WO2010151747A1 - Pyrimine compounds and methods of making and using same - Google Patents
Pyrimine compounds and methods of making and using same Download PDFInfo
- Publication number
- WO2010151747A1 WO2010151747A1 PCT/US2010/039963 US2010039963W WO2010151747A1 WO 2010151747 A1 WO2010151747 A1 WO 2010151747A1 US 2010039963 W US2010039963 W US 2010039963W WO 2010151747 A1 WO2010151747 A1 WO 2010151747A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- pyrimidin
- amine
- alkyl
- cycloalkyl
- cyclohexyl
- Prior art date
Links
- 0 CC(c1c(*)c(C)nc(NI/C2=C/C=C/C=C/C=C2)n1)=**=**=* Chemical compound CC(c1c(*)c(C)nc(NI/C2=C/C=C/C=C/C=C2)n1)=**=**=* 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/32—One oxygen, sulfur or nitrogen atom
- C07D239/42—One nitrogen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/04—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/10—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a carbon chain containing aromatic rings
Abstract
Disclosed herein are pyrimidinyl compounds that are contemplated to be modulators of cystic fibrosis transmembrane regulators (CFTR), and methods of making and using same. Also provided are pharmaceutical compositions and methods of treating disorders associated with cystic fibrosis transmembrane regulators, such as airway inflammation, cystic fibrosis, and the like.
Description
PYRIMIDINE COMPOUNDS AND METHODS OF MAKING AND USING SAME
RELATED APPLICATIONS
[0001] This application claims the benefit of and priority to United States Provisional Patent Application Serial No. 61/220,689, filed June 26, 2009, the contents of which are hereby incorporated by reference.
BACKGROUND [0002] The cystic fibrosis transmembrane regulator (CFTR), is a protein of approximately 1480 amino acids made up of two repeated elements, each having six transmembrane segments and a nucleotide binding domain. Based on its predicted domain structure, CFTR is a member or a class of related proteins which includes the multi-drug resistance (MDR) or P-glycoprotein, bovine adenyl cyclase, the yeast STE6 protein as well as several bacterial amino acid transport proteins. Proteins in this group, characteristically, are involved in pumping molecules into or out of cells. CFTR has been postulated to regulate the outward flow of anions from epithelial cells in response to phosphorylation by cyclic AMP-dependent protein kinase or protein kinase C. [0003] Cystic fibrosis (CF) is a lethal hereditary autosomal recessive disease which is caused by mutations in the gene coding for the CFTR Cl -channel. By far the most common disease- causing mutation is the deletion of the codon for phenylalanine 508 (ΔF508) in the primary sequence of wild type CFTR. Over 90% of patients carry at least one allele of the ΔF508 CFTR mutant gene. The gene product from this mutant gene is a CFTR Cl -channel that is poorly processed within the cell: most of the mutant protein is incorrectly or incompletely folded and becomes targeted to endoplasmic reticulum-associated degradation (ERAD). The few mutant Cl -channels that pass the quality control or simply escape the ER before they are degraded will mature through the golgi and eventually are incorporated into the plasma membrane. These are thought to represent <5% of the level observed in cells expressing wild type CFTR, resulting in a commensurate low total whole-cell Cl"-conductance. In addition to the much lower number of channels in the plasma membrane, the open probability of the individual channel proteins is ~3-fold reduced compared to wild type CFTR.
[0004] For over a decade, efforts have been ongoing to identify small molecule drugs that can restore the cell CFTR Cl"-conductance to levels high enough to ameliorate the effects of CF. These include correctors of ΔF508 CFTR, compounds that can improve the intracellular processing, and potentiators, compounds which increase the open probability of mutant CFTR channels at the cell surface.
[0005] A small molecule dual-acting potentiator-corrector is expected to be of great benefit for the treatment of most CF patients. To date, it has proven difficult to develop compounds acting solely by correction of the intracellular processing that can sufficiently increase the number of channels in the cell surface to overcome the disease-causing deficiency in Cl"-conductance. On the other hand, potentiation, i.e., increase of open probability, of only the mutant channels at the cell surface will not sufficiently restore Cl"-conductance for most CF patients. A dual- acting potentiator-corrector molecule would mechanistically combine aspects of both corrector and potentiator compounds: the number of CFTR channels at the surface and the channel open probability are increased in parallel.
SUMMARY
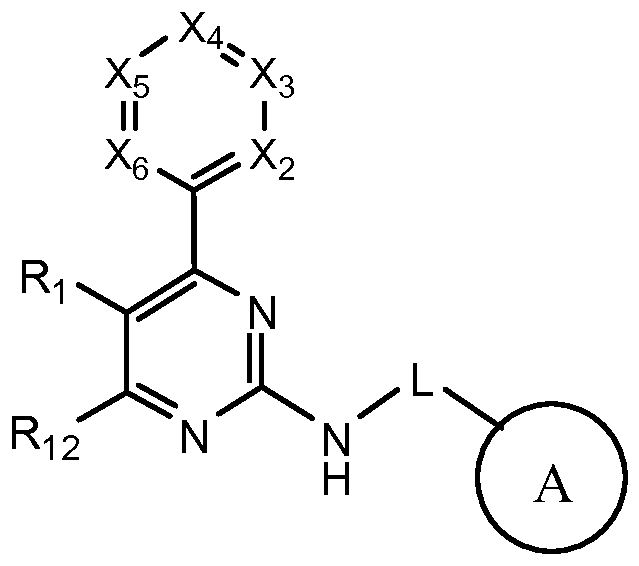
[0006] Provided herein are compounds contemplated to be CFTR modulators, and their use as, for example, medicinal agents. Also provided are pharmaceutical compositions comprising at least one disclosed compound, or a pharmaceutically acceptable salt or N-oxide thereof, and a pharmaceutically acceptable carrier. [0007] Accordingly, one aspect of the invention provides a compound of formula I:
I including a pharmaceutically acceptable salt or N-oxide thereof; wherein:
X2 is CR2 or N, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5 or N, and X6 is CR6 or N, where no more than two OfX2-X6 are N; where if X3 is N, L is a bond, and A is cyclohexyl, then R2 is not methoxy; and if X5 is N, L is a bond, and A is cyclohexyl, then R6 is not methoxy; L is a bond or a Ci_2alkylidene chain optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of Ci-C6alkyl, C3-C6cycloalkyl, -OCi-C6alkyl, -OC3-C6cycloalkyl, and F;
A is C4-Ciocycloalkyl, C3-Cioheterocycloalkyl, or phenyl, each of which is optionally substituted with one, two, or three substituents independently, for each occurrence, selected from the group consisting of F, Cl, -CF3, -OCi-C6alkyl, -OH, Ci-C6alkyl, Ci-C6alkoxycarbonyl, C3-C5cycloalkyl, aryl, -C(O)-aryl, -C(O)-heteroaralkyl, -C(O)-C i-C6alkyl, and -C(O)N(H)(C1- C6alkyl);
Ri and Ri2 are each independently hydrogen, CN, Ci-C6alkyl, -OCi-C6alkyl, C3. locycloalkyl, -OC3_i0cycloalkyl, -OCF3, -OCF2H, -OCH2F, halogen, -NR7Ri0, -NR7COR8, -NR7SO2R9 , or -SO2R9, where if Ri2 is -OCF2H, then R4 is not methyl;
R3 and R5 are each independently hydrogen, halogen, -CF3, -OH, -OCF3, -OCHF2, -OCH2F, Ci-Cgalkyl, C3-C8cycloalkyl, C3-C8heterocyclyl, -OCi-Ci0alkyl, -OC3-Ci0cycloalkyl, -NR7COR8, -NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, or -SO2R9;
R4 is hydrogen, halogen, Ci-C3alkyl, -OCi-C6alkyl, -O-aryl, -OH, -OCHF2, -OCH2F, - CN, heteroaryl, -NR7Ri0, or -SO2NR7Ri0;
R6 is hydrogen, halogen, -CN, -OCi-Ci0alkyl, -Oaryl, -CF3, -OCHF2, -OCH2F, -NR7Ri0,
where any two adjacent variables selected from R2, R3, R4, R5, and R6 can be taken together to form a cycloalkyl, aryl, heteroaryl, or heterocyclyl, each of which is optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl, where R3 and R4 cannot be taken together to form a dioxolanyl when L is a bond and A is cyclohexyl;
- A -
where at least one of R2, R3, R4, R5, and R6 is not hydrogen; and if R4 is -OCH3, then R3 and R5 are not -OCH3;
R7 and Rio each represent independently for each occurrence hydrogen, alkyl, or cycloalkyl, wherein the alkyl and cycloalkyl are optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of halogen, cyano, hydroxy, nitro, and alkoxy; or R7 and Rio are taken together to form a heterocyclyl optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl, where the heterocyclyl is not dihydro-2H-benzo [b] [ 1 ,4] dioxepinyl;
Rs is alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl;
R9 represents independently for each occurrence alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydroxyl; and Rn is alkyl, alkenyl, alkynyl, amido, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydrogen.
[0008] Also provided herein are methods of treating airway inflammation, such as cystic fibrosis, comprising administering to a subject in need thereof a therapeutically effective amount of a compound described herein, such as a compound of formula I, IA, IB, II, III, or IIIA. Also contemplated herein are compositions that include a compound described herein, such as a compound formula I, IA, IB, II, III, or IIIA, and a pharmaceutically acceptable carrier.
[0009] The compound of formula III is represented by:
III including a pharmaceutically acceptable salt or N-oxide thereof; wherein:
X2 is CR2 or N, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5 or N, and X6 is CR6 or N, where no more than two OfX2-X6 are N;
L is a bond or a Ci_2alkylidene chain optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of Ci-C6alkyl, C3-C6cycloalkyl, -OCi-C6alkyl, -OC3-C6cycloalkyl, and F;
A is Ci-Cioalkyl, C3-Ciocycloalkyl, C3-Cioheterocycloalkyl, or phenyl; each of which is optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of -CF3, -OCi-C6alkyl, -OH, Ci-C6alkyl, Ci-C6alkoxycarbonyl, C3- C6cycloalkyl, aryl, halogen, -C(O)-aryl, -C(O)-heteroaralkyl, -C(O)-CrC6alkyl, and -C(O)N(H)(Ci-C6alkyl);
Ri and Ri2 are each independently hydrogen, CN, Ci-C6alkyl, -OCi-C6alkyl, C3. locycloalkyl, -OC3_i0cycloalkyl, -OCF3, -OCF2H, -OCH2F, halogen, -NR7Ri0, -NR7COR8, -NR7SO2R9, or -SO2R9;
R2, R3, R4, R5, and R6 are each independently hydrogen, halogen, -CF3, -OH, -OCF3, -OCHF2, -OCH2F, Ci-Cioalkyl, C3-Ci0cycloalkyl, C3-C8heterocyclyl, heteroaryl, -OCi- Cioalkyl, -O-Cs-Ciocycloalkyl, -OH, -O-aryl, -NR7COR8, -NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, -SO2R9, or -CO2Rn; or where any two adjacent variables selected from R2, R3, R4, R5, and R6 can be taken together to form a cycloalkyl, aryl, heteroaryl, or heterocyclyl, each of which is optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl;
R7 and Ri0 each represent independently for each occurrence hydrogen, alkyl, or cycloalkyl, wherein the alkyl or cycloalkyl are optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of halogen, cyano, hydroxy, nitro, and alkoxy; or
R7 and Ri0 are taken together to form a heterocyclyl optionally substituted by one, two, or three substituents selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl;
R8 is alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl;
Rg represents independently for each occurrence alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl; and
Rn is alkyl, alkenyl, alkynyl, amido, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydrogen. [0010] The disclosure further provides methods of modulating the activity of one or more cystic fibrosis transmembrane regulators comprising, for example, exposing said receptor to a compound described herein, e.g., a compound of formula I, IA, IB, II, III, or IIIA.
[0011] Also provided herein are methods of treating a disease associated with expression or activity of one or more cystic fibrosis transmembrane regulators in a subject comprising administering to the subject a therapeutically effective amount of a disclosed compound. For example, provided herein are methods of treating chronic obstructive pulmonary disease, dry eye disease, and Sjogren's syndrome, comprising administering a compound described herein, e.g., a compound of formula I, IA, IB, II, III, or IIIA. Also provided are use of the compounds described herein for therapy and/or the manufacture of a medicament for the treatment of disease associated with cystic fibrosis transmembrane regulators.
DETAILED DESCRIPTION
[0012] The features and other details of the disclosure will now be more particularly described. Before further description of the present invention, certain terms employed in the specification, examples and appended claims are collected here. These definitions should be read in light of the remainder of the disclosure and understood as by a person of skill in the art. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by a person of ordinary skill in the art.
I. Definitions
[0013] "Treating" includes any effect, e.g., lessening, reducing, modulating, or eliminating, that results in the improvement of the condition, disease, disorder and the like.
[0014] The term "aldehyde" or "formyl" as used herein refers to the radical -CHO. [0015] The term "alkanoyl" as used herein refers to a radical -O-CO-alkyl.
[0016] The term "alkenyl" as used herein refers to an unsaturated straight or branched hydrocarbon having at least one carbon-carbon double bond, such as a straight or branched
group of 2-12, 2-10, or 2-6 carbon atoms, referred to herein as C2-Ci2alkenyl, C2_Cioalkenyl, and C2-C6alkenyl, respectively. Exemplary alkenyl groups include, but are not limited to, vinyl, allyl, butenyl, pentenyl, hexenyl, butadienyl, pentadienyl, hexadienyl, 2-ethylhexenyl, 2- propyl-2-butenyl, 4-(2-methyl-3-butene)-pentenyl, etc. [0017] The term "alkoxy" as used herein refers to an alkyl group attached to an oxygen (-O- alkyl). Exemplary alkoxy groups include, but are not limited to, groups with an alkyl, alkenyl or alkynyl group of 1-12, 1-8, or 1-6 carbon atoms, referred to herein as Ci-Ci2alkoxy, Ci- Cgalkoxy, and Ci-Cβalkoxy, respectively. Exemplary alkoxy groups include, but are not limited to methoxy, ethoxy, etc. Similarly, exemplary "alkenoxy" groups include, but are not limited to vinyloxy, allyloxy, butenoxy, etc.
[0018] The term "alkyl" as used herein refers to a saturated straight or branched hydrocarbon, such as a straight or branched group of 1-12, 1-10, or 1-6 carbon atoms, referred to herein as Ci-Ci2alkyl, Ci-CiOalkyl, and Ci-C6alkyl, respectively. Exemplary alkyl groups include, but are not limited to, methyl, ethyl, propyl, isopropyl, 2 -methyl- 1 -propyl, 2-methyl-2-propyl, 2- methyl- 1 -butyl, 3 -methyl- 1 -butyl, 2-methyl-3 -butyl, 2,2-dimethyl-l -propyl, 2-methyl-l-pentyl, 3-methyl-l-pentyl, 4-methyl-l-pentyl, 2-methyl-2-pentyl, 3-methyl-2-pentyl, 4-methyl-2- pentyl, 2,2-dimethyl-l -butyl, 3,3-dimethyl-l-butyl, 2-ethyl-l -butyl, butyl, isobutyl, t-butyl, pentyl, isopentyl, neopentyl, hexyl, heptyl, octyl, etc. Unless specified otherwise, alkyl groups are optionally substituted by one or two substituents independently selected from the group consisting of alkanoyl, alkoxy, amino, carboxy, cycloalkyl, ester, ether, halogen, heterocycloalkyl, and hydroxyl. In certain embodiments, the alkyl group is not substituted, i.e., it is unsubstituted.
[0019] The term "alkynyl" as used herein refers to an unsaturated straight or branched hydrocarbon having at least one carbon-carbon triple bond, such as a straight or branched group of 2-12, 2-8, or 2-6 carbon atoms, referred to herein as C2-Ci2alkynyl, C2-C8alkynyl, and C2-Cealkynyl, respectively. Exemplary alkynyl groups include, but are not limited to, ethynyl, propynyl, butynyl, pentynyl, hexynyl, methylpropynyl, 4-methyl-l-butynyl, 4-propyl-2- pentynyl, and 4-butyl-2-hexynyl, etc.
[0020] Unless specified otherwise, alkenyl and alkynyl groups are optionally substituted by at least one group selected from alkanoyl, alkoxy, alkyl, alkenyl, alkynyl, amido, amidino, amino, aryl, arylalkyl, azido, carbamate, carbonate, carboxy, cyano, cycloalkyl, ester, ether, formyl,
halogen, haloalkyl, heteroaryl, heterocyclyl, hydroxyl, imino, ketone, nitro, phosphate, phosphonato, phosphinato, sulfate, sulfide, sulfonamido, sulfonyl and thiocarbonyl. In certain embodiments, the alkenyl and alkynyl groups are not substituted, i.e., they are unsubstituted.
[0021] The term "amide" or "amido" as used herein refers to a radical of the form -RaC(O)N(Rb)-, -RaC(O)N(Rb)Rc-, -C(0)NRbRc, or -C(O)NH2, wherein Ra, Rb and R0 are each independently selected from alkoxy, alkyl, alkenyl, alkynyl, amide, amino, aryl, arylalkyl, carbamate, cycloalkyl, ester, ether, formyl, halogen, haloalkyl, heteroaryl, heterocyclyl, hydrogen, hydroxyl, ketone, and nitro. The amide can be attached to another group through the carbon, the nitrogen, Rb, Rc, or Ra. The amide also may be cyclic, for example Rb and Rc, Ra and Rb, or Ra and Rc may be joined to form a 3- to 12-membered ring, such as a 3- to 10- membered ring or a 5- to 6-membered ring. The term "carboxamido" refers to the structure -C(O)NRbRc.
[0022] The term "amidino" as used herein refers to a radical of the form -C(=NR)NR'R" where R, R', and R" can each independently be selected from alkyl, alkenyl, alkynyl, amide, aryl, arylalkyl, cyano, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydroxyl, ketone and nitro.
[0023] The term "amine" or "amino" as used herein refers to a radical of the form -NRdRg, -N(Rj)Re-, or -RgN(Rd)Rf- where Rj, Rg, and Rf are independently selected from alkoxy, alkyl, alkenyl, alkynyl, amide, amino, aryl, arylalkyl, carbamate, cycloalkyl, ester, ether, formyl, halogen, haloalkyl, heteroaryl, heterocyclyl, hydrogen, hydroxyl, ketone, and nitro. The amino can be attached to the parent molecular group through the nitrogen, Rj, R6 or Rf. The amino also may be cyclic, for example any two of Rd, Re or Rf may be joined together or with the N to form a 3- to 12-membered ring, e.g., morpholino or piperidinyl. The term amino also includes the corresponding quaternary ammonium salt of any amino group, e.g., -[N(Rd)(Re)(Rf)]+. Exemplary amino groups include aminoalkyl groups, wherein at least one of RJ, Rg, or Rf is an alkyl group.
