WO2011141515A1 - Diagnostic agents for amyloid beta imaging - Google Patents
Diagnostic agents for amyloid beta imaging Download PDFInfo
- Publication number
- WO2011141515A1 WO2011141515A1 PCT/EP2011/057630 EP2011057630W WO2011141515A1 WO 2011141515 A1 WO2011141515 A1 WO 2011141515A1 EP 2011057630 W EP2011057630 W EP 2011057630W WO 2011141515 A1 WO2011141515 A1 WO 2011141515A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- vinyl
- group
- tert
- phenyl
- compound
- Prior art date
Links
- SFUPOZWNKXSOEZ-DHZHZOJOSA-N CC(C)(C)OC(N(C)c1ccc(/C=C/c2cccc(F)n2)cc1)=O Chemical compound CC(C)(C)OC(N(C)c1ccc(/C=C/c2cccc(F)n2)cc1)=O SFUPOZWNKXSOEZ-DHZHZOJOSA-N 0.000 description 1
- RHAXBTFYTFVTTN-AATRIKPKSA-N CC(C)(C)OC(N(C)c1ccc(/C=C/c2ccnc(I)c2)cc1)=O Chemical compound CC(C)(C)OC(N(C)c1ccc(/C=C/c2ccnc(I)c2)cc1)=O RHAXBTFYTFVTTN-AATRIKPKSA-N 0.000 description 1
- QIEDQDUGSSASID-UHFFFAOYSA-N CCOP(Cc(cc1)ccc1N(C)C(OC(C)(C)C)=O)(OCC)=O Chemical compound CCOP(Cc(cc1)ccc1N(C)C(OC(C)(C)C)=O)(OCC)=O QIEDQDUGSSASID-UHFFFAOYSA-N 0.000 description 1
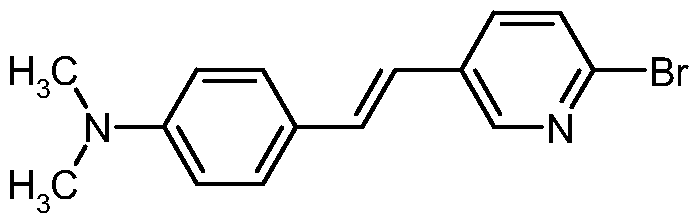
- KCYFPNFNXGONDM-ONEGZZNKSA-N CN(C)c1ccc(/C=C/c(cn2)ccc2Br)cc1 Chemical compound CN(C)c1ccc(/C=C/c(cn2)ccc2Br)cc1 KCYFPNFNXGONDM-ONEGZZNKSA-N 0.000 description 1
- VZUWJJFIJCSOTE-ONEGZZNKSA-N CN(C)c1ccc(/C=C/c(cn2)ccc2I)cc1 Chemical compound CN(C)c1ccc(/C=C/c(cn2)ccc2I)cc1 VZUWJJFIJCSOTE-ONEGZZNKSA-N 0.000 description 1
- CSQQRPCKCXCDSA-ONEGZZNKSA-N CN(C)c1ccc(/C=C/c(cn2)ccc2[N+]([O-])=O)cc1 Chemical compound CN(C)c1ccc(/C=C/c(cn2)ccc2[N+]([O-])=O)cc1 CSQQRPCKCXCDSA-ONEGZZNKSA-N 0.000 description 1
- XYKZFXKBECCMLE-RMKNXTFCSA-N CN(C)c1ccc(/C=C/c2cccc(Cl)n2)cc1 Chemical compound CN(C)c1ccc(/C=C/c2cccc(Cl)n2)cc1 XYKZFXKBECCMLE-RMKNXTFCSA-N 0.000 description 1
- FWJZWEJAPOELBZ-RMKNXTFCSA-N CN(C)c1ccc(/C=C/c2cccc(I)n2)cc1 Chemical compound CN(C)c1ccc(/C=C/c2cccc(I)n2)cc1 FWJZWEJAPOELBZ-RMKNXTFCSA-N 0.000 description 1
- OPHWDOUTXOPPHM-NSCUHMNNSA-N CNc1ccc(/C=C/c2cc(F)ncc2)cc1 Chemical compound CNc1ccc(/C=C/c2cc(F)ncc2)cc1 OPHWDOUTXOPPHM-NSCUHMNNSA-N 0.000 description 1
- CKSGZHPQYOQIFB-JXMROGBWSA-N CNc1ccc(/C=C/c2cccc(F)n2)cc1 Chemical compound CNc1ccc(/C=C/c2cccc(F)n2)cc1 CKSGZHPQYOQIFB-JXMROGBWSA-N 0.000 description 1
- PVUKGNBRJFTFNJ-UHFFFAOYSA-N O=Cc(cc1)cnc1Br Chemical compound O=Cc(cc1)cnc1Br PVUKGNBRJFTFNJ-UHFFFAOYSA-N 0.000 description 1
- HENWRHPVXMPQNF-UHFFFAOYSA-N O=Cc1cccc(F)n1 Chemical compound O=Cc1cccc(F)n1 HENWRHPVXMPQNF-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/61—Halogen atoms or nitro radicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/041—Heterocyclic compounds
- A61K51/044—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine, rifamycins
- A61K51/0455—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine, rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/72—Nitrogen atoms
Definitions
- This invention relates to compounds suitable for labeling or already labeled by F- 18, methods of preparing such a compound, compositions comprising such compounds, kits comprising such compounds or compositions and uses of such compounds, compositions or kits for diagnostic imaging.
- AD Alzheimer's Disease
- ⁇ beta-amyloid peptide
- APP amyloid precursor protein
- ⁇ peptides are released as soluble proteins and are detected at low level in the cerebrospinal fluid (CSF) in normal aging brain.
- CSF cerebrospinal fluid
- the ⁇ peptides aggregate and form amyloid deposits in the parenchyma and vasculature of the brain, which can be detected post mortem as diffuse and senile plaques and vascular amyloid during histological examination (for a recent review see: Blennow et al. Lancet. 2006 Jul 29;368(9533):387-403).
- AD Alzheimers disease
- MRI magnetic resonance imaging
- SPECT positron emission computed tomography
- positron emitting isotopes include e.g. carbon, iodine, nitrogen, and oxygen. These isotopes can replace their non-radioactive counterparts in target compounds to produce PET tracers that have similar biological properties.
- F-18 is a preferred labeling isotope due to its half life of 1 10 min, which permits the preparation of diagnostic tracers and subsequent study of biochemical processes.
- its low ⁇ + energy (634 keV) is also advantageous.
- the nucleophilic aromatic and aliphatic [F-18]fluoro-fluorination reaction is of great importance for [F-18]fluoro-labeled radiopharmaceuticals which are used as in vivo imaging agents for diseases.
- the half-life of F-18 is about 1 10 minutes, which demands quick preparation and administration of the radioactive compound.
- amyloid deposits are also known to play a role in amyloidoses, in which amyloid proteins (e.g. tau) are abnormally deposited in different organs and/or tissues, causing disease.
- amyloid proteins e.g. tau
- PET tracers which were already investigated in humans regarding their accumulation in the brain of AD patients are e.g. [F-18]FDDNP (A) (Shoghi- Jadid et al., Am J Geriatr Psychiatry 2002; 10:24-35), [C-1 1]PIB (B) (Klunk et al. Ann Neurol.
- the PET ligand should enter the brain rapidly in sufficient amounts. A fraction of tracer molecules should then bind specifically to the target. Subsequently, those molecules which have not bound should be eliminated rapidly from the surrounding area ("wash-out" from the brain) in order to achieve an image with a high signal to background ratio.
- Styrylpyridine derivatives (D) have also been labeled with PET isotopes and are covered by patent application WO2007126733 and members of the corresponding patent families.
- non-fluoropegylated styrylpyridine derivative described herein showed excellent brain uptake and higher brain wash out ratios (18.6) in healthy mice, indicating a lower background signal, which is advantageous for high contrast imaging of amyloid beta.
- figure 1 overview on aspects of the present invention
- the present invention provides novel compounds of Formulae I and II encompassing single isomers and mixtures thereof, or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates and prodrugs thereof.
- the present invention also provides pharmaceutical compositions comprising a radiolabeled compound of Formula I or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates and prodrugs thereof and optionally a pharmaceutically acceptable carrier, diluent, adjuvant or excipient.
- the present invention provides a compound of Formula II which is a precursor (starting material) for PET-isotope labeling compounds of Formula I.
- the present invention provides a method of imaging or diagnosing diseases, the method comprising introducing into a patient a detectable quantity of a labeled compound of Formula I or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates and prodrugs thereof.
- a preferred method of imaging or diagnosing a disease is imaging or diagnosing Alzheimer's disease.
- the present invention is directed to the use of compounds of Formula I for the manufacture of a pharmaceutical for imaging.
- the present invention provides the compounds of Formula I for use as medicament.
- the present invention also provides methods for producing compounds of Formula I by reacting a) compounds of Formula II with [F-18]fluoride, optionally including a subsequent deprotection step; b) compounds of Formula IV, being generated from compounds of Formula III, with compounds of Formula V optionally including a subsequent deprotection step.
- the present invention also provides a kit for preparing a radiopharmaceutical preparation, said kit comprising a sealed vial containing a predetermined quantity of
- a further aspect of this invention is directed to methods and intermediates useful for producing the imaging compound of Formula I. More specifically the compound of this invention is useful for the imaging of diseases including but not limited to Alzheimer's disease, other forms of dementias (e.g. Lewy body dementia) and/or amyloidoses. The invention, therefore, also relates to the use of imaging compounds for diagnosing these diseases as well as for stratification of therapy and therapy monitoring.
- diseases including but not limited to Alzheimer's disease, other forms of dementias (e.g. Lewy body dementia) and/or amyloidoses.
- the invention therefore, also relates to the use of imaging compounds for diagnosing these diseases as well as for stratification of therapy and therapy monitoring.
- the present invention also relates to a method of imaging amyloid aggregates using radioactively labeled compounds of the invention.
- F is a fluorine atom, preferably, F is a [F-18]fluorine atom.
- R' is selected from the group comprising:
- R A , R B , R c are selected from the group comprising: a) hydrogen,
- R' is selected from the group comprising:
- R' is selected from the group comprising:
- a preferred compounds is:
- Another preferred compounds is:
- Another preferred compounds is:
- Another preferred compounds is:
- Another preferred compounds is:
- Another preferred compounds is:
- Another referred compounds is:
- Another preferred compounds is:
- Another preferred compounds is:
- the present invention is directed to compounds of Formula II or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates or prodrugs thereof, encompassing also single isomers and mixtures thereof,
- R is 0-A 1A and LG is a trialkylammonium group
- R is 0-A 1A and LG is a aryl iodonium group
- R is NA 1B A C and LG is a trialkylammonium group
- R is NA B A 1 C and LG is a aryl iodonium group
- a 1A A 1B , A 1C are selected from the group comprising:
- PG is a protecting group which is known or obvious to someone skilled in the art, which is chosen from but not limited to a class of protecting groups namely ethers, benzyl ethers, silyl ethers, esters, carbonates, sulfonates, acetals, ketals, ortho esters and boronates and which is chosen from but not limited to those described in the textbook Greene and Wuts, Protecting groups in Organic Synthesis, third edition, page 17-245, included herewith by reference.
- PG is selected from the group comprising:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another preferred compounds is: 4-[(E)-2-(2-chloropyridin-4-yl)vinyl]-N,N-dimethylaniline
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- Another referred compounds is:
- the first method comprises a straight forward fluoro labeling reaction i.e. one-step method from compounds of Formula II, as described above, for obtaining compound of Formula I.
- the fluorination agent is a fluorine radioactive isotope derivative.
- the fluorine radioactive isotope derivative is a F-18 derivative. More preferably, the F-18 radioactive isotope derivative is 4,7,13,16,21 ,24- Hexaoxa-1 ,10-diazabicyclo[8.8.8]-hexacosane K[F-18]F (crownether salt Kryptofix K[F-18]F), K[F-18]F, H[F-18]F, KH[F-18]F 2 , Cs[F-18]F, Na[F-18]F or tetraalkylammonium salt of F-18 (e.g. [F-18] tetrabutylammonium fluoride).
- the F-18 radioactive isotope derivative is 4,7,13,16,21 ,24- Hexaoxa-1 ,10-diazabicyclo[8.8.8]-hexacosane K[F-18]F (crownether salt Kryptofix K[F
- the fluorination agent is K[F-18]F, H[F-18]F, or KH[F-18]F 2 , [F-18] tetrabutylammonium fluoride, most preferably K[F-18]F.
- the second method comprises a straight forward radioisotope labeling reaction i.e. one-step method from compounds of Formula III using [F-18]fluoride anions and subsequent reaction of F-18 labeled compound of Formula IV with compound of Formula V.
- the method for obtaining compound of Formula I comprises the steps of:
- the fluorination agent is defined as above.
- the compound of Formula III is:
- LG is a leaving group
- Preferred compounds are:
- the compound of Formula IV is:
- Preferred compound are:
- LG'" is selected from the group comprising:
- a 3A , A 3B , A 3C are selected from the group comprising:
- a 1 is selected from the group comprising:
- PG is a protecting group which is known or obvious to someone skilled in the art, which is chosen from but not limited to a class of protecting groups namely ethers, benzyl ethers, silyl ethers, esters, carbonates, sulfonates, acetals, ketals, ortho esters and boronates and which is chosen from but not limited to those described in the textbook Greene and Wuts, Protecting groups in Organic Synthesis, third edition, page 17-245, included herewith by reference; in a preferred embodiment, PG is selected from the group comprising:
- a 3 is selected from the group comprising:
- X is selected from the group comprising:
- Preferred compound are:
- the invention relates also to the use of compound of Formula I for the manufacture of medicament or pharmaceutical for treatment.
- compounds according to Formula I are provided as diagnostic imaging agent or imaging agent, preferably as imaging agent for PET applications.
- the invention relates also to the use of compound of Formula for the manufacture of imaging agent.
- the use concerns the imaging of CNS diseases.
- CNS diseases include but are not limited to Alzheimer's disease, dementia with Lewy bodies, frontotemporal dementia, amyloidoses and diseases of unidentified cause.
- the present invention is also directed to a method of imaging comprising the step of introducing into a patient a detectable quantity of an F-18 labeled compound of Formula I and imaging said patient.
- the compounds as described above and herein are, in a preferred embodiment of the invention, bound to a tau filament or tangle.
