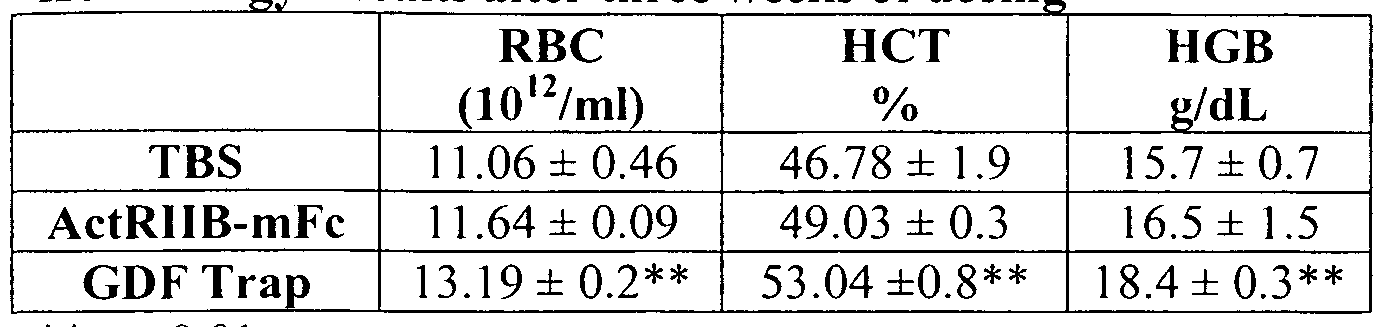WO2010019261A1 - Use of gdf traps to increase red blood cell levels - Google Patents
Use of gdf traps to increase red blood cell levels Download PDFInfo
- Publication number
- WO2010019261A1 WO2010019261A1 PCT/US2009/004659 US2009004659W WO2010019261A1 WO 2010019261 A1 WO2010019261 A1 WO 2010019261A1 US 2009004659 W US2009004659 W US 2009004659W WO 2010019261 A1 WO2010019261 A1 WO 2010019261A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- seq
- amino acid
- polypeptide
- sequence
- actriib
- Prior art date
Links
- 210000003743 erythrocyte Anatomy 0.000 title claims abstract description 124
- 238000000034 method Methods 0.000 claims abstract description 77
- 102000001554 Hemoglobins Human genes 0.000 claims abstract description 50
- 108010054147 Hemoglobins Proteins 0.000 claims abstract description 50
- 230000001965 increasing effect Effects 0.000 claims abstract description 42
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 332
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 328
- 229920001184 polypeptide Polymers 0.000 claims description 325
- 150000001413 amino acids Chemical group 0.000 claims description 179
- 235000001014 amino acid Nutrition 0.000 claims description 165
- 230000027455 binding Effects 0.000 claims description 93
- 210000004027 cell Anatomy 0.000 claims description 91
- 108020001507 fusion proteins Proteins 0.000 claims description 75
- 102000037865 fusion proteins Human genes 0.000 claims description 75
- 208000007502 anemia Diseases 0.000 claims description 72
- 239000000488 activin Substances 0.000 claims description 63
- 108010059616 Activins Proteins 0.000 claims description 61
- 238000011282 treatment Methods 0.000 claims description 49
- 150000007523 nucleic acids Chemical class 0.000 claims description 41
- 210000004369 blood Anatomy 0.000 claims description 37
- 239000008280 blood Substances 0.000 claims description 37
- 102000039446 nucleic acids Human genes 0.000 claims description 30
- 108020004707 nucleic acids Proteins 0.000 claims description 30
- 239000003795 chemical substances by application Substances 0.000 claims description 25
- 238000001727 in vivo Methods 0.000 claims description 25
- 210000001519 tissue Anatomy 0.000 claims description 21
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 18
- 108010023082 activin A Proteins 0.000 claims description 18
- 125000000539 amino acid group Chemical group 0.000 claims description 16
- 238000000746 purification Methods 0.000 claims description 16
- 230000002378 acidificating effect Effects 0.000 claims description 15
- 208000020832 chronic kidney disease Diseases 0.000 claims description 13
- 230000015572 biosynthetic process Effects 0.000 claims description 11
- 238000002512 chemotherapy Methods 0.000 claims description 9
- 102000005606 Activins Human genes 0.000 claims description 8
- 108060003951 Immunoglobulin Proteins 0.000 claims description 8
- 102000018358 immunoglobulin Human genes 0.000 claims description 8
- 238000009826 distribution Methods 0.000 claims description 7
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 6
- 210000004962 mammalian cell Anatomy 0.000 claims description 6
- 210000002966 serum Anatomy 0.000 claims description 6
- 210000002027 skeletal muscle Anatomy 0.000 claims description 6
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 claims description 4
- 102000007474 Multiprotein Complexes Human genes 0.000 claims description 4
- 108010085220 Multiprotein Complexes Proteins 0.000 claims description 4
- 235000003704 aspartic acid Nutrition 0.000 claims description 4
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 claims description 4
- 210000004978 chinese hamster ovary cell Anatomy 0.000 claims description 4
- 235000013922 glutamic acid Nutrition 0.000 claims description 4
- 239000004220 glutamic acid Substances 0.000 claims description 4
- 208000017169 kidney disease Diseases 0.000 claims description 4
- 230000004807 localization Effects 0.000 claims description 4
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Chemical compound NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 claims description 3
- 239000000710 homodimer Substances 0.000 claims description 3
- 238000009396 hybridization Methods 0.000 claims description 3
- 108010071390 Serum Albumin Proteins 0.000 claims description 2
- 102000007562 Serum Albumin Human genes 0.000 claims description 2
- 229940123237 Taxane Drugs 0.000 claims description 2
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 claims description 2
- 101100437153 Rattus norvegicus Acvr2b gene Proteins 0.000 claims 12
- 210000004748 cultured cell Anatomy 0.000 claims 7
- 125000003473 lipid group Chemical group 0.000 claims 3
- 201000006370 kidney failure Diseases 0.000 claims 1
- DKPFODGZWDEEBT-QFIAKTPHSA-N taxane Chemical class C([C@]1(C)CCC[C@@H](C)[C@H]1C1)C[C@H]2[C@H](C)CC[C@@H]1C2(C)C DKPFODGZWDEEBT-QFIAKTPHSA-N 0.000 claims 1
- 239000000203 mixture Substances 0.000 abstract description 31
- 241000251539 Vertebrata <Metazoa> Species 0.000 abstract description 11
- 241000288906 Primates Species 0.000 abstract description 4
- 241000283984 Rodentia Species 0.000 abstract description 3
- 108090000623 proteins and genes Proteins 0.000 description 114
- 229940024606 amino acid Drugs 0.000 description 99
- 102000004169 proteins and genes Human genes 0.000 description 88
- 235000018102 proteins Nutrition 0.000 description 86
- 239000003446 ligand Substances 0.000 description 78
- 230000000694 effects Effects 0.000 description 74
- 102220479051 CD59 glycoprotein_L79D_mutation Human genes 0.000 description 67
- 102100026818 Inhibin beta E chain Human genes 0.000 description 55
- 125000003275 alpha amino acid group Chemical group 0.000 description 55
- 150000001875 compounds Chemical class 0.000 description 50
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 44
- 230000014509 gene expression Effects 0.000 description 36
- 230000035772 mutation Effects 0.000 description 33
- 239000002773 nucleotide Substances 0.000 description 28
- 125000003729 nucleotide group Chemical group 0.000 description 28
- 241000699670 Mus sp. Species 0.000 description 27
- 102000005962 receptors Human genes 0.000 description 27
- 108020003175 receptors Proteins 0.000 description 27
- 239000003981 vehicle Substances 0.000 description 26
- 230000002489 hematologic effect Effects 0.000 description 25
- 239000013598 vector Substances 0.000 description 25
- 230000004927 fusion Effects 0.000 description 24
- 238000005534 hematocrit Methods 0.000 description 23
- 108020001756 ligand binding domains Proteins 0.000 description 23
- 229910052742 iron Inorganic materials 0.000 description 22
- 230000004075 alteration Effects 0.000 description 21
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 20
- 230000000925 erythroid effect Effects 0.000 description 20
- 210000000952 spleen Anatomy 0.000 description 20
- 230000002829 reductive effect Effects 0.000 description 19
- 238000006467 substitution reaction Methods 0.000 description 19
- 239000003814 drug Substances 0.000 description 18
- 238000004519 manufacturing process Methods 0.000 description 18
- -1 aspartic acid Chemical class 0.000 description 17
- 238000003556 assay Methods 0.000 description 17
- 230000036772 blood pressure Effects 0.000 description 17
- 230000013595 glycosylation Effects 0.000 description 17
- 238000006206 glycosylation reaction Methods 0.000 description 17
- 108010007726 Bone Morphogenetic Proteins Proteins 0.000 description 16
- 102000007350 Bone Morphogenetic Proteins Human genes 0.000 description 16
- 206010028980 Neoplasm Diseases 0.000 description 16
- 102000003978 Tissue Plasminogen Activator Human genes 0.000 description 16
- 108090000373 Tissue Plasminogen Activator Proteins 0.000 description 16
- 210000001185 bone marrow Anatomy 0.000 description 16
- 229940112869 bone morphogenetic protein Drugs 0.000 description 16
- 230000011664 signaling Effects 0.000 description 16
- 230000001225 therapeutic effect Effects 0.000 description 16
- 229960000187 tissue plasminogen activator Drugs 0.000 description 16
- 102100039939 Growth/differentiation factor 8 Human genes 0.000 description 15
- 108010056852 Myostatin Proteins 0.000 description 15
- 102000003951 Erythropoietin Human genes 0.000 description 13
- 108090000394 Erythropoietin Proteins 0.000 description 13
- 229940105423 erythropoietin Drugs 0.000 description 13
- 239000012634 fragment Substances 0.000 description 13
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 description 13
- 210000001995 reticulocyte Anatomy 0.000 description 13
- 238000012360 testing method Methods 0.000 description 13
- 102100040897 Embryonic growth/differentiation factor 1 Human genes 0.000 description 12
- 101000937269 Homo sapiens Activin receptor type-2B Proteins 0.000 description 12
- 229930012538 Paclitaxel Natural products 0.000 description 12
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 12
- 229940009098 aspartate Drugs 0.000 description 12
- 210000004899 c-terminal region Anatomy 0.000 description 12
- 201000011510 cancer Diseases 0.000 description 12
- 230000010437 erythropoiesis Effects 0.000 description 12
- 102000045412 human ACVR2B Human genes 0.000 description 12
- 229960001592 paclitaxel Drugs 0.000 description 12
- 239000013612 plasmid Substances 0.000 description 12
- 108091033319 polynucleotide Proteins 0.000 description 12
- 102000040430 polynucleotide Human genes 0.000 description 12
- 239000002157 polynucleotide Substances 0.000 description 12
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 12
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 11
- 230000007423 decrease Effects 0.000 description 11
- 208000035475 disorder Diseases 0.000 description 11
- 210000003013 erythroid precursor cell Anatomy 0.000 description 11
- 230000001154 acute effect Effects 0.000 description 10
- 230000008859 change Effects 0.000 description 10
- 238000012217 deletion Methods 0.000 description 10
- 230000037430 deletion Effects 0.000 description 10
- 238000000338 in vitro Methods 0.000 description 10
- 230000004048 modification Effects 0.000 description 10
- 238000012986 modification Methods 0.000 description 10
- 230000001105 regulatory effect Effects 0.000 description 10
- 108010090296 Growth Differentiation Factor 1 Proteins 0.000 description 9
- 102220542870 Presenilins-associated rhomboid-like protein, mitochondrial_L79E_mutation Human genes 0.000 description 9
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 9
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 9
- 241000269370 Xenopus <genus> Species 0.000 description 9
- 238000007792 addition Methods 0.000 description 9
- 210000002960 bfu-e Anatomy 0.000 description 9
- 230000003247 decreasing effect Effects 0.000 description 9
- 201000010099 disease Diseases 0.000 description 9
- 229940079593 drug Drugs 0.000 description 9
- 210000003527 eukaryotic cell Anatomy 0.000 description 9
- 239000013604 expression vector Substances 0.000 description 9
- 239000003112 inhibitor Substances 0.000 description 9
- 239000002502 liposome Substances 0.000 description 9
- 230000001177 retroviral effect Effects 0.000 description 9
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 9
- 210000000689 upper leg Anatomy 0.000 description 9
- 102220525561 Coiled-coil domain-containing protein 200_A24N_mutation Human genes 0.000 description 8
- 241000282412 Homo Species 0.000 description 8
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 8
- 241001465754 Metazoa Species 0.000 description 8
- 241000699666 Mus <mouse, genus> Species 0.000 description 8
- 108010076504 Protein Sorting Signals Proteins 0.000 description 8
- 241000700159 Rattus Species 0.000 description 8
- 238000004458 analytical method Methods 0.000 description 8
- 238000000423 cell based assay Methods 0.000 description 8
- 230000002401 inhibitory effect Effects 0.000 description 8
- 239000002609 medium Substances 0.000 description 8
- 229920000609 methyl cellulose Polymers 0.000 description 8
- 239000001923 methylcellulose Substances 0.000 description 8
- 238000010172 mouse model Methods 0.000 description 8
- 239000002243 precursor Substances 0.000 description 8
- UBWXUGDQUBIEIZ-UHFFFAOYSA-N (13-methyl-3-oxo-2,6,7,8,9,10,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-17-yl) 3-phenylpropanoate Chemical compound CC12CCC(C3CCC(=O)C=C3CC3)C3C1CCC2OC(=O)CCC1=CC=CC=C1 UBWXUGDQUBIEIZ-UHFFFAOYSA-N 0.000 description 7
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 7
- 241000282567 Macaca fascicularis Species 0.000 description 7
- 235000004279 alanine Nutrition 0.000 description 7
- CKLJMWTZIZZHCS-REOHCLBHSA-L aspartate group Chemical group N[C@@H](CC(=O)[O-])C(=O)[O-] CKLJMWTZIZZHCS-REOHCLBHSA-L 0.000 description 7
- 238000004820 blood count Methods 0.000 description 7
- 230000006870 function Effects 0.000 description 7
- 230000011132 hemopoiesis Effects 0.000 description 7
- 210000003205 muscle Anatomy 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- 230000004044 response Effects 0.000 description 7
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 6
- 102000014914 Carrier Proteins Human genes 0.000 description 6
- 241000699802 Cricetulus griseus Species 0.000 description 6
- 108020004414 DNA Proteins 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 102000005720 Glutathione transferase Human genes 0.000 description 6
- 108010070675 Glutathione transferase Proteins 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- 239000007983 Tris buffer Substances 0.000 description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 6
- 108091008324 binding proteins Proteins 0.000 description 6
- 230000004071 biological effect Effects 0.000 description 6
- 150000001720 carbohydrates Chemical group 0.000 description 6
- 230000001684 chronic effect Effects 0.000 description 6
- 238000003776 cleavage reaction Methods 0.000 description 6
- 235000018417 cysteine Nutrition 0.000 description 6
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 6
- 238000001514 detection method Methods 0.000 description 6
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 6
- 230000003993 interaction Effects 0.000 description 6
- 210000003734 kidney Anatomy 0.000 description 6
- 210000000265 leukocyte Anatomy 0.000 description 6
- 229910052760 oxygen Inorganic materials 0.000 description 6
- 239000001301 oxygen Substances 0.000 description 6
- 239000002953 phosphate buffered saline Substances 0.000 description 6
- 229920001223 polyethylene glycol Polymers 0.000 description 6
- 210000001236 prokaryotic cell Anatomy 0.000 description 6
- 238000011084 recovery Methods 0.000 description 6
- 230000007017 scission Effects 0.000 description 6
- 239000011780 sodium chloride Substances 0.000 description 6
- 235000000346 sugar Nutrition 0.000 description 6
- 230000004083 survival effect Effects 0.000 description 6
- 229940124597 therapeutic agent Drugs 0.000 description 6
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 6
- 238000011740 C57BL/6 mouse Methods 0.000 description 5
- 108091006020 Fc-tagged proteins Proteins 0.000 description 5
- 102000012673 Follicle Stimulating Hormone Human genes 0.000 description 5
- 108010079345 Follicle Stimulating Hormone Proteins 0.000 description 5
- 230000004988 N-glycosylation Effects 0.000 description 5
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 5
- 239000004473 Threonine Substances 0.000 description 5
- 108010023079 activin B Proteins 0.000 description 5
- 239000005557 antagonist Substances 0.000 description 5
- 230000003021 clonogenic effect Effects 0.000 description 5
- 230000001086 cytosolic effect Effects 0.000 description 5
- 230000004069 differentiation Effects 0.000 description 5
- 230000000913 erythropoietic effect Effects 0.000 description 5
- 235000019441 ethanol Nutrition 0.000 description 5
- 239000012091 fetal bovine serum Substances 0.000 description 5
- 229940028334 follicle stimulating hormone Drugs 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 238000001415 gene therapy Methods 0.000 description 5
- 229930195712 glutamate Natural products 0.000 description 5
- 230000002209 hydrophobic effect Effects 0.000 description 5
- 150000002632 lipids Chemical group 0.000 description 5
- 230000007246 mechanism Effects 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 230000037257 muscle growth Effects 0.000 description 5
- 239000008194 pharmaceutical composition Substances 0.000 description 5
- 230000001737 promoting effect Effects 0.000 description 5
- 230000001603 reducing effect Effects 0.000 description 5
- 239000000523 sample Substances 0.000 description 5
- 238000012216 screening Methods 0.000 description 5
- 210000000130 stem cell Anatomy 0.000 description 5
- 238000010254 subcutaneous injection Methods 0.000 description 5
- 239000007929 subcutaneous injection Substances 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 239000003656 tris buffered saline Substances 0.000 description 5
- 241000701447 unidentified baculovirus Species 0.000 description 5
- 239000004475 Arginine Substances 0.000 description 4
- 206010013801 Duchenne Muscular Dystrophy Diseases 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 239000004471 Glycine Substances 0.000 description 4
- 241000238631 Hexapoda Species 0.000 description 4
- 101000869690 Homo sapiens Protein S100-A8 Proteins 0.000 description 4
- 206010020772 Hypertension Diseases 0.000 description 4
- 206010061218 Inflammation Diseases 0.000 description 4
- 206010022971 Iron Deficiencies Diseases 0.000 description 4
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 4
- 241001529936 Murinae Species 0.000 description 4
- 206010028289 Muscle atrophy Diseases 0.000 description 4
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 4
- 125000000729 N-terminal amino-acid group Chemical group 0.000 description 4
- 102100032442 Protein S100-A8 Human genes 0.000 description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 4
- 229930006000 Sucrose Natural products 0.000 description 4
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 4
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 4
- 241000700605 Viruses Species 0.000 description 4
- 230000002411 adverse Effects 0.000 description 4
- 238000001042 affinity chromatography Methods 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 4
- 239000001506 calcium phosphate Substances 0.000 description 4
- 238000004587 chromatography analysis Methods 0.000 description 4
- 230000000295 complement effect Effects 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 230000003111 delayed effect Effects 0.000 description 4
- 235000005911 diet Nutrition 0.000 description 4
- 230000037213 diet Effects 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 229940088598 enzyme Drugs 0.000 description 4
- 235000011187 glycerol Nutrition 0.000 description 4
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 4
- 239000003102 growth factor Substances 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- VDXZNPDIRNWWCW-JFTDCZMZSA-N melittin Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O)CC1=CNC2=CC=CC=C12 VDXZNPDIRNWWCW-JFTDCZMZSA-N 0.000 description 4
- 201000000585 muscular atrophy Diseases 0.000 description 4
- 239000000816 peptidomimetic Substances 0.000 description 4
- 239000000825 pharmaceutical preparation Substances 0.000 description 4
- 238000012163 sequencing technique Methods 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 241000894007 species Species 0.000 description 4
- 239000003381 stabilizer Substances 0.000 description 4
- 230000000638 stimulation Effects 0.000 description 4
- 239000005720 sucrose Substances 0.000 description 4
- 208000011580 syndromic disease Diseases 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 230000008685 targeting Effects 0.000 description 4
- 239000004474 valine Substances 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 3
- UZOVYGYOLBIAJR-UHFFFAOYSA-N 4-isocyanato-4'-methyldiphenylmethane Chemical compound C1=CC(C)=CC=C1CC1=CC=C(N=C=O)C=C1 UZOVYGYOLBIAJR-UHFFFAOYSA-N 0.000 description 3
- 102000018918 Activin Receptors Human genes 0.000 description 3
- 108010052946 Activin Receptors Proteins 0.000 description 3
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 3
- 108010049870 Bone Morphogenetic Protein 7 Proteins 0.000 description 3
- 102100022544 Bone morphogenetic protein 7 Human genes 0.000 description 3
- 101100297347 Caenorhabditis elegans pgl-3 gene Proteins 0.000 description 3
- 241000282693 Cercopithecidae Species 0.000 description 3
- 102220578642 Chorion-specific transcription factor GCMb_K74M_mutation Human genes 0.000 description 3
- 108091026890 Coding region Proteins 0.000 description 3
- 102220575187 Egl nine homolog 1_F82A_mutation Human genes 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 102000016970 Follistatin Human genes 0.000 description 3
- 108010014612 Follistatin Proteins 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- 208000032843 Hemorrhage Diseases 0.000 description 3
- 101000970954 Homo sapiens Activin receptor type-2A Proteins 0.000 description 3
- 101000893552 Homo sapiens Embryonic growth/differentiation factor 1 Proteins 0.000 description 3
- 208000015710 Iron-Deficiency Anemia Diseases 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 3
- 240000007472 Leucaena leucocephala Species 0.000 description 3
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 3
- 206010027540 Microcytosis Diseases 0.000 description 3
- 102100025751 Mothers against decapentaplegic homolog 2 Human genes 0.000 description 3
- 101710143123 Mothers against decapentaplegic homolog 2 Proteins 0.000 description 3
- 102000044547 Nodal Human genes 0.000 description 3
- 108700024442 Nodal Proteins 0.000 description 3
- 102000035195 Peptidases Human genes 0.000 description 3
- 108091005804 Peptidases Proteins 0.000 description 3
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- 241000714474 Rous sarcoma virus Species 0.000 description 3
- 229920002684 Sepharose Polymers 0.000 description 3
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 3
- 102000043168 TGF-beta family Human genes 0.000 description 3
- 108091085018 TGF-beta family Proteins 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 3
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 3
- 230000000692 anti-sense effect Effects 0.000 description 3
- 235000009582 asparagine Nutrition 0.000 description 3
- 229960001230 asparagine Drugs 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 210000000988 bone and bone Anatomy 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 238000012754 cardiac puncture Methods 0.000 description 3
- 230000022159 cartilage development Effects 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 238000009643 clonogenic assay Methods 0.000 description 3
- 231100000096 clonogenic assay Toxicity 0.000 description 3
- 238000001246 colloidal dispersion Methods 0.000 description 3
- 230000002950 deficient Effects 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 230000002255 enzymatic effect Effects 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 239000005090 green fluorescent protein Substances 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 230000003394 haemopoietic effect Effects 0.000 description 3
- 125000001165 hydrophobic group Chemical group 0.000 description 3
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 3
- 230000001976 improved effect Effects 0.000 description 3
- 238000012423 maintenance Methods 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 201000006938 muscular dystrophy Diseases 0.000 description 3
- 231100000350 mutagenesis Toxicity 0.000 description 3
- 238000013059 nephrectomy Methods 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 3
- 210000005259 peripheral blood Anatomy 0.000 description 3
- 239000011886 peripheral blood Substances 0.000 description 3
- 150000003904 phospholipids Chemical class 0.000 description 3
- 239000006187 pill Substances 0.000 description 3
- 229920002704 polyhistidine Polymers 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 230000003389 potentiating effect Effects 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 238000000159 protein binding assay Methods 0.000 description 3
- 230000017854 proteolysis Effects 0.000 description 3
- 238000001959 radiotherapy Methods 0.000 description 3
- 230000002441 reversible effect Effects 0.000 description 3
- 206010039073 rheumatoid arthritis Diseases 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 238000007423 screening assay Methods 0.000 description 3
- 210000004989 spleen cell Anatomy 0.000 description 3
- 230000000087 stabilizing effect Effects 0.000 description 3
- 150000008163 sugars Chemical class 0.000 description 3
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 3
- 239000000375 suspending agent Substances 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 238000011285 therapeutic regimen Methods 0.000 description 3
- 238000011269 treatment regimen Methods 0.000 description 3
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 3
- 239000000080 wetting agent Substances 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- UUUHXMGGBIUAPW-UHFFFAOYSA-N 1-[1-[2-[[5-amino-2-[[1-[5-(diaminomethylideneamino)-2-[[1-[3-(1h-indol-3-yl)-2-[(5-oxopyrrolidine-2-carbonyl)amino]propanoyl]pyrrolidine-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-methylpentanoyl]pyrrolidine-2-carbon Chemical compound C1CCC(C(=O)N2C(CCC2)C(O)=O)N1C(=O)C(C(C)CC)NC(=O)C(CCC(N)=O)NC(=O)C1CCCN1C(=O)C(CCCN=C(N)N)NC(=O)C1CCCN1C(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C1CCC(=O)N1 UUUHXMGGBIUAPW-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- 208000030090 Acute Disease Diseases 0.000 description 2
- 102220556625 Acyl-coenzyme A thioesterase 11_K55A_mutation Human genes 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- 208000030760 Anaemia of chronic disease Diseases 0.000 description 2
- 102220638160 Arylamine N-acetyltransferase 1_R64K_mutation Human genes 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 102100027522 Baculoviral IAP repeat-containing protein 7 Human genes 0.000 description 2
- 201000006935 Becker muscular dystrophy Diseases 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 206010006895 Cachexia Diseases 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 102220578645 Chorion-specific transcription factor GCMb_N65A_mutation Human genes 0.000 description 2
- 108020004705 Codon Proteins 0.000 description 2
- 102000008186 Collagen Human genes 0.000 description 2
- 108010035532 Collagen Proteins 0.000 description 2
- 206010009944 Colon cancer Diseases 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 101100179813 Drosophila melanogaster Actbeta gene Proteins 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 108010013369 Enteropeptidase Proteins 0.000 description 2
- 102100029727 Enteropeptidase Human genes 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 208000037149 Facioscapulohumeral dystrophy Diseases 0.000 description 2
- 102100029379 Follistatin-related protein 3 Human genes 0.000 description 2
- 241000206672 Gelidium Species 0.000 description 2
- 102000005744 Glycoside Hydrolases Human genes 0.000 description 2
- 108010031186 Glycoside Hydrolases Proteins 0.000 description 2
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 2
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 2
- 208000036581 Haemorrhagic anaemia Diseases 0.000 description 2
- 241000713858 Harvey murine sarcoma virus Species 0.000 description 2
- 101001062529 Homo sapiens Follistatin-related protein 3 Proteins 0.000 description 2
- 101001059454 Homo sapiens Serine/threonine-protein kinase MARK2 Proteins 0.000 description 2
- 108091006905 Human Serum Albumin Proteins 0.000 description 2
- 102000008100 Human Serum Albumin Human genes 0.000 description 2
- 201000002980 Hyperparathyroidism Diseases 0.000 description 2
- 206010021137 Hypovolaemia Diseases 0.000 description 2
- 102000000646 Interleukin-3 Human genes 0.000 description 2
- 108010002386 Interleukin-3 Proteins 0.000 description 2
- 102000004889 Interleukin-6 Human genes 0.000 description 2
- 108090001005 Interleukin-6 Proteins 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- 102100022745 Laminin subunit alpha-2 Human genes 0.000 description 2
- 201000009342 Limb-girdle muscular dystrophy Diseases 0.000 description 2
- 108060001084 Luciferase Proteins 0.000 description 2
- 239000005089 Luciferase Substances 0.000 description 2
- 101710175625 Maltose/maltodextrin-binding periplasmic protein Proteins 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 241000713869 Moloney murine leukemia virus Species 0.000 description 2
- 208000034578 Multiple myelomas Diseases 0.000 description 2
- 101100392292 Mus musculus Gdf1 gene Proteins 0.000 description 2
- 101100369076 Mus musculus Tdgf1 gene Proteins 0.000 description 2
- 208000029578 Muscle disease Diseases 0.000 description 2
- 206010068871 Myotonic dystrophy Diseases 0.000 description 2
- 230000004989 O-glycosylation Effects 0.000 description 2
- 201000009110 Oculopharyngeal muscular dystrophy Diseases 0.000 description 2
- 102220567352 Ornithine decarboxylase antizyme 1_K74F_mutation Human genes 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 102000004270 Peptidyl-Dipeptidase A Human genes 0.000 description 2
- 108090000882 Peptidyl-Dipeptidase A Proteins 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 description 2
- 102220496976 Platelet-activating factor acetylhydrolase 2, cytoplasmic_L79A_mutation Human genes 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 102220495689 Putative uncharacterized protein FLJ43944_R40A_mutation Human genes 0.000 description 2
- 108091081021 Sense strand Proteins 0.000 description 2
- 102100028904 Serine/threonine-protein kinase MARK2 Human genes 0.000 description 2
- 102000007374 Smad Proteins Human genes 0.000 description 2
- 108010007945 Smad Proteins Proteins 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 239000012190 activator Substances 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 2
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 2
- 235000011130 ammonium sulphate Nutrition 0.000 description 2
- 208000022400 anemia due to chronic disease Diseases 0.000 description 2
- 239000002246 antineoplastic agent Substances 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 239000000440 bentonite Substances 0.000 description 2
- 235000012216 bentonite Nutrition 0.000 description 2
- 229910000278 bentonite Inorganic materials 0.000 description 2
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- 108010005774 beta-Galactosidase Proteins 0.000 description 2
- 230000003115 biocidal effect Effects 0.000 description 2
- 208000034158 bleeding Diseases 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 238000010322 bone marrow transplantation Methods 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 239000006143 cell culture medium Substances 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 208000022831 chronic renal failure syndrome Diseases 0.000 description 2
- 229920001436 collagen Polymers 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 230000009918 complex formation Effects 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 239000003636 conditioned culture medium Substances 0.000 description 2
- 201000006815 congenital muscular dystrophy Diseases 0.000 description 2
- 239000000470 constituent Substances 0.000 description 2
- 239000013068 control sample Substances 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 230000022811 deglycosylation Effects 0.000 description 2
- 238000000502 dialysis Methods 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 201000009338 distal myopathy Diseases 0.000 description 2
- 230000004064 dysfunction Effects 0.000 description 2
- 230000013020 embryo development Effects 0.000 description 2
- 210000002257 embryonic structure Anatomy 0.000 description 2
- 208000028208 end stage renal disease Diseases 0.000 description 2
- 201000000523 end stage renal failure Diseases 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 208000008570 facioscapulohumeral muscular dystrophy Diseases 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 2
- 150000002333 glycines Chemical class 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 238000013537 high throughput screening Methods 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 230000013632 homeostatic process Effects 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 230000002806 hypometabolic effect Effects 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000005462 in vivo assay Methods 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 239000003701 inert diluent Substances 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 239000000893 inhibin Substances 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 229940076264 interleukin-3 Drugs 0.000 description 2
- 229940100601 interleukin-6 Drugs 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 238000005304 joining Methods 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000008297 liquid dosage form Substances 0.000 description 2
- 208000019423 liver disease Diseases 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 210000001161 mammalian embryo Anatomy 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 210000003716 mesoderm Anatomy 0.000 description 2
- 239000000693 micelle Substances 0.000 description 2
- 208000010555 moderate anemia Diseases 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 238000002703 mutagenesis Methods 0.000 description 2
- 210000003643 myeloid progenitor cell Anatomy 0.000 description 2
- 230000004766 neurogenesis Effects 0.000 description 2
- 210000002569 neuron Anatomy 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 230000009437 off-target effect Effects 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 239000004006 olive oil Substances 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 230000011164 ossification Effects 0.000 description 2
- 210000001672 ovary Anatomy 0.000 description 2
- 238000000059 patterning Methods 0.000 description 2
- 125000001151 peptidyl group Chemical group 0.000 description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 2
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920000747 poly(lactic acid) Polymers 0.000 description 2
- 239000004626 polylactic acid Substances 0.000 description 2
- 102000054765 polymorphisms of proteins Human genes 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 230000001323 posttranslational effect Effects 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 238000011321 prophylaxis Methods 0.000 description 2
- 230000006337 proteolytic cleavage Effects 0.000 description 2
- 238000011552 rat model Methods 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 210000003705 ribosome Anatomy 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 238000002864 sequence alignment Methods 0.000 description 2
- 230000022379 skeletal muscle tissue development Effects 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 239000001509 sodium citrate Substances 0.000 description 2
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 235000010487 tragacanth Nutrition 0.000 description 2
- 239000000196 tragacanth Substances 0.000 description 2
- 229940116362 tragacanth Drugs 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 102000035160 transmembrane proteins Human genes 0.000 description 2
- 108091005703 transmembrane proteins Proteins 0.000 description 2
- 229910000391 tricalcium phosphate Inorganic materials 0.000 description 2
- 229940078499 tricalcium phosphate Drugs 0.000 description 2
- 235000019731 tricalcium phosphate Nutrition 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 description 1
- NRJAVPSFFCBXDT-HUESYALOSA-N 1,2-distearoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCCCC NRJAVPSFFCBXDT-HUESYALOSA-N 0.000 description 1
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 1
- 229940058015 1,3-butylene glycol Drugs 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- GZCWLCBFPRFLKL-UHFFFAOYSA-N 1-prop-2-ynoxypropan-2-ol Chemical compound CC(O)COCC#C GZCWLCBFPRFLKL-UHFFFAOYSA-N 0.000 description 1
- INGWEZCOABYORO-UHFFFAOYSA-N 2-(furan-2-yl)-7-methyl-1h-1,8-naphthyridin-4-one Chemical compound N=1C2=NC(C)=CC=C2C(O)=CC=1C1=CC=CO1 INGWEZCOABYORO-UHFFFAOYSA-N 0.000 description 1
- JNODDICFTDYODH-UHFFFAOYSA-N 2-hydroxytetrahydrofuran Chemical compound OC1CCCO1 JNODDICFTDYODH-UHFFFAOYSA-N 0.000 description 1
- HVCOBJNICQPDBP-UHFFFAOYSA-N 3-[3-[3,5-dihydroxy-6-methyl-4-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyoxan-2-yl]oxydecanoyloxy]decanoic acid;hydrate Chemical compound O.OC1C(OC(CC(=O)OC(CCCCCCC)CC(O)=O)CCCCCCC)OC(C)C(O)C1OC1C(O)C(O)C(O)C(C)O1 HVCOBJNICQPDBP-UHFFFAOYSA-N 0.000 description 1
- OSJPPGNTCRNQQC-UWTATZPHSA-N 3-phospho-D-glyceric acid Chemical compound OC(=O)[C@H](O)COP(O)(O)=O OSJPPGNTCRNQQC-UWTATZPHSA-N 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N 4-hydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 1
- 102000013563 Acid Phosphatase Human genes 0.000 description 1
- 108010051457 Acid Phosphatase Proteins 0.000 description 1
- 102100034111 Activin receptor type-1 Human genes 0.000 description 1
- 102100034134 Activin receptor type-1B Human genes 0.000 description 1
- 102100034135 Activin receptor type-1C Human genes 0.000 description 1
- 101710173005 Activin receptor type-1C Proteins 0.000 description 1
- 208000026872 Addison Disease Diseases 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 102000008873 Angiotensin II receptor Human genes 0.000 description 1
- 108050000824 Angiotensin II receptor Proteins 0.000 description 1
- 235000003276 Apios tuberosa Nutrition 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000010744 Arachis villosulicarpa Nutrition 0.000 description 1
- CKLJMWTZIZZHCS-UHFFFAOYSA-N Aspartic acid Chemical compound OC(=O)C(N)CC(O)=O CKLJMWTZIZZHCS-UHFFFAOYSA-N 0.000 description 1
- 244000186140 Asperula odorata Species 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 241000714235 Avian retrovirus Species 0.000 description 1
- 208000000412 Avitaminosis Diseases 0.000 description 1
- 101710177963 Baculoviral IAP repeat-containing protein 7 Proteins 0.000 description 1
- 206010065553 Bone marrow failure Diseases 0.000 description 1
- 101000937267 Bos taurus Activin receptor type-2B Proteins 0.000 description 1
- 241000701822 Bovine papillomavirus Species 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 229940127291 Calcium channel antagonist Drugs 0.000 description 1
- 101710132601 Capsid protein Proteins 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 108090000317 Chymotrypsin Proteins 0.000 description 1
