002683 Glycosaminoglycan Polymers 0.000 description 1
- WYCLKVQLVUQKNZ-UHFFFAOYSA-N Halazepam Chemical compound N=1CC(=O)N(CC(F)(F)F)C2=CC=C(Cl)C=C2C=1C1=CC=CC=C1 WYCLKVQLVUQKNZ-UHFFFAOYSA-N 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 102000000543 Histamine Receptors Human genes 0.000 description 1
- 108010002059 Histamine Receptors Proteins 0.000 description 1
- 101000728661 Homo sapiens Beta-arrestin-2 Proteins 0.000 description 1
- 101000582950 Homo sapiens Platelet factor 4 Proteins 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical class ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- STECJAGHUSJQJN-GAUPFVANSA-N Hyoscine Natural products C1([C@H](CO)C(=O)OC2C[C@@H]3N([C@H](C2)[C@@H]2[C@H]3O2)C)=CC=CC=C1 STECJAGHUSJQJN-GAUPFVANSA-N 0.000 description 1
- 208000035150 Hypercholesterolemia Diseases 0.000 description 1
- 208000031226 Hyperlipidaemia Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- MPBVHIBUJCELCL-UHFFFAOYSA-N Ibandronate Chemical compound CCCCCN(C)CCC(O)(P(O)(O)=O)P(O)(O)=O MPBVHIBUJCELCL-UHFFFAOYSA-N 0.000 description 1
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 1
- JJKOTMDDZAJTGQ-DQSJHHFOSA-N Idoxifene Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN2CCCC2)=CC=1)/C1=CC=C(I)C=C1 JJKOTMDDZAJTGQ-DQSJHHFOSA-N 0.000 description 1
- 101000668058 Infectious salmon anemia virus (isolate Atlantic salmon/Norway/810/9/99) RNA-directed RNA polymerase catalytic subunit Proteins 0.000 description 1
- 108700022013 Insecta cecropin B Proteins 0.000 description 1
- 108010054698 Interferon Alfa-n3 Proteins 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- WTDRDQBEARUVNC-LURJTMIESA-N L-DOPA Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C(O)=C1 WTDRDQBEARUVNC-LURJTMIESA-N 0.000 description 1
- WTDRDQBEARUVNC-UHFFFAOYSA-N L-Dopa Natural products OC(=O)C(N)CC1=CC=C(O)C(O)=C1 WTDRDQBEARUVNC-UHFFFAOYSA-N 0.000 description 1
- 239000002211 L-ascorbic acid Substances 0.000 description 1
- 235000000069 L-ascorbic acid Nutrition 0.000 description 1
- QAQJMLQRFWZOBN-LAUBAEHRSA-N L-ascorbyl-6-palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](O)[C@H]1OC(=O)C(O)=C1O QAQJMLQRFWZOBN-LAUBAEHRSA-N 0.000 description 1
- 239000011786 L-ascorbyl-6-palmitate Substances 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- KJQFBVYMGADDTQ-CVSPRKDYSA-N L-buthionine-(S,R)-sulfoximine Chemical compound CCCCS(=N)(=O)CC[C@H](N)C(O)=O KJQFBVYMGADDTQ-CVSPRKDYSA-N 0.000 description 1
- GSDBGCKBBJVPNC-BYPYZUCNSA-N L-lombricine Chemical compound NC(=[NH2+])NCCOP([O-])(=O)OC[C@H]([NH3+])C([O-])=O GSDBGCKBBJVPNC-BYPYZUCNSA-N 0.000 description 1
- 108010043135 L-methionine gamma-lyase Proteins 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- ZHTRILQJTPJGNK-FYBAATNNSA-N Leinamycin Chemical compound N([C@@H](C=1SC=C(N=1)\C=C/C=C/C(=O)[C@H](O)/C=C(C)/CC1)C)C(=O)C[C@@]21S(=O)SC(=O)[C@]2(C)O ZHTRILQJTPJGNK-FYBAATNNSA-N 0.000 description 1
- ZHTRILQJTPJGNK-UHFFFAOYSA-N Leinamycin Natural products C1CC(C)=CC(O)C(=O)C=CC=CC(N=2)=CSC=2C(C)NC(=O)CC21S(=O)SC(=O)C2(C)O ZHTRILQJTPJGNK-UHFFFAOYSA-N 0.000 description 1
- 108010062867 Lenograstim Proteins 0.000 description 1
- 229920001491 Lentinan Polymers 0.000 description 1
- LMVRPBWWHMVLPC-KBPJCXPTSA-N Leptolstatin Natural products CC(CC=CC(=CC(C)C(=O)C(C)C(O)C(C)CC(=CCO)C)C)C=C(C)/C=C/C1CC=CC(=O)O1 LMVRPBWWHMVLPC-KBPJCXPTSA-N 0.000 description 1
- HLFSDGLLUJUHTE-SNVBAGLBSA-N Levamisole Chemical compound C1([C@H]2CN3CCSC3=N2)=CC=CC=C1 HLFSDGLLUJUHTE-SNVBAGLBSA-N 0.000 description 1
- 239000000232 Lipid Bilayer Substances 0.000 description 1
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- BLOFGONIVNXZME-UHFFFAOYSA-N Mannostatin A Natural products CSC1C(N)C(O)C(O)C1O BLOFGONIVNXZME-UHFFFAOYSA-N 0.000 description 1
- 102000004318 Matrilysin Human genes 0.000 description 1
- 108090000855 Matrilysin Proteins 0.000 description 1
- 102000000422 Matrix Metalloproteinase 3 Human genes 0.000 description 1
- 206010061285 Mental disorder due to a general medical condition Diseases 0.000 description 1
- 108700021154 Metallothionein 3 Proteins 0.000 description 1
- 102100028708 Metallothionein-3 Human genes 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- DUGOZIWVEXMGBE-UHFFFAOYSA-N Methylphenidate Chemical compound C=1C=CC=CC=1C(C(=O)OC)C1CCCCN1 DUGOZIWVEXMGBE-UHFFFAOYSA-N 0.000 description 1
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- PCZOHLXUXFIOCF-UHFFFAOYSA-N Monacolin X Natural products C12C(OC(=O)C(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 PCZOHLXUXFIOCF-UHFFFAOYSA-N 0.000 description 1
- 206010048723 Multiple-drug resistance Diseases 0.000 description 1
- HFPXYDFQVINJBV-UHFFFAOYSA-N Mycaperoxide B Natural products O1OC(C(C)C(O)=O)CCC1(C)CCC1(O)C2(C)CCCC(C)(C)C2CCC1C HFPXYDFQVINJBV-UHFFFAOYSA-N 0.000 description 1
- USVMJSALORZVDV-SDBHATRESA-N N(6)-(Delta(2)-isopentenyl)adenosine Chemical compound C1=NC=2C(NCC=C(C)C)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O USVMJSALORZVDV-SDBHATRESA-N 0.000 description 1
- WUKZPHOXUVCQOR-UHFFFAOYSA-N N-(1-azabicyclo[2.2.2]octan-3-yl)-6-chloro-4-methyl-3-oxo-1,4-benzoxazine-8-carboxamide Chemical compound C1N(CC2)CCC2C1NC(=O)C1=CC(Cl)=CC2=C1OCC(=O)N2C WUKZPHOXUVCQOR-UHFFFAOYSA-N 0.000 description 1
- BNQSTAOJRULKNX-UHFFFAOYSA-N N-(6-acetamidohexyl)acetamide Chemical compound CC(=O)NCCCCCCNC(C)=O BNQSTAOJRULKNX-UHFFFAOYSA-N 0.000 description 1
- QJMCKEPOKRERLN-UHFFFAOYSA-N N-3,4-tridhydroxybenzamide Chemical compound ONC(=O)C1=CC=C(O)C(O)=C1 QJMCKEPOKRERLN-UHFFFAOYSA-N 0.000 description 1
- STECJAGHUSJQJN-UHFFFAOYSA-N N-Methyl-scopolamin Natural products C1C(C2C3O2)N(C)C3CC1OC(=O)C(CO)C1=CC=CC=C1 STECJAGHUSJQJN-UHFFFAOYSA-N 0.000 description 1
- AHUHJYKCOJAGGQ-UHFFFAOYSA-N NCCNc(cccc1O)c1O Chemical compound NCCNc(cccc1O)c1O AHUHJYKCOJAGGQ-UHFFFAOYSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 108010021717 Nafarelin Proteins 0.000 description 1
- GTEXXGIEZVKSLH-UHFFFAOYSA-N Naphterpin Natural products O=C1C2=CC(O)=C(C)C(O)=C2C(=O)C2=C1C1C=C(C)CCC1C(C)(C)O2 GTEXXGIEZVKSLH-UHFFFAOYSA-N 0.000 description 1
- 108090000028 Neprilysin Proteins 0.000 description 1
- 102000003729 Neprilysin Human genes 0.000 description 1
- 102400000058 Neuregulin-1 Human genes 0.000 description 1
- 108090000556 Neuregulin-1 Proteins 0.000 description 1
- BUSGWUFLNHIBPT-UHFFFAOYSA-N Nisamycin Natural products O=C1C2OC2C(C=CC=CC=CC(=O)O)(O)C=C1NC(=O)C=CC=CC1CCCCC1 BUSGWUFLNHIBPT-UHFFFAOYSA-N 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- KYRVNWMVYQXFEU-UHFFFAOYSA-N Nocodazole Chemical compound C1=C2NC(NC(=O)OC)=NC2=CC=C1C(=O)C1=CC=CS1 KYRVNWMVYQXFEU-UHFFFAOYSA-N 0.000 description 1
- KGTDRFCXGRULNK-UHFFFAOYSA-N Nogalamycin Natural products COC1C(OC)(C)C(OC)C(C)OC1OC1C2=C(O)C(C(=O)C3=C(O)C=C4C5(C)OC(C(C(C5O)N(C)C)O)OC4=C3C3=O)=C3C=C2C(C(=O)OC)C(C)(O)C1 KGTDRFCXGRULNK-UHFFFAOYSA-N 0.000 description 1
- QDKJBAVJBLOQGR-UHFFFAOYSA-N O=C(N1)OC2C1=CC=CC2N1CCN(CCCCOc2ccc(cn[nH]3)c3c2)CC1 Chemical compound O=C(N1)OC2C1=CC=CC2N1CCN(CCCCOc2ccc(cn[nH]3)c3c2)CC1 QDKJBAVJBLOQGR-UHFFFAOYSA-N 0.000 description 1
- TVOVTKQHQISTHP-UHFFFAOYSA-N O=C(c1c2)NCCc1ccc2OCCCCBr Chemical compound O=C(c1c2)NCCc1ccc2OCCCCBr TVOVTKQHQISTHP-UHFFFAOYSA-N 0.000 description 1
- WKXGCJMHUWZCIC-UHFFFAOYSA-N O=C1Nc(cc(cc2)OCCCCN(CC3)CCN3c3cccc4c3OCCO4)c2C=C1 Chemical compound O=C1Nc(cc(cc2)OCCCCN(CC3)CCN3c3cccc4c3OCCO4)c2C=C1 WKXGCJMHUWZCIC-UHFFFAOYSA-N 0.000 description 1
- CRARFJICFRVCGK-UHFFFAOYSA-N O=C1Nc2cc(OCC3CCC(CN(CC4)CCN4c(cccc4Cl)c4Cl)CC3)ccc2CC1 Chemical compound O=C1Nc2cc(OCC3CCC(CN(CC4)CCN4c(cccc4Cl)c4Cl)CC3)ccc2CC1 CRARFJICFRVCGK-UHFFFAOYSA-N 0.000 description 1
- 108010016076 Octreotide Proteins 0.000 description 1
- VTAZRSXSBIHBMH-UHFFFAOYSA-N Ophiocordin Natural products OC1=CC(C(=O)O)=CC(O)=C1C(=O)C1=C(O)C=CC=C1C(=O)NC1C(OC(=O)C=2C=CC(O)=CC=2)CCCNC1 VTAZRSXSBIHBMH-UHFFFAOYSA-N 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- LKBBOPGQDRPCDS-UHFFFAOYSA-N Oxaunomycin Natural products C12=C(O)C=3C(=O)C4=C(O)C=CC=C4C(=O)C=3C(O)=C2C(O)C(CC)(O)CC1OC1CC(N)C(O)C(C)O1 LKBBOPGQDRPCDS-UHFFFAOYSA-N 0.000 description 1
- WYNCHZVNFNFDNH-UHFFFAOYSA-N Oxazolidine Chemical compound C1COCN1 WYNCHZVNFNFDNH-UHFFFAOYSA-N 0.000 description 1
- VYOQBYCIIJYKJA-UHFFFAOYSA-N Palauamine Natural products C1N2C(=O)C3=CC=CN3C3N=C(N)NC32C2C1C(CN)C(Cl)C12NC(N)=NC1O VYOQBYCIIJYKJA-UHFFFAOYSA-N 0.000 description 1
- FRCJDPPXHQGEKS-UHFFFAOYSA-N Parabactin Natural products CC1OC(=NC1C(=O)N(CCCCNC(=O)c1cccc(O)c1O)CCCNC(=O)c1cccc(O)c1O)c1ccccc1O FRCJDPPXHQGEKS-UHFFFAOYSA-N 0.000 description 1
- 208000018737 Parkinson disease Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 108010057150 Peplomycin Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 229940083963 Peptide antagonist Drugs 0.000 description 1
- 241000009328 Perro Species 0.000 description 1
- APNRZHLOPQFNMR-UHFFFAOYSA-N Phenazinomycin Natural products C12=CC=CC=C2N=C(C(C=CC=2)=O)C=2N1CC=C(C)CCC1C(=C)CCCC1(C)C APNRZHLOPQFNMR-UHFFFAOYSA-N 0.000 description 1
- RMUCZJUITONUFY-UHFFFAOYSA-N Phenelzine Chemical compound NNCCC1=CC=CC=C1 RMUCZJUITONUFY-UHFFFAOYSA-N 0.000 description 1
- CXOFVDLJLONNDW-UHFFFAOYSA-N Phenytoin Chemical compound N1C(=O)NC(=O)C1(C=1C=CC=CC=1)C1=CC=CC=C1 CXOFVDLJLONNDW-UHFFFAOYSA-N 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- KMSKQZKKOZQFFG-HSUXVGOQSA-N Pirarubicin Chemical compound O([C@H]1[C@@H](N)C[C@@H](O[C@H]1C)O[C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1CCCCO1 KMSKQZKKOZQFFG-HSUXVGOQSA-N 0.000 description 1
- 102000010752 Plasminogen Inactivators Human genes 0.000 description 1
- 108010077971 Plasminogen Inactivators Proteins 0.000 description 1
- 102100030304 Platelet factor 4 Human genes 0.000 description 1
- 229920005372 Plexiglas® Polymers 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 102000004257 Potassium Channel Human genes 0.000 description 1
- HFVNWDWLWUCIHC-GUPDPFMOSA-N Prednimustine Chemical compound O=C([C@@]1(O)CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)[C@@H](O)C[C@@]21C)COC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 HFVNWDWLWUCIHC-GUPDPFMOSA-N 0.000 description 1
- 102000003946 Prolactin Human genes 0.000 description 1
- 108010057464 Prolactin Proteins 0.000 description 1
- ZGUGWUXLJSTTMA-UHFFFAOYSA-N Promazinum Chemical compound C1=CC=C2N(CCCN(C)C)C3=CC=CC=C3SC2=C1 ZGUGWUXLJSTTMA-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 229940079156 Proteasome inhibitor Drugs 0.000 description 1
- 102100032420 Protein S100-A9 Human genes 0.000 description 1
- PICZCWCKOLHDOJ-UHFFFAOYSA-N Pseudoaxinellin Natural products N1C(=O)C2CCCN2C(=O)C(CC(N)=O)NC(=O)C(C(C)C)NC(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C(C(C)C)NC(=O)C1CC1=CC=CC=C1 PICZCWCKOLHDOJ-UHFFFAOYSA-N 0.000 description 1
- XESARGFCSKSFID-UHFFFAOYSA-N Pyrazofurin Natural products OC1=C(C(=O)N)NN=C1C1C(O)C(O)C(CO)O1 XESARGFCSKSFID-UHFFFAOYSA-N 0.000 description 1
- 102000003901 Ras GTPase-activating proteins Human genes 0.000 description 1
- 108090000231 Ras GTPase-activating proteins Proteins 0.000 description 1
- 229940078123 Ras inhibitor Drugs 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- LCQMZZCPPSWADO-UHFFFAOYSA-N Reserpilin Natural products COC(=O)C1COCC2CN3CCc4c([nH]c5cc(OC)c(OC)cc45)C3CC12 LCQMZZCPPSWADO-UHFFFAOYSA-N 0.000 description 1
- QEVHRUUCFGRFIF-SFWBKIHZSA-N Reserpine Natural products O=C(OC)[C@@H]1[C@H](OC)[C@H](OC(=O)c2cc(OC)c(OC)c(OC)c2)C[C@H]2[C@@H]1C[C@H]1N(C2)CCc2c3c([nH]c12)cc(OC)cc3 QEVHRUUCFGRFIF-SFWBKIHZSA-N 0.000 description 1
- OWPCHSCAPHNHAV-UHFFFAOYSA-N Rhizoxin Natural products C1C(O)C2(C)OC2C=CC(C)C(OC(=O)C2)CC2CC2OC2C(=O)OC1C(C)C(OC)C(C)=CC=CC(C)=CC1=COC(C)=N1 OWPCHSCAPHNHAV-UHFFFAOYSA-N 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 108010005173 SERPIN-B5 Proteins 0.000 description 1
- YADVRLOQIWILGX-MIWLTHJTSA-N Sarcophytol A Chemical compound CC(C)C/1=C/C=C(C)/CC\C=C(C)\CC\C=C(C)\C[C@@H]\1O YADVRLOQIWILGX-MIWLTHJTSA-N 0.000 description 1
- 239000002262 Schiff base Substances 0.000 description 1
- 150000004753 Schiff bases Chemical class 0.000 description 1
- 206010039897 Sedation Diseases 0.000 description 1
- 102100030333 Serpin B5 Human genes 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- 229920000519 Sizofiran Polymers 0.000 description 1
- OCOKWVBYZHBHLU-UHFFFAOYSA-N Sobuzoxane Chemical compound C1C(=O)N(COC(=O)OCC(C)C)C(=O)CN1CCN1CC(=O)N(COC(=O)OCC(C)C)C(=O)C1 OCOKWVBYZHBHLU-UHFFFAOYSA-N 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- UIRKNQLZZXALBI-MSVGPLKSSA-N Squalamine Chemical compound C([C@@H]1C[C@H]2O)[C@@H](NCCCNCCCCN)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@H](C)CC[C@H](C(C)C)OS(O)(=O)=O)[C@@]2(C)CC1 UIRKNQLZZXALBI-MSVGPLKSSA-N 0.000 description 1
- UIRKNQLZZXALBI-UHFFFAOYSA-N Squalamine Natural products OC1CC2CC(NCCCNCCCCN)CCC2(C)C2C1C1CCC(C(C)CCC(C(C)C)OS(O)(=O)=O)C1(C)CC2 UIRKNQLZZXALBI-UHFFFAOYSA-N 0.000 description 1
- ZSJLQEPLLKMAKR-UHFFFAOYSA-N Streptozotocin Natural products O=NN(C)C(=O)NC1C(O)OC(CO)C(O)C1O ZSJLQEPLLKMAKR-UHFFFAOYSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- CYQFCXCEBYINGO-UHFFFAOYSA-N THC Natural products C1=C(C)CCC2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3C21 CYQFCXCEBYINGO-UHFFFAOYSA-N 0.000 description 1
- 206010043118 Tardive Dyskinesia Diseases 0.000 description 1
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- WXZSUBHBYQYTNM-UHFFFAOYSA-N Tetrazomine Natural products C1=CC=2CC(N34)C(N5C)C(CO)CC5C4OCC3C=2C(OC)=C1NC(=O)C1NCCCC1O WXZSUBHBYQYTNM-UHFFFAOYSA-N 0.000 description 1
- UPGGKUQISSWRJJ-XLTUSUNSSA-N Thiocoraline Chemical compound O=C([C@H]1CSSC[C@@H](N(C(=O)CNC2=O)C)C(=O)N(C)[C@@H](C(SC[C@@H](C(=O)NCC(=O)N1C)NC(=O)C=1C(=CC3=CC=CC=C3N=1)O)=O)CSC)N(C)[C@H](CSC)C(=O)SC[C@@H]2NC(=O)C1=NC2=CC=CC=C2C=C1O UPGGKUQISSWRJJ-XLTUSUNSSA-N 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-M Thiocyanate anion Chemical compound [S-]C#N ZMZDMBWJUHKJPS-UHFFFAOYSA-M 0.000 description 1
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 1
- GFBKORZTTCHDGY-UWVJOHFNSA-N Thiothixene Chemical compound C12=CC(S(=O)(=O)N(C)C)=CC=C2SC2=CC=CC=C2\C1=C\CCN1CCN(C)CC1 GFBKORZTTCHDGY-UWVJOHFNSA-N 0.000 description 1
- 108010078233 Thymalfasin Proteins 0.000 description 1
- 102000011923 Thyrotropin Human genes 0.000 description 1
- 108010061174 Thyrotropin Proteins 0.000 description 1
- 229910003074 TiCl4 Inorganic materials 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- IVTVGDXNLFLDRM-HNNXBMFYSA-N Tomudex Chemical compound C=1C=C2NC(C)=NC(=O)C2=CC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)S1 IVTVGDXNLFLDRM-HNNXBMFYSA-N 0.000 description 1
- KJADKKWYZYXHBB-XBWDGYHZSA-N Topiramic acid Chemical compound C1O[C@@]2(COS(N)(=O)=O)OC(C)(C)O[C@H]2[C@@H]2OC(C)(C)O[C@@H]21 KJADKKWYZYXHBB-XBWDGYHZSA-N 0.000 description 1
- IWEQQRMGNVVKQW-OQKDUQJOSA-N Toremifene citrate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C1=CC(OCCN(C)C)=CC=C1C(\C=1C=CC=CC=1)=C(\CCCl)C1=CC=CC=C1 IWEQQRMGNVVKQW-OQKDUQJOSA-N 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- HWHLPVGTWGOCJO-UHFFFAOYSA-N Trihexyphenidyl Chemical group C1CCCCC1C(C=1C=CC=CC=1)(O)CCN1CCCCC1 HWHLPVGTWGOCJO-UHFFFAOYSA-N 0.000 description 1
- 102000001742 Tumor Suppressor Proteins Human genes 0.000 description 1
- 108010040002 Tumor Suppressor Proteins Proteins 0.000 description 1
- VGQOVCHZGQWAOI-UHFFFAOYSA-N UNPD55612 Natural products N1C(O)C2CC(C=CC(N)=O)=CN2C(=O)C2=CC=C(C)C(O)=C12 VGQOVCHZGQWAOI-UHFFFAOYSA-N 0.000 description 1
- 102000003990 Urokinase-type plasminogen activator Human genes 0.000 description 1
- 108090000435 Urokinase-type plasminogen activator Proteins 0.000 description 1
- 108010003205 Vasoactive Intestinal Peptide Proteins 0.000 description 1
- 102400000015 Vasoactive intestinal peptide Human genes 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- MHDDZDPNIDVLNK-ZGIWMXSJSA-N Zanoterone Chemical compound C1C2=NN(S(C)(=O)=O)C=C2C[C@]2(C)[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CC[C@H]21 MHDDZDPNIDVLNK-ZGIWMXSJSA-N 0.000 description 1
- OGQICQVSFDPSEI-UHFFFAOYSA-N Zorac Chemical compound N1=CC(C(=O)OCC)=CC=C1C#CC1=CC=C(SCCC2(C)C)C2=C1 OGQICQVSFDPSEI-UHFFFAOYSA-N 0.000 description 1
- ZZWKZQDOSJAGGF-VRSYWUPDSA-N [(1s,2e,7s,10e,12r,13r,15s)-12-hydroxy-7-methyl-9-oxo-8-oxabicyclo[11.3.0]hexadeca-2,10-dien-15-yl] 2-(dimethylamino)acetate Chemical compound O[C@@H]1\C=C\C(=O)O[C@@H](C)CCC\C=C\[C@@H]2C[C@H](OC(=O)CN(C)C)C[C@H]21 ZZWKZQDOSJAGGF-VRSYWUPDSA-N 0.000 description 1
- VUPBDWQPEOWRQP-RTUCOMKBSA-N [(2R,3S,4S,5R,6R)-2-[(2R,3S,4S,5S,6S)-2-[(1S,2S)-3-[[(2R,3S)-5-[[(2S,3R)-1-[[2-[4-[4-[[4-amino-6-[3-(4-aminobutylamino)propylamino]-6-oxohexyl]carbamoyl]-1,3-thiazol-2-yl]-1,3-thiazol-2-yl]-1-[(2S,3R,4R,5S,6S)-5-amino-3,4-dihydroxy-6-methyloxan-2-yl]oxy-2-hydroxyethyl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-3-hydroxy-5-oxopentan-2-yl]amino]-2-[[6-amino-2-[(1S)-3-amino-1-[[(2S)-2,3-diamino-3-oxopropyl]amino]-3-oxopropyl]-5-methylpyrimidine-4-carbonyl]amino]-1-(1H-imidazol-5-yl)-3-oxopropoxy]-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl] carbamate Chemical compound C[C@@H](O)[C@H](NC(=O)C[C@H](O)[C@@H](C)NC(=O)[C@@H](NC(=O)c1nc(nc(N)c1C)[C@H](CC(N)=O)NC[C@H](N)C(N)=O)[C@H](O[C@@H]1O[C@@H](CO)[C@@H](O)[C@H](O)[C@@H]1O[C@H]1O[C@H](CO)[C@@H](O)[C@H](OC(N)=O)[C@@H]1O)c1cnc[nH]1)C(=O)NC(O[C@@H]1O[C@@H](C)[C@@H](N)[C@@H](O)[C@H]1O)C(O)c1nc(cs1)-c1nc(cs1)C(=O)NCCCC(N)CC(=O)NCCCNCCCCN VUPBDWQPEOWRQP-RTUCOMKBSA-N 0.000 description 1
- SPKNARKFCOPTSY-XWPZMVOTSA-N [(2r,3s)-2-[(2s,3r)-3-methyloxiran-2-yl]-6-oxo-2,3-dihydropyran-3-yl] acetate Chemical compound C[C@H]1O[C@@H]1[C@H]1[C@@H](OC(C)=O)C=CC(=O)O1 SPKNARKFCOPTSY-XWPZMVOTSA-N 0.000 description 1
- IVCRCPJOLWECJU-XQVQQVTHSA-N [(7r,8r,9s,10r,13s,14s,17s)-7,13-dimethyl-3-oxo-2,6,7,8,9,10,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-17-yl] acetate Chemical compound C1C[C@]2(C)[C@@H](OC(C)=O)CC[C@H]2[C@@H]2[C@H](C)CC3=CC(=O)CC[C@@H]3[C@H]21 IVCRCPJOLWECJU-XQVQQVTHSA-N 0.000 description 1
- PQNNIEWMPIULRS-SUTYWZMXSA-N [(8e,10e,12e)-7-hydroxy-6-methyl-2-(3-methyl-6-oxo-2,3-dihydropyran-2-yl)tetradeca-8,10,12-trien-5-yl] dihydrogen phosphate Chemical compound C\C=C\C=C\C=C\C(O)C(C)C(OP(O)(O)=O)CCC(C)C1OC(=O)C=CC1C PQNNIEWMPIULRS-SUTYWZMXSA-N 0.000 description 1
- IFJUINDAXYAPTO-UUBSBJJBSA-N [(8r,9s,13s,14s,17s)-17-[2-[4-[4-[bis(2-chloroethyl)amino]phenyl]butanoyloxy]acetyl]oxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-yl] benzoate Chemical compound C([C@@H]1[C@@H](C2=CC=3)CC[C@]4([C@H]1CC[C@@H]4OC(=O)COC(=O)CCCC=1C=CC(=CC=1)N(CCCl)CCCl)C)CC2=CC=3OC(=O)C1=CC=CC=C1 IFJUINDAXYAPTO-UUBSBJJBSA-N 0.000 description 1
- KMLCRELJHYKIIL-UHFFFAOYSA-N [1-(azanidylmethyl)cyclohexyl]methylazanide;platinum(2+);sulfuric acid Chemical compound [Pt+2].OS(O)(=O)=O.[NH-]CC1(C[NH-])CCCCC1 KMLCRELJHYKIIL-UHFFFAOYSA-N 0.000 description 1
- JJULHOZRTCDZOH-JGJFOBQESA-N [1-[[[(2r,3s,4s,5r)-5-(4-amino-2-oxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-3-octadecylsulfanylpropan-2-yl] hexadecanoate Chemical compound O[C@H]1[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OCC(CSCCCCCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC)O[C@H]1N1C(=O)N=C(N)C=C1 JJULHOZRTCDZOH-JGJFOBQESA-N 0.000 description 1
- XSMVECZRZBFTIZ-UHFFFAOYSA-M [2-(aminomethyl)cyclobutyl]methanamine;2-oxidopropanoate;platinum(4+) Chemical compound [Pt+4].CC([O-])C([O-])=O.NCC1CCC1CN XSMVECZRZBFTIZ-UHFFFAOYSA-M 0.000 description 1
- NAFFDQVVNWTDJD-UHFFFAOYSA-L [4-(azanidylmethyl)oxan-4-yl]methylazanide;cyclobutane-1,1-dicarboxylate;platinum(4+) Chemical compound [Pt+4].[NH-]CC1(C[NH-])CCOCC1.[O-]C(=O)C1(C([O-])=O)CCC1 NAFFDQVVNWTDJD-UHFFFAOYSA-L 0.000 description 1
- JURAJLFHWXNPHG-UHFFFAOYSA-N [acetyl(methylcarbamoyl)amino] n-methylcarbamate Chemical compound CNC(=O)ON(C(C)=O)C(=O)NC JURAJLFHWXNPHG-UHFFFAOYSA-N 0.000 description 1
- GZOSMCIZMLWJML-VJLLXTKPSA-N abiraterone Chemical compound C([C@H]1[C@H]2[C@@H]([C@]3(CC[C@H](O)CC3=CC2)C)CC[C@@]11C)C=C1C1=CC=CN=C1 GZOSMCIZMLWJML-VJLLXTKPSA-N 0.000 description 1
- 229960000853 abiraterone Drugs 0.000 description 1
- IPBVNPXQWQGGJP-UHFFFAOYSA-N acetic acid phenyl ester Natural products CC(=O)OC1=CC=CC=C1 IPBVNPXQWQGGJP-UHFFFAOYSA-N 0.000 description 1
- RUGAHXUZHWYHNG-NLGNTGLNSA-N acetic acid;(4r,7s,10s,13r,16s,19r)-10-(4-aminobutyl)-n-[(2s,3r)-1-amino-3-hydroxy-1-oxobutan-2-yl]-19-[[(2r)-2-amino-3-naphthalen-2-ylpropanoyl]amino]-16-[(4-hydroxyphenyl)methyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-7-propan-2-yl-1,2-dithia-5, Chemical compound CC(O)=O.CC(O)=O.CC(O)=O.CC(O)=O.CC(O)=O.C([C@H]1C(=O)N[C@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](N)CC=1C=C2C=CC=CC2=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)=O)C(C)C)C1=CC=C(O)C=C1.C([C@H]1C(=O)N[C@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](N)CC=1C=C2C=CC=CC2=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)=O)C(C)C)C1=CC=C(O)C=C1 RUGAHXUZHWYHNG-NLGNTGLNSA-N 0.000 description 1
- IGCAUIJHGNYDKE-UHFFFAOYSA-N acetic acid;1,4-bis[2-(2-hydroxyethylamino)ethylamino]anthracene-9,10-dione Chemical compound CC([O-])=O.CC([O-])=O.O=C1C2=CC=CC=C2C(=O)C2=C1C(NCC[NH2+]CCO)=CC=C2NCC[NH2+]CCO IGCAUIJHGNYDKE-UHFFFAOYSA-N 0.000 description 1
- PBCJIPOGFJYBJE-UHFFFAOYSA-N acetonitrile;hydrate Chemical compound O.CC#N PBCJIPOGFJYBJE-UHFFFAOYSA-N 0.000 description 1
- WETWJCDKMRHUPV-UHFFFAOYSA-N acetyl chloride Chemical compound CC(Cl)=O WETWJCDKMRHUPV-UHFFFAOYSA-N 0.000 description 1
- 239000012346 acetyl chloride Substances 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- QAWIHIJWNYOLBE-OKKQSCSOSA-N acivicin Chemical compound OC(=O)[C@@H](N)[C@@H]1CC(Cl)=NO1 QAWIHIJWNYOLBE-OKKQSCSOSA-N 0.000 description 1
- 229950008427 acivicin Drugs 0.000 description 1
- 229950000616 acronine Drugs 0.000 description 1
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 125000002252 acyl group Chemical group 0.000 description 1
- HLAKJNQXUARACO-UHFFFAOYSA-N acylfulvene Natural products CC1(O)C(=O)C2=CC(C)=CC2=C(C)C21CC2 HLAKJNQXUARACO-UHFFFAOYSA-N 0.000 description 1
- 125000004423 acyloxy group Chemical group 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- DPGOLRILOKERAV-AAWJQDODSA-N adecypenol Chemical compound OC1C(CO)=CCC1(O)N1C(N=CNC[C@H]2O)C2N=C1 DPGOLRILOKERAV-AAWJQDODSA-N 0.000 description 1
- WJSAFKJWCOMTLH-UHFFFAOYSA-N adecypenol Natural products OC1C(O)C(CO)=CC1N1C(NC=NCC2O)=C2N=C1 WJSAFKJWCOMTLH-UHFFFAOYSA-N 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- WNLRTRBMVRJNCN-UHFFFAOYSA-L adipate(2-) Chemical compound [O-]C(=O)CCCCC([O-])=O WNLRTRBMVRJNCN-UHFFFAOYSA-L 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 125000003158 alcohol group Chemical group 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 229920013820 alkyl cellulose Polymers 0.000 description 1
- 125000000304 alkynyl group Chemical group 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 229950010817 alvocidib Drugs 0.000 description 1
- BIIVYFLTOXDAOV-YVEFUNNKSA-N alvocidib Chemical compound O[C@@H]1CN(C)CC[C@@H]1C1=C(O)C=C(O)C2=C1OC(C=1C(=CC=CC=1)Cl)=CC2=O BIIVYFLTOXDAOV-YVEFUNNKSA-N 0.000 description 1
- 229960003805 amantadine Drugs 0.000 description 1
- DKNWSYNQZKUICI-UHFFFAOYSA-N amantadine Chemical compound C1C(C2)CC3CC2CC1(N)C3 DKNWSYNQZKUICI-UHFFFAOYSA-N 0.000 description 1
- 229950010949 ambamustine Drugs 0.000 description 1
- 229950004821 ambomycin Drugs 0.000 description 1
- 125000003368 amide group Chemical group 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- JKOQGQFVAUAYPM-UHFFFAOYSA-N amifostine Chemical compound NCCCNCCSP(O)(O)=O JKOQGQFVAUAYPM-UHFFFAOYSA-N 0.000 description 1
- 229960001097 amifostine Drugs 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229960002749 aminolevulinic acid Drugs 0.000 description 1
- 229960002519 amoxapine Drugs 0.000 description 1
- QWGDMFLQWFTERH-UHFFFAOYSA-N amoxapine Chemical compound C12=CC(Cl)=CC=C2OC2=CC=CC=C2N=C1N1CCNCC1 QWGDMFLQWFTERH-UHFFFAOYSA-N 0.000 description 1
- 229960002550 amrubicin Drugs 0.000 description 1
- VJZITPJGSQKZMX-XDPRQOKASA-N amrubicin Chemical compound O([C@H]1C[C@](CC2=C(O)C=3C(=O)C4=CC=CC=C4C(=O)C=3C(O)=C21)(N)C(=O)C)[C@H]1C[C@H](O)[C@H](O)CO1 VJZITPJGSQKZMX-XDPRQOKASA-N 0.000 description 1
- 229960001694 anagrelide Drugs 0.000 description 1
- OTBXOEAOVRKTNQ-UHFFFAOYSA-N anagrelide Chemical compound N1=C2NC(=O)CN2CC2=C(Cl)C(Cl)=CC=C21 OTBXOEAOVRKTNQ-UHFFFAOYSA-N 0.000 description 1
- ASLUCFFROXVMFL-UHFFFAOYSA-N andrographolide Natural products CC1(CO)C(O)CCC2(C)C(CC=C3/C(O)OCC3=O)C(=C)CCC12 ASLUCFFROXVMFL-UHFFFAOYSA-N 0.000 description 1
- 239000004037 angiogenesis inhibitor Substances 0.000 description 1
- 229940121369 angiogenesis inhibitor Drugs 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 230000008485 antagonism Effects 0.000 description 1
- 108010070670 antarelix Proteins 0.000 description 1
- VGQOVCHZGQWAOI-HYUHUPJXSA-N anthramycin Chemical compound N1[C@@H](O)[C@@H]2CC(\C=C\C(N)=O)=CN2C(=O)C2=CC=C(C)C(O)=C12 VGQOVCHZGQWAOI-HYUHUPJXSA-N 0.000 description 1
- 230000002280 anti-androgenic effect Effects 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 230000001430 anti-depressive effect Effects 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 239000000051 antiandrogen Substances 0.000 description 1
- 239000002518 antifoaming agent Substances 0.000 description 1
- 102000025171 antigen binding proteins Human genes 0.000 description 1
- 108091000831 antigen binding proteins Proteins 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 229940005529 antipsychotics Drugs 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- IOASYARYEYRREA-LQAJYKIKSA-N aphidicolin glycinate Chemical compound C1[C@]23[C@]4(C)CC[C@H](O)[C@](C)(CO)[C@H]4CC[C@@H]3C[C@@H]1[C@@](COC(=O)CN)(O)CC2 IOASYARYEYRREA-LQAJYKIKSA-N 0.000 description 1
- 229960004046 apomorphine Drugs 0.000 description 1
- VMWNQDUVQKEIOC-CYBMUJFWSA-N apomorphine Chemical compound C([C@H]1N(C)CC2)C3=CC=C(O)C(O)=C3C3=C1C2=CC=C3 VMWNQDUVQKEIOC-CYBMUJFWSA-N 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 108010055530 arginyl-tryptophyl-N-methylphenylalanyl-tryptophyl-leucyl-methioninamide Proteins 0.000 description 1
- 239000000823 artificial membrane Substances 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 235000010385 ascorbyl palmitate Nutrition 0.000 description 1
- 229960003272 asparaginase Drugs 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-M asparaginate Chemical compound [O-]C(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-M 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- TWHSQQYCDVSBRK-UHFFFAOYSA-N asulacrine Chemical compound C12=CC=CC(C)=C2N=C2C(C(=O)NC)=CC=CC2=C1NC1=CC=C(NS(C)(=O)=O)C=C1OC TWHSQQYCDVSBRK-UHFFFAOYSA-N 0.000 description 1
- 229950011088 asulacrine Drugs 0.000 description 1
- PEPMWUSGRKINHX-TXTPUJOMSA-N atamestane Chemical compound C1C[C@@H]2[C@@]3(C)C(C)=CC(=O)C=C3CC[C@H]2[C@@H]2CCC(=O)[C@]21C PEPMWUSGRKINHX-TXTPUJOMSA-N 0.000 description 1
- 229950004810 atamestane Drugs 0.000 description 1
- 229960002274 atenolol Drugs 0.000 description 1
- 229960002430 atomoxetine Drugs 0.000 description 1
- VHGCDTVCOLNTBX-QGZVFWFLSA-N atomoxetine Chemical compound O([C@H](CCNC)C=1C=CC=CC=1)C1=CC=CC=C1C VHGCDTVCOLNTBX-QGZVFWFLSA-N 0.000 description 1
- 229950006933 atrimustine Drugs 0.000 description 1
- 108010093161 axinastatin 1 Proteins 0.000 description 1
- PICZCWCKOLHDOJ-GHTSNYPWSA-N axinastatin 1 Chemical compound C([C@H]1C(=O)N[C@H](C(=O)N[C@H](C(=O)N2CCC[C@H]2C(=O)N[C@H](C(N[C@@H](CC(N)=O)C(=O)N2CCC[C@H]2C(=O)N1)=O)C(C)C)C(C)C)C(C)C)C1=CC=CC=C1 PICZCWCKOLHDOJ-GHTSNYPWSA-N 0.000 description 1
- 108010093000 axinastatin 2 Proteins 0.000 description 1
- OXNAATCTZCSVKR-AVGVIDKOSA-N axinastatin 2 Chemical compound C([C@H]1C(=O)N[C@H](C(=O)N[C@H](C(N2CCC[C@H]2C(=O)N[C@@H](C(=O)N[C@@H](CC(N)=O)C(=O)N2CCC[C@H]2C(=O)N1)C(C)C)=O)CC(C)C)C(C)C)C1=CC=CC=C1 OXNAATCTZCSVKR-AVGVIDKOSA-N 0.000 description 1
- UZCPCRPHNVHKKP-UHFFFAOYSA-N axinastatin 2 Natural products CC(C)CC1NC(=O)C2CCCN2C(=O)C(NC(=O)C(CC(=O)N)NC(=O)C3CCCN3C(=O)C(Cc4ccccc4)NC(=O)C(NC1=O)C(C)C)C(C)C UZCPCRPHNVHKKP-UHFFFAOYSA-N 0.000 description 1
- 108010092978 axinastatin 3 Proteins 0.000 description 1
- ANLDPEXRVVIABH-WUUSPZRJSA-N axinastatin 3 Chemical compound C([C@H]1C(=O)N[C@H](C(N[C@@H](CC(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](C(=O)N[C@@H](CC(N)=O)C(=O)N2CCC[C@H]2C(=O)N1)C(C)C)=O)[C@@H](C)CC)C1=CC=CC=C1 ANLDPEXRVVIABH-WUUSPZRJSA-N 0.000 description 1
- RTGMQVUKARGBNM-UHFFFAOYSA-N axinastatin 3 Natural products CCC(C)C1NC(=O)C(CC(C)C)NC(=O)C2CCCN2C(=O)C(NC(=O)C(CC(=O)N)NC(=O)C3CCCN3C(=O)C(Cc4ccccc4)NC1=O)C(C)C RTGMQVUKARGBNM-UHFFFAOYSA-N 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- OPWOOOGFNULJAQ-UHFFFAOYSA-L azane;cyclopentanamine;2-hydroxybutanedioate;platinum(2+) Chemical compound N.[Pt+2].NC1CCCC1.[O-]C(=O)C(O)CC([O-])=O OPWOOOGFNULJAQ-UHFFFAOYSA-L 0.000 description 1
- KLNFSAOEKUDMFA-UHFFFAOYSA-N azanide;2-hydroxyacetic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OCC(O)=O KLNFSAOEKUDMFA-UHFFFAOYSA-N 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- 229950005951 azasetron Drugs 0.000 description 1
- HRXVDDOKERXBEY-UHFFFAOYSA-N azatepa Chemical compound C1CN1P(=O)(N1CC1)N(CC)C1=NN=CS1 HRXVDDOKERXBEY-UHFFFAOYSA-N 0.000 description 1
- MIXLRUYCYZPSOQ-HXPMCKFVSA-N azatoxin Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=C(C4=CC=CC=C4N3)C[C@@H]3N2C(OC3)=O)=C1 MIXLRUYCYZPSOQ-HXPMCKFVSA-N 0.000 description 1
- IVRMZWNICZWHMI-UHFFFAOYSA-N azide group Chemical group [N-]=[N+]=[N-] IVRMZWNICZWHMI-UHFFFAOYSA-N 0.000 description 1
- 229950004295 azotomycin Drugs 0.000 description 1
- 150000004200 baccatin III derivatives Chemical class 0.000 description 1
- 229960000794 baclofen Drugs 0.000 description 1
- XYUFCXJZFZPEJD-PGRDOPGGSA-N balanol Chemical compound OC(=O)C1=CC=CC(O)=C1C(=O)C1=C(O)C=C(C(=O)O[C@H]2[C@H](CNCCC2)NC(=O)C=2C=CC(O)=CC=2)C=C1O XYUFCXJZFZPEJD-PGRDOPGGSA-N 0.000 description 1
- 230000003542 behavioural effect Effects 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 229960001081 benzatropine Drugs 0.000 description 1
- GIJXKZJWITVLHI-PMOLBWCYSA-N benzatropine Chemical compound O([C@H]1C[C@H]2CC[C@@H](C1)N2C)C(C=1C=CC=CC=1)C1=CC=CC=C1 GIJXKZJWITVLHI-PMOLBWCYSA-N 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 125000005873 benzo[d]thiazolyl group Chemical group 0.000 description 1
- 229940050390 benzoate Drugs 0.000 description 1
- 229950005567 benzodepa Drugs 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 125000005872 benzooxazolyl group Chemical group 0.000 description 1
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 1
- 239000012965 benzophenone Substances 0.000 description 1
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 1
- 125000004196 benzothienyl group Chemical group S1C(=CC2=C1C=CC=C2)* 0.000 description 1
- MMIMIFULGMZVPO-UHFFFAOYSA-N benzyl 3-bromo-2,6-dinitro-5-phenylmethoxybenzoate Chemical compound [O-][N+](=O)C1=C(C(=O)OCC=2C=CC=CC=2)C([N+](=O)[O-])=C(Br)C=C1OCC1=CC=CC=C1 MMIMIFULGMZVPO-UHFFFAOYSA-N 0.000 description 1
- PRZWPYRSPPIOGO-UHFFFAOYSA-N benzyl 4-(2,3-dichlorophenyl)piperidine-1-carboxylate Chemical compound ClC1=CC=CC(C2CCN(CC2)C(=O)OCC=2C=CC=CC=2)=C1Cl PRZWPYRSPPIOGO-UHFFFAOYSA-N 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- 229960004217 benzyl alcohol Drugs 0.000 description 1
- HSDAJNMJOMSNEV-UHFFFAOYSA-N benzyl chloroformate Chemical compound ClC(=O)OCC1=CC=CC=C1 HSDAJNMJOMSNEV-UHFFFAOYSA-N 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- VFIUCBTYGKMLCM-UHFFFAOYSA-N benzyl n-[bis(aziridin-1-yl)phosphoryl]carbamate Chemical compound C=1C=CC=CC=1COC(=O)NP(=O)(N1CC1)N1CC1 VFIUCBTYGKMLCM-UHFFFAOYSA-N 0.000 description 1
- 235000021028 berry Nutrition 0.000 description 1
- 108010032967 beta-Arrestin 2 Proteins 0.000 description 1
- QGJZLNKBHJESQX-FZFNOLFKSA-N betulinic acid Chemical compound C1C[C@H](O)C(C)(C)[C@@H]2CC[C@@]3(C)[C@]4(C)CC[C@@]5(C(O)=O)CC[C@@H](C(=C)C)[C@@H]5[C@H]4CC[C@@H]3[C@]21C QGJZLNKBHJESQX-FZFNOLFKSA-N 0.000 description 1
- 125000002619 bicyclic group Chemical group 0.000 description 1
- YSXKPIUOCJLQIE-UHFFFAOYSA-N biperiden Chemical compound C1C(C=C2)CC2C1C(C=1C=CC=CC=1)(O)CCN1CCCCC1 YSXKPIUOCJLQIE-UHFFFAOYSA-N 0.000 description 1
- 229960003003 biperiden Drugs 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- QKSKPIVNLNLAAV-UHFFFAOYSA-N bis(2-chloroethyl) sulfide Chemical compound ClCCSCCCl QKSKPIVNLNLAAV-UHFFFAOYSA-N 0.000 description 1
- 229950002370 bisnafide Drugs 0.000 description 1
- NPSOIFAWYAHWOH-UHFFFAOYSA-N bistratene A Natural products O1C(CC(=O)C=CC)CCC(O2)(O)CC(C)C2CCCNC(=O)C(C)C2OC(CCC(C)C=C(C)C(C)O)CCCCC(C)C1CC(=O)NC2 NPSOIFAWYAHWOH-UHFFFAOYSA-N 0.000 description 1
- 229960004395 bleomycin sulfate Drugs 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 229910021538 borax Inorganic materials 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- PZOHOALJQOFNTB-UHFFFAOYSA-M brequinar sodium Chemical compound [Na+].N1=C2C=CC(F)=CC2=C(C([O-])=O)C(C)=C1C(C=C1)=CC=C1C1=CC=CC=C1F PZOHOALJQOFNTB-UHFFFAOYSA-M 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 229960002802 bromocriptine Drugs 0.000 description 1
- OZVBMTJYIDMWIL-AYFBDAFISA-N bromocriptine Chemical compound C1=CC(C=2[C@H](N(C)C[C@@H](C=2)C(=O)N[C@]2(C(=O)N3[C@H](C(N4CCC[C@H]4[C@]3(O)O2)=O)CC(C)C)C(C)C)C2)=C3C2=C(Br)NC3=C1 OZVBMTJYIDMWIL-AYFBDAFISA-N 0.000 description 1
- 229950002361 budotitane Drugs 0.000 description 1
- 244000309464 bull Species 0.000 description 1
- QWCRAEMEVRGPNT-UHFFFAOYSA-N buspirone Chemical compound C1C(=O)N(CCCCN2CCN(CC2)C=2N=CC=CN=2)C(=O)CC21CCCC2 QWCRAEMEVRGPNT-UHFFFAOYSA-N 0.000 description 1
- 229960002495 buspirone Drugs 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- LFLBHTZRLVHUQC-UHFFFAOYSA-N butyl methanesulfonate Chemical compound CCCCOS(C)(=O)=O LFLBHTZRLVHUQC-UHFFFAOYSA-N 0.000 description 1
- 230000001275 ca(2+)-mobilization Effects 0.000 description 1
- 229950009908 cactinomycin Drugs 0.000 description 1
- 108700002839 cactinomycin Proteins 0.000 description 1
- 229960002882 calcipotriol Drugs 0.000 description 1
- LWQQLNNNIPYSNX-UROSTWAQSA-N calcipotriol Chemical compound C1([C@H](O)/C=C/[C@@H](C)[C@@H]2[C@]3(CCCC(/[C@@H]3CC2)=C\C=C\2C([C@@H](O)C[C@H](O)C/2)=C)C)CC1 LWQQLNNNIPYSNX-UROSTWAQSA-N 0.000 description 1
- 229960005084 calcitriol Drugs 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- LSUTUUOITDQYNO-UHFFFAOYSA-N calphostin C Chemical compound C=12C3=C4C(CC(C)OC(=O)C=5C=CC=CC=5)=C(OC)C(O)=C(C(C=C5OC)=O)C4=C5C=1C(OC)=CC(=O)C2=C(O)C(OC)=C3CC(C)OC(=O)OC1=CC=C(O)C=C1 LSUTUUOITDQYNO-UHFFFAOYSA-N 0.000 description 1
- IVFYLRMMHVYGJH-PVPPCFLZSA-N calusterone Chemical compound C1C[C@]2(C)[C@](O)(C)CC[C@H]2[C@@H]2[C@@H](C)CC3=CC(=O)CC[C@]3(C)[C@H]21 IVFYLRMMHVYGJH-PVPPCFLZSA-N 0.000 description 1
- 229950009823 calusterone Drugs 0.000 description 1
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical class C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 1
- 229960004117 capecitabine Drugs 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 229950009338 caracemide Drugs 0.000 description 1
- FFGPTBGBLSHEPO-UHFFFAOYSA-N carbamazepine Chemical compound C1=CC2=CC=CC=C2N(C(=O)N)C2=CC=CC=C21 FFGPTBGBLSHEPO-UHFFFAOYSA-N 0.000 description 1
- 229960000623 carbamazepine Drugs 0.000 description 1
- 229950005155 carbetimer Drugs 0.000 description 1
- 229960004205 carbidopa Drugs 0.000 description 1
- TZFNLOMSOLWIDK-JTQLQIEISA-N carbidopa (anhydrous) Chemical compound NN[C@@](C(O)=O)(C)CC1=CC=C(O)C(O)=C1 TZFNLOMSOLWIDK-JTQLQIEISA-N 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 125000005518 carboxamido group Chemical group 0.000 description 1
- WNRZHQBJSXRYJK-UHFFFAOYSA-N carboxyamidotriazole Chemical compound NC1=C(C(=O)N)N=NN1CC(C=C1Cl)=CC(Cl)=C1C(=O)C1=CC=C(Cl)C=C1 WNRZHQBJSXRYJK-UHFFFAOYSA-N 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 150000001733 carboxylic acid esters Chemical class 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- XREUEWVEMYWFFA-CSKJXFQVSA-N carminomycin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=C(O)C=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XREUEWVEMYWFFA-CSKJXFQVSA-N 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 229950001725 carubicin Drugs 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 229950010667 cedefingol Drugs 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 108700008462 cetrorelix Proteins 0.000 description 1
- SBNPWPIBESPSIF-MHWMIDJBSA-N cetrorelix Chemical compound C([C@@H](C(=O)N[C@H](CCCNC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)N[C@H](C)C(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](CC=1C=NC=CC=1)NC(=O)[C@@H](CC=1C=CC(Cl)=CC=1)NC(=O)[C@@H](CC=1C=C2C=CC=CC2=CC=1)NC(C)=O)C1=CC=C(O)C=C1 SBNPWPIBESPSIF-MHWMIDJBSA-N 0.000 description 1
- 229960003230 cetrorelix Drugs 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- HZCWPKGYTCJSEB-UHFFFAOYSA-N chembl118841 Chemical compound C12=CC(OC)=CC=C2NC2=C([N+]([O-])=O)C=CC3=C2C1=NN3CCCN(C)C HZCWPKGYTCJSEB-UHFFFAOYSA-N 0.000 description 1
- OWSKEUBOCMEJMI-KPXOXKRLSA-N chembl2105946 Chemical compound [N-]=[N+]=CC(=O)CC[C@H](NC(=O)[C@@H](N)C)C(=O)N[C@H](CCC(=O)C=[N+]=[N-])C(O)=O OWSKEUBOCMEJMI-KPXOXKRLSA-N 0.000 description 1
- UKTAZPQNNNJVKR-KJGYPYNMSA-N chembl2368925 Chemical compound C1=CC=C2C(C(O[C@@H]3C[C@@H]4C[C@H]5C[C@@H](N4CC5=O)C3)=O)=CNC2=C1 UKTAZPQNNNJVKR-KJGYPYNMSA-N 0.000 description 1
- ZWVZORIKUNOTCS-OAQYLSRUSA-N chembl401930 Chemical compound C1([C@H](O)CNC2=C(C(NC=C2)=O)C=2NC=3C=C(C=C(C=3N=2)C)N2CCOCC2)=CC=CC(Cl)=C1 ZWVZORIKUNOTCS-OAQYLSRUSA-N 0.000 description 1
- DCKFXSZUWVWFEU-JECTWPLRSA-N chembl499423 Chemical compound O1[C@@H](CC)CCCC[C@]11NC(N23)=N[C@]4(O[C@H](C)CCC4)[C@@H](C(=O)OCCCCCCCCCCCCCCCC(=O)N(CCCN)C[C@@H](O)CCN)[C@@]3(O)CC[C@H]2C1 DCKFXSZUWVWFEU-JECTWPLRSA-N 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 150000004035 chlorins Chemical class 0.000 description 1
- 125000000068 chlorophenyl group Chemical group 0.000 description 1
- 229960001552 chlorprothixene Drugs 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 229960004407 chorionic gonadotrophin Drugs 0.000 description 1
- ARUGKOZUKWAXDS-SEWALLKFSA-N cicaprost Chemical compound C1\C(=C/COCC(O)=O)C[C@@H]2[C@@H](C#C[C@@H](O)[C@@H](C)CC#CCC)[C@H](O)C[C@@H]21 ARUGKOZUKWAXDS-SEWALLKFSA-N 0.000 description 1
- 229950000634 cicaprost Drugs 0.000 description 1
- 229950011359 cirolemycin Drugs 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960001653 citalopram Drugs 0.000 description 1
- JKNIRLKHOOMGOJ-UHFFFAOYSA-N cladochrome D Natural products COC1=C(CC(C)OC(=O)Oc2ccc(O)cc2)c3c4C(=C(OC)C(=O)c5c(O)cc(OC)c(c45)c6c(OC)cc(O)c(C1=O)c36)CC(C)OC(=O)c7ccc(O)cc7 JKNIRLKHOOMGOJ-UHFFFAOYSA-N 0.000 description 1
- SRJYZPCBWDVSGO-UHFFFAOYSA-N cladochrome E Natural products COC1=CC(O)=C(C(C(OC)=C(CC(C)OC(=O)OC=2C=CC(O)=CC=2)C2=3)=O)C2=C1C1=C(OC)C=C(O)C(C(C=2OC)=O)=C1C=3C=2CC(C)OC(=O)C1=CC=CC=C1 SRJYZPCBWDVSGO-UHFFFAOYSA-N 0.000 description 1
- GKIRPKYJQBWNGO-OCEACIFDSA-N clomifene Chemical class C1=CC(OCCN(CC)CC)=CC=C1C(\C=1C=CC=CC=1)=C(\Cl)C1=CC=CC=C1 GKIRPKYJQBWNGO-OCEACIFDSA-N 0.000 description 1
- 229960004606 clomipramine Drugs 0.000 description 1
- DGBIGWXXNGSACT-UHFFFAOYSA-N clonazepam Chemical compound C12=CC([N+](=O)[O-])=CC=C2NC(=O)CN=C1C1=CC=CC=C1Cl DGBIGWXXNGSACT-UHFFFAOYSA-N 0.000 description 1
- 229960003120 clonazepam Drugs 0.000 description 1
- 229960004362 clorazepate Drugs 0.000 description 1
