WO2008062472A2 - Process for the preparation of memantine - Google Patents
Process for the preparation of memantine Download PDFInfo
- Publication number
- WO2008062472A2 WO2008062472A2 PCT/IN2007/000500 IN2007000500W WO2008062472A2 WO 2008062472 A2 WO2008062472 A2 WO 2008062472A2 IN 2007000500 W IN2007000500 W IN 2007000500W WO 2008062472 A2 WO2008062472 A2 WO 2008062472A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- memantine
- dimethyl
- base
- adamantine
- Prior art date
Links
- 0 CI(C=CN)[*+]=C Chemical compound CI(C=CN)[*+]=C 0.000 description 4
- CWNOIUTVJRWADX-UHFFFAOYSA-N CC1(CC(C2)C3)CC3(C)CC2C1 Chemical compound CC1(CC(C2)C3)CC3(C)CC2C1 CWNOIUTVJRWADX-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C209/00—Preparation of compounds containing amino groups bound to a carbon skeleton
- C07C209/62—Preparation of compounds containing amino groups bound to a carbon skeleton by cleaving carbon-to-nitrogen, sulfur-to-nitrogen, or phosphorus-to-nitrogen bonds, e.g. hydrolysis of amides, N-dealkylation of amines or quaternary ammonium compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C211/00—Compounds containing amino groups bound to a carbon skeleton
- C07C211/33—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of rings other than six-membered aromatic rings
- C07C211/34—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of rings other than six-membered aromatic rings of a saturated carbon skeleton
- C07C211/38—Compounds containing amino groups bound to a carbon skeleton having amino groups bound to carbon atoms of rings other than six-membered aromatic rings of a saturated carbon skeleton containing condensed ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2603/00—Systems containing at least three condensed rings
- C07C2603/56—Ring systems containing bridged rings
- C07C2603/58—Ring systems containing bridged rings containing three rings
- C07C2603/70—Ring systems containing bridged rings containing three rings containing only six-membered rings
- C07C2603/74—Adamantanes
Definitions
- the present invention relates to a process for the preparation of Memantine of formula (I) ' and its pharmaceutically acceptable salts. More particularly, the present invention provides the process for the preparation of Memantine hydrochloride. The present invention further provides an improved process for the preparation of 1- Acetamido-3 ,5 -dimethyl adamantine of formula (II), which is a key intermediate for the preparation of Memantine.
- Memantine is chemically known as 3,5-Dimethyl-l- adamantylamine and represented by below mentioned formula (I), which is useful for the treatment of Alzheimer's and Parkinson's disease.
- 1,3-Dimethyl adamantane is reacted with Br 2 to give 1-Bromo- 3,5-dimethyl adamantine.
- l-bromo-3,5-dimethyladamantane reacts with 17 moles of acetonitrile and 35 moles of sulphuric acid at room temperature to give the crude intermediate product in 100 percent yield.
- the intermediate product is subjected to alkaline hydrolysis with sodium hydroxide in diethylene glycol by refluxing at a temperature of greater than 19O 0 C. for six hours.
- the hydrolyzed product is diluted with water, followed by several benzene extractions, and the memantine free base is recovered by solvent distillation. The free base is then diluted with ether, and the addition of hydrogen chloride gas provides crude memantine hydrochloride. Crystallization of the hydrochloride from an ethanol- ether mixture gives pure memantine hydrochloride in 69.8 %yield.
- the said process may not be suitable for commercial production considering the bromination is carried out at reflux conditions, which leads to generation of toxic bromine vapors. Moreover, the use of ether in the process is hazardous because of its highly inflammable nature and tendency to form peroxides.
- JP 2002 275142A2 discloses the synthesis of n- acetyl-memantine according to the following scheme with a yield of 45 percent.
- U.S. Pat. No. 4,122,193 discloses pharmacological compositions and methods for treating living mammals suffering from hyperkinesias, as well as memantine hydrochloride, hydrobromide, and sulphate synthesis, where the hydrochloride is prepared according to the following scheme with a yield of 78 percent.
- U.S. Pat. No. 5,599,998 discloses memantine synthesis with a yield of 48 percent according to the following scheme.
- the disclosed method is cumbersome on an industrial scale, using metallic lithium and chloramine, which can b6 hazardous if not handled properly.
- the deficiencies of the prior art processes described above include, but are not limited to the production of undesirable byproducts, such as Br 2 and SO 2 , very high reaction temperatures, the need for high pressures, and the use of dangerous reactants.
- the undesirable byproducts are produced in the Ritter reaction of l-bromo-3,5- dimethyladamantane and acetonitrile in presence of 96 percent sulphuric acid.
- the very high reaction temperatures typically from 19O 0 C to 225 0 C.
- WO 2005/062724 discloses a novel process for the preparation of compound of below mentioned formula
- Memantine is prepared as shown below:
- a Group IA e.g., sodium hydroxide
- HA hydroxide e.g., 1- methoxy-2-propanol
- an alkoxy ale. solvent e.g., 1- methoxy-2-propanol
- aminoadamantane e.g., l-amino-3,5- dimethyladamantane
- acids e.g., HCl
- an acid- addn. salts e.g., memantine hydrochloride
- Patent WO 2006/122238 relates to process for preparing memantine or an acid addition salt of memantine comprises reaction of l-bromo-3 5 5-dimethyladamantane with formamide to form l-N-formylamino-3,5-dimethyladamantane.
- 1-bromo- 3,5-dimethyladamantane prepn. given
- H2NCHO H2NCHO
- H2NCHO 1-bromo- 3,5-dimethyladamantane
- H2NCHO 157° for 14 h
- 76.4% pure l-N-forniylamino-3,5-dimethyladamantane was heated in cone.
- HCl at 104° for 6.5 h to give 99.9% pure memantine hydrochloride.
- the patent discloses gas chromatographic method for Memantine Hydrochloride and impurities. Further, patent provides bulk density and particle size distribution for Memantine Hydrochloride.
- It is an object of the present invention is to prepare memantine of formula (I) and its pharmaceutically acceptable salts.
- Yet another object of the present invention is to provide a one-pot process for the preparation of memantine of formula (I) and its pharmaceutically acceptable salts.
- Still another object of the present invention is to provide a process for the preparation of l-Acetamido-3,5-dimethyl adamantine of formula (II) DESCRIPTION OF THE INVNETION:
- Memantine of formula (I) or its pharmaceutically acceptable salts which comprises reacting halogenated-3,5-dimethyl adamantine of formula (III), wherein X is Cl or Br; with acetamide in presence of sulfuric acid to provide l-Acetamido-3,5-dimethyl adamantine of formula (II) and treating said l-Acetamido-3, 5 -dimethyl adamantine of. formula (II) with base in presence of solvent to obtain Memantine of formula (I), optionally converting Memantine of formula (I) in to pharmaceutically acceptable salts.
- the process according to the present invention is carried out in a single pot.
- the present invention further provides the improved process for the preparation of l-Acetamido-3, 5-dimethyl adamantine of formula (II) comprising reacting halogenated-3,5-dimethyl adamantine of formula (III), wherein X is Cl or Br; with acetamide in presence of sulfuric acid to provide l-Acetamido-3, 5-dimethyl adamantine of formula (II).
- the deacetylation of acetamido compound may be carried in a solvent selected from acyclic and cyclic poly ether to yield Memantine of formula (I).
- the acyclic or cyclic polyether used in the present invention may be selected from the group comprising 1,2-dimethoxyethane, 1,4-dimethoxybutane, poly (alkylene glycol) such as poly (ethylene glycol), (PEGs) 5 1, 4-dioxane, crown ethers, mono alkylated ordialkylated poly (alkylene glycol) wherein the alkyl group is selected from C 1 to C 6 linear or branched alkyl, diethylene glycol dimethyl ether (diglyme), triethylene glycol dimethyl ether,triethylene glycol diethyl ether, tetraethylene glycol dimethyl ether(tetraglyme) and the like and mixtures thereof.
- the poly (alkylene glycol) and monoalkylated or dialkylated poly (alkylene glycol) being mixtures are defined by an average molecular weight and do not have specific boiling point.
