WO2007128985A2 - Skin dressing - Google Patents
Skin dressing Download PDFInfo
- Publication number
- WO2007128985A2 WO2007128985A2 PCT/GB2007/001309 GB2007001309W WO2007128985A2 WO 2007128985 A2 WO2007128985 A2 WO 2007128985A2 GB 2007001309 W GB2007001309 W GB 2007001309W WO 2007128985 A2 WO2007128985 A2 WO 2007128985A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- dressing
- enzyme
- papain
- component
- gelatin
- Prior art date
Links
- 102000004190 Enzymes Human genes 0.000 claims abstract description 62
- 108090000790 Enzymes Proteins 0.000 claims abstract description 62
- 239000012530 fluid Substances 0.000 claims abstract description 28
- 108091005804 Peptidases Proteins 0.000 claims abstract description 22
- 102000035195 Peptidases Human genes 0.000 claims abstract description 22
- 108090000526 Papain Proteins 0.000 claims description 59
- 239000004365 Protease Substances 0.000 claims description 58
- 229940088598 enzyme Drugs 0.000 claims description 56
- 229940055729 papain Drugs 0.000 claims description 56
- 235000019834 papain Nutrition 0.000 claims description 56
- 108010010803 Gelatin Proteins 0.000 claims description 41
- 239000008273 gelatin Substances 0.000 claims description 41
- 229920000159 gelatin Polymers 0.000 claims description 41
- 235000019322 gelatine Nutrition 0.000 claims description 41
- 235000011852 gelatine desserts Nutrition 0.000 claims description 41
- 239000004372 Polyvinyl alcohol Substances 0.000 claims description 30
- 229920002451 polyvinyl alcohol Polymers 0.000 claims description 30
- 238000001804 debridement Methods 0.000 claims description 18
- 230000002745 absorbent Effects 0.000 claims description 13
- 239000002250 absorbent Substances 0.000 claims description 13
- 230000003750 conditioning effect Effects 0.000 claims description 3
- 238000010276 construction Methods 0.000 claims description 3
- 239000000853 adhesive Substances 0.000 claims description 2
- 230000001070 adhesive effect Effects 0.000 claims description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 102
- 206010052428 Wound Diseases 0.000 description 50
- 208000027418 Wounds and injury Diseases 0.000 description 50
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 33
- 229940068984 polyvinyl alcohol Drugs 0.000 description 29
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 29
- 239000000243 solution Substances 0.000 description 22
- 239000010410 layer Substances 0.000 description 18
- 239000000463 material Substances 0.000 description 16
- 230000006870 function Effects 0.000 description 9
- 239000000843 powder Substances 0.000 description 9
- 238000000034 method Methods 0.000 description 8
- 230000001338 necrotic effect Effects 0.000 description 8
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 239000000203 mixture Substances 0.000 description 7
- 239000011942 biocatalyst Substances 0.000 description 5
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 5
- 235000018417 cysteine Nutrition 0.000 description 5
- 210000000416 exudates and transudate Anatomy 0.000 description 5
- 229940024999 proteolytic enzymes for treatment of wounds and ulcers Drugs 0.000 description 5
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 239000004202 carbamide Substances 0.000 description 4
- 230000015556 catabolic process Effects 0.000 description 4
- 230000003247 decreasing effect Effects 0.000 description 4
- 238000006731 degradation reaction Methods 0.000 description 4
- 230000029087 digestion Effects 0.000 description 4
- 238000004090 dissolution Methods 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 230000002255 enzymatic effect Effects 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 230000009471 action Effects 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 230000007062 hydrolysis Effects 0.000 description 3
- 238000006460 hydrolysis reaction Methods 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 238000003860 storage Methods 0.000 description 3
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 2
- 239000012790 adhesive layer Substances 0.000 description 2
- 230000002358 autolytic effect Effects 0.000 description 2
- 239000013020 final formulation Substances 0.000 description 2
- 108010025899 gelatin film Proteins 0.000 description 2
- 229920002674 hyaluronan Polymers 0.000 description 2
- 229960003160 hyaluronic acid Drugs 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- -1 streptodormase Proteins 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 125000003396 thiol group Chemical group [H]S* 0.000 description 2
- 102000004400 Aminopeptidases Human genes 0.000 description 1
- 108090000915 Aminopeptidases Proteins 0.000 description 1
- 108010004032 Bromelains Proteins 0.000 description 1
- 102000005367 Carboxypeptidases Human genes 0.000 description 1
- 108010006303 Carboxypeptidases Proteins 0.000 description 1
- GHXZTYHSJHQHIJ-UHFFFAOYSA-N Chlorhexidine Chemical compound C=1C=C(Cl)C=CC=1NC(N)=NC(N)=NCCCCCCN=C(N)N=C(N)NC1=CC=C(Cl)C=C1 GHXZTYHSJHQHIJ-UHFFFAOYSA-N 0.000 description 1
- 108090001069 Chymopapain Proteins 0.000 description 1
- 108090000317 Chymotrypsin Proteins 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 102000029816 Collagenase Human genes 0.000 description 1
- 108060005980 Collagenase Proteins 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 108010088842 Fibrinolysin Proteins 0.000 description 1
- 108090000270 Ficain Proteins 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- PWKSKIMOESPYIA-BYPYZUCNSA-N L-N-acetyl-Cysteine Chemical compound CC(=O)N[C@@H](CS)C(O)=O PWKSKIMOESPYIA-BYPYZUCNSA-N 0.000 description 1
- 239000004201 L-cysteine Substances 0.000 description 1
- 235000013878 L-cysteine Nutrition 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- HSHXDCVZWHOWCS-UHFFFAOYSA-N N'-hexadecylthiophene-2-carbohydrazide Chemical compound CCCCCCCCCCCCCCCCNNC(=O)c1cccs1 HSHXDCVZWHOWCS-UHFFFAOYSA-N 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 108010023197 Streptokinase Proteins 0.000 description 1
- ISWQCIVKKSOKNN-UHFFFAOYSA-L Tiron Chemical compound [Na+].[Na+].OC1=CC(S([O-])(=O)=O)=CC(S([O-])(=O)=O)=C1O ISWQCIVKKSOKNN-UHFFFAOYSA-L 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 229960004308 acetylcysteine Drugs 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 150000001398 aluminium Chemical class 0.000 description 1
- 239000005030 aluminium foil Substances 0.000 description 1
- 229940124326 anaesthetic agent Drugs 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 229960002976 chymopapain Drugs 0.000 description 1
- 229960002376 chymotrypsin Drugs 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 229960002424 collagenase Drugs 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000009088 enzymatic function Effects 0.000 description 1
- 239000003248 enzyme activator Substances 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 229940001501 fibrinolysin Drugs 0.000 description 1
- 235000019836 ficin Nutrition 0.000 description 1
- POTUGHMKJGOKRI-UHFFFAOYSA-N ficin Chemical compound FI=CI=N POTUGHMKJGOKRI-UHFFFAOYSA-N 0.000 description 1
- 239000003193 general anesthetic agent Substances 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 229960000502 poloxamer Drugs 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 239000013047 polymeric layer Substances 0.000 description 1
- 229920006264 polyurethane film Polymers 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 239000001253 polyvinylpolypyrrolidone Substances 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 235000019419 proteases Nutrition 0.000 description 1
- 235000018102 proteins Nutrition 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 229960005202 streptokinase Drugs 0.000 description 1
- 238000011477 surgical intervention Methods 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 229960001322 trypsin Drugs 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000003357 wound healing promoting agent Substances 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0203—Adhesive bandages or dressings with fluid retention members
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/00051—Accessories for dressings
- A61F13/00063—Accessories for dressings comprising medicaments or additives, e.g. odor control, PH control, debriding, antimicrobic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/38—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00365—Plasters use
- A61F2013/00531—Plasters use for exfoliation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00365—Plasters use
- A61F2013/0054—Plasters use for deep wounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00544—Plasters form or structure
- A61F2013/00604—Multilayer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00727—Plasters means for wound humidity control
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00902—Plasters containing means
- A61F2013/0091—Plasters containing means with disinfecting or anaesthetics means, e.g. anti-mycrobic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00902—Plasters containing means
- A61F2013/00927—Plasters containing means with biological activity, e.g. enzymes for debriding wounds or others, collagen or growth factors
Definitions
- the present invention relates to a skin dressing for delivery of proteolytic enzyme to skin e.g. to aid removal of dead and necrotic skin for therapeutic purposes, particularly in the form of wound debridement dressings.