- 101710094648 Coat protein Proteins 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 206010010356 Congenital anomaly Diseases 0.000 description 1
- 108091035707 Consensus sequence Proteins 0.000 description 1
- 206010010904 Convulsion Diseases 0.000 description 1
- 102000004420 Creatine Kinase Human genes 0.000 description 1
- 108010042126 Creatine kinase Proteins 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 241000701022 Cytomegalovirus Species 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 241000701959 Escherichia virus Lambda Species 0.000 description 1
- 206010015548 Euthanasia Diseases 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 108010054218 Factor VIII Proteins 0.000 description 1
- 102000001690 Factor VIII Human genes 0.000 description 1
- 108010074860 Factor Xa Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 101150050927 Fcgrt gene Proteins 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 108010012820 Follistatin-Related Proteins Proteins 0.000 description 1
- 102000019203 Follistatin-Related Proteins Human genes 0.000 description 1
- 235000008526 Galium odoratum Nutrition 0.000 description 1
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 1
- KOSRFJWDECSPRO-WDSKDSINSA-N Glu-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(O)=O KOSRFJWDECSPRO-WDSKDSINSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 1
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 1
- 229930186217 Glycolipid Natural products 0.000 description 1
- 102100021181 Golgi phosphoprotein 3 Human genes 0.000 description 1
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 1
- 102100039619 Granulocyte colony-stimulating factor Human genes 0.000 description 1
- 102100040898 Growth/differentiation factor 11 Human genes 0.000 description 1
- 206010059484 Haemodilution Diseases 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000799140 Homo sapiens Activin receptor type-1 Proteins 0.000 description 1
- 101000799189 Homo sapiens Activin receptor type-1B Proteins 0.000 description 1
- 101000893545 Homo sapiens Growth/differentiation factor 11 Proteins 0.000 description 1
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- 206010020880 Hypertrophy Diseases 0.000 description 1
- 206010021135 Hypovitaminosis Diseases 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 102000009490 IgG Receptors Human genes 0.000 description 1
- 108010073807 IgG Receptors Proteins 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 102000000589 Interleukin-1 Human genes 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 102100021592 Interleukin-7 Human genes 0.000 description 1
- 108010002586 Interleukin-7 Proteins 0.000 description 1
- 239000007760 Iscove's Modified Dulbecco's Medium Substances 0.000 description 1
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 1
- 208000007177 Left Ventricular Hypertrophy Diseases 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 101710125418 Major capsid protein Proteins 0.000 description 1
- 206010025476 Malabsorption Diseases 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 102100026632 Mimecan Human genes 0.000 description 1
- 102100025748 Mothers against decapentaplegic homolog 3 Human genes 0.000 description 1
- 101710143111 Mothers against decapentaplegic homolog 3 Proteins 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 102100038895 Myc proto-oncogene protein Human genes 0.000 description 1
- 101710135898 Myc proto-oncogene protein Proteins 0.000 description 1
- PHSRRHGYXQCRPU-AWEZNQCLSA-N N-(3-oxododecanoyl)-L-homoserine lactone Chemical compound CCCCCCCCCC(=O)CC(=O)N[C@H]1CCOC1=O PHSRRHGYXQCRPU-AWEZNQCLSA-N 0.000 description 1
- OVRNDRQMDRJTHS-CBQIKETKSA-N N-Acetyl-D-Galactosamine Chemical compound CC(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-CBQIKETKSA-N 0.000 description 1
- OVRNDRQMDRJTHS-UHFFFAOYSA-N N-acelyl-D-glucosamine Natural products CC(=O)NC1C(O)OC(CO)C(O)C1O OVRNDRQMDRJTHS-UHFFFAOYSA-N 0.000 description 1
- MBLBDJOUHNCFQT-UHFFFAOYSA-N N-acetyl-D-galactosamine Natural products CC(=O)NC(C=O)C(O)C(O)C(O)CO MBLBDJOUHNCFQT-UHFFFAOYSA-N 0.000 description 1
- OVRNDRQMDRJTHS-FMDGEEDCSA-N N-acetyl-beta-D-glucosamine Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-FMDGEEDCSA-N 0.000 description 1
- MBLBDJOUHNCFQT-LXGUWJNJSA-N N-acetylglucosamine Natural products CC(=O)N[C@@H](C=O)[C@@H](O)[C@H](O)[C@H](O)CO MBLBDJOUHNCFQT-LXGUWJNJSA-N 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 101710141454 Nucleoprotein Proteins 0.000 description 1
- 208000011623 Obstructive Lung disease Diseases 0.000 description 1
- 240000007817 Olea europaea Species 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 101800002327 Osteoinductive factor Proteins 0.000 description 1
- 206010031252 Osteomyelitis Diseases 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 101150012394 PHO5 gene Proteins 0.000 description 1
- 102220633854 PRKCA-binding protein_D80A_mutation Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 102000011755 Phosphoglycerate Kinase Human genes 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 102220577876 Poly(rC)-binding protein 1_W78A_mutation Human genes 0.000 description 1
- 229920002732 Polyanhydride Polymers 0.000 description 1
- 208000008601 Polycythemia Diseases 0.000 description 1
- 208000018525 Postpartum Hemorrhage Diseases 0.000 description 1
- 101710083689 Probable capsid protein Proteins 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102220469872 Protein argonaute-3_E37A_mutation Human genes 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 206010062237 Renal impairment Diseases 0.000 description 1
- 108700008625 Reporter Genes Proteins 0.000 description 1
- 235000004443 Ricinus communis Nutrition 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- SSZBUIDZHHWXNJ-UHFFFAOYSA-N Stearinsaeure-hexadecylester Natural products CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCC SSZBUIDZHHWXNJ-UHFFFAOYSA-N 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 101710137500 T7 RNA polymerase Proteins 0.000 description 1
- 108020005038 Terminator Codon Proteins 0.000 description 1
- 208000002903 Thalassemia Diseases 0.000 description 1
- 108090001109 Thermolysin Proteins 0.000 description 1
- 101001099217 Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8) Triosephosphate isomerase Proteins 0.000 description 1
- 102000002933 Thioredoxin Human genes 0.000 description 1
- 108090000190 Thrombin Proteins 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- 101710150448 Transcriptional regulator Myc Proteins 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 206010046865 Vaccinia virus infection Diseases 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 108010003533 Viral Envelope Proteins Proteins 0.000 description 1
- 102220469729 Voltage-dependent L-type calcium channel subunit beta-2_D54K_mutation Human genes 0.000 description 1
- 102220479829 Voltage-dependent L-type calcium channel subunit beta-2_R56K_mutation Human genes 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- ATBOMIWRCZXYSZ-XZBBILGWSA-N [1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC ATBOMIWRCZXYSZ-XZBBILGWSA-N 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000003655 absorption accelerator Substances 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 208000038016 acute inflammation Diseases 0.000 description 1
- 230000006022 acute inflammation Effects 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 230000011759 adipose tissue development Effects 0.000 description 1
- 230000001800 adrenalinergic effect Effects 0.000 description 1
- 238000001261 affinity purification Methods 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 150000001350 alkyl halides Chemical class 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 239000002160 alpha blocker Substances 0.000 description 1
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 1
- 229940124308 alpha-adrenoreceptor antagonist Drugs 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- 150000004645 aluminates Chemical class 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 125000003368 amide group Chemical group 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 208000022531 anorexia Diseases 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940045719 antineoplastic alkylating agent nitrosoureas Drugs 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 1
- 239000000823 artificial membrane Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 1
- 238000002820 assay format Methods 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- 239000002876 beta blocker Substances 0.000 description 1
- 229940097320 beta blocking agent Drugs 0.000 description 1
- WQZGKKKJIJFFOK-FPRJBGLDSA-N beta-D-galactose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-FPRJBGLDSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 102000005936 beta-Galactosidase Human genes 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 239000003462 bioceramic Substances 0.000 description 1
- 238000002306 biochemical method Methods 0.000 description 1
- 239000005312 bioglass Substances 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 238000010241 blood sampling Methods 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 210000002798 bone marrow cell Anatomy 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 235000019437 butane-1,3-diol Nutrition 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000000480 calcium channel blocker Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 238000005251 capillar electrophoresis Methods 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 230000021523 carboxylation Effects 0.000 description 1
- 238000006473 carboxylation reaction Methods 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 238000005277 cation exchange chromatography Methods 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 210000004671 cell-free system Anatomy 0.000 description 1
- 230000007541 cellular toxicity Effects 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 229930183167 cerebroside Natural products 0.000 description 1
- 150000001784 cerebrosides Chemical class 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 229940044683 chemotherapy drug Drugs 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 230000006020 chronic inflammation Effects 0.000 description 1
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 1
- 229960002376 chymotrypsin Drugs 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000003920 cognitive function Effects 0.000 description 1
- 239000000084 colloidal system Substances 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 230000001332 colony forming effect Effects 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000001447 compensatory effect Effects 0.000 description 1
- 230000036461 convulsion Effects 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- ATDGTVJJHBUTRL-UHFFFAOYSA-N cyanogen bromide Chemical compound BrC#N ATDGTVJJHBUTRL-UHFFFAOYSA-N 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 206010061428 decreased appetite Diseases 0.000 description 1
- 231100001020 decreased hemoglobin concentration Toxicity 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 230000003412 degenerative effect Effects 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 230000000994 depressogenic effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 235000018823 dietary intake Nutrition 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- UGMCXQCYOVCMTB-UHFFFAOYSA-K dihydroxy(stearato)aluminium Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Al](O)O UGMCXQCYOVCMTB-UHFFFAOYSA-K 0.000 description 1
- 230000000447 dimerizing effect Effects 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 229940030606 diuretics Drugs 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- VJECBOKJABCYMF-UHFFFAOYSA-N doxazosin mesylate Chemical compound [H+].CS([O-])(=O)=O.C1OC2=CC=CC=C2OC1C(=O)N(CC1)CCN1C1=NC(N)=C(C=C(C(OC)=C2)OC)C2=N1 VJECBOKJABCYMF-UHFFFAOYSA-N 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 238000007877 drug screening Methods 0.000 description 1
- 241001493065 dsRNA viruses Species 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 230000000431 effect on proliferation Effects 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 230000002124 endocrine Effects 0.000 description 1
- 210000001900 endoderm Anatomy 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000001976 enzyme digestion Methods 0.000 description 1
- 230000009786 epithelial differentiation Effects 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 229940093499 ethyl acetate Drugs 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000003631 expected effect Effects 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 210000003414 extremity Anatomy 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 238000002875 fluorescence polarization Methods 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 239000004052 folic acid antagonist Substances 0.000 description 1
- 150000002270 gangliosides Chemical class 0.000 description 1
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- 238000001641 gel filtration chromatography Methods 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 1
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- 230000002414 glycolytic effect Effects 0.000 description 1
- 229930182470 glycoside Natural products 0.000 description 1
- 150000002338 glycosides Chemical class 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 1
- 210000000777 hematopoietic system Anatomy 0.000 description 1
- 208000034737 hemoglobinopathy Diseases 0.000 description 1
- 108060003558 hepcidin Proteins 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 1
- 238000012203 high throughput assay Methods 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 206010020718 hyperplasia Diseases 0.000 description 1
- 230000001631 hypertensive effect Effects 0.000 description 1
- 201000009939 hypertensive encephalopathy Diseases 0.000 description 1
- 230000002989 hypothyroidism Effects 0.000 description 1
- 208000003532 hypothyroidism Diseases 0.000 description 1
- 230000007954 hypoxia Effects 0.000 description 1
- 230000036737 immune function Effects 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 230000000415 inactivating effect Effects 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 206010022000 influenza Diseases 0.000 description 1
- 208000018337 inherited hemoglobinopathy Diseases 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 229940100994 interleukin-7 Drugs 0.000 description 1
- 230000031146 intracellular signal transduction Effects 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 229940082629 iron antianemic preparations Drugs 0.000 description 1
- 229960002725 isoflurane Drugs 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 230000017687 left/right axis specification Effects 0.000 description 1
- 210000000982 limb bud Anatomy 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 230000004777 loss-of-function mutation Effects 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 238000003670 luciferase enzyme activity assay Methods 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 206010025135 lupus erythematosus Diseases 0.000 description 1
- 210000004324 lymphatic system Anatomy 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 210000003738 lymphoid progenitor cell Anatomy 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 210000004779 membrane envelope Anatomy 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 230000029115 microtubule polymerization Effects 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 238000012900 molecular simulation Methods 0.000 description 1
- CQDGTJPVBWZJAZ-UHFFFAOYSA-N monoethyl carbonate Chemical compound CCOC(O)=O CQDGTJPVBWZJAZ-UHFFFAOYSA-N 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- 238000002887 multiple sequence alignment Methods 0.000 description 1
- 230000020763 muscle atrophy Effects 0.000 description 1
- 230000003387 muscular Effects 0.000 description 1
- 230000003039 myelosuppressive effect Effects 0.000 description 1
- 210000003098 myoblast Anatomy 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 210000004165 myocardium Anatomy 0.000 description 1
- 229950006780 n-acetylglucosamine Drugs 0.000 description 1
- 239000002088 nanocapsule Substances 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 230000004770 neurodegeneration Effects 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 208000018360 neuromuscular disease Diseases 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 239000012457 nonaqueous media Substances 0.000 description 1
- 210000003458 notochord Anatomy 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 210000001706 olfactory mucosa Anatomy 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 235000010603 pastilles Nutrition 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 125000001095 phosphatidyl group Chemical group 0.000 description 1
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 230000008288 physiological mechanism Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 230000001817 pituitary effect Effects 0.000 description 1
- 230000003169 placental effect Effects 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 208000037920 primary disease Diseases 0.000 description 1
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 229960004063 propylene glycol Drugs 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 239000002510 pyrogen Substances 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 230000007261 regionalization Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 238000003571 reporter gene assay Methods 0.000 description 1
- 210000003019 respiratory muscle Anatomy 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 239000003340 retarding agent Substances 0.000 description 1
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 1
- 229960004641 rituximab Drugs 0.000 description 1
- 102220125997 rs141863326 Human genes 0.000 description 1
- 102220288227 rs141863326 Human genes 0.000 description 1
- 102220140258 rs146572379 Human genes 0.000 description 1
- 102200145357 rs1555341957 Human genes 0.000 description 1
- 102220036189 rs273585616 Human genes 0.000 description 1
- 102220005147 rs34173382 Human genes 0.000 description 1
- 102200158858 rs34435255 Human genes 0.000 description 1
- 102200089383 rs57977969 Human genes 0.000 description 1
- 208000001076 sarcopenia Diseases 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 208000007056 sickle cell anemia Diseases 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 150000003408 sphingolipids Chemical class 0.000 description 1
- 210000003594 spinal ganglia Anatomy 0.000 description 1
- 230000003393 splenic effect Effects 0.000 description 1
- 238000013222 sprague-dawley male rat Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 238000012353 t test Methods 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 108060008226 thioredoxin Proteins 0.000 description 1
- 229940094937 thioredoxin Drugs 0.000 description 1
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 229960004072 thrombin Drugs 0.000 description 1
- 210000002303 tibia Anatomy 0.000 description 1
- MNRILEROXIRVNJ-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=NC=N[C]21 MNRILEROXIRVNJ-UHFFFAOYSA-N 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 208000037816 tissue injury Diseases 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 230000010474 transient expression Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 102000003390 tumor necrosis factor Human genes 0.000 description 1
- 238000003160 two-hybrid assay Methods 0.000 description 1
- 238000010396 two-hybrid screening Methods 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- 238000002562 urinalysis Methods 0.000 description 1
- 208000007089 vaccinia Diseases 0.000 description 1
- 229940124549 vasodilator Drugs 0.000 description 1
- 239000003071 vasodilator agent Substances 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 238000011100 viral filtration Methods 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 210000002845 virion Anatomy 0.000 description 1
- 208000030401 vitamin deficiency disease Diseases 0.000 description 1
- 230000003313 weakening effect Effects 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/10—Transferases (2.)
- C12N9/12—Transferases (2.) transferring phosphorus containing groups, e.g. kinases (2.7)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
- A61K38/179—Receptors; Cell surface antigens; Cell surface determinants for growth factors; for growth regulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
- A61K38/1796—Receptors; Cell surface antigens; Cell surface determinants for hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/475—Growth factors; Growth regulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/71—Receptors; Cell surface antigens; Cell surface determinants for growth factors; for growth regulators
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6897—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids involving reporter genes operably linked to promoters
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y207/00—Transferases transferring phosphorus-containing groups (2.7)
- C12Y207/11—Protein-serine/threonine kinases (2.7.11)
- C12Y207/1103—Receptor protein serine/threonine kinase (2.7.11.30)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
Definitions
- the mature red blood cell or erythrocyte, is responsible for oxygen transport in the circulatory systems of vertebrates.
- Red blood cells carry high concentrations of hemoglobin, a protein that binds oxygen in the lungs at relatively high partial pressure of oxygen (p ⁇ 2 ) and delivers oxygen to areas of the body with a relatively low p ⁇ 2 .
- Mature red blood cells are produced from pluripotent hematopoietic stem cells in a process termed erythropoiesis. Postnatal erythropoiesis occurs primarily in the bone marrow and in the red pulp of the spleen. The coordinated action of various signaling pathways control the balance of cell proliferation, differentiation, survival and death. Under normal conditions, red blood cells are produced at a rate that maintains a constant red cell mass in the body, and production may increase or decrease in response to various stimuli, including increased or decreased oxygen tension or tissue demand. The process of erythropoiesis begins with the formation of lineage committed precursor cells and proceeds through a series of distinct precursor cell types.
- Erythropoietin is widely recognized as the most significant positive regulator of erythropoiesis in post-natal vertebrates. Epo regulates the compensatory erythropoietic response to reduced tissue oxygen tension (hypoxia) and low red blood cell levels or low hemoglobin levels.
- Epo levels promote red blood cell formation by stimulating the generation of erythroid progenitors in the bone marrow and spleen.
- Epo enhances erythropoiesis primarily in the spleen.
- Anemia is a broadly-defined condition characterized by lower than normal levels of hemoglobin or red blood cells in the blood.
- anemia is caused by a primary disorder in the production or survival of red blood cells. More commonly, anemia is secondary to diseases of other systems (Weatherall & Provan (2000) Lancet 355, 1 169-1 175).
- Anemia may result from a reduced rate of production or increased rate of destruction of red blood cells or by loss of red blood cells due to bleeding.
- Anemia may result from a variety of disorders that include, for example, chronic renal failure, chemotherapy treatment, myelodysplastic syndrome, rheumatoid arthritis, and bone marrow transplantation.
- Epo typically causes a rise in hemoglobins by about 1 -3 g/dL in healthy humans over a period of weeks. When administered to anemic individuals, this treatment regimen often provides substantial increases in hemoglobin and red blood cell levels and leads to improvements in quality of life and prolonged survival. Epo is not uniformly effective, and many individuals are refractory to even high doses (Horl et al. (2000) Nephrol Dial Transplant 15, 43-50). Over 50% of patients with cancer have an inadequate response to Epo, approximately 10% with end-stage renal disease are hyporesponsive (Glaspy et al. (1997) J Clin Oncol 15, 1218-1234; Demetri et al.
- GDF Traps may be used to increase red blood cell and hemoglobin levels.
- Variant ActRIIB polypeptides having a significantly decreased affinity for activin (e.g., activin A and/or activin B) relative to other ActRIIB ligands, such as GDFl 1 and/or myostatin are referred to as GDF Traps.
- ActRIIB variants described herein are GDF Traps unless otherwise stated.
- the disclosure demonstrates that a GDF Trap which is a soluble form of ActRlIB polypeptide having an acidic residue at position 79 of SEQ ID NO: 1 , when administered in vivo, increases red blood cell levels in the blood.
- the disclosure provides methods for using GDF Traps to increase red blood cell and hemoglobin levels in patients and to treat disorders associated with low red blood cell or hemoglobin levels in patients in need thereof.
- GDF Traps can be used to increase muscle mass and decrease fat mass.
- the present disclosure provides GDF Traps that are variant ActRIIB polypeptides, including ActRIIB polypeptides having amino- and carboxy-terminal truncations and sequence alterations.
- GDF Traps of the invention may be designed to preferentially antagonize one or more ligands of ActRIIB receptors, such as GDF8 (also called myostatin), GDFl 1 , Nodal, and BMP7 (also called OP-I ).
- GDF8 also called myostatin
- GDFl 1 also called Nodal
- BMP7 also called OP-I
- Examples of GDF Traps include a set of variants derived from ActRIIB that have greatly diminished affinity for activin. These variants exhibit desirable effects on red blood cells while reducing effects on other tissues.
- the GDF Trap polypeptide comprises an amino acid sequence that comprises, consists of, or consists essentially of, the amino acid sequence of SEQ ID NO: 7, 26, 28, 29, 32, 37 or 38, and polypeptides that are at least 80%, 85%, 90%, 95%, 97%, 98%, or 99% identical to any of the foregoing.
- the disclosure provides pharmaceutical preparations comprising a GDF Trap that binds to an ActRIIB ligand such as GDF8, GDFl 1 , activin (e.g., activin B), BMP7 or nodal, and a pharmaceutically acceptable carrier.
- a GDF Trap that binds to an ActRIIB ligand such as GDF8, GDFl 1 , activin (e.g., activin B), BMP7 or nodal, and a pharmaceutically acceptable carrier.
- the GDF Trap binds to an ActRIIB ligand with a Kd less than 10 micromolar, less than 1 micromolar, less than
- a GDF Trap for use in such a preparation may be any of those disclosed herein, including, for example, GDF Traps having an amino acid sequence selected from SEQ ID NOs: 2, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38 or 40, or GDF Traps having an amino acid sequence that is at least 80%, 85%, 90%, 95%, 97% or 99% identical to an amino acid sequence selected from SEQ ID NOs: 2, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38 or 40, or GDF Traps having an amino acid sequence that is at least 80%, 85%, 90%, 95%, 97% or 99% identical to an amino acid sequence selected from SEQ ID NOs: 2, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38 or 40 wherein the position corresponding to L79 in SEQ ID NOs: 2, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38 or 40 wherein the position corresponding to L79 in SEQ ID NOs: 2, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38 or 40 where
- a preferred GDF Trap for use in such a preparation consists of, or consists essentially of, the amino acid sequence of SEQ ID NO: 26.
- a GDF Trap may include a functional fragment of a natural ActRIIB polypeptide, such as one comprising at least 10, 20 or 30 amino acids of a sequence selected from SEQ ID NOs: 2, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38 or 40 or a sequence of SEQ ID NO: 2, lacking the C-terminal 1 , 2, 3, 4, 5 or 10 to 15 amino acids and lacking 1 , 2, 3, 4 or 5 amino acids at the N-terminus.
- a preferred polypeptide will comprise a truncation relative to SEQ ID NO: 2 or 40 of between 2 and 5 amino acids at the N-terminus and no more than 3 amino acids at the C-terminus.
- a GDF Trap may include one or more alterations in the amino acid sequence of an ActRIIB polypeptide (e.g., in the ligand-binding domain) relative to a naturally occurring ActRIIB polypeptide.
- the alteration in the amino acid sequence may, for example, alter glycosylation of the polypeptide when produced in a mammalian, insect or other eukaryotic cell or alter proteolytic cleavage of the polypeptide relative to the naturally occurring ActRIIB polypeptide.
- a GDF Trap may be a fusion protein that has, as one domain, an ActRIIB polypeptide (e.g., a ligand-binding domain of an ActRIIB with one or more sequence variations) and one or more additional domains that provide a desirable property, such as improved pharmacokinetics, easier purification, targeting to particular tissues, etc.
- a domain of a fusion protein may enhance one or more of in vivo stability, in vivo half life, uptake/administration, tissue localization or distribution, formation of protein complexes, multimerization of the fusion protein, and/or purification.
- GDF Trap fusion proteins may include an immunoglobulin Fc domain (wild-type or mutant) or a serum albumin.
- a GDF Trap fusion comprises a relatively unstructured linker positioned between the Fc domain and the extracellular ActRIIB domain.
- This unstructured linker may correspond to the roughly 15 amino acid unstructured region at the C-terminal end of the extracellular domain of ActRIIB (the "tail"), or it may be an artificial sequence of between 3 and 5, 15. 20, 30, 50 or more amino acids that are relatively free of secondary structure.
- a linker may be rich in glycine and proline residues and may, for example, contain repeating sequences of threonine/serine and glycines (e.g., TG 4 (SEQ ID NO: 13) or SG 4 (SEQ ID NO: 14) singlets or repeats) or a series of three glycines.
- a fusion protein may include a purification subsequence, such as an epitope tag, a FLAG tag, a polyhistidine sequence, and a GST fusion.
- a GDF Trap fusion comprises a leader sequence.
- the leader sequence may be a native ActRlIB leader sequence or a heterologous leader sequence.
- the leader sequence is a Tissue Plasminogen Activator (TPA) leader sequence.
- TPA Tissue Plasminogen Activator
- a GDF Trap fusion protein comprises an amino acid sequence as set forth in the formula A-B-C.
- the B portion is an N- and C-terminally truncated ActRIIB polypeptide consisting of the amino acid sequence corresponding to amino acids 25-131 of SEQ ID NO: 2 or 40.
- the A and C portions may be independently zero, one or more than one amino acids, and both A and C portions are heterologous to B.
- the A and/or C portions may be attached to the B portion via a linker sequence.
- a GDF Trap includes a variant ActRIIB polypeptide having one or more modified amino acid residues selected from: a glycosylated amino acid, a PEGylated amino acid, a farnesylated amino acid, an acetylated amino acid, a biotinylated amino acid, an amino acid conjugated to a lipid moiety, and an amino acid conjugated to an organic derivatizing agent.
- a pharmaceutical preparation may also include one or more additional compounds such as a compound that is used to treat an ActRIIB-associated disorder.
- a pharmaceutical preparation is substantially pyrogen free.
- a GDF Trap be expressed in a mammalian cell line that mediates suitably natural glycosylation of the GDF Trap so as to diminish the likelihood of an unfavorable immune response in a patient.
- Human and CHO cell lines have been used successfully, and it is expected that other common mammalian expression vectors will be useful.
- the disclosure provides packaged pharmaceuticals comprising a pharmaceutical preparation described herein and labeled for use in increasing red blood cell levels in a human.
- the disclosure provides GDF Traps which are soluble ActRIIB polypeptides comprising an altered ligand-binding (e.g., GDF8-binding) domain.
- GDF Traps with altered ligand-binding domains may comprise, for example, one or more mutations at amino acid residues such as E37, E39, R40, K55, R56, Y60, A64, K74, W78, L79, D80, F82 and Fl Ol of human ActRIIB (numbering is relative to SEQ ID NO: 1 ).
- the altered ligand-binding domain can have increased selectivity for a ligand such as GDF8/GDF1 1 relative to a wild-type ligand-binding domain of an ActRIIB receptor.
- a ligand such as GDF8/GDF1 1 relative to a wild-type ligand-binding domain of an ActRIIB receptor.
- these mutations are demonstrated herein to increase the selectivity of the altered ligand-binding domain for GDFl 1 (and therefore, presumably, GDF8) over activin: K.74Y, K74F, K74I, L79D, L79E, and D80I .
- the following mutations have the reverse effect, increasing the ratio of activin binding over GDFl 1 : D54A, K55A, L79A and F82A.
- the overall (GDFl 1 and activin) binding activity can be increased by inclusion of the "tail" region or, presumably, an unstructured linker region, and also by use of a K74A mutation.
- Other mutations that caused an overall decrease in ligand binding affinity include: R40A, E37A, R56A, W78A, D80K, D80R, D80A, D80G, D80F, D80M and D80N. Mutations may be combined to achieve desired effects. For example, many of the mutations that affect the ratio of GDFl 1 : Activin binding have an overall negative effect on ligand binding, and therefore, these may be combined with mutations that generally increase ligand binding to produce an improved binding protein with ligand selectivity.
- a GDF Trap is an ActRIIB polypeptide comprising an L79D or L79E mutation, optionally in combination with additional amino acid substitutions, additions or deletions.
- a GDF Trap comprising an altered ligand-binding domain has a ratio of K d for activin binding to K ⁇ for GDF8 binding that is at least 2, 5, 10, or even 100 fold greater relative to the ratio for the wild-type ligand-binding domain.
- the GDF Trap comprising an altered ligand-binding domain has a ratio of IC 50 for inhibiting activin to IC 50 for inhibiting GDF8/GDF11 that is at least 2, 5, 10, or even 100 fold greater relative to the wild-type ActRIIB ligand-binding domain.
- the GDF Trap comprising an altered ligand-binding domain inhibits GDF8/GDF1 1 with an IC 50 at least 2, 5, 10, or even 100 times less than the IC 50 for inhibiting activin.
- These GDF Traps can be fusion proteins that include an immunoglobulin Fc domain (either wild-type or mutant).
- the subject soluble GDF Traps are antagonists (inhibitors) of GDF8 and/or GDFl 1.
- a GDF Trap fusion protein comprising a portion derived from the ActRIIB sequence of SEQ ID NO: 1 or 39 and a second polypeptide portion, wherein the portion derived from ActRIIB corresponds to a sequence beginning at any of amino acids 21-29 of SEQ ID NO: 1 or 39 (optionally beginning at 22-25 of SEQ ID NO: 1 or 39) and ending at any of amino acids 109-134 of SEQ ID NO: 1 or 39, and wherein the GDF Trap fusion protein inhibits signaling by activin, myostatin and/or GDFl 1 in a cell-based assay.
- the GDF Trap fusion protein above wherein the portion derived from ActRIIB corresponds to a sequence beginning at any of amino acids 20-29 of SEQ ID NO: 1 and ending at any of amino acids 128- 133 of SEQ ID NO: 1 or 39.
- constructs beginning at 22-25 of SEQ ID NO: 1 or 39 have activity levels greater than proteins having the full extracellular domain of human ActRIIB.
- the GDF Trap fusion protein comprises, consists essentially of, or consists of, an amino acid sequence beginning at amino acid position 25 of SEQ ID NO: 1 or 39 and ending at amino acid position 131 of SEQ ID NO: 1 or 39.
- the GDF Trap polypeptide consists of, or consists essentially of, the amino acid sequence of SEQ ID NO: 7, 26, 28, 29, 32, 37 or 38.
- Any of the above GDF Trap fusion proteins may be produced as a homodimer.
- Any of the above GDF Trap fusion proteins may have a heterologous portion that comprises a constant region from an IgG heavy chain, such as an Fc domain.
- Any of the above GDF Trap fusion proteins may comprise an acidic amino acid at the position corresponding to position 79 of SEQ ID NO: 1 , optionally in combination with one or more additional amino acid substitutions, deletions or insertions relative to SEQ ID NO: 1.
- GDF Trap proteins are contemplated, such as the following.
- a GDF Trap protein comprising an amino acid sequence that is at least 80% identical to the sequence of amino acids 29-109 of SEQ ID NO: 1 or 39, wherein the position corresponding to 64 of SEQ ID NO: 1 is an R or K, and wherein the GDF Trap protein inhibits signaling by activin, myostatin and/or GDFl 1 in a cell-based assay.
- the GDF Trap protein above, wherein at least one alteration with respect to the sequence of SEQ ID NO: 1 or 39 is positioned outside of the ligand binding pocket.
- the GDF Trap protein above wherein at least one alteration with respect to the sequence of SEQ ID NO: 1 or 39 is a conservative alteration positioned within the ligand binding pocket.
- the GDF Trap protein above, wherein at least one alteration with respect to the sequence of SEQ ID NO: 1 or 39 is an alteration at one or more positions selected from the group consisting of K74, R40, Q53, K55, F82 and L79.
- the GDF Trap protein above, wherein the protein comprises at least one N-X-S/T sequence at a position other than an endogenous N-X-S/T sequence of ActRIIB, and at a position outside of the ligand binding pocket.
- GDF Trap protein comprising an amino acid sequence that is at least 80% identical to the sequence of amino acids 29-109 of SEQ ID NO: 1 or 39, and wherein the protein comprises at least one N-X-S/T sequence at a position other than an endogenous N-X-S/T sequence of ActRIIB, and at a position outside of the ligand binding pocket.
- the GDF Trap above wherein the GDF Trap protein comprises an N at the position corresponding to position 24 of SEQ ID NO: 1 or 39 and an S or T at the position corresponding to position 26 of SEQ ID NO: 1 or 39, and wherein the GDF Trap inhibits signaling by activin, myostatin and/or GDFl 1 in a cell-based assay.
- the GDF Trap above wherein the ActRIIB protein comprises a D or E at the position corresponding to position 79 of SEQ ID NO: 1 or 39 and wherein the GDF Trap inhibits signaling by activin, myostatin and/or GDF l 1 in a cell-based assay.
- the GDF Trap above, wherein at least one alteration with respect to the sequence of SEQ ID NO: 1 or 39 is a conservative alteration positioned within the ligand binding pocket.
