WO2008117062A1 - Bioeffective krill oil compositions - Google Patents
Bioeffective krill oil compositions Download PDFInfo
- Publication number
- WO2008117062A1 WO2008117062A1 PCT/GB2008/001080 GB2008001080W WO2008117062A1 WO 2008117062 A1 WO2008117062 A1 WO 2008117062A1 GB 2008001080 W GB2008001080 W GB 2008001080W WO 2008117062 A1 WO2008117062 A1 WO 2008117062A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- krill
- composition
- phospholipids
- oil
- fatty acids
- Prior art date
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 227
- 229940106134 krill oil Drugs 0.000 title claims abstract description 170
- 241000239366 Euphausiacea Species 0.000 claims abstract description 164
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims abstract description 92
- 150000003904 phospholipids Chemical class 0.000 claims abstract description 79
- 235000012054 meals Nutrition 0.000 claims abstract description 78
- 238000000034 method Methods 0.000 claims abstract description 74
- 235000020660 omega-3 fatty acid Nutrition 0.000 claims abstract description 67
- 150000002632 lipids Chemical class 0.000 claims abstract description 63
- 239000003921 oil Substances 0.000 claims abstract description 43
- 239000000284 extract Substances 0.000 claims abstract description 41
- 230000007935 neutral effect Effects 0.000 claims abstract description 36
- 150000001514 astaxanthins Chemical class 0.000 claims abstract description 26
- 230000008569 process Effects 0.000 claims abstract description 25
- 238000000194 supercritical-fluid extraction Methods 0.000 claims abstract description 23
- 239000000463 material Substances 0.000 claims abstract description 20
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims abstract description 18
- 210000004369 blood Anatomy 0.000 claims abstract description 16
- 239000008280 blood Substances 0.000 claims abstract description 16
- 239000006184 cosolvent Substances 0.000 claims abstract description 13
- 206010022489 Insulin Resistance Diseases 0.000 claims abstract description 11
- 206010061218 Inflammation Diseases 0.000 claims abstract description 10
- 230000004054 inflammatory process Effects 0.000 claims abstract description 10
- 229940012843 omega-3 fatty acid Drugs 0.000 claims description 62
- 239000006014 omega-3 oil Substances 0.000 claims description 59
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 50
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 claims description 47
- 239000001168 astaxanthin Substances 0.000 claims description 46
- 229940022405 astaxanthin Drugs 0.000 claims description 46
- -1 ether phospholipids Chemical class 0.000 claims description 46
- JEBFVOLFMLUKLF-IFPLVEIFSA-N Astaxanthin Natural products CC(=C/C=C/C(=C/C=C/C1=C(C)C(=O)C(O)CC1(C)C)/C)C=CC=C(/C)C=CC=C(/C)C=CC2=C(C)C(=O)C(O)CC2(C)C JEBFVOLFMLUKLF-IFPLVEIFSA-N 0.000 claims description 44
- 235000013793 astaxanthin Nutrition 0.000 claims description 44
- MQZIGYBFDRPAKN-ZWAPEEGVSA-N astaxanthin Chemical compound C([C@H](O)C(=O)C=1C)C(C)(C)C=1/C=C/C(/C)=C/C=C/C(/C)=C/C=C/C=C(C)C=CC=C(C)C=CC1=C(C)C(=O)[C@@H](O)CC1(C)C MQZIGYBFDRPAKN-ZWAPEEGVSA-N 0.000 claims description 44
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 43
- 150000004665 fatty acids Chemical class 0.000 claims description 43
- 239000000194 fatty acid Substances 0.000 claims description 42
- 229930195729 fatty acid Natural products 0.000 claims description 42
- 238000000605 extraction Methods 0.000 claims description 36
- 241000239370 Euphausia superba Species 0.000 claims description 33
- 229910002092 carbon dioxide Inorganic materials 0.000 claims description 31
- 239000000047 product Substances 0.000 claims description 31
- 150000003626 triacylglycerols Chemical class 0.000 claims description 28
- 235000005911 diet Nutrition 0.000 claims description 24
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims description 20
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 claims description 20
- 102000004169 proteins and genes Human genes 0.000 claims description 19
- 108090000623 proteins and genes Proteins 0.000 claims description 19
- 238000011282 treatment Methods 0.000 claims description 19
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 claims description 18
- 210000003205 muscle Anatomy 0.000 claims description 18
- 238000004925 denaturation Methods 0.000 claims description 17
- 230000036425 denaturation Effects 0.000 claims description 17
- 239000001569 carbon dioxide Substances 0.000 claims description 16
- 230000037213 diet Effects 0.000 claims description 16
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims description 13
- 230000003247 decreasing effect Effects 0.000 claims description 13
- 230000001965 increasing effect Effects 0.000 claims description 13
- 102000011690 Adiponectin Human genes 0.000 claims description 12
- 108010076365 Adiponectin Proteins 0.000 claims description 12
- 230000015556 catabolic process Effects 0.000 claims description 11
- CYQFCXCEBYINGO-IAGOWNOFSA-N delta1-THC Chemical group C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@@H]21 CYQFCXCEBYINGO-IAGOWNOFSA-N 0.000 claims description 11
- 235000019197 fats Nutrition 0.000 claims description 11
- 230000009965 odorless effect Effects 0.000 claims description 11
- 210000002966 serum Anatomy 0.000 claims description 11
- 239000002904 solvent Substances 0.000 claims description 10
- 102000004877 Insulin Human genes 0.000 claims description 9
- 108090001061 Insulin Proteins 0.000 claims description 9
- 235000012000 cholesterol Nutrition 0.000 claims description 9
- 229940125396 insulin Drugs 0.000 claims description 9
- 239000002798 polar solvent Substances 0.000 claims description 9
- 230000009467 reduction Effects 0.000 claims description 9
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 claims description 8
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 claims description 8
- 206010060378 Hyperinsulinaemia Diseases 0.000 claims description 8
- YZXBAPSDXZZRGB-DOFZRALJSA-N arachidonic acid Chemical compound CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O YZXBAPSDXZZRGB-DOFZRALJSA-N 0.000 claims description 8
- 235000015872 dietary supplement Nutrition 0.000 claims description 8
- 235000021588 free fatty acids Nutrition 0.000 claims description 8
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 claims description 8
- 206010019708 Hepatic steatosis Diseases 0.000 claims description 7
- 239000002775 capsule Substances 0.000 claims description 7
- 150000001875 compounds Chemical class 0.000 claims description 7
- 230000003451 hyperinsulinaemic effect Effects 0.000 claims description 7
- 201000008980 hyperinsulinism Diseases 0.000 claims description 7
- 241000894007 species Species 0.000 claims description 7
- 208000004880 Polyuria Diseases 0.000 claims description 6
- 238000010438 heat treatment Methods 0.000 claims description 6
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 claims description 5
- 206010020880 Hypertrophy Diseases 0.000 claims description 5
- 241001465754 Metazoa Species 0.000 claims description 5
- 230000035619 diuresis Effects 0.000 claims description 5
- 150000008104 phosphatidylethanolamines Chemical class 0.000 claims description 5
- 230000002265 prevention Effects 0.000 claims description 5
- 102000004190 Enzymes Human genes 0.000 claims description 4
- 108090000790 Enzymes Proteins 0.000 claims description 4
- 229940114079 arachidonic acid Drugs 0.000 claims description 4
- 235000021342 arachidonic acid Nutrition 0.000 claims description 4
- 150000002170 ethers Chemical class 0.000 claims description 4
- 239000003607 modifier Substances 0.000 claims description 4
- 230000036542 oxidative stress Effects 0.000 claims description 4
- 239000002028 Biomass Substances 0.000 claims description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 3
- 102000015439 Phospholipases Human genes 0.000 claims description 3
- 108010064785 Phospholipases Proteins 0.000 claims description 3
- 241000324401 Superba Species 0.000 claims description 3
- 239000004784 Superba Substances 0.000 claims description 3
- 230000001939 inductive effect Effects 0.000 claims description 3
- 240000008791 Antiaris toxicaria Species 0.000 claims description 2
- 208000004930 Fatty Liver Diseases 0.000 claims description 2
- 238000002481 ethanol extraction Methods 0.000 claims description 2
- 238000000227 grinding Methods 0.000 claims description 2
- 238000005086 pumping Methods 0.000 claims description 2
- 230000004083 survival effect Effects 0.000 claims description 2
- 208000010706 fatty liver disease Diseases 0.000 claims 1
- 231100000240 steatosis hepatitis Toxicity 0.000 claims 1
- 230000003078 antioxidant effect Effects 0.000 abstract description 7
- 235000019198 oils Nutrition 0.000 description 37
- 230000000694 effects Effects 0.000 description 15
- 210000000577 adipose tissue Anatomy 0.000 description 14
- 235000021323 fish oil Nutrition 0.000 description 14
- 238000012545 processing Methods 0.000 description 13
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 11
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 11
- 238000011680 zucker rat Methods 0.000 description 11
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 10
- 239000008103 glucose Substances 0.000 description 10
- 235000009200 high fat diet Nutrition 0.000 description 10
- KSMVZQYAVGTKIV-UHFFFAOYSA-N decanal Chemical compound CCCCCCCCCC=O KSMVZQYAVGTKIV-UHFFFAOYSA-N 0.000 description 9
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Chemical compound CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 8
- 208000008589 Obesity Diseases 0.000 description 8
- 239000003963 antioxidant agent Substances 0.000 description 8
- 235000006708 antioxidants Nutrition 0.000 description 8
- 230000000378 dietary effect Effects 0.000 description 8
- 210000004185 liver Anatomy 0.000 description 8
- 235000020824 obesity Nutrition 0.000 description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 8
- 238000004458 analytical method Methods 0.000 description 7
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 6
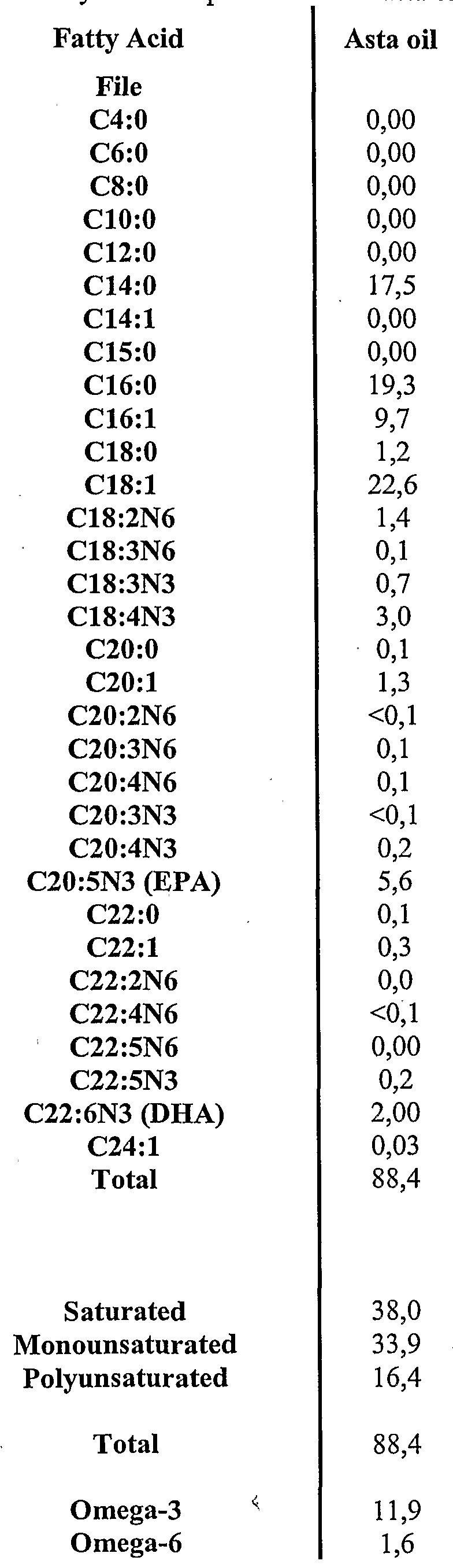
- 241001132374 Asta Species 0.000 description 6
- 208000001145 Metabolic Syndrome Diseases 0.000 description 6
- 241000699670 Mus sp. Species 0.000 description 6
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 6
- WTEVQBCEXWBHNA-JXMROGBWSA-N geranial Chemical compound CC(C)=CCC\C(C)=C\C=O WTEVQBCEXWBHNA-JXMROGBWSA-N 0.000 description 6
- 229910052500 inorganic mineral Inorganic materials 0.000 description 6
- 235000010755 mineral Nutrition 0.000 description 6
- 239000011707 mineral Substances 0.000 description 6
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 6
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Chemical compound [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 description 6
- 238000003860 storage Methods 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 5
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 5
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 description 5
- 229940088597 hormone Drugs 0.000 description 5
- 239000005556 hormone Substances 0.000 description 5
- 239000004615 ingredient Substances 0.000 description 5
- 230000006372 lipid accumulation Effects 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- SATCULPHIDQDRE-UHFFFAOYSA-N piperonal Chemical compound O=CC1=CC=C2OCOC2=C1 SATCULPHIDQDRE-UHFFFAOYSA-N 0.000 description 5
- 230000009469 supplementation Effects 0.000 description 5
- 210000001519 tissue Anatomy 0.000 description 5
- 229940088594 vitamin Drugs 0.000 description 5
- 229930003231 vitamin Natural products 0.000 description 5
- 235000013343 vitamin Nutrition 0.000 description 5
- 239000011782 vitamin Substances 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 4
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 4
- 240000004160 Capsicum annuum Species 0.000 description 4
- 235000009421 Myristica fragrans Nutrition 0.000 description 4
- 206010033307 Overweight Diseases 0.000 description 4
- LQKRYVGRPXFFAV-UHFFFAOYSA-N Phenylmethylglycidic ester Chemical compound CCOC(=O)C1OC1(C)C1=CC=CC=C1 LQKRYVGRPXFFAV-UHFFFAOYSA-N 0.000 description 4
- AUNGANRZJHBGPY-SCRDCRAPSA-N Riboflavin Chemical compound OC[C@@H](O)[C@@H](O)[C@@H](O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-SCRDCRAPSA-N 0.000 description 4
- 244000178231 Rosmarinus officinalis Species 0.000 description 4
- 235000010323 ascorbic acid Nutrition 0.000 description 4
- HUMNYLRZRPPJDN-UHFFFAOYSA-N benzaldehyde Chemical compound O=CC1=CC=CC=C1 HUMNYLRZRPPJDN-UHFFFAOYSA-N 0.000 description 4
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 description 4
- ULDHMXUKGWMISQ-UHFFFAOYSA-N carvone Chemical compound CC(=C)C1CC=C(C)C(=O)C1 ULDHMXUKGWMISQ-UHFFFAOYSA-N 0.000 description 4
- RMRCNWBMXRMIRW-BYFNXCQMSA-M cyanocobalamin Chemical compound N#C[Co+]N([C@]1([H])[C@H](CC(N)=O)[C@]\2(CCC(=O)NC[C@H](C)OP(O)(=O)OC3[C@H]([C@H](O[C@@H]3CO)N3C4=CC(C)=C(C)C=C4N=C3)O)C)C/2=C(C)\C([C@H](C/2(C)C)CCC(N)=O)=N\C\2=C\C([C@H]([C@@]/2(CC(N)=O)C)CCC(N)=O)=N\C\2=C(C)/C2=N[C@]1(C)[C@@](C)(CC(N)=O)[C@@H]2CCC(N)=O RMRCNWBMXRMIRW-BYFNXCQMSA-M 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 238000001704 evaporation Methods 0.000 description 4
- 230000008020 evaporation Effects 0.000 description 4
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 4
- 235000013305 food Nutrition 0.000 description 4
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 4
- 229920006395 saturated elastomer Polymers 0.000 description 4
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 3
- 235000008534 Capsicum annuum var annuum Nutrition 0.000 description 3
- 240000008574 Capsicum frutescens Species 0.000 description 3
- 235000002568 Capsicum frutescens Nutrition 0.000 description 3
- WTEVQBCEXWBHNA-UHFFFAOYSA-N Citral Natural products CC(C)=CCCC(C)=CC=O WTEVQBCEXWBHNA-UHFFFAOYSA-N 0.000 description 3
- 235000015655 Crocus sativus Nutrition 0.000 description 3
- 244000124209 Crocus sativus Species 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- SQUHHTBVTRBESD-UHFFFAOYSA-N Hexa-Ac-myo-Inositol Natural products CC(=O)OC1C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C1OC(C)=O SQUHHTBVTRBESD-UHFFFAOYSA-N 0.000 description 3
- 238000008214 LDL Cholesterol Methods 0.000 description 3
- 239000005913 Maltodextrin Substances 0.000 description 3
- 229920002774 Maltodextrin Polymers 0.000 description 3
- 244000270834 Myristica fragrans Species 0.000 description 3
- 238000005481 NMR spectroscopy Methods 0.000 description 3
- 229930003427 Vitamin E Natural products 0.000 description 3
- 235000006886 Zingiber officinale Nutrition 0.000 description 3
- 244000273928 Zingiber officinale Species 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 230000003110 anti-inflammatory effect Effects 0.000 description 3
- 239000011668 ascorbic acid Substances 0.000 description 3
- 229960005070 ascorbic acid Drugs 0.000 description 3
- 230000037396 body weight Effects 0.000 description 3
- 210000004556 brain Anatomy 0.000 description 3
- 239000001506 calcium phosphate Substances 0.000 description 3
- 239000001511 capsicum annuum Substances 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 235000020940 control diet Nutrition 0.000 description 3
- 229940109239 creatinine Drugs 0.000 description 3
- 239000012467 final product Substances 0.000 description 3
- 235000019152 folic acid Nutrition 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 229960003692 gamma aminobutyric acid Drugs 0.000 description 3
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 3
- 239000000499 gel Substances 0.000 description 3
- 235000008397 ginger Nutrition 0.000 description 3
- 230000004968 inflammatory condition Effects 0.000 description 3
- 229960000367 inositol Drugs 0.000 description 3
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 description 3
- 230000003907 kidney function Effects 0.000 description 3
- CDOSHBSSFJOMGT-UHFFFAOYSA-N linalool Chemical compound CC(C)=CCCC(C)(O)C=C CDOSHBSSFJOMGT-UHFFFAOYSA-N 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 229940035034 maltodextrin Drugs 0.000 description 3
- 230000002503 metabolic effect Effects 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 150000003905 phosphatidylinositols Chemical class 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 230000004844 protein turnover Effects 0.000 description 3
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 description 3
- 235000019165 vitamin E Nutrition 0.000 description 3
- 229940046009 vitamin E Drugs 0.000 description 3
- 239000011709 vitamin E Substances 0.000 description 3
- 239000013585 weight reducing agent Substances 0.000 description 3
- GHOKWGTUZJEAQD-ZETCQYMHSA-N (D)-(+)-Pantothenic acid Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-ZETCQYMHSA-N 0.000 description 2
- KJPRLNWUNMBNBZ-QPJJXVBHSA-N (E)-cinnamaldehyde Chemical compound O=C\C=C\C1=CC=CC=C1 KJPRLNWUNMBNBZ-QPJJXVBHSA-N 0.000 description 2
- RYCNUMLMNKHWPZ-SNVBAGLBSA-N 1-acetyl-sn-glycero-3-phosphocholine Chemical compound CC(=O)OC[C@@H](O)COP([O-])(=O)OCC[N+](C)(C)C RYCNUMLMNKHWPZ-SNVBAGLBSA-N 0.000 description 2
- FPIPGXGPPPQFEQ-UHFFFAOYSA-N 13-cis retinol Natural products OCC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-UHFFFAOYSA-N 0.000 description 2
- WVXRAFOPTSTNLL-NKWVEPMBSA-N 2',3'-dideoxyadenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@H]1CC[C@@H](CO)O1 WVXRAFOPTSTNLL-NKWVEPMBSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 2
- 244000016163 Allium sibiricum Species 0.000 description 2
- 235000001270 Allium sibiricum Nutrition 0.000 description 2
- 241000189429 Angostura Species 0.000 description 2
- 235000007258 Anthriscus cerefolium Nutrition 0.000 description 2
- 240000002022 Anthriscus cerefolium Species 0.000 description 2
- 235000011330 Armoracia rusticana Nutrition 0.000 description 2
- 240000003291 Armoracia rusticana Species 0.000 description 2
- 235000003092 Artemisia dracunculus Nutrition 0.000 description 2
- 240000001851 Artemisia dracunculus Species 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 240000001432 Calendula officinalis Species 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- 235000005747 Carum carvi Nutrition 0.000 description 2
- 240000000467 Carum carvi Species 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- 244000037364 Cinnamomum aromaticum Species 0.000 description 2
- 235000014489 Cinnamomum aromaticum Nutrition 0.000 description 2
- 235000002787 Coriandrum sativum Nutrition 0.000 description 2
- 244000018436 Coriandrum sativum Species 0.000 description 2
- 235000007129 Cuminum cyminum Nutrition 0.000 description 2
- 244000304337 Cuminum cyminum Species 0.000 description 2
- 244000163122 Curcuma domestica Species 0.000 description 2
- 235000003405 Curcuma zedoaria Nutrition 0.000 description 2
- 240000009138 Curcuma zedoaria Species 0.000 description 2
- AUNGANRZJHBGPY-UHFFFAOYSA-N D-Lyxoflavin Natural products OCC(O)C(O)C(O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-UHFFFAOYSA-N 0.000 description 2
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 description 2
- 240000002943 Elettaria cardamomum Species 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 241000195955 Equisetum hyemale Species 0.000 description 2
- 244000024675 Eruca sativa Species 0.000 description 2
- 239000004258 Ethoxyquin Substances 0.000 description 2
- 241001625807 Euphausia frigida Species 0.000 description 2
- 241000212919 Euphausia vallentini Species 0.000 description 2
- 240000006927 Foeniculum vulgare Species 0.000 description 2
- 235000004204 Foeniculum vulgare Nutrition 0.000 description 2
- GLZPCOQZEFWAFX-UHFFFAOYSA-N Geraniol Chemical compound CC(C)=CCCC(C)=CCO GLZPCOQZEFWAFX-UHFFFAOYSA-N 0.000 description 2
- 235000010650 Hyssopus officinalis Nutrition 0.000 description 2
- 240000001812 Hyssopus officinalis Species 0.000 description 2
- 235000008227 Illicium verum Nutrition 0.000 description 2
- 240000007232 Illicium verum Species 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- 150000000994 L-ascorbates Chemical class 0.000 description 2
- 244000165082 Lavanda vera Species 0.000 description 2
- 235000010663 Lavandula angustifolia Nutrition 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- 235000014837 Malpighia glabra Nutrition 0.000 description 2
- 240000003394 Malpighia glabra Species 0.000 description 2
- 244000137850 Marrubium vulgare Species 0.000 description 2
- 235000005321 Marrubium vulgare Nutrition 0.000 description 2
- 240000004658 Medicago sativa Species 0.000 description 2
- 241000212925 Meganyctiphanes norvegica Species 0.000 description 2
- 244000062730 Melissa officinalis Species 0.000 description 2
- 235000010654 Melissa officinalis Nutrition 0.000 description 2
- 235000014749 Mentha crispa Nutrition 0.000 description 2
- 235000004357 Mentha x piperita Nutrition 0.000 description 2
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 2
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 2
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 2
- DFPAKSUCGFBDDF-UHFFFAOYSA-N Nicotinamide Chemical compound NC(=O)C1=CC=CN=C1 DFPAKSUCGFBDDF-UHFFFAOYSA-N 0.000 description 2
- 235000010676 Ocimum basilicum Nutrition 0.000 description 2
- 240000007926 Ocimum gratissimum Species 0.000 description 2
- 240000000783 Origanum majorana Species 0.000 description 2
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 2
- 235000006990 Pimenta dioica Nutrition 0.000 description 2
- 240000008474 Pimenta dioica Species 0.000 description 2
- 235000012550 Pimpinella anisum Nutrition 0.000 description 2
- 240000004760 Pimpinella anisum Species 0.000 description 2
- 235000008184 Piper nigrum Nutrition 0.000 description 2
- 244000203593 Piper nigrum Species 0.000 description 2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 241000220317 Rosa Species 0.000 description 2
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 2
- 244000000231 Sesamum indicum Species 0.000 description 2
- 235000003434 Sesamum indicum Nutrition 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- 244000269722 Thea sinensis Species 0.000 description 2
- 235000007303 Thymus vulgaris Nutrition 0.000 description 2
- 240000002657 Thymus vulgaris Species 0.000 description 2
- 241001534900 Thysanoessa inermis Species 0.000 description 2
- 241000680651 Thysanoessa raschii Species 0.000 description 2
- 235000001484 Trigonella foenum graecum Nutrition 0.000 description 2
- 244000250129 Trigonella foenum graecum Species 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 244000290333 Vanilla fragrans Species 0.000 description 2
- 235000009499 Vanilla fragrans Nutrition 0.000 description 2
- FPIPGXGPPPQFEQ-BOOMUCAASA-N Vitamin A Natural products OC/C=C(/C)\C=C\C=C(\C)/C=C/C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-BOOMUCAASA-N 0.000 description 2
- 229930003268 Vitamin C Natural products 0.000 description 2