- XDDJGVMJFWAHJX-UHFFFAOYSA-N clorazepic acid Chemical compound C12=CC(Cl)=CC=C2NC(=O)C(C(=O)O)N=C1C1=CC=CC=C1 XDDJGVMJFWAHJX-UHFFFAOYSA-N 0.000 description 1
- 229960004022 clotrimazole Drugs 0.000 description 1
- VNFPBHJOKIVQEB-UHFFFAOYSA-N clotrimazole Chemical compound ClC1=CC=CC=C1C(N1C=NC=C1)(C=1C=CC=CC=1)C1=CC=CC=C1 VNFPBHJOKIVQEB-UHFFFAOYSA-N 0.000 description 1
- GVPFVAHMJGGAJG-UHFFFAOYSA-L cobalt dichloride Chemical compound [Cl-].[Cl-].[Co+2] GVPFVAHMJGGAJG-UHFFFAOYSA-L 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 208000010877 cognitive disease Diseases 0.000 description 1
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 230000002301 combined effect Effects 0.000 description 1
- 229960005537 combretastatin A-4 Drugs 0.000 description 1
- HVXBOLULGPECHP-UHFFFAOYSA-N combretastatin A4 Natural products C1=C(O)C(OC)=CC=C1C=CC1=CC(OC)=C(OC)C(OC)=C1 HVXBOLULGPECHP-UHFFFAOYSA-N 0.000 description 1
- 150000004814 combretastatins Chemical class 0.000 description 1
- 239000007891 compressed tablet Substances 0.000 description 1
- GLESHRYLRAOJPS-DHCFDGJBSA-N conagenin Chemical compound C[C@@H](O)[C@H](C)[C@@H](O)C(=O)N[C@@](C)(CO)C(O)=O GLESHRYLRAOJPS-DHCFDGJBSA-N 0.000 description 1
- 230000037011 constitutive activity Effects 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- SBRXTSOCZITGQG-UHFFFAOYSA-N crisnatol Chemical compound C1=CC=C2C(CNC(CO)(CO)C)=CC3=C(C=CC=C4)C4=CC=C3C2=C1 SBRXTSOCZITGQG-UHFFFAOYSA-N 0.000 description 1
- 229950007258 crisnatol Drugs 0.000 description 1
- PSNOPSMXOBPNNV-VVCTWANISA-N cryptophycin 1 Chemical class C1=C(Cl)C(OC)=CC=C1C[C@@H]1C(=O)NC[C@@H](C)C(=O)O[C@@H](CC(C)C)C(=O)O[C@H]([C@H](C)[C@@H]2[C@H](O2)C=2C=CC=CC=2)C/C=C/C(=O)N1 PSNOPSMXOBPNNV-VVCTWANISA-N 0.000 description 1
- 108010090203 cryptophycin 8 Proteins 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- 125000004802 cyanophenyl group Chemical group 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000000640 cyclooctyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- PESYEWKSBIWTAK-UHFFFAOYSA-N cyclopenta-1,3-diene;titanium(2+) Chemical compound [Ti+2].C=1C=C[CH-]C=1.C=1C=C[CH-]C=1 PESYEWKSBIWTAK-UHFFFAOYSA-N 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 108010041566 cypemycin Proteins 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- YJTVZHOYBAOUTO-URBBEOKESA-N cytarabine ocfosfate Chemical compound O[C@H]1[C@H](O)[C@@H](COP(O)(=O)OCCCCCCCCCCCCCCCCCC)O[C@H]1N1C(=O)N=C(N)C=C1 YJTVZHOYBAOUTO-URBBEOKESA-N 0.000 description 1
- 229950006614 cytarabine ocfosfate Drugs 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- YCWXIQRLONXJLF-PFFGJIDWSA-N d06307 Chemical compound OS(O)(=O)=O.C([C@]1([C@@H]2O1)CC)N(CCC=1C3=CC=CC=C3NC=11)C[C@H]2C[C@]1(C(=O)OC)C1=CC([C@]23[C@H]([C@@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC.C([C@]1([C@@H]2O1)CC)N(CCC=1C3=CC=CC=C3NC=11)C[C@H]2C[C@]1(C(=O)OC)C1=CC([C@]23[C@H]([C@@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC YCWXIQRLONXJLF-PFFGJIDWSA-N 0.000 description 1
- 229960003901 dacarbazine Drugs 0.000 description 1
- 229960000640 dactinomycin Drugs 0.000 description 1
- 229960003109 daunorubicin hydrochloride Drugs 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- CYQFCXCEBYINGO-IAGOWNOFSA-N delta1-THC Chemical compound C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@@H]21 CYQFCXCEBYINGO-IAGOWNOFSA-N 0.000 description 1
- 229960003914 desipramine Drugs 0.000 description 1
- 108700025485 deslorelin Proteins 0.000 description 1
- 229960005408 deslorelin Drugs 0.000 description 1
- 125000004431 deuterium atom Chemical group 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229950001640 dexormaplatin Drugs 0.000 description 1
- VPOCYEOOFRNHNL-RQDPQJJXSA-J dexormaplatin Chemical compound Cl[Pt](Cl)(Cl)Cl.N[C@@H]1CCCC[C@H]1N VPOCYEOOFRNHNL-RQDPQJJXSA-J 0.000 description 1
- 229960000605 dexrazoxane Drugs 0.000 description 1
- SGTNSNPWRIOYBX-HHHXNRCGSA-N dexverapamil Chemical compound C1=C(OC)C(OC)=CC=C1CCN(C)CCC[C@@](C#N)(C(C)C)C1=CC=C(OC)C(OC)=C1 SGTNSNPWRIOYBX-HHHXNRCGSA-N 0.000 description 1
- 229950005878 dexverapamil Drugs 0.000 description 1
- 229950010621 dezaguanine Drugs 0.000 description 1
- NIJJYAXOARWZEE-UHFFFAOYSA-N di-n-propyl-acetic acid Natural products CCCC(C(O)=O)CCC NIJJYAXOARWZEE-UHFFFAOYSA-N 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 229960003529 diazepam Drugs 0.000 description 1
- AAOVKJBEBIDNHE-UHFFFAOYSA-N diazepam Chemical compound N=1CC(=O)N(C)C2=CC=C(Cl)C=C2C=1C1=CC=CC=C1 AAOVKJBEBIDNHE-UHFFFAOYSA-N 0.000 description 1
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 1
- WGLUMOCWFMKWIL-UHFFFAOYSA-N dichloromethane;methanol Chemical compound OC.ClCCl WGLUMOCWFMKWIL-UHFFFAOYSA-N 0.000 description 1
- KYHUYMLIVQFXRI-UHFFFAOYSA-N didemnin B Natural products CC1OC(=O)C(CC=2C=CC(OC)=CC=2)N(C)C(=O)C2CCCN2C(=O)C(CC(C)C)NC(=O)C(C)C(=O)C(C(C)C)OC(=O)CC(O)C(C(C)CC)NC(=O)C1NC(=O)C(CC(C)C)N(C)C(=O)C1CCCN1C(=O)C(C)O KYHUYMLIVQFXRI-UHFFFAOYSA-N 0.000 description 1
- 108010061297 didemnins Proteins 0.000 description 1
- PZXJOHSZQAEJFE-UHFFFAOYSA-N dihydrobetulinic acid Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C(O)=O)CCC(C(C)C)C5C4CCC3C21C PZXJOHSZQAEJFE-UHFFFAOYSA-N 0.000 description 1
- 125000005045 dihydroisoquinolinyl group Chemical group C1(NC=CC2=CC=CC=C12)* 0.000 description 1
- 125000005044 dihydroquinolinyl group Chemical group N1(CC=CC2=CC=CC=C12)* 0.000 description 1
- 125000000597 dioxinyl group Chemical group 0.000 description 1
- 150000002016 disaccharides Chemical class 0.000 description 1
- CZLKTMHQYXYHOO-QTNFYWBSSA-L disodium;(2s)-2-[(2-phosphonatoacetyl)amino]butanedioic acid Chemical compound [Na+].[Na+].OC(=O)C[C@@H](C(O)=O)NC(=O)CP([O-])([O-])=O CZLKTMHQYXYHOO-QTNFYWBSSA-L 0.000 description 1
- SVJSWELRJWVPQD-KJWOGLQMSA-L disodium;(2s)-2-[[4-[2-[(6r)-2-amino-4-oxo-5,6,7,8-tetrahydro-1h-pyrido[2,3-d]pyrimidin-6-yl]ethyl]benzoyl]amino]pentanedioate Chemical compound [Na+].[Na+].C([C@@H]1CC=2C(=O)N=C(NC=2NC1)N)CC1=CC=C(C(=O)N[C@@H](CCC([O-])=O)C([O-])=O)C=C1 SVJSWELRJWVPQD-KJWOGLQMSA-L 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 229960000735 docosanol Drugs 0.000 description 1
- 229960003413 dolasetron Drugs 0.000 description 1
- 229960003530 donepezil Drugs 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- ZWAOHEXOSAUJHY-ZIYNGMLESA-N doxifluridine Chemical compound O[C@@H]1[C@H](O)[C@@H](C)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ZWAOHEXOSAUJHY-ZIYNGMLESA-N 0.000 description 1
- 229950005454 doxifluridine Drugs 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 229960002918 doxorubicin hydrochloride Drugs 0.000 description 1
- NOTIQUSPUUHHEH-UXOVVSIBSA-N dromostanolone propionate Chemical compound C([C@@H]1CC2)C(=O)[C@H](C)C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](OC(=O)CC)[C@@]2(C)CC1 NOTIQUSPUUHHEH-UXOVVSIBSA-N 0.000 description 1
- 229960004242 dronabinol Drugs 0.000 description 1
- 229960000394 droperidol Drugs 0.000 description 1
- RMEDXOLNCUSCGS-UHFFFAOYSA-N droperidol Chemical compound C1=CC(F)=CC=C1C(=O)CCCN1CC=C(N2C(NC3=CC=CC=C32)=O)CC1 RMEDXOLNCUSCGS-UHFFFAOYSA-N 0.000 description 1
- 229950004683 drostanolone propionate Drugs 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 229950005133 duazomycin Drugs 0.000 description 1
- 229930192837 duazomycin Natural products 0.000 description 1
- VQNATVDKACXKTF-XELLLNAOSA-N duocarmycin Chemical compound COC1=C(OC)C(OC)=C2NC(C(=O)N3C4=CC(=O)C5=C([C@@]64C[C@@H]6C3)C=C(N5)C(=O)OC)=CC2=C1 VQNATVDKACXKTF-XELLLNAOSA-N 0.000 description 1
- 229960005510 duocarmycin SA Drugs 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 229950010033 ebselen Drugs 0.000 description 1
- 229950005678 ecomustine Drugs 0.000 description 1
- 229950006700 edatrexate Drugs 0.000 description 1
- FSIRXIHZBIXHKT-MHTVFEQDSA-N edatrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CC(CC)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FSIRXIHZBIXHKT-MHTVFEQDSA-N 0.000 description 1
- 229950011461 edelfosine Drugs 0.000 description 1
- 229960001776 edrecolomab Drugs 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- VLCYCQAOQCDTCN-UHFFFAOYSA-N eflornithine Chemical compound NCCCC(N)(C(F)F)C(O)=O VLCYCQAOQCDTCN-UHFFFAOYSA-N 0.000 description 1
- 229960002759 eflornithine Drugs 0.000 description 1
- 229960002046 eflornithine hydrochloride Drugs 0.000 description 1
- 230000003028 elevating effect Effects 0.000 description 1
- MGQRRMONVLMKJL-KWJIQSIXSA-N elsamitrucin Chemical compound O1[C@H](C)[C@H](O)[C@H](OC)[C@@H](N)[C@H]1O[C@@H]1[C@](O)(C)[C@@H](O)[C@@H](C)O[C@H]1OC1=CC=CC2=C(O)C(C(O3)=O)=C4C5=C3C=CC(C)=C5C(=O)OC4=C12 MGQRRMONVLMKJL-KWJIQSIXSA-N 0.000 description 1
- 229950002339 elsamitrucin Drugs 0.000 description 1
- 229950005450 emitefur Drugs 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 230000002124 endocrine Effects 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- JOZGNYDSEBIJDH-UHFFFAOYSA-N eniluracil Chemical compound O=C1NC=C(C#C)C(=O)N1 JOZGNYDSEBIJDH-UHFFFAOYSA-N 0.000 description 1
- 229950010625 enloplatin Drugs 0.000 description 1
- 229950001022 enpromate Drugs 0.000 description 1
- JRURYQJSLYLRLN-BJMVGYQFSA-N entacapone Chemical compound CCN(CC)C(=O)C(\C#N)=C\C1=CC(O)=C(O)C([N+]([O-])=O)=C1 JRURYQJSLYLRLN-BJMVGYQFSA-N 0.000 description 1
- 229960003337 entacapone Drugs 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 229950004926 epipropidine Drugs 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- 229960003265 epirubicin hydrochloride Drugs 0.000 description 1
- 229950009537 epristeride Drugs 0.000 description 1
- 229950001426 erbulozole Drugs 0.000 description 1
- KLEPCGBEXOCIGS-QPPBQGQZSA-N erbulozole Chemical compound C1=CC(NC(=O)OCC)=CC=C1SC[C@@H]1O[C@@](CN2C=NC=C2)(C=2C=CC(OC)=CC=2)OC1 KLEPCGBEXOCIGS-QPPBQGQZSA-N 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 229960001842 estramustine Drugs 0.000 description 1
- 229960001766 estramustine phosphate sodium Drugs 0.000 description 1
- IIUMCNJTGSMNRO-VVSKJQCTSA-L estramustine sodium phosphate Chemical compound [Na+].[Na+].ClCCN(CCCl)C(=O)OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)OP([O-])([O-])=O)[C@@H]4[C@@H]3CCC2=C1 IIUMCNJTGSMNRO-VVSKJQCTSA-L 0.000 description 1
- HYSIJEPDMLSIQJ-UHFFFAOYSA-N ethanolate;1-phenylbutane-1,3-dione;titanium(4+) Chemical compound [Ti+4].CC[O-].CC[O-].CC(=O)[CH-]C(=O)C1=CC=CC=C1.CC(=O)[CH-]C(=O)C1=CC=CC=C1 HYSIJEPDMLSIQJ-UHFFFAOYSA-N 0.000 description 1
- XPGDODOEEWLHOI-GSDHBNRESA-N ethyl (2s)-2-[[(2s)-2-[[(2s)-2-amino-3-(4-fluorophenyl)propanoyl]amino]-3-[3-[bis(2-chloroethyl)amino]phenyl]propanoyl]amino]-4-methylsulfanylbutanoate Chemical compound C([C@@H](C(=O)N[C@@H](CCSC)C(=O)OCC)NC(=O)[C@@H](N)CC=1C=CC(F)=CC=1)C1=CC=CC(N(CCCl)CCCl)=C1 XPGDODOEEWLHOI-GSDHBNRESA-N 0.000 description 1
- ADCTYGVBLFHKDL-UHFFFAOYSA-N ethyl 3-[2-(2,2-dimethylpropanoylamino)-6-(4-phenylmethoxybutoxy)pyridin-3-yl]prop-2-enoate Chemical compound N1=C(NC(=O)C(C)(C)C)C(C=CC(=O)OCC)=CC=C1OCCCCOCC1=CC=CC=C1 ADCTYGVBLFHKDL-UHFFFAOYSA-N 0.000 description 1
- FCEYOLCDAIZMCL-UHFFFAOYSA-N ethyl 3-[2-(2,2-dimethylpropanoylamino)-6-(4-phenylmethoxybutoxy)pyridin-3-yl]propanoate Chemical compound N1=C(NC(=O)C(C)(C)C)C(CCC(=O)OCC)=CC=C1OCCCCOCC1=CC=CC=C1 FCEYOLCDAIZMCL-UHFFFAOYSA-N 0.000 description 1
- RIFGWPKJUGCATF-UHFFFAOYSA-N ethyl chloroformate Chemical compound CCOC(Cl)=O RIFGWPKJUGCATF-UHFFFAOYSA-N 0.000 description 1
- HZQPPNNARUQMJA-IMIWJGOWSA-N ethyl n-[4-[[(2r,4r)-2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methylsulfanyl]phenyl]carbamate;hydrochloride Chemical compound Cl.C1=CC(NC(=O)OCC)=CC=C1SC[C@@H]1O[C@@](CN2C=NC=C2)(C=2C(=CC(Cl)=CC=2)Cl)OC1 HZQPPNNARUQMJA-IMIWJGOWSA-N 0.000 description 1
- ISVXIZFUEUVXPG-UHFFFAOYSA-N etiopurpurin Chemical compound CC1C2(CC)C(C(=O)OCC)=CC(C3=NC(C(=C3C)CC)=C3)=C2N=C1C=C(N1)C(CC)=C(C)C1=CC1=C(CC)C(C)=C3N1 ISVXIZFUEUVXPG-UHFFFAOYSA-N 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 238000000105 evaporative light scattering detection Methods 0.000 description 1
- 229960000255 exemestane Drugs 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 229960004177 filgrastim Drugs 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- DBEPLOCGEIEOCV-WSBQPABSSA-N finasteride Chemical compound N([C@@H]1CC2)C(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](C(=O)NC(C)(C)C)[C@@]2(C)CC1 DBEPLOCGEIEOCV-WSBQPABSSA-N 0.000 description 1
- 229960004039 finasteride Drugs 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- 229950006000 flezelastine Drugs 0.000 description 1
- ODKNJVUHOIMIIZ-RRKCRQDMSA-N floxuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ODKNJVUHOIMIIZ-RRKCRQDMSA-N 0.000 description 1
- 229960000961 floxuridine Drugs 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- 229960005304 fludarabine phosphate Drugs 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 125000001207 fluorophenyl group Chemical group 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 229960002690 fluphenazine Drugs 0.000 description 1
- 229950005682 flurocitabine Drugs 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 229950004217 forfenimex Drugs 0.000 description 1
- 229960004421 formestane Drugs 0.000 description 1
- OSVMTWJCGUFAOD-KZQROQTASA-N formestane Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CCC2=C1O OSVMTWJCGUFAOD-KZQROQTASA-N 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- UXTSQCOOUJTIAC-UHFFFAOYSA-N fosquidone Chemical compound C=1N2CC3=CC=CC=C3C(C)C2=C(C(C2=CC=C3)=O)C=1C(=O)C2=C3OP(O)(=O)OCC1=CC=CC=C1 UXTSQCOOUJTIAC-UHFFFAOYSA-N 0.000 description 1
- 229950005611 fosquidone Drugs 0.000 description 1
- 229950010404 fostriecin Drugs 0.000 description 1
- 229960004783 fotemustine Drugs 0.000 description 1
- YAKWPXVTIGTRJH-UHFFFAOYSA-N fotemustine Chemical compound CCOP(=O)(OCC)C(C)NC(=O)N(CCCl)N=O YAKWPXVTIGTRJH-UHFFFAOYSA-N 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 229960002870 gabapentin Drugs 0.000 description 1
- 229960003980 galantamine Drugs 0.000 description 1
- ASUTZQLVASHGKV-UHFFFAOYSA-N galanthamine hydrochloride Natural products O1C(=C23)C(OC)=CC=C2CN(C)CCC23C1CC(O)C=C2 ASUTZQLVASHGKV-UHFFFAOYSA-N 0.000 description 1
- 229940044658 gallium nitrate Drugs 0.000 description 1
- 229950004410 galocitabine Drugs 0.000 description 1
- 108700032141 ganirelix Proteins 0.000 description 1
- GJNXBNATEDXMAK-PFLSVRRQSA-N ganirelix Chemical compound C([C@@H](C(=O)N[C@H](CCCCN=C(NCC)NCC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN=C(NCC)NCC)C(=O)N1[C@@H](CCC1)C(=O)N[C@H](C)C(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](CC=1C=NC=CC=1)NC(=O)[C@@H](CC=1C=CC(Cl)=CC=1)NC(=O)[C@@H](CC=1C=C2C=CC=CC2=CC=1)NC(C)=O)C1=CC=C(O)C=C1 GJNXBNATEDXMAK-PFLSVRRQSA-N 0.000 description 1
- 229960003794 ganirelix Drugs 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000002406 gelatinase inhibitor Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 229960005144 gemcitabine hydrochloride Drugs 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 229960003180 glutathione Drugs 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 229960002158 halazepam Drugs 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 208000018578 heart valve disease Diseases 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 239000008241 heterogeneous mixture Substances 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 150000002430 hydrocarbons Chemical group 0.000 description 1
- 229910000043 hydrogen iodide Inorganic materials 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N hydrogen thiocyanate Natural products SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 1
- 229940071870 hydroiodic acid Drugs 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- SOCGJDYHNGLZEC-UHFFFAOYSA-N hydron;n-methyl-n-[4-[(7-methyl-3h-imidazo[4,5-f]quinolin-9-yl)amino]phenyl]acetamide;chloride Chemical compound Cl.C1=CC(N(C(C)=O)C)=CC=C1NC1=CC(C)=NC2=CC=C(NC=N3)C3=C12 SOCGJDYHNGLZEC-UHFFFAOYSA-N 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 229920013821 hydroxy alkyl cellulose Polymers 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 229960000930 hydroxyzine Drugs 0.000 description 1
- ZQDWXGKKHFNSQK-UHFFFAOYSA-N hydroxyzine Chemical compound C1CN(CCOCCO)CCN1C(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 ZQDWXGKKHFNSQK-UHFFFAOYSA-N 0.000 description 1
- 201000001421 hyperglycemia Diseases 0.000 description 1
- MPGWGYQTRSNGDD-UHFFFAOYSA-N hypericin Chemical compound OC1=CC(O)=C(C2=O)C3=C1C1C(O)=CC(=O)C(C4=O)=C1C1=C3C3=C2C(O)=CC(C)=C3C2=C1C4=C(O)C=C2C MPGWGYQTRSNGDD-UHFFFAOYSA-N 0.000 description 1
- 229940005608 hypericin Drugs 0.000 description 1
- PHOKTTKFQUYZPI-UHFFFAOYSA-N hypericin Natural products Cc1cc(O)c2c3C(=O)C(=Cc4c(O)c5c(O)cc(O)c6c7C(=O)C(=Cc8c(C)c1c2c(c78)c(c34)c56)O)O PHOKTTKFQUYZPI-UHFFFAOYSA-N 0.000 description 1
- 229960005236 ibandronic acid Drugs 0.000 description 1
- 229960000908 idarubicin Drugs 0.000 description 1
- 229960001176 idarubicin hydrochloride Drugs 0.000 description 1
- 229950002248 idoxifene Drugs 0.000 description 1
- TZBDEVBNMSLVKT-UHFFFAOYSA-N idramantone Chemical compound C1C(C2)CC3CC1(O)CC2C3=O TZBDEVBNMSLVKT-UHFFFAOYSA-N 0.000 description 1
- 229950009926 idramantone Drugs 0.000 description 1
- NITYDPDXAAFEIT-DYVFJYSZSA-N ilomastat Chemical compound C1=CC=C2C(C[C@@H](C(=O)NC)NC(=O)[C@H](CC(C)C)CC(=O)NO)=CNC2=C1 NITYDPDXAAFEIT-DYVFJYSZSA-N 0.000 description 1
- 229960003696 ilomastat Drugs 0.000 description 1
- 229960003162 iloperidone Drugs 0.000 description 1
- XMXHEBAFVSFQEX-UHFFFAOYSA-N iloperidone Chemical compound COC1=CC(C(C)=O)=CC=C1OCCCN1CCC(C=2C3=CC=C(F)C=C3ON=2)CC1 XMXHEBAFVSFQEX-UHFFFAOYSA-N 0.000 description 1
- 238000010191 image analysis Methods 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 150000002466 imines Chemical class 0.000 description 1
- BCGWQEUPMDMJNV-UHFFFAOYSA-N imipramine Chemical compound C1CC2=CC=CC=C2N(CCCN(C)C)C2=CC=CC=C21 BCGWQEUPMDMJNV-UHFFFAOYSA-N 0.000 description 1
- 229960004801 imipramine Drugs 0.000 description 1
- 229960002751 imiquimod Drugs 0.000 description 1
- DOUYETYNHWVLEO-UHFFFAOYSA-N imiquimod Chemical compound C1=CC=CC2=C3N(CC(C)C)C=NC3=C(N)N=C21 DOUYETYNHWVLEO-UHFFFAOYSA-N 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 229960001438 immunostimulant agent Drugs 0.000 description 1
- 239000003022 immunostimulating agent Substances 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 125000001041 indolyl group Chemical group 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 102000028416 insulin-like growth factor binding Human genes 0.000 description 1
- 108091022911 insulin-like growth factor binding Proteins 0.000 description 1
- 229960003521 interferon alfa-2a Drugs 0.000 description 1
- 229960003507 interferon alfa-2b Drugs 0.000 description 1
- 229940109242 interferon alfa-n3 Drugs 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- VBUWHHLIZKOSMS-RIWXPGAOSA-N invicorp Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)C(C)C)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=C(O)C=C1 VBUWHHLIZKOSMS-RIWXPGAOSA-N 0.000 description 1
- 229960003795 iobenguane (123i) Drugs 0.000 description 1
- 229950010897 iproplatin Drugs 0.000 description 1
- 229960000779 irinotecan hydrochloride Drugs 0.000 description 1
- GURKHSYORGJETM-WAQYZQTGSA-N irinotecan hydrochloride (anhydrous) Chemical compound Cl.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 GURKHSYORGJETM-WAQYZQTGSA-N 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 229950000855 iroplact Drugs 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 229950010984 irsogladine Drugs 0.000 description 1
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 229960002672 isocarboxazid Drugs 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- RWXRJSRJIITQAK-ZSBIGDGJSA-N itasetron Chemical compound C12=CC=CC=C2NC(=O)N1C(=O)N[C@H](C1)C[C@H]2CC[C@@H]1N2C RWXRJSRJIITQAK-ZSBIGDGJSA-N 0.000 description 1
- 229950007654 itasetron Drugs 0.000 description 1
- GQWYWHOHRVVHAP-DHKPLNAMSA-N jaspamide Chemical compound C1([C@@H]2NC(=O)[C@@H](CC=3C4=CC=CC=C4NC=3Br)N(C)C(=O)[C@H](C)NC(=O)[C@@H](C)C/C(C)=C/[C@H](C)C[C@@H](OC(=O)C2)C)=CC=C(O)C=C1 GQWYWHOHRVVHAP-DHKPLNAMSA-N 0.000 description 1
- 108010052440 jasplakinolide Proteins 0.000 description 1
- GQWYWHOHRVVHAP-UHFFFAOYSA-N jasplakinolide Natural products C1C(=O)OC(C)CC(C)C=C(C)CC(C)C(=O)NC(C)C(=O)N(C)C(CC=2C3=CC=CC=C3NC=2Br)C(=O)NC1C1=CC=C(O)C=C1 GQWYWHOHRVVHAP-UHFFFAOYSA-N 0.000 description 1
- 108010091711 kahalalide F Proteins 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 229940001447 lactate Drugs 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 229960001848 lamotrigine Drugs 0.000 description 1
- PYZRQGJRPPTADH-UHFFFAOYSA-N lamotrigine Chemical compound NC1=NC(N)=NN=C1C1=CC=CC(Cl)=C1Cl PYZRQGJRPPTADH-UHFFFAOYSA-N 0.000 description 1
- 229960002437 lanreotide Drugs 0.000 description 1
- 229960001739 lanreotide acetate Drugs 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 229960002618 lenograstim Drugs 0.000 description 1
- 229940115286 lentinan Drugs 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- KDQAABAKXDWYSZ-SDCRJXSCSA-N leurosidine sulfate Chemical compound OS(O)(=O)=O.C([C@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 KDQAABAKXDWYSZ-SDCRJXSCSA-N 0.000 description 1
- 229960001614 levamisole Drugs 0.000 description 1
- 229960004002 levetiracetam Drugs 0.000 description 1
- HPHUVLMMVZITSG-ZCFIWIBFSA-N levetiracetam Chemical compound CC[C@H](C(N)=O)N1CCCC1=O HPHUVLMMVZITSG-ZCFIWIBFSA-N 0.000 description 1
- 229960004502 levodopa Drugs 0.000 description 1
- UGFHIPBXIWJXNA-UHFFFAOYSA-N liarozole Chemical compound ClC1=CC=CC(C(C=2C=C3NC=NC3=CC=2)N2C=NC=C2)=C1 UGFHIPBXIWJXNA-UHFFFAOYSA-N 0.000 description 1
- 229950007056 liarozole Drugs 0.000 description 1
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 1
- 108010020270 lissoclinamide 7 Proteins 0.000 description 1
- RBBBWKUBQVARPL-SWQMWMPHSA-N lissoclinamide 7 Chemical compound C([C@H]1C(=O)N2CCC[C@H]2C2=N[C@@H]([C@H](O2)C)C(=O)N[C@@H](C=2SC[C@H](N=2)C(=O)N[C@H](CC=2C=CC=CC=2)C=2SC[C@H](N=2)C(=O)N1)C(C)C)C1=CC=CC=C1 RBBBWKUBQVARPL-SWQMWMPHSA-N 0.000 description 1
- RBBBWKUBQVARPL-UHFFFAOYSA-N lissoclinamide 7 Natural products N1C(=O)C(N=2)CSC=2C(CC=2C=CC=CC=2)NC(=O)C(N=2)CSC=2C(C(C)C)NC(=O)C(C(O2)C)N=C2C2CCCN2C(=O)C1CC1=CC=CC=C1 RBBBWKUBQVARPL-UHFFFAOYSA-N 0.000 description 1
- 229960001078 lithium Drugs 0.000 description 1
- 229950008991 lobaplatin Drugs 0.000 description 1
- 229950000909 lometrexol Drugs 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- 229960003538 lonidamine Drugs 0.000 description 1
- WDRYRZXSPDWGEB-UHFFFAOYSA-N lonidamine Chemical compound C12=CC=CC=C2C(C(=O)O)=NN1CC1=CC=C(Cl)C=C1Cl WDRYRZXSPDWGEB-UHFFFAOYSA-N 0.000 description 1
- 229960004391 lorazepam Drugs 0.000 description 1
- YROQEQPFUCPDCP-UHFFFAOYSA-N losoxantrone Chemical compound OCCNCCN1N=C2C3=CC=CC(O)=C3C(=O)C3=C2C1=CC=C3NCCNCCO YROQEQPFUCPDCP-UHFFFAOYSA-N 0.000 description 1
- 229950008745 losoxantrone Drugs 0.000 description 1
- XDMHALQMTPSGEA-UHFFFAOYSA-N losoxantrone hydrochloride Chemical compound Cl.Cl.OCCNCCN1N=C2C3=CC=CC(O)=C3C(=O)C3=C2C1=CC=C3NCCNCCO XDMHALQMTPSGEA-UHFFFAOYSA-N 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- PCZOHLXUXFIOCF-BXMDZJJMSA-N lovastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 PCZOHLXUXFIOCF-BXMDZJJMSA-N 0.000 description 1
- 229960004844 lovastatin Drugs 0.000 description 1
- QLJODMDSTUBWDW-UHFFFAOYSA-N lovastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(C)C=C21 QLJODMDSTUBWDW-UHFFFAOYSA-N 0.000 description 1
- 229960000423 loxapine Drugs 0.000 description 1
- XJGVXQDUIWGIRW-UHFFFAOYSA-N loxapine Chemical compound C1CN(C)CCN1C1=NC2=CC=CC=C2OC2=CC=C(Cl)C=C12 XJGVXQDUIWGIRW-UHFFFAOYSA-N 0.000 description 1
- 229950005634 loxoribine Drugs 0.000 description 1
- RVFGKBWWUQOIOU-NDEPHWFRSA-N lurtotecan Chemical compound O=C([C@]1(O)CC)OCC(C(N2CC3=4)=O)=C1C=C2C3=NC1=CC=2OCCOC=2C=C1C=4CN1CCN(C)CC1 RVFGKBWWUQOIOU-NDEPHWFRSA-N 0.000 description 1
- 229950002654 lurtotecan Drugs 0.000 description 1
- 230000002101 lytic effect Effects 0.000 description 1
- NCBZRJODKRCREW-UHFFFAOYSA-N m-anisidine Chemical compound COC1=CC=CC(N)=C1 NCBZRJODKRCREW-UHFFFAOYSA-N 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 229950001474 maitansine Drugs 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- BLOFGONIVNXZME-YDMGZANHSA-N mannostatin A Chemical compound CS[C@@H]1[C@@H](N)[C@@H](O)[C@@H](O)[C@H]1O BLOFGONIVNXZME-YDMGZANHSA-N 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 229950008959 marimastat Drugs 0.000 description 1
- OCSMOTCMPXTDND-OUAUKWLOSA-N marimastat Chemical compound CNC(=O)[C@H](C(C)(C)C)NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO OCSMOTCMPXTDND-OUAUKWLOSA-N 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- 239000003771 matrix metalloproteinase inhibitor Substances 0.000 description 1
- 229940121386 matrix metalloproteinase inhibitor Drugs 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- QZIQJVCYUQZDIR-UHFFFAOYSA-N mechlorethamine hydrochloride Chemical compound Cl.ClCCN(C)CCCl QZIQJVCYUQZDIR-UHFFFAOYSA-N 0.000 description 1
- 229960002868 mechlorethamine hydrochloride Drugs 0.000 description 1
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 1
- 229960004296 megestrol acetate Drugs 0.000 description 1
- 229960003846 melengestrol acetate Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 230000004630 mental health Effects 0.000 description 1
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 229960000300 mesoridazine Drugs 0.000 description 1
- SLVMESMUVMCQIY-UHFFFAOYSA-N mesoridazine Chemical compound CN1CCCCC1CCN1C2=CC(S(C)=O)=CC=C2SC2=CC=CC=C21 SLVMESMUVMCQIY-UHFFFAOYSA-N 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 108700025096 meterelin Proteins 0.000 description 1
- KPQJSSLKKBKWEW-RKDOVGOJSA-N methanesulfonic acid;5-nitro-2-[(2r)-1-[2-[[(2r)-2-(5-nitro-1,3-dioxobenzo[de]isoquinolin-2-yl)propyl]amino]ethylamino]propan-2-yl]benzo[de]isoquinoline-1,3-dione Chemical compound CS(O)(=O)=O.CS(O)(=O)=O.[O-][N+](=O)C1=CC(C(N([C@@H](CNCCNC[C@@H](C)N2C(C=3C=C(C=C4C=CC=C(C=34)C2=O)[N+]([O-])=O)=O)C)C2=O)=O)=C3C2=CC=CC3=C1 KPQJSSLKKBKWEW-RKDOVGOJSA-N 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- BKBBTCORRZMASO-ZOWNYOTGSA-M methotrexate monosodium Chemical compound [Na+].C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C([O-])=O)C=C1 BKBBTCORRZMASO-ZOWNYOTGSA-M 0.000 description 1
- 229960003058 methotrexate sodium Drugs 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- 229960001344 methylphenidate Drugs 0.000 description 1
- 229960004584 methylprednisolone Drugs 0.000 description 1
- 229960004503 metoclopramide Drugs 0.000 description 1
- TTWJBBZEZQICBI-UHFFFAOYSA-N metoclopramide Chemical compound CCN(CC)CCNC(=O)C1=CC(Cl)=C(N)C=C1OC TTWJBBZEZQICBI-UHFFFAOYSA-N 0.000 description 1
- VQJHOPSWBGJHQS-UHFFFAOYSA-N metoprine, methodichlorophen Chemical compound CC1=NC(N)=NC(N)=C1C1=CC=C(Cl)C(Cl)=C1 VQJHOPSWBGJHQS-UHFFFAOYSA-N 0.000 description 1
- QTFKTBRIGWJQQL-UHFFFAOYSA-N meturedepa Chemical compound C1C(C)(C)N1P(=O)(NC(=O)OCC)N1CC1(C)C QTFKTBRIGWJQQL-UHFFFAOYSA-N 0.000 description 1
- 229950009847 meturedepa Drugs 0.000 description 1
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- BMGQWWVMWDBQGC-IIFHNQTCSA-N midostaurin Chemical class CN([C@H]1[C@H]([C@]2(C)O[C@@H](N3C4=CC=CC=C4C4=C5C(=O)NCC5=C5C6=CC=CC=C6N2C5=C43)C1)OC)C(=O)C1=CC=CC=C1 BMGQWWVMWDBQGC-IIFHNQTCSA-N 0.000 description 1
- VKHAHZOOUSRJNA-GCNJZUOMSA-N mifepristone Chemical compound C1([C@@H]2C3=C4CCC(=O)C=C4CC[C@H]3[C@@H]3CC[C@@]([C@]3(C2)C)(O)C#CC)=CC=C(N(C)C)C=C1 VKHAHZOOUSRJNA-GCNJZUOMSA-N 0.000 description 1
- 229960003248 mifepristone Drugs 0.000 description 1
- 229960003775 miltefosine Drugs 0.000 description 1
- PQLXHQMOHUQAKB-UHFFFAOYSA-N miltefosine Chemical compound CCCCCCCCCCCCCCCCOP([O-])(=O)OCC[N+](C)(C)C PQLXHQMOHUQAKB-UHFFFAOYSA-N 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 229950008541 mirimostim Drugs 0.000 description 1
- CFCUWKMKBJTWLW-BKHRDMLASA-N mithramycin Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@H](O)[C@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@@H](O)[C@H](O)[C@@H](C)O1 CFCUWKMKBJTWLW-BKHRDMLASA-N 0.000 description 1
- DRCJGCOYHLTVNR-ZUIZSQJWSA-N mitindomide Chemical compound C1=C[C@@H]2[C@@H]3[C@H]4C(=O)NC(=O)[C@H]4[C@@H]3[C@H]1[C@@H]1C(=O)NC(=O)[C@H]21 DRCJGCOYHLTVNR-ZUIZSQJWSA-N 0.000 description 1
- 229950001314 mitindomide Drugs 0.000 description 1
- 229950002137 mitocarcin Drugs 0.000 description 1
- 229950000911 mitogillin Drugs 0.000 description 1
- 229960003539 mitoguazone Drugs 0.000 description 1
- MXWHMTNPTTVWDM-NXOFHUPFSA-N mitoguazone Chemical compound NC(N)=N\N=C(/C)\C=N\N=C(N)N MXWHMTNPTTVWDM-NXOFHUPFSA-N 0.000 description 1
- VFKZTMPDYBFSTM-GUCUJZIJSA-N mitolactol Chemical compound BrC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-GUCUJZIJSA-N 0.000 description 1
- 229950010913 mitolactol Drugs 0.000 description 1
- 229950007612 mitomalcin Drugs 0.000 description 1
- 108010026677 mitomalcin Proteins 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 229950001745 mitonafide Drugs 0.000 description 1
- 229950005715 mitosper Drugs 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- ZAHQPTJLOCWVPG-UHFFFAOYSA-N mitoxantrone dihydrochloride Chemical compound Cl.Cl.O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO ZAHQPTJLOCWVPG-UHFFFAOYSA-N 0.000 description 1
- 229960004169 mitoxantrone hydrochloride Drugs 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 229960001165 modafinil Drugs 0.000 description 1
- 229950008012 mofarotene Drugs 0.000 description 1
- 239000007932 molded tablet Substances 0.000 description 1
- VOWOEBADKMXUBU-UHFFFAOYSA-J molecular oxygen;tetrachlorite;hydrate Chemical compound O.O=O.[O-]Cl=O.[O-]Cl=O.[O-]Cl=O.[O-]Cl=O VOWOEBADKMXUBU-UHFFFAOYSA-J 0.000 description 1
- 229960003063 molgramostim Drugs 0.000 description 1
- 108010032806 molgramostim Proteins 0.000 description 1
- 229940035032 monophosphoryl lipid a Drugs 0.000 description 1
- 230000036651 mood Effects 0.000 description 1
- FOYWNSCCNCUEPU-UHFFFAOYSA-N mopidamol Chemical compound C12=NC(N(CCO)CCO)=NC=C2N=C(N(CCO)CCO)N=C1N1CCCCC1 FOYWNSCCNCUEPU-UHFFFAOYSA-N 0.000 description 1
- 229950010718 mopidamol Drugs 0.000 description 1
- 230000001002 morphogenetic effect Effects 0.000 description 1
- AARXZCZYLAFQQU-UHFFFAOYSA-N motexafin gadolinium Chemical compound [Gd].CC(O)=O.CC(O)=O.C1=C([N-]2)C(CC)=C(CC)C2=CC(C(=C2C)CCCO)=NC2=CN=C2C=C(OCCOCCOCCOC)C(OCCOCCOCCOC)=CC2=NC=C2C(C)=C(CCCO)C1=N2 AARXZCZYLAFQQU-UHFFFAOYSA-N 0.000 description 1
- WIQKYZYFTAEWBF-UHFFFAOYSA-L motexafin lutetium hydrate Chemical compound O.[Lu+3].CC([O-])=O.CC([O-])=O.C1=C([N-]2)C(CC)=C(CC)C2=CC(C(=C2C)CCCO)=NC2=CN=C2C=C(OCCOCCOCCOC)C(OCCOCCOCCOC)=CC2=NC=C2C(C)=C(CCCO)C1=N2 WIQKYZYFTAEWBF-UHFFFAOYSA-L 0.000 description 1
- 235000010460 mustard Nutrition 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- PAVKBQLPQCDVNI-UHFFFAOYSA-N n',n'-diethyl-n-(9-methoxy-5,11-dimethyl-6h-pyrido[4,3-b]carbazol-1-yl)propane-1,3-diamine Chemical compound N1C2=CC=C(OC)C=C2C2=C1C(C)=C1C=CN=C(NCCCN(CC)CC)C1=C2C PAVKBQLPQCDVNI-UHFFFAOYSA-N 0.000 description 1
- QTIICTFBNRXNRF-UHFFFAOYSA-N n-(6-chloro-3-formylpyridin-2-yl)-2,2-dimethylpropanamide Chemical compound CC(C)(C)C(=O)NC1=NC(Cl)=CC=C1C=O QTIICTFBNRXNRF-UHFFFAOYSA-N 0.000 description 1
- YPWKLBCTOUEZKE-UHFFFAOYSA-N n-(6-chloropyridin-2-yl)-2,2-dimethylpropanamide Chemical compound CC(C)(C)C(=O)NC1=CC=CC(Cl)=N1 YPWKLBCTOUEZKE-UHFFFAOYSA-N 0.000 description 1
- CRJGESKKUOMBCT-PMACEKPBSA-N n-[(2s,3s)-1,3-dihydroxyoctadecan-2-yl]acetamide Chemical compound CCCCCCCCCCCCCCC[C@H](O)[C@H](CO)NC(C)=O CRJGESKKUOMBCT-PMACEKPBSA-N 0.000 description 1
- NKFHKYQGZDAKMX-PPRKPIOESA-N n-[(e)-1-[(2s,4s)-4-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-3,4-dihydro-1h-tetracen-2-yl]ethylideneamino]benzamide;hydrochloride Chemical compound Cl.O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(\C)=N\NC(=O)C=1C=CC=CC=1)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 NKFHKYQGZDAKMX-PPRKPIOESA-N 0.000 description 1
- TVYPSLDUBVTDIS-FUOMVGGVSA-N n-[1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxopyrimidin-4-yl]-3,4,5-trimethoxybenzamide Chemical compound COC1=C(OC)C(OC)=CC(C(=O)NC=2C(=CN(C(=O)N=2)[C@H]2[C@@H]([C@H](O)[C@@H](C)O2)O)F)=C1 TVYPSLDUBVTDIS-FUOMVGGVSA-N 0.000 description 1
- OUIJVCLVIVBGEK-UHFFFAOYSA-N n-[3-formyl-6-(4-phenylmethoxybutoxy)pyridin-2-yl]-2,2-dimethylpropanamide Chemical compound C1=C(C=O)C(NC(=O)C(C)(C)C)=NC(OCCCCOCC=2C=CC=CC=2)=C1 OUIJVCLVIVBGEK-UHFFFAOYSA-N 0.000 description 1
- ARKYUICTMUZVEW-UHFFFAOYSA-N n-[5-[[5-[(3-amino-3-iminopropyl)carbamoyl]-1-methylpyrrol-3-yl]carbamoyl]-1-methylpyrrol-3-yl]-4-[[4-[bis(2-chloroethyl)amino]benzoyl]amino]-1-methylpyrrole-2-carboxamide Chemical compound C1=C(C(=O)NCCC(N)=N)N(C)C=C1NC(=O)C1=CC(NC(=O)C=2N(C=C(NC(=O)C=3C=CC(=CC=3)N(CCCl)CCCl)C=2)C)=CN1C ARKYUICTMUZVEW-UHFFFAOYSA-N 0.000 description 1
- UMJJGDUYVQCBMC-UHFFFAOYSA-N n-ethyl-n'-[3-[3-(ethylamino)propylamino]propyl]propane-1,3-diamine Chemical compound CCNCCCNCCCNCCCNCC UMJJGDUYVQCBMC-UHFFFAOYSA-N 0.000 description 1
- WRINSSLBPNLASA-FOCLMDBBSA-N n-methyl-n-[(e)-(n-methylanilino)diazenyl]aniline Chemical compound C=1C=CC=CC=1N(C)\N=N\N(C)C1=CC=CC=C1 WRINSSLBPNLASA-FOCLMDBBSA-N 0.000 description 1
- RWHUEXWOYVBUCI-ITQXDASVSA-N nafarelin Chemical compound C([C@@H](C(=O)N[C@H](CC=1C=C2C=CC=CC2=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 RWHUEXWOYVBUCI-ITQXDASVSA-N 0.000 description 1
- 229960002333 nafarelin Drugs 0.000 description 1
- UZHSEJADLWPNLE-GRGSLBFTSA-N naloxone Chemical compound O=C([C@@H]1O2)CC[C@@]3(O)[C@H]4CC5=CC=C(O)C2=C5[C@@]13CCN4CC=C UZHSEJADLWPNLE-GRGSLBFTSA-N 0.000 description 1
- 229960004127 naloxone Drugs 0.000 description 1
- JZGDNMXSOCDEFQ-UHFFFAOYSA-N napavin Chemical compound C1C(CC)(O)CC(C2)CN1CCC(C1=CC=CC=C1N1)=C1C2(C(=O)OC)C(C(=C1)OC)=CC2=C1N(C)C1C2(C23)CCN3CC=CC2(CC)C(O)C1(O)C(=O)NCCNC1=CC=C(N=[N+]=[N-])C=C1[N+]([O-])=O JZGDNMXSOCDEFQ-UHFFFAOYSA-N 0.000 description 1
- 125000004593 naphthyridinyl group Chemical group N1=C(C=CC2=CC=CN=C12)* 0.000 description 1
- 108010032539 nartograstim Proteins 0.000 description 1
- 229950010676 nartograstim Drugs 0.000 description 1
- 229950007221 nedaplatin Drugs 0.000 description 1
- VRBKIVRKKCLPHA-UHFFFAOYSA-N nefazodone Chemical compound O=C1N(CCOC=2C=CC=CC=2)C(CC)=NN1CCCN(CC1)CCN1C1=CC=CC(Cl)=C1 VRBKIVRKKCLPHA-UHFFFAOYSA-N 0.000 description 1
- 229960001800 nefazodone Drugs 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- CTMCWCONSULRHO-UHQPFXKFSA-N nemorubicin Chemical compound C1CO[C@H](OC)CN1[C@@H]1[C@H](O)[C@H](C)O[C@@H](O[C@@H]2C3=C(O)C=4C(=O)C5=C(OC)C=CC=C5C(=O)C=4C(O)=C3C[C@](O)(C2)C(=O)CO)C1 CTMCWCONSULRHO-UHQPFXKFSA-N 0.000 description 1
- 229950010159 nemorubicin Drugs 0.000 description 1
- QZGIWPZCWHMVQL-UIYAJPBUSA-N neocarzinostatin chromophore Chemical compound O1[C@H](C)[C@H](O)[C@H](O)[C@@H](NC)[C@H]1O[C@@H]1C/2=C/C#C[C@H]3O[C@@]3([C@@H]3OC(=O)OC3)C#CC\2=C[C@H]1OC(=O)C1=C(O)C=CC2=C(C)C=C(OC)C=C12 QZGIWPZCWHMVQL-UIYAJPBUSA-N 0.000 description 1
- MQYXUWHLBZFQQO-UHFFFAOYSA-N nepehinol Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C)CCC(C(=C)C)C5C4CCC3C21C MQYXUWHLBZFQQO-UHFFFAOYSA-N 0.000 description 1
- PUUSSSIBPPTKTP-UHFFFAOYSA-N neridronic acid Chemical compound NCCCCCC(O)(P(O)(O)=O)P(O)(O)=O PUUSSSIBPPTKTP-UHFFFAOYSA-N 0.000 description 1
- 229950010733 neridronic acid Drugs 0.000 description 1
- 229960003512 nicotinic acid Drugs 0.000 description 1
- 229960002653 nilutamide Drugs 0.000 description 1
- XWXYUMMDTVBTOU-UHFFFAOYSA-N nilutamide Chemical compound O=C1C(C)(C)NC(=O)N1C1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 XWXYUMMDTVBTOU-UHFFFAOYSA-N 0.000 description 1
- 229940125745 nitric oxide modulator Drugs 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 239000012299 nitrogen atmosphere Substances 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 229950006344 nocodazole Drugs 0.000 description 1
- KGTDRFCXGRULNK-JYOBTZKQSA-N nogalamycin Chemical compound CO[C@@H]1[C@@](OC)(C)[C@@H](OC)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=C(O)C=C4[C@@]5(C)O[C@H]([C@H]([C@@H]([C@H]5O)N(C)C)O)OC4=C3C3=O)=C3C=C2[C@@H](C(=O)OC)[C@@](C)(O)C1 KGTDRFCXGRULNK-JYOBTZKQSA-N 0.000 description 1
- 229950009266 nogalamycin Drugs 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 238000012758 nuclear staining Methods 0.000 description 1
- 239000007764 o/w emulsion Substances 0.000 description 1
- 229960002700 octreotide Drugs 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 229950011093 onapristone Drugs 0.000 description 1
- 239000003605 opacifier Substances 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 239000012074 organic phase Substances 0.000 description 1
- ZLLOIFNEEWYATC-XMUHMHRVSA-N osaterone Chemical compound C1=C(Cl)C2=CC(=O)OC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2 ZLLOIFNEEWYATC-XMUHMHRVSA-N 0.000 description 1
- 229950006466 osaterone Drugs 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 229960001756 oxaliplatin Drugs 0.000 description 1
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 1
- ADIMAYPTOBDMTL-UHFFFAOYSA-N oxazepam Chemical compound C12=CC(Cl)=CC=C2NC(=O)C(O)N=C1C1=CC=CC=C1 ADIMAYPTOBDMTL-UHFFFAOYSA-N 0.000 description 1
- 229960004535 oxazepam Drugs 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 229950000370 oxisuran Drugs 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- VYOQBYCIIJYKJA-VORKOXQSSA-N palau'amine Chemical compound N([C@@]12[C@@H](Cl)[C@@H]([C@@H]3[C@@H]2[C@]24N=C(N)N[C@H]2N2C=CC=C2C(=O)N4C3)CN)C(N)=N[C@H]1O VYOQBYCIIJYKJA-VORKOXQSSA-N 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- ZFYKZAKRJRNXGF-XRZRNGJYSA-N palmitoyl rhizoxin Chemical compound O1C(=O)C2OC2CC(CC(=O)O2)CC2C(C)\C=C\C2OC2(C)C(OC(=O)CCCCCCCCCCCCCCC)CC1C(C)C(OC)C(\C)=C\C=C\C(\C)=C\C1=COC(C)=N1 ZFYKZAKRJRNXGF-XRZRNGJYSA-N 0.000 description 1
- WRUUGTRCQOWXEG-UHFFFAOYSA-N pamidronate Chemical compound NCCC(O)(P(O)(O)=O)P(O)(O)=O WRUUGTRCQOWXEG-UHFFFAOYSA-N 0.000 description 1
- 229960003978 pamidronic acid Drugs 0.000 description 1
- RDIMTXDFGHNINN-IKGGRYGDSA-N panaxytriol Chemical compound CCCCCCC[C@H](O)[C@@H](O)CC#CC#C[C@H](O)C=C RDIMTXDFGHNINN-IKGGRYGDSA-N 0.000 description 1
- ZCKMUKZQXWHXOF-UHFFFAOYSA-N panaxytriol Natural products CCC(C)C(C)C(C)C(C)C(C)C(O)C(O)CC#CC#CC(O)C=C ZCKMUKZQXWHXOF-UHFFFAOYSA-N 0.000 description 1
- 229950003440 panomifene Drugs 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 235000010603 pastilles Nutrition 0.000 description 1
- LPHSYQSMAGVYNT-UHFFFAOYSA-N pazelliptine Chemical compound N1C2=CC=NC=C2C2=C1C(C)=C1C=CN=C(NCCCN(CC)CC)C1=C2 LPHSYQSMAGVYNT-UHFFFAOYSA-N 0.000 description 1
- 229950006361 pazelliptine Drugs 0.000 description 1
- DOHVAKFYAHLCJP-UHFFFAOYSA-N peldesine Chemical compound C1=2NC(N)=NC(=O)C=2NC=C1CC1=CC=CN=C1 DOHVAKFYAHLCJP-UHFFFAOYSA-N 0.000 description 1
- 229950000039 peldesine Drugs 0.000 description 1
- 229950006960 peliomycin Drugs 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- VOKSWYLNZZRQPF-GDIGMMSISA-N pentazocine Chemical compound C1C2=CC=C(O)C=C2[C@@]2(C)[C@@H](C)[C@@H]1N(CC=C(C)C)CC2 VOKSWYLNZZRQPF-GDIGMMSISA-N 0.000 description 1
- 229960005301 pentazocine Drugs 0.000 description 1