[0024] The term "aryl" as used herein refers to refers to a mono-, bi-, or other multi- carbocyclic, aromatic ring system. Unless specified otherwise, the aromatic ring is optionally substituted at one or more ring positions with substituents selected from alkanoyl, alkoxy, alkyl, alkenyl, alkynyl, amido, amidino, amino, aryl, arylalkyl, azido, carbamate, carbonate, carboxy, cyano, cycloalkyl, ester, ether, formyl, halogen, haloalkyl, heteroaryl, heterocyclyl,
hydroxyl, imino, ketone, nitro, phosphate, phosphonato, phosphinato, sulfate, sulfide, sulfonamido, sulfonyl and thiocarbonyl. The term "aryl" also includes polycyclic ring systems having two or more cyclic rings in which two or more carbons are common to two adjoining rings (the rings are "fused rings") wherein at least one of the rings is aromatic, e.g., the other cyclic rings may be cycloalkyls, cycloalkenyls, cycloalkynyls, and/or aryls. Exemplary aryl groups include, but are not limited to, phenyl, tolyl, anthracenyl, fluorenyl, indenyl, azulenyl, and naphthyl, as well as benzo-fused carbocyclic moieties such as 5,6,7,8-tetrahydronaphthyl. In certain embodiments, the aryl group is not substituted, i.e., it is unsubstituted.
[0025] The term "arylalkyl" as used herein refers to an aryl group having at least one alkyl substituent, e.g. -aryl-alkyl-. Exemplary arylalkyl groups include, but are not limited to, arylalkyls having a monocyclic aromatic ring system, wherein the ring comprises 6 carbon atoms. For example, "phenylalkyl" includes phenylC4alkyl, benzyl, 1-phenylethyl, 2- phenylethyl, etc.
[0026] The term "azido" as used herein refers to the radical -N3. [0027] The term "carbamate" as used herein refers to a radical of the form -RgOC(O)N(Rt1)-, -RgOC(O)N(Rh)Ri-, or -OC(O)NRhRi, wherein Rg? Rh and Ri are each independently selected from alkoxy, aryloxy, alkyl, alkenyl, alkynyl, amide, amino, aryl, arylalkyl, carbamate, carboxy, cyano, cycloalkyl, ester, ether, formyl, halogen, haloalkyl, heteroaryl, heterocyclyl, hydroxyl, ketone, nitro, sulfide, sulfonyl, and sulfonamide. Exemplary carbamates include, but are not limited to, arylcarbamates or heteroaryl carbamates, e.g., wherein at least one of Rg Rh and Ri are independently selected from aryl or heteroaryl, such as phenyl and pyridinyl.
[0028] The term "carbonyl" as used herein refers to the radical -C(O)-.
[0029] The term "carboxamido" as used herein refers to the radical -C(O)NRR', where R and R' may be the same or different. R and R' may be selected from, for example, alkyl, aryl, arylalkyl, cycloalkyl, formyl, haloalkyl, heteroaryl and heterocyclyl.
[0030] The term "carboxy" as used herein refers to the radical -COOH or its corresponding salts, e.g. -COONa, etc.
[0031] The term "cyano" as used herein refers to the radical -CN.
[0032] The term "cycloalkoxy" as used herein refers to a cycloalkyl group attached to an oxygen.
[0033] The term "cycloalkyl" as used herein refers to a monovalent saturated or unsaturated cyclic, bicyclic, or bridged cyclic (e.g., adamantyl) hydrocarbon group of 3-12, 3-8, 4-8, or 4-6 carbons, referred to herein, e.g., as "C4_8cycloalkyl," derived from a cycloalkane. Exemplary cycloalkyl groups include, but are not limited to, cyclohexanes, cyclohexenes, cyclopentanes, cyclopentenes, cyclobutanes and cyclopropanes. Unless specified otherwise, cycloalkyl groups are optionally substituted with alkanoyl, alkoxy, alkyl, alkenyl, alkynyl, amido, amidino, amino, aryl, arylalkyl, azido, carbamate, carbonate, carboxy, cyano, cycloalkyl, ester, ether, formyl, halogen, haloalkyl, heteroaryl, heterocyclyl, hydroxyl, imino, ketone, nitro, phosphate, phosphonato, phosphinato, sulfate, sulfide, sulfonamido, sulfonyl and thiocarbonyl. Cycloalkyl groups can be fused to other cycloalkyl, aryl, or heterocyclyl groups. In certain embodiments, the cycloalkyl group is not substituted, i.e., it is unsubstituted.
[0034] The term "ether" refers to a radical having the structure -RiO-Rm-, where Ri and Rm can independently be alkyl, aryl, cycloalkyl, heterocyclyl, or ether. The ether can be attached to the parent molecular group through Ri or Rm. Exemplary ethers include, but are not limited to, alkoxyalkyl and alkoxyaryl groups. Ether also includes polyethers, e.g., where one or both of Ri and Rm are ethers.
[0035] The terms "halo" or "halogen" or "Hal" as used herein refer to F, Cl, Br, or I. [0036] The term "haloalkyl" as used herein refers to an alkyl group substituted with one or more halogen atoms.
[0037] The terms "heteroaryl" as used herein refers to a 5-15 membered mono-, bi-, or other multi-cyclic, aromatic ring system containing one or more heteroatoms, for example one to four heteroatoms, such as nitrogen, oxygen, and sulfur. Heteroaryls can also be fused to non- aromatic rings. Unless specified otherwise, the heteroaryl ring is optionally substituted at one or more positions with such substituents as described above, as for example, alkanoyl, alkoxy, alkyl, alkenyl, alkynyl, amido, amidino, amino, aryl, arylalkyl, azido, carbamate, carbonate, carboxy, cyano, cycloalkyl, ester, ether, formyl, halogen, haloalkyl, heteroaryl, heterocyclyl, hydroxyl, imino, ketone, nitro, phosphate, phosphonato, phosphinato, sulfate, sulfide, sulfonamido, sulfonyl and thiocarbonyl. Illustrative examples of heteroaryl groups include, but are not limited to, acridinyl, benzimidazolyl, benzofuryl, benzothiazolyl, benzothienyl,
benzoxazolyl, carbazolyl, carbolinyl, cinnolinyl, furazanyl, furyl, imidazolyl, indazolyl, indolizinyl, indolyl, isobenzofuryl, isoindolyl, isoquinolinyl, isothiazolyl, isoxazolyl, naphthyridinyl, oxadiazolyl, oxazolyl, phenanthridinyl, phenanthrolinyl, phenarsazinyl, phenazinyl, phenothiazinyl, phenoxazinyl, phthalazinyl, pteridinyl, purinyl, pyrazinyl, pyrazolyl, pyrazyl, pyridazinyl, pyridinyl, pyrimidilyl, pyrimidyl, pyrrolyl, quinolinyl, quinolizinyl, quinoxalinyl, quinoxaloyl, quinazolinyl, tetrazolyl, thiadiazolyl, thianthrenyl, thiazolyl, thienyl, thiophenyl, triazinyl, (1,2,3,)- and (l,2,4)-triazolyl, and the like. Exemplary heteroaryl groups include, but are not limited to, a monocyclic aromatic ring, wherein the ring comprises 2 to 5 carbon atoms and 1 to 3 heteroatoms. In certain embodiments, the heteroaryl group is not substituted, i.e., it is unsubstituted.
[0038] The terms "heterocyclyl" or "heterocyclic group" are art-recognized and refer to saturated or partially unsaturated 3- to 10-membered ring structures, alternatively 3- to 7- membered rings, whose ring structures include one to four heteroatoms, such as nitrogen, oxygen, and sulfur. Heterocycles may also be mono-, bi-, or other multi-cyclic ring systems. A heterocycle may be fused to one or more aryl, partially unsaturated, or saturated rings.
Heterocyclyl groups include, for example, biotinyl, chromenyl, dihydrofuryl, dihydroindolyl, dihydropyranyl, dihydrothienyl, dithiazolyl, homopiperidinyl, imidazolidinyl, isoquinolyl, isothiazolidinyl, isoxazolidinyl, morpholinyl, oxolanyl, oxazolidinyl, phenoxanthenyl, piperazinyl, piperidinyl, pyranyl, pyrazolidinyl, pyrazolinyl, pyridyl, pyrimidinyl, pyrrolidinyl, pyrrolidin-2-onyl, pyrrolinyl, tetrahydrofuryl, tetrahydroisoquinolyl, tetrahydropyranyl, tetrahydroquinolyl, thiazolidinyl, thiolanyl, thiomorpholinyl, thiopyranyl, xanthenyl, lactones, lactams such as azetidinones and pyrrolidinones, sultams, sultones, and the like. Unless specified otherwise, the heterocyclic ring is optionally substituted at one or more positions with substituents such as alkanoyl, alkoxy, alkyl, alkenyl, alkynyl, amido, amidino, amino, aryl, arylalkyl, azido, carbamate, carbonate, carboxy, cyano, cycloalkyl, ester, ether, formyl, halogen, haloalkyl, heteroaryl, heterocyclyl, hydroxyl, imino, ketone, nitro, phosphate, phosphonato, phosphinato, sulfate, sulfide, sulfonamido, sulfonyl and thiocarbonyl. In certain embodiments, the heterocyclcyl group is not substituted, i.e., it is unsubstituted.
[0039] The term "heterocycloalkyl" is art-recognized and refers to a saturated heterocyclyl group as defined above.
[0040] The term "heterocyclylalkoxy" as used herein refers to a heterocyclyl attached to an alkoxy group.
[0041] The term "heterocyclyloxyalkyl" refers to a heterocyclyl attached to an oxygen (-O-), which is attached to an alkyl group. [0042] The terms "hydroxy" and "hydroxyl" as used herein refers to the radical -OH.
[0043] The term "hydroxyalkyl" as used herein refers to a hydroxy radical attached to an alkyl group.
[0044] The term "imino" as used herein refers to the radical -C(=N)-R", where R" can be, for example, alkyl, alkenyl, alkynyl, aryl, arylalkyl, cycloalkyl, ether, haloalkyl, heteroaryl, heterocyclyl, and ketone.
[0045] The term "nitro" as used herein refers to the radical -NO2.
[0046] The term "phosphate" as used herein refers to the radical -OP(O)(ORaa)2 or its anions. The term "phosphanato" refers to the radical - P(O)(ORaa)2 or its anions. The term "phosphinato" refers to the radical -PRaa(O)(ORaa) or its anion, where each R321 can be selected from, for example, alkyl, alkenyl, alkynyl, aryl, arylalkyl, cycloalkyl, hydrogen, haloalkyl, heteroaryl, and heterocyclyl.
[0047] The term "sulfate" as used herein refers to the radical -OS(O)(ORaa)2 or its anions, where Raa is defined above.
[0048] The term "sulfonamide" or "sulfonamido" as used herein refers to a radical having the structure -N(Rr)-S(O)2-Rs- or -S(O)2-N(Rr)Rs, where Rr, and R8 can be, for example, hydrogen, alkyl, aryl, cycloalkyl, and heterocyclyl. Exemplary sulfonamides include alkylsulfonamides (e.g., where R8 is alkyl), arylsulfonamides (e.g., where R8 is aryl), cycloalkyl sulfonamides (e.g., where R8 is cycloalkyl), and heterocyclyl sulfonamides (e.g., where R8 is heterocyclyl), etc.
[0049] The term "sulfonyl" as used herein refers to a radical having the structure RuSθ2-, where Ru can be alkyl, aryl, cycloalkyl, and heterocyclyl, e.g., alkylsulfonyl. The term "alkylsulfonyl" as used herein refers to an alkyl group attached to a sulfonyl group.
[0050] The term "sulfide" as used herein refers to the radical having the structure R2S-, where R2 can be alkoxy, alkyl, alkenyl, alkynyl, amide, amino, aryl, arylalkyl, carbamate, carboxy, cycloalkyl, ester, ether, formyl, haloalkyl, heteroaryl, heterocyclyl, and ketone. The term "alkylsulfide" as used herein refers to an alkyl group attached to a sulfur atom. Exemplary sulfides include "thio," which as used herein refers to an -SH radical.
[0051] The term "thiocarbonyl" or "thiocarboxy" as used herein refers to compounds and moieties which contain a carbon connected with a double bond to a sulfur atom.
[0052] "Pharmaceutically or pharmacologically acceptable" include molecular entities and compositions that do not produce an adverse, allergic or other untoward reaction when administered to an animal, or a human, as appropriate. "For human administration, preparations should meet sterility, pyrogenicity, general safety and purity standards as required by FDA Office of Biologies standards.
[0053] The term "pharmaceutically acceptable carrier" or "pharmaceutically acceptable excipient" as used herein refers to any and all solvents, dispersion media, coatings, isotonic and absorption delaying agents, and the like, that are compatible with pharmaceutical administration. The use of such media and agents for pharmaceutically active substances is well known in the art. The compositions may also contain other active compounds providing supplemental, additional, or enhanced therapeutic functions.
[0054] The term "pharmaceutical composition" as used herein refers to a composition comprising at least one compound as disclosed herein formulated together with one or more pharmaceutically acceptable carriers.
[0055] "Individual," "patient," or "subject" are used interchangeably and include to any animal, including mammals, preferably mice, rats, other rodents, rabbits, dogs, cats, swine, cattle, sheep, horses, or primates, and most preferably humans. The compounds of the invention can be administered to a mammal, such as a human, but can also be other mammals such as an animal in need of veterinary treatment, e.g., domestic animals (e.g., dogs, cats, and the like), farm animals (e.g., cows, sheep, pigs, horses, and the like) and laboratory animals (e.g., rats, mice, guinea pigs, and the like). The mammal treated in the methods of the invention is desirably a mammal in whom modulation of cystic fibrosis transmembrane regulators is desired.
[0056] "Modulation" includes antagonism (e.g., inhibition), agonism, partial antagonism and/or partial agonism. Modulators may be dual acting corrector/potentiator compounds. In one embodiment, a modulator is a corrector compound. In another embodiment, a modulator is a potentiator compound. [0057] In the present specification, the term "therapeutically effective amount" means the amount of the subject compound that will elicit the biological or medical response of a tissue, system, animal or human that is being sought by the researcher, veterinarian, medical doctor or other clinician. The compounds of the invention are administered in therapeutically effective amounts to treat a disease. Alternatively, a therapeutically effective amount of a compound is the quantity required to achieve a desired therapeutic and/or prophylactic effect, such as an amount which results in the prevention of or a decrease in the symptoms associated with a disease associated with cystic fibrosis transmembrane regulators.
[0058] The term "pharmaceutically acceptable salt(s)" as used herein refers to salts of acidic or basic groups that may be present in compounds used in the present compositions. Compounds included in the present compositions that are basic in nature are capable of forming a wide variety of salts with various inorganic and organic acids. The acids that may be used to prepare pharmaceutically acceptable acid addition salts of such basic compounds are those that form non-toxic acid addition salts, i.e., salts containing pharmacologically acceptable anions, including but not limited to malate, oxalate, chloride, bromide, iodide, nitrate, sulfate, bisulfate, phosphate, acid phosphate, isonicotinate, acetate, lactate, salicylate, citrate, tartrate, oleate, tannate, pantothenate, bitartrate, ascorbate, succinate, maleate, gentisinate, fumarate, gluconate, glucaronate, saccharate, formate, benzoate, glutamate, methanesulfonate, ethanesulfonate, benzenesulfonate,/?-toluenesulfonate and pamoate (i.e., l,l'-methylene-bis-(2-hydroxy-3- naphthoate)) salts. Compounds included in the present compositions that include an amino moiety may form pharmaceutically acceptable salts with various amino acids, in addition to the acids mentioned above. Compounds included in the present compositions that are acidic in nature are capable of forming base salts with various pharmacologically acceptable cations. Examples of such salts include alkali metal or alkaline earth metal salts and, particularly, calcium, magnesium, sodium, lithium, zinc, potassium, and iron salts.
[0059] The compounds of the disclosure may contain one or more chiral centers and/or double bonds and, therefore, exist as stereoisomers, such as geometric isomers, enantiomers or diastereomers. The term "stereoisomers" when used herein consist of all geometric isomers, enantiomers or diastereomers. These compounds may be designated by the symbols "R" or "S," depending on the configuration of substituents around the stereogenic carbon atom. The present invention encompasses various stereoisomers of these compounds and mixtures thereof. Stereoisomers include enantiomers and diastereomers. Mixtures of enantiomers or diastereomers may be designated "(±)" in nomenclature, but the skilled artisan will recognize that a structure may denote a chiral center implicitly. [0060] Individual stereoisomers of compounds of the present invention can be prepared synthetically from commercially available starting materials that contain asymmetric or stereogenic centers, or by preparation of racemic mixtures followed by resolution methods well known to those of ordinary skill in the art. These methods of resolution are exemplified by (1) attachment of a mixture of enantiomers to a chiral auxiliary, separation of the resulting mixture of diastereomers by recrystallization or chromatography and liberation of the optically pure product from the auxiliary, (2) salt formation employing an optically active resolving agent, or (3) direct separation of the mixture of optical enantiomers on chiral chromatographic columns. Stereoisomeric mixtures can also be resolved into their component stereoisomers by well known methods, such as chiral-phase gas chromatography, chiral-phase high performance liquid chromatography, crystallizing the compound as a chiral salt complex, or crystallizing the compound in a chiral solvent. Stereoisomers can also be obtained from stereomerically-pure intermediates, reagents, and catalysts by well known asymmetric synthetic methods.
[0061] Geometric isomers can also exist in the compounds of the present invention. The symbol ^≡≡r denotes a bond that may be a single, double or triple bond as described herein. The present invention encompasses the various geometric isomers and mixtures thereof resulting from the arrangement of substituents around a carbon-carbon double bond or arrangement of substituents around a carbocyclic ring. Substituents around a carbon-carbon double bond are designated as being in the "Z" or "E" configuration wherein the terms "Z" and "E" are used in accordance with IUPAC standards. Unless otherwise specified, structures depicting double bonds encompass both the "E" and "Z" isomers.