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- PNNCWTXUWKENPE-UHFFFAOYSA-N [N].NC(N)=O Chemical compound [N].NC(N)=O PNNCWTXUWKENPE-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- OENHQHLEOONYIE-UKMVMLAPSA-N all-trans beta-carotene Natural products CC=1CCCC(C)(C)C=1/C=C/C(/C)=C/C=C/C(/C)=C/C=C/C=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C OENHQHLEOONYIE-UKMVMLAPSA-N 0.000 description 2
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 2
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 2
- DTOSIQBPPRVQHS-PDBXOOCHSA-N alpha-linolenic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCC(O)=O DTOSIQBPPRVQHS-PDBXOOCHSA-N 0.000 description 2
- RDOXTESZEPMUJZ-UHFFFAOYSA-N anisole Chemical compound COC1=CC=CC=C1 RDOXTESZEPMUJZ-UHFFFAOYSA-N 0.000 description 2
- 239000001387 apium graveolens Substances 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 235000013734 beta-carotene Nutrition 0.000 description 2
- 239000011648 beta-carotene Substances 0.000 description 2
- TUPZEYHYWIEDIH-WAIFQNFQSA-N beta-carotene Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CCCC1(C)C)C=CC=C(/C)C=CC2=CCCCC2(C)C TUPZEYHYWIEDIH-WAIFQNFQSA-N 0.000 description 2
- 229960002747 betacarotene Drugs 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 229910000019 calcium carbonate Inorganic materials 0.000 description 2
- 235000010216 calcium carbonate Nutrition 0.000 description 2
- FDSDTBUPSURDBL-LOFNIBRQSA-N canthaxanthin Chemical compound CC=1C(=O)CCC(C)(C)C=1/C=C/C(/C)=C/C=C/C(/C)=C/C=C/C=C(C)C=CC=C(C)C=CC1=C(C)C(=O)CCC1(C)C FDSDTBUPSURDBL-LOFNIBRQSA-N 0.000 description 2
- 239000001728 capsicum frutescens Substances 0.000 description 2
- KJPRLNWUNMBNBZ-UHFFFAOYSA-N cinnamic aldehyde Natural products O=CC=CC1=CC=CC=C1 KJPRLNWUNMBNBZ-UHFFFAOYSA-N 0.000 description 2
- 229940117916 cinnamic aldehyde Drugs 0.000 description 2
- 230000001276 controlling effect Effects 0.000 description 2
- 235000003373 curcuma longa Nutrition 0.000 description 2
- VFLDPWHFBUODDF-FCXRPNKRSA-N curcumin Chemical compound C1=C(O)C(OC)=CC(\C=C\C(=O)CC(=O)\C=C\C=2C=C(OC)C(O)=CC=2)=C1 VFLDPWHFBUODDF-FCXRPNKRSA-N 0.000 description 2
- 229960002104 cyanocobalamin Drugs 0.000 description 2
- 235000000639 cyanocobalamin Nutrition 0.000 description 2
- 239000011666 cyanocobalamin Substances 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- FMGSKLZLMKYGDP-USOAJAOKSA-N dehydroepiandrosterone Chemical compound C1[C@@H](O)CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC=C21 FMGSKLZLMKYGDP-USOAJAOKSA-N 0.000 description 2
- 239000002702 enteric coating Substances 0.000 description 2
- 238000009505 enteric coating Methods 0.000 description 2
- 235000019285 ethoxyquin Nutrition 0.000 description 2
- 229940093500 ethoxyquin Drugs 0.000 description 2
- DECIPOUIJURFOJ-UHFFFAOYSA-N ethoxyquin Chemical compound N1C(C)(C)C=C(C)C2=CC(OCC)=CC=C21 DECIPOUIJURFOJ-UHFFFAOYSA-N 0.000 description 2
- CBOQJANXLMLOSS-UHFFFAOYSA-N ethyl vanillin Chemical compound CCOC1=CC(C=O)=CC=C1O CBOQJANXLMLOSS-UHFFFAOYSA-N 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000000796 flavoring agent Substances 0.000 description 2
- 235000019634 flavors Nutrition 0.000 description 2
- 235000013312 flour Nutrition 0.000 description 2
- 229960000304 folic acid Drugs 0.000 description 2
- 239000011724 folic acid Substances 0.000 description 2
- 238000004108 freeze drying Methods 0.000 description 2
- 238000002290 gas chromatography-mass spectrometry Methods 0.000 description 2
- HIGQPQRQIQDZMP-DHZHZOJOSA-N geranyl acetate Chemical compound CC(C)=CCC\C(C)=C\COC(C)=O HIGQPQRQIQDZMP-DHZHZOJOSA-N 0.000 description 2
- 230000002641 glycemic effect Effects 0.000 description 2
- 125000005456 glyceride group Chemical group 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N glycerol group Chemical group OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 235000013761 grape skin extract Nutrition 0.000 description 2
- 229940060367 inert ingredients Drugs 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 150000002576 ketones Chemical class 0.000 description 2
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 2
- UWKAYLJWKGQEPM-UHFFFAOYSA-N linalool acetate Natural products CC(C)=CCCC(C)(C=C)OC(C)=O UWKAYLJWKGQEPM-UHFFFAOYSA-N 0.000 description 2
- UWKAYLJWKGQEPM-LBPRGKRZSA-N linalyl acetate Chemical compound CC(C)=CCC[C@](C)(C=C)OC(C)=O UWKAYLJWKGQEPM-LBPRGKRZSA-N 0.000 description 2
- OYHQOLUKZRVURQ-IXWMQOLASA-N linoleic acid Natural products CCCCC\C=C/C\C=C\CCCCCCCC(O)=O OYHQOLUKZRVURQ-IXWMQOLASA-N 0.000 description 2
- KQQKGWQCNNTQJW-UHFFFAOYSA-N linolenic acid Natural products CC=CCCC=CCC=CCCCCCCCC(O)=O KQQKGWQCNNTQJW-UHFFFAOYSA-N 0.000 description 2
- 229960004488 linolenic acid Drugs 0.000 description 2
- 235000019359 magnesium stearate Nutrition 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 229960003966 nicotinamide Drugs 0.000 description 2
- 235000005152 nicotinamide Nutrition 0.000 description 2
- 239000011570 nicotinamide Substances 0.000 description 2
- 235000001968 nicotinic acid Nutrition 0.000 description 2
- 229960003512 nicotinic acid Drugs 0.000 description 2
- 239000011664 nicotinic acid Substances 0.000 description 2
- 208000008338 non-alcoholic fatty liver disease Diseases 0.000 description 2
- 230000035764 nutrition Effects 0.000 description 2
- 235000016709 nutrition Nutrition 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- 239000011574 phosphorus Substances 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- SHUZOJHMOBOZST-UHFFFAOYSA-N phylloquinone Natural products CC(C)CCCCC(C)CCC(C)CCCC(=CCC1=C(C)C(=O)c2ccccc2C1=O)C SHUZOJHMOBOZST-UHFFFAOYSA-N 0.000 description 2
- 235000020777 polyunsaturated fatty acids Nutrition 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- LXNHXLLTXMVWPM-UHFFFAOYSA-N pyridoxine Chemical compound CC1=NC=C(CO)C(CO)=C1O LXNHXLLTXMVWPM-UHFFFAOYSA-N 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 235000019192 riboflavin Nutrition 0.000 description 2
- 239000002151 riboflavin Substances 0.000 description 2
- 229960002477 riboflavin Drugs 0.000 description 2
- 239000004248 saffron Substances 0.000 description 2
- 235000013974 saffron Nutrition 0.000 description 2
- 235000002020 sage Nutrition 0.000 description 2
- 239000011669 selenium Substances 0.000 description 2
- 229910052711 selenium Inorganic materials 0.000 description 2
- 229940091258 selenium supplement Drugs 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 239000000377 silicon dioxide Substances 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- JZRWCGZRTZMZEH-UHFFFAOYSA-N thiamine Chemical compound CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N JZRWCGZRTZMZEH-UHFFFAOYSA-N 0.000 description 2
- 239000001585 thymus vulgaris Substances 0.000 description 2
- RUVINXPYWBROJD-ONEGZZNKSA-N trans-anethole Chemical compound COC1=CC=C(\C=C\C)C=C1 RUVINXPYWBROJD-ONEGZZNKSA-N 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- 235000001019 trigonella foenum-graecum Nutrition 0.000 description 2
- 235000019155 vitamin A Nutrition 0.000 description 2
- 239000011719 vitamin A Substances 0.000 description 2
- 235000019154 vitamin C Nutrition 0.000 description 2
- 239000011718 vitamin C Substances 0.000 description 2
- 229940045997 vitamin a Drugs 0.000 description 2
- 230000004584 weight gain Effects 0.000 description 2
- 235000019786 weight gain Nutrition 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- XLOMVQKBTHCTTD-UHFFFAOYSA-N zinc oxide Inorganic materials [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 2
- OENHQHLEOONYIE-JLTXGRSLSA-N β-Carotene Chemical compound CC=1CCCC(C)(C)C=1\C=C\C(\C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C OENHQHLEOONYIE-JLTXGRSLSA-N 0.000 description 2
- OGNSCSPNOLGXSM-UHFFFAOYSA-N (+/-)-DABA Natural products NCCC(N)C(O)=O OGNSCSPNOLGXSM-UHFFFAOYSA-N 0.000 description 1
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- SBHCLVQMTBWHCD-METXMMQOSA-N (2e,4e,6e,8e,10e)-icosa-2,4,6,8,10-pentaenoic acid Chemical compound CCCCCCCCC\C=C\C=C\C=C\C=C\C=C\C(O)=O SBHCLVQMTBWHCD-METXMMQOSA-N 0.000 description 1
- MJYQFWSXKFLTAY-OVEQLNGDSA-N (2r,3r)-2,3-bis[(4-hydroxy-3-methoxyphenyl)methyl]butane-1,4-diol;(2r,3r,4s,5s,6r)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O.C1=C(O)C(OC)=CC(C[C@@H](CO)[C@H](CO)CC=2C=C(OC)C(O)=CC=2)=C1 MJYQFWSXKFLTAY-OVEQLNGDSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- BNDAMTLLUULVLF-ZIMISOLQSA-N (3E,6E)-octa-3,6-dien-1-ol Chemical compound C\C=C\C\C=C\CCO BNDAMTLLUULVLF-ZIMISOLQSA-N 0.000 description 1
- 239000001490 (3R)-3,7-dimethylocta-1,6-dien-3-ol Substances 0.000 description 1
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- KSEBMYQBYZTDHS-HWKANZROSA-M (E)-Ferulic acid Natural products COC1=CC(\C=C\C([O-])=O)=CC=C1O KSEBMYQBYZTDHS-HWKANZROSA-M 0.000 description 1
- CDOSHBSSFJOMGT-JTQLQIEISA-N (R)-linalool Natural products CC(C)=CCC[C@@](C)(O)C=C CDOSHBSSFJOMGT-JTQLQIEISA-N 0.000 description 1
- ULDHMXUKGWMISQ-VIFPVBQESA-N (S)-(+)-Carvone Natural products CC(=C)[C@H]1CC=C(C)C(=O)C1 ULDHMXUKGWMISQ-VIFPVBQESA-N 0.000 description 1
- SLKDGVPOSSLUAI-PGUFJCEWSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine zwitterion Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OCCN)OC(=O)CCCCCCCCCCCCCCC SLKDGVPOSSLUAI-PGUFJCEWSA-N 0.000 description 1
- HNAGHMKIPMKKBB-UHFFFAOYSA-N 1-benzylpyrrolidine-3-carboxamide Chemical compound C1C(C(=O)N)CCN1CC1=CC=CC=C1 HNAGHMKIPMKKBB-UHFFFAOYSA-N 0.000 description 1
- PZNPLUBHRSSFHT-RRHRGVEJSA-N 1-hexadecanoyl-2-octadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[C@@H](COP([O-])(=O)OCC[N+](C)(C)C)COC(=O)CCCCCCCCCCCCCCC PZNPLUBHRSSFHT-RRHRGVEJSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- LKUDTTGAGPETJH-UHFFFAOYSA-N 2-amino-3-methylbenzoic acid;methyl 2-aminobenzoate Chemical compound COC(=O)C1=CC=CC=C1N.CC1=CC=CC(C(O)=O)=C1N LKUDTTGAGPETJH-UHFFFAOYSA-N 0.000 description 1
- CFWRDBDJAOHXSH-SECBINFHSA-N 2-azaniumylethyl [(2r)-2,3-diacetyloxypropyl] phosphate Chemical compound CC(=O)OC[C@@H](OC(C)=O)COP(O)(=O)OCCN CFWRDBDJAOHXSH-SECBINFHSA-N 0.000 description 1
- PBQSTXCCVHCVHS-UHFFFAOYSA-N 2-methylnona-2,7-diene Chemical compound CC=CCCCC=C(C)C PBQSTXCCVHCVHS-UHFFFAOYSA-N 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- MIDXCONKKJTLDX-UHFFFAOYSA-N 3,5-dimethylcyclopentane-1,2-dione Chemical compound CC1CC(C)C(=O)C1=O MIDXCONKKJTLDX-UHFFFAOYSA-N 0.000 description 1
- PMYDPQQPEAYXKD-UHFFFAOYSA-N 3-hydroxy-n-naphthalen-2-ylnaphthalene-2-carboxamide Chemical compound C1=CC=CC2=CC(NC(=O)C3=CC4=CC=CC=C4C=C3O)=CC=C21 PMYDPQQPEAYXKD-UHFFFAOYSA-N 0.000 description 1
- YLWILEVNDBDRIU-UHFFFAOYSA-N 3-hydroxybutan-2-one;4-hydroxybutan-2-one Chemical compound CC(O)C(C)=O.CC(=O)CCO YLWILEVNDBDRIU-UHFFFAOYSA-N 0.000 description 1
- 238000004679 31P NMR spectroscopy Methods 0.000 description 1
- AJBZENLMTKDAEK-UHFFFAOYSA-N 3a,5a,5b,8,8,11a-hexamethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysene-4,9-diol Chemical compound CC12CCC(O)C(C)(C)C1CCC(C1(C)CC3O)(C)C2CCC1C1C3(C)CCC1C(=C)C AJBZENLMTKDAEK-UHFFFAOYSA-N 0.000 description 1
- AUNGANRZJHBGPY-MBNYWOFBSA-N 7,8-dimethyl-10-[(2R,3R,4S)-2,3,4,5-tetrahydroxypentyl]benzo[g]pteridine-2,4-dione Chemical compound OC[C@H](O)[C@H](O)[C@H](O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-MBNYWOFBSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- JBYXPOFIGCOSSB-GOJKSUSPSA-N 9-cis,11-trans-octadecadienoic acid Chemical compound CCCCCC\C=C\C=C/CCCCCCCC(O)=O JBYXPOFIGCOSSB-GOJKSUSPSA-N 0.000 description 1
- 208000004611 Abdominal Obesity Diseases 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 240000002234 Allium sativum Species 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 244000061520 Angelica archangelica Species 0.000 description 1
- 235000007070 Angelica archangelica Nutrition 0.000 description 1
- 240000007087 Apium graveolens Species 0.000 description 1
- 235000002764 Apium graveolens Nutrition 0.000 description 1
- 240000005528 Arctium lappa Species 0.000 description 1
- 235000003130 Arctium lappa Nutrition 0.000 description 1
- 235000008078 Arctium minus Nutrition 0.000 description 1
- 206010003210 Arteriosclerosis Diseases 0.000 description 1
- 235000016425 Arthrospira platensis Nutrition 0.000 description 1
- 240000002900 Arthrospira platensis Species 0.000 description 1
- 241000512259 Ascophyllum nodosum Species 0.000 description 1
- 108010011485 Aspartame Proteins 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 241000972773 Aulopiformes Species 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 239000004135 Bone phosphate Substances 0.000 description 1
- 241000219198 Brassica Species 0.000 description 1
- 235000006463 Brassica alba Nutrition 0.000 description 1
- 235000003351 Brassica cretica Nutrition 0.000 description 1
- 244000140786 Brassica hirta Species 0.000 description 1
- 235000011371 Brassica hirta Nutrition 0.000 description 1
- 244000178993 Brassica juncea Species 0.000 description 1
- 235000011332 Brassica juncea Nutrition 0.000 description 1
- 235000014700 Brassica juncea var napiformis Nutrition 0.000 description 1
- 235000011291 Brassica nigra Nutrition 0.000 description 1
- 244000180419 Brassica nigra Species 0.000 description 1
- 235000011299 Brassica oleracea var botrytis Nutrition 0.000 description 1
- 235000017647 Brassica oleracea var italica Nutrition 0.000 description 1
- 240000003259 Brassica oleracea var. botrytis Species 0.000 description 1
- 235000003343 Brassica rupestris Nutrition 0.000 description 1
- 241000273930 Brevoortia tyrannus Species 0.000 description 1
- 238000011740 C57BL/6 mouse Methods 0.000 description 1
- 235000014161 Caesalpinia gilliesii Nutrition 0.000 description 1
- 244000003240 Caesalpinia gilliesii Species 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 235000003880 Calendula Nutrition 0.000 description 1
- 235000005881 Calendula officinalis Nutrition 0.000 description 1
- 235000002566 Capsicum Nutrition 0.000 description 1
- 235000002567 Capsicum annuum Nutrition 0.000 description 1
- 235000002283 Capsicum annuum var aviculare Nutrition 0.000 description 1
- 235000013303 Capsicum annuum var. frutescens Nutrition 0.000 description 1
- 235000002284 Capsicum baccatum var baccatum Nutrition 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 229920000623 Cellulose acetate phthalate Polymers 0.000 description 1
- 206010065941 Central obesity Diseases 0.000 description 1
- 235000007866 Chamaemelum nobile Nutrition 0.000 description 1
- 240000003538 Chamaemelum nobile Species 0.000 description 1
- GHOKWGTUZJEAQD-UHFFFAOYSA-N Chick antidermatitis factor Natural products OCC(C)(C)C(O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-UHFFFAOYSA-N 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 229910021555 Chromium Chloride Inorganic materials 0.000 description 1
- 235000007871 Chrysanthemum coronarium Nutrition 0.000 description 1
- 244000067456 Chrysanthemum coronarium Species 0.000 description 1
- 235000021511 Cinnamomum cassia Nutrition 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- OCUCCJIRFHNWBP-IYEMJOQQSA-L Copper gluconate Chemical compound [Cu+2].OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O OCUCCJIRFHNWBP-IYEMJOQQSA-L 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- 241000195493 Cryptophyta Species 0.000 description 1
- 235000003392 Curcuma domestica Nutrition 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 241000238557 Decapoda Species 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 235000018602 Elettaria cardamomum Nutrition 0.000 description 1
- 241001632410 Eleutherococcus senticosus Species 0.000 description 1
- 241000239368 Euphausia Species 0.000 description 1
- DKKCQDROTDCQOR-UHFFFAOYSA-L Ferrous lactate Chemical compound [Fe+2].CC(O)C([O-])=O.CC(O)C([O-])=O DKKCQDROTDCQOR-UHFFFAOYSA-L 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 241000748691 Furia Species 0.000 description 1
- 239000005792 Geraniol Substances 0.000 description 1
- GLZPCOQZEFWAFX-YFHOEESVSA-N Geraniol Natural products CC(C)=CCC\C(C)=C/CO GLZPCOQZEFWAFX-YFHOEESVSA-N 0.000 description 1
- 102000034354 Gi proteins Human genes 0.000 description 1
- 108091006101 Gi proteins Proteins 0.000 description 1
- 235000008100 Ginkgo biloba Nutrition 0.000 description 1
- 244000194101 Ginkgo biloba Species 0.000 description 1
- 206010018429 Glucose tolerance impaired Diseases 0.000 description 1
- 229920002527 Glycogen Polymers 0.000 description 1
- 235000005206 Hibiscus Nutrition 0.000 description 1
- 235000007185 Hibiscus lunariifolius Nutrition 0.000 description 1
- 244000284380 Hibiscus rosa sinensis Species 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 235000004185 Hyptis suaveolens Nutrition 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 235000017858 Laurus nobilis Nutrition 0.000 description 1
- 244000147568 Laurus nobilis Species 0.000 description 1
- 235000010701 Lavanda vera Nutrition 0.000 description 1
- OYHQOLUKZRVURQ-HZJYTTRNSA-N Linoleic acid Chemical compound CCCCC\C=C/C\C=C/CCCCCCCC(O)=O OYHQOLUKZRVURQ-HZJYTTRNSA-N 0.000 description 1
- 102000004882 Lipase Human genes 0.000 description 1
- 108090001060 Lipase Proteins 0.000 description 1
- 239000004367 Lipase Substances 0.000 description 1
- UPYKUZBSLRQECL-UKMVMLAPSA-N Lycopene Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1C(=C)CCCC1(C)C)C=CC=C(/C)C=CC2C(=C)CCCC2(C)C UPYKUZBSLRQECL-UKMVMLAPSA-N 0.000 description 1
- JEVVKJMRZMXFBT-XWDZUXABSA-N Lycophyll Natural products OC/C(=C/CC/C(=C\C=C\C(=C/C=C/C(=C\C=C\C=C(/C=C/C=C(\C=C\C=C(/CC/C=C(/CO)\C)\C)/C)\C)/C)\C)/C)/C JEVVKJMRZMXFBT-XWDZUXABSA-N 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 235000010624 Medicago sativa Nutrition 0.000 description 1
- 235000017587 Medicago sativa ssp. sativa Nutrition 0.000 description 1
- ABSPRNADVQNDOU-UHFFFAOYSA-N Menaquinone 1 Natural products C1=CC=C2C(=O)C(CC=C(C)C)=C(C)C(=O)C2=C1 ABSPRNADVQNDOU-UHFFFAOYSA-N 0.000 description 1
- 244000024873 Mentha crispa Species 0.000 description 1
- 244000246386 Mentha pulegium Species 0.000 description 1
- 235000016257 Mentha pulegium Nutrition 0.000 description 1
- 244000078639 Mentha spicata Species 0.000 description 1
- 241001479543 Mentha x piperita Species 0.000 description 1
- 235000005135 Micromeria juliana Nutrition 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- 235000007265 Myrrhis odorata Nutrition 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 235000011203 Origanum Nutrition 0.000 description 1
- 235000006297 Origanum majorana Nutrition 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 241000206754 Palmaria palmata Species 0.000 description 1
- 235000008753 Papaver somniferum Nutrition 0.000 description 1
- 244000062780 Petroselinum sativum Species 0.000 description 1
- OOUTWVMJGMVRQF-DOYZGLONSA-N Phoenicoxanthin Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)C(=O)C(O)CC1(C)C)C=CC=C(/C)C=CC2=C(C)C(=O)CCC2(C)C OOUTWVMJGMVRQF-DOYZGLONSA-N 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 235000016311 Primula vulgaris Nutrition 0.000 description 1
- 244000028344 Primula vulgaris Species 0.000 description 1
- 240000007726 Pritchardia pacifica Species 0.000 description 1
- 241000241413 Propolis Species 0.000 description 1
- REFJWTPEDVJJIY-UHFFFAOYSA-N Quercetin Chemical compound C=1C(O)=CC(O)=C(C(C=2O)=O)C=1OC=2C1=CC=C(O)C(O)=C1 REFJWTPEDVJJIY-UHFFFAOYSA-N 0.000 description 1
- 240000007164 Salvia officinalis Species 0.000 description 1
- 235000002912 Salvia officinalis Nutrition 0.000 description 1
- 244000151637 Sambucus canadensis Species 0.000 description 1
- 235000018735 Sambucus canadensis Nutrition 0.000 description 1
- 235000007315 Satureja hortensis Nutrition 0.000 description 1
- 240000002114 Satureja hortensis Species 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 241000320380 Silybum Species 0.000 description 1
- 235000010841 Silybum marianum Nutrition 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 235000009337 Spinacia oleracea Nutrition 0.000 description 1
- 244000300264 Spinacia oleracea Species 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 235000012308 Tagetes Nutrition 0.000 description 1
- 241000736851 Tagetes Species 0.000 description 1
- 240000001949 Taraxacum officinale Species 0.000 description 1
- 235000005187 Taraxacum officinale ssp. officinale Nutrition 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- HXWJFEZDFPRLBG-UHFFFAOYSA-N Timnodonic acid Natural products CCCC=CC=CCC=CCC=CCC=CCCCC(O)=O HXWJFEZDFPRLBG-UHFFFAOYSA-N 0.000 description 1
- 235000015724 Trifolium pratense Nutrition 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 238000010162 Tukey test Methods 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- AXMVYSVVTMKQSL-UHFFFAOYSA-N UNPD142122 Natural products OC1=CC=C(C=CC=O)C=C1O AXMVYSVVTMKQSL-UHFFFAOYSA-N 0.000 description 1
- 235000009108 Urtica dioica Nutrition 0.000 description 1
- 241000218215 Urticaceae Species 0.000 description 1
- 235000017537 Vaccinium myrtillus Nutrition 0.000 description 1
- 244000078534 Vaccinium myrtillus Species 0.000 description 1
- 235000012036 Vanilla tahitensis Nutrition 0.000 description 1
- 229920001938 Vegetable gum Polymers 0.000 description 1
- 229930003316 Vitamin D Natural products 0.000 description 1
- 229930003448 Vitamin K Natural products 0.000 description 1
- 235000009754 Vitis X bourquina Nutrition 0.000 description 1
- 235000012333 Vitis X labruscana Nutrition 0.000 description 1
- 240000006365 Vitis vinifera Species 0.000 description 1
- 235000014787 Vitis vinifera Nutrition 0.000 description 1
- 201000010390 abdominal obesity-metabolic syndrome 1 Diseases 0.000 description 1
- 230000035508 accumulation Effects 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 125000003158 alcohol group Chemical group 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- MBMBGCFOFBJSGT-KUBAVDMBSA-N all-cis-docosa-4,7,10,13,16,19-hexaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O MBMBGCFOFBJSGT-KUBAVDMBSA-N 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 235000002783 ambrette Nutrition 0.000 description 1
- 244000096712 ambrette Species 0.000 description 1
- 235000005550 amino acid supplement Nutrition 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 229940011037 anethole Drugs 0.000 description 1
- UIERETOOQGIECD-ARJAWSKDSA-N angelic acid group Chemical group C(\C(\C)=C/C)(=O)O UIERETOOQGIECD-ARJAWSKDSA-N 0.000 description 1
- 229940105969 annatto extract Drugs 0.000 description 1
- 238000009360 aquaculture Methods 0.000 description 1
- 244000144974 aquaculture Species 0.000 description 1
- 239000001181 artemisia dracunculus Substances 0.000 description 1
- 208000011775 arteriosclerosis disease Diseases 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 230000002917 arthritic effect Effects 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 239000000605 aspartame Substances 0.000 description 1
- IAOZJIPTCAWIRG-QWRGUYRKSA-N aspartame Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 IAOZJIPTCAWIRG-QWRGUYRKSA-N 0.000 description 1
- 235000010357 aspartame Nutrition 0.000 description 1
- 229960003438 aspartame Drugs 0.000 description 1
