WO2010116385A2 - Pharmaceutical compositions for alleviating unpleasant taste - Google Patents
Pharmaceutical compositions for alleviating unpleasant taste Download PDFInfo
- Publication number
- WO2010116385A2 WO2010116385A2 PCT/IN2010/000228 IN2010000228W WO2010116385A2 WO 2010116385 A2 WO2010116385 A2 WO 2010116385A2 IN 2010000228 W IN2010000228 W IN 2010000228W WO 2010116385 A2 WO2010116385 A2 WO 2010116385A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- eszopiclone
- taste
- composition
- cyclodextrin
- glyceryl
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4745—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having nitrogen as a ring hetero atom, e.g. phenantrolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
- A61K9/0056—Mouth soluble or dispersible forms; Suckable, eatable, chewable coherent forms; Forms rapidly disintegrating in the mouth; Lozenges; Lollipops; Bite capsules; Baked products; Baits or other oral forms for animals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1664—Compounds of unknown constitution, e.g. material from plants or animals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2009—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
- A61K9/2018—Sugars, or sugar alcohols, e.g. lactose, mannitol; Derivatives thereof, e.g. polysorbates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2077—Tablets comprising drug-containing microparticles in a substantial amount of supporting matrix; Multiparticulate tablets
- A61K9/2081—Tablets comprising drug-containing microparticles in a substantial amount of supporting matrix; Multiparticulate tablets with microcapsules or coated microparticles according to A61K9/50
Definitions
- the present invention relates to oral pharmaceutical compositions that alleviate the bitter, unpleasant or metallic taste associated with eszopiclone therapy.
- the invention relates to pharmaceutical compositions that mask the unpleasant or objectionable taste of eszopiclone and alleviate the immediate bitter taste associated with eszopiclone therapy comprising eszopiclone, at least one taste-masking agent and at least one pharmaceutically acceptable excipient.
- the invention also relates to compositions devoid of the active agent that alleviate the bitter, metallic or unpleasant taste experienced by a patient after administration of eszopiclone.
- compositions of the present invention are suitable for oral administration in the form of orally disintegrating tablets, bite-dispersion tablets, dispersible tablets, effervescent tablets or the like.
- Methods of countering the unpleasant after-taste of eszopiclone comprising administering to the patients in need thereof a composition devoid of the active agent either along with or subsequently at an appropriate time after administration of taste-masked eszopiclone formulation are also provided.
- Insomnia or sleeplessness, or disturbed sleep is a common condition affecting millions of people worldwide. It is characterized by persistent difficulty in falling asleep or staying asleep despite the opportunity. Women have approximately 1.5 times higher risk of insomnia than men and the overall prevalence of insomnia increases with age. The most common complaints related to insomnia are difficulty in initiating sleep (sleep-onset insomnia), difficulty in maintaining sleep due to prolonged nocturnal awakenings (sleep- maintenance insomnia), early morning awakenings (sleep-offset insomnia), and excessive daytime sleepiness resulting from lack of sleep. Insomnia may be primary (caused by biologic or psychosocial mechanisms independent of a disease process) or secondary (brought on or exacerbated by a disease process, disorder or substance).
- Secondary insomnia may constitute up to 70% of sleep problems seen in the general population.
- Further insomnia can be (a) transient insomnia which lasts from days to weeks (b) acute insomnia with inability to consistently sleep well for a period of between three weeks to six months or (c) chronic insomnia that lasts for years at a time, with various primary or secondary causes for each.
- Insomnia is generally managed using both non-pharmacologic and pharmacologic interventions, and where possible the treatment is directed to underlying cause of the condition.
- Pharmacotherapy has been found to be effective in the management of insomnia.
- Benzodiazepines have been the drugs of choice for the treatment of insomnia over the past three decades.
- Eszopiclone (+)-(5S)-6-(chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrroIo[3,4-b] pyrazin- 5-yl 4-methyl- piperazine-1-carboxylate, is a non-benzodiazepine hypnotic agent that belongs to the class of drugs known as cyclopyrrolones. This class of compounds appears to cause less residual sedation than the benzodiazepines and provide an improved therapeutic index over them.
- Eszopiclone is the S-isomer of racemic zopiclone and is indicated for the treatment of transient and chronic insomnia in adult patients ⁇ 18 years of age.
- GABA gamma aminobutyric acid
- Eszopiclone sold under the brand name Lunesta® in the U.S., received approval from the FDA in December 2004.
- U.S Patent Nos. 6,319,926, 6,444,673, 6,864,257 and 7,381 ,724 disclose the molecule and its use as a tranquilizer, sedative and hypnotic, mainly for improving sleep quality or time.
- Eszopiclone generally administered at bedtime for treatment of insomnia, is rapidly absorbed after oral administration. Peak plasma concentrations are achieved within approximately 1 hour after oral administration. It is widely distributed in body tissues, including brain, and is eliminated in urine, saliva and breast milk. Approximately 52% to 59% of a dose is weakly bound to plasma protein. The elimination half-life of eszopiclone is approximately 6 hours and it is extensively metabolized by oxidation and demethylation. Cytochrome P450 (CYP) isozymes CYP3A4 and CYP2E1 are involved in the metabolism of eszopiclone. The primary plasma metabolites are CS ⁇ -zopiclone-N- oxide and (Sj-N-desmethyl zopiclone. (Lunesta - Pack insert, Marlborough, MA, Sepracor, December 2004).
- eszopiclone has been evaluated in healthy adults, including elderly patients, for the treatment of transient and chronic insomnia. Compared with placebo, eszopiclone has been shown to considerably reduce sleep induction, improve sleep maintenance, duration, quality, and depth, as well as next-day functioning. Further, U.S. Patent No.6,436,936 discloses that (+) zopiclone is also useful in treating convulsive disorders such as epilepsy, anxiety, aggressive behavior, muscle spasms, behavioral disorders, depression, schizophrenia or endocrine disorders.
- Eszopiclone has an extremely bitter and unpleasant taste. Reviews from patients consuming eszopiclone indicate that bitter, metallic or unpleasant taste is felt immediately on consuming the medication. Further, similar unpleasant taste is also experienced after administration of the active over a time period ranging from about a few minutes to a few hours or persists even longer and is felt the next day as well. Consumption of this medication is also said to result in dysguesia or altered sensation of taste, often unpleasant taste. Unpleasant after-taste is generally considered as the most common adverse effect with eszopiclone administration.
- Eszopiclone is generally well tolerated; however discontinuation of therapy due to adverse events has been reported in some patients.
- drug discontinuation due to adverse events occurred in 1.4% of 72 patients receiving eszopiclone 1 mg, 2.3% of 215 patients receiving eszopiclone 2 mg, and 3.8% of 208 patients receiving placebo.
- the most commonly reported adverse event in these trials was headache (15%, 13%, and 14%, respectively), followed by unpleasant taste (8%, 12%, and 0%) (Jadwiga Najib, Clinical Therapeutics, 28(4), 491-516 (2006)).
- eszopiclone is associated with adverse events of bitter or unpleasant taste which has in many instances also lead to discontinuation of therapy.
- the unpleasant taste with eszopiclone is experienced at different time intervals by different patients.
- Some patients experience the bitterness immediately while some patients who consume eszopiclone at night, experience bitter taste not only immediately upon administration of the drug but also in the mornings.
- Still another group of patients experience the bitter after-taste immediately and over a period of time after administration and in the mornings and throughout the next day until the next dose as well.
- U.S. Patent No.5,206, 025 discloses porous, unitary freeze-dried, solid pharmaceutical dosage forms that consist of an inclusion compound comprising active substance and cyclodextrin; and at least one substance chosen from diluents or binders. Freeze-drying employed herein, however, is a cumbersome, lengthy and an expensive process that can present challenges during scale-up of the formulation. Further, such porous freeze-dried pharmaceuticals tend to lack mechanical strength and fracture easily during handling and packaging.
- eszopiclone can be then incorporated into palatable formulations suitable for oral administration such as orally disintegrating tablets, bite-dispersion tablets, dispersible tablets, effervescent tablets or the like which alleviates any immediate bitterness normally experienced by the patient on oral intake of eszopiclone.
- palatable formulations suitable for oral administration such as orally disintegrating tablets, bite-dispersion tablets, dispersible tablets, effervescent tablets or the like which alleviates any immediate bitterness normally experienced by the patient on oral intake of eszopiclone.
- Such formulations also have adequate mechanical strength, desired taste and in vitro release profile.
- the present invention provides compositions devoid of the active agent that alleviate the bitter or unpleasant after-taste experienced by a patient after administration of eszopiclone.
- the inventors after in-depth research have also arrived at a method of alleviating the bitter or metallic or unpleasant after taste reported subsequent to the administration of eszopiclone comprising administering to a patient in need thereof a composition devoid of the active agent either along with or subsequently at an appropriate time.
- the present invention relates to oral pharmaceutical compositions that alleviate the bitter, unpleasant or metallic taste associated with eszopiclone therapy.
- the present invention relates to pharmaceutical compositions for alleviating immediate unpleasant taste associated with eszopiclone therapy comprising eszopiclone, at least one taste-masking agent and at least one pharmaceutically acceptable excipient.
- the invention also relates to compositions devoid of the active agent that alleviate the bitter, metallic or unpleasant taste experienced by a patient after administration of eszopiclone.
- the invention further provides compositions of the present invention for alleviating the unpleasant taste of eszopiclone for oral administration in the form of orally disintegrating tablets, bite-dispersion tablets, dispersible tablets, effervescent tablets or the like.
- a kit and method of alleviating unpleasant taste associated with eszopiclone therapy comprising administering to the patient in need thereof a taste- masked eszopiclone composition to alleviate any immediate bitterness associated with oral intake of eszopiclone followed by optional administration of at least one placebo composition devoid of the eszopiclone either along with or subsequently at an appropriate time after the administration of the taste-masked eszopiclone composition to counter unpleasant after-taste reported subsequent to the administration of eszopiclone is provided.
- Palatability and mouth feel are important factors for achieving total compliance of patients who are being administered the unpleasant, disagreeable or objectionable tasting active pharmaceutical agent.
- Several processes of masking the taste of actives are known. Not all taste-masking technologies, however, work with every drug. Processes and technologies employed to mask the taste of an active can, in certain instances, interfere with disintegration, affect stability, provide inadequate taste-masking for a given active or interfere with the bioavailability or pharmacokinetic properties of the drug. Therefore, it becomes important to develop a process for masking the taste of an active agent that not only enhances the organoleptic properties of the dosage form containing the same, but also does not interfere with the bioavailability of the drug. Further, patient compliance is also an important factor when using an active agent which is excreted in the saliva leading to the perception of bitter or unpleasant taste, which sometimes lasts for several hours after administration of the active.
- the present invention provides compositions for reducing the bitterness associated with eszopiclone therapy.
- the present invention provides compositions to alleviate immediate bitter taste normally experienced by a patient on oral intake of eszopiclone, wherein the bitter or unpleasant taste of eszopiclone is masked and taste-masked eszopiclone is incorporated into palatable formulations that have desired taste without compromising on the in-vitro profile and manufactured by using simple, cost-effective processes. Such processes of masking the unpleasant taste of eszopiclone have also been provided herein.
- the present invention provides a pharmaceutical composition for alleviating unpleasant taste associated with eszopiclone therapy comprising (a) eszopiclone; (b) at least one taste-masking agent; and (c) at least one pharmaceutically acceptable excipient.
- the present invention also provides compositions devoid of the active agent for alleviating any unpleasant after-taste associated with eszopiclone therapy and methods for using the compositions of the present invention.
- the present invention thereby provides compositions and methods of alleviating bitter or unpleasant taste experienced by a patient after eszopiclone administration.
- eszopiclone as used in the present invention encompasses the (S) - form of racemic zopiclone, its pharmaceutically acceptable salts, prodrugs, solvates, hydrates, polymorphs, enantiomer, metabolites, clathrates or derivatives thereof.
- Pharmaceutically acceptable salt refers to salts prepared from pharmaceutically acceptable non-toxic acids, including organic acids or inorganic acids.
- Suitable non-toxic acids include inorganic and organic acids such as, but not limited to, acetic, alginic, anthranilic, benzenesulfonic, benzoic, citric, ethenesulfonic, camphorsulfonic, fumaric, furoic, gluconic, glutamic, glucorenic, galacturonic, glycidic, hydrobromic, hydrochloric, formic, isethionic, lactic, maleic, malic, mandelic, nitric, pamoic, pantothenic, methanesulfonic, mucic, phenylacetic, propionic, phosphoric, salicylic, sulfanilic, sulfuric, stearic, succinic, tartaric acid, p-toluenesulfonic and the like.
- inorganic and organic acids such as, but not limited to, acetic, alginic, anthranilic, benzenesulfonic, be
- the various features and embodiments of the present invention can also be applied to other stereoisomers of eszopiclone, racemic or stereoisomeric mixtures of eszopiclone and its stereoisomers like zopiclone, their pharmaceutically acceptable salts, prodrugs, solvates, hydrates, polymorphs, enantiomer, metabolites, clatharates or derivatives thereof.
- a pharmaceutically effective amount of eszopiclone is employed in the formulations of the present invention.
- the term "effective amount" refers to an amount effective to achieve desired therapeutic and/or beneficial effect.
- Therapeutic or beneficial effect may be desired in various conditions such as insomnia, epilepsy, anxiety, aggressive behavior, muscle spasms, behavioral disorders, depression, schizophrenia, movement disorders, gastric disorders, cognitive disorders or endocrine disorders.
- the concentration of eszopiclone in the formulation can vary from about 0.01 weight % to about 60 weight % based on the total weight of the composition.
- the concentration of eszopiclone in the formulation can vary from about 0.05 weight % to about 50 weight % based on the total weight of the composition.
- the concentration of eszopiclone in the formulation can vary from about 0.1 weight % to about 35 weight % based on the total weight of the composition.
- the formulations of the present invention may be administered at a dose of about 0.001 mg to about 50 mg of eszopiclone.
- the formulations of the present invention may be administered at a dose of about 0.01 mg to about 50 mg of eszopiclone.
- the formulations of the present invention may be administered at a dose of about 0.05 mg to about 40 mg of eszopiclone.
- Eszopiclone may be in the form of, but not limited to, powder, granules, pellets, beads, minitablets or the like. Eszopiclone granules may be prepared by wet granulation, dry granulation or roll compaction or the like. In one aspect of the present invention, pellets of eszopiclone may be prepared using extrusion spheronization. In another aspect of the present invention, eszopiclone can be loaded on an inert carrier before taste-masking.
- the inert carrier can be selected from, but not limiting to, beads, pellets, spheres or similar particles that do not contain an active ingredient. Non-limiting examples of inert carriers include microcrystalline cellulose, sugar or silicon dioxide.
- eszopiclone in the powder form, may be treated with a taste-masking agent.
- taste-masking agent employed in the compositions of the present invention include, but are not limited to, a polymeric pharmaceutically acceptable excipient, a non- polymeric pharmaceutically acceptable excipient, an adsorbent, a carbomer, an ion- exchange resin, a cyclodextrin or a derivative thereof, or a pH-modifier, or a combination thereof.
- the bitter taste of eszopiclone according to the present invention is masked by a process of treating the drug with at least one taste-masking agent.
- treating or “treatment” or “treated” as used in the present invention in the context of taste-masking encompasses any method known to a person skilled in the art whereby the bitter or objectionable taste of the active can be masked using pharmaceutically acceptable taste-masking agent, such as but not limited to, coating, physical mixing, melt granulation, complexation, adsorption or the like.
- taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is a polymeric pharmaceutically acceptable excipient or non-polymeric pharmaceutically acceptable excipient or any combination thereof.
- the bitter taste of the active is masked by coating or by physical mixing using as taste-masking agents polymeric or non-polymeric pharmaceutically acceptable excipients or any combinations thereof.
- Polymeric pharmaceutically acceptable excipients for taste-masking eszopiclone as employed in the present invention include, but are not limited to, cellulose derivatives, saccharides or polysaccharides, polyhydric alcohols, poly(oxyethylene)- poly(oxypropylene) block copolymers (poloxamers), vinyl derivatives or polymers or copolymers thereof, acrylic acid derivatives or the like or any combinations thereof.
- Cellulose derivatives include, but are not limited to, ethyl cellulose, methylcellulose, hydroxypropylmethylcellulose (HPMC), hydroxypropyl cellulose (HPC), hydroxyethyl cellulose, hydroxymethyl cellulose, hydroxypropyl ethylcellulose, carboxymethylethyl cellulose, carboxy ethylcellulose, carboxymethyl hydroxyethylcellulose, hydroxyethylmethyl carboxymethyl cellulose, hydroxyethyl methyl cellulose, carboxymethyl cellulose, methylhydroxyethyl cellulose, methylhydroxypropyl cellulose, carboxymethyl sulfoethyl cellulose, sodium carboxymethyl cellulose, cellulose acetate, cellulose acetate phthalate, cellulose acetate butyrate, hydroxypropylmethylcellulose acetate succinate, hydroxypropylmethylcellulose phthalate, hydroxymethyl ethylcellulose phthalate, cellulose acetate phthalate, cellulose acetate succinate,
- Saccharides or polysaccharides include, but are not limited to, guar gum, xanthan gum, gum arabic, tragacanth or combinations thereof.
- Polyhydric alcohols include, but are not limited to, polyethylene glycol (PEG) or polypropylene glycol.
- Vinyl derivatives, polymers and copolymers thereof include, but are not limited to, polyvinylacetate aqueous dispersion (Kollicoat ® SR 30D) 1 copolymers of vinyl pyrrolidone, copolymers of polyvinyl alcohol (Kollicoat ® IR), polyvinyl alcohol phthalate, polyvinylacetal phthalate, polyvinyl butylate phthalate, polyvinylacetoacetal phthalate, polyvinylpyrrolidone (PVP) 1 or combinations thereof.
- polyvinylacetate aqueous dispersion Kercoat ® SR 30D
- Polyvinyl alcohol phthalate copolymers of polyvinyl alcohol
- PVP polyvinylacetoacetal phthalate
- PVP polyvinylpyrrolidone
- Acrylic acid derivatives include, but are not limited to, methacrylic acids, polymethacrylic acids, polyacrylates, especially polymethacrylates like a) copolymer formed from monomers selected from methacrylic acid, methacrylic acid esters, acrylic acid and acrylic acid esters b) copolymer formed from monomers selected from butyl methacrylate, (2-dimethylaminoethyl)methacrylate and methyl methacrylate c) copolymer formed from monomers selected from ethyl acrylate, methyl methacrylate and trimethylammonioethyl methacrylate chloride or d) copolymers of acrylate and methacrylates with/without quarternary ammonium group in combination with sodium carboxymethylcellulose, e.g.
- Eudragit ® those available from Rohm GmbH under the trademark Eudragit ® like Eudragit EPO (dimethylaminoethyl methacrylate copolymer; basic butylated methacrylate copolymer), Eudragit RL and RS (trimethylammonioethyl methacrylate copolymer), Eudragit NE30D and Eudragit NE40D (ethylacrylate methymethacrylate copolymer), Eudragit RD 100 (ammoniomethacrylate copolymer with sodium carboxymethylcellulose); or the like or any combinations thereof.
