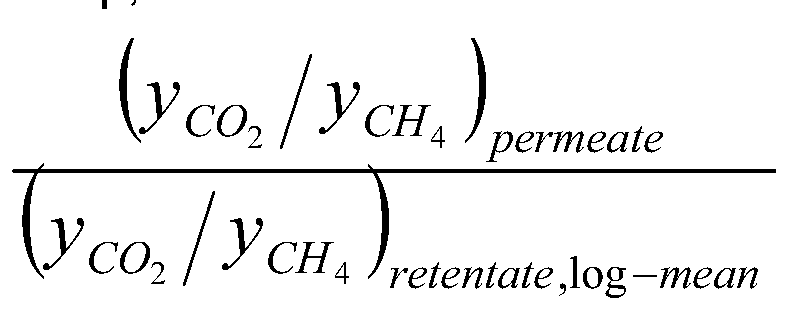WO2011072215A1 - High-flux sapo-34 membranes for co2/ch4 separations - Google Patents
High-flux sapo-34 membranes for co2/ch4 separations Download PDFInfo
- Publication number
- WO2011072215A1 WO2011072215A1 PCT/US2010/059874 US2010059874W WO2011072215A1 WO 2011072215 A1 WO2011072215 A1 WO 2011072215A1 US 2010059874 W US2010059874 W US 2010059874W WO 2011072215 A1 WO2011072215 A1 WO 2011072215A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- sapo
- membrane
- porous support
- support
- membranes
- Prior art date
Links
- 239000012528 membrane Substances 0.000 title claims abstract description 371
- 238000000926 separation method Methods 0.000 title claims abstract description 37
- 238000000034 method Methods 0.000 claims abstract description 95
- 238000002791 soaking Methods 0.000 claims abstract description 89
- 238000003618 dip coating Methods 0.000 claims abstract description 23
- 239000013078 crystal Substances 0.000 claims description 217
- 229910002092 carbon dioxide Inorganic materials 0.000 claims description 107
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 claims description 83
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical group O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 claims description 79
- 239000011148 porous material Substances 0.000 claims description 65
- 239000001569 carbon dioxide Substances 0.000 claims description 58
- 239000003795 chemical substances by application Substances 0.000 claims description 56
- 239000000203 mixture Substances 0.000 claims description 51
- 239000000463 material Substances 0.000 claims description 45
- 239000007789 gas Substances 0.000 claims description 41
- 229910001868 water Inorganic materials 0.000 claims description 26
- 229940073455 tetraethylammonium hydroxide Drugs 0.000 claims description 24
- LRGJRHZIDJQFCL-UHFFFAOYSA-M tetraethylazanium;hydroxide Chemical group [OH-].CC[N+](CC)(CC)CC LRGJRHZIDJQFCL-UHFFFAOYSA-M 0.000 claims description 24
- 238000010438 heat treatment Methods 0.000 claims description 21
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 claims description 19
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 19
- 229910052782 aluminium Inorganic materials 0.000 claims description 14
- 238000001354 calcination Methods 0.000 claims description 14
- 229910052710 silicon Inorganic materials 0.000 claims description 14
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical group O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 claims description 12
- 239000010703 silicon Substances 0.000 claims description 11
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 claims description 10
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 10
- 239000012466 permeate Substances 0.000 claims description 10
- 229910052698 phosphorus Inorganic materials 0.000 claims description 9
- 239000000919 ceramic Substances 0.000 claims description 8
- 229910052760 oxygen Inorganic materials 0.000 claims description 8
- 238000007598 dipping method Methods 0.000 claims description 7
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 claims description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 6
- 239000011574 phosphorus Substances 0.000 claims description 6
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims description 5
- 125000004432 carbon atom Chemical group C* 0.000 claims description 5
- 239000001301 oxygen Substances 0.000 claims description 5
- 125000003277 amino group Chemical group 0.000 claims 1
- 238000010899 nucleation Methods 0.000 abstract description 37
- 239000002808 molecular sieve Substances 0.000 abstract description 29
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 abstract description 29
- 238000001027 hydrothermal synthesis Methods 0.000 abstract description 26
- 238000011282 treatment Methods 0.000 abstract description 26
- 230000004907 flux Effects 0.000 abstract description 15
- 241000269350 Anura Species 0.000 abstract 2
- 239000000499 gel Substances 0.000 description 142
- 230000015572 biosynthetic process Effects 0.000 description 104
- 239000010410 layer Substances 0.000 description 84
- 238000003786 synthesis reaction Methods 0.000 description 82
- 239000000243 solution Substances 0.000 description 50
- 239000010457 zeolite Substances 0.000 description 41
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 37
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 37
- 229910021536 Zeolite Inorganic materials 0.000 description 36
- 230000032683 aging Effects 0.000 description 24
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 16
- 150000001412 amines Chemical class 0.000 description 16
- 238000001878 scanning electron micrograph Methods 0.000 description 14
- WEHWNAOGRSTTBQ-UHFFFAOYSA-N dipropylamine Chemical compound CCCNCCC WEHWNAOGRSTTBQ-UHFFFAOYSA-N 0.000 description 13
- 238000011065 in-situ storage Methods 0.000 description 13
- 239000002245 particle Substances 0.000 description 13
- 238000009826 distribution Methods 0.000 description 12
- 239000000377 silicon dioxide Substances 0.000 description 12
- 230000000694 effects Effects 0.000 description 11
- LPSKDVINWQNWFE-UHFFFAOYSA-M tetrapropylazanium;hydroxide Chemical compound [OH-].CCC[N+](CCC)(CCC)CCC LPSKDVINWQNWFE-UHFFFAOYSA-M 0.000 description 11
- 238000002425 crystallisation Methods 0.000 description 10
- 230000008025 crystallization Effects 0.000 description 10
- 230000012010 growth Effects 0.000 description 10
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 229910052681 coesite Inorganic materials 0.000 description 9
- 229910052906 cristobalite Inorganic materials 0.000 description 9
- 229910052682 stishovite Inorganic materials 0.000 description 9
- 229910052905 tridymite Inorganic materials 0.000 description 9
- 125000004430 oxygen atom Chemical group O* 0.000 description 8
- 125000001453 quaternary ammonium group Chemical group 0.000 description 8
- VDZOOKBUILJEDG-UHFFFAOYSA-M tetrabutylammonium hydroxide Chemical compound [OH-].CCCC[N+](CCCC)(CCCC)CCCC VDZOOKBUILJEDG-UHFFFAOYSA-M 0.000 description 8
- 229910052799 carbon Inorganic materials 0.000 description 7
- 150000001768 cations Chemical class 0.000 description 7
- 230000007423 decrease Effects 0.000 description 7
- 230000007547 defect Effects 0.000 description 7
- 238000009792 diffusion process Methods 0.000 description 7
- -1 quaternary ammonium cations Chemical class 0.000 description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 6
- 229910019142 PO4 Inorganic materials 0.000 description 6
- 235000021317 phosphate Nutrition 0.000 description 6
- 239000004809 Teflon Substances 0.000 description 5
- 229920006362 Teflon® Polymers 0.000 description 5
- 239000011230 binding agent Substances 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 239000013335 mesoporous material Substances 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 230000006911 nucleation Effects 0.000 description 5
- 239000002904 solvent Substances 0.000 description 5
- 239000010935 stainless steel Substances 0.000 description 5
- 229910001220 stainless steel Inorganic materials 0.000 description 5
- 239000000758 substrate Substances 0.000 description 5
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical group OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 4
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 4
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 4
- 239000007864 aqueous solution Substances 0.000 description 4
- HUCVOHYBFXVBRW-UHFFFAOYSA-M caesium hydroxide Chemical compound [OH-].[Cs+] HUCVOHYBFXVBRW-UHFFFAOYSA-M 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- 239000002178 crystalline material Substances 0.000 description 4
- PAFZNILMFXTMIY-UHFFFAOYSA-N cyclohexylamine Chemical compound NC1CCCCC1 PAFZNILMFXTMIY-UHFFFAOYSA-N 0.000 description 4
- 230000000873 masking effect Effects 0.000 description 4
- 239000012229 microporous material Substances 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 235000015097 nutrients Nutrition 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 4
- 239000010452 phosphate Substances 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- CPRMKOQKXYSDML-UHFFFAOYSA-M rubidium hydroxide Chemical compound [OH-].[Rb+] CPRMKOQKXYSDML-UHFFFAOYSA-M 0.000 description 4
- 230000035040 seed growth Effects 0.000 description 4
- 241000894007 species Species 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- JRMUNVKIHCOMHV-UHFFFAOYSA-M tetrabutylammonium bromide Chemical compound [Br-].CCCC[N+](CCCC)(CCCC)CCCC JRMUNVKIHCOMHV-UHFFFAOYSA-M 0.000 description 4
- HWCKGOZZJDHMNC-UHFFFAOYSA-M tetraethylammonium bromide Chemical compound [Br-].CC[N+](CC)(CC)CC HWCKGOZZJDHMNC-UHFFFAOYSA-M 0.000 description 4
- YMBCJWGVCUEGHA-UHFFFAOYSA-M tetraethylammonium chloride Chemical compound [Cl-].CC[N+](CC)(CC)CC YMBCJWGVCUEGHA-UHFFFAOYSA-M 0.000 description 4
- DLYUQMMRRRQYAE-UHFFFAOYSA-N tetraphosphorus decaoxide Chemical compound O1P(O2)(=O)OP3(=O)OP1(=O)OP2(=O)O3 DLYUQMMRRRQYAE-UHFFFAOYSA-N 0.000 description 4
- BGQMOFGZRJUORO-UHFFFAOYSA-M tetrapropylammonium bromide Chemical compound [Br-].CCC[N+](CCC)(CCC)CCC BGQMOFGZRJUORO-UHFFFAOYSA-M 0.000 description 4
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 3
- 238000002441 X-ray diffraction Methods 0.000 description 3
- COHDHYZHOPQOFD-UHFFFAOYSA-N arsenic pentoxide Inorganic materials O=[As](=O)O[As](=O)=O COHDHYZHOPQOFD-UHFFFAOYSA-N 0.000 description 3
- 230000004888 barrier function Effects 0.000 description 3
- 230000002902 bimodal effect Effects 0.000 description 3
- 230000001186 cumulative effect Effects 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 3
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 3
- 229940071676 hydroxypropylcellulose Drugs 0.000 description 3
- 238000007654 immersion Methods 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 239000002923 metal particle Substances 0.000 description 3
- 239000003345 natural gas Substances 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 235000011007 phosphoric acid Nutrition 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 239000002356 single layer Substances 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 229910011255 B2O3 Inorganic materials 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- MWRWFPQBGSZWNV-UHFFFAOYSA-N Dinitrosopentamethylenetetramine Chemical compound C1N2CN(N=O)CN1CN(N=O)C2 MWRWFPQBGSZWNV-UHFFFAOYSA-N 0.000 description 2
- FUJCRWPEOMXPAD-UHFFFAOYSA-N Li2O Inorganic materials [Li+].[Li+].[O-2] FUJCRWPEOMXPAD-UHFFFAOYSA-N 0.000 description 2
- DJEQZVQFEPKLOY-UHFFFAOYSA-N N,N-dimethylbutylamine Chemical compound CCCCN(C)C DJEQZVQFEPKLOY-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- BOTDANWDWHJENH-UHFFFAOYSA-N Tetraethyl orthosilicate Chemical compound CCO[Si](OCC)(OCC)OCC BOTDANWDWHJENH-UHFFFAOYSA-N 0.000 description 2
- 239000004964 aerogel Substances 0.000 description 2
- 125000003158 alcohol group Chemical group 0.000 description 2
- LTPBRCUWZOMYOC-UHFFFAOYSA-N beryllium oxide Inorganic materials O=[Be] LTPBRCUWZOMYOC-UHFFFAOYSA-N 0.000 description 2
- 229910002056 binary alloy Inorganic materials 0.000 description 2
- 244000309464 bull Species 0.000 description 2
- UNYSKUBLZGJSLV-UHFFFAOYSA-L calcium;1,3,5,2,4,6$l^{2}-trioxadisilaluminane 2,4-dioxide;dihydroxide;hexahydrate Chemical compound O.O.O.O.O.O.[OH-].[OH-].[Ca+2].O=[Si]1O[Al]O[Si](=O)O1.O=[Si]1O[Al]O[Si](=O)O1 UNYSKUBLZGJSLV-UHFFFAOYSA-L 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- IVMYJDGYRUAWML-UHFFFAOYSA-N cobalt(II) oxide Inorganic materials [Co]=O IVMYJDGYRUAWML-UHFFFAOYSA-N 0.000 description 2
- 239000008119 colloidal silica Substances 0.000 description 2
- 238000000151 deposition Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- QZQVBEXLDFYHSR-UHFFFAOYSA-N gallium(III) oxide Inorganic materials O=[Ga]O[Ga]=O QZQVBEXLDFYHSR-UHFFFAOYSA-N 0.000 description 2
- 230000014509 gene expression Effects 0.000 description 2
- PVADDRMAFCOOPC-UHFFFAOYSA-N germanium monoxide Inorganic materials [Ge]=O PVADDRMAFCOOPC-UHFFFAOYSA-N 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N iron oxide Inorganic materials [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 2
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 2
- VASIZKWUTCETSD-UHFFFAOYSA-N manganese(II) oxide Inorganic materials [Mn]=O VASIZKWUTCETSD-UHFFFAOYSA-N 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012856 packing Methods 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 150000003856 quaternary ammonium compounds Chemical group 0.000 description 2
- 238000010900 secondary nucleation Methods 0.000 description 2
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 2
- 238000007569 slipcasting Methods 0.000 description 2
- 238000001179 sorption measurement Methods 0.000 description 2
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N zinc oxide Inorganic materials [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- ZOAMZFNAPHWBEN-UHFFFAOYSA-N 2-$l^{1}-oxidanylpropane Chemical compound CC(C)[O] ZOAMZFNAPHWBEN-UHFFFAOYSA-N 0.000 description 1
- BSFODEXXVBBYOC-UHFFFAOYSA-N 8-[4-(dimethylamino)butan-2-ylamino]quinolin-6-ol Chemical compound C1=CN=C2C(NC(CCN(C)C)C)=CC(O)=CC2=C1 BSFODEXXVBBYOC-UHFFFAOYSA-N 0.000 description 1
- 235000007119 Ananas comosus Nutrition 0.000 description 1
- 244000099147 Ananas comosus Species 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- 229920001479 Hydroxyethyl methyl cellulose Polymers 0.000 description 1
- 241001112867 Jafar Species 0.000 description 1
- UEEJHVSXFDXPFK-UHFFFAOYSA-N N-dimethylaminoethanol Chemical compound CN(C)CCO UEEJHVSXFDXPFK-UHFFFAOYSA-N 0.000 description 1
- 241000201976 Polycarpon Species 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 229910052910 alkali metal silicate Inorganic materials 0.000 description 1
- 150000003973 alkyl amines Chemical group 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- SMZOGRDCAXLAAR-UHFFFAOYSA-N aluminium isopropoxide Chemical compound [Al+3].CC(C)[O-].CC(C)[O-].CC(C)[O-] SMZOGRDCAXLAAR-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical compound Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 1
- VXAUWWUXCIMFIM-UHFFFAOYSA-M aluminum;oxygen(2-);hydroxide Chemical compound [OH-].[O-2].[Al+3] VXAUWWUXCIMFIM-UHFFFAOYSA-M 0.000 description 1
- ANBBXQWFNXMHLD-UHFFFAOYSA-N aluminum;sodium;oxygen(2-) Chemical compound [O-2].[O-2].[Na+].[Al+3] ANBBXQWFNXMHLD-UHFFFAOYSA-N 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000001680 brushing effect Effects 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 238000005341 cation exchange Methods 0.000 description 1
- 229920003086 cellulose ether Polymers 0.000 description 1
- 229910052676 chabazite Inorganic materials 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 239000000306 component Substances 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 239000007822 coupling agent Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- XUCJHNOBJLKZNU-UHFFFAOYSA-M dilithium;hydroxide Chemical compound [Li+].[Li+].[OH-] XUCJHNOBJLKZNU-UHFFFAOYSA-M 0.000 description 1
- 239000012972 dimethylethanolamine Substances 0.000 description 1
- KZHJGOXRZJKJNY-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Si]=O.O=[Al]O[Al]=O.O=[Al]O[Al]=O.O=[Al]O[Al]=O KZHJGOXRZJKJNY-UHFFFAOYSA-N 0.000 description 1
- 238000000921 elemental analysis Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 229920002313 fluoropolymer Polymers 0.000 description 1
- 239000004811 fluoropolymer Substances 0.000 description 1
- 229910021485 fumed silica Inorganic materials 0.000 description 1
- 229910001679 gibbsite Inorganic materials 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 229910000856 hastalloy Inorganic materials 0.000 description 1
- 239000012456 homogeneous solution Substances 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 238000005216 hydrothermal crystallization Methods 0.000 description 1
- 238000010335 hydrothermal treatment Methods 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 229940071826 hydroxyethyl cellulose Drugs 0.000 description 1
- 229920003063 hydroxymethyl cellulose Polymers 0.000 description 1
- 229940031574 hydroxymethyl cellulose Drugs 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 229910001026 inconel Inorganic materials 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 239000011147 inorganic material Substances 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 229910052863 mullite Inorganic materials 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 238000005373 pervaporation Methods 0.000 description 1
- 230000010287 polarization Effects 0.000 description 1
- 229920005597 polymer membrane Polymers 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 239000011164 primary particle Substances 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000004626 scanning electron microscopy Methods 0.000 description 1
- 150000003335 secondary amines Chemical group 0.000 description 1
- 230000034655 secondary growth Effects 0.000 description 1
- 238000010942 self-nucleation Methods 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910052604 silicate mineral Inorganic materials 0.000 description 1
- HBMJWWWQQXIZIP-UHFFFAOYSA-N silicon carbide Chemical compound [Si+]#[C-] HBMJWWWQQXIZIP-UHFFFAOYSA-N 0.000 description 1
- 229910010271 silicon carbide Inorganic materials 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 229910001388 sodium aluminate Inorganic materials 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 238000004528 spin coating Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- 150000003512 tertiary amines Chemical group 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000007669 thermal treatment Methods 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- DQWPFSLDHJDLRL-UHFFFAOYSA-N triethyl phosphate Chemical compound CCOP(=O)(OCC)OCC DQWPFSLDHJDLRL-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D71/00—Semi-permeable membranes for separation processes or apparatus characterised by the material; Manufacturing processes specially adapted therefor
- B01D71/02—Inorganic material
- B01D71/028—Molecular sieves
- B01D71/0281—Zeolites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D71/00—Semi-permeable membranes for separation processes or apparatus characterised by the material; Manufacturing processes specially adapted therefor
- B01D71/02—Inorganic material
- B01D71/028—Molecular sieves
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/22—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by diffusion
- B01D53/228—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by diffusion characterised by specific membranes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D67/00—Processes specially adapted for manufacturing semi-permeable membranes for separation processes or apparatus
- B01D67/0039—Inorganic membrane manufacture
- B01D67/0051—Inorganic membrane manufacture by controlled crystallisation, e,.g. hydrothermal growth
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2256/00—Main component in the product gas stream after treatment
- B01D2256/24—Hydrocarbons
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/50—Carbon oxides
- B01D2257/504—Carbon dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2323/00—Details relating to membrane preparation
- B01D2323/08—Specific temperatures applied
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2323/00—Details relating to membrane preparation
- B01D2323/08—Specific temperatures applied
- B01D2323/081—Heating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2323/00—Details relating to membrane preparation
- B01D2323/12—Specific ratios of components used
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A40/00—Adaptation technologies in agriculture, forestry, livestock or agroalimentary production
- Y02A40/10—Adaptation technologies in agriculture, forestry, livestock or agroalimentary production in agriculture
- Y02A40/28—Adaptation technologies in agriculture, forestry, livestock or agroalimentary production in agriculture specially adapted for farming
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02C—CAPTURE, STORAGE, SEQUESTRATION OR DISPOSAL OF GREENHOUSE GASES [GHG]
- Y02C20/00—Capture or disposal of greenhouse gases
- Y02C20/40—Capture or disposal of greenhouse gases of CO2
Definitions
- This invention is in the field of molecular sieve membranes, in particular silicoaluminophosphate (SAPO) membranes, prepared on a porous support.
- SAPO silicoaluminophosphate
- the invention provides supported molecular sieve membranes having improved gas separation properties as well as methods for making and using them.
- Molecular sieve membranes including SAPO membranes, are often used in gas separations.
- an important parameter is the separation selectivity.
- a separation selectivity S, / j greater than one implies that the membrane is selectively permeable to component i. If a feedstream containing both components is applied to one side of the membrane, the permeate stream exiting the other side of the membrane will be enriched in component i and depleted in component j. The greater the separation selectivity, the greater the enrichment of the permeate stream in component i.
- Engstrom and co-workers prepared silicalite-1 film on gold surface using 60, 165 and 320 nm zeolite seeds, respectively. They found that the grain packing was better and the film was less rough when the smaller seeds were used (Engstrom et al, Micropor. Mesopor. Mater. 38 (2000) 51 ).
- Zhang et al prepared silicalite-1 membranes on porous a-alumina supports using silicalite-1 zeolite seeds of around 100 nm, 600 nm, 1 .5 ⁇ , 3.0 ⁇ and 7.5 ⁇ .
- the seed layers and membranes prepared from smaller seeds had smoother surfaces and fewer defects.
- Oversized seeds (7.5 ⁇ ) led to the formation of a discontinuous seed layer, which resulted in the formation of an discontinuous and uneven zeolite membrane (Zhang et al, Materials Chemistry and Physics 96 (2006) 42-50).
- Zhang et al investigated the formation of silicalite-1 seed layers on a porous carbon support of 0.5 ⁇ pore size and a-A ⁇ Os supports with different pore sizes (0.1 ⁇ and 4 ⁇ ) via the slip-casting technique. It was found that a continuous seed layer was obtained on the smooth support of 0.1 ⁇ pore size by using any seed of 100 nm, 600 nm or 2.2 ⁇ in size, whereas, on the coarse supports with either 0.5 ⁇ or 4 ⁇ pore size, a
- Hasegawa et al prepared MFI-type zeolite membranes on porous mullite tubes by secondary growth from seeds with different particle sizes (100 ⁇ 1000 nm)
- n-C 4 Hio/i-C 4 Hio permselectivity was as high as 220 for single-components.
- the separation factor for binary system was up to 58.
- Chen et al used one-step and two-step in situ hydrothermal synthesis to prepare high-performance silicalite-1 membranes on porous silica tubes (Chen et al, Microporous and Mesoporous Materials 102 (2007) 249-257). It was found that both aging temperature and aging time showed significant influence on the membrane performance.
- suitable aging conditions for example, aging synthesis solution and silica tubes together at 348 K for 8 h, a silicalite-1 membrane with ethanol/water separation factor of 81 and total flux of 0.39 kg/m 2 h at 333 K was obtained by only one step in situ hydrothermal synthesis.
- the membrane thickness with two-step synthesis was much higher than that obtained by one-step synthesis, the former had higher flux than that of the latter.
- Li et al used in situ aging, a method utilizing microwave heating, to prepare high quality LTA zeolite membranes without seeding (Li et al, Journal of Membrane Science 277 (2006) 230-239). It was found that in situ aging temperature and microwave heating time had the most significant influences on the synthesis. LTA zeolite membranes showed superior gas permeation properties (permeance of h
- LTA zeolite membranes with good pervaporation performance were prepared with high reproducibility (water/ethanol selectivity>10000).
- Li et al found that a gel layer is first formed on the support after in situ aging, which contains a suitable amount of pre-nuclei. During the following microwave assisted crystallization, it is believed these pre-nuclei rapidly and simultaneously develop into crystal nuclei, crystal growth occurs by propagation through the amorphous primary particles (with size of ca. 50 nm), and finally, the amorphous particles transform into LTA crystal particles with the same size (Li et al, Journal of Membrane Science 281 (2006) 646-657).
- Barri et al disclosed supported zeolite membranes (U.S. Patent 5,567,664) and methods for the production of zeolite membranes on porous supports (U.S. Patent 5,362,522).
- Barri et al. state that any type of zeolite-type material may be used, including silicoaluminophosphates (SAPOs).
- SAPOs are largely composed of Si, Al, P and O and can have a three-dimensional microporous crystal framework structure of PO 2 + , AIO 2 " and SiO 2 tetrahedral units.
- the cages, channels and cavities created by the crystal framework can permit separation of mixtures of molecules based on their effective sizes.
