WO2008026217A1 - Improved and simplified process for the preparation of 1,2-benzisoxazole-3-acetic acid - Google Patents
Improved and simplified process for the preparation of 1,2-benzisoxazole-3-acetic acid Download PDFInfo
- Publication number
- WO2008026217A1 WO2008026217A1 PCT/IN2006/000313 IN2006000313W WO2008026217A1 WO 2008026217 A1 WO2008026217 A1 WO 2008026217A1 IN 2006000313 W IN2006000313 W IN 2006000313W WO 2008026217 A1 WO2008026217 A1 WO 2008026217A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- process according
- benzisoxazole
- acid
- acetic acid
- reaction
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D261/00—Heterocyclic compounds containing 1,2-oxazole or hydrogenated 1,2-oxazole rings
- C07D261/20—Heterocyclic compounds containing 1,2-oxazole or hydrogenated 1,2-oxazole rings condensed with carbocyclic rings or ring systems
Definitions
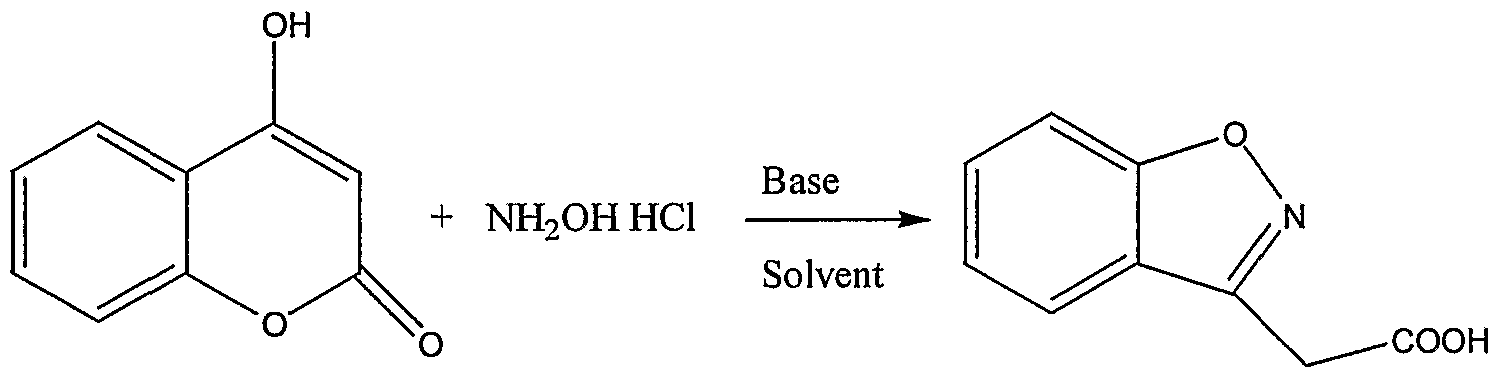
- the present invention relates to a process for preparation of l,2-benzisoxazole-3-acetic acid.
- l,2-benzisoxazole-3-acetic acid is a key material for the preparation of l,2-benzisoxazole-3-methane sulfonamide ( Zonisamide) anti-epileptic agent which possesses anti-convulant and anti neurotoxic effects.
- Zonisamide l,2-benzisoxazole-3-acetic acid
- the l,2-benzisoxazole-3-acetic acid (BOA) preparation was reported in literature using 4- hydroxyl coumarin and hydroxylamine acid salt using different bases Scheme -1
- the present invention relates to a process for the preparation of 1,2-benzisoxazole- 3-acetic acid .by reacting 4-hydroxy coumarin with hydroxylamine in water as solvent.
- l,2-benzisoxazole-3 -acetic acid is obtained in quantitative yield by reacting 4-hydroxy coumarin with hydroxylamine in water as solvent.
- decomposition of hydroxylamine which will occur during the reaction. It is reported that such decomposition is controlled by addition of chelating agents by which the reaction temperatures can be controlled.
- the present invented process does not involve neither high temperature nor chelating agent.
