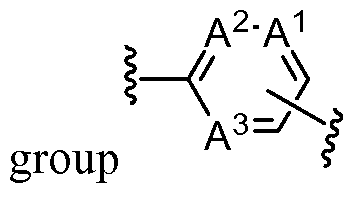WO2010141768A2 - Polycyclic antagonists of lysophosphatidic acid receptors - Google Patents
Polycyclic antagonists of lysophosphatidic acid receptors Download PDFInfo
- Publication number
- WO2010141768A2 WO2010141768A2 PCT/US2010/037316 US2010037316W WO2010141768A2 WO 2010141768 A2 WO2010141768 A2 WO 2010141768A2 US 2010037316 W US2010037316 W US 2010037316W WO 2010141768 A2 WO2010141768 A2 WO 2010141768A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- substituted
- compound
- unsubstituted
- phenyl
- isoxazol
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
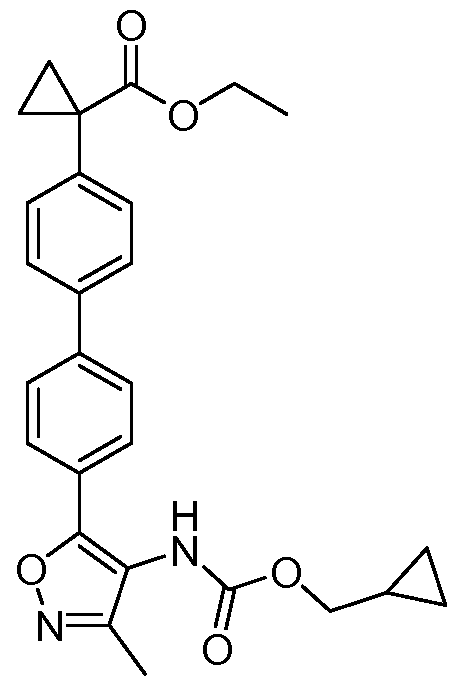
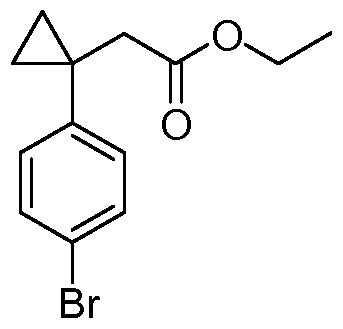
- 0 Cc1n[o]c(C)c1* Chemical compound Cc1n[o]c(C)c1* 0.000 description 15
- WGSQKRJZCUVTMJ-UHFFFAOYSA-N CC(C)(c1ccccc1)OC(Nc1c(-c(cc2)ccc2Br)[o]nc1C)=O Chemical compound CC(C)(c1ccccc1)OC(Nc1c(-c(cc2)ccc2Br)[o]nc1C)=O WGSQKRJZCUVTMJ-UHFFFAOYSA-N 0.000 description 1
- ZNDXSPMPMUZYQU-UHFFFAOYSA-N CC1(CCCC1)OC(Nc1c(-c(cc2)ccc2-c2ccc(C3(CC3)C(O)=O)cc2)[o]nc1C)=O Chemical compound CC1(CCCC1)OC(Nc1c(-c(cc2)ccc2-c2ccc(C3(CC3)C(O)=O)cc2)[o]nc1C)=O ZNDXSPMPMUZYQU-UHFFFAOYSA-N 0.000 description 1
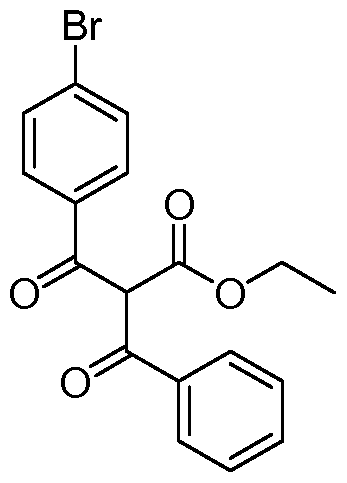
- VCZJJXIKTPSTOF-UHFFFAOYSA-N CCC(C(C(c(cc1)ccc1Br)=O)C(OC)=O)=O Chemical compound CCC(C(C(c(cc1)ccc1Br)=O)C(OC)=O)=O VCZJJXIKTPSTOF-UHFFFAOYSA-N 0.000 description 1
- GEZLURMHGQNNBP-UHFFFAOYSA-N CCC1OC1C(C1)C2C1CCC2 Chemical compound CCC1OC1C(C1)C2C1CCC2 GEZLURMHGQNNBP-UHFFFAOYSA-N 0.000 description 1
- QJUJVTPYIMWKHX-DSQCSBQDSA-N CCOC(C(C1)(C1[Si+](C)(C)C)c(cc1)ccc1-c(cc1)ccc1-c([o]nc1C)c1NC(O[C@H](C)C1CC1)=O)=O Chemical compound CCOC(C(C1)(C1[Si+](C)(C)C)c(cc1)ccc1-c(cc1)ccc1-c([o]nc1C)c1NC(O[C@H](C)C1CC1)=O)=O QJUJVTPYIMWKHX-DSQCSBQDSA-N 0.000 description 1
- UJIFZFHUSUFJTE-RHKGEPBNSA-N CCOC(C(C1)C1c(cc1)ccc1-c(cc1)ccc1-c([o]nc1C)c1NC(O[C@H](C)c1ccccc1)=O)=O Chemical compound CCOC(C(C1)C1c(cc1)ccc1-c(cc1)ccc1-c([o]nc1C)c1NC(O[C@H](C)c1ccccc1)=O)=O UJIFZFHUSUFJTE-RHKGEPBNSA-N 0.000 description 1
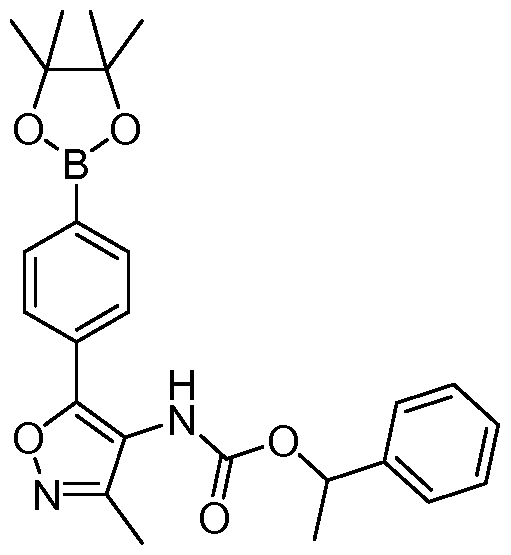
- HJZXPOFSSGHGRO-UHFFFAOYSA-N CCOC(C(C1)C1c1ccc(B2OC(C)(C)C(C)(C)O2)cc1)=O Chemical compound CCOC(C(C1)C1c1ccc(B2OC(C)(C)C(C)(C)O2)cc1)=O HJZXPOFSSGHGRO-UHFFFAOYSA-N 0.000 description 1
- FBESNYWIDDWGCA-GOSISDBHSA-N CCOC(C1(CC1)c(cc1)ccc1-c(cc1)ccc1-c([o]nc1C)c1NC(O[C@H](C)C1CC1)=O)=O Chemical compound CCOC(C1(CC1)c(cc1)ccc1-c(cc1)ccc1-c([o]nc1C)c1NC(O[C@H](C)C1CC1)=O)=O FBESNYWIDDWGCA-GOSISDBHSA-N 0.000 description 1
- DQUPQENRTDQBLK-HXUWFJFHSA-N CCOC(C1(CC1)c(cc1)ccc1-c(cc1)ccc1-c([o]nc1C)c1NC(O[C@H](C)c1cccc(C(F)(F)F)c1)=O)=O Chemical compound CCOC(C1(CC1)c(cc1)ccc1-c(cc1)ccc1-c([o]nc1C)c1NC(O[C@H](C)c1cccc(C(F)(F)F)c1)=O)=O DQUPQENRTDQBLK-HXUWFJFHSA-N 0.000 description 1
- UHJGNMJYLBTLCM-UHFFFAOYSA-N CCOC(C1(CC1)c(cc1)ccc1Oc(cc1)ccc1-c([o]nc1C)c1NC(OC(C)c1ccccc1)=O)=O Chemical compound CCOC(C1(CC1)c(cc1)ccc1Oc(cc1)ccc1-c([o]nc1C)c1NC(OC(C)c1ccccc1)=O)=O UHJGNMJYLBTLCM-UHFFFAOYSA-N 0.000 description 1
- IGHBVTWJGNIBBY-UHFFFAOYSA-N CCOC(CC1(CC1)c(cc1)ccc1Br)=O Chemical compound CCOC(CC1(CC1)c(cc1)ccc1Br)=O IGHBVTWJGNIBBY-UHFFFAOYSA-N 0.000 description 1
- SZQREWWARAFVLC-OAQYLSRUSA-N CCc1n[o]c(-c(cc2)ccc2-c2ccc(C3(CC3)C(OCC)=O)cc2)c1NC(O[C@H](C)c1ccccc1)=O Chemical compound CCc1n[o]c(-c(cc2)ccc2-c2ccc(C3(CC3)C(OCC)=O)cc2)c1NC(O[C@H](C)c1ccccc1)=O SZQREWWARAFVLC-OAQYLSRUSA-N 0.000 description 1
- MJJFPKWHYNJAKG-UHFFFAOYSA-N COc(cc(cc1)Br)c1C(Cl)=O Chemical compound COc(cc(cc1)Br)c1C(Cl)=O MJJFPKWHYNJAKG-UHFFFAOYSA-N 0.000 description 1
- CVMICEFROSSHQB-YFKPBYRVSA-N C[C@@H](C1CC1)OC(NC)=O Chemical compound C[C@@H](C1CC1)OC(NC)=O CVMICEFROSSHQB-YFKPBYRVSA-N 0.000 description 1
- SITXNCHAZUHTTN-QMMMGPOBSA-N C[C@@H](c1ccccc1)OC(NC)=O Chemical compound C[C@@H](c1ccccc1)OC(NC)=O SITXNCHAZUHTTN-QMMMGPOBSA-N 0.000 description 1
- YIZLYJGOEIDGMD-ZDUSSCGKSA-N C[C@@H](c1ccccc1)OC(Nc1c(-c(cc2)ccc2Br)[o]nc1C)=O Chemical compound C[C@@H](c1ccccc1)OC(Nc1c(-c(cc2)ccc2Br)[o]nc1C)=O YIZLYJGOEIDGMD-ZDUSSCGKSA-N 0.000 description 1
- CVMICEFROSSHQB-RXMQYKEDSA-N C[C@H](C1CC1)OC(NC)=O Chemical compound C[C@H](C1CC1)OC(NC)=O CVMICEFROSSHQB-RXMQYKEDSA-N 0.000 description 1
- USQHGVWPVLVNCN-SNVBAGLBSA-N C[C@H](C1CC1)OC(Nc1c(-c(cc2)ccc2Br)[o]nc1C)=O Chemical compound C[C@H](C1CC1)OC(Nc1c(-c(cc2)ccc2Br)[o]nc1C)=O USQHGVWPVLVNCN-SNVBAGLBSA-N 0.000 description 1
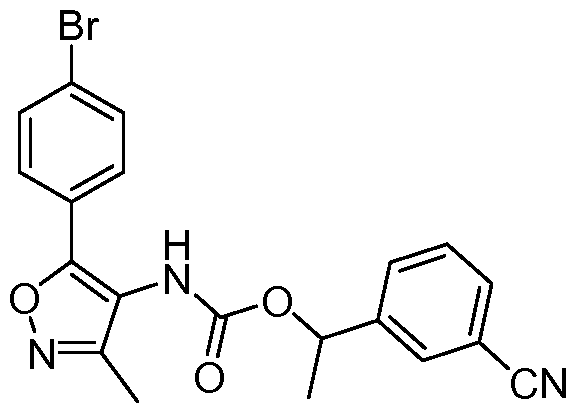
- XGAVOODMMBMCKV-SSDOTTSWSA-N C[C@H](c(cc1)ccc1C#N)O Chemical compound C[C@H](c(cc1)ccc1C#N)O XGAVOODMMBMCKV-SSDOTTSWSA-N 0.000 description 1
- OQEHWVRLAYOJNP-LJQANCHMSA-N C[C@H](c(cccc1)c1C#N)OC(Nc1c(-c(cc2)ccc2-c2ccc(C3(CC3)C(O)=O)cc2)[o]nc1C)=O Chemical compound C[C@H](c(cccc1)c1C#N)OC(Nc1c(-c(cc2)ccc2-c2ccc(C3(CC3)C(O)=O)cc2)[o]nc1C)=O OQEHWVRLAYOJNP-LJQANCHMSA-N 0.000 description 1
- YNVXCOKNHXMBQC-ZCFIWIBFSA-N C[C@H](c1cccc(C(F)(F)F)c1)O Chemical compound C[C@H](c1cccc(C(F)(F)F)c1)O YNVXCOKNHXMBQC-ZCFIWIBFSA-N 0.000 description 1
- GFZBMGYFPQFACY-LJQANCHMSA-N C[C@H](c1cccc(C(F)(F)F)c1)OC(Nc1c(-c(cc2)ccc2-c2ccc(C3(CC3)C(NS(C)(=O)=O)=O)cc2)[o]nc1C)=O Chemical compound C[C@H](c1cccc(C(F)(F)F)c1)OC(Nc1c(-c(cc2)ccc2-c2ccc(C3(CC3)C(NS(C)(=O)=O)=O)cc2)[o]nc1C)=O GFZBMGYFPQFACY-LJQANCHMSA-N 0.000 description 1
- SVALBCHNKAJLSW-LJQANCHMSA-N C[C@H](c1cccc(C(F)(F)F)c1)OC(Nc1c(-c(cc2)ccc2-c2ccc(CC3(CC3)C(O)=O)cc2)[o]nc1C)=O Chemical compound C[C@H](c1cccc(C(F)(F)F)c1)OC(Nc1c(-c(cc2)ccc2-c2ccc(CC3(CC3)C(O)=O)cc2)[o]nc1C)=O SVALBCHNKAJLSW-LJQANCHMSA-N 0.000 description 1
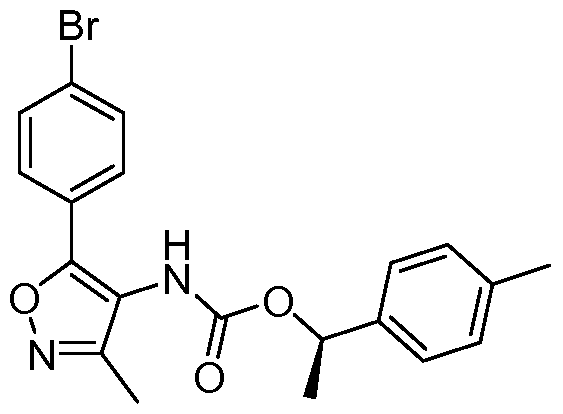
- MTKYDRFFKYSTSW-GFCCVEGCSA-N C[C@H](c1cccc(C(F)(F)F)c1)OC(Nc1c(-c(ccc(Br)c2)c2OC)[o]nc1C)=O Chemical compound C[C@H](c1cccc(C(F)(F)F)c1)OC(Nc1c(-c(ccc(Br)c2)c2OC)[o]nc1C)=O MTKYDRFFKYSTSW-GFCCVEGCSA-N 0.000 description 1
- ZUBPFBWAXNCEOG-SSDOTTSWSA-N C[C@H](c1cccc(OC)c1)O Chemical compound C[C@H](c1cccc(OC)c1)O ZUBPFBWAXNCEOG-SSDOTTSWSA-N 0.000 description 1
- SITXNCHAZUHTTN-MRVPVSSYSA-N C[C@H](c1ccccc1)OC(NC)=O Chemical compound C[C@H](c1ccccc1)OC(NC)=O SITXNCHAZUHTTN-MRVPVSSYSA-N 0.000 description 1
- IRHZOCZBTRHKAO-HXUWFJFHSA-N C[C@H](c1ccccc1)OC(Nc1c(-c(cc2)ccc2-c2ccc(C3(CCC3)C(O)=O)cc2)[o]nc1C)=O Chemical compound C[C@H](c1ccccc1)OC(Nc1c(-c(cc2)ccc2-c2ccc(C3(CCC3)C(O)=O)cc2)[o]nc1C)=O IRHZOCZBTRHKAO-HXUWFJFHSA-N 0.000 description 1
- XCCMYOKFTITMTC-HXUWFJFHSA-N C[C@H](c1ccccc1)OC(Nc1c(-c(cc2)ccc2-c2ccc(CC3(CC3)C(O)=O)cc2)[o]nc1C)=O Chemical compound C[C@H](c1ccccc1)OC(Nc1c(-c(cc2)ccc2-c2ccc(CC3(CC3)C(O)=O)cc2)[o]nc1C)=O XCCMYOKFTITMTC-HXUWFJFHSA-N 0.000 description 1
- URHNEVXQWRVMJO-UHFFFAOYSA-N Cc1n[o]c(-c(cc2)ccc2-c2ccc(C3(CC3)C(O)=O)cc2)c1NC(OC(c1ccccc1Cl)=C)=O Chemical compound Cc1n[o]c(-c(cc2)ccc2-c2ccc(C3(CC3)C(O)=O)cc2)c1NC(OC(c1ccccc1Cl)=C)=O URHNEVXQWRVMJO-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D261/00—Heterocyclic compounds containing 1,2-oxazole or hydrogenated 1,2-oxazole rings
- C07D261/02—Heterocyclic compounds containing 1,2-oxazole or hydrogenated 1,2-oxazole rings not condensed with other rings
- C07D261/06—Heterocyclic compounds containing 1,2-oxazole or hydrogenated 1,2-oxazole rings not condensed with other rings having two or more double bonds between ring members or between ring members and non-ring members
- C07D261/10—Heterocyclic compounds containing 1,2-oxazole or hydrogenated 1,2-oxazole rings not condensed with other rings having two or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D261/14—Nitrogen atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/42—Oxazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/04—Drugs for skeletal disorders for non-specific disorders of the connective tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/10—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a carbon chain containing aromatic rings
Definitions
- Lysophospholipids are membrane-derived bioactive lipid mediators. Lysophospholipids affect fundamental cellular functions that include proliferation, differentiation, survival, migration, adhesion, invasion, and morphogensis. These functions influence many biological processes that include, but are not limited to, neurogensis, angiogenesis, wound healing, fibrosis, immunity, and carcinogenesis.
- Lysophosphatidic acid is a lysophospholipid that has been shown to act through sets of specific G protein-coupled receptors (GPCRs) in an autocrine and paracrine fashion. LPA binding to its cognate GPCRs (LPAi, LPA 2 , LPA 3 , LPA 4 , LPA 5 , LPA 6 ) activates intracellular signaling pathways to produce a variety of biological responses. Antagonists of the LPA receptors find use in the treatment of diseases, disorders or conditions in which LPA plays a role.
- GPCRs G protein-coupled receptors
- the compounds of Formula (I), (II), (III), (IV) and (V) are useful for the treatment of fibrosis of organs (liver, kidney, lung, heart and the like), liver diseases (acute hepatatis, chronic hepatitis, liver fibrosis, liver cirrhosis, portal hypertension, regenerative failure, non-alcoholic steatohepatitis (NASH), liver hypofunction, hepatic blood flow disorder, and the like), cell proliferative disease (cancer (solid tumor, solid tumor metastasis, vascular fibroma, myeloma, multiple myeloma, Kaposi's sarcoma, leukemia, chronic lymphocytic leukemia (CLL) and the like) and invasive metastasis of cancer cell, and the like), inflammatory disease (psoriasis, nephropathy, pneumonia and the like), gastrointestinal tract disease (irritable bowel syndrome (IBS), inflammatory bowel disease
- IBS irritable
- compounds of Formula (I), (II), (III), (IV) and (V) are antagonists of at least one of the LPA receptors selected from LPAi, LPA 2 , LPA 3 , LPA 4 LPA 5 and LPA 6 .
- compounds of Formula (I), (II), (III), (IV) and (V) are antagonists of LPAi.
- compounds of Formula (I), (II), (III), (IV) and (V) are antagonists of LPAi and/or LPA 3 .
- Compounds of Formula (I), (II), (III), (IV) and (V) are used in the treatment of diseases, disorders, or conditions in which activation of at least one LPA receptor by LPA contributes to the symptomology or progression of the disease, disorder or condition. These diseases, disorders, or conditions may arise from one or more of a genetic, iatrogenic, immunological, infectious, metabolic, oncological, toxic, surgical, and/or traumatic etiology.
- the methods, compounds, pharmaceutical compositions, and medicaments described herein comprise antagonists of LPA receptors.
- the methods, compounds, pharmaceutical compositions, and medicaments described herein comprise antagonists of LPAi.
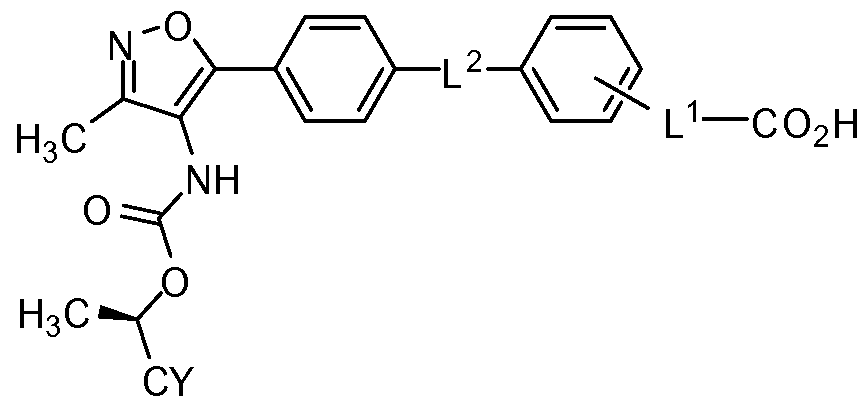
- provided herein is a compound having the structure of Formula (I), pharmaceutically acceptable salt, pharmaceutically acceptable solvate, or prodrug thereof:
- R D is H or C 1 -
- L 1 is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted - alkylene-cycloalkylene, or a substituted or unsubstituted -cycloalkylene-alkylene-;
- ring A is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring A is substituted then ring A is substituted with 1, 2, 3, or 4 R A ;
- ring B is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring B is substituted then ring
- R 2 is H, Ci-C 4 alkyl, or Ci-C 4 fluoroalkyl
- R 3 is H, Ci-C 4 alkyl, C 3 -C 6 cycloalkyl, Ci-C 4 fluoroalkyl or a substituted or unsubstituted phenyl
- R 4 is -NR 7 C(O)0C(R 8 )(R 8a )-CY, or -NR 7 C(O)O-CY;
- R 7 is H or C r C 4 alkyl;
- R 8 is H, Ci-C 4 alkyl, C 3 -C 7 cycloalkyl or Ci-C 4 fluoroalkyl;
- R 8a is H or Ci-C 4 alkyl
- CY is a substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, a substituted or unsubstituted heterocycloalkyl, a substituted or unsubstituted aryl, a substituted or unsubstituted benzyl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then CY is substituted with 1, 2, 3, or 4 R c ; or
- R 10 is d-C 6 alkyl, C 3 - Cecycloalkyl, or a substituted or unsubstituted phenyl. In some embodiments, R 10 is Ci-C ⁇ alkyl or a substituted or unsubstituted phenyl. In some embodiments, R D is H, methyl, ethyl, propyl, isopropyl, n-butyl, iso-butyl, or tert-butyl.
- R 8 is H, Ci-C 4 alkyl, or Ci-C 4 fluoroalkyl. In some embodiments, R 8 is H or -CH 3 . In some embodiments, R 8 is-CH 3 . [0014] In some embodiments, R 8a is H Or -CH 3 . In some embodiments, R 8a is H.
- CY is a substituted or unsubstituted C 3 -Ciocycloalkyl, a substituted or unsubstituted C 2 -Ci 0 heterocycloalkyl, a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then CY is substituted with 1, 2, or 3 R c ; or R 8 and CY are taken together with the carbon atom to which they are attached to form a substituted or unsubstituted carbocycle or a substituted or unsubstituted heterocycle.
- CY is a substituted or unsubstituted heteroaryl, wherein if CY is substituted then CY is substituted with 1, 2, or 3 R c .
- CY is a substituted or unsubstituted monocyclic heteroaryl, wherein if CY is substituted then CY is substituted with 1, 2, or 3 R c .
- CY is a substituted or unsubstituted 6-membered heteroaryl, wherein if CY is substituted then CY is substituted with 1 , 2, or 3 R c .
- L 1 is -C 3 -C 6 cycloalkylene-, -Ci-C 4 alkylene-C 3 -C 6 cycloalkylene-, Or -C 3 - C6cycloalkylene-Ci-C 4 alkylene-;
- L 2 is absent, Ci-C ⁇ alkylene, Ci-Cefluoroalkylene, -C 1 - C 6 alkylene-O-, -Ci-C 3 alkylene-O-Ci-C 3 alkylene
- ring A is a substituted or unsubstituted phenylene, a substituted or unsubstituted napthylene, or a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring A is substituted then ring A is substituted with 1 or 2 R A .
- ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring A is substituted then ring A is substituted with 1 or 2 R A .
- ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted pyridinylene, where if ring A is substituted then ring A is substituted with 1 or 2 R A .
- ring B is a substituted or unsubstituted monocyclic cycloalkylene, a substituted or unsubstituted bicyclic cycloalkylene, a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted bicyclic heterocycloalkylene, a substituted or unsubstituted phenylene, a substituted or unsubstituted naphthylene, a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then ring B is substituted with 1 or 2 R B .
- each of R A , R B , and R c are independently selected from halogen, -CN, -NO 2 , -OH, -OR 10 , -N(R 9 ) 2 , d-C 4 alkyl, Ci-C 4 fluoroalkyl, Ci-C 4 fluoroalkoxy, C 1 - C 4 alkoxy, and Ci-C 4 heteroalkyl.
- ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring A is substituted then ring A is substituted with 1 or 2 R A ;
- ring B is a substituted or unsubstituted monocyclic cycloalkylene, a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene, where if ring B is substituted then ring B is substituted with 1 or 2 R B ;
- CY is a substituted or unsubstituted C3-Cecycloalkyl, a substituted or unsubstituted phen
- each of R A , R B , and R c are independently selected from halogen, -CN, -OH, C r C 4 alkyl, C r C 4 fluoroalkyl, C r C 4 fluoroalkoxy, C r C 4 alkoxy, and C 1 - C 4 heteroalkyl.
- L 1 is cyclopropyl- 1 , 1 -diyl, cyclopropyl-l,2-diyl, cycloprop-2- enyl-l,l-diyl, cyclobutyl- 1,1 -diyl, cyclopentyl- 1 , 1 -diyl, cyclohexyl- 1 , 1 -diyl, -CH 2 C(CH 2 CH 2 )- or -C(CH 2 CH 2 )CH 2 -;
- L 2 is absent, -CH 2 -, -CH 2 O-, -OCH 2 -, -CH 2 S-, -SCH 2 -, -CH 2 NH-, - NHCH 2 -, -O-, -S-, or -NH-;
- ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocycl
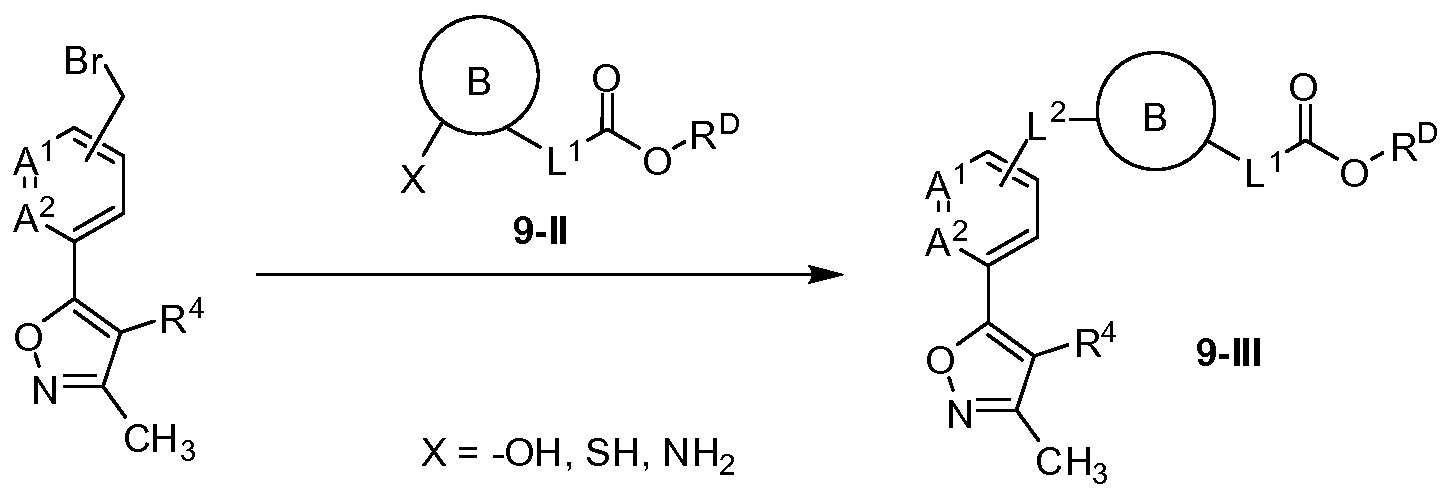
- the compound of Formula (I) has the structure of Formula (II):
- each of A 1 , A 2 , and A 3 is CH or CR A ; or one of A 1 , A 2 , and A 3 is N. In some embodiments, each of A 1 , A 2 , and A 3 is CH or CR A . In some embodiments, one of A 1 , A 2 , and A 3 is N. In some embodiments, each of A 1 , A 2 , and A 3 is CH.
- ring A is a substituted or unsubstituted phen-l,3-ylene, or a substituted or unsubstituted phen- 1 ,4-ylene, where if ring A is substituted then ring A is substituted with R A . In some embodiments, ring A is a substituted or unsubstituted phen- 1 ,4- ylene, where if ring A is substituted then ring A is substituted with R A .
- ring B is a substituted or unsubstituted phen-l,2-ylene, a substituted or unsubstituted phen-l,3-ylene, or a substituted or unsubstituted phen-l,4-ylene, where if ring B is substituted then ring B is substituted with R B .
- ring B is a substituted or unsubstituted phen-l,3-ylene, or a substituted or unsubstituted phen- 1,4- ylene, where if ring B is substituted then ring B is substituted with R B .
- ring B is a substituted or unsubstituted phen- 1 ,4-ylene, where if ring B is substituted then ring B is substituted with R B .
- each of A 1 , A 2 , and A 3 is CH or CR A ; or one of A 1 , A 2 , and A 3 is
- each R A is independently selected from halogen, -OH, Ci-C 4 alkyl, Ci-C 4 fluoroalkyl, C 1 -
- R D is H, -CH 3 , or -CH 2 CH 3 ;
- R 8 is H, -CH 3 , -CH 2 CH 3 or -CF 3 .
- R 8 is H or -CH 3 .
- R 8 is-CH 3 .
- R D is H,
- R 7 is H;
- R 8 is H, C r C 4 alkyl or C 1 -
- R 10 is a Ci-C ⁇ alkyl, Ci-Cefiuoroalkyl, C 3 -Cecycloalkyl, or a substituted or unsubstituted phenyl.
- R 1 is -CO 2 H; each R A is independently selected from halogen, -
- R 3 is H, -CH 3 or -CH 2 CH 3 ;
- R 7 is H; and
- R 8 is -CH 3 or -
- R 7 is H.
- R 8 is H, Ci-C 4 alkyl or C 1 - C 4 fluoroalkyl.
- R 8 is CrC 4 alkyl or C 1 -C 4 fluoroalkyl.
- R 8 is H, -CH 3 or -CF 3 .
- R 8 is -CH 3 or -CF 3 .
- R 8 is H or -CH 3 .
- R 8 is -CH 3 .
- CY is a substituted or unsubstituted cycloalkyl, a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then CY is substituted with 1 or 2 R c .
- CY is C 3 -Cecycloalkyl, a substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 0-4 N atoms, wherein if CY is substituted then CY is substituted with 1 or 2 R c .
- CY is CrC ⁇ alkyl, substituted or unsubstituted C 3 -Cecycloalkyl, a substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 0-4 N atoms, wherein if CY is substituted then CY is substituted with 1 or 2 R c .
- ring A is a substituted or unsubstituted phen- 1 ,4-ylene, where if ring A is substituted then ring A is substituted with R A ;
- ring B is a substituted or unsubstituted phenyl- 1, 3 -ene or a substituted or unsubstituted phenyl- 1 ,4-ene, where if ring B is substituted then ring B is substituted with R B .
- A is unsubstituted or monosubstituted with R A .
- B is unsubstituted or monosubstituted with R B .
- CY is unsubstituted or monosubstituted with R c .
- L 2 is a absent, Ci-C 6 alkylene, Ci-C 6 fluoroalkylene, -C 1 -
- L 1 is cyclopropyl- 1 , 1 -diyl, cyclopropyl-l,2-diyl, cycloprop-2- enyl-l,l-diyl, cyclobutyl- 1,1 -diyl, cyclopentyl- 1 , 1 -diyl, cyclohexyl- 1 , 1 -diyl, -CH 2 C(CH 2 CH 2 )- or -C(CH 2 CH 2 )CH 2 -.
- L 1 is cyclopropyl- 1,1 -diyl, cyclopropyl- 1 ,2-diyl, cyclobutyl- 1,1 -diyl, cyclopentyl- 1 , 1 -diyl or cyclohexyl- 1,1 -diyl. In some embodiments, L 1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl- 1 , 1 -diyl, cyclopentyl- 1 , 1 -diyl or cyclohexyl- 1 , 1 -diyl.
- ring B is a substituted or unsubstituted cyclopentylene, a substituted or unsubstituted cyclopentenylene, a substituted or unsubstituted cyclohexylene, a substituted or unsubstituted cyclohexenylene, or a substituted or unsubstituted phenylene, where if ring B is substituted then ring B is substituted with 1 or 2 R B .
- ring B is a substituted or unsubstituted phenylene, where if ring
- ring B is substituted then ring B is substituted with 1 or 2 R B .
- ring B is a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted bicyclic heterocycloalkylene, a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then ring B is substituted with 1 or 2 R B .
- ring B is a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then ring B is substituted with 1 or 2 R B .
- ring B is a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring B is substituted then ring B is substituted with 1 or 2 R B .
- ring B is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring B is substituted then ring B is substituted with 1 or 2 R B .
- R 4 is H or H
- R 8 is -CH3 or -CF3. In some embodiments, R 8 is -CH3. In some embodiments, R 8 is H or -CH3. In some embodiments, R 8 is H.
- R 4 is . In some embodiments, R 4 is
- R 4 is H . In some embodiments, R 4 is
- CY is a substituted or unsubstituted phenyl, where if CY is substituted then CY is substituted with 1 or 2 R c ; each R c is independently selected from halogen, Ci-C 4 alkyl, Ci-C 4 fluoroalkyl, Ci-C 4 fluoroalkoxy, Ci-C 4 alkoxy, and Ci-C 4 heteroalkyl. [0063] In some embodiments, each R c is independently selected from F, Cl, -CH 3 , -CF 3 , - OCF 3 , -OCH 3 . [0064] In some embodiments, CY is phenyl, 2-fluorophenyl or 2-chloro-phenyl.
- L 2 is absent; ring A is a substituted or unsubstituted phen-1,4- ylene, where if ring A is substituted then ring A is substituted with R A ; ring B is a substituted or unsubstituted phen-l,4-ylene, where if ring B is substituted then ring B is substituted with R B ; R 8 is -CH 3 ; each R c is independently selected from F, Cl, Br, I, -CH 3 , -CF 3 , -OH, -OCF 3 , and - OCH 3 .
- R D is H, -CH 3 , or -CH 2 CH 3
- ring A is a substituted or unsubstituted phen- 1 ,4-ylene, where if ring A is substituted then ring A is substituted with R A
- ring B is a substituted or unsubstituted phen- 1,4- ylene, where if ring B is substituted then ring B is substituted with R B
- R 3 is H, or -CH 3
- R 7 is H;
- R 8 is -CH 3
- CY is a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with R c ;
- R 10 is H,
- L 1 is cyclopropyl- 1 , 1 -diyl; L 2 is absent; ring B is a substituted or unsubstituted phen-l,4-ylene, where if ring B is substituted then ring B is substituted with R B ; each R A is independently selected from H, F, Cl, -CH 3 , and -OH; each R B is independently selected from H, F, Cl, -CH 3 , and -OH; each R c is independently selected from H, F, Cl, -CH 3 , - CF 3 , -OH, -OCF 3 , and -OCH 3 .
- the compound of Formula (I) has the structure of Formula (III):
- a 1 is CR A and N;
- R A is H, F, Cl, Br, I, -CH 3 , or -CF 3 ;
- a 4 is CH or N;
- L 1 is C 3 -C 6 cycloalkylene;
- R 2 is H or -CH 3 ;
- CY is C 3 -C 6 cycloalkyl or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with R c ; R c is H, F, Cl, Br, I, -OH, C r C 4 alkyl,
- Ci-C 4 fluoroalkyl Ci-C 4 fluoroalkoxy, or Ci-C 4 alkoxy.
- L 1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl- 1 , 1 -diyl, cyclopentyl-1,1- diyl or cyclohexyl- 1,1 -diyl. In some embodiments, L 1 is cyclopropyl- 1,1 -diyl.
- L 2 is absent, -CH 2 -, -CH 2 O-, -OCH 2 -, -CH 2 S-, -SCH 2 -, -0-, or - S-. In some embodiments, L 2 is absent.
- a 1 is CH. In some embodiments, A 4 is CH. [0072] In some embodiments, CY is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, a substituted or unsubstituted pentenyl, a substituted or unsubstituted cyclohexenyl, or a substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with R c ; R c is F, Cl, -CH 3 , or CF 3 .
- CY is a substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with R c ; R c is F, Cl, -CH 3 , or CF 3 .
- CY is cyclopropyl.
- CY is phenyl.
- CY is phenyl, 2-fluorophenyl or 2-chloro-phenyl.
- L 1 is cyclopropyl- 1 , 1 -diyl; L 2 is absent; CY is cyclopropyl.
- L 1 is cyclopropyl- 1 , 1 -diyl; L 2 is absent; CY is phenyl.
- a 1 is CH; and A 4 is CH.
- the compound of Formula (I) has the structure of Formula (IV):
- a 1 is CR A and N;
- R A is H, F, Cl, Br, I, -CH 3 , or -CF 3 ;
- L 2 is absent, C r C 4 alkylene, C r C 4 heteroalkylene, -O-, -S-, -SO-, -SO 2 -, -NR 2 -, or -
- R 2 is H or -CH 3 ;
- R 8 is H or -CH 3 ;
- CY is Ci-C ⁇ alkyl, C 3 -C 6 cycloalkyl, or substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with R c ;
- R c is H, F, Cl, Br, I, -OH, Ci-C 4 alkyl, Ci-C 4 fluoroalkyl, Ci-C 4 fluoroalkoxy, or Ci-C 4 alkoxy;
- n is 1, 2, or 3.
- a 1 is CR A . In some embodiments, A 1 is CH. [0082] In some embodiments, A 1 is N. [0083] In some embodiments, CY is -CH 3 , -CH 2 CH 3 , -CH 2 CH 2 CH 3 , -CH 2 CH 2 CH 2 CH 3 , - CH(CH 3 ) 2 , -CH(CH 2 CH 3 ) 2 , -CH 2 CH(CH 3 ) 2 , -C(CH 3 ) 3 , -CH 2 (CH 3 ) 3 , C 3 -C 6 cycloalkyl, substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with R c ;
- R c is F, Cl, -CH 3 , or CF 3 .
- n is 1.
- CY is -CH 2 CH 3 , -CH 2 CH 2 CH 3 , -CH 2 CH 2 CH 2 CH 3 , -CH(CH 3 ) 2 , -CH(CH 2 CH 3 ) 2 , -CH 2 CH(CH 3 ) 2 , -C(CH 3 ) 3 , - CH 2 (CH 3 ) 3 , or C 3 -C 6 cycloalkyl.
- CY is -C(CH 3 ) 3 or C 3 -C 6 cycloalkyl.
- CY is -C(CH 3 ) 3 .
- R D is H, -CH 3 , or -CH 2 CH 3
- R 7 is H
- R 8 is H, Ci-C 4 alkyl or C 1 - C 4 fluoroalkyl
- R 10 is a Ci-C 6 alkyl, Ci-C 6 fluoroalkyl, C 3 -C 6 cycloalkyl, or a substituted or unsubstituted phenyl.
- a 1 is CH, CR A or N;
- R A is F, Cl, Br, I, -CH 3 , or -CF 3 ;
- L 2 is absent, -CH 2 -, -CH 2 O-, -OCH 2 -, -CH 2 S-, -SCH 2 -, -0-, or -S-;
- R 8 is H or -CH 3 ;
- CY is Ci-C ⁇ alkyl, substituted or unsubstituted C 3 -Cecycloalkyl, or substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with 1 or 2 R c ;
- R c is F, Cl, Br, I, -OH, Ci-C 4 alkyl, Ci-C 4 fluoroalkyl, Ci-C 4 fluoroalkoxy, or C 1 - C 4 alkoxy;
- n is 1, 2, or 3.
- the compound of Formula (I) has the structure of Formula (V) or a pharmaceutically acceptable salt thereof:
- R D is H or Ci-C 4 alkyl;
- R 3 is H, C r C 4 alkyl, C 3 -C 6 cycloalkyl, or C r C 4 fluoroalkyl;
- R 7 is H or Ci-C 4 alkyl
- R 8 is H, C r C 4 alkyl, or C r C 4 fluoroalkyl
- CY is a substituted or unsubstituted C 3 -Cecycloalkyl or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with 1 or 2 R c ;
- Ci-C 4 fluoroalkyl Ci-C 4 fluoroalkoxy, Ci-C 4 alkoxy, and Ci-C 4 heteroalkyl
- m is 0, 1, or 2
- n is 1, 2, 3 or 4
- p is 0, 1, or 2.
- R 3 is H or Ci-C 4 alkyl
- R 7 is H
- R 8 is H, -CH 3 or -CF 3
- R 10 is a Ci-C 6 alkyl or a substituted or unsubstituted phenyl
- each R A is independently selected from F, Cl, Br, I, -OH, -CH 3 , -CF 3 , - OCF 3 , and -OCH 3
- each R B is independently selected from F, Cl, Br, I, -OH, -CH 3 , -CF 3 , -OCF 3 , and -OCH 3
- each R c is independently selected from F, Cl, Br, I, -OH, -CH 3 , -CF 3 , -OCF 3 , and - OCH 3
- m is O or 1
- R 1 is -CO 2 H or -CO 2 R D ;
- R D is H, -CH 3 , or -CH 2 CH 3 ;
- R 3 is H, - CH 3 or -CH 2 CH 3 ;
- R 8 is H, or -CH 3 ;
- CY is a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with 1 or 2 R c .
- the compound of Formula (I) or Formula (V) has the following structure:
- the compound of Formula (I) or Formula (V) has the following structure:
- R 3 is H, -CH 3 or -CH 2 CH 3 ;
- R 8 is H, or -CH 3 ;
- R 10 is -CH 3 , or -CH 2 CH 3 .
- n 1.
- R 4 is phenyl, wherein if CY is substituted phenyl then the phenyl is substituted with 1 or 2 R c ; R c is H, F, Cl, -OH, -CH 3 , -CF 3 , or -OCH 3 ; n is 1.
- R 8 is -CH 3 ;
- CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3-hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fiuoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanopheny
- the compound of Formula I) or Formula (V) has the following structure:
- R 1 is -CO 2 H
- CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl,
- 2,4-difluorophenyl 2,5-difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3 -hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl.
- CY is phenyl.
- R 1 is -CO 2 H;
- CY is phenyl, 2-fluorophenyl, 3 -fluorophenyl, 2-chlorophenyl, 3-chlorophenyl, 2-methylphenyl, 3- methylphenyl, 2-trifluoromethylphenyl, or 3-trifluoromethylphenyl.
- R 1 is -CO 2 H;
- CY is phenyl.
- R c is F, Cl, -CH 3 , or CF 3 .
- ring B is a substituted or unsubstituted phenylene; R c is F or Cl.
- R c is F
- the compound of Formula (I) or Formula (V) has the following structure:
- the compound of Formula (I) or Formula (V) has one of the following structures:
- CY is cyclopropyl, cyclobutyl, cyclohexyl, 2-chlorocyclohex- 1 - enyl, phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6- difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4- dichlorophenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl
- CY is cyclopropyl, cyclobutyl, cyclohexyl, 2-chlorocyclohex- 1 - enyl, phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6- difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4- dichlorophenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2-hydroxyphenyl, 3- hydroxyphenyl, 4-hydroxyphenyl, 2-trifluoromethylphenyl, 3-trifluoromethylphenyl, 4- trifluoromethylphenyl, 2-fluoro-4-methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-
- R 3 is H or C r C 4 alkyl;
- R 7 is H;
- R 8 is H, or -CH 3 ;
- R 10 is a C 1 - C ⁇ alkyl or a substituted or unsubstituted phenyl;
- CY is cyclopropyl, cyclobutyl, cyclopentyl, cyclopent-1-enyl, 2-chlorocyclopent- 1 -enyl, cyclohexyl, cyclohex-1-enyl, 2-chlorocyclohex- 1 - enyl, phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-d
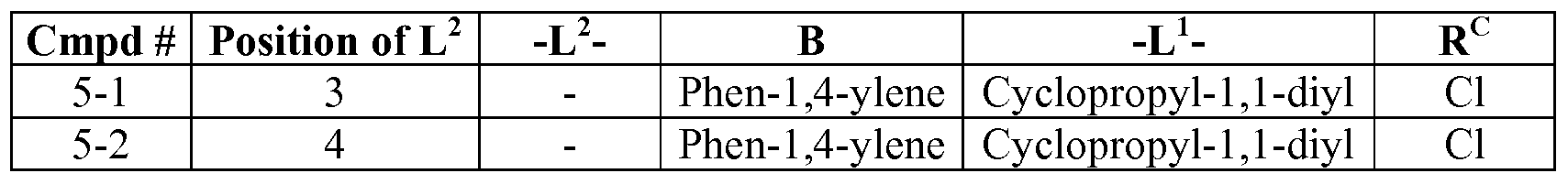
- compounds of Formula (I), (II), (III), (IV) and (V) include compounds described in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6.
- the compound of Formula (I), (II), (III), (IV) or (V) is an antagonist of a LPA receptor. In some embodiments, the compound of Formula (I), (II), (III), (IV) or (V) is an antagonist of LPA 1 . In some embodiments, the compound of Formula (I), (II), (III), (IV) or (V) is an antagonist Of LPA 2 . In some embodiments, the compound of Formula (I), (II), (III), (IV) or (V) is an antagonist of LP A 3 .
- a pharmaceutical composition comprising a therapeutically effective amount of a compound of Formula (I), (II), (III), (IV) or (V).
- the pharmaceutical composition also contains at least one pharmaceutically acceptable inactive ingredient.
- a pharmaceutical composition comprising a therapeutically effective amount of a compound of Formula (I), (II), (III), (IV) or (V), or a pharmaceutically acceptable salt thereof, and at least one pharmaceutically acceptable inactive ingredient.
- the pharmaceutical composition is formulated for intravenous injection, subcutaneous injection, oral administration, inhalation, nasal administration, topical administration, ophthalmic administration or otic administration.
- the pharmaceutical composition is a tablet, a pill, a capsule, a liquid, an inhalant, a nasal spray solution, a suppository, a suspension, a gel, a colloid, a dispersion, a suspension, a solution, an emulsion, an ointment, a lotion, an eye drop or an ear drop.
- the pharmaceutical composition further comprises one or more additional therapeutically active agents selected from: corticosteroids, immunosuppresants, analgesics, anti-cancer agent, antiinflammatories, chemokine receptor antagonists, bronchodilators, leukotriene receptor antagonists, leukotriene formation inhibitors, monoacylglycerol kinase inhibitors, phospholipase A 1 inhibitors, phospholipase A 2 inhibitors, and lysophospholipase D (lysoPLD) inhibitors, autotaxin inhibitors, decongestants, antihistamines, mucolytics, anticholinergics, antitussives, expectorants, and ⁇ -2 agonists.
- additional therapeutically active agents selected from: corticosteroids, immunosuppresants, analgesics, anti-cancer agent, antiinflammatories, chemokine receptor antagonists, bronchodilators, leukotriene receptor antagonists, leukotriene formation
- a method comprising administering a compound of Formula (I), (II), (III), (IV) or (V) to a human with a LPA-dependent or LPA-mediated disease or condition.
- the human is already being administered one or more additional therapeutically active agents other than a compound of Formula (I), (II), (III), (IV) or (V).
- the method further comprises administering one or more additional therapeutically active agents other than a compound of Formula (I), (II), (III), (IV) or (V).
- the one or more additional therapeutically active agents other than a compound of Formula (I), (II), (III), (IV) or (V) are selected from: corticosteroids, immunosuppresants, analgesics, anti-cancer agent, antiinflammatories, chemokine receptor antagonists, bronchodilators, leukotriene receptor antagonists, leukotriene formation inhibitors, monoacylglycerol kinase inhibitors, phospholipase Ai inhibitors, phospholipase A 2 inhibitors, and lysophospho lipase D (lysoPLD) inhibitors, autotaxin inhibitors, decongestants, antihistamines, mucolytics, anticholinergics, antitussives, expectorants, and ⁇ -2 agonists.
- compositions described herein are administerable to a subject in a variety of ways by multiple administration routes, including but not limited to, oral, parenteral (e.g., intravenous, subcutaneous, intramuscular), intranasal, buccal, topical or transdermal administration routes.
- the pharmaceutical formulations described herein include, but are not limited to, aqueous liquid dispersions, self-emulsifying dispersions, solid solutions, liposomal dispersions, aerosols, solid dosage forms, powders, immediate release formulations, controlled release formulations, fast melt formulations, tablets, capsules, pills, delayed release formulations, extended release formulations, pulsatile release formulations, multiparticulate formulations, and mixed immediate and controlled release formulations.
- the compound of Formula (I), (II), (III), (IV) or (V) is administered orally.
- the compound of Formula (I), (II), (III), (IV) or (V) is administered topically.
- the compound of Formula (I), (II), (III), (IV) or (V) is formulated into a variety of topically administrable compositions, such as solutions, suspensions, lotions, gels, pastes, shampoos, scrubs, rubs, smears, medicated sticks, medicated bandages, balms, creams or ointments.
- Such pharmaceutical compounds can contain solubilizers, stabilizers, tonicity enhancing agents, buffers and preservatives.
- the compound of Formula (I), (II), (III), (IV) or (V) is administered topically to the skin. [00131] In another aspect, the compound of Formula (I), (II), (III), (IV) or (V) is administered by inhalation. In one embodiment, the compound of Formula (I), (II), (III), (IV) or (V) is administered by inhalation that directly targets the pulmonary system.
- the compound of Formula (I), (II), (III), (IV) or (V) is formulated for intranasal adminstration. Such formulations include nasal sprays, nasal mists, and the like. [00133] In another aspect, the compound of Formula (I), (II), (III), (IV) or (V) is formulated as eye drops.
- the LPA is selected from LPAi, LPA 2 , LPA 3 , LPA 4 , LPA5 and LPA ⁇ .
- the LPA receptor is LPAi.
- the disease or condition is any of the diseases or conditions specified herein.
- any of the aforementioned aspects are further embodiments in which: (a) the effective amount of the compound of Formula (I), (II), (III), (IV) or (V) is systemically administered to the mammal; and/or (b) the effective amount of the compound is administered orally to the mammal; and/or (c) the effective amount of the compound is intravenously administered to the mammal; and/or (d) the effective amount of the compound is administered by inhalation; and/or (e) the effective amount of the compound is administered by nasal administration; or and/or (f) the effective amount of the compound is administered by injection to the mammal; and/or (g) the effective amount of the compound is administered topically to the mammal; and/or (h) the effective amount of the compound is administered by ophthalmic administration; and/or (i) the effective amount of the compound is administered rectally to the mammal; and/or (j) the effective amount is adminstered non-systemically or locally to the mammal.
- any of the aforementioned aspects are further embodiments comprising single administrations of the effective amount of the compound, including further embodiments in which (i) the compound is administered once; (ii) the compound is administered to the mammal multiple times over the span of one day; (iii) continually; or (iv) continuously.
- any of the aforementioned aspects are further embodiments comprising multiple administrations of the effective amount of the compound, including further embodiments in which (i) the compound is administered continuously or intermittently: as in a a single dose; (ii) the time between multiple administrations is every 6 hours; (iii) the compound is administered to the mammal every 8 hours; (iv) the compound is administered to the mammal every 12 hours; (v) the compound is administered to the mammal every 24 hours.
- the method comprises a drug holiday, wherein the administration of the compound is temporarily suspended or the dose of the compound being administered is temporarily reduced; at the end of the drug holiday, dosing of the compound is resumed.
- the length of the drug holiday varies from 2 days to 1 year.
- a method of inhibiting the physiological activity of LPA in a mammal comprising administering a therapuetically effective amount of a compound of Formula (I), (II), (III), (IV) or (V) or a pharmaceutically acceptable salt thereof to the mammal in need thereof.
- a medicament for treating a LPA-dependent or LPA-mediated disease or condition in a mammal comprising a therapeutically effective amount of a compound of Formula (I), (II), (III), (IV) or (V).
- a compound of Formula (I), (II), (III), (IV) or (V) in the treatment or prevention of a LPA-dependent or LPA-mediated disease or condition.
- a method for treating or preventing a LPA-dependent or LPA-mediated disease or condition in a mammal comprising administering a therapuetically effective amount of a compound of Formula (I), (II), (III), (IV) or (V).
- LPA-dependent or LPA-mediated diseases or conditions include, but are not limited to, fibrosis of organs or tissues, scarring, liver diseases, dermatological conditions, cancer, cardiovascular disease, respiratory diseases or conditions, inflammatory disease, gastrointestinal tract disease, renal disease, urinary tract-associated disease, inflammatory disease of lower urinary tract, dysuria, frequent urination, pancreas disease, arterial obstruction, cerebral infarction, cerebral hemorrhage, pain, peripheral neuropathy, and fibromyalgia.
- the LPA-dependent or LPA-mediated disease or condition is a respiratory disease or condition.
- the respiratory disease or condition is asthma, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, pulmonary arterial hypertension or acute respiratory distress syndrome.
- COPD chronic obstructive pulmonary disease
- the LPA-dependent or LPA-mediated disease or condition is selected from idiopathic pulmonary fibrosis; other diffuse parenchymal lung diseases of different etiologies including iatrogenic drug-induced fibrosis, occupational and/or environmental induced fibrosis, granulomatous diseases (sarcoidosis, hypersensitivity pneumonia), collagen vascular disease, alveolar proteinosis, langerhans cell granulomatosis, lymphangioleiomyomatosis, inherited diseases (Hermansky-Pudlak Syndrome, tuberous sclerosis, neurofibromatosis, metabolic storage disorders, familial interstitial lung disease); radiation induced fibrosis; chronic obstructive pulmonary disease (COPD); scleroderma; bleomycin induced pulmonary fibrosis; chronic asthma; silicosis; asbestos induced pulmonary fibrosis; acute respiratory distress syndrome (ARDS); kidney fibrosis; tubulointerstitium fibros
- the LPA-dependent or LPA-mediated disease or condition is described herein.
- a method for the treatment or prevention of organ fibrosis in a mammal comprising administering a therapeutically effective amount of a compound of
- the organ fibrosis comprises lung fibrosis, renal fibrosis, or hepatic fibrosis.
- a method of improving lung function in a mammal comprising administering a therapeutically effective amount of a compound of Formula (I), (II),
- the mammal has been diagnosed as having lung fibrosis.
- compounds disclosed herein are used to treat idiopathic pulmonary fibrosis (usual interstitial pneumonia) in a mammal.
- compounds disclosed herein are used to treat diffuse parenchymal interstitial lung diseases in mammal: iatrogenic drug induced, occupational/environmental (Farmer lung), granulomatous diseases (sarcoidosis, hypersensitivity pneumonia), collagen vascular disease (scleroderma and others), alveolar proteinosis, langerhans cell granulonmatosis, lymphangioleiomyomatosis, Hermansky-Pudlak
- Tuberous sclerosis Tuberous sclerosis, neurofibromatosis, metabolic storage disorders, familial interstitial lung disease.
- compounds disclosed herein are used to treat post-transplant fibrosis associated with chronic rejection in a mammal: Bronchiolitis obliterans for lung transplant.
- compounds disclosed herein are used to treat cutaneous fibrosis in a mammal: cutaneous scleroderma, Dupuytren disease, keloids.
- compounds disclosed herein are used to treat renal fibrosis in a mammal: tubulointerstitium fibrosis, glomerular sclerosis.
- a mammal is a human.
- compounds provided herein are administered to a human.
- compounds provided herein are orally administered.
- compounds provided herein are used as antagonists of at least one LPA receptor. In some embodiments, compounds provided herein are used for inhibiting the activity of at least one LPA receptor or for the treatment of a disease or condition that would benefit from inhibition of the activity of at least one LPA receptor. In one aspect, the LPA receptor is LPA 1 .
- compounds provided herein are used for the formulation of a medicament for the inhibition of LPAi activity.
- Articles of manufacture which include packaging material, a compound of Formula (I),
- Lysophospholipids are membrane-derived bioactive lipid mediators. Lysophospholipids include, but are not limited to, lysophosphatidic acid (l-acyl-2-hydroxy-s «-glycero-3- phosphate; LPA), sphingosine 1 -phosphate (SlP), lysophosphatidylcholine (LPC), and sphingosylphosphoryl choline (SPC). Lysophospholipids affect fundamental cellular functions that include cellular proliferation, differentiation, survival, migration, adhesion, invasion, and morphogensis. These functions influence many biological processes that include neurogensis, angiogenesis, wound healing, immunity, and carcinogenesis.
- LPA acts through sets of specific G protein-coupled receptors (GPCRs) in an autocrine and paracrine fashion. LPA binding to its cognate GPCRs (LPAi, LPA 2 , LPA 3 , LPA 4 , LPA 5 , LP Ae) activates intracellular signaling pathways to produce a variety of biological responses.
- GPCRs G protein-coupled receptors
- Lysophospholipids, such as LPA are quantitatively minor lipid species compared to their major phospholipid counterparts (e.g., phosphatidylcholine, phosphatidylethanolamine, and sphingomyelin).
- LPA has a role as a biological effector molecule, and has a diverse range of physiological actions such as, but not limited to, effects on blood pressure, platelet activation, and smooth muscle contraction, and a variety of cellular effects, which include cell growth, cell rounding, neurite retraction, and actin stress fiber formation and cell migration.
- the effects of LPA are predominantly receptor mediated.
- LPA receptor activation include, but are not limited to, cyclic adenosine monophosphate (cAMP), cell division cycle 42/GTP-binding protein (Cdc42) , proto-oncogene serine/threonine-protein kinase Raf (c-RAF), proto-oncogene tyrosine-protein kinase Src (c-src), extracellular signal-regulated kinase (ERK), focal adhesion kinase (FAK), guanine nucleotide exchange factor (GEF), glycogen synthase kinase 3b (GSK3b), c-jun amino- terminal kinase (JNK), MEK, myosin light chain II (MLC II), nuclear factor kB (NF-kB), N- methyl-D-aspartate (NMDA) receptor activation, phosphatidylinositol 3-kinase (PI3K), protein kinase (PI
- Serum LPA is produced by multiple enzymatic pathways that involve monoacylglycerol kinase, phospho lipase A 1 , secretory phospho lipase A 2 , and lysophospho lipase D (lysoPLD), including autotaxin.
- lysophospholipase Several enzymes are involved in LPA degradation: lysophospholipase, lipid phosphate phosphatase, and LPA acyl transferase such as endophilin.
- LPA concentrations in human serum are estimated to be 1-5 ⁇ M.
- Serum LPA is bound to albumin, low-density lipoproteins, or other proteins, which possibly protect LPA from rapid degradation.
- LPA induces a range of cellular responses through LPAi including but not limited to: cell proliferation, serum-response element (SRE) activation, mitogen-activated protein kinase (MAPK) activation, adenylyl cyclase (AC) inhibition, phospholipase C (PLC) activation, Ca 2+ mobilization, Akt activation, and Pvho activation.
- SRE serum-response element
- MAPK mitogen-activated protein kinase
- AC adenylyl cyclase
- PLC phospholipase C
- LPA 2 (EDG-4) also couples with three types of G proteins, G 1 /,,, G q , and G 12 / 1 3, to mediate LPA-induced cellular signaling. Expression Of LPA 2 is observed in the testis, kidney, lung, thymus, spleen, and stomach of adult mice and in the human testis, pancreas, prostate, thymus, spleen, and peripheral blood leukocytes.
- LPA 2 is upregulated in various cancer cell lines, and several human LPA 2 transcriptional variants with mutations in the 3'- untranslated region have been observed. Targeted deletion of LPA 2 in mice has not shown any obvious phenotypic abnormalities, but has demonstrated a significant loss of normal LPA signaling (e.g., PLC activation, Ca 2+ mobilization, and stress fiber formation) in primary cultures of mouse embryonic fibroblasts (MEFs).
- LPA signaling e.g., PLC activation, Ca 2+ mobilization, and stress fiber formation
- LPA-induced responses which include cell proliferation, AC inhibition, PLC activation, Ca 2+ mobilization, JNK and Akt activation, and stress fiber formation, are absent or severely reduced in double-null MEFs. All these responses, except for AC inhibition (AC inhibition is nearly abolished in LPAi (-/-) MEFs), are only partially affected in either LPAi (-/-) or LPA 2 (-/-) MEFs.
- LPA 2 contributes to normal LPA-mediated signaling responses in at least some cell types (Choi et al, Biochemica et Biophysica Acta 2008, 1781, p531-539).
- LPA 4 (p2y 9 /GPR23) is of divergent sequence compared to LPA 1 , LPA 2 , and LPA 3 with closer similarity to the platelet-activating factor (PAF) receptor.
- LPA 4 mediates LPA induced Ca 2+ mobilization and cAMP accumulation, and functional coupling to the G protein Gs for AC activation, as well as coupling to other G proteins.
- the LPA 4 gene is expressed in the ovary, pancreas, thymus, kidney and skeletal muscle.
- LPA 5 (GPR92) is a member of the purinocluster of GPCRs and is structurally most closely related to LPA 4 .
- LPA 5 is expressed in human heart, placenta, spleen, brain, lung and gut.
- LPA 5 also shows very high expression in the CD8+ lymphocyte compartment of the gastrointestinal tract.
- LPA is one such mediator that is released from activated platelets; this induces platelet aggregation along with mitogenic/migration effects on the surrounding cells, such as endothelial cells, smooth muscle cells, fibroblasts, and keratinocytes.
- Topical application of LPA to cutaneous wounds in mice promotes repair processes (wound closure and increased neoepithelial thickness) by increasing cell proliferation/ migration without affecting secondary inflammation.
- ECM dermal extracellular matrix
- LPA regulates many important functions of fibroblasts in wound healing, including proliferation, migration, differentiation and contraction. Fibroblast proliferation is required in wound healing in order to fill an open wound. In contrast, fibrosis is characterized by intense proliferation and accumulation of myofibroblasts that actively synthesize ECM and proinflammatory cytokines. LPA can either increase or suppress the proliferation of cell types important in wound healing, such as epithelial and endothelial cells (EC),macrophages, keratinocytes, and fibroblasts.
- EC epithelial and endothelial cells
- keratinocytes keratinocytes
- fibroblasts A role for LPAi in LPA-induced proliferation was provided by the observation that LPA-stimulated proliferation of fibroblasts isolated from LPAi receptor null mice was attenuated (Mills et al, Nat Rev. Cancer 2003; 3: 582-591). LPA induces cytoskeletal changes that are integral to fibroblast adhesion, migration, differentiation and contraction
- Tissue injury initiates a complex series of host wound-healing responses; if successful, these responses restore normal tissue structure and function. If not, these responses can lead to tissue fibrosis and loss of function.
- fibrosis For the majority of organs and tissues the development of fibrosis involves a multitude of events and factors. Molecules involved in the development of fibrosis include proteins or peptides (profibrotic cytokines, chemokines, metalloproteinases etc.) and phospholipids. Phospholipids involved in the development of fibrosis include platelet activating factor (PAF), phosphatidyl choline, sphingosine- 1 phosphate (SlP) and lysophosphatidic acid (LPA).
- PAF platelet activating factor
- SlP phosphatidyl choline
- SlP sphingosine- 1 phosphate
- LPA lysophosphatidic acid
- CTGF connective tissue growth factor
- LPA is associated with the progression of liver fibrosis.
- LPA induces stellate cell and hepatocyte proliferation. These activated cells are the main cell type responsible for the accumulation of ECM in the liver.
- LPA plasma levels rise during CCU-induced liver fibrosis in rodents, or in hepatitis C virus-induced liver fibrosis in human (N. Watanabe, et al., Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity, Life Sci. 81 (2007) 1009-1015; N.Watanabe, et al, J. Clin. Gastroenterol. 41 (2007) 616-623).
- Fibrotic lung diseases such as idiopathic pulmonary fibrosis (IPF) are associated with high morbidity and mortality.
- LPA is an important mediator of fibroblast recruitment in pulmonary fibrosis.
- LPA and LPAi play key pathogenic roles in pulmonary fibrosis.
- Fibroblast chemoattractant activity plays an important role in the lungs in patients with pulmonary fibrosis.
- Profibrotic effects of LPAi- receptor stimulation is explained by LPAi -receptor-mediated vascular leakage and increased fibroblast recruitment, both profibrotic events.
- the LPA-LPAi pathway has a role in mediating fibroblast migration and vascular leakage in IPF. The end result is the aberrant healing process that characterises this fibrotic condition.
- the LPAi receptor is the LPA receptor most highly expressed on fibroblasts obtained from patients with IPF. Furthermore, BAL obtained from IPF patients induced chemotaxis of human foetal lung fibroblasts that was blocked by the dual LPA r LPA 3 receptor antagonist Ki 16425. In an experimental bleomycin-induced lung injury mouse model, it was shown that LPA levels were high in bronchoalveolar lavage samples compared with unexposed controls. LPAi knockout mice are protected from fibrosis after bleomycin challenge with reduced fibroblast accumulation and vascular leakage. In human subjects with IPF, high LPA levels were observed in bronchoalveolar lavage samples compared with healthy controls.
- Activation of latent TGF- ⁇ by the av ⁇ 6 integrin plays a critical role in the development of lung injury and fibrosis (Munger et al. Cell, vol. 96, 319-328, 1999).
- LPA induces av ⁇ 6- mediated TGF- ⁇ activation on human lung epithelial cells (Xu et al. Am. J. Pathology, vol. 174, no. 2, 1264-1279, 2009).
- the LPA-induced av ⁇ 6-mediated TGF- ⁇ activation is mediated by the LP A2 receptor. Expression of the LP A2 receptor is increased in epithelial cells and mesenchymal cells in areas of lung fibrosis from IPF patients compared to normal human lung tissue.
- the LP A-LP A2 pathway contributes to the activation of the TGF- ⁇ pathway in pulmonary fibrosis.
- compounds that inhibit LPA2 show efficacy in the treatment of lung fibrosis.
- compounds that inhibit both LPAl and LP A2 show improved efficacy in the treatment of lung fibrosis compared to compounds which inhibit only LPAl or LPA2. Renal Fibrosis
- LPA and LPAi are involved in the etiology of kidney fibrosis.
- LPA has effetcs on both proliferation and contraction of glomerular mesangial cells and thus has been implicated in proliferative glomerulonephritis (CN. Inoue, et al, Clin. Sci. (Colch.) 96, 431- 436, 1999).
- UUO unilateral ureteral obstruction
- LPAi LPAi receptor
- Ki 16425 LPA receptor antagonist Ki 16425
- LPA can participate in intraperitonial accumulation of monocyte/macrophages and that LPA can induce expression of the profibrotic cytokine CTGF in primary cultures of human fibroblasts (J.S. Koh,e£ ah, J. CHn. Invest. 102 (1998) 716-727).
- LPA treatment of a mouse epithelial renal cell line, MCT induced a rapid increase in the expression of the profibrotic cytokine CTGF.
- CTGF plays a crucial role in UUO-induced tubulointerstitial fibrosis (TIF), and is involved in the profibrotic activity of TGF ⁇ . This induction was almost completely suppressed by co-treatment with the LPA-receptor antagonist Ki 16425.
- the profibrotic activity of LPA in kidney results from a direct action of LPA on kidney cells involving induction of CTGF.
- Hepatic fibrosis [00196] LPA is implicated in liver disease and fibrosis.
- Plasma LPA levels and serum autotoxin are elevated in hepatitis patients and animal models of liver injury in correlation with increased fibrosis. LPA also regulates liver cell function. LPAi and LPA 2 receptors are expressed by mouse hepatic stellate cells and LPA stimulates migration of hepatic myofibroblasts. Ocular Fibrosis
- LPA is in involved in wound healing in the eye.
- LPAi and LP A3 receptors are detectable in the normal rabbit corneal epithelial cells, keratocytes and endothelial cells and LPAi and LPA 3 expression are increased in corneal epithelial cells following injury.
- LPA and its homologues are present in the aqueous humor and the lacrimal gland fluid of the rabbit eye and these levels are increased in a rabbit corneal injury model.
- LPA induces actin stress fiber formation in rabbit corneal endothelial and epithelial cells and promotes contraction corneal fibroblasts. LPA also stimulates proliferation of human retinal pigmented epithelial cells Cardiac fibrosis [00200] LPA is implicated in myocardial infarction and cardiac fibrosis. Serum LPA levels are increased in patients following mycocardial infarction (MI) and LPA stimulates proliferation and collagen production (fibrosis) by rat cardiac fibroblasts. Both LPAl and LPA3 receptors are highly expressed in human heart tissue. Treatment of Fibrosis
- the compound of Formula (I), (II), (III), (IV) or (V) is used to treat or prevent fibrosis in a mammal.
- the compound of Formula (I), (II), (III), (IV) or (V) is used to treat fibrosis of an organ or tissue in a mammal.
- a method for preventing a fibrosis condition in a mammal comprising administering to the mammal at risk of developing one or more fibrosis conditions a therapeutically effective amount of a compound of Formula (I), (II), (III), (IV) or (V).
- the mammal has been exposed to one or more environmental conditions that are known to increase the risk of fibrosis of an organ or tissue. In one aspect, the mammal has been exposed to one or more environmental conditions that are known to increase the risk of lung, liver or kidney fibrosis. In one aspect, the mammal has a genetic predisposition of developing fibrosis of an organ or tissue. In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is adminstered to a mammal to prevent or minimize scarring following injury. In one aspect, injury includes surgery.
- fibrosis refers to conditions that are associated with the abnormal accumulation of cells and/or fibronectin and/or collagen and/or increased fibroblast recruitment and include but are not limited to fibrosis of individual organs or tissues such as the heart, kidney, liver, joints, lung, pleural tissue, peritoneal tissue, skin, cornea, retina, musculoskeletal and digestive tract.
- Exemplary diseases, disorders, or conditions that involve fibrosis include, but are not limited to: Lung diseases associated with fibrosis, e.g., idiopathic pulmonary fibrosis, pulmonary fibrosis secondary to systemic inflammatory disease such as rheumatoid arthritis, scleroderma, lupus, cryptogenic fibrosing alveolitis, radiation induced fibrosis, chronic obstructive pulmonary disease (COPD), scleroderma, chronic asthma, silicosis, asbestos induced pulmonary or pleural fibrosis, acute lung injury and acute respiratory distress (including bacterial pneumonia induced, trauma induced, viral pneumonia induced, ventilator induced, non-pulmonary sepsis induced, and aspiration induced); Chronic nephropathies associated with injury/fibrosis (kidney fibrosis), e.g., glomerulonephritis secondary to systemic inflammatory diseases such as lupus and sclero
- a mammal suffering from one of the following non- limiting exemplary diseases, disorders, or conditions will benefit from therapy with a compound of Formula (I), (II), (III), (IV) or (V): atherosclerosis, thrombosis, heart disease, vasculitis, formation of scar tissue, restenosis, phlobitis, COPD (chronic obstructive pulmonary disease), pulmonary hypertension, pulmonary fibrosis, pulmonary inflammation, bowel adhesions, bladder fibrosis and cystitis, fibrosis of the nasal passages, sinusitis, inflammation mediated by neutrophils, and fibrosis mediated by fibroblasts.
- atherosclerosis atherosclerosis, thrombosis, heart disease, vasculitis, formation of scar tissue, restenosis, phlobitis, COPD (chronic obstructive pulmonary disease), pulmonary hypertension, pulmonary fibrosis, pulmonary inflammation, bowel adhesions, bladder fibrosis and cystit
- a compound of Formula (I), (II), (III), (IV) or (V) is adminstered to a mammal with fibrosis of an organ or tissue or with a predisposition of developing fibrosis of an organ or tissue with one or more other agents that are used to treat fibrosis.
- the one or more agents include corticosteroids.
- the one or more agents include immunosuppresants.
- the one or more agents include B-cell antagonists.
- the one or more agents include uteroglobin.
- a compound of Formula (I), (II), (III), (IV) or (V) is used to treat a dermatological disorders in a mammal.
- dermatological disorder refers to a skin disorder.
- Such dermatological disorders include, but are not limited to, proliferative or inflammatory disorders of the skin such as, atopic dermatitis, bullous disorders, collagenoses, psoriasis, psoriatic lesions, dermatitis, contact dermatitis, eczema, urticaria, rosacea, scleroderma, wound healing, scarring, hypertrophic scarring, keloids, Kawasaki Disease, rosacea, Sjogren-Larsso Syndrome, urticaria.
- proliferative or inflammatory disorders of the skin such as, atopic dermatitis, bullous disorders, collagenoses, psoriasis, psoriatic lesions, dermatitis, contact dermatitis, eczema, urticaria, rosacea, scleroderma, wound healing, scarring, hypertrophic scarring, keloids, Kawasaki Disease, rosacea, Sjogren-Larsso Syndrome
- a method of reducing lung injury, vascular leakage, inflammation and fibrosis in a mammal comprising administering to the mammal a selective LPAl receptor antagonist.
- a method of attenuating fibrosis in a mammal comprising administering a selective LPAl receptor antagonist.
- a method of decreasing cytokine production in a mammal comprising administering a selective LPAl receptor antagonist.
- the method of decreasing cytokine production in a mammal comprising administering a selective LPAl receptor antagonist results in a reduction of tissue damage and fibrosis in a mammal.
- a method of treating fibrosis is a mammal comprising administering to the mammal a selective LPAl receptor antagonist. In some embodiments, provided is a method of treating fibrosis in a mammal while maintaining body weight in the mammal comprising administering to the mammal a selective LPAl receptor antagonist. In some embodiments, provided is a method of treating respiratory disease in a mammal comprising administering to the mammal a selective LPAl receptor antagonist.
- a method of treating fibrosis in a mammal with a selective LPAl receptor anatgonist wherein the fibrosis in the mammal is not responsive to treatment with pirfenidone.
- the LPAl receptor antagonist is a compound of Formula (I), (II), (III), (IV) or (V).
- a selective LPAl receptor antagonist reduced lung fibrosis, kidney fibrosis and liver fibrosis in various animal models of fibrosis.
- a selective LPAl receptor antagonist e.g. Compound 1-1
- reduced lung injury vascular leakage, inflammation and fibrosis at multiple timepoints following intratracheal bleomycin instillation.
- a selective LPAl receptor antagonist e.g. Compound 1-1
- Pain [00213] Since LPA is released following tissue injury, LPAi plays an important role in the initiation of neuropathic pain.
- LPA 1 unlike LPA 2 or LPA 3 , is expressed in both dorsal root ganglion (DRG) and dorsal root neurons.
- DRG dorsal root ganglion
- AS-ODN antisense oligodeoxynucleotide
- LPAi and downstream Rho-ROCK activation play a role in the initiation of neuropathic pain signaling.
- neuropathic pain markers such as protein kinase C ⁇ (PKC ⁇ ) and a voltage-gated calcium channel ⁇ 2 ⁇ l subunit (Ca ⁇ 2 ⁇ l) in an LPAi and Rho-dependent manner
- PLC ⁇ protein kinase C ⁇
- Ca ⁇ 2 ⁇ l voltage-gated calcium channel ⁇ 2 ⁇ l subunit
- a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of pain in a mammal.
- the pain is acute pain or chronic pain.
- the pain is neuropathic pain.
- the pain is cancer pain.
- a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of fibromylagia.
- fibromyalgia stems from the formation of fibrous scar tissue in contractile (voluntary) muscles. Fibrosis binds the tissue and inhibits blood flow, resulting in pain.
- Lysophospholipid receptor signaling plays a role in the etiology of cancer.
- Lysophosphatidic acid (LPA) and its G protein-coupled receptors (GPCRs) LPAi, LPA 2 , and/or LPA 3 play a role in the development of several types of cancers.
- the initiation, progression and metastasis of cancer involve several concurrent and sequential processes including cell proliferation and growth, survival and anti-apoptosis, migration of cells, penetration of foreign cells into defined cellular layers and/or organs, and promotion of angiogenesis.
- LPA signaling in physiological and pathophysiological conditions underscores the potential therapeutic usefulness of modulating LPA signaling pathways for the treatment of cancer, especially at the level of the LPA receptors or ATX/lysoPLD.
- Autotaxin (ATX) is a prometastatic enzyme initially isolated from the conditioned medium of human melanoma cells that stimulates a myriad of biological activities, including angiogenesis and the promotion of cell growth, migration, survival, and differentiation through the production of LPA (MoI Cancer Ther 2008;7(10):3352-62).
- LPA signals through its own GPCRs leading to activation of multiple downstream effector pathways. Such downstream effector pathways play a role in cancer.
- LPA and its GPCRs are linked to cancer through major oncogenic signaling pathways.
- LPA contributes to tumorigenesis by increasing motility and invasiveness of cells. LPA has been implicated in the initiation or progression of ovarian cancer. LPA is present at significant concentrations (2-80 ⁇ M) in the ascitic fluid of ovarian cancer patients. Ovarian cancer cells constitutively produce increased amounts of LPA as compared to normal ovarian surface epithelial cells, the precursor of ovarian epithelial cancer. Elevated LPA levels are also detected in plasma from patients with early-stage ovarian cancers compared with controls.
- LPA receptors (LP A2 and LPA3) are also overexpressed in ovarian cancer cells as compared to normal ovarian surface epithelial cells. LPA stimulates Cox-2 expression through transcriptional activation and post-transcriptional enhancement of Cox-2 mRNA in ovarian cancer cells. Prostaglandins produced by Cox-2 have been implicated in a number of human cancers and pharmacological inhibition of Cox-2 activity reduces colon cancer development and decreases the size and number of adenomas in patients with familial adenomatous polyposis.
- LPA has also been implicated in the initiation or progression of prostate cancer, breast cancer, melanoma, head and neck cancer, bowel cancer (colorectal cancer), thyroid cancer, glioblastoma, and other cancers
- Gardell et al Trends in Molecular Medicine, vol. 12, no. 2, p 65-75, 2006; Ishii et al, Annu. Rev. Biochem, 73, 321-354, 2004; Mills et al, Nat. Rev. Cancer, 3, 582-591, 2003; Murph et al, Biochimica et Biophysica Acta, 1781, 547-557, 2008; Kishi et al, J. Biol. Chem., 281, 17492-17500, 2006; ).
- LPA receptors mediate both migration of and invasion by pancreatic cancer cell lines: an antagonist of LPAi and LPA 3 (Kil6425) and LPAi-specific siRNA effectively blocked in vitro migration in response to LPA and peritoneal fluid (ascites) from pancreatic cancer patients; in addition, Ki 16425 blocked the LPA-induced and ascites-induced invasion activity of a highly peritoneal metastatic pancreatic cancer cell line (Yamada et al, J. Biol. Chem., 279, 6595-6605, 2004).
- Colorectal carcinoma cell lines show significant expression of LPAi mRNA and respond to LPA by cell migration and production of angiogenic factors. Overexpression of LPA receptors has a role in the pathogenesis of thyroid cancer. LPA 3 was originally cloned from prostate cancer cells, concordant with the ability of LPA to induce autocrine proliferation of prostate cancer cells.
- LPA has stimulatory roles in cancer progression in many types of cancer.
- LPA is produced from and induces proliferation of prostate cancer cell lines.
- LPA induces human colon carcinoma DLDl cell proliferation, migration, adhesion, and secretion of angiogenic factors through LPAi signalling.
- LPA enhances cell proliferation and secretion of angiogenic factors.
- LPA 2 and LPA 3 receptor activation results in proliferation of the cells.
- LPAi is implicated in bone metastasis and the LPAi/LPA 3 dual antagonist Ki 16425 has been shown to inhibit metastasis to bone in vivo (Boucharaba et al, Proc. Natl. Acad. Sci USA, 103, 9643-9648, 2006).
- the genetic or pharmacological manipulation of LPA metabolism, specific blockade of receptor signaling, and/or inhibition of downstream signal transduction pathways, represent approaches for cancer therapies.
- a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of cancer.
- a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of malignant and benign proliferative disease.
- a compound of Formula (I), (II), (III), (IV) or (V) is used to prevent or reduce proliferation of tumor cells, invasion and metastasis of carcinomas, pleural mesothelioma (Yamada, Cancer Sci., 2008, 99(8), 1603-1610) or peritoneal mesothelioma, cancer pain, bone metastases (Boucharaba et al, J. Clin.
- a method of treating cancer in a mammal comprising administering to the mammal a compound of Formula (I), (II), (III), (IV) or (V) and a second therapeutic agent, wherein the second therapeutic agent is an anti-cancer agent.
- cancer refers to an abnormal growth of cells which tend to proliferate in an uncontrolled way and, in some cases, to metastasize (spread).