- Eudragit EPO dimethylaminoethyl methacrylate copolymer; basic butylated methacrylate copolymer
- Eudragit RL and RS trimethylammonioethyl methacrylate copolymer
- Eudragit NE30D and Eudragit NE40D ethylacrylate methymethacrylate copolymer
- non-polymeric pharmaceutically acceptable excipients for taste-masking eszopiclone include, ' but are not limited to, fats, oils, waxes, fatty acids, fatty acid esters, long chain monohydric alcohols and their esters, phospholipids, terpenes or combinations thereof.
- Waxes are esters of fatty acids with long chain monohydric alcohols. Natural waxes are often mixtures of such esters, and may also contain hydrocarbons.
- Waxes employed in the present invention include, but are not limited to, natural waxes, such as animal waxes, vegetable waxes, and petroleum waxes ⁇ i.e., paraffin waxes, microcrystalline waxes, petrolatum waxes, mineral waxes), and synthetic waxes. Specific examples include but are not limited to spermaceti wax, carnauba wax, Japan wax, bayberry wax, flax wax, beeswax, yellow wax, Chinese wax, shellac wax, lanolin wax, sugarcane wax, candelilla wax, castor wax paraffin wax, microcrystalline wax, petrolatum wax, carbowax, and the like, and mixtures thereof.
- natural waxes such as animal waxes, vegetable waxes, and petroleum waxes ⁇ i.e., paraffin waxes, microcrystalline waxes, petrolatum waxes, mineral waxes
- synthetic waxes include but are not limited to spermaceti wax, carnauba wax, Japan wax,
- Waxes are also monoglyceryl esters, diglyceryl esters, or triglyceryl esters (glycerides) and derivatives thereof formed from a fatty acid having from about 10 to about 22 carbon atoms and glycerol, wherein one or more of the hydroxyl groups of glycerol are substituted by a fatty acid.
- Glycerides employed in the present invention include, but are not limited to, glyceryl monostearate, glyceryl distearate, glyceryl tristearate, glyceryl dipalmitate, glyceryl tripalmitate, glyceryl monopalmitate, glyceryl palmitostearate, glyceryl dilaurate, glyceryl trilaurate, glyceryl monolaurate, glyceryl didocosanoate, glyceryl tridocosanoate, glyceryl monodocosanoate, glyceryl monocaproate, glyceryl dicaproate, glyceryl tricaproate, glyceryl monomyristate, glyceryl dimyristate, glyceryl trimyristate, glyceryl monodecenoate, glyceryl didecenoate, glyceryl tridecen
- Fatty acids include, but are not limited to, hydrogenated palm kernel oil, hydrogenated peanut oil, hydrogenated palm oil, hydrogenated rapeseed oil, hydrogenated rice bran oil, hydrogenated soybean oil, hydrogenated sunflower oil, hydrogenated castor oil, hydrogenated cottonseed oil, and mixtures thereof.
- Other fatty acids include, but are not limited to, decenoic acid, docosanoic acid, stearic acid, palmitic acid, lauric acid, myristic acid, and the like, and mixtures thereof.
- the fatty acids employed include, but are not limited to, hydrogenated palm oil, hydrogenated castor oil, stearic acid, hydrogenated cottonseed oil, palmitic acid, and mixtures thereof.
- Long chain monohydric alcohols include, but are not limited to, cetyl alcohol, and stearyl alcohol, and mixtures thereof.
- the non-polymeric pharmaceutically acceptable excipients employed for taste-masking eszopiclone include, but are not limited to, Cutina (hydrogenated castor oil), Hydrobase (hydrogenated soybean oil), Castorwax (hydrogenated castor oil), Croduret (hydrogenated castor oil), Carbowax, Compritol (glyceryl behenate), Sterotex (hydrogenated cottonseed oil), Lubritab (hydrogenated cottonseed oil), Capmul (glyceryl mono- and dicaprate), Apifil (wax yellow), Akofine (hydrogenated cottonseed oil), Softtisan (hydrogenated palm oil), Hydrocote (hydrogenated soybean oil), Corona (lanolin), Gelucire (macrogolglycerides lauriques), Precirol (glyceryl palmitostearate), Emulcire (cet
- lipids or waxes are employed in the form of an aqueous dispersion or emulsion stabilized by surfactants and suitable stabilizers.
- the active ingredient is physically mixed or blended with these polymeric or non- polymeric pharmaceutically acceptable excipients or is partially or completely coated with these excipients by any of the techniques known in the art, such as microencapsulation, melt granulation, melt extrusion, fluid bed coating, wet granulation, spray drying, dry granulation or roll compaction. Further, coating, based on lipids or waxes or other non- polymeric pharmaceutically acceptable excipients require that the melting point of the lipid should be sufficiently high to prevent melting in the mouth and should not be so high that active ingredient itself melts or is chemically degraded while preparation of the formulation. Coating can be carried out in the range from about 1% to about 150% weight gain. In one embodiment, coating is carried out in the range of from about preferably from about 2% to about 120% weight gain. In another embodiment, coating is carried out in the range of from about 5% to about 100% weight gain.
- the objectionable taste of eszopiclone is masked by melt granulation, which involves dispersing the active in a mass of molten polymeric or non- polymeric pharmaceutically acceptable excipients, followed by cooling and granulation.
- eszopiclone is granulated using molten mass of polymeric and/or pharmaceutically acceptable excipients.
- the polymeric or non-polymeric pharmaceutically acceptable excipient can be applied alone or in combination with other suitable pharmaceutical excipients, to eszopiclone, in the form of, but not limited to, powder, granules, beads, pellets, minitablets or the like to achieve the desired taste-masking.
- the amount of taste-masking agent employed can be in the range of about 1% to about 90% by weight of the composition. In one embodiment, the amount of taste-masking agent employed can be in the range of about 1 % to about 80% by weight of the composition. In one embodiment, the amount of taste-masking agent is in the range of about 2% to about 60% by weight of the composition. In another embodiment, the amount of taste-masking agent is in the range of about 5 to about 50% by weight of the composition.
- taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is a cyclodextrin or a derivative thereof.
- the bitter taste of eszopiclone is masked by complexation with cyclodextrins or derivatives thereof.
- Cyclodextrins are cyclic oligosaccharides formed from ⁇ -(1 , 4)-linked D-glucopyranose units, ⁇ , ⁇ and ⁇ -cyclodextrins consist of six, seven and eight units respectively.
- the molecules have a toroidal shape, with a hydrophobic central cavity and a relatively hydrophilic outer surface. This structure enables cyclodextrins to bind appropriately sized non-polar guest molecules, or moieties of guest molecules, within the hydrophobic central cavity, to form clathrate complexes.
- Suitable cyclodextrins for use in the compositions of the present invention include, but are not limited to, ⁇ , ⁇ and ⁇ cyclodextrins, or alkylated, hydroxyalkylated, esterified, glycosylated or substituted derivatives thereof, such as (2,6-di-o-methyl)- ⁇ -cyclodextrin (DIMEB) (dimethyl- ⁇ - cyclodextrin), randomly methylated- ⁇ -cyclodextrin (RAMEB), and hydroxypropyl- ⁇ - cyclodextrin (HP ⁇ CD), hydroxyethyl- ⁇ -cyclodextrin, dihydroxypropyl- ⁇ -cyclodextrin, trimethyl- ⁇ -cyclodextrin, hydroxymethyl- ⁇ -cyclodextrin, ⁇ -cyclodextrin sulfate, ⁇ - cyclodextrin sulfonate, methyl- ⁇ -cyclodextrin,
- the complex of active with cyclodextrin can be prepared by various methods such as solution method, co-precipitation method, co-evaporation/solid dispersion method, neutralization method, slurry method, kneading method, and grinding method.
- compositions of the present invention comprise eszopiclone and cyclodextrin or a derivative thereof in an uncomplexed form along with suitable pharmaceutically acceptable excipients.
- taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is a carbomer or a derivative thereof.
- the bitter taste of eszopiclone is masked by complexation with carbomers or derivatives thereof.
- Eszopiclone can be taste-masked by complexation with carbomers such as carbomer 934, carbomer 971 , carbomer 974 or the like wherein the complex is held together by ionic bonding and gel properties of the carbomer, providing stable and palatable compositions.
- These complexes can be prepared by mixing, blending or slurrying eszopiclone and carbomer together to allow the desired complex formation.
- taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is an adsorbent.
- the bitter taste of eszopiclone is masked by adsorption using as taste-masking agents, adsorbents that form adsorbates with the active.
- Adsorbates is formed by adsorbing or partially or significantly blending eszopiclone with an adsorbent selected from, but not limited to, magnesium aluminum silicate, zeolite, activated granular carbon, silica gel, active aluminum, clay and mixtures thereof.
- adsorbent materials surround the drug particles by forming a physical bond, by Van der Waals interactions, and hydrogen bonding force of attraction, so that the bitter taste of the drug is not perceived.
- the adsorbate of eszopiclone is formed by mixing or blending the active with the adsorbent in high or moderate shear mixers like planetary mixer or rapid mixer granulator. Alternatively, adsorbate is formed by wet granulation involving the adsorbent and eszopiclone in any conventional granulation equipment.
- taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is an ion exchange resin.
- the bitter taste of eszopiclone is masked by complexation with an ion-exchange resin.
- Ion exchange resins are solid and water insoluble high molecular weight polyelectrolytes that can exchange their mobile ions of equal charge with the surrounding medium and are not absorbed by the body. The resulting ion exchange is reversible and stoichiometric with the displacement of one ionic species by another.
- the drug-resinates effectively mask the taste of a bitter or unpleasant tasting drug within the matrix of the ion exchange material.
- the correct selection of ion exchange resin is important so that the drug is not released in the mouth leading to perception of the bitter taste of drug.
- the present invention provides taste- masking of eszopiclone by reversibly adsorbing the active compound on to an ion exchange resin
- the polymeric matrix of the ion exchange resin can have functional groups including anionic groups, e.g., weakly acidic- carboxylic, esteric and phosphonic; strongly acidic-sulfonic or cationic groups, e.g., weakly basic- tertiary amine; strongly basic- quaternary amine.
- suitable polymeric matrices include copolymers of acrylic and substituted acrylic acids; styrene and styrene derivatives; cellulose esters; vinyl and substituted vinyl esters; and polysulfonic acids and polysulfonic acid esters.
- the ion exchange resin having the polymeric matrix with an anionic functional group is a cation exchange resin and that having a cationic functional group is an anionic exchange resin.
- the mobile or exchangeable moieties depending on the type of resin can be but not limiting to sodium, hydrogen, potassium, chloride and the like.
- a cationic exchange resin is employed to mask the bitter taste of eszopiclone.
- suitable cation exchange resin that may be employed include Amberlite ® IRP64 (porous copolymer of methacrylic acid and divinylbenzene), Amberlite ® IRP69 (sulfonated copolymer of styrene and divinylbenzene), Amberlite ® IRP88 (cross linked polymer of methacrylic acid and divinylbenzene), DOWEX ® RTM.
- taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is a pH modifier.
- a pH-modif ⁇ er is employed in the compositions of the present invention include, but is not limited to, disodium hydrogen phosphate, sodium triphosphate, monosodium phosphate, sodium carbonate, potassium dihydrogen phosphate, sodium bicarbonate, meglumine, citric acid, benzoic acid, tartaric acid or the like or any combinations thereof.
- sweetening agent including, but not limited to, aspartame, stevia extract, glycyrrhiza, saccharine, saccharine sodium, acesulfame, sucralose and dipotassium glycyrrhizinate; and one or more flavors, e.g., mint flavour, orange flavour, lemon flavors, strawberry aroma, vanilla flavour, raspberry aroma, cherry flavor, tutty frutty flavor, magnasweet 135, key lime flavor, grape flavor, trusil art 511815, and fruit extracts.
- polyols such as, but not limited to, mannitol, xylitol, sorbitol or the like are also used as sweeteners.
- alleviation of bitter or unpleasant taste associated with eszopiclone is achieved using taste-modifying agents that are capable of competitively binding or interacting with the taste buds or taste receptors or coating them thereby alleviating the perception of bitter or unpleasant taste such as, but not limited to, hydrogenated castor oil (Cremophor RH40), thaumatin, arginine, ornithine, lysine, citrulline, adenosine 5 1 monophosphate, thymidine 5 r monophosphate, adenosine 5' diphosphate, adenosine 3' monophosphate, adenosine 5'-succinate, adenosine 5 1 triphosphate, adenosine 2' monophosphate, ⁇ '-cytidylic acid, inosinic acid, monellin, ferulic acid or caffeic acid or the like or any combinations thereof.
- taste-modifying agents that are capable of competitively binding or interacting with the taste buds or
- the immediate bitter taste associated with eszopiclone therapy is alleviated by masking the objectionable taste of eszopiclone by use of taste-masking agents as discussed above followed by incorporation of such taste-masked eszopiclone, in the form of, but not limited to, powder, granules, pellets, beads, minitablets or the like in various palatable formulations including, but not limited to, orally disintegrating, bite-dispersion, dispersible, chewable or effervescent tablets, sprinkle granules, quick melt wafers, lozenge, dry suspensions or syrups for reconstitution, chewing gum, buccal tablet or the like.
- the pharmaceutical compositions comprising taste-masked eszopiclone are in the form of rapidly disintegrating dosage forms such as, but are not limited to, orally disintegrating, bite-dispersion, dispersible, chewable or effervescent tablets, sprinkle granules, quick melt wafers, lozenges or the like.
- the various taste- masking approaches discussed above can also be applied for the development of immediate release tablets.
- These oral formulations may contain from about 5% to about 95% of tastemasked eszopiclone.
- the taste-masked formulations may be so designed that the bioavailability of the eszopiclone is preferably not compromised.
- compositions of the present invention in addition to eszopiclone and a taste-masking agent further comprise at least one pharmaceutically acceptable excipient. Based on the final form of the palatable pharmaceutical composition to be prepared suitable pharmaceutically acceptable excipients are incorporated.
- taste-masked eszopiclone is incorporated in an orally disintegrating tablet.
- Orally disintegrating tablets ODTs
- ODTs disintegrate/dissolve in the mouth rapidly without administering extra water, providing the convenience of a tablet formulation while allowing the ease of swallowing provided by a liquid formulation.
- the orally disintegrating tablets comprising eszopiclone can further comprise directly compressible coprocessed excipient.
- PCT Application WO2007052289 describes directly compressible coprocessed excipient comprising of at least one water soluble excipient and water insoluble inorganic excipient such as calcium silicate.
- the water soluble carbohydrate can be a monosaccharide, disaccharide, oligosaccharide or polysaccharide.
- carbohydrates include, but are not limited to, monosaccharides such as sorbitol, glucose, dextrose, fructose, maltose or xylitol, disaccharides such as sucrose, trehalose, lactose, glucose, galactose or mannitol, and oligosaccharides and polysaccharides such as dextrates and maltodextrins.
- the water soluble and water insoluble excipients in the directly compressible coprocessed excipient can be in a ratio of water-soluble excipient to water insoluble excipient of from about 50:1 to about 1 :50. In one embodiment of the present invention, this ratio is about 30:1 to about 1 :30.
- this ratio is from about 20:1 to about 1:20.
- the directly compressible coprocessed excipient is available commercially as PanExceaTM ODT from Mallinckrodt Baker, US.
- the amount of directly compressible co-processed excipient employed in the orally disintegrating tablet compositions comprising eszopiclone and a taste-masking agent is about 5% to about 95 % by weight of the said dosage form.
- the orally disintegrating tablet compositions comprising eszopiclone dissolve or disintegrate in the oral cavity within about 60 seconds.
- the orally disintegrating tablet compositions can be prepared by any of the known non-limiting techniques such as freeze-drying, molding and sublimation, compression, cotton candy process, mass extrusion, etc or with use of specialized excipients such as effervescent couple, highly micronized agents, coprocessed excipients or the like.
- compositions of the present invention may include, in addition to eszopiclone, taste- masking agent and directly compressible co-processed excipient, one or more binders, disintegrants, superdisintegrants, diluents, salivating agents, surfactants, flavors, sweeteners, colorants, diluents, souring agents, viscolizers, glidants or lubricants, solubilizers, or stabilizers.
- superdisintegrants include, but are not limited to, natural, modified or pregelatinized starch, crospovidone, croscarmellose sodium, sodium starch glycolate, low-substituted hydroxypropyl cellulose as well as effervescent disintegrating systems. Further, the disintegrants can be selected from, but not limiting to, crospovidone, calcium silicate and starch. The amount of disintegrant or superdisintegrant employed in the composition is about 2% to about 30 % by weight of the said dosage form.
- binders include, but are not limited to, starch, pregelatinized starch, polyvinyl pyrrolidone (PVP), copovidone, cellulose derivatives, such as hydroxypropylmethyl cellulose (HPMC), hydroxypropyl cellulose (HPC) and carboxymethyl cellulose (CMC) and their salts.
- suitable diluents include, but are not limited to, starch, microcrystalline cellulose, lactose, xylitol, mannitol, maltose, polyols, fructose, guar gum, sorbitol, magnesium hydroxide, dicalcium phosphate, and the like or any combinations thereof.
- lubricant examples include, but are not limited to, magnesium stearate, calcium stearate, stearic acid, talc, and sodium stearyl fumarate.
- the tablet compositions of the invention may also include a glidant such as, but not limited to, colloidal silica, silica gel, precipitated silica, or combinations thereof.
- the compositions of the present invention may also include salivating agents including, but not limited to, micronised polyethylene glycol, sodium chloride or precipitated micronised silica to improve the disintegration properties of the said compositions.
- solubilizers include, but are not limited to cetostearyl alcohol, cholesterol, diethanolamine, ethyl oleate, ethylene glycol palmitostearate, glycerin, glyceryl monostearate, isopropyl myristate, lecithin, medium-chain glyceride, monoethanolamine, oleic acid, propylene glycol, polyoxyethylene alkyl ether, polyoxyethylene castor oil glycoside, polyethylene sorbitan fatty acid ester, polyoxyethylene stearate, propylene glycol alginate, sorbitan fatty acid ester, stearic acid, sunflower oil, triethanolmine.or combinations thereof.
- compositions of the present invention may also include stabilizers including, but not limited to, benzoic acid, sodium benzoate, citric acid, and the like.
- stabilizers including, but not limited to, benzoic acid, sodium benzoate, citric acid, and the like.
- surfactants include, but are not limited to, sodium docusate, glyceryl monooleate, polyethylene alkyl ether, polyoxyethylene sorbitan fatty acid ester, sodium lauryl sulfate, sorbic acid, sorbitan fatty acid ester, and mixtures thereof.
- Souring agents include, but are not limited to, monosodium fumarate and/or citric acid.
- compositions of the present invention may optionally include viscolizers such as polyalkylene oxides; polyols; starch and starch-based polymers; chitosan; polysaccharide gums; polyethylene oxide, hydroxypropyl cellulose, hydroxypropy! methyl cellulose, hydroxyethyl cellulose, sodium carboxy methylcellulose, calcium carboxymethyl cellulose, methyl cellulose, polyacrylic acid, gum acacia, gum tragacanth, xanthan gum, guar gum and polyvinyl alcohol and copolymers and mixtures thereof.