- the base used in the reaction can be selected from selected from inorganic base (s) such as an alkoxide, wherein the alkyl residue is C 1 to C 6 linear, branched or cyclic alkyl and the counter ion is an alkali or alkaline earth metal, alkali or alkaline earth metal oxide, hydroxide, carbonate or bicarbonate preferably sodium hydroxide, potassium hydroxide, potassium tertiary butoxide and the like; an organic base (s) such as amine base selected from aliphatic or aromatic amines, cyclic or acyclic amines, for example isoquinolines, quinolines,dialkylarylamines, pyridine, substituted pyridines preferably alkanolamine bases like ethanolamine and the like; and mixtures of inorganic base (s) with organic base (s).
- inorganic base s
- inorganic base such as an alkoxide, wherein the alkyl residue is C 1 to C 6 linear, branched or
- one pot process for preparing compounds of formula I or its pharmaceutically acceptable salts by reacting compound of formula lib with ammonium acetate without using solvent. It is then extracted with organic solvent preferably toluene. Then treated with alkali solution and diethylene glycol to obtain compound of formula I, which is free base of memantine, which can be optionally converted to its pharmaceutically acceptable salts.
- Formula II b Formula I (where in Ri, R 2 are selected from Cj -4 linear and branched alkyl and R 3 is selected from hydrogen, C 1-4 linear and branched alkyl).
- R 2 are selected from Cj -4 linear and branched alkyl
- R 3 is selected from hydrogen, C 1-4 linear and branched alkyl.
- the present invention describes a process for preparing memantine hydrochloride having formula :
- deacetylation reaction of acetamido derivative is carried out by heating of compound of formula Hc with sodium hydroxide in 2-8 volumes of Diethylene glycol at 80-180 0 C temperature for 6 to 15 hours to give compound of formula I.
- Formula I
- R 1 , R 2 are selected from C 1-4 linear and branched alkyl and R 3 is selected from hydrogen, C 1-4 linear and branched alkyl
- R 15 R 2 is CH 3 and R 3 is hydrogen.
- Compound formula Hc can be obtain by converting compound of formula lib to compound of formula Hc by using acetamide without any solvent.
- R 2 are selected from C 1-4 linear and branched alkyl and R 3 is selected from hydrogen, C 1-4 linear and branched alkyl.
- R 15 R 2 is CH 3 and R 3 is hydrogen.
- Compound of formula lib can be prepared by converting compound of formula Ha to compound of formula lib via bromination.
- Formula II a (where in Rj, R 2 are selected from Cj -4 linear and branched alkyl and R 3 is selected from hydrogen, C 1-4 linear and branched alkyl).
- Rj R 2 are selected from Cj -4 linear and branched alkyl
- R 3 is selected from hydrogen, C 1-4 linear and branched alkyl.
- R 15 R 2 is CH 3 and R 3 is hydrogen.
- Compound of formula lib is prepared with about 4 to 8 mole ratio of Bromine , preferably 4.5 molar equivalent and obtained good yield. To our surprise the reaction occurred at ambient conditions with bromine liquid to give bromoadamantane, compound of formula lib, making the process safe.
- Another aspect of the invention is the one pot process for preparing compound of formula I by reacting compound of formula lib with ammonium acetate without using solvent. It is then extracted with organic solvent preferably toluene. Then treated with alkali solution and diethylene glycol to obtain compound of formula I, which is optionally converted to its pharmaceutically acceptable salts.
- R 2 are selected from Ci -4 linear and branched alkyl and R 3 is selected from hydrogen, C 1-4 linear and branched alkyl).
- the pharmaceutically acceptable salts may be selected from mineral acid salts such as hydrochloride, hydrobromide, sulfate and the like; organic acid salts such as oxalate, citrate, succinate, maleate, fumarate, malate, tartrate, and the like ; and sulfonates such as methanesulfonate, benzenesulfonate, toluenesulfonate and the like ; preferably hydrochloride.
- mineral acid salts such as hydrochloride, hydrobromide, sulfate and the like
- organic acid salts such as oxalate, citrate, succinate, maleate, fumarate, malate, tartrate, and the like
- sulfonates such as methanesulfonate, benzenesulfonate, toluenesulfonate and the like ; preferably hydrochloride.
- the present invention further provides a process for purification of Memantine hydrochloride, which comprises treating memantine hydrochloride with aqueous solution of base selected from sodium hydroxide, potassium hydrochloride, sodium bicarbonate, in solvent selected from toluene, xylene, methylene dichloride, acetone or mixtures thereof to obtain memantine base, extracting memantine base in organic later, treating said memantine base with hydrochloride acid to obtain pure Memantine hydrochloride.
- Example 1 (a): Preparation of l-Bromo-3,5-dimethyl adamantane with 4.5 equivalent of bromine
- 1,3 -Dimethyl adamantane (48.0 g) was added to reaction vessel followed by addition of bromine (67.36 mL, 4.5 equivalent) at 25-30 0 C within 30 minutes.
- the reaction mixture was heated at 48-51 C and maintained for 12 Hrs. After heating for 12 Hrs, the reaction mixture was cooled to 10-15 0 C and then methylene chloride (800 mL) was added at 5-10 0 C. Reaction mixture was stirred at 5-10 0 C for 30 minutes. To the reaction mixture water (1.5 L) was poured at 5-10 C within 1 Hr and stirred for 30 minutes. Sodium metabisulf ⁇ te (70.0 g) was added within 2-3 Hrs and stirred for 30 minutes at 5-10 0 C .
- Example 1 (b) : Preparation of l-Bromo-3,5-dimethyl adamantane with 6.5 equivalent of bromine 1,3 -Dimethyl adamantane (48.0 g) was added to reaction vessel followed by addition of bromine (97.36 mL, 6.5 equivalent) at 25-30 0 C within 30 minutes. The reaction mixture was heated at 48-51 0 C and maintained for 12 Hrs.
- reaction mixture was cooled to 20-25 0 C and then methylene chloride (800 mL) was added at 5-10 0 C. Reaction mixture was stirred at 5-10 0 C for 30 minutes. The reaction mixture was poured into water (1.5 L) at 5-10 0 C within 1 Hr and stirred for 30 minutes. Sodium metabisulfite (70.0 g) was added within 2-3 Hrs and stirred for 30 minutes at 5-10 0 C. After stirring the layers were settled for 30 minutes and then separated out. To the organic (MDC) layer solution of 1% sodium bicarbonate (500 mL) was added at 5-10 0 C and stirred for 30 minutes and after stirring layers were settled and separated out.
- MDC organic
- Example 2 (a): Preparation of l-Acetamido-3,5-dimethyl adamantane with 10.0 equivalent of acetamide l-Bromo-3,5-dimethyl adamantane (60.0 g) was added to reaction vessel followed by addition of acetamide (145.72 g) at 25-30 0 C. The reaction mixture was stirred and then heated at 130-140 0 C and heating was maintained for 3-4 Hrs. The reaction mixture was cooled to 20-25 0 C and toluene (300 mL) was added. Reaction mixture was stirred at 25-30 0 C for 1 Hr. Water (600 mL) was added at 25-30 0 C and stirred for 1 Hr.
- Example 2 (b): Preparation of l-Acetamido-3,5-dimethyI adamantane with 5.0 equivalent of acetamide l-Bromo-3 ,5 -dimethyl adamantane (60.0 g) was added to reaction vessel followed by addition of acetamide (72.85 g) at 25-30 0 C. The reaction mixture was stirred and then heated at 130-140 0 C and heating was maintained for 3-5 Hrs. The reaction mixture was cooled to 80-85 0 C and toluene (300 mL) was added. Reaction mixture was stirred at 25-30 0 C for 1 Hr.
- Example 3 (a): Preparation of Memantine Base l-Acetamido-3,5-dimethyl adamantane (40.0 g) was added to reaction vessel followed by addition of sodium hydroxide (57.80 g) and DEG (200 mL) at 25-30 0 C. The reaction mixture was stirred and then heated at 155-170 0 C and heating was maintained for 10 Hrs. The reaction mixture was cooled to 80-85 0 C and water (1600 mL) was added within 1 Hr. Reaction mixture was stirred for 30 minutes and toluene (500 mL) was added to the reaction mixture and further stirred for 1 Hr. After stirring the layers were settled for 30 minutes and then separated out.