- 230000000778 atheroprotective effect Effects 0.000 description 1
- IRERQBUNZFJFGC-UHFFFAOYSA-L azure blue Chemical compound [Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Al+3].[Al+3].[Al+3].[Al+3].[Al+3].[Al+3].[S-]S[S-].[O-][Si]([O-])([O-])[O-].[O-][Si]([O-])([O-])[O-].[O-][Si]([O-])([O-])[O-].[O-][Si]([O-])([O-])[O-].[O-][Si]([O-])([O-])[O-].[O-][Si]([O-])([O-])[O-] IRERQBUNZFJFGC-UHFFFAOYSA-L 0.000 description 1
- 229940038481 bee pollen Drugs 0.000 description 1
- 235000012677 beetroot red Nutrition 0.000 description 1
- 235000021028 berry Nutrition 0.000 description 1
- 229940000635 beta-alanine Drugs 0.000 description 1
- WHGYBXFWUBPSRW-FOUAGVGXSA-N beta-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO WHGYBXFWUBPSRW-FOUAGVGXSA-N 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- QKSKPIVNLNLAAV-UHFFFAOYSA-N bis(2-chloroethyl) sulfide Chemical compound ClCCSCCCl QKSKPIVNLNLAAV-UHFFFAOYSA-N 0.000 description 1
- 235000019481 bixa orellana extract Nutrition 0.000 description 1
- 235000013614 black pepper Nutrition 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 210000005013 brain tissue Anatomy 0.000 description 1
- 235000021329 brown rice Nutrition 0.000 description 1
- OBNCKNCVKJNDBV-UHFFFAOYSA-N butanoic acid ethyl ester Natural products CCCC(=O)OCC OBNCKNCVKJNDBV-UHFFFAOYSA-N 0.000 description 1
- 235000014121 butter Nutrition 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- FAPWYRCQGJNNSJ-UBKPKTQASA-L calcium D-pantothenic acid Chemical compound [Ca+2].OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O.OCC(C)(C)[C@@H](O)C(=O)NCCC([O-])=O FAPWYRCQGJNNSJ-UBKPKTQASA-L 0.000 description 1
- 239000001639 calcium acetate Substances 0.000 description 1
- 235000011092 calcium acetate Nutrition 0.000 description 1
- 229960005147 calcium acetate Drugs 0.000 description 1
- 229960003563 calcium carbonate Drugs 0.000 description 1
- 229960002079 calcium pantothenate Drugs 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 235000012682 canthaxanthin Nutrition 0.000 description 1
- 239000001659 canthaxanthin Substances 0.000 description 1
- 229940008033 canthaxanthin Drugs 0.000 description 1
- 239000001325 capsicum annuum l. var. longum oleoresin Substances 0.000 description 1
- 239000001390 capsicum minimum Substances 0.000 description 1
- 235000013736 caramel Nutrition 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 235000005300 cardamomo Nutrition 0.000 description 1
- 235000012730 carminic acid Nutrition 0.000 description 1
- 229960004203 carnitine Drugs 0.000 description 1
- 150000001745 carotenals Chemical class 0.000 description 1
- 235000015190 carrot juice Nutrition 0.000 description 1
- 235000013709 carrot oil Nutrition 0.000 description 1
- 235000011472 cat’s claw Nutrition 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 229940081734 cellulose acetate phthalate Drugs 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 229940099352 cholate Drugs 0.000 description 1
- BHQCQFFYRZLCQQ-OELDTZBJSA-M cholate Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 BHQCQFFYRZLCQQ-OELDTZBJSA-M 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- QSWDMMVNRMROPK-UHFFFAOYSA-K chromium(3+) trichloride Chemical compound [Cl-].[Cl-].[Cl-].[Cr+3] QSWDMMVNRMROPK-UHFFFAOYSA-K 0.000 description 1
- 229940043350 citral Drugs 0.000 description 1
- WTEVQBCEXWBHNA-YFHOEESVSA-N citral B Natural products CC(C)=CCC\C(C)=C/C=O WTEVQBCEXWBHNA-YFHOEESVSA-N 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000013065 commercial product Substances 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 229940108924 conjugated linoleic acid Drugs 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 229940108925 copper gluconate Drugs 0.000 description 1
- 229910000365 copper sulfate Inorganic materials 0.000 description 1
- ARUVKPQLZAKDPS-UHFFFAOYSA-L copper(II) sulfate Chemical compound [Cu+2].[O-][S+2]([O-])([O-])[O-] ARUVKPQLZAKDPS-UHFFFAOYSA-L 0.000 description 1
- 235000013712 corn endosperm oil Nutrition 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 229960001681 croscarmellose sodium Drugs 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 239000001812 curcuma zedoaria berg. rosc. Substances 0.000 description 1
- 235000012754 curcumin Nutrition 0.000 description 1
- 239000004148 curcumin Substances 0.000 description 1
- 229940109262 curcumin Drugs 0.000 description 1
- 230000020176 deacylation Effects 0.000 description 1
- 238000005947 deacylation reaction Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000015961 delipidation Effects 0.000 description 1
- 230000000779 depleting effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- VFLDPWHFBUODDF-UHFFFAOYSA-N diferuloylmethane Natural products C1=C(O)C(OC)=CC(C=CC(=O)CC(=O)C=CC=2C=C(OC)C(O)=CC=2)=C1 VFLDPWHFBUODDF-UHFFFAOYSA-N 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- ZPWVASYFFYYZEW-UHFFFAOYSA-L dipotassium hydrogen phosphate Chemical compound [K+].[K+].OP([O-])([O-])=O ZPWVASYFFYYZEW-UHFFFAOYSA-L 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- BVTBRVFYZUCAKH-UHFFFAOYSA-L disodium selenite Chemical compound [Na+].[Na+].[O-][Se]([O-])=O BVTBRVFYZUCAKH-UHFFFAOYSA-L 0.000 description 1
- 230000001882 diuretic effect Effects 0.000 description 1
- 235000013760 dried algae meal Nutrition 0.000 description 1
- 239000003651 drinking water Substances 0.000 description 1
- 235000020188 drinking water Nutrition 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 239000001921 dulse Substances 0.000 description 1
- JAZBEHYOTPTENJ-UHFFFAOYSA-N eicosapentaenoic acid Natural products CCC=CCC=CCC=CCC=CCC=CCCCC(O)=O JAZBEHYOTPTENJ-UHFFFAOYSA-N 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 229940073505 ethyl vanillin Drugs 0.000 description 1
- 235000008995 european elder Nutrition 0.000 description 1
- 235000008524 evening primrose extract Nutrition 0.000 description 1
- 229940089020 evening primrose oil Drugs 0.000 description 1
- 239000010475 evening primrose oil Substances 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 239000011552 falling film Substances 0.000 description 1
- 150000002191 fatty alcohols Chemical class 0.000 description 1
- 235000013924 ferrous gluconate Nutrition 0.000 description 1
- 239000004222 ferrous gluconate Substances 0.000 description 1
- 229960001645 ferrous gluconate Drugs 0.000 description 1
- 235000013925 ferrous lactate Nutrition 0.000 description 1
- 239000004225 ferrous lactate Substances 0.000 description 1
- 229940037907 ferrous lactate Drugs 0.000 description 1
- 229940114124 ferulic acid Drugs 0.000 description 1
- KSEBMYQBYZTDHS-HWKANZROSA-N ferulic acid Chemical compound COC1=CC(\C=C\C(O)=O)=CC=C1O KSEBMYQBYZTDHS-HWKANZROSA-N 0.000 description 1
- KSEBMYQBYZTDHS-UHFFFAOYSA-N ferulic acid Natural products COC1=CC(C=CC(O)=O)=CC=C1O KSEBMYQBYZTDHS-UHFFFAOYSA-N 0.000 description 1
- 235000001785 ferulic acid Nutrition 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 235000019688 fish Nutrition 0.000 description 1
- 229930003935 flavonoid Natural products 0.000 description 1
- 150000002215 flavonoids Chemical class 0.000 description 1
- 235000017173 flavonoids Nutrition 0.000 description 1
- 235000004426 flaxseed Nutrition 0.000 description 1
- 150000002224 folic acids Chemical class 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 235000015203 fruit juice Nutrition 0.000 description 1
- 235000011389 fruit/vegetable juice Nutrition 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 235000004611 garlic Nutrition 0.000 description 1
- 210000005095 gastrointestinal system Anatomy 0.000 description 1
- 239000007897 gelcap Substances 0.000 description 1
- HIGQPQRQIQDZMP-UHFFFAOYSA-N geranil acetate Natural products CC(C)=CCCC(C)=CCOC(C)=O HIGQPQRQIQDZMP-UHFFFAOYSA-N 0.000 description 1
- 229940113087 geraniol Drugs 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 150000002314 glycerols Chemical class 0.000 description 1
- 229940096919 glycogen Drugs 0.000 description 1
- 235000002532 grape seed extract Nutrition 0.000 description 1
- 229940087603 grape seed extract Drugs 0.000 description 1
- 229940107702 grapefruit seed extract Drugs 0.000 description 1
- 235000009569 green tea Nutrition 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 235000008216 herbs Nutrition 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 235000001050 hortel pimenta Nutrition 0.000 description 1
- 235000006486 human diet Nutrition 0.000 description 1
- 150000002430 hydrocarbons Chemical group 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 230000035879 hyperinsulinaemia Effects 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000006362 insulin response pathway Effects 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 235000013980 iron oxide Nutrition 0.000 description 1
- WBJZTOZJJYAKHQ-UHFFFAOYSA-K iron(3+) phosphate Chemical compound [Fe+3].[O-]P([O-])([O-])=O WBJZTOZJJYAKHQ-UHFFFAOYSA-K 0.000 description 1
- 229910000399 iron(III) phosphate Inorganic materials 0.000 description 1
- VRIVJOXICYMTAG-IYEMJOQQSA-L iron(ii) gluconate Chemical compound [Fe+2].OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O VRIVJOXICYMTAG-IYEMJOQQSA-L 0.000 description 1
- 229930013032 isoflavonoid Natural products 0.000 description 1
- 150000003817 isoflavonoid derivatives Chemical class 0.000 description 1
- 235000012891 isoflavonoids Nutrition 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 239000001102 lavandula vera Substances 0.000 description 1
- 235000018219 lavender Nutrition 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 102000005861 leptin receptors Human genes 0.000 description 1
- 108010019813 leptin receptors Proteins 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 235000001510 limonene Nutrition 0.000 description 1
- 229940087305 limonene Drugs 0.000 description 1
- 229930007744 linalool Natural products 0.000 description 1
- 239000000865 liniment Substances 0.000 description 1
- 235000020778 linoleic acid Nutrition 0.000 description 1
- 235000019421 lipase Nutrition 0.000 description 1
- 230000037356 lipid metabolism Effects 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 235000020845 low-calorie diet Nutrition 0.000 description 1
- 235000012661 lycopene Nutrition 0.000 description 1
- 239000001751 lycopene Substances 0.000 description 1
- 229960004999 lycopene Drugs 0.000 description 1
- OAIJSZIZWZSQBC-GYZMGTAESA-N lycopene Chemical compound CC(C)=CCC\C(C)=C\C=C\C(\C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\C=C(/C)CCC=C(C)C OAIJSZIZWZSQBC-GYZMGTAESA-N 0.000 description 1
- 239000001115 mace Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 1
- 229940057948 magnesium stearate Drugs 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical class [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000001771 mentha piperita Substances 0.000 description 1
- 239000001220 mentha spicata Substances 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 230000010120 metabolic dysregulation Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 208000011661 metabolic syndrome X Diseases 0.000 description 1
- UZKWTJUDCOPSNM-UHFFFAOYSA-N methoxybenzene Substances CCCCOC=C UZKWTJUDCOPSNM-UHFFFAOYSA-N 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 235000010460 mustard Nutrition 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 239000000974 natural food coloring agent Substances 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 239000012454 non-polar solvent Substances 0.000 description 1
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 239000001702 nutmeg Substances 0.000 description 1
- 239000002417 nutraceutical Substances 0.000 description 1
- 235000021436 nutraceutical agent Nutrition 0.000 description 1
- 235000021238 nutrient digestion Nutrition 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 235000021313 oleic acid Nutrition 0.000 description 1
- 239000008601 oleoresin Substances 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 230000001151 other effect Effects 0.000 description 1
- 235000019161 pantothenic acid Nutrition 0.000 description 1
- 229940055726 pantothenic acid Drugs 0.000 description 1
- 239000011713 pantothenic acid Substances 0.000 description 1
- 235000012658 paprika extract Nutrition 0.000 description 1
- DYUUPIKEWLHQGQ-SDXBLLFJSA-N paprika oleoresin Chemical compound C(\[C@]12[C@@](O1)(C)C[C@@H](O)CC2(C)C)=C/C(/C)=C/C=C/C(/C)=C/C=C/C=C(C)C=CC=C(C)C=C[C@H]1C(C)=C[C@H](O)CC1(C)C DYUUPIKEWLHQGQ-SDXBLLFJSA-N 0.000 description 1
- QNGNSVIICDLXHT-UHFFFAOYSA-N para-ethylbenzaldehyde Natural products CCC1=CC=C(C=O)C=C1 QNGNSVIICDLXHT-UHFFFAOYSA-N 0.000 description 1
- RUVINXPYWBROJD-UHFFFAOYSA-N para-methoxyphenyl Natural products COC1=CC=C(C=CC)C=C1 RUVINXPYWBROJD-UHFFFAOYSA-N 0.000 description 1
- 235000011197 perejil Nutrition 0.000 description 1
- 210000003024 peritoneal macrophage Anatomy 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- MBWXNTAXLNYFJB-NKFFZRIASA-N phylloquinone Chemical compound C1=CC=C2C(=O)C(C/C=C(C)/CCC[C@H](C)CCC[C@H](C)CCCC(C)C)=C(C)C(=O)C2=C1 MBWXNTAXLNYFJB-NKFFZRIASA-N 0.000 description 1
- 235000019175 phylloquinone Nutrition 0.000 description 1
- 239000011772 phylloquinone Substances 0.000 description 1
- 229960001898 phytomenadione Drugs 0.000 description 1
- 229940068065 phytosterols Drugs 0.000 description 1
- 239000001909 pimpinella anisum Substances 0.000 description 1
- 229940081310 piperonal Drugs 0.000 description 1
- 230000007505 plaque formation Effects 0.000 description 1
- 229920001197 polyacetylene Polymers 0.000 description 1
- 150000008442 polyphenolic compounds Chemical class 0.000 description 1
- 235000013824 polyphenols Nutrition 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Substances [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 1
- 235000011056 potassium acetate Nutrition 0.000 description 1
- 239000001103 potassium chloride Substances 0.000 description 1
- 235000011164 potassium chloride Nutrition 0.000 description 1
- 229940069328 povidone Drugs 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 229940069949 propolis Drugs 0.000 description 1
- 235000008160 pyridoxine Nutrition 0.000 description 1
- 239000011677 pyridoxine Substances 0.000 description 1
- ZUFQODAHGAHPFQ-UHFFFAOYSA-N pyridoxine hydrochloride Chemical compound Cl.CC1=NC=C(CO)C(CO)=C1O ZUFQODAHGAHPFQ-UHFFFAOYSA-N 0.000 description 1
- 229960004172 pyridoxine hydrochloride Drugs 0.000 description 1
- 235000019171 pyridoxine hydrochloride Nutrition 0.000 description 1
- 239000011764 pyridoxine hydrochloride Substances 0.000 description 1
- 235000005875 quercetin Nutrition 0.000 description 1
- 150000004053 quinones Chemical class 0.000 description 1
- 238000011552 rat model Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 235000013526 red clover Nutrition 0.000 description 1
- 230000010282 redox signaling Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 235000015639 rosmarinus officinalis Nutrition 0.000 description 1
- 229940109850 royal jelly Drugs 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 235000019204 saccharin Nutrition 0.000 description 1
- 229940081974 saccharin Drugs 0.000 description 1
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 description 1
- 235000019515 salmon Nutrition 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000000741 silica gel Substances 0.000 description 1
- 229910002027 silica gel Inorganic materials 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000011684 sodium molybdate Substances 0.000 description 1
- 235000015393 sodium molybdate Nutrition 0.000 description 1
- TVXXNOYZHKPKGW-UHFFFAOYSA-N sodium molybdate (anhydrous) Chemical compound [Na+].[Na+].[O-][Mo]([O-])(=O)=O TVXXNOYZHKPKGW-UHFFFAOYSA-N 0.000 description 1
- 239000011655 sodium selenate Substances 0.000 description 1
- 235000018716 sodium selenate Nutrition 0.000 description 1
- 229960001881 sodium selenate Drugs 0.000 description 1
- 239000011781 sodium selenite Substances 0.000 description 1
- 235000015921 sodium selenite Nutrition 0.000 description 1
- 229960001471 sodium selenite Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 235000003687 soy isoflavones Nutrition 0.000 description 1
- 239000008347 soybean phospholipid Substances 0.000 description 1
- 229940082787 spirulina Drugs 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 229960004274 stearic acid Drugs 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 235000013616 tea Nutrition 0.000 description 1
- 241001223854 teleost fish Species 0.000 description 1
- 150000003505 terpenes Chemical class 0.000 description 1
- 235000007586 terpenes Nutrition 0.000 description 1
- 235000021195 test diet Nutrition 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 1
- 230000008646 thermal stress Effects 0.000 description 1
- 235000019157 thiamine Nutrition 0.000 description 1
- 239000011721 thiamine Substances 0.000 description 1
- UIERGBJEBXXIGO-UHFFFAOYSA-N thiamine mononitrate Chemical compound [O-][N+]([O-])=O.CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N UIERGBJEBXXIGO-UHFFFAOYSA-N 0.000 description 1
- 210000001541 thymus gland Anatomy 0.000 description 1
- 230000009092 tissue dysfunction Effects 0.000 description 1
- QURCVMIEKCOAJU-UHFFFAOYSA-N trans-isoferulic acid Natural products COC1=CC=C(C=CC(O)=O)C=C1O QURCVMIEKCOAJU-UHFFFAOYSA-N 0.000 description 1
- ZCIHMQAPACOQHT-ZGMPDRQDSA-N trans-isorenieratene Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/c1c(C)ccc(C)c1C)C=CC=C(/C)C=Cc2c(C)ccc(C)c2C ZCIHMQAPACOQHT-ZGMPDRQDSA-N 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- 229940078499 tricalcium phosphate Drugs 0.000 description 1
- 229910000391 tricalcium phosphate Inorganic materials 0.000 description 1
- 235000019731 tricalcium phosphate Nutrition 0.000 description 1
- 125000005457 triglyceride group Chemical group 0.000 description 1
- 150000003648 triterpenes Chemical class 0.000 description 1
- 235000013976 turmeric Nutrition 0.000 description 1
- 230000007306 turnover Effects 0.000 description 1
- 235000013799 ultramarine blue Nutrition 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 235000012141 vanillin Nutrition 0.000 description 1
- MWOOGOJBHIARFG-UHFFFAOYSA-N vanillin Chemical compound COC1=CC(C=O)=CC=C1O MWOOGOJBHIARFG-UHFFFAOYSA-N 0.000 description 1
- FGQOOHJZONJGDT-UHFFFAOYSA-N vanillin Natural products COC1=CC(O)=CC(C=O)=C1 FGQOOHJZONJGDT-UHFFFAOYSA-N 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 235000015192 vegetable juice Nutrition 0.000 description 1
- 235000020852 very low calorie diet Nutrition 0.000 description 1
- 235000019166 vitamin D Nutrition 0.000 description 1
- 239000011710 vitamin D Substances 0.000 description 1
- 150000003710 vitamin D derivatives Chemical class 0.000 description 1
- QYSXJUFSXHHAJI-YRZJJWOYSA-N vitamin D3 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-YRZJJWOYSA-N 0.000 description 1
- 235000005282 vitamin D3 Nutrition 0.000 description 1
- 239000011647 vitamin D3 Substances 0.000 description 1
- 235000019168 vitamin K Nutrition 0.000 description 1
- 239000011712 vitamin K Substances 0.000 description 1
- 150000003721 vitamin K derivatives Chemical class 0.000 description 1
- 229940011671 vitamin b6 Drugs 0.000 description 1
- 229940046008 vitamin d Drugs 0.000 description 1
- 229940021056 vitamin d3 Drugs 0.000 description 1
- 150000003722 vitamin derivatives Chemical class 0.000 description 1
- 229940046010 vitamin k Drugs 0.000 description 1
- 239000001717 vitis vinifera seed extract Substances 0.000 description 1
- 235000019509 white turmeric Nutrition 0.000 description 1
- NWONKYPBYAMBJT-UHFFFAOYSA-L zinc sulfate Chemical compound [Zn+2].[O-]S([O-])(=O)=O NWONKYPBYAMBJT-UHFFFAOYSA-L 0.000 description 1
- 229910000368 zinc sulfate Inorganic materials 0.000 description 1
- 229960001763 zinc sulfate Drugs 0.000 description 1
- 239000001841 zingiber officinale Substances 0.000 description 1
- 238000011684 zucker rat (obese) Methods 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/56—Materials from animals other than mammals
- A61K35/612—Crustaceans, e.g. crabs, lobsters, shrimps, krill or crayfish; Barnacles
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/115—Fatty acids or derivatives thereof; Fats or oils
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/12—Ketones
- A61K31/122—Ketones having the oxygen directly attached to a ring, e.g. quinones, vitamin K1, anthralin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/20—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/20—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids
- A61K31/202—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids having three or more double bonds, e.g. linolenic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/21—Esters, e.g. nitroglycerine, selenocyanates
- A61K31/215—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/21—Esters, e.g. nitroglycerine, selenocyanates
- A61K31/215—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids
- A61K31/22—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids of acyclic acids, e.g. pravastatin
- A61K31/23—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids of acyclic acids, e.g. pravastatin of acids having a carboxyl group bound to a chain of seven or more carbon atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/21—Esters, e.g. nitroglycerine, selenocyanates
- A61K31/215—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids
- A61K31/235—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids having an aromatic ring attached to a carboxyl group
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/575—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids substituted in position 17 beta by a chain of three or more carbon atoms, e.g. cholane, cholestane, ergosterol, sitosterol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/683—Diesters of a phosphorus acid with two hydroxy compounds, e.g. phosphatidylinositols
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/683—Diesters of a phosphorus acid with two hydroxy compounds, e.g. phosphatidylinositols
- A61K31/685—Diesters of a phosphorus acid with two hydroxy compounds, e.g. phosphatidylinositols one of the hydroxy compounds having nitrogen atoms, e.g. phosphatidylserine, lecithin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4816—Wall or shell material
- A61K9/4825—Proteins, e.g. gelatin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4858—Organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/02—Nutrients, e.g. vitamins, minerals
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11B—PRODUCING, e.g. BY PRESSING RAW MATERIALS OR BY EXTRACTION FROM WASTE MATERIALS, REFINING OR PRESERVING FATS, FATTY SUBSTANCES, e.g. LANOLIN, FATTY OILS OR WAXES; ESSENTIAL OILS; PERFUMES
- C11B3/00—Refining fats or fatty oils
- C11B3/006—Refining fats or fatty oils by extraction
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
Definitions
- This invention relates to extracts from Antarctic krill that comprise bioactive fatty acids.