- Hydroxylamine decomposition is avoided by maintained the reaction at temperature below 50 C and a pH of between 4.5 to 5.5. It is found by the applicant that use of temperatures above 50 C ( 55 to 95C) and pH above 6.0 ( 6.0 to basic) may lead to formation of the oxime impurity to the extent of about 25 % in some cases. Also at pH below 4.5 the reaction incomplete ( formation of product is not observed) even after maintaining more than 50 hours.
- Example 1 100 gms 4-hydroxy coumarin is added to hydroxy 1 amine solution in water (from 200 gms Hydroxylamine Hydrochloride and 1200 ml 10% sodium carbonate solution ) at room temperature. pH of the reaction mass maintained in the range of 4.5 to 5.5. Reaction mass is warmed to 40 0 C and maintained at 40-45 0 C for 12 hours till the reaction completes . Reaction mass is cooled to 10 0 C and pH of the mass is adjusted to 1 to 1.5 with dilute hydrochloric acid. Precipitated l,2-Benzisoxazole-3-acetic acid is cooled to 0-5 °Cand maintained at 0-5° C for lhour and isolated and dried at 60-70 0 C.
- Dry weight 105 gms ( 96% by theory on 4-Hydroxy coumarin, 1.05 wt/wt)
- Precipitated l,2-benzisoxazole-3 ⁇ acetic acid is further cooled to 0-5° C, maintained for one hour at 0-5 C, isolated by centrifuging and dried at 60-70 0 C.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Plural Heterocyclic Compounds (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Nitrogen And Oxygen As The Only Ring Hetero Atoms (AREA)
Abstract
Description
Claims
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/IN2006/000313 WO2008026217A1 (en) | 2006-08-28 | 2006-08-28 | Improved and simplified process for the preparation of 1,2-benzisoxazole-3-acetic acid |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/IN2006/000313 WO2008026217A1 (en) | 2006-08-28 | 2006-08-28 | Improved and simplified process for the preparation of 1,2-benzisoxazole-3-acetic acid |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2008026217A1 true WO2008026217A1 (en) | 2008-03-06 |
Family
ID=37963708
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IN2006/000313 WO2008026217A1 (en) | 2006-08-28 | 2006-08-28 | Improved and simplified process for the preparation of 1,2-benzisoxazole-3-acetic acid |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2008026217A1 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018229197A1 (en) | 2017-06-14 | 2018-12-20 | European Molecular Biology Laboratory | Bicyclic heteroaromatic urea or carbamate compounds for use in therapy |
| WO2018229195A1 (en) | 2017-06-14 | 2018-12-20 | European Molecular Biology Laboratory | Bicyclic heteroaromatic amide compounds for use in therapy |
| CN113651767A (en) * | 2021-09-18 | 2021-11-16 | 江西中医药大学 | Benzisoxazole heterocyclic compound and preparation method and application thereof |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002070495A1 (en) * | 2001-03-02 | 2002-09-12 | Teva Pharmaceutical Industries Ltd. | Process for the preparation of 1,2-benzisoxazole-3-acetic acid |
| US20050215796A1 (en) * | 2004-03-25 | 2005-09-29 | Yoshikazu Ueno | One-pot process for the preparation of 1,2-benzisoxazole-3-methanesulfonamide |
| US20060084814A1 (en) * | 2004-05-20 | 2006-04-20 | Siva Kumar Bobba V | Process for the preparation of zonisamide |
-
2006
- 2006-08-28 WO PCT/IN2006/000313 patent/WO2008026217A1/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002070495A1 (en) * | 2001-03-02 | 2002-09-12 | Teva Pharmaceutical Industries Ltd. | Process for the preparation of 1,2-benzisoxazole-3-acetic acid |
| US20050215796A1 (en) * | 2004-03-25 | 2005-09-29 | Yoshikazu Ueno | One-pot process for the preparation of 1,2-benzisoxazole-3-methanesulfonamide |