- Example 3 (b): Preparation of Memantine Base (One pot synthesis) l ⁇ Bromo-3,5-dimethyl adamantane (60.0 g) was added to reaction vessel followed addition of acetamide (87.42 g) the reaction mixture was stirred and heated at 130-140 0 C for 3 to 5 Hrs. The reaction mixture was cooled up to 80- 85 0 C and then toluene (300 mL) was added to reaction mixture and stirred for 1 Hr. The reaction mixture was treated with water (600 mL) and stirred for lhr. The reaction mixture was filtered and washed with toluene.
- the organic layer was separated and toluene was distilled out at temperature range 105-130°C.Cool to 105-115 0 C and the reaction mass was again treated with DEG(270 mL) at 105-115 0 C maintaing 105-115 0 C for 2 hrs. Again distill out toluene at 130-140°C.Cool at 105-115 0 C and add sodium hydroxide (78 g) was added at 105-115 0 C. The reaction mixture was stirred and then heated at 155-170 0 C and heating was maintained for 10 Hrs. The reaction mixture was cooled to 80-85 0 C and water (200 mL) was added within 1 Hr.
- Memantine Base (21.0 g) was added to reaction vessel followed by addition of methyl-tert-butyl ether (300 mL). The reaction mixture was stirred and then cooled at 10-15 0 C and this was maintained for 30 minutes. Then IPA/HC1 (20%) (23.40 g) was added within 1 Hr at 10-15 0 C and stirred for 1 Hr at 20-25 0 C. The reaction mixture was distilled under reduced pressure below 60 0 C. The crude solid (35-40 g) was obtained. Methanol (35-40 mL) was added and further stirred at 30-40 0 C for 30 minutes.
- Memantine Base oil (21.0 g) was added to reaction vessel followed by addition of toluene (300 mL) at 25-30 0 C . The reaction mixture was stirred and then cooled at 10-15 0 C and maintained for 30 minutes. Then IPA/HC1 (20%) (23.40 g) was added within 1 Hr at 10-15 0 C and stirred for 1 Hr at 20-25 0 C. The reaction mixture was distilled under reduced pressure below 60 C. The crude solid (36-40 g) was obtained and methanol (35-40 mL) was added and further stirred at 30-40 0 C for 30 minutes.
- reaction mixture was cooled up to 80- 85 0 C and then toluene (300 mL) was added to reaction mixture and stirred for 1 Hr.
- the reaction mixture was treated with water (600 mL) and stirred for lhr.
- the reaction mixture was filtered and washed with toluene.
- the organic layer was separated and toluene was distilled out at temperature range 105-130 0 C and then cool to 105-115 0 C and the reaction mass was again treated with DEG(260 mL) at 105-115 0 C maintaining 105- 115 0 C for 2 hrs.
- the toluene layer was cooled to 10-15 0 C and aqueous HCl (18-22 mL) was added drop wise with in 1 hr at 10-15 0 C and stirred for 30 minutes.
- the reaction mixture was cooled 5-1O 0 C and maintain for lhr.
- the product was filtered and washed with chilled acetone (5 x 50 mL), the product was dried under vacuum at 80-90 0 C for 15 hrs - 20 lirs. to obtain 25- 35g of the title compound i.e.
- memantine hydrochloride with more than 99% purity and having individual known impurities like l-Bromo-3,5-dimethyladamantane, 1- Hydroxy-3,5-dimethyladamantane, l-(N)-Acetamido-3,5-dimethyl adamantane, 1- Amino-3,5-dimethyladamantane less than 0.10% and unknown impurities less than 0.10%. The total impurities is less than 0.5%.
- Example 7 Preparation of Memantine Hydrochloride.
- Memantine Base (5.0 g) was added to reaction vessel followed by addition of IPA (10 mL) at 25-30 0 C. The reaction mixture was stirred and then cooled at 10-15 0 C and this was maintained for 30 minutes. Then IPA/HC1 (5.1 g) was added within 1 Hr at 10-15 0 C and stirred for 1 Hr. The solid was filtered at 5-10 0 C and washed with chilled IPA (3 x 5.0 mL) and dried the solid to obtain (4.5 g) title compound memantine hydrochloride.
- Example 8 Preparation of Memantine Hydrochloride.
- Memantine Base (15.0 g) was added to reaction vessel followed by addition of cyclohexane (150 mL) at 10-15 0 C. The reaction mixture was stirred and then cooled at 10-15 0 C and this was maintained for 30 minutes. Then IPA/HC1 (15.25 g) was added within 1 Hr at 10-15 0 C and stirred for 1 Hr. The solid was filtered at 5-10 0 C and washed with chilled cyclohexane (3 x 15 mL) and dried the solid to afford title compound memantine hydrochloride (25 g).
- Example 9 Purification of Memantine hydrochloride:
- Memantine Hydrochloride 100 gm was added to 300ml of toluene. Aqueous solution of sodium hydroxide (35gm) was added to reaction mass and heated to 60- 65oC for about 90 minutes. Organic layer was isolated by extraction and hydrochloric acid was added to organic layer containing memantine base to form Memantine hydrochloride. The reaction mass is cooled to precipitate Memantine hydrochloride and isolated by filtration and dried.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
A process for the preparation of Memantine of formula (I) or its pharmaceutically acceptable salts is disclosed. The process comprises formula (I): (a) reacting halogenated-3, 5 -dimethyl adamantine of formula (III) with acetamide in presence of sulfuric acid to provide l-Acetamido-3,5-dimethyl adamantine of formula (II), wherein X is Cl or Br. Formula (III), (II): (b) treating l-Acetamido-3,5-dimethyl adamantine of formula (II) with base in presence of solvent to obtain Memantine of formula (I) (c) optionally converting Memantine of formula (I) in to pharmaceutically acceptable salts.
Description
PROCESS FOR THE PREPARATION OF MEMANTINE FIELD OF THE INVENTION
The present invention relates to a process for the preparation of Memantine of formula (I) ' and its pharmaceutically acceptable salts. More particularly, the present invention provides the process for the preparation of Memantine hydrochloride. The present invention further provides an improved process for the preparation of 1- Acetamido-3 ,5 -dimethyl adamantine of formula (II), which is a key intermediate for the preparation of Memantine. Memantine is chemically known as 3,5-Dimethyl-l- adamantylamine and represented by below mentioned formula (I), which is useful for the treatment of Alzheimer's and Parkinson's disease.
(I) ' (H)
BACKGROUND OF THE INVENTION: United States Patent No 3,391. 142 (Assigned to: Eli Lilly and Company, referred to herein as US' 142) discloses a process for the preparation of memantine hydrochloride as shown below:
Step: 1
1-Bromo, 1 -Acetamido
3,5-dimethyl 3,5-dimethyl adamantane adamantane
1 -Acetamido Memantine 3,5-dimethyl Memantine-HCI adamantane
Accordingly, 1,3-Dimethyl adamantane is reacted with Br2 to give 1-Bromo- 3,5-dimethyl adamantine. Further, l-bromo-3,5-dimethyladamantane reacts with 17 moles of acetonitrile and 35 moles of sulphuric acid at room temperature to give the crude intermediate product in 100 percent yield. The intermediate product is subjected to alkaline hydrolysis with sodium hydroxide in diethylene glycol by refluxing at a temperature of greater than 19O0C. for six hours.
The hydrolyzed product is diluted with water, followed by several benzene extractions, and the memantine free base is recovered by solvent distillation. The free base is then diluted with ether, and the addition of hydrogen chloride gas provides
crude memantine hydrochloride. Crystallization of the hydrochloride from an ethanol- ether mixture gives pure memantine hydrochloride in 69.8 %yield.
The said process may not be suitable for commercial production considering the bromination is carried out at reflux conditions, which leads to generation of toxic bromine vapors. Moreover, the use of ether in the process is hazardous because of its highly inflammable nature and tendency to form peroxides.
Czech Patent Publication No. CZ 282398B6 discloses the synthesis of memantine according to the following scheme, which is carried out under pressure.
Japanese Patent Publication No. JP 2002 275142A2 discloses the synthesis of n- acetyl-memantine according to the following scheme with a yield of 45 percent.
U.S. Pat. No. 4,122,193 discloses pharmacological compositions and methods for treating living mammals suffering from hyperkinesias, as well as memantine hydrochloride, hydrobromide, and sulphate synthesis, where the hydrochloride is prepared according to the following scheme with a yield of 78 percent.
U.S. Pat. No. 5,599,998 discloses memantine synthesis with a yield of 48 percent according to the following scheme.