- Another aspect of the invention is the use of a compound of Formula I as described above and herein for diagnosing and/or treating Alzheimer's disease and/or amyloidoses in a patient, in particular in a mammal, such as a human.
- the use of a compound of the invention in the diagnosis is performed using positron emission tomography (PET).
- PET positron emission tomography
- Another aspect of the invention is directed to a method of imaging amyloid deposits.
- a method of imaging amyloid deposits comprises a) administering to a mammal a compound as described above and herein containing a detectable label, and b) detecting the signal stemming from the compound that is specifically bound to the amyloid deposits.
- the specific binding is a result of the high binding affinity of the compounds of the present invention to the amyloid deposits.
- the invention is directed to a method of diagnosing a patient with Alzheimer's disease or amyloidoses.
- This method comprises a) administering to a human in need of such diagnosis a compound of the invention with a detectable label for detecting the compound in the human as described above and herein, and b) measuring the signal from the detectable label arising from the administration of the compound to the human, preferably by positron emission tomography (PET).
- PET positron emission tomography
- a further embodiment of the invention includes a diagnostic method for other neurological disorders than Alzheimer's disease comprising the exclusion of Alzheimer's disease in a patient, that method comprising administering a compound of the invention to a patient and applying an imaging method of the invention.
- the invention is directed to a kit comprising one vial or more than one vial comprising a predetermined quantity of a compound having any one of the following general chemical Formulae or mixture thereof
- the kit comprises a compound having general chemical Formula as disclosed above along with an acceptable carrier, diluent, excipient or adjuvant or mixture thereof.
- alkyl refers to a linear or branched chain monovalent or divalent radical consisting of solely carbon and hydrogen, containing no unsaturation and having the specified number of carbons, such as methyl (Ci ), ethyl (C2), n-propyl (C3), 1 -methlyethyl ((C3) iso-propyl), n-butyl (C 4 ), n-pentyl (C5) and the like. More preferably alkyl is Ci-C 4 alkyl.
- alkenyl is similarly defined as for alkyl, but contains at least one carbon-carbon double bond, respectively. More preferably alkenyl is C2-C 4 alkenyl.
- alkynyl is similarly defined as for alkyl, but contain at least one carbon-carbon triple bond, respectively. More preferably alkynyl is C 2 -C 4 alkynyl.
- aryl as employed herein by itself or as part of another group refers to monocyclic or bicyclic aromatic or heteroaromatic groups containing from 5-10 atoms (C, N, S) in the ring portion, such as phenyl, naphthyl, thiophenyl or tetrahydronaphthyl.
- halogen or halo refers to CI, Br, F or I .
- alkyloxy or "alkoxy” refers to alkyl groups respectively linked by an oxygen atom, with the alkyl being as defined above.
- prodrug means any covalently bonded compound, which releases the active parent pharmaceutical according to Formula II .
- prodrug as used throughout this text means the pharmacologically acceptable derivatives such as esters, amides and phosphates, such that the resulting in vivo biotransformation product of the derivative is the active drug as defined in the compounds of Formula (! ⁇ .
- the reference by Goodman and GiSman The Pharmaco- logical Basis of Therapeutics, 8 ed, McGraw- ⁇ , Int. Ed. 1992, “Biotransformation of Drugs", p 13-15) describing prodrugs generally is hereby incorporated.
- Prodrugs of a compound of the present invention are prepared by modifying functional groups present in the compound in such a way that the modifications are cleaved, either in routine manipulation or in vivo, to the parent compound.
- Prodrugs of the compounds of the present invention include those compounds wherein for instance a hydroxy group, such as the hydroxy group on the asymmetric carbon atom, or an amino group is bonded to any group that, when the prodrug is administered to a patient, cleaves to form a free hydroxyl or free amino, respectively.
- prodrugs are described for instance in WO 99/33795, WO 99/33815, WO 99/33793 and WO 99/33792 all incorporated herein by reference. Prodrugs are characterized by excellent aqueous solubility, increased bioavailability and are readily metabolized into the active inhibitors in vivo.
- inorganic acid and “organic acid” refer to mineral acids, including, but not being limited to: acids such as carbonic, nitric, phosphoric, hydrochloric, perchloric or sulphuric acid or the acidic salts thereof such as potassium hydrogen sulphate, or to appropriate organic acids which include, but are not limited to: acids such as aliphatic, cycloaliphatic, aromatic, araliphatic, heterocyclic, carboxylic and sulphonic acids, examples of which are formic, acetic, trifluoracetic, propionic, succinic, glycolic, gluconic, lactic, malic, fumaric, pyruvic, benzoic, anthranilic, mesylic, fumaric, salicylic, phenylacetic, mandelic, embonic, methansulfonic, ethanesulfonic, benz
- the term "pharmaceutically acceptable salt” relates to salts of inorganic and organic acids, such as mineral acids, including, but not limited to, acids such as carbonic, nitric or sulfuric acid, or organic acids, including, but not limited to, acids such as aliphatic, cycloaliphatic, aromatic, araliphatic, heterocyclic, carboxylic and sulphonic acids, examples of which are formic, acetic, trifluoroacetic, propionic, succinic, glycolic, gluconic, lactic, malic, fumaric, pyruvic, benzoic, anthranilic, mesylic, salicylic, phenylacetic, mandelic, embonic, methansulfonic, ethanesulfonic, benzenesulfonic, phantothenic, toluenesulfonic and sulfanilic acid.
- mineral acids including, but not limited to, acids such as carbonic, nitric or sulfuric acid,
- the invention relates to
- R' is selected from the group comprising:
- R A , R B , R c are selected from the group comprising:
- R' is selected from the group comprising: a) Hydroxyl
- R' is selected from the group comprising: a) OMe,
- a compound according to count 1 selected from the group of compounds consisting of:
- R is O-A and LG is a halogen
- R is 0-A A and LG is a nitro group
- R is O-A 1A and LG is a trialkylammonium group
- R is 0-A 1A and LG is a aryl iodonium group
- R is 0-A 1A and LG is a diaryl sulfonium group
- R is NA B A 1 C and LG is a halogen
- R is NA 1B A 1 C and LG is a nitro group
- R is NA 1B A 1 C and LG is a trialkylammonium group
- R is NA 1B A 1 C and LG is a aryl iodonium group
- R is NA 1B A 1 C and LG is a diaryl sulfonium group
- a 1A , A 1B , A 1C are selected from the group consisting of:
- the compound is 2-bromo-6-[(E)-2-(4-methoxyphenyl)vinyl]pyridine or; d) the compound is 2-bromo-3-[(E)-2-(4-methoxyphenyl)vinyl]pyridine or; e) the compound is 2-bromo-5-[(E)-2-(4-methoxyphenyl)vinyl]pyridine or; f) ((R" is OPG or OMe) and (LG is a nitro group)) or;
- a compound according to counts 4 or 5 as a diagnostic compound 12.
- a compound according to counts 4 or 5 as a diagnostic compound for diagnosing Alzheimer's disease is a compound according to counts 4 or 5 as a diagnostic compound for diagnosing Alzheimer's disease.
- a kit comprising at least one sealed vial comprising a compound according to counts 6 - 9.
- kits according to count 14 comprising at least one sealed vial comprising a compound according to count 9.
- a pharmaceutical or diagnostic composition comprising a compound according to counts 4 or 5.
- FIG. 1 Analytical HPLC: top: [F-18]1a (gamma), bottom: co-injection of reference 1a (UV).
- Figure 2 Autoradiographical analysis of binding of compound [F-18]1 a to brain sections from cortex of Alzheimer ' s disease patients (AD) and controls without ⁇ plaques (HC) (healthy control). Blocking of specific signals was performed with an excess of cold compound. Arrows point to plaque-specific signals.
- phosphonate of formula VI in the presence of a base known to the expert in the field like lithium hydroxide, sodium hydride, butyl lithium or preferably potassium tert. butylate.
- Compound 1a was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Boc-protecting group.
- Compound 2a is radiofluorinated with [F-18]fluoride, potassium carbonate and crown ether (kryptofix) in dimethyl formamide or dimethyl sulfoxide to obtain compound [F-18]1a'.
- This radiofluorination can carried by a single operator by "hand” or on a module (see above) by automated or semi-automated methods (Krasikowa 2006).
- Compound [F-18]1a' is deprotected using acid, preferably mineral acid, more preferably hydrogen chloride, perchloric acid or sulfuric acid. After deprotection of compound [F-18]1a' compound [F-18]1a is obtained which is typically purified using cartridges or HPLC-columns.
- the crude product was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 20%) followed by thin layer chromatography on silica gel yield (10% ethyl acetate in hexane) to yield 66 mg of the title compound.
- Aqueous [ 18 F]Fluoride (15410 MBq) was trapped on a QMA cartridge (Waters) and eluted with 1 .5 ml_ Kryptofix solution (5 mg K2.2.2 ' n 0.95ml_ MeCN + 1 mg K2CO3 in 50 ⁇ _ water) into the reactor. The solvent was removed by heating at 120°C for 10 min under a stream of nitrogen. Anhydrous MeCN (1 ml_) was added and evaporated as before. A solution of 3 mg precursor 2a in 400 ⁇ anhydrous DMSO was added.
- the collected HPLC fraction was diluted with 40ml water and immobilized on a Sep-Pak plus short tC18 cartridge (Waters), which was washed with 10mL 20% EtOH in H 2 O and eluted with 1 mL EtOH to deliver 3678 MBq of the F-18 labeled product (45% rc. yield, corrected for decay; >96% TLC, >95% HPLC) in a overall synthesis time of -100 min.
- Compound 1 b was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Boc-protecting group.
- the crude product was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 20%) followed by thin layer chromatography on silica gel yield (10% ethyl acetate in hexane) to yield 47 mg of the title compound.
- Compound 1c was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Boc-protecting group.
- the crude product was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 20%) followed by thin layer chromatography on silica gel yield (10% ethyl acetate in hexane) to yield 10 mg of the title compound.
- Compound 1d was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Boc-protecting group.
- Labeling precursor 2d was synthesized from bromo derivative 8 and phosphonate
- the residue was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 50%) to yield 60 mg tert-butyl ⁇ 4-[(E)-2-(2-fluoropyridin-4- yl)vinyl]phenyl ⁇ methylcarbamate, which was solved in dichloromethane (3 mL) and treated with a 4 M HCI in dioxane (180 ⁇ ) for 72 hours at room temperature.
- the residue was purified by chromatography on silica gel (methanol in dichloromethane 0 to 20%) followed by thin layer chromatography on silica gel yield (30% ethyl acetate in hexane) to yield 13.9 mg of the title compound.
- Compound 1c was synthesized by Horner-Wadsworth-Emmons reaction using phosphanate 9 and aldehyde 4.
- Labeling precursor 2d was synthesized from bromo derivative 8 and phosphonate
- Compound 1f was synthesized by Horner-Wadsworth-Emmons.
- Compound 1 h was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Boc-protecting group.
- a competition assay with a tritiated amyloid ligand was performed in 96-well plates (Greiner bio-one; Cat. 651201 ; Lot. 06260130) using brain homogenate from AD patients.
- Homogenates were prepared by homogenizing (Ultra-Turrax, setting 2, 30 s, 24000 rpm) dissected frontal cortex containing grey matter and white matter from AD patients in phosphate buffered saline (PBS, pH 7.4). The homogenate with a concentration of 100 mg wet tissue/ml was divided into aliquots of 300 ⁇ and stored at -80°C.
- Varying concentrations of the unlabeled test substances were incubated with 100 g/ml homogenate and 10 nM of the tritiated ligand in PBS, 0.1 % BSA (final volume 200 ⁇ ) for 3 h at room temperature. Subsequently the binding mixture was filtered through Whatman GF/B filters (wetted with PBS, 0.1 % BSA) using a Filtermate 196 harvester (Packard). Filters were then washed twice with PBS, 0.1 % BSA and 40 ⁇ scintillator was added to each well before the bound radioactivity was measured in a TopCount devise (Perkin Elmer). Non-specific binding was assessed by adding an excess of 1000x of the reference ligand to the reaction mixture. Finally IC50 values were calculated with the help of appropriate analysis software. Table 1 IC50 to AD brain homagenate
- Frozen sections sliced at 18 ⁇ thickness on a cryostate (Leica, Germany) and paraffin sections, sliced on a sliding microtom (Leica) at a thickness of 6 ⁇ , were mounted onto glass slides (Superfrost Plus, Fa.Menzel, Braunschweig Germany). Frozen sections were allowed to adhere to the slides for several nights at -20°C. The paraffin sections were deparaffinized using routine histological methods. For binding studies sections were incubated with the F-18 labeled test compound at 10 Bq/ ⁇ diluted in 25mM Hepes buffer, pH 7.4, 0,1 % (BSA) (200-300 ⁇ /slide) for 1 ,5 hour at room temperature in a humidified chamber.
- BSA 0,1 %
- Biodistribution and excretion studies were performed in male NMRI mice (body weight approx. 30 g; 3 animals per time point). During an acclimation period of at least 3 days before the beginning of the study, animals were clinically examined to ascertain the absence of abnormal clinical signs.
Abstract
This invention relates to compounds suitable for labeling or already labeled by F-18, methods of preparing such a compound, compositions comprising such compounds, kits comprising such compounds or compositions and uses of such compounds, compositions or kits for diagnostic imaging.
Description
Diagnostic agents for amyloid beta imaging Field of Invention
This invention relates to compounds suitable for labeling or already labeled by F- 18, methods of preparing such a compound, compositions comprising such compounds, kits comprising such compounds or compositions and uses of such compounds, compositions or kits for diagnostic imaging.
Background
Alzheimer's Disease (AD) is a progressive neurodegenerative disorder marked by loss of memory, cognition, and behavioral stability. AD is defined pathologically by extracellular senile plaques comprised of fibrillar deposits of the beta-amyloid peptide (Αβ) and neurofibrillary tangles comprised of paired helical filaments of hyperphosphorylated tau. The 39 - 43 amino acids comprising Αβ peptides are derived from the larger amyloid precursor protein (APP). In the amyloidogenic pathway, Αβ peptides are cleaved from APP by the sequential proteolysis by beta- and gamma-secretases. Αβ peptides are released as soluble proteins and are detected at low level in the cerebrospinal fluid (CSF) in normal aging brain. During the progress of AD the Αβ peptides aggregate and form amyloid deposits in the parenchyma and vasculature of the brain, which can be detected post mortem as diffuse and senile plaques and vascular amyloid during histological examination (for a recent review see: Blennow et al. Lancet. 2006 Jul 29;368(9533):387-403).