- 229960003820 pentosan polysulfate sodium Drugs 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- QIMGFXOHTOXMQP-GFAGFCTOSA-N peplomycin Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCCN[C@@H](C)C=1C=CC=CC=1)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1NC=NC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C QIMGFXOHTOXMQP-GFAGFCTOSA-N 0.000 description 1
- 229950003180 peplomycin Drugs 0.000 description 1
- WTWWXOGTJWMJHI-UHFFFAOYSA-N perflubron Chemical compound FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)Br WTWWXOGTJWMJHI-UHFFFAOYSA-N 0.000 description 1
- 229960001217 perflubron Drugs 0.000 description 1
- UWCVGPLTGZWHGS-ZORIOUSZSA-N pergolide mesylate Chemical compound CS(O)(=O)=O.C1=CC([C@H]2C[C@@H](CSC)CN([C@@H]2C2)CCC)=C3C2=CNC3=C1 UWCVGPLTGZWHGS-ZORIOUSZSA-N 0.000 description 1
- 229960001511 pergolide mesylate Drugs 0.000 description 1
- 229960000769 periciazine Drugs 0.000 description 1
- LUALIOATIOESLM-UHFFFAOYSA-N periciazine Chemical compound C1CC(O)CCN1CCCN1C2=CC(C#N)=CC=C2SC2=CC=CC=C21 LUALIOATIOESLM-UHFFFAOYSA-N 0.000 description 1
- 235000005693 perillyl alcohol Nutrition 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- JRKICGRDRMAZLK-UHFFFAOYSA-L peroxydisulfate Chemical compound [O-]S(=O)(=O)OOS([O-])(=O)=O JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- LCADVYTXPLBAGB-GNCBHIOISA-N phenalamide A1 Natural products CC(CO)NC(=O)C(=CC=CC=C/C=C/C(=C/C(C)C(O)C(=CC(C)CCc1ccccc1)C)/C)C LCADVYTXPLBAGB-GNCBHIOISA-N 0.000 description 1
- 229960000964 phenelzine Drugs 0.000 description 1
- 229940049953 phenylacetate Drugs 0.000 description 1
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 1
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N phenylbenzene Natural products C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 1
- 229960002036 phenytoin Drugs 0.000 description 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical group CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 1
- 150000008105 phosphatidylcholines Chemical class 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 229960002139 pilocarpine hydrochloride Drugs 0.000 description 1
- RNAICSBVACLLGM-GNAZCLTHSA-N pilocarpine hydrochloride Chemical compound Cl.C1OC(=O)[C@@H](CC)[C@H]1CC1=CN=CN1C RNAICSBVACLLGM-GNAZCLTHSA-N 0.000 description 1
- NJBFOOCLYDNZJN-UHFFFAOYSA-N pipobroman Chemical compound BrCCC(=O)N1CCN(C(=O)CCBr)CC1 NJBFOOCLYDNZJN-UHFFFAOYSA-N 0.000 description 1
- 229960000952 pipobroman Drugs 0.000 description 1
- NUKCGLDCWQXYOQ-UHFFFAOYSA-N piposulfan Chemical compound CS(=O)(=O)OCCC(=O)N1CCN(C(=O)CCOS(C)(=O)=O)CC1 NUKCGLDCWQXYOQ-UHFFFAOYSA-N 0.000 description 1
- 229950001100 piposulfan Drugs 0.000 description 1
- 229960001221 pirarubicin Drugs 0.000 description 1
- XESARGFCSKSFID-FLLFQEBCSA-N pirazofurin Chemical compound OC1=C(C(=O)N)NN=C1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 XESARGFCSKSFID-FLLFQEBCSA-N 0.000 description 1
- 229950001030 piritrexim Drugs 0.000 description 1
- 229950010765 pivalate Drugs 0.000 description 1
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 1
- 239000002797 plasminogen activator inhibitor Substances 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229960003171 plicamycin Drugs 0.000 description 1
- 229950008499 plitidepsin Drugs 0.000 description 1
- 108010049948 plitidepsin Proteins 0.000 description 1
- UUSZLLQJYRSZIS-LXNNNBEUSA-N plitidepsin Chemical compound CN([C@H](CC(C)C)C(=O)N[C@@H]1C(=O)N[C@@H]([C@H](CC(=O)O[C@H](C(=O)[C@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N2CCC[C@H]2C(=O)N(C)[C@@H](CC=2C=CC(OC)=CC=2)C(=O)O[C@@H]1C)C(C)C)O)[C@@H](C)CC)C(=O)[C@@H]1CCCN1C(=O)C(C)=O UUSZLLQJYRSZIS-LXNNNBEUSA-N 0.000 description 1
- JKPDEYAOCSQBSZ-OEUJLIAZSA-N plomestane Chemical compound O=C1CC[C@]2(CC#C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CCC2=C1 JKPDEYAOCSQBSZ-OEUJLIAZSA-N 0.000 description 1
- 229950004541 plomestane Drugs 0.000 description 1
- 229920000191 poly(N-vinyl pyrrolidone) Polymers 0.000 description 1
- 229920000768 polyamine Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000002952 polymeric resin Substances 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 235000015320 potassium carbonate Nutrition 0.000 description 1
- 108020001213 potassium channel Proteins 0.000 description 1
- ZNNZYHKDIALBAK-UHFFFAOYSA-M potassium thiocyanate Chemical compound [K+].[S-]C#N ZNNZYHKDIALBAK-UHFFFAOYSA-M 0.000 description 1
- 229940116357 potassium thiocyanate Drugs 0.000 description 1
- 229960003089 pramipexole Drugs 0.000 description 1
- FASDKYOPVNHBLU-ZETCQYMHSA-N pramipexole Chemical compound C1[C@@H](NCCC)CCC2=C1SC(N)=N2 FASDKYOPVNHBLU-ZETCQYMHSA-N 0.000 description 1
- 229960004694 prednimustine Drugs 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- 229960001233 pregabalin Drugs 0.000 description 1
- AYXYPKUFHZROOJ-ZETCQYMHSA-N pregabalin Chemical compound CC(C)C[C@H](CN)CC(O)=O AYXYPKUFHZROOJ-ZETCQYMHSA-N 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 229960001586 procarbazine hydrochloride Drugs 0.000 description 1
- 229960005253 procyclidine Drugs 0.000 description 1
- 239000000186 progesterone Substances 0.000 description 1
- 229960003387 progesterone Drugs 0.000 description 1
- 229940097325 prolactin Drugs 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 229960003598 promazine Drugs 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- UQOQENZZLBSFKO-POPPZSFYSA-N prostaglandin J2 Chemical compound CCCCC[C@H](O)\C=C\[C@@H]1[C@@H](C\C=C/CCCC(O)=O)C=CC1=O UQOQENZZLBSFKO-POPPZSFYSA-N 0.000 description 1
- 239000003207 proteasome inhibitor Substances 0.000 description 1
- 239000003528 protein farnesyltransferase inhibitor Substances 0.000 description 1
- 239000003806 protein tyrosine phosphatase inhibitor Substances 0.000 description 1
- BWPIARFWQZKAIA-UHFFFAOYSA-N protriptyline Chemical compound C1=CC2=CC=CC=C2C(CCCNC)C2=CC=CC=C21 BWPIARFWQZKAIA-UHFFFAOYSA-N 0.000 description 1
- 229960002601 protriptyline Drugs 0.000 description 1
- SSKVDVBQSWQEGJ-UHFFFAOYSA-N pseudohypericin Natural products C12=C(O)C=C(O)C(C(C=3C(O)=CC(O)=C4C=33)=O)=C2C3=C2C3=C4C(C)=CC(O)=C3C(=O)C3=C(O)C=C(O)C1=C32 SSKVDVBQSWQEGJ-UHFFFAOYSA-N 0.000 description 1
- 239000000784 purine nucleoside phosphorylase inhibitor Substances 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- MKSVFGKWZLUTTO-FZFAUISWSA-N puromycin dihydrochloride Chemical compound Cl.Cl.C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO MKSVFGKWZLUTTO-FZFAUISWSA-N 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 238000005956 quaternization reaction Methods 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 229960004432 raltitrexed Drugs 0.000 description 1
- NTHPAPBPFQJABD-LLVKDONJSA-N ramosetron Chemical compound C12=CC=CC=C2N(C)C=C1C(=O)[C@H]1CC(NC=N2)=C2CC1 NTHPAPBPFQJABD-LLVKDONJSA-N 0.000 description 1
- 229950001588 ramosetron Drugs 0.000 description 1
- 229960000245 rasagiline Drugs 0.000 description 1
- RUOKEQAAGRXIBM-GFCCVEGCSA-N rasagiline Chemical compound C1=CC=C2[C@H](NCC#C)CCC2=C1 RUOKEQAAGRXIBM-GFCCVEGCSA-N 0.000 description 1
- 229940044601 receptor agonist Drugs 0.000 description 1
- 239000000018 receptor agonist Substances 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 230000033300 receptor internalization Effects 0.000 description 1
- BJOIZNZVOZKDIG-MDEJGZGSSA-N reserpine Chemical compound O([C@H]1[C@@H]([C@H]([C@H]2C[C@@H]3C4=C([C]5C=CC(OC)=CC5=N4)CCN3C[C@H]2C1)C(=O)OC)OC)C(=O)C1=CC(OC)=C(OC)C(OC)=C1 BJOIZNZVOZKDIG-MDEJGZGSSA-N 0.000 description 1
- 229960003147 reserpine Drugs 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 229950002225 retelliptine Drugs 0.000 description 1
- 229940100552 retinamide Drugs 0.000 description 1
- OWPCHSCAPHNHAV-LMONGJCWSA-N rhizoxin Chemical compound C/C([C@H](OC)[C@@H](C)[C@@H]1C[C@H](O)[C@]2(C)O[C@@H]2/C=C/[C@@H](C)[C@]2([H])OC(=O)C[C@@](C2)(C[C@@H]2O[C@H]2C(=O)O1)[H])=C\C=C\C(\C)=C\C1=COC(C)=N1 OWPCHSCAPHNHAV-LMONGJCWSA-N 0.000 description 1
- 229960004356 riboprine Drugs 0.000 description 1
- 108091092562 ribozyme Proteins 0.000 description 1
- 238000006798 ring closing metathesis reaction Methods 0.000 description 1
- 229950003733 romurtide Drugs 0.000 description 1
- 108700033545 romurtide Proteins 0.000 description 1
- 229960001879 ropinirole Drugs 0.000 description 1
- UHSKFQJFRQCDBE-UHFFFAOYSA-N ropinirole Chemical compound CCCN(CCC)CCC1=CC=CC2=C1CC(=O)N2 UHSKFQJFRQCDBE-UHFFFAOYSA-N 0.000 description 1
- 229960003522 roquinimex Drugs 0.000 description 1
- MDMGHDFNKNZPAU-UHFFFAOYSA-N roserpine Natural products C1C2CN3CCC(C4=CC=C(OC)C=C4N4)=C4C3CC2C(OC(C)=O)C(OC)C1OC(=O)C1=CC(OC)=C(OC)C(OC)=C1 MDMGHDFNKNZPAU-UHFFFAOYSA-N 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- YADVRLOQIWILGX-UHFFFAOYSA-N sarcophytol N Natural products CC(C)C1=CC=C(C)CCC=C(C)CCC=C(C)CC1O YADVRLOQIWILGX-UHFFFAOYSA-N 0.000 description 1
- 229960002530 sargramostim Drugs 0.000 description 1
- 108010038379 sargramostim Proteins 0.000 description 1
- 238000004626 scanning electron microscopy Methods 0.000 description 1
- STECJAGHUSJQJN-FWXGHANASA-N scopolamine Chemical compound C1([C@@H](CO)C(=O)O[C@H]2C[C@@H]3N([C@H](C2)[C@@H]2[C@H]3O2)C)=CC=CC=C1 STECJAGHUSJQJN-FWXGHANASA-N 0.000 description 1
- 229960002646 scopolamine Drugs 0.000 description 1
- 238000007790 scraping Methods 0.000 description 1
- 230000036280 sedation Effects 0.000 description 1
- 230000009758 senescence Effects 0.000 description 1
- 229940076279 serotonin Drugs 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 229950009089 simtrazene Drugs 0.000 description 1
- 229950001403 sizofiran Drugs 0.000 description 1
- 229950010372 sobuzoxane Drugs 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 235000011083 sodium citrates Nutrition 0.000 description 1
- 239000000176 sodium gluconate Substances 0.000 description 1
- 235000012207 sodium gluconate Nutrition 0.000 description 1
- 229940005574 sodium gluconate Drugs 0.000 description 1
- 239000001540 sodium lactate Substances 0.000 description 1
- 235000011088 sodium lactate Nutrition 0.000 description 1
- 229940005581 sodium lactate Drugs 0.000 description 1
- 235000010288 sodium nitrite Nutrition 0.000 description 1
- 229940006198 sodium phenylacetate Drugs 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 235000011008 sodium phosphates Nutrition 0.000 description 1
- 235000010339 sodium tetraborate Nutrition 0.000 description 1
- QUCDWLYKDRVKMI-UHFFFAOYSA-M sodium;3,4-dimethylbenzenesulfonate Chemical compound [Na+].CC1=CC=C(S([O-])(=O)=O)C=C1C QUCDWLYKDRVKMI-UHFFFAOYSA-M 0.000 description 1
- MIXCUJKCXRNYFM-UHFFFAOYSA-M sodium;diiodomethanesulfonate;n-propyl-n-[2-(2,4,6-trichlorophenoxy)ethyl]imidazole-1-carboxamide Chemical compound [Na+].[O-]S(=O)(=O)C(I)I.C1=CN=CN1C(=O)N(CCC)CCOC1=C(Cl)C=C(Cl)C=C1Cl MIXCUJKCXRNYFM-UHFFFAOYSA-M 0.000 description 1
- 239000002195 soluble material Substances 0.000 description 1
- 229950004225 sonermin Drugs 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 229950004796 sparfosic acid Drugs 0.000 description 1
- 229950009641 sparsomycin Drugs 0.000 description 1
- XKLZIVIOZDNKEQ-CLQLPEFOSA-N sparsomycin Chemical compound CSC[S@](=O)C[C@H](CO)NC(=O)\C=C\C1=C(C)NC(=O)NC1=O XKLZIVIOZDNKEQ-CLQLPEFOSA-N 0.000 description 1
- XKLZIVIOZDNKEQ-UHFFFAOYSA-N sparsomycin Natural products CSCS(=O)CC(CO)NC(=O)C=CC1=C(C)NC(=O)NC1=O XKLZIVIOZDNKEQ-UHFFFAOYSA-N 0.000 description 1
- YBZRLMLGUBIIDN-NZSGCTDASA-N spicamycin Chemical compound O1[C@@H](C(O)CO)[C@H](NC(=O)CNC(=O)CCCCCCCCCCCCC(C)C)[C@@H](O)[C@@H](O)[C@H]1NC1=NC=NC2=C1N=CN2 YBZRLMLGUBIIDN-NZSGCTDASA-N 0.000 description 1
- YBZRLMLGUBIIDN-UHFFFAOYSA-N spicamycin Natural products O1C(C(O)CO)C(NC(=O)CNC(=O)CCCCCCCCCCCCC(C)C)C(O)C(O)C1NC1=NC=NC2=C1NC=N2 YBZRLMLGUBIIDN-UHFFFAOYSA-N 0.000 description 1
- DKGZKTPJOSAWFA-UHFFFAOYSA-N spiperone Chemical compound C1=CC(F)=CC=C1C(=O)CCCN1CCC2(C(NCN2C=2C=CC=CC=2)=O)CC1 DKGZKTPJOSAWFA-UHFFFAOYSA-N 0.000 description 1
- 229950001675 spiperone Drugs 0.000 description 1
- 229950004330 spiroplatin Drugs 0.000 description 1
- 108010032486 splenopentin Proteins 0.000 description 1
- ICXJVZHDZFXYQC-UHFFFAOYSA-N spongistatin 1 Natural products OC1C(O2)(O)CC(O)C(C)C2CCCC=CC(O2)CC(O)CC2(O2)CC(OC)CC2CC(=O)C(C)C(OC(C)=O)C(C)C(=C)CC(O2)CC(C)(O)CC2(O2)CC(OC(C)=O)CC2CC(=O)OC2C(O)C(CC(=C)CC(O)C=CC(Cl)=C)OC1C2C ICXJVZHDZFXYQC-UHFFFAOYSA-N 0.000 description 1
- HAOCRCFHEPRQOY-JKTUOYIXSA-N spongistatin-1 Chemical compound C([C@@H]1C[C@@H](C[C@@]2(C[C@@H](O)C[C@@H](C2)\C=C/CCC[C@@H]2[C@H](C)[C@@H](O)C[C@](O2)(O)[C@H]2O)O1)OC)C(=O)[C@@H](C)[C@@H](OC(C)=O)[C@H](C)C(=C)C[C@H](O1)C[C@](C)(O)C[C@@]1(O1)C[C@@H](OC(C)=O)C[C@@H]1CC(=O)O[C@H]1[C@H](O)[C@@H](CC(=C)C(C)[C@H](O)\C=C\C(Cl)=C)O[C@@H]2[C@@H]1C HAOCRCFHEPRQOY-JKTUOYIXSA-N 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 229950001248 squalamine Drugs 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000024642 stem cell division Effects 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 108091007196 stromelysin Proteins 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 125000000565 sulfonamide group Chemical group 0.000 description 1
- 125000001273 sulfonato group Chemical group [O-]S(*)(=O)=O 0.000 description 1
- 229950007841 sulofenur Drugs 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- FIAFUQMPZJWCLV-UHFFFAOYSA-N suramin Chemical compound OS(=O)(=O)C1=CC(S(O)(=O)=O)=C2C(NC(=O)C3=CC=C(C(=C3)NC(=O)C=3C=C(NC(=O)NC=4C=C(C=CC=4)C(=O)NC=4C(=CC=C(C=4)C(=O)NC=4C5=C(C=C(C=C5C(=CC=4)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C)C=CC=3)C)=CC=C(S(O)(=O)=O)C2=C1 FIAFUQMPZJWCLV-UHFFFAOYSA-N 0.000 description 1
- 229960005314 suramin Drugs 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 229960005566 swainsonine Drugs 0.000 description 1
- FXUAIOOAOAVCGD-FKSUSPILSA-N swainsonine Chemical compound C1CC[C@H](O)[C@H]2[C@H](O)[C@H](O)CN21 FXUAIOOAOAVCGD-FKSUSPILSA-N 0.000 description 1
- FXUAIOOAOAVCGD-UHFFFAOYSA-N swainsonine Natural products C1CCC(O)C2C(O)C(O)CN21 FXUAIOOAOAVCGD-UHFFFAOYSA-N 0.000 description 1
- VAZAPHZUAVEOMC-UHFFFAOYSA-N tacedinaline Chemical compound C1=CC(NC(=O)C)=CC=C1C(=O)NC1=CC=CC=C1N VAZAPHZUAVEOMC-UHFFFAOYSA-N 0.000 description 1
- 229960001685 tacrine Drugs 0.000 description 1
- YLJREFDVOIBQDA-UHFFFAOYSA-N tacrine Chemical compound C1=CC=C2C(N)=C(CCCC3)C3=NC2=C1 YLJREFDVOIBQDA-UHFFFAOYSA-N 0.000 description 1
- 229950002687 talisomycin Drugs 0.000 description 1
- 108700003774 talisomycin Proteins 0.000 description 1
- 108010021891 tallimustine Proteins 0.000 description 1
- 229950005667 tallimustine Drugs 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 229950010168 tauromustine Drugs 0.000 description 1
- 150000004579 taxol derivatives Chemical class 0.000 description 1
- 229960000565 tazarotene Drugs 0.000 description 1
- URLYINUFLXOMHP-HTVVRFAVSA-N tcn-p Chemical compound C=12C3=NC=NC=1N(C)N=C(N)C2=CN3[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O URLYINUFLXOMHP-HTVVRFAVSA-N 0.000 description 1
- 239000003277 telomerase inhibitor Substances 0.000 description 1
- RNVNXVVEDMSRJE-UHFFFAOYSA-N teloxantrone hydrochloride Chemical compound Cl.Cl.OCCNCCN1NC2=C3C(=O)C=CC(=O)C3=C(O)C3=C2C1=CC=C3NCCNC RNVNXVVEDMSRJE-UHFFFAOYSA-N 0.000 description 1
- 229960004964 temozolomide Drugs 0.000 description 1
- 229950008703 teroxirone Drugs 0.000 description 1
- RHNYZBOYJIUYKF-UHFFFAOYSA-N tert-butyl 4-(2,3-dichlorophenyl)piperazine-1-carboxylate Chemical compound C1CN(C(=O)OC(C)(C)C)CCN1C1=CC=CC(Cl)=C1Cl RHNYZBOYJIUYKF-UHFFFAOYSA-N 0.000 description 1
- LYCDXZQZEMBMLU-UHFFFAOYSA-N tert-butyl 4-(2-methoxyphenyl)-1,4-diazepane-1-carboxylate Chemical compound COC1=CC=CC=C1N1CCN(C(=O)OC(C)(C)C)CCC1 LYCDXZQZEMBMLU-UHFFFAOYSA-N 0.000 description 1
- RUTRVXDBOSVFFT-UHFFFAOYSA-N tert-butyl n-(2-amino-4-methoxyphenyl)carbamate Chemical compound COC1=CC=C(NC(=O)OC(C)(C)C)C(N)=C1 RUTRVXDBOSVFFT-UHFFFAOYSA-N 0.000 description 1
- 229960005353 testolactone Drugs 0.000 description 1
- BPEWUONYVDABNZ-DZBHQSCQSA-N testolactone Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(OC(=O)CC4)[C@@H]4[C@@H]3CCC2=C1 BPEWUONYVDABNZ-DZBHQSCQSA-N 0.000 description 1
- 229960005333 tetrabenazine Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 125000003831 tetrazolyl group Chemical group 0.000 description 1
- WXZSUBHBYQYTNM-WMDJANBXSA-N tetrazomine Chemical compound C=1([C@@H]2CO[C@@H]3[C@H]4C[C@@H](CO)[C@H](N4C)[C@@H](N23)CC=1C=C1)C(OC)=C1NC(=O)C1NCCC[C@H]1O WXZSUBHBYQYTNM-WMDJANBXSA-N 0.000 description 1
- ZCTJIMXXSXQXRI-UHFFFAOYSA-N thaliblastine Natural products CN1CCC2=CC(OC)=C(OC)C3=C2C1CC1=C3C=C(OC)C(OC2=C(CC3C4=CC(OC)=C(OC)C=C4CCN3C)C=C(C(=C2)OC)OC)=C1 ZCTJIMXXSXQXRI-UHFFFAOYSA-N 0.000 description 1
- ZCTJIMXXSXQXRI-KYJUHHDHSA-N thalicarpine Chemical compound CN1CCC2=CC(OC)=C(OC)C3=C2[C@@H]1CC1=C3C=C(OC)C(OC2=C(C[C@H]3C4=CC(OC)=C(OC)C=C4CCN3C)C=C(C(=C2)OC)OC)=C1 ZCTJIMXXSXQXRI-KYJUHHDHSA-N 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 125000002813 thiocarbonyl group Chemical group *C(*)=S 0.000 description 1
- 108010062880 thiocoraline Proteins 0.000 description 1
- UPGGKUQISSWRJJ-UHFFFAOYSA-N thiocoraline Natural products CN1C(=O)CNC(=O)C(NC(=O)C=2C(=CC3=CC=CC=C3N=2)O)CSC(=O)C(CSC)N(C)C(=O)C(N(C(=O)CNC2=O)C)CSSCC1C(=O)N(C)C(CSC)C(=O)SCC2NC(=O)C1=NC2=CC=CC=C2C=C1O UPGGKUQISSWRJJ-UHFFFAOYSA-N 0.000 description 1
- 125000005505 thiomorpholino group Chemical group 0.000 description 1
- 229960001196 thiotepa Drugs 0.000 description 1
- NZVYCXVTEHPMHE-ZSUJOUNUSA-N thymalfasin Chemical compound CC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O NZVYCXVTEHPMHE-ZSUJOUNUSA-N 0.000 description 1
- 229960004231 thymalfasin Drugs 0.000 description 1
- 108010013515 thymopoietin receptor Proteins 0.000 description 1
- 229950010183 thymotrinan Drugs 0.000 description 1
- YFTWHEBLORWGNI-UHFFFAOYSA-N tiamiprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC(N)=NC2=C1NC=N2 YFTWHEBLORWGNI-UHFFFAOYSA-N 0.000 description 1
- 229950011457 tiamiprine Drugs 0.000 description 1
- 229960003723 tiazofurine Drugs 0.000 description 1
- FVRDYQYEVDDKCR-DBRKOABJSA-N tiazofurine Chemical compound NC(=O)C1=CSC([C@H]2[C@@H]([C@H](O)[C@@H](CO)O2)O)=N1 FVRDYQYEVDDKCR-DBRKOABJSA-N 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- MNRILEROXIRVNJ-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=NC=N[C]21 MNRILEROXIRVNJ-UHFFFAOYSA-N 0.000 description 1
- 229960005013 tiotixene Drugs 0.000 description 1
- XJDNKRIXUMDJCW-UHFFFAOYSA-J titanium tetrachloride Chemical compound Cl[Ti](Cl)(Cl)Cl XJDNKRIXUMDJCW-UHFFFAOYSA-J 0.000 description 1
- 229930003799 tocopherol Natural products 0.000 description 1
- 239000011732 tocopherol Substances 0.000 description 1
- 235000019149 tocopherols Nutrition 0.000 description 1
- MIQPIUSUKVNLNT-UHFFFAOYSA-N tolcapone Chemical compound C1=CC(C)=CC=C1C(=O)C1=CC(O)=C(O)C([N+]([O-])=O)=C1 MIQPIUSUKVNLNT-UHFFFAOYSA-N 0.000 description 1
- 229960004603 tolcapone Drugs 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 125000003944 tolyl group Chemical group 0.000 description 1
- 229960004394 topiramate Drugs 0.000 description 1
- ONYVJPZNVCOAFF-UHFFFAOYSA-N topsentin Natural products Oc1ccc2cc([nH]c2c1)C(=O)c3ncc([nH]3)c4c[nH]c5ccccc45 ONYVJPZNVCOAFF-UHFFFAOYSA-N 0.000 description 1
- 229960005026 toremifene Drugs 0.000 description 1
- XFCLJVABOIYOMF-QPLCGJKRSA-N toremifene Chemical compound C1=CC(OCCN(C)C)=CC=C1C(\C=1C=CC=CC=1)=C(\CCCl)C1=CC=CC=C1 XFCLJVABOIYOMF-QPLCGJKRSA-N 0.000 description 1
- 229960004167 toremifene citrate Drugs 0.000 description 1
- 210000003014 totipotent stem cell Anatomy 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- 229960003741 tranylcypromine Drugs 0.000 description 1
- PHLBKPHSAVXXEF-UHFFFAOYSA-N trazodone Chemical compound ClC1=CC=CC(N2CCN(CCCN3C(N4C=CC=CC4=N3)=O)CC2)=C1 PHLBKPHSAVXXEF-UHFFFAOYSA-N 0.000 description 1
- 229960003991 trazodone Drugs 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 229950003873 triciribine Drugs 0.000 description 1
- HOGVTUZUJGHKPL-HTVVRFAVSA-N triciribine Chemical compound C=12C3=NC=NC=1N(C)N=C(N)C2=CN3[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O HOGVTUZUJGHKPL-HTVVRFAVSA-N 0.000 description 1
- GGUBFICZYGKNTD-UHFFFAOYSA-N triethyl phosphonoacetate Chemical compound CCOC(=O)CP(=O)(OCC)OCC GGUBFICZYGKNTD-UHFFFAOYSA-N 0.000 description 1
- ZEWQUBUPAILYHI-UHFFFAOYSA-N trifluoperazine Chemical compound C1CN(C)CCN1CCCN1C2=CC(C(F)(F)F)=CC=C2SC2=CC=CC=C21 ZEWQUBUPAILYHI-UHFFFAOYSA-N 0.000 description 1
- 229960002324 trifluoperazine Drugs 0.000 description 1
- 229960003904 triflupromazine Drugs 0.000 description 1
- XSCGXQMFQXDFCW-UHFFFAOYSA-N triflupromazine Chemical compound C1=C(C(F)(F)F)C=C2N(CCCN(C)C)C3=CC=CC=C3SC2=C1 XSCGXQMFQXDFCW-UHFFFAOYSA-N 0.000 description 1
- 229960000538 trimetrexate glucuronate Drugs 0.000 description 1
- ZSCDBOWYZJWBIY-UHFFFAOYSA-N trimipramine Chemical compound C1CC2=CC=CC=C2N(CC(CN(C)C)C)C2=CC=CC=C21 ZSCDBOWYZJWBIY-UHFFFAOYSA-N 0.000 description 1
- 229960002431 trimipramine Drugs 0.000 description 1
- VLPFTAMPNXLGLX-UHFFFAOYSA-N trioctanoin Chemical group CCCCCCCC(=O)OCC(OC(=O)CCCCCCC)COC(=O)CCCCCCC VLPFTAMPNXLGLX-UHFFFAOYSA-N 0.000 description 1
- BSVBQGMMJUBVOD-UHFFFAOYSA-N trisodium borate Chemical compound [Na+].[Na+].[Na+].[O-]B([O-])[O-] BSVBQGMMJUBVOD-UHFFFAOYSA-N 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- ZNRGQMMCGHDTEI-ITGUQSILSA-N tropisetron Chemical compound C1=CC=C2C(C(=O)O[C@H]3C[C@H]4CC[C@@H](C3)N4C)=CNC2=C1 ZNRGQMMCGHDTEI-ITGUQSILSA-N 0.000 description 1
- 229960003688 tropisetron Drugs 0.000 description 1
- WMPQMBUXZHMEFZ-YJPJVVPASA-N turosteride Chemical compound CN([C@@H]1CC2)C(=O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](C(=O)N(C(C)C)C(=O)NC(C)C)[C@@]2(C)CC1 WMPQMBUXZHMEFZ-YJPJVVPASA-N 0.000 description 1
- 229950007816 turosteride Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 229950009811 ubenimex Drugs 0.000 description 1
- ZDPHROOEEOARMN-UHFFFAOYSA-N undecanoic acid Chemical compound CCCCCCCCCCC(O)=O ZDPHROOEEOARMN-UHFFFAOYSA-N 0.000 description 1
- 229960001055 uracil mustard Drugs 0.000 description 1
- SPDZFJLQFWSJGA-UHFFFAOYSA-N uredepa Chemical compound C1CN1P(=O)(NC(=O)OCC)N1CC1 SPDZFJLQFWSJGA-UHFFFAOYSA-N 0.000 description 1
- 229950006929 uredepa Drugs 0.000 description 1
- AUFUWRKPQLGTGF-FMKGYKFTSA-N uridine triacetate Chemical compound CC(=O)O[C@@H]1[C@H](OC(C)=O)[C@@H](COC(=O)C)O[C@H]1N1C(=O)NC(=O)C=C1 AUFUWRKPQLGTGF-FMKGYKFTSA-N 0.000 description 1
- 229960005356 urokinase Drugs 0.000 description 1
- 229950008261 velaresol Drugs 0.000 description 1
- XLQGICHHYYWYIU-UHFFFAOYSA-N veramine Natural products O1C2CC3C4CC=C5CC(O)CCC5(C)C4CC=C3C2(C)C(C)C21CCC(C)CN2 XLQGICHHYYWYIU-UHFFFAOYSA-N 0.000 description 1
- 229960001722 verapamil Drugs 0.000 description 1
- KDQAABAKXDWYSZ-PNYVAJAMSA-N vinblastine sulfate Chemical compound OS(O)(=O)=O.C([C@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 KDQAABAKXDWYSZ-PNYVAJAMSA-N 0.000 description 1
- 229960004982 vinblastine sulfate Drugs 0.000 description 1
- AQTQHPDCURKLKT-JKDPCDLQSA-N vincristine sulfate Chemical compound OS(O)(=O)=O.C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C=O)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 AQTQHPDCURKLKT-JKDPCDLQSA-N 0.000 description 1
- 229960002110 vincristine sulfate Drugs 0.000 description 1
- UGGWPQSBPIFKDZ-KOTLKJBCSA-N vindesine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1N=C1[C]2C=CC=C1 UGGWPQSBPIFKDZ-KOTLKJBCSA-N 0.000 description 1
- 229960004355 vindesine Drugs 0.000 description 1
- 229960005212 vindesine sulfate Drugs 0.000 description 1
- BCXOZISMDZTYHW-IFQBWSDRSA-N vinepidine sulfate Chemical compound OS(O)(=O)=O.C([C@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C=O)C=2)OC)C[C@@H](C2)CC)N2CCC2=C1NC1=CC=CC=C21 BCXOZISMDZTYHW-IFQBWSDRSA-N 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 229960002166 vinorelbine tartrate Drugs 0.000 description 1
- GBABOYUKABKIAF-IWWDSPBFSA-N vinorelbinetartrate Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC(C23[C@H]([C@@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-IWWDSPBFSA-N 0.000 description 1
- 239000007762 w/o emulsion Substances 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- DVPVGSLIUJPOCJ-XXRQFBABSA-N x1j761618a Chemical compound OS(O)(=O)=O.OS(O)(=O)=O.OS(O)(=O)=O.C([C@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(=O)CN(C)C)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21.C([C@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(=O)CN(C)C)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 DVPVGSLIUJPOCJ-XXRQFBABSA-N 0.000 description 1
- 229950005561 zanoterone Drugs 0.000 description 1
- UZVNCLCLJHPHIF-NOJKMYKQSA-J zinc;(1e)-2-(ethylcarbamoylamino)-n-methoxy-2-oxoethanimidoyl cyanide;manganese(2+);n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[Zn+2].[S-]C(=S)NCCNC([S-])=S.[S-]C(=S)NCCNC([S-])=S.CCNC(=O)NC(=O)C(\C#N)=N\OC UZVNCLCLJHPHIF-NOJKMYKQSA-J 0.000 description 1
- 229950009268 zinostatin Drugs 0.000 description 1
- FYQZGCBXYVWXSP-STTFAQHVSA-N zinostatin stimalamer Chemical compound O1[C@H](C)[C@H](O)[C@H](O)[C@@H](NC)[C@H]1OC1C/2=C/C#C[C@H]3O[C@@]3([C@H]3OC(=O)OC3)C#CC\2=C[C@H]1OC(=O)C1=C(C)C=CC2=C(C)C=C(OC)C=C12 FYQZGCBXYVWXSP-STTFAQHVSA-N 0.000 description 1
- 229950009233 zinostatin stimalamer Drugs 0.000 description 1
- 229960002911 zonisamide Drugs 0.000 description 1
- UBQNRHZMVUUOMG-UHFFFAOYSA-N zonisamide Chemical compound C1=CC=C2C(CS(=O)(=O)N)=NOC2=C1 UBQNRHZMVUUOMG-UHFFFAOYSA-N 0.000 description 1
- 229960004141 zuclopenthixol Drugs 0.000 description 1
- WFPIAZLQTJBIFN-DVZOWYKESA-N zuclopenthixol Chemical compound C1CN(CCO)CCN1CC\C=C\1C2=CC(Cl)=CC=C2SC2=CC=CC=C2/1 WFPIAZLQTJBIFN-DVZOWYKESA-N 0.000 description 1
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 1
- 150000003952 β-lactams Chemical class 0.000 description 1
- QUEDXNHFTDJVIY-UHFFFAOYSA-N γ-tocopherol Chemical class OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1 QUEDXNHFTDJVIY-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4523—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems
- A61K31/454—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. pimozide, domperidone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/472—Non-condensed isoquinolines, e.g. papaverine
- A61K31/4725—Non-condensed isoquinolines, e.g. papaverine containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4965—Non-condensed pyrazines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/538—1,4-Oxazines, e.g. morpholine ortho- or peri-condensed with carbocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/20—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D217/00—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems
- C07D217/22—Heterocyclic compounds containing isoquinoline or hydrogenated isoquinoline ring systems with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to carbon atoms of the nitrogen-containing ring
- C07D217/24—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D231/00—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings
- C07D231/54—Heterocyclic compounds containing 1,2-diazole or hydrogenated 1,2-diazole rings condensed with carbocyclic rings or ring systems
- C07D231/56—Benzopyrazoles; Hydrogenated benzopyrazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
- C07D235/04—Benzimidazoles; Hydrogenated benzimidazoles
- C07D235/24—Benzimidazoles; Hydrogenated benzimidazoles with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached in position 2
- C07D235/26—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D241/00—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings
- C07D241/02—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings
- C07D241/04—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D277/00—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings
- C07D277/60—Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings condensed with carbocyclic rings or ring systems
- C07D277/62—Benzothiazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/04—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms
- C07D295/08—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly bound oxygen or sulfur atoms
- C07D295/084—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly bound oxygen or sulfur atoms with the ring nitrogen atoms and the oxygen or sulfur atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings
- C07D295/088—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly bound oxygen or sulfur atoms with the ring nitrogen atoms and the oxygen or sulfur atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings to an acyclic saturated chain
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
Definitions
- the present invention relates to novel functionally selective ligands of dopamine D 2 receptors, including agonists, antagonists, and inverse agonists.
- the invention further relates to the use of these compounds in research and for treating central nervous system disorders related to D 2 receptors.
- a partial agonist possesses a lower degree of intrinsic efficacy and partially activates all cellular responses induced by an agonist Antagonists, according to this schema, are neutral entities which possess no intrinsic activity but are able to block the receptor and preclude activation by full or partial agonists (Furchgott, Advances in Drug Research (Harper N and Simmonds A eds) Academic Press, New York., pp 21- 55 (1966)).
- GPCR G-protein coupled receptor
- GPCR G-protein coupled receptor
- the present invention relates to novel, functionally selective ligands of dopamine D 2 receptors.
- the compounds of the invention When evaluated in multiple D 2 binding and functional assays, the compounds of the invention showed different activity profiles ranging from full agonists to antagonists to inverse agonists.
- the compounds of the invention are ⁇ -arrestin biased and, in certain embodiments, also are inverse agonists of Gj-regulated pathways. These pharmacologically active compounds exhibit fewer side effects and are useful in research to study D 2 receptor function and its role in psychosis and for the treatment of CNS disorders.
- the invention provides a compound of formula I:
- m is 0 or 1 ;
- n 0, 1, or 2;
- q 1 or 2;
- X is O, NH, or CH 2 ;
- Y is N or CH
- Z is C, CH, CH 2 , cycloalkyl, aryl, or heteroaryl
- Aii and Ar 2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
- the invention provides a compound of formula II:
- n 0, 1, or 2;
- q 1 or 2;
- X is O, NH, or CH 2 ;
- Y is N or CH
- Z is C, CH, CH 2 , cycloalkyl, aryl, or heteroaryl
- Ari and Ar 2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl; or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
- the invention provides a compound of formula VI:
- n 0, 1, or 2;
- q 1 or 2;
- X is O, NH, or CH 2 ;
- Y is N or CH
- Z is C, CH, CH 2 , cycloalkyl, aryl, or heteroaryl
- Ari and Ar 2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
- the invention further relates to a pharmaceutical composition
- a pharmaceutical composition comprising the compound of the invention and a pharmaceutically acceptable carrier.
- the invention also relates to a method of modulating the activity of a D 2 dopamine receptor comprising contacting the receptor with a compound of the invention.
- the invention additionally relates to a method of treating a central nervous system disorder associated with D 2 dopamine receptors in a subject (e.g., a psychiatric, neurological, pituitary, or endocrine disorder), comprising delivering to the subject a therapeutically effective amount of the compound of the invention.
- a central nervous system disorder associated with D 2 dopamine receptors e.g., a psychiatric, neurological, pituitary, or endocrine disorder
- the invention further relates to the use of a compound of the invention for treating a central nervous system disorder associated with D 2 dopamine receptors in a subject.
- the invention further relates to the use of a compound of the invention for stimulating D 2 dopamine receptors in a ⁇ -arrestin biased manner.
- Figures 1A-1F show that UNC 9975 exhibits potent antipsychotic-like activity in mouse hyperlocomotion studies.
- Figure 2 shows that UNC9975 and UNC0006 induce catalepsy in p-arrestin-2 knockout mice but not in wild-type littermates.
- a can mean one or more than one.
- a cell can mean a single cell or a multiplicity of cells.
- compositions of this invention means the composition can contain additional components as long as the additional components do not materially alter the composition.
- materially altered refers to an increase or decrease in the therapeutic effectiveness of the composition of at least about 20% or more as compared to the effectiveness of a composition consisting of the recited components.
- terapéuticaally effective amount refers to that amount of a composition, compound, or agent of this invention that imparts a modulating effect, which, for example, can be a beneficial effect, to a subject afflicted with a disorder, disease or illness, including improvement in the condition of the subject (e.g., in one or more symptoms), delay or reduction in the progression of the condition, prevention or delay of the onset of the disorder, and/or change in clinical parameters, disease or illness, etc., as would be well known in the art.
- a therapeutically effective amount or effective amount can refer to the amount of a composition, compound, or agent that improves a condition in a subject by at least 5%, e.g., at least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, or at least 100%.
- Treat” or “treating” or “treatment” refers to any type of action that imparts a modulating effect, which, for example, can be a beneficial effect, to a subject afflicted with a disorder, disease or illness, including improvement in the condition of the subject (e.g., in one or more symptoms), delay or reduction in the progression of the condition, and/or change in clinical parameters, disease or illness, etc., as would be well known in the art.
- a "central nervous system disorder associated with D 2 dopamine receptors” refers to any disorder in which modulation (either an increase or a decrease) of one or more activities of a D 2 dopamine receptor in a subject results in the treatment of one or more symptoms of the disorder in the subject,
- “Pharmaceutically acceptable,” as used herein, means a material that is not biologically or otherwise undesirable, i.e., the material can be administered to an individual along with the compositions of this invention, without causing substantial deleterious biological effects or interacting in a deleterious manner with any of the other components of the composition in which it is contained.
- the material would naturally be selected to minimize any degradation of the active ingredient and to minimize any adverse side effects in the subject, as would be well known to one of skill in the art ⁇ see, e.g., Remington's Pharmaceutical Science; 21 st ed. 2005).
- Exemplary pharmaceutically acceptable carriers for the compositions of this invention include, but are not limited to, sterile pyrogen-free water and sterile pyrogen-free physiological saline solution.
- Concurrently means sufficiently close in time to produce a combined effect (that is, concurrently can be simultaneously, or it can be two or more events occurring within a short time period before or after each other).
- the administration of two or more compounds "concurrently” means that the two compounds are administered closely enough in time that the presence of one alters the biological effects of the other.
- the two compounds can be administered in the same or different formulations or sequentially. Concurrent administration can be carried out by mixing the compounds prior to administration, or by administering the compounds in two different formulations, for example, at the same point in time but at different anatomic sites or using different routes of administration.
- ⁇ -arrestin biased refers to a compound that binds the D 2 dopamine receptor, is a partial or full agonist of the ⁇ -arrestin pathway, and is either neutral or an inverse agonist of the Gj-regulated cAMP pathway.
- neutral refers to a compound that binds to a receptor but does not stimulate a particular receptor-linked pathway.
- inverse agonist refers to a compound that binds to a receptor and reduces constitutive activity of a particular receptor-linked pathway.
- alkyl denotes a straight or branched hydrocarbon chain containing 1- 12 carbon atoms, e.g., 1-6 or 1-4 carbon atoms.
- alkyl groups include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl, and the like.
- substituted alkyl an alkyl in which an atom of the alkyl is substituted with, for example, a carbon, nitrogen, sulfur, oxygen, silicon, or halogen atom, or alternatively a nitrogen, sulfur, oxygen, or halogen atom.
- the term encompasses substituents on alkyl, alkenyl, alkynyl, and cycloalkyl groups.
- substituents that can be attached to any atom of the alkyl group in a "substituted alkyl” include cyclyl groups, heterocyclyl groups; aryl groups, heteroaryl groups, amino groups, amido groups, nitro groups, cyano groups, azide groups, hydroxy groups, alkoxy groups, acyloxy groups, thioalkoxy groups, acyl thioalkoxy groups, halogen groups, sulfonate groups, sulfonamide groups, ester groups, carboxylic acids, oxygen ⁇ e.g., a carbonyl group), and sulfur ⁇ e.g., a thiocarbonyl group).
- Substituents also include any chemical functional group that imparts improved water-solubility to the molecule ⁇ e.g., carboxylic acid, carboxylic ester, carboxamido, morpholino, piperazinyl, imidazolyl, thiomorpholino, or tetrazolyl groups; both unsubstituted and substituted).
- cycloalkyl denotes a monocyclic saturated carbocyclic group containing 3-8 carbon atoms, e.g., 3-6 carbon atoms.
- Examples of cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, and the like.
- alkoxy denotes an oxygen linked to an alkyl or substituted alkyl as defined above.
- halo and halogen refer to any radical of fluorine, chlorine, bromine or iodine.
- ring and "ring system” refer to a ring comprising the delineated number of atoms, said atoms being carbon or, where indicated, a heteroatom such as nitrogen, oxygen or sulfur.
- the ring itself, as well as any substituents thereon, can be attached at any atom that allows a stable compound to be formed.
- aryl refers to an aromatic 5-8 membered monocyclic or 8-12 membered bicyclic ring system wherein 0, 1, 2, or 3 atoms of each ring can be substituted by a substituent.
- the term also includes aromatic bicyclic ring systems in which a hydrogen atom has been added to one, two, or three of the ring carbons in one of the rings (e.g., a partially saturated ring). Examples of aryl groups include phenyl, naphthyl and the like.
- heteroaryl refers to an aromatic 5-8 membered monocyclic or 8-12 membered bicyclic ring system comprising 1-3 heteroatoms if monocyclic or 1-6 heteroatoms if bicyclic, said heteroatoms selected from O, N, or S, wherein 0, 1, 2 or 3 atoms of each ring can be substituted by a substituent.
- the term also includes aromatic bicyclic ring systems in which a hydrogen atom has been added to one, two, or three of the ring carbons in one of the rings (e.g., a partially saturated ring).