- Debridement of necrotic tissue from e.g. burns and traumatic wounds is an essential step before the wound can begin to heal effectively.
- Enzymatic debridement using proteolytic enzymes is a convenient method of removing the tissue because it enhances the body's natural autolytic debridement process (whereby endogenous enzymes and leukocytes clean up the dead tissue) and does not require mechanical or surgical intervention.
- Commercial debridement products are traditionally available in the form of creams, sprays, lotions or ointments. With these product forms only a fraction of the total applied enzyme actually reaches the necrotic tissue at any one time and are often painful to apply.
- Enzymatic debridement dressings are known in the art. However, the manufacture of a dressing which can deliver an effective enzyme dose in the right time frame and in the right condition is not a simple matter. A particular difficulty arises from the management of water: proteolytic enzymes require the presence of water in order to perform their function, yet the same water can cause the enzyme to deactivate over a few hours due to such enzymes digesting themselves. Several technical solutions have been suggested which overcome this technical dilemma.
- US 6,043,407 solves the problem by providing an anhydrous debridement pad which requires rehydration in sterile water before application to the wound. Such a technique suffers from the inconvenience of waiting for the pad to hydrate.
- a common technical solution in the prior art is to maintain the dressing in an anhydrous state and rely on the wound itself to supply the moisture to activate the enzyme.
- US 4,668,228 discloses a debriding tape comprising a polyurethane film and an adhesive layer.
- the adhesive layer contains the proteolytic enzyme in dry or powdered form.
- the tape does not release enzyme in a sustained manner. Instead most or all of the enzyme is applied to the skin surface all at once with moisture from a wound activating the enzyme.
- US 5,206,026 discloses a dry enzymatic debridement film which gives immediate release of enzyme by dissolution of the film in wound exudate. It is intended to be used in conjunction with conventional dressings.
- US 6,548,556 discloses a wound debrider comprising anhydrous hydrophilic poloxamer carrier and proteolytic enzymes. The dry environment keeps the enzyme stable and the moisture is supplied by wound exudate.
- WO 2005/035010 discloses a dressing having a polymeric layer comprising proteolytic enzymes which are released over a prolonged period of time.
- the dressing may be multi- layered with a first degradable polymeric wound contacting layer and a second absorbent layer.
- the absorbent layer may be a hydrogel.
- the water for activation of the enzyme comes from wound exudate.
- the dressing provided sustained release of enzymes, optionally with a "burst" at the beginning.
- the invention provides a skin dressing for delivery of proteolytic enzyme to skin, comprising a lower dressing component comprising releasable proteolytic enzyme and an upper dressing component which is impermeable to the passage of fluid and the proteolytic enzyme and is degradable by being digested by the enzyme, following application of the dressing onto a moist wound site, to allow the passage of fluids and the proteolytic enzyme away from the wound site.
- the lower dressing component releases its enzyme into the wound site e.g. to perform a debridement function.
- the enzyme and moisture in the wound are prevented from leaving the wound site by the presence of the upper dressing component.
- the upper component degrades with the aid of enzyme action, to allow the enzyme and wound fluid to leave the wound site, optionally being absorbed by an absorbing dressing material possibly forming part of the dressing.
- the dressing provides enzyme to the skin e.g. to a wound site and allows it to remain on the skin, e.g. in the wound site, for an appropriate time to perform a desired function, e.g. provide an effective debridement function, whilst also subsequently providing for removal of fluids e.g. including wound exudate, liquefied slough and degraded necrotic tissue after an appropriate period of time as is discussed below.
- a desired function e.g. provide an effective debridement function
- the moisture in the wound site may be purely wound exudate, however it is preferred that the wound site is first moistened with a separate sterile aqueous fluid.
- the invention is equally applicable to naturally moist wounds as well as naturally dry wounds e.g. dry necrotic wounds, provided such dry wounds are suitably moistened before application of the dressing.
- the dressing is preferably of layered construction with each dressing component in the form of a layer, wherein in use, the lower component is in contact or near to the skin, and the upper component is remote from the skin surface.
- the lower and/or upper components may be in the form of a solid layer, sheet, slab or film of material.
- the size and shape of the layer, sheet, slab or film can be selected to suit the intended use of the dressing. Thicknesses in the range 0.01 to 1.0 mm, preferably 0.05 to 0.5 mm are particularly suitable.
- the lower enzyme-containing component is the lower enzyme-containing component
- the lower component may be in a relatively dry or even anhydrous condition, e.g. being in the form of an anhydrous film. Being in anhydrous form has a number of benefits, including improved storage stability of the enzyme. In addition it is possible for the enzyme to be present in activated condition, as will be discussed below.
- the lower component may be either water soluble or water permeable in order that it can effectively deliver its enzyme payload.
- the lower component be a hydrophilic, film-forming material.
- the lower component is not susceptible to rapid enzyme activity as this allows preparation via a drying step to be employed.
- water soluble film-forming polymers examples include those listed in US 5,206,026, column 3 lines 19-27.
- a preferred material for the first layer is polyvinyl alcohol (PVA).
- PVA is commercially available in a range of different grades of different chain length and molecular weight and water solubility, some of which are water soluble and some of which are water insoluble but water permeable.
- Other preferred materials are hyaluronic acid, a long chain water soluble film-forming polymer, polyvinylpolypyrrolidone, polymeric celluloses, e.g. hydroxypropyl cellulose and hydroxypropyl methyl cellulose, carbomers and the like.
- Proteolytic enzymes are able to hydrolyse peptide amide bonds and so are capable of digesting dead skin, such as necrotic skin.
- Suitable enzymes are well-known to a person skilled in the art.
- suitable enzymes for use in accordance with the invention include papain, trypsin, chymo-trypsin, streptokinase, streptodormase, ficin, pepsin, carboxypeptidase, aminopeptidase, chymopapain, bromelin, collagenase, lysozyme, fibrinolysin, n-acetyl cysteine.
- a mixture of enzymes may be used.
- the enzyme comprises papain.
- papain In its native state, papain may exist in a relatively inactive form. This is due to its free sulfhydryl group being oxidised. To fully activate the papain, the sulfhydryl group requires mild reduction.
- papain may be present in active or inactive form, but is conveniently pre-activated by pre-treatment with a reducing agent, e.g. cysteine.