Alzheimers disease (AD) is becoming a great health and social economical problem all over the world. There are great efforts to develop techniques and methods for the early detection and effective treatment of the disease. Currently, diagnosis of AD in an academic memory-disorders clinic setting is approximately 85-90% accurate (Petrella JR et al. Radiology. 2003 226:315-36). It is based on the exclusion of a variety of diseases causing similar symptoms and the careful neurological and psychiatric examination, as well as neuropsychological testing.
Molecular imaging has the potential to detect disease progression or therapeutic effectiveness earlier than most conventional methods in the fields of neurology, oncology and cardiology. Among the several promising molecular imaging technologies, such as optical imaging, MRI, SPECT and PET, PET is of particular interest for drug development because of its high sensitivity and ability to provide quantitative and kinetic data.
For example positron emitting isotopes include e.g. carbon, iodine, nitrogen, and oxygen. These isotopes can replace their non-radioactive counterparts in target compounds to produce PET tracers that have similar biological properties. Among these isotopes F-18 is a preferred labeling isotope due to its half life of 1 10 min, which permits the preparation of diagnostic tracers and subsequent study of biochemical processes. In addition, its low β+ energy (634 keV) is also advantageous.
The nucleophilic aromatic and aliphatic [F-18]fluoro-fluorination reaction is of great importance for [F-18]fluoro-labeled radiopharmaceuticals which are used as in vivo imaging agents for diseases. The half-life of F-18 is about 1 10 minutes, which demands quick preparation and administration of the radioactive compound.
A couple of methods are known to introduce F-18 e.g. to an aromatic ring (Coenen, Fluorine-18 Labeling Methods: Features and Possibilities of Basic Reactions, (2006), in: Schubiger P.A., Friebe M., Lehmann L, (eds), PET- Chemistry - The Driving Force in Molecular Imaging. Springer, Berlin Heidelberg, pp.15-50). One of the later discoveries is the replacement of an iodonium leaving group with [F-18]fluoride (compare also e.g. WO2005061415(A1 ), WO2005097713(A1 ), WO2007010534(A2), WO2007073200(A1 ) and WO2007141529(A1 )).
Post-mortem histological examination of the brain is still the only definite diagnosis of Alzheimer's disease. Thus, the in vivo detection of one pathological feature of the disease - the amyloid aggregate deposition in the brain - is thought to have a strong impact on the early detection of AD and differentiating it from other forms of dementia. Additionally, most disease modifying therapies which are in development are aiming at lowering of the amyloid load in the brain.
Thus, imaging the amyloid load in the brain may provide an essential tool for patient stratification and treatment monitoring (for a recent review see: Nordberg. Eur J Nucl Med Mol Imaging. 2008 Mar;35 Suppl 1 :S46-50).
In addition, amyloid deposits are also known to play a role in amyloidoses, in which amyloid proteins (e.g. tau) are abnormally deposited in different organs and/or tissues, causing disease. For a recent review see Chiti et al. Annu Rev Biochem. 2006;75:333-66.
Potential ligands for visualizing amyloid aggregates in the brain must show a high binding affinity to amyloid aggregates and must cross the blood brain barrier. PET tracers which were already investigated in humans regarding their accumulation in the brain of AD patients are e.g. [F-18]FDDNP (A) (Shoghi- Jadid et al., Am J Geriatr Psychiatry 2002; 10:24-35), [C-1 1]PIB (B) (Klunk et al. Ann Neurol. 2004 55:306-319), [C-1 1]SB-13 (C) (Verhoeff et al., Am J Geriatr Psychiatry 2004; 12:584-595, [C-11]BF227 (Kudo et al., J Nucl. Med 2007; 49:554-561 ), and [F-18]PIB (Farrar et al. Turku PET Symposium, Abstract 49).
It is an important goal for the design of a suitable CNS-PET tracer that the brain pharmacokinetics are optimized. Thus, the PET ligand should enter the brain
rapidly in sufficient amounts. A fraction of tracer molecules should then bind specifically to the target. Subsequently, those molecules which have not bound should be eliminated rapidly from the surrounding area ("wash-out" from the brain) in order to achieve an image with a high signal to background ratio.
Styrylpyridine derivatives (D) have also been labeled with PET isotopes and are covered by patent application WO2007126733 and members of the corresponding patent families.
AV-45 (D)
Reports in the literature (Zhang et al., Nucl Med Biol. 2007 Jan;34(1 ):89-97; Stephenson et al., Bioconjug Chem. 2007 Jan-Feb;18(1 ):238-46) describe the advantages of fluoropegylated tracers for detection of Αβ plaques. In healthy mice, AV-45 shows a only a moderate brain wash out ratio ([%ID/g at 2min] / [%ID/g at 60min]) of 3.9 (Choi et al., J Nucl Med. 2009 Nov;50(1 1 ):1887-94). Surprisingly, non-fluoropegylated styrylpyridine derivative described herein (e.g. 1a) showed excellent brain uptake and higher brain wash out ratios (18.6) in healthy mice, indicating a lower background signal, which is advantageous for high contrast imaging of amyloid beta.
Summary of the Invention
III IV V
figure 1 : overview on aspects of the present invention
• The present invention provides novel compounds of Formulae I and II encompassing single isomers and mixtures thereof, or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates and prodrugs thereof.
• The present invention also provides pharmaceutical compositions comprising a radiolabeled compound of Formula I or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates and prodrugs thereof and optionally a pharmaceutically acceptable carrier, diluent, adjuvant or excipient.
· The present invention provides a compound of Formula II which is a precursor (starting material) for PET-isotope labeling compounds of Formula I.
• The present invention provides a method of imaging or diagnosing diseases, the method comprising introducing into a patient a detectable quantity of a labeled compound of Formula I or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates and prodrugs thereof.
A preferred method of imaging or diagnosing a disease is imaging or diagnosing Alzheimer's disease.
The present invention is directed to the use of compounds of Formula I for the manufacture of a pharmaceutical for imaging.
The present invention provides the compounds of Formula I for use as medicament.
The present invention also provides methods for producing compounds of Formula I by reacting a) compounds of Formula II with [F-18]fluoride, optionally including a subsequent deprotection step; b) compounds of Formula IV, being generated from compounds of Formula III, with compounds of Formula V optionally including a subsequent deprotection step.
The present invention also provides a kit for preparing a radiopharmaceutical preparation, said kit comprising a sealed vial containing a predetermined quantity of
a) the compound of Formula II;
b) the compounds of Formula III and V;
A further aspect of this invention is directed to methods and intermediates useful for producing the imaging compound of Formula I. More specifically the compound of this invention is useful for the imaging of diseases including but not limited to Alzheimer's disease, other forms of dementias (e.g. Lewy body dementia) and/or amyloidoses. The invention, therefore,
also relates to the use of imaging compounds for diagnosing these diseases as well as for stratification of therapy and therapy monitoring.
• The present invention also relates to a method of imaging amyloid aggregates using radioactively labeled compounds of the invention.
Description of the Invention: In a first aspect the present invention is directed to compounds of Formula I
or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates or prodrugs thereof, encompassing also single isomers and mixtures thereof,
wherein
F is a fluorine atom, preferably, F is a [F-18]fluorine atom.
R' is selected from the group comprising:
a) 0-RA,
b) NRBRC
RA, RB, Rc are selected from the group comprising:
a) hydrogen,
b) branched or non-branched (C1 -C5)alkyl, c) branched or non-branched (C3-C5)alkenyl, with the proviso that RA ,R' and Rc are not attached to O or N with a sp2 hybridized carbon atom, d) branched or non-branched (C3-C5)alkynyl, with the proviso that RA ,R' and Rc are not attached to O or N with a sp hybridized carbon atom, e) (C1 -C5)alkyl-[0-(C1 -C5)alkyl]n wherein n is 1-5, or RB and Rc together are a group that is selected from groups comprising: a) -(CH2)m-,
b) -(CH2)2-0-(CH2)2-, c) -(CH2)2-NRA-(CH2)2-. wherein m is 2-5.
in a preferred embodiment R' is selected from the group comprising:
a) Hydroxyl,
b) OMe,
c) NH2,
d) NHMe,
e) NMe2. in a more preferred embodiment R' is selected from the group comprising:
a) OMe,
b) NHMe,
c) NMe2.
4-{(E)-2-[6-(F-18)fluoropyridin-3-yl]vinyl}phenol
2-(F-18)fluoro-5-[(E)-2-(4-methoxyphenyl)vinyl]pyridine
4-{(E)-2-[6-(F-18)fluoropyridin-3-yl]vinyl}aniline
4-{(E)-2-[6-(F-18)fluoropyridin-3-yl]vinyl}-N-methylaniline
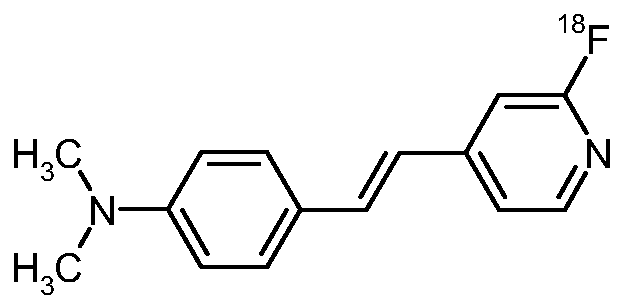
4-{(E)-2-[6-(F-18)fluoropyridin-3-yl]vinyl}-N,N-dimethylaniline Another preferred compounds is:
4-{(E)-2-[2-(F-18)fluoropyridin-4-yl]vinyl}-N-methylaniline Another preferred compounds is:
4-{(E)-2-[2-(F-18)fluoropyridin-4-yl]vinyl}aniline
4-{(E)-2-[2-(F-18)fluoropyridin-4-yl]vinyl}phenol Another preferred compounds is:
4-{(E)-2-[6-(F-18)fluoropyridin-2-yl]vinyl}-N-methylaniline
4-{(E)-2-[6-(F-18)fluoropyridin-2-yl]vinyl}-N,N-dimethylaniline
4-{(E)-2-[2-(F-18)fluoropyridin-3-yl]vinyl}-N-methylaniline
4-{(E)-2-[2-(F-18)fluoropyridin-3-yl]vinyl}-N,N-dimethylaniline
In second aspect the present invention is directed to compounds of Formula II
or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates or prodrugs thereof, encompassing also single isomers and mixtures thereof,
wherein:
(R" is 0-A A and LG is a halogen) or;
(R" is 0-A1A and LG is a nitro group) or;
(R" is 0-A1A and LG is a trialkylammonium group) or;
(R" is 0-A1A and LG is a aryl iodonium group) or;
(R" is 0-A A and LG is a diaryl sulfonium group) or;
(R" is NA1BA1 C and LG is a halogen) or;
(R" is NA1BA C and LG is a nitro group) or;
(R" is NA1BA C and LG is a trialkylammonium group) or;
(R" is NA BA1 C and LG is a aryl iodonium group) or;
(R" is NA BA1 C and LG is a diaryl sulfonium group).
A1A A1B, A1C are selected from the group comprising:
a) hydrogen,
b) branched or non-branched (C1 -C5)alkyl,
c) branched or non-branched (C3-C5)alkenyl, with the proviso that A1A A1 B, A1C are not attached to O or N with a sp2 hybridized carbon atom, d) branched or non-branched (C3-C5)alkynyl, with the proviso that A1A A1 B, A1C are not attached to O or N with a sp hybridized carbon atom, e) PG
f) (C1-C5)alkyl-[0-(C1 -C5)alkyl]n wherein n is 1-5
Or A1B and A1C together are group that is selected from groups comprising:
d) -(CH2)m-,
e) -(CH2)2-O-(CH2)2-, f) -(CH2)2-NA1A-(CH2)2-. wherein m is 2-5.
PG is a protecting group which is known or obvious to someone skilled in the art, which is chosen from but not limited to a class of protecting groups namely ethers, benzyl ethers, silyl ethers, esters, carbonates, sulfonates, acetals, ketals, ortho esters and boronates and which is chosen from but not limited to those described in the textbook Greene and Wuts, Protecting groups in Organic Synthesis, third edition, page 17-245, included herewith by reference.
In a preferred embodiment, PG is selected from the group comprising:
a) Boc,
b) Methoxymethyl,
c) Acetyl,
d) Trityl,
e) Fmoc.