[0062] Substituents around a carbon-carbon double bond alternatively can be referred to as "cis" or "trans," where "cis" represents substituents on the same side of the double bond and "trans" represents substituents on opposite sides of the double bond. The arrangement of substituents around a carbocyclic ring are designated as "cis" or "trans." The term "cis" represents substituents on the same side of the plane of the ring and the term "trans" represents substituents on opposite sides of the plane of the ring. Mixtures of compounds wherein the substituents are disposed on both the same and opposite sides of plane of the ring are designated "cis/trans."
[0063] The compounds disclosed herein can exist in solvated as well as unsolvated forms with pharmaceutically acceptable solvents such as water, ethanol, and the like, and it is intended that the invention embrace both solvated and unsolvated forms. In one embodiment, the compound is amorphous. In one embodiment, the compound is a polymorph. In another embodiment, the compound is in a crystalline form.
[0064] The invention also embraces isotopically labeled compounds of the invention which are identical to those recited herein, except that one or more atoms are replaced by an atom having an atomic mass or mass number different from the atomic mass or mass number usually found in nature. Examples of isotopes that can be incorporated into compounds of the invention include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorus, fluorine and chlorine, such as 2H, 3H, 13C, 14C, 15N, 18O, 170, 31P, 32P, 35S, 18F, and 36Cl, respectively. [0065] Certain isotopically-labeled disclosed compounds (e.g., those labeled with 3H and 14C) are useful in compound and/or substrate tissue distribution assays. Tritiated (i.e., 3H) and carbon- 14 (i.e., 14C) isotopes are particularly preferred for their ease of preparation and detectability. Further, substitution with heavier isotopes such as deuterium (i.e., 2H) may afford certain therapeutic advantages resulting from greater metabolic stability (e.g., increased in vivo half-life or reduced dosage requirements) and hence may be preferred in some circumstances. Isotopically labeled compounds of the invention can generally be prepared by following procedures analogous to those disclosed in the e.g., Examples herein by substituting an isotopically labeled reagent for a non-isotopically labeled reagent.
[0066] Prodrugs of the compounds described herein are specifically contemplated. The term "prodrug" refers to compounds that are transformed in vivo to yield a disclosed compound or a pharmaceutically acceptable salt, hydrate or solvate of the compound. The transformation may
occur by various mechanisms, such as through hydrolysis in blood. For example, if a compound of the invention or a pharmaceutically acceptable salt, hydrate or solvate of the compound contains a carboxylic acid functional group, a prodrug can comprise an ester formed by the replacement of the hydrogen atom of the acid group with a group such as (Ci-Cg)alkyl, (C2-C i2)alkanoyloxymethyl, l-(alkanoyloxy)ethyl having from 4 to 9 carbon atoms, 1-methyl- l-(alkanoyloxy)-ethyl having from 5 to 10 carbon atoms, alkoxycarbonyloxymethyl having from 3 to 6 carbon atoms, l-(alkoxycarbonyloxy)ethyl having from 4 to 7 carbon atoms, 1- methyl-l-(alkoxycarbonyloxy)ethyl having from 5 to 8 carbon atoms, N- (alkoxycarbonyl)aminomethyl having from 3 to 9 carbon atoms, l-(N-(alkoxycarbonyl)amino)ethyl having from 4 to 10 carbon atoms, 3-phthalidyl,
4-crotonolactonyl, gamma-butyrolacton-4-yl, di-N,N-(Ci-C2)alkylamino(C2-C3)alkyl (such as β-dimethylaminoethyl), carbamoyl-(Ci-C2)alkyl, N,N-di(Ci-C2)alkylcarbamoyl-(Ci-C2)alkyl and piperidino-, pyrrolidino- or morpholino(C2-C3)alkyl.
[0067] Similarly, if a compound of the invention contains an alcohol functional group, a prodrug can be formed by the replacement of the hydrogen atom of the alcohol group with a group such as (Ci-C6)alkanoyloxymethyl, l-((Ci-C6)alkanoyloxy)ethyl, 1 -methyl- 1 -((C i-C6)alkanoyloxy)ethyl (C i -C6)alkoxycarbonyloxymethyl, N-(Ci-C6)alkoxycarbonylaminomethyl, succinoyl, (Ci-Ce)alkanoyl, α-amino(Ci-C4)alkanoyl, arylacyl and α-aminoacyl, or α-aminoacyl-α-aminoacyl, where each α-aminoacyl group is independently selected from the naturally occurring L-amino acids, P(O)(OH)2,
-P(O)(O(ci-C6)alkyl)2 or glycosyl (the radical resulting from the removal of a hydroxyl group of the hemiacetal form of a carbohydrate).
[0068] If a compound of the invention incorporates an amine functional group, a prodrug can be formed by the replacement of a hydrogen atom in the amine group with a group such as R-carbonyl, RO-carbonyl, NRR'-carbonyl where R and R' are each independently (Ci- Cio)alkyl, (C3-Cy)cycloalkyl, benzyl, or R-carbonyl is a natural α-aminoacyl or natural α- aminoacyl-natural α-aminoacyl, — C(OH)C(O)OY1 wherein Y1 is H, (Ci-Ce)alkyl or benzyl, -C(OY2)Y3 wherein Y2 is (Ci-C4) alkyl and Y3 is (Ci-C6)alkyl, carboxy(Ci-C6)alkyl, amino(Ci- C4)alkyl or mono-N— or di-N,N— (Ci-C6)alkylaminoalkyl, — C(Y4)Y5 wherein Y4 is H or methyl and Y5 is mono-N — or di-N,N — (Ci-C6)alkylamino, morpholino, piperidin-1-yl or pyrrolidin-1-yl.
II. Pyrimidinyl Compounds & Pharmaceutical Compositions
[0069] One aspect of the invention provides a compound of formula I:
I including a pharmaceutically acceptable salt or N-oxide thereof; wherein:
X2 is CR2 or N, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5 or N, and X6 is CR6 or N, where no more than two OfX2-X6 are N; where if X3 is N, L is a bond, and A is cyclohexyl, then R2 is not methoxy; and if X5 is N, L is a bond, and A is cyclohexyl, then R6 is not methoxy; L is a bond or a Ci_2alkylidene chain optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of Ci-C6alkyl, C3-C6cycloalkyl, -OCi-C6alkyl, -OC3-C6cycloalkyl, and F;
A is C4-Ciocycloalkyl, C3-Cioheterocycloalkyl, or phenyl, each of which is optionally substituted with one, two, or three substituents independently, for each occurrence, selected from the group consisting of F, Cl, -CF3, -OCi-C6alkyl, -OH, Ci-C6alkyl, Ci-C6alkoxycarbonyl, C3-C5cycloalkyl, aryl, -C(O)-aryl, -C(O)-heteroaralkyl, -C(O)-C i-C6alkyl, and -C(O)N(H)(C1- C6alkyl);
Ri and Ri2 are each independently hydrogen, CN, Ci-C6alkyl, -OCi-C6alkyl, C3. locycloalkyl, -OC3_i0cycloalkyl, -OCF3, -OCF2H, -OCH2F, halogen, -NR7Ri0, -NR7COR8, -NR7SO2R9 , or -SO2R9, where if Ri2 is -OCF2H, then R4 is not methyl;
R3 and R5 are each independently hydrogen, halogen, -CF3, -OH, -OCF3, -OCHF2, -OCH2F, Ci-C8alkyl, C3-C8cycloalkyl, C3-C8heterocyclyl, -OCi-Ci0alkyl, -OC3-Ci0cycloalkyl, -NR7COR8, -NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, or -SO2R9;
R4 is hydrogen, halogen, d-C3alkyl, -OCi-C6alkyl, -O-aryl, -OH, -OCHF2, -OCH2F, -CN, heteroaryl, -NR7Ri0, or -SO2NR7Ri0;
R6 is hydrogen, halogen, -CN, -OCi-Ci0alkyl, -Oaryl, -CF3, -OCHF2, -OCH2F, -NR7Ri0,
where any two adjacent variables selected from R2, R3, R4, R5, and R6 can be taken together to form a cycloalkyl, aryl, heteroaryl, or heterocyclyl, each of which is optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl, where R3 and R4 cannot be taken together to form a dioxolanyl when L is a bond and A is cyclohexyl; where at least one of R2, R3, R4, R5, and R6 is not hydrogen; and if R4 is -OCH3, then R3 and R5 are not -OCH3;
R7 and Ri0 each represent independently for each occurrence hydrogen, alkyl, or cycloalkyl, wherein the alkyl and cycloalkyl are optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of halogen, cyano, hydroxy, nitro, and alkoxy; or R7 and Ri0 are taken together to form a heterocyclyl optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl, where the heterocyclyl is not dihydro-2H-benzo[b] [ 1 ,4]dioxepinyl;
Rg is alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl;
R9 represents independently for each occurrence alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydroxyl; and Rn is alkyl, alkenyl, alkynyl, amido, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydrogen.
[0070] In certain embodiments, Ri and Ri2 are independently hydrogen or methyl. In certain embodiments, at least one of R2 and R6 is selected from the group consisting of F, Cl, -CF3, - OCH3, and -OCF3. In certain embodiments, R2 and R6 is independently hydrogen, F, Cl, -CF3, -OCH3, or -OCF3. In certain embodiments, at least one of R3 and R5 is selected from the group consisting of F, Cl, -OH, -OCH3, -OiPr, -Osec-butyl, -OCF3, -Ophenyl, -Ocyclohexyl, -SO2Me, pyrrolidinylsulfonyl, morpholinylsulfonyl, -CON(H)-cyclopropyl, 5-methyl-l,3,4-oxadiazolyl,
-NHSθ2cyclopropyl, and -NHCOcyclopropyl. In certain embodiments, R4 is selected from the group consisting of -NH2, -NMe2, -Ophenyl, -OCH3, and -OCF3. In certain embodiments, R4 is selected from the group consisting of -NH2, -NMe2, -Ophenyl, -OCH3, -OCH2CH3, -OCH(CH3)2, -OCF3, Cl, and F. In certain embodiments, R2 is hydrogen, -CN, -OCi-Cioalkyl, -Oaryl, -CF3, -OCHF2, -OCH2F, -NR7Ri0, -CO2Rn, or -SO2NR7Ri0. In certain embodiments, R3 and R4 are taken together to form a heterocyclyl selected from the group consisting of dioxanyl, oxazolyl, pyrazinyl, and thiazolyl. In certain embodiments, R4 is hydrogen. In certain embodiments, R4 is hydrogen or fluoro.
[0071] In certain embodiments, A is C4-Ci0cycloalkyl. In certain embodiments, A is selected from the group consisting of cyclopentyl, cyclohexyl, 1-methylcyclohexyl, 4- methylcyclohexyl, 4-ethylcyclohexyl, 4-phenylcyclohexyl, 4,4-difluorocyclohexyl, 4,4- dimethylcyclohexyl, cycloheptyl, bicyclo[2.2.1]heptan-2-yl, adamantanyl, and 1,2,3,4- tetrahydronaphthalenyl. In certain embodiments, A is cis-4-methylcyclohexyl, cis-4- ethylcyclohexyl; cis-4-trifluoromethylcyclohexyl; 4,4-dimethylcyclohexyl; or 4,4- difluorocyclohexyl. In certain embodiments, A is cis-4-methylcyclohexyl. In certain embodiments, R5 and R4 cannot be taken together to form a dioxolanyl when L is a bond and A is cyclohexyl.
[0072] In certain embodiments, R2 is fluoro or chloro. In certain embodiments, R2 is fluoro. In certain embodiments, X2 is CR2, X3 is CR3, X4 is CR4, X5 is CR5, and X6 is CR6. In certain embodiments, L is a bond.
[0073] In certain other embodiments, X2 is CR2, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5, and X6 is CR6; where if X3 is N, L is a bond, and A is cyclohexyl, then R2 is not methoxy. In certain embodiments, L is a bond. In certain other embodiments, A is C4-Ci0cycloalkyl optionally substituted with one, two, or three substituents independently, for each occurrence, selected from the group consisting of F and Ci-Cβalkyl. In certain other embodiments, Ri and R12 are each independently hydrogen and Ci-Cβalkyl. In certain other embodiments, R2 is hydrogen, -OCi-Ci0alkyl, or -CF3. In certain other embodiments, R6 is hydrogen, halogen, or -OCi-Ci0alkyl. In certain other embodiments, R3 and R5 are each independently hydrogen, halogen, -CF3, -OH, -OCF3, Ci-C8alkyl, C3-C8cycloalkyl, -OCi-Ci0alkyl, -OCs-Ciocycloalkyl, -NR7COR8, -NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, or -SO2R9. In certain embodiment, R3 and R5 are each independently hydrogen, halogen, -CF3, -
OCF3, or -OCi-Cioalkyl. In certain other embodiments, R4 is halogen, Ci-C3alkyl, -OCi- Cβalkyl, -O-aryl, -OH, -NR7R10, or -SO2NR7R10. In certain other embodiments, R4 is hydrogen, wherein at least one of R2, R3, R5, and Re is not hydrogen. In certain other embodiments, Rs is cycloalkyl. In certain other embodiments, R9 represents independently for each occurrence alkyl or cycloalkyl. In certain other embodiments, Rn is alkyl.
[0074] Another aspect of the invention provides a compound of formula IA:
(IA) including a pharmaceutically acceptable salt thereof; wherein: A is C4-Ciocycloalkyl optionally substituted with one, two, or three substituents independently, for each occurrence, selected from the group consisting of F and Ci-Cβalkyl; Ri and Ri2 are each independently hydrogen or d-C6alkyl; R2 is hydrogen, -OCi-Cioalkyl, or -CF3;
R3 and R5 are each independently hydrogen, halogen, -CF3, -OH, -OCF3, Ci-Csalkyl, C3-C8cycloalkyl, -OCi-Cioalkyl, -OCs-Ciocycloalkyl, -NR7COR8, -NR7SO2R9, -CONR7R10, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, or -SO2R9.
Re is hydrogen, halogen, or -OCi-Ci0alkyl; R7 and Ri0 each represent independently for each occurrence hydrogen, d-C6alkyl, or
C3-Cgcycloalkyl;
Rs is C3-Cgcycloalkyl;
R9 represents independently for each occurrence Ci-Cβalkyl or C3-Cgcycloalkyl; and
Rn is Ci-C6alkyl.
[0075] In certain embodiments, Ri and Ri2 are hydrogen or methyl. In certain embodiment, R3 and R5 are each independently hydrogen, halogen, -CF3, -OCF3, or -OCi-Cioalkyl. In certain embodiment, R4 is hydrogen, halogen, or Ci-C3alkyl. In certain embodiment, R6 is hydrogen or halogen.
[0076] Another aspect of the invention provides a compound of formula IB:
(IB) including a pharmaceutically acceptable salt thereof; wherein:
Ri is hydrogen, methyl or ethyl;
R2 is -O-methyl, -O-ethyl, -O-propyl, or -CF3;
R3 and R5 are each independently hydrogen, halogen, -CF3, -OCF3, or -OCi-Ci0alkyl;
R4 is hydrogen, halogen, or Ci-C3alkyl; and
R6 is hydrogen, halogen, or Ci-C3alkyl.
[0077] In certain embodiments, Ri is hydrogen. In certain embodiments, Ri is methyl. In certain embodiments, R2 is -O-methyl. In certain embodiments, R3 is hydrogen or halogen. In certain embodiments, R5 is halogen. In certain embodiments, R4 is hydrogen or methyl. In certain embodiments, R6 is hydrogen or methyl.
[0078] Another aspect of the invention provides a compound of formula II:
II
wherein X2 is CR2 or N, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5 or N, and X6 is CR6 or N, where no more than two OfX2-X6 are N; where if X3 is N, L is a bond, and A is cyclohexyl, then R2 is not methoxy; and if X5 is N, L is a bond, and A is cyclohexyl, then R6 is not methoxy; L is a bond or a Ci_2alkylidene chain optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of Ci-C6alkyl, C3-C6cycloalkyl, -OCi-C6alkyl, -OC3-C6cycloalkyl, and F;
A is a C4-Ciocycloalkyl, optionally substituted with one, two, or three substituents independently, for each occurrence, selected from the group consisting of F, Cl, -CF3, -OCi- C6alkyl, Ci-C6alkyl, Ci-C6alkoxycarbonyl, C3-C5cycloalkyl, and aryl;
Ri and Ri2 are each independently selected from the group consisting of hydrogen, CN, Ci-C6alkyl, -OCi-C6alkyl, C3_i0cycloalkyl, -OC3_iocycloalkyl, -OCF3, -OCF2H, -OCH2F, halogen, -NR7Ri0, -NR7COR8, -NR7SO2R9 , and -SO2R9, where if Ri2 is -OCF2H, R4 is not methyl; R2 is independently selected from the group consisting of hydrogen, -CN, -OCi-C6alkyl,
-Oaryl, -CF3, -OCHF2, -OCH2F, -NR7Ri0, -CO2Rn, and -SO2NR7Ri0;
R3 and R5 are each independently selected from the group consisting of hydrogen, halogen, -CF3, -OH, -OCF3, -OCHF2, -OCH2F, C2-C8alkyl, C3-C8cycloalkyl, C3- C8heterocyclyl, -OCi-Ci0alkyl, -OC3-Ci0cycloalkyl, -NR7COR8, NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, and -SO2R9;
R4 is selected from the group consisting of hydrogen, halogen, Ci-C3alkyl, -OC2- C6alkyl, -CN, -OCHF2, -OCH2F, -NR7Ri0, and -SO2NR7Ri0;
R6 is independently selected from the group consisting of hydrogen, halogen, -CN, -OCi-C6alkyl, -Oaryl, -CF3, -OCHF2, -OCH2F, -NR7Ri0, and -SO2NR7Ri0; where any two adjacent variables selected from R2, R3, R4, R5, and R6 can be taken together to form a cycloalkyl, aryl, heteroaryl, or heterocyclyl, each of which is optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl, where R3 and R4 cannot be taken together to form a dioxolanyl when L is a bond and A is cyclohexyl; where at least one of R2, R3, R4, R5, and R6 is not hydrogen; and if R4 is -CH3, then R3 and R5 are not both -OCH3;
R7 and Rio are each independently selected from the group consisting of hydrogen, alkyl, and cycloalkyl, wherein the alkyl and cycloalkyl are optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of halogen, cyano, hydroxy, nitro, and alkoxy, or R7 and Rio are taken together to form a heterocyclyl optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl, where the heterocyclyl is not dihydro-2H-benzo[b][l,4]dioxepinyl;
Rg is selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, and hydroxyl;
R9 is selected from the group consisting of alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, and hydroxyl; and
Rn is selected from the group consisting of alkyl, alkenyl, alkynyl, amido, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, and hydrogen; or pharmaceutically acceptable salts or N-oxides thereof.