- SAPO crystals can be synthesized by hydrothermal crystallization from a reaction mixture containing reactive sources of silica, alumina, and phosphate, and an organic templating agent.
- Lok et al (U.S. 4,440,871 ) report gel compositions and procedures for forming several types of SAPO crystals, including SAPO-5, SAPO-1 1 , SAPO-16, SAPO-17, SAPO-20, SAPO-31 , SAPO-34, SAPO-35, SAPO-37, SAPO-40, SAPO 41 , SAPO-42, and SAPO-44 crystals.
- SAPO-5 SAPO-1 1
- SAPO-16 SAPO-17
- SAPO-20 SAPO-31
- SAPO-34 SAPO-35
- SAPO-37 SAPO-40
- SAPO 41 SAPO-42
- SAPO-44 crystals can be synthesized by hydrothermal crystallization from a reaction mixture containing reactive sources of silica, alumina, and phosphate, and an organic templating agent.
- Lixiong et al. reported single gas permeances for H 2 , N 2 , CO 2 , and n-C 4 Hi 0 .
- Poshuta et al. (Ind. Eng. Chem. Res., 37 (1998), p. 3924-3929; and AIChE Journal, 46(4) (2000), 779-789) reported hydrothermal synthesis of SAPO-34 membranes on the inside surface of asymmetric, porous a-AI 2 O3 tubes.
- Poshuta et al reported single gas and mixture permeances and ideal and mixture selectivities for several gases, including CO 2 and CH 4 .
- the CO 2 /CH 4 selectivities reported for a 50/50 CO 2 /CH 4 mixture at 300K were between 14 and 36 for a feed pressure of 270 kPa and a pressure drop of 138 kPa (Poshusta et al, AIChE Journal, 46(4) (2000), pp 779-789).
- the CO 2 /CH 4 selectivity was attributed to both competitive absorption (at lower temperatures) and differences in diffusivity.
- Li et al. reported an average CO 2 /CH 4 selectivity of 76+/- 19 for a 50/50 CO 2 /CH 4 mixture at 295 K with a feed pressure of 222 kPa and a pressure drop of 138 kPa.
- Patent Application Publication 2007-0265484 to Li et al. discloses methods for making SAPO- 34 membranes including methods where the membrane forming gel is aged prior to contact with the porous support and hydrothermal synthesis. [0017] Several U.S. patents report processes for the manufacture of mo
- the present invention provides molecular sieve membranes, particularly silicoaluminophosphate (SAPO) membranes such as SAPO-34 membranes, prepared on porous supports and methods of making such membranes.
- SAPO silicoaluminophosphate
- the membranes of the present invention provide increased permeance and selectivity properties for use in gas separations, particularly carbon dioxide (CO2) and methane (CH 4 ) separations.
- CO2 carbon dioxide
- CH 4 methane
- Inorganic membranes such as SAPO membranes can have superior thermal, mechanical and chemical stability, good erosion resistance, and high pressure stability as compared to conventional polymeric membranes.
- SAPO membranes are generally prepared by contacting a membrane forming gel with a porous support and heating for several hours at temperatures in excess of 420 K.
- the membrane forming gel generally comprises AI2O3, P2O5, S1O2, H 2 O and one or more organic templates.
- the organic template is a quaternary organic ammonium templating agent. Where more than one organic templating agent is used, the organic template preferably includes at least one quaternary organic ammonium templating agent and one or more amines.
- membranes are typically washed, dried and calcined to remove the organic template.
- the membrane forming gel is aged several hours to several days prior to contacting the membrane forming gel with the porous support.
- SAPO crystals able to make molecular sieve membranes according to the methods discussed herein include, but are not limited to, SAPO-5, SAPO-11 , SAPO-16, SAPO-17, SAPO-20
- the present invention provides SAPO membranes, particularly SAPO-34 membranes, prepared using hydrothermal synthesis where an additional soaking treatment is performed.
- a porous support preferably a seeded porous support, is soaked in the membrane forming gel for several hours prior to the hydrothermal synthesis.
- the porous support is soaked in the membrane forming gel at a temperature between 10° C and 100° C, more preferably between 20° C and 30° C.
- the porous support is soaked in the membrane forming gel at room temperature (defined herein as 25° C to 30° C).
- SAPO membranes prepared with this additional soaking treatment have higher permeance, higher selectivity and improved reproducibility than membranes prepared without this soaking treatment.
- One side or both sides of the porous support may be soaked in the membrane forming gel prior to the hydrothermal synthesis.
- a SAPO membranes prepared with this additional soaking treatment have higher permeance, higher selectivity and improved reproducibility than membranes prepared without this soaking treatment.
- crystalline layer is only formed on one surface of the porous support during
- the present invention provides a method for making a crystalline silicoaluminophosphate (SAPO) membrane comprising the steps of: a) providing a porous support having a first and a second side, which are optionally seeded; b) preparing an aqueous SAPO forming membrane forming gel, wherein the membrane forming gel comprises aluminum, phosphorus, silicon, oxygen, an organic templating agent and water; c) soaking at least one side of the porous support in the membrane forming gel for two or more hours at a temperature between 10° C and 100° C; d) following the soaking step, heating the porous support and the membrane forming gel between about 420 K and about 540 K to form a layer of SAPO crystals on the porous support; and e) calcining the SAPO layer to remove the templating agent. At least one side of the porous support may be soaked in the membrane fori
- the porous support is soaked in the membrane forming gel between one and fourteen hours prior to heating, between two and five hours, or between three and four hours.
- both sides of the porous support are soaked in the membrane for two or more hours, preferably between two and fourteen hours, preferably between three and five hours, even more preferably between three and four hours.
- the SAPO forming membrane forming gel is a SAPO-34 forming membrane forming gel and the resulting molecular sieve membrane is a SAPO-34 membrane.
- the porous support and the membrane forming gel are heated between about 453 K and about 533 K, preferably between about 470 K and about 515 K, more preferably between about 463 K and about 493 K.
- the porous support and membrane forming gel are preferably heated for a time greater than two hours, preferably between 2 and 25 hours.
- the porous support and membrane forming gel are heated between about 15 and about 25 hours, preferably between about 20 and 25 hours.
- the porous support and membrane forming gel are heated between about 3 and about 8 hours, preferably between about 4 and 6 hours.
- the porous support and membrane forming gel are heated between about 4 and 6 hours at a temperature between about 463 K to about 493 K.
- At least part of the surface of the porous support is seeded with SAPO crystal material prior to contact with the membrane forming gel.
- seeding it is meant that a small amount of crystals, preferably crystals of the same material that is to be grown or formed, is applied to the porous support prior to contact with the membrane forming gel.
- the size of the crystals applied to the support surface can vary.
- the average size of these seed crystals or particles is less than or equal to 5 microns, less than or equal to 3 microns, less than or equal to 1 micron, or less than or equal to 0.8 microns. In further embodiments, the average size o
- crystals or particles is less than 1 micron, from 25 nm to 1 .0 micron, from bU nm to 1 UUU nm, from 100 nm to 1000 nm, from 25 nm to 500 nm, from 50 nm to 500 nm or from 100 nm to 500 nm.
- the seed crystals or particles can exhibit cubic, cuboid and rectangular plate morphology. If the crystals are cubic, the characteristic size of the crystals is the average length or width of the cubes.
- the characteristic size of the crystals may be considered to be the average of the longest dimension of the crystals, the shortest dimensions of the crystals, or the average of the longest and shortest dimensions.
- the average size of the longest and shortest dimensions of cubic and cuboid crystals may be estimated through measurement of the length and width, respectively, of one face of each crystal.
- the characteristic size is considered to be the average of the longest and shortest dimensions. If the crystals exhibit rectangular plate morphology, the characteristic size may be considered to be the average of the longest dimension (length) while the shortest dimension can be considered to be the plate's thickness.
- seed crystals with rectangular plate morphology have a length less than or equal to 5 microns, less than or equal to 3 microns, less than or equal to 1 micron, or less than or equal to 0.8 microns, and a thickness less than or equal to 1 micron, less than or equal to .6 microns, less than or equal to .2 micron, or less than or equal to 0.4 microns.
- seed crystals with rectangular plate morphology have a length less than or equal to 5 microns, less than or equal to 3 microns, less than or equal to 1 micron, or less than or equal to 0.8 microns, and a thickness less than or equal to 1 micron, less than or equal to .6 microns, less than or equal to .2 micron, or less than or equal to 0.4 microns.
- seed crystals with rectangular plate morphology have a length less than or equal to 5 microns, less than or equal to 3 microns, less than or equal to 1 micron, or less than or
- the average of the width and length can be less than 1 micron, from 25 nm to 1 .0 micron, from 50 nm to 1000 nm, from 100 nm to 1000 nm, from 50 nm to 500 nm or from 100 nm to 500 nm.
- the standard deviation of the size distribution is less than or equal to 0.5 microns, less than or equal to 0.1 microns, less than or equal to 20% of the average, less than or equal to 15% of the average, or less than or equal to 10% of the average.
- the ratio of the average longest dimension to the average shortest dimension of cuboid crystals is less than or equal to 2 or less than or equal to 1 .5.
- Seeding can be accomplished using a variety of methods, including but not limited to dip coating and rubbing. Without wishing to be bound by theory, it is believed that the deposited seed crystals provide nucleation sites for crystal growtl
- a "low density" with regard to “seeding” and “seed crystals” means that the seed crystals are deposited on the porous support or other surface so that the seed crystals do not form a continuous layer of crystals across the support or surface. More specifically, a low density of seed crystals deposited on the porous support is less than 1 g/m 2 (where this value is calculated as the weight of deposited crystals divided by the approximate surface area over which the particles are applied) and would cover approximately 5% or less of the support surface excluding the area occupied by the pores.
- a low density of seed crystals is provided on the porous support for improved performance.
- dip coating is used to provide a low seed density on the porous support.
- a low density of seed crystals is applied to the porous support followed by soaking the seeded porous support in the membrane forming gel; however, the lower seed density on the support surface is a key factor in determining membrane quality, even without a soaking treatment.
- the average amount of seed particles deposited on the support is 0.6 g/m 2 or less, where this value is calculated as the weight of deposited crystals divided by the approximate surface area over which the particles are applied.
- the average amount of seed particles deposited on the support is 0.4 g/m 2 or less, 0.2 g/m 2 or less, or 0.1 g/m 2 or less.
- the seed crystals deposited on the porous support cover approximately 1 % or less of the support surface excluding the area occupied by the pores.
- the seed crystals deposited on the porous support cover approximately 0.1 % or less, preferably 0.01 % or less, even more preferably 0.001 % or less of the support surface excluding the area occupied by the pores.
- the porous support is dipped one or more times in a SAPO crystal seed solution comprising between approximately 0.005 wt% and 0.67 wt% of the SAPO crystal seed material.
- the SAPO crystal seed solution comprises between approximately 0.02 wt% and 0.042 wt% of SAPO crystal seed material.
- the SAPO crystal seed solution is a SAPO-34 seed solution and contains between approximately 0.005 wt% and 0.67 wt% of SAPO-34 crystal seed material, preferably between approximately 0.02 wt% and 0.042 wt% of SAPO-34 crystal seed material.
- the porous support can be dipped in the seed solution for any amount of time suitable to transfer the desired amount of seed crystals to the porous support.
- the porous support is dipped in the seed solution for a time between 1 second and 10 minutes, more preferably between 5 seconds and 1 minute, even more preferably between 5 seconds and 15 seconds.
- the porous supports are dipped one or more times in a SAPO crystal seed solution, preferably a SAPO-34 seed solution, for 10 seconds and withdrawn from the seed solution at a rate between about 0.1 and 5 cm/second, preferably at a rate of between about 0.8 and 1 .2 cm/second, even more preferably at a rate of about 1 cm/second.
- porous support can be dipped multiple times in the seed solution, good results can be obtained with only a single dip coating.
- the porous support is seeded and soaked in the membrane forming gel prior to synthesis.
- SAPO membranes with improved selectivity may be formed using the dip coating treatment without soaking, or using the soaking treatment without the dip coating treatment.
- the soaking treatment improved permeance significantly.
- the porous support is a body capable of supporting the SAPO m
- porous support may be of any suitable shape, including disks, flat panels, and tubes.
- the porous support is a metal, ceramic or an inorganic material.
- the porous support does not appreciably dissolve or form reaction products at the interface when placed in contact with the synthesis gel.
- Suitable inorganic porous supports include, but are not limited to, a-alumina, glass, titania, zirconia, carbon, silicon carbide, clays or silicate minerals, aerogels, supported aerogels, and supported silica, titania and zirconia.
- the porous support is aluminum oxide (AI2O3).
- Suitable porous metal supports include, but are not limited to, stainless steel, nickel based alloys (Inconel, Hastalloy), Fecralloy, chromium and titanium.
- the metal may be in the form of a fibrous mesh (woven or non-woven), a combination of fibrous metal with sintered metal particles, and sintered metal particles.
- the metal support is formed of sintered metal particles.
- Ceramic supports having different porosity layers are commercially available (for example Membralox ceramic membranes available from Pall Corp.)
- the average pore size of the support can range from 2 nm to 500 nm, preferably between 5 nm to 200 nm. In further embodiments, the pore size is between 100 nm to 200 nm, 25 nm to 500 nm, or 50 nm to 300 nm.
- the pore size of the support is relatively uniform throughout the support.
- the pore size at the surface of the support can be
- the pore size characteristic of the surface of the support may be taken as the pore size characteristic of the support as a whole.
- the support may have a different pore size at or near the surface on which the membrane is to be formed than the pore size away from the surface.
- the support may have two well-defined regions, a first layer with a smaller average pore size (on which the membrane is to be formed) and a second layer with a larger average pore size.
- the pore size at the surface can be characterized by pore size of the region or layer nearest the surface on which the membrane is to be formed.
- the pore size characteristic of the surface of the support ma
- the pore diameter of the support or the surface region of the support is large enough to allow the synthesis gel to penetrate the support.
- the pore size of the support or of its surface region can be smaller than, equal to, or greater than the characteristic size of the particles. In an embodiment, the average
- characteristic size of the loose SAPO crystals is larger than the average pore size of the support. This limits the extent of penetration of the crystals inside the support. Often, a porous support will have a distribution of pore sizes.
- the pore diameter of the support or the surface region of the support is greater than about 0.1 microns.
- the pore diameter of the support being greater than about 0.1 microns does not require that every single pore in the support is greater than about 0.1 microns, but it does exclude supports having regions where the characteristic pore size is about 0.1 microns (for example, a support having a layer with an 0.1 micron average pore size).
- the characteristic pore size may be taken as the average, median or largest pore size.
- the SAPO layer is formed on just one side of the porous support.
- the SAPO layer is typically formed on either the upper surface or the lower surface.
- the support is in the form of a tube having an inner and an outer surface, the SAPO layer is formed on either the inner or the outer surface.
- the SAPO layer is formed on both sides of the porous support, such as both the upper and lower surface of a disk or panel and both the inner and outer surfaces of a tube.
- the membranes of the present invention have a SAPO layer having a thickness of less than about 10 ⁇ , preferably between approximately 5 ⁇ and 6 ⁇ .
- the present invention provides a method for making a crystalline silicoaluminophosphate-34 (SAPO-34) membrane comprising the steps of: a) providing a porous support having a first and a second side; b) preparing an aqueous SAPO-34 forming membrane forming gel, wherein the membrane forming gel comprises aluminum, phosphorus, silicon, oxygen, an organic templating agent and
- the membrane forming gel comprises a quaternary ammonium templating agent and at least on amine templating agent.
- SAPO-34 seed material is also applied to the second side of the porous support.
- 0.6 g/m 2 or less of SAPO- 34 seed material is applied to at least a portion of the first side and/or second side of the porous support.
- Another embodiment of the invention comprises applying 0.4 g/m 2 or less of SAPO-34 seed material to at least a portion of first side and/or second side of the porous support.
- Another embodiment of the invention comprises applying 0.2 g/m 2 or less of SAPO-34 seed material to at least a portion of the first side and/or second side of the porous support.
- Another embodiment of the invention comprises applying 0.1 g/m 2 or less of SAPO-34 seed material to at least a portion of the first side and/or second side of the porous support.
- the first and second sides of the porous support are contacted with the membrane forming gel.
- the first side or the first and second sides of the porous support are soaked in the membrane forming gel between one and fourteen hours.
- a further embodiment comprises soaking the first side or the first and second sides of the porous support between two and five hours in the membrane forming gel.
- a further embodiment comprises soaking the first side or the first and second sides of the porous support between three and four hours in the membrane forming gel.
- the first side or the first and second sides of the porous support are soaked in the membrane forming gel at a temperature between 10° C and 100° C, at a temperature between 20° C and 30° C, or at a temperature between 250° C and 30° C.
- the seed crystal material may be applied by dipping the porous support one or more times in a SAPO-34 seed solution comprising between approximately 0.005 wt% and 0.67 wt% of SAPO-34 seed material, prefe
- a further embodiment comprises dipping the porous supports one or more times in a SAPO-34 seed solution for 10 seconds and
- the aqueous SAPO-34 forming membrane forming gel has the formula:
- R is a templating agent
- a is between about 0.01 and about 52
- b is between about 0.03 and about 196
- c is between about 0.2 and about 5
- d is between about 20 and about 300.
- c is less than about 2.
- a is about 1
- b is 0.03-0.6,
- c is 1.07-1.2
- d is 55-56.
- the ratio of silicon to aluminum is greater than 0.1 , greater than 0.10 and less than or equal to 0.6, between 0.10 and 0.6, between 0.15 and 0.45, from 0.15 to 0.3, between 0.15 and 0.3, from 0.15 to 0.2, or is about 0.15.
- R is a quaternary ammonium templating agent.
- the quaternary ammonium templating agent is selected from the group consisting of tetrapropyl ammonium hydroxide (TPAOH), tetrapropyl ammonium bromide, tetrabutyl ammonium hydroxide, tetrabutyl ammonium bromide, tetraethyl ammonium hydroxide (TEAOH), or tetraethyl ammonium bromide.
- TPAOH tetrapropyl ammonium hydroxide
- TEAOH tetrabutyl ammonium hydroxide
- TEAOH tetraethyl ammonium hydroxide
- the aqueous SAPO-34 forming membrane forming gel has the formula:
- AI 2 O 3 aP 2 O 5 : bSiO 2 : cRi: dR 2 : eH 2 O Formula 2,
- the ratio of silicon to aluminum is greater than 0.1 , between 0.15 and 0.45, from 0.15-0.45, between 0.15 and 0.3, from 0.15-0.3, between 0.15 and 0.2, from 0.15 to 0.2 and is about 0.15.
- Ri is a quaternary organic ammonium templating agent, and R 2 is an ami
- Mn molecular weight
- the value of parameter c is greater than or equal to 1 and less than or equal to 2. In another embodiment, the value of parameter c is greater than or equal to 1 and less than or equal to 1 .6.
- the aqueous SAPO-34 forming membrane forming gel has the formula:
- the porous support and the membrane forming gel are heated between about 453 K and about 533 K, preferably between about 470 K and about 515 K, more preferably between about 463 K and about 493 K.
- the porous support and membrane forming gel are preferably heated for a time greater than two hours, preferably between 2 and 25 hours.
- the porous support and membrane forming gel are heated between about 15 and about 25 hours, preferably between about 20 and 25 hours.
- the porous support and membrane forming gel are heated between about 3 and about 8 hours, preferably between about 4 and 6 hours.
- the porous support and membrane forming gel are heated between about 4 and 6 hours at a temperature between about 463 K to about 493 K.
- membranes can be described by several parameters. As used herein, the flux, J,, through a membrane is the number of moles of a specified component i p
- I he permeance or pressure normalized flux, P, is the flux of component i per unit transmembrane driving force.
- the transmembrane driving force is the gradient in chemical potential for the component (Karger et al, Diffusion in
- the selectivity of a membrane for components i over j, S, j is the permeance of component i divided by the permeance of component j.
- the ideal selectivity is the ratio of the permeances obtained from single gas permeation experiments.
- the actual selectivity (also called separation selectivity) for a gas mixture may differ from the ideal selectivity.
- the separation of C02 from CH 4 is important in natural gas processing because CO2 reduces the energy content of natural gas.
- the methods of the present invention provides SAPO membranes, particularly SAPO-34 membranes, with equal or improved CO2/CH4 selectivity and CO2 permeance as compared to previously reported SAPO-34 membranes.
- the membranes of the present invention can have a CO2 CH 4 selectivity of 180 or greater for a 50/50 CO2 CH 4 mixture at a pressure drop of 0.14 MPa, preferably a CO 2 /CH 4 selectivity of 200 or greater, even more preferably a CO 2 /CH 4 selectivity of 250 or greater.
- the SAPO-34 membranes have a CO2 CH 4 selectivity of 50 or greater, preferably a CO2 CH 4 selectivity of 70 or greater, even more preferably a CO2 CH 4 selectivity of 85 or greater.
- a percent mixture of a combination of gases refers to the molar percent of the gases.
- One embodiment of the invention provides a SAPO-34 membrane made according to the methods described above having a CO2 CH 4 separation selectivity of 274 or greater for an approximately 50/50 CO2 CH 4 mixture with a pressure differential across the membrane of 0.14 MPa, and a has a CO 2 /CH 4 separation selectivity of 78 or greater for an approximately 50/50 CO2 CH 4 mixture with a pressure differential across the membrane of 4.6 MPa.
- membranes provided by the present invention can h
- the membranes provided by the present invention also can have a C0 2 permeance of 1.8 x 10 "7 mol/(m 2 • s ⁇ Pa) or greater at a pressure drop of 4.6 MPa, preferably a C0 2 permeance of 3.8 x 10 "7 mol/(m 2 ⁇ s ⁇ Pa) or greater, even more preferably a C0 2 permeance of 4.7 x 10 "7 mol/(m 2 ⁇ s ⁇ Pa) or greater.
- One embodiment of the invention provides a SAPO-34 membrane made according to the methods described above having a CO 2 permeance of 2.9 x 10 " 6 mol/(m 2 ⁇ s ⁇ Pa) or greater for an approximately 50/50 CO 2 /CH 4 mixture with a pressure differential across the membrane of 0.14 MPa, and a CO 2 permeance of 4.7 x 10 "7 mol/(m 2 ⁇ s ⁇ Pa) or greater for an approximately 50/50 CO 2 /CH 4 mixture with a pressure differential across the membrane of 4.6 MPa.
- One embodiment of the present invention provides methods for separating a first gas component from a gas mixture containing at least a first and a second gas component using the SAPO membranes, particularly SAPO-34 membranes, described herein.
- the first gas component is carbon dioxide and the second gas component is methane.
- the separating method comprises the steps of: a) providing a membrane generated using a soaking treatment, a seeding treatment or both as described above, the membrane having a feed and a permeate side and being selectively permeable to the first gas component over the second gas component; b) applying a feed stream including the first and the second gas components to the feed side of the membrane; and c) providing a driving force sufficient for permeation of the first gas component through the membrane, thereby producing a permeate stream enriched in the first gas component from the permeate side of the membrane.
- the soaking and seeding treatments improve the permeance and selectivity of SAPO-34 membranes.
- Scanning electron microscope (SEM) images, X-ray diffraction (XRD) and high pressure C0 2 /CH 4 mixtui experiments were used to characterize SAPO-34 membranes.
- Figure 1 shows an SEM image of SAPO-34 seed crystals. Average seed crystal size was approximately 300 nm in length and 60 nm in thickness.
- Figure 2 shows the X-ray diffraction (XRD) pattern of a SAPO-34 seed crystal.
- Figures 3a-3d show SEM images of SAPO-34 membranes prepared using different conditions.
- Figure 3a shows a membrane grown on a glazed area and forming a free-standing membrane.
- Figures 3b-3d shown membranes prepared on non-glazed support surfaces with 4 hours soaking of one side of the support (Figure 3b), 4 hours soaking on both sides of the support ( Figure 3c), and no soaking ( Figure 3d).
- Figures 4a-4c show cross-sectional SEM images of membranes prepared with 4 hours soaking of one side of the support (Figure 4a), 4 hours soaking on both sides of the support ( Figure 4b), and no soaking ( Figure 4c).
- Figures 5a-5f show cross-sectional SEM images of a SAPO-34 membrane prepared by soaking both sides of the support.
- Figure 5a illustrates that the porous support contains three layers with different pore sizes (layers labeled A, B and C).
- Figures 5b-5d show increasingly magnified views of one section shown in Figure 5a.
- Figure 5e shows a magnified view of layer B from Figure 5a
- Figure 5f shows a magnified view of layer C from Figure 5a.
- Figures 6a-6c show SEM images of powder SAPO-34 crystals (not part of a membrane) collected at the bottom of an autoclave.