- heteroaryl groups include pyridyl, furyl or furanyl, benzofuranyl, imidazolyl, benzimidazolyl, pyrimidinyl, thiophenyl or thienyl, benzothiophenyl, quinolinyl, isoquinolinyl, dihydroquinolinyl, dihydroisoquinolinyl, naphthyridinyl, dihydronaphthyridinyl, quinazolinyl, indolyl, indazolyl, thiazolyl, benzothiazolyl, oxazinyl, benzooxazinyl, oxazolyl, benzooxazolyl, dihydrobenzodioxinyl, and the like.
- Suitable substituents for aryl and heteroaryl groups are the same as the substituents for alkyl groups.
- the present invention provides novel, functionally selective ligands of dopamine D 2 receptors.
- the compounds of the invention are ⁇ -arrestin biased.
- the compounds are at least partial agonists of the ⁇ -arrestin pathway.
- the compounds are full agonists of the ⁇ -arrestin pathway.
- the compounds also are neutral or inverse agonists of the Gj-regulated cAMP pathway. These compounds represent the first functionally selective ⁇ -arrestin-biased dopamine D 2 receptor ligands to exhibit antipsychotic activity in vivo.
- the invention provides a compound of formula I:
- n 0 or 1
- q 1 or 2;
- n 0, 1, or 2;
- X is O, NH, or CH 2 ;
- Y is N or CH
- Z is C, CH, CH 2 , cycloalkyl, aryl, or heteroaryl
- Ari and Ar 2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
- Ar 2 is an unsubstituted or substituted bicyclic aryl or heteroaryl.
- Z is C, CH, CH 2 , cycloalkyl, monocyclic aryl, or monocyclic heteroaryl.
- the bond indicated as refers to the bond attached to Z and not to any other bond, e.g., not between the two carbon atoms that are present when q is 2.
- the compound of the invention has the structure of formula II:
- n 0, 1, or 2;
- q 1 or 2;
- X is O, NH, or CH 2 ; Y is N or CH;
- Z is C, CH, CH 2 , cycloalkyl, aryl, or heteroaryl
- Arj and Ar 2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
- the compound of the invention has the structure of formula
- n 0, 1, or 2;
- X is O, NH, or CH 2 ;
- Y is N or CH
- Ari and Ar 2 are independently an unsubstituted or substituted monocyclic or bicyclic heteroaryl
- n is 1. In a further embodiment, n is 0. In another embodiment, X is O. In another embodiment, X is NH.
- Ax ⁇ is selected from the group consisting of phenyl, substituted phenyl (e.g., chlorophenyl, dichlorophenyl, fluorophenyl, trifluoromethylphenyl, methylphenyl, cyanophenyl, methoxyphenyl, ethoxyphenyl, isopropoxyphenyl), 2,3-dihydro-lH-benzo[d]imidazolyl, benzo[d]oxazol-2(3H)-one, 1H- benzo[d]imidazolyl, benzo[d]oxazolyl, IH-indazolyl, IH-indazolyl, IH-indolyl, benzofuranyl, benzo[b]thiophenyl, benzo[d]thiazolyl, naphthalenyl, isoquinolinyl, quinazolinyl, quinolin
- Ar 2 is selected from the group consisting of 3,4-dihydroquinolin-2(lH)-one, 3,4-dihydro-l ,8-naphthyridin-2(lH)-one, quinolin-2(lH)-one, 3,4-dihydroisoquinolin-l(2H)-one, benzo jthiazole, lH-benzo[ ⁇ /Jimidazol- 2(3H)-one, lH-indazole, and benzo[d]oxazol-2(3H)-one, or any subgroup selected from the list.
- Ari is dichlorophenyl or methoxyphenyl and Ar 2 is quinolinone, dihydroquinolinone, naphthyridinone, or benzothiazole.
- ⁇ is dichlorophenyl, methoxyphenyl, ethoxyphenyl, or isopropyloxyphenyl and Ar 2 is quinolinone, dihydroquinolinone, naphthyridinone, benzothiazole, or benzoimidazolone.
- Ar 2 is not naphthyridinone, dihydronaphthyridinone, quinolinone, dihydroquinolinone, tetrahydropyridoazepinone, or iso indole.
- the compounds of the invention have the structure of formula
- the compound of formula II is selected from the group of compounds shown in Tables 1 and 2.
- the compound of the invention has the structure of formula
- n 0, 1, or 2;
- q 1 or 2;
- X is O, NH, or C3 ⁇ 4;
- Y is N or CH
- Z is C, CH, CH 2 , cycloalkyl, aryl, or heteroaryl
- Ari and Ar 2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
- Ar 2 is an unsubstituted or substituted bicyclic aryl or heteroaryl.
- Z is C, CH, CH 2 , cycloalkyl, monocyclic aryl, or monocyclic heteroaryl.
- n is 0. In a different embodiment, n is 1. In another embodiment, X is O or NH.
- Ari is selected from the group consisting of substituted phenyl ⁇ e.g., dichlorophenyl, methoxyphenyl), 2H- benzo[b][l,4]oxazin-3(4H)-one, 2,3-dihydrobenzo[b][l,4]dioxinyl, and benzo[d]oxazol-2(3H)- one, or any subgroup selected from the list.
- Ax ⁇ is dichlorophenyl.
- Ar 2 is selected from the group consisting of IH-indazole, benzo[d]thiazole, imidazol-2(3H)-one, 3,4-dihydroisoquinolin- l(2H)-one, 3,4-dihydroquinolin-2(lH)-one, lH-benzo[d]imidazol-2(3H)-one, 3,4-dihydro-l,8- naphthyridin-2(lH)-one, quinolin-2(lH)-one, 2-methylphenylacetamide, and benzo[d]oxazol- 2(3H)-one, or any subgroup selected from the list.
- Ar 2 is selected from the group consisting of IH-indazole, benzo[d]thiazole, and imidazol-2(3H)-one.
- Ari is dichlorophenyl or dihydrobenzodioxinyl and Ar 2 is dihydroquinolinone, benzothiazole, or indazole.
- Ari is dichlorophenyl, methoxyphenyl, or dihydrobenzodioxinyl and Ar 2 is dihydroquinolinone, benzothiazole, indazole, benzoimidazolone, or quinolinone.
- Y is N or CH
- n is 0 or 1
- X is O
- Ari is dichlorophenyl
- Ar 2 is selected from the group consisting of IH-indazole, benzo[d]thiazole, and lH-benzoimidazol-2(3H)-one.
- Ar 2 is not naphthyridinone, dihydronaphthyridinone, quinolinone, dihydroquinolinone, tetrahydropyridoazepinone, or isoindole.
- the compound of formula VI is selected from the group of compounds shown in Tables 3 and 4.
- the compounds of this invention include all pharmaceutically acceptable salt forms thereof.
- such salts include those derived from pharmaceutically acceptable inorganic and organic acids and bases.
- suitable acid salts include, without limitation, acetate, adipate, alginate, aspartate, benzoate, butyrate, citrate, fumarate, glycolate, hemisulfate, heptanoate, hexanoate, hydrochloride, hydrobromide, hydroiodide, 2- hydroxyethanesulfonate, lactate, maleate, malonate, methanesulfonate, nicotinate, nitrate, oxalate, palmoate, pectinate, persulfate, hydroxynapthoate, pivalate, propionate, salicylate, succinate, sulfate, tartrate, thiocyanate, tosylate and undecanoate.
- Other acids such as oxalic, while not in themselves pharmaceutically acceptable, can be
- Salts derived from appropriate bases include, without limitation, alkali metal (e.g., sodium, potassium), alkaline earth metal (e.g., magnesium and calcium), ammonium and N- (alkyl)4 + salts.
- the compounds of this invention include deuterium-substituted versions of the disclosed compounds, e.g., compounds comprising 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 or more deuterium atoms.
- the compounds of the invention include prodrugs of the compounds that are converted to the active compound in vivo.
- the compound can be modified to enhance cellular permeability (e.g., by esterification of polar groups) and then converted by cellular enzymes to produce the active agent.
- Methods of masking charged or reactive moieties as a pro-drug are known by those skilled in the art ⁇ see, e.g., P. Korgsgaard-Larsen and H. Bundgaard, A Textbook of Drug Design and Development, Reading U.K., Harwood Academic Publishers, 1991).
- prodrug refers to compounds that are rapidly transformed in vivo to yield the parent compound of the above formula, for example, by hydrolysis in blood, see, e.g., T. Higuchi and V. Stella, Prodrugs as Novel delivery Systems, Vol. 14 of the A.C.S. Symposium Series and in Edward B. Roche, ed., Bioreversible Carriers in Drug Design, American Pharmaceutical Association and Pergamon Press, 1987, both of which are incorporated by reference herein. See also U.S. Patent No. 6,680,299.
- Exemplary prodrugs include a prodrug that is metabolized in vivo by a subject to an active drug having an activity of the compounds as described herein, wherein the prodrug is an ester of an alcohol or carboxylic acid group, if such a group is present in the compound; an amide of an amine group or carboxylic acid group, if such groups are present in the compound; a urethane of an amine group, if such a group is present in the compound; an acetal or ketal of an alcohol group, if such a group is present in the compound; a N-Mannich base or an imine of an amine group, if such a group is present in the compound; or a Schiff base, oxime, acetal, enol ester, oxazolidine, or thiazolidine of a carbonyl group, if such a group is present in the compound, such as described, for example, in U.S. Patent No. 6,680,324 and U.S
- prodrug refers to those prodrugs of the compounds of the present invention which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of humans and/or other animals without undue toxicity, irritation, allergic response and the like, commensurate with a reasonable risk/benefit ratio, and effective for their intended use, as well as the zwitterionic forms, where possible, of the compounds of the invention.
- the compounds in Tables 1 and 3 were designed and synthesized as novel, functionally selective ligands of dopamine D 2 receptors to elucidate the key signal transduction pathways essential for antipsychotic efficacy and the undesired ones associated with side-effects. These novel compounds were evaluated in multiple D 2 binding and functional assays: D 2 radioligand binding, D 2 cAMP GloSensor, D 2 ERK phosphorylation (p-ERK), and/or D 2 ⁇ - arrestin 2 translocation (Tango). In the D 2 cAMP GloSensor assay, these compounds showed different activity profiles ranging from full agonists to antagonists to inverse agonists of the Gj- regulated cAMP pathway.
- these compounds showed significantly attenuated antipsychotic-like activity and increased catalepsy in p-arrestin-2 knockout mice.
- These novel, pharmacologically active compounds are useful agents for studying dopamine receptor function and signaling pathways, as well as for treating CNS disorders.
- the compounds may have advantageous pharmacokinetic characteristics for treating CNS disorders, including a longer half life in brain and higher brain :plasma ratio over 24 h compared to other antipsychotic compounds.
- the receptor binding pattern of the compounds e.g., relative low potency at histamine receptors suggests that the compounds may exhibit a lower risk for side effects, such as sedation and/or cognitive impairment.
- one aspect of the invention relates to a method of modulating the activity of a D 2 dopamine receptor comprising contacting the receptor with a compound of the invention.
- the D 2 dopamine receptor may be present in a cell, an isolated cell membrane or an artificial membrane.
- the cell may be a cultured cell, a cell isolated from a subject, or a cell in a subject (e.g., an animal model of disease or a patient).
- the receptor may be a wild-type or modified receptor and may be naturally-occurring or recombinantly expressed.
- modulating is intended to encompass any type of change in one or more activities of the receptor, including increasing or decreasing the activity of one or more receptor-linked signaling pathways.
- Many of the compounds of the invention display potent antipsychotic activity in animal models in vivo and are thus predicted to possess antipsychotic actions in humans and other animals.
- the compounds would thus be effective for treating diseases including, but not limited to, a variety of psychiatric disorders including: schizophrenia, schizoaffective disorder, schizophreniform disorder, bipolar disorder, mania, manic psychosis, Tourette's syndrome and Tourette-like disorders, obsessive-compulsive disorder, depression with psychotic features, psychosis not otherwise specified (Psychosis NOS) and additional affective and non-affective psychiatric disorders.
- the compounds would be effective against a number of neurological disorders including Huntington's Disease, Parkinsonian Psychosis, Alzheimer's Disease and associated psychosis and various organic brain syndromes. Further, the compounds would be effective at a variety of autism spectrum and autistic disorders. Given the D -receptor functional selectivity, the compounds would also be effective against pituitary diseases including pituitary adenomas and prolactinomas as well as endocrinological disorders including, but not limited to, galactorrhea.
- the compounds have significant and unexpected advantages over conventional antipsychotic drugs in that they are predicted to effectively treat psychotic disorders without appreciable extra-pyramidal side effects (EPS), having minimal propensity for elevating serum prolactin and with a potentially lower risk of inducing tardive dyskinesia.
- EPS extra-pyramidal side effects
- the compounds Given the low affinities for Hi -histamine and partial agonism for 5-HT 2C serotonin receptors the compounds are expected to differ from approved atypical antipsychotic drugs by virtue of a lower propensity to induce weight gain and associated metabolic adverse consequences (diabetes, hyperlipidemia, hyperglycemia, hypercholesterolemia, hypertension and so on (Kroeze et al, Neuropsychopharmacology 28:519 (2003)).
- the compounds also lack agonist activity at 5-HT 2 B serotonin receptors and hERG potassium channels and are thus predicted to have a low incidence of cardiovascular side-effects including prolongation of the Q-T interval and drug-induced valvular heart disease (Roth, N. Engl. J. Med. 356:6 (2007)). Given the affinities of certain compounds for 5-HT 6 and 5-HT 7 receptors the compounds are expected to have potential cognition-enhancing and antidepressant actions (Abbas et al, Psychopharmacology (Berl) 205: 119 (2009); Woolley et al, Neuropharmacology 41 :210 (2001)). Thus, these compounds are likely to also be effective in treating bipolar depression, unipolar depression, and a variety of anxiety disorders as well as disorders such as Alzheimer's Disease, dementia, Niemann-Pick Disorder, and other late-life dementias.
- one aspect of the invention relates to a method of treating a central nervous system disorder associated with D 2 dopamine receptors in a subject, comprising delivering to the subject a therapeutically effective amount of the compound of the invention.
- the disorder associated with D 2 dopamine receptors is a psychiatric, neurological, pituitary, or endocrine disorder.
- one or more of the compounds of the invention is administered to the subject as needed to treat a disorder.
- the compound can be administered continuously or intermittently.
- the compound is administered to the subject more than once a day or once every 1, 2, 3, 4, 5, 6, or 7 days.
- the compound is administered to the subject no more than once a week, e.g., no more than once every two weeks, once a month, once every two months, once every three months, once every four months, once every five months, once every six months, or longer.
- the compound is administered using two or more different schedules, e.g., more frequently initially (for example to build up to a certain level, e.g., once a day or more) and then less frequently (e.g., once a week or less).
- the compound can be administered by any discontinuous administration regimen.
- the compound can be administered not more than once every three days, every four days, every five days, every six days, every seven days, every eight days, every nine days, or every ten days, or longer.
- the administration can continue for one, two, three, or four weeks or one, two, or three months, or longer.
- the compound after a period of rest, can be administered under the same or a different schedule.
- the period of rest can be one, two, three, or four weeks, or longer, according to the pharmacodynamic effects of the compound on the subject.
- the compound of the invention can be delivered to the subject by any suitable route, e.g., oral, rectal, buccal (e.g., sub-lingual), vaginal, parenteral (e.g., subcutaneous, intramuscular, intradermal, or intravenous), topical (i.e., both skin and mucosal surfaces, including airway surfaces) and transdermal administration.
- the compound is delivered to the subject at a dose that is effective to treat the disorder.
- the effective dosage will depend on many factors including the gender, age, weight, and general physical condition of the subject, the severity of the disorder, the particular compound or composition being administered, the duration of the treatment, the nature of any concurrent treatment, the carrier used, and like factors within the knowledge and expertise of those skilled in the art.
- the compound is administered at a dose of about 0.001 to about 10 mg/kg body weight, e.g., about 0.001, 0.005, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 mg/kg.
- the dose can be even lower, e.g., as low as 0.0005 or 0.0001 mg/kg or lower.
- the dose can be even higher, e.g., as high as 20, 50, 100, 500, or 1000 mg/kg or higher.
- the present invention encompasses every sub-range within the cited ranges and amounts.
- the compound of the invention is delivered to a subject concurrently with an additional therapeutic agent.
- the additional therapeutic agent can be delivered in the same composition as the compound or in a separate composition.
- the additional therapeutic agent can be delivered to the subject on a different schedule or by a different route as compared to the compound.
- the additional therapeutic agent can be any agent that provides a benefit to the subject.
- Further agents include, without limitation, anti-psychotics, antidepressants, agents for neurological disorders, and chemotherapeutic agents.
- anti-psychotic agents include, without limitation, for psychosis: prochloroperazine, chlorpromazine, droperidol, fluphenazine, periciazine, perphenazine, thiothixene, triflupromazine, haloperidol, molindone, pimozide, and thioridazine: and for schizophrenia: olanzapine, paliperidone, aripiprazole, quetiapine, risperidone, clozapine, flupenthixene, iloperidone, loxapine, mesoridazine, promazine, reserpine, thioridazine, zuclopenthixol, asenapine, levomepromazine, ziprasidone, molindone, pimozide, and thioridazine.
- agents for depression and related disorders include, without limitation, for depression: modafinil, desvenlafaxine, nortriptyline, seleginline, amoxapine, citalopram, clomipramine, fluoxetine, isocarboxazid, mapriotiline, nefazodone, niacin, nortriptyline, phenelzine, protriptyline, tranylcypromine, trazodone, trimipramine, desipramine, imipramine, methylphenidate, buproprion, alprazolam, amitriptyline, chlordiazepoxide, perphenazine, doxepin, mirtazapine, 5-hydroxytryptophan, duloxetine, escitalopram, venlafaxine, desvenlafaxine, paroxetine, fluoxetine, olanzapine, 1-methylfolate, amitriptyline, sertraline, fluvoxamine
- agents for neurological disorders include, without limitation, for Alzheimer's disease: caprylidene, donepezil, galantamine, tacrine, vitamin E, ergoloid mesylates, rivastigmine; for Parkinson's disease: nadolol, zonisamide, amantadine, apomorphine, belladonna, benztropine, biperiden, bromocriptine, carbidopa, entacapone, levodopa, pergolide mesylate, pramipexole, procyclidine, rasagiline, ropinirole, rotiotine, scopolamine, tolcapone, trihexylphenidyl, rivastigmine, seleginline; for Huntington's disease: baclofen, pregabalin, tetrabenazine, methylprednisolone, desvenlafaxine, nortriptyline; and for dementia: haloperidol and
- chemotherapeutic agents include, without limitation, acivicin, aclarubicin, acodazole hydrochloride, acronine, adozelesin, aldesleukin, altretamine, ambomycin, ametantrone acetate, aminoglutethimide, amsacrine, anastrozole, anthramycin, asparaginase, asperlin, azacytidine, azetepa, azotomycin, batimastat, benzodepa, bicalutamide, bisantrene hydrochloride, bisnafide dimesylate, bizelesin, bleomycin sulfate, brequinar sodium, bropirimine, busulfan, cactinomycin, calusterone, caracemide, carbetimer, carboplatin, carmustine, carubicin hydrochloride, carzelesin, cedefingol, chloramb
- chemotherapeutic agents include, but are not limited to, 20-epi- 1,25 dihydroxyvitamin D3; 5-ethynyluracil; abiraterone; aclarubicin; acylfulvene; adecypenol; adozelesin; aldesleukin; ALL-TK antagonists; altretamine; ambamustine; amidox; amifostine; aminolevulinic acid; amrubicin; amsacrine; anagrelide; anastrozole; andrographolide; angiogenesis inhibitors; antagonist D; antagonist G; antarelix; anti-dorsalizing morphogenetic protein- 1; prostatic carcinoma antiandrogen; antiestrogen; antineoplaston; antisense oligonucleotides; aphidicolin glycinate; apoptosis gene modulators; apoptosis regulators; apurinic acid; ara-C
- Suitable subjects are generally mammalian subjects.
- mammalian subjects includes, but is not limited to, humans, non-human primates, cattle, sheep, goats, pigs, horses, cats, dog, rabbits, rodents (e.g., rats or mice), etc.
- Human subjects include neonates, infants, juveniles, adults and geriatric subjects.
- the subject is a human subject that has a CNS disorder associated with D 2 dopamine receptors.
- the subject used in the methods of the invention is an animal model of a CNS disorder associated with D 2 dopamine receptors.
- the subject can be a subject "in need of the methods of the present invention, e.g., in need of the therapeutic effects of the inventive methods.
- the subject can be a subject that is experiencing a CNS disorder associated with D 2 dopamine receptors and/or is anticipated to experience a CNS disorder associated with D 2 dopamine receptors, and the methods and compositions of the invention are used for therapeutic and/or prophylactic treatment.
- the compounds of the invention described above can be formulated for administration in a pharmaceutical carrier in accordance with known techniques. See, e.g., Remington, The Science And Practice of Pharmacy (21 st ed. 2005).
- the compound is typically admixed with, inter alia, an acceptable carrier.
- the carrier must, of course, be acceptable in the sense of being compatible with any other ingredients in the formulation and must not be deleterious to the patient.
- the carrier can be a solid or a liquid, or both, and can be formulated with the compound as a unit-dose formulation, for example, a tablet, which can contain from 0.01% or 0.5% to 95% or 99% by weight of the compound.
- One or more compounds can be incorporated in the formulations of the invention, which can be prepared by any of the well known techniques of pharmacy comprising admixing the components, optionally including one or more accessory ingredients.
- compositions of the invention include those suitable for oral, rectal, topical, buccal (e.g., sub-lingual), vaginal, parenteral (e.g., subcutaneous, intramuscular, intradermal, or intravenous), topical (i.e., both skin and mucosal surfaces, including airway surfaces) and transdermal administration, although the most suitable route in any given case will depend on the nature and severity of the condition being treated and on the nature of the particular active compound which is being used.
- Formulations suitable for oral administration can be presented in discrete units, such as capsules, cachets, lozenges, or tablets, each containing a predetermined amount of the active compound; as a powder or granules; as a solution or a suspension in an aqueous or nonaqueous liquid; or as an oil-in-water or water-in-oil emulsion.
- Such formulations can be prepared by any suitable method of pharmacy which includes the step of bringing into association the compound and a suitable carrier (which can contain one or more accessory ingredients as noted above).
- the formulations of the invention are prepared by uniformly and intimately admixing the compound with a liquid or finely divided solid carrier, or both, and then, if necessary, shaping the resulting mixture.
- a tablet can be prepared by compressing or molding a powder or granules containing the compound, optionally with one or more accessory ingredients.
- Compressed tablets can be prepared by compressing, in a suitable machine, the compound in a free-flowing form, such as a powder or granules optionally mixed with a binder, lubricant, inert diluent, and/or surface active/dispersing agent(s).
- Molded tablets can be made by molding, in a suitable machine, the powdered compound moistened with an inert liquid binder.
- Formulations suitable for buccal (sub-lingual) administration include lozenges comprising the compound in a flavored base, usually sucrose and acacia or tragacanth; and pastilles comprising the compound in an inert base such as gelatin and glycerin or sucrose and acacia.
- Formulations of the present invention suitable for parenteral administration comprise sterile aqueous and non-aqueous injection solutions of the compound, which preparations are preferably isotonic with the blood of the intended recipient. These preparations can contain anti-oxidants, buffers, bacteriostats and solutes which render the formulation isotonic with the blood of the intended recipient.
- Aqueous and non-aqueous sterile suspensions can include suspending agents and thickening agents.
- the formulations can be presented in unit/dose (e.g., in a syringe or other injection device) or multi-dose containers, for example sealed ampoules and vials, and can be stored in a freeze-dried (lyophilized) condition requiring only the addition of the sterile liquid carrier, for example, saline or water-for-injection immediately prior to use.
- sterile liquid carrier for example, saline or water-for-injection immediately prior to use.
- Extemporaneous injection solutions and suspensions can be prepared from sterile powders, granules and tablets of the kind previously described.
- an injectable, stable, sterile composition comprising one or more compounds, in a unit dosage form in a sealed container.
- the compound is provided in the form of a lyophilizate which is capable of being reconstituted with a suitable pharmaceutically acceptable carrier to form a liquid composition suitable for injection thereof into a subject.
- the unit dosage form typically comprises from about 0.001 mg to about 10 grams of the compound.
- a sufficient amount of emulsifying agent which is physiologically acceptable can be employed in sufficient quantity to emulsify the compound in an aqueous carrier.
- emulsifying agent is phosphatidyl choline.
- Formulations suitable for rectal administration are preferably presented as unit dose suppositories. These can be prepared by admixing the compound with one or more conventional solid carriers, for example, cocoa butter, and then shaping the resulting mixture.
- one or more conventional solid carriers for example, cocoa butter
- Formulations suitable for topical application to the skin preferably take the form of an ointment, cream, lotion, paste, gel, spray, aerosol, or oil.
- Carriers which can be used include petroleum jelly, lanoline, polyethylene glycols, alcohols, transdermal enhancers, and combinations of two or more thereof.
- Formulations suitable for transdermal administration can be presented as discrete patches adapted to remain in intimate contact with the epidermis of the recipient for a prolonged period of time. Formulations suitable for transdermal administration can also be delivered by iontophoresis (see, for example, Pharm. Res. 3:318 (1986)) and typically take the form of an optionally buffered aqueous solution of the compound. Suitable formulations comprise citrate or bis ⁇ tris buffer (pH 6) or ethanol/water and contain from 0.1 to 0.2 M active ingredient.
- compositions can be prepared from the compounds disclosed herein, such as aqueous base emulsions.
- the composition will contain a sufficient amount of pharmaceutically acceptable emulsifying agent to emulsify the desired amount of the compound.
- Particularly useful emulsifying agents include phosphatidyl cholines and lecithin.
- the pharmaceutical compositions can contain other additives, such as pH-adjusting additives.
- useful pH-adjusting agents include acids, such as hydrochloric acid, bases or buffers, such as sodium lactate, sodium acetate, sodium phosphate, sodium citrate, sodium borate, or sodium gluconate.
- the compositions can contain microbial preservatives.
- Useful microbial preservatives include methylparaben, propylparaben, and benzyl alcohol. The microbial preservative is typically employed when the formulation is placed in a vial designed for multidose use.
- additives that are well known in the art include, e.g., detackifiers, anti-foaming agents, antioxidants (e.g., ascorbyl palmitate, butyl hydroxy anisole (BHA), butyl hydroxy toluene (BHT) and tocopherols, e.g., a-tocopherol (vitamin E)), preservatives, chelating agents (e.g., EDTA and/or EGTA), viscomodulators, tonicifiers (e.g., a sugar such as sucrose, lactose, and/or mannitol), flavorants, colorants, odorants, opacifiers, suspending agents, binders, fillers, plasticizers, lubricants, and mixtures thereof.
- detackifiers e.g., anti-foaming agents
- antioxidants e.g., ascorbyl palmitate, butyl hydroxy anisole (BHA), butyl hydroxy to
- the additive can also comprise a thickening agent.
- suitable thickening agents can be those known and employed in the art, including, e.g., pharmaceutically acceptable polymeric materials and inorganic thickening agents.
- Exemplary thickening agents for use in the present pharmaceutical compositions include polyacrylate and polyacrylate co-polymer resins, for example poly-acrylic acid and poly-acrylic acid/methacrylic acid resins; celluloses and cellulose derivatives including: alkyl celluloses, e.g., methyl-, ethyl- and propyl-celluloses; hydroxyalkyl- celluloses, e.g., hydroxypropyl-celluloses and hydroxypropylalkyl-celluloses such as hydroxypropyl-methyl-celluloses; acylated celluloses, e.g., cellulose-acetates, cellulose- acetatephthallates, cellulose-acetatesuccinates and hydroxypropylmethyl-cellulose phthallates; and salts thereof
- thickening agents as described above can be included, e.g., to provide a sustained release effect.
- the use of thickening agents as aforesaid will generally not be required and is generally less preferred.
- Use of thickening agents is, on the other hand, indicated, e.g., where topical application is foreseen.
- the present invention provides liposomal formulations of the compounds disclosed herein.
- the technology for forming liposomal suspensions is well known in the art.
- the compound When the compound is in the form of an aqueous-soluble material, using conventional liposome technology, the same can be incorporated into lipid vesicles. In such an instance, due to the water solubility of the compound, the compound will be substantially entrained within the hydrophilic center or core of the liposomes.
- the lipid layer employed can be of any conventional composition and can either contain cholesterol or can be cholesterol-free.
- the compound of interest is water-insoluble, again employing conventional liposome formation technology, the compound can be substantially entrained within the hydrophobic lipid bilayer which forms the structure of the liposome.
- the liposomes which are produced can be reduced in size, as through the use of standard sonication and homogenization techniques.
- the liposomal formulations containing the compound disclosed herein can be lyophilized to produce a lyophilizate which can be reconstituted with a pharmaceutically acceptable carrier, such as water, to regenerate a liposomal suspension.
- Mass spectra (MS) and HPLC (UV 254 nM or ELSD) data for all compounds were recorded on an 1100 LC/MS system (Agilent Technology Corporation) using a 4.6x50 mm column (CenturySIL C-18 AQ + , 5 ⁇ ) with a linear gradient 30-90% (v/v) acetonitrile-water with 0.035% trifluoroacetic acid over 5 min with a flow rate of 3.5 mL/min.
- Analytical TLC was performed using 2.5 x 5 cm plates coated with 0.25 mm of silica gel GF254. Column chromatography was performed on silica gel G (200-300 mesh) with reagent grade solvents.
- a well-dried flask was first charged with l-bromo-2,3-dichlorobenzene (10 g, 44 mmol) and piperazine (4.55 g, 53 mmol), which was evacuated and backfilled with N 2 through a balloon under gentle warming (40°C). Toluene was charged and the mixture was bubbled with N 2 for 10 min, then BINAP (822 mg, 1.32 mmol) and Pd 2 dba 3 (403 mg, 0.44 mmol) were added to the mixture.
- Triethylamine in acetone was added dropwise to the solution of 2,3- dihydrobenzo[b][l,4]dioxine-5-carboxylic acid (360 mg, 2 mmol) in water and acetone at 0°C.
- ethyl carbonochloridate was added dropwise.
- the reaction mixture was stirred for 1 h then it was poured into ice water and extracted with Et 2 0. The combined organic extracts were washed with brine, dried with MgS0 4 , and concentrated in vacuo.
- Cells stably expressing D 2 receptors were plated in 15-cm dishes (in DMEM containing 10% fetal bovine serum and grown to 90% confluence. Then, cells were washed with PBS, pH 7.4, and harvested by scraping into PBS, pH 7.4. Harvested cells were centrifuged at 1,000 x g for 10 min, then hypotonically lysed by resuspension in ice-cold 50 mM HEPES, 1% BSA, pH 7.4. Membranes were isolated by centrifugation at 21,000 x g for 20 min. The supernatant was removed and the membrane pellets were assayed for D2 ligand-stimulated GTP-gamma-S binding or stored at -80°C until used for radioligand binding assays.
- Membranes prepared as above were resuspended to 1 ⁇ g protein/ ⁇ (measured by Bradford assay using BSA as standard), and 50 ⁇ were added to each well of a polypropylene 96-well plate containing (per well): 50 ⁇ of buffer (20 mM HEPES, 10 mM MgCl 2 , 1 mM EDTA, 1 mM EGTA, 100 mM N-methyl-D-gluconate, pH 7.4), 50 ⁇ of 1.5 nM [ 3 H]N- methylspiperone (final concentration 0.3 nM) and reference or D2 test ligand at various concentrations ranging from 50 pM to 50 ⁇ (final concentrations ranging from 10 pM to 10 ⁇ , triplicate determinations for each concentration of D 2 test ligand).
- buffer 20 mM HEPES, 10 mM MgCl 2 , 1 mM EDTA, 1 mM EGTA, 100 mM N-methyl-D-gluconate
- Residual [ 3 H]N-methylspiperone binding to filtered membranes was plotted as a function of log [reference] or log [D 2 test ligand] and the data were regressed using the one-site competition model built into Prism 4.0 (GraphPad software).
- HEK293T cells co-expressing the cAMP biosensor GloSensor-22F (Promega) and hD 2 receptors were seeded (10,000 cells/20 ⁇ /well) into white, clear-bottom, tissue culture plates in HBSS, 10% FBS, 20 mM HEPES, pH 7.4. After a one- to two-hour recoveiy, cells were treated with 10 ⁇ of 3X test or reference drug prepared in HBSS, 10% FBS, 20 mM HEPES, pH 7.4. After 30 minutes, cells were challenged with 10 ⁇ of 1,200 nM (4X) isoproterenol in 8% (4X) GloSensor reagent.
- Luminescence per well per second was read on a Wallac TriLux microbeta plate counter. Data were normalized to the isoproterenol response (100%) and the maximal quinpirole-induced inhibition thereof (0%) and regressed using the sigmoidal dose- response function built into GraphPad Prism 4.0. Notably, HEK293T cells expressing the GloSensor-22F alone (no hD 2 ) were assayed in parallel and displayed no inhibition of isoproterenol-stimulated cAMP, either by quinpirole or by the test compounds, suggesting that the effect observed in hD 2 -expressing cells was due to compound acting via the recombinant receptor.
- Immunofluorescence Automatic multichannel pipetters were used for liquid handling and multichannel vacuum manifolds for aspirations. Each tested concentration was typically measured in triplicate or quadruplicate. For concentration curves, half-log-dilutions were used. Drug dilutions were prepared in stimulation medium (serum-free medium, 100 mg/L ascorbic acid). 100 ⁇ dopamine and 1 ⁇ PMA (phorbol 12-myristate 13 -acetate) were used as positive controls. Medium only was used as a negative control. Specificity of the response was confirmed by pretreatment for 5 min with 10 ⁇ of the antagonist spiperone and in experiments using wild type CHO cells.
- stimulation medium serum-free medium, 100 mg/L ascorbic acid
- PMA phorbol 12-myristate 13 -acetate
- Plates were incubated in 100 ⁇ , ⁇ blocking buffer (PBS/Ca, 5% goat serum, 0.1% Triton X-100) for 1 h at room temperature and then in blocking buffer containing rabbit phospho-Thr202/p-Tyr204-ERK antibody (Cell Signaling 9101) at 1 :1000 at 4°C overnight. Cells were washed 3X for 5-10 min with 250 ⁇ wash buffer (PBS/Ca, 0.03% Triton X-100).
- PBS/Ca 5% goat serum, 0.1% Triton X-100
- Plates were incubated with 50 ⁇ Alexa-594 coupled goat anti-rabbit IgG (Invitrogen) at 1 :250, 5 ⁇ g/mL Hoechst 33342 nuclear stain, and 25 ⁇ g/mL concanavaline-Alexa488 conjugate (ConA, Invitrogen) in blocking buffer for 2 h at room temperature. Plates were washed three times with 250 ⁇ ⁇ ⁇ washing buffer, post-fixed for 10 min in fixing buffer, washed with 250 ⁇ PBS/Ca, filled with 200 nL/well PBS/Ca, sealed with transparent adhesive plate seals, and stored at 4°C.
- Alexa-594 coupled goat anti-rabbit IgG Invitrogen
- ConA concanavaline-Alexa488 conjugate
- Microscopy and Image Analysis Plates were scanned with a high-content automated microscopic system (BD Pathway 855) using a 20x objective and a 2 x 2 image montage setting.
- the Alexa594 light path was used for the target signal, Alexa488 for whole cell staining, and Hoechst for the nuclear staining.
- Well-averaged individual cell-based measurements were exported to Excel and cell- free background intensity was subtracted from the whole cell intensity. Concentration curves were analyzed in GraphPad Prism by fitting against a sigmoidal dose-response model and normalization to the dopamine curve.
- the cAMP (Glo-Sensor) assay shows that the majority of the tested compounds are not agonists for the Gj-coupled signaling pathway and that 14 of the 43 compounds function as inverse agonists for the Gj-coupled signaling pathway (i.e., have a negative E max ).
- the p-ERK assay confirms that the majority of compounds lack agonism via canonical G-protein-dependent pathways.
- 2 and 3 had moderate to high binding affinity ( j's: 0.6 - 250 nM) at 5HT 2A , 5HT 2B , 5HT 2C , and 5HTi A , but were significantly less potent in functional assays (Ca 2+ mobilization fluorometric imaging plate reader (FLIPR) or cAMP biosensor).
- 2 and 3 were antagonists at 5HT 2A , 5HT 2B , and 5HT 2C and weak agonists (EC 50 's > 1 ⁇ ) at 5HTi A .
- 2 and 3 had moderate affinities to Hi -histamine receptor (Xj's ⁇ 10 nM) but were less potent in Hi functional assays.
- 2 and 3 display a similar G protein-coupled receptor selectivity profile as aripiprazole (Table 7).
- Neutral ligands are either antagonists or are inactive; NA: not applicable.
- Kj, IC 50 , pA 2 , or EC 50 values are the average of at least two duplicate experiments with standard deviation (SD) values that are 3-fold less than the average.
- mice All experiments were approved by the Institutional Animal Care and Use Committees at the University of North Carolina, Chapel Hill and Duke University. C57BL/6J and P-arrestin-2 knockout (Bohn et al, Science 286:2495 (1999)) mice were housed under standard conditions - 12 hour light/dark cycle and food and water provided ad libitum. Adult, age-matched male and female C57BL/6J and P-arrestin-2 knockout drug naive mice were used for all behavioral testing.
- Locomotor activity was assessed in photocell-based activity chambers under standardized environmental conditions, using an AccuScan activity monitor (AccuScan Instruments, Columbus, OH) with a 25.8 x 25.8 cm Plexiglas chamber and a beam spacing of 1.52 cm as described (Abbas et al, J. Neurosci. 29:7124 (2009)). Mice were injected (i.p.) with vehicle (0.9% saline/0.2% acetic acid) , aripiprazole (0.10, 0.25, 0.50 or 2.0 mg/kg), or UNC9975 (0.25, 0.5 and 2.0 mg/kg) and placed into the open field.
- vehicle i.p.
- aripiprazole 0.10, 0.25, 0.50 or 2.0 mg/kg
- UNC9975 0.25, 0.5 and 2.0 mg/kg
- mice were initially injected (i.p.) with vehicle (0.9% saline/0.2% acetic acid), aripiprazole, U C0006 (5 mg/kg), UNC9975 (5 mg/kg) or haloperidol (2 mg/kg) and returned to their home cage.
- vehicle 0.9% saline/0.2% acetic acid
- U C0006 5 mg/kg
- UNC9975 5 mg/kg
- haloperidol haloperidol
- UNC9975 exhibited potent antipsychotic-like activity in mouse hyperlocomotion studies which is attenuated in p-arrestin-2 knockout mice .
- locomotion responses in inbred C57BL/6 mice are shown as 5 minute binned intervals to different doses (i.p.) of UNC9975 or vehicle followed 30 min later by 3 mg/kg D-Amphetamine (AMPH, i.p.).
- AMPH i.p.
- Fig. IB total distance traveled after D-Amphetamine administration (30-70 min time interval) is shown.
- C57BL/6 mice were given vehicle or different doses of UNC9975 or aripiprazole 30 min prior to AMPH treatment.
- n 8 animals/group. *, p ⁇ 0.05 versus vehicle + 3 mg/kg D- Amphetamine group.
- locomotor activities are shown as 5 min binned intervals of wild-type (WT) or P-arrestin-2 knockout ( ⁇ - ⁇ 2 KO) littermate mice given vehicle or different doses of UNC9975 followed 30 min later with 6 mg/kg phencyclidine (PCP, i.p.).
- UNC9975 and U C0006 induced catalepsy in p-arrestin-2 knockout mice but not in wild-type littermates.
- wild-type and p-Arrestin-2 knockout littermate mice were administered (i.p.) vehicle, 5.0 mg/kg UNC9975, 5.0 mg/kg UNC0006, 5.0 mg/kg aripiprazole, or 2.0 mg/kg haloperidol.
- novel D 2 ⁇ -arrestin-biased agonists were designed, synthesized and characterized, and demonstrated to have unique atypical antipsychotic drug-like activities in vivo. These novel compounds represent the first functionally selective ⁇ -arrestin- biased dopamine D 2 receptor ligands to exhibit antipsychotic activity in vivo. These findings show that based on the arrestin-bias of these compounds, ⁇ -arrestin may be an important contributor to both antipsychotic drug efficacy and antipsychotic side effects.
- the disclosed compounds were discovered as unique, ⁇ -arrestin-biased functionally selective D 2 ligands.
- both UNC0006 and UNC9975 are potent partial agonists which induce D 2 receptors-mediated ⁇ - arrestin recruitment and signaling, and simultaneously are potent inverse agonists at Gi-dependent signaling.
- the in vivo atypical antipsychotic drug-like activity of UNC9975 indicated a strict requirement for p-arrestin-2 for both full antipsychotic activity and protection against motoric side-effects.
- this ⁇ -arrestin-biased ligand shows a potent ability to suppress both D-amphetamine and phencyclidine-induced hyperlocomotion in mice, indicating that the compound possesses antipsychotic drug-like activities in vivo.
- the antipsychotic drug-like activity of UNC9975 was attenuated in ⁇ -3 ⁇ 8 ⁇ -2 knockout mice, indicating p-arrestin-2 is required in vivo for full activity.
- aripiprazole With the exception of aripiprazole, all FDA approved typical and atypical antipsychotic medications (e.g., haloperidol, chlorpromazine, clozapine, risperidone) share the common property of antagonizing D 2 -mediated G protein-dependent and independent signaling (Masri et al, Proc. Natl. Acad. Sci. USA 105:13656 (2008)). Indeed, typical and atypical antipsychotic drugs, with the exception of aripiprazole, are inverse agonists at Gj-mediated signaling and antagonists at arrestin-ergic pathways.
- typical and atypical antipsychotic drugs are inverse agonists at Gj-mediated signaling and antagonists at arrestin-ergic pathways.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Abstract
The present invention relates to novel functionally selective ligands of dopamine D2 receptors, including agonists, antagonists, and inverse agonists. The invention further relates to the use of these compounds for treating central nervous system disorders related to D2 receptors.
Description
Functionally Selective Ligands of Dopamine D2 Receptors
Statement of Priority
[0001] This application claims the benefit of U.S. Provisional Application Serial No. 61/360,951, filed July 2, 2010, the entire contents of which is incorporated by reference herein.
Statement of Government Support
[0002] Aspects of this invention were made with government support under National Institute of Mental Health Grant Nos. U19MH082441S1 and U19MH082441. The United States Government has certain rights to this invention.
Field of the Invention
[0003] The present invention relates to novel functionally selective ligands of dopamine D2 receptors, including agonists, antagonists, and inverse agonists. The invention further relates to the use of these compounds in research and for treating central nervous system disorders related to D2 receptors.
Background of the Invention
[0004] Classical notions of receptor pharmacology imply that when a ligand interacts with a receptor, only a unitary outcome is possible. According to these models of receptor pharmacology, a full or partial agonist can activate only a single signal transduction pathway while an antagonist can only block the actions of an agonist. The key theoretical construct underlying this model was the concept of intrinsic efficacy (Furchgott, Advances in Drug Research (Harper N and Simmonds A eds) Academic Press, New York, pp 21-55 (1966)). According to this conceptualization, a full agonist has maximum intrinsic efficacy and maximally stimulates all cellular responses induced by ligand binding. A partial agonist possesses a lower degree of intrinsic efficacy and partially activates all cellular responses induced by an agonist Antagonists, according to this schema, are neutral entities which possess no intrinsic activity but are able to block the receptor and preclude activation by full or partial agonists (Furchgott, Advances in Drug Research (Harper N and Simmonds A eds) Academic Press, New York., pp 21- 55 (1966)). An extension of this model has been that a single G-protein coupled receptor (GPCR) interacts with a single G protein subtype (e.g., Gi, Gs, Gq, Go and so on) and that full and partial agonists activate only a single transduction pathway.
[0005] For many decades, however, it has been clear that these simplistic notions of GPCR (also known as 7-transmembrane receptor or 7-TM) function cannot account for the myriad of actions induced by agonist or antagonist binding. This was first convincingly
demonstrated for the serotonin (5-hydroxytryptamine; 5-HT) and dopamine (DA) families of receptors where it has been demonstrated that: (1) agonists differentially activate distinct signal transduction pathways (Beaulieu et al, J. Neurosci. 27:881 (2007); Berg et al, Mol. Pharmacol. 54:94 (1998); Ghosh et al, J. Med. Chem. 39:549 (1996); Kilts et al, J. Pharmacol. Exp. Ther. 307: 1179 (2002); Mottola et al, J. Pharmacol. Exp. Ther. 307: 1166 (2002); Parrish et al, J. Neurochem. 95:1575 (2005); Roth and Chuang, Life Sci. ¥7:1051 (1987); Urban et al, J. Pharmacol. Exp. Ther. 320:1 (2007)); (2) antagonists can possess negative intrinsic activity (e.g., function as inverse agonists) or be silent and thereby block the actions of an inverse agonist (e.g., neutral antagonists) (Barker et al, J. Biol. Chem. 269:11687 (1994); Burstein et al, J. Pharmacol. Exp. Ther. 375:1278 (2005); Chidiac et al, Mol Pharmacol. 45:490 (1994); Gilliland and Alper, Naunyn Schmiedebergs Arch. Pharmacol. 3(57:498 (2000); Rauser et al, J. Pharmacol. Exp. Ther. 299:83 (2001)); (3) antagonists can also induce receptor down regulation (Peroutka and Snyder, Science 270:88 (1980)) and receptor internalization in vitro (Berry et al, Mol. Pharmacol. 50:306 (1996)) and in vivo (Gray and Roth, Brain Res. Bull 56:441 (2001); Willins et al, Ann. NY Acad. Sci. 861:121 (1998); Willins et al, euroscience 91:599 (1999)) - properties typically associated with agonists.
[0006] The processes by which agonists and antagonists differentially modulate signaling pathways and receptor trafficking have been variously dubbed 'functional selectivity', 'biased agonism' or 'agonist-directed trafficking'. Currently, the preferred term for the process by which ligands can differentially activate signaling pathways mediated via a single GPCR is functional selectivity (Urban et al, J. Pharmacol. Exp. Ther. 320:1 (2007)). Functional selectivity has been demonstrated for more than a dozen GPCRs (reviewed in (Urban et al, Neuropsychopharmacology 32:67 (2007)) and is, likely, a ubiquitous phenomenon. In particular, functional selectivity has been widely observed among DA receptor subtypes.
[0007] It is desirable to identify novel, functionally selective ligands of dopamine D2 receptors to elucidate the key signal transduction pathways essential for antipsychotic efficacy and the undesired ones associated with side-effects. Such compounds would also be useful agents for treating central nervous system (CNS) disorders including schizophrenia and depression.
Summary of the Invention
[0008] The present invention relates to novel, functionally selective ligands of dopamine D2 receptors. When evaluated in multiple D2 binding and functional assays, the compounds of the invention showed different activity profiles ranging from full agonists to antagonists to inverse agonists. In particular, the compounds of the invention are β-arrestin biased and, in
certain embodiments, also are inverse agonists of Gj-regulated pathways. These pharmacologically active compounds exhibit fewer side effects and are useful in research to study D2 receptor function and its role in psychosis and for the treatment of CNS disorders.
[0009] Accordingly, as one aspect, the invention provides a compound of formula I:
wherein m is 0 or 1 ;
n is 0, 1, or 2;
q is 1 or 2;
X is O, NH, or CH2;
Y is N or CH;
Z is C, CH, CH2, cycloalkyl, aryl, or heteroaryl;
is a single, double, or triple bond as valencies permit; and
Aii and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
[0010] In another aspect, the invention provides a compound of formula II:
wherein n is 0, 1, or 2;
q is 1 or 2;
X is O, NH, or CH2;
Y is N or CH;
Z is C, CH, CH2, cycloalkyl, aryl, or heteroaryl;
is a single, double, or triple bond as valencies permit; and
Ari and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
[0011] In a further aspect, the invention provides a compound of formula VI:
wherein n is 0, 1, or 2;
q is 1 or 2;
X is O, NH, or CH2;
Y is N or CH;
Z is C, CH, CH2, cycloalkyl, aryl, or heteroaryl;
is a single, double, or triple bond as valencies permit; and
Ari and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
[0012] The invention further relates to a pharmaceutical composition comprising the compound of the invention and a pharmaceutically acceptable carrier.
[0013] The invention also relates to a method of modulating the activity of a D2 dopamine receptor comprising contacting the receptor with a compound of the invention.
[0014] The invention additionally relates to a method of treating a central nervous system disorder associated with D2 dopamine receptors in a subject (e.g., a psychiatric, neurological, pituitary, or endocrine disorder), comprising delivering to the subject a therapeutically effective amount of the compound of the invention.
[0015] The invention further relates to the use of a compound of the invention for treating a central nervous system disorder associated with D2 dopamine receptors in a subject.
[0016] The invention further relates to the use of a compound of the invention for stimulating D2 dopamine receptors in a β-arrestin biased manner.
[0017] The present invention is explained in greater detail in the drawings herein and the specification set forth below.
Brief Description of the Drawings
[0018] Figures 1A-1F show that UNC 9975 exhibits potent antipsychotic-like activity in mouse hyperlocomotion studies.
[0019] Figure 2 shows that UNC9975 and UNC0006 induce catalepsy in p-arrestin-2 knockout mice but not in wild-type littermates.
Detailed Description of the Invention
[0020] The present invention can be embodied in different forms and should not be construed as limited to the embodiments set forth herein. Rather, these embodiments are provided so that this disclosure will be thorough and complete, and will fully convey the scope of the invention to those skilled in the art. For example, features illustrated with respect to one embodiment can be incorporated into other embodiments, and features illustrated with respect to a particular embodiment can be deleted from that embodiment. In addition, numerous variations and additions to the embodiments suggested herein will be apparent to those skilled in the art in light of the instant disclosure, which do not depart from the instant invention.
[0021] Unless otherwise defined, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. The terminology used in the description of the invention herein is for the purpose of describing particular embodiments only and is not intended to be limiting of the invention.
[0022] Unless the context indicates otherwise, it is specifically intended that the various features of the invention described herein can be used in any combination.
[0023] Moreover, the present invention also contemplates that in some embodiments of the invention, any feature or combination of features set forth herein can be excluded or omitted.
[0024] To illustrate, if the specification states that a complex comprises components A, B and C, it is specifically intended that any of A, B or C, or a combination thereof, can be omitted and disclaimed singularly or in any combination.
[0025] All publications, patent applications, patents, and other references mentioned herein are incorporated by reference herein in their entirety.
Definitions.
[0026] As used herein, "a," "an," or "the" can mean one or more than one. For example, "a" cell can mean a single cell or a multiplicity of cells.
[0027] Also as used herein, "and/or" refers to and encompasses any and all possible combinations of one or more of the associated listed items, as well as the lack of combinations when interpreted in the alternative ("or").
[0028] Furthermore, the term "about," as used herein when referring to a measurable value such as an amount of a compound or agent of this invention, dose, time, temperature, and the like, is meant to encompass variations of ± 20%, ± 10%, ± 5%, ± 1%, ± 0.5%), or even + 0.1% of the specified amount.
[0029] The term "consists essentially of (and grammatical variants), as applied to the compositions of this invention, means the composition can contain additional components as long as the additional components do not materially alter the composition. The term "materially altered," as applied to a composition, refers to an increase or decrease in the therapeutic effectiveness of the composition of at least about 20% or more as compared to the effectiveness of a composition consisting of the recited components.
[0030] The term "therapeutically effective amount" or "effective amount," as used herein, refers to that amount of a composition, compound, or agent of this invention that imparts a modulating effect, which, for example, can be a beneficial effect, to a subject afflicted with a disorder, disease or illness, including improvement in the condition of the subject (e.g., in one or more symptoms), delay or reduction in the progression of the condition, prevention or delay of the onset of the disorder, and/or change in clinical parameters, disease or illness, etc., as would be well known in the art. For example, a therapeutically effective amount or effective amount can refer to the amount of a composition, compound, or agent that improves a condition in a subject by at least 5%, e.g., at least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, or at least 100%.
[0031] "Treat" or "treating" or "treatment" refers to any type of action that imparts a modulating effect, which, for example, can be a beneficial effect, to a subject afflicted with a disorder, disease or illness, including improvement in the condition of the subject (e.g., in one or more symptoms), delay or reduction in the progression of the condition, and/or change in clinical parameters, disease or illness, etc., as would be well known in the art.