- the GDF Trap above, wherein at least one alteration with respect to the sequence of SEQ ID NO: 1 or 39 is an alteration at one or more positions selected from the group consisting of K74, R40, Q53, K55, F82 and L79.
- the protein is a fusion protein further comprising a heterologous portion.
- Any of the above GDF Trap fusion proteins may be produced as a homodimer.
- Any of the above GDF Trap fusion proteins may have a heterologous portion that comprises a constant region from an IgG heavy chain, such as an Fc domain.
- the disclosure provides nucleic acids encoding a GDF Trap polypeptide.
- An isolated polynucleotide may comprise a coding sequence for a soluble GDF Trap polypeptide, such as described above.
- an isolated nucleic acid may include a sequence coding for a GDF Trap comprising an extracellular domain (e.g., ligand- binding domain) of an ActRIIB polypeptide having one or more sequence variations and a sequence that would code for part or all of the transmembrane domain and/or the cytoplasmic domain of an ActRIIB polypeptide, but for a stop codon positioned within the transmembrane domain or the cytoplasmic domain, or positioned between the extracellular domain and the transmembrane domain or cytoplasmic domain.
- a GDF Trap comprising an extracellular domain (e.g., ligand- binding domain) of an ActRIIB polypeptide having one or more sequence variations and a sequence that would code for part or all of the transmembrane domain and/or the cytoplasmic domain of an ActRIIB polypeptide, but for a stop codon positioned within the transmembrane domain or the cytoplasmic domain, or positioned between the extracellular domain and
- an isolated polynucleotide coding for a GDF Trap may comprise a full-length ActRIIB polynucleotide sequence such as SEQ ID NO: 4 having one or more variations, or a partially truncated version, said isolated polynucleotide further comprising a transcription termination codon at least six hundred nucleotides before the 3 '-terminus or otherwise positioned such that translation of the polynucleotide gives rise to an extracellular domain optionally fused to a truncated portion of a full-length ActRIIB.
- Nucleic acids disclosed herein may be operably linked to a promoter for expression, and the disclosure provides cells transformed with such recombinant polynucleotides.
- the cell is a mammalian cell such as a CHO cell.
- the disclosure provides methods for making a GDF Trap polypeptide.
- a method may include expressing any of the nucleic acids (e.g., SEQ ID NO: 5, 25, 27, 30 or 31 ) disclosed herein in a suitable cell, such as a Chinese hamster ovary (CHO) cell.
- a suitable cell such as a Chinese hamster ovary (CHO) cell.
- Such a method may comprise: a) culturing a cell under conditions suitable for expression of the GDF Trap polypeptide, wherein said cell is transformed with a GDF Trap expression construct; and b) recovering the GDF Trap polypeptide so expressed.
- GDF Trap polypeptides may be recovered as crude, partially purified or highly purified fractions using any of the well known techniques for obtaining protein from cell cultures.
- a GDF Trap polypeptide disclosed herein may be used in a method for promoting red blood cell production or increasing red blood cell levels in a subject.
- the disclosure provides methods for treating a disorder associated with low red blood cell counts or low hemoglobin levels (e.g., an anemia), or to promote red blood cell production, in patients in need thereof.
- a method may comprise administering to a subject in need thereof an effective amount of a GDF Trap polypeptide.
- the disclosure provides uses of GDF Trap polypeptides for making a medicament for the treatment of a disorder or condition as described herein.
- the disclosure provides methods for administering a GDF Trap polypeptide to a patient.
- GDF Trap polypeptides can be used to increase red blood cell and hemoglobin levels.
- GDF Trap polypeptides may also be used for treating or preventing other therapeutic uses such as promoting muscle growth.
- it may be desirable to monitor the effects on red blood cells during administration of the GDF Trap polypeptide, or to determine or adjust the dosing of the GDF Trap polypeptide, in order to reduce undesired effects on red blood cells. For example, increases in red blood cell levels, hemoglobin levels, or hematocrit levels may cause increases in blood pressure.
- Figure 1 shows an alignment of the extracellular domains of human ActRIIA (SEQ ID NO: 15) and human ActRIIB (SEQ ID NO: 2) with the residues that are deduced herein, based on composite analysis of multiple ActRIIB and ActRIIA crystal structures to directly contact ligand (the ligand binding pocket) indicated with boxes.
- Figure 2 shows a multiple sequence alignment of various vertebrate ActRIIB proteins and human ActRIIA (SEQ ID NOs: 16-23).
- Figure 3 shows the full amino acid sequence for the GDF Trap ActRIIB(L79D 20- 134)-hFc (SEQ ID NO: 1 1), including the TPA leader sequence (double underlined), ActRIIB extracellular domain (residues 20-134 in SEQ ID NO: 1 ; underlined), and hFc domain.
- the aspartate substituted at position 79 in the native sequence is double underlined and highlighted, as is the glycine revealed by sequencing to be the N-terminal residue in the mature fusion protein.
- Figure 4 shows a nucleotide sequence encoding ActRIlB(L79D 20-134)-hFc.
- SEQ ID NO: 25 corresponds to the sense strand
- SEQ ID NO 33 corresponds to the antisense strand.
- the TPA leader (nucleotides 1-66) is double underlined
- the ActRIIB extracellular domain (nucleotides 76-420) is underlined.
- Figure 5 shows the full amino acid sequence for the truncated GDF Trap ActRIIB(L79D 25-131 )-hFc (SEQ ID NO 26), including the TPA leader (double underlined), truncated ActRIIB extracellular domain (residues 25-131 in SEQ ID NO: 1 ; underlined), and hFc domain.
- the aspartate substituted at position 79 in the native sequence is double underlined and highlighted, as is the glutamate revealed by sequencing to be the N- terminal residue in the mature fusion protein.
- Figure 6 shows a nucleotide sequence encoding ActRIIB(L79D 25-131)-hFc.
- SEQ ID NO: 27 corresponds to the sense strand
- SEQ ID NO. 34 corresponds to the antisense strand.
- the TPA leader (nucleotides 1-66) is double underlined, and the truncated ActRIIB extracellular domain (nucleotides 76-396) is underlined.
- the amino acid sequence for the ActRIIB extracellular domain is also shown.
- Figure 7 shows the amino acid sequence for the truncated GDF Trap ActRIIB(L79D 25-131)-hFc without a leader (SEQ ID NO: 28).
- the truncated ActRIIB extracellular domain (residues 25-131 in SEQ ID NO: 1 ) is underlined.
- the aspartate substituted at position 79 in the native sequence is double underlined and highlighted, as is the glutamate revealed by sequencing to be the N-terminal residue in the mature fusion protein.
- Figure 8 shows the amino acid sequence for the truncated GDF Trap ActRIIB(L79D 25-131 ) without the leader, hFc domain, and linker (SEQ ID NO 29).
- the aspartate substituted at position 79 in the native sequence is underlined and highlighted, as is the glutamate revealed by sequencing to be the N-terminal residue in the mature fusion protein.
- Figure 9 shows an alternative nucleotide sequence encoding ActRIIB(L79D 25-131)- hFc.
- SEQ ID NO 30 corresponds to the sense stiand
- SEQ ID NO- 35 corresponds to the antisense strand
- the TPA leader (nucleotides 1 -66) is double underlined
- the truncated ActRIIB extracellular domain (nucleotides 76-396) is undei lined
- substitutions in the wildtype nucleotide sequence of the extracellular domain are double underlined and highlighted (compare with SEQ ID NO: 27, Figure 6).
- the amino acid sequence for the ActRlIB extracellular domain is also shown.
- Figure 10 shows nucleotides 76-396 (SEQ ID NO: 31 ) of the alternative nucleotide sequence shown in Figure 9 (SEQ ID NO: 30). The same nucleotide substitutions indicated in Figure 9 are also underlined and highlighted here.
- SEQ ID NO: 31 encodes only the truncated ActRlIB extracellular domain (corresponding to residues 25-131 in SEQ ID NO: 1) with a L79D substitution, e.g., ActRIIB(L79D 25-131).
- Figure 1 1 shows the effect of ActRIIB(L79D 25-131)-hFc on hemoglobin concentration in a mouse model of chemotherapy-induced anemia. Data are means ⁇ SEM. **, P ⁇ 0.01 vs. paclitaxel at the same time point. This GDF Trap offset the anemia induced by paclitaxel treatment.
- Figure 12 shows the effect of ActRIIB(L79D 25-13 I)-IiFc on red blood cell (RBC) levels in a unilaterally nephrectomized (NEPHX) mouse model of chronic kidney disease. Data are means ⁇ SEM. ***, P ⁇ 0.001 vs. baseline. This GDF Trap reversed the nephrectomy-induced anemia observed in control mice.
- Figure 13 shows the effect of ActRIIB(L79D 25-131)-hFc on red blood cell (RBC), hemoglobin (HGB), and hematocrit (HCT) levels in a unilaterally nephrectomized (NEPHX) mouse model of chronic kidney disease. Data are mean changes from baseline over 4 weeks ( ⁇ SEM). *, P ⁇ 0.05; **, P ⁇ 0.01 ; ***, P ⁇ 0.001 vs. NEPHX controls. This GDF Trap prevented the nephrectomy-associated decline in these erythrocytic parameters, increasing each by a magnitude similar to that in kidney-intact (sham) mice .
- Figure 14 shows the effect of ActRIIB(L79D 25-13 I )-IiFc on red blood cell (RBC) levels in a rat model of anemia induced by acute blood loss. Blood removal occurred on Day -1 , with dosing on Days 0 and 3. Data are means ⁇ SEM. **, P ⁇ 0.01 ; ***, P ⁇ 0.001 vs. vehicle at same time point. This GDF Trap improved the rate and extent of recovery from blood-loss-induced anemia.
- Figure 1 5 shows the effect of treatment with ActRIIB(L79D 20-134)-hFc (gray) or ActRlIB(L79D 25-13 I )-IiFc (black) on the absolute change in red blood cell concentration from baseline in cynomolgus monkey.
- Figure 16 shows the effect of treatment with ActRIlB(L79D 20-134)-hFc (gray) or ActRIIB(L79D 25-13 I )-IiFc (black) on the absolute change in hematocrit from baseline in cynomolgus monkey.
- Figure 17 shows the effect of treatment with ActRIIB(L79D 20-134)-hFc (gray) or ActRIIB(L79D 25-131)-hFc (black) on the absolute change in hemoglobin concentration from baseline in cynomolgus monkey.
- VEH vehicle.
- Data are means + SEM.
- n 4-8 per group.
- Figure 18 shows the effect of treatment with ActRIIB(L79D 20-134)-hFc (gray) or ActRIIB(L79D 25-131)-hFc (black) on the absolute change in circulating reticulocyte concentration from baseline in cynomolgus monkey.
- VEH vehicle.
- Data are means + SEM.
- n 4-8 per group.
- TGF-beta transforming growth factor-beta
- BMP/GDF BMP/GDF
- TGF-beta/Activin/BMPIO branches whose members have diverse, often complementary effects.
- TGF- ⁇ signals are mediated by heteromeric complexes of type 1 and type II serine/threonine kinase receptors, which phosphorylate and activate downstream Smad proteins upon ligand stimulation (Massague, 2000, Nat. Rev. MoI. Cell Biol. 1 : 169- 178).
- type I and type Il receptors are transmembrane proteins, composed of a ligand-binding extracellular domain with cysteine-rich region, a transmembrane domain, and a cytoplasmic domain with predicted serine/threonine specificity.
- Type I receptors are essential for signaling.
- Type 11 receptors are required for binding ligands and for expression of Type I receptors.
- Type I and Il activin receptors form a stable complex after ligand binding, resulting in phosphorylation of Type I receptors by Type II receptors.
- ActRII Two related Type II receptors (ActRII), ActRIIA and ActRIIB, have been identified as the Type II receptors for activins (Mathews and Vale, 1991 , Cell 65:973-982; Attisano et al., 1992, Cell 68: 97-108). Besides activins, ActRIIA and ActRIIB can biochemically interact with several other TGF- ⁇ family proteins, including BMP7, Nodal, GDF8, and GDFI l (Yamashita et al., 1995, J. Cell Biol. 130:217-226; Lee and McPherron, 2001 , Proc. Natl. Acad. Sci.
- ALK4 is the primary type I receptor for activins, particularly for activin A, and ALK-7 may serve as a receptor for activins as well, particularly for activin B.
- the present invention relates to antagonizing a ligand of ActRIIB receptors (also referred to as an ActRIIB ligand) with a subject GDF Trap polypeptide.
- exemplary ligands of ActRIIB receptors include some TGF- ⁇ family members, such as activin, Nodal, GDF8, GDFl 1 , and BMP7.
- Activins are dimeric polypeptide growth factors that belong to the TGF-beta superfamily. There are three principal activin forms (A, B, and AB) that are homo/heterodimers of two closely related ⁇ subunits ( ⁇ A ⁇ A, ⁇ , and ⁇ A ⁇ , respectively). The human genome also encodes an activin C and an activin E, which are primarily expressed in the liver, and heterodimeric forms containing ⁇ c or ⁇ e are also known.
- activins are unique and multifunctional factors that can stimulate hormone production in ovarian and placental cells, support neuronal cell survival, influence cell-cycle progress positively or negatively depending on cell type, and induce mesodermal differentiation at least in amphibian embryos (DePaolo et al., 1991 , Proc Soc Ep Biol Med. 198:500-512; Dyson et al., 1997, Curr Biol. 7:81 -84; Woodruff, 1998, Biochem Pharmacol. 55:953-963).
- erythroid differentiation factor isolated from the stimulated human monocytic leukemic cells was found to be identical to activin A (Murata et al., 1988, PNAS, 85:2434). It has been suggested that activin A promotes erythropoiesis in the bone marrow. In several tissues, activin signaling is antagonized by its related heterodimer, inhibin. For example, during the release of follicle-stimulating hormone (FSH) from the pituitary, activin promotes FSH secretion and synthesis, while inhibin prevents FSH secretion and synthesis.
- FSH follicle-stimulating hormone
- Other proteins that may regulate activin bioactivity and/or bind to activin include follistatin (FS), follistatin-related protein (FSRP) and ⁇ 2 -macroglobulin.
- Nodal proteins have functions in mesoderm and endoderm induction and formation, as well as subsequent organization of axial structures such as heart and stomach in early embryogenesis. It has been demonstrated that dorsal tissue in a developing vertebrate embryo contributes predominantly to the axial structures of the notochord and pre-chordal plate while it recruits surrounding cells to form non-axial embryonic structures. Nodal appears to signal through both type I and type II receptors and intracellular effectors known as Smad proteins. Recent studies support the idea that ActRIIA and ActRIIB serve as type II receptors for Nodal (Sakuma et al., Genes Cells. 2002, 7:401 -12).
- Nodal ligands interact with their co-factors (e.g., cripto) to activate activin type I and type II receptors, which phosphorylate Smad2.
- Nodal proteins are implicated in many events critical to the early vertebrate embryo, including mesoderm formation, anterior patterning, and left- right axis specification. Experimental evidence has demonstrated that Nodal signaling activates pAR3-Lux, a luciferase reporter previously shown to respond specifically to activin and TGF-beta. However, Nodal is unable to induce pTlx2-Lux, a reporter specifically responsive to bone morphogenetic proteins.
- GDF8 Growth and Differentiation Factor-8
- GDF8 is a negative regulator of skeletal muscle mass. GDF8 is highly expressed in the developing and adult skeletal muscle. The GDF8 null mutation in transgenic mice is characterized by a marked hypertrophy and hyperplasia of the skeletal muscle (McPherron et al., Nature, 1997, 387:83-90). Similar increases in skeletal muscle mass are evident in naturally occurring mutations of GDF8 in cattle (Ashmore et al., 1974, Growth, 38:501 -507; Swatland and Kieffer, J. Anim. Sci., 1994, 38:752-757; McPherron and Lee, Proc. Natl. Acad.
- GDF8 can modulate the production of muscle-specific enzymes (e.g., creatine kinase) and modulate myoblast cell proliferation (WO 00/43781 ).
- the GDF8 propeptide can noncovalently bind to the mature GDF8 domain dimer, inactivating its biological activity (Miyazono et al. (1988) J. Biol. Chem., 263: 6407-6415; Wakefield et al. (1988) J. Biol. Chem., 263; 7646-7654; and Brown et al. (1990) Growth Factors, 3: 35-43).
- Other proteins which bind to GDF8 or structurally related proteins and inhibit their biological activity include follistatin, and potentially, follistatin-related proteins (Gamer et al. (1999) Dev. Biol., 208: 222-232).
- GDFl 1 Growth and Differentiation Factor- 1 1
- BMP l 1 also known as BMP l 1
- GDFl 1 is a secreted protein (McPherron et al., 1999, Nat. Genet. 22: 260-264). GDFl 1 is expressed in the tail bud, limb bud, maxillary and mandibular arches, and dorsal root ganglia during mouse development (Nakashima et al., 1999, Mech. Dev. 80: 185-189). GDFl 1 plays a unique role in patterning both mesodermal and neural tissues (Gamer et al., 1999, Dev Biol., 208:222- 32).
- GDFl 1 was shown to be a negative regulator of chondrogenesis and myogenesis in developing chick limb (Gamer et al., 2001 , Dev Biol. 229:407-20).
- the expression of GDFl 1 in muscle also suggests its role in regulating muscle growth in a similar way to GDF8.
- the expression of GDFl 1 in brain suggests that GDFl 1 may also possess activities that relate to the function of the nervous system.
- GDFl 1 was found to inhibit neurogenesis in the olfactory epithelium (Wu et al., 2003, Neuron. 37:197-207).
- GDFl 1 may have in vitro and in vivo applications in the treatment of diseases such as muscle diseases and neurodegenerative diseases (e.g., amyotrophic lateral sclerosis).
- Bone morphogenetic protein also called osteogenic protein- 1 (OP-I)
- BMP7 also called osteogenic protein- 1 (OP-I)
- BMP7 regulates a wide array of physiological processes.
- BMP7 may be the osteoinductive factor responsible for the phenomenon of epithelial osteogenesis. It is also found that BMP7 plays a role in calcium regulation and bone homeostasis.
- BMP7 binds to Type II receptors, ActRIIA and ActRIIB.
- BMP7 and activin recruit distinct Type I receptors into heteromeric receptor complexes.
- the major BMP7 Type I receptor observed was ALK2, while activin bound exclusively to ALK.4 (ActRIIB).
- BMP7 and activin elicited distinct biological responses and activated different Smad pathways (Macias-Silva et al., 1998, J Biol Chem. 273:25628-36).
- a GDF Trap polypeptide which is a variant ActRIIB polypeptide (ActRIIB) is more effective at increasing red blood cell levels in vivo as compared to a wild-type soluble ActRIIB polypeptide and has beneficial effects in a variety of models for anemias.
- ActRIIB ActRIIB polypeptide
- hematopoiesis is a complex process, regulated by a variety of factors, including erythropoietin, G-CSF and iron homeostasis.
- the terms "increase red blood cell levels” and “promote red blood cell formation” refer to clinically observable metrics, such as hematocrit, red blood cell counts and hemoglobin measurements, and are intended to be neutral as to the mechanism by which such changes occur.
- GDF Trap polypeptides are useful for a variety of therapeutic applications, including, for example, promoting muscle growth (see PCT Publication Nos. WO 2006/012627 and WO 2008/097541 , which are hereby incorporated by reference in their entirety).
- a GDF Trap polypeptide when administering a GDF Trap polypeptide for the purpose of increasing muscle, it may be desirable to reduce or minimize effects on red blood cells.
- appropriate dosing including amounts and frequency of administration
- therapeutic progress and effects on one or more hematologic parameters over time may be useful in managing patients being dosed with a GDF Trap polypeptide by facilitating patient care, determining appropriate maintenance dosing (both amounts and frequency), etc.
- the terms “about' “ and “approximately” may mean values that are within an order of magnitude, preferably within 5- fold and more preferably within 2-fold of a given value Numerical quantities given herein are approximate unless stated otherwise, meaning that the term '"about” or “approximately” can be inferred when not expressly stated
- the methods of the invention may include steps of comparing sequences to each other, including wild-type sequence to one or more mutants (sequence va ⁇ ants)
- Such comparisons typically comprise alignments of polymer sequences, e g , using sequence alignment programs and/or algorithms that are well known in the art (for example, BLAST, FASTA and MEGALlGN, to name a few)
- sequence alignment programs and/or algorithms that are well known in the art (for example, BLAST, FASTA and MEGALlGN, to name a few)
- sequence alignment programs and/or algorithms that are well known in the art (for example, BLAST, FASTA and MEGALlGN, to name a few)
- a mutation contains a residue insertion or deletion
- the sequence alignment will introduce a "gap" (typically represented by a dash, or "A " ') in the polymer sequence not containing the inserted or deleted residue
- homologous in all its grammatical forms and spelling variations, refers to the relationship between two proteins that possess a “common evolutionary origin, "including proteins from superfamilies in the same species of organism, as well as homologous proteins from different species of organism Such proteins (and their encoding nucleic acids) have sequence homology, as reflected by their sequence similarity, whether in terms of percent identity or by the presence of specific residues or motifs and conserved positions
- sequence similarity in all its grammatical forms, refers to the degree of identity or correspondence between nucleic acid or amino acid sequences that may or may not share a common evolutionary origin Howevei, in common usage and in the instant application, the term “homologous,” when modified with an adverb such as “highly,” may refer to sequence similarity and may oi may not relate to a common evolutionary origin
- the invention relates to GDF Ti ap polypeptides, e g . soluble variant ActRIlB polypeptides, including, for example, fiagments, functional valiants, and modified forms of ActRIIB polypeptides.
- the GDF Trap polypeptides have at least one similar or same biological activity as a corresponding wild- type ActRIIB polypeptide.
- a GDF Trap polypeptide of the invention may bind to and inhibit the function of an ActRIIB ligand (e.g., activin A, activin AB, activin B, Nodal, GDF8, GDFl 1 or BMP7).
- a GDF Trap polypeptide increases red blood cell levels.
- GDF Trap polypeptides include human ActRIIB precursor polypeptides (SEQ ID NO: 1 or 39) having one or more sequence variations, and soluble human ActRIIB polypeptides (e.g., SEQ ID NOs: 2, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38, 40 and 41 ) having one or more sequence variations.
- a GDF Trap refers to an ActRIIB polypeptide having a decreased affinity for activin relative to other ActRIIB ligands, including for example GDFl 1 and/or myostatin.
- ActRIIB refers to a family of activin receptor type Hb (ActRIIB) proteins from any species and variants derived from such ActRIIB proteins by mutagenesis or other modification. Reference to ActRIIB herein is understood to be a reference to any one of the currently identified forms. Members of the ActRIIB family are generally transmembrane proteins, composed of a ligand-binding extracellular domain with a cysteine-rich region, a transmembrane domain, and a cytoplasmic domain with predicted serine/threonine kinase activity. Amino acid sequences of human ActRIIA soluble extracellular domain (provided for comparison) and ActRIIB soluble extracellular domain are illustrated in Figure 1.
- ActRIIB polypeptide includes polypeptides comprising any naturally occurring polypeptide of an ActRIIB family member as well as any variants thereof (including mutants, fragments, fusions, and peptidomimetic forms) that retain a useful activity. See, for example, WO 2006/012627.
- ActRIIB polypeptides include polypeptides derived from the sequence of any known ActRIIB having a sequence at least about 80% identical to the sequence of an ActRIIB polypeptide, and optionally at least 85%, 90%, 95%, 97%, 99% or greater identity.
- an ActRIIB polypeptide may bind to and inhibit the function of an ActRIIB protein and/or activin.
- ActRIIB polypeptide which is a GDF Trap may be selected for activity in promoting red blood cell formation in vivo.
- Examples of ActRIIB polypeptides include human ActRIIB precursor polypeptide (SEQ ID NO: 1 and 39) and soluble human ActRIIB polypeptides (e.g., SEQ ID NO: 2, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38, 40 and 41 ). Numbering of amino acids for all ActRIIB-related polypeptides described herein is based on the numbering for SEQ ID NO: 1 , unless specifically designated otherwise.
- the human ActRllB precursor protein sequence is as follows:
- the protein may be produced with an "SGR" sequence at the N- terminus.
- the C-terminal "tail” of the extracellular domain is underlined.
- the sequence with the "tail” deleted is as follows:
- the protein may be produced with an "SGR" sequence at the N- terminus.
- the nucleic acid sequence encoding a human ActRIIB precursor protein is as follows: (nucleotides 5-1543 of Genbank entry N M_001 106)(the sequence as shown provides an alanine at position 64, and may be modified to provide an arginine instead)
- nucleic acid sequence encoding a human ActRllA soluble (extracellular) polypeptide is as follows (the sequence as shown provides an alanine at position 64, and may be modified to provide an arginine instead):
- the invention relates to GDF Trap polypeptides which are variant forms of soluble ActRIIB polypeptides.
- soluble ActRIIB polypeptide generally refers to polypeptides comprising an extracellular domain of an ActRIIB protein.
- soluble ActRIIB polypeptide includes any naturally occurring extracellular domain of an ActRIIB protein as well as any variants thereof (including mutants, fragments and peptidomimetic forms) that retain a useful activity.
- the extracellular domain of an ActRIIB protein binds to a ligand and is generally soluble.
- soluble ActRIIB polypeptides examples include ActRIIB soluble polypeptides (e.g., SEQ ID NOs: 22, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38, 40 and 41).
- Other examples of soluble ActRIIB polypeptides comprise a signal sequence in addition to the extracellular domain of an ActRIIB protein, see Example 1.
- the signal sequence can be a native signal sequence of an ActRIIB, or a signal sequence from another protein, such as a tissue plasminogen activator (TPA) signal sequence or a honey bee melittin (HBM) signal sequence.
- TPA tissue plasminogen activator
- HBM honey bee melittin
- the disclosure identifies functionally active portions and variants of ActRIIB.
- Applicants have ascertained that an Fc fusion protein having the sequence disclosed by Hilden et al. (Blood. 1994 Apr 15;83(8):2163-70), which has an Alanine at the position corresponding to amino acid 64 of SEQ ID NO: 1 (A64), has a relatively low affinity for activin and GDF- 1 1.
- the same Fc fusion protein with an Arginine at position 64 (R64) has an affinity for activin and GDF-1 1 in the low nanomolar to high picomolar range. Therefore, a sequence with an R64 is used as the wild-type reference sequence for human ActRIIB in this disclosure.
- Attisano et al. (Cell. 1992 Jan 10;68( 1 ):97- 108) showed that a deletion of the proline knot at the C-terminus of the extracellular domain of ActRIIB reduced the affinity of the receptor for activin.
- An ActRIlB-Fc fusion protein containing amino acids 20-1 19 of SEQ ID NO: 1 “ActRlIB(20-l 19)-Fc”
- ActRlIB(20-l 19)-Fc has reduced binding to GDF- I l and activin relative to an ActRIIB(20- 134)-Fc, which includes the proline knot region and the complete juxtamembrane domain.
- an ActRlIB(20-129)-Fc protein retains similar but somewhat reduced activity relative to the wild type, even though the proline knot region is disrupted.
- ActRlIB extracellular domains that stop at amino acid 134, 133, 132, 131 , 130 and 129 are all expected to be active, but constructs stopping at 134 or 133 may be most active.
- mutations at any of residues 129-134 are not expected to alter ligand binding affinity by large margins. In support of this, mutations of P 129 and P 130 do not substantially decrease ligand binding.
- a GDF Trap polypeptide which is an ActRIIB-Fc fusion protein may end as early as amino acid 109 (the final cysteine), however, forms ending at or between 109 and 1 19 are expected to have reduced ligand binding.
- Amino acid 1 19 is poorly conserved and so is readily altered or truncated.
- Forms ending at 128 or later retain ligand binding activity.
- Forms ending at or between 1 19 and 127 will have an intermediate binding ability. Any of these forms may be desirable to use, depending on the clinical or experimental setting.
- an active portion of ActRIIB comprises amino acids 29-109 of SEQ ID NO: 1
- GDF Trap constructs may, for example, comprise a portion of ActRIIB beginning at a residue corresponding to amino acids 20-29 of SEQ ID NO: 1 or 39 and ending at a position corresponding to amino acids 109-134 of SEQ ID NO: 1 or 39.
- Other examples include constructs that begin at a position from 20-29 or 21 -29 and end at a position from 1 19- 134, 1 19- 133, 129- 134, or 129-133 of SEQ ID NO: 1 or 39.
- constructs that begin at a position from 20-24 (or 21-24, or 22-25) and end at a position from 109- 134 (or 109- 133), 1 19- 134 (or 1 19-133) or 129-134 (or 129- 133) of SEQ ID NO: 1 or 39. Variants within these ranges are also contemplated, particularly those having at least 80%, 85%, 90%, 95% or 99% identity to the corresponding portion of SEQ ID NO: 1 or 39.
- the GDF Trap polypeptide comprises, consists essentially of, or consists of, a polypeptide having an amino acid sequence that is at least 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99% or 100% identical to amino acid residues 25- 131 of SEQ ID NO: 1 or 39.
- the GDF Trap polypeptide comprises, consists essentially of, or consists of, a polypeptide having an amino acid sequence that is at least 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99% or 100% identical to SEQ ID NOs: 7, 26, 28, 29, 32, 37 or 38.
- the GDF Trap polypeptide consists of, or consists essentially of, the amino acid sequence of SEQ ID NO: 7, 26, 28, 29, 32, 37 or 38.
- the disclosure includes the results of an analysis of composite ActRIIB structures, shown in Figure 1 , demonstrating that the ligand binding pocket is defined by residues Y31 , N33, N35, L38 through T41 , E47, E50, Q53 through K55, L57, H58, Y60, S62, K74, W78 through N83, Y85, R87, A92, and E94 through FlOl .
- R40 is a K in Xenopus, indicating that basic amino acids at this position will be tolerated.
- Q53 is R in bovine ActRIIB and K in
- a general formula for a GDF Trap protein is one that comprises amino acids 29-109 of SEQ ID NO: 1 or 39, but optionally beginning at a position ranging from 20- 24 or 22-25 and ending at a position ranging from 129-134, and comprising no more than 1 , 2, 5, 10 or 15 conservative amino acid changes in the ligand binding pocket, and zero, one or more non-conservative alterations at positions 40, 53, 55, 74, 79 and/or 82 in the ligand binding pocket.
- Such a protein may retain greater than 80%, 90%, 95% or 99% sequence identity to the sequence of amino acids 29-109 of SEQ ID NO: 1 or 39.
- Sites outside the binding pocket, at which variability may be particularly well tolerated, include the amino and carboxy termini of the extracellular domain (as noted above), and positions 42-46 and 65-73.
- An asparagine to alanine alteration at position 65 (N65A) actually improves ligand binding in the A64 background, and is thus expected to have no detrimental effect on ligand binding in the R64 background. This change probably eliminates glycosylation at N65 in the A64 background, thus demonstrating that a significant change in this region is likely to be tolerated.
- an active, human ActRIIB variant polypeptide useful as a GDF Trap may include one or more amino acids at corresponding positions from the sequence of another vertebrate ActRIIB, or may include a residue that is similar to that in the human or other vertebrate sequence.
- L46 is a valine in Xenopus ActRIIB, and so this position may be altered, and optionally may be altered to another hydrophobic residue, such as V, I or F, or a non-polar residue such as A.
- E52 is a K in Xenopus, indicating that this site may be tolerant of a wide variety of changes, including polar residues, such as E, D, K, R, H, S, T, P, G, Y and probably A.
- T93 is a K in Xenopus, indicating that a wide structural variation is tolerated at this position, with polar residues favored, such as S, K, R, E, D, H, G, P, G and Y.
- F 108 is a Y in Xenopus, and therefore Y or other hydrophobic group, such as I, V or L should be tolerated.
- El I l is K in Xenopus, indicating that charged residues will be tolerated at this position, including D, R, K and H, as well as Q and N.
- Rl 12 is K in Xenopus, indicating that basic residues are tolerated at this position, including R and H.
- a at position 119 is relatively poorly conserved, and appears as P in rodents and V in Xenopus, thus essentially any amino acid should be tolerated at this position.
- N-X-S/T N-linked glycosylation site
- N-X-S/T sequences include amino acids 20-29, 20-24, 22-25, 109-134, 120- 134 or 129-134.
- N-X-S/T sequences may also be introduced into the linker between the ActRIIB sequence and the Fc or other fusion component. Such a site may be introduced with minimal effort by introducing an N in the correct position with respect to a pre-existing S or T, or by introducing an S or T at a position corresponding to a pre-existing N.
- alterations that would create an N-linked glycosylation site are: A24N, R64N, S67N (possibly combined with an N65A alteration), E106N, R l 12N, Gl 2ON, E 123N, P129N, A 132N, Rl 12S and Rl 12T.
- Any S that is predicted to be glycosylated may be altered to a T without creating an immunogenic site, because of the protection afforded by the glycosylation.
- any T that is predicted to be glycosylated may be altered to an S.
- S67T and S44T are contemplated.
- an S26T alteration may be used.
- a GDF Trap may be an ActRIIB variant having one or more additional, non-endogenous N-linked glycosylation consensus sequences.
- Position L79 of ActRIIB may be altered to confer altered activin - myostatin (GDF- 1 1 ) binding properties.
- L79A or L79P reduces GDF-1 1 binding to a greater extent than activin binding.
- L79E or L79D retains GDF-1 1 binding.
- the L79E and L79D variants have greatly reduced activin binding.
- In vivo experiments indicate that these non- activin receptors retain significant ability to increase red blood cells but show decreased effects on other tissues.
- the methods described herein utilize a GDF Trap polypeptide which is a variant ActRIIB polypeptide comprising an acidic amino acid (e.g., D or E) at the position corresponding to position 79 of SEQ ID NO: 1 or 39, optionally in combination with one or more additional amino acid substitutions, additions, or deletions.
- an acidic amino acid e.g., D or E
- the results of the mutagenesis program described herein indicate that there are amino acid positions in ActRIIB that are often beneficial to conserve. These include position 64 (basic amino acid), position 80 (acidic or hydrophobic amino acid), position 78 (hydrophobic, and particularly tryptophan), position 37 (acidic, and particularly aspartic or glutamic acid), position 56 (basic amino acid), position 60 (hydrophobic amino acid, particularly phenylalanine or tyrosine).
- the disclosure provides a framework of amino acids that may be conserved.
- Other positions that may be desirable to conserve are as follows: position 52 (acidic amino acid), position 55 (basic amino acid), position 81 (acidic), 98 (polar or charged, particularly E, D, R or K).
- isolated fragments of ActRIIB polypeptides can be obtained by screening polypeptides recombinantly produced from the corresponding fragment of the nucleic acid encoding an ActRIIB polypeptide (e.g., SEQ ID NOs: 4 and 5).