- the enzyme can get to work on the skin to be treated in a short timescale. However it is believed that it is better that the enzyme is not deposited instantaneously and instead is delivered in a controlled manner over a period of minutes. This is believed to give the optimal balance between effective earlier enzyme action and not over-dosing the skin at the initial stage. Thus it is preferred that, in use, 90% of the available enzyme is delivered to the skin within 2 hours of application, preferably between 1 minute and 1 hour, more preferably between 2 minutes and 30 minutes.
- the types and amounts of the enzymes can be readily determined by experiment according to the desired application.
- the upper degradable component is
- the upper component is initially impermeable to both fluids and the proteolytic enzyme, thus acting as a barrier keeping the wound moist and rich in enzyme e.g. in order to maximise the process of autolytic debridement.
- the upper component physically degrades to allow the passage of fluids and enzyme away from the wound site. This provides further benefit to the wound and assists in the healing process.
- the appropriate length of time required for enzyme function, and hence the time delay before completion of degradation of the upper component, will depend on factors including the nature of treatment required, as will be known to those skilled in the art.
- typical enzyme treatment times are up to 24 hours, typically from 12-24 or from 18-24 hours.
- Dressings may be tailored to provide the desired time delay before completion of degradation.
- the time taken for initial degradation to occur, to result in the upper component being permeable to the passage of fluids, may be significantly less than this, for example, 1-4 hours.
- the enzyme will typically continue to provide benefit to the wound over time periods mentioned above.
- the upper component degrades by being digested by the proteolytic enzyme.
- the time taken to reach the stage of allowing the passage of fluids and enzyme is dependent upon e.g. the thickness of the component, the availability of water and the ability of the enzyme to digest the component.
- Suitable materials for this purpose are proteins which are not cold water soluble, susceptible to protease action, e.g. gelatin and collagen.
- the upper component may also be cross-linked using techniques readily available to a person skilled in the art. It will be apparent that the upper degradable layer could eventually degrade completely and no longer be present as a distinct entity.
- dressings of the present invention may be combined with an absorbent component, typically covering the dressing so as to be further away from the wound than the dressing.
- an absorbent layer can conveniently absorb said fluids.
- Any suitable absorbent material may be used and can be a simple bandage material, cotton wool or other conventional absorbent material.
- a further benefit of the present invention is the possibility of including materials which can be tailored to provide an ideal environment for the enzymes to do their job of skin digestion.
- Preferred enhancing materials include, but are not limited to, urea, hyaluronic acid, glycerol, antimicrobial agents (e.g. chlorhexadine), anaesthetic agents, wound healing agents, enzyme activators (e.g. cysteine). These may be present in either or both of the upper and lower components.
- antimicrobial agents e.g. chlorhexadine
- anaesthetic agents e.g. cysteine
- wound healing agents e.g. cysteine
- a separate conditioning fluid e.g. a sterile aqueous fluid
- a separate conditioning fluid e.g. a sterile aqueous fluid
- a fluid could be used to prepare a wound, either a moist or a dry necrotic wound, so that when the dressing is applied to the wound, the enzyme is released into an improved aqueous environment.
- Urea is particularly beneficial because it helps to prepare the target proteins for digestion by the enzyme.
- urea is preferably not present in the lower component because it is known that it can denature enzymes over time. Urea may therefore conveniently be located in the conditioning fluid discussed above.
- Dressings according to the invention may also comprise an ionising radiation protection system, as described in co-pending application EP 05255825.1. They may also comprise a thermal stability system as described in co-pending application GB 0513653.6.
- a dressing of the present invention comprises a layered construction comprising a lower component comprised of dry polyvinylalcohol containing papain as the enzyme (e.g. a mixture of papain and cysteine activator) and an upper component made of gelatin.
- a lower component comprised of dry polyvinylalcohol containing papain as the enzyme (e.g. a mixture of papain and cysteine activator)
- an upper component made of gelatin.
- the dressing is preferably in the form of a wound debridement dressing.
- the dressing of the invention finds particular application in wound debridement and can be used in treatment of a wide range of wounds and conditions where debridement is beneficial, including exuding wounds and those exhibiting dry necrosis.
- the dressings are used by being applied to an area of skin to be treated, for therapeutic purposes.
- the lower component can be selectively applied only to a wound area, e.g. with a sheet of material being cut to a suitable size and shape.
- the upper component can then be applied, possibly followed by an optional outer absorbent material.
- the dressing may be applied in a constructed, laminated form whereby the upper and lower components are pre-assembled prior to use.
- This may be conveniently achieved as an all-in-one dressing, which may also include an absorbent outer layer as part of the complete dressing.
- the dressings of the present invention only need to be in place for a short period of time, for example less than 24 hours. This is because the dressing is intended to deliver the enzyme quickly and effectively so that it can get to work on the wound.
- a second dressing of the invention can be used to deliver a fresh amount of effective enzyme. This cycle of treatment can be continued for as long as is required.
- the components of the dressings in accordance with the invention may each be suitably supplied in respective sterile, sealed, water-impervious packages, e.g. laminated aluminium foil pouches.
- a pre-assembled dressing comprising both upper and lower components in laminated form, optionally including an absorbent layer, which may also be adhesive to attach to skin, can be supplied in a sterile, sealed, water-impervious package.
- the components of the dressing in accordance with the invention can be manufactured in a range of different sizes and shapes for treatment of areas of skin, e.g. wounds, of different sizes and shapes.
- the invention also provides a kit comprising a lower component and an upper component each sealed in a respective package.
- PVA poly vinyl alcohol (Sigma- Aldrich 363073 (31-50K molecular weight, 87-89% hydrolysed)) was dissolved to 5%w/w in water (analytical grade, supplier Fisher).
- Papain was prepared as follows: papain powder (Biocatalysts (Promod 144P, 700TU)) was dissolved to 200mg/g in analytical water. This solution was then buffer exchanged into analytical water using a PDlO column (Amersham Biosciences 17-0851-01). The final concentration of papain powder was 140mg/g.
- the PVA and papain stock solutions were mixed 50:50. Prior to mixing, the papain was first activated. To achieve this, lOOmg of L-cysteine (Fluka,
- the papain/cysteine mix can either be used directly, or heated to 6O 0 C for lOmins (which allows for a greater recovery of activity after drying). If a softer film was required, glycerol was included at l%w/w. Film thickness was varied, depending on the quantity of PVA/papain mix used. Typically, a minimum of 7.Og of the mix was prepared, and poured into a suitable flat dish (e.g. 8.3cm diameter petri dish, surface area of 54cm 2 ). The mix was placed in an incubator, at 4O 0 C, for at least 3 hours, or until the film had dried.
- a suitable flat dish e.g. 8.3cm diameter petri dish, surface area of 54cm 2 .
- Gelatin (Sigma, Gl 890, porcine origin) was dissolved at elevated temperature into analytical water to a final concentration of 10% w/w. Glycerol was added if required, typically to a final concentration of 5% w/w. Gelatin films were then prepared by pouring either 9g of the molten gelatin/glycerol into 8.3cm diameter petri dishes or a range of weights into 10cm x 10cm square petri dishes, and drying at 4O 0 C overnight (or until the sheets were dry).