In a preferred embodiment compounds of Formula II are selected from group consisting of:
a) ((R" is OPG) and (LG is chloro, iodo or bromo)) or; b) ((R" is OMe) and (LG is chloro, iodo)) or;
c) 2-bromo-6-[(E)-2-(4-methoxyphenyl)vinyl]pyridine or;
d) 2-bromo-3-[(E)-2-(4-methoxyphenyl)vinyl]pyridine or;
e) 2-bromo-5-[(E)-2-(4-methoxyphenyl)vinyl]pyridine or;
f) ((R" is OPG or OMe) and (LG is a nitro group)) or;
g) t(R" is OPG or OMe) and (LG is a trimethylammonium group)) or; h) ;(R" is OPG or OMe) and (LG is a aryl iodonium group)) or; i) ;(R" is OPG or OMe) and (LG is a diaryl sulfonium group)) or; j) ((R" is NPGH) and (LG is chloro, iodo or bromo)) or;
k) ((R" is NPGH) and (LG is a nitro group)) or;
1) ((R" is NPGH) and (LG is a trimethylammonium group)) or;
m) ((R" is NPGH) and (LG is a aryl iodonium group)) or;
n) ((R" is NPGH) and (LG is a diaryl sulfonium group)) or;
o) ((R" is NPG2) and (LG is chloro, iodo or bromo)) or;
P) ((R" is NPG2) and (LG is a nitro group)) or;
q) ((R" is NPG2) and (LG is a trimethylammonium group)) or;
r) ((R" is NPG2) and (LG is a aryl iodonium group)) or;
s) ((R" is NPG2) and (LG is a diaryl sulfonium group)) or;
t) ((R" is NPGMe) and (LG is chloro, iodo or bromo)) or;
u) ((R" is NPGMe) and (LG is a nitro group)) or;
v) ((R" is NPGMe) and (LG is a trimethylammonium group)) or; w) ((R" is NPGMe) and (LG is a aryl iodonium group)) or; x) ((R" is NPGMe) and (LG is a diaryl sulfonium group)) or; y) ((R" is NMe2) and (LG is chloro, iodo or bromo)) or; z) ((R" is NMe2) and (LG is a nitro group)) or;
aa) ((R" is NMe2) and (LG is a trimethylammonium group)) or;
bb) ((R" is NMe2) and (LG is a aryl iodonium group)) or;
tert-butyl {4-[(E)-2-(6-bromopyridin-3-yl)vinyl]phenyl}methylcarbamate Another referred compounds is:
tert-butyl {4-[(E)-2-(6-chloropyridin-3-yl)vinyl]phenyl}methylcarbamate
tert-butyl {4-[(E)-2-(6-iodopyridin-3-yl)vinyl]phenyl}methylcarbamate
N,N-dimethyl-4-[(E)-2-(6-bromopyridin-3-yl)vinyl]aniline
N,N-dimethyl-4-[(E)-2-(6-chloropyridin-3-yl)vinyl]aniline
N,N-dimethyl-4-[(E)-2-(6-iodopyridin-3-yl)vinyl]aniline
N,N-dimethyl-4-[(E)-2-(6-nitropyridin-3-yl)vinyl]aniline
tert-butyl {4-[(E)-2-(2-bromopyridin-4-yl)vinyl]phenyl}methylcarbamate Another preferred compounds is:
tert-butyl {4-[(E)-2-(2-chloropyridin-4-yl)vinyl]phenyl}methylcarbamate
tert-butyl {4-[(E)-2-(2-iodopyridin-4-yl)vinyl]phenyl}methylcarbamate
Another referred compounds is:
tert-butyl {4-[(E)-2-(2-nitropyridin-4-yl)vinyl]phenyl}methylcarbamate Another referred compounds is:
4-[(E)-2-(2-bromopyridin-4-yl)vinyl]-N,N-dimethylaniline
4-[(E)-2-(2-iodopyridin-4-yl)vinyl]-N,N-dimethylaniline
4-[(E)-2-(2-nitropyridin-4-yl)vinyl]-N,N-dimethylaniline
Another referred compounds is:
H
tert-butyl {4-[(E)-2-(6-bromopyridin-2-yl)vinyl]phenyl}methylcarbamate Another referred compounds is:
H
tert-butyl {4-[(E)-2-(6-chloropyridin-2-yl)vinyl]phenyl}methylcarbamate Another preferred compounds is:
O
H3C^(
H3C CH3
tert-butyl {4-[(E)-2-(6-iodopyridin-2-yl)vinyl]phenyl}methylcarbamate Another referred compounds is:
H
tert-butyl {4-[(E)-2-(6-nitropyridin-2-yl)vinyl]phenyl}methylcarbamate Another referred compounds is:
4-[(E)-2-(6-bromopyridin-2-yl)vinyl]-N,N-dimethylaniline
4-[(E)-2-(6-chloropyridin-2-yl)vinyl]-N,N-dimethylaniline
4-[(E)-2-(6-nitropyridin-2-yl)vinyl]-N,N-dimethylaniline
Another referred compounds is:
H
tert-butyl {4-[(E)-2-(2-bromopyridin-3-yl)vinyl]phenyl}methylcarbamate Another referred compounds is:
H
tert-butyl {4-[(E)-2-(2-chloropyridin-3-yl)vinyl]phenyl}methylcarbamate
tert-butyl {4-[(E)-2-(2-iodopyridin-3-yl)vinyl]phenyl}methylcarbamate Another preferred compounds is:
tert-butyl {4-[(E)-2-(2-nitropyridin-3-yl)vinyl]phenyl}methylcarbamate
4-[(E)-2-(2-bromopyridin-3-yl)vinyl]-N,N-dimethylaniline
4-[(E)-2-(2-chloropyridin-3-yl)vinyl]-N,N-dimethylaniline
4-[(E)-2-(2-iodopyridin-3-yl)vinyl]-N,N-dimethylaniline
4-[(E)-2-(2-nitropyridin-3-yl)vinyl]-N,N-dimethylaniline
In a third aspect of the invention is directed to a method for obtaining compounds of Formula I, are as defined above. This includes in particular all preferred embodiments mentioned above.
Two methods have been identified for obtaining compounds of Formula I.
The first method comprises a straight forward fluoro labeling reaction i.e. one-step method from compounds of Formula II, as described above, for obtaining compound of Formula I.
The radiolabeling method for obtaining compound of Formula I comprises the step of:
• Reacting a compound of Formula II with a fluorinating agent for obtaining a compound of Formula I.
• Optionally, a subsequent deprotection leads to compound of Formula I after reacting a compound of Formula II with a fluorinating agent.
Wherein compound of Formula II is described above.
In a preferred embodiment, the fluorination agent is a fluorine radioactive isotope derivative.
More preferably the fluorine radioactive isotope derivative is a F-18 derivative. More preferably, the F-18 radioactive isotope derivative is 4,7,13,16,21 ,24- Hexaoxa-1 ,10-diazabicyclo[8.8.8]-hexacosane K[F-18]F (crownether salt Kryptofix K[F-18]F), K[F-18]F, H[F-18]F, KH[F-18]F2, Cs[F-18]F, Na[F-18]F or tetraalkylammonium salt of F-18 (e.g. [F-18] tetrabutylammonium fluoride). More preferably, the fluorination agent is K[F-18]F, H[F-18]F, or KH[F-18]F2, [F-18] tetrabutylammonium fluoride, most preferably K[F-18]F.
The second method comprises a straight forward radioisotope labeling reaction i.e. one-step method from compounds of Formula III using [F-18]fluoride anions and subsequent reaction of F-18 labeled compound of Formula IV with compound of Formula V.
The method for obtaining compound of Formula I comprises the steps of:
• Reacting a compound of Formula III with fluorination agent to obtain compound of Formula IV.
• Reacting of compound of Formula IV with compound of Formula V.
· Optionally, a deprotection reaction.
The fluorination agent is defined as above.
The compound of Formula III is:
III
or salts of an inorganic or organic acid thereof, wherein
LG is a leaving group.
In a preferred embodiment LG is selected from the group comprising:
a) halogen,
b) nitro,
c) trimethylammonium,
d) diaryl-sulfonium,
e) aryl-iodonium.
IV
or salts of an inorganic or organic acid thereof, wherein
The compound of Formula V is
v
or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates or prodrugs thereof,
wherein
LG'" is selected from the group comprising:
a) 0-A3A,
b) NA3BA3C
A3A, A3B, A3C are selected from the group comprising:
a) hydrogen,
b) branched or non-branched (C1 -C5)alkyl,
c) branched or non-branched (C3-C5)alkenyl, with the proviso that A1A, A1 B, A1C are not attached to O or N with a sp2 hybridized carbon atom, d) branched or non-branched (C3-C5)alkynyl, with the proviso that A1A, A1 B, A1C are not attached to O or N with a sp hybridized carbon atom, e) PG
f) (C1-C5)alkyl-[0-(C1 -C5)alkyl]n wherein n is 1-5 in a preferred embodiment A1 is selected from the group comprising:
a) hydroxyl
b) O-PG,
c) OMe,
d) NHPG,
e) N(PG)2,
f) NHMe,
g) NMePG,
h) NMe2. PG is a protecting group which is known or obvious to someone skilled in the art, which is chosen from but not limited to a class of protecting groups namely
ethers, benzyl ethers, silyl ethers, esters, carbonates, sulfonates, acetals, ketals, ortho esters and boronates and which is chosen from but not limited to those described in the textbook Greene and Wuts, Protecting groups in Organic Synthesis, third edition, page 17-245, included herewith by reference; in a preferred embodiment, PG is selected from the group comprising:
a) Boc,
b) Methoxymethyl,
c) Acetyl,
d) Trityl,
e) Fmoc. in a more preferred embodiment A3 is selected from the group comprising:
a) OMe,
b) NHMe,
c) NMePG,
d) NMe2.
X is selected from the group comprising:
a) P(O)(O-[C1 -C5]alkyl)2,
b) P+(aryl)3.
Preferred compound are:
In a fourth aspect of the invention compounds according to Formula I are provided as medicament or pharmaceutical.
The invention relates also to the use of compound of Formula I for the manufacture of medicament or pharmaceutical for treatment.
In a fifth aspect of the invention, compounds according to Formula I are provided as diagnostic imaging agent or imaging agent, preferably as imaging agent for PET applications.
The invention relates also to the use of compound of Formula for the manufacture of imaging agent. In a more preferred embodiment the use concerns the imaging of CNS diseases. CNS diseases include but are not limited to Alzheimer's disease,
dementia with Lewy bodies, frontotemporal dementia, amyloidoses and diseases of unidentified cause.
The present invention is also directed to a method of imaging comprising the step of introducing into a patient a detectable quantity of an F-18 labeled compound of Formula I and imaging said patient.
The compounds as described above and herein are, in a preferred embodiment of the invention, bound to Αβ.
The compounds as described above and herein are, in a preferred embodiment of the invention, bound to a tau filament or tangle.
Another aspect of the invention is the use of a compound of Formula I as described above and herein for diagnosing and/or treating Alzheimer's disease and/or amyloidoses in a patient, in particular in a mammal, such as a human.
Preferably, the use of a compound of the invention in the diagnosis is performed using positron emission tomography (PET).
Another aspect of the invention is directed to a method of imaging amyloid deposits. Such a method comprises a) administering to a mammal a compound as described above and herein containing a detectable label, and b) detecting the signal stemming from the compound that is specifically bound to the amyloid deposits. The specific binding is a result of the high binding affinity of the compounds of the present invention to the amyloid deposits.
In a further aspect, the invention is directed to a method of diagnosing a patient with Alzheimer's disease or amyloidoses. This method comprises a) administering to a human in need of such diagnosis a compound of the invention with a detectable label for detecting the compound in the human as described above and herein, and b) measuring the signal from the detectable label arising
from the administration of the compound to the human, preferably by positron emission tomography (PET).
A further embodiment of the invention includes a diagnostic method for other neurological disorders than Alzheimer's disease comprising the exclusion of Alzheimer's disease in a patient, that method comprising administering a compound of the invention to a patient and applying an imaging method of the invention.
In a sixt aspect, the invention is directed to a kit comprising one vial or more than one vial comprising a predetermined quantity of a compound having any one of the following general chemical Formulae or mixture thereof
a) compounds of Formula II or Formula Ma;
b) compounds of Formula III and V.
Further, according to this aspect of the present invention the kit comprises a compound having general chemical Formula as disclosed above along with an acceptable carrier, diluent, excipient or adjuvant or mixture thereof.
Definitions
The term "alkyl" refers to a linear or branched chain monovalent or divalent radical consisting of solely carbon and hydrogen, containing no unsaturation and having the specified number of carbons, such as methyl (Ci ), ethyl (C2), n-propyl (C3), 1 -methlyethyl ((C3) iso-propyl), n-butyl (C4), n-pentyl (C5) and the like. More preferably alkyl is Ci-C4 alkyl.
The term "Alkenyl" is similarly defined as for alkyl, but contains at least one carbon-carbon double bond, respectively. More preferably alkenyl is C2-C4 alkenyl.
The term "Alkynyl" is similarly defined as for alkyl, but contain at least one carbon-carbon triple bond, respectively. More preferably alkynyl is C2-C4 alkynyl.
The term "aryl" as employed herein by itself or as part of another group refers to monocyclic or bicyclic aromatic or heteroaromatic groups containing from 5-10 atoms (C, N, S) in the ring portion, such as phenyl, naphthyl, thiophenyl or tetrahydronaphthyl.
The term halogen or halo refers to CI, Br, F or I .
The term "alkyloxy" or "alkoxy" refers to alkyl groups respectively linked by an oxygen atom, with the alkyl being as defined above.
As used hereinafter in the description of the invention and in the claims, the term "prodrug" means any covalently bonded compound, which releases the active parent pharmaceutical according to Formula II .
The term "prodrug" as used throughout this text means the pharmacologically acceptable derivatives such as esters, amides and phosphates, such that the resulting in vivo biotransformation product of the derivative is the active drug as defined in the compounds of Formula (!}. The reference by Goodman and GiSman (The Pharmaco- logical Basis of Therapeutics, 8 ed, McGraw-ΗΐΜ, Int. Ed. 1992, "Biotransformation of Drugs", p 13-15) describing prodrugs generally is hereby incorporated. Prodrugs of a compound of the present invention are prepared by modifying functional groups present in the compound in such a way that the modifications are cleaved, either in routine manipulation or in vivo, to the parent compound. Prodrugs of the compounds of the present invention include those compounds wherein for instance a hydroxy group, such as the hydroxy group on the asymmetric carbon atom, or an amino group is bonded to any group that, when the prodrug is administered to a patient, cleaves to form a free hydroxyl or free amino, respectively.
Typical examples of prodrugs are described for instance in WO 99/33795, WO 99/33815, WO 99/33793 and WO 99/33792 all incorporated herein by reference.
Prodrugs are characterized by excellent aqueous solubility, increased bioavailability and are readily metabolized into the active inhibitors in vivo.
As used hereinafter in the description of the invention and in the claims, the terms "salts of inorganic or organic acids", "inorganic acid" and "organic acid" refer to mineral acids, including, but not being limited to: acids such as carbonic, nitric, phosphoric, hydrochloric, perchloric or sulphuric acid or the acidic salts thereof such as potassium hydrogen sulphate, or to appropriate organic acids which include, but are not limited to: acids such as aliphatic, cycloaliphatic, aromatic, araliphatic, heterocyclic, carboxylic and sulphonic acids, examples of which are formic, acetic, trifluoracetic, propionic, succinic, glycolic, gluconic, lactic, malic, fumaric, pyruvic, benzoic, anthranilic, mesylic, fumaric, salicylic, phenylacetic, mandelic, embonic, methansulfonic, ethanesulfonic, benzenesulfonic, phantothenic, toluenesulfonic, trifluormethansulfonic and sulfanilic acid, respectively.
As used hereinafter in the description of the invention and in the claims, the term "pharmaceutically acceptable salt" relates to salts of inorganic and organic acids, such as mineral acids, including, but not limited to, acids such as carbonic, nitric or sulfuric acid, or organic acids, including, but not limited to, acids such as aliphatic, cycloaliphatic, aromatic, araliphatic, heterocyclic, carboxylic and sulphonic acids, examples of which are formic, acetic, trifluoroacetic, propionic, succinic, glycolic, gluconic, lactic, malic, fumaric, pyruvic, benzoic, anthranilic, mesylic, salicylic, phenylacetic, mandelic, embonic, methansulfonic, ethanesulfonic, benzenesulfonic, phantothenic, toluenesulfonic and sulfanilic acid.