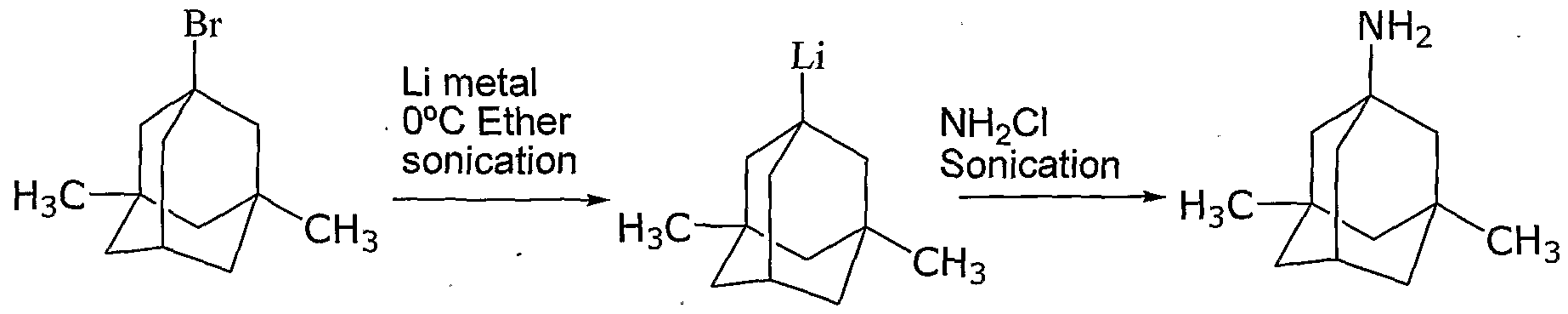
In addition to the low yield, the disclosed method is cumbersome on an industrial scale, using metallic lithium and chloramine, which can b6 hazardous if not handled properly. The deficiencies of the prior art processes described above include, but are not limited to the production of undesirable byproducts, such as Br2 and SO2, very high reaction temperatures, the need for high pressures, and the use of dangerous reactants. The undesirable byproducts are produced in the Ritter reaction of l-bromo-3,5- dimethyladamantane and acetonitrile in presence of 96 percent sulphuric acid. The very high reaction temperatures, typically from 19O0C to 2250C. are required in the alkaline hydrolysis of n-acetyl-memantine in diethylene glycol or ethylene glycol and in the reaction between l-halo-3,5-dimethyl adamantane and urea. Where the alkaline hydrolysis is performed in low boiling alcohols, elevated pressure is required to attain the reaction temperatures required for an acceptable reaction time. Moreover, as discussed above, the dangerous reactants lithium and chloramine are required in the electrophilic animation reaction.
WO 2005/062724 discloses a novel process for the preparation of compound of below mentioned formula,
Accordingly to the process, Memantine is prepared as shown below:
Patent US 2006/258885 discloses synthesis of acetamidoadamantanes [I; Rl5 R2 = H, or a (un)branched C 1-6 alkyl; e.g., l-acetamido-3,5-dimethyladamantane] comprises the amidation of haloadamantanes (II; X = F, Cl, Br, I; e.g., l-chloro-3,5- dimethyladamantane) with acetonitrile in the presence of glacial acetic acid, and coned. H2SO4 is then added. It may be hydrolyzed in the presence of a Group IA (e.g., sodium hydroxide) or HA hydroxide in the presence of an alkoxy ale. solvent (e.g., 1- methoxy-2-propanol) to give the corresponding aminoadamantane (e.g., l-amino-3,5- dimethyladamantane) which may then be salifϊed with acids (e.g., HCl) to give an acid- addn. salts (e.g., memantine hydrochloride).
Patent WO 2006/122238 relates to process for preparing memantine or an acid addition salt of memantine comprises reaction of l-bromo-355-dimethyladamantane with formamide to form l-N-formylamino-3,5-dimethyladamantane. Thus, 1-bromo- 3,5-dimethyladamantane (prepn. given) was heated in H2NCHO at 157° for 14 h to
give 76.4% pure l-N-forniylamino-3,5-dimethyladamantane. The latter was heated in cone. HCl at 104° for 6.5 h to give 99.9% pure memantine hydrochloride. The patent discloses gas chromatographic method for Memantine Hydrochloride and impurities. Further, patent provides bulk density and particle size distribution for Memantine Hydrochloride.
Patent WO 2007/096124 relates to a process for the preparation of an adamantanamine of formula I [R and Rl each = H or Me; X = halo, in particular Cl] (i) reacting a compd. of formula II with thiourea; (ii) subjecting the resulting thiourea deriv. Ill to an acid treatment and (iii) isolating the resulting adamantanamine or a hydrohalogenide thereof.
Overall, there is a need in the art to provide improved processes for preparing memantine hydrochloride which is eco-friendly and applicable for industrial scale. OBJECTS OF THE INVENTION:
It is an object of the present invention is to prepare memantine of formula (I) and its pharmaceutically acceptable salts.
Yet another object of the present invention is to provide a one-pot process for the preparation of memantine of formula (I) and its pharmaceutically acceptable salts.
Still another object of the present invention is to provide a process for the preparation of l-Acetamido-3,5-dimethyl adamantine of formula (II) DESCRIPTION OF THE INVNETION:
According to the present invention, there is provided a process for the preparation of Memantine of formula (I) or its pharmaceutically acceptable salts, which comprises
(I)
(a) reacting halogenated-3,5-dimethyl adamantine of formula (III) with acetamide in presence of sulfuric acid to provide l-Acetamido-3, 5 -dimethyl adamantine of formula (II), wherein X is Cl or Br.
(b) treating l-Acetamido-3, 5 -dimethyl adamantine of formula (II) with base in presence of solvent to obtain Memantine of formula (I)
(c) optionally converting Memantine of formula (I) in to pharmaceutically acceptable salts. The present invention further provides a one-pot process for preparing
Memantine of formula (I) or its pharmaceutically acceptable salts, which comprises reacting halogenated-3,5-dimethyl adamantine of formula (III), wherein X is Cl or Br; with acetamide in presence of sulfuric acid to provide l-Acetamido-3,5-dimethyl adamantine of formula (II) and treating said l-Acetamido-3, 5 -dimethyl adamantine of. formula (II) with base in presence of solvent to obtain Memantine of formula (I), optionally converting Memantine of formula (I) in to pharmaceutically acceptable salts. The process according to the present invention is carried out in a single pot.
The present invention further provides the improved process for the preparation of l-Acetamido-3, 5-dimethyl adamantine of formula (II) comprising reacting halogenated-3,5-dimethyl adamantine of formula (III), wherein X is Cl or Br; with acetamide in presence of sulfuric acid to provide l-Acetamido-3, 5-dimethyl adamantine of formula (II).
The deacetylation of acetamido compound may be carried in a solvent selected from acyclic and cyclic poly ether to yield Memantine of formula (I). The acyclic or cyclic polyether used in the present invention may be selected from the group comprising 1,2-dimethoxyethane, 1,4-dimethoxybutane, poly (alkylene glycol) such as poly (ethylene glycol), (PEGs)5 1, 4-dioxane, crown ethers, mono alkylated ordialkylated poly (alkylene glycol) wherein the alkyl group is selected from C1 to C6 linear or branched alkyl, diethylene glycol dimethyl ether (diglyme), triethylene glycol dimethyl ether,triethylene glycol diethyl ether, tetraethylene glycol dimethyl ether(tetraglyme) and the like and mixtures thereof. The poly (alkylene glycol) and monoalkylated or dialkylated poly (alkylene glycol) being mixtures are defined by an average molecular weight and do not have specific boiling point.
The base used in the reaction can be selected from selected from inorganic base (s) such as an alkoxide, wherein the alkyl residue is C1 to C6 linear, branched or cyclic alkyl and the counter ion is an alkali or alkaline earth metal, alkali or alkaline earth metal oxide, hydroxide, carbonate or bicarbonate preferably sodium hydroxide, potassium hydroxide, potassium tertiary butoxide and the like; an organic base (s) such as amine base selected from aliphatic or aromatic amines, cyclic or acyclic amines, for example isoquinolines, quinolines,dialkylarylamines, pyridine, substituted pyridines preferably alkanolamine bases like ethanolamine and the like; and mixtures of inorganic base (s) with organic base (s).