[0079] In certain embodiments, the compound is selected from the group consisting of: bicyclo[2.2.1 ]hept-2-yl-[4-(5-chloro-2-methoxy-phenyl)-pyrimidin-2-yl]-amine; [4-(5-Chloro- 2-methoxy-phenyl)-pyrimidin-2-yl]-(l,2,3,4-tetrahydro-naphthalen-2-yl)-amine; N-(3-(2- (cyclohexylamino)pyrimidin-4-yl)phenyl)-N-methylcyclopropane-carboxamide; N-(3-(2- (cyclohexylamino)pyrimidin-4-yl)phenyl)-N-methylcyclopropane-sulfonamide; N-cyclohexyl- 4-(6-methylpyridin-3-yl)pyrimidin-2-amine; N-cyclohexyl-4-(4-methoxyphenyl)pyrimidin-2- amine; N-cyclohexyl-4-(4-methoxy-2-(trifluoromethyl)henyl)pyrimidin-2-amine; N- cyclohexyl-4-(3-methoxyphenyl)pyrimidin-2-amine; N-cyclohexyl-4-(4- fluorophenyl)pyrimidin-2-amine; ethyl 2-(2-(cyclohexylamino)pyrimidin-4-yl)benzoate; N- cyclohexyl-4-(4-ethoxyphenyl)pyrimidin-2-amine; N-cyclohexyl-4-(2-methylpyridin-4- yl)pyrimidin-2-amine; 4-(5 -chloro-2-methoxyphenyl)-N-cyclohexylpyrimidin-2-amine; N- cyclohexyl-4-(2,4-difluorophenyl)pyrimidin-2-amine; N-cyclohexyl-4-(2,5- dichlorophenyl)pyrimidin-2-amine; 4-(3 -chlorophenyl)-N-cyclohexylpyrimidin-2-amine; 4-(4- chloro-3 -fluorophenyl)-N-cyclohexylpyrimidin-2-amine; 4-(5 -chloro-2-methoxyphenyl)-N- ((lR,4R)-4-methylcyclohexyl)pyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)-N- cyclohexyl-6-methylpyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)-N-((lS,4S)-4- methylcyclohexyl)pyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)-N-(4,4-
difluorocyclohexyl)pyrimidin-2-amine; tert-butyl 4-(4-(5-chloro-2-methoxyphenyl)pyrimidin- 2-ylamino)piperidine- 1 -carboxylate; N-cyclohexyl-4-(2-fluoro-3-methoxyphenyl)pyrimidin-2- amine; 4-(3-chlorophenyl)-N-((lR,4R)-4-methylcyclohexyl)pyrimidin-2-amine; N-cyclohexyl- 4-(3-fluorophenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3-isopropoxyphenyl)-pyrimidin-2- amine; N-cyclohexyl-4-(4-ethoxy-3-fluorophenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3,5- difluorophenyl)pyrimidin-2-amine; N-cyclohexyl-4-(2,3-dihydrobenzo[b][l,4]dioxin-6- yl)pyrimidin-2-amine; N-cyclohexyl-4-(2,3-dihydrobenzofuran-5-yl)pyrimidin-2-amine; 4-(4- chlorophenyl)-N-cyclohexylpyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)-N-(4,4- dimethylcyclohexyl)pyrimidin-2-amine; 3 -(2-(cyclohexylamino)pyrimidin-4-yl)benzonitrile; 4- (5 -chloro-2-methoxyphenyl)-N-cycloheptylpyrimidin-2-amine; 4-(5 -chloro-2-methoxyphenyl)- N-cyclopentylpyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)-N-phenylpyrimidin-2-amine; 4-(benzo[d] [ 1 ,3]dioxol-5-yl)-N-cyclohexylpyrimidin-2-amine; 4-(3-chlorophenyl)-N-((l S,4S)- 4-methylcyclohexyl)pyrimidin-2-amine; N-cyclohexyl-4-(4-fluoro-3- methoxyphenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3,5-dichlorophenyl)pyrimidin-2-amine; N-cycloheptyl-4-(3-isopropoxyphenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3- (methylsulfonyl)phenyl)pyrimidin-2-amine; N-cyclohexyl-4-(5-fluoro-2- methoxyphenyl)pyrimidin-2-amine; 4-(3-chlorophenyl)-N-cycloheptylpyrimidin-2-amine; N- cyclopentyl-4-(3-isopropoxyphenyl)pyrimidin-2-amine; (4-(4-(5-chloro-2- methoxyphenyl)pyrimidin-2-ylamino)piperidin- 1 -yl)(phenyl)methanone; N-cyclohexyl-4-(5 - fluoropyridin-3-yl)pyrimidin-2-amine; 1 -(4-(4-(5-chloro-2-methoxyphenyl)pyrimidin-2- ylamino)piperidin- 1 -yl)-2,2-dimethylpropan- 1 -one; N-cyclohexyl-4-(2,5- difluorophenyl)pyrimidin-2-amine; N-cyclohexyl-4-(2,5-dimethoxyphenyl)pyrimidin-2-amine; 4-(3-sec-butoxyphenyl)-N-cyclohexylpyrimidin-2-amine; N-cyclohexyl-4-(2-methoxypyridin- 4-yl)pyrimidin-2-amine; 4-(3-isopropoxyphenyl)-N-((lS,4S)-4-methylcyclohexyl)pyrimidin-2- amine; 4-(5-chloro-2-methoxyphenyl)-N-((lS,4S)-4-phenylcyclohexyl)pyrimidin-2-amine; 4- (5-chloro-2-methoxyphenyl)-N-((lr,4r)-4-phenylcyclohexyl)pyrimidin-2-amine; 3-(2- (cyclohexylamino)pyrimidin-4-yl)benzamide; 3-(2-(cyclohexylamino)pyrimidin-4-yl)-N,N- dimethylbenzamide; 3 -(2-(cyclohexylamino)pyrimidin-4-yl)-N-cyclopropylbenzamide; N- cyclohexyl-4-(3-(trifluoromethoxy)phenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3- phenoxyphenyl)pyrimidin-2-amine; N-cyclohexyl-4-(4-phenoxyphenyl)pyrimidin-2-amine; 3- (2-(cyclohexylamino)pyrimidin-4-yl)phenol; N-cyclohexyl-4-(3-(cyclohexyloxy)phenyl)- pyrimidin-2-amine; 4-(2-(cyclohexylamino)pyrimidin-4-yl)phenol; N-cyclohexyl-4-(3-
(pyrrolidin-l-ylsulfonyl)phenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3 -(5 -methyl- 1,3,4- oxadiazol-2-yl)phenyl)pyrimidin-2-amine; N-cyclohexyl-4-(4-(5 -methyl- 1, 3, 4-oxadiazol-2- yl)phenyl)pyrimidin-2-amine; 4-(2-(cyclohexylamino)pyrimidin-4-yl)-N- cyclopropylbenzenesulfonamide; methyl 4-(4-(5-chloro-2-methoxyphenyl)pyrimidin-2- ylamino)piperidine-l-carboxylate; N-cyclohexyl-4-(quinoxalin-6-yl)pyrimidin-2-amine; N- (bicyclo[2.2.1 ]heptan-2-yl)-4-(5-chloro-2-methoxyphenyl)pyrimidin-2-amine; 4-(5-chloro-2- methoxyphenyl)-N-( 1 ,2,3 ,4-tetrahydronaphthalen-2-yl)pyrimidin-2-amine; N-tert-butyl-4-(4- (5-chloro-2-methoxyphenyl)pyrimidin-2-ylamino)piperidine- 1 -carboxamide; 1 -(4-(4-(5-chloro- 2-methoxyphenyl)pyrimidin-2-ylamino)piperidin- 1 -yl)-2-(pyridin-3 -yl)ethanone; 1 -(4-(4-(5 - chloro-2-methoxyphenyl)pyrimidin-2-ylamino)piperidin- 1 -yl)-2-(tetrahydrofuran-3 - yl)ethanone; 4-(4-aminophenyl)-N-cyclohexylpyrimidin-2-amine; N-cyclohexyl-4-(3- morpholinosulfonyl)phenyl) pyrimidin-2-amine; N-(3 -(2-(cyclohexylamino)pyrimidin-4- yl)phenyl)cyclopropanesulfonamide; N-(3-(2-(cyclohexylamino)pyrimidin-4- yl)phenyl)cyclopropanecarboxamide; (3-(2-(cyclohexylamino)pyrimidin-4- yl)phenyl)(piperidin- 1 -yl)methanone; 3 -(2-(cyclohexylamino)pyrimidin-4-yl)-N- cyclopropylbenzenesulfonamide; and N-cyclohexyl-4-(2-methylbenzo[d]oxazol-6- yl)pyrimidin-2-amine; or a pharmaceutically acceptable salt or N-oxide thereof.
[0080] Exemplary procedures for making compounds described herein are provided below with reference to Schemes 1-4. The first three schemes show alternative procedures for making variously substituted 4-phenylpyrimidin-2-amine compounds. In particular, Scheme 1 illustrates reacting a dichloropyrimidine with an amine to form a chloropyrimidinylamino synthetic intermediate that is used in a subsequent Suzuki coupling reaction with an aryl boronic acid to form a 4-phenylpyrimidin-2-amine compound. The first step in this sequence, i.e., the amine coupling step, can be performed by reacting a 2,4-dichloropyrimidine compound and a desired amine in the presence of triethylamine in ethanol at about 750C for about 8-48 hours. The Suzuki coupling reaction can be performed according to standard, known Suzuki coupling conditions using a desired boronic acid or its pinacol ester. This synthetic sequence is contemplated to be amenable to a variety of dichloropyrimidine compounds, aryl boronic acids, and/or aryl boronic esters, which are commercially available or can be readily prepared from commercially available materials.
SCHEME 1
[0081] Scheme 2 illustrates reacting a 2,4-dichloropyrimidine with an aryl boronic acid under Suzuki coupling conditions to form a 2-chloro-4-phenylpyrimidine synthetic intermediate that can be reacted with an amine to form a 4-phenylpyrimidin-2-amine compound. The first step in this sequence, i.e., the Suzuki coupling reaction, can be performed according to standard, known Suzuki coupling conditions using a desired boronic acid or its pinacol ester. The amine coupling step can be performed by reacting the 2-chloro-4-phenylpyrimidine synthetic intermediate with an amine in the presence of triethylamine in isopropanol at about 1000C for about 24-48 hours or heating in a microwave oven at 1000C for about 0.5-1 hours. This synthetic sequence is contemplated to be amenable to a variety of dichloropyrimidine compounds, aryl boronic acids, and/or aryl boronic esters, which are commercially available or can be readily prepared from commercially available materials.
[0082] Scheme 3 illustrates reacting a 4-chloro-2-(methylthio)pyrimidine with an aryl boronic acid under Suzuki coupling conditions to form a 2-(methylthio)-4-phenylpyrimidine synthetic intermediate that can be reacted with an oxidant to form a methylsulfone that undergoes reaction with an amine to form the 4-phenylpyrimidin-2-amine product. The first step in this sequence, i.e., the Suzuki coupling reaction, can be performed according to standard, known Suzuki coupling conditions using a desired boronic acid or its pinacol ester. The thiomethyl ether can be oxidized to the methylsulfone by reaction with meta-chloroperbenzoic acid (mCPBA) in dichloromethane at room temperature for about 12-24 hours. Reaction of the methylsulfone intermediate with a desired amine R-NH2 in the presence of triethylamine in
isopropanol at about 1000C for about 24-48 hours or heating in a microwave oven at 1000C for about 0.5-1 hours provides the final 4-phenylpyrimidin-2-amine compound.
SCHEME 3
[0083] Scheme 4 illustrates a procedure for alkylating a phenolic hydroxylic group. The procedure involves reacting the phenol with an alkyl halide (RX) in the presence of alkali metal base, such as potassium carbonate, in an organic solvent (such as acetone) at elevated temperature (such as ~ 700C) for about 12-24 hours.
SCHEME 4
[0084] The present disclosure also provides pharmaceutical compositions comprising compounds as disclosed herein formulated together with one or more pharmaceutically acceptable carriers. These formulations include those suitable for oral, rectal, topical, buccal and parenteral (e.g., subcutaneous, intramuscular, intradermal, or intravenous) administration, although the most suitable form of administration in any given case will depend on the degree and severity of the condition being treated and on the nature of the particular compound being used.
III. Therapeutic Applications
[0085] The invention further provides methods of modulating the activity of one or more cystic fibrosis transmembrane regulators comprising exposing said receptor to a compound of the invention. The invention further provides methods of treating a disease associated with expression or activity of one or more cystic fibrosis transmembrane regulators in a patient
comprising administering to the patient a therapeutically effective amount of a compound of the invention.
[0086] These compounds and pharmaceutically acceptable compositions are useful for treating or lessening the severity of a variety of diseases, disorders, or conditions, including, but not limited to, cystic fibrosis, hereditary emphysema, hereditary hemochromatosis, coagulation- cibrinolysis deficiencies, such as protein C deficiency, Type 1 hereditary angioedema, lipid processing deficiencies, such as familial hypercholesterolemia, Type 1 chylomicronemia, abetalipoproteinemia, lysosomal storage diseases, such as I- cell disease/pseudo-Hurler, secretory diarrhea or polycystic kidney disease, mucopolysaccharidoses, Sandhof/T ay-Sachs, Crigler-Najjar type II, polyendocrinopathy/hyperinsulemia, Diabetes mellitus, Laron dwarfism, myleoperoxidase deficiency, primary hypoparathyroidism, melanoma, glycanosis CDG type 1 , hereditary emphysema, congenital hyperthyroidism, osteogenesis imperfecta, hereditary hypofibrinogenemia, ACT deficiency, Diabetes insipidus (DI), neurophyseal DI, neprogenic DI, Charcot-Marie Tooth syndrome, Perlizaeus-Merzbacher disease, neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, progressive supranuclear plasy, Pick's disease, several polyglutamine neurological disorders asuch as Huntington, spinocerebullar ataxia type I, spinal and bulbar muscular atrophy, dentatorubal pallidoluysian, and myotonic dystrophy, as well as spongiform encephalopathies, such as hereditary Creutzfeldt- Jakob disease (due to prion protein processing defect), Fabry disease, Straussler-Scheinker syndrome, COPD, dry eye disease, or Sjogren's disease.
[0087] One embodiment of the invention provides a method of treating airway inflammation comprising administering to a subject in need thereof a therapeutically effective amount of a compound described herein, such as a compound of Formula I, IA, IB, II, III, or IIIA, as described herein. Another embodiment of the invention provides a method of treating airway inflammation comprising administering to a subject in need thereof a therapeutically effective amount of a compound described herein, such as a compound of Formula I, IA, IB, II, III, or IIIA, as described herein.
[0088] Accordingly, one aspect of the invention provides a method of treating a condition selected from the group consisting of airway inflammation and cystic fibrosis, comprising administering to a subject in need thereof a therapeutically effective amount of a compound of formula III:
III including a pharmaceutically acceptable salt or N-oxide thereof; wherein: X2 is CR2 or N, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5 or N, and X6 is CR6 or N, where no more than two of X2-X6 are N;
L is a bond or a Ci_2alkylidene chain optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of Ci-C6alkyl, C3-C6cycloalkyl, -OCi-C6alkyl, -OC3-C6cycloalkyl, and F;
A is Ci-Cioalkyl, C3-Ciocycloalkyl, C3-Cioheterocycloalkyl, or phenyl; each of which is optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of -CF3, -OCi-C6alkyl, -OH, Ci-C6alkyl, Ci-C6alkoxycarbonyl, C3- C6cycloalkyl, aryl, halogen, -C(O)-aryl, -C(O)-heteroaralkyl, -C(O)-Ci-C6alkyl, and -C(O)N(H)(d-C6alkyl);
Ri and Ri2 are each independently hydrogen, CN, Ci-C6alkyl, -OCi-C6alkyl, C3. locycloalkyl,
-OCF3, -OCF2H, -OCH2F, halogen, -NR7Ri0, -NR7COR8, -NR7SO2R9, or -SO2R9;
R2, R3, R4, R5, and R6 are each independently hydrogen, halogen, -CF3, -OH, -OCF3, -OCHF2, -OCH2F, Ci-Cioalkyl, C3-Ci0cycloalkyl, C3-C8heterocyclyl, heteroaryl, -OCi- Cioalkyl, -O-C3-Ci0cycloalkyl, -OH, -O-aryl, -NR7COR8, -NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, -SO2R9, or -CO2Rn; or where any two adjacent variables selected from R2, R3, R4, R5, and R6 can be taken together to form a cycloalkyl, aryl, heteroaryl, or heterocyclyl, each of which is optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro, and sulfonyl;
R7 and Rio each represent independently for each occurrence hydrogen, alkyl, or cycloalkyl, wherein the alkyl or cycloalkyl are optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of halogen, cyano, hydroxy, nitro, and alkoxy; or R7 and Rio are taken together to form a heterocyclyl optionally substituted by one, two, or three substituents selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl;
Rg is alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl;
R9 represents independently for each occurrence alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl; and
Rn is alkyl, alkenyl, alkynyl, amido, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydrogen. [0089] In certain embodiments, Ri and R12 are independently hydrogen or methyl. In certain embodiments, at least one of R2 and R6 is selected from the group consisting of F, Cl, -CF3, Me, -OMe, -OCF3, and -CO2Et. In certain embodiments, R2 and R6 are independently selected from the group consisting of F, Cl, -CF3, Me, -OMe, -OCF3, and -CO2Et. In certain embodiments, at least one of R3 and R5 is selected from the group consisting of F, Cl, -OH, -OMe, -OiPr, -Osec-butyl, -OCF3, -Ophenyl, -Ocyclohexyl, -SO2Me, pyrrolidinylsulfonyl, morpholinylsulfonyl, -CON(H)(cyclopropyl), 5-methyl-l,3,4-oxadiazolyl, -NHSO2cyclopropyl, and -NHCOcyclopropyl. In certain embodiments, R2 is -OMe, and R5 is chloro. In certain embodiments, R4 is selected from the group consisting of F, Cl, -OH, -OMe, -OEt, -OiPr, -OCF3, -Ophenyl, -Ocyclohexyl, -NH2, -NMe2, -CN, and 5-methyl-l,3,4-oxadiazolyl. In certain embodiments, R3 and R4 are taken together to form a heterocyclyl selected from the group consisting of dioxanyl, dioxolanyl, oxazolyl, pyrazinyl, and thiazolyl. In certain embodiments, R4 is hydrogen.