- Figures 6a and 6b show images of crystals collected during membrane synthesis with no soaking ( Figure 6a) and 4 hr soaking ( Figure 6b).
- Figure 6c shows an image of crystals collected from non- membrane synthesis (no soaking and without addition of a seeded support).
- Figures 7a-7c show SEM images of porous supports dipcoated ir
- Figures 8a-8b show the top view and cross-section view of SEM images of a SAPO-34 membrane prepared with the dip coating and soaking method.
- SAPOs are molecular sieve materials, having a tetrahedral crystal structure joined together through oxygen atoms to produce an extended network of channels of molecular dimensions.
- the SAPO crystals have a three-dimensional crystal framework structure of PO2+, AIO2- and S1O2 tetrahedral units, the framework structure defining a structure of regular cages, cavities, and channels.
- the dimensions of the channels and cavities in these crystal layers are generally microporous.
- microporous refers to pore diameters less than about 2 nanometers.
- Crystalline SAPO-34 has the CHA structure and is an analog of the natural zeolite chabazite.
- the CHA framework structure contains single eight ring, double six ring, and single four ring secondary building units.
- SAPO-34 adsorption measurements have determined that n-C 4 Hio (0.43 nm diameter) can fit the pores formed in the SAPO- 34 framework structure, but i-C 4 Hi 0 (0.5 nm diameter) cannot, thus the pore size is believed to be between 0.43 and 0.5 nm (Lok et al., in Lok. et al. (eds.) Crystalline Silicoalumino Phosphates, US, 1984).
- SAPOs have different crystalline structures and different pore sizes and can be classified as small, medium, or large-pore molecular sieves based on the size of the largest oxygen rings in the structure.
- Crystalline SAPO-5 has the AFI structure which contains rings of 12 oxygen atoms, 6 oxygen atoms, and 4 oxygen atoms.
- SAPO-5 is typically considered a large-pore molecular sieve.
- crystalline SAPO-1 1 has the AEL structure which contains rings of 10 oxygen atoms, 6 oxygen atoms, and 4 oxygen atoms.
- SAPO-11 is typically considered a medium- sieve. Structures where the largest ring contains 8 or fewer oxygen atoms are typically considered small-pore molecular sieves.
- SAPO crystals able to make molecular sieve membranes include, but are not limited to, SAPO-5, SAPO-1 1 , SAPO-16, SAPO-17, SAPO-20, SAPO-31 , SAPO-34, SAPO-35, SAPO-37, SAPO-40, SAPO 41 , SAPO-42, and SAPO-44 crystals.
- SAPO structures are available in Baerlocher, W.M. Meier and D.H. Olson, "Atlas of Zeolite Framework Types", 5th ed., Elsevier: Amsterdam, 2001 and online at http://www.iza-strucures.org/databases.
- SAPOs exhibit cation exchange properties.
- the excess negative charge in the lattice may be compensated by protons or by compensating cations located in the cavities of the structural framework.
- Acid hydrogen forms of SAPOs e.g. H-SAPO-314
- Other forms of SAPO-34 include, but are not limited to Na- SAPO-34, Cu-SAPO-34, Li-SAPO-34, K-SAPO-34, Rb-SAPO-34, and Ca-SAPO-34. These may be made through ion-exchange of H-SAPO-34 or by including the
- the membranes of the invention are formed through in situ crystallization of an aqueous silicoaluminophosphate-forming gel.
- the membrane forming gel contains one or more organic templating agents.
- templating agent or “template” is a term of art and refers to a species added to the synthesis media to aid in and/or guide the polymerization and/or organization of the building blocks that form the crystal
- the template is a quaternary organic ammonium templating agent including but not limited to tetrapropyl ammonium hydroxide (TPAOH), tetrapropyl ammonium bromide, tetrabutyl ammonium hydroxide, tetrabutyl ammonium bromide, tetraethyl ammonium hydroxide (TEAOH), tetraethyl ammonium bromide,
- TPAOH tetrapropyl ammonium hydroxide
- TEAOH tetrapropyl ammonium bromide
- TEAOH tetraethyl ammonium hydroxide
- TEACI tetraethylammonium chloride
- the additional templating agents can include amines.
- Membrane forming gels for forming SAPO crystals are known to the art, but preferred gel compositions for forming membranes may differ from preferred compositions for forming loose crystals or granules.
- the preferred gel composition may v.
- the membrane forming gel is prepared by mixing sources of aluminum, phosphorus, silicon, and oxygen in the presence of a templating agent and water.
- the gel comprises Al, P, Si, O, at least one templating agent and water.
- the composition of the mixture may be expressed in terms of the following molar ratios as:
- R is a templating agent.
- R is a quaternary ammonium templating agent.
- the quaternary ammonium templating agent is selected from the group consisting of tetrapropyl ammonium hydroxide (TPAOH), tetrapropyl ammonium bromide, tetrabutyl ammonium hydroxide, tetrabutyl ammonium bromide, tetraethyl ammonium hydroxide (TEAOH), or tetraethyl ammonium bromide.
- the gel composition can also include Li 2 0, BeO, MgO, CoO, FeO, MnO, ZnO, B 2 0 3 , Ga 2 0 3 , Fe 2 0 3 , GeO, TiO, As 2 05 or combinations thereof.
- the gel composition can also include sources of the compensating cations (for example, NaOH for Na + , LiOH for Li + , KOH for K + , RbOH for Rb + , and CsOH for Cs + ).
- sources of the compensating cations for example, NaOH for Na + , LiOH for Li + , KOH for K + , RbOH for Rb + , and CsOH for Cs + ).
- c is less than about 2.
- a is about 1
- b is 0.03-0.6
- c is 1 .07-1 .2
- d is 55-56.
- the ratio of silicon to aluminum is between 0.3 and 0.15, is between 0.2 and 0.15, and is 0.15.
- R is a quaternary organic ammonium templating agent selected from the group consisting of tetrapropyl ammonium hydroxide, tetraethyl ammonium hydroxide
- TAAOH TEAOH
- Si Si
- the ratio of Si to Al is high enough so that AIPO5 is not formed.
- the ratio of silicon to aluminum is greater than 0.1 , greater than 0.10 and less than or equal to 0.6, between 0.10 and 0.6, between 0.15 and 0.45, from 0.15 to 0.3, between 0.15 and 0.3, from 0.15 to 0.2, or is about 0.15.
- the gel comprises at least two templating agents. Any templating agent or each templating agent, independently of one another, may comprise nitrogen. In an embodiment, the gel includes only two templating agents, where optionally one templating agent is a quaternary ammonium compound and the second templating agent is an amine. In another embodiment, the gel includes three templating agents, where optionally the first templating agent is a quaternary ammonium
- a given templating agent may form ionic species in the gel, so that the gel also contains ionic species derived from the templating agent.
- quaternary ammonium compounds may produce quaternary ammonium cations in the gel.
- the gel comprises Al, P, Si, O, at least two templating agents and water.
- the composition of the mixture may be expressed in terms of the following molar ratios as:
- AI 2 O 3 aP 2 O 5 : bSiO 2 : cRi: dR 2 : eH 2 O Formula 2,
- the gel composition can also include Li 2 O, BeO, MgO, CoO, FeO, MnO, ZnO, B 2 O 3 , Ga 2 O 3 , Fe 2 O 3 , GeO, TiO, As 2 O 5 or
- the gel composition can also include sources of the compensating cations (for example, NaOH for Na + , LiOH for Li + , KOH for K + , RbOH for Rb + , and CsOH for Cs + ).
- sources of the compensating cations for example, NaOH for Na + , LiOH for Li + , KOH for K + , RbOH for Rb + , and CsOH for Cs + ).
- a is greater than 0.5 and less than 1.5
- b is greater than 0.2 and less than 1 .0
- c is greater than or equal to 1 and less than 2
- d is greater than zero and less than 4.0 and e is greater than 50 and less than 1 10.
- the ratio of silicon to aluminum is greater than 0.1 , between 0.15 and 0.4i
- 0.45 between 0.15 and 0.3, from 0.15-0.3, between 0.15 and 0.2, from 0.1 b to ⁇ . ⁇ and is about 0.15.
- Ri is a quaternary organic ammonium templating agent.
- the quaternary ammonium templating agent is selected from the group consisting of tetrapropyl ammonium hydroxide (TPAOH), tetrapropyl ammonium bromide, tetrabutyl ammonium hydroxide, tetrabutyl ammonium bromide, tetraethyl ammonium hydroxide (TEAOH), tetraethyl ammonium bromide, tetraethylammonium chloride (TEACI) or combinations thereof.
- TPAOH tetrapropyl ammonium hydroxide
- TEAOH tetrapropyl ammonium bromide
- TEAOH tetrabutyl ammonium hydroxide
- TEACI tetraethyl ammonium bromide
- TEACI tetraethylammonium chloride
- the quaternary ammonium templating agent is selected from the group consisting of TPAOH, TEAOH or combinations thereof.
- the templating agent is TEAOH.
- the value of parameter c is greater than or equal to 1 and less than or equal to 2. In another embodiment, the value of parameter c is greater than or equal to 1 and less than or equal to 1 .6.
- R 2 is an amine, which may be a "small amine".
- small amines means amines and organic amines having a molecular weight (Mn) of less than or equal to 300 and/or equal to or less than 12 carbon atoms.
- the amine may be a neutral amine.
- R 2 is a primary, secondary or tertiary amine.
- R 2 may be an aliphatic or a cyclic amine.
- R 2 is an alkyl amine such as dipropylamine (DPA) or N,N- dimethylbutylamine (DMBA).
- R 2 may have both an amine and an alcohol functionality, such as ⁇ , ⁇ -dimtheylethanolamine (DMEA).
- DMEA ⁇ , ⁇ -dimtheylethanolamine
- R 2 may also be morpholine (MOR).
- R 2 is selected from the group consisting of dipropylamine (DPA) and cyclohexylamine(CHA).
- R 2 is DPA.
- the value of parameter d is greater than or equal to 1 and less than or equal to 4, between 1 .0 and 3.0, from 1.0 to 3.0, between 1 .0 and 2.0, or from 1 .0 to 2.0.
- the initial pH of a gel combining TEAOH with DPA is between about 9 and about 10
- the initial pH of a gel combining TEAOH with CHA is between about 8 and about 8.5.
- the comj mixture may be expressed in terms of the following molar ratios as:
- the value of parameter di in Formula 3 is between 0.5 and 1 .5 and the value of parameter d 2 is between 0.5 andl .5.
- R 2 and R 3 are dipropylamine (DPA) and cyclohexylamine(CHA).
- di and d 2 are both between 0.5 andl .0.
- the initial pH of a gel combining TEAOH with DPA and CHA is between about 8.5 and about 9.0.
- the values of the other parameters (a, b, c, e) may be as specified for Formulas 1 and 2.
- the amount of water in the synthesis gel is also an important parameter.
- the amount of water used in the membrane synthesis gel is significantly greater than that which would typically be used in a gel for synthesis of loose crystals of the same zeolite or other molecular sieve material.
- the value of the parameter e in Formulas 1 -3 is greater than 50, between 50 and 1 10, from 50 to 1 10, between 60 and 100, from 60-100, between 70 and 90, from 70-90, between 70 and 80, or from 70-80.
- the synthesis gel composition is 1.0 Al 2 0 3 : 1 .0 P 2 0 5 : 0.3 Si0 2 : 1.0 TEAOH: 1.6 DPA: x H 2 O, where x is between 70 and 80.
- the synthesis gel composition is 1 .0 AI 2 O 3 : 1 .0 P 2 Os: 0.32 SiO 2 : 1 .0 TEAOH: 1 .6 DPA: x H 2 O, where x is from 70 to 80.
- the gel is prepared by mixing sources of phosphate and alumina with water for several hours before adding the template. The mixture is then stirred before adding the source of silica.
- the source of phosphate is phosphoric acid.
- Suitable phosphate sources also include organic phosphates such as triethyl phosphate, and crystalline or amorphous aluminophosphates.
- the source of alumina is an aluminum alkoxide, such as aluminum isopropoxide.
- Suitable alumina sources also include pseudoboehmite and crystalline or amorphous
- aluminophosphates (gibbsite, sodium aluminate, aluminum trichloride).
- the source of silica is a silica sol.
- Suitable silica sources also include fumed silica, reactive solid amorphous precipitated silica, silica gel, alkoxi (silicic acid or alkali metal silicate).
- Na-SAPO-34 can be made by incorporating NaOH into the synthesis gel.
- the gel composition can be expressed by: AI2O3: aP20s: bSi02: eNa20: cR: dH 2 0.
- a is 0.77
- b is 0.46
- e is 0.23
- c is 0.77
- d is 46.
- the gel is aged prior to contact with the porous support.
- an “aged” gel is a gel that is held (not used) for a specific period of time after all the components of the gel are mixed together or a gel that is maintained at a
- the gel is sealed and stirred during storage to prevent settling and the formation of a solid cake.
- aging of the gel affects subsequent crystallization of the gel by generating nucleation sites. In general, it is believed that longer aging times lead to formation of more nucleation sites.
- the preferred aging time will depend upon the aging temperature selected. Preferably, crystal precipitation is not observed during the aging period.
- the viscosity of the aged gel is such that the gel is capable of penetrating the pores of the porous support.
- the aging time is greater than two hours, greater than five hours, greater than ten hours, or greater than twenty four hours.
- the aging time at room temperature is at least about twenty-four hours, greater than about twenty-four hours, at least about forty-eight hours, and at least about seventy-two hours.
- the aging time at room temperature or above can be at least twenty four hours, greater than about twenty-four hours at least about forty-eight hours, at least about seventy-two hours, between about three days and about seven days or between four days and 28 days.
- the gel is not aged longer than one month. In different e
- the aging temperature is between 10 °C and 75 °C or between 25 °C and biru. in different embodiments, the aging time is at least 24 hours between 290 K and 350K, between 290K and 335K, or between 290 K and 300 K.
- aging of the gel is not required to obtain the desired quality of membrane.
- gel aging may not be required if SAPO-34 crystals are applied to the support prior to in situ synthesis.
- gel aging may not be required for certain types of silica sources.
- aging is not required if tetraethyl orthosilicate (TEOS) is used as the silica source.
- TEOS tetraethyl orthosilicate
- the present invention provides SAPO membranes, particularly SAPO-34 membranes, prepared using hydrothermal synthesis where an additional soaking treatment, seeding treatment, or both is performed.
- soaking it is meant that the membrane forming gel is brought into continuous contact with at least one surface of the porous support for one or more hours, preferably between one and fourteen hours, preferably between three and five hours, even more preferably between three and four hours. If SAPO seed crystals have been applied to at least part of the surface of the support, the gel is brought into contact with at least this part of the surface.
- the porous support has two sides (e.g.
- the gel is brought into contact with only one side of the support.
- One side of the support may be masked to limit its contact with the gel.
- Suitable masking techniques are known to the art.
- One known masking technique involves covering the surface with a polymer layer, for example covering it with Teflon or fluoropolymer tape.
- Another masking technique involves infiltrating the pores of the support with an organic masking agent, such as a polymer or a wax, which can later be removed through thermal treatment.
- the porous support may be immersed in the gel so that more than one surface of the porous support contacts the gel.
- at least some of the gel penetrates the pores of the support.
- the pores of the support need not be completely filled with gel.
- the porous support is brought into contact with a sufficient quantity of gel such that growth of the SAPO membrane i
- the porous support may be seeded prior to hydrothermal synthesis.
- a first quantity of a SAPO crystalline seed material in the form of loose SAPO crystals (such as SAPO-34 crystals) is applied to at least part of the surface of the porous support prior to bringing the support in contact with the membrane forming gel.
- loose crystals refers to crystals which are largely unagglomerated or interlocking, in contrast to the interlocking crystals formed during in situ synthesis of the membrane.
- the surface of the support can include both non-porous portions and porous portions where the pores of the support open to the surface.
- crystals can be applied to the surface by contacting crystals with the surface or with crystals already associated with the surface. Since the surface has porous and non-porous portions, contacting the crystals with the surface can include contacting crystals with non-porous portions of the surface or lodging crystals wholly or partially within the pores which open to the surface.
- the crystals may also be applied to the surface by using coupling agents or binding agents to form covalent linkages between the crystals and the support surface.
- the porous support is treated with a barrier layer to prevent the crystals from preferentially entering the pores of the support as described in U.S. 6,090,289. In another embodiment, no barrier layer is used.
- the seed crystals used herein do not form a continuous or nearly continuous layer over the support surface.
- the seed crystals may be applied in dry form.
- various types of brushes or other applicators may be used to apply the crystals.
- the crystals may be rubbed onto the surface of the support. In an embodiment where a stainless steel support is used, sufficient crystals are rubbed onto the surface of the support that the support appears uniformly white to the eye.
- the crystals may also be suspended in solution and the solution applied to the support surface.
- a variety of techniques are known to the art for applying solutions of colloidal particles including, but not limited to, spin-coating, wash-coating, spray-coating, brushing, slip-casting, dip coating, and immersion for longer periods of tin
- the seeding methods of the present invention such as dip coating, provide seed crystals that are well bonded to the support and are present in low densities.
- the seed solution can be any solution known in the art able to transfer seed crystals within the solution to a surface placed in contact with the solution.
- the seed solution will comprise a solvent, the seed crystals and optionally an organic binding agent to provide improved adhesion of the seed crystals to the contacted surface.
- the solvent can be any solvent known in the art which can be removed from the porous solvent after the seed crystals are deposited and which does not react with the porous support.
- the solvent is an alcohol.
- Organic binders according to the present invention include cellulose ether type binders and/or their derivatives. Some typical organic binders according to the present invention are methylcellulose, hydroxybutylcellulose, hydrobutyl methylcellulose,
- applying the seed solution to the porous support is able to deposit seed crystals on the porous support, where the seed crystals do not form a continuous monolayer.
- SAPO crystal synthesis step can lead to formation of SAPO crystalline material on and in the porous support.
- crystalline material includes both newly formed crystals and crystalline material grown on previously formed crystals. If SAPO crystals have been applied to the support prior to the synthesis step, the synthesis step results in the formation of a second quantity of crystalline material which may take the form of new crystals and/or growth of the applied crystals.
- a layer of SAPO crystals can be said to form on the surface of the porous support and/or on previously formed SAPO crystals.
- the layer of SAPO crystals formed during each synthesis step may not be continuous.
- crystals may also precipitate from the membrane forming gel without being incorporated inti
- the heating temperature for the synthesis step is between about 420K and about 540 K.
- the heating temperature is between about 453 K and about 533 K, between about 470 K and about 515 K, or between about 463 K to about 493 K.
- the crystallization time is greater than two hours, preferably between 2 and 25 hours.
- the porous support and membrane forming gel are heated between about 15 and about 25 hours, preferably between about 20 and 25 hours.
- the porous support and membrane forming gel are heated between about 3 and about 8 hours, preferably between about 4 and 6 hours.
- the porous support and membrane forming gel are heated between about 4 and 6 hours at a temperature between about 463 K to about 493 K. Synthesis typically occurs under autogenous pressure.
- Excess membrane forming gel is removed from the support and the SAPO crystals after each synthesis step.
- the excess gel may be removed by washing with water. After washing with water, the support and SAPO crystals may then be dried.
- the synthesis step may be repeated in order to form a greater amount of SAPO crystals.
- the excess synthesis gel is removed and then the porous support is brought into contact with synthesis gel before performing the next synthesis step.
- Sufficient synthesis steps are performed so that the cumulative layer formed on the support surface by the synthesis steps and any crystal application steps forms a continuous layer.
- the SAPO membrane is formed by the cumulative layer(s) of SAPO crystals on the support surface(s) and the (interconnected) SAPO crystals formed inside the porous support.
- the SAPO crystals inside the support are substantially interconnected.
- the interconnected SAPO crystals are connected to the layers of SAPO crystals formed on the support surface.
- sufficient synthesis steps are performed that the membrane is impermeable to nitrogen after preparation (but before calcination).
- the membrane becomes a semi-permeable barrier between two phases that is capable of restricting the movement of molecules across it in a very specific manner.
- the calcination temperature is between about 600 K and about 900K, and between about 623 K and about 773 K.
- the calcining temperature can be between about 623 K and about 673 K.
- the calcination time is between about 5 hours and about 25 hours. Longer times may be required at lower temperatures in order to substantially remove the template material. Use of lower calcining temperatures can reduce the formation of calcining-related defects in the membrane.
- the heating rate during calcination should be slow enough to limit formation of defects such as cracks.
- the heating rate is less than about 2.0 K/min. In a different embodiment, the heating rate is about 0.6 K/min.
- the cooling rate must be sufficiently slow to limit membrane defect formation. In an embodiment, the cooling rate is less than about 2.0 K/min. In a different embodiment, the cooling rate is about 0.9 K/min.
- the SAPO membranes of the present invention comprise SAPO crystals which form a layer on at least one side of the porous support. SAPO crystals may also be present within at least some of the pores of the support.
- the thickness of the SAPO layer depends in part on the number of synthesis steps performed. In embodiment where synthesis steps are performed until the membrane is impermeable to nitrogen, the thickness of the cumulative SAPO layer is less than about 20 microns. When the layer thicknesses are measured from cross-sections with scanning electron microscopy, the uncertainty in the thickness measurement is believed to be on the order of +/-10%. In other embodiments, the thickness of the SAPO layer is about 5 microns, less than 5 microns or about 2.5 microns.
- immersion of a porous support in the synthesis gel can lead to formation of SAPO crystals within the support as well as on both sides of the support.
- immersion of a porous tube in the synthesis gel can lead to formation of SAPO crystals within the tube as well as formation of a SAPO layer on the inside and the outside of the tube.
- the SAPO crystals may form throughout the thic
- SAPO crystals throughout the thickness of the support indicates that the synthesis gel has penetrated to the center of the support.
- formation of SAPO crystals throughout the support does not require that SAPO crystals completely fill the pore space of the support.
- SAPO-34 membranes with high CO 2 permeance and CO 2 /CH 4 selectivity were successfully generated on supports utilizing the soaking methods and/or seeding methods described above. After 2-4 hours of soaking the support in the membrane forming gel prior to hydrothermal synthesis, the CO 2 permeance increased >100% to 2.2 10 "6 mol/(m2xsxPa) at 0.14 MPa. The CO 2 / CH 4 selectivities are greater than 200 at 0.14 MPa. Real improvement was seen at high pressure drop. The CO 2 / CH 4 selectivity at 4.6 MPa was greatly improved from 14 (0 hr soaking) to 74 (2 hr soaking) and 59 (4 hr soaking), respectively, which is >400% increase.
- All supports 200 nm and 100 nm AI2O3 and 5 nm ⁇ 2 tubes, outside diameter: 1 1 mm and inside diameter: 7 mm) were provided by Inopor Gmbh. All supports were cut into 5 cm long tubes, with the two ends glazed with high temperature ceramic glaze. Before seeding, the supports were boiled in deionized (Dl) water for 3 hours and dried at 373 K under vacuum for 30 min.
- Dl deionized
- the SAPO-34 membranes were prepared on seeded support.
- the seed gel molar ratio was 1.0 Al 2 0 3 : 2.0 P 2 0 5 : 0.6 Si0 2 : 4.0 TEAOH : 75 H 2 0.
- AI(i-C3H 7 0)3 (98%) H3PO4 (85 wt% aqueous solution) and deionized H 2 0 were stirred for 3 hours to form an homogeneous solution, and then Ludox AS-40 colloidal silica (40 wt % Si0 2 suspension in water) was added and the resulting solution stirred for another 3 hours.
- the membrane synthesis gel molar ratio was 1.0 Al 2 03: 1 .0 P 2 Os: 0.3 Si0 2 TEAOH: 1 .6 DPA: 150 H 2 O. All chemicals were purchased from Sigma-Aldrich and used as received. In a typical synthesis, Al source (AI(i-C3H 7 O)3 ,98%), H3PO4 (85 wt% aqueous solution) and deionized H 2 0 were stirred for 3 hours to form an I
- a seed suspension was prepared by mixing 0.05 wt% hydroxy propyl cellulose and 0.67 wt% SAPO-34 seed crystals with anhydrous ethanol.
- the seeded supports were then placed in an autoclave and the autoclave was filled with the membrane synthesis gel.
- the outside of the tubes were first wrapped with Teflon tape.
- the seeded supports were soaked in the membrane synthesis gel at room temperature from 0-14 hours before hydrothermal synthesis at 493 K for 6 hours, and the membranes were then washed with Dl water and dried for ⁇ 2 h at 340 K.
- the membranes were calcined in air at 670 K for 4 hours to remove the templates.