- viscolizers such as polyalkylene oxides; polyols; starch and starch-based polymers; chitosan; polysaccharide gums; polyethylene oxide, hydroxypropyl cellulose, hydroxypropy! methyl cellulose, hydroxyethyl cellulose, sodium carboxy methylcellulose, calcium carboxymethyl cellulose, methyl cellulose, polyacrylic acid, gum acacia, gum trag
- taste-masked eszopiclone is incorporated in bite-dispersion tablets.
- Bite-dispersion tablets are meant to be taken without water and disperse easily, and quickly, after a gentle bite when taken orally enabling the taste- masked eszopiclone to be orally administered.
- These tablets comprise in addition to eszopiclone and taste-masking agent, various pharmaceutically acceptable excipients as have been discussed under orally disintegrating tablets in addition to excipients which may be specifically employed for bite-dispersion tablets.
- taste-masked eszopiclone may also be incorporated in chewable tablets.
- Chewable tablets are taken slowly by chewing or sucking in the mouth, and enable taste-masked active contained therein to be administered without water.
- These chewable tablets comprise in addition to eszopiclone and taste-masking agent, various pharmaceutically acceptable excipients as have been discussed under orally disintegrating tablets in addition to excipients which may be specifically employed for chewable tablets.
- taste-masked eszopiclone is incorporated in effervescent tablets.
- Effervescent tablets are intended to be dissolved or dispersed in water before administration and generally contain acid substances and carbonates or bicarbonates, which react rapidly in the presence of water releasing carbon dioxide. These tablets comprise in addition to eszopiclone and taste-masking agent, various pharmaceutically acceptable excipients as have been discussed under dispersible tablets and orally disintegrating tablets.
- the effervescent tablets can comprise effervescent couples selected from, but not limited to, thermolabile gas generating agents such as sodium bicarbonate, sodium glycine carbonate, potassium bicarbonate, ammonium bicarbonate, sodium bisulfite, sodium metabisulfite, and an acid source such as citric acid, maleic acid or tartaric acid.
- thermolabile gas generating agents such as sodium bicarbonate, sodium glycine carbonate, potassium bicarbonate, ammonium bicarbonate, sodium bisulfite, sodium metabisulfite, and an acid source such as citric acid, maleic acid or tartaric acid.
- taste-masked eszopiclone is incorporated in dispersible tablets.
- Dispersible tablet refers to a tablet which disperses in aqueous phase, e.g. in water before administration.
- a water-dispersible tablet according to the British Pharmacopoeia and European Pharmacopoeia, should meet the requirements of the test for dispersible tablets as regards dispersion time ( ⁇ 3 minutes) and dispersion quality (i.e. to pass through a 710 ⁇ m sieve).
- the dispersible tablet compositions comprising eszopiclone and taste-masking agent can further comprise in addition to pharmaceutically acceptable excipients as disclosed under orally disintegrating tablets, one or more viscolizers.
- viscolizers which can be used include, but are not limited to, polyalkylene oxides such as polyethylene oxide; cellulose ethers such as hydroxyethyl cellulose, hydroxypropylcellulose, hydroxypropyl methyl cellulose, methyl cellulose, ethyl cellulose, sodium carboxy methylcellulose, calcium carboxymethyl cellulose, microcrystalline cellulose; gums such as gum arabic alginates, agar, guar gum, locust bean, carrageenan, tara, gum arabic, tragacanth, pectin, xanthan, gellan, maltodextrin, galactomannan, pusstulan, laminarin, scleroglucan, gum arabic, inulin, karaya, whelan; polyols
- viscolizers include hydroxypropyl cellulose, hydroxypropyl methylcellulose, hydroxyethyl cellulose, polyethylene oxide, sodium carboxy methylcellulose, microcrystalline cellulose, guar gum, xanthan gum, alginates, and combinations thereof.
- the weight percent of the viscolizer in the dosage form is about 2 to about 75 weight percent. In another embodiment of the present invention, the viscolizer in the dosage form is about 10 to about 70 weight percent. In yet another embodiment of the present invention, the viscolizer in the dosage form is about 5 to about 50 weight percent.
- Viscolizers act to control sedimentation rate of dispersed eszopiclone thereby producing homogeneous dispersions when the dispersible tablets are dispersed in water before administration thus ensuring substantially uniform dosing. They rapidly generate viscosity when the dispersible tablets come in contact with water, and a homogenous suspension is formed, which can be easily swallowed by children and the elderly, with minimal effect of the release properties of the biologically active ingredient.
- tablette and “tablet composition” are used synonymously within the context of the present invention. These terms should be construed to include a compacted or compressed powder composition obtained by compressing or otherwise forming the composition to form a solid having a defined shape. Tablets in accordance with the invention may be manufactured using conventional techniques of common tableting methods known in the art such as direct compression, wet granulation, dry granulation and extrusion/ melt granulation. In one embodiment, the process is direct compression which involves compression of taste-masked drug-excipient blend after mixing them for a definite time period. The tablet may vary in shape such as oval, triangle, almond, peanut, parallelogram, round, pentagonal, hexagonal, and trapezoidal. The preferred shapes are round, oval and parallelogram forms.
- the tablets may optionally be coated.
- Surface coating may be employed for aesthetic purposes or for dimensionally stabilizing the dosage form.
- the coating may be carried out using any conventional technique employing conventional ingredients suitable for enteral use.
- a surface coating can for example be in the form of film using conventional polymers such as hydroxypropyl methyl cellulose, hydroxypropyl cellulose, carboxymethyl cellulose, polyvinyl alcohol polymethacrylates and the like.
- the composition may optionally be coated with a functional coat.
- the coat can be employed using polymeric or non-polymeric excipients as described above either alone or in combination, along with plasticizers, colorants, opacifiers etc.
- the taste-masked eszopiclone is incorporated in sprinkle granules, quick melt wafers, lozenge, dry suspensions or syrups for reconstitution, chewing gum, orally dissolvable film or the like.
- process for preparing a pharmaceutical composition for alleviating unpleasant taste associated with eszopiclone therapy comprises: a. treating eszopiclone with at least one taste-masking agent, optionally along with at least one pharmaceutically acceptable excipient to form taste-masked eszopiclone; b. blending taste-masked eszopiclone of step (a) with other pharmaceutically acceptable excipients, except lubricant, to form a uniform powder mix; c. lubricating the powder mix of step (b); and d.
- step (c) forming the powder mix of step (c) or of step (b) into an orally disintegrating tablet, dispersible tablet, effervescent tablet, chewable tablet, sprinkle granules, suspension, powder for suspension, quick melt wafers, lozenge, or chewing gum.
- the various dosage forms of the present invention for alleviation of any immediate bitter taste perceived by a patient on eszopiclone therapy comprising eszopiclone, at least one taste-masking agent and at least one pharmaceutically acceptable excipient are immediate release dosage forms that release taste-masked eszopiclone instantly upon reaching either the stomach or the intestines.
- the in vitro release of eszopiclone is measured by dissolution using 0.1 N hydrochloric acid (HCI) (500 ml) in USP apparatus Il (paddle) at 50 rpm speed.
- HCI hydrochloric acid
- the pharmaceutical composition of the present invention comprising eszopiclone releases not less than 70% of the active within about 45 minutes in vitro.
- the pharmaceutical composition of the present invention comprising eszopiclone releases not less than 80% of the active within about 45 minutes in vitro.
- the formulations disclosed in the present invention can also be adapted to develop a composition wherein taste-masked eszopiclone is released in a controlled manner over a period of time, for example, from about 2 to about 24 hours.
- eszopiclone is treated with polymeric, non-polymeric pharmaceutically acceptable excipients described above or any combinations thereof. The amount of such polymeric or non-polymeric excipients can not only ensure masking of the objectionable taste of the active but also provide any desired control over the release of eszopiclone.
- pellets, granules or the like of eszopiclone are prepared comprising at least one release retardant in combination with one or more pharmaceutically acceptable excipients.
- Suitable release retardants can be polymeric or non-polymeric pharmaceutically acceptable excipients or agents and include, but are not limited to, cellulose ethers, such as hydroxypropylmethylcellulose (HPMC), hydroxypropylcellulose (HPC), hydroxyethylcellulose, ethyl cellulose and carboxymethylcellulose sodium; polysaccharides, such as carageenan, guar gum, xanthan gum, tragacanth and ceratonia; polymethacrylates, such as copolymers of acrylic and methacrylic acid esters containing quarternary ammonium groups; cellulose esters, such as cellulose acetate; acrylic acid polymers, such as carbomers; waxes, such as hydrogenated castor oil, hydrogenated vegetable oil, carnauba wax and micro
- pellets or granules or the like can be further coated using excipients described above in order to achieve taste-masking and controlled release of eszopiclone.
- the amount of release retardant in the formulation is from about 1 to about 90% by weight of the dosage form. In one embodiment, the amount of release retardant in the formulation is about 5 to about 80% by weight of the dosage form.
- compositions devoid of the active agent that alleviate the bitter or metallic or unpleasant taste experienced by a patient after administration of eszopiclone.
- Such placebo compositions devoid of the active agent comprise various taste-masking agents and/or pharmaceutically acceptable excipients described above.
- these placebo compositions can be provided in the form of orally disintegrating tablet, chewable tablet, bite-dispersion tablet, dispersible tablet, effervescent tablet, chewing gum, orally dissolvable film, quick melt wafers, lozenge, buccal tablet, immediate release tablet or the like, as discussed above, and can be prepared by any of the aforementioned methods and processes.
- the placebo compositions when in the form of orally disintegrating tablets, bite- dispersion tablets, chewable tablets, dispersible tablets, effervescent tablets or the like comprise sweeteners, flavourants, taste-modifiers or any taste-masking agents including, but not limited to, polymeric or non-polymeric pharmaceutically acceptable excipients, cyclodextrins, carbomers, adsorbents, pH modifiers or the like described above.
- the placebo composition may be in the form of an orally dissolvable film or a strip dosage form comprising at least one film-forming polymer, flavor and sweetener.
- the film forming polymer includes, but is not limited to, methyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose, ethyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, carboxymethyl cellulose, cellulose acetate phtalate, cellulose acetate butyrate, amylose, dextran, casein, pullulan, gelatine, pectin, agar, carrageenan, xanthan gum, tragacanth, guar gum, acacia gum, arabic gum, polyethylene glycol, polyethylene oxide, polyvinyl pyrrolidone, polyvinyl alcohol, carboxyvinyl polymers, sodium alginate, polyacrylic acid, methylmethacrylate, or the like or any combinations thereof.
- compositions can optionally comprise any additional mucoadhesive agent including, but not limited to, chitosan, carbopol or the like.
- any additional mucoadhesive agent including, but not limited to, chitosan, carbopol or the like.
- the placebo composition may be in the form of chewing gum that comprises at least one chewing gum base along with sweeteners or flavourants.
- Chewing gum base that may be employed includes, but is not limited to, styrene-diene block copolymers, isobutylene-isoprene copolymers or polymers such as polyethylene, polyisobutylene or polyvinyl acetate, or their derivatives or combinations thereof.
- the placebo composition may be in the form of a buccal tablet.
- the tablet is held between the cheek and the gum or adhered to buccal mucosa and may be designed to alleviate bitter or unpleasant taste over a prolonged period of time.
- Such compositions may comprise in addition to sweeteners, flavourants, pH-modifiers or taste-modifiers, mucoadhesive agents including, but not limited to, chitosan, carbopol, or the like, that can hold the tablet in the oral cavity for a longer duration of time.
- the placebo composition comprises a displacing agent which competitively displaces any eszopiclone from the taste receptors so that the bitter taste is not experienced.
- the displacing agent in one embodiment may be a taste-modifier such as, but not limited to, hydrogenated castor oil (Cremophor RH40), thaumatin, arginine, ornithine, lysine, citrulline, adenosine 5" monophosphate, thymidine 5 1 monophosphate, adenosine 5' diphosphate, adenosine 3' monophosphate, adenosine ⁇ '-succinate, adenosine 5' triphosphate, adenosine 2' monophosphate, 5'-cytidylic acid, inosinic acid, monellin, ferulic acid or caffeic acid or the like or any combinations thereof.
- a taste-modifier such as, but not limited to, hydrogenated castor
- Placebo composition is provided to counter the bitter or metallic after taste either instantaneously or over a prolonged period of time.
- the placebo composition in such a case can release the sweeteners, flavors, pH-modifier or taste-modifying agents either immediately or over a controlled manner in the oral cavity of the patient based on the level of polymers and other ingredients used in the placebo composition.
- the present invention also relates to a method of countering the unpleasant taste experienced by a patient after administration of eszopiclone comprising administering to the patients in need thereof compositions devoid of the active agent that alleviate the bitter or unpleasant after-taste either along with or subsequently at an appropriate time after administration of a composition wherein the objectionable taste of eszopiclone has been masked.
- bitter or metallic or unpleasant after taste reported subsequent to the administration of eszopiclone compositions as mentioned in the present invention intends to encompass any unpleasant taste perceived by a patient receiving eszopiclone compositions either within a minute after the intake of the eszopiclone formulation or after a period of time ranging from about one minute to about 24 hours after the consumption of the eszopiclone composition.
- appropriate time refers to the time when the patient who has been administered eszopiclone composition perceives a bitter, unpleasant or metallic after-taste, which could be any time ranging from within about one minute to about 24 hours after administration or consumption of the eszopiclone medication.
- the present invention also relates to methods of alleviating the immediate bitter or unpleasant taste as well as the objectionable after-taste associated with eszopiclone therapy.
- Such a method comprises administering to the patient in need thereof compositions of the present invention wherein the objectionable taste of eszopiclone has been masked, to alleviate any immediate bitterness generally associated with the drug, followed by optional administration of a composition devoid of the active either along with or subsequently at an appropriate time after the administration of the eszopiclone formulation, to counter any bitter or unpleasant or metallic after-taste reported subsequent to the administration of eszopiclone.
- the method of alleviating unpleasant taste associated with eszopiclone therapy comprises administering to the patient in need thereof a pharmaceutical composition comprising eszopiclone, at least one taste-masking agent and at least one pharmaceutically acceptable excipient to alleviate any immediate bitterness otherwise associated with oral intake of eszopiclone followed by optional administration of at least one placebo composition devoid of the eszopiclone either along with or subsequently at an appropriate time after the administration of the pharmaceutical composition comprising taste-masked eszopiclone to counter unpleasant after-taste reported subsequent to the administration of eszopiclone.
- compositions of the present invention for alleviating bitter or unpleasant taste associated with eszopiclone therapy are provided in the form of a kit.
- a kit may comprise a composition wherein the objectionable taste of eszopiclone has been masked and a placebo composition devoid of the active agent along with instructions for their use either together or separately, wherein when consumed separately the placebo composition is taken at an appropriate time subsequent to the intake of taste-masked eszopiclone composition.
- the appropriate time as discussed above can vary from patient to patient and could be any time ranging from within about one minute to about 24 hours after administration or consumption of the taste-masked eszopiclone medication.
- a kit for alleviating unpleasant taste associated with eszopiclone therapy comprises (a) a pharmaceutical composition comprising eszopiclone; at least one taste-masking agent; and at least one pharmaceutically acceptable excipient; (b) a placebo composition devoid of eszopiclone, and (c) instructions for use of (a) and (b) either together or separately.
- one or more than one unit placebo dosage forms can be provided or taken, subsequently after administration of taste-masked eszopiclone composition to counter the bitter or unpleasant after-taste experienced by patients consuming the medication.
- the taste-masked eszopiclone composition and the placebo composition can be packed together or separately in a suitable primary pack for the ease of administration of the patient.
- a kit for alleviating unpleasant taste associated with eszopiclone therapy comprises (a) a pharmaceutical composition comprising eszopiclone that releases eszopiclone in a controlled manner over a period of about 2 to about 24 hours; (b) a placebo composition devoid of eszopiclone, and (c) instructions for use of (a) and (b) either together or separately.
- the taste-masked eszopiclone composition to counter any immediate bitter taste experienced by the patient upon administration of eszopiclone and the placebo composition to alleviate any bitter or unpleasant taste perceived after administration of eszopiclone can be administered together in the form of a dosage form, such as a bilayer tablet.
- the placebo composition in such a bilayered tablet can optionally comprise an additional mucoadhesive component including, but not limited to, carbopol, chitosan, or the like, that help the dosage form to remain adhered to the buccal mucosa and release the flavor or sweetener slowly such that unpleasant taste or bitter after taste due to eszopiclone is not experienced.
- the taste-masked eszopiclone component of such a bilayered dosage form dissolves rapidly thereby delivering the taste-masked drug to the patient.
- the taste-masked eszopiclone compositions may be adapted to deliver one or more active agents in addition to eszopiclone.
- the active agent includes, but is not limited to, omeprazole, hydroxyomeprazole, esomeprazole, tenatoprazole, lansoprazole, pantoprazole, rabeprazole, dontoprazole, dontoprazole, dontoprazole, dontoprazole, dontoprazole, dontoprazole, dontoprazole, donprazole, periprazole, ransoprazole, pariprazole, leminoprazole, acetaminophen, ibuprofen, aspirin, naproxen, doxepin, escitalopram, milnacipran, melatonin, agomelatine, GR196429, S20242, S23478, S24268, S25150, BMS-214778,
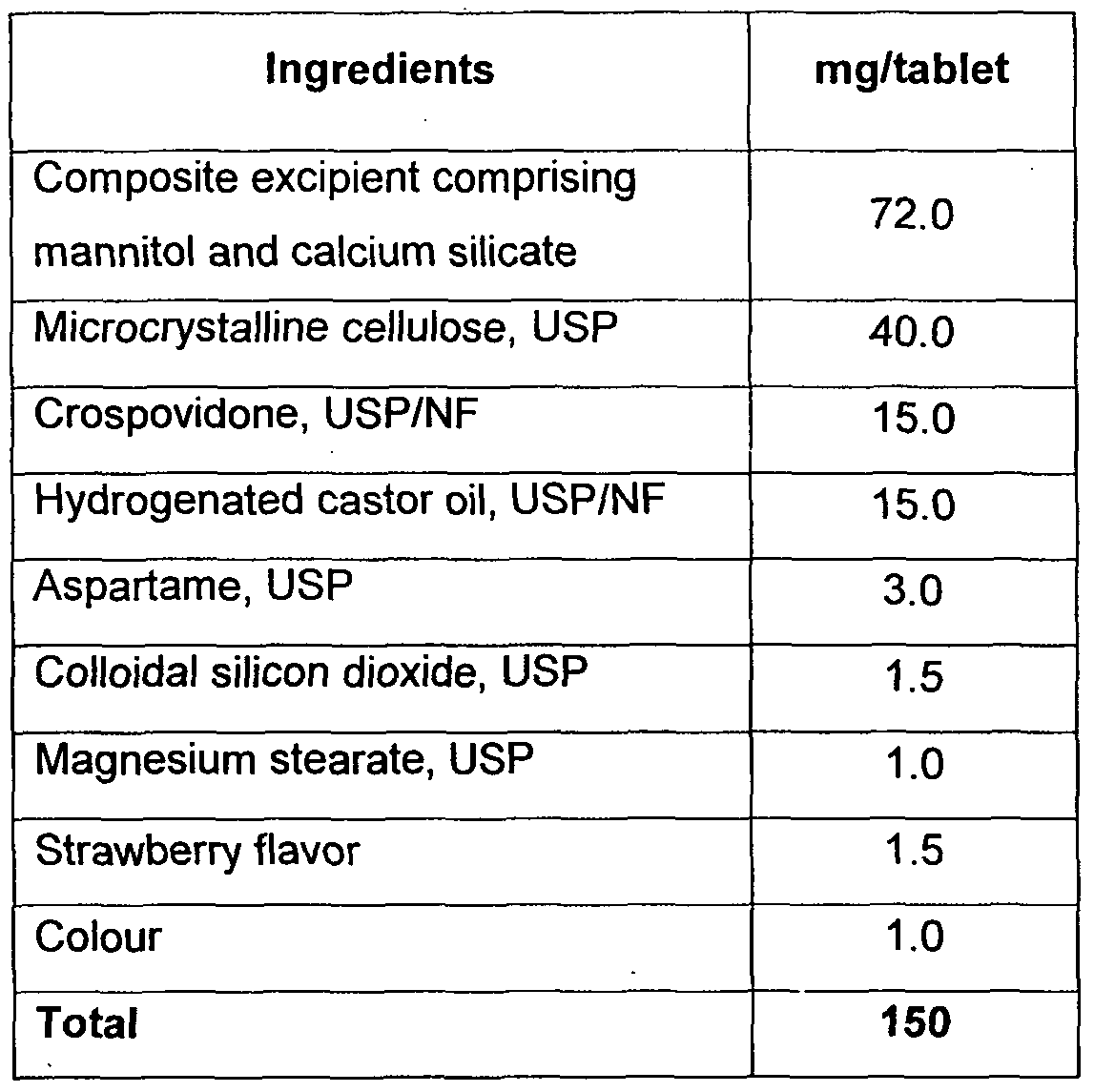
- Example 1 Taste-masked eszopiclone orally disintegrating tablets
- Table 1 Composition of orally disintegrating tablets of eszopiclone
- Eszopiclone was dry mixed with glyceryl behenate and then blended with other excipients, except lubricant, in a blender to get a uniform powder mix.