[0032] A "central nervous system disorder associated with D2 dopamine receptors" refers to any disorder in which modulation (either an increase or a decrease) of one or more activities of a D2 dopamine receptor in a subject results in the treatment of one or more symptoms of the disorder in the subject,
[0033] "Pharmaceutically acceptable," as used herein, means a material that is not biologically or otherwise undesirable, i.e., the material can be administered to an individual along with the compositions of this invention, without causing substantial deleterious biological effects or interacting in a deleterious manner with any of the other components of the composition in which it is contained. The material would naturally be selected to minimize any degradation of
the active ingredient and to minimize any adverse side effects in the subject, as would be well known to one of skill in the art {see, e.g., Remington's Pharmaceutical Science; 21st ed. 2005). Exemplary pharmaceutically acceptable carriers for the compositions of this invention include, but are not limited to, sterile pyrogen-free water and sterile pyrogen-free physiological saline solution.
[0034] "Concurrently" means sufficiently close in time to produce a combined effect (that is, concurrently can be simultaneously, or it can be two or more events occurring within a short time period before or after each other). In some embodiments, the administration of two or more compounds "concurrently" means that the two compounds are administered closely enough in time that the presence of one alters the biological effects of the other. The two compounds can be administered in the same or different formulations or sequentially. Concurrent administration can be carried out by mixing the compounds prior to administration, or by administering the compounds in two different formulations, for example, at the same point in time but at different anatomic sites or using different routes of administration.
[0035] The term "β-arrestin biased" refers to a compound that binds the D2 dopamine receptor, is a partial or full agonist of the β-arrestin pathway, and is either neutral or an inverse agonist of the Gj-regulated cAMP pathway.
[0036] The term "neutral" refers to a compound that binds to a receptor but does not stimulate a particular receptor-linked pathway.
[0037] The term "inverse agonist" refers to a compound that binds to a receptor and reduces constitutive activity of a particular receptor-linked pathway.
[0038] The term "alkyl" denotes a straight or branched hydrocarbon chain containing 1- 12 carbon atoms, e.g., 1-6 or 1-4 carbon atoms. Examples of alkyl groups include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl, and the like.
[0039] By "substituted alkyl" is meant an alkyl in which an atom of the alkyl is substituted with, for example, a carbon, nitrogen, sulfur, oxygen, silicon, or halogen atom, or alternatively a nitrogen, sulfur, oxygen, or halogen atom. The term encompasses substituents on alkyl, alkenyl, alkynyl, and cycloalkyl groups.
[0040] Examples of substituents that can be attached to any atom of the alkyl group in a "substituted alkyl" include cyclyl groups, heterocyclyl groups; aryl groups, heteroaryl groups, amino groups, amido groups, nitro groups, cyano groups, azide groups, hydroxy groups, alkoxy groups, acyloxy groups, thioalkoxy groups, acyl thioalkoxy groups, halogen groups, sulfonate groups, sulfonamide groups, ester groups, carboxylic acids, oxygen {e.g., a carbonyl group), and sulfur {e.g., a thiocarbonyl group). Substituents also include any chemical functional group that imparts improved water-solubility to the molecule {e.g., carboxylic acid, carboxylic ester,
carboxamido, morpholino, piperazinyl, imidazolyl, thiomorpholino, or tetrazolyl groups; both unsubstituted and substituted).
[0041] The term "cycloalkyl" denotes a monocyclic saturated carbocyclic group containing 3-8 carbon atoms, e.g., 3-6 carbon atoms. Examples of cycloalkyl groups include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, and the like.
[0042] The term "alkoxy" denotes an oxygen linked to an alkyl or substituted alkyl as defined above.
[0043] The terms "halo" and "halogen" refer to any radical of fluorine, chlorine, bromine or iodine.
[0044] The terms "ring" and "ring system" refer to a ring comprising the delineated number of atoms, said atoms being carbon or, where indicated, a heteroatom such as nitrogen, oxygen or sulfur. The ring itself, as well as any substituents thereon, can be attached at any atom that allows a stable compound to be formed.
[0045] The term "aryl" refers to an aromatic 5-8 membered monocyclic or 8-12 membered bicyclic ring system wherein 0, 1, 2, or 3 atoms of each ring can be substituted by a substituent. The term also includes aromatic bicyclic ring systems in which a hydrogen atom has been added to one, two, or three of the ring carbons in one of the rings (e.g., a partially saturated ring). Examples of aryl groups include phenyl, naphthyl and the like.
[0046] The term "heteroaryl" refers to an aromatic 5-8 membered monocyclic or 8-12 membered bicyclic ring system comprising 1-3 heteroatoms if monocyclic or 1-6 heteroatoms if bicyclic, said heteroatoms selected from O, N, or S, wherein 0, 1, 2 or 3 atoms of each ring can be substituted by a substituent. The term also includes aromatic bicyclic ring systems in which a hydrogen atom has been added to one, two, or three of the ring carbons in one of the rings (e.g., a partially saturated ring). Examples of heteroaryl groups include pyridyl, furyl or furanyl, benzofuranyl, imidazolyl, benzimidazolyl, pyrimidinyl, thiophenyl or thienyl, benzothiophenyl, quinolinyl, isoquinolinyl, dihydroquinolinyl, dihydroisoquinolinyl, naphthyridinyl, dihydronaphthyridinyl, quinazolinyl, indolyl, indazolyl, thiazolyl, benzothiazolyl, oxazinyl, benzooxazinyl, oxazolyl, benzooxazolyl, dihydrobenzodioxinyl, and the like.
[0047] Suitable substituents for aryl and heteroaryl groups are the same as the substituents for alkyl groups.
Compounds.
[0048] The present invention provides novel, functionally selective ligands of dopamine D2 receptors. In particular, the compounds of the invention are β-arrestin biased. In certain embodiments, the compounds are at least partial agonists of the β-arrestin pathway. In other
embodiments, the compounds are full agonists of the β-arrestin pathway. In further embodiments, the compounds also are neutral or inverse agonists of the Gj-regulated cAMP pathway. These compounds represent the first functionally selective β-arrestin-biased dopamine D2 receptor ligands to exhibit antipsychotic activity in vivo.
[0049] In one aspect the invention provides a compound of formula I:
wherein m is 0 or 1;
q is 1 or 2;
n is 0, 1, or 2;
X is O, NH, or CH2;
Y is N or CH;
Z is C, CH, CH2, cycloalkyl, aryl, or heteroaryl;
is optionally a single, double, or triple bond as valencies permit; and Ari and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
[0050] In one embodiment, Ar2 is an unsubstituted or substituted bicyclic aryl or heteroaryl. In another embodiment, Z is C, CH, CH2, cycloalkyl, monocyclic aryl, or monocyclic heteroaryl. The bond indicated as refers to the bond attached to Z and not to any other bond, e.g., not between the two carbon atoms that are present when q is 2.
[0051] In one aspect the compound of the invention has the structure of formula II:
wherein n is 0, 1, or 2;
q is 1 or 2;
X is O, NH, or CH2;
Y is N or CH;
Z is C, CH, CH2, cycloalkyl, aryl, or heteroaryl;
is optionally a single, double, or triple bond as valencies permit; and Arj and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
[0052] In one embodiment, the compound of the invention has the structure of formula
III:
wherein n is 0, 1, or 2;
X is O, NH, or CH2;
Y is N or CH; and
Ari and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
[0053] In another e structure of formula IV:
[0054] In one embodiment for the compound of formula II-IV, n is 1. In a further embodiment, n is 0. In another embodiment, X is O. In another embodiment, X is NH.
[0055] In one embodiment for the compound of formula II-IV, Ax\ is selected from the group consisting of phenyl, substituted phenyl (e.g., chlorophenyl, dichlorophenyl, fluorophenyl, trifluoromethylphenyl, methylphenyl, cyanophenyl, methoxyphenyl, ethoxyphenyl, isopropoxyphenyl), 2,3-dihydro-lH-benzo[d]imidazolyl, benzo[d]oxazol-2(3H)-one, 1H- benzo[d]imidazolyl, benzo[d]oxazolyl, IH-indazolyl, IH-indazolyl, IH-indolyl, benzofuranyl, benzo[b]thiophenyl, benzo[d]thiazolyl, naphthalenyl, isoquinolinyl, quinazolinyl, quinolinyl, and pyridinyl, or any subgroup selected from the list. In a further embodiment, Ari is dichlorophenyl.
[0056] In one embodiment for the compound of formula II-IV, Ar2 is selected from the group consisting of 3,4-dihydroquinolin-2(lH)-one, 3,4-dihydro-l ,8-naphthyridin-2(lH)-one,
quinolin-2(lH)-one, 3,4-dihydroisoquinolin-l(2H)-one, benzo jthiazole, lH-benzo[</Jimidazol- 2(3H)-one, lH-indazole, and benzo[d]oxazol-2(3H)-one, or any subgroup selected from the list.
[0057] In one embodiment for the compound of formula II-IV, Ari is dichlorophenyl or methoxyphenyl and Ar2 is quinolinone, dihydroquinolinone, naphthyridinone, or benzothiazole.
[0058] In one embodiment for the compound of formula II-IV, Α is dichlorophenyl, methoxyphenyl, ethoxyphenyl, or isopropyloxyphenyl and Ar2 is quinolinone, dihydroquinolinone, naphthyridinone, benzothiazole, or benzoimidazolone.
[0059] In certain embodiments for the compound of formula II-IV, Ar2 is not naphthyridinone, dihydronaphthyridinone, quinolinone, dihydroquinolinone, tetrahydropyridoazepinone, or iso indole.
[0060] In a further embodi
[0061] In certain embodiments, the compounds of the invention have the structure of formula
[0062] In one embodiment, the compound of formula II is selected from the group of compounds shown in Tables 1 and 2.
Table 1
[0063] In one embodiment, the compound of the invention has the structure of formula
wherein n is 0, 1, or 2;
q is 1 or 2;
X is O, NH, or C¾;
Y is N or CH;
Z is C, CH, CH2, cycloalkyl, aryl, or heteroaryl;
is optionally a single, double, or triple bond as valencies permit; and
Ari and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
[0064] In one embodiment, Ar2 is an unsubstituted or substituted bicyclic aryl or heteroaryl. In another embodiment, Z is C, CH, CH2, cycloalkyl, monocyclic aryl, or monocyclic heteroaryl.
[0065] In one embodiment for the compound of formula VI, n is 0. In a different embodiment, n is 1. In another embodiment, X is O or NH.
[0066] In one embodiment for the compound of formula VI, Ari is selected from the group consisting of substituted phenyl {e.g., dichlorophenyl, methoxyphenyl), 2H- benzo[b][l,4]oxazin-3(4H)-one, 2,3-dihydrobenzo[b][l,4]dioxinyl, and benzo[d]oxazol-2(3H)- one, or any subgroup selected from the list. In a further embodiment, Ax\ is dichlorophenyl.
[0067] In another embodiment for the compound of formula VI, Ar2 is selected from the group consisting of IH-indazole, benzo[d]thiazole, imidazol-2(3H)-one, 3,4-dihydroisoquinolin- l(2H)-one, 3,4-dihydroquinolin-2(lH)-one, lH-benzo[d]imidazol-2(3H)-one, 3,4-dihydro-l,8- naphthyridin-2(lH)-one, quinolin-2(lH)-one, 2-methylphenylacetamide, and benzo[d]oxazol- 2(3H)-one, or any subgroup selected from the list. In a further embodiment, Ar2 is selected from the group consisting of IH-indazole, benzo[d]thiazole, and imidazol-2(3H)-one.
[0068] In another embodiment for the compound of formula VI, Ari is dichlorophenyl or dihydrobenzodioxinyl and Ar2 is dihydroquinolinone, benzothiazole, or indazole.
[0069] In another embodiment for the compound of formula V, Ari is dichlorophenyl, methoxyphenyl, or dihydrobenzodioxinyl and Ar2 is dihydroquinolinone, benzothiazole, indazole, benzoimidazolone, or quinolinone.
[0070] In another embodiment for the compound of formula VI, Y is N or CH, n is 0 or 1, X is O, Ari is dichlorophenyl, and Ar2 is selected from the group consisting of IH-indazole, benzo[d]thiazole, and lH-benzoimidazol-2(3H)-one.
[0071] In certain embodiments for the compound of formula VI, Ar2 is not naphthyridinone, dihydronaphthyridinone, quinolinone, dihydroquinolinone, tetrahydropyridoazepinone, or isoindole.
[0072] In one embodiment, the compound of formula VI is selected from the group of compounds shown in Tables 3 and 4.
Table 3
Table 4
[0073] The compounds of the invention can be synthesized in a similar way as Examples 1 - 51 below and/or according to Feenstra et al, Bioorg. Med. Chem. Lett. 11:2345 (2001) (incorporated herein by reference in its entirety).
[0074] The compounds of this invention include all pharmaceutically acceptable salt forms thereof. Examples of such salts include those derived from pharmaceutically acceptable inorganic and organic acids and bases. Examples of suitable acid salts include, without limitation, acetate, adipate, alginate, aspartate, benzoate, butyrate, citrate, fumarate, glycolate, hemisulfate, heptanoate, hexanoate, hydrochloride, hydrobromide, hydroiodide, 2- hydroxyethanesulfonate, lactate, maleate, malonate, methanesulfonate, nicotinate, nitrate, oxalate, palmoate, pectinate, persulfate, hydroxynapthoate, pivalate, propionate, salicylate, succinate, sulfate, tartrate, thiocyanate, tosylate and undecanoate. Other acids, such as oxalic, while not in themselves pharmaceutically acceptable, can be employed in the preparation of salts useful as intermediates in obtaining the compounds of the invention and their pharmaceutically acceptable acid addition salts.
[0075] Salts derived from appropriate bases include, without limitation, alkali metal (e.g., sodium, potassium), alkaline earth metal (e.g., magnesium and calcium), ammonium and N- (alkyl)4+ salts.
[0076] Compounds of the formulae herein include those having quaternization of any basic nitrogen-containing group therein.
[0077] The compounds of this invention include deuterium-substituted versions of the disclosed compounds, e.g., compounds comprising 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 or more deuterium atoms.
[0078] The discussion herein is, for simplicity, provided without reference to stereoisomerism. Those skilled in the art will appreciate that the compounds of the invention can contain one or more asymmetric centers and thus occur as racemates and racemic mixtures, single optical isomers, individual diastereomers, and diastereomeric mixtures. All such isomeric forms of these compounds are expressly included in the present invention.
[0079] Similarly, compounds of the invention containing a double bond can exist in the form of geometric isomers, which can be readily separated and recovered by conventional procedures. Such isomeric forms are included in the scope of this invention.
[0080] Further, the compounds of the invention include prodrugs of the compounds that are converted to the active compound in vivo. For example, the compound can be modified to enhance cellular permeability (e.g., by esterification of polar groups) and then converted by cellular enzymes to produce the active agent. Methods of masking charged or reactive moieties
as a pro-drug are known by those skilled in the art {see, e.g., P. Korgsgaard-Larsen and H. Bundgaard, A Textbook of Drug Design and Development, Reading U.K., Harwood Academic Publishers, 1991).
[0081] The term "prodrug" refers to compounds that are rapidly transformed in vivo to yield the parent compound of the above formula, for example, by hydrolysis in blood, see, e.g., T. Higuchi and V. Stella, Prodrugs as Novel delivery Systems, Vol. 14 of the A.C.S. Symposium Series and in Edward B. Roche, ed., Bioreversible Carriers in Drug Design, American Pharmaceutical Association and Pergamon Press, 1987, both of which are incorporated by reference herein. See also U.S. Patent No. 6,680,299. Exemplary prodrugs include a prodrug that is metabolized in vivo by a subject to an active drug having an activity of the compounds as described herein, wherein the prodrug is an ester of an alcohol or carboxylic acid group, if such a group is present in the compound; an amide of an amine group or carboxylic acid group, if such groups are present in the compound; a urethane of an amine group, if such a group is present in the compound; an acetal or ketal of an alcohol group, if such a group is present in the compound; a N-Mannich base or an imine of an amine group, if such a group is present in the compound; or a Schiff base, oxime, acetal, enol ester, oxazolidine, or thiazolidine of a carbonyl group, if such a group is present in the compound, such as described, for example, in U.S. Patent No. 6,680,324 and U.S. Patent No. 6,680,322.
[0082] The term "pharmaceutically acceptable prodrug" (and like terms) as used herein refers to those prodrugs of the compounds of the present invention which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of humans and/or other animals without undue toxicity, irritation, allergic response and the like, commensurate with a reasonable risk/benefit ratio, and effective for their intended use, as well as the zwitterionic forms, where possible, of the compounds of the invention.
Methods.
[0083] The compounds in Tables 1 and 3 were designed and synthesized as novel, functionally selective ligands of dopamine D2 receptors to elucidate the key signal transduction pathways essential for antipsychotic efficacy and the undesired ones associated with side-effects. These novel compounds were evaluated in multiple D2 binding and functional assays: D2 radioligand binding, D2 cAMP GloSensor, D2 ERK phosphorylation (p-ERK), and/or D2 β- arrestin 2 translocation (Tango). In the D2 cAMP GloSensor assay, these compounds showed different activity profiles ranging from full agonists to antagonists to inverse agonists of the Gj- regulated cAMP pathway. In the D2 β-arrestin recruitment (Tango) assay, these compounds showed activity profiles ranging from little agonist activity to full agonist activity of the β-
arrestin pathway. In the D2 ERK phosphorylation (p-ERK) assay, these compounds again showed different activity profiles ranging from antagonists to partial agonists that are more efficacious compared to aripiprazole with similar potency. Several β-arrestin biased, functionally selective D2 ligands were efficacious in D-amphetamine-induced and phencyclidine (PCP)- induced hyperlocomotion mouse models and did not induce catalepsy in wild-type mice at pharmacologically active doses. Interestingly, these compounds showed significantly attenuated antipsychotic-like activity and increased catalepsy in p-arrestin-2 knockout mice. These novel, pharmacologically active compounds are useful agents for studying dopamine receptor function and signaling pathways, as well as for treating CNS disorders. Furthermore, the compounds may have advantageous pharmacokinetic characteristics for treating CNS disorders, including a longer half life in brain and higher brain :plasma ratio over 24 h compared to other antipsychotic compounds. Additionally, the receptor binding pattern of the compounds (e.g., relative low potency at histamine receptors) suggests that the compounds may exhibit a lower risk for side effects, such as sedation and/or cognitive impairment.
[0084] Thus, one aspect of the invention relates to a method of modulating the activity of a D2 dopamine receptor comprising contacting the receptor with a compound of the invention. The D2 dopamine receptor may be present in a cell, an isolated cell membrane or an artificial membrane. The cell may be a cultured cell, a cell isolated from a subject, or a cell in a subject (e.g., an animal model of disease or a patient). The receptor may be a wild-type or modified receptor and may be naturally-occurring or recombinantly expressed. The term "modulating" is intended to encompass any type of change in one or more activities of the receptor, including increasing or decreasing the activity of one or more receptor-linked signaling pathways.
[0085] Many of the compounds of the invention display potent antipsychotic activity in animal models in vivo and are thus predicted to possess antipsychotic actions in humans and other animals. The compounds would thus be effective for treating diseases including, but not limited to, a variety of psychiatric disorders including: schizophrenia, schizoaffective disorder, schizophreniform disorder, bipolar disorder, mania, manic psychosis, Tourette's syndrome and Tourette-like disorders, obsessive-compulsive disorder, depression with psychotic features, psychosis not otherwise specified (Psychosis NOS) and additional affective and non-affective psychiatric disorders. Additionally, the compounds would be effective against a number of neurological disorders including Huntington's Disease, Parkinsonian Psychosis, Alzheimer's Disease and associated psychosis and various organic brain syndromes. Further, the compounds would be effective at a variety of autism spectrum and autistic disorders. Given the D -receptor functional selectivity, the compounds would also be effective against pituitary diseases including
pituitary adenomas and prolactinomas as well as endocrinological disorders including, but not limited to, galactorrhea.
[0086] The compounds have significant and unexpected advantages over conventional antipsychotic drugs in that they are predicted to effectively treat psychotic disorders without appreciable extra-pyramidal side effects (EPS), having minimal propensity for elevating serum prolactin and with a potentially lower risk of inducing tardive dyskinesia. Given the low affinities for Hi -histamine and partial agonism for 5-HT2C serotonin receptors the compounds are expected to differ from approved atypical antipsychotic drugs by virtue of a lower propensity to induce weight gain and associated metabolic adverse consequences (diabetes, hyperlipidemia, hyperglycemia, hypercholesterolemia, hypertension and so on (Kroeze et al, Neuropsychopharmacology 28:519 (2003)). The compounds also lack agonist activity at 5-HT2B serotonin receptors and hERG potassium channels and are thus predicted to have a low incidence of cardiovascular side-effects including prolongation of the Q-T interval and drug-induced valvular heart disease (Roth, N. Engl. J. Med. 356:6 (2007)). Given the affinities of certain compounds for 5-HT6 and 5-HT7 receptors the compounds are expected to have potential cognition-enhancing and antidepressant actions (Abbas et al, Psychopharmacology (Berl) 205: 119 (2009); Woolley et al, Neuropharmacology 41 :210 (2001)). Thus, these compounds are likely to also be effective in treating bipolar depression, unipolar depression, and a variety of anxiety disorders as well as disorders such as Alzheimer's Disease, dementia, Niemann-Pick Disorder, and other late-life dementias.
[0087] Thus, one aspect of the invention relates to a method of treating a central nervous system disorder associated with D2 dopamine receptors in a subject, comprising delivering to the subject a therapeutically effective amount of the compound of the invention. In certain embodiments, the disorder associated with D2 dopamine receptors is a psychiatric, neurological, pituitary, or endocrine disorder.
[0088] In one embodiment of the invention, one or more of the compounds of the invention is administered to the subject as needed to treat a disorder. The compound can be administered continuously or intermittently. In one embodiment, the compound is administered to the subject more than once a day or once every 1, 2, 3, 4, 5, 6, or 7 days. In another embodiment, the compound is administered to the subject no more than once a week, e.g., no more than once every two weeks, once a month, once every two months, once every three months, once every four months, once every five months, once every six months, or longer. In a further embodiment, the compound is administered using two or more different schedules, e.g., more frequently initially (for example to build up to a certain level, e.g., once a day or more) and then less frequently (e.g., once a week or less). In other embodiments, the compound can be
administered by any discontinuous administration regimen. In one example, the compound can be administered not more than once every three days, every four days, every five days, every six days, every seven days, every eight days, every nine days, or every ten days, or longer. The administration can continue for one, two, three, or four weeks or one, two, or three months, or longer. Optionally, after a period of rest, the compound can be administered under the same or a different schedule. The period of rest can be one, two, three, or four weeks, or longer, according to the pharmacodynamic effects of the compound on the subject.
[0089] The compound of the invention can be delivered to the subject by any suitable route, e.g., oral, rectal, buccal (e.g., sub-lingual), vaginal, parenteral (e.g., subcutaneous, intramuscular, intradermal, or intravenous), topical (i.e., both skin and mucosal surfaces, including airway surfaces) and transdermal administration. The compound is delivered to the subject at a dose that is effective to treat the disorder. The effective dosage will depend on many factors including the gender, age, weight, and general physical condition of the subject, the severity of the disorder, the particular compound or composition being administered, the duration of the treatment, the nature of any concurrent treatment, the carrier used, and like factors within the knowledge and expertise of those skilled in the art. As appropriate, a treatment effective amount in any individual case can be determined by one of ordinary skill in the art by reference to the pertinent texts and literature and/or by using routine experimentation (see, e.g., Remington, The Science and Practice of Pharmacy (21st ed. 2005)). In one embodiment, the compound is administered at a dose of about 0.001 to about 10 mg/kg body weight, e.g., about 0.001, 0.005, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 mg/kg. In some instances, the dose can be even lower, e.g., as low as 0.0005 or 0.0001 mg/kg or lower. In some instances, the dose can be even higher, e.g., as high as 20, 50, 100, 500, or 1000 mg/kg or higher. The present invention encompasses every sub-range within the cited ranges and amounts.
[0090] In one aspect of the invention, the compound of the invention is delivered to a subject concurrently with an additional therapeutic agent. The additional therapeutic agent can be delivered in the same composition as the compound or in a separate composition. The additional therapeutic agent can be delivered to the subject on a different schedule or by a different route as compared to the compound. The additional therapeutic agent can be any agent that provides a benefit to the subject. Further agents include, without limitation, anti-psychotics, antidepressants, agents for neurological disorders, and chemotherapeutic agents.
[0091] Examples of anti-psychotic agents include, without limitation, for psychosis: prochloroperazine, chlorpromazine, droperidol, fluphenazine, periciazine, perphenazine, thiothixene, triflupromazine, haloperidol, molindone, pimozide, and thioridazine: and for schizophrenia: olanzapine, paliperidone, aripiprazole, quetiapine, risperidone, clozapine,
flupenthixene, iloperidone, loxapine, mesoridazine, promazine, reserpine, thioridazine, zuclopenthixol, asenapine, levomepromazine, ziprasidone, molindone, pimozide, and thioridazine.
[0092] Examples of agents for depression and related disorders include, without limitation, for depression: modafinil, desvenlafaxine, nortriptyline, seleginline, amoxapine, citalopram, clomipramine, fluoxetine, isocarboxazid, mapriotiline, nefazodone, niacin, nortriptyline, phenelzine, protriptyline, tranylcypromine, trazodone, trimipramine, desipramine, imipramine, methylphenidate, buproprion, alprazolam, amitriptyline, chlordiazepoxide, perphenazine, doxepin, mirtazapine, 5-hydroxytryptophan, duloxetine, escitalopram, venlafaxine, desvenlafaxine, paroxetine, fluoxetine, olanzapine, 1-methylfolate, amitriptyline, sertraline, fluvoxamine, paliperidone, aripiprazole, quetiapine, risperidone, amisulpride; for anxiety: atomoxetine, aspirin, meprobamate, atenolol, buspirone, chlorodiazepoxide, chlorprothixene, clorazepate, diazepam, gabapentin, halazepam, hydroxyzine, lorazepam, meprobamate, nadolol, oxazepam, phenytoin, trifluoperazine, clonidine, clonazepam, oxcarbazepine, prochloroperazine, alprazolam, amitriptyline, chlordiazepoxide, perphenazine, doxepin, mirtazapine, 5- hydroxytryptophan, duloxetine, escitalopram, venlafaxine, desvenlafaxine, paroxetine, sertraline, fluvoxamine, amisulpride; and for bipolar disorder: clonidine, clonazapam, oxcarbazepine, carbamazepine, divalproex, lamotrigine, levetiracetam, lithium, topiramate, valproic acid, verapamil, buproprion, duloxetine, escitalopram, venlafaxine, 1-methylfolate, fluoxetine, olanzapine, sertraline, olanzapine, paliperidone, aripiprazole, quetiapine, risperidone, asenapine, levomepromazine, ziprasidone.
[0093] Examples of agents for neurological disorders include, without limitation, for Alzheimer's disease: caprylidene, donepezil, galantamine, tacrine, vitamin E, ergoloid mesylates, rivastigmine; for Parkinson's disease: nadolol, zonisamide, amantadine, apomorphine, belladonna, benztropine, biperiden, bromocriptine, carbidopa, entacapone, levodopa, pergolide mesylate, pramipexole, procyclidine, rasagiline, ropinirole, rotiotine, scopolamine, tolcapone, trihexylphenidyl, rivastigmine, seleginline; for Huntington's disease: baclofen, pregabalin, tetrabenazine, methylprednisolone, desvenlafaxine, nortriptyline; and for dementia: haloperidol and ergoloid mesylates.
[0094] Examples of chemotherapeutic agents include, without limitation, acivicin, aclarubicin, acodazole hydrochloride, acronine, adozelesin, aldesleukin, altretamine, ambomycin, ametantrone acetate, aminoglutethimide, amsacrine, anastrozole, anthramycin, asparaginase, asperlin, azacytidine, azetepa, azotomycin, batimastat, benzodepa, bicalutamide, bisantrene hydrochloride, bisnafide dimesylate, bizelesin, bleomycin sulfate, brequinar sodium, bropirimine, busulfan, cactinomycin, calusterone, caracemide, carbetimer, carboplatin, carmustine, carubicin
hydrochloride, carzelesin, cedefingol, chlorambucil, cirolemycin, cisplatin, cladribine, crisnatol mesylate, cyclophosphamide, cytarabine, dacarbazine, dactinomycin, daunorubicin hydrochloride, decitabine, dexormaplatin, dezaguanine, dezaguanine mesylate, diaziquone, docetaxel, doxorubicin, doxorubicin hydrochloride, droloxifene, droloxifene citrate, dromostanolone propionate, duazomycin, edatrexate, eflornithine hydrochloride, elsamitrucin, enloplatin, enpromate, epipropidine, epirubicin hydrochloride, erbulozole, esorubicin hydrochloride, estramustine, estramustine phosphate sodium, etanidazole, etoposide, etoposide phosphate, etoprine, fadrozole hydrochloride, fazarabine, fenretinide, floxuridine, fludarabine phosphate, fluorouracil, flurocitabine, fosquidone, fostriecin sodium, gemcitabine, gemcitabine hydrochloride, hydroxyurea, idarubicin hydrochloride, ifosfarnide, ilmofosine, interleukin II (including recombinant interleukin II or rIL2), interferon alfa-2a, interferon alfa-2b, interferon alfa-nl, interferon alfa-n3, interferon beta-la, interferon gamma-lb, iproplatin, irinotecan hydrochloride, lanreotide acetate, letrozole, leuprolide acetate, liarozole hydrochloride, lometrexol sodium, lomustine, losoxantrone hydrochloride, masoprocol, maytansine, mechlorethamine hydrochloride, megestrol acetate, melengestrol acetate, melphalan, menogaril, mercaptopurine, methotrexate, methotrexate sodium, metoprine, meturedepa, mitindomide, mitocarcin, mitocromin, mitogillin, mitomalcin, mitomycin, mitosper, mitotane, mitoxantrone hydrochloride, mycophenolic acid, nocodazole, nogalamycin, ormaplatin, oxisuran, paclitaxel, pegaspargase, peliomycin, pentamustine, peplomycin sulfate, perfosfamide, pipobroman, piposulfan, piroxantrone hydrochloride, plicamycin, plomestane, porfimer sodium, porfiromycin, prednimustine, procarbazine hydrochloride, puromycin, puromycin hydrochloride, pyrazofurin, riboprine, rogletimide, safingol, safingol hydrochloride, semustine, simtrazene, sparfosate sodium, sparsomycin, spirogermanium hydrochloride, spiromustine, spiroplatin, streptonigrin, streptozotocin, sulofenur, talisomycin, tecogalan sodium, tegafur, teloxantrone hydrochloride, temoporfin, teniposide, teroxirone, testolactone, thiamiprine, thioguanine, thiotepa, tiazofurin, tirapazamine, toremifene citrate, trestolone acetate, triciribine phosphate, trimetrexate, trimetrexate glucuronate, triptorelin, tubulozole hydrochloride, uracil mustard, uredepa, vapreotide, verteporfin, vinblastine sulfate, vincristine sulfate, vindesine, vindesine sulfate, vinepidine sulfate, vinglycinate sulfate, vinleurosine sulfate, vinorelbine tartrate, vinrosidine sulfate, vinzolidine sulfate, vorozole, zeniplatin, zinostatin, zorubicin hydrochloride.
[0095] Examples of other chemotherapeutic agents include, but are not limited to, 20-epi- 1,25 dihydroxyvitamin D3; 5-ethynyluracil; abiraterone; aclarubicin; acylfulvene; adecypenol; adozelesin; aldesleukin; ALL-TK antagonists; altretamine; ambamustine; amidox; amifostine; aminolevulinic acid; amrubicin; amsacrine; anagrelide; anastrozole; andrographolide; angiogenesis inhibitors; antagonist D; antagonist G; antarelix; anti-dorsalizing morphogenetic
protein- 1; prostatic carcinoma antiandrogen; antiestrogen; antineoplaston; antisense oligonucleotides; aphidicolin glycinate; apoptosis gene modulators; apoptosis regulators; apurinic acid; ara-CDP-DL-PTBA; arginine deaminase; asulacrine; atamestane; atrimustine; axinastatin 1; axinastatin 2; axinastatin 3; azasetron; azatoxin; azatyrosine; baccatin III derivatives; balanol; batimastat; BCR/ABL antagonists; benzochlorins; benzoylstaurosporine; beta lactam derivatives; beta-alethine; betaclamycin B; betulinic acid; bFGF inhibitor; bicalutamide; bisantrene; bisaziridinylspermine; bisnafide; bistratene A; bizelesin; breflate; bropirimine; budotitane; buthionine sulfoximine; calcipotriol; calphostin C; camptothecin derivatives; canarypox IL-2; capecitabine; carboxamide-amino-triazole; carboxyamidotriazole; CaRest M3; CARN 700; cartilage derived inhibitor; carzelesin; casein kinase inhibitors (ICOS); castanospermine; cecropin B; cetrorelix; chlorins; chloroquinoxaline sulfonamide; cicaprost; cis-porphyrin; cladribine; clomifene analogues; clotrimazole; collismycin A; collismycin B; combretastatin A4; combretastatin analogue; conagenin; crambescidin 816; crisnatol; cryptophycin 8; cryptophycin A derivatives; curacin A; cyclopentanthraquinones; cycloplatam; cypemycin; cytarabine ocfosfate; cytolytic factor; cytostatin; dacliximab; decitabine; dehydrodidemnin B; deslorelin; dexamethasone; dexifosfamide; dexrazoxane; dexverapamil; diaziquone; didemnin B; didox; diethylnorspermine; dihydro-5-azacytidine; dihydrotaxol, 9-; dioxamycin; diphenyl spiromustine; docetaxel; docosanol; dolasetron; doxifluridine; droloxifene; dronabinol; duocarmycin SA; ebselen; ecomustine; edelfosine; edrecolomab; eflornithine; elemene; emitefur; epirubicin; epristeride; estramustine analogue; estrogen agonists; estrogen antagonists; etanidazole; etoposide phosphate; exemestane; fadrozole; fazarabine; fenretinide; filgrastim; finasteride; flavopiridol; flezelastine; fluasterone; fludarabine; fluorodaunorunicin hydrochloride; forfenimex; formestane; fostriecin; fotemustine; gadolinium texaphyrin; gallium nitrate; galocitabine; ganirelix; gelatinase inhibitors; gemcitabine; glutathione inhibitors; hepsulfam; heregulin; hexamethylene bisacetamide; hypericin; ibandronic acid; idarubicin; idoxifene; idramantone; ilmofosine; ilomastat; imidazoacridones; imiquimod; immunostimulant peptides; insulin-like growth factor-1 receptor inhibitor; interferon agonists; interferons; interleukins; iobenguane; iododoxorubicin; 4- ipomeanol; iroplact; irsogladine; isobengazole; isohomohalicondrin B; itasetron; jasplakinolide; kahalalide F; lamellarin-N triacetate; lanreotide; leinamycin; lenograstim; lentinan sulfate; leptolstatin; letrozole; leukemia inhibiting factor; leukocyte alpha interferon; leuprolide+estrogen+progesterone; leuprorelin; levamisole; liarozole; linear polyamine analogue; lipophilic disaccharide peptide; lipophilic platinum compounds; lissoclinamide 7; lobaplatin; lombricine; lometrexol; lonidamine; losoxantrone; lovastatin; loxoribine; lurtotecan; lutetium texaphyrin; lysofylline; lytic peptides; maitansine; mannostatin A; marimastat; masoprocol; maspin; matrilysin inhibitors; matrix metalloproteinase inhibitors; menogaril; merbarone;
meterelin; methioninase; metoclopramide; MIF inhibitor; mifepristone; miltefosine; mirimostim; mismatched double stranded RNA; mitoguazone; mitolactol; mitomycin analogues; mitonafide; mitotoxin fibroblast growth factor-saporin; mitoxantrone; mofarotene; molgramostim; monoclonal antibody, human chorionic gonadotrophin; monophosphoryl lipid A+myobacterium cell wall sk; mopidamol; multiple drug resistance gene inhibitor; multiple tumor suppressor 1- based therapy; mustard anticancer agent; mycaperoxide B; mycobacterial cell wall extract; myriaporone; N-acetyldinaline; N-substituted benzamides; nafarelin; nagrestip; naloxone+pentazocine; napavin; naphterpin; nartograstim; nedaplatin; nemorubicin; neridronic acid; neutral endopeptidase; nilutamide; nisamycin; nitric oxide modulators; nitroxide antioxidant; nitrullyn; 06-benzylguanine; octreotide; okicenone; oligonucleotides; onapristone; odansteron; oracin; oral cytokine inducer; ormaplatin; osaterone; oxaliplatin; oxaunomycin; paclitaxel; paclitaxel analogues; paclitaxel derivatives; palauamine; palmitoylrhizoxin; pamidronic acid; panaxytriol; panomifene; parabactin; pazelliptine; pegaspargase; peldesine; pentosan polysulfate sodium; pentostatin; pentrozole; perflubron; perfosfamide; perillyl alcohol; phenazinomycin; phenylacetate; phosphatase inhibitors; picibanil; pilocarpine hydrochloride; pirarubicin; piritrexim; placetin A; placetin B; plasminogen activator inhibitor; platinum complex; platinum compounds; platinum-triamine complex; porfimer sodium; porfiromycin; prednisone; propyl bis-acridone; prostaglandin J2; proteasome inhibitors; protein A-based immune modulator; protein kinase C inhibitor; protein kinase C inhibitors, microalgal; protein tyrosine phosphatase inhibitors; purine nucleoside phosphorylase inhibitors; purpurins; pyrazoloacridine; pyridoxylated hemoglobin polyoxyethylene conjugate; raf antagonists; raltitrexed; ramosetron; ras farnesyl protein transferase inhibitors; ras inhibitors; ras-GAP inhibitor; retelliptine demethylated; rhenium Re 186 etidronate; rhizoxin; ribozymes; RJI retinamide; rogletimide; rohitukine; romurtide; roquinimex; rubiginone Bl; ruboxyl; safingol; saintopin; SarCNU; sarcophytol A; sargramostim; Sdi 1 mimetics; semustine; senescence derived inhibitor 1 ; sense oligonucleotides; signal transduction inhibitors; signal transduction modulators; single chain antigen binding protein; sizofiran; sobuzoxane; sodium borocaptate; sodium phenylacetate; solverol; somatomedin binding protein; sonermin; sparfosic acid; spicamycin D; spiromustine; splenopentin; spongistatin 1; squalamine; stem cell inhibitor; stem-cell division inhibitors; stipiamide; stromelysin inhibitors; sulfinosine; superactive vasoactive intestinal peptide antagonist; suradista; suramin; swainsonine; synthetic glycosaminoglycans; tallimustine; tamoxifen methiodide; tauromustine; tazarotene; tecogalan sodium; tegafur; tellurapyrylium; telomerase inhibitors; temoporfin; temozolomide; teniposide; tetrachlorodecaoxide; tetrazomine; thaliblastine; thiocoraline; thrombopoietin; thrombopoietin mimetic; thymalfasin; thymopoietin receptor agonist; thymotrinan; thyroid stimulating hormone; tin ethyl etiopurpurin; tirapazamine;
titanocene bichloride; topsentin; toremifene; totipotent stem cell factor; translation inhibitors; tretinoin; triacetyluridine; triciribine; trimetrexate; triptorelin; tropisetron; turosteride; tyrosine kinase inhibitors; tyrphostins; UBC inhibitors; ubenimex; urogenital sinus-derived growth inhibitory factor; urokinase receptor antagonists; vapreotide; variolin B; vector system, erythrocyte gene therapy; velaresol; veramine; verdins; verteporfin; vinorelbine; vinxaltine; vitaxin; vorozole; zanoterone; zeniplatin; zilascorb; and zinostatin stimalamer.
[0096] The present invention finds use in research as well as veterinary and medical applications. Suitable subjects are generally mammalian subjects. The term "mammal" as used herein includes, but is not limited to, humans, non-human primates, cattle, sheep, goats, pigs, horses, cats, dog, rabbits, rodents (e.g., rats or mice), etc. Human subjects include neonates, infants, juveniles, adults and geriatric subjects.
[0097] In particular embodiments, the subject is a human subject that has a CNS disorder associated with D2 dopamine receptors. In other embodiments, the subject used in the methods of the invention is an animal model of a CNS disorder associated with D2 dopamine receptors.
[0098] The subject can be a subject "in need of the methods of the present invention, e.g., in need of the therapeutic effects of the inventive methods. For example, the subject can be a subject that is experiencing a CNS disorder associated with D2 dopamine receptors and/or is anticipated to experience a CNS disorder associated with D2 dopamine receptors, and the methods and compositions of the invention are used for therapeutic and/or prophylactic treatment.
[0099] The compounds of the invention described above can be formulated for administration in a pharmaceutical carrier in accordance with known techniques. See, e.g., Remington, The Science And Practice of Pharmacy (21st ed. 2005). In the manufacture of a pharmaceutical formulation according to the invention, the compound is typically admixed with, inter alia, an acceptable carrier. The carrier must, of course, be acceptable in the sense of being compatible with any other ingredients in the formulation and must not be deleterious to the patient. The carrier can be a solid or a liquid, or both, and can be formulated with the compound as a unit-dose formulation, for example, a tablet, which can contain from 0.01% or 0.5% to 95% or 99% by weight of the compound. One or more compounds can be incorporated in the formulations of the invention, which can be prepared by any of the well known techniques of pharmacy comprising admixing the components, optionally including one or more accessory ingredients.
[0100] The formulations of the invention include those suitable for oral, rectal, topical, buccal (e.g., sub-lingual), vaginal, parenteral (e.g., subcutaneous, intramuscular, intradermal, or intravenous), topical (i.e., both skin and mucosal surfaces, including airway surfaces) and transdermal administration, although the most suitable route in any given case will depend on the
nature and severity of the condition being treated and on the nature of the particular active compound which is being used.
[0101] Formulations suitable for oral administration can be presented in discrete units, such as capsules, cachets, lozenges, or tablets, each containing a predetermined amount of the active compound; as a powder or granules; as a solution or a suspension in an aqueous or nonaqueous liquid; or as an oil-in-water or water-in-oil emulsion. Such formulations can be prepared by any suitable method of pharmacy which includes the step of bringing into association the compound and a suitable carrier (which can contain one or more accessory ingredients as noted above). In general, the formulations of the invention are prepared by uniformly and intimately admixing the compound with a liquid or finely divided solid carrier, or both, and then, if necessary, shaping the resulting mixture. For example, a tablet can be prepared by compressing or molding a powder or granules containing the compound, optionally with one or more accessory ingredients. Compressed tablets can be prepared by compressing, in a suitable machine, the compound in a free-flowing form, such as a powder or granules optionally mixed with a binder, lubricant, inert diluent, and/or surface active/dispersing agent(s). Molded tablets can be made by molding, in a suitable machine, the powdered compound moistened with an inert liquid binder.
[0102] Formulations suitable for buccal (sub-lingual) administration include lozenges comprising the compound in a flavored base, usually sucrose and acacia or tragacanth; and pastilles comprising the compound in an inert base such as gelatin and glycerin or sucrose and acacia.
[0103] Formulations of the present invention suitable for parenteral administration comprise sterile aqueous and non-aqueous injection solutions of the compound, which preparations are preferably isotonic with the blood of the intended recipient. These preparations can contain anti-oxidants, buffers, bacteriostats and solutes which render the formulation isotonic with the blood of the intended recipient. Aqueous and non-aqueous sterile suspensions can include suspending agents and thickening agents. The formulations can be presented in unit/dose (e.g., in a syringe or other injection device) or multi-dose containers, for example sealed ampoules and vials, and can be stored in a freeze-dried (lyophilized) condition requiring only the addition of the sterile liquid carrier, for example, saline or water-for-injection immediately prior to use. Extemporaneous injection solutions and suspensions can be prepared from sterile powders, granules and tablets of the kind previously described. For example, in one aspect of the present invention, there is provided an injectable, stable, sterile composition comprising one or more compounds, in a unit dosage form in a sealed container. The compound is provided in the form of a lyophilizate which is capable of being reconstituted with a suitable pharmaceutically
acceptable carrier to form a liquid composition suitable for injection thereof into a subject. The unit dosage form typically comprises from about 0.001 mg to about 10 grams of the compound. When the compound is substantially water-insoluble (e.g., when conjugated to a lipid), a sufficient amount of emulsifying agent which is physiologically acceptable can be employed in sufficient quantity to emulsify the compound in an aqueous carrier. One such useful emulsifying agent is phosphatidyl choline.
[0104] Formulations suitable for rectal administration are preferably presented as unit dose suppositories. These can be prepared by admixing the compound with one or more conventional solid carriers, for example, cocoa butter, and then shaping the resulting mixture.
[0105] Formulations suitable for topical application to the skin preferably take the form of an ointment, cream, lotion, paste, gel, spray, aerosol, or oil. Carriers which can be used include petroleum jelly, lanoline, polyethylene glycols, alcohols, transdermal enhancers, and combinations of two or more thereof.
[0106] Formulations suitable for transdermal administration can be presented as discrete patches adapted to remain in intimate contact with the epidermis of the recipient for a prolonged period of time. Formulations suitable for transdermal administration can also be delivered by iontophoresis (see, for example, Pharm. Res. 3:318 (1986)) and typically take the form of an optionally buffered aqueous solution of the compound. Suitable formulations comprise citrate or bis\tris buffer (pH 6) or ethanol/water and contain from 0.1 to 0.2 M active ingredient.
[0107] Other pharmaceutical compositions can be prepared from the compounds disclosed herein, such as aqueous base emulsions. In such an instance, the composition will contain a sufficient amount of pharmaceutically acceptable emulsifying agent to emulsify the desired amount of the compound. Particularly useful emulsifying agents include phosphatidyl cholines and lecithin.
[0108] In addition to compound, the pharmaceutical compositions can contain other additives, such as pH-adjusting additives. In particular, useful pH-adjusting agents include acids, such as hydrochloric acid, bases or buffers, such as sodium lactate, sodium acetate, sodium phosphate, sodium citrate, sodium borate, or sodium gluconate. Further, the compositions can contain microbial preservatives. Useful microbial preservatives include methylparaben, propylparaben, and benzyl alcohol. The microbial preservative is typically employed when the formulation is placed in a vial designed for multidose use. Other additives that are well known in the art include, e.g., detackifiers, anti-foaming agents, antioxidants (e.g., ascorbyl palmitate, butyl hydroxy anisole (BHA), butyl hydroxy toluene (BHT) and tocopherols, e.g., a-tocopherol (vitamin E)), preservatives, chelating agents (e.g., EDTA and/or EGTA), viscomodulators, tonicifiers (e.g., a sugar such as sucrose, lactose, and/or mannitol), flavorants, colorants, odorants,
opacifiers, suspending agents, binders, fillers, plasticizers, lubricants, and mixtures thereof. The amounts of such additives can be readily determined by one skilled in the art, according to the particular properties desired.
[0109] The additive can also comprise a thickening agent. Suitable thickening agents can be those known and employed in the art, including, e.g., pharmaceutically acceptable polymeric materials and inorganic thickening agents. Exemplary thickening agents for use in the present pharmaceutical compositions include polyacrylate and polyacrylate co-polymer resins, for example poly-acrylic acid and poly-acrylic acid/methacrylic acid resins; celluloses and cellulose derivatives including: alkyl celluloses, e.g., methyl-, ethyl- and propyl-celluloses; hydroxyalkyl- celluloses, e.g., hydroxypropyl-celluloses and hydroxypropylalkyl-celluloses such as hydroxypropyl-methyl-celluloses; acylated celluloses, e.g., cellulose-acetates, cellulose- acetatephthallates, cellulose-acetatesuccinates and hydroxypropylmethyl-cellulose phthallates; and salts thereof such as sodium-carboxymethyl-celluloses; polyvinylpyrrolidones, including for example poly-N-vinylpyrrolidones and vinylpyrrolidone co-polymers such as vinylpyrrolidone- vinylacetate co-polymers; polyvinyl resins, e.g., including polyvinylacetates and alcohols, as well as other polymeric materials including gum traganth, gum arabicum, alginates, e.g., alginic acid, and salts thereof, e.g., sodium alginates; and inorganic thickening agents such as atapulgite, bentonite and silicates including hydrophilic silicon dioxide products, e.g., alkylated (for example methylated) silica gels, in particular colloidal silicon dioxide products. Such thickening agents as described above can be included, e.g., to provide a sustained release effect. However, where oral administration is intended, the use of thickening agents as aforesaid will generally not be required and is generally less preferred. Use of thickening agents is, on the other hand, indicated, e.g., where topical application is foreseen.
[0110] Further, the present invention provides liposomal formulations of the compounds disclosed herein. The technology for forming liposomal suspensions is well known in the art. When the compound is in the form of an aqueous-soluble material, using conventional liposome technology, the same can be incorporated into lipid vesicles. In such an instance, due to the water solubility of the compound, the compound will be substantially entrained within the hydrophilic center or core of the liposomes. The lipid layer employed can be of any conventional composition and can either contain cholesterol or can be cholesterol-free. When the compound of interest is water-insoluble, again employing conventional liposome formation technology, the compound can be substantially entrained within the hydrophobic lipid bilayer which forms the structure of the liposome. In either instance, the liposomes which are produced can be reduced in size, as through the use of standard sonication and homogenization techniques. The liposomal formulations containing the compound disclosed herein, can be lyophilized to produce a
lyophilizate which can be reconstituted with a pharmaceutically acceptable carrier, such as water, to regenerate a liposomal suspension.
[0111] The present invention is explained in greater detail in the following non-limiting Examples.
EXAMPLE 1
Synthesis of Compounds
[0112] The compounds shown in Table 5 were synthesized as described below.
General Procedures.
[0113] Unless stated to the contrary, where applicable, the following conditions apply: all commercial grade reagents were used without further purification. MeCN and CH2C12 were distilled from CaH2 under a N2 atmosphere prior to use; THF was distilled from Na/benzophenone under N2. All other dry solvents were of anhydrous quality purchased from Sigma-Aldrich. Brine (NaCl), NaHC03, and NH4C1 refer to saturated aqueous solutions. Melting points were uncorrected. Mass spectra (MS) and HPLC (UV 254 nM or ELSD) data for
all compounds were recorded on an 1100 LC/MS system (Agilent Technology Corporation) using a 4.6x50 mm column (CenturySIL C-18 AQ+, 5μ) with a linear gradient 30-90% (v/v) acetonitrile-water with 0.035% trifluoroacetic acid over 5 min with a flow rate of 3.5 mL/min. Analytical TLC was performed using 2.5 x 5 cm plates coated with 0.25 mm of silica gel GF254. Column chromatography was performed on silica gel G (200-300 mesh) with reagent grade solvents. ¾ NMR spectra (300 MHz) are reported as follows: chemical shifts in ppm (δ scale) downfield from TMS as the internal standard with either CDC13 or DMSO-i/6 as the solvent. Multiplicities are indicated as the following: bs=broad singlet, s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet.
Synthesis of Compound 1
Scheme 1. Synthesis of intermediate 2
Intermediate 3
Scheme 3. Synthesis of compound 1
[0114] A well-dried flask was first charged with l-bromo-2,3-dichlorobenzene (4 g, 17.7 mmol) and 1,4-diazepane (2.1 g, 20.9 mmol), which was evacuated and backfilled with N2 through a balloon under gentle warming (40°C). Toluene was charged and the mixture was bubbled with N2 for 10 min, then BINAP (318 mg, 0.51 mmol) and Pd2dba3 (156 mg, 0.17 mmol) was added to the mixture, followed by DBU (3.4 mL). The resulting mixture was warmed at 60-
70°C while fine powder of tBuONa was added in one portion to start the amination. After the reaction mixture cooled to rt, (Boc)20 (11 g, 51 mmol) solution in DCM was added dropwise to the reaction mixture, then stirred for 3 h at rt. The reaction mixture was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 10:1) to give tert-butyl 4-(2,3-dichlorophenyl)-l,4-diazepane- 1-carboxylate (intermediate 1) (4.1 g, 70%) as a yellow oil.
[0115] Excess HC1 in EtOAc was added dropwise to a solution of intermediate 1 (4.0 g, 11.6 mmol) in EtOAc and the reaction mixture was stirred at rt for 1.5 h. The reaction mixture was filtered to give l-(2,3-dichlorophenyl)-l,4-diazepane hydrochloride salt (intermediate 2) (3.0 g, 90%) as a white solid. HPLC: 99%, RT 2.108 min. MS (ESI) m/z 245.0 [M + H]+. mp: 186- 187°C.