- Fluxes were measured with bubble flow meters and compositions were analyzed by a gas chromatograph (GC) (SRI 8610C) with a Hayesep D column at 373 K and a TC detector.
- GC gas chromatograph
- SRI 8610C gas chromatograph
- Hayesep D column at 373 K
- TC detector a gas chromatograph
- An automated sample loop took samples from the feed and permeates streams.
- the tubular membranes were sealed in a stainless steel module with silicone O-rings. The leak integrity of the module was verified by replacing the membrane with a solid stainless steel tube.
- the leak rate for an 8 MPa pressure drop across the O-ring was ⁇ 0.2 % of the measured CH 4 flux for a 50/50 CO2 mixture at the same pressure drop.
- a Teflon spacer was used to reduce the cross section for feed flow and thus increasing gas velocity;
- a high pressure gas booster was used to increase the feed pressure up to 7 MPa as maintaining high flux velocity in the feed side.
- J (mol/m 2 s) is the steady-state flux of C0 2 or CH 4 and Api og- mean i
- SAPO-34 seeds used herein were prepared by microwave heating.
- the crystals exhibit rectangular plate morphology, with approximately 300 nm in length and 60 nm in thickness, as shown in Figure 1 .
- the X-ray diffraction (XRD) patterns indicated that crystals had the chabazite structure of SAPO-34 (shown in Figure 2).
- the size of SAPO-34 crystals can be controlled from 100nm to micron size, as well as the morphology, such as cubic, rectangular, and spherical. Moises and others found smaller, homogeneous cubic and rectangular crystals ( ⁇ 1 ⁇ ) had superior separation performance. Smaller crystals with narrow size distributions pack better than larger crystals, and thus the size of non-zeolite pores can decrease so that higher CO 2 /CH 4 selectivities result.
- hydrothermal synthesis was utilized to prepare homogeneous SAPO-34 crystals with rectangular plate morphology.
- Table 1 shows the separation performance of SAPO-34 membranes prepared with different soaking time at 0.14 MPa and 4.6 MPa pressure drops.
- the SAPO-34 membranes prepared without any soaking treatment exhibit a wide distribution of CO 2 /CH 4 selectivity, which indicates poor reproducibility.
- membranes 1 and 2 represent two extreme cases (the other non-treated membranes are not shown here). Both M1 and M2 have high C0 2 permeance, approximi
- the C0 2 /CH 4 selectivity of M1 is 217 at 0.14 MPa pressure drop, and it dramatically decreases to 14 at 4.6 MPa. M2 is much worse than M1 and it was not tested at high pressure.
- the C0 2 /CH 4 selectivities are greater than 150 at 0.14 MPa.
- the C0 2 /CH 4 selectivity at low pressure drop is not a good indicator of membrane quality, as shown in previous publications (Adolfo et al, Journal of Membrane Science, 335 (2009), p. 32-36).
- the C0 2 /CH 4 selectivity at 4.6 MPa was greatly improved from 14 (no soaking) to 74 (soaking for 2 hours) and 59 (soaking for 4 hours), respectively, which is a >400% increase.
- the C0 2 permeance also increased >400% from 1.1 * 10 "7 mol/(m 2 s Pa) (no soaking) to 5.1 * 10 " 7 (soaking for 2 hours) and 4. Ox 10 "7 mol/(m 2 s Pa) (soaking for 4 hours). Soaking for 2 to 4 hours produced the best results at high pressure. Between 4-8 hours soaking, C0 2 /CH 4 selectivity starts to decrease, but the C0 2 permeance remains unchanged. After 14 hours soaking, the obtained SAPO-34 membrane exhibited no selectivity toward C0 2 /CH 4 separation and C0 2 permeance also decreased significantly.
- Membranes 10, 11 and 12 were soaked in the membrane synthesis gel without Teflon tape wrapping, which means the membrane synthesis gel have free access and was able to soak both sides of the seeded support surface. All three of these membranes have high C0 2 permeance and decent C0 2 /CH 4 selectivity at 0.14 MPa, with an average of 2.3x10 "6 mol/(m 2 s Pa) for C0 2 permeance and 259 for C0 2 /CH 4 selectivity. The average C0 2 permeance and C0 2 /CH 4 selectivity at 4.6 MPa are 4.1 ⁇ 10 "7 mol/(m 2 s Pa) and 72 respectively.
- M12 has C0 2 permeance of 2.9 ⁇ 10 "6 and 4.7x10 "7 mol/(m 2 s Pa) at 0.14 and 4.6 MPa, with C0 2 /CH 4 selectivity of 78 at 4.6 MPa. This provides high permeability for C0 2 /CH 4 separation while also providing decent high pressure C0 2 /CH 4 selectivity. Perhaps the biggest improvement is the reproducibility. All three membranes prepared at similar conditions show consistent high permeance and selectivity.
- Example 4 Effect of soaking and support surface on crystal size and str
- FIGS 3a-3d show SEM images of SAPO-34 membranes prepared under different conditions.
- Figure 3a was taken on a membrane grown on a glazed support area prepared with 4 hours of soaking. Membranes grown on glazed areas tend to fall off to form free-standing membranes.
- Figures 3b, 3c and 3d were taken on membranes prepared on unglazed support surfaces with 4 hours of soaking one side, 4 hours of soaking both sides, and no soaking, respectively.
- All four SEM images in Figure 3 show cubic crystals, which is typical for SAPO-34. The crystal coverage and intergrowth are excellent for all four samples.
- the crystals grown on the glazed area are approximately 13 ⁇ (Figure 3a), much bigger than crystals grown on porous areas.
- the crystals grown on non-glazed supports ( Figures 3b, 3c and 3d) show similar bimodal distribution, with crystal size of approximately 1.5 ⁇ and 6 ⁇ . This size difference might be caused by the smooth surface of the glaze, which is difficult for the seeds to attach, thus leading to fewer nuclei and bigger crystals. It appears that there is a greater amount of small crystals when a soaking treatment is used. This result is similar with Kong and Chen's results, which can be attributed to the increase in generated nuclei (Kong et al, Journal of Membrane Science 285 (2006), p. 258-264; and Chen et al, Microporous and Mesoporous Materials 102 (2007), p.249-257).
- FIGS 4a-4c show the cross-section SEM images of membranes prepared with 4 hours of soaking of one side of the support, 4 hours of soaking on both sides, and no soaking, respectively. Surprisingly, there is not a big difference in membrane thickness. Each membrane had a thickness of approximately 5-6 ⁇ . The CO2 permeance of membranes prepared with 4 hours soaking is about 100% higher than membranes prepared without soaking, which suggests the membranes should be much thinner. Kong et al (supra) obtained thinner silicalite-1 membranes when using two-stage hydrothermal synthesis; however, no permeation data were provided.
- the top layer of the molecular sieve membrane is not the only crystal layer.
- the crystals of the molecular sieve material may penetrate into the support, which contributes substantially to the overall diffusion resistance.
- Figures 5a-5f show the cross-section SEM images of SAPO-34 membrane prepared with 4 hours of soaking on both sides of the support.
- the ceramic support composes of three layers with descending pore sizes from bottom to top, marked as A, B and C.
- the thicknesses of layers C and B are approximately 8 ⁇ and 30 ⁇ , respectively.
- layers A and B many micron sized cubic SAPO-34 crystals were observed, which can be seen in Figures 5a and 5e.
- the small pores in layer B also limit the crystal size. No cubic crystals were found in layer C. However, elemental analysis verified the presence of plenty phosphorus and silicon in layer C, as well as in layer B, which indicated the presence of SAPO-34 crystals.
- the small pores (200 nm) in layer C may limit the growth of SAPO-34 crystals, which makes it difficult to find typical cubic SAPO-34 crystals with SEM.
- seed crystals When seed crystals are added to a synthesis mixture, they may (a) remain inert, (b) dissolve, (c) act as pure seeds, in that mass is deposited upon them and they grow, or (d) give rise to secondary nuclei and hence a new crop of crystals (Cundy et al, Microporous and Mesoporous Materials 82 (2005), p. 1-78). In the SAPO-34 membrane system, seeding is indispensable for high quality membranes,
- Figures 6a-6c show SEM images of powder SAPO-34 crystals collected at the bottom of an autoclave after synthesis.
- Figures 6a and 6b shows images of crystals collected during membrane synthesis with no soaking and 4 hours of soaking, respectively.
- Bimodal crystal size distribution was observed for both systems, which is consistent with crystal size distribution in top layer zeolite membranes, as shown in Figures 3a-3d. More small crystals were observed in Figure 6b, which suggests that soaking helps to generate more nuclei and leads to a greater number of small crystals.
- the bimodal crystal size distribution might be caused by the non-homogeneity of the membrane forming gel.
- seed growth and secondary nucleation are parallel reactions.
- the balance between seed growth and secondary nucleation depends on the nature of the system, the quantity of material added and the degree of agitation. If the surface area of seed crystals present is sufficient to absorb most of the available flux of growth species and thus prevent solution supersaturation from reaching high levels, then most of the growth in the system will take place on the seed crystals, whose size and growth rate can be closely controlled and predicted by kinetic modeling. As the quantity of seed material is reduced, then the natural supersaturation and
- Seeding is a powerful technique in the synthesis of zeolite and other molecular sieve membranes. Many seeding techniques, like rubbing, dip coating, and filtration have been developed for zeolite membrane synthesis. Among these seeding
- dip coating seems the easiest for large scale application. Rubbing is a simple seeding method, but rubbing cannot generate a uniformly distributed seed layer on the support surface. It is also difficult to apply rubbing to large scale membrane synthesis. Accordingly, dip coating was used to check the relationship be
- the quality of seed layer determines the quality of the molecular sieve membrane.
- a densely packed seed layer is a must for high quality membranes.
- a lower seed density was surprisingly shown to be more effective to grow optimal membranes for the present SAPO-34 membranes. This is surprising in that previously it was believed that greater seed crystal density would require less seed growth during hydrothermal synthesis and would more likely provide a thinner, uniform and interconnected crystalline layer.
- solution F 0.67 g SAPO-34 seeds and 0.05 g hydroxyl propyl cellulose were dispersed in 100 g ethanol to make a dip coating solution (0.67wt% seed concentration, designated as solution F).
- the seed concentration commonly used for dip coating is between 0.5-2.0 wt%.
- the addition of hydroxyl propyl cellulose is to increase the bonding between seed crystals and the support.
- Table 2 (shown below) lists the effect of seed concentration on separation performance of SAPO-34 membranes.
- the first six membranes were dip coated in solution F (0.05 wt% hydroxy propyl cellulose and 0.67wt% seed concentration in ethanol) from 1 to 4 times. All six membranes show relatively poor selectivity, especially at high pressure.
- the best membrane, membrane A4 has CO2/CH4 selectivities of 160 and 13 at 0.14 MPa and 4.6 MPa, respectively, and the CO2 permeances are 1 1 and 2.1 x10 "7 mol/(m 2 s Pa).
- Figures 7a-7c show SEM images of supports dip coated in seed s different concentrations. No dense-packed seed layer was observed even when undiluted solution F was used. When seed solution was diluted 4 times (Figure 7a), many seeds were observed easily on the support surface and the seed coverage is around 50%. Further dilution of seed concentration greatly reduces the seed density on the support surface. Only 17 seed crystals were observed in an area of 9 ⁇ 7 ⁇ when seed concentration was diluted 16 times ( Figure 7b) and only several crystals were found with 128 times dilution (Figure 7c). Lower seed density is a key to high quality SAPO-34 membranes.
- Figures 8a-8b show the top view and cross-section view of a SAPO-34 membrane prepared with a dip coating seeding method. Optimal coverage and intergrowth were observed. The crystal size and membrane thickness are 10 ⁇ and 8 ⁇ , respectively. Both are larger than membranes prepared with a rubbing seeding method. This can be explained by the lower seed density on the support, which leads to fewer secondary nuclei and bigger crystals and thickness. This also explains the lower permeance of dipcoated membranes.
- Table 3 shows the effect of soaking time and seeding on membrane performance.
- Membranes A8 and A14 were soaked in membrane forming gel on one side (inside surface) and both sides, respectively. Prior to soaking, both membranes were dipcoated in seed solution F diluted 8 times. Soaking both sides of the membrane in the membrane forming gel resulted in the C0 2 /CH 4 sele
- Membrane A15 prepared by dipocating in a seed solution F diluted 16 times but without soaking treatment, exhibits decent C0 2 permeance of 12 x10 "7 mol/(m 2 s Pa) and C0 2 /CH 4 selectivity of 69 at 4.6 MPa pressure drop. This result suggests that low seed density is a key factor in determining membrane quality.
- the soaking treatment significantly improves the C0 2 permanence (by 58%) and C0 2 /CH 4 selectivity (by 39%) at 4.6 MPa.
- the increase of permeance can be explained as the secondary nuclei generated during soaking treatment, which leads to a thinner membrane.
- Table 4 shows the effect of support pore size on the membrane performance. Besides 200 nm AI2O3 support, 100 nm AI2O3 and 5 nm T1O2 supports were also tried. Similar results were obtained for all supports. Despite the limited number of
Abstract
SAPO molecular sieve membranes, particularly SAPO-34 membranes, with high flux are prepared using hydrothermal synthesis. Additional soaking and seeding treatments are performed to improve the CO2/CH4 separation performance of the SAPO membranes. Dip coating and other methods are used to seed the porous support with a low seed density prior to hydrothermal synthesis for improved performance. In the soaking treatment, the support is soaked in the membrane forming gel for several hours before hydrothermal synthesis. SAPO-34 membranes prepared with these new treatments have higher permeance, higher selectivity and improved reproducibility. Soaking the support from both sides also leads to better selectivity.
Description
HIGH-FLUX SAPO-34 MEMBRANES FOR C02/CH4 SEPARATIONS
CROSS-REFERENCETO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Provisional Application No. 61/285,703, filed December 1 1 , 2009, which is incorporated by reference in its entirety.
BACKGROUND OF THE INVENTION
[0002] This invention is in the field of molecular sieve membranes, in particular silicoaluminophosphate (SAPO) membranes, prepared on a porous support. The invention provides supported molecular sieve membranes having improved gas separation properties as well as methods for making and using them.
[0003] Molecular sieve membranes, including SAPO membranes, are often used in gas separations. For these applications, an important parameter is the separation selectivity. For two gas components i and j, a separation selectivity S, / j greater than one implies that the membrane is selectively permeable to component i. If a feedstream containing both components is applied to one side of the membrane, the permeate stream exiting the other side of the membrane will be enriched in component i and depleted in component j. The greater the separation selectivity, the greater the enrichment of the permeate stream in component i.
[0004] In the past two decades, extensive research has been devoted to SAPO and zeolite molecular sieve membranes because they have higher thermal and chemical properties compared with those of polymer membranes. Many types of zeolite and zeolite-type membranes have been studied such as MFI, LTA, MOR, and FAU-type membranes. (See Sano et al, Chem. Lett. 12 (1992), p. 2413; Bakker et al, J. Membr. Sci. 1 17 (1996), p. 57; Lai et al, Ind. Eng. Chem. Res. 37 (1998), p. 4275; Liu et al,
Chem. Commun. (2000), p. 1889; Bernal et al, Catal. Today 67 (2001 ), p.
et al, J. Membr. Sci. 52 (2002), p. 179; Lai et al, Science 300 (2003), p. 4bb; Li et al, Ind. Eng. Chem. Res. 40 (2001 ), p. 4577; Kita et al, J. Mater. Sci. Lett. 14 (1995), p. 206; Kondo et al, J. Membr. Sci. 133 (1997), p. 133; Jafar and Budd, Microporous Mater. 12 (1997), p. 305; Aoki et al, J. Membr. Sci 141 (1998), p. 197; Kumakiri et al, Ind. Eng. Chem. Res. 38 (1999), p. 4689; Okamoto et al, Ind. Eng. Chem. Res. 40 (2001 ), p. 163; Morigami et al, Sep. Purif. Technol. 25 (2001 ), p. 251 ; Van den Berg et al, J. Membr. Sci. 224 (2003), p. 29; Pina et al, J. Membr. Sci. 244 (2004), p. 141 ;
Huang et al, J. Membr. Sci. 245 (2004), p. 41 ; Sato et al, J. Membr. Sci. 301 (2007), p. 151 ; Nishiyama et al, J. Chem. Soc. Chem. Commun. (1995), p. 1967; Tavolaro et al, J. Mater. Chem. 10 (2000), p. 1 131 ; Zhang et al, J. Membr. Sci. 210 (2002), p. 361 ; Li et al, Microporous Mater. 62 (2003), p. 211 ; Nikolakis et al, J. Membr. Sci. 184 (2001 ), p. 209; Kita et al, Sep. Purif. Technol. 25 (2001 ), p. 261 ; Matsukata and Kikuchi, Bull.
Chem. Soc. Jpn. 70 (1997), p. 2341 ; Caro et al, Microporous Mesoporous Mater. 38 (2000), p. 3; and Bowen et al, J. Membr. Sci. 245 (2004), p. 1 ).
[0005] However, to date it is believed there is only one reported commercial
application of zeolite membranes, namely Na-A membranes for dehydration of alcohol (Morigami et al, Sep. Purif. Technol. 25 (2001 ), p. 251 ). One of the reasons why it is so difficult to commercialize zeolite and zeolite-type membranes is the incomplete development of fabrication technology and poor reproducibility. In particular, few studies have reviewed reproducibility in order to evaluate current fabrication methods (Hedlund et al, J. Membr. Sci. 52 (2002), p. 179; Sato et al, J. Membr. Sci. 301 (2007), p. 151 ; and Jolinde et al, Ind. Eng. Chem. Res. 37 (1998), p. 4071 ).
[0006] Although it is a great challenge to make defect-free zeolite and zeolite-type membranes in commercial scale, many new techniques including but not limited to support pre-treatment, seeding, gel recipe, calcination, and defect blocking have been developed to conquer this problem. In general, seeding is a powerful technique and many seeding techniques, such as rubbing, dip coating and filtration, have been developed for the synthesis of zeolite membranes. The use of seeds can help to control the growth of the zeolite layer, crystal size and intergrowth. It is believed that the seed
size, morphology, seed density and packing on the support, support size,
pretreatment are important in determining the quality of zeolite membranes (Matsukata and Kikuchi, Bull. Chem. Soc. Jpn. 70 (1997), p. 2341 ; Caro et al, Microporous
Mesoporous Mater. 38 (2000), p. 3; and Bowen et al, J. Membr. Sci. 245 (2004), p. 1 .26-28).
[0007] Engstrom and co-workers prepared silicalite-1 film on gold surface using 60, 165 and 320 nm zeolite seeds, respectively. They found that the grain packing was better and the film was less rough when the smaller seeds were used (Engstrom et al, Micropor. Mesopor. Mater. 38 (2000) 51 ).
[0008] Zhang et al prepared silicalite-1 membranes on porous a-alumina supports using silicalite-1 zeolite seeds of around 100 nm, 600 nm, 1 .5 μηπ, 3.0 μηι and 7.5 μηπ. The seed layers and membranes prepared from smaller seeds had smoother surfaces and fewer defects. Oversized seeds (7.5 μηπ) led to the formation of a discontinuous seed layer, which resulted in the formation of an discontinuous and uneven zeolite membrane (Zhang et al, Materials Chemistry and Physics 96 (2006) 42-50). Zhang et al investigated the formation of silicalite-1 seed layers on a porous carbon support of 0.5 μιη pore size and a-A^Os supports with different pore sizes (0.1 μπι and 4 μηι) via the slip-casting technique. It was found that a continuous seed layer was obtained on the smooth support of 0.1 μηι pore size by using any seed of 100 nm, 600 nm or 2.2 μιη in size, whereas, on the coarse supports with either 0.5 μηι or 4 μηι pore size, a
continuous seed layer was not formed using the above seed sizes and the same seeding time. The seed layers and membranes grown from the smaller seeds are more uniform and continuous and possessed smoother surfaces than those from the larger seed. However, the conclusions from this study were mainly based on SEM images, not gas permeation or separation studies (Zhang et al, Front. Chem. Eng. China, 1 (2) (2007), 172-177).
[0009] Hasegawa et al prepared MFI-type zeolite membranes on porous mullite tubes by secondary growth from seeds with different particle sizes (100 ~ 1000 nm)
(Hasegawa et al, Journal of Membrane Science 280 (2006) 397-405). Morphologies of
the membranes were almost the same. The size and amount of the seed
influenced the i-C4Hio permeances for pure and binary systems, while the n-u4Hio permeances were almost constant. The n-C4Hio/i-C4Hio permselectivity was as high as 220 for single-components. The separation factor for binary system was up to 58.
Those results indicate that the pore structures of the membranes can be controlled by the density of seed particles.
[0010] Besides the advance of seeding techniques, recently, a two-stage hydrothermal synthesis method was used to make MFI, A and Y membranes. Kong et al used a two- stage varying-temperature in situ synthesis (TSVTS) for Silicalite-1 membranes (Kong et al, Journal of Membrane Science 285 (2006) 258-264). Compared with one-stage synthesis, it was suggested that thinner membranes can be generated using a two- stage synthesis for these particular types of membranes. Since a large number of new nuclei can form at low temperature while the crystallization temperature rapidly changes to a high temperature after completion of the nucleation period, a thin and continuous membrane can quickly form on the support by the nuclei re-growth.
[0011] Chen et al used one-step and two-step in situ hydrothermal synthesis to prepare high-performance silicalite-1 membranes on porous silica tubes (Chen et al, Microporous and Mesoporous Materials 102 (2007) 249-257). It was found that both aging temperature and aging time showed significant influence on the membrane performance. By choosing suitable aging conditions, for example, aging synthesis solution and silica tubes together at 348 K for 8 h, a silicalite-1 membrane with ethanol/water separation factor of 81 and total flux of 0.39 kg/m2 h at 333 K was obtained by only one step in situ hydrothermal synthesis. Although the membrane thickness with two-step synthesis was much higher than that obtained by one-step synthesis, the former had higher flux than that of the latter.
[0012] Li et al used in situ aging, a method utilizing microwave heating, to prepare high quality LTA zeolite membranes without seeding (Li et al, Journal of Membrane Science 277 (2006) 230-239). It was found that in situ aging temperature and microwave heating time had the most significant influences on the synthesis. LTA zeolite
membranes showed superior gas permeation properties (permeance of h
molm"2 s"1 Pa-1, permselectivities for H2/N2 and H2/C3H8 were 5.60 and 9.1 ,
respectively). Through this method, LTA zeolite membranes with good pervaporation performance were prepared with high reproducibility (water/ethanol selectivity>10000). Li et al found that a gel layer is first formed on the support after in situ aging, which contains a suitable amount of pre-nuclei. During the following microwave assisted crystallization, it is believed these pre-nuclei rapidly and simultaneously develop into crystal nuclei, crystal growth occurs by propagation through the amorphous primary particles (with size of ca. 50 nm), and finally, the amorphous particles transform into LTA crystal particles with the same size (Li et al, Journal of Membrane Science 281 (2006) 646-657).
[0013] In situ microwave synthesis of high-quality FAU-type zeolite membranes were achieved by the aging method. In situ aging was able to suppress the formation of LTA phase and facilitated the nucleation of FAU-type zeolite crystals. Water/ethanol selectivity >10000 was obtained (Zhu et al, Journal of Membrane Science 337 (2009) 47-54).
[0014] Barri et al disclosed supported zeolite membranes (U.S. Patent 5,567,664) and methods for the production of zeolite membranes on porous supports (U.S. Patent 5,362,522). Barri et al. state that any type of zeolite-type material may be used, including silicoaluminophosphates (SAPOs). SAPOs are largely composed of Si, Al, P and O and can have a three-dimensional microporous crystal framework structure of PO2 +, AIO2 " and SiO2 tetrahedral units. The cages, channels and cavities created by the crystal framework can permit separation of mixtures of molecules based on their effective sizes.
[0015] SAPO crystals can be synthesized by hydrothermal crystallization from a reaction mixture containing reactive sources of silica, alumina, and phosphate, and an organic templating agent. Lok et al (U.S. 4,440,871 ) report gel compositions and procedures for forming several types of SAPO crystals, including SAPO-5, SAPO-1 1 , SAPO-16, SAPO-17, SAPO-20, SAPO-31 , SAPO-34, SAPO-35, SAPO-37, SAPO-40,
SAPO 41 , SAPO-42, and SAPO-44 crystals. However, Lok et al do not a
disclose formation of molecular sieve membranes from SAPO crystals. Similarly, Prakash and Unnikrishnan report gel compositions and procedures for forming SAPO- 34 crystals. (Prakash and Unnikrishnan, J. Chem. Sc. Faraday Trans., 90(15) (1994), p. 2291 -2296). In several of Prakash and Unnikrishnan's reported procedures, the gel was aged for 24 hours at 27 °C (300 K). However, Prakash and Unnikrishnan also do not appear to disclose formation of SAPO-34 membranes.