- the blend was lubricated and compressed to form tablets with the following parameters:
- the tablets had acceptable taste and pleasant mouth feel.
- Example 2 Taste-masked eszopiclone bite-dispersion tablets
- Eszopiclone was blended with microcrystalline cellulose (1 :2 parts).
- Coating solution was prepared by melting hydrogenated vegetable oil in water bath and adding glyceryl mono- & dicaprate in molten wax. Aspartame was dissolved in a hot solution of hypromellose and this aqueous phase was added to above oily phase. The system was homogenized and cooled to room temperature, to which flavor was added to get a coating solution. Drug blend was coated with coating composition using top spray assembly to weight gain of 40 %.
- Table 4 Composition of bite -dispersion tablets of eszopiclone
- the coated taste-masked eszopiclone granules were blended with other excipients like spray dried mannitol, microcrystalline cellulose, sodium starch glycolate, citric acid, aspartame, colloidal silicon dioxide, talc and flavor and finally lubricated using magnesium stearate.
- the blend was compressed to get a bite-dispersion tablet.
- the tablets had pleasant and acceptable mouth feel along with desirable disintegration time.
- Example 3 Kit comprising taste masked eszopiclone formulation and placebo orally disintegrating tablet composition
- Eszopiclone was thoroughly mixed with basic butylated methacrylate copolymer and granulated using povidone dissolved in water. These granules were dried and then blended with other excipients, except lubricant, to get a uniform mass. The mass was lubricated and compressed into tablets having the following parameters:
- Eszopiclone orally disintegrating tablets with desirable pleasant taste and acceptable wicking time were obtained.
- Table 6 Composition of placebo orally disintegrating tablet
- the placebo orally disintegrating composition was consumed by the patient after half an hour subsequent to the intake of taste-masked eszopiclone.
- the placebo was pleasant to taste and could mask unpleasant bitter taste perceived due to administration of eszopiclone formulation.
- Example 4 Kit comprising taste masked eszopiclone and placebo buccal tablet composition
- Eszopiclone was layered on non-pareil beads and these drug-loaded beads were further coated with a combination of ethyl cellulose and hydroxypropyl methylcellulose (20:80) to a weight gain of about 20%.
- Table 7 Composition of orally disintegrating tablet of eszopiclone
- Table 8 Composition of placebo buccal tablet
- the placebo buccal tablet composition was adhered to the buccal mucosa by the patient subsequent to the administration of eszopiclone formulation while going to bed.
- the tablet slowly and gradually releases the sweetener, flavor and taste modifying agent such that the bitter taste of drug due to its excretion in saliva is not experienced in the morning.
- Example S Kit comprising taste masked eszopiclone and placebo chewing gum composition
- Table 9 Composition of taste masked eszopiclone granules
- Table 10 Composition of orally disintegrating tablets of eszopiclone
- Eszopiclone granules were blended with other excipients and the lubricated blend was compressed to form tablets with the following parameters:
- butadiene Styrene 50:50 Rubber was melted and collected in a vessel having molten polyvinyl acetate and microcrystalline wax at a temperature of about 80 0 C. This was mixed with butyl hydroxy toluene and stirred well until it had the consistency of a thick syrup. This mass was filtered through a fine mesh screen. The clear base was then mixed well with glyceryl monostearate under hot conditions. The mixture was further mixed with calcium carbonate and sucralose. Flavor and color were added to the above mass at low temerature. The homogenized mixture was then poured onto cooling belts and cooled with cold air. The cooled mass was cut into square shape chunks and packed in suitable packing wrapper.
- the placebo chewing gum composition was consumed by the patient in the morning when the unpleasant taste of eszopiclone was felt.
- the placebo composition was chewed over extended time period as desired by the patient and it was pleasant to taste and could mask unpleasant bitter taste perceived due to administration of eszopiclone formulation.
- Example 6 Taste-masked eszopiclone orally disintegrating tablets
- Table 12 Composition of orally disintegrating tablets of eszopiclone
- Eszopiclone beads of controlled size and density were prepared using the melt extrusion technique by passing / forcing a molten mixture of eszopiclone, microcrystalline cellulose and glyceryl behenate through a fine nozzle. The extruded mass was then cut and shaped, in a spheronization process, to produce beads suitable for formulation as controlled release multiparticulates. The resultant beads were further coated with Kollicoat ® SR 30 D to a weight gain of 20% by weight for additional release rate control and taste masking. These taste masked eszopiclone beads was mixed with other excipients and compressed into orally disintegrating tablets. Tablets with desired taste and disintegration time were obtained.
Abstract
The present invention relates to oral pharmaceutical compositions that alleviate the bitter, unpleasant or metallic taste associated with eszopiclone therapy. The invention relates to pharmaceutical compositions comprising eszopiclone, at least one taste-masking agent and at least one pharmaceutically acceptable excipient. This taste-masked eszopiclone formulation that masks the immediate unpleasant or objectionable taste of eszopiclone is provided in dosage forms suitable for oral administration such as orally disintegrating tablets, bite-dispersion tablets, dispersible tablets, or the like. The invention further relates to a method of countering this unpleasant after-taste of eszopiclone comprising administering to the patients in need thereof a composition devoid of the active agent either along with or subsequently at an appropriate time after administration of taste-masked eszopiclone.
Description
PHARMACEUTICAL COMPOSITIONS FOR ALLEVIATING UNPLEASANT TASTE
Field of the Invention
The present invention relates to oral pharmaceutical compositions that alleviate the bitter, unpleasant or metallic taste associated with eszopiclone therapy. Particularly, the invention relates to pharmaceutical compositions that mask the unpleasant or objectionable taste of eszopiclone and alleviate the immediate bitter taste associated with eszopiclone therapy comprising eszopiclone, at least one taste-masking agent and at least one pharmaceutically acceptable excipient. The invention also relates to compositions devoid of the active agent that alleviate the bitter, metallic or unpleasant taste experienced by a patient after administration of eszopiclone. Further the compositions of the present invention are suitable for oral administration in the form of orally disintegrating tablets, bite-dispersion tablets, dispersible tablets, effervescent tablets or the like. Methods of countering the unpleasant after-taste of eszopiclone comprising administering to the patients in need thereof a composition devoid of the active agent either along with or subsequently at an appropriate time after administration of taste-masked eszopiclone formulation are also provided.
Background of the Invention
Insomnia or sleeplessness, or disturbed sleep, is a common condition affecting millions of people worldwide. It is characterized by persistent difficulty in falling asleep or staying asleep despite the opportunity. Women have approximately 1.5 times higher risk of insomnia than men and the overall prevalence of insomnia increases with age. The most common complaints related to insomnia are difficulty in initiating sleep (sleep-onset insomnia), difficulty in maintaining sleep due to prolonged nocturnal awakenings (sleep- maintenance insomnia), early morning awakenings (sleep-offset insomnia), and excessive daytime sleepiness resulting from lack of sleep. Insomnia may be primary (caused by biologic or psychosocial mechanisms independent of a disease process) or secondary (brought on or exacerbated by a disease process, disorder or substance). Secondary insomnia may constitute up to 70% of sleep problems seen in the general population. Further insomnia can be (a) transient insomnia which lasts from days to weeks (b) acute insomnia with inability to consistently sleep well for a period of between three weeks to six months or (c) chronic insomnia that lasts for years at a time, with various primary or secondary causes for each.
Insomnia is generally managed using both non-pharmacologic and pharmacologic interventions, and where possible the treatment is directed to underlying cause of the condition. Pharmacotherapy has been found to be effective in the management of insomnia. Benzodiazepines have been the drugs of choice for the treatment of insomnia over the past three decades. But they have abuse potential and are capable of causing changes in sleep structure, as well as physical dependence, tolerance, anterograde amnesia, respiratory depression, cognitive and psychomotor impairement, rebound insomnia and withdrawal reactions on discontinuation. Due to these effects, non- benzodiazepines having shorter half lives, limited duration of action and reduced affinity for benzodiazepine receptors were developed and are preferred.
Eszopiclone, (+)-(5S)-6-(chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrroIo[3,4-b] pyrazin- 5-yl 4-methyl- piperazine-1-carboxylate, is a non-benzodiazepine hypnotic agent that belongs to the class of drugs known as cyclopyrrolones. This class of compounds appears to cause less residual sedation than the benzodiazepines and provide an improved therapeutic index over them. Eszopiclone is the S-isomer of racemic zopiclone and is indicated for the treatment of transient and chronic insomnia in adult patients ≥18 years of age. Although the exact mechanism of action of eszopiclone is unknown, its effects are thought to be related to its interaction with gamma aminobutyric acid (GABA) receptor complexes at binding sites located close or allosterically coupled to benzodiazepine receptors (Pandi-Perumal et al., Sleep Disorders: Diagnosis and Therapeutics, Chapter 29, p. 324, lnforma UK Ltd, 2008).
Eszopiclone, sold under the brand name Lunesta® in the U.S., received approval from the FDA in December 2004. U.S Patent Nos. 6,319,926, 6,444,673, 6,864,257 and 7,381 ,724 disclose the molecule and its use as a tranquilizer, sedative and hypnotic, mainly for improving sleep quality or time.
Eszopiclone, generally administered at bedtime for treatment of insomnia, is rapidly absorbed after oral administration. Peak plasma concentrations are achieved within approximately 1 hour after oral administration. It is widely distributed in body tissues, including brain, and is eliminated in urine, saliva and breast milk. Approximately 52% to 59% of a dose is weakly bound to plasma protein. The elimination half-life of eszopiclone is approximately 6 hours and it is extensively metabolized by oxidation and
demethylation. Cytochrome P450 (CYP) isozymes CYP3A4 and CYP2E1 are involved in the metabolism of eszopiclone. The primary plasma metabolites are CS^-zopiclone-N- oxide and (Sj-N-desmethyl zopiclone. (Lunesta - Pack insert, Marlborough, MA, Sepracor, December 2004).
The efficacy of eszopiclone has been evaluated in healthy adults, including elderly patients, for the treatment of transient and chronic insomnia. Compared with placebo, eszopiclone has been shown to considerably reduce sleep induction, improve sleep maintenance, duration, quality, and depth, as well as next-day functioning. Further, U.S. Patent No.6,436,936 discloses that (+) zopiclone is also useful in treating convulsive disorders such as epilepsy, anxiety, aggressive behavior, muscle spasms, behavioral disorders, depression, schizophrenia or endocrine disorders.
Eszopiclone has an extremely bitter and unpleasant taste. Reviews from patients consuming eszopiclone indicate that bitter, metallic or unpleasant taste is felt immediately on consuming the medication. Further, similar unpleasant taste is also experienced after administration of the active over a time period ranging from about a few minutes to a few hours or persists even longer and is felt the next day as well. Consumption of this medication is also said to result in dysguesia or altered sensation of taste, often unpleasant taste. Unpleasant after-taste is generally considered as the most common adverse effect with eszopiclone administration.
The incidence of treatment-emergent adverse events was evaluated in placebo- controlled Phase III studies in elderly patients (age range, 65-86 years) which indicated that the incidence of unpleasant taste occurred in a dose-response manner (8% eszopiclone 1 mg, 12% eszopiclone 2 mg) and no unpleasant taste was reported with placebo. This observation was also supported by another Phase III placebo-controlled study in non-elderly adults for 6 weeks which also suggested dose-response relationship for unpleasant taste (2mg: 16.3%; 3mg: 33.3%; placebo: 3.0%) (Jadwiga Najib, Clinical Therapeutics, 28(4), 491-516 (2006)). Overall, the most frequently reported adverse event associated with eszopiclone administration was bitter after taste, and similar taste disturbances (bitter or metallic taste, taste perversion) that have also been reported with racemic zopiclone. A study conducted by G. Hempel and G. Blaschke, showed that a bitter taste that occurred after 7.5 mg zopiclone dose is due to excretion of zopiclone in
the saliva which was confirmed by analysis of saliva samples (G. Hempel et a/, Journal of Chromatography B, 675, 139-146 (1996)). In another study involving administration of 7.5 mg zopiclone, the volunteers reported a bitter taste at an average of 24 minutes after zopiclone administration at which time concentrations in saliva were approximately 50ng/ml. (G. Caille et al, Biopharmaceutics and Drug Disposition, 5(2), 117-125 (1983)). Thus excretion of eszopiclone occurs in saliva and renders the bitter taste perceived after administration of the drug.
Eszopiclone is generally well tolerated; however discontinuation of therapy due to adverse events has been reported in some patients. In placebo controlled, parallel- group, Phase III trials in the elderly, drug discontinuation due to adverse events occurred in 1.4% of 72 patients receiving eszopiclone 1 mg, 2.3% of 215 patients receiving eszopiclone 2 mg, and 3.8% of 208 patients receiving placebo. The most commonly reported adverse event in these trials was headache (15%, 13%, and 14%, respectively), followed by unpleasant taste (8%, 12%, and 0%) (Jadwiga Najib, Clinical Therapeutics, 28(4), 491-516 (2006)).
Thus prior art indicates that use of eszopiclone is associated with adverse events of bitter or unpleasant taste which has in many instances also lead to discontinuation of therapy. The unpleasant taste with eszopiclone is experienced at different time intervals by different patients. Some patients experience the bitterness immediately while some patients who consume eszopiclone at night, experience bitter taste not only immediately upon administration of the drug but also in the mornings. Still another group of patients experience the bitter after-taste immediately and over a period of time after administration and in the mornings and throughout the next day until the next dose as well. Further, the use of such a drug in the conventional swallow tablet formulation combined with the unpleasant/bitter taste or dysguesia or taste alteration that it produces over a longer period of time, can lead to abatement of the process of management of insomnia. Moreover such a dosage form may not be suitable for geriatric or pediatric populations who may have difficulty in swallowing tablets or capsules. A need thus exists to provide patient friendly dosage forms that alleviate the bitter or unpleasant or metallic taste associated with eszopiclone therapy. A need also exists to provide a method of alleviating the immediate bitter or unpleasant taste as well as the objectionable aftertaste reported subsequent to the administration of eszopiclone compositions.
Attempts have been made in the prior art to provide zopiclone in the form of dosage forms that disintegrate or dissolve rapidly in an aqueous medium or in saliva and are easy to administer. U.S. Patent No.5,206, 025 discloses porous, unitary freeze-dried, solid pharmaceutical dosage forms that consist of an inclusion compound comprising active substance and cyclodextrin; and at least one substance chosen from diluents or binders. Freeze-drying employed herein, however, is a cumbersome, lengthy and an expensive process that can present challenges during scale-up of the formulation. Further, such porous freeze-dried pharmaceuticals tend to lack mechanical strength and fracture easily during handling and packaging.
There thus exists a need to develop oral pharmaceutical compositions for alleviating the unpleasant taste associated with eszopiclone therapy by simple, cost-effective, easy to scale-up processes. The process employed to provide desired reduction in the perception of bitter taste should not alter the desired disintegration or dissolution requirements. It is also required that oral formulations for reducing bitterness be presented in dosage forms such as orally disintegrating tablets, bite-dispersion tablets, dispersible tablets, effervescent tablets or the like that are easy to administer and increase patient compliance. Need also exists to provide a method of countering the unpleasant after taste experienced by a patient post administration of eszopiclone. Thus an urgent need exists for patient friendly, pharmaceutical compositions with excellent taste characteristics, which alleviate both the immediate bitter taste as well as the unpleasant after-taste, associated with eszopiclone therapy.
After rigorous experimentation, it was surprisingly found that it is possible to mask the unpleasant or objectionable taste of eszopiclone without the use of any expensive or specialized equipment or process. Such taste-masked eszopiclone can be then incorporated into palatable formulations suitable for oral administration such as orally disintegrating tablets, bite-dispersion tablets, dispersible tablets, effervescent tablets or the like which alleviates any immediate bitterness normally experienced by the patient on oral intake of eszopiclone. Such formulations also have adequate mechanical strength, desired taste and in vitro release profile. Further, the present invention provides compositions devoid of the active agent that alleviate the bitter or unpleasant after-taste experienced by a patient after administration of eszopiclone. The inventors after in-depth
research have also arrived at a method of alleviating the bitter or metallic or unpleasant after taste reported subsequent to the administration of eszopiclone comprising administering to a patient in need thereof a composition devoid of the active agent either along with or subsequently at an appropriate time.
Summary of the Invention
The present invention relates to oral pharmaceutical compositions that alleviate the bitter, unpleasant or metallic taste associated with eszopiclone therapy. Particularly, the present invention relates to pharmaceutical compositions for alleviating immediate unpleasant taste associated with eszopiclone therapy comprising eszopiclone, at least one taste-masking agent and at least one pharmaceutically acceptable excipient. The invention also relates to compositions devoid of the active agent that alleviate the bitter, metallic or unpleasant taste experienced by a patient after administration of eszopiclone. The invention further provides compositions of the present invention for alleviating the unpleasant taste of eszopiclone for oral administration in the form of orally disintegrating tablets, bite-dispersion tablets, dispersible tablets, effervescent tablets or the like. Further a kit and method of alleviating unpleasant taste associated with eszopiclone therapy is also provided. Particularly, a method of alleviating unpleasant taste associated with eszopiclone therapy comprising administering to the patient in need thereof a taste- masked eszopiclone composition to alleviate any immediate bitterness associated with oral intake of eszopiclone followed by optional administration of at least one placebo composition devoid of the eszopiclone either along with or subsequently at an appropriate time after the administration of the taste-masked eszopiclone composition to counter unpleasant after-taste reported subsequent to the administration of eszopiclone is provided.