- fragments can be chemically synthesized using techniques known in the art such as conventional Merrifield solid phase f-Moc or t-Boc chemistry. The fragments can be produced (recombinantly or by chemical synthesis) and tested to identify those peptidyl fragments that can function, for example, as antagonists (inhibitors) or agonists (activators) of an ActRlIB protein or an ActRlIB ligand.
- GDF Trap polypeptide is a. variant ActRlIB polypeptide having an amino acid sequence that is at least 75% identical to an amino acid sequence selected from SEQ ID NOs: 2, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38, 40 or 41 .
- the GDF Trap has an amino acid sequence at least 80%, 85%, 90%, 95%, 97%, 98%, 99% or 100% identical to an amino acid sequence selected from SEQ ID NOs: 2, 3, 7, 1 1 , 26, 28, 29, 32, 37, 38, 40 or 41.
- the GDF Trap comprises, consists essentially of, or consists of, an amino acid sequence at least 80%, 85%, 90%, 95%, 97%, 98%, 99% or 100% identical to an amino acid sequence selected from SEQ ID NOs: 2, 3, 7, 1 1, 26, 28, 29, 32, 37, 38, 40 or 41 , wherein the position corresponding to L79 of SEQ ID NO: 1 is an acidic amino acid (e.g., a D or E amino acid residue).
- the present invention contemplates making functional variants by modifying the structure of a GDF Trap polypeptide for such purposes as enhancing therapeutic efficacy, or stability (e.g., ex vivo shelf life and resistance to proteolytic degradation in vivo).
- GDF Trap polypeptides can also be produced by amino acid substitution, deletion, or addition. For instance, it is reasonable to expect that an isolated replacement of a leucine with an isoleucine or valine, an aspartate with a glutamate, a threonine with a serine, or a similar replacement of an amino acid with a structurally related amino acid (e.g., conservative mutations) will not have a major effect on the biological activity of the resulting molecule.
- Conservative replacements are those that take place within a family of amino acids that are related in their side chains. Whether a change in the amino acid sequence of a GDF Trap polypeptide results in a functional variant can be readily determined by assessing the ability of the GDF Trap polypeptide to produce a response in cells relative to the unmodified GDF Trap polypeptide or a wild-type ActRlIB polypeptide, or to bind to one or more ligands, such as activin, GDF-1 1 or myostatin as compared to the unmodified GDF Trap polypeptide or a wild-type ActRlIB polypeptide.
- ligands such as activin, GDF-1 1 or myostatin
- the present invention contemplates making mutations in the extracellular domain (also referred to as ligand-binding domain) of an ActRlIB polypeptide such that the ActRlIB polypeptide has altered ligand-binding activities (e.g., binding affinity or binding specificity).
- ligand-binding activities e.g., binding affinity or binding specificity.
- GDF Trap polypeptides have altered (elevated or reduced) binding affinity for a specific ligand.
- the GDF Trap polypeptides have altered binding specificity for ActRIIB ligands.
- the disclosure provides GDF Trap polypeptides that preferentially bind to GDF8/GDF1 1 relative to activins.
- the disclosure further establishes the desirability of such polypeptides for reducing off-target effects, although such selective variants may be less desirable for the treatment of severe diseases where very large gains in red blood cell levels may be needed for therapeutic effect and where some level of off-target effect is acceptable.
- amino acid residues of the ActRIIB protein such as E39, K55, Y60, K74, W78, D80, and FlOl , are in the ligand-binding pocket and mediate binding to its ligands such as activin and GDF8.
- the present invention provides a GDF Trap comprising an altered ligand-binding domain (e.g., GDF8-binding domain) of an ActRIIB receptor, which comprises one or more mutations at those amino acid residues.
- the altered ligand-binding domain can have increased selectivity for a ligand such as GDF8 relative to a wild-type ligand-binding domain of an ActRIIB receptor.
- these mutations increase the selectivity of the altered ligand-binding domain for GDF8 over activin.
- the altered ligand-binding domain has a ratio of Kj for activin binding to K d for GDF8 binding that is at least 2, 5, 10, or even 100 fold greater relative to the ratio for the wild-type ligand-binding domain.
- the altered ligand-binding domain has a ratio of IC 5 o for inhibiting activin to IC 50 for inhibiting GDF8 that is at least 2, 5, 10, or even 100 fold greater relative to the wild-type ligand-binding domain.
- the altered ligand- binding domain inhibits GDF8 with an IC 50 at least 2, 5, 10, or even 100 times less than the IC 5 o for inhibiting activin.
- the positively-charged amino acid residue Asp (D80) of the ligand-binding domain of ActRIIB can be mutated to a different amino acid residue to produce a GDF Trap polypeptide that preferentially binds to GDF8, but not activin.
- the D80 residue is changed to an amino acid residue selected from the group consisting of: an uncharged amino acid residue, a negative amino acid residue, and a hydrophobic amino acid residue.
- the hydrophobic residue, L79 can be altered to the acidic amino acids aspartic acid or glutamic acid to greatly reduce activin binding while retaining GDF l 1 binding.
- most of the described mutations, variants or modifications may be made at the nucleic acid level or, in some cases, by post translational modification or chemical synthesis Such techniques are well known in the art
- the present invention contemplates GDF Trap polypeptides having specific mutations in ActRlIB so as to alter the glycosylation of the ActRIIB polypeptide
- Exemplary glycosylation sites in GDF Trap polypeptides are illustrated in Figure 1 (e g , the underlined NX(S/T) sites)
- Such mutations may be selected so as to introduce or eliminate one or more glycosylation sites, such as O-linked or N-linked glycosylation sites
- Asparagine-hnked glycosylation recognition sites generally comp ⁇ se a t ⁇ peptide sequence, asparagine-X-threonine (where "X" is any amino acid) which is specifically recognized by appropriate cellular glycosylation enzymes
- the alteration may also be made by the addition of, or substitution by, one or more se ⁇ ne or threonine residues to the sequence of the wild-type ActRIIB polypeptide (for O-linked glycosylation sites)
- GDF Trap polypeptide may be adjusted, as appiop ⁇ ate, depending on the type of expression system used, as mammalian, yeast, insect and plant cells may all introduce differing glycosylation patterns that can be affected by the amino acid sequence of the peptide.
- GDF Trap polypeptides for use in humans will be expressed in a mammalian cell line that provides proper glycosylation, such as HEK293 or CHO cell lines, although other mammalian expression cell lines are expected to be useful as well.
- This disclosure further contemplates a method of generating variants, particularly sets of combinatorial variants of a GDF Trap polypeptide, including, optionally, truncation variants; pools of combinatorial mutants are especially useful for identifying GDF Trap sequences.
- the purpose of screening such combinatorial libraries may be to generate, for example, GDF Trap polypeptide variants which have altered properties, such as altered pharmacokinetics, or altered ligand binding.
- a variety of screening assays are provided below, and such assays may be used to evaluate variants.
- a GDF Trap polypeptide variant may be screened for the ability to bind to an ActRIlB polypeptide, to prevent binding of an ActRIIB ligand to an ActRIIB polypeptide or to interfere with signaling caused by an ActRIIB ligand.
- the activity of a GDF Trap polypeptide or its variants may also be tested in a cell- based or in vivo assay.
- the effect of a GDF Trap polypeptide variant on the expression of genes involved in hematopoiesis may be assessed. This may, as needed, be performed in the presence of one or more recombinant ActRIIB ligand proteins (e.g., activin), and cells may be transfected so as to produce a GDF Trap polypeptide and/or variants thereof, and optionally, an ActRIIB ligand.
- ActRIIB ligand proteins e.g., activin
- a GDF Trap polypeptide may be administered to a mouse or other animal, and one or more blood measurements, such as an RBC count, hemoglobin levels, hematocrit levels, iron stores, or reticulocyte count may be assessed using art recognized methods.
- Combinatorially-derived variants can be generated which have a selective potency relative to a reference GDF Trap polypeptide.
- Such variant proteins when expressed from recombinant DNA constructs, can be used in gene therapy protocols.
- mutagenesis can give rise to variants which have intracellular half-lives dramatically different than the corresponding unmodified GDF Trap polypeptide.
- the altered protein can be rendered either more stable or less stable to proteolytic degradation or other processes which result in destruction of, or otherwise inactivation of an unmodified GDF Trap polypeptide.
- Such variants, and the genes which encode them can be utilized to alter GDF Trap polypeptide levels by modulating the half-life of the GDF Trap polypeptides
- a short half-life can give rise to more transient biological effects and, when part of an inducible expression system, can allow tighter control of recombinant GDF Trap polypeptide levels within the cell
- mutations may be made in the linker (if any) and/or the Fc portion to alter the half-life of the protein
- the GDF Trap polypeptides of the invention may further comprise post-translational modifications in addition to any that are naturally present in the ActRIIB polypeptides Such modifications include, but are not limited to, acetylation, carboxylation, glycosylation, phosphorylation, hpidation, and acylation
- GDF Trap polypeptides may contain non-amino acid elements, such as polyethylene glycols, lipids, poly- or mono-saccha ⁇ de, and phosphates Effects of such non-amino acid elements on the functionality of a GDF Trap polypeptide may be tested as desc ⁇ bed herein for other GDF Trap polypeptide variants
- post-translational processing may also be important for correct folding and/or function of the protein Different cells (such as CHO, HeLa, MDCK, 293, WI38,
- GDF Trap polypeptides include fusion proteins having at least a portion of an ActRIIB polypeptide and one or more fusion domains
- fusion domains include, but are not limited to, polyhistidine, Glu-Glu, glutathione S transferase (GST), thioredoxin, protein A, protein G, an immunoglobulin heavy chain constant region (e g , an Fc), maltose binding protein (MBP), or human serum albumin
- GFP glutathione S transferase
- MBP maltose binding protein
- human serum albumin A fusion domain may be selected so as to confer a desired property
- some fusion domains are particularly useful foi isolation of the fusion proteins by affinity chromatography
- relevant matrices for affinity chromatography such as glutathione-, amylase-, and nickel- or cobalt- conjugated resins are used Many of such mati ices aie available in "kit' " form, such as the Pharmacia GST purification system and
- the fusion domains have a protease cleavage site, such as for Factor Xa or Thrombin, which allows the relevant protease to partially digest the fusion proteins and thereby liberate the recombinant proteins therefrom. The liberated proteins can then be isolated from the fusion domain by subsequent chromatographic separation.
- a GDF Trap polypeptide is fused with a domain that stabilizes the GDF Trap polypeptide in vivo (a "stabilizer” domain).
- stabilizing is meant anything that increases serum half life, regardless of whether this is because of decreased destruction, decreased clearance by the kidney, or other pharmacokinetic effect. Fusions with the Fc portion of an immunoglobulin are known to confer desirable pharmacokinetic properties on a wide range of proteins. Likewise, fusions to human serum albumin can confer desirable properties. Other types of fusion domains that may be selected include multimerizing (e.g., dimerizing, tetramerizing) domains and functional domains (that confer an additional biological function, such as further increasing red blood cell levels).
- the present invention provides GDF Trap that is an ActRIIB- Fc fusion protein which comprises an extracellular (e.g., ligand-binding) domain of ActRIIB polypeptide fused to an Fc domain.
- GDF Trap that is an ActRIIB- Fc fusion protein which comprises an extracellular (e.g., ligand-binding) domain of ActRIIB polypeptide fused to an Fc domain.
- the sequence of an exemplary Fc domain is shown below (SEQ ID NO: 6).
- the Fc domain has one or more mutations at residues such as Asp-265, lysine 322, and Asn-434.
- the mutant Fc domain having one or more of these mutations has reduced ability of binding to the Fc ⁇ receptor relative to a wildtype Fc domain.
- the mutant Fc domain having one or more of these mutations e.g., Asn-434 mutation
- a GDF Trap polypeptide may be placed C-terminal to a heterologous domain, or, alternatively, a heterologous domain may be placed C-terminal to a GDF Trap polypeptide.
- the GDF Trap polypeptide domain and the heterologous domain need not be adjacent in a fusion protein, and additional domains or amino acid sequences may be included C- or N-terminal to either domain or between the domains.
- a GDF Trap fusion protein comprises an amino acid sequence as set forth in the formula A-B-C.
- the B portion is an N- and C-terminally truncated ActRIIB polypeptide consisting of the amino acid sequence corresponding to amino acids 26-132 of SEQ ID NO: 26.
- the A and C portions may be independently zero, one or more than one amino acids, and both the A and C portions when present are heterologous to B.
- the A and/or C portions may be attached to the B portion via a linker sequence.
- Exemplary linkers are include short polypeptide linkers such as 2-10, 2-5, 2-4, 2-3 Glycine residues, such as, for example, a Gly-Gly-Gly linker. Other suitable linkers are described herein above.
- a GDF Trap fusion protein comprises an amino acid sequence as set forth in the formula A-B-C, wherein A is a leader sequence, B consists of amino acids 26-132 of SEQ ID NO: 26, and C is a polypeptide portion that enhances one or more of in vivo stability, in vivo half life, uptake/administration, tissue localization or distribution, formation of protein complexes, and/or purification.
- a GDF Trap fusion protein comprises an amino acid sequence as set forth in the formula A-B- C, wherein A is a TPA leader sequence, B consists of amino acids 26-132 of SEQ ID NO: 26, and C is an immunoglobulin Fc domain.
- a preferred GDF Trap fusion protein comprises the amino acid sequence set forth in SEQ ID NO: 26.
- the GDF Trap polypeptides of the present invention contain one or more modifications that are capable of stabilizing the GDF Trap polypeptides.
- modifications enhance the in vitro half life of the GDF Trap polypeptides, enhance circulatory half life of the GDF Trap polypeptides or reducing proteolytic degradation of the GDF Trap polypeptides.
- Such stabilizing modifications include, but are not limited to, fusion proteins (including, for example, fusion proteins comprising an GDF Trap polypeptide and a stabilizer domain), modifications of a glycosylation site (including, for example, addition of a glycosylation site to a GDF Trap polypeptide), and modifications of carbohydrate moiety (including, for example, removal of carbohydrate moieties from a GDF Trap polypeptide).
- fusion proteins including, for example, fusion proteins comprising an GDF Trap polypeptide and a stabilizer domain
- modifications of a glycosylation site including, for example, addition of a glycosylation site to a GDF Trap polypeptide
- modifications of carbohydrate moiety including, for example, removal of carbohydrate moieties from a GDF Trap polypeptide.
- a GDF Trap polypeptide is fused to a stabilizer domain such as an IgG molecule (e.g., an Fc domain).
- stabilizer domain not only refers to a fusion domain (e.g., Fc) as in the case of fusion proteins, but also includes nonproteinaceous modifications such as a carbohydrate moiety, or nonproteinaceous polymer, such as polyethylene glycol.
- the present invention makes available isolated and/or purified forms of the GDF Trap polypeptides, which are isolated from, or otherwise substantially free of, other proteins.
- GDF Trap polypeptides (unmodified or modified) of the invention can be produced by a variety of art-known techniques.
- GDF Trap polypeptides can be synthesized using standard protein chemistry techniques such as those described in Bodansky, M. Principles of Peptide Synthesis, Springer Verlag, Berlin (1993) and Grant G. A. (ed.), Synthetic Peptides: A User's Guide, W. H. Freeman and Company, New York (1992).
- automated peptide synthesizers are commercially available (e.g., Advanced ChemTech Model 396; Milligen/Biosearch 9600).
- the GDF Trap polypeptides, fragments or variants thereof may be recombinantly produced using various expression systems (e.g., E. coli, Chinese Hamster Ovary (CHO) cells, COS cells, baculovirus) as is well known in the art.
- the modified or unmodified GDF Trap polypeptides may be produced by digestion of recombinantly produced full-length GDF Trap polypeptides by using, for example, a protease, e.g., trypsin, thermolysin, chymotrypsin, pepsin, or paired basic amino acid converting enzyme (PACE).
- a protease e.g., trypsin, thermolysin, chymotrypsin, pepsin, or paired basic amino acid converting enzyme (PACE).
- PACE paired basic amino acid converting enzyme
- GDF Trap polypeptides may be produced from recombinantly produced full-length GDF Trap polypeptides such as standard techniques known in the art, such as by chemical cleavage (e.g., cyanogen bromide, hydroxylamine).
- the invention provides isolated and/or recombinant nucleic acids encoding any of the GDF Trap polypeptides disclosed herein.
- SEQ ID NO: 4 encodes a naturally occurring ActRIIB precursor polypeptide
- SEQ ID NO: 5 encodes a soluble ActRlIB polypeptide
- SEQ ID NOs: 25, 27, 30 and 31 encode soluble GDF Traps.
- the subject nucleic acids may be single-stranded or double stranded.
- Such nucleic acids may be DNA or RNA molecules. These nucleic acids may be used, for example, in methods for making GDF Trap polypeptides or as direct therapeutic agents (e.g., in a gene therapy approach).
- the subject nucleic acids encoding GDF Trap polypeptides are further understood to include nucleic acids that are variants of SEQ ID NOs: 5, 25, 27, 30 and 31.
- Variant nucleotide sequences include sequences that differ by one or more nucleotide substitutions, additions or deletions, such as allelic variants; and will, therefore, include coding sequences that differ from the nucleotide sequence of the coding sequence designated in SEQ ID NOs: 5, 25, 27, 30 and 31.
- the invention provides isolated or recombinant nucleic acid sequences that are at least 80%, 85%, 90%, 95%, 97%, 98%, 99% or 100% identical to SEQ ID NO: 5, 25, 27, 30 or 31.
- nucleic acid sequences complementary to SEQ ID NO: 5, 25, 27, 30 or 31 and variants of SEQ ID NO: 5, 25, 27, 30 or 31 , are also within the scope of this invention.
- the nucleic acid sequences of the invention can be isolated, recombinant, and/or fused with a heterologous nucleotide sequence, or in a DNA library.
- nucleic acids of the invention also include nucleotide sequences that hybridize under highly stringent conditions to the nucleotide sequence designated in SEQ ID NO: 5, 25, 27, 30 or 31 , complement sequence of SEQ ID NO: 5, 25, 27, 30 or 31 , or fragments thereof.
- appropriate stringency conditions which promote DNA hybridization can be varied. For example, one could perform the hybridization at 6.0 x sodium chloride/sodium citrate (SSC) at about 45 0 C, followed by a wash of 2.0 x SSC at 50 °C.
- the salt concentration in the wash step can be selected from a low stringency of about 2.0 x SSC at 50 °C to a high stringency of about 0.2 x SSC at 50 0 C.
- the temperature in the wash step can be increased from low stringency conditions at room temperature, about 22 °C. to high stringency conditions at about 65 0 C. Both temperature and salt may be varied, or temperature or salt concentration may be held constant while the other variable is changed.
- the invention provides nucleic acids which hybridize under low stringency conditions of 6 x SSC at room temperature followed by a wash at 2 x SSC at room temperature.
- Isolated nucleic acids which differ from the nucleic acids as set forth in SEQ ID NO: 5, 25, 27, 30 or 31 due to degeneracy in the genetic code are also within the scope of the invention.
- a number of amino acids are designated by more than one triplet. Codons that specify the same amino acid, or synonyms (for example, CAU and CAC are synonyms for histidine) may result in "silent" mutations which do not affect the amino acid sequence of the protein.
- the GDF Trap polypeptide will be encoded by an alternative nucleotide sequence.
- Alternative nucleotide sequences are degenerate with respect to the native GDF Trap nucleic acid sequence but still encode for the same fusion protein.
- the GDF Trap having SEQ ID NO: 26 is encoded by an alternative nucleic acid sequence comprising SEQ ID NO: 30.
- DNA sequence polymorphisms that do lead to changes in the amino acid sequences of the subject proteins will exist among mammalian cells.
- these variations in one or more nucleotides (up to about 3-5% of the nucleotides) of the nucleic acids encoding a particular protein may exist among individuals of a given species due to natural allelic variation. Any and all such nucleotide variations and resulting amino acid polymorphisms are within the scope of this invention.
- the recombinant nucleic acids of the invention may be operably linked to one or more regulatory nucleotide sequences in an expression construct.
- Regulatory nucleotide sequences will generally be appropriate to the host cell used for expression. Numerous types of appropriate expression vectors and suitable regulatory sequences are known in the art for a variety of host cells.
- said one or more regulatory nucleotide sequences may include, but are not limited to, promoter sequences, leader or signal sequences, ribosomal binding sites, transcriptional start and termination sequences, translational start and termination sequences, and enhancer or activator sequences. Constitutive or inducible promoters as known in the art are contemplated by the invention.
- the promoters may be either naturally occurring promoters, or hybrid promoters that combine elements of more than one promoter.
- An expression construct may be present in a cell on an episome, such as a plasmid, or the expression construct may be inserted in a chromosome.
- the expression vector contains a selectable marker gene to allow the selection of transformed host cells. Selectable marker genes are well known in the art and will vary with the host cell used.
- the subject nucleic acid is provided in an expression vector comprising a nucleotide sequence encoding a GDF Trap polypeptide and operably linked to at least one regulatory sequence Regulatory sequences are art-recognized and are selected to direct expression of the GDF Trap polypeptide
- regulatory sequence includes promoters, enhancers, and other expression control elements Exemplary regulatory sequences are desc ⁇ bed in Goeddel, Gene Expression Technology Methods in Enzymology, Academic Press, San Diego, CA (1990)
- any of a wide variety of expression control sequences that control the expression of a DNA sequence when operatively linked to it may be used in these vectors to express DNA sequences encoding a GDF Trap polypeptide
- Such useful expression control sequences include, for example, the early and late promoters of SV40, tet promoter, adenovirus or cytomegalovirus immediate early promoter, RSV promoters, the lac system, the tip system, the T
- a recombinant nucleic acid of the invention can be produced by ligating the cloned gene, or a portion thereof, into a vector suitable for expression in either prokaryotic cells, eukaryotic cells (yeast, avian, insect or mammalian), or both Expression vehicles for production of a recombinant GDF Trap polypeptide include plasmids and other vectors Foi instance, suitable vectors include plasmids of the types pBR322-de ⁇ ved plasmids, pEMBL- de ⁇ ved plasmids, pEX-de ⁇ ved plasmids, pBTac-de ⁇ ved plasmids and pUC-de ⁇ ved plasmids foi expression in prokaryotic cells, such as E coli
- Some mammalian expression vectors contain both prokaryotic sequences to facilitate the propagation of the vectoi in bacteria, and one or more eukaryotic ti ansc ⁇ ption units that are expressed in eukaryotic cells.
- the pcDNAI/amp, pcDNAI/neo, pRc/CMV, pSV2gpt, pSV2neo, pSV2-dhfr, pTk2, pRSVneo, pMSG, pSVT7, pko-neo and pHyg derived vectors are examples of mammalian expression vectors suitable for transfection of eukaryotic cells.
- vectors are modified with sequences from bacterial plasmids, such as pBR322, to facilitate replication and drug resistance selection in both prokaryotic and eukaryotic cells.
- bacterial plasmids such as pBR322
- derivatives of viruses such as the bovine papilloma virus (BPV-I ), or Epstein- Barr virus (pHEBo, pREP-derived and p205) can be used for transient expression of proteins in eukaryotic cells.
- BBV-I bovine papilloma virus
- pHEBo Epstein- Barr virus
- examples of other viral (including retroviral) expression systems can be found below in the description of gene therapy delivery systems.
- the various methods employed in the preparation of the plasmids and in transformation of host organisms are well known in the art.
- baculovirus expression systems include pVL-derived vectors (such as pVL1392, pVL1393 and pVL941 ), pAcUW-derived vectors (such as pAcUWl), and pBlueBac-derived vectors (such as the ⁇ -gal containing pBlueBac III).
- pVL-derived vectors such as pVL1392, pVL1393 and pVL941
- pAcUW-derived vectors such as pAcUWl
- pBlueBac-derived vectors such as the ⁇ -gal containing pBlueBac III.
- a vector will be designed for production of the subject GDF Trap polypeptides in CHO cells, such as a Pcmv-Script vector (Stratagene, La Jolla, Calif), pcDNA4 vectors (Invitrogen, Carlsbad, Calif.) and pCl-neo vectors (Promega, Madison, Wise).
- a vector will be designed for production of the subject GDF Trap polypeptides in CHO cells, such as a Pcmv-Script vector (Stratagene, La Jolla, Calif), pcDNA4 vectors (Invitrogen, Carlsbad, Calif.) and pCl-neo vectors (Promega, Madison, Wise).
- the subject gene constructs can be used to cause expression of the subject GDF Trap polypeptides in cells propagated in culture, e.g., to produce proteins, including fusion proteins or variant proteins, for purification.
- This invention also pertains to a host cell transfected with a recombinant gene including a coding sequence (e.g., SEQ ID NO: 4, 5, 25, 27, 30 or 3 1 ) for one or more of the subject GDF Trap polypeptides.
- the host cell may be any prokaryotic or eukaryotic cell.
- a GDF Trap polypeptide of the invention may be expressed in bacterial cells such as E. coli, insect cells (e.g., using a baculovirus expression system), yeast, or mammalian cells. Other suitable host cells are known to those skilled in the art.
- the present invention further pertains to methods of producing the subject GDF Trap polypeptides.
- a host cell transfected with an expression vector encoding a GDF Trap polypeptide can be cultured under appropriate conditions to allow expression of the GDF Trap polypeptide to occur.
- the GDF Trap polypeptide may be secreted and isolated from a mixture of cells and medium containing the GDF Trap polypeptide.
- the GDF Trap polypeptide may be retained cytoplasmically or in a membrane fraction and the cells harvested, lysed and the protein isolated.
- a cell culture includes host cells, media and other byproducts. Suitable media for cell culture are well known in the art.
- the subject GDF Trap polypeptides can be isolated from cell culture medium, host cells, or both, using techniques known in the art for purifying proteins, including ion-exchange chromatography, gel filtration chromatography, ultrafiltration, electrophoresis, and immunoaffinity purification with antibodies specific for particular epitopes of the GDF Trap polypeptides.
- the GDF Trap polypeptide is a fusion protein containing a domain which facilitates its purification.
- a fusion gene coding for a purification leader sequence such as a poly-(His)/enterokinase cleavage site sequence at the N-terminus of the desired portion of the recombinant GDF Trap polypeptide, can allow purification of the expressed fusion protein by affinity chromatography using a Ni + metal resin.
- the purification leader sequence can then be subsequently removed by treatment with enterokinase to provide the purified GDF Trap polypeptide (e.g., see Hochuli et al., (1987) J. Chromatography 41 1 :177; and Janknecht et al., PNAS USA 88:8972). Techniques for making fusion genes are well known.
- the joining of various DNA fragments coding for different polypeptide sequences is performed in accordance with conventional techniques, employing blunt-ended or stagger-ended termini for ligation, restriction enzyme digestion to provide for appropriate termini, filling-in of cohesive ends as appropriate, alkaline phosphatase treatment to avoid undesirable joining, and enzymatic ligation.
- the fusion gene can be synthesized by conventional techniques including automated DNA synthesizers.
- PCR amplification of gene fragments can be earned out using anchor primers which give rise to complementary overhangs between two consecutive gene fragments which can subsequently be annealed to generate a chimeric gene sequence (see, for example, Current Protocols in Molecular Biology, eds. Ausubel et al., John Wiley & Sons: 1992). 4. Screening Assays
- the present invention relates to the use of the subject GDF Trap polypeptides (e.g., soluble variant ActRIIB polypeptides) to identify compounds (agents) which are agonist or antagonists of ActRIIB polypeptides.
- Compounds identified through this screening can be tested to assess their ability to modulate red blood cell, hemoglobin and/or reticulocyte levels in vivo or in vitro. These compounds can be tested, for example, in animal models.
- high-throughput screening of compounds can be carried out to identify agents that perturb ActRIIB-mediated effects on a selected cell line.
- the assay is carried out to screen and identify compounds that specifically inhibit or reduce binding of an ActRIIB polypeptide to its binding partner, such as an ActRIIB ligand (e.g., activin, Nodal, GDF8, GDFl 1 or BMP7).
- an ActRIIB ligand e.g., activin, Nodal, GDF8, GDFl 1 or BMP7
- the assay can be used to identify compounds that enhance binding of an ActRIIB polypeptide to its binding partner such as an ActRIIB ligand.
- the compounds can be identified by their ability to interact with an ActRIIB polypeptide.
- test compounds (agents) of the invention may be created by any combinatorial chemical method.
- the subject compounds may be naturally occurring biomolecules synthesized in vivo or in vitro.
- Compounds (agents) to be tested for their ability to act as modulators of tissue growth can be produced, for example, by bacteria, yeast, plants or other organisms (e.g., natural products), produced chemically (e.g., small molecules, including peptidomimetics), or produced recombinantly.
- Test compounds contemplated by the present invention include non-peptidyl organic molecules, peptides, polypeptides, peptidomimetics, sugars, hormones, and nucleic acid molecules.
- the test agent is a small organic molecule having a molecular weight of less than about 2,000 Daltons.
- the test compounds of the invention can be provided as single, discrete entities, or provided in libraries of greater complexity, such as made by combinatorial chemistry. These libraries can comprise, for example, alcohols, alkyl halides, amines, amides, esters, aldehydes, ethers and other classes of organic compounds. Presentation of test compounds to the test system can be in either an isolated form or as mixtures of compounds, especially in initial screening steps.
- the compounds may be optionally derivatized with other compounds and have derivatizing groups that facilitate isolation of the compounds.
- derivatizing groups include biotin, fluorescein, digoxygenin, green fluorescent protein, isotopes, polyhistidine, magnetic beads, glutathione S transferase (GST), photoactivatible crosslinkers or any combinations thereof.
- the effects of cellular toxicity or bioavailability of the test compound can be generally ignored in the in vitro system, the assay instead being focused primarily on the effect of the drug on the molecular target as may be manifest in an alteration of binding affinity between an ActRIIB polypeptide and its binding partner (e.g., an ActRIIB ligand).
- an ActRIIB polypeptide and its binding partner e.g., an ActRIIB ligand
- the compound of interest is contacted with an isolated and purified ActRIIB polypeptide which is ordinarily capable of binding to an ActRIIB ligand, as appropriate for the intention of the assay.
- an isolated and purified ActRIIB polypeptide which is ordinarily capable of binding to an ActRIIB ligand, as appropriate for the intention of the assay.
- To the mixture of the compound and ActRIIB polypeptide is then added to a composition containing an ActRIIB ligand. Detection and quantification of
- ActRIIB/ActRIIB ligand complexes provides a means for determining the compound's efficacy at inhibiting (or potentiating) complex formation between the ActRIIB polypeptide and its binding protein.
- the efficacy of the compound can be assessed by generating dose response curves from data obtained using various concentrations of the test compound.
- a control assay can also be performed to provide a baseline for comparison. For example, in a control assay, isolated and purified ActRIIB ligand is added to a composition containing the ActRIIB polypeptide, and the formation of ActRIIB/ActRIIB ligand complex is quantitated in the absence of the test compound.
- the order in which the reactants may be admixed can be varied, and can be admixed simultaneously.
- cellular extracts and lysates may be used to render a suitable cell-free assay system.
- Complex formation between the ActRIIB polypeptide and its binding protein may be detected by a variety of techniques.
- modulation of the formation of complexes can be quantitated using, for example, detectably labeled proteins such as radiolabeled (e.g., 32 P, 35 S, 14 C or 3 H), fluorescently labeled (e.g., FITC), or enzymatically labeled ActRIIB polypeptide or its binding protein, by immunoassay, or by chromatographic detection.
- detectably labeled proteins such as radiolabeled (e.g., 32 P, 35 S, 14 C or 3 H), fluorescently labeled (e.g., FITC), or enzymatically labeled ActRIIB polypeptide or its binding protein
- the present invention contemplates the use of fluorescence polarization assays and fluorescence resonance energy transfer (FRET) assays in measuring, either directly or indirectly, the degree of interaction between an ActRIIB polypeptide and its binding protein.
- FRET fluorescence resonance energy transfer
- an interaction trap assay also known as the "two hybrid assay," for identifying agents that disrupt or potentiate interaction between an ActRIIB polypeptide and its binding partner. See for example, U.S. Pat. No. 5,283,317; Zervos et al. (1993) Cell 72:223-232; Madura et al. (1993) J Biol Chem
- the present invention contemplates the use of reverse two hybrid systems to identify compounds (e.g., small molecules or peptides) that dissociate interactions between an ActRIIB polypeptide and its binding protein. See for example, Vidal and Legrain, (1999) Nucleic Acids Res 27:919-29; Vidal and Legrain, ( 1999) Trends Biotechnol 17:374-81 ; and U.S. Pat. Nos. 5,525,490; 5,955,280; and 5,965,368.
- the subject compounds are identified by their ability to interact with an ActRIIB polypeptide.
- the interaction between the compound and the ActRIIB polypeptide may be covalent or non-covalent.
- such interaction can be identified at the protein level using in vitro biochemical methods, including photo- crosslinking, radiolabeled ligand binding, and affinity chromatography (Jakoby WB et al.,
- the compounds may be screened in a mechanism based assay, such as an assay to detect compounds which bind to an ActRlIB polypeptide.
- a mechanism based assay such as an assay to detect compounds which bind to an ActRlIB polypeptide. This may include a solid phase or fluid phase binding event.
- the gene encoding an ActRlIB polypeptide can be transfected with a reporter system (e.g., ⁇ - galactosidase, luciferase, or green fluorescent protein) into a cell and screened against the library preferably by a high throughput screening or with individual members of the library.
- a reporter system e.g., ⁇ - galactosidase, luciferase, or green fluorescent protein
- Other mechanism based binding assays may be used, for example, binding assays which detect changes in free energy.
- Binding assays can be performed with the target fixed to a well, bead or chip or captured by an immobilized antibody or resolved by capillary electrophoresis.
- the bound compounds may be detected usually using colorimetric or fluorescence or surface plasmon resonance.
- the GDF Trap polypeptides of the present invention can be used to increase red blood cell levels in mammals such as rodents and primates, and particularly human patients.
- the present invention provides methods of treating or preventing anemia in an individual in need thereof by administering to the individual a therapeutically effective amount of a GDF Trap polypeptide. These methods may be used for therapeutic and prophylactic treatments of mammals, and particularly humans.
- a therapeutic that "prevents" a disorder or condition refers to a compound that, in a statistical sample, reduces the occurrence of the disorder or condition in the treated sample relative to an untreated control sample, or delays the onset or reduces the severity of one or more symptoms of the disorder or condition relative to the untreated control sample.
- treating includes prophylaxis of the named condition or amelioration or elimination of the condition once it has been established. In either case, prevention or treatment may be discerned in the diagnosis provided by a physician or other health care provider and the intended result of administration of the therapeutic agent.