[0090] In certain embodiments, A is C3-Ciocycloalkyl. In certain embodiments, A is selected from the group consisting of t-butyl, cyclopentyl, cyclohexyl, 1-methylcyclohexyl, 4- methylcyclohexyl, 4-ethylcyclohexyl, 4-phenylcyclohexyl, 4,4-difluorocyclohexyl, 4,4- dimethylcyclohexyl, cycloheptyl, bicyclo[2.2.1]heptan-2-yl, adamantanyl, and 1,2,3,4-
tetrahydronaphthalenyl. In certain embodiments, A is cis-4-methylcyclohexyl. In certain embodiments, R2 is fluoro.
[0091] In certain embodiments, X2 is CR2, X3 is CR3, X4 is CR4, X5 is CR5, and X6 is CR6. In certain embodiments, L is a bond. In certain embodiments, the subject is human. [0092] Formula IIIA is represented by:
(MA) wherein X2 is CR2 or N, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5 or N, and X6 is CR6 or N, where one or two OfX2-X6 can be N;
L is a bond or a Ci_2alkylidene chain optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of Ci-C6alkyl, C3-C6cycloalkyl, -OCrC6alkyl, -OC3-C6cycloalkyl, and F;
A is selected from the group consisting of Ci-Cioalkyl and C3-Ciocycloalkyl optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of -CF3, -OCi-C6alkyl, Ci-C6alkyl, Ci-C6alkoxycarbonyl, C3-C6cycloalkyl, aryl, and halogen; Ri and Ri2 are each independently selected from the group consisting of hydrogen, CN,
Ci-C6alkyl, -OCi-C6alkyl, C3_i0cycloalkyl, -OC3_iocycloalkyl, -OCF3, -OCF2H, -OCH2F, halogen, -NR7Ri0, -NR7COR8, -NR7SO2R9 , and -SO2R9,
R2, R3, R4, R5, and R6 are each independently selected from the group consisting of hydrogen, halogen, -CF3, -OH, -OCF3, -OCHF2, -OCH2F, Ci-CiOalkyl, C3-Ci0cycloalkyl, C3-C8heterocyclyl, -OCi-Cioalkyl, -O-C3-Ci0cycloalkyl, -NR7COR8, -NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, -SO2R9, and -CO2Rn; or where any two adjacent variables selected from R2, R3, R4, R5, and R6 can be taken together to form a cycloalkyl, aryl, heteroaryl, or heterocyclyl, optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group
consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl;
R7 and Rio are each independently selected from the group consisting of hydrogen, alkyl, and cycloalkyl, optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of halogen, cyano, hydroxy, nitro, and alkoxy, or
R7 and Rio can be taken together to form a heterocyclyl optionally substituted by one, two, or three substituents selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl;
Rs is selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, and hydroxyl;
Rg is selected from the group consisting of alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, and hydroxyl; Rn is selected from the group consisting of alkyl, alkenyl, alkynyl, amido, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, and hydrogen; or pharmaceutically acceptable salts or N-oxides thereof.
[0093] In one embodiment, at least one of R2 and R6 may be selected from the group consisting of F, Cl, -CF3, Me, -OMe, -OCF3, and -CO2Et. In another embodiment, at least one of R3 and R5 may be selected from the group consisting of F, Cl, -OH, -OMe, -OiPr, -Osec-butyl, -OCF3, -Ophenyl, -Ocyclohexyl, -SO2Me, pyrrolidinylsulfonyl, morpholinylsulfonyl, -CONHR10 where Rio is cyclopropyl, 5-methyl-l,3,4-oxadiazolyl, -NHSO2cyclopropyl, and -NHCOcyclopropyl. In another embodiment, R2 may be -OMe, and R5 may be chloro. In a further embodiment, R4 may be selected from the group consisting of F, Cl, -OH, -OMe, -OEt, -OiPr, -OCF3, -Ophenyl, -Ocyclohexyl, -NH2, -NMe2, -CN, and 5-methyl-l,3,4-oxadiazolyl.
[0094] In one embodiment, R3 and R4 may be taken together to form a heterocyclyl selected from the group consisting of dioxanyl, dioxolanyl, oxazolyl, pyrazinyl, and thiazolyl. In another embodiment, A is selected from the group consisting of t-butyl, cyclopentyl, cyclohexyl, 1-methylcyclohexyl, 4-methylcyclohexyl, 4-ethylcyclohexyl, 4-phenylcyclohexyl, 4,4-difluorocyclohexyl, 4,4-dimethylcyclohexyl, cycloheptyl, bicyclo[2.2.1]heptan-2-yl, adamantanyl, and 1,2,3,4-tetrahydronaphthalenyl, such as cis-4-methylcyclohexyl. In a further embodiment, R2 may be fluoro.
[0095] In another embodiment, X2 may be CR2, X3 may be CR3, X4 may be CR4, X5 may be CR5, and X6 may be CR6. In another embodiment, L is be a bond.
[0096] Another aspect of the invention provides a method of modulating the activity of a cystic fibrosis transmembrance regulator protein, comprising exposing a cystic fibrosis transmembrance regulator protein to a compound of Formula III:
III including a pharmaceutically acceptable salt or N-oxide thereof; wherein: X2 is CR2 or N, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5 or N, and X6 is CR6 or N, where no more than two OfX2-X6 are N;
L is a bond or a Ci_2alkylidene chain optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of Ci-C6alkyl, C3-C6cycloalkyl, -OCi-C6alkyl, -OC3-C6cycloalkyl, and F;
A is Ci-CiOalkyl, C3-Ci0cycloalkyl, C3-Ci0heterocycloalkyl, or phenyl; each of which is optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of -CF3, -OCi-C6alkyl, -OH, Ci-C6alkyl, Ci-C6alkoxycarbonyl, C3- C6cycloalkyl, aryl, halogen, -C(O)-aryl, -C(O)-C i-C6alkyl, and -C(O)N(H)(C i-C6alkyl); Ri and Ri2 are each independently hydrogen, CN, Ci-C6alkyl, -OCi-C6alkyl, C3. locycloalkyl, -OC3_i0cycloalkyl, -OCF3, -OCF2H, -OCH2F, halogen, -NR7R10, -NR7COR8, -NR7SO2R9 , or -SO2R9;
R2, R3, R4, R5, and R6 are each independently hydrogen, halogen, -CF3, -OH, -OCF3, -OCHF2, -OCH2F, Ci-Cioalkyl, C3-Ci0cycloalkyl, C3-C8heterocyclyl, heteroaryl, -OCr Cioalkyl, -O-Cs-Ciocycloalkyl, -OH, -O-aryl, -NR7COR8, -NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, -SO2R9, or -CO2Rn; or where any two adjacent variables selected from R2, R3, R4, R5, and R6 can be taken together to form a cycloalkyl, aryl, heteroaryl, or heterocyclyl, each of which is optionally
substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl;
R7 and Rio each represent independently for each occurrence hydrogen, alkyl, or cycloalkyl, wherein the alkyl or cycloalkyl are optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of halogen, cyano, hydroxy, nitro, and alkoxy; or
R7 and Rio are taken together to form a heterocyclyl optionally substituted by one, two, or three substituents selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl;
Rs is alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl;
R9 represents independently for each occurrence alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl; and
Rn is alkyl, alkenyl, alkynyl, amido, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydrogen.
[0097] The compounds of the invention may be administered to patients (animals and humans) in need of such treatment in dosages that will provide optimal pharmaceutical efficacy. It will be appreciated that the dose required for use in any particular application will vary from patient to patient, not only with the particular compound or composition selected, but also with the route of administration, the nature of the condition being treated, the age and condition of the patient, concurrent medication or special diets then being followed by the patient, and other factors which those skilled in the art will recognize, with the appropriate dosage ultimately being at the discretion of the attendant physician. For treating clinical conditions and diseases noted above, the compound of this invention may be administered orally, topically, parenterally, by inhalation spray or rectally in dosage unit formulations containing conventional non-toxic pharmaceutically acceptable carriers, adjuvants and vehicles. The term parenteral as used herein includes subcutaneous injections, intravenous, intramuscular, intrasternal injection or infusion techniques.
[0098] Exemplary pharmaceutical compositions of this invention may be used in the form of a pharmaceutical preparation, for example, in solid, semisolid or liquid form, which contains one or more of the compound of the invention, as an active ingredient, in admixture with an organic or inorganic carrier or excipient suitable for external, enteral or parenteral applications. The active ingredient may be compounded, for example, with the usual non-toxic, pharmaceutically acceptable carriers for tablets, pellets, capsules, suppositories, solutions, emulsions, suspensions, and any other form suitable for use. The carriers which can be used are water, glucose, lactose, gum acacia, gelatin, mannitol, starch paste, magnesium trisilicate, talc, corn starch, keratin, colloidal silica, potato starch, urea and other carriers suitable for use in manufacturing preparations, in solid, semisolid, or liquid form, and in addition auxiliary, stabilizing, thickening and coloring agents and perfumes may be used. The active object compound is included in the pharmaceutical composition in an amount sufficient to produce the desired effect upon the process or condition of the disease.
[0099] For preparing solid compositions such as tablets, the principal active ingredient may bemixed with a pharmaceutical carrier, e.g., conventional tableting ingredients such as corn starch, lactose, sucrose, sorbitol, talc, stearic acid, magnesium stearate, dicalcium phosphate or gums, and other pharmaceutical diluents, e.g., water, to form a solid preformulation composition containing a homogeneous mixture of a compound of the invention, or a non-toxic pharmaceutically acceptable salt thereof. When referring to these preformulation compositions as homogeneous, it is meant that the active ingredient is dispersed evenly throughout the composition so that the composition may be readily subdivided into equally effective unit dosage forms such as tablets, pills and capsules.
[0100] The liquid forms in which the compositions of the invention may be incorporated for administration orally or by injection include aqueous solution, suitably flavored syrups, aqueous or oil suspensions, and emulsions with acceptable oils such as cottonseed oil, sesame oil, coconut oil or peanut oil, or with a solubilizing or emulsifying agent suitable for intravenous use, as well as elixirs and similar pharmaceutical vehicles. Suitable dispersing or suspending agents for aqueous suspensions include synthetic and natural gums such as tragacanth, acacia, alginate, dextran, sodium carboxymethylcellulose, methylcellulose, polyvinylpyrrolidone or gelatin. Compositions for inhalation or insufflation include solutions and suspensions in pharmaceutically acceptable, aqueous or organic solvents, or mixtures thereof, and powders.
[0101] Advantageously, the invention also provides kits for use by a consumer having, or at risk of having, a disease or condition associated with cystic fibrosis transmembrane regulators. Such kits include a suitable dosage form such as those described above and instructions describing the method of using such dosage form to mediate, reduce or prevent inflammation. The instructions would direct the consumer or medical personnel to administer the dosage form according to administration modes known to those skilled in the art. Such kits could advantageously be packaged and sold in single or multiple kit units. An example of such a kit is a so-called blister pack. Blister packs are well known in the packaging industry and are being widely used for the packaging of pharmaceutical unit dosage forms (tablets, capsules, and the like). Blister packs generally consist of a sheet of relatively stiff material covered with a foil of a preferably transparent plastic material. During the packaging process recesses are formed in the plastic foil. The recesses have the size and shape of the tablets or capsules to be packed. Next, the tablets or capsules are placed in the recesses and the sheet of relatively stiff material is sealed against the plastic foil at the face of the foil which is opposite from the direction in which the recesses were formed. As a result, the tablets or capsules are sealed in the recesses between the plastic foil and the sheet. Preferably the strength of the sheet is such that the tablets or capsules can be removed from the blister pack by manually applying pressure on the recesses whereby an opening is formed in the sheet at the place of the recess. The tablet or capsule can then be removed via said opening. [0102] It may be desirable to provide a memory aid on the kit, e.g., in the form of numbers next to the tablets or capsules whereby the numbers correspond with the days of the regimen which the tablets or capsules so specified should be ingested. Another example of such a memory aid is a calendar printed on the card, e.g., as follows "First Week, Monday, Tuesday, . . . etc. . . . Second Week, Monday, Tuesday, . . ." etc. Other variations of memory aids will be readily apparent. A "daily dose" can be a single tablet or capsule or several pills or capsules to be taken on a given day. Also, a daily dose of a first compound can consist of one tablet or capsule while a daily dose of the second compound can consist of several tablets or capsules and vice versa. The memory aid should reflect this.
EXAMPLES
[0103] The compounds of the present invention can be prepared in a number of ways well known to one skilled in the art of organic synthesis. More specifically, compounds of the invention may be prepared using the reactions and techniques described herein. In the description of the synthetic methods described below, it is to be understood that all proposed reaction conditions, including choice of solvent, reaction atmosphere, reaction temperature, duration of the experiment and workup procedures, can be chosen to be the conditions standard for that reaction, unless otherwise indicated. It is understood by one skilled in the art of organic synthesis that the functionality present on various portions of the molecule should be compatible with the reagents and reactions proposed. Substituents not compatible with the reaction conditions will be apparent to one skilled in the art, and alternate methods are therefore indicated. The starting materials for the examples are either commercially available or are readily prepared by standard methods from known materials.
General Experimental Procedures: [0104] NMR spectra were recorded on a Varian AS 400 (Varian Inc., Palo Alto, CA) at room temperature at 400 MHz for proton, or a Bruker Avance 300 UltraShield™ (Bruker BioSpin Corp., Billerica, MA) at 300 MHz for proton and at 282 MHz for 19F. Chemical shifts are expressed in parts per million (d) relative to residual solvent as an internal reference. The peak shapes are denoted as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; bs, broad singlet; bd, broad doublet. Liquid chromatography electrospray ionization mass spectra
(LCMS) were obtained on an Agilent HP 1100 instrument (Agilent Technologies, Foster City, CA). Where the intensity of chlorine or bromine-containing ions are described, the expected intensity ratio was observed (approximately 3 : 1 for 35Cl/37Cl-containing ions and 1 : 1 for 79Br/81Br-containing ions) and the intensity of only the lower mass ion is given. MS peaks are reported for all examples. Microwave reactions were performed on a Biotage Emiys™ Optimizer (Biotage, Charlottesville, VA), Column chromatography was performed on a CombiFlash Companion™ (Teledyne ISCO Inc., Lincoln, NE) with different size of RcdiSep Rf columns. Preparative thin-layer chromatography was performed using Analtech silica gel GF with UV254 indicator (Analtech Inc., Newark, DE) on 20 cm x 20 cm x 1 mm plates. When needed multiple plates are used. After eluting the plates with the indicated solvent, the desired band is marked under UV light, and scrapped off. The desire product is extracted from the
silica using a polar solvent system (e.g., 20% methanol in methylene chloride or 100% EtOAc). Preparative HPLC was performed on a Varian Dynamax instrument (Varian Inc., Palo Alto, CA) using a Kromasil 100- 10-Cl 8 250 mm x 20 mm column (EKA Chemicals, 80 Bohus, Sweden).
EXAMPLE 1
General Procedure for Preparation of 4-Chloro-N-((ls,4s)-4-methylcyclohexyl) pyrimidin-2-amine (1):
[0105] To a solution of 2,4-dichloropyrimidine (1.76 g, 11.8 mmol) in EtOH (10.0 mL) was treated at room temperature with 4-methylcyclohexanamine (mixture of cis and trans isomers, 1.56 g, 11.8 mmol) followed by triethylamine (2.39 g, 23.6 mmol). The mixture was heated in oil-bath at 70 0C for 18 hours. After cooling to room temperature, the reaction mixture was diluted with CH2Cl2, and washed with saturated NaCl solution. The organic layer was separated, dried (Na2SO4) and concentrated in vacuo. The residue was purified by ISCO chromatography (330 g silica column, 0 - 100% EtOAc in hexanes; Rf - 0.80 with hexanes : EtOAc = 1 : 1 for 1, less polar isomer) to give 331 mg (12%) of 1 as a white solid. 1H NMR (CDCl3, 300 MHz) δ 8.11 (b, 1 H), 6.55 (d, 1 H, J= 5.3 Hz), 4.12 (m, 1 H), 1.83-1.76 (m, 2 H), 1.71-1.47 (m, 5 H), 1.26-1.14 (m, 2 H), 0.94 (d, 3 H, J= 6.5 Hz); MS (ESI, M + H+) CHHI7CIN3, calcd. 226.1, found 226.0.
EXAMPLE 2
General Procedure for Preparation of 4-(5-Chloro-2-methoxyphenyl)-N-((ls,4s)-4- methylcyclohexyl)pyrimidin-2-amine
[0106] To a solution of compound 1 (90.0 mg, 399 μmol, from Example 1) in CH3CN (6.00 mL) was added S-chloro^-methoxyphenylboronic acid (149 mg, 799 μmol) followed by Pd(PPh3)4 (23.0 mg, 19.9 μmol) and 2 M Na2CO3 solution (3.00 mL). The mixture was heated in oil-bath at 100 0C for 6 hours. After cooling to room temperature, the reaction mixture was diluted with EtOAc, and washed with saturated NaCl solution. The organic layer was separated, dried (Na2SO4) and concentrated in vacuo. The residue was purified by ISCO chromatography (40 g silica column, 0 - 100% EtOAc in hexanes; Rf ~ 0.70 with hexanes : EtOAc = 1 : 1) to give 75.0 mg (57%) of of the title compound as a white solid. 1H NMR (CDCl3, 300 MHz) δ 8.29 (b, 1 H), 7.89 (s, 1 H), 7.36 (dd, 1 H, J= 8.8, 2.7 Hz), 7.14 (b, 1 H), 6.93 (d, 1 H, J= 8.8 Hz), 5.62 (b, 1 H), 4.20 (b, 1 H), 3.88 (s, 3 H), 1.89-1.83 (m, 2 H), 1.74-1.52 (m, 5 H), 1.33- 1.20 (m, 2 H), 0.95 (d, 3 H, J= 6.4 Hz); MS (ESI, M + H+) Ci8H23ClN3O, calcd. 332.2, found 332.2.