Detailed Description of the Invention
Palatability and mouth feel are important factors for achieving total compliance of patients who are being administered the unpleasant, disagreeable or objectionable tasting active pharmaceutical agent. Several processes of masking the taste of actives are known. Not all taste-masking technologies, however, work with every drug. Processes and technologies employed to mask the taste of an active can, in certain instances, interfere with disintegration, affect stability, provide inadequate taste-masking for a given active or interfere with the bioavailability or pharmacokinetic properties of the
drug. Therefore, it becomes important to develop a process for masking the taste of an active agent that not only enhances the organoleptic properties of the dosage form containing the same, but also does not interfere with the bioavailability of the drug. Further, patient compliance is also an important factor when using an active agent which is excreted in the saliva leading to the perception of bitter or unpleasant taste, which sometimes lasts for several hours after administration of the active.
The present invention provides compositions for reducing the bitterness associated with eszopiclone therapy. The present invention provides compositions to alleviate immediate bitter taste normally experienced by a patient on oral intake of eszopiclone, wherein the bitter or unpleasant taste of eszopiclone is masked and taste-masked eszopiclone is incorporated into palatable formulations that have desired taste without compromising on the in-vitro profile and manufactured by using simple, cost-effective processes. Such processes of masking the unpleasant taste of eszopiclone have also been provided herein. Particularly the present invention provides a pharmaceutical composition for alleviating unpleasant taste associated with eszopiclone therapy comprising (a) eszopiclone; (b) at least one taste-masking agent; and (c) at least one pharmaceutically acceptable excipient. The present invention also provides compositions devoid of the active agent for alleviating any unpleasant after-taste associated with eszopiclone therapy and methods for using the compositions of the present invention. The present invention thereby provides compositions and methods of alleviating bitter or unpleasant taste experienced by a patient after eszopiclone administration.
The term eszopiclone as used in the present invention encompasses the (S) - form of racemic zopiclone, its pharmaceutically acceptable salts, prodrugs, solvates, hydrates, polymorphs, enantiomer, metabolites, clathrates or derivatives thereof. Pharmaceutically acceptable salt refers to salts prepared from pharmaceutically acceptable non-toxic acids, including organic acids or inorganic acids. Suitable non-toxic acids include inorganic and organic acids such as, but not limited to, acetic, alginic, anthranilic, benzenesulfonic, benzoic, citric, ethenesulfonic, camphorsulfonic, fumaric, furoic, gluconic, glutamic, glucorenic, galacturonic, glycidic, hydrobromic, hydrochloric, formic, isethionic, lactic, maleic, malic, mandelic, nitric, pamoic, pantothenic, methanesulfonic, mucic, phenylacetic, propionic, phosphoric, salicylic, sulfanilic, sulfuric, stearic, succinic, tartaric acid, p-toluenesulfonic and the like. Further, the various features and
embodiments of the present invention can also be applied to other stereoisomers of eszopiclone, racemic or stereoisomeric mixtures of eszopiclone and its stereoisomers like zopiclone, their pharmaceutically acceptable salts, prodrugs, solvates, hydrates, polymorphs, enantiomer, metabolites, clatharates or derivatives thereof.
A pharmaceutically effective amount of eszopiclone is employed in the formulations of the present invention. The term "effective amount" refers to an amount effective to achieve desired therapeutic and/or beneficial effect. Therapeutic or beneficial effect may be desired in various conditions such as insomnia, epilepsy, anxiety, aggressive behavior, muscle spasms, behavioral disorders, depression, schizophrenia, movement disorders, gastric disorders, cognitive disorders or endocrine disorders. In one embodiment the concentration of eszopiclone in the formulation can vary from about 0.01 weight % to about 60 weight % based on the total weight of the composition. In another embodiment the concentration of eszopiclone in the formulation can vary from about 0.05 weight % to about 50 weight % based on the total weight of the composition. In a still another embodiment the concentration of eszopiclone in the formulation can vary from about 0.1 weight % to about 35 weight % based on the total weight of the composition. In one embodiment the formulations of the present invention may be administered at a dose of about 0.001 mg to about 50 mg of eszopiclone. In another embodiment the formulations of the present invention may be administered at a dose of about 0.01 mg to about 50 mg of eszopiclone. In yet another embodiment the formulations of the present invention may be administered at a dose of about 0.05 mg to about 40 mg of eszopiclone.
Eszopiclone may be in the form of, but not limited to, powder, granules, pellets, beads, minitablets or the like. Eszopiclone granules may be prepared by wet granulation, dry granulation or roll compaction or the like. In one aspect of the present invention, pellets of eszopiclone may be prepared using extrusion spheronization. In another aspect of the present invention, eszopiclone can be loaded on an inert carrier before taste-masking. The inert carrier can be selected from, but not limiting to, beads, pellets, spheres or similar particles that do not contain an active ingredient. Non-limiting examples of inert carriers include microcrystalline cellulose, sugar or silicon dioxide. In a yet another embodiment, eszopiclone, in the powder form, may be treated with a taste-masking agent.
Further, taste-masking agent employed in the compositions of the present invention include, but are not limited to, a polymeric pharmaceutically acceptable excipient, a non- polymeric pharmaceutically acceptable excipient, an adsorbent, a carbomer, an ion- exchange resin, a cyclodextrin or a derivative thereof, or a pH-modifier, or a combination thereof. The bitter taste of eszopiclone according to the present invention is masked by a process of treating the drug with at least one taste-masking agent. The term "treating" or "treatment" or "treated" as used in the present invention in the context of taste-masking encompasses any method known to a person skilled in the art whereby the bitter or objectionable taste of the active can be masked using pharmaceutically acceptable taste-masking agent, such as but not limited to, coating, physical mixing, melt granulation, complexation, adsorption or the like.
In one embodiment, taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is a polymeric pharmaceutically acceptable excipient or non-polymeric pharmaceutically acceptable excipient or any combination thereof. In another embodiment, the bitter taste of the active is masked by coating or by physical mixing using as taste-masking agents polymeric or non-polymeric pharmaceutically acceptable excipients or any combinations thereof.
Polymeric pharmaceutically acceptable excipients for taste-masking eszopiclone as employed in the present invention include, but are not limited to, cellulose derivatives, saccharides or polysaccharides, polyhydric alcohols, poly(oxyethylene)- poly(oxypropylene) block copolymers (poloxamers), vinyl derivatives or polymers or copolymers thereof, acrylic acid derivatives or the like or any combinations thereof.
Cellulose derivatives include, but are not limited to, ethyl cellulose, methylcellulose, hydroxypropylmethylcellulose (HPMC), hydroxypropyl cellulose (HPC), hydroxyethyl cellulose, hydroxymethyl cellulose, hydroxypropyl ethylcellulose, carboxymethylethyl cellulose, carboxy ethylcellulose, carboxymethyl hydroxyethylcellulose, hydroxyethylmethyl carboxymethyl cellulose, hydroxyethyl methyl cellulose, carboxymethyl cellulose, methylhydroxyethyl cellulose, methylhydroxypropyl cellulose, carboxymethyl sulfoethyl cellulose, sodium carboxymethyl cellulose, cellulose acetate, cellulose acetate phthalate, cellulose acetate butyrate, hydroxypropylmethylcellulose
acetate succinate, hydroxypropylmethylcellulose phthalate, hydroxymethyl ethylcellulose phthalate, cellulose acetate phthalate, cellulose acetate succinate, cellulose acetate maleate, cellulose acetate trimelliate, cellulose benzoate phthalate, cellulose propionate phthalate, methylcellulose phthalate, ethylhydroxy ethylcellulose phthalate, or combinations thereof.
Saccharides or polysaccharides include, but are not limited to, guar gum, xanthan gum, gum arabic, tragacanth or combinations thereof. Polyhydric alcohols include, but are not limited to, polyethylene glycol (PEG) or polypropylene glycol. Vinyl derivatives, polymers and copolymers thereof include, but are not limited to, polyvinylacetate aqueous dispersion (Kollicoat® SR 30D)1 copolymers of vinyl pyrrolidone, copolymers of polyvinyl alcohol (Kollicoat® IR), polyvinyl alcohol phthalate, polyvinylacetal phthalate, polyvinyl butylate phthalate, polyvinylacetoacetal phthalate, polyvinylpyrrolidone (PVP)1 or combinations thereof.
Acrylic acid derivatives include, but are not limited to, methacrylic acids, polymethacrylic acids, polyacrylates, especially polymethacrylates like a) copolymer formed from monomers selected from methacrylic acid, methacrylic acid esters, acrylic acid and acrylic acid esters b) copolymer formed from monomers selected from butyl methacrylate, (2-dimethylaminoethyl)methacrylate and methyl methacrylate c) copolymer formed from monomers selected from ethyl acrylate, methyl methacrylate and trimethylammonioethyl methacrylate chloride or d) copolymers of acrylate and methacrylates with/without quarternary ammonium group in combination with sodium carboxymethylcellulose, e.g. those available from Rohm GmbH under the trademark Eudragit ® like Eudragit EPO (dimethylaminoethyl methacrylate copolymer; basic butylated methacrylate copolymer), Eudragit RL and RS (trimethylammonioethyl methacrylate copolymer), Eudragit NE30D and Eudragit NE40D (ethylacrylate methymethacrylate copolymer), Eudragit RD 100 (ammoniomethacrylate copolymer with sodium carboxymethylcellulose); or the like or any combinations thereof.
According to the present invention, non-polymeric pharmaceutically acceptable excipients for taste-masking eszopiclone include,' but are not limited to, fats, oils, waxes, fatty acids, fatty acid esters, long chain monohydric alcohols and their esters, phospholipids, terpenes or combinations thereof.
Waxes are esters of fatty acids with long chain monohydric alcohols. Natural waxes are often mixtures of such esters, and may also contain hydrocarbons. Waxes employed in the present invention include, but are not limited to, natural waxes, such as animal waxes, vegetable waxes, and petroleum waxes {i.e., paraffin waxes, microcrystalline waxes, petrolatum waxes, mineral waxes), and synthetic waxes. Specific examples include but are not limited to spermaceti wax, carnauba wax, Japan wax, bayberry wax, flax wax, beeswax, yellow wax, Chinese wax, shellac wax, lanolin wax, sugarcane wax, candelilla wax, castor wax paraffin wax, microcrystalline wax, petrolatum wax, carbowax, and the like, and mixtures thereof. Waxes are also monoglyceryl esters, diglyceryl esters, or triglyceryl esters (glycerides) and derivatives thereof formed from a fatty acid having from about 10 to about 22 carbon atoms and glycerol, wherein one or more of the hydroxyl groups of glycerol are substituted by a fatty acid. Glycerides employed in the present invention include, but are not limited to, glyceryl monostearate, glyceryl distearate, glyceryl tristearate, glyceryl dipalmitate, glyceryl tripalmitate, glyceryl monopalmitate, glyceryl palmitostearate, glyceryl dilaurate, glyceryl trilaurate, glyceryl monolaurate, glyceryl didocosanoate, glyceryl tridocosanoate, glyceryl monodocosanoate, glyceryl monocaproate, glyceryl dicaproate, glyceryl tricaproate, glyceryl monomyristate, glyceryl dimyristate, glyceryl trimyristate, glyceryl monodecenoate, glyceryl didecenoate, glyceryl tridecenoate, glyceryl behenate, polyglyceryl diisostearate, lauroyl macrogolglycerides, oleoyl macrogolglycerides, stearoyl macrogolglycerides, and combinations thereof.
Fatty acids include, but are not limited to, hydrogenated palm kernel oil, hydrogenated peanut oil, hydrogenated palm oil, hydrogenated rapeseed oil, hydrogenated rice bran oil, hydrogenated soybean oil, hydrogenated sunflower oil, hydrogenated castor oil, hydrogenated cottonseed oil, and mixtures thereof. Other fatty acids include, but are not limited to, decenoic acid, docosanoic acid, stearic acid, palmitic acid, lauric acid, myristic acid, and the like, and mixtures thereof. In one embodiment the fatty acids employed include, but are not limited to, hydrogenated palm oil, hydrogenated castor oil, stearic acid, hydrogenated cottonseed oil, palmitic acid, and mixtures thereof. Long chain monohydric alcohols include, but are not limited to, cetyl alcohol, and stearyl alcohol, and mixtures thereof.
In one embodiment, the non-polymeric pharmaceutically acceptable excipients employed for taste-masking eszopiclone include, but are not limited to, Cutina (hydrogenated castor oil), Hydrobase (hydrogenated soybean oil), Castorwax (hydrogenated castor oil), Croduret (hydrogenated castor oil), Carbowax, Compritol (glyceryl behenate), Sterotex (hydrogenated cottonseed oil), Lubritab (hydrogenated cottonseed oil), Capmul (glyceryl mono- and dicaprate), Apifil (wax yellow), Akofine (hydrogenated cottonseed oil), Softtisan (hydrogenated palm oil), Hydrocote (hydrogenated soybean oil), Corona (lanolin), Gelucire (macrogolglycerides lauriques), Precirol (glyceryl palmitostearate), Emulcire (cetyl alcohol), Plurol diisostearique (polyglyceryl diisostearate), and Geleol (glyceryl stearate), and mixtures thereof.
In another embodiment, lipids or waxes are employed in the form of an aqueous dispersion or emulsion stabilized by surfactants and suitable stabilizers.
The active ingredient is physically mixed or blended with these polymeric or non- polymeric pharmaceutically acceptable excipients or is partially or completely coated with these excipients by any of the techniques known in the art, such as microencapsulation, melt granulation, melt extrusion, fluid bed coating, wet granulation, spray drying, dry granulation or roll compaction. Further, coating, based on lipids or waxes or other non- polymeric pharmaceutically acceptable excipients require that the melting point of the lipid should be sufficiently high to prevent melting in the mouth and should not be so high that active ingredient itself melts or is chemically degraded while preparation of the formulation. Coating can be carried out in the range from about 1% to about 150% weight gain. In one embodiment, coating is carried out in the range of from about preferably from about 2% to about 120% weight gain. In another embodiment, coating is carried out in the range of from about 5% to about 100% weight gain.
In one embodiment, the objectionable taste of eszopiclone is masked by melt granulation, which involves dispersing the active in a mass of molten polymeric or non- polymeric pharmaceutically acceptable excipients, followed by cooling and granulation. In another embodiment, eszopiclone is granulated using molten mass of polymeric and/or pharmaceutically acceptable excipients.
The polymeric or non-polymeric pharmaceutically acceptable excipient can be applied alone or in combination with other suitable pharmaceutical excipients, to eszopiclone, in the form of, but not limited to, powder, granules, beads, pellets, minitablets or the like to achieve the desired taste-masking. The amount of taste-masking agent employed can be in the range of about 1% to about 90% by weight of the composition. In one embodiment, the amount of taste-masking agent employed can be in the range of about 1 % to about 80% by weight of the composition. In one embodiment, the amount of taste-masking agent is in the range of about 2% to about 60% by weight of the composition. In another embodiment, the amount of taste-masking agent is in the range of about 5 to about 50% by weight of the composition.
In one embodiment, taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is a cyclodextrin or a derivative thereof. In another embodiment, the bitter taste of eszopiclone is masked by complexation with cyclodextrins or derivatives thereof.
Cyclodextrins are cyclic oligosaccharides formed from α-(1 , 4)-linked D-glucopyranose units, α, β and γ-cyclodextrins consist of six, seven and eight units respectively. The molecules have a toroidal shape, with a hydrophobic central cavity and a relatively hydrophilic outer surface. This structure enables cyclodextrins to bind appropriately sized non-polar guest molecules, or moieties of guest molecules, within the hydrophobic central cavity, to form clathrate complexes. Suitable cyclodextrins for use in the compositions of the present invention include, but are not limited to, α, β and γ~ cyclodextrins, or alkylated, hydroxyalkylated, esterified, glycosylated or substituted derivatives thereof, such as (2,6-di-o-methyl)-β-cyclodextrin (DIMEB) (dimethyl-β- cyclodextrin), randomly methylated-β-cyclodextrin (RAMEB), and hydroxypropyl-β- cyclodextrin (HPβCD), hydroxyethyl-β-cyclodextrin, dihydroxypropyl-β-cyclodextrin, trimethyl-β-cyclodextrin, hydroxymethyl-β-cyclodextrin, β-cyclodextrin sulfate, β- cyclodextrin sulfonate, methyl-β-cyclodextrin, sulfobutyl ether cyclodextrin (SBE-CD) (β- cyclodextrin sulfobutyl ether), glucosyl-α-cyclodextrin, glucosyl-β-cyclodextrin, diglucosyl- β-cyclodextrin, maltosyl-γ-cyclodextrin, maltosyl-γ-cyclodextrin, maltosyl-γ-cyclodextrin, maltotriosyl-β cyclodextrin, maltotriosyl-γ-cyclodextrin, dimaltosyl-β-cyclodextrin and mixtures thereof such as maltosyl-β-cyclodextrin/dimaltosyl-β-cyclodextrin. The complex of active with cyclodextrin can be prepared by various methods such as solution method,
co-precipitation method, co-evaporation/solid dispersion method, neutralization method, slurry method, kneading method, and grinding method.
In one embodiment a physical mixture of active and cyclodextrin or a derivative thereof is employed in the composition of the present invention. In one embodiment, the compositions of the present invention comprise eszopiclone and cyclodextrin or a derivative thereof in an uncomplexed form along with suitable pharmaceutically acceptable excipients.
In one embodiment, taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is a carbomer or a derivative thereof. In another embodiment, the bitter taste of eszopiclone is masked by complexation with carbomers or derivatives thereof. Eszopiclone can be taste-masked by complexation with carbomers such as carbomer 934, carbomer 971 , carbomer 974 or the like wherein the complex is held together by ionic bonding and gel properties of the carbomer, providing stable and palatable compositions. These complexes can be prepared by mixing, blending or slurrying eszopiclone and carbomer together to allow the desired complex formation.
In one embodiment, taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is an adsorbent. The bitter taste of eszopiclone is masked by adsorption using as taste-masking agents, adsorbents that form adsorbates with the active. Adsorbates is formed by adsorbing or partially or significantly blending eszopiclone with an adsorbent selected from, but not limited to, magnesium aluminum silicate, zeolite, activated granular carbon, silica gel, active aluminum, clay and mixtures thereof. These adsorbent materials surround the drug particles by forming a physical bond, by Van der Waals interactions, and hydrogen bonding force of attraction, so that the bitter taste of the drug is not perceived. The adsorbate of eszopiclone is formed by mixing or blending the active with the adsorbent in high or moderate shear mixers like planetary mixer or rapid mixer granulator. Alternatively, adsorbate is formed by wet granulation involving the adsorbent and eszopiclone in any conventional granulation equipment.