In another aspect of the invention, there is provided one pot process for preparing compounds of formula I or its pharmaceutically acceptable salts by reacting compound of formula lib with ammonium acetate without using solvent. It is then extracted with organic solvent preferably toluene. Then treated with alkali solution and diethylene glycol to obtain compound of formula I, which is free base of memantine, which can be optionally converted to its pharmaceutically acceptable salts.
Formula II b Formula I
(where in Ri, R2 are selected from Cj-4 linear and branched alkyl and R3 is selected from hydrogen, C1-4 linear and branched alkyl). Compound of formula I, where in Ri, R2 are methyl and R3 is hydrogen, commonly known as memantine and converting it to
Memantine hydrochloride.
(where in Ri, R2 are selected from Cj-4 linear and branched alkyl and R3 is selected from hydrogen, C1-4 linear and branched alkyl). Compound of formula I, where in Ri, R2 are methyl and R3 is hydrogen, commonly known as memantine.
The present invention describes a process for preparing memantine hydrochloride having formula :
Memantine hydrochloride
by reacting compound of formula lie with base, diethylene glycol solution, then extracted with toluene to give compound of formula I.
Preferably, deacetylation reaction of acetamido derivative is carried out by heating of compound of formula Hc with sodium hydroxide in 2-8 volumes of Diethylene glycol at 80-1800C temperature for 6 to 15 hours to give compound of formula I.
Formula I
(where in R1, R2, are selected from C1-4 linear and branched alkyl and R3 is selected from hydrogen, C1-4 linear and branched alkyl) Here in formula I and formula Hc R15R2 is CH3 and R3 is hydrogen.
Preferably, Compound formula Hc can be obtain by converting compound of formula lib to compound of formula Hc by using acetamide without any solvent. The
Formula II b
compound of formula Hc is isolated by addition of toluene in the reaction mass followed by addition of water to precipitate out compound
(where in Ri, R2, are selected from C1-4 linear and branched alkyl and R3 is selected from hydrogen, C1-4 linear and branched alkyl). Here in formula lib and formula Hc R15R2 is CH3 and R3 is hydrogen.
Compound of formula lib can be prepared by converting compound of formula Ha to compound of formula lib via bromination.
Formula II a Formula II b
(where in Rj, R2 are selected from Cj-4 linear and branched alkyl and R3 is selected from hydrogen, C1-4 linear and branched alkyl). Here in formula Ha and formula Hb, R15R2 is CH3 and R3 is hydrogen.
Compound of formula lib is prepared with about 4 to 8 mole ratio of Bromine , preferably 4.5 molar equivalent and obtained good yield. To our surprise the reaction occurred at ambient conditions with bromine liquid to give bromoadamantane, compound of formula lib, making the process safe.
Another aspect of the invention is the one pot process for preparing compound of formula I by reacting compound of formula lib with ammonium acetate without using solvent. It is then extracted with organic solvent preferably toluene. Then treated with alkali solution and diethylene glycol to obtain compound of formula I, which is optionally converted to its pharmaceutically acceptable salts.
Formula II b Formula I
(where in R), R2 are selected from Ci-4 linear and branched alkyl and R3 is selected from hydrogen, C1-4 linear and branched alkyl). Compound of formula I, where in Ri, R2 are methyl and R3 is hydrogen, commonly known as memantine.
The pharmaceutically acceptable salts may be selected from mineral acid salts such as hydrochloride, hydrobromide, sulfate and the like; organic acid salts such as oxalate, citrate, succinate, maleate, fumarate, malate, tartrate, and the like ; and sulfonates such as methanesulfonate, benzenesulfonate, toluenesulfonate and the like ; preferably hydrochloride.
The present invention further provides a process for purification of Memantine hydrochloride, which comprises treating memantine hydrochloride with aqueous solution of base selected from sodium hydroxide, potassium hydrochloride, sodium bicarbonate, in solvent selected from toluene, xylene, methylene dichloride, acetone or mixtures thereof to obtain memantine base, extracting memantine base in organic later, treating said memantine base with hydrochloride acid to obtain pure Memantine hydrochloride.
Having thus described the invention with reference to particular preferred embodiments and illustrative examples, those in the art would appreciate modifications to the invention as described and illustrated that do not depart from the spirit and scope of the invention as disclosed in the specification. The examples are set forth to aid in understanding the invention but are not intended to, and should not be construed to limit its scope in any way. The examples do not include detailed descriptions of conventional methods. Such methods are well known to those of ordinary skill in the art and are described in numerous publications. Examples: Example 1 (a): Preparation of l-Bromo-3,5-dimethyl adamantane with 4.5 equivalent of bromine
1,3 -Dimethyl adamantane (48.0 g) was added to reaction vessel followed by addition of bromine (67.36 mL, 4.5 equivalent) at 25-300C within 30 minutes. The reaction mixture was heated at 48-51 C and maintained for 12 Hrs. After heating for 12 Hrs, the reaction mixture was cooled to 10-150C and then methylene chloride (800 mL) was added at 5-100C. Reaction mixture was stirred at 5-100C for 30 minutes. To the reaction mixture water (1.5 L) was poured at 5-10 C within 1 Hr and stirred for 30 minutes. Sodium metabisulfϊte (70.0 g) was added within 2-3 Hrs and stirred for 30 minutes at 5-100C . After stirring the layers were settled for 30 minutes and then separated out. To the organic (MDC) layer solution of 1% sodium bicarbonate (500 mL) was added at 5-100C and stirred for 30 minutes and after stirring layers were settled and separated out. Further to the organic layer (MDC) Brine solution (500 mL) was added and stirred for 30 minutes at 5-100C. After stirring the layers were settled for 30 minutes and then separated out. Organic layer (MDC) was dried over anhydrous sodium sulfate and wash the bed with MDC (48 mL). Further MDC layer was distilled atmospherically below 600C and further traces of MDC was removed under vacuum for 1 Hr below 40 C to afford the title compound l-bromo-3,5-dimethyl adamantane. Example 1 (b) : Preparation of l-Bromo-3,5-dimethyl adamantane with 6.5 equivalent of bromine 1,3 -Dimethyl adamantane (48.0 g) was added to reaction vessel followed by addition of bromine (97.36 mL, 6.5 equivalent) at 25-300C within 30 minutes. The reaction mixture was heated at 48-510C and maintained for 12 Hrs. After heating for 12 Hrs, the reaction mixture was cooled to 20-250C and then methylene chloride (800 mL) was added at 5-100C. Reaction mixture was stirred at 5-100C for 30 minutes. The
reaction mixture was poured into water (1.5 L) at 5-100C within 1 Hr and stirred for 30 minutes. Sodium metabisulfite (70.0 g) was added within 2-3 Hrs and stirred for 30 minutes at 5-100C. After stirring the layers were settled for 30 minutes and then separated out. To the organic (MDC) layer solution of 1% sodium bicarbonate (500 mL) was added at 5-100C and stirred for 30 minutes and after stirring layers were settled and separated out. Further to the organic layer (MDC) Brine solution (500 mL) was added and stirred for 30 minutes at 5-100C. After stirring the layers were settled for 30 minutes and then separated out. Organic layer (MDC) was dried over anhydrous sodium sulfate and wash the bed with MDC (48 mL). Further MDC layer was distilled atmospherically below 600C and further traces of MDC was removed under vacuum for 1 Hr below 400C to afford the title compound l-bromo-3, 5 -dimethyl adamantane. Example 2 (a): Preparation of l-Acetamido-3,5-dimethyl adamantane with 10.0 equivalent of acetamide l-Bromo-3,5-dimethyl adamantane (60.0 g) was added to reaction vessel followed by addition of acetamide (145.72 g) at 25-300C. The reaction mixture was stirred and then heated at 130-1400C and heating was maintained for 3-4 Hrs. The reaction mixture was cooled to 20-250C and toluene (300 mL) was added. Reaction mixture was stirred at 25-300C for 1 Hr. Water (600 mL) was added at 25-300C and stirred for 1 Hr. After stirring the layers were settled for 30 minutes and then separated out. The organic (Toluene) layer was kept and to the aqueous layer, toluene (150 mL) was added at 25-300C and stirred for 1 Hr and after stirring layers were settled and separated out. Further, to the aqueous layer, toluene (150 mL) was added at 25-300C and stirred for 1 Hr and after stirring layers were settled and separated out. Total toluene layers were collected and dried over anhydrous sodium sulfate and toluene layer was distilled under pressure below 600C. Methanol (300 mL) was added to the residue and methanol was distilled under pressure below 600C. The residue was cooled upto 25-300C and then stirred vigorously and water (500 mL) was added during vigorous stirring. The reaction mixture was cooled upto 5-100C for 2 Hrs. The solid was filtered under reduced pressure at 10-150C and washed with chilled (10-150C) water and then dried under reduced pressure at 50-550C for 2 Hrs to afford the title compound l-acetamido-3,5-dimethyl adamantane.