- Antarctic krill In the Southern Ocean, off the coast of Antarctica, Antarctic krill (Euphausia superba) can be found in large quantities, ranging from 300-500 million metric tons of biomass. It feeds on phytoplankton during the short Antarctic summer. During winter, however, its food supply is limited to ice algae, bacteria, marine detritus as well as depleting body protein for energy.
- krill oil In order to isolate the krill oil from the krill, solvent extraction methods have been used. See, e.g., WO 00/23546.
- Krill lipids have been extracted by placing the material in a ketone solvent (e.g. acetone) in order to extract the lipid soluble fraction.
- a ketone solvent e.g. acetone
- This method involves separating the liquid and solid contents and recovering a lipid rich fraction from the liquid fraction by evaporation. Further processing steps include extracting and recovering by evaporation the remaining soluble lipid fraction from the solid contents by using a solvent such as ethanol. See, e.g., WO 00/23546.
- compositions produced by these methods are characterized by containing at least 75 ⁇ g/g astaxanthin, preferably 90 ⁇ g/g astaxanthin.
- Another krill lipid extract disclosed contained at least 250 ⁇ g/g canastaxanthin, preferably 270 ⁇ g/g canastaxanthin.
- Krill oil compositions have been described as being effective for decreasing cholesterol, inhibiting platelet adhesion, inhibiting artery plaque formation, preventing hypertension, controlling arthritis symptoms, preventing skin cancer, enhancing transdermal transport, reducing the symptoms of premenstrual symptoms or controlling blood glucose levels in a patient. See, e.g., WO 02/102394.
- a krill oil composition has been disclosed comprising a phospholipid and/or a flavonoid.
- the phospholipid content in the krill lipid extract could be as high as 60% w/w and the EPA/DHA content as high as 35% (w/w). See, e.g., WO 03/011873.
- nutraceuticals, pharmaceuticals and cosmetics comprising the phospholipid extract were disclosed.
- supercritical fluid extraction using neat CO 2 could be used to prevent the extraction of phospholipids in order to extract the neutral lipid fraction from krill, which comprised of esterified and free astaxanthin.
- Supercritical fluid extraction with solvent modifier has previously been used to extract marine phospholipids from salmon roe, but has not been previously used to extract phospholipids from krill meal. See, e.g., Tanaka et al., J. Oleo Sci. (2004), 53(9), 417-424.
- composition characterized by comprising at least 65% (w/w) phospholipids.
- composition obtained from aquatic or marine sources characterized by comprising 65% (w/w) phospholipids.
- composition obtained from krill characterized by comprising at least 65% (w/w) phospholipids.
- composition obtained from krill characterized by comprising at least 65% (w/w) phospholipids and at least 39% omega-3 fatty acids (w/w).
- composition obtained from krill characterized by comprising at least 65% (w/w) phospholipids, at least 39% omega-3 fatty acids (w/w) and at least 580 mg/kg astaxanthin esters.
- composition obtained from krill characterized by comprising at least 39% omega-3 fatty acids (w/w) and at least 580 mg/kg astaxanthin esters.
- composition obtained from krill characterized by comprising at least 65% (w/w) phospholipids and at least 580mg/kg astaxanthin esters.
- present invention provides a krill oil effective for reducing insulin resistance, improving blood lipid profile, reducing inflammation or reducing oxidative stress.
- the present invention provides compositions comprising: from about 3% to 10% ether phospholipids on a w/w basis; from about 35% to 50% non-ether phospholipids on w/w basis, so that the total amount of ether phospholipids and non-ether phospholipids in the composition is from about 48% to 60% on a w/w basis; from about 20% to 45% triglycerides on a w/w basis; and from about 400 to about 2500 mg/kg astaxanthin.
- the ether phospholipids are selected from the group consisting of alkylacylphosphatidylcholine, lyso-alkylacylphosphatidylcholine, alkylacylphosphatidylethanolamine, and combinations thereof. In some embodiments, the ether lipids are greater than 90% alkylacylphosphatidylcholine. In some embodiments, the non-ether phospholipids are selected from the group consisting of phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine and combinations thereof. In some embodiments, krill oil composition comprises a blend of lipid fractions obtained from krill.
- krill is Euphausia superba, although other krill species also find use in the present invention.
- Other krill species include, but are not limited to E. paci ⁇ ca, E. frigida, E. longirostris, E. triacantha, E. vallentini, Meganyctiphanes norvegica, Thysanoessa raschii and Thysanoessa inermis.
- the compositions comprise from about 25% to 30% omega-3 fatty acids as a percentage of total fatty acids and wherein from about 80% to 90% of said omega-3 fatty acids are attached to said phospholipids.
- the present invention provides a capsule containing the foregoing compositions.
- the present inventions provide compositions comprising: from about 3% to 10% ether phospholipids on a w/w basis; and from about 400 to about 2500 mg/kg astaxanthin.
- the compositions further comprise from about 35% to 50% non-ether phospholipids on w/w basis, so that the total amount of ether phospholipids and non-ether phospholipids in the composition is from about 38% to 60% on a w/w basis.
- the compositions further comprise from about 20% to 45% triglycerides on a w/w basis.
- the ether phospholipids are selected from the group ' consisting of alkylacylphosphatidylcholine, lyso-alkylacylphosphatidylcholine, alkylacylphosphatidylethanolamine, and combinations thereof. In some embodiments, the ether lipids are greater than 90% alkylacylphosphatidylcholine. In some embodiments, the non-ether phospholipids are selected from the group consisting of phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine and combinations thereof. In some embodiments, krill oil composition comprises a blend of lipid fractions obtained from krill.
- krill is Euphausia superba, although other krill species also find use in the present invention.
- Other krill species include, but are not limited to E. pacifica, E. frigida, E. longirostris, E. triacantha, E. vallentini, Meganyctiphanes norvegica, Thysanoessa raschii and Thysanoessa inermis.
- the compositions comprise about
- omega-3 fatty acids 25% to 30% omega-3 fatty acids as a percentage of total fatty acids and wherein from about
- the present invention provides a capsule containing the foregoing compositions.
- the present invention provides a composition comprising at least 65% (w/w) of phospholipids, said phospholipids characterized in containing at least 35% omega-3 fatty acid residues.
- the composition is derived from a marine or aquatic biomass. In some further preferred embodiments, the composition is derived from krill. In some embodiments, the composition comprises less than 2% free fatty acids. In some embodiments, composition comprises less than 10% triglycerides. In some preferred embodiments, the phospholipids comprise greater than 50% phosphatidylcholine. In some embodiments, the composition comprises at least 500 mg/kg astaxanthin esters.
- the composition comprises at least 500 mg/kg astaxanthin esters and at least 36% (w/w) omega-3 fatty acids. In some embodiments, the composition comprises less than about 0.5g/100g total cholesterol. In some embodiments, the composition comprises less than about 0.45% arachidonic acid (w/w).
- the present invention provides a krill lipid extract comprising at least 500, 100, 1500, 2000, 2100, or 2200 mg/kg astaxanthin esters and at least 36% (w/w) omega-3 fatty acids. In further embodiments, the present invention provides a krill lipid extract comprising at least 100 mg/kg astaxanthin esters, at least 20% (w/w) omega-3 fatty acids, and less than about 0.45% arachidonic acid (w/w). 1
- the present invention provides methods comprising administering the foregoing compositions to a subject in an amount effective for reducing insulin resistance, reducing inflammation, improving blood lipid profile and reducing oxidative stress.
- the present invention provides a krill lipid extract comprising greater than about 80% triglycerides and greater than about 90, 100, 500, 1000, 1500, 200, 2100 or 2200 mg/kg astaxanthin esters, hi some embodiments, the krill lipid extract is characterized in containing from about 5% to about 15% omega-3 fatty acid residues. In some embodiments, the krill lipid extract is characterized in containing less than about 5% phospholipids. In some embodiments, the krill lipid extract is characterized in comprising from about 5% to about 10% cholesterol.
- the present invention provides a krill meal composition comprising less than about 50g/kg total fat. In some embodiments, the krill meal composition comprises from about 5 to about 20 mg/kg astaxanthin esters. In some embodiments, the krill meal composition comprises greater than about 65% protein. In some embodiments, the krill meal composition of comprises greater than about 70% protein. In some further embodiments, the present invention provides an animal feed comprising the krill meal composition. In some embodiments, the present invention provides methods of increasing flesh coloration in an aquatic species comprising feeding said aquatic species a composition comprising the krill meal described above. In some embodiments, the present invention provides methods of increasing growth and overall survival rate of aquatic species by feeding the krill meal described above.
- the present invention provides methods of producing krill oil comprising: a) providing krill meal; and b) extracting oil from said krill meal.
- the krill meal is produced by heat-treating krill.
- the krill meal is stored prior to the extraction step.
- the extracting step comprises extraction by supercritical fluid extraction.
- the supercritical fluid extraction is a two step process comprising a first extraction step with carbon dioxide and a low concentration of a co-solvent (e.g., from about 1-10% co-solvent) and a second extraction step with carbon dioxide and a high concentration of a co-solvent (e.g., from about 10-30% co-solvent).
- the co-solvent is a C 1 -C 3 monohydric alcohol, preferably ethanol.
- the present invention provides oil produced by the foregoing method.
- the present invention provides methods of production of krill oil comprising: a) providing fresh krill; b) treating said fresh krill to denature Upases and phospholipases in said fresh krill to provide a denatured krill product; and c) extracting oil from said denatured krill product.
- the denaturation step comprises heating of said fresh krill.
- the denaturation step comprises heating said fresh krill after grinding.
- the methods further comprise storing said denatured krill product at room temperature or below between the denaturation step and the extraction step.
- the enzyme denaturation step is achieved by application of heat.
- the extraction step comprises use of supercritical carbon dioxide, with or without use of a polar modifier. In some embodiments, the extraction step comprises use of ethanol. In some embodiments, the extraction step is comprises ethanol extraction followed by acetone to precipitation of phospholipids. In some embodiments, the denatured krill product is a meal. In some embodiments, the present invention provides oil produced by the foregoing method.
- the present invention provides a composition comprising oil extracted from krill having a phosphatidylcholine content of greater then about 50% (w/w). In some embodiments, the oil has a phosphatidylcholine content of greater then about 70% (w/w). In some embodiments, the oil has a phosphatidylcholine content of greater then about 80% (w/w). In some embodiments, the composition comprises less than 2% free fatty acids. In some embodiments, the composition comprises less than 10% triglycerides. In some embodiments, the composition comprises at least 500 mg/kg astaxanthin esters, hi some embodiments, the composition comprises less than about 0.45% arachidonic acid (w/w).
- the present invention provides composition comprising odorless krill oil.
- the odorless krill oil comprises less than about 10 mg/kg (w/w) trimethylamine.
- the present invention provides an odorless krill oil produced by the method comprising: extracting a neutral krill oil from a krill oil containing material by supercritical fluid extraction to provide a deodorized krill material, wherein said neutral krill oil contains odor causing compounds and extracting a polar krill oil from said deodorized krill material by supercritical fluid extraction with a polar entrainer to provide an essentially odorless krill oil.
- the present invention provides a composition comprising krill oil containing less than about 70 micrograms/kilogram (w/w) astaxanthin esters. In some embodiments, the compositions comprise less than about 50 micrograms/kilogram (w/w) astaxanthin esters. In some embodiments, the compositions comprise less than about 20 micrograms/kilogram (w/w) astaxanthin esters. In some embodiments, the compositions comprise less than about 5 micrograms/kilogram (w/w) astaxanthin esters.
- the present invention provides a krill oil produced by the process comprising: pumping fresh krill from a trawl onto a ship, heating the krill to provide a krill material, and extracting oil from the krill material.
- the present invention provides a blended krill oil composition
- a blended krill oil composition comprising: from about 45% to 55% w/w phospholipids; 'from about 20% to 45% w/w triglycerides; and from about 400 to about 2500 mg/kg astaxanthin.
- the blended krill oil product comprises a blend of lipid fractions obtained from Euphausia superba.
- the composition comprises from about 25% to 30% omega-3 fatty acids as a percentage of total fatty acids and wherein from about 80% to 90% of said omega-3 fatty acids are attached to said phospholipids.
- the present invention provides a Euphausia superba krill oil composition comprising: from about 30% to 60% w/w phospholipids; from about 20% to
- omega-3 fatty acids as a percentage of total fatty acids in said composition, wherein from about 70% to 95% of said omega-3 fatty acids are attached to said phospholipids.
- the present invention provides a dietary supplement comprising encapsulated Euphausia superba krill oil comprising from about 30% to 60% w/w phospholipids; from about 20% to 50% triglycerides; from about 400 to about 2500 mg/kg astaxanthin; and from about 20% to 35% omega-3 fatty acids as a percentage of total fatty acids in said composition, wherein from about 70% to 95% of said omega-3 fatty acids are attached to said phospholipids.
- the present invention provides methods of making a Euphausia superba krill oil composition comprising: contacting Euphausia superba with a polar solvent to provide a polar extract comprising phospholipids; contacting Euphausia superba with a neutral solvent to provide a neutral extract comprising triglycerides and astaxanthin; combining said polar extract and said neutral extract to provide Euphausia superba krill oil comprising from about 30% to 60% w/w phospholipids; from about 20% to 50% triglycerides; from about 400 to about 2500 mg/kg astaxanthin; and from about 20% to 35% omega-3 fatty acids as a percentage of total fatty acids in said composition, wherein from about 70% to 95% of said omega-3 fatty acids are attached to said phospholipids.
- the methods further comprise the step of encapsulating the Euphausia superba krill oil.
- the present invention provides a Euphausia superba krill oil.
- the present invention provides methods of producing a dietary supplement comprising; contacting Euphausia superba with a polar solvent to provide an polar extract comprising phospholipids; contacting Euphausia superba with a neutral solvent to provide a neutral extract, comprising triglycerides and astaxanthin; combining said polar extract and said neutral extract to provide Euphausia superba krill oil comprising from about 30% to 60% w/w phospholipids; from about 20% to 50% triglycerides; from about 400 to about 2500 mg/kg astaxanthin; and from about 20% to 35% omega-3 fatty acids as a percentage of total fatty acids in said composition, wherein from about 70% to 95% of said omega-3 fatty acids are attached to said phospholipids; and encapsulating said Euphausia superba krill oil.
- the present invention provides methods of reducing diet- induced hyperinsulinemia, insulin insensitivity, muscle mass hypertrophy, serum adiponectin reduction or hepatic steatosis comprising in a subject exposed to a high fat diet: administering to said subject exposed to a high fat diet an effective amount of a krill oil composition under conditions such that a condition selected from the group consisting of diet-induced hyperinsulinemia, insulin insensitivity, muscle mass hypertrophy, serum adiponectin reduction and hepatic steatosis is reduced.
- the present invention is not limited to any particular krill oil composition.
- the krill oil composition is a Euphausia superba krill oil composition.
- the present invention is not limited to any particular formulation of krill oil.
- the krill oil composition is encapsulated.
- the effective amount of a krill oil composition is from 0.2 grams to 10 grams of said krill oil composition.
- the krill oil composition comprises: from about 45% to 55% w/w phospholipids; from about 20% to 45% w/w triglycerides; and from about 400 to about 2500 mg/kg astaxanthin.
- the krill oil composition comprises a blend of lipid fractions obtained from Euphausia superba.
- the krill oil composition comprises from about 25% to 30% omega-3 fatty acids as a percentage of total fatty acids and wherein from about 80% to 90% of said omega-3 fatty acids are attached to said phospholipids
- the krill oil composition comprises from about 30% to 60% w/w phospholipids; from about 20% to 50% triglycerides; from about 400 to about 2500 mg/kg astaxanthin; and from about 20% to 35% omega-3 fatty acids as a percentage of total fatty acids in said composition, and wherein from about 70% to 95% of said omega-3 fatty acids are attached to said phospholipids.
- the present invention provides methods of reducing diet- induced hyperinsulinemia, insulin insensitivity, muscle mass hypertrophy, serum adiponectin reduction or hepatic steatosis comprising in a subject consuming a high fat diet or a normal fat diet: administering to said subject consuming a high fat diet or a normal fat diet an effective amount of a krill oil composition under conditions such that a condition selected from the group consisting of diet-induced hyperinsulinemia, insulin insensitivity, muscle mass hypertrophy, serum adiponectin reduction and hepatic steatosis is reduced.