In particular, the invention relates to
or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates or prodrugs thereof, encompassing also single isomers and mixtures thereof,
wherein
R' is selected from the group comprising:
a) 0-RA,
b) NRBRC and wherein RA, RB, Rc are selected from the group comprising:
a) hydrogen,
b) branched or non-branched (C1 -C5)alkyl, c) branched or non-branched (C3-C5)alkenyl, with the proviso that RA ,R' and Rc are not attached to O or N with a sp2 hybridized carbon atom, d) branched or non-branched (C3-C5)alkynyl, with the proviso that RA ,R' and Rc are not attached to O or N with a sp hybridized carbon atom, e) (C1 -C5)alkyl-[0-(C1 -C5)alkyl]n wherein n is 1-5, or RB and Rc together are a group that is selected from groups comprising:
a) -(CH2)m-,
b) -(CH2)2-0-(CH2)2-,
c) -(CH2)2-NRA-(CH2)2- wherein m is 2-5.
2. A compound according to count 1 , wherein R' is selected from the group comprising: a) Hydroxyl,
b) OMe,
c) NH2,
d) NHMe,
e) NMe2.
3. A compound according to count 1 , wherein R' is selected from the group comprising: a) OMe,
b) NHMe,
c) NMe2.
4. A compound according to counts 1 -3, wherein F has the meaning of 18F.
4-{(E)-2-[6-(F-18)fluoropyridin-3-yl]vinyl}phenol,
4-{(E)-2-[6-(F-18)fluoropyridin-3-yl]vinyl}aniline,
4-{(E)-2-[2-(F-18)fluoropyridin-4-yl]vinyl}-N-methylaniline,
4-{(E)-2-[2-(F-18)fluoropyridin-4-yl]vinyl}-N,N-dimethylaniline,
4-{(E)-2-[2-(F-18)fluoropyridin-4-yl]vinyl}phenol,
4-{(E)-2-[6-(F-18)fluoropyridin-2-yl]vinyl}-N,N-dimethylaniline,
4-{(E)-2-[2-(F-18)fluoropyridin-3-yl]vinyl}-N-methylaniline, and
4-{(E)-2-[2-(F-18)fluoropyridin-3-yl]vinyl}-N,N-dimethylaniline.
6. A compound according to formula II
or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates or prodrugs thereof, encompassing also single isomers and mixtures thereof,
wherein:
(R" is O-A and LG is a halogen), or (R" is 0-A A and LG is a nitro group), or (R" is O-A1A and LG is a trialkylammonium group), or (R" is 0-A1A and LG is a aryl iodonium group), or (R" is 0-A1A and LG is a diaryl sulfonium group), or (R" is NA BA1 C and LG is a halogen), or (R" is NA1BA1 C and LG is a nitro group), or (R" is NA1BA1 C and LG is a trialkylammonium group), or (R" is NA1BA1 C and LG is a aryl iodonium group), or
(R" is NA1BA1 C and LG is a diaryl sulfonium group); and wherein A1A, A1B, A1C are selected from the group consisting of:
a) hydrogen,
b) branched or non-branched (C1 -C5)alkyl,
c) branched or non-branched (C3-C5)alkenyl, with the proviso that A1A, A1 B, A1C are not attached to O or N with a sp2 hybridized carbon atom,
d) branched or non-branched (C3-C5)alkynyl, with the proviso that RA ,RB and Rc are not attached to O or N with a sp hybridized carbon atom, e) PG, being a protecting group,
f) (C1 -C5)alkyl-[0-(C1 -C5)alkyl]n, wherein n is 1-5; or A1B and A1C together are group that is selected from groups comprising:
a) -(CH2)m-,
b) -(CH2)2-0-(CH2)2-, c) -(CH2)2-NA1A-(CH2)2-. wherein m is 2-5.
7. A compound according to count 6, wherein: a) ((R" is OPG) and (LG is chloro, iodo or bromo)) or; b) ((R" is OMe) and (LG is chloro, iodo)) or;
c) the compound is 2-bromo-6-[(E)-2-(4-methoxyphenyl)vinyl]pyridine or; d) the compound is 2-bromo-3-[(E)-2-(4-methoxyphenyl)vinyl]pyridine or; e) the compound is 2-bromo-5-[(E)-2-(4-methoxyphenyl)vinyl]pyridine or;
f) ((R" is OPG or OMe) and (LG is a nitro group)) or;
g) ((R" is OPG or OMe) and (LG is a trimethylammonium group)) or; h) ((R" is OPG or OMe) and (LG is a aryl iodonium group)) or;
i) ((R" is OPG or OMe) and (LG is a diaryl sulfonium group)) or; j) ((R" is NPGH) and (LG is chloro, iodo or bromo)) or;
k) ((R" is NPGH) and (LG is a nitro group)) or;
I) ((R" is NPGH) and (LG is a trimethylammonium group)) or;
m) ((R" is NPGH) and (LG is a aryl iodonium group)) or;
n) ((R" is NPGH) and (LG is a diaryl sulfonium group)) or;
o) ((R" is NPG2) and (LG is chloro, iodo or bromo)) or;
p) ((R" is NPG2) and (LG is a nitro group)) or;
q) ((R" is NPG2) and (LG is a trimethylammonium group)) or;
r) ((R" is NPG2) and (LG is a aryl iodonium group)) or;
s) ((R" is NPG2) and (LG is a diaryl sulfonium group)) or;
t) ((R" is NPGMe) and (LG is chloro, iodo or bromo)) or;
u) ((R" is NPGMe) and (LG is a nitro group)) or;
v) ((R" is NPGMe) and (LG is a trimethylammonium group)) or; w) ((R" is NPGMe) and (LG is a aryl iodonium group)) or;
x) ((R" is NPGMe) and (LG is a diaryl sulfonium group)) or;
y) ((R" is NMe2) and (LG is chloro, iodo or bromo)) or;
z ((R" is NMe2) and (LG is a nitro group)) or;
aa) ((R" is NMe2) and (LG is a trimethylammonium group)) or;
bb) ((R" is NMe2) and (LG is a aryl iodonium group)) or;
cc) ((R" is NMe2) and (LG is a diaryl sulfonium group)).
8. A compound according to count 6 or 7, wherein PG is selected from the group comprising: a) Boc,
b) Methoxymethyl,
c) Acetyl,
d) Trityl,
e) Fmoc. 9. A compound according to count 6, selected from the group of compounds consisting of
tert-butyl {4-[(E)-2-(6-bromopyridin-3-yl)vinyl]phenyl}methylcarbamate,
tert-butyl {4-[(E)-2-(6-chloropyridin-3-yl)vinyl]phenyl}methylcarbamate,
tert-butyl {4-[(E)-2-(6-iodopyridin-3-yl)vinyl]phenyl}methylcarbamate,
O
H3C^<
H3C CH3
N,N-dimethyl-4-[(E)-2-(6-bromopyridin-3-yl)vinyl]aniline,
N,N-dimethyl-4-[(E)-2-(6-chloropyridin-3-yl)vinyl]aniline,
N,N-dimethyl-4-[(E)-2-(6-iodopyridin-3-yl)vinyl]aniline,
N,N-dimethyl-4-[(E)-2-(6-nitropyridin-3-yl)vinyl]aniline,
tert-butyl {4-[(E)-2-(2-bromopyridin-4-yl)vinyl]phenyl}methylcarbamate,
4-[(E)-2-(2-bromopyridin-4-yl)vinyl]-N,N-dimethylaniline,
tert-butyl {4-[(E)-2-(6-bromopyridin-2-yl)vinyl]phenyl}methylcarbamate,
H
4-[(E)-2-(6-bromopyridin-2-yl)vinyl]-N,N-dimethylaniline,
4-[(E)-2-(6-chloropyridin-2-yl)vinyl]-N,N-dimethylaniline,
4-[(E)-2-(6-iodopyridin-2-yl)vinyl]-N,N-dimethylaniline,
tert-butyl {4-[(E)-2-(2-bromopyridin-3-yl)vinyl]phenyl}methylcarbamate,
O
H3C^<
H3C CH3
4-[(E)-2-(2-bromopyridin-3-yl)vinyl]-N,N-dimethylaniline,
4-[(E)-2-(2-iodopyridin-3-yl)vinyl]-N,N-dimethylaniline,
4-[(E)-2-(2-nitropyridin-3-yl)vinyl]-N,N-dimethylaniline.
10. A method of manufacturing a compound of count 4 or 5, wherein a suitable precursor compound of counts 6-9 is reacted with a 18F fluorination agent. 1 1 . A method according to count 10, wherein a compound of count 5 is prepared by reacting a suitable precursor compound of count 9 with a 18F fluorination agent.
12. A compound according to counts 4 or 5 as a diagnostic compound.
13. A compound according to counts 4 or 5 as a diagnostic compound for diagnosing Alzheimer's disease.
14. A kit comprising at least one sealed vial comprising a compound according to counts 6 - 9.
15. A kit according to count 14, comprising at least one sealed vial comprising a compound according to count 9. 16. A pharmaceutical or diagnostic composition, comprising a compound according to counts 4 or 5.
17. A diagnostic composition according to count 16 for the imaging and/or diagnosis of amyloidoses, preferably Alzheimer's disease.
18. Use of a compound according to claims 4 or 5 in a method for manufacturing a diagnostic composition useful for imaging or diagnosing an amyloidodsis, preferably Alzheimer's disease.
Brief description of the figures
Figure 1 : Analytical HPLC: top: [F-18]1a (gamma), bottom: co-injection of reference 1a (UV).
Figure 2: Autoradiographical analysis of binding of compound [F-18]1 a to brain sections from cortex of Alzheimer's disease patients (AD) and controls without Αβ plaques (HC) (healthy control). Blocking of specific signals was performed with an excess of cold compound. Arrows point to plaque-specific signals.
General synthetic access Compounds of the general formula I or II are synthetically accessible via a Horner-Wadsworth-Emmons reaction reaction (Kelly, S. E. Comp. Org. Syn. 1991 , 1, 729-817; B. E. Maryanoff, Reitz, A. B. Chem. Rev. 1989, 89, 863-927) of a pyridyl aldehyde like compound of formula VII with a benzyl
phosphonate of formula VI in the presence of a base known to the expert in the field like lithium hydroxide, sodium hydride, butyl lithium or preferably potassium tert. butylate.
VI VII
Compounds of the general formula I or II are generally accessible via a Suzuki reaction (Miyaura, N.; Suzuki, A Chem Rev. 1995, 95, 2457-2483) of a pyridyl halogenid like compound of formula VIII with a vinyl boronic acid of formula IX in the presence of a catalyst known to the expert in the field like tetrakis triphenylphosphin palladium and a base known to the expert in the field like potassium carbonate.
Abbreviations d doublet
dd doublet of doublet
DMF Λ/,/V-dimethylformamide
DMSO dimethyl sulphoxide
dt doublet of triplet
EtOH ethanol
HCL hydrochloric acid
MS mass spectrometry
m multiplet
MeCN acetonitrile
NaOH sodium hydroxide
NMR nuclear magnetic resonance
spectroscopy : chemical shifts (δ) are given in ppm.
s singlet
t triplet
THF tetrahydrofurane
Examples
Example 1 Synthesis of 4-[(E)-2-(6-fluoropyridin-3-yl)vinyl]-N- methylaniline (1a)
Compound 1a was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Boc-protecting group.
1a' 1a Labeling precursor 2a was synthesized from bromo derivative 5 and phosphonate
Compound 2a is radiofluorinated with [F-18]fluoride, potassium carbonate and crown ether (kryptofix) in dimethyl formamide or dimethyl sulfoxide to obtain compound [F-18]1a'. This radiofluorination can carried by a single operator by "hand" or on a module (see above) by automated or semi-automated methods (Krasikowa 2006). Compound [F-18]1a' is deprotected using acid, preferably mineral acid, more preferably hydrogen chloride, perchloric acid or sulfuric acid. After deprotection of compound [F-18]1a' compound [F-18]1a is obtained which is typically purified using cartridges or HPLC-columns.
To a solution of diethyl {4-[(tertbutoxycarbonyl)(methyl)amino]benzyl} phosphonate (520 mg, 1 .45 mmol) and 6-fluoronicotinaldehyde (200 mg, 1 .60 mmol) in DMF (14.5 mL) was added to a solution of potassium tert.butylat (408 mg, 3.63 mmol) in DMF (36 mL). After stirring for one hour the reaction mixture was quenched with ice water, extracted with dichloromethane, washed with brine and dried over sodium sulfate. The residue was purified by chromatography on silica gel (ethyl acetate in hexane 5 to 10%) to yield 153 mg tert-butyl {4-[(E)-2-(6-fluoropyridin-3-yl)vinyl]phenyl}methylcarbamate, which was solved in trifluoroacetic acid (1 .9 mL) and stirred for 20 min at room temperature. The solvent was removed under reduced pressure, and the residue was dissolved in dichloromethane and extracted with brine and water. The organic layer was dried over sodium sulphate, filtrated and concentrated. The crude product was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 20%) followed by thin layer chromatography on silica gel yield (10% ethyl acetate in hexane) to yield 66 mg of the title compound.
1 H-NMR (300 MHz, CHLOROFORM-d) δ = 2.88 (s, 3H), 6.61 (d, 2H), 6.83 (d, 1 H), 6.90 (dd, 1 H), 7.00 (d, 1 H), 7.37 (d, 2H), 7.90 (td, 1 H), 8.23 (m, 1 H) ppm. 19F-NMR (376 MHz, CHLOROFORM-d) δ = -70.96 (s, 1 F) ppm. MS ES+ m/z = 229 (M+1 ).
Synthesis of tert- -[(E)-2-(6-bromopyridin-3-yl)vinyl1phenyl) methyl-
[(tert-butoxycarbonyl)(methyl)amino]benzyl} phosphonate (250 mg, 0.70 mmol) and 6-bromopyridine-3-carbaldehyde (130 mg, 0.70 mmol) in DMF (2 mL) was
added a solution of potassium tert.butylat (196 mg, 1 .75 mmol) in DMF (8 ml_). After stirring for one hour the reaction mixture was quenched with saturated aqueous ammonium chloride solution, extracted with dichloromethane, washed with brine and dried over sodium sulfate. The residue was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 25%) to yield 197 mg (72 %) of the title compound.