- types of cancer include, but is not limited to, solid tumors (such as those of the bladder, bowel, brain, breast, endometrium, heart, kidney, lung, lymphatic tissue (lymphoma), ovary, pancreas or other endocrine organ (thyroid), prostate, skin (melanoma or basal cell cancer) or hematological tumors (such as the leukemias) at any stage of the disease with or without metastases.
- LPA is a contributor to the pathogenesis of respiratory diseases.
- the respiratory disease is asthma.
- LPA lipoprotein kinase
- IL-8 is found in increased concentrations in BAL fluids from patients with asthma, chronic obstructive lung disease, pulmonary sarcoidosis and acute respiratory distress syndrome and 11-8 has been shown to exacerbate airway inflammation and airway remodeling of asthmatics.
- LPAl, LP A2 and LPA3 receptors have all been shown to contribute to the LPA-induced IL-8 production.
- the release of normal repair mediators, including LPA is exaggerated or the actions of the repair mediators are inappropriately prolonged leading to inappropriate airway remodeling.
- Major structural features of the remodeled airway observed in asthma include a thickened lamina reticularis (the basement membrane-like structure just beneath the airway epithelial cells), increased numbers and activation of myofibroblasts, thickening of the smooth muscle layer, increased numbers of mucus glands and mucus secretions, and alterations in the connective tissue and capillary bed throughout the airway wall.
- LPA contributes to these structural changes in the airway.
- LPA is involved in acute airway hyperresponsiveness in asthma.
- LPA contributes to the long-term structural remodeling and the acute hyperresponsiveness of the asthmatic airway. In one aspect, LPA contributes to the hyper-responsiveness that is a primary feature of acute exacerbations of asthma. [00228] In addition to the cellular responses mediated by LPA, several of the LPA signaling pathway components leading to these responses are relevant to asthma. EGF receptor upregulation is induced by LPA and is also seen in asthmatic airways (M. Amishima, et ah, Am. J. Respir. Crit. Care Med. 157, 1907- 1912, 1998).
- Chronic inflammation is a contributor to asthma, and several of the transcription factors that are activated by LPA are known to be involved in inflammation (Ediger et ah, Eur Respir J 21 :159-169, 2003).
- the fibroblast proliferation and contraction and extracellular matrix secretion stimulated by LPA contributes to the fibroproliferative features of other airway diseases, such as the peribronchiolar fibrosis present in chronic bronchitis, emphysema, and interstitial lung disease.
- Emphysema is also associated with a mild fibrosis of the alveolar wall, a feature which is believed to represent an attempt to repair alveolar damage.
- LPA plays a role in the fibrotic interstitial lung diseases and obliterative bronchiolitis, where both collagen and myofibroblasts are increased.
- LPA is involved in several of the various syndromes that constitute chronic obstructive pulmonary disease.
- LPA administration of LPA in vivo induces airway hyper-responsiveness, itch-scratch responses, infiltration and activation of eosinophils and neutrophils, vascular remodeling, and nociceptive flexor responses.
- LPA also induces histamine release from mouse and rat mast cells. In an acute allergic reaction, histamine induces various responses, such as contraction of smooth muscle, plasma exudation, and mucus production. Plasma exudation is important in the airway, because the leakage and subsequent airway-wall edema contribute to the development of airway hyperresponsiveness.
- Plasma exudation progresses to conjunctival swelling in ocular allergic disorder and nasal blockage in allergic rhinitis (Hashimoto et ah, J Pharmacol 100, 82 - 87, 2006).
- plasma exudation induced by LPA is mediated by histamine release from mast cells via one or more LPA receptors.
- the LPA receptor(s) include LPAi and/or LPA 3 .
- a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of various allergic disorders in a mammal.
- a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of respiratory diseases, disorders or conditions in a mammal. In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of asthma in a mammal. In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of chronic asthma in a mammal.
- respiratory disease refers to diseases affecting the organs that are involved in breathing, such as the nose, throat, larynx, eustachian tubes, trachea, bronchi, lungs, related muscles (e.g., diaphram and intercostals), and nerves.
- Respiratory diseases include, but are not limited to, asthma, adult respiratory distress syndrome and allergic (extrinsic) asthma, non-allergic (intrinsic) asthma, acute severe asthma, chronic asthma, clinical asthma, nocturnal asthma, allergen-induced asthma, aspirin-sensitive asthma, exercise-induced asthma, isocapnic hyperventilation, child-onset asthma, adult-onset asthma, cough-variant asthma, occupational asthma, steroid-resistant asthma, seasonal asthma, seasonal allergic rhinitis, perennial allergic rhinitis, chronic obstructive pulmonary disease, including chronic bronchitis or emphysema, pulmonary hypertension, interstitial lung fibrosis and/or airway inflammation and cystic fibrosis, and hypoxia.
- asthma refers to any disorder of the lungs characterized by variations in pulmonary gas flow associated with airway constriction of whatever cause (intrinsic, extrinsic, or both; allergic or non-allergic).
- the term asthma may be used with one or more adjectives to indicate cause.
- chronic obstructive pulmonary disease includes, but is not limited to, chronic bronchitis or emphysema, pulmonary hypertension, interstitial lung fibrosis and/or airway inflammation, and cystic fibrosis.
- the nervous system is a major locus for LPA 1 expression; there it is spatially and temporally regulated throughout brain development.
- Oligodendrocytes the myelinating cells in the central nervous system (CNS)
- CNS central nervous system
- Schwann cells the myelinating cells of the peripheral nervous system
- LPAi which is involved in regulating Schwann cell survival and morphology.
- neural system disorder refers to conditions that alter the structure or function of the brain, spinal cord or peripheral nervous system, including but not limited to Alzheimer's Disease, cerebral edema, cerebral ischemia, stroke, multiple sclerosis, neuropathies, Parkinson's Disease, those found after blunt or surgical trauma (including post-surgical cognitive dysfunction and spinal cord or brain stem injury), as well as the neurological aspects of disorders such as degenerative disk disease and sciatica.
- CNS disorders include, but are not limited to, multiple sclerosis, Parkinson's disease, Alzheimer's disease, stroke, cerebral ischemia, retinal ischemia, post-surgical cognitive dysfunction, migraine, peripheral neuropathy/neuropathic pain, spinal cord injury, cerebral edema and head injury.
- Cardiovascular Disorders include, but are not limited to, multiple sclerosis, Parkinson's disease, Alzheimer's disease, stroke, cerebral ischemia, retinal ischemia, post-surgical cognitive dysfunction, migraine, peripheral neuropathy/neuropathic pain, spinal cord injury, cerebral edema and head injury.
- Angiogenesis the formation of new capillary networks from pre-existing vasculature, is normally invoked in wound healing, tissue growth and myocardial angiogenesis after ischemic injury.
- Peptide growth factors e.g.
- LPA contributes to both the early phase (barrier dysfunction and monocyte adhesion of the endothelium) and the late phase (platelet activation and intra-arterial thrombus formation) of atherosclerosis, in addition to its overall progression.
- LPA LPA from numerous sources accumulates in lesions and activates its cognate GPCRs (LPAi and LP A3) expressed on platelets (Siess, W. Biochim. Biophys. Acta 1582, 204-215, 2002; Rother, E. et al. Circulation 108, 741-747, 2003). This triggers platelet shape change and aggregation, leading to intra- arterial thrombus formation and, potentially, myocardial infarction and stroke.
- LPA can also be a mitogen and motogen to VSMCs and an activator of endothelial cells and macrophages.
- LPA has been shown to be involved in ischemia- reperfussion injury. Blockade of the LP A 3 receptor in a murine model of renal ischemia- reperfusion injury reduced the severity of injury. This effect was reversed in the presence of the selective LPA 3 receptor agonist OMPT (Okusa et al, Am. J. Physiol. Renal Physiol., 285, F565- F574, 2003).
- mammals with cardiovascular disease benefit from LPA receptor antagonists that prevent thrombus and neointima plaque formation.
- the specific effects of LPA are receptor-mediated.
- the compound of Formula (I), (II), (III), (IV) or (V) is used to treat or prevent cardiovascular disease in mammal.
- cardiac disease refers to diseases affecting the heart or blood vessels or both, including but not limited to: arrhythmia (atrial or ventricular or both); atherosclerosis and its sequelae; angina; cardiac rhythm disturbances; myocardial ischemia; myocardial infarction; cardiac or vascular aneurysm; vasculitis, stroke; peripheral obstructive arteriopathy of a limb, an organ, or a tissue; reperfusion injury following ischemia of the brain, heart, kidney or other organ or tissue; endotoxic, surgical, or traumatic shock; hypertension, valvular heart disease, heart failure, abnormal blood pressure; shock; vasoconstriction (including that associated with migraines); vascular abnormality, inflammation, insufficiency limited to a single organ or tissue.
- kits for preventing or treating vasoconstriction, atherosclerosis and its sequelae myocardial ischemia, myocardial infarction, aortic aneurysm, vasculitis and stroke comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V) or pharmaceutical composition or medicament which includes a compound of Formula (I), (II), (III), (IV) or (V).
- provided herein are methods for reducing cardiac reperfusion injury following myocardial ischemia and/or endotoxic shock comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
- methods for reducing the constriction of blood vessels in a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
- LPA has been shown to regulate immunological responses by modulating activities/functions of immune cells such as T-/B-lymphocytes and macrophages.
- T cells T-/B-lymphocytes and macrophages.
- LPA activates IL-2 production/cell proliferation through LPAi (Gardell et al, TRENDS in Molecular Medicine Vol.12 No.2 February 2006).
- LPA-induced inflammatory response genes is mediated by LPAi and LPA 3 ⁇ Biochem Biophys Res Commun. 363(4): 1001-8, 2007).
- LPA modulates the chemotaxis of inflammatory cells ⁇ Biochem Biophys Res Commun., 1993, 15;193(2), 497).
- LPA lymphoid protein
- a compound of Formula (I), (II), (III), (IV) or (V) is used to treat or prevent inflammation in a mammal.
- antagonists of LPAi and/or LPA 3 find use in the treatment or prevention of inflammatory/immune disorders in a mammal.
- the antagonist of LPAi is a compound of Formula (I), (II), (III), (IV) or (V).
- inflammatory/immune disorders include psoriasis, rheumatoid arthritis, vasculitis, inflammatory bowel disease, dermatitis, osteoarthritis, asthma, inflammatory muscle disease, allergic rhinitis, vaginitis, interstitial cystitis, scleroderma, eczema, allogeneic or xenogeneic transplantation (organ, bone marrow, stem cells and other cells and tissues) graft rejection, graft- versus-host disease, lupus erythematosus, inflammatory disease, type I diabetes, pulmonary fibrosis, dermatomyositis, Sjogren's syndrome, thyroiditis (e.g., Hashimoto's and autoimmune thyroiditis), myasthenia gravis, autoimmune hemolytic anemia, multiple sclerosis, cystic fibrosis, chronic relapsing hepatitis, primary biliary cirrhosis, allergic conjun
- the subject already has a LPA-dependent or LPA-mediated disease or condition at the time of administration, or is at risk of developing a LPA-dependent or LPA-mediated disease or condition.
- the activity of LPAi in a mammal is directly or indirectly modulated by the administration of (at least once) a therapeutically effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
- modulation includes, but is not limited to, reducing and/or inhibiting the activity of LPAi.
- the activity of LPA in a mammal is directly or indirectly modulated, including reducing and/or inhibiting, by the administration of (at least once) a therapeutically effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
- modulation includes, but is not limited to, reducing and/or inhibiting the amount and/or activity of a LPA receptor.
- the LPA receptor In one aspect, the LPA receptor
- LPA has a contracting action on bladder smooth muscle cell isolated from bladder, and promotes growth of prostate-derived epithelial cell ⁇ The Journal of Urology , 1999, vol. 162, pp. 1779-1784; The Journal of Urology , 2000, vol. 163, pp. 1027-1032).
- LPA contracts the urinary tract and prostate in vitro and increases intraurethral pressure in vivo (WO 02/062389).
- eosinophil and/or basophil and/or dendritic cell and/or neutrophil and/or monocyte and/or T-cell recruitment comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
- methods for the treatment of cystitis comprising administering at least once to the mammal a therapeutically effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
- methods described herein include the diagnosis or determination of whether or not a patient is suffering from a LPA-dependent or LPA-mediated disease or condition by administering to the subject a therapeutically effective amount of a compound of Formula (I), (II), (III), (IV) or (V) and determining whether or not the patient responds to the treatment.
- LPA-dependent conditions or diseases include those wherein an absolute or relative excess of LPA is present and/or observed.
- the LPA-dependent or LPA-mediated diseases or conditions include, but are not limited to, organ fibrosis, asthma, allergic disorders, chronic obstructive pulmonary disease, pulmonary hypertension, lung or pleural fibrosis, peritoneal fibrosis, arthritis, allergy, cancer, cardiovascular disease, ult respiratory distress syndrome, myocardial infarction, aneurysm, stroke, and cancer.
- a compound of Formula (I), (II), (III), (IV) or (V) is used to improve the corneal sensitivity decrease caused by corneal operations such as laser-assisted in situ keratomileusis (LASIK) or cataract operation, corneal sensitivity decrease caused by corneal degeneration, and dry eye symptom caused thereby.
- corneal operations such as laser-assisted in situ keratomileusis (LASIK) or cataract operation
- corneal sensitivity decrease caused by corneal degeneration corneal sensitivity decrease caused by corneal degeneration
- dry eye symptom caused thereby.
- a compound of Formula (I), (II), (III), (IV) or (V) in the treatment or prevention of ocular inflammation and allergic conjunctivitis, vernal keratoconjunctivitis, and papillary conjunctivitis in a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
- a compound of Formula (I), (II), (III), (IV) or (V) in the treatment or prevention of Sjogren disease or inflammatory disease with dry eyes in a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
- LPA and LPA receptors e.g. LPA 1
- LPA 1 are involved in the pathogenesis of osteoarthritis (Kotani et al, Hum. MoI. Genet., 2008, 17, 1790-1797).
- LPA receptors e.g. LPAi, LPA 3
- LPAi, LPA 3 contribute to the pathogenesis of rheumatoid arthritis (Zhao et al, MoI. Pharmacol, 2008, 73(2), 587-600).
- LPA receptors e.g. LPAi
- LPAi contribute to adipogenesis.
- a compound of Formula (I), (II), (III), (IV) or (V) in the promotion of adipose tissue formation in a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
- compounds disclosed herein are used to treat Raynaud's phenomenon.
- Raynaud's phenomenon comprises both Raynaud's disease (where the phenomenon is idiopathic) and Raynaud's syndrome, where it is caused by some instigating factor.
- R D is H or Ci-C 6 alkyl
- ring A is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring A is substituted then each substituent on ring A is H or R A
- ring B is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring B is substituted then each substituent on ring B is H or R B ;
- L 1 is a substituted or unsubstituted cycloalkylene
- L 2 is absent, a substituted or unsubstituted alkylene, a substituted or unsubstituted fluoroalkylene, a substituted or unsubstituted heteroalkylene, -0-, -S-, -SO-, -SO 2 -, - NR 2 -, or -C(O)-;
- R 2 is H, C r C 4 alkyl, or CrC 4 fluoroalkyl
- R 3 is H, Ci-C 4 alkyl, or Ci-C 4 fluoroalkyl
- R 7 is H or C r C 4 alkyl;
- R 8 is H, Ci-C 4 alkyl, or Ci-C 4 fluoroalkyl;
- R 8a is H or Ci-C 4 alkyl
- CY is a substituted or unsubstituted alkyl, a substituted or unsubstituted cycloalkyl a substituted or unsubstituted heterocycloalkyl, a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then each substituent on CY is H or R c ; or
- each R 9 is independently selected from H, Ci-C ⁇ alkyl, Ci-Ceheteroalkyl, C 1 - Cefluoroalkyl, a substituted or unsubstituted cycloalkyl, a substituted or unsubstituted heterocycloalkyl, a substituted or unsubstituted aryl,
- R 3 is H, Ci-C 4 alkyl, or C 1 - C 4 fluoroalkyl.
- R 3 is Ci-C 4 alkyl, C 3 -C 6 cycloalkyl, or Ci-C 4 fluoroalkyl.
- R 3 is H, Ci-C 4 alkyl, cyclopropyl, or Ci-C 4 fluoroalkyl.
- R 3 is Ci-C 4 alkyl, cyclopropyl, or Ci-C 4 fluoroalkyl.
- R 3 is Ci-C 4 alkyl, or Ci-C 4 fluoroalkyl. In yet other embodiments, R 3 is Ci-C 4 alkyl. In some embodiments, R 3 is -CH 3 or -CH 2 CH 3 . In some embodiments, R 3 is -CH 3 , -CF 3 , -CH 2 CH 3 , - CHCH 2 , or cyclopropyl. In some embodiments, R 3 is -CH 3 , -CF 3 , -CH 2 CH 3 , or -CHCH 2 . In one aspect, R 3 is -CH 3 .
- ring A is a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring A is substituted then each substituent on ring A is H or R A .
- ring A is a substituted or unsubstituted phenylene, a substituted or unsubstituted napthylene, or a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring A is substituted then each substituent on ring A is H or R A .
- ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring A is substituted then each substituent on ring A is H or R A .
- ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted pyridinylene, where if ring A is substituted then each substituent on ring A is H or R A .
- ring B is a substituted or unsubstituted monocyclic cycloalkylene, a substituted or unsubstituted bicyclic cycloalkylene, a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted bicyclic heterocycloalkylene, a substituted or unsubstituted phenylene, a substituted or unsubstituted naphthylene, a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then each substituent on ring B is H or R B .
- each of R A , R B , and R c are independently selected from halogen, -CN, -NO 2 , -OH, -OR 10 , -N(R 9 ) 2 , C r C 4 alkyl, C r C 4 fluoroalkyl, C r C 4 fluoroalkoxy, C 1 - C 4 alkoxy, and Ci-C 4 heteroalkyl.
- each of R A , R B , and R c are independently selected from halogen, Ci-C 4 alkyl, C r C 4 fluoroalkyl, C r C 4 fluoroalkoxy, C r C 4 alkoxy, and C r C 4 heteroalkyl.
- the compounds of Formula (I) has the structure of Formula (II):
- each of A 1 , A 2 , and A 3 is independently selected from CR A and N.
- each R A is independently selected from H, halogen, -CH 3 , -CF 3 , - OCF 3 , and -OCH 3 .
- each of A 1 , A 2 , and A 3 is CR A .
- one of A 1 , A 2 , and A 3 is N.
- a 1 is N. In some embodiments, A 2 is N. In some embodiments, A 3 is N.
- R 1 is -CO 2 H.
- CY is a substituted or unsubstituted cycloalkyl, a substituted or unsubstituted heterocycloalkyl, a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then each substituent on CY is H or R c ; or R 8 and CY are taken together with the carbon atom to which they are attached to form a substituted or unsubstituted carbocycle or a substituted or unsubstituted heterocycle [00289] In some embodiments, CY is a substituted or unsubstituted cycloalkyl, a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then each substituent on CY is H or R c
- CY is a substituted or unsubstituted monocyclic cycloalkyl, a substituted or unsubstituted bicyclic cycloalkyl, a substituted or unsubstituted phenyl, a substituted or unsubstituted napthyl, a substituted or unsubstituted monocyclic heteroaryl containing 0-4 N atoms, or a substituted or unsubstituted bicyclic heteroaryl containing 0-4 N atoms, wherein if CY is substituted then each substituent on CY is H or R c .
- CY is a substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 1-3 N atoms, wherein if CY is substituted then each substituent on CY is H or R c .
- each R c is independently selected from H, halogen, Ci-C 4 alkyl, Ci-C 4 fluoroalkyl, Ci-C 4 fluoroalkoxy, Ci-C 4 alkoxy, and Ci-C 4 heteroalkyl.
- A is unsubstituted or monosubstituted with R A . In one aspect, in any of the embodiments described herein, A is unsubstituted.
- B is unsubstituted or monosubstituted with R B . In one aspect, in any of the embodiments described herein, B is unsubstituted.
- CY is unsubstituted or monosubstituted with R c .
- L 1 is a substituted or unsubstituted C 3 -Cealkylene. In some embodiments, L 1 is
- L 2 is absent, Ci-C ⁇ alkylene, Ci-Cefluoroalkylene, C 1 - C 6 heteroalkylene, -O-, -S-, -SO-, -SO 2 - or -NR 2 -.
- L 2 is absent, C r C 6 alkylene, -O-, -S-, -SO-, -SO 2 - or -NR 2 -.
- L 2 is absent, -CH 2 -, -O-, -S-, -SO-, -SO 2 - or -NR 2 -.
- L 2 is absent, -CH 2 -, -O-, -S-, -SO-, or -SO 2 -. In some embodiments, L 2 is absent. In yet other embodiments, L 2 is -CH 2 -. In some embodiments, L 2 is -0-, -S-, -SO-, or -SO 2 -.
- ring B is a substituted or unsubstituted monocyclic cycloalkylene, a substituted or unsubstituted bicyclic cycloalkylene, a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted bicyclic heterocycloalkylene, a substituted or unsubstituted phenylene, a substituted or unsubstituted naphthylene, a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then each substituent on ring B is H or R B .
- each R B is independently selected from H, halogen, Ci-C 4 alkyl, Ci-C 4 fluoroalkyl, Ci-C 4 fluoroalkoxy, Ci-C 4 alkoxy, and Ci-C 4 heteroalkyl.
- L 2 is a absent, Ci-C 6 alkylene, Ci-C 6 fluoroalkylene, -C 1 - C 6 alkylene-O-, -Ci-C 3 alkylene-O-Ci-C 3 alkylene -, -O-Ci-C 6 alkylene -, -Ci-C 6 alkylene-S-, -C 1 - C 3 alkylene-S-Ci-C 3 alkylene -, -S-Ci-C 6 alkylene -, -Ci-Cgalkylene-SO-, -Ci-C 3 alkylene-SO-Ci- C 3 alkylene -, -SO-Ci-C 6 alkylene -, -Ci-C 6 alkylene-SO 2 -, -Ci-C 3 alkylene-SO 2 -Ci-C 3 alkylene -, - SO 2 -Ci-C 6 alkylene -, -SO 2 -Ci-
- L 1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl-l,l-diyl, cyclopentyl- 1 , 1 - diyl or cyclohexyl- 1,1 -diyl. In some embodiments, L 1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl- 1 , 1 - diyl, or cyclopentyl- 1,1 -diyl. In some embodiments, L 1 is cyclopropyl- 1,1 -diyl.
- ring B is a substituted or unsubstituted monocyclic cycloalkylene, or a substituted or unsubstituted phenylene, where if ring B is substituted then each substituent on ring B is H or R B .
- ring B is a substituted or unsubstituted cyclopentylene, a substituted or unsubstituted cyclopentenylene, a substituted or unsubstituted cyclohexylene, a substituted or unsubstituted cyclohexenylene, or a substituted or unsubstituted phenylene, where if ring B is substituted then each substituent on ring B is H or R B .
- ring B is a substituted or unsubstituted phenylene, where if ring B is substituted then each substituent on ring B is H or R B . In other embodiments, ring B is an unsubstituted phenylene.
- ring B is a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted bicyclic heterocycloalkylene, a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then each substituent on ring B is H or R B .
- ring B is a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then each substituent on ring B is H or R B .
- ring B is a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring B is substituted then each substituent on ring B is H or R B .
- ring B is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring B is substituted then each substituent on ring B is H or R B .
- ring B is a unsubstituted or substitituted divalent ring selected from phenylene, naphthylene, furanylene, pyrrolylene, oxazolylene, thiazolylene, imidazolylene, pyrazolylene, triazolylene, tetrazolylene, isoxazolylene, isothiazolylene, oxadiazolylene, thiadiazolylene, pyridinylene, pyrimidinylene, pyrazinylene, pyridazinylene, triazinylene, quinolinylene, isoquinolinylene, quinazolinylene, quinoxalinylene, naphthyridinylene, indolylene, indazolylene, benzoxazolylene, benzisoxazolylene, benzofuranylene, benzothienylene, benzothiazolylene, benzimidazolylene, purinylene, isoxazolylene
- ring B is a unsubstituted or substitituted divalent ring selected from phenylene, furanylene, pyrrolylene, oxazolylene, thiazolylene, imidazolylene, pyrazolylene, triazolylene, tetrazolylene, isoxazolylene, isothiazolylene, oxadiazolylene, thiadiazolylene, pyridinylene, pyrimidinylene, pyrazinylene, pyridazinylene, triazinylene, cyclopentylene, cyclopentenylene, cycloalkylene, and cyclalkenylene.
- ring B is a unsubstituted or substitituted divalent ring selected from pyrrolylene, oxazolylene, thiazolylene, imidazolylene, pyrazolylene, triazolylene, tetrazolylene, isoxazolylene, isothiazolylene, oxadiazolylene, thiadiazolylene, pyridinylene, pyrimidinylene, pyrazinylene, pyridazinylene, triazinylene, cyclopentylene, cyclopentenylene, cycloalkylene, and cyclalkenylene.
- R 4 is H or H
- R 8 is -CH 3 or -CF 3 . In other embodiments, R 8 is -CH 3 .
- R 4 is ⁇ . In other embodiments, R 4 is
- CY is a substituted or unsubstituted phenyl, wherein if CY is substituted then each substituent on CY is H or R c ; each R c is independently selected from halogen, Ci-C 4 alkyl, Ci-C 4 fluoroalkyl, Ci-C 4 fluoroalkoxy, Ci-C 4 alkoxy, and Ci-C 4 heteroalkyl.
- CY is a substituted or unsubstituted C 3 -C6cycloalkyl. In some embodiments, CY is cyclopropyl.
- each R c is independently selected from F, Cl, -CH 3 , -CF 3 , -
- CY is phenyl, 2-fluorophenyl or 2-chloro-phenyl.
- the compound of Formula (I) has the structure of Formula (III):
- a 1 is CR A and N;
- R A is H, F, Cl, Br, I, -CH 3 , or -CF 3 ;
- a 4 is CH or N
- L 1 is C 3 -C6cycloalkylene
- L 2 is absent, C r C 4 alkylene, C r C 4 heteroalkylene, -O-, -S-, -SO-, -SO 2 -, -NR 2 -, or -
- R 2 is H or -CH 3 ;
- CY is C 3 -C 6 Cycloalkyl or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with R c ;
- R c is F, Cl, Br, I, -OH, Ci-C 4 alkyl, C 1 - C 4 fluoroalkyl, Ci-C 4 fluoroalkoxy, or Ci-C 4 alkoxy.
- L 2 is absent, -CH 2 -, -CH 2 O-, -OCH 2 -, -CH 2 S-, -SCH 2 -, -O-, or - S-. In some embodiments, L 2 is absent, -CH 2 -, -O-, or -S-. In some embodiments, L 2 is absent or -O-. In some embodiments, L 2 is absent. [00323] In some embodiments, A 1 is CH. In some embodiments, A 4 is CH.
- CY is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, a substituted or unsubstituted cyclohexeneyl, or a substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with R c ; R c is F, Cl, -CH 3 , or CF 3 .
- CY is a substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with R c ; R c is F, Cl, -CH 3 , or CF 3 .
- CY is cyclopropyl [00325] In some embodiments, CY is phenyl, 2-fluorophenyl or 2-chloro-phenyl.
- CY is cyclopropyl, cyclobutyl, cyclohexyl, 2-chlorocyclohex- 1 - enyl, phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6- difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4- dichlorophenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanopheny
- CY is cyclopropyl, cyclobutyl, cyclopentyl, cyclopent- 1 -enyl, 2- chlorocyclopent-1-enyl, cyclohexyl, cyclohex-1-enyl, 2-chlorocyclohex- 1 -enyl, phenyl, 2- fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2- chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2- hydroxyphenyl, 3- hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4- methoxyphenyl, 2-trifluoromethylphenyl, 3-
- CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4- difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3- hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2-trifluoromethylphenyl, 3-trifluoromethylphenyl, 4- trifluoromethylphenyl, 2-fluoro-4-methoxyphenyl, 2-methylphenyl, 3-methylphenyl, or 4- methylphenyl.
- CY is phenyl, 2-fluorophenyl, 3-fluorophenyl, 2-chlorophenyl, 3-chlorophenyl, 2-methylphenyl, 3-methylphenyl, 2-trifluoromethylphenyl, or 3- trifluoromethylphenyl.
- CY is phenyl.
- CY is phenyl, 2-hydroxyphenyl, 3- hydroxyphenyl, or 4- hydroxyphenyl.
- L 1 is cyclopropyl- 1 , 1 -diyl, cyclopropyl-l,2-diyl, cycloprop-2- enyl-l,l-diyl, cyclobutyl- 1,1 -diyl, cyclopentyl- 1,1 -diyl, cyclohexyl- 1,1 -diyl, -CH 2 C(CH 2 CH 2 )- or -C(CH 2 CH 2 )CH 2 -;
- L 2 is absent, -CH 2 -, -CH 2 O-, -OCH 2 -, -CH 2 S-, -SCH 2 -, -CH 2 NH-, - NHCH 2 -, -O-, -S-, or -NH-;
- ring A is a substituted or unsubstituted phen-l,4-ylene, where if ring A is substituted then ring A
- L 1 is cyclopropyl- 1 , 1 -diyl; L 2 is absent. In some embodiments, L 1 is cyclopropyl- 1,1 -diyl; L 2 is absent; CY is cyclopropyl. In some embodiments, L 1 is cyclopropyl- 1,1 -diyl; L 2 is absent; CY is a substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with R c ; R c is F, Cl, -OH, -CH 3 , or CF 3 . [00332] In some embodiments, A 1 is CH; and A 4 is CH.
- the compound of Formula (III) has the structure:
- the compound of Formula (III) has the structure:
- the compound of Formula (I) has the structure of Formula (IV):
- a 1 is CR A and N;
- R A is H, F, Cl, Br, I, -CH 3 , or -CF 3 ;
- R 8 is H or -CH 3 ;
- CY is Ci-C ⁇ alkyl, C 3 -Cecycloalkyl, or substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with R c ; R c is F, Cl, Br, I, -OH, C 1 - C 4 alkyl, Ci-C 4 fluoroalkyl, Ci-C 4 fluoroalkoxy, or Ci-C 4 alkoxy; n is 1, 2, or 3.
- L 2 is absent, -CH 2 -, -CH 2 O-, -OCH 2 -, -CH 2 S-, -SCH 2 -, -0-, or - S-. In some embodiments, L is absent. [00337] In some embodiments, A 1 is CR A . [00338] In some embodiments, A 1 is N.
- CY is -CH 3 , -CH 2 CH 3 , -CH 2 CH 2 CH 3 , -CH 2 CH 2 CH 2 CH 3 , - CH(CH 3 ) 2 , -CH(CH 2 CH 3 ) 2 , -CH 2 CH(CH 3 ) 2 , -C(CH 3 ) 3 , -CH 2 (CH 3 ) 3 , C 3 -C 6 cycloalkyl, substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with R c ; R c is F, Cl, -CH 3 , or CF 3 .
- n is 1.
- CY is -C(CH 3 ) 3 or C 3 -C 6 cycloalkyl.
- the compound has the following structure:
- the compound has the following structure:
- R 4 is
- R 4 is . In one aspect, R 4 is
- the compound of Formula (I) has the structure of Formula (V) or a pharmaceutically acceptable salt thereof:
- Ci-C 4 alkyl is H, Ci-C 4 alkyl, C 3 -C 6 cycloalkyl, or Ci-C 4 fluoroalkyl;
- R 7 is H or Ci-C 4 alkyl;
- R 8 is H, Ci-C 4 alkyl, or Ci-C 4 fluoroalkyl;
- CY is a substituted or unsubstituted C 3 -C 6 cycloalkyl or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with 1 or 2 R c ;
- R 9 is H, Ci-C 6 alkyl, Ci-C 6 fluoroalkyl, C 3 -C 6 cycloalkyl, or a substituted or unsubstituted phenyl;
- R 10 is a Ci-C ⁇ alkyl, Ci-Ceflu
- Ci-C 4 fluoroalkyl Ci-C 4 fluoroalkoxy, Ci-C 4 alkoxy, and Ci-C 4 heteroalkyl
- m is 0, 1, or 2
- n is i, 2, 3 or 4
- p is 0, 1, or 2.
- R 3 is H or Ci-C 4 alkyl
- R 7 is H
- R 8 is H, -CH 3 or -CF 3
- R 10 is a Ci-C 6 alkyl or a substituted or unsubstituted phenyl
- each R A is independently selected from F, Cl, Br, I, -OH, -CH 3 , -CF 3 , - OCF 3 , and -OCH 3
- each R B is independently selected from F, Cl, Br, I, -OH, -CH 3 , -CF 3 , -OCF 3 , and -OCH 3
- each R c is independently selected from F, Cl, Br, I, -OH, -CH 3 , -CF 3 , -OCF 3 , and - OCH 3
- m is O or
- R 1 is -CO 2 H or -CO 2 R D ;
- R D is H, -CH 3 , or -CH 2 CH 3 ;
- R 3 is H, - CH 3 or -CH 2 CH 3 ;
- R 8 is H, or -CH 3 ;
- CY is a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with 1 or 2 R c .
- the compound of Formula (I) or Formula (V) has the following structure:
- the compound of Formula (I) or Formula (V) has the following structure:
- R 3 is H, -CH 3 or -CH 2 CH 3 ;
- R 8 is H, or -CH 3 ;
- R 10 is -CH 3 , or -CH 2 CH 3 .
- R 4 is H ;
- CY is a substituted or unsubstituted phenyl, wherein if CY is substituted phenyl then the phenyl is substituted with 1 or 2 R c ;
- R c is H, F, Cl, -OH, -CH 3 , -CF 3 , or -OCH 3 ;
- n is 1.
- R 8 is -CH 3 ;
- CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3-hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl
- the compound of Formula I) or Formula (V) has the following structure:
- R 1 is -CO 2 H;
- CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3-hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl
- CY is phenyl.
- R 1 is -CO 2 H;
- CY is phenyl, 2-fluorophenyl, 3 -fluorophenyl, 2-chlorophenyl, 3-chlorophenyl, 2-methylphenyl, 3- methylphenyl, 2-trifluoromethylphenyl, or 3-trifluoromethylphenyl.
- R 1 is -CO 2 H;
- CY is phenyl.
- R c is F, Cl, -CH 3 , or CF 3 .
- ring B is a substituted or unsubstituted phenylene; R c is F or Cl.
- the compound of Formula (I) or Formula (V) has the following structure:
- the compound of Formula (I) or Formula (V) has one of the following structures:
- the compound of Formula (I) is a 3-substituted- ⁇ 4-[l-(2-R c -phenyl)- ethoxycarbonylamino]-3-methyl-isoxazol-5-yl ⁇ -benzene compound, wherein 3-substituent is - i ⁇ B-l ⁇ R 1 , or pharmaceutically acceptable salts, pharmaceutically acceptable solvates, or prodrugs thereof;
- R A is H or Ci-C ⁇ alkyl;
- B is a divalent group selected from a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted
- R A is H or Ci-C ⁇ alkyl;
- B is a divalent group selected from a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, and a substituted or unsubstituted heteroarylene, where if ring B is substituted then each substituent on ring B is H or R B ; each R B is selected from H, halogen, -OH, Ci-C 4 alkyl, Ci-C 4 fluoroalky
- R c is H, F, Cl, -CH 3 , or CF 3 . In some other embodiments, R c is F or Cl. In other embodiments, R c is Cl. In yet other embodiments, R c is F. [00369] In some embodiments, L 1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl- 1 , 1 -diyl, cyclopentyl-1,1- diyl or cyclohexyl- 1 , 1 -diyl. In some embodiments, L 1 is cyclopropyl- 1 , 1 -diyl.
- the compound of Formula (I) is a 4-substituted- ⁇ 4-[l-(CY)- ethoxycarbonylamino]-3-methyl-isoxazol-5-yl ⁇ -benzene compound, wherein the 4-substituent is -i ⁇ B-l ⁇ R 1 , and CY is as described herein.
- the compound of Formula (I) is a 3-Substituted- ⁇ 4-[l-(CY)- ethoxycarbonylamino]-3-methyl-isoxazol-5-yl ⁇ -benzene compound, wherein the 3-substituent is -i ⁇ B-l ⁇ R 1 , and CY is as described herein
- ring B is a substituted or unsubstituted phenyl- 1,3 -ene or a substituted or unsubstituted phenyl- 1,4-ene, where ring B is unsubstituted or monosubstituted with R B .
- ring B is a substituted or unsubstituted phenyl- 1,3-ene, where ring B is unsubstituted or monosubstituted with R B .
- ring B is a substituted or unsubstituted phenyl- 1,4-ene, where ring B is unsubstituted or monosubstituted with R B .
- L 2 is absent.
- ring A is as described in any one of Tables 1 through 6.
- ring B is as described in any one of Tables 1 through 6.
- CY is as described in any one of Tables 1 through 6.
- L 2 is as described in any one of Tables 1 through 6.
- L 1 is as described in any one of Tables 1 through 6.
- R 8 is as described in any one of Tables 1 through 6.
- R 1 is as described in any one of Tables 1 through 6.
- the compound of Formula (I) has a structure selected from:
- compounds of Formula (I) include, but are not limited to, those described in the following Tables: Table 1:
- Phen-l,4-ylene i.e. ring B in Tables 4 through 6 is replaced with a group selected from Phen-l,2-ylene, Phen-l,3-ylene, Pyridin-2,3-ylene, Pyridin-2,4-ylene, Pyridin- 2,5-ylene, Pyridin-2,6-ylene, Pyridin-3 ,4-ylene, Pyridin-3,5-ylene, Pyridin-3,6-ylene, Pyridin- 4,5-ylene, Pyridin-4,6-ylene, Pyridin-5,6-ylene, Pyrimidin-2,4-ylene, Pyrimidin-2,5-ylene, Pyrimidin-4,5-ylene, Pyrimidin-4,6-ylene, Pyrazin-2,3-ylene, Pyrazin-2,5-ylene, Pyrazin-2,6- ylene, Pyridazin-3,4-ylene, Pyridazin-3,5-ylene,5-ylene,5-
- Phen-l,4-ylene (i.e. ring B) in Tables 4 through 6 is replaced with a group selected from Phen-l,2-ylene, Phen-l,3-ylene, Pyridin-2,3-ylene, Pyridin-2,4-ylene, Pyridin- 2,5-ylene, Pyridin-2,6-ylene, Pyridin-3 ,4-ylene, Pyridin-3, 5-ylene, Pyridin-3, 6-ylene, Pyridin- 4,5-ylene, Pyridin-4,6-ylene, Pyridin-5, 6-ylene, Pyrimidin-2,4-ylene, Pyrimidin-2,5-ylene, Pyrimidin-4,5-ylene, Pyrimidin-4,6-ylene, Pyrazin-2,3-ylene, Pyrazin-2,5-ylene, Pyrazin-2,6- ylene, Pyridazin-3,4-ylene, Pyridazin-3, 5-ylene, Pyrid
- Cl (i.e. R c ) in Tables 4 through 6 is replaced with F. In one aspect, Cl (i.e. R c ) in Tables 4 through 6 is replaced with ⁇ . In one aspect, Cl (i.e. R c ) in Tables 4 through 6 is replaced with -CH 3 . In one aspect, Cl (i.e. R c ) in Tables 4 through 6 is replaced with -CF 3 .
- the starting material used for the synthesis of the compounds of Formula (I), (II), (III), (IV) and (V) are either synthesized or obtained from commercial sources, such as, but not limited to, Sigma-Aldrich, Fluka, Acros Organics, Alfa Aesar, and the like.
- the compounds described herein, and other related compounds having different substituents are synthesized using techniques and materials described herein or otherwise known, including those found in March, ADVANCED ORGANIC CHEMISTRY 4 th Ed., (Wiley 1992); Carey and Sundberg, ADVANCED ORGANIC CHEMISTRY 4 th Ed., VoIs.
- the synthesis of compounds of Formula (I), (II), (III), (IV) and (V) described herein begins with the reaction of an alkyl acetoacetate with methylamine to provide a compound of structure l-II.
- Compounds of structure l-II are reacted with a substituted or unsubstituted 4-halo-benzoyl chloride (structure l-III, where A 1 and A 2 are both CH) to provide compounds of structure 1-TV.
- n is 0, 1, or 2.
- substituted napthylene carbonyl chlorides or susbtituted heteroaryl carbonyl chlorides are used instead of compounds
- n is 0. In other embodiments, n is 1.
- V provides carboxylic acids of structure 1-VI.
- a Curtius rearrangement of carboxylic acids of structure 1-VI in the presence of hydroxy compounds of structure 1-VII provides carbamate compounds of structure 1-VIII.
- the preparation of carbamates includes procedures described in Scemem 2.
- a Curtius rearrangement of carboxylic acids of structure 2-1 in the presence of tert- butanol provides carbamate compounds of structure 2-II. Removal of the tert-butyl group with acid provides amines of structure 2-III. Amines of structure 2-III are reacted with chloro formates to provide carbamates of structure 2-IV , where R4 is a carbamate as described herein.
- metal mediated couplings are used to provide compounds of Formula (I), (II), (III), (IV) and (V) as described in Scheme 4, Scheme 5, Scheme 6, and Scheme 7.
- Scheme 4
- a Suzuki reaction between compounds of structure 4-III and compounds of structure 4-1 or 4-11 is used to provide compounds of structure 4-IV or 4-V.
- the phenyl ring of compounds of structure 4-III is replaced with other cyclic rings (e.g. napthyl, cycloalkyl, monocyclic heteroaryl, bicyclic heteroaryl).
- metal mediated coupling reactions contemplated for the preparation of compounds of structure 4-IV and 4-V include, but are not limited to Suzuki reactions, Stille cross couplings, Negishi couplings, Kumada couplings, Ullmann reactions, Hiyama Coupling, and variants thereof (Metal-Catalyzed Cross- Coupling Reactions, Armin de Meijere (Editor), Francois Diederich (Editor), John Wiley & Sons; 2nd edition, 2004; Ozdemir, et al, Tetrahedron, 2005, 61, 9791-9798; Ackermann, et al, Org. Lett., 2006, 8, 3457-3460; Blakey, et al, J. Am. Chem.
- the coupling partners in the metal mediated reaction condition includes those outlined in Scheme 5.
- halides of structure 4-1 are reacted with a borylating agent using transition metal mediated reaction conditions to form boronate compounds of structure 5-II.
- Boronate compounds of structure 5-11 are reacted with compounds of structure 5-III under palladium mediated coupling conditions to form biaryl aldehydes of structure 5-IV.
- boronate esters of structure 5-11 are oxidized to provide hydroxy compounds of structure 6-11.
- An Ullman ether reaction between hydroxy compounds of structure 6-11 and boronic acid compounds of structure 6-III provides compounds of structure 6- IV.
- Methods of forming diaryl ethers, such as metal mediated reactions include but are not limited to the Ulman Ether synthesis, Chan-Lam coupling, and Buchwald-Hartwig synthesis (D. Ma, Q. Cai, Org. Lett., 2003, 5, 3799-3802; C. G. Bates, et ah, Org. Lett., 2002, 4, 2803-2806; C. H. Burgos, et al, Angew. Chem. Int.
- a prodrug is enzymatically metabolized by one or more steps or processes to the biologically, pharmaceutically or therapeutically active form of the compound.
- prodrugs are designed to alter the metabolic stability or the transport characteristics of a drug, to mask side effects or toxicity, to improve the flavor of a drug or to alter other characteristics or properties of a drug.
- Prodrug forms of the herein described compounds, wherein the prodrug is metabolized in vivo to produce a compound of Formula (I), (II), (III), (IV) or (V) as set forth herein are included within the scope of the claims. In some cases, some of the herein-described compounds may be a prodrug for another derivative or active compound.
- sites on the aromatic ring portion of compounds of Formula (I), (II), (III), (IV) or (V) are susceptible to various metabolic reactions Therefore incorporation of appropriate substituents on the aromatic ring structures will reduce, minimize or eliminate this metabolic pathway.
- the appropriate substituent to decrease or eliminate the susceptibility of the aromatic ring to metabolic reactions is, by way of example only, a halogen, or an alkyl group.
- the compounds described herein are labeled isotopically (e.g. with a radioisotope) or by another other means, including, but not limited to, the use of chromophores or fluorescent moieties, bioluminescent labels, or chemiluminescent labels.
- Compounds described herein include isotopically-labeled compounds, which are identical to those recited in the various formulae and structures presented herein, but for the fact that one or more atoms are replaced by an atom having an atomic mass or mass number different from the atomic mass or mass number usually found in nature.
- isotopes that can be incorporated into the present compounds include isotopes of hydrogen, carbon, nitrogen, oxygen, fluorine and chlorine, such as, for example, 2 H, 3 H, 13 C, 14 C, 15 N, 18 0, 17 O,
- pharmaceutically acceptable salt refers to a formulation of a compound that does not cause significant irritation to an organism to which it is administered and does not abrogate the biological activity and properties of the compound.
- pharmaceutically acceptable salts are obtained by reacting a compound of Formula (I), (II), (III), (IV) or (V) with acids.
- Pharmaceutically acceptable salts are also obtained by reacting a compound of Formula (I), (II), (III), (IV) or (V) with a base to form a salt.
- Compounds described herein may be formed as, and/or used as, pharmaceutically acceptable salts.
- compounds described herein may coordinate with an organic base, such as, but not limited to, ethanolamine, diethanolamine, triethanolamine, tromethamine, N- methylglucamine, dicyclohexylamine, tris(hydroxymethyl)methylamine.
- compounds described herein may form salts with amino acids such as, but not limited to, arginine, lysine, and the like.
- Acceptable inorganic bases used to form salts with compounds that include an acidic proton include, but are not limited to, aluminum hydroxide, calcium hydroxide, potassium hydroxide, sodium carbonate, sodium hydroxide, and the like.
- a reference to a pharmaceutically acceptable salt includes the solvent addition forms or crystal forms thereof, particularly solvates or polymorphs. Solvates contain either stoichiometric or non-stoichiometric amounts of a solvent, and may be formed during the process of crystallization with pharmaceutically acceptable solvents such as water, ethanol, and the like.
- the "alkyl” group may have 1 to 10 carbon atoms (whenever it appears herein, a numerical range such as “1 to 10" refers to each integer in the given range; e.g., "1 to 10 carbon atoms” means that the alkyl group may consist of 1 carbon atom, 2 carbon atoms, 3 carbon atoms, etc. , up to and including 10 carbon atoms, although the present definition also covers the occurrence of the term "alkyl” where no numerical range is designated).
- the alkyl group of the compounds described herein may be designated as "Ci-C 6 alkyl" or similar designations.
- Ci-C 6 alkyl indicates that there are one, two, three, four, five, or six carbon atoms in the alkyl chain.
- the alkyl is selected from the group consisting of methyl, ethyl, propyl, iso-propyl, n-butyl, iso-butyl, sec-butyl, and t-butyl.
- Typical alkyl groups include, but are in no way limited to, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tertiary butyl, pentyl, neopentyl, hexyl, allyl, but-2-enyl, but-3-enyl, and the like.
- an alkyl is a C r C 6 alkyl.
- Typical alkylene groups include, but are not limited to, -CH 2 -, -CH(CH 3 )-, - C(CHs) 2 -, -CH 2 CH 2 -, -CH 2 CH(CH 3 )-, -CH 2 C(CH 3 ) 2 -, -CH 2 CH 2 CH 2 -, -CH 2 CH 2 CH 2 CH 2 -, and the like.
- Carbocyclic or “carbocycle” refers to a ring or ring system where the atoms forming the backbone of the ring are all carbon atoms. The term thus distinguishes carbocyclic from heterocyclic rings in which the ring backbone contains at least one atom which is different from carbon.
- an aryl group can be a monoradical or a diradical (i.e., an arylene group).
- Examplary arylenes include, but are not limited to, phenyl- 1,2-ene, phenyl- 1,3 -ene, and phenyl- 1,4-ene.
- cycloalkyl refers to a monocyclic or polycyclic aliphatic, non-aromatic radical, wherein each of the atoms forming the ring (i.e. skeletal atoms) is a carbon atom. Cycloalkyls may be saturated, or partially unsaturated.
- Cycloalkyls may be fused with an aromatic ring, and the point of attachment is at a carbon that is not an aromatic ring carbon atom.
- Cycloalkyl groups include groups having from 3 to 10 ring atoms. In some embodiments, cycloalkyl groups are selected from among cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cycloheptyl, and cyclooctyl. Cycloalkyl groups may be substituted or unsubstituted.
- a cycloalkyl group can be a monoradical or a diradical (i.e., an cycloalkylene group, such as, but not limited to, cyclopropan-l,l-diyl, cyclobutan-l,l-diyl, cyclopentan-l,l-diyl, cyclohexan-l,l-diyl, cyclohexan-l,4-diyl, cycloheptan-l,l-diyl, and the like).
- a cycloalkyl is a C3-Cecycloalkyl.
- haloalkyl refers to an alkyl group in which one or more hydrogen atoms are replaced by one or more halide atoms.
- a haloalkyl is a Ci-C 4 haloalkyl.
- haloalkylene refers to an alkylene group in which one or more hydrogen atoms are replaced by one or more halide atoms.
- a haloalkylene is a C 1 - C ⁇ haloalkylene.
- a haloalkylene is a Ci-C 4 haloalkylene.
- fluoroalkyl refers to an alkyl in which one or more hydrogen atoms are replaced by a fluorine atom.
- a fluoralkyl is a Ci-C 4 iluoroalkyl.
- fluoroalkylene refers to an alkylene in which one or more hydrogen atoms are replaced by a fluorine atom.
- a fluoralkylene is a Ci-Cefluoroalkylene.
- a fluoralkylene is a Ci-C 4 iluoroalkylene.
- heteroalkyl refers to an alkyl group in which one or more skeletal atoms of the alkyl are selected from an atom other than carbon, e.g., oxygen, nitrogen, sulfur, phosphorus or combinations thereof.
- a heteroalkyl is a Ci-Ceheteroalkyl.
- heteroalkylene refers to an alkylene group in which one or more skeletal atoms of the alkyl are selected from an atom other than carbon, e.g., oxygen, nitrogen, sulfur, phosphorus or combinations thereof.
- a heteroalkylene is a Ci-Ceheteroalkylene.
- a heteroalkylene is a Ci-C 4 heteroalkylene.
- Examplary heteroalkylenes include, but are not limited to, -OCH 2 -, -OCH(CH 3 )-, -OC(CH 3 ) 2 -, -OCH 2 CH 2 -, -CH 2 O-, - CH(CH 3 )O-, -C(CHs) 2 O-, -CH 2 CH 2 O-, -CH 2 OCH 2 -, -CH 2 OCH 2 CH 2 -, -CH 2 CH 2 OCH 2 -, -SCH 2 -, -SCH(CH 3 )-, -SC(CHs) 2 -, -SCH 2 CH 2 -, -CH 2 S-, -CH(CH 3 )S-, -C(CH 3 ) 2 S-, -CH 2 CH 2 S-, - CH 2 SCH 2 -, -CH 2 SCH 2 CH 2 -, -CH 2 CH 2
- heterocycle refers to heteroaromatic rings (also known as heteroaryls) and heterocycloalkyl rings (also known as heteroalicyclic groups) containing one to four heteroatoms in the ring(s), where each heteroatom in the ring(s) is selected from O, S and N, wherein each heterocyclic group has from 4 to 10 atoms in its ring system, and with the proviso that the any ring does not contain two adjacent O or S atoms.
- Non-aromatic heterocyclic groups also known as heterocycloalkyls
- the heterocyclic groups include benzo-fused ring systems.
- An example of a 3-membered heterocyclic group is aziridinyl.
- An example of a 4-membered heterocyclic group is azetidinyl.
- An example of a 5-membered heterocyclic group is thiazolyl.
- An example of a 6-membered heterocyclic group is pyridyl, and an example of a 10-membered heterocyclic group is quinolinyl.
- non-aromatic heterocyclic groups are pyrrolidinyl, tetrahydrofuranyl, dihydrofuranyl, tetrahydrothienyl, oxazolidinonyl, tetrahydropyranyl, dihydropyranyl, tetrahydrothiopyranyl, piperidinyl, morpholinyl, thiomorpholinyl, thioxanyl, piperazinyl, aziridinyl, azetidinyl, oxetanyl, thietanyl, homopiperidinyl, oxepanyl, thiepanyl, oxazepinyl, diazepinyl, thiazepinyl, 1,2,3,6-tetrahydropyridinyl, pyrrolin-2-yl, pyrrolin-3-yl, indolinyl, 2H- pyranyl, 4H-pyranyl, dioxanyl
- aromatic heterocyclic groups are pyridinyl, imidazolyl, pyrimidinyl, pyrazolyl, triazolyl, pyrazinyl, tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl, pyrrolyl, quinolinyl, isoquinolinyl, indolyl, benzimidazolyl, benzofuranyl, cinnolinyl, indazolyl, indolizinyl, phthalazinyl, pyridazinyl, triazinyl, isoindolyl, pteridinyl, purinyl, oxadiazolyl, thiadiazolyl, furazanyl, benzofurazanyl, benzothiophenyl, benzothiazolyl, benzoxazolyl, quinazolinyl, quinox
- the foregoing groups may be C- attached or N-attached where such is possible.
- a group derived from pyrrole may be pyrrol- 1-yl (N-attached) or pyrrol-3-yl (C-attached).
- a group derived from imidazole may be imidazol-1-yl or imidazol-3-yl (both N-attached) or imidazol-2-yl, imidazol-4-yl or imidazol-5-yl (all C-attached).
- the heterocyclic groups include benzo-fused ring systems.
- heteroaryl or, alternatively, “heteroaromatic” refers to an aryl group that includes one or more ring heteroatoms selected from nitrogen, oxygen and sulfur.
- heteroaryl groups include the following moieties:
- Monocyclic heteroaryls include pyridinyl, imidazolyl, pyrimidinyl, pyrazolyl, triazolyl, pyrazinyl, tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl, pyrrolyl, pyridazinyl, triazinyl, oxadiazolyl, thiadiazolyl, and furazanyl.
- a heteroaryl contains 0-3 N atoms.
- a heteroaryl contains 1-3 N atoms.
- a heteroaryl contains 0-3 N atoms, 0-1 O atoms, and 0-1 S atoms.
- a heteroaryl is a monocyclic or bicyclic heteroaryl.
- heteroaryl is a Ci-Cgheteroaryl.
- monocyclic heteroaryl is a Ci-Csheteroaryl.
- monocyclic heteroaryl is a 5-membered or 6-membered heteroaryl.
- bicyclic heteroaryl is a Ce-Cgheteroaryl.
- a heteroaryl group can be a monoradical or a diradical (i.e., a heteroarylene group).
- heterocycloalkyl or “heteroalicyclic” group refers to a cycloalkyl group that includes at least one heteroatom selected from nitrogen, oxygen and sulfur.
- the radicals may be fused with an aryl or heteroaryl.
- heterocycloalkyl groups also referred to as non-aromatic heterocycles, include:
- the heterocycloalkyl is selected from oxazolidinonyl, pyrrolidinyl, tetrahydrofuranyl, tetrahydrothienyl, tetrahydropyranyl, tetrahydrothiopyranyl, piperidinyl, morpholinyl, thiomorpholinyl, piperazinyl, and indolinyl.
- the term heteroalicyclic also includes all ring forms of the carbohydrates, including but not limited to the monosaccharides, the disaccharides and the oligosaccharides.
- a heterocycloalkyl is a C 2 -Cioheterocycloalkyl.
- a heterocycloalkyl is a C 4 -Cioheterocycloalkyl. In one aspect, a heterocycloalkyl contains 0-2 N atoms. In another aspect, a heterocycloalkyl contains 0-2 N atoms, 0-2 O atoms or 0-1 S atoms. [00451]
- the term "bond” or “single bond” refers to a chemical bond between two atoms, or two moieties when the atoms joined by the bond are considered to be part of larger substructure. In one aspect, when a group described herein is a bond, the referenced group is absent thereby allowing a bond to be formed between the remaining identified groups.
- membered ring includes any cyclic structure.
- membered is meant to denote the number of skeletal atoms that constitute the ring.
- cyclohexyl, pyridinyl, pyranyl and thiopyranyl are 6-membered rings and cyclopentyl, pyrrolyl, furanyl, and thienyl are 5-membered rings.
- moiety refers to a specific segment or functional group of a molecule. Chemical moieties are often recognized chemical entities embedded in or appended to a molecule.
- carboxylic acid bioisostere refers to a functional group or moiety that exhibits similar physical, biological and/or chemical properties as a carboxylic acid moiety.
- Examples of carboxylic acid bioisosteres include, but are not limited to,
- the term "optionally substituted” or “substituted” means that the referenced group may be substituted with one or more additional group(s) individually and independently selected from alkyl, cycloalkyl, aryl, heteroaryl, heteroalicyclic, hydroxy, alkoxy, aryloxy, alkylthio, arylthio, alkylsulfoxide, arylsulfoxide, alkylsulfone, arylsulfone, cyano, halo, nitro, haloalkyl, fluoroalkyl, fluoroalkoxy, and amino, including mono- and di-substituted amino groups, and the protected derivatives thereof.
- substituents are selected from halogen, -CN, -NH 2 , -OH, -N(CH 3 ) 2 , alkyl, fluoroalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkoxy, aryloxy, alkylthio, arylthio, alkylsulfoxide, arylsulfoxide, alkylsulfone, and arylsulfone.
- an optional substituent is selected from halogen, -CN, -NH 2 , -OH, -NH(CH 3 ), - N(CH 3 ) 2 , -CH 3 , -CH 2 CH 3 , -CF 3 , -OCH 3 , and -OCF 3 .
- substituted groups are substituted with one or two of the preceding groups.
- substituted groups are substituted with one of the preceding groups.
- the compounds presented herein possess one or more stereocenters and each center independently exists in either the R or S configuration.
- the compounds presented herein include all diastereomeric, enantiomeric, and epimeric forms as well as the appropriate mixtures thereof.
- Stereoisomers are obtained, if desired, by methods such as, stereoselective synthesis and/or the separation of stereoisomers by chiral chromatographic columns.
- the methods and formulations described herein include the use of N-oxides (if appropriate), crystalline forms (also known as polymorphs), or pharmaceutically acceptable salts of compounds having the structure of Formula (I), (II), (III), (IV) or (V), as well as active metabolites of these compounds having the same type of activity.
- compounds may exist as tautomers. All tautomers are included within the scope of the compounds presented herein.
- the compounds described herein exist in solvated forms with pharmaceutically acceptable solvents such as water, ethanol, and the like. In other embodiments, the compounds described herein exist in unsolvated form.
- acceptable with respect to a formulation, composition or ingredient, as used herein, means having no persistent detrimental effect on the general health of the subject being treated.
- modulate means to interact with a target either directly or indirectly so as to alter the activity of the target, including, by way of example only, to enhance the activity of the target, to inhibit the activity of the target, to limit the activity of the target, or to extend the activity of the target.
- modulator refers to a molecule that interacts with a target either directly or indirectly.
- the interactions include, but are not limited to, the interactions of an agonist, partial agonist, an inverse agonist and antagonist.
- a modulator is an antagonist.
- agonist refers to a molecule such as a compound, a drug, an enzyme activator or a hormone modulator that binds to a specific receptor and triggers a response in the cell.
- An agonist mimics the action of an endogenous ligand (such as LPA, prostaglandin, hormone or neurotransmitter) that binds to the same receptor.
- Antagonist refers to a molecule such as a compound, which diminishes, inhibits, or prevents the action of another molecule or the activity of a receptor site. Antagonists include, but are not limited to, competitive antagonists, non-competitive antagonists, uncompetitive antagonists, partial agonists and inverse agonists.
- LPA-dependent refers to conditions or disorders that would not occur, or would not occur to the same extent, in the absence of LPA.
- LPA-mediated refers to conditions or disorders that might occur in the absence of LPA but can occur in the presence of LPA.
- selectivity for one LPA receptor versus other LPA receptors means that the compound has an IC 50 (Ca Flux assay) for the indicated LPA receptor that is at least 10-fold less than the IC50 for other LPA receptors.
- selectivity for one LPA receptor versus other LPA receptor means that the compound has an IC50 for the indicated LPA receptor that is at least 10-fold, at least 20-fold, at least 40-fold, at least 50-fold, at least 100-fold, at least 200-fold, at least 500-fold, or at least 1000-fold, less than the IC 50 for other LPA receptors.
- a selective LPAi receptor antagonist has an IC50 that is at least 10-fold, at least 20- fold, at least 40-fold, at least 50-fold, at least 100-fold, at least 200-fold, at least 500-fold, or at least 1000-fold, less than the IC 50 for other LPA receptors (e.g. LPA 2 , LPA 3 ).
- LPA 2 , LPA 3 LPA 2 , LPA 3
- co-administration or the like, as used herein, are meant to encompass administration of the selected therapeutic agents to a single patient, and are intended to include treatment regimens in which the agents are administered by the same or different route of administration or at the same or different time.
- an “effective amount” or “therapeutically effective amount,” as used herein, refer to a sufficient amount of an agent or a compound being administered which will relieve to some extent one or more of the symptoms of the disease or condition being treated. The result can be reduction and/or alleviation of the signs, symptoms, or causes of a disease, or any other desired alteration of a biological system.
- an “effective amount” for therapeutic uses is the amount of the composition comprising a compound as disclosed herein required to provide a clinically significant decrease in disease symptoms.
- An appropriate “effective” amount in any individual case may be determined using techniques, such as a dose escalation study.
- the terms “enhance” or “enhancing,” as used herein, means to increase or prolong either in potency or duration a desired effect.
- the term “enhancing” refers to the ability to increase or prolong, either in potency or duration, the effect of other therapeutic agents on a system.
- An “enhancing-effective amount,” as used herein, refers to an amount adequate to enhance the effect of another therapeutic agent in a desired system.
- a “metabolite” of a compound disclosed herein is a derivative of that compound that is formed when the compound is metabolized.
- active metabolite refers to a biologically active derivative of a compound that is formed when the compound is metabolized.
- metabolism refers to the sum of the processes (including, but not limited to, hydrolysis reactions and reactions catalyzed by enzymes) by which a particular substance is changed by an organism. Thus, enzymes may produce specific structural alterations to a compound.
- cytochrome P450 catalyzes a variety of oxidative and reductive reactions while uridine diphosphate glucuronyltransferases catalyze the transfer of an activated glucuronic-acid molecule to aromatic alcohols, aliphatic alcohols, carboxylic acids, amines and free sulphydryl groups.
- carboxylic acid containing compounds form taurine conjugates in vivo.
- Metabolites of the compounds disclosed herein are optionally identified either by administration of compounds to a host and analysis of tissue samples from the host, or by incubation of compounds with hepatic cells in vitro and analysis of the resulting compounds.
- the term "pharmaceutical combination” as used herein, means a product that results from the mixing or combining of more than one active ingredient and includes both fixed and non- fixed combinations of the active ingredients.
- the term “fixed combination” means that the active ingredients, e.g. a compound of Formula (I), (II), (III), (IV) or (V) and a co-agent, are both administered to a patient simultaneously in the form of a single entity or dosage.
- non- fixed combination means that the active ingredients, e.g.
- a compound of Formula (I), (II), (III), (IV) or (V) and a co-agent are administered to a patient as separate entities either simultaneously, concurrently or sequentially with no specific intervening time limits, wherein such administration provides effective levels of the two compounds in the body of the patient.
- cocktail therapy e.g. the administration of three or more active ingredients.
- mammals include, but are not limited to, any member of the Mammalian class: humans, non- human primates such as chimpanzees, and other apes and monkey species; farm animals such as cattle, horses, sheep, goats, swine; domestic animals such as rabbits, dogs, and cats; laboratory animals including rodents, such as rats, mice and guinea pigs, and the like.
- the mammal is a human.
- treat include alleviating, abating or ameliorating at least one symptom of a disease disease or condition, preventing additional symptoms, inhibiting the disease or condition, e.g., arresting the development of the disease or condition, relieving the disease or condition, causing regression of the disease or condition, relieving a condition caused by the disease or condition, or stopping the symptoms of the disease or condition either prophylactically and/or therapeutically.
- Routes of Administration include alleviating, abating or ameliorating at least one symptom of a disease disease or condition, preventing additional symptoms, inhibiting the disease or condition, e.g., arresting the development of the disease or condition, relieving the disease or condition, causing regression of the disease or condition, relieving a condition caused by the disease or condition, or stopping the symptoms of the disease or condition either prophylactically and/or therapeutically.
- Suitable routes of administration include, but are not limited to, oral, intravenous, rectal, aerosol, parenteral, ophthalmic, pulmonary, transmucosal, transdermal, vaginal, otic, nasal, and topical administration.
- parenteral delivery includes intramuscular, subcutaneous, intravenous, intramedullary injections, as well as intrathecal, direct intraventricular, intraperitoneal, intralymphatic, and intranasal injections.
- a compound as described herein is administered in a local rather than systemic manner, for example, via injection of the compound directly into an organ, often in a depot preparation or sustained release formulation.
- long acting formulations are administered by implantation (for example subcutaneous Iy or intramuscularly) or by intramuscular injection.
- the drug is delivered in a targeted drug delivery system, for example, in a liposome coated with organ-specific antibody.
- the liposomes are targeted to and taken up selectively by the organ.
- the compound as described herein is provided in the form of a rapid release formulation, in the form of an extended release formulation, or in the form of an intermediate release formulation.
- the compound described herein is administered topically.
- the compounds described herein are formulated into pharmaceutical compositions.
- Pharmaceutical compositions are formulated in a conventional manner using one or more pharmaceutically acceptable inactive ingredients that facilitate processing of the active compounds into preparations that can be used pharmaceutically. Proper formulation is dependent upon the route of administration chosen.
- a summary of pharmaceutical compositions described herein can be found, for example, in Remington: The Science and Practice of Pharmacy, Nineteenth Ed (Easton, Pa.: Mack Publishing Company, 1995); Hoover, John E., Remington's Pharmaceutical Sciences, Mack Publishing Co., Easton, Pennsylvania 1975; Liberman, H.A.
- compositions that include a compound of Formula (I), (II), (III), (IV) or (V) and at least one pharmaceutically acceptable inactive ingredient.
- the compounds described herein are administered as pharmaceutical compositions in which a compound of Formula (I), (II), (III), (IV) or (V) is mixed with other active ingredients, as in combination therapy.
- the pharmaceutical compositions include other medicinal or pharmaceutical agents, carriers, adjuvants, preserving, stabilizing, wetting or emulsifying agents, solution promoters, salts for regulating the osmotic pressure, and/or buffers.
- the pharmaceutical compositions include other therapeutically valuable substances.
- a pharmaceutical composition refers to a mixture of a compound of Formula (I), (II), (III), (IV) or (V) with other chemical components (i.e. pharmaceutically acceptable inactive ingredients), such as carriers, excipients, binders, filling agents, suspending agents, flavoring agents, sweetening agents, disintegrating agents, dispersing agents, surfactants, lubricants, colorants, diluents, solubilizers, moistening agents, plasticizers, stabilizers, penetration enhancers, wetting agents, anti-foaming agents, antioxidants, preservatives, or one or more combination thereof.
- the pharmaceutical composition facilitates administration of the compound to an organism.
- therapeutically effective amounts of compounds described herein are administered in a pharmaceutical composition to a mammal having a disease, disorder, or condition to be treated.
- the mammal is a human.
- a therapeutically effective amount can vary widely depending on the severity of the disease, the age and relative health of the subject, the potency of the compound used and other factors.
- the compounds can be used singly or in combination with one or more therapeutic agents as components of mixtures.
- the pharmaceutical formulations described herein are administered to a subject by appropriate administration routes, including but not limited to, oral, parenteral (e.g., intravenous, subcutaneous, intramuscular), intranasal, buccal, topical, rectal, or transdermal administration routes.
- the pharmaceutical formulations described herein include, but are not limited to, aqueous liquid dispersions, self-emulsifying dispersions, solid solutions, liposomal dispersions, aerosols, solid dosage forms, powders, immediate release formulations, controlled release formulations, fast melt formulations, tablets, capsules, pills, delayed release formulations, extended release formulations, pulsatile release formulations, multiparticulate formulations, and mixed immediate and controlled release formulations.
- compositions including a compound of Formula (I), (II), (III), (IV) or (V) are manufactured in a conventional manner, such as, by way of example only, by means of conventional mixing, dissolving, granulating, dragee-making, levigating, emulsifying, encapsulating, entrapping or compression processes.
- the pharmaceutical compositions will include at least one compound of Formula (I), (II), (III), (IV) or (V) as an active ingredient in free-acid or free-base form, or in a pharmaceutically acceptable salt form.
- the methods and pharmaceutical compositions described herein include the use of N-oxides (if appropriate), crystalline forms, amorphous phases, as well as active metabolites of these compounds having the same type of activity.
- compounds described herein exist in unsolvated form or in solvated forms with pharmaceutically acceptable solvents such as water, ethanol, and the like. The solvated forms of the compounds presented herein are also considered to be disclosed herein.
- compositions provided herein include one or more preservatives to inhibit microbial activity.
- Suitable preservatives include mercury-containing substances such as merfen and thiomersal; stabilized chlorine dioxide; and quaternary ammonium compounds such as benzalkonium chloride, cetyltrimethylammonium bromide and cetylpyridinium chloride.
- formulations described herein benefit from antioxidants, metal chelating agents, thiol containing compounds and other general stabilizing agents.
- stabilizing agents include, but are not limited to: (a) about 0.5% to about 2% w/v glycerol, (b) about 0.1% to about 1% w/v methionine, (c) about 0.1% to about 2% w/v monothioglycerol, (d) about 1 mM to about 10 mM EDTA, (e) about 0.01% to about 2% w/v ascorbic acid, (f) 0.003% to about 0.02% w/v polysorbate 80, (g) 0.001% to about 0.05% w/v.
- polysorbate 20 (h) arginine, (i) heparin, (j) dextran sulfate, (k) cyclodextrins, (1) pentosan polysulfate and other heparinoids, (m) divalent cations such as magnesium and zinc; or (n) combinations thereof.
- compositions described herein which include a compound of Formula (I), (II), (III), (IV) or (V) are formulated into any suitable dosage form, including but not limited to, aqueous oral dispersions, liquids, gels, syrups, elixirs, slurries, suspensions, solid oral dosage forms, aerosols, controlled release formulations, fast melt formulations, effervescent formulations, lyophilized formulations, tablets, powders, pills, dragees, capsules, delayed release formulations, extended release formulations, pulsatile release formulations, multiparticulate formulations, and mixed immediate release and controlled release formulations.
- aqueous oral dispersions liquids, gels, syrups, elixirs, slurries, suspensions, solid oral dosage forms, aerosols, controlled release formulations, fast melt formulations, effervescent formulations, lyophilized formulations, tablets, powders, pills, dragees, capsules, delayed release formulations, extended release formulation
- compositions for oral use are obtained by mixing one or more solid excipient with one or more of the compounds described herein, optionally grinding the resulting mixture, and processing the mixture of granules, after adding suitable auxiliaries, if desired, to obtain tablets or dragee cores.
- Suitable excipients include, for example, fillers such as sugars, including lactose, sucrose, mannitol, or sorbitol; cellulose preparations such as, for example, maize starch, wheat starch, rice starch, potato starch, gelatin, gum tragacanth, methylcellulose, microcrystalline cellulose, hydroxypropylmethylcellulose, sodium carboxymethylcellulose; or others such as: polyvinylpyrrolidone (PVP or povidone) or calcium phosphate.
- disintegrating agents are added, such as the cross-linked croscarmellose sodium, polyvinylpyrrolidone, agar, or alginic acid or a salt thereof such as sodium alginate.
- dyestuffs or pigments are added to the tablets or dragee coatings for identification or to characterize different combinations of active compound doses.
- compositions that are administered orally include push-fit capsules made of gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer, such as glycerol or sorbitol.
- the push-fit capsules contain the active ingredients in admixture with filler such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers.
- the active compounds are dissolved or suspended in suitable liquids, such as fatty oils, liquid paraffin, or liquid polyethylene glycols. In some embodiments, stabilizers are added.
- All formulations for oral administration are in dosages suitable for such administration.
- solid oral soage forms are prepared by mixing a compound of Formula (I), (II), (III), (IV) or (V) with one or more of the following: antioxidants, flavoring agents, and carrier materials such as binders, suspending agents, disintegration agents, filling agents, surfactants, solubilizers, stabilizers, lubricants, wetting agents, and diluents.
- antioxidants such as binders, suspending agents, disintegration agents, filling agents, surfactants, solubilizers, stabilizers, lubricants, wetting agents, and diluents.
- the solid dosage forms disclosed herein are in the form of a tablet, (including a suspension tablet, a fast-melt tablet, a bite-disintegration tablet, a rapid- disintegration tablet, an effervescent tablet, or a caplet), a pill, a powder, a capsule, solid dispersion, solid solution, bioerodible dosage form, controlled release formulations, pulsatile release dosage forms, multiparticulate dosage forms, beads, pellets, granules.
- the pharmaceutical formulation is in the form of a powder.
- the pharmaceutical formulation is in the form of a tablet.
- pharmaceutical formulations of the compound of Formula (I), (II), (III), (IV) or (V) is in the form of a capsule.
- solid dosage forms e.g., tablets, effervescent tablets, and capsules, are prepared by mixing particles of a compound of Formula (I), (II), (III), (IV) or (V) with one or more pharmaceutical excipients to form a bulk blend composition.
- the bulk blend is readily subdivided into equally effective unit dosage forms, such as tablets, pills, and capsules.
- the individual unit dosages include film coatings. These formulations are manufactured by conventional formulation techniques.
- Conventional formulation techniques include, e.g., one or a combination of methods: (1) dry mixing, (2) direct compression, (3) milling, (4) dry or non-aqueous granulation, (5) wet granulation, or (6) fusion.
- Other methods include, e.g., spray drying, pan coating, melt granulation, granulation, fluidized bed spray drying or coating (e.g., wurster coating), tangential coating, top spraying, tableting, extruding and the like.
- Suitable carriers for use in the solid dosage forms described herein include, but are not limited to, acacia, gelatin, colloidal silicon dioxide, calcium glycerophosphate, calcium lactate, maltodextrin, glycerine, magnesium silicate, sodium caseinate, soy lecithin, sodium chloride, tricalcium phosphate, dipotassium phosphate, sodium stearoyl lactylate, carrageenan, monoglyceride, diglyceride, pregelatinized starch, hydroxypropylmethylcellulose, hydroxypropylmethylcellulose acetate stearate, sucrose, microcrystalline cellulose, lactose, mannitol and the like.
- Suitable filling agents for use in the solid dosage forms described herein include, but are not limited to, lactose, calcium carbonate, calcium phosphate, dibasic calcium phosphate, calcium sulfate, microcrystalline cellulose, cellulose powder, dextrose, dextrates, dextran, starches, pregelatinized starch, hydroxypropylmethycellulose (HPMC), hydroxypropylmethycellulose phthalate, hydroxypropylmethylcellulose acetate stearate (HPMCAS), sucrose, xylitol, lactitol, mannitol, sorbitol, sodium chloride, polyethylene glycol, and the like.
- Suitable disintegrants for use in the solid dosage forms described herein include, but are not limited to, natural starch such as corn starch or potato starch, a pregelatinized starch, or sodium starch glycolate, a cellulose such as methylcrystalline cellulose, methylcellulose, microcrystalline cellulose, croscarmellose, or a cross-linked cellulose, such as cross-linked sodium carboxymethylcellulose, cross-linked carboxymethylcellulose, or cross-linked croscarmellose, a cross-linked starch such as sodium starch glycolate, a cross-linked polymer such as crospovidone, a cross-linked polyvinylpyrrolidone, alginate such as alginic acid or a salt of alginic acid such as sodium alginate, a gum such as agar, guar, locust bean, Karaya, pectin, or tragacanth, sodium starch glycolate, bentonite, sodium lauryl sulfate, sodium lauryl
- Binders impart cohesiveness to solid oral dosage form formulations: for powder filled capsule formulation, they aid in plug formation that can be filled into soft or hard shell capsules and for tablet formulation, they ensure the tablet remaining intact after compression and help assure blend uniformity prior to a compression or fill step.
- binders in the solid dosage forms described herein include, but are not limited to, carboxymethylcellulose, methylcellulose, hydroxypropylmethylcellulose, hydroxypropylmethylcellulose acetate stearate, hydroxyethylcellulose, hydroxypropylcellulose, ethylcellulose, and microcrystalline cellulose, microcrystalline dextrose, amylose, magnesium aluminum silicate, polysaccharide acids, bentonites, gelatin, polyvinylpyrrolidone/vinyl acetate copolymer, crospovidone, povidone, starch, pregelatinized starch, tragacanth, dextrin, a sugar, such as sucrose, glucose, dextrose, molasses, mannitol, sorbitol, xylitol, lactose, a natural or synthetic gum such as acacia, tragacanth, ghatti gum, mucilage of isapol husks, starch, polyvin
- binder levels of 20-70% are used in powder- filled gelatin capsule formulations. Binder usage level in tablet formulations varies whether direct compression, wet granulation, roller compaction, or usage of other excipients such as fillers which itself can act as moderate binder. Binder levels of up to 70% in tablet formulations is common.
- Suitable lubricants or glidants for use in the solid dosage forms described herein include, but are not limited to, stearic acid, calcium hydroxide, talc, corn starch, sodium stearyl fumerate, alkali-metal and alkaline earth metal salts, such as aluminum, calcium, magnesium, zinc, stearic acid, sodium stearates, magnesium stearate, zinc stearate, waxes, Stearowet ® , boric acid, sodium benzoate, sodium acetate, sodium chloride, leucine, a polyethylene glycol or a methoxypolyethylene glycol such as CarbowaxTM, PEG 4000, PEG 5000, PEG 6000, propylene glycol, sodium oleate, glyceryl behenate, glyceryl palmitostearate, glyceryl benzoate, magnesium or sodium lauryl sulfate, and the like.
- stearic acid calcium hydroxide, talc
- Suitable diluents for use in the solid dosage forms described herein include, but are not limited to, sugars (including lactose, sucrose, and dextrose), polysaccharides (including dextrates and maltodextrin), polyols (including mannitol, xylitol, and sorbitol), cyclodextrins and the like.
- Suitable wetting agents for use in the solid dosage forms described herein include, for example, oleic acid, glyceryl monostearate, sorbitan monooleate, sorbitan monolaurate, triethanolamine oleate, polyoxyethylene sorbitan monooleate, polyoxyethylene sorbitan monolaurate, quaternary ammonium compounds (e.g., Polyquat 10 ® ), sodium oleate, sodium lauryl sulfate, magnesium stearate, sodium docusate, triacetin, vitamin E TPGS and the like.
- quaternary ammonium compounds e.g., Polyquat 10 ®
- sodium oleate sodium lauryl sulfate
- magnesium stearate sodium docusate
- triacetin vitamin E TPGS and the like.
- Suitable surfactants for use in the solid dosage forms described herein include, for example, sodium lauryl sulfate, sorbitan monooleate, polyoxyethylene sorbitan monooleate, polysorbates, polaxomers, bile salts, glyceryl monostearate, copolymers of ethylene oxide and propylene oxide, e.g., Pluronic ® (BASF), and the like.
- Suitable suspending agents for use in the solid dosage forms described here include, but are not limited to, polyvinylpyrrolidone, e.g., polyvinylpyrrolidone K12, polyvinylpyrrolidone Kl 7, polyvinylpyrrolidone K25, or polyvinylpyrrolidone K30, polyethylene glycol, e.g., the polyethylene glycol can have a molecular weight of about 300 to about 6000, or about 3350 to about 4000, or about 7000 to about 5400, vinyl pyrrolidone/vinyl acetate copolymer (S630), sodium carboxymethylcellulose, methylcellulose, hydroxy-propylmethylcellulose, polysorbate- 80, hydroxyethylcellulose, sodium alginate, gums, such as, e.g., gum tragacanth and gum acacia, guar gum, xanthans, including xanthan gum, sugars, cellulosics,
- Suitable antioxidants for use in the solid dosage forms described herein include, for example, e.g., butylated hydroxytoluene (BHT), sodium ascorbate, and tocopherol.
- BHT butylated hydroxytoluene
- sodium ascorbate sodium ascorbate
- tocopherol sodium ascorbate
- BHT butylated hydroxytoluene
- tocopherol sodium ascorbate
- Compressed tablets are solid dosage forms prepared by compacting the bulk blend of the formulations described above.
- the formulations are placed in a soft gelatin capsule.
- the formulations are placed in standard gelatin capsules or non-gelatin capsules such as capsules comprising HPMC.
- the formulation is placed in a sprinkle capsule, wherein the capsule is swallowed whole or the capsule is opened and the contents sprinkled on food prior to eating.
- a powder including a compound of Formula (I), (II), (III), (IV) or (V) is formulated to include one or more pharmaceutical excipients and flavors.
- Such a powder is prepared, for example, by mixing the the compound of Formula (I), (II), (III), (IV) or (V) and optional pharmaceutical excipients to form a bulk blend composition. Additional embodiments also include a suspending agent and/or a wetting agent. This bulk blend is uniformly subdivided into unit dosage packaging or multi-dosage packaging units.
- effervescent powders are also prepared. Effervescent salts have been used to disperse medicines in water for oral administration.
- the pharmaceutical solid oral dosage forms are formulated to provide a controlled release of the compound of Formula (I), (II), (III), (IV) or (V).
- Controlled release refers to the release of the compound of Formula (I), (II), (III), (IV) or (V) from a dosage form in which it is incorporated according to a desired profile over an extended period of time.
- Controlled release profiles include, for example, sustained release, prolonged release, pulsatile release, and delayed release profiles.
- controlled release compositions allow delivery of an agent to a subject over an extended period of time according to a predetermined profile.
- liquid formulation dosage forms for oral administration are in the form of aqueous suspensions selected from the group including, but not limited to, pharmaceutically acceptable aqueous oral dispersions, emulsions, solutions, elixirs, gels, and syrups. See, e.g., Singh et al.., Encyclopedia of Pharmaceutical Technology, 2nd Ed., pp. 754-757 (2002).