- the present invention is not limited to any particular krill oil composition.
- the krill oil composition is a Euphausia superba krill oil composition.
- the present invention is not limited to any particular formulation of krill oil.
- the krill oil composition is encapsulated.
- the effective amount of a krill oil composition is from 0.2 grams to 10 grams of said krill oil composition.
- the krill oil composition comprises: from about 45% to 55% w/w phospholipids; from about 20% to 45% w/w triglycerides; and from about 400 to about 2500 mg/kg astaxanthin.
- the krill oil composition comprises a blend of lipid fractions obtained from Euphausia superba.
- the krill oil composition comprises from about 25% to 30% omega-3 fatty acids as a percentage of total fatty acids and wherein from about 80% to 90% of said omega-3 fatty acids are attached to said phospholipids. In some embodiments, the krill oil composition comprises from about 30% to 60% w/w phospholipids; from about 20% to 50% triglycerides; from about 400 to about 2500 mg/kg astaxanthin; and from about 20% to 35% omega-3 fatty acids as a percentage of total fatty acids in said composition, and wherein from about 70% to 95% of said omega-3 fatty acids are attached to said phospholipids.
- the present invention provides methods of inducing diuresis in a subject comprising: administering to said subject an effective amount of a krill oil composition under conditions such that diuresis is induced.
- the present invention provides methods of increasing muscle mass in a subject, comprising: administering to said subject an effective amount of a krill oil composition under conditions such that muscle mass is increased.
- the present invention provides methods of decreasing protein catabolism in a subject, comprising: administering to said subject an effective amount of a krill oil composition under conditions such that protein catabolism is decreased.
- the present invention provides methods of decreasing lipid content in the heart of a subject, comprising: administering to said subject an effective amount of a krill oil composition under conditions such that lipid content in the heart of the subject is decreased. In some embodiments, the present invention provides methods of decreasing lipid content in the liver of a subject, comprising: administering to said subject an effective amount of a krill oil composition under conditions such that lipid content in the liver of the subject is decreased.
- Figure 3. Plasma glucose concentration in Zucker rats fed different forms of omega-3 fatty acids.
- Figure 7 The effect of dietary omega-3 fatty acids on lipid accumulation in the liver.
- Figure 8 The effect of dietary omega-3 fatty acids on lipid accumulation in the muscle.
- Figure 9 The effect of dietary omega-3 fatty acids on lipid accumulation in the heart.
- Figure 10 Relative concentrations of DHA in the brain in Zucker rats supplemented with omega-3 fatty acids.
- FIG. 1 Body weight for the various treatment groups.
- FIG. 1 HOMA-IR values for the various treatment groups. 25 Figure 19. Liver triglyceride levels ( ⁇ mol/g) for the various treatment groups.
- phospholipid refers to an organic compound having the following 30 general structure:
- Rl is a fatty acid residue
- R2 is a fatty .acid residue or -OH
- R3 is a -H or nitrogen containing compound choline (HOCH 2 CH 2 N + (CH S ) 3 OH " ), ethanolamine (HOCH 2 CH 2 NH 2 ), inositol or serine.
- Rl and R2 cannot simultaneously be OH.
- R3 is an -OH
- the compound is a diacylglycerophosphate
- R3 is a nitrogen-containing compound
- the compound is a phosphatide such as lecithin, cephalin, phosphatidyl serine or plasmalogen.
- ether phospholipid refers to a phospholipid having an ether bond at position 1 the glycerol backbone.
- ether phospholipids include, but are not limited to, alkylacylphosphatidylcholine (AAPC), lyso-alkylacylphosphatidylcholine
- a "non-ether phospholipid” is a phospholipid that does not have an ether bond at position 1 of the glycerol backbone.
- omega-3 fatty acid refers to polyunsaturated fatty acids that have the final double bond in the hydrocarbon chain between the third and fourth carbon atoms from the methyl end of the molecule.
- Non-limiting examples of omega-3 fatty acids include, 5,8,11,14,17-eicosapentaenoic acid (EPA), 4,7;10,13,16,19-docosahexanoic acid
- DHA 7,10,13,16,19-docosapentanoic acid
- astaxanthin refers to the following chemical structure:
- astaxanthin esters refer to the fatty acids esterif ⁇ ed to OH group in the astaxanthin molecule.
- w/w refers to the amount of a given substance in a composition on weight basis.
- a composition comprising 50% w/w phospholipids means that the mass of the phospholipids is 50% of the total mass of the composition (i.e., 50 grams of phospholipids in 100 grams of the composition, such as an oil).
- This invention discloses novel krill oil compositions characterized by containing high levels of astaxanthin, phospholipids, included an enriched quantities of ether phospholipids, and omega-3 fatty acids.
- the krill oils compositions are extracted from krill meal using supercritical fluid extraction (SFE) with a co-solvent modifier.
- SFE supercritical fluid extraction
- the krill meal has been processed on board a ship in Antarctica using live krill as starting material in order to ensure the highest possible quality of the krill meal.
- the krill oils are extracted from the krill meal in two stages, in step 1 the neutral fraction is extracted using neat supercritical CO 2 or in combination with 5% ethanol.
- the neutral fraction consisted mostly of triglycerides and cholesterol.
- the polar lipids phospholipids
- the present invention provides methods to avoid decomposition of glycerides and phospholipids in krill oil and compositions produced by those methods.
- the product obtained by these new methods is virtually free of enzymatically decomposed oil constituents.
- the solution to the problem is to incorporate a protein denaturation step on fresh krill prior to use of any extraction technology. Denaturation can be achieved by thermal stress or by other means. After denaturation, the oil can be extracted by an optional selection of nonpolar and polar solvents including use of supercritical carbon dioxide.
- Krill is adapted to a very efficient nutrient digestion at very low temperatures. Therefore the enzymes are sensitive to heat and the step of applying thermal denaturation of lipases and phospholipases does not imply use of very high temperatures.
- A. Krill Processing provides methods for processing freshly caught krill at the site of capture and preferably on board a ship. After processing on board, the krill can be further subjected to extraction processes on board the ship or at a remote location away from the ship.
- the processing steps described herein also allow for the storage of krill material, preferably a krill meal for from about 1,2, 3, 4, 5, 6, 8, 9, 10, 11, or 12 months to about 24 to 36 months prior to processing.
- freshly caught krill is first subjected to a protein denaturation step.
- the present invention is not limited to any particular method of protein denaturation.
- the denaturation is accomplished by application of chemicals, heat, or combinations thereof.
- freshly caught krill is wet pressed to obtain oil and meal.
- the meal is then heated to a temperature of about 50 0 C to about 100°C for about 20 minutes to about an hour, preferably about 40 minutes to denature the proteins.
- this material is then pressed to yield a press cake. When this method is used on krill, only a small amount of oil is released. Most of the oil is still present in the denatured meal.
- antioxidants such as ethoxyquin or Vitamin E are added to the meal.
- the resulting meal is surprisingly stable.
- the stability can only partly be explained by addition of an antioxidant to the meal.
- This antioxidant can, after extraction of the oil from denatured meal, be removed by further processing steps. Alternatively the oil can be extracted rather shortly after production of the meal without any addition of antioxidant in the process. Further, storage conditions at a low to very low temperature can be applied if addition of antioxidant is not desired.
- This product turned out to be substantially different from samples of krill oil available in the market today.
- Previously described commercial krill processing procedures utilize krill that has been frozen immediately after catching followed by freeze drying and extraction at low temperatures. However, these processes only yield a suitable product if the time the krill is kept frozen is very short or the temperature is extremely low (-60°to -80°C).
- data provided herein clearly shows that if a step of denaturation of the proteins is added in front of an optional extraction method, an excellent krill oil can be produced even after a long time of storage.
- the denatured material should preferably be stored at low temperature preferably at -2O 0 C.
- krill oil is extracted from the denatured krill meal. In some embodiments, the krill oil is extracted by contacting the krill meal with ethanol. In some embodiments, krill is then extracted with a ketone solvent such as acetone. In other embodiments, the krill oil is extracted by one or two step supercritical fluid extraction. In some embodiments, the supercritical fluid extraction uses carbon dioxide and neutral krill oil is produced. In some embodiments, the supercritical fluid extraction uses carbon dioxide with the addition of a polar entrainer, such as ethanol, to produce a polar krill oil. In some embodiments, the krill meal is first extracted with carbon dioxide followed by carbon dioxide with a polar entrainer, or vice versa.
- the krill meal is first extracted with CO 2 supplemented with a low amount of a polar co-solvent (e.g., from about 1 % to about 10%, preferably about 5%) such a C 1 -C 3 monohydric alcohol, preferably ethanol, followed by extraction with CO 2 supplemented with a high amount of a polar co-solvent (from about 10% to about 30%, preferably about 23%) such as such a C 1 -C 3 monohydric alcohol, preferably ethanol, or vice versa.
- a low amount of polar solvent in the CO 2 as an entrainer facilitates the extraction of neutral lipid components and astaxanthin in a single step.
- Use of the high of polar solvent as an entrainer in the other step facilitates extraction of ether phospholipids, as well as non-ether phospholipids.
- the present invention is distinguished from previously described krill oil products, such as those described in U.S. Pat. No. 6,800,299 or WO 03/011873 and Neptune brand krill oil, by having substantially higher levels of non-ether phospholipids, ether phospholipids, and astaxanthin.
- the krill oils of the present invention also have, unexpected and superior properties as compared to previously available krill oils.
- the krill oil of the present invention has been demonstrated to reduce blood LDL cholesterol levels, improve DHA transfer to the brain as well as reduce lipid accumulation in the liver and muscle while the previously described krill oil compositions do not have such a properties.
- the present invention provides a krill oil composition, preferably a
- Euphausia superba krill oil composition comprising from about 40% to about 60% w/w phospholipids, preferably from about 45% to 55% w/w phospholipids and from about 300 mg/kg astaxanthin to about 2500 mg/kg astaxanthin, preferably from about 1000 to about 2200 mg/kg astaxanthin, more preferably from about 1500 to about 2200 mg/kg astaxanthin.
- the compositions comprise greater than about 1000, 1500, 1800, 1900, 2000, or 2100 mg/kg astaxanthin.
- the krill oil compositions of the present invention comprise from about 1%, 2%, 3% or 4% to about 8%, 10%, 12% or 15% w/w ether phospholipids or greater than about 4%, 5%, 6%, 7%, 8%, 9% or 10% ether phospholipids.
- the ether phospholipids are preferably alkylacylphosphatidylcholine, lyso-alkylacylphosphatidylcholine, alkylacylphosphatidyl- ethanolamine or combinations thereof.
- the krill oil compositions comprise from about 1%, 2%, 3% or 4% to about 8%, 10%, 12% or 15% w/w ether phospholipids and from about 30%, 33%, 40%, 42%, 45%, 48%, 50%, 52%, 54%, 55% 56%, 58% to about 60% non-ether phospholipids so that the total amount of phospholipids (both ether and non-ether phospholipids) ranges from about 40% to about 60%.
- the range of 40% to 60% total phospholipids, as well as the other ranges of ether and non-ether phospholipids can include other values not specifically listed within the range.
- the compositions comprise from about 20% to 45% w/w triglycerides; and from about 400 to about 2500 mg/kg astaxanthin.
- the compositions comprise from about 20% to 35%, preferably from about 25% to 35%, omega-3 fatty acids as a percentage of total fatty acids in the composition, wherein from about 70% to 95%, or preferably from about 80% to 90% of the omega-3 fatty acids are attached to the phospholipids.
- the present invention provides encapsulated Euphausia superba krill oil compositions.
- the present invention provides a method of making a Euphausia superba krill oil composition
- a method of making a Euphausia superba krill oil composition comprising contacting Euphausia superba with a polar solvent to provide an polar extract comprising phospholipids, contacting Euphausia superba with a neutral solvent to provide a neutral extract comprising triglycerides and astaxanthin, and combining said polar extract and said neutral extract to provide the Euphausia superba krill oils described above.
- fractions from polar and non-polar extractions are combined to provide a final product comprising the desired ether phospholipids, non-ether phospholipids, omega-3 moieties and astaxanthin.
- the present invention provides methods of making a Euphausia superba (or other krill species) krill oil comprising contacting a Euphausia superba preparation such as Euphausia superba krill meal under supercritical conditions with CO 2 containing a low amount of a polar solvent such as ethanol to extract neutral lipids and astaxanthin; contacting meal remaining from the first extraction step under supercritical conditions with CO 2 containing a high amount of a polar solvent such as ethanol to extract a polar lipid fraction containing ether and non-ether phospholipids; and then blending the neutral and polar lipid extracts to provide the compositions described above.
- the krill oil extracted by the methods of the present invention contains few enzymatic breakdown products.
- Examples of the krill oil compositions of the present invention are provided in Tables 9-24.
- the present invention provides a polar krill oil comprising at least 65% (w/w) of phospholipids, wherein the phospholipids are characterized in containing at least 35% omega-3 fatty acid residues.
- the present invention is not limited to the presence of any particular omega-3 fatty acid residues in the krill oil composition.
- the krill oil comprises EPA and DHA residues.
- the krill oil compositions comprise less than about 5%, 4%, 3% or preferably 2% free fatty acids on a weight/weight (w/w) basis. In some embodiments, the krill oil compositions comprise less than about 25%, 20%, 15%, 10% or 5% triglycerides
- the krill oil compositions comprise greater than about 30%, 40%, 45%, 50%, 55%, 60%, or 65% phosphatidyl choline (w/w). In some embodiments, the krill oil compositions comprise greater than about 100, 200, 300, 400, or 500 mg/kg astaxanthin esters and up to about 700 mg/kg astaxanthin esters. In some embodiments, the present invention provides krill oil compositions comprising at least 500, 1000, 1500, 2000, 2100, or 2200 mg/kg astaxanthin esters and at least 36% (w/w) omega-3 fatty acids.
- the krill oil compositions of the present invention comprise less than about l.Og/lOOg, 0.5g/100g, 0.2g/100g or O.lg/lOOg total cholesterol. In some embodiments, the krill oil compositions of the present invention comprise less than about .0.45
- the present invention provides a neutral krill oil extract comprising greater than about 70%, 75% 80%, 85% or 90% triglycerides.
- the krill oil compositions comprise from about 50 to about 2500 mg/kg astaxanthin esters.
- the krill oil compositions comprise from about 50, 100, 200, or 500 to about 750, 1000, 1500 or 2500 mg/kg astaxanthin esters.
- the compositions comprise from about 1% to about 30% omega-3 fatty acid residues, and preferably from about 5%-15% omega-3 fatty acid residues.
- the krill oil compositions comprise less than about 20%, 15%, 10% or 5% phospholipids.
- the present invention provides krill oil containing less than about 70, 60, 50, 40, 30, 20, 10, 5 or 1 micrograms/kilogram (w/w) astaxanthin esters.
- the krill oil is clear or only has a pale red color.
- the low-astaxanthin krill oil is obtained by first extracting a krill material, such as krill oil, by supercritical fluid extraction with neat carbon dioxide. It is contemplated that this step removes astaxanthin from the krill material, hi some embodiments, the krill material is then subjected to supercritical fluid extraction with carbon dioxide and a polar entrainer such as ethanol, preferably about 20% ethanol. The oil extracted during this step is characterized in containing low amounts of astaxanthin.
- krill oil comprising astaxanthin is extracted by countercurrent supercritical fluid extraction with neat carbon dioxide to provide a low-astaxanthin krill oil.
- the present invention provides krill oil that is substantially odorless.
- substantially odorless it is meant that the krill oil lacks an appreciable odor as determined by a test panel.
- the substantially odorless krill oil comprises less than about 10, 5 or 1 milligrams/kilogram trimethylamine.
- the odorless krill oil is produced by first subjecting krill material to supercritical fluid extraction with neat carbon dioxide to remove odor causing compounds such as trimethylamine, followed by extraction with carbon dioxide with a polar entrainer such as ethanol.
- the present invention provides a delipidated krill meal produced after extraction of lipids from the krill meal.
- the delipidated krill meal comprises krill protein.
- the delipidated krill meal comprises less than about 200, 150, 120, 100, 75, 65, 60, 55, or 50 g/kg total fat.
- the delipidated krill meal comprises from about 1 to about 100 mg/kg astaxanthin esters, and preferably from about 5 to about 20 mg/kg astaxanthin esters.
- the delipidated krill meal comprises greater than about 60%, 65%, 70% or 75% krill protein.
- the present invention provides animal feeds comprising the delipidated krill meal.
- the animal feed is a fish feed or aquatic organism feed, such as shrimp feed, crab feed, or crawfish feed.
- the krill meal is incorporated into complete ration for the target organism.
- the feed is provided in pelleted form.
- compounds such as astaxanthin are removed during delipidation.
- the methods of the present invention provide a delipidated krill meal that retains significant amounts of astaxanthin. Accordingly, in some embodiments, the present invention provides methods of feeding aquatic organisms, comprising providing to the aquatic organism a feed comprising the delipidated krill meal described above. In other embodiments, the present invention provides methods of increasing flesh coloration in an aquatic species comprising feeding the aquatic species a comprising the delipidated krill meal described above.
- compositions Containing Krill Oil are contained in acceptable excipients and/or carriers for oral consumption.
- the actual form of the carrier, and thus, the composition itself, is not critical.
- the carrier may be a liquid, gel, gelcap, capsule, powder, solid tablet (coated or non-coated),
- the composition is. preferably in the form of a .tablet or capsule and most preferably in the form of a soft gel capsule.
- Suitable excipient and/or carriers include maltodextrin, calcium carbonate, dicalcium phosphate, tricalcium phosphate, macrocrystalline . cellulose, dextrose, rice flour, magnesium stearate, stearic acid, croscarmellose sodium, sodium starch glycolate, crospovidone, sucrose, vegetable gums, lactose, methylcellulose, povidone, carboxymethylcellulose, corn starch, and the like (including mixtures thereof).
- Preferred carriers include calcium carbonate, magnesium stearate, maltodextrin, and mixtures thereof.
- the various ingredients and the excipient and/or carrier are mixed and formed into the desired form using conventional techniques.
- the tablet or capsule of the present invention may be coated with an enteric coating that dissolves at a pH of about 6.0 to 7.0.
- a suitable enteric coating that dissolves in the small intestine but not in the stomach is cellulose acetate phthalate. Further details on techniques for formulation for and administration may be found in the latest edition of Remington's Pharmaceutical Sciences (Maack Publishing Co., Easton, PA).
- the dietary supplement may comprise one or more inert ingredients, especially if it is desirable to limit the number of calories added to the diet by the dietary supplement.
- the dietary supplement of the present invention may also contain optional ingredients including, for example, herbs, vitamins, minerals, enhancers, colorants, sweeteners, flavorants, inert ingredients, and the like.
- the dietary supplement of the present invention may contain one or more of the following: ascorbates (ascorbic acid, mineral ascorbate salts, rose hips, acerola, and the like), dehydroepiandosterone (DHEA), Fo- Ti or Ho Shu Wu (herb common to traditional Asian treatments), Cat's Claw ( ancient herbal ingredient), green tea (polyphenols), inositol, kelp, dulse, bioflavinoids, maltodextrin, nettles, niacin, niacinamide, rosemary, selenium, silica (silicon dioxide, silica gel, horsetail, shavegrass, and the like), spirulina, zinc, and the like.
- ascorbates ascorbic acid, mineral ascorbate salts, rose hips, acerola, and the like
- DHEA dehydroepiandosterone
- Fo- Ti or Ho Shu Wu herb common to traditional Asian treatments
- the dietary supplements further comprise vitamins and minerals including, but not limited to, calcium phosphate or acetate, tribasic; potassium phosphate, dibasic; magnesium sulfate or oxide; salt (sodium chloride); potassium chloride or acetate; ascorbic acid; ferric orthophosphate; niacinamide; zinc sulfate or oxide; calcium pantothenate; copper gluconate; riboflavin; beta-carotene; pyridoxine hydrochloride; thiamin mononitrate; folic acid; biotin; chromium chloride or picolonate; potassium iodide; sodium selenate; sodium molybdate; phylloquinone; vitamin D3; cyanocobalamin; sodium selenite; copper sulfate; vitamin A; vitamin C; inositol; potassium iodide.
- vitamins and minerals including, but not limited to, calcium phosphate or acetate, tribasic; potassium
- compositions comprise at least one food flavoring such as acetaldehyde (ethanal), acetoin (acetyl methylcarbinol), anethole (parapropenyl anisole), benzaldehyde (benzoic aldehyde), N butyric acid (butanoic acid), d or 1 carvone (carvol), cinnamaldehyde (cinnamic aldehyde), citral (2,6 dimethyloctadien 2,6 al 8, gera nial, neral), decanal (N decylaldehyde, capraldehyde, capric aldehyde, caprinaldehyde, aldehyde C 10), ethyl acetate, ethyl butyrate, 3 methyl 3 phenyl glycidic acid ethyl
- food flavoring such as acetaldehyde (ethanal), acetoin (acetyl methylcar
- compositions comprise at least one synthetic or natural food coloring (e.g., annatto extract, astaxanthin, beet powder, ultramarine blue, canthaxanthin, caramel, carotenal, beta carotene, carmine, toasted cottonseed flour, ferrous gluconate, ferrous lactate, grape color extract, grape skin extract, iron oxide, fruit juice, vegetable juice, dried algae meal, tagetes meal, carrot oil, corn endosperm oil, paprika, paprika oleoresin, riboflavin, saffron, tumeric, tumeric and oleoresin).
- synthetic or natural food coloring e.g., annatto extract, astaxanthin, beet powder, ultramarine blue, canthaxanthin, caramel, carotenal, beta carotene, carmine, toasted cottonseed flour, ferrous gluconate, ferrous lactate, grape color extract, grape skin extract, iron oxide, fruit juice, vegetable juice, dried algae
- compositions comprise at least one phytonutrient (e.g., soy isoflavonoids, oligomeric proanthcyanidins, indol 3 carbinol, sulforaphone, fibrous ligands, plant phytosterols, ferulic acid, anthocyanocides, triterpenes, omega 3/6 fatty acids, conjugated fatty acids such as conjugated linoleic acid and conjugated linolenic acid, polyacetylene, quinones, terpenes, cathechins, gallates, and quercitin).
- phytonutrient e.g., soy isoflavonoids, oligomeric proanthcyanidins, indol 3 carbinol, sulforaphone, fibrous ligands, plant phytosterols, ferulic acid, anthocyanocides, triterpenes, omega 3/6 fatty acids, conjugated fatty acids such as conjugated linoleic acid
- Sources of plant phytonutrients include, but are not limited to, soy lecithin, soy isoflavones, brown rice germ, royal jelly, bee propolis, acerola berry juice powder, Japanese green tea, grape seed extract, grape skin extract, carrot juice, bilberry, flaxseed meal, bee pollen, ginkgo biloba, primrose (evening primrose oil), red clover, burdock root, dandelion, parsley, rose hips, milk thistle, ginger, Siberian ginseng, rosemary, curcumin, garlic, lycopene, grapefruit seed extract, spinach, and broccoli.