EXAMPLE 3
Preparation of Bicyclo [2.2.1] hept-2-yl- [4-(5-chloro-2-methoxy-phenyl)-pyrimidin-2-yl] - amine Part I: Synthesis of2-Chloro-4-(5-chloro-2-methoxy-phenyl)-pyrimidine
[0107] To a solution of 5-chloro-2-methoxyphenylboronic acid (560 mg, 3 mmol) and 2,4- dichloro pyrimidine (300 mg, 2 mmol) in a mixture of EtOH (6 mL) and toluene (18 mL) was added IM K2CO3 (6 mL), followed by tetrakis(triphenylphosphine)palladium (110 mg, 0.1 mmol). The reaction mixture was refluxed for 1 h, cooled to room temperature, and extracted with EtOAc (2x). The combined organic layers were washed with brine, dried (Na2SO4), and then concentrated in vacuo. The crude compound was purified by ISCO (20 to 40 % EtOAc//?- hexane) to provide the title compound (310 mg, 61%) as a pale yellow solid. 1H NMR(CDCl3, 300MHz) δ 3.91(s, 3H), 6.95(d, J=9Hz, IH), 7.41(dd, J=9Hz, J =3Hz, IH), 7.95(d, J=5.1Hz, IH), 8.08 (d, J=2.7Hz, IH), 8.59(d, J=5.1Hz, IH); MS: Found: ES+ 255(M+1); Calcd: 254.0
Part II: Synthesis ofBicyclo[2.2.1] hept-2-yl-[4-(5-chloro-2-methoxy-phenyl)-pyrimidin-2-yl] - amine
[0108] A reaction mixture of 2-chloro-4-(5-chloro-2-methoxyphenyl)pyrimidine (75 mg, 0.3 mmol), bicyclo[2.2.1]heptan-2-amine hydrochloride (90 mg, 0.6 mmol), and DIPEA (300 μL, 1.7 mmol) in isopropanol (4 mL) was heated at 100°C for 2 days. After cooling to room temperature, the solution was concentrated in vacuo, and purified by ISCO (10 to 20 % EtOAc/n-hexane) to provide the title compound (60 mg, 60% yield). 1H NMR(CDCl3, 300MHz) δ 0.84 (ddd, IH), 1.26~1.75(set of m, 6H), 2.14~2.25(set ofm, 2H), 2.57(s, IH), 3.86(s, 3H), 4.20~4.26(m, IH), 5.39(br, IH), 6.91(d, J=9Hz, IH), 7.11(d, J=5.1Hz, IH), 7.34(dd, J=9Hz, J =2.7Hz, IH), 7.88(s, IH), 8.27 (d, J=5.1Hz, IH). MS: Found: ES+ 330 (M+l); Calcd: 329.1
EXAMPLE 4
Preparation of [4-(5-Chloro-2-methoxy-phenyl)-pyrimidin-2-yl]-(l,2,3,4-tetrahydro- naphthalen-2-yl)-amine
Part I: Synthesis of4-(5-Chloro-2-methoxy-phenyl)-2-methylsulfanyl-pyrimidine
[0109] To a solution of 5-chloro-2-methoxyphenylboronic acid (420 mg, 2.6 mmol) and 4- chloro-2-methylsulfanyl-pyrimidine (700 mg, 3.7 mmol) in a mixture of EtOH (6 mL) and toluene (18 mL) was added IM K2CO3 (6 mL) followed by tetrakis(triphenylphosphine) palladium (142 mg, 0.12 mmol). The reaction mixture was refluxed for 1 hour, cooled to room
temperature, and extracted with EtOAc (2x). The combined organic layers were washed with brine, dried (Na2SO4), and then concentrated in vacuo. The crude compound was purified by ISCO (20 to 40 % EtOAc/n-hexane) to provide the title compound (670 mg, 97%) as pale yellow solid. 1H NMR(CDCl3, 300MHz) δ 2.63(s, 3H), 3.89(s, 3H), 6.94(d, J=9Hz, IH), 7.38(dd, J=9Hz, J =2.7Hz, IH), 7.65(d, J=5.1Hz, IH), 8.06(d, J=3Hz, IH), 8.51(d, J=5.1Hz, IH); MS: Found: ES+ 267 (M+l); Calcd: 266.0
Part II: Synthesis of4-(5-Chloro-2-methoxy-phenyl)-2-methanesulfonyl-pyrimidine
[0110] To a solution of 4-(5-Chloro-2-methoxy-phenyl)-2-methylsulfanyl-pyrimidine (670 mg, 2.5 mmol, from Part I of this Example) in dichloromethane (15 mL) was added mCPBA (2.1 g, 12 mmol). The reaction mixture was stirred at room temperature under nitrogen for 19 h. When the reaction was finished, it was neutralized with NaHCO3, washed with brine, and dried (Na2SO4), The solution was concentrated under the reduced pressure to obtain the title compound (665 mg, 89%) as a pale yellow solid which was used in the next step without further purification. 1H NMR(CDCl3, 300MHz) δ 3.42(s, 3H), 3.94(s, 3H), 6.99(d, J=8.7Hz, IH), 7.46(dd, J=9Hz, J =3Hz, IH), 8.16(d, J=3Hz, IH), 8.24(d, J=5.1Hz, IH), 8.88(d, J=5.7Hz, IH); MS: Found: ES+ 299 (M+l); Calcd: 298.0
P art III: Synthesis of [4-(5-Chloro-2-methoxy-phenyl)-pyrimidin-2-yl] -(1 ,2,3,4-tetrahydro- naphthalen-2-yl)-amine
[0111] A reaction mixture of 4-(5-Chloro-2-methoxy-phenyl)-2-methanesulfonyl-pyrimidine (100 mg, 0.33 mmol, from Part II of this Example), l,2,3,4-tetrahydronaphthalen-2-amine hydrochloride (121 mg, 0.66 mmol), and DIPEA (300 μL, 1.7 mmol) in isopropanol (4 mL) was heated at 100°C for overnight. After cooling to room temperature, the solution was concentrated under the reduced pressure, purified by ISCO (20% EtOAc/n-hexane), and then recrystalized in MeOH to provide the title compound (33 mg, 27% yield) as a solid. 1H NMR(CDCl3, 300 MHz) δ 1.84~1.96(m, IH), 2.18~2.26(m, IH), 2.78(dd, J=16.2Hz, J =8. IHz, IH), 2.95(t, J=6.6Hz, 2H), 3.27(dd, J=16.2Hz, J =4.8Hz, IH), 3.87(s, 3H), 4.41~4.46(m, IH), 5.36(d, J=7.5Hz, IH), 6.91(d, J=8.7Hz, IH), 7.08~7.13(m, IH), 7.16(d, J=5.4Hz, IH), 7.34 (dd, J=9Hz, J=2.7Hz, IH), 7.90(s, IH), 8.29(d, J=5.1Hz, IH); MS: Found: ES+ 366(M+1); Calcd:365.1.
EXAMPLE 5
Preparation of7V-Cyclohexyl-4-(3-(cyclohexyloxy)phenyl)pyrimidin-2-amine
[0112] To a solution of 3-(2-(cyclohexylamino)pyrimidin-4-yl)phenol (47 mg, 0.17 mmol) in acetone (4 mL) was added iodocyclohexane (0.45 mL, 3.49 mmol) and potassium carbonate (242 mg, 1.75 mL). The reaction mixture was stirred at 750C for 24 h. The mixture was poured into water (20 mL) and the product was extracted with dichloromethane (3x15 mL). The combined organic layer was dried (Na2SO4) and concentrated under reduced pressure. The crude compound was purified by preparative TLC (35% EtOAC in hexane) and then it was re- crystallized from methanol and dichloromethane to provide title product (12 mg, 20%) as pale
yellow solid. 1H NMR (CDCl3, 300MHz) δ 8.3 (s, IH), 7.6 (m, 2H), 7.3 (m, IH), 7.0 (m, IH), 6.9 (m, IH), 5.1 (m, IH), 4.3 (m, IH), 3.9 (m, IH), 2.1 (m, 4H), 1.8 (m, 4H), 1.2-1.4 (m, 12H); MS (ESI, M + H+) C22H29N3O, calcd. 351.2, found 352.2.
EXAMPLE 6 Preparation of N-(3-(2-(cyclohexylamino)pyrimidin-4-yl)phenyl)-N-methylcyclopropane- carboxamide
Part I : Synthesis ofN-(3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)phenyl) cyclopropanecarboxamide (I)
1
[0113] To a solution of 3-aminophenylboronic acid pinacol ester (2.19 g, 10 mmol), TEA (1.46 rnL, 10.5 mmol) in DCM (20 mL), cooled in a ice-water bath, was added cyclopropyl carbonyl chloride (1.1 g, 10.5 mmol). The temperature was allowed to rise to room temperature and stirred at room temperature for 5 hr. The reaction was quenched with water, and extracted with dichloromethane (DCM, 2 x 10 mL). The combined organic layers were dried over MgSO4 and evaporated to give the title compound (2.85 g) as an off-white solid which was used for the next reaction without further purification. 1HNMR (CDCl3, S): 7.85 (d, J=7.6Hz, IH), 7.70 (d, J=2.0Hz, IH), 7.52 (d, J=7.2Hz, IH), 7.34 (t, J=7.2Hz, 2H), 1.46 (br, IH), 1.34 (s, 12H), 1.08(m, 2H), 0.84 (m, 2H).
Part II : Synthesis ofN-methyl-N-(3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2- yl)phenyl)cyclopropane-carboxamide (2)
[0114] To a solution of N-(3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2- yl)phenyl)cyclopropane-carboxamide (574.3 mg, 2 mmol) in THF (10 niL), was added, under argon, sodium hydride (60% oil dispersion, 100 mg, 2.5 mmol). The mixture was stirred at room temperature for 1 hr. To which was added dropwise iodomethane (156 μL, 1.25 mmol). The resulting mixture was stirred for 19 hr. The reaction was quenched with water and solvent was removed under vacuum. The aqueous solution was extracted with DCM (3 x10 mL). The combined organic layers were dried over MgSO4 and evaporated. The residue was purified by ISCO silica gel flash column, eluting with with gradient ethyl acetate (0-30%) in hexane to give the title compound (359 mg) as a light yellow oil. 1HNMR (CDCl3, S): 7.75 (d, J=6.8Hz, IH), 7.71 (s, IH), 7.42 (t, J=7.2Hz, IH), 7.36 (d, J=7.2Hz, IH), 3.29 (s, 3H), 1.36 (s, 13H), 1.02(m, 2H), 0.60 (m, 2H).
Part III : Synthesis ofN-(3-(2-(cyclohexylamino)pyrimidin-4-yl)phenyl)-N- methylcyclopropane-carboxamide
[0115] A mixture of N-methyl-N-(3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)phenyl) cyclopropanecarboxamide 2 (135 mg, 0.448 mmol), 4-chloro-N-cyclohexylpyrimidin-2-amine (104.4 mg, 0.493 mmol), tetrakis(triphenylphosphine) palladium (25.9 mg, 0.05 eq.), Na2CO3 (95 mg, 0.9 mmol) in dioxane/H2O (3:1, 2 mL) under argon, was irradiated in a microwave at 1200C for 30 min. Water (3 mL) was added to the reaction mixture. The mixture was extracted with ethyl acetate (3 x 5 mL), the combined organic solution was dried (MgSO4) and evaporated under vacuum. The residue was purified by ISCO silica gel flash column, eluting with with gradient ethyl acetate (0-50%) in hexane to give light yellow oily residue (175 mg) containing significant amount of pinacol. The residue was dissolved in diethyl ether, and 2N HCl was added. The mixture was extracted with hexane (3 times) and ethyl acetate (3 times). The combined EA solution was dried over MgSO4 and evaporated to dry. The residue was washed with hexane to give the title compound as a white solid (38 mg). 1HNMR (CDCl3, δ):
8.35 (d, J=4.8Hz, IH), 7.98 (s, IH), 7.94 (d, J=7.6Hz, , IH), 7.52 (t, J=7.6Hz, IH), 7.38 (dm, J=7.6Hz, IH), 6.92 (d, J=4.8Hz, IH), 5.14 (d, J=7.6Hz, IH), 3.92 (br, IH), 3.35 (s, 3H), 2.10 (m, 2H), 1.78 (m, 2H), 1.66 (m, IH), 1.44 (m, 3H), 1.27 (m, 3H), 1.05 (m, 2H), 0.64 (m, 2H). MS (ESI, M + H+), Found 351.2; Calcd: 350.2
EXAMPLE 7
Preparation of7V-(3-(2-(cyclohexylamino)pyrimidin-4-yl)phenyl)-N-methylcyclopropane- sulfonamide
Part I : Synthesis ofN-(3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2- yl)phenyl)cyclopropanesulfonamide (I)
[0116] To a mixture of 3-aminophenylboronic acid pinacol ester (2.19 g, 10 mmol), Na2CO3 (2.12 g, 20 mmol) in a mixture of water (10 mL)/DCM (10 mL) at room temperature was added dropwise cyclopropylsulfonyl chloride (1.41 g, 10 mmol). The mixture was stirred at room temperature for 5 hr. The reaction mixture was extracted with DCM (2 x 10 mL). The combined organic layers were dried over MgSO4 and evaporated to give a light brown residue which was purified by ISCO silica gel flash column, eluting with with gradient ethyl acetate (0- 50%) in hexane to give the title compound 3 (1.46 g) as an off-white solid. 1HNMR (CDCl3, S): 7.63 (d, J=7.2Hz, IH), 7.54 (d, J=2.0Hz, IH), 7.48 (ddd, J=8.4, 2.4, 1.2Hz, IH), 7.36 (t, J=7.2Hz, IH), 6.26 (s, IH), 2.48 (m, IH), 1.35 (s, 12H), 1.17 (m, 2H), 0.96 (m, 2H).
Part II: Synthesis ofN-methyl-N-(3-(4, 4, 5, 5-tetramethyl-l, 3, 2-dioxaborolan-2- yl)phenyl)cyclopropane-sulfonamide (2)
[0117] To a solution of N-(3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2- yl)phenyl)cyclopropane-sulfonamide (1) (646.4 mg, 2 mmol, from Part I of this Example) in THF (10 mL), was added, under argon, sodium hydride (60% oil dispersion, 100 mg, 2.5 mmol). The mixture was stirred at room temperature for lhr, and iodomethane (156 μL, 1.25 mmol) was added dropwise. The mixture was stirred at room temperature for 19 hr. Water was introduced to the reaction mixture and solvent was removed under vacuum. The resulting mixture was extracted with DCM (3 x 10 mL), the combined organic layers were dried over MgSO4 and evaporated. The residue was purified by ISCO silica gel flash column, eluted with gradient ethyl acetate (0-30%) in hexane to give the title compound (490 mg) as a light yellow oil. 1HNMR (CDCl3, δ): 7.79 (d, J=2.0Hz, IH), 7.72 (d, J=7.6Hz, , IH), 7.54 (d, J=7.6Hz, IH), 7.38 (d, J=7.6Hz, IH), 3.37 (s, 3H), 2.39 (m, IH), 1.34 (s, 12H), 1.08(m, 2H), 0.94 (m, 2H).
Part III: Synthesis ofN-(3-(2-(cyclohexylamino)pyrimidin-4-yl)phenyl)-N- methylcyclopropane-sulfonamide
[0118] A mixture of N-methyl-N-(3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)phenyl)- cyclopropanesulfonamide (2) (190 mg, 0.563 mmol, from Part II of this Example), 4-chloro-N- cyclohexylpyrimidin-2-amine (131.2 mg, 0.62 mmol), tetrakis(triphenylphosphine)palladium (32.5 mg, 0.05 eq.), Na2CO3 (120 mg, 0.9 mmol) in dioxane/H2O (3:1, 2 mL) under argon, was irradiated in a microwave at 1200C for 30 min. Water (3 mL) was added to the reaction mixture. The mixture was extracted with ethyl acetate (3 x 5 mL), and the combined organic solution was dried (MgSO4) and evaporated under vacuum. The residue was purified by ISCO silica gel flash column, eluted with gradient ethyl acetate (0-50%) in hexane to give the title compound as a light yellow oil which became a white solid (175 mg) after washing with hexane to remove pinacol. 1HNMR (CDCl3, δ): 8.34 (d, J=4.8Hz, IH), 8.11 (s, IH), 7.90 (d, J=8.0Hz, , IH), 7.52 (ddd, J=7.6, 2.0, 1.2Hz, IH), 7.47 (t, J=8.0Hz, IH), 6.92 (d, J=4.8Hz, IH),
5.10 (d, J=7.6Hz, IH), 3.91 (br, IH), 3.42 (s, 3H), 2.40 (m, IH), 2.10 (m, 2H), 1.79 (m, 2H), 1.66 (m, IH), 1.44 (m, 2H), 1.10 (m, 3H), 1.05 (m, 2H), 0.94 (m, 2H). MS (ESI, M + H+), Found 387.1; Calcd: 386.2
EXAMPLES 8-86
[0119] Compounds listed in Table 1 below were prepared using procedures analogous to those described above for the synthesis of Examples 1-7 using appropriate starting materials which are available commercially, prepared using procedures known in the art, or prepared in a manner analogous to routes described above for other compounds.
TABLE 1
Dual Corrector Potentiator Assay
[0120] The ability of exemplary compounds to correct the processing defect of ΔF508 CTFR, i.e., increase the surface expression of CFTR channels, and potentiate existing channels was demonstrated in an FRT cell electrophysiological (Ussing chamber) assay. FRT epithelial cell monolayers were grown on Snapwell filter inserts and optionally treated with a reference corrector N-(2-(5-chloro-2-methoxyphenylamino)-4'-methyl-4,5'-bithiazol-2'-yl)benzamide. The cells were exposed to a compound of the invention for 24 hours prior to the assay. The inserts were transferred to a Navicyte Ussing recording chamber and superfused with a HEPES buffered physiological saline (HB-PS) with composition (in mM): NaCl, 137; KCl, 4.0; CaCl2, 1.8; MgCl2, 1; HEPES, 10; Glucose, 10; pH adjusted to 7.4 with NaOH. The mucosal solution was lOCF-PS (composition in mM: Na-gluconate, 137; KCl, 4; CaCl2, 1.8; MgCl2, 1; HEPES, 10; Mannitol, 10; pH adjusted to 7.4 with N-methyl-D-glucamine) to create a transepithelial Cl ion gradient. A Physiologic Instruments VCC MC6 epithelial voltage clamp (Physiologic Instruments, Inc., San Diego, CA) was used to record the short circuit current (ISC).