[0116] 7-hydroxy-3,4-dihydroquinolin-2(lH)-one (195 mg, 1.2 mmol), 1,3- dibromopropane (960 mg, 4.8 mmol) and anhydrous K2C03 (166 mg, 1.2 mmol) were dissolved in EtOH and the solution was heated to reflux overnight. The solution was diluted with water and extracted with EtOAc. The combined organic layers were washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 2:1) to give 7-(3-bromopropoxy)-3,4- dihydroquinolin-2(lH)-one (intermediate 3) (300 mg, 88%) as a white solid. HPLC: 99%, RT 2.775 min. MS (ESI) m/z 284.0 [M + H]+.
[0117] A mixture of intermediate 3 (142 mg, 0.5 mmol) and Nal (150 mg, 1.0 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 2 (196 mg, 0.7 mmol) and anhydrous K2C03 (173 mg, 1.25 mmol) were added to the mixture. The resulting mixture
was heated to reflux for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30: 1) to give 7-(3-(4- (2,3-dichlorophenyl)-l,4-diazepan-l-yl)propoxy)-3,4-dihydroquinolin-2(lH)-one (compound 1) (140 mg, 63%). ¾ NMR (300 MHz, CDC13): δ 8.16 (s, 1H), 7.10-6.98 (m, 4H), 6.53 (dd, J = 8.1 Hz, 2.4 Hz, 1H), 6.34 (d, J = 2.1 Hz, 1H), 4.01 (t, J = 6.6 Hz, 2H), 3.32-3.28 (m, 4H), 2.92-2.87 (m, 6H), 2.76 (t, J = 6.6 Hz, 2H), 2.62 (t, J = 6.6 Hz, 2H), 2.04-1.97 (m, 4H). HPLC: 99%, RT 2.421 min. MS (ESI) m/z 448.1 [M + H]+. mp: 130-131°C.
Synthesis of Compound 2
Intermediate 4
Scheme 5. Synthesis of compound 2
[0118] 7-hydroxy-3,4-dihydroquinolin-2(lH)-one (163 mg, 1.0 mmol), 1,4- dibromobutane (0.36 mL, 3.0 mmol) and anhydrous K2C03 (138 mg, 1.0 mmol) were dissolved in EtOH and the solution was heated to reflux overnight. The solution was diluted with water and extracted with EtOAc. The combined organic layers were washed with saturated NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE EtOAc = 2: 1) to give 7-(4-bromobutoxy)-3,4-dihydroquinolin- 2(lH)-one (intermediate 4) (220 mg, 74%) as a white solid, mp: 106-109°C.
Compound 2:
[0119] A mixture of intermediate 4 (156 mg, 0.52 mmol) and Nal (120 mg, 0.8 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 2 (119 mg, 0.4 mmol) and anhydrous K2C03 (189 mg, 1.37 mmol) were then added to the mixture. The resulting mixture was heated to reflux for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 7-(4-(4-(2,3-dichlorophenyl)-l ,4-diazepan-l-yl)butoxy)-3,4-dihydroquinolin-2(lH)-one
(compound 2) (75 mg, 41%). ¾ NMR (300 MHz, CDC13): δ 8.08 (s, 1H), 7.10-6.99 (m, 4H), 6.52 (dd, J = 6.6 Hz, 2.1 Hz, 1H), 6.33 (s, 1H), 3.96 (t, J= 6.0 Hz, 2H), 3.31 -3.27 (m, 4H), 2.92- 2.84 (m, 6H), 2.64-2.60 (m, 4H), 2.02-1.98 (m, 2H), 1.84-1.70 (m, 4H). HPLC: 99%, RT 2.435 min. MS (ESI) m/z 462.2 [M + H]+. mp: 88-89°C.
Synthesis of Compound 3
Scheme 6. Synthesis of intermediate 12
ntermediate 11 intermediate 12
Scheme 7. Synthesis of compound 3
[0120] Pivaloyl chloride (18.7 mL, 151.6 mmol) was slowly added to an ice-cooled solution of 6-chloropyridin-2-amine (15 g, 116.7 mmol) and triethylamine (24.25 mL, 124.96 mmol) in DCM (180 mL). The mixture was stirred in an ice-bath for 15 min and then at rt for 6 h. The mixture was poured into water. The organic layer was washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04; filtered and concentrated in vacuo to give N-(6- chloropyridin-2-yl)pivalamide (intermediate 5) (20.01 g, 81%) as a yellow solid.
[0121] A solution of intermediate 5 (19.28 g, 90.6 mmol) in THF (181 mL) was treated with 77-BuLi (108.8 mL, 272 mmol) and the resulting mixture was stirred at -20°C for 3 h. After addition of DMF (20.81 mL, 271.86 mmol), the reaction was allowed to warm to rt. The reaction was poured into cold 6N HC1 and stirred for 15 min. The mixture was then neutralized with anhydrous K2C03 to pH=7 and extracted with Et20. The combined organic layers were washed with water, brine, dried over anhydrous Na2SC>4, concentrated in vacuo and purified by recrystallization from EtOAc and hexanes to afford N-(6-chloro-3-formylpyridin-2-yl)pivalamide (intermediate 6) (12.84 g, 59%).
[0122] To a solution of 4-(benzyloxy)butan-l-ol (15.42 g, 85.6 mmol) in DMF (120 mL) was added sodium hydride (4.11 g, 171 mmol) at 0°C. The mixture was stirred for 20 min, then intermediate 6 (10.28 g, 42.7 mmol) was added portion-wise and the resulting mixture was stirred overnight. The mixture was quenched with saturated aq NH CI and extracted with EtOAc. The combined organic layers were washed with water, brine, dried over anhydrous Na2S04 and concentrated in vacuo. The residue was purified by flash chromatography on silica gel column
(elution with PE EtOAc = 8: 1 - 4: 1) to give N-(6-(4-(benzyloxy)butoxy)-3-formylpyridin-2- yl)pivalamide (intermediate 7) (5.04 g, 31%).
[0123] Under N2, an ice-cooled flask containing THF (100 mL) was charged with sodium hydride (5.7 g, 142.5 mmol) to which ethyl 2-(diethoxyphosphoryl)acetate (32 g, 142 mmol) was added. The ice bath was removed and a solution of intermediate 7 (21.8 g, 56.7 mmol) in THF (120 mL) was slowly added to the preformed anion. The reaction was heated to reflux overnight. The reaction mixture was cooled to rt and partitioned between EtOAc and water. The organic layer was separated and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried over anhydrous Na2S04 and concentrated in vacuo to give ethyl 3-(6-(4-(benzyloxy)butoxy)-2-pivalamidopyridin-3-yl)acrylate (intermediate 8) (13.3 g, 52%) as a white solid, mp: 75-76°C.
[0124] Intermediate 8 (2.46 g, 5.4 mmol) was hydrogenated under an atmosphere of ¾ (40 psi) using Raney Ni (1.59 g, 27.1 mmol) in THF (120 mL). The reaction was filtered to remove the Raney Ni and the filtrate was concentrated to afford ethyl 3-(6-(4- (benzyloxy)butoxy)-2-pivalamidopyridin-3-yl)propanoate (intermediate 9) (1.76 g, 71.5% crude) as an oil.
[0125] To a solution of intermediate 9 (1.76 g, 3.9 mmol) in 1,4-dioxane (14 mL) was added 3N HC1 (7.1 mL) and the mixture was then heated to reflux for 3 h. The mixture was cooled to rt and then anhydrous K2C03 was slowly added to neutralize the reaction mixture to pH=8, then extracted with EtOAc. The combined organic layers were washed with water, brine, dried over anhydrous Na2S04 and concentrated in vacuo. The crude product was purified by flash chromatography on silica gel column (elution with PE EtOAc = 4: 1 - 1 : 1) to give 7-(4- (benzyloxy)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one (intermediate 10) (671 mg, 54%) as an oil.
Intermediate 11:
[0126] To the solution of intermediate 10 (900 mg, 2.8 mmol) in EtOH (25 mL) was added 10% Pd/C (400 mg) under an atmosphere of H2 overnight. The reaction was filtered to remove the Pd/C and the filtrate was concentrated and washed with Et20 to give 7-(4- hydroxybutoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one (intermediate 11) (360 mg, 55%) as a white solid.
[0127] A mixture of intermediate 11 (140 mg, 0.593 mmol), Et3N (240 mg, 2.37 mmol) and methylsulfonyl chloride (102 mg, 0.89 mmol) was dissolved in DCM and the solution was stirred for 6 h at rt. The solution was then washed with water, brine, and dried over anhydrous Na2S04. After filtration, the solvent was evaporated in vacuo to give 4-(7-oxo-5,6,7,8- tetrahydro-l,8-naphthyridin-2-yloxy)butyl methanesulfonate (intermediate 12) (180 mg, 96%) as a yellow solid.
[0128] Intermediate 12 (85 mg, 0.27 mmol) was dissolved in CH3CN then Nal (55 mg, 0.37 mmol), Et3N (91 mg, 0.9 mmol), and intermediate 2 (102 mg, 0.36 mmol) were added. The resulting mixture was stirred at 70°C overnight. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous a2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 7-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8- naphthyridin-2(lH)-one (compound 3) (78 mg, 63%). ¾ MR (300 MHz, CDC13): δ 7.64 (s, 1H), 7.38 (d, J = 8.1 Hz, 1H), 7.27-7.15 (m, 2H), 7.06 (d, J = 8.1 Hz, 1H), 6.37 (d, J = 8.1 Hz, 1H), 4.36 (t, J- 6.0 Hz, 2H), 3.61-3.49 (m, 7H), 3.32-3.20 (m, 5H), 2.90-2.85 (m, 2H), 2.67-2.62 (m, 2H), 2.21-2.16 (m, 2H), 1.90-1.85 (m, 2H). HPLC: 99%, RT 2.682 min. MS (ESI) m/z 463.1 [M + H]+. mp: 110-111°C.
Synthesis of Compound 4
Scheme 8. Synthesis of intermediate 15
[0129] A solution of cinnamoyl chloride (4.25 g, 25.6 mmol) in acetone (25 mL) was added to a solution of 3-methoxyaniline (2.74 mL, 24.4 mmol) and anhydrous K2CO3 (3.7 g, 26.8 mmol) in acetone-ice (28 mL-28 g) and the resulting reaction mixture was stirred at rt for 3 h. The reaction mixture was then poured into ice water, filtered, and washed with water. Recrystallization from EtOH gave N-(3-methoxyphenyl)cinnamamide (intermediate 13) (4.56 g, 74%) as a white solid.
[0130] AICI3 (11.5 g, 86.3 mmol) was added portion-wise to a suspension of intermediate 13 (4.2 g, 16.6 mmol) in chlorobenzene (90 mL) at 0°C. The reaction mixture was gradually warmed to 120°C and stirred for 3 h. The mixture was poured into ice water and the resulting precipitate was collected by filtration, washed with water, and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 60:1) to give 7-hydroxyquinolin-2(lH)-one (intermediate 14) (1.41 g, 53%) as a white solid.
Intermediate 15:
[0131] A mixture of intermediate 14 (193 mg, 1.2 mmol), 1,4-dibromobutane (0.43 mL, 3.6 mmol) and anhydrous K2C03 (166 mg, 1.2 mmol) was dissolved in EtOH and the solution was heated to reflux overnight. The solution was diluted with water and extracted with EtOAc. The combined organic layers was washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 2:1) to give 7-(4-bromobutoxy)quinolin-2(lH)-one (intermediate 15) (147 mg, 41%) as a yellow solid.
[0132] A mixture of intermediate 15 (120 mg, 0.41 mmol) and al (123 mg, 0.82 mmol) in C¾CN was heated to reflux for 30 min and then cooled to rt. Intermediate 2 (175 mg, 0.62 mmol) and anhydrous K2C03 (226 mg, 1.64 mmol) were added to the mixture. The resulting mixture was heated to reflux overnight. The mixture was evaporated under reduced pressure and the residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 7-(4-(4-(2,3-dichlorophenyl)-l,4- diazepan-l-yl)butoxy)quinolin-2(lH)-one (compound 4) (50 mg, 26%). Ή NMR (300 MHz, CDC13) δ 11.54 (br„ 1H), 7.71 (d, J = 6.9 Hz, 1H) 7.44 (d, J = 8.1 Hz, 1H), 7.09-7.07 (m, 2H), 7.01-6.97 (m, 1H), 6.82-6.78 (m, 2H), 6.53 (d, J= 9.3 Hz, 1H), 4.10 (t, J= 6.6 Hz, 2H), 3.30 (d, J = 5.1 Hz, 4H), 2.87-2.81 (m, 4H), 2.62 (t, J= 7.5 Hz, 2H), 2.01-1.99 (m, 2H), 1.92-1.82 (m, 2H), 1.76-1.68 (m, 2H). HPLC: 99%, RT 2.461 min. MS (ESI) m/z 460.2 [M + H]+. mp: 55-57°C.
Synthesis of Compound 5
BF3/MeOH MsCl then TiCI4
Scheme 11. Synthesis of compound 5
[0133] To the solution of 6-methoxy-2,3-dihydro-lH-inden-l-one (6.5 g, 40 mmol) in 100 mL of MeOH was added Et3N (11.1 mL, 80 mmol) and hydroxylamine hydrochloride (3.9 g, 56 mmol). The mixture was stirred at rt for 2 h. The mixture was evaporated and redissolved in EtOAc, the organic phase was washed with water, brine, dried over anhydrous a2S04, filtered and concentrated to give (£)-6-methoxy-2,3-dihydro-lH-inden-l-one oxime (intermediate 16) (6.6 g, 93%).
[0134] To the solution of intermediate 16 (6.6 g, 37.2 mmol) in CH2C12 (120 mL) was added Et3N (20.6 mL, 149 mmol), DMAP (455 mg, 3.73 mmol) and MsCl (4.35 mL, 55.87 mmol). The mixture was stirred at rt overnight. The reaction mixture was washed successively with water, saturated aq NH4C1, brine, dried over anhydrous MgS04, filtered and concentrated.
The crude product was purified by flash chromatography on silica gel column (elution with PE/CH2C12 = 1 : 1) to give (£)-6-methoxy-2,3-dihydro-lH-inden-l-one O-methylsulfonyl oxime (intermediate 17) (7.76 g, 82%) as a white solid.
[0135] To the solution of intermediate 17 (7.76 g, 30.4 mmol) in 1,2-dichloroethane (150 mL) was added BF3 (5.52 mL, 48.7 mmol, in MeOH), MsCl (3.8 mL, 48.7 mmol) and TiCl4 (5.35 mL, 48.7 mmol). The mixture was stirred at rt for 5 h. CH2C12 (150 mL) was then added to the reaction mixture. The solution was washed with water and brine. The water layer was neutralized by aqueous Na2C03 to pH = 7 and was extracted with CH2C12. The combined organic layers were dried over anhydrous MgS04, filtered and concentrated. The crude product was purified by flash chromatography on silica gel column (elution with PE EtOAc = 4: 1) to give 7- methoxy-3,4-dihydroisoquinolin-l(2H)-one (intermediate 18) (5.1 g, 95%) as a white solid.
[0136] BBr3 (4.75 mL, 47.5 mmol, in CH2C12) was added to a cold solution (ice-bath) of intermediate 18 (3.36 g, 18.98 mmol) in CH2C12 (100 mL). The mixture was then warmed to rt and stirred overnight. Water was carefully added dropwise to quench the reaction. EtOAc was added and the mixture was washed with water, brine, dried over anhydrous MgS04, filtered and concentrated to afford 7-hydroxy-3,4-dihydroisoquinolin-l(2H)-one (intermediate 19) (2.46 g, 80%) as a white solid, mp: 201-203°C.
[0137] A mixture of intermediate 19 (326 mg, 2 mmol), 1,3-dibromopropane (0.62 mL, 6 mmol) and anhydrous K2C03 (276 mg, 2 mmol) was dissolved in 4 mL of EtOH and the solution was heated to reflux and stirred overnight. The mixture was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with water, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE EtOAc = 5:1) to give 7-(3-bromopropoxy)-3,4-dihydroisoquinolin-l(2H)-one (intermediate 20) (360 mg, 63%) as a white solid.
Compound 5:
[0138] A mixture of intermediate 20 (110 mg, 0.39 mmol) and Nal (117 mg, 0.78 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 2 (165 mg, 0.58 mmol) and anhydrous K2C03 (216 mg, 1.56 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 7-(3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l- yl)propoxy)-3,4-dihydroisoquinolin-l(2H)-one (compound 5) (125 mg, 71%). !H NMR (300 MHz, CDCI3) δ 7.60 (d, J = 2.1 Hz, 1H), 7.14-7.08 (m, 3H), 7.02-7.00 (m, 2H), 6.02 (br., 1H), 4.10 (t, J = 6.3 Hz, 2H), 3.56-3.53 (m, 2H), 3.31-3.27 (m, 4H), 2.96-2.88 (m, 6H), 2.77-2.72 (m, 2H), 2.01(m, 4H). HPLC: 99%, RT 2.578 min. MS (ESI) m/z 448.1 [M + H]+. mp: 116-117°C.
Synthesis of Compound 6
Scheme 12. Synthesis of intermediate 21
Scheme 13. Synthesis of compound 6
[0139] A mixture of intermediate 19 (196 mg, 1.2 mmol), 1,4-dibromobutane (0.43 mL, 3.6 mmol) and anhydrous K2C03 (166 mg, 1.22 mmol) was dissolved in EtOH (4 mL) and the solution was heated to reflux and stirred overnight. The mixture was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with water, brine, dried over
anhydrous Na2SC>4, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 3 : 1) to give 7-(4-bromobutoxy)-3,4-dihydroisoquinolin-l(2H)- one (intermediate 21) (230 mg, 63%) as a white solid.
Compound 6:
[0140] A mixture of intermediate 21 (100 mg, 0.34 mmol) and Nal (102 mg, 0.68 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 2 (144 mg, 0.51 mmol) and anhydrous K2CO3 (188 mg, 1.36 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous a2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30: 1) to give 7-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l- yl)butoxy)-3,4-dihydroisoquinolin-l(2H)-one (compound 6) (107 mg, 68%). ¾ NMR (300 MHz, CDCI3) δ 7.58 (s, 1H), 7.20-7.09 (m, 3H), 7.02-6.98 (m, 2H), 6.04 (br„ 1H), 4.05 (t, J = 6.6 Hz, 2H), 3.54-3.52 (m, 2H), 3.32-3.28 (m, 4H), 2.95-2.91 (m, 6H), 2.70-2.68 (m, 2H), 2.06-2.04 (m, 2H), 1.84-1.77 (m, 4H). HPLC: 99%, RT 2.676 min. MS (ESI) m/z 462.4 [M + H]+. mp: 86-88°C.
Synthesis of Compound 7
Scheme 14. Synthesis of intermediate 26
intermediate 26 intermediate 25 intermediate 24
Scheme 15. Synthesis of compound 7
[0141] A sample of 3-nitro-4-aminoanisole (13.5 g, 80 mmol) was dissolved in water (100 mL) and concentrated H2S04 (44 mL), cooled to 0°C, and diazotized with a solution of sodium nitrite (14.3 g, 207.2 mmol) in water (25 mL) while maintaining a temperature no greater than 5°C. The resulting solution was added dropwise with vigorous stirring to a solution of crystalline cobalt chloride (42 g, 176 mmol) and potassium thiocyanate (25.6 g, 264 mmol) in water (100 mL), after which the mixture was stirred at 0-10°C for 3 h and allowed to stand overnight. It was then stirred at 50°C for 16 h until nitrogen evolution had ceased. The precipitate was removed by filtration, washed with water, and dried at 80°C to give 4-methoxy-2- nitro-l-thiocyanatobenzene (intermediate 22) (9.82 g, 58%). mp: 127-128°C.
[0142] A solution of intermediate 22 (4 g, 19 mmol) in benzene was added dropwise with stirring and cooling to a suspension of technical-grade lithium aluminum hydride (9.4 g, 247 mmol) in absolute ether (90 mL), after which the mixture was heated to reflux and stirred under a stream of dry nitrogen for 8 h. The excess lithium aluminum hydride was quenched by ice water. Water (25 mL) was then added, the mixture acidified to pH = 6 with dilute HC1, and then extracted with benzene. The extract was dried with MgS04, evaporated, and purified by flash column chromatography to afford 2-amino-4-methoxybenzenethiol hydrochloride (intermediate 23) (1.43 g, 49%) as a light yellow solid.
[0143] A mixture of intermediate 23 (1.33 g, 6.9 mmol), boric acid (2.3 g, 37.2 mmol) and formic acid (17 mL) was heated to reflux and stirred for 6 h. The reaction mixture was
cooled to rt and diluted with water. The solution was made alkaline and extracted with ethyl acetate. Further purification by column chromatography (elution with PE EtOAc = 3:1) gave 5- methoxybenzo[JJthiazole (intermediate 24) (290 mg, 15%). HPLC: 99%, RT 2.646 min. MS (ESI) m/z 166.0 [M + H]+.
[0144] A mixture of intermediate 24 (272 mg, 1.65 mmol) and hydroiodic acid (45%, 2 mL) was heated to reflux for 5 h. The mixture was diluted with water and filtered. The filtrate was neutralized and the solids were filtered, washed with water, and combined to give benzo[c ]thiazol-5-ol (intermediate 25) (206 mg, 83%). HPLC: 99%, RT 2.038 min. MS (ESI) m/z 152.1 [M + H]+.
[0145] A mixture of intermediate 25 (206 mg, 1.4 mmol), 1,4-dibromobutane (0.5 mL, 4.2 mmol) and anhydrous K2C03 (193 mg, 1.4 mmol) in EtOH (20 mL) was heated to reflux and stirred overnight. The yellow solid was filtered and purified by column chromatography (elution with PE/EtOAc = 4:1) to afford 5-(4-bromobutoxy)benzo[if]thiazole (intermediate 26) (108 mg, 39%) as a yellow oil. HPLC: 99%, RT 3.426 min. MS (ESI) m/z 288.0 [M + H]+.
[0146] A mixture of intermediate 26 (47 mg, 0.16 mmol) and Nal (48 mg, 0.32 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 2 (70 mg, 0.25 mmol) and anhydrous K2C03 (88 mg, 0.64 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 5-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l- yl)butoxy)benzo[if]thiazole (compound 7) (31 mg, 42%). ¾ NMR (300 MHz, CDC13) δ 8.97 (s, 1H), 7.80 (d, J = 8.7 Hz, 1H), 7.60 (d, J = 3.0 Hz, 1H), 7.11-7.07 (m, 3H), 7.01-6.98 (m, 1H), 4.10 (t, J= 5.7 Hz, 2H),), 3.33-3.27 (m, 4H), 2.96-2.95 (m, 4H), 2.73-2.71 (m, 2H), 2.06-2.04 (m,
2H), 1.92-1.80 (m, 4H), HPLC: 99%, RT 2.702 min. MS (ESI) m/z 450.1 [M + H]+. mp: 82- 84°C.
Synthesis of Compound 8
Scheme 17. Synthesis of compound 8
[0147] A mixture of intermediate 25 (1 g, 6.6 mmol), 1,3-dibromopropane (1.83 mL, 18 mmol), anhydrous K2C03 (828 mg, 6.0 mmol) and EtOH (20 mL) was heated to reflux and stirred overnight. The yellow solid was filtered and purified by column chromatography (elution with PE/EtOAc = 3:1) to afford 5-(3-bromopropoxy)benzo[d]thiazole (intermediate 27) (543 mg, 33%) as a yellow oil.
[0148] A mixture of intermediate 27 (106 mg, 0.39 mmol) and Nal (140 mg, 0.78 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 2 (165 mg, 0.59 mmol) and anhydrous K2C03 (215 mg, 1.56 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 5-(3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l- yl)propoxy)benzo[<fJthiazole (compound 8) (98 mg, 60%) as a yellow solid. 'H NMR (300 MHz, DMSO-d6) δ 9.37 (s, 1H), 8.05 (d, J= 10.5 Hz, 1H), 7.66 (d, J= 4.8 Hz, 1H), 7.32-7.30 (m, 2H),
7.25-7.23 (m, 1H), 7.14 (dd, J = 9.0 Hz, 2.4 Hz, 1H), 4.19 (t, J = 6.0 Hz, 2H), 3.61-3.29 (m, 10H), 2.31-2.26 (m, 4H). HPLC: 99%, RT 2.601 min. MS (ESI) m/z 436.3 [M + H]+. mp: 93- 94°C.
Synthesis of Compound 9
Scheme 18. Synthesis of intermediate 29
Scheme 19. Synthesis of compound 9
[0149] A well-dried flask was first charged with l-bromo-2-methoxybenzene (2.5 g, 13.4 mmol) and 1,4-diazepane (1.6 g, 16.1 mmol), which was then evacuated and backfilled with N2 through a balloon under gentle warming (40°C). Toluene was added and the mixture was bubbled with N2 for 10 min, then BINAP (250 mg, 0.4 mmol) and Pd2dba3 (156 mg, 0.17 mmol) was added to the mixture. After the addition of DBU (2.4 mL), the solution was warmed at 60- 70°C while tBuONa (2.25 g, 20.1 mmol) was added in one portion to start the amination. After the reaction mixture cooled to rt, (Boc)20 (7.3 g, 33.5 mmol) was dissolved in DCM and added dropwise to the reaction mixture and stirred for 3 h at rt. The reaction mixture was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 30:1) to give tert-butyl 4-(2-methoxyphenyl)- 1,4-diazepane- 1- carboxylate (intermediate 28) (396 mg, 10%) as a yellow oil.
Intermediate 29:
[0150] Excess HC1 in EtOAc was added dropwise to a solution of intermediate 28 (396 mg, 1.29 mmol) in EtOAc and the reaction mixture was stirred at rt for 1.5 h. Filtration gave 1- (2-methoxyphenyl)-l,4-diazepane hydrochloride (intermediate 29) (200 mg, 64%) as a white solid.
Compound 9:
[0151] A mixture of intermediate 4 (150 mg, 0.51 mmol) and Nal (150 mg, 1.02 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 29 (121 mg, 0.5 mmol) and anhydrous K2C03 (276 mg, 2.0 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30: 1) to give 7-(4-(4-(2-methoxyphenyl)-l,4-diazepan-l- yl)butoxy)-3,4-dihydroquinolin-2(lH)-one (compound 9) (25 mg, 11%). HPLC: 95%, RT 2.314 min. MS (ESI) m/z 424.2 [M + H]+.
Synthesis of Compound 10
Scheme 20. Synthesis of compound 10
Compound 10:
[0152] A mixture of intermediate 15 (150 mg, 0.51 mmol) and Nal (150 mg, 1.02 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 29 (124 mg, 0.51 mmol) and anhydrous K2C03 (282 mg, 2.04 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried Over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30: 1) to give 7-(4-(4-(2-methoxyphenyl)-l,4-diazepan-l- yl)butoxy)quinolin-2(lH)-one (compound 10) (61 mg, 26%). *H NMR (300 MHz, CDC13) δ 7.69 (d, J = 9.6 Hz, 1H), 7.43 (d, J= 8.7 Hz, 1H), 6.91-6.85 (m, 5H), 6.79 (d, J= 9.0 Hz, 1H), 6.65 (s, 1H) 6.49 (d, J= 9.3 Hz, 1H), 4.07 (t, J = 6.0 Hz, 2H), 3.83 (s, 3H), 3.34-3.29 (m, 4H), 2.93-2.86 (m, 4H), 2.69-2.65 (m, 2H), 2.06-2.02 (m, 2H), 1.86-1.84 (m, 2H), 1.77-1.73 (m, 2H). HPLC: 95%, RT 1.902 min. MS (ESI) m/z 422.3 [M + H]+.
Synthesis of Compound 11
Scheme 21. Synthesis of compound 11
[0153] A mixture of intermediate 12 (150 mg, 0.48 mmol) and Nal (141 mg, 0.94 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 29 (114 mg, 0.47 mmol) and anhydrous K2C03 (260 mg, 1.88 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 7-(4-(4-(2-methoxyphenyl)-l,4-diazepan-l - yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one (compound 11) (40 mg, 17%). HPLC: 95%, RT 2.400 min. MS (ESI) m/z 422.2 [M + H]+.
Synthesis of Compound 12
Scheme 22. S nthesis of intermediate 29
Scheme 23. Synthesis of compound 12
Intermediate 30:
[0154] A well-dried flask was first charged with l-bromo-2-ethoxybenzene (2.5 g, 12.4 mmol) and 1,4-diazepane (1.5 g, 15.0 mmol), which was evacuated and backfilled with N2 through a balloon under gentle warming (40°C). Toluene was charged and the mixture was bubbled with N2 for 10 min, then BINAP (234 mg, 0.38 mmol) and Pd2dba3 (1 19 mg, 0.13 mmol) was added to the mixture. After the addition of DBU (2.25 mL), the solution was warmed at 60- 70°C while a fine powder of ffluONa (2.1 g, 18.75 mmol) was added in one portion to start the amination. After the reaction mixture cooled to rt, (Boc)20 (6.9 g, 31.25 mmol) was dissolved in DCM and added dropwise to the reaction mixture, then stirred for 3 h at rt. The reaction mixture was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 30:1) to give tert-bvXy\ 4-(2- ethoxyphenyl)-l,4-diazepane-l-carboxylate (intermediate 30) (800 mg, 20%). HPLC: 99%, RT 2.485 min. MS (ESI) m/z 321.0 [M + H]+.
Intermediate 31 :
[0155] Excess HC1 in EtOAc was added dropwise to a solution of intermediate 30 (800 mg, 2.5 mmol) in EtOAc and the reaction mixture was stirred at rt for 1.5 h. Filtration gave l-(2- ethoxyphenyl)-l,4-diazepane hydrochloride (intermediate 31) (382 mg, 59%) as a white solid.
Compound 12:
[0156] A mixture of intermediate 4 (150 mg, 0.5 mmol) and Nal (150 mg, 1.0 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 31 (129 mg, 0.5 mmol) and anhydrous K2CO3 (276 mg, 2.0 mmol) were then added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give 7-(4-(4-(2-ethoxyphenyl)-l,4-diazepan-l- yl)butoxy)-3,4-dihydroquinolin-2(lH)-one (compound 12) (95 mg, 44%). ¾ MR (300 MHz, CDC13) δ 7.75 (bs, 1H), 7.04 (d, J= 8.1 Hz, 1H), 6.92-6.83 (m, 4H), 6.52 (dd, J = 8.4 Hz, 2.1 Hz, 1H), 6.30 (d, J = 2.7 Hz, 1H), 4.04 (q, J = 6.9 Hz, 2H), 3.96-3.94 (m, 2H), 3.38-3.29 (m, 4H), 3.02-2.93 (m, 4H), 2.89 (t, J= 6.9 Hz, 2H), 2.74-2.70 (m, 2H), 2.61 (t, J= 8.1 Hz, 2H), 2.14-2.10 (m, 2H), 1.83-1.79 (m, 4H), 1.45 (t, J= 6.9 Hz, 3H). HPLC: 99%, RT 2.438 min. MS (ESI) m/z 438.2 [M + H]+.
Synthesis of Compound 13
Scheme 24. Synthesis of compound 13
[0157] A mixture of intermediate 12 (150 mg, 0.48 mmol) and Nal (150 mg, 1.0 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 31 (124 mg, 0.48 mmol) and anhydrous Κ2003 (265 mg, 1.92 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give 7-(4-(4-(2-ethoxyphenyl)-l,4-diazepan-l- yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one (compound 13) (50 mg, 24%). XR NMR (300 MHz, CDCI3) δ 7.55 (bs, 1H), 7.35 (d, J= 8.1 Hz, 1H), 6.89-6.85 (m, 4H), 6.34 (d, J= 8.1 Hz, 1H), 4.24-4.20 (m, 2H), 4.04 (q, J= 6.9 Hz, 2H), 3.41-3.37 (m, 2H), 3.31 (t, J= 5.7 Hz, 2H), 3.15-2.95 (m, 4H), 2.86 (t, J= 6.9 Hz, 2H), 2.79-2.69 (m, 2H), 2.64 (t, J= 7.5 Hz, 2H), 2.20-2.12 (s, 2H), 1.82-1.76 (m, 4H), 1.45 (t, J = 6.9 Hz, 3H). HPLC: 99%, RT 2.393 min. MS (ESI) m/z 439.2 [M + H]+.
Synthesis of Compound 14
Scheme 25. Synthesis of compound 14
Compound 14:
[0158] A mixture of intermediate 15 (150 mg, 0.51 mmol) and Nal (150 mg, 1.0 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 31 (130 mg, 0.51
mmol) and anhydrous K2C03 (282 mg, 2.04 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give 7-(4-(4-(2-ethoxyphenyl)-l,4-diazepan-l- yl)butoxy)quinolin-2(lH)-one (compound 14) (50 mg, 24%). lH NMR (300 MHz, CDC13) δ 10.89 (bs, 1H), 7.69 (d, J= 9.6 Hz, 1H), 7.44 (d, J= 9.0 Hz, 1H), 6.92-6.78 (m, 5H), 6.71 (d, J =2.7 Hz, 1H), 6.51 (d, J=9.6 Hz, 1H), 4.10-4.01 (m, 4H), 3.36-3.30 (m, 4H), 2.89-2.79 (m, 4H), 2.62-2.59 (m, 4H), 2.02-2.01 (m, 2H), 1.89-1.82 (m, 2H), 1.45 (t, J= 6.9 Hz, 3H). HPLC: 99%, RT 2.375 min. MS (ESI) m/z 436.2 [M + H]+.
Synthesis of Compound 15
Scheme 27. Synthesis of compound 15
[0159] A mixture of intermediate 14 (193 mg, 1.2 mmol), 1,3-dibromopropane (0.37 mL, 3.6 mmol) and anhydrous K2C03 (166 mg, 1.2 mmol) was dissolved in EtOH and the solution was heated to reflux overnight. The solution was diluted with water and extracted with EtOAc. The combined organic layers were washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE EtOAc = 2:1) to give 7-(3-bromopropoxy)quinolin-2(lH)-one (intermediate 32) (185 mg, 55%) as a white solid.
Compound 15:
[0160] A mixture of intermediate 32 (217 mg, 0.77 mmol) and Nal (231 mg, 1.54 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 2 (217 mg, 0.77 mmol) and anhydrous K2C03 (425 mg, 3.08 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30: 1) to give 7-(3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l- yl)propoxy)quinolin-2(lH)-one (compound 15) (135 mg, 39%). 1H NMR (300 MHz, CDC13) δ 11.96 (bs., 1H), 7.72 (d, J = 9.3 Hz, 1H) 7.45 (d, J = 8.7 Hz, 1H), 7.09-7.07 (m, 2H), 7.01-6.99 (m, 1H), 6.83-6.81 (m, 2H), 6.54 (d, J= 9.0 Hz, 1H), 4.15 (t, J= 5.7 Hz, 2H), 3.31 (d, J= 5.1 Hz, 4H), 2.92-2.96 (m, 4H), 2.76 (t, J= 6.6 Hz, 2H), 2.03-1.01 (m, 4H). HPLC: 99%, RT 2.348 min. MS (ESI) m/z 446.1 [M + H]+. mp: 117-118°C.
Synthesis of Compound 16
Scheme 28. S nthesis of intermediate 34
Scheme 29. Synthesis of compound 16
Intermediate 33:
[0161] A well-dried flask was first charged with l-bromo-2-isopropoxybenzene (500 mg, 2.32 mmol) and 1,4-diazepane (348 mg, 3.48 mmol), which was evacuated and backfilled with N2 through a balloon under gentle warming (40°C). Toluene was charged and the mixture was bubbled with N2 for 10 min, then BINAP (44 mg, 0.07 mmol) and Pd2dba3 (14 mg, 0.02 mmol) were added to the mixture. After the addition of DBU (0.5 mL), the solution was warmed at 60- 70°C while a fine powder of iBuONa (334 mg, 3.48 mmol) was added in one portion to start the amination. After the reaction mixture cooled to rt, (Boc)20 (1.265 g, 5.8 mmol) was dissolved in DCM and added dropwise to the reaction mixture then stirred for 3 h at rt. The reaction mixture was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 30:1) to give tert-butyl 4-(2-isopropoxyphenyl)- 1,4-diazepane-l-carboxylate (intermediate 33) (172 mg, 22%).
Intermediate 34:
[0162] Excess HC1 in EtOAc was added dropwise to a solution of intermediate 33 (172 mg, 0.51 mmol) in EtOAc and the reaction mixture was stirred at rt for 1.5 h. Filtration gave 1- (2-isopropoxyphenyl)- 1,4-diazepane hydrochloride (intermediate 34) (175 mg, 100%) as a white solid.
Compound 16:
[0163] A mixture of intermediate 4 (75 mg, 0.25 mmol) and Nal (75 mg, 0.5 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 34 (100 mg, 0.37 mmol) and anhydrous K2C03 (138 mg, 1.0 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give 7-(4-(4-(2-isopropoxyphenyl)-l,4-diazepan-l- yl)butoxy)-3,4-dihydroquinolin-2(lH)-one (compound 16) (81 mg, 72%). 1H NMR (300 MHz, CDC13) δ 7.72 (bs, 1H), 7.03 (d, J=8.1 Hz, 1H), 6.89-6.85 (m, 4H), 6.51 (d, J=8.1 Hz, 1H), 6.30
(s, 1H), 4.62-4.57 (m, 1H), 4.02-3.92 (m, 2H), 3.42-3.36 (m, 2H ), 3.32-3.28 (m, 2H), 3.07-3.00 (m, 4H), 2.89 (t, J = 7.2 Hz, 2H), 2.80-2.74 (m, 2H), 2.61 (t, J= 6.9 Hz, 2H), 2.20-2.10 (m, 2H), 1.90-1.76 (m, 4H), 1.35 (d, J = 6.0 Hz, 6H). HPLC: 99%, RT 2.506 min. MS (ESI) m/z 452.4 [M + H]+.
Synthesis of Compound 17
Scheme 30. Synthesis of compound 17
Compound 17:
[0164] A mixture of intermediate 12 (114 mg, 0.36 mmol) and Nal (109 mg, 0.73 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 34 (98 mg, 0.36 mmol) and anhydrous K2C03 (200 mg, 1.45 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give 7-(4-(4-(2-isopropoxyphenyl)-l,4-diazepan-l- yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one (compound 17) (52 mg, 32%). Ή NMR (300 MHz, CDCI3) δ 7.58 (bs., 1H), 7.35 (d, J=8.1 Hz, 1H), 6.89-6.86 (m, 4H,), 6.34 (d, J=8.1 Hz, 1H), 4.62-4.57 (m, 1H), 4.22 (m, 2H), 3.42-3.36 (m, 2H), 3.30 (t, J = 6.3 Hz, 2H), 3.08-3.01 (m, 4H), 2.89-2.77 (m, 4H), 2.64 (t, J= 7.2 Hz, 2H), 2.21-2.11 (m, 2H), 1.90-1.72 (m., 4H), 1.35 (d, J= 6.0 Hz, 6H). HPLC: 99%, RT 2.463 min. MS (ESI) m/z 453.3 [M + H]+.
Synthesis of Compound 18
Scheme 31. Synthesis of compound 18
Compound 18:
[0165] A mixture of intermediate 15 (120 mg, 0.41 mmol) and Nal (123 mg, 0.82 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 34 (111 mg, 0.41 mmol) and anhydrous K2C03 (226 mg, 1.64 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give 7-(4-(4-(2-isopropoxyphenyl)-l,4-diazepan-l- yl)butoxy)quinolin-2(lH)-one (compound 18) (115 mg, 62%). 1H NM (300 MHz, CDC13) δ 11.68 (bs., 1H), 7.71 (d, J= 9.3 Hz, 1H), 7.44 (d, J= 9.3 Hz, 1H), 6.89-6.79 (m, 6H), 6.53 (d, J= 9.6 Hz, 1H), 4.62-4.54 (m, 1H), 4.11-4.07 (m, 2H), 3.34-3.29 (m, 4H), 2.94-2.86 (m, 4H), 2.72- 2.62 (m, 2H), 2.12-1.96 (m, 2H), 1.87-1.76 (m, 4H), 1.35 (d, J= 6.0 Hz, 6H). HPLC: 99%, RT 2.434 min. MS (ESI) m/z 450.3 [M + H]+.
\
Synthesis of Compound 19
intermediate 35 intermediate 36
Scheme 33. Synthesis of compound 19
[0166] To a solution of 4-methoxy-2-nitroaniline (10 g, 59.4 mmol) in EtOH (200 mL) and water (80 mL) was added iron (12 g, 210 mmol) and ammonium chloride (9.63 g, 180 mmol). The mixture was then heated to reflux and stirred overnight. The mixture was filtered and the filtrate was evaporated to remove the EtOH and the residue was purified by flash chromatography on silica gel column (elution with DCM/MeOH = 80:1) to give 4- methoxybenzene-l,2-diamine (intermediate 35) (5.65 g, 69%) as a yellow solid.
[0167] Intermediate 35 (5.6 g, 40.5 mmol) was dissolved in MeOH (10 mL). To this solution was added (Boc)20 (8.86 g, 40.6 mmol) followed by iodine (515 mg, 4.06 mmol), which was then stirred at rt for 1 h. The solvent was evaporated, and the residue was dissolved in EtOAc and washed with 5% aq Na2S203 and saturated aq NaHC03. The organic layer was dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel
column (elution with DCM/MeOH = 100:1) to give tert-butyl 2-amino-4- methoxyphenylcarbamate (intermediate 36) (7.4 g, 69%) as a brown solid, mp: 108-110°C.
[0168] Intermediate 36 (7.3 g, 30.6 mmol) was dissolved in DMF (70 mL). To this solution was added anhydrous K2C03 (4.7 g, 33.7 mmol) and the mixture was heated to reflux (130°C) for 5 h. The solvent was removed under pressure, the residue was poured into ice water, and the aq phase was extracted with EtOAc. The combined organic layers were dried over anhydrous Na2S04, concentrated under vacuum, and the resulting yellow solid was recrystallized from EtOH to give 5-methoxy-lH-benzo[^imidazol-2(3H)-one (intermediate 37) (3.6 g, 72%) as a yellow solid, mp: 249-251°C.
[0169] A mixture of intermediate 37 (3.6 g, 22 mmol) and 48% hydrogen iodide (30 mL) was heated to reflux (130°C) and stirred for 12 h. The reaction mixture was neutralized to pH = 8 with aq NaOH and then extracted with EtOAc. The combined organic layers were dried over anhydrous Na2S04, concentrated under vacuum and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 80: 1) to give 5-hydroxy-lH-benzo[cf]imidazol-2(3H)- one (intermediate 38) (16.9 g, 80%). HPLC: 99%, RT 1.377 min. MS (ESI) m/z 151.0 [M + H]+. mp: 186-187°C
[0170] A solution of intermediate 38 (5 g, 33.3 mmol), 1,3-dibromopropane (4 mL, 33.3 mmol) and anhydrous K2C03 (4.6 g, 33.3 mmol) in EtOH (25 mL) was heated to reflux and stirred overnight. The solvent was removed under vacuum. The residue was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with water, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 100: 1) to give 5-(3-bromopropoxy)-lH-benzo[d]imidazol- 2(3H)-one (intermediate 39) (240 mg, 12%) as a yellow solid.
Compound 19:
[0171] Intermediate 39 (80 mg, 0.29 mmol) was dissolved in CH3CN (9 mL), then Nal (89 mg, 0.59 mmol) was added and the mixture was heated to reflux for 30 min, then cooled to rt. Intermediate 2 (43 mg, 0.15 mmol) and anhydrous K2C03 (163 mg, 1.19 mmol) were added. The resulting mixture was heated to reflux and stirred for 6 h. The reaction solution was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 5:1) to give 5-(3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l- yl)propoxy)-lH-benzo[iflimidazol-2(3H)-one (compound 19) (41mg, 32%). Ή NMR (300 MHz, CDC13): δ 9.37 (bs, 1H), 9.05 (bs., 1H), 7.09 - 7.07 (m, 2H), 7.00 - 6.97 (m, 1H), 6.91 - 6.88 (m, 1H), 6.66 - 6.60 (m, 2H), 4.10 - 3.90 (m, 2H), 3.31 - 3.29 (m, 4H), 2.95 - 2.80 (m, 4H), 2.76 - 2.71 (m, 2H), 2.04 - 1.92 (m, 4H). HPLC: 99%, RT 2.241 min. MS (ESI) m/z 436.9 [M + H]+. mp: 148-150°C.
Synthesis of Compound 20
Intermediate 43
[0172] A well-dried flask was first charged with l-bromo-2,3-dichlorobenzene (10 g, 44 mmol) and piperazine (4.55 g, 53 mmol), which was evacuated and backfilled with N2 through a balloon under gentle warming (40°C). Toluene was charged and the mixture was bubbled with N2 for 10 min, then BINAP (822 mg, 1.32 mmol) and Pd2dba3 (403 mg, 0.44 mmol) were added to the mixture. After the addition of DBU (8.0 mL), the solution was warmed at 60-70°C while a fine powder of ffluONa (7.39 g, 66 mmol) was added in one portion to start the amination. After the reaction mixture cooled to rt, (Boc)20 (24 g, 110 mmol) was dissolved in DCM and added dropwise to the reaction mixture, then stirred at rt for 3 h. The reaction mixture was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 10:1) to give tert-butyl 4-(2,3-dichlorophenyl)piperazine-l- carboxylate (intermediate 40) (11.1 g, 76%) as a white solid.
[0173] Excess HC1 in EtOAc was added dropwise to a solution of intermediate 40 (11.1 g, 33.5 mmol) in EtOAc and the reaction mixture was stirred at rt for 1.5 h. Filtration gave 1- (2,3-dichlorophenyl)piperazine hydrochloride salt (intermediate 41) (7.5 g, 78%) as a white solid. HPLC: 99%, RT 2.052 min. MS (ESI) m/z 231.0[M + H]+.
[0174] A mixture of 2-fluoro-4-methoxybenzaldehyde (1 g, 6.5 mmol) and hydrazine (7 mL) was heated to reflux for 4 h. The mixture was extracted with CH2(¾. The combined organic layers were washed with water, brine, concentrated in vacuo, and purified by column chromatography (elution with PE/EtOAc=5: l) to afford 6-methoxy-lH-indazole (intermediate 42) (568 mg, 59%) as a yellow solid. HPLC: 99%, RT 2.159 min. MS (ESI) m/z 149.1[M + H]+. Intermediate 43:
[0175] A mixture of 6-methoxy-lH-indazole (560 mg, 3.8 mmol) and HI (2.4 mL 48%) was heated to reflux for 4 h. The mixture was extracted with CH2C12. The combined organic
layers were washed with water, brine, concentrated in vacuo, and purified by column chromatography (CH2Cl2 MeOH = 60/1) to give lH-indazol-6-ol (intermediate 43) (436 mg, 86%).
[0176] A mixture of lH-indazol-6-ol (600 mg, 4.5 mmol), 1,3-dibromopropane (1.4 mL, 13.4 mmol) and anhydrous K2C03 (618 mg, 4.5 mmol) in EtOH (20 mL) was heated to reflux and stirred overnight. Filtration gave a yellow solid which was purified by column chromatography (elution with PE/EtOAc = 3:1) to afford 6-(3-bromopropoxy)-lH-indazole (intermediate 44) (84 mg, 7%) as a yellow oil.
Compound 20:
[0177] A mixture of intermediate 44 (84 mg, 0.31 mmol) and Nal (94 mg, 0.63 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (125 mg, 0.47 mmol) and anhydrous K2C03 (172 mg, 1.25 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 40:1) to give 6-(3-(4-(2,3-dichlorophenyl)piperazin-l-yl)propoxy)-lH-indazole (compound 20)
(90 mg, 71%). 'H NMR(300 MHZ, CDC13): δ 7.97 (s, 1H), 7.61 (d, J - 8.7 Hz, 1H), 7.16 - 7.14 (m, 2H), 6.98 - 6.95 (m, 1H), 6.87 - 6.82 (m, 2H), 4.10 (t, J = 6.6Hz, 2H), 3.10 (m, 4H), 2.71 - 2.63 (m, 6H), 2.10 - 2.04 (m, 2H). HPLC: 99%, RT 2.462 min. MS (ESI) m/z 405.2 [M + H]+. mp: 159-161°C.
Synthesis of Compound 21
Scheme 37. Synthesis of intermediate 47
H2,RhCI(PPh3)3
96%
intermediate 47 Intermediate 46 38. Synthesis of compound 21
[0178] To a -30°C solution of l-bromo-2,3-dichlorobenzene (10 g, 44 mmol) in THF (120 mL) was added z-PrMgCl (2.0 M in THF, 35 mL, 70 mmol) at a rate such that the temperature < -20°C. Meanwhile, to a -10°C solution of Cul (420 mg, 2.2 mmol) in THF (120 mL) was added pyridine (7.1 mL, 88 mmol) and then benzyl carbonochloridate (9.7 mL, 68 mmol) such that the temperature < 0°C. To this heterogeneous mixture was added the initially formed Grignard at a rate such that the temperature < 0°C. The resulting solution was stirred at 0°C for 30 min and then allowed to warm to rt. The reaction was then quenched with 10% aq
NH4C1. EtOAc was added and the blue aq layer was removed. The organic layer was washed with 10% aq H4CI, 1 N HC1, and a 20% aq NaCl solution. The organic layer was then concentrated and the residue was dissolved and crystallized from MeOH. The slurry was filtered and the filtercake washed with MeOH to give benzyl 4-(2,3-dichlorophenyl)pyridine-l(4H)- carboxylate (intermediate 45) (12 g, 76%) as an off-white solid. HPLC: 99%, RT 4.118 min. MS (ESI) m/z 382.0 [M + Na]+. mp: 69-70°C.
[0179] To a solution of intermediate 45 (7.0 g, 19.5 mmol) in toluene (150 mL) was added R Cl(PPh3)3 (2.1 g, 2.0 mmol) as a slurry in toluene (50 mL). The reaction was subjected to an atmosphere of H2 at 40 psi and heated to 70°C. After 6 h, the reaction was filtered through silica gel and the silica gel was washed with 1 :9 EtO Ac/toluene. The filtrate was dissolved in toluene, concentrated in vacuo, and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 50:1) to give benzyl 4-(2,3-dichlorophenyl)piperidine-l-carboxylate (intermediate 46) (6.8 g, 96%) as an oil. HPLC: 99%, RT 30843 min. MS (ESI) m/z 386.1 [M + Na]+.
[0180] To 6 N HC1 (30 mL) was added a solution of intermediate 46 (5.2 g, 14 mmol) in THF (10 mL). The mixture was heated to reflux for 3 h and then concentrated in vacuo. The residue was washed with Et20 to give 4-(2,3-dichlorophenyl)piperidine (intermediate 47) (3.5 g, 92%) as a white solid. HPLC: 99%, RT 2.149 min. MS (ESI) m/z 230.1 [M + H]+
Compound 21:
[0181] A mixture of intermediate 26 (114 mg, 0.4 mmol) and Nal (120 mg, 0.8 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 47 (160 mg, 0.6 mmol) and anhydrous K2C03 (221 mg, 1.6 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH =
30: 1) to give 5-(4-(4-(2,3-dichlorophenyl)piperidin-l-yl)butoxy)benzo[d]thiazole (compound 21) (144 mg, 66%). ¾ MR (300 MHz, CDC13): δ 8.97 (s, 1H), 7.80 (d, J = 8.7 Hz, 1H), 7.61 (d, J = 2.1 Hz, 1H), 7.33-7.29 (m, 1H), 7.26-7.16 (m, 1H), 7.09 (dd, J= 2.1 Hz, 8.7 Hz, 3H), 4.10 (t, J = 6.6 Hz, 2H), 3.13-3.09 (m, 2H), 2.50 (t, J=7.5 Hz, 2H), 2.14-2.10 (m, 2H), 1.91-1.74 (m, 10H). HPLC: 99%, RT 2.689 min. MS (ESI) m/z 435.3 [M + H]+. mp: 79-81°C.
Synthesis of Compound 22
Scheme 39. Synthesis of compound 22
[0182] A mixture of intermediate 26 (218 mg, 0.8 mmol) and Nal (240 mg, 1.6 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 47 (320 mg, 1.2 mmol) and anhydrous K2C03 (442 mg, 3.8 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30: 1) to give 5-(3-(4-(2,3-dichlorophenyl)piperidin-l-yl)propoxy)benzo[d]thiazole (compound 22) (21 1 mg, 56%). Ή NMR (300 MHz, CDC13): δ 8.98 (s, 1H), 7.80 (d, J = 8.7 Hz, 1H), 7.62 (d, J = 3.0 Hz, 1H), 7.34-7.31 (m, 1H), 7.24-7.18 (m, 3H), 7.15-7.09 (m, 1H), 4.15 (t, J = 5.7Hz, 2H), 3.18-3.12 (m, 3H), 2.70-2.66 (m, 2H), 2.27-2.19 (m, 2H), 2.17-2.10 (m, 2H), 1.92-1.81 (m, 4H). HPLC: 99%, RT 2.622 min. MS (ESI) m/z 421.1 [M + H]+. mp: 114-1 16°C.