- the compositions comprise at least one vitamin (e.g., vitamin A, thiamin (Bl), riboflavin (B2), pyridoxine (B6), cyanocobalamin (B 12), biotin, ascorbic acid (vitamin C), retinoic acid (vitamin D), vitamin E, folic acid and other folates, vitamin K, niacin, and pantothenic acid).
- the particles comprise at least one mineral (e.g., sodium, potassium, magnesium, calcium, phosphorus, chlorine, iron, zinc, manganese, flourine, copper, molybdenum, chromium, selenium, and iodine).
- a dosage of a plurality of particles includes vitamins or minerals in the range of the recommended daily allowance (RDA) as specified by the United States Department of Agriculture.
- the particles comprise an amino acid supplement formula in which at least one amino acid is included (e.g., 1-carnitine or tryptophan).
- omega-3 fatty acids have anti-inflammatory properties. See, e.g., Calder. Am. J. Clin. Nutr. 83 (2006) 1505S.
- a phospholipid emulsion derived from a marine and/or synthetic origin comprising polyunsaturated fatty acids have anti-inflammatory and/or immuno-suppressive effects. See, e.g., 5,434,183.
- An embodiment of this invention is a krill oil composition effective for reducing inflammation i.e. reducing the levels of TNF- ⁇ , IL-I beta, IL-6, IL-10, TGF beta and fibrinogen in the blood.
- Type 2 diabetes is a metabolic disorder characterized by impaired glycemic control (high blood glucose levels). In type 2 diabetes, it is the tissue wide insulin resistance that contributes to the development of the disease. Strategies reducing insulin resistance or improving tissue sensitivity to insulin are recognized as beneficial in preventing type 2 diabetes.
- a 3-week supplementation with fish oil 1.1 g EPA/d and 0.7 g DHA/d
- Omega-3 PUFA dietary enrichment resulted in lower glucose oxidation, higher fat oxidation, and increased glycogen storage; the glycemic response was unchanged, however, which indicates an improved sensitivity to insulin
- hi another embodiment of this invention is a krill oil composition effective for reducing the insulin resistance.
- Omega-3 fatty acid supplementation may alleviate the inflammatory condition in adipose tissue and thus ideally complement the principal strategies of weight reduction i.e. low calorie diet and exercise.
- omega-3 enhance the effect of very low calorie diet and exercise in reducing body fat mass.
- omega-3 fatty acids alleviating the inflammatory condition in the adipose tissue may persist generating a condition that can be described as "healthy adipose tissue".
- dietary omega-3 fatty acids can be used to reduce inflammation in adipose tissue without influencing level of obesity.
- Reduction in adipose tissue inflammation was demonstrated by an increase in circulating levels of adiponectin.
- Adiponectin is an adipose tissue derived antiinflammatory hormone.
- An embodiment of the invention is the use of krill oil to increase serum adiponectin levels.
- Adiponectin is a protein hormone that modulates a number of metabolic processes, including glucose regulation and fatty acid catabolism. Adiponectin is exclusively secreted from adipose tissue into the bloodstream and is very abundant in plasma relative to many hormones. Levels of the hormone are inversely correlated with body mass index (BMI). The hormone plays a role in alleviating the metabolic dysregulation that may result in type 2 diabetes, obesity, atherosclerosis and non-alcoholic fatty liver disease (NAFLD). Diez et al., Eur. J. Endocrinol. 148 (3): 293-300; Ukkola et al., J. MoI. Med. 80 (11): 696-702.
- Another embodiment of the invention is to use krill oil in an overweight and obese subjects for alleviating diet induced adipose tissue dysfunction and diet induced changes in the lipid metabolism.
- krill oil is effective in reducing risk factors of type 2 diabetes such as hyperinsulinemia and insulin resistance and cardiovascular disease risk factors in overweight subjects.
- this invention discloses that krill oil is effective in preventing accumulation of fat in muscles and in the liver (liver steatosis).
- the obese Zucker rat is a useful rat model to study metabolic Syndrome X and non-insulin dependent diabetes mellitus, including glucose tolerance, insulin resistance and hyperinsulinaemia. It has also been shown previously that astaxanthin is a powerful antioxidant, useful for prevention of oxidative stress in vivo and in Zucker rats using vitamin E. See, e.g., Aoi et al., (2003). Antioxidants & Redox Signaling. 5(l):139-44; Laight et al., Eur. J. Pharmacol. 377 (1999) 89.
- a krill oil composition effective of improving the blood lipid profile by increasing the HDL cholesterol levels, decreasing the LDL cholesterol and triglyceride levels.
- novel krill oil composition is effective for treating metabolic syndrome.
- Metabolic syndrome is defined as the coexistence of 3 or more components selected from the group: abdominal obesity, high serum triglyceride levels, low HDL levels, elevated blood pressure and high fasting plasma glucose levels.
- the krill oil compositions are found to be effective and safe for the treatment of metabolic syndrome in humans.
- the krill oil compositions of the present invention find use in increasing or inducing diuresis. In some embodiments, the krill oil compositions of the present invention find use in decreasing protein catabolism and increasing the muscle mass of a subject. In some embodiments, the kill oil composition of the present invention find use in the treatment of fatty heart disease and non-alcoholic fatty acid liver disease. Thus, the krill oil compositions are useful for decreasing the lipid content of the heart and/or liver and/or muscle of a subject.
- In yet another embodiment of the invention is a method to increase the transfer of DHA to the brain.
- 3-aminopropanoic acid is also known as ⁇ -alanine
- 4-aminobutanoic acid is alos known as ⁇ -aminobutyric acid or GABA
- EXAMPLE 2 The krill meal obtained in example 1 was then ethanol extracted according to the method disclosed in JP02215351. The results showed that around 22% fat from the meal could be extracted, somewhat lower than was extracted using Folch (25%).
- Table 6 shows the fatty acid composition of the krill meal and the krill oil extracted from the meal using ethanol.
- Table 7 shows the composition and properties of the krill meal and products before and after extraction, whereas table 8 shows the lipid composition.
- the krill meal obtained in example 1 was then subjected to a supercritical fluid extraction method in two stages. During stage 1, 12.1% fat (neutral krill oil) was removed using neat CO 2 only at 300 bars, 60° C and for 30 minutes, hi stage 2, the pressure was increased to 400 bar and 20% ethanol was added (v/v) for 90 minutes. This resulted in further extraction of 9% polar fat which hereafter is called polar krill oil.
- the total fatty acid composition of the polar krill oil, the neutral krill oil and a commercial product obtained from Neptune Biotech (Laval, Quebec, Canada) are listed in Table 9.
- fatty acid composition for the phospholipids (Table 10), the neutral lipids (Table 11), the free fatty acids, diglycerides (Table 12), triglycerides, lyso-phosphatidylcholine (LPC) (Table 13), phosphatidylcholine (PC), phosphatidylethanolamine (PE) (Table 14), phosphatidylinositol (PI) and phosphatidylserine (PS) (Table 15) are shown.
- Table 16 shows the level of astaxanthin and cholesterol for the different fractions.
- Neutral lipids were extracted from krill meal (138 kg) using SFE with neat CO 2 (solvent ratio 25 kg/kg) at 500 bar and 75 °C.
- the neutral lipids were fractionated at 200 bar (75 0 C) and at 60 bar (35 0 C) at separator Sl and S2, respectively.
- the extract obtained in Sl (19,6 kg) were characterized and the results can be found in Tables 17A-C.
- the extract in table S2 (0,4 kg) were rich in water and were not further used.
- the polar lipids were extracted using CO 2 at 500 bar, 20% ethanol and at a temperature of 75 0 C.
- Table 18B Lipid class composition of the extract collected after CO 2 and 20% ethanol in Sl.
- Table 19A Fatty acid composition of the final blended product obtained in Example 4 in Sl .
- the asta oil obtained in example 1 was blended with the polar lipids obtained in example 4 in a ratio of 46:54 (v/v). Next the ethanol was removed by evaporation and a dark red and transparent product was obtained. The product was analyzed and the results can be found in Tables 20A-C. Furthermore, the product was encapsulated into soft gels successfully. During the encapsulation it was observed that any further increase in phospholipids and thereby viscosity will make it very difficult to encapsulate the final product.
- Table 2OA Fatty acid composition of the final blended product obtained in Example 5.
- Fresh krill was pumped from the harvesting trawl directly into an indirect steam cooker, and heated to 9OC. Water and a small amount of oil were removed in a screw press before ethoxyquin (antioxidant) was added and the denatured meal was dried under vacuum at a temperature not exceeding 8OC. After 19 months storage in room temperature, a sample of the denatured meal was extracted in two steps with supercritical CO 2 in laboratory scale at a flow rate of 2ml/min at IOOC and a pressure of 7500 psi. In the second step 20% ethanol was added to the CO 2 . The two fractions collected were combined and analyzed by HPLC using ELS detection.
- the phosphatidylcholine was measured to 42.22% whereas the partly decomposed phosphatidylcholine was 1.68%. This data strongly contrasts the data obtained by analysis of a krill oil sample in the marketplace that showed a content of 9.05% of phosphatidylcholine and 4.60% of partly decomposed phosphatidylcholine.
- Krill lipids were extracted from krill meal (a food grade powder) using supercritical fluid extraction with co-solvent. Initially, 300 bar pressure, 333°K and 5% ethanol (ethanol .'CO 2 , w/w) were utilized for 60 minutes in order to remove neutral lipids and astaxanthin from the krill meal. Next, the ethanol content was increased to 23% and the extraction was maintained for 3 hours and 40 minutes. The extract was then evaporated using a falling film evaporator and the resulting krill oil was finally filtered. The product obtained was then analyzed and the results can be found in Table 21. Table 21. Analysis of the krill oil obtained using supercritical fluid extraction.
- Krill oil was prepared according to the method described in example 7 extracting from the same krill meal. The oil was subjected to 31 P NMR analysis for the identification and quantification of the various forms of phospholipids. The analysis was performed according to the following methods: Samples (20 - 40 mg) were weighed into 1.5 ml centrifuge tubes. Next, NMR detergent (750 ⁇ l -10% Na cholate, 1% EDTA, pH 7.0 in H 2 O+D 2 O, 0.3 g L-I PMG internal standard) was added. Next, the tube was placed in a oven at 6O 0 C and periodically shaken/sonicated until completely dispersed. The solution was then transferred to a 5 ml NMR tube for analysis.
- NMR detergent 750 ⁇ l -10% Na cholate, 1% EDTA, pH 7.0 in H 2 O+D 2 O, 0.3 g L-I PMG internal standard
- Phosphorus NMR spectra were recorded on the two-channel Bruker Avance300 with the following instrument settings: spectrometer frequency 121.498MHz, sweep width 24,271 Hz, 64,000 data points, 30 degree excitation pulse, 576 transients were normally taken, each with an 8 second delay time and f.i.d. acquisition time of 1.35 sec. Spectra were processed with a standard exponential weighting function with 0.2 Hz line broadening before Fourier transformation.
- Peaks were identified using known chemical shifts. Deacylation of samples with monomethylamine was also used on two samples for confirmation of peak identity and to achieve better peak resolution.
- the main polar ether lipids of the krill meal are alkylacylphosphatidylcholine (AAPC) at 7-9 % of total polar lipids, lyso-alkylacylphosphatidylcholine (LAAPC) at 1 % of total polar lipids (TPL) and alkylacylphosphatidyl-ethanolamine (AAPE) at ⁇ 1 % of TPL.
- AAPC alkylacylphosphatidylcholine
- LAAPC lyso-alkylacylphosphatidylcholine
- TPL total polar lipids
- AAPE alkylacylphosphatidyl-ethanolamine
- Table 23 Fatty acid profile of the alkylacylphosphatidylcholine.
- the purpose of this experiment was to investigate the effect of different omega-3 fatty acid sources on metabolic parameters in the Zucker rat.
- the Zucker rat is a widely used model, of obesity and insulin resistance. Obesity is due to a mutation in the leptin receptor which impairs the regulation of intake.
- Omega-3 sources compared in this study were fish oil (FO) and two types of krill oil.
- the krill oil were either from a commercial supplier (Neptune Krill oil) or prepared according to example 7 (SuperbaTM).
- This example describes the effect of the supplementation of human diets with krill oil, fish oil (positive control), or a negative control oil (no omega-3 fatty acids) on blood urea nitrogen (BUN).
- BUN measures the amount of nitrogen in the blood that comes from urea.
- BUN is used as a measure of renal function. Serum creatinine is, however, considered to be a more specific measure of renal function.
- krill oil decreased BUN by 11.8% while creatinine levels were unchanged. Thus, it is likely that the decrease in BUN is due to some other effect than improved renal function.
- BUN decreases if krill oil induced diuresis i.e. excretion of urine (diuretic effect).
- BUN also decreases if body protein catabolism is reduced.
- Protein catabolism is a normal feature of body protein turnover. Many tissues express high protein turnover rates. For example the gastrointestinal system expresses high rates of protein turnover. In growing animals a reduction in GI protein catabolism improves weight gain. Mice supplemented with krill oil grew at a faster rate than mice supplemented with fish oil or control diet (Figure 11).
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Emergency Medicine (AREA)
- Engineering & Computer Science (AREA)
- Nutrition Science (AREA)
- Insects & Arthropods (AREA)
- Marine Sciences & Fisheries (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Wood Science & Technology (AREA)
- Microbiology (AREA)
- Physiology (AREA)
- Mycology (AREA)
- Food Science & Technology (AREA)
- Polymers & Plastics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Diabetes (AREA)
- General Chemical & Material Sciences (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
Description
Claims
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2008231570A AU2008231570C1 (en) | 2007-03-28 | 2008-03-28 | Bioeffective krill oil compositions |
| EP08718910.6A EP2144618B1 (en) | 2007-03-28 | 2008-03-28 | Bioeffective krill oil compositions |
| SI200830977T SI2144618T1 (en) | 2007-03-28 | 2008-03-28 | Bioeffective krill oil compositions |
| PL08718910T PL2144618T3 (en) | 2007-03-28 | 2008-03-28 | Bioeffective krill oil compositions |
| DK08718910.6T DK2144618T3 (en) | 2007-03-28 | 2008-03-28 | BIO-EFFECTIVE KRILL OIL COMPOSITIONS |
| ES08718910T ES2415684T3 (en) | 2007-03-28 | 2008-03-28 | Biologically effective krill oil compositions |
| CA2682068A CA2682068C (en) | 2007-03-28 | 2008-03-28 | Bioeffective krill oil compositions |
| HRP20130551AT HRP20130551T1 (en) | 2007-03-28 | 2013-06-17 | Bioeffective krill oil compositions |
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US92048307P | 2007-03-28 | 2007-03-28 | |
| US60/920,483 | 2007-03-28 | ||
| US97505807P | 2007-09-25 | 2007-09-25 | |
| US60/975,058 | 2007-09-25 | ||
| US98344607P | 2007-10-29 | 2007-10-29 | |
| US60/983,446 | 2007-10-29 | ||
| US2407208P | 2008-01-28 | 2008-01-28 | |
| US61/024,072 | 2008-01-28 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2008117062A1 true WO2008117062A1 (en) | 2008-10-02 |
Family
ID=39577593
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB2008/001080 WO2008117062A1 (en) | 2007-03-28 | 2008-03-28 | Bioeffective krill oil compositions |
Country Status (10)
| Country | Link |
|---|---|
| US (16) | US9034388B2 (en) |
| EP (2) | EP2612672A1 (en) |
| AU (1) | AU2008231570C1 (en) |
| CA (1) | CA2682068C (en) |
| DK (1) | DK2144618T3 (en) |
| ES (1) | ES2415684T3 (en) |
| HR (1) | HRP20130551T1 (en) |
| PL (1) | PL2144618T3 (en) |
| PT (1) | PT2144618E (en) |
| WO (1) | WO2008117062A1 (en) |
Cited By (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2094823A1 (en) * | 2006-11-16 | 2009-09-02 | Pronova Biopharma Norge AS | Process for production of omega-3 rich marine phospholipids from krill |
| WO2010097699A1 (en) * | 2009-02-26 | 2010-09-02 | Aker Biomarine Asa | Phospholipid and protein tablets |
| WO2010097701A1 (en) * | 2009-02-26 | 2010-09-02 | Aker Biomarine Asa | Low viscosity phospholipid compositions |
| KR100992995B1 (en) | 2010-05-03 | 2010-11-09 | 연세대학교 산학협력단 | Novel uses of piperonal |
| WO2011018096A1 (en) * | 2009-08-10 | 2011-02-17 | K.D. Pharma Bexbach Gmbh | Phytanic acid fractionation process, fatty acid products and use thereof |
| WO2011050474A1 (en) | 2009-10-29 | 2011-05-05 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions |
| WO2010136900A3 (en) * | 2009-05-28 | 2011-06-16 | Aker Biomarine Asa | Methods of using krill oil to treat risk factors for metabolic disorders and obesity |
| DE202011050351U1 (en) | 2010-06-04 | 2011-11-24 | Sana Pharma As | Dietetic products or formulations |
| CN102603791A (en) * | 2012-02-21 | 2012-07-25 | 山东师范大学 | Preparation method of Antarctic krill phosphatidyl ethanolamine product |
| WO2011124895A3 (en) * | 2010-04-09 | 2012-11-22 | Ayanda Group As | Phospholipid-containing pharmaceutical and nutraceutical compositions |
| WO2012172411A1 (en) * | 2011-06-15 | 2012-12-20 | Stable Solutions Llc | Therapeutic application of parenteral krill oil |
| WO2013136183A2 (en) | 2012-03-12 | 2013-09-19 | Innolipid, As | Oxidixable fatty acid composition delivery form |
| US8609157B2 (en) | 2009-10-30 | 2013-12-17 | Tharos Ltd. | Solvent-free process for obtaining phospholipids and neutral enriched krill oils |
| WO2014013335A2 (en) | 2012-07-17 | 2014-01-23 | Aker Biomarine As | Concentration of omega-3 polyunsaturated fatty acids in krill oil |
| AU2013100812B4 (en) * | 2009-10-29 | 2014-03-06 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions (III) |
| WO2014045127A2 (en) | 2012-09-19 | 2014-03-27 | Aker Biomarine As | Omega-3 phospholipid supplements for females |
| US8697138B2 (en) | 2007-03-28 | 2014-04-15 | Aker Biomarine As | Methods of using krill oil to treat risk factors for cardiovascular, metabolic, and inflammatory disorders |
| US8784921B2 (en) | 2008-09-26 | 2014-07-22 | Nippon Suisan Kaisha, Ltd. | Method for concentrating lipids |
| WO2014140873A2 (en) | 2013-03-14 | 2014-09-18 | Aker Biomarine As | Omega- 3 phospholipid supplements for improved brain maturity |
| US8846604B2 (en) | 2011-09-02 | 2014-09-30 | Artic Nutrition AS | Lipid compositions with high DHA content |
| US8895074B2 (en) | 2011-06-15 | 2014-11-25 | Stable Solutions Llc | Therapeutic application of parenteral krill oil |
| US9028877B2 (en) | 2007-03-28 | 2015-05-12 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| WO2016128838A2 (en) | 2015-02-11 | 2016-08-18 | Aker Biomarine Antarctic As | Lipid compositions |
| WO2016128830A1 (en) | 2015-02-11 | 2016-08-18 | Aker Biomarine Antarctic As | Lipid extraction processes |
| EP2988759A4 (en) * | 2013-03-14 | 2016-11-30 | Inst Rech Sur Les Zones Cotieres Irzc | Method for extracting organic solids and oil from marine organisms enriched with astaxanthin |
| WO2016190748A1 (en) | 2015-05-27 | 2016-12-01 | Rimfrost As | A flowable concentrated phospholipid krill oil composition |
| US9867856B2 (en) | 2014-01-10 | 2018-01-16 | Aker Biomarine Antarctic As | Phospholipid compositions and their preparation |
| EP2800481B1 (en) | 2012-01-03 | 2018-01-24 | Rimfrost Technologies AS | Method for processing crustaceans to produce low fluoride/low trimethyl amine products thereof |
| IT201600124013A1 (en) * | 2016-12-06 | 2018-06-06 | Solarpharm Srl | Astaxanthin based food product |
| US10052352B2 (en) | 2011-06-15 | 2018-08-21 | Stable Solutions Llc | Therapeutic application of parenteral krill oil |
| US10080803B2 (en) | 2014-04-25 | 2018-09-25 | Aker Biomarine Antartic As | Emulsified krill phospholipid compositions |