[0121] Inserts were voltage clamped at 0 mV to measure the ISC. 1 OCF-PS solution (5 ml) was added to the mucosal (top) side of the Snapwell filter and HB-PS solution (5 mL) was added to the serosal (bottom) side of the Snapwell filter insert to permeabilize the serosal membrane. Solution additions and replacements in the Navicyte chambers were performed in a way to maintain a hydrostatic pressure gradient from mucosal to serosal sides of the filters by maintaining a solution level greater or equal in the mucosal chamber relative to the serosal chamber during solution changes. After acquisition of at least 10 minutes of baseline current, agonists (final concentrations: 10 μM forskolin, 100 μM 3-isobutyl-l-methylxanthine [IBMX] and 20 μM genistein) and antagonist (final concentration: 10 μM CFTRinh-172) were applied sequentially and cumulatively at 10 minute intervals for forskolin and IBMX, and at 15 minute intervals for genistein and CFTRinh-172, to both serosal and mucosal epithelial surfaces. [0122] Agonists were prepared as 200X-1000X concentrated solutions in HP-PS and 10CF-PS. Agonist stocks prepared in HB-PS were added to the serosal surface, while agonist stocks prepared in 1 OCF-PS were added to the mucosal surface. In potentiator assays, appropriate volumes from 10 mM test compound solution in DMSO were added to the mucosal 1 OCF-PS solution. Agonists were diluted to the final working concentration in the Navicyte chamber by removal of chamber solution and addition of the concentrated stock solution. Order of solution
removal was serosal then mucosal and for solution additions mucosal then serosal in order to maintain a hydrostatic pressure gradient from mucosal to serosal during solution changes. Transepithelial resistance was monitored every 20 s with 10 mV voltage steps.
[0123] EC50 values are defined as the concentration of compound that gives a >25% increase in whole cell Cl" conductance (compared to DMSO at 37°C as a vehicle) at 10 μM. The corrector efficacy was measured as a percentage change in agonist + compound vs. agonist: ΔICompound(forskolin+IBMX+genistein) / ΔIvehicie(forskolin+IBMX+genistein). The potentiator efficacy was measured as a percentage change in forskolin activity: ΔI(forskolin+compound) / ΔI(forskolin+IBMX+genistein) .
[0124] Table 2 provides results for several exemplary compounds. Corrector efficacy ranges correspond to + = <2, ++ = 2-3, and +++ = >3. Potentiator efficacy ranges correspond to + = <0.3, ++ = 0.3-0.6, and +++ = >0.6.
TABLE 2
[0125] All publications and patents mentioned herein, including those items listed below, are hereby incorporated by reference in their entirety as if each individual publication or patent was specifically and individually incorporated by reference. In case of conflict, the present application, including any definitions herein, will control.
Equivalents
[0126] While specific embodiments of the subject invention have been discussed, the above specification is illustrative and not restrictive. Many variations of the invention will become apparent to those skilled in the art upon review of this specification. The full scope of the invention should be determined by reference to the claims, along with their full scope of equivalents, and the specification, along with such variations.
[0127] Unless otherwise indicated, all numbers expressing quantities of ingredients, reaction conditions, and so forth used in the specification and claims are to be understood as being modified in all instances by the term "about." Accordingly, unless indicated to the contrary, the numerical parameters set forth in this specification and attached claims are approximations that may vary depending upon the desired properties sought to be obtained by the present invention. [0128] What is claimed is:
Claims
1. A compound of formula I :
including a pharmaceutically acceptable salt or N-oxide thereof; wherein:
X2 is CR2 or N, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5 or N, and X6 is CR6 or N, where no more than two OfX2-X6 are N; where if X3 is N, L is a bond, and A is cyclohexyl, then R2 is not methoxy; and if X5 is N, L is a bond, and A is cyclohexyl, then R6 is not methoxy;
L is a bond or a Ci_2alkylidene chain optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of Ci-C6alkyl, C3-C6cycloalkyl, -OCi-C6alkyl, -OC3-C6cycloalkyl, and F;
A is C4-Ci0cycloalkyl, C3-Ci0heterocycloalkyl, or phenyl, each of which is optionally substituted with one, two, or three substituents independently, for each occurrence, selected from the group consisting of F, Cl, -CF3, -OCi-C6alkyl, -OH, Ci-C6alkyl, Ci-C6alkoxycarbonyl, C3-C5cycloalkyl, aryl, -C(O)-aryl, -C(O)-heteroaralkyl, -C(O)-C i-C6alkyl, and -C(O)N(H)(C1- C6alkyl);
Ri and Ri2 are each independently hydrogen, CN, Ci-C6alkyl, -OCi-C6alkyl, C3. locycloalkyl, -OC3_i0cycloalkyl, -OCF3, -OCF2H, -OCH2F, halogen, -NR7R10, -NR7COR8, -NR7SO2R9 , or -SO2R9, where if Ri2 is -OCF2H, then R4 is not methyl;
R2 is hydrogen, halogen, -CN, -OCi-Cioalkyl, -Oaryl, -CF3, -OCHF2, -OCH2F, -NR7Ri0, -CO2Rii, or -SO2NR7Ri0;
R3 and R5 are each independently hydrogen, halogen, -CF3, -OH, -OCF3, -OCHF2, -OCH2F, Ci-Csalkyl, C3-C8cycloalkyl, C3-C8heterocyclyl, -OCi-Ci0alkyl, -OC3-Ci0cycloalkyl, -NR7COR8, -NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, or -SO2R9; R4 is hydrogen, halogen, Ci-C3alkyl, -OCi-C6alkyl, -O-aryl, -OH, -OCHF2, -OCH2F, -CN, heteroaryl, -NR7Ri0, or -SO2NR7Ri0; R6 is hydrogen, halogen, -CN, -OCrCi0alkyl, -Oaryl, -CF3, -OCHF2, -OCH2F, -NR7Ri0, where any two adjacent variables selected from R2, R3, R4, R5, and R6 can be taken together to form a cycloalkyl, aryl, heteroaryl, or heterocyclyl, each of which is optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl, where R3 and R4 cannot be taken together to form a dioxolanyl when L is a bond and A is cyclohexyl; where at least one of R2, R3, R4, R5, and R6 is not hydrogen; and if R4 is -OCH3, then R3 and R5 are not -OCH3; R7 and Ri0 each represent independently for each occurrence hydrogen, alkyl, or cycloalkyl, wherein the alkyl and cycloalkyl are optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of halogen, cyano, hydroxy, nitro, and alkoxy; or R7 and Ri0 are taken together to form a heterocyclyl optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl, where the heterocyclyl is not dihydro-2H-benzo[b] [ 1 ,4]dioxepinyl; Rs is alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl; R9 represents independently for each occurrence alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydroxyl; and Rn is alkyl, alkenyl, alkynyl, amido, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydrogen.
2. The compound according to claim 1, wherein Ri and Ri2 are independently hydrogen or methyl.
3. The compound according to claim 1 or 2, wherein at least one of R2 and R6 is selected from the group consisting of F, Cl, -CF3, -OCH3, and -OCF3.
4. The compound according to any one of claims 1 to 3, wherein at least one of R3 and R5 is selected from the group consisting of F, Cl, -OH, -OCH3, -OiPr, -Osec-butyl, -OCF3, -Ophenyl, -Ocyclohexyl, -SO2Me, pyrrolidinylsulfonyl, morpholinylsulfonyl, -CON(H)-cyclopropyl, 5- methyl- 1, 3, 4-oxadiazo IyI, -NHSO2cyclopropyl, and -NHCOcyclopropyl.
5. The compound according to any one of claims 1 to 4, wherein R4 is selected from the group consisting of -NH2, -NMe2, -Ophenyl, -OCH3, -OCH2CH3, -OCH(CH3)2, -OCF3, Cl, and F.
6. The compound according to any one of claims 1 to 3, wherein R3 and R4 are taken together to form a heterocyclyl selected from the group consisting of dioxanyl, oxazolyl, pyrazinyl, and thiazolyl.
7. The compound according to any one of claims 1 to 4, wherein R4 is hydrogen or fluoro.
8. The compound according to any one of claims 1 to 7, wherein A is C4-Ciocycloalkyl.
9. The compound according to any one of claims 1 to 7, wherein A is selected from the group consisting of cyclopentyl, cyclohexyl, 1-methylcyclohexyl, 4-methylcyclohexyl, 4- ethylcyclohexyl, 4-phenylcyclohexyl, 4,4-difluorocyclohexyl, 4,4-dimethylcyclohexyl, cycloheptyl, bicyclo[2.2.1]heptan-2-yl, adamantanyl, and 1,2,3,4-tetrahydronaphthalenyl.
10. The compound according to any one of claims 1 to 7, wherein A is cis-4- methylcyclohexyl, cis-4-ethylcyclohexyl; cis-4-trifluoromethylcyclohexyl; 4,4- dimethylcyclohexyl; or 4,4-difluorocyclohexyl.
11. The compound according to any one of claims 1 to 10, wherein R2 is fluoro or chloro.
12. The compound according to any one of claims 1 to 11, wherein X2 is CR2, X3 is CR3, X4 is CR4, X5 is CR5, and X6 is CR6.
13. The compound according to any one of claims 1 to 12, wherein L is a bond.
14. A compound selected from the group consisting of: bicyclo[2.2.1]hept-2-yl-[4-(5-chloro- 2-methoxy-phenyl)-pyrimidin-2-yl]-amine; [4-(5-Chloro-2-methoxy-phenyl)-pyrimidin-2-yl]- (1 ,2,3,4-tetrahydro-naphthalen-2-yl)-amine; N-(3-(2-(cyclohexylamino)pyrimidin-4-yl)phenyl)- N-methylcyclopropane-carboxamide; N-(3-(2-(cyclohexylamino)pyrimidin-4-yl)phenyl)-N- methylcyclopropane-sulfonamide; N-cyclohexyl-4-(6-methylpyridin-3 -yl)pyrimidin-2-amine; N-cyclohexyl-4-(4-methoxyphenyl)pyrimidin-2-amine; N-cyclohexyl-4-(4-methoxy-2- (trifluoromethyl)henyl)pyrimidin-2-amine; N-cyclohexyl-4-(3 -methoxyphenyl)pyrimidin-2- amine; N-cyclohexyl-4-(4-fluorophenyl)pyrimidin-2-amine; ethyl 2-(2- (cyclohexylamino)pyrimidin-4-yl)benzoate; N-cyclohexyl-4-(4-ethoxyphenyl)pyrimidin-2- amine; N-cyclohexyl-4-(2-methylpyridin-4-yl)pyrimidin-2-amine; 4-(5-chloro-2- methoxyphenyl)-N-cyclohexylpyrimidin-2-amine; N-cyclohexyl-4-(2,4- difluorophenyl)pyrimidin-2-amine; N-cyclohexyl-4-(2,5-dichlorophenyl)pyrimidin-2-amine; A- (3-chlorophenyl)-N-cyclohexylpyrimidin-2-amine; 4-(4-chloro-3-fluorophenyl)-N- cyclohexylpyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)-N-((lR,4R)-4- methylcyclohexyl)pyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)-N-cyclohexyl-6- methylpyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)-N-((l S,4S)-4- methylcyclohexyl)pyrimidin-2-amine; 4-(5 -chloro-2-methoxyphenyl)-N-(4,4- difluorocyclohexyl)pyrimidin-2-amine; tert-butyl 4-(4-(5-chloro-2-methoxyphenyl)pyrimidin- 2-ylamino)piperidine- 1 -carboxylate; N-cyclohexyl-4-(2-fluoro-3-methoxyphenyl)pyrimidin-2- amine; 4-(3-chlorophenyl)-N-((lR,4R)-4-methylcyclohexyl)pyrimidin-2-amine; N-cyclohexyl- 4-(3-fluorophenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3-isopropoxyphenyl)-pyrimidin-2- amine; N-cyclohexyl-4-(4-ethoxy-3-fluorophenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3,5- difluorophenyl)pyrimidin-2-amine; N-cyclohexyl-4-(2,3-dihydrobenzo[b][l,4]dioxin-6- yl)pyrimidin-2-amine; N-cyclohexyl-4-(2,3-dihydrobenzofuran-5-yl)pyrimidin-2-amine; 4-(4- chlorophenyl)-N-cyclohexylpyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)-N-(4,4- dimethylcyclohexyl)pyrimidin-2-amine; 3-(2-(cyclohexylamino)pyrimidin-4-yl)benzonitrile; A- (5-chloro-2-methoxyphenyl)-N-cycloheptylpyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)- N-cyclopentylpyrimidin-2-amine; 4-(5-chloro-2-methoxyphenyl)-N-phenylpyrimidin-2-amine; 4-(benzo[d] [ 1 ,3]dioxol-5-yl)-N-cyclohexylpyrimidin-2-amine; 4-(3-chlorophenyl)-N-((l S,4S)- 4-methylcyclohexyl)pyrimidin-2-amine; N-cyclohexyl-4-(4-fluoro-3- methoxyphenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3,5-dichlorophenyl)pyrimidin-2-amine; N-cycloheptyl-4-(3-isopropoxyphenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3- (methylsulfonyl)phenyl)pyrimidin-2-amine; N-cyclohexyl-4-(5 -fluoro-2- methoxyphenyl)pyrimidin-2-amine; 4-(3-chlorophenyl)-N-cycloheptylpyrimidin-2-amine; N- cyclopentyl-4-(3-isopropoxyphenyl)pyrimidin-2-amine; (4-(4-(5-chloro-2- methoxyphenyl)pyrimidin-2-ylamino)piperidin- 1 -yl)(phenyl)methanone; N-cyclohexyl-4-(5 - fluoropyridin-3-yl)pyrimidin-2-amine; 1 -(4-(4-(5-chloro-2-methoxyphenyl)pyrimidin-2- ylamino)piperidin- 1 -yl)-2,2-dimethylpropan- 1 -one; N-cyclohexyl-4-(2,5- difluorophenyl)pyrimidin-2-amine; N-cyclohexyl-4-(2,5-dimethoxyphenyl)pyrimidin-2-amine; 4-(3-sec-butoxyphenyl)-N-cyclohexylpyrimidin-2-amine; N-cyclohexyl-4-(2-methoxypyridin- 4-yl)pyrimidin-2-amine; 4-(3-isopropoxyphenyl)-N-((lS,4S)-4-methylcyclohexyl)pyrimidin-2- amine; 4-(5-chloro-2-methoxyphenyl)-N-((lS,4S)-4-phenylcyclohexyl)pyrimidin-2-amine; 4- (5-chloro-2-methoxyphenyl)-N-((lr,4r)-4-phenylcyclohexyl)pyrimidin-2-amine; 3-(2- (cyclohexylamino)pyrimidin-4-yl)benzamide; 3 -(2-(cyclohexylamino)pyrimidin-4-yl)-N,N- dimethylbenzamide; 3-(2-(cyclohexylamino)pyrimidin-4-yl)-N-cyclopropylbenzamide; N- cyclohexyl-4-(3-(trifluoromethoxy)phenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3- phenoxyphenyl)pyrimidin-2-amine; N-cyclohexyl-4-(4-phenoxyphenyl)pyrimidin-2-amine; 3- (2-(cyclohexylamino)pyrimidin-4-yl)phenol; N-cyclohexyl-4-(3-(cyclohexyloxy)phenyl)- pyrimidin-2-amine; 4-(2-(cyclohexylamino)pyrimidin-4-yl)phenol; N-cyclohexyl-4-(3- (pyrrolidin-l-ylsulfonyl)phenyl)pyrimidin-2-amine; N-cyclohexyl-4-(3 -(5 -methyl- 1,3,4- oxadiazol-2-yl)phenyl)pyrimidin-2-amine; N-cyclohexyl-4-(4-(5 -methyl- 1 ,3, 4-oxadiazol-2- yl)phenyl)pyrimidin-2-amine; 4-(2-(cyclohexylamino)pyrimidin-4-yl)-N- cyclopropylbenzenesulfonamide; methyl 4-(4-(5-chloro-2-methoxyphenyl)pyrimidin-2- ylamino)piperidine-l-carboxylate; N-cyclohexyl-4-(quinoxalin-6-yl)pyrimidin-2-amine; N- (bicyclo[2.2. l]heptan-2-yl)-4-(5-chloro-2-methoxyphenyl)pyrimidin-2-amine; 4-(5-chloro-2- methoxyphenyl)-N-(l ,2,3,4-tetrahydronaphthalen-2-yl)pyrimidin-2-amine; N-tert-butyl-4-(4- (5-chloro-2-methoxyphenyl)pyrimidin-2-ylamino)piperidine- 1 -carboxamide; 1 -(4-(4-(5-chloro- 2-methoxyphenyl)pyrimidin-2-ylamino)piperidin- 1 -yl)-2-(pyridin-3 -yl)ethanone; 1 -(4-(4-(5 - chloro-2-methoxyphenyl)pyrimidin-2-ylamino)piperidin- 1 -yl)-2-(tetrahydrofuran-3 - yl)ethanone; 4-(4-aminophenyl)-N-cyclohexylpyrimidin-2-amine; N-cyclohexyl-4-(3- morpholinosulfonyl)phenyl) pyrimidin-2-amine; N-(3-(2-(cyclohexylamino)pyrimidin-4- yl)phenyl)cyclopropanesulfonamide; N-(3-(2-(cyclohexylamino)pyrimidin-4- yl)phenyl)cyclopropanecarboxamide; (3-(2-(cyclohexylamino)pyrimidin-4- yl)phenyl)(piperidin- 1 -yl)methanone; 3 -(2-(cyclohexylamino)pyrimidin-4-yl)-N- cyclopropylbenzenesulfonamide; and N-cyclohexyl-4-(2-methylbenzo[d]oxazol-6- yl)pyrimidin-2-amine; or a pharmaceutically acceptable salt or N-oxide thereof.
15. A pharmaceutical composition comprising a compound of any one of claims 1-14 and a pharmaceutically acceptable carrier.