Synthesis of Compound 23
Scheme 40. Synthesis of compound 23
Intermediate 27 xamp e
Compound 23:
[0183] A mixture of intermediate 27 (218 mg, 0.8 mmol) and Nal (240 mg, 1.6 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (320 mg, 1.2 mmol) and anhydrous K2C03 (442 mg, 3.2 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 5-(3-(4-(2,3-dichlorophenyl)piperazin-l-yl)propoxy)benzo[d]thiazole (compound 23) (209 mg, 62%). 1H NMR(300 MHz, CDC13): δ 8.97 (s, 1H), 7.80 (d, J= 8.7 Hz, 1H), 7.63 (d, J = 2.1 Hz, 1H), 7.16-7.09 (m, 3H), 6.94 (dd, J = 3.6 Hz, 6.0 Hz, 1H), 4.15(t, J = 5.7Hz, 2H), 3.09 (m, 4H), 2.68-2.63 (m, 6H), 2.10-2.05 (m, 2H). HPLC: 99%, RT 2.607 min. MS (ESI) m/z 422.0 [M + H]+. mp: 127-129°C.
Synthesis of Compound 24
Intermediate 38 reflux
Scheme 42. Synthesis of compound 24
[0184] A mixture of intermediate 38 (253 mg, 1.68 mmol), 1 ,4-dibromobutane (0.6 mL, 5.04 mmol) and anhydrous K2C03 (232 mg, 1.68 mmol) was dissolved in EtOH and the solution was heated to reflux overnight. The solution was diluted with water and extracted with EtOAc. The combined organic layers were washed with saturated NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 4:1) to give 7-(4-bromobutoxy)quinolin-2(lH)-one (intermediate 48) (120 mg, 25%) as a yellow solid.
Compound 24:
[0185] A mixture of intermediate 48 (200 mg, 0.7 mmol) and Nal (210 mg, 1.4 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 47 (167 mg, 0.63 mmol) and anhydrous K2C03 (386 mg, 2.8 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous a2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 5:1) to give 5-(4-(4-(2,3-dichlorophenyl)piperidin-l-yl)butoxy)-lH-benzo[d]imidazol-2(3H)-one
(compound 24) (61 mg, 20%). !H NMR(300 MHz, CDC13): δ 9.59 (brs, 1H), 9.29 (brs, 1H), 7.33-7.30 (m, 2H), 7.20-7.13 (m, 1H), 6.92 (d, J = 8.7 Hz, 1H), 6.65-6.59 (m, 2H), 3.94-3.91 (m, 2H), 3.15-3.04 (m, 3H), 2.51-2.49 (m, 2H), 2.15(t, J = 11.1Hz, 2H), 1.90-1.74 (m, 8H). HPLC: 99%, RT 2.332 min. MS (ESI) m/z 434.0 [M + H]+. mp: 180-182°C.
Synthesis of Compound 25
Scheme 43. Synthesis of compound 25
Compound 25:
[0186] A mixture of intermediate 21 (220 mg, 0.74 mmol) and Nal (222 mg, 1.48 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (278 mg, 1.04 mmol) and anhydrous K2C03 (1409 mg, 2.96 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give 7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-3,4-dihydroisoquinolin-l(2H)- one (compound 25) (215 mg, 65%). Ή NMR (300 MHz, CDC13): δ 7.58 (d, J = 2.7 Hz, 1H), 7.14-7.11 (m, 3H), 7.01-6.94 (m, 2H), 6.18 (brs, 1H), 4.05 (t, J= 6.3 Hz, 2H), 3.56-3.51 (m, 2H), 3.07 (m, 4H), 2.90 (t, J = 8.1 Hz, 2H), 2.66 (m, 4H), 2.49-2.46 (m, 2H), 1.86-1.68 (m, 4H). HPLC: 99%, RT 2.374 min. MS (ESI) m/z 448.3 [M + H]+.
Synthesis of Compound 26
Scheme 44. Synthesis of intermediate 49
Intermediate 49
Scheme 45. Synthesis of compound 26
Intermediate 49 CH3CN Example 26
[0187] A mixture of 7-hydroxy-3,4-dihydroquinolin-2(lH)-one (196 mg, 1.2 mmol), 1,4- bis(bromomethyl)cyclohexane (972 mg, 3.6 mmol) and anhydrous K2CO3 (166 mg, 1.2 mmol) was dissolved in EtOH and the solution was heated to reflux overnight. The solution was diluted with water and extracted with EtOAc. The combined organic layers were washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column to give 7-((4-(bromomethyl)cyclohexyl)methoxy)-3,4- dihydroquinolin-2(lH)-one (intermediate 49) (130 mg, 30%) as a white solid.
[0188] A mixture of intermediate 49 (70 mg, 0.2 mmol) and Nal (60 mg, 0.4 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (81 mg, 0.3 mmol) and anhydrous K2C03 (110 mg, 0.8 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give 7-((4-((4-(2,3-dichlorophenyl)piperazin-l-yl)methyl)cyclohexyl)methoxy)-3,4-dihydroquinolin-
2(lH)-one (compound 26) (63 mg, 63%). 1H NMR (300 MHz, CDC13): δ 8.06 (s, 1H), 7.13-7.15 (m, 2H), 7.04 (d, J= 8.4 Hz, 1H), 6.94-6.97 (m, 1H), 6.52 (dd, J= 2.4 Hz, 8.4 Hz, 1H), 6.32 (d, J = 2.1 Hz, 1H), 3.71-3.74 (m, 2H), 3.05-3.07 (m, 4H), 2.87-2.92 (m, 2H), 2.59-2.64 (m, 6H), 2.24 (d, J= 6.9 Hz, 2H), 3.83 (d, J= 9.9 Hz, 2H), 1.53-1.69 (m, 6H), 0.94-1.10 (m, 4H). HPLC: 99%, RT 2.652 min. MS (ESI) m/z 502.2 [M + H]+.
Synthesis of Compound 27
Intermediate 50
Scheme 47. Synthesis of compound 27
[0189] A mixture of 7-hydroxy-3,4-dihydroquinolin-2(lH)-one (930 mg, 6 mmol), 1,4- dichlorobut-2-yne (326 mg, 2 mmol) and anhydrous K2C03 (276 mg, 2 mmol) was dissolved in EtOH and the solution was heated to reflux overnight. The solution was diluted with water and extracted with EtOAc. The combined organic layers were washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column to give 7-(4-chlorobut-2-ynyloxy)-3,4-dihydroquinolin-2(lH)-one (intermediate 50) (330 mg, 66%) as a yellow solid.
Compound 27:
[0190] A mixture of intermediate 50 (200 mg, 0.8 mmol) and Nal (240 mg, 1.6 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (320 mg, 1.2 mmol) and anhydrous K2C03 (442 mg, 3.2 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give 7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)but-2-ynyloxy)-3,4-dihydroquinolin- 2(lH)-one (compound 27) (280 mg, 79%). Ή NMR (300 MHz, CDC13): δ 7.85 (s, 1H), 7.20- 7.13 (m, 2H), 7.08 (d, J= 8.4 Hz, 1H), 6.97 (dd, J = 3.0Hz, 6.6Hz, 1H), 4.73 (s, 2H), 3.42 (s, 2H),
3.10 (s, 4H), 2.93-2.88 (m, 2H), 2.75 (s, 4H), 2.64-2.59 (m, 2H), 2.06 (s, lH), 1.70 (s, 2H), 1.29- 1.24 (m, 1H). HPLC: 99%, RT 2.403 min. MS (ESI) m/z 444.1 [M + H]+.
Synthesis of Compound 28
Scheme 49. Synthesis of compound 28
[0191] A mixture of lH-indazol-6-ol (intermediate 43) (320 g, 2.39 mmol), 1 ,4- dibromobutane (0.9 mL, 7.2 mmol), anhydrous K2C03 (55 mg, 2.39 mmol) and EtOH (5 mL) was heated to reflux and stirred overnight. A yellow solid was filtered and purified by column chromatography (elution with PE/EtOAc = 3 :1) to afford 5-(3-bromopropoxy)benzo[d]thiazole (intermediate 51) (70 mg, 17%) as a yellow oil.
Compound 28:
[0192] A mixture of intermediate 51 (65 mg, 0.24 mmol) and Nal (72 mg, 0.48 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (97 mg, 0.36 mmol) and anhydrous K2C03 (132 mg, 0.96 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30: 1) to give 6-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-lH-indazole (compound 28) (35 mg, 35%). Ή MR (300 MHz, CDC13): δ 7.97 (s, 1H), 7.61 (d, J = 8.7 Hz 1H), 7.17-7.15 (m,
2H), 6.96(dd, J = 3.0 Hz, 6.6 Hz, 1H), 6.86-6.81 (m, 2H), 4.05 (t, J = 5.4 Hz, 2H), 3.16 (s, 4H), 2.79 (s, 4H), 2.64-2.60 (m, 2H), 1.89-1.83 (m, 4H). HPLC: 99%, RT 2.491 min. MS (ESI) m/z 419.2 [M + H]+. mp: 101-103°C.
Synthesis of Compound 29
Intermediate 52
Scheme 51. Synthesis of compound 29
[0193] A mixture of 7-hydroxy-3,4-dihydroquinolin-2(lH)-one (196 mg, 1.2 mmol), (£)- 1 ,4-dichlorobut-2-ene (450 mg, 3.6 mmol) and anhydrous K2C03 (166 mg, 1.2 mmol) was dissolved in EtOH and the solution was heated to reflux and stirred overnight. The solution was diluted with water and extracted with EtOAc. The combined organic layers were washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column to give (£)-7-(4-chlorobut-2-enyloxy)-3,4- dihydroquinolin-2(lH)-one (intermediate 52) (102 mg, 34%) as a white solid.
Compound 29:
[0194] A mixture of intermediate 52 (75 mg, 0.25 mmol) and Nal (75 mg, 0.5 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (102 mg, 0.38 mmol) and anhydrous K2C03 (138 mg, 1 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The
combined EtOAc layers were washed with brine, dried over anhydrous Na2SC>4, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give (E)-7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)but-2-enyloxy)-3,4-dihydroquinolin- 2(lH)-one (compound 29) (79 mg, 71%). ]H MR (300 MHz, CDC13): δ 7.77 (s, 1H), 7.16-7.14 (m, 2H), 7.05 (d, J= 8.1 Hz, 1H), 6.98-6.94 (m, 1H), 6.53 (d, J- 8.1 Hz, 1H), 6.33 (s, 1H), 5.94- 5.89 (m, 2H), 4.51 (d, J= 4.2 Hz, 2H), 3.19-3.08 (m, 6H), 2.90 (t, J= 6.9 Hz, 2H), 2.67-2.59 (m, 6H). HPLC: 99%, RT 2.468 min. MS (ESI) m/z 446.1 [M + H]+. mp: 151-152°C.
Synthesis of Compound 30
Intermediate 41 Intermediate 53
Scheme 53. Synthesis of compound 30
[0195] A mixture of l-(2,3-dichlorophenyl)piperazine hydrochloride (intermediate 41) (294 mg, 1.1 mmol), l-bromo-3-(bromomethyl)benzene (250 mg, 1 mmol) and anhydrous triethylamine (253 mg, 2.5 mmol) was dissolved in CH3CN and the solution was heated to reflux for 4 h. The solution was diluted with water and extracted with EtOAc. The combined organic layers were washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 8:1) to give l-(3-bromobenzyl)-4-(2,3-dichlorophenyl)piperazine (intermediate 53) (220 mg, 74%) as a white solid.
Compound 30:
[0196] To a solution of 7-hydroxy-3,4-dihydroquinolin-2(lH)-one (196 mg, 1.2 mmol in NMP was added Cs2C03 (391 mg, 1.2 mmol). The slurry was degassed by evacuating and filling the reaction flask with N2 three times. Intermediate 53 (240 mg, 0.6 mmol) and TMHD (11 mg, 0.06 mmol) were added followed by the addition of CuCl (60 mg, 0.6mmol). The reaction mixture was degassed by evacuating and filling the reaction flask with N2 three times, and then warmed to 120°C under N2 for 7.5 h. The reaction mixture was cooled to rt and diluted with Et20. The slurry was filtered and the filtercake was washed with Et20. Combined filtrates were washed with 2 N HC1, 0.6 N HC1, 2 M NaOH, and 10% aq NaCl. The resulting organic layer was dried, concentrated, and purified by flash chromatography on a silica gel column (elution with PE/EtOAc = 1 :1) to give 7-(3-((4-(2,3-dichlorophenyl)piperazin-l-yl)methyl)phenoxy)-3,4- dihydro quinolin-2(lH)-one (compound 30) (110 mg, 38%). 'H NMR (300 MHz, CDC13): δ 7.62 (s, 1H), 7.31 (d, J = 7.2 Hz, 2H), 7.15-7.05 (m, 4H), 6.96-6.89 (m, 2H), 6.62 (dd, J = 2.1 Hz, 8.1Hz, 1H), 6.41 (d, J= 2.1Hz, 1H), 3.57 (s, 2H), 3.06 (m, 4H), 2.94 (t, J= 7.5Hz, 2H), 2.66-2.61 (m, 5H). HPLC: 99%, RT 2.637 min. MS (ESI) m/z 482.1 [M + H]+. mp: 182-183°C.
Synthesis of Compound 31
Scheme 54. Synthesis of intermediate 54
[0197] A mixture of intermediate 41 (294 mg, 1.1 mmol), l-bromo-4-(bromomethyl) benzene (250 mg, 1.0 mmol) and anhydrous triethylamine (253 mg, 2.5 mmol) was dissolved in CH3CN and the solution was heated to reflux for 4 h. The solution was diluted with water and extracted with EtOAc. The combined organic layers were washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 8:1) to give l-(4-bromobenzyl)-4-(2,3- dichlorophenyl)piperazine (intermediate 54) (220 mg, 74%) as a white solid.
[0198] To a solution of 7-hydroxy-3,4-dihydroquinolin-2(lH)-one (228 mg, 1.4 mmol) in NMP was added Cs2C03 (456 mg, 1.4 mmol). The slurry was degassed by evacuating and filling the reaction flask with N2 three times. Intermediate 54 (280 mg, 0.7 mmol) and TMHD (13 mg, 0.07 mmol) were added followed by the addition of CuCl (70 mg, 0.7 mmol). The reaction mixture was degassed by evacuating and filling the reaction flask with N2 three times, and then warmed to 120°C under N2 for 7.5 h. The reaction mixture was cooled to rt and diluted with Et20. The slurry was filtered and the filtercake was washed with Et20. Combined filtrates were washed with 2 N HC1, 0.6 N HC1, 2 M NaOH, and 10% aq NaCl. The resulting organic layer was dried, concentrated, and purified by flash chromatography on a silica gel column (elution with PE EtOAc = 1:1) to give 7-(4-((4-(2,3-dichlorophenyl)piperazin-l-yl)methyl)phenoxy)-3,4- dihydro quinolin-2(lH)-one (compound 31) (120 mg, 35%). !H NMR (300 MHz, CDC13): δ 7.67 (s, 1H), 7.32 (d, J = 8.7 Hz, 2H), 7.16-7.09 (m, 3H), 6.98-6.94 (m, 3H), 6.64-6.61 (m, 1H), 6.41 (d, J = 3.0Hz, 1H), 3.56 (s, 2H), 3.07 (m, 4H), 2.94 (t, J = 6.6 Hz, 2H), 2.66-2.61 (m, 6H). HPLC: 99%, RT 2.626 min. MS (ESI) m/z 482.1 [M + H]+. mp: 185-186°C.
Synthesis of Compound 32
Scheme 56. Synthesis of compound 32
[0199] A mixture of intermediate 20 (110 mg, 0.39 mmol) and Nal (117 mg, 0.78 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (157 mg, 0.59 mmol) and anhydrous K2C03 (216 mg, 1.56 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 6 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 7-(3-(4-(2,3-dichlorophenyl)piperazin-l-yl)propoxy)-3,4-dihydroisoquinolin-l(2H)- one (compound 32) (88 mg, 52%). ¾ NMR (300 MHz, CDC13): δ 7.61 (s, 1H), 7.14-7.11 (m, 3H), 7.03-6.98(m, 2H), 5.93 (brs, 1H), 4.11 (m, 2H), 3.55 (m, 2H), 3.09-2.94 (m, 6H), 2.65-2.61 (m, 6H), 2.02 (s, 2H). HPLC: 99%, RT 2.612 min. MS (ESI) m/z 434.1 [M + H]+. mp: 162- 164°C.
Synthesis of Compound 33
Scheme 57. Synthesis of compound 33
[0200] A mixture of intermediate 21 (103 mg, 0.35 mmol) and Nal (105 mg, 0.7 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 47 (142 mg, 0.53 mmol) and anhydrous K2C03 (194 mg, 1.4 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30: 1) to give 7-(4-(4-(2,3-dichlorophenyl)piperidin-l-yl)butoxy)-3,4-dihydroisoquinolin-l(2H)- one (compound 33) (108 mg, 69%). *H NMR (300 MHz, CDC13): δ 7.58 (d, J = 2.1Hz, 1H), 7.23-7.11 (m, 5H), 7.02-6.99 (m, 1H), 6.05 (s, 1H), 4.05 (t, J = 6.0 Hz, 2H), 3.56-3.53 (m, 2H), 3.15-3.06 (m, 3H), 2.93 (t, J = 6.6Hz, 2H), 2.51 (m, 2H), 2.18 (m, 2H), 1.82 (m, 8H). HPLC: 99%, RT 2.746 min. MS (ESI) m/z 447.2 [M + H]+. mp: 113-114°C.
Synthesis of Compound 34
Scheme 58. Synthesis of compound 34
in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (188 mg, 0.71 mmol) and anhydrous K2C03 (259 mg, 1.88 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. Precipitated crystals were filtered off and the
filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 15:1) to give 5-(3-(4-(2,3-dichlorophenyl)piperazin-l-yl)propoxy)-lH-benzo[d]imidazol-2(3H)- one (compound 34) (54 mg, 27%). 'H MR (300 MHz, DMSO): δ 10.60 (s, 1H), 10.36 (s, 1H), 7.32 (d, J = 5.1 Hz, 2H), 7.16-7.18 (m, 1H), 6.80 (d, J = 9.6 Hz, 1H), 6.51-6.53 (d, J = 6.6 Hz, 2H), 3.99-3.95 (m, 2H), 2.81-3.26 (s, 5H), 2.80-2.60 (m, 5H), 1.80-2.01 (m, 2H). HPLC: 99%, RT 2.228 min. MS (ESI) m/z 421.1 [M + H]+.
Synthesis of Compound 35
Scheme 59. Synthesis of compound 35
Compound 35:
[0202] A mixture of intermediate 48 (110 mg, 0.39 mmol) and al (117 mg, 0.78 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (156 mg, 0.59 mmol) and anhydrous K2C03 (215 mg, 1.56 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred overnight. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 15:1) to give 5-(3-(4-(2,3-dichlorophenyl)piperazin-l-yl)propoxy)-lH-benzo[d]imidazol-2(3H)- one (compound 35) (54 mg, 32%). *H NMR (300 MHz, CDC13): δ 9.53 (s, 1H), 9.24 (s, 1H), 7.17-7.13 (m, 2H), 6.97-6.91 (m, 2H), 6.66-6.60 (m, 2H), 3.95 (t, J= 5.7 Hz, 2H), 3.20-3.01 (m, 4H), 2.80-2.61 (m, 4H), 2.52 (t, J= 7.5 Hz, 2H), 1.82-1.73(m, 4H). HPLC: 99%, RT 2.325 min. MS (ESI) m/z 435.1 [M + H]+.
Synthesis of Compound 36
[0203] A mixture of intermediate 26 (108 mg, 0.38 mmol) and Nal (114 mg, 0.76 mmol) in C¾CN was heated to reflux for 30 min and then cooled to rt. Intermediate 41 (152 mg, 0.57 mmol) and anhydrous K2C03 (210 mg, 1.52 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 5-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)benzo[d]thiazole (compound 36) (98 mg, 60%). ¾ NM (300 MHz, CDC13): δ 8.97 (s, 1H), 7.79 (d, J = 8.7 Hz, 1H), 7.61 (s, 1H), 7.16-7.08 (m, 3H), 6.97-6.94 (m, 1H), 4.11 (t, J= 5.7 Hz, 2H), 3.09 (br, 4H), 2.68 (brs, 4H), 2.55-2.50 (m, 2H), 1.92-1.76 ( m, 5H). HPLC: 99%, RT 2.651 min. MS (ESI) m/z 436.3 [M+H]+. mp: 93-94.5°C.
Synthesis of Compound 37
Scheme 62. S nthesis of com ound 37
[0204] A well-dried flask was first charged with l-bromo-2-methoxybenzene (4.0 g, 21.3 mmol) and piperazine (2.2 g, 25.6 mmol), which was evacuated and backfilled with N2 through a balloon under gentle warming (40°C). Toluene was charged and the mixture was bubbled with N2 for 10 min, then BINAP (398 mg, 0.64 mmol) and Pd2dba3 (195 mg, 0.21 mmol) was added to the mixture. After the addition of DBU (3.8 mL), the solution was warmed at 60-70°C while a fine powder of tBuONa (3.5 g, 31.9 mmol) was added in one portion to start the amination. After the reaction mixture cooled to rt, (Boc)20 (11.6 g, 53.2 mmol) was dissolved in DCM and added dropwise to the reaction mixture, then stirred at rt for 3 h. The reaction mixture was diluted with water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with PE/EtOAc = 10:1) to give tert-butyl 4-(2-methoxyphenyl)piperazine-l- carboxylate as a white solid. Excess HC1 in EtOAc was added dropwise to a solution of tert- butyl 4-(2-methoxyphenyl)piperazine-l-carboxylate in EtOAc and the reaction mixture was stirred at rt for 1.5 h. Filtration gave l-(2-methoxyphenyl)piperazine hydrochloride salt (intermediate 55) (2.6 g, 42%) as a white solid. HPLC: 99%, RT 1.586 min. MS (ESI) m/z 193.1 [M + H]+.
Compound 37:
[0205] A mixture of intermediate 12 (314 mg, 0.48 mmol) and Nal (150 mg, 0.96 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 55 (228 mg, 0.48 mmol) and anhydrous K2C03 (138 mg, 1.92 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 7-(4-(4-(2-methoxyphenyl)piperazin-l-yl)butoxy)-3,4-dihydroquinolin-2(lH)-one (compound 37) (98 mg, 60%). ¾ NMR (300 MHz, CDC13): δ 7.55 (s, 1H), 7.35 (d, J= 8.1 Hz, 1H), 7.02-6.85 (m, 3H), 6.37-6.34(m, 1H), 4.29-4.20 (m, 2H), 3.86 (s, 3H), 3.11 (brs, 4H), 2.86
(t, J = 6.9 Hz, 2H), 2.67-2.61(m, 5H), 2.48 (t, J = 7.2 Hz, 1H), 1.82-1.62(m, 6H). HPLC: 99%, RT 2.109 min. MS (ESI) m/z 411.2 [M + H]+.
Synthesis of Compound 38
Scheme 63. S nthesis of intermediate 59
Intermediate 57 Intermediate 58 Intermediate 59
Scheme 64. Synthesis of compound 38
[0206] To a cooled (0°C) solution of 2-amino-4-chlorophenol (30 g, 209 mmol) in DCM (200 mL) and pyridine (17 mL) was added a solution of chloroacetyl chloride in DCM (23.6 g, 230 mmol in 75 mL). The mixture was allowed to reach rt slowly and was subsequently stirred for 18 h. The precipitate was filtered, washed with DCM, and dried overnight at 50°C to give crude 2-chloro-N-(5-chloro-2-hydroxyphenyl)acetamide (intermediate 56) (26 g, 55%), as a brown solid which was used in the next step without any further purification.
[0207] Intermediate 56 (26 g, 118 mmol) was suspended in water at 0°C. To suspension was added HN03 (1.5 equiv, 15 mL, 174 mmol) over a period of 0.75 h.
resulting red solution was allowed to reach rt after 1 h, then water was added to give a dark brown solid which was isolated by filtration. The resulting filtrate was washed with cold water (3x100 mL) and dried to give a yellow solid. Ring closure to intermediate 57 was established by heating the yellow solid with K2C03 in toluene/water (50/1, v/v) for 2 h. The reaction mixture was cooled to rt and the resulting brown precipitate was filtered and purified by silica gel chromatography (EtOAc/PA, 1/1, v/v to 1/0, v/v) to give 6-chloro-8-nitro-2H- benzo[b][l,4]oxazin-3(4H)-one (intermediate 57) (21 g, 78%) as a brown solid.
[0208] Intermediate 57 (326 mg, 1.4 mmol) was dissolved in EtOH, then Pd/C (0.03 g) was added. The mixture was hydrogenated under H2 for 4 h at 50°C. The reaction mixture was filtered over Hyflo and the filtrate was concentrated in vacuo to give 8-amino-2H- benzo[b][l,4]oxazin-3(4H)-one (intermediate 58) (83%) as a brown solid, which was used in the next step without any further purification.
Intermediate 59:
[0209] To a solution of intermediate 58 (164 mg, 1.0 mmol) in chlorobenzene (5 mL) was added bis(2-chloroethyl)amine hydrochloric acid (285 mg, 1.6 mmol) and the mixture was heated to reflux for 100 h. The reaction mixture was concentrated in vacuo and the residue was stirred in EtOAc for 2 h. The resulting brown solid was filtered and purified by silica gel chromatography to give 8-(piperazin-l-yl)-2H-benzo[b][l,4] oxazin-3(4H)-one (intermediate 59) (150 mg, 64%). HPLC: 99%, RT 1.344 min. MS(ESI) m/z 234.1 [M + H]+.
Compound 38:
[0210] A mixture of intermediate 4 (110 mg, 0.37 mmol) and Nal (110 mg, 0.74 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 59 (118 mg, 0.5 mmol) and anhydrous K2C03 (138 mg, 1.92 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the
filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 8-(4-(4-(2-oxo- 1,2,3, 4-tetrahydroquinolin-7-yloxy)butyl)piperazin-l-yl)-2H- benzo[b][l,4]oxazin-3(4H)-one (compound 38) (55 mg, 34%). ]H NMR (300 MHz, CDC13): δ 10.56 (s, 1H), 9.96 (s, 1H), 7.04 (d, J= 8.4 Hz, 1H), 6.85 (t, 1H), 6.57-6.43 (m, 4H), 4.53 (s, 2H), 3.92 (t, J = 5.7 Hz, 2H), 2.97 (br, 4H), 2.78 (t, J= 6.9 Hz, 2H), 2.43-2.38 (m, 4H), 1.72-1.58 (m, 4H). HPLC: 99%, RT 1.970 min. MS (ESI) m/z 451.0 [M + H]+.
Synthesis of Compound 39
Scheme 65. Synthesis of compound 39
Compound 39:
[0211] A mixture of intermediate 4 (110 mg, 0.34 mmol) and Nal (102 mg, 1.36 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 55 (117 mg, 0.51 mmol) and anhydrous K2C03 (188 mg, 1.36 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 7-(5-(4-(2-methoxyphenyl)piperazin-l-yl)pentyloxy)-3,4-dihydroquinolin-2(lH)- one (compound 39) (112 mg, 81%). 1H MR (300 MHz, CDC13): δ 7.92 (brs, 1H), 7.06-6.85 (m, 5H), 6.52 (d, J = 6.6 Hz, 1H), 6.31 (s, 1H), 3.98-3.94 (m, 2H), 3.86 (s, 3H), 3.12 (br, 4H), 2.92- 2.87 (m, 2H), 2.69-2.59 (m, 6H), 2.52-2.47 (m, 2H), 1.82-1.72 (m, 4H). HPLC: 99%, RT 2.100 min. MS (ESI) m/z 410.2 [M + H]+.
Synthesis of Compound 40
Scheme 66, Synthesis of intermediate 61
[0212] Triethylamine in acetone was added dropwise to the solution of 2,3- dihydrobenzo[b][l,4]dioxine-5-carboxylic acid (360 mg, 2 mmol) in water and acetone at 0°C. To the mixture was added ethyl carbonochloridate in acetone slowly. The mixture was stirred for 4 h then a solution of NaN3 (196 mg, 3.02 mmol) in water was added dropwise. The reaction mixture was stirred for 1 h then it was poured into ice water and extracted with Et20. The combined organic extracts were washed with brine, dried with MgS04, and concentrated in vacuo. The residue was dissolved in anhydrous toluene and the mixture was heated on a steam bath until nitrogen evolution had ceased. The toluene was then removed in vacuo and 20% aq HCI (4 mL) was added and the solution was heated to reflux and stirred overnight. The reaction was concentrated under reduced pressure. The residue was dissolved in water, made strongly alkaline by the addition of 40% aq NaOH, then extracted with Et20. The combined organic extracts were washed with brine, dried by MgS04, and purified by flash chromatography on silica gel column (PE:EtOAc=5:l) to give 2,3-dihydrobenzo[b][l,4]dioxin-5-amine (intermediate 60) (270 mg, 70%). HPLC: 99%, RT 1.214 min. MS (ESI) m/z 152.1 [M + H]+.
Intermediate 61:
[0213] Bis(2-chloroethyl)amine hydrochloride salt (452 mg, 2.54 mmol) was added to a solution of intermediate 60 (320 mg, 2.12 mmol) in chlorobenzene (4 mL) and the mixture was heated to reflux and stirred overnight. The solvent was removed under reduced pressure and the residue was purified by flash chromatography on silica gel column (DCM:MeOH=30: 1-20:1), followed by a wash with EtOAc, to give l-(2,3-dihydrobenzo[b][l,4]dioxin-5-yl)piperazine (intermediate 61) (236 mg, 51%). HPLC: 99%, T 1.673 min. MS (ESI) m/z 221.1 [M + H]+.
Compound 40:
[0214] A mixture of intermediate 4 (100 mg, 0.34 mmol) and Nal (102 mg, 0.68 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 61 (112 mg, 0.51 mmol) and anhydrous K2C03 (188 mg, 1.36 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 7-(4-(4-(2,3-dihydrobenzo[b][l,4]dioxin-5-yl)piperazin-l-yl)butoxy)-3,4- dihydroquinolin-2(lH)-one (compound 40) (125 mg, 83%). ¾ NMR (300 MHz, CDC13): δ 7.88 (s, 1H), 7.04 (d, J=8.1Hz, 1H), 6.80-6.77(m, 1H), 6.61-6.51 (m, 3H), 6.31 (d, J = 1.8 Hz, 1H), 4.33-4.24 (m, 4H),3.96(m, 2H), 3.10(m, 4H), 2.89 (t, J = 7.5 Hz, 2H), 2.66-2.59 (m, 6H), 2.50- 2.46(m, 2H), 1.81-1.71(m, 6H). HPLC: 99%, RT 2.468 min. MS (ESI) m/z 438.2 [M + H]+.
Synthesis of Compound 41
Scheme 68. S nthesis of com ound 41
xamp e
Compound 41:
[0215] A mixture of intermediate 12 (100 mg, 0.32 mmol) and Nal (95 mg, 0.64 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 61 (105 mg, 0.48 mmol) and anhydrous K2C03 (176 mg, 1.27 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 30:1) to give 7-(4-(4-(2,3-dihydrobenzo[b][l,4]dioxin-5-yl)piperazin-l-yl)butoxy)-3,4-dihydro- l,8-naphthyridin-2(lH)-one (compound 41) (115 mg, 83%). !H MR (300 MHz, CDC13): δ 7.60 (s, 1H), 7.36 (d, J=8.1Hz,lH), 7.27 (s, 1H), 6.78 (t, J= 8.1 Hz, lH),6.61-6.53(m, 2H), 6.35 (d, J = 8.1 Hz, lH),4.33-4.20 (m, 6H), 3.10 (s, 4H), 2.86 (dd, J=7.5 Hz, 2H), 2.67-2.62 (m, 6H),2.51- 2.46 (m, 2H), 1.82-1.69(m, 6H). HPLC: 99%, RT 2.084 min. MS (ESI) m/z 439.2 [M + H]+.
Synthesis of Compound 42
Scheme 69. S nthesis of com ound 42
Compound 42:
[0216] A mixture of intermediate 15 (70 mg, 0.24 mmol) and Nal (70 mg, 0.48 mmol) in CH3CN was heated to reflux for 30 min and then cooled to rt. Intermediate 61 (78 mg, 0.36 mmol) and anhydrous K2C03 (162 mg, 0.96 mmol) were added to the mixture. The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOA. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH =
30:1) to give 7-(4-(4-(2,3-dihydrobenzo[b][l,4]dioxin-5-yl)piperazin-l-yl)butoxy)quinolin- 2(lH)-one (compound 42) (52 mg, 50%). !H NMR (300 MHz, CDC13): δ 11.65 (brs, 1H), 7.72 (d, J = 9.6 Hz, 1H), 7.45 (d, J = 8.4 Hz, 1H), 6.82-6.75 (m, 3H), 6.61- 6.52 (m, 3H), 4.33-4.26 (m, 4H), 4.12-4.08 (m, 2H), 3.12 (br, 4H), 2.70 (br, 4H), 2.52(br, 2H), 2.04-1.68 (m, 6H). HPLC: 99%, RT 2.167 min. MS (ESI) m/z 436.0 [M + H]+.
Synthesis of Compound 43
Scheme 70. Synthesis of compound 43
[0217] A mixture of 4-(4-bromobutoxy)-l-methyl-2-nitrobenzene (200 mg, 0.7 mmol), intermediate 41 (186 mg, 0.7 mmol) and anhydrous K2C03 (195 mg, 1.4 mmol) was dissolved in CH3CN and the solution was heated to reflux and stirred overnight. The solution was concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 50:1) to give l-(2,3-dichlorophenyl)-4-(4-(4-methyl-3- nitrophenoxy)butyl)piperazine (intermediate 62) (180 mg, 59%) as a yellow solid.
Intermediate 63:
[0218] A mixture of intermediate 62 (180 mg, 0.41 mmol), Fe (70 mg, 1.23 mmol) and ammonium chloride (25 mg, 0.41 mmol) was dissolved in EtOH and ¾0, and the solution was heated to reflux and stirred overnight. The solution was filtered, diluted with water, and then extracted with DCM. The combined organic layers were washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04, and concentrated in vacuo to give 5-(4-(4-(2,3-
dichlorophenyl)piperazin-l-yl)butoxy)-2-methylaniline (intermediate 63) (150 mg, 90%) as a yellow solid. HPLC: 99%, RT 3.197 min. MS(ESI) m/z 407.9 [M + H]+.
Compound 43:
[0219] A mixture of intermediate 63 (120 mg, 0.29 mmol), acetyl chloride (35 mg, 0.44 mmol) and Et3N (54 mg, 0.54 mmol) was dissolved in DCM and the solution was stirred at rt. The solution was diluted with water and extracted with DCM. The combined organic layers were washed with saturated aq NaHC03, brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by flash chromatography on silica gel column (elution with DCM/MeOH = 100:1- 30: 1) to give N-(5-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-methylphenyl)acetamide (compound 43) (45 mg, 35%) as a yellow solid. ¾ NMR (300 MHz, CDC13): δ 7.56 (s, 1H), 7.18-7.13 (m, 2H), 7.07-6.88 (m, 3H), 6.62 (d, J= 8.1 Hz, 1H), 3.99 (br, 2H), 3.26 (br, 4H), 2.92- 2.75 (br, 6H), 2.21 (s, 3H), 2.19 (s, 3H), 1.86 (br, 6H). HPLC: 99%, RT 2.464 min. MS (ESI) m/z 449.8 [M + H]+.
Synthesis of Compound 44
Scheme 71. Synthesis of compound 44
[0220] A mixture of intermediate 44 (41 mg, 0.16 mmol) and Nal (48.1 mg, 0.32 mmol) in CH3CN (3 mL) was heated to reflux for 30 min and then cooled to rt. To this mixture was added intermediate 47 (64.3 mg, 0.24 mmol), followed then by DIEA (0.062 mL, 0.35 mmol). The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with
EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by ISCO to give 6-(3-(4-(2,3-dichlorophenyl)piperidin-l- yl)propoxy)-lH-indazole (compound 44) (42, 1 mg, 65%). 1H NMR (400 MHz, CDC13): δ 7.92 (d, J = 0.8 Hz, 1H), 7.57 (d, J = 8.8 Hz, 1H), 7.33 (dd, J = 7.8, 1.6 Hz, 1H), 7.28 - 7.24 (dd, J = 7.8, 1 Hz, 1H), 7.18 (t, J = 7.8 Hz, 1H), 6.98 (s, 1H), 6.77 (dd, J = 8.8, 2.1 Hz, 1H), 4.10 (t, J = 6.0 Hz, 2H), 3.56 - 3.40 (m, 2H), 3.39 - 3.26 (m, 1H), 3.11 - 2.97 (m, 2H), 2.73 (s, 2H), 2.39 - 2.15 (m, 4H), 2.04 - 1.94 (dd, J = 16.8, 9.4 Hz, 2H). HPLC: 99%, RT 4.397 min. MS (ESI) m/z 404.15 [M + H]+.
Synthesis of Compound 45
Scheme 72. Synthesis of compound 45
[0221] A mixture of intermediate 51 (40.6 mg, 0.15 mmol) and Nal (45.2 mg, 0.30 mmol) in CH3CN (3 mL) was heated to reflux for 30 min and then cooled to rt. To this mixture was added intermediate 47 (60.3 mg, 0.23 mmol), followed then by DIEA (0.058 mL, 0.33 mmol). The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by ISCO to give 6-(4-(4-(2,3- dichlorophenyl)piperidin-l-yl)butoxy)-lH-indazole (compound 45) (37.6 mg, 60%). lH NMR (400 MHz, CDC13): δ 7.93 (s, 1H), 7.54 (d, J= 8.8 Hz, 1H), 7.33 (dd, J= 7.9, 1.2 Hz, 1H), 7.25 - 7.20 (m, 1H), 7.15 (t, J= 7.9 Hz, 1H), 6.93 (s, 1H), 6.74 (dd, J = 8.8, 1.9 Hz, 1H), 3.94 (t, J = 6.0 Hz, 2H), 3.75 - 3.67 (m, 2H), 3.46 - 3.36 (m, 1H), 3.15 - 3.08 (m, 2H), 3.03 - 2.91 (m, 2H), 2.59 - 2.43 (m, 2H), 2.18 - 2.06 (m, 2H), 2.06 - 1.96 (m, 2H), 1.88 - 1.75 (m, 2H). HPLC: 99%, RT 4.447 min. MS (ESI) m/z 418.10 [M + H]+.
Synthesis of Compound 46
Scheme 73. Synthesis of compound 46
[0222] A mixture of intermediate 44 (43.7 mg, 0.17 mmol) and Nal (51.3 mg, 0.34 mmol) in CH3CN (3 mL) was heated to reflux for 30 min and then cooled to rt. To this mixture was added intermediate 2 (57.9 mg, 0.21 mmol), followed then by DIEA (0.066 mL, 0.38 mmol). The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by ISCO to give 6-(3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l- yl)propoxy)-lH-indazole (compound 46) (43.6 mg, 61%). !H NMR (400 MHz, CDC13): δ 7.96 (d, J= 0.8 Hz, 1H), 7.59 (d, J= 8.8 Hz, 1H), 7.15 - 7.07 (m, 2H), 7.00 (dd, J= 7.4, 2.3 Hz, 1H), 6.88 (s, 1H), 6.80 (dd, J= 8.8, 2.1 Hz, 1H), 4.09 (t, J= 6.1 Hz, 2H), 3.43 - 3.37 (m, 2H), 3.29 (t, J= 6.1 Hz, 2H), 3.14 - 3.05 (m, 4H), 3.00 - 2.92 (m, 2H), 2.22 - 2.10 (m, 4H). HPLC: 99%, RT 4.201 min. MS(ESI) m/z 419.10 [M + H]+.
Synthesis of Compound 47
Scheme 74. Synthesis of compound 47
[0223] A mixture of intermediate 51 (54.2 mg, 0.20 mmol) and Nal (60.3 mg, 0.40 mmol) in CH3CN (3 mL) was heated to reflux for 30 min and then cooled to rt. To this mixture was added intermediate 2 (62.4 mg, 0.22 mmol), followed then by DIEA (0.077 mL, 0.44 mmol). The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by ISCO to give 6-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l- yl)butoxy)-lH-indazole (compound 47) (50.8 mg, 59%). HPLC: 99%, RT 4.526 min. MS(ESI) m/z 433.20 [M + H]+.
Synthesis of Compound 48
Scheme 75. Synthesis of compound 48
Intermediate 44 Intermediate 64 CH3CN Example 48
Compound 48:
[0224] A mixture of intermediate 44 (43.6 mg, 0.17 mmol) and Nal (51.2 mg, 0.34 mmol) in CH3CN (3 mL) was heated to reflux for 30 min and then cooled to rt. To this mixture was added intermediate 64 (45.0 mg, 0.21 mmol, prepared following the procedure in US Publication No. 2010/0119622) followed by DIEA (0.066 mL, 0.38 mmol). The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by ISCO to give 7-(4-(3-(lH-indazol-6-yloxy)propyl)piperazin-l-
yl)benzo[d]oxazol-2(3H)-one (compound 48) (43.7 mg, 65%). HPLC: 99%, RT 3.551 min. MS (ESI) m/z 394.20 [M + H]+.
Synthesis of Compound 49
Scheme 76. Synthesis of compound 49
Compound 49:
[0225] A mixture of intermediate 51 (47.3 mg, 0.18 mmol) and Nal (52.6 mg, 0.35 mmol) in CH3CN (3 mL) was heated to reflux for 30 min and then cooled to rt. To this mixture was added intermediate 64 (42.4 mg, 0.19 mmol), followed then by DIEA (0.067 mL, 0.39 mmol). The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by ISCO to give 7-(4-(4-(lH-indazol-6- yloxy)butyl)piperazin-l-yl)benzo[d]oxazol-2(3H)-one (compound 49) (43.5 mg, 61%). HPLC: 99%, RT 3.680 min. MS(ESI) m/z 408.20 [M + H]+.
Synthesis of Compound 50
Scheme 77. Synthesis of intermediate 65
Intermediate 44 Intermediate 65 CH3CN Example 50
[0226] To a solution of l-bromo-2-methoxybenzene (0.623 mL, 5.0 mmol) and piperazine (1722.8 mg, 20.0 mmol) in diglyme (5 mL) was added t-BuONa. The resulting mixture was stirred at rt for a few minutes before the addition of dichlorobis(tri-0- tolyphosphine)palladium (235.8 mg, 0.3 mmol). The resulting mixture was then stirred at 170°C under microwave irradiation for 30 min. Water was added and the mixture was extracted with EtOAc. The combined organic layers were dried, concentrated, and purified by ISCO to give 1- (2-methoxyphenyl)piperazine (intermediate 65) (758 mg, 79%) as an off-white solid. HPLC: 95%, RT 2.641 min. MS (ESI) m/z 193.10 [M + H]+.
[0227] A mixture of intermediate 44 (42.2 mg, 0.17 mmol) and Nal (49.5 mg, 0.33 mmol) in CH3CN (3 mL) was heated to reflux for 30 min and then cooled to rt. To this mixture was added intermediate 65 (35.0 mg, 0.18 mmol), followed then by DIEA (0.063 mL, 0.36 mmol). The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by ISCO to give 6-(3-(4-(2- methoxyphenyl)piperazin-l-yl)propoxy)-lH-indazole (compound 50) (36.6 mg, 60%). HPLC: 99%, RT 3.907 min. MS(ESI) m/z 367.20 [M + H]+.
Synthesis of Compound 51
Scheme 79. Synthesis of compound 51
[0228] A mixture of intermediate 51 (41.6 mg, 0.15 mmol) and Nal (46.3 mg, 0.31 mmol) in CH3CN (3 mL) was heated to reflux for 30 min and then cooled to rt. To this mixture was added intermediate 65 (32.7 mg, 0.17 mmol), followed then by DIEA (0.059 mL, 0.34 mmol). The resulting mixture was heated to reflux and stirred for 4 h. Precipitated crystals were filtered off and the filtrate was evaporated under reduced pressure. The residue was extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2S04, concentrated in vacuo and purified by ISCO to give 6-(4-(4-(2- methoxyphenyl)piperazin-l-yl)butoxy)-lH-indazole (compound 51) (35.6 mg, 62%). HPLC: 99%, RT 4.000 min. MS (ESI) m/z 381.20 [M + H]+.
EXAMPLE 2
In Vitro Studies
[0229] Each of the synthesized compounds were tested in a variety of in vitro assays for D2 receptor activity. Results are summarized in Table 6.
CHO-D? Membrane Preparation for GTP-gamma-S and Radioligand Binding Assays
[0230] Cells stably expressing D2 receptors (CHO-D2) were plated in 15-cm dishes (in DMEM containing 10% fetal bovine serum and grown to 90% confluence. Then, cells were washed with PBS, pH 7.4, and harvested by scraping into PBS, pH 7.4. Harvested cells were centrifuged at 1,000 x g for 10 min, then hypotonically lysed by resuspension in ice-cold 50 mM HEPES, 1% BSA, pH 7.4. Membranes were isolated by centrifugation at 21,000 x g for 20 min. The supernatant was removed and the membrane pellets were assayed for D2 ligand-stimulated GTP-gamma-S binding or stored at -80°C until used for radioligand binding assays.
CHO-D? Radioligand Binding Assay
[0231] Membranes prepared as above were resuspended to 1 μg protein/μΐ (measured by Bradford assay using BSA as standard), and 50 μΐ were added to each well of a polypropylene
96-well plate containing (per well): 50 μΐ of buffer (20 mM HEPES, 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 100 mM N-methyl-D-gluconate, pH 7.4), 50 μΐ of 1.5 nM [3H]N- methylspiperone (final concentration 0.3 nM) and reference or D2 test ligand at various concentrations ranging from 50 pM to 50 μΜ (final concentrations ranging from 10 pM to 10 μΜ, triplicate determinations for each concentration of D2 test ligand). After a 1.5-hr incubation in the dark at room temperature, the reactions were harvested onto 0.3% PEI-soaked Filtermax GF/A filters (Wallac) and washed three times with ice-cold 50 mM Tris, pH 7.4 using a Perkin- Elmer Filtermate 96-well harvester. The filters were subsequently dried, placed on a hot plate (100°C), and Melitilex-A (Wallac) scintillant was applied. The filters were then removed from the hot plate and allowed to cool. The filters were counted on a Wallac TriLux microbeta counter (3 min per well). Residual [3H]N-methylspiperone binding to filtered membranes was plotted as a function of log [reference] or log [D2 test ligand] and the data were regressed using the one-site competition model built into Prism 4.0 (GraphPad software).
D? mediated cAMP GloSensor Assay
[0232] HEK293T cells co-expressing the cAMP biosensor GloSensor-22F (Promega) and hD2 receptors were seeded (10,000 cells/20 μΐ/well) into white, clear-bottom, tissue culture plates in HBSS, 10% FBS, 20 mM HEPES, pH 7.4. After a one- to two-hour recoveiy, cells were treated with 10 μΐ of 3X test or reference drug prepared in HBSS, 10% FBS, 20 mM HEPES, pH 7.4. After 30 minutes, cells were challenged with 10 μΐ of 1,200 nM (4X) isoproterenol in 8% (4X) GloSensor reagent. Luminescence per well per second was read on a Wallac TriLux microbeta plate counter. Data were normalized to the isoproterenol response (100%) and the maximal quinpirole-induced inhibition thereof (0%) and regressed using the sigmoidal dose- response function built into GraphPad Prism 4.0. Notably, HEK293T cells expressing the GloSensor-22F alone (no hD2) were assayed in parallel and displayed no inhibition of isoproterenol-stimulated cAMP, either by quinpirole or by the test compounds, suggesting that the effect observed in hD2-expressing cells was due to compound acting via the recombinant receptor.
D? β-Arrestin Recruitment (Tango) assay
[0233] Recruitment of β-arrestin to agonist-stimulated D2 receptors was performed using a previously described "Tango"-type assay (Barnea et at, Proc. Natl. Acad. Sci. USA 105:64 (2008)). Briefly, HTLA cells stably expressing β-arrestin-TEV protease and a tetracycline transactivator-driven luciferase were plated in 15-cm dishes in DMEM containing 10% fetal bovine serum and transfected (via calcium phosphate) with 20 μg of a D2V2-TCS-tTA construct (Barnea et al, Proc. Natl. Acad. Sci. USA 105:64 (2008)). The next day, cells were plated in white, clear-bottom, 384-well plates (Greiner, 10,000 cells/well, 50 μΐ/well) in DMEM containing
1% dialyzed fetal bovine serum. The following day, the cells were challenged with 10 μΐ/well of reference agonist (6 μΜ) or D2 test ligand (6 μΜ) ± reference agonist prepared in HBS, 20 mM HEPES, pH 7.4, 18% DMSO (final ligand concentrations were 1 μΜ, final DMSO concentration was 3%). After 18 hours, the medium was removed and replaced with IX BriteGlo reagent (Promega), and luminescence/well was read using a TriLux plate reader (1 sec/well). Data were normalized to vehicle (0%) and quinpirole (100%) controls and regressed using the sigmoidal dose-response function built into GraphPad Prism 4.0.
pERK High-Content Assay
[0234] Cell Culture: Chinese Hamster Ovary (CHO) cells stably expressing the hD2L dopamine receptor (Urban et al, Neuropsychopharmacology 32:67 (2007)) were maintained in Ham's F12 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.5 μg/ml G418. On day 1 of the assay, cells were seeded onto black clear-bottom tissue culture-treated 96-well plates (Greiner, BioOne). On day 2 of the assay, cells were washed with serum-free medium (Ham's F-12, penicillin and streptomycin) and incubated in 100 μΐ serum-free medium overnight .
[0235] Immunofluorescence: Automatic multichannel pipetters were used for liquid handling and multichannel vacuum manifolds for aspirations. Each tested concentration was typically measured in triplicate or quadruplicate. For concentration curves, half-log-dilutions were used. Drug dilutions were prepared in stimulation medium (serum-free medium, 100 mg/L ascorbic acid). 100 μΜ dopamine and 1 μΜ PMA (phorbol 12-myristate 13 -acetate) were used as positive controls. Medium only was used as a negative control. Specificity of the response was confirmed by pretreatment for 5 min with 10 μΜ of the antagonist spiperone and in experiments using wild type CHO cells.
[0236] Cells were stimulated on day 3 by fast addition of 50 μΐ^ equilibrated 3X drug dilutions in a tissue culture incubator for 5 min. Plates were placed on ice, the medium was aspirated, and 100 μΕΛνεΙΙ of freshly prepared ice-cold fixing buffer (4% formaldehyde and 0.5 mM CaCl2 in PBS) solution was added. After 30 min fixing at room temperature, the plates were washed with 350 μΐ ννεΐΐ PBS/Ca (0.5 mM CaCl2 in PBS) and permeabilized for 20 min in 100 μΙ,ΛνεΙΙ 0.3% Triton X-100 in PBS/Ca. Plates were incubated in 100 μΙ,ΛνεΙΙ blocking buffer (PBS/Ca, 5% goat serum, 0.1% Triton X-100) for 1 h at room temperature and then in blocking buffer containing rabbit phospho-Thr202/p-Tyr204-ERK antibody (Cell Signaling 9101) at 1 :1000 at 4°C overnight. Cells were washed 3X for 5-10 min with 250 μΕΛνεΙΙ wash buffer (PBS/Ca, 0.03% Triton X-100). Plates were incubated with 50 μΕΛνεΙΙ Alexa-594 coupled goat anti-rabbit IgG (Invitrogen) at 1 :250, 5 μg/mL Hoechst 33342 nuclear stain, and 25 μg/mL
concanavaline-Alexa488 conjugate (ConA, Invitrogen) in blocking buffer for 2 h at room temperature. Plates were washed three times with 250 μΙ^ΛνεΙΙ washing buffer, post-fixed for 10 min in fixing buffer, washed with 250 μΐ PBS/Ca, filled with 200 nL/well PBS/Ca, sealed with transparent adhesive plate seals, and stored at 4°C.