[0016] Synthesis of SAPO-34 membranes on porous supports has been reported in the scientific literature. Lixiong et al. (Stud. Surf. Sci. Catl., 105 (1997), p 221 1 ) reported synthesis of a SAPO-34 membrane on one side of a porous a-AI2O3 disk by immersing the substrate surface in a hydrogel and heating the substrate and gel.
Lixiong et al. reported single gas permeances for H2, N2, CO2, and n-C4Hi0. Poshuta et al. (Ind. Eng. Chem. Res., 37 (1998), p. 3924-3929; and AIChE Journal, 46(4) (2000), 779-789) reported hydrothermal synthesis of SAPO-34 membranes on the inside surface of asymmetric, porous a-AI2O3 tubes. Poshuta et al reported single gas and mixture permeances and ideal and mixture selectivities for several gases, including CO2 and CH4. The CO2/CH4 selectivities reported for a 50/50 CO2/CH4 mixture at 300K were between 14 and 36 for a feed pressure of 270 kPa and a pressure drop of 138 kPa (Poshusta et al, AIChE Journal, 46(4) (2000), pp 779-789). The CO2/CH4 selectivity was attributed to both competitive absorption (at lower temperatures) and differences in diffusivity. Li et al. reported an average CO2/CH4 selectivity of 76+/- 19 for a 50/50 CO2/CH4 mixture at 295 K with a feed pressure of 222 kPa and a pressure drop of 138 kPa. The average CO2 permeance was (2.3 +/- 0.2) X 10"7 mol/(m2sPa) and the average CH4 permeance was (3.1 +/- 0.8) x 10"9 mol/(m2sPa). (Li et al, Ind. Eng. Chem. Res. 44 (2005), 3220-3228.) U.S. Patent Application Publication 2005-0204916-A1 to Li et al. reports CO2/CH4 separation selectivities of 67-93 for a 50/50 CO2/CH4 mixture at 297 K with a feed pressure of 222 kPa and a pressure drop of 138 kPa. U.S. Patent Application Publication 2007-0265484 to Li et al. discloses methods for making SAPO- 34 membranes including methods where the membrane forming gel is aged prior to contact with the porous support and hydrothermal synthesis.
[0017] Several U.S. patents report processes for the manufacture of mo
layers on a support which involve depositing or forming molecular sieve crystals on the support prior to an in situ synthesis step. U.S. Patent 6,090,289 to Verduijn et al.
reports a process which involves forming an intermediate layer by applying molecular sieve crystals to the support or forming such crystals on the support, contacting the resulting coated support with a molecular sieve synthesis mixture and then subjecting the mixture to hydrothermal treatment in order to deposit an upper layer of a crystalline molecular sieve of crystals. U.S. Patent 6,177,373 to Sterte et al. reports a process which involves depositing a monolayer on a substrate where the monolayer comprises molecular sieve monocrystals which are capable of nucleating the growth of a molecular sieve film. U.S. Patent 5,871 ,650 to Lai et al reports a process for preparing a zeolite membrane exhibiting a columnar cross-sectional morphology.
[0018] Moises et al prepared SAPO-34 membranes on ceramic support (Moises et al, J. Am. Chem. Soc, 130 (16) (2008), pp 5412-5413; and Moises et al, Advanced Materials, 20(4) (2008), p. 729-732). It was found that membranes made with smaller, homogeneous cubic and rectangular crystals (< 1 μηπ) had superior separation performance. It is believed that smaller crystals with narrow size distributions pack better than larger crystals, and thus the size of non-zeolite pores can decrease so that higher CO2/CH4 selectivities result. The best membrane had CO2 permeance of 2x10"6 mol/(m2 s Pa) with CO2/CH4 selectivity of -170 at a 0.14 MPa pressure drop.
[0019] In previous studies, it has been shown that polycrystalline SAPO-34 molecular sieve membranes can have high selectivities for CO2/CH4 separations, even at high pressures (Moises et al, J. Am. Chem. Soc, 130 (16) (2008), pp 5412-5413; Moises et al, Advanced Materials, 20(4) (2008), p. 729-732; and Adolfo et al, Journal of
Membrane Science, 335 (2009), p. 32-36). The separation of CO2 from CH4 with membranes has potential applications in natural gas processes where CO2 has to be removed at pressures of 7 MPa or higher to increase the heating value and minimize corrosion in the pipelines. The selectivity of the SAPO-34 membranes decreases as the feed pressure increased because flux is not only through the SAPO-34 pores but also through defects between crystals. Knudsen diffusion and viscous flow through these
defects contribute proportionally more to the flux at higher pressures. Thi adsorption isotherm for SAPO-34 indicates that the flux through the SAP -34 pores is not expected to increase linearly with pressure because the pores approach saturation loading.
[0020] Therefore, despite advances in this field, there remains a need in the art for improved methods for making molecular sieve membranes, in particular SAPO membranes, with improved gas separation selectivities and methods.
SUMMARY OF THE INVENTION
[0021] The present invention provides molecular sieve membranes, particularly silicoaluminophosphate (SAPO) membranes such as SAPO-34 membranes, prepared on porous supports and methods of making such membranes. The membranes of the present invention provide increased permeance and selectivity properties for use in gas separations, particularly carbon dioxide (CO2) and methane (CH4) separations.
Inorganic membranes such as SAPO membranes can have superior thermal, mechanical and chemical stability, good erosion resistance, and high pressure stability as compared to conventional polymeric membranes.
[0022] SAPO membranes are generally prepared by contacting a membrane forming gel with a porous support and heating for several hours at temperatures in excess of 420 K. The membrane forming gel generally comprises AI2O3, P2O5, S1O2, H2O and one or more organic templates. Preferably the organic template is a quaternary organic ammonium templating agent. Where more than one organic templating agent is used, the organic template preferably includes at least one quaternary organic ammonium templating agent and one or more amines. After hydrothermal synthesis, the
membranes are typically washed, dried and calcined to remove the organic template. Optionally, the membrane forming gel is aged several hours to several days prior to contacting the membrane forming gel with the porous support. SAPO crystals able to make molecular sieve membranes according to the methods discussed herein include,
but are not limited to, SAPO-5, SAPO-11 , SAPO-16, SAPO-17, SAPO-20
SAPO-34, SAPO-35, SAPO-37, SAPO-40, SAPO 41 , SAPO-42, and SAPu-44 crystals.
[0023] In one embodiment, the present invention provides SAPO membranes, particularly SAPO-34 membranes, prepared using hydrothermal synthesis where an additional soaking treatment is performed. In an embodiment, a porous support, preferably a seeded porous support, is soaked in the membrane forming gel for several hours prior to the hydrothermal synthesis. In one embodiment, the porous support is soaked in the membrane forming gel at a temperature between 10° C and 100° C, more preferably between 20° C and 30° C. Optionally, the porous support is soaked in the membrane forming gel at room temperature (defined herein as 25° C to 30° C). SAPO membranes prepared with this additional soaking treatment have higher permeance, higher selectivity and improved reproducibility than membranes prepared without this soaking treatment. One side or both sides of the porous support may be soaked in the membrane forming gel prior to the hydrothermal synthesis. Typically, a SAPO
crystalline layer is only formed on one surface of the porous support during
hydrothermal synthesis; however, soaking both sides of the porous support prior to synthesis of the layer can lead to increased selectivity. Without wishing to be bound by theory, where the porous support is also seeded, it is believed the soaking treatment dissolves excessive seed crystals and generates more nuclei on the support surface, which leads to better coverage and higher permeance.
[0024] In one embodiment, the present invention provides a method for making a crystalline silicoaluminophosphate (SAPO) membrane comprising the steps of: a) providing a porous support having a first and a second side, which are optionally seeded; b) preparing an aqueous SAPO forming membrane forming gel, wherein the membrane forming gel comprises aluminum, phosphorus, silicon, oxygen, an organic templating agent and water; c) soaking at least one side of the porous support in the membrane forming gel for two or more hours at a temperature between 10° C and 100° C; d) following the soaking step, heating the porous support and the membrane forming gel between about 420 K and about 540 K to form a layer of SAPO crystals on the porous support; and e) calcining the SAPO layer to remove the templating agent. At
least one side of the porous support may be soaked in the membrane fori
temperature between 20° C and 30° C, or at room temperature. Optionally, at least one side of the porous support is soaked in the membrane forming gel between one and fourteen hours prior to heating, between two and five hours, or between three and four hours. In a further embodiment, both sides of the porous support are soaked in the membrane for two or more hours, preferably between two and fourteen hours, preferably between three and five hours, even more preferably between three and four hours. Preferably, the SAPO forming membrane forming gel is a SAPO-34 forming membrane forming gel and the resulting molecular sieve membrane is a SAPO-34 membrane.
[0025] The porous support and the membrane forming gel are heated between about 453 K and about 533 K, preferably between about 470 K and about 515 K, more preferably between about 463 K and about 493 K. Following the soaking step, the porous support and membrane forming gel are preferably heated for a time greater than two hours, preferably between 2 and 25 hours. In one embodiment, the porous support and membrane forming gel are heated between about 15 and about 25 hours, preferably between about 20 and 25 hours. In another embodiment, the porous support and membrane forming gel are heated between about 3 and about 8 hours, preferably between about 4 and 6 hours. In a further embodiment, the porous support and membrane forming gel are heated between about 4 and 6 hours at a temperature between about 463 K to about 493 K.
[0026] In one embodiment, at least part of the surface of the porous support is seeded with SAPO crystal material prior to contact with the membrane forming gel. By
"seeding" it is meant that a small amount of crystals, preferably crystals of the same material that is to be grown or formed, is applied to the porous support prior to contact with the membrane forming gel.
[0027] The size of the crystals applied to the support surface can vary. In different embodiments, the average size of these seed crystals or particles is less than or equal to 5 microns, less than or equal to 3 microns, less than or equal to 1 micron, or less
than or equal to 0.8 microns. In further embodiments, the average size o
crystals or particles is less than 1 micron, from 25 nm to 1 .0 micron, from bU nm to 1 UUU nm, from 100 nm to 1000 nm, from 25 nm to 500 nm, from 50 nm to 500 nm or from 100 nm to 500 nm. The seed crystals or particles can exhibit cubic, cuboid and rectangular plate morphology. If the crystals are cubic, the characteristic size of the crystals is the average length or width of the cubes. If the crystals are cuboid (a parallelepiped of which all the faces are rectangular) the characteristic size of the crystals may be considered to be the average of the longest dimension of the crystals, the shortest dimensions of the crystals, or the average of the longest and shortest dimensions. The average size of the longest and shortest dimensions of cubic and cuboid crystals may be estimated through measurement of the length and width, respectively, of one face of each crystal. In an embodiment, the characteristic size is considered to be the average of the longest and shortest dimensions. If the crystals exhibit rectangular plate morphology, the characteristic size may be considered to be the average of the longest dimension (length) while the shortest dimension can be considered to be the plate's thickness. In different embodiments, seed crystals with rectangular plate morphology have a length less than or equal to 5 microns, less than or equal to 3 microns, less than or equal to 1 micron, or less than or equal to 0.8 microns, and a thickness less than or equal to 1 micron, less than or equal to .6 microns, less than or equal to .2 micron, or less than or equal to 0.4 microns. In further embodiments, seed crystals with
rectangular plate morphology have where the width and length of the prism being greater than the thickness, the average of the width and length can be less than 1 micron, from 25 nm to 1 .0 micron, from 50 nm to 1000 nm, from 100 nm to 1000 nm, from 50 nm to 500 nm or from 100 nm to 500 nm. In different embodiments, the standard deviation of the size distribution is less than or equal to 0.5 microns, less than or equal to 0.1 microns, less than or equal to 20% of the average, less than or equal to 15% of the average, or less than or equal to 10% of the average. In an embodiment, the ratio of the average longest dimension to the average shortest dimension of cuboid crystals is less than or equal to 2 or less than or equal to 1 .5.
[0028] Seeding can be accomplished using a variety of methods, including but not limited to dip coating and rubbing. Without wishing to be bound by theory, it is believed
that the deposited seed crystals provide nucleation sites for crystal growtl
synthesis of the SAPO layer. However, in the present invention, a lower seed density than currently used in similar seeding methods on the porous support provides membranes having improved gas separation properties. This is surprising in that previously it was believed that greater seed crystal density would require less seed growth during hydrothermal synthesis and would more likely provide a thinner, uniform and interconnected crystalline layer. Providing a low seed density on the porous support is itself challenging in that it is often problematic to uniformly disperse the seed crystals at low density.
[0029] As used herein, a "low density" with regard to "seeding" and "seed crystals" means that the seed crystals are deposited on the porous support or other surface so that the seed crystals do not form a continuous layer of crystals across the support or surface. More specifically, a low density of seed crystals deposited on the porous support is less than 1 g/m2 (where this value is calculated as the weight of deposited crystals divided by the approximate surface area over which the particles are applied) and would cover approximately 5% or less of the support surface excluding the area occupied by the pores.
[0030] In one embodiment of the present invention, a low density of seed crystals is provided on the porous support for improved performance. In a further embodiment, dip coating is used to provide a low seed density on the porous support. In some
embodiments, a low density of seed crystals is applied to the porous support followed by soaking the seeded porous support in the membrane forming gel; however, the lower seed density on the support surface is a key factor in determining membrane quality, even without a soaking treatment. In one embodiment, the average amount of seed particles deposited on the support is 0.6 g/m2 or less, where this value is calculated as the weight of deposited crystals divided by the approximate surface area over which the particles are applied. In a further embodiment, the average amount of seed particles deposited on the support is 0.4 g/m2 or less, 0.2 g/m2 or less, or 0.1 g/m2 or less.
[0031] In another embodiment, the seed crystals deposited on the poroi
cover approximately 1 % or less of the support surface excluding the area occupied by the pores. In another embodiment, the seed crystals deposited on the porous support cover approximately 0.1 % or less, preferably 0.01 % or less, even more preferably 0.001 % or less of the support surface excluding the area occupied by the pores.
[0032] Dip coating in a solution having a limited concentration of seed crystals has been found to deposit seed crystals with suitable density and uniformity. In an embodiment, the porous support is dipped one or more times in a SAPO crystal seed solution comprising between approximately 0.005 wt% and 0.67 wt% of the SAPO crystal seed material. Preferably, the SAPO crystal seed solution comprises between approximately 0.02 wt% and 0.042 wt% of SAPO crystal seed material. In one embodiment, the SAPO crystal seed solution is a SAPO-34 seed solution and contains between approximately 0.005 wt% and 0.67 wt% of SAPO-34 crystal seed material, preferably between approximately 0.02 wt% and 0.042 wt% of SAPO-34 crystal seed material. The porous support can be dipped in the seed solution for any amount of time suitable to transfer the desired amount of seed crystals to the porous support. In one embodiment, the porous support is dipped in the seed solution for a time between 1 second and 10 minutes, more preferably between 5 seconds and 1 minute, even more preferably between 5 seconds and 15 seconds. In a further embodiment, the porous supports are dipped one or more times in a SAPO crystal seed solution, preferably a SAPO-34 seed solution, for 10 seconds and withdrawn from the seed solution at a rate between about 0.1 and 5 cm/second, preferably at a rate of between about 0.8 and 1 .2 cm/second, even more preferably at a rate of about 1 cm/second. While the porous support can be dipped multiple times in the seed solution, good results can be obtained with only a single dip coating. Preferably, the porous support is seeded and soaked in the membrane forming gel prior to synthesis. However, SAPO membranes with improved selectivity may be formed using the dip coating treatment without soaking, or using the soaking treatment without the dip coating treatment. For dip coated supports, the soaking treatment improved permeance significantly.
[0033] The porous support is a body capable of supporting the SAPO m
porous support may be of any suitable shape, including disks, flat panels, and tubes. The porous support is a metal, ceramic or an inorganic material. In one embodiment, the porous support does not appreciably dissolve or form reaction products at the interface when placed in contact with the synthesis gel. Suitable inorganic porous supports include, but are not limited to, a-alumina, glass, titania, zirconia, carbon, silicon carbide, clays or silicate minerals, aerogels, supported aerogels, and supported silica, titania and zirconia. In one embodiment, the porous support is aluminum oxide (AI2O3). Suitable porous metal supports include, but are not limited to, stainless steel, nickel based alloys (Inconel, Hastalloy), Fecralloy, chromium and titanium. The metal may be in the form of a fibrous mesh (woven or non-woven), a combination of fibrous metal with sintered metal particles, and sintered metal particles. In an embodiment, the metal support is formed of sintered metal particles. Ceramic supports having different porosity layers are commercially available (for example Membralox ceramic membranes available from Pall Corp.) The average pore size of the support can range from 2 nm to 500 nm, preferably between 5 nm to 200 nm. In further embodiments, the pore size is between 100 nm to 200 nm, 25 nm to 500 nm, or 50 nm to 300 nm.
[0034] In an embodiment, the pore size of the support is relatively uniform throughout the support. In this case, the pore size at the surface of the support can be
characterized by the pore size of the support as a whole. In an embodiment, the pore size characteristic of the surface of the support may be taken as the pore size characteristic of the support as a whole.
[0035] In an embodiment, the support may have a different pore size at or near the surface on which the membrane is to be formed than the pore size away from the surface. For example, the support may have two well-defined regions, a first layer with a smaller average pore size (on which the membrane is to be formed) and a second layer with a larger average pore size. When the support has regions or layers which differ in pore size, the pore size at the surface can be characterized by pore size of the region or layer nearest the surface on which the membrane is to be formed. In an
embodiment, the pore size characteristic of the surface of the support ma
the pore size characteristic of the surface layer or region of the support.
[0036] In an embodiment, the pore diameter of the support or the surface region of the support is large enough to allow the synthesis gel to penetrate the support. When SAPO-34 crystals are applied to the surface of the support prior to in situ synthesis, the pore size of the support or of its surface region can be smaller than, equal to, or greater than the characteristic size of the particles. In an embodiment, the average
characteristic size of the loose SAPO crystals is larger than the average pore size of the support. This limits the extent of penetration of the crystals inside the support. Often, a porous support will have a distribution of pore sizes. In an embodiment, the pore diameter of the support or the surface region of the support is greater than about 0.1 microns. The pore diameter of the support being greater than about 0.1 microns does not require that every single pore in the support is greater than about 0.1 microns, but it does exclude supports having regions where the characteristic pore size is about 0.1 microns (for example, a support having a layer with an 0.1 micron average pore size). The characteristic pore size may be taken as the average, median or largest pore size.
[0037] Typically, the SAPO layer is formed on just one side of the porous support. When the porous support is a disk or panel having an upper and lower surface, the SAPO layer is typically formed on either the upper surface or the lower surface. When the support is in the form of a tube having an inner and an outer surface, the SAPO layer is formed on either the inner or the outer surface. In some embodiments, the SAPO layer is formed on both sides of the porous support, such as both the upper and lower surface of a disk or panel and both the inner and outer surfaces of a tube. The membranes of the present invention have a SAPO layer having a thickness of less than about 10 μηπ, preferably between approximately 5 μηι and 6 μηπ.
[0038] In one embodiment, the present invention provides a method for making a crystalline silicoaluminophosphate-34 (SAPO-34) membrane comprising the steps of: a) providing a porous support having a first and a second side; b) preparing an aqueous SAPO-34 forming membrane forming gel, wherein the membrane forming gel comprises
aluminum, phosphorus, silicon, oxygen, an organic templating agent and
applying a low density of SAPO-34 seed material to at least the first side of the porous support; d) contacting at least the first side of the porous support with the membrane forming gel; e) heating the porous support and the membrane forming gel between about 420 K and about 540 K to form a layer of SAPO-34 crystals on the porous support; and f) calcining the SAPO-34 layer to remove the templating agent. Optionally, the membrane forming gel comprises a quaternary ammonium templating agent and at least on amine templating agent. Optionally, SAPO-34 seed material is also applied to the second side of the porous support. In one embodiment, 0.6 g/m2 or less of SAPO- 34 seed material is applied to at least a portion of the first side and/or second side of the porous support. Another embodiment of the invention comprises applying 0.4 g/m2 or less of SAPO-34 seed material to at least a portion of first side and/or second side of the porous support. Another embodiment of the invention comprises applying 0.2 g/m2 or less of SAPO-34 seed material to at least a portion of the first side and/or second side of the porous support. Another embodiment of the invention comprises applying 0.1 g/m2 or less of SAPO-34 seed material to at least a portion of the first side and/or second side of the porous support. In a further embodiment, the first and second sides of the porous support are contacted with the membrane forming gel.
[0039] In a further embodiment, prior to the heating of step e), the first side or the first and second sides of the porous support are soaked in the membrane forming gel between one and fourteen hours. A further embodiment comprises soaking the first side or the first and second sides of the porous support between two and five hours in the membrane forming gel. A further embodiment comprises soaking the first side or the first and second sides of the porous support between three and four hours in the membrane forming gel. The first side or the first and second sides of the porous support are soaked in the membrane forming gel at a temperature between 10° C and 100° C, at a temperature between 20° C and 30° C, or at a temperature between 250° C and 30° C.
[0040] In certain embodiments, the seed crystal material may be applied by dipping the porous support one or more times in a SAPO-34 seed solution comprising between
approximately 0.005 wt% and 0.67 wt% of SAPO-34 seed material, prefe
in a SAPO-34 seed solution comprising between approximately 0.02 wt% and Ό .Ό4Ζ wt% of SAPO-34 seed material. A further embodiment comprises dipping the porous supports one or more times in a SAPO-34 seed solution for 10 seconds and
withdrawing the porous supports from the seed solution at a rate of about 1 cm/second. Such a dipping procedure has been found to provide suitable surface densities and surface distribution of seed crystals.
[0041] In one embodiment, the aqueous SAPO-34 forming membrane forming gel has the formula:
1 .0 AI2O3: aP2O5: bSiO2: cR: dH2O Formula 1 ,
where R is a templating agent, a is between about 0.01 and about 52, b is between about 0.03 and about 196, c is between about 0.2 and about 5 and d is between about 20 and about 300. Preferably, c is less than about 2. In a further embodiment a is about 1 , b is 0.03-0.6, c is 1.07-1.2 and d is 55-56. In other embodiments, the ratio of silicon to aluminum is greater than 0.1 , greater than 0.10 and less than or equal to 0.6, between 0.10 and 0.6, between 0.15 and 0.45, from 0.15 to 0.3, between 0.15 and 0.3, from 0.15 to 0.2, or is about 0.15. Preferably, R is a quaternary ammonium templating agent. In a further embodiment, the quaternary ammonium templating agent is selected from the group consisting of tetrapropyl ammonium hydroxide (TPAOH), tetrapropyl ammonium bromide, tetrabutyl ammonium hydroxide, tetrabutyl ammonium bromide, tetraethyl ammonium hydroxide (TEAOH), or tetraethyl ammonium bromide.
[0042] In one embodiment, the aqueous SAPO-34 forming membrane forming gel has the formula:
AI2O3: aP2O5: bSiO2: cRi: dR2: eH2O Formula 2,
where a is greater than 0.5 and less than 1 .5, b is greater than 0.2 and less than 1 .0, c is greater than or equal to 1 and less than 2, and d is greater than zero and less than 4.0 and e is greater than 50 and less than 1 10. In different embodiments, the ratio of silicon to aluminum is greater than 0.1 , between 0.15 and 0.45, from 0.15-0.45, between 0.15 and 0.3, from 0.15-0.3, between 0.15 and 0.2, from 0.15 to 0.2 and is about 0.15.
Ri is a quaternary organic ammonium templating agent, and R2 is an ami
an amine having a molecular weight (Mn) of less than or equal to 300 and/or equal to or less than 12 carbon atoms. In an embodiment, the value of parameter c is greater than or equal to 1 and less than or equal to 2. In another embodiment, the value of parameter c is greater than or equal to 1 and less than or equal to 1 .6.
[0043] In one embodiment, the aqueous SAPO-34 forming membrane forming gel has the formula:
Al203: aP205: bSi02: cRi : diR2: d2R3: eH20 Formula 3, where the values of a, b, c, and e areas specified for Formulas 1 and 2 above. The value of parameter di in Formula 3 is between 0.5 and 1.5 and the value of parameter d2 is between 0.5 andl .5. Ri is a quaternary organic ammonium templating agent, and R2 and R3 are amines, preferably amines having a molecular weight (Mn) of less than or equal to 300 and/or equal to or less than 12 carbon atoms. The value of parameter di in Formula 3 is between 0.5 and 1.5 and the value of parameter d2 is between 0.5 and 1 .5.