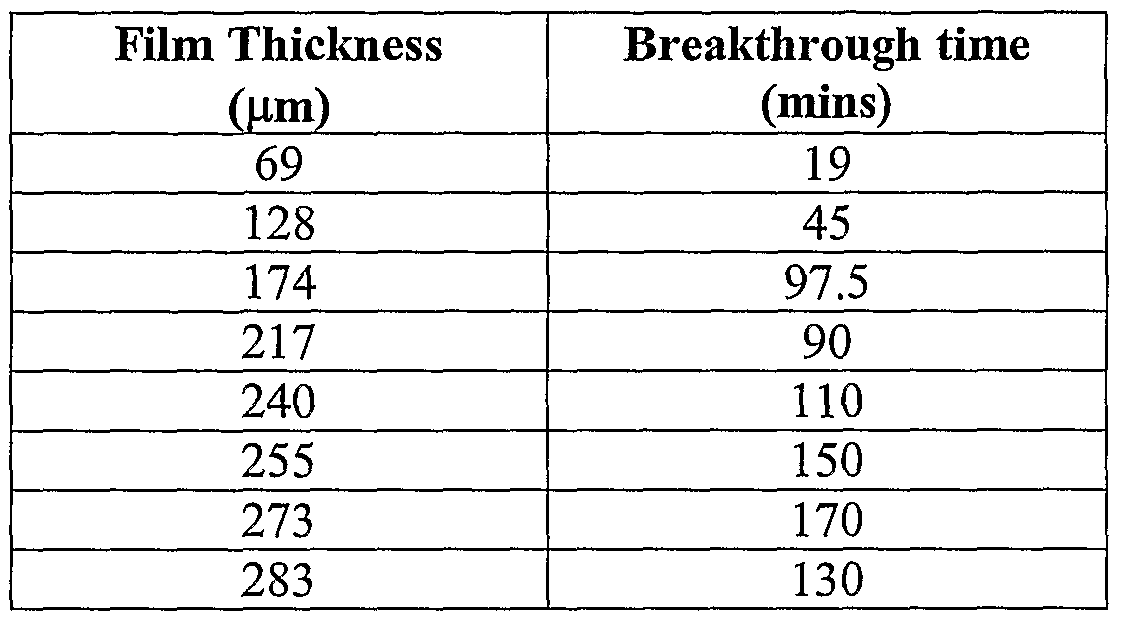
- Gelatin was prepared as described above. To form different thickness of films, the molten gelatin/glycerol solution was poured into 10cm x 10cm petri dishes, in the range of 5-20 grams, in 2.5g increments. The dishes were cooled on a level bench before being placed into a 4O 0 C oven, overnight. The gelatin sheets were cut into 2cm x 2cm sections. These were placed onto white tissue paper. A 10mg/ml papain solution was prepared (papain powder (Biocatalysts (Promod 144P, 700TU)) in analytical water and activated with cysteine. 30 microlitres of papain was applied to the gelatin surfaces, and a further 30 microlitres re-applied every 30mins. A control experiment was performed in parallel, applying PBS (Sigma) instead of papain. The time taken for the fluid to break through onto the tissue paper as a function of the original casting weight was recorded and is shown in Table 1.
- PBS Sigma
- the gelatin sheet was not breached when PBS was used as the wetting agent.
- the time taken for the gelatin sheet to allow flow of fluid was controlled by (i) the introduction of papain and (ii) the thickness of the gelatin sheet.
- the PVA/papain + gelatin/glycerol stacked sheet system demonstrated the ability to (i) prevent fluid flow to an absorbent material due to the presence of the gelatin sheet, and (ii) release activated papain from a dry sheet, that then degraded the gelatin sheet to allow fluid flow.
- PVA Polyvinyl Alcohol
- H 2 O analytical grade
- Papain enzyme powder supplied by Biocatalysts, product code Promod 144P, 700TU
- Glycerol supplied by Biocatalysts, product code Promod 144P, 700TU
- Glycerol supplied by Biocatalysts, product code Promod 144P, 700TU
- Glycerol supplied by Biocatalysts, product code Promod 144P, 700TU
- Glycerol supplied to the PVA/papain mixture.
- the final solution had the following formula: 9wt% PVA, 36wt% H 2 O, 40wt% Papain powder and 15wt% Glycerol.
- a second solution was prepared with gelatin (supplied by Sigma, code Gl 890) dissolved in H 2 O (analytical grade) to 10% w/w, with glycerol added to 5% w/w.
- gelatin supplied by Sigma, code Gl 890
- H 2 O analytical grade
- glycerol added to 5% w/w.
- a laminated film featuring a layer of dry gelatin/glycerol film and a further layer of dry PVA/papain/glycerol film was prepared in two ways.
- Gelatin/glycerol was cast at a suitable coat weight and air dried at 40°C to give a film of approximately 400 ⁇ m. This may be increased or decreased as desired, depending on the time required for the film to dissolve.
- the PVA/papain/glycerol solution was cast at a suitable coat weight and air dried to give a film of approximately 200 ⁇ m. This may also be increased or decreased as desired, depending on the quantity of papain that is required to be delivered.
- the separate dried films are subsequently bought into contact with each other, where under suitable pressure, they adhere together. Because the films are dry, the papain will not degrade the gelatin sheet during subsequent storage.
- the laminated sheet can then be further processed, for example cut into any shape required (square, rectangular, circular, ovoid etc).
- the sheets may then be stored, preferably under controlled, low humidity or dry (desiccated) conditions where the water content of the films is below that required for activation of the papain.
- the gelatin/glycerol solution was cast at a suitable coat weight and air dried at 40 0 C to give a film with a thickness of approximately 400 ⁇ m. This may be higher or lower, depending on the time required for the film to dissolve.
- PVA/papain/glycerol solution was then cast directly onto the gelatin sheet, at a suitable coat weight to give a dried PVA/papain/glycerol film thickness of approximately 200 ⁇ m. This may also be increased or decreased, depending on the quantity of papain that is required to be delivered.
- the wet PVA/papain/glycerol layer was then air dried quickly (within minutes of application, using film drying techniques as apparent to those skilled in the art) to yield a laminated sheet constructed of a layer of gelatin/glycerol and a further layer of PVA/papain/glycerol. Because the sheet was dry, the papain did not degrade the gelatin during storage.
- the laminated sheet can then be further processed, for example cut into any shape required (square, rectangular, circular, ovoid etc).
- the sheets may then be stored, preferably under controlled, low humidity or dry (desiccated) conditions where the water content of the films is below that required for activation of the papain.
- Example 5 Example 5:
- the final formulation was 22wt% PVA, 57wt% papain powder and 21wt% glycerol.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- Vascular Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Hematology (AREA)
- Materials Engineering (AREA)
- Epidemiology (AREA)
- Medicinal Preparation (AREA)
- Materials For Medical Uses (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
A skin dressing for delivery of proteolytic enzyme to skin, comprising a lower dressing component comprising releasable proteolytic enzyme and an upper dressing component which is impermeable to the passage of fluid and the proteolytic enzyme and is degradable by being digested by the enzyme following application of the dressing onto a moist wound site, to allow the passage of fluids and the proteolytic enzyme away from the wound site is provided.
Description
Skin Dressinfi
TECHNICAL FIELD
The present invention relates to a skin dressing for delivery of proteolytic enzyme to skin e.g. to aid removal of dead and necrotic skin for therapeutic purposes, particularly in the form of wound debridement dressings.