1 H NMR (300 MHz, CHLOROFORM-d) δ = 1 .48 (s, 9H), 3.29 (s, 3H), 6.97 (d, 1 H), 7.13 (d, 1 H), 7.27 (d, 2H), 7.47 (d, 1 H), 7.48 (d, 2H), 7.70 (dd, 1 H), 8.45 (d, 1 H) ppm. LC/MS ES+ m/z = 388 / 390 (M+1 ).
Aqueous [18F]Fluoride (15410 MBq) was trapped on a QMA cartridge (Waters) and eluted with 1 .5 ml_ Kryptofix solution (5 mg K2.2.2 'n 0.95ml_ MeCN + 1 mg K2CO3 in 50μΙ_ water) into the reactor. The solvent was removed by heating at 120°C for 10 min under a stream of nitrogen. Anhydrous MeCN (1 ml_) was added and evaporated as before. A solution of 3 mg precursor 2a in 400 μΙ anhydrous DMSO was added. After heating at 180°C for 20 min the crude reaction mixture was cooled down to 50°C and to cleave the protective group a mixture of 1 ml_ MeCN and 1 ml_ 2M HCI was added and heated for 5 more min at 80°C. After cooling to room temperature the crude reaction mixture was diluted with a mixture of 1 .5 ml_ 0.1 M ammonium formate + 1 ml_ 2M NaOH + 10mg sodium ascorbate and purified by preparative HPLC: ACE 5-C18 250mmx10mm; 5μηι Advanced Chromatography Technologies; Cat.No.: ACE 121-2510; isocratic, 0.1 M ammonium formate in H20 /MeCN= 56 / 44, flow: 4 mL/min; tR~26.5 min. The collected HPLC fraction was diluted with 40ml water and immobilized on a Sep-Pak plus short tC18 cartridge (Waters), which was washed with 10mL 20% EtOH in H2O and eluted with 1 mL EtOH to deliver 3678 MBq of the F-18 labeled product (45% rc. yield, corrected for decay; >96% TLC, >95% HPLC) in a overall synthesis time of -100 min. The desired F-18 labeled
product was analyzed using analytical HPLC: ACE3-C18 50 mm x 4,6 mm; ACE-1 1 1 -0546; S /N: A56904, Advanced Chromatography Technologies; solvent gradient: start 5% acetonitrile - 95% acetonitrile in 0.1 % trifluoroacetic acid in 7 min., flow: 2 mL/min and confirmed by co-injection with the corresponding non-radioactive F-19 fluoro-standard 1a on the analytical HPLC (tR=2.8 min), see figure 1 .
Example 2 Synthesis of 4-[(E)-2-(2-fluoropyridin-3-yl)vinyl]-N-methyl- aniline (1 b)
Compound 1 b was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Boc-protecting group.
lb- lb
Synthesis of 4-[(E)-2-(2-fluoropyridin-3-vnvinyll-N-methylaniline (1b)
To a solution of diethyl {4-[(tertbutoxycarbonyl)(methyl)amino]benzyl} phosphonate (520 mg, 1 .45 mmol) and 2-fluoronicotinaldehyde (200 mg, 1 .60 mmol) in DMF (14.5 ml_) was added to a solution of potassium tert.butylat (408 mg, 3.63 mmol) in DMF (36 ml_). After stirring for one hour the reaction mixture was quenched with ice water, extracted with dichloromethane, washed with brine and dried over sodium sulfate. The residue was purified by chromatography on silica gel (ethyl acetate in hexane 5 to 10%) to yield 121 mg tert-butyl {4-[(E)-2-(2-fluoropyridin-3-yl)vinyl]phenyl}methylcarbamate, which was solved in trifluoroacetic acid (1 .5 ml_) and stirred for 25 min at room temperature. The solvent was removed under reduced pressure, and the residue was
dissolved in dichloromethane and extracted with brine and water. The organic layer was dried over sodium sulphate, filtrated and concentrated. The crude product was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 20%) followed by thin layer chromatography on silica gel yield (10% ethyl acetate in hexane) to yield 47 mg of the title compound.
1 H-NMR (300 MHz, CHLOROFORM-d) δ = 2.88 (s, 3H), 6.61 (d, 2H), 6.93 (d, 1 H), 7.15 (m, 2H), 7.40 (d, 2H), 7.94 (m, 1 H), 8.02 (m, 1 H) ppm. 19F-NMR (376 MHz, CHLOROFORM-d) δ = -72.0 (s, 1 F) ppm. MS ES+ m/z = 229 (M+1 ).
Example 3 Synthesis of 4-[(E)-2-(6-fluoropyridin-2-yl)vinyl]-N-methyl- aniline (1c)
Compound 1c was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Boc-protecting group.
Synthesis of 4-[(E)-2-(6-fluoropyridin-2-vnvinyll-N-methylaniline (1c)
To a solution of diethyl {4-[(tertbutoxycarbonyl)(methyl)amino]benzyl} phosphonate (480 mg, 1 .34 mmol) and 6-fluoropyridine-2-carbaldehyde (185 mg, 1.48 mmol) in DMF (10 mL) was added to a solution of potassium tert.butylat (377 mg, 3.36 mmol) in DMF (20 mL). After stirring for one hour the reaction mixture was quenched with ice water, extracted with dichloromethane, washed with brine and dried over sodium sulfate. The residue was purified by chromatography on silica gel (ethyl acetate in hexane 5 to 10%) to yield tert- butyl {4-[(E)-2-(6-fluoropyridin-2-yl)vinyl]phenyl}methylcarbamate, which was solved in trifluoroacetic acid (1 .5 mL) and stirred for 25 min at room temperature. The solvent was removed under reduced pressure, and the residue was
dissolved in dichloromethane and extracted with brine and water. The organic layer was dried over sodium sulphate, filtrated and concentrated. The crude product was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 20%) followed by thin layer chromatography on silica gel yield (10% ethyl acetate in hexane) to yield 10 mg of the title compound.
1 H-NMR (300 MHz, CHLOROFORM-d) δ = 2.87 (s, 3H), 6.60 (d, 2H), 6.69 (dd, 1 H), 6.87 (d, 1 H), 7.13 (m, 1 H), 7.94 (m, 1 H), 7.43 (d, 2H), 7.60 (d, 1 H), 7.68 (q, 1 H) ppm. 19F-NMR (376 MHz, CHLOROFORM-d) δ = -66.9 (s, 1 F) ppm. Example 4 Synthesis of 4-[(E)-2-(2-fluoropyridin-4-yl)vinyl]-N-methyl- aniline (1d)
Compound 1d was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Boc-protecting group.
"Id1 1d
Labeling precursor 2d was synthesized from bromo derivative 8 and phosphonate
To a solution of diethyl {4-[(tertbutoxycarbonyl)(methyl)amino]benzyl} phosphonate (250 mg, 0.70 mmol) and 2-fluoropyridine-4-carbaldehyde (88 mg,
0.70 mmol) in DMF (2 mL) was added a solution of potassium tert.butylat (196 mg, 1 .75 mmol) in DMF (8 mL). After stirring for one hour the reaction mixture was quenched with saturated aqueous ammonium chloride solution, extracted with dichloromethane, washed with brine and dried over sodium sulfate. The residue was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 50%) to yield 60 mg tert-butyl {4-[(E)-2-(2-fluoropyridin-4- yl)vinyl]phenyl}methylcarbamate, which was solved in dichloromethane (3 mL) and treated with a 4 M HCI in dioxane (180 μί) for 72 hours at room temperature. The residue was purified by chromatography on silica gel (methanol in dichloromethane 0 to 20%) followed by thin layer chromatography on silica gel yield (30% ethyl acetate in hexane) to yield 13.9 mg of the title compound.
1 H-NMR (300 MHz, DMSO-d6) δ = 2.87 (s, 3H), 6.93 (d, 1 H), 7.34 (d, 2H), 7.43 (m, 2H), 7.54 (d, 1 H), 7.66 (d, 1 H), 7.74 (d, 2H), 8.21 (d, 1 H) ppm. 19F-NMR (376 MHz, CHLOROFORM-d) δ = -70.06 (s, 1 F) ppm. LC/MS ES+ m/z = 228.9 (M+1 ).
Synthesis of tert-but -[(E)-2-(2-bromopyridin-4-yl)vinyllphenyl)methyl-
To a solution of diethyl diethyl {4-[(tert-butoxycarbonyl)(methyl)amino]benzyl} phosphonate (384 mg, 1 .08 mmol) in THF (4.4 mL) was added a 1 M solution of potassium tert.butylat (1 .6 mL, 1 .6 mmol) in THF at 0°C. After stirring for 15 minutes at 0°C 2-bromopyridine-4-carbaldehyde (200 mg, 1 .08 mmol) in THF (3.5 mL) was added and stirring was continued for 30 minutes at 0°C. The reaction mixture was quenched with saturated aqueous ammonium chloride solution, extracted with diethyl ether, washed with brine and dried over sodium sulfate. The residue was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 20%) to yield 41 mg (9.8%) of the title compound and 42 mg of the Z isomer.
1 H-NMR (300 MHz, CDCI3) δ =1 .48 (s, 9H), 3.30 (s, 3H), 6.90 (d, 1 H), 7.25 - 7.35 (m, 4H), 7.50 (d, 1 H), 7.56 (d, 2H), 8.32 (d, 1 H) ppm. MS ES+ m/z = 388 / 390 (M+1 ). Example 5 Synthesis of 4-[(E)-2-(6-fluoropyridin-3-yl)vinyl]-N,N-dimethyl- aniline (1e)
Compound 1c was synthesized by Horner-Wadsworth-Emmons reaction using phosphanate 9 and aldehyde 4.
9 4 1e
Labeling precursor 2d was synthesized from bromo derivative 8 and phosphonate
To a solution of diethyl [4-(dimethylamino)benzyl]phosphonate (208 mg, 0.77 mmol) and 6-fluoropyridine-3-carbaldehyde (96 mg, 0.77 mmol) in DMF (2 mL) was added a solution of potassium tert.butylat (215.1 mg, 1 .92 mmol) in DMF (8 mL). After stirring for one hour the reaction mixture was quenched with saturated aqueous ammonium chloride solution, extracted with dichloromethane, washed with brine and dried over sodium sulfate. The residue was purified by
chromatography on silica gel (ethyl acetate in hexane 0 to 50%) to yield 6 mg (3%) of the title compound. 1H NMR (400 MHz, CHLOROFORM-d) δ = 3.02 (s, 6H), 6.78 (br. , 2H), 6.87 (d, 1 H), 6.91 (dd, 1 H), 7.02 (d, 1 H), 7.43 (d, 2H), 7.92 (ddd, 1 H), 8.25 (d, 1 H) ppm. 19F NMR (400 MHz, CHLOROFORM-d) δ = -71 .06 (s, 1 F). MS ES+ m/z = 243.14 (M+1 ).
Example 6 Synthesis of 2-fluoro-5-[(E)-2-(4-methoxyphenyl)vinyl]pyridine (1f)
Compound 1f was synthesized by Horner-Wadsworth-Emmons.
10
To a solution of diethyl [4-(meththoxy)benzyl]phosphonate (500 mg, 1 .94 mmol) and 6-fluoropyridine-3-carbaldehyde (242 mg, 1 .94 mmol) in DMF (4 mL) was added a solution of potassium tert.butylat (543 mg, 4.8 mmol) in DMF (16 mL). After stirring for one hour the reaction mixture was quenched with saturated aqueous ammonium chloride solution, extracted with dichloromethane, washed with brine and dried over sodium sulfate. The residue was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 50%) to yield 50.6 mg (10.3%) of the title compound.
1 H-NMR (400 MHz, CHLOROFORM-d) d = 3.85 (s, 3H), 6.92 (d, 1 H), 6.92 (dd, 1 H), 6.93 (d, 2H), 7.05 (d, 1 H), 7.46 (d, 2H), 7.93 (ddd, 1 H), 8.28 (d, 1 H) ppm. 19F-NMR (376 MHz, CHLOROFORM-d) δ = -70.13 (d, 1 F) ppm. MS ES+ m/z = 230.13 (M+1 ).
Example 7 Synthesis of 4-[(E)-2-(6-fluoropyridin-3-yl)vinyl]phenol (1g)
Compound 1d was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Mom-protecting group.
To a solution of diethyl [4-(methoxymethoxy)benzyl]phosphonate (250 mg, 0.87 mmol) and 6-fluoropyridine-3-carbaldehyde (108.5 mg, 0.87 mmol) in DMF (2 ml_) was added a solution of potassium tert.butylat (243 mg, 2.17 mmol) in DMF (8 ml_). After stirring for one hour the reaction mixture was quenched with saturated aqueous ammonium chloride solution, extracted with dichloromethane, washed with brine and dried over sodium sulfate. The residue was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 50%) to yield 32.8 mg 2-fluoro-5-{(E)-2-[4-(methoxymethoxy)phenyl]vinyl}pyridine, which was solved in THF (2 ml_) and treated with aqueous 18.5% HCI (182 μΙ_) at 50°C for one hour. The solution was concentrated under reduced pressure to yield 25.4 mg of the title compound as a yellow solid.
1 H-NMR (400 MHz, DMSO-d6) δ = 6.79 (d, 2H), 7.05 (d, 1 H), 7.18 (dd, 1 H), 7.24 (d, 1 H), 7.43 (d, 2H), 8.20 (ddd, 1 H), 8.34 (d, 1 H) ppm. 19F-NMR (376 MHz, CHLOROFORM-d) δ = -71.20 (d, 1 F) ppm. LC/MS ES+ m/z = (M+1 ).
Example 8 Synthesis of 4-[(E)-2-(6-fluoropyridin-3-yl)vinyl]aniline (1 h)
Compound 1 h was synthesized by Horner-Wadsworth-Emmons reaction and subsequent acidic cleavage of the Boc-protecting group.