Example 2 (b): Preparation of l-Acetamido-3,5-dimethyI adamantane with 5.0 equivalent of acetamide
l-Bromo-3 ,5 -dimethyl adamantane (60.0 g) was added to reaction vessel followed by addition of acetamide (72.85 g) at 25-300C. The reaction mixture was stirred and then heated at 130-1400C and heating was maintained for 3-5 Hrs. The reaction mixture was cooled to 80-850C and toluene (300 mL) was added. Reaction mixture was stirred at 25-300C for 1 Hr. Water (600 mL) was added to the reaction mixture at 25-300C and stirred for 1 Hr. After stirring the layers were settled for 30 minutes and then separated out. The organic (Toluene) layer was kept and to the aqueous layer, toluene (150 mL) was added at 25-300C and stirred for 1 Hr and after stirring layers were settled and separated out. Further, to the aqueous layer, toluene (150 mL) was added at 25-300C and stirred for 1 Hr and after stirring layers were settled and separated out. Total toluene layers were collected and dried over anhydrous sodium sulfate and toluene layer was distilled under pressure below 60 C. Methanol (300 mL) was added to the residue and methanol was distilled under pressure below 600C. The residue was cooled upto 25-300C and then stirred vigrously and water (500 mL) was added during vigorous stirring. The reaction mixture was cooled upto 5-100C for 2 Hrs. The solid was filtered under reduced pressure at 10-150C and washed with chilled (10-150C) water and then dried under reduced pressure at 50-550C for 2 Hrs to afford the title compound l-acetamido-3,5-dimethyl adamantane. Example 3 (a): Preparation of Memantine Base l-Acetamido-3,5-dimethyl adamantane (40.0 g) was added to reaction vessel followed by addition of sodium hydroxide (57.80 g) and DEG (200 mL) at 25-300C. The reaction mixture was stirred and then heated at 155-1700C and heating was maintained for 10 Hrs. The reaction mixture was cooled to 80-850C and water (1600 mL) was added within 1 Hr. Reaction mixture was stirred for 30 minutes and toluene (500 mL) was added to the reaction mixture and further stirred for 1 Hr. After stirring the layers were settled for 30 minutes and then separated out. The organic (Toluene) layer was kept and to the aqueous layer, toluene (300 mL) was added and stirred for 1 Hr and after stirring layers were settled and separated out. Total toluene layers were collected and water (500 mL) was added to it and stirred for 1 Hr at 25-300C. After stirring the layers were settled for 30 minutes and then separated out. Organic (toluene) layer was dried over anhydrous sodium sulfate and wash the bed with toluene (50 mL)and then toluene was distilled under reduced pressure below 600C to afford the title compound Memantine Base.
Example 3 (b): Preparation of Memantine Base (One pot synthesis) l~Bromo-3,5-dimethyl adamantane (60.0 g) was added to reaction vessel followed addition of acetamide (87.42 g) the reaction mixture was stirred and heated at 130-1400C for 3 to 5 Hrs. The reaction mixture was cooled up to 80- 850C and then toluene (300 mL) was added to reaction mixture and stirred for 1 Hr. The reaction mixture was treated with water (600 mL) and stirred for lhr. The reaction mixture was filtered and washed with toluene. The organic layer was separated and toluene was distilled out at temperature range 105-130°C.Cool to 105-1150C and the reaction mass was again treated with DEG(270 mL) at 105-1150C maintaing 105-1150C for 2 hrs. Again distill out toluene at 130-140°C.Cool at 105-1150C and add sodium hydroxide (78 g) was added at 105-1150C. The reaction mixture was stirred and then heated at 155-1700C and heating was maintained for 10 Hrs. The reaction mixture was cooled to 80-850C and water (200 mL) was added within 1 Hr. Reaction mixture was stirred for 30 minutes and toluene (400 mL) was added to the reaction mixture and further stirred for 1 Hr. After stirring the layers were settled for 30 minutes and then separated out. The organic (Toluene) layer was kept and to the aqueous layer, toluene (50 mL) was added and stirred for 1 Hr and after stirring layers were settled and separated out. Total toluene layers were collected and water (500 ml) was added to it and stirred for 1 Hr at 25-300C. After stirring the layers were settled for 30 minutes and then separated out to afford the title compound Memantine Base.
Example 4 (a) : Preparation of Memantine Hydrochloride using IPA/HC1
Memantine Base (21.0 g) was added to reaction vessel followed by addition of methyl-tert-butyl ether (300 mL). The reaction mixture was stirred and then cooled at 10-150C and this was maintained for 30 minutes. Then IPA/HC1 (20%) (23.40 g) was added within 1 Hr at 10-150C and stirred for 1 Hr at 20-250C. The reaction mixture was distilled under reduced pressure below 600C. The crude solid (35-40 g) was obtained. Methanol (35-40 mL) was added and further stirred at 30-400C for 30 minutes. After stirring the reaction mixture was cooled at 10-15 C and methyl-tert-butyl ether (500 mL) was added within 1-2 Hrs at 10-150C. The reaction mixture was further cooled at 5-100C for 2 Hrs. The solid was filtered at 5-100C and washed with methyl-tert-butyl ether (2 x 21.0 mL) and dried the solid under reduced pressure at 60-700C for 8-10 Hrs to afford the title compound Memantine Hydrochloride technical - 1.
Example 4 (b): Preparation of Memantine Hydrochloride using Toluene and IPA/HC1
Memantine Base oil (21.0 g) was added to reaction vessel followed by addition of toluene (300 mL) at 25-300C . The reaction mixture was stirred and then cooled at 10-150C and maintained for 30 minutes. Then IPA/HC1 (20%) (23.40 g) was added within 1 Hr at 10-150C and stirred for 1 Hr at 20-250C. The reaction mixture was distilled under reduced pressure below 60 C. The crude solid (36-40 g) was obtained and methanol (35-40 mL) was added and further stirred at 30-400C for 30 minutes. After stirring the reaction mixture was cooled at 10-150C and methyl-tert-butyl ether (500 mL) was added within 1-2 Hrs at 10-150C. The reaction mixture was further cooled at 5-100C for 2 Hrs. The solid was filtered at 5-100C and washed with methyl- tert-butyl ether (2 x 21.0 mL) and dried the solid under reduced pressure at 60-700C for 8-10 Hrs to afford the title compound Memantine Hydrochloride technical - 1. Example 5: Preparation of Memantine Hydrochloride. As above Example 4(a) and 4(b) Memantine Hydrochloride technical - 1 (21.0 g
1.0 mole) was added to reaction vessel followed by addition of methanol (42 mL) at 25- 300C. Reflux at 55-600C for 1 hr. Add methyl-tert-butyl ether (500 mL) was added within 1-1.5 Hrs at 40-500C. The reaction mixture was further stirred at 40-450C for 1 Hr. The reaction mixture was cooled at 25-300C for 2 Hrs, further cooled at 5-100C for 2 Hrs. The solid was filtered at 5-100C and washed with methyl-tert-butyl ether (2 x 21.0 mL) and dried the solid under reduced pressure at 80-90° for 15 Hrs to afford the title compound Memantine Hydrochloride. Example 6: Preparation of Memantine Hydrochloride (One pot synthesis). l-Bromo-3, 5 -dimethyl adamantane (60.0 g) was added to reaction vessel followed addition of acetamide (87.42 g) the reaction mixture was stirred and heated at 130-1400C for 3 to 5 Hrs. The reaction mixture was cooled up to 80- 850C and then toluene (300 mL) was added to reaction mixture and stirred for 1 Hr. The reaction mixture was treated with water (600 mL) and stirred for lhr. The reaction mixture was filtered and washed with toluene. The organic layer was separated and toluene was distilled out at temperature range 105-1300C and then cool to 105-1150C and the reaction mass was again treated with DEG(260 mL) at 105-1150C maintaining 105- 1150C for 2 hrs. Again distill out toluene at temperature 130-140°C.Cool at 105-1150C and add sodium hydroxide (78 g) was added at 105-1150C. The reaction mixture was stirred and then heated at 155-1700C and heating was maintained for 10 Hrs. The
reaction mixture was cooled to 80-850C and water (200 niL) was added within 1 Hr. Reaction mixture was stirred for 30 minutes and toluene (400 mL) was added to the reaction mixture and further stirred for 1 Hr. After stirring the layers were settled for 30 minutes and then separated out. The organic (Toluene) layer was kept and to the aqueous layer, toluene (50 mL) was added and stirred for 1 Hr and after stirring layers were settled and separated out. Total toluene layers were collected and water (500 ml) was added to it and stirred for 1 Hr at 25-300C. After stirring the layers were settled for 30 minutes and then separated out. Distilled out toluene (350 mL) under reduced pressure below 6O0C, add (1.2 g) charcoal and heat at 50-600C for 20 to 30 minutes. The reaction mixture was filtered and washed with toluene (2 X 50 mL). The toluene layer was cooled to 10-150C and aqueous HCl (18-22 mL) was added drop wise with in 1 hr at 10-150C and stirred for 30 minutes. The reaction mixture was cooled 5-1O0C and maintain for lhr. The product was filtered and washed with chilled acetone (5 x 50 mL), the product was dried under vacuum at 80-900C for 15 hrs - 20 lirs. to obtain 25- 35g of the title compound i.e. memantine hydrochloride with more than 99% purity and having individual known impurities like l-Bromo-3,5-dimethyladamantane, 1- Hydroxy-3,5-dimethyladamantane, l-(N)-Acetamido-3,5-dimethyl adamantane, 1- Amino-3,5-dimethyladamantane less than 0.10% and unknown impurities less than 0.10%. The total impurities is less than 0.5%. Example 7: Preparation of Memantine Hydrochloride.