BACKGROUND AND PRIOR ART
Debridement of necrotic tissue from e.g. burns and traumatic wounds is an essential step before the wound can begin to heal effectively. Enzymatic debridement using proteolytic enzymes is a convenient method of removing the tissue because it enhances the body's natural autolytic debridement process (whereby endogenous enzymes and leukocytes clean up the dead tissue) and does not require mechanical or surgical intervention. Commercial debridement products are traditionally available in the form of creams, sprays, lotions or ointments. With these product forms only a fraction of the total applied enzyme actually reaches the necrotic tissue at any one time and are often painful to apply. Wounds in need of debridement are often sore and painful, so it is important that the medium in which the enzymes are applied is comforting and able to minimise pain. Additionally, such known products are often not sterile because known sterilisation processes kill off the enzyme. A more convenient product form for enzymatic debridement is a dressing, however this presents significant technical difficulties.
Enzymatic debridement dressings are known in the art. However, the manufacture of a dressing which can deliver an effective enzyme dose in the right time frame and in the right condition is not a simple matter. A particular difficulty arises from the management of water: proteolytic enzymes require the presence of water in order to perform their function, yet the same water can cause the enzyme to deactivate over a few hours due to such enzymes
digesting themselves. Several technical solutions have been suggested which overcome this technical dilemma.
US 6,043,407 solves the problem by providing an anhydrous debridement pad which requires rehydration in sterile water before application to the wound. Such a technique suffers from the inconvenience of waiting for the pad to hydrate.
A common technical solution in the prior art is to maintain the dressing in an anhydrous state and rely on the wound itself to supply the moisture to activate the enzyme.
US 4,668,228 discloses a debriding tape comprising a polyurethane film and an adhesive layer. The adhesive layer contains the proteolytic enzyme in dry or powdered form. The tape does not release enzyme in a sustained manner. Instead most or all of the enzyme is applied to the skin surface all at once with moisture from a wound activating the enzyme.
US 5,206,026 discloses a dry enzymatic debridement film which gives immediate release of enzyme by dissolution of the film in wound exudate. It is intended to be used in conjunction with conventional dressings.
US 6,548,556 discloses a wound debrider comprising anhydrous hydrophilic poloxamer carrier and proteolytic enzymes. The dry environment keeps the enzyme stable and the moisture is supplied by wound exudate.
WO 2005/035010 discloses a dressing having a polymeric layer comprising proteolytic enzymes which are released over a prolonged period of time. The dressing may be multi- layered with a first degradable polymeric wound contacting layer and a second absorbent layer. The absorbent layer may be a hydrogel. The water for activation of the enzyme comes from wound exudate. The dressing provided sustained release of enzymes, optionally with a "burst" at the beginning.
Although provision of an absorbing layer helps in the removal of wound fluids, liquefied slough and degraded necrotic tissue, it can also result in absorption of enzyme from the wound before it has performed its function, thus reducing the effectiveness of the dressing.
SUMMARY OF INVENTION
The invention provides a skin dressing for delivery of proteolytic enzyme to skin, comprising a lower dressing component comprising releasable proteolytic enzyme and an upper dressing component which is impermeable to the passage of fluid and the proteolytic enzyme and is degradable by being digested by the enzyme, following application of the dressing onto a moist wound site, to allow the passage of fluids and the proteolytic enzyme away from the wound site.
Thus, on application to a moist wound the lower dressing component releases its enzyme into the wound site e.g. to perform a debridement function. The enzyme and moisture in the wound are prevented from leaving the wound site by the presence of the upper dressing component. After a period of time the upper component degrades with the aid of enzyme action, to allow the enzyme and wound fluid to leave the wound site, optionally being absorbed by an absorbing dressing material possibly forming part of the dressing.
Thus the dressing provides enzyme to the skin e.g. to a wound site and allows it to remain on the skin, e.g. in the wound site, for an appropriate time to perform a desired function, e.g. provide an effective debridement function, whilst also subsequently providing for removal of fluids e.g. including wound exudate, liquefied slough and degraded necrotic tissue after an appropriate period of time as is discussed below.
The moisture in the wound site may be purely wound exudate, however it is preferred that the wound site is first moistened with a separate sterile aqueous fluid. In this manner, the invention is equally applicable to naturally moist wounds as well as naturally dry wounds e.g. dry necrotic wounds, provided such dry wounds are suitably moistened before application of the dressing.
Structure
The dressing is preferably of layered construction with each dressing component in the form of a layer, wherein in use, the lower component is in contact or near to the skin, and the upper component is remote from the skin surface.
The lower and/or upper components may be in the form of a solid layer, sheet, slab or film of material. The size and shape of the layer, sheet, slab or film can be selected to suit the intended use of the dressing. Thicknesses in the range 0.01 to 1.0 mm, preferably 0.05 to 0.5 mm are particularly suitable.
The lower enzyme-containing component
The lower component may be in a relatively dry or even anhydrous condition, e.g. being in the form of an anhydrous film. Being in anhydrous form has a number of benefits, including improved storage stability of the enzyme. In addition it is possible for the enzyme to be present in activated condition, as will be discussed below.
The lower component may be either water soluble or water permeable in order that it can effectively deliver its enzyme payload.
It is preferable that the lower component be a hydrophilic, film-forming material. Desirably the lower component is not susceptible to rapid enzyme activity as this allows preparation via a drying step to be employed.
Examples of water soluble film-forming polymers include those listed in US 5,206,026, column 3 lines 19-27. A preferred material for the first layer is polyvinyl alcohol (PVA). PVA is commercially available in a range of different grades of different chain length and molecular weight and water solubility, some of which are water soluble and some of which are water insoluble but water permeable. Other preferred materials are hyaluronic acid, a long chain water soluble film-forming polymer, polyvinylpolypyrrolidone, polymeric
celluloses, e.g. hydroxypropyl cellulose and hydroxypropyl methyl cellulose, carbomers and the like.
Enzymes
Proteolytic enzymes are able to hydrolyse peptide amide bonds and so are capable of digesting dead skin, such as necrotic skin. Suitable enzymes are well-known to a person skilled in the art. Examples of suitable enzymes for use in accordance with the invention include papain, trypsin, chymo-trypsin, streptokinase, streptodormase, ficin, pepsin, carboxypeptidase, aminopeptidase, chymopapain, bromelin, collagenase, lysozyme, fibrinolysin, n-acetyl cysteine. A mixture of enzymes may be used. Preferably the enzyme comprises papain.
In its native state, papain may exist in a relatively inactive form. This is due to its free sulfhydryl group being oxidised. To fully activate the papain, the sulfhydryl group requires mild reduction.
In embodiments where the lower component is in anhydrous condition papain may be present in active or inactive form, but is conveniently pre-activated by pre-treatment with a reducing agent, e.g. cysteine.
It is beneficial if the enzyme can get to work on the skin to be treated in a short timescale. However it is believed that it is better that the enzyme is not deposited instantaneously and instead is delivered in a controlled manner over a period of minutes. This is believed to give the optimal balance between effective earlier enzyme action and not over-dosing the skin at the initial stage. Thus it is preferred that, in use, 90% of the available enzyme is delivered to the skin within 2 hours of application, preferably between 1 minute and 1 hour, more preferably between 2 minutes and 30 minutes.
The types and amounts of the enzymes can be readily determined by experiment according to the desired application.
The upper degradable component
The upper component is initially impermeable to both fluids and the proteolytic enzyme, thus acting as a barrier keeping the wound moist and rich in enzyme e.g. in order to maximise the process of autolytic debridement.
After a time, once the enzyme has had an opportunity to perform a desired function e.g. debride the wound, the upper component physically degrades to allow the passage of fluids and enzyme away from the wound site. This provides further benefit to the wound and assists in the healing process.