- GDF Trap polypeptides may be used to increase red blood cell, hemoglobin or reticulocyte levels in healthy individuals, and such GDF Trap polypeptides may be used in selected patient populations.
- appropriate patient populations include those with undesirably low red blood cell or hemoglobin levels, such as patients having an anemia, and those that are at ⁇ sk for developing undesirably low red blood cell or hemoglobin levels, such as those patients that are about to undergo major surgery or other procedures that may result in substantial blood loss
- a patient with adequate red blood cell levels is treated with a GDF Trap polypeptide to increase red blood cell levels, and then blood is drawn and stored for later use in transfusions
- GDF Trap polypeptides disclosed herein may be used to increase red blood cell levels in patients having an anemia
- a level of less than normal for the appropnate age and gender category may be indicative of anemia, although individual variations aie taken into account
- a hemoglobin level of 12 g/dl is generally considered the lower limit of normal in the general adult population
- Potential causes include blood-loss, nut ⁇ tional deficits, medication reaction, va ⁇ ous problems with the bone marrow and many diseases
- anemia has been associated with a va ⁇ ety of disorders that include, for example, chronic renal failure, myelodysplasia syndrome, rheumatoid arthritis, bone marrow transplantation
- Anemia may also be associated with the following conditions solid tumors (e g breast cancer, lung cancer, colon cancer), tumors of the lymphatic system (e g chronic lymphocyte leukemia, non-Hodgkins and Hodgkins lymphomas), tumors of the hematopoi
- Hypoproliferative anemias include: 1 ) anemia of chronic disease, 2) anemia of kidney disease, and 3) anemia associated with hypometabolic states. In each of these types, endogenous erythropoietin levels are inappropriately low for the degree of anemia observed. Other hypoproliferative anemias include: 4) early-stage iron-deficient anemia, and 5) anemia caused by damage to the bone marrow. In these types, endogenous erythropoietin levels are appropriately elevated for the degree of anemia observed.
- the most common type is anemia of chronic disease, which encompasses inflammation, infection, tissue injury, and conditions such as cancer, and is distinguished by both low erythropoietin levels and an inadequate response to erythropoietin in the bone marrow (Adamson, 2008, Harrison's Principles of Internal Medicine, 17th ed.; McGraw Hill, New York, pp 628-634). Many factors can contribute to cancer-related anemia. Some are associated with the disease process itself and the generation of inflamatory cytokines such as interleukin-1 , interferon-gamma, and tumor necrosis factor (Bron et al., 2001 , Semin Oncol 28(Suppl 8):1 -6).
- inflammation induces the key iron-regulatory peptide hepcidin, thereby inhibiting iron export from macrophages and generally limiting iron availability for erythropoiesis (Ganz, 2007, J Am Soc Nephrol 18:394-400).
- Blood loss through various routes can also contribute to cancer-related anemia.
- the prevalence of anemia due to cancer progression varies with cancer type, ranging from 5% in prostate cancer up to 90% in multiple myeloma. Cancer-related anemia has profound consequences for patients, including fatigue and reduced quality of life, reduced treatment efficacy, and increased mortality.
- Chronic kidney disease is associated with hypoproliferative anemia that varies in severity with the degree of renal impairment. Such anemia is primarily due to inadequate production of erythropoietin and reduced survival of red blood cells.
- Chronic kidney disease usually proceeds gradually over a period of years or decades to end-stage (Stage-5) disease, at which point dialysis or kidney transplantation is required for patient survival. Anemia often develops early in this process and worsens as disease progresses.
- kidney disease The clinical consequences of anemia of kidney disease are well-documented and include development of left ventricular hypertrophy, impaired cognitive function, reduced quality of life, and altered immune function (Levin et al., 1999, Am J Kidney Dis 27:347-354; Nissenson, 1992, Am J Kidney Dis 20(Suppl l):21-24; Revicki et al., 1995, Am J Kidney Dis 25:548-554; Gafter et al., 1994, Kidney Int 45:224-231 ). As demonstrated by the Applicants in a mouse model of chronic kidney disease (see Example below), a GDF Trap polypeptide can be used to treat anemia of kidney disease.
- hypometabolic rate can produce a mild-to-moderate hypoproliferative anemia.
- endocrine deficiency states For example, anemia can occur in Addison's disease, hypothyroidism, hyperparathyroidism, or males who are castrated or treated with estrogen. Mild-to-moderate anemia can also occur with reduced dietary intake of protein, a condition particularly prevalent in the elderly.
- anemia can develop in patients with chronic liver disease arising from nearly any cause (Adamson, 2008, Harrison's Principles of Internal Medicine, 17th ed.; McGraw Hill, New York, pp 628-634).
- Acute blood loss initially causes hypovolemia without anemia since there is proportional depletion of RBCs along with other blood constituents.
- hypovolemia will rapidly trigger physiologic mechanisms that shift fluid from the extravascular to the vascular compartment, which results in hemodilution and anemia. If chronic, blood loss gradually depletes body iron stores and eventually leads to iron deficiency.
- a GDF Trap polypeptide can be used to speed recovery from anemia of acute blood loss.
- Iron-deficiency anemia is the final stage in a graded progression of increasing iron deficiency which includes negative iron balance and iron -deficient erythropoiesis as intermediate stages. Iron deficiency can result from increased iron demand, decreased iron intake, or increased iron loss, as exemplified in conditions such as pregnancy, inadequate diet, intestinal malabsorption, acute or chronic inflammation, and acute or chronic blood loss.
- An GDF Trap polypeptide could be used to treat chronic iron-deficiency anemias alone or in combination with conventional therapeutic approaches, particularly to treat anemias of multifactorial origin.
- Hypoproliferative anemias can result from primary dysfunction or failure of the bone marrow, instead of dysfunction secondary to inflammation, infection, or cancer progression.
- Prominent examples would be myelosuppression caused by cancer chemotherapeutic drugs or cancer radiation therapy.
- a broad review of clinical trials found that mild anemia can occur in 100% of patients after chemotherapy, while more severe anemia can occur in up to 80% of such patients (Groopman et al., 1999, J Natl Cancer Inst 91 : 1616-1634).
- Myelosuppressive drugs include: 1) alkylating agents such as nitrogen mustards (e.g., melphalan) and nitrosoureas (e.g., streptozocin); 2) antimetabolites such as folic acid antagonists (e.g., methotrexate), purine analogs (e.g., thioguanine), and pyrimidine analogs (e.g., gemcitabine); 3) cytotoxic antibotics such as anthracyclines (e.g., doxorubicin); 4) kinase inhibitors (e.g., gefitinib); 5) mitotic inhibitors such as taxanes (e.g., paclitaxel) and vinca alkaloids (e.g., vinorelbine); 6) monoclonal antibodies (e.g., rituximab); and 7) topoisomerase inhibitors (e.g., topotecan and etoposide).
- GDF Trap polypeptide can be used to treat anemia caused by chemotherapeutic agents and/or radiation therapy. GDF Trap polypeptides would also be appropriate for treating anemias of disordered
- RBC maturation which are characterized in part by undersized (microcytic), oversized (macrocytic), misshapen, or abnormally colored (hypochromic) RBCs.
- Patients may be treated with a dosing regimen intended to restore the patient to a target hemoglobin level, usually between about 10 g/dl and about 12.5 g/dl, and typically about 1 1.0 g/dl (see also Jacobs et al. (2000) Nephrol Dial Transplant 15, 15- 19), although lower target levels may cause fewer cardiovascular side effects.
- hematocrit levels percentage of the volume of a blood sample occupied by the cells
- Hematocrit levels for healthy individuals range from 41 to 51 % for adult males and from 35 to 45% for adult females.
- Target hematocrit levels are usually around 30-33%.
- hemoglobin/hematocrit levels vary from person to person.
- the target hemoglobin/hematocrit level can be individualized for each patient.
- the rapid effect on red blood cell levels of the GDF Trap polypeptides disclosed herein indicate that these agents act by a different mechanism than Epo. Accordingly, these antagonists may be useful for increasing red blood cell and hemoglobin levels in patients that do not respond well to Epo.
- a GDF Trap polypeptide may be beneficial for a patient in which administration of a normal to increased (>300 lU/kg/week) dose of Epo does not result in the increase of hemoglobin level up to the target level.
- Epo patients with an inadequate Epo response are found for all types of anemia, but higher numbers of non- responders have been observed particularly frequently in patients with cancers and patients with end-stage renal disease.
- An inadequate response to Epo can be either constitutive (i.e. observed upon the first treatment with Epo) or acquired (e.g. observed upon repeated treatment with Epo).
- the GDF Trap polypeptides may also be used to treat patients that are susceptible to adverse effects of Epo.
- the primary adverse effects of Epo are an excessive increase in the hematocrit or hemoglobin levels and polycythemia. Elevated hematocrit levels can lead to hypertension (more particularly aggravation of hypertension) and vascular thrombosis.
- Other adverse effects of Epo which have been reported, some of which related to hypertension, are headaches, influenza-like syndrome, obstruction of shunts, myocardial infarctions and cerebral convulsions due to thrombosis, hypertensive encephalopathy, and red cell blood cell applasia (Singibarti, (1994) J.
- GDF traps may also be used in combination with Epo and other agents that activate the erythropoietin pathway. In some instances, this may permit lower dosing of each drug in the combination.
- the present invention provides methods for managing a patient that has been treated with, or is a candidate to be treated with, a GDF Trap polypeptide by measuring one or more hematologic parameters in the patient.
- the hematologic parameters may be used to evaluate appropriate dosing for a patient who is a candidate to be treated with a GDF Trap polypeptide, to monitor the hematologic parameters during treatment with a GDF Trap polypeptide, to evaluate whether to adjust the dosage during treatment with a GDF Trap polypeptide, and/or to evaluate an appropriate maintenance dose of a GDF Trap polypeptide. If one or more of the hematologic parameters are outside the normal level, dosing with a GDF Trap polypeptide may be reduced, delayed or terminated.
- Hematologic parameters that may be measured in accordance with the methods provided herein include, for example, red blood cell levels, blood pressure, iron stores, and other agents found in bodily fluids that correlate with increased red blood cell levels, using art recognized methods. Such parameters may be determined using a blood sample from a patient. Increases in red blood cell levels, hemoglobin levels, and/or hematocrit levels may cause increases in blood pressure.
- hematologic parameters are outside the normal range, or on the high side of normal, in a patient who is a candidate to be treated with a GDF Trap polypeptide then onset of administration of the GDF Trap polypeptide may be delayed until the hematologic parameters have returned to a normal or acceptable level either naturally or via therapeutic intervention.
- a candidate patient is hypertensive or prehypertensive, then the patient may be treated with a blood pressure lowering agent in order to reduce the patient's blood pressure.
- Any blood pressure lowering agent appropriate for the individual patient's condition may be used including, for example, diuretics, adrenergic inhibitors (including alpha blockers and beta blockers), vasodilators, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin II receptor blockers.
- Blood pressure may alternatively be treated using a diet and exercise regimen.
- the patient may be treated with an appropriate regimen of diet and/or iron supplements until the patient's iron stores have returned to a normal or acceptable level.
- administration of the GDF Trap polypeptide may be delayed until the levels have returned to a normal or acceptable level.
- the dosage amount or frequency of dosing of the GDF Trap polypeptide may be set at an amount that would reduce the risk of an unacceptable increase in the hematologic parameters arising upon administration of the GDF Trap polypeptide.
- a therapeutic regimen may be developed for the patient that combines a GDF Trap polypeptide with a therapeutic agent that addresses the undesirable level of the hematologic parameter.
- a therapeutic regimen involving administration of a GDF Trap polypeptide and a blood pressure lowering agent may be designed.
- a therapeutic regimen of a GDF Trap polypeptide and iron supplementation may be developed.
- baseline parameter(s) for one or more hematologic parameters may be established for a patient who is a candidate to be treated with a GDF Trap polypeptide and an appropriate dosing regimen establish for that patient based on the baseline value(s).
- established baseline parameters based on a patient's medical history could be used to inform an appropriate GDF Trap polypeptide dosing regimen for a patient. For example, if a healthy patient has an established baseline blood pressure reading that is above the defined normal range it may not be necessary to bring the patient's blood pressure into the range that is considered normal for the general population prior to treatment with the GDF Trap polypeptide.
- a patient's baseline values for one or more hematologic parameters prior to treatment with a GDF Trap polypeptide may also be used as the relevant comparative values for monitoring any changes to the hematologic parameters during treatment with the GDF Trap polypeptide.
- one or more hematologic parameters are measured in patients who are being treated with a GDF Trap polypeptide.
- the hematologic parameters may be used to monitor the patient during treatment and permit adjustment or termination of the dosing with the GDF Trap polypeptide or additional dosing with another therapeutic agent. For example, if administration of a GDF Trap polypeptide results in an increase in blood pressure, red blood cell level, or hemoglobin level, or a reduction in iron stores, then the dose of the GDF Trap polypeptide may be reduced in amount or frequency in order to decrease the effects of the GDF Trap polypeptide on the one or more hematologic parameters.
- the dosing of the GDF Trap polypeptide may be terminated either temporarily, until the hematologic parameter(s) return to an acceptable level, or permanently. Similarly, if one or more hematologic parameters are not brought within an acceptable range after reducing the dose or frequency of administration of the GDF Trap polypeptide then the dosing may be terminated.
- the patient may be dosed with an additional therapeutic agent that addresses the undesirable level in the hematologic parameter(s), such as, for example, a blood pressure lowering agent or an iron supplement.
- an additional therapeutic agent that addresses the undesirable level in the hematologic parameter(s), such as, for example, a blood pressure lowering agent or an iron supplement.
- dosing with the GDF Trap polypeptide may continue at the same level and a blood pressure lowering agent is added to the treatment regimen, dosing with the GDF Trap polypeptide may be reduce (e.g., in amount and/or frequency) and a blood pressure lowering agent is added to the treatment regimen, or dosing with the GDF Trap polypeptide may be terminated and the patient may be treated with a blood pressure lowering agent.
- patients being treated with a GDF Trap polypeptide, or candidate patients to be treated with a GDF Trap polypeptide are patients in need of muscle growth, such as patients suffering from, or at risk of developing, a neuromuscular disorder or musculogenerative disorder.
- patients or candidate patients may be suffering from, or at risk for developing, Lou Gehrig's disease (ALS), cancer anorexia-cachexia syndrome, muscular dystrophy, muscle atrophy, congestive obstructive pulmonary disease (and muscle wasting associated with COPD), muscle wasting syndrome, sarcopenia, or cachexia.
- ALS Lou Gehrig's disease
- cancer anorexia-cachexia syndrome muscular dystrophy
- muscle atrophy muscle atrophy
- congestive obstructive pulmonary disease and muscle wasting associated with COPD
- muscle wasting syndrome sarcopenia, or cachexia.
- Muscular dystrophy refers to a group of degenerative muscle diseases characterized by gradual weakening and deterioration of skeletal muscles and sometimes the heart and respiratory muscles.
- Exemplary muscular dystrophies that can be treated with a regimen including the subject GDF Trap polypeptides include: Duchenne Muscular Dystrophy (DMD), Becker Muscular Dystrophy (BMD), Emery-Dreifuss Muscular
- EDMD Limb-Girdle Muscular Dystrophy
- LGMD Limb-Girdle Muscular Dystrophy
- FSH or FSHD Facioscapulohumeral Muscular Dystrophy
- MMD Myotonic Dystrophy
- OPMD Oculopharyngeal Muscular Dystrophy
- DD Congenital Muscular Dystrophy
- CMD Congenital Muscular Dystrophy
- compounds (e.g., GDF Trap polypeptides) of the present invention are formulated with a pharmaceutically acceptable carrier.
- a GDF Trap polypeptide can be administered alone or as a component of a pharmaceutical formulation (therapeutic composition).
- the subject compounds may be formulated for administration in any convenient way for use in human or veterinary medicine.
- the therapeutic method of the invention includes administering the composition systemically, or locally as an implant or device
- the therapeutic composition for use in this invention is, of course, in a pyrogen- free, physiologically acceptable form
- Therapeutically useful agents other than the GDF Trap polypeptides which may also optionally be included in the composition as described above, may be administered simultaneously or sequentially with the subject compounds (e g , GDF Trap polypeptides) in the methods of the invention
- compositions suitable for parenteral administration may compnse one or more GDF Trap polypeptides in combination with one or more pharmaceutically acceptable ste ⁇ le isotonic aqueous or nonaqueous solutions, dispersions, suspensions or emulsions, or sterile powders which may be reconstituted into ste ⁇ le injectable solutions or dispersions just prior to use, which may contain antioxidants, buffers, bacte ⁇ ostats, solutes which render the formulation isotonic with the blood of the intended recipient or suspending or thickening agents
- suitable aqueous and nonaqueous earners which may be employed in the pharmaceutical compositions of the invention include water, ethanol, polyols (such as glycerol, propylene glycol, polyethylene glycol, and the like), and suitable mixtures thereof, vegetable oils, such as olive oil, and injectable organic esters, such as ethyl oleate Proper fluidity can be maintained, for example, by the use
- compositions of the present invention may include a mat ⁇ x capable of delivering one or more therapeutic compounds (e g , GDF Trap polypeptides) to a target tissue site (e g , bone marrow), providing a structure for the developing tissue and optimally capable of being resorbed into the body
- the matrix may provide slow release of the GDF Trap polypeptides
- Such matrices may be formed of materials presently in use for other implanted medical applications
- compositions may be biodegradable and chemically defined calcium sulfate, tricalciumphosphate, hydroxyapatite, polylactic acid and polyanhydrides.
- potential materials are biodegradable and biologically well defined, such as bone or dermal collagen.
- Further matrices are comprised of pure proteins or extracellular matrix components.
- Other potential matrices are non-biodegradable and chemically defined, such as sintered hydroxyapatite, bioglass, aluminates, or other ceramics.
- Matrices may be comprised of combinations of any of the above mentioned types of material, such as polylactic acid and hydroxyapatite or collagen and tricalciumphosphate.
- the bioceramics may be altered in composition, such as in calcium-aluminate-phosphate and processing to alter pore size, particle size, particle shape, and biodegradability.
- methods of the invention can be administered for orally, e.g., in the form of capsules, cachets, pills, tablets, lozenges (using a flavored basis, usually sucrose and acacia or tragacanth), powders, granules, or as a solution or a suspension in an aqueous or non-aqueous liquid, or as an oil-in-water or water-in-oil liquid emulsion, or as an elixir or syrup, or as pastilles (using an inert base, such as gelatin and glycerin, or sucrose and acacia) and/or as mouth washes and the like, each containing a predetermined amount of an agent as an active ingredient.
- An agent may also be administered as a bolus, electuary or paste.
- one or more therapeutic compounds of the present invention may be mixed with one or more pharmaceutically acceptable carriers, such as sodium citrate or dicalcium phosphate, and/or any of the following: (1) fillers or extenders, such as starches, lactose, sucrose, glucose, mannitol, and/or silicic acid; (2) binders, such as, for example, carboxymethylcellulose, alginates, gelatin, polyvinyl pyrrolidone, sucrose, and/or acacia; (3) humectants, such as glycerol; (4) disintegrating agents, such as agar-agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and sodium carbonate; (5) solution retarding agents, such as paraffin; (6) absorption accelerators, such as quaternary ammonium compounds; (7) wetting agents, such as
- compositions may also comprise buffering agents.
- Solid compositions of a similar type may also be employed as fillers in soft and hard-filled gelatin capsules using such excipients as lactose or milk sugars, as well as high molecular weight polyethylene glycols and the like.
- Liquid dosage forms for oral administration include pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions, syrups, and elixirs.
- the liquid dosage forms may contain inert diluents commonly used in the art, such as water or other solvents, solubilizing agents and emulsifiers, such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1 ,3-butylene glycol, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and sesame oils), glycerol, tetrahydrofuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof.
- the oral compositions can also include adjuvants such as wetting agents, emulsifying and suspending
- Suspensions in addition to the active compounds, may contain suspending agents such as ethoxylated isostearyl alcohols, polyoxyethylene sorbitol, and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar and tragacanth, and mixtures thereof.
- suspending agents such as ethoxylated isostearyl alcohols, polyoxyethylene sorbitol, and sorbitan esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar and tragacanth, and mixtures thereof.
- compositions of the invention may also contain adjuvants, such as preservatives, wetting agents, emulsifying agents and dispersing agents. Prevention of the action of microorganisms may be ensured by the inclusion of various antibacterial and antifungal agents, for example, paraben, chlorobutanol, phenol sorbic acid, and the like. It may also be desirable to include isotonic agents, such as sugars, sodium chloride, and the like into the compositions. In addition, prolonged absorption of the injectable pharmaceutical form may be brought about by the inclusion of agents which delay absorption, such as aluminum monostearate and gelatin.
- the dosage regimen will be determined by the attending physician considering various factors which modify the action of the subject compounds of the invention (e.g., GDF Trap polypeptides).
- the various factors include, but are not limited to, the patient's red blood cell count, hemoglobin level or other diagnostic assessments, the desired target red blood cell count, the patient's age, sex, and diet, the severity of any disease that may be contributing to a depressed red blood cell level, time of administration, and other clinical factors.
- the addition of other known growth factors to the final composition may also affect the dosage. Progress can be monitored by periodic assessment of red blood cell and hemoglobin levels, as well as assessments of reticulocyte levels and other indicators of the hematopoietic process.
- the present invention also provides gene therapy for the in vivo production of GDF Trap polypeptides.
- Such therapy would achieve its therapeutic effect by introduction of the GDF Trap polynucleotide sequences into cells or tissues having the disorders as listed above.
- Delivery of GDF Trap polynucleotide sequences can be achieved using a recombinant expression vector such as a chimeric virus or a colloidal dispersion system.
- Preferred for therapeutic delivery of GDF Trap polynucleotide sequences is the use of targeted liposomes.
- Various viral vectors which can be utilized for gene therapy as taught herein include adenovirus, herpes virus, vaccinia, or an RNA virus such as a retrovirus.
- the retroviral vector may be a derivative of a murine or avian retrovirus.
- retroviral vectors in which a single foreign gene can be inserted include, but are not limited to: Moloney murine leukemia virus (MoMuLV), Harvey murine sarcoma virus (HaMuSV), murine mammary tumor virus (MuMTV), and Rous Sarcoma Virus (RSV).
- MoMuLV Moloney murine leukemia virus
- HaMuSV Harvey murine sarcoma virus
- MuMTV murine mammary tumor virus
- RSV Rous Sarcoma Virus
- retroviral vectors can incorporate multiple genes. All of these vectors can transfer or incorporate a gene for a selectable marker so that transduced cells can be identified and generated.
- Retroviral vectors can be made target-specific by attaching, for example, a sugar, a glycolipid, or a protein. Preferred targeting is accomplished by using an antibody.
- tissue culture cells can be directly transfected with plasmids encoding the retroviral structural genes gag, pol and env, by conventional calcium phosphate transfection. These cells are then transfected with the vector plasmid containing the genes of interest. The resulting cells release the retroviral vector into the culture medium.
- colloidal dispersion systems include macromolecule complexes, nanocapsules, microspheres, beads, and lipid-based systems including oil-in-water emulsions, micelles, mixed micelles, and liposomes.
- the preferred colloidal system of this invention is a liposome.
- Liposomes are artificial membrane vesicles which are useful as delivery vehicles in vitro and in vivo. RNA, DNA and intact virions can be encapsulated within the aqueous interior and be delivered to cells in a biologically active form (see e.g., Fraley, et al., Trends Biochem. ScL, 6:77, 1981 ).
- compositions of the liposome is usually a combination of phospholipids, usually in combination with steroids, especially cholesterol. Other phospholipids or other lipids may also be used.
- the physical characteristics of liposomes depend on pH, ionic strength, and the presence of divalent cations.
- lipids useful in liposome production include phosphatidyl compounds, such as phosphatidylglycerol, phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, sphingolipids, cerebrosides, and gangliosides.
- Illustrative phospholipids include egg phosphatidylcholine, dipalmitoylphosphatidylcholine, and distearoylphosphatidylcholine.
- the targeting of liposomes is also possible based on, for example, organ-specificity, cell-specificity, and organelle-specificity and is known in the art.
- Example 1 Generation of a GDF Trap.
- a polypeptide having a modified extracellular domain of ActRIIB with greatly reduced activin A binding relative to GDFl 1 and/or myostatin (as a consequence of a leucine-to-aspartate substitution at position 79 in SEQ ID NO: 1 ) was fused to a human or mouse Fc domain with a minimal linker (three glycine amino acids) in between.
- the constructs are referred to as ActRIIB(L79D 20-134)- hFc and ActRIIB(L79D 20- 134)-mFc, respectively.
- Alternative forms with a glutamate rather than an aspartate at position 79 performed similarly (L79E).
- the GDF Trap ActRIIB(L79D 20-134)-hFc is shown below as purified from CHO cell lines (SEQ ID NO: 7).
- GRGEAETRECIYYNANWELERTNQSGLERCEGEQDKRLHCYASWRNSSGTIELVKK GCWDDDFNCYDRQECVATEENPQVYFCCCEGNFCNERFTHLPEAGGPEVTYEPPPT APTGGGTHTCPPCP APELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVK FNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHODWLNGKEYKCKVSNKAL PIPIEKTISKAKGOPREPQVYTLPPSREEMTKNOVSLTCLVKGFYPSDIAVEWESNGO PENNYKTTPPVLDSDGSFFLYSKLTVDKSRWOOGNVFSCSVMHEALHNHYTOKSLS
- the ActRIIB-derived portion of the GDF Trap has an amino acid sequence set forth below (SEQ ID NO: 32), and that portion could be used as a monomer or as a non-Fc fusion protein as a monomer, dimer or greater order complex.
- SEQ ID NO: 32 amino acid sequence set forth below
- the GDF Trap protein was expressed in CHO cell lines. Three different leader sequences were considered: (i) Honey bee melittin (HBML): MKFLVNVALVFMVVYISYIYA (SEQ ID NO: 8)
- Tissue Plasminogen Activator TPA: MDAMKRGLCCVLLLCGAVFVSP (SEQ ID NO: 9)
- the selected form employs the TPA leader and has the following unprocessed amino acid sequence:
- This polypeptide is encoded by the following nucleic acid sequence (SEQ ID NO: 12):
- Purification could be achieved by a series of column chromatography steps, including, for example, three or more of the following, in any order: protein A chromatography, Q sepharose chromatography, phenylsepharose chromatography, size exclusion chromatography, and cation exchange chromatography.
- the purification could be completed with viral filtration and buffer exchange.
- the cell culture medium is passed over a protein A column, washed in 150 mM Tris/NaCl (pH 8.0), then washed in 50 mM Tris/NaCl (pH 8.0) and eluted with 0.1 M glycine, pH 3.0.
- the low pH eluate is kept at room temperature for 30 minutes as a viral clearance step.
- the eluate is then neutralized and passed over a Q sepharose ion exchange column and washed in 50 mM Tris pH 8.0, 50 mM NaCl, and eluted in 50 mM Tris pH 8.0, with an NaCl concentration between 150 mM and 300 mM.
- the eluate is then changed into 50 mM Tris pH 8.0, 1.1 M ammonium sulfate and passed over a phenyl sepharose column, washed, and eluted in 50 mM Tris pH 8.0 with ammonium sulfate between 150 and 300 mM.
- the eluate is dialyzed and filtered for use.
- Day 1 Split A-204 cells into 48-well plate.
- Activin A showed 10 fold stimulation of reporter gene expression and an ED50 ⁇ 2 ng/ml.
- GDF-1 1 16 fold stimulation, ED50: ⁇ 1 5 ng/ml.
- ActRIIB(20-134) is a potent inhibitor of activin, GDF-8 and GDF-1 1 activity in this assay Vanants were tested in this assay as well
- Vanants of ActRIIB(20-134)-hFc with truncations at the N-terminus and/or C- terminus were generated and tested foi activity as inhibitors of GDF-1 1 and activin. The activities are shown below (as measured in conditioned media)'
- truncations of three (ending with ...PPT), six (ending with ...YEP) or more amino acids at the C-terminus causes a threefold or greater decrease in the activity of the molecule.
- the truncation of the final 15 amino acids of the ActRIIB portion causes a greater loss of activity (see WO2006/012627).
- Amino terminal truncations were made in the background of an ActRlIB(20- 131 )-hFc protein. The activities are shown below (as measured in conditioned media):
- ActRIIB(20-134)-Fc has a serum half-life of approximately 70 hours.
- ActRIIB(A24N 20-134)-Fc has a serum half-life of approximately 100-150 hours.
- the A24N variant has activity in the cell-based assay (above) and in vivo assays (below) that are equivalent to the wild-type molecule. Coupled with the longer half-life, this means that over time an A24N variant will give greater effect per unit of protein than the wild-type molecule.
- the A24N variant, and any of the other variants tested above, may be combined with the GDF Trap molecules, such as the L79D or L79E variants.
- the ActRIIB-Fc variants or wild-type protein were captured onto the system using an anti-hFc antibody. Ligands were injected and flowed over the captured receptor proteins. Results are summarized in the tables, below.
- ActRIIB(20-134)-hFc (IgGl) was administered once a week for 1 month to male and female cynomolgus monkeys by subcutaneous injection. Forty-eight cynomolgus monkeys (24/sex) were assigned to one of four treatment groups (6 animals/sex/group) and were administered subcutaneous injections of either vehicle or ActRIIB-hFc at 3, 10, or 30 mg/kg once weekly for 4 weeks (total of 5 doses). Parameters evaluated included general clinical pathology (hematology, clinical chemistry, coagulation, and urinalysis). ActRIIB-hFc caused statistically significant elevated mean absolute reticulocyte values by day 15 in treated animals.
- ActRIIB-hFc caused several hematological changes, including elevated mean absolute reticulocyte and red blood cell distribution width values and lower mean corpuscular hemoglobin concentration. All treated groups and both sexes were affected. These effects are consistent with a positive effect of ActRIIB-hFc on the release of immature reticulocytes from the bone marrow. This effect was reversed after drug was washed out of the treated animals (by study day 56). Accordingly, we conclude that ActRIIB-hFc stimulates erythropoiesis.
- Example 7 ActRIlB-mFc Promotes Aspects of Erythropoiesis in Mice by Stimulation of Splenic Erythropoietic Activities
- ActRIlB(20-134)-mFc the effects of the in vivo administration of ActRIlB(20-134)-mFc on the frequency of hematopoietic progenitors in bone marrow and spleen was analyzed.
- One group of C57BL/6 mice was injected with PBS as a control and a second group of mice administered two doses of ActRIIB-mFc at 10 mg/kg and both groups sacrificed after 8 days.
- Peripheral blood was used to perform complete blood counts and femurs and spleens were used to perform in vitro clonogenic assays to assess the lymphoid, erythroid and myeloid progenitor cell content in each organ.
- no significant changes were seen in red blood cell, hemoglobin or white blood cell levels in treated mice.
- the femurs there was no difference in the nucleated cell numbers or progenitor content between the control and treated groups.
- the compound treated group experienced a statistically significant increase in the mature erythroid progenitor (CFU-E) colony number per dish, frequency and total progenitor number per spleen.
- CFU-GM mature erythroid progenitor
- BFU-E immature erythroid
- total progenitor number per spleen was seen in the number of myeloid (CFU-GM), immature erythroid (BFU-E) and total progenitor number per spleen.
- mice Sixteen C57BL/6 female mice 6-8 weeks of age were used in the study. Eight mice were injected subcutaneously with test compound ActRIIB-mFc at days 1 and 3 at a dose of 10 mg/kg and eight mice were injected subcutaneously with vehicle control, phosphate buffered saline (PBS), at a volume of 100 ⁇ L per mouse. All mice were sacrificed 8 days after first injection in accordance with the relevant Animal Care Guidelines. Peripheral blood (PB) samples from individual animals were collected by cardiac puncture and used for complete blood counts and differential (CBC/Diff). Femurs and spleens were harvested from each mouse.
- PB peripheral blood
- CBC/Diff Counts PB from each mouse was collected via cardiac puncture and placed into the appropriate microtainer tubes. Samples were sent to CLV for analysis on a CellDyn 3500 counter.
- Clonogenic progenitors of the myeloid, erythroid and lymphoid lineages were assessed using the in vitro methylcellulose-based media systems described below.
- Clonogenic progenitors of the mature erythroid (CFU-E) lineages were cultured in MethoCultTM 3334, a methylcellulose-based medium containing recombinant human (rh) Erythropoietin (3 U/mL). Lymphoid Progenitors:
- Clonogenic progenitors of the lymphoid (CFU-pre-B) lineage were cultured in MethoCult® 3630, a methylcellulose-based medium containing rh Interleukin 7 ( 10 ng/mL).
- Myeloid and Immature Erythroid Progenitors Clonogenic progenitors of the granulocyte-monocyte (CFU-GM), erythroid (BFU-E) and multipotential (CFU-GEMM) lineages were cultured in MethoCultTM 3434, a methylcellulose-based medium containing recombinant murine (rm) Stem Cell Factor (50 ng/mL), rh Interleukin 6 (10 ng/mL), rm Interleukin 3 (10 ng/mL) and rh Erythropoietin (3 LVmL).
- MethoCultTM 3434 a methylcellulose-based medium containing recombinant murine (rm) Stem Cell Factor (50 ng/mL), rh Interleukin 6 (10 ng/mL), rm Interleukin 3 (10 ng/mL) and rh Erythropoietin (3 LVmL).
- Mouse femurs and spleens were processed by standard protocols. Briefly, bone marrow was obtained by flushing the femoral cavity with Iscove's Modified Dulbecco's Media containing 2% fetal bovine serum (IMDM 2% FBS) using a 21 gauge needle and 1 cc syringe. Spleen cells were obtained by crushing spleens through a 70 ⁇ M filter and rinsing the filter with IMDM 2% FBS. Nucleated cell counts in 3% glacial acetic acid were then performed on the single cells suspensions using a Neubauer counting chamber so that the total cells per organ could be calculated.
- IMDM 2% FBS Iscove's Modified Dulbecco's Media containing 2% fetal bovine serum
- Spleen cells were obtained by crushing spleens through a 70 ⁇ M filter and rinsing the filter with IMDM 2% FBS. Nucleated cell counts in 3% glacial acetic
- total spleen cells were then diluted with 3 times the volume of ammonium chloride lysis buffer and incubated on ice 10 minutes. The cells were then washed and resuspended in IMDM 2% FBS and a second cell count were performed to determine the cell concentration of cells after lysis.