[0237] Microscopy and Image Analysis: Plates were scanned with a high-content automated microscopic system (BD Pathway 855) using a 20x objective and a 2 x 2 image montage setting. The Alexa594 light path was used for the target signal, Alexa488 for whole cell staining, and Hoechst for the nuclear staining. Images were analyzed using the CellProfiler software. Well-averaged individual cell-based measurements were exported to Excel and cell- free background intensity was subtracted from the whole cell intensity. Concentration curves were analyzed in GraphPad Prism by fitting against a sigmoidal dose-response model and normalization to the dopamine curve.
[0238] The results in Table 6 show that, of the 43 compounds tested, 33 are partial agonists (Emax of 10% - 80%) of the β-arrestin pathway and another 8 compounds are full agonists (Emax > 80%) of the β-arrestin pathway. The compounds are also exceedingly potent, with 39 having an EC5o less than 100 nM and 23 having an EC50 less than 10 nM.
[0239] The cAMP (Glo-Sensor) assay shows that the majority of the tested compounds are not agonists for the Gj-coupled signaling pathway and that 14 of the 43 compounds function as inverse agonists for the Gj-coupled signaling pathway (i.e., have a negative Emax). The p-ERK assay confirms that the majority of compounds lack agonism via canonical G-protein-dependent pathways.
[0240] Compounds 2 and 3 were tested for receptor binding specificity. Consistent with their high potency in D2 functional assays, 2 and 3 displayed high affinities (Kfs < 10 nM) in the D2 radioligand competition binding assay (Table 7). Although 2 and 3 also had high affinity for D3-dopamine receptor, they displayed low affinities for other dopamine receptors (i.e., Dl5 D4, and D5). At serotonin receptors, 2 and 3 had moderate to high binding affinity ( j's: 0.6 - 250 nM) at 5HT2A, 5HT2B, 5HT2C, and 5HTiA, but were significantly less potent in functional assays (Ca2+ mobilization fluorometric imaging plate reader (FLIPR) or cAMP biosensor). 2 and 3 were antagonists at 5HT2A, 5HT2B, and 5HT2C and weak agonists (EC50's > 1 μΜ) at 5HTiA. In addition, 2 and 3 had moderate affinities to Hi -histamine receptor (Xj's < 10 nM) but were less potent in Hi functional assays. In general, 2 and 3 display a similar G protein-coupled receptor selectivity profile as aripiprazole (Table 7).
ATTORNEY DOCKET NO. 5470-568WO
Table 6
ATTORNEY DOCKET NO. 5470-568WO
Neutral ligands are either antagonists or are inactive; NA: not applicable.
I l l
Table 7
* Kj, IC50, pA2, or EC50 values are the average of at least two duplicate experiments with standard deviation (SD) values that are 3-fold less than the average.
[0241] In mouse pharmacokinetic (PK) studies, both 3 and aripiprazole displayed high exposure levels in brain and excellent CNS penetration (Table 8). Although the brain exposure level of 3 was about 3-fold lower, 3 had a longer half life in brain and higher brain plasma ratio over 24 h compared to aripiprazole.
Table 8
EXAMPLE 3
In Vivo Studies
[0242] Compounds 2 (UNC10000006A, or UNC0006) and 3 (UNC10099975A, or UNC9975) were evaluated in D-amphetamine-induced hyperlocomotion, phencyclidine (PCP)- induced hyperlocomotion, and/or catalepsy mouse models.
[0243] Mice. All experiments were approved by the Institutional Animal Care and Use Committees at the University of North Carolina, Chapel Hill and Duke University. C57BL/6J and P-arrestin-2 knockout (Bohn et al, Science 286:2495 (1999)) mice were housed under standard conditions - 12 hour light/dark cycle and food and water provided ad libitum. Adult, age-matched male and female C57BL/6J and P-arrestin-2 knockout drug naive mice were used for all behavioral testing.
[0244] Locomotor activity. Mouse locomotor activity was assessed in photocell-based activity chambers under standardized environmental conditions, using an AccuScan activity monitor (AccuScan Instruments, Columbus, OH) with a 25.8 x 25.8 cm Plexiglas chamber and a beam spacing of 1.52 cm as described (Abbas et al, J. Neurosci. 29:7124 (2009)). Mice were
injected (i.p.) with vehicle (0.9% saline/0.2% acetic acid) , aripiprazole (0.10, 0.25, 0.50 or 2.0 mg/kg), or UNC9975 (0.25, 0.5 and 2.0 mg/kg) and placed into the open field. Thirty min later D-amphetamine (3 mg/kg) or phencyclidine (6.0 mg/kg) was administered and they were immediately returned to the open field for 80 min. Activity was monitored throughout this entire period . Horizontal activity was measured as the total distance traveled in centimeters. The means ± SEMs of the locomotor responses of animals were analyzed using Graphpad Prism 5.0. To estimate the half-maximal inhibitory concentration (ED50), dose responses of total locomotor activity during the 90 minutes after D-amphetamine or phencyclidine administration were plotted and best-fit decay curves were determined using a nonlinear regression one-phase decay equation. Cumulative locomotor responses were analyzed using a one-way ANOVA followed by Newman- Keuls multiple comparison test using Graphpad Prism 5.0. Statistical significance was set at p<0.05.
[0245] Catalepsy testing. In this testing paradigm, mice were initially injected (i.p.) with vehicle (0.9% saline/0.2% acetic acid), aripiprazole, U C0006 (5 mg/kg), UNC9975 (5 mg/kg) or haloperidol (2 mg/kg) and returned to their home cage. The latency of movement was assessed 30, 60, 90 and 120 min after drug injection; the maximal latency to move in the mice was observed 60 min after drug treatments. Mice were placed upright on a screen placed at a 45 degree angle. The time required for the animal to move all four paws was scored in seconds (maximum of 5 minutes) and is reported as the latency to movement. An extended delay to voluntarily move on the inclined screen test is indicative of drug-induced catalepsy. The data are displayed as mean ± SEM. Catalepsy data were analyzed for statistical significance using a oneway ANOVA followed by Newman-Keuls multiple comparison test using Graphpad Prism 5.0. Differences were considered significant at p<0.05.
[0246] UNC9975 exhibited potent antipsychotic-like activity in mouse hyperlocomotion studies which is attenuated in p-arrestin-2 knockout mice . In Fig. 1A, locomotion responses in inbred C57BL/6 mice are shown as 5 minute binned intervals to different doses (i.p.) of UNC9975 or vehicle followed 30 min later by 3 mg/kg D-Amphetamine (AMPH, i.p.). In Fig. IB, total distance traveled after D-Amphetamine administration (30-70 min time interval) is shown. C57BL/6 mice were given vehicle or different doses of UNC9975 or aripiprazole 30 min prior to AMPH treatment. n=8 animals/group. *, p<0.05 versus vehicle + 3 mg/kg D- Amphetamine group.
[0247] In Figs. 1C and ID, locomotor activities are shown as 5 min binned intervals of wild-type (WT) or P-arrestin-2 knockout (β-ΑΓΓ<2 KO) littermate mice given vehicle or different doses of UNC9975 followed 30 min later with 6 mg/kg phencyclidine (PCP, i.p.). Fig. IE shows the total distance traveled by WT and P-ARR2 KO mice after PCP administration (30 to 70 min
time interval), shown in Figs. 1C and ID - different doses of UNC9975 were given to mice 30 min before PCP treatment, n = 14 WT and P-ARR2 KO pairs/group. *p<0.05, versus vehicle + 6 mg/kg PCP group. Fig. IF shows the total distance traveled by WT and -ARR2 KO mice after aripiprazole followed by PCP treatments (30 to 70 min time interval), n = 8 littermate WT and β- ARR2 KO pairs/group. *p<0.05, versus vehicle + 6 mg/kg PCP group.
[0248] UNC9975 and U C0006 induced catalepsy in p-arrestin-2 knockout mice but not in wild-type littermates. In Figs. 2A and 2B, wild-type and p-Arrestin-2 knockout littermate mice were administered (i.p.) vehicle, 5.0 mg/kg UNC9975, 5.0 mg/kg UNC0006, 5.0 mg/kg aripiprazole, or 2.0 mg/kg haloperidol. Following 30 and 60 min after drug injection, catalepsy was assessed using the inclined screen test where latency to move was scored, n = 8 littermate wild-type and knockout animal pairs/group. *p<0.05 versus vehicle controls.
[0249] Through a combined medicinal chemistry and comprehensive in vitro and in vivo pharmacological profiling approach, novel D2 β-arrestin-biased agonists were designed, synthesized and characterized, and demonstrated to have unique atypical antipsychotic drug-like activities in vivo. These novel compounds represent the first functionally selective β-arrestin- biased dopamine D2 receptor ligands to exhibit antipsychotic activity in vivo. These findings show that based on the arrestin-bias of these compounds, β-arrestin may be an important contributor to both antipsychotic drug efficacy and antipsychotic side effects.
[0250] The disclosed compounds were discovered as unique, β-arrestin-biased functionally selective D2 ligands. In addition to possessing high affinity for D2 receptors, both UNC0006 and UNC9975 are potent partial agonists which induce D2 receptors-mediated β- arrestin recruitment and signaling, and simultaneously are potent inverse agonists at Gi-dependent signaling. More significantly, the in vivo atypical antipsychotic drug-like activity of UNC9975 indicated a strict requirement for p-arrestin-2 for both full antipsychotic activity and protection against motoric side-effects. Similar to aripiprazole, this β-arrestin-biased ligand shows a potent ability to suppress both D-amphetamine and phencyclidine-induced hyperlocomotion in mice, indicating that the compound possesses antipsychotic drug-like activities in vivo. Significantly, the antipsychotic drug-like activity of UNC9975 was attenuated in β-3ΓΓε8ΐϊη-2 knockout mice, indicating p-arrestin-2 is required in vivo for full activity.
[0251] With the exception of aripiprazole, all FDA approved typical and atypical antipsychotic medications (e.g., haloperidol, chlorpromazine, clozapine, risperidone) share the common property of antagonizing D2-mediated G protein-dependent and independent signaling (Masri et al, Proc. Natl. Acad. Sci. USA 105:13656 (2008)). Indeed, typical and atypical antipsychotic drugs, with the exception of aripiprazole, are inverse agonists at Gj-mediated
signaling and antagonists at arrestin-ergic pathways. This combination of inverse agonism and antagonism is thought to underlie both the therapeutic benefit to prevent psychotic symptoms but can also cause serious extrapyramidal side-effects including catalepsy and other motor diskenesias (Roth et al, Nat. Rev. Drug Discov. 3:353 (2004)). Although aripiprazole, UNC0006 and UNC9975 do not induce catalepsy in wild-type mice at doses at which antipsychotic druglike therapeutic activities are measured, UNC0006 and UNC9975 resemble haloperidol in inducing catalepsy in p-arrestin-2 knockout mice. This propensity to cause catalepsy in the absence of P-arrestin-2 suggests that UNC0006 and UNC9975 signal through P-arrestin-2 in vivo and that this signaling may protect against motoric side-effects due to inverse agonism at D2 receptor Gj pathways.
[0252] These findings have obvious implications for the development of novel therapeutic approaches for treating schizophrenia and related disorders. Atypical antipsychotic drugs, which differ from older medications {e.g. , haloperidol and chlorpromazine) by virtue of their reduced propensity to induce motoric side-effects, are among the most widely prescribed medications. Many pharmacologic strategies which aim to target a multiplicity of non-D2 receptor molecular targets {e.g., 5-HT2A inverse agonists, 5-HT2C agonists, mGluR2/3 agonists, NK-3 antagonists, sigma antagonists, Dp or D4-selective antagonists and so forth) have led to a growing number of unsuccessful attempts to create safer and more effective atypical antipsychotic drugs (Roth et al, Nat. Rev. Drug Discov. 3:353 (2004); Conn et al, Neuropsychopharmacology 33:2048 (2008); Gray et al, Mol. Psychiatry 12:904 (2007)). The present results suggest that novel atypical antipsychotic drugs with a unique mechanism of action may arise from the disclosed β-arrestin-biased D2 ligands. These compounds may demonstrate special efficacies in psychotic disorders with a mood dysfunction given the prominent role of arrestin-ergic signaling in the action of lithium and related compounds.
[0253] The foregoing is illustrative of the present invention, and is not to be construed as limiting thereof. The invention is defined by the following claims, with equivalents of the claims to be included therein. All publications, patent applications, patents, patent publications, and any other references cited herein are incorporated by reference in their entireties for the teachings relevant to the sentence and/or paragraph in which the reference is presented.
Claims
What is Claimed is:
1. A compound of formula I:
wherein m is 0 or 1;
n is 0, 1, or 2;
q is 1 or 2;
X is O, NH, or C¾;
Y is N or CH;
Z is C, CH, C¾, cycloalkyl, aryl, or heteroaryl;
is a single, double, or triple bond as valencies permit; and
Ari and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
2. The compound of claim 1, which is β-arrestin biased.
3. The compound of claim 1, having the structure of formula II:
wherein n is 0, 1 , or 2;
q is 1 or 2;
X is O, NH, or CH2;
Y is N or CH;
Z is C, CH, CH2, cycloalkyl, aryl, or heteroaryl;
is a single, double, or triple bond as valencies permit; and
Art and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
4. The compound of claim 3, having the structure of formula III:
wherein n is 0, 1, or 2;
X is O, NH, or CH2;
Y is N or CH; and
Ari and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof. compound of claim 3, having the structure of formula IV
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
6. The compound of claim 3, wherein n is 1.
7. The compound of claim 3, wherein X is O.
8. The compound of claim 3 wherein Ar2 is:
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof. 10. The compound of claim 3, selected from the group consisting of:
120
11. The compound of claim 3, selected from the group consisting of:
12. The compound of claim 3, selected from the group consisting of:
-(3-(4-(2,3-dichloiOphenyl)-l,4-diazepan-l -yl)propoxy)-3,4-dihydroquinolin-2(lH)-one;
-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydroquinolin-2(lH)-one;
-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;-(4-(4-(2,3-dichlorophenyl)-l ,4-diazepan-l-yl)butoxy)quinolin-2(lH)-one;
-(3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)propoxy)-3,4-dihydroisoquinolin-l(2H)-one;-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydroisoquinolin-l (2H)-one;
5-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)butoxy)benzo[< ]thiazole;
5-(3-(4-(2,3-dichlorophenyl)-l ,4-diazepan-l-yl)propoxy)benzo[ ]thiazole;
7-(4-(4-(2-methoxyphenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydroquinolin-2(lH)-one;
7-(4-(4-(2-methoxyphenyl)-l,4-diazepan-l-yl)butoxy)quinolin-2(lH)-one;
7-(4-(4-(2-methoxyphenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(2-ethoxyphenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydroquinolin-2(lH)-one;
7-(4-(4-(2-ethoxyphenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(2-ethoxyphenyl)- 1 ,4-diazepan- 1 -yl)butoxy)quinolin-2(lH)-one;
7-(3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l -yl)propoxy)quinolin-2(lH)-one;
7-(4-(4-(2-isopropoxyphenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydroquinolin-2(lH)-one;
7-(4-(4-(2-isopropoxyphenyl)-l ,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)- one;
7-(4-(4-(2-isopropoxyphenyl)-l,4-diazepan-l-yl)butoxy)quinolin-2(lH)-one;
5- (3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)propoxy)-lH-benzo[^imidazol-2(3H)-one;
6- (3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)propoxy)-lH-indazole; and
6- (4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)butoxy)-lH-indazole.
13. The compound of claim 3, selected from the group consisting of:
7- (4-(4-(2-oxo-2,3-dihydro-lH-benzo[d]imidazol-4-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro- 1 ,8-naphthyridin-2(lH)-one;
7-(4-(4-(7-oxo-5,6,7,8-tetrahydro-l,8-naphthyridin-2-yloxy)butyl)-l,4-diazepan-l- yl)benzo[d]oxazol-2(3H)-one;
4-(4-(4-(7-oxo-5,6,7,8-tetrahydro-l,8-naphthyridin-2-yloxy)butyl)-l,4-diazepan-l- yl)benzo[d]oxazol-2(3H)-one;
7-(4-(4-(lH-benzo[d]imidazol-7-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin- 2(lH)-one;
7-(4-(4-(benzo[d]oxazol-7-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)- one;
7-(4-(4-(benzo[d]oxazol-4-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)- one;
7-(4-(4-(lH-indazol-7-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one; 7-(4-(4-(lH-indol-7-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one; 7-(4-(4-(benzofuran-7-yl)- 1 ,4-diazepan- 1 -yl)butoxy)-3 ,4-dihydro- 1 , 8-naphthyridin-2(l H)-one; 7-(4-(4-(benzo[b]thiophen-7-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)- one;
7-(4-(4-(benzo[d]thiazol-7-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)- one;
7-(4-(4-(benzo[d]thiazol-4-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)- one;
7-(4-(4-( 1 H-indol-4-yl)- 1 ,4-diazepan- 1 -yl)butoxy)-3 ,4-dihydro- 1 , 8-naphthyridin-2( 1 H)-one; 7-(4-(4-(lH-indazol-4-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one; 7-(4-(4-(benzofuran-4-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one; 7-(4-(4-(benzo[b]thiophen-4-yl)- 1 ,4-diazepan- 1 -yl)butoxy)-3 ,4-dihydro- 1 , 8-naphthyridin-2( 1 H)- one;
7-(4-(4-(naphthalen- 1 -yl)- 1 ,4-diazepan- 1 -yl)butoxy)-3 ,4-dihydro- 1 ,8-naphthyridin-2( 1 H)-one;
7-(4-(4-(isoquinolin-l-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(quinazolin-4-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(isoquinolin-4-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(quinolin-4-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(quinazolin-5-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(quinazolin-8-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(quinolin-5-yl)- 1 ,4-diazepan- 1 -yl)butoxy)-3,4-dihydro- 1 ,8-naphthyridin-2(lH)-one;
7-(4-(4-(isoquinolin-5-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(isoquinolin-8-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(quinolin-8-yl)- 1 ,4-diazepan- 1 -yl)butoxy)-3,4-dihydro- 1 ,8-naphthyridin-2(lH)-one;
7-(4-(4-phenyl-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(pyridin-2-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(pyridin-3-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(pyridin-4-yl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(2-fluorophenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(2-chlorophenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
7-(4-(4-(2-(trifluoromethyl)phenyl)-l,4-diazepan-l-yl)butoxy)-3,4-dihydroquinolin-2(lH)-one;
2-(4-(4-(7-oxo-5,6,7,8-tetrahydro- 1 ,8-naphthyridin-2-yloxy)butyl)- 1 ,4-diazepan- 1 - yl)benzonitrile;
7-(4-(4-o-tolyl-l,4-diazepan-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
N-(3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)propyl)-lH-indazol-6-amine;
N-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)butyl)-lH-indazol-6-amine;
N-(3-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)propyl)benzo[d]thiazol-5-amine;
N-(4-(4-(2,3-dichlorophenyl)- 1 ,4-diazepan- 1 -yl)butyl)benzo[d]thiazol-5-amine;
5-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)butoxy)-lH-benzo[d]imidazol-2(3H)-one;
5 -(3 -(4-(2,3 -dichlorophenyl)- 1 ,4-diazepan- 1 -yl)propylamino)- 1 H-benzo [d] imidazol-2(3H)-one; and
5-(4-(4-(2,3-dichlorophenyl)-l,4-diazepan-l-yl)butylamino)-lH-benzo[d]imidazol-2(3H)-one. 14. The compound of claim 1 , having the structure of formula VI:
wherein n is 0, 1, or 2;
q is 1 or 2;
X is O, H, or CH2;
Y is N or CH;
Z is C, CH, CH2, cycloalkyl, aryl, or heteroaryl;
is a single, double, or triple bond as valencies permit; and
Ari and Ar2 are independently an unsubstituted or substituted monocyclic or bicyclic aryl or heteroaryl;
or a pharmaceutically acceptable salt, prodrug, or optical isomer thereof.
15. The compound of claim 14, wherein n is 0 or 1.
16. The compound of claim 14, wherein X is O or NH.
17. The compound of claim 14, wherein Ari is dichlorophenyl.
18. The compound of claim 14, wherein Ar2 is selected from the group consisting of lH-indazole, benzo[d]thiazole, and imidazol-2(3H)-one.
19. The compound of claim 14, wherein Ar2 is not naphthyridinone, dihydronaphthyridinone, quinolinone, dihydroquinolinone, tetrahydropyridoazepinone, or isoindole.
130
22. The compound of claim 14, selected from the group consisting of:
6- (3-(4-(2,3-dichlorophenyl)piperazin-l-yl)propoxy)-lH-indazole;
5-(4-(4-(2,3-dichlorophenyl)piperidin-l-yl)butoxy)benzo[d]thiazole;
5-(3-(4-(2,3-dichlorophenyl)piperidin-l-yl)propoxy)benzo[d]thiazole;
5-(3-(4-(2,3-dichlorophenyl)piperazin-l-yl)propoxy)benzo[d]thiazole;
5-(4-(4-(2,3-dichlorophenyl)piperidin-l-yl)butoxy)-lH-benzo[d]imidazol-2(3H)-one;
7- (4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-3,4-dihydroisoquinolin-l(2H)-one;
7-((4-((4-(2,3-dichlorophenyl)piperazin-l-yl)methyl)cyclohexyl)methoxy)-3,4-dihydroquinoli 2(lH)-one;
7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)but-2-ynyloxy)-3,4-dihydroquinolin-2(lH)-one;
6- (4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-lH-indazole;
(E)-7-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)but-2-enyloxy)-3,4-dihydroquinolin-2(lH)-one;
7- (3-((4-(2,3-dichlorophenyl)piperazin-l-yl)methyl)phenoxy)-3,4-dihydroquinolin-2(lH)-one; 7-(4-((4-(2,3-dichlorophenyl)piperazin-l-yl)methyl)phenoxy)-3,4-dihydroquinolin-2(lH)-one; 7-(3-(4-(2,3-dichlorophenyl)piperazin-l-yl)propoxy)-3,4-dihydroisoquinolin-l(2H)-one;
7-(4-(4-(2,3-dichlorophenyl)piperidin-l-yl)butoxy)-3,4-dihydroisoquinolin-l(2H)-one;
5 -(3 -(4-(2,3 -dichlorophenyl)piperazin- 1 -yl)propoxy)- 1 H-benzo [d] imidazol-2(3H)-one;
5-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-lH-benzo[d]imidazol-2(3H)-one;
5- (4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)benzo[d]thiazole;
7- (4-(4-(2-methoxyphenyl)piperazin-l-yl)butoxy)-3,4-dihydro-l,8-naphthyridin-2(lH)-one;
8- (4-(4-(2-oxo-l,2,3,4-tetrahydroquinolin-7-yloxy)butyl)piperazin-l-yl)-2H-benzo[b][l,4]oxazin- 3(4H)-one;
7-(4-(4-(2-methoxyphenyl)piperazin-l-yl)butoxy)-3,4-dihydroquinolin-2(lH)-one;
7-(4-(4-(2,3-dihydrobenzo[b][l,4]dioxin-5-yl)piperazin-l-yl)butoxy)-3,4-dihydroquinolin-2(lH)- one;
7-(4-(4-(2,3-dihydrobenzo[b][l,4]dioxin-5-yl)piperazin-l-yl)butoxy)-3,4-dihydro-l,8- naphthyridin-2(lH)-one;
7-(4-(4-(2,3-dihydrobenzo[b][l,4]dioxin-5-yl)piperazin-l-yl)butoxy)quinolin-2(lH)-one;
N-(5-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butoxy)-2-methylphenyl)acetamide;
6- (3-(4-(2,3-dichlorophenyl)piperidin-l-yl)propoxy)-lH-indazole;
6- (4-(4-(2,3-dichlorophenyl)piperidin-l-yl)butoxy)-lH-indazole;
7- (4-(3-(lH-indazol-6-yloxy)propyl)piperazin-l-yl)benzo[d]oxazol-2(3H)-one;
7-(4-(4-(lH-indazol-6-yloxy)butyl)piperazin-l-yl)benzo[d]oxazol-2(3H)-one;
6-(3-(4-(2-methoxyphenyl)piperazin-l-yl)propoxy)-lH-indazole; and
6-(4-(4-(2-methoxyphenyl)piperazin-l-yl)butoxy)-lH-indazole.
23. The compound of claim 14, selected from the group consisting of:
N-(3-(4-(2,3-dichlorophenyl)piperidin-l -yl)propyl)- lH-indazol-6-amine;
N-(4-(4-(2,3-dichlorophenyl)piperidin-l-yl)butyl)-lH-indazol-6-amine;
N-(3-(4-(2,3-dichlorophenyl)piperazin-l-yl)propyl)-lH-indazol-6-amine;
N-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butyl)-lH-indazol-6-amine;
N-(3-(4-(2-methoxyphenyl)piperazin-l-yl)propyl)-lH-indazol-6-amine;
N-(4-(4-(2-methoxyphenyl)piperazin- 1 -yl)butyl)- lH-indazol-6-amine;
7-(4-(3 -( 1 H-indazol-6-ylamino)propyl)piperazin- 1 -yl)benzo [d]oxazol-2(3H)-one;
7-(4-(4-(lH-indazol-6-ylamino)butyl)piperazin-l-yl)benzo[d]oxazol-2(3H)-one;
N-(3-(4-(2,3-dichlorophenyl)piperidin-l-yl)propyl)benzo[d]thiazol-5-amine;
N-(4-(4-(2,3-dichlorophenyl)piperidin-l-yl)butyl)benzo[d]thiazol-5-amine;
N-(3-(4-(2,3-dichlorophenyl)piperazin-l-yl)propyl)benzo[d]thiazol-5-amine;
N-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butyl)benzo[d]thiazol-5-amine;
5 -(3 -(4-(2-methoxyphenyl)piperazin- 1 -yl)propoxy)benzo [d]thiazole;
5-(4-(4-(2-methoxyphenyl)piperazin-l-yl)butoxy)benzo[d]thiazole;
N-(3-(4-(2-methoxyphenyl)piperazin-l-yl)propyl)benzo[d]thiazol-5-amine;
N-(4-(4-(2-methoxyphenyl)piperazin-l-yl)butyl)benzo[d]thiazol-5-amine;
7-(4-(3-(benzo[d]thiazol-5-yloxy)propyl)piperazin-l-yl)benzo[d]oxazol-2(3H)-one;
7-(4-(4-(benzo[d]thiazol-5-yloxy)butyl)piperazin-l-yl)benzo[d]oxazol-2(3H)-one;
7-(4-(3-(benzo[d]thiazol-5-ylamino)propyl)piperazin-l-yl)benzo[d]oxazol-2(3H)-one;
7-(4-(4-(benzo[d]thiazol-5-ylamino)butyl)piperazin-l-yl)benzo[d]oxazol-2(3H)-one;
5 -(3 -(4-(2,3 -dichlorophenyl)piperidin- 1 -yl)propylamino)- 1 H-benzo [d] imidazol-2(3H)-one;
5-(4-(4-(2,3-dichlorophenyl)piperidin-l-yl)butylamino)-lH-benzo[d]imidazol-2(3H)-one;
5 -(3 -(4-(2,3 -dichlorophenyl)piperazin- 1 -yl)propylamino)- 1 H-benzo [d]imidazol-2(3H)-one;
5-(4-(4-(2,3-dichlorophenyl)piperazin-l-yl)butylamino)-lH-benzo[d]imidazol-2(3H)-one;
5-(3-(4-(2-methoxyphenyl)piperazin-l-yl)propoxy)-lH-benzo[d]imidazol-2(3H)-one;
5-(4-(4-(2-methoxyphenyl)piperazin-l-yl)butoxy)-lH-benzo[d]imidazol-2(3H)-one;
5-(3-(4-(2-methoxyphenyl)piperazin-l-yl)propylamino)-lH-benzo[d]imidazol-2(3H)-one;
5-(4-(4-(2-methoxyphenyl)piperazin-l-yl)butylamino)-lH-benzo[d]imidazol-2(3H)-one;
7-(4-(3-(2-oxo-2,3-dihydro-lH-benzo[d]imidazol-5-yloxy)propyl)piperazin-l-yl)benzo[d]oxazol-
2(3H)-one;
7-(4-(4-(2-oxo-2,3-dihydro-lH-benzo[d]imidazol-5-yloxy)butyl)piperazin-l-yl)benzo[d]oxazol- 2(3H)-one;
7-(4-(3-(2-oxo-2,3-dihydro-lH-benzo[d]imidazol-5-ylamino)propyl)piperazin-l- yl)benzo[d]oxazol-2(3H)-one; and
7-(4-(4-(2-oxo-2,3-dihydro-lH-benzo[d]imidazol-5-ylamino)butyl)piperazin-l- yl)benzo [d] oxazol-2(3H)-one.
24. A pharmaceutical composition comprising the compound of any one of claims 1- 23 and a pharmaceutically acceptable carrier.
25. A method of modulating the activity of a D2 dopamine receptor comprising contacting the receptor with a compound of any one of claims 1-23.
26. A method of treating a central nervous system disorder associated with D2 dopamine receptors in a subject, comprising delivering to the subject a therapeutically effective amount of the compound of any one of claims 1-23.
27. The method of claim 26, wherein the disorder associated with D2 dopamine receptors is a psychiatric disorder.
28. The method of claim 27, wherein the psychiatric disorder is selection from the group consisting of schizophrenia, schizoaffective disorder, schizophreniform disorder, bipolar disorder, mania, manic psychosis, Tourette's syndrome and Tourette-like disorders, obsessive- compulsive disorder, depression, bipolar depression, unipolar depression, depression with psychotic features, psychosis not otherwise specified, anxiety disorders, affective disorders, and non-affective disorders.
29. The method of claim 26, wherein the disorder associated with D2 dopamine receptors is a neurological disorder.
30. The method of claim 29, wherein the neurological disorder is selected from the group consisting of Huntington's Disease, Parkinsonian Psychosis, autism spectrum and autistic disorders, Alzheimer's Disease, dementia, and iemann-Pick Disorder.
31. The method of claim 26, wherein the disorder associated with D2 dopamine receptors is a pituitary disorder.
32. The method of claim 31, wherein the pituitary disorder is selected from the group consisting of pituitary adenoma and prolactinoma.
33. The method of claim 26, wherein the disorder associated with D2 dopamine receptors is an endocrine disorder.
34. The method of claim 33, wherein the endocrine disorder is galactorrhea.
35. The method of claim 26, further comprising delivering to the subject an additional therapeutic agent.
36. The method of claim 35, wherein the compound is administered in the same pharmaceutical composition as the additional therapeutic agent.
37. The method of claim 35, wherein the compound is administered in a different pharmaceutical composition than the additional therapeutic agent.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/807,347 US9156822B2 (en) | 2010-07-02 | 2011-07-01 | Functionally selective ligands of dopamine D2 receptors |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US36095110P | 2010-07-02 | 2010-07-02 | |
| US61/360,951 | 2010-07-02 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2012003418A2 true WO2012003418A2 (en) | 2012-01-05 |
| WO2012003418A3 WO2012003418A3 (en) | 2012-05-18 |
Family
ID=45402673
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2011/042734 WO2012003418A2 (en) | 2010-07-02 | 2011-07-01 | Functionally selective ligands of dopamine d2 receptors |
Country Status (2)
| Country | Link |
|---|---|
| US (1) | US9156822B2 (en) |
| WO (1) | WO2012003418A2 (en) |
Cited By (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013169964A1 (en) | 2012-05-09 | 2013-11-14 | Sunovion Pharmaceuticals Inc. | Heteroaryl compounds and methods of use thereof |
| WO2014031635A1 (en) * | 2012-08-21 | 2014-02-27 | Ortho-Clinical Diagnostics, Inc | Antibodies to aripiprazole haptens and use thereof |
| WO2015069110A1 (en) * | 2013-11-07 | 2015-05-14 | Aapa B.V. | Multiple d2 a(nta)gonists/h3 antagonists for treatment of cns-related disorders |
| WO2016096686A1 (en) | 2014-12-17 | 2016-06-23 | Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.P.A. | Antibacterial compounds having broad spectrum of activity |
| US9410972B2 (en) | 2012-08-21 | 2016-08-09 | Janssen Pharmaceutica Nv | Antibodies to quetiapine and use thereof |
| US9465041B2 (en) | 2012-08-21 | 2016-10-11 | Janssen Pharmaceutica Nv | Antibodies to paliperidone and use thereof |
| US9494607B2 (en) | 2012-08-21 | 2016-11-15 | Janssen Pharmaceutica Nv | Antibodies to aripiprazole and use thereof |
| US9494608B2 (en) | 2012-08-21 | 2016-11-15 | Janssen Pharmaceutica Nv | Antibodies to olanzapine and use thereof |
| WO2017084627A1 (en) * | 2015-11-20 | 2017-05-26 | 江苏恩华药业股份有限公司 | Lactam compound derivative and application thereof |
| US9664700B2 (en) | 2012-08-21 | 2017-05-30 | Janssen Pharmaceutica Nv | Antibodies to risperidone and use thereof |
| CN106749007A (en) * | 2017-04-05 | 2017-05-31 | 沧州那瑞化学科技有限公司 | A kind of method for preparing the quinolone of 7 hydroxyl 2 |
| WO2017115287A1 (en) | 2015-12-28 | 2017-07-06 | Honour (R&D) | Process for the preparation of quinoline-2(1h)-one derivatives |
| CN106995410A (en) * | 2016-01-26 | 2017-08-01 | 江苏恩华药业股份有限公司 | A kind of lactam derivative and its application |
| CN107011288A (en) * | 2017-04-20 | 2017-08-04 | 齐鲁天和惠世制药有限公司 | A kind of preparation method of aripiprazole intermediate 1 (2,3 dichlorophenyl) piperazine hydrochloride |
| CN107098855A (en) * | 2017-04-05 | 2017-08-29 | 上海诺星医药科技有限公司 | A kind of method for preparing the quinolinone of 7 hydroxyl 2 |
| US9751953B2 (en) | 2012-08-21 | 2017-09-05 | Janssen Pharmaceutica Nv | Antibodies to risperidone haptens and use thereof |
| WO2017172368A1 (en) | 2016-03-31 | 2017-10-05 | Oncternal Therapeutics, Inc. | Indoline analogs and uses thereof |
| US9795685B2 (en) | 2012-08-21 | 2017-10-24 | Janssen Pharmaceutica Nv | Haptens of aripiprazole |
| WO2017211760A1 (en) | 2016-06-08 | 2017-12-14 | Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.P.A. | New antibacterial compounds |
| US9850318B2 (en) | 2012-08-21 | 2017-12-26 | Janssen Pharmaceutica Nv | Antibodies to quetiapine haptens and use thereof |
| CN108218771A (en) * | 2018-03-12 | 2018-06-29 | 钦州学院 | Deuterated Aripiprazole and its preparation method and application |
| WO2019119481A1 (en) * | 2017-12-21 | 2019-06-27 | 中国科学院合肥物质科学研究院 | Carbazole derivative kinase inhibitor |
| US10370457B2 (en) | 2012-08-21 | 2019-08-06 | Janssen Pharmaceutica Nv | Antibodies to paliperidone haptens and use thereof |
| US10369130B2 (en) | 2014-12-17 | 2019-08-06 | AZIEN DE CHIMICHE RIUNITE ANGELINI FRANCESCO A.C.R.A.F. S.p.A. | Antibacterial compounds |
| CN110204547A (en) * | 2019-07-04 | 2019-09-06 | 绍兴市精益生物化工有限公司 | Synthesis method of 1, 3-dimethyl uric acid |
| US10435478B2 (en) | 2015-12-17 | 2019-10-08 | Janssen Pharmaceutica Nv | Antibodies to quetiapine and use thereof |
| US10444250B2 (en) | 2015-12-17 | 2019-10-15 | Janssen Pharmaceutica Nv | Antibodies to risperidone and use thereof |
| WO2020114514A1 (en) * | 2018-12-07 | 2020-06-11 | 江苏恩华药业股份有限公司 | Crystalline form of propanamide derivative and preparation method therefor |
| US10712353B2 (en) | 2012-08-21 | 2020-07-14 | Janssen Pharmaceutica Nv | Antibodies to olanzapine haptens and use thereof |
| WO2021234082A1 (en) * | 2020-05-21 | 2021-11-25 | Syngenta Crop Protection Ag | Chemical process |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016100940A1 (en) | 2014-12-19 | 2016-06-23 | The Broad Institute, Inc. | Dopamine d2 receptor ligands |
| US10752588B2 (en) | 2014-12-19 | 2020-08-25 | The Broad Institute, Inc. | Dopamine D2 receptor ligands |
| CN111320582A (en) * | 2018-12-17 | 2020-06-23 | 江苏恩华药业股份有限公司 | Preparation method of amide-like derivative and intermediate thereof |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060223820A1 (en) * | 2006-03-21 | 2006-10-05 | Chemagis Ltd. | Crystalline aripiprazole salts and processes for preparation and purification thereof |
| US20060270683A1 (en) * | 2003-04-25 | 2006-11-30 | Lohray Braj B | Polymorphs of aripiprazole |
| US7160888B2 (en) * | 2003-08-22 | 2007-01-09 | Warner Lambert Company Llc | [1,8]naphthyridin-2-ones and related compounds for the treatment of schizophrenia |
| US7491726B2 (en) * | 2003-03-21 | 2009-02-17 | Hetero Drugs Limited | Crystalline forms of aripiprazole |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5006528A (en) | 1988-10-31 | 1991-04-09 | Otsuka Pharmaceutical Co., Ltd. | Carbostyril derivatives |
| DE19600934A1 (en) * | 1996-01-12 | 1997-07-17 | Basf Ag | Substituted aza and diazacycloheptane and cyclooctane compounds and their use |
| WO2006090273A2 (en) | 2005-02-22 | 2006-08-31 | Warner-Lambert Company Llc | [1,8]naphthyridin-2-ones and related compounds with keto or hydroxyl linkers for the treatment of schizophrenia |
| WO2006090272A1 (en) | 2005-02-22 | 2006-08-31 | Warner-Lambert Company Llc | Isoquinoline [1,8]naphthyridin-2-ones and related compounds for treatment of schizophrenia |
| JP4109709B1 (en) | 2005-04-01 | 2008-07-02 | ワーナー−ランバート カンパニー リミテッド ライアビリティー カンパニー | Tetrahydro-pyridazepin-8-one and related compounds for the treatment of schizophrenia |
| WO2008020306A2 (en) | 2006-08-18 | 2008-02-21 | Pfizer Products Inc. | Isoindole derivatives |
| WO2008084324A1 (en) | 2007-01-04 | 2008-07-17 | Pfizer Products Inc. | Naphthyridinone compound |
-
2011
- 2011-07-01 US US13/807,347 patent/US9156822B2/en not_active Expired - Fee Related
- 2011-07-01 WO PCT/US2011/042734 patent/WO2012003418A2/en active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7491726B2 (en) * | 2003-03-21 | 2009-02-17 | Hetero Drugs Limited | Crystalline forms of aripiprazole |
| US20060270683A1 (en) * | 2003-04-25 | 2006-11-30 | Lohray Braj B | Polymorphs of aripiprazole |
| US7160888B2 (en) * | 2003-08-22 | 2007-01-09 | Warner Lambert Company Llc | [1,8]naphthyridin-2-ones and related compounds for the treatment of schizophrenia |
| US20060223820A1 (en) * | 2006-03-21 | 2006-10-05 | Chemagis Ltd. | Crystalline aripiprazole salts and processes for preparation and purification thereof |
Cited By (73)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013169964A1 (en) | 2012-05-09 | 2013-11-14 | Sunovion Pharmaceuticals Inc. | Heteroaryl compounds and methods of use thereof |
| US20190031685A1 (en) * | 2012-05-09 | 2019-01-31 | Sunovion Pharmaceuticals Inc. | Heteroaryl compounds and methods of use thereof |
| US20150072974A1 (en) * | 2012-05-09 | 2015-03-12 | Sunovion Pharmaceuticals Inc. | Heteroaryl compounds and methods of use thereof |
| US9951088B2 (en) | 2012-05-09 | 2018-04-24 | Sunovion Pharmaceuticals Inc. | D2 receptor modulators and methods of use thereof in the treatment of diseases and disorders |
| JP2015516432A (en) * | 2012-05-09 | 2015-06-11 | スノビオン プハルマセウトイカルス インコーポレイテッド | Heteroaryl compounds and methods of use thereof |
| AU2013259551B2 (en) * | 2012-05-09 | 2017-11-02 | Sunovion Pharmaceuticals Inc. | Heteroaryl compounds and methods of use thereof |
| US10379105B2 (en) | 2012-08-21 | 2019-08-13 | Janssen Pharmaceutica Nv | Antibodies to aripiprazole haptens and use thereof |
| WO2014031635A1 (en) * | 2012-08-21 | 2014-02-27 | Ortho-Clinical Diagnostics, Inc | Antibodies to aripiprazole haptens and use thereof |
| US9494607B2 (en) | 2012-08-21 | 2016-11-15 | Janssen Pharmaceutica Nv | Antibodies to aripiprazole and use thereof |
| US9494608B2 (en) | 2012-08-21 | 2016-11-15 | Janssen Pharmaceutica Nv | Antibodies to olanzapine and use thereof |
| US9611332B2 (en) | 2012-08-21 | 2017-04-04 | Janssen Pharmaceutica Nv | Antibodies to aripiprazole haptens and use thereof |
| US10712353B2 (en) | 2012-08-21 | 2020-07-14 | Janssen Pharmaceutica Nv | Antibodies to olanzapine haptens and use thereof |
| US9664700B2 (en) | 2012-08-21 | 2017-05-30 | Janssen Pharmaceutica Nv | Antibodies to risperidone and use thereof |
| US11385246B2 (en) | 2012-08-21 | 2022-07-12 | Saladax Biomedical Inc. | Antibodies to paliperidone and use thereof |
| US10690686B2 (en) | 2012-08-21 | 2020-06-23 | Janssen Pharmaceutica Nv | Antibodies to risperidone and use thereof |
| US10816561B2 (en) | 2012-08-21 | 2020-10-27 | Janssen Pharmaceutica Nv | Antibodies to aripiprazole and use thereof |
| US10488401B2 (en) | 2012-08-21 | 2019-11-26 | Janssen Pharmaceutica Nv | Antibodies to aripiprazole haptens and use thereof |
| US10465013B2 (en) | 2012-08-21 | 2019-11-05 | Janssen Pharmaceutica Nv | Antibodies to quetiapine haptens and use thereof |
| US9751953B2 (en) | 2012-08-21 | 2017-09-05 | Janssen Pharmaceutica Nv | Antibodies to risperidone haptens and use thereof |
| US9410972B2 (en) | 2012-08-21 | 2016-08-09 | Janssen Pharmaceutica Nv | Antibodies to quetiapine and use thereof |
| US9795685B2 (en) | 2012-08-21 | 2017-10-24 | Janssen Pharmaceutica Nv | Haptens of aripiprazole |
| US10379129B2 (en) | 2012-08-21 | 2019-08-13 | Janssen Pharmaceutica Nv | Antibodies to paliperidone and use thereof |
| US11226345B2 (en) | 2012-08-21 | 2022-01-18 | Janssen Pharmaceutica Nv | Antibodies to olanzapine haptens and use thereof |
| US11225527B2 (en) | 2012-08-21 | 2022-01-18 | Janssen Pharmaceutica Nv | Antibodies to paliperidone haptens and use thereof |
| US9850318B2 (en) | 2012-08-21 | 2017-12-26 | Janssen Pharmaceutica Nv | Antibodies to quetiapine haptens and use thereof |
| US10793644B2 (en) | 2012-08-21 | 2020-10-06 | Janssen Pharmaceutica Nv | Antibodies to risperidone haptens and use thereof |
| US10370457B2 (en) | 2012-08-21 | 2019-08-06 | Janssen Pharmaceutica Nv | Antibodies to paliperidone haptens and use thereof |
| US10344098B2 (en) | 2012-08-21 | 2019-07-09 | Janssen Pharmaceutica Nv | Antibodies to olanzapine and use thereof |
| US10166296B2 (en) | 2012-08-21 | 2019-01-01 | Janssen Pharmaceutica Nv | Haptens of aripiprazole |
| US10175257B2 (en) | 2012-08-21 | 2019-01-08 | Janssen Pharmaceutica Nv | Antibodies to aripiprazole and use thereof |
| US11046786B2 (en) | 2012-08-21 | 2021-06-29 | Janssen Pharmaceutica Nv | Antibodies to olanzapine and use thereof |
| US9465041B2 (en) | 2012-08-21 | 2016-10-11 | Janssen Pharmaceutica Nv | Antibodies to paliperidone and use thereof |
| US11105793B2 (en) | 2012-08-21 | 2021-08-31 | Janssen Pharmaceutica Nv | Antibodies to aripiprazole haptens and use thereof |
| US10288631B2 (en) | 2012-08-21 | 2019-05-14 | Janssen Pharmaceutica Nv | Antibodies to quetiapine and use thereof |
| WO2015069110A1 (en) * | 2013-11-07 | 2015-05-14 | Aapa B.V. | Multiple d2 a(nta)gonists/h3 antagonists for treatment of cns-related disorders |
| US10369130B2 (en) | 2014-12-17 | 2019-08-06 | AZIEN DE CHIMICHE RIUNITE ANGELINI FRANCESCO A.C.R.A.F. S.p.A. | Antibacterial compounds |
| US10221144B2 (en) | 2014-12-17 | 2019-03-05 | Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.P.A. | Antibacterial compounds having broad spectrum of activity |
| WO2016096686A1 (en) | 2014-12-17 | 2016-06-23 | Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.P.A. | Antibacterial compounds having broad spectrum of activity |
| JP7035118B2 (en) | 2015-11-20 | 2022-03-14 | エヌエイチダブリュエイ、ファーマ.コーポレイション | Lactam compound derivatives and their applications |
| CN108290880A (en) * | 2015-11-20 | 2018-07-17 | 江苏恩华药业股份有限公司 | Lactam analog compound derivative and its application |
| CN112851656A (en) * | 2015-11-20 | 2021-05-28 | 江苏恩华药业股份有限公司 | Lactam compound derivative and application thereof |
| CN108290880B (en) * | 2015-11-20 | 2021-03-09 | 江苏恩华药业股份有限公司 | Lactam compound derivative and application thereof |
| JP2019500329A (en) * | 2015-11-20 | 2019-01-10 | エヌエイチダブリュエイ、ファーマ.コーポレイションNhwa Pharma. Corporation | Lactam compound derivatives and applications thereof |
| WO2017084627A1 (en) * | 2015-11-20 | 2017-05-26 | 江苏恩华药业股份有限公司 | Lactam compound derivative and application thereof |
| CN112851656B (en) * | 2015-11-20 | 2022-07-12 | 江苏恩华药业股份有限公司 | Lactam compound derivative and application thereof |
| US10501452B2 (en) | 2015-11-20 | 2019-12-10 | Nhwa Pharma. Corporation | Lactam compound derivative and application thereof |
| JP2020158520A (en) * | 2015-11-20 | 2020-10-01 | エヌエイチダブリュエイ、ファーマ.コーポレイションNhwa Pharma. Corporation | Lactam compound derivative and application thereof |
| US11104742B2 (en) | 2015-12-17 | 2021-08-31 | Janssen Pharmaceutica Nv | Antibodies to quetiapine and use thereof |
| US10435478B2 (en) | 2015-12-17 | 2019-10-08 | Janssen Pharmaceutica Nv | Antibodies to quetiapine and use thereof |
| US10444250B2 (en) | 2015-12-17 | 2019-10-15 | Janssen Pharmaceutica Nv | Antibodies to risperidone and use thereof |
| US10852313B2 (en) | 2015-12-17 | 2020-12-01 | Janssen Pharmaceutica Nv | Antibodies to risperidone and use thereof |
| EP3397636A4 (en) * | 2015-12-28 | 2019-10-09 | Honour (R&D) | Process for the preparation of quinoline-2(1h)-one derivatives |
| WO2017115287A1 (en) | 2015-12-28 | 2017-07-06 | Honour (R&D) | Process for the preparation of quinoline-2(1h)-one derivatives |
| CN106995410A (en) * | 2016-01-26 | 2017-08-01 | 江苏恩华药业股份有限公司 | A kind of lactam derivative and its application |
| CN106995410B (en) * | 2016-01-26 | 2021-03-02 | 江苏恩华药业股份有限公司 | Lactam derivative and application thereof |
| WO2017172368A1 (en) | 2016-03-31 | 2017-10-05 | Oncternal Therapeutics, Inc. | Indoline analogs and uses thereof |
| EP3795563A1 (en) | 2016-03-31 | 2021-03-24 | Oncternal Therapeutics, Inc. | Indoline analogs and uses thereof |
| WO2017211760A1 (en) | 2016-06-08 | 2017-12-14 | Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.P.A. | New antibacterial compounds |
| US10640488B2 (en) | 2016-06-08 | 2020-05-05 | Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.P.A. | Antibacterial compounds |
| US10633366B2 (en) | 2016-06-08 | 2020-04-28 | Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.P.A. | Antibacterial compounds |
| WO2017211759A1 (en) | 2016-06-08 | 2017-12-14 | Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.P.A. | New antibacterial compounds |
| CN106749007B (en) * | 2017-04-05 | 2019-07-02 | 沧州那瑞化学科技有限公司 | A method of preparing 7- hydroxyl -2- quinolone |
| CN106749007A (en) * | 2017-04-05 | 2017-05-31 | 沧州那瑞化学科技有限公司 | A kind of method for preparing the quinolone of 7 hydroxyl 2 |
| CN107098855A (en) * | 2017-04-05 | 2017-08-29 | 上海诺星医药科技有限公司 | A kind of method for preparing the quinolinone of 7 hydroxyl 2 |
| CN107011288A (en) * | 2017-04-20 | 2017-08-04 | 齐鲁天和惠世制药有限公司 | A kind of preparation method of aripiprazole intermediate 1 (2,3 dichlorophenyl) piperazine hydrochloride |
| CN109942544A (en) * | 2017-12-21 | 2019-06-28 | 中国科学院合肥物质科学研究院 | A kind of novel indazole analog derivative kinase inhibitor |
| WO2019119481A1 (en) * | 2017-12-21 | 2019-06-27 | 中国科学院合肥物质科学研究院 | Carbazole derivative kinase inhibitor |
| CN109942544B (en) * | 2017-12-21 | 2021-06-11 | 中国科学院合肥物质科学研究院 | Novel indazole derivative kinase inhibitor |
| CN108218771A (en) * | 2018-03-12 | 2018-06-29 | 钦州学院 | Deuterated Aripiprazole and its preparation method and application |
| WO2020114514A1 (en) * | 2018-12-07 | 2020-06-11 | 江苏恩华药业股份有限公司 | Crystalline form of propanamide derivative and preparation method therefor |
| AU2019394523B2 (en) * | 2018-12-07 | 2022-03-10 | Nhwa Pharma. Corporation | Crystalline form of propanamide derivative and preparation method therefor |
| CN110204547A (en) * | 2019-07-04 | 2019-09-06 | 绍兴市精益生物化工有限公司 | Synthesis method of 1, 3-dimethyl uric acid |
| WO2021234082A1 (en) * | 2020-05-21 | 2021-11-25 | Syngenta Crop Protection Ag | Chemical process |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2012003418A3 (en) | 2012-05-18 |
| US20130137679A1 (en) | 2013-05-30 |
| US9156822B2 (en) | 2015-10-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9156822B2 (en) | Functionally selective ligands of dopamine D2 receptors | |
| US20210060040A1 (en) | Cannabinoid receptor modulators | |
| ES2434946T3 (en) | Isoindol imide compounds and compositions comprising them and methods for using it | |
| US9447070B2 (en) | 5-substituted isoindoline compounds | |
| CA2660806C (en) | 5-substituted isoindoline compounds | |
| US20040102467A1 (en) | Neuroprotective and anti-proliferative compounds | |
| TW200823192A (en) | 5-substituted quinazolinone derivatives and compositions comprising and methods of using the same | |
| US20240083904A1 (en) | Antagonists of the adenosine a2a receptor | |
| CA3234693A1 (en) | Novel modulators of ehmt1 and ehmt2 and therapeutic use thereof | |
| US20060199835A1 (en) | Neuroprotective and anti-proliferative compounds | |
| WO2024034652A1 (en) | Tertiary amide derivative substituted by quaternary carbon | |
| US20230331693A1 (en) | Gspt1 compounds and methods of use of the novel compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11801470 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13807347 Country of ref document: US |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11801470 Country of ref document: EP Kind code of ref document: A2 |