16. A method of treating a condition selected from the group consisting of airway inflammation and cystic fibrosis, comprising administering to a subject in need thereof a therapeutically effective amount of a compound of formula III:
III including a pharmaceutically acceptable salt or N-oxide thereof; wherein: X2 is CR2 or N, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5 or N, and X6 is CR6 or N, where no more than two of X2-X6 are N; L is a bond or a Ci_2alkylidene chain optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of Ci-C6alkyl, C3-C6cycloalkyl, -OCi-C6alkyl, -OC3-C6cycloalkyl, and F; A is Ci-Cioalkyl, C3-Ciocycloalkyl, C3-Cioheterocycloalkyl, or phenyl; each of which is optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of -CF3, -OCi-C6alkyl, -OH, Ci-C6alkyl, Ci-C6alkoxycarbonyl, C3- C6cycloalkyl, aryl, halogen, -C(O)-aryl, -C(O)-heteroaralkyl, -C(O)-Ci-C6alkyl, and -C(O)N(H)(Ci-C6alkyl); Ri and Ri2 are each independently hydrogen, CN, Ci-C6alkyl, -OCi-C6alkyl, C3. locycloalkyl, -OC3_i0cycloalkyl, -OCF3, -OCF2H, -OCH2F, halogen, -NR7Ri0, -NR7COR8, -NR7SO2R95 Or -SO2R9; R2, R3, R4, R5, and R6 are each independently hydrogen, halogen, -CF3, -OH, -OCF3, -OCHF2, -OCH2F, Ci-Cioalkyl, C3-C10cycloalkyl, C3-C8heterocyclyl, heteroaryl, -OC1- Cioalkyl, -O-C3-Ci0cycloalkyl, -OH, -O-aryl, -NR7COR8, -NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, -SO2R9, or -CO2Rn; or any two adjacent variables selected from R2, R3, R4, R5, and R6 can be taken together to form a cycloalkyl, aryl, heteroaryl, or heterocyclyl, each of which is optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl; R7 and Rio each represent independently for each occurrence hydrogen, alkyl, or cycloalkyl, wherein the alkyl or cycloalkyl are optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of halogen, cyano, hydroxy, nitro, and alkoxy; or R7 and Rio are taken together to form a heterocyclyl optionally substituted by one, two, or three substituents selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl; R8 is alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl; R9 represents independently for each occurrence alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl; and Rn is alkyl, alkenyl, alkynyl, amido, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydrogen.
17. The method according to claim 16, wherein Ri and Ri2 are independently hydrogen or methyl.
18. The method according to claim 16 or 17, wherein at least one of R2 and R6 is selected from the group consisting of F, Cl, -CF3, Me, -OMe, -OCF3, and -CO2Et.
19. The method according to any one of claims 16-18, wherein at least one OfR3 and R5 is selected from the group consisting of F, Cl, -OH, -OMe, -OiPr, -Osec-butyl, -OCF3, -Ophenyl, -Ocyclohexyl, -SO2Me, pyrrolidinylsulfonyl, morpholinylsulfonyl, -CON(H)(cyclopropyl), 5- methyl- 1, 3, 4-oxadiazo IyI, -NHSO2Cyclopropyl, and -NHCOcyclopropyl.
20. The method according to any one of claims 16 to 19, wherein R2 is -OMe, and R5 is chloro.
21. The method according to any one of claims 16 to 20, wherein R4 is selected from the group consisting of F, Cl, -OH, -OMe, -OEt, -OiPr, -OCF3, -Ophenyl, -Ocyclohexyl, -NH2, -NMe2, -CN, and 5-methyl-l,3,4-oxadiazolyl.
22. The method according to claim 16 or 18, wherein R3 and R4 are taken together to form a heterocyclyl selected from the group consisting of dioxanyl, dioxolanyl, oxazolyl, pyrazinyl, and thiazolyl.
23. The method according to any one of claims 16 to 20, wherein R4 is hydrogen.
24. The method according to any one of claims 16 to 23, wherein A is C3-Ciocycloalkyl.
25. The method according to any one of claims 16 to 23, wherein A is selected from the group consisting of t-butyl, cyclopentyl, cyclohexyl, 1-methylcyclohexyl, 4-methylcyclohexyl, 4- ethylcyclohexyl, 4-phenylcyclohexyl, 4,4-difluorocyclohexyl, 4,4-dimethylcyclohexyl, cycloheptyl, bicyclo[2.2.1]heptan-2-yl, adamantanyl, and 1,2,3,4-tetrahydronaphthalenyl.
26. The method according to any one of claims 16 to 23, wherein A is cis-4-methylcyclohexyl.
27. The method according to claim 26, wherein R2 is fluoro.
28. The method according to any one of claims 16 to 27, wherein X2 is CR2, X3 is CR3, X4 is CR4, X5 is CR5, and X6 is CR6.
29. The method according to any one of claims 16-28, wherein L is a bond.
30. The method according to any one of claims 16-29, wherein the subject is human.
31. A method of modulating the activity of a cystic fibrosis transmembrance regulator protein, comprising exposing a cystic fibrosis transmembrance regulator protein to a compound of Formula III:
III including a pharmaceutically acceptable salt or N-oxide thereof; wherein: X2 is CR2 or N, X3 is CR3 or N, X4 is CR4 or N, X5 is CR5 or N, and X6 is CR6 or N, where no more than two of X2-X6 are N; L is a bond or a Ci_2alkylidene chain optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of Ci-C6alkyl, C3-C6cycloalkyl, -OCrC6alkyl, -OC3-C6cycloalkyl, and F; A is Ci-Cioalkyl, C3-Ciocycloalkyl, C3-Cioheterocycloalkyl, or phenyl; each of which is optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of -CF3, -OCi-C6alkyl, -OH, Ci-C6alkyl, Ci-C6alkoxycarbonyl, C3- Cecycloalkyl, aryl, halogen, -C(O)-aryl, -C(O)-heteroaralkyl, -C(O)-Ci-C6alkyl, and -C(O)N(H)(Ci-C6alkyl); Ri and Ri2 are each independently hydrogen, CN, Ci-C6alkyl, -OCi-C6alkyl, C3. locycloalkyl, -OC3_i0cycloalkyl, -OCF3, -OCF2H, -OCH2F, halogen, -NR7Ri0, -NR7COR8, -NR7SO2R9, or -SO2R9; R2, R3, R4, R5, and R6 are each independently hydrogen, halogen, -CF3, -OH, -OCF3, -OCHF2, -OCH2F, Ci-Cioalkyl, C3-Ci0cycloalkyl, C3-C8heterocyclyl, heteroaryl, -OC1- Cioalkyl, -O-C3-Ci0cycloalkyl, -OH, -O-aryl, -NR7COR8, -NR7SO2R9, -CONR7Ri0, -SO2NR7Ri0, -CN, aryl, -Oaryl, heteroaryl, -NR7Ri0, -SO2R9, or -CO2Rn; or any two adjacent variables selected from R2, R3, R4, R5, and R6 can be taken together to form a cycloalkyl, aryl, heteroaryl, or heterocyclyl, each of which is optionally substituted by one, two, or three substituents independently, for each occurrence, selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl; R7 and Rio each represent independently for each occurrence hydrogen, alkyl, or cycloalkyl, wherein the alkyl or cycloalkyl are optionally substituted with one or two substituents independently, for each occurrence, selected from the group consisting of halogen, cyano, hydroxy, nitro, and alkoxy; or R7 and Rio are taken together to form a heterocyclyl optionally substituted by one, two, or three substituents selected from the group consisting of alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cyano, cycloalkyl, haloalkyl, halogen, heteroaryl, heterocyclyl, hydroxyl, nitro and sulfonyl; Rs is alkoxy, alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl; R9 represents independently for each occurrence alkyl, alkenyl, alkynyl, amido, amino, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, hydrogen, or hydroxyl; and Rn is alkyl, alkenyl, alkynyl, amido, aryl, cycloalkyl, haloalkyl, heteroaryl, heterocyclyl, or hydrogen.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US22068909P | 2009-06-26 | 2009-06-26 | |
| US61/220,689 | 2009-06-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010151747A1 true WO2010151747A1 (en) | 2010-12-29 |
Family
ID=43386907
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2010/039963 WO2010151747A1 (en) | 2009-06-26 | 2010-06-25 | Pyrimine compounds and methods of making and using same |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2010151747A1 (en) |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8334292B1 (en) | 2010-06-14 | 2012-12-18 | Cystic Fibrosis Foundation Therapeutics, Inc. | Pyrimidine compounds and methods of making and using same |
| WO2013034238A1 (en) * | 2011-09-09 | 2013-03-14 | Merck Patent Gmbh | Benzonitrile derivatives as kinase inhibitors |
| US8513242B2 (en) | 2008-12-12 | 2013-08-20 | Cystic Fibrosis Foundation Therapeutics, Inc. | Pyrimidine compounds and methods of making and using same |
| US8685993B2 (en) | 2010-12-21 | 2014-04-01 | Novartis Ag | Bi-heteroaryl compounds as Vps34 inhibitors |
| WO2014086687A1 (en) | 2012-12-03 | 2014-06-12 | Universita' Degli Studi Di Padova | A cftr corrector for the teatment of genetic disorders affecting striated muscle |
| JP2014526500A (en) * | 2011-09-16 | 2014-10-06 | ノバルティス アーゲー | Heterocyclic compounds for the treatment of cystic fibrosis |
| US9365552B2 (en) | 2010-03-19 | 2016-06-14 | Novartis Ag | Pyridine and pyrazine derivative for the treatment of CF |
| US9951069B1 (en) | 2017-01-11 | 2018-04-24 | Rodin Therapeutics, Inc. | Bicyclic inhibitors of histone deacetylase |
| US10421756B2 (en) | 2015-07-06 | 2019-09-24 | Rodin Therapeutics, Inc. | Heterobicyclic N-aminophenyl-amides as inhibitors of histone deacetylase |
| US10457669B2 (en) | 2015-10-21 | 2019-10-29 | Otsuka Pharmaceutical Co., Ltd. | Benzolactam compounds as protein kinase inhibitors |
| WO2020049189A1 (en) | 2018-09-09 | 2020-03-12 | Qanatpharma Gmbh | Use of cftr modulators for treating cerebrovascular conditions |
| US10919902B2 (en) | 2015-07-06 | 2021-02-16 | Alkermes, Inc. | Hetero-halo inhibitors of histone deacetylase |
| US11142518B2 (en) | 2017-04-20 | 2021-10-12 | Otsuka Pharmaceutical Co., Ltd. | 6-pyrimidin-isoindole derivative as ERK1/2 inhibitor |
| US11225475B2 (en) | 2017-08-07 | 2022-01-18 | Alkermes, Inc. | Substituted pyridines as inhibitors of histone deacetylase |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003056329A2 (en) * | 2001-12-21 | 2003-07-10 | 7Tm Pharma A/S | Use of a signal transduction complex in drug discovery processes |
| US20060079543A1 (en) * | 2004-10-13 | 2006-04-13 | Wyeth | Anilino-pyrimidine analogs |
-
2010
- 2010-06-25 WO PCT/US2010/039963 patent/WO2010151747A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003056329A2 (en) * | 2001-12-21 | 2003-07-10 | 7Tm Pharma A/S | Use of a signal transduction complex in drug discovery processes |
| US20060079543A1 (en) * | 2004-10-13 | 2006-04-13 | Wyeth | Anilino-pyrimidine analogs |
Non-Patent Citations (1)
| Title |
|---|
| NAGARAJAN ET AL.: "IKKbeta inhibitors identification part I: Homology model assisted structure based virtual screening", BIOORGANIC & MEDICINAL CHEMISTRY, vol. 17, no. 7, 1 April 2009 (2009-04-01), pages 2759 - 2766, XP026073626, DOI: doi:10.1016/j.bmc.2009.02.041 * |
Cited By (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8513242B2 (en) | 2008-12-12 | 2013-08-20 | Cystic Fibrosis Foundation Therapeutics, Inc. | Pyrimidine compounds and methods of making and using same |
| US10117858B2 (en) | 2010-03-19 | 2018-11-06 | Novartis Ag | Pyridine and pyrazine derivative for the treatment of CF |
| US9365552B2 (en) | 2010-03-19 | 2016-06-14 | Novartis Ag | Pyridine and pyrazine derivative for the treatment of CF |
| US11911371B2 (en) | 2010-03-19 | 2024-02-27 | Novartis Ag | Pyridine and pyrazine derivative for the treatment of chronic bronchitis |
| USRE46757E1 (en) | 2010-03-19 | 2018-03-20 | Novartis Ag | Pyridine and pyrazine derivative for the treatment of CF |
| US8334292B1 (en) | 2010-06-14 | 2012-12-18 | Cystic Fibrosis Foundation Therapeutics, Inc. | Pyrimidine compounds and methods of making and using same |
| US8685993B2 (en) | 2010-12-21 | 2014-04-01 | Novartis Ag | Bi-heteroaryl compounds as Vps34 inhibitors |
| US8901147B2 (en) | 2010-12-21 | 2014-12-02 | Novartis Ag | Bi-heteroaryl compounds as Vps34 inhibitors |
| EA025038B1 (en) * | 2011-09-09 | 2016-11-30 | Мерк Патент Гмбх | Benzonitrile derivatives as kinase inhibitors |
| US8969335B2 (en) | 2011-09-09 | 2015-03-03 | Merck Patent Gmbh | Benzonitrile derivatives as kinase inhibitors |
| WO2013034238A1 (en) * | 2011-09-09 | 2013-03-14 | Merck Patent Gmbh | Benzonitrile derivatives as kinase inhibitors |
| JP2014526500A (en) * | 2011-09-16 | 2014-10-06 | ノバルティス アーゲー | Heterocyclic compounds for the treatment of cystic fibrosis |
| WO2014086687A1 (en) | 2012-12-03 | 2014-06-12 | Universita' Degli Studi Di Padova | A cftr corrector for the teatment of genetic disorders affecting striated muscle |
| US10919902B2 (en) | 2015-07-06 | 2021-02-16 | Alkermes, Inc. | Hetero-halo inhibitors of histone deacetylase |
| US10421756B2 (en) | 2015-07-06 | 2019-09-24 | Rodin Therapeutics, Inc. | Heterobicyclic N-aminophenyl-amides as inhibitors of histone deacetylase |
| US11858939B2 (en) | 2015-07-06 | 2024-01-02 | Alkermes, Inc. | Hetero-halo inhibitors of histone deacetylase |
| US10457669B2 (en) | 2015-10-21 | 2019-10-29 | Otsuka Pharmaceutical Co., Ltd. | Benzolactam compounds as protein kinase inhibitors |
| US11001575B1 (en) | 2015-10-21 | 2021-05-11 | Otsuka Pharmaceutical Co., Ltd. | Benzolactam compounds as protein kinase inhibitors |
| US11939321B2 (en) | 2015-10-21 | 2024-03-26 | Otsuka Pharmaceutical Co., Ltd. | Benzolactam compounds as protein kinase inhibitors |
| US10696673B2 (en) | 2017-01-11 | 2020-06-30 | Rodin Therapeutics, Inc. | Bicyclic inhibitors of histone deacetylase |
| US10793567B2 (en) | 2017-01-11 | 2020-10-06 | Rodin Therapeutics, Inc. | Bicyclic inhibitors of histone deacetylase |
| US11225479B2 (en) | 2017-01-11 | 2022-01-18 | Alkermes, Inc. | Bicyclic inhibitors of histone deacetylase |
| US11286256B2 (en) | 2017-01-11 | 2022-03-29 | Alkermes, Inc. | Bicyclic inhibitors of histone deacetylase |
| US10519149B2 (en) | 2017-01-11 | 2019-12-31 | Rodin Therapeutics, Inc. | Bicyclic inhibitors of histone deacetylase |
| US9951069B1 (en) | 2017-01-11 | 2018-04-24 | Rodin Therapeutics, Inc. | Bicyclic inhibitors of histone deacetylase |
| US11142518B2 (en) | 2017-04-20 | 2021-10-12 | Otsuka Pharmaceutical Co., Ltd. | 6-pyrimidin-isoindole derivative as ERK1/2 inhibitor |
| US11225475B2 (en) | 2017-08-07 | 2022-01-18 | Alkermes, Inc. | Substituted pyridines as inhibitors of histone deacetylase |
| US11912702B2 (en) | 2017-08-07 | 2024-02-27 | Alkermes, Inc. | Substituted pyridines as inhibitors of histone deacetylase |
| WO2020049189A1 (en) | 2018-09-09 | 2020-03-12 | Qanatpharma Gmbh | Use of cftr modulators for treating cerebrovascular conditions |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2010151747A1 (en) | Pyrimine compounds and methods of making and using same | |
| WO2010068863A2 (en) | Pyrimidine compounds and methods of making and using same | |
| WO2011008931A2 (en) | Arylpyrimidine compounds and combination therapy comprising same for treating cystic fibrosis & related disorders | |
| AU2019271928B2 (en) | Prodrugs of pyridone amides useful as modulators of sodium channels | |
| US11919887B2 (en) | Substituted tetrahydrofurans as modulators of sodium channels | |
| CN108164457B (en) | Pyridone amides as sodium channel modulators | |
| US20230062053A1 (en) | Carboxamides as modulators of sodium channels | |
| CN108558889A (en) | Bu Luomo structural domain inhibitor | |
| CN104684899A (en) | Substituted sulfonamide compounds | |
| EA019085B1 (en) | 1,3-disubstituted 4-(aryl-x-phenyl)-1h-pyridin-2-ones | |
| EP3344632A1 (en) | Heterobicyclic pyrimidinone compounds and their use in the treatment of medical disorders | |
| JP2010530405A (en) | Condensed quinoline derivatives useful as GABA modulators | |
| AU2014230215A1 (en) | Acylaminocycloalkyl compounds suitable for treating disorders that respond to modulation of dopamine D3 receptor | |
| EP2445877B1 (en) | Bicyclic compounds and methods of making and using same | |
| CN109496213A (en) | Spiro-compound and its preparation and application | |
| WO2021097054A1 (en) | 6-membered heteroarylaminosulfonamides for treating diseases and conditions mediated by deficient cftr activity | |
| IL308953A (en) | N-(hydroxyalkyl (hetero)aryl) tetrahydrofuran carboxamides as modulators of sodium channels | |
| EP4347033A1 (en) | Hydroxy and (halo)alkoxy substituted tetrahydrofurans as modulators of sodium channels | |
| CN103857670B (en) | 2-oxo-piperidinyl derivatives | |
| US8334292B1 (en) | Pyrimidine compounds and methods of making and using same | |
| PT1659112E (en) | Arylpiperazuine derivatives as selective ligand for the dopamine d3 receptor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10792712 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10792712 Country of ref document: EP Kind code of ref document: A1 |