- Bone marrow cells were plated at 1 x10 5 cells per dish in MethoCultTM 3334 to assess mature erythroid progenitors, 2x10 5 cells per dish in MethoCultTM 3630 to assess lymphoid progenitors and 3x10 4 cells per dish in MethoCultTM 3434 to assess immature erythroid and myeloid progenitors.
- Spleen cells were plated at 4x 10 5 cells per dish in MethoCultTM 3334 to assess mature erythroid progenitors, 4x 10 ' cells per dish in MethoCultTM 3630 to assess lymphoid progenitors and 2x 10 5 cells per dish in MethoCultTM 3434 to assess immature erythroid and myeloid progenitors. Cultures plated in triplicate dishes were incubated at 4x 10 5 cells per dish in MethoCultTM 3334 to assess mature erythroid progenitors, 4x 10 ' cells per dish in MethoCultTM 3630 to assess lymphoid progenitors and 2x 10 5 cells per dish in MethoCultTM 3434 to assess immature erythroid and myeloid progenitors. Cultures plated in triplicate dishes were incubated at
- Mature erythroid progenitors were cultured for 2 days, lymphoid progenitors were cultured for 7 days and mature erythroid and myeloid progenitors were cultured for 12 days.
- the mean +/- 1 standard deviation was calculated for the triplicate cultures of the clonogenic assays and for the control and treatment groups for all data sets.
- CFC colony forming cells
- mice with ActRIIB(20-134)-mFc did not result in significant increases in red blood cell or hemoglobin content.
- the effect on progenitor cell content was notable.
- the compound treated group experienced a statistically significant increase in the nucleated cell number before red blood cell lysis and in the mature erythroid progenitor (CFU-E) colony number per dish, frequency and total progenitor number per spleen.
- CFU-GM myeloid
- BFU-E immature erythroid
- total progenitor number per spleen an increase was seen in the number of myeloid (CFU-GM), immature erythroid (BFU-E) and total progenitor number per spleen. Accordingly, it is expected that over a longer time course, ActRIIB(20-134)-mFc treatment may result in elevated red blood cell and hemoglobin content.
- Example 8 A GDF Trap Increases Red Blood Cell Levels in vivo
- GDF Trap ActRIIB polypeptide
- SC subcutaneous injection
- whole blood was collected by cardiac puncture into EDTA containing tubes and analyzed for cell distribution using an HM2 hematology analyzer (Abaxis, Inc).
- Treatment with the GDF Trap did not have a statistically significant effect on the number of white blood cells (WBC) compared to the vehicle controls.
- Red blood cell (RBC) numbers were increased in the treated group relative to the controls (see table below). Both the hemoglobin content (HGB) and hematocrit (HCT) were also increased due to the additional red blood cells.
- the average width of the red blood cells (RDWc) was higher in the treated animals, indicating an increase in the pool of immature red blood cells. Therefore, treatment with the GDF Trap leads to increases in red blood cells, with no distinguishable effects on white blood cell populations.
- Example 9 A GDF Trap is Superior to ActRIIB-Fc for Increasing Red Blood Cell Levels In vivo.
- mice Nineteen-week-old male C57BL/6NTac mice were randomly assigned to one of three groups. Mice were dosed with vehicle (10 mM Tris Buffered Saline, TBS), wild-type ActRIIB(20-134)-mFc, or GDF trap ActRIIB(L79D 20-134)-hFc by subcutaneous injection twice per week for three weeks. Blood was collected cheek bleed at baseline and after three weeks of dosing and analyzed for cell distribution using a hematology analyzer (HM2, Abaxis, Inc.)
- HM2 hematology analyzer
- a GDF Trap referred to as ActRIIB(L79D 20-134)-hFc was generated by N-terminal fusion of TPA leader to the ActRIIB extracellular domain (residues 20-134 in SEQ ID NO: 1 ) containing a leucine-to-aspartate substitution (at residue 79 in SEQ ID NO: 1) and C-terminal fusion of human Fc domain with minimal linker (three glycine residues) (Figure 3).
- a nucleotide sequence corresponding to this fusion protein is shown in Figure 4.
- a GDF Trap with truncated ActRIIB extracellular domain was generated by N-terminal fusion of TPA leader to truncated extracellular domain (residues 25-131 in SEQ ID NO: 1) containing a leucine-to-aspartate substitution (at residue 79 in SEQ ID NO: 1 ) and C-terminal fusion of human Fc domain with minimal linker (three glycine residues) (Figure 5).
- a nucleotide sequence corresponding to this fusion protein is shown in Figure 6.
- Example 12 Generation of ActRIIB(L79D 25-131)-hFc with Alternative Nucleotide Sequences
- the human ActRIIB extracellular domain with an aspartate substitution at native position 79 (SEQ ID NO: 1) and with N-terminal and C-terminal truncations (residues 25-131 in SEQ ID NO: 1 ) was fused N-terminally with a TPA leader sequence instead of the native ActRIIB leader and C-terminally with a human Fc domain via a minimal linker (three glycine residues) (Figure 5).
- ActRIIB(L79D 25-13 I )-IiFc was evaluated to determine its effect on proliferation of erythroid progenitors.
- CFU-E colony-forming unit- erythroid
- BFU-E burst forming unit-erythroid
- mice included recombinant murine stem cell factor, interleukin-3, and interleukin-6, which were not present in methylcellulose medium for CFU-E determination (MethoCult M3334, Stem Cell Technologies), while both media contained erythropoietin, among other constituents.
- MethodhoCult M3334, Stem Cell Technologies included recombinant murine stem cell factor, interleukin-3, and interleukin-6, which were not present in methylcellulose medium for CFU-E determination (MethoCult M3334, Stem Cell Technologies), while both media contained erythropoietin, among other constituents.
- BFU-E and CFU-E the number of colonies were determined in duplicate culture plates derived from each tissue sample, and statistical analysis of the results was based on the number of mice per treatment group.
- Male Sprague-Dawley rats (approximately 300 g) received a chronic jugular catheter at the vendor (Harlan).
- On Day -1 20% of total blood volume was withdrawn from each rat over a 5-minute period via the catheter under isoflurane anesthesia.
- the volume of blood removed was based on a value for total blood volume calculated according to the following relationship derived by Lee and co-workers (J Nucl Med 25:72-76, 1985) for rats with body weight greater than 120 g:
- ActRIIB5 soluble form of ActRIIB
- exon 4 including the ActRIIB transmembrane domain
- WO2007/053775 The sequence of native human ActRIIB5 without its leader is as follows:
- This variant may be connected to human Fc with a TGGG linker to generate a human ActRlIB5(L79D)-hFc fusion protein with the following sequence:
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Wood Science & Technology (AREA)
- Gastroenterology & Hepatology (AREA)
- Immunology (AREA)
- Biophysics (AREA)
- General Engineering & Computer Science (AREA)
- Cell Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Toxicology (AREA)
- Epidemiology (AREA)
- Biomedical Technology (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Neurology (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Endocrinology (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Physical Education & Sports Medicine (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Urology & Nephrology (AREA)
Abstract
Description
Claims
Priority Applications (77)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES09806981T ES2738543T3 (en) | 2008-08-14 | 2009-08-13 | GDF traps for use in the treatment of anemia |
| EP18198713.2A EP3494986B1 (en) | 2008-08-14 | 2009-08-13 | Gdf traps |
| CA2733911A CA2733911C (en) | 2008-08-14 | 2009-08-13 | Use of gdf traps comprising variant actriib polypeptides to increase red blood cell levels |
| DK09806981.8T DK2340031T3 (en) | 2008-08-14 | 2009-08-13 | GDF CUSTOMER FOR USE OF ANALYSIS TREATMENT |
| SI200931986T SI2340031T1 (en) | 2008-08-14 | 2009-08-13 | Gdf traps for use to treat anemia |
| LTEP09806981.8T LT2340031T (en) | 2008-08-14 | 2009-08-13 | Gdf traps for use to treat anemia |
| PL18198713T PL3494986T3 (en) | 2008-08-14 | 2009-08-13 | Gdf traps |
| JP2011523000A JP5922928B2 (en) | 2008-08-14 | 2009-08-13 | Use of GDF traps to increase red blood cell levels |
| AU2009282441A AU2009282441B2 (en) | 2008-08-14 | 2009-08-13 | Use of GDF traps to increase red blood cell levels |
| PL09806981T PL2340031T4 (en) | 2008-08-14 | 2009-08-13 | Gdf traps for use to treat anemia |
| EP09806981.8A EP2340031B1 (en) | 2008-08-14 | 2009-08-13 | Gdf traps for use to treat anemia |
| EP20173397.9A EP3750552B1 (en) | 2008-08-14 | 2009-08-13 | Gdf traps |
| KR1020197027722A KR102170682B1 (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| CN202110879546.9A CN113577291A (en) | 2009-08-13 | 2010-08-13 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| BR122020019169-4A BR122020019169B1 (en) | 2009-08-13 | 2010-08-13 | POLYPEPTIDE |
| PCT/US2010/045509 WO2011020045A1 (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| AU2010282361A AU2010282361B2 (en) | 2009-08-13 | 2010-08-13 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| EP20197940.8A EP3838919A1 (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| NZ712943A NZ712943A (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| CN202110421547.9A CN113171442A (en) | 2009-08-13 | 2010-08-13 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| CA2770822A CA2770822C (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| KR1020207030283A KR20200124322A (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| NZ598348A NZ598348A (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| ES16186033T ES2844123T3 (en) | 2009-08-13 | 2010-08-13 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| KR1020127004678A KR101882521B1 (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| CN201510979490.9A CN105535938B (en) | 2009-08-13 | 2010-08-13 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| ES10808838T ES2796121T3 (en) | 2009-08-13 | 2010-08-13 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| CN201080045618.XA CN102655872B (en) | 2009-08-13 | 2010-08-13 | GDF catches and erythropoietin receptor activator use in conjunction are to increase hematocrit level |
| EP16186033.3A EP3117829B1 (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| CN201510979350.1A CN105561295B (en) | 2009-08-13 | 2010-08-13 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| RU2016123808A RU2642302C1 (en) | 2009-08-13 | 2010-08-13 | Combined administration of gdf traps and erythropoetin receptor activators for increasing content of erythrocytes |
| BR112012003232-1A BR112012003232B1 (en) | 2009-08-13 | 2010-08-13 | Using a polypeptide in conjunction with an erythropoietin receptor activator to increase red blood cell levels or treat anemia |
| JP2012524910A JP5909446B2 (en) | 2009-08-13 | 2010-08-13 | Combined use of GDF trap and erythropoietin receptor activator to increase red blood cell levels |
| CN201510979291.8A CN105412908B (en) | 2009-08-13 | 2010-08-13 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| RU2012109393/10A RU2592670C2 (en) | 2009-08-13 | 2010-08-13 | Combined application of traps of gdf and erythropoietin receptor activators for increasing erythrocyte content |
| CN202110417494.3A CN113082194A (en) | 2009-08-13 | 2010-08-13 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| KR1020187020803A KR20180085825A (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| NZ623113A NZ623113A (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| US12/856,420 US8216997B2 (en) | 2008-08-14 | 2010-08-13 | Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators |
| MX2012001916A MX2012001916A (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels. |
| KR1020227045338A KR102606494B1 (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| EP10808838.6A EP2464369B1 (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| IL287990A IL287990B (en) | 2009-08-13 | 2010-08-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| IL218052A IL218052A (en) | 2009-08-13 | 2012-02-12 | Use of erythropoietin receptor activator and a polypeptide in the preparation of medicaments for combined administration for increasing red blood cell levels or treating or preventing anemia |
| MX2019010341A MX2019010341A (en) | 2009-08-13 | 2012-02-13 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels. |
| US13/542,269 US8703927B2 (en) | 2008-08-14 | 2012-07-05 | Isolated nucleotide sequences encoding GDF traps |
| US14/201,192 US9439945B2 (en) | 2008-08-14 | 2014-03-07 | Isolated nucleotide sequences encoding GDF traps |
| AU2015202035A AU2015202035B2 (en) | 2008-08-14 | 2015-04-22 | Use of GDF traps to increase red blood cell levels |
| JP2015236828A JP2016034981A (en) | 2009-08-13 | 2015-12-03 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| US15/221,341 US9932379B2 (en) | 2008-08-14 | 2016-07-27 | Isolated nucleotide sequences encoding GDF traps |
| HK16110720.8A HK1223818A1 (en) | 2009-08-13 | 2016-09-09 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels gdf |
| HK16112523.3A HK1224199A1 (en) | 2009-08-13 | 2016-11-01 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels gdf |
| IL252046A IL252046B (en) | 2009-08-13 | 2017-05-01 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| IL25204417A IL252044B (en) | 2009-08-13 | 2017-05-01 | Actriib variant polypeptides |
| IL252045A IL252045B (en) | 2009-08-13 | 2017-05-01 | An isolated nucleic acid, an expression vector and a cultured cell comprising the isolated nucleic acid |
| AU2017204230A AU2017204230B2 (en) | 2008-08-14 | 2017-06-22 | Use of GDF traps to increase red blood cell levels |
| US15/790,400 US10131700B2 (en) | 2008-08-14 | 2017-10-23 | Methods for treating refractory anemia with ringed sideroblasts |
| RU2017145301A RU2732229C3 (en) | 2017-12-22 | COMBINED USE OF GDF TRAPS AND ERYTHROPOIETIN RECEPTOR ACTIVATORS TO INCREASE RED CELL CONTENT | |
| JP2018153528A JP6860533B2 (en) | 2009-08-13 | 2018-08-17 | Use of GDF trap in combination with erythropoietin receptor activator to increase red blood cell levels |
| US16/157,261 US10689427B2 (en) | 2008-08-14 | 2018-10-11 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| HRP20191109TT HRP20191109T1 (en) | 2008-08-14 | 2019-06-18 | Gdf traps for use to treat anemia |
| CY20191100755T CY1121971T1 (en) | 2008-08-14 | 2019-07-16 | GDF TRAPS FOR USE IN THE THERAPEUTIC TREATMENT OF ANEMIA |
| AU2019222789A AU2019222789B2 (en) | 2008-08-14 | 2019-08-26 | Use of GDF traps to increase red blood cell levels |
| US16/795,015 US10889626B2 (en) | 2008-08-14 | 2020-02-19 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| US16/795,026 US10829533B2 (en) | 2008-08-14 | 2020-02-19 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| US16/795,021 US10829532B2 (en) | 2008-08-14 | 2020-02-19 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| IL275851A IL275851B (en) | 2009-08-13 | 2020-07-05 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| CY20201100705T CY1123217T1 (en) | 2008-08-14 | 2020-07-29 | GDF TRAPS |
| RU2020127574A RU2814047C2 (en) | 2009-08-13 | 2020-08-19 | Combined use of gdf traps and erythropoietin receptor activators to increase erythrocyte count |
| US17/032,706 US20210253658A1 (en) | 2008-08-14 | 2020-09-25 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| JP2020163806A JP2020203953A (en) | 2009-08-13 | 2020-09-29 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| CY2020043C CY2020043I1 (en) | 2008-08-14 | 2020-12-17 | GDF TRAPS FOR USE IN THE THERAPEUTIC TREATMENT OF ANEMIA |
| LTPA2020006C LTC2340031I2 (en) | 2008-08-14 | 2020-12-17 | GDF TRAPS FOR USE IN THE TREATMENT OF ANEMIA |
| NO2020045C NO2020045I1 (en) | 2008-08-14 | 2020-12-17 | Luspatercept |
| FR20C1066C FR20C1066I2 (en) | 2008-08-14 | 2020-12-19 | GDF TRAPS FOR USE IN THE TREATMENT OF ANEMIA |
| NL301082C NL301082I2 (en) | 2008-08-14 | 2020-12-21 | loop patercept |
| AU2022200078A AU2022200078A1 (en) | 2008-08-14 | 2022-01-07 | Use of GDF traps to increase red blood cell levels |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US18909408P | 2008-08-14 | 2008-08-14 | |
| US61/189,094 | 2008-08-14 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/583,177 Continuation-In-Part US8058229B2 (en) | 2008-08-14 | 2009-08-13 | Method of increasing red blood cell levels or treating anemia in a patient |
Related Child Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/583,177 Continuation-In-Part US8058229B2 (en) | 2008-08-14 | 2009-08-13 | Method of increasing red blood cell levels or treating anemia in a patient |
| US12/856,420 Continuation-In-Part US8216997B2 (en) | 2008-08-14 | 2010-08-13 | Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators |
| US12/856,420 Continuation US8216997B2 (en) | 2008-08-14 | 2010-08-13 | Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010019261A1 true WO2010019261A1 (en) | 2010-02-18 |
Family
ID=41669153
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2009/004659 WO2010019261A1 (en) | 2008-08-14 | 2009-08-13 | Use of gdf traps to increase red blood cell levels |
Country Status (21)
| Country | Link |
|---|---|
| US (10) | US8058229B2 (en) |
| EP (3) | EP2340031B1 (en) |
| JP (6) | JP5922928B2 (en) |
| AU (1) | AU2009282441B2 (en) |
| CA (1) | CA2733911C (en) |
| CY (3) | CY1121971T1 (en) |
| DK (3) | DK2340031T3 (en) |
| ES (3) | ES2949049T3 (en) |
| FI (1) | FI3750552T3 (en) |
| FR (1) | FR20C1066I2 (en) |
| HR (3) | HRP20230761T1 (en) |
| HU (4) | HUE045456T2 (en) |
| LT (4) | LT3494986T (en) |
| NL (1) | NL301082I2 (en) |
| NO (1) | NO2020045I1 (en) |
| PL (3) | PL3494986T3 (en) |
| PT (3) | PT3494986T (en) |
| SI (3) | SI2340031T1 (en) |
| TR (1) | TR201910890T4 (en) |
| TW (7) | TWI748373B (en) |
| WO (1) | WO2010019261A1 (en) |
Cited By (48)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011020045A1 (en) * | 2009-08-13 | 2011-02-17 | Acceleron Pharma Inc. | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| US8128933B2 (en) | 2005-11-23 | 2012-03-06 | Acceleron Pharma, Inc. | Method of promoting bone growth by an anti-activin B antibody |
| US8138142B2 (en) | 2009-01-13 | 2012-03-20 | Acceleron Pharma Inc. | Methods for increasing adiponectin in a patient in need thereof |
| EP2440577A1 (en) * | 2009-06-12 | 2012-04-18 | Acceleron Pharma, Inc. | Truncated actriib-fc fusion proteins |
| US8173601B2 (en) | 2007-02-09 | 2012-05-08 | Acceleron Pharma, Inc. | Activin-ActRIIa antagonists and uses for treating multiple myeloma |
| US8178488B2 (en) | 2009-06-08 | 2012-05-15 | Acceleron Pharma, Inc. | Methods for increasing thermogenic adipocytes |
| US8216997B2 (en) | 2008-08-14 | 2012-07-10 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators |
| US8343933B2 (en) | 2007-02-02 | 2013-01-01 | Acceleron Pharma, Inc. | Variants derived from ActRIIB and uses therefor |
| US8361957B2 (en) | 2008-08-14 | 2013-01-29 | Acceleron Pharma, Inc. | Isolated GDF trap polypeptide |
| US8367611B2 (en) | 2007-09-18 | 2013-02-05 | Acceleron Pharma Inc. | Activin-actriia antagonists for inhibiting germ cell maturation |
| WO2013106175A1 (en) * | 2011-12-19 | 2013-07-18 | Amgen Inc. | Variant activin receptor polypeptides, alone or in combination with chemotherapy, and uses thereof |
| US8501678B2 (en) | 2007-03-06 | 2013-08-06 | Atara Biotherapeutics, Inc. | Variant activin receptor polypeptides and uses thereof |
| US8629109B2 (en) | 2005-11-23 | 2014-01-14 | Acceleron Pharma Inc. | Method for promoting bone growth using activin-actriia antagonists |
| US8710016B2 (en) | 2009-11-17 | 2014-04-29 | Acceleron Pharma, Inc. | ActRIIB proteins and variants and uses therefore relating to utrophin induction for muscular dystrophy therapy |
| US8716459B2 (en) | 2007-03-06 | 2014-05-06 | Amgen Inc. | Isolated nucleic acid molecules encoding variant activin receptor polypeptides |
| CN103987403A (en) * | 2011-10-17 | 2014-08-13 | 阿塞勒隆制药公司 | Methods and compositions for treating ineffective erythropoiesis |
| US8895016B2 (en) | 2006-12-18 | 2014-11-25 | Acceleron Pharma, Inc. | Antagonists of activin-actriia and uses for increasing red blood cell levels |
| US9138459B2 (en) | 2004-07-23 | 2015-09-22 | Acceleron Pharma Inc. | ACTRIIB-FC polynucleotides, polypeptides, and compositions |
| WO2015143403A1 (en) * | 2014-03-21 | 2015-09-24 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating ineffective erythropoiesis by inhibiting activin b and/or gdf11 |
| US9273114B2 (en) | 2008-11-26 | 2016-03-01 | Amgen Inc. | Stabilized receptor polypeptides and uses thereof |
| US9284364B2 (en) | 2005-11-01 | 2016-03-15 | Amgen Inc. | Isolated nucleic acid molecule encoding a fusion protein comprising an activin receptor |
| WO2016090188A1 (en) | 2014-12-03 | 2016-06-09 | Acceleron Pharma Inc. | Methods for treating myelodysplastic syndromes and sideroblastic anemias |
| WO2016164497A1 (en) * | 2015-04-06 | 2016-10-13 | Acceleron Pharma Inc. | Alk4:actriib heteromultimers and uses thereof |
| US9493556B2 (en) | 2010-11-08 | 2016-11-15 | Acceleron Pharma Inc. | Actriia binding agents and uses thereof |
| US9526759B2 (en) | 2007-02-01 | 2016-12-27 | Acceleron Pharma Inc. | Activin-actriia antagonists and uses for treating or preventing breast cancer |
| WO2017079591A2 (en) | 2015-11-04 | 2017-05-11 | Acceleron Pharma Inc. | Methods for increasing red blood cell levels and treating ineffective erythropoiesis |
| WO2017091706A1 (en) | 2015-11-23 | 2017-06-01 | Acceleron Pharma Inc. | Methods for treating eye disorders |
| US9850298B2 (en) | 2014-06-13 | 2017-12-26 | Acceleron Pharma Inc. | Methods for treating ulcers in thalassemia syndrome with an ActRIIB polypeptide |
| WO2018067874A1 (en) | 2016-10-05 | 2018-04-12 | Acceleron Pharma Inc. | Variant actriib proteins and uses thereof |
| EP3308796A1 (en) | 2012-11-02 | 2018-04-18 | Celgene Corporation | Activin-actrii antagonists and uses for treating bone and other disorders |
| EP3351260A1 (en) | 2012-04-06 | 2018-07-25 | Acceleron Pharma Inc. | Methods and compositions for increasing red blood cells |
| WO2018192973A1 (en) | 2017-04-18 | 2018-10-25 | Vifor (International) Ag | Ferroportin-inhibitor salts |
| US10227393B2 (en) | 2015-04-06 | 2019-03-12 | Acceleron Pharma Inc. | TGF-beta superfamily type I and type II receptor heteromultimers and uses thereof |
| EP3527219A1 (en) | 2012-10-24 | 2019-08-21 | Celgene Corporation | Methods for treating anemia |
| US10421969B2 (en) | 2011-10-04 | 2019-09-24 | Royal Holloway And Bedford New College | Oligomers |
| US10548976B2 (en) | 2015-05-20 | 2020-02-04 | Celgene Corporation | In vitro cell culture methods for beta-thalassemia using activin type II receptor ligand traps |
| EP3608419A1 (en) | 2012-10-24 | 2020-02-12 | Celgene Corporation | Biomarker for use in treating anemia |
| US10626396B2 (en) | 2005-02-09 | 2020-04-21 | Sarepta Therapeutics, Inc. | Antisense composition and method for treating muscle atrophy |
| US10695405B2 (en) | 2016-07-15 | 2020-06-30 | Acceleron Pharma Inc. | Compositions and methods for treating pulmonary hypertension |
| US10934532B2 (en) | 2016-10-05 | 2021-03-02 | Acceleron Pharma Inc. | ALK4.ActRIIB heteromultimers |
| EP3808778A1 (en) | 2014-04-18 | 2021-04-21 | Acceleron Pharma Inc. | Methods for increasing red blood cell levels and treating sickle-cell disease |
| US11015200B2 (en) | 2015-03-18 | 2021-05-25 | Sarepta Therapeutics, Inc. | Antisense-induced exon exclusion in myostatin |
| EP3858993A1 (en) | 2015-10-09 | 2021-08-04 | Sarepta Therapeutics, Inc. | Compositions and methods for treating duchenne muscular dystrophy and related disorders |
| WO2021158675A1 (en) * | 2020-02-03 | 2021-08-12 | Acceleron Pharma Inc. | Variant actriib proteins and uses thereof |
| AU2019204127B2 (en) * | 2009-08-13 | 2021-08-12 | Acceleron Pharma Inc. | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| US11471510B2 (en) | 2014-12-03 | 2022-10-18 | Celgene Corporation | Activin-ActRII antagonists and uses for treating anemia |
| US11541070B2 (en) | 2013-02-01 | 2023-01-03 | Atara Biotherapeutics, Inc. | Administration of an anti-activin-A compound to a subject |
| US11813308B2 (en) | 2014-10-09 | 2023-11-14 | Celgene Corporation | Treatment of cardiovascular disease using ActRII ligand traps |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100028332A1 (en) * | 2006-12-18 | 2010-02-04 | Acceleron Pharma Inc. | Antagonists of actriib and uses for increasing red blood cell levels |
| CA2729100C (en) * | 2008-06-26 | 2018-01-02 | Acceleron Pharma Inc. | Methods for dosing an activin-actriia antagonist and monitoring of treated patients |
| BR112012005225B8 (en) * | 2009-09-09 | 2023-01-10 | Acceleron Pharma Inc | USE OF AN ACTRIIB-FC FUSION PROTEIN FOR THE TREATMENT OF A DISORDER RELATED TO BONE OR ASSOCIATED WITH MUSCLE LOSS DUE TO FAULT MUSCLE GROWTH |
| EP3818988A1 (en) * | 2009-11-03 | 2021-05-12 | Acceleron Pharma Inc. | Methods for treating fatty liver disease |
| JP2013512674A (en) * | 2009-12-02 | 2013-04-18 | アクセルロン ファーマ, インコーポレイテッド | Compositions and methods for increasing the serum half-life of an Fc fusion protein |
| JO3340B1 (en) | 2010-05-26 | 2019-03-13 | Regeneron Pharma | Antibodies to human gdf8 |
| ES2671347T3 (en) * | 2010-06-21 | 2018-06-06 | Kyowa Hakko Kirin Co., Ltd. | Procedure to purify a protein that uses an amino acid |
| EP2718328A4 (en) * | 2011-06-08 | 2014-12-24 | Acceleron Pharma Inc | Compositions and methods for increasing serum half-life |
| EP2780368B1 (en) | 2011-11-14 | 2018-01-03 | Regeneron Pharmaceuticals, Inc. | Compositions and methods for increasing muscle mass and muscle strength by specifically antagonizing gdf8 and/or activin a |
| WO2014030683A1 (en) * | 2012-08-21 | 2014-02-27 | 国立大学法人九州大学 | Biomarker for detecting factor for anemia in anemic patient |
| TWI655207B (en) | 2013-07-30 | 2019-04-01 | 再生元醫藥公司 | Anti-activin A antibody and use thereof |
| WO2016069925A1 (en) | 2014-10-30 | 2016-05-06 | Acceleron Pharma Inc. | Methods and compositions using gdf15 polypeptides for increasing red blood cells |
| WO2016128523A1 (en) | 2015-02-12 | 2016-08-18 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for predicting the responsiveness of a patient affected with malignant hematological disease to chemotherapy treatment and methods of treatment of such disease |
| KR20170141215A (en) | 2015-04-06 | 2017-12-22 | 악셀레론 파마 인코포레이티드 | Single-cancer type I and type II receptor fusion proteins and their uses |
| CA2982810A1 (en) | 2015-04-15 | 2016-10-20 | Regeneron Pharmaceuticals, Inc. | Methods of increasing strength and functionality with gdf8 inhibitors |
| RU2733492C2 (en) | 2015-04-22 | 2020-10-02 | Байоджен Ма Инк. | Novel hybrid actriib ligand trap proteins for treating diseases associated with muscular atrophy |
| WO2016183280A1 (en) * | 2015-05-13 | 2016-11-17 | Celgene Corporation | Treatment of beta-thalassemia using actrii ligand traps |
| US20170306027A1 (en) | 2016-04-06 | 2017-10-26 | Acceleron Pharma Inc. | Alk7 antagonists and uses thereof |
| EP3487868B1 (en) | 2016-07-25 | 2024-08-28 | Cephalon, Inc. | Affinity chromatography wash buffer |
| FI3490582T3 (en) * | 2016-07-27 | 2024-08-01 | Acceleron Pharma Inc | Compositions for use in treating myelofibrosis |
| WO2018089715A1 (en) | 2016-11-10 | 2018-05-17 | Keros Therapeutics, Inc. | Activin receptor type iia variants and methods of use thereof |
| CA3082146A1 (en) | 2017-11-09 | 2019-05-16 | Keros Therapeutics, Inc. | Activin receptor type iia variants and methods of use thereof |
| CN112292144A (en) | 2018-01-12 | 2021-01-29 | 科乐斯疗法公司 | Activin receptor type IIB variants and methods of use thereof |
| EP3758800A1 (en) | 2018-03-01 | 2021-01-06 | Regeneron Pharmaceuticals, Inc. | Methods for altering body composition |
| JP7405772B2 (en) * | 2018-05-09 | 2023-12-26 | ケロス セラピューティクス インコーポレイテッド | Activin type IIA receptor mutants and pharmaceutical compositions containing the same mutants |
| WO2023141724A1 (en) | 2022-01-28 | 2023-08-03 | 35Pharma Inc. | Activin receptor type iib variants and uses thereof |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1265208A (en) | 1915-09-07 | 1918-05-07 | Edward C Kahn | Liquid-fuel burner. |
| WO1987005330A1 (en) | 1986-03-07 | 1987-09-11 | Michel Louis Eugene Bergh | Method for enhancing glycoprotein stability |
| US5283317A (en) | 1987-08-03 | 1994-02-01 | Ddi Pharmaceuticals, Inc. | Intermediates for conjugation of polypeptides with high molecular weight polyalkylene glycols |
| US5525490A (en) | 1994-03-29 | 1996-06-11 | Onyx Pharmaceuticals, Inc. | Reverse two-hybrid method |
| WO1996026432A1 (en) | 1995-02-23 | 1996-08-29 | University Of Utah Research Foundation | Composite waveguide for solid phase binding assays |
| US5677196A (en) | 1993-05-18 | 1997-10-14 | University Of Utah Research Foundation | Apparatus and methods for multi-analyte homogeneous fluoro-immunoassays |
| US5955280A (en) | 1995-04-11 | 1999-09-21 | The General Hospital Corporation | Reverse two-hybrid system |
| WO2000043781A2 (en) | 1999-01-21 | 2000-07-27 | Metamorphix, Inc. | Growth differentiation factor inhibitors and uses therefor |
| WO2006012627A2 (en) | 2004-07-23 | 2006-02-02 | Acceleron Pharma Inc. | Actrii receptor polypeptides, methods and compositions |