- Examples of disintegrating agents for use in the aqueous suspensions and dispersions include, but are not limited to, a starch, e.g., a natural starch such as corn starch or potato starch, a pregelatinized starch, or sodium starch glycolate; a cellulose such as methylcrystalline cellulose, methylcellulose, croscarmellose, or a cross-linked cellulose, such as cross-linked sodium carboxymethylcellulose, cross-linked carboxymethylcellulose, or cross-linked croscarmellose; a cross-linked starch such as sodium starch glycolate; a cross-linked polymer such as crospovidone; a cross-linked polyvinylpyrrolidone; alginate such as alginic acid or a salt of alginic acid such as sodium alginate; a gum such as agar, guar, locust bean, Karaya, pectin, or tragacanth; sodium starch glycolate; bentonite; a starch,
- the dispersing agents suitable for the aqueous suspensions and dispersions described herein include, for example, hydrophilic polymers, electrolytes, Tween ® 60 or 80, PEG, polyvinylpyrrolidone, and the carbohydrate-based dispersing agents such as, for example, hydroxypropylcellulose and hydroxypropyl cellulose ethers, hydroxypropyl methylcellulose and hydroxypropyl methylcellulose ethers, carboxymethylcellulose sodium, methylcellulose, hydroxyethylcellulose, hydroxypropylmethyl-cellulose phthalate, hydroxypropylmethyl-cellulose acetate stearate, noncrystalline cellulose, magnesium aluminum silicate, triethanolamine, polyvinyl alcohol (PVA), polyvinylpyrrolidone/vinyl acetate copolymer, 4-(l,l,3,3-tetramethylbutyl)-phenol polymer with ethylene oxide and formaldehyde (also known as tyloxapol
- Wetting agents suitable for the aqueous suspensions and dispersions described herein include, but are not limited to, cetyl alcohol, glycerol monostearate, polyoxyethylene sorbitan fatty acid esters (e.g., the commercially available Tweens ® such as e.g., Tween 20 ® and Tween 80 ® , and polyethylene glycols, oleic acid, glyceryl monostearate, sorbitan monooleate, sorbitan monolaurate, triethanolamine oleate, polyoxyethylene sorbitan monooleate, polyoxyethylene sorbitan monolaurate, sodium oleate, sodium lauryl sulfate, sodium docusate, triacetin, vitamin E TPGS, sodium taurocholate, simethicone, phosphotidylcholine and the like
- Tweens ® such as e.g., Tween 20 ® and Tween 80 ®
- Suitable preservatives for the aqueous suspensions or dispersions described herein include, for example, potassium sorbate, parabens (e.g., methylparaben and propylparaben), benzoic acid and its salts, other esters of parahydroxybenzoic acid such as butylparaben, alcohols such as ethyl alcohol or benzyl alcohol, phenolic compounds such as phenol, or quaternary compounds such as benzalkonium chloride.
- Preservatives, as used herein, are incorporated into the dosage form at a concentration sufficient to inhibit microbial growth.
- Suitable viscosity enhancing agents for the aqueous suspensions or dispersions described herein include, but are not limited to, methyl cellulose, xanthan gum, carboxymethyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose, Plasdon ® S-630, carbomer, polyvinyl alcohol, alginates, acacia, chitosans and combinations thereof.
- concentration of the viscosity enhancing agent will depend upon the agent selected and the viscosity desired.
- the liquid formulations also include inert diluents commonly used in the art, such as water or other solvents, solubilizing agents, and emulsifiers.
- emulsifiers are ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propyleneglycol, 1,3-butyleneglycol, dimethylformamide, sodium lauryl sulfate, sodium doccusate, cholesterol, cholesterol esters, taurocholic acid, phosphotidylcholine, oils, such as cottonseed oil, groundnut oil, corn germ oil, olive oil, castor oil, and sesame oil, glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols, fatty acid esters of sorbitan, or mixtures of these substances, and the like.
- Formulations that include a compound of Formula (I), (II), (HI), (IV) or (V) are prepared as solutions in saline, employing benzyl alcohol or other suitable preservatives, fluorocarbons, and/or other solubilizing or dispersing agents known in the art. See, for example, Ansel, H. C. et ah, Pharmaceutical Dosage Forms and Drug Delivery Systems, Sixth Ed. (1995).
- these compositions and formulations are prepared with suitable nontoxic pharmaceutically acceptable ingredients. These ingredients are known to those skilled in the preparation of nasal dosage forms and some of these can be found in
- Nasal dosage forms generally contain large amounts of water in addition to the active ingredient. Minor amounts of other ingredients such as pH adjusters, emulsifiers or dispersing agents, preservatives, surfactants, gelling agents, or buffering and other stabilizing and solubilizing agents are optionally present.
- the nasal dosage form should be isotonic with nasal secretions.
- Capsules and cartridges of, such as, by way of example only, gelatin for use in an inhaler or insufflator may be formulated containing a powder mix of the compound described herein and a suitable powder base such as lactose or starch.
- buccal formulations that include a compound of Formula (I), (II), (III), (IV) or (V) are administered using a variety of formulations known in the art.
- formulations include, but are not limited to, U.S. Pat. Nos. 4,229,447, 4,596,795, 4,755,386, and 5,739,136.
- the buccal dosage forms described herein can further include a bioerodible (hydrolysable) polymeric carrier that also serves to adhere the dosage form to the buccal mucosa.
- the compositions may take the form of tablets, lozenges, or gels formulated in a conventional manner.
- a compound of Formula (I), (II), (III), (IV) or (V) is prepared as transdermal dosage forms.
- the transdermal formulations described herein include at least three components: (1) a formulation of a compound of Formula (I), (II), (III), (IV) or (V); (2) a penetration enhancer; and (3) an aqueous adjuvant.
- the transdermal formulations include additional components such as, but not limited to, gelling agents, creams and ointment bases, and the like.
- the transdermal formulation further include a woven or non-woven backing material to enhance absorption and prevent the removal of the transdermal formulation from the skin.
- the transdermal formulations described herein can maintain a saturated or supersaturated state to promote diffusion into the skin.
- formulations suitable for transdermal administration of compounds described herein employ transdermal delivery devices and transdermal delivery patches and can be lipophilic emulsions or buffered, aqueous solutions, dissolved and/or dispersed in a polymer or an adhesive.
- patches are constructed for continuous, pulsatile, or on demand delivery of pharmaceutical agents.
- transdermal delivery of the compounds described herein can be accomplished by means of iontophoretic patches and the like.
- transdermal patches provide controlled delivery of the compound of Formula (I), (II), (III), (IV) or (V).
- transdermal devices are in the form of a bandage comprising a backing member, a reservoir containing the compound optionally with carriers, optionally a rate controlling barrier to deliver the compound to the skin of the host at a controlled and predetermined rate over a prolonged period of time, and means to secure the device to the skin.
- a compound of Formula (I), (II), (III), (IV) or (V) is formulated into a pharmaceutical composition suitable for intramuscular, subcutaneous, or intravenous injection.
- formulations suitable for intramuscular, subcutaneous, or intravenous injection include physiologically acceptable sterile aqueous or non-aqueous solutions, dispersions, suspensions or emulsions, and sterile powders for reconstitution into sterile injectable solutions or dispersions.
- suitable aqueous and non-aqueous carriers, diluents, solvents, or vehicles include water, ethanol, polyols (propyleneglycol, polyethylene-glycol, glycerol, cremophor and the like), suitable mixtures thereof, vegetable oils (such as olive oil) and injectable organic esters such as ethyl oleate.
- formulations suitable for subcutaneous injection also contain additives such as preserving, wetting, emulsifying, and dispensing agents. Prevention of the growth of microorganisms can be ensured by various antibacterial and antifungal agents, such as parabens, chlorobutanol, phenol, sorbic acid, and the like. In some cases it is desirable to include isotonic agents, such as sugars, sodium chloride, and the like.
- Parenteral injections may involve bolus injection or continuous infusion.
- Formulations for injection may be presented in unit dosage form, e.g., in ampoules or in multi-dose containers, with an added preservative.
- the pharmaceutical composition described herein may be in a form suitable for parenteral injection as a sterile suspensions, solutions or emulsions in oily or aqueous vehicles, and may contain formulatory agents such as suspending, stabilizing and/or dispersing agents.
- the active ingredient is in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use.
- compositions provided herein can also include an mucoadhesive polymer, selected from among, for example, carboxymethylcellulose, carbomer (acrylic acid polymer), poly(methylmethacrylate), polyacrylamide, polycarbophil, acrylic acid/butyl acrylate copolymer, sodium alginate and dextran.
- an mucoadhesive polymer selected from among, for example, carboxymethylcellulose, carbomer (acrylic acid polymer), poly(methylmethacrylate), polyacrylamide, polycarbophil, acrylic acid/butyl acrylate copolymer, sodium alginate and dextran.
- I l l - during a drug holiday is, by way of example only, by 10%- 100%, including by way of example only 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, and 100%.
- the dose reduction during a drug diversion is, by way of example only, by 10%- 100%, including by way of example only 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, and 100%.
- the normal dosing schedule is optionally reinstated.
- a maintenance dose is administered if necessary.
- the dosage or the frequency of administration, or both, is reduced, as a function of the symptoms, to a level at which the improved disease, disorder or condition is retained.
- the patient requires intermittent treatment on a long-term basis upon any recurrence of symptoms.
- the amount of a given agent that corresponds to such an amount varies depending upon factors such as the particular compound, disease condition and its severity, the identity ⁇ e.g., weight, sex) of the subject or host in need of treatment, but can nevertheless be determined according to the particular circumstances surrounding the case, including, e.g., the specific agent being administered, the route of administration, the condition being treated, and the subject or host being treated.
- doses employed for adult human treatment are typically in the range of 0.01mg-5000 mg per day. In one aspect, doses employed for adult human treatment are from about lmg to about 1000 mg per day.
- the desired dose is conveniently presented in a single dose or in divided doses administered simultaneously (or over a short period of time) or at appropriate intervals, for example as two, three, four or more sub-doses per day.
- the daily dosages appropriate for the compound of Formula (I), (II), (III), (IV) or (V) described herein are from about 0.01 to about 10 mg/kg per body weight.
- an indicated daily dosage in a large mammal including, but not limited to, humans, is in the range from about 0.5 mg to about 1000 mg, conveniently administered in divided doses, including, but not limited to, up to four times a day.
- the daily dosage is administered in extended release form.
- suitable unit dosage forms for oral administration comprise from about 1 to 500 mg active ingredient.
- the daily dosage or the amount of active in the dosage form are lower or higher than the ranges indicated herein, based on a number of variables in regard to an individual treatment regime.
- the daily and unit dosages are altered depending on a number of variables including, but not limited to, the activity of the compound used, the disease or condition to be treated, the mode of administration, the requirements of the individual subject, the severity of the disease or condition being treated, and the judgment of the practitioner. [00554] Toxicity and therapeutic efficacy of such therapeutic regimens are determined by standard pharmaceutical procedures in cell cultures or experimental animals, including, but not limited to, the determination of the LD 50 and the ED 50 .
- the dose ratio between the toxic and therapeutic effects is the therapeutic index and it is expressed as the ratio between LD 50 and ED50.
- the data obtained from cell culture assays and animal studies are used in formulating the therapeutically effective daily dosage range and/or the therapeutically effective unit dosage amount for use in mammals, including humans.
- the daily dosage amount of the compounds described herein lies within a range of circulating concentrations that include the ED 50 with minimal toxicity.
- the daily dosage range and/or the unit dosage amount varies within this range depending upon the dosage form employed and the route of administration utilized. Patient Selection
- CLL chronic lymphocytic leukemia
- LPA can protect some CLL cells from apoptosis and the cells that are protected by LPA have high levels of LPAi mRNA.
- CLL patients are selected based on the expression of the LPAlR. LPA receptor expression are determined by methods including, but not limited to, northern blotting, western blotting, quantitative PCR (qPCR), flow cytometry, autoradiography (using a small molecule radioligand or PET ligand).
- a compound of Formula (I), (II), (III), (IV) or (V) is coadministered with a second therapeutic agent, wherein the compound of Formula (I), (II), (III), (IV) or (V) and the second therapeutic agent modulate different aspects of the disease, disorder or condition being treated, thereby providing a greater overall benefit than administration of either therapeutic agent alone.
- dosages of the co-administered compounds vary depending on the type of co-drug employed, on the specific drug employed, on the disease or condition being treated and so forth.
- the compound provided herein when co-administered with one or more other therapeutic agents, is administered either simultaneously with the one or more other therapeutic agents, or sequentially.
- the multiple therapeutic agents are administered in any order or even simultaneously. If administration is simultaneous, the multiple therapeutic agents are, by way of example only, provided in a single, unified form, or in multiple forms (e.g., as a single pill or as two separate pills).
- one of the therapeutic agents is given in multiple doses, and in another, two (or more if present) are given as multiple doses.
- the timing between the multiple doses vary from more than zero weeks to less than four weeks.
- the combination methods, compositions and formulations are not to be limited to the use of only two agents; the use of multiple therapeutic combinations is also envisioned. [00565]
- the compounds of Formula (I), (II), (III), (IV) or (V) and combination therapies are administered before, during or after the occurrence of a disease or condition, and the timing of administering the composition containing a compound varies.
- the compounds described herein are used as a prophylactic and are administered continuously to subjects with a propensity to develop conditions or diseases in order to prevent the occurrence of the disease or condition.
- the compounds and compositions are administered to a subject during or as soon as possible after the onset of the symptoms.
- a compound described herein is administered as soon as is practicable after the onset of a disease or condition is detected or suspected, and for a length of time necessary for the treatment of the disease. In some embodiments, the length required for treatment varies, and the treatment length is adjusted to suit the specific needs of each subject.
- a compound described herein or a formulation containing the compound is administered for at least 2 weeks, about 1 month to about 5 years.
- therapies which combine a compound of Formula (I), (II), (III), (IV) or (V) with inhibitors of LPA synthesis or LPA receptor antagonists, either acting at the same or other points in the LPA synthesis or signalling pathway, are encompassed herein for treating LPA-dependent or LPA-mediated diseases or conditions.
- the compound of Formula (I), (II), (III), (IV) or (V) is administered or formulated in combination with one or more anti-cancer agents.
- one or more of the anti-cancer agents are proapoptotic agents.
- anti-cancer agents include, but are not limited to, any of the following: gossypol, genasense, polyphenol E, Chlorofusin, all trans-retinoic acid (ATRA), bryostatin, tumor necrosis factor-related apoptosis-inducing ligand
- TRAIL 5-aza-2'-deoxycytidine
- all trans retinoic acid doxorubicin
- vincristine doxorubicin
- etoposide gemcitabine
- imatinib geldanamycin
- TaxolTM paclitaxel
- anti-cancer agents for use in combination with a compound of Formula (I), (II), (III), (IV) or (V) include inhibitors of mitogen-activated protein kinase signaling, e.g., U0126, PD98059, PD184352, PD0325901, ARRY-142886, SB239063,
- anti-cancer agents for use in combination with a compound of Formula (I), (II), (III), (IV) or (V) include one or more of the following: abiraterone; abarelix; adriamycin; aactinomycin; acivicin; aclarubicin; acodazole hydrochloride; acronine; adozelesin; aldesleukin; alemtuzumab; allopurinol; alitretinoin; altretamine; ambomycin; ametantrone acetate; aminoglutethimide; aminolevulinic acid; amifostine; amsacrine; anastrozole; anthramycin; aprepitant; arsenic trioxide; asparaginase; asperlin; azacitidine; azetepa; azotomycin; batimastat; bendamustine hydrochloride; benzodepa
- (III), (IV) or (V) include alkylating agents, antimetabolites, natural products, or hormones, e.g., nitrogen mustards (e.g., mechloroethamine, cyclophosphamide, chlorambucil, etc.), alkyl sulfonates (e.g., busulfan), nitrosoureas (e.g., carmustine, lomusitne, ete.), or triazenes (decarbazine, etc.).
- nitrogen mustards e.g., mechloroethamine, cyclophosphamide, chlorambucil, etc.
- alkyl sulfonates e.g., busulfan
- nitrosoureas e.g., carmustine, lomusitne, ete.
- triazenes decarbazine, etc.
- Examples of natural products for use in combination with a compound of Formula (I), (II), (III), (IV) or (V) include but are not limited to vinca alkaloids (e.g., vinblastin, vincristine), epipodophyllotoxins (e.g., etoposide), antibiotics (e.g., daunorubicin, doxorubicin, bleomycin), enzymes (e.g., L-asparaginase), or biological response modifiers (e.g., interferon alpha).
- vinca alkaloids e.g., vinblastin, vincristine
- epipodophyllotoxins e.g., etoposide
- antibiotics e.g., daunorubicin, doxorubicin, bleomycin
- enzymes e.g., L-asparaginase
- biological response modifiers e.g., interferon alpha
- alkylating agents for use in combination with a compound of Formula (I), (II), (III), (IV) or (V) include, but are not limited to, nitrogen mustards (e.g., mechloroethamine, cyclophosphamide, chlorambucil, meiphalan, etc.), ethylenimine and methylmelamines (e.g., hexamethlymelamine, thiotepa), alkyl sulfonates (e.g., busulfan), nitrosoureas (e.g., carmustine, lomusitne, semustine, streptozocin, etc.), or triazenes (decarbazine, ete.).
- nitrogen mustards e.g., mechloroethamine, cyclophosphamide, chlorambucil, meiphalan, etc.
- ethylenimine and methylmelamines e.g., hex
- antimetabolites include, but are not limited to folic acid analog (e.g., methotrexate), or pyrimidine analogs (e.g., fluorouracil, floxouridine, Cytarabine), purine analogs (e.g., mercaptopurine, thioguanine, pentostatin.
- folic acid analog e.g., methotrexate
- pyrimidine analogs e.g., fluorouracil, floxouridine, Cytarabine
- purine analogs e.g., mercaptopurine, thioguanine, pentostatin.
- hormones and antagonists for use in combination with a compound of Formula (I), (II), (III), (IV) or (V) include, but are not limited to, adrenocorticosteroids (e.g., prednisone), progestins (e.g., hydroxyprogesterone caproate, megestrol acetate, medroxyprogesterone acetate), estrogens (e.g., diethlystilbestrol, ethinyl estradiol), antiestrogen (e.g., tamoxifen), androgens (e.g., testosterone propionate, fluoxymesterone), antiandrogen (e.g., flutamide), gonadotropin releasing hormone analog (e.g., leuprolide).
- adrenocorticosteroids e.g., prednisone
- progestins e.g., hydroxyprogesterone caproate, megestrol acetate,
- Examples of anti-cancer agents which act by arresting cells in the G2-M phases due to stabilized microtubules include without limitation the following marketed drugs and drugs in development: Erbulozole, Dolastatin 10, Mivobulin isethionate, Vincristine, NSC-639829, Discodermolide, ABT-751 , Altorhyrtins (such as Altorhyrtin A and Altorhyrtin C), Spongistatins (such as Spongistatin 1, Spongistatin 2, Spongistatin 3, Spongistatin 4, Spongistatin 5, Spongistatin 6, Spongistatin 7, Spongistatin 8, and Spongistatin 9), Cemadotin hydrochloride, Epothilones (such as Epothilone A, Epothilone B, Epothilone C, Epothilone D, Epothilone E, Epothilone F, Epothilone B N-oxide
- a compound of Formula (I), (II), (III), (IV) or (V) is co-administered with thrombolytic agents (e.g., alteplase anistreplase, streptokinase, urokinase, or tissue plasminogen activator), heparin, tinzaparin, warfarin, dabigatran (e.g., dabigatran etexilate), factor Xa inhibitors (e.g., fondaparinux, draparinux, rivaroxaban, DX-9065a, otamixaban, LY517717, or YM150), ticlopidine, clopidogrel, CS-747 (prasugrel, LY640315), ximelagatran, or BIBR 1048.
- thrombolytic agents e.g., alteplase anistreplase, streptokinase, urokinase
- a compound of Formula (I), (II), (III), (IV) or (V) is used in combination with anti-emetic agents to treat nausea or emesis, which may result from the use of a compound of Formula (I), (II), (III), (IV) or (V), anti-cancer agent(s) and/or radiation therapy.
- Anti-emetic agents include, but are not limited to: neurokinin-1 receptor antagonists, 5HT3 receptor antagonists (such as ondansetron, granisetron, tropisetron, Palonosetron, and zatisetron), GABA B receptor agonists (such as baclofen), corticosteroids (such as dexamethasone, prednisone, prednisolone, or others), dopamine antagonists (such as, but not limited to, domperidone, droperidol, haloperidol, chlorpromazine, promethazine, prochlorperazine, metoclopramide), antihistamines (Hl histamine receptor antagonists, such as but not limited to, cyclizine, diphenhydramine, dimenhydrinate, meclizine, promethazine, hydroxyzine), cannabinoids (such as but not limited to, cannabis, marinol, dronabinol), and others (such as,
- a compound of Formula (I), (II), (III), (IV) or (V) is used in combination with an agent useful in the treatment of anemia.
- an anemia treatment agent is, for example, a continuous eythropoiesis receptor activator (such as epoetin- ⁇ ).
- a compound of Formula (I), (II), (III), (IV) or (V) is used in combination with an agent useful in the treatment of neutropenia.
- agents useful in the treatment of neutropenia include, but are not limited to, a hematopoietic growth factor which regulates the production and function of neutrophils such as a human granulocyte colony stimulating factor, (G-CSF).
- G-CSF human granulocyte colony stimulating factor
- a G-CSF include filgrastim.
- Radiation therapy is the treatment of cancer and other diseases with ionizing radiation. Radiation therapy can be used to treat localized solid tumors, such as cancers of the skin, tongue, larynx, brain, breast, prostate, colon, uterus and/or cervix. It can also be used to treat leukemia and lymphoma (cancers of the blood- forming cells and lymphatic system, respectively).
- a technique for delivering radiation to cancer cells is to place radioactive implants directly in a tumor or body cavity. This is called internal radiotherapy (brachytherapy, interstitial irradiation, and intracavitary irradiation are types of internal radiotherapy.) Using internal radiotherapy, the radiation dose is concentrated in a small area, and the patient stays in the hospital for a few days. Internal radiotherapy is frequently used for cancers of the tongue, uterus, prostate, colon, and cervix. [00583] The term "radiotherapy” or “ionizing radiation” include all forms of radiation, including but not limited to ⁇ , ⁇ , and ⁇ radiation and ultraviolet light. Immunosuppresants
- a compound of Formula (I), (II), (III), (IV) or (V) is used to treat or reduce fibrosis in a mammal.
- a compound of Formula (I), (II), (III), (IV) or (V) is administered in combination with one or more immunosuppresants.
- Immunosuppressive therapy is clinically used to treat or prevent the rejection of transplanted organs and tissues (e.g. bone marrow, heart, kidney, liver); treatment of autoimmune diseases or diseases that are most likely of autoimmune origin (e.g.
- a compound of Formula (I), (II), (III), (IV) or (V) is adminsitered with corticosteroids.
- a compound of Formula (I), (II), (III), (IV) or (V) is adminsitered with an a therapeutic agent selected from among: Calcineurin inhibitors (such as, but not limited to, cyclosporin, tacrolimus); mTOR inhibitors (such as, but not limited to, sirolimus, everolimus); anti-proliferatives (such as, but not limited to, azathioprine, mycophenolic acid); corticosteroids (such as, but not limited to, prednisone, cortisone acetate, prednisolone, methylprednisolone, dexamethasone, betamethasone, triamcinolone, beclometasone, fludrocortisone acetate, deoxycorticosterone acetate, ald
- Calcineurin inhibitors such as, but
- NSAIDs include, but are not limited to: aspirin, salicylic acid, gentisic acid, choline magnesium salicylate, choline salicylate, choline magnesium salicylate, choline salicylate, magnesium salicylate, sodium salicylate, diflunisal, carprofen, fenoprofen, fenoprofen calcium, flurobiprofen, ibuprofen, ketoprofen, nabutone, ketolorac, ketorolac tromethamine, naproxen, oxaprozin, diclofenac, etodolac, indomethacin, sulindac, tolmetin, meclofenamate, meclofenamate sodium, mefenamic acid, piroxicam, meloxicam, COX-2 specific inhibitors (such as, but not limited to, celecoxib, rofecoxib, valdecoxib, parecoxib, e
- methods for treatment of LPA-dependent or LPA-mediated conditions or diseases comprises administration to a patient compounds, pharmaceutical compositions, or medicaments described herein in combination with at least one additional agent selected, by way of example only, HMG-CoA reductase inhibitors (e.g., statins in their lactonized or dihydroxy open acid forms and pharmaceutically acceptable salts and esters thereof, including but not limited to lovastatin; simvastatin; dihydroxy open-acid simvastatin, particularly the ammonium or calcium salts thereof; pravastatin, particularly the sodium salt thereof; fluvastatin, particularly the sodium salt thereof; atorvastatin, particularly the calcium salt thereof; nisvastatin, also referred to as NK- 104; rosuvastatin); agents that have both lipid-altering effects and other pharmaceutical activities; HMG-CoA synthase inhibitors; cholesterol absorption inhibitors such as ezetimibe; cholesterol
- KRP-297 vitamin B6 (also known as pyridoxine) and the pharmaceutically acceptable salts thereof such as the HCl salt
- vitamin B 12 also known as cyanocobalamin
- folic acid or a pharmaceutically acceptable salt or ester thereof such as the sodium salt and the methylglucamine salt
- anti-oxidant vitamins such as vitamin C and E and beta carotene
- beta-blockers angiotensin II antagonists such as losartan
- angiotensin converting enzyme inhibitors such as enalapril and captopril
- calcium channel blockers such as nifedipine and diltiazam
- endothelian antagonists agents that enhance ABCl gene expression
- bisphosphonate compounds such as alendronate sodium
- cyclooxygenase-2 inhibitors such as rofecoxib and celecoxib.
- methods for treatment of LPA-dependent or LPA-mediated conditions or diseases comprises administration to a patient compounds, pharmaceutical compositions, or medicaments described herein in combination with at least one additional agent selected from, by way of example only, COX-2 inhibitors; nitric oxide synthase inhibitors, such as N-(3-(aminomethyl)benzyl) acetamidine; Rho kinase inhibitors, such as fasudil; angiotension II type-1 receptor antagonists, including candesartan, losartan, irbesartan, eprosartan, telmisartan and valsartan; glycogen synthase kinase 3 inhibitors; sodium or calcium channel blockers, including crobenetine; p38 MAP kinase inhibitors, including SKB 239063; thromboxane AX- synthetase inhibitors, including isbogrel, ozagrel
- methods for treatment of LPA-dependent or LPA-mediated conditions or diseases comprises administration to a patient compounds, pharmaceutical compositions, or medicaments described herein in combination with at least one additional agent selected from, by way of example only, dimethylsulfoxide, omalizumab, and pentosan polysulfate.
- methods for treating LPA-dependent or LPA-mediated conditions or diseases comprises administration to a patient anti-inflammatory agents.
- methods for treating LPA-dependent or LPA-mediated conditions or diseases, such as the therapy of asthma and/or COPD comprise administration to a patient compounds, pharmaceutical compositions, or medicaments described herein in combination with at least one additional agent selected from, but not limited to, epinephrine, isoproterenol, orciprenaline, bronchodilators, glucocorticoids, leukotriene modifiers, mast-cell stabilizers, xanthines, anticholinergics, ⁇ -2 agonists, FLAP inhibitors, FLAP modulators or 5-LO inhibitors, ⁇ -2 agonists include, but are not limited to, short-acting ⁇ -2 agonists (e.g., salbutamol (albuterol), levalbuterol, terbuta
- Anticholinergics include, but are not limited to, ipratropium and tiotropium.
- Mast cell stabilizers include, but are not limited to, cromoglicate and nedocromil.
- Xanthines include, but are not limited to, amminophylline, theobromine and theophylline.
- Leukotriene antagonists include, but are not limited to, montelukast, tomelukast, pranlukast and zafirlukast.
- 5-LO inhibitors include, but are not limited to, zileuton, VIA-2291 (ABT761), AZ-4407 and ZD-2138 and compounds found in US 2007/0149579, WO2007/016784.
- Non- limiting examples of such materials include, but not limited to, buffers, diluents, filters, needles, syringes; carrier, package, container, vial and/or tube labels listing contents and/or instructions for use, and package inserts with instructions for use. A set of instructions will also typically be included.
- Step 3 5-(4-Bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid methyl ester
- Step 5 [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-phenyl-ethyl ester
- Step 6 l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid
- [00616] [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl-ethyl ester (0.248g, 0.62mmol), 4-(l'-carboxyl-cyclopropyl)phenylboronic acid (0.16Og, 0.62mmol), and sodium carbonate (0.155g, 1.85mmol) were combined in 2:1 DME:H 2 O. The solution was purged with N 2 for 10 minutes, and then bis(triphenylphosphine)palladium(II) dichloride (0.047g, O.O ⁇ mmol) was added.
- the reaction was purged with N 2 for an additional 10 minutes, and then stirred in a sealed tube at 80 0 C for 2 hours.
- the mixture was partitioned between EtOAc and H 2 O, and the aqueous layer was extracted with EtOAc. The combined organic layers were dried over MgSO 4 , filtered, and concentrated, and the residue was purified by silica gel chromatography to give the title compound.
- Example Ia Alternate synthesis of l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl- ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl ⁇ -cyclopropanecarboxylic acid (Compound 1-1)
- Step 1 l-(biphenyl-4-yl)cyclopropanecarbonitrile
- 4-phenyl-phenylacetonitrile VWR scientific, 55.7 g, 289 mmol
- KOH 161.6 g, 2890 mmol
- Tetrabutyl ammonium bromide 9.2g, 29 mmol
- 1,2 dibromoethane 64.9 g, 347 mmol
- TLC 10% EtOAc/hex
- the organic layer was extracted 2 times with dilute hydrochloric acid, dried and evaporated to yield 63g of l-(biphenyl-4-yl)cyclopropanecarbonitrile.
- Step 2 l-(Biphenyl-4-yl)cyclopropanecarboxylic acid
- Step 3 l-(Biphenyl-4-yl)cyclopropanecarboxylic acid ethyl ester
- Step 4 l-(4'-Acetylbiphenyl-4-yl)cyclopropanecarboxylic acid ethyl ester
- Step 5 4'-(l-(Ethoxycarbonyl)cyclopropyl)biphenyl-4-carboxylic acid [00621] To l-(4'-acetylbiphenyl-4-yl)cyclopropanecarboxylic acid ethyl ester (10.1 g, 33 mmol) in dioxane (200 mL) at ⁇ 10 0 C was added a solution of bromine (26.4 g, 165 mmol), sodium hydroxide (22.4 g, 561 mmol) in water (150 mL). The solution was stirred at room temperature for 30 minutes, poured into water (500 mL) and acidified with dilute hydrochloric acid.
- Step 6 3-Methylamino-but-2-enoic acid benzyl ester
- benzyl acetoacetate 29 g, 151 mmol
- ethanol 30 mL
- methyl amine 33% in ethanol, 7.02 g, 226 mmol
- the solution was stirred for 2 hours at room temperature followed by evaporation to yield a yellow oil ( ⁇ 30 g).
- Step 7 Ethyl l-(4'-(2-(benzyloxycarbonyl)-3-(methylamino)but-2-enoyl)biphenyl-4- yl)cyclopropanecarboxylate
- Step 8 Benzyl 5-(4'-(l-(ethoxycarbonyl)cyclopropyl)biphenyl-4-yl)-3-methylisoxazole-4- carboxylate
- hydroxyl amine hydrochloride 1.5 g, 21.6 mmol
- acetic acid 50 mL
- the solution was heated to 95°C for 30 minutes cooled to room temperature, extracted with CH 2 CI 2 and water (4 times, second and third time made basic with sodium bicarbonate). Dried, evaporated and purified on column 0 to 20% EtOAc/hexanes to yield 3.3 g of product.
- Step 9 5-(4'-(l-(ethoxycarbonyl)cyclopropyl)biphenyl-4-yl)-3-methylisoxazole-4- carboxylic acid
- Step 10 l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid ethyl ester
- Step 11 l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid
- Example Ib Alternate synthesis of l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl- ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl ⁇ -cyclopropanecarboxylic acid (Compound 1-1)
- Step 1 l-(Biphenyl-4-yl)cyclopropanecarboxylic acid isopropyl ester
- Step 2 l-(4'-Acetylbiphenyl-4-yl)cyclopropanecarboxylic acid isopropyl ester
- Step 3 4'-(l-(isopropoxycarbonyl)cyclopropyl)biphenyl-4-carboxylic acid
- Step 4 Isopropyl l-(4'-(2-(benzyloxycarbonyl)-3-(methylamino)but-2-enoyl)biphenyl-4- yl)cyclopropanecarboxylate
- Step 5 Methyl 5-(4'-(l-(isopropoxycarbonyl)cyclopropyl)biphenyl-4-yl)-3- methylisoxazole-4-carboxylate
- Step 6 5-(4'-(l-(propoxycarbonyl)cyclopropyl)biphenyl-4-yl)-3-methylisoxazole-4- carboxylic acid
- Step 7 l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid isopropyl ester
- Step 8 l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid
- Step 1 l-(4-Bromo-phenyl)-cyclopropanecarbonitrile
- Step 2 l-(4-Bromo-phenyl)-cyclopropanecarboxylic acid [00637] l-(4-Bromo-phenyl)-cyclopropanecarbonitrile (5g, 22.5mmol) and potassium hydroxide (5g, 89.3mmol) were combined in ethylene glycol (7OmL), and the reaction was stirred at 180 0 C for 4 hours. The mixture was poured into H 2 O, acidified, and filtered to give the title compound.
- Step 3 l-(4-Bromo-phenyl)-cyclopropanecarboxylic acid ethyl ester
- Step 4 l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester
- Step 6 [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l- ⁇ -tolyl-ethyl ester
- Step 7 l- ⁇ 4'-[3-Methyl-4-((R)-l-o-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid ethyl ester
- Step 8 l- ⁇ 4'-[3-Methyl-4-((R)-l-o-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid
- Example 3a Synthesis of l- ⁇ 4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl- isoxazol-5-yl]-biphenyl-4-yl ⁇ -cyclopropanecarboxylic acid (Compound 1-10)
- Step 1 [5-(4-Bromo-phenyl)-3-met yl-isoxazol-4-yl]-carbamic acid 1-cyclopropyl-ethyl ester
- Step 2 l- ⁇ 4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid ethyl ester
- Step 3 (R)-l- ⁇ 4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl ⁇ -cyclopropanecarboxylic acid
- Step 2 l-(R)-[5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-cyclopropyl- ethyl ester
- Step 3 (R)-l- ⁇ 4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl ⁇ -cyclopropanecarboxylic acid ethyl ester [00649] Prepared according to the procedure described in Example 1, Step 6 using the following starting materials: l-(R)-[5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1- cyclopropyl-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester; the enantiomeric excess of the isolated material was determined by chiral HPLC to be 92% (Chiracel OD column (97:3 hexanes
- Step 4 (R)-l- ⁇ 4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl ⁇ -cyclopropanecarboxylic acid
- Step 1 [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(2-chloro- phenyl)-ethyl ester
- Step 2 l-(4'- ⁇ 4-[(R)-l-(2-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl ⁇ - biphenyl-4-yl)-cyclopropanecarboxylic acid
- Step 1 [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid tert-butyl ester
- Step 2 l-[4'-(4-t ⁇ rt-Butoxycarbonylam no-3-meth Vyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester
- [00654] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid tert-butyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
- Step 3 l-[4'-(4-t ⁇ rt-Butoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
- Step 2 [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(2- trifluoromethyl-phenyl)-ethyl ester [00657] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: (R)- 1 -(2-trifluoromethyl-phenyl)-ethanol and 5-(4-bromo-phenyl)-3 -methyl - isoxazole-4-carboxylic acid; the isolated material was purified by preparative HPLC, using a Chiracel OD column (98.6:1.4 hexanes:EtOH) to give the title compound.
- Step 3 l-(4'- ⁇ 3-Methyl-4-[(R)-l-(2-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl ⁇ -biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
- Step 4 l-(4'- ⁇ 3-Methyl-4-[(R)-l-(2-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl ⁇ -biphenyl-4-yl)-cyclopropanecarboxylic acid
- Step 1 l-(4-Bromo-phenyl)-cyclopentanecarboxylic acid ethyl ester
- Step 2 l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopentanecarboxylic acid ethyl ester
- Step 3 l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopentanecarboxylic acid ethyl ester
- Step 4 l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopentanecarboxylic acid
- Step 1 l-(4-Bromo-phenyl)-cyclobutanecarboxylic acid ethyl ester
- Step 2 l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclobutanecarboxylic acid ethyl ester
- Step 3 l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclobutanecarboxylic acid ethyl ester
- [00666] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl- ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclobutanecarboxylic acid ethyl ester.
- Step 4 l- ⁇ 4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclobutanecarboxylic acid
- Step 2 l-[4'-(4-Isopropoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
- Step 1 [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-cyclohexyl-ethyl ester
- Step 2 l- ⁇ 4'-[4-(l-Cyclohexyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid ethyl ester
- Step 3 l- ⁇ 4'-[4-(l-Cyclohexyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid
- Step 1 [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid benzyl ester
- Step 2 l-[4'-(4-Benzyloxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester
- Step 3 l-[4'-(4-Benzyloxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
- Step 1 [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid ethyl ester
- Step 2 l-[4'-(4-Ethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester
- [00677] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
- Step 3 l-[4'-(4-Ethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
- Step 2 l-[4'-(4-Methoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester
- Step 3 l-[4'-(4-Methoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
- Step 1 [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-methyl-l-phenyl- ethyl ester
- Step 2 l- ⁇ 4'-[3-Methyl-4-(l-methyl-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl ⁇ -cyclopropanecarboxylic acid ethyl ester
- Step 3 l- ⁇ 4'-[3-Methyl-4-(l-methyl-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl ⁇ -cyclopropanecarboxylic acid
- Step 1 l- ⁇ 4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl ⁇ -cyclopropanecarboxylic acid
- Step 1 [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid cyclopropylmethyl ester [00686] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and cyclopropyl carbinol.
- Step 2 l-[4'-(4-Cyclopropylmethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4- yl]-cyclopropanecarboxylic acid ethyl ester
- Step 3 l-[4'-(4-Cyclopropylmethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4- yl]-cyclopropanecarboxylic acid
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Pulmonology (AREA)
- Biomedical Technology (AREA)
- Oncology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Urology & Nephrology (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Hematology (AREA)
- Vascular Medicine (AREA)
- Neurology (AREA)
- Physical Education & Sports Medicine (AREA)
- Transplantation (AREA)
- Neurosurgery (AREA)
- Ophthalmology & Optometry (AREA)
- Dermatology (AREA)
- Gastroenterology & Hepatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Described herein are compounds that are antagonists of lysophosphatidic receptor(s). Also described are pharmaceutical compositions and medicaments that include the compounds described herein, as well as methods of using such antagonists, alone and in combination with other compounds, for treating LPA-dependent or LPA-mediated conditions or diseases.
Description
POLYCYCLIC ANTAGONISTS OF LYSOPHOSPHATIDIC ACID RECEPTORS
RELATED APPLICATIONS
[0001] This application claims benefit of U.S. Provisional Application No. 61/183,785, entitled "ANTAGONISTS OF LYS OPHO SPHATIDIC ACID RECEPTORS" filed on June 3, 2009, which is herein incorporated by reference.
FIELD OF THE INVENTION
[0002] Described herein are compounds, methods of making such compounds, pharmaceutical compositions and medicaments comprising such compounds, and methods of using such compounds to treat, prevent or diagnose diseases, disorders or conditions associated with one or more of the lysophosphatidic acid (LPA) receptors.
BACKGROUND OF THE INVENTION
[0003] Lysophospholipids are membrane-derived bioactive lipid mediators. Lysophospholipids affect fundamental cellular functions that include proliferation, differentiation, survival, migration, adhesion, invasion, and morphogensis. These functions influence many biological processes that include, but are not limited to, neurogensis, angiogenesis, wound healing, fibrosis, immunity, and carcinogenesis.
[0004] Lysophosphatidic acid (LPA) is a lysophospholipid that has been shown to act through sets of specific G protein-coupled receptors (GPCRs) in an autocrine and paracrine fashion. LPA binding to its cognate GPCRs (LPAi, LPA2, LPA3, LPA4, LPA5, LPA6) activates intracellular signaling pathways to produce a variety of biological responses. Antagonists of the LPA receptors find use in the treatment of diseases, disorders or conditions in which LPA plays a role.
SUMMARY OF THE INVENTION
[0005] In one aspect, presented herein are compounds of Formula (I), (II), (III), (IV) and (V) that inhibit the physiological activity of lysophosphatidic acid (LPA), and therefore, are useful as agents for the treatment or prevention of diseases in which inhibition of the physiological activity of LPA is useful, such as diseases in which an LPA receptor participates, is involved in the etiology or pathology of the disease, or is otherwise associated with at least one symptom of the disease. [0006] In one aspect, the compounds of Formula (I), (II), (III), (IV) and (V) are useful for the treatment of fibrosis of organs (liver, kidney, lung, heart and the like), liver diseases (acute hepatatis, chronic hepatitis, liver fibrosis, liver cirrhosis, portal hypertension, regenerative
failure, non-alcoholic steatohepatitis (NASH), liver hypofunction, hepatic blood flow disorder, and the like), cell proliferative disease (cancer (solid tumor, solid tumor metastasis, vascular fibroma, myeloma, multiple myeloma, Kaposi's sarcoma, leukemia, chronic lymphocytic leukemia (CLL) and the like) and invasive metastasis of cancer cell, and the like), inflammatory disease (psoriasis, nephropathy, pneumonia and the like), gastrointestinal tract disease (irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), abnormal pancreatic secretion, and the like), renal disease, urinary tract-associated disease (benign prostatic hyperplasia or symptoms associated with neuropathic bladder disease, spinal cord tumor, hernia of intervertebral disk, spinal canal stenosis, symptoms derived from diabetes, lower urinary tract disease (obstruction of lower urinary tract, and the like), inflammatory disease of lower urinary tract, dysuria, frequent urination, and the like), pancreas disease, abnormal angiogenesis- associated disease (arterial obstruction and the like), scleroderma, brain-associated disease (cerebral infarction, cerebral hemorrhage, and the like), neuropathic pain, peripheral neuropathy, and the like, ocular disease (age-related macular degeneration (AMD), diabetic retinopathy, proliferative vitreoretinopathy (PVR), cicatricial pemphigoid, glaucoma filtration surgery scarring, and the like). In one aspect, the compounds of Formula (I), (II), (III), (IV) and (V) are used in the treatment of fibrotic diseases or conditions.
[0007] In one aspect, described herein are compounds of Formula (I), (II), (III), (IV) and (V), pharmaceutically acceptable salts, pharmaceutically acceptable solvates, and prodrugs thereof. Compounds of Formula (I), (II), (III), (IV) and (V) are antagonists of at least one of the LPA receptors selected from LPAi, LPA2, LPA3, LPA4 LPA5 and LPA6. In one embodiment, compounds of Formula (I), (II), (III), (IV) and (V) are antagonists of LPAi. In one embodiment, compounds of Formula (I), (II), (III), (IV) and (V) are antagonists of LPAi and/or LPA3. In some embodiments, compounds of Formula (I), (II), (III), (IV) and (V) are antagonists of LPAi and/or LPA2. In some embodiments, compounds of Formula (I), (II), (III), (IV) and (V) are selective antagonists for one of the LPA receptors relative to the other LPA receptors. In some embodiments, such a selective antagonist is selective for the LPAi receptor. In some embodiments, such a selective antagonist is selective for the LPA2 receptor. In some embodiments, such a selective antagonist is selective for the LPA3 receptor. [0008] Compounds of Formula (I), (II), (III), (IV) and (V) are used in the treatment of diseases, disorders, or conditions in which activation of at least one LPA receptor by LPA contributes to the symptomology or progression of the disease, disorder or condition. These diseases, disorders, or conditions may arise from one or more of a genetic, iatrogenic, immunological, infectious, metabolic, oncological, toxic, surgical, and/or traumatic etiology. In one aspect, the methods, compounds, pharmaceutical compositions, and medicaments described herein
comprise antagonists of LPA receptors. In one aspect, the methods, compounds, pharmaceutical compositions, and medicaments described herein comprise antagonists of LPAi. [0009] In one aspect, provided herein is a compound having the structure of Formula (I), pharmaceutically acceptable salt, pharmaceutically acceptable solvate, or prodrug thereof:
Formula (I) wherein
R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -C(=O)N(R9)2, -C(O)NH-OH, -
C(=0)NH-CN, -P(O)(OH)2, -P(=O)(ORD)2, -OPO3H2, -SO2NHC(=O)R10, -CN, - C(=NH)-NH2, -C(=NH)-NHC(=O)RD, -C(O)NHCH2CH2SO3H, tetrazolyl, 5-oxo-
2,5-dihydro-[l,2,4]oxadiazol-3-yl, or carboxylic acid bioisostere; RD is H or C1-
C6alkyl; L1 is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted - alkylene-cycloalkylene, or a substituted or unsubstituted -cycloalkylene-alkylene-; ring A is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring A is substituted then ring A is substituted with 1, 2, 3, or 4 RA; ring B is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring B is substituted then ring B is substituted with 1, 2, 3, or 4 RB; L2 is absent, a substituted or unsubstituted alkylene, a substituted or unsubstituted fluoroalkylene, a substituted or unsubstituted heteroalkylene, -0-, -S-, -SO-, -SO2-, - NR2-, or -C(O)-;
R2 is H, Ci-C4alkyl, or Ci-C4fluoroalkyl; R3 is H, Ci-C4alkyl, C3-C6cycloalkyl, Ci-C4fluoroalkyl or a substituted or unsubstituted phenyl;
R4 is -NR7C(O)0C(R8)(R8a)-CY, or -NR7C(O)O-CY; R7 is H or CrC4alkyl;
R8 is H, Ci-C4alkyl, C3-C7cycloalkyl or Ci-C4fluoroalkyl;
R8a is H or Ci-C4alkyl;
CY is a substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, a substituted or unsubstituted heterocycloalkyl, a substituted or unsubstituted aryl, a substituted or unsubstituted benzyl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then CY is substituted with 1, 2, 3, or 4 Rc; or
R8 and CY are taken together with the carbon atom to which they are attached to form a substituted or unsubstituted carbocycle or a substituted or unsubstituted heterocycle; each of RA, RB, and Rc are independently selected from halogen, -CN, -NO2, -OH, - OR10, -SR10, -S(=O)R10, -S(=O)2R10, -N(R9)S(=O)2R10, -S(=O)2N(R9)2, -C(=O)R10, -
OC(=O)R10, -CO2R9, -OCO2R10, -N(R9)2, -C(=O)N(R9)2, -OC(=O)N(R9)2, - NR9C(=O)N(R9)2, -NR9C(=O)R10, -NR9C(O)OR10, d-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl; each R9 is independently selected from H, Ci-C6alkyl, Ci-C6heteroalkyl, C1- Cefluoroalkyl, a substituted or unsubstituted C3-Ciocycloalkyl, a substituted or unsubstituted C2-Cioheterocycloalkyl, a substituted or unsubstituted aryl, a substituted or unsubstituted heteroaryl, a substituted or unsubstituted -C1- C4alkylene-C3-Ciocycloalkyl, a substituted or unsubstituted -Ci-C4alkylene-C2- Cioheterocycloalkyl, a substituted or unsubstituted -Ci-C4alkylene-aryl, or a substituted or unsubstituted -Ci-C4alkylene-heteroaryl; or two R9 groups attached to the same N atom are taken together with the N atom to which they are attached to form a substituted or unsubstituted heterocycle; R10 is selected from Ci-C6alkyl, Ci-C6heteroalkyl, Ci-C6fluoroalkyl, a substituted or unsubstituted C3-Ciocycloalkyl, a substituted or unsubstituted C2- Cioheterocycloalkyl, a substituted or unsubstituted aryl, a substituted or unsubstituted heteroaryl, a substituted or unsubstituted -Ci-C4alkylene-C3- Ciocycloalkyl, a substituted or unsubstituted -Ci-C4alkylene-C2- Cioheterocycloalkyl, a substituted or unsubstituted -Ci-C4alkylene-aryl, and a substituted or unsubstituted -Ci-C4alkylene-heteroaryl. [0010] For any and all of the embodiments, substituents are selected from among from a subset of the listed alternatives. For example, in some embodiments, R1 is -CO2H, -CO2RD, - C(=O)NHSO2R10, -C(=O)N(R9)2, -CN, -C(=NH)-NH2, -C(=NH)-NHC(=0)RD, or tetrazolyl. In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -C(=O)N(R9)2, -CN, or tetrazolyl. In some embodiments, R1 is -CO2H, -CO2RD, or -C(=O)NHSO2R10. In some embodiments, R1 is -CO2H or -CO2RD. In some embodiments, R1 is -CO2H. In some
embodiments, R1 is -C(=O)NHSO2R10. In some embodiments, R10 is d-C6alkyl, C3- Cecycloalkyl, or a substituted or unsubstituted phenyl. In some embodiments, R10 is Ci-Cβalkyl or a substituted or unsubstituted phenyl. In some embodiments, RD is H, methyl, ethyl, propyl, isopropyl, n-butyl, iso-butyl, or tert-butyl. [0011] In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -C(=O)N(R9)2, -CN, -CC=O)NHCH2CH2SO3H, tetrazolyl, or 5-oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl. In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -C(=O)N(R9)2, -CN, tetrazolyl, or 5- oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl. In some embodiments, R1 is -CO2H, -CO2RD, or - C(=O)NHSO2R10. In some embodiments, R1 tetrazolyl or 5-oxo-2,5-dihydro-[l,2,4]oxadiazol- 3-yl.
[0012] In some embodiments, R4 is -NR7C(=O)OCH(R8)-CY, or -NR7C(=O)O-CY. In some embodiments, R4 is -NR7C(=O)OCH(R8)-CY.
[0013] In some embodiments, R8 is H, Ci-C4alkyl, or Ci-C4fluoroalkyl. In some embodiments, R8 is H or -CH3. In some embodiments, R8 is-CH3. [0014] In some embodiments, R8a is H Or -CH3. In some embodiments, R8a is H.
[0015] In some embodiments, CY is a substituted or unsubstituted C3-Ciocycloalkyl, a substituted or unsubstituted C2-Ci0heterocycloalkyl, a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then CY is substituted with 1, 2, or 3 Rc; or R8 and CY are taken together with the carbon atom to which they are attached to form a substituted or unsubstituted carbocycle or a substituted or unsubstituted heterocycle. [0016] In some embodiments, CY is a substituted or unsubstituted heteroaryl, wherein if CY is substituted then CY is substituted with 1, 2, or 3 Rc. In some embodiments, CY is a substituted or unsubstituted monocyclic heteroaryl, wherein if CY is substituted then CY is substituted with 1, 2, or 3 Rc. In some embodiments, CY is a substituted or unsubstituted 6-membered heteroaryl, wherein if CY is substituted then CY is substituted with 1 , 2, or 3 Rc.
[0017] In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -C(=O)N(R9)2, - SO2NHC(=O)R10, -CN, -CC=O)NHCH2CH2SO3H, tetrazolyl or 5-oxo-2,5-dihydro- [l,2,4]oxadiazol-3-yl; L1 is -C3-C6cycloalkylene-, -Ci-C4alkylene-C3-C6cycloalkylene-, Or -C3- C6cycloalkylene-Ci-C4alkylene-; L2 is absent, Ci-Cβalkylene, Ci-Cefluoroalkylene, -C1- C6alkylene-O-, -Ci-C3alkylene-O-Ci-C3alkylene -, -O-Ci-C6alkylene -, -Ci-C6alkylene-S-, -C1- C3alkylene-S-CrC3alkylene -, -S-CrC6alkylene -, -d-Cgalkylene-SO-, -C1-C3alkylene-SO-C1- C3alkylene -, -SO-Ci-C6alkylene -, -Ci-C6alkylene-SO2-, -Ci-C3alkylene-SO2-Ci-C3alkylene -, - SO2-Ci-C6alkylene -, -Ci-C4alkylene-NRπ-, -Ci-C2alkylene-NRπ-Ci-C2alkylene -, - NRπ-Ci- C4alkylene -, -O-, -S-, -SO-, -SO2- or -NR2-; R11 is H or CrC2alkyl; ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene, where if
ring A is substituted then ring A is substituted with 1 or 2 RA, where the groups
R
or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene, where if ring B is substituted then ring B is substituted with 1 or 2 RB; R3 is H or C1-C4alkyl; R7 is H; R8a is H; CY is Ci-Cβalkyl, substituted or unsubstituted Cs-Cβcycloalkyl, substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 1-3 N atoms, wherein if CY is substituted then CY is substituted with 1, 2, or 3 Rc; each of RA, RB, and Rc are independently selected from halogen, -CN, -NO2, -OH, -OR10, -N(R9)2, d-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl. [0018] In some embodiments, L1 is -Cs-Cβcycloalkylene-, -Ci^alkylene-Cs-Cecycloalkylene-, or -Cs^cycloalkylene-Ci^alkylene-. In some embodiments, L1 is -C3-C6cycloalkylene-. [0019] In some embodiments, ring A is a substituted or unsubstituted monocyclic ring wherein
the groups
and
in a 1,3 -relationship on ring A (i.e. an meta relationship). [0020] In some embodiments, ring A is a substituted or unsubstituted monocyclic ring wherein
[0021] In some embodiments, ring A is a substituted or unsubstituted phenylene, a substituted or unsubstituted napthylene, or a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring A is substituted then ring A is substituted with 1 or 2 RA.
[0022] In some embodiments, ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring A is substituted then ring A is substituted with 1 or 2 RA.
[0023] In some embodiments, ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted pyridinylene, where if ring A is substituted then ring A is substituted with 1 or 2 RA.
[0024] In some embodiments, ring B is a substituted or unsubstituted monocyclic cycloalkylene, a substituted or unsubstituted bicyclic cycloalkylene, a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted bicyclic heterocycloalkylene, a substituted or unsubstituted phenylene, a substituted or unsubstituted naphthylene, a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then ring B is substituted with 1 or 2 RB.
[0025] In some embodiments, each of RA, RB, and Rc are independently selected from halogen, -CN, -NO2, -OH, -OR10, -N(R9)2, d-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, C1- C4alkoxy, and Ci-C4heteroalkyl.
[0026] In some embodiments, each of RA, RB, and Rc are independently selected from halogen, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl. In some embodiments, each of RA, RB, and Rc are independently selected from halogen, Ci-C2alkyl, C1- C2fluoroalkyl, Ci-C2fluoroalkoxy, and Ci-C2alkoxy. [0027] In some embodiments, ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring A is substituted then ring A is substituted with 1 or 2 RA; ring B is a substituted or unsubstituted monocyclic cycloalkylene, a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene, where if ring B is substituted then ring B is substituted with 1 or 2 RB; CY is a substituted or unsubstituted C3-Cecycloalkyl, a substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 0-4 N atoms, wherein if CY is substituted then CY is substituted with 1 or 2 Rc; each of RA, RB, and Rc are independently selected from halogen, -CN, -NO2, -OH, -OR10, -N(R9)2, CrC4alkyl, CrC4fluoroalkyl, CrC4fluoroalkoxy, C1- C4alkoxy, and Ci-C4heteroalkyl.
[0028] In some embodiments, each of RA, RB, and Rc are independently selected from halogen, -CN, -OH, CrC4alkyl, CrC4fluoroalkyl, CrC4fluoroalkoxy, CrC4alkoxy, and C1- C4heteroalkyl. [0029] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl, cyclopropyl-l,2-diyl, cycloprop-2- enyl-l,l-diyl, cyclobutyl- 1,1 -diyl, cyclopentyl- 1 , 1 -diyl, cyclohexyl- 1 , 1 -diyl, -CH2C(CH2CH2)- or -C(CH2CH2)CH2-; L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -CH2NH-, - NHCH2-, -O-, -S-, or -NH-; ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring A is substituted then ring A is
substituted with 1 or 2 RA; ring B is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing O or 1 O atoms, O or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring B is substituted then ring B is substituted with 1 or 2 RB. (-CH2C(CH2CH2)- is -CH2-(cyclopropyl-l,l-diyl)-. -C(CH2CH2)CH2- is - (cyclopropyl- 1 , 1 -diyl)CH2-) . [0030] In some embodiments, the compound of Formula (I) has the structure of Formula (II):
Formula (II) wherein each of A1, A2, and A3 is independently selected from CH, CRA and N.
[0031] In some embodiments, each of A1, A2, and A3 is CH or CRA; or one of A1, A2, and A3 is N. In some embodiments, each of A1, A2, and A3 is CH or CRA. In some embodiments, one of A1, A2, and A3 is N. In some embodiments, each of A1, A2, and A3 is CH. [0032] In some embodiments, ring A is a substituted or unsubstituted phen-l,3-ylene, or a substituted or unsubstituted phen- 1 ,4-ylene, where if ring A is substituted then ring A is substituted with RA. In some embodiments, ring A is a substituted or unsubstituted phen- 1 ,4- ylene, where if ring A is substituted then ring A is substituted with RA. [0033] In some embodiments, ring B is a substituted or unsubstituted phen-l,2-ylene, a substituted or unsubstituted phen-l,3-ylene, or a substituted or unsubstituted phen-l,4-ylene, where if ring B is substituted then ring B is substituted with RB. In some embodiments, ring B is a substituted or unsubstituted phen-l,3-ylene, or a substituted or unsubstituted phen- 1,4- ylene, where if ring B is substituted then ring B is substituted with RB. In some embodiments, ring B is a substituted or unsubstituted phen- 1 ,4-ylene, where if ring B is substituted then ring B is substituted with RB.
[0034]
embodiments, the . In some embodiments, the
[0035] In some embodiments, each of A1, A2, and A3 is CH or CRA; or one of A1, A2, and A3 is
N; each RA is independently selected from halogen, -OH, Ci-C4alkyl, Ci-C4fluoroalkyl, C1-
C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl.
[0036] In some embodiments, R1 is -CO2H, -CO2RD, or -C(=O)NHSO2R10; RD is H, -CH3, or -CH2CH3; R8 is H, -CH3, -CH2CH3 or -CF3. In some embodiments, R8 is H or -CH3. In some embodiments, R8 is-CH3.
[0037] In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, or tetrazolyl; RD is H,
-CH3, or -CH2CH3; R4 is -NR7C(=O)OCH(R8)-CY; R7 is H; R8 is H, CrC4alkyl or C1-
C4fluoroalkyl; R10 is a Ci-Cβalkyl, Ci-Cefiuoroalkyl, C3-Cecycloalkyl, or a substituted or unsubstituted phenyl.
[0038] In some embodiments, R1 is -CO2H; each RAis independently selected from halogen, -
OH, -CH3, -CF3, -OCF3, and -OCH3; R3 is H, -CH3 or -CH2CH3; R7 is H; and R8 is -CH3 or -
CF3.
[0039] In some embodiments, R7 is H. In some embodiments, R8 is H, Ci-C4alkyl or C1- C4fluoroalkyl. In some embodiments, R8 is CrC4alkyl or C1-C4fluoroalkyl. In some other embodiments, R8 is H, -CH3 or -CF3. In yet other embodiments, R8 is -CH3 or -CF3. In some embodiments, R8 is H or -CH3. In some embodiments, R8 is -CH3.
[0040] In some embodiments, CY is a substituted or unsubstituted cycloalkyl, a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then CY is substituted with 1 or 2 Rc.
[0041] In some embodiments, CY is C3-Cecycloalkyl, a substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 0-4 N atoms, wherein if CY is substituted then CY is substituted with 1 or 2 Rc.
[0042] In some embodiments, CY is CrCβalkyl, substituted or unsubstituted C3-Cecycloalkyl, a substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 0-4 N atoms, wherein if CY is substituted then CY is substituted with 1 or 2 Rc.
[0043] In some embodiments, ring A is a substituted or unsubstituted phen- 1 ,4-ylene, where if ring A is substituted then ring A is substituted with RA; ring B is a substituted or unsubstituted phenyl- 1, 3 -ene or a substituted or unsubstituted phenyl- 1 ,4-ene, where if ring B is substituted then ring B is substituted with RB.
[0044] In one aspect, in any of the embodiments described herein, A is unsubstituted or monosubstituted with RA.
[0045] In one aspect, in any of the embodiments described herein, B is unsubstituted or monosubstituted with RB.
[0046] In one aspect, in any of the embodiments described herein, CY is unsubstituted or monosubstituted with Rc.
[0047] In some embodiments, L2 is absent, Ci-Cβalkylene, Ci-Cefluoroalkylene, C1-
C6heteroalkylene, -O-, -S-, -SO-, -SO2- or -NR2-. [0048] In some embodiments, L2 is a absent, Ci-C6alkylene, Ci-C6fluoroalkylene, -C1-
C6alkylene-O-, -Ci-C3alkylene-O-Ci-C3alkylene -, -O-Ci-C6alkylene -, -Ci-C6alkylene-S-, -C1-
C3alkylene-S-Ci-C3alkylene -, -S-Ci-C6alkylene -, -Ci-Cgalkylene-SO-, -Ci-C3alkylene-SO-Ci-
C3alkylene -, -SO-CrC6alkylene -, -C1-C6alkylene-Sθ2-, -C1-C3alkylene-SO2-C1-C3alkylene -, -
SO2-Ci-C6alkylene -, -Ci-C4alkylene-NRπ-, -Ci-C2alkylene-NRπ-Ci-C2alkylene -, - NRπ-Ci- C4alkylene -, -O-, -S-, -SO-, -SO2- or -NR2-; R11 is H or Ci-C2alkyl.
[0049] In some embodiments, L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -CH2NH-
, -NHCH2-, -O-, -S-, or -NH-.
[0050] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl, cyclopropyl-l,2-diyl, cycloprop-2- enyl-l,l-diyl, cyclobutyl- 1,1 -diyl, cyclopentyl- 1 , 1 -diyl, cyclohexyl- 1 , 1 -diyl, -CH2C(CH2CH2)- or -C(CH2CH2)CH2-. In some embodiments, L1 is cyclopropyl- 1,1 -diyl, cyclopropyl- 1 ,2-diyl, cyclobutyl- 1,1 -diyl, cyclopentyl- 1 , 1 -diyl or cyclohexyl- 1,1 -diyl. In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl- 1 , 1 -diyl, cyclopentyl- 1 , 1 -diyl or cyclohexyl- 1 , 1 -diyl. In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl- 1 , 1 -diyl, or cyclopentyl- 1,1 -diyl. In some embodiments, L1 is cyclopropyl- 1,1 -diyl. [0051] In some embodiments, ring B is a substituted or unsubstituted monocyclic cycloalkylene, a substituted or unsubstituted phenylene, where if ring B is substituted then ring
B is substituted with 1 or 2 RB.
[0052] In some embodiments, ring B is a substituted or unsubstituted cyclopentylene, a substituted or unsubstituted cyclopentenylene, a substituted or unsubstituted cyclohexylene, a substituted or unsubstituted cyclohexenylene, or a substituted or unsubstituted phenylene, where if ring B is substituted then ring B is substituted with 1 or 2 RB.
[0053] In some embodiments, ring B is a substituted or unsubstituted phenylene, where if ring
B is substituted then ring B is substituted with 1 or 2 RB.
[0054] In some embodiments, ring B is a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted bicyclic heterocycloalkylene, a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then ring B is substituted with 1 or 2 RB.
[0055] In some embodiments, ring B is a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then ring B is substituted with 1 or 2 RB.
[0056] In some embodiments, ring B is a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring B is substituted then ring B is substituted with 1 or 2 RB. [0057] In some embodiments, ring B is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring B is substituted then ring B is substituted with 1 or 2 RB.
O H R8 O H, R8
[0058] In some embodiments, R4 is H or H
[0059] In some embodiments, R8 is -CH3 or -CF3. In some embodiments, R8 is -CH3. In some embodiments, R8 is H or -CH3. In some embodiments, R8 is H.
O H CH3
^N^O^CY [0061] In some embodiments, R4 is H . In some embodiments, R4 is
[0062] In some embodiments, CY is a substituted or unsubstituted phenyl, where if CY is substituted then CY is substituted with 1 or 2 Rc; each Rc is independently selected from halogen, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl. [0063] In some embodiments, each Rc is independently selected from F, Cl, -CH3, -CF3, - OCF3, -OCH3. [0064] In some embodiments, CY is phenyl, 2-fluorophenyl or 2-chloro-phenyl.
[0065] In some embodiments, L2 is absent; ring A is a substituted or unsubstituted phen-1,4- ylene, where if ring A is substituted then ring A is substituted with RA; ring B is a substituted or unsubstituted phen-l,4-ylene, where if ring B is substituted then ring B is substituted with RB; R8 is -CH3; each Rcis independently selected from F, Cl, Br, I, -CH3, -CF3, -OH, -OCF3, and - OCH3.
[0066] In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, or tetrazolyl; RD is H, -CH3, or -CH2CH3; ring A is a substituted or unsubstituted phen- 1 ,4-ylene, where if ring A is substituted then ring A is substituted with RA; ring B is a substituted or unsubstituted phen- 1,4- ylene, where if ring B is substituted then ring B is substituted with RB; R3 is H, or -CH3; R4 is - NR7C(=O)OCH(R8)-CY; R7 is H; R8 is -CH3; CY is a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with Rc; R10 is a Ci-C6alkyl, C3- Cecycloalkyl, or a substituted or unsubstituted phenyl; each Rc is independently selected from H, F, Cl, Br, I, -OH, -CH3, -CF3, -OH, -OCF3, and -OCH3.
[0067] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl; L2 is absent; ring B is a substituted or unsubstituted phen-l,4-ylene, where if ring B is substituted then ring B is substituted with RB; each RAis independently selected from H, F, Cl, -CH3, and -OH; each RB is independently selected from H, F, Cl, -CH3, and -OH; each Rc is independently selected from H, F, Cl, -CH3, - CF3, -OH, -OCF3, and -OCH3. [0068] In one aspect, the compound of Formula (I) has the structure of Formula (III):
Formula (III) wherein
A1 is CRA and N; RA is H, F, Cl, Br, I, -CH3, or -CF3; A4 is CH or N; L1 is C3-C6cycloalkylene;
L2 is absent, Ci-C4alkylene, d-C4heteroalkylene, -0-, -S-, -SO-, -SO2-, -NR2-, or - C(=0)-;
R2 is H or -CH3;
CY is C3-C6cycloalkyl or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with Rc; Rc is H, F, Cl, Br, I, -OH, CrC4alkyl,
Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, or Ci-C4alkoxy.
[0069] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl- 1 , 1 -diyl, cyclopentyl-1,1- diyl or cyclohexyl- 1,1 -diyl. In some embodiments, L1 is cyclopropyl- 1,1 -diyl. [0070] In some embodiments, L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -0-, or - S-. In some embodiments, L2 is absent.
[0071] In some embodiments, A1 is CH. In some embodiments, A4 is CH.
[0072] In some embodiments, CY is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, a substituted or unsubstituted pentenyl, a substituted or unsubstituted cyclohexenyl, or a substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -CH3, or CF3. In some embodiments, CY is a substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -CH3, or CF3. In some embodiments, CY is cyclopropyl. In some embodiments, CY is phenyl. [0073] In some embodiments, CY is phenyl, 2-fluorophenyl or 2-chloro-phenyl. [0074] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl; L2 is absent; CY is cyclopropyl. [0075] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl; L2 is absent; CY is phenyl. [0076] In some embodiments, A1 is CH; and A4 is CH.
[0077] In one aspect, the compound of Formula (III) has the structure:
[0078] In one aspect, the compound of Formula (III) has the structure:
Formula (IV) wherein
A1 is CRA and N; RA is H, F, Cl, Br, I, -CH3, or -CF3; L2 is absent, CrC4alkylene, CrC4heteroalkylene, -O-, -S-, -SO-, -SO2-, -NR2-, or -
C(=O)-;
R2 is H or -CH3;
R4 is -NHC(=O)OCH(R8)-CY, or -NHC(=O)O-CY; R8 is H or -CH3;
CY is Ci-Cβalkyl, C3-C6cycloalkyl, or substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with Rc; Rc is H, F, Cl, Br, I, -OH, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, or Ci-C4alkoxy; n is 1, 2, or 3. [0080] In some embodiments, L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -O-, or - S-. In some embodiments, L2 is absent.
[0081] In some embodiments, A1 is CRA. In some embodiments, A1 is CH. [0082] In some embodiments, A1 is N. [0083] In some embodiments, CY is -CH3, -CH2CH3, -CH2CH2CH3, -CH2CH2CH2CH3, - CH(CH3)2, -CH(CH2CH3)2, -CH2CH(CH3)2, -C(CH3)3, -CH2(CH3)3, C3-C6cycloalkyl, substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with Rc;
Rc is F, Cl, -CH3, or CF3.
[0084] In some embodiments, R4 is -NHC(=O)OCH(R8)-CY.
[0085] In some embodiments, n is 1. [0086] In some embodiments, R4 is -NHC(=O)OCH2-(cyclopropyl), -NHC(=O)OCH(CH3)- (cyclopropyl), -NHC(=O)OCH2-(substituted or unsubstituted phenyl) or - NHC(=O)OCH(CH3)-(substituted or unsubstituted phenyl); wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -CH3, or CF3. In some embodiments, R4 is - NHC(=O)OCH(CH3)-(cyclopropyl). In some embodiments, R4 is -NHC(=O)OCH2-(substituted or unsubstituted phenyl) or -NHC(=O)OCH(CH3)-(substituted or unsubstituted phenyl); wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -CH3, or CF3. In some embodiments, R4 is -NHC(=O)OCH(CH3)-(substituted or unsubstituted phenyl); wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -CH3, or CF3. [0087] In some embodiments, R4 is -NHC(=O)O-CY. In some embodiments, CY is -CH2CH3, -CH2CH2CH3, -CH2CH2CH2CH3, -CH(CH3)2, -CH(CH2CH3)2, -CH2CH(CH3)2, -C(CH3)3, - CH2(CH3)3, or C3-C6cycloalkyl. In some embodiments, CY is -C(CH3)3 or C3-C6cycloalkyl. In some embodiments, CY is -C(CH3)3.
[0088] In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, or tetrazolyl; RD is H, -CH3, or -CH2CH3; R4 is -NR7C(=O)OCH(R8)-CY; R7 is H; R8 is H, Ci-C4alkyl or C1- C4fluoroalkyl; R10 is a Ci-C6alkyl, Ci-C6fluoroalkyl, C3-C6cycloalkyl, or a substituted or unsubstituted phenyl.
[0089] In some embodiments, R1 is -CO2H; L1 is cyclopropyl- 1 , 1 -diyl, cyclopropyl- 1 ,2-diyl, cyclobutyl-l,l-diyl, cyclopentyl- 1,1 -diyl, or cyclohexyl- 1,1 -diyl; L2 is absent; ring B is a substituted or unsubstituted phenyl- 1,4-ene, where if ring B is substituted then ring B is substituted with RB; R3 is H, -CH3 or -CH2CH3; R8 is H, -CH3 or -CF3; each RA is
independently selected from halogen, -OH, -CH3, -CF3, -OCF3, and -OCH3; each RB is independently selected from halogen, -OH, -CH3, -CF3, -OCF3, and -OCH3; each Rc is independently selected from halogen -OH, -CH3, -CF3, -OCF3, and -OCH3. [0090] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl; R3 is -CH3; R8 is -CH3; CY is substituted or unsubstituted phenyl, wherein if CY is substituted then CY is monosubstituted with Rc.
[0091] In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -CN, - C(=O)NHCH2CH2SO3H, tetrazolyl, or 5-oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl; L1 is -C3- Cecycloalkylene-; L2 is absent; ring A is a substituted or unsubstituted phen-l,4-ylene, where if ring A is substituted then ring A is substituted with RA; ring B is a substituted or unsubstituted phenyl- 1 ,4-ene, where if ring B is substituted then ring B is substituted with RB; R3 is H or Ci- C4alkyl; R4 is -NR7C(=O)OCH(R8)-CY; R7 is H; R8 is H or Ci-C4alkyl, CY is substituted or unsubstituted C3-Cecycloalkyl, substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 1-3 N atoms, wherein if CY is substituted then CY is substituted with Rc; each of RA, RB, and Rc are independently selected from H, halogen, - OH, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, CrC4alkoxy, and Ci-C4heteroalkyl. [0092] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl; R3 is H or -CH3; R4 is O H R8
H ; R8 is H or -CH3; CY is substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with Rc. [0093] In some embodiments, R1 is -CO2H; R3 is -CH3; R8 is -CH3; CY is substituted or unsubstituted phenyl, where CY is unsubstituted or monosubstituted with Rc. [0094] In some embodiments, the compound of Formula (I) has the following structure:
A1 is CH, CRA or N; RA is F, Cl, Br, I, -CH3, or -CF3;
L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -0-, or -S-;
R8 is H or -CH3;
CY is Ci-Cβalkyl, substituted or unsubstituted C3-Cecycloalkyl, or substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with 1 or 2 Rc; Rc is F, Cl, Br, I, -OH, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, or C1- C4alkoxy; n is 1, 2, or 3.
[0095] In some embodiments, A1 is CH; L2 is absent; CY is -CH3, -CH2CH3, -CH2CH2CH3, - CH2CH2CH2CH3, -CH(CH3)2, -CH(CH2CH3)2, -CH2CH(CH3)2, -C(CH3)3, -CH2(CH3)3, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, a substituted or unsubstituted pentenyl, a substituted or unsubstituted cyclohexenyl, or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -OH, -CH3, or CF3; n is 1.
[0096] In some embodiments, the compound of Formula (I) has the structure of Formula (V) or a pharmaceutically acceptable salt thereof:
Formula (V) wherein
R1 is -CO2H, -CO2RD, -CN, -C(=O)N(R9)2, -C(=O)NHCH2CH2SO3H, or -
C(=O)NHSO2R10, tetrazolyl, or 5-oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl; RD is H or Ci-C4alkyl;
R3 is H, CrC4alkyl, C3-C6cycloalkyl, or CrC4fluoroalkyl; R4 is -NR7C(=O)OCH(R8)-CY;
R7 is H or Ci-C4alkyl; R8 is H, CrC4alkyl, or CrC4fluoroalkyl; CY is a substituted or unsubstituted C3-Cecycloalkyl or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with 1 or 2 Rc;
R9 is H, Ci-Cβalkyl, Ci-Cefluoroalkyl, C3-Cecycloalkyl, or a substituted or unsubstituted phenyl; R10 is a Ci-Cβalkyl, Ci-Cefluoroalkyl, C3-Cecycloalkyl, or a substituted or unsubstituted phenyl; each of RA, RB, and Rc are independently selected from F, Cl, Br, I, -CN, -OH, C1-
C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl; m is 0, 1, or 2;
n is 1, 2, 3 or 4; p is 0, 1, or 2.
[0097] In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10 or tetrazolyl; R3 is H or Ci-C4alkyl; R7 is H; R8 is H, -CH3 or -CF3; R10 is a Ci-C6alkyl or a substituted or unsubstituted phenyl; each RAis independently selected from F, Cl, Br, I, -OH, -CH3, -CF3, - OCF3, and -OCH3; each RB is independently selected from F, Cl, Br, I, -OH, -CH3, -CF3, -OCF3, and -OCH3; each Rc is independently selected from F, Cl, Br, I, -OH, -CH3, -CF3, -OCF3, and - OCH3; m is O or 1; [0098] n is 1, 2, or 3; p is O or 1. [0099] In some embodiments, R1 is -CO2H or -CO2RD; RD is H, -CH3, or -CH2CH3; R3 is H, - CH3 or -CH2CH3; R4 is -NHC(=O)OCH(R8)-CY; R8 is H, or -CH3; CY is a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with 1 or 2 Rc. [00100] In some embodiments, the compound of Formula (I) or Formula (V) has the following structure:
[00101] In some embodiments, the compound of Formula (I) or Formula (V) has the following structure:
[00102] In some embodiments, R1 is -C(=O)NHSO2R10; R3 is H, -CH3 or -CH2CH3; R8 is H, or -CH3; R10 is -CH3, or -CH2CH3.
[00103] In some embodiments, R4 is -NHC(=O)OCH(CH3)-(substituted or unsubstituted phenyl); wherein if the phenyl is substituted then the phenyl is substituted with Rc; Rc is F, Cl, -
CH3, or CF3; n is 1.
[00104] In some embodiments, R4 is
phenyl, wherein if CY is substituted phenyl then the phenyl is substituted with 1 or 2 Rc; Rc is H, F, Cl, -OH, -CH3, -CF3, or -OCH3; n is 1.
[00105] In some embodiments, R8 is -CH3; CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3-hydroxyphenyl, 4-
hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fiuoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl.
[00106] In some embodiments, the compound of Formula I) or Formula (V) has the following structure:
[00107] In some embodiments, R1 is -CO2H; CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl,
2,4-difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3 -hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl. [00108] In some embodiments, CY is phenyl. In some embodiments, R1 is -CO2H; CY is phenyl, 2-fluorophenyl, 3 -fluorophenyl, 2-chlorophenyl, 3-chlorophenyl, 2-methylphenyl, 3- methylphenyl, 2-trifluoromethylphenyl, or 3-trifluoromethylphenyl. In some embodiments, R1 is -CO2H; CY is phenyl.
[00109] In some embodiments, Rc is F, Cl, -CH3, or CF3. [00110] In some embodiments, ring B is a substituted or unsubstituted phenylene; Rc is F or Cl.
[00111] In some embodiments, Rc is Cl.
[00112] In some embodiments, Rc is F.
[00113] In some embodiments, the compound of Formula (I) or Formula (V) has the following structure:
[00114] In some embodiments, the compound of Formula (I) or Formula (V) has one of the following structures:
[00115] In some embodiments, CY is cyclopropyl, cyclobutyl, cyclohexyl, 2-chlorocyclohex- 1 - enyl, phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6- difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4- dichlorophenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl.
[00116] In some embodiments, CY is cyclopropyl, cyclobutyl, cyclohexyl, 2-chlorocyclohex- 1 - enyl, phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6- difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4- dichlorophenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2-hydroxyphenyl, 3- hydroxyphenyl, 4-hydroxyphenyl, 2-trifluoromethylphenyl, 3-trifluoromethylphenyl, 4- trifluoromethylphenyl, 2-fluoro-4-methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4- methylphenyl, 2-cyanophenyl, 3-cyanophenyl, or 4-cyanophenyl.
[00117] In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, tetrazolyl, or 5-oxo- 2,5-dihydro-[l,2,4]oxadiazol-3-yl; R3 is H or CrC4alkyl; R7 is H; R8 is H, or -CH3; R10 is a C1- Cβalkyl or a substituted or unsubstituted phenyl; CY is cyclopropyl, cyclobutyl, cyclopentyl, cyclopent-1-enyl, 2-chlorocyclopent- 1 -enyl, cyclohexyl, cyclohex-1-enyl, 2-chlorocyclohex- 1 - enyl, phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6- difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4- dichlorophenyl, 2-hydroxyphenyl, 3- hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3- methoxyphenyl, 4-methoxyphenyl, 2-trifluoromethylphenyl, 3-trifluoromethylphenyl, 4- trifluoromethylphenyl, 2-fluoro-4-methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4- methylphenyl, 2-cyanophenyl, 3-cyanophenyl, or 4-cyanophenyl.
[00118] In some embodiments, each RAis independently selected from F, Cl, -CH3, -CF3, -OH, - OCF3, and -OCH3; each RB is independently selected from F, Cl, -CH3, -CF3, -OH, -OCF3, and - OCH3; m is 0 or 1 ; p is 0 or 1. In some embodiments, n is 1.
[00119] Any combination of the groups described above for the various variables is contemplated herein. Throughout the specification, groups and substituents thereof are chosen by one skilled in the field to provide stable moieties and compounds.
[00120] In one aspect, compounds of Formula (I), (II), (III), (IV) and (V) include compounds described in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6.
[00121] The compound of Formula (I), (II), (III), (IV) or (V) is an antagonist of a LPA receptor. In some embodiments, the compound of Formula (I), (II), (III), (IV) or (V) is an antagonist of LPA1. In some embodiments, the compound of Formula (I), (II), (III), (IV) or (V) is an antagonist Of LPA2. In some embodiments, the compound of Formula (I), (II), (III), (IV) or (V) is an antagonist of LP A3.
[00122] In some embodiments, presented herein are compounds selected from active metabolites, tautomers, pharmaceutically acceptable solvates, pharmaceutically acceptable salts or prodrugs of a compound of Formula (I), (II), (III), (IV) or (V). [00123] In some embodiments, provided is a pharmaceutical composition comprising a therapeutically effective amount of a compound of Formula (I), (II), (III), (IV) or (V). In some embodiments, the pharmaceutical composition also contains at least one pharmaceutically acceptable inactive ingredient.
[00124] In some embodiments, provided is a pharmaceutical composition comprising a therapeutically effective amount of a compound of Formula (I), (II), (III), (IV) or (V), or a pharmaceutically acceptable salt thereof, and at least one pharmaceutically acceptable inactive ingredient. In one aspect, the pharmaceutical composition is formulated for intravenous injection, subcutaneous injection, oral administration, inhalation, nasal administration, topical administration, ophthalmic administration or otic administration. In some embodiments, the pharmaceutical composition is a tablet, a pill, a capsule, a liquid, an inhalant, a nasal spray solution, a suppository, a suspension, a gel, a colloid, a dispersion, a suspension, a solution, an emulsion, an ointment, a lotion, an eye drop or an ear drop.