Memantine Base (5.0 g) was added to reaction vessel followed by addition of IPA (10 mL) at 25-300C. The reaction mixture was stirred and then cooled at 10-150C and this was maintained for 30 minutes. Then IPA/HC1 (5.1 g) was added within 1 Hr at 10-150C and stirred for 1 Hr. The solid was filtered at 5-100C and washed with chilled IPA (3 x 5.0 mL) and dried the solid to obtain (4.5 g) title compound memantine hydrochloride. Example 8: Preparation of Memantine Hydrochloride.
Memantine Base (15.0 g) was added to reaction vessel followed by addition of cyclohexane (150 mL) at 10-150C. The reaction mixture was stirred and then cooled at 10-150C and this was maintained for 30 minutes. Then IPA/HC1 (15.25 g) was added within 1 Hr at 10-150C and stirred for 1 Hr. The solid was filtered at 5-100C and washed with chilled cyclohexane (3 x 15 mL) and dried the solid to afford title compound memantine hydrochloride (25 g).
Example 9: Purification of Memantine hydrochloride:
100 gm of Memantine Hydrochloride was added to 300ml of toluene. Aqueous solution of sodium hydroxide (35gm) was added to reaction mass and heated to 60- 65oC for about 90 minutes. Organic layer was isolated by extraction and hydrochloric acid was added to organic layer containing memantine base to form Memantine hydrochloride. The reaction mass is cooled to precipitate Memantine hydrochloride and isolated by filtration and dried.
Claims
We claim:
1. A process for the preparation of Memantine of formula (I) or its pharmaceutically acceptable salts, which comprises
(I)
(a) reacting halogenated-3, 5 -dimethyl adamantine of formula (III) with acetamide in presence of sulfuric acid to provide l-Acetamido-3,5-dimethyl adamantine of formula (II), wherein X is Cl or Br.
(IH) (II) (b) treating l-Acetamido-3,5-dimethyl adamantine of formula (II) with base in presence of solvent to obtain Memantine of formula (I)
(c) optionally converting Memantine of formula (I) in to pharmaceutically acceptable salts.
2. A one pot process for preparation of Memantine of formula (I) or its pharmaceutically acceptable salts, which comprises
which comprises of steps:
a) reacting 1,3 -dimethyl adamantane with bromine in suitable solvent to provide 1- bromo-3,5-dimethyl adamantine of formula (lib). b) reacting l-bromo-3,5-dimethyl adamantine of formula (lib) with acetamide in presence of mineral acid to provide l-Acetamido-3, 5 -dimethyl adamantine of formula (II)
(lib) (II) c) treating l-Acetamido-3,5-dimethyl adamantine of formula (II) with base in presence of solvent to obtain Memantine of formula (I)
(i) d) optionally converting Memantine of formula (I) in to pharmaceutically acceptable salts by treating Memantine base with hydrochloric acid in a suitable solvent.
3. A process as claimed in claim 2, wherein said bromine is employed in 4.0 to 6.5 molar equivalent of 1,3-dimethyl adamantine, preferably 4.5 molar equivalent.
4. A process as claimed in claim 1, wherein said mineral acid is selected from the group of hydrochloric acid, nitric acid, sulphuric acid, preferably sulphuric acid.
5. A process as claimed in claim 1, wherein said base in step (c) is selected from the group of inorganic base or organic base selected from sodium hydroxide, potassium hydroxide, potassium tertiary butoxide, aliphatic or aromatic amines, cyclic or acyclic amines, such as isoquinolines, quinolines, dialkylarylamines, pyridine, substituted pyridines preferably alkanolamine bases like ethanolamine and mixtures there of.
6. A process as claimed in claim 5, wherein said inorganic base is sodium hydroxide.
7. A process as claimed in claim 5, wherein said preferred organic base is ethanolamine.
8. A process as claimed in claim 1 or 2, wherein the solvent is selected from the group of acyclic or cyclic polyether such as 1,2-dimethoxyethane, 1,4-dimethoxybutane, poly (alkylene glycol) such as poly (ethylene glycol), (PEGs), 1, 4-dioxane, crown ethers, mono alkylated or dialkylated poly (alkylen glycol) such as diethylene glycol dimethyl ether (diglyme), triethylene glycol dimethyl ether,triethylene glycol diethyl ether, tetraethylene glycol dimethyl ether(tetraglyme) or a mixtures thereof.
10. A novel one pot process for preparation of l-Acetamido-3,5-dimethyl adamantine of formula (II) which comprises of steps:
a) reacting 1,3 -dimethyl adamantane with bromine in suitable solvent to provide 1- bromo-3,5-dimethyl adamantine of formula (lib). b) reacting l-bromo-3,5-dimethyl adamantine of formula (lib) with acetamide in presence of mineral acid to provide l-Acetamido-3,5-dimethyl adamantine of formula (II)
(Hb) (II) 11. A process as claimed in claim 10, wherein bromine used in 4.0 to 6.5 molar equivalent of 1,3 -dimethyl adamantine, preferably 4.5 molar equivalent.
12. A process as claimed in claim 10, wherein the mineral acid is selected from the group of hydrochloric acid, nitric acid, sulphuric acid, preferably sulphuric acid.
13. A process for purification of purification of Memantine hydrochloride, which comprises treating memantine hydrochloride with aqueous solution of base selected from sodium hydroxide, potassium hydrochloride, sodium bicarbonate, in solvent selected from toluene, xylene, methylene dichloride, acetone or mixtures thereof to obtain memantine base, extracting memantine base in organic later, treating said memantine base with hydrochloride acid to obtain pure Memantine hydrochloride.