| US10105376B2 (en) | 2012-09-24 | 2018-10-23 | Aker Biomarine Antarctic As | Omega-3 compositions |
| EP3137070B1 (en) | 2014-04-30 | 2020-04-15 | Aker BioMarine Antarctic AS | Krill oil preparations and their uses |
| US10653725B2 (en) | 2015-08-17 | 2020-05-19 | Aker Biomarine Antarctic As | Methods for improving health of farmed fish |
| US10704011B2 (en) | 2013-06-14 | 2020-07-07 | Aker Biomarine Antarctic As | Lipid extraction processes |
| US10806742B2 (en) | 2014-02-12 | 2020-10-20 | Aker Biomarine Antarctic As | Liquid phospholipid-containing compositions for the preparation of pharmaceuticals |
| US10856566B2 (en) | 2015-02-27 | 2020-12-08 | Rb Health (Us) Llc | Co-Q10, krill oil and vitamin D |
| US10960016B2 (en) | 2014-02-12 | 2021-03-30 | Aker Biomarine Antarctic As | Capsules containing high doses of krill phospholipids |
| US11147841B2 (en) | 2014-12-19 | 2021-10-19 | Aker Biomarine Antarctic As | Enhanced omega-3 formulations |
| EP3749339B1 (en) | 2018-01-30 | 2022-08-03 | Aker Biomarine Antarctic As | Marine protein hydrolysate with low fluoride and trimethylamin content |
| WO2023198752A1 (en) * | 2022-04-12 | 2023-10-19 | Aker Biomarine Antarctic As | Supplemented krill meal and uses thereof |
| US12023359B2 (en) | 2022-11-01 | 2024-07-02 | Aker Biomarine Human Ingredients As | Phospholipid compositions for delivery of therapeutic compounds |
Families Citing this family (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE168097T1 (en) * | 1993-10-15 | 1998-07-15 | Fina Research | METHOD FOR PURIFYING MEDIUM OLEFINS |
| AU2014100741B4 (en) * | 2007-08-29 | 2014-09-11 | Aker Biomarine Antarctic As | Processes and products thereof |
| EP3777831B1 (en) | 2008-03-26 | 2023-11-29 | Oramed Ltd. | Methods and compositions for oral administration of proteins |
| US9814256B2 (en) * | 2009-09-14 | 2017-11-14 | Rimfrost Technologies As | Method for processing crustaceans to produce low fluoride/low trimethyl amine products thereof |
| ES2639959T3 (en) | 2008-09-12 | 2017-10-30 | Rimfrost Technologies As | Fluoride content reduction procedure when protein concentrates are produced from krill |
| RU2563995C2 (en) * | 2009-01-05 | 2015-09-27 | Каланус Ас | Composition containing biological fat, medicinal products containing composition containing biological fat, and using them for preventing or treating cardiovascular diseases |
| JP2012529503A (en) * | 2009-06-12 | 2012-11-22 | カラナス エーエス | Oil compositions, formulations containing oil compositions, and their use to prevent or treat obesity-related diseases and disorders by reducing the accumulation of built-in fats and improving glucose tolerance |
| US20130295171A1 (en) | 2009-07-23 | 2013-11-07 | U.S NUTRACEUTICALS, LLC d/b/a Valensa International | Krill oil and reacted astaxanthin composition and associated method |
| US20110223246A1 (en) * | 2010-03-10 | 2011-09-15 | Joar Opheim | Docosahexaenoic acid bound in phospholipids and method of recovering same from a natural source |
| US8273248B1 (en) | 2010-04-06 | 2012-09-25 | Heliae Development, Llc | Extraction of neutral lipids by a two solvent method |
| JP2013523156A (en) | 2010-04-06 | 2013-06-17 | ヘリアエ デベロップメント、 エルエルシー | Selective extraction of proteins from freshwater algae |
| US8663704B2 (en) | 2010-04-30 | 2014-03-04 | U.S. Nutraceuticals, LLC | Composition and method to improve blood lipid profiles and optionally reduce low density lipoprotein (LDL) per-oxidation in humans |
| US9763897B2 (en) | 2010-04-30 | 2017-09-19 | U.S. Nutraceuticals, LLC | Therapeutic astaxanthin and phospholipid composition and associated method |
| JP5271454B2 (en) | 2010-09-01 | 2013-08-21 | 日本水産株式会社 | Alcoholic disorder alleviator |
| US20120231087A1 (en) | 2011-03-07 | 2012-09-13 | Olympic Seafood | Compositions And Methods for Nutritional Supplementation |
| PT2697345T (en) * | 2011-04-14 | 2016-07-07 | Polar Omega As | A process for the isolation of a phospholipid |
| CN103608022A (en) * | 2011-05-31 | 2014-02-26 | 日本水产株式会社 | Brain function improving agent |
| WO2013075116A2 (en) | 2011-11-17 | 2013-05-23 | Heliae Development, Llc | Omega 7 rich compositions and methods of isolating omega 7 fatty acids |
| AU2012347606A1 (en) * | 2011-12-08 | 2014-06-26 | Metaproteomics, Llc | Supplemented oil compositions and methods for improved health |
| AU2015100022B4 (en) * | 2012-01-03 | 2016-01-07 | Rimfrost Technologies As | Method for processing crustaceans to produce low fluoride/low trimethyl amine products thereof |
| US20140088047A1 (en) * | 2012-09-24 | 2014-03-27 | Aker Bioassist As | Use of long chain polyunsaturated fatty acid derivatives to treat sickle cell disease |
| EP4215205A1 (en) | 2013-01-03 | 2023-07-26 | Oramed Ltd. | Methods and compositions for treating nafld, hepatic steatosis, and sequelae thereof |
| WO2014184655A1 (en) | 2013-02-07 | 2014-11-20 | Olympic Seafood As | Methods for using crustacean phospholipid-peptide-protein complexes |
| CN104208117A (en) * | 2014-09-15 | 2014-12-17 | 天津蒙特立医疗科技有限责任公司 | Plant extract compound product with function of maintaining liver |
| AU2015347014B2 (en) | 2014-11-14 | 2019-01-17 | Tharos Ltd. | Solvent-free process for obtaining phospholipids and neutral enriched krill oils using melting and evaporation |
| US20180243345A1 (en) * | 2015-08-20 | 2018-08-30 | Avoca, Inc. | Method for increasing muscle growth using krill extract |
| CN111432654A (en) | 2017-12-04 | 2020-07-17 | 雾凇科技有限公司 | Method for producing protein phospholipid complex from crustacean capture |
| KR20200102914A (en) * | 2017-12-22 | 2020-09-01 | 파르마링크 인터내셔날 리미티드 | Lipid combination |
| CN108179053B (en) * | 2018-01-26 | 2021-07-20 | 日照职业技术学院 | Preparation method of high-quality euphausia superba oil |
| JP7188028B2 (en) * | 2018-11-30 | 2022-12-13 | 株式会社リコー | Cabinet-integrated image forming device |
| JP7438216B2 (en) * | 2018-11-30 | 2024-02-26 | エボニック オペレーションズ ゲーエムベーハー | Preparations containing dispersions of phospholipids and fatty acid salts |
| AU2020220816A1 (en) * | 2019-02-12 | 2021-09-23 | Acasti Pharma Inc. | Process of producing concentrated therapeutic phospholipid composition from krill extracts containing high level of free fatty acids |
| CN110575680A (en) * | 2019-09-19 | 2019-12-17 | 福建启元堂生物技术有限公司 | Method for double super-extraction of marine nutrients |
| CN111876246B (en) * | 2020-08-05 | 2023-06-20 | 赵福江 | Krill oil extraction method |
| CN114073864B (en) * | 2022-01-19 | 2022-05-24 | 华南理工大学 | Method for synchronously extracting and separating multiple components in raw material by four-liquid-phase system |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4119619A (en) * | 1976-12-16 | 1978-10-10 | Sergei Vasilievich Rogozhin | Emulsification method for the processing of kriel to produce protein, lipids and chitin |
| WO2000023546A1 (en) * | 1998-10-21 | 2000-04-27 | Universite De Sherbrooke | Method of extracting lipids from marine and aquatic animal tissues |
| EP1127497A1 (en) * | 1998-11-02 | 2001-08-29 | Nippon Suisan Kaisha, Ltd. | Dry krill powder |
| WO2002102394A2 (en) * | 2001-06-18 | 2002-12-27 | Neptune Technologies & Bioressources Inc. | Krill and/or marine extracts for prevention and/or treatment of cardiovascular diseases, arthritis, skin cancer, diabetes, premenstrual syndrome and transdermal transport |
| WO2003011873A2 (en) | 2001-07-27 | 2003-02-13 | Neptune Technologies & Bioressources Inc. | Natural marine source phospholipids comprising flavonoids, polyunsaturated fatty acids and their applications |
Family Cites Families (126)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2652235A (en) * | 1951-11-29 | 1953-09-15 | Beatrice L Samuelsen | Heating attachment for electric food mixers |
| GB921537A (en) | 1959-05-22 | 1963-03-20 | Mario Meroni | Process for extracting fatty substances from animal material |
| JPS58850B2 (en) | 1975-03-14 | 1983-01-08 | 日魯漁業株式会社 | Processing method for krill from the Antarctic Ocean |
| JPS51125773A (en) * | 1975-04-25 | 1976-11-02 | Amao Suisan Kk | Method of producing fish meat extract |
| JPS51125774A (en) | 1975-04-25 | 1976-11-02 | Nichiro Gyogyo Kk | Method of processing euphausiid from antarctic ocean |
| JPS5842741B2 (en) | 1976-03-19 | 1983-09-21 | 日研化学株式会社 | Method for producing protein liquid and protein powder |
| PL110567B1 (en) | 1976-09-14 | 1980-07-31 | Przedsieb Polowow Dalekom | Method of obtaining the meat from marine crustaceans,specially from antarctic krill |
| CA1098900A (en) | 1977-12-14 | 1981-04-07 | Sergei V. Rogozhin | Method for the processing of krill to produce protein, lipids and chitin |
| JPS54160789A (en) * | 1978-06-12 | 1979-12-19 | Shimose Shokuhin Kk | Fish meal producing system plant by batch type and complete closed system |
| WO1982002819A1 (en) | 1981-02-16 | 1982-09-02 | Hansen Bent Schiott | A food product on the basis of fish flesh and a process for the preparation thereof |
| GB2097014B (en) | 1981-04-13 | 1984-08-01 | Baikoff Eugene Marc Alexandre | Ultrasonic extraction of vegetable oil |
| US4505936A (en) * | 1983-09-14 | 1985-03-19 | Louisiana State University | Process for the utilization of shellfish waste |
| JPS60153779A (en) | 1984-01-24 | 1985-08-13 | Hohnen Oil Co Ltd | Nutrient-assisting food |
| US4814111A (en) | 1984-02-13 | 1989-03-21 | Air Products And Chemicals, Inc. | Process for purification of phospholipids |
| US4714571A (en) * | 1984-02-13 | 1987-12-22 | The Liposome Company, Inc. | Process for purification of phospholipids |
| JPS611222A (en) | 1984-06-08 | 1986-01-07 | 日本電気株式会社 | Power source switch circuit |
| US5006281A (en) * | 1985-03-26 | 1991-04-09 | Century Laboratories, Inc. | Process for the production of a marine animal oil |
| DK157452C (en) | 1985-04-12 | 1990-05-21 | Matcon Radgivende Ing Firma | PROCEDURE FOR THE EXTRACTION OF CHITIN FROM CHITINE SOURCES, WHICH IT IS TOGETHER WITH OR CONNECTED TO PROTEIN SUBSTANCES |
| JPS61281159A (en) | 1985-06-06 | 1986-12-11 | Shiseido Co Ltd | Production of orange pigment |
| US4749522A (en) | 1985-10-31 | 1988-06-07 | Angio-Medical Corporation | Supercritical fluid extraction of animal derived materials |
| FR2596060B1 (en) | 1986-03-20 | 1988-07-08 | Guillot Bernard | OIL SEED OIL EXTRACTION BY ULTRASOUND |
| JPS6323819A (en) | 1986-07-16 | 1988-02-01 | Kao Corp | Inhibitor of blood platelet aggregation |
| JPS642665A (en) | 1987-06-25 | 1989-01-06 | Matsushita Electric Works Ltd | Warm bath device |
| SE8703064D0 (en) | 1987-08-06 | 1987-08-06 | Viggo Mohr | METHOD FOR ISOLATING ACTIVE ENZYME PREPARATIONS FROM ANIMAL TISSUES |
| JP2524217B2 (en) | 1988-04-18 | 1996-08-14 | マルハ株式会社 | Brain function improving composition, learning ability enhancing agent, memory enhancing agent, dementia preventive agent or dementia therapeutic agent |
| SE8801701D0 (en) | 1988-05-05 | 1988-05-05 | Pharmacia Ab | PRODUCTION TECHNOLOGY BASED ON ENZYMIC METHOD |
| JP2619491B2 (en) | 1988-08-11 | 1997-06-11 | サントリー株式会社 | Astaxanthin-containing composition |
| NO176323C (en) | 1988-11-18 | 1995-03-15 | Mikalsen Ester | Process for Extraction of Astaxanthin, Related Carotenoids and Astaxanthin Esters from Krill, Shrimp and Other Crustaceans |
| JP2909508B2 (en) | 1989-02-14 | 1999-06-23 | マルハ株式会社 | Krill phospholipid fractionation method |
| JPH02215352A (en) | 1989-02-16 | 1990-08-28 | Advance Co Ltd | Auxiliary material for cooking |
| DE3918082A1 (en) * | 1989-06-02 | 1991-01-24 | Max Planck Gesellschaft | AGENT FOR AUTOIMMUNE DISEASES |
| JPH0381692A (en) | 1989-08-23 | 1991-04-08 | Hitachi Ltd | Fuel supply device of magnetic field confinment type nuclear fusion device |
| JP2861246B2 (en) | 1990-04-26 | 1999-02-24 | 松下電器産業株式会社 | Switching power supply |
| JP2963152B2 (en) * | 1990-06-28 | 1999-10-12 | クロリンエンジニアズ株式会社 | Extraction and separation method of pigment from krill |
| JP2524217Y2 (en) | 1990-12-29 | 1997-01-29 | ニチバン株式会社 | Medical adhesive tape |
| SE9101642D0 (en) | 1991-05-30 | 1991-05-30 | Kabi Pharmacia Ab | phospholipids |
| JP3081692B2 (en) | 1991-11-28 | 2000-08-28 | クロリンエンジニアズ株式会社 | Extraction and separation method of pigment from krill |
| SE9201628D0 (en) | 1992-05-22 | 1992-05-22 | Phairson Medical Ab | PHARMACEUTICAL COMPOSITION |
| GB9301629D0 (en) | 1993-01-27 | 1993-03-17 | Scotia Holdings Plc | Formulations containing unsaturated fatty acids |
| JP3467794B2 (en) | 1993-03-09 | 2003-11-17 | 日本油脂株式会社 | Learning ability improver |
| JP3486778B2 (en) | 1993-04-02 | 2004-01-13 | 三菱ウェルファーマ株式会社 | Alzheimer's disease preventive / treatment agent |
| JPH07242621A (en) | 1994-03-02 | 1995-09-19 | Nippon Oil Co Ltd | Method for extracting carotenoid compound |
| JPH08231391A (en) | 1995-02-24 | 1996-09-10 | Kanagawa Kagaku Kenkyusho:Kk | Dementia-improving medicine |
| FR2731015B1 (en) * | 1995-02-24 | 1997-05-30 | Sci Sartone | PROCESS FOR THE ENZYMATIC ENRICHMENT OF OILS OF MARINE ORIGIN AND THE TRIGLYCERIDES OF POLYUNSATURATED FATTY ACIDS THUS OBTAINED |
| JP3344887B2 (en) | 1995-12-22 | 2002-11-18 | 池田食研株式会社 | Method for concentrating fats and oils containing highly unsaturated fatty acids |
| JP3611222B2 (en) | 1996-01-26 | 2005-01-19 | 池田食研株式会社 | Method for reducing peroxides of highly unsaturated fatty acids |
| US5672370A (en) | 1996-04-16 | 1997-09-30 | The University Of British Columbia | Method of producing a dried krill product |
| WO1997039759A2 (en) | 1996-04-24 | 1997-10-30 | Brigham And Women's Hospital | Omega-3 fatty acids and omega-3 phosphatidylcholine in the treatment of bipolar disorder |
| JP3678317B2 (en) | 1996-05-21 | 2005-08-03 | クロリンエンジニアズ株式会社 | Method for concentrating eicosapentaenoic acid-containing material |
| US6555155B2 (en) | 1996-10-21 | 2003-04-29 | Biozyme Systems, Inc. | Method and apparatus for harvesting, digestion and dehydrating of krill hydrolysates and co-drying and processing of such hydrolysates |
| CA2197137A1 (en) | 1997-02-07 | 1998-08-07 | Biozyme Systems Inc. | Method and apparatus for co-drying krill hydrolysate, liquid marine protein and dry carrier |
| FR2757021B1 (en) * | 1996-12-17 | 1999-01-22 | Sea Oil | PROCESS AND PLANT FOR EXTRACTING FISH OIL AND PRODUCTS OBTAINED |
| IL121320A (en) | 1997-02-23 | 2000-12-06 | Ibr Ltd | Extracts from cells or tissue of organisms which are capable of entering dormancy for inhibition of proliferation of target cells or tissue |
| JP3408958B2 (en) * | 1997-10-24 | 2003-05-19 | 旭化成株式会社 | Composition containing useful substance derived from fish and shellfish and method for producing the useful substance |
| US20030113432A1 (en) * | 1998-11-02 | 2003-06-19 | Nippon Suisan Kaisha, Ltd. | Process for making dried powdery and granular krill |
| WO2000038708A1 (en) | 1998-12-24 | 2000-07-06 | Phairson Medical Inc. | Treatment and prevention of immune rejection reactions |
| IT1315252B1 (en) | 1999-10-21 | 2003-02-03 | Truffini & Regge Farmaceutici | GASTRORESISTANT TABLETS FOR FOOD OR DIET USE, OBTAINED THROUGH GROUND MIXED FATS |
| JP2001158736A (en) | 1999-11-30 | 2001-06-12 | Snow Brand Milk Prod Co Ltd | Agent for preventing and improving osteoarthropathy |
| GB2358862B (en) * | 1999-12-21 | 2004-07-21 | Fermentron Ltd | Processes for Extracting Carotenoids from Biomass Carotenoid Sources |
| WO2001076385A1 (en) | 2000-04-12 | 2001-10-18 | Westfalia Separator Industry Gmbh | Method for the fractionation of oil and polar lipid-containing native raw materials using alcohol and centrifugation |
| DK1292294T3 (en) | 2000-05-01 | 2009-06-22 | Accera Inc | Use of medium chain triglycerides for the treatment and prevention of Alzheimer's disease |
| EP1333077A4 (en) * | 2000-10-20 | 2004-07-07 | Nippon Kayaku Kk | Varnish containing polyamide resin and use thereof |
| IL142535A0 (en) | 2001-04-11 | 2002-03-10 | Yeda Res & Dev | Pharmaceutical compositions for the treatment of inflammation |
| JP3081692U (en) | 2001-05-11 | 2001-11-16 | 鶴進 陳 | Shower rod structure with detergent |
| MX281182B (en) | 2001-05-14 | 2010-11-22 | Martek Biosciences Boulder Corp | Production and use of a polar lipid-rich fraction containing omega-3 and/or omega-6 highly unsatruated fatty acids from microbes, genetically modified plant seeds and marine organisms. |
| JP2003003192A (en) | 2001-06-20 | 2003-01-08 | Unitika Ltd | Method for extracting sphingolipid or sphingoglycolipid |
| AR034680A1 (en) | 2001-07-04 | 2004-03-03 | Angulas Aguinaga S A | PROCEDURE FOR OBTAINING THERMOCONFORMED PRODUCTS FROM THE LIQUID AND DENSA FRACTIONS OF ANTARCTIC KRILL |
| JP2003048831A (en) | 2001-08-02 | 2003-02-21 | Suntory Ltd | Composition having preventing and ameliorating action on symptom or disease caused by decrease in brain function |
| JP4568464B2 (en) | 2001-11-07 | 2010-10-27 | 雪印乳業株式会社 | Memory disorder prevention and treatment |
| US20050014722A1 (en) * | 2001-12-13 | 2005-01-20 | Gil-Ja Jhon | Process for preparing n-acylated lysophosphatidylcholine and pharmaceutical composition for treatment of metabolic bone disease comprising said compounds |
| CN100418519C (en) | 2002-09-24 | 2008-09-17 | 三得利株式会社 | Composition containing arachidonic acid or the combination of docosahexenoic acid therewith for enhancing cognitive abilities |
| EP1569525A1 (en) | 2002-11-26 | 2005-09-07 | Phares Pharmaceutical Research N.V. | Marine lipid compositions |
| US7488503B1 (en) | 2003-03-31 | 2009-02-10 | Mccormick & Company, Inc. | Encapsulation compositions and processes for preparing the same |
| CN1771046A (en) * | 2003-04-11 | 2006-05-10 | 协和发酵工业株式会社 | Preventive or remedy for arthritis |
| GB0311081D0 (en) | 2003-05-14 | 2003-06-18 | Btg Internat Limted | Treatment of neurodegenerative conditions |
| US9072311B2 (en) * | 2003-06-19 | 2015-07-07 | Advanced Bionutrition Corporation | Absorption of fat-soluble nutrients |
| WO2005004393A1 (en) | 2003-07-03 | 2005-01-13 | Koninklijke Philips Electronics N.V. | Secure indirect addressing |
| NO20033198D0 (en) | 2003-07-15 | 2003-07-15 | Norway Seafoods As | Device by trawl |
| US7964641B2 (en) | 2003-08-18 | 2011-06-21 | Btg International Limited | Treatment of neurodegenerative conditions |
| IL158552A0 (en) | 2003-10-22 | 2004-05-12 | Enzymotec Ltd | Lipids containing omega-3 fatty acids |
| IL158553A0 (en) | 2003-10-22 | 2004-05-12 | Enzymotec Ltd | Method for preparing phosphatidylserine containing omega-3 acid moieties |
| ITMI20040069A1 (en) | 2004-01-21 | 2004-04-21 | Tiberio Bruzzese | USE OF HIGH CONCENTRATION N-3 FATTY ACID COMPOSITIONS FOR THE TREATMENT OF DISORDERS OF THE CENTRAL NERVOUS SYSTEM |
| JP2005245379A (en) | 2004-03-08 | 2005-09-15 | Nippon Suisan Kaisha Ltd | Aggregate of eyeball of crustaceans, method for using the same, food containing the same, and method for producing the same |
| JP2006069948A (en) | 2004-09-01 | 2006-03-16 | Yukihiro Hirose | Anti-aging composition and cosmetics, beverages and foods containing the same |
| JP2006083136A (en) | 2004-09-17 | 2006-03-30 | Suntory Ltd | Composition having action for preventing or ameliorating lowering of cerebral function caused by stress and symptom or disease involving the same lowering |
| US7666447B2 (en) * | 2004-10-08 | 2010-02-23 | Pharmanutrients | Compositions including Krill extracts and conjugated linoleic acid and methods of using same |
| US20060088574A1 (en) * | 2004-10-25 | 2006-04-27 | Manning Paul B | Nutritional supplements |
| CA2587730A1 (en) * | 2004-11-17 | 2006-05-26 | Natural Asa | Enzymatically synthesized marine phospholipids |
| JP2006290784A (en) | 2005-04-08 | 2006-10-26 | Yukihiro Hirose | Composition having bloodstream aggravation-preventing action and food and beverage containing the same composition |
| US7759325B2 (en) | 2005-04-18 | 2010-07-20 | Sc Dicophar | Use of lecithin as a medication for the treatment of psoriasis |
| JP2006328014A (en) | 2005-05-30 | 2006-12-07 | Yukihiro Hirose | Composition having prevention action on blood flow deterioration |
| JP2007246404A (en) | 2006-03-14 | 2007-09-27 | Snow Brand Milk Prod Co Ltd | Learning ability-improving agent |
| JP2007126455A (en) | 2005-10-07 | 2007-05-24 | Fuji Chem Ind Co Ltd | Cerebral dysfunction improving agent |
| WO2007080514A2 (en) | 2006-01-13 | 2007-07-19 | Krill A/S | A method for the extraction of lipid fractions from krill |
| WO2007080515A1 (en) | 2006-01-13 | 2007-07-19 | Aker Biomarine Asa | Thrombosis preventing krill extract |