To a solution of diethyl diethyl {4-[(tert-butoxycarbonyl)amino]benzyl} phosphonate (272 mg, 0.79 mmol) and 6-fluoropyridine-3-carbaldehyde (99 mg, 0.79 mmol) in DMF (2 mL) was added a solution of potassium tert.butylat (196 mg, 1 .75 mmol) in DMF (8 mL). After stirring for one hour the reaction mixture was quenched with saturated aqueous ammonium chloride solution, extracted with dichloromethane, washed with brine and dried over sodium sulfate. The residue was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 50%) to yield 21 mg of tert-butyl {4-[(E)-2-(6-fluoropyridin-3-yl)vinyl]phenyl} carbamate, which was solved in dichloromethane (1 mL) and treated with a 4 M HCI in dioxane (68 μί) for 24 under reflux. The solution was concentrated under reduced pressure and purified by thin layer chromatography on silica gel (30% ethyl acetate in hexane) to yield 9 mg of the title compound.
1 H NMR (400 MHz, CHLOROFORM-d) δ = 3.81 (br., 2H), 6.69 (d, 2H), 6.86 (d, 1 H), 6.91 (dd, 1 H), 7.00 (d, 1 H), 7.34 (d, 2H), 7.91 (ddd, 1 H), 8.25 (d, 1 H) ppm. 19F-NMR (376 MHz, CHLOROFORM-d) δ = -70.70 (d, 1 F) ppm. MS ES+ m/z = 214.9 (M+1 ).
Synthesis of 5-[(E)-2-{4-[(tert-butoxycarbonyl)(methyl)aminolphenyl)vinyl1- N,N,N-trimethylpyridin-2-aminium acetate (2i)
To a solution of diethyl {4-[(tert-butoxycarbonyl)(methyl)amino]benzyl} phosphonate (1 .5 g, 4.2 mmol) and 6-fluoropyridine-3-carbaldehyde ( 525 mg, 4.2 mmol) in DMF (20 ml_) was added a solution of potassium tert.butylat (1 .18 mg, 10.5 mmol) in DMF (40 ml_). After stirring for 6 hours the reaction mixture was quenched with saturated aqueous ammonium chloride solution, extracted with diethyl ether, washed with brine and dried over sodium sulfate. The residue was purified by chromatography on silica gel (ethyl acetate in hexane 0 to 25%) to yield 800 mg of tert-butyl (4-{(E)-2-[6-(dimethylamino)pyridin-3- yl]vinyl}phenyl)methylcarbamate, which (100 mg, 0.28 mmol) was suspended in toluene (3 ml_) and treated with methyl trifluormethansulfonate (31 μ, 0.28 mmol). Stirred for 20 hours while the methyl trifluormethansulfonate addition was repeated after 4 hours.The mixture was filtered, the filtrate concentrated and purified by HPLC on a XBrigde C18 5μηι 150x19 mm column (methanol in 0.1 M ammonium acetate / acetic acid buffer pH 5.8 hexane 40 to 90%) to yield 26 mg of the title compound.
1 H NMR (400 MHz, DMSO-d6) δ = 1 .38 (s, 9H), 1 .71 (s, 3H), 3.17 (s, 3H), 3.55 (s, 9H), 7.32 (d, 2H), 7.33 (d, 1 H), 7.53 (d, 1 H), 7.58 (d, 2H), 8.06 (d, 1 H), 8.41 (dd, 1 H), 8.78 (d, 1 H) ppm. MS ES+ m/z = 368.1 (M - COOCH3).
Example 9 Biological evaluation of compounds 1a, 1d, 1e, 1f
Binding studies using human brain homogenate
A competition assay with a tritiated amyloid ligand was performed in 96-well plates (Greiner bio-one; Cat. 651201 ; Lot. 06260130) using brain homogenate from AD patients.
Homogenates were prepared by homogenizing (Ultra-Turrax, setting 2, 30 s, 24000 rpm) dissected frontal cortex containing grey matter and white matter from AD patients in phosphate buffered saline (PBS, pH 7.4). The homogenate with a concentration of 100 mg wet tissue/ml was divided into aliquots of 300 μΙ and stored at -80°C.
Varying concentrations of the unlabeled test substances were incubated with 100 g/ml homogenate and 10 nM of the tritiated ligand in PBS, 0.1 % BSA (final
volume 200 μΙ) for 3 h at room temperature. Subsequently the binding mixture was filtered through Whatman GF/B filters (wetted with PBS, 0.1 % BSA) using a Filtermate 196 harvester (Packard). Filters were then washed twice with PBS, 0.1 % BSA and 40 μΙ scintillator was added to each well before the bound radioactivity was measured in a TopCount devise (Perkin Elmer). Non-specific binding was assessed by adding an excess of 1000x of the reference ligand to the reaction mixture. Finally IC50 values were calculated with the help of appropriate analysis software. Table 1 IC50 to AD brain homagenate
Compound IC50 [nM]
4-[(E)-2-(6-fluoropyridin-3-yl)vinyl]-N-methylaniline (1a) 31
4-[(E)-2-(2-fluoropyridin-4-yl)vinyl]-N-methylaniline (1d) 40
4-[(E)-2-(6-fluoropyridin-3-yl)vinyl]-N,N-dimethylaniline (1e) 31
2-fluoro-5-[(E)-2-(4-methoxyphenyl)vinyl]pyridine (1f) 88
4-[(E)-2-(6-fluoropyridin-3-yl)vinyl]phenol (1g) 108
Autoradiographical analysis
Fresh frozen as well as paraffin embedded sections of the frontal lobe from Alzheimer's dementia patients (AD) and age matched controls (HC) were used for the study.
Frozen sections, sliced at 18 μηι thickness on a cryostate (Leica, Germany) and paraffin sections, sliced on a sliding microtom (Leica) at a thickness of 6 μηπ, were mounted onto glass slides (Superfrost Plus, Fa.Menzel, Braunschweig Germany). Frozen sections were allowed to adhere to the slides for several nights at -20°C. The paraffin sections were deparaffinized using routine histological methods. For binding studies sections were incubated with the F-18 labeled test compound at 10 Bq/μΙ diluted in 25mM Hepes buffer, pH 7.4, 0,1 %
(BSA) (200-300 μΙ/slide) for 1 ,5 hour at room temperature in a humidified chamber. For blocking experiments an excess of the unlabeled test substance was added to the incubation mixture. After hybridization, sections were washed four times with Hepes buffer, 0.1 % BSA (or alternatively two times with 40% ethanol) and finally dipped two times into dest. water for 10 sec. The air-dried sections were exposed to imaging plates and signals were detected by a phosphoimager device (Fuji BAS5000).
Biodistribution experiments in mice
Biodistribution and excretion studies were performed in male NMRI mice (body weight approx. 30 g; 3 animals per time point). During an acclimation period of at least 3 days before the beginning of the study, animals were clinically examined to ascertain the absence of abnormal clinical signs.
At 2, 5, 30, 60, 240 min post intravenous injection via the tail vein of ca. 150 kBq of the test compound (ca. 100 μΙ), urine and feces were quantitatively collected. At the same time points, animals were sacrificed by decapitation and under isoflurane anaesthesia and organs and tissues of interest were removed for the determination of radioactivity using a gamma-counter. For analysis the decay corrected percentage of the injected dose per tissue weight (%ID/g ± standard deviation) was calculated.
Table 2 Brain uptake of compound [F-18]1a in % of injected dose per gram tissue [%ID/g]. Distribution of F-18 signal after administration of compound [F-18]1a in mice at 2 min and 60 min after compound administration
1a 5.6 0.3 18.6
PAGE INTENTIONALLY LEFT BLANK
Claims
1 . A compound according to formula I
or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates or prodrugs thereof, encompassing single isomers and mixtures thereof,
wherein
R' is selected from the group comprising:
a) 0-RA,
b) NRBRC and wherein
RA, RB, Rc are selected from the group comprising:
a) hydrogen,
b) branched or non-branched (C1 -C5)alkyl, c) branched or non-branched (C3-C5)alkenyl, with the proviso that RA ,RB and Rc are not attached to O or N with a sp2 hybridized carbon atom, d) branched or non-branched (C3-C5)alkynyl, with the proviso that RA ,RB and Rc are not attached to O or N with a sp hybridized carbon atom, e) (C1 -C5)alkyl-[0-(C1 -C5)alkyl]n wherein n is 1-5, or RB and Rc together are group that is selected from groups comprising: a) -(CH2)m-,
b) -(CH2)2-0-(CH2)2-,
c) -(CH2)2-NRA-(CH2)2-. wherein m is 2-5.
2. A compound according to claim 1 , wherein R' is selected from the group comprising: a) Hydroxy I,
b) OMe,
c) NH2,
d) NHMe,
e) NMe2.
3. A compound according to claim 1 , wherein R' is selected from the group comprising: a) OMe,
b) NHMe,
c) NMe2.
4. A compound according to claims 1 -3, wherein F has the meaning of 8F.
4-{(E)-2-[6-(F-18)fluoropyridin-3-yl]vinyl}-N-methylaniline,
4-{(E)-2-[2-(F-18)fluoropyridin-4-yl]vinyl}phenol,
4-{(E)-2-[2-(F-18)fluoropyridin-3-yl]vinyl}-N-methylaniline,
and
4-{(E)-2-[2-(F-18)fluoropyridin-3-yl]vinyl}-N,N-dimethylaniline.
6. A compound according to formula II
or pharmaceutically acceptable salts of an inorganic or organic acid thereof, hydrates, complexes, esters, amides, solvates or prodrugs thereof, encompassing single isomers and mixtures thereof,
wherein:
(R" is 0-A1A and LG is a halogen), or (R" is 0-A1A and LG is a nitro group), or (R" is 0-A1A and LG is a trialkylammonium group), or (R" is 0-A1A and LG is a aryl iodonium group), or (R" is 0-A1A and LG is a diaryl sulfonium group), or (R" is NA BA C and LG is a halogen), or (R" is NA1BA C and LG is a nitro group), or (R" is NA1BA1 C and LG is a trialkylammonium group), or (R" is NA1BA1 C and LG is a aryl iodonium group), or (R" is NA BA C and LG is a diaryl sulfonium group); and wherein
A1A, A1B, A1C are selected from the group consisting of:
a) hydrogen,
b) branched or non-branched (C1 -C5)alkyl,
c) branched or non-branched (C3-C5)alkenyl, with the proviso that A1A, A A1C are not attached to O or N with a sp2 hybridized carbon atom, d) branched or non-branched (C3-C5)alkynyl, with the proviso that A1A, A A1C are not attached to O or N with a sp hybridized carbon atom, e) PG, being a protecting group,
f) (C1 -C5)alkyl-[0-(C1 -C5)alkyl]n, wherein n is 1-5; or A1B and A1C together are group that is selected from groups comprising: a) -(CH2)m-,
b) -(CH2)2-0-(CH2)2-, c) -(CH2)2-NA A-(CH2)2-. wherein m is 2-5.
7. A compound according to claim 6, wherein:
((R" is OPG) and (LG is chloro, iodo or bromo)), or (R" is OMe) and (LG is chloro, iodo)), or
he compound is 2-bromo-6-[(E)-2-(4-methoxyphenyl)vinyl]pyridine, or he compound is 2-bromo-3-[(E)-2-(4-methoxyphenyl)vinyl]pyridine, or he compound is 2-bromo-5-[(E)-2-(4-methoxyphenyl)vinyl]pyridine, or
((R' ' is OPG or OMe) and (LG is a nitro group)), or
((R" is OPG or OMe) and (LG is a trimethylammonium group)), or
((R" is OPG or OMe) and (LG is a aryl iodonium group)), or
((R" is OPG or OMe) and (LG is a diaryl sulfonium group)), or
((R" is NPGH) and (LG is chloro, iodo or bromo)), or
((R" is NPGH) and (LG is a nitro group)), or
((R" is NPGH) and (LG is a trimethylammonium group)), or
((R" is NPGH) and (LG is a aryl iodonium group)), or
((R" is NPGH) and (LG is a diaryl sulfonium group)), or
((R" is NPG2) and (LG is chloro, iodo or bromo)), or
((R" is NPG2) and (LG is a nitro group)), or
((R" is NPG2) and (LG is a trimethylammonium group)), or
((R" is NPG2) and (LG is a aryl iodonium group)), or
((R" is NPG2) and (LG is a diaryl sulfonium group)), or
((R" is NPGMe) and (LG is chloro, iodo or bromo)), or
((R" is NPGMe) and (LG is a nitro group)), or
((R" is NPGMe) and (LG is a trimethylammonium group)), or
((R" is NPGMe) and (LG is a aryl iodonium group)), or
((R" is NPGMe) and (LG is a diaryl sulfonium group)), or
((R" is NMe2) and (LG is chloro, iodo or bromo)), or
((R" is NMe2) and (LG is a nitro group)), or ((R" is NMe2) and (LG is a trimethylammonium group)), or ((R" is ΝΜθ2) and (LG is a aryl iodonium group)), or ((R" is NMe2) and (LG is a diaryl sulfonium group)).
8. A compound according to claim 6 or 7, wherein PG is selected from the group comprising: a) Boc,
b) Methoxymethyl,
c) Acetyl,
d) Trityl,
e) Fmoc.
9. A compound according to claim 6, selected from the group of compounds consisting of
tert-butyl {4-[(E)-2-(6-bromopyridin-3-yl)vinyl]phenyl}methylcarbamate,
N,N-dimethyl-4-[(E)-2-(6-bromopyridin-3-yl)vinyl]aniline,
N,N-dimethyl-4-[(E)-2-(6-nitropyridin-3-yl)vinyl]aniline, O
H3C^<
H3C CH3
tert-butyl {4-[(E)-2-(2-bromopyridin-4-yl)vinyl]phenyl}methylcarbamate,
H
4-[(E)-2-(2-bromopyridin-4-yl)vinyl]-N,N-dimethylaniline,
tert-butyl {4-[(E)-2-(6-chloropyridin-2-yl)vinyl]phenyl}methylcarbamate, O
H3C^(
H3C CH3
4-[(E)-2-(6-iodopyridin-2-yl)vinyl]-N,N-dimethylaniline,
4-[(E)-2-(2-chloropyridin-3-yl)vinyl]-N,N-dimethylaniline,
4-[(E)-2-(2-iodopyridin-3-yl)vinyl]-N,N-dimethylaniline,
4-[(E)-2-(2-nitropyridin-3-yl)vinyl]-N,N-dimethylaniline.
10. A method of manufacturing a compound of claim 4 or 5, wherein a suitable precursor compound of claims 6-9 is reacted with a 18F fluorination agent.
1 1 . A method according to claim 10, wherein a compound of claim 5 is prepared by reacting a suitable precursor compound of claim 9 with a 18F fluorination agent.