| WO2007053775A1 (en) | 2005-11-01 | 2007-05-10 | Amgen Inc. | Novel activin receptor and uses thereof |
| WO2008076437A2 (en) * | 2006-12-18 | 2008-06-26 | Acceleron Pharma Inc. | Activin-actrii antagonists and uses for increasing red blood cell levels |
| WO2008097541A2 (en) | 2007-02-02 | 2008-08-14 | Acceleron Pharma Inc. | Variants derived from actriib and uses therefor |
Family Cites Families (198)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0637520B2 (en) | 1985-07-03 | 1994-05-18 | 味の素株式会社 | Polypeptide |
| US4973577A (en) | 1986-04-04 | 1990-11-27 | The Salk Institute For Biological Studies | FSH-releasing peptides |
| WO1992004913A1 (en) * | 1990-09-13 | 1992-04-02 | Children's Hospital Medical Center Of Northern California | Method for increasing red blood cell production by treatment with activin or activin-related peptides |
| US5118667A (en) | 1991-05-03 | 1992-06-02 | Celtrix Pharmaceuticals, Inc. | Bone growth factors and inhibitors of bone resorption for promoting bone formation |
| US20050186593A1 (en) | 1991-05-10 | 2005-08-25 | The Salk Institute For Biological Studies | Cloning and recombinant production of CRF receptor(s) |
| US6162896A (en) | 1991-05-10 | 2000-12-19 | The Salk Institute For Biological Studies | Recombinant vertebrate activin receptors |
| US5885794A (en) | 1991-05-10 | 1999-03-23 | The Salk Institute For Biological Studies | Recombinant production of vertebrate activin receptor polypeptides and identification of receptor DNAs in the activin/TGF-β superfamily |
| JPH06500574A (en) | 1991-05-10 | 1994-01-20 | ザ ソーク インスティテュート フォア バイオロジカル スタディーズ | Cloning and recombinant production of receptors of the activin/TGF-β superfamily |
| US6287816B1 (en) | 1991-06-25 | 2001-09-11 | Genetics Institute, Inc. | BMP-9 compositions |
| DK0592562T3 (en) | 1991-06-25 | 1999-08-30 | Genetics Inst | BMP-9 compositions |
| US6692925B1 (en) | 1992-11-17 | 2004-02-17 | Ludwig Institute For Cancer Research | Proteins having serine/threonine kinase domains, corresponding nucleic acid molecules, and their use |
| EP0679163A4 (en) | 1993-01-12 | 1997-07-16 | Univ Johns Hopkins Med | Growth differentiation factor-3. |
| US5354934A (en) | 1993-02-04 | 1994-10-11 | Amgen Inc. | Pulmonary administration of erythropoietin |
| US5637480A (en) | 1993-05-12 | 1997-06-10 | Genetics Institute, Inc. | DNA molecules encoding bone morphogenetic protein-10 |
| BR9406716A (en) | 1993-05-12 | 1996-02-06 | Genetics Inst | Isolated DNA molecule host cell vector method to produce a purified bone morphogenetic protein-BMP-10 (BMP-10) polypeptide and chimeric DNA molecule |
| US5831050A (en) | 1993-06-07 | 1998-11-03 | Creative Biomolecules, Inc. | Morphogen cell surface receptor |
| DE69434934D1 (en) | 1993-10-14 | 2007-04-12 | Harvard College | PROCESS FOR INDUCING AND CONSERVING NEURONAL CELLS |
| US5658876A (en) | 1994-04-28 | 1997-08-19 | The General Hospital Corporation | Activin antagonists as novel contraceptives |
| US5760010A (en) | 1995-01-01 | 1998-06-02 | Klein; Ira | Method of treating liver disorders with a macrolide antibiotic |
| US6132988A (en) | 1995-10-27 | 2000-10-17 | Takeda Chemical Industries, Ltd. | DNA encoding a neuronal cell-specific receptor protein |
| GB9526131D0 (en) | 1995-12-21 | 1996-02-21 | Celltech Therapeutics Ltd | Recombinant chimeric receptors |
| US20050244867A1 (en) | 1996-03-26 | 2005-11-03 | Human Genome Sciences, Inc. | Growth factor HTTER36 |
| US6004780A (en) | 1996-03-26 | 1999-12-21 | Human Genome Sciences, Inc. | Growth factor HTTER36 |
| US6372480B1 (en) * | 1996-04-19 | 2002-04-16 | Mycogen Corporation | Pesticidal proteins |
| BR9712675A (en) | 1996-10-25 | 1999-10-19 | Searle & Co | Circularly exchanged erythropoietin receptor agonists. |
| US6017534A (en) * | 1996-11-20 | 2000-01-25 | Ecogen, Inc. | Hybrid Bacillus thuringiensis δ-endotoxins with novel broad-spectrum insecticidal activity |
| US6605699B1 (en) | 1997-01-21 | 2003-08-12 | Human Genome Sciences, Inc. | Galectin-11 polypeptides |
| US6034062A (en) | 1997-03-13 | 2000-03-07 | Genetics Institute, Inc. | Bone morphogenetic protein (BMP)-9 compositions and their uses |
| US6231880B1 (en) | 1997-05-30 | 2001-05-15 | Susan P. Perrine | Compositions and administration of compositions for the treatment of blood disorders |
| US6891082B2 (en) | 1997-08-01 | 2005-05-10 | The Johns Hopkins University School Of Medicine | Transgenic non-human animals expressing a truncated activintype II receptor |
| WO1999006559A1 (en) | 1997-08-01 | 1999-02-11 | The Johns Hopkins University School Of Medicine | Methods to identify growth differentiation factor (gdf) receptors |
| US6696260B1 (en) | 1997-08-01 | 2004-02-24 | The Johns Hopkins University School Of Medicine | Methods to identify growth differentiation factor (GDF) binding proteins |
| US6656475B1 (en) | 1997-08-01 | 2003-12-02 | The Johns Hopkins University School Of Medicine | Growth differentiation factor receptors, agonists and antagonists thereof, and methods of using same |
| US6953662B2 (en) | 1997-08-29 | 2005-10-11 | Human Genome Sciences, Inc. | Follistatin-3 |
| JP2001513982A (en) | 1997-08-29 | 2001-09-11 | ヒューマン ジノーム サイエンシーズ, インコーポレイテッド | Follistatin-3 |
| DE69838454T2 (en) | 1997-10-03 | 2008-02-07 | Chugai Seiyaku K.K. | NATURAL HUMAN ANTIBODY |
| US6696411B1 (en) | 1998-04-22 | 2004-02-24 | Cornell Research Foundation, Inc. | Canine erythropoietin gene and recombinant protein |
| CN1317967A (en) | 1998-09-17 | 2001-10-17 | 伊莱利利公司 | Protein formulations |
| AU5502799A (en) | 1998-09-22 | 2000-04-10 | Long Yu | New human growth differentiation factor encoding sequence and polypeptide encoded by such dna sequence and producing method thereof |
| US6472179B2 (en) | 1998-09-25 | 2002-10-29 | Regeneron Pharmaceuticals, Inc. | Receptor based antagonists and methods of making and using |
| US6548634B1 (en) | 1998-09-30 | 2003-04-15 | Chiron Corporation | Synthetic peptides having FGF receptor affinity |
| AU774419C (en) | 1998-10-30 | 2005-03-03 | Genetics Institute, Llc | Inhibition of differentiation of cytotoxic T-cells by P-selectin ligand (PSGL) antagonists |
| US6238860B1 (en) | 1998-11-05 | 2001-05-29 | Dyax Corp. | Binding moieties for human parvovirus B19 |
| US6777205B1 (en) | 1998-11-06 | 2004-08-17 | Sterrenbeld Biotechnologie North America, Inc. | Host cells expressing recombinant human erythropoietin |
| US20040009535A1 (en) | 1998-11-27 | 2004-01-15 | Celltech R&D, Inc. | Compositions and methods for increasing bone mineralization |
| US6914128B1 (en) | 1999-03-25 | 2005-07-05 | Abbott Gmbh & Co. Kg | Human antibodies that bind human IL-12 and methods for producing |
| CA2365449A1 (en) | 1999-04-19 | 2000-10-26 | Kyowa Hakko Kogyo Co., Ltd. | Inhibitor of the growth of androgen-independent tumor |
| US6468543B1 (en) | 1999-05-03 | 2002-10-22 | Zymogenetics, Inc. | Methods for promoting growth of bone using ZVEGF4 |
| SK8292002A3 (en) | 1999-11-12 | 2002-12-03 | Maxygen Holdings Ltd | A conjugate exhibiting interferon gamma activity, nucleotide sequence encoding for a polypeptide fraction of conjugate, an expression vector and a host cell containing nucleotide sequence, pharmaceutical composition comprising the same and use thereof |
| EP1237565B1 (en) | 1999-12-15 | 2006-03-08 | Research Development Foundation | Betaglycan as an inhibin receptor and uses thereof |
| US20030224501A1 (en) | 2000-03-17 | 2003-12-04 | Young Paul E. | Bone morphogenic protein polynucleotides, polypeptides, and antibodies |
| JP4487376B2 (en) | 2000-03-31 | 2010-06-23 | 味の素株式会社 | Kidney disease treatment |
| DK1311285T4 (en) | 2000-05-15 | 2017-07-24 | Hoffmann La Roche | Liquid pharmaceutical composition containing an erythropoietin derivative |
| US6627424B1 (en) | 2000-05-26 | 2003-09-30 | Mj Bioworks, Inc. | Nucleic acid modifying enzymes |
| AU2001269709A1 (en) | 2000-07-19 | 2002-02-05 | Eli Lilly And Company | Nucleic acids, vectors, host cells, polypeptides, and uses thereof |
| US6632180B1 (en) | 2000-09-07 | 2003-10-14 | John H. Laragh | Method for evaluating and treating hypertension |
| DE10045591A1 (en) | 2000-09-15 | 2002-04-04 | Klaus Pfizenmaier | Site-specific, antibody-mediated activation of proapoptotic cytokines - AMAIZe (Antibody-Mediated Apoptosis Inducing Cytokines) |
| US7087224B2 (en) | 2000-10-31 | 2006-08-08 | Amgen Inc. | Method of treating anemia by administering IL-1ra |
| AU2567002A (en) | 2000-11-20 | 2002-05-27 | Univ Illinois | Membrane scaffold proteins |
| US20030082233A1 (en) | 2000-12-01 | 2003-05-01 | Lyons Karen M. | Method and composition for modulating bone growth |
| EP1370287A2 (en) | 2000-12-01 | 2003-12-17 | Wyeth | Method and composition for modulating bone growth |
| TW200526779A (en) | 2001-02-08 | 2005-08-16 | Wyeth Corp | Modified and stabilized GDF propeptides and uses thereof |
| US20040132675A1 (en) | 2002-02-08 | 2004-07-08 | Calvin Kuo | Method for treating cancer and increasing hematocrit levels |
| US7294472B2 (en) | 2001-03-14 | 2007-11-13 | Caden Biosciences | Method for identifying modulators of G protein coupled receptor signaling |
| EP1369130A1 (en) | 2001-03-16 | 2003-12-10 | Takeda Chemical Industries, Ltd. | Process for producing sustained release preparation |
| ATE542545T1 (en) | 2001-05-24 | 2012-02-15 | Zymogenetics Inc | TACI-IMMUNOGLOBULIN FUSION PROTEINS |
| WO2002094864A2 (en) | 2001-05-25 | 2002-11-28 | Genset S.A. | Human cdnas and proteins and uses thereof |
| AUPR638101A0 (en) | 2001-07-13 | 2001-08-09 | Bioa Pty Limited | Composition and method for treatment of disease |
| US6855344B2 (en) | 2001-07-17 | 2005-02-15 | Integrated Chinese Medicine Holdings, Ltd. | Compositions and methods for prostate and kidney health and disorders, an herbal preparation |
| ATE448481T1 (en) | 2001-07-17 | 2009-11-15 | Teijin Ltd | SELECTION PROCESS FOR A SUBSTANCE AND MEDICINAL CHARACTERIZED BY TESTING A PPARD-ACTIVATE EFFECT |
| KR100453877B1 (en) | 2001-07-26 | 2004-10-20 | 메덱스젠 주식회사 | METHOD OF MANUFACTURING Ig-FUSION PROTEINS BY CONCATAMERIZATION, TNFR/Fc FUSION PROTEINS MANUFACTURED BY THE METHOD, DNA CODING THE PROTEINS, VECTORS INCLUDING THE DNA, AND CELLS TRANSFORMED BY THE VECTOR |
| US7320789B2 (en) | 2001-09-26 | 2008-01-22 | Wyeth | Antibody inhibitors of GDF-8 and uses thereof |
| US6784154B2 (en) | 2001-11-01 | 2004-08-31 | University Of Utah Research Foundation | Method of use of erythropoietin to treat ischemic acute renal failure |
| ES2414706T3 (en) | 2001-12-06 | 2013-07-22 | Fibrogen, Inc. | Methods to increase endogenous erythropoietin |
| US20030144203A1 (en) | 2001-12-19 | 2003-07-31 | Voyager Pharmaceutical Corporation | Methods for slowing senescence and treating and preventing diseases associated with senescence |
| US20060234918A1 (en) | 2001-12-19 | 2006-10-19 | Voyager Pharmaceutical Corporation | Methods for treating and preventing cancers that express the hypothalamic-pituitary-gonadal axis of hormones and receptors |
| US6998118B2 (en) | 2001-12-21 | 2006-02-14 | The Salk Institute For Biological Studies | Targeted retrograde gene delivery for neuronal protection |
| KR20040082421A (en) | 2002-02-11 | 2004-09-24 | 제넨테크, 인크. | Antibody Variants with Faster Antigen Association Rates |
| MXPA04008150A (en) | 2002-02-21 | 2005-06-17 | Wyeth Corp | A follistatin domain containing protein. |
| US20030219846A1 (en) | 2002-02-28 | 2003-11-27 | Pfizer Inc. | Assay for activity of the ActRIIB kinase |
| AU2003232485A1 (en) | 2002-04-18 | 2003-10-27 | Mtm Laboratories Ag | Neopeptides and methods useful for detection and treatment of cancer |
| DE10234192B4 (en) | 2002-07-26 | 2009-11-26 | Epoplus Gmbh Co.Kg | Use of erythropoietin |
| CN1753904A (en) | 2002-08-16 | 2006-03-29 | 惠氏公司 | Bmp-2 estrogen responsive element and methods of using the same |
| KR20050056247A (en) | 2002-10-15 | 2005-06-14 | 셀진 코포레이션 | Method of using and compositions comprising selective cytokine inhibitory drugs for the treatment and management of myelodysplastic syndromes |
| US7261893B2 (en) | 2002-10-22 | 2007-08-28 | Wyeth | Neutralizing antibodies against GDF-8 and uses therefor |
| US20040223966A1 (en) | 2002-10-25 | 2004-11-11 | Wolfman Neil M. | ActRIIB fusion polypeptides and uses therefor |
| AU2002953327A0 (en) | 2002-12-12 | 2003-01-09 | Monash University | Methods of diagnosing prognosing and treating activin associated diseases and conditions |
| DK1592416T3 (en) | 2003-02-07 | 2009-04-20 | Prometic Biosciences Inc | Medium chain fatty acids, glycerides and analogues as stimulators of erythropoiesis |
| US20070155661A1 (en) | 2003-02-14 | 2007-07-05 | The Board Of Trustees Of The Leland Standord Junior University | Methods and compositions for modulating the development of stem cells |
| GB0304424D0 (en) | 2003-02-26 | 2003-04-02 | Glaxosmithkline Biolog Sa | Novel compounds |
| US20040197828A1 (en) | 2003-03-26 | 2004-10-07 | Gaddy Dana P. | Method for diagnosis and treatment of bone turnover |
| US20070184052A1 (en) | 2003-05-09 | 2007-08-09 | Lin Herbert Y | Soluble tgf-b type III receptor fusion proteins |
| CN1829532A (en) | 2003-06-02 | 2006-09-06 | 惠氏公司 | Use of myostatin (GDF8) inhibitors in conjunction with corticosteroids for treating neuromuscular disorders |
| CA2529623A1 (en) | 2003-06-16 | 2005-02-17 | Celltech R & D, Inc. | Antibodies specific for sclerostin and methods for increasing bone mineralization |
| WO2005009460A2 (en) | 2003-07-25 | 2005-02-03 | Medexis, S.A. | Pharmaceutical composition comprising activin a, alk-4 or derivatives thereof for the treatment of ophthalmic disorders or cancer |
| US8895540B2 (en) | 2003-11-26 | 2014-11-25 | DePuy Synthes Products, LLC | Local intraosseous administration of bone forming agents and anti-resorptive agents, and devices therefor |
| DE602005016773D1 (en) | 2004-01-22 | 2009-11-05 | Merck Patent Gmbh | ANTIBODY ANTIBODIES WITH REDUCED COMPLEMENT FIXATION |
| US20050197292A1 (en) | 2004-01-30 | 2005-09-08 | Glennda Smithson | Compositions and methods for treating T-cell mediated pathological conditions |
| CA2561317A1 (en) | 2004-03-26 | 2005-10-13 | Acceleron Pharma Inc. | Bmp-3 propeptides and related methods |
| AU2005230854A1 (en) | 2004-03-31 | 2005-10-20 | Xencor, Inc | BMP-7 variants with improved properties |
| WO2005113590A2 (en) | 2004-05-12 | 2005-12-01 | Acceleron Pharma Inc. | Bmp10 propeptides and related methods |
| AU2005258286A1 (en) | 2004-06-24 | 2006-01-05 | Acceleron Pharma Inc. | GDF3 propeptides and related methods |
| US8288557B2 (en) | 2004-07-23 | 2012-10-16 | Endocyte, Inc. | Bivalent linkers and conjugates thereof |
| US8617815B2 (en) | 2004-08-05 | 2013-12-31 | The Regents Of The University Of California | Molecules with effects on cellular development and function |
| US7095608B2 (en) | 2004-08-12 | 2006-08-22 | Audiovox Corporation | Video display mounting system and method |
| AU2005272646A1 (en) | 2004-08-12 | 2006-02-23 | Wyeth | Combination therapy for diabetes, obesity, and cardiovascular diseases using GDF-8 inhibitors |
| US8435948B2 (en) | 2004-09-29 | 2013-05-07 | Mount Sinai School Of Medicine Of New York University | Methods for inhibiting osteoclastic bone resorption and bone loss comprising administration of an anti-FSH or anti-FSHR antibody |
| US20070003576A1 (en) | 2004-12-09 | 2007-01-04 | Andrea Gambotto | Vaccines for the rapid response to pandemic avian influenza |
| NZ538097A (en) | 2005-02-07 | 2006-07-28 | Ovita Ltd | Method and compositions for improving wound healing |
| ES2547866T3 (en) | 2005-02-16 | 2015-10-09 | The General Hospital Corporation | Use of hemojuvelin fusion proteins to regulate iron metabolism mediated by hepcidin |
| JPWO2006115274A1 (en) | 2005-04-26 | 2008-12-18 | 味の素株式会社 | Bone marrow erythroid progenitor cell differentiation promoter |
| JP2007099764A (en) | 2005-09-09 | 2007-04-19 | Ajinomoto Co Inc | Hypoglycaemic agent |
| AU2006294511B2 (en) | 2005-09-28 | 2011-11-17 | Zymogenetics, Inc | IL-17A and IL-17F antagonists and methods of using the same |
| WO2007042169A2 (en) | 2005-10-07 | 2007-04-19 | Istituto Di Ricerche Di Biologia Molecolare P Angeletti Spa | Matrix metalloproteinase 11 vaccine |
| US8128933B2 (en) | 2005-11-23 | 2012-03-06 | Acceleron Pharma, Inc. | Method of promoting bone growth by an anti-activin B antibody |
| CN105001320A (en) | 2005-11-23 | 2015-10-28 | 阿塞勒隆制药公司 | Activin-ActRIIa antagonists and uses for promoting bone growth |
| US20070149458A1 (en) | 2005-12-06 | 2007-06-28 | Amgen Inc. | Uses of myostatin antagonists |
| US7799787B2 (en) | 2005-12-20 | 2010-09-21 | Merck Frosst Canada Ltd. | Heteroaromatic compounds as inhibitors of stearoyl-coenzyme a delta-9 desaturase |
| MX2008008340A (en) | 2005-12-21 | 2008-09-03 | Schering Corp | Treatment of nonalcoholic fatty liver disease using cholesterol lowering agents and h3 receptor antagonist/inverse agonist. |
| EP1973909A2 (en) | 2005-12-22 | 2008-10-01 | Biogen Idec MA Inc. | Transforming growth factor modulators |
| US7361512B2 (en) | 2006-01-20 | 2008-04-22 | Beckman Coulter, Inc. | Low hemoglobin concentration cell percentage and method of use in detection of iron deficiency |
| US7820721B2 (en) | 2006-01-25 | 2010-10-26 | Wellstat Therapeutics Corporation | Compounds for the treatment of metabolic disorders |
| CN101394738A (en) | 2006-02-28 | 2009-03-25 | 维尔斯达医疗公司 | Compounds for the treatment of metabolic disorders |
| EP2007813A2 (en) | 2006-04-14 | 2008-12-31 | Amgen Inc. | Agonist erythropoietin receptor antibodies |
| WO2007123391A1 (en) | 2006-04-20 | 2007-11-01 | Academisch Ziekenhuis Leiden | Therapeutic intervention in a genetic disease in an individual by modifying expression of an aberrantly expressed gene. |
| AU2007249827A1 (en) | 2006-05-09 | 2007-11-22 | Colorado State University Research Foundation | Methods for treating blood disorders |
| AU2007275606B9 (en) | 2006-07-21 | 2013-09-26 | Lyne Laboratories, Inc. | Liquid compositions of calcium acetate |
| GB0615129D0 (en) | 2006-07-29 | 2006-09-06 | Univ Cardiff | Anti-cancer activity of BMP-9 and BMP-10 and their use in cancer therapies |
| CL2007002567A1 (en) | 2006-09-08 | 2008-02-01 | Amgen Inc | ISOLATED PROTEINS FROM LINK TO ACTIVINE TO HUMAN. |
| US7547781B2 (en) | 2006-09-11 | 2009-06-16 | Curis, Inc. | Quinazoline based EGFR inhibitors containing a zinc binding moiety |
| WO2008060139A1 (en) | 2006-11-17 | 2008-05-22 | Erasmus University Medical Center Rotterdam | Methods for controlling mineralization of extracellular matrix, therapeutic methods based thereon and medicaments for use therein |
| US20100003190A1 (en) | 2006-12-08 | 2010-01-07 | Caritas St. Elizabeth's Medical Center Of Boston, Inc. | Method for protecting renal tubular epithelial cells from radiocontrast nephropathy (RCN) |
| CA2672581A1 (en) | 2006-12-14 | 2008-06-19 | Forerunner Pharma Research Co., Ltd. | Anti-claudin 3 monoclonal antibody and treatment and diagnosis of cancer using the same |
| US8895016B2 (en) | 2006-12-18 | 2014-11-25 | Acceleron Pharma, Inc. | Antagonists of activin-actriia and uses for increasing red blood cell levels |
| US20100028332A1 (en) | 2006-12-18 | 2010-02-04 | Acceleron Pharma Inc. | Antagonists of actriib and uses for increasing red blood cell levels |
| CN101835485B (en) | 2007-02-01 | 2016-10-26 | 阿塞勒隆制药公司 | Activin-ACTRIIA antagonist and the purposes in treatment or Breast Cancer Prevention |
| ME02333B (en) | 2007-02-09 | 2013-04-30 | Acceleron Pharma Inc | PHARMACEUTICAL COMPOSITIONS COMPRISING ACTIVIN-ActRIIA ANTAGONISTS AND USE THEREOF IN PREVENTING OR TREATING MULTIPLE MYELOMA |
| US8501678B2 (en) | 2007-03-06 | 2013-08-06 | Atara Biotherapeutics, Inc. | Variant activin receptor polypeptides and uses thereof |
| TWI454479B (en) | 2007-03-06 | 2014-10-01 | Amgen Inc | Variant activin receptor polypeptides and uses thereof |
| US20090017019A1 (en) | 2007-06-01 | 2009-01-15 | Wyeth | Methods and compositions for modulating bmp-10 activity |
| WO2009009059A1 (en) | 2007-07-09 | 2009-01-15 | Biogen Idec Ma Inc. | Spiro compounds as antagonists of tgf-beta |
| GB0715087D0 (en) | 2007-08-03 | 2007-09-12 | Summit Corp Plc | Drug combinations for the treatment of duchenne muscular dystrophy |
| CN101678107A (en) | 2007-08-03 | 2010-03-24 | 萨米特公开有限公司 | Drug combinations for the treatment of duchenne muscular dystrophy |
| GB0715938D0 (en) | 2007-08-15 | 2007-09-26 | Vastox Plc | Method of treatment of duchenne muscular dystrophy |
| WO2009025651A1 (en) | 2007-08-17 | 2009-02-26 | University Of Maine System Board Of Trustees | Biologically active peptide and method of using the same |
| US20100279409A1 (en) | 2007-09-13 | 2010-11-04 | Neil Robson | Method for modifying celluar immune resonse by modulating activin activity |
| CN101861161B (en) | 2007-09-18 | 2017-04-19 | 阿塞勒隆制药公司 | Activin-ACTRIIA antagonists and uses for decreasing or inhibiting FSH secretion |
| PE20091163A1 (en) | 2007-11-01 | 2009-08-09 | Wyeth Corp | ANTIBODIES FOR GDF8 |
| WO2009070243A2 (en) | 2007-11-21 | 2009-06-04 | Amgen Inc. | Wise binding agents and epitopes |
| JP5638961B2 (en) | 2008-03-13 | 2014-12-10 | ザ ジェネラル ホスピタル コーポレイション | Inhibitors of BMP signaling pathway |
| WO2009137613A2 (en) | 2008-05-06 | 2009-11-12 | Joslin Diabetes Center, Inc. | Methods and compositions for inducing brown adipogenesis |
| WO2009137075A1 (en) | 2008-05-06 | 2009-11-12 | Acceleron Pharma Inc. | Anti-activin antibodies and uses for promoting bone growth |
| CA2729100C (en) | 2008-06-26 | 2018-01-02 | Acceleron Pharma Inc. | Methods for dosing an activin-actriia antagonist and monitoring of treated patients |
| WO2009158033A2 (en) * | 2008-06-26 | 2009-12-30 | Acceleron Pharma Inc. | Antagonists of activin-actriia and uses for increasing red blood cell levels |
| US8216997B2 (en) | 2008-08-14 | 2012-07-10 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators |
| TWI748373B (en) | 2008-08-14 | 2021-12-01 | 美商艾瑟勒朗法瑪公司 | Use of gdf traps to increase red blood cell levels |
| US20110293526A1 (en) | 2008-11-20 | 2011-12-01 | University Of Southern California | Compositions and methods to modulate hair growth |
| LT2370463T (en) | 2008-11-26 | 2016-12-12 | Amgen Inc. | A stabilized variant of activin iib receptor |
| EP2387412A4 (en) | 2009-01-13 | 2013-04-03 | Acceleron Pharma Inc | Methods for increasing adiponectin |
| US8110355B2 (en) | 2009-02-20 | 2012-02-07 | GenRemedy, LLC | Methods for identifying agents that inhibit cell migration, promote cell adhesion and prevent metastasis |
| LT2424895T (en) | 2009-04-27 | 2017-12-27 | Novartis Ag | Compositions and methods for increasing muscle growth |
| MX2011013172A (en) | 2009-06-08 | 2012-04-02 | Acceleron Pharma Inc | Methods for increasing thermogenic adipocytes. |
| EP3805259A1 (en) | 2009-06-12 | 2021-04-14 | Acceleron Pharma Inc. | Truncated actriib-fc fusion proteins |
| CN102655872B (en) | 2009-08-13 | 2016-01-20 | 阿塞勒隆制药公司 | GDF catches and erythropoietin receptor activator use in conjunction are to increase hematocrit level |
| BR112012005225B8 (en) | 2009-09-09 | 2023-01-10 | Acceleron Pharma Inc | USE OF AN ACTRIIB-FC FUSION PROTEIN FOR THE TREATMENT OF A DISORDER RELATED TO BONE OR ASSOCIATED WITH MUSCLE LOSS DUE TO FAULT MUSCLE GROWTH |
| EP3818988A1 (en) | 2009-11-03 | 2021-05-12 | Acceleron Pharma Inc. | Methods for treating fatty liver disease |
| EP3332796A1 (en) | 2009-11-17 | 2018-06-13 | Acceleron Pharma Inc. | Actriib proteins and variants and uses therefore relating to utrophin induction for muscular dystrophy therapy |
| WO2012027065A2 (en) | 2010-08-27 | 2012-03-01 | Celgene Corporation | Combination therapy for treatment of disease |
| US9595813B2 (en) | 2011-01-24 | 2017-03-14 | Soraa Laser Diode, Inc. | Laser package having multiple emitters configured on a substrate member |
| US8580922B2 (en) | 2011-03-04 | 2013-11-12 | Shire Human Genetic Therapies, Inc. | Peptide linkers for polypeptide compositions and methods for using same |
| HUE040276T2 (en) | 2011-07-01 | 2019-02-28 | Novartis Ag | Method for treating metabolic disorders |
| BR122019023174B1 (en) | 2011-10-17 | 2021-02-23 | Acceleron Pharma, Inc | USE OF A POLYPEPTIDE FOR THE MANUFACTURE OF A MEDICINAL PRODUCT TO TREAT ANEMIA ASSOCIATED WITH SPLENOMEGALIA |
| US8765385B2 (en) | 2011-10-27 | 2014-07-01 | Ravindra Kumar | Method of detection of neutralizing anti-actriib antibodies |
| WO2013063536A1 (en) | 2011-10-27 | 2013-05-02 | Acceleron Pharma, Inc. | Actriib binding agents and uses thereof |
| US20140303068A1 (en) | 2011-10-28 | 2014-10-09 | Paranta Biosciences Limited | Method of treating mucus hypersecretion |
| AU2012364736A1 (en) | 2011-12-19 | 2014-07-24 | Amgen Inc. | Variant activin receptor polypeptides, alone or in combination with chemotherapy, and uses thereof |
| US9303079B2 (en) | 2012-04-02 | 2016-04-05 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of cytoplasmic and cytoskeletal proteins |
| US9663569B2 (en) | 2012-06-14 | 2017-05-30 | The Medical Research, Infrastructure, And Health Services Fund Of The Tel Aviv Medical Center | Use of blocking agents of bone morphogenic protein (BMP) signalling for the treatment of neuroinflammatory and neurodegenerative diseases |
| JP6298762B2 (en) | 2012-07-02 | 2018-03-20 | 協和発酵キリン株式会社 | Therapeutic agent for anemia such as renal anemia and cancer anemia comprising anti-BMP9 antibody as an active ingredient |
| EP3608419A1 (en) | 2012-10-24 | 2020-02-12 | Celgene Corporation | Biomarker for use in treating anemia |
| CN112933223A (en) | 2012-10-24 | 2021-06-11 | 细胞基因公司 | Methods for treating anemia |
| WO2014064292A1 (en) | 2012-10-26 | 2014-05-01 | Universite Pierre Et Marie Curie (Paris 6) | A method for preventing or treating atrial fibrillation |
| CA2890217C (en) | 2012-11-02 | 2021-07-20 | Yifu FANG | Activin-actrii antagonists and uses for treating bone and other disorders |
| MX2015005839A (en) | 2012-11-08 | 2015-12-17 | Clearside Biomedical Inc | Methods and devices for the treatment of ocular diseases in human subjects. |
| WO2014093531A1 (en) | 2012-12-11 | 2014-06-19 | Los Angeles Biomedical Research Institute At Harbor-Ucla Medical Center | Modulation of myofiber repair by anti-myostatin in strategies with stem cells |
| US20140220033A1 (en) | 2013-02-01 | 2014-08-07 | Santa Maria Biotherapeutics, Inc. | Administration of an Anti-Activin-A Compound to a Subject |
| US20160184458A1 (en) | 2013-03-14 | 2016-06-30 | Shire Human Genetic Therapies, Inc. | Mrna therapeutic compositions and use to treat diseases and disorders |
| TWI655207B (en) | 2013-07-30 | 2019-04-01 | 再生元醫藥公司 | Anti-activin A antibody and use thereof |
| AU2014307589A1 (en) | 2013-08-14 | 2016-02-11 | Novartis Ag | Methods of treating sporadic inclusion body myositis |
| AU2014366827A1 (en) | 2013-12-16 | 2016-06-23 | Paranta Biosciences Limited | Method of diagnosis and treatment |
| WO2015108972A1 (en) | 2014-01-14 | 2015-07-23 | Santa Maria Biotherapeutics, Inc. | Activin inhibitor response prediction and uses for treatment |
| JP2017510622A (en) | 2014-01-27 | 2017-04-13 | ノバルティス アーゲー | Biomarkers, methods and uses for predicting muscle atrophy |
| JP6601687B2 (en) | 2014-03-31 | 2019-11-06 | 大日本住友製薬株式会社 | Preventive and therapeutic agent for progressive ossification fibrodysplasia |
| AP2016009549A0 (en) | 2014-04-18 | 2016-11-30 | Acceleron Pharma Inc | Methods for increasing red blood cell levels and treating sickle-cell disease |
| TW201622746A (en) | 2014-04-24 | 2016-07-01 | 諾華公司 | Methods of improving or accelerating physical recovery after surgery for hip fracture |
| SG11201610432UA (en) | 2014-06-13 | 2017-01-27 | Santa Maria Biotherapeutics Inc | Formulated receptor polypeptides and related methods |
| EP3154566B1 (en) | 2014-06-13 | 2022-08-03 | Acceleron Pharma Inc. | Actrii antagonist for the treatment or prevention of a cutaneous ulcer in a subject that has anemia |
| WO2016183280A1 (en) | 2015-05-13 | 2016-11-17 | Celgene Corporation | Treatment of beta-thalassemia using actrii ligand traps |
-
2009
- 2009-08-13 TW TW109108617A patent/TWI748373B/en active
- 2009-08-13 JP JP2011523000A patent/JP5922928B2/en active Active
- 2009-08-13 EP EP09806981.8A patent/EP2340031B1/en active Active
- 2009-08-13 HU HUE09806981A patent/HUE045456T2/en unknown
- 2009-08-13 DK DK09806981.8T patent/DK2340031T3/en active
- 2009-08-13 AU AU2009282441A patent/AU2009282441B2/en active Active
- 2009-08-13 CA CA2733911A patent/CA2733911C/en active Active
- 2009-08-13 TW TW98127213A patent/TWI472336B/en active
- 2009-08-13 PL PL18198713T patent/PL3494986T3/en unknown
- 2009-08-13 HU HUE20173397A patent/HUE063136T2/en unknown
- 2009-08-13 TW TW110118496A patent/TWI784538B/en active
- 2009-08-13 ES ES20173397T patent/ES2949049T3/en active Active
- 2009-08-13 SI SI200931986T patent/SI2340031T1/en unknown
- 2009-08-13 SI SI200932184T patent/SI3750552T1/en unknown
- 2009-08-13 DK DK18198713.2T patent/DK3494986T3/en active
- 2009-08-13 DK DK20173397.9T patent/DK3750552T5/en active
- 2009-08-13 ES ES09806981T patent/ES2738543T3/en active Active
- 2009-08-13 PL PL09806981T patent/PL2340031T4/en unknown
- 2009-08-13 ES ES18198713T patent/ES2808139T3/en active Active
- 2009-08-13 LT LTEP18198713.2T patent/LT3494986T/en unknown
- 2009-08-13 TW TW105103260A patent/TWI617316B/en active
- 2009-08-13 EP EP18198713.2A patent/EP3494986B1/en active Active