In one embodiment, taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is an ion exchange resin. In another embodiment, the bitter taste of eszopiclone is masked by complexation with an ion-exchange resin. Ion exchange resins are solid and water insoluble high molecular weight polyelectrolytes that can exchange their mobile ions of equal charge with the surrounding medium and are not absorbed by the body. The resulting ion exchange is reversible and stoichiometric with the displacement of one ionic species by another. The drug-resinates effectively mask the taste of a bitter or unpleasant tasting drug within the matrix of the ion exchange material. The correct selection of ion exchange resin is important so that the drug is not released in the mouth leading to perception of the bitter taste of drug. The present invention provides taste- masking of eszopiclone by reversibly adsorbing the active compound on to an ion exchange resin wherein the polymeric matrix of the ion exchange resin can have functional groups including anionic groups, e.g., weakly acidic- carboxylic, esteric and phosphonic; strongly acidic-sulfonic or cationic groups, e.g., weakly basic- tertiary amine; strongly basic- quaternary amine. Additionally, suitable polymeric matrices include copolymers of acrylic and substituted acrylic acids; styrene and styrene derivatives; cellulose esters; vinyl and substituted vinyl esters; and polysulfonic acids and polysulfonic acid esters. The ion exchange resin having the polymeric matrix with an anionic functional group is a cation exchange resin and that having a cationic functional group is an anionic exchange resin. The mobile or exchangeable moieties depending on the type of resin can be but not limiting to sodium, hydrogen, potassium, chloride and the like.
In one embodiment of the present invention a cationic exchange resin is employed to mask the bitter taste of eszopiclone. Non limiting examples of suitable cation exchange resin that may be employed include Amberlite® IRP64 (porous copolymer of methacrylic acid and divinylbenzene), Amberlite® IRP69 (sulfonated copolymer of styrene and divinylbenzene), Amberlite® IRP88 (cross linked polymer of methacrylic acid and divinylbenzene), DOWEX® RTM. resins (strong cationic exchangers based upon polystyrenesulphonic acid with variable crosslinking (1-12% divinylbenzene)), Tulsion® 335 - (Polacrilex/{Polacirilex S), Tulsion® 339 (Polacrilin potassium USP), Tulsion® 344 (Sodium polystyrene sulfonate BP), Indion® 204 (crosslinked polyacrylic acid), Indion® 214 (crosslinked polyacrylic acid), Indion® 234 (crosslinked polyacrylic acid), Indion®
234S (crosslinked polyacrylic acid), Indion® 294 (crosslinked polyacrylic acid), Purolite® C115 HMR (carboxylic acid functional group), Purolite® C115 E (carboxylic acid functional group), Purolite® C100 HMR (sulfonic acid functional group), Purolite® 100 MR (sulfonic acid functional group) or cation exchange resins having phosphonic functional groups. In one embodiment, ion exchange resin can be used for complexation with eszopiclone in a ratio of eszopiclone to resin of about 1:0.1 to about 1 :20.
In another embodiment, taste-masking agent employed in the compositions of the present invention for alleviating bitter taste associated with eszopiclone therapy is a pH modifier. A pH-modifιer is employed in the compositions of the present invention include, but is not limited to, disodium hydrogen phosphate, sodium triphosphate, monosodium phosphate, sodium carbonate, potassium dihydrogen phosphate, sodium bicarbonate, meglumine, citric acid, benzoic acid, tartaric acid or the like or any combinations thereof.
Further, reduction in the bitter taste associated with eszopiclone is also achieved by using at least one sweetening agent including, but not limited to, aspartame, stevia extract, glycyrrhiza, saccharine, saccharine sodium, acesulfame, sucralose and dipotassium glycyrrhizinate; and one or more flavors, e.g., mint flavour, orange flavour, lemon flavors, strawberry aroma, vanilla flavour, raspberry aroma, cherry flavor, tutty frutty flavor, magnasweet 135, key lime flavor, grape flavor, trusil art 511815, and fruit extracts. In one embodiment, polyols such as, but not limited to, mannitol, xylitol, sorbitol or the like are also used as sweeteners.
In one embodiment, alleviation of bitter or unpleasant taste associated with eszopiclone is achieved using taste-modifying agents that are capable of competitively binding or interacting with the taste buds or taste receptors or coating them thereby alleviating the perception of bitter or unpleasant taste such as, but not limited to, hydrogenated castor oil (Cremophor RH40), thaumatin, arginine, ornithine, lysine, citrulline, adenosine 51 monophosphate, thymidine 5r monophosphate, adenosine 5' diphosphate, adenosine 3' monophosphate, adenosine 5'-succinate, adenosine 51 triphosphate, adenosine 2' monophosphate, δ'-cytidylic acid, inosinic acid, monellin, ferulic acid or caffeic acid or the like or any combinations thereof.
The immediate bitter taste associated with eszopiclone therapy is alleviated by masking the objectionable taste of eszopiclone by use of taste-masking agents as discussed above followed by incorporation of such taste-masked eszopiclone, in the form of, but not limited to, powder, granules, pellets, beads, minitablets or the like in various palatable formulations including, but not limited to, orally disintegrating, bite-dispersion, dispersible, chewable or effervescent tablets, sprinkle granules, quick melt wafers, lozenge, dry suspensions or syrups for reconstitution, chewing gum, buccal tablet or the like. In one embodiment the pharmaceutical compositions comprising taste-masked eszopiclone are in the form of rapidly disintegrating dosage forms such as, but are not limited to, orally disintegrating, bite-dispersion, dispersible, chewable or effervescent tablets, sprinkle granules, quick melt wafers, lozenges or the like. The various taste- masking approaches discussed above can also be applied for the development of immediate release tablets. These oral formulations may contain from about 5% to about 95% of tastemasked eszopiclone. The taste-masked formulations may be so designed that the bioavailability of the eszopiclone is preferably not compromised. Further the pharmaceutical compositions of the present invention in addition to eszopiclone and a taste-masking agent further comprise at least one pharmaceutically acceptable excipient. Based on the final form of the palatable pharmaceutical composition to be prepared suitable pharmaceutically acceptable excipients are incorporated.
In one embodiment, taste-masked eszopiclone is incorporated in an orally disintegrating tablet. Orally disintegrating tablets (ODTs) disintegrate/dissolve in the mouth rapidly without administering extra water, providing the convenience of a tablet formulation while allowing the ease of swallowing provided by a liquid formulation.
The orally disintegrating tablets comprising eszopiclone can further comprise directly compressible coprocessed excipient. PCT Application WO2007052289 describes directly compressible coprocessed excipient comprising of at least one water soluble excipient and water insoluble inorganic excipient such as calcium silicate. The water soluble carbohydrate can be a monosaccharide, disaccharide, oligosaccharide or polysaccharide. Examples of carbohydrates include, but are not limited to, monosaccharides such as sorbitol, glucose, dextrose, fructose, maltose or xylitol, disaccharides such as sucrose, trehalose, lactose, glucose, galactose or mannitol, and oligosaccharides and polysaccharides such as dextrates and maltodextrins. The water
soluble and water insoluble excipients in the directly compressible coprocessed excipient can be in a ratio of water-soluble excipient to water insoluble excipient of from about 50:1 to about 1 :50. In one embodiment of the present invention, this ratio is about 30:1 to about 1 :30. In a further embodiment of the present invention, this ratio is from about 20:1 to about 1:20. In one embodiment the directly compressible coprocessed excipient is available commercially as PanExcea™ ODT from Mallinckrodt Baker, US. The amount of directly compressible co-processed excipient employed in the orally disintegrating tablet compositions comprising eszopiclone and a taste-masking agent is about 5% to about 95 % by weight of the said dosage form.
The orally disintegrating tablet compositions comprising eszopiclone dissolve or disintegrate in the oral cavity within about 60 seconds. The orally disintegrating tablet compositions can be prepared by any of the known non-limiting techniques such as freeze-drying, molding and sublimation, compression, cotton candy process, mass extrusion, etc or with use of specialized excipients such as effervescent couple, highly micronized agents, coprocessed excipients or the like.
The compositions of the present invention may include, in addition to eszopiclone, taste- masking agent and directly compressible co-processed excipient, one or more binders, disintegrants, superdisintegrants, diluents, salivating agents, surfactants, flavors, sweeteners, colorants, diluents, souring agents, viscolizers, glidants or lubricants, solubilizers, or stabilizers.
Examples of superdisintegrants include, but are not limited to, natural, modified or pregelatinized starch, crospovidone, croscarmellose sodium, sodium starch glycolate, low-substituted hydroxypropyl cellulose as well as effervescent disintegrating systems. Further, the disintegrants can be selected from, but not limiting to, crospovidone, calcium silicate and starch. The amount of disintegrant or superdisintegrant employed in the composition is about 2% to about 30 % by weight of the said dosage form. Examples of suitable binders include, but are not limited to, starch, pregelatinized starch, polyvinyl pyrrolidone (PVP), copovidone, cellulose derivatives, such as hydroxypropylmethyl cellulose (HPMC), hydroxypropyl cellulose (HPC) and carboxymethyl cellulose (CMC) and their salts. Examples of suitable diluents include, but are not limited to, starch, microcrystalline cellulose, lactose, xylitol, mannitol, maltose, polyols, fructose, guar gum,
sorbitol, magnesium hydroxide, dicalcium phosphate, and the like or any combinations thereof. Examples of the lubricant include, but are not limited to, magnesium stearate, calcium stearate, stearic acid, talc, and sodium stearyl fumarate. The tablet compositions of the invention may also include a glidant such as, but not limited to, colloidal silica, silica gel, precipitated silica, or combinations thereof. The compositions of the present invention may also include salivating agents including, but not limited to, micronised polyethylene glycol, sodium chloride or precipitated micronised silica to improve the disintegration properties of the said compositions. Examples of solubilizers include, but are not limited to cetostearyl alcohol, cholesterol, diethanolamine, ethyl oleate, ethylene glycol palmitostearate, glycerin, glyceryl monostearate, isopropyl myristate, lecithin, medium-chain glyceride, monoethanolamine, oleic acid, propylene glycol, polyoxyethylene alkyl ether, polyoxyethylene castor oil glycoside, polyethylene sorbitan fatty acid ester, polyoxyethylene stearate, propylene glycol alginate, sorbitan fatty acid ester, stearic acid, sunflower oil, triethanolmine.or combinations thereof. The compositions of the present invention may also include stabilizers including, but not limited to, benzoic acid, sodium benzoate, citric acid, and the like. Examples of surfactants include, but are not limited to, sodium docusate, glyceryl monooleate, polyethylene alkyl ether, polyoxyethylene sorbitan fatty acid ester, sodium lauryl sulfate, sorbic acid, sorbitan fatty acid ester, and mixtures thereof. Souring agents include, but are not limited to, monosodium fumarate and/or citric acid. The compositions of the present invention may optionally include viscolizers such as polyalkylene oxides; polyols; starch and starch-based polymers; chitosan; polysaccharide gums; polyethylene oxide, hydroxypropyl cellulose, hydroxypropy! methyl cellulose, hydroxyethyl cellulose, sodium carboxy methylcellulose, calcium carboxymethyl cellulose, methyl cellulose, polyacrylic acid, gum acacia, gum tragacanth, xanthan gum, guar gum and polyvinyl alcohol and copolymers and mixtures thereof.
In one embodiment of the present invention, taste-masked eszopiclone is incorporated in bite-dispersion tablets. Bite-dispersion tablets are meant to be taken without water and disperse easily, and quickly, after a gentle bite when taken orally enabling the taste- masked eszopiclone to be orally administered. These tablets comprise in addition to eszopiclone and taste-masking agent, various pharmaceutically acceptable excipients as have been discussed under orally disintegrating tablets in addition to excipients which may be specifically employed for bite-dispersion tablets.
In another embodiment of the present invention, taste-masked eszopiclone may also be incorporated in chewable tablets. Chewable tablets are taken slowly by chewing or sucking in the mouth, and enable taste-masked active contained therein to be administered without water. These chewable tablets comprise in addition to eszopiclone and taste-masking agent, various pharmaceutically acceptable excipients as have been discussed under orally disintegrating tablets in addition to excipients which may be specifically employed for chewable tablets.
In yet another embodiment of the present invention, taste-masked eszopiclone is incorporated in effervescent tablets. Effervescent tablets are intended to be dissolved or dispersed in water before administration and generally contain acid substances and carbonates or bicarbonates, which react rapidly in the presence of water releasing carbon dioxide. These tablets comprise in addition to eszopiclone and taste-masking agent, various pharmaceutically acceptable excipients as have been discussed under dispersible tablets and orally disintegrating tablets. The effervescent tablets can comprise effervescent couples selected from, but not limited to, thermolabile gas generating agents such as sodium bicarbonate, sodium glycine carbonate, potassium bicarbonate, ammonium bicarbonate, sodium bisulfite, sodium metabisulfite, and an acid source such as citric acid, maleic acid or tartaric acid.
In a further embodiment, taste-masked eszopiclone is incorporated in dispersible tablets. Dispersible tablet refers to a tablet which disperses in aqueous phase, e.g. in water before administration. A water-dispersible tablet, according to the British Pharmacopoeia and European Pharmacopoeia, should meet the requirements of the test for dispersible tablets as regards dispersion time (< 3 minutes) and dispersion quality (i.e. to pass through a 710 μm sieve).
The dispersible tablet compositions comprising eszopiclone and taste-masking agent can further comprise in addition to pharmaceutically acceptable excipients as disclosed under orally disintegrating tablets, one or more viscolizers. Examples of viscolizers which can be used include, but are not limited to, polyalkylene oxides such as polyethylene oxide; cellulose ethers such as hydroxyethyl cellulose, hydroxypropylcellulose, hydroxypropyl methyl cellulose, methyl cellulose, ethyl cellulose, sodium carboxy methylcellulose, calcium carboxymethyl cellulose, microcrystalline cellulose; gums such
as gum arabic alginates, agar, guar gum, locust bean, carrageenan, tara, gum arabic, tragacanth, pectin, xanthan, gellan, maltodextrin, galactomannan, pusstulan, laminarin, scleroglucan, gum arabic, inulin, karaya, whelan; polyols such as dipropylene glycol, polypropylene glycol, propylene glycol, polyethylene glycol (PEG), sorbitol and glycerol; carbopol, starch and starch-based polymers such as pregelatinized starch, acrylic acid and methacrylic acid polymers, and esters thereof, maleic anhydride polymers; polymaleic acid; poly(acrylamides); poly(olefinic alcohol)s; poly(N-vinyl lactams); polyoxyethylated saccharides; polyoxazolines; polyvinylamines; polyvinylacetates; polyimines; povidone vinylpyrrolidone/vinyl acetate copolymer and polyvinyl acetate, mixture of polyvinyl acetate and polyvinylpyrrolidone, chitin, cyclodextrin, gelatin, chitosan, and combinations thereof. In one embodiment of the present invention, viscolizers include hydroxypropyl cellulose, hydroxypropyl methylcellulose, hydroxyethyl cellulose, polyethylene oxide, sodium carboxy methylcellulose, microcrystalline cellulose, guar gum, xanthan gum, alginates, and combinations thereof. The weight percent of the viscolizer in the dosage form is about 2 to about 75 weight percent. In another embodiment of the present invention, the viscolizer in the dosage form is about 10 to about 70 weight percent. In yet another embodiment of the present invention, the viscolizer in the dosage form is about 5 to about 50 weight percent. Viscolizers act to control sedimentation rate of dispersed eszopiclone thereby producing homogeneous dispersions when the dispersible tablets are dispersed in water before administration thus ensuring substantially uniform dosing. They rapidly generate viscosity when the dispersible tablets come in contact with water, and a homogenous suspension is formed, which can be easily swallowed by children and the elderly, with minimal effect of the release properties of the biologically active ingredient.
The terms "tablet" and "tablet composition" are used synonymously within the context of the present invention. These terms should be construed to include a compacted or compressed powder composition obtained by compressing or otherwise forming the composition to form a solid having a defined shape. Tablets in accordance with the invention may be manufactured using conventional techniques of common tableting methods known in the art such as direct compression, wet granulation, dry granulation and extrusion/ melt granulation. In one embodiment, the process is direct compression which involves compression of taste-masked drug-excipient blend after mixing them for a definite time period. The tablet may vary in shape such as oval, triangle, almond, peanut, parallelogram, round, pentagonal, hexagonal, and trapezoidal. The preferred shapes are
round, oval and parallelogram forms. In one embodiment the tablets may optionally be coated. Surface coating may be employed for aesthetic purposes or for dimensionally stabilizing the dosage form. The coating may be carried out using any conventional technique employing conventional ingredients suitable for enteral use. A surface coating can for example be in the form of film using conventional polymers such as hydroxypropyl methyl cellulose, hydroxypropyl cellulose, carboxymethyl cellulose, polyvinyl alcohol polymethacrylates and the like. In another embodiment of the present invention the composition may optionally be coated with a functional coat. The coat can be employed using polymeric or non-polymeric excipients as described above either alone or in combination, along with plasticizers, colorants, opacifiers etc.
In one embodiment of the present invention, the taste-masked eszopiclone is incorporated in sprinkle granules, quick melt wafers, lozenge, dry suspensions or syrups for reconstitution, chewing gum, orally dissolvable film or the like.
In one embodiment of the present invention process for preparing a pharmaceutical composition for alleviating unpleasant taste associated with eszopiclone therapy comprises: a. treating eszopiclone with at least one taste-masking agent, optionally along with at least one pharmaceutically acceptable excipient to form taste-masked eszopiclone; b. blending taste-masked eszopiclone of step (a) with other pharmaceutically acceptable excipients, except lubricant, to form a uniform powder mix; c. lubricating the powder mix of step (b); and d. forming the powder mix of step (c) or of step (b) into an orally disintegrating tablet, dispersible tablet, effervescent tablet, chewable tablet, sprinkle granules, suspension, powder for suspension, quick melt wafers, lozenge, or chewing gum.
The various dosage forms of the present invention for alleviation of any immediate bitter taste perceived by a patient on eszopiclone therapy, comprising eszopiclone, at least one taste-masking agent and at least one pharmaceutically acceptable excipient are immediate release dosage forms that release taste-masked eszopiclone instantly upon reaching either the stomach or the intestines. In one aspect of the present invention the in vitro release of eszopiclone is measured by dissolution using 0.1 N hydrochloric acid (HCI) (500 ml) in USP apparatus Il (paddle) at 50 rpm speed. In one embodiment the
pharmaceutical composition of the present invention comprising eszopiclone releases not less than 70% of the active within about 45 minutes in vitro. In another embodiment the pharmaceutical composition of the present invention comprising eszopiclone releases not less than 80% of the active within about 45 minutes in vitro. In a further embodiment, the formulations disclosed in the present invention can also be adapted to develop a composition wherein taste-masked eszopiclone is released in a controlled manner over a period of time, for example, from about 2 to about 24 hours. In such a formulation, eszopiclone is treated with polymeric, non-polymeric pharmaceutically acceptable excipients described above or any combinations thereof. The amount of such polymeric or non-polymeric excipients can not only ensure masking of the objectionable taste of the active but also provide any desired control over the release of eszopiclone.
In another embodiment of the present invention, pellets, granules or the like of eszopiclone are prepared comprising at least one release retardant in combination with one or more pharmaceutically acceptable excipients. Suitable release retardants, as discussed above, can be polymeric or non-polymeric pharmaceutically acceptable excipients or agents and include, but are not limited to, cellulose ethers, such as hydroxypropylmethylcellulose (HPMC), hydroxypropylcellulose (HPC), hydroxyethylcellulose, ethyl cellulose and carboxymethylcellulose sodium; polysaccharides, such as carageenan, guar gum, xanthan gum, tragacanth and ceratonia; polymethacrylates, such as copolymers of acrylic and methacrylic acid esters containing quarternary ammonium groups; cellulose esters, such as cellulose acetate; acrylic acid polymers, such as carbomers; waxes, such as hydrogenated castor oil, hydrogenated vegetable oil, carnauba wax and microcrystalline wax; alginates, such as alginic acid and sodium alginate; and fatty acid derivatives, such as glyceryl monostearate and glyceryl palmitostearate. These pellets or granules or the like can be further coated using excipients described above in order to achieve taste-masking and controlled release of eszopiclone. The amount of release retardant in the formulation is from about 1 to about 90% by weight of the dosage form. In one embodiment, the amount of release retardant in the formulation is about 5 to about 80% by weight of the dosage form.