14. A process for preparation of Memantine Hydrochloride as claimed in any preceding claims, wherein Memantine hydrochloride having purity more than 99.5% and individual known impurities like l-Bromo-3,5-dimethyladarnantane, 1-Hydroxy- 3,5-dimethyladamantane, l-(N)-Acetamido-3,5-dimethyl adamantane, 1-Amino- 3,5-dimethyladamantane less than 0.10% and unknown impurities less than 0.10% and the total impurities content is less than 0.5%.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN1761/MUM/2006 | 2006-10-24 | ||
| IN1761MU2006 | 2006-10-24 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2008062472A2 true WO2008062472A2 (en) | 2008-05-29 |
| WO2008062472A3 WO2008062472A3 (en) | 2008-07-10 |
Family
ID=39277330
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IN2007/000500 WO2008062472A2 (en) | 2006-10-24 | 2007-10-22 | Process for the preparation of memantine |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2008062472A2 (en) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010007351A1 (en) * | 2008-07-14 | 2010-01-21 | Cipla Limited | Process for the preparation of memantine and its salts and intermediate for use therein |
| WO2010015415A1 (en) | 2008-08-08 | 2010-02-11 | Merz Pharma Gmbh & Co. Kgaa | Process for manufacturing adamantane derivatives with high yield |
| WO2010067252A1 (en) * | 2008-12-12 | 2010-06-17 | Alembic Limited | An improved process for the preparation of 1-bromo-3,5-dimethyl adamantane |
| CN102093228A (en) * | 2011-01-18 | 2011-06-15 | 广东工业大学 | Method for synthesizing 1, 3-adamantane diamine |
| CN102617277A (en) * | 2012-03-12 | 2012-08-01 | 浙江洪波化工有限公司 | Synthesis method of 1-boro-3,5-dimethyladamantane |
| EP2555616A1 (en) * | 2010-04-08 | 2013-02-13 | Hetero Research Foundation | Process for the preparation of memantine hydrochloride |
| WO2014115638A1 (en) | 2013-01-23 | 2014-07-31 | 三菱瓦斯化学株式会社 | Manufacturing process for memantine |
| CN104529791A (en) * | 2014-12-10 | 2015-04-22 | 哈药集团技术中心 | Preparation method of memantine hydrochloride |
| CN105503718A (en) * | 2015-12-18 | 2016-04-20 | 重庆康乐制药有限公司 | Continuous preparing method for amodiaquine hydrochloride |
| JP2017114820A (en) * | 2015-12-25 | 2017-06-29 | 宇部興産株式会社 | Method for producing 1-amino-3,5-dimethyl adamantane hydrochloride |
| CN113387815A (en) * | 2021-07-23 | 2021-09-14 | 刘振洋 | Green preparation method of memantine |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0392059A1 (en) * | 1989-04-14 | 1990-10-17 | Merz & Co. GmbH & Co. | Use of adamantane derivatives in the prevention and treatment of cerebral ischemia |
| WO2006122238A1 (en) * | 2005-05-11 | 2006-11-16 | Dr. Reddy's Laboratories Ltd. | Process for preparing memantine |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CZ288445B6 (en) * | 1996-06-20 | 2001-06-13 | Lachema Np | Process for preparing hydrochloride of 5-amino-1,3-dimethyltricyclo(3,3,1,1 3,7 )decane |
-
2007
- 2007-10-22 WO PCT/IN2007/000500 patent/WO2008062472A2/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0392059A1 (en) * | 1989-04-14 | 1990-10-17 | Merz & Co. GmbH & Co. | Use of adamantane derivatives in the prevention and treatment of cerebral ischemia |
| WO2006122238A1 (en) * | 2005-05-11 | 2006-11-16 | Dr. Reddy's Laboratories Ltd. | Process for preparing memantine |
Non-Patent Citations (2)
| Title |
|---|
| DATABASE WPI Week 200125 Derwent Publications Ltd., London, GB; AN 2001-235873 XP002479004 & CZ 9 601 813 A3 (LACHEMA AS) 14 March 2001 (2001-03-14) * |
| HENKEL J G ET AL: "Structure-Anti-Parkinson Activity Relationships in the Aminoadamantanes. Influence of Bridgehead Substitution" JOURNAL OF MEDICINAL CHEMISTRY, AMERICAN CHEMICAL SOCIETY. WASHINGTON, US, vol. 25, no. 1, 1 January 1982 (1982-01-01), pages 51-56, XP002381694 ISSN: 0022-2623 * |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010007351A1 (en) * | 2008-07-14 | 2010-01-21 | Cipla Limited | Process for the preparation of memantine and its salts and intermediate for use therein |
| WO2010015415A1 (en) | 2008-08-08 | 2010-02-11 | Merz Pharma Gmbh & Co. Kgaa | Process for manufacturing adamantane derivatives with high yield |
| US8796491B2 (en) | 2008-08-08 | 2014-08-05 | Merz Pharma Gmbh & Co. Kgaa | Process for manufacturing adamantane derivatives with high yield |
| WO2010067252A1 (en) * | 2008-12-12 | 2010-06-17 | Alembic Limited | An improved process for the preparation of 1-bromo-3,5-dimethyl adamantane |
| EP2555616A4 (en) * | 2010-04-08 | 2014-04-02 | Hetero Research Foundation | Process for the preparation of memantine hydrochloride |
| EP2555616A1 (en) * | 2010-04-08 | 2013-02-13 | Hetero Research Foundation | Process for the preparation of memantine hydrochloride |
| CN102093228A (en) * | 2011-01-18 | 2011-06-15 | 广东工业大学 | Method for synthesizing 1, 3-adamantane diamine |
| CN102093228B (en) * | 2011-01-18 | 2013-12-11 | 广东工业大学 | Method for synthesizing 1, 3-adamantane diamine |
| CN102617277A (en) * | 2012-03-12 | 2012-08-01 | 浙江洪波化工有限公司 | Synthesis method of 1-boro-3,5-dimethyladamantane |
| WO2014115638A1 (en) | 2013-01-23 | 2014-07-31 | 三菱瓦斯化学株式会社 | Manufacturing process for memantine |
| US9452971B2 (en) | 2013-01-23 | 2016-09-27 | Mitsubishi Gas Chemical Company, Inc. | Manufacturing process for memantine |
| CN104529791A (en) * | 2014-12-10 | 2015-04-22 | 哈药集团技术中心 | Preparation method of memantine hydrochloride |
| CN105503718A (en) * | 2015-12-18 | 2016-04-20 | 重庆康乐制药有限公司 | Continuous preparing method for amodiaquine hydrochloride |
| JP2017114820A (en) * | 2015-12-25 | 2017-06-29 | 宇部興産株式会社 | Method for producing 1-amino-3,5-dimethyl adamantane hydrochloride |
| CN113387815A (en) * | 2021-07-23 | 2021-09-14 | 刘振洋 | Green preparation method of memantine |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2008062472A3 (en) | 2008-07-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2008062472A2 (en) | Process for the preparation of memantine | |
| EP1836157B1 (en) | Process for the preparation of 1-amino-3,5-dimethyladamantane hydrochloride | |
| EP1820792B1 (en) | Process for the preparation of adamantanamines | |
| CN107074738B (en) | Process for preparing 2-substituted-1, 4-phenylenediamines and salts thereof | |
| KR20100029332A (en) | New preparation of hydroxychloroquine | |
| JP5916721B2 (en) | Synthetic method of ferroquine by intensive reductive amination | |
| WO2009057140A2 (en) | Improved process for memantine hydrochloride | |
| CN106543026A (en) | A kind of preparation method of methyl hydrazine | |
| JP2012526802A (en) | Method for producing alkylamine derivative | |
| NZ526370A (en) | A process for the preparation of 1-(aminomethyl) cyclohexaneacetic acid | |
| US9902693B2 (en) | Preparation method for pyrrolidine-2-carboxylic acid derivatives | |
| JPWO2011001976A1 (en) | Process for producing threo-3- (3,4-dihydroxyphenyl) -L-serine | |
| WO2008074887A1 (en) | Process of producing amorolfine | |
| US20240228441A1 (en) | Method for preparing intermediate for synthesis of sphingosine-1-phosphate receptor agonist | |
| WO2009153806A2 (en) | Process for preparing memantine hydrochloride substantially free of !mpurities | |
| CN112194585A (en) | Synthetic method of bromhexine hydrochloride | |
| US20100063292A1 (en) | Process for the preparation of trifluoroethoxytoluenes. | |
| WO2013150020A1 (en) | Process for making bendamustine | |
| JP4356111B2 (en) | Process for producing N- (2-amino-1,2-dicyanovinyl) formamidine | |
| WO2011097490A1 (en) | Method of producing polyalkylated oligoalkylenepolyamines | |
| EP2555616A1 (en) | Process for the preparation of memantine hydrochloride | |
| WO2010007351A1 (en) | Process for the preparation of memantine and its salts and intermediate for use therein | |
| JP2015166383A (en) | Method of preparing neramexane or salt thereof | |
| KR101325589B1 (en) | Process for the preparation of 1-alkyl-2-(2-aminoethyl)pyrrolidines | |
| EP2834215B1 (en) | Process for making bendamustine |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 07866733 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 07866733 Country of ref document: EP Kind code of ref document: A2 |