[00125] In some embodiments, the pharmaceutical composition further comprises one or more additional therapeutically active agents selected from: corticosteroids, immunosuppresants, analgesics, anti-cancer agent, antiinflammatories, chemokine receptor antagonists, bronchodilators, leukotriene receptor antagonists, leukotriene formation inhibitors, monoacylglycerol kinase inhibitors, phospholipase A1 inhibitors, phospholipase A2 inhibitors, and lysophospholipase D (lysoPLD) inhibitors, autotaxin inhibitors, decongestants, antihistamines, mucolytics, anticholinergics, antitussives, expectorants, and β-2 agonists. [00126] In some embodiments, provided is a method comprising administering a compound of Formula (I), (II), (III), (IV) or (V) to a human with a LPA-dependent or LPA-mediated disease
or condition. In some embodiments, the human is already being administered one or more additional therapeutically active agents other than a compound of Formula (I), (II), (III), (IV) or (V). In some embodiments, the method further comprises administering one or more additional therapeutically active agents other than a compound of Formula (I), (II), (III), (IV) or (V). [00127] In some embodiments, the one or more additional therapeutically active agents other than a compound of Formula (I), (II), (III), (IV) or (V) are selected from: corticosteroids, immunosuppresants, analgesics, anti-cancer agent, antiinflammatories, chemokine receptor antagonists, bronchodilators, leukotriene receptor antagonists, leukotriene formation inhibitors, monoacylglycerol kinase inhibitors, phospholipase Ai inhibitors, phospholipase A2 inhibitors, and lysophospho lipase D (lysoPLD) inhibitors, autotaxin inhibitors, decongestants, antihistamines, mucolytics, anticholinergics, antitussives, expectorants, and β-2 agonists. [00128] Pharmaceutical formulations described herein are administerable to a subject in a variety of ways by multiple administration routes, including but not limited to, oral, parenteral (e.g., intravenous, subcutaneous, intramuscular), intranasal, buccal, topical or transdermal administration routes. The pharmaceutical formulations described herein include, but are not limited to, aqueous liquid dispersions, self-emulsifying dispersions, solid solutions, liposomal dispersions, aerosols, solid dosage forms, powders, immediate release formulations, controlled release formulations, fast melt formulations, tablets, capsules, pills, delayed release formulations, extended release formulations, pulsatile release formulations, multiparticulate formulations, and mixed immediate and controlled release formulations.
[00129] In some embodiments, the compound of Formula (I), (II), (III), (IV) or (V) is administered orally.
[00130] In some embodiments, the compound of Formula (I), (II), (III), (IV) or (V) is administered topically. In such embodiments, the compound of Formula (I), (II), (III), (IV) or (V) is formulated into a variety of topically administrable compositions, such as solutions, suspensions, lotions, gels, pastes, shampoos, scrubs, rubs, smears, medicated sticks, medicated bandages, balms, creams or ointments. Such pharmaceutical compounds can contain solubilizers, stabilizers, tonicity enhancing agents, buffers and preservatives. In one aspect, the compound of Formula (I), (II), (III), (IV) or (V) is administered topically to the skin. [00131] In another aspect, the compound of Formula (I), (II), (III), (IV) or (V) is administered by inhalation. In one embodiment, the compound of Formula (I), (II), (III), (IV) or (V) is administered by inhalation that directly targets the pulmonary system.
[00132] In another aspect, the compound of Formula (I), (II), (III), (IV) or (V) is formulated for intranasal adminstration. Such formulations include nasal sprays, nasal mists, and the like.
[00133] In another aspect, the compound of Formula (I), (II), (III), (IV) or (V) is formulated as eye drops.
[00134] In another aspect is the use of a compound of Formula (I), (II), (III), (IV) or (V) in the manufacture of a medicament for treating a disease, disorder or conditions in which the activity of at least one LPA receptor contributes to the pathology and/or symptoms of the disease or condition. In one embodiment of this aspect, the LPA is selected from LPAi, LPA2, LPA3, LPA4, LPA5 and LPAβ. In one aspect, the LPA receptor is LPAi. In one aspect, the disease or condition is any of the diseases or conditions specified herein. [00135] In any of the aforementioned aspects are further embodiments in which: (a) the effective amount of the compound of Formula (I), (II), (III), (IV) or (V) is systemically administered to the mammal; and/or (b) the effective amount of the compound is administered orally to the mammal; and/or (c) the effective amount of the compound is intravenously administered to the mammal; and/or (d) the effective amount of the compound is administered by inhalation; and/or (e) the effective amount of the compound is administered by nasal administration; or and/or (f) the effective amount of the compound is administered by injection to the mammal; and/or (g) the effective amount of the compound is administered topically to the mammal; and/or (h) the effective amount of the compound is administered by ophthalmic administration; and/or (i) the effective amount of the compound is administered rectally to the mammal; and/or (j) the effective amount is adminstered non-systemically or locally to the mammal. [00136] In any of the aforementioned aspects are further embodiments comprising single administrations of the effective amount of the compound, including further embodiments in which (i) the compound is administered once; (ii) the compound is administered to the mammal multiple times over the span of one day; (iii) continually; or (iv) continuously. [00137] In any of the aforementioned aspects are further embodiments comprising multiple administrations of the effective amount of the compound, including further embodiments in which (i) the compound is administered continuously or intermittently: as in a a single dose; (ii) the time between multiple administrations is every 6 hours; (iii) the compound is administered to the mammal every 8 hours; (iv) the compound is administered to the mammal every 12 hours; (v) the compound is administered to the mammal every 24 hours. In further or alternative embodiments, the method comprises a drug holiday, wherein the administration of the compound is temporarily suspended or the dose of the compound being administered is temporarily reduced; at the end of the drug holiday, dosing of the compound is resumed. In one embodiment, the length of the drug holiday varies from 2 days to 1 year.
[00138] Also provided is a method of inhibiting the physiological activity of LPA in a mammal comprising administering a therapuetically effective amount of a compound of Formula (I), (II), (III), (IV) or (V) or a pharmaceutically acceptable salt thereof to the mammal in need thereof. [00139] In one aspect, provided is a medicament for treating a LPA-dependent or LPA-mediated disease or condition in a mammal comprising a therapeutically effective amount of a compound of Formula (I), (II), (III), (IV) or (V).
[00140] In some cases disclosed herein is the use of a compound of Formula (I), (II), (III), (IV) or (V) in the treatment or prevention of a LPA-dependent or LPA-mediated disease or condition. [00141] In one aspect, is a method for treating or preventing a LPA-dependent or LPA-mediated disease or condition in a mammal comprising administering a therapuetically effective amount of a compound of Formula (I), (II), (III), (IV) or (V).
[00142] In one aspect, LPA-dependent or LPA-mediated diseases or conditions include, but are not limited to, fibrosis of organs or tissues, scarring, liver diseases, dermatological conditions, cancer, cardiovascular disease, respiratory diseases or conditions, inflammatory disease, gastrointestinal tract disease, renal disease, urinary tract-associated disease, inflammatory disease of lower urinary tract, dysuria, frequent urination, pancreas disease, arterial obstruction, cerebral infarction, cerebral hemorrhage, pain, peripheral neuropathy, and fibromyalgia. [00143] In one aspect, the LPA-dependent or LPA-mediated disease or condition is a respiratory disease or condition. In some embodiments, the respiratory disease or condition is asthma, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, pulmonary arterial hypertension or acute respiratory distress syndrome.
[00144] In some embodiments, the LPA-dependent or LPA-mediated disease or condition is selected from idiopathic pulmonary fibrosis; other diffuse parenchymal lung diseases of different etiologies including iatrogenic drug-induced fibrosis, occupational and/or environmental induced fibrosis, granulomatous diseases (sarcoidosis, hypersensitivity pneumonia), collagen vascular disease, alveolar proteinosis, langerhans cell granulomatosis, lymphangioleiomyomatosis, inherited diseases (Hermansky-Pudlak Syndrome, tuberous sclerosis, neurofibromatosis, metabolic storage disorders, familial interstitial lung disease); radiation induced fibrosis; chronic obstructive pulmonary disease (COPD); scleroderma; bleomycin induced pulmonary fibrosis; chronic asthma; silicosis; asbestos induced pulmonary fibrosis; acute respiratory distress syndrome (ARDS); kidney fibrosis; tubulointerstitium fibrosis; glomerular nephritis; focal segmental glomerular sclerosis; IgA nephropathy; hypertension; Alport; gut fibrosis; liver fibrosis; cirrhosis; alcohol induced liver fibrosis; toxic/drug induced liver fibrosis; hemochromatosis; nonalcoholic steatohepatitis (NASH);
biliary duct injury; primary biliary cirrhosis; infection induced liver fibrosis; viral induced liver fibrosis; and autoimmune hepatitis; corneal scarring; hypertrophic scarring; Duputren disease, keloids, cutaneous fibrosis; cutaneous scleroderma; spinal cord injury/fibrosis; myelofibrosis; vascular restenosis; atherosclerosis; arteriosclerosis; Wegener's granulomatosis; Peyronie's disease, chronic lymphocytic leukemia, tumor metastasis, transplant organ rejection, endometreosis, neonatal respiratory distress syndrome and neuropathic pain.
[00145] In one aspect, the LPA-dependent or LPA-mediated disease or condition is described herein.
[00146] In one aspect, provided is a method for the treatment or prevention of organ fibrosis in a mammal comprising administering a therapeutically effective amount of a compound of
Formula (I), (II), (III), (IV) or (V) or a pharmaceutically acceptable salt thereof to a mammal in need thereof.
[00147] In one aspect, the organ fibrosis comprises lung fibrosis, renal fibrosis, or hepatic fibrosis. [00148] In one aspect, provided is a method of improving lung function in a mammal comprising administering a therapeutically effective amount of a compound of Formula (I), (II),
(III), (IV) or (V) or a pharmaceutically acceptable salt thereof to the mammal in need thereof. In one aspect, the mammal has been diagnosed as having lung fibrosis.
[00149] In one aspect, compounds disclosed herein are used to treat idiopathic pulmonary fibrosis (usual interstitial pneumonia) in a mammal.
[00150] In some embodiments, compounds disclosed herein are used to treat diffuse parenchymal interstitial lung diseases in mammal: iatrogenic drug induced, occupational/environmental (Farmer lung), granulomatous diseases (sarcoidosis, hypersensitivity pneumonia), collagen vascular disease (scleroderma and others), alveolar proteinosis, langerhans cell granulonmatosis, lymphangioleiomyomatosis, Hermansky-Pudlak
Syndrome, Tuberous sclerosis, neurofibromatosis, metabolic storage disorders, familial interstitial lung disease.
[00151] In some embodiments, compounds disclosed herein are used to treat post-transplant fibrosis associated with chronic rejection in a mammal: Bronchiolitis obliterans for lung transplant.
[00152] In some embodiments, compounds disclosed herein are used to treat cutaneous fibrosis in a mammal: cutaneous scleroderma, Dupuytren disease, keloids.
[00153] In one aspect, compounds disclosed herein are used to treat hepatic fibrosis with or without cirrhosis in a mammal: toxic/drug induced (hemochromatosis), alcoholic liver disease,
viral hepatitis (hepatitis B virus, hepatitis C virus, HCV), nonalcoholic liver disease (NASH), metabolic and auto-immune.
[00154] In one aspect, compounds disclosed herein are used to treat renal fibrosis in a mammal: tubulointerstitium fibrosis, glomerular sclerosis. [00155] In any of the aforementioned aspects involving the treatment of LPA dependent diseases or conditions are further embodiments comprising administering at least one additional agent in addition to the administartion of a compound having the structure of Formula (I), (II), (III), (IV) or (V). In various embodiments, each agent is administered in any order, including simultaneously. [00156] In any of the embodiments disclosed herein, the mammal is a human.
[00157] In some embodiments, compounds provided herein are administered to a human.
[00158] In some embodiments, compounds provided herein are orally administered.
[00159] In some embodiments, compounds provided herein are used as antagonists of at least one LPA receptor. In some embodiments, compounds provided herein are used for inhibiting the activity of at least one LPA receptor or for the treatment of a disease or condition that would benefit from inhibition of the activity of at least one LPA receptor. In one aspect, the LPA receptor is LPA1.
[00160] In other embodiments, compounds provided herein are used for the formulation of a medicament for the inhibition of LPAi activity. [00161] Articles of manufacture, which include packaging material, a compound of Formula (I),
(II), (III), (IV) or (V) within the packaging material, and a label that indicates that the compound or composition, or pharmaceutically acceptable salt, tautomers, pharmaceutically acceptable N-oxide, pharmaceutically active metabolite, pharmaceutically acceptable prodrug, or pharmaceutically acceptable solvate thereof, is used for inhibiting the activity of at least one LPA receptor, or for the treatment, prevention or amelioration of one or more symptoms of a disease or condition that would benefit from inhibition of the activity of at least one LPA receptor, are provided.
[00162] Other objects, features and advantages of the compounds, methods and compositions described herein will become apparent from the following detailed description. It should be understood, however, that the detailed description and the specific examples, while indicating specific embodiments, are given by way of illustration only, since various changes and modifications within the spirit and scope of the instant disclosure will become apparent to those skilled in the art from this detailed description
DETAILED DESCRIPTION OF THE INVENTION
[00163] Lysophospholipids are membrane-derived bioactive lipid mediators. Lysophospholipids include, but are not limited to, lysophosphatidic acid (l-acyl-2-hydroxy-s«-glycero-3- phosphate; LPA), sphingosine 1 -phosphate (SlP), lysophosphatidylcholine (LPC), and sphingosylphosphoryl choline (SPC). Lysophospholipids affect fundamental cellular functions that include cellular proliferation, differentiation, survival, migration, adhesion, invasion, and morphogensis. These functions influence many biological processes that include neurogensis, angiogenesis, wound healing, immunity, and carcinogenesis.
[00164] LPA acts through sets of specific G protein-coupled receptors (GPCRs) in an autocrine and paracrine fashion. LPA binding to its cognate GPCRs (LPAi, LPA2, LPA3, LPA4, LPA5, LP Ae) activates intracellular signaling pathways to produce a variety of biological responses. [00165] Lysophospholipids, such as LPA, are quantitatively minor lipid species compared to their major phospholipid counterparts (e.g., phosphatidylcholine, phosphatidylethanolamine, and sphingomyelin). LPA has a role as a biological effector molecule, and has a diverse range of physiological actions such as, but not limited to, effects on blood pressure, platelet activation, and smooth muscle contraction, and a variety of cellular effects, which include cell growth, cell rounding, neurite retraction, and actin stress fiber formation and cell migration. The effects of LPA are predominantly receptor mediated.
[00166] Activation of the LPA receptors (LPA1, LPA2, LPA3, LPA4, LPA5, LPA6) with LPA mediates a range of downstream signaling cascades. These include, but are not limited to, mitogen-activated protein kinase (MAPK) activation, adenylyl cyclase (AC) inhibition/activation, phospholipase C (PLC) activation/Ca2+ mobilization, arachidonic acid release, Akt/PKB activation, and the activation of small GTPases, Rho, ROCK, Rac, and Ras. Other pathways that are affected by LPA receptor activation include, but are not limited to, cyclic adenosine monophosphate (cAMP), cell division cycle 42/GTP-binding protein (Cdc42) , proto-oncogene serine/threonine-protein kinase Raf (c-RAF), proto-oncogene tyrosine-protein kinase Src (c-src), extracellular signal-regulated kinase (ERK), focal adhesion kinase (FAK), guanine nucleotide exchange factor (GEF), glycogen synthase kinase 3b (GSK3b), c-jun amino- terminal kinase (JNK), MEK, myosin light chain II (MLC II), nuclear factor kB (NF-kB), N- methyl-D-aspartate (NMDA) receptor activation, phosphatidylinositol 3-kinase (PI3K), protein kinase A (PKA), protein kinase C (PKC), ras-related C3 botulinum toxin substrate 1 (RACl). The actual pathway and realized end point are dependent on a range of variables that include receptor usage, cell type, expression level of a receptor or signaling protein, and LPA concentration. Nearly all mammalian cells, tissues and organs co-express several LPA-receptor subtypes, which indicates that LPA receptors signal in a cooperative manner. LPA1, LPA2, and LPA3 share high amino acid sequence similarity.
[00167] LPA is produced from activated platelets, activated adipocytes, neuronal cells, and other cell types. Serum LPA is produced by multiple enzymatic pathways that involve monoacylglycerol kinase, phospho lipase A1, secretory phospho lipase A2, and lysophospho lipase D (lysoPLD), including autotaxin. Several enzymes are involved in LPA degradation: lysophospholipase, lipid phosphate phosphatase, and LPA acyl transferase such as endophilin. LPA concentrations in human serum are estimated to be 1-5 μM. Serum LPA is bound to albumin, low-density lipoproteins, or other proteins, which possibly protect LPA from rapid degradation. LPA molecular species with different acyl chain lengths and saturation are naturally occurring, including 1-palmitoyl (16:0), 1 -palmitoleoyl (16: 1), 1-stearoyl (18:0), 1- oleoyl (18: 1), 1-linoleoyl (18:2), and 1-arachidonyl (20:4) LPA. Quantitatively minor alkyl LPA has biological activities similar to acyl LPA, and different LPA species activate LPA receptor subtypes with varied efficacies. LPA RECEPTORS [00168] LPA1 (previously called VZG- l/EDG-2/mrec 1.3) couples with three types of G proteins, G1/,,, Gq, and G12/13. Through activation of these G proteins, LPA induces a range of cellular responses through LPAi including but not limited to: cell proliferation, serum-response element (SRE) activation, mitogen-activated protein kinase (MAPK) activation, adenylyl cyclase (AC) inhibition, phospholipase C (PLC) activation, Ca2+ mobilization, Akt activation, and Pvho activation. [00169] Wide expression of LPAi is observed in adult mice, with clear presence in testis, brain, heart, lung, small intestine, stomach, spleen, thymus, and skeletal muscle. Similarly, human tissues also express LPAi; it is present in brain, heart, lung, placenta, colon, small intestine, prostate, testis, ovary, pancreas, spleen, kidney, skeletal muscle, and thymus. [00170] LPA2 (EDG-4) also couples with three types of G proteins, G1/,,, Gq, and G12/13, to mediate LPA-induced cellular signaling. Expression Of LPA2 is observed in the testis, kidney, lung, thymus, spleen, and stomach of adult mice and in the human testis, pancreas, prostate, thymus, spleen, and peripheral blood leukocytes. Expression Of LPA2 is upregulated in various cancer cell lines, and several human LPA2 transcriptional variants with mutations in the 3'- untranslated region have been observed. Targeted deletion of LPA2 in mice has not shown any obvious phenotypic abnormalities, but has demonstrated a significant loss of normal LPA signaling (e.g., PLC activation, Ca2+ mobilization, and stress fiber formation) in primary cultures of mouse embryonic fibroblasts (MEFs). Creation of lpa\(-l-) lpal (-/-) double-null mice has revealed that many LPA-induced responses, which include cell proliferation, AC inhibition, PLC activation, Ca2+ mobilization, JNK and Akt activation, and stress fiber formation, are absent or severely reduced in double-null MEFs. All these responses, except for
AC inhibition (AC inhibition is nearly abolished in LPAi (-/-) MEFs), are only partially affected in either LPAi (-/-) or LPA2 (-/-) MEFs. LPA2 contributes to normal LPA-mediated signaling responses in at least some cell types (Choi et al, Biochemica et Biophysica Acta 2008, 1781, p531-539). [00171] LPA3 (EDG-7) is distinct from LPAx and LPA2 in its ability to couple with Gl/o and Gq but not Gi2/i3 and is much less responsive to LPA species with saturated acyl chains. LPA3 can mediate pleiotropic LPA-induced signaling that includes PLC activation, Ca + mobilization, AC inhibition/activation, and MAPK activation. Overexpression of LPA3 in neuroblastoma cells leads to neurite elongation, whereas that of LPAi or LPA2 results in neurite retraction and cell rounding when stimulated with LPA. Expression Of LPA3 is observed in adult mouse testis, kidney, lung, small intestine, heart, thymus, and brain. In humans, it is found in the heart, pancreas, prostate, testis, lung, ovary, and brain (frontal cortex, hippocampus, and amygdala). [00172] LPA4 (p2y9/GPR23) is of divergent sequence compared to LPA1, LPA2, and LPA3 with closer similarity to the platelet-activating factor (PAF) receptor. LPA4 mediates LPA induced Ca2+ mobilization and cAMP accumulation, and functional coupling to the G protein Gs for AC activation, as well as coupling to other G proteins. The LPA4 gene is expressed in the ovary, pancreas, thymus, kidney and skeletal muscle.
[00173] LPA5 (GPR92) is a member of the purinocluster of GPCRs and is structurally most closely related to LPA4. LPA5 is expressed in human heart, placenta, spleen, brain, lung and gut. LPA5 also shows very high expression in the CD8+ lymphocyte compartment of the gastrointestinal tract.
[00174] LPA6 (p2y5) is a member of the purinocluster of GPCRs and is structurally most closely related to LPA4. LPA6 is an LPA receptor coupled to the G12/13-Rho signaling pathways and is expressed in the inner root sheaths of human hair follicles. Illustrative Biological Activity Wound Healing
[00175] Normal wound healing occurs by a highly coordinated sequence of events in which cellular, soluble factors and matrix components act in concert to repair the injury. The healing response can be described as taking place in four broad, overlapping phases — hemostasis, inflammation, proliferation, and remodeling. Many growth factors and cytokines are released into a wound site to initiate and perpetuate wound healing processes.
[00176] When wounded, damaged blood vessels activate platelets. The activated platelets play pivotal roles in subsequent repair processes by releasing bioactive mediators to induce cell proliferation, cell migration, blood coagulation, and angiogenesis. LPA is one such mediator that is released from activated platelets; this induces platelet aggregation along with
mitogenic/migration effects on the surrounding cells, such as endothelial cells, smooth muscle cells, fibroblasts, and keratinocytes.
[00177] Topical application of LPA to cutaneous wounds in mice promotes repair processes (wound closure and increased neoepithelial thickness) by increasing cell proliferation/ migration without affecting secondary inflammation.
[00178] Activation of dermal fibroblasts by growth factors and cytokines leads to their subsequent migration from the edges of the wound into the provisional matrix formed by the fibrin clot whereupon the fibroblasts proliferate and start to restore the dermis by secreting and organizing the characteristic dermal extracellular matrix (ECM). The increasing number of fibroblasts within the wound and continuous precipitation of ECM enhances matrix rigidity by applying small tractional forces to the newly formed granulation tissue. The increase in mechanical stress, in conjunction with transforming growth factor β (TGFβ), induces α-smooth muscle actin (α-SMA) expression and the subsequent transformation of fibroblasts into myofibroblasts. Myofibroblasts facilitate granulation tissue remodeling via myofibroblast contraction and through the production of ECM components.
[00179] LPA regulates many important functions of fibroblasts in wound healing, including proliferation, migration, differentiation and contraction. Fibroblast proliferation is required in wound healing in order to fill an open wound. In contrast, fibrosis is characterized by intense proliferation and accumulation of myofibroblasts that actively synthesize ECM and proinflammatory cytokines. LPA can either increase or suppress the proliferation of cell types important in wound healing, such as epithelial and endothelial cells (EC),macrophages, keratinocytes, and fibroblasts. A role for LPAi in LPA-induced proliferation was provided by the observation that LPA-stimulated proliferation of fibroblasts isolated from LPAi receptor null mice was attenuated (Mills et al, Nat Rev. Cancer 2003; 3: 582-591). LPA induces cytoskeletal changes that are integral to fibroblast adhesion, migration, differentiation and contraction. Fibrosis
[00180] Tissue injury initiates a complex series of host wound-healing responses; if successful, these responses restore normal tissue structure and function. If not, these responses can lead to tissue fibrosis and loss of function.
[00181] For the majority of organs and tissues the development of fibrosis involves a multitude of events and factors. Molecules involved in the development of fibrosis include proteins or peptides (profibrotic cytokines, chemokines, metalloproteinases etc.) and phospholipids. Phospholipids involved in the development of fibrosis include platelet activating factor (PAF), phosphatidyl choline, sphingosine- 1 phosphate (SlP) and lysophosphatidic acid (LPA).
[00182] A number of muscular dystrophies are characterized by a progressive weakness and wasting of musculature, and by extensive fibrosis. It has been shown that LPA treatment of cultured myoblasts induced significant expression of connective tissue growth factor (CTGF). CTGF subsequently induces collagen, fibronectin and integrin expression and induces dedifferentiation of these myoblasts. Treatment of a variety of cell types with LPA induces reproducible and high level induction of CTGF (J.P. Pradere, et al, LPAi receptor activation promotes renal interstitial fibrosis, J. Am. Soc. Nephrol. 18 (2007) 3110-3118; N. Wiedmaier, et al, Int J Med Microbiol; 298(3-4):231-43, 2008). CTGF is a profibrotic cytokine, signaling down-stream and in parallel with TGFβ. [00183] CTGF expression by gingival epithelial cells, which are involved in the development of gingival fibromatosis, was found to be exacerbated by LPA treatment (A. Kantarci, et al, J. Pathol. 210 (2006) 59-66).
[00184] LPA is associated with the progression of liver fibrosis. In vitro, LPA induces stellate cell and hepatocyte proliferation. These activated cells are the main cell type responsible for the accumulation of ECM in the liver. Furthermore, LPA plasma levels rise during CCU-induced liver fibrosis in rodents, or in hepatitis C virus-induced liver fibrosis in human (N. Watanabe, et al., Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity, Life Sci. 81 (2007) 1009-1015; N.Watanabe, et al, J. Clin. Gastroenterol. 41 (2007) 616-623). [00185] An increase of phospholipid concentrations in the bronchoalveolar lavage fluid in rabbits and rodents injected with bleomycin has been reported (K. Kuroda, et al., Phospholipid concentration in lung lavage fluid as biomarker for pulmonary fibrosis, Inhal. Toxicol. 18 (2006) 389-393; K. Yasuda, et al, Lung 172 (1994) 91-102). [00186] LPA is associated with heart disease and mycocardial remodeling. Serum LPA levels are increased after myocardial infarction in patients and LPA stimulates rat cardiac fibroblast proliferation and collagen production {Chen et al FEBS Lett. 2006 Aug 21;580(19):4737-45). Pulmonary Fibrosis
[00187] In the lung, aberrant wound healing responses to injury contribute to the pathogenesis of fibrotic lung diseases. Fibrotic lung diseases, such as idiopathic pulmonary fibrosis (IPF), are associated with high morbidity and mortality.
[00188] LPA is an important mediator of fibroblast recruitment in pulmonary fibrosis. LPA and LPAi play key pathogenic roles in pulmonary fibrosis. Fibroblast chemoattractant activity plays an important role in the lungs in patients with pulmonary fibrosis. Profibrotic effects of LPAi- receptor stimulation is explained by LPAi -receptor-mediated vascular leakage and increased fibroblast recruitment, both profibrotic events. The LPA-LPAi pathway has a role in mediating
fibroblast migration and vascular leakage in IPF. The end result is the aberrant healing process that characterises this fibrotic condition.
[00189] The LPAi receptor is the LPA receptor most highly expressed on fibroblasts obtained from patients with IPF. Furthermore, BAL obtained from IPF patients induced chemotaxis of human foetal lung fibroblasts that was blocked by the dual LPAr LPA3 receptor antagonist Ki 16425. In an experimental bleomycin-induced lung injury mouse model, it was shown that LPA levels were high in bronchoalveolar lavage samples compared with unexposed controls. LPAi knockout mice are protected from fibrosis after bleomycin challenge with reduced fibroblast accumulation and vascular leakage. In human subjects with IPF, high LPA levels were observed in bronchoalveolar lavage samples compared with healthy controls. Increased fibroblast chemotactic activity in these samples was inhibited by the Ki 16425 indicating that fibroblast migration is mediated by the LPA-LPA receptor(s) pathway (Tager et al. Nature Medicine, vol. 14, no. 1, 45-54, 2008). [00190] The LPA-LPAi pathway is crucial in fibroblast recruitment and vascular leakage in pulmonary fibrosis.
[00191] Activation of latent TGF-β by the avβ6 integrin plays a critical role in the development of lung injury and fibrosis (Munger et al. Cell, vol. 96, 319-328, 1999). LPA induces avβ6- mediated TGF-β activation on human lung epithelial cells (Xu et al. Am. J. Pathology, vol. 174, no. 2, 1264-1279, 2009). The LPA-induced avβ6-mediated TGF-β activation is mediated by the LP A2 receptor. Expression of the LP A2 receptor is increased in epithelial cells and mesenchymal cells in areas of lung fibrosis from IPF patients compared to normal human lung tissue. The LP A-LP A2 pathway contributes to the activation of the TGF-β pathway in pulmonary fibrosis. In some embodiments, compounds that inhibit LPA2 show efficacy in the treatment of lung fibrosis. In some embodiments, compounds that inhibit both LPAl and LP A2 show improved efficacy in the treatment of lung fibrosis compared to compounds which inhibit only LPAl or LPA2. Renal Fibrosis
[00192] LPA and LPAi are involved in the etiology of kidney fibrosis. LPA has effetcs on both proliferation and contraction of glomerular mesangial cells and thus has been implicated in proliferative glomerulonephritis (CN. Inoue, et al, Clin. Sci. (Colch.) 96, 431- 436, 1999). In an animal model of renal fibrosis, unilateral ureteral obstruction (UUO), it was found that renal LPA receptors are expressed under basal conditions with an expression order of LPA2>LPA3=LPAi»LPA4. This model mimics in an accelerated manner the development of renal fibrosis including renal inflammation, fibroblast activation and accumulation of
extracellular matrix in the tubulointerstitium. UUO significantly induced LPAi -receptor expression. This was paralleled by renal LPA production (3.3 fold increase) in conditioned media from kidney explants. Contra-lateral kidneys exhibited no significant changes in LPA release and LPA-receptors expression. This shows that a prerequisite for an action of LPA in fibrosis is met: production of a ligand (LPA) and induction of one of its receptors (the LPAi receptor) (J.P. Pradere et ah, Biochim Biophys Acta. 2008 Sep;1781(9):582-7). [00193] In mice invalidated for the LPAi receptor (LPAi (~/~), the development of renal fibrosis was significantly attenuated. UUO mice treated with the LPA receptor antagonist Ki 16425 closely resembled the LPAi (~/~) mice. [00194] LPA can participate in intraperitonial accumulation of monocyte/macrophages and that LPA can induce expression of the profibrotic cytokine CTGF in primary cultures of human fibroblasts (J.S. Koh,e£ ah, J. CHn. Invest. 102 (1998) 716-727).
[00195] LPA treatment of a mouse epithelial renal cell line, MCT, induced a rapid increase in the expression of the profibrotic cytokine CTGF. CTGF plays a crucial role in UUO-induced tubulointerstitial fibrosis (TIF), and is involved in the profibrotic activity of TGFβ. This induction was almost completely suppressed by co-treatment with the LPA-receptor antagonist Ki 16425. In one aspect, the profibrotic activity of LPA in kidney results from a direct action of LPA on kidney cells involving induction of CTGF. Hepatic fibrosis [00196] LPA is implicated in liver disease and fibrosis. Plasma LPA levels and serum autotoxin (enzyme responsible for LPA production) are elevated in hepatitis patients and animal models of liver injury in correlation with increased fibrosis. LPA also regulates liver cell function. LPAi and LPA2 receptors are expressed by mouse hepatic stellate cells and LPA stimulates migration of hepatic myofibroblasts. Ocular Fibrosis
[00197] LPA is in involved in wound healing in the eye. LPAi and LP A3 receptors are detectable in the normal rabbit corneal epithelial cells, keratocytes and endothelial cells and LPAi and LPA3 expression are increased in corneal epithelial cells following injury. [00198] LPA and its homologues are present in the aqueous humor and the lacrimal gland fluid of the rabbit eye and these levels are increased in a rabbit corneal injury model.
[00199] LPA induces actin stress fiber formation in rabbit corneal endothelial and epithelial cells and promotes contraction corneal fibroblasts. LPA also stimulates proliferation of human retinal pigmented epithelial cells Cardiac fibrosis
[00200] LPA is implicated in myocardial infarction and cardiac fibrosis. Serum LPA levels are increased in patients following mycocardial infarction (MI) and LPA stimulates proliferation and collagen production (fibrosis) by rat cardiac fibroblasts. Both LPAl and LPA3 receptors are highly expressed in human heart tissue. Treatment of Fibrosis
[00201] In one aspect, the compound of Formula (I), (II), (III), (IV) or (V) is used to treat or prevent fibrosis in a mammal. In one aspect, the compound of Formula (I), (II), (III), (IV) or (V) is used to treat fibrosis of an organ or tissue in a mammal. In one aspect is a method for preventing a fibrosis condition in a mammal, the method comprising administering to the mammal at risk of developing one or more fibrosis conditions a therapeutically effective amount of a compound of Formula (I), (II), (III), (IV) or (V). In one aspect, the mammal has been exposed to one or more environmental conditions that are known to increase the risk of fibrosis of an organ or tissue. In one aspect, the mammal has been exposed to one or more environmental conditions that are known to increase the risk of lung, liver or kidney fibrosis. In one aspect, the mammal has a genetic predisposition of developing fibrosis of an organ or tissue. In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is adminstered to a mammal to prevent or minimize scarring following injury. In one aspect, injury includes surgery. [00202] The terms "fibrosis" or "fibrosing disorder," as used herein, refers to conditions that are associated with the abnormal accumulation of cells and/or fibronectin and/or collagen and/or increased fibroblast recruitment and include but are not limited to fibrosis of individual organs or tissues such as the heart, kidney, liver, joints, lung, pleural tissue, peritoneal tissue, skin, cornea, retina, musculoskeletal and digestive tract. [00203] Exemplary diseases, disorders, or conditions that involve fibrosis include, but are not limited to: Lung diseases associated with fibrosis, e.g., idiopathic pulmonary fibrosis, pulmonary fibrosis secondary to systemic inflammatory disease such as rheumatoid arthritis, scleroderma, lupus, cryptogenic fibrosing alveolitis, radiation induced fibrosis, chronic obstructive pulmonary disease (COPD), scleroderma, chronic asthma, silicosis, asbestos induced pulmonary or pleural fibrosis, acute lung injury and acute respiratory distress (including bacterial pneumonia induced, trauma induced, viral pneumonia induced, ventilator induced, non-pulmonary sepsis induced, and aspiration induced); Chronic nephropathies associated with injury/fibrosis (kidney fibrosis), e.g., glomerulonephritis secondary to systemic inflammatory diseases such as lupus and scleroderma, diabetes,, glomerular nephritis, focal segmental glomerular sclerosis, IgA nephropathy, hypertension, allograft and Alport; Gut fibrosis, e.g., scleroderma, and radiation induced gut fibrosis; Liver fibrosis, e.g., cirrhosis, alcohol induced
liver fibrosis, nonalcoholic steatohepatitis (NASH), biliary duct injury, primary biliary cirrhosis, infection or viral induced liver fibrosis (e.g., chronic HCV infection), and autoimmune hepatitis; Head and neck fibrosis, e.g., radiation induced; Corneal scarring, e.g., LASIK (laser-assisted in situ keratomileusis), corneal transplant, and trabeculectomy; Hypertrophic scarring and keloids, e.g., burn induced or surgical; and Other fibrotic diseases, e.g., sarcoidosis, scleroderma, spinal cord injury/fibrosis, myelofibrosis, vascular restenosis, atherosclerosis, arteriosclerosis, Wegener's granulomatosis, mixed connective tissue disease, and Peyronie's disease. [00204] In one aspect, a mammal suffering from one of the following non- limiting exemplary diseases, disorders, or conditions will benefit from therapy with a compound of Formula (I), (II), (III), (IV) or (V): atherosclerosis, thrombosis, heart disease, vasculitis, formation of scar tissue, restenosis, phlobitis, COPD (chronic obstructive pulmonary disease), pulmonary hypertension, pulmonary fibrosis, pulmonary inflammation, bowel adhesions, bladder fibrosis and cystitis, fibrosis of the nasal passages, sinusitis, inflammation mediated by neutrophils, and fibrosis mediated by fibroblasts. [00205] In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is adminstered to a mammal with fibrosis of an organ or tissue or with a predisposition of developing fibrosis of an organ or tissue with one or more other agents that are used to treat fibrosis. In one aspect, the one or more agents include corticosteroids. In one aspect, the one or more agents include immunosuppresants. In one aspect, the one or more agents include B-cell antagonists. In one aspect, the one or more agents include uteroglobin.
[00206] In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used to treat a dermatological disorders in a mammal. The term "dermatological disorder," as used herein refers to a skin disorder. Such dermatological disorders include, but are not limited to, proliferative or inflammatory disorders of the skin such as, atopic dermatitis, bullous disorders, collagenoses, psoriasis, psoriatic lesions, dermatitis, contact dermatitis, eczema, urticaria, rosacea, scleroderma, wound healing, scarring, hypertrophic scarring, keloids, Kawasaki Disease, rosacea, Sjogren-Larsso Syndrome, urticaria.
[00207] In some embodiments, provided is a method of reducing lung injury, vascular leakage, inflammation and/or fibrosis in a mammal comprising administering to the mammal a selective LPAl receptor antagonist. In some embodiments, provided is a method of reducing lung injury, vascular leakage, inflammation and fibrosis in a mammal comprising administering to the mammal a selective LPAl receptor antagonist. In some embodiments, provided is a method of attenuating fibrosis in a mammal comprising administering a selective LPAl receptor antagonist. In some embodiments, provided is a method of attenuating tissue remodeling and fibrosis in a mammal comprising administering a selective LPAl receptor antagonist.
[00208] In some embodiments, provided is a method of decreasing cytokine production in a mammal comprising administering a selective LPAl receptor antagonist. In some embodiments, the method of decreasing cytokine production in a mammal comprising administering a selective LPAl receptor antagonist results in a reduction of tissue damage and fibrosis in a mammal.
[00209] In some embodiments, provided is a method of treating fibrosis is a mammal comprising administering to the mammal a selective LPAl receptor antagonist. In some embodiments, provided is a method of treating fibrosis in a mammal while maintaining body weight in the mammal comprising administering to the mammal a selective LPAl receptor antagonist. In some embodiments, provided is a method of treating respiratory disease in a mammal comprising administering to the mammal a selective LPAl receptor antagonist. [00210] In some embodiments, provided is a method of treating fibrosis in a mammal with a selective LPAl receptor anatgonist, wherein the fibrosis in the mammal is not responsive to treatment with pirfenidone. In some embodiments, the LPAl receptor antagonist is a compound of Formula (I), (II), (III), (IV) or (V).
[00211] As shown in the Examples, a selective LPAl receptor antagonist reduced lung fibrosis, kidney fibrosis and liver fibrosis in various animal models of fibrosis.
[00212] In a mouse bleomycin lung fibrosis model, a selective LPAl receptor antagonist (e.g. Compound 1-1), reduced lung injury, vascular leakage, inflammation and fibrosis at multiple timepoints following intratracheal bleomycin instillation. In the acute setting (3 day), a selective LPAl receptor antagonist (e.g. Compound 1-1) reduced BALF collagen, protein, TGFβl, MMP-7, hyaluronan, and inflammatory cell influx. Pain [00213] Since LPA is released following tissue injury, LPAi plays an important role in the initiation of neuropathic pain. LPA1, unlike LPA2 or LPA3, is expressed in both dorsal root ganglion (DRG) and dorsal root neurons. Using the antisense oligodeoxynucleotide (AS-ODN) for LPAi and LPAi-null mice, it was found that LPA-induced mechanical allodynia and hyperalgesia is mediated in an LPArdependent manner. LPAi and downstream Rho-ROCK activation play a role in the initiation of neuropathic pain signaling. Pretreatment with Clostridium botulinum C3 exoenzyme (BoTXC3, Rho inhibitor) or Y-27632 (ROCK inhibitor) completely abolished the allodynia and hyperalgesia in nerve-injured mice. LPA also induced demyelination of the dorsal root, which was prevented by BoTXC3. The dorsal root demyelination by injury was not observed in LPAi -null mice or AS-ODN injected wild-type mice. LPA signaling appears to induce important neuropathic pain markers such as protein kinase Cγ (PKCγ) and a voltage-gated calcium channel α2δl subunit (Caα2δl) in an LPAi and
Rho-dependent manner (M. Inoue, et ah, Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling, Nat. Med. 10 (2004) 712-718). [00214] In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of pain in a mammal. In one aspect, the pain is acute pain or chronic pain. In another aspect, the pain is neuropathic pain. In another aspect, the pain is cancer pain.
[00215] In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of fibromylagia. In one aspect, fibromyalgia stems from the formation of fibrous scar tissue in contractile (voluntary) muscles. Fibrosis binds the tissue and inhibits blood flow, resulting in pain. Cancer
[00216] Lysophospholipid receptor signaling plays a role in the etiology of cancer. Lysophosphatidic acid (LPA) and its G protein-coupled receptors (GPCRs) LPAi, LPA2, and/or LPA3 play a role in the development of several types of cancers. The initiation, progression and metastasis of cancer involve several concurrent and sequential processes including cell proliferation and growth, survival and anti-apoptosis, migration of cells, penetration of foreign cells into defined cellular layers and/or organs, and promotion of angiogenesis. The control of each of these processes by LPA signaling in physiological and pathophysiological conditions underscores the potential therapeutic usefulness of modulating LPA signaling pathways for the treatment of cancer, especially at the level of the LPA receptors or ATX/lysoPLD. Autotaxin (ATX) is a prometastatic enzyme initially isolated from the conditioned medium of human melanoma cells that stimulates a myriad of biological activities, including angiogenesis and the promotion of cell growth, migration, survival, and differentiation through the production of LPA (MoI Cancer Ther 2008;7(10):3352-62). [00217] LPA signals through its own GPCRs leading to activation of multiple downstream effector pathways. Such downstream effector pathways play a role in cancer. LPA and its GPCRs are linked to cancer through major oncogenic signaling pathways. [00218] LPA contributes to tumorigenesis by increasing motility and invasiveness of cells. LPA has been implicated in the initiation or progression of ovarian cancer. LPA is present at significant concentrations (2-80 μM) in the ascitic fluid of ovarian cancer patients. Ovarian cancer cells constitutively produce increased amounts of LPA as compared to normal ovarian surface epithelial cells, the precursor of ovarian epithelial cancer. Elevated LPA levels are also detected in plasma from patients with early-stage ovarian cancers compared with controls. LPA receptors (LP A2 and LPA3) are also overexpressed in ovarian cancer cells as compared to normal ovarian surface epithelial cells. LPA stimulates Cox-2 expression through transcriptional activation and post-transcriptional enhancement of Cox-2 mRNA in ovarian
cancer cells. Prostaglandins produced by Cox-2 have been implicated in a number of human cancers and pharmacological inhibition of Cox-2 activity reduces colon cancer development and decreases the size and number of adenomas in patients with familial adenomatous polyposis. LPA has also been implicated in the initiation or progression of prostate cancer, breast cancer, melanoma, head and neck cancer, bowel cancer (colorectal cancer), thyroid cancer, glioblastoma, and other cancers (Gardell et al, Trends in Molecular Medicine, vol. 12, no. 2, p 65-75, 2006; Ishii et al, Annu. Rev. Biochem, 73, 321-354, 2004; Mills et al, Nat. Rev. Cancer, 3, 582-591, 2003; Murph et al, Biochimica et Biophysica Acta, 1781, 547-557, 2008; Kishi et al, J. Biol. Chem., 281, 17492-17500, 2006; ). [00219] The cellular responses to LPA are mediated through the lysophosphatidic acid receptors. For example, LPA receptors mediate both migration of and invasion by pancreatic cancer cell lines: an antagonist of LPAi and LPA3 (Kil6425) and LPAi-specific siRNA effectively blocked in vitro migration in response to LPA and peritoneal fluid (ascites) from pancreatic cancer patients; in addition, Ki 16425 blocked the LPA-induced and ascites-induced invasion activity of a highly peritoneal metastatic pancreatic cancer cell line (Yamada et al, J. Biol. Chem., 279, 6595-6605, 2004).
[00220] Colorectal carcinoma cell lines show significant expression of LPAi mRNA and respond to LPA by cell migration and production of angiogenic factors. Overexpression of LPA receptors has a role in the pathogenesis of thyroid cancer. LPA3 was originally cloned from prostate cancer cells, concordant with the ability of LPA to induce autocrine proliferation of prostate cancer cells.
[00221] LPA has stimulatory roles in cancer progression in many types of cancer. LPA is produced from and induces proliferation of prostate cancer cell lines. LPA induces human colon carcinoma DLDl cell proliferation, migration, adhesion, and secretion of angiogenic factors through LPAi signalling. In other human colon carcinoma cells lines (HT29 and WiDR), LPA enhances cell proliferation and secretion of angiogenic factors. In other colon cancer cell lines, LPA2 and LPA3 receptor activation results in proliferation of the cells. LPAi is implicated in bone metastasis and the LPAi/LPA3 dual antagonist Ki 16425 has been shown to inhibit metastasis to bone in vivo (Boucharaba et al, Proc. Natl. Acad. Sci USA, 103, 9643-9648, 2006). The genetic or pharmacological manipulation of LPA metabolism, specific blockade of receptor signaling, and/or inhibition of downstream signal transduction pathways, represent approaches for cancer therapies.
[00222] In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of cancer. In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of malignant and benign proliferative disease. In one aspect, a compound of Formula
(I), (II), (III), (IV) or (V) is used to prevent or reduce proliferation of tumor cells, invasion and metastasis of carcinomas, pleural mesothelioma (Yamada, Cancer Sci., 2008, 99(8), 1603-1610) or peritoneal mesothelioma, cancer pain, bone metastases (Boucharaba et al, J. Clin. Invest., 2004, 114(12), 1714-1725; Boucharaba et al, Proc. Natl. acad. Sci., 2006, 103(25) 9643-9648). In one aspect is a method of treating cancer in a mammal, the method comprising administering to the mammal a compound of Formula (I), (II), (III), (IV) or (V) and a second therapeutic agent, wherein the second therapeutic agent is an anti-cancer agent.
[00223] The term "cancer," as used herein refers to an abnormal growth of cells which tend to proliferate in an uncontrolled way and, in some cases, to metastasize (spread). The types of cancer include, but is not limited to, solid tumors (such as those of the bladder, bowel, brain, breast, endometrium, heart, kidney, lung, lymphatic tissue (lymphoma), ovary, pancreas or other endocrine organ (thyroid), prostate, skin (melanoma or basal cell cancer) or hematological tumors (such as the leukemias) at any stage of the disease with or without metastases. [00224] The increased concentrations of LPA and vesicles in ascites from ovarian cancer patients and breast cancer effussions indicate that it could be an early diagnostic marker, a prognostic indicator or an indicator of response to therapy (Mills et al, Nat. Rev. Cancer., 3, 582-591, 2003; Sutphen et al, Cancer Epidemiol. Biomarkers Prev. 13, 1185-1191, 2004). LPA concentrations are consistently higher in ascites samples than in matched plasma samples. Respiratory and Allergic Disorders [00225] In one aspect, LPA is a contributor to the pathogenesis of respiratory diseases. In one aspect the respiratory disease is asthma. Proinflammatory effects of LPA include degranulation of mast cells, contraction of smooth-muscle cells and release of cytokines from dendritic cells. Airway smooth muscle cells, epithelial cells and lung fibroblasts all show responses to LPA. LPA induces the secretion of IL-8 from human bronchial epithelial cells. IL-8 is found in increased concentrations in BAL fluids from patients with asthma, chronic obstructive lung disease, pulmonary sarcoidosis and acute respiratory distress syndrome and 11-8 has been shown to exacerbate airway inflammation and airway remodeling of asthmatics. LPAl, LP A2 and LPA3 receptors have all been shown to contribute to the LPA-induced IL-8 production. Studies cloning multiple GPCRs that are activated by LPA allowed the demonstration of the presence of mPvNA for the LPA1, LPA2 and LPA3 in the lung (J.J.A. Contos, et al, MoI. Pharmacol. 58, 1188-1196, 2000).
[00226] The release of LPA from platelets activated at a site of injury and its ability to promote fibroblast proliferation and contraction are features of LPA as a mediator of wound repair. In the context of airway disease, asthma is an inflammatory disease where inappropriate airway "repair" processes lead to structural "remodeling" of the airway. In asthma, the cells of the
airway are subject to ongoing injury due to a variety of insults, including allergens, pollutants, other inhaled environmental agents, bacteria and viruses, leading to the chronic inflammation that characterizes asthma.
[00227] In one aspect, in the asthmatic individual, the release of normal repair mediators, including LPA, is exaggerated or the actions of the repair mediators are inappropriately prolonged leading to inappropriate airway remodeling. Major structural features of the remodeled airway observed in asthma include a thickened lamina reticularis (the basement membrane-like structure just beneath the airway epithelial cells), increased numbers and activation of myofibroblasts, thickening of the smooth muscle layer, increased numbers of mucus glands and mucus secretions, and alterations in the connective tissue and capillary bed throughout the airway wall. In one aspect, LPA contributes to these structural changes in the airway. In one aspect, LPA is involved in acute airway hyperresponsiveness in asthma. The lumen of the remodeled asthmatic airway is narrower due to the thickening of the airway wall, thus decreasing airflow. In one aspect, LPA contributes to the long-term structural remodeling and the acute hyperresponsiveness of the asthmatic airway. In one aspect, LPA contributes to the hyper-responsiveness that is a primary feature of acute exacerbations of asthma. [00228] In addition to the cellular responses mediated by LPA, several of the LPA signaling pathway components leading to these responses are relevant to asthma. EGF receptor upregulation is induced by LPA and is also seen in asthmatic airways (M. Amishima, et ah, Am. J. Respir. Crit. Care Med. 157, 1907- 1912, 1998). Chronic inflammation is a contributor to asthma, and several of the transcription factors that are activated by LPA are known to be involved in inflammation (Ediger et ah, Eur Respir J 21 :159-169, 2003). [00229] In one aspect, the fibroblast proliferation and contraction and extracellular matrix secretion stimulated by LPA contributes to the fibroproliferative features of other airway diseases, such as the peribronchiolar fibrosis present in chronic bronchitis, emphysema, and interstitial lung disease. Emphysema is also associated with a mild fibrosis of the alveolar wall, a feature which is believed to represent an attempt to repair alveolar damage. In another aspect, LPA plays a role in the fibrotic interstitial lung diseases and obliterative bronchiolitis, where both collagen and myofibroblasts are increased. In another aspect, LPA is involved in several of the various syndromes that constitute chronic obstructive pulmonary disease.
[00230] Administration of LPA in vivo induces airway hyper-responsiveness, itch-scratch responses, infiltration and activation of eosinophils and neutrophils, vascular remodeling, and nociceptive flexor responses. LPA also induces histamine release from mouse and rat mast cells. In an acute allergic reaction, histamine induces various responses, such as contraction of smooth muscle, plasma exudation, and mucus production. Plasma exudation is important in the airway,
because the leakage and subsequent airway-wall edema contribute to the development of airway hyperresponsiveness. Plasma exudation progresses to conjunctival swelling in ocular allergic disorder and nasal blockage in allergic rhinitis (Hashimoto et ah, J Pharmacol
100, 82 - 87, 2006). In one aspect, plasma exudation induced by LPA is mediated by histamine release from mast cells via one or more LPA receptors. In one aspect, the LPA receptor(s) include LPAi and/or LPA3. In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of various allergic disorders in a mammal. In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of respiratory diseases, disorders or conditions in a mammal. In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of asthma in a mammal. In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used in the treatment of chronic asthma in a mammal.
[00231] The term "respiratory disease," as used herein, refers to diseases affecting the organs that are involved in breathing, such as the nose, throat, larynx, eustachian tubes, trachea, bronchi, lungs, related muscles (e.g., diaphram and intercostals), and nerves. Respiratory diseases include, but are not limited to, asthma, adult respiratory distress syndrome and allergic (extrinsic) asthma, non-allergic (intrinsic) asthma, acute severe asthma, chronic asthma, clinical asthma, nocturnal asthma, allergen-induced asthma, aspirin-sensitive asthma, exercise-induced asthma, isocapnic hyperventilation, child-onset asthma, adult-onset asthma, cough-variant asthma, occupational asthma, steroid-resistant asthma, seasonal asthma, seasonal allergic rhinitis, perennial allergic rhinitis, chronic obstructive pulmonary disease, including chronic bronchitis or emphysema, pulmonary hypertension, interstitial lung fibrosis and/or airway inflammation and cystic fibrosis, and hypoxia.
[00232] The term "asthma" as used herein refers to any disorder of the lungs characterized by variations in pulmonary gas flow associated with airway constriction of whatever cause (intrinsic, extrinsic, or both; allergic or non-allergic). The term asthma may be used with one or more adjectives to indicate cause.
[00233] In one aspect, presented herein is the use of a compound of Formula (I), (II), (III), (IV) or (V) in the treatment or prevention of chronic obstructive pulmonary disease in a mammal comprising administering to the mammal at least once an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V). In addition, chronic obstructive pulmonary disease includes, but is not limited to, chronic bronchitis or emphysema, pulmonary hypertension, interstitial lung fibrosis and/or airway inflammation, and cystic fibrosis. Nervous System [00234] The nervous system is a major locus for LPA1 expression; there it is spatially and temporally regulated throughout brain development. Oligodendrocytes, the myelinating cells in
the central nervous system (CNS), express LPAi in mammals. In addition, Schwann cells, the myelinating cells of the peripheral nervous system, also express LPAi, which is involved in regulating Schwann cell survival and morphology. These observations identify important functions for receptor-mediated LPA signaling in neurogenesis, cell survival, and myelination. [00235] Exposure of peripheral nervous system cell lines to LPA produces a rapid retraction of their processes resulting in cell rounding, which was, in part, mediated by polymerization of the actin cytoskeleton. In one aspect, LPA causes neuronal degeneration under pathological conditions when the blood-brain barrier is damaged and serum components leak into the brain (Moolenaar, Curr. Opin. Cell Biol. 7:203-10, 1995). Immortalized CNS neuroblast cell lines from the cerebral cortex also display retraction responses to LPA exposure through Rho activation and actomyosin interactions. In one aspect, LPA is associated with post-ischemic neural damage (J. Neurochem. 61, 340, 1993; J. Neurochem., 70:66, 1998). [00236] In one aspect, provided is a compound of Formula (I), (II), (III), (IV) or (V) for use in the treatment or prevention of a nervous system disorder in a mammal. The term "nervous system disorder," as used herein, refers to conditions that alter the structure or function of the brain, spinal cord or peripheral nervous system, including but not limited to Alzheimer's Disease, cerebral edema, cerebral ischemia, stroke, multiple sclerosis, neuropathies, Parkinson's Disease, those found after blunt or surgical trauma (including post-surgical cognitive dysfunction and spinal cord or brain stem injury), as well as the neurological aspects of disorders such as degenerative disk disease and sciatica.
[00237] In one aspect, provided is a compound of Formula (I), (II), (III), (IV) or (V) for use in the treatment or prevention of a CNS disorder in a mammal. CNS disorders include, but are not limited to, multiple sclerosis, Parkinson's disease, Alzheimer's disease, stroke, cerebral ischemia, retinal ischemia, post-surgical cognitive dysfunction, migraine, peripheral neuropathy/neuropathic pain, spinal cord injury, cerebral edema and head injury. Cardiovascular Disorders
[00238] Cardiovascular phenotypes observed after targeted deletion of lysophospho lipid receptors reveal important roles for lysophospholipid signaling in the development and maturation of blood vessels, formation of atherosclerotic plaques and maintenance of heart rate (Ishii, I. et al. Annu. Rev. Biochem. 73, 321-354, 2004). Angiogenesis, the formation of new capillary networks from pre-existing vasculature, is normally invoked in wound healing, tissue growth and myocardial angiogenesis after ischemic injury. Peptide growth factors (e.g. vascular endothelial growth factor (VEGF)) and lysophospholipids control coordinated proliferation, migration, adhesion, differentiation and assembly of vascular endothelial cells (VECs) and surrounding vascular smooth-muscle cells (VSMCs). In one aspect, dysregulation of the
processes mediating angiogenesis leads to atherosclerosis, hypertension, tumor growth, rheumatoid arthritis and diabetic retinopathy (Osborne, N. and Stainier, D.Y. Annu. Rev. Physiol. 65, 23-43, 2003).
[00239] Downstream signaling pathways evoked by lysophospholipid receptors include Rac- dependent lamellipodia formation (e.g. LPAi) and Rho-dependent stress-fiber formation (e.g. LPAi), which is important in cell migration and adhesion. Dysfunction of the vascular endothelium can shift the balance from vasodilatation to vasoconstriction and lead to hypertension and vascular remodeling, which are risk factors for atherosclerosis (Maguire, JJ. et al., Trends Pharmacol. Sci. 26, 448^54, 2005). [00240] LPA contributes to both the early phase (barrier dysfunction and monocyte adhesion of the endothelium) and the late phase (platelet activation and intra-arterial thrombus formation) of atherosclerosis, in addition to its overall progression. In the early phase, LPA from numerous sources accumulates in lesions and activates its cognate GPCRs (LPAi and LP A3) expressed on platelets (Siess, W. Biochim. Biophys. Acta 1582, 204-215, 2002; Rother, E. et al. Circulation 108, 741-747, 2003). This triggers platelet shape change and aggregation, leading to intra- arterial thrombus formation and, potentially, myocardial infarction and stroke. In support of its atherogenic activity, LPA can also be a mitogen and motogen to VSMCs and an activator of endothelial cells and macrophages. LPA has been shown to be involved in ischemia- reperfussion injury. Blockade of the LP A3 receptor in a murine model of renal ischemia- reperfusion injury reduced the severity of injury. This effect was reversed in the presence of the selective LPA3 receptor agonist OMPT (Okusa et al, Am. J. Physiol. Renal Physiol., 285, F565- F574, 2003). In one aspect, mammals with cardiovascular disease benefit from LPA receptor antagonists that prevent thrombus and neointima plaque formation. [00241] The specific effects of LPA are receptor-mediated. [00242] In one aspect, the compound of Formula (I), (II), (III), (IV) or (V) is used to treat or prevent cardiovascular disease in mammal.
[00243] The term "cardiovascular disease," as used herein refers to diseases affecting the heart or blood vessels or both, including but not limited to: arrhythmia (atrial or ventricular or both); atherosclerosis and its sequelae; angina; cardiac rhythm disturbances; myocardial ischemia; myocardial infarction; cardiac or vascular aneurysm; vasculitis, stroke; peripheral obstructive arteriopathy of a limb, an organ, or a tissue; reperfusion injury following ischemia of the brain, heart, kidney or other organ or tissue; endotoxic, surgical, or traumatic shock; hypertension, valvular heart disease, heart failure, abnormal blood pressure; shock; vasoconstriction (including that associated with migraines); vascular abnormality, inflammation, insufficiency limited to a single organ or tissue..
[00244] In one aspect, provided herein are methods for preventing or treating vasoconstriction, atherosclerosis and its sequelae myocardial ischemia, myocardial infarction, aortic aneurysm, vasculitis and stroke comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V) or pharmaceutical composition or medicament which includes a compound of Formula (I), (II), (III), (IV) or (V).
[00245] In one aspect, provided herein are methods for reducing cardiac reperfusion injury following myocardial ischemia and/or endotoxic shock comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V). [00246] In one aspect, provided herein are methods for reducing the constriction of blood vessels in a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
[00247] In one aspect, provided herein are methods for lowering or preventing an increase in blood pressure of a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V). Inflammation
[00248] LPA has been shown to regulate immunological responses by modulating activities/functions of immune cells such as T-/B-lymphocytes and macrophages. In activated T cells, LPA activates IL-2 production/cell proliferation through LPAi (Gardell et al, TRENDS in Molecular Medicine Vol.12 No.2 February 2006). Expression of LPA-induced inflammatory response genes is mediated by LPAi and LPA3 {Biochem Biophys Res Commun. 363(4): 1001-8, 2007). In addition, LPA modulates the chemotaxis of inflammatory cells {Biochem Biophys Res Commun., 1993, 15;193(2), 497). The proliferation and cytokine-secreting activity in response to LPA of immune cells ( J. Imuunol. 1999, 162, 2049), platelet aggregation activity in response to LPA, acceleration of migration activity in monocytes, activation of NF-κB in fibroblast, enhancement of fibronectin-binding to the cell surface, and the like are known. Thus, LPA is associated with various inflammatory/immune diseases .
[00249] In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used to treat or prevent inflammation in a mammal. In one aspect, antagonists of LPAi and/or LPA3 find use in the treatment or prevention of inflammatory/immune disorders in a mammal. In one aspect, the antagonist of LPAi is a compound of Formula (I), (II), (III), (IV) or (V). [00250] Examples of inflammatory/immune disorders include psoriasis, rheumatoid arthritis, vasculitis, inflammatory bowel disease, dermatitis, osteoarthritis, asthma, inflammatory muscle disease, allergic rhinitis, vaginitis, interstitial cystitis, scleroderma, eczema, allogeneic or xenogeneic transplantation (organ, bone marrow, stem cells and other cells and tissues) graft
rejection, graft- versus-host disease, lupus erythematosus, inflammatory disease, type I diabetes, pulmonary fibrosis, dermatomyositis, Sjogren's syndrome, thyroiditis (e.g., Hashimoto's and autoimmune thyroiditis), myasthenia gravis, autoimmune hemolytic anemia, multiple sclerosis, cystic fibrosis, chronic relapsing hepatitis, primary biliary cirrhosis, allergic conjunctivitis and atopic dermatitis.
Other Diseases, Disorders or Conditions
[00251] In accordance with one aspect, are methods for treating, preventing, reversing, halting or slowing the progression of LPA-dependent or LPA-mediated diseases or conditions once it becomes clinically evident, or treating the symptoms associated with or related to LPA- dependent or LPA-mediated diseases or conditions, by administering to the mammal a compound of Formula (I), (II), (III), (IV) or (V). In certain embodiments, the subject already has a LPA-dependent or LPA-mediated disease or condition at the time of administration, or is at risk of developing a LPA-dependent or LPA-mediated disease or condition. [00252] In certain aspects, the activity of LPAi in a mammal is directly or indirectly modulated by the administration of (at least once) a therapeutically effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V). Such modulation includes, but is not limited to, reducing and/or inhibiting the activity of LPAi. In additional aspects, the activity of LPA in a mammal is directly or indirectly modulated, including reducing and/or inhibiting, by the administration of (at least once) a therapeutically effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V). Such modulation includes, but is not limited to, reducing and/or inhibiting the amount and/or activity of a LPA receptor. In one aspect, the LPA receptor
[00253] In one aspect, LPA has a contracting action on bladder smooth muscle cell isolated from bladder, and promotes growth of prostate-derived epithelial cell {The Journal of Urology , 1999, vol. 162, pp. 1779-1784; The Journal of Urology , 2000, vol. 163, pp. 1027-1032). In another aspect, LPA contracts the urinary tract and prostate in vitro and increases intraurethral pressure in vivo (WO 02/062389).
[00254] In certain aspects, are methods for preventing or treating eosinophil and/or basophil and/or dendritic cell and/or neutrophil and/or monocyte and/or T-cell recruitment comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
[00255] In certain aspects, are methods for the treatment of cystitis, including, e.g., interstitial cystitis, comprising administering at least once to the mammal a therapeutically effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V).
[00256] In accordance with one aspect, methods described herein include the diagnosis or determination of whether or not a patient is suffering from a LPA-dependent or LPA-mediated disease or condition by administering to the subject a therapeutically effective amount of a compound of Formula (I), (II), (III), (IV) or (V) and determining whether or not the patient responds to the treatment.
[00257] In one aspect provided herein are compounds of Formula (I), (II), (III), (IV) and (V), pharmaceutically acceptable salts, pharmaceutically acceptable prodrugs, and pharmaceutically acceptable solvates thereof, which are antagonists of LPAi, and are used to treat patients suffering from one or more LPA-dependent or LPA-mediated conditions or diseases, including, but not limited to, lung fibrosis, kindney fibrosis, liver fibrosis, scarring, asthma, rhinitis, chronic obstructive pulmonary disease, pulmonary hypertension, interstitial lung fibrosis, arthritis, allergy, psoriasis, inflammatory bowel disease, adult respiratory distress syndrome, myocardial infarction, aneurysm, stroke, cancer, pain, proliferative disorders and inflammatory conditions. In some embodiments, LPA-dependent conditions or diseases include those wherein an absolute or relative excess of LPA is present and/or observed.
[00258] In any of the aforementioned aspects the LPA-dependent or LPA-mediated diseases or conditions include, but are not limited to, organ fibrosis, asthma, allergic disorders, chronic obstructive pulmonary disease, pulmonary hypertension, lung or pleural fibrosis, peritoneal fibrosis, arthritis, allergy, cancer, cardiovascular disease, ult respiratory distress syndrome, myocardial infarction, aneurysm, stroke, and cancer.
[00259] In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used to improve the corneal sensitivity decrease caused by corneal operations such as laser-assisted in situ keratomileusis (LASIK) or cataract operation, corneal sensitivity decrease caused by corneal degeneration, and dry eye symptom caused thereby. [00260] In one aspect, presented herein is the use of a compound of Formula (I), (II), (III), (IV) or (V) in the treatment or prevention of ocular inflammation and allergic conjunctivitis, vernal keratoconjunctivitis, and papillary conjunctivitis in a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V). [00261] In one aspect, presented herein is the use of a compound of Formula (I), (II), (III), (IV) or (V) in the treatment or prevention of Sjogren disease or inflammatory disease with dry eyes in a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V). [00262] In one aspect, LPA and LPA receptors (e.g. LPA1) are involved in the pathogenesis of osteoarthritis (Kotani et al, Hum. MoI. Genet., 2008, 17, 1790-1797). In one aspect, presented
herein is the use of a compound of Formula (I), (II), (III), (IV) or (V) in the treatment or prevention of osteoarthritis in a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V). [00263] In one aspect, LPA receptors (e.g. LPAi, LPA3) contribute to the pathogenesis of rheumatoid arthritis (Zhao et al, MoI. Pharmacol, 2008, 73(2), 587-600). In one aspect, presented herein is the use of a compound of Formula (I), (II), (III), (IV) or (V) in the treatment or prevention of rheumatoid arthritis in a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V). [00264] In one aspect, LPA receptors (e.g. LPAi) contribute to adipogenesis. (Simon et al, J.Biol. Chem., 2005, vol. 280, no. 15, p.14656). In one aspect, presented herein is the use of a compound of Formula (I), (II), (III), (IV) or (V) in the promotion of adipose tissue formation in a mammal comprising administering at least once to the mammal an effective amount of at least one compound of Formula (I), (II), (III), (IV) or (V). [00265] In one aspect, compounds disclosed herein are used to treat Raynaud's phenomenon. Raynaud's phenomenon comprises both Raynaud's disease (where the phenomenon is idiopathic) and Raynaud's syndrome, where it is caused by some instigating factor. Compounds
[00266] Compounds of Formula (I), (II), (III), (IV) and (V), including pharmaceutically acceptable salts, pharmaceutically acceptable prodrugs, and pharmaceutically acceptable solvates thereof, antagonize or modulate LPAi and are used to treat patients suffering from LPAi-dependent or LPAi-mediated conditions or diseases.
[00267] In one aspect, provided herein is a compound of Formula (I), pharmaceutically acceptable salt, pharmaceutically acceptable solvate, metabolite, or prodrugs thereof:
R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -C(=O)N(R9)2, -C(O)NH-OH, -
C(=O)NH-CN, -P(O)(OH)2, -P(=O)(ORD)2, -OPO3H2, -SO2NHC(=O)R10, -CN, - C(=NH)-NH2, -C(=NH)-NHC(=O)RD, -C(O)NHCH2CH2SO3H, tetrazolyl, 5-oxo- 2,5-dihydro-[l,2,4]oxadiazol-3-yl, or carboxylic acid bioisostere;
RD is H or Ci-C6alkyl; ring A is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, or a substituted or
unsubstituted heteroarylene, where if ring A is substituted then each substituent on ring A is H or RA; ring B is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring B is substituted then each substituent on ring B is H or RB;
L1 is a substituted or unsubstituted cycloalkylene; L2 is absent, a substituted or unsubstituted alkylene, a substituted or unsubstituted fluoroalkylene, a substituted or unsubstituted heteroalkylene, -0-, -S-, -SO-, -SO2-, - NR2-, or -C(O)-;
R2 is H, CrC4alkyl, or CrC4fluoroalkyl; R3 is H, Ci-C4alkyl, or Ci-C4fluoroalkyl;
R4 is -NR7C(=O)OCH(R8)-CY, -NR7C(=O)OC(R8)(R8a)-CY, or -NR7C(=O)O-CY; R7 is H or CrC4alkyl; R8 is H, Ci-C4alkyl, or Ci-C4fluoroalkyl;
R8a is H or Ci-C4alkyl;
CY is a substituted or unsubstituted alkyl, a substituted or unsubstituted cycloalkyl a substituted or unsubstituted heterocycloalkyl, a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then each substituent on CY is H or Rc; or
R8 and CY are taken together with the carbon atom to which they are attached to form a substituted or unsubstituted carbocycle or a substituted or unsubstituted heterocycle; each of RA, RB, and Rc are independently selected from H, halogen, -CN, -NO2, -OH, - OR10, -S(=O)2R10, -SR10, -S(=O)R10, -N(R9)S(=O)2R10, -S(=O)2N(R9)2, -C(=O)R10, -
OC(=O)R10, -CO2R9, -OCO2R10, -N(R9)2, -C(=O)N(R9)2, -OC(=O)N(R9)2, - NR9C(=O)N(R9)2, -NR9C(O)R10, -NR9C(O)OR10, d-C4alkyl, CrC4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl; each R9 is independently selected from H, Ci-Cβalkyl, Ci-Ceheteroalkyl, C1- Cefluoroalkyl, a substituted or unsubstituted cycloalkyl, a substituted or unsubstituted heterocycloalkyl, a substituted or unsubstituted aryl, a substituted or unsubstituted heteroaryl, a substituted or unsubstituted -Ci-C4alkylene- cycloalkyl, a substituted or unsubstituted -Ci-Qalkylene-heterocycloalkyl, a substituted or unsubstituted -CrQalkylene-aryl, or a substituted or unsubstituted -Ci-C/jalkylene-heteroaryl; or
two R9 groups attached to the same N atom are taken together with the N atom to which they are attached to form a substituted or unsubstituted heterocycle; R10 is selected from Ci-Cβalkyl, Ci-Ceheteroalkyl, Ci-Cefluoroalkyl, a substituted or unsubstituted cycloalkyl, a substituted or unsubstituted heterocycloalkyl, a substituted or unsubstituted aryl, a substituted or unsubstituted heteroaryl, a substituted or unsubstituted -Ci-C4alkylene-cycloalkyl, a substituted or unsubstituted -Ci-C4alkylene-heterocycloalkyl, a substituted or unsubstituted - Ci-C4alkylene-aryl, and a substituted or unsubstituted -Ci-C4alkylene- heteroaryl. [00268] For any and all of the embodiments, substituents are selected from among from a subset of the listed alternatives. For example, in some embodiments, R3 is H, Ci-C4alkyl, or C1- C4fluoroalkyl. In other embodiments, R3 is Ci-C4alkyl, C3-C6cycloalkyl, or Ci-C4fluoroalkyl. In some embodiments, R3 is H, Ci-C4alkyl, cyclopropyl, or Ci-C4fluoroalkyl. In some embodiments, R3 is Ci-C4alkyl, cyclopropyl, or Ci-C4fluoroalkyl. In other embodiments, R3 is Ci-C4alkyl, or Ci-C4fluoroalkyl. In yet other embodiments, R3 is Ci-C4alkyl. In some embodiments, R3 is -CH3 or -CH2CH3. In some embodiments, R3 is -CH3, -CF3, -CH2CH3, - CHCH2, or cyclopropyl. In some embodiments, R3 is -CH3, -CF3, -CH2CH3, or -CHCH2. In one aspect, R3 is -CH3.
[00269] In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -C(=O)N(R9)2, - C(=O)NH-OH, -C(=O)NH-CN, -CN, tetrazolyl or carboxylic acid bioisostere. In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, tetrazolyl, 5-oxo-2,5-dihydro- [l,2,4]oxadiazol-3-yl, or carboxylic acid bioisostere. In some embodiments, R1 is -CO2H, - CO2RD, -C(=O)NHSO2R10, or tetrazolyl. In some embodiments, R1 is -CO2H, -CO2RD, or - C(=O)NHSO2R10. In some embodiments, R1 is -CO2H. In some embodiments, R1 is - C(=O)NHSO2R10. In some embodiments, R1 is a carboxylic acid bioisostere.
[00270] In some embodiments, ring A is a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring A is substituted then each substituent on ring A is H or RA.
[00271] In some embodiments, ring A is a substituted or unsubstituted phenylene, a substituted or unsubstituted napthylene, or a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring A is substituted then each substituent on ring A is H or RA.
[00272] In some embodiments, ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S
atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring A is substituted then each substituent on ring A is H or RA.
[00273] In some embodiments, ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted pyridinylene, where if ring A is substituted then each substituent on ring A is H or RA.
[00274] In some embodiments, ring B is a substituted or unsubstituted monocyclic cycloalkylene, a substituted or unsubstituted bicyclic cycloalkylene, a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted bicyclic heterocycloalkylene, a substituted or unsubstituted phenylene, a substituted or unsubstituted naphthylene, a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then each substituent on ring B is H or RB.
[00275] In some embodiments, each of RA, RB, and Rc are independently selected from halogen, -CN, -NO2, -OH, -OR10, -N(R9)2, CrC4alkyl, CrC4fluoroalkyl, CrC4fluoroalkoxy, C1- C4alkoxy, and Ci-C4heteroalkyl.
[00276] In some embodiments, each of RA, RB, and Rc are independently selected from halogen, Ci-C4alkyl, CrC4fluoroalkyl, CrC4fluoroalkoxy, CrC4alkoxy, and CrC4heteroalkyl.
[00277] In one aspect, the
are on non-adjacent ring atoms of ring A. [00278] In one aspect, the compound of Formula (I) has the structure of Formula (II):
Formula (II) wherein each of A1, A2, and A3 is independently selected from CRA and N. [00279] In some embodiments, each RAis independently selected from H, halogen, -CH3, -CF3, - OCF3, and -OCH3.
[00280] In some embodiments, each of A1, A2, and A3 is CRA. [00281] In some embodiments, one of A1, A2, and A3 is N.
[00282] In some embodiments, A1 is N. In some embodiments, A2 is N. In some embodiments, A3 is N.
[00283] In some embodiments, R4 is -NR7C(=O)OCH(R8)-CY or -NR7C(=O)O-CY.
[00284] In some embodiments, R4 is -NR7C(=O)O-CY. In some embodiments, R4 is - NHC(=O)O-CY. In some embodiments, R4 is -NR7C(=O)OCH(R8)-CY. In some embodiments, R4 is -NHC(=O)OCH(R8)-CY.
[00285] In one aspect, R7 is H. In some embodiments, R8 is H, Ci-C4alkyl or Ci-C4fluoroalkyl. In some embodiments, R8 is Ci-C4alkyl or Ci-C4fluoroalkyl. In some other embodiments, R8 is H, -CH3 or -CF3. In yet other embodiments, R8 is -CH3 or -CF3. In one aspect, R8 is-CH3. [00286] In some embodiments, R1 is -CO2H or -CO2RD. In some embodiments, RD is H, -CH3, Or -CH2CH3. [00287] In one aspect, R1 is -CO2H. [00288] In some embodiments, CY is a substituted or unsubstituted cycloalkyl, a substituted or unsubstituted heterocycloalkyl, a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then each substituent on CY is H or Rc; or R8 and CY are taken together with the carbon atom to which they are attached to form a substituted or unsubstituted carbocycle or a substituted or unsubstituted heterocycle [00289] In some embodiments, CY is a substituted or unsubstituted cycloalkyl, a substituted or unsubstituted aryl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then each substituent on CY is H or Rc.
[00290] In some embodiments, CY is a substituted or unsubstituted monocyclic cycloalkyl, a substituted or unsubstituted bicyclic cycloalkyl, a substituted or unsubstituted phenyl, a substituted or unsubstituted napthyl, a substituted or unsubstituted monocyclic heteroaryl containing 0-4 N atoms, or a substituted or unsubstituted bicyclic heteroaryl containing 0-4 N atoms, wherein if CY is substituted then each substituent on CY is H or Rc. [00291] In some embodiments, CY is a substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 1-3 N atoms, wherein if CY is substituted then each substituent on CY is H or Rc.
[00292] In some embodiments, each Rc is independently selected from H, halogen, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl. [00293] In one aspect, in any of the embodiments described herein, A is unsubstituted or monosubstituted with RA. In one aspect, in any of the embodiments described herein, A is unsubstituted.
[00294] In one aspect, in any of the embodiments described herein, B is unsubstituted or monosubstituted with RB. In one aspect, in any of the embodiments described herein, B is unsubstituted.
[00295] In one aspect, in any of the embodiments described herein, CY is unsubstituted or monosubstituted with Rc.
[00296] In some embodiments, L1 is a substituted or unsubstituted C3-Cealkylene. In some embodiments, L1 is
[00297] In alternative embodiments, L2 is absent, Ci-Cβalkylene, Ci-Cefluoroalkylene, C1- C6heteroalkylene, -O-, -S-, -SO-, -SO2- or -NR2-. [00298] In some other embodiments, L2 is absent, CrC6alkylene, -O-, -S-, -SO-, -SO2- or -NR2-. In yet other embodiments, L2 is absent, -CH2-, -O-, -S-, -SO-, -SO2- or -NR2-. In yet other embodiments, L2 is absent, -CH2-, -O-, -S-, -SO-, or -SO2-. In some embodiments, L2 is absent. In yet other embodiments, L2 is -CH2-. In some embodiments, L2 is -0-, -S-, -SO-, or -SO2-. [00299] In one aspect, ring B is a substituted or unsubstituted monocyclic cycloalkylene, a substituted or unsubstituted bicyclic cycloalkylene, a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted bicyclic heterocycloalkylene, a substituted or unsubstituted phenylene, a substituted or unsubstituted naphthylene, a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then each substituent on ring B is H or RB. [00300] In some embodiments, each RB is independently selected from H, halogen, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl. [00301] In some embodiments, L2 is a absent, Ci-C6alkylene, Ci-C6fluoroalkylene, -C1- C6alkylene-O-, -Ci-C3alkylene-O-Ci-C3alkylene -, -O-Ci-C6alkylene -, -Ci-C6alkylene-S-, -C1- C3alkylene-S-Ci-C3alkylene -, -S-Ci-C6alkylene -, -Ci-Cgalkylene-SO-, -Ci-C3alkylene-SO-Ci- C3alkylene -, -SO-Ci-C6alkylene -, -Ci-C6alkylene-SO2-, -Ci-C3alkylene-SO2-Ci-C3alkylene -, - SO2-Ci-C6alkylene -, -Ci-C4alkylene-NRπ-, -Ci-C2alkylene-NRπ-Ci-C2alkylene -, - NRπ-Ci- C4alkylene -, -O-, -S-, -SO-, -SO2- or -NR2-; R11 is H or Ci-C2alkylene.
[00302] In some embodiments, L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -CH2NH- , -NHCH2-, -O-, -S-, or -NH-. In some other embodiments, L2 is -CH2O-, -OCH2-, -CH2S-, - SCH2-, -CH2NH-, or -NHCH2-. In some embodiments, L2 is absent, -CH2-, or -CH2O-. In some embodiments, L2 is absent.
[00303] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl-l,l-diyl, cyclopentyl- 1 , 1 - diyl or cyclohexyl- 1,1 -diyl. In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl- 1 , 1 - diyl, or cyclopentyl- 1,1 -diyl. In some embodiments, L1 is cyclopropyl- 1,1 -diyl. [00304] In some embodiments, ring B is a substituted or unsubstituted monocyclic cycloalkylene, or a substituted or unsubstituted phenylene, where if ring B is substituted then each substituent on ring B is H or RB.
[00305] In some other embodiments, ring B is a substituted or unsubstituted cyclopentylene, a substituted or unsubstituted cyclopentenylene, a substituted or unsubstituted cyclohexylene, a
substituted or unsubstituted cyclohexenylene, or a substituted or unsubstituted phenylene, where if ring B is substituted then each substituent on ring B is H or RB.
[00306] In other embodiments, ring B is a substituted or unsubstituted phenylene, where if ring B is substituted then each substituent on ring B is H or RB. In other embodiments, ring B is an unsubstituted phenylene.
[00307] In some embodiments, ring B is a substituted or unsubstituted monocyclic heterocycloalkylene, a substituted or unsubstituted bicyclic heterocycloalkylene, a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then each substituent on ring B is H or RB. [00308] In some embodiments, ring B is a substituted or unsubstituted monocyclic heteroarylene, or a substituted or unsubstituted bicyclic heteroarylene, where if ring B is substituted then each substituent on ring B is H or RB.
[00309] In some embodiments, ring B is a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring B is substituted then each substituent on ring B is H or RB.
[00310] In some embodiments, ring B is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing 0 or 1 O atoms, 0 or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring B is substituted then each substituent on ring B is H or RB. [00311] In one aspect, ring B is a unsubstituted or substitituted divalent ring selected from phenylene, naphthylene, furanylene, pyrrolylene, oxazolylene, thiazolylene, imidazolylene, pyrazolylene, triazolylene, tetrazolylene, isoxazolylene, isothiazolylene, oxadiazolylene, thiadiazolylene, pyridinylene, pyrimidinylene, pyrazinylene, pyridazinylene, triazinylene, quinolinylene, isoquinolinylene, quinazolinylene, quinoxalinylene, naphthyridinylene, indolylene, indazolylene, benzoxazolylene, benzisoxazolylene, benzofuranylene, benzothienylene, benzothiazolylene, benzimidazolylene, purinylene, cinnolinylene, phthalazinylene, pteridinylene, pyridopyrimidinylene, pyrazolopyrimidinylene, azaindolylene, cyclopentylene, cyclopentenylene, cycloalkylene, and cyclalkenylene. [00312] In one aspect, ring B is a unsubstituted or substitituted divalent ring selected from phenylene, furanylene, pyrrolylene, oxazolylene, thiazolylene, imidazolylene, pyrazolylene, triazolylene, tetrazolylene, isoxazolylene, isothiazolylene, oxadiazolylene, thiadiazolylene, pyridinylene, pyrimidinylene, pyrazinylene, pyridazinylene, triazinylene, cyclopentylene, cyclopentenylene, cycloalkylene, and cyclalkenylene. [00313] In one aspect, ring B is a unsubstituted or substitituted divalent ring selected from pyrrolylene, oxazolylene, thiazolylene, imidazolylene, pyrazolylene, triazolylene, tetrazolylene,
isoxazolylene, isothiazolylene, oxadiazolylene, thiadiazolylene, pyridinylene, pyrimidinylene, pyrazinylene, pyridazinylene, triazinylene, cyclopentylene, cyclopentenylene, cycloalkylene, and cyclalkenylene.