Further, the present invention also relates to compositions devoid of the active agent that alleviate the bitter or metallic or unpleasant taste experienced by a patient after
administration of eszopiclone. Such placebo compositions devoid of the active agent comprise various taste-masking agents and/or pharmaceutically acceptable excipients described above. Further, these placebo compositions can be provided in the form of orally disintegrating tablet, chewable tablet, bite-dispersion tablet, dispersible tablet, effervescent tablet, chewing gum, orally dissolvable film, quick melt wafers, lozenge, buccal tablet, immediate release tablet or the like, as discussed above, and can be prepared by any of the aforementioned methods and processes.
The placebo compositions when in the form of orally disintegrating tablets, bite- dispersion tablets, chewable tablets, dispersible tablets, effervescent tablets or the like comprise sweeteners, flavourants, taste-modifiers or any taste-masking agents including, but not limited to, polymeric or non-polymeric pharmaceutically acceptable excipients, cyclodextrins, carbomers, adsorbents, pH modifiers or the like described above.
In one embodiment of the present invention, the placebo composition may be in the form of an orally dissolvable film or a strip dosage form comprising at least one film-forming polymer, flavor and sweetener. The film forming polymer includes, but is not limited to, methyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose, ethyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, carboxymethyl cellulose, cellulose acetate phtalate, cellulose acetate butyrate, amylose, dextran, casein, pullulan, gelatine, pectin, agar, carrageenan, xanthan gum, tragacanth, guar gum, acacia gum, arabic gum, polyethylene glycol, polyethylene oxide, polyvinyl pyrrolidone, polyvinyl alcohol, carboxyvinyl polymers, sodium alginate, polyacrylic acid, methylmethacrylate, or the like or any combinations thereof. Such compositions can optionally comprise any additional mucoadhesive agent including, but not limited to, chitosan, carbopol or the like. By adjusting levels of polymers and other ingredients within the matrix of the films, release of the flavor or sweetener from within the matrix of the films can be controlled. Additionally based on the levels of the different ingredients employed, the time period over which the film or the strip dosage form dissolves may be also be controlled.
In another embodiment, the placebo composition may be in the form of chewing gum that comprises at least one chewing gum base along with sweeteners or flavourants. Chewing gum base that may be employed includes, but is not limited to, styrene-diene
block copolymers, isobutylene-isoprene copolymers or polymers such as polyethylene, polyisobutylene or polyvinyl acetate, or their derivatives or combinations thereof.
In yet another embodiment of the present invention, the placebo composition may be in the form of a buccal tablet. The tablet is held between the cheek and the gum or adhered to buccal mucosa and may be designed to alleviate bitter or unpleasant taste over a prolonged period of time. Such compositions may comprise in addition to sweeteners, flavourants, pH-modifiers or taste-modifiers, mucoadhesive agents including, but not limited to, chitosan, carbopol, or the like, that can hold the tablet in the oral cavity for a longer duration of time.
In one embodiment, the placebo composition comprises a displacing agent which competitively displaces any eszopiclone from the taste receptors so that the bitter taste is not experienced. The displacing agent in one embodiment may be a taste-modifier such as, but not limited to, hydrogenated castor oil (Cremophor RH40), thaumatin, arginine, ornithine, lysine, citrulline, adenosine 5" monophosphate, thymidine 51 monophosphate, adenosine 5' diphosphate, adenosine 3' monophosphate, adenosine δ'-succinate, adenosine 5' triphosphate, adenosine 2' monophosphate, 5'-cytidylic acid, inosinic acid, monellin, ferulic acid or caffeic acid or the like or any combinations thereof.
Placebo composition is provided to counter the bitter or metallic after taste either instantaneously or over a prolonged period of time. The placebo composition in such a case can release the sweeteners, flavors, pH-modifier or taste-modifying agents either immediately or over a controlled manner in the oral cavity of the patient based on the level of polymers and other ingredients used in the placebo composition.
Further, the present invention also relates to a method of countering the unpleasant taste experienced by a patient after administration of eszopiclone comprising administering to the patients in need thereof compositions devoid of the active agent that alleviate the bitter or unpleasant after-taste either along with or subsequently at an appropriate time after administration of a composition wherein the objectionable taste of eszopiclone has been masked. The bitter or metallic or unpleasant after taste reported subsequent to the administration of eszopiclone compositions as mentioned in the present invention intends to encompass any unpleasant taste perceived by a patient receiving eszopiclone
compositions either within a minute after the intake of the eszopiclone formulation or after a period of time ranging from about one minute to about 24 hours after the consumption of the eszopiclone composition. Further, the term "appropriate time" refers to the time when the patient who has been administered eszopiclone composition perceives a bitter, unpleasant or metallic after-taste, which could be any time ranging from within about one minute to about 24 hours after administration or consumption of the eszopiclone medication.
The present invention also relates to methods of alleviating the immediate bitter or unpleasant taste as well as the objectionable after-taste associated with eszopiclone therapy. Such a method comprises administering to the patient in need thereof compositions of the present invention wherein the objectionable taste of eszopiclone has been masked, to alleviate any immediate bitterness generally associated with the drug, followed by optional administration of a composition devoid of the active either along with or subsequently at an appropriate time after the administration of the eszopiclone formulation, to counter any bitter or unpleasant or metallic after-taste reported subsequent to the administration of eszopiclone. In one embodiment of the present invention, the method of alleviating unpleasant taste associated with eszopiclone therapy comprises administering to the patient in need thereof a pharmaceutical composition comprising eszopiclone, at least one taste-masking agent and at least one pharmaceutically acceptable excipient to alleviate any immediate bitterness otherwise associated with oral intake of eszopiclone followed by optional administration of at least one placebo composition devoid of the eszopiclone either along with or subsequently at an appropriate time after the administration of the pharmaceutical composition comprising taste-masked eszopiclone to counter unpleasant after-taste reported subsequent to the administration of eszopiclone.
In one embodiment, the compositions of the present invention for alleviating bitter or unpleasant taste associated with eszopiclone therapy are provided in the form of a kit. Such a kit may comprise a composition wherein the objectionable taste of eszopiclone has been masked and a placebo composition devoid of the active agent along with instructions for their use either together or separately, wherein when consumed separately the placebo composition is taken at an appropriate time subsequent to the intake of taste-masked eszopiclone composition. The appropriate time as discussed
above can vary from patient to patient and could be any time ranging from within about one minute to about 24 hours after administration or consumption of the taste-masked eszopiclone medication. A kit for alleviating unpleasant taste associated with eszopiclone therapy comprises (a) a pharmaceutical composition comprising eszopiclone; at least one taste-masking agent; and at least one pharmaceutically acceptable excipient; (b) a placebo composition devoid of eszopiclone, and (c) instructions for use of (a) and (b) either together or separately. Further, one or more than one unit placebo dosage forms can be provided or taken, subsequently after administration of taste-masked eszopiclone composition to counter the bitter or unpleasant after-taste experienced by patients consuming the medication. Both, the taste-masked eszopiclone composition and the placebo composition can be packed together or separately in a suitable primary pack for the ease of administration of the patient. In another embodiment a kit for alleviating unpleasant taste associated with eszopiclone therapy comprises (a) a pharmaceutical composition comprising eszopiclone that releases eszopiclone in a controlled manner over a period of about 2 to about 24 hours; (b) a placebo composition devoid of eszopiclone, and (c) instructions for use of (a) and (b) either together or separately.
In another embodiment of the present invention, the taste-masked eszopiclone composition to counter any immediate bitter taste experienced by the patient upon administration of eszopiclone and the placebo composition to alleviate any bitter or unpleasant taste perceived after administration of eszopiclone can be administered together in the form of a dosage form, such as a bilayer tablet. The placebo composition in such a bilayered tablet can optionally comprise an additional mucoadhesive component including, but not limited to, carbopol, chitosan, or the like, that help the dosage form to remain adhered to the buccal mucosa and release the flavor or sweetener slowly such that unpleasant taste or bitter after taste due to eszopiclone is not experienced. The taste-masked eszopiclone component of such a bilayered dosage form dissolves rapidly thereby delivering the taste-masked drug to the patient.
In a further embodiment of the present invention, the taste-masked eszopiclone compositions may be adapted to deliver one or more active agents in addition to eszopiclone. The active agent includes, but is not limited to, omeprazole, hydroxyomeprazole, esomeprazole, tenatoprazole, lansoprazole, pantoprazole, rabeprazole, dontoprazole, habeprazole, periprazole, ransoprazole, pariprazole,
leminoprazole, acetaminophen, ibuprofen, aspirin, naproxen, doxepin, escitalopram, milnacipran, melatonin, agomelatine, GR196429, S20242, S23478, S24268, S25150, BMS-214778, luzindole, GG-012, enol-3-IPA, ML-23, SL-18.1616, IP-100-9, AH-017, AH-002, IP-101 , modafinil, apomorphine, bromocriptine, cabergoline, optically pure (S)- didesmethylsibutramine, lisuride, pergolide, pramipexole, ropinirole, amantadine, apomorphine, bromocriptine, cabergoline, carmoxirole, optically pure (S)- didesmethylsibutramine, dopexamine, fenoldopam, ibopamine, lergotrile, lisuride, memantine, mesulergine, pergolide, piribedil, pramipexole, quinagolide, roxindole, talipexole, eplivanserin, temazepam, clonazepam, gaboxadol and pregabaline, citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, milnacipran, paroxetine, sertraline, clominpramine, femoxetine, indapline, alaprolclate, cericlamine, ifoxetine, desipramine, maprotiline, lofepramine, reboxetine, oxaprotiline, fezolamine, tomoxetine, O-desmethylvenlafaxine, amantadine, apomorphine, bromocriptine, or the like.
While the present invention has been described in terms of its specific embodiments, certain modifications and equivalents will be apparent to those skilled in the art and are intended to be included within the scope of the present invention. Details of the present invention, including its objects and advantages, are provided in the non-limiting exemplary illustrations below.
Examples
Example 1: Taste-masked eszopiclone orally disintegrating tablets
Table 1: Composition of orally disintegrating tablets of eszopiclone
Procedure: Eszopiclone was dry mixed with glyceryl behenate and then blended with other excipients, except lubricant, in a blender to get a uniform powder mix. The blend was lubricated and compressed to form tablets with the following parameters:
Hardness (N) : 28-38
Friability (%) : 0.45
Disintegration time (sec) : 10-15
Disintegration time in oral cavity (sec) : 30-40
The tablets had acceptable taste and pleasant mouth feel.
Example 2: Taste-masked eszopiclone bite-dispersion tablets
Table 2: Formulation of blend eszopiclone with microcrystalline cellulose
Procedure: Eszopiclone was blended with microcrystalline cellulose (1 :2 parts). Coating solution was prepared by melting hydrogenated vegetable oil in water bath and adding glyceryl mono- & dicaprate in molten wax. Aspartame was dissolved in a hot solution of hypromellose and this aqueous phase was added to above oily phase. The system was homogenized and cooled to room temperature, to which flavor was added to get a coating solution. Drug blend was coated with coating composition using top spray assembly to weight gain of 40 %.
Table 4: Composition of bite -dispersion tablets of eszopiclone
Procedure: The coated taste-masked eszopiclone granules were blended with other excipients like spray dried mannitol, microcrystalline cellulose, sodium starch glycolate, citric acid, aspartame, colloidal silicon dioxide, talc and flavor and finally lubricated using magnesium stearate. The blend was compressed to get a bite-dispersion tablet. The tablets had pleasant and acceptable mouth feel along with desirable disintegration time.
Example 3: Kit comprising taste masked eszopiclone formulation and placebo orally disintegrating tablet composition
A. Taste-masked eszopiclone formulation
Table 5: Composition of orally disintegrating tablets of eszopiclone
Procedure: Eszopiclone was thoroughly mixed with basic butylated methacrylate copolymer and granulated using povidone dissolved in water. These granules were dried and then blended with other excipients, except lubricant, to get a uniform mass. The mass was lubricated and compressed into tablets having the following parameters:
Hardness (N) : 30-40
Friability (%) : 0.25
Disintegration time (sec) : 10-20
Disintegration time in oral cavity (sec) : 20-30
Eszopiclone orally disintegrating tablets with desirable pleasant taste and acceptable wicking time were obtained.
B. Placebo orally disintegrating tablet composition
Table 6: Composition of placebo orally disintegrating tablet
Procedure: All the ingredients were mixed well in a blender and lubricated. Placebo orally disintegrating tablets were compressed which had acceptable wicking time and friability along with pleasant taste.
The placebo orally disintegrating composition was consumed by the patient after half an hour subsequent to the intake of taste-masked eszopiclone. The placebo was pleasant to taste and could mask unpleasant bitter taste perceived due to administration of eszopiclone formulation.
Example 4: Kit comprising taste masked eszopiclone and placebo buccal tablet composition
A. Taste-masked eszopiclone formulation
Eszopiclone was layered on non-pareil beads and these drug-loaded beads were further coated with a combination of ethyl cellulose and hydroxypropyl methylcellulose (20:80) to a weight gain of about 20%.
Table 7: Composition of orally disintegrating tablet of eszopiclone
Procedure: Coated eszopiclone pellets were blended with other excipients except lubricant in a blender to obtain a uniform mass. This mass was lubricated and compressed into tablets having the following parameters:
Hardness (N) : 40-50
Friability (%) : 0.35
Disintegration time (sec) : 15-20
Disintegration time in oral cavity (sec) : 40-50
Tablets with desired taste-masking, friability and disintegration time were obtained.
B. Placebo buccal tablet composition
Table 8: Composition of placebo buccal tablet
Procedure: All the excipients, including Carbopol®934 and hypromellose, were blended well. The mass was lubricated and compressed to get a buccal tablet.
The placebo buccal tablet composition was adhered to the buccal mucosa by the patient subsequent to the administration of eszopiclone formulation while going to bed. The tablet slowly and gradually releases the sweetener, flavor and taste modifying agent such that the bitter taste of drug due to its excretion in saliva is not experienced in the morning.
Example S: Kit comprising taste masked eszopiclone and placebo chewing gum composition
Table 9: Composition of taste masked eszopiclone granules
Procedure: Weighed quantity of eszopicloπe was added to molten hydrogenated castor oil to form a homogenous mixture that was allowed to cool to room temperature and sifted to obtain a granular mass.
Table 10: Composition of orally disintegrating tablets of eszopiclone
Eszopiclone granules were blended with other excipients and the lubricated blend was compressed to form tablets with the following parameters:
Hardness (N) : 30-40
Friability (%) : 0.40
Disintegration time (sec) : 15-20
Disintegration time in oral cavity (sec) : 40-50
Tablets with desired taste-masking, friability and disintegration time were obtained.
B. Placebo chewing gum composition
Table 11: Composition of placebo chewing gum
Procedure: Butadiene Styrene 50:50 Rubber was melted and collected in a vessel having molten polyvinyl acetate and microcrystalline wax at a temperature of about 800C. This was mixed with butyl hydroxy toluene and stirred well until it had the consistency of a thick syrup. This mass was filtered through a fine mesh screen. The clear base was then mixed well with glyceryl monostearate under hot conditions. The mixture was further mixed with calcium carbonate and sucralose. Flavor and color were added to the above mass at low temerature. The homogenized mixture was then poured onto cooling belts and cooled with cold air. The cooled mass was cut into square shape chunks and packed in suitable packing wrapper.
The placebo chewing gum composition was consumed by the patient in the morning when the unpleasant taste of eszopiclone was felt. The placebo composition was chewed over extended time period as desired by the patient and it was pleasant to taste and could mask unpleasant bitter taste perceived due to administration of eszopiclone formulation.
Example 6: Taste-masked eszopiclone orally disintegrating tablets
Table 12: Composition of orally disintegrating tablets of eszopiclone
Procedure: Eszopiclone beads of controlled size and density were prepared using the melt extrusion technique by passing / forcing a molten mixture of eszopiclone, microcrystalline cellulose and glyceryl behenate through a fine nozzle. The extruded mass was then cut and shaped, in a spheronization process, to produce beads suitable for formulation as controlled release multiparticulates. The resultant beads were further coated with Kollicoat® SR 30 D to a weight gain of 20% by weight for additional release rate control and taste masking. These taste masked eszopiclone beads was mixed with other excipients and compressed into orally disintegrating tablets. Tablets with desired taste and disintegration time were obtained.
Claims
1. A pharmaceutical composition for alleviating unpleasant taste associated with eszopiclone therapy comprising:
(a) eszopiclone;
(b) at least one taste-masking agent; and
(c) at least one pharmaceutically acceptable excipient.
2. The composition of claim 1 wherein said taste-masking agent is a polymeric pharmaceutically acceptable excipient, a non-polymeric pharmaceutically acceptable excipient, an adsorbent, a carbomer, an ion exchange resin, a cyclodextrin or a derivative thereof, or a pH-modifier, or a combination thereof.
3. The composition of claim 2 wherein said polymeric pharmaceutically acceptable excipient is a cellulose derivative, a saccharide or polysaccharide, a polyhydric alcohol, a poly(oxyethylene)-poly(oxypropylene) block copolymer, a vinyl derivative or polymer or copolymer thereof, or a acrylic acid derivative, or a combination thereof.
4. The composition of claim 3 wherein said cellulose derivative is ethyl cellulose, methylcellulose, hydroxypropylmethylcellulose, hydroxypropyl cellulose, hydroxyethyl cellulose, hydroxymethyl cellulose, hydroxypropyl ethylcellulose, carboxy ethylcellulose, carboxymethyl ethylcellulose, carboxymethyl hydroxyethylcellulose, hydroxyethylmethyl carboxymethyl cellulose, hydroxyethyl methyl cellulose, carboxymethyl cellulose, methylhydroxyethyl cellulose, methylhydroxypropyl cellulose, carboxymethyl sulfoethyl cellulose, sodium carboxy methyl cellulose, cellulose acetate, cellulose acetate phthalate, cellulose acetate butyrate, hydroxypropylmethylcellulose acetate succinate, hydroxypropylmethylcellulose phthalate, hydroxymethyl ethylcellulose phthalate, cellulose acetate phthalate, cellulose acetate succinate, cellulose acetate maleate, cellulose acetate trimelliate, cellulose benzoate phthalate, cellulose propionate phthalate, methylcellulose phthalate, or ethylhydroxy ethylcellulose phthalate, or a combination thereof.
5. The composition of claim 3 wherein said vinyl derivative or polymer or copolymer thereof is polyvinylacetate aqueous dispersion (Kollicoat® SR 30D), copolymers of vinyl pyrrolidone, polyvinyl alcohol phthalate, polyvinylacetal phthalate, polyvinyl butylate phthalate, polyvinylacetoacetal phthalate, or polyvinylpyrrolidone, or a combination thereof.