[0044] In a further embodiment, the porous support and the membrane forming gel are heated between about 453 K and about 533 K, preferably between about 470 K and about 515 K, more preferably between about 463 K and about 493 K. The porous support and membrane forming gel are preferably heated for a time greater than two hours, preferably between 2 and 25 hours. In one embodiment, the porous support and membrane forming gel are heated between about 15 and about 25 hours, preferably between about 20 and 25 hours. In another embodiment, the porous support and membrane forming gel are heated between about 3 and about 8 hours, preferably between about 4 and 6 hours. In a further embodiment, the porous support and membrane forming gel are heated between about 4 and 6 hours at a temperature between about 463 K to about 493 K.
[0045] Transport of gases through a zeolite-type and other molecular sieve
membranes can be described by several parameters. As used herein, the flux, J,,
through a membrane is the number of moles of a specified component i p
time through a unit of membrane surface area normal to the thickness direction. I he permeance or pressure normalized flux, P,, is the flux of component i per unit transmembrane driving force. For a diffusion process, the transmembrane driving force is the gradient in chemical potential for the component (Karger et al, Diffusion in
Zeolites, John Wiley and Sons: New York, 1992, pp. 9-10). The selectivity of a membrane for components i over j, S, j is the permeance of component i divided by the permeance of component j. The ideal selectivity is the ratio of the permeances obtained from single gas permeation experiments. The actual selectivity (also called separation selectivity) for a gas mixture may differ from the ideal selectivity.
[0046] The separation of C02 from CH4 is important in natural gas processing because CO2 reduces the energy content of natural gas. In one embodiment, the methods of the present invention provides SAPO membranes, particularly SAPO-34 membranes, with equal or improved CO2/CH4 selectivity and CO2 permeance as compared to previously reported SAPO-34 membranes. The membranes of the present invention can have a CO2 CH4 selectivity of 180 or greater for a 50/50 CO2 CH4 mixture at a pressure drop of 0.14 MPa, preferably a CO2/CH4 selectivity of 200 or greater, even more preferably a CO2/CH4 selectivity of 250 or greater. At a pressure drop of 4.6 MPa, the SAPO-34 membranes have a CO2 CH4 selectivity of 50 or greater, preferably a CO2 CH4 selectivity of 70 or greater, even more preferably a CO2 CH4 selectivity of 85 or greater.
[0047] As used herein, a percent mixture of a combination of gases, such as a 50/50 CO2/CH4 mixture, refers to the molar percent of the gases.
[0048] One embodiment of the invention provides a SAPO-34 membrane made according to the methods described above having a CO2 CH4 separation selectivity of 274 or greater for an approximately 50/50 CO2 CH4 mixture with a pressure differential across the membrane of 0.14 MPa, and a has a CO2/CH4 separation selectivity of 78 or greater for an approximately 50/50 CO2 CH4 mixture with a pressure differential across the membrane of 4.6 MPa.
[0049] Additionally, membranes provided by the present invention can h
permeance of 1 .3 x 10"6 mol/(m2 · s · Pa) or greater at a pressure drop of U.14 MPa, preferably a CO2 permeance of 2.2 x 10"6 mol/(m2 · s · Pa) or greater, even more preferably a CO2 permeance of 2.9 x 10"6 mol/(m2 · s · Pa) or greater. The membranes provided by the present invention also can have a C02 permeance of 1.8 x 10"7 mol/(m2 • s · Pa) or greater at a pressure drop of 4.6 MPa, preferably a C02 permeance of 3.8 x 10"7 mol/(m2 · s · Pa) or greater, even more preferably a C02 permeance of 4.7 x 10"7 mol/(m2 · s · Pa) or greater.
[0050] One embodiment of the invention provides a SAPO-34 membrane made according to the methods described above having a CO2 permeance of 2.9 x 10" 6mol/(m2 · s · Pa) or greater for an approximately 50/50 CO2/CH4 mixture with a pressure differential across the membrane of 0.14 MPa, and a CO2 permeance of 4.7 x 10"7mol/(m2 · s · Pa) or greater for an approximately 50/50 CO2/CH4 mixture with a pressure differential across the membrane of 4.6 MPa.
[0051] One embodiment of the present invention provides methods for separating a first gas component from a gas mixture containing at least a first and a second gas component using the SAPO membranes, particularly SAPO-34 membranes, described herein. Preferably the first gas component is carbon dioxide and the second gas component is methane. The separating method comprises the steps of: a) providing a membrane generated using a soaking treatment, a seeding treatment or both as described above, the membrane having a feed and a permeate side and being selectively permeable to the first gas component over the second gas component; b) applying a feed stream including the first and the second gas components to the feed side of the membrane; and c) providing a driving force sufficient for permeation of the first gas component through the membrane, thereby producing a permeate stream enriched in the first gas component from the permeate side of the membrane.
[0052] As described herein, the soaking and seeding treatments improve the permeance and selectivity of SAPO-34 membranes. Scanning electron microscope
(SEM) images, X-ray diffraction (XRD) and high pressure C02/CH4 mixtui experiments were used to characterize SAPO-34 membranes.
BRIEF DESCRIPTION OF THE DRAWINGS
[0053] Figure 1 shows an SEM image of SAPO-34 seed crystals. Average seed crystal size was approximately 300 nm in length and 60 nm in thickness.
[0054] Figure 2 shows the X-ray diffraction (XRD) pattern of a SAPO-34 seed crystal.
[0055] Figures 3a-3d show SEM images of SAPO-34 membranes prepared using different conditions. Figure 3a shows a membrane grown on a glazed area and forming a free-standing membrane. Figures 3b-3d shown membranes prepared on non-glazed support surfaces with 4 hours soaking of one side of the support (Figure 3b), 4 hours soaking on both sides of the support (Figure 3c), and no soaking (Figure 3d).
[0056] Figures 4a-4c show cross-sectional SEM images of membranes prepared with 4 hours soaking of one side of the support (Figure 4a), 4 hours soaking on both sides of the support (Figure 4b), and no soaking (Figure 4c).
[0057] Figures 5a-5f show cross-sectional SEM images of a SAPO-34 membrane prepared by soaking both sides of the support. Figure 5a illustrates that the porous support contains three layers with different pore sizes (layers labeled A, B and C).
Figures 5b-5d show increasingly magnified views of one section shown in Figure 5a. Figure 5e shows a magnified view of layer B from Figure 5a, and Figure 5f shows a magnified view of layer C from Figure 5a.
[0058] Figures 6a-6c show SEM images of powder SAPO-34 crystals (not part of a membrane) collected at the bottom of an autoclave. Figures 6a and 6b show images of crystals collected during membrane synthesis with no soaking (Figure 6a) and 4 hr soaking (Figure 6b). Figure 6c shows an image of crystals collected from non- membrane synthesis (no soaking and without addition of a seeded support).
[0059] Figures 7a-7c show SEM images of porous supports dipcoated ir
solutions having different dilutions of an initial seed solution (F).
[0060] Figures 8a-8b show the top view and cross-section view of SEM images of a SAPO-34 membrane prepared with the dip coating and soaking method.
DETAILED DESCRIPTION OF THE INVENTION
[0061] The methods of the invention provide silicoaluminophosphate (SAPO) membranes, particularly SAPO-34 membranes. SAPOs are molecular sieve materials, having a tetrahedral crystal structure joined together through oxygen atoms to produce an extended network of channels of molecular dimensions. The SAPO crystals have a three-dimensional crystal framework structure of PO2+, AIO2- and S1O2 tetrahedral units, the framework structure defining a structure of regular cages, cavities, and channels. The dimensions of the channels and cavities in these crystal layers are generally microporous. As used herein, "microporous" refers to pore diameters less than about 2 nanometers.
[0062] Crystalline SAPO-34 has the CHA structure and is an analog of the natural zeolite chabazite. The CHA framework structure contains single eight ring, double six ring, and single four ring secondary building units. SAPO-34 adsorption measurements have determined that n-C4Hio (0.43 nm diameter) can fit the pores formed in the SAPO- 34 framework structure, but i-C4Hi0 (0.5 nm diameter) cannot, thus the pore size is believed to be between 0.43 and 0.5 nm (Lok et al., in Lok. et al. (eds.) Crystalline Silicoalumino Phosphates, US, 1984).
[0063] Other SAPOs have different crystalline structures and different pore sizes and can be classified as small, medium, or large-pore molecular sieves based on the size of the largest oxygen rings in the structure. Crystalline SAPO-5 has the AFI structure which contains rings of 12 oxygen atoms, 6 oxygen atoms, and 4 oxygen atoms.
SAPO-5 is typically considered a large-pore molecular sieve. In contrast, crystalline SAPO-1 1 has the AEL structure which contains rings of 10 oxygen atoms, 6 oxygen
atoms, and 4 oxygen atoms. SAPO-11 is typically considered a medium- sieve. Structures where the largest ring contains 8 or fewer oxygen atoms are typically considered small-pore molecular sieves. SAPO crystals able to make molecular sieve membranes include, but are not limited to, SAPO-5, SAPO-1 1 , SAPO-16, SAPO-17, SAPO-20, SAPO-31 , SAPO-34, SAPO-35, SAPO-37, SAPO-40, SAPO 41 , SAPO-42, and SAPO-44 crystals. Further information regarding SAPO structures is available in Baerlocher, W.M. Meier and D.H. Olson, "Atlas of Zeolite Framework Types", 5th ed., Elsevier: Amsterdam, 2001 and online at http://www.iza-strucures.org/databases.
[0064] SAPOs exhibit cation exchange properties. The excess negative charge in the lattice may be compensated by protons or by compensating cations located in the cavities of the structural framework. Acid hydrogen forms of SAPOs (e.g. H-SAPO-34) have protons that are loosely attached to their framework structure in lieu of inorganic compensating cations. Other forms of SAPO-34 include, but are not limited to Na- SAPO-34, Cu-SAPO-34, Li-SAPO-34, K-SAPO-34, Rb-SAPO-34, and Ca-SAPO-34. These may be made through ion-exchange of H-SAPO-34 or by including the
appropriate cation in the synthesis gel.
[0065] The membranes of the invention are formed through in situ crystallization of an aqueous silicoaluminophosphate-forming gel. The membrane forming gel contains one or more organic templating agents. The term "templating agent" or "template" is a term of art and refers to a species added to the synthesis media to aid in and/or guide the polymerization and/or organization of the building blocks that form the crystal
framework. Preferably the template is a quaternary organic ammonium templating agent including but not limited to tetrapropyl ammonium hydroxide (TPAOH), tetrapropyl ammonium bromide, tetrabutyl ammonium hydroxide, tetrabutyl ammonium bromide, tetraethyl ammonium hydroxide (TEAOH), tetraethyl ammonium bromide,
tetraethylammonium chloride (TEACI) and combinations thereof. Where more than one templating agent is used, the additional templating agents can include amines.
Membrane forming gels for forming SAPO crystals are known to the art, but preferred gel compositions for forming membranes may differ from preferred compositions for
forming loose crystals or granules. The preferred gel composition may v.
upon the desired crystallization temperature and time.
[0066] The membrane forming gel is prepared by mixing sources of aluminum, phosphorus, silicon, and oxygen in the presence of a templating agent and water.
Generally, the gel comprises Al, P, Si, O, at least one templating agent and water. In one embodiment, the composition of the mixture may be expressed in terms of the following molar ratios as:
1 .0 Al203: aP205: bSi02: cR: dH20 Formula 1 ,
where R is a templating agent. Preferably, R is a quaternary ammonium templating agent. In a further embodiment, the quaternary ammonium templating agent is selected from the group consisting of tetrapropyl ammonium hydroxide (TPAOH), tetrapropyl ammonium bromide, tetrabutyl ammonium hydroxide, tetrabutyl ammonium bromide, tetraethyl ammonium hydroxide (TEAOH), or tetraethyl ammonium bromide. In one embodiment suitable for crystallization between about 420 K and about 540 K , a is between about 0.01 and about 52, b is between about 0.03 and about 196, c is between about 0.2 and about 5 and d is between about 20 and about 300. If other elements are to be substituted into the structural framework of the SAPO, the gel composition can also include Li20, BeO, MgO, CoO, FeO, MnO, ZnO, B203, Ga203, Fe203, GeO, TiO, As205 or combinations thereof. If compensating cations are to be included in the cavities of the structural framework, the gel composition can also include sources of the compensating cations (for example, NaOH for Na+, LiOH for Li+, KOH for K+ , RbOH for Rb+ , and CsOH for Cs+).
[0067] In an embodiment suitable for crystallization of SAPO-34, c is less than about 2. In an embodiment suitable for crystallization of SAPO-34 at 453K to 533K for 20-24 hours, a is about 1 , b is 0.03-0.6, c is 1 .07-1 .2 and d is 55-56. In other embodiments, the ratio of silicon to aluminum is between 0.3 and 0.15, is between 0.2 and 0.15, and is 0.15. R is a quaternary organic ammonium templating agent selected from the group consisting of tetrapropyl ammonium hydroxide, tetraethyl ammonium hydroxide
(TEAOH), or combinations thereof.
[0068] One important gel composition parameter is the ratio of Si to Al.
the ratio of Si to Al is high enough so that AIPO5 is not formed. In different
embodiments, the ratio of silicon to aluminum is greater than 0.1 , greater than 0.10 and less than or equal to 0.6, between 0.10 and 0.6, between 0.15 and 0.45, from 0.15 to 0.3, between 0.15 and 0.3, from 0.15 to 0.2, or is about 0.15.
[0069] In one embodiment, the gel comprises at least two templating agents. Any templating agent or each templating agent, independently of one another, may comprise nitrogen. In an embodiment, the gel includes only two templating agents, where optionally one templating agent is a quaternary ammonium compound and the second templating agent is an amine. In another embodiment, the gel includes three templating agents, where optionally the first templating agent is a quaternary ammonium
compound, and the second and third templating agents are amines. A given templating agent may form ionic species in the gel, so that the gel also contains ionic species derived from the templating agent. For example, quaternary ammonium compounds may produce quaternary ammonium cations in the gel.
[0070] In one embodiment, the gel comprises Al, P, Si, O, at least two templating agents and water. The composition of the mixture may be expressed in terms of the following molar ratios as:
AI2O3: aP2O5: bSiO2: cRi: dR2: eH2O Formula 2,
where Ri and R2 are both templating agents. If other elements are to be substituted into the structural framework of the SAPO, the gel composition can also include Li2O, BeO, MgO, CoO, FeO, MnO, ZnO, B2O3, Ga2O3, Fe2O3, GeO, TiO, As2O5 or
combinations thereof. If compensating cations are to be included in the cavities of the structural framework, the gel composition can also include sources of the compensating cations (for example, NaOH for Na+, LiOH for Li+, KOH for K+ , RbOH for Rb+ , and CsOH for Cs+). In an embodiment suitable for crystallization of SAPO-34 at 453K to 533K for 20-24 hours, a is greater than 0.5 and less than 1.5, b is greater than 0.2 and less than 1 .0, c is greater than or equal to 1 and less than 2, and d is greater than zero and less than 4.0 and e is greater than 50 and less than 1 10. In different embodiments,
the ratio of silicon to aluminum is greater than 0.1 , between 0.15 and 0.4i
0.45, between 0.15 and 0.3, from 0.15-0.3, between 0.15 and 0.2, from 0.1 b to Ό.ζ and is about 0.15.
[0071] In an embodiment, Ri is a quaternary organic ammonium templating agent. Preferably, the quaternary ammonium templating agent is selected from the group consisting of tetrapropyl ammonium hydroxide (TPAOH), tetrapropyl ammonium bromide, tetrabutyl ammonium hydroxide, tetrabutyl ammonium bromide, tetraethyl ammonium hydroxide (TEAOH), tetraethyl ammonium bromide, tetraethylammonium chloride (TEACI) or combinations thereof. In another embodiment, the quaternary ammonium templating agent is selected from the group consisting of TPAOH, TEAOH or combinations thereof. In an embodiment, the templating agent is TEAOH. In an embodiment, the value of parameter c is greater than or equal to 1 and less than or equal to 2. In another embodiment, the value of parameter c is greater than or equal to 1 and less than or equal to 1 .6.
[0072] In an embodiment, R2 is an amine, which may be a "small amine". As used herein, the term "small amines" means amines and organic amines having a molecular weight (Mn) of less than or equal to 300 and/or equal to or less than 12 carbon atoms. The amine may be a neutral amine. In an embodiment, R2 is a primary, secondary or tertiary amine. In different embodiments, R2 may be an aliphatic or a cyclic amine. In an embodiment, R2 is an alkyl amine such as dipropylamine (DPA) or N,N- dimethylbutylamine (DMBA). In another embodiment, R2 may have both an amine and an alcohol functionality, such as Ν,Ν-dimtheylethanolamine (DMEA). R2 may also be morpholine (MOR). In an embodiment, R2 is selected from the group consisting of dipropylamine (DPA) and cyclohexylamine(CHA). In an embodiment, R2 is DPA. In different embodiments, the value of parameter d is greater than or equal to 1 and less than or equal to 4, between 1 .0 and 3.0, from 1.0 to 3.0, between 1 .0 and 2.0, or from 1 .0 to 2.0. In an embodiment, the initial pH of a gel combining TEAOH with DPA is between about 9 and about 10, the initial pH of a gel combining TEAOH with CHA is between about 8 and about 8.5.
[0073] When a combination of three templating agents is used, the comj mixture may be expressed in terms of the following molar ratios as:
Al203: aP205: bSi02: cRi: di R2: d2R3: eH20 Formula 3,
In an embodiment, the value of parameter di in Formula 3 is between 0.5 and 1 .5 and the value of parameter d2 is between 0.5 andl .5. In an embodiment, R2 and R3 are dipropylamine (DPA) and cyclohexylamine(CHA). In another embodiment, di and d2 are both between 0.5 andl .0. In an embodiment, the initial pH of a gel combining TEAOH with DPA and CHA is between about 8.5 and about 9.0. The values of the other parameters (a, b, c, e) may be as specified for Formulas 1 and 2.
[0074] The amount of water in the synthesis gel is also an important parameter. In an embodiment, the amount of water used in the membrane synthesis gel is significantly greater than that which would typically be used in a gel for synthesis of loose crystals of the same zeolite or other molecular sieve material. In different embodiments, the value of the parameter e in Formulas 1 -3 is greater than 50, between 50 and 1 10, from 50 to 1 10, between 60 and 100, from 60-100, between 70 and 90, from 70-90, between 70 and 80, or from 70-80. In an embodiment, the synthesis gel composition is 1.0 Al203: 1 .0 P205: 0.3 Si02: 1.0 TEAOH: 1.6 DPA: x H2O, where x is between 70 and 80. In another embodiment, the synthesis gel composition is 1 .0 AI2O3: 1 .0 P2Os: 0.32 SiO2: 1 .0 TEAOH: 1 .6 DPA: x H2O, where x is from 70 to 80.
[0075] Typically, the gel is prepared by mixing sources of phosphate and alumina with water for several hours before adding the template. The mixture is then stirred before adding the source of silica. In an embodiment, the source of phosphate is phosphoric acid. Suitable phosphate sources also include organic phosphates such as triethyl phosphate, and crystalline or amorphous aluminophosphates. In an embodiment, the source of alumina is an aluminum alkoxide, such as aluminum isopropoxide. Suitable alumina sources also include pseudoboehmite and crystalline or amorphous
aluminophosphates (gibbsite, sodium aluminate, aluminum trichloride). In an
embodiment, the source of silica is a silica sol. Suitable silica sources also include
fumed silica, reactive solid amorphous precipitated silica, silica gel, alkoxi (silicic acid or alkali metal silicate).
[0076] Na-SAPO-34 can be made by incorporating NaOH into the synthesis gel. The gel composition can be expressed by: AI2O3: aP20s: bSi02: eNa20: cR: dH20. In a further embodiment, a is 0.77, b is 0.46, e is 0.23, c is 0.77, and d is 46.
[0077] Optionally, the gel is aged prior to contact with the porous support. As used herein, an "aged" gel is a gel that is held (not used) for a specific period of time after all the components of the gel are mixed together or a gel that is maintained at a
temperature below the membrane synthesis temperature for a specific period of time after all the components are mixed. In an embodiment, the gel is sealed and stirred during storage to prevent settling and the formation of a solid cake. Without wishing to be bound by any particular theory, it is believed that aging of the gel affects subsequent crystallization of the gel by generating nucleation sites. In general, it is believed that longer aging times lead to formation of more nucleation sites. The preferred aging time will depend upon the aging temperature selected. Preferably, crystal precipitation is not observed during the aging period. In an embodiment, the viscosity of the aged gel is such that the gel is capable of penetrating the pores of the porous support. After initial mixing of the components of the synthesis gel in a container, material can settle to the bottom of the container. In an embodiment, the gel is stirred and aged until no settled material is visible at the bottom of the container and the gel appears translucent and substantially uniform to the eye.
[0078] In different embodiments, the aging time is greater than two hours, greater than five hours, greater than ten hours, or greater than twenty four hours. In different embodiments, the aging time at room temperature is at least about twenty-four hours, greater than about twenty-four hours, at least about forty-eight hours, and at least about seventy-two hours. For SAPO-34 membranes, in different embodiments the aging time at room temperature or above can be at least twenty four hours, greater than about twenty-four hours at least about forty-eight hours, at least about seventy-two hours, between about three days and about seven days or between four days and 28 days. In
an embodiment, the gel is not aged longer than one month. In different e
the aging temperature is between 10 °C and 75 °C or between 25 °C and biru. in different embodiments, the aging time is at least 24 hours between 290 K and 350K, between 290K and 335K, or between 290 K and 300 K.
[0079] In other embodiments, aging of the gel is not required to obtain the desired quality of membrane. For example, gel aging may not be required if SAPO-34 crystals are applied to the support prior to in situ synthesis. In addition, gel aging may not be required for certain types of silica sources. In an embodiment, aging is not required if tetraethyl orthosilicate (TEOS) is used as the silica source.
[0080] In one embodiment, the present invention provides SAPO membranes, particularly SAPO-34 membranes, prepared using hydrothermal synthesis where an additional soaking treatment, seeding treatment, or both is performed. By "soaking" it is meant that the membrane forming gel is brought into continuous contact with at least one surface of the porous support for one or more hours, preferably between one and fourteen hours, preferably between three and five hours, even more preferably between three and four hours. If SAPO seed crystals have been applied to at least part of the surface of the support, the gel is brought into contact with at least this part of the surface. In an embodiment, the porous support has two sides (e.g. the inside and outside of a tube or the top or bottom of a plate or disk) and the gel is brought into contact with only one side of the support. One side of the support may be masked to limit its contact with the gel. Suitable masking techniques are known to the art. One known masking technique involves covering the surface with a polymer layer, for example covering it with Teflon or fluoropolymer tape. Another masking technique involves infiltrating the pores of the support with an organic masking agent, such as a polymer or a wax, which can later be removed through thermal treatment. In another embodiment, the porous support may be immersed in the gel so that more than one surface of the porous support contacts the gel. In an embodiment, at least some of the gel penetrates the pores of the support. The pores of the support need not be completely filled with gel. In an embodiment, the porous support is brought into contact
with a sufficient quantity of gel such that growth of the SAPO membrane i
substantially limited by the amount of gel available.
[0081] As discussed above, the porous support may be seeded prior to hydrothermal synthesis. In seeding, a first quantity of a SAPO crystalline seed material in the form of loose SAPO crystals (such as SAPO-34 crystals) is applied to at least part of the surface of the porous support prior to bringing the support in contact with the membrane forming gel. As used herein, the term "loose crystals" refers to crystals which are largely unagglomerated or interlocking, in contrast to the interlocking crystals formed during in situ synthesis of the membrane. As used herein, the surface of the support can include both non-porous portions and porous portions where the pores of the support open to the surface. In the present invention crystals can be applied to the surface by contacting crystals with the surface or with crystals already associated with the surface. Since the surface has porous and non-porous portions, contacting the crystals with the surface can include contacting crystals with non-porous portions of the surface or lodging crystals wholly or partially within the pores which open to the surface. The crystals may also be applied to the surface by using coupling agents or binding agents to form covalent linkages between the crystals and the support surface. In an embodiment, the porous support is treated with a barrier layer to prevent the crystals from preferentially entering the pores of the support as described in U.S. 6,090,289. In another embodiment, no barrier layer is used. Preferably, the seed crystals used herein do not form a continuous or nearly continuous layer over the support surface.