O H R8 O H. R8
[00314] In some embodiments, R4 is H or H [00315] In some embodiments, R8 is -CH3 or -CF3. In other embodiments, R8 is -CH3.
O H PH3
[00316] In some embodiments, R4 is ^ . In other embodiments, R4 is
O H CH3
H
[00317] In some embodiments, CY is a substituted or unsubstituted phenyl, wherein if CY is substituted then each substituent on CY is H or Rc; each Rc is independently selected from halogen, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl.
In some embodiments, CY is a substituted or unsubstituted C3-C6cycloalkyl. In some embodiments, CY is cyclopropyl.
[00318] In some embodiments, each Rc is independently selected from F, Cl, -CH3, -CF3, -
OCF3, -OCH3. [00319] In some embodiments, CY is phenyl, 2-fluorophenyl or 2-chloro-phenyl.
[00320] In one aspect, the compound of Formula (I) has the structure of Formula (III):
Formula (III) wherein A1 is CRA and N; RA is H, F, Cl, Br, I, -CH3, or -CF3;
A4 is CH or N;
L1 is C3-C6cycloalkylene;
L2 is absent, CrC4alkylene, CrC4heteroalkylene, -O-, -S-, -SO-, -SO2-, -NR2-, or -
C(=O)-; R2 is H or -CH3;
CY is C3-C6Cycloalkyl or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, Br, I, -OH, Ci-C4alkyl, C1- C4fluoroalkyl, Ci-C4fluoroalkoxy, or Ci-C4alkoxy.
[00321] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl- 1 , 1 -diyl, cyclopentyl- 1,1- diyl or cyclohexyl- 1 , 1 -diyl. In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl.
[00322] In some embodiments, L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -O-, or - S-. In some embodiments, L2 is absent, -CH2-, -O-, or -S-. In some embodiments, L2 is absent or -O-. In some embodiments, L2 is absent. [00323] In some embodiments, A1 is CH. In some embodiments, A4 is CH. [00324] In some embodiments, CY is cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, a substituted or unsubstituted cyclohexeneyl, or a substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -CH3, or CF3. In some embodiments, CY is a substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -CH3, or CF3. In some embodiments, CY is cyclopropyl [00325] In some embodiments, CY is phenyl, 2-fluorophenyl or 2-chloro-phenyl.
[00326] In some embodiments, CY is cyclopropyl, cyclobutyl, cyclohexyl, 2-chlorocyclohex- 1 - enyl, phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6- difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4- dichlorophenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl.
[00327] In some embodiments, CY is cyclopropyl, cyclobutyl, cyclopentyl, cyclopent- 1 -enyl, 2- chlorocyclopent-1-enyl, cyclohexyl, cyclohex-1-enyl, 2-chlorocyclohex- 1 -enyl, phenyl, 2- fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2- chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2- hydroxyphenyl, 3- hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4- methoxyphenyl, 2-trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2- fluoro-4-methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl.
[00328] In some embodiments, CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4- difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3- hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2-trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-
trifluoromethylphenyl, 2-fluoro-4-methoxyphenyl, 2-methylphenyl, 3-methylphenyl, or 4- methylphenyl.
[00329] In some embodiments, CY is phenyl, 2-fluorophenyl, 3-fluorophenyl, 2-chlorophenyl, 3-chlorophenyl, 2-methylphenyl, 3-methylphenyl, 2-trifluoromethylphenyl, or 3- trifluoromethylphenyl. In some embodiments, CY is phenyl. In some embodiments, CY is phenyl, 2-hydroxyphenyl, 3- hydroxyphenyl, or 4- hydroxyphenyl.
[00330] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl, cyclopropyl-l,2-diyl, cycloprop-2- enyl-l,l-diyl, cyclobutyl- 1,1 -diyl, cyclopentyl- 1,1 -diyl, cyclohexyl- 1,1 -diyl, -CH2C(CH2CH2)- or -C(CH2CH2)CH2-; L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -CH2NH-, - NHCH2-, -O-, -S-, or -NH-; ring A is a substituted or unsubstituted phen-l,4-ylene, where if ring A is substituted then ring A is substituted with RA; ring B is a substituted or unsubstituted phenyl- 1,4-ene, where if ring B is substituted then ring B is substituted with RB; R4 is - NHC(=O)OCH(R8)-CY; CY is cyclopropyl, cyclobutyl, cyclopentyl, cyclopent-1-enyl, 2- chlorocyclopent- 1 -enyl, cyclohexyl, cyclohex-1-enyl, 2-chlorocyclohex-l-enyl, phenyl, 2- fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2- chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2- hydroxyphenyl, 3- hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4- methoxyphenyl, 2-trifluoromethylphenyl, 3 -trifluoromethylphenyl, 4-trifluoromethylphenyl, 2- fluoro-4-methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl.
[00331] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl; L2 is absent. In some embodiments, L1 is cyclopropyl- 1,1 -diyl; L2 is absent; CY is cyclopropyl. In some embodiments, L1 is cyclopropyl- 1,1 -diyl; L2 is absent; CY is a substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -OH, -CH3, or CF3. [00332] In some embodiments, A1 is CH; and A4 is CH.
[00333] In one aspect, the compound of Formula (III) has the structure:
[00335] In one aspect, the compound of Formula (I) has the structure of Formula (IV):
Formula (IV) wherein
A1 is CRA and N; RA is H, F, Cl, Br, I, -CH3, or -CF3;
L2 is absent, Ci-C4alkylene, d-C4heteroalkylene, -0-, -S-, -SO-, -SO2-, -NR2-, or - C(=0)-;
R2 is H or -CH3; R4 is -NHC(=O)OCH(R8)-CY, or -NHC(=O)O-CY;
R8 is H or -CH3;
CY is Ci-Cβalkyl, C3-Cecycloalkyl, or substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, Br, I, -OH, C1- C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, or Ci-C4alkoxy; n is 1, 2, or 3.
[00336] In some embodiments, L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -0-, or - S-. In some embodiments, L is absent. [00337] In some embodiments, A1 is CRA. [00338] In some embodiments, A1 is N. [00339] In some embodiments, CY is -CH3, -CH2CH3, -CH2CH2CH3, -CH2CH2CH2CH3, - CH(CH3)2, -CH(CH2CH3)2, -CH2CH(CH3)2, -C(CH3)3, -CH2(CH3)3, C3-C6cycloalkyl, substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -CH3, or CF3.
[00340] In some embodiments, R4 is -NHC(=O)OCH(R8)-CY. [00341] In some embodiments, n is 1.
[00342] In some embodiments, R4 is -NHC(=O)OCH2-(cyclopropyl), -NHC(=O)OCH(CH3)- (cyclopropyl), -NHC(=O)OCH2-(substituted or unsubstituted phenyl) or - NHC(=O)OCH(CH3)-(substituted or unsubstituted phenyl); wherein if CY is substituted then
CY is substituted with Rc; Rc is F, Cl, -CH3, or CF3. In some embodiments, R4 is -
NHC(=O)OCH(CH3)-(cyclopropyl).
[00343] In some embodiments, R4 is -NHC(=O)O-CY. In some embodiments, CY is -C(CH3)3 or C3-C6cycloalkyl.
[00344] In some embodiments, the compound has the following structure:
[00346] In one aspect, R4 is
[00348] In some embodiments, the compound of Formula (I) has the structure of Formula (V) or a pharmaceutically acceptable salt thereof:
Formula (V) wherein R1 is -CO2H, -CO2RD, -CN, -C(=O)N(R9)2, -C(=O)NHCH2CH2SO3H, or -
C(=O)NHSO2R10, tetrazolyl, or 5-oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl; RD is H or
Ci-C4alkyl;
R3 is H, Ci-C4alkyl, C3-C6cycloalkyl, or Ci-C4fluoroalkyl; R4 is -NR7C(=O)OCH(R8)-CY; R7 is H or Ci-C4alkyl; R8 is H, Ci-C4alkyl, or Ci-C4fluoroalkyl; CY is a substituted or unsubstituted C3-C6cycloalkyl or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with 1 or 2 Rc; R9 is H, Ci-C6alkyl, Ci-C6fluoroalkyl, C3-C6cycloalkyl, or a substituted or unsubstituted phenyl; R10 is a Ci-Cβalkyl, Ci-Cefluoroalkyl, C3-Cecycloalkyl, or a substituted or unsubstituted phenyl; each of RA, RB, and Rc are independently selected from F, Cl, Br, I, -CN, -OH, C1-
C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl; m is 0, 1, or 2; n is i, 2, 3 or 4; p is 0, 1, or 2.
[00349] In some embodiments, R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10 or tetrazolyl; R3 is H or Ci-C4alkyl; R7 is H; R8 is H, -CH3 or -CF3; R10 is a Ci-C6alkyl or a substituted or unsubstituted phenyl; each RAis independently selected from F, Cl, Br, I, -OH, -CH3, -CF3, - OCF3, and -OCH3; each RB is independently selected from F, Cl, Br, I, -OH, -CH3, -CF3, -OCF3, and -OCH3; each Rc is independently selected from F, Cl, Br, I, -OH, -CH3, -CF3, -OCF3, and - OCH3; m is O or 1; [00350] n is 1, 2, or 3; p is 0 or 1.
[00351] In some embodiments, R1 is -CO2H or -CO2RD; RD is H, -CH3, or -CH2CH3; R3 is H, - CH3 or -CH2CH3; R4 is -NHC(=O)OCH(R8)-CY; R8 is H, or -CH3; CY is a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with 1 or 2 Rc. [00352] In some embodiments, the compound of Formula (I) or Formula (V) has the following structure:
[00353] In some embodiments, the compound of Formula (I) or Formula (V) has the following structure:
[00354] In some embodiments, R1 is -C(=O)NHSO2R10; R3 is H, -CH3 or -CH2CH3; R8 is H, or -CH3; R10 is -CH3, or -CH2CH3.
[00355] In some embodiments, R4 is -NHC(=O)OCH(CH3)-(substituted or unsubstituted phenyl); wherein if the phenyl is substituted then the phenyl is substituted with Rc; Rc is F, Cl, - CH3, or CF3; n is 1.
O H R8
[00356] In some embodiments, R4 is H ; CY is a substituted or unsubstituted phenyl, wherein if CY is substituted phenyl then the phenyl is substituted with 1 or 2 Rc; Rcis H, F, Cl, -OH, -CH3, -CF3, or -OCH3; n is 1. [00357] In some embodiments, R8 is -CH3; CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3-hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl.
[00358] In some embodiments, the compound of Formula I) or Formula (V) has the following structure:
[00359] In some embodiments, R1 is -CO2H; CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5-difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2-bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3-hydroxyphenyl, 4- hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl.
[00360] In some embodiments, CY is phenyl. In some embodiments, R1 is -CO2H; CY is phenyl, 2-fluorophenyl, 3 -fluorophenyl, 2-chlorophenyl, 3-chlorophenyl, 2-methylphenyl, 3- methylphenyl, 2-trifluoromethylphenyl, or 3-trifluoromethylphenyl. In some embodiments, R1 is -CO2H; CY is phenyl.
[00361] In some embodiments, Rc is F, Cl, -CH3, or CF3.
[00362] In some embodiments, ring B is a substituted or unsubstituted phenylene; Rc is F or Cl.
[00363] In some embodiments, Rc is Cl. In some embodiments, Rc is F.
[00364] In some embodiments, the compound of Formula (I) or Formula (V) has the following structure:
[00365] In some embodiments, the compound of Formula (I) or Formula (V) has one of the following structures:
[00366] In one aspect, the compound of Formula (I) is a 3-substituted-{4-[l-(2-Rc-phenyl)- ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -benzene compound, wherein 3-substituent is - iΛB-lΛR1, or pharmaceutically acceptable salts, pharmaceutically acceptable solvates, or prodrugs thereof; R1 is -CO2H, -CO2RA, -C(=O)NHSO2R10, -C(=O)N(R9)2, -C(=O)NH-OH, - C(=O)NH-CN, tetrazolyl or carboxylic acid bioisostere; RA is H or Ci-Cβalkyl; B is a divalent group selected from a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, and a substituted or unsubstituted heteroarylene, where if ring B is substituted then each substituent on ring B is H or RB; each RB is selected from H, halogen, -OH, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci- C4alkoxy, and Ci-C4heteroalkyl; L1 is a substituted or unsubstituted cycloalkylene; L2 is absent, a substituted or unsubstituted alkylene, a substituted or unsubstituted fluoroalkylene, a substituted or unsubstituted heteroalkylene, -O-, -S-, -SO-, -SO2-, -NR2-, or -C(=O)-; R2 is H,
Ci-C4alkyl, or Ci-C4fluoroalkyl; Rc is selected from H, halogen, -OH, CrC4alkyl, C1- C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl. [00367] In one aspect, the compound of Formula (I) is a 4-substituted-{4-[l-(2-Rc-phenyl)- ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -benzene compound, wherein 4-substituent is - I^-B-L1-!?.1, or pharmaceutically acceptable salts, pharmaceutically acceptable solvates, or prodrugs thereof; R1 is -CO2H, -CO2RA, -C(=O)NHSO2R10, -C(=O)N(R9)2, -C(O)NH-OH, - C(=0)NH-CN, tetrazolyl or carboxylic acid bioisostere; RA is H or Ci-Cβalkyl; B is a divalent group selected from a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, and a substituted or unsubstituted heteroarylene, where if ring B is substituted then each substituent on ring B is H or RB; each RB is selected from H, halogen, -OH, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, C1- C4alkoxy, and Ci-C4heteroalkyl; L1 is a substituted or unsubstituted cycloalkylene; L2 is absent, a substituted or unsubstituted alkylene, a substituted or unsubstituted fluoroalkylene, a substituted or unsubstituted heteroalkylene, -0-, -S-, -SO-, -SO2-, -NR2-, or -C(O)-; R2 is H, Ci-C4alkyl, or C1-C4£luoroalkyl; Rc is selected from H, halogen, -OH, C1-C4alkyl, C1- C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl.
[00368] In some embodiments, Rc is H, F, Cl, -CH3, or CF3. In some other embodiments, Rc is F or Cl. In other embodiments, Rc is Cl. In yet other embodiments, Rc is F. [00369] In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl, cyclobutyl- 1 , 1 -diyl, cyclopentyl-1,1- diyl or cyclohexyl- 1 , 1 -diyl. In some embodiments, L1 is cyclopropyl- 1 , 1 -diyl.
[00370] In some embodiments, cyclopropyl- 1,1 -diyl, cyclobutyl- 1 , 1 -diyl, cyclopentyl- 1 , 1 -diyl or cyclohexyl- 1,1 -diyl; L2 is absent; ring B is a substituted or unsubstituted phenyl- 1, 3 -ene or a substituted or unsubstituted phenyl- 1,4-ene, where ring B is unsubstituted or monosubstituted with RB; each Rc is independently selected from H, F, Cl, Br, I, -CH3, -CF3, -OH, -OCF3, and - OCH3.
[00371] In one aspect, the compound of Formula (I) is a 4-substituted-{4-[l-(CY)- ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -benzene compound, wherein the 4-substituent is -iΛB-lΛR1, and CY is as described herein. [00372] In one aspect, the compound of Formula (I) is a 3-Substituted-{4-[l-(CY)- ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -benzene compound, wherein the 3-substituent is -iΛB-lΛR1, and CY is as described herein
[00373] In some embodiments, ring B is a substituted or unsubstituted phenyl- 1,3 -ene or a substituted or unsubstituted phenyl- 1,4-ene, where ring B is unsubstituted or monosubstituted with RB. In some embodiments, ring B is a substituted or unsubstituted phenyl- 1,3-ene, where ring B is unsubstituted or monosubstituted with RB. In some embodiments, ring B is a
substituted or unsubstituted phenyl- 1,4-ene, where ring B is unsubstituted or monosubstituted with RB.
[00374] In some embodiments, L2 is absent.
[00375] In one aspect, ring A is as described in any one of Tables 1 through 6. In one aspect, ring B is as described in any one of Tables 1 through 6. In one aspect, CY is as described in any one of Tables 1 through 6. In one aspect, L2 is as described in any one of Tables 1 through 6. In one aspect, L1 is as described in any one of Tables 1 through 6. In one aspect, R8 is as described in any one of Tables 1 through 6. In one aspect, R1 is as described in any one of Tables 1 through 6.
[00376] In one aspect, the compound of Formula (I) has a structure selected from:
[00377] Any combination of the groups described above for the various variables is contemplated herein. Throughout the specification, groups and substituents thereof are chosen by one skilled in the field to provide stable moieties and compounds.
[00378] In one aspect, compounds of Formula (I) include, but are not limited to, those described in the following Tables: Table 1:
# represent individual stereoisomers; absolute configuration not determined
* mass spectrometric data
[00379] Compounds in Table 1 are named: l-(4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-1); l-[4'-(4-Isopropoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-2); l-{4'-[4-(l-Cyclohexyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-3); l-{4'-[3-Methyl-4-((R)-l-o-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-4); l-[4'-(4-Benzyloxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-5); l-[4'-(4-Ethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]-cyclopropanecarboxylic acid (Compound 1-6); l-[4'-(4-Methoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-7); l-{4'-[3-Methyl-4-(l -methyl- l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-8); l-{4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-9);
1 - (4'-[4-((R)- 1 -Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl} - cyclopropanecarboxylic acid (Compound 1-10);
1 -(4'- (4-[(R)- 1 -(2-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-4- yl)-cyclopropanecarboxylic acid (Compound 1-12);
l-[4'-(4-?ert-Butoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-13);
1 -(4'- (3-Methyl-4-[(R)- 1 -(2-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-14); l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclobutanecarboxylic acid (Compound 1-15); l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopentanecarboxylic acid (Compound 1-16); l-[4'-(4-Cyclopropylmethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-11); l-[4'-(4-?ert-Butoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclobutanecarboxylic acid (Compound 1-17);
1 -(4'- {4-[ 1 -(2-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-4- yl)-cyclopropanecarboxylic acid (Compound 1-18); 1 -(4'- {3-Methyl-4-[ 1 -(4-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-19);
1 -(4'- {3-Methyl-4-[ 1 -(4-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-20); l-{4'-[3-Methyl-4-(l-pyrazin-2-yl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-21);
1 -(4'- {4-[ 1 -(3-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-4-yl)- cyclopropanecarboxylic acid (Compound 1-22); l-[4-(4- (4-[(R)-I -(2-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}-phenoxy)- phenylj-cyclopropanecarboxylic acid (Compound 1-23); l-(4-{4-[3-Methyl-4-(l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-phenoxy}-phenyl)- cyclopropanecarboxylic acid (Compound 1-24); l-{4'-[3-Methyl-4-((R)-l-/)-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-25); l-{4'-[3-Methyl-4-((R)-l-m-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-26);
1 -(4- (4-[3-Methyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-phenoxy} -phenyl)- cyclopropanecarboxylic acid (Compound 1-27);
1 -(4'- (4-[(R)- 1 -(4-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-4- yl)-cyclopropanecarboxylic acid (Compound 1-28);
1 -(4'- (4-[(R)- 1 -(2-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-4- yl)-cyclopropanecarboxylic acid (Compound 1-29); l-{4'-[3-Methyl-4-((R)-l,2,2-trimethyl-propoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-30); 1 - (4'-[4-((R)- 1 -Cyclobutyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl} - cyclopropanecarboxylic acid (Compound 1-31);
1 - (4'-[4-((R)- 1 ,2-Dimethyl-propoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl} - cyclopropanecarboxylic acid (Compound 1-32); l-(4'-[4-(l-Ethyl-propoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-33);
1 -(4'- (4-[ 1 -(2-Chloro-cyclohex- 1 -enyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-34);
1 -(4'- (3-Methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-35); 1 -(4'- (4-[(R)- 1 -(3-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-
4-yl)-cyclopropanecarboxylic acid (Compound 1-36);
1 -(4'- (4-[(R)- 1 -(4-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-
4-yl)-cyclopropanecarboxylic acid (Compound 1-37);
1 - (4'-[4-((R)- 1 -Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl} - cycloprop-2-enecarboxylic acid (Compound 1-38);
2-(4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-39);
1 -(4- (5-[3-Methyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-pyridin-2-yl} - phenyl)-cyclopropanecarboxylic acid (Compound 1-40); 1 -(4'- (4-[ 1 -(3-Bromo-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-4-yl)- cyclopropanecarboxylic acid (Compound 1-41);
1 -(4'- (4-[ 1 -(3-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-4-yl)- cyclopropanecarboxylic acid (Compound 1-42);
1 - (4'-[3-Methyl-4-((S)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl} - cyclopropanecarboxylic acid (Compound 1-43);
1 -(4'- (4-[ 1 -(3-Hydroxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-4-yl)- cyclopropanecarboxylic acid (Compound 1-44);
[ 1 -(4'- (3-Methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropyl]-acetic acid (Compound 1-45);
l-{4'-[3-Ethyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-46);
1 - (4'-[3-Phenyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl} - cyclopropanecarboxylic acid (Compound 1-47); l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-ylmethyl}- cyclopropanecarboxylic acid (Compound 1-48);
1 -(4'- (3-Methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl} - biphenyl-4-ylmethyl)-cyclopropanecarboxylic acid (Compound 1-49);
1 -(4'- (3-Ethyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-50);
1 -(4'- (3-Phenyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-51); l-{4'-[3-Methyl-4-(l-phenyl-ethoxy-d9-carbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-52); l-(4'-{3-Methyl-4-[l-methyl-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-53);
1 -(3'-Methoxy-4!- (3-methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-54); l-(4'-{4-[(R)-l-(3,5-Dibromo-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-55); l-{4'-[4-((R)-l-Phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-56); l-{4'-[3-Methyl-4-(l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-57);
Table 2:
* mass spectrometric data
[00380] Compounds in Table 2 are named:
{5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazol-4-yl}- carbamic acid (R)- 1 -phenyl-ethyl ester (Compound 2-1); {5-[4'-(l-
Benzenesulfonylaminocarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazol-4-yl}-carbamic acid (R)-I -phenyl-ethyl ester (Compound 2-2); {5-[4'-(l-Cyano-cyclopropyl)-biphenyl-4-yl]-3- methyl-isoxazol-4-yl}-carbamic acid (R)- 1 -phenyl-ethyl ester (Compound 2-3); (3-Methyl-5- (4'-[l-(5-oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl)-cyclopropyl]-biphenyl-4-yl}-isoxazol-4-yl)- carbamic acid (R)- 1 -phenyl-ethyl ester (Compound 2-4); (3-Methyl-5-{4'-[l-(lH-tetrazol-5-yl)- cyclopropyl]-biphenyl-4-yl}-isoxazol-4-yl)-carbamic acid (R)- 1 -phenyl-ethyl ester (Compound 2-5); {5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazol- 4-yl}-carbamic acid (R)-l-(3-trifluoromethyl-phenyl)-ethyl ester (Compound 2-6); {5-[4'-(l- Methanesulfonylaminocarbonyl-cyclopropyl)-3-methoxy-biphenyl-4-yl]-3-methyl-isoxazol-4- yl}-carbamic acid (R)-l-(3-trifluoromethyl-phenyl)-ethyl ester (Compound 2-7).
Table 3:
* mass spectrometric data [00381] Compounds in Table 3 are named: l-{4'-[3-Methyl-4-(l-methyl-cyclopentyloxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 3-1); and l-[4'-(4-Cyclobutoxycarbonylamino-3- methyl-isoxazol-5-yl)-biphenyl-4-yl]-cyclopropanecarboxylic acid (Compound 3-2).
[00382] Additional non- limiting examples of compounds include those described in Tables 4 to
6.
Table 4:
Table 5:
[00383] In one aspect, Phen-l,4-ylene (i.e. ring B) in Tables 4 through 6 is replaced with a group selected from Phen-l,2-ylene, Phen-l,3-ylene, Pyridin-2,3-ylene, Pyridin-2,4-ylene, Pyridin- 2,5-ylene, Pyridin-2,6-ylene, Pyridin-3 ,4-ylene, Pyridin-3,5-ylene, Pyridin-3,6-ylene, Pyridin- 4,5-ylene, Pyridin-4,6-ylene, Pyridin-5,6-ylene, Pyrimidin-2,4-ylene, Pyrimidin-2,5-ylene, Pyrimidin-4,5-ylene, Pyrimidin-4,6-ylene, Pyrazin-2,3-ylene, Pyrazin-2,5-ylene, Pyrazin-2,6- ylene, Pyridazin-3,4-ylene, Pyridazin-3,5-ylene, Pyridazin-3,6-ylene, Pyridazin-4,5-ylene, IH- Imidazol-l,2-ylene, lH-Imidazol-l,4-ylene, lH-Imidazol-l,5-ylene, lH-Pyrazol-l,3-ylene, IH- Pyrazol-l,4-ylene, lH-Pyrazol-l,5-ylene, IH-[1, 2,3]Triazol-l,4-ylene, lH-[l,2,3]Triazol-l,5- ylene, IH-[1, 2,4]Triazol-l,3-ylene, and IH-[1, 2,4]Triazol-l,5-ylene.
[00384] In one aspect, Phen-l,4-ylene (i.e. ring B) in Tables 4 through 6 is replaced with a group selected from Phen-l,2-ylene, Phen-l,3-ylene, Pyridin-2,3-ylene, Pyridin-2,4-ylene, Pyridin- 2,5-ylene, Pyridin-2,6-ylene, Pyridin-3 ,4-ylene, Pyridin-3, 5-ylene, Pyridin-3, 6-ylene, Pyridin- 4,5-ylene, Pyridin-4,6-ylene, Pyridin-5, 6-ylene, Pyrimidin-2,4-ylene, Pyrimidin-2,5-ylene, Pyrimidin-4,5-ylene, Pyrimidin-4,6-ylene, Pyrazin-2,3-ylene, Pyrazin-2,5-ylene, Pyrazin-2,6- ylene, Pyridazin-3,4-ylene, Pyridazin-3, 5-ylene, Pyridazin-3, 6-ylene, Pyridazin-4,5-ylene, IH- Imidazol-l,2-ylene, lH-Imidazol-l,4-ylene, lH-Imidazol-1, 5-ylene, lH-Pyrazol-l,3-ylene, IH- Pyrazol-l,4-ylene, lH-Pyrazol-1, 5-ylene, IH-[1, 2,3]Triazol-l,4-ylene, lH-[l,2,3]Triazol-l,5- ylene, IH-[1, 2,4]Triazol-l,3-ylene, and lH-[l,2,4]Triazol-l,5-ylene; and Cl (i.e. Rc) is replaced with F.
[00385] In one aspect, Cl (i.e. Rc) in Tables 4 through 6 is replaced with F. In one aspect, Cl (i.e. Rc) in Tables 4 through 6 is replaced with Η. In one aspect, Cl (i.e. Rc) in Tables 4 through 6 is replaced with -CH3. In one aspect, Cl (i.e. Rc) in Tables 4 through 6 is replaced with -CF3. Synthesis of Compounds [00386] Compounds of Formula (I), (II), (III), (IV) and (V) described herein are synthesized using standard synthetic techniques or using methods known in the art in combination with methods described herein. In additions, solvents, temperatures and other reaction conditions presented herein may vary. [00387] The starting material used for the synthesis of the compounds of Formula (I), (II), (III), (IV) and (V) are either synthesized or obtained from commercial sources, such as, but not limited to, Sigma-Aldrich, Fluka, Acros Organics, Alfa Aesar, and the like. The compounds described herein, and other related compounds having different substituents are synthesized using techniques and materials described herein or otherwise known, including those found in March, ADVANCED ORGANIC CHEMISTRY 4th Ed., (Wiley 1992); Carey and Sundberg,
ADVANCED ORGANIC CHEMISTRY 4th Ed., VoIs. A and B (Plenum 2000, 2001), and Green and Wuts, PROTECTIVE GROUPS IN ORGANIC SYNTHESIS 3rd Ed., (Wiley 1999). General methods for the preparation of compounds can be modified by the use of appropriate reagents and conditions for the introduction of the various moieties found in the formulae as provided herein. [00388] In one aspect, the compounds of Formula (I), (II), (III), (IV) and (V) are prepared as outlined in the following Schemes. Scheme 1:
1 -VI 1 -VIII
[00389] In one aspect, the synthesis of compounds of Formula (I), (II), (III), (IV) and (V) described herein begins with the reaction of an alkyl acetoacetate with methylamine to provide a compound of structure l-II. Compounds of structure l-II are reacted with a substituted or unsubstituted 4-halo-benzoyl chloride (structure l-III, where A1 and A2 are both CH) to provide compounds of structure 1-TV. In one aspect, n is 0, 1, or 2. In one aspect, substituted napthylene carbonyl chlorides or susbtituted heteroaryl carbonyl chlorides are used instead of compounds
of structure l-III, resulting in the synthesis of compounds of structure 1-VIII, wherein
is replaced with other monocyclic or bicyclic rings. In some embodiments, n is 0. In other embodiments, n is 1.
[00390] Treatment of compounds of structure 1-IV with hydroxyl amine and acetic acid provides isoxazoles of structure 1-V. Hydrolysis of the ester group of isoxazoles of structure 1-
V provides carboxylic acids of structure 1-VI. A Curtius rearrangement of carboxylic acids of
structure 1-VI in the presence of hydroxy compounds of structure 1-VII provides carbamate compounds of structure 1-VIII.
[00391] In some embodiments, the preparation of carbamates includes procedures described in Scemem 2.
[00392] A Curtius rearrangement of carboxylic acids of structure 2-1 in the presence of tert- butanol provides carbamate compounds of structure 2-II. Removal of the tert-butyl group with acid provides amines of structure 2-III. Amines of structure 2-III are reacted with chloro formates to provide carbamates of structure 2-IV , where R4 is a carbamate as described herein.
[00393] In some embodiments, carbamates of structure 3-11 are prepared as outlined in Scheme 3. Scheme 3
[00394] A Curtius rearrangement of carboxylic acids of structure 3-1 in the presence of hydroxyl compounds CY-OH provides carbamates of structure 3-II.
[00395] In one aspect, metal mediated couplings are used to provide compounds of Formula (I), (II), (III), (IV) and (V) as described in Scheme 4, Scheme 5, Scheme 6, and Scheme 7. Scheme 4
[00396] In one aspect, a Suzuki reaction between compounds of structure 4-III and compounds of structure 4-1 or 4-11 is used to provide compounds of structure 4-IV or 4-V. In one aspect, the phenyl ring of compounds of structure 4-III is replaced with other cyclic rings (e.g. napthyl, cycloalkyl, monocyclic heteroaryl, bicyclic heteroaryl). Other metal mediated coupling reactions contemplated for the preparation of compounds of structure 4-IV and 4-V include, but are not limited to Suzuki reactions, Stille cross couplings, Negishi couplings, Kumada couplings, Ullmann reactions, Hiyama Coupling, and variants thereof (Metal-Catalyzed Cross- Coupling Reactions, Armin de Meijere (Editor), Francois Diederich (Editor), John Wiley & Sons; 2nd edition, 2004; Ozdemir, et al, Tetrahedron, 2005, 61, 9791-9798; Ackermann, et al, Org. Lett., 2006, 8, 3457-3460; Blakey, et al, J. Am. Chem. Soc, 2003, 125, 6046-6047; Dai, et al, Org. Lett., 2004, 6, 221-224; Yoshikai, et al, J. Am. Chem. Soc, 2005, 127, 17978-17979; Tang, et al, J. Org. Chem., 2006, 71, 2167-2169; Murata, et al, Synthesis, 2001, 2231-2233). [00397] In one aspect, the coupling partners in the metal mediated reaction condition includes those outlined in Scheme 5. Scheme 5
[00398] In one aspect, halides of structure 4-1 are reacted with a borylating agent using transition metal mediated reaction conditions to form boronate compounds of structure 5-II. Boronate compounds of structure 5-11 are reacted with compounds of structure 5-III under palladium mediated coupling conditions to form biaryl aldehydes of structure 5-IV.
[00399] In one aspect, compounds of Formula (I) where L2 is -O- are prepared as outlined in
Scheme 6.
Scheme 6
[00400] In one aspect, boronate esters of structure 5-11 are oxidized to provide hydroxy compounds of structure 6-11. An Ullman ether reaction between hydroxy compounds of structure 6-11 and boronic acid compounds of structure 6-III provides compounds of structure 6- IV. Methods of forming diaryl ethers, such as metal mediated reactions, include but are not limited to the Ulman Ether synthesis, Chan-Lam coupling, and Buchwald-Hartwig synthesis (D. Ma, Q. Cai, Org. Lett., 2003, 5, 3799-3802; C. G. Bates, et ah, Org. Lett., 2002, 4, 2803-2806; C. H. Burgos, et al, Angew. Chem. Int. Ed., 2006, 45, 4321-4326; C. H. Burgos, et al, Angew. Chem. Int. Ed., 2006, 45, 4321-4326; D. M. T. Chan, et al, Tetrahedron Lett., 1998, 39, 2933-
2936; Z. Liu, R. C. Larock, J. Org. Chem., 2006, 71, 3198-3209; Y.-J. Chen, H.-H. Chen, Org. Lett., 2006, 8, 5609-5612; F. Li, Q. et al., Org. Lett., 2003, 5, 2169-2171; D. A. Evans, et al., Tetrahedron Letters, 1998, 39, 2937-2940; C-E. Yeom, et al., Synlett, 2007, 146-150). [00401] In one aspect, compounds of Formula (I) where L2 is -O-, -S-, or -NH- are prepared as outlined Scheme 7. Scheme 7
[00402] An Ullman ether reaction is used to couple boronic esters, or boronoc acids, or structure 5-11 with compounds of structure 7-11 to provide compounds of structure 7-III. As described for Scheme 6, other coupling conditions exist.
[00403] In one aspect, benzyl halides of structure 8-11 are reacted with hydroxy compounds of structure 6-11 to provide ethers of structure 8-III. Scheme 8
[00404] Treatment of hydroxy compounds of structure 6-11 with alkyl halides of structure 8-11 in the presence of a suitable base and a suitable solvent provide ethers of structure 8-III. In one aspect, the suitable base is selected from potassium carbonate, sodium carbonate, triethylamine and the like.
[00405] In one aspect, compounds of Formula (I) where L2 is a heteroalkyl are prepared as outlined in Scheme 9. Scheme 9
9-I RD = Et D RDϋ _= H
[00406] Treatment of alkyl halides of structure 9-1 with compounds of 9-11 in the presence of a suitable base and a suitable solvent provides compounds of structure 9-III. In one aspect, L2 is - CH2-O-, -CH2-S-, -CH2-NH-, and the like.
[00407] In yet another aspect, a compound of Formula (I), (II), (III), (IV) or (V) is prepared as outlined in Scheme 10. Scheme 10
DPPA, NEt3, toluene 8O0C
10-IV
[00408] Following the same procedures as described in Scheme 1, compounds of structure 10-1 are used to prepare a compound of Formula (I), (II), (III), (IV) or (V). In one aspect, compounds of structure 10-1 are prepared using the methodology described herein or known in the art. [00409] A non-limiting example of the synthesis of compounds of structure 10-1 is shown in Scheme 11.
Scheme 11
[00410] In yet another aspect, a compound of Formula (I), (II), (III), (IV) or (V) is prepared as outlined in Scheme 12. Scheme 12.
[00411] In some embodiments, biphenyl compounds of structure 12-1 are elaborated into the polycyclic compounds as shown in scheme 12. Biphenyl compounds of structure 12-1 are treated with a dihalo alkyl compound, such as 1 ,2-dibromoethane, to form a cycloalkyl group. The cyano group is hydrolysed to the acid and an ester is formed from the acid to provide tricyclic compounds of structure 12-11. In some embodiments, RD is ethyl. In some embodiments, RD is isopropyl. Tricyclic compounds of structure 12-11 are then treated with acetyl chloride in the presence of a suitable Lewis acid, follow by conversion of the acetyl group to the carboxylic acid and treatment of the carboxylic acid with thionyl chloride to provide acid chlorides of structure 12-111. Acid chlorides of structure 12-111 are then used to prepare isoxazoles of structure 12-V as described in Scheme 1. In some embodiments, R is an alkyl group. In some embodiments, R is methyl and R is removed from isoxazoles of structure 12-V under hydrolysis conditions. In some embodiments, R is benzyl and R is removed from isoxazoles of structure 12-V under hydrogenation conditions (e.g. H2, Pd/C). A Curtius rearrangement of carboxylic acids of structure 12-VI in the presence of hydroxy compounds CY-CH(R8)-0H provides carbamate compounds of structure 12-VII.
[00412] In any of the schemes,
can be replaced with other cyclic groups as described herein.
[00413] Representative synthetic procedures for compounds of Formula (I), (II), (III), (IV) and (V) are outlined in the Examples. [00414] Throughout the specification, groups and substituents thereof are chosen by one skilled in the field to provide stable moieties and compounds.
[00415] A detailed description of techniques applicable to the creation of protecting groups and their removal are described in Greene and Wuts, Protective Groups in Organic Synthesis, 3rd Ed., John Wiley & Sons, New York, NY, 1999, and Kocienski, Protective Groups, Thieme Verlag, New York, NY, 1994, which are incorporated herein by reference for such disclosure. Further Forms of Compounds
[00416] In one aspect, the compound of Formula (I), (II), (III), (IV) or (V) possesses one or more stereocenters and each stereocenter exists independently in either the R or S configuration. The compounds presented herein include all diastereomeric, enantiomeric, and epimeric forms as well as the appropriate mixtures thereof. The compounds and methods provided herein include all cis, trans, syn, anti, entgegen (E), and zusammen (Z) isomers as well as the appropriate mixtures thereof. In certain embodiments, compounds of Formula (I), (II), (III), (IV) and (V) are prepared as their individual stereoisomers by reacting a racemic mixture of the compound with an optically active resolving agent to form a pair of diastereoisomeric compounds/salts, separating the diastereomers and recovering the optically pure enantiomers. In some embodiments, resolution of enantiomers is carried out using covalent diastereomeric derivatives of the compounds described herein. In another embodiment, diastereomers are seprated by separation/resolution techniques based upon differences in solubility. In other embodiments, separation of steroisomers is performed by chromatography or by the forming diastereomeric salts and separation by recrystallization, or chromatography, or any combination thereof. Jean Jacques, Andre Collet, Samuel H. Wilen, "Enantiomers, Racemates and Resolutions", John Wiley And Sons, Inc., 1981. In one aspect, stereoisomers are obtained by stereoselective synthesis. [00417] The methods and compositions described herein include the use of amorphous forms as well as crystalline forms (also known as polymorphs). In one aspect, compounds described herein are in the form of pharmaceutically acceptable salts. As well, active metabolites of these compounds having the same type of activity are included in the scope of the present disclosure.
In addition, the compounds described herein can exist in unsolvated as well as solvated forms with pharmaceutically acceptable solvents such as water, ethanol, and the like. The solvated forms of the compounds presented herein are also considered to be disclosed herein. [00418] In some embodiments, compounds described herein are prepared as prodrugs. A "prodrug" refers to an agent that is converted into the parent drug in vivo. Prodrugs are often useful because, in some situations, they may be easier to administer than the parent drug. They may, for instance, be bioavailable by oral administration whereas the parent is not. The prodrug may also have improved solubility in pharmaceutical compositions over the parent drug. In some embodiments, the design of a prodrug increases the effective water solubility. An example, without limitation, of a prodrug is a compound described herein, which is administered as an ester (the "prodrug") to facilitate transmittal across a cell membrane where water solubility is detrimental to mobility but which then is metabolically hydrolyzed to the carboxylic acid, the active entity, once inside the cell where water-solubility is beneficial. A further example of a prodrug might be a short peptide (polyaminoacid) bonded to an acid group where the peptide is metabolized to reveal the active moiety. In certain embodiments, upon in vivo administration, a prodrug is chemically converted to the biologically, pharmaceutically or therapeutically active form of the compound. In certain embodiments, a prodrug is enzymatically metabolized by one or more steps or processes to the biologically, pharmaceutically or therapeutically active form of the compound. [00419] In one aspect, prodrugs are designed to alter the metabolic stability or the transport characteristics of a drug, to mask side effects or toxicity, to improve the flavor of a drug or to alter other characteristics or properties of a drug. By virtue of knowledge of pharmacokinetic, pharmacodynamic processes and drug metabolism in vivo, once a pharmaceutically active compound is known, the design prodrugs of the compound is possible, (see, for example, Nogrady (1985) Medicinal Chemistry A Biochemical Approach, Oxford University Press, New York, pages 388-392; Silverman (1992), The Organic Chemistry of Drug Design and Drug Action, Academic Press, Inc., San Diego, pages 352-401, Rooseboom et ah, Pharmacological Reviews, 56:53-102, 2004; Aesop Cho, "Recent Advances in Oral Prodrug Discovery", Annual Reports in Medicinal Chemistry, Vol. 41, 395-407, 2006; T. Higuchi and V. Stella, Pro-drugs as Novel Delivery Systems, Vol. 14 of the A. C. S. Symposium Series).
[00420] Prodrug forms of the herein described compounds, wherein the prodrug is metabolized in vivo to produce a compound of Formula (I), (II), (III), (IV) or (V) as set forth herein are included within the scope of the claims. In some cases, some of the herein-described compounds may be a prodrug for another derivative or active compound.
[00421] In some embodiments, sites on the aromatic ring portion of compounds of Formula (I), (II), (III), (IV) or (V) are susceptible to various metabolic reactions Therefore incorporation of appropriate substituents on the aromatic ring structures will reduce, minimize or eliminate this metabolic pathway. In specific embodiments, the appropriate substituent to decrease or eliminate the susceptibility of the aromatic ring to metabolic reactions is, by way of example only, a halogen, or an alkyl group.
[00422] In another embodiment, the compounds described herein are labeled isotopically (e.g. with a radioisotope) or by another other means, including, but not limited to, the use of chromophores or fluorescent moieties, bioluminescent labels, or chemiluminescent labels. [00423] Compounds described herein include isotopically-labeled compounds, which are identical to those recited in the various formulae and structures presented herein, but for the fact that one or more atoms are replaced by an atom having an atomic mass or mass number different from the atomic mass or mass number usually found in nature. Examples of isotopes that can be incorporated into the present compounds include isotopes of hydrogen, carbon, nitrogen, oxygen, fluorine and chlorine, such as, for example, 2H, 3H, 13C, 14C, 15N, 180, 17O,
35S, 18F, 36Cl. In one aspect, isotopically-labeled compounds described herein, for example those into which radioactive isotopes such as 3H and 14C are incorporated, are useful in drug and/or substrate tissue distribution assays. In one aspect, substitution with isotopes such as deuterium affords certain therapeutic advantages resulting from greater metabolic stability, such as, for example, increased in vivo half-life or reduced dosage requirements.
[00424] In additional or further embodiments, the compounds described herein are metabolized upon administration to an organism in need to produce a metabolite that is then used to produce a desired effect, including a desired therapeutic effect. [00425] "Pharmaceutically acceptable," as used herein, refers a material, such as a carrier or diluent, which does not abrogate the biological activity or properties of the compound, and is relatively nontoxic, i.e., the material may be administered to an individual without causing undesirable biological effects or interacting in a deleterious manner with any of the components of the composition in which it is contained. [00426] The term "pharmaceutically acceptable salt" refers to a formulation of a compound that does not cause significant irritation to an organism to which it is administered and does not abrogate the biological activity and properties of the compound. In some embodiments, pharmaceutically acceptable salts are obtained by reacting a compound of Formula (I), (II), (III), (IV) or (V) with acids. Pharmaceutically acceptable salts are also obtained by reacting a compound of Formula (I), (II), (III), (IV) or (V) with a base to form a salt.
[00427] Compounds described herein may be formed as, and/or used as, pharmaceutically acceptable salts. The type of pharmaceutical acceptable salts, include, but are not limited to: (1) acid addition salts, formed by reacting the free base form of the compound with a pharmaceutically acceptable: inorganic acid, such as, for example, hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid, metaphosphoric acid, and the like; or with an organic acid, such as, for example, acetic acid, propionic acid, hexanoic acid, cyclopentanepropionic acid, glycolic acid, pyruvic acid, lactic acid, malonic acid, succinic acid, malic acid, maleic acid, fumaric acid, trifluoroacetic acid, tartaric acid, citric acid, benzoic acid, 3-(4-hydroxybenzoyl)benzoic acid, cinnamic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, 1 ,2-ethanedisulfonic acid, 2-hydroxyethanesulfonic acid, benzenesulfonic acid, toluenesulfonic acid, 2-naphthalenesulfonic acid, 4-methylbicyclo-[2.2.2]oct-2-ene-l- carboxylic acid, glucoheptonic acid, 4,4'-methylenebis-(3-hydroxy-2-ene-l-carboxylic acid), 3- phenylpropionic acid, trimethylacetic acid, tertiary butylacetic acid, lauryl sulfuric acid, gluconic acid, glutamic acid, hydroxynaphthoic acid, salicylic acid, stearic acid, muconic acid, butyric acid, phenylacetic acid, phenylbutyric acid, valproic acid, and the like; (2) salts formed when an acidic proton present in the parent compound is replaced by a metal ion, e.g., an alkali metal ion (e.g. lithium, sodium, potassium), an alkaline earth ion (e.g. magnesium, or calcium), or an aluminum ion. In some embodiments, where the compound of Formula (I), (II), (III), (IV) or (V) has an acidic proton, a sodium salt of the compound of Formula (I), (II), (III), (IV) or (V) is formed. In some cases, compounds described herein may coordinate with an organic base, such as, but not limited to, ethanolamine, diethanolamine, triethanolamine, tromethamine, N- methylglucamine, dicyclohexylamine, tris(hydroxymethyl)methylamine. In other cases, compounds described herein may form salts with amino acids such as, but not limited to, arginine, lysine, and the like. Acceptable inorganic bases used to form salts with compounds that include an acidic proton, include, but are not limited to, aluminum hydroxide, calcium hydroxide, potassium hydroxide, sodium carbonate, sodium hydroxide, and the like. [00428] It should be understood that a reference to a pharmaceutically acceptable salt includes the solvent addition forms or crystal forms thereof, particularly solvates or polymorphs. Solvates contain either stoichiometric or non-stoichiometric amounts of a solvent, and may be formed during the process of crystallization with pharmaceutically acceptable solvents such as water, ethanol, and the like. Hydrates are formed when the solvent is water, or alcoholates are formed when the solvent is alcohol. Solvates of compounds described herein can be conveniently prepared or formed during the processes described herein. In addition, the compounds provided herein can exist in unsolvated as well as solvated forms. In general, the
solvated forms are considered equivalent to the unsolvated forms for the purposes of the compounds and methods provided herein.
[00429] Compounds described herein, such as compounds of Formula (I), (II), (III), (IV) and (V), may be in various forms, including but not limited to, amorphous forms, milled forms and nano-particulate forms. In addition, compounds described herein include crystalline forms, also known as polymorphs. Polymorphs include the different crystal packing arrangements of the same elemental composition of a compound. Polymorphs usually have different X-ray diffraction patterns, melting points, density, hardness, crystal shape, optical properties, stability, and solubility. Various factors such as the recrystallization solvent, rate of crystallization, and storage temperature may cause a single crystal form to dominate.
[00430] Throughout the specification, groups and substituents thereof can be chosen by one skilled in the field to provide stable moieties and compounds.
Certain Terminology
[00431] Unless otherwise stated, the following terms used in this application, including the specification and claims, have the definitions given below. It must be noted that, as used in the specification and the appended claims, the singular forms "a," "an" and "the" include plural referents unless the context clearly dictates otherwise. Unless otherwise indicated, conventional methods of mass spectroscopy, NMR, HPLC, protein chemistry, biochemistry, recombinant DNA techniques and pharmacology are employed. In this application, the use of "or" or "and" means "and/or" unless stated otherwise. Furthermore, use of the term "including" as well as other forms, such as "include", "includes," and "included," is not limiting. The section headings used herein are for organizational purposes only and are not to be construed as limiting the subject matter described. [00432] An "alkyl" group refers to an aliphatic hydrocarbon group. The alkyl group may be a saturated alkyl group (which means that it does not contain any carbon-carbon double bonds or carbon-carbon triple bonds) or the the alkyl group may be an unsaturated alkyl group (which means that it contains at least one carbon-carbon double bonds or carbon-carbon triple bond). The alkyl moiety, whether saturated or unsaturated, may be branched or straight chain. [00433] The "alkyl" group may have 1 to 10 carbon atoms (whenever it appears herein, a numerical range such as "1 to 10" refers to each integer in the given range; e.g., "1 to 10 carbon atoms" means that the alkyl group may consist of 1 carbon atom, 2 carbon atoms, 3 carbon atoms, etc. , up to and including 10 carbon atoms, although the present definition also covers the occurrence of the term "alkyl" where no numerical range is designated). The alkyl group of the compounds described herein may be designated as "Ci-C6 alkyl" or similar designations. By way of example only, "Ci-C6 alkyl" indicates that there are one, two, three, four, five, or six
carbon atoms in the alkyl chain. In one aspect the alkyl is selected from the group consisting of methyl, ethyl, propyl, iso-propyl, n-butyl, iso-butyl, sec-butyl, and t-butyl. Typical alkyl groups include, but are in no way limited to, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tertiary butyl, pentyl, neopentyl, hexyl, allyl, but-2-enyl, but-3-enyl, and the like. In one aspect, an alkyl is a CrC6alkyl.
[00434] The term "alkyl ene" refers to a divalent alkyl radical. Any of the above mentioned monovalent alkyl groups may be an alkyl ene by abstraction of a second hydrogen atom from the alkyl. In one aspect, an alkelene is a Ci-Cealkylene. In another apsect, an alkylene is a C1- C4alkylene. Typical alkylene groups include, but are not limited to, -CH2-, -CH(CH3)-, - C(CHs)2-, -CH2CH2-, -CH2CH(CH3)-, -CH2C(CH3)2-, -CH2CH2CH2-, -CH2CH2CH2CH2-, and the like.
[00435] An "alkoxy" group refers to a (alkyl)O- group, where alkyl is as defined herein. [00436] The term "alkylamine" refers to the -N(alkyl)xHy group, where x and y are selected from the group x=l, y=l and x=2, y=0. In some embodiments, when x=2 and y=0, the alkyl groups taken together with the ntrogen atom to which they are attached form a cyclic ring system.
[00437] The term "aromatic" refers to a planar ring having a delocalized π-electron system containing 4n+2 π electrons, where n is an integer. Aromatic rings can be formed from five, six, seven, eight, nine, ten, or more than ten atoms. Aromatics are optionally substituted. The term "aromatic" includes both carbocyclic aryl ("aryl", e.g., phenyl) and heterocyclic aryl (or "heteroaryl" or "heteroaromatic") groups (e.g., pyridine). The term includes monocyclic or fused-ring polycyclic (i.e., rings which share adjacent pairs of carbon atoms) groups. [00438] The term "carbocyclic" or "carbocycle" refers to a ring or ring system where the atoms forming the backbone of the ring are all carbon atoms. The term thus distinguishes carbocyclic from heterocyclic rings in which the ring backbone contains at least one atom which is different from carbon.
[00439] As used herein, the term "aryl" refers to an aromatic ring wherein each of the atoms forming the ring is a carbon atom. Aryl rings are formed by five, six, seven, eight, nine, or more than nine carbon atoms. Aryl groups are optionally substituted. In one aspect, an aryl is a phenyl or a naphthalenyl. In one aspect, an aryl is a phenyl. In one aspect, an aryl is a C6-Cioaryl.
Depending on the structure, an aryl group can be a monoradical or a diradical (i.e., an arylene group). Examplary arylenes include, but are not limited to, phenyl- 1,2-ene, phenyl- 1,3 -ene, and phenyl- 1,4-ene. [00440] The term "cycloalkyl" refers to a monocyclic or polycyclic aliphatic, non-aromatic radical, wherein each of the atoms forming the ring (i.e. skeletal atoms) is a carbon atom.
Cycloalkyls may be saturated, or partially unsaturated. Cycloalkyls may be fused with an aromatic ring, and the point of attachment is at a carbon that is not an aromatic ring carbon atom. Cycloalkyl groups include groups having from 3 to 10 ring atoms. In some embodiments, cycloalkyl groups are selected from among cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cycloheptyl, and cyclooctyl. Cycloalkyl groups may be substituted or unsubstituted. Depending on the structure, a cycloalkyl group can be a monoradical or a diradical (i.e., an cycloalkylene group, such as, but not limited to, cyclopropan-l,l-diyl, cyclobutan-l,l-diyl, cyclopentan-l,l-diyl, cyclohexan-l,l-diyl, cyclohexan-l,4-diyl, cycloheptan-l,l-diyl, and the like). In one aspect, a cycloalkyl is a C3-Cecycloalkyl. [00441] The term "halo" or, alternatively, "halogen" or "halide" means fluoro, chloro, bromo or iodo.
[00442] The term "haloalkyl" refers to an alkyl group in which one or more hydrogen atoms are replaced by one or more halide atoms. In one aspect, a haloalkyl is a Ci-C4haloalkyl. [00443] The term "haloalkylene" refers to an alkylene group in which one or more hydrogen atoms are replaced by one or more halide atoms. In one aspect, a haloalkylene is a C1- Cβhaloalkylene. In another aspect, a haloalkylene is a Ci-C4haloalkylene. [00444] The term "fluoroalkyl" refers to an alkyl in which one or more hydrogen atoms are replaced by a fluorine atom. In one aspect, a fluoralkyl is a Ci-C4iluoroalkyl. [00445] The term "fluoroalkylene" refers to an alkylene in which one or more hydrogen atoms are replaced by a fluorine atom. In one aspect, a fluoralkylene is a Ci-Cefluoroalkylene. In another aspect, a fluoralkylene is a Ci-C4iluoroalkylene.
[00446] The term "heteroalkyl" refers to an alkyl group in which one or more skeletal atoms of the alkyl are selected from an atom other than carbon, e.g., oxygen, nitrogen, sulfur, phosphorus or combinations thereof. In one aspect, a heteroalkyl is a Ci-Ceheteroalkyl. [00447] The term "heteroalkylene" refers to an alkylene group in which one or more skeletal atoms of the alkyl are selected from an atom other than carbon, e.g., oxygen, nitrogen, sulfur, phosphorus or combinations thereof. In one aspect, a heteroalkylene is a Ci-Ceheteroalkylene. In another aspect, a heteroalkylene is a Ci-C4heteroalkylene. Examplary heteroalkylenes include, but are not limited to, -OCH2-, -OCH(CH3)-, -OC(CH3)2-, -OCH2CH2-, -CH2O-, - CH(CH3)O-, -C(CHs)2O-, -CH2CH2O-, -CH2OCH2-, -CH2OCH2CH2-, -CH2CH2OCH2-, -SCH2-, -SCH(CH3)-, -SC(CHs)2-, -SCH2CH2-, -CH2S-, -CH(CH3)S-, -C(CH3)2S-, -CH2CH2S-, - CH2SCH2-, -CH2SCH2CH2-, -CH2CH2SCH2-, -SO2CH2-, -SO2CH(CH3)-, -SO2C(CH3)2-, - SO2CH2CH2-, -CH2SO2-, -CH(CH3)SO2-, -C(CH3)2SO2-, -CH2CH2SO2-, -CH2SO2CH2-, - CH2SO2CH2CH2-, -CH2CH2SO2CH2-, -NHCH2-, -NHCH(CH3)-, -NHC(CH3)2-, -NHCH2CH2-, -
CH2NH-, -CH(CH3)NH-, -C(CH3)2NH-, -CH2CH2NH-, -CH2NHCH2-, -CH2NHCH2CH2-, - CH2CH2NHCH2-, and the like.
[00448] The term "heterocycle" or "heterocyclic" refers to heteroaromatic rings (also known as heteroaryls) and heterocycloalkyl rings (also known as heteroalicyclic groups) containing one to four heteroatoms in the ring(s), where each heteroatom in the ring(s) is selected from O, S and N, wherein each heterocyclic group has from 4 to 10 atoms in its ring system, and with the proviso that the any ring does not contain two adjacent O or S atoms. Non-aromatic heterocyclic groups (also known as heterocycloalkyls) include groups having only 3 atoms in their ring system, but aromatic heterocyclic groups must have at least 5 atoms in their ring system. The heterocyclic groups include benzo-fused ring systems. An example of a 3-membered heterocyclic group is aziridinyl. An example of a 4-membered heterocyclic group is azetidinyl. An example of a 5-membered heterocyclic group is thiazolyl. An example of a 6-membered heterocyclic group is pyridyl, and an example of a 10-membered heterocyclic group is quinolinyl. Examples of non-aromatic heterocyclic groups are pyrrolidinyl, tetrahydrofuranyl, dihydrofuranyl, tetrahydrothienyl, oxazolidinonyl, tetrahydropyranyl, dihydropyranyl, tetrahydrothiopyranyl, piperidinyl, morpholinyl, thiomorpholinyl, thioxanyl, piperazinyl, aziridinyl, azetidinyl, oxetanyl, thietanyl, homopiperidinyl, oxepanyl, thiepanyl, oxazepinyl, diazepinyl, thiazepinyl, 1,2,3,6-tetrahydropyridinyl, pyrrolin-2-yl, pyrrolin-3-yl, indolinyl, 2H- pyranyl, 4H-pyranyl, dioxanyl, 1,3-dioxolanyl, pyrazolinyl, dithianyl, dithiolanyl, dihydropyranyl, dihydrothienyl, dihydrofuranyl, pyrazolidinyl, imidazolinyl, imidazolidinyl, 3- azabicyclo[3.1.0]hexanyl, 3-azabicyclo[4.1.0]heptanyl, 3H-indolyl and quinolizinyl. Examples of aromatic heterocyclic groups are pyridinyl, imidazolyl, pyrimidinyl, pyrazolyl, triazolyl, pyrazinyl, tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl, pyrrolyl, quinolinyl, isoquinolinyl, indolyl, benzimidazolyl, benzofuranyl, cinnolinyl, indazolyl, indolizinyl, phthalazinyl, pyridazinyl, triazinyl, isoindolyl, pteridinyl, purinyl, oxadiazolyl, thiadiazolyl, furazanyl, benzofurazanyl, benzothiophenyl, benzothiazolyl, benzoxazolyl, quinazolinyl, quinoxalinyl, naphthyridinyl, and furopyridinyl. The foregoing groups may be C- attached or N-attached where such is possible. For instance, a group derived from pyrrole may be pyrrol- 1-yl (N-attached) or pyrrol-3-yl (C-attached). Further, a group derived from imidazole may be imidazol-1-yl or imidazol-3-yl (both N-attached) or imidazol-2-yl, imidazol-4-yl or imidazol-5-yl (all C-attached). The heterocyclic groups include benzo-fused ring systems. Νon- aromatic heterocycles may ber substituted with one or two oxo (=0) moieties, such as pyrrolidin-2-one.
[00449] The terms "heteroaryl" or, alternatively, "heteroaromatic" refers to an aryl group that includes one or more ring heteroatoms selected from nitrogen, oxygen and sulfur. Illustrative examples of heteroaryl groups include the following moieties:
and the like. Monocyclic heteroaryls include pyridinyl, imidazolyl, pyrimidinyl, pyrazolyl, triazolyl, pyrazinyl, tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl, pyrrolyl, pyridazinyl, triazinyl, oxadiazolyl, thiadiazolyl, and furazanyl. In one aspect, a heteroaryl contains 0-3 N atoms. In another aspect, a heteroaryl contains 1-3 N atoms. In another aspect, a heteroaryl contains 0-3 N atoms, 0-1 O atoms, and 0-1 S atoms. In another aspect, a heteroaryl is a monocyclic or bicyclic heteroaryl. In one aspect, heteroaryl is a Ci-Cgheteroaryl. In one aspect, monocyclic heteroaryl is a Ci-Csheteroaryl. In one aspect, monocyclic heteroaryl is a 5-membered or 6-membered heteroaryl. In one aspect, bicyclic heteroaryl is a Ce-Cgheteroaryl. Depending on the structure, a heteroaryl group can be a monoradical or a diradical (i.e., a heteroarylene group). [00450] A "heterocycloalkyl" or "heteroalicyclic" group refers to a cycloalkyl group that includes at least one heteroatom selected from nitrogen, oxygen and sulfur. The radicals may be fused with an aryl or heteroaryl. Illustrative examples of heterocycloalkyl groups, also referred to as non-aromatic heterocycles, include:
and the like. In some embodiments, the heterocycloalkyl is selected from oxazolidinonyl, pyrrolidinyl, tetrahydrofuranyl, tetrahydrothienyl, tetrahydropyranyl, tetrahydrothiopyranyl, piperidinyl, morpholinyl, thiomorpholinyl, piperazinyl, and indolinyl. The term heteroalicyclic also includes all ring forms of the carbohydrates, including but not limited to the monosaccharides, the disaccharides and the oligosaccharides. In one aspect, a heterocycloalkyl is a C2-Cioheterocycloalkyl. In another aspect, a heterocycloalkyl is a C4-Cioheterocycloalkyl. In one aspect, a heterocycloalkyl contains 0-2 N atoms. In another aspect, a heterocycloalkyl contains 0-2 N atoms, 0-2 O atoms or 0-1 S atoms. [00451] The term "bond" or "single bond" refers to a chemical bond between two atoms, or two moieties when the atoms joined by the bond are considered to be part of larger substructure. In one aspect, when a group described herein is a bond, the referenced group is absent thereby allowing a bond to be formed between the remaining identified groups. [00452] The term "membered ring" includes any cyclic structure. The term "membered" is meant to denote the number of skeletal atoms that constitute the ring. Thus, for example, cyclohexyl, pyridinyl, pyranyl and thiopyranyl are 6-membered rings and cyclopentyl, pyrrolyl, furanyl, and thienyl are 5-membered rings.
[00453] The term "moiety" refers to a specific segment or functional group of a molecule. Chemical moieties are often recognized chemical entities embedded in or appended to a molecule.
[00454] As used herein, "carboxylic acid bioisostere" refers to a functional group or moiety that exhibits similar physical, biological and/or chemical properties as a carboxylic acid moiety. Examples of carboxylic acid bioisosteres include, but are not limited to,
[00455] The term "optionally substituted" or "substituted" means that the referenced group may be substituted with one or more additional group(s) individually and independently selected from alkyl, cycloalkyl, aryl, heteroaryl, heteroalicyclic, hydroxy, alkoxy, aryloxy, alkylthio,
arylthio, alkylsulfoxide, arylsulfoxide, alkylsulfone, arylsulfone, cyano, halo, nitro, haloalkyl, fluoroalkyl, fluoroalkoxy, and amino, including mono- and di-substituted amino groups, and the protected derivatives thereof. By way of example an optional substituents may be halide, -CN, - NO2, or LSRS, wherein each Ls is independently selected from a bond, -O-, -C(=O)-, -C(=O)O-, - S-, -S(=O)-, -S(=O)2-, -NH-, -NHC(=O)-, -C(=O)NH-, S(=O)2NH-, -NHS(=O)2, -OC(=O)NH-, -NHC(=O)O-, or -(Ci-Cβ alkylene)-; and each Rs is selected from H, alkyl, fluoroalkyl, heteroalkyl, cycloalkyl, aryl, heteroaryl, or heterocycloalkyl. The protecting groups that may form the protective derivatives of the above substituents may be found in sources such as Greene and Wuts, above. In some embodiments, optional substituents are selected from halogen, -CN, -NH2, -OH, -N(CH3)2, alkyl, fluoroalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, alkoxy, aryloxy, alkylthio, arylthio, alkylsulfoxide, arylsulfoxide, alkylsulfone, and arylsulfone. In some embodiments, an optional substituent is halogen, -CN, -NH2, -OH, -NH(CH3), -N(CH3)2, -CO2H, -C02alkyl, -C(=O)NH2, - C(=O)NHalkyl, -C(=O)N(alkyl)2, -S(=O)2NH2, -S(=O)2NH(alkyl), -S(=O)2N(alkyl)2, alkyl, cycloalkyl, fluoroalkyl, heteroalkyl, alkoxy, fluoroalkoxy, -S-alkyl, or -S(=O)2alkyl. In some embodiments, an optional substituent is selected from halogen, -CN, -NH2, -OH, -NH(CH3), - N(CH3)2, -CH3, -CH2CH3, -CF3, -OCH3, and -OCF3. In some embodiments, substituted groups are substituted with one or two of the preceding groups. In some embodiments, substituted groups are substituted with one of the preceding groups. In some embodiments, an optional substituent on an aliphatic carbon atom (acyclic or cyclic, saturated or unsaturated carbon atoms, excluding aromatic carbon atoms) includes oxo (=0).
[00456] As used herein, "4-substituted {4-[l-(2-Rc-phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl} -benzene compound" refers to the following structure:
,, w whiieerree t mhee p poυiiniiti o υfi a atntaachment of the 4-substitutent is denoted by "1^ , [00457] As used herein, "3 -substituted {4-[l-(2-R c -phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl} -benzene compound" refers to the following structure:
by ^" . [00458] "4-Substituted {4-[ 1 -(CY)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -benzene compound" refers to the following structure:
, where the point of attachment of the 4-substitutent is denoted by ^" .
[00459] "3 -Substituted {4-[ 1 -(CY)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -benzene compound" refers to the following structure:
, where the point of attachment of the 3-substitutent is denoted by ^" .
[00460] In certain embodiments, the compounds presented herein possess one or more stereocenters and each center independently exists in either the R or S configuration. The compounds presented herein include all diastereomeric, enantiomeric, and epimeric forms as well as the appropriate mixtures thereof. Stereoisomers are obtained, if desired, by methods such as, stereoselective synthesis and/or the separation of stereoisomers by chiral chromatographic columns. [00461] The methods and formulations described herein include the use of N-oxides (if appropriate), crystalline forms (also known as polymorphs), or pharmaceutically acceptable salts of compounds having the structure of Formula (I), (II), (III), (IV) or (V), as well as active metabolites of these compounds having the same type of activity. In some situations, compounds may exist as tautomers. All tautomers are included within the scope of the compounds presented herein. In specific embodiments, the compounds described herein exist in solvated forms with pharmaceutically acceptable solvents such as water, ethanol, and the like. In other embodiments, the compounds described herein exist in unsolvated form.
[00462] The term "acceptable" with respect to a formulation, composition or ingredient, as used herein, means having no persistent detrimental effect on the general health of the subject being treated.
[00463] The term "modulate," as used herein, means to interact with a target either directly or indirectly so as to alter the activity of the target, including, by way of example only, to enhance the activity of the target, to inhibit the activity of the target, to limit the activity of the target, or to extend the activity of the target.
[00464] The term "modulator," as used herein, refers to a molecule that interacts with a target either directly or indirectly. The interactions include, but are not limited to, the interactions of an agonist, partial agonist, an inverse agonist and antagonist. In one embodiment, a modulator is an antagonist.
[00465] The term "agonist," as used herein, refers to a molecule such as a compound, a drug, an enzyme activator or a hormone modulator that binds to a specific receptor and triggers a response in the cell. An agonist mimics the action of an endogenous ligand (such as LPA, prostaglandin, hormone or neurotransmitter) that binds to the same receptor.
[00466] The term "antagonist," as used herein, refers to a molecule such as a compound, which diminishes, inhibits, or prevents the action of another molecule or the activity of a receptor site. Antagonists include, but are not limited to, competitive antagonists, non-competitive antagonists, uncompetitive antagonists, partial agonists and inverse agonists. [00467] The term "LPA-dependent", as used herein, refers to conditions or disorders that would not occur, or would not occur to the same extent, in the absence of LPA. [00468] The term "LPA-mediated", as used herein, refers to conditions or disorders that might occur in the absence of LPA but can occur in the presence of LPA. [00469] "Selectivity" for one LPA receptor versus other LPA receptors means that the compound has an IC50 (Ca Flux assay) for the indicated LPA receptor that is at least 10-fold less than the IC50 for other LPA receptors. In some embodiments, selectivity for one LPA receptor versus other LPA receptor means that the compound has an IC50 for the indicated LPA receptor that is at least 10-fold, at least 20-fold, at least 40-fold, at least 50-fold, at least 100-fold, at least 200-fold, at least 500-fold, or at least 1000-fold, less than the IC50 for other LPA receptors. For example, a selective LPAi receptor antagonist has an IC50 that is at least 10-fold, at least 20- fold, at least 40-fold, at least 50-fold, at least 100-fold, at least 200-fold, at least 500-fold, or at least 1000-fold, less than the IC50 for other LPA receptors (e.g. LPA2, LPA3). [00470] The terms "co-administration" or the like, as used herein, are meant to encompass administration of the selected therapeutic agents to a single patient, and are intended to include
treatment regimens in which the agents are administered by the same or different route of administration or at the same or different time.
[00471] The terms "effective amount" or "therapeutically effective amount," as used herein, refer to a sufficient amount of an agent or a compound being administered which will relieve to some extent one or more of the symptoms of the disease or condition being treated. The result can be reduction and/or alleviation of the signs, symptoms, or causes of a disease, or any other desired alteration of a biological system. For example, an "effective amount" for therapeutic uses is the amount of the composition comprising a compound as disclosed herein required to provide a clinically significant decrease in disease symptoms. An appropriate "effective" amount in any individual case may be determined using techniques, such as a dose escalation study.
[00472] The terms "enhance" or "enhancing," as used herein, means to increase or prolong either in potency or duration a desired effect. Thus, in regard to enhancing the effect of therapeutic agents, the term "enhancing" refers to the ability to increase or prolong, either in potency or duration, the effect of other therapeutic agents on a system. An "enhancing-effective amount," as used herein, refers to an amount adequate to enhance the effect of another therapeutic agent in a desired system.
[00473] The terms "kit" and "article of manufacture" are used as synonyms. [00474] A "metabolite" of a compound disclosed herein is a derivative of that compound that is formed when the compound is metabolized. The term "active metabolite" refers to a biologically active derivative of a compound that is formed when the compound is metabolized. The term "metabolized," as used herein, refers to the sum of the processes (including, but not limited to, hydrolysis reactions and reactions catalyzed by enzymes) by which a particular substance is changed by an organism. Thus, enzymes may produce specific structural alterations to a compound. For example, cytochrome P450 catalyzes a variety of oxidative and reductive reactions while uridine diphosphate glucuronyltransferases catalyze the transfer of an activated glucuronic-acid molecule to aromatic alcohols, aliphatic alcohols, carboxylic acids, amines and free sulphydryl groups. In some embodiments, carboxylic acid containing compounds form taurine conjugates in vivo. Metabolites of the compounds disclosed herein are optionally identified either by administration of compounds to a host and analysis of tissue samples from the host, or by incubation of compounds with hepatic cells in vitro and analysis of the resulting compounds.
[00475] The term "pharmaceutical combination" as used herein, means a product that results from the mixing or combining of more than one active ingredient and includes both fixed and non- fixed combinations of the active ingredients. The term "fixed combination" means that the
active ingredients, e.g. a compound of Formula (I), (II), (III), (IV) or (V) and a co-agent, are both administered to a patient simultaneously in the form of a single entity or dosage. The term "non- fixed combination" means that the active ingredients, e.g. a compound of Formula (I), (II), (III), (IV) or (V) and a co-agent, are administered to a patient as separate entities either simultaneously, concurrently or sequentially with no specific intervening time limits, wherein such administration provides effective levels of the two compounds in the body of the patient. The latter also applies to cocktail therapy, e.g. the administration of three or more active ingredients. [00476] The term "subject" or "patient" encompasses mammals and non-mammals. Examples of mammals include, but are not limited to, any member of the Mammalian class: humans, non- human primates such as chimpanzees, and other apes and monkey species; farm animals such as cattle, horses, sheep, goats, swine; domestic animals such as rabbits, dogs, and cats; laboratory animals including rodents, such as rats, mice and guinea pigs, and the like. In one embodiment, the mammal is a human. [00477] The terms "treat," "treating" or "treatment," as used herein, include alleviating, abating or ameliorating at least one symptom of a disease disease or condition, preventing additional symptoms, inhibiting the disease or condition, e.g., arresting the development of the disease or condition, relieving the disease or condition, causing regression of the disease or condition, relieving a condition caused by the disease or condition, or stopping the symptoms of the disease or condition either prophylactically and/or therapeutically. Routes of Administration
[00478] Suitable routes of administration include, but are not limited to, oral, intravenous, rectal, aerosol, parenteral, ophthalmic, pulmonary, transmucosal, transdermal, vaginal, otic, nasal, and topical administration. In addition, by way of example only, parenteral delivery includes intramuscular, subcutaneous, intravenous, intramedullary injections, as well as intrathecal, direct intraventricular, intraperitoneal, intralymphatic, and intranasal injections. [00479] In certain embodiments, a compound as described herein is administered in a local rather than systemic manner, for example, via injection of the compound directly into an organ, often in a depot preparation or sustained release formulation. In specific embodiments, long acting formulations are administered by implantation (for example subcutaneous Iy or intramuscularly) or by intramuscular injection. Furthermore, in other embodiments, the drug is delivered in a targeted drug delivery system, for example, in a liposome coated with organ-specific antibody. In such embodiments, the liposomes are targeted to and taken up selectively by the organ. In yet other embodiments, the compound as described herein is provided in the form of a rapid release formulation, in the form of an extended release
formulation, or in the form of an intermediate release formulation. In yet other embodiments, the compound described herein is administered topically. Pharmaceutical Compositions/Formulations
[00480] In some embodiments, the compounds described herein are formulated into pharmaceutical compositions. Pharmaceutical compositions are formulated in a conventional manner using one or more pharmaceutically acceptable inactive ingredients that facilitate processing of the active compounds into preparations that can be used pharmaceutically. Proper formulation is dependent upon the route of administration chosen. A summary of pharmaceutical compositions described herein can be found, for example, in Remington: The Science and Practice of Pharmacy, Nineteenth Ed (Easton, Pa.: Mack Publishing Company, 1995); Hoover, John E., Remington's Pharmaceutical Sciences, Mack Publishing Co., Easton, Pennsylvania 1975; Liberman, H.A. and Lachman, L., Eds., Pharmaceutical Dosage Forms, Marcel Decker, New York, N.Y., 1980; and Pharmaceutical Dosage Forms and Drug Delivery Systems, Seventh Ed. (Lippincott Williams & Wilkins 1999), herein incorporated by reference for such disclosure.
[00481] Provided herein are pharmaceutical compositions that include a compound of Formula (I), (II), (III), (IV) or (V) and at least one pharmaceutically acceptable inactive ingredient. In some embodiments, the compounds described herein are administered as pharmaceutical compositions in which a compound of Formula (I), (II), (III), (IV) or (V) is mixed with other active ingredients, as in combination therapy. In other embodiments, the pharmaceutical compositions include other medicinal or pharmaceutical agents, carriers, adjuvants, preserving, stabilizing, wetting or emulsifying agents, solution promoters, salts for regulating the osmotic pressure, and/or buffers. In yet other embodiments, the pharmaceutical compositions include other therapeutically valuable substances. [00482] A pharmaceutical composition, as used herein, refers to a mixture of a compound of Formula (I), (II), (III), (IV) or (V) with other chemical components (i.e. pharmaceutically acceptable inactive ingredients), such as carriers, excipients, binders, filling agents, suspending agents, flavoring agents, sweetening agents, disintegrating agents, dispersing agents, surfactants, lubricants, colorants, diluents, solubilizers, moistening agents, plasticizers, stabilizers, penetration enhancers, wetting agents, anti-foaming agents, antioxidants, preservatives, or one or more combination thereof. The pharmaceutical composition facilitates administration of the compound to an organism. In practicing the methods of treatment or use provided herein, therapeutically effective amounts of compounds described herein are administered in a pharmaceutical composition to a mammal having a disease, disorder, or condition to be treated. In some embodiments, the mammal is a human. A therapeutically effective amount can vary
widely depending on the severity of the disease, the age and relative health of the subject, the potency of the compound used and other factors. The compounds can be used singly or in combination with one or more therapeutic agents as components of mixtures. [00483] The pharmaceutical formulations described herein are administered to a subject by appropriate administration routes, including but not limited to, oral, parenteral (e.g., intravenous, subcutaneous, intramuscular), intranasal, buccal, topical, rectal, or transdermal administration routes. The pharmaceutical formulations described herein include, but are not limited to, aqueous liquid dispersions, self-emulsifying dispersions, solid solutions, liposomal dispersions, aerosols, solid dosage forms, powders, immediate release formulations, controlled release formulations, fast melt formulations, tablets, capsules, pills, delayed release formulations, extended release formulations, pulsatile release formulations, multiparticulate formulations, and mixed immediate and controlled release formulations. [00484] Pharmaceutical compositions including a compound of Formula (I), (II), (III), (IV) or (V) are manufactured in a conventional manner, such as, by way of example only, by means of conventional mixing, dissolving, granulating, dragee-making, levigating, emulsifying, encapsulating, entrapping or compression processes.
[00485] The pharmaceutical compositions will include at least one compound of Formula (I), (II), (III), (IV) or (V) as an active ingredient in free-acid or free-base form, or in a pharmaceutically acceptable salt form. In addition, the methods and pharmaceutical compositions described herein include the use of N-oxides (if appropriate), crystalline forms, amorphous phases, as well as active metabolites of these compounds having the same type of activity. In some embodiments, compounds described herein exist in unsolvated form or in solvated forms with pharmaceutically acceptable solvents such as water, ethanol, and the like. The solvated forms of the compounds presented herein are also considered to be disclosed herein.
[00486] In certain embodiments, compositions provided herein include one or more preservatives to inhibit microbial activity. Suitable preservatives include mercury-containing substances such as merfen and thiomersal; stabilized chlorine dioxide; and quaternary ammonium compounds such as benzalkonium chloride, cetyltrimethylammonium bromide and cetylpyridinium chloride.
[00487] In some embodiments, formulations described herein benefit from antioxidants, metal chelating agents, thiol containing compounds and other general stabilizing agents. Examples of such stabilizing agents, include, but are not limited to: (a) about 0.5% to about 2% w/v glycerol, (b) about 0.1% to about 1% w/v methionine, (c) about 0.1% to about 2% w/v monothioglycerol, (d) about 1 mM to about 10 mM EDTA, (e) about 0.01% to about 2% w/v ascorbic acid, (f)
0.003% to about 0.02% w/v polysorbate 80, (g) 0.001% to about 0.05% w/v. polysorbate 20, (h) arginine, (i) heparin, (j) dextran sulfate, (k) cyclodextrins, (1) pentosan polysulfate and other heparinoids, (m) divalent cations such as magnesium and zinc; or (n) combinations thereof. [00488] The pharmaceutical compositions described herein, which include a compound of Formula (I), (II), (III), (IV) or (V) are formulated into any suitable dosage form, including but not limited to, aqueous oral dispersions, liquids, gels, syrups, elixirs, slurries, suspensions, solid oral dosage forms, aerosols, controlled release formulations, fast melt formulations, effervescent formulations, lyophilized formulations, tablets, powders, pills, dragees, capsules, delayed release formulations, extended release formulations, pulsatile release formulations, multiparticulate formulations, and mixed immediate release and controlled release formulations. [00489] Pharmaceutical preparations for oral use are obtained by mixing one or more solid excipient with one or more of the compounds described herein, optionally grinding the resulting mixture, and processing the mixture of granules, after adding suitable auxiliaries, if desired, to obtain tablets or dragee cores. Suitable excipients include, for example, fillers such as sugars, including lactose, sucrose, mannitol, or sorbitol; cellulose preparations such as, for example, maize starch, wheat starch, rice starch, potato starch, gelatin, gum tragacanth, methylcellulose, microcrystalline cellulose, hydroxypropylmethylcellulose, sodium carboxymethylcellulose; or others such as: polyvinylpyrrolidone (PVP or povidone) or calcium phosphate. If desired, disintegrating agents are added, such as the cross-linked croscarmellose sodium, polyvinylpyrrolidone, agar, or alginic acid or a salt thereof such as sodium alginate. In some embodiments, dyestuffs or pigments are added to the tablets or dragee coatings for identification or to characterize different combinations of active compound doses.
[00490] Pharmaceutical preparations that are administered orally include push-fit capsules made of gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer, such as glycerol or sorbitol. The push-fit capsules contain the active ingredients in admixture with filler such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers. In soft capsules, the active compounds are dissolved or suspended in suitable liquids, such as fatty oils, liquid paraffin, or liquid polyethylene glycols. In some embodiments, stabilizers are added. [00491] All formulations for oral administration are in dosages suitable for such administration. [00492] In one aspect, solid oral soage forms are prepared by mixing a compound of Formula (I), (II), (III), (IV) or (V) with one or more of the following: antioxidants, flavoring agents, and carrier materials such as binders, suspending agents, disintegration agents, filling agents, surfactants, solubilizers, stabilizers, lubricants, wetting agents, and diluents.
[00493] In some embodiments, the solid dosage forms disclosed herein are in the form of a tablet, (including a suspension tablet, a fast-melt tablet, a bite-disintegration tablet, a rapid- disintegration tablet, an effervescent tablet, or a caplet), a pill, a powder, a capsule, solid dispersion, solid solution, bioerodible dosage form, controlled release formulations, pulsatile release dosage forms, multiparticulate dosage forms, beads, pellets, granules. In other embodiments, the pharmaceutical formulation is in the form of a powder. In still other embodiments, the pharmaceutical formulation is in the form of a tablet. In other embodiments, pharmaceutical formulations of the compound of Formula (I), (II), (III), (IV) or (V) is in the form of a capsule. [00494] In some embodiments, solid dosage forms, e.g., tablets, effervescent tablets, and capsules, are prepared by mixing particles of a compound of Formula (I), (II), (III), (IV) or (V) with one or more pharmaceutical excipients to form a bulk blend composition. The bulk blend is readily subdivided into equally effective unit dosage forms, such as tablets, pills, and capsules. In some embodiments, the individual unit dosages include film coatings. These formulations are manufactured by conventional formulation techniques.
[00495] Conventional formulation techniques include, e.g., one or a combination of methods: (1) dry mixing, (2) direct compression, (3) milling, (4) dry or non-aqueous granulation, (5) wet granulation, or (6) fusion. Other methods include, e.g., spray drying, pan coating, melt granulation, granulation, fluidized bed spray drying or coating (e.g., wurster coating), tangential coating, top spraying, tableting, extruding and the like.