12. A compound according to claims 4 or 5 as a diagnostic compound.
13. A compound according to claims 4 or 5 as a diagnostic compound for diagnosing Alzheimer's disease.
14. A kit comprising at least one sealed vial comprising a compound according to claims 6 - 9.
15. A kit according to claim 14, comprising at least one sealed vial comprising a compound according to claim 9.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP10162836 | 2010-05-14 | ||
| EP10162836.0 | 2010-05-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011141515A1 true WO2011141515A1 (en) | 2011-11-17 |
Family
ID=44245070
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2011/057630 WO2011141515A1 (en) | 2010-05-14 | 2011-05-11 | Diagnostic agents for amyloid beta imaging |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2011141515A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013144301A3 (en) * | 2012-03-30 | 2013-11-21 | Ge Healthcare Limited | Easily purifiable synthon composition |
| JP2019531299A (en) * | 2016-09-30 | 2019-10-31 | オシェラ インコーポレイテッドOzchela Inc. | Stilbene derivative and method for producing the same |
| CN111499570A (en) * | 2019-01-31 | 2020-08-07 | 中国医学科学院放射医学研究所 | Synthetic method of AV-45 intermediate |
| CN112375042A (en) * | 2020-10-27 | 2021-02-19 | 安徽医科大学 | Trimethoxy styryl six-membered ring and pyrazolopyrimidine compound, preparation and application thereof |
Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS61210363A (en) * | 1985-03-15 | 1986-09-18 | Canon Inc | Electrophotographic sensitive body |
| WO1999033793A2 (en) | 1997-12-24 | 1999-07-08 | Vertex Pharmaceuticals Incorporated | Prodrugs of aspartyl protease inhibitors |
| WO1999033815A1 (en) | 1997-12-24 | 1999-07-08 | Vertex Pharmaceuticals Incorporated | Sulphonamide derivatives as prodrugs of aspartyl protease inhibitors |
| WO1999033795A1 (en) | 1997-12-24 | 1999-07-08 | Vertex Pharmaceuticals Incorporated | Prodrugs of aspartyl protease inhibitors |
| WO1999033792A2 (en) | 1997-12-24 | 1999-07-08 | Vertex Pharmaceuticals Incorporated | Prodrugs os aspartyl protease inhibitors |
| WO2003018070A1 (en) * | 2001-08-27 | 2003-03-06 | The Trustees Of The University Of Pennsylvania | Stilbene derivatives and their use for binding and imaging amyloid plaques |
| WO2005061415A1 (en) | 2003-12-23 | 2005-07-07 | Ge Healthcare Limited | Radical trap in fluoridation of iodonium salt |
| WO2005097713A1 (en) | 2004-04-08 | 2005-10-20 | Ge Healthcare Limited | Fluoridation method |
| WO2007010534A2 (en) | 2005-07-19 | 2007-01-25 | Spectrum Dynamics Llc | Imaging protocols |
| WO2007073200A1 (en) | 2005-12-21 | 2007-06-28 | Hammersmith Imanet Limited | Pet radiotracers |
| WO2007126733A2 (en) | 2006-03-30 | 2007-11-08 | The Trustees Of The University Of Pennsylvania | Styrylpyridine derivatives and their use for binding and imaging amyloid plaques |
| WO2007141529A1 (en) | 2006-06-09 | 2007-12-13 | Ge Healthcare Limited | Fluoridation method |
| WO2010011964A2 (en) * | 2008-07-24 | 2010-01-28 | Siemens Medical Solutions Usa, Inc. | Imaging agents useful for identifying ad pathology |
| US20100101643A1 (en) * | 2007-03-29 | 2010-04-29 | Sumitomo Chemical Company, Limited | Compound, photoelectric converter and photoelectrochemical cell |
| KR20100121204A (en) * | 2009-05-08 | 2010-11-17 | 고려대학교 산학협력단 | Novel amphiphilic compounds, methods of preparing the compounds and dye-sensitized solar cells using dyes comprising the compounds |
-
2011
- 2011-05-11 WO PCT/EP2011/057630 patent/WO2011141515A1/en active Application Filing
Patent Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS61210363A (en) * | 1985-03-15 | 1986-09-18 | Canon Inc | Electrophotographic sensitive body |
| WO1999033793A2 (en) | 1997-12-24 | 1999-07-08 | Vertex Pharmaceuticals Incorporated | Prodrugs of aspartyl protease inhibitors |
| WO1999033815A1 (en) | 1997-12-24 | 1999-07-08 | Vertex Pharmaceuticals Incorporated | Sulphonamide derivatives as prodrugs of aspartyl protease inhibitors |
| WO1999033795A1 (en) | 1997-12-24 | 1999-07-08 | Vertex Pharmaceuticals Incorporated | Prodrugs of aspartyl protease inhibitors |
| WO1999033792A2 (en) | 1997-12-24 | 1999-07-08 | Vertex Pharmaceuticals Incorporated | Prodrugs os aspartyl protease inhibitors |
| WO2003018070A1 (en) * | 2001-08-27 | 2003-03-06 | The Trustees Of The University Of Pennsylvania | Stilbene derivatives and their use for binding and imaging amyloid plaques |
| WO2005061415A1 (en) | 2003-12-23 | 2005-07-07 | Ge Healthcare Limited | Radical trap in fluoridation of iodonium salt |
| WO2005097713A1 (en) | 2004-04-08 | 2005-10-20 | Ge Healthcare Limited | Fluoridation method |
| WO2007010534A2 (en) | 2005-07-19 | 2007-01-25 | Spectrum Dynamics Llc | Imaging protocols |
| WO2007073200A1 (en) | 2005-12-21 | 2007-06-28 | Hammersmith Imanet Limited | Pet radiotracers |
| WO2007126733A2 (en) | 2006-03-30 | 2007-11-08 | The Trustees Of The University Of Pennsylvania | Styrylpyridine derivatives and their use for binding and imaging amyloid plaques |
| WO2007141529A1 (en) | 2006-06-09 | 2007-12-13 | Ge Healthcare Limited | Fluoridation method |
| US20100101643A1 (en) * | 2007-03-29 | 2010-04-29 | Sumitomo Chemical Company, Limited | Compound, photoelectric converter and photoelectrochemical cell |
| WO2010011964A2 (en) * | 2008-07-24 | 2010-01-28 | Siemens Medical Solutions Usa, Inc. | Imaging agents useful for identifying ad pathology |
| KR20100121204A (en) * | 2009-05-08 | 2010-11-17 | 고려대학교 산학협력단 | Novel amphiphilic compounds, methods of preparing the compounds and dye-sensitized solar cells using dyes comprising the compounds |
Non-Patent Citations (23)
| Title |
|---|
| B. E. MARYANOFF, REITZ, A. B., CHEM. REV., vol. 89, 1989, pages 863 - 927 |
| BLENNOW ET AL., LANCET, vol. 368, no. 9533, 29 July 2006 (2006-07-29), pages 387 - 403 |
| CHITI ET AL., ANNU REV BIOCHEM., vol. 75, 2006, pages 333 - 66 |
| CHOI ET AL., J NUCL MED., vol. 50, no. 11, pages 1887 - 94 |
| COENEN: "PET-Chemistry - The Driving Force in Molecular Imaging", 2006, SPRINGER, article "Fluorine-18 Labeling Methods: Features and Possibilities of Basic Reactions", pages: 15 - 50 |
| DATABASE CA [online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 18 September 1986 (1986-09-18), MATSUMOTO, M.: "Electrophotographic photoreceptor", XP002651245, retrieved from STN Database accession no. 1987:147085 * |
| DATABASE CA [online] CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; 8 May 2009 (2009-05-08), KO ET AL: "Production of ruthenium complex amphipathic compound as dye for dye-sensitized solar cell", XP002651244, retrieved from STN Database accession no. 2010:1450746 * |
| FARRAR ET AL., TURKU PET SYMPOSIUM |
| GOODMAN, GILMAN: "Biotransformation of Drugs", 1992, MCGRAW-HIM, article "The Pharmaco- logical Basis of Therapeutics", pages: 13 - 15 |
| GREENE, WUTS: "Protecting groups in Organic Synthesis", pages: 17 - 245 |
| KELLY, S. E., COMP. ORG. SYN., vol. 1, 1991, pages 729 - 817 |
| KLUNK ET AL., ANN NEUROL., vol. 55, 2004, pages 306 - 319 |
| KUDO ET AL., J NUCL. MED, vol. 49, 2007, pages 554 - 561 |
| M. HOOPER ET AL: "Preparation and antibacterial activity of isatogens and related compounds", JOURNAL OF PHARMACY AND PHARMACOLOGY, vol. 17, no. 11, 1 November 1965 (1965-11-01), pages 734 - 741, XP055002988, ISSN: 0022-3573, DOI: 10.1111/j.2042-7158.1965.tb07596.x * |
| MIYAURA, N., SUZUKI, A, CHEM REV., vol. 95, 1995, pages 2457 - 2483 |
| NORDBERG, EUR J NUCL MED MOL IMAGING, vol. 35, no. 1, March 2008 (2008-03-01), pages 46 - 50 |
| ONO M ET AL: "Synthesis and biological evaluation of (E)-3-styrylpyridine derivatives as amyloid imaging agents for Alzheimer's disease", NUCLEAR MEDICINE AND BIOLOGY, ELSEVIER, NY, US, vol. 32, no. 4, 1 May 2005 (2005-05-01), pages 329 - 335, XP004881117, ISSN: 0969-8051, DOI: DOI:10.1016/J.NUCMEDBIO.2005.01.006 * |
| PETRELLA JR ET AL., RADIOLOGY, vol. 226, 2003, pages 315 - 36 |
| SHOGHI-JADID ET AL., AM J GERIATR PSYCHIATRY, vol. 10, 2002, pages 24 - 35 |
| STEPHENSON ET AL., BIOCONJUG CHEM., vol. 18, no. 1, January 2007 (2007-01-01), pages 238 - 46 |
| VERHOEFF ET AL., AM J GERIATR PSYCHIATRY, vol. 12, 2004, pages 584 - 595 |
| YIYUAN PENG ET AL: "Highly Efficient N -Monomethylation of Primary Aryl Amines", CHINESE JOURNAL OF CHEMISTRY, vol. 27, no. 7, 1 July 2009 (2009-07-01), pages 1339 - 1344, XP055002998, ISSN: 1001-604X, DOI: 10.1002/cjoc.200990224 * |
| ZHANG ET AL., NUCL MED BIOL., vol. 34, no. 1, January 2007 (2007-01-01), pages 89 - 97 |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013144301A3 (en) * | 2012-03-30 | 2013-11-21 | Ge Healthcare Limited | Easily purifiable synthon composition |
| JP2019531299A (en) * | 2016-09-30 | 2019-10-31 | オシェラ インコーポレイテッドOzchela Inc. | Stilbene derivative and method for producing the same |
| CN111499570A (en) * | 2019-01-31 | 2020-08-07 | 中国医学科学院放射医学研究所 | Synthetic method of AV-45 intermediate |
| CN111499570B (en) * | 2019-01-31 | 2023-05-16 | 中国医学科学院放射医学研究所 | Synthesis method of AV-45 intermediate |
| CN112375042A (en) * | 2020-10-27 | 2021-02-19 | 安徽医科大学 | Trimethoxy styryl six-membered ring and pyrazolopyrimidine compound, preparation and application thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2009278279B2 (en) | DAA-pyridine as peripheral benzodiazepine receptor ligand for diagnostic imaging and pharmaceutical treatment | |
| EP2501696B1 (en) | Imaging agents and their use for the diagnostic in vivo of neurodegenerative diseases, notably alzheimer's disease and derivative diseases | |
| Pichika et al. | Nicotinic α4β2 receptor imaging agents: part II. Synthesis and biological evaluation of 2-[18F] fluoro-3-[2-((S)-3-pyrrolinyl) methoxy] pyridine (18F-nifene) in rodents and imaging by PET in nonhuman primate | |
| US7772256B2 (en) | 2-heteroaryl substituted benzothiophenes and benzofuranes 709 | |
| EP3055308A1 (en) | Diazacarbazole derivatives as tau-pet-ligands | |
| CN109475648B (en) | Compounds for imaging TAU protein aggregates | |
| CN111712265B (en) | Diagnostic composition for PET imaging, method for producing the same and use thereof in diagnosis | |
| US20120264943A1 (en) | Compounds Suitable As Precursors to Compounds that are Useful for Imaging Amyloid Deposits | |
| WO2011141515A1 (en) | Diagnostic agents for amyloid beta imaging | |
| JP2010520275A (en) | Novel 2-heteroaryl substituted indole 695 | |
| JP2021512064A (en) | A new method for preparing imaging compounds | |
| JP7059270B2 (en) | Compounds for imaging tau protein aggregates | |
| CA2911307C (en) | Use of fluorinated derivatives of 4-aminopyridine in therapeutics and medical imaging | |
| Mori et al. | Radiosynthesis and evaluation of 4-(6-[18 F] Fluoro-4-(5-isopropoxy-1 H-indazol-3-yl) pyridin-2-yl) morpholine as a novel radiotracer candidate targeting leucine-rich repeat kinase 2 | |
| Milicevic Sephton et al. | Synthesis and biological evaluation of quinoxaline derivatives for PET imaging of the NMDA receptor | |
| JP7284490B2 (en) | Monoamine oxidase B imaging probe | |
| RU2778739C2 (en) | Compounds for imaging of tau protein aggregates | |
| US20100278751A1 (en) | Radiolabeled 2-amino-4-alkyl-6-(haloalkyl)pyridine compounds and their use in diagnostic imaging | |
| EP4329826A1 (en) | Deuterated compounds and imaging agents for imaging huntingtin protein | |
| RU2472789C2 (en) | Novel 2-heteroaryl-substituted benzothiophenes and benzofurans 709 | |
| WO2013040183A1 (en) | Beta-amyloid imaging agents, methods of manufacture, and methods of use thereof | |
| EA042728B1 (en) | A NEW METHOD FOR OBTAINING A VISUALIZING COMPOUND | |
| KR20130088117A (en) | Method for production of f-18 labeled amyloid beta ligand | |
| JP2019528249A (en) | Tau PET imaging ligand | |
| US20120237446A1 (en) | Compounds for binding and imaging amyloid plaques and their use |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11719009 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11719009 Country of ref document: EP Kind code of ref document: A1 |