[0082] The seed crystals may be applied in dry form. For example, various types of brushes or other applicators may be used to apply the crystals. The crystals may be rubbed onto the surface of the support. In an embodiment where a stainless steel support is used, sufficient crystals are rubbed onto the surface of the support that the support appears uniformly white to the eye.
[0083] The crystals may also be suspended in solution and the solution applied to the support surface. A variety of techniques are known to the art for applying solutions of colloidal particles including, but not limited to, spin-coating, wash-coating, spray-coating,
brushing, slip-casting, dip coating, and immersion for longer periods of tin
used in dip coating. Preferably, the seeding methods of the present invention, such as dip coating, provide seed crystals that are well bonded to the support and are present in low densities.
[0084] The seed solution can be any solution known in the art able to transfer seed crystals within the solution to a surface placed in contact with the solution. Typically the seed solution will comprise a solvent, the seed crystals and optionally an organic binding agent to provide improved adhesion of the seed crystals to the contacted surface. The solvent can be any solvent known in the art which can be removed from the porous solvent after the seed crystals are deposited and which does not react with the porous support. In an embodiment, the solvent is an alcohol. Organic binders according to the present invention include cellulose ether type binders and/or their derivatives. Some typical organic binders according to the present invention are methylcellulose, hydroxybutylcellulose, hydrobutyl methylcellulose,
hydroxyethylcellulose, hydroxymethylcellulose, hydroxypropylcellulose, hydroxypropyl methylcellulose, hydroxyethyl methylcellulose, sodium carboxy methylcellulose, and combinations thereof. In one embodiment of the present invention, applying the seed solution to the porous support is able to deposit seed crystals on the porous support, where the seed crystals do not form a continuous monolayer.
[0085] After the porous support and the membrane forming gel are brought into contact, the support and gel are heated in a SAPO crystal synthesis step. This synthesis step can lead to formation of SAPO crystalline material on and in the porous support. As used herein, crystalline material includes both newly formed crystals and crystalline material grown on previously formed crystals. If SAPO crystals have been applied to the support prior to the synthesis step, the synthesis step results in the formation of a second quantity of crystalline material which may take the form of new crystals and/or growth of the applied crystals. During each synthesis step a layer of SAPO crystals can be said to form on the surface of the porous support and/or on previously formed SAPO crystals. The layer of SAPO crystals formed during each synthesis step may not be continuous. During the synthesis step, crystals may also
precipitate from the membrane forming gel without being incorporated inti
membrane.
[0086] The heating temperature for the synthesis step is between about 420K and about 540 K. Preferably, the heating temperature is between about 453 K and about 533 K, between about 470 K and about 515 K, or between about 463 K to about 493 K. The crystallization time is greater than two hours, preferably between 2 and 25 hours. In one embodiment, the porous support and membrane forming gel are heated between about 15 and about 25 hours, preferably between about 20 and 25 hours. In another embodiment, the porous support and membrane forming gel are heated between about 3 and about 8 hours, preferably between about 4 and 6 hours. In a further embodiment, the porous support and membrane forming gel are heated between about 4 and 6 hours at a temperature between about 463 K to about 493 K. Synthesis typically occurs under autogenous pressure.
[0087] Excess membrane forming gel is removed from the support and the SAPO crystals after each synthesis step. The excess gel may be removed by washing with water. After washing with water, the support and SAPO crystals may then be dried.
[0088] The synthesis step may be repeated in order to form a greater amount of SAPO crystals. After each synthesis step, the excess synthesis gel is removed and then the porous support is brought into contact with synthesis gel before performing the next synthesis step. Sufficient synthesis steps are performed so that the cumulative layer formed on the support surface by the synthesis steps and any crystal application steps forms a continuous layer. The SAPO membrane is formed by the cumulative layer(s) of SAPO crystals on the support surface(s) and the (interconnected) SAPO crystals formed inside the porous support. In an embodiment, the SAPO crystals inside the support are substantially interconnected. In an embodiment, the interconnected SAPO crystals are connected to the layers of SAPO crystals formed on the support surface. In an embodiment, sufficient synthesis steps are performed that the membrane is impermeable to nitrogen after preparation (but before calcination).
[0089] After synthesis of the SAPO layers is complete, the SAPO memb
calcined to substantially remove the organic template material. After calcination, the membrane becomes a semi-permeable barrier between two phases that is capable of restricting the movement of molecules across it in a very specific manner. In different embodiments, the calcination temperature is between about 600 K and about 900K, and between about 623 K and about 773 K. For membranes made using TEAOH and TPAOH as a templating agent, the calcining temperature can be between about 623 K and about 673 K. In an embodiment, the calcination time is between about 5 hours and about 25 hours. Longer times may be required at lower temperatures in order to substantially remove the template material. Use of lower calcining temperatures can reduce the formation of calcining-related defects in the membrane. The heating rate during calcination should be slow enough to limit formation of defects such as cracks. In an embodiment, the heating rate is less than about 2.0 K/min. In a different embodiment, the heating rate is about 0.6 K/min. Similarly, the cooling rate must be sufficiently slow to limit membrane defect formation. In an embodiment, the cooling rate is less than about 2.0 K/min. In a different embodiment, the cooling rate is about 0.9 K/min.
[0090] In one embodiment, the SAPO membranes of the present invention comprise SAPO crystals which form a layer on at least one side of the porous support. SAPO crystals may also be present within at least some of the pores of the support. The thickness of the SAPO layer depends in part on the number of synthesis steps performed. In embodiment where synthesis steps are performed until the membrane is impermeable to nitrogen, the thickness of the cumulative SAPO layer is less than about 20 microns. When the layer thicknesses are measured from cross-sections with scanning electron microscopy, the uncertainty in the thickness measurement is believed to be on the order of +/-10%. In other embodiments, the thickness of the SAPO layer is about 5 microns, less than 5 microns or about 2.5 microns. In an embodiment, immersion of a porous support in the synthesis gel can lead to formation of SAPO crystals within the support as well as on both sides of the support. For example, immersion of a porous tube in the synthesis gel can lead to formation of SAPO crystals within the tube as well as formation of a SAPO layer on the inside and the outside of the
tube. In an embodiment, the SAPO crystals may form throughout the thic
support. When both sides of the support are immersed and capable of being
penetrated by the gel, formation of SAPO crystals throughout the thickness of the support indicates that the synthesis gel has penetrated to the center of the support. However, formation of SAPO crystals throughout the support does not require that SAPO crystals completely fill the pore space of the support.
EXAMPLES
[0091] As described below, SAPO-34 membranes with high CO2 permeance and CO2/CH4 selectivity were successfully generated on supports utilizing the soaking methods and/or seeding methods described above. After 2-4 hours of soaking the support in the membrane forming gel prior to hydrothermal synthesis, the CO2 permeance increased >100% to 2.2 10"6 mol/(m2xsxPa) at 0.14 MPa. The CO2/ CH4 selectivities are greater than 200 at 0.14 MPa. Real improvement was seen at high pressure drop. The CO2/ CH4 selectivity at 4.6 MPa was greatly improved from 14 (0 hr soaking) to 74 (2 hr soaking) and 59 (4 hr soaking), respectively, which is >400% increase. At 4.6 MPa, CO2 permeance increased >400% to 4-5 χ 10"7 mol/(m2xsxPa). Soaking for 2-4 hours gives the best results at high pressure. Soaking the support at both sides also substantially improves the selectivity and permeance. An exemplary membrane generated in the Examples below has CO2 permeance of 2.9x10"6 and 4.7x10"7 mol/(m2xsxPa) at 0.14 and 4.6 MPa, with CO2/ CH4 selectivity of 78 at 4.6 MPa. SAPO-34 membranes with improved performance were also prepared with a dip coating method in a seed solution. Lower seed density on the support is also a key factor in determining membrane quality, even without a soaking treatment.
Example 1 - Synthesis of membranes
Support pretreatment:
[0092] All supports (200 nm and 100 nm AI2O3 and 5 nm ΤΊΟ2 tubes, outside diameter: 1 1 mm and inside diameter: 7 mm) were provided by Inopor Gmbh. All supports were cut into 5 cm long tubes, with the two ends glazed with high temperature ceramic glaze. Before seeding, the supports were boiled in deionized (Dl) water for 3 hours and dried at 373 K under vacuum for 30 min.
Seed material synthesis:
[0093] The SAPO-34 membranes were prepared on seeded support. The seed gel molar ratio was 1.0 Al203 : 2.0 P205 : 0.6 Si02 : 4.0 TEAOH : 75 H20. In a typical seed synthesis, AI(i-C3H70)3 (98%) H3PO4 (85 wt% aqueous solution) and deionized H20 were stirred for 3 hours to form an homogeneous solution, and then Ludox AS-40 colloidal silica (40 wt % Si02 suspension in water) was added and the resulting solution stirred for another 3 hours. The template, tetra-ethyl ammonium hydroxide (35 wt% aqueous solution), was then added, and the solution stirred overnight at room
temperature (RT). The solution was then placed in an autoclave and held at 453 K for 7 hours under microwave heating (CEM Mars Microwave Reaction System with XP-1500 plus reactor). After the solution was cooled to room temperature, it was centrifuged at 6000 rpm for 30 min to separate the seeds, which were then washed with Dl water. This procedure was repeated three times. The resulting precipitate was dried at 373 K overnight.
Membrane forming gel synthesis:
[0094] The membrane synthesis gel molar ratio was 1.0 Al203: 1 .0 P2Os: 0.3 Si02 TEAOH: 1 .6 DPA: 150 H2O. All chemicals were purchased from Sigma-Aldrich and used as received. In a typical synthesis, Al source (AI(i-C3H7O)3 ,98%), H3PO4 (85 wt%
aqueous solution) and deionized H20 were stirred for 3 hours to form an I
solution, and then Ludox AS-40 colloidal silica (40 wt % Si02 suspension in water) was added and the resulting solution was stirred for another 3 hours. The tetra-ethyl ammonium hydroxide (35 wt% aqueous solution) was added and the solution was stirred for 1 hour. After the addition of dipropyl amine (99%), the solution was stirred for 4 days at 318 - 323 K before membrane synthesis.
Seeding:
[0095] Two methods, rubbing and dip coating, were used for seeding the support. With rubbing, the seeds were physically attached to the inside surface of support by contacting the support with a tube cleaner coated with the seeds.
[0096] With dip coating, a seed suspension was prepared by mixing 0.05 wt% hydroxy propyl cellulose and 0.67 wt% SAPO-34 seed crystals with anhydrous ethanol.
Extensive sonication was used to disperse the SAPO-34 crystals. The obtained seed solution (F) was then diluted with ethanol. Ceramic supports, wrapped with Teflon tape, were dipped into the seed solution for 10 s and withdrawn at a speed of 1 cm/s. Then the supports were dried in air for 10 minutes at room temperature. The seeded support was calcined in air at 673 K for 4 hours, with a heating and cooling rate of 1 K/min.
Membrane synthesis:
[0097] The seeded supports were then placed in an autoclave and the autoclave was filled with the membrane synthesis gel. For embodiments where only one side of the support is to be exposed to the synthesis gel, the outside of the tubes were first wrapped with Teflon tape. For methods comprising a soaking step, the seeded supports were soaked in the membrane synthesis gel at room temperature from 0-14 hours before hydrothermal synthesis at 493 K for 6 hours, and the membranes were then washed with Dl water and dried for ~2 h at 340 K. The membranes were calcined
in air at 670 K for 4 hours to remove the templates. The calcination heati
rates were 0.7 and 0.9 K/min, respectively.
Example 2 - Permeation and separations measurements
[0098] 50/50 C02/CH4 mixture (molar percent) permeations were measured at 295 K in a flow system whose flow rates were adjusted with mass flow controllers. Feed pressures ranged from 0.22 to 7.1 MPa, and it was controlled with a back pressure regulator. Feed flows ranged from 250 seem at low pressure to 1500 seem at high pressure. The permeate pressure was fixed at 84 kPa. No sweep gas was used.
Fluxes were measured with bubble flow meters and compositions were analyzed by a gas chromatograph (GC) (SRI 8610C) with a Hayesep D column at 373 K and a TC detector. An automated sample loop took samples from the feed and permeates streams. The tubular membranes were sealed in a stainless steel module with silicone O-rings. The leak integrity of the module was verified by replacing the membrane with a solid stainless steel tube. The leak rate for an 8 MPa pressure drop across the O-ring was < 0.2 % of the measured CH4 flux for a 50/50 CO2 mixture at the same pressure drop. In order to minimize concentration polarization of the mixtures, the following issues were taken into account: i) a Teflon spacer was used to reduce the cross section for feed flow and thus increasing gas velocity; ii) a high pressure gas booster was used to increase the feed pressure up to 7 MPa as maintaining high flux velocity in the feed side.
[0099] Single CF4 permeation tests were done with dead end module at 295K and feed pressure from 0.9 to 5.8 MPa. For the separation quantification, the permeance selectivity a and the separation factor SF were used and defined as follows:
partial pressure drop; and
where y is the molar fraction in the gas phase.
Example 3 - Characterization of resulting membranes
[0100] The SAPO-34 seeds used herein were prepared by microwave heating. The crystals exhibit rectangular plate morphology, with approximately 300 nm in length and 60 nm in thickness, as shown in Figure 1 . The X-ray diffraction (XRD) patterns indicated that crystals had the chabazite structure of SAPO-34 (shown in Figure 2). The size of SAPO-34 crystals can be controlled from 100nm to micron size, as well as the morphology, such as cubic, rectangular, and spherical. Moises and others found smaller, homogeneous cubic and rectangular crystals (< 1 μηπ) had superior separation performance. Smaller crystals with narrow size distributions pack better than larger crystals, and thus the size of non-zeolite pores can decrease so that higher CO2/CH4 selectivities result.
[0101 ] In certain embodiments of the present invention, microwave assisted
hydrothermal synthesis was utilized to prepare homogeneous SAPO-34 crystals with rectangular plate morphology.
[0102] Table 1 (shown below) shows the separation performance of SAPO-34 membranes prepared with different soaking time at 0.14 MPa and 4.6 MPa pressure drops. As shown in Table 1 , the SAPO-34 membranes prepared without any soaking treatment exhibit a wide distribution of CO2/CH4 selectivity, which indicates poor reproducibility. Among ten membranes made under the same conditions, membranes 1 and 2 (M1 and M2) represent two extreme cases (the other non-treated membranes are
not shown here). Both M1 and M2 have high C02 permeance, approximi
mol/(m2 s Pa), which is much higher than membranes grown on stainless steel supports.
The C02/CH4 selectivity of M1 is 217 at 0.14 MPa pressure drop, and it dramatically decreases to 14 at 4.6 MPa. M2 is much worse than M1 and it was not tested at high pressure.
Table 1 - The effect of aging time on the C02/CH4 separation performance of SAPO-34 membranes at different pressure drops.
[0103] After the soaking treatment described herein, both C02 permeance and
C02/CH4 selectivity exhibit significant increase and the best result was obtained at 2-4
hours of soaking time. At 0.14 MPa, the C02 permeance increased more
2.2* 10"6 mol/(m2 s Pa) when soaking time was increased to 4 hours. The C02/CH4 selectivities are greater than 150 at 0.14 MPa. The C02/CH4 selectivity at low pressure drop is not a good indicator of membrane quality, as shown in previous publications (Adolfo et al, Journal of Membrane Science, 335 (2009), p. 32-36). The C02/CH4 selectivity at 4.6 MPa was greatly improved from 14 (no soaking) to 74 (soaking for 2 hours) and 59 (soaking for 4 hours), respectively, which is a >400% increase. The C02 permeance also increased >400% from 1.1 * 10"7 mol/(m2 s Pa) (no soaking) to 5.1 * 10" 7 (soaking for 2 hours) and 4. Ox 10"7 mol/(m2 s Pa) (soaking for 4 hours). Soaking for 2 to 4 hours produced the best results at high pressure. Between 4-8 hours soaking, C02/CH4 selectivity starts to decrease, but the C02 permeance remains unchanged. After 14 hours soaking, the obtained SAPO-34 membrane exhibited no selectivity toward C02/CH4 separation and C02 permeance also decreased significantly.
[0104] Membranes 10, 11 and 12 (M10, M11 and M12) were soaked in the membrane synthesis gel without Teflon tape wrapping, which means the membrane synthesis gel have free access and was able to soak both sides of the seeded support surface. All three of these membranes have high C02 permeance and decent C02/CH4 selectivity at 0.14 MPa, with an average of 2.3x10"6 mol/(m2 s Pa) for C02 permeance and 259 for C02/CH4 selectivity. The average C02 permeance and C02/CH4 selectivity at 4.6 MPa are 4.1 χ 10"7 mol/(m2 s Pa) and 72 respectively. M12 has C02 permeance of 2.9χ10"6 and 4.7x10"7 mol/(m2 s Pa) at 0.14 and 4.6 MPa, with C02/CH4 selectivity of 78 at 4.6 MPa. This provides high permeability for C02/CH4 separation while also providing decent high pressure C02/CH4 selectivity. Perhaps the biggest improvement is the reproducibility. All three membranes prepared at similar conditions show consistent high permeance and selectivity.
Example 4 - Effect of soaking and support surface on crystal size and str
[0105] Soaking the seeded support in membrane forming gel prior to hydrothermal synthesis has great effect on the physical characteristics and quality of SAPO-34 membranes. Figures 3a-3d show SEM images of SAPO-34 membranes prepared under different conditions. Figure 3a was taken on a membrane grown on a glazed support area prepared with 4 hours of soaking. Membranes grown on glazed areas tend to fall off to form free-standing membranes. Figures 3b, 3c and 3d were taken on membranes prepared on unglazed support surfaces with 4 hours of soaking one side, 4 hours of soaking both sides, and no soaking, respectively. All four SEM images in Figure 3 show cubic crystals, which is typical for SAPO-34. The crystal coverage and intergrowth are excellent for all four samples. The crystals grown on the glazed area are approximately 13 μηι (Figure 3a), much bigger than crystals grown on porous areas. The crystals grown on non-glazed supports (Figures 3b, 3c and 3d) show similar bimodal distribution, with crystal size of approximately 1.5 μηι and 6 μηπ. This size difference might be caused by the smooth surface of the glaze, which is difficult for the seeds to attach, thus leading to fewer nuclei and bigger crystals. It appears that there is a greater amount of small crystals when a soaking treatment is used. This result is similar with Kong and Chen's results, which can be attributed to the increase in generated nuclei (Kong et al, Journal of Membrane Science 285 (2006), p. 258-264; and Chen et al, Microporous and Mesoporous Materials 102 (2007), p.249-257).
[0106] Figures 4a-4c show the cross-section SEM images of membranes prepared with 4 hours of soaking of one side of the support, 4 hours of soaking on both sides, and no soaking, respectively. Surprisingly, there is not a big difference in membrane thickness. Each membrane had a thickness of approximately 5-6 μηπ. The CO2 permeance of membranes prepared with 4 hours soaking is about 100% higher than membranes prepared without soaking, which suggests the membranes should be much thinner. Kong et al (supra) obtained thinner silicalite-1 membranes when using two-stage hydrothermal synthesis; however, no permeation data were provided. Chen et al (supra) obtained much thicker Silicalite-1 membranes with higher permeance when using two-step hydrothermal synthesis. Chen et al concluded that the main factors
affecting the membrane flux are (1 ) the crystal thickness in b-axis which c
diffusion path of permeable molecules, and (2) the formation of zeolite or other molecular sieve crystals in the support pores, which further enhances the diffusion resistance. Apparently, the top layer of the molecular sieve membrane is not the only crystal layer. The crystals of the molecular sieve material may penetrate into the support, which contributes substantially to the overall diffusion resistance.
[0107] Figures 5a-5f show the cross-section SEM images of SAPO-34 membrane prepared with 4 hours of soaking on both sides of the support. As shown in Figure 5a, it is obvious that the ceramic support composes of three layers with descending pore sizes from bottom to top, marked as A, B and C. The thicknesses of layers C and B are approximately 8 μηι and 30 μηπ, respectively. In layers A and B, many micron sized cubic SAPO-34 crystals were observed, which can be seen in Figures 5a and 5e.
These cubic crystals are much smaller than the crystals formed at the top layer of the SAPO-34 membrane, which have an approximate size of 5-6 μηπ. This phenomenon might be attributed to the limited nutrients in layers A and B. Once the hydrothermal synthesis started, the formation of the top layer of the SAPO-34 membrane blocked the flow of nutrients from the membrane forming gel to layers A and B. The crystals in top layer can grow bigger because of unlimited supply of nutrients from membrane forming gel. The crystals in layers A and B would stop growing once the nutrients are
completely consumed. The small pores in layer B also limit the crystal size. No cubic crystals were found in layer C. However, elemental analysis verified the presence of plenty phosphorus and silicon in layer C, as well as in layer B, which indicated the presence of SAPO-34 crystals. The small pores (200 nm) in layer C may limit the growth of SAPO-34 crystals, which makes it difficult to find typical cubic SAPO-34 crystals with SEM. The crystals in the support, especially in layers B and C, might exhibit substantial diffusion resistance.
[0108] When seed crystals are added to a synthesis mixture, they may (a) remain inert, (b) dissolve, (c) act as pure seeds, in that mass is deposited upon them and they grow, or (d) give rise to secondary nuclei and hence a new crop of crystals (Cundy et al, Microporous and Mesoporous Materials 82 (2005), p. 1-78). In the SAPO-34
membrane system, seeding is indispensable for high quality membranes,
indicates that the seeds should not remain inert or dissolve completely. So the seeds may act as pure seeds and/or induce secondary nuclei. Hasegawa et al (Journal of Membrane Science 280 (2006), p. 397-405) and Zhang et al (Materials Chemistry and Physics 96 (2006) 42-50; and Front. Chem. Eng. China, 1 (2) (2007), p. 172-177) found the presence of a seed layer (several microns in thickness) under the membrane layer in the synthesis of Silicalite-1 membranes, which indicated that the seeds generated secondary nuclei and formed new crystals on top of the seed layer. However, no seed layer was observed under the membrane layer in the present SAPO-34 membranes (Figure 5 d).
Example 5 - Effects of seeding on crystal size and membrane quality
[0109] Figures 6a-6c show SEM images of powder SAPO-34 crystals collected at the bottom of an autoclave after synthesis. Figures 6a and 6b shows images of crystals collected during membrane synthesis with no soaking and 4 hours of soaking, respectively. Bimodal crystal size distribution was observed for both systems, which is consistent with crystal size distribution in top layer zeolite membranes, as shown in Figures 3a-3d. More small crystals were observed in Figure 6b, which suggests that soaking helps to generate more nuclei and leads to a greater number of small crystals. The bimodal crystal size distribution might be caused by the non-homogeneity of the membrane forming gel. However, powder collected from non-membrane synthesis (same condition without the addition of seeded support) shows very uniform crystal size distribution, approximately 1 .5 μηπ, as shown in Figure 6c. This result suggests that the big crystals (~6 μηπ) in Figures 6a and 6b might have a different synthesis path compared with the micron size small crystals. The small crystals might grow from the nuclei in the membrane forming gel (original nuclei in the gel and secondary nuclei), while the big crystals might grow from the seed crystals, which are much bigger than the nuclei.
[0110] This result is consistent with Baerlocher et al, Atlas of Zeolite Stri
fifth ed., Elsevier, Amsterdam, 2001 . In the synthesis of the zeolite EUO, the addition of seeds generated large "parent" and small "daughter" crystals with 18 and 4 μηι in diameter, respectively. The spawning of daughter crystals from a partially dissolving calcined seed crystal was observed. It was believed the large parent crystals grow from seeds while the small daughter crystals grow from secondary nuclei. During
hydrothermal synthesis, the seed growth and secondary nucleation are parallel reactions. The balance between seed growth and secondary nucleation depends on the nature of the system, the quantity of material added and the degree of agitation. If the surface area of seed crystals present is sufficient to absorb most of the available flux of growth species and thus prevent solution supersaturation from reaching high levels, then most of the growth in the system will take place on the seed crystals, whose size and growth rate can be closely controlled and predicted by kinetic modeling. As the quantity of seed material is reduced, then the natural supersaturation and
consequent self-nucleation will no longer be suppressed and the product crystal size will deviate more and more from that predicted on the basis of linear growth on seed crystals only (see Cundy et al, Microporous and Mesoporous Materials 82 (2005), 1-78; Baerlocher et al, Atlas of Zeolite Structure Types, fifth ed., Elsevier, Amsterdam, 2001 ; Cundy et al, Micropor. Mesopor. Mater. 66 (2003) 143; Cundy et al, Chem. Commun. (1998) 1465; Cundy et al, Zeolites and Mesoporous Materials at the Dawn of the 21 st Century, Studies in Surface Science and Catalysis, vol. 135, Elsevier, Amsterdam, 2000, p. 192, Paper No. 02-P-08; Thompson and Dyer, Zeolites 5 (1985) 202;
Thompson and Dyer, Zeolites 5 (1985) 292; and R.W. Thompson in: Modelling and Reactivity in Zeolites, Academic Press, London, 1992, p. 231 ).