[00496] Suitable carriers for use in the solid dosage forms described herein include, but are not limited to, acacia, gelatin, colloidal silicon dioxide, calcium glycerophosphate, calcium lactate, maltodextrin, glycerine, magnesium silicate, sodium caseinate, soy lecithin, sodium chloride, tricalcium phosphate, dipotassium phosphate, sodium stearoyl lactylate, carrageenan, monoglyceride, diglyceride, pregelatinized starch, hydroxypropylmethylcellulose, hydroxypropylmethylcellulose acetate stearate, sucrose, microcrystalline cellulose, lactose, mannitol and the like.
[00497] Suitable filling agents for use in the solid dosage forms described herein include, but are not limited to, lactose, calcium carbonate, calcium phosphate, dibasic calcium phosphate, calcium sulfate, microcrystalline cellulose, cellulose powder, dextrose, dextrates, dextran, starches, pregelatinized starch, hydroxypropylmethycellulose (HPMC), hydroxypropylmethycellulose phthalate, hydroxypropylmethylcellulose acetate stearate (HPMCAS), sucrose, xylitol, lactitol, mannitol, sorbitol, sodium chloride, polyethylene glycol, and the like.
[00498] Suitable disintegrants for use in the solid dosage forms described herein include, but are not limited to, natural starch such as corn starch or potato starch, a pregelatinized starch, or sodium starch glycolate, a cellulose such as methylcrystalline cellulose, methylcellulose, microcrystalline cellulose, croscarmellose, or a cross-linked cellulose, such as cross-linked sodium carboxymethylcellulose, cross-linked carboxymethylcellulose, or cross-linked croscarmellose, a cross-linked starch such as sodium starch glycolate, a cross-linked polymer such as crospovidone, a cross-linked polyvinylpyrrolidone, alginate such as alginic acid or a salt of alginic acid such as sodium alginate, a gum such as agar, guar, locust bean, Karaya, pectin, or tragacanth, sodium starch glycolate, bentonite, sodium lauryl sulfate, sodium lauryl sulfate in combination starch, and the like.
[00499] Binders impart cohesiveness to solid oral dosage form formulations: for powder filled capsule formulation, they aid in plug formation that can be filled into soft or hard shell capsules and for tablet formulation, they ensure the tablet remaining intact after compression and help assure blend uniformity prior to a compression or fill step. Materials suitable for use as binders in the solid dosage forms described herein include, but are not limited to, carboxymethylcellulose, methylcellulose, hydroxypropylmethylcellulose, hydroxypropylmethylcellulose acetate stearate, hydroxyethylcellulose, hydroxypropylcellulose, ethylcellulose, and microcrystalline cellulose, microcrystalline dextrose, amylose, magnesium aluminum silicate, polysaccharide acids, bentonites, gelatin, polyvinylpyrrolidone/vinyl acetate copolymer, crospovidone, povidone, starch, pregelatinized starch, tragacanth, dextrin, a sugar, such as sucrose, glucose, dextrose, molasses, mannitol, sorbitol, xylitol, lactose, a natural or synthetic gum such as acacia, tragacanth, ghatti gum, mucilage of isapol husks, starch, polyvinylpyrrolidone, larch arabogalactan, polyethylene glycol, waxes, sodium alginate, and the like. [00500] In general, binder levels of 20-70% are used in powder- filled gelatin capsule formulations. Binder usage level in tablet formulations varies whether direct compression, wet granulation, roller compaction, or usage of other excipients such as fillers which itself can act as moderate binder. Binder levels of up to 70% in tablet formulations is common. [00501] Suitable lubricants or glidants for use in the solid dosage forms described herein include, but are not limited to, stearic acid, calcium hydroxide, talc, corn starch, sodium stearyl fumerate, alkali-metal and alkaline earth metal salts, such as aluminum, calcium, magnesium, zinc, stearic acid, sodium stearates, magnesium stearate, zinc stearate, waxes, Stearowet®, boric acid, sodium benzoate, sodium acetate, sodium chloride, leucine, a polyethylene glycol or a methoxypolyethylene glycol such as Carbowax™, PEG 4000, PEG 5000, PEG 6000, propylene
glycol, sodium oleate, glyceryl behenate, glyceryl palmitostearate, glyceryl benzoate, magnesium or sodium lauryl sulfate, and the like.
[00502] Suitable diluents for use in the solid dosage forms described herein include, but are not limited to, sugars (including lactose, sucrose, and dextrose), polysaccharides (including dextrates and maltodextrin), polyols (including mannitol, xylitol, and sorbitol), cyclodextrins and the like.
[00503] Suitable wetting agents for use in the solid dosage forms described herein include, for example, oleic acid, glyceryl monostearate, sorbitan monooleate, sorbitan monolaurate, triethanolamine oleate, polyoxyethylene sorbitan monooleate, polyoxyethylene sorbitan monolaurate, quaternary ammonium compounds (e.g., Polyquat 10®), sodium oleate, sodium lauryl sulfate, magnesium stearate, sodium docusate, triacetin, vitamin E TPGS and the like. [00504] Suitable surfactants for use in the solid dosage forms described herein include, for example, sodium lauryl sulfate, sorbitan monooleate, polyoxyethylene sorbitan monooleate, polysorbates, polaxomers, bile salts, glyceryl monostearate, copolymers of ethylene oxide and propylene oxide, e.g., Pluronic® (BASF), and the like.
[00505] Suitable suspending agents for use in the solid dosage forms described here include, but are not limited to, polyvinylpyrrolidone, e.g., polyvinylpyrrolidone K12, polyvinylpyrrolidone Kl 7, polyvinylpyrrolidone K25, or polyvinylpyrrolidone K30, polyethylene glycol, e.g., the polyethylene glycol can have a molecular weight of about 300 to about 6000, or about 3350 to about 4000, or about 7000 to about 5400, vinyl pyrrolidone/vinyl acetate copolymer (S630), sodium carboxymethylcellulose, methylcellulose, hydroxy-propylmethylcellulose, polysorbate- 80, hydroxyethylcellulose, sodium alginate, gums, such as, e.g., gum tragacanth and gum acacia, guar gum, xanthans, including xanthan gum, sugars, cellulosics, such as, e.g., sodium carboxymethylcellulose, methylcellulose, sodium carboxymethylcellulose, hydroxypropylmethylcellulose, hydroxyethylcellulose, polysorbate-80, sodium alginate, polyethoxylated sorbitan monolaurate, polyethoxylated sorbitan monolaurate, povidone and the like.
[00506] Suitable antioxidants for use in the solid dosage forms described herein include, for example, e.g., butylated hydroxytoluene (BHT), sodium ascorbate, and tocopherol. [00507] It should be appreciated that there is considerable overlap between additives used in the solid dosage forms described herein. Thus, the above-listed additives should be taken as merely exemplary, and not limiting, of the types of additives that can be included in solid dosage forms of the pharmaceutical compositions described herein. The amounts of such additives can be readily determined by one skilled in the art, according to the particular properties desired.
[00508] Compressed tablets are solid dosage forms prepared by compacting the bulk blend of the formulations described above.
[00509] In various embodiments, tablets will include one or more flavoring agents. [00510] In other embodiments, the tablets will include a film surrounding the final compressed tablet. In some embodiments, the film coating can provide a delayed release of the compound of Formula (I), (II), (III), (IV) or (V) from the formulation. In other embodiments, the film coating aids in patient compliance (e.g., Opadry® coatings or sugar coating). Film coatings including Opadry® typically range from about 1% to about 3% of the tablet weight. [00511] A capsule may be prepared, for example, by placing the bulk blend of the formulation of the compound described above, inside of a capsule. In some embodiments, the formulations (non-aqueous suspensions and solutions) are placed in a soft gelatin capsule. In other embodiments, the formulations are placed in standard gelatin capsules or non-gelatin capsules such as capsules comprising HPMC. In other embodiments, the formulation is placed in a sprinkle capsule, wherein the capsule is swallowed whole or the capsule is opened and the contents sprinkled on food prior to eating.
[00512] In various embodiments, the particles of the compound of Formula (I), (II), (III), (IV) or (V) and one or more excipients are dry blended and compressed into a mass, such as a tablet, having a hardness sufficient to provide a pharmaceutical composition that substantially disintegrates within less than about 30 minutes, less than about 35 minutes, less than about 40 minutes, less than about 45 minutes, less than about 50 minutes, less than about 55 minutes, or less than about 60 minutes, after oral administration, thereby releasing the formulation into the gastrointestinal fluid.
[00513] In other embodiments, a powder including a compound of Formula (I), (II), (III), (IV) or (V) is formulated to include one or more pharmaceutical excipients and flavors. Such a powder is prepared, for example, by mixing the the compound of Formula (I), (II), (III), (IV) or (V) and optional pharmaceutical excipients to form a bulk blend composition. Additional embodiments also include a suspending agent and/or a wetting agent. This bulk blend is uniformly subdivided into unit dosage packaging or multi-dosage packaging units. [00514] In still other embodiments, effervescent powders are also prepared. Effervescent salts have been used to disperse medicines in water for oral administration.
[00515] In some embodiments, the pharmaceutical solid oral dosage forms are formulated to provide a controlled release of the compound of Formula (I), (II), (III), (IV) or (V). Controlled release refers to the release of the compound of Formula (I), (II), (III), (IV) or (V) from a dosage form in which it is incorporated according to a desired profile over an extended period of time. Controlled release profiles include, for example, sustained release, prolonged release,
pulsatile release, and delayed release profiles. In contrast to immediate release compositions, controlled release compositions allow delivery of an agent to a subject over an extended period of time according to a predetermined profile. Such release rates can provide therapeutically effective levels of agent for an extended period of time and thereby provide a longer period of pharmacologic response while minimizing side effects as compared to conventional rapid release dosage forms. Such longer periods of response provide for many inherent benefits that are not achieved with the corresponding short acting, immediate release preparations. [00516] In some embodiments, the solid dosage forms described herein are formulated as enteric coated delayed release oral dosage forms, i.e., as an oral dosage form of a pharmaceutical composition as described herein which utilizes an enteric coating to affect release in the small intestine or large intestine. In one aspect, the enteric coated dosage form is a compressed or molded or extruded tablet/mold (coated or uncoated) containing granules, powder, pellets, beads or particles of the active ingredient and/or other composition components, which are themselves coated or uncoated. In one aspect, the enteric coated oral dosage form is in the form of a capsule containing pellets, beads or granules, which include a compound of Formula (I), (II), (III), (IV) or (V) that are coated or uncoated.
[00517] Any coatings should be applied to a sufficient thickness such that the entire coating does not dissolve in the gastrointestinal fluids at pH below about 5, but does dissolve at pH about 5 and above. Coatings are typically selected from any of the following: [00518] Shellac - this coating dissolves in media of pH >7; Acrylic polymers - examples of suitable acrylic polymers include methacrylic acid copolymers and ammonium methacrylate copolymers. The Eudragit series E, L, S, RL, RS and NE (Rohm Pharma) are available as solubilized in organic solvent, aqueous dispersion, or dry powders. The Eudragit series RL, NE, and RS are insoluble in the gastrointestinal tract but are permeable and are used primarily for colonic targeting. The Eudragit series E dissolve in the stomach. The Eudragit series L, L-30D and S are insoluble in stomach and dissolve in the intestine; Poly Vinyl Acetate Phthalate (PVAP) - PVAP dissolves in pH >5, and it is much less permeable to water vapor and gastric fluids. [00519] Conventional coating techniques such as spray or pan coating are employed to apply coatings. The coating thickness must be sufficient to ensure that the oral dosage form remains intact until the desired site of topical delivery in the intestinal tract is reached. [00520] In other embodiments, the formulations described herein are delivered using a pulsatile dosage form. A pulsatile dosage form is capable of providing one or more immediate release pulses at predetermined time points after a controlled lag time or at specific sites. Exemplary pulsatile dosage forms and methods of their manufacture are disclosed in U.S. Pat. Nos.
5,011,692, 5,017,381, 5,229,135, 5,840,329 and 5,837,284. In one embodiment, the pulsatile dosage form includes at least two groups of particles, (i.e. multiparticulate) each containing the formulation described herein. The first group of particles provides a substantially immediate dose of the compound of Formula (I), (II), (III), (IV) or (V) upon ingestion by a mammal. The first group of particles can be either uncoated or include a coating and/or sealant. In one aspect, the second group of particles comprises coated particles. The coating on the second group of particles provides a delay of from about 2 hours to about 7 hours following ingestion before release of the second dose. Suitable coatings for pharmaceutical compositions are described herein or known in the art. [00521] In some embodiments, pharmaceutical formulations are provided that include particles of a compound of Formula (I), (II), (III), (IV) or (V) and at least one dispersing agent or suspending agent for oral administration to a subject. The formulations may be a powder and/or granules for suspension, and upon admixture with water, a substantially uniform suspension is obtained. [00522] In one aspect, liquid formulation dosage forms for oral administration are in the form of aqueous suspensions selected from the group including, but not limited to, pharmaceutically acceptable aqueous oral dispersions, emulsions, solutions, elixirs, gels, and syrups. See, e.g., Singh et al.., Encyclopedia of Pharmaceutical Technology, 2nd Ed., pp. 754-757 (2002). In addition to the particles of the compound of Formula (I), (II), (III), (IV) or (V), the liquid dosage forms include additives, such as: (a) disintegrating agents; (b) dispersing agents; (c) wetting agents; (d) at least one preservative, (e) viscosity enhancing agents, (f) at least one sweetening agent, and (g) at least one flavoring agent. In some embodiments, the aqueous dispersions can further include a crystalline inhibitor. [00523] Furthermore, pharmaceutical compositions optionally include one or more pH adjusting agents or buffering agents, including acids such as acetic, boric, citric, lactic, phosphoric and hydrochloric acids; bases such as sodium hydroxide, sodium phosphate, sodium borate, sodium citrate, sodium acetate, sodium lactate and tris-hydroxymethylaminomethane; and buffers such as citrate/dextrose, sodium bicarbonate and ammonium chloride. Such acids, bases and buffers are included in an amount required to maintain pH of the composition in an acceptable range. [00524] Additionally, pharmaceutical compositions optionally include one or more salts in an amount required to bring osmolality of the composition into an acceptable range. Such salts include those having sodium, potassium or ammonium cations and chloride, citrate, ascorbate, borate, phosphate, bicarbonate, sulfate, thiosulfate or bisulfite anions; suitable salts include sodium chloride, potassium chloride, sodium thiosulfate, sodium bisulfite and ammonium sulfate.
[00525] Other pharmaceutical compositions optionally include one or more preservatives to inhibit microbial activity. Suitable preservatives include mercury-containing substances such as merfen and thiomersal; stabilized chlorine dioxide; and quaternary ammonium compounds such as benzalkonium chloride, cetyltrimethylammonium bromide and cetylpyridinium chloride. [00526] In one embodiment, the aqueous suspensions and dispersions described herein remain in a homogenous state, as defined in The USP Pharmacists' Pharmacopeia (2005 edition, chapter 905), for at least 4 hours. In one embodiment, an aqueous suspension is re-suspended into a homogenous suspension by physical agitation lasting less than 1 minute. In still another embodiment, no agitation is necessary to maintain a homogeneous aqueous dispersion. [00527] Examples of disintegrating agents for use in the aqueous suspensions and dispersions include, but are not limited to, a starch, e.g., a natural starch such as corn starch or potato starch, a pregelatinized starch, or sodium starch glycolate; a cellulose such as methylcrystalline cellulose, methylcellulose, croscarmellose, or a cross-linked cellulose, such as cross-linked sodium carboxymethylcellulose, cross-linked carboxymethylcellulose, or cross-linked croscarmellose; a cross-linked starch such as sodium starch glycolate; a cross-linked polymer such as crospovidone; a cross-linked polyvinylpyrrolidone; alginate such as alginic acid or a salt of alginic acid such as sodium alginate; a gum such as agar, guar, locust bean, Karaya, pectin, or tragacanth; sodium starch glycolate; bentonite; a natural sponge; a surfactant; a resin such as a cation-exchange resin; citrus pulp; sodium lauryl sulfate; sodium lauryl sulfate in combination starch; and the like.
[00528] In some embodiments, the dispersing agents suitable for the aqueous suspensions and dispersions described herein include, for example, hydrophilic polymers, electrolytes, Tween ® 60 or 80, PEG, polyvinylpyrrolidone, and the carbohydrate-based dispersing agents such as, for example, hydroxypropylcellulose and hydroxypropyl cellulose ethers, hydroxypropyl methylcellulose and hydroxypropyl methylcellulose ethers, carboxymethylcellulose sodium, methylcellulose, hydroxyethylcellulose, hydroxypropylmethyl-cellulose phthalate, hydroxypropylmethyl-cellulose acetate stearate, noncrystalline cellulose, magnesium aluminum silicate, triethanolamine, polyvinyl alcohol (PVA), polyvinylpyrrolidone/vinyl acetate copolymer, 4-(l,l,3,3-tetramethylbutyl)-phenol polymer with ethylene oxide and formaldehyde (also known as tyloxapol), poloxamers; and poloxamines. In other embodiments, the dispersing agent is selected from a group not comprising one of the following agents: hydrophilic polymers; electrolytes; Tween® 60 or 80; PEG; polyvinylpyrrolidone (PVP); hydroxypropylcellulose and hydroxypropyl cellulose ethers; hydroxypropyl methylcellulose and hydroxypropyl methylcellulose ethers; carboxymethylcellulose sodium; methylcellulose; hydroxyethylcellulose; hydroxypropylmethyl-cellulose phthalate; hydroxypropylmethyl-
cellulose acetate stearate; non-crystalline cellulose; magnesium aluminum silicate; triethanolamine; polyvinyl alcohol (PVA); 4-(l,l,3,3-tetramethylbutyl)-phenol polymer with ethylene oxide and formaldehyde; poloxamers; or poloxamines.
[00529] Wetting agents suitable for the aqueous suspensions and dispersions described herein include, but are not limited to, cetyl alcohol, glycerol monostearate, polyoxyethylene sorbitan fatty acid esters (e.g., the commercially available Tweens® such as e.g., Tween 20® and Tween 80®, and polyethylene glycols, oleic acid, glyceryl monostearate, sorbitan monooleate, sorbitan monolaurate, triethanolamine oleate, polyoxyethylene sorbitan monooleate, polyoxyethylene sorbitan monolaurate, sodium oleate, sodium lauryl sulfate, sodium docusate, triacetin, vitamin E TPGS, sodium taurocholate, simethicone, phosphotidylcholine and the like
[00530] Suitable preservatives for the aqueous suspensions or dispersions described herein include, for example, potassium sorbate, parabens (e.g., methylparaben and propylparaben), benzoic acid and its salts, other esters of parahydroxybenzoic acid such as butylparaben, alcohols such as ethyl alcohol or benzyl alcohol, phenolic compounds such as phenol, or quaternary compounds such as benzalkonium chloride. Preservatives, as used herein, are incorporated into the dosage form at a concentration sufficient to inhibit microbial growth. [00531] Suitable viscosity enhancing agents for the aqueous suspensions or dispersions described herein include, but are not limited to, methyl cellulose, xanthan gum, carboxymethyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose, Plasdon® S-630, carbomer, polyvinyl alcohol, alginates, acacia, chitosans and combinations thereof. The concentration of the viscosity enhancing agent will depend upon the agent selected and the viscosity desired. [00532] Examples of sweetening agents suitable for the aqueous suspensions or dispersions described herein include, for example, acacia syrup, acesulfame K, alitame, aspartame, chocolate, cinnamon, citrus, cocoa, cyclamate, dextrose, fructose, ginger, glycyrrhetinate, glycyrrhiza (licorice) syrup, monoammonium glyrrhizinate (MagnaSweet®), maltol, mannitol, menthol, neohesperidine DC, neotame, Prosweet® Powder, saccharin, sorbitol, stevia, sucralose, sucrose, sodium saccharin, saccharin, aspartame, acesulfame potassium, mannitol, sucralose, tagatose, thaumatin, vanilla, xylitol, or any combination thereof. [00533] In some embodiments, the liquid formulations also include inert diluents commonly used in the art, such as water or other solvents, solubilizing agents, and emulsifiers. Exemplary emulsifiers are ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propyleneglycol, 1,3-butyleneglycol, dimethylformamide, sodium lauryl sulfate, sodium doccusate, cholesterol, cholesterol esters, taurocholic acid, phosphotidylcholine, oils, such as cottonseed oil, groundnut oil, corn germ oil, olive oil, castor oil, and sesame oil,
glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols, fatty acid esters of sorbitan, or mixtures of these substances, and the like.
[00534] Representative intranasal formulations are described in, for example, U.S. Pat. Nos. 4,476,116, 5,116,817 and 6,391,452. Formulations that include a compound of Formula (I), (II), (HI), (IV) or (V) are prepared as solutions in saline, employing benzyl alcohol or other suitable preservatives, fluorocarbons, and/or other solubilizing or dispersing agents known in the art. See, for example, Ansel, H. C. et ah, Pharmaceutical Dosage Forms and Drug Delivery Systems, Sixth Ed. (1995). Preferably these compositions and formulations are prepared with suitable nontoxic pharmaceutically acceptable ingredients. These ingredients are known to those skilled in the preparation of nasal dosage forms and some of these can be found in
REMINGTON: THE SCIENCE AND PRACTICE OF PHARMACY, 21st edition, 2005. The choice of suitable carriers is dependent upon the exact nature of the nasal dosage form desired, e.g., solutions, suspensions, ointments, or gels. Nasal dosage forms generally contain large amounts of water in addition to the active ingredient. Minor amounts of other ingredients such as pH adjusters, emulsifiers or dispersing agents, preservatives, surfactants, gelling agents, or buffering and other stabilizing and solubilizing agents are optionally present. Preferably, the nasal dosage form should be isotonic with nasal secretions.
[00535] For administration by inhalation, a compound of Formula (I), (II), (III), (IV) or (V) is formulated for use as an aerosol, a mist or a powder. Pharmaceutical compositions described herein are conveniently delivered in the form of an aerosol spray presentation from pressurized packs or a nebuliser, with the use of a suitable propellant, e.g., dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other suitable gas. In the case of a pressurized aerosol, the dosage unit may be determined by providing a valve to deliver a metered amount. Capsules and cartridges of, such as, by way of example only, gelatin for use in an inhaler or insufflator may be formulated containing a powder mix of the compound described herein and a suitable powder base such as lactose or starch.
[00536] Buccal formulations that include a compound of Formula (I), (II), (III), (IV) or (V) are administered using a variety of formulations known in the art. For example, such formulations include, but are not limited to, U.S. Pat. Nos. 4,229,447, 4,596,795, 4,755,386, and 5,739,136. In addition, the buccal dosage forms described herein can further include a bioerodible (hydrolysable) polymeric carrier that also serves to adhere the dosage form to the buccal mucosa. For buccal or sublingual administration, the compositions may take the form of tablets, lozenges, or gels formulated in a conventional manner. [00537] In some embodiments, a compound of Formula (I), (II), (III), (IV) or (V) is prepared as transdermal dosage forms. In one embodiment, the transdermal formulations described herein
include at least three components: (1) a formulation of a compound of Formula (I), (II), (III), (IV) or (V); (2) a penetration enhancer; and (3) an aqueous adjuvant. In some embodiments the transdermal formulations include additional components such as, but not limited to, gelling agents, creams and ointment bases, and the like. In some embodiments, the transdermal formulation further include a woven or non-woven backing material to enhance absorption and prevent the removal of the transdermal formulation from the skin. In other embodiments, the transdermal formulations described herein can maintain a saturated or supersaturated state to promote diffusion into the skin. [00538] In one aspect, formulations suitable for transdermal administration of compounds described herein employ transdermal delivery devices and transdermal delivery patches and can be lipophilic emulsions or buffered, aqueous solutions, dissolved and/or dispersed in a polymer or an adhesive. In one aspect, such patches are constructed for continuous, pulsatile, or on demand delivery of pharmaceutical agents. Still further, transdermal delivery of the compounds described herein can be accomplished by means of iontophoretic patches and the like. In one aspect, transdermal patches provide controlled delivery of the compound of Formula (I), (II), (III), (IV) or (V). In one aspect, transdermal devices are in the form of a bandage comprising a backing member, a reservoir containing the compound optionally with carriers, optionally a rate controlling barrier to deliver the compound to the skin of the host at a controlled and predetermined rate over a prolonged period of time, and means to secure the device to the skin. [00539] In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is formulated into a pharmaceutical composition suitable for intramuscular, subcutaneous, or intravenous injection. In one aspect, formulations suitable for intramuscular, subcutaneous, or intravenous injection include physiologically acceptable sterile aqueous or non-aqueous solutions, dispersions, suspensions or emulsions, and sterile powders for reconstitution into sterile injectable solutions or dispersions. Examples of suitable aqueous and non-aqueous carriers, diluents, solvents, or vehicles include water, ethanol, polyols (propyleneglycol, polyethylene-glycol, glycerol, cremophor and the like), suitable mixtures thereof, vegetable oils (such as olive oil) and injectable organic esters such as ethyl oleate. Proper fluidity can be maintained, for example, by the use of a coating such as lecithin, by the maintenance of the required particle size in the case of dispersions, and by the use of surfactants. In some embodiments, formulations suitable for subcutaneous injection also contain additives such as preserving, wetting, emulsifying, and dispensing agents. Prevention of the growth of microorganisms can be ensured by various antibacterial and antifungal agents, such as parabens, chlorobutanol, phenol, sorbic acid, and the like. In some cases it is desirable to include isotonic agents, such as sugars, sodium chloride,
and the like. Prolonged absorption of the injectable pharmaceutical form can be brought about by the use of agents delaying absorption, such as aluminum monostearate and gelatin. [00540] For intravenous injections, compounds described herein are formulated in aqueous solutions, preferably in physiologically compatible buffers such as Hank's solution, Ringer's solution, or physiological saline buffer. For transmucosal administration, penetrants appropriate to the barrier to be permeated are used in the formulation. Such penetrants are generally known in the art. For other parenteral injections, appropriate formulations include aqueous or nonaqueous solutions, preferably with physiologically compatible buffers or excipients. Such excipients are known. [00541] Parenteral injections may involve bolus injection or continuous infusion. Formulations for injection may be presented in unit dosage form, e.g., in ampoules or in multi-dose containers, with an added preservative. The pharmaceutical composition described herein may be in a form suitable for parenteral injection as a sterile suspensions, solutions or emulsions in oily or aqueous vehicles, and may contain formulatory agents such as suspending, stabilizing and/or dispersing agents. In one aspect, the active ingredient is in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use. [00542] In certain embodiments, delivery systems for pharmaceutical compounds may be employed, such as, for example, liposomes and emulsions. In certain embodiments, compositions provided herein can also include an mucoadhesive polymer, selected from among, for example, carboxymethylcellulose, carbomer (acrylic acid polymer), poly(methylmethacrylate), polyacrylamide, polycarbophil, acrylic acid/butyl acrylate copolymer, sodium alginate and dextran.
[00543] In some embodiments, the compounds described herein may be administered topically and can be formulated into a variety of topically administrable compositions, such as solutions, suspensions, lotions, gels, pastes, medicated sticks, balms, creams or ointments. Such pharmaceutical compounds can contain solubilizers, stabilizers, tonicity enhancing agents, buffers and preservatives.
[00544] In some embodiments, the compound of Formula (I), (II), (III), (IV) or (V) is formulated in rectal compositions such as enemas, rectal gels, rectal foams, rectal aerosols, suppositories, jelly suppositories, or retention enemas, containing conventional suppository bases such as cocoa butter or other glycerides, as well as synthetic polymers such as polyvinylpyrrolidone, PEG, and the like. In suppository forms of the compositions, a low-melting wax such as, but not limited to, a mixture of fatty acid glycerides, optionally in combination with cocoa butter is first melted. Methods of Dosing and Treatment Regimens
[00545] In one embodiment, a compound of Formula (I), (II), (III), (IV) or (V) is used in the preparation of medicaments for the treatment of LPA-dependent or LPA-mediated diseases or conditions. In addition, a method for treating any of the diseases or conditions described herein in a subject in need of such treatment, involves administration of pharmaceutical compositions that include at least one compound of Formula (I), (II), (III), (IV) or (V) or a pharmaceutically acceptable salt, pharmaceutically active metabolite, pharmaceutically acceptable prodrug, or pharmaceutically acceptable solvate thereof, in therapeutically effective amounts to said subject. [00546] In certain embodiments, the compositions containing the compound(s) described herein are administered for prophylactic and/or therapeutic treatments. In certain therapeutic applications, the compositions are administered to a patient already suffering from a disease or condition, in an amount sufficient to cure or at least partially arrest at least one of the symptoms of the disease or condition. Amounts effective for this use depend on the severity and course of the disease or condition, previous therapy, the patient's health status, weight, and response to the drugs, and the judgment of the treating physician. Therapeutically effective amounts are optionally determined by methods including, but not limited to, a dose escalation clinical trial. [00547] In prophylactic applications, compositions containing the compounds described herein are administered to a patient susceptible to or otherwise at risk of a particular disease, disorder or condition. Such an amount is defined to be a "prophylactically effective amount or dose." In this use, the precise amounts also depend on the patient's state of health, weight, and the like. When used in a patient, effective amounts for this use will depend on the severity and course of the disease, disorder or condition, previous therapy, the patient's health status and response to the drugs, and the judgment of the treating physician. In one aspect, prophylactic treatments include admistering to a mammal, who previously experienced at least one symtom of the disease being treated and is currently in remission, a pharmaceutical composition comprising a compound of Formula (I), (II), (III), (IV) or (V) in order to prevent a return of the symptoms of the disease or condition.
[00548] In certain embodiments wherein the patient's condition does not improve, upon the doctor's discretion the administration of the compounds are administered chronically, that is, for an extended period of time, including throughout the duration of the patient's life in order to ameliorate or otherwise control or limit the symptoms of the patient's disease or condition.
[00549] In certain embodiments wherein a patient's status does improve, the dose of drug being administered may be temporarily reduced or temporarily suspended for a certain length of time (i.e., a "drug holiday"). In specific embodiments, the length of the drug holiday is between 2 days and 1 year, including by way of example only, 2 days, 3 days, 4 days, 5 days, 6 days, 7 days, 10 days, 12 days, 15 days, 20 days, 28 days, or more than 28 days. The dose reduction
- I l l -
during a drug holiday is, by way of example only, by 10%- 100%, including by way of example only 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, and 100%.
[00550] In certain embodiments in which the patient is presented with a situation in which the activity of LPA needs to be enhanced, for example, to assist with wound healing, the dose of drug being administered may be temporarily reduced or temporarily suspended for a certain length of time {i.e., a "drug diversion"). In specific embodiments, the length of the drug diversion is between 2 days and 1 year, including by way of example only, 2 days, 3 days, 4 days, 5 days, 6 days, 7 days, 10 days, 12 days, 15 days, 20 days, 28 days, or more than 28 days. The dose reduction during a drug diversion is, by way of example only, by 10%- 100%, including by way of example only 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, and 100%. Once the situation requiring enhanced activity of LPA is alleviated, the normal dosing schedule is optionally reinstated. [00551] Once improvement of the patient's conditions has occurred, a maintenance dose is administered if necessary. Subsequently, in specific embodiments, the dosage or the frequency of administration, or both, is reduced, as a function of the symptoms, to a level at which the improved disease, disorder or condition is retained. In certain embodiments, however, the patient requires intermittent treatment on a long-term basis upon any recurrence of symptoms. [00552] The amount of a given agent that corresponds to such an amount varies depending upon factors such as the particular compound, disease condition and its severity, the identity {e.g., weight, sex) of the subject or host in need of treatment, but can nevertheless be determined according to the particular circumstances surrounding the case, including, e.g., the specific agent being administered, the route of administration, the condition being treated, and the subject or host being treated. In general, however, doses employed for adult human treatment are typically in the range of 0.01mg-5000 mg per day. In one aspect, doses employed for adult human treatment are from about lmg to about 1000 mg per day. In one embodiment, the desired dose is conveniently presented in a single dose or in divided doses administered simultaneously (or over a short period of time) or at appropriate intervals, for example as two, three, four or more sub-doses per day. [00553] In one embodiment, the daily dosages appropriate for the compound of Formula (I), (II), (III), (IV) or (V) described herein are from about 0.01 to about 10 mg/kg per body weight. In specific embodiments, an indicated daily dosage in a large mammal, including, but not limited to, humans, is in the range from about 0.5 mg to about 1000 mg, conveniently administered in divided doses, including, but not limited to, up to four times a day. In one embodiment, the daily dosage is administered in extended release form. In certain embodiments, suitable unit
dosage forms for oral administration comprise from about 1 to 500 mg active ingredient. In other embodiments, the daily dosage or the amount of active in the dosage form are lower or higher than the ranges indicated herein, based on a number of variables in regard to an individual treatment regime. In various embodiments, the daily and unit dosages are altered depending on a number of variables including, but not limited to, the activity of the compound used, the disease or condition to be treated, the mode of administration, the requirements of the individual subject, the severity of the disease or condition being treated, and the judgment of the practitioner. [00554] Toxicity and therapeutic efficacy of such therapeutic regimens are determined by standard pharmaceutical procedures in cell cultures or experimental animals, including, but not limited to, the determination of the LD50 and the ED50. The dose ratio between the toxic and therapeutic effects is the therapeutic index and it is expressed as the ratio between LD50 and ED50. In certain embodiments, the data obtained from cell culture assays and animal studies are used in formulating the therapeutically effective daily dosage range and/or the therapeutically effective unit dosage amount for use in mammals, including humans. In some embodiments, the daily dosage amount of the compounds described herein lies within a range of circulating concentrations that include the ED50 with minimal toxicity. In certain embodiments, the daily dosage range and/or the unit dosage amount varies within this range depending upon the dosage form employed and the route of administration utilized. Patient Selection
[00555] In any of the aforementioned aspects involving the prevention or treatment of LPA- mediated diseases or conditions are further embodiments comprising identifying patients by screening for LPA receptor gene SNPs. A SNP located in the promoter region of LPAi showed significant association with knee osteoarthritis in two independent populations (Mototani et al. Hum. MoI. Genetics, vol. 17, no. 12, 2008). Patients can be further selected based on increased LPA receptor expression in the tissue of interest. For example, chronic lymphocytic leukemia (CLL) is characterized by the accumulation of CD19+/CD5+ B -lymphocytes in the peripheral blood, bone marrow and lymphoid organs which occurs as a result of a block in B-lymphocyte apoptosis. LPA can protect some CLL cells from apoptosis and the cells that are protected by LPA have high levels of LPAi mRNA. In some embodiments, CLL patients are selected based on the expression of the LPAlR. LPA receptor expression are determined by methods including, but not limited to, northern blotting, western blotting, quantitative PCR (qPCR), flow cytometry, autoradiography (using a small molecule radioligand or PET ligand). In some embodiments, patients are selected based on the concentration of serum or tissue LPA measured by mass spectrometry. LPA concentrations are high in ovarian cancer ascites and in some breast
cancer effusions. In some embodiments, patients are selected based on a combination of the above markers (increased LPA concentrations and increased LPA receptor expression). Combination Treatments
[00556] In certain instances, it is appropriate to administer at least one compound of Formula (I), (II), (III), (IV) or (V) in combination with another therapeutic agent. By way of example only, if one of the side effects experienced by a patient upon receiving one of the compounds herein is inflammation, then it may be appropriate to administer an anti-inflammatory agent in combination with the initial therapeutic agent. Or in another example, a patient is presented with a situation in which antagonism of LPA receptors provides potential harm, for example, if the patient is wounded, antagonism of LPA receptors may lead to a delay in wound healing. In such an event, in certain embodiments, the patient benefits by administration of a local wound- healing agent (at the site of the wound) in combination with the co-existing administration of a compound of Formula (I), (II), (III), (IV) or (V). [00557] Or, in one embodiment, the therapeutic effectiveness of one of the compounds described herein is enhanced by administration of an adjuvant (i.e., by itself the adjuvant may have minimal therapeutic benefit, but in combination with another therapeutic agent, the overall therapeutic benefit to the patient is enhanced). Or, in some embodiments, the benefit experienced by a patient is increased by administering one of the compounds described herein with another therapeutic agent (which also includes a therapeutic regimen) that also has therapeutic benefit.
[00558] In one specific embodiment, a compound of Formula (I), (II), (III), (IV) or (V) is coadministered with a second therapeutic agent, wherein the compound of Formula (I), (II), (III), (IV) or (V) and the second therapeutic agent modulate different aspects of the disease, disorder or condition being treated, thereby providing a greater overall benefit than administration of either therapeutic agent alone.
[00559] In any case, regardless of the disease, disorder or condition being treated, the overall benefit experienced by the patient may simply be additive of the two therapeutic agents or the patient may experience a synergistic benefit. [00560] In certain embodiments, different therapeutically-effective dosages of the compounds disclosed herein will be utilized in formulating pharmaceutical composition and/or in treatment regimens when the compounds disclosed herein are administered in combination with one or more additional agent, such as an additional therapeutically effective drug, an adjuvant or the like. Therapeutically-effective dosages of drugs and other agents for use in combination treatment regimens can be determined by means similar to those set forth hereinabove for the actives themselves. Furthermore, the methods of prevention/treatment described herein
encompasses the use of metronomic dosing, i.e., providing more frequent, lower doses in order to minimize toxic side effects. In some embodiments, a combination treatment regimen encompasses treatment regimens in which administration of a compound of Formula (I), (II), (III), (IV) or (V) is initiated prior to, during, or after treatment with a second agent described herein, and continues until any time during treatment with the second agent or after termination of treatment with the second agent. It also includes treatments in which a compound of Formula (I), (II), (III), (IV) or (V) and the second agent being used in combination are administered simultaneously or at different times and/or at decreasing or increasing intervals during the treatment period. Combination treatment further includes periodic treatments that start and stop at various times to assist with the clinical management of the patient.
[00561] Compositions and methods for combination therapy are provided herein. In accordance with one aspect, the pharmaceutical compositions disclosed herein are used to treat LPA- dependent or LPA-mediated conditions. [00562] It is understood that the dosage regimen to treat, prevent, or ameliorate the condition(s) for which relief is sought, is modified in accordance with a variety of factors. These factors include the disease, disorder or condition from which the subject suffers, as well as the age, weight, sex, diet, and medical condition of the subject. Thus, in some instances, the dosage regimen actually employed varies and, in some embodiments, deviates from the dosage regimens set forth herein. [00563] For combination therapies described herein, dosages of the co-administered compounds vary depending on the type of co-drug employed, on the specific drug employed, on the disease or condition being treated and so forth. In additional embodiments, when co-administered with one or more other therapeutic agents, the compound provided herein is administered either simultaneously with the one or more other therapeutic agents, or sequentially. [00564] In combination therapies, the multiple therapeutic agents (one of which is one of the compounds described herein) are administered in any order or even simultaneously. If administration is simultaneous, the multiple therapeutic agents are, by way of example only, provided in a single, unified form, or in multiple forms (e.g., as a single pill or as two separate pills). In one embodiment, one of the therapeutic agents is given in multiple doses, and in another, two (or more if present) are given as multiple doses. In some embodiments of non- simultaneous administration, the timing between the multiple doses vary from more than zero weeks to less than four weeks. In addition, the combination methods, compositions and formulations are not to be limited to the use of only two agents; the use of multiple therapeutic combinations is also envisioned.
[00565] The compounds of Formula (I), (II), (III), (IV) or (V) and combination therapies are administered before, during or after the occurrence of a disease or condition, and the timing of administering the composition containing a compound varies. Thus, in one embodiment, the compounds described herein are used as a prophylactic and are administered continuously to subjects with a propensity to develop conditions or diseases in order to prevent the occurrence of the disease or condition. In another embodiment, the compounds and compositions are administered to a subject during or as soon as possible after the onset of the symptoms. In specific embodiments, a compound described herein is administered as soon as is practicable after the onset of a disease or condition is detected or suspected, and for a length of time necessary for the treatment of the disease. In some embodiments, the length required for treatment varies, and the treatment length is adjusted to suit the specific needs of each subject. For example, in specific embodiments, a compound described herein or a formulation containing the compound is administered for at least 2 weeks, about 1 month to about 5 years. [00566] By way of example, therapies which combine a compound of Formula (I), (II), (III), (IV) or (V) with inhibitors of LPA synthesis or LPA receptor antagonists, either acting at the same or other points in the LPA synthesis or signalling pathway, are encompassed herein for treating LPA-dependent or LPA-mediated diseases or conditions. Exemplary Agent for use in Combination Therapy [00567] In another embodiment described herein, methods for treatment of LPA-dependent or LPA-mediated conditions or diseases, such as proliferative disorders, including cancer, comprises administration to a mammal a compound of Formula (I), (II), (III), (IV) or (V) in combination with at least one additional agent selected, by way of example only, alemtuzumab, arsenic trioxide, asparaginase (pegylated or non-), bevacizumab, cetuximab, platinum-based compounds such as cisplatin, cladribine, daunorubicin/doxorubicin/idarubicin, irinotecan, fludarabine, 5-fluorouracil, gemtuzumab, methotrexate, Paclitaxel™, taxol, temozolomide, thioguanine, or classes of drugs including hormones (an antiestrogen, an antiandrogen, or gonadotropin releasing hormone analogues, interferons such as alpha interferon, nitrogen mustards such as busulfan or melphalan or mechlorethamine, retinoids such as tretinoin, topoisomerase inhibitors such as irinotecan or topotecan, tyrosine kinase inhibitors such as gefϊnitinib or imatinib, or agents to treat signs or symptoms induced by such therapy including allopurinol, filgrastim, granisetron/ondansetron/palonosetron, dronabinol. [00568] In one aspect, the compound of Formula (I), (II), (III), (IV) or (V) is administered or formulated in combination with one or more anti-cancer agents. In some embodiments, one or more of the anti-cancer agents are proapoptotic agents. Examples of anti-cancer agents include, but are not limited to, any of the following: gossypol, genasense, polyphenol E, Chlorofusin, all
trans-retinoic acid (ATRA), bryostatin, tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL), 5-aza-2'-deoxycytidine, all trans retinoic acid, doxorubicin, vincristine, etoposide, gemcitabine, imatinib, geldanamycin, 17-N-Allylamino- 17-Demethoxygeldanamycin
(17-AAG), flavopiridol, LY294002, bortezomib, trastuzumab, BAY 11-7082, PKC412, or PD184352, Taxol™ (paclitaxel), and analogs of Taxol™, such as Taxotere™. Compounds that have the basic taxane skeleton as a common structure feature, have also been shown to have the ability to arrest cells in the G2-M phases due to stabilized microtubules and may be useful for treating cancer in combination with the compounds described herein.
[00569] Further examples of anti-cancer agents for use in combination with a compound of Formula (I), (II), (III), (IV) or (V) include inhibitors of mitogen-activated protein kinase signaling, e.g., U0126, PD98059, PD184352, PD0325901, ARRY-142886, SB239063,
SP600125, BAY 43-9006, wortmannin, or LY294002; Syk inhibitors; mTOR inhibitors; and antibodies (e.g., rituxan).
[00570] Other anti-cancer agents for use in combination with a compound of Formula (I), (II), (III), (IV) or (V) include one or more of the following: abiraterone; abarelix; adriamycin; aactinomycin; acivicin; aclarubicin; acodazole hydrochloride; acronine; adozelesin; aldesleukin; alemtuzumab; allopurinol; alitretinoin; altretamine; ambomycin; ametantrone acetate; aminoglutethimide; aminolevulinic acid; amifostine; amsacrine; anastrozole; anthramycin; aprepitant; arsenic trioxide; asparaginase; asperlin; azacitidine; azetepa; azotomycin; batimastat; bendamustine hydrochloride; benzodepa; bevacizumab; bexarotene; bicalutamide; bisantrene hydrochloride; bisnafide dimesylate; bizelesin; bleomycin; bleomycin sulfate; bortezomib; brequinar sodium; bropirimine; busulfan; cactinomycin; calusterone; caracemide; carbetimer; carboplatin; carmustine; carubicin hydrochloride; carzelesin; capecitabine; cedefingol; cetuximab; chlorambucil; cirolemycin; cisplatin; cladribine; clofarabine; crisnatol mesylate; cyclophosphamide; cytarabine; dacarbazine; dasatinib; daunorubicin hydrochloride; dactinomycin; darbepoetin alfa; decitabine; degarelix; denileukin diftitox; dexormaplatin; dexrazoxane hydrochloride; dezaguanine; dezaguanine mesylate; diaziquone; docetaxel; doxorubicin; doxorubicin hydrochloride; droloxifene; droloxifene citrate; dromostanolone propionate; duazomycin; edatrexate; eflornithine hydrochloride; elsamitrucin; eltrombopag olamine; enloplatin; enpromate; epipropidine; epirubicin hydrochloride; epoetin alfa; erbulozole; erlotinib hydrochloride; esorubicin hydrochloride; estramustine; estramustine phosphate sodium; etanidazole; etoposide; etoposide phosphate; etoprine; everolimus; exemestane; fadrozole hydrochloride; fazarabine; fenretinide; filgrastim; floxuridine; fludarabine phosphate; fluorouracil; flurocitabine; fosquidone; fostriecin sodium; fulvestrant; gefitinib; gemcitabine; gemcitabine hydrochloride; gemcitabine -cisplatin; gemtuzumab
ozogamicin; goserelin acetate; histrelin acetate; hydroxyurea; idarubicin hydrochloride; ifosfamide; iimofosine; ibritumomab tiuxetan; idarubicin; ifosfamide; imatinib mesylate; imiquimod; interleukin Il (including recombinant interleukin II, or rlL2), interferon alfa-2a; interferon alfa-2b; interferon alfa-nl; interferon alfa-n3; interferon beta-1 a; interferon gamma-1 b; iproplatin; irinotecan hydrochloride; ixabepilone; lanreotide acetate; lapatinib; lenalidomide; letrozole; leuprolide acetate; leucovorin calcium; leuprolide acetate; levamisole; liposomal cytarabine; liarozole hydrochloride; lometrexol sodium; lomustine; losoxantrone hydrochloride; masoprocol; maytansine; mechlorethamine hydrochloride; megestrol acetate; melengestrol acetate; melphalan; menogaril; mercaptopurine; methotrexate; methotrexate sodium; methoxsalen; metoprine; meturedepa; mitindomide; mitocarcin; mitocromin; mitogillin; mitomalcin; mitomycin C; mitosper; mitotane; mitoxantrone hydrochloride; mycophenolic acid; nandrolone phenpropionate; nelarabine; nilotinib; nocodazoie; nofetumomab; nogalamycin; ofatumumab; oprelvekin; ormaplatin; oxaliplatin;oxisuran; paclitaxel; palifermin; palonosetron hydrochloride; pamidronate; pegfilgrastim; pemetrexed disodium; pentostatin; panitumumab; pazopanib hydrochloride; pemetrexed disodium; plerixafor; pralatrexate; pegaspargase; peliomycin; pentamustine; peplomycin sulfate; perfosfamide; pipobroman; piposulfan; piroxantrone hydrochloride; plicamycin; plomestane; porfimer sodium; porfiromycin; prednimustine; procarbazine hydrochloride; puromycin; puromycin hydrochloride; pyrazofurin; quinacrine; raloxifene hydrochloride; rasburicase; recombinant HPV bivalent vaccine; recombinant HPV quadrivalent vaccine; riboprine; rogletimide; rituximab; romidepsin; romiplostim; safingol; safingol hydrochloride; sargramostim; semustine; simtrazene; sipuleucel-
T; sorafenib; sparfosate sodium; sparsomycin; spirogermanium hydrochloride; spiromustine; spiroplatin; streptonigrin; streptozocin; sulofenur; sunitinib malate; talisomycin; tamoxifen citrate; tecogalan sodium; tegafur; teloxantrone hydrochloride; temozolomide; temoporfin; temsirolimus; teniposide; teroxirone; testolactone; thalidomide;thiamiprine; thioguanine; thiotepa; tiazofurin; tirapazamine; topotecan hydrochloride; toremifene; tositumomab; tositumomab and 1 131 Iodine tositumomab; trastuzumab; trestolone acetate; tretinoin; triciribine phosphate; trimetrexate; trimetrexate glucuronate; triptorelin; tubulozole hydrochloride; uracil mustard; uredepa; valrubicin; vapreotide; verteporfin; vinblastine; vinblastine sulfate; vincristine sulfate; vindesine; vindesine sulfate; vinepidine sulfate; vinglycinate sulfate; vinleurosine sulfate; vinorelbine tartrate; vinrosidine sulfate; vinzolidine sulfate; vorinostat; vorozole; zeniplatin; zinostatin; zoledronic acid; zorubicin hydrochloride.
[00571] Yet other anticancer agents for use in combination with a compound of Formula (I), (II),
(III), (IV) or (V) include alkylating agents, antimetabolites, natural products, or hormones, e.g., nitrogen mustards (e.g., mechloroethamine, cyclophosphamide, chlorambucil, etc.), alkyl
sulfonates (e.g., busulfan), nitrosoureas (e.g., carmustine, lomusitne, ete.), or triazenes (decarbazine, etc.). Examples of antimetabolites include but are not limited to folic acid analog (e.g., methotrexate), or pyrimidine analogs (e.g., Cytarabine), purine analogs (e.g., mercaptopurine, thioguanine, pentostatin). [00572] Examples of natural products for use in combination with a compound of Formula (I), (II), (III), (IV) or (V) include but are not limited to vinca alkaloids (e.g., vinblastin, vincristine), epipodophyllotoxins (e.g., etoposide), antibiotics (e.g., daunorubicin, doxorubicin, bleomycin), enzymes (e.g., L-asparaginase), or biological response modifiers (e.g., interferon alpha). [00573] Examples of alkylating agents for use in combination with a compound of Formula (I), (II), (III), (IV) or (V) include, but are not limited to, nitrogen mustards (e.g., mechloroethamine, cyclophosphamide, chlorambucil, meiphalan, etc.), ethylenimine and methylmelamines (e.g., hexamethlymelamine, thiotepa), alkyl sulfonates (e.g., busulfan), nitrosoureas (e.g., carmustine, lomusitne, semustine, streptozocin, etc.), or triazenes (decarbazine, ete.). Examples of antimetabolites include, but are not limited to folic acid analog (e.g., methotrexate), or pyrimidine analogs (e.g., fluorouracil, floxouridine, Cytarabine), purine analogs (e.g., mercaptopurine, thioguanine, pentostatin.
[00574] Examples of hormones and antagonists for use in combination with a compound of Formula (I), (II), (III), (IV) or (V) include, but are not limited to, adrenocorticosteroids (e.g., prednisone), progestins (e.g., hydroxyprogesterone caproate, megestrol acetate, medroxyprogesterone acetate), estrogens (e.g., diethlystilbestrol, ethinyl estradiol), antiestrogen (e.g., tamoxifen), androgens (e.g., testosterone propionate, fluoxymesterone), antiandrogen (e.g., flutamide), gonadotropin releasing hormone analog (e.g., leuprolide). Other agents that can be used in the methods and compositions described herein for the treatment or prevention of cancer include platinum coordination complexes (e.g., cisplatin, carboblatin), anthracenedione (e.g., mitoxantrone), substituted urea (e.g., hydroxyurea), methyl hydrazine derivative (e.g., procarbazine), adrenocortical suppressant (e.g., mitotane, aminoglutethimide). [00575] Examples of anti-cancer agents which act by arresting cells in the G2-M phases due to stabilized microtubules include without limitation the following marketed drugs and drugs in development: Erbulozole, Dolastatin 10, Mivobulin isethionate, Vincristine, NSC-639829, Discodermolide, ABT-751 , Altorhyrtins (such as Altorhyrtin A and Altorhyrtin C), Spongistatins (such as Spongistatin 1, Spongistatin 2, Spongistatin 3, Spongistatin 4, Spongistatin 5, Spongistatin 6, Spongistatin 7, Spongistatin 8, and Spongistatin 9), Cemadotin hydrochloride, Epothilones (such as Epothilone A, Epothilone B, Epothilone C, Epothilone D, Epothilone E, Epothilone F, Epothilone B N-oxide, Epothilone A N-oxide, 16-aza-epothilone B, 21 -aminoepothilone B, 21 -hydroxyepothilone D, 26-fluoroepothilone, Auristatin PE,
Soblidotin, Vincristine sulfate, Cryptophycin 52, Vitilevuamide, Tubulysin A, Canadensol, Centaureidin, Oncocidin Al Fijianolide B, Laulimalide, Narcosine, Nascapine, Hemiasterlin, Vanadocene acetylacetonate, Indanocine Eleutherobins (such as Desmethyleleutherobin, Desaetyleleutherobin, lsoeleutherobin A, and Z-Eleutherobin), Caribaeoside, Caribaeolin, Halichondrin B, Diazonamide A, Taccalonolide A, Diozostatin, (-)-Phenylahistin, Myoseverin B, Resverastatin phosphate sodium.
[00576] In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is co-administered with thrombolytic agents (e.g., alteplase anistreplase, streptokinase, urokinase, or tissue plasminogen activator), heparin, tinzaparin, warfarin, dabigatran (e.g., dabigatran etexilate), factor Xa inhibitors (e.g., fondaparinux, draparinux, rivaroxaban, DX-9065a, otamixaban, LY517717, or YM150), ticlopidine, clopidogrel, CS-747 (prasugrel, LY640315), ximelagatran, or BIBR 1048. [00577] In some embodiments, a compound of Formula (I), (II), (III), (IV) or (V) is used in combination with anti-emetic agents to treat nausea or emesis, which may result from the use of a compound of Formula (I), (II), (III), (IV) or (V), anti-cancer agent(s) and/or radiation therapy. [00578] Anti-emetic agents include, but are not limited to: neurokinin-1 receptor antagonists, 5HT3 receptor antagonists (such as ondansetron, granisetron, tropisetron, Palonosetron, and zatisetron), GABAB receptor agonists (such as baclofen), corticosteroids (such as dexamethasone, prednisone, prednisolone, or others), dopamine antagonists (such as, but not limited to, domperidone, droperidol, haloperidol, chlorpromazine, promethazine, prochlorperazine, metoclopramide), antihistamines (Hl histamine receptor antagonists, such as but not limited to, cyclizine, diphenhydramine, dimenhydrinate, meclizine, promethazine, hydroxyzine), cannabinoids (such as but not limited to, cannabis, marinol, dronabinol), and others (such as, but not limited to, trimethobenzamide; ginger, emetrol, propofol). [00579] In some embodiments, a compound of Formula (I), (II), (III), (IV) or (V) is used in combination with an agent useful in the treatment of anemia. Such an anemia treatment agent is, for example, a continuous eythropoiesis receptor activator (such as epoetin-α). [00580] In some embodiments, a compound of Formula (I), (II), (III), (IV) or (V) is used in combination with an agent useful in the treatment of neutropenia. Examples of agents useful in the treatment of neutropenia include, but are not limited to, a hematopoietic growth factor which regulates the production and function of neutrophils such as a human granulocyte colony stimulating factor, (G-CSF). Examples of a G-CSF include filgrastim. [00581] In some embodiments, a compound of Formula (I), (II), (III), (IV) or (V) is used in combination with radiation therapy (or radiotherapy). Radiation therapy is the treatment of cancer and other diseases with ionizing radiation. Radiation therapy can be used to treat localized solid tumors, such as cancers of the skin, tongue, larynx, brain, breast, prostate, colon,
uterus and/or cervix. It can also be used to treat leukemia and lymphoma (cancers of the blood- forming cells and lymphatic system, respectively).
[00582] A technique for delivering radiation to cancer cells is to place radioactive implants directly in a tumor or body cavity. This is called internal radiotherapy (brachytherapy, interstitial irradiation, and intracavitary irradiation are types of internal radiotherapy.) Using internal radiotherapy, the radiation dose is concentrated in a small area, and the patient stays in the hospital for a few days. Internal radiotherapy is frequently used for cancers of the tongue, uterus, prostate, colon, and cervix. [00583] The term "radiotherapy" or "ionizing radiation" include all forms of radiation, including but not limited to α, β, and γ radiation and ultraviolet light. Immunosuppresants
[00584] In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is used to treat or reduce fibrosis in a mammal. In one aspect, a compound of Formula (I), (II), (III), (IV) or (V) is administered in combination with one or more immunosuppresants. Immunosuppressive therapy is clinically used to treat or prevent the rejection of transplanted organs and tissues (e.g. bone marrow, heart, kidney, liver); treatment of autoimmune diseases or diseases that are most likely of autoimmune origin (e.g. rheumatoid arthritis, myasthenia gravis, systemic lupus erythematosus, Crohn's disease, and ulcerative colitis); and treatment of some other non- autoimmune inflammatory diseases (e.g. long term allergic asthma control), and in the treatment of fibrotic conditions.
[00585] In some embodiments, a compound of Formula (I), (II), (III), (IV) or (V) is adminsitered with corticosteroids. In some embodiments, a compound of Formula (I), (II), (III), (IV) or (V) is adminsitered with an a therapeutic agent selected from among: Calcineurin inhibitors (such as, but not limited to, cyclosporin, tacrolimus); mTOR inhibitors (such as, but not limited to, sirolimus, everolimus); anti-proliferatives (such as, but not limited to, azathioprine, mycophenolic acid); corticosteroids (such as, but not limited to, prednisone, cortisone acetate, prednisolone, methylprednisolone, dexamethasone, betamethasone, triamcinolone, beclometasone, fludrocortisone acetate, deoxycorticosterone acetate, aldosterone, hydrocortisone); antibodies (such as, but not limited to, monoclonal anti-IL-2Rα receptor antibodies (basiliximab, daclizumab), polyclonal anti-T-cell antibodies (anti-thymocyte globulin (ATG), anti-lymphocyte globulin (ALG)), B-cell antagonists, rituximab, natalizumab. [00586] Other therapeutic agents include, but are not limited to: cyclophosphamide, penicillamine, cyclosporine, nitrosoureas, cisplatin, carboplatin, oxaliplatin, methotrexate, azathioprine, mercaptopurine, pyrimidine analogues, protein synthesis inhibitors, dactinomycin, anthracyclines, mitomycin C, bleomycin, mithramycin, Atgarr/R), Thymoglobuline®, OKT3®,
basiliximab, daclizumab, cyclosporin, tacrolimus, sirolimus, Interferons (IFN-β, IFN-γ), opioids, TNF binding proteins (infliximab, etanercept, adalimumab, golimumab), leflunomide, gold thioglucose, gold thiomalate, aurofin, sulfasalazine, hydroxychloroquinine, minocycline, rapamicin, mycophenolic acid, mycophenolate mofetil, FTY720, as well as those listed in US 7,060,697.
[00587] In one embodiment, a compound of Formula (I), (II), (III), (IV) or (V) is administered in combination with Cyclosporin A (CsA) or tacrolimus (FK506). In one embodiment, a compound of Formula (I), (II), (III), (IV) or (V) is administered to a mammal in combination with an anti-inflammatory agent including, but not limited to, non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids (glucocorticoids).
[00588] NSAIDs include, but are not limited to: aspirin, salicylic acid, gentisic acid, choline magnesium salicylate, choline salicylate, choline magnesium salicylate, choline salicylate, magnesium salicylate, sodium salicylate, diflunisal, carprofen, fenoprofen, fenoprofen calcium, flurobiprofen, ibuprofen, ketoprofen, nabutone, ketolorac, ketorolac tromethamine, naproxen, oxaprozin, diclofenac, etodolac, indomethacin, sulindac, tolmetin, meclofenamate, meclofenamate sodium, mefenamic acid, piroxicam, meloxicam, COX-2 specific inhibitors (such as, but not limited to, celecoxib, rofecoxib, valdecoxib, parecoxib, etoricoxib, lumiracoxib, CS-502, JTE-522, L-745,337 and NS398). [00589] Corticosteroids, include, but are not limited to: betamethasone, prednisone, alclometasone, aldosterone, amcinonide, beclometasone, betamethasone, budesonide, ciclesonide, clobetasol, clobetasone, clocortolone, cloprednol, cortisone, cortivazol, deflazacort, deoxycorticosterone, desonide, desoximetasone, desoxycortone, dexamethasone, diflorasone, diflucortolone, difluprednate, fluclorolone, fludrocortisone, fludroxycortide, flumetasone, flunisolide, fluocinolone acetonide, fluocinonide, fluocortin, fluocortolone, fluorometholone, fluperolone, fluprednidene, fluticasone, formocortal, halcinonide, halometasone, hydrocortisone/cortisol, hydrocortisone aceponate, hydrocortisone buteprate, hydrocortisone butyrate, loteprednol, medrysone, meprednisone, methylprednisolone, methylprednisolone aceponate, mometasone furoate, paramethasone, prednicarbate, prednisone/prednisolone, rimexolone, tixocortol, triamcinolone, and ulobetasol. [00590] In one embodiment, a compound of Formula (I), (II), (III), (IV) or (V) is administered in combination with leukotriene receptor antagonists including, but are not limited to, BAY u9773 (see EP 00791576; published 27 Aug 1997), DUO-LT (Tsuji et al, Org. Biomol. Chem., 1, 3139-3141, 2003), zafirlukast, montelukast, prankulast, and derivatives or analogs thereof.
Other Combination Therapies
[00591] In another embodiment described herein, methods for treatment of LPA-dependent or LPA-mediated conditions or diseases, such as atherosclerosis, comprises administration to a patient compounds, pharmaceutical compositions, or medicaments described herein in combination with at least one additional agent selected, by way of example only, HMG-CoA reductase inhibitors (e.g., statins in their lactonized or dihydroxy open acid forms and pharmaceutically acceptable salts and esters thereof, including but not limited to lovastatin; simvastatin; dihydroxy open-acid simvastatin, particularly the ammonium or calcium salts thereof; pravastatin, particularly the sodium salt thereof; fluvastatin, particularly the sodium salt thereof; atorvastatin, particularly the calcium salt thereof; nisvastatin, also referred to as NK- 104; rosuvastatin); agents that have both lipid-altering effects and other pharmaceutical activities; HMG-CoA synthase inhibitors; cholesterol absorption inhibitors such as ezetimibe; cholesterol ester transfer protein (CETP) inhibitors, for example JTT-705 and CP529, 414; squalene epoxidase inhibitors; squalene synthetase inhibitors (also known as squalene synthase inhibitors); acyl-coenzyme A: cholesterol acyltransferase (ACAT) inhibitors including selective inhibitors of ACAT-I or ACAT-2 as well as dual inhibitors of ACAT-I and-2; microsomal triglyceride transfer protein (MTP) inhibitors; probucol; niacin; bile acid sequestrants; LDL (low density lipoprotein) receptor inducers; platelet aggregation inhibitors, for example glycoprotein Ilb/IIIa fibrinogen receptor antagonists and aspirin; human peroxisome proliferator activated receptor gamma (PPARγ) agonists, including the compounds commonly referred to as glitazones, for example troglitazone, pioglitazone and rosiglitazone and including those compounds included within the structural class known as thiazolidinediones as well as those PPARγ agonists outside the thiazolidinedione structural class; PP ARa agonists such as clofibrate, fenofibrate including micronized fenofibrate, and gemfibrozil ; PPAR dual α/γ agonists such as 5-[(2, 4-dioxo-5-thiazolidinyl)methyl]-2-methoxy-N-[[4-
(trifluoromethyl)phenyl]methyl]-benzamide, known as KRP-297; vitamin B6 (also known as pyridoxine) and the pharmaceutically acceptable salts thereof such as the HCl salt; vitamin B 12 (also known as cyanocobalamin); folic acid or a pharmaceutically acceptable salt or ester thereof such as the sodium salt and the methylglucamine salt; anti-oxidant vitamins such as vitamin C and E and beta carotene; beta-blockers; angiotensin II antagonists such as losartan; angiotensin converting enzyme inhibitors such as enalapril and captopril ; calcium channel blockers such as nifedipine and diltiazam; endothelian antagonists; agents that enhance ABCl gene expression; FXR and LXR ligands including both inhibitors and agonists; bisphosphonate compounds such as alendronate sodium; and cyclooxygenase-2 inhibitors such as rofecoxib and celecoxib.
[00592] In another embodiment described herein, methods for treatment of LPA-dependent or LPA-mediated conditions or diseases, such as the therapy of stroke, comprises administration to a patient compounds, pharmaceutical compositions, or medicaments described herein in combination with at least one additional agent selected from, by way of example only, COX-2 inhibitors; nitric oxide synthase inhibitors, such as N-(3-(aminomethyl)benzyl) acetamidine; Rho kinase inhibitors, such as fasudil; angiotension II type-1 receptor antagonists, including candesartan, losartan, irbesartan, eprosartan, telmisartan and valsartan; glycogen synthase kinase 3 inhibitors; sodium or calcium channel blockers, including crobenetine; p38 MAP kinase inhibitors, including SKB 239063; thromboxane AX- synthetase inhibitors, including isbogrel, ozagrel, ridogrel and dazoxiben; statins (HMG CoA reductase inhibitors), including lovastatin, simvastatin, dihydroxy open-acid simvastatin, pravastatin, fluvastatin, atorvastatin, nisvastatin, and rosuvastatin; neuroprotectants, including free radical scavengers, calcium channel blockers, excitatory amino acid antagonists, growth factors, antioxidants, such as edaravone, vitamin C, TROLOX™, citicoline and minicycline, and reactive astrocyte inhibitors, such as (2R)-2-propyloctanoic acid; beta andrenergic blockers, such as propranolol, nadolol, timolol, pindolol, labetalol, metoprolol, atenolol, esmolol and acebutolol; NMDA receptor antagonists, including memantine; NR2B antagonists, such as traxoprodil; 5-HT1A agonists; receptor platelet fibrinogen receptor antagonists, including tirofiban and lamifiban; thrombin inhibitors; antithrombotics, such as argatroban; antihypertensive agents, such as enalapril; vasodilators, such as cyclandelate; nociceptin antagonists; DPIV antagonists; GABA 5 inverse agonists; and selective androgen receptor modulators.
[00593] In another embodiment described herein, methods for treatment of LPA-dependent or LPA-mediated conditions or diseases, such as the therapy of interstitial cystitis, comprises administration to a patient compounds, pharmaceutical compositions, or medicaments described herein in combination with at least one additional agent selected from, by way of example only, dimethylsulfoxide, omalizumab, and pentosan polysulfate.
[00594] In yet another embodiment described herein, methods for treating LPA-dependent or LPA-mediated conditions or diseases, such as the therapy of respiratory disorders (e.g., asthma, COPD and rhinitis), comprises administration to a patient compounds, pharmaceutical compositions, or medicaments described herein in combination with at least one agent used in the treatment of respiratory conditions. Agents used in the treatment of respiratory conditions include, but are not limited to, bronchodilators (e.g., sympathomimetic agents and xanthine derivatives), leukotriene receptor antagonists, leukotriene formation inhibitors, leukotriene modulators, nasal decongestants, respiratory enzymes, lung surfactants, antihistamines (e.g., mepyramine (pyrilamine), antazoline, diphenhydramine, carbinoxamine, doxylamine,
clemastine, dimenhydrinate, pheniramine, chlorphenamine (chlorpheniramine), dexchlorpheniramine, brompheniramine, triprolidine, cetirizine, cyclizine, chlorcyclizine, hydroxyzine, meclizine, loratadine, desloratidine, promethazine, alimemazine (trimeprazine), cyproheptadine, azatadine, ketotifen, acrivastine, astemizole, cetirizine, mizolastine, terfenadine, azelastine, levocabastine, olopatadine, levocetirizine, fexofenadine), mucolytics, corticosteroids, anticholinergics, antitussives, analgesics, expectorants, albuterol, ephedrine, epinephrine, fomoterol, metaproterenol, terbutaline, budesonide, ciclesonide, dexamethasone, flunisolide, fluticasone propionate, triamcinolone acetonide, ipratropium bromide, pseudoephedrine, theophylline, montelukast, zafirlukast, ambrisentan, bosentan, enrasentan, sitaxsentan, tezosentan, iloprost, treprostinil, pirfenidone, 5-lipoxygenase-activating protein (FLAP) inhibitors, FLAP modulators and 5-LO inhibitors.
[00595] In a specific embodiment described herein, methods for treating LPA-dependent or LPA-mediated conditions or diseases, such as the therapy of asthma and/or COPD, comprises administration to a patient anti-inflammatory agents. In certain embodiments, methods for treating LPA-dependent or LPA-mediated conditions or diseases, such as the therapy of asthma and/or COPD, comprise administration to a patient compounds, pharmaceutical compositions, or medicaments described herein in combination with at least one additional agent selected from, but not limited to, epinephrine, isoproterenol, orciprenaline, bronchodilators, glucocorticoids, leukotriene modifiers, mast-cell stabilizers, xanthines, anticholinergics, β-2 agonists, FLAP inhibitors, FLAP modulators or 5-LO inhibitors, β-2 agonists include, but are not limited to, short-acting β-2 agonists (e.g., salbutamol (albuterol), levalbuterol, terbutaline, pirbuterol, procaterol, metaproterenol, fenoterol and bitolterol mesylate) and long-acting β-2 agonists (e.g., salmeterol, formoterol, bambuterol and clenbuterol). FLAP inhibitors and/or FLAP modulators include, but are not limited to, 3-[3-tert-butylsulfanyl-l-[4-(6-methoxy- pyridin-3-yl)-benzyl]-5-(pyridin-2-ylmethoxy)-lH-indol-2-yl]-2,2-dimethyl-propionic acid, 3- [3-fert-butylsulfanyl-l-[4-(6-ethoxy-pyridin-3-yl)-benzyl]-5-(5-methyl-pyridin-2-ylmethoxy)- lH-indol-2-yl]-2,2-dimethyl-propionic acid, MK-886, MK-0591, BAY-xl005 and compounds found in US 2007/0225285, US 2007/0219206, US 2007/0173508, US 2007/0123522 and US 2007/0105866 (each of which are hereby incorporated by reference). Glucocorticoids include, but are not limited to, beclometasone, budesonide, ciclesonide, fluticasone and mometasone.
Anticholinergics include, but are not limited to, ipratropium and tiotropium. Mast cell stabilizers include, but are not limited to, cromoglicate and nedocromil. Xanthines include, but are not limited to, amminophylline, theobromine and theophylline. Leukotriene antagonists include, but are not limited to, montelukast, tomelukast, pranlukast and zafirlukast. 5-LO inhibitors include,
but are not limited to, zileuton, VIA-2291 (ABT761), AZ-4407 and ZD-2138 and compounds found in US 2007/0149579, WO2007/016784.
[00596] In another specific embodiment described herein, methods for treating LPA-dependent or LPA-mediated conditions or diseases, such as the therapy of allergic diseases or conditions, comprises administration to a patient compounds, pharmaceutical compositions, or medicaments described herein in combination with at least one additional agent selected from, by way of example only, antihistamines, leukotriene antagonists, corticosteroids and decongestants. Leukotriene antagonists include, but are not limited to, montelukast, tomelukast, pranlukast and zafirlukast. [00597] In one aspect, LPA receptor antagonists described herein are admistered in combination with one or more agents used to treat used to treat asthma, including, but not limited to: combination inhalers (fluticasone and salmeterol oral inhalation (e.g. Advair)); inhaled Beta-2 agonists (albuterol inhaler; albuterol nebulizer solution; formoterol; isoproterenol oral inhalation; levalbuterol; metaproterenol inhalation; pirbuterol acetate oral inhalation; salmeterol aerosol inhalation; salmeterol powder inhalation; terbutaline inhaler); inhaled corticosteroids
(beclomethasone oral inhalation; budesonide inhalation solution; budesonide inhaler; flunisolide oral inhalation; fluticasone inhalation aerosol; fluticasone powder for oral inhalation; mometasone inhalation powder; triamcinolone oral inhalation); leukotriene modifiers (montelukast; zafirlukast; zileuton); mast cell stabilizers (cromolyn inhaler; nedocromil oral inhalation); monoclonal antibodies (omalizumab); oral Beta-2 agonists (albuterol oral syrup; albuterol oral tablets; metaproterenol; terbutaline); bronchodilator (aminophylline; oxtriphylline; theophylline).
[00598] In one aspect, LPA receptor anatogonists described herein are admistered in combination with one or more agents used to treat allergy, including, but not limited to: antihistamine and decongestant combinations (cetirizine and pseudoephedrine; desloratadine and pseudoephedrine ER; fexofenadine and pseudoephedrine; loratadine and pseudoephedrine); antihistamines (azelastine nasal spray; brompheniramine; brompheniramine oral suspension; carbinoxamine; cetirizine; chlorpheniramine; clemastine; desloratadine; dexchlorpheniramine ER; dexchlorpheniramine oral syrup; diphenhydramine oral; fexofenadine; loratadine; promethazine); decongestants (pseudoephedrine); leukotriene modifiers (montelukast; montelukast granules); nasal anticholinergics (ipratropium); nasal corticosteroids (beclomethasone nasal inhalation; budesonide nasal inhaler; flunisolide nasal inhalation; fluticasone nasal inhalation; mometasone nasal spray; triamcinolone nasal inhalation; triamcinolone nasal spray); nasal decongestants (phenylephrine); nasal mast cell stabilizers (cromolyn nasal spray).
[00599] In one aspect, LPA receptor anatogonists described herein are admistered in combination with one or more agents used to treat chronic obstructive pulmonary disease (COPD), including, but not limited to: anticholinergics - ipratropium bromide oral inhalation); combination Inhalers (albuterol and ipratropium (e.g. Combivent, DuoNeb); fluticasone and salmeterol oral inhalation (e.g. Advair)); corticosteroids (dexamethasone tablets; fludrocortisone acetate; hydrocortisone tablets; methylprednisolone; prednisolone liquid; prednisone oral; triamcinolone oral); inhaled Beta-2 Agonists (albuterol inhaler; albuterol nebulizer solution; formoterol; isoproterenol oral inhalation; levalbuterol; metaproterenol inhalation; pirbuterol acetate oral inhalation; salmeterol aerosol inhalation; salmeterol powder inhalation; terbutaline inhaler); inhaled Corticosteroids (beclomethasone oral inhalation; budesonide inhalation solution; budesonide inhaler; flunisolide oral inhalation; fluticasone inhalation aerosol; fluticasone powder for oral inhalation; triamcinolone oral inhalation); mukolytics (guaifenesin); oral Beta-2 agonists (albuterol oral syrup; albuterol oral tablets; metaproterenol; terbutaline); bronchodilator (aminophylline; oxtriphylline; theophylline). [00600] In one embodiment, a compound of Formula (I), (II), (III), (IV) or (V) described herein is administered to a patient in combination with inhaled corticosteroids.
[00601] In one embodiment, a compound of Formula (I), (II), (III), (IV) or (V) described herein is administered to a patient in combination with beta2-adrenergic receptor agonists. In one embodiment, a compound of Formula (I), (II), (III), (IV) or (V) is administered to a patient in combination with short acting beta2-adrenergic receptor agonists. In one embodiment, a compound of Formula (I), (II), (III), (IV) or (V) are administered to a patient in combination with long-acting beta2-adrenergic receptor agonists.
Kits/ Articles of Manufacture
[00602] For use in the therapeutic applications described herein, kits and articles of manufacture are also described herein. Such kits can comprise a carrier, package, or container that is compartmentalized to receive one or more containers such as vials, tubes, and the like, each of the container(s) comprising one of the separate elements to be used in a method described herein. Suitable containers include, for example, bottles, vials, syringes, and test tubes. The containers are formed from any acceptable material including, e.g., glass or plastic. [00603] For example, the container(s) can comprise one or more compounds described herein, optionally in a composition or in combination with another agent as disclosed herein. The container(s) optionally have a sterile access port (for example the container can be an intravenous solution bag or a vial having a stopper pierceable by a hypodermic injection needle). Such kits optionally comprising a compound with an identifying description or label or instructions relating to its use in the methods described herein.
[00604] A kit will typically comprise one or more additional containers, each with one or more of various materials (such as reagents, optionally in concentrated form, and/or devices) desirable from a commercial and user standpoint for use of a compound described herein. Non- limiting examples of such materials include, but not limited to, buffers, diluents, filters, needles, syringes; carrier, package, container, vial and/or tube labels listing contents and/or instructions for use, and package inserts with instructions for use. A set of instructions will also typically be included.
[00605] A label can be on or associated with the container. A label can be on a container when letters, numbers or other characters forming the label are attached, molded or etched into the container itself; a label can be associated with a container when it is present within a receptacle or carrier that also holds the container, e.g., as a package insert. A label can be used to indicate that the contents are to be used for a specific therapeutic application. The label can also indicate directions for use of the contents, such as in the methods described herein.
EXAMPLES
[00606] These examples are provided for illustrative purposes only and not to limit the scope of the claims provided herein.
Synthesis of Compounds
(R)-2 '-chloro-alpha-methylbenzyl alcohol
[00607] Using the procedure of Meier et al (Tetrahedron, 1996, 52, 589; Method 3), T- chloroacetophenone (Aldrich) was reduced to give (R)-2'-chloro-alpha-methylbenzylalcohol in % 92e.e. (HPLC analysis of the acetate derivative (made by reacting the benzyl alcohol with acetyl chloride and triethylamine in methylene chloride) using Chiralcel OD eluted with 99: 1 Hexane:Ethanol. R isomer retention time 4.3 minutes) (S)-2 '-chloro-alpha-methylbenzyl alcohol
[00608] Using the procedure of Meier et al (Tetrahedron, 1996, 52, 589; Method 3), T- chloroacetophenone (Aldrich) was reduced to give (R)-2'-chloro-alpha-methylbenzylalcohol in % e.e. (HPLC analysis of the acetate derivative (made by reacting the benzyl alcohol with acetyl
chloride and triethylamine in methylene chloride) using Chiralcel OD eluted with 99: 1 Hexane:Ethanol. S isomer retention time 5.3 minutes) (R)-2 '-fluoro-alpha-methylbenzyl alcohol
[00609] Using the procedure of Meier et al (Tetrahedron, 1996, 52, 589; Method 3), T- fluoroacetophenone (Aldrich) was reduced to give (R)-2'-fluoro-alpha-methylbenzylalcohol in % e.e. (HPLC analysis of the acetate derivative (made by reacting the benzyl alcohol with acetyl chloride and triethylamine in methylene chloride) using Chiralcel OD eluted with 99.8:0.2 Hexane:Ethanol. R isomer retention time 5.9 minutes) (S)-2 '-fluoro-alpha-methylbenzyl alcohol
[00610] Using the procedure of Meier et al (Tetrahedron, 1996, 52, 589; Method 3), T- fluoroacetophenone (Aldrich) was reduced to give (R)-2'-fluoro-alpha-methylbenzylalcohol in % e.e. (HPLC analysis of the acetate derivative (made by reacting the benzyl alcohol with acetyl chloride and triethylamine in methylene chloride) using Chiralcel OD eluted with 99.8:0.2 Hexane:Ethanol. S isomer retention time 6.7 minutes)
Example 1: Synthesis of l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol- 5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-1)
^NH O
Step 1: 3-Methylamino-but-2-enoic acid methyl ester
[00611] To a solution of methyl acetoacetate (29.4g, 253mmol) in MeOH (3OmL) was added methylamine (33 wt% in EtOH; 48mL, 385mmol) dropwise at room temperature. The reaction was stirred for 1 hour, and then concentrated and dried to give the title compound as a white crystalline solid.
[00612] To 3-methylamino-but-2-enoic acid methyl ester (5.Og, 39.1mmol) in THF (7OmL) was added pyridine (3.7mL). The mixture was cooled to 00C, and 4-bromobenzoyl chloride (8.55g, 39.1mmol) in THF (3OmL) was added dropwise over 2 minutes. The reaction was warmed to room temperature over 1 hour and then stirred at room temperature overnight. Aqueous workup gave the title compound.
Step 3: 5-(4-Bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid methyl ester
[00613] 2-(4-Bromo-benzoyl)-3-oxo-butyric acid methyl ester (1 Ig, 39mmol) and hydroxylamine hydrochloride (2.66g, 39mmol) were combined in acetic acid (5OmL), and the reaction was stirred at 115°C for 1 hour. After cooling, aqueous work-up gave the title compound.
Step 4: 5-(4-Bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid
[00614] Lithium hydroxide (2g, 47.7mmol) was added to a solution of 5-(4-bromo-phenyl)-3- methyl-isoxazole-4-carboxylic acid methyl ester (7g, 23.6mmol) in MeOH (5OmL) and H2O (1OmL), and the reaction was stirred at 6O0C for 1 hour. Acidic work-up the title compound.
Step 5: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-phenyl-ethyl ester
[00615] 5-(4-Bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid (2.Og, 7.09mmol) and triethylamine (0.99mL, 7.09mmol) were dissolved in toluene (5OmL). Diphenylphosphoryl
azide (1.5mL, 7.09mmol) was added, followed by (R)-(+)-l-phenylethyl alcohol (0.865g, 7.09mmol; commercially available or prepared using procedures desribed herein or in the literature: e.g. EJ. Corey et al. J. Am. Chem. 1987, 109, 5551-5553), and the reaction was stirred at 800C for 4 hours. The mixture was concentrated, and the residue was purified by silica gel chromatography to give the title compound.
Step 6: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00616] [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl-ethyl ester (0.248g, 0.62mmol), 4-(l'-carboxyl-cyclopropyl)phenylboronic acid (0.16Og, 0.62mmol), and sodium carbonate (0.155g, 1.85mmol) were combined in 2:1 DME:H2O. The solution was purged with N2 for 10 minutes, and then bis(triphenylphosphine)palladium(II) dichloride (0.047g, O.Oόmmol) was added. The reaction was purged with N2 for an additional 10 minutes, and then stirred in a sealed tube at 800C for 2 hours. The mixture was partitioned between EtOAc and H2O, and the aqueous layer was extracted with EtOAc. The combined organic layers were dried over MgSO4, filtered, and concentrated, and the residue was purified by silica gel chromatography to give the title compound.
Example Ia: Alternate synthesis of l-{4'-[3-Methyl-4-((R)-l-phenyl- ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-1)
Step 1: l-(biphenyl-4-yl)cyclopropanecarbonitrile
[00617] 4-phenyl-phenylacetonitrile (VWR scientific, 55.7 g, 289 mmol) was added to a solution of KOH (161.6 g, 2890 mmol) in water (170 mL) and toluene (550 mL) at room temperature. Tetrabutyl ammonium bromide (9.2g, 29 mmol) followed by 1,2 dibromoethane (64.9 g, 347 mmol) were added and the solution was heated to 65°C overnight. Reaction complete by TLC (10% EtOAc/hex). The organic layer was extracted 2 times with dilute hydrochloric acid, dried and evaporated to yield 63g of l-(biphenyl-4-yl)cyclopropanecarbonitrile.