- 2009-08-13 PT PT181987132T patent/PT3494986T/en unknown
- 2009-08-13 TW TW108100646A patent/TW201919685A/en unknown
- 2009-08-13 TW TW103139735A patent/TWI626945B/en active
- 2009-08-13 LT LTEP09806981.8T patent/LT2340031T/en unknown
- 2009-08-13 WO PCT/US2009/004659 patent/WO2010019261A1/en active Application Filing
- 2009-08-13 SI SI200932078T patent/SI3494986T1/en unknown
- 2009-08-13 TW TW106133717A patent/TW201803586A/en unknown
- 2009-08-13 LT LTEP20173397.9T patent/LT3750552T/en unknown
- 2009-08-13 US US12/583,177 patent/US8058229B2/en active Active
- 2009-08-13 FI FIEP20173397.9T patent/FI3750552T3/en active
- 2009-08-13 EP EP20173397.9A patent/EP3750552B1/en active Active
- 2009-08-13 TR TR2019/10890T patent/TR201910890T4/en unknown
- 2009-08-13 PT PT201733979T patent/PT3750552T/en unknown
- 2009-08-13 PT PT09806981T patent/PT2340031T/en unknown
- 2009-08-13 HR HRP20230761TT patent/HRP20230761T1/en unknown
- 2009-08-13 HU HUE18198713A patent/HUE051137T2/en unknown
- 2009-08-13 PL PL20173397.9T patent/PL3750552T3/en unknown
-
2011
- 2011-09-28 US US13/247,748 patent/US8361957B2/en active Active
-
2013
- 2013-01-25 US US13/750,249 patent/US9505813B2/en active Active
-
2014
- 2014-08-27 JP JP2014172279A patent/JP2014224154A/en not_active Withdrawn
-
2016
- 2016-10-19 JP JP2016204858A patent/JP2017019860A/en not_active Withdrawn
- 2016-10-27 US US15/336,363 patent/US10377996B2/en active Active
-
2018
- 2018-05-31 JP JP2018104584A patent/JP6649432B2/en active Active
-
2019
- 2019-06-18 HR HRP20191109TT patent/HRP20191109T1/en unknown
- 2019-06-27 US US16/455,301 patent/US20200165583A1/en not_active Abandoned
- 2019-07-16 CY CY20191100755T patent/CY1121971T1/en unknown
-
2020
- 2020-01-16 JP JP2020005252A patent/JP6968917B2/en active Active
- 2020-02-19 US US16/795,088 patent/US11155791B2/en active Active
- 2020-02-19 US US16/795,076 patent/US20200199546A1/en not_active Abandoned
- 2020-02-19 US US16/795,083 patent/US11168311B2/en active Active
- 2020-03-03 US US16/808,010 patent/US11162085B2/en active Active
- 2020-07-29 CY CY20201100705T patent/CY1123217T1/en unknown
- 2020-07-29 HR HRP20201185TT patent/HRP20201185T1/en unknown
- 2020-12-17 CY CY2020043C patent/CY2020043I1/en unknown
- 2020-12-17 LT LTPA2020006C patent/LTC2340031I2/en unknown
- 2020-12-17 NO NO2020045C patent/NO2020045I1/en unknown
- 2020-12-17 HU HUS2000054C patent/HUS2000054I1/en unknown
- 2020-12-19 FR FR20C1066C patent/FR20C1066I2/en active Active
- 2020-12-21 NL NL301082C patent/NL301082I2/en unknown
-
2021
- 2021-04-28 US US17/243,049 patent/US20220098559A1/en not_active Abandoned
- 2021-10-27 JP JP2021175400A patent/JP7329573B2/en active Active
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1265208A (en) | 1915-09-07 | 1918-05-07 | Edward C Kahn | Liquid-fuel burner. |
| WO1987005330A1 (en) | 1986-03-07 | 1987-09-11 | Michel Louis Eugene Bergh | Method for enhancing glycoprotein stability |
| US5283317A (en) | 1987-08-03 | 1994-02-01 | Ddi Pharmaceuticals, Inc. | Intermediates for conjugation of polypeptides with high molecular weight polyalkylene glycols |
| US5677196A (en) | 1993-05-18 | 1997-10-14 | University Of Utah Research Foundation | Apparatus and methods for multi-analyte homogeneous fluoro-immunoassays |
| US5525490A (en) | 1994-03-29 | 1996-06-11 | Onyx Pharmaceuticals, Inc. | Reverse two-hybrid method |
| WO1996026432A1 (en) | 1995-02-23 | 1996-08-29 | University Of Utah Research Foundation | Composite waveguide for solid phase binding assays |
| US5955280A (en) | 1995-04-11 | 1999-09-21 | The General Hospital Corporation | Reverse two-hybrid system |
| US5965368A (en) | 1995-04-11 | 1999-10-12 | The General Hospital Corporation | Reverse two-hybrid systems |
| WO2000043781A2 (en) | 1999-01-21 | 2000-07-27 | Metamorphix, Inc. | Growth differentiation factor inhibitors and uses therefor |
| WO2006012627A2 (en) | 2004-07-23 | 2006-02-02 | Acceleron Pharma Inc. | Actrii receptor polypeptides, methods and compositions |
| WO2007053775A1 (en) | 2005-11-01 | 2007-05-10 | Amgen Inc. | Novel activin receptor and uses thereof |
| WO2008076437A2 (en) * | 2006-12-18 | 2008-06-26 | Acceleron Pharma Inc. | Activin-actrii antagonists and uses for increasing red blood cell levels |
| WO2008097541A2 (en) | 2007-02-02 | 2008-08-14 | Acceleron Pharma Inc. | Variants derived from actriib and uses therefor |
Non-Patent Citations (72)
| Title |
|---|
| "Synthetic Peptides: A User's Guide", 1992, W. H. FREEMAN AND COMPANY |
| ADAMSON: "Harrison's Principles of Internal Medicine", 2008, MCGRAW HILL, pages: 628 - 634 |
| APLIN; WRISTON, CRC CRIT. REV. BIOCHEM., 1981, pages 259 - 306 |
| ASHMORE ET AL., GROWTH, vol. 38, 1974, pages 501 - 507 |
| ATTISANO ET AL., CELL, vol. 68, 1992, pages 97 - 108 |
| ATTISANO ET AL., CELL, vol. 68, no. 1, 10 January 1992 (1992-01-10), pages 97 - 108 |
| AUSUBEL ET AL: "Current Protocols in Molecular Biology", 1992, JOHN WILEY & SONS |
| BARTEL ET AL., BIOTECHNIQUES, vol. 14, 1993, pages 920 - 924 |
| BODANSKY, M.: "Principles of Peptide Synthesis", 1993, SPRINGER VERLAG |
| BRON ET AL., SEMIN ONCOL, vol. 28, no. 8, 2001, pages 1 - 6 |
| BROWN ET AL., GROWTH FACTORS, vol. 3, 1990, pages 35 - 43 |
| BUNN, N ENGL J MED, vol. 346, no. 7, 2002, pages 522 - 523 |
| DELANTY ET AL., NEUROLOGY, vol. 49, 1997, pages 686 - 689 |
| DEMETRI ET AL., J CLIN ONCOL, vol. 16, 1998, pages 3412 - 3425 |
| DENNLER ET AL., EMBO, vol. 17, 1998, pages 3091 - 3100 |
| DEPAOLO ET AL., PROC SOC EP BIOL MED, vol. 198, 1991, pages 500 - 512 |
| DYSON ET AL., CURR BIOL., vol. 7, 1997, pages 81 - 84 |
| EDGE ET AL., ANAL. BIOCHEM., vol. 118, 1981, pages 131 |
| ESTEY, CURR OPIN HEMATOL, vol. 10, 2003, pages 60 - 67 |
| FRALEY ET AL., TRENDS BIOCHEM. SCI., vol. 6, 1981, pages 77 |
| GAFTER ET AL., KIDNEY INT, vol. 45, 1994, pages 224 - 231 |
| GAMER ET AL., DEV BIOL., vol. 208, 1999, pages 222 - 232 |
| GAMER ET AL., DEV BIOL., vol. 229, 2001, pages 407 - 420 |
| GAMER ET AL., DEV. BIOL., vol. 208, 1999, pages 222 - 232 |
| GANZ, J AM SOC NEPHROL, vol. 18, 2007, pages 394 - 400 |
| GLASPY ET AL., J CLIN ONCOL, vol. 15, 1997, pages 1218 - 1234 |
| GOEDDEL: "Gene Expression Technology: Methods in Enzymology", 1990, ACADEMIC PRESS |
| GONZALEZ-CADAVID ET AL., PNAS, vol. 95, 1998, pages 14938 - 14943 |
| GROBET ET AL., NAT GENET., vol. 17, no. L, 1997, pages 71 - 74 |
| GROOPMAN ET AL., J NATL CANCER INST, vol. 91, 1999, pages 1616 - 1634 |
| HAKIMUDDIN ET AL., ARCH. BIOCHEM. BIOPHYS., vol. 259, 1987, pages 52 |
| HILDEN ET AL., BLOOD, vol. 83, no. 8, 15 April 1994 (1994-04-15), pages 2163 - 2170 |
| HOCHULI ET AL., J. CHROMATOGRAPHY, vol. 411, 1987, pages 177 |
| HORL ET AL., NEPHROL DIAL TRANSPLANT, vol. 15, 2000, pages 43 - 50 |
| HORL ET AL., NEPHROL DIAL TRANSPLANT, vol. 15, no. 4, 2000, pages 51 - 56 |
| IWABUCHI ET AL., ONCOGENE, vol. 8, 1993, pages 1693 - 1696 |
| J NUCL MED, vol. 25, 1985, pages 72 - 76 |
| JACOBS ET AL., NEPHROL DIAL TRANSPLANT, vol. 15, 2000, pages 15 - 19 |
| JAKOBY WB ET AL., METHODS IN ENZYMOLOGY, vol. 46, 1974, pages 1 |
| JANKNECHT ET AL., PNAS USA, vol. 88, pages 8972 |
| KAMBADUR ET AL., GENOME RES., vol. 7, 1997, pages 910 - 915 |
| LEE; MCPHERRON, PROC. NATL. ACAD. SCI., vol. 98, 2001, pages 9306 - 9311 |
| LEVIN ET AL., AM J KIDNEY DIS, vol. 27, 1999, pages 347 - 354 |
| MACIAS-SILVA ET AL., J BIOL CHEM., vol. 273, 1998, pages 25628 - 25636 |
| MADURA ET AL., J BIOL CHEM, vol. 268, 1993, pages 12046 - 12054 |
| MANNINO ET AL., BIOTECHNIQUES, vol. 6, 1988, pages 682 |
| MASSAGUE, NAT. REV. MOL. CELL BIOL., vol. 1, 2000, pages 169 - 178 |
| MATHEWS; VALE, CELL, vol. 65, 1991, pages 973 - 982 |
| MCPHERRON ET AL., NAT. GENET., vol. 22, 1999, pages 260 - 264 |
| MCPHERRON ET AL., NATURE, vol. 387, 1997, pages 83 - 90 |
| MCPHERRON; LEE, PROC. NATL. ACAD. SCI. USA, vol. 94, 1997, pages 12457 - 12461 |
| MIYAZONO ET AL., J. BIOL. CHEM., vol. 263, 1988, pages 6407 - 6415 |
| MURATA ET AL., PNAS, vol. 85, 1988, pages 2434 |
| NAKASHIMA ET AL., MECH. DEV., vol. 80, 1999, pages 185 - 189 |
| NISSENSON, AM J KIDNEY DIS, vol. 20, no. 1, 1992, pages 21 - 24 |
| OH ET AL., GENES DEV, vol. 16, 2002, pages 2749 - 2754 |
| REVICKI ET AL., AM J KIDNEY DIS, vol. 25, 1995, pages 548 - 554 |
| SAKUMA ET AL., GENES CELLS, vol. 7, 2002, pages 401 - 412 |
| SAMBROOK ET AL: "Molecular Cloning A Laboratory Manual", 1989, COLD SPRING HARBOR LABORATORY PRESS |
| SCHUELKE ET AL., N ENGL J MED, vol. 350, 2004, pages 2682 - 2688 |
| SINGIBARTI, J. CLIN INVESTIG, vol. 72, no. 6, 1994, pages S36 - S43 |
| SWATLAND; KIEFFER, J. ANIM. SCI, vol. 38, 1994, pages 752 - 757 |
| THOTAKURA ET AL., METH. ENZYMOL., vol. 138, 1987, pages 350 |
| VIDAL; LEGRAIN, NUCLEIC ACIDS RES, vol. 27, 1999, pages 919 - 929 |
| VIDAL; LEGRAIN, TRENDS BIOTECHNOL, vol. 17, 1999, pages 374 - 381 |
| WAKEFIELD ET AL., J. BIOL. CHEM., vol. 263, 1988, pages 7646 - 7654 |
| WEATHERALL; PROVAN, LANCET, vol. 355, 2000, pages 1169 - 1175 |
| WOODRUFF, BIOCHEM PHARMACOL., vol. 55, 1998, pages 953 - 963 |
| WU ET AL., NEURON, vol. 37, 2003, pages 197 - 207 |
| YAMASHITA ET AL., J. CELL BIOL., vol. 130, 1995, pages 217 - 226 |
| YEO; WHITMAN, MOL. CELL, vol. 7, 2001, pages 949 - 957 |
| ZERVOS ET AL., CELL, vol. 72, 1993, pages 223 - 232 |
Cited By (146)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9138459B2 (en) | 2004-07-23 | 2015-09-22 | Acceleron Pharma Inc. | ACTRIIB-FC polynucleotides, polypeptides, and compositions |
| US10626396B2 (en) | 2005-02-09 | 2020-04-21 | Sarepta Therapeutics, Inc. | Antisense composition and method for treating muscle atrophy |
| US9284364B2 (en) | 2005-11-01 | 2016-03-15 | Amgen Inc. | Isolated nucleic acid molecule encoding a fusion protein comprising an activin receptor |
| US10071135B2 (en) | 2005-11-23 | 2018-09-11 | Acceleron Pharma Inc. | Method of identifying an agent that promotes bone growth or increases bone density |
| US8486403B2 (en) | 2005-11-23 | 2013-07-16 | Acceleron Pharma, Inc. | Method of promoting bone growth by an anti-activin A antibody |
| US9480742B2 (en) | 2005-11-23 | 2016-11-01 | Acceleron Pharma Inc. | Method of promoting bone growth by an anti-actriia antibody |
| US11129873B2 (en) | 2005-11-23 | 2021-09-28 | Acceleron Pharma Inc. | Method for promoting bone growth using activin-actriia antagonists |
| US8629109B2 (en) | 2005-11-23 | 2014-01-14 | Acceleron Pharma Inc. | Method for promoting bone growth using activin-actriia antagonists |
| US9572865B2 (en) | 2005-11-23 | 2017-02-21 | Acceleron Pharma Inc. | Activin-actriia antagonists and uses for treating multiple myeloma |
| US9163075B2 (en) | 2005-11-23 | 2015-10-20 | Acceleron Pharma Inc. | Isolated polynucleotide that encodes an ActRIIa-Fc fusion polypeptide |
| US10239940B2 (en) | 2005-11-23 | 2019-03-26 | Acceleron Pharma Inc. | Method of promoting bone growth by an anti-actriia antibody |
| US8128933B2 (en) | 2005-11-23 | 2012-03-06 | Acceleron Pharma, Inc. | Method of promoting bone growth by an anti-activin B antibody |
| US10093707B2 (en) | 2006-12-18 | 2018-10-09 | Acceleron Pharma Inc. | Antagonists of activin-ActRIIa and uses for increasing red blood cell levels |
| US8895016B2 (en) | 2006-12-18 | 2014-11-25 | Acceleron Pharma, Inc. | Antagonists of activin-actriia and uses for increasing red blood cell levels |
| US9526759B2 (en) | 2007-02-01 | 2016-12-27 | Acceleron Pharma Inc. | Activin-actriia antagonists and uses for treating or preventing breast cancer |
| US10259861B2 (en) | 2007-02-02 | 2019-04-16 | Acceleron Pharma Inc. | Variants derived from ActRIIB and uses therefor |
| US9399669B2 (en) | 2007-02-02 | 2016-07-26 | Acceleron Pharma Inc. | Variants derived from ActRIIB |
| US8343933B2 (en) | 2007-02-02 | 2013-01-01 | Acceleron Pharma, Inc. | Variants derived from ActRIIB and uses therefor |
| US8173601B2 (en) | 2007-02-09 | 2012-05-08 | Acceleron Pharma, Inc. | Activin-ActRIIa antagonists and uses for treating multiple myeloma |
| US9809638B2 (en) | 2007-03-06 | 2017-11-07 | Amgen Inc. | Variant activin receptor |
| US8999917B2 (en) | 2007-03-06 | 2015-04-07 | Amgen Inc. | Variant activin receptor polypeptides and uses thereof |
| US9447165B2 (en) | 2007-03-06 | 2016-09-20 | Amgen Inc. | Variant activin IIB receptor |
| US8716459B2 (en) | 2007-03-06 | 2014-05-06 | Amgen Inc. | Isolated nucleic acid molecules encoding variant activin receptor polypeptides |
| US8501678B2 (en) | 2007-03-06 | 2013-08-06 | Atara Biotherapeutics, Inc. | Variant activin receptor polypeptides and uses thereof |
| US10407487B2 (en) | 2007-03-06 | 2019-09-10 | Amgen Inc. | Variant activin receptor |
| US9610327B2 (en) | 2007-03-06 | 2017-04-04 | Amgen Inc. | Variant activin receptor polypeptides, alone or in combination with chemotherapy, and uses thereof |
| US9353356B2 (en) | 2007-09-18 | 2016-05-31 | Acceleron Pharma Inc. | Activin-actriia antagonists for treating a follicle-stimulating horomone-secreting pituitary tumor |
| US8367611B2 (en) | 2007-09-18 | 2013-02-05 | Acceleron Pharma Inc. | Activin-actriia antagonists for inhibiting germ cell maturation |
| US9439945B2 (en) | 2008-08-14 | 2016-09-13 | Acceleron Pharma Inc. | Isolated nucleotide sequences encoding GDF traps |
| US8216997B2 (en) | 2008-08-14 | 2012-07-10 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators |
| US8703927B2 (en) | 2008-08-14 | 2014-04-22 | Acceleron Pharma Inc. | Isolated nucleotide sequences encoding GDF traps |
| US10829532B2 (en) | 2008-08-14 | 2020-11-10 | Acceleron Pharma Inc. | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| US8361957B2 (en) | 2008-08-14 | 2013-01-29 | Acceleron Pharma, Inc. | Isolated GDF trap polypeptide |
| US10829533B2 (en) | 2008-08-14 | 2020-11-10 | Acceleron Pharma Inc. | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| US10889626B2 (en) | 2008-08-14 | 2021-01-12 | Acceleron Pharma Inc. | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| US9932379B2 (en) | 2008-08-14 | 2018-04-03 | Acceleron Pharma Inc. | Isolated nucleotide sequences encoding GDF traps |
| US11155791B2 (en) | 2008-08-14 | 2021-10-26 | Acceleron Pharma Inc. | Methods for treating anemia in a subject in need thereof |
| US9505813B2 (en) | 2008-08-14 | 2016-11-29 | Acceleron Pharma Inc. | Use of GDF traps to treat anemia |
| US11162085B2 (en) | 2008-08-14 | 2021-11-02 | Acceleron Pharma Inc. | Methods for treating anemia in a subject in need thereof |
| US10131700B2 (en) | 2008-08-14 | 2018-11-20 | Acceleron Pharma Inc. | Methods for treating refractory anemia with ringed sideroblasts |
| US10377996B2 (en) | 2008-08-14 | 2019-08-13 | Acceleron Pharma Inc. | Methods of identifying ActRIIB variants |
| US11168311B2 (en) | 2008-08-14 | 2021-11-09 | Acceleron Pharma Inc. | Methods for treating anemia in a subject in need thereof |
| US9273114B2 (en) | 2008-11-26 | 2016-03-01 | Amgen Inc. | Stabilized receptor polypeptides and uses thereof |
| US11685770B2 (en) | 2008-11-26 | 2023-06-27 | Amgen Inc. | Stabilized receptor polypeptides and uses thereof |
| US10308704B2 (en) | 2008-11-26 | 2019-06-04 | Amgen Inc. | Isolated nucleic acid molecules encoding stabilized receptor polypeptides and uses thereof |
| US8138142B2 (en) | 2009-01-13 | 2012-03-20 | Acceleron Pharma Inc. | Methods for increasing adiponectin in a patient in need thereof |
| US8765663B2 (en) | 2009-01-13 | 2014-07-01 | Acceleron Pharma Inc. | Methods for increasing adiponectin |
| US10968282B2 (en) | 2009-06-08 | 2021-04-06 | Acceleron Pharma Inc. | Methods for screening compounds for increasing thermogenic adipocytes |
| US9790284B2 (en) | 2009-06-08 | 2017-10-17 | Acceleron Pharma Inc. | Methods for increasing thermogenic adipocytes |
| US8703694B2 (en) | 2009-06-08 | 2014-04-22 | Acceleron Pharma, Inc. | Methods for increasing thermogenic adipocytes |
| US8178488B2 (en) | 2009-06-08 | 2012-05-15 | Acceleron Pharma, Inc. | Methods for increasing thermogenic adipocytes |
| CN102656187A (en) * | 2009-06-12 | 2012-09-05 | 阿塞勒隆制药公司 | Truncated ActRIIB-FC fusion proteins |
| US10358633B2 (en) | 2009-06-12 | 2019-07-23 | Acceleron Pharma Inc. | Method for producing an ActRIIB-Fc fusion polypeptide |
| EP2440577A1 (en) * | 2009-06-12 | 2012-04-18 | Acceleron Pharma, Inc. | Truncated actriib-fc fusion proteins |
| US9181533B2 (en) | 2009-06-12 | 2015-11-10 | Acceleron Pharma, Inc. | Truncated ACTRIIB-FC fusion protein |
| AU2016213725B2 (en) * | 2009-06-12 | 2018-02-22 | Acceleron Pharma Inc. | Truncated ActRIIB-FC fusion proteins |
| US8293881B2 (en) | 2009-06-12 | 2012-10-23 | Acceleron Pharma Inc. | Isolated nucleic acid encoding a truncated ActRIIB fusion protein |
| US9745559B2 (en) | 2009-06-12 | 2017-08-29 | Acceleron Pharma Inc. | Method for decreasing the body fat content in a subject by administering an ActRIIB protein |
| EP2440577A4 (en) * | 2009-06-12 | 2013-01-23 | Acceleron Pharma Inc | Truncated actriib-fc fusion proteins |
| CN107267520A (en) * | 2009-06-12 | 2017-10-20 | 阿塞勒隆制药公司 | The ACTRIIB FC fusion proteins of truncation |
| CN104805105A (en) * | 2009-06-12 | 2015-07-29 | 阿塞勒隆制药公司 | Truncated ACTRIIB-FC fusion proteins |
| AU2010263182B2 (en) * | 2009-06-12 | 2016-05-12 | Acceleron Pharma Inc. | Truncated ActRIIB-Fc fusion proteins |
| EP3805259A1 (en) * | 2009-06-12 | 2021-04-14 | Acceleron Pharma Inc. | Truncated actriib-fc fusion proteins |
| US11066654B2 (en) | 2009-06-12 | 2021-07-20 | Acceleron Pharma Inc. | Methods and compositions for reducing serum lipids |
| AU2010282361B2 (en) * | 2009-08-13 | 2015-03-19 | Acceleron Pharma Inc. | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| EP2464369A1 (en) * | 2009-08-13 | 2012-06-20 | Acceleron Pharma, Inc. | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| AU2019204127B2 (en) * | 2009-08-13 | 2021-08-12 | Acceleron Pharma Inc. | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| KR102170682B1 (en) * | 2009-08-13 | 2020-10-28 | 악셀레론 파마 인코포레이티드 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| EP2464369A4 (en) * | 2009-08-13 | 2013-07-17 | Acceleron Pharma Inc | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| CN105561295A (en) * | 2009-08-13 | 2016-05-11 | 阿塞勒隆制药公司 | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels |
| KR20190111158A (en) * | 2009-08-13 | 2019-10-01 | 악셀레론 파마 인코포레이티드 | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| EP3838919A1 (en) * | 2009-08-13 | 2021-06-23 | Acceleron Pharma Inc. | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| WO2011020045A1 (en) * | 2009-08-13 | 2011-02-17 | Acceleron Pharma Inc. | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| EP3117829A1 (en) * | 2009-08-13 | 2017-01-18 | Acceleron Pharma Inc. | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels |
| US10968262B2 (en) | 2009-11-17 | 2021-04-06 | Acceleron Pharma Inc. | Methods of increasing sarcolemmal utrophin |
| US8710016B2 (en) | 2009-11-17 | 2014-04-29 | Acceleron Pharma, Inc. | ActRIIB proteins and variants and uses therefore relating to utrophin induction for muscular dystrophy therapy |
| US9617319B2 (en) | 2009-11-17 | 2017-04-11 | Acceleron Pharma Inc. | ActRIIB proteins and variants and uses therefore relating to utrophin induction for muscular dystrophy therapy |
| US9493556B2 (en) | 2010-11-08 | 2016-11-15 | Acceleron Pharma Inc. | Actriia binding agents and uses thereof |
| US10421969B2 (en) | 2011-10-04 | 2019-09-24 | Royal Holloway And Bedford New College | Oligomers |
| US10947536B2 (en) | 2011-10-04 | 2021-03-16 | Royal Holloway And Bedford New College | Oligomers |
| US10662431B2 (en) | 2011-10-04 | 2020-05-26 | Royal Holloway And Bedford New College | Oligomers |
| US20210207107A1 (en) * | 2011-10-17 | 2021-07-08 | Acceleron Pharma Inc. | Methods and compositions for treating ineffective erythropoiesis |
| CN103987403A (en) * | 2011-10-17 | 2014-08-13 | 阿塞勒隆制药公司 | Methods and compositions for treating ineffective erythropoiesis |
| EP3520805A1 (en) * | 2011-10-17 | 2019-08-07 | Acceleron Pharma Inc. | Compositions for treating myelofibrosis |
| CN103987403B (en) * | 2011-10-17 | 2017-12-01 | 阿塞勒隆制药公司 | Methods and compositions for treating ineffective erythropoiesis |
| EP3875104A1 (en) * | 2011-10-17 | 2021-09-08 | Acceleron Pharma Inc. | Compositions for treating myelofibrosis |
| EP2797620A4 (en) * | 2011-10-17 | 2015-05-20 | Acceleron Pharma Inc | Methods and compositions for treating ineffective erythropoiesis |
| KR20190105113A (en) * | 2011-10-17 | 2019-09-11 | 악셀레론 파마 인코포레이티드 | Methods and compositions for treating ineffective erythropoiesis |
| JP2017101060A (en) * | 2011-10-17 | 2017-06-08 | アクセルロン ファーマ, インコーポレイテッド | Methods and compositions for treating ineffective erythropoiesis |
| CN107837390A (en) * | 2011-10-17 | 2018-03-27 | 阿塞勒隆制药公司 | Methods And Compositions For Treating Ineffective Erythropoiesis |
| JP2014530253A (en) * | 2011-10-17 | 2014-11-17 | アクセルロンファーマ, インコーポレイテッド | Methods and compositions for treating ineffective erythropoiesis |
| KR102079482B1 (en) | 2011-10-17 | 2020-02-19 | 악셀레론 파마 인코포레이티드 | Methods and compositions for treating ineffective erythropoiesis |
| CN107693776A (en) * | 2011-10-17 | 2018-02-16 | 阿塞勒隆制药公司 | Methods and compositions for treating ineffective erythropoiesis |
| WO2013106175A1 (en) * | 2011-12-19 | 2013-07-18 | Amgen Inc. | Variant activin receptor polypeptides, alone or in combination with chemotherapy, and uses thereof |
| EP3351260A1 (en) | 2012-04-06 | 2018-07-25 | Acceleron Pharma Inc. | Methods and compositions for increasing red blood cells |
| EP3608419A1 (en) | 2012-10-24 | 2020-02-12 | Celgene Corporation | Biomarker for use in treating anemia |
| EP3527219A1 (en) | 2012-10-24 | 2019-08-21 | Celgene Corporation | Methods for treating anemia |
| CN112933223A (en) * | 2012-10-24 | 2021-06-11 | 细胞基因公司 | Methods for treating anemia |
| EP3964224A1 (en) | 2012-11-02 | 2022-03-09 | Celgene Corporation | Activin-actrii antagonists and uses for use in treating renal disease |
| EP3308796A1 (en) | 2012-11-02 | 2018-04-18 | Celgene Corporation | Activin-actrii antagonists and uses for treating bone and other disorders |
| US10195249B2 (en) | 2012-11-02 | 2019-02-05 | Celgene Corporation | Activin-ActRII antagonists and uses for treating bone and other disorders |
| US11541070B2 (en) | 2013-02-01 | 2023-01-03 | Atara Biotherapeutics, Inc. | Administration of an anti-activin-A compound to a subject |
| WO2015143403A1 (en) * | 2014-03-21 | 2015-09-24 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating ineffective erythropoiesis by inhibiting activin b and/or gdf11 |
| CN106659769A (en) * | 2014-03-21 | 2017-05-10 | 阿塞勒隆制药公司 | Methods for increasing red blood cell levels and treating ineffective erythropoiesis by inhibiting activin B and/or GDF11 |
| AU2015231022B2 (en) * | 2014-03-21 | 2021-02-04 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating ineffective erythropoiesis by inhibiting activin B and/or GDF11 |
| EP3808778A1 (en) | 2014-04-18 | 2021-04-21 | Acceleron Pharma Inc. | Methods for increasing red blood cell levels and treating sickle-cell disease |
| US9850298B2 (en) | 2014-06-13 | 2017-12-26 | Acceleron Pharma Inc. | Methods for treating ulcers in thalassemia syndrome with an ActRIIB polypeptide |
| US11260107B2 (en) | 2014-06-13 | 2022-03-01 | Acceleron Pharma Inc. | Methods and compositions for treating ulcers |
| US10487144B2 (en) | 2014-06-13 | 2019-11-26 | Acceleron Pharma Inc. | Methods for treating ulcers in a hemoglobinopathy anemia with a soluble actRIIB polypeptide |
| US11813308B2 (en) | 2014-10-09 | 2023-11-14 | Celgene Corporation | Treatment of cardiovascular disease using ActRII ligand traps |
| WO2016090188A1 (en) | 2014-12-03 | 2016-06-09 | Acceleron Pharma Inc. | Methods for treating myelodysplastic syndromes and sideroblastic anemias |
| US11471510B2 (en) | 2014-12-03 | 2022-10-18 | Celgene Corporation | Activin-ActRII antagonists and uses for treating anemia |
| US10829531B2 (en) | 2014-12-03 | 2020-11-10 | Acceleron Pharma Inc. | Methods for treating myelodysplastic syndromes and sideroblastic anemias |
| EP4233889A2 (en) | 2014-12-03 | 2023-08-30 | Celgene Corporation | Activin-actrii antagonists and uses for treating myelodysplastic syndrome |
| US10189882B2 (en) | 2014-12-03 | 2019-01-29 | Acceleron Pharma Inc. | Methods for treating myelodysplastic syndromes and sideroblastic anemias |
| US11015200B2 (en) | 2015-03-18 | 2021-05-25 | Sarepta Therapeutics, Inc. | Antisense-induced exon exclusion in myostatin |
| US11279746B2 (en) | 2015-04-06 | 2022-03-22 | Acceleron Pharma Inc. | ALK4:ActRIIB heteromultimers and uses thereof |
| AU2016246703B2 (en) * | 2015-04-06 | 2021-02-04 | Acceleron Pharma Inc. | ALK4:ActRIIB heteromultimers and uses thereof |
| WO2016164497A1 (en) * | 2015-04-06 | 2016-10-13 | Acceleron Pharma Inc. | Alk4:actriib heteromultimers and uses thereof |
| US10227393B2 (en) | 2015-04-06 | 2019-03-12 | Acceleron Pharma Inc. | TGF-beta superfamily type I and type II receptor heteromultimers and uses thereof |
| US10906958B2 (en) | 2015-04-06 | 2021-02-02 | Acceleron Pharma Inc. | TGF-beta superfamily type I and type II receptor heteromultimers and uses thereof |
| US10738098B2 (en) | 2015-04-06 | 2020-08-11 | Acceleron Pharma Inc. | ALK4:ActRIIB heteromultimers and uses thereof |
| US10196434B2 (en) | 2015-04-06 | 2019-02-05 | Acceleron Pharma Inc. | ALK4:ActRIIB heteromultimers and uses thereof |
| EP4190805A2 (en) | 2015-05-20 | 2023-06-07 | Celgene Corporation | In vitro cell culture methods for beta-thalassemia using activin type ii receptor ligand traps |
| US10548976B2 (en) | 2015-05-20 | 2020-02-04 | Celgene Corporation | In vitro cell culture methods for beta-thalassemia using activin type II receptor ligand traps |
| EP3858993A1 (en) | 2015-10-09 | 2021-08-04 | Sarepta Therapeutics, Inc. | Compositions and methods for treating duchenne muscular dystrophy and related disorders |
| WO2017079591A2 (en) | 2015-11-04 | 2017-05-11 | Acceleron Pharma Inc. | Methods for increasing red blood cell levels and treating ineffective erythropoiesis |
| WO2017091706A1 (en) | 2015-11-23 | 2017-06-01 | Acceleron Pharma Inc. | Methods for treating eye disorders |
| US11065303B2 (en) | 2016-07-15 | 2021-07-20 | Acceleron Pharma Inc. | Compositions and methods for treating pulmonary hypertension |
| US11497794B2 (en) | 2016-07-15 | 2022-11-15 | Acceleron Pharma Inc. | Compositions and methods for treating pulmonary hypertension |
| US11219666B2 (en) | 2016-07-15 | 2022-01-11 | Acceleron Pharma Inc. | Compositions and methods for treating pulmonary hypertension |
| US10695405B2 (en) | 2016-07-15 | 2020-06-30 | Acceleron Pharma Inc. | Compositions and methods for treating pulmonary hypertension |
| US10722558B2 (en) | 2016-07-15 | 2020-07-28 | Acceleron Pharma Inc. | Compositions and methods for treating pulmonary hypertension |
| US11622992B2 (en) | 2016-07-15 | 2023-04-11 | Acceleron Pharma Inc. | Compositions and methods for treating pulmonary hypertension |
| US10946067B2 (en) | 2016-07-15 | 2021-03-16 | Acceleron Pharma Inc. | Compositions and methods for treating pulmonary hypertension |
| US11318188B2 (en) | 2016-07-15 | 2022-05-03 | Acceleron Pharma Inc. | Compositions and methods for treating pulmonary hypertension |
| US10973880B2 (en) | 2016-07-15 | 2021-04-13 | Acceleron Pharma Inc. | Compositions and methods for treating pulmonary hypertension |
| EP3523328A4 (en) * | 2016-10-05 | 2020-04-01 | Acceleron Pharma Inc. | Variant actriib proteins and uses thereof |
| WO2018067874A1 (en) | 2016-10-05 | 2018-04-12 | Acceleron Pharma Inc. | Variant actriib proteins and uses thereof |
| KR20190073414A (en) * | 2016-10-05 | 2019-06-26 | 악셀레론 파마 인코포레이티드 | Mutant ACTRIIB protein and uses thereof |
| US10934532B2 (en) | 2016-10-05 | 2021-03-02 | Acceleron Pharma Inc. | ALK4.ActRIIB heteromultimers |
| US11267865B2 (en) | 2016-10-05 | 2022-03-08 | Acceleron Pharma Inc. | Variant ActRIIB proteins and uses thereof |
| KR102595559B1 (en) | 2016-10-05 | 2023-10-30 | 악셀레론 파마 인코포레이티드 | Variant ACTRIIB proteins and uses thereof |
| US12054753B2 (en) | 2016-10-05 | 2024-08-06 | Acceleron Pharma Inc. | Methods of treatment of muscular dystrophy with ALK4:ActRIIB heteromultimers |
| WO2018192973A1 (en) | 2017-04-18 | 2018-10-25 | Vifor (International) Ag | Ferroportin-inhibitor salts |
| WO2021158675A1 (en) * | 2020-02-03 | 2021-08-12 | Acceleron Pharma Inc. | Variant actriib proteins and uses thereof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11168311B2 (en) | Methods for treating anemia in a subject in need thereof | |
| US10889626B2 (en) | Combined use of GDF traps and erythropoietin receptor activators to increase red blood cell levels | |
| WO2011020045A9 (en) | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels | |
| AU2017204230B2 (en) | Use of GDF traps to increase red blood cell levels |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 09806981 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2011523000 Country of ref document: JP Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2733911 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2009282441 Country of ref document: AU |
|
| ENP | Entry into the national phase |
Ref document number: 2009282441 Country of ref document: AU Date of ref document: 20090813 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2009806981 Country of ref document: EP |