The appropriate length of time required for enzyme function, and hence the time delay before completion of degradation of the upper component, will depend on factors including the nature of treatment required, as will be known to those skilled in the art. For wound debridement, typical enzyme treatment times are up to 24 hours, typically from 12-24 or from 18-24 hours. Dressings may be tailored to provide the desired time delay before completion of degradation.
However, the time taken for initial degradation to occur, to result in the upper component being permeable to the passage of fluids, may be significantly less than this, for example, 1-4 hours. Following an initial degradation, the enzyme will typically continue to provide benefit to the wound over time periods mentioned above.
As discussed, the upper component degrades by being digested by the proteolytic enzyme. The time taken to reach the stage of allowing the passage of fluids and enzyme is dependent upon e.g. the thickness of the component, the availability of water and the ability of the enzyme to digest the component. Suitable materials for this purpose are proteins which are not cold water soluble, susceptible to protease action, e.g. gelatin and collagen. The upper component may also be cross-linked using techniques readily available to a person skilled in the art.
It will be apparent that the upper degradable layer could eventually degrade completely and no longer be present as a distinct entity.
Optional absorbent component
Optionally, dressings of the present invention may be combined with an absorbent component, typically covering the dressing so as to be further away from the wound than the dressing.
Once the dressing begins to allow fluid and/or enzyme to pass upwards away from the wound, an absorbent layer can conveniently absorb said fluids.
Any suitable absorbent material may be used and can be a simple bandage material, cotton wool or other conventional absorbent material.
Additional materials
A further benefit of the present invention is the possibility of including materials which can be tailored to provide an ideal environment for the enzymes to do their job of skin digestion.
Preferred enhancing materials include, but are not limited to, urea, hyaluronic acid, glycerol, antimicrobial agents (e.g. chlorhexadine), anaesthetic agents, wound healing agents, enzyme activators (e.g. cysteine). These may be present in either or both of the upper and lower components.
Additionally, a separate conditioning fluid, e.g. a sterile aqueous fluid, may be provided which may contain any of the above mentioned materials. Such a fluid could be used to prepare a wound, either a moist or a dry necrotic wound, so that when the dressing is applied to the wound, the enzyme is released into an improved aqueous environment.
Urea is particularly beneficial because it helps to prepare the target proteins for digestion by the enzyme. However urea is preferably not present in the lower component because it is
known that it can denature enzymes over time. Urea may therefore conveniently be located in the conditioning fluid discussed above.
Dressings according to the invention may also comprise an ionising radiation protection system, as described in co-pending application EP 05255825.1. They may also comprise a thermal stability system as described in co-pending application GB 0513653.6.
Preferred embodiments
In one preferred embodiment, a dressing of the present invention comprises a layered construction comprising a lower component comprised of dry polyvinylalcohol containing papain as the enzyme (e.g. a mixture of papain and cysteine activator) and an upper component made of gelatin.
The dressing is preferably in the form of a wound debridement dressing.
Treatment regime
The dressing of the invention finds particular application in wound debridement and can be used in treatment of a wide range of wounds and conditions where debridement is beneficial, including exuding wounds and those exhibiting dry necrosis.
The dressings are used by being applied to an area of skin to be treated, for therapeutic purposes. The lower component can be selectively applied only to a wound area, e.g. with a sheet of material being cut to a suitable size and shape. The upper component can then be applied, possibly followed by an optional outer absorbent material.
Alternatively, the dressing may be applied in a constructed, laminated form whereby the upper and lower components are pre-assembled prior to use. This may be conveniently achieved as an all-in-one dressing, which may also include an absorbent outer layer as part of the complete dressing.
Because of the rapid delivery of enzyme the dressings of the present invention only need to be in place for a short period of time, for example less than 24 hours. This is because the dressing is intended to deliver the enzyme quickly and effectively so that it can get to work on the wound. If necessary, a second dressing of the invention can be used to deliver a fresh amount of effective enzyme. This cycle of treatment can be continued for as long as is required.
Packaging and form
The components of the dressings in accordance with the invention may each be suitably supplied in respective sterile, sealed, water-impervious packages, e.g. laminated aluminium foil pouches. Alternatively a pre-assembled dressing comprising both upper and lower components in laminated form, optionally including an absorbent layer, which may also be adhesive to attach to skin, can be supplied in a sterile, sealed, water-impervious package.
The components of the dressing in accordance with the invention can be manufactured in a range of different sizes and shapes for treatment of areas of skin, e.g. wounds, of different sizes and shapes.
The invention also provides a kit comprising a lower component and an upper component each sealed in a respective package.
The invention will be further described, by way of illustration, in the following examples.
Examples:
Preparation of a typical PVA film containing papain
PVA (poly vinyl alcohol (Sigma- Aldrich 363073 (31-50K molecular weight, 87-89% hydrolysed)) was dissolved to 5%w/w in water (analytical grade, supplier Fisher). Papain was prepared as follows: papain powder (Biocatalysts (Promod 144P, 700TU)) was dissolved to 200mg/g in analytical water. This solution was then buffer exchanged into
analytical water using a PDlO column (Amersham Biosciences 17-0851-01). The final concentration of papain powder was 140mg/g.
To make a high activity papain film, the PVA and papain stock solutions were mixed 50:50. Prior to mixing, the papain was first activated. To achieve this, lOOmg of L-cysteine (Fluka,
30090) was added per 3.5g of the papain solution and allowed to dissolve. The papain/cysteine mix can either be used directly, or heated to 6O0C for lOmins (which allows for a greater recovery of activity after drying). If a softer film was required, glycerol was included at l%w/w. Film thickness was varied, depending on the quantity of PVA/papain mix used. Typically, a minimum of 7.Og of the mix was prepared, and poured into a suitable flat dish (e.g. 8.3cm diameter petri dish, surface area of 54cm2). The mix was placed in an incubator, at 4O0C, for at least 3 hours, or until the film had dried.
To prepare a low activity film, the method above was repeated, but the quantity of papain added was reduced as necessary with de-ionised water added to compensate.
Preparation of a typical gelatin film
Gelatin (Sigma, Gl 890, porcine origin) was dissolved at elevated temperature into analytical water to a final concentration of 10% w/w. Glycerol was added if required, typically to a final concentration of 5% w/w. Gelatin films were then prepared by pouring either 9g of the molten gelatin/glycerol into 8.3cm diameter petri dishes or a range of weights into 10cm x 10cm square petri dishes, and drying at 4O0C overnight (or until the sheets were dry).
Example 1:
Demonstration of the effect of papain solution on the dissolution of gelatin sheets of varying thicknesses
1) Gelatin was prepared as described above. To form different thickness of films, the molten gelatin/glycerol solution was poured into 10cm x 10cm petri dishes, in the range of 5-20 grams, in 2.5g increments. The dishes were cooled on a level bench before being placed into a 4O0C oven, overnight.
The gelatin sheets were cut into 2cm x 2cm sections. These were placed onto white tissue paper. A 10mg/ml papain solution was prepared (papain powder (Biocatalysts (Promod 144P, 700TU)) in analytical water and activated with cysteine. 30 microlitres of papain was applied to the gelatin surfaces, and a further 30 microlitres re-applied every 30mins. A control experiment was performed in parallel, applying PBS (Sigma) instead of papain. The time taken for the fluid to break through onto the tissue paper as a function of the original casting weight was recorded and is shown in Table 1.
Table 1
The gelatin sheet was not breached when PBS was used as the wetting agent. The time taken for the gelatin sheet to allow flow of fluid was controlled by (i) the introduction of papain and (ii) the thickness of the gelatin sheet.