Step 2: l-(Biphenyl-4-yl)cyclopropanecarboxylic acid
[00618] l-(Biphenyl-4-yl)cyclopropanecarbonitrile (63 g, 288 mmol), KOH (1130 mmol) and ethylene glycol (350 mL) were heated to 1600C for 6 hours (reaction complete by LCMS). The solution was cooled to room temperature, water (1.5 L) was added and the solution acidified to precipitate the product. The product was filtered overnight on a large Buchner (product formed a gel like suspension). The resulting wet solid was extracted with CH2Cl2 (~2 L) and water, dried and evaporated to yield ~60 g of 1 -(biphenyl-4-yl)cyclopropanecarboxylic acid that was used as such in the next step.
Step 3: l-(Biphenyl-4-yl)cyclopropanecarboxylic acid ethyl ester
[00619] l-(Biphenyl-4-yl)cyclopropanecarboxylic acid (10 g, 42 mmol), ethanol (100 mL) and sulfuric acid (40 mL) were heated to 65°C for 4 hours. The product was extracted with CH2Cl2 and water (2X), dried and evaporated to yield 9.5g of 1 -(biphenyl-4-yl)cyclopropanecarboxylic acid ethyl ester.
Step 4: l-(4'-Acetylbiphenyl-4-yl)cyclopropanecarboxylic acid ethyl ester
[00620] To l-(biphenyl-4-yl)cyclopropanecarboxylic acid ethyl ester (9 g, 33.8 mmol) in CH2Cl2 (100 mL) was added aluminum chloride (9.4 g, 71 mmol) followed by acetyl chloride (5.5 g, 71 mmol). The solution was stirred at room temperature for 1.5 hours then slowly poured into water. The organic layer was separated and extracted 2 times with water. The organic layer was dried and evaporated to yield 11.3 g of the title compound.
Step 5: 4'-(l-(Ethoxycarbonyl)cyclopropyl)biphenyl-4-carboxylic acid [00621] To l-(4'-acetylbiphenyl-4-yl)cyclopropanecarboxylic acid ethyl ester (10.1 g, 33 mmol) in dioxane (200 mL) at ~ 100C was added a solution of bromine (26.4 g, 165 mmol), sodium hydroxide (22.4 g, 561 mmol) in water (150 mL). The solution was stirred at room temperature for 30 minutes, poured into water (500 mL) and acidified with dilute hydrochloric acid. Sodium metabisulfite was added until the brown bromine color dissipated. The product was filtered and dried in a vacuum over overnight at 400C to yield 1O g of 4'-(I- (ethoxycarbonyl)cyclopropyl)biphenyl-4-carboxylic acid.
Step 6: 3-Methylamino-but-2-enoic acid benzyl ester
[00622] To benzyl acetoacetate (29 g, 151 mmol) in ethanol (30 mL) was added methyl amine (33% in ethanol, 7.02 g, 226 mmol). The solution was stirred for 2 hours at room temperature followed by evaporation to yield a yellow oil (~30 g).
Step 7: Ethyl l-(4'-(2-(benzyloxycarbonyl)-3-(methylamino)but-2-enoyl)biphenyl-4- yl)cyclopropanecarboxylate
[00623] 4'-(l-(Ethoxycarbonyl)cyclopropyl)biphenyl-4-carboxylic acid (4.47 g, 14.4 mmol), dichloroethane (50 mL), DMF (0.1 mL), thionyl choride (2.3 mL, 32 mmol) were heated to 800C for 1 hours, (acid chloride formation was monitored by adding small aliquot (100 μL) to a solution of benyl amine in acetonitrile and analyzing for the benzyl amide by LCMS; no starting material was observed by LCMS). The solution was evaporated on a rotavap and THF (10 mL) was added. The solution of the acid chloride in THF was added via syringe to a solution of 3- methylamino-but-2-enoic acid benzyl ester (3.23 g, 15.8 mmol) and pyridine (2.4 mL, 30.2 mmol) in THF (50 mL). The solution was stirred at 500C for 2 hours then the volatiles were evaporated using a rotavap to yield the crude product.
Step 8: Benzyl 5-(4'-(l-(ethoxycarbonyl)cyclopropyl)biphenyl-4-yl)-3-methylisoxazole-4- carboxylate
[00624] To the crude material from the previous reaction was added hydroxyl amine hydrochloride (1.5 g, 21.6 mmol) and acetic acid (50 mL). The solution was heated to 95°C for 30 minutes cooled to room temperature, extracted with CH2CI2 and water (4 times, second and third time made basic with sodium bicarbonate). Dried, evaporated and purified on column 0 to 20% EtOAc/hexanes to yield 3.3 g of product.
Step 9: 5-(4'-(l-(ethoxycarbonyl)cyclopropyl)biphenyl-4-yl)-3-methylisoxazole-4- carboxylic acid
[00625] The benzyl ester from Step 8 (1 g, 2.1 mmol) in ethyl acetate (10 mL) was degassed with nitrogen for 10 minutes. 10% Palladium on activated carbon (0.2 g, 0.2 mmol) was added and the solution was sparged with hydrogen via balloon. The balloon of hydrogen was maintained on the head space and the solution stirred for 1.5 hours. The reaction was diluted with ethanol and actone (to solublize the product), filtered through celite and evaporated to yield 700 mg product.
Step 10: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester
[00626] To the acid from Step 9 (0.5 g, 1.28 mmol) in toluene (5 mL) was added (R)-I -phenyl ethanol (0.16 g, 1.34 mmol), triethyl amine (0.26 g, 2.56 mmol) and diphenyl phosphoryl azide
(0.39 g, 1.4 mmol). The solution was heated to 800C for 1 hour, cooled to room temperature and extracted with water 3 times. The organic layer wad dried and evaporated to yield 0.61 g. The product was further purified by column 0 to 40% EtOAc/hex to yield 0.42 g of pure product (65%) as an oil that foams on drying under vacuum.
Step 11: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00627] To ethyl ester (22.7 g, 44 mmol) in methanol (300 mL) was added lithium hydroxide (9.1 g, 222 mmol). The solution was heated to 65 0C for 2 hours, extracted into methylene choride and washed with diluted hydrochloric acid. The organic layer was dried and evaporated to yield 20.8 grams of product.
Example Ib: Alternate synthesis of l-{4'-[3-Methyl-4-((R)-l-phenyl- ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-1)
Step 1: l-(Biphenyl-4-yl)cyclopropanecarboxylic acid isopropyl ester
[00628] l-(Biphenyl-4-yl)cyclopropanecarboxylic acid (10 g, 42 mmol), isopropanol (100 mL), thionyl chloride (6.8 mL, 92 mmol) were heated to 65°C for 4 hours. Sulfuric acid (20 mL) was added and heated at 65°C overnight. The product is extracted with CH2CI2 and water (2X) dried and evaporated to yield 10.8 g of product.
Step 2: l-(4'-Acetylbiphenyl-4-yl)cyclopropanecarboxylic acid isopropyl ester
[00629] To l-(biphenyl-4-yl)cyclopropanecarboxylic acid isopropyl ester (10.2 g, 36 mmol) in CH2CI2 (100 mL) was added aluminum chloride (10.2 g, 76.5 mmol) followed by acetyl chloride (5.97 g, 76.5 mmol). The solution was stirred at room temperature for 1.5 hours then slowly poured into water. The organic layer was separated and extracted 1 time with sodium potassium tartrate solution (20 g in 250 mL water). The organic layer was dried and evaporated to yield 12.6 g of product.
[00630] To l-(4'-acetylbiphenyl-4-yl)cyclopropanecarboxylic acid isopropyl ester (11.6 g, 36 mmol) in dioxane (200 mL) at ~ 100C was added a solution of bromine (28.8 g, 180 mmol), sodium hydroxide (24.5 g, 612 mmol) in water (150 mL). The solution was stirred at room temperature for 30 minutes poured into water (500 mL) and acidified with dilute hydrochloric acid. Sodium metabisulfite was added until the brown bromine color dissipated. The product was filtered and dried in a vacuum over overnight at 400C to yield 1O g of product.
Step 4: Isopropyl l-(4'-(2-(benzyloxycarbonyl)-3-(methylamino)but-2-enoyl)biphenyl-4- yl)cyclopropanecarboxylate
[00631] 4'-(l-(Isopropoxycarbonyl)cyclopropyl)biphenyl-4-carboxylic acid (9.2 g, 28 mmol), dichloroethane (50 niL), DMF (0.1 niL), thionyl choride (5.5 niL, 62 mmol) were heated to 75°C for 1.5 hours, (acid chloride formation was monitored by adding small aliquot (100 μL) to a solution of benyl amine in acetonitrile and analyzing for the benzyl amide by LCMS; no starting material was observed by LCMS). The solution was evaporated on a rotavap and THF (10 mL) was added. The solution of the acid chloride in THF was added via syringe to a solution of 3-methylamino-but-2-enoic acid methyl ester (4.0 g, 31.2 mmol) and pyridine (5.5 mL, 70 mmol) in THF (50 mL). The solution was stirred at room temperature overnight. The volatiles were evaporated on a rotavap to yield the crude product.
Step 5: Methyl 5-(4'-(l-(isopropoxycarbonyl)cyclopropyl)biphenyl-4-yl)-3- methylisoxazole-4-carboxylate
[00632] To the crude material from the previous reaction was added hydroxyl amine hydrochloride (2.9 g, 42 mmol) and acetic acid (50 mL). The solution was heated to 1000C for 30 minutes cooled to room temperature, extracted with CH2CI2 and water (4 times, second and
third time made basic with sodium bicarbonate). The organic phase was dried, evaporated and purified on column (220 g silica; 0 to 20% EtOAc/hexanes) to yield 6 g of product.
Step 6: 5-(4'-(l-(propoxycarbonyl)cyclopropyl)biphenyl-4-yl)-3-methylisoxazole-4- carboxylic acid
[00633] To the methyl ester from Step 5 (5.2 g, 12.4 mmol) in THF (100 niL) and ethanol (20 mL) was added a solution of sodium hydroxide (1.5 g, 37.2 mmol) in water (4OmL). The solution was stirred at room temperature 3 hours. ~ 50 mL solvent evaporated and 200 mL water added. The product was precipitated out of solution with dilute hydrochloric acid to pH 2. The product was isolated by filtration to yield 4.6 grams of product.
Step 7: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid isopropyl ester
[00634] To the acid from Step 6 (4.0 g, 10 mmol) in toluene (50 mL) was added R-1-pheynyl ethanol (1.33 g, 11 mmol), triethyl amine (2.02 g, 20 mmol) and diphenyl phosphoryl azide (3.16 g, 11.5 mmol). The solution was heated to 800C for 1 hour cooled to room temperature and extracted with water 3 times. The organic layer wad dried and evaporated to yield 5.7g of product.
Step 8: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00635] To the isopropyl ester from Step 7 (5.2 g, 10 mmol) in THF (30 mL), MeOH (10 mL) was added NaOH (2 g, 50 mmol) in water (10 mL). The solution is heated to 65°C for 5 hours. The solution was cooled to room temperature, extracted with methylene chloride and dilute hydrochloric acid. The organic was dried and evaporated and the product was purified by column chromatography (0 to 60% EtOAc/hexanes) to yield ~3.5 grams of product. Example 2: Synthesis of l-{4'-[3-Methyl-4-((R)-l-o-tolyl-ethoxycarbonylamino)-isoxazol-5- yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-4)
Step 1: l-(4-Bromo-phenyl)-cyclopropanecarbonitrile
[00636] Potassium hydroxide (14.3g, 255mmol) was dissolved in H2O (5mL) and toluene (4OmL). 4-Bromophenylacetonitrile (5.Og, 25.5mmol) and tetrabutylammonium bromide (0.41g, 1.3mmol) was added, followed by 1 ,2-dibromoethane (3.25mL, 38mmol) dropwise over 10 minutes. The reaction was stirred at room temperature for 2 hours and then worked-up to give the title compound.
Step 2: l-(4-Bromo-phenyl)-cyclopropanecarboxylic acid
[00637] l-(4-Bromo-phenyl)-cyclopropanecarbonitrile (5g, 22.5mmol) and potassium hydroxide (5g, 89.3mmol) were combined in ethylene glycol (7OmL), and the reaction was stirred at 1800C for 4 hours. The mixture was poured into H2O, acidified, and filtered to give the title compound.
[00638] l-(4-Bromo-phenyl)-cyclopropanecarboxylic acid (5g, 20.7mmol) in EtOH (5OmL) was treated with sulfuric acid (2mL), and the reaction was stirred at 75°C for 1 hour. The mixture was worked up to give the title compound.
Step 4: l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester
[00639] l-(4-Bromo-phenyl)-cyclopropanecarboxylic acid ethyl ester (3.6g, 13.4mmol), bis(pinacolato)diboron (3.37g, lό.lmmol), and potassium acetate (2.8g, 29mmol) were combined in 1,4-dioxane (3OmL). The solution was purged with N2 for 10 minutes, and then (l,l'-bis(diphenylphosphino)ferrocene)-dichloropalladium(II) (0.5Og, 0.65mmol) was added and the reaction was heated to 800C for 2 hours. Aqueous work-up, followed by silica gel chromatography (0-30% EtOAc in hexanes), gave the title compound.
Step 5: (R)-l-ø-Tolyl-ethanol [00640] (S)-(-)-2-Methyl-CBS-oxazaborolidine (3.72g, 13.4mmol) was dissolved in THF
(6OmL). Borane methyl sulfide complex (2M in THF; 36.6mL, 73.3mmol) was added, and the mixture was cooled to 00C. 2'-Methylacetophenone (15g, 11 lmmol) in THF (3OmL) was added over 1 hour, and the mixture was then worked-up to yield a liquid with a white precipitate.
Hexanes was added, the suspension was filtered to remove the precipitate, and the resulting filtrate was concentrated to give the title compound in 93% e.e.
Step 6: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-ø-tolyl-ethyl ester
[00641] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)- 1 -o-tolyl- ethanol.
Step 7: l-{4'-[3-Methyl-4-((R)-l-o-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester
[00642] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -o-tolyl- ethyl ester, l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester, and tetrakis(triphenylphosphine)palladium(0).
Step 8: l-{4'-[3-Methyl-4-((R)-l-o-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00643] To 1 - (4'-[3-methyl-4-((R)- 1 -o-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester (0.36mmol) in 2:1 MeOH:H2θ was added lithium hydroxide (l.lmmol), and the reaction was stirred at room temperature until no starting material was seen by analytical LCMS. The mixture was acidified with IN aqueous HCl and extracted with EtOAc. The combined organic layers were dried, filtered, and concentrated to give the title compound.
Example 3a: Synthesis of l-{4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl- isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-10)
Step 1: [5-(4-Bromo-phenyl)-3-met yl-isoxazol-4-yl]-carbamic acid 1-cyclopropyl-ethyl ester
[00644] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and alpha- methylcyclopropanemethanol .
Step 2: l-{4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester
[00645] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1 -cyclopropyl- ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester; the isolated material was then purified by preparative HPLC, using a Chiracel OD column (97:3 hexanes:EtOH) to provide enantiomer A and enantiomer B. Enantiomer A had a retention time of 27 minutes, enantiomer B had a retention time of 33 minutes .
Step 3: (R)-l-{4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid
[00646] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: enantiomer B from Example 3a, Step 2 (l-{4'-[4-(l-cyclopropyl- ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester).
Example 3b: Alternative Synthesis of (R)-l-{4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)- 3-methyl-isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-10)
Step 1: (RJ-alpha-methylcyclopropanemethanol
[00647] Using an analogous procedure of Meier et al (Tetrahedron, 1996, 52, 589; Method 3), cyclopropyl methyl ketone (Aldrich) was reduced to give (R)-alpha- methylcyclopropanemethanol.
Step 2: l-(R)-[5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-cyclopropyl- ethyl ester
[00648] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)-alpha- methylcyclopropanemethanol .
Step 3: (R)-l-{4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester [00649] Prepared according to the procedure described in Example 1, Step 6 using the following starting materials: l-(R)-[5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1- cyclopropyl-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester; the enantiomeric excess of the isolated material was determined by chiral HPLC to be 92% (Chiracel OD column (97:3 hexanes:EtOH, lml/min, minor isomer retention time 27 min, major isomer retention time 32 minutes).
Step 4: (R)-l-{4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid
[00650] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: (R)-(I -{4'-[4-(l -cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester).
Example 4: Synthesis of l-(4'-{4-[(R)-l-(2-Chloro-phenyl)-ethoxycarbonylamino]-3- methyl-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-12)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(2-chloro- phenyl)-ethyl ester
[00651] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)-I -(2- chloro-phenyl)-ethanol.
Step 2: l-(4'-{4-[(R)-l-(2-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid
[00652] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(2-chloro- phenyl)-ethyl ester and 4-(l'-carboxyl-cyclopropyl)phenylboronic acid. Example 5: Synthesis of l-[4'-(4-tørt-Butoxycarbonylamino-3-methyl-isoxazol-5-yl)- biphenyl-4-yl]-cyclopropanecarboxylic acid (Compound 1-13)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid tert-butyl ester
[00653] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and tert-butanol.
Step 2: l-[4'-(4-tørt-Butoxycarbonylam no-3-meth Vyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester
[00654] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid tert-butyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 3: l-[4'-(4-tørt-Butoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
[00655] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -[4'-(4-?ert-butoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester.
Example 6: Synthesis of l-(4'-{3-Methyl-4-[(R)-l-(2-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-14)
Step 1: (R)-l-(2-Trifluoromethyl-phenyl)-ethanol
[00656] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: 2'-(trifluoromethyl)acetophenone.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(2- trifluoromethyl-phenyl)-ethyl ester
[00657] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: (R)- 1 -(2-trifluoromethyl-phenyl)-ethanol and 5-(4-bromo-phenyl)-3 -methyl - isoxazole-4-carboxylic acid; the isolated material was purified by preparative HPLC, using a Chiracel OD column (98.6:1.4 hexanes:EtOH) to give the title compound.
Step 3: l-(4'-{3-Methyl-4-[(R)-l-(2-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00658] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-I -(2- trifluoromethyl-phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)- phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 4: l-(4'-{3-Methyl-4-[(R)-l-(2-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
[00659] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -(4'- (3-methyl-4-[(R)- 1 -(2-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester.
Example 7: Synthesis of l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol- 5-yl]-biphenyl-4-yl}-cyclopentanecarboxylic acid (Compound 1-16)
Step 1: l-(4-Bromo-phenyl)-cyclopentanecarboxylic acid ethyl ester
[00660] To a solution of ethyl 4-bromophenylacetate (2g, 8.2mmol) in DMF (2OmL) at 00C was added sodium hydride (60% in mineral oil; 0.72g, 18.1mmol), and the mixture was stirred for 10 minutes. 1 ,4-Dibromobutane (1.07mL, 9.0mmol) was added, and the reaction was stirred at room temperature for 30 minutes. Once no starting material was seen by analytical tic, the mixture was worked up with EtOAc and aqueous 10% HCl, and the crude material was purified by silica gel chromatography to give the title compound.
Step 2: l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopentanecarboxylic acid ethyl ester
[00661] Prepared according to the procedure described in Example 2, Step 4 using the following starting materials: l-(4-bromo-phenyl)-cyclopentanecarboxylic acid ethyl ester and bis(pinacolato)diboron.
Step 3: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopentanecarboxylic acid ethyl ester
[00662] [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl-ethyl ester (0.077g, 0.19mmol), l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopentanecarboxylic acid ethyl ester (0.079g, 0.23mmol), and potassium carbonate (0.066g, 0.48mmol) were combined in 2: 1 DME:H2O (3mL). The solution was purged with N2 for 5 minutes, and then tetrakis(triphenylphosphine)palladium(0) (0.022g, 0.02mmol) was added. The mixture was purged with N2 for an additional 5 minutes, and then the reaction was stirred at 900C in a sealed tube for 1.5 hours. Aqueous work-up, followed by silica gel chromatography, provided the title compound.
Step 4: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopentanecarboxylic acid
[00663] 1 - (4'-[3-methyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl} - cyclopentanecarboxylic acid ethyl ester (0.06Og, 0.1 lmmol) in 1,4-dioxane (2mL) was treated with IN aqueous LiOH (ImL), and the reaction was stirred at 600C overnight. Acidic work-up, followed by silica gel chromatography (0-50% EtOAc in hexanes) gave the title compound. Example 8: Synthesis of l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol- 5-yl]-biphenyl-4-yl}-cyclobutanecarboxylic acid (Compound 1-15)
Step 1: l-(4-Bromo-phenyl)-cyclobutanecarboxylic acid ethyl ester
[00664] Prepared according to the procedure described in Example 7, Step 1 using the following starting materials: ethyl 4-bromophenylacetate and 1,3-dibromopropane.
Step 2: l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclobutanecarboxylic acid ethyl ester
[00665] Prepared according to the procedure described in Example 2, Step 4 using the following starting materials: l-(4-bromo-phenyl)-cyclobutanecarboxylic acid ethyl ester and bis(pinacolato)diboron.
Step 3: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclobutanecarboxylic acid ethyl ester [00666] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl- ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclobutanecarboxylic acid ethyl ester.
Step 4: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclobutanecarboxylic acid
[00667] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: 1 - (4'-[3-methyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclobutanecarboxylic acid ethyl ester.
Example 9: Synthesis of l-[4'-(4-Isopropoxycarbonylamino-3-methyl-isoxazol-5-yl)- biphenyl-4-yl]-cyclopropanecarboxylic acid (Compound 1-2)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid isopropyl ester
[00668] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and isopropanol.
Step 2: l-[4'-(4-Isopropoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
[00669] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid isopropyl ester and 4-(l-carboxycyclopropyl)phenylboronic acid.
Example 10: Synthesis of l-{4'-[4-(l-Cyclohexyl-ethoxycarbonylamino)-3-methyl-isoxazol- 5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-3)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-cyclohexyl-ethyl ester
[00670] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and 1- cyclohexylethanol.
Step 2: l-{4'-[4-(l-Cyclohexyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester [00671] Prepared according to the procedure described in Example 1, Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-cyclohexyl- ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 3: l-{4'-[4-(l-Cyclohexyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00672] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: l-{4'-[4-(l-cyclohexyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 11: Synthesis of l-[4'-(4-Benzyloxycarbonylamino-3-methyl-isoxazol-5-yl)- biphenyl-4-yl]-cyclopropanecarboxylic acid (Compound 1-5)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid benzyl ester
[00673] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and benzyl alcohol.
Step 2: l-[4'-(4-Benzyloxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester
[00674] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid benzyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 3: l-[4'-(4-Benzyloxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
[00675] Prepared according to the procedure described in Example 2, Step 8 using the following starting materials: 1 -[4'-(4-benzyloxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester.
Example 12: Synthesis of l-[4'-(4-Ethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl- 4-yl]-cyclopropanecarboxylic acid (Compound 1-6)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid ethyl ester
[00676] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and ethanol.
Step 2: l-[4'-(4-Ethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester
[00677] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 3: l-[4'-(4-Ethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
[00678] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -[4'-(4-ethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester.
Example 13: Synthesis of l-[4'-(4-Methoxycarbonylamino-3-methyl-isoxazol-5-yl)- biphenyl-4-yl]-cyclopropanecarboxylic acid (Compound 1-7)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid methyl ester
[00679] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and methanol.
Step 2: l-[4'-(4-Methoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester
[00680] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid methyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 3: l-[4'-(4-Methoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
[00681] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -[4'-(4-methoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester.
Example 14: Synthesis of l-{4'-[3-Methyl-4-(l-methyl-l-phenyl-ethoxycarbonylamino)- isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-8)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-methyl-l-phenyl- ethyl ester
[00682] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and 2-phenyl-2- propanol.
Step 2: l-{4'-[3-Methyl-4-(l-methyl-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester [00683] Prepared according to the procedure described in Example 1, Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1 -methyl- 1- phenyl-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 3: l-{4'-[3-Methyl-4-(l-methyl-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid
[00684] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 - {4'-[3-methyl-4-(l -methyl- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 15: Synthesis of (S)-l-{4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl- isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-9)
Step 1 : l-{4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00685] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: enantiomer A from Example 3a, Step 2 (l-{4'-[4-(l-cyclopropyl- ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester). Example 16: Synthesis of l-[4'-(4-Cyclopropylmethoxycarbonylamino-3-methyl-isoxazol- 5-yl)-biphenyl-4-yl]-cyclopropanecarboxylic acid (Compound 1-11)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid cyclopropylmethyl ester [00686] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and cyclopropyl carbinol.
Step 2: l-[4'-(4-Cyclopropylmethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4- yl]-cyclopropanecarboxylic acid ethyl ester
[00687] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid cyclopropylmethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 3: l-[4'-(4-Cyclopropylmethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4- yl]-cyclopropanecarboxylic acid
[00688] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -[4'-(4-cyclopropylmethoxycarbonylamino-3-methyl-isoxazol-5-yl)- biphenyl-4-yl]-cyclopropanecarboxylic acid ethyl ester.
Example 17: Synthesis of l-[4'-(4-tørt-Butoxycarbonylamino-3-methyl-isoxazol-5-yl)- biphenyl-4-yl]-cyclobutanecarboxylic acid (Compound 1-17)
Step 1: 4-(l-Carboxy-cyclobutyl)phenylboronic acid
[00689] l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclobutanecarboxylic acid ethyl ester (0.30Og, 0.91mmol) in 1,4-dioxane was treated with IN aqueous LiOH at 65°C overnight. Purification by preparative HPLC gave the title compound.
Step 2: l-[4'-(4-tørt-Butoxycarbonylam no-3-meth Vyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
[00690] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid tert-butyl ester and 4-(l-carboxy-cyclobutyl)phenylboronic acid.
Example 18: Synthesis of l-(4'-{4-[l-(2-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-18)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid l-(2-methoxy-phenyl)- ethyl ester
[00691] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and l-(2- methoxyphenyl)ethanol .
Step 2: l-(4'-{4-[l-(2-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00692] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1 -(2-methoxy- phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 3: l-(4'-{4-[l-(2-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid
[00693] Prepared according to the procedure described in Example 7, Step 4 using the following starting materials: 1 -(4'- {4-[ 1 -(2-methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester Example 19: Synthesis of l-(4'-{3-Methyl-4-[l-(4-trifluoromethyl-phenyl)-
ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-19)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid l-(4-trifluoromethyl- phenyl)-ethyl ester
[00694] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and l-[4- (trifluoromethyl)phenyl]ethanol.
Step 2: l-(4'-{3-Methyl-4-[l-(4-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00695] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid l-(4- trifluoromethyl-phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)- phenylj-cyclopropanecarboxylic acid ethyl ester; the isolated material was then purified by preparative HPLC, using a chiral column (95:5 hexanes:EtOAc) to provide enantiomer A and enantiomer B. Enantiomer A had a retention time of 30 minutes, enantiomer B had a retention time of 50 minutes.
Step 3: l-(4'-{3-Methyl-4-[l-(4-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
[00696] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: enantiomer A from Example 19, Step 2 (l-(4'-{3-methyl-4-[l-(4- trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)- cyclopropanecarboxylic acid ethyl ester).
Example 20: Synthesis of l-(4'-{3-Methyl-4-[l-(4-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
(Compound 1-20)
Step 1: l-(4'-{3-Methyl-4-[l-(4-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
[00697] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: enantiomer B from Example 19, Step 2 (l-(4'-{3-Methyl-4-[l-(4- trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)- cyclopropanecarboxylic acid ethyl ester).
Example 21: Synthesis of l-{4'-[3-Methyl-4-(l-pyrazin-2-yl-ethoxycarbonylamino)- isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-21)
Step 1: l-Pyrazin-2-yl-ethanol
[00698] To a solution of 2-acetylpyrazine (4.2g, 34.4mmol) in MeOH (10OmL) at room temperature was added sodium borohydride (2.Og, 57.6mmol), and the reaction was stirred for 20 minutes. Aqueous work-up provided the title compound.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid l-pyrazin-2-yl-ethyl ester
[00699] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and l-pyrazin-2- yl-ethanol.
Step 3: l-{4'-[3-Methyl-4-(l-pyrazin-2-yl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester
[00700] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1 -pyrazin-2-yl- ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-{4'-[3-Methyl-4-(l-pyrazin-2-yl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00701] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: l-{4'-[3-methyl-4-(l-pyrazin-2-yl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 22: Synthesis of l-(4'-{4-[l-(3-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-22)
Step 1: 3-(l-Hydroxy-ethyl)-benzonitrile
[00702] Prepared according to the procedure described in Example 21, Step 1 using the following starting material: 3-acetylbenzonitrile.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid l-(3-cyano-phenyl)- ethyl ester
[00703] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and 3-(l-hydroxy- ethyl)-benzonitrile .
Step 3: l-(4'-{4-[l-(3-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00704] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid l-(3-cyano- phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-(4'-{4-[l-(3-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid
[00705] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: l-(4'-{4-[l-(3-cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester.
Example 23: Synthesis of l-[4-(4-{4-[(R)-l-(2-Chloro-phenyl)-ethoxycarbonylamino]-3- methyl-isoxazol-5-yl}-phenoxy)-phenyl]-cyclopropanecarboxylic acid (Compound 1-23)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(2-chloro- phenyl)-ethyl ester
[00706] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)-I -(2- chloro-phenyl)-ethanol.
Step 2: {3-Methyl-5-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-isoxazol-4- yl}-carbamic acid (R)-l-(2-chloro-phenyl)-ethyl ester
[00707] Prepared according to the procedure described in Example 2, Step 4 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(2-chloro- phenyl)-ethyl ester and bis(pinacolato)diboron.
Step 3: [5-(4-Hydroxy-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(2-chloro- phenyl)-ethyl ester
[00708] A solution of {3-methyl-5-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- isoxazol-4-yl}-carbamic acid (R)- 1 -(2-chloro-phenyl)-ethyl ester (3.2g, 6.64mmol) in MeOH was treated with IN aqueous NaOH (33mL, 33mmol) and H2O2 (30% in H2O; 3.8g, 33mmol), and the reaction was stirred at room temperature for 2 hours. The mixture was diluted with H2O
(5OmL), and IN aqueous HCl was added to acidify the solution to pH 3. The mixture was stirred for 30 minutes, and the white solid precipitate that formed was isolated by filtration and washed 5 times with H2O to give the title compound.
[00709] l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester (0.50Og, 1.58mmol), sodium periodate (0.745g, 3.48mmol), and ammonium acetate (0.365g, 4.74mmol) were combined in 1 : 1 acetone:H2O (15mL), and the reaction was stirred at room temperature until no starting material was seen by analytical LCMS. Aqueous work-up provided the title compound.
Step 5: l-[4-(4-{4-[(R)-l-(2-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5- yl}-phenoxy)-phenyl]-cyclopropanecarboxylic acid ethyl ester
[00710] [5-(4-Hydroxy-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -(2-chloro-phenyl)- ethyl ester (0.12Og, 0.32mmol), 4-(l-ethoxycarbonyl-cyclopropyl)phenylboronic acid (0.075g, 0.32mmol), pyridine (0.1OmL, 1.28mmol), and copper(II) acetate (0.175g, 0.96mmol) were combined in CH2Cl2 and stirred at room temperature for 3 days. The mixture was extracted with EtOAc, and the combined organic layers were purified by preparative HPLC to give the title compound.
Step 6: l-[4-(4-{4-[(R)-l-(2-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5- yl}-phenoxy)-phenyl]-cyclopropanecarboxylic acid
[00711] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -[4-(4- (4-[(R)- 1 -(2-chloro-phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl}-phenoxy)-phenyl]-cyclopropanecarboxylic acid ethyl ester; the crude material was purified by preparative HPLC.
Example 24: Synthesis of l-(4-{4-[3-Methyl-4-(l-phenyl-ethoxycarbonylamino)-isoxazol-5- yl]-phenoxy}-phenyl)-cyclopropanecarboxylic acid (Compound 1-24)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-phenyl-ethyl ester
[00712] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-Bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and 1- phenylethanol.
Step 2: {3-Methyl-5-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-isoxazol-4- yl}-carbamic acid 1-phenyl-ethyl ester
[00713] Prepared according to the procedure described in Example 2, Step 4 using the following starting materials: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1 -phenyl-ethyl ester and bis(pinacolato)diboron.
Step 3: [5-(4-Hydroxy-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-phenyl-ethyl ester
[00714] Prepared according to the procedure described in Example 23, Step 3 using the following starting material: (3-methyl-5-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)- phenyl]-isoxazol-4-yl}-carbamic acid 1-phenyl-ethyl ester.
Step 4: l-(4-{4-[3-Methyl-4-(l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-phenoxy}- phenyl)-cyclopropanecarboxylic acid ethyl ester
[00715] Prepared according to the procedure described in Example 23, Step 5 using the following starting materials: [5-(4-hydroxy-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1- phenyl-ethyl ester and 4-(l-ethoxycarbonyl-cyclopropyl)phenylboronic acid.
Step 5: l-(4-{4-[3-Methyl-4-(l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-phenoxy}- phenyl)-cyclopropanecarboxylic acid
[00716] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: l-(4-{4-[3-methyl-4-(l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- phenoxy}-phenyl)-cyclopropanecarboxylic acid ethyl ester.
Example 25: Synthesis of l-{4'-[3-Methyl-4-((R)-l-p-tolyl-ethoxycarbonylamino)-isoxazol- 5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-25)
Step 1: (R)-l-p-Tolyl-ethanol
[00717] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: 4'-methylacetophenone.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-p-tolyl-ethyl ester
[00718] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)- 1 -p-tolyl- ethanol.
Step 3: l-{4'-[3-Methyl-4-((R)-l-p-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester
[00719] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -p-tolyl-
ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-{4'-[3-Methyl-4-((R)-l-p-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00720] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: 1 - (4'-[3-methyl-4-((R)- 1 -/?-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 26: Synthesis of l-{4'-[3-Methyl-4-((R)-l-#w-tolyl-ethoxycarbonylamino)-isoxazol- 5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-26)
Step 1: (R)-l-ιw-Tolyl-ethanol
[00721] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: 3'-methylacetophenone.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-#w-tolyl-ethyl ester
[00722] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)- 1 -m-to IyI- ethanol.
Step 3: l-{4'-[3-Methyl-4-((R)-l-ι«-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester
[00723] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -m-tolyl- ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-{4'-[3-Methyl-4-((R)-l-ι«-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00724] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: 1 - (4'-[3-methyl-4-((R)- 1 -m-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 27: Synthesis of l-(4-{4-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)- isoxazol-5-yl]-phenoxy}-phenyl)-cyclopropanecarboxylic acid (Compound 1-27)
Step 1: {3-Methyl-5-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-isoxazol-4- yl}-carbamic acid (R)-l-phenyl-ethyl ester
[00725] Prepared according to the procedure described in Example 2, Step 4 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl- ethyl ester and bis(pinacolato)diboron.
Step 2: [5-(4-Hydroxy-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-phenyl-ethyl ester
[00726] Prepared according to the procedure described in Example 23, Step 3 using the following starting material: (3-methyl-5-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)- phenyl]-isoxazol-4-yl}-carbamic acid (R)- 1 -phenyl-ethyl ester.
Step 3: l-(4-{4-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-phenoxy}- phenyl)-cyclopropanecarboxylic acid ethyl ester
[00727] Prepared according to the procedure described in Example 23, Step 5 using the following starting materials: [5-(4-hydroxy-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl-ethyl ester and 4-(l-ethoxycarbonyl-cyclopropyl)phenylboronic acid.
Step 4: l-(4-{4-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-phenoxy}- phenyl)-cyclopropanecarboxylic acid
[00728] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -(4- (4-[3-methyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- phenoxy}-phenyl)-cyclopropanecarboxylic acid ethyl ester.
Example 28: Synthesis of l-(4'-{4-[(R)-l-(4-Cyano-phenyl)-ethoxycarbonylamino]-3- methyl-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-28)
Step 1: 4-((R)-l-Hydroxy-ethyl)-benzonitrile
[00729] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: 4-acetylbenzonitrile.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(4-cyano- phenyl)-ethyl ester
[00730] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and 4-((R)-I- hydroxy-ethyl)-benzonitrile.
Step 3: l-(4'-{4-[(R)-l-(4-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00731] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(4-cyano- phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-(4'-{4-[(R)-l-(4-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid
[00732] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: 1 -(4'- (4-[(R)- 1 -(4-cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester.
Example 29: Synthesis of l-(4'-{4-[(R)-l-(2-Cyano-phenyl)-ethoxycarbonylamino]-3- methyl-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-29)
Step 1: 2-((R)-l-Hydroxy-ethyl)-benzonitrile
[00733] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: 2-acetylbenzenecarbonitrile.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(2-cyano- phenyl)-ethyl ester
[00734] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and 2-((R)-I- hydroxy-ethyl)-benzonitrile.
Step 3: l-(4'-{4-[(R)-l-(2-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00735] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(2-cyano- phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-(4'-{4-[(R)-l-(2-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid
[00736] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: 1 -(4'- (4-[(R)- 1 -(2-cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester.
Example 30: Synthesis of l-{4'-[3-Methyl-4-((R)-l,2,2-trimethyl-propoxycarbonylamino)- isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-30)
Step 1: (R)-3,3-Dimethyl-butan-2-ol
[00737] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: pinacolone.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l,2,2-trimethyl- propyl ester
[00738] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)-3,3- dimethyl-butan-2-ol.
Step 3: l-{4'-[3-Methyl-4-((R)-l,2,2-trimethyl-propoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester
[00739] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1,2,2- trimethyl-propyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-{4'-[3-Methyl-4-((R)-l,2,2-trimethyl-propoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid
[00740] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 - (4'-[3-methyl-4-((R)- 1 ,2,2-trimethyl-propoxycarbonylamino)-isoxazol-5- yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 31: Synthesis of l-{4'-[4-((R)-l-Cyclobutyl-ethoxycarbonylamino)-3-methyl- isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-31)
Step 1: (R)-l-Cyclobutyl-ethanol
[00741] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: cyclobutyl methyl ketone.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-cyclobutyl-ethyl ester
[00742] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)-I- cyclobutyl-ethanol.
Step 3: l-{4'-[4-((R)-l-Cyclobutyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester
[00743] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-I- cyclobutyl-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-{4'-[4-((R)-l-Cyclobutyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid
[00744] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 - (4'-[4-((R)- 1 -cyclobutyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 32: Synthesis of l-{4'-[4-((R)-l,2-Dimethyl-propoxycarbonylamino)-3-methyl- isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-32)
Step 1: (R)-3-Methyl-butan-2-ol
[00745] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: 3-methyl-2-butanone.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l,2-dimethyl- propyl ester
[00746] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)-3-methyl- butan-2-ol.
Step 3: l-{4'-[4-((R)-l,2-Dimethyl-propoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester
[00747] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1,2- dimethyl-propyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-{4'-[4-((R)-l,2-Dimethyl-propoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid
[00748] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 - (4'-[4-((R)- 1 ,2-cimethyl-propoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 33: Synthesis of l-{4'-[4-(l-Ethyl-propoxycarbonylamino)-3-methyl-isoxazol-5- yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-33)
Step 1: Pentan-3-ol
[00749] Prepared according to the procedure described in Example 21, Step 1 using the following starting material: 3-pentanone.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-ethyl-propyl ester
[00750] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and pentan-3-ol.
Step 3: l-{4'-[4-(l-Ethyl-propoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid ethyl ester [00751] Prepared according to the procedure described in Example 1, Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-ethyl-propyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 4: l-{4'-[4-(l-Ethyl-propoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid
[00752] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: l-{4'-[4-(l-ethyl-propoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester.
Example 34: Synthesis of l-(4'-{4-[l-(2-Chloro-cyclohex-l-enyl)-ethoxycarbonylamino]-3- methyl-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-34)
Step 1: 2-Chloro-cyclohex-l-enecarbaldehyde [00753] To a solution of cyclohexanone (1.34g, 13.6mmol) in toluene at room temperature was added DMF (1.58mL, 20.5mmol) and phosphorus oxychloride (1.88mL, 20.5mmol). The reaction was stirred overnight at room temperature, and then diluted with H2O and stirred for 30 minutes. 4N Aqueous NaOH (1OmL) was added, and the mixture was extracted with EtOAc. The combined organic layers were washed with saturated aqueous NH4Cl, dried over MgSO4, filtered, and concentrated to give the title compound.
Step 2: l-(2-Chloro-cyclohex-l-enyl)-ethanol
[00754] To a solution of 2-chloro-cyclohex- 1 -enecarbaldehyde (13.6mmol) in THF at 00C was added methyl magnesium bromide (3M in THF; 5.4mL, 16.32mmol). The reaction was stirred under N2 for 1 hour, and then /PrOH (2mL) was added. The mixture was concentrated, and the residue was diluted with IN aqueous HCl and extracted with EtOAc. The combined organic layers were washed with saturated aqueous NH4Cl, dried over MgSO4, filtered, and concentrated, and the crude material was purified by silica gel chromatography to give the title compound.
Step 3: 5-[4'-(l-Ethoxycarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazole-4- carboxylic acid
[00755] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and l-[4-(4,4,5,5- tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 4: l-(4'-{4-[l-(2-Chloro-cyclohex-l-enyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester [00756] Prepared according to the procedure described in Example 1, Step 5 using the following starting materials: 5-[4'-(l-ethoxycarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazole-4- carboxylic acid and l-(2-chloro-cyclohex-l-enyl)-ethanol.
Step 5: l-(4'-{4-[l-(2-Chloro-cyclohex-l-enyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
[00757] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -(4'- {4-[ 1 -(2-chloro-cyclohex- 1 -enyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester. Example 35: Synthesis of l-(4'-{3-Methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-35)
[00758] Step 1: (R)-l-(3-Trifluoromethyl-phenyl)-ethanol
Prepared according to the procedure described in Example 2, Step 5 using the following starting material: 3'-(trifluoromethyl)acetophenone.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(3- trifluoromethyl-phenyl)-ethyl ester
[00759] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)- 1 -(3- trifluoromethyl-phenyl)-ethanol.
Step 3: l-(4'-{3-Methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00760] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -(3- trifluoromethyl-phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)- phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 4: l-(4'-{3-Methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
[00761] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: 1 -(4'- (3-methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester. Example 36: Synthesis of l-(4'-{4-[(R)-l-(3-Methoxy-phenyl)-ethoxycarbonylamino]-3- methyl-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-36)
Step 1: (R)-l-(3-Methoxy-phenyl)-ethanol
[00762] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: 3'-methoxyacetophenone.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(3-methoxy- phenyl)-ethyl ester
[00763] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)- 1 -(3- methoxy-phenyl)-ethanol.
Step 3: l-(4'-{4-[(R)-l-(3-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00764] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-I -(3- methoxy-phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-(4'-{4-[(R)-l-(3-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid
[00765] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: 1 -(4'- (4-[(R)- 1 -(3-methoxy-phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester. Example 37: Synthesis of l-(4'-{4-[(R)-l-(4-Methoxy-phenyl)-ethoxycarbonylamino]-3- methyl-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-37)
Step 1: (R)-l-(4-Methoxy-phenyl)-ethanol
[00766] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: 4'-methoxyacetophenone.
Step 2: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(4-methoxy- phenyl)-ethyl ester
[00767] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)-I -(4- methoxy-phenyl)-ethanol.
Step 3: l-(4'-{4-[(R)-l-(4-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00768] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-I -(4- methoxy-phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: l-(4'-{4-[(R)-l-(4-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid
[00769] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: 1 -(4'- (4-[(R)- 1 -(4-methoxy-phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester.
Example 38: Synthesis of l-{4'-[4-((R)-l-Cyclopropyl-ethoxycarbonylamino)-3-methyl- isoxazol-5-yl]-biphenyl-4-yl}-cycloprop-2-enecarboxylic acid (Compound 1-38)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-cyclopropyl- ethyl ester
[00770] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)-alpha- methylcyclopropanemethanol .
Step 2: [4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-acetic acid ethyl ester
[00771] Ethyl 4-bromophenylacetate (12g, 49mmol), bis(pinacolato)diboron (12.4g, 59mmol), l,l'-bis(diphenylphosphino)ferrocene)-dichloropalladium(II) (2g, 2.4mmol), and potassium acetate (9.6g, 98mmol) were combined in 1,4-dioxane (10OmL), and the reaction was heated to 800C for 6 hours. The mixture was worked-up to give the title compound.
Step 3: {4'-[4-((R)-l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl- 4-yl} -acetic acid ethyl ester
[00772] Prepared according to the procedure described in Example 7, Step 4 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-I-
cyclopropyl-ethyl ester and [4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-acetic acid ethyl ester.
Step 4: {4'-[4-((R)-l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl- 4-yl}-diazo-acetic acid ethyl ester
[00773] To a solution of {4'-[4-((R)-l-cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5- yl]-biphenyl-4-yl} -acetic acid ethyl ester (0.73g, 1.63mmol) in CH3CN (2OmL) at room temperature was added l,8-diazabicyclo[5.4.0]undec-7-ene (0.37mL, 2.44mmol). 4- Acetamidobenzenesulfonyl azide (0.524g, 2.18mmol) was added dropwise over 20 minutes, and the reaction was stirred overnight at room temperature. The mixture was concentrated to dryness, 5% aqueous KOH was added, and the solution was extracted with EtOAc. The combined organic layers were washed with brine, dried, filtered, and concentrated to give the title compound.
Step 5: l-{4'-[4-((R)-l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-2-trimethylsilanyl-cycloprop-2-enecarboxylic acid ethyl ester
[00774] To a suspension of rhodium(II) acetate dimer (0.036g, 0.08mmol) in (trimethylsilyl)acetylene (1OmL) at 53°C was added {4'-[4-((R)-l-cyclopropyl- ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl}-diazo-acetic acid ethyl ester
(0.55Og, 1.15mmol) as a solution in CH2CI2 (15mL) and (trimethylsilyl)acetylene (5mL) over 16 hours by syringe pump. The reaction was refluxed for an additional 2 hours, and then filtered and concentrated to give the title compound.
Step 6: l-{4'-[4-((R)-l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cycloprop-2-enecarboxylic acid ethyl ester
[00775] 1- (4'-[4-((R)-I -Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl- 4-yl}-2-trimethylsilanyl-cycloprop-2-enecarboxylic acid ethyl ester (1.15mmol) in THF (1OmL) was treated with 10% aqueous Na2COs dropwise. The reaction was stirred at room temperature, and then aqueous work-up provided the title compound.
Step 7: l-{4'-[4-((R)-l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]- biphenyl-4-yl}-cycloprop-2-enecarboxylic acid
[00776] 1- (4'-[4-((R)-I -Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl- 4-yl}-cycloprop-2-enecarboxylic acid ethyl ester (1.15mmol) in MeOH (5mL) was treated with IN aqueous LiOH (ImL), and the reaction was stirred at 8O0C for 2 hours. The mixture was worked up with EtOAc and 10% aqueous HCl, and the crude material was purified by preparative HPLC to give the title compound.
Example 39: Synthesis of 2-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol- 5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-39)
Step 1: 2-(4-Bromo-phenyl)-cyclopropanecarboxylic acid ethyl ester [00777] To copper(II) acetylacetonate (0.156g, O.όmmol) in CH2C12 (4OmL) was added phenylhydrazine (3 drops), followed by 4-bromostyrene (2.6mL, 20mmol). The solution was stirred at 400C, and ethyl diazoacetate (3.ImL, 30mmol) in CH2C12 (4OmL) was added dropwise via syringe pump. The reaction was stirred overnight at 400C, and then cooled to room temperature for work-up. The mixture was washed twice with aqueous NaHCCβ, twice with H2O, dried over Na2SO4, filtered, and concentrated, and the crude material was purified by silica gel chromatography to give a mixture of the cis and trans isomers. The isolated material was repurified by silica gel chromatography (0- 10% EtOAc in hexanes) to give the cis and trans isomers as separate products.
Step 2: rrøns-2-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester
[00778] Prepared according to the procedure described in Example 2, Step 4 using the following starting materials: £rø«s-2-(4-bromo-phenyl)-cyclopropanecarboxylic acid ethyl ester and bis(pinacolato)diboron.
Step 3: rr«ns-2-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester
[00779] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl- ethyl ester and £rø«s-2-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 4: rr«ns-2-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid
[00780] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: £rø«s-2-{4'-[3-methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 40: Synthesis of l-(4-{5-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)- isoxazol-5-yl]-pyridin-2-yl}-phenyl)-cyclopropanecarboxylic acid (Compound 1-40)
Step 1: 2-(6-Chloro-pyridine-3-carbonyl)-3-oxo-butyric acid methyl ester
[00781] Prepared according to the procedure described in Example 1 , Step 2 using the following starting materials: 6-chloronicotinoyl chloride and 3-methylamino-but-2-enoic acid methyl ester.
Step 2: 5-(6-Chloro-pyridin-3-yl)-3-methyl-isoxazole-4-carboxylic acid methyl ester
[00782] Prepared according to the procedure described in Example 1 , Step 3 using the following starting materials: 2-(6-chloro-pyridine-3-carbonyl)-3-oxo-butyric acid methyl ester and hydroxylamine hydrochloride.
Step 3: 5-(6-Chloro-pyridin-3-yl)-3-methyl-isoxazole-4-carboxylic acid
[00783] Prepared according to the procedure described in Example 1 , Step 4 using the following starting material: 5-(6-chloro-pyridin-3-yl)-3-methyl-isoxazole-4-carboxylic acid methyl ester.
Step 4: [5-(6-Chloro-pyridin-3-yl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-phenyl- ethyl ester
[00784] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(6-chloro-pyridin-3-yl)-3-methyl-isoxazole-4-carboxylic acid and (R)-I- phenyl-ethanol.
Step 5: l-(4-{5-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-pyridin-2- yl}-phenyl)-cyclopropanecarboxylic acid ethyl ester
[00785] Prepared according to the procedure described in Example 7, Step 3 using the following starting materials: [5-(6-chloro-pyridin-3-yl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-I- phenyl-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 6: l-(4-{5-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-pyridin-2- yl}-phenyl)-cyclopropanecarboxylic acid
[00786] Prepared according to the procedure described in Example 7, Step 4 using the following starting material: 1 -(4- (5-[3-methyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- pyridin-2-yl}-phenyl)-cyclopropanecarboxylic acid ethyl ester.
Example 41: Synthesis of l-(4'-{4-[l-(3-Bromo-phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-41)'
Step 1: l-(4'-{4-[l-(3-Bromo-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00787] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-[4'-(l-ethoxycarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazole-4- carboxylic acid and 3-bromo-alpha-methylbenzyl alcohol.
Step 2: l-(4'-{4-[l-(3-Bromo-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid [00788] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -(4'- {4-[ 1 -(3-bromo-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester.
Example 42: Synthesis of l-(4'-{4-[l-(3-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-42)
Step 1: l-(4'-{4-[l-(3-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00789] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-[4'-(l-ethoxycarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazole-4- carboxylic acid and l-(3-chlorophenyl)ethanol.
Step 2: l-(4'-{4-[l-(3-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid
[00790] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: l-(4'-{4-[l-(3-chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester.
Example 43: Synthesis of l-{4'-[3-Methyl-4-((S)-l-phenyl-ethoxycarbonylamino)-isoxazol- 5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-43)
[00791] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (S)-(-)-l- phenylethanol (commercially available or prepared using procedures desribed herein or in the literature: e.g. EJ. Corey et al. J. Am. Chem. 1987, 109, 5551-5553).
Step 2: l-{4'-[3-Methyl-4-((S)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester
[00792] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: (S)-[5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-phenyl- ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 3: l-{4'-[3-Methyl-4-((S)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00793] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 - (4'-[3-methyl-4-((S)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 44: Synthesis of l-(4'-{4-[l-(3-Hydroxy-phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-44)
Step 1: l-[3-(tert-Butyl-dimethyl-silanyloxy)-phenyl]-ethanone
[00794] To a solution of 3'-hydroxyacetophenone (0.50Og, 3.67mmol) and imidazole (0.50Og, 7.34mmol) in CH2Cl2 (5mL) was added tert-butyldimethylsilyl chloride (0.609g, 4.04mmol), and the reaction was stirred for 1 hour at room temperature. The mixture was partitioned between CH2Cl2 and H2O, and the aqueous layer was separated and extracted with CH2Cl2. The combined organic layers were dried over MgSO4, filtered, and concentrated to give the title compound.
Step 2: l-[3-(tert-Butyl-dimethyl-silanyloxy)-phenyl]-ethanol
[00795] l-[3-(tert-Butyl-dimethyl-silanyloxy)-phenyl]-ethanone (3.67mmol) in MeOH (5mL) was treated with sodium borohydride (0.139g, 3.67mmol). The reaction was stirred for 20 minutes, and then standard work-up provided the title compound.
Step 3: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid l-[3-(tert-butyl- dimethyl-silanyloxy)-phenyl] -ethyl ester
[00796] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and l-[3-(tert- butyl-dimethyl-silanyloxy)-phenyl]-ethanol.
Step 4: l-[4'-(4-{l-[3-(terr-Butyl-dimethyl-silanyloxy)-phenyl]-ethoxycarbonylamino}-3- methyl-isoxazol-5-yl)-biphenyl-4-yl]-cyclopropanecarboxylic acid ethyl ester
[00797] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid l-[3-(tert-butyl- dimethyl-silanyloxy)-phenyl]-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2- yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 5: l-(4'-{4-[l-(3-Hydroxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid
[00798] 1 -[4'-(4- { 1 -[3-(?ert-Butyl-dimethyl-silanyloxy)-phenyl]-ethoxycarbonylamino} -3- methyl-isoxazol-5-yl)-biphenyl-4-yl]-cyclopropanecarboxylic acid ethyl ester (0.40Og, 0.63mmol) in 3: 1 MeOHiH^O (1OmL) was treated with excess lithium hydroxide. The reaction was stirred overnight at 600C, and then acidified and extracted with EtOAc. The combined organic layers were dried over MgSO4, filtered, and concentrated, and the residue was purified by preparative HPLC to give the title compound.
Example 45: Synthesis of [l-(4'-{3-Methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropyl]-acetic acid (Compound 1-45)
[00799] To l-(4-bromo-phenyl)-cyclopropanecarboxylic acid ethyl ester (25g, 92.9mmol) in THF (175mL) at -4°C was added lithium aluminum hydride (4.58g, 120.8mmol) portionwise over 1 hour. The reaction was stirred for 30 minutes, and then H2O (1OmL) was added, followed by 4N aqueous NaOH (1OmL) and additional H2O (3OmL). The mixture was stirred for 15 minutes and filtered. The filtrate was washed with brine, dried over MgSO4, filtered, and concentrated to give the title compound.
[00800] [l-(4-Bromo-phenyl)-cyclopropyl] -methanol (11.42g, 50.3mmol) in CH2Cl2 (22OmL) at -78°C was treated with triethylamine (8.4ImL, 60.4mmol), followed by methanesulfonyl chloride (4.3OmL, 55.3mmol). The reaction was stirred for 1 hour at -78°C, and then warmed to 00C and stirred for 2 hours. The mixture was diluted with IN aqueous HCl and extracted twice with CH2Cl2. The combined organic layers were washed with aqueous NaHCOs, brine, and then dried over MgSO4, filtered, and concentrated to give the title compound.
Step 3: [l-(4-Bromo-phenyl)-cyclopropyl]-acetonitrile
[00801] Methanesulfonic acid l-(4-bromo-phenyl)-cyclopropylmethyl ester (50.3mmol) in DMF (HOmL) was treated with sodium cyanide (7.39g, 150.9mmol), and the reaction was stirred at 700C for 3 hours. The mixture was diluted with EtOAc and H2O, and the organic layer was
washed once with brine and three times with H2O, and then dried, filtered, and concentrated to give the title compound.
[00802] [l-(4-Bromo-phenyl)-cyclopropyl]-acetonitrile (7.98, 33.8mmol) and potassium hydroxide (7.56g, 135mmol) were combined in EtOH (54mL) and H2O (6.75mL), and the reaction was stirred at 1100C for 16 hours. The mixture was diluted with EtOAc and acidified with HCl to pH 3-4, and then extracted with EtOAc. The combined organic layers were washed brine, dried, filtered, and concentrated to give the title compound.
Step 5: [l-(4-Bromo-phenyl)-cyclopropyl]-acetic acid ethyl ester
[00803] [l-(4-Bromo-phenyl)-cyclopropyl] -acetic acid (8.54g, 33.3mmol) in EtOH (8OmL) was treated with sulfuric acid (2mL), and the reaction was stirred at 8O0C overnight. The mixture was concentrated, and the residue was diluted with EtOAc, washed with saturated aqueous NaHCθ3 and brine, dried, filtered, and concentrated, to give the title compound.
Step 6: {l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropyl}-acetic acid ethyl ester
[00804] Prepared according to the procedure described in Example 2, Step 4 using the following starting materials: [l-(4-bromo-phenyl)-cyclopropyl]-acetic acid ethyl ester and bis(pinacolato)diboron.
Step 7: [l-(4'-{3-Methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropyl]-acetic acid ethyl ester
[00805] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -(3- trifluoromethyl-phenyl)-ethyl ester and (l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)- phenyl]-cyclopropyl} -acetic acid ethyl ester.
Step 8: [l-(4'-{3-Methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropyl]-acetic acid
[00806] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: [ 1 -(4'- (3-methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropyl]-acetic acid ethyl ester.
Example 46: Synthesis of l-{4'-[3-Ethyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5- yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-46)
[00807] Prepared according to the procedure described in Example 1 , Step 2 using the following starting materials: 4-bromobenzoyl chloride and methyl 3-oxovalerate; sodium tert-butoxide was used in place of pyridine.
Step 2: 5-(4-Bromo-phenyl)-3-ethyl-isoxazole-4-carboxylic acid methyl ester
[00808] Prepared according to the procedure described in Example 1 , Step 3 using the following starting materials: 2-(4-bromo-benzoyl)-3-oxo-pentanoic acid methyl ester and hydroxylamine hydrochloride.
Step 3: 5-(4-Bromo-phenyl)-3-ethyl-isoxazole-4-carboxylic acid
[00809] Prepared according to the procedure described in Example 1 , Step 4 using the following starting material: 5-(4-bromo-phenyl)-3-ethyl-isoxazole-4-carboxylic acid methyl ester.
Step 4: [5-(4-Bromo-phenyl)-3-ethyl-isoxazol-4-yl]-carbamic acid (R)-l-phenyl-ethyl ester
[00810] Prepared according to the procedure described in Example 1, Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-ethyl-isoxazole-4-carboxylic acid and (R)- 1 -phenyl- ethanol.
Step 5: l-{4'-[3-Ethyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester
[00811] Prepared according to the procedure described in Example 1, Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-ethyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 6: l-{4'-[3-Ethyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00812] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 - (4'-[3-ethyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 47: Synthesis of l-{4'-[3-Phenyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol- 5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-47)
Step 1: 2-Benzoyl-3-(4-bromo-phenyl)-3-oxo-propionic acid ethyl ester
[00813] Prepared according to the procedure described in Example 1, Step 2 using the following starting materials: bromobenzoyl chloride and ethyl benzoylacetate; sodium tert-butoxide was used in place of pyridine.
Step 2: 5-(4-Bromo-phenyl)-3-phenyl-isoxazole-4-carboxylic acid ethyl ester
[00814] Prepared according to the procedure described in Example 1, Step 3 using the following starting materials: 2-benzoyl-3-(4-bromo-phenyl)-3-oxo-propionic acid ethyl ester and hydroxylamine hydrochloride.
Step 3: 5-(4-Bromo-phenyl)-3-phenyl-isoxazole-4-carboxylic acid
[00815] Prepared according to the procedure described in Example 1, Step 4 using the following starting material: 5-(4-bromo-phenyl)-3-phenyl-isoxazole-4-carboxylic acid ethyl ester.
Step 4: [5-(4-Bromo-phenyl)-3-phenyl-isoxazol-4-yl]-carbamic acid (R)-l-phenyl-ethyl ester
[00816] Prepared according to the procedure described in Example 1, Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-phenyl-isoxazole-4-carboxylic acid and (R)- 1 -phenyl - ethanol.
Step 5: l-{4'-[3-Phenyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester [00817] Prepared according to the procedure described in Example 1, Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-phenyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl - ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 6: l-{4'-[3-Phenyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid
[00818] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: l-{4'-[3-phenyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 48: Synthesis of l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol- 5-yl]-biphenyl-4-ylmethyl}-cyclopropanecarboxylic acid (Compound 1-48)
[00819] To diisopropylamine (3.39mL, 24mmol) in THF (4OmL) at 00C was added n- butyllithium (1.6M in hexanes; 15mL, 24mmol), and the solution was stirred for 15 minutes. Cyclopropanecarbonitrile (1.66g, 24mmol) was added, and the mixture was stirred for 1 hour at 00C. 4-Bromobenzyl bromide (5g, 20mmol) in THF (5mL) was then added, and the reaction was stirred at 00C for 30 minutes, and at room temperature for 2 hours. The mixture was diluted with EtOAc (5OmL) and washed with H2O (2OmL), and then dried over MgSO4, filtered, and concentrated. The crude material was purified by silica gel chromatography (0-25% EtOAc in hexanes) to give the title compound.
Step 2: l-(4-Bromo-benzyl)-cyclopropanecarboxylic acid
[00820] l-(4-Bromo-benzyl)-cyclopropanecarbonitrile (3g, 12.7mmol) was dissolved in ethylene glycol (25mL). Potassium hydroxide (2.12g, 37.8mmol) was added, and the reaction was stirred overnight at 1600C. After cooling to room temperature, H2O (10OmL) was added, and the mixture was washed 3 times with CHCI3. The aqueous layer was acidified with IN aqueous HCl and extracted 3 times with EtOAc (75mL portions). The combined organic layers were dried over MgSO4, filtered, and concentrated to give the title compound.
[00821] l-(4-Bromo-benzyl)-cyclopropanecarboxylic acid (12.7mmol) was dissolved in EtOH (5OmL) and treated with sulfuric acid (ImL). The reaction was refluxed overnight, and then cooled to room temperature and poured into H2O (10OmL). The mixture was extracted 3 times with EtOAc (75mL portions), and the combined organic layers were washed with saturated aqueous NaHCOs, dried over MgSO4, filtered, and concentrated. The crude material was purified by silica gel chromatography to give the title compound.
Step 4: l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-benzyl]- cyclopropanecarboxylic acid ethyl ester [00822] Prepared according to the procedure described in Example 2, Step 4 using the following starting materials: l-(4-bromo-benzyl)-cyclopropanecarboxylic acid ethyl ester and bis(pinacolato)diboron.
Step 5: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- ylmethyl}-cyclopropanecarboxylic acid ethyl ester
[00823] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl- ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-benzyl]- cyclopropanecarboxylic acid ethyl ester.
Step 6: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- ylmethyl}-cyclopropanecarboxylic acid
[00824] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 - (4'-[3-methyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-ylmethyl}-cyclopropanecarboxylic acid ethyl ester.
Example 49: Synthesis of l-(4'-{3-Methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-ylmethyl)-cyclopropanecarboxylic acid
Step 1: l-(4'-{3-Methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-ylmethyl)-cyclopropanecarboxylic acid ethyl ester
[00825] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -(3- trifluoromethyl-phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)- benzyl]-cyclopropanecarboxylic acid ethyl ester.
Step 2: l-(4'-{3-Methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-ylmethyl)-cyclopropanecarboxylic acid
[00826] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -(4'- (3-methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-ylmethyl)-cyclopropanecarboxylic acid ethyl ester. Example 50: Synthesis of l-(4'-{3-Ethyl-4-[(R)-l-(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-50)
Step 1: [5-(4-Bromo-phenyl)-3-ethyl-isoxazol-4-yl]-carbamic acid (R)-l-(3-trifluoromethyl- phenyl)-ethyl ester
[00827] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-ethyl-isoxazole-4-carboxylic acid and (R)-I -(3- trifluoromethyl-phenyl)-ethanol.
Step 2: l-(4'-{3-Ethyl-4-[(R)-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol- 5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester [00828] Prepared according to the procedure described in Example 1, Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-ethyl-isoxazol-4-yl]-carbamic acid (R)-I -(3- trifluoromethyl-phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)- phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 3: l-(4'-{3-Ethyl-4-[(R)-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol- 5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
[00829] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -(4'- (3-ethyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester. Example 51: Synthesis of l-(4'-{3-Phenyl-4-[(R)-l-(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-51)
Step 1: [5-(4-Bromo-phenyl)-3-phenyl-isoxazol-4-yl]-carbamic acid (R)-l-(3- trifluoromethyl-phenyl)-ethyl ester
[00830] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-phenyl-isoxazole-4-carboxylic acid and (R)-l-(3- trifluoromethyl-phenyl)-ethanol.
Step 2: l-(4'-{3-Phenyl-4-[(R)-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00831] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-phenyl-isoxazol-4-yl]-carbamic acid (R)- 1 -(3- trifluoromethyl-phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)- phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 3: l-(4'-{3-Phenyl-4-[(R)-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
[00832] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -(4'- (3-phenyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester. Example 52: Synthesis of l-{4'-[3-Methyl-4-(l-phenyl-ethoxy-d9-carbonylamino)-isoxazol- 5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-52)
Step 1 : l-{4'-[3-Methyl-4-(l-phenyl-ethoxy-d9-carbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid ethyl ester
[00833] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-[4'-(l-ethoxycarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazole-4- carboxylic acid and 1 -phenylethanol-d9 (obtained from Carbocore).
Step 2: l-{4'-[3-Methyl-4-(l-phenyl-ethoxy-d9-carbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid [00834] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: l-{4'-[3-methyl-4-(l-phenyl-ethoxy-d9-carbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 53: Synthesis of l-(4'-{3-Methyl-4-[l-methyl-l-(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-53)
Step 1: 2-(3-Trifluoromethyl-phenyl)-propan-2-ol
[00835] To a stirring solution of 3'-(trifluoromethyl)acetophenone (2.77g, 14.7mmol) in THF at 00C was slowly added methylmagnesium chloride (3M in THF; 7AmL, 22.2mmol). The reaction was stirred at room temperature for 2 hours, and then quenched with saturated aqueous NH4CI and extracted with EtOAc. The crude material was purified by silica gel chromatography to give the title compound.
Step 2: l-(4'-{3-Methyl-4-[l-methyl-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00836] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-[4'-(l-ethoxycarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazole-4- carboxylic acid and 2-(3-trifluoromethyl-phenyl)-propan-2-ol.
Step 3: l-(4'-{3-Methyl-4-[l-methyl-l-(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
[00837] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -(4'- {3-methyl-4-[ 1 -methyl- 1 -(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester.
Example 54: Synthesis of l-(3'-Methoxy-4'-{3-methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-54)
[00838] To a suspension of 4-bromo-2-methoxybenzoic acid (2.5g, 1 1.04mmol) in CHC13 (2OmL) was added DMF (catalytic) and thionyl chloride (1.6mL, 22.08mmol). The reaction was stirred at 55°C for 1 hour and then concentrated to dryness to give the title compound.
[00839] Prepared according to the procedure described in Example 1 , Step 2 using the following starting materials: 4-bromo-2-methoxy-benzoyl chloride and 3-methylamino-but-2-enoic acid methyl ester.
[00840] Prepared according to the procedure described in Example 1 , Step 3 using the following starting materials: 2-(4-bromo-2-methoxy-benzoyl)-3-oxo-butyric acid methyl ester and hydroxylamine hydrochloride.
Step 4: 5-(4-Bromo-2-methoxy-phenyl)-3-methyl-isoxazole-4-carboxylic acid
[00841] Prepared according to the procedure described in Example 1 , Step 4 using the following starting material: 5-(4-bromo-2-methoxy-phenyl)-3-methyl-isoxazole-4-carboxylic acid methyl ester.
Step 5: [5-(4-Bromo-2-methoxy-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(3- trifluoromethyl-phenyl)-ethyl ester
[00842] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-2-methoxy-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)- 1 -(3-trifluoromethyl-phenyl)-ethanol.
Step 6: l-(3'-Methoxy-4'-{3-methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00843] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-2-methoxy-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-
l-(3-trifluoromethyl-phenyl)-ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2- yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 7: l-(3'-Methoxy-4'-{3-methyl-4-[(R)-l-(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
[00844] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -(3'-methoxy-4!- (3-methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester. Example 55: Synthesis of l-(4'-{4-[(R)-l-(3,5-Dibromo-phenyl)-ethoxycarbonylamino]-3- methyl-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-55)
Step 1: l-(3,5-Dibromo-phenyl)-ethanone
[00845] To a solution of 3,5-dibromobenzoic acid (2.5g, 8.9mmol) in Et2O (3OmL) at 00C was added methyllithium (1.6M in Et2O; 12.3mL, 19.6mmol) dropwise. The reaction was stirred at 00C for 2 hours, and then worked-up with EtOAc and 10% aqueous HCl. The crude material was purified by silica gel chromatography to give the title compound.
Step 2: (R)-l-(3,5-Dibromo-phenyl)-ethanol
[00846] Prepared according to the procedure described in Example 2, Step 5 using the following starting material: l-(3,5-dibromo-phenyl)-ethanone.
Step 3: l-(4'-{4-[(R)-l-(3,5-Dibromo-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester
[00847] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-[4'-(l-ethoxycarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazole-4- carboxylic acid and (R)-l-(3,5-cibromo-phenyl)-ethanol.
Step 4: l-(4'-{4-[(R)-l-(3,5-Dibromo-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid [00848] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: l-(4'-{4-[(R)-l-(3,5-dibromo-phenyl)-ethoxycarbonylamino]-3-methyl- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid ethyl ester.
Example 56: Synthesis of {5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-biphenyl- 4-yl]-3-methyl-isoxazol-4-yl}-carbamic acid (R)-l-phenyl-ethyl ester (Compound 2-1)
Step 1 : {5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl- isoxazol-4-yl}-carbamic acid (R)-l-phenyl-ethyl ester
[00849] l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (O.lg, 0.2mmol), methanesulfonamide (0.08g, 0.8mmol), and N,N'-carbonyldiimidazole (0.15g, O.όmmol) were combined in THF (4mL). Diisopropylethylamine (0.5mL) was added, and the reaction was stirred at 65°C overnight. The mixture was acidified and extracted with CH2Cl2. The crude material was purified by silica gel chromatography (0-50% EtOAc in hexanes) to give the title compound.
Example 57: Synthesis of {5-[4'-(l-Benzenesulfonylaminocarbonyl-cyclopropyl)-biphenyl- 4-yl]-3-methyl-isoxazol-4-yl}-carbamic acid (R)-l-phenyl-ethyl ester (Compound 2-2)
Step 1 : {5-[4'-(l-Benzenesulfonylaminocarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl- isoxazol-4-yl}-carbamic acid (R)-l-phenyl-ethyl ester
[00850] Prepared according to the procedure described in Example 56, Step 1 using the following starting materials: l-{4'-[3-methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol- 5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid and benzenesulfonamide. Example 58: Synthesis of {5-[4'-(l-Cyano-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazol-4- yl}-carbamic acid (R)-l-phenyl-ethyl ester (Compound 2-3)
Step l: l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarbonitrile
[00851] Prepared according to the procedure described in Example 2, Step 4 using the following starting materials: l-(4-bromo-phenyl)-cyclopropanecarbonitrile and bis(pinacolato)diboron.
Step 2: {5-[4'-(l-Cyano-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazol-4-yl}-carbamic acid (R)-l-phenyl-ethyl ester
[00852] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl- ethyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarbonitrile.
Example 59: Synthesis of (3-Methyl-5-{4'-[l-(5-oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl)- cyclopropyl]-biphenyl-4-yl}-isoxazol-4-yl)-carbamic acid (R)-l-phenyl-ethyl ester (Compound 2-4)
Step 1 : (5-{4'-[l-(iV-Hydroxycarbamimidoyl)-cyclopropyl]-biphenyl-4-yl}-3-methyl- isoxazol-4-yl)-carbamic acid (R)-l-phenyl-ethyl ester
[00853] {5-[4'-(l-Cyano-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazol-4-yl}-carbamic acid (R)-l-phenyl-ethyl ester (0.307g, 0.66mmol), hydroxylamine hydrochloride (0.046g, 0.67mmol), and triethylamine (0.097mL, 0.67mmol) were combined in EtOH (7mL), and the reaction was stirred at 500C overnight. Additional hydroxylamine hydrochloride (0.10Og, 1.45mmol) and triethylamine (0.3OmL, 2.15mmol) were added, and the reaction was stirred overnight. The mixture was then concentrated to provide the title compound.
Step 2: (3-Methyl-5-{4'-[l-(5-oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl)-cyclopropyl]- biphenyl-4-yl}-isoxazol-4-yl)-carbamic acid (R)-l-phenyl-ethyl ester
[00854] To (5-{4'-[l-(N-hydroxycarbamimidoyl)-cyclopropyl]-biphenyl-4-yl}-3-methyl- isoxazol-4-yl)-carbamic acid (R)- 1 -phenyl-ethyl ester (0.66mmol) in CH2CI2 (5mL) was added triethylamine (0.19mL, 1.32mmol) and ethyl chloroformate (0.127mL, 1.32mmol), and the reaction was stirred overnight at room temperature. Additional triethylamine (0.19mL, 1.32mmol) was added, and the reaction was stirred for 6 hours. Additional triethylamine (0.19mL, 1.32mmol) was added, and the reaction was stirred until complete. The mixture was diluted with CH2Cl2 (2OmL) and washed with 10% aqueous citric acid, and then dried over
MgSO4, filtered, and concentrated. The residue was dissolved in toluene (1OmL), and the solution was refluxed for 3 days. After concentrating, the crude material was purified by silica gel chromatography (0-100% EtOAc in hexanes) to give the title compound. Example 60: Synthesis of (3-Methyl-5-{4'-[l-(lH-tetrazol-5-yl)-cyclopropyl]-biphenyl-4- yl}-isoxazol-4-yl)-carbamic acid (R)-l-phenyl-ethyl ester (Compound 2-5)
Step 1 : (3-Methyl-5-{4'-[l-(lH-tetrazol-5-yl)-cyclopropyl]-biphenyl-4-yl}-isoxazol-4-yl)- carbamic acid (R)-l-phenyl-ethyl ester
[00855] {5-[4'-(l-Cyano-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazol-4-yl}-carbamic acid (R)-l-phenyl-ethyl ester (0.385g, 0.83mmol) and N,N-dimethylethanolamine (O.lOlmL, l.Ommol) were combined in diglyme (diethylene glycol dimethyl ether; 2mL). Hydrochloric acid (4M in 1,4-dioxane; 4.2mL) was added, and the reaction was stirred for 15 minutes. Additional N,N-dimethylethanolamine (0.22ImL, 2.2mmol) was added, followed by sodium azide (0.098g, 1.5mmol), and the reaction was stirred at 1200C for 24 hours. After cooling to room temperature, the mixture was diluted with CH2CI2 (2OmL) and H2O (1OmL). The organic layer was dried over MgSO4, filtered, and concentrated, and the residue was purified by silica gel chromatography to give the title compound.
Example 61: Synthesis of {5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-biphenyl- 4-yl]-3-methyl-isoxazol-4-yl}-carbamic acid (R)-l-(3-trifluoromethyl-phenyl)-ethyl ester (Compound 2-6)
Step 1: iV-[l-(4-Bromo-phenyl)-cyclopropanecarbonyl]-methanesulfonamide
[00856] To a solution of l-(4-bromo-phenyl)-cyclopropanecarboxylic acid (5.Og, 20.7mmol) in toluene (3OmL) was slowly added thionyl chloride (17.7mL, 243mmol), and the reaction was refluxed for 4 hours. The mixture was concentrated, and the crude material was dissolved in toluene (5OmL). Methanesulfonamide (11.4Ig, 120mmol) was added, followed by triethylamine (15mL), and the reaction was refluxed for 3 hours. After cooling to room temperature, the mixture was poured in CH2CI2 (20OmL) and washed with H2O (15OmL). The organic layer was dried over MgSO4, filtered, and concentrated, and the crude material was purified by silica gel chromatography to give the title compound.
Step 2: iV-{l-[4-(4,4,5,5-Tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarbonyl}-methanesulfonamide
[00857] Prepared according to the procedure described in Example 2, Step 4 using the following starting materials: N-[l-(4-bromo-phenyl)-cyclopropanecarbonyl]-methanesulfonamide and bis(pinacolato)diboron.
Step 3: {5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl- isoxazol-4-yl}-carbamic acid (R)-l-(3-trifluoromethyl-phenyl)-ethyl ester
[00858] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-I -(3- trifluoromethyl-phenyl)-ethyl ester and N-{l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)- phenyl]-cyclopropanecarbonyl} -methanesulfonamide.
Example 62: Synthesis of {5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-3- methoxy-biphenyl-4-yl]-3-methyl-isoxazol-4-yl}-carbamic acid (R)-l-(3-trifluoromethyl- phenyl)-ethyl ester (Compound 2-7)
Step 1: [5-(4-Bromo-2-methoxy-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-l-(3- trifluoromethyl-phenyl)-ethyl ester
[00859] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-2-methoxy-phenyl)-3-methyl-isoxazole-4-carboxylic acid and (R)- 1 -(3-trifluoromethyl-phenyl)-ethanol.
Step 2: {5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-3-methoxy-biphenyl-4-yl]- 3-methyl-isoxazol-4-yl}-carbamic acid (R)-l-(3-trifluoromethyl-phenyl)-ethyl ester
[00860] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-2-methoxy-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)- l-(3-trifluoromethyl-phenyl)-ethyl ester and N-{l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-
2-yl)-phenyl]-cyclopropanecarbonyl}-methanesulfonamide.
Example 63: Synthesis of l-{4'-[3-Methyl-4-(l-methyl-cyclopentyloxycarbonylamino)- isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 3-1)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-methyl-cyclopentyl ester
[00861] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and 1- methylcyclopentanol.
Step 2: l-{4'-[3-Methyl-4-(l-methyl-cyclopentyloxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester [00862] Prepared according to the procedure described in Example 1, Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid 1-methyl- cyclopentyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]- cyclopropanecarboxylic acid ethyl ester.
Step 3: l-{4'-[3-Methyl-4-(l-methyl-cyclopentyloxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid
[00863] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: l-{4'-[3-methyl-4-(l-methyl-cyclopentyloxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid ethyl ester.
Example 64: Synthesis of l-[4'-(4-Cyclobutoxycarbonylamino-3-methyl-isoxazol-5-yl)- biphenyl-4-yl]-cyclopropanecarboxylic acid (Compound 3-2)
Step 1: [5-(4-Bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid cyclobutyl ester
[00864] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-3-methyl-isoxazole-4-carboxylic acid and cyclobutanol.
Step 2: l-[4'-(4-Cyclobutoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester
[00865] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid cyclobutyl ester and l-[4-(4,4,5,5-tetramethyl-[l,3,2]dioxaborolan-2-yl)-phenyl]-cyclopropanecarboxylic acid ethyl ester.
Step 3: l-[4'-(4-Cyclobutoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid
[00866] Prepared according to the procedure described in Example 2, Step 8 using the following starting material: 1 -[4'-(4-cyclobutoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid ethyl ester.
Example 65: Synthesis of l-{4'-[4-((R)-l-Phenyl-ethoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-56)
[00867] A solution of ethyl (4-bromobenzoyl)acetate (1.19g, 4.39mmol) in N,N- dimethylformamide dimethyl acetal (1OmL) was stirred at 1000C for 1 hour. The mixture was concentrated, and the residue was dissolved in EtOH (1OmL). Hydroxylamine hydrochloride (0.454g, 6.57mmol) was added, and the reaction was stirred at 1000C for 1 hour. After cooling to room temperature, the mixture was partitioned between EtOAc and H2O, and the organic layer was separated, dried over MgSO4, filtered, and concentrated. The crude material was purified by silica gel chromatography to give the title compound.
Step 4: 5-(4-Bromo-phenyl)-isoxazole-4-carboxylic acid
[00868] 5-(4-Bromo-phenyl)-isoxazole-4-carboxylic acid ethyl ester (0.50Og, 1.69mmol) was dissolved in concentrated hydrochloric acid (2mL), acetic acid (5mL), and H2O (5mL), and the reaction was stirred at 1000C overnight. The mixture was partitioned between EtOAc and H2O, and the organic layer was separated, dried over MgSO4, filtered, and concentrated to give the title compound.
Step 5: [5-(4-Bromo-phenyl)-isoxazol-4-yl]-carbamic acid (R)-l-phenyl-ethyl ester
[00869] Prepared according to the procedure described in Example 1 , Step 5 using the following starting materials: 5-(4-bromo-phenyl)-isoxazole-4-carboxylic acid and (R)-l-phenyl-ethanol.
Step 6: l-{4'-[4-((R)-l-Phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid
[00870] Prepared according to the procedure described in Example 1 , Step 6 using the following starting materials: [5-(4-bromo-phenyl)-isoxazol-4-yl]-carbamic acid (R)- 1 -phenyl-ethyl ester and 4-(l-carboxycyclopropyl)phenylboronic acid.
Example 66: Synthesis of l-{4'-[3-Methyl-4-(l-phenyl-ethoxycarbonylamino)-isoxazol-5- yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-57)
[00871] Prepared according to the procedure described in Example 1 for Compound 1-1 but using racemic 1 -phenylethyl alcohol in place of (R)-(+)- 1 -phenylethyl alcohol. [00872] In some embodiments, Mass spectrometric data (mass spec, data) is obtained on with a Shimadzu LCMS 201 OA.