| NO20061315L (en) | 2006-03-23 | 2007-09-24 | Aker Seafoods Holding As | Device by trolley |
| TW200811280A (en) | 2006-04-20 | 2008-03-01 | Owen John Catchpole | Product and process |
| NZ547429A (en) | 2006-05-24 | 2009-09-25 | Ind Res Ltd | Extraction of highly unsaturated lipids with liquid dimethyl ether |
| AU2007271900B2 (en) | 2006-07-14 | 2013-05-23 | Nattopharma As | Pharmaceutical and nutraceutical products comprising vitamin K2 |
| CN101652462A (en) | 2006-11-16 | 2010-02-17 | 普罗诺瓦生物医药挪威公司 | Process for production of omega-3 rich marine phospholipids from krill |
| WO2008072563A1 (en) | 2006-12-11 | 2008-06-19 | Nippon Suisan Kaisha, Ltd. | Feed using peeled krill as the starting material and method of preventing decrease in fish growth rate by using the same |
| US20080166420A1 (en) | 2007-01-04 | 2008-07-10 | Sones Scott F | Krill Oil Compositions |
| US20080166419A1 (en) * | 2007-01-04 | 2008-07-10 | Sones Scott F | Krill oil compositions |
| EP2612672A1 (en) | 2007-03-28 | 2013-07-10 | Aker BioMarine AS | Bioeffective krill oil compositions |
| US8697138B2 (en) | 2007-03-28 | 2014-04-15 | Aker Biomarine As | Methods of using krill oil to treat risk factors for cardiovascular, metabolic, and inflammatory disorders |
| US20080268117A1 (en) | 2007-04-30 | 2008-10-30 | David Rubin | Method of purifying oils containing epa and dha |
| US20100226977A1 (en) | 2007-08-29 | 2010-09-09 | Aker Biomarine Asa | Low viscosity phospholipid compositions |
| EP2732709A1 (en) | 2007-08-29 | 2014-05-21 | Aker BioMarine AS | A new method for making krill meal |
| BRPI0912749A2 (en) | 2008-05-15 | 2017-05-23 | Pronova Biopharma Norge As | Krill oil process. |
| US8557297B2 (en) | 2008-09-12 | 2013-10-15 | Olympic Seafood, As | Method for processing crustaceans and products thereof |
| WO2010097701A1 (en) | 2009-02-26 | 2010-09-02 | Aker Biomarine Asa | Low viscosity phospholipid compositions |
| US8372812B2 (en) | 2009-02-26 | 2013-02-12 | Aker Biomarine Asa | Phospholipid and protein tablets |
| PT2493478T (en) | 2009-10-29 | 2018-04-03 | Acasti Pharma Inc | Concentrated therapeutic phospholipid compositions |
| KR101616446B1 (en) | 2009-10-30 | 2016-04-28 | 샤로스 리미티드 | Solvent-free process for obtaining phospholipids and neutral enriched krill oils |
| US20110223246A1 (en) | 2010-03-10 | 2011-09-15 | Joar Opheim | Docosahexaenoic acid bound in phospholipids and method of recovering same from a natural source |
| WO2011130487A1 (en) | 2010-04-14 | 2011-10-20 | Ganeden Biotech, Inc. | Probiotic confection and lipid compositions |
| KR101835326B1 (en) | 2010-09-26 | 2018-03-07 | 엘지전자 주식회사 | Method and apparatus for efficient feedback in a wireless communication system supporting multiple antenna |
| PT2697345T (en) | 2011-04-14 | 2016-07-07 | Polar Omega As | A process for the isolation of a phospholipid |
| US20150030718A1 (en) | 2012-03-12 | 2015-01-29 | Aker Biomarine Antarctic As | Oxidixable fatty acid composition delivery form |
| EP2874500A2 (en) | 2012-07-17 | 2015-05-27 | Aker Biomarine Antarctic AS | Concentration of omega-3 polyunsaturated fatty acids in krill oil |
| CN102746941B (en) | 2012-07-18 | 2014-01-15 | 山东师范大学 | Method for enriching phosphatidyl inositol from antarctic krill |
| US20140080791A1 (en) | 2012-09-19 | 2014-03-20 | Aker Biomarine As | Omega-3 phospholipid supplements for females |
| WO2014057362A2 (en) | 2012-09-24 | 2014-04-17 | Aker Biopharma As | Omega -3 compositions |
| US20140088047A1 (en) | 2012-09-24 | 2014-03-27 | Aker Bioassist As | Use of long chain polyunsaturated fatty acid derivatives to treat sickle cell disease |
| WO2014140873A2 (en) | 2013-03-14 | 2014-09-18 | Aker Biomarine As | Omega- 3 phospholipid supplements for improved brain maturity |
| AU2014203179C1 (en) | 2013-06-14 | 2017-05-04 | Aker Biomarine Antarctic As | Lipid extraction processes |
-
2008
- 2008-03-28 EP EP12187516.5A patent/EP2612672A1/en not_active Withdrawn
- 2008-03-28 US US12/057,775 patent/US9034388B2/en not_active Expired - Fee Related
- 2008-03-28 ES ES08718910T patent/ES2415684T3/en active Active
- 2008-03-28 AU AU2008231570A patent/AU2008231570C1/en active Active
- 2008-03-28 DK DK08718910.6T patent/DK2144618T3/en active
- 2008-03-28 CA CA2682068A patent/CA2682068C/en active Active
- 2008-03-28 PL PL08718910T patent/PL2144618T3/en unknown
- 2008-03-28 EP EP08718910.6A patent/EP2144618B1/en active Active
- 2008-03-28 PT PT87189106T patent/PT2144618E/en unknown
- 2008-03-28 WO PCT/GB2008/001080 patent/WO2008117062A1/en active Application Filing
-
2013
- 2013-06-17 HR HRP20130551AT patent/HRP20130551T1/en unknown
- 2013-09-06 US US14/020,162 patent/US9375453B2/en active Active
- 2013-09-06 US US14/020,155 patent/US9320765B2/en not_active Expired - Fee Related
-
2014
- 2014-09-18 US US14/490,176 patent/US9028877B2/en not_active Expired - Fee Related
- 2014-09-18 US US14/490,221 patent/US9078905B2/en not_active Expired - Fee Related
-
2015
- 2015-02-12 US US14/620,784 patent/US9072752B1/en not_active Expired - Fee Related
- 2015-02-12 US US14/620,779 patent/US9119864B2/en not_active Expired - Fee Related
-
2016
- 2016-06-13 US US15/180,431 patent/US9644169B2/en active Active
- 2016-06-13 US US15/180,439 patent/US9644170B2/en active Active
-
2017
- 2017-05-08 US US15/589,605 patent/US10010567B2/en active Active
- 2017-05-08 US US15/589,572 patent/US9816046B2/en active Active
- 2017-05-08 US US15/589,591 patent/US9889163B2/en active Active
-
2018
- 2018-03-08 US US15/915,439 patent/US11865143B2/en active Active
- 2018-05-10 US US15/976,130 patent/US20180256650A1/en not_active Abandoned
- 2018-06-29 US US16/023,146 patent/US10543237B2/en not_active Expired - Fee Related
-
2023
- 2023-12-15 US US18/541,250 patent/US20240189366A1/en active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4119619A (en) * | 1976-12-16 | 1978-10-10 | Sergei Vasilievich Rogozhin | Emulsification method for the processing of kriel to produce protein, lipids and chitin |
| WO2000023546A1 (en) * | 1998-10-21 | 2000-04-27 | Universite De Sherbrooke | Method of extracting lipids from marine and aquatic animal tissues |
| US6800299B1 (en) | 1998-10-21 | 2004-10-05 | Universite De Sherbrooke | Method of extracting lipids from marine and aquatic animal tissues |
| EP1127497A1 (en) * | 1998-11-02 | 2001-08-29 | Nippon Suisan Kaisha, Ltd. | Dry krill powder |
| WO2002102394A2 (en) * | 2001-06-18 | 2002-12-27 | Neptune Technologies & Bioressources Inc. | Krill and/or marine extracts for prevention and/or treatment of cardiovascular diseases, arthritis, skin cancer, diabetes, premenstrual syndrome and transdermal transport |
| WO2003011873A2 (en) | 2001-07-27 | 2003-02-13 | Neptune Technologies & Bioressources Inc. | Natural marine source phospholipids comprising flavonoids, polyunsaturated fatty acids and their applications |
Non-Patent Citations (4)
| Title |
|---|
| SAETHER ET AL., COMP. BIOCHEM PHYS. B, vol. 83B, no. 1, 1986, pages 51 - 55 |
| TANAKA ET AL., J. OLEO SCI., vol. 53, no. 9, 2004, pages 417 - 424 |
| YAMAGUCHI ET AL., J AGRIC. FOOD CHEM., vol. 34, no. 5, 1986, pages 904 - 7 |
| YAMAGUCHI K ET AL: "Supercritical carbon dioxide extraction of oils from antarctic krill", JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY, AMERICAN CHEMICAL SOCIETY. WASHINGTON, US, vol. 34, 1 January 1986 (1986-01-01), pages 904 - 907, XP002430955, ISSN: 0021-8561 * |
Cited By (102)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2094823A4 (en) * | 2006-11-16 | 2011-02-02 | Pronova Biopharma Norge As | Process for production of omega-3 rich marine phospholipids from krill |
| EP2094823A1 (en) * | 2006-11-16 | 2009-09-02 | Pronova Biopharma Norge AS | Process for production of omega-3 rich marine phospholipids from krill |
| US9644169B2 (en) | 2007-03-28 | 2017-05-09 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US9119864B2 (en) | 2007-03-28 | 2015-09-01 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US8697138B2 (en) | 2007-03-28 | 2014-04-15 | Aker Biomarine As | Methods of using krill oil to treat risk factors for cardiovascular, metabolic, and inflammatory disorders |
| US11865143B2 (en) | 2007-03-28 | 2024-01-09 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US9028877B2 (en) | 2007-03-28 | 2015-05-12 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US9034388B2 (en) | 2007-03-28 | 2015-05-19 | Aker Biomarine Antartic As | Bioeffective krill oil compositions |
| US9889163B2 (en) | 2007-03-28 | 2018-02-13 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US9816046B2 (en) | 2007-03-28 | 2017-11-14 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US9730966B2 (en) | 2007-03-28 | 2017-08-15 | Aker Biomarine Antartic As | Method of reducing appetite in a human subject comprising administering krill oil composition |
| US10543237B2 (en) | 2007-03-28 | 2020-01-28 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US9072752B1 (en) | 2007-03-28 | 2015-07-07 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US9078905B2 (en) | 2007-03-28 | 2015-07-14 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US9644170B2 (en) | 2007-03-28 | 2017-05-09 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US9375453B2 (en) | 2007-03-28 | 2016-06-28 | Aker Biomarine Antarctic As | Methods for producing bioeffective krill oil compositions |
| US9320765B2 (en) | 2007-03-28 | 2016-04-26 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US9220735B2 (en) | 2007-03-28 | 2015-12-29 | Aker Biomarine Antarctic As | Methods of using krill oil to treat risk factors for cardiovascular, metabolic, and inflammatory disorders |
| US10010567B2 (en) | 2007-03-28 | 2018-07-03 | Aker Biomarine Antarctic As | Bioeffective krill oil compositions |
| US8784921B2 (en) | 2008-09-26 | 2014-07-22 | Nippon Suisan Kaisha, Ltd. | Method for concentrating lipids |
| WO2010097701A1 (en) * | 2009-02-26 | 2010-09-02 | Aker Biomarine Asa | Low viscosity phospholipid compositions |
| WO2010097699A1 (en) * | 2009-02-26 | 2010-09-02 | Aker Biomarine Asa | Phospholipid and protein tablets |
| US8372812B2 (en) | 2009-02-26 | 2013-02-12 | Aker Biomarine Asa | Phospholipid and protein tablets |
| WO2010136900A3 (en) * | 2009-05-28 | 2011-06-16 | Aker Biomarine Asa | Methods of using krill oil to treat risk factors for metabolic disorders and obesity |
| WO2011018096A1 (en) * | 2009-08-10 | 2011-02-17 | K.D. Pharma Bexbach Gmbh | Phytanic acid fractionation process, fatty acid products and use thereof |
| US10617702B2 (en) | 2009-10-29 | 2020-04-14 | Acasti Pharma Inc. | Concentrated therapeutic phospholipid compositions |
| AU2010312238B2 (en) * | 2009-10-29 | 2016-08-11 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions |
| JP2018138612A (en) * | 2009-10-29 | 2018-09-06 | アカスティ ファーマ インコーポレイテッド | Concentrated therapeutic phospholipid compositions |
| EP3335713A1 (en) * | 2009-10-29 | 2018-06-20 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions |
| AU2013100813B4 (en) * | 2009-10-29 | 2014-03-06 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions (IV) |
| AU2013100811B4 (en) * | 2009-10-29 | 2014-03-06 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions (II) |
| WO2011050474A1 (en) | 2009-10-29 | 2011-05-05 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions |
| RU2642653C2 (en) * | 2009-10-29 | 2018-01-25 | Акасти Фарма, Инк. | Concentrated therapeutic phospholipide compositions |
| US10130644B2 (en) | 2009-10-29 | 2018-11-20 | Acasti Pharma Inc. | Concentrated therapeutic phospholipid compositions |
| KR101788342B1 (en) * | 2009-10-29 | 2017-10-19 | 아카스티 파마, 인크. | Concentrated therapeutic phospholipid compositions |
| AU2010312238C1 (en) * | 2009-10-29 | 2017-03-23 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions |
| AU2013100810B4 (en) * | 2009-10-29 | 2014-03-06 | Acasti Pharma, Inc | Concentrated therapeutic phospholipid compositions (I) |
| AU2013100814B4 (en) * | 2009-10-29 | 2014-03-06 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions (V) |
| AU2013100812B4 (en) * | 2009-10-29 | 2014-03-06 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions (III) |
| TWI573591B (en) * | 2009-10-29 | 2017-03-11 | 阿卡斯提製藥公司 | Concentrated therapeutic phospholipid compositions |
| TWI494110B (en) * | 2009-10-29 | 2015-08-01 | Acasti Pharma Inc | Concentrated therapeutic phospholipid compositions |
| JP2015147793A (en) * | 2009-10-29 | 2015-08-20 | アカスティ ファーマ インコーポレイテッド | concentrated therapeutic phospholipid composition |
| JP2013508431A (en) * | 2009-10-29 | 2013-03-07 | アカスティ ファーマ インコーポレイテッド | Concentrated therapeutic phospholipid composition |
| US9475830B2 (en) | 2009-10-29 | 2016-10-25 | Acasti Pharma Inc. | Concentrated therapeutic phospholipid compositions |
| US8586567B2 (en) | 2009-10-29 | 2013-11-19 | Acasti Pharma, Inc. | Concentrated therapeutic phospholipid compositions |
| RU2755902C2 (en) * | 2009-10-29 | 2021-09-22 | Акасти Фарма, Инк. | Concentrated therapeutic phospholipid compositions |
| US9150815B2 (en) | 2009-10-30 | 2015-10-06 | Tharos Ltd. | Solvent-free process for obtaining phospholipids and neutral enriched krill oils |
| US8772516B2 (en) | 2009-10-30 | 2014-07-08 | Tharos. Ltd. | Solvent-free process for obtaining phospholipids and neutral enriched krill oils |
| US8865236B2 (en) | 2009-10-30 | 2014-10-21 | Tharos Ltd. | Solvent-Free Process for Obtaining Phospholipids and Neutral Enriched Krill Oils |
| US9011942B2 (en) | 2009-10-30 | 2015-04-21 | Tharos, Ltd. | Solvent-free process for obtaining phospholipids and neutral enriched krill oils |
| US8609157B2 (en) | 2009-10-30 | 2013-12-17 | Tharos Ltd. | Solvent-free process for obtaining phospholipids and neutral enriched krill oils |
| WO2011124895A3 (en) * | 2010-04-09 | 2012-11-22 | Ayanda Group As | Phospholipid-containing pharmaceutical and nutraceutical compositions |
| KR100992995B1 (en) | 2010-05-03 | 2010-11-09 | 연세대학교 산학협력단 | Novel uses of piperonal |
| DE202011050351U1 (en) | 2010-06-04 | 2011-11-24 | Sana Pharma As | Dietetic products or formulations |
| WO2011151632A1 (en) | 2010-06-04 | 2011-12-08 | Sana Pharma As | Dietary formulations |
| AT12966U1 (en) * | 2010-06-04 | 2013-03-15 | Sana Pharma As | FOOD SUPPLEMENT FORMULATIONS |
| AU2011260037B2 (en) * | 2010-06-04 | 2014-05-01 | Sana Pharma As | Dietary formulations |
| CN107648268A (en) * | 2011-06-15 | 2018-02-02 | 稳定解决方案有限责任公司 | The parenteral therapy application of krill oil |
| US10052352B2 (en) | 2011-06-15 | 2018-08-21 | Stable Solutions Llc | Therapeutic application of parenteral krill oil |
| WO2012172411A1 (en) * | 2011-06-15 | 2012-12-20 | Stable Solutions Llc | Therapeutic application of parenteral krill oil |
| RU2625760C2 (en) * | 2011-06-15 | 2017-07-18 | СТЕЙБЛ СОЛЮШНЗ ЭлЭлСи | Therapeutic application of krill oil |
| US8895074B2 (en) | 2011-06-15 | 2014-11-25 | Stable Solutions Llc | Therapeutic application of parenteral krill oil |
| US10076530B2 (en) | 2011-09-02 | 2018-09-18 | Arctic Nutrition As | Lipid compositions with high DHA content |
| US9458409B2 (en) | 2011-09-02 | 2016-10-04 | Arctic Nutrition As | Lipid compositions with high DHA content |
| US8846604B2 (en) | 2011-09-02 | 2014-09-30 | Artic Nutrition AS | Lipid compositions with high DHA content |
| US11135230B2 (en) | 2011-09-02 | 2021-10-05 | Arctic Nutrition As | Lipid compositions with high DHA content |
| EP2800481B1 (en) | 2012-01-03 | 2018-01-24 | Rimfrost Technologies AS | Method for processing crustaceans to produce low fluoride/low trimethyl amine products thereof |
| EP3311679B1 (en) | 2012-01-03 | 2021-11-24 | Rimfrost Technologies AS | Low fluoride/low trimethyl amine krill oil |
| CN102603791A (en) * | 2012-02-21 | 2012-07-25 | 山东师范大学 | Preparation method of Antarctic krill phosphatidyl ethanolamine product |
| CN102603791B (en) * | 2012-02-21 | 2014-12-24 | 山东师范大学 | Preparation method of Antarctic krill phosphatidyl ethanolamine product |
| WO2013136183A2 (en) | 2012-03-12 | 2013-09-19 | Innolipid, As | Oxidixable fatty acid composition delivery form |
| WO2014013335A2 (en) | 2012-07-17 | 2014-01-23 | Aker Biomarine As | Concentration of omega-3 polyunsaturated fatty acids in krill oil |
| WO2014045127A2 (en) | 2012-09-19 | 2014-03-27 | Aker Biomarine As | Omega-3 phospholipid supplements for females |
| US10105376B2 (en) | 2012-09-24 | 2018-10-23 | Aker Biomarine Antarctic As | Omega-3 compositions |
| EP2988759A4 (en) * | 2013-03-14 | 2016-11-30 | Inst Rech Sur Les Zones Cotieres Irzc | Method for extracting organic solids and oil from marine organisms enriched with astaxanthin |
| WO2014140873A2 (en) | 2013-03-14 | 2014-09-18 | Aker Biomarine As | Omega- 3 phospholipid supplements for improved brain maturity |
| US9295683B2 (en) | 2013-03-14 | 2016-03-29 | Aker Biomarine Antarctic As | Omega-3 phospholipid supplements for improved brain maturity |
| EP3008156B1 (en) | 2013-06-14 | 2023-06-07 | Aker Biomarine Antarctic As | Lipid extraction processes |
| US11578289B2 (en) | 2013-06-14 | 2023-02-14 | Aker Biomarine Antarctic As | Lipid extraction processes |
| US10704011B2 (en) | 2013-06-14 | 2020-07-07 | Aker Biomarine Antarctic As | Lipid extraction processes |
| US9867856B2 (en) | 2014-01-10 | 2018-01-16 | Aker Biomarine Antarctic As | Phospholipid compositions and their preparation |
| US10960016B2 (en) | 2014-02-12 | 2021-03-30 | Aker Biomarine Antarctic As | Capsules containing high doses of krill phospholipids |
| US10806742B2 (en) | 2014-02-12 | 2020-10-20 | Aker Biomarine Antarctic As | Liquid phospholipid-containing compositions for the preparation of pharmaceuticals |
| US10080803B2 (en) | 2014-04-25 | 2018-09-25 | Aker Biomarine Antartic As | Emulsified krill phospholipid compositions |
| EP3137070B1 (en) | 2014-04-30 | 2020-04-15 | Aker BioMarine Antarctic AS | Krill oil preparations and their uses |
| US11147841B2 (en) | 2014-12-19 | 2021-10-19 | Aker Biomarine Antarctic As | Enhanced omega-3 formulations |
| US11819509B2 (en) | 2015-02-11 | 2023-11-21 | Aker Biomarine Antarctic As | Lipid compositions |
| US10456412B2 (en) | 2015-02-11 | 2019-10-29 | Aker Biomarine Antarctic As | Lipid extraction processes |
| US10864223B2 (en) | 2015-02-11 | 2020-12-15 | Aker Biomarine Antarctic As | Lipid compositions |
| WO2016128830A1 (en) | 2015-02-11 | 2016-08-18 | Aker Biomarine Antarctic As | Lipid extraction processes |
| WO2016128838A2 (en) | 2015-02-11 | 2016-08-18 | Aker Biomarine Antarctic As | Lipid compositions |
| EP3256003B1 (en) | 2015-02-11 | 2022-11-09 | Aker Biomarine Antarctic AS | Lipid extraction processes |
| US11490644B2 (en) | 2015-02-27 | 2022-11-08 | Rb Health (Us) Llc | Co-Q10, krill oil and vitamin D |
| US10856566B2 (en) | 2015-02-27 | 2020-12-08 | Rb Health (Us) Llc | Co-Q10, krill oil and vitamin D |
| US10525087B2 (en) | 2015-05-27 | 2020-01-07 | Rimfrost Technologies As | Flowable concentrated phospholipid krill oil composition |
| WO2016190748A1 (en) | 2015-05-27 | 2016-12-01 | Rimfrost As | A flowable concentrated phospholipid krill oil composition |
| US10328105B2 (en) | 2015-05-27 | 2019-06-25 | Rimfrost Technologies As | Flowable concentrated phospholipid krill oil composition |
| US10653725B2 (en) | 2015-08-17 | 2020-05-19 | Aker Biomarine Antarctic As | Methods for improving health of farmed fish |
| IT201600124013A1 (en) * | 2016-12-06 | 2018-06-06 | Solarpharm Srl | Astaxanthin based food product |
| EP3749339B1 (en) | 2018-01-30 | 2022-08-03 | Aker Biomarine Antarctic As | Marine protein hydrolysate with low fluoride and trimethylamin content |
| WO2023198752A1 (en) * | 2022-04-12 | 2023-10-19 | Aker Biomarine Antarctic As | Supplemented krill meal and uses thereof |
| US12023359B2 (en) | 2022-11-01 | 2024-07-02 | Aker Biomarine Human Ingredients As | Phospholipid compositions for delivery of therapeutic compounds |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20240189366A1 (en) | Bioeffective krill oil compositions | |
| AU2019202956A1 (en) | Bio effective krill oil compositions | |
| AU2013227998C1 (en) | Bioeffective krill oil compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 08718910 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2682068 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2008231570 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2008718910 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2008231570 Country of ref document: AU Date of ref document: 20080328 Kind code of ref document: A |