2) Gelatin sheets were prepared as described above and cast at varying weights. Prior to use, the films were cut into circles of 3.8cm diameter and weighed. To each film circle, 200 microlitres of either 10mg/ml papain solution (as in example (I)) or PBS was added, and the time taken for fluid break through to occur measured. This is shown in Table 2.
Table 2
A very good linear relationship between film thickness and breakthrough time was demonstrated. The time taken for breakthrough also decreased, with the increase in papain quantity applied to the gelatin/glycerol sheets.
Example 2:
Demonstration of the effect of papain delivered from a dry film on the dissolution of gelatin sheets of varying thicknesses
20% PVA (supplied by Sigma-Aldrich, code 363073, 31-50K MW and 87-89% hydrolysis) was prepared in H2O (analytical grade). Papain enzyme powder (supplied by Biocatalysts, product code Promod 144P, 700TU) was dissolved in H2O (analytical grade) to give a 200mg/g solution. This was subsequently buffer exchanged using a PDlO column (supplied by Amersham) to yield a 140mg/ml solution in H2O. 3.05g 20% PVA was mixed with 12.2g exchanged papain solution and 0.6g glycerol and 9.15g H2O. When thoroughly mixed, 12g was poured into a 10x10cm plate and the film dried at 40°C until dry (minimum of 2 hours). The final formulation was 22wt% PVA, 57wt% papain powder and 21wt% glycerol. A 10% gelatin + 5% glycerol solution, as described in example 4, was prepared and poured into several different 10x10cm dishes to provide a range of dry film thicknesses. The films were dried at 400C until dry. 1.5cm x 1.5cm PVA/papain/glycerol sections were placed on top of 3cm x 3cm sections of the gelatin/glycerol sheets. 40μl PBS was applied to dissolve and activate the papain sheet. Every 20mins a further 40μl PBS was applied. The time taken for fluid to breakthrough the gelatin sheet (as a marker for gelatin digestion) was recorded.
Table 3
From Table 3, as the gelatin/glycerol film thickness increases, the time required for the papain to digest the film also increases. The quantity of papain added per experiment was kept constant, thus the difference in time taken to digest through the gelatin/glycerol film is a function of the film thickness.
The PVA/papain + gelatin/glycerol stacked sheet system, demonstrated the ability to (i) prevent fluid flow to an absorbent material due to the presence of the gelatin sheet, and (ii) release activated papain from a dry sheet, that then degraded the gelatin sheet to allow fluid flow.
Example 3: Laminated Gelatin and Enzyme Layer
Polyvinyl Alcohol (PVA, supplied by Sigma-Aldrich, code 363073, 31-50K MW and 87- 89% hydrolysis) was dissolved into H2O (analytical grade) to 20% w/w. Papain enzyme powder (supplied by Biocatalysts, product code Promod 144P, 700TU) was added to the 20% PVA solution with constant stirring and allowed to dissolve. Glycerol (supplier Fisher) was further added to the PVA/papain mixture. The final solution had the following formula: 9wt% PVA, 36wt% H2O, 40wt% Papain powder and 15wt% Glycerol.
A second solution was prepared with gelatin (supplied by Sigma, code Gl 890) dissolved in H2O (analytical grade) to 10% w/w, with glycerol added to 5% w/w.
A laminated film featuring a layer of dry gelatin/glycerol film and a further layer of dry PVA/papain/glycerol film was prepared in two ways.
1) Gelatin/glycerol was cast at a suitable coat weight and air dried at 40°C to give a film of approximately 400μm. This may be increased or decreased as desired, depending on the time required for the film to dissolve. The PVA/papain/glycerol solution was cast at a suitable coat weight and air dried to give a film of approximately 200μm. This may also be increased or decreased as desired, depending on the quantity of papain that is required to be delivered. The separate dried films are subsequently bought into contact with each other, where under suitable pressure, they adhere together. Because the films are dry, the papain will not degrade the gelatin sheet during subsequent storage. The laminated sheet can then be further processed, for example cut into any shape required (square, rectangular, circular, ovoid etc). The sheets may then be stored, preferably under controlled, low humidity or dry (desiccated) conditions where the water content of the films is below that required for activation of the papain.
2) The gelatin/glycerol solution was cast at a suitable coat weight and air dried at 400C to give a film with a thickness of approximately 400μm. This may be higher or lower, depending on the time required for the film to dissolve. PVA/papain/glycerol solution was then cast directly onto the gelatin sheet, at a suitable coat weight to give a dried PVA/papain/glycerol film thickness of approximately 200μm. This may also be increased or decreased, depending on the quantity of papain that is required to be delivered. The wet PVA/papain/glycerol layer was then air dried quickly (within minutes of application, using film drying techniques as apparent to those skilled in the art) to yield a laminated sheet constructed of a layer of gelatin/glycerol and a further layer of PVA/papain/glycerol. Because the sheet was dry, the papain did not degrade the gelatin during storage. The laminated sheet can then be further processed, for example cut into any shape required (square, rectangular, circular, ovoid etc). The sheets may then be stored, preferably under controlled, low humidity or dry (desiccated) conditions where the water content of the films is below that required for activation of the papain.
Example 5:
Effect of Gelatin Film Thickness and Dissolution Time
20% PVA (supplied by Sigma-Aldrich, code 363073, 31-50K MW and 87-89% hydrolysis) was prepared in H2O (analytical grade). Papain enzyme powder (supplied by Biocatalysts, product code Promod 144P, 700TU) was dissolved in H2O (analytical grade) to give a
200mg/ml solution. This was subsequently buffer exchanged using a PDlO column (supplied by Amersham) to yield a 140mg/ml solution in H2O. 3.05g 20% PVA was mixed with 12.2g exchanged papain solution and 0.6g glycerol and 9.15g H2O. When thoroughly mixed, 12g was poured into a 10x1 Ocm plate and the film dried at 40°C until dry (minimum of 2 hours).
The final formulation was 22wt% PVA, 57wt% papain powder and 21wt% glycerol. A 10% gelatin + 5% glycerol solution, as described in example 4, was prepared and poured into several different 10x10cm dishes to provide a range of dry film thicknesses. The films were dried at 40°C until dry. 1.5cm2 PVA/papain/glycerol sections were placed on top of 3cm2 sections of the gelatin/glycerol sheets. 40μl PBS was applied to dissolve and activate the papain sheet. Every 20mins a further 40μl PBA was applied. The time taken for fluid to breakthrough the gelatin sheet (as a marker for gelatin digestion) was recorded.
Table 3
As seen in Table 3, as the gelatin/glycerol film thickness increases, the time required for the papain to digest the film also increases. The quantity of papain added per experiment was kept constant, thus the difference in time taken to digest through the gelatin/glycerol film is a function of the film thickness.
Claims
1. A skin dressing for delivery of proteolytic enzyme to skin, comprising a lower dressing component comprising releasable proteolytic enzyme and an upper dressing component which is impermeable to the passage of fluid and the proteolytic enzyme and is degradable by being digested by the enzyme, following application of the dressing onto a moist wound site, to allow the passage of fluids and the proteolytic enzyme away from the wound site.
2. A dressing according to claim 1, which is in the form of a wound debridement dressing.
3. A dressing according to claim 1 or claim 2, which is of layered construction with each dressing component in the form of a layer.