6. The composition of claim 3 wherein said acrylic acid derivative is methacrylic acid, polymethacrylic acid, polyacrylate, or polymethacrylate, or a combination thereof.
7. The composition of claim 6 wherein said polyacrylate is a) copolymer formed from monomers selected from the group consisting of methacrylic acid, methacrylic acid esters, acrylic acid and acrylic acid esters; b) copolymer formed from monomers selected from the group consisting of butyl methacrylate, (2- dimethylaminoethyl)methacrylate and methyl methacrylate; c) copolymer formed from monomers selected from the group consisting of ethyl acrylate, methyl methacrylate and trimethylammonioethyl methacrylate chloride; or d) copolymer of acrylate and methacrylates with/without quarternary ammonium group in combination with sodium carboxymethylcellulose.
8. The composition of claim 2 wherein said non-polymeric pharmaceutically acceptable excipient is spermaceti wax, carnauba wax, Japan wax, bayberry wax, flax wax, beeswax, yellow wax, Chinese wax, shellac wax, lanolin wax, sugarcane wax, candelilla wax, castor wax, paraffin wax, microcrystalline wax, petrolatum wax, carbowax, mineral waxes, glyceryl monostearate, glyceryl distearate, glyceryl tristearate, glyceryl dipalmitate, glyceryl tripalmitate, glyceryl monopalmitate, glyceryl patmitostearate, glyceryl dilaurate, glyceryl trilaurate, glyceryl monolaurate, glyceryl didocosanoate, glyceryl tridocosanoate, glyceryl monodocosanoate, glyceryl monocaproate, glyceryl dicaproate, glyceryl tricaproate, glyceryl monomyristate, glyceryl dimyristate, glyceryl trimyristate, glyceryl monodecenoate, glyceryl didecenoate, glyceryl tridecenoate, glyceryl behenate, polyglyceryl diisostearate, lauroyl macrogolglycerides, oleoyl macrogolglycerides, stearoyl macrogolglycerides, hydrogenated palm kernel oil, hydrogenated peanut oil, hydrogenated palm oil, hydrogenated rapeseed oil, hydrogenated rice bran oil, hydrogenated soybean oil, hydrogenated sunflower oil, hydrogenated castor oil, hydrogenated cottonseed oil, decenoic acid, docosanoic acid, stearic acid, palmitic acid, lauric acid, myristic acid, cetyl alcohol, or stearyl alcohol, or a combination thereof.
9. The composition of claim 2 wherein said adsorbent is bentonite, kaolite, zeolite, sodium alginate, magnesium aluminium silicate, silica gel, or activated charcoal, or a combination thereof.
10. The composition of claim 2 wherein said carbomer is carbomer 934, carbomer 971, or carbomer 974, or a combination thereof.
11. The composition of claim 2 wherein said ion exchange resin is a cationic ion exchange resin.
12. The composition of claim 2 wherein said cyclodextrin is α-cyclodextrin, β- cyclodextrin, γ-cyclodextrin, dimethyi-β-cyc!odextrin, trimethyl-β-cyclodextrin, hydroxymethyl-β-cyclodextrin, hydroxypropyl-β-cyclodextrin, hydroxyethyl-β- cyclodextrin, β-cyclodextrin sulfate, β-cyclodextrin sulfonate, or β-cyclodextrin sulfobutyl ether, dihydroxypropyl-β-cyclodextrin, glucosyl-α-cyclodextrin, glucosyl-β- cyclodextrin, diglucosyl-β-cyclodextrin, maltosyl-γ-cyclodextrin, maltosyl-γ- cyclodextrin, maltosyl-γ-cyclodextrin, maltotriosyl-β cyclodextrin, maltotriosyl-γ- cyclodextrin, dimaltosyl-β-cyclodextrin, maltosyl-β-cyclodextrin/dimaltosyl-β- cyclodextrin or a combination thereof.
13. The composition of claim 2 wherein said pH-modifier is disodium hydrogen phosphate, sodium triphosphate, monosodium phosphate, sodium carbonate, potassium dihydrogen phosphate, sodium bicarbonate, meglumine, citric acid, benzoic acid or tartaric acid or a combination thereof.
14. The composition of claim 1 wherein said taste-masking agent is a taste-modifier, said taste-modifier being hydrogenated castor oil, thaumatin, arginine, ornithine, lysine, citrulline, adenosine 51 monophosphate, thymidine 5' monophosphate, adenosine 51 diphosphate, adenosine 3' monophosphate, adenosine 5'-succinate, adenosine 5' triphosphate, adenosine 2' monophosphate, δ'-cytidylic acid, inosinic acid, monellin, ferulic acid, or caffeic acid, or a combination thereof.
15. The composition of claim 1 wherein said excipient is a directly compressible excipient, binder, disintegrant, superdisintegrant, diluent, salivating agent, surfactant, flavor, sweetener, colorant, souring agent, viscolizer, glidant, lubricant, solubilizer, or stabilizer.
16. The composition of claim 15 wherein said directly compressible excipient comprises mannitol and calcium silicate.
17. The composition of claim 1 wherein said eszopiclone is in the form of powder, granules, pellets or beads, or a combination thereof.
18. The composition of claim 1 wherein said composition is in the form of a suspension, powder for suspension, dispersible tablet, orally disintegrating tablet, effervescent tablet, chewable tablet, sprinkle granules, quick melt wafers, lozenge, or chewing gum.
19. The composition of claim 1 wherein said composition alleviates the immediate bitter taste otherwise experienced by a patient on oral intake of eszopiclone.
20. The composition of claim 1 wherein said composition releases not less than 70% of eszopiclone within about 45 minutes.
21. The composition of claim 1 wherein said composition releases eszopiclone in a controlled manner over a period of about 2 to about 24 hours.
22. A process for preparing a pharmaceutical composition for alleviating unpleasant taste associated with eszopiclone therapy comprising: a. treating eszopiclone with at least one taste-masking agent, optionally along with at least one pharmaceutically acceptable excipient to form taste-masked eszopiclone; b. blending taste-masked eszopiclone of step a. with other pharmaceutically acceptable excipients, except lubricant, to form a uniform powder mix; c. lubricating the powder mix of step b.; and d. forming the powder mix of step c. or of step b. into an orally disintegrating tablet, dispersible tablet, effervescent tablet, chewable tablet, sprinkle granules, suspension, powder for suspension, quick melt wafers, lozenge, or chewing gum.
23. A kit for alleviating unpleasant taste associated with eszopiclone therapy comprising:
(a) the pharmaceutical composition of claim 1 ;
(b) a placebo composition devoid of eszopiclone; and
(c) instructions for use of a. and b. either together or separately, wherein when consumed separately, b. is taken at an appropriate time subsequent to the intake of a., said appropriate time being from about one minute to about 24 hours after administration of a.
24. The kit of claim 23 wherein said placebo composition is in the form of a suspension, powder for suspension, dispersible tablet, effervescent tablet, orally disintegrating tablet, chewable tablet, sprinkle granules, quick melt wafers, lozenge, chewing gum, buccal tablet, orally dissolvable film or strip.
25. A method of alleviating unpleasant taste associated with eszopiclone therapy comprising: administering to a patient in need thereof the pharmaceutical composition of claim 1 to alleviate any immediate bitterness otherwise associated with oral intake of eszopiclone; and optionally administering at least one placebo composition devoid of the eszopiclone either along with or subsequently at an appropriate time after the administration of the pharmaceutical composition of claim 1 to counter unpleasant after-taste associated with administration of eszopiclone, wherein said appropriate time is from about one minute to about 24 hours after administration of the pharmaceutical composition of claim 1.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN931MU2009 | 2009-04-08 | ||
| IN931/MUM/2009 | 2009-04-08 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2010116385A2 true WO2010116385A2 (en) | 2010-10-14 |
| WO2010116385A3 WO2010116385A3 (en) | 2011-01-20 |
Family
ID=42936653
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IN2010/000228 WO2010116385A2 (en) | 2009-04-08 | 2010-04-08 | Pharmaceutical compositions for alleviating unpleasant taste |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2010116385A2 (en) |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010116385A3 (en) * | 2009-04-08 | 2011-01-20 | Rubicon Research Private Limited | Pharmaceutical compositions for alleviating unpleasant taste |
| CN102727452A (en) * | 2011-04-01 | 2012-10-17 | 成都康弘药业集团股份有限公司 | Eszopiclone-containing particle and its preparation method |
| WO2013038323A1 (en) * | 2011-09-13 | 2013-03-21 | Unimark Remedies Ltd. | Taste masked pharmaceutical compositions of cefuroxime axetil |
| WO2014012571A1 (en) * | 2012-07-16 | 2014-01-23 | Ratiopharm Gmbh | Complex of agomelatine and cyclodextrin |
| WO2014148883A1 (en) * | 2013-03-20 | 2014-09-25 | Natureceuticals Sdn Bhd | Herbaceutical formulations |
| WO2014184663A3 (en) * | 2013-05-15 | 2015-01-22 | Apr Applied Pharma Research Sa | Orally dispersible drug formulations |
| CN104922687A (en) * | 2015-04-21 | 2015-09-23 | 襄阳市中心医院 | Eszopiclone-cyclodextrin inclusion compound and preparation method thereof |
| TWI594765B (en) * | 2012-04-30 | 2017-08-11 | 大塚製藥股份有限公司 | Oral formulation |
| US10010638B2 (en) | 2016-06-14 | 2018-07-03 | S. C. Johnson & Son, Inc. | Wax melt with filler |
| US10076494B2 (en) | 2016-06-16 | 2018-09-18 | Dexcel Pharma Technologies Ltd. | Stable orally disintegrating pharmaceutical compositions |
| CN109925331A (en) * | 2019-04-19 | 2019-06-25 | 中国农业科学院农产品加工研究所 | A kind of peanut stem leaf extract and the preparation method and application thereof |
| US10342886B2 (en) | 2016-01-26 | 2019-07-09 | S.C. Johnson & Son, Inc. | Extruded wax melt and method of producing same |
| JP2020090464A (en) * | 2018-12-06 | 2020-06-11 | 日本ケミファ株式会社 | Tablet containing any of zopiclone, its optical isomer or salts thereof as active ingredient |
| US11077055B2 (en) | 2015-04-29 | 2021-08-03 | Dexcel Pharma Technologies Ltd. | Orally disintegrating compositions |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5206025A (en) | 1989-05-24 | 1993-04-27 | Rhone-Poulenc Sante | Porous pharmaceutical form and its preparation |
| US6319926B1 (en) | 1991-01-17 | 2001-11-20 | Sepracor Inc. | Optically active 5H-pyrrolo[3, 4-B]pyrazine derivative, its preparation and pharmaceutical compositions containing it |
| US6436936B1 (en) | 1991-12-02 | 2002-08-20 | Sepracor Inc. | Methods and compositions for treating disorders, using optically pure (+) zopiclone |
| WO2007052289A2 (en) | 2005-07-22 | 2007-05-10 | Rubicon Research Pvt Ltd. | Novel dispersible tablet composition |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7632517B2 (en) * | 1997-10-01 | 2009-12-15 | Novadel Pharma Inc. | Buccal, polar and non-polar spray containing zolpidem |
| US20040258752A1 (en) * | 2003-01-31 | 2004-12-23 | Paruthi Manoj Kumar | Taste masking pharmaceutical composition and process for its preparation |
| CA2548917C (en) * | 2003-12-11 | 2014-09-23 | Sepracor Inc. | Combination of a sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression |
| US7465729B2 (en) * | 2004-04-05 | 2008-12-16 | Sepracor Inc. | Methods of treatment of menopause and perimenopause using eszopiclone |
| US8242131B2 (en) * | 2005-05-25 | 2012-08-14 | Transcept Pharmaceuticals, Inc. | Methods of treating middle-of-the-night insomnia |
| CA2614244A1 (en) * | 2005-07-06 | 2007-01-11 | Sepracor Inc. | Combinations of eszopiclone and an antidepressant |
| WO2010116385A2 (en) * | 2009-04-08 | 2010-10-14 | Rubicon Research Private Limited | Pharmaceutical compositions for alleviating unpleasant taste |
-
2010
- 2010-04-08 WO PCT/IN2010/000228 patent/WO2010116385A2/en active Application Filing
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5206025A (en) | 1989-05-24 | 1993-04-27 | Rhone-Poulenc Sante | Porous pharmaceutical form and its preparation |
| US6319926B1 (en) | 1991-01-17 | 2001-11-20 | Sepracor Inc. | Optically active 5H-pyrrolo[3, 4-B]pyrazine derivative, its preparation and pharmaceutical compositions containing it |
| US6444673B1 (en) | 1991-01-17 | 2002-09-03 | Sepracor Inc. | Optically active 5H-pyrrolo[3,4-b]pyrazine derivative, its preparation and pharmaceutical compositions containing it |
| US6864257B2 (en) | 1991-01-17 | 2005-03-08 | Sepracor Inc. | Optically active 5H-pyrrolo[3,4-b]pyrazine derivative, its preparation and pharmaceutical compositions containing it |
| US7381724B2 (en) | 1991-01-17 | 2008-06-03 | Sepracor Inc. | Optically active 5H-pyrrolo[3,4-b]pyrazine derivative, its preparation and pharmaceutical compositions containing same |
| US6436936B1 (en) | 1991-12-02 | 2002-08-20 | Sepracor Inc. | Methods and compositions for treating disorders, using optically pure (+) zopiclone |
| WO2007052289A2 (en) | 2005-07-22 | 2007-05-10 | Rubicon Research Pvt Ltd. | Novel dispersible tablet composition |
Non-Patent Citations (3)
| Title |
|---|
| G. CAILLE ET AL., BIOPHARMACEUTICS AND DRUG DISPOSITION, vol. 5, no. 2, 1983, pages 117 - 125 |
| G. HEMPEL ET AL., JOURNAL OF CHROMATOGRAPHY B, vol. 675, 1996, pages 139 - 146 |
| JADWIGA NAJIB, CLINICAL THERAPEUTICS, vol. 28, no. 4, 2006, pages 491 - 516 |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010116385A3 (en) * | 2009-04-08 | 2011-01-20 | Rubicon Research Private Limited | Pharmaceutical compositions for alleviating unpleasant taste |
| CN102727452A (en) * | 2011-04-01 | 2012-10-17 | 成都康弘药业集团股份有限公司 | Eszopiclone-containing particle and its preparation method |
| CN102727452B (en) * | 2011-04-01 | 2014-12-24 | 成都康弘药业集团股份有限公司 | Eszopiclone-containing particle and its preparation method |
| WO2013038323A1 (en) * | 2011-09-13 | 2013-03-21 | Unimark Remedies Ltd. | Taste masked pharmaceutical compositions of cefuroxime axetil |
| TWI594765B (en) * | 2012-04-30 | 2017-08-11 | 大塚製藥股份有限公司 | Oral formulation |
| WO2014012571A1 (en) * | 2012-07-16 | 2014-01-23 | Ratiopharm Gmbh | Complex of agomelatine and cyclodextrin |
| WO2014148883A1 (en) * | 2013-03-20 | 2014-09-25 | Natureceuticals Sdn Bhd | Herbaceutical formulations |
| WO2014184663A3 (en) * | 2013-05-15 | 2015-01-22 | Apr Applied Pharma Research Sa | Orally dispersible drug formulations |
| CN104922687A (en) * | 2015-04-21 | 2015-09-23 | 襄阳市中心医院 | Eszopiclone-cyclodextrin inclusion compound and preparation method thereof |
| US11077055B2 (en) | 2015-04-29 | 2021-08-03 | Dexcel Pharma Technologies Ltd. | Orally disintegrating compositions |
| US10342886B2 (en) | 2016-01-26 | 2019-07-09 | S.C. Johnson & Son, Inc. | Extruded wax melt and method of producing same |
| US10010638B2 (en) | 2016-06-14 | 2018-07-03 | S. C. Johnson & Son, Inc. | Wax melt with filler |
| US10076494B2 (en) | 2016-06-16 | 2018-09-18 | Dexcel Pharma Technologies Ltd. | Stable orally disintegrating pharmaceutical compositions |
| US10835488B2 (en) | 2016-06-16 | 2020-11-17 | Dexcel Pharma Technologies Ltd. | Stable orally disintegrating pharmaceutical compositions |
| JP2020090464A (en) * | 2018-12-06 | 2020-06-11 | 日本ケミファ株式会社 | Tablet containing any of zopiclone, its optical isomer or salts thereof as active ingredient |
| JP7304691B2 (en) | 2018-12-06 | 2023-07-07 | 日本ケミファ株式会社 | Tablets containing Zopiclone, its optical isomers, or salts thereof as active ingredients |
| CN109925331A (en) * | 2019-04-19 | 2019-06-25 | 中国农业科学院农产品加工研究所 | A kind of peanut stem leaf extract and the preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2010116385A3 (en) | 2011-01-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2010116385A2 (en) | Pharmaceutical compositions for alleviating unpleasant taste | |
| JP5845173B2 (en) | Orally disintegrating tablet composition comprising a combination of a non-opioid and an opioid analgesic | |
| US8840924B2 (en) | Compositions and methods of making rapidly dissolving ionically masked formulations | |
| ES2739888T3 (en) | Compositions and pharmaceutical tablets with compressible coating and manufacturing methods | |
| WO2011030351A2 (en) | Taste - masked pharmaceutical compositions | |
| US20120093738A1 (en) | Taste-masked oral formulations of influenza antivirals | |
| US20090155360A1 (en) | Orally disintegrating tablets comprising diphenhydramine | |
| US20050169986A1 (en) | Fast disintegrating tablets | |
| US20110212171A1 (en) | Taste masked topiramate composition and an orally disintegrating tablet comprising the same | |
| US20110268808A1 (en) | Dual-release pharmaceutical suspension | |
| JP2013545746A (en) | Sustained release composition | |
| WO2009084017A2 (en) | Taste-masked orally disintegrating tablets of memantine hydrochloride | |
| WO2011110939A2 (en) | Pharmaceutical compositions of substituted benzhydrylpiperazines | |
| BRPI0608853B1 (en) | pharmaceutical compositions and process for the manufacture of gastro-resistant rifaximin microgranules | |
| WO2013183062A2 (en) | Palatable formulations of ibuprofen | |
| CA2574303A1 (en) | Modafinil oral lyophilizate | |
| JP2013534242A (en) | Formulations containing nalbuphine and their use | |
| WO2009078034A2 (en) | Oral disintegrating tablets of ropinirole hydrochloride | |
| BRPI0407116B1 (en) | ACTIVE PRINCIPLE COATED PARTICLE, PHARMACEUTICAL OR COSMETIC COMPOSITION, AND METHOD FOR PRODUCTION OF ACTIVE PRINCIPLE COATED PARTICLE | |
| WO2010046933A2 (en) | Pharmaceutical compositions of taste-masked linezolid | |
| US20190125792A1 (en) | Pharmaceutical compositions comprising ferric citrate and methods for the production thereof | |
| JP6723229B2 (en) | Pharmaceutical composition containing alpericib | |
| EP1621186A1 (en) | Modafinil oral lyophilizate | |
| CN117355296A (en) | Prolonged release pharmaceutical composition for oral administration Shu Sai mex |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10747682 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10747682 Country of ref document: EP Kind code of ref document: A2 |