| US20060084814A1 (en) * | 2004-05-20 | 2006-04-20 | Siva Kumar Bobba V | Process for the preparation of zonisamide |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018229197A1 (en) | 2017-06-14 | 2018-12-20 | European Molecular Biology Laboratory | Bicyclic heteroaromatic urea or carbamate compounds for use in therapy |
| WO2018229195A1 (en) | 2017-06-14 | 2018-12-20 | European Molecular Biology Laboratory | Bicyclic heteroaromatic amide compounds for use in therapy |
| CN113651767A (en) * | 2021-09-18 | 2021-11-16 | 江西中医药大学 | Benzisoxazole heterocyclic compound and preparation method and application thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6514398B2 (en) | Process for the preparation and medicinal use of methyl {4,6-diamino-2- [1- (2-fluorobenzyl) -1H-pyrazolo [3,4-b] pyridin-3-yl] pyrimidin-5-yl} methyl carbamate Process for its purification for use as active compound | |
| US7081539B2 (en) | One-pot process for the preparation of 1,2-benzisoxazole-3-methanesulfonamide | |
| KR20120098815A (en) | Method for producing methyl- {4,6-diamino-2-[1-(2-fluorobenzyl)-1h-pyrazolo [3,4-b]pyridino-3-yl] pyrimidino-5-yl} carbamate and its purification for use thereof as pharmaceutical substance | |
| US20240317773A1 (en) | High-purity thienopyrimidine compound and preparation method therefor | |
| WO2005105755A1 (en) | Process for the production of 5-difluoromethoxy -4-thiomethylpyrazoles | |
| WO2008026217A1 (en) | Improved and simplified process for the preparation of 1,2-benzisoxazole-3-acetic acid | |
| WO2011021218A2 (en) | Process for the preparation of 4-[3,5-bis(2-hydroxyphenyl)-1h-1,2,4-triazol-1-yl]-benzoic acid and its amine salts | |
| US6936720B2 (en) | Method for preparing benzisoxazole methane sulfonyl chloride and its amidation to form zonisamide | |
| WO2002070495A1 (en) | Process for the preparation of 1,2-benzisoxazole-3-acetic acid | |
| TW202102477A (en) | Production method of quinolinecarboxamide derivative or production intermediate thereof | |
| CN110878101A (en) | Novel method for preparing cefixime mother nucleus 7-AMOCA | |
| EP4232442A1 (en) | A process for the preparation of chlorantraniliprole | |
| US20080058550A1 (en) | Chemical process | |
| EP1728786B1 (en) | One-pot process for producing 1,2-benzisoxazole-3-methanesulfonamide | |
| CN101180289B (en) | Method for the production of substituted 2-alkoxycarbonyl-3-aminothiophenes | |
| US10259770B2 (en) | Process for the preparation of ethacrynic acid | |
| US20050014739A1 (en) | Aztreonam beta polymorph with very low residual solvent content | |
| WO2008092410A1 (en) | Preparation method of thioureas or salts thereof | |
| JP5414163B2 (en) | Process for producing 5- {4- [2- (5-ethyl-2-pyridyl) ethoxy] benzyl} thiazolidine-2,4-dione hydrochloride | |
| SE124934C1 (en) | ||
| JPS6117591A (en) | Manufacture of cephalosporin compound | |
| CN109912673A (en) | Cangrelor intermediate and preparation method thereof | |
| JP2005139098A (en) | Method for producing 1-thio-2-propanone derivative and 5-methyl-5-thiomethylhydantoin derivative | |
| JP2006056832A (en) | Method for producing and purifying 4-hydroxyquinolines | |
| JPH0211580B2 (en) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 06796192 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1316/CHENP/2009 Country of ref document: IN |
|
| NENP | Non-entry into the national phase |
Ref country code: RU |
|
| 32PN | Ep: public notification in the ep bulletin as address of the adressee cannot be established |
Free format text: "NOTING OF LOSS OF RIGHTS PURSUANT TO RULE 112(1) EPC, FORM 1205A DATED 11.08.09 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 06796192 Country of ref document: EP Kind code of ref document: A1 |