Example 67. Establishment of a CHO Cell Line Stably Expressing Human LPA1 [00873] A 1.1 kb cDNA encoding the human LPA1 receptor was cloned from human lung. Human lung RNA (Clontech Laboratories, Inc. USA) was reverse transcribed using the RETROscript kit (Ambion, Inc.) and the full-length cDNA for human LPA1 was obtained by PCR of the reverse transcription reaction. The nucleotide sequence of the cloned human LPA1 was determined by sequencing and confirmed to be identical to the published human LPA1 sequence (An et al. Biochem. Biophys. Res. Commun. 231 :619 (1997). The cDNA was cloned into the pCDNA5/FRT expression plasmid and transfected in CHO cells using lipofectamine 2000 (Invitrogen Corp., USA). Clones stably expressing human LPA1 were selected using hygromycin and identified as cells that show Ca-influx in response to LPA. Example 68. Generation of Cells Transiently Expressing Human LPA2 [00874] A vector containing the human LPA2 receptor cDNA was obtained from the Missouri S&T cDNA Resource Center (www.cdna.org). The full-length cDNA fragment for human LPA2 was obtained by PCR from the vector. The nucleotide sequence of the cloned human LPA2 was determined by sequencing and confirmed to be identical to the published human LPA2 sequence (NCBI accession number NM_004720). The cDNA was cloned into the pCDNA3.1 expression plasmid and transfected into B 103 cells (Invitrogen Corp., USA) by seeding cells in a 96-well poly-D-lysine coated plate at 30,000-35,000 cells per well together with 0.2 μl lipofectamine 2000 and 0.2 μg of the LPA2 expression vector. Cells were cultured overnight in complete media before being assayed for LPA-induced Ca-influx.
Example 69. Establishment of a CHO Cell Line Stably Expressing Human LPA3
[00875] A vector containing the human LP A3 receptor cDNA was obtained from the Missouri S&T cDNA Resource Center (www.cdna.org). The full-length cDNA fragment for human LPA3 was obtained by PCR from the vector. The nucleotide sequence of the cloned human LPA3 was determined by sequencing and confirmed to be identical to the published human LPA3 sequence (NCBI accession number NM_012152). The cDNA was cloned into the pCDNA5/FRT expression plasmid and trans fected in CHO cells using lipofectamine 2000 (Invitrogen Corp., USA). Clones stably expressing human LPA3 were selected using hygromycin and identified as cells that show Ca-influx in response to LPA. Example 70. LPAl and LPA3 Calcium Flux Assays. [00876] Human LPAi or LPA3 expressing CHO cells are seeded at 20,000-45,000 cells per well in a 96-well poly-D-lysine coated plate one or two days before the assay. Prior to the assay, the cells are washed once with PBS and then cultured in serum- free media overnight. On the day of the assay, a calcium indicator dye (Calcium 4, Molecular Devices) in assay buffer (HBSS with Ca2+ and Mg2+ and containing 20 mM Hepes and 0.3% fatty-acid free human serum albumin) is added to each well and incubation continued for 1 hour at 37°C. 10 μl of test compounds in 2.5% DMSO are added to the cells and incubation continued at room temperature for 30 minutes. Cells are the stimulated by the addition of 10 nM LPA and intracellular Ca2+ measured using the Flexstation 3 (Molecular Devices). IC50S are determined using Graphpad prism analysis of drug titration curves. Example 71. LPA2 Calcium Flux Assay
[00877] BT-20 human breast cancer cells are seeded at 25,000-35,000 cells per well in 150 μl complete media on Poly-D-Lysine coated black-wall clear-bottom plates. Following an overnight culture, cells are washed once with PBS then serum starved for 4-6 hours prior to the assay. On the day of the assay, a calcium indicator dye (Calcium 5, Molecular Devices) in assay buffer (HBSS with Ca2+ and Mg2+ and containing 20 mM Hepes and 0.3% fatty-acid free human serum albumin) is added to each well and incubation continued for 15 minutes at 37°C. 25 μl of test compounds in 2.5% DMSO are added to the cells and incubation continued at 37°C for 15 minutes. Cells are the stimulated by the addition of 100 nM LPA and intracellular Ca2+ measured using the Flexstation 3 (Molecular Devices). IC50S are determined using Symyx Assay Explorer analysis of drug titration curves. Example 72. GTPγS Binding Assay
[00878] The ability of a compound to inhibit binding of GTP to LPA1 is assessed via a membrane GTPγS assay. CHO cells stably expressing the recombinant human LPA1 receptor are resuspended in 10 mM Hepes, 7.4 containing 1 mM DTT, lysed and centrifuged at 75,000 xg to pellet the membranes. The membranes are resuspended in 10 mM Hepes, 7.4 containing 1
niM DTT and 10% glycerol. Membranes (~25 μg per well) are incubated in 96-well plates with 0.1 nM [35S]-GTPyS, 900 nM LPA, 5 μM GDP, and test compound in Assay Buffer (50 mM Hepes, pH 7.4, 100 mM NaCl, 10 mM MgCl2 , 50 μg/ml saponin and 0.2% fatty-acid free human serum albumin) for 30 minutes at 300C. The reactions are terminated by rapid filtration through Whatman GF/B glass fibre filter plates. The filter plates are washed 3 times with 1 ml cold Wash Buffer (50 mM Hepes, 7.5, 100 mM NaCl and 10 mM MgCl2) and dried. Scintillant is then added to the plates and the radioactivity retained on the filters is determined on a Packard TopCount (Perkin Elmer). Specific binding is determined as total radioactive binding minus non-specific binding in the absence of the ligand (900 nM LPA). IC50S were determined using Graphpad prism analysis of drug titration curves.
[00879] Illustrative in vitro biological data for representative compounds described herein is presented in the Table below.
A = less than 0.3 μM; B = greater than 0.3 μM and less than 1 μM; C= greater than 1 μM and less than 10 μM; D= greater than 10 μM. ND = not determined
Example 73. LPAl Chemotaxis Assay.
[00880] Chemotaxis of the A2058 human melanoma cells was measured using the Neuroprobe ChemoTx® System plates (8 μm pore size, 5.7 mm diameter sites). The filter sites were coated with 0.001% fibronectin (Sigma) in 20 mM Hepes, pH 7.4 and allowed to dry. A2058 cells were serum-starved for 24 hours , then harvested with Cell Stripper and resuspended in DMEM containing 0.1% fatty-acid-free bovine serum albumin (BSA) to a concentration of 1 x 106/ml.
Cells were mixed with an equal volume of test compound (2X) in DMEM containing 0.1% fatty-acid-free BSA and incubated at 37°C for 15 minutes. LPA (100 nM in DMEM containing 0.1% fatty-acid-free BSA) or vehicle was added to each well of the lower chamber and 50 μl of the cell suspension/test compound mix was applied to the upper portion of the ChemoTx plate. Plates were incubated at 37°C for three hours and then the cells removed from the upper portion by rinsing with PBS and scraping. The filter was dried then stained with HEMA 3 Staining System (Fisher Scientific). The absorbance of the filter was read at 590 nM and IC50S were determined using Symyx Assay Explorer. [00881] Compounds 1-1, 1-5, 1-12, 1-25, 1-26, 1-29, 1-34, 1-46, 2-1, 2-2, 2-4, 2-5, inhibited LPA-driven chemotaxis (IC50 less than 100 nM) of human A2058 melanoma cells Example 74: Bleomycin-induced Lung fibrosis model in mice
[00882] Female C57B1/6 mice (Harlan, 25-3Og) are housed 4 per cage, given free access to food and water and allowed to acclimate for at least 7 days prior to test initiation. After the habituation phase, mice are lightly anesthetized with isoflurane (5% in 100% O2) and administered with bleomycin sulfate (0.01-5 U/kg, Henry Schein) via intratracheal instillation (Cuzzocrea S et al. Am J Physiol Lung Cell MoI Physiol. 2007 May;292(5):L 1095- 104. Epub 2007 Jan 12.). Mice are returned to their cages and monitored daily for the duration of the experiment. Test compound or vehicle is delivered po, ip or sc daily. The route and frequency of dosing is based on previously determined pharmacokinetic properties. All animals are sacrificed using inhaled isoflurane 3, 7, 14, 21 or 28 days after bleomycin instillation. Following sacrifice, mice are intubated with a 20 gauge angiocatheter attached to a 1 ml syringe. Lungs are lavaged with saline to obtain bronchoalveolar lavage fluid (BALF) and then removed and fixed in 10% neutral buffered formalin for subsequent histopathological analysis. BALF is centrifuged for 10 min at 800 x g to pellet the cells and the cell supernatant removed and frozen at -80 0C for subsequent protein analysis using the DC protein assay kit (Biorad, Hercules, CA.) and soluble collagen analysis using Sircol (Biocolor Ltd, UK). BALF is analyzed for concentrations of inflammatory, pro-fibrotic and tissue injury biomarkers including transforming growth factor βl, hyaluronic acid, tissue inhibitor of metalloproteinase- 1 , matrix matelloproteinase-7, connective tissue growth factor and lactate dehydrogenase activity, using commercially available ELISA. The cell pellet is re-suspended in PBS. Total cell counts are then obtained using a Hemavet hematology system (Drew Scientific, Wayne, PA.) and differential cells counts are determined using Shandon cytospin (Thermo Scientific, Waltham, MA.). Lung tissue is stained using hematoxylin and eosin (H&E) and trichrome and lung fibrosis. is determined by semiquantitative histopathological scoring (Ashcroft T. et al. J. Clin. Path. 1988;41;4, 467-470) using light microscopy (10x magnification) and quantitative, computer-assisted densitometry of
collagen in lung tissue sections using light microscopy. The data are plotted using Graphpad prism and statistical differences between groups determined.
[00883] In the acute setting (3 day), Compound 1-1 significantly reduced total protein and collagen concentrations in broncheoalveolar lavage fluid (BALF). In a 7-day bleomycin model compound 1-1 reduced BALF collagen, protein, TGFβl, MMP-7, hyaluronan, and inflammatory cell influx. In the chronic setting (14 day bleomycin model), Compound 1-1 decreased total lung collagen when dosed either propylactically (day 0 - day 14) or therapeutically (day 3 - day 14). Example 75: Mouse carbon tetrachloride (CCLt)-induced liver fibrosis model [00884] Female C57BL/6 mice (Harlan, 20-25g) housed 4/cage are given free access to food and water and allowed to acclimate for at least 7 days prior to test initiation. After the habituation phase, mice receive CCl4 (1.0 ml/kg body weight) diluted in corn oil vehicle (100 μL volume) via i.p. injection twice a week for 8 weeks. (Higazi, A. A. et ah, Clin Exp Immunol. 2008 Apr;152(l): 163-73. Epub 2008 Feb 14.). Control mice receive an equivalent volume of corn oil vehicle only. Test compound or vehicle is delivered po, ip or sc daily. At the end of the study (8 weeks after first i.p. injection Of CCl4), mice are sacrificed using inhaled isoflurane and blood is drawn via cardiac puncture for subsequent analysis of ALT/AST levels. The liver is harvested, and one half of the liver is frozen at -800C and the other half is fixed in 10% neutral buffered formalin for histological assessment of liver fibrosis using light microscopy (10x magnification). Liver tissue homogenates are analyzed for collagen levels using Sircol
(Biocolor Ltd, UK). Fixed Liver tissue is stained using hematoxylin and eosin (H&E) and trichrome and liver fibrosis is determined by quantitative, computer-assisted densitometry of collagen in liver tissue sections using light microscopy. Plasma and liver tissue lysates are also analyzed for concentrations of inflammatory, pro-fibrotic and tissue injury biomarkers including transforming growth factor βl, hyaluronic acid, tissue inhibitor of metalloproteinase- 1 , matrix matelloproteinase-7, connective tissue growth factor and lactate dehydrogenase activity, using commercially available ELISA. The resulting data are plotted using Graphpad prism and statistical differences between groups determined. [00885] In this experiment, Compound 1-1 significantly reduced liver weight increase and collagen deposition in the liver as compared to the untreated group. Example 76: Mouse intravenous LPA-induced histamine release
[00886] A mouse intravenous LPA-induced histamine release model is utilized to determine the in vivo potency of LPAi and LPA3 receptor antagonists. Female CD-I mice (weighing 25 - 35 grams) are administered compound (i.p., s.c. or p.o.) in a volume of 10ml/kg 30 minutes to 24 hours prior to intravenous LPA challenge (300 μg/mouse in 0.1% FAF BSA). Immediately
following LPA challenge mice are placed into an enclosed Plexiglas chamber and exposed to an isoflurane for a period of 2 minutes. They are removed, decapitated and trunk blood collected into tubes containing EDTA. Blood is then centrifuged at 10,000 X g for 10 minutes at 4°C. Histamine concentrations in the plasma are determined by EIA. Drug concentrations in plasma are determined by mass spectrometry. The dose to achieve 50% inhibition of blood histamine release is calculated by nonlinear regression (Graphpad Prism) and plotted as the ED50. The plasma concentration associated with this dose is plotted as the EC50. Example 77: Mouse dermal vascular leak assay [00887] Female BALB/c mice (Harlan) weighing 20-25 grams were given free access to standard mouse chow and water and were allowed to acclimate for two weeks prior to study initiation. Compound 1-1 was prepared in water vehicle at a concentration of 3 mg/ml and delivered by oral gavage at a volume of 10 ml/kg to yield a dose of 30 mg/kg. Three hours following dose, mice were placed into a restraining device and given Evan's blue dye intravenously by tail vein injection (0.2 ml of a 0.5 % solution). Mice were then anesthetized using 3% isoflurane anaesthesia to allow for intradermal injection of LPA (30 μg in 20 μl 0.1 % fatty acid free BSA). Thirty minutes after LPA injection mice were sacrificed by CO2 inhalation and the skin removed from the challenge site and placed into 2 ml formamide for overnight extraction of Evan's blue dye. [00888] Following extraction, a 150 μl aliquot of formamide for each tissue sample was placed into a 96 well plate and read at 610 nm using a photospectometer. The resulting data (OD units) were plotted using GraphPad Prizm. In this experiment compound 1-1 reduced LPA-induced Evan's blue dye leak into the skin.
Example 78: Mouse unilateral ureteral obstruction kidney fibrosis model [00889] Female C57BL/6 mice (Harlan, 20-25g) housed 4/cage will be given free access to food and water and allowed to acclimate for at least 7 days prior to test initiation. After the habituation phase, mice undergo unilateral ureteral obstruction (UUO) surgery or sham to left kidney. Briefly, a longitudinal, upper left incision is performed to expose the left kidney. The renal artery is located and 6/0 silk thread is passed between the artery and the ureter. The thread is looped around the ureter and knotted 3 times insuring full ligation of ureter. The kidney is returned to abdomen, the abdominal muscle is sutured and the skin is stapled closed. Mice are returned to their cages and monitored daily for the duration of the experiment. Test compound or vehicle is delivered po, ip or sc daily. The route and frequency of dosing is based on previously determined pharmacokinetic properties. All animals are sacrificed using inhaled isoflurane 4, 8 or 14 days after UUO surgery. Following sacrifice blood is drawn via cardiac puncture, the kidneys are harvested and one half of the kidney is frozen at -8O0C and the other
half is fixed in 10% neutral buffered formalin for histological assessment of kidney fibrosis using light microscopy (1Ox magnification). Kidney tissue homogenates are analyzed for collagen levels using Sircol (Biocolor Ltd, UK). Fixed kidney tissue is also stained using hematoxylin and eosin (H&E) and trichrome and kidney fibrosis is determined by quantitative, computer-assisted densitometry of collagen in liver tissue sections using light microscopy and collagen content in kidney lysate. Plasma and kidney tissue lysates are also analyzed for concentrations of inflammatory, pro-fibrotic and tissue injury biomarkers including transforming growth factor βl, hyaluronic acid, tissue inhibitor of metalloproteinase- 1 , and plasminogen activator inhibitor -1,, using commercially available ELISA. The resulting data are plotted using Graphpad prism and statistical differences between groups determined.
[00890] In this experiment, Compound 1-1 reduced total kidney collogen, collagen Type 1, transforming growth factor βl, hyaluronic acid, tissue inhibitor of metalloproteinase- 1 and plasminogen activator inhibitor -1 compared to untreated group. Example 79: Clinical Trial in Humans with Idiopathic Pulmonary Fibrosis (IPF) Purpose
[00891] The purposes of this study is to assess the efficacy of treatment with a compound of Formula (I), (II), (III), (IV) or (V) compared with placebo in patients with idiopathic pulmonary fibrosis (IPF) and to assess the safety of treatment with a compound of Formula (I), (II), (III), (IV) or (V) compared with placebo in patients with IPF. [00892] The primary outcome variable is the absolute change in percent predicted forced vital capacity (FVC) from baseline to Week 72.
[00893] Secondary outcome measures include: composite outcomes of important IPF-related events; progression- free survival; categorical assessment of absolute change in percent predicted FVC from baseline to Week 72; change in Shortness-of-Breath from baseline to Week 72; change in percent predicted hemoglobin (Hb)-corrected carbon monoxide diffusing capacity (DLco) of the lungs from baseline to Week 72; change in oxygen saturation during the 6 minute walk test (6MWT) from baseline to Week 72; change in high-resolution computed tomography (HRCT) assessment from baseline to Week 72; change in distance walked in the 6MWT from baseline to Week 72. Criteria
[00894] Patients eligible for this study include those patients that satisfy the following inclusion criteria: diagnosis of IPF; 40 to 80 years of age; FVC > 50% predicted value; DLco > 35% predicted value; either FVC or DLco < 90% predicted value; no improvement in past year; able to walk 150 meters in 6 minutes and maintain saturation > 83% while on no more than 6 L/min supplemental oxygen.
[00895] Patients are excluded from this study if they satisfy any of the following criteria: unable to undergo pulmonary function testing; evidence of significant obstructive lung disease or airway hyper-responsiveness; in the clinical opinion of the investigator, the patient is expected to need and be eligible for a lung transplant within 72 weeks of randomization; active infection; liver disease; cancer or other medical condition likely to result in death within 2 years; diabetes; pregnancy or lactation; substance abuse; personal or family history of long QT syndrome; other IPF treatment; unable to take study medication; withdrawal from other IPF trials. [00896] Patients are orally dosed with either placebo or an amount of compound of Formula (I), (II), (III), (IV) or (V) (1 mg/day - 1000 mg/day). The primary outcome variable will be the absolute change in percent predicted FVC from Baseline to Week 72. Patients will receive blinded study treatment from the time of randomization until the last patient randomized has been treated for 72 weeks. A Data Monitoring Committee (DMC) will periodically review safety and efficacy data to ensure patient safety. [00897] After week 72, patients who meet the Progression of Disease (POD) definition, which is a > 10% absolute decrease in percent predicted FVC or a > 15% absolute decrease in percent predicted DLco, will be eligible to receive permitted IPF therapies in addition to their blinded study drug. Permitted IPF therapies include corticosteroids, azathioprine, cyclophosphamide and N-acetyl-cysteine. Example 80: Parenteral Composition [00898] To prepare a parenteral pharmaceutical composition suitable for administration by injection (subcutaneous, intravenous, and the like), 100 mg of a water-soluble salt of a compound of Formula (I), (II), (III), (IV) or (V) is dissolved in sterile water and then mixed with 10 mL of 0.9% sterile saline. The mixture is incorporated into a dosage unit form suitable for administration by injection [00899] In another embodiment, the following ingredients are mixed to form an injectable formulation: 1.2 g of a compound of Formulas (I), 2.0 mL of sodium acetate buffer solution (0.4 M), HCl (1 N) or NaOH (1 M) (q.s. to suitable pH), water (distilled, sterile) (q.s.to 20 mL). All of the above ingredients, except water, are combined and stirred and if necessary, with slight heating if necessary. A sufficient quantity of water is then added. Example 81: Oral Composition
[00900] To prepare a pharmaceutical composition for oral delivery, 100 mg of a compound of Formula (I), (II), (III), (IV) or (V) is mixed with 750 mg of starch. The mixture is incorporated into an oral dosage unit for, such as a hard gelatin capsule, which is suitable for oral administration. Example 82: Sublingual (Hard Lozenge) Composition
[00901] To prepare a pharmaceutical composition for buccal delivery, such as a hard lozenge, mix 100 mg of a compound of Formula (I), (II), (III), (IV) or (V) with 420 mg of powdered sugar mixed, with 1.6 mL of light corn syrup, 2.4 mL distilled water, and 0.42 mL mint extract.
The mixture is gently blended and poured into a mold to form a lozenge suitable for buccal administration.
Example 83: Fast-Disintegrating Sublingual Tablet
[00902] A fast-disintegrating sublingual tablet is prepared by mixing 48.5% by weigh of a compound of Formula (I), (II), (III), (IV) or (V), 44.5% by weight of microcrystalline cellulose
(KG-802), 5% by weight of low-substituted hydroxypropyl cellulose (50 μm), and 2% by weight of magnesium stearate. Tablets are prepared by direct compression (AAPS
PharmSciTech. 2006;7(2):E41). The total weight of the compressed tablets is maintained at 150 mg. The formulation is prepared by mixing the amount of compound of Formula (I), (II), (III),
(IV) or (V) with the total quantity of microcrystalline cellulose (MCC) and two-thirds of the quantity of low-substituted hydroxypropyl cellulose (L-HPC) by using a three dimensional manual mixer (lnversina®, Bioengineering AG, Switzerland) for 4.5 minutes. All of the magnesium stearate (MS) and the remaining one-third of the quantity of L-HPC are added 30 seconds before the end of mixing.
Example 84: Inhalation Composition
[00903] To prepare a pharmaceutical composition for inhalation delivery, 20 mg of a compound of Formula (I), (II), (III), (IV) or (V) is mixed with 50 mg of anhydrous citric acid and 100 mL of 0.9% sodium chloride solution. The mixture is incorporated into an inhalation delivery unit, such as a nebulizer, which is suitable for inhalation administration.
[00904] In another embodiment, a compound of Formula (I), (II), (III), (IV) or (V) (500 mg) is suspended in sterile water (100 mL), Span 85 (1 g) is added followed by addition of dextrose (5.5 g) and ascorbic acid (10 mg). Benzalkonium chloride (3 mL of a 1 :750 aqueous solution) is added and the pH is adjusted to 7 with phosphate buffer. The suspension is packaged in sterile nebulizers.
Example 85: Rectal Gel Composition
[00905] To prepare a pharmaceutical composition for rectal delivery, 100 mg of a compound of Formula (I), (II), (III), (IV) or (V) is mixed with 2.5 g of methylcelluose (1500 mPa), 100 mg of methylparapen, 5 g of glycerin and 100 mL of purified water. The resulting gel mixture is then incorporated into rectal delivery units, such as syringes, which are suitable for rectal administration.
Example 86: Topical Gel Composition
[00906] To prepare a pharmaceutical topical gel composition, 100 mg of a compound of Formula (I), (II), (III), (IV) or (V) is mixed with 1.75 g of hydroxypropyl celluose, 10 mL of propylene glycol, 10 mL of isopropyl myristate and 100 mL of purified alcohol USP. The resulting gel mixture is then incorporated into containers, such as tubes, which are suitable for topicl administration.
Example 87: Ophthalmic Solution Composition
[00907] To prepare a pharmaceutical opthalmic solution composition, 100 mg of a compound of Formula (I), (II), (III), (IV) or (V) is mixed with 0.9 g of NaCl in 100 mL of purified water and filterd using a 0.2 micron filter. The resulting isotonic solution is then incorporated into ophthalmic delivery units, such as eye drop containers, which are suitable for ophthalmic administration.
Example 88: Nasal spray solution
[00908] To prepare a pharmaceutical nasal spray solution, 1O g of a compound of Formula (I), (II), (III), (IV) or (V) is mixed with 30 mL of a 0.05M phosphate buffer solution (pH 4.4). The solution is placed in a nasal administrator designed to deliver 100 μl of spray for each application.
[00909] The examples and embodiments described herein are for illustrative purposes only and various modifications or changes suggested to persons skilled in the art are to be included within the spirit and purview of this application and scope of the appended claims.
Claims
1. A compound having the structure of Formula (I), or pharmaceutically acceptable salt, pharmaceutically acceptable solvate, prodrug thereof:
Formula (I) wherein
R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -C(=O)N(R9)2, -C(O)NH-OH, -
C(=O)NH-CN, -P(O)(OH)2, -P(=O)(ORD)2, -OPO3H2, -SO2NHC(=O)R10, -CN, - C(=NH)-NH2, -C(=NH)-NHC(=O)RD, -C(O)NHCH2CH2SO3H, tetrazolyl, 5-oxo-
2,5-dihydro-[l,2,4]oxadiazol-3-yl, or carboxylic acid bioisostere; RD is H or C1-
C6alkyl; L1 is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted - alkylene-cycloalkylene, or a substituted or unsubstituted -cycloalkylene-alkylene-; ring A is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring A is substituted then ring A is substituted with 1, 2, 3, or 4 RA; ring B is a substituted or unsubstituted cycloalkylene, a substituted or unsubstituted heterocycloalkylene, a substituted or unsubstituted arylene, or a substituted or unsubstituted heteroarylene, where if ring B is substituted then ring B is substituted with 1, 2, 3, or 4 RB; L2 is absent, a substituted or unsubstituted alkylene, a substituted or unsubstituted fluoroalkylene, a substituted or unsubstituted heteroalkylene, -0-, -S-, -SO-, -SO2-, - NR2-, or -C(O)-;
R2 is H, Ci-C4alkyl, or Ci-C4fluoroalkyl; R3 is H, Ci-C4alkyl, C3-C6cycloalkyl, Ci-C4fluoroalkyl or a substituted or unsubstituted phenyl;
R4 is -NR7C(O)0C(R8)(R8a)-CY, or -NR7C(O)O-CY; R7 is H or CrC4alkyl;
R8 is H, Ci-C4alkyl, C3-C7cycloalkyl or Ci-C4fluoroalkyl;
R8a is H or Ci-C4alkyl; CY is a substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, a substituted or unsubstituted heterocycloalkyl, a substituted or unsubstituted aryl, a substituted or unsubstituted benzyl, or a substituted or unsubstituted heteroaryl, wherein if CY is substituted then CY is substituted with 1, 2,
3, or 4 Rc; or
R8 and CY are taken together with the carbon atom to which they are attached to form a substituted or unsubstituted carbocycle or a substituted or unsubstituted heterocycle; each of RA, RB, and Rc are independently selected from halogen, -CN, -NO2, -OH, - OR10, -SR10, -S(=O)R10, -S(=O)2R10, -N(R9)S(=O)2R10, -S(=O)2N(R9)2, -C(=O)R10, -
OC(=O)R10, -CO2R9, -OCO2R10, -N(R9)2, -C(=O)N(R9)2, -OC(=O)N(R9)2, - NR9C(=O)N(R9)2, -NR9C(=O)R10, -NR9C(O)OR10, d-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl; each R9 is independently selected from H, Ci-C6alkyl, Ci-C6heteroalkyl, C1- Cefluoroalkyl, a substituted or unsubstituted C3-Ciocycloalkyl, a substituted or unsubstituted C2-Cioheterocycloalkyl, a substituted or unsubstituted aryl, a substituted or unsubstituted heteroaryl, a substituted or unsubstituted -C1- C4alkylene-C3-Ciocycloalkyl, a substituted or unsubstituted -Ci-C4alkylene-C2- Cioheterocycloalkyl, a substituted or unsubstituted -Ci-C4alkylene-aryl, or a substituted or unsubstituted -Ci-C4alkylene-heteroaryl; or two R9 groups attached to the same N atom are taken together with the N atom to which they are attached to form a substituted or unsubstituted heterocycle; R10 is selected from Ci-C6alkyl, Ci-C6heteroalkyl, Ci-C6fluoroalkyl, a substituted or unsubstituted C3-Ciocycloalkyl, a substituted or unsubstituted C2- Cioheterocycloalkyl, a substituted or unsubstituted aryl, a substituted or unsubstituted heteroaryl, a substituted or unsubstituted -Ci-C4alkylene-C3- Ciocycloalkyl, a substituted or unsubstituted -Ci-C4alkylene-C2- Cioheterocycloalkyl, a substituted or unsubstituted -Ci-C4alkylene-aryl, and a substituted or unsubstituted -Ci-C4alkylene-heteroaryl. The compound of claim 1, wherein:
R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -C(=O)N(R9)2, -SO2NHC(=O)R10, -CN, -
CC=O)NHCH2CH2SO3H, tetrazolyl or 5-oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl; L1 is -C3-C6cycloalkylene-, -Ci-Qalkylene-Cs-Cecycloalkylene-, or -C3- C6cycloalkylene-Ci-C4alkylene-; L2 is absent, Ci-Cβalkylene, Ci-Cefluoroalkylene, -Ci-Cβalkylene-O-, -Ci-C3alkylene-O- Ci-C3alkylene -, -O-Ci-C6alkylene -, -Ci-Cgalkylene-S-, -Ci-C3alkylene-S-Ci- C3alkylene -, -S-Ci-C6alkylene -, -Ci-Cgalkylene-SO-, -Ci-Csalkylene-SO-Ci- C3alkylene -, -SO-Ci-C6alkylene -, -Ci-C6alkylene-SO2-, -Ci-C3alkylene-SO2-Ci- C3alkylene -, -SO2-CrC6alkylene -, -C1-C4alkylene-NR11-, -C1-C2alkylene-NR11-C1-
C2alkylene -, - NRπ-Ci-C4alkylene -, -O-, -S-, -SO-, -SO2- or -NR2-; R11 is H or Ci-C2alkyl; ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene, where if ring A is substituted then ring A is substituted
with 1 or 2 RA, where the groups on non-adjacent ring atoms of ring A; ring B is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene, where if ring B is substituted then ring B is substituted with 1 or 2 RB; R3 is H or CrC4alkyl;
R7 is H; R8a is H; CY is Ci-Cβalkyl, substituted or unsubstituted C3-Cecycloalkyl, substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 1-3 N atoms, wherein if CY is substituted then CY is substituted with 1,
2, or 3 Rc; each of RA, RB, and Rc are independently selected from halogen, -CN, -NO2, -OH, - OR10, -N(R9)2, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl. The compound of claim 2, wherein:
L1 is cyclopropyl-l,l-diyl, cyclopropyl-l,2-diyl, cycloprop-2-enyl-l,l-diyl, cyclobutyl- 1,1-diyl, cyclopentyl-l,l-diyl, cyclohexyl- 1 , 1 -diyl, -CH2C(CH2CH2)- or - C(CH2CH2)CH2-;
L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -CH2NH-, -NHCH2-, -0-, -S-, or -NH-; ring A is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing O or 1 O atoms, O or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring A is substituted then ring A is substituted with 1 or 2 RA; ring B is a substituted or unsubstituted phenylene, or a substituted or unsubstituted monocyclic heteroarylene containing O or 1 O atoms, O or 1 S atoms, 0-3 N atoms, and at least 2 carbon atoms in the ring, where if ring B is substituted then ring B is substituted with 1 or 2 RB.
4. The compound of claim 3, wherein the compound of Formula (I) has the structure of Formula (II):
5. The compound of claim 4, wherein: each ofA1, A2, and A3 is CH or CRA; or one of A1, A2, and A3 is N; each RA is independently selected from halogen, -OH, Ci-C4alkyl, Ci-C4fluoroalkyl, C1- C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl.
6. The compound of claim 3, wherein: ring A is a substituted or unsubstituted phen-l,4-ylene, where if ring A is substituted then ring A is substituted with RA; ring B is a substituted or unsubstituted phenyl- 1, 3 -ene or a substituted or unsubstituted phenyl- 1 ,4-ene, where if ring B is substituted then ring B is substituted with RB;
7. The compound of any one of claims 1-6, wherein:
R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, or tetrazolyl; RD is H, -CH3, or -CH2CH3;
R4 is -NR7C(=O)OCH(R8)-CY;
R7 is H;
R8 is H, Ci-C4alkyl or Ci-C4fluoroalkyl;
R10 is a Ci-C6alkyl, Ci-C6fluoroalkyl, C3-C6cycloalkyl, or a substituted or unsubstituted phenyl.
8. The compound of claim 7, wherein: R1 is -CO2H; L1 is cyclopropyl-l,l-diyl, cyclopropyl-l,2-diyl, cyclobutyl-l,l-diyl, cyclopentyl-1,1- diyl, or cyclohexyl-l,l-diyl; L2 is absent; ring B is a substituted or unsubstituted phenyl- 1 ,4-ene, where if ring B is substituted then ring B is substituted with RB;
R3 is H, -CH3 or -CH2CH3; R8 is H, -CH3 or -CF3; each RAis independently selected from halogen, -OH, -CH3, -CF3, -OCF3, and -OCH3; each RB is independently selected from halogen, -OH, -CH3, -CF3, -OCF3, and -OCH3; each Rc is independently selected from halogen -OH, -CH3, -CF3, -OCF3, and -OCH3.
9. The compound of claim 8, wherein: L1 is cyclopropyl-l,l-diyl
R3 is -CH3; R8 is -CH3; CY is substituted or unsubstituted phenyl, wherein if CY is substituted then CY is monosubstituted with Rc.
10. The compound of claim 1, wherein:
R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10, -CN, tetrazolyl, or 5-oxo-2,5-dihydro-
[l,2,4]oxadiazol-3-yl; L1 is -C3-C6cycloalkylene-;
L2 is absent; ring A is a substituted or unsubstituted phen-l,4-ylene, where if ring A is substituted then ring A is substituted with RA; ring B is a substituted or unsubstituted phenyl- 1 ,4-ene, where if ring B is substituted then ring B is substituted with RB;
R3 is H or Ci-C4alkyl; R4 is -NR7C(=O)OCH(R8)-CY; R7 is H;
R8 is H or Ci-C4alkyl, CY is substituted or unsubstituted C3-Cecycloalkyl, substituted or unsubstituted phenyl, or a substituted or unsubstituted monocyclic heteroaryl containing 1-3 N atoms, wherein if CY is substituted then CY is substituted with Rc; each of RA, RB, and Rc are independently selected from halogen, -OH, Ci-C4alkyl, Ci-
C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl.
11. The compound of claim 10, wherein: L1 is cyclopropyl-l,l-diyl; R3 is H or -CH3;
O H R8
R4 is H .
R8 is H or -CH3; CY is substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with Rc.
12. The compound of claim 11 , wherein: R1 is -CO2H;
R3 is -CH3; R8 is -CH3;
CY is substituted or unsubstituted phenyl, where CY is unsubstituted or monosubstituted with Rc.
13. The compound of claim 1 , wherein the compound of Formula (I) has the following structure:
A1 is CH, CRA or N; RA is F, Cl, Br, I, -CH3, or -CF3; L2 is absent, -CH2-, -CH2O-, -OCH2-, -CH2S-, -SCH2-, -0-, or -S-; R8 is H or -CH3;
CY is Ci-Cβalkyl, substituted or unsubstituted C3-Cecycloalkyl, or substituted or unsubstituted phenyl; wherein if CY is substituted then CY is substituted with 1 or 2 Rc; Rc is F, Cl, Br, I, -OH, Ci-C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, or C1- C4alkoxy; n is i, 2, or 3.
14. The compound of claim 13, wherein: A1 is CH; L2 is absent; CY is -CH3, -CH2CH3, -CH2CH2CH3, -CH2CH2CH2CH3, -CH(CH3)2, -CH(CH2CH3)2, - CH2CH(CH3)2, -C(CHs)3, -CH2(CH3)3, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, a substituted or unsubstituted pentenyl, a substituted or unsubstituted cyclohexenyl, or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with Rc; Rc is F, Cl, -OH, -CH3, or CF3; n is 1.
15. The compound of claim 1, wherein the compound of Formula (I) has the structure of Formula (V) or a pharmaceutically acceptable salt thereof: n Formula (V) wherein R1 is -CO2H, -CO2RD, -CN, -C(=O)N(R9)2, -C(=O)NHCH2CH2SO3H, -
C(=O)NHSO2R10, tetrazolyl, or 5-oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl; RD is H or Ci-C4alkyl; R3 is H, Ci-C4alkyl, C3-C6cycloalkyl, or Ci-C4fluoroalkyl;
R4 is -NR7C(=O)OCH(R8)-CY; R7 is H or Ci-C4alkyl; R8 is H, Ci-C4alkyl, or Ci-C4fluoroalkyl;
CY is a substituted or unsubstituted C3-C6cycloalkyl or a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with 1 or 2 Rc; R9 is H, Ci-C6alkyl, Ci-C6fluoroalkyl, C3-C6cycloalkyl, or a substituted or unsubstituted phenyl;
R10 is a Ci-Cβalkyl, Ci-Cefluoroalkyl, C3-Cecycloalkyl, or a substituted or unsubstituted phenyl; each of RA, RB, and Rc are independently selected from F, Cl, Br, I, -CN, -OH, C1-
C4alkyl, Ci-C4fluoroalkyl, Ci-C4fluoroalkoxy, Ci-C4alkoxy, and Ci-C4heteroalkyl; m is O, 1, or 2; n is 1, 2, 3 or 4; p is 0, 1, or 2.
16. The compound of claim 15, wherein:
R1 is -CO2H, -CO2RD, -C(=O)NHSO2R10 or tetrazolyl; R3 is H or Ci-C4alkyl; R7 is H;
R8 is H, -CH3 or -CF3;
R10 is a Ci-Cβalkyl or a substituted or unsubstituted phenyl; each RA is independently selected from F, Cl, Br, I, -OH, -CH3, -CF3, -OCF3, and -
OCH3; each RB is independently selected from F, Cl, Br, I, -OH, -CH3, -CF3, -OCF3, and - OCH3; each Rcis independently selected from F, Cl, Br, I, -OH, -CH3, -CF3, -OCF3, and - OCH3; m is O or 1 ; n is 1, 2, or 3; p is O or 1.
17. The compound of claim 16, wherein: R1 is -CO2H or -CO2RD; RD is H, -CH3, or -CH2CH3;
R3 is H, -CH3 or -CH2CH3;
R4 is -NHC(=0)0CH(R8)-CY;
R8 is H, or -CH3;
CY is a substituted or unsubstituted phenyl, wherein if CY is substituted then CY is substituted with 1 or 2 Rc.
18. The compound of claim 17, wherein the compound of Formula (V) has the following structure:
19. The compound of claim 18, wherein: R1 is -C(=O)NHSO2R10;
R3 is H, -CH3 or -CH2CH3;
R8 is H, or -CH3;
R10 is -CH3, or -CH2CH3.
20. The compound of any one of claims 15-19, wherein: R4 is -NHC(=O)OCH(CH3)-(substituted or unsubstituted phenyl); wherein if the phenyl is substituted then the phenyl is substituted with Rc; Rc is F, Cl, -CH3, or CF3; n is 1.
CY is a substituted or unsubstituted phenyl, wherein if CY is substituted phenyl then the phenyl is substituted with 1 or 2 Rc;
Rc is H, F, Cl, -OH, -CH3, -CF3, or -OCH3; n is 1.
22. The compound of claim 21 , wherein:
R8 is -CH3;
CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5- difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2- bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3-hydroxyphenyl,
4-hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2- trifluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 2-fluoro-4- methoxyphenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-cyanophenyl, 3- cyanophenyl, or 4-cyanophenyl.
23. The compound of any one of claims 15-19, wherein the compound of Formula (V) has the following structure:
24. The compound of 23, wherein: R1 is -CO2H; CY is phenyl, 2-fluorophenyl, 2,3-difluorophenyl, 2,4-difluorophenyl, 2,5- difluorophenyl, 2,6-difluorophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, 2- bromophenyl, 3-bromophenyl, 2,4-dichlorophenyl, 2-hydroxyphenyl, 3- hydroxyphenyl, 4-hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4- methoxyphenyl, 2-trifluoromethylphenyl, 3-trifluoromethylphenyl, 4- trifluoromethylphenyl, 2-fluoro-4-methoxyphenyl, 2-methylphenyl, 3-methylphenyl,
4-methylphenyl, 2-cyanophenyl, 3-cyanophenyl, or 4-cyanophenyl.
25. The compound of claim 1 selected from: l-{4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-1); l-[4'-(4-Isopropoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-2); l-{4'-[4-(l-Cyclohexyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-3); l-{4'-[3-Methyl-4-((R)-l-o-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-4); l-[4'-(4-Benzyloxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-5); l-[4'-(4-Ethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-6); l-[4'-(4-Methoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-7); l-{4'-[3-Methyl-4-(l -methyl- l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-
4-yl} -cyclopropanecarboxylic acid (Compound 1-8); l-{4'-[4-(l-Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl} -cyclopropanecarboxylic acid (Compound 1-9);
1- (4'-[4-((R)-I -Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-
4-yl} -cyclopropanecarboxylic acid (Compound 1-10); l-[4'-(4-Cyclopropylmethoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-11);
1 -(4'- (4-[(R)- 1 -(2-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-12); l-[4'-(4-?ert-Butoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 1-13); 1 -(4'- (3-Methyl-4-[(R)- 1 -(2-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-14); l-(4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclobutanecarboxylic acid (Compound 1-15); l-(4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopentanecarboxylic acid (Compound 1-16); l-[4'-(4-?ert-Butoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclobutanecarboxylic acid (Compound 1-17);
1 -(4'- (4-[ 1 -(2-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-18); 1 -(4'- {3-Methyl-4-[ 1 -(4-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-19);
1 -(4'- {3-Methyl-4-[ 1 -(4-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-20); l-{4'-[3-Methyl-4-(l-pyrazin-2-yl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid (Compound 1-21);
1 -(4'- {4-[ 1 -(3-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} -biphenyl-
4-yl)-cyclopropanecarboxylic acid (Compound 1-22); l-[4-(4- (4-[(R)-I -(2-Chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} - phenoxy)-phenyl]-cyclopropanecarboxylic acid (Compound 1-23); l-(4-{4-[3-Methyl-4-(l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-phenoxy}- phenyl)-cyclopropanecarboxylic acid (Compound 1-24); l-{4'-[3-Methyl-4-((R)-l-/»-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-25); l-{4'-[3-Methyl-4-((R)-l-m-tolyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-26);
1 -(4- (4-[3-Methyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-phenoxy} - phenyl)-cyclopropanecarboxylic acid (Compound 1-27);
1 -(4'- (4-[(R)- 1 -(4-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-28);
1 -(4'- (4-[(R)- 1 -(2-Cyano-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-29); l-(4'-[3-Methyl-4-((R)-l,2,2-trimethyl-propoxycarbonylamino)-isoxazol-5-yl]- biphenyl-4-yl}-cyclopropanecarboxylic acid (Compound 1-30); 1 - (4'-[4-((R)- 1 -Cyclobutyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid (Compound 1-31);
1 - (4'-[4-((R)- 1 ,2-Dimethyl-propoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-
4-yl}-cyclopropanecarboxylic acid (Compound 1-32); l-(4'-[4-(l-Ethyl-propoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-33);
1 -(4'- (4-[ 1 -(2-Chloro-cyclohex- 1 -enyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-34);
1 -(4'- (3-Methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-35); 1 -(4'- (4-[(R)- 1 -(S-Methoxy-pheny^-ethoxycarbonylaminoJ-S-methyl-isoxazol-S-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-36);
1 -(4'- (4-[(R)- 1 -(4-Methoxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-37); 1- (4'-[4-((R)-I -Cyclopropyl-ethoxycarbonylamino)-3-methyl-isoxazol-5-yl]-biphenyl-
4-yl}-cycloprop-2-enecarboxylic acid (Compound 1-38);
2-(4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-39);
1 -(4- (5-[3-Methyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-pyridin-2-yl} - phenyl)-cyclopropanecarboxylic acid (Compound 1-40); l-(4'-(4-[l-(3-Bromo-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-41); l-(4'-(4-[l-(3-Cmoro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-42); 1 - (4'-[3-Methyl-4-((S)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl} - cyclopropanecarboxylic acid (Compound 1-43);
1 -(4'- (4-[ 1 -(3-Hydroxy-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl} - biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-44);
[ 1 -(4'- (3-Methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol- 5-yl}-biphenyl-4-yl)-cyclopropyl]-acetic acid (Compound 1-45); l-(4'-[3-Ethyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-46);
1 - (4'-[3-Phenyl-4-((R)- 1 -phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl} - cyclopropanecarboxylic acid (Compound 1-47); l-(4'-[3-Methyl-4-((R)-l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- ylmethyl} -cyclopropanecarboxylic acid (Compound 1-48);
1 -(4'- (3-Methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5- yl}-biphenyl-4-ylmethyl)-cyclopropanecarboxylic acid (Compound 1-49);
1 -(4'- (3-Ethyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-50);
1 -(4'- (3-Phenyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]-isoxazol-5- yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-51);
1 -(4'- (3 -Methyl-4-[l -methyl- 1 -(3-trifluoromethyl-phenyl)-ethoxycarbonylamino]- isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-53); 1 -(3'-Methoxy-4!- (3-methyl-4-[(R)- 1 -(3-trifluoromethyl-phenyl)- ethoxycarbonylamino]-isoxazol-5-yl}-biphenyl-4-yl)-cyclopropanecarboxylic acid
(Compound 1-54); l-(4'-{4-[(R)-l-(3,5-Dibromo-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}- biphenyl-4-yl)-cyclopropanecarboxylic acid (Compound 1-55); l-{4'-[4-((R)-l-Phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-56); l-{4'-[3-Methyl-4-(l-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- cyclopropanecarboxylic acid (Compound 1-57); {5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl- isoxazol-4-yl}-carbamic acid (R)- 1 -phenyl-ethyl ester (Compound 2-1);
{5-[4'-(l-Benzenesulfonylaminocarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl- isoxazol-4-yl}-carbamic acid (R)- 1 -phenyl-ethyl ester (Compound 2-2);
{5-[4'-(l-Cyano-cyclopropyl)-biphenyl-4-yl]-3-methyl-isoxazol-4-yl}-carbamic acid (R)-I -phenyl-ethyl ester (Compound 2-3);
(3-Methyl-5-{4'-[l-(5-oxo-2,5-dihydro-[l,2,4]oxadiazol-3-yl)-cyclopropyl]-biphenyl-4- yl}-isoxazol-4-yl)-carbamic acid (R)- 1 -phenyl-ethyl ester (Compound 2-4);
(3-Methyl-5-{4'-[l-(lH-tetrazol-5-yl)-cyclopropyl]-biphenyl-4-yl}-isoxazol-4-yl)- carbamic acid (R)- 1 -phenyl-ethyl ester (Compound 2-5); {5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-biphenyl-4-yl]-3-methyl- isoxazol-4-yl}-carbamic acid (R)-l-(3-trifluoromethyl-phenyl)-ethyl ester (Compound
2-6);
{5-[4'-(l-Methanesulfonylaminocarbonyl-cyclopropyl)-3-methoxy-biphenyl-4-yl]-3- methyl-isoxazol-4-yl}-carbamic acid (R)-l-(3-trifluoromethyl-phenyl)-ethyl ester (Compound 2-7); l-{4'-[3-Methyl-4-(l-methyl-cyclopentyloxycarbonylamino)-isoxazol-5-yl]-biphenyl-4- yl}-cyclopropanecarboxylic acid (Compound 3-1); and l-[4'-(4-Cyclobutoxycarbonylamino-3-methyl-isoxazol-5-yl)-biphenyl-4-yl]- cyclopropanecarboxylic acid (Compound 3-2); or a pharmaceutically acceptable salt thereof.
26. A pharmaceutical composition comprising a therapeutically effective amount of a compound of any one of claims 1-25, or a pharmaceutically acceptable salt thereof.
27. The pharmaceutical composition of claim 26, wherein: the pharmaceutical composition is formulated for intravenous injection, subcutaneous injection, oral administration, inhalation, nasal administration, topical administration, ophthalmic administration or otic administration; or the pharmaceutical composition is a tablet, a pill, a capsule, a liquid, an inhalant, a nasal spray solution, a suppository, a suspension, a gel, a colloid, a dispersion, a suspension, a solution, an emulsion, an ointment, a lotion, an eye drop or an ear drop.
28. A method of inhibiting the physiological activity of LPA in a mammal comprising administering a therapeutically effective amount of a compound according to any one of claims 1-25 or a pharmaceutically acceptable salt thereof to the mammal in need thereof.
29. A method for treating or preventing a LPA-dependent or LPA-mediated disease or condition in a mammal comprising administering a therapuetically effective amount of a compound according to any one of claims 1 -25 or a pharmaceutically acceptable salt thereof to the mammal in need thereof.
30. The method of claim 29, wherein the LPA-dependent or LPA-mediated disease or condition is selected from lung fibrosis, asthma, chronic obstructive pulmonary disease (COPD), renal fibrosis, acute kidney injury, chronic kidney disease, liver fibrosis, skin fibrosis, fibrosis of the gut, breast cancer, pancreatic cancer, ovarian cancer, prostate cancer, glioblastoma, bone cancer, colon cancer, bowel cancer, head and neck cancer, melanoma, multiple myeloma, chronic lymphocytic leukemia, cancer pain, tumor metastasis, transplant organ rejection, scleroderma, ocular fibrosis, age related macular degeneration (AMD), diabetic retinopathy, collagen vascular disease, atherosclerosis, Raynaud's phenomenom, or neuropathic pain.
31. A method of controlling the activation of LPA receptors in a tissue in a mammal comprising administering a compound of any one of claims 1-25 to mammal.
32. The method of claim 31 , wherein the activation of LPA receptors in a tissue in a mammal results in fibrosis.
33. A method for the treatment or prevention of fibrosis in a mammal comprising administering a therapeutically effective amount of a compound of any one of claims 1 - 25 to the mammal in need thereof.
34. The method of claim 33, wherein the fibrosis comprises lung fibrosis, renal fibrosis, hepatic fibrosis or cutaneous fibrosis.
35. A method of improving lung function in a mammal comprising administering a therapeutically effective amount of a compound of any one of claims 1-25 to the mammal in need thereof.
36. The method of claim 35, wherein the mammal has been diagnosed as having lung fibrosis.
37. A method of treating idopathic pulmonary fibrosis in a mammal comprising administering a therapeutically effective amount of a compound of any one of claims 1 - 25 to the mammal in need thereof.
38. A method of controlling an abnormal accumulation or activation of cells, fibronectin, collagen or increased fibroblast recruitment in a tissue of a mammal comprising administering a compound of any one of claims 1-25 to mammal.
39. The method of claim 38, wherein the abnormal accumulation or activation of cells, fibronectin, collagen or increased fibroblast recruitment in the tissue results in fibrosis.
40. The method of any one of claims 28-39 further comprising administereing to the mammal one or more additional therapeutically active agents selected from: corticosteroids, immunosuppresant, analgesics, anti-cancer agent, antiinflammatories, chemokine receptor antagonists, bronchodilators, leukotriene receptor antagonists, leukotriene formation inhibitors, monoacylglycerol kinase inhibitors, phospho lipase Ai inhibitors, phospholipase A2 inhibitors, and lysophospholipase D (lysoPLD) inhibitors, autotaxin inhibitors, decongestants, antihistamines, mucolytics, anticholinergics, antitussives, expectorants, and β-2 agonists.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US18378509P | 2009-06-03 | 2009-06-03 | |
| US61/183,785 | 2009-06-03 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2010141768A2 true WO2010141768A2 (en) | 2010-12-09 |
| WO2010141768A3 WO2010141768A3 (en) | 2011-03-31 |
Family
ID=42471045
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2010/037316 Ceased WO2010141768A2 (en) | 2009-06-03 | 2010-06-03 | Polycyclic antagonists of lysophosphatidic acid receptors |
| PCT/US2010/037309 Ceased WO2010141761A2 (en) | 2009-06-03 | 2010-06-03 | Polycyclic antagonists of lysophosphatidic acid receptors |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2010/037309 Ceased WO2010141761A2 (en) | 2009-06-03 | 2010-06-03 | Polycyclic antagonists of lysophosphatidic acid receptors |
Country Status (28)
| Country | Link |
|---|---|
| US (2) | US8058300B2 (en) |
| EP (1) | EP2438048B1 (en) |
| JP (1) | JP5599457B2 (en) |
| KR (2) | KR101628706B1 (en) |
| CN (2) | CN102459204B (en) |
| AU (2) | AU2010256501B2 (en) |
| BR (1) | BRPI1014582A2 (en) |
| CA (1) | CA2764445C (en) |
| CL (1) | CL2011003072A1 (en) |
| CO (1) | CO6480918A2 (en) |
| DK (1) | DK2438048T3 (en) |
| EA (1) | EA020139B1 (en) |
| ES (1) | ES2445714T3 (en) |
| GB (1) | GB2470833B (en) |
| HR (1) | HRP20140036T1 (en) |
| IL (2) | IL216220A (en) |
| MX (1) | MX2011012839A (en) |
| MY (1) | MY163055A (en) |
| NZ (1) | NZ596826A (en) |
| PE (1) | PE20120936A1 (en) |
| PL (1) | PL2438048T3 (en) |
| PT (1) | PT2438048E (en) |
| SG (1) | SG176112A1 (en) |
| SI (1) | SI2438048T1 (en) |
| SM (1) | SMT201400031B (en) |
| TW (1) | TWI405757B (en) |
| WO (2) | WO2010141768A2 (en) |
| ZA (1) | ZA201108421B (en) |
Cited By (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8048902B2 (en) | 2008-12-15 | 2011-11-01 | Amira Pharmaceuticals, Inc. | Antagonists of lysophosphatidic acid receptors |
| US8058300B2 (en) | 2009-06-03 | 2011-11-15 | Amira Pharmaceuticals, Inc. | Polycyclic antagonists of lysophosphatidic acid receptors |
| US8217066B2 (en) | 2009-10-01 | 2012-07-10 | Amira Pharmaceuticals, Inc. | Compounds as lysophosphatidic acid receptor antagonists |
| WO2013070879A1 (en) | 2011-11-10 | 2013-05-16 | Bristol-Myers Squibb Company | Methods for treating spinal cord injury with lpa receptor antagonists |
| WO2013078283A1 (en) | 2011-11-22 | 2013-05-30 | Intermune, Inc. | Methods of diagnosing and treating idiopathic pulmonary fibrosis |
| US8455499B2 (en) | 2008-12-11 | 2013-06-04 | Amira Pharmaceuticals, Inc. | Alkyne antagonists of lysophosphatidic acid receptors |
| US8541587B2 (en) | 2011-04-05 | 2013-09-24 | Amira Pharmaceuticals, Inc. | Lysophosphatidic acid receptor antagonists |
| US8592402B2 (en) | 2009-08-04 | 2013-11-26 | Amira Pharmaceuticals, Inc. | Compounds as lysophosphatidic acid receptor antagonists |
| US8664220B2 (en) | 2009-10-01 | 2014-03-04 | Amira Pharmaceuticals, Inc. | Polycyclic compounds as lysophosphatidic acid receptor antagonists |
| WO2014085373A1 (en) * | 2012-11-27 | 2014-06-05 | Glaxosmithkline Llc | Combination |
| WO2014104372A1 (en) | 2012-12-28 | 2014-07-03 | 宇部興産株式会社 | Halogen-substituted heterocyclic compound |
| EP2744807A4 (en) * | 2011-08-15 | 2015-03-04 | Intermune Inc | Lysophosphatidic acid receptor antagonists |
| WO2016197009A1 (en) | 2015-06-05 | 2016-12-08 | Vertex Pharmaceuticals Incorporated | Triazoles for the treatment of demyelinating diseases |
| KR20170016988A (en) | 2014-06-27 | 2017-02-14 | 우베 고산 가부시키가이샤 | Salt of halogen-substituted heterocyclic compound |
| WO2018106646A1 (en) | 2016-12-06 | 2018-06-14 | Vertex Pharmaceuticals Incorporated | Aminotriazoles for the treatment of demyelinating diseases |
| WO2018106643A1 (en) | 2016-12-06 | 2018-06-14 | Vertex Pharmaceuticals Incorporated | Heterocyclic azoles for the treatment of demyelinating diseases |
| WO2018106641A1 (en) | 2016-12-06 | 2018-06-14 | Vertex Pharmaceuticals Incorporated | Pyrazoles for the treatment of demyelinating diseases |
| US10000459B2 (en) | 2013-03-15 | 2018-06-19 | Epigen Biosciences, Inc. | Heterocyclic compounds useful in the treatment of disease |
| WO2020257138A1 (en) * | 2019-06-18 | 2020-12-24 | Bristol-Myers Squibb Company | Isoxazole carboxylic acids as lpa antagonists |
| US11548871B2 (en) | 2019-11-15 | 2023-01-10 | Gilead Sciences, Inc. | Triazole carbamate pyridyl sulfonamides as LPA receptor antagonists and uses thereof |
| US11584738B2 (en) | 2020-06-03 | 2023-02-21 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
| US11702407B2 (en) | 2020-06-03 | 2023-07-18 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
| US11939318B2 (en) | 2021-12-08 | 2024-03-26 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
| US11980609B2 (en) | 2021-05-11 | 2024-05-14 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
| US12435057B2 (en) | 2019-06-18 | 2025-10-07 | Bristol-Myers Squibb Company | Triazole carboxylic acids as LPA antagonists |
| US12503454B2 (en) | 2021-05-13 | 2025-12-23 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5708936B2 (en) * | 2009-09-25 | 2015-04-30 | アステラス製薬株式会社 | Substituted amide compounds |
| EP2509802B1 (en) * | 2009-12-11 | 2016-05-04 | Pirelli Tyre S.p.A. | Tire for a heavy duty vehicle |
| CN107721940A (en) * | 2010-12-07 | 2018-02-23 | 阿米拉制药公司 | Polycyclic LPA1Antagonist and its use |
| BR112014031108A2 (en) * | 2012-06-20 | 2017-06-27 | Hoffmann La Roche | substituted pyrazole compounds as lpar antagonists |
| MA37764A1 (en) * | 2012-06-20 | 2016-01-29 | Hoffmann La Roche | N-alkyltriazole compounds used as antagonists of lpar |
| TW201437200A (en) * | 2013-01-15 | 2014-10-01 | Intermune Inc | Lysophosphatidic acid receptor antagonists |
| JP7467361B2 (en) * | 2018-06-18 | 2024-04-15 | エピゲン バイオサイエンシズ,インコーポレイテッド | Heterocyclic compounds useful in the treatment of diseases |
| EP3062827A1 (en) * | 2013-10-31 | 2016-09-07 | Bristol-Myers Squibb Company | Radioligands for imaging the lpa-1 receptor |
| CA3059273A1 (en) * | 2017-04-10 | 2018-10-18 | Bayer Aktiengesellschaft | Substituted n-arylethyl-2-arylquinoline-4-carboxamides and use thereof |
| TWI770157B (en) * | 2017-04-10 | 2022-07-11 | 德商拜耳廠股份有限公司 | Substituted n-arylethyl-2-aminoquinoline-4-carboxamides and use thereof |
| CA3106692A1 (en) * | 2017-07-19 | 2019-01-24 | Aazeintx Inc. | Method to abate acute airway hypersensitivity and asthma attacks |
| CA3082108A1 (en) | 2017-11-14 | 2019-05-23 | Merck Sharp & Dohme Corp. | Novel substituted biaryl compounds as indoleamine 2,3-dioxygenase (ido) inhibitors |
| EP3709986B1 (en) | 2017-11-14 | 2023-11-01 | Merck Sharp & Dohme LLC | Novel substituted biaryl compounds as indoleamine 2,3-dioxygenase (ido) inhibitors |
| US11447475B2 (en) | 2017-12-19 | 2022-09-20 | Bristol-Myers Squibb Company | Isoxazole N-linked carbamoyl cyclohexyl acids as LPA antagonists |
| KR20210061383A (en) | 2018-09-18 | 2021-05-27 | 브리스톨-마이어스 스큅 컴퍼니 | Cyclopentyl acid as an LPA antagonist |
| WO2020257139A1 (en) * | 2019-06-18 | 2020-12-24 | Bristol-Myers Squibb Company | Cyclobutyl carboxylic acids as lpa antagonists |
| KR20220039718A (en) | 2019-07-30 | 2022-03-29 | 다이쇼 세이야꾸 가부시끼가이샤 | Urea compounds that antagonize the LPA1 receptor |
| IL293474A (en) | 2019-12-04 | 2022-08-01 | Idorsia Pharmaceuticals Ltd | Combination of an azetidine lpa1 receptor antagonist with pirfenidone and/or nintedanib for use in the treatment of fibrotic diseases |
| KR102393079B1 (en) | 2020-02-28 | 2022-04-29 | 한국화학연구원 | Composition for preventing or treating respiratory disease of domestic animals |
| WO2022006470A1 (en) | 2020-07-01 | 2022-01-06 | Vanderbilt University | Methods of treatment for a kidney disease |
| WO2022100625A1 (en) * | 2020-11-10 | 2022-05-19 | 武汉人福创新药物研发中心有限公司 | Nitrogen-substituted aminocarbonate thiophene compound and use thereof |
| TW202432132A (en) | 2022-12-23 | 2024-08-16 | 美商必治妥美雅史谷比公司 | Lpa1 antagonists for treating interstitial lung disease |
| WO2024249867A1 (en) * | 2023-06-02 | 2024-12-05 | Am Sciences | Compounds for treating fibrotic diseases |
Family Cites Families (58)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5612359A (en) | 1994-08-26 | 1997-03-18 | Bristol-Myers Squibb Company | Substituted biphenyl isoxazole sulfonamides |
| ATE236890T1 (en) | 1996-12-23 | 2003-04-15 | Bristol Myers Squibb Pharma Co | OXYGEN OR SULFUR CONTAINING 5-MEMBED HETEROAROMATIC DERIVATIVES AS FACTOR XA INHIBITORS |
| JP3237608B2 (en) | 1997-04-21 | 2001-12-10 | 住友製薬株式会社 | Isoxazole derivative |
| EP1175419B1 (en) | 1999-04-02 | 2003-05-28 | Bristol-Myers Squibb Pharma Company | Aryl sulfonyls as factor xa inhibitors |
| WO2001060819A1 (en) | 2000-02-18 | 2001-08-23 | Kirin Beer Kabushiki Kaisha | Novel isoxazole and thiazole compounds and use thereof as drugs |
| AU2001273040A1 (en) | 2000-06-27 | 2002-01-08 | Du Pont Pharmaceuticals Company | Factor xa inhibitors |
| DE60234318D1 (en) | 2001-02-08 | 2009-12-24 | Ono Pharmaceutical Co | FOR THE TREATMENT OF RESPIRATORY DISEASES, COMPRISING MEANS FOR THE CONTROL OF THE LPA RECEPTOR |
| US7521192B2 (en) | 2001-04-18 | 2009-04-21 | Rigel Pharmaceuticals, Inc. | EDG: modulators of lymphocyte activation and migration |
| JP2004526803A (en) * | 2001-04-25 | 2004-09-02 | バイオサイナーゲン、インコーポレーテッド | Pharmaceutical composition comprising lysophosphatidic acid (APHARAMEUTICALCOMPOSITIONCOMPLYSINGLYSOPHOSPHATIDICACID) |
| WO2003007991A1 (en) | 2001-07-17 | 2003-01-30 | Ono Pharmaceutical Co., Ltd. | Pancreatic juice secretion regulators comprising lpa receptor controller |
| MY151199A (en) | 2001-11-02 | 2014-04-30 | Rigel Pharmaceuticals Inc | Substituted diphenyl heterocycles useful for treating hcv infection |
| EP1466602A4 (en) | 2001-12-25 | 2006-03-01 | Ajinomoto Kk | Organ fibrosis inhibitors |
| US20050101518A1 (en) | 2002-01-18 | 2005-05-12 | David Solow-Cordero | Methods of treating conditions associated with an EDG-2 receptor |
| JP2005519915A (en) | 2002-01-18 | 2005-07-07 | セレテック・リミテッド・ライアビリティ・カンパニー | Method for treating symptoms associated with Edg receptors |
| US20050261298A1 (en) | 2002-01-18 | 2005-11-24 | David Solow-Cordero | Methods of treating conditions associated with an Edg-7 receptor |
| US20050113283A1 (en) | 2002-01-18 | 2005-05-26 | David Solow-Cordero | Methods of treating conditions associated with an EDG-4 receptor |
| AU2003233119A1 (en) | 2002-05-08 | 2003-11-11 | Neuronova Ab | Modulation of neural stem cells with s1p or lpa receptor agonists |
| US7229987B2 (en) | 2002-05-13 | 2007-06-12 | Eli Lilly And Company | Multicyclic compounds for use as melanin concentrating hormone antagonists in the treatment of obesity and diabetes |
| JP4596314B2 (en) | 2002-05-28 | 2010-12-08 | 小野薬品工業株式会社 | β-alanine derivatives and uses thereof |
| US7300917B2 (en) | 2002-06-26 | 2007-11-27 | Ono Pharmaceuticals Co., Ltd. | Remedy for chronic disease |
| TW200408393A (en) | 2002-10-03 | 2004-06-01 | Ono Pharmaceutical Co | Antagonist of lysophosphatidine acid receptor |
| WO2004046332A2 (en) | 2002-11-19 | 2004-06-03 | Amgen Inc. | Amplified genes involved in cancer |
| US20040192739A1 (en) | 2003-01-16 | 2004-09-30 | David Solow-Cordero | Methods of treating conditions associated with an Edg-2 receptor |
| US20050065194A1 (en) | 2003-01-16 | 2005-03-24 | Geetha Shankar | Methods of treating conditions associated with an Edg-2 receptor |
| US20040167132A1 (en) | 2003-01-16 | 2004-08-26 | Geetha Shankar | Methods of treating conditions associted with an Edg-2 receptor |
| US7135469B2 (en) | 2003-03-18 | 2006-11-14 | Bristol Myers Squibb, Co. | Linear chain substituted monocyclic and bicyclic derivatives as factor Xa inhibitors |
| WO2004091496A2 (en) | 2003-04-11 | 2004-10-28 | The University Of Tennessee Research Foundation | Lysophosphatidic acid analogs and inhibition of neointima formation |
| US7115642B2 (en) | 2003-05-02 | 2006-10-03 | Rigel Pharmaceuticals, Inc. | Substituted diphenyl isoxazoles, pyrazoles and oxadiazoles useful for treating HCV infection |
| WO2005012269A1 (en) * | 2003-08-05 | 2005-02-10 | Ajinomoto Co., Inc. | Novel azole compound |
| AU2004278042B2 (en) | 2003-10-09 | 2012-02-09 | University Of Tennessee Research Foundation | LPA receptor agonists and antagonists and methods of use |
| WO2005058790A1 (en) | 2003-12-19 | 2005-06-30 | Ono Pharmaceutical Co., Ltd. | Compounds having lysophosphatidic acid receptor antagonism and uses thereof |
| DE10360369A1 (en) | 2003-12-22 | 2005-07-14 | Bayer Cropscience Ag | amides |
| US7794713B2 (en) * | 2004-04-07 | 2010-09-14 | Lpath, Inc. | Compositions and methods for the treatment and prevention of hyperproliferative diseases |
| JP2006096712A (en) | 2004-09-30 | 2006-04-13 | Senju Pharmaceut Co Ltd | Corneal perception improving agent containing LPA receptor antagonist |
| GB0511684D0 (en) * | 2005-06-08 | 2005-07-13 | Novartis Ag | Organic compounds |
| BRPI0612028A2 (en) | 2005-06-08 | 2010-10-13 | Novartis Ag | polycyclic oxadiazoles or isodiazoles and their use as s1p receptor ligands |
| JP2008546651A (en) * | 2005-06-14 | 2008-12-25 | メルク フロスト カナダ リミテツド | Reversible inhibitors of monoamine oxidase A and B |
| WO2007007588A1 (en) | 2005-07-08 | 2007-01-18 | Ono Pharmaceutical Co., Ltd. | Compound having cyclic group with planarity as core |
| CN101291908A (en) | 2005-08-23 | 2008-10-22 | Irm责任有限公司 | Immunosuppressant compounds and compositions |
| WO2007096647A2 (en) * | 2006-02-27 | 2007-08-30 | Sterix Limited | Diaryl compounds as non-steroidal inhibitors of 17-beta hydroxysteroid dehydrogenase and/or steroid sulphatase for the treatment of oestrogen-related diseases such as hormone dependent breast cancer |
| US20090029949A1 (en) | 2006-05-25 | 2009-01-29 | Parrill-Baker Abby L | GPCR Ligands Identified by Computational Modeling |
| JP2009545531A (en) | 2006-07-24 | 2009-12-24 | ユニバーシティ オブ バージニア パテント ファンデーション | Phosphonate vinyl lysophosphatidic acid receptor antagonist |
| US20080051372A1 (en) | 2006-08-24 | 2008-02-28 | The Scripps Research Institute | Methods utilizing cell-signaling lysophospholipids |
| WO2008024979A2 (en) | 2006-08-24 | 2008-02-28 | The Scripps Research Institute | Methods utilizing cell-signaling lysophospholipids |
| RU2009127095A (en) * | 2006-12-15 | 2011-01-20 | Эбботт Лэборетриз (Us) | NEW OXADIAZOLE COMPOUNDS |
| WO2008112201A2 (en) | 2007-03-12 | 2008-09-18 | The General Hospital Corporation | Lysophosphatidic acid receptor targeting for lung disease |
| US9163091B2 (en) | 2007-05-30 | 2015-10-20 | Lpath, Inc. | Compositions and methods for binding lysophosphatidic acid |
| US20090069288A1 (en) | 2007-07-16 | 2009-03-12 | Breinlinger Eric C | Novel therapeutic compounds |
| SI2303270T1 (en) | 2008-05-05 | 2018-01-31 | Sanofi | Acylamino-substituted fused cyclopentanecarboxylic acid derivatives and their use as pharmaceuticals |
| US8455499B2 (en) | 2008-12-11 | 2013-06-04 | Amira Pharmaceuticals, Inc. | Alkyne antagonists of lysophosphatidic acid receptors |
| GB2466121B (en) | 2008-12-15 | 2010-12-08 | Amira Pharmaceuticals Inc | Antagonists of lysophosphatidic acid receptors |
| GB2470833B (en) | 2009-06-03 | 2011-06-01 | Amira Pharmaceuticals Inc | Polycyclic antagonists of lysophosphatidic acid receptors |
| EP2462128B1 (en) | 2009-08-04 | 2016-09-21 | Amira Pharmaceuticals, Inc. | Compounds as lysophosphatidic acid receptor antagonists |
| GB2474120B (en) | 2009-10-01 | 2011-12-21 | Amira Pharmaceuticals Inc | Compounds as Lysophosphatidic acid receptor antagonists |
| GB2474748B (en) | 2009-10-01 | 2011-10-12 | Amira Pharmaceuticals Inc | Polycyclic compounds as lysophosphatidic acid receptor antagonists |
| WO2011159633A1 (en) | 2010-06-15 | 2011-12-22 | Amira Pharmaceuticals, Inc. | Inhalable formulations of lysophosphatdic acid receptor antagonists |
| WO2011159635A1 (en) | 2010-06-15 | 2011-12-22 | Amira Pharmaceuticals, Inc. | Lysophosphatidic acid receptor antagonist for the treatment of dermal conditions |
| WO2011159632A1 (en) | 2010-06-15 | 2011-12-22 | Amira Pharmaceuticals, Inc. | Lysophosphatidic acid receptor antagonists for the treatment of conditions or diseases of the eye |
-
2010
- 2010-06-02 GB GB1009231A patent/GB2470833B/en not_active Expired - Fee Related
- 2010-06-03 TW TW099117993A patent/TWI405757B/en not_active IP Right Cessation
- 2010-06-03 KR KR1020127000028A patent/KR101628706B1/en not_active Expired - Fee Related
- 2010-06-03 US US12/793,440 patent/US8058300B2/en active Active
- 2010-06-03 EP EP10784122.3A patent/EP2438048B1/en active Active
- 2010-06-03 CA CA2764445A patent/CA2764445C/en not_active Expired - Fee Related
- 2010-06-03 PT PT107841223T patent/PT2438048E/en unknown
- 2010-06-03 CN CN201080034419.9A patent/CN102459204B/en not_active Expired - Fee Related
- 2010-06-03 BR BRPI1014582A patent/BRPI1014582A2/en not_active Application Discontinuation
- 2010-06-03 SI SI201030496T patent/SI2438048T1/en unknown
- 2010-06-03 MY MYPI2011005870A patent/MY163055A/en unknown
- 2010-06-03 PE PE2011002041A patent/PE20120936A1/en active IP Right Grant
- 2010-06-03 NZ NZ596826A patent/NZ596826A/en not_active IP Right Cessation
- 2010-06-03 AU AU2010256501A patent/AU2010256501B2/en not_active Ceased
- 2010-06-03 KR KR1020167014623A patent/KR101774722B1/en not_active Expired - Fee Related
- 2010-06-03 PL PL10784122T patent/PL2438048T3/en unknown
- 2010-06-03 CN CN201510490659.4A patent/CN105061346A/en active Pending
- 2010-06-03 DK DK10784122.3T patent/DK2438048T3/en active
- 2010-06-03 JP JP2012514144A patent/JP5599457B2/en not_active Expired - Fee Related
- 2010-06-03 MX MX2011012839A patent/MX2011012839A/en active IP Right Grant
- 2010-06-03 SG SG2011084589A patent/SG176112A1/en unknown
- 2010-06-03 WO PCT/US2010/037316 patent/WO2010141768A2/en not_active Ceased
- 2010-06-03 HR HRP20140036AT patent/HRP20140036T1/en unknown
- 2010-06-03 EA EA201101709A patent/EA020139B1/en not_active IP Right Cessation
- 2010-06-03 WO PCT/US2010/037309 patent/WO2010141761A2/en not_active Ceased
- 2010-06-03 ES ES10784122.3T patent/ES2445714T3/en active Active
-
2011
- 2011-09-27 US US13/246,746 patent/US8273780B2/en active Active
- 2011-11-08 IL IL216220A patent/IL216220A/en active IP Right Grant
- 2011-11-16 ZA ZA2011/08421A patent/ZA201108421B/en unknown
- 2011-12-02 CL CL2011003072A patent/CL2011003072A1/en unknown
- 2011-12-21 CO CO11176424A patent/CO6480918A2/en active IP Right Grant
-
2014
- 2014-03-14 SM SM201400031T patent/SMT201400031B/en unknown
-
2015
- 2015-08-23 IL IL240749A patent/IL240749B/en not_active IP Right Cessation
-
2016
- 2016-04-27 AU AU2016202676A patent/AU2016202676B2/en not_active Ceased
Cited By (46)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8455499B2 (en) | 2008-12-11 | 2013-06-04 | Amira Pharmaceuticals, Inc. | Alkyne antagonists of lysophosphatidic acid receptors |
| US8048902B2 (en) | 2008-12-15 | 2011-11-01 | Amira Pharmaceuticals, Inc. | Antagonists of lysophosphatidic acid receptors |
| US8440707B2 (en) | 2008-12-15 | 2013-05-14 | Amira Pharmaceuticals, Inc. | Antagonists of lysophosphatidic acid receptors |
| US8058300B2 (en) | 2009-06-03 | 2011-11-15 | Amira Pharmaceuticals, Inc. | Polycyclic antagonists of lysophosphatidic acid receptors |
| US8273780B2 (en) | 2009-06-03 | 2012-09-25 | Amira Pharmaceuticals, Inc. | Polycyclic antagonists of lysophosphatidic acid receptors |
| US8592402B2 (en) | 2009-08-04 | 2013-11-26 | Amira Pharmaceuticals, Inc. | Compounds as lysophosphatidic acid receptor antagonists |
| US9624182B2 (en) | 2009-10-01 | 2017-04-18 | Amira Pharmaceuticals, Inc. | Compounds as lysophosphatidic acid receptor antagonists |
| US8217066B2 (en) | 2009-10-01 | 2012-07-10 | Amira Pharmaceuticals, Inc. | Compounds as lysophosphatidic acid receptor antagonists |
| EP2483252A4 (en) * | 2009-10-01 | 2013-04-03 | Amira Pharmaceuticals Inc | Compounds as lysophosphatidic acid receptor antagonists |
| US8664220B2 (en) | 2009-10-01 | 2014-03-04 | Amira Pharmaceuticals, Inc. | Polycyclic compounds as lysophosphatidic acid receptor antagonists |
| US8778983B2 (en) | 2009-10-01 | 2014-07-15 | Amira Pharmaceuticals, Inc. | Polycyclic compounds as lysophosphatidic acid receptor antagonists |
| US9090573B2 (en) | 2009-10-01 | 2015-07-28 | Amira Pharmaceuticals, Inc. | Compounds as lysophosphatidic acid receptor antagonists |
| US10000456B2 (en) | 2009-10-01 | 2018-06-19 | Amira Pharmaceuticals, Inc. | Polycyclic compounds as lysophosphatidic acid receptor antagonists |
| US8975235B2 (en) | 2011-03-20 | 2015-03-10 | Intermune, Inc. | Lysophosphatidic acid receptor antagonists |
| US8541587B2 (en) | 2011-04-05 | 2013-09-24 | Amira Pharmaceuticals, Inc. | Lysophosphatidic acid receptor antagonists |
| EP2744807A4 (en) * | 2011-08-15 | 2015-03-04 | Intermune Inc | Lysophosphatidic acid receptor antagonists |
| WO2013070879A1 (en) | 2011-11-10 | 2013-05-16 | Bristol-Myers Squibb Company | Methods for treating spinal cord injury with lpa receptor antagonists |
| WO2013078283A1 (en) | 2011-11-22 | 2013-05-30 | Intermune, Inc. | Methods of diagnosing and treating idiopathic pulmonary fibrosis |
| WO2014085373A1 (en) * | 2012-11-27 | 2014-06-05 | Glaxosmithkline Llc | Combination |
| WO2014104372A1 (en) | 2012-12-28 | 2014-07-03 | 宇部興産株式会社 | Halogen-substituted heterocyclic compound |
| US10597375B2 (en) | 2012-12-28 | 2020-03-24 | Ube Industries, Ltd. | Halogen-substituted heterocyclic compound |
| KR20150100756A (en) | 2012-12-28 | 2015-09-02 | 우베 고산 가부시키가이샤 | Halogen-substituted heterocyclic compound |
| US10000463B2 (en) | 2012-12-28 | 2018-06-19 | Ube Industries, Ltd. | Halogen-substituted heterocyclic compound |
| EP3360869A1 (en) | 2012-12-28 | 2018-08-15 | Ube Industries, Ltd. | Halogen-substituted heterocyclic compound useful for the treatment of diseases caused by lpa |
| US10570103B2 (en) | 2013-03-15 | 2020-02-25 | Epigen Biosciences, Inc. | Heterocyclic compounds useful in the treatment of disease |
| US11427552B2 (en) | 2013-03-15 | 2022-08-30 | Epigen Biosciences, Inc. | Heterocyclic compounds useful in the treatment of disease |
| US10000459B2 (en) | 2013-03-15 | 2018-06-19 | Epigen Biosciences, Inc. | Heterocyclic compounds useful in the treatment of disease |
| US10526298B2 (en) | 2013-03-15 | 2020-01-07 | Epigen Biosciences, Inc. | Heterocyclic compounds useful in the treatment of disease |
| KR20170016988A (en) | 2014-06-27 | 2017-02-14 | 우베 고산 가부시키가이샤 | Salt of halogen-substituted heterocyclic compound |
| US10023554B2 (en) | 2014-06-27 | 2018-07-17 | Ube Industries, Ltd. | Halogen-substituted heterocyclic compound salt |
| WO2016197009A1 (en) | 2015-06-05 | 2016-12-08 | Vertex Pharmaceuticals Incorporated | Triazoles for the treatment of demyelinating diseases |
| WO2018106646A1 (en) | 2016-12-06 | 2018-06-14 | Vertex Pharmaceuticals Incorporated | Aminotriazoles for the treatment of demyelinating diseases |
| WO2018106641A1 (en) | 2016-12-06 | 2018-06-14 | Vertex Pharmaceuticals Incorporated | Pyrazoles for the treatment of demyelinating diseases |
| WO2018106643A1 (en) | 2016-12-06 | 2018-06-14 | Vertex Pharmaceuticals Incorporated | Heterocyclic azoles for the treatment of demyelinating diseases |
| WO2020257138A1 (en) * | 2019-06-18 | 2020-12-24 | Bristol-Myers Squibb Company | Isoxazole carboxylic acids as lpa antagonists |
| US12435057B2 (en) | 2019-06-18 | 2025-10-07 | Bristol-Myers Squibb Company | Triazole carboxylic acids as LPA antagonists |
| US12384768B2 (en) | 2019-06-18 | 2025-08-12 | Bristol-Myers Squibb Company | Isoxazole carboxylic acids as LPA antagonists |
| US11999717B2 (en) | 2019-11-15 | 2024-06-04 | Gilead Sciences, Inc. | Triazole carbamate pyridyl sulfonamides as LPA receptor antagonists and uses thereof |
| US11548871B2 (en) | 2019-11-15 | 2023-01-10 | Gilead Sciences, Inc. | Triazole carbamate pyridyl sulfonamides as LPA receptor antagonists and uses thereof |
| US11912686B2 (en) | 2020-06-03 | 2024-02-27 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
| US11702407B2 (en) | 2020-06-03 | 2023-07-18 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
| US12139474B2 (en) | 2020-06-03 | 2024-11-12 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
| US11584738B2 (en) | 2020-06-03 | 2023-02-21 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
| US11980609B2 (en) | 2021-05-11 | 2024-05-14 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
| US12503454B2 (en) | 2021-05-13 | 2025-12-23 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
| US11939318B2 (en) | 2021-12-08 | 2024-03-26 | Gilead Sciences, Inc. | LPA receptor antagonists and uses thereof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2648714B1 (en) | Lysophosphatidic acid receptor antagonists and uses thereof | |
| US8592402B2 (en) | Compounds as lysophosphatidic acid receptor antagonists | |
| WO2010141768A2 (en) | Polycyclic antagonists of lysophosphatidic acid receptors | |
| US8664220B2 (en) | Polycyclic compounds as lysophosphatidic acid receptor antagonists | |
| US9624182B2 (en) | Compounds as lysophosphatidic acid receptor antagonists | |
| US8455499B2 (en) | Alkyne antagonists of lysophosphatidic acid receptors | |
| US8541587B2 (en) | Lysophosphatidic acid receptor antagonists | |
| US20110301142A1 (en) | Antagonists of lysophosphatidic acid receptors | |
| EP2714680B1 (en) | Heterocyclic autotaxin inhibitors and uses thereof | |
| AU2011338417B2 (en) | Polycyclic LPA1 antagonist and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10784125 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10784125 Country of ref document: EP Kind code of ref document: A2 |