[0111] Seeding is a powerful technique in the synthesis of zeolite and other molecular sieve membranes. Many seeding techniques, like rubbing, dip coating, and filtration have been developed for zeolite membrane synthesis. Among these seeding
techniques, dip coating seems the easiest for large scale application. Rubbing is a simple seeding method, but rubbing cannot generate a uniformly distributed seed layer on the support surface. It is also difficult to apply rubbing to large scale membrane
synthesis. Accordingly, dip coating was used to check the relationship be
layer and membrane quality.
[0112] It is believed that the quality of seed layer determines the quality of the molecular sieve membrane. For the synthesis of MFI zeolite membranes, a densely packed seed layer is a must for high quality membranes. However, a lower seed density was surprisingly shown to be more effective to grow optimal membranes for the present SAPO-34 membranes. This is surprising in that previously it was believed that greater seed crystal density would require less seed growth during hydrothermal synthesis and would more likely provide a thinner, uniform and interconnected crystalline layer.
[0113] In one embodiment, 0.67 g SAPO-34 seeds and 0.05 g hydroxyl propyl cellulose were dispersed in 100 g ethanol to make a dip coating solution (0.67wt% seed concentration, designated as solution F). The seed concentration commonly used for dip coating is between 0.5-2.0 wt%. The addition of hydroxyl propyl cellulose is to increase the bonding between seed crystals and the support.
[0114] Table 2 (shown below) lists the effect of seed concentration on separation performance of SAPO-34 membranes. The first six membranes were dip coated in solution F (0.05 wt% hydroxy propyl cellulose and 0.67wt% seed concentration in ethanol) from 1 to 4 times. All six membranes show relatively poor selectivity, especially at high pressure. The best membrane, membrane A4, has CO2/CH4 selectivities of 160 and 13 at 0.14 MPa and 4.6 MPa, respectively, and the CO2 permeances are 1 1 and 2.1 x10"7mol/(m2 s Pa). As the seed solution was diluted (1/n F means solution F was diluted n times with ethanol), the CO2/CH4 selectivity and permeance exhibit significant increase at both low pressure and high pressure. The best result (membrane A9) was obtained when the seed solution was diluted 16 times. The CO2/CH4 selectivities of A9 are 255 and 96 at 0.14 MPa and 4.6 MPa respectively, and the CO2 permeances are 19 and 4.0 x10"7mol/(m2 s Pa) at 0.14 MPa and 4.6 MPa. When the seed concentration was further diluted to 1/128 F (membrane A11 ), permeance starts to decrease while the selectivity only shows slight decrease. Even
the seed concentration was diluted 512 times (A12), C02/CH4 selectivity < MPa was obtained, which is still better than membranes dipcoated in undiluted solution F. This result clearly indicates that low seed density on the support surface is the key for the formation of high quality SAPO-34 membrane. However, no seeding leads to poor membrane quality (A13), which suggests that seeding is still indispensable for a high quality membrane.
Table 2 - The effect of seed concentration on the C02/CH4 separation performance of SAPO-34 membranes at different pressure drops.
Supports: 200 nm Al203 from Inopor Gmbh.
[0115] Figures 7a-7c show SEM images of supports dip coated in seed s different concentrations. No dense-packed seed layer was observed even when undiluted solution F was used. When seed solution was diluted 4 times (Figure 7a), many seeds were observed easily on the support surface and the seed coverage is around 50%. Further dilution of seed concentration greatly reduces the seed density on the support surface. Only 17 seed crystals were observed in an area of 9χ7μηι when seed concentration was diluted 16 times (Figure 7b) and only several crystals were found with 128 times dilution (Figure 7c). Lower seed density is a key to high quality SAPO-34 membranes. In zeolite A membrane synthesis, Sato et al claimed sparse distribution of seed crystal on the substrate surface was preferable for zeolite A membranes (Sato et al, Journal of Membrane Science 301 (2007) 151-161 ). Best results were obtained when 0.5 wt% seed concentration was used while less desirable results were obtained when using 2 wt% seed concentration.
[0116] Figures 8a-8b show the top view and cross-section view of a SAPO-34 membrane prepared with a dip coating seeding method. Optimal coverage and intergrowth were observed. The crystal size and membrane thickness are 10 μηι and 8 μηπ, respectively. Both are larger than membranes prepared with a rubbing seeding method. This can be explained by the lower seed density on the support, which leads to fewer secondary nuclei and bigger crystals and thickness. This also explains the lower permeance of dipcoated membranes.
[0117] However, it is believed that a densely packed seed layer is the pre-requisite for high quality MFI membranes. Zhang et al (supra) even observed a dense seed layer under zeolite membrane after hydrothermal synthesis. This suggests that the membrane formation of MFI membrane might be quite different with SAPO-34 and zeolite A membranes.
[0118] Table 3 (shown below) shows the effect of soaking time and seeding on membrane performance. Membranes A8 and A14 were soaked in membrane forming gel on one side (inside surface) and both sides, respectively. Prior to soaking, both membranes were dipcoated in seed solution F diluted 8 times. Soaking both sides of
the membrane in the membrane forming gel resulted in the C02/CH4 sele
increasing approximately 50% without sacrificing permeance. Soaking both sides of the support introduces more membrane forming gel into the support, which might grow more crystals in the support and thus leads to better coverage. This result is similar to membranes prepared with rubbing, as shown in Table 1 . Membrane A15, prepared by dipocating in a seed solution F diluted 16 times but without soaking treatment, exhibits decent C02 permeance of 12 x10"7mol/(m2 s Pa) and C02/CH4 selectivity of 69 at 4.6 MPa pressure drop. This result suggests that low seed density is a key factor in determining membrane quality. The soaking treatment significantly improves the C02 permanence (by 58%) and C02/CH4 selectivity (by 39%) at 4.6 MPa. The increase of permeance can be explained as the secondary nuclei generated during soaking treatment, which leads to a thinner membrane.
Table 3 - The effect of soaking and seeding on the C02/CH4 separation performance of SAPO- 34 membranes at different pressure drops.
[0119] Based on the obtained result, it is clear that low seed concentration on the support surface is a key factor in determining the membrane quality. Rubbing provides higher seed density on the support, which leads to lower membrane quality. Proper soaking treatment dissolves excessive seeds on the support and generates secondary nuclei which leads to membranes with high permeance and selectivity. For dip coating
methods, low seed density on the support can result into good membrane even without soaking treatment.
Example 6 - Effect of support pore size
[0120] Table 4 shows the effect of support pore size on the membrane performance. Besides 200 nm AI2O3 support, 100 nm AI2O3 and 5 nm T1O2 supports were also tried. Similar results were obtained for all supports. Despite the limited number of
membranes in this study, the results indicate that good membranes can be made on 100 nm and 5 nm supports. Again, soaking both sides of the support improved selectivity.
Table 4 - The effect of support pore size on the C02/CH4 separation performance of SAPO-34 membranes at different pressure drops.
Rubbing was used to seed supports
[0121] Having now fully described the present invention in some detail by way of illustration and examples for purposes of clarity of understanding, it will be obvious to one of ordinary skill in the art that the same can be performed by modifying or changing the invention within a wide and equivalent range of conditions, formulations and other parameters without affecting the scope of the invention or any specific embodiment
thereof, and that such modifications or changes are intended to be encon
the scope of the appended claims.
[0122] When a group of materials, compositions, components or compounds is disclosed herein, it is understood that all individual members of those groups and all subgroups thereof are disclosed separately. When a Markush group or other grouping is used herein, all individual members of the group and all combinations and
subcombinations possible of the group are intended to be individually included in the disclosure. Every formulation or combination of components described or exemplified herein can be used to practice the invention, unless otherwise stated. Whenever a range is given in the specification, for example, a temperature range, a time range, or a composition range, all intermediate ranges and subranges, as well as all individual values included in the ranges given are intended to be included in the disclosure.
Additionally, the end points in a given range are to be included within the range. In the disclosure and the claims, "and/or" means additionally or alternatively. Moreover, any use of a term in the singular also encompasses plural forms.
[0123] One of ordinary skill in the art will appreciate that starting materials, reagents, purification methods, materials, substrates, device elements, analytical methods, assay methods, mixtures and combinations of components other than those specifically exemplified can be employed in the practice of the invention without resort to undue experimentation. All art-known functional equivalents, of any such materials and methods are intended to be included in this invention. The terms and expressions which have been employed are used as terms of description and not of limitation, and there is no intention that in the use of such terms and expressions of excluding any equivalents of the features shown and described or portions thereof, but it is recognized that various modifications are possible within the scope of the invention claimed. The invention illustratively described herein suitably may be practiced in the absence of any element or elements, limitation or limitations which is not specifically disclosed herein.
[0124] All publications referred to herein are incorporated herein to the extent not inconsistent herewith. Some references provided herein are incorporated by reference
to provide details of additional uses of the invention. All patents and publ
mentioned in the specification are indicative of the levels of skill of those skilled in the art to which the invention pertains. References cited herein are incorporated by reference herein in their entirety to indicate the state of the art as of their filing date and it is intended that this information can be employed herein, if needed, to exclude specific embodiments that are in the prior art. For example, when a compound is claimed, it should be understood that compounds known in the art including the compounds disclosed in the references disclosed herein are not intended to be included in the claim.
Claims
1 . A method for making a crystalline silicoaluminophosphate-34 (SAPO-34) membrane, the method comprising the steps of:
a) providing a porous support having a first and a second side;
b) preparing an aqueous SAPO-34 forming membrane forming gel, wherein the membrane forming gel comprises aluminum, phosphorus, silicon, oxygen, an organic templating agent and water;
c) applying a low density of SAPO-34 seed material to at least the first side of the porous support;
d) following step c), contacting at least the first side of the porous support with the membrane forming gel;
e) heating the porous support and the membrane forming gel to about 453 K to about 533 K to form a layer of SAPO-34 crystals on the porous support; and f) calcining the SAPO layer to remove the templating agent.
2. The method of claim 1 wherein 0.4 g/m2 or less of SAPO-34 seed material is applied to at least the first side of the porous support.
3. The method of claim 1 wherein 0.2 g/m2 or less of SAPO-34 seed material is applied to at least the first side of the porous support.
4. The method of claim 1 wherein 0.1 g/m2 or less of SAPO-34 seed material is applied to at least the first side of the porous support.
5. The method of claims 1 -4 further comprising applying a low density of SAPO-34 seed material to the second side of the porous support.
6. The method of claims 1-5 wherein application of said SAPO-34 sei
comprises dipping the porous support one or more times in a SAPO-34 seed solution comprising between approximately 0.005 wt% and 0.67 wt% of SAPO-34 seed material.
7. The method of claims 1-6 wherein application of said SAPO-34 seed material comprises dipping the porous support one or more times in a SAPO-34 seed solution comprising between approximately 0.02 wt% and 0.042 wt% of SAPO-34 seed material
8. The method of claims 1-7 wherein application of said SAPO-34 seed material comprises dipping the porous supports one or more times in a SAPO-34 seed solution for 10 seconds and withdrawing the porous supports from the seed solution at a rate of about 1 cm/second.
9. The method of claims 1 -8 wherein the porous support has a pore size between 2 nm to 500 nm.
10. The method of claims 1 -9 wherein the membrane forming gel has the formula:
1 .0 AI2O3: aP2O5: bSiO2: cR: dH2O ,
where R is a templating agent, a is between about 0.01 and about 52, b is between about 0.03 and about 196, c is between about 0.2 and about 5 and d is between about 20 and about 300.
1 1 . The method of claims 1 -9 wherein the membrane forming gel has the formula:
AI2O3: aP2O5: bSiO2: cRi: dR2: eH2O ,
where Ri is a quaternary organic ammonium templating agent, and R2 is an amine having less than 12 carbon atoms, a is greater than 0.5 and less than 1 .5, b is greater than 0.2 and less than 1 .0, c is greater than or equal to 1 and less than 2, d is greater than zero and less than 4.0 and e is greater than 50 and less than 1 10.
12. The method of claims 1 -9 wherein the membrane forming gel has the formula:
AI2O3: aP2O5: bSiO2: cRi : diR2: d2R3: eH2O ,
where Ri is a quaternary organic ammonium templating agent, R2 and R
having less than 12 carbon atoms, a is greater than 0.5 and less than 1 .5, b is greater than 0.2 and less than 1 .0, c is greater than or equal to 1 and less than 2, d is greater than zero and less than 4.0, e is greater than 50 and less than 110, di is between 0.5 and 1 .5, and d2 is between 0.5 and 1 .5.
13. The method of claims 1 -12 wherein in step e) the porous support and the membrane forming gel are heated between 463 K and 493K.
14. The method of claims 1 -13 wherein the templating agent is a quaternary organic ammonium templating agent.
15. The method of claims 1 -14 wherein the templating agent is tetraethyl ammonium hydroxide (TEAOH).
16. The membrane of claims 1 -15 wherein the membrane has a C02/CH4 separation selectivity of 274 or greater for an approximately 50/50 C02/CH4 mixture with a pressure differential across the membrane of 0.14 MPa.
17. The membrane of claims 1 -16 wherein the membrane has a C02/CH4 separation selectivity of 78 or greater for an approximately 50/50 C02/CH4 mixture with a pressure differential across the membrane of 4.6 MPa.
18. The method of claims 1 -17, prior to the heating of step e), further comprising soaking the first side or the first and second sides of the porous support in the membrane forming gel between one and fourteen hours.
19. The method of claim 18 further comprising soaking the first side or the first and second sides of the porous support between two and five hours in the membrane forming gel.
20. The method of claim 18 further comprising soaking the first side or
second sides of the porous support between three and four hours in the membrane forming gel.
21 . The method of claims 1-20 wherein the membrane has a C02 permeance of 2.9 x 10"6mol/(m2 · s · Pa) or greater for an approximately 50/50 C02/CH4 mixture with a pressure differential across the membrane of 0.14 MPa.
22. The method of claims 1-21 wherein the membrane has a C02 permeance of 4.7 x 10"7mol/(m2 · s · Pa) or greater for an approximately 50/50 C02/CH4 mixture with a pressure differential across the membrane of 4.6 MPa.
23. A method for making a crystalline silicoaluminophosphate (SAPO) membrane, the method comprising the steps of:
a) providing a porous support having a first and a second side;
b) preparing an aqueous SAPO forming membrane forming gel, wherein the membrane forming gel comprises aluminum, phosphorus, silicon, oxygen, an organic templating agent and water;
c) soaking at least one side of the porous support in the membrane forming gel between one and fourteen hours at room temperature;
d) following the soaking step in step c), heating the porous support and the membrane forming gel to about 453 K to about 533 K to form a layer of SAPO crystals on the porous support; and
e) calcining the SAPO layer to remove the templating agent.
24. The method of claim 23 wherein, prior to heating, at least one side of the porous support is soaked in the membrane forming gel between 2 and 5 hours.
25. The method of claims 23-24 wherein both sides of the porous support are soaked in the membrane forming gel.
26. The method of claims 23-25 wherein one or both sides of the poroi soaked in the membrane forming gel between 2 and 5 hours.
27. The method of claims 23-26 wherein in step d) the porous support and the membrane forming gel are heated between 463 K and 493K.
28. The method of claims 23-27 wherein said porous support is aluminum oxide (Al203).
29. The method of claims 23-27 wherein said porous support is a ceramic support.
30. The method of claims 23-29, wherein the support has an upper and a lower surface and the SAPO layer is formed on either the upper surface or the lower surface.
31 . The method of claims 23-29, wherein the support is in the form of a tube having an inner and an outer surface, and the SAPO layer is formed on either the inner or the outer surface.
32. The method of claims 23-31 wherein the membrane forming gel is aged at least 24 hours prior to the soaking step of step c).
33. The method of claims 23-32 wherein the SAPO is SAPO-34.
34. The membrane of claims 23-33 wherein the thickness of the SAPO-34 layer is less than about 10 μηπ.
35. The membrane of claims 23-34 wherein the thickness of the SAPO-34 layer is between approximately 5 μηι and 6 μηπ.
36. The method of claims 23-35 further comprising applying a SAPO s
at least part of the surface of the porous support prior to contacting the porous support with the membrane forming gel.
37. The method of claim 36 wherein the seed material is applied through dip coating.
38. The method of claim 36 further comprising dipping the porous support one or more times in a SAPO-34 seed solution comprising between approximately 0.02 wt% and 0.042 wt% of SAPO-34 seed material.
39. A method for separating a first gas component from a gas mixture containing at least a first and a second gas component, the method comprising the steps of:
a) providing the membrane of any of claims 1 -17 and 23-35, the membrane having a feed and a permeate side and being selectively permeable to the first gas component over the second gas component;
b) applying a feed stream including the first and the second gas components to the feed side of the membrane; and
c) providing a driving force sufficient for permeation of the first gas
component through the membrane, thereby producing a permeate stream enriched in the first gas component from the permeate side of the membrane.
40. The method of claim 39, wherein the first gas component is carbon dioxide and the second gas component is methane.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US28570309P | 2009-12-11 | 2009-12-11 | |
| US61/285,703 | 2009-12-11 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011072215A1 true WO2011072215A1 (en) | 2011-06-16 |
Family
ID=44145932
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2010/059874 WO2011072215A1 (en) | 2009-12-11 | 2010-12-10 | High-flux sapo-34 membranes for co2/ch4 separations |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2011072215A1 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012095405A1 (en) * | 2011-01-12 | 2012-07-19 | Shell Internationale Research Maatschappij B.V. | Gas separation membrane and method of manufacture and use |
| WO2013020968A3 (en) * | 2011-08-09 | 2013-06-06 | Shell Internationale Research Maatschappij B.V. | Large surface supported molecular sieve membrane |
| CN103896300A (en) * | 2012-12-28 | 2014-07-02 | 中国科学院上海高等研究院 | Preparation method of high-performance SAPO (silicoaluminophosphate)-34 molecular sieve membrane |
| US8926732B2 (en) | 2009-07-24 | 2015-01-06 | The Regents Of The University Of Colorado, A Body Corporate | Imidazolium-based room-temperature ionic liquids, polymers, monomers, and membranes incorporating same |
| CN104340993A (en) * | 2013-08-06 | 2015-02-11 | 中国科学院上海高等研究院 | Preparation method of SAPO-34 molecular sieve membrane |
| CN104525250A (en) * | 2015-01-09 | 2015-04-22 | 中国科学院上海高等研究院 | SAPO-34 molecular sieve based catalyst of hierarchical pore structure and preparation and application thereof |
| CN110548538A (en) * | 2018-06-04 | 2019-12-10 | 中国科学院大连化学物理研究所 | Preparation method and application of metal modified SAPO-34 molecular sieve |
| JPWO2020175247A1 (en) * | 2019-02-28 | 2020-09-03 | ||
| US11969692B2 (en) | 2019-02-28 | 2024-04-30 | Zeon Corporation | Method of producing separation membrane |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050139065A1 (en) * | 2003-12-24 | 2005-06-30 | Chevron U.S.A. Inc. | Mixed matrix membranes with low silica-to-alumina ratio molecular sieves and methods for making and using the membranes |
| US20050204916A1 (en) * | 2004-03-19 | 2005-09-22 | Falconer John L | High-selectivity supported SAPO membranes |
| US20070265484A1 (en) * | 2006-05-15 | 2007-11-15 | The Regents Of The University Of Colorado, A Body Corporate | High flux and selectivity sapo-34 membranes for co2/ch4 separations |
| US20080216650A1 (en) * | 2007-03-09 | 2008-09-11 | The Regents Of The University Of Colorado, A Body Corporate | Synthesis of Zeolites and Zeolite Membranes Using Multiple Structure Directing Agents |
-
2010
- 2010-12-10 WO PCT/US2010/059874 patent/WO2011072215A1/en active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050139065A1 (en) * | 2003-12-24 | 2005-06-30 | Chevron U.S.A. Inc. | Mixed matrix membranes with low silica-to-alumina ratio molecular sieves and methods for making and using the membranes |
| US20050204916A1 (en) * | 2004-03-19 | 2005-09-22 | Falconer John L | High-selectivity supported SAPO membranes |
| US7316727B2 (en) * | 2004-03-19 | 2008-01-08 | The Regents Of The University Of Colorado | High-selectivity supported SAPO membranes |
| US20070265484A1 (en) * | 2006-05-15 | 2007-11-15 | The Regents Of The University Of Colorado, A Body Corporate | High flux and selectivity sapo-34 membranes for co2/ch4 separations |
| US20080216650A1 (en) * | 2007-03-09 | 2008-09-11 | The Regents Of The University Of Colorado, A Body Corporate | Synthesis of Zeolites and Zeolite Membranes Using Multiple Structure Directing Agents |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8926732B2 (en) | 2009-07-24 | 2015-01-06 | The Regents Of The University Of Colorado, A Body Corporate | Imidazolium-based room-temperature ionic liquids, polymers, monomers, and membranes incorporating same |
| US9446348B2 (en) | 2009-07-24 | 2016-09-20 | The Regents Of The University Of Colorado, A Body Corporate | Imidazolium-based room-temperature ionic liquids, polymers, monomers and membranes incorporating same |
| WO2012095405A1 (en) * | 2011-01-12 | 2012-07-19 | Shell Internationale Research Maatschappij B.V. | Gas separation membrane and method of manufacture and use |
| WO2013020968A3 (en) * | 2011-08-09 | 2013-06-06 | Shell Internationale Research Maatschappij B.V. | Large surface supported molecular sieve membrane |
| CN103896300A (en) * | 2012-12-28 | 2014-07-02 | 中国科学院上海高等研究院 | Preparation method of high-performance SAPO (silicoaluminophosphate)-34 molecular sieve membrane |
| CN104340993A (en) * | 2013-08-06 | 2015-02-11 | 中国科学院上海高等研究院 | Preparation method of SAPO-34 molecular sieve membrane |
| CN104525250A (en) * | 2015-01-09 | 2015-04-22 | 中国科学院上海高等研究院 | SAPO-34 molecular sieve based catalyst of hierarchical pore structure and preparation and application thereof |
| CN110548538A (en) * | 2018-06-04 | 2019-12-10 | 中国科学院大连化学物理研究所 | Preparation method and application of metal modified SAPO-34 molecular sieve |
| JPWO2020175247A1 (en) * | 2019-02-28 | 2020-09-03 | ||
| EP3932530A4 (en) * | 2019-02-28 | 2022-11-16 | Zeon Corporation | Separation membrane manufacturing method |
| JP7464037B2 (en) | 2019-02-28 | 2024-04-09 | 日本ゼオン株式会社 | Separation membrane manufacturing method |
| US11969692B2 (en) | 2019-02-28 | 2024-04-30 | Zeon Corporation | Method of producing separation membrane |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2650846C (en) | High flux and selectivity sapo-34 membranes for co2/ch4 separations | |
| US8302782B2 (en) | Synthesis of zeolites and zeolite membranes using multiple structure directing agents | |
| US7316727B2 (en) | High-selectivity supported SAPO membranes | |
| US8679227B2 (en) | High flux SAPO-34 membranes for CO2/CH4 separation and template removal method | |
| US7828875B2 (en) | Membranes for highly selective separations | |
| Yu et al. | Highly permeable CHA membranes prepared by fluoride synthesis for efficient CO 2/CH 4 separation | |
| WO2011072215A1 (en) | High-flux sapo-34 membranes for co2/ch4 separations | |
| Wu et al. | Alumina-supported AlPO-18 membranes for CO2/CH4 separation | |
| US20140352533A1 (en) | Seeded-gel synthesis of high flux and high selectivity sapo-34 membranes for co2/ch4 separations | |
| JP2014508643A (en) | Microporous UZM-5 inorganic zeolite membrane for gas, vapor and liquid separation | |
| Shi | Organic template-free synthesis of SAPO-34 molecular sieve membranes for CO 2–CH 4 separation | |
| US8221525B2 (en) | Oxygen enrichment using small-pore silicoaluminophosphate membranes | |
| US11964242B2 (en) | Zeolite membranes, molecular separation methods, and manufacturing processes for zeolite membranes | |
| Li et al. | Membrane Processes for N2–CH4 Separation | |
| Zong | Synthesis of Small Pore Zeolite Membranes for Nitrogen/Methane Separation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10836745 Country of ref document: EP Kind code of ref document: A1 |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10836745 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10836745 Country of ref document: EP Kind code of ref document: A1 |