4. A dressing according to any one preceding claim, wherein the lower component is in anhydrous condition.
5. A dressing according to any one preceding claim, wherein the lower component comprises polyvinyl alcohol.
6. A dressing according to any one preceding claim, wherein the enzyme comprises papain.
7. A dressing according to any one preceding claim, wherein the upper component comprises gelatin.
8. A dressing according to any one preceding claim, which is covered with an absorbent component.
9. A dressing according to any one preceding claim, which is supplied with a separate conditioning fluid.
10. A dressing according to any preceding claim, wherein the upper and lower components are each sealed in a respective package.
11. A dressing according to any one of claims 1 to 9, wherein the upper and lower components are laminated together and sealed in a single package.
12. A dressing according to claim 11, wherein the dressing includes an absorbent layer and optionally an adhesive surface for attachment to the skin.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0606921.5 | 2006-04-06 | ||
| GB0606921A GB0606921D0 (en) | 2006-04-06 | 2006-04-06 | Skin dressing |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2007128985A2 true WO2007128985A2 (en) | 2007-11-15 |
| WO2007128985A3 WO2007128985A3 (en) | 2008-05-15 |
Family
ID=36539434
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/GB2007/001309 WO2007128985A2 (en) | 2006-04-06 | 2007-04-05 | Skin dressing |
Country Status (2)
| Country | Link |
|---|---|
| GB (1) | GB0606921D0 (en) |
| WO (1) | WO2007128985A2 (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014150857A1 (en) * | 2013-03-15 | 2014-09-25 | Smith & Nephew, Inc. | Dissolvable gel-forming film for delivery of active agents |
| WO2017140017A1 (en) * | 2016-02-20 | 2017-08-24 | 深圳市圣必智科技开发有限公司 | Wound hemostatic device having repeated drug administration function |
| CN109260465A (en) * | 2012-11-14 | 2019-01-25 | 史密夫和内修公司 | Stable thermolysin hydrogel |
| WO2019083827A1 (en) * | 2017-10-24 | 2019-05-02 | Kci Licensing, Inc. | Debridement wound dressings and systems using the same |
| CN111432761A (en) * | 2017-11-03 | 2020-07-17 | 凯希特许有限公司 | Dressing for extending wearing time |
| US12016907B2 (en) | 2022-08-15 | 2024-06-25 | Smith & Nephew, Inc. | Stable thermolysin hydrogel |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4668228A (en) * | 1985-03-12 | 1987-05-26 | Johnson & Johnson Products, Inc. | Debriding tape |
| US5206026A (en) * | 1988-05-24 | 1993-04-27 | Sharik Clyde L | Instantaneous delivery film |
| US20020114798A1 (en) * | 2000-12-27 | 2002-08-22 | Hobson David W. | Stable enzymatic wound debrider |
| WO2004108176A1 (en) * | 2003-06-09 | 2004-12-16 | Insense Limited | Skin dressings containing oxidoreductase enzyme |
| WO2005035010A1 (en) * | 2003-10-10 | 2005-04-21 | Coloplast A/S | Wound dressing containing proteolytic enzymes |
-
2006
- 2006-04-06 GB GB0606921A patent/GB0606921D0/en not_active Ceased
-
2007
- 2007-04-05 WO PCT/GB2007/001309 patent/WO2007128985A2/en active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4668228A (en) * | 1985-03-12 | 1987-05-26 | Johnson & Johnson Products, Inc. | Debriding tape |
| US5206026A (en) * | 1988-05-24 | 1993-04-27 | Sharik Clyde L | Instantaneous delivery film |
| US20020114798A1 (en) * | 2000-12-27 | 2002-08-22 | Hobson David W. | Stable enzymatic wound debrider |
| WO2004108176A1 (en) * | 2003-06-09 | 2004-12-16 | Insense Limited | Skin dressings containing oxidoreductase enzyme |
| WO2005035010A1 (en) * | 2003-10-10 | 2005-04-21 | Coloplast A/S | Wound dressing containing proteolytic enzymes |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109260465A (en) * | 2012-11-14 | 2019-01-25 | 史密夫和内修公司 | Stable thermolysin hydrogel |
| WO2014150857A1 (en) * | 2013-03-15 | 2014-09-25 | Smith & Nephew, Inc. | Dissolvable gel-forming film for delivery of active agents |
| EP3659630A1 (en) * | 2013-03-15 | 2020-06-03 | Smith & Nephew, Inc. | Dissolvable gel-forming film for delivery of active agents |
| US11452698B2 (en) | 2013-03-15 | 2022-09-27 | Smith & Nephew, Inc. | Dissolvable gel-forming film for delivery of active agents |
| WO2017140017A1 (en) * | 2016-02-20 | 2017-08-24 | 深圳市圣必智科技开发有限公司 | Wound hemostatic device having repeated drug administration function |
| WO2019083827A1 (en) * | 2017-10-24 | 2019-05-02 | Kci Licensing, Inc. | Debridement wound dressings and systems using the same |
| CN111447901A (en) * | 2017-10-24 | 2020-07-24 | 凯希特许有限公司 | Debridement wound dressing and system for using same |
| US11850124B2 (en) | 2017-10-24 | 2023-12-26 | 3M Innovative Properties Company | Debridement wound dressings and systems and methods using the same |
| CN111432761A (en) * | 2017-11-03 | 2020-07-17 | 凯希特许有限公司 | Dressing for extending wearing time |
| CN111432761B (en) * | 2017-11-03 | 2022-09-09 | 3M创新知识产权公司 | Dressing for extending wearing time |
| US12016907B2 (en) | 2022-08-15 | 2024-06-25 | Smith & Nephew, Inc. | Stable thermolysin hydrogel |
Also Published As
| Publication number | Publication date |
|---|---|
| GB0606921D0 (en) | 2006-05-17 |
| WO2007128985A3 (en) | 2008-05-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1693073B1 (en) | Wound dressings comprising hydrated hydrogels and enzymes | |
| US4668228A (en) | Debriding tape | |
| US7368128B2 (en) | Controlled release dressing for enzymatic debridement of necrotic and non-viable tissue in a wound | |
| EP2442835B1 (en) | Hemostatic sponge | |
| CA2706828C (en) | Skin dressings generating nitric oxide to improve healing | |
| US20060018955A1 (en) | Method for preparing medical dressings | |
| US20050256437A1 (en) | Absorbent wound dressing containing a hydrogel layer | |
| US20020172709A1 (en) | Medical dressing comprising an antimicrobial silver compound and a method for enhancing wound healing | |
| US6033684A (en) | Compositions and methods for wound management | |
| US5441741A (en) | Freeze-dried pad | |
| JP2005511147A (en) | Controlled release wound dressing | |
| JPH04502569A (en) | Gel formulation for wound treatment | |
| MX2011000131A (en) | Temperature reducing, healing wound dressing. | |
| JP2011067632A (en) | Cushioned adhesive bandage | |
| WO2007128985A2 (en) | Skin dressing | |
| GB2399289A (en) | Hydrogel wound dressing | |
| JP2003225298A (en) | Adhesive plaster | |
| Wang et al. | Tissue adhesives based on chitosan for skin wound healing: Where do we stand in this era? A review | |
| GB2589549A (en) | Composition for delivering nitric oxide to skin | |
| JP2022552825A (en) | Compositions that deliver nitric oxide to the skin | |
| GB2588748A (en) | Composition for delivering nitric oxide to skin | |
| EP2000119A1 (en) | Wound dressings |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 07732353 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 07732353 Country of ref document: EP Kind